This draft registration
statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly
confidential.
As confidentially submitted to the Securities and Exchange
Commission on October 9, 2018
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
FORM 20-F
| x | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| ¨ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from __________ to __________
OR
| ¨ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Date of event requiring this shell company report _________
Commission file number: ●
ITAMAR MEDICAL LTD.
(Exact name of registrant as specified in its charter)
N/A
(Translation of registrant’s name into English)
Israel
(Jurisdiction of incorporation or organization)
9 Halamish Street
Caesarea 3088900, Israel
(Address of principal executive offices)
Shy Basson, CFO
Tel: +972-4-6177000; Fax: +972-4-6275598
Itamar Medical Ltd., 9 Halamish Street, Caesarea 3088900, Israel
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on which Registered |
American
Depository Shares, Ordinary Shares, par value NIS 0.01 per share (1) |
The Nasdaq Capital Market |
| Ordinary Shares, par value NIS 0.01 per share (2) | The Nasdaq Capital Market |
| (1) | Evidenced by American Depositary Receipts. |
| (2) | Not for trading, but only in connection with the listing of the American Depositary Shares. |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
Securities registered or to be registered pursuant to Section 12(g) of the Act: None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
Indicate the number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report: N/A (see Item 7)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
¨ Yes x No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. ¨ Yes ¨ No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. ¨ Yes x No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). ¨ Yes ¨ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
Large Accelerated Filer ¨ Accelerated Filer ¨
Non-Accelerated Filer x Emerging growth company x
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 13(a) of the Exchange Act. ¨
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| ¨ | U.S. GAAP |
| x | International Financial Reporting Standards as issued by the International Accounting Standards Board |
| ¨ | Other |
If “Other” has been checked in response to the previous question indicate by check mark which financial statements the registrant has elected to follow:
¨ Item 17 ¨ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). o Yes o No
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
INTRODUCTION
We are Itamar Medical Ltd., an Israeli company. We are a medical technology company that designs, develops, manufactures and sells sleep apnea diagnostic ambulatory products and related services.
Unless indicated otherwise by the context, all references in this registration statement to “Itamar Medical”, the “Company””, “our Company”, “we”, “us”, “our” or the “Registrant” are to Itamar Medical Ltd. and its subsidiaries.
When the following terms and abbreviations appear in the text of this registration statement, they have the meanings indicated below:
| · | “ADSs” means our American Depositary Shares, each representing ● ordinary shares. |
| · | “Companies Law” means the Israeli Companies Law, 1999. |
| · | “convertible notes” or “Series L convertible notes” mean the convertible notes we issued as part of a public offering we conducted in 2013, all of which notes were fully repaid in February 2018. |
| · | “CPAP” means continuous positive airway pressure. CPAP devices are therapy devices used to treat certain sleep apnea conditions. |
| · | “CPT” means Cost per Test, which is a service offered as part of our TSS program, whereby the customer pays a fixed fee per HSAT that includes all the components associated with the test, including the disposable biosensor, hardware rental fees and access to CloudPAT. |
| · | “dollars”, “U.S. dollars” or “$” mean United States dollars. |
| · | “Endo PAT” means our device that enables testing of endothelial dysfunctions (the failure of the normal function of the inner lining of blood vessels). |
| · | “HSAT” means home sleep apnea test. |
| · | “Israeli CPI” means the Israeli consumer price index published by the Israeli Central Bureau of Statistics. |
| · | “MADs” means mandibular advancement devices. MADs are therapy devices used to treat certain sleep apnea conditions, also known as sleep apnea oral or dental appliances. |
| · | “Nasdaq” means the Nasdaq Stock Market. |
| · | “NIS” means New Israeli Shekels, the official currency of the State of Israel. |
| · | “ordinary shares” means our ordinary shares, par value NIS 0.01 per share. |
| · | “OSA” means obstructive sleep apnea. |
| · | “PAMS” means patient adherence management services, the purpose of which is to increase sleep apnea and respiratory patients’ adherence rate. |
| · | “PAT” or “PAT signal” means Peripheral Arterial Tonometry, or Peripheral Arterial Tone, which measures the arterial volume changes at the fingertip, reflecting the sympathetic nervous system activation. |
| · | “PSG” means polysomnography. PSG is the process of monitoring, recording and analyzing physiologic data during sleep and wakefulness to assist in the assessment and diagnosis of sleep disorders. |
| · | “SEC” means the United States Securities and Exchange Commission. |
| · | “TASE” means the Tel Aviv Stock Exchange. |
| - iii - |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
| · | “TaaS” means Test as a Service, also known as CPT (see above). |
| · | “TSS,” “TSS marketing program” or “TSS program” means our Total Sleep Solution. TSS is our marketing program that is designed to allow any medical practice or physician that is not a sleep physician by specialty, easy access to a comprehensive suite of products and services for the diagnosis, treatment and management of patients they suspect suffer from sleep apnea. |
| · | “U.S. Subsidiary” means Itamar Medical, Inc. |
| · | “Viola” means, collectively, Viola Growth II A.V. LP, a limited partnership registered in Israel, Viola Growth II (A) L.P., a limited partnership registered in Cayman Islands, and Viola Growth II (B) L.P., a limited partnership registered in Cayman Islands. |
| · | “Viola Transaction” means the private placement transaction pursuant to the share purchase agreement we entered into with Viola, dated as of August 26, 2015. |
| · | “Viola Warrants” means warrants issued to Viola in November 2015 and January 2016 as part of the Viola Transaction. |
| · | “Warrants (Series 4)” means the warrants issued to certain of our shareholders as part of a rights offering in December 2015. |
| · | “WatchPAT” means our portable diagnostic device that enables HSATs. |
EMERGING GROWTH COMPANY
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable to public companies that are not emerging growth companies. For example, we have elected to rely on the following exemptions:
| · | an exemption from complying with any requirement that may be adopted by the Public Company Accounting Oversight Board, or the PCAOB, regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis); and |
| · | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002; |
We may take advantage of the exemptions available for emerging growth companies for up to five years or such earlier time that we are no longer an emerging growth company. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenue, have more than $700 million in market value of the ADSs held by non-affiliates, or issue more than $1.0 billion of non-convertible debt over a three-year period. We may choose to take advantage of some, but not all of these reduced burdens.
It should be noted that the JOBS Act permits an emerging growth company like us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period is irrevocable.
| - iv - |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
PRESENTATION OF FINANCIAL INFORMATION
Our consolidated financial statements appearing in this registration statement are prepared in dollars and in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board, or IASB, and are audited in accordance with the standards of the PCAOB.
On August 1, 2018, the exchange rate between the NIS and the dollar, as quoted by the Bank of Israel, was NIS 3.677 to $1.00. Unless derived from our financial statements or indicated otherwise by the context, statements in this registration statement that provide the dollar equivalent of NIS amounts or provide the NIS equivalent of dollar amounts are based on such exchange rate.
MARKET, INDUSTRY AND OTHER DATA
Unless otherwise indicated, information contained in this registration statement concerning our industry and the markets in which we operate, including our competitive position and market opportunity, is based on information from our own management estimates and research, as well as from industry and general publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. Our management estimates have not been verified by any independent source, and we have not independently verified any third-party information. In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in Item 3.D “Risk Factors” below.
Statements made in this registration statement concerning the contents of any contract, agreement or other document are summaries of such contracts, agreements or documents and are not complete descriptions of all of their terms. If we filed any of these documents as an exhibit to this registration statement, you may read the document itself for a complete description of its terms, and the summary included herein is qualified by reference to the full text of the document which is incorporated by reference into this registration statement.
TRADEMARKS
We have obtained trademark registrations in the U.S. for, among others, PAT, Endo PAT, WatchPAT, EndoScore, ITAMAR, CloudPAT and SLEEPATH and some of them are also registered in additional jurisdictions, including Europe, Japan, Canada, China, India, Russia, Mexico, Korea and Singapore. Although we have omitted the “®” and “TM” trademark designations for such marks in this registration statement, all rights to such trademarks and service marks are nevertheless reserved. Unless indicated otherwise by the context, any other trademarks and trade names appearing in this registration statement are owned by their respective holders.
| - v - |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Except for the historical information contained in this registration statement, the statements contained in this registration statement are “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the Private Securities Litigation Reform Act of 1995, as amended, and other federal securities laws with respect to our business, financial condition and results of operations. We urge you to consider that statements which use the terms “anticipate,” “believe,” “expect,” “plan,” “intend,” “estimate,” and similar expressions are intended to identify forward-looking statements. Such forward-looking statements reflect our current view with respect to future events and financial results.
We remind readers that forward-looking statements are merely predictions and therefore inherently subject to uncertainties and other factors and involve known and unknown risks that could cause the actual results, including revenues from agreements we signed, expansion of our operations, development and release of new products, performance, levels of activity, our achievements, or industry results, to be materially different from any future results, plans to expand our operations, plans to develop and release new products, performance, levels of activity, or our achievements, or industry results, expressed or implied by such forward-looking statements. Such forward-looking statements appear in Item 3.D “Risk Factors”, Item 4 “Information on the Company” and Item 5 “Operating and Financial Review and Prospects” as well as elsewhere in this registration statement. The forward-looking statements contained in this registration statement are subject to risks and uncertainties, including those discussed under Item 3.D “Risk Factors” and in our other filings with the SEC. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof.
Except as required by applicable law, including the securities laws of the United States, we undertake no obligation to publicly release any update or revision to any forward-looking statements to reflect new information, future events or circumstances, or otherwise after the date hereof.
| - vi - |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
TABLE OF CONTENTS
| - vii - |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
| - viii - |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
| ITEM 1. | IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
| A. | Directors and Senior Management |
For the names, business addresses and functions of our directors and senior management, see “Item 6. Directors, Senior Management and Employees – A. Directors and Senior Management” and “Item 6. Directors, Senior Management and Employees – C. Board Practices.”
| B. | Advisers |
Our principal legal advisers are Goldfarb Seligman & Co., Ampa Tower, 98 Yigal Alon Street, Tel Aviv 6789141, Israel.
| C. | Auditors |
Somekh Chaikin, Certified Public Accountants (Israel), a member of KPMG International, or KPMG, audited our consolidated financial statements for the fiscal years ended December 31, 2015, 2016 and 2017. The address of KPMG is KPMG Millennium Tower, 17 Ha’arba’a Street, Tel Aviv, 6473917, Israel.
| ITEM 2. | OFFER STATISTICS AND EXPECTED TIMETABLE |
Not applicable.
| ITEM 3. | KEY INFORMATION |
| A. | Selected Financial Data |
The following selected consolidated statements of operations data for the years ended December 31, 2017, 2016 and 2015 and the selected consolidated balance sheet data as of December 31, 2017 and 2016, are derived from our audited consolidated financial statements set forth elsewhere in this registration statement. The selected consolidated balance sheet data as of December 31, 2015, have been derived from our audited consolidated financial statements which are not included in this registration statement. The selected consolidated statements of operations data for the six months ended June 30, 2018 and 2017 and the selected consolidated balance sheet data as of June 30, 2018 are derived from our unaudited consolidated financial statements included elsewhere in this registration statement.
You should read the following selected financial data in conjunction with, and it is qualified in its entirety by reference to, our historical financial information and other information provided in this registration statement, including Item 5. “Operating and Financial Review and Prospects” and our consolidated financial statements and notes thereto set forth elsewhere in this registration statement. The historical results set forth below are not necessarily indicative of the results to be expected in future periods.
| 1 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||
| 2018 | 2017 | 2017 | 2016 | 2015 | ||||||||||||||||
| (in thousands, except per share and share data) | ||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Consolidated Statements of Operations Data: | ||||||||||||||||||||
| Revenues | $ | 11,546 | $ | 9,403 | $ | 20,701 | $ | 18,440 | $ | 16,807 | ||||||||||
| Cost of revenues | 2,680 | 2,286 | 5,002 | 4,979 | 4,401 | |||||||||||||||
| Gross profit | 8,866 | 7,117 | 15,699 | 13,461 | 12,406 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Selling and marketing expenses | 6,078 | 6,091 | 12,140 | 14,035 | 10,684 | |||||||||||||||
| Research and development expenses | 1,856 | 1,962 | 4,129 | 3,225 | 2,831 | |||||||||||||||
| General and administrative expenses | 2,724 | 2,710 | 5,278 | 6,213 | 4,350 | |||||||||||||||
| Total operating expenses | 10,658 | 10,763 | 21,547 | 23,473 | 17,865 | |||||||||||||||
| Operating loss | (1,792 | ) | (3,646 | ) | (5,848 | ) | (10,012 | ) | (5,459 | ) | ||||||||||
| Financial income (expenses) from cash and investments | 182 | 1,454 | 1,591 | 716 | (354 | ) | ||||||||||||||
| Financial expenses from notes and loans | (761 | ) | (3,291 | ) | (4,884 | ) | (4,760 | ) | (4,229 | ) | ||||||||||
| Gain (loss) from derivatives instruments, net | 2,110 | 3,835 | 3,925 | (216 | ) | 7,930 | ||||||||||||||
| Financial income (expenses), net | 1,531 | 1,998 | 632 | (4,260 | ) | 3,347 | ||||||||||||||
| Loss before taxes on income | (261 | ) | (1,648 | ) | (5,216 | ) | (14,272 | ) | (2,112 | ) | ||||||||||
| Taxes on income | (51 | ) | (42 | ) | (85 | ) | (131 | ) | (135 | ) | ||||||||||
| Net loss | $ | (312 | ) | $ | (1,690 | ) | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||||
| Loss per share: | ||||||||||||||||||||
| Basic | $ | (0.00 | ) | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.01 | ) | |||||
| Diluted | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.02 | ) | |||||
| As of June 30, | As of December 31, | |||||||||||||||
| 2018 | 2017 | 2016 | 2015 | |||||||||||||
| (in thousands) | ||||||||||||||||
| (Unaudited) | ||||||||||||||||
| Consolidated Balance Sheet Data: | ||||||||||||||||
| Cash and cash equivalents | $ | 8,534 | $ | 7,643 | $ | 23,358 | $ | 33,019 | ||||||||
| Investment in marketable securities | - | 3,173 | 2,781 | 2,710 | ||||||||||||
| Working capital | 7,376 | 3,356 | 18,843 | 36,989 | ||||||||||||
| Total assets | 17,335 | 21,227 | 35,547 | 43,740 | ||||||||||||
| Total non-current liabilities | 2,044 | 4,133 | 15,986 | 22,169 | ||||||||||||
| Accumulated deficit | (104,807 | ) | (105,004 | ) | (100,885 | ) | (88,151 | ) | ||||||||
| Total equity | 7,372 | 1,377 | 5,241 | 16,951 | ||||||||||||
| B. | Capitalization and Indebtedness |
The following table sets forth our capitalization as of June 30, 2018. You should read this information together with our historical financial information and other information provided in this registration statement, including Item 5. “Operating and Financial Review and Prospects” and our unaudited consolidated financial statements and notes thereto set forth elsewhere in this registration statement.
| 2 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| As of June 30, 2018 | ||||
| (in thousands) | ||||
| (Unaudited) | ||||
| Liabilities | ||||
| Short-term bank loan (secured) | $ | 5,000 | ||
| Derivative instruments relating to warrants (unsecured) | 765 | |||
| Other long-term liabilities (unsecured) | 956 | |||
| Total liabilities | 6,721 | |||
| Equity | ||||
| Ordinary share capital | 746 | |||
| Additional paid-in capital | 111,433 | |||
| Accumulated deficit | (104,807 | ) | ||
| Total equity | 7,372 | |||
| Total Capitalization (debt and equity) | $ | 14,093 | ||
| C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
| D. | Risk Factors |
The following risk factors, among others, could in the future affect our actual results of operations and could cause our actual results to differ materially from those expressed in any forward-looking statements made by us. These forward-looking statements are based on current expectations and we assume no obligation to update this information, except as may be required by applicable law. Before you decide to buy, hold, or sell our ordinary shares or ADSs, you should carefully consider the risks described below, in addition to the other information contained elsewhere in this registration statement. The following risk factors are not the only risk factors facing our Company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business. Our business, financial condition and results of operations could be seriously harmed if any of the events underlying any of these risks or uncertainties actually occurs. In that event, the price for our ordinary shares and ADSs could decline, and you may lose all or part of your investment.
Risks Related to Our Business and Operations
We have a history of losses, may incur future losses and may never achieve profitability.
Since our incorporation in 1997, we have incurred operating and net losses in most of our years of operation. In particular, we incurred operating losses of approximately $5.8 million, $10.0 million and $5.5 million for the years ended December 31, 2017, 2016 and 2015 respectively, and our operating losses for the six months period ended June 30, 2018 were approximately $1.8 million. We expect to continue to incur operating and net losses for the foreseeable future, as we continue to invest in research and development and marketing and sales operations aimed at growing our business. The extent of our future operating and net losses is highly uncertain and we may never achieve or sustain profitability. Even if we reach and maintain profitability, we cannot assure that future net income will offset our cumulative losses, which, as of December 31, 2017 and June 30, 2018, were approximately $105.0 million and $104.8 million, respectively. In addition, there is no guarantee that we will be able to benefit from our losses for tax purposes.
| 3 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
There is no certainty that our WatchPAT device and related services will be accepted by the international medical community, in general, and specifically by the cardiology community.
Building upon our WatchPAT device and related services, one of the key elements of our business strategy and success is to focus on and sell a one-stop sleep apnea solution for the cardiology market. Our success in doing so depends, to a large extent, on the recognition by the international medical community, in general, and by the cardiology community in particular, of:
| · | the linkage between sleep apnea and cardiovascular disease; |
| · | the advantages of shifting the point of care for sleep apnea, mainly in the U.S., from sleep centers to the cardiology care point; and |
| · | the advantages of our WatchPAT product and related services. |
Recognition by the cardiology community of the linkage between sleep apnea and cardiovascular disease depends, among other things, on our ability to promote awareness amongst physicians, primarily cardiologists, to such linkage, including by providing supporting clinical data and studies demonstrating the said linkage and benefits of sleep apnea diagnosis and treatment to their cardiology patients. Recognition by the cardiology community of the advantages of shifting the point of care for sleep apnea, mainly in the U.S., from sleep centers to the cardiology care point, in general, and of the advantages of our WatchPAT product and related services in particular, depends to a large extent on our ability to demonstrate that (1) our WatchPAT device is efficient, cost effective and provides significant improvement in performance and data compared to other diagnostic tools available in the sleep market and (2) our WatchPAT related services, such as our TSS program, provide cardiologists with an easy access to prescribe HSATs to patients and increases the diagnosis rate with an effective management and monitoring of sleep apnea.
