Trillium Therapeutics Inc. - Exhibit 99.2 - Filed by newsfilecorp.com

MANAGEMENT’S DISCUSSION AND ANALYSIS
FOR THE YEARS ENDED
DECEMBER 31, 2018 AND 2017
Dated: March 7, 2019
2488 Dunwin Drive
Mississauga, Ontario, L5L 1J9
www.trilliumtherapeutics.com
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
ABOUT THIS MANAGEMENT’S DISCUSSION AND ANALYSIS
All references in this management’s discussion and analysis, or
MD&A to “the Company”, “Trillium”, “we”, “us”, or “our” refer to Trillium
Therapeutics Inc. and the subsidiaries through which it conducts its business,
unless otherwise indicated or the context requires otherwise.
The following MD&A is prepared as of March 8, 2019 for
Trillium Therapeutics Inc. for the years ended December 31, 2018 and 2017, and
should be read in conjunction with the audited consolidated financial statements
for the years ended December 31, 2018 and 2017, which have been prepared by
management in accordance with International Financial Reporting Standards, or
IFRS as issued by the International Accounting Standards Board, or IASB. Our
IFRS accounting policies are set out in note 3 of the annual audited
consolidated financial statements for the years ended December 31, 2018 and
2017. All amounts are in thousands of Canadian dollars, except per share amounts
and unless otherwise indicated. References to “U.S. $” are to United States
dollars.
CAUTIONARY STATEMENT ABOUT FORWARD-LOOKING STATEMENTS
This MD&A contains forward-looking statements within the
meaning of applicable securities laws. All statements contained herein that are
not clearly historical in nature are forward-looking, and the words
“anticipate”, “believe”, “expect”, “estimate”, “may”, “will”, “could”,
“leading”, “intend”, “contemplate”, “shall” and similar expressions are
generally intended to identify forward-looking statements. Forward-looking
statements in this MD&A include, but are not limited to, statements with
respect to:
- our expected future loss and accumulated deficit levels;
- our projected financial position and estimated cash burn rate;
- our requirements for, and the ability to obtain, future funding on
favorable terms or at all;
- our projections for the SIRPαFc development plans and progress of each of
our products and technologies, particularly with respect to the timely and
successful completion of studies and trials and availability of results from
such studies and trials;
- our plans to focus development of TTI-621 on patients with cutaneous
T-cell lymphoma based on our early clinical results;
- our expectations about our products’ safety and efficacy;
- our expectations regarding our ability to arrange for and scale up the
manufacturing of our products and technologies;
- our expectations regarding the progress, and the successful and timely
completion, of the various stages of the regulatory approval process;
- our expectations about the timing of achieving milestones and the cost of
our development programs;
- our observations and expectations regarding the relative low binding of
SIRPαFc to red blood cells, or RBCs, compared to anti-CD47 monoclonal
antibodies and proprietary CD47-blocking agents and the potential benefits to
patients;
- our ability to intensify the dose of TTI-621 with the goal of achieving
increased blockade of CD47;
- our expectation that we will achieve levels of TTI-622 in patients
sufficient to obtain sustained CD47 blockade;
- our expectation that TTI-622 is likely to be more effective in combination
with agents that provide additional “eat” signals to macrophages or other
forms of immune activation;
- our plans to market, sell and distribute our products and technologies;
- our expectations regarding the acceptance of our products and technologies
by the market;
- our ability to retain and access appropriate staff, management and expert
advisers;
- our expectations about the differentiated nature and discovery research
capabilities of Fluorinov Pharma Inc., or Fluorinov;
- our ability to generate future product development programs with improved
pharmacological properties and acceptable safety profiles using Fluorinov
technology;
- 1 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
- our expectations about whether various clinical and regulatory milestones
with an existing Fluorinov compound will be achieved;
- our expectations of the final quantum and form of any future contingent
milestone payments related to the Fluorinov acquisition;
- our expectations of the ability to secure the requisite approvals
(including approvals from the Toronto Stock Exchange, or TSX, and the NASDAQ
Stock Market, or NASDAQ) with respect to the issuance of any common shares in
satisfaction of future milestone payments;
- our ability to secure strategic partnerships with larger pharmaceutical
and biotechnology companies;
- our strategy to acquire and develop new products and technologies and to
enhance the safety and efficacy of existing products and technologies;
- our expectations with respect to existing and future corporate alliances
and licensing transactions with third parties, and the receipt and timing of
any payments to be made by us or to us in respect of such arrangements; and
- our strategy with respect to the protection of our intellectual property.
All forward-looking statements reflect our beliefs and
assumptions based on information available at the time the assumption was made.
These forward-looking statements are not based on historical facts but rather on
management’s expectations regarding future activities, results of operations,
performance, future capital and other expenditures (including the amount, nature
and sources of funding thereof), competitive advantages, business prospects and
opportunities.
By its nature, forward-looking information involves numerous
assumptions, inherent risks and uncertainties, both general and specific, known
and unknown, that contribute to the possibility that the predictions, forecasts,
projections or other forward-looking statements will not occur. In evaluating
forward-looking statements, readers should specifically consider various
factors, including the risks outlined under the heading “Risk Factors” in this
MD&A. Some of these risks and assumptions include, among others:
- substantial fluctuation of losses from quarter to quarter and year to year
due to numerous external risk factors, and anticipation that we will continue
to incur significant losses in the future;
- uncertainty as to our ability to raise additional funding to support
operations;
- our ability to generate product revenue to maintain our operations without
additional funding;
- the risks associated with the development of our product candidates which
are at early stages of development;
- positive results from preclinical and early clinical research are not
necessarily predictive of the results of later-stage clinical trials;
- reliance on third parties to plan, conduct and monitor our preclinical
studies and clinical trials;
- our product candidates may fail to demonstrate safety and efficacy to the
satisfaction of regulatory authorities or may not otherwise produce positive
results;
- risks related to filing Investigational New Drug applications, or INDs, to
commence clinical trials and to continue clinical trials if approved;
- the risks of delays and inability to complete clinical trials due to
difficulties enrolling patients;
- competition from other biotechnology and pharmaceutical companies;
- our reliance on the capabilities and experience of our key executives and
scientists and the resulting loss of any of these individuals;
- our ability to fully realize the benefits of acquisitions;
- our ability to adequately protect our intellectual property and trade
secrets;
- our ability to source and maintain licenses from third-party owners;
- the risk of patent-related litigation; and
- our expectations regarding our status as a passive foreign investment
company, or PFIC,
all as further and more fully described under the heading “Risk
Factors” in this MD&A.
- 2 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Although the forward-looking statements contained in this
MD&A are based upon what our management believes to be reasonable
assumptions, we cannot assure readers that actual results will be consistent
with these forward-looking statements.
Any forward-looking statements represent our estimates only as
of the date of this MD&A and should not be relied upon as representing our
estimates as of any subsequent date. We undertake no obligation to update any
forward-looking statement or statements to reflect events or circumstances after
the date on which such statement is made or to reflect the occurrence of
unanticipated events, except as may be required by securities legislation.
BUSINESS
Overview
We are a clinical stage immuno-oncology company developing
innovative therapies for the treatment of cancer. Our lead program, TTI-621, is
a SIRPαFc fusion protein that consists of the extracellular CD47-binding domain
of human signal regulatory protein alpha, or SIRPα, linked to the Fc region of a
human immunoglobulin G1, or IgG1. It is designed to act as a soluble decoy
receptor, preventing CD47 from delivering its inhibitory (“do not eat”) signal.
Neutralization of the inhibitory CD47 signal enables the activation of
macrophage anti-tumor effects by pro-phagocytic (“eat”) signals. The IgG1 Fc
region of TTI-621 may also assist in the activation of macrophages by engaging
Fc receptors. Two phase 1 clinical trials evaluating TTI-621 are ongoing. In
these trials, TTI-621 has shown single agent activity by both local and/or
systemic delivery in multiple B- and T-cell lymphoma indications and has been
well tolerated in over 200 patients to date.
We are also developing a second SIRPαFc fusion protein,
TTI-622. TTI-622 consists of the extracellular CD47-binding domain of human
SIRPα linked to a human immunoglobulin G4, or IgG4 Fc region, which has a
decreased ability to engage Fc receptors than an IgG1 Fc. We initiated a phase 1
clinical trial for TTI-622 in June 2018. Both SIRPαFc fusion proteins enable
CD47 blockade with different levels of Fc receptor engagement on macrophages and
thus may find unique applications.
We also have a medicinal chemistry platform that uses
proprietary fluorine-based chemistry to yield new chemical entities. Our most
advanced preclinical program stemming from this platform is an epidermal growth
factor receptor, or EGFR antagonist with increased uptake and retention in the
brain. In addition, a number of compounds directed at undisclosed
immuno-oncology targets are currently in the discovery phase.
Our Strategy
Our goal is to become a leading innovator in the field of
oncology by targeting immune-regulatory pathways that tumor cells exploit to
evade the host immune system. We believe we have the most differentiated and
comprehensive approach to targeting CD47, with the development of two SIRPαFc
fusion proteins, monotherapy and combination therapy approaches, and both
intravenous and intratumoral administration. We intend to:
-
Rapidly advance the clinical development of TTI-621. Because
CD47 is highly expressed by multiple liquid and solid tumors, and high
expression is correlated with worse clinical outcomes, we believe SIRPαFc has
potential to be effective in a variety of cancers. In our clinical trials to
date, we have enrolled over 200 patients with multiple tumor types where
TTI-621 may provide clinical benefit to find specific malignancies of interest
for further development.
-
Focus our TTI-621 clinical program on promising cancer indications.
From our broad clinical approach, we found a number of cancers where
we saw positive responses in patients. We are particularly interested in our
initial results treating mycosis fungoides, a predominant form of cutaneous
T-cell lymphoma, or CTCL, and we are focusing our near-term efforts on
patients with T-cell malignancies broadly to include both CTCL and peripheral
T-cell lymphoma, or PTCL, patients.
- 3 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
-
Expand our portfolio of SIRPαFc
constructs through advancement of TTI-622. Our expertise in designing
fusion proteins allows us to explore alternative approaches to blocking CD47
that may be advantageous for certain applications. We began testing TTI-622 in
a phase 1 clinical trial in June 2018 as a second and differentiated approach
to block CD47. We expect TTI-622 may be of particular interest when used in
combination with other anti-cancer drugs, including immunomodulatory agents.
-
Build a pipeline of novel oncology products using our proprietary
medicinal chemistry platform. We have several preclinical and
discovery stage assets developed using our proprietary fluorine chemistry
platform. We plan to advance these novel oncology products for internal
development or out-license.
Our CD47 Clinical Pipeline
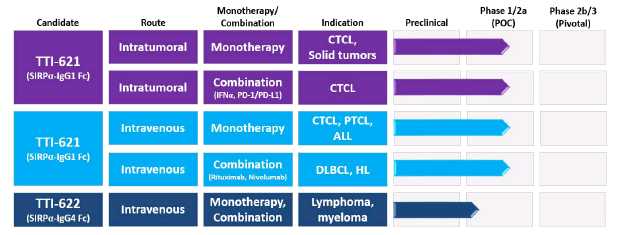
SIRPαFc
Blocking the CD47 “do not eat” signal using a
SIRPαFc decoy receptor
The immune system is the body’s mechanism to identify and
eliminate pathogens, and can be divided into the innate immune system and the
adaptive immune system. The innate immune system is the body’s first line of
defense to identify and eliminate pathogens and consists of proteins and cells,
such as macrophages, that identify and provide an immediate response to
pathogens. The adaptive immune system is activated by, and adapts to, pathogens,
creating a targeted and durable response. Cancer cells often have the ability to
reduce the immune system’s ability to recognize and destroy them.
Macrophages are a type of white blood cell that can ingest and
destroy (phagocytose) other cells. Macrophage activity is controlled by both
positive “eat” and negative “do not eat” signals. Recently, a role for
macrophages in the control of tumors has been described. Tumor cells may express
“eat” signals (e.g., calreticulin) that make themselves visible to macrophages.
To counterbalance this increased visibility the tumor cells often express high
levels of CD47, which transmits a “do not eat” signal by binding SIRPα on the
surface of macrophages. Elevated expression of CD47 has been observed across a
range of hematological and solid tumors. In many cases, high CD47 expression was
shown to have negative clinical consequences, correlating with more aggressive
disease and poor survival.
Our lead program, TTI-621, is a novel SIRPαFc fusion protein
that harnesses the innate immune system by blocking the activity of CD47.
TTI-621 is a protein that consists of the CD47-binding domain of human SIRPα
linked to the Fc region of IgG1. It is designed to act as a soluble decoy
receptor, preventing CD47 from delivering its inhibitory signal. Neutralization
of the inhibitory CD47 signal enables the activation of macrophage anti-tumor
effects by the pro-phagocytic “eat” signals. The IgG1 Fc region of TTI-621 may
also assist in the activation of macrophages by engaging Fc receptors. A second
SIRPαFc fusion protein, TTI-622, entered phase 1 testing in June 2018. TTI-622
consists of the same CD47-binding domain of human SIRPα and is linked to the Fc
region of IgG4. The IgG4 Fc region of TTI-622 is expected to have a decreased ability to
engage activating Fc receptors compared to an IgG1 Fc, and thus provide a more
modest “eat” signal to macrophages, allowing for greater tolerability and higher
CD47 blockade but lower potency. TTI-622 will allow us to assess how higher CD47
blockade with an IgG4-based agent in patients compares to lower CD47 blockade
with an IgG1-based drug (TTI-621).
- 4 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
In preclinical studies, TTI-621 and TTI-622 frequently
triggered significant macrophage-mediated tumor cell phagocytosis in vitro
compared to control treatment. In vivo, both fusion proteins exhibited
anti-tumor activity in human xenograft models.
In addition to their direct anti-tumor activity, macrophages
can also function as antigen-presenting cells and stimulate antigen-specific
T-cells. Thus, it is possible that increasing tumor cell phagocytosis after
SIRPαFc exposure may result in enhanced adaptive immunity. In support of this,
CD47 antibody blockade has been recently shown to augment antigen presentation
and prime an anti-tumor cytotoxic T-cell response in immune-competent mice. In
2016, we presented data demonstrating that TTI-621 can augment antigen-specific
T-cell responses in vitro. CD47 blockade has also been reported to promote
tumor-specific T-cell responses through a dendritic cell-based mechanism,
although the effect of SIRPαFc on dendritic cells is currently unknown.
The figure below illustrates how SIRPαFc blocks the CD47 “do
not eat” signal and engages activating Fc receptors on macrophages, leading to
tumor cell phagocytosis, increased antigen presentation and enhanced T-cell
responses.
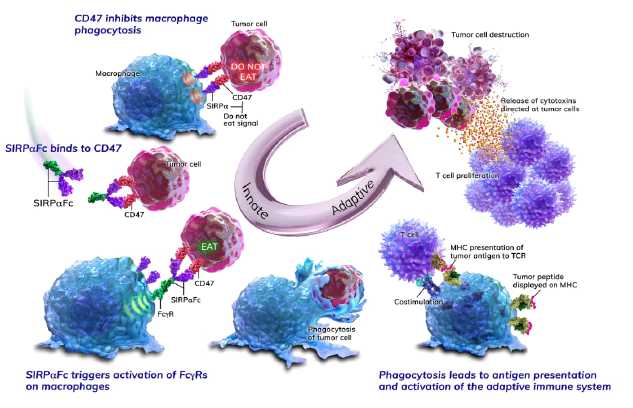
By inhibiting the CD47 “do not eat” signal, we believe SIRPαFc
has the ability to promote the macrophage-mediated killing of tumor cells in a
broad variety of cancers both as a monotherapy and in combination with other
immune therapies. Both SIRPαFc fusion proteins enable CD47 blockade with
different levels of Fc receptor engagement on macrophages and thus may find
unique applications.