Even if we succeed in promoting awareness to the linkage between sleep apnea and cardiovascular disease and in proving the advantages of shifting the point of care to the cardiology care point and the advantages of our WatchPAT product and related services, there is a risk that healthcare service providers and other prospective customers will avoid purchasing our products and related services for any number of other reasons. For example, they may continue to use PSG tests in sleep centers or other, traditional HSAT devices because such diagnostic tools are already widely accepted. The failure to gain wide market acceptance of the linkage between sleep apnea and cardiovascular disease and in proving the advantages of shifting the point of care to the cardiology care point or the failure of our WatchPAT product and related services to otherwise gain market acceptance would adversely affect our business, financial condition and results of operations.
If healthcare providers are not adequately reimbursed for procedures conducted in connection with the use of our products and related services, we may not be successful in marketing and selling our products.
We market our products and related services primarily to healthcare providers, including health facilities and physicians, many of whom rely on reimbursement for the healthcare services they receive or provide to their patients, from third-party payors, such as Medicare and Medicaid in the U.S., as well as private insurance plans, managed care programs and other domestic and international government programs. These healthcare providers as well as government agencies are unlikely to purchase our products if they are not adequately reimbursed for the procedures conducted using our products. Unless a sufficient amount of conclusive, peer-reviewed clinical data about our products has been published and regular clinical use is documented, third-party payors, including insurance companies and government agencies, may refuse to provide reimbursement.
| 4 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Even if reimbursement is provided for our products, it may not be adequate to fully compensate the health facilities and physicians. For example, in the U.S., our largest market, the American Medical Association, or AMA, assigns specific Current Procedural Terminology, or CPT codes, which describe medical, surgical, and diagnostic services and are necessary for establishing reimbursement of any medical service. Once the CPT code is established, the Centers for Medicare and Medicaid Services establish reimbursement payment levels and coverage rules under Medicaid and Medicare, and private payors establish rates and coverage rules independently. In 2010, AMA has assigned a category I CPT code to the Peripheral Arterial Tone, or PAT-based technology utilized in our WatchPAT product. Nevertheless, generally, medical institutions in the United States that use our WatchPAT test for diagnosis of sleep apnea may be able to receive only partial reimbursement for the use of our WatchPAT products. In addition, most Medicaid payors currently do not cover HSATs, such as our WatchPAT. In Japan, our second largest market in the past two years, our WatchPAT product was approved by local authorities and medical institutions that use our WatchPAT test for diagnosis of sleep apnea are entitled to a fixed reimbursement per test. Nevertheless, local authorities have limited such clearance to diagnose obstructive sleep apnea, or OSA, for the purpose of prescribing therapy only to those patients who are categorized as severe and, to our knowledge, PSG tests remain the dominant means of sleep apnea diagnosis.
In addition, some third-party payors may also impose restrictions on the procedures for which they will provide reimbursement, such as guidelines and standards for the dispatch, prescription and billing procedures for medical products. For example, Medicare has issued guidelines that generally require the billing physician prescribing a sleep test to be a board-certified sleep physician or a staff member of accredited sleep centers only.
If healthcare providers cannot obtain sufficient reimbursement from third-party payors for our products or the tests conducted with our products, or if third-party payors impose restrictions on the procedures for which they will provide reimbursement as described above, we may not achieve significant market acceptance of our products and related services. Acceptance of our products and related services in the U.S. and in international markets depends to a large extent upon the availability of adequate reimbursement or funding within prevailing healthcare payment systems. Reimbursement, funding, and healthcare payment systems vary significantly by country and we may not obtain approvals for reimbursement in a timely manner or at all.
Even if we are successful in obtaining third party reimbursement or coverage for our products, adverse changes in reimbursement policies in general could harm our business. We are unable to predict changes in the reimbursement methods used by third-party healthcare payors. In addition, some payors are moving toward a managed care system in which providers contract to provide comprehensive healthcare for a fixed cost per person. In a managed care system, the cost of our products may not be incorporated into the overall payment for patient care or there may not be adequate reimbursement for our products separate from reimbursement for other procedures.
In addition, our TSS program relies, to some extent, on the reimbursement available for sleep apnea treatment devices, such as CPAP devices and on the desire of durable mobile equipment providers, or DMEs, to sell such devices. If healthcare providers cannot obtain sufficient reimbursement from third-party payors for such third-party treatment devices, our business may suffer. For example, in the past several years, Medicare has gradually reduced the reimbursement levels of CPAP devices. A further reduction of reimbursement levels or institution of burdensome restrictions and procedures on such reimbursement may also cause DMEs to lose interest in selling such devices, in which case, our TSS program and business would suffer.
| 5 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We will require additional funds to support our strategy and long-term operational plans, and, if additional funds are not available, we may need to significantly scale back or even cease our planned operations.
We plan to expand our business, which would require us to increase our investment in research and development as well as require expansion of our sales and marketing activities, including investing significant resources in further developing our sales work force and in obtaining insurance reimbursement of our product in additional territories, to support and drive our sales and marketing efforts. Our ability to take these and other actions may be limited by our available liquidity. As a consequence, in the future, we intend to seek additional financing.
Additional debt or equity financing that we may need may not be available on terms favorable to us, or at all, and, if additional funds are raised through an equity financing, the percentage ownership of our then current shareholders would be diluted. If we are unable to obtain such additional financing on a timely basis, we may have to curtail our development activities and growth plans or be forced to sell assets, perhaps on unfavorable terms, which would have a material adverse effect on our business, financial condition and results of operations. Further, we may not be able to continue operating if we do not generate sufficient revenues to finance our operations. In addition, we may incur substantial costs in pursuing capital financing, including investment banking fees, legal fees, accounting fees, securities law compliance fees, printing and distribution expenses and other costs.
There is no certainty that our WatchPAT device will be included in, or recommended by, any clinical practice guidelines or other guidelines and standards relevant to our business.
Professional associations publish, from time to time, clinical practice guidelines, suggesting processes and procedures intended for various medical conditions, as well as other guidelines and standards for the dispatch, prescription and billing procedures for medical devices. Such guidelines and standards have significant importance and influence on decisions by various health plans administrators, clinicians, government agencies and hospital administrators. In addition, many physicians consider clinical practice guidelines and act according to the recommendations included therein. For example, the clinical practice guidelines for the diagnosis of OSA, published by the American Academy of Sleep Medicine, or AASM, included the PAT-based technology used by our WatchPAT product only in March 2017, whereas AIM Specialty Health, or AIM, an organization which manages the insurance reimbursement policy for some of the insurance companies and payors in the U.S., updated its guidelines to medical insurers to include sleep apnea diagnostic tests using the PAT-based technology, only in November 2017. However, there is no assurance that all medical insurers will follow the foregoing AAMS and AIM guidelines and provide reimbursement for our WatchPAT product and related services. There is also no certainty that our product will continue to be included in such guidelines, or recommended by, additional clinical guidelines or that the methods by which we offer our products and related services for sale will be consistent with guidelines and standards related to the dispatch, prescription and billing procedures for medical devices.
If we fail to have our products included in such clinical practice guidelines (or, in the case of the AAMS and AIM guidelines, continue to be included in such guidelines) or if we fail to offer our products and related services for sale in a manner consistent with guidelines and standards related to the dispatch, prescription and billing, it could have an adverse affect on our business, financial condition and results of operations.
| 6 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We depend on the sales of our WatchPAT device.
Building upon our WatchPAT device and related services, one of the key elements of our business strategy and success is to focus on and sell a one-stop sleep apnea solution for the cardiology market. In order to do so, we have, among other things, focused and invested substantial time and resources in the past years on developing various solutions and WatchPAT related services, such as our TSS program, and, at the same time, limited our sales and marketing efforts for our legacy Endo PAT product, whose sales remained relatively constant in the past three fiscal years. However, the sales of the WatchPAT as a stand-alone product remain our main source of revenue, representing approximately 87.5%, 85.1% and 73.9% of our total revenues in the years ended December 31, 2017, 2016 and 2015, respectively. If we are not successful in implementing our business strategy to sell a one-stop sleep apnea solution for the cardiology market and increase the sales of our WatchPAT and related consumables and services or able to use our technologies to further develop and enhance our products and services with significant commercial potential, we will not be able to achieve our objectives or build a sustainable or profitable business.
The loss of one or more of our material customers or a decline in demand from one or more of these customers could harm our business.
Historically, a limited number of customers accounted for a substantial portion of our total sales. For example, in the year ended December 31, 2017, our three largest customers for that year, namely, Kaiser Foundation Health Plan, Inc., or Kaiser, Philips Respironics GK, a subsidiary of Koninklijke Philips NV (also known as Royal Philips), or Philips Japan, and the Department of Veterans Affairs, or VA, accounted for 17.5%, 12.7% and 12.1%, respectively, of our total revenues. There can be no assurance that such customers will continue to order our products in the same level or at all. A reduction or delay in orders from such customers, including reductions or delays due to market, economic or competitive conditions, could have a material adverse effect on our business, operating results and financial condition.
We depend on our proprietary PAT-based technology.
Our PAT-based technology is designed to provide a non-invasive window to the cardiovascular system and autonomic nervous system by monitoring the PAT signal and analyzing it for diagnostic purposes. Since our products are mainly based on our PAT-based technology, we are dependent on such technology that has taken us many years to develop. We have benefited from the fact that the type of proprietary technology equivalent to our PAT-based technology has not been widely available to or used by our competitors. If our technology becomes more widely available to our current or future competitors for any reason, our operating results may be adversely affected. Additionally, adoption or development of similar or more advanced technologies by our competitors may require that we devote substantial resources to the development of more advanced technology to remain competitive.
The market for our WatchPAT device and related services is highly competitive. If we are unable to compete successfully, this would adversely impact our business, revenues and results of operation.
The market for our WatchPAT device is highly competitive and is characterized by frequent product improvements and evolving technology. Our competitors range from small privately held companies to multinational corporations and their product offerings vary in scope and breadth, and some of our competitors may have certain competitive advantages, including:
| · | significant brand name recognition; |
| · | established relationships with healthcare professionals, customers and third-party payors; |
| · | established distribution networks and channels; |
| 7 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | additional product lines and the ability to offer rebates or bundle products to offer higher discounts or other incentives to gain a competitive advantage; and |
| · | greater financial and human resources for product development, sales and marketing, customer support and intellectual property litigation. |
Also, while we are not aware of a service offering similar to our TSS program and other WatchPAT related services that are targeted at the cardiology community, we believe that competitors who possess robust financial resources and sales and regulatory personnel may be able to overcome the barriers to entry into this market and offer products and service models similar to our TSS program.
Our ability to compete successfully depends, in part, on our ability to continuously develop, improve and market our WatchPAT device and related services. Consequently, we may need to increase our efforts, and related expenses for research and development, clinical studies and sales and marketing, to maintain or improve our market position. Additionally, our efforts to educate the medical community, specifically the cardiology community, and third-party payors on the linkage between sleep apnea and cardiovascular conditions, the advantages of shifting the point of care for sleep apnea from sleep centers to the cardiology care point as well as on the benefits of the WatchPAT and related services may require significant resources and may not be successful.
The development of innovative new products and services by our competitors for the same or similar indications as our offering, which competitive products and services may be less costly, more effective, or more widely accepted by the medical community, may also adversely affect the sales of our products and related services and could result in our products and services being noncompetitive or obsolete. In addition, our WatchPAT device may be subject to pricing pressures as a result of competition with other HSATs or with PSG tests.
We depend on strategic relationships with our distributors and other business partners and our revenues may be reduced if such relationships are not successful or are terminated.
Our products and services are offered through both direct and indirect channels, including distributors, and other business partners. Specifically, we rely on strategic relationships with distributors and other business partners, such as Philips Japan who acts as the exclusive distributor of our WatchPAT products in Japan, to sell our products, and these relationships account for a large portion of our revenues. In addition, in order to promote our TSS program, we are also developing partnerships with various business partners whose products or services are complimentary to ours. For example, we have entered into agreements with Philips Respironics, Inc., or Philips U.S., an affiliate of Philips Japan, under which we were granted non-exclusive rights to distribute its sleep apnea treatment devices, such as CPAP devices, to DMEs that participate in our TSS program to cardiology centers in the United States. Any failure of these relationships, whether to market our products effectively or generate significant revenues for us or our inability to sell products and services that are complimentary to ours, a termination of any of these relationships, or if we are unable to form additional strategic alliances in the future that will prove beneficial to us, could have a material adverse effect on our business, operating results and financial condition.
We are dependent on a single facility that houses the majority of our manufacturing operations.
We are dependent on the uninterrupted and efficient operations of our leased manufacturing facility, located in Caesarea, Israel. If operations at the plant were to be disrupted as a result of equipment failures, earthquakes and other natural disasters, fires, accidents, work stoppages, power outages, acts of war or terrorism or other reasons, we will likely need to use subcontractors until we are able to set up an alternative facility and our business could be materially adversely affected. Lost sales or increased costs that we may experience during the disruption of operations may not be recoverable under our insurance policies, and longer-term business disruptions could result in a loss of customers. If this were to occur, our business could be materially negatively impacted.
| 8 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We are dependent upon third-party manufacturers and suppliers, which make us vulnerable to supply disruptions.
In addition to manufacturing our products in our own manufacturing facility, we also engage third party manufacturers and suppliers for the assembly or manufacturing of our products as well as to provide us with software licenses for information technology, or IT platforms and other applications which we use as part of our CloudPAT and related services. Some of our suppliers and third-party manufacturers are not obligated to continue to supply us. We have relatively few sources of supply for some of the components and materials used in our products and IT platforms and other applications and in some cases we rely entirely on sole-source suppliers. In addition, the lead-time involved in the manufacturing of some of these components can be lengthy. Our third-party suppliers and manufacturers may encounter problems during manufacturing or supply due to a variety of reasons, including, among others, failure to follow specific protocols and procedures, failure to comply with applicable regulations, equipment or software malfunctions, environmental factors, or work force stoppages, any of which could delay or impede their ability to meet our demand for components or ongoing support. Our sole-source suppliers, and any of our other suppliers or our third-party contract manufacturers, may be unwilling or unable to supply the necessary materials and components or manufacture and assemble our products reliably and at the levels we anticipate or that are required by the market. Our ability to supply our products and related services commercially and to develop any future products and related services depends, in part, on our ability to obtain these materials, components and products in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. For example, we rely upon a single supplier who provides us with development services and database management services used for our CloudPAT platform.
While our suppliers and other contract manufacturers have generally met our demand for their products and services on a timely basis in the past, we cannot guarantee that they will in the future be able to meet our demand for their products and services, either because of acts of nature, the nature of our agreements with those suppliers and other manufacturers or our relative importance to them as a customer, and our suppliers and other manufacturers may decide in the future to discontinue or reduce the level of business they conduct with us. While we believe we can engage alternative suppliers, license or purchase our requirements or develop an alternative independently, changing suppliers or contract manufacturers due to any change in or termination of our relationships with these third parties may be a lengthy and expensive process and, consequently, we may lose sales, experience manufacturing or other delays, incur increased costs or otherwise experience impairment to our customer relationships. We cannot guarantee that we will be able to establish alternative relationships on adequate terms and without delays.
Our reliance on third-party suppliers also subjects us to additional risks that could harm our business, including, among others:
| · | our third-party suppliers or third-party manufacturers, especially new suppliers or manufacturers, may make manufacturing errors that may not be detected by our quality assurance testing, which could negatively affect the efficacy or safety of our products or cause shipment delays due to such errors; |
| 9 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | our suppliers or third-party manufacturers may encounter financial or other hardships unrelated to our demand, which could inhibit their ability to fulfill our orders and meet our requirements; and |
| · | our suppliers or third-party manufacturers may not maintain their regulatory approvals and as a result we may not be able use their products or services, which may result in delays and reduction of our production capacity. |
In addition, replacement or alternative sources might not be readily obtainable due to regulatory requirements and other factors applicable to our manufacturing operations. Incorporation of components or services from new suppliers or new third-party manufacturers into our products and related services may require a new or supplemental filing with applicable regulatory authorities and clearance or approval of the filing before we could resume product manufacturing. This process may take a substantial period of time, and we may not be able to obtain the necessary regulatory clearance or approval. This could also create supply disruptions that would harm our ability to meet our delivery obligations to our customers and may impede product sales and could have a material adverse effect on our business, financial condition and results of operations.
Our freedom to operate our business is limited as a result of certain restrictive covenants contained in our credit facility.
In March 2017, we secured a line of credit of up to $10 million from an Israeli bank. In order to secure our obligations to the bank, we pledged and granted to the bank a first priority floating charge on all of our assets and a first priority fixed charge on (i) our intellectual property, goodwill, holdings in our subsidiaries and certain other, immaterial, assets; and (ii) all of the assets of the U.S. Subsidiary. We refer to the agreements relating to such charges and other security interests as the Security Agreements.
The Security Agreements contain a number of customary restrictive terms and covenants that limit our operating flexibility, such as (i) limitations on the creation of additional liens, on the incurrence of indebtedness, on the provision of loans and guarantees and on distribution of dividends; and (ii) the ability of the bank to accelerate repayment in certain events, such as breach of covenants, liquidation, and a change of control of our Company. In addition, our right to make any draws under the credit line is conditioned upon us having cash balances with the bank of not less than 40% of the total amount drawn in our account with the lending bank. Such provisions may hinder our future operations or the manner in which we operate our business, which could have a material adverse effect on our business, financial condition or results of operations.
Defects or failures associated with our products or our quality system could lead to the filing of adverse event reports, product recalls or safety alerts with associated negative publicity and could also subject us to regulatory actions.
Manufacturing flaws, component failures, design defects, off-label uses or inadequate disclosure of product-related information could result in an unsafe condition or bodily injury of a patient. These problems could lead to a recall of, or issuance of a safety alert relating to, our products and result in significant costs and negative publicity. An adverse event involving one of our products could result in reduced market acceptance and demand for all products within that brand, and could harm our reputation and our ability to market our products in the future. In some circumstances, adverse events arising from or associated with the design, manufacture or marketing of our products could result in the suspension of current regulatory approvals of our products or delays in regulatory reviews of our applications for new product approvals or clearances. We may also voluntarily undertake a recall of our products, temporarily shut down production lines, or place products on a shipping hold based on internal safety and quality monitoring. We may also face litigation brought against us as a result of any of the foregoing instances, by customers and patients and there is no assurance that our insurance policies will fully cover such claims.
| 10 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Our future operating results will depend on our ability to sustain an effective quality control system and effectively train and manage our employee base, suppliers and subcontractors with respect to our quality system. Our quality system plays an essential role in determining and meeting customer requirements, preventing defects and improving our products and services. While we have a network of quality systems throughout our business lines and facilities, quality and safety issues may occur with respect to any of our products. A quality or safety issue may result in a public warning letter or potentially a consent decree from the U.S. Food and Drug Administration, or the FDA, in the U.S. and from similar regulatory bodies elsewhere. In addition, we may be subject to product recalls or seizures, monetary sanctions, injunctions to halt manufacturing and distribution of products, civil or criminal sanctions, import detentions of our products, and restrictions on operations. Any of the foregoing events could disrupt our business and have an adverse effect on our results of operations and financial condition.