- 5 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Combination Therapy
We believe that SIRPαFc enhancement of macrophage activity, and
possibly T-cell responses, could be synergistic with other immune-mediated
therapies. Since many cancer antibodies work at least in part by activating
cells of the innate immune system, it may be possible to enhance the potency of
these agents by blocking the negative “do not eat” CD47 signal that tumor cells
deliver to macrophages. In fact, we have observed anti-tumor activity when
combining SIRPαFc with rituximab in both preclinical studies and in B lymphoma
patients. We hypothesize that SIRPαFc may act synergistically with other
immunological agents, including T-cell checkpoint inhibitors (e.g. pembrolizumab
and nivolumab), cancer vaccines, oncolytic viruses or chimeric antigen receptor,
or CAR T-cells.
SIRPαFc Clinical Development
– TTI-621
We are recruiting patients in two ongoing phase 1 clinical
trials; one with intratumoral injection and one with intravenous infusion. These
trials were designed to establish a safe dosing level, characterize safety,
pharmacokinetics, and pharmacodynamics and treat a broad range of malignancies
searching for evidence of antitumor activity.
Intratumoral administration – TTI-621
In a multi-center, open-label phase 1 trial, TTI-621 is being
delivered by intratumoral injection in patients with relapsed and refractory,
percutaneously-accessible cancers. In the escalation phase, patients were
enrolled in sequential dose cohorts to receive intratumoral injections of
TTI-621 that increased in dose and dosing frequency to characterize safety,
pharmacokinetics, pharmacodynamics and preliminary evidence of antitumor
activity. In addition, detailed evaluation of serial, on-treatment tumor
biopsies of both injected and non-injected cancer lesions is being performed to
help characterize the tumor microenvironment. The most recent data from the
ongoing study were reported at the American Society of Hematology
60th Annual Meeting in December 2018. Local delivery of TTI-621 was
well tolerated, with no treatment-related > Grade 3 adverse events or
dose-limiting toxicity observed in 27 mycosis fungoides patients. Among 22
evaluable patients, reductions in Composite Assessment of Index Lesion Severity,
or CAILS, scores, which measure local lesion responses, were observed in 91% of
patients, with 41% exhibiting reductions of 50% or greater. These responses
occurred rapidly within the 2-week induction period. Similar CAILS scores
changes were seen in adjacent non-injected lesions, suggesting locoregional
effects that were not confined to the site of injection. Evidence of a systemic
effect was observed in 1 of 2 patients receiving continuation monotherapy beyond
the 2-week induction therapy. In addition, data suggest a combination effect
with pegylated IFN-alpha-2a. Collectively, the data demonstrate that CTCL
appears biologically responsive to intratumoral injections of TTI-621.
We have amended the protocol for this trial to focus on
recruiting additional patients with T-cell malignancies, and specifically CTCL,
to determine if the preliminary results will be seen in a larger patient
population. Patients are currently being enrolled in the expansion phase of the
trial in which they receive 10 mg of TTI-621 three times per week for two weeks
followed by weekly dosing, to further characterize safety and efficacy. In
addition, patients may receive intratumoral TTI-621 in combination with other
anti-cancer therapies (anti-PD-1 or anti-PD-L1, pegylated interferon α2a,
talimogene laherparepvec or radiation). We have modified this trial to allow for
the increase in the size of each cohort from 12 to 40 patients based on early
signs of clinical benefit.
- 6 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Intravenous administration – TTI-621
We are enrolling patients with advanced hematologic
malignancies in a phase 1b clinical trial with intravenous administration of
TTI-621. The most recent data from the ongoing expansion phase were reported at
the
T-Cell Lymphoma Forum in January 2019. Based on an expanded data set of 179
patients, weekly infusions of TTI-621 were shown to be well tolerated.
Thrombocytopenia was the most frequent grade 3 or higher treatment-emergent
adverse event, occurring in 18% of patients. Platelet reductions, however, were
shown to be transient and pre-dose platelet levels remained steady during the
course of the study. Notably, the reversible thrombocytopenia did not lead to an
increased risk of bleeding and had no impact on drug delivery, nor was there a
significant impact of TTI-621 on hemoglobin levels. Monotherapy efficacy was
observed in patients with mycosis fungoides (17% overall response rate, or ORR,
n=24), peripheral T-cell lymphoma, or PTCL (18% ORR, n=11), Sézary Syndrome (20%
ORR, n=5). As reported at the 16th Annual Discovery on Target conference in
September 2018, monotherapy efficacy was also observed in diffuse large B-cell
lymphoma, or DLBCL patients (25% ORR, n=8), and in DLBCL patients when combined
with rituximab (25% ORR, n=24). This clinical activity was observed in patients
receiving relatively low doses of drug (0.2 mg/kg for monotherapy or 0.1 mg/kg
in combination with rituximab). Dose intensification beyond 0.2 mg/kg is
currently ongoing, and doses of 0.5 mg/kg have been well tolerated for up to 27
weeks.
We are focusing our near-term efforts on patients with CTCL and
PTCL, following the early signals of efficacy observed in the intratumoral
trial. These patients are being enrolled in separate cohorts that will be
evaluated using a Simon 2-stage design where we must achieve predefined target
responses in the first stage (18 patients) to continue recruiting patients for a
second stage of 17 patients. We also introduced a standardized intra-subject dose
intensification schedule for all newly enrolled subjects to increase drug
exposure.
- 7 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
TTI-621 has recently been granted an Orphan Drug Designation by
the U.S. Food and Drug Administration, or FDA for the treatment of cutaneous
T-cell lymphoma. Orphan Drug Designation qualifies the sponsor of the drug
candidate for various development incentives, which may include tax credits for
qualified clinical testing, an exemption from fees under the Prescription Drug
User Fee Act, and a seven-year marketing exclusivity period following approval.
SIRPαFc Clinical Development
– TTI-622
A second SIRPαFc fusion protein, TTI-622, is in clinical
development. A two-part, multicenter, open-label, phase 1a/1b study of TTI-622
in patients with advanced relapsed or refractory lymphoma or multiple myeloma
was initiated in June 2018. In the phase 1a dose-escalation part, patients will
be enrolled in sequential dose cohorts to receive TTI-622 once weekly to
characterize safety, tolerability, pharmacokinetics, and to determine the
maximum tolerated dose. In the phase 1b part, patients will be treated with
TTI-622 in combination with rituximab, a proteasome inhibitor-containing
regimen, or a PD-1 inhibitor. Rituximab and proteasome inhibitors may provide
additional “eat” signals that could enhance the efficacy of TTI-622. A PD-1
inhibitor may help amplify any anti-tumor T-cell response generated by TTI-622.
TTI-622 consists of the same extracellular CD47-binding domain
of human SIRPα as TTI-621 but has a different Fc region (IgG4 Fc instead of IgG1
Fc), which provides a more modest “eat” signal than IgG1 due to more limited
interactions with activating Fc receptors. Preclinical studies suggest that
IgG4-based SIRPαFc fusion proteins have greater tolerability but lower potency
than IgG1-based fusion proteins. We therefore expect to achieve higher levels of
TTI-622 in patients compared to TTI-621, leading to greater and more sustained
CD47 blockade. Thus, TTI-622 will allow us to assess how higher CD47 blockade
with an IgG4-based agent in patients compares to lower CD47 blockade with the
IgG1-based TTI-621. Due to the lower potency of the IgG4 Fc, we expect that
TTI-622 is likely to be more effective in combination with agents that provide
additional “eat” signals to macrophages or other forms of immune activation.
Preclinical TTI-622 data were reported at the 2018 Annual
Meeting of the American Association for Cancer Research. The data demonstrate
that TTI-622 induces the phagocytosis of a broad panel of tumor cells derived
from patients with both hematological and solid tumors. As a monotherapy,
TTI-622 treatment resulted in decreased tumor growth and improved survival in a
B cell lymphoma xenograft model, and enhanced the efficacy of cetuximab
(anti-EGFR) and daratumumab (anti-CD38) antibodies in solid and hematological
xenograft models, respectively. Unlike CD47-blocking antibodies, TTI-622 bound
minimally to RBCs and did not induce hemagglutination in vitro. We believe that
this property could give TTI-622 best-in-class status among IgG4-based blocking
agents currently in clinical development.
- 8 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
SIRPαFc Key Takeaways
-
Multiple clinical approaches. We believe we have the most
systematic and comprehensive approach to CD47 with two decoy receptors in
development with different Fc functions, monotherapy and combination therapy
approaches, and intravenous and intratumoral delivery modalities.
-
Demonstrated clear signals of activity. TTI-621 monotherapy
has produced positive signals of activity in CTCL, PTCL and DLBCL patients. A
signal of activity was also seen in DLBCL patients when combined with
rituximab.
-
Tolerability and safety. TTI-621 has been well tolerated in
over 180 patients to date.
-
Clear paths forward. We are focusing our development on
intratumoral monotherapy and combination therapy in CTCL; intravenous
monotherapy in both CTCL and PTCL; and intravenous combination therapy in
B-cell lymphoma.
SIRPαFc
Competition
There are a number of companies developing blocking agents to
the CD47-SIRPα axis, which can be broadly classified into six groups which
include, but are not limited to:
- CD47-specific antibodies: Forty Seven Inc (phase 2); Celgene
Corporation, Surface Oncology, Innovent Biologics (Suzhou) Co. (phase 1); Arch
Oncology, I-Mab Biopharma, Phanes Therapeutics, ImmuneOncia (preclinical)
- CD47 bispecific antibodies: Novimmune SA, Hummingbird
BioSciences, Pharmabcine (preclinical)
- Mutated high affinity SIRPαFc:
ALX Oncology (phase 1)
- SIRPα-specific antibody: Celgene
(phase 1), OSE Immunotherapeutics (preclinical), Forty Seven Inc (preclinical)
- SIRPαFc-agonist fusion protein:
Shattuck Labs (preclinical)
- Small molecule inhibitor: Aurigene Discovery Technologies
(preclinical)
We believe that TTI-621 has advantages over other CD47 blocking
agents. TTI-621’s IgG1 Fc maximizes potency by delivering an activating signal
to macrophages through Fc receptors. With this higher potency, we believe that
TTI-621 has a higher likelihood of monotherapy activity and therefore is not
dependent upon a combination with another IgG1 antibody. TTI-621 could also have
the potential to be used to treat tumors where no anti-cancer antibody is
available.
- 9 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
We have also demonstrated that our SIRPαFc fusion proteins
exhibit minimal binding to RBCs in contrast to CD47-specific antibodies and a
mutated high affinity SIRPαFc. We believe that this property confers several
possible advantages including avoidance of drug-induced anemia, avoidance of the
“antigen sink effect” (i.e. removal of drug from circulation by RBCs) and
non-interference with laboratory blood typing tests. It should be noted that
TTI-622 shares the same CD47-binding domain as TTI-621 and preclinical studies
have shown that it also exhibits minimal binding to human RBCs. Thus, we
anticipate that TTI-622, like TTI-621, will not induce anemia in patients.
Fluorine Chemistry Platform
Our medicinal chemistry platform uses proprietary
fluorine-based chemistry yield new chemical entities. We believe the potency
and/or safety of both existing pharmacophores and historically inaccessible
chemical structures may be enhanced using our technology. This chemistry
platform has been utilized to establish several preclinical programs including
an EGFR inhibitor, and a number of compounds directed at undisclosed
immuno-oncology targets are currently in the discovery phase.
Plan of Operations
We are advancing our intratumoral and intravenous clinical
trials of TTI-621 with a focus on CTCL and PTCL and our TTI-622 phase 1 trial
has been initiated. We also continue to advance our small molecule program in
internal development and pursue partnering activities.
Legal Proceedings
To our knowledge, there have not been any legal or arbitration
proceedings, including those relating to bankruptcy, receivership or similar
proceedings, those involving any third party, and governmental proceedings
pending or known to be contemplated, which may have, or have had in the recent
past, significant effect on our financial position or profitability.
Also, to our knowledge, there have been no material proceedings
in which any director, any member of senior management, or any of our affiliates
is either a party adverse to us or any of our subsidiaries or has a material
interest adverse to us or any of our subsidiaries.
- 10 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
RESULTS OF OPERATIONS
For the three months and years ended December 31, 2018 and
2017
Overview
Since inception, we have incurred losses while advancing the
research and development of our products. Net loss for the year ended December
31, 2018 of $42,486 was lower than the loss of $45,088 for the year ended
December 31, 2017. The net loss was lower due mainly to a net foreign currency
gain of $3,489 for the current year compared to a net foreign currency loss of
$4,742 in the prior year, and lower manufacturing costs, partially offset by
higher clinical trial expenses and an amendment to the SIRPαFc license
agreement, where the sublicense revenue sharing provisions were removed in
return for a payment to the licensors of $3,000 in the form of 369,621 common
shares.
Net loss for the three months ended December 31, 2018 of $8,553
was lower than the loss of $10,658 for the three months ended December 31, 2017
mainly due to a higher net foreign currency gain of $2,005 compared to $167 in
the prior year, partially offset by higher research and development expenses of
$10,611 with two active phase 1 trials for TTI-621, and the initiation of a
phase 1 trial for TTI-622.
Research and Development
Research and development expenses by program for the three
months and years ended December 31, 2018 and 2017 were as follows:
| |
|
Three months |
|
|
Three months |
|
|
|
|
|
|
|
| |
|
ended |
|
|
ended |
|
|
Year ended |
|
|
Year ended |
|
| |
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
|
December 31, |
|
| |
|
2018 |
|
|
2017 |
|
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
| SIRPαFc |
|
10,128 |
|
|
9,003 |
|
|
39,748 |
|
|
31,052 |
|
| Small molecule programs |
|
483 |
|
|
805 |
|
|
3,653 |
|
|
6,041 |
|
| Other |
|
- |
|
|
3 |
|
|
25 |
|
|
42 |
|
| Total(1) |
|
10,611 |
|
|
9,811 |
|
|
43,426 |
|
|
37,135 |
|
|
Note: |
|
| (1) |
Research and development expenditures in the above table
include all direct and indirect costs for the programs, personnel costs,
intellectual property, amortization, share-based compensation and research
and development overhead, and is net of government assistance. Research
and development overhead costs have been allocated to the programs based
mainly on personnel time spent on the programs. |
During 2018 and 2017, most of our resources were focused on the
development of our SIRPαFc program. For the year ended December 31, 2018,
SIRPαFc research and development costs were higher than the prior year due to
higher clinical trial expenses with three active phase 1 clinical trials and the
amendment to the SIRPαFc license agreement, partially offset by lower SIRPαFc
manufacturing and reduced activity relating to academic research
collaborations.