We face the risk of product liability claims that could be expensive, divert management attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices. This risk exists even if a device is cleared or approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our products are designed to test, and future products may be designed to test, important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our devices could result in patient injury. The medical device industry has historically been subject to extensive litigation over product liability claims, and we cannot offer any assurance that we will not face product liability suits. We may be subject to product liability claims if our devices cause, or merely appear to have caused, patient injury. In addition, an injury that is caused by the activities of our suppliers, such as those who provide us with components and raw materials, may be the basis for a claim against us either as manufacturers or resellers of third party devices. Product liability claims may be brought against us by patients, physicians, healthcare providers or others selling or otherwise coming into contact with our products or, while less likely, the products we resell.
If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| · | cost of litigation; |
| · | distraction of management’s attention from our primary business; |
| · | the inability to commercialize our products and related services; |
| · | decreased demand for our products and related services; |
| · | damage to our business reputation; |
| · | product recalls or withdrawals from the market; |
| · | withdrawal of clinical trial participants; |
| 11 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | substantial monetary awards to patients or other claimants; and |
| · | loss of sales. |
While we may attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. We can provide no assurance that we will be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products, either of which could have a material adverse effect on our business, financial condition and results of operations.
Although we have product liability and clinical study liability insurance that we believe is appropriate, this insurance is subject to deductibles and coverage limitations. Our current product liability insurance may not continue to be available to us on acceptable terms, if at all, and, if available, coverage may not be adequate to protect us against any future product liability claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms or otherwise protect against potential product liability claims, we could be exposed to significant liabilities. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations.
If we receive a significant number of warranty claims or our products require significant post-sale support, our costs will increase and our business and financial results will be adversely affected.
Sales of our products generally include a warranty on our part, generally for a period of twelve months from the date the product is delivered to the customer’s facility. While we have not experienced many warranty claims in the past and the cost of repairing or replacing our products has not been material thus far, if product returns or warranty claims are significant or exceed our expectations, we could incur unanticipated reductions in sales or additional expenditures for parts and service. In addition, our reputation could be damaged and our products may not achieve market acceptance.
Long lead-times required by certain suppliers could prevent us from meeting the demand for our products. As such, if we do not accurately forecast such demand, our operating results could be adversely affected.
Market uncertainty makes it difficult for us, our customers, our distributors and our suppliers to accurately forecast future product demand trends, which could cause us to order or produce excess products that can increase our inventory costs and result in obsolete inventory. Alternatively, this forecasting difficulty could cause a shortage of products, or components and materials used in our products that could result in an inability to satisfy demand within a timeframe acceptable by our customers for our products and a resulting material loss of potential revenue.
In addition, some of our suppliers, such as suppliers of components, may require extensive advance notice of our requirements in order to produce products in the quantities we desire. This long lead-time, which in some cases can be more than six months, may require us to place orders far in advance of the time when certain products will be offered for sale, thereby also making it difficult for us to accurately forecast demand for our products, exposing us to risks relating to shifts in consumer demand and trends and adversely affecting our operating results.
| 12 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The risks and uncertainties inherent in conducting clinical trials could delay or prevent the development and commercialization of new products, or new indications for our products, which could have a material adverse effect on our results of operations, financial condition, and growth prospects.
Manufacturers are generally required to conduct clinical trials prior to obtaining regulatory authorizations to market and sell a medical device in any given territory. Clinical trials are experiments conducted or observations made in clinical research of our medical devices on human participants. Such trials are designed to answer particular questions about novel medical devices or new indications that require further study and provide data about the product’s safety and efficacy and can be conducted only after approval of the proposed clinical trial by the health authority or institute ethics committee.
There are a number of risks and uncertainties associated with conducting clinical trials. Clinical trials vary in scale and scope and may entail significant costs. They are also often conducted with patients having advanced stages of disease and, as a result, during the course of the trial, these patients may suffer adverse medical effects for reasons that may not be related to the product being tested, but which nevertheless affect the clinical trial results. In addition, side effects experienced by the patients may cause a delay of approval or limited profile of an approved product. Moreover, clinical trials may not demonstrate sufficient safety and efficacy to obtain FDA approval or the approval of applicable foreign regulatory authorities.
Failure can occur at any time during the clinical trial process, the results from early clinical trials may not be predictive of results obtained in later and larger clinical trials and product candidates in later clinical trials may fail to show the desired safety or efficacy despite having progressed successfully through earlier clinical testing. In the future, the completion of clinical trials, if required, for our new products or new indications of current products may be delayed or halted for many reasons, including:
| · | delays in patient enrollment, and variability in the number and types of patients available for clinical trials; |
| · | regulators or institutional review boards or ethics committees may not allow us to commence or continue a clinical trial; |
| · | risks associated with trial design, which may result in a failure of the trial to show statistically significant results even if the product candidate is effective; |
| · | poor effectiveness of product candidates during clinical trials; |
| · | safety issues, including adverse events associated with product candidates, occurring during clinical trials; |
| · | the failure of patients to complete clinical trials due to adverse side effects, dissatisfaction with the product candidate, or other reasons; and |
| · | governmental or regulatory delays or changes in regulatory requirements, policy, guidelines or interpretations. |
The FDA or foreign regulatory authorities may require us to conduct unanticipated additional clinical trials as part of future product submissions, which could result in additional expenses and delays in bringing new products to the market. Any failure or delay in completing clinical trials for new products or new indications of our products, would prevent or delay the commercialization of our product or the introduction of new indications for our products. There is no certainty that our expenses related to clinical trials will lead to the development of products or new product indications that will receive regulatory approval and generate revenues in the near future, or ever.
| 13 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Delays or failure in the development and commercialization of our products could have a material adverse effect on our results of operations, liquidity, financial condition, and our growth prospects. Negative results of clinical trials performed by us or by third parties regarding the use of our products may also adversely affect the medical community’s and customers’ acceptance of our products.
Our operating results may decline if we fail to develop additional products and applications, or enhance existing products.
We plan to develop and manufacture additional products and applications using our PAT-based technology, and continue enhancing our existing line of products. There is no certainty that we will meet the technological, clinical and regulatory requirements or any other requirements applicable to the development process of such new products or applications. In addition, we may not have the financial resources necessary for the completion of such development. If we fail to develop additional products and applications, or enhance our existing products, it may have an adverse effect on our reputation, our growth prospects and our business results, and our operating results may decline or fail to grow as expected.
If we are unable to support our plans for continued growth, our business could suffer.
We intend to increase our investment in research and development activities and expand our sales and marketing activities. If we continue to grow, the complexity of our operations is likely to increase, placing greater demands on our management. Our ability to manage our growth effectively depends on our ability to implement and improve our financial and management information systems on a timely basis and to effect other changes in our business including, the ability to monitor and improve our manufacturing systems, and align our information, quality and regulatory compliance systems, among others. Unexpected difficulties during expansion, the failure to attract and retain qualified employees and subcontractors, the failure to successfully replace or upgrade our management information systems, the failure to manage costs or our inability to respond effectively to growth or plan for future expansion could halt our growth. If we fail to manage our growth effectively and efficiently, our costs could increase faster than our revenues and our business results could suffer.
Our revenues and operating income could fluctuate significantly.
Our revenues and operating results may vary significantly from year-to-year and quarter-to-quarter. Variations may result from, among other factors:
| · | the timing of product launches, and market acceptance of such products launched; |
| · | changes in the amount we spend to research, develop, acquire, license or promote new products; |
| · | the outcome of our research, development and clinical trial programs, as well as independent trials conducted without our involvement which could be published in peer-reviewed journals; |
| · | serious or unexpected health or safety concerns related to our products or our product candidates; |
| 14 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | the introduction of new products by others that render our products obsolete or noncompetitive; |
| · | the ability to maintain selling prices and high gross margins on our products; |
| · | changes in coverage and reimbursement policies of health plans and other health insurers, including changes to Medicare, Medicaid and similar state programs; |
| · | increases in the cost of components or raw materials used to manufacture our products; |
| · | manufacturing and supply interruptions, including product rejections or recalls due to failure to comply with manufacturing specifications; |
| · | the timing of FDA or any other foreign regulatory authority approvals; |
| · | the ability to protect our intellectual property and avoid infringing the intellectual property of others; |
| · | the timing and quantities of our customers’ purchases of our products, which may be affected by factors out of our control including, among others, their budget constraints; and |
| · | the outcome and cost of possible litigation over patents with third parties. |
We are an international business, and we are exposed to various global risks that could have a material adverse effect on our financial condition and results of operations.
As an international business, which operates in multiple jurisdictions, we are exposed to trends and financial risks of international markets, and are also required to comply with varying legal and regulatory requirements in such multiple jurisdictions. Profitability from international operations may be limited by risks and uncertainties related to regional economic conditions, regulatory and reimbursement approvals, and our ability to implement our overall business strategy in various jurisdictions. We expect these risks will increase as we pursue our strategy to expand operations into new geographic markets. We may not succeed in developing and implementing effective policies and strategies in each location where we conduct business. Any failure to do so may harm our business, results of operations and financial condition.
International sales and operations are subject to a variety of risks, including:
| · | foreign currency exchange rate fluctuations; |
| · | potential adverse changes in laws and regulatory practices, including export license requirements, trade barriers, tariffs and tax laws; |
| · | burdens and costs of compliance with a variety of foreign laws; |
| · | foreign tax laws and potential increased costs associated with overlapping tax structures; |
| · | greater difficulty in staffing and managing foreign operations; |
| · | greater risk of uncollectible accounts; |
| 15 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | longer collection cycles; |
| · | logistical and communications challenges; |
| · | changes in labor conditions; |
| · | political and economic instability; |
| · | greater difficulty in protecting intellectual property; |
| · | the risk of third party disputes over ownership of intellectual property and infringement of third party intellectual property by our products; and |
| · | general economic and political conditions in these foreign markets. |
International markets are also affected by economic pressure to contain reimbursement levels and healthcare costs. Profitability from international operations may be limited by risks and uncertainties related to regional economic conditions, regulatory and reimbursement approvals, competing products, infrastructure development, intellectual property rights protection and our ability to implement our overall business strategy. We expect these risks will increase as we pursue our strategy to expand operations into new geographic markets. We may not succeed in developing and implementing effective policies and strategies in each location where we conduct business. Any failure to do so may harm our business, results of operations and financial condition.
Exchange rate fluctuations, primarily between the dollar and the NIS, may negatively affect our liquidity, financial condition and results of operation.
We currently generate a substantial portion of our revenues in dollars whereas we currently incur a significant portion of our expenses in other currencies, predominantly NIS. Since our functional and reporting currency is the dollar, our financial results may be affected by fluctuations in the exchange rates of currencies in the countries in which we transact business. For example, during 2017, we witnessed a strengthening of the average exchange rate of the NIS against the dollar, which increased the dollar value of Israeli expenses. If the NIS strengthens against the dollar, as it did in 2017, the dollar value of our Israeli expenses, mainly personnel and facility-related, will increase. While we engage, from time to time, in currency hedging transactions intended to reduce the effect of fluctuations in foreign currency exchange rates on our results of operations, we cannot guarantee that such measures will adequately protect us against currency fluctuations in the future. Although exposure to currency fluctuations to date has not had a material adverse effect on our business, there can be no assurance such fluctuations in the future will not have a material adverse effect on our operating results and financial condition.
Changing or severe global economic conditions may materially adversely affect our business.
Our business and financial condition are affected by global economic conditions and their impact on levels of spending by customers, which may be disproportionately affected by economic downturns. The global economy is still subject to uncertainties surrounding its strength in many regions. For example, the recent escalating disagreements between the U.S. and certain European states, as well between the U.S. and China, with respect to placing tariffs and other trade barriers, may adversely affect international trade and we cannot predict the implications of such barriers on our business. Uncertainty about current global economic conditions continues to pose a risk as customers may postpone or reduce spending in response to restraints on credit. Should the economic slowdown resume and/or companies in our target markets reduce capital expenditures, it may cause our customers to reduce or postpone their spending significantly, which could result in reductions in sales of our products, longer sales cycles, slower adoption of new technologies and increased price competition. In addition, if the market is flat and customers experience low visibility we may not be able to increase our sales (whether direct sales or indirect sales through our distributors). Each of the above scenarios would have a material adverse effect on our business, operating results and financial condition.
| 16 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Our ability to retain and attract qualified senior management, including our President and Chief Executive Officer, as well as employees with the expertise required for our business is key to our success.
Our success largely depends on our ability to retain and attract qualified senior management, in particular Mr. Gilad Glick, our President and Chief Executive Officer, who also acts as our VP marketing and as acting President and Chief Executive Officer of the U.S. Subsidiary, as well as on our ability to retain and attract qualified personnel, including personnel with expertise in research and development and sales and marketing, and to effectively provide for the succession of senior management. There is intense competition from numerous biotechnology, medical device and other companies seeking to employ qualified individuals in the business fields in which we operate, and we may not be able to attract and retain the qualified personnel necessary for the achievement of our business objectives.
We do not maintain life insurance on any of our personnel. Regardless, the loss of senior management employees, the failure of any senior management employee to perform or our inability to attract and retain skilled employees, as needed, or an inability to effectively plan for and implement a succession plan for senior management could harm our business. In particular, the loss of the services of Mr. Glick could result in a significant loss in the knowledge and experience that we possess and could significantly delay or prevent successful implementation of our business objectives.
Under applicable employment laws, we may not be able to enforce covenants not to compete.
Our employment agreements generally include covenants not to compete. These agreements prohibit our employees, if they cease working for us, from competing directly with us or working for our competitors for a limited period. We may be unable to enforce these agreements under the laws of the jurisdictions in which our employees work. For example, Israeli courts have required employers seeking to enforce covenants not to compete to demonstrate that the competitive activities of a former employee will harm one of a limited number of material interests of the employer, such as the secrecy of a company’s confidential commercial information or the protection of its intellectual property. If we cannot demonstrate that such an interest will be harmed, we may be unable to prevent our competitors from benefiting from the expertise of our former employees or consultants and our competitiveness may be diminished, which could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
We may face both reputational and SEC enforcement risks with respect to conflict minerals obligations.
Upon the listing of our ADSs on the Nasdaq Capital Market, we will become subject to disclosure requirements under section 102 of the Dodd-Frank Wall Street Reform and Consumer Protection Act regarding the source of certain minerals for which such conflict minerals are necessary to the functionality or production of a product manufactured, or contracted to be manufactured which are mined from the Democratic Republic of Congo, and adjoining countries, including: Angola, Burundi, Central African Republic, the Republic of the Congo, Rwanda, South Sudan, Tanzania, Uganda, and Zambia. These rules require reporting companies to file a conflict minerals report as an exhibit to a Form SD report with the SEC. The conflict minerals report is required to set out the due diligence efforts and procedures exercised on the source and chain of custody of such conflict minerals, in accordance with internationally recognized due diligence framework, and a description of our products containing such conflict minerals. Although we expect that we will be able to comply with the SEC rules and timely file our initial Form SD report with the SEC, in preparing to do so we are dependent upon information supplied by certain suppliers of products that contain, or potentially contain, conflict minerals. Such preparation may be costly. To the extent that the information that we receive from our suppliers is inaccurate or inadequate or our processes in obtaining that information do not fulfill the SEC’s requirements, we could face both reputational and SEC enforcement risks.
| 17 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Cyber security attacks or breaches of our data could adversely affect our reputation and business.
Risks to cyber security and privacy, including the activities of criminal hackers, hacktivists, state-sponsored intrusions, industrial espionage, employee malfeasance and human or technological error are constantly evolving. Computer hackers’ and others routinely attempt to breach the security of high profile companies, governmental agencies, technology products, services and systems.
A cyber incident is considered to be any event that threatens the confidentiality, integrity or availability of information resources. More specifically, a cyber incident is an intentional attack or an unintentional event that can include gaining unauthorized access to IT systems to disrupt operations, corrupt data or steal confidential information.
In the ordinary course of our business, we collect and store personal, financial, proprietary and other confidential information related to our business, employees, customers and partners on our IT systems. We rely on said systems to manage our business, operations and research and development and, in some cases, to provide services to our customers. For example, sensitive data is stored using our CloudPAT platform. This includes, where required or permitted by applicable laws, personally identifiable information. Certain third parties with whom we collaborate with also collect and store such data. The secure maintenance of this information is important to our operations and business strategy. Despite security measures employed by us, our information technology and infrastructure may be vulnerable to attacks by hackers or breached due to employee error, malfeasance or other disruptions. Any such breach could compromise information stored on our networks or those of our partners.
We are subject to strict data privacy laws and regulations in the U.S., European Union and other jurisdictions in which we operate, governing the collection, transmission, storage and use of data and personally identifying information, such as the Health Insurance Portability and Accountability Act, or HIPAA, in the U.S and the General Data Protection Regulation, or the GDPR, in Europe. Any breach, unauthorized access, disclosure or other loss of information could result in legal claims or proceedings, liability under data privacy laws and regulations, disruption of our operations, including delays in our regulatory approval efforts, criminal penalties or civil liabilities, any of which would damage our reputation and adversely affect our business. See also below under “Risks Related to Our Industry - Privacy regulations may impose costs and liabilities on us, limit our use of information, and adversely affect our business.”
We can provide no assurance that our current IT systems are fully protected against cyber security threats. Even when a security breach is detected, the full extent of the breach may not be determined immediately. An increasing number of companies have disclosed security breaches of their IT systems and networks. We believe such incidents are likely to continue, and we are unable to predict the direct or indirect impact of these future attacks on us. In addition, although we maintain cyber-security insurance that we believe is appropriate, this insurance is subject to deductibles and coverage limitations and there can be no assurances that our insurance coverage will be sufficient, or that insurance proceeds will be paid to us in a timely manner.
| 18 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Consolidation in the healthcare industry or group purchasing organizations could lead to demands for price concessions.
Healthcare costs have risen significantly over the past decade, which has resulted in or led to numerous cost reform initiatives by legislators, regulators and third-party payors. Cost reform may also trigger a consolidation trend in the healthcare industry to aggregate purchasing power, which may create more requests for pricing concessions in the future. Additionally, group purchasing organizations, independent delivery networks and large single accounts may continue to use their market power to consolidate purchasing decisions for hospitals and our other targeted customers. We expect that market demand, government regulation, third-party coverage and reimbursement policies and social pressures will continue to change the healthcare industry worldwide, resulting in further business consolidations and alliances among our customers, which may exert further downward pressure on the prices of our products.
We face risks associated with acquisition of businesses and technologies.
As part of our growth strategy, we intend to evaluate and may pursue acquisitions of, or significant investments in, complementary companies or technologies to increase our technological capabilities and expand our product offerings. Acquisitions and the successful integration of new technologies, products, assets or businesses that we may acquire in the future, will require significant attention from our management and could result in a diversion of resources from our existing business, which in turn could have an adverse effect on our business operations. Other risks typically encountered with acquisitions include disruption of our ongoing business; difficulties in integration of the acquired operations and personnel; inability of our management to maximize our financial and strategic position by the successful implementation or integration of the acquired technology into our product offerings; being subject to known or unknown contingent liabilities, including taxes, expenses and litigation costs; and inability to realize expected synergies or other anticipated benefits which may, among other things, also lead to goodwill impairments or other write offs. We cannot assure you that we will be successful in overcoming these risks or any other problems we may encounter in connection with potential future acquisitions. Our inability to successfully integrate the operations of an acquired business, including a successful implementation of the technologies we acquire, and realize anticipated benefits associated with an acquisition, could have a material adverse effect on our business, financial condition, results of operations and cash flows. Acquisitions or other strategic transactions may also result in dilution to our existing shareholders if we issue additional equity securities as consideration or partial consideration as well as in the incurrence of indebtedness if we borrow funds to finance such transactions.
Risks Related to Our Intellectual Property
We depend on our intellectual property, and our future success is dependent on our ability to protect our intellectual property and not infringe on the rights of others.
Our success depends, in part, on our ability to obtain patent protection for our products, protect against any infringement or misuse of our trademarks, maintain the confidentiality of our trade secrets and know how, operate without infringing on the proprietary rights of others and prevent others from infringing our proprietary rights. We try to protect our proprietary rights by, among other things, filing world-wide patent applications related to our products, inventions and improvements that may be important to the continuing development of our products and applying for the registration of our trademark in certain geographic locations in which we operate. However, we cannot assure you that:
| · | any of our future processes or products will be patentable; |
| · | our processes or products will not infringe upon the patents of third parties; |
| 19 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | our patents will protect us worldwide; or |
| · | we will have the resources to defend against charges of patent infringement or other violation or misappropriation of intellectual property by third parties or to protect our own intellectual property rights against infringement, misappropriation or violation by third parties. |
Because the patent position of medical device companies involves complex legal and factual questions, we cannot predict the validity and enforceability of patents with certainty. Our issued patents may not provide us with any competitive advantages, may be held invalid or unenforceable as a result of legal challenges by third parties or could be circumvented. Our competitors may also independently develop formulations, processes and technologies or products similar to ours or design around or otherwise circumvent patents issued to, or licensed by, us. Thus, any patents that we own or license from others may not provide adequate protection against competitors. Our pending patent applications, those we may file in the future or those we may license from third parties may not result in patents being issued. If these patents are issued, they may not provide us with proprietary protection or competitive advantages. The degree of future protection to be afforded by our proprietary rights is uncertain because legal means afford relatively limited protection and may not adequately protect our rights or permit us to gain or keep our competitive advantage.
After the completion of development and registration of our patents, third parties may still manufacture or market our products despite our patent protected rights. Such infringement of our patent protected rights is likely to cause us damage and lead to a reduction in the prices of our products, thereby reducing our anticipated profits.
In addition, due to the extensive time needed to develop, test and obtain regulatory approval for our products, any patents that protect our products may expire early during commercialization. For example, our original U.S. patent and corresponding international patents, covering our PAT-based technology and certain embodiments thereof expired during 2017. Since our products have undergone substantial development since then, we believe they should be protected by newer supplemental patents. However, we cannot be sure that these patents will be commercially useful in protecting our technology and, even if they are, such patents are scheduled to expire between 2022 and 2030. This may reduce or eliminate any market advantages that such patents may give us. Following patent expiration, we may face increased competition through the entry of competing products into the market and a subsequent decline in market share and profits.
International patent protection is particularly uncertain, and if we are involved in opposition proceedings in foreign countries, we may have to expend substantial sums and management resources.
Patent rights are territorial; thus, the patent protection we currently have will extend only to those countries in which we have issued patents. Even so, the laws of certain countries do not protect our intellectual property rights to the same extent as do the laws of the United States and the European Union. For example, certain countries do not grant patent claims that are directed to the treatment of humans. Competitors may successfully challenge our patents, produce similar devices that circumvent and do not infringe our patents, or manufacture devices in countries where we have not applied for patent protection or that do not respect our patents. Furthermore, it is difficult to predict the scope of claims that will be allowed in published applications and it is also difficult to predict which claims of granted patents, if any, will be deemed enforceable in a court of law. We may participate in opposition proceedings to determine the validity of our foreign patents or our competitors’ foreign patents, which would result in substantial costs and diversion of our management’s efforts, thus adversely affecting our results of operations.
| 20 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
If we are unable to protect the confidentiality of our trade secrets or know-how, such proprietary information may be used by others to compete against us.
In addition to filing patents, we protect our trade secrets, know-how and technology by entering into confidentiality or non-disclosure agreements with parties that have access to our proprietary information, such as our development or commercialization partners, employees, contractors and consultants. We also enter into agreements that purport to require the disclosure and assignment to us of the rights to the ideas, developments, discoveries and inventions of our employees, advisors, research collaborators, contractors and consultants while we employ or engage them. However, these agreements can be difficult and costly to enforce or may not provide adequate remedies. Any of these parties may breach the confidentiality agreements and willfully or unintentionally disclose our confidential information, or our competitors might learn of the information in some other way. The disclosure to, or independent development by, a competitor of any trade secret, know-how or other technology not protected by a patent could materially adversely affect any competitive advantage we may have over any such competitor.
To the extent that any of our employees, advisors, research collaborators, contractors or consultants independently develop, or use independently developed, intellectual property in connection with any of our projects, disputes may arise as to the proprietary rights to this type of information. If a dispute arises with respect to any proprietary right, enforcement of our rights can be costly and unpredictable and a court may determine that the right belongs to a third party, which could materially adversely affect our business, results of operations and ability to capitalize on our proprietary information.
Legal proceedings or third-party claims of intellectual property infringement and other challenges may require us to spend substantial time and money and could prevent us from developing or commercializing our products.
The development, manufacture, use, sale or importation of our products may infringe third-party patents or other intellectual property rights. The nature of claims contained in unpublished patent filings around the world is unknown to us and it is not possible to know which countries patent holders may choose for the extension of their filings under the Patent Cooperation Treaty, or other mechanisms. We may also be subject to claims based on the actions of employees and consultants with respect to the usage or disclosure of intellectual property learned at other employers. The cost to us of any intellectual property litigation or other infringement proceeding, even if resolved in our favor, could be substantial. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively because of their greater financial resources. Uncertainties resulting from the initiation and continuation or defense of intellectual property litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace. Intellectual property litigation and other proceedings may also absorb significant financial resources and management time. Consequently, there is no assurance that we will be able to develop or commercialize in line with our business objectives, in the event of an infringement action.
In the event of patent infringement claims, or to avoid potential claims, we may choose or be required to seek a license from a third party and would most likely be required to pay license fees or royalties, or both. These licenses may not be available on acceptable terms, or at all. Even if we were able to obtain a license, the rights may be non-exclusive, which could potentially limit our competitive advantage. Ultimately, we could be prevented from completing the development or commercialization of a product if, as a result of actual or threatened patent infringement or other claims, we are unable to enter into licenses on acceptable terms. This inability to enter into licenses could harm our business significantly.
| 21 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We may become subject to claims for remuneration or royalties for assigned service invention rights by our employees, which could result in litigation and adversely affect our business.
A significant portion of our intellectual property has been developed by our employees in the course of their employment for us. Under the Israeli Patent Law, 1967, or the Patent Law, inventions conceived by an employee in the course and as a result of or arising from his or her employment with a company are regarded as “service inventions”, which belong to the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patent Law also provides that if there is no agreement between an employer and an employee regarding consideration for service inventions, the Israeli Compensation and Royalties Committee, or the Committee, a body constituted under the Patent Law, shall determine whether the employee is entitled to remuneration for his inventions. Prior decisions by the Committee (in which the Israeli Supreme Court refused to intervene on appeal), created uncertainty, as it was held that employees may be entitled to remuneration for their service inventions despite having waived any such rights. However, more recent decisions by the Committee held that such right can be waived by the employee. The Committee further held that an explicit reference to the waived right is not necessary in every circumstance in order for the employee’s waiver of such right to be valid. Such waiver can be formalized in writing or orally or be implied by the actions of the parties in accordance with the rules of interpretation of Israeli contract law. However, the Israeli Supreme Court’s position on this matter remains uncertain. We generally enter into assignment-of-invention agreements with our employees pursuant to which such individuals assign to us all rights to any inventions created in the scope of their employment or engagement with us, without further compensation. Although our employees have agreed to assign to us service invention rights and have specifically waived their right to receive any special remuneration for such assignment beyond their regular salary and benefits, we may face claims demanding remuneration in consideration for assigned inventions. If such claims are successful we may be required to pay remuneration to our employees which could negatively affect our results of operations.
Risks Related to our Industry
Our ability to market and sell products depends upon receipt of domestic and foreign regulatory approvals of our products and manufacturing operations. Our failure to obtain or maintain regulatory approvals and compliance could negatively affect our business.
Our products and manufacturing operations are subject to extensive regulation by governmental authorities such as the FDA in the U.S., the European Union National Competent Authorities, or NCAs of the Member States of the European Economic Area, or EEA, and numerous other national or state governmental authorities in the countries in which we manufacture and sell our products. These regulations govern, among other things: the research, testing, manufacturing, safety, clinical efficacy, effectiveness and performance, product standards, packaging requirements, labeling requirements, import/export restrictions, storage, recordkeeping, promotion, distribution, production, post marketing surveillance and handling of complaints, tariffs, duties and tax requirements. Our products and operations are also often subject to the rules or norms of industrial standards bodies, such as the International Standards Organization, or ISO, or the rules of associations of healthcare professionals.
| 22 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In the U.S., our products are subject to regulation by the FDA pursuant to its authority under the federal Food, Drug and Cosmetic Act, or the FDCA, and its implementing regulations. In addition, future products, or components thereof, may also be subject to regulation by the Federal Communications Commission, or the FCC. Many of the laws and regulations applicable to our products in other countries, such as the new EU Medical Devices Regulation, or the MDR, are generally comparable to those of the FDCA in their aim to ensure safety and effectiveness of medical devices, but the applicable standards and proceedings are not globally harmonized. Such regulations are subject to continuous revision, which may entail increased requirements, and, more generally, there appears to be a trend toward more stringent regulatory oversight throughout the world. We do not anticipate this trend to diminish in the near future. Due to the movement towards harmonization of standards in the European Union, we expect a changing regulatory environment in Europe characterized by a shift from a country-by-country regulatory system to a European Union-wide harmonized regulatory system, while such harmonized regulatory system would not necessarily preclude state specific requirements which we may have to comply with. The timing of this harmonization and its effect on us cannot currently be predicted. The changing regulatory environment may have a material impact on existing device marketing authorizations as well as future device registration applications, requirements and timings, which may, in turn, have material impacts upon our ability to continue or begin to market existing and new devices. Our failure to obtain or maintain regulatory approvals and compliance could negatively affect our business.
Modifications to our currently FDA-cleared products or the introduction of new products may require new regulatory clearances or approvals or require us to recall or cease marketing of our current products until clearances or approvals are obtained.
In general, unless an exemption applies, each medical device to be marketed in the U.S. must first receive one of the following types of FDA premarket review authorizations:
| · | clearance via Section 510(k) of the FDCA; or |
| · | premarket approval via Section 515 of the FDCA if the FDA has determined that the medical device in question poses a greater risk of injury. The applicant may also submit a De novo application, in which case the regulator shall determine whether the device shall be classified from class III to class II or class I, with new classification or regulation. |
Generally, all of our products (excluding one exempt product) received a 510(k) clearance. The FDA will clear marketing of a medical device through the 510(k) process if it is demonstrated that the new product is substantially equivalent to other 510(k)-cleared products. The premarket approval application process is much more costly, lengthy and uncertain than the 510(k) process, and must be supported by extensive data from clinical trials. The FDA may not grant either 510(k) clearance or premarket approval for any product we propose to market. Further, any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or, possibly, approval of a premarket approval application. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. If the FDA requires us to seek 510(k) clearance or premarket approval for modification of a previously cleared product for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties. Further, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective.
The application process to receive clearances or approvals of our products by the pertinent regulatory authorities is costly and generally lasts between approximately three to twenty four months. Delays in receipt of, or failure to receive, clearances or approvals, the loss of previously received clearances or approvals, or the failure to comply with existing or future regulatory requirements could adversely impact our operating results. If the FDA or a foreign regulatory authority finds that we have failed to comply with these requirements, such authority may institute a wide variety of enforcement actions, ranging from a public warning letter to more severe sanctions such as:
| · | fines, injunctions and civil penalties; |
| 23 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | recall or seizure of our products; |
| · | issuance of public notices or warnings; |
| · | imposition of operating restrictions, partial suspension, or total shutdown of production; |
| · | refusal of our requests for Section 510(k) clearance or premarket approval of new products; |
| · | withdrawal of Section 510(k) clearance or premarket approvals already granted; or |
| · | criminal prosecution. |
Countries outside of the U.S. regulate medical devices in a manner similar to that of the FDA. The marketing and distribution of our products in the European Union, for example, is subject to the European Union’s Medical Device Directive described above. Devices that comply with the requirements of the Medical Devices Directive are entitled to bear the CE conformity mark, or the CE Mark, indicating that the device meets minimum standards of performance, safety and quality (i.e., the essential requirements) and, accordingly, can be commercially distributed throughout the EEA, Turkey and other countries outside Europe that have accepted the CE marking as a certification of efficiency and safety of medical devices. In Japan, we must comply with Japans Pharmaceuticals and Medical Devices Act, or the PMD Act, and are subject to the Pharmaceutical Medical Devices Authority, or the PMDA, the regulatory body supervising and regulating the marketing and sale of medical devices such as our products. We currently hold PMDA authorizations to market and sell our WatchPAT200/U and Endo PAT 2000 in Japan. Such authorizations are held by a local MAH/D-MAH with whom we maintain a contractual engagement.
Even though we have received FDA clearance, CE Mark certification, PMDA authorizations and other regulatory approvals for our products, there can be no assurance that we will be able to continue to comply with the required annual auditing requirements or other international regulatory requirements that may be applicable. For example, we must comply with medical device reporting, or MDR, requirements, including the reporting of adverse events and malfunctions related to our products. Adverse events, manufacturing faults, or failures to comply with regulatory requirements may result in voluntary actions as well as actions imposed by regulators, such as voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension of regulatory clearances or approvals, product seizures, injunctions or the imposition of civil or criminal penalties which would adversely affect our business, operating results and prospects.
In addition, there can be no assurance that government regulations applicable to our products or the interpretation of those regulations will not change or that we will be able to obtain required regulatory approvals for our new products. The extent of potentially adverse government regulation that might arise from future legislation or administrative action and the impact on our business and results of operations cannot be predicted.
We expect the healthcare industry to face increased limitations on reimbursement as a result of healthcare reform, which could adversely affect third-party coverage of our products and how much or under what circumstances healthcare providers will prescribe or administer our products.
In both the United States and other countries, sales of our products will depend in part upon the availability of reimbursement from third-party payors, which include governmental authorities, managed care organizations and other private health insurers. Third-party payors are increasingly challenging the price and examining the cost effectiveness of medical products and services.
Increasing expenditures for healthcare have been the subject of considerable public attention in the United States. Both private and government entities are seeking ways to reduce or contain healthcare costs. Numerous proposals that would effect changes in the U.S. healthcare system have been introduced or proposed in Congress and in some state legislatures, including reducing reimbursement for prescription products.
| 24 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In the U.S., President Obama signed into law in 2010 the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 designed to reform the American healthcare system. However, in 2017, President Trump signed an executive order in anticipation of a repeal or partial repeal of the 2010 Patient Protection and Affordable Care Act and introduced the American Health Care Act, or the AHCA, which was passed in the House of Representatives and was introduced to the Senate, where it has been the subject of extensive debate. It is difficult to assess the full long-term impact of proposed healthcare reforms stemming from the AHCA, if signed into law, and other proposals, on our business. However, certain adverse effects of such reforms may include imposition of new taxes on medical device providers, and a decrease in our products’ pricing. It is uncertain at this point what negative consequences these provisions may have on patient access to new technologies. We cannot predict the nature of healthcare programs and regulations which will ultimately be implemented at the federal or state level, or the effect of any future legislation or regulation on our results of operations. However, any regulatory changes that lower reimbursement for our products or reduce medical procedure volumes could adversely affect our business and results of operations.
Although we cannot predict the full effect on our business of the implementation of existing legislation, including the Affordable Care Act or the enactment of additional legislation, we believe that legislation or regulations that reduce reimbursement for, or restrict coverage of our products could adversely affect how much or under what circumstances healthcare providers will prescribe or administer our products. This could materially and adversely affect our business by reducing our ability to generate revenue, raise capital, obtain additional collaborators and market our products. In addition, we believe the increasing emphasis on managed care in the United States has and will continue to put pressure on the price and usage of pharmaceutical products, which may adversely impact our product sales.
We may be subject to U.S. and foreign anti-kickback laws and regulations. Our failure to comply with these laws and regulations could have adverse consequences.
There are extensive U.S. federal and state laws and regulations prohibiting fraud and abuse in the healthcare industry that can result in significant criminal and civil penalties. These federal laws include: the anti-kickback statute, which prohibits certain business practices and relationships, including the payment or receipt of remuneration for the referral of patients whose care will be paid by Medicare or other federal healthcare programs; the physician self-referral prohibition, commonly referred to as the Stark Law; the anti-inducement law, which prohibits providers from offering anything to a Medicare or Medicaid beneficiary to induce that beneficiary to use items or services covered by either program; the False Claims Act, which prohibits any person from knowingly presenting or causing to be presented false or fraudulent claims for payment by the federal government, including the Medicare and Medicaid programs; and the Civil Monetary Penalties Law, which authorizes the U.S. Department of Health and Human Services to impose civil penalties administratively for fraudulent or abusive acts.