Small molecule program expenses were lower than the prior year
as we completed most of our targeted preclinical development studies for the
bromodomain and EGFR inhibitors in 2017, while 2018 activities were focused on
our immuno-oncology discovery programs.
- 11 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Components of research and development expenses for the three
months ended December 31, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| Research and development programs excluding
the below items |
|
7,643 |
|
|
6,241 |
|
| Salaries, fees and short-term benefits |
|
2,114 |
|
|
2,675 |
|
| Share-based compensation |
|
755 |
|
|
713 |
|
| Amortization of intangible assets |
|
584 |
|
|
965 |
|
| Change in fair value of contingent
consideration |
|
(674 |
) |
|
(1,012 |
) |
| Depreciation of property and equipment |
|
202 |
|
|
243 |
|
| Tax credits |
|
(13 |
) |
|
(14 |
) |
| |
|
10,611 |
|
|
9,811
|
|
Components of research and development expenses for the years
ended December 31, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| Research and development programs excluding
the below items |
|
27,493 |
|
|
22,831 |
|
| Salaries, fees and short-term benefits |
|
8,510 |
|
|
7,969 |
|
| License agreement amendment |
|
3,000 |
|
|
- |
|
| Share-based compensation |
|
2,148 |
|
|
2,911 |
|
| Amortization of intangible assets |
|
2,338 |
|
|
3,860 |
|
| Change in fair value of contingent consideration |
|
(674 |
) |
|
(1,158 |
) |
| Depreciation of property and equipment |
|
808 |
|
|
849 |
|
| Tax credits |
|
(197 |
) |
|
(127 |
) |
| |
|
43,426 |
|
|
37,135 |
|
The increase in research and development program expenses for
the three months ended December 31, 2018 compared to the same period last year
was due mainly to higher SIRPαFc clinical trial expenses by $744 and higher
manufacturing costs by $860. Salaries, fees and short-term benefits decreased in
the three months ended December 31, 2018 due to lower incentive compensation
expenses compared to the same period in 2017. Share-based compensation costs
were comparable to the same period in the prior year. Amortization of intangible
assets decreased as we extended our estimate of the life of our small molecule
platform intangible asset. The change in fair value of contingent consideration
reflected an increase in the time estimate and increased risk of reaching the
potential milestones. Depreciation of property and equipment and tax credits
were comparable to the prior year period.
The increase in research and development program expenses for
the year ended December 31, 2018 over the prior year was due mainly to an
increase in SIRPαFc clinical trial expenses of $6,432, partially offset by a
decrease in SIRPαFc manufacturing costs of $178 and lower activity related to
academic research collaborations. Salaries, fees, and short-term benefits
increased in the year ended December 31, 2018 due to higher staffing and
salaries compared to 2017. For the year ended December 31, 2018, we incurred an
expense of $3,000 relating to the SIRPαFc license agreement amendment.
Share-based compensation costs decreased mainly due to an increase in stock
option forfeitures and in the expected forfeiture rate. Amortization of
intangible assets decreased as we extended our estimate of the life of our small
molecule platform intangible asset. The change in fair value of contingent
consideration reflected an increase in the time estimate and lowered the
likelihood of
reaching the potential milestones.
- 12 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Depreciation of property and equipment was comparable to the
prior year. Tax credits increased compared to the prior year due to an increase
in eligible expenses.
General and Administrative
Components of general and administrative expenses for the three
months ended December 31, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| General and administrative expenses
excluding the below items |
|
470 |
|
|
407 |
|
| Salaries, fees and short-term benefits |
|
829 |
|
|
603 |
|
| Change in fair value of deferred share
units |
|
(1,197 |
) |
|
154 |
|
| Share-based
compensation |
|
104 |
|
|
90
|
|
| |
|
206 |
|
|
1,254 |
|
Components of general and administrative expenses for the years
ended December 31, 2018 and 2017 were as follows:
| |
|
2018 |
|
|
2017 |
|
| |
|
$ |
|
|
$ |
|
| |
|
|
|
|
|
|
| General and administrative expenses
excluding the below items |
|
1,905 |
|
|
1,469 |
|
| Salaries, fees and short-term benefits |
|
2,716 |
|
|
2,038 |
|
| Change in fair value of deferred share
units |
|
(1,401 |
) |
|
10 |
|
| Share-based
compensation |
|
362 |
|
|
344
|
|
| |
|
3,582 |
|
|
3,861 |
|
General and administrative expenses for the three months ended
December 31, 2018 of $470 were higher than the prior year comparable period
mainly due to higher professional fees incurred. Salaries, fees and short-term
benefits increased mainly due to higher staffing levels and higher
director-related fees. The change in the fair value of deferred share units was
a result of fluctuations in our share price during the respective periods.
Share-based compensation expense was comparable to the prior year period.
General and administrative expenses for the year ended December
31, 2018 of $1,905 were higher than the prior year mainly due to higher
professional fees and listing fees incurred related to a prospectus supplement
filing and the 2018 Stock Option Plan. Salaries, fees and short-term benefits
increased mainly due to higher staffing levels and the issuance of director
DSUs. The change in the fair value of deferred share units was a result of
fluctuations in our share price during the respective periods. Share-based
compensation expense was comparable to the prior year.
Finance income and costs, foreign exchange gains and
losses, and income taxes
Finance income for the three months and year ended December 31,
2018 of $270 and $1,084, respectively, were higher than the prior year
comparable period amounts of $254 and $722 due mainly to higher interest rates.
Finance costs for the three months and year ended December 31,
2018 were comparable to the prior year periods.
- 13 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
During the three months ended December 31, 2018, we recorded a
net foreign currency gain of $2,005, compared to $167 for the comparative period
in 2017. The net foreign currency gain in the current period reflected a
strengthening of the U.S. dollar versus the Canadian dollar while holding net
U.S. dollar denominated assets. During the year ended December 31, 2018, we
recorded a net foreign currency gain of $3,489, compared to a net foreign
currency loss of $4,742 for the year ended December 31, 2017.
Liquidity and Capital Resources
Cash, working capital, and debt
Since inception, we have financed our operations primarily from
sales of equity, proceeds from the exercise of warrants and stock options and
from interest income on funds available for investment. Our primary capital
needs are for funds to support our scientific research and development
activities including staffing, facilities, manufacturing, preclinical studies,
clinical trials, administrative costs and for working capital.
We have experienced operating losses and cash outflows from
operations since incorporation, will require ongoing financing in order to
continue our research and development activities and we have not earned
significant revenue or reached successful commercialization of our products. Our
future operations are dependent upon our ability to finance our cash
requirements which will allow us to continue our research and development
activities and the commercialization of our products. There can be no assurance
that we will be successful in continuing to finance our operations.
In June 2017, we completed an underwritten public offering of
common shares and non-voting convertible preferred shares in the United States.
In the offering, we sold 2,949,674 common shares and 3,250,000 Series II
Non-Voting Convertible First Preferred Shares at a price of U.S. $5.00 per
share. The gross proceeds from this offering were $41,847 (U.S. $30,998) before
deducting offering expenses of $2,856.
The Series II Non-Voting Convertible First Preferred Shares
sold in the offering are non-voting and are convertible into common shares, on a
one-for-one basis (subject to adjustment), at any time at the option of the
holder, subject to certain restrictions on conversion. Holders may not convert
Series II Non-Voting Convertible First Preferred Shares into common shares if,
after giving effect to the exercise of conversion, the holder and its joint
actors would have beneficial ownership or direction or control over common
shares in excess of 4.99% of the then outstanding common shares. This limit may
be raised at the option of the holder on 61 days’ prior written notice: (i) up
to 9.99%, (ii) up to 19.99%, subject to clearance of a personal information form
submitted by the holder to the TSX, and (iii) above 19.99%, subject to approval
by the TSX and shareholder approval.
In connection with the acquisition of Series II First Preferred
Shares in this offering at the public offering price by an existing
institutional shareholder, we entered into an investment agreement with such
shareholder. The investment agreement provides this shareholder the right, but
not the obligation, for so long as it beneficially owns at least 10% of the
adjusted share capital of the Company, calculated on a fully-diluted basis, to
nominate one person for election to our board of directors, subject to meeting
applicable legal and stock exchange requirements and we have the obligation to
appoint such director, whose term will run until the next annual meeting of
shareholders. Thereafter, we are required to nominate such director to be a
director at any meeting of shareholders called for the purposes of electing
directors and to use commercially reasonable efforts to ensure that such
director is elected to the board of directors, including soliciting proxies in
support of his or her election and taking the same actions taken by us to ensure
the election of the other nominees selected by the board of directors for
election to the board of directors. In addition, until such time as the existing
shareholder exercises its right to nominate a member of our board of directors,
and so long as the existing shareholder’s nominee is not an employee, officer,
director or limited partner of such shareholder, then such shareholder shall
have the right, but not the obligation, to appoint an observer to our board of
directors, who must be an employee, officer or director of such shareholder. The
observer will have the right to receive notice of and attend the meetings of the
board of directors, and will have the right to address the board of directors at
any of its meetings, but will not have any right to vote at any meeting of the
board of directors. In addition, we have agreed to provide this existing
shareholder with certain registration rights in the event that such shareholder
and its joint actors are deemed to be “affiliates” for purposes of applicable
U.S. securities laws.
- 14 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
In December 2017, we completed a non-brokered private placement
financing and sold 1,950,000 common shares and 400,000 Series II Non-Voting
Convertible Preferred Shares at a price of U.S. $8.50 per share yielding gross
proceeds of $25,338 (U.S. $19,975) before deducting offering expenses of
$1,784.
On January 5, 2018, we filed a base shelf prospectus with the
British Columbia, Alberta, Manitoba, Ontario and Nova Scotia securities
commissions in Canada and a Form F-10 registration statement with the United
States Securities and Exchange Commission, or SEC, that provides that we may
sell under the prospectus from time to time over the following 25 months up to
U.S. $150 million, in one or more offerings, of common shares, First Preferred
shares, warrants to purchase common shares, subscription receipts, or units
comprising a combination of common shares, First Preferred shares and/or
warrants.
On June 19, 2018 we filed a prospectus supplement to the base
prospectus included in our U.S. registration statement on Form F-10 declared
effective on January 8, 2018. We also entered into a sales agreement with Cowen
and Company, LLC, or the Agent, pursuant to which we may, at our discretion and
from time to time during the term of the sales agreement, sell, through the
Agent, acting as agent and/or principal, such number of common shares of
Trillium as would result in aggregate gross proceeds to us of up to U.S. $25
million. Sales of common shares through the Agent, acting as agent, will be made
through “at the market” issuances on NASDAQ at the market price prevailing at
the time of each sale, and, as a result, sale prices may vary. No common shares
will be offered or sold on the TSX or any other trading markets in Canada.
Our cash and cash equivalents and marketable securities, and
working capital at December 31, 2018 were $45,409 and $34,185, respectively
compared to $81,791 and $68,900, respectively at December 31, 2017. The decrease
in cash and cash equivalents and marketable securities was due mainly to cash
used in operations of $39,295, net of an unrealized foreign exchange gain of
$3,108. The decrease in working capital was due mainly to cash used in
operations and a decrease to accounts payable and accrued liabilities due to
timing of clinical trial related payments.
We are indebted to the Federal
Economic Development Agency for Southern Ontario, or FedDev, under a
non-interest bearing contribution agreement and are making monthly repayments of
$10 through November 2019. As at December 31, 2018 and 2017, the balances
repayable were $96 and $211 respectively. The loan payable was discounted using
an estimated market interest rate of 15%. Interest expense accretes on the
discounted loan amount until it reaches its face value at maturity.
As at December 31, 2018 and 2017, we had a deferred lease
inducement of $375 and $407, respectively, for our facility lease. The
inducement benefit is being recognized over the expected term of the lease.
As at December 31, 2018 and 2017, we had a long-term liability
of $127 and $801, respectively, related to contingent consideration on the
acquisition of Fluorinov. For the year ended December 31, 2018, the
remeasurement of the fair value of the contingent consideration recognized an
increase in the time estimate and increased risk of reaching the potential
milestones, resulting in an expense reduction of $674 which is included in
research and development expenses.
Cash flows from operating activities
Cash used in operating activities increased to $39,295 for the
year ended December 31, 2018, compared to $27,038 for the year ended December
31, 2017, due mainly to higher research and development costs, a decrease in
accounts payable and accrued liabilities of $1,196 compared to an increase of
$8,165 in the prior year, and by an unrealized foreign exchange gain of $3,108
compared to an unrealized loss of $3,748 in the prior year.
Cash flows from investing activities
Cash provided from investing activities totaled $30,632 for the
year ended December 31, 2018, compared to cash used of $57,465 for the year
ended December 31, 2017. The change was due to the maturities of marketable
securities in the year ended December 31, 2018 compared to purchases of
marketable securities in the prior year.
- 15 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Cash flows from financing activities
Cash used in financing activities totaled $115 for the year
ended December 31, 2018, compared to cash provided by financing activities of
$62,575 for the year ended December 31, 2017. The decrease was due to an
underwritten public offering of common shares and non-voting convertible
preferred shares in June 2017 and financing activity in December 2017.
Contractual Obligations and Contingencies
We enter into research, development and license agreements in
the ordinary course of business where we receive research services and rights to
proprietary technologies. Milestone and royalty payments that may become due
under various agreements are dependent on, among other factors, clinical trials,
regulatory approvals and ultimately the successful development of a new drug,
the outcome and timing of which is uncertain.
Under the license agreement for SIRPαFc, we have future
contingent milestones payable of $25 related to successful patent grants, $200
and $300 on the first patient dosed in phase 2 and 3 clinical trials
respectively, and regulatory milestones on their first achievement totaling
$5,000, and low single digit royalties payable on net sales.
Under two agreements with Catalent pursuant to which we
acquired the right to use a proprietary expression system for the manufacture of
two SIRPαFc constructs, we have future contingent milestones on pre-marketing
approval of up to U.S. $875 and aggregate sales milestone payments of up to U.S.
$28,750 for each agreement.
In connection with our acquisition of all the outstanding
shares of Fluorinov, we are obligated to pay up to $35,000 of additional future
payments that are contingent on us achieving certain clinical and regulatory
milestones with an existing Fluorinov compound. We will also have an obligation
to pay royalty payments on future sales of such compounds.
We periodically enter into research and license agreements with
third parties that include indemnification provisions customary in the industry.
These guarantees generally require us to compensate the other party for certain
damages and costs incurred as a result of claims arising from research and
development activities undertaken by or on our behalf. In some cases, the
maximum potential amount of future payments that could be required under these
indemnification provisions could be unlimited. These indemnification provisions
generally survive termination of the underlying agreement. The nature of the
indemnification obligations prevents us from making a reasonable estimate of the
maximum potential amount we could be required to pay. Historically, we have not
made any indemnification payments under such agreements and no amount has been
accrued in our consolidated financial statements with respect to these
indemnification obligations.