Sanctions for violating these federal laws include criminal and civil penalties that range from punitive sanctions, damage assessments, money penalties, imprisonment, denial of Medicare and Medicaid payments, or exclusion from the Medicare and Medicaid programs, or both, and debarment. As federal and state budget pressures continue, federal and state administrative agencies may also continue to escalate investigation and enforcement efforts to root out waste and to control fraud and abuse in governmental healthcare programs. Private enforcement of healthcare fraud has also increased, due in large part to amendments to the civil False Claims Act in 1986 that were designed to encourage private persons to sue on behalf of the government. A violation of any of these federal and state fraud and abuse laws and regulations or any similar law in a different jurisdiction which is applicable to us could have a material adverse effect on our liquidity and financial condition. An investigation into the use by physicians of any of our products once commercialized may dissuade physicians from either purchasing or using them, and could have a material adverse effect on our ability to commercialize those products.
| 25 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Privacy regulations may impose costs and liabilities on us, limit our use of information, and adversely affect our business.
Our products generate medical information about patients and certain of our services are provided by way of a cloud service. Personal privacy has become a significant issue in the United States, Europe, Israel and many other countries where we operate. Many federal, state, and foreign legislatures and government agencies have imposed or are considering imposing restrictions and requirements about the collection, use, safeguarding and disclosure of personal information obtained from individuals.
In the U.S. the privacy rule established under HIPAA sets forth national standards to protect patients’ medical records and other personal health information and applies, among others, to health-care providers. The said rule requires appropriate safeguards to protect the privacy of personal health information and sets limits and conditions on the permissible uses and disclosures of such information. Said rule also provides patients with certain rights over their health information, including rights to examine and obtain a copy of their health records, and to request corrections. In May 2018, the GDPR came into force in the EU and in June 2018, in the EEA. GDPR is designed to set forth a harmonized framework for the regulations of privacy across the EU and EEA, while it also allows members states to enact state specific requirements. The provisions of the GDPR set forth requirements as to the permitted uses and disclosures of personal information, including personal medical information and appropriate safeguards to protect the privacy and security of such information. While the interpretation of the GDPR by European regulators remains to be seen and the full impact of such regulation is difficult to assess at this time, the GDPR may impose on us additional compliance costs and limitations on how we store and use information. In addition, we may be subject to requests for information, amendments of personal information records, data portability requests or law suits, from data subject in Europe. The maximum sanctions under the GDPR are the higher of 20 million Euros and 4% of a company’s worldwide annual revenues.
Changes to laws or regulations affecting privacy in the U.S. and in other locations we operate in could impose additional costs and liability on us and could limit our use of such information to add value to our customers. If we were required to change our business activities or revise or eliminate services, or to implement burdensome compliance measures, we may face additional expenditures. In addition, we may be subject to fines, penalties, and potential litigation if we fail to comply with applicable privacy regulations. Regulatory burdens of this sort increase our costs and harm our financial results.
We are subject to various laws relating to trade, export controls, and foreign corrupt practices, the violation of which could adversely affect our reputation, operations, business, prospects, operating results and financial condition.
We must comply with all applicable international trade, export and import laws and regulations of the United States and other countries, and we are subject to export controls and economic sanctions laws and embargoes imposed by the U.S. Government. Changes in trade sanctions laws may restrict our business practices, including cessation of business activities in sanctioned countries or with sanctioned entities, and may result in modifications to compliance programs. Among others, we are subject to the Foreign Corrupt Practices Act, or the FCPA, and other anti-bribery and anti-corruption laws that generally prohibit the offering, promising, giving, or authorizing others to give anything of value, either directly or indirectly, to a non-U.S. government official in order to influence official action, or otherwise obtain or retain business. The FCPA also requires public companies to make and keep books and records that accurately and fairly reflect the transactions of the corporation and to devise and maintain an adequate system of internal accounting controls.
| 26 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Our business is heavily regulated and therefore involves significant interaction with public officials, including officials of non-U.S. governments. We have implemented safeguards and policies to discourage prohibited practices by our employees and agents that would violate applicable anti-bribery and anti-corruption laws. However, we cannot ensure that our compliance controls, policies, and procedures will in every instance protect us from acts committed by our employees, agents, contractors, or collaborators that may violate the laws or regulations of the jurisdictions in which we operate.
Violations of these laws and regulations could result in significant fines, criminal sanctions against us, our officers, or our employees, requirements to obtain export licenses, disgorgement of profits, cessation of business activities in sanctioned countries, implementation of compliance programs, exclusion from government programs, prohibitions on the conduct of our business, and our inability to market and sell our products in one or more countries. Additionally, any such violations could materially damage our reputation, our brand, our international expansion efforts, our ability to attract and retain employees, and our business, prospects, operating results, and financial condition.
If we do not comply with laws regulating the protection of the environment and health and human safety, our business could be adversely affected.
Our research and development and manufacturing involve the use of hazardous materials and chemicals and related equipment. If an adverse safety incident occurs, we could be held liable for resulting damages, which could be substantial. We are also subject to numerous environmental, health and workplace safety laws and regulations, including those governing laboratory procedures and the handling of biohazardous materials. Insurance may not provide adequate coverage against these potential liabilities and we do not maintain insurance for environmental liability claims that may be asserted against us. Moreover, additional foreign and local laws and regulations affecting our operations may be adopted in the future. We may incur substantial costs to comply with such regulations and pay substantial fines or penalties if we violate any of these laws or regulations.
With respect to environmental, safety and health laws and regulations, we cannot accurately predict the outcome or timing of future expenditures that we may be required to make in order to comply with such laws as they apply to our operations and facilities. We are also subject to potential liability for the remediation of contamination associated with both present and past hazardous waste generation, handling, and disposal activities. We will be periodically subject to environmental compliance reviews by environmental, safety, and health regulatory agencies. Environmental laws are subject to change and we may become subject to stricter environmental standards in the future and face larger capital expenditures in order to comply with environmental laws which could have a material adverse effect on our business.
Risks Related to Our Ordinary Shares and ADSs
There has been no prior public market in the United States for our ordinary shares and ADSs, and an active trading market in the United States may not develop.
We plan to list our ADSs on the Nasdaq Capital Market. Prior to such listing, there has been no public market in the United States for our ordinary shares and ADSs. An active trading market in the United States may not develop following the aforementioned listing or, if developed, may not be sustained. The lack of an active market may impair the ability of our shareholders to sell their shares at the time they wish to sell them or at a price that they would consider reasonable. The lack of an active market may also reduce the fair market value of such shares. An inactive market may also impair our ability to raise capital by selling shares of capital stock and may impair our ability to acquire other companies by using our shares as consideration.
| 27 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Our ADSs and ordinary shares will be traded on different markets and this may result in price variations.
Our ordinary shares have been traded on the Tel Aviv Stock Exchange Ltd., or the TASE, since March 2007. We plan to list our ADSs on the Nasdaq Capital Market. Price variations may result due to this dual listing. Trading in our ordinary shares and ADSs on these markets will be in different currencies, dollars on the Nasdaq Capital Market and NIS on the TASE and at different times (resulting from different time zones, different trading days and different public holidays in the United States and Israel). Given these and other factors, such as differences in exchange rates, our ordinary shares and ADSs may trade at different prices on the TASE and the Nasdaq Capital Market. In addition, market influences in one market may influence the price at which our shares are traded on the other.
Our share price may be volatile and could be substantially affected by various factors.
The market price of our ordinary shares and ADSs could be highly volatile and may fluctuate substantially. Numerous factors, many of which are beyond our control, may cause our market price and trade volume to fluctuate and decrease in the future, including the following factors:
| · | actual or anticipated fluctuations in our results of operations; |
| · | changes in expectations as to our future financial performance and cash position, including financial estimates by securities analysts and investors; |
| · | announcements of technological innovations, medical findings or new products by us or our competitors; |
| · | announcements by us or our competitors of significant business developments, changes in distributor relationships, strategic partnerships, joint ventures, capital commitments, acquisitions or expansion plans; |
| · | changes in the prices of our raw materials or the products we sell; |
| · | changes in the status of our intellectual property rights; |
| · | our involvement in significant claims or proceedings; |
| · | our sales of ordinary shares and ADSs or other securities in the future; |
| · | market conditions in our industry; |
| · | changes in key personnel; |
| · | the trading volume of our ordinary shares and ADSs; |
| · | changes in the estimation of the future size and growth rate of our markets; |
| · | general economic and market conditions; and |
| 28 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | any of the events underlying any of the other risks or uncertainties set forth elsewhere in this registration statement actually occurs. |
In addition, the stock markets have experienced extreme price and volume fluctuations. Broad market and industry factors may materially harm the market price of our ordinary shares and ADSs, regardless of our operating performance. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been instituted against that company. If we were involved in any similar litigation, we could incur substantial costs and our management’s attention and resources could be diverted.
Low trading volume may also increase the price volatility of our ordinary shares and ADSs. A thin trading market could cause the price of our ordinary shares and ADSs to fluctuate significantly more than the stock market as a whole. In addition, domestic and international stock markets and electronic trading platforms often experience extreme price and volume fluctuations. Market fluctuations, as well as general political and economic conditions, such as a recession or interest rate or currency rate fluctuations or political events or hostilities in or surrounding Israel, could also adversely affect the price of our ordinary shares and ADSs.
Holders of our ADSs are not treated as shareholders of our Company.
Holders of our ADSs are not treated as shareholders of our Company unless they withdraw the ordinary shares underlying the ADSs from the depositary, which holds the ordinary shares underlying the ADSs. Holders of ADSs therefore do not have any rights as shareholders of our Company, other than the rights that they have pursuant to the deposit agreement with the depositary. For example, under the deposit agreement, if a holder of our ADSs does not provide the depositary with voting instructions for an agenda item in our shareholders meeting in a timely manner, we may instruct the depositary, if we reasonably do not know of any substantial opposition to such agenda item and the matter is not materially adverse to the interests of shareholders, to treat the holder as giving a discretionary proxy to a person designated by us as to that matter.
Our directors and executive officers own a substantial percentage of our ordinary shares.
As of August 1, 2018, our directors and executive officers beneficially own approximately 18.5% of our outstanding ordinary shares (or, when taken together with the holdings of Viola and MS Pace, which are associated with some of these directors, approximately 61.3% of our outstanding ordinary shares). As a result, if these shareholders acted together, they could exert significant influence on the election of our directors and on decisions by our shareholders on matters submitted to shareholder vote, including mergers, consolidations and the sale of all or substantially all of our assets. This concentration of ownership of our ordinary shares could delay or prevent proxy contests, mergers, tender offers or other purchases of our ordinary shares that might otherwise give our shareholders the opportunity to realize a premium over the then-prevailing market price for our ordinary shares and, as a result, may also adversely affect our share price.
If equity research analysts do not publish research or reports about our business or if they issue unfavorable commentary or downgrade our ordinary shares and ADSs, the price of our ordinary shares and ADSs could decline.
The trading market for our ordinary shares and ADSs will rely in part on the research and reports that equity research analysts publish about us and our business. The price of our ordinary shares and ADSs could decline if one or more securities analysts downgrade our ordinary shares and ADSs or if those analysts issue other unfavorable commentary or cease publishing reports about us or our business.
| 29 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
As a foreign private issuer whose shares are listed on the Nasdaq Capital Market we intend to follow certain home country corporate governance practices instead of certain Nasdaq requirements.
As a foreign private issuer whose shares will be listed on the Nasdaq Capital Market, we are permitted to follow certain home country corporate governance practices instead of certain requirements of the Nasdaq rules. As permitted under the Companies Law, our articles of association provide that the quorum for any meeting of shareholders is 33 1/3% of the issued share capital, as required under Nasdaq requirements, however, if the meeting is adjourned for lack of quorum, the quorum for such adjourned meeting will be two shareholders who hold or represent between them at least 10% of the issued and outstanding share capital, instead of 33 1/3% of the issued share capital. We also intend to adopt and approve material changes to equity incentive plans in accordance with the Companies Law, which does not impose a requirement of shareholder approval for such actions. In addition, we intend to follow the Companies Law in respect of private placements (see under Item 10B. “Memorandum and Articles of Association – Private Placements”) instead of Nasdaq requirements to obtain shareholder approval for certain dilutive events (such as issuances that will result in a change of control, certain transactions other than a public offering involving issuances of a 20% or greater interest in us and certain acquisitions of the stock or assets of another company). Accordingly, our shareholders may not be afforded the same protection as provided under Nasdaq corporate governance rules for domestic issuers.
Following our home country governance practices as opposed to the requirements that would otherwise apply to a U.S. company listed on the Nasdaq Capital Market may provide less protection than is accorded to investors of domestic issuers.
As a “foreign private issuer” our disclosure and reporting requirements will be different than those of a U.S. domestic reporting company.
In addition, as a foreign private issuer, we will be exempt from the rules and regulations under the United States Securities Exchange Act of 1934, as amended, or the Exchange Act, related to the furnishing and content of proxy statements, and our officers, directors, and principal shareholders will be exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file annual, quarterly and current reports and financial statements with the Securities and Exchange Commission, or the SEC, as frequently or as promptly as domestic companies whose securities are registered under the Exchange Act.
We will incur additional increased costs as a result of the planned listing of our ADSs for trading on the Nasdaq Capital Market, and our management will be required to devote substantial time to compliance initiatives and reporting requirements associated therewith.
As a public company in the United States, we will incur additional significant accounting, legal and other expenses as a result of the planned listing of our ADSs on the Nasdaq Capital Market. These include costs associated with corporate governance requirements of the SEC and the Marketplace Rules of Nasdaq, as well as requirements under Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act. These rules and regulations will increase our legal and financial compliance costs, introduce new costs such as investor relations, stock exchange listing fees and shareholder reporting, and make some activities more time consuming and costly. Any future changes in the laws and regulations affecting public companies in the United States and Israel, including Section 404 and other provisions of the Sarbanes-Oxley Act, the rules and regulations adopted by the SEC and the rules of Nasdaq, as well as applicable Israeli reporting requirements, for so long as they apply to us, will result in increased costs to us as we respond to such changes. These laws, rules and regulations could make it more difficult or more costly for us to obtain certain types of insurance, including director and officer liability insurance, and we may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our Board of Directors, our committees of our Board of Directors or as executive officers.
| 30 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
If we are unable to satisfy the requirements of Section 404 as they apply to a foreign private issuer and emerging growth company that is listing on a U.S. exchange for the first time, or our internal controls over financial reporting are not effective, the reliability of our financial statements may be questioned and our share price may suffer.
We will become subject to the requirements of the Sarbanes-Oxley Act if our ordinary shares and ADSs are listed on the Nasdaq Capital Market. Section 404 of the Sarbanes-Oxley Act, or Section 404, requires companies subject to the reporting requirements of the U.S. securities laws to complete a comprehensive evaluation of its and its subsidiaries’ internal controls over financial reporting. To comply with this statute, we will be required to document and test our internal control procedures and our management will be required to assess and issue a report concerning our internal controls over financial reporting. Pursuant to the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, we will be classified as an “emerging growth company.” Under the JOBS Act, emerging growth companies are exempt from certain reporting requirements, including the independent auditor attestation requirements of Section 404(b) of the Sarbanes-Oxley Act. Under this exemption, our independent auditor will not be required to attest to and report on management’s assessment of our internal controls over financial reporting during a five year transition period. We will need to prepare for compliance with Section 404 by strengthening, assessing and testing our system of internal controls to provide the basis for our report. However, the continuous process of strengthening our internal controls and complying with Section 404 is complicated and time-consuming. Furthermore, we believe that our business will grow both domestically and internationally, in which case our internal controls will become more complex and will require significantly more resources and attention to ensure our internal controls remain effective overall. During the course of its testing, our management may identify material weaknesses or significant deficiencies, which may not be remedied in a timely manner to meet the deadline imposed by the Sarbanes-Oxley Act. If our management cannot favorably assess the effectiveness of our internal controls over financial reporting, or our independent registered public accounting firm identifies material weaknesses in our internal controls, investor confidence in our financial results may weaken, and the market price of our securities may suffer.
Our U.S. shareholders may suffer adverse tax consequences if we are characterized as a passive foreign investment company.
Generally, if for any taxable year 75% or more of our gross income is passive income, or at least 50% of our assets are held for the production of, or produce, passive income, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. Based on certain estimates of our gross income and gross assets and the nature of our business, we do not expect that we will be classified as a PFIC for the taxable year ending December 31, 2018. However, because PFIC status is based on our income, assets and activities for the entire taxable year, it is not possible to determine whether we will be characterized as a PFIC for the 2018 taxable year until after the close of the year. Moreover, we must determine our PFIC status annually based on tests which are factual in nature, and our status in future years will depend on our income, assets and activities in those years. Moreover, because the value of our gross assets may be determined in part by reference to our market capitalization, a decline in the value of our ordinary shares and ADSs may result in our becoming a PFIC. There can be no assurance that we will not be considered a PFIC for any taxable year. If we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. Holder, as defined in Item 10.E “Taxation —United States Federal Income Tax Consequences”, owns ordinary shares and ADSs, such U.S. Holder could face adverse U.S. federal income tax consequences, including having gains realized on the sale of our ordinary shares and ADSs classified as ordinary income, rather than as capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares and ADSs by individuals who are U.S. Holders, and having interest charges apply to distributions by us and the proceeds of share sales. Certain elections exist that may alleviate some of the adverse consequences of PFIC status and would result in an alternative treatment (such as mark-to-market treatment) of our ordinary shares and ADSs. Although we have no obligation to do so, we currently intend to notify U.S. Holders, if we believe we will be treated as a PFIC for any tax year in order to enable U.S. Holders to consider whether to elect to treat us as a qualified electing fund by making a so-called “QEF election”.
| 31 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The market price of our ordinary shares and ADSs could be negatively affected by future sales of our ordinary shares and ADSs.
As of August 1, 2018, we had approximately 286.7 million ordinary shares issued and outstanding and approximately 77.7 million of additional ordinary shares which are issuable upon exercise of outstanding warrants, stock options and vesting of outstanding restricted share units, or RSUs. The issuance of a significant amount of additional ordinary shares or ADSs on account of these outstanding securities will dilute our current shareholders’ holdings and may depress our share price.
If our existing shareholders or holders of our warrants, options or RSUs sell substantial amounts of our ordinary shares or ADSs, either on the TASE or Nasdaq, the market price of our ordinary shares (and, once listed on the Nasdaq Capital Market, our ADSs) may be adversely affected. Any substantial sales of our ordinary shares or ADSs in the public market might also make it more difficult for us to sell equity or equity related securities in the future at a time and on terms we deem appropriate. Even if there are not a substantial number of sales, the mere existence of this “market overhang” could have a negative impact on the market for, and the market price of, our ordinary shares.
In 2018, we issued a total of approximately 22.0 million ordinary shares to several investors, including several of our major shareholders (listed in Item 7.A under “Security Ownership of Certain Beneficial Owners and Management”). These shares are subject to resale restrictions under Israeli law as applicable to private placements, including an initial six month full lockup resale restriction that expires in November 2018. In addition, in 2015, in connection with the Viola Investment, we agreed to grant Viola, our largest shareholder, registration rights that require that we register under the Securities Act the resale of their shares into the public markets. The market price of our ordinary shares and ADSs may drop when the restrictions on resale by our existing shareholders lapse and these shareholders are able to sell our ordinary shares and ADSs into the market or, in the case of Viola, if Viola were to exercise its registration rights.
Provisions of our Amended and Restated Articles of Association and Israeli law as well as the terms of some of our equity-based grants and our credit facility may delay, prevent or make difficult an acquisition of us, which could prevent a change of control and negatively affect the price of our ordinary shares and ADSs.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for certain transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to these types of transactions. These provisions of Israeli law may delay, prevent or make difficult an acquisition of us, which could prevent a change of control and therefore depress the price of our shares. For example, under the Companies Law, upon the request of a creditor of either party to a proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that as a result of the merger the surviving company will be unable to satisfy the obligations of any of the parties to the merger. In addition, our executive officers and certain other key employees are entitled to certain benefits in connection with a change of control of our Company and our credit facility allows the bank to accelerate repayment of outstanding debt upon a change of control of our Company (see under “Our freedom to operate our business is limited as a result of certain restrictive covenants contained in our credit facility” above). These provisions could cause our ordinary shares to trade at prices below the price for which third parties might be willing to pay to gain control of us. Third parties who are otherwise willing to pay a premium over prevailing market prices to gain control of us may be unable or unwilling to do so because of these provisions of Israeli law.
| 32 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders, especially for those shareholders whose country of residence does not have a tax treaty with Israel which exempts such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred.
We have never paid cash dividends on our share capital, and we do not anticipate paying any cash dividends in the foreseeable future.
We have never declared or paid cash dividends on our share capital, nor do we anticipate paying any cash dividends on our share capital in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. As a result, capital appreciation, if any, of our ordinary shares and ADSs will be investors’ sole source of gain for the foreseeable future. In addition, Israeli law limits our ability to declare and pay dividends, and may subject our dividends to Israeli withholding taxes. Furthermore, our payment of dividends (out of tax-exempt income) may retroactively subject us to certain Israeli corporate income taxes, to which we would not otherwise be subject.
Risks Related to Our Operations in Israel
Our headquarters, manufacturing and other significant operations are located in Israel and, therefore, our business and operation may be adversely affected by political, economic and military conditions in Israel.
We are incorporated under the laws of the State of Israel, and our principal offices and research and development and production facilities are located in Israel. In addition, the majority of our key employees, officers and directors are residents of Israel. Accordingly, political, economic and security conditions in the Middle East in general, and in Israel in particular, directly affect our business.
Over the past several decades, a number of armed conflicts have taken place between Israel and its Arab neighbors and a state of hostility, varying in degree and intensity, has led to security and economic problems for Israel. Since late 2000, there has also been a high level of violence between Israel and the Palestinians including during the summer of 2014, when Israel was engaged in armed conflicts with Hamas, a militia group and political party operating in the Gaza Strip. This violence has strained Israel’s relationship with its Arab citizens, Arab countries and, to some extent, with other countries around the world. Since the end of 2010, several countries in the region have been experiencing increased political instability, which led to changes in government in some of these countries and the ongoing war in Syria, the effects of which are currently difficult to assess. In addition, Israel faces threats from more distant neighbors, such as Iran (which is believed to be an ally of Hamas in Gaza and Hezbollah in Lebanon) and the militant group known as the Islamic State of Iraq and Syria. This situation may potentially escalate in the future and may also lead to deterioration of the political and trade relationships that exist between the State of Israel and these countries. In addition, this instability in the region may affect the global economy and marketplace. Any armed conflicts or political instability in the region, including acts of terrorism as well as cyber-attacks or any other hostilities involving or threatening Israel, would likely negatively affect business conditions and could make it more difficult for us to conduct our operations in Israel, which could increase our costs and adversely affect our financial results. Our commercial insurance does not cover losses that may occur as a result of events associated with the security situation in the Middle East, such as damages to our facilities resulting in disruption of our operations. Although the Israeli government currently covers the reinstatement value of direct damages that are caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained or will be adequate in the event we submit a claim. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflict involving Israel could adversely affect our operations and results of operations.
| 33 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Furthermore, some neighboring countries, as well as certain companies, organizations and movements, continue to participate in a boycott of Israeli firms and others doing business with Israel or with Israeli companies. In the past several years, there have been increased efforts by activists to cause companies and consumers to boycott Israeli goods based on Israeli government policies. Similarly, Israeli companies are limited in conducting business with entities from several countries. For example, in 2008, the Israeli legislature passed a law forbidding any investments in entities that transact business with Iran. Restrictive laws, policies or practices directed towards Israel or Israeli businesses could have an adverse impact on the expansion of our business.
In addition, we could be adversely affected by the interruption or curtailment of trade between Israel and its trading partners, a significant increase in the rate of inflation, or a significant downturn in the economic or financial condition of Israel.
Some of our officers and employees are obligated to perform annual military reserve duty, and in the event of a military conflict, these persons could be called to active duty at any time, for extended periods of time and on very short notice. The absence of a number of our officers and employees for significant periods could materially adversely affect our business and results of operations. We cannot assess the full impact of these obligations on our workforce or business if conditions should change.
Our operations may be affected by negative labor conditions in Israel.
Strikes and work-stoppages occur relatively frequently in Israel. If Israeli trade unions threaten additional strikes or work-stoppages and such strikes or work-stoppages occur, those may, if prolonged, have a material adverse effect on the Israeli economy and on our business, including our ability to deliver products to our customers and to receive raw materials from our suppliers in a timely manner.
The Israeli government grants that we have received require us to meet several conditions and restrict our ability to manufacture products and transfer know-how outside of Israel and require us to satisfy specified conditions.
We have in the past received, and in the future may apply for, royalty-bearing grants from the Israel Innovation Authority (formerly known as the Office of the Chief Scientist of the Israeli Ministry of Economy), or the IIA, for research and development programs that meet specified criteria pursuant to the Law for the Encouragement of Research, Development and Technological Innovation, 1984 (formerly known as the Law for Encouragement of Research and Development in the Industry, 1984), and the regulations promulgated thereunder, or the R&D Law. The terms of the IIA grants limit our ability to manufacture products or transfer technologies outside of Israel if such products or technologies were developed using know-how developed with or based upon IIA grants. In addition, any non-Israeli who, among other things, becomes an “interested party” in Itamar (e.g., becomes a holder of 5% or more of our share capital or voting rights), is generally required to undertake to observe the law governing the grant programs of the IIA, some of the principal restrictions and penalties of which are the transferability limits described above and elsewhere in this registration statement.
| 34 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Further, the IIA grants may be terminated in the future or the available benefits may be reduced or impacted, including, among other possible circumstances, should we transfer certain research and development or manufacturing activities outside the State of Israel. The termination or curtailment of these programs or the loss or reduction of such benefits could have a material adverse effect on our business, financial condition and results of operations. In addition, the IIA may establish new guidelines regarding the R&D Law, which may affect our existing and/or future IIA programs and incentives for which we may be eligible. We cannot predict what changes, if any, the IIA may make.
To date, we have received royalty-bearing grants from the IIA in a total amount of approximately NIS 3.8 million (equivalent to $1.03 million) for the development of Endo PAT 3000, a new generation of our Endo PAT product. Since we have ceased our development efforts of Endo PAT 3000, we believe that the terms of these IIA royalty-bearing grants mean that we are not required to repay these grants to the IIA. However, in 2009 the IIA informed us that we must pay royalties on the sale of all of our products since 2012 and, since then, we have been in discussions with the IIA in an attempt to resolve this disagreement. While we disagree with the IIA demand, there is no assurance that we will necessarily prevail in our efforts to oppose this demand. See also in Item 4.B under “Business Overview – Government Regulations – The Israeli Market - Grants from the IIA.”
Enforcing a U.S. judgment against our Company and our executive officers and directors, or asserting U.S. securities law claims in Israel may be difficult.
We are incorporated in Israel. Service of process upon us, our Israeli subsidiaries, our directors and officers and the Israeli experts, if any, named in this registration statement, substantially all of whom reside outside the United States, may be difficult to obtain within the United States. Furthermore, because the majority of our assets and investments, and substantially all of our directors, officers and such Israeli experts are located outside the United States, any judgment obtained in the United States against us or any of them may be difficult to collect within the United States.
| 35 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We have been informed by our legal counsel in Israel that it may also be difficult to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws if they determine that Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. There is little binding case law in Israel addressing these matters. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact, which can be a time-consuming and costly process. Certain matters of procedure will also be governed by Israeli law.
Subject to specified time limitations and legal procedures, under the rules of private international law currently prevailing in Israel, Israeli courts may enforce a U.S. judgment in a civil matter, including a judgment based upon the civil liability provisions of the U.S. securities laws, as well as a monetary or compensatory judgment in a non-civil matter, provided that the following key conditions are met:
| · | subject to limited exceptions, the judgment is final and non-appealable; |
| · | the judgment was given by a court competent under the laws of the state of the court and is otherwise enforceable in such state; |
| · | the judgment was rendered by a court competent under the rules of private international law applicable in Israel; |
| · | the laws of the state in which the judgment was given provides for the enforcement of judgments of Israeli courts; |
| · | adequate service of process has been effected and the defendant has had a reasonable opportunity to present his arguments and evidence; |
| · | the judgment and its enforcement are not contrary to the law, public policy, security or sovereignty of the State of Israel; |
| · | the judgment was not obtained by fraud and does not conflict with any other valid judgment in the same matter between the same parties; and |
| · | an action between the same parties in the same matter was not pending in any Israeli court at the time the lawsuit was instituted in the U.S. court. |
Your rights and responsibilities as a shareholder will be governed by Israeli law, which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our ordinary shares and ADSs are governed by our amended and restated articles of association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in U.S. based corporations. For example, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain from abusing its power in the company, including, among other things, voting at a general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares and ADSs that are not typically imposed on shareholders of U.S. corporations.
| 36 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| ITEM 4. | INFORMATION ON THE COMPANY |
| A. | History and Development of the Company |
Corporate History and Details
We, Itamar Medical Ltd., were incorporated under the laws of the State of Israel under the name “Itamar Medical (CM) 1997 Ltd.” on January 15, 1997 as a company limited by shares. We changed our name to our current name in July 2000.
In 2001, the first generation of our WatchPAT device received its initial FDA clearance and, in 2003, the first generation of our Endo PAT device received its initial FDA clearance.
In March 2007, we completed our initial public offering in Israel on the TASE, where our ordinary shares are traded under the symbol “ITMR.”
In August 2018, we applied for the listing of our ADSs on the Nasdaq Capital Market under the symbol “ITMR.” We make no representation, however, that such application will be approved or that the ADSs will trade on such market either now or at any time in the future.
Our principal executive offices are located at 9 Halamish St., Caesarea, 3088900 Israel, and our telephone number is +972 (4) 617-7000.
Our authorized representative and agent in the U.S. is Itamar Medical, Inc., which maintains its principal offices at 3290 Cumberland Club Drive, Atlanta, GA 30339, telephone number 1-888-748-2627.
Our address on the Internet is http://www.itamar-medical.com. The information on our website is not incorporated by reference into this registration statement and should not be considered a part of this registration statement, and the reference to our website in this registration statement is an inactive textual reference only.
Recent Major Business Developments
Below is a summary of the major business developments in Itamar Medical since January 1, 2018:
| · | On October 9, 2018, we held a special general meeting of our shareholders at which our shareholders approved amendments to our compensation policy for our executive officers and directors, or the Compensation Policy, relating to the criteria for our purchase of directors and officers liability insurance. For additional details, see Item 6.B “Directors, Senior Management and Employees – Compensation.” |
| · | On August 12, 2018, we reported our unaudited financial results for the six and three months ended June 30, 2018, which were approved by our Board of Directors on August 9, 2018. For additional details, see Item 5.A “Operating Results” and our unaudited consolidated interim financial statements included elsewhere in this registration statement. |
| 37 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | On July 30, 2018, we publicly announced that the framework agreement for the sale of our products to one of our material customers, a U.S. hospital and clinics chain, was extended by five years, until June 2023. For additional details, see Item 4.B “Business Overview – Sales and Marketing”. |
| · | On May 27, 2018, we completed a private placement of 22,013,893 ordinary shares, resulting in aggregate proceeds (before expenses) of NIS 20.8 million (equivalent to approximately $6.0 million). For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| · | On May 23, 2018, we held our annual meeting of shareholders for 2018, at which our shareholders approved all of the items on the agenda of the meeting, namely, approval of the following matters: (1) reelection of all of our directors (other than our external directors whose term are scheduled to expire in June 2019); (2) the private placement described in the preceding paragraph; (3) an increase of the base salary of our President and Chief Executive Officer (see Item 6.B “Directors, Senior Management and Employees – Compensation – Individual Compensation of Covered Executives”); (4) modification of the performance criteria related to the vesting of stock options and RSUs previously granted to our President and Chief Executive Officer (see Item 6.B “Directors, Senior Management and Employees – Compensation – Individual Compensation of Covered Executives”); (5) an annual cash bonus to our President and Chief Executive Officer for the years 2018 through 2022 (see Item 6.B “Directors, Senior Management and Employees – Compensation – Individual Compensation of Covered Executives”); (6) once our ADSs will become listed on the Nasdaq Capital Market, we will comply with the Israeli regime for dual listed companies under Chapter E3 of the Israeli Securities Law, 1968, or the ISL, which will allow us to use in Israel the same periodic reports, financial and other relevant disclosure information (in English) that we submit to the SEC and Nasdaq; and (7) the reappointment of Somekh Chaikin, a member of KPMG International, as our independent auditors. |
| · | On May 7, 2018, we publicly announced the launch of SleePath, an integrated e-health sleep apnea care pathway monitoring system that is designed to allow cardiologists to monitor patients with atrial fibrillation (AF) sleep apnea management status and compliance with CPAP devices on demand. For additional details, see Item 4.B “Business Overview – Our Products and Services”. |
| · | On April 8, 2018, we publicly announced that we made our preliminary submission to the FDA for clearance of WatchPAT300, a new generation of the WatchPAT line of products. On August 17, 2018, we obtained the FDA clearance. For additional details, see Item 4.B “Business Overview – Our Products and Services”. |
| · | On February 28, 2018, we repaid the entire outstanding principal amount and accrued interest of our then outstanding convertible notes. For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
Principal Capital Expenditure and Divestitures
See Item 5.B “Operating and Financial Review and Prospects –Liquidity and Capital Resources –Principal Capital Expenditure and Divestitures.”
| 38 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| B. | Business Overview |
Overview
We are a medical technology company that designs, develops, manufactures and sells sleep apnea diagnostic ambulatory products and related services.
We believe a key competitive differentiator for us is the use of the Peripheral Arterial Tone (PAT) biological signal along with other measurements, such as actigraphy, heart rate, chest motion, body position and snoring. All of these inputs are analyzed by our proprietary technology and algorithms.
Our PAT-based technology is implemented in a simple to use non-invasive watch-like wrist worn device called WatchPAT that uses a finger mount bio-sensor to measure and record the PAT signal, which is then transferred to either a local (zzzPAT) or cloud-based (CloudPAT) software for analysis and reporting of sleep apnea diagnosis. The results of our proprietary analysis are automatically populated into an easy to read report that allows physicians to make accurate diagnosis of sleep apnea.
Our Total Sleep Solution (TSS) is a comprehensive marketing program we offer to physicians that combines products and services, including our proprietary diagnostic test and data analytics as well as access to resale of third party sleep apnea treatment devices and a network of independent diagnostics testing facilities (IDTFs) and durable mobile equipment (DMEs) providers. TSS is designed to allow any medical practice or physician that is not a sleep physician by specialty, easy access to a comprehensive suite of products and services for the diagnosis, treatment and management of patients they suspect suffer from sleep apnea. We believe the combination of our proprietary test combined with the ease of single point of contact management of the diagnosis and treatment of sleep apnea provided by TSS has been a driver of the increased usage of our tests. Specific products and services included in the TSS program include CloudPAT and SleePath for cloud-based data and information mobilization solutions, access to the resale of sleep apnea therapeutic products such as CPAP devices, PAMS and MADs, related services and logistical solutions such as WatchPAT Direct.
Since 2015, we have focused on offering TSS to the cardiology market through various business models; however the Test as a Service (TaaS), also known as Cost Per Test (CPT) model, is the primary model we utilized to date. In the TaaS model, the medical practice or physician ordering the TaaS pays a fixed fee per home sleep apnea test (HSAT) that includes all the components associated with the test, including the disposable biosensor, hardware rental fees and access to our CloudPAT platform.
Market Overview and Our Solutions
Cardiovascular Disease
Cardiovascular disease, to which we sometimes refer to as CVD or cardiac disease, is a class of diseases that involves the heart or blood vessels, such as hypertension, heart disease, arrhythmias (including atrial fibrillation) and congestive heart failure.
CVD is highly prevalent and, quite often, a severe and potentially fatal, medical condition. According to reports published by the American Heart Association, approximately 92.1 million (nearly 37.0%) American adults are living with some form of CVD or the after-effects of stroke, and, by 2035, 130 million adults in the U.S. are projected to have some form of CVD.