Other than as disclosed below, we did not have any contractual
obligations relating to long-term debt obligations, capital (finance) lease
obligations, operating lease obligations, purchase obligations or other
long-term liabilities reflected on our balance sheet as at December 31, 2018:
| |
|
|
Payment due by period |
|
| |
|
|
|
|
|
Less than |
|
|
1 to 3 |
|
|
3 to 5 |
|
|
More than |
|
| Contractual Obligations(1)(2) |
|
|
Total |
|
|
1
year |
|
|
years |
|
|
years |
|
|
5
years |
|
| Long-Term Debt
Obligations(3) |
|
$ |
105 |
|
$ |
105 |
|
$ |
- |
|
$ |
- |
|
$ |
- |
|
| Operating Lease Obligations(4) |
|
|
1,982 |
|
|
398 |
|
|
830 |
|
|
708 |
|
|
46 |
|
| Purchase
Obligations(5) |
|
|
30,694 |
|
|
23,308 |
|
|
7,290 |
|
|
96 |
|
|
- |
|
| Other Long-Term Liabilities Reflected on our
Balance Sheet(6) |
|
|
467 |
|
|
340 |
|
|
- |
|
|
- |
|
|
127 |
|
| |
|
$ |
33,248 |
|
$ |
24,151 |
|
$ |
8,120 |
|
$ |
804 |
|
$ |
173 |
|
- 16 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
|
|
|
|
|
|
|
|
|
Notes: |
|
|
| |
(1) |
Contractual obligations in the above table do not include
amounts in accounts payable and accrued liabilities on our balance sheet
as at December 31, 2018. |
| |
(2) |
Contingent milestones under the SIRPαFc license agreement
and the Catalent expression system agreements are not included in the
above table. |
| |
(3) |
Amounts due to FedDev repayable in equal monthly
installments of $10 through November 2019. |
| |
(4) |
Includes operating lease obligations for laboratory and
office facilities. |
| |
(5) |
Purchase obligations include all non-cancellable
contracts, and all cancellable contracts with $100 or greater remaining
committed at the period end including agreements related to the conduct of
our clinical trials, preclinical studies and manufacturing
activities. |
| |
(6) |
Includes $127 of contingent consideration related to
potential future payments of up to $35,000 based on the achievement of
clinical and regulatory milestones with an existing Fluorinov
compound. |
Description of Share Capital
The continuity of the number of our issued and outstanding
common and preferred shares from December 31, 2017 to the date of this MD&A
is presented below:
|
|
|
Number
of Series I |
|
|
Number
of Series II |
|
|
Number
of |
|
| |
|
Preferred Shares(1 |
) |
|
Preferred Shares(2 |
) |
|
Common Shares |
|
| |
|
|
|
|
|
|
|
|
|
| Balance at December 31, 2017
|
|
52,325,827 |
|
|
4,368,403 |
|
|
13,147,404 |
|
| Issued to amend SIRPαFc license |
|
- |
|
|
- |
|
|
369,621 |
|
| Preferred share conversion |
|
(35,154,286 |
) |
|
- |
|
|
1,171,806 |
|
| Balance at December 31, 2018 |
|
17,171,541 |
|
|
4,368,403 |
|
|
14,688,831 |
|
| Public offering |
|
- |
|
|
12,200,000 |
|
|
6,550,000 |
|
| Balance at the date of this MD&A |
|
17,171,541 |
|
|
16,568,403 |
|
|
21,238,831 |
|
|
Notes: |
|
|
| |
(1) |
Convertible at a ratio of 30 Series I Preferred Shares
for one common share. |
| |
(2) |
Convertible at a ratio of one Series II Preferred Share
for one common share. |
Share capital issued – issued subsequent to the year
ended December 31, 2018
In February 2019, we completed an underwritten public offering
for gross proceeds of U.S. $15 million comprised of 6,550,000 common share units
and 12,200,000 Series II Non-Voting Convertible First Preferred Share units,
each issued at U.S. $0.80 per unit. Each common share unit is comprised of one
common share of the Company and one common share purchase warrant. Each common
share purchase warrant will be exercisable for one common share at a price of
U.S. $0.96 per common share purchase warrant for sixty months. Each preferred
share unit is comprised of one Series II First Preferred Share of the Company
and one Series II First Preferred Share purchase warrant. Each Series II First
Preferred Share purchase warrant will be exercisable for one Series II First
Preferred Share at a price of U.S. $0.96 per Series II First Preferred Share
purchase warrant for sixty months.
Share capital issued – for the year ended December 31,
2018
During the year ended December 31, 2018, 369,621 common shares
were issued on the renegotiation of the SIRPαFc license agreement, and 1,171,806
common shares were issued on the conversion of preferred shares.
- 17 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Share capital issued – for the year ended December 31,
2017
In June 2017, we completed an underwritten public offering of
common shares and non-voting convertible preferred shares in the United States.
In the offering, we sold 2,949,674 common shares and 3,250,000 Series II
Non-Voting Convertible First Preferred Shares at a price of U.S. $5.00 per
share. The gross proceeds from this offering were $41,847 (U.S. $30,998) before
deducting offering expenses of $2,856.
In December 2017, the Company completed a non-brokered private
placement financing and sold 1,950,000 common shares and 400,000 Series II
Non-Voting Convertible Preferred Shares at a price of U.S. $8.50 per share
yielding gross proceeds of $25,338 (U.S. $19,975) before deducting offering
expenses of $1,784.
During the year ended December 31, 2017, 13,332 common shares
were issued on the exercise of 399,980 warrants for proceeds of $159; 900,364
Series I First Preferred Shares were converted into 30,012 common shares; and
359,202 Series II First Preferred Shares were converted into 359,202 common
shares.
Warrants
The continuity of the number of issued and outstanding warrants
for the years ended December 31, 2017 and 2018, and to the date of this MD&A
is presented below:
| |
|
Preferred |
|
|
Common
Share |
|
| |
|
Warrants |
|
|
Warrants |
|
| |
|
|
|
|
|
|
| Balance at December 31, 2017 |
|
1,190,476 |
(1) |
|
69,073,031 |
(2)
|
| Expired |
|
(1,190,476 |
) |
|
(69,073,031 |
) |
| Balance at December 31, 2018 |
|
- |
|
|
- |
|
| Issued in public
offering |
|
12,200,000 |
(3) |
|
6,550,000 |
(4) |
| Balance at the date of this MD&A |
|
12,200,000 |
|
|
6,550,000 |
|
|
Notes: |
|
|
| |
(1) |
These Preferred Warrants were exercisable at $8.40 per
warrant for one common share or one Series II Preferred Share. |
| |
(2) |
These warrants were exercisable at a ratio of 30 warrants
for one common share. |
| |
(3) |
Each preferred share warrant is exercisable for one
Series II First Preferred Share at an exercise price of U.S. $0.96 per
Series II First Preferred Share. |
| |
(4) |
Each common share warrant is exercisable for one common
share at an exercise price of U.S. $0.96 per common
share. |
Stock Options
The 2018 Stock Option Plan was approved by our shareholders at
the annual meeting held on June 1, 2018. Stock options granted are
equity-settled, have a vesting period of four years and have a maximum term of
ten years. The total number of common shares available for issuance under the
2018 Stock Option Plan is 3,894,501. As at December 31, 2018, we were entitled
to issue an additional 1,195,296 stock options under the 2018 Stock Option Plan.
- 18 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
The continuity of the number of issued and outstanding stock
options from December 31, 2017 to the date of this MD&A is presented below:
|
|
|
Number
of |
|
|
Weighted
Average |
|
| |
|
Options |
|
|
Exercise Price |
|
| |
|
|
|
|
|
|
| Balance at December 31, 2017
|
|
1,746,982 |
|
|
$12.87 |
|
| Granted |
|
1,082,600 |
|
|
4.95 |
|
| Forfeited |
|
(128,356 |
) |
|
12.98 |
|
| Expired |
|
(2,021 |
) |
|
14.08
|
|
| Balance at December 31, 2018
|
|
2,699,205 |
|
|
9.69 |
|
| Expired |
|
(116,309 |
) |
|
20.26
|
|
| Balance at the date of this MD&A |
|
2,582,896 |
|
|
9.21 |
|
Deferred Share Unit Plan
The board of directors approved a Cash-Settled DSU Plan on
November 9, 2016. For the years ended December 31, 2018 and 2017, there were
189,393 and 46,187 DSUs issued, respectively. The fair value of DSUs as at
December 31, 2018 and 2017 was $806 and $1,349, respectively. The number of DSUs
outstanding as at December 31, 2018 and 2017 were 334,982 and 145,589,
respectively.
Fully Diluted Share Capital
The number of issued and outstanding common shares, Series I
First Preferred Shares, Series II First Preferred Shares, and stock options on a
fully converted basis as at December 31, 2018 was as follows:
| |
|
Number of Common |
|
| |
|
Share Equivalents |
|
| |
|
|
|
| Common shares |
|
14,688,831 |
|
| Series I First Preferred Shares |
|
572,385 |
|
| Series II First Preferred Shares |
|
4,368,403 |
|
| Stock options |
|
2,699,205 |
|
| Total |
|
22,328,824 |
|
Trend Information
Historical patterns of expenditures cannot be taken as an
indication of future expenditures. The amount and timing of expenditures and
therefore liquidity and capital resources vary substantially from period to
period depending on the number of research and development programs being
undertaken at any one time, the stage of the development programs, the timing of
significant expenditures for manufacturing, toxicology and pharmacology studies
and clinical trials, and the availability of funding from investors and
prospective commercial partners.
- 19 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Selected Annual Financial Information
|
2018
$ |
2017
$ |
2016
$ |
Revenue
|
-
|
-
|
-
|
| Net loss and comprehensive loss |
42,486 |
45,088 |
31,733 |
| Fully diluted net loss per common share |
3.06 |
4.61 |
4.06 |
Total long-term liabilities
|
502
|
1,306
|
2,588
|
| Total assets |
55,459 |
94,403 |
66,623 |
Net loss for the year ended December 31, 2017 was higher than
the net loss of 2016 due mainly to higher clinical development expenses with two
active TTI-621 phase I trials and manufacturing expenses for TTI-622, the
recognition of a deferred tax recovery in the year ended December 31, 2016
related to the acquisition of Fluorinov of $3,690, and a higher net foreign
currency loss of $2,715 in 2017. Total long term liabilities decreased from
December 31, 2016 to December 31, 2018 due mainly to the reduction in the
contingent consideration related to the Fluorinov acquisition. Total assets
increased in 2017 over 2016 due mainly to two financings completed in 2017, net
of cash used in operations. In 2018, total assets decreased due mainly to the
use of cash in operations for the year.
Selected Quarterly Financial Information
2018 |
Q4-2018
$ |
Q3-2018
$ |
Q2-2018
$ |
Q1-2018
$ |
| Revenue |
- |
- |
- |
- |
| Research and development expenses |
10,611 |
10,752 |
12,722 |
9,341 |
| General and administrative expenses |
206 |
1,100 |
1,247 |
1,029 |
| Net loss for the period |
8,553 |
13,052 |
12,316 |
8,565 |
| Basic and diluted net loss per share |
0.56 |
0.91 |
0.92 |
0.65 |
| Cash and cash equivalents and marketable
securities |
45,409 |
52,095 |
64,698 |
73,920 |
2017 |
Q4-2017
$ |
Q3-2017
$ |
Q2-2017
$ |
Q1-2017
$ |
| Revenue |
- |
- |
- |
- |
| Research and development expenses |
9,811 |
8,275 |
8,851 |
10,199 |
| General and administrative expenses |
1,254 |
969 |
684 |
954 |
| Net loss for the period |
10,658 |
11,337 |
11,642 |
11,451 |
| Basic and diluted net loss per share |
0.91 |
1.05 |
1.33 |
1.46 |
| Cash and cash equivalents and marketable
securities |
81,791 |
64,297 |
72,618 |
41,347 |
- 20 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
The net losses for the first and second quarters of 2017 were
higher due to higher personnel costs, SIRPαFc clinical trial costs, and
preclinical work on the bromodomain inhibitor and EGFR inhibitor programs. The
net loss for the third and fourth quarters of 2017 reflected continued focus on
the SIRPαFc development program, and lower small molecule expenses relative to
the first and second quarters of 2017. The decrease in net loss in the first
quarter of 2018 reflected a higher net foreign currency gain. The increase in
net loss in the second quarter of 2018 reflected higher clinical development
expenses and the license agreement amendment payment, partially offset by a net
foreign currency gain. The increase in net loss in the third quarter of 2018
reflected higher clinical development costs. The decrease in net loss in the
fourth quarter of 2018 was mainly due to a net foreign currency gain and change
in fair value of deferred share units.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have, or
are reasonably likely to have, a current or future effect on our financial
condition, revenues or expenses, results of operations, liquidity, capital
expenditures or capital resources that are material to investors.
Implications of Being an Emerging Growth Company
We are an “emerging growth company” under the U.S. Jumpstart
Our Business Startups Act of 2012, or the JOBS Act, and will continue to qualify
as an “emerging growth company” until the earliest to occur of: (a) the last day
of the fiscal year during which we have total annual gross revenues of $1.07
billion (as such amount is indexed for inflation every 5 years by the SEC) or
more; (b) the last day of our fiscal year following the fifth anniversary of the
date of the first sale of our common shares pursuant to an effective
registration statement under the U.S. Securities Act of 1933 which is December
31, 2019; (c) the date on which we have, during the previous 3-year period,
issued more than $1.0 billion in non-convertible debt; or (d) the date on which
we are deemed to be a “large accelerated filer”, as defined in Rule 12b–2 of the
U.S. Securities Exchange Act of 1934, or the Exchange Act.
Generally, a company that registers any class of its securities
under Section 12 of the Exchange Act is required to include in the second and
all subsequent annual reports filed by it under the Exchange Act, a management
report on internal control over financial reporting and, subject to an exemption
available to companies that meet the definition of a “smaller reporting company”
in Rule 12b-2 under the Exchange Act, an auditor attestation report on
management’s assessment of the company’s internal control over financial
reporting. However, for so long as we continue to qualify as an emerging growth
company, we will be exempt from the requirement to include an auditor
attestation report in our annual reports filed under the Exchange Act, even if
we do not qualify as a “smaller reporting company”. In addition, Section
103(a)(3) of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, has been
amended by the JOBS Act to provide that, among other things, auditors of an
emerging growth company are exempt from any rules of the Public Company
Accounting Oversight Board requiring mandatory audit firm rotation or a
supplement to the auditor’s report in which the auditor would be required to
provide additional information about the audit and the financial statements of
the company.
Any U.S. domestic issuer that is an emerging growth company is
able to avail itself of the reduced disclosure obligations regarding executive
compensation in periodic reports and proxy statements, and to not present to its
shareholders a non-binding advisory vote on executive compensation, obtain
approval of any golden parachute payments not previously approved, or present
the relationship between executive compensation actually paid and our financial
performance. So long as we are a foreign private issuer, we are not subject to
such requirements, and will not become subject to such requirements even if we
were to cease to be an emerging growth company.
As a reporting issuer under the securities legislation of the
Canadian provinces of Ontario, British Columbia, Manitoba, Nova Scotia and
Alberta, we are required to comply with all new or revised accounting standards
that apply to Canadian public companies. Pursuant to Section 107(b) of the JOBS
Act, an emerging growth company may elect to utilize an extended transition
period for complying with new or revised accounting standards for public
companies until such standards apply to private companies. We have elected not
to utilize this extended transition period.
- 21 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Critical Accounting Estimates
The preparation of financial statements in conformity with IFRS
requires management to make judgments, estimates and assumptions that affect the
application of accounting policies and the reported amounts of assets and
liabilities, revenue and expenses, related disclosures of contingent assets and
liabilities and the determination of our ability to continue as a going concern.