It has been shown through several published, peer reviewed, studies that sleep apnea is a direct contributing factor to the incidence of various forms of CVD. Accordingly, cardiologists have become increasingly aware and focused on the diagnosis and treatment of sleep apnea. In addition, according to various published reports, there were approximately 32,000 cardiologists in the U.S. in 2017 that work in approximately 7,800 cardiology offices.
| 39 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Sleep Apnea
Sleep apnea is a serious and chronic sleep breathing disorder that negatively impacts a patient’s sleep, health and quality of life. There are two types of sleep apnea:
| · | OSA: Obstructive sleep apnea, or OSA, the most common form of sleep apnea, occurs when a person’s breathing is interrupted during sleep by a partially or completely blocked airway. When the airway becomes blocked, the brain detects a stress signal from various biological sources including the chest muscles, lungs and, at times, also a drop in blood oxygen content, which causes the individual to awaken unconsciously (a micro-arousal), just enough to tighten the airway muscles and allow normal breathing to resume. While regular breathing is restored temporarily, the obstruction typically occurs again which restarts the apnea cycle. This cycle of obstructions and waking can repeat dozens of times per hour throughout the night, disrupting the rapid eye movement, or REM, and deep, restorative sleep that are critical to good health as well as creating negative pressure in the abdomen that causes damage to the organs; and |
| · | CSA: Central sleep apnea, or CSA, a less common form of sleep apnea, occurs when a person’s breathing is impacted by lack of brain stimulation of the lungs and diaphragm muscles rather than obstruction. CSA is usually mixed with OSA and rarely appears in a pure form. This condition is known to be prevalent in heart failure patients as well as residence of high altitudes and opiates addicts and a specific pattern of it is called Cheyne-Stokes Respiration. To our knowledge, there is a continuing debate in the scientific community and among clinical practitioners whether this diagnosis impacts the treatment pathway. |
A 2013 study of the American Academy of Sleep Medicine (AASM) reported that 25% of adults worldwide suffer from sleep apnea. According to a 2018 study published by the American Journal of Respiratory and Critical Care Medicine, the prevalence of sleep apnea impacts more than 936 million people worldwide. At the same time, and despite the growing awareness of the consequences of OSA, the most common form of sleep apnea, it was estimated in a 2018 study published by the American Thoracic Society that over 80% of patients with clinically significant and treatable OSA have never been diagnosed.
A 2010 report published by Harvard Medical School estimated the annual economic costs (including the cost of diagnosis and treatment, public safety costs from OSA-related traffic accidents, and the incremental medical costs of OSA co-morbidities) of untreated moderate to severe OSA in the U.S. to be between $65 billion and $165 billion annually, potentially greater than the cost of asthma, heart failure, stroke or hypertensive disease, which range from $20 billion to $80 billion according to estimates. At the same time, according to an estimate published by Fisher & Paykel Healthcare, the sleep apnea diagnostic and treatment worldwide market was estimated to exceed $3 billion.
The severity of sleep apnea is typically measured by:
| · | the number of partial or complete airway blockages that a patient experiences in an hour, referred to as the apnea-hypopnea index, or AHI. For example, moderate OSA patients have an AHI of 15 to 30 events per hour, while severe OSA patients have an AHI of more than 30 events per hour; or |
| 40 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | the average number of respiratory disturbances and related arousals (RERAs) per hour of sleep, referred to as the respiratory disturbance index, or RDI. |
Left untreated, sleep apnea increases the risk of serious chronic conditions, such as high blood pressure, cardiac arrhythmias (such as atrial fibrillation) and other cardiovascular disease, metabolic disease, adult type II diabetes and other life-threatening diseases. In particular, published research shows that, if sleep apnea is untreated (1) the risk of stroke or of death from sudden cardiac arrest doubles; (2) the risk of death from CVD is five times greater; and (3) the risk of recurrence of atrial fibrillation following ablation increases by 42%. In addition, a 2010 study published in Anesthesiology Clinics illustrates the following co-morbidities associated with sleep apnea: drug resistant hypertension (83% of the studied patients with drug resistant hypertension were diagnosed with OSA); congestive heart failure (76%); diabetes type 2 (72%); stroke (63%); pacemakers (59%); arrhythmias (58%); coronary heart disease (57%); and atrial fibrillation (49%).
There are several treatments options for sleep apnea, including (1) CPAP machines that are used with a variety of breathing masks - the mask, worn snugly over the nose or mouth during sleep, uses the CPAP machine to supply pressurized air that flows continuously or intermittently into the throat and the increased air pressure prevents the airway from collapsing; (2) MADs, also known as sleep apnea oral or dental appliances – the device is designed to position the lower jaw slightly forward of its usual rest position, which may be enough to keep the airway open during sleep for patients with mild to moderate OSA; and (3) other treatment options, such as positional pillows, upper airway neurostimulation devices, tongue ablation and even surgery.
Linkage between Sleep Apnea and Cardiovascular Disease
There is increasing awareness among cardiologists and the general population of the importance of sleep apnea in the causation or promotion of hypertension, coronary artery disease, heart failure, atrial arrhythmias, and stroke, and, consequently, as a predictor of premature cardiovascular death.
A 2013 study published in American Journal of Epidemiology estimated that sleep apnea is evident in approximately 25% of adults in the general population, but in certain cardiovascular diseases its prevalence can be approximately 50%. Similarly, according to research published in the Journal of the American College of Cardiology in 2017, sleep apnea is highly prevalent in patients with cardiovascular disease and evidence supports a causal association of sleep apnea with the incidence and morbidity of hypertension, coronary heart disease, arrhythmia, heart failure, and stroke. In many cases, sleep apnea was demonstrated to increase the risk for cardiovascular disease or its recurrence post treatment – such as in atrial fibrillation, high blood pressures and myocardial infarction. The 2017 research also indicates that patients undergoing surgery or other invasive procedures who suffer from sleep apnea are at a greater risk to develop post-operative complications and recurrence of the disease.
Other studies also support the linkage between sleep apnea and cardiovascular disease. For example, a 2017 study published in Circulation: Arrhythmia and Electrophysiology concluded, among other things, that OSA is associated with structural and functional atrial remodeling, and that in the sleep apnea cohort, post pulmonary vein (PV) isolation, additional non-PV triggers elimination improves ablation outcome, compared with the cohort with no sleep apnea where PV isolation only was sufficient. In addition, this 2017 study calls for conducting sleep studies before ablation, which lead us to believe that the presence of sleep apnea may help define the ablation strategy.
| 41 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Current Alternatives for Sleep Apnea Diagnosis and their Limitations
A definitive diagnosis of sleep apnea can be made with either a sleep study conducted during an overnight visit to a sleep laboratory or sleep center, known as polysomnography (PSG) studies or PSG tests, or through a HSAT:
| · | In-lab PSG Tests. PSG tests have been the standard method of diagnosing sleep apnea. They are performed in a sleep lab while the patient is constantly monitored by medical professionals, usually sleep technicians. Patients are hooked up to a web of sensors, electrodes and wires attached to various body parts, as well as chest and abdomen belts and air tubes in the nostrils. PSG tests typically record 12 or more channels of measurements, including brain waves, eye and chin movements that signal the different stages of sleep; heart rate and rhythm; respiration, such as nasal air flow; abdominal and chest belts; and oxygen levels in the blood. |
| · | Home Sleep Apnea Test (HSAT). HSATs are low cost, portable devices that allow patients to be tested in the comfort of their home for diagnosing sleep apnea. They vary in terms of the number of channels or parameters that they measure, the simplicity of the technology to set up and use, and the comfort to the patient. Most HSATs are self-administered with the patient returning the equipment to the physician where the data is then downloaded and interpreted by a board-certified sleep physician. |
We believe that there are several shortcomings to in-lab PSG testing, including:
| · | Convenience. Patients must travel to a sleep lab to conduct the sleep test, spending an overnight away from their home, and, if they are residents of a non-metropolitan area, the patients typically must travel to the closest networked center; |
| · | Cost. The reimbursement rate of PSG by third party payors is higher compared to HSAT devices. We estimate that PSG testing in the U.S. is reimbursed at a range of between $750 and $2,000 compared to HSAT that is reimbursed at a range of between $160 and $240 and, consequently, the deductible to the patient for HSATs is lower; |
| · | Access to Care. Due to a limited number of sleep centers and higher denial rates by medical insurance companies (requiring an HSAT prior to approving an in-lab PSG test), patients often have to wait longer for their scheduled appointment; and |
| · | Patient Discomfort and Quality of Sleep During Test. Patients are less likely to have a typical night’s sleep in a sleep lab, compared to sleeping in their own home. This is primarily because they are in an unfamiliar setting and being watched by strangers, not subject to their regular home environment that may include allergens (such as pollens and animal particles) and are also hooked up to a web of sensors and wires. |
As more fully described below, we believe that HSATs address many of these PSG shortcomings, primarily because HSATs are more convenient for the patient; provide easy access to care; are less expensive; and provide a more typical night of sleep recorded in the comfort of the patient’s home.
The importance of HSATs has been recognized by various medical organizations and associations, including AASM that approved the usage of home sleep testing for the diagnosis of sleep apnea with a portable sleep device in 2009. In addition, in 2008, the Centers for Medicare and Medicaid Services (CMS) approved HSATs as a new technology alternative and, in 2011, AASM issued new CPT codes (“Current Procedural Terminology” (CPT) and “Healthcare Common Procedure Coding” (HCPC)) for HSAT reimbursement. As such, various third party payors have been implementing prior authorization programs which stipulate that reimbursement requests for in-lab PSG testing would be rejected, unless an HSAT was first conducted. These all contribute to increased use of HSATs and we believe that it will become the predominant form of sleep testing in the future, at least in the U.S.
| 42 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Despite the advantages of HSATs over PSG tests, we believe there are several deficiencies in cardio-pulmonary HSAT devices, to which we sometimes refer herein as traditional HSAT devices. These deficiencies include:
| · | Possible Misdiagnosis. Most HSATs use Total Recording Time (TRT) as their denominator to calculate the most critical criteria of the Apnea Hypopnea Index (AHI), the index used by physicians to analyze sleep apnea, compared with PSG tests that use Total Sleep Time (TST). TST is similar to TRT, but deducts the total time that the patient was awake, such as the time it takes the patient to fall asleep, insomniac episodes and trips to the restroom. The use of TRT without manual scoring of the data by a sleep technician has been proven in recent studies to result in a net misdiagnosis of up to 19% of patients; and |
| · | Completion Rates. The rates of completion of traditional HSATs are relatively low in the first night due to technical challenges, such as disconnection of sensors, mainly due to finger oximetry and nasal probes, and patient self-setup errors. For example, according to a 2014 study published by Frost & Sullivan, approximately 80% of HSATs fail in the first night. |
Our Solutions
Our WatchPAT proprietary product, which utilizes the PAT signal, is designed to enable patients to easily conduct sleep tests in the comfort of their home while delivering the treating physicians with accurate and reliable results for diagnosis of sleep apnea. We believe that WatchPAT provides several key advantages over both in-lab PSG testing as well as other HSAT devices by offering the following key benefits: ease of use and patient comfort; accuracy; low cost; and immediate and easy-to-read results (see additional details under “Our Products and Services - The WatchPAT - Key Benefits of WatchPAT” below).
We believe these advantages enable a shift in the “point of care” (the focal point at which the disease is being managed) of sleep apnea from sleep centers to the cardiology care point. In particular, through our WatchPAT related services, including CloudPAT, our cloud-based IT platform, and our TSS program, we offer physicians in the cardiology market what we believe to be an effective solution to manage the entire care pathway for patients suffering from sleep apnea by covering both the screening and diagnosis stage of sleep apnea, using our WatchPAT product, as well as, through the resale of devices of our business partners, treatment thereof (see additional details under “Our Products and Services - WatchPAT Related Services and Accessories” below).
Our Strategy
Our goal is to become a world leader in sleep apnea management solutions for the cardiology market. The key elements of our strategy to achieve our goal include:
| · | Position WatchPAT as the Leading Platform for Cardiologists. We plan to educate, market and make available the WatchPAT device to cardiologists, leveraging on its innovative features and benefits enabled by our PAT-based technology. We believe that the main advantages to cardiologists include ease of use to end-users at home, differentiated clinical value with True Sleep Time (as opposed to Total Recording Time, as explained below) and sleep architecture as well as the ability to accurately autoscore both obstructive and central sleep apnea events, all leading to both scalability and operational efficiency. With our CloudPAT data transfer and SleePath monitoring platforms, we believe we are further positioned to enable cardiologists to integrate sleep apnea management into the cardiology care continuum. |
| 43 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | Focus on a One-Stop Sleep Apnea Solution for the Cardiology Market. We intend to capitalize on the linkage between sleep apnea and cardiovascular disease as well as benefit from the advantages of our WatchPAT products compared to traditional HSAT solutions, by shifting the point of care for sleep apnea from sleep centers to the cardiology care point and by focusing our sales and marketing efforts on cardiologists. We intend to do so by, among other things, promoting our TSS program to the cardiology market, a program which is designed to allow us to offer a comprehensive solution, covering both the screening (performed by the clinics) and diagnosis stage of sleep apnea as well as treatment thereof by access to reselling of therapeutic product lines such as CPAPs and MADs. |
| · | Continue to Commercialize our WatchPAT Solution. We currently maintain direct and indirect sales channels (through distributors) in the United States, Europe, Japan and Asia Pacific. We intend to continue to focus on commercializing our WatchPAT product and related services by expanding our sales and marketing infrastructure, primarily in the United States. |
| · | Expand to New Customers. We plan to expand our sales to new customers by introducing and promoting flexible sales models, such as our Cost per Test (CPT) model to clinical customers. |
| · | Broaden Medical Insurer Coverage. We plan to continue our efforts in obtaining wide insurance reimbursement for our WatchPAT products. |
| · | Expand and Leverage our Strategic Relationships. We believe that a significant market opportunity exists to sell our solutions as complementary to the products and services provided by other organizations with whom we wish to collaborate. To that end, we have already established strategic relationships with various third parties, including leading global partners, where our products are sold as complementary products to their product offering or their products are sold as complementary products to our product offering. We plan to extend our existing strategic relationships and develop new alliances with other partners, in order to increase sales. Doing so will also allow us to leverage the sales and marketing capabilities of our alliance partners and facilitate the wider adoption of our products. |
| · | Promote Awareness to Our Products. We believe that many patients, physicians and cardiologists are still unaware of our TSS program and WatchPAT offerings. We intend to continue to promote awareness of our products through training and educating physicians (including cardiologists), sleep centers, referring physicians, key opinion leaders and various medical societies. We also plan to continue building awareness through our various marketing initiatives. |
| · | Invest in Research and Development. We will continue to make investments in research and development, including investments to enhance our WatchPAT product, and to develop additional applications and indications using our proprietary technologies. |
| 44 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Our Products and Services
The WatchPAT
Overview. Our WatchPAT sleep apnea test line of products, the first generation of which received its initial FDA clearance in 2001, is a watch-like wrist-mounted device with one or two (depending on the model of the WatchPAT product) single-use disposable bio-sensors connected to the patient’s fingers, designed to non-invasively record, measure and analyze digital pulse volume change, or changes in arterial blood volume, primarily in a patient’s finger.
The product is based on our proprietary, clinically validated, technology using the PAT signal, which technology is capable of monitoring the PAT signal and to analyze it for diagnostic purposes. The PAT signal measures changes in the patient’s peripheral arterial pulse volumes as well as various parameters of arterial activity. These arterial activity parameters accurately reflect the patient’s sympathetic nervous system (autonomous (involuntary) nervous system) activity. The WatchPAT continuously records and interprets the autonomic or involuntary nervous system activation during sleep, including that which occurs upon every sleep breathing disorder, as measured through the PAT signal. The PAT probe uses optical sensors to non-invasively measure the changes in arterial blood volume while applying sub-diastolic pressure on the distal two thirds of the finger, including the tip. The pressure fields reduce the arterial wall tension and generate a greater dynamic range of the measured PAT signal and improved sensitivity to changes in the signal amplitude.
With the original models of the WatchPAT, the patient had an additional oximetry sensor attached to another finger measuring blood oxygen saturation. In 2014, we introduced WatchPAT 200 Unified, which allows our proprietary sleep apnea test to be performed using only a single finger to collect both oximetry and PAT data in a unified probe. The WatchPAT 200 Unified is currently our main product offering and, unless otherwise indicated, we refer to it in this registration statement as WatchPAT or WatchPAT200/U.