Actual results could differ materially from these estimates and assumptions. We
review our estimates and underlying assumptions on an ongoing basis. Revisions
are recognized in the period in which the estimates are revised and may impact
future periods.
The areas involving a higher degree of judgment or complexity,
or areas where assumptions and estimates are significant to the financial
statements have been set out in note 2 of our annual audited consolidated
financial statements for the year ended December 31, 2018.
Accounting Policies
Our significant accounting policies are outlined in our annual
audited consolidated financial statements for the year ended December 31, 2018.
This MD&A should be read in conjunction with the annual audited consolidated
financial statements for the year ended December 31, 2018.
New standards, amendments and interpretations adopted
during 2018
IFRS 9 Financial Instruments
As at January 1, 2018, we adopted IFRS 9 Financial
Instruments, or IFRS 9. We have elected to not restate comparative periods
in the year of initial application of IFRS 9 relating to the transition for
classification, measurement and impairment. As a result, the comparative
information provided continues to be accounted for on a basis consistent with
those followed in the December 31, 2017 consolidated financial statements.
IFRS 9 replaces the provisions of IAS 39 Financial
Instruments: Recognition and Measurement that relate to the recognition,
classification and measurement of financial assets and financial liabilities,
derecognition of financial instruments, impairment of financial assets and hedge
accounting. IFRS 9 also significantly amends other standards dealing with
financial instruments such as IFRS 7 Financial Instruments: Disclosures.
We assessed the classification and measurement of financial
instruments we held at the date of initial application of IFRS 9 (January 1,
2018) and have classified our financial instruments into the appropriate IFRS 9
categories. There were no changes to the carrying value of our financial
instruments resulting from this reclassification and accordingly there was no
impact to our opening balance of deficit as at January 1, 2018 as a result of
the adoption of IFRS 9.
IFRS 15 Revenue from Contracts with Customers
As at January 1, 2018, we adopted IFRS 15 Revenue from
Contracts with Customers, or IFRS 15, which covers principles for reporting
about the nature, amount, timing and uncertainty of revenue and cash flows
arising from contracts with customers. The adoption of this standard did not
have an impact on the audited consolidated financial statements.
- 22 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
New standards and interpretations not yet
effective
IFRS 16 Leases
In January 2016 the IASB issued IFRS 16 Leases, or IFRS
16, which replaces IAS 17 Leases. IFRS 16 sets out the principles for the
recognition, measurement, presentation and disclosure of leases and requires
lessees to account for all leases under a single on-balance sheet model, with
certain exemptions. The standard includes two recognition exemptions for lessees
– leases of “low-value” assets and short-term leases with a lease term of 12
months or less. At the commencement date of a lease, a lessee will recognise a
liability to make lease payments and an asset representing the right to use the
underlying asset during the lease term. Lessees will be required to separately
recognise the interest expense on the lease liability and the depreciation
expense on the right-of-use asset. The new standard will be effective for annual periods beginning
on or after January 1, 2019.
We plan to adopt IFRS 16 retrospectively and will elect to use
the exemption proposed by the standard on lease contracts where the underlying
asset is of low value. We have leases of certain office equipment (i.e.
photocopying machines) that are considered of low value. Management are in the process of assessing the impact of IFRS
16. Management’s preliminary assessment is that the impact on the financial
statements is unlikely to be significant.
Other accounting standards or amendments to existing accounting
standards that have been issued, but have future effective dates, are either not
applicable or are not expected to have a significant impact on our consolidated
financial statements.
RISK FACTORS
The following information sets forth material risks and
uncertainties that may affect our business, including our future financing and
operating results and could cause our actual results to differ materially from
those contained in forward-looking statements we have made in this MD&A. The
risks and uncertainties below are not the only ones we face. Additional risks
and uncertainties not presently known to us or that we believe to be immaterial
may also adversely affect our business. Further, if we fail to meet the
expectations of the public market in any given period, the market price of our
common shares could decline. We operate in a highly competitive environment that
involves significant risks and uncertainties, some of which are outside of our
control.
Risks Related to Our Financial Position and Need for
Additional Capital
We expect to incur future losses and we may never become
profitable.
We have incurred losses of $42,486, $45,088 and $31,733 for the
years ended December 31, 2018, 2017, and 2016, respectively, and expect to incur
an operating loss for the year ending December 31, 2019. We have an accumulated
deficit since inception through December 31, 2018 of $184,597. We believe that
operating losses will continue as we are planning to incur significant costs
associated with the clinical development of SIRPαFc. Our net losses have had and
will continue to have an adverse effect on, among other things, our
shareholders’ equity, total assets and working capital. We expect that losses
will fluctuate from quarter to quarter and year to year, and that such
fluctuations may be substantial. We cannot predict when we will become
profitable, if at all.
We will require additional capital to finance our
operations, which may not be available to us on acceptable terms, or at all. As
a result, we may not complete the development and commercialization of our
product candidates or develop new product candidates.
- 23 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
As a research and development company, our operations have
consumed substantial amounts of cash since inception. We expect to spend
substantial funds to continue the research, development and testing of our
product candidates and to prepare to commercialize products subject to approval
of the FDA, in the U.S. and similar approvals in other jurisdictions. We will
also require significant additional funds if we expand the scope of our current
clinical plans or if we were to acquire any new assets and advance their
development. Therefore, for the foreseeable future, we will have to fund all of
our operations and development expenditures from cash on hand, equity or debt
financings, through collaborations with other biotechnology or pharmaceutical
companies or through financings from other sources. We expect that our existing
cash and cash equivalents and marketable securities as at December 31, 2018 of
$45,409 will enable us to fund our current operating plan requirements for at
least the next twelve months. Additional financing will be required to meet our
longer term liquidity needs. If we do not succeed in raising additional funds on
acceptable terms, we might not be able to complete planned preclinical studies
and clinical trials or pursue and obtain approval of any product candidates from
the FDA and other regulatory authorities. It is possible that future financing
will not be available or, if available, may not be on favorable terms. The
availability of financing will be affected by the achievement of our corporate
goals, the results of scientific and clinical research, the ability to obtain
regulatory approvals, the state of the capital markets generally and with
particular reference to drug development companies, the status of strategic
alliance agreements and other relevant commercial considerations. If adequate
funding is not available, we may be required to delay, reduce or eliminate one
or more of our product development programs, or obtain funds through corporate
partners or others who may require us to relinquish significant rights to
product candidates or obtain funds on less favorable terms than we would
otherwise accept. To the extent that external sources of capital become limited
or unavailable or available on onerous terms, our intangible assets and our
ability to continue our clinical development plans may become impaired, and our
assets, liabilities, business, financial condition and results of operations may
be materially or adversely affected.
We currently have no product revenue and will not be able
to maintain our operations and research and development without additional
funding.
To date, we have generated no product revenue and cannot
predict when and if we will generate product revenue. Our ability to generate
product revenue and ultimately become profitable depends upon our ability, alone
or with partners, to successfully develop our product candidates, obtain
regulatory approval, and commercialize products, including any of our current
product candidates, or other product candidates that we may develop, in-license
or acquire in the future. We do not anticipate generating revenue from the sale
of products for the foreseeable future. We expect our research and development
expenses to increase in connection with our ongoing activities, particularly as
we advance our product candidates through clinical trials.
If the price of our common shares continues to trade
below US$1.00 per share on the Nasdaq Capital Market for a sustained period, or
we do not meet other continued listing requirements, our common shares may be
delisted from the Nasdaq Capital Market, which could affect the market price and
liquidity for our common shares and reduce our ability to raise additional
capital.
Our common shares are listed on the Nasdaq Capital Market. In order to maintain
that listing, we must satisfy minimum financial and other requirements
including, without limitation, a requirement that our closing bid price be at
least US$1.00 per share. Since February 22, 2019, the share price of our common
shares has been below US$1.00. If our common shares continue to trade below
US$1.00 per share for 30 consecutive business days since February 22, 2019, we
will receive a notification letter from the Nasdaq Capital Market and will have
180 calendar days (subject to extension in some circumstances) to regain
compliance with the minimum bid price rule. To regain compliance, the closing
bid price of our common shares must be at least US$1.00 per share for a minimum
of ten consecutive business days (or such longer period of time as the Nasdaq
Capital Market may require in some circumstances). If we fail to regain
compliance with the minimum bid price rule or fail to maintain compliance with
all other applicable Nasdaq Capital Market continued listing requirements, the
Nasdaq Capital Market may determine to delist our common shares, at which time
our common shares would likely trade in the United States only on the
over-the-counter market (the “OTC”). The delisting of our common shares from the
Nasdaq Capital Market could adversely impact us by, among other things, reducing
the liquidity and market price of our common shares in the United States,
reducing the number of investors willing to hold or acquire our common shares,
limiting our ability to issue additional securities in the future, and limiting
our ability to fund our operations.
We are exposed to the financial risk related to the
fluctuation of foreign exchange rates and the degrees of volatility of those
rates.
We may be adversely affected by foreign currency fluctuations.
To date, we have been primarily funded through issuances of equity, proceeds
from the exercise of warrants and stock options and from interest income on
funds available for investment, which are denominated both in Canadian and U.S.
dollars. Also, a significant portion of our expenditures are in U.S. dollars,
and we are therefore subject to foreign currency fluctuations which may, from
time to time, impact our financial position and results of operations.
- 24 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Risks Related to Our Business and Our Industry
Our prospects depend on the success of our product
candidates which are at early stages of development, and we may not generate
revenue for several years, if at all, from these products.
Given the early stage of our product development, we can make
no assurance that our research and development programs will result in
regulatory approval or commercially viable products. To achieve profitable
operations, we, alone or with others, must successfully develop, gain regulatory
approval, and market our future products. We currently have no products that
have been approved by the FDA, Health Canada, or HC, or any similar regulatory
authority. To obtain regulatory approvals for our product candidates being
developed and to achieve commercial success, clinical trials must demonstrate
that the product candidates are safe for human use and that they demonstrate
efficacy. While we have commenced clinical trials for SIRPαFc, we have not yet
completed later stage clinical trials for any of our product candidates.
Many product candidates never reach the stage of clinical
testing and even those that do have only a small chance of successfully
completing clinical development and gaining regulatory approval. Product
candidates may fail for a number of reasons, including, but not limited to,
being unsafe for human use or due to the failure to provide therapeutic benefits
equal to or better than the standard of treatment at the time of testing.
Unsatisfactory results obtained from a particular study relating to a research
and development program may cause us or our collaborators to abandon commitments
to that program.
We acquired several preclinical and discovery research programs
in our acquisition of Fluorinov, including certain assets relating to the
treatment of CNS disorders. While we conducted extensive due diligence before
making this acquisition, our assessment of the Fluorinov technologies may not be
accurate. Therefore, our expectations about whether various clinical and
regulatory milestones with an existing Fluorinov compound or development of a
future program on the Fluorinov development platform will be achieved may not be
borne out fully or at all. We have made a commitment to use commercially
reasonable efforts to monetize the Fluorinov CNS assets and, if successful, to
share the net proceeds with the Fluorinov vendors. As this is not our core
competency, our efforts to monetize these assets or any other Fluorinov assets
may not be successful. We can make no assurances that toxicology, or other
preclinical, studies will yield results that will allow us to proceed with
clinical trials in humans.
The early stage of our product development makes it
particularly uncertain whether any of our product development efforts will prove
to be successful and meet applicable regulatory requirements, and whether any of
our product candidates will receive the requisite regulatory approvals, be
capable of being manufactured at a reasonable cost or be successfully marketed.
If we are successful in developing our current and future product candidates
into approved products, we will still experience many potential obstacles such
as the need to develop or obtain manufacturing, marketing and distribution
capabilities. If we are unable to successfully commercialize any of our
products, our financial condition and results of operations may be materially
and adversely affected.
Positive results from preclinical and early clinical
research of TTI-621 and TTI-622 are not necessarily predictive of the results of
later clinical trials of TTI-621 or TTI-622. If we cannot replicate the positive
results from preclinical and early clinical research in our later clinical
trials, we may be unable to successfully develop, obtain regulatory approval for
and commercialize TTI-621 or TTI-622.
Positive results of preclinical and early clinical research of
TTI-621 and TTI-622 may not be indicative of the results that will be obtained
in later-stage clinical trials. For example, we have focused our near-term
clinical product development on T-cell malignancies based on preliminary results
of our intratumoral trial which were presented at the American Society of
Hematology meeting in December 2017 and updated results presented at the EORTC
CLTF meeting in September 2018. There can be no assurance that the preliminary
results we have seen in a small number of mycosis fungoides patients will be
reproducible in a larger population of patients. We can make no assurance that
any future studies, if undertaken, will yield favorable results.
- 25 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Many companies in the pharmaceutical and biotechnology
industries have suffered significant setbacks in later-stage clinical trials
after achieving positive results in early-stage development, and we cannot be
certain that we will not face similar setbacks. These setbacks have been caused
by, among other things, preclinical findings made while clinical trials were
underway or safety or efficacy observations made in clinical trials, including
previously unreported adverse events. Moreover, preclinical and clinical data
are often susceptible to varying interpretations and analyses, and many
companies that believed their product candidates performed satisfactorily in
preclinical studies and clinical trials nonetheless failed to obtain FDA
approval. If we fail to produce positive results in our future clinical trials
of TTI-621 or TTI-622, the development timeline and regulatory approval and
commercialization prospects for our leading product candidates, and,
correspondingly, our business and financial prospects, would be materially
adversely affected.
We rely and will continue to rely on third parties to
plan, conduct and monitor our preclinical studies and clinical trials, and their
failure to perform as required could cause substantial harm to our
business.
We rely and will continue to rely on third parties to conduct a
significant portion of our preclinical and clinical development activities.
Preclinical activities include in vivo studies providing access to specific
disease models, pharmacology and toxicology studies, and assay development.
Clinical development activities include trial design, regulatory submissions,
clinical patient recruitment, clinical trial monitoring, clinical data
management and analysis, safety monitoring and project management. If there is
any dispute or disruption in our relationship with third parties, or if they are
unable to provide quality services in a timely manner and at a feasible cost,
our active development programs will face delays. Further, if any of these third
parties fails to perform as we expect or if their work fails to meet regulatory
requirements, our testing could be delayed, cancelled or rendered
ineffective.
We rely on contract manufacturers over whom we have
limited control. If we are subject to quality, cost or delivery issues with the
preclinical and clinical grade materials supplied by contract manufacturers, our
business operations could suffer significant harm.
We have limited manufacturing experience and rely on contract
manufacturing organizations, or CMOs to manufacture our product candidates for
larger preclinical studies and clinical trials. We rely on CMOs for
manufacturing, filling, packaging, storing and shipping of drug product in
compliance with current Good Manufacturing Practice, or cGMP, regulations
applicable to our products. The FDA ensures the quality of drug products by
carefully monitoring drug manufacturers’ compliance with cGMP regulations. The
cGMP regulations for drugs contain minimum requirements for the methods,
facilities and controls used in manufacturing, processing and packing of a drug
product.
We contracted with Catalent for the manufacture of the SIRPαFc
protein to supply drug substance for our phase 1 clinical trials. The
manufacture of recombinant proteins uses well established processes including a
protein expression system. Catalent is producing SIRPαFc using their proprietary
GPEx® expression system. We believe that Catalent has the capacity, the systems,
and the experience to supply SIRPαFc for our phase 1 clinical trials and we may
consider using Catalent for manufacturing for later clinical trials. However,
since the Catalent manufacturing facility where SIRPαFc is being produced does
not support commercial manufacturing, it has not yet been inspected by the FDA.
Any manufacturing failures, delays or compliance issues could cause delays in
the conduct of SIRPαFc preclinical studies and clinical trials.
There can be no assurances that CMOs will be able to meet our
timetable and requirements. We have not contracted with alternate suppliers for
SIRPαFc drug substance production in the event Catalent is unable to scale up
production, or if Catalent otherwise experiences any other significant problems.
If we are unable to arrange for alternative third-party manufacturing sources on
commercially reasonable terms or in a timely manner, we may be delayed in the
development of our product candidates. Further, CMOs must operate in compliance
with cGMP and failure to do so could result in, among other things, the
disruption of product supplies. Our dependence upon third parties for the
manufacture of our products may adversely affect our profit margins and our
ability to develop and deliver products on a timely and competitive basis.
- 26 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
We require commercial scale and quality manufactured
product to be available for pivotal or registration clinical trials. If we do
not have commercial grade drug supply when needed, we may face delays in
initiating or completing pivotal trials and our business operations could suffer
significant harm.
To date, our product has been manufactured in small quantities
for pre-clinical studies and clinical trials by third-party manufacturers. In
order to commercialize our product, we need to manufacture commercial quality
drug supply for use in registration clinical trials. Most, if not all, of the
clinical material used in phase 3/pivotal/registration studies must be derived
from the defined commercial process including scale, manufacturing site, process
controls and batch size. If we have not scaled up and validated the commercial
production of our product prior to the commencement of pivotal clinical trials,
we may have to employ a bridging strategy during the trial to demonstrate
equivalency of early stage material to commercial drug product, or potentially
delay the initiation or completion of the trial until drug supply is available.
The manufacturing of commercial quality drug product requires significant
efforts including, but not limited to scale-up of production to anticipated
commercial scale, process characterization and validation, analytical method
validation, identification of critical process parameters and product quality
attributes, multiple process performance and validation runs, has long lead
times and is very expensive. If we do not have commercial drug supply available
when needed for pivotal clinical trials, our regulatory and commercial progress
may be delayed and we may incur increased product development cost. This may
have a material adverse effect on our business, financial condition and
prospects, and may delay marketing of the product.
If clinical trials of our product candidates fail to
demonstrate safety and efficacy to the satisfaction of regulatory authorities or
do not otherwise produce positive results, we would incur additional costs or
experience delays in completing, or ultimately be unable to complete, the
development and commercialization of our product candidates.
Before obtaining marketing approval from regulatory authorities
for the sale of our product candidates, we must conduct preclinical studies in
animals and extensive clinical trials in humans to demonstrate the safety and
efficacy of the product candidates. Clinical testing is expensive and difficult
to design and implement, can take many years to complete and has uncertain
outcomes. The outcome of preclinical studies and early clinical trials may not
predict the success of later clinical trials, and interim results of a clinical
trial do not necessarily predict final results. A number of companies in the
pharmaceutical and biotechnology industries have suffered significant setbacks
in advanced clinical trials due to lack of efficacy or unacceptable safety
profiles, notwithstanding promising results in earlier trials. We do not know
whether the clinical trials we may conduct will demonstrate adequate efficacy
and safety to result in regulatory approval to market any of our product
candidates in any jurisdiction. A product candidate may fail for safety or
efficacy reasons at any stage of the testing process. A major risk we face is
the possibility that none of our product candidates under development will
successfully gain market approval from the FDA or other regulatory authorities,
resulting in us being unable to derive any commercial revenue from them after
investing significant amounts of capital in their development.
If we experience delays in clinical testing, we will be
delayed in commercializing our product candidates, and our business may be
substantially harmed.
We cannot predict whether any clinical trials will begin as
planned, will need to be restructured, or will be completed on schedule, or at
all. Our product development costs will increase if we experience delays in
clinical testing. Significant clinical trial delays could shorten any periods
during which we may have the exclusive right to commercialize our product
candidates or allow our competitors to bring products to market before us, which
would impair our ability to successfully commercialize our product candidates
and may harm our financial condition, results of operations and prospects. The
commencement and completion of clinical trials for our products may be delayed
for a number of reasons, including delays related, but not limited, to:
- failure by regulatory authorities to grant permission to proceed or
placing the clinical trial on hold;
- patients failing to enroll or remain in our trials at the rate we expect;
- suspension or termination of clinical trials by regulators for many
reasons, including concerns about patient safety or failure of our CMOs to
comply with cGMP requirements;
- 27 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
- any changes to our manufacturing process that may be necessary or desired;
- delays or failure to obtain clinical supply from CMOs of our products
necessary to conduct clinical trials;
- product candidates demonstrating a lack of safety or efficacy during
clinical trials;
- patients choosing an alternative treatment for the indications for which
we are developing any of our product candidates or participating in competing
clinical trials;
- patients failing to complete clinical trials due to dissatisfaction with
the treatment, side effects or other reasons;
- reports of clinical testing on similar technologies and products raising
safety and/or efficacy concerns;
- competing clinical trials and scheduling conflicts with participating
clinicians;
- clinical investigators not performing our clinical trials on their
anticipated schedule, dropping out of a trial, or employing methods not
consistent with the clinical trial protocol, regulatory requirements or other
third parties not performing data collection and analysis in a timely or
accurate manner;
- failure of our contract research organizations, or CROs, to satisfy their
contractual duties or meet expected deadlines;
- inspections of clinical trial sites by regulatory authorities or
Institutional Review Boards, or IRBs, or ethics committees finding regulatory
violations that require us to undertake corrective action, resulting in
suspension or termination of one or more sites or the imposition of a clinical
hold on the entire study;
- one or more IRBs or ethics committees rejecting, suspending or terminating
the study at an investigational site, precluding enrollment of additional
subjects, or withdrawing its approval of the trial; or
- failure to reach agreement on acceptable terms with prospective clinical
trial sites.
Our product development costs will increase if we experience
delays in testing or approval or if we need to perform more or larger clinical
trials than planned. Additionally, changes in regulatory requirements and
policies may occur, and we may need to amend study protocols to reflect these
changes. Amendments may require us to resubmit our study protocols to regulatory
authorities or IRBs or ethics committees for re-examination, which may impact
the cost, timing or successful completion of that trial. Delays or increased
product development costs may have a material adverse effect on our business,
financial condition and prospects.
We may not be able to file INDs to commence additional
clinical trials on the timelines we expect, and even if we are able to, the FDA
may not permit us to proceed in a timely manner, or at all.
Prior to commencing clinical trials in the United States for
any of our product candidates, we may be required to have an allowed IND for
each product candidate and to file additional INDs prior to initiating any
additional clinical trials for SIRPαFc. We believe that the data from previous
studies will support the filing of additional INDs, to enable us to undertake
additional clinical studies as we have planned. However, submission of an IND
may not result in the FDA allowing further clinical trials to begin and, once
begun, issues may arise that will require us to suspend or terminate such
clinical trials. Additionally, even if relevant regulatory authorities agree
with the design and implementation of the clinical trials set forth in an IND,
these regulatory authorities may change their requirements in the future.
Failure to submit or have effective INDs and commence or continue clinical
programs will significantly limit our opportunity to generate revenue.
If we have difficulty enrolling patients in clinical
trials, the completion of the trials may be delayed or cancelled.
As our product candidates advance from preclinical testing to
clinical testing, and then through progressively larger and more complex
clinical trials, we will need to enroll an increasing number of patients that
meet our eligibility criteria. There is significant competition for recruiting
cancer patients in clinical trials, and we may be unable to enroll the patients
we need to complete clinical trials on a timely basis or at all. The factors
that affect our ability to enroll patients are largely uncontrollable and
include, but are not limited to, the following:
- size and nature of the patient population;
- eligibility and exclusion criteria for the trial;
- design of the study protocol;
- competition with other companies for clinical sites or patients;
- the perceived risks and benefits of the product candidate under study;
- 28 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
- the patient referral practices of physicians; and
- the number, availability, location and accessibility of clinical trial
sites.
If we are unable to successfully develop companion
diagnostics for our therapeutic product candidates, or experience significant
delays in doing so, we may not achieve marketing approval or realize the full
commercial potential of our therapeutic product candidates.
We may develop companion diagnostics for our therapeutic
product candidates. We expect that, at least in some cases, regulatory
authorities may require the development and regulatory approval of a companion
diagnostic as a condition to approving our therapeutic product candidates. We
have limited experience and capabilities in developing or commercializing
diagnostics and plan to rely in large part on third parties to perform these
functions. We have not begun to develop companion diagnostics for any of our
therapeutic product candidates.
Companion diagnostics are subject to regulation by the FDA, HC,
and comparable foreign regulatory authorities as medical devices and may require
separate regulatory approval or clearance prior to commercialization. If we, or
any third parties that we engage to assist us, are unable to successfully
develop companion diagnostics for our therapeutic product candidates, or
experience delays in doing so, our business may be substantially harmed.
Regulatory approval processes are lengthy, expensive and
inherently unpredictable. Our inability to obtain regulatory approval for our
product candidates would substantially harm our business.
Our development and commercialization activities and product
candidates are significantly regulated by a number of governmental entities,
including the FDA, HC, and comparable authorities in other countries. Regulatory
approvals are required prior to each clinical trial and we may fail to obtain
the necessary approvals to commence or continue clinical testing. We must comply
with regulations concerning the manufacture, testing, safety, effectiveness,
labeling, documentation, advertising, and sale of products and product
candidates and ultimately must obtain regulatory approval before we can
commercialize a product candidate. The time required to obtain approval by such
regulatory authorities is unpredictable but typically takes many years following
the commencement of preclinical studies and clinical trials. Any analysis of
data from clinical activities we perform is subject to confirmation and
interpretation by regulatory authorities, which could delay, limit or prevent
regulatory approval. Even if we believe results from our clinical trials are
favorable to support the marketing of our product candidates, the FDA or other
regulatory authorities may disagree. In addition, approval policies,
regulations, or the type and amount of clinical data necessary to gain approval
may change during the course of a product candidate’s clinical development and
may vary among jurisdictions. We have not obtained regulatory approval for any
product candidate and it is possible that none of our existing product
candidates or any future product candidates will ever obtain regulatory
approval.
We could fail to receive regulatory approval for our product
candidates for many reasons, including, but not limited to:
- disagreement with the design or implementation of our clinical trials;
- failure to demonstrate that a product candidate is safe and effective for
its proposed indication;
- failure of clinical trials to meet the level of statistical significance
required for approval;
- failure to demonstrate that a product candidate’s clinical and other
benefits outweigh its safety risks;
- disagreement with our interpretation of data from preclinical studies or
clinical trials;
- the insufficiency of data collected from clinical trials of our product
candidates to support the submission and filing of a biologic license
application, or BLA, or other submission to obtain regulatory approval;
- deficiencies in the manufacturing processes or the failure of facilities
of CMOs with whom we contract for clinical and commercial supplies to pass a
pre-approval inspection; or
- changes in the approval policies or regulations that render our
preclinical and clinical data insufficient for approval.
- 29 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
A regulatory authority may require more information, including
additional preclinical or clinical data to support approval, which may delay or
prevent approval and our commercialization plans, or we may decide to abandon
the development program. If we were to obtain approval, regulatory authorities
may approve any of our product candidates for fewer or more limited indications
than we request, may grant approval contingent on the performance of costly
post-marketing clinical trials, or may approve a product candidate with a label
that does not include the labeling claims necessary or desirable for the
successful commercialization of that product candidate. Moreover, depending on
any safety issues associated with our product candidates that garner approval,
the FDA may impose a risk evaluation and mitigation strategy, thereby imposing
certain restrictions on the sale and marketability of such products.
We may not achieve our publicly announced milestones
according to schedule, or at all.
From time to time, we may announce the timing of certain events
we expect to occur, such as the anticipated timing of results from our clinical
trials. These statements are forward-looking and are based on the best estimates
of management at the time relating to the occurrence of such events. However,
the actual timing of such events may differ from what has been publicly
disclosed. The timing of events such as initiation or completion of a clinical
trial, filing of an application to obtain regulatory approval, or announcement
of additional clinical trials for a product candidate may ultimately vary from
what is publicly disclosed. These variations in timing may occur as a result of
different events, including the nature of the results obtained during a clinical
trial or during a research phase, timing of the completion of clinical trials,
problems with a CMO or a CRO or any other event having the effect of delaying
the publicly announced timeline. We undertake no obligation to update or revise
any forward-looking information, whether as a result of new information, future
events or otherwise, except as otherwise required by law. Any variation in the
timing of previously announced milestones could have a material adverse effect
on our business plan, financial condition or operating results and the trading
price of common shares.
We face competition from other biotechnology and
pharmaceutical companies and our financial condition and operations will suffer
if we fail to effectively compete.
The biotechnology and pharmaceutical industries are intensely
competitive and subject to rapid and significant technological change. Our
competitors include large, well-established pharmaceutical companies,
biotechnology companies, and academic and research institutions developing
cancer therapeutics for the same indications we are targeting and competitors
with existing marketed therapies. Many other companies are developing or
commercializing therapies to treat the same diseases or indications for which
our product candidates may be useful. Although there are no approved therapies
that specifically target the CD47 pathway, some competitors use therapeutic
approaches that may compete directly with our product candidates. For example,
SIRPαFc is in direct competition with CD47 blocking antibodies from Forty Seven
Inc., Celgene Corporation, Novimmune SA and others.
Many of our competitors have substantially greater financial,
technical and human resources than we do and have significantly greater
experience than us in conducting preclinical testing and human clinical trials
of product candidates, scaling up manufacturing operations and obtaining
regulatory approvals of products. Accordingly, our competitors may succeed in
obtaining regulatory approval for products more rapidly than we do. Our ability
to compete successfully will largely depend on:
- the efficacy and safety profile of our product candidates relative to
marketed products and other product candidates in development;
- our ability to develop and maintain a competitive position in the product
categories and technologies on which we focus;
- the time it takes for our product candidates to complete clinical
development and receive marketing approval;
- our ability to obtain required regulatory approvals;
- our ability to commercialize any of our product candidates that receive
regulatory approval;
- our ability to establish, maintain and protect intellectual property
rights related to our product candidates; and
- 30 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
- acceptance of any of our product candidates that receive regulatory
approval by physicians and other healthcare providers and payers.
Competitors have developed and may develop technologies that
could be the basis for products that challenge the discovery research
capabilities of Fluorinov. Some of those products may have an entirely different
approach or means of accomplishing the desired therapeutic effect than our
product candidates and may be more effective or less costly than our product
candidates. The success of our competitors and their products and technologies
relative to our technological capabilities and competitiveness could have a
material adverse effect on the future preclinical studies and clinical trials of
our product candidates, including our ability to obtain the necessary regulatory
approvals for the conduct of such clinical trials. This may further negatively
impact our ability to generate future product development programs using
Fluorinov technology.
If we are not able to compete effectively against our current
and future competitors, our business will not grow and our financial condition
and operations will substantially suffer.
We heavily rely on the capabilities and experience of our
key executives and scientists and the loss of any of them could affect our
ability to develop our products.
The loss of Dr. Niclas Stiernholm, our President and Chief
Executive Officer, or other key members of our staff, could harm us. We have
employment agreements with Dr. Stiernholm and other key members of our staff,
although such employment agreements do not guarantee their retention. We also
depend on our scientific and clinical collaborators and advisors, all of whom
have outside commitments that may limit their availability to us. In addition,
we believe that our future success will depend in large part upon our ability to
attract and retain highly skilled scientific, managerial, medical,
manufacturing, clinical and regulatory personnel, particularly as we expand our
activities and seek regulatory approvals for clinical trials. We enter into
agreements with our scientific and clinical collaborators and advisors, key
opinion leaders and academic partners in the ordinary course of our business. We
also enter into agreements with physicians and institutions who will recruit
patients into our clinical trials on our behalf in the ordinary course of our
business. Notwithstanding these arrangements, we face significant competition
for these types of personnel from other companies, research and academic
institutions, government entities and other organizations. We cannot predict our
success in hiring or retaining the personnel we require for continued growth.
The loss of the services of any of our executive officers or other key personnel
could potentially harm our business, operating results or financial condition.
Our employees may engage in misconduct or other improper
activities, including noncompliance with regulatory standards and requirements,
which could have a material adverse effect on our business.
We are exposed to the risk of employee fraud or other
misconduct. Misconduct by employees could include failures to comply with FDA
regulations, provide accurate information to the FDA, comply with manufacturing
standards we have established, comply with federal and state healthcare fraud
and abuse laws and regulations, report financial information or data accurately
or disclose unauthorized activities to us. In particular, sales, marketing and
business arrangements in the healthcare industry are subject to extensive laws
and regulations intended to prevent fraud, kickbacks, self-dealing, and other
abusive practices. These laws and regulations may restrict or prohibit a wide
range of pricing, discounting, marketing and promotion, sales commission,
customer incentive programs and other business arrangements. Employee misconduct
could also involve the improper use of information obtained in the course of
clinical trials, which could result in regulatory sanctions and serious harm to
our reputation. If any such actions are instituted against us, and we are not
successful in defending ourselves or asserting our rights, those actions could
have a substantial impact on our business and results of operations, including
the imposition of substantial fines or other sanctions.
The failure to fully realize the benefits of our
acquisition of Fluorinov may adversely affect our future results.
- 31 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
In January 2016, we acquired all of the outstanding capital
stock of Fluorinov, a small molecule medicinal chemistry company with
preclinical oncology assets and a potential discovery platform. The success of
our acquisition of Fluorinov will depend, in part, on our ability to fully
realize the anticipated benefits from combining our business with Fluorinov’s
business. However, to realize these anticipated benefits, we must continue the
research and development activities previously undertaken by Fluorinov as a
stand-alone company. If we are unable to achieve these objectives, the
anticipated benefits of our acquisition of Fluorinov may not be realized fully
or at all or may take longer to realize than expected.
We may expand our business through the acquisition of
companies or businesses or by entering into collaborations or by in-licensing
product candidates, each of which could disrupt our business and harm our
financial condition.
We have in the past and may in the future seek to expand our
pipeline and capabilities by acquiring one or more companies or businesses,
entering into collaborations, or in-licensing one or more product candidates.
Acquisitions, collaborations and in-licenses involve numerous risks, including,
but not limited to:
- substantial cash expenditures;
- technology development risks;
- potentially dilutive issuances of equity securities;
- incurrence of debt and contingent liabilities, some of which may be
difficult or impossible to identify at the time of acquisition;
- difficulties in assimilating the operations of the acquired companies;
- potential disputes regarding contingent consideration;
- diverting our management’s attention away from other business concerns;
- entering markets in which we have limited or no direct experience; and
- potential loss of our key employees or key employees of the acquired
companies or businesses.
We have experience in making acquisitions, entering
collaborations, and in-licensing product candidates, however, we cannot provide
assurance that any acquisition, collaboration or in-license will result in
short-term or long-term benefits to us. We may incorrectly judge the value or
worth of an acquired company or business or in-licensed product candidate. In
addition, our future success would depend in part on our ability to manage the
rapid growth associated with some of these acquisitions, collaborations and
in-licenses. We cannot provide assurance that we would be able to successfully
combine our business with that of acquired businesses, manage a collaboration or
integrate in-licensed product candidates. Furthermore, the development or
expansion of our business may require a substantial capital investment by
us.
Negative results from clinical trials or studies of
others and adverse safety events involving the targets of our products may have
an adverse impact on our future commercialization efforts.
From time to time, studies or clinical trials on various
aspects of biopharmaceutical products are conducted by academic researchers,
competitors or others. The results of these studies or trials, when published,
may have a significant effect on the market for the biopharmaceutical product
that is the subject of the study. The publication of negative results of studies
or clinical trials or adverse safety events related to our product candidates,
or the therapeutic areas in which our product candidates compete, could
adversely affect our share price and our ability to finance future development
of our product candidates, and our business and financial results could be
materially and adversely affected.
- 32 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
We face the risk of product liability claims, which could
exceed our insurance coverage and produce recalls, each of which could deplete
our cash resources.
We are exposed to the risk of product liability claims alleging
that use of our product candidates caused an injury or harm. These claims can
arise at any point in the development, testing, manufacture, marketing or sale
of our product candidates and may be made directly by patients involved in
clinical trials of our product candidates, by consumers or healthcare providers
or by individuals, organizations or companies selling our products. Product
liability claims can be expensive to defend, even if the product or product
candidate did not actually cause the alleged injury or harm.
Insurance covering product liability claims becomes
increasingly expensive as a product candidate moves through the development
pipeline to commercialization. We currently maintain clinical trial liability
insurance coverage of $10,000. However, there can be no assurance that such
insurance coverage is or will continue to be adequate or available to us at a
cost acceptable to us or at all. We may choose or find it necessary under our
collaborative agreements to increase our insurance coverage in the future. We
may not be able to secure greater or broader product liability insurance
coverage on acceptable terms or at reasonable costs when needed. Any liability
for damages resulting from a product liability claim could exceed the amount of
our coverage, require us to pay a substantial monetary award from our own cash
resources and have a material adverse effect on our business, financial
condition and results of operations. Moreover, a product recall, if required,
could generate substantial negative publicity about our products and business,
inhibit or prevent commercialization of other products and product candidates or
negatively impact existing or future collaborations.
If we are unable to maintain product liability insurance
required by our third parties, the corresponding agreements would be subject to
termination, which could have a material adverse impact on our
operations.
Some of our licensing and other agreements with third parties
require or might require us to maintain product liability insurance. If we
cannot maintain acceptable amounts of coverage on commercially reasonable terms
in accordance with the terms set forth in these agreements, the corresponding
agreements would be subject to termination, which could have a material adverse
impact on our operations.
Risks Related to Intellectual Property
If we are unable to adequately protect and enforce our
intellectual property, our competitors may take advantage of our development
efforts or acquired technology and compromise our prospects of marketing and
selling our key products.
We control two patent families relating to SIRPα. One family
relates to the use of SIRPα to treat cancer. The other family relates to our
drug as a composition of matter, SIRPαFc. We have also recently filed for patent
protection covering additional inventions relating to SIRPα, including
anti-cancer drug combination therapies that utilize SIRPαFc.
Our small molecule portfolio embraces patent filings that cover
numerous different inventions. With the exception of one process scheme, these
patent filings each claim a family of small molecule drugs as compositions of
matter, together with claims for their production and their medical uses. These
drugs target cancer for the most part, and some related medical end-uses.
Our success will depend in part upon our ability to protect our
intellectual property and proprietary technologies and upon the nature and scope
of the intellectual property protection we receive. For example, some of our
patent portfolio covers primarily methods of medical use but not compositions of
matter. The ability to compete effectively and to achieve partnerships will
depend on our ability to develop and maintain proprietary aspects of our
technology and to operate without infringing on the proprietary rights of
others. The presence of such proprietary rights of others could severely limit
our ability to develop and commercialize our products, to conduct our existing
research and could require financial resources to defend litigation, which may
be in excess of our ability to raise such funds. There is no assurance that our
pending patent applications or those that we intend to acquire will be approved
in a form that will be sufficient to protect our proprietary technology and gain
or keep any competitive advantage that we may have or, once approved, will be
upheld in any post-grant proceedings brought by any third parties.
- 33 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
The patent positions of pharmaceutical companies can be highly
uncertain and involve complex legal, scientific and factual questions for which
important legal principles remain unresolved. Patents issued to us or our
respective licensors may be challenged, invalidated or circumvented. To the
extent our intellectual property, including licensed intellectual property,
offers inadequate protection, or is found to be invalid or unenforceable, we are
exposed to a greater risk of direct competition. If our intellectual property
does not provide adequate protection against our competitors’ products, our
competitive position could be adversely affected, as could our business,
financial condition and results of operations. Both the patent application
process and the process of managing patent disputes can be time consuming and
expensive, and the laws of some foreign countries may not protect our
intellectual property rights to the same extent as do the laws of Canada and the
United States.
We will be able to protect our intellectual property from
unauthorized use by third parties only to the extent that our proprietary
technologies, key products, and any future products are covered by valid and
enforceable intellectual property rights including patents or are effectively
maintained as trade secrets, and provided we have the funds to enforce our
rights, if necessary.
If we lose our licenses from third-party owners we may be
unable to continue a substantial part of our business.
We are party to licenses that give us rights to intellectual
property that is necessary or useful for a substantial part of our business.
Pursuant to our exclusive license agreement with the University Health Network
and the Hospital for Sick Children under which we license certain patent rights
for our key products and their uses, we are required to use commercially
reasonable efforts to commercialize products based on the licensed rights and
pay milestone payments, royalties on net sales, and an annual maintenance
fee.
We have also entered into agreements allowing us to manufacture
SIRPαFc using Catalent’s proprietary GPEx® expression system. The consideration
includes payments at the time we successfully reach a series of development and
sales milestones. We may also enter into licenses in the future to access
additional third-party intellectual property.
If we fail to pay annual maintenance fees, development and
sales milestones, or it is determined that we did not use commercially
reasonable efforts to commercialize licensed products, we could lose our
licenses which could have a material adverse effect on our business and
financial condition.
We may require additional third-party licenses to
effectively develop and manufacture our key products and are currently unable to
predict the availability or cost of such licenses.
A substantial number of patents have already been issued to
other biotechnology and pharmaceutical companies. To the extent that valid
third-party patent rights cover our products or services, we or our strategic
collaborators would be required to seek licenses from the holders of these
patents in order to manufacture, use or sell these products and services, and
payments under them would reduce our profits from these products and services.
We are currently unable to predict the extent to which we may wish or be
required to acquire rights under such patents, the availability and cost of
acquiring such rights, and whether a license to such patents will be available
on acceptable terms or at all. There may be patents in the U.S. or in foreign
countries or patents issued in the future that are unavailable to license on
acceptable terms. Our inability to obtain such licenses may hinder or eliminate
our ability to manufacture and market our products.
Changes in patent law and its interpretation could
diminish the value of patents in general, thereby impairing our ability to
protect our product candidates.
- 34 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
As is the case with other biotechnology and pharmaceutical
companies, our success is heavily dependent on intellectual property rights,
particularly patents. Obtaining and enforcing patents in the biopharmaceutical
industry involves technological and legal complexity, and obtaining and
enforcing biopharmaceutical patents is costly, time-consuming and inherently
uncertain. The U.S. Supreme Court has ruled on several patent cases in recent
years, either narrowing the scope of patent protection available in certain
circumstances or weakening the rights of patent owners in certain situations. In
addition to increasing uncertainty with regard to our and our licensors’ or
collaborators’ ability to obtain patents in the future, this combination of
events has created uncertainty with respect to the value of patents, once
obtained. Depending on decisions by the U.S. Congress, the federal courts, and
the U.S. Patent and Trademark Office, or USPTO, the laws and regulations
governing patents could change in unpredictable ways that would weaken our and
our licensors’ or collaborators’ ability to obtain new patents or to enforce
existing patents and patents we and our licensors or collaborators may obtain in
the future.
Recent patent reform legislation could increase the
uncertainties and costs surrounding the prosecution of our and our licensors’ or
collaborators’ patent applications and the enforcement or defense of our or our
licensors’ or collaborators’ issued patents. On September 16, 2011, the
Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law.
The Leahy-Smith Act includes a number of significant changes to U.S. patent law.
These include provisions that affect the way patent applications are prosecuted
and may also affect patent litigation. The USPTO recently developed new
regulations and procedures to govern administration of the Leahy-Smith Act, and
many of the substantive changes to patent law associated with the Leahy-Smith
Act, and in particular, the first to file provisions, only became effective on
March 16, 2013. Accordingly, it is not clear what, if any, impact the
Leahy-Smith Act will have on the operation of our business. However, the
Leahy-Smith Act and its implementation could increase the uncertainties and
costs surrounding the prosecution of our or our licensors’ or collaborators’
patent applications and the enforcement or defense of our or our licensors’ or
collaborators’ issued patents, all of which could have a material adverse effect
on our business and financial condition.
Litigation regarding patents, patent applications, and
other proprietary rights may be expensive, time consuming and cause delays in
the development and manufacturing of our key products.
Our success will depend in part on our ability to operate
without infringing the proprietary rights of third parties. The pharmaceutical
industry is characterized by extensive patent litigation. Other parties may
have, or obtain in the future, patents and allege that the use of our
technologies infringes these patent claims or that we are employing their
proprietary technology without authorization.
In addition, third parties may challenge or infringe upon our
existing or future patents. Proceedings involving our patents or patent
applications or those of others could result in adverse decisions regarding:
- the patentability of our inventions relating to our key products; and/or
- the enforceability, validity, or scope of protection offered by our
patents relating to our key products.
If we are unable to avoid infringing the patent rights of
others, we may be required to seek a license, defend an infringement action, or
challenge the validity of the patents in court. Regardless of the outcome,
patent litigation is costly and time consuming. In some cases, we may not have
sufficient resources to bring these actions to a successful conclusion. In
addition, if we do not obtain a license, develop or obtain non-infringing
technology, fail to defend an infringement action successfully or have infringed
patents declared invalid, we may:
- incur substantial monetary damages;
- encounter significant delays in bringing our key products to market;
and/or
- be precluded from participating in the manufacture, use or sale of our key
products or methods of treatment requiring licenses.
Even if we are successful in these proceedings, we may incur
substantial costs and divert management time and attention in pursuing these
proceedings, which could have a material adverse effect on us.
- 35 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Our reliance on third parties requires us to share our
trade secrets, which increases the possibility that a competitor will discover
them.
Because we rely on third parties to develop our products, we
must share trade secrets with them. We seek to protect our proprietary
technology in part by entering into confidentiality agreements and, if
applicable, material transfer agreements, collaborative research agreements,
consulting agreements or other similar agreements with our collaborators,
advisors, employees and consultants prior to beginning research or disclosing
proprietary information. These agreements typically restrict the ability of our
collaborators, advisors, employees and consultants to publish data potentially
relating to our trade secrets. Our academic and clinical collaborators typically
have rights to publish data, provided that we are notified in advance and may
delay publication for a specified time in order to secure our intellectual
property rights arising from the collaboration. In other cases, publication
rights are controlled exclusively by us, although in some cases we may share
these rights with other parties. We may also conduct joint research and
development programs which may require us to share trade secrets under the terms
of research and development collaboration or similar agreements. Despite our
efforts to protect our trade secrets, our competitors may discover our trade
secrets, either through breach of these agreements, independent development or
publication of information including our trade secrets in cases where we do not
have proprietary or otherwise protected rights at the time of publication. A
competitor’s discovery of our trade secrets may impair our competitive position
and could have a material adverse effect on our business and financial
condition.
Risks Related to Our Common Shares
Our common share price has been volatile in recent years,
and may continue to be volatile.
The market prices for securities of biopharmaceutical
companies, including ours, have historically been volatile. In the year ended
December 31, 2018, our common shares traded on the TSX at a high of $11.44 and a
low of $1.99 per share and on the NASDAQ at a high of U.S. $9.16 and a low of
U.S. $1.46 per share. In the year ended December 31, 2017, our common shares
traded on the TSX at a high of $15.68 and a low of $5.26 per share and on the
NASDAQ at a high of U.S. $13.30 and a low of U.S. $4.15 per share. A number of
factors could influence the volatility in the trading price of our common
shares, including changes in the economy or in the financial markets, industry
related developments, the results of product development and commercialization,
changes in government regulations, and developments concerning proprietary
rights, litigation and cash flow. Our quarterly losses may vary because of the
timing of costs for manufacturing, preclinical studies and clinical trials.
Also, the reporting of adverse safety events involving our products and public
rumors about such events could cause our share price to decline or experience
periods of volatility. Each of these factors could lead to increased volatility
in the market price of our common shares. In addition, changes in the market
prices of the securities of our competitors may also lead to fluctuations in the
trading price of our common shares.
We have never paid dividends and do not expect to do so
in the foreseeable future.
We have not declared or paid any cash dividends on our common
or preferred shares to date. The payment of dividends in the future will be
dependent on our earnings and financial condition in addition to such other
factors as our board of directors considers appropriate. Unless and until we pay
dividends, shareholders may not receive a return on their shares. There is no
present intention by our board of directors to pay dividends on our shares.
We may issue additional common shares to the former
shareholders of Fluorinov as a result of our satisfaction of certain milestones,
resulting in share ownership dilution.
Under the terms of our agreements with Fluorinov and its former
shareholders, at our discretion up to 50% of any future contingent payments can
be satisfied through the issuance of our common shares, provided that the
aggregate number of common shares issuable under such payments will not exceed
1,558,447 common shares, which amount represented 19.99% of the outstanding
common shares at the time of execution of the acquisition, unless shareholder
approval has first been obtained.
- 36 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Issuing additional common shares to the former shareholders of
Fluorinov in satisfaction of contingent consideration dilutes the ownership
interests of holders of our common shares on the dates of such issuances. If we
are unable to realize the strategic, operational and financial benefits
anticipated from our acquisition of Fluorinov, our shareholders may experience
dilution of their ownership interests in our company upon any such future
issuances of our common shares without receiving any commensurate benefit.
Future sales or issuances of equity securities and the
conversion of outstanding securities to common shares could decrease the value
of the common shares, dilute investors’ voting power, and reduce our earnings
per share.
We may sell additional equity securities in future offerings,
including through the sale of securities convertible into equity securities, to
finance operations, acquisitions or projects, and issue additional common shares
if outstanding warrants or stock options are exercised, or preferred shares are
converted to common shares, which may result in dilution. See the information in
the section of this MD&A entitled “Description of Share Capital” for details
of our outstanding securities convertible into common shares. Subject to receipt
of any required regulatory approvals, subscribers of the December 2013 private
placement who purchased a minimum of 10% of the securities sold under the
offering received rights to purchase our securities in future financings to
enable each such shareholder to maintain their percentage holding in our common
shares for so long as the subscriber holds at least 10% of the outstanding
common shares on a fully-diluted basis. Shareholders who do not have this future
financing participation right may be disadvantaged in participating in such
financings.
Our board of directors has the authority to authorize certain
offers and sales of additional securities without the vote of, or prior notice
to, shareholders. Based on the need for additional capital to fund expected
expenditures and growth, it is likely that we will issue additional securities
to provide such capital. Such additional issuances may involve the issuance of a
significant number of common shares at prices less than the current market price
for our common shares.
Sales of substantial amounts of our securities, or the
availability of such securities for sale, as well as the issuance of substantial
amounts of our common shares upon conversion of outstanding convertible equity
securities, could adversely affect the prevailing market prices for our
securities and dilute investors’ earnings per share. A decline in the market
prices of our securities could impair our ability to raise additional capital
through the sale of securities should we desire to do so.
U.S. holders of 10% or more of the voting power of our
common shares may be subject to U.S. federal income taxation at ordinary income
tax rates on undistributed earnings and profits.
There is a risk that we will be classified as a controlled
foreign corporation, or CFC, for U.S. federal income tax purposes. We will
generally be classified as a CFC if more than 50% of our outstanding shares,
measured by reference to voting power or value, are owned (directly, indirectly
or by attribution) by “U.S. Shareholders.” For this purpose, a “U.S.
Shareholder” is any U.S. person that owns directly, indirectly or by
attribution, 10% or more of the voting power of our outstanding shares. If we
are classified as a CFC, a U.S. Shareholder may be subject to U.S. income
taxation at ordinary income tax rates on all or a portion of our undistributed
earnings and profits attributable to “subpart F income” and may also be subject
to tax at ordinary income tax rates on any gain realized on a sale of common
shares, to the extent of our current and accumulated earnings and profits
attributable to such shares. The CFC rules are complex and U.S. Shareholders of
our common shares are urged to consult their own tax advisors regarding the
possible application of the CFC rules to them in their particular circumstances.
- 37 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
We are likely a “passive foreign investment company,”
which may have adverse U.S. federal income tax consequences for U.S.
shareholders.
U.S. investors should be aware that we believe we were
classified as a PFIC during the tax years ended December 31, 2017 and 2016, and
based on current business plans and financial expectations, we believe that we
will be a PFIC for the current tax year and may be a PFIC in future tax years.
If we are a PFIC for any year during a U.S. shareholder’s holding period of our
common shares, then such U.S. shareholder generally will be required to treat
any gain realized upon a disposition of our common shares, or any so-called
“excess distribution” received on our common shares, as ordinary income, and to
pay an interest charge on a portion of such gain or distributions, unless the
shareholder makes a timely and effective “qualified electing fund” election, or
QEF Election, or a “mark-to-market” election with respect to our shares.
A U.S. shareholder who makes a QEF Election generally must report on a current
basis its share of our net capital gain and ordinary earnings for any year in
which we are a PFIC, whether or not we distribute any amounts to our
shareholders. A U.S. shareholder who makes the mark-to-market election generally
must include as ordinary income each year the excess of the fair market value of
the common shares over the shareholder’s adjusted tax basis therein. Each U.S.
shareholder should consult its own tax advisors regarding the PFIC rules and the
U.S. federal income tax consequences of the acquisition, ownership and
disposition of our common shares.
The effect of comprehensive U.S. tax reform legislation
on the Company is uncertain.
On December 22, 2017, the U.S. government enacted H.R. 1, “An
Act to provide for reconciliation pursuant to titles II and V of the concurrent
resolution on the budget for fiscal year 2018” (informally titled the “Tax Cuts
and Jobs Act”). Among a number of significant changes to the U.S. federal income
tax rules, the Tax Cuts and Jobs Act reduces the marginal U.S. corporate income
tax rate from 35% to 21%, limits the deduction for net interest expense, shifts
the United States toward a more territorial tax system, and imposes new taxes to
combat erosion of the U.S. federal income tax base, such as a one-time tax on
earnings of certain foreign subsidiaries that were previously tax deferred and a
new minimum tax on foreign earnings. The effects of the Tax Cuts and Jobs Act on
our company, whether adverse or favorable, are uncertain, and may not become
evident for some period of time, but could have a material adverse effect on our
business, financial position or results from operations.
It may be difficult for non-Canadian investors to obtain
and enforce judgments against us because of our Canadian incorporation and
presence.
We are a corporation existing under the laws of the Province of
Ontario, Canada. Several of our directors and officers, and several of the
experts are residents of Canada, and all or a substantial portion of their
assets, and a substantial portion of our assets, are located outside the United
States. Consequently, although we have appointed an agent for service of process
in the United States, it may be difficult for holders of our securities who
reside in the United States to effect service within the United States upon
those directors and officers, and the experts who are not residents of the
United States. It may also be difficult for holders of our securities who reside
in the United States to realize in the United States upon judgments of courts of
the United States predicated upon our civil liability and the civil liability of
our directors, officers and experts under the United States federal securities
laws. Investors should not assume that Canadian courts (i) would enforce
judgments of United States courts obtained in actions against us or such
directors, officers or experts predicated upon the civil liability provisions of
the United States federal securities laws or the securities or “blue sky” laws
of any state or jurisdiction of the United States or (ii) would enforce, in
original actions, liabilities against us or such directors, officers or experts
predicated upon the United States federal securities laws or any securities or
“blue sky” laws of any state or jurisdiction of the United States. In addition,
the protections afforded by Canadian securities laws may not be available to
investors in the United States.
If there are substantial sales of our common shares, the
market price of our common shares could decline.
Sales of substantial numbers of our common shares could cause a
decline in the market price of our common shares. Any sales by existing
shareholders or holders who exercise their warrants or stock options may have an
adverse effect on our ability to raise capital and may adversely affect the
market price of our common shares.
- 38 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
We are an “emerging growth company,” and we cannot be
certain if the reduced reporting requirements applicable to emerging growth
companies will make our common shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS
Act. For as long as we continue to be an emerging growth company, we may take
advantage of exemptions from various reporting requirements that are applicable
to other public companies that are not emerging growth companies, including not
being required to comply with the auditor attestation requirements of Section
404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding
executive compensation in our periodic reports and exemptions from the
requirements of holding a nonbinding advisory vote on executive compensation and
shareholder approval of any golden parachute payments not previously approved.
We could be an emerging growth company until the last day of our fiscal year
following the fifth anniversary of the date of the first sale of our common
shares pursuant to an effective registration statement under the U.S. Securities
Act of 1933, which is December 31, 2019, although circumstances could cause us
to lose that status earlier. We cannot predict if investors will find our common
shares less attractive because we may rely on these exemptions. If some
investors find our common shares less attractive as a result, there may be a
less active trading market for our common shares and our share price may be more
volatile.
Any failure to maintain an effective system of internal
controls may result in material misstatements of our consolidated financial
statements or cause us to fail to meet our reporting obligations or fail to
prevent fraud; and in that case, our shareholders could lose confidence in our
financial reporting, which would harm our business and could negatively impact
the price of our common shares.
Effective internal controls are necessary for us to provide
reliable financial reports and prevent fraud. If we fail to maintain an
effective system of internal controls, we might not be able to report our
financial results accurately or prevent fraud; and in that case, our
shareholders could lose confidence in our financial reporting, which would harm
our business and could negatively impact the price of our common shares. While
we believe that we have sufficient personnel and review procedures to allow us
to maintain an effective system of internal controls, we cannot provide
assurance that we will not experience potential material weaknesses in our
internal control. Even if we conclude that our internal control over financial
reporting provides reasonable assurance regarding the reliability of financial
reporting and the preparation of consolidated financial statements for external
purposes in accordance with IFRS as issued by the IASB, because of its inherent
limitations, internal control over financial reporting may not prevent or detect
fraud or misstatements. Failure to implement required new or improved controls,
or difficulties encountered in their implementation, could harm our results of
operations or cause us to fail to meet our future reporting obligations.
If we fail to timely achieve and maintain the adequacy of our
internal control over financial reporting, we may not be able to produce
reliable financial reports or help prevent fraud. Our failure to achieve and
maintain effective internal control over financial reporting could prevent us
from complying with our reporting obligations on a timely basis, which could
result in the loss of investor confidence in the reliability of our consolidated
financial statements, harm our business and negatively impact the trading price
of our common shares.
As a foreign private issuer, we are not subject to
certain United States securities law disclosure requirements that apply to a
domestic United States issuer, which may limit the information which would be
publicly available to our shareholders.
As a foreign private issuer, we are not required to comply with
all the periodic disclosure requirements of the Exchange Act, and therefore,
there may be less publicly available information about us than if we were a
United States domestic issuer. For example, we are not subject to the proxy
rules in the United States and disclosure with respect to our annual meetings
will be governed by Canadian requirements.
- 39 -
| TRILLIUM THERAPEUTICS INC.
|
| Management’s
Discussion and Analysis |
Our charter documents and certain Canadian legislation
could delay or deter a change of control, limit attempts by our shareholders to
replace or remove our current management and limit the market price of our
common shares.
Our authorized preferred shares are available for issuance from
time to time at the discretion of our board of directors, without shareholder
approval. Our articles grant our board of directors the authority to determine
the special rights and restrictions granted to or imposed on any unissued series
of preferred shares, and those rights may be superior to those of our common
shares. Further, the Investment Canada Act subjects any acquisition of control
of a company by a non-Canadian to government review if the value of the assets
as calculated pursuant to the legislation exceeds a threshold amount or in other
circumstances determined at the discretion of the Canadian government. A
reviewable acquisition may not proceed unless the relevant minister is satisfied
that the investment is likely to be of net benefit to Canada and the Canadian
government is satisfied that no other important concerns arise from the
acquisition of control. Any of the foregoing could prevent or delay a change of
control and may deprive or limit strategic opportunities to our shareholders to
sell their shares.
DISCLOSURE CONTROLS AND INTERNAL CONTROLS OVER FINANCIAL
REPORTING
We have implemented a system of internal controls that we
believe adequately protects our assets and is appropriate for the nature of our
business and the size of our operations. Our internal control system was
designed to provide reasonable assurance that all transactions are accurately
recorded, that transactions are recorded as necessary to permit preparation of
financial statements in accordance with IFRS, and that our assets are
safeguarded.
These internal controls include disclosure controls and
procedures designed to ensure that information required to be disclosed by us is
accumulated and communicated as appropriate to allow timely decisions regarding
required disclosure.
Internal control over financial reporting means a process
designed by or under the supervision of the Chief Executive Officer and the
Chief Financial Officer to provide reasonable assurance regarding the
reliability of financial reporting and the preparation of financial statements
for external purposes in accordance with IFRS as issued by the IASB. The
internal controls are not expected to prevent and detect all misstatements due
to error or fraud.
There were no changes in our internal control over financial
reporting that occurred during the three months ended December 31, 2018 that
have materially affected, or are reasonably likely to materially affect, our
internal control over financial reporting. As at December 31, 2018, we have
assessed the effectiveness of our internal control over financial reporting and
disclosure controls and procedures using the Committee of Sponsoring
Organizations of the Treadway Commission’s 2013 framework. Based on their
evaluation, the Chief Executive Officer and the Chief Financial Officer have
concluded that these controls and procedures are effective.
ADDITIONAL INFORMATION
Additional information regarding our company can be found on
SEDAR at www.sedar.com, and on EDGAR at www.sec.gov/edgar.shtml.
- 40 -