The following picture depicts the WatchPAT 200 Unified device:
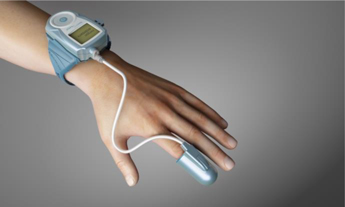
Key Features. The key features of WatchPAT are as follows:
| · | Single-use disposable bio-sensor. WatchPAT employs a single-use disposable finger bio-sensor to measure the PAT signal. The bio-sensor is built of external hard shell and sensitive internal membrane that create blood pooling in the finger circulation as well as sensitive optical sensors to measure accurately changes in blood volumes and oxygen levels. |
| 45 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | Reusable device. Except for the bio-sensor, the WatchPAT device itself is reusable and returned to the physician or clinic after patient use. |
| · | Data processing. The data acquired by the various sensors is automatically processed by our proprietary algorithm and a final report is automatically generated to the physicians through local or cloud-based software. |
| · | Seven channels. WatchPAT is designed to measure seven unique parameters, also known as channels: |
| o | PAT— Peripheral Arterial Tone, which is a physiological signal that mirrors changes in the autonomic nervous system caused by respiratory disturbances during sleep. |
| o | Oximetry— the measurement of oxygen levels in the blood. |
| o | Actigraphy— the measurement of body movement while sleeping. WatchPAT actigraphy is equipped with adaptive algorithms that prevent detection of severe apneic events, such as wakefulness. |
| o | Heart Rate— the number of heart beats per minute while sleeping. |
| o | Body Position— notes whether the patient is asleep on back (supine), front (prone) or side, all of which influence sleep apnea. |
| o | Snoring Intensity— loud snoring is a major indicator of sleep apnea. |
| o | Chest Motion— three axial movement of a point on the chest, just under the sternum notch, during the breathing cycle. |
| · | Rich Data Output. WatchPAT uses the seven channels of patient data and our proprietary algorithms to process and provide the physicians various data outputs for their diagnosis, including: |
| o | Total Sleep Time (TST) — WatchPAT reports both TST and basic Hypnogram (also known as sleep architecture), even though it does not employ the traditional airflow and chest and abdominal effort belts channels nor the electroencephalograph (EEG) and eye movement detectors used in some other HSAT devices. |
| o | Apnea/Hypopnea Index (AHI) and Respiratory Disturbance Index (RDI) — WatchPAT provides information on AHI and RDI, the clinically accepted indices that determine the severity of sleep apnea. |
| o | Central Sleep Apnea (CSA) diagnosis. With our Central Plus module, WatchPAT can also provide quantification of cAHI, which is the portion of events per hour that are identified as CSA and percent of sleep time with Cheyne-Stokes Respiration. |
| o | Oxygen Desaturation Index (ODI) — WatchPAT provides information on ODI, which is the total number of blood oxygen saturation drops per hour of sleep, as well as statistics about the blood oxygen saturation statistics throughout the night. |
| 46 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| o | Sleep Stages and Architecture — WatchPAT provides information on the cyclical pattern of sleep stages, summarized in a chart called a hypnogram, which differentiates between light sleep, deep sleep and REM (rapid eye movement) sleep. REM related sleep disorders are associated with significant higher risk for hypertension. |
| o | Sleep Fragmentation — WatchPAT detects repeated short interruptions of sleep throughout the night. |
| o | Other — WatchPAT provides data on outputs of various other channels, including heart rate, body position and snoring intensity. |
Key Benefits of WatchPAT. We believe that WatchPAT has several key advantages over in-lab PSG testing and competing HSAT products by providing the following key benefits:
| · | Ease of Use and Patient Comfort. Composed of a simple wrist worn watch-like device with one “on” button and one finger probe (in the WatchPAT 200 Unified model), WatchPAT was specifically designed for home sleep apnea testing with minimal patient education. As such, we believe it is easy and intuitive to operate for patients in all ages, including by way of offering a durable and easy to clean device (which supports infection prevention), equipped with validated automated scoring algorithms that reduces time of processing and analyzing the raw data collected to few minutes, compared with manual scoring that we estimate takes on average between 30 to 40 minutes. We estimate that these advantages also translate to a test completion rate of 99%, compared with other HSAT devices that have estimated completion rates of 80%. |
| · | Accuracy. Based upon, among other things, several studies, including a meta-analysis study (see below under “Business Overview - Clinical Results and Studies”), we believe that the WatchPAT offers diagnosis accuracy that is comparable to in-lab PSG. The WatchPAT device has several features that are provided by in-lab PSG but we believe are lacking in most traditional HSATs, including: |
| o | The ability to accurately report TST (Total Sleep Time), not just TRT (Total Recording Time) like in traditional HSATs. The TST detection is important for patients who tend to wake up frequently during the night or suffer from insomnia; and |
| o | The ability to detect sleep stages, with a focus on REM sleep. The diagnosis of REM related sleep patients can be missed because their overall AHI is low, whereas their REM-related AHI is high. According to one study from 2015, if REM related sleep apnea is left untreated, it is associated with up to a 24% increase in risk for hypertension. |
| · | Cost. The total cost of a WatchPAT test is less expensive for third party payors than an overnight sleep center test. We estimate that PSG testing is reimbursed in the U.S. at a range of between $750 and $2,000, compared to WatchPAT that is reimbursed at a range of between $160 and $240 and, consequently, the deductible to the patient for HSATs is lower. We believe this cost advantage to payors and patients will help drive market penetration. |
| · | Provides Immediate and Easy-to-Read Results. Most in-lab PSG and HSATs require a sleep technician to review and interpret the raw data recording and identify areas of poor signals, wakefulness and other technical issues related to nasal cannula motion. The WatchPAT is designed to provide validated automated reports, without the need for the additional step of a sleep technician’s review. |
| 47 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
WatchPAT300. In April 2018, we publicly announced that we made our preliminary submission to the FDA for clearance of WatchPAT300, a new generation of the WatchPAT line of products that is designed to expedite data transfer, allow the use of a lighter and smaller watch and reduce manufacturing costs. The WatchPAT300 also lays the foundation for possible additional future capabilities, such as wireless communication embedded in the device. On August 17, 2018, we obtained the FDA clearance.
WatchPAT Related Services and Accessories
Total Sleep Solution (TSS). Our Total Sleep Solution, or TSS, marketing program aims to provide a complete sleep apnea management solution to cardiology customers, either at the cardiology center or through third party service providers. The key components of our TSS program, which is currently offered only in the United States, include:
| · | Screening – We provide information that helps clinics to implement patients’ systematic screening for high pre-test probability into the cardiology practice patient flow routine by using validated questionnaires, such as STOP-Bang (Snoring, Tired, Observed stop breathing, high blood Pressure, BMI, Age, Neck size and Gender). This initial screening is a required documentation step by most insurance companies to qualify for HSAT reimbursement; |
| · | Diagnostics – Following initial screening, we help the diagnostic stage by offering (1) home sleep testing using our WatchPAT devices; use of our CloudPAT solution, as described below, to transfer the test results to a board-certified sleep physician for interpreting the test results and access to our WatchPAT Direct platform described below for customers who prefer outsourcing the logistics; or (2) for those customers who prefer to prescribe for the test only, access to a network of Independent Diagnostic Testing Facilities (IDTF) for patient diagnostic services using the WatchPAT or other HSAT devices; |
| · | Treatment – Through arrangements between the clinics and Durable Medical Equipment (DME) service providers, patients diagnosed with sleep apnea can be provided with sleep apnea therapy devices, such as CPAP, or, by the clinic referring to dentists specializing in sleep medicine, MADs. Those service providers may use devices that we acquire from third parties and sell to the service providers or use other therapy devices (and, in turn, delivers them to the patient and claims the reimbursement), if the certified sleep physician assigned to interpret the test results prescribes such devices; and |
| · | Reporting – Using our CloudPAT and SleePath solutions, as described below, we facilitate the cardiology customers' receipt of status reports and to otherwise monitor the patients’ sleep apnea management status and compliance, if their DME service providers use devices compatible with our SleePath solutions. |
WatchPAT Direct. WatchPAT Direct is a set of logistical support services that we offer from our service center in Atlanta, Georgia, that include coordination, delivery and collection of WatchPAT devices, based on orders of prescribed sleep tests, from a customer to the patient and back. WatchPAT Direct is currently offered only in the mainland of the United States.
CloudPAT. CloudPAT is a cloud-based information technology (IT) platform, designed to allow customers to transfer the WatchPAT test results primarily to board-certified sleep physicians, IDTF and DMEs. The board-certified sleep physicians receive and interpret the test results, make a diagnosis and potentially prescribe therapy. In the U.S., the signing off on the diagnostic report by a board-certified sleep physician is required by the reimbursement guidelines of AASM and CMS. Recently, CloudPAT was expanded to include also most of the zzzPAT’s analytical tools.
| 48 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
zzzPAT. zzzPAT is an analysis software used in conjunction with our WatchPAT devices. This software stores the recorded raw signals and provides a set of both automated as well as manual scoring and analytical functions for interpretation and reporting purposes used in the diagnosis of sleep apnea.
SleePath. SleePath is an integrated e-health sleep apnea care pathway monitoring module, included as part of our CloudPAT system, that is designed to allow cardiologists to monitor a patient’s sleep apnea management status and compliance with CPAP therapeutic devices on demand. Key features of SleePath include (1) utilizing data from both the CloudPAT and the cloud-based data transmitted and stored by leading CPAP devices manufacturers, including Philips U.S., to provide a “cardio sleep dashboard”, which is designed to allow physicians to track the sleep care pathway status of both the physician practice and the individual patient; and (2) the system monitors and reports CPAP device compliance (the number of days and hours on CPAP and residual sleep apnea), with the data being presented in a user-friendly visual format that is designed to show progress or deviation toward specific treatment goals and changes in metrics over time.
The Endo PAT
The Endo PAT device, the first generation of which received FDA clearance in 2003, was designed to diagnose endothelial function by measuring the ability of blood vessels to dilate as a response to shear stress, or other stimuli, in order to accommodate increased blood flow. The endothelium is the inner lining of all blood vessels regulating their function and ability to dilate or constrict. The Endo PAT device uses our PAT-based technology to measure the ability of blood vessels to dilate after an artificially created ischemic situation. Endothelial dysfunction is a proven independent functional marker for most types of CVD.
In the United States, Endo PAT has no reimbursement and is sold primarily for research purposes.
Clinical Results and Studies
We have invested in and developed a significant body of clinical studies and data that demonstrates the effectiveness and safety of our WatchPAT product by validating it against “gold-standard” PSG tests. The effectiveness and safety of our WatchPAT product has been consistent across both funded and independent clinical studies that have evaluated tests of more than a thousand patients, all of which have been published in peer-reviewed publications.
The following is a summary that highlights key findings from certain of these studies. In our discussion of the results of these studies, we have indicated, where applicable, the relevant p-values which demonstrate the statistical significance, all of which are less than 0.05, which is the commonly accepted threshold for statistical significance. This follows the convention used by the authors of the relevant study as well as what we believe is standard clinical practice.
Diagnosis of Obstructive Sleep Apnea by Peripheral Arterial Tonometry
This meta-analysis study, which was published with the above title in JAMA Otolaryngology—Head & Neck Surgery in December 2013, aimed to assess the correlation between sleep indexes (namely, the respiratory disturbance index (RDI), apnea hypopnea index (AHI), and oxygen desaturation index (ODI), which indexes are described above under “Marketing Overview and Our Solutions”) measured by a PAT-based portable sleep testing device (using our WatchPAT device) and those measured by PSG tests, by conducting a review of multiple studies and articles that, overall, examined 909 patients.
The key results of this study were that (1) studies comparing the RDI between the PAT-based tests and PSG tests had a correlation of 0.879 (P<0.001), where 1.00 would indicate the highest correlation; (2) studies comparing the AHI between the PAT-based tests and PSG tests had a correlation of 0.893 (P<0.001); and (3) studies comparing the ODI between the PAT-based tests and PSG tests had a correlation of 0.942 (P<0.001).
| 49 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Based on the results, we interpret this study to show that PAT-based portable devices, such as our WatchPAT device, present a viable alternative to PSG for confirmation of clinically suspected sleep apnea.
Sleep Staging Based on Autonomic Signals: A Multi-Center Validation Study
This multi-center study, which was published with the above title in the Journal of Clinical Sleep Medicine in June 2011, aimed to assess the WatchPAT-based algorithm for determining wake, light sleep, deep sleep, and REM sleep based on epoch-by-epoch comparisons to PSG tests, by monitoring a total of 237 patients (of which 38 were normal and 189 were diagnosed with OSA) that underwent simultaneous, synchronized overnight recordings with PSG and the WatchPAT. It should be noted that this study, which was authored by, among others, certain of our current or former employees and consultants, was partially sponsored by us.
The key results of this study were that the overall agreement between PSG tests and WatchPAT in (1) detecting light/deep sleep was 88.6% ± 5.9%; (2) detecting REM sleep was 88.7% ± 5.5%; and (3) quantifying sleep efficiency was 78.4% ± 9.9%, compared with 78.8% ± 13.4%, respectively. In addition, according to this study, OSA severity did not affect the sensitivity and specificity of the WatchPAT algorithm.
Based on the results, we interpret this study to show that WatchPAT is capable of detecting sleep stages with moderate agreement to PSG tests in normal subjects and OSA patients and that sleep staging based on actigraphy and signals recorded by the WatchPAT is of reasonable accuracy.
A Novel Adaptive Wrist Actigraphy Algorithm for Sleep-Wake Assessment in Sleep Apnea Patients
This study, which was published with the above title in Sleep in December 2004, aimed to validate an automatic algorithm, developed for actigraphic studies in normal subjects and patients with OSA, by comparing it on an epoch-by-epoch basis to PSG tests, by monitoring a total of 228 subjects from three different sleep centers that underwent simultaneous, synchronized recordings with PSG and the WatchPAT (a model with a built-in actigraph).
The key results of this study were that (1) the overall agreement between PSG and WatchPAT ranged from 86% in normal subjects to 86%, 84%, and 80% in the patients with mild, moderate, and severe OSA, respectively; and (2) while for most subjects differences between PSG and WatchPAT100 actigraphy were relatively small, but for some subjects there was a substantial disagreement.
Based on the results, we interpret this study to show that the WatchPAT actigraphy algorithm provides a reasonably accurate estimation of sleep and wakefulness in normal subjects as well as in OSA patients.
The Effect of the Transition to Home Monitoring for the Diagnosis of OSAs on Test Availability, Waiting time, Patients’ Satisfaction, and Outcome in a Large Health Provider System
This study, which was published with the above title in Sleep Disorders in April 2014, aimed to assess the effects of the transition of one of the leading health insurance providers in Israel from PSG tests to HSATs in terms of accessibility, waiting time, patient satisfaction, costs and CPAP device purchases by patients, by comparing data that was retrieved from the insurance providers database of 650,000 patients between the period of 2007-2008 and 2010-2011 (2009 was excluded during the transition from PSG to HSAT).
| 50 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The key results of this study were that (1) there was a 90% increase of the number of sleep study tests following the transition to HSAT (while the increase in total insured people during same period was less than 5%); (2) despite an increase in the number of tests, the shift to HSAT was accompanied by a decrease of over 20% in overall expense of OSA diagnosis; (3) the average waiting time decreased from 9.9 weeks during 2007-2008 to 1.1 week during 2010-2011 (p<0.05); (4) CPAP device purchases increased by 39%, from 597 devices in 2007-2008 to 831 devices in 2010-2011; (5) there were similar outcomes for both HSAT and PSG tests of compliance to CPAP treatment, daily CPAP usage, improvement in daytime sleepiness and quality of life, and patient satisfaction; and (6) in retrospect, 56% of patients who underwent PSG tests indicated that they preferred HSAT and 72% of patients who underwent HSATs indicated that they preferred HSAT.
Based on the results, we interpret this study to show that a transition from in-lab testing to unattended home sleep testing improved OSA diagnosis test accessibility reduced waiting time and reduced overall OSA diagnosis costs, while maintaining patient satisfaction.
Sales and Marketing
General. Our WatchPAT products and related services are sold and marketed through both direct and indirect channels, including distributors, primarily to hospitals, medical centers (including sleep centers), HMOs, physicians (including sleep specialists), research institutions and cardiology departments. The targeted customers for our WatchPAT technology are primarily cardiologists and electrophysiologists who are interested in integrating sleep medicine into their practice, as well as physicians who specialize in sleep medicine. Sleep specialists represent a variety of medical backgrounds, including pulmonologists (lung specialists), otolaryngologists (ears, nose, and throat), neurologists, primary care physicians and dentists. Our physician customers typically practice in an office setting, clinics, or hospitals. Our Endo PAT products and related services are sold primarily through indirect channels to research institutions and directly to pharmaceutical companies.
We offer our WatchPAT products to customers in two main business models:
| · | Test as a Service (TaaS), also named as Cost per Test (CPT), whereby our customers pay a fixed fee per each home sleep test conducted with our product. The fee per test includes all the components associated with the test, including the disposable bio-sensor, the hardware (the WatchPAT device itself) and access to our CloudPAT platform; and |
| · | Capital purchase, whereby our customers purchase and own the hardware (the WatchPAT device itself), the disposables bio-sensor and other related accessories. We also offer our customers capital purchase through a lease model, whereby the customer leases the product for monthly lease payments, typically over a period of between 18 to 24 months, and becomes the owner of the product at the end of the lease period in consideration for a nominal amount. |
While sleep physicians and traditional sleep business represent the majority of our U.S. customers today, consistent with our strategy, our plan is that cardiologists will represent the majority of our growth and will become an increasingly larger component of our U.S. sleep business over time.
| 51 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In the past three fiscal years, a substantial majority of our revenues were derived from our WatchPAT products and related services (87% in the year ended December 31, 2017, 85% in the year ended December 31, 2016 and 74% in the year ended December 31, 2015). In terms of geographic markets in the past three fiscal years, a substantial majority of our revenues were from sales in the United States (71% in the year ended December 31, 2017, 72% in the year ended December 31, 2016 and 62% in the year ended December 31, 2015). For additional details regarding the breakdown of our revenues in the past three fiscal years by type of products and geographical distribution, see Item 5.A “Operating and Financial Review and Prospects – Operating Results – Results of Operations”.
For the year ended December 31, 2017, (1) Kaiser, one of the largest medical insurers and hospital system in the U.S., accounted for approximately 17.5% of our total revenues (compared with 19.2% in the year ended December 31, 2016 and 17.4% in the year ended December 31, 2015); (2) Philips Japan, a leading global provider of solutions to the sleep and respiratory market, accounted for approximately 12.7% of our total revenues (compared with 11.5% in the year ended December 31, 2016 and less than 10% in the year ended December 31, 2015); and (3) VA, one of the largest U.S. hospital and clinics chains, accounted for approximately 12.1% of our total revenues (compared with 11.4% in the year ended December 31, 2016 and less than 10% in the year ended December 31, 2015). See also Note 16 to our audited consolidated financial statements included elsewhere in this registration statement.
Direct Sales. We continue to develop our sales and marketing organization that consists of a dedicated sales team that is complemented by a marketing team as well as sales and marketing support personnel. Our sales force (including marketing, sales and sales and marketing support personnel) as of December 31, 2017 was comprised of a total of 42 persons, of which 35 persons were located in the United States (in the U.S., we have 17 distinct geographic territories) and 7 persons were located in other locations. See also Item 6.E “Directors, Senior Management and Employees – Employees.”
Indirect Sales and Strategic Collaborations. Over the course of the past several years, we have focused on developing long-term strategic partnerships with distributors and other business partners, including leading global partners such as Medtronic and Philips Respironics:
| · | Co-Marketing Agreement with Medtronic, Inc. - In April 2015, we entered into a co-marketing agreement with Medtronic, Inc., an indirect wholly owned subsidiary of Medtronic plc. Medtronic currently markets and sells its cardiac ablation products for the treatment of cardiac arrhythmias, including atrial fibrillation condition. Under the co-marketing agreement, Medtronic was granted exclusive rights to co-market, with us, our WatchPAT products within our Total Sleep Solution framework to electrophysiologists (physicians who specialize in cardiology arrhythmias) in the United States. See also Item 7.B “Related Party Transactions – Medtronic Co-Marketing Agreement.” |
| · | Distribution Agreement with Philips Respironics GK - In February 2014, we entered into a distribution agreement with Philips Respironics GK, a subsidiary of Koninklijke Philips NV (also known as Royal Philips), or Philips Japan. Under the distribution agreement, Philips Japan was granted exclusive rights to distribute our WatchPAT products and ancillary accessories in Japan. According to this agreement, we may terminate the agreement if Philips Japan does not meet certain minimum purchase requirements of our products. See also Item 5.A “Operating Results - Critical Accounting Policies and Significant Judgments and Estimates - Revenue Recognition” and Item 10.C “Material Agreements – Philips Japan Distribution Agreement” below. |
| 52 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In order to promote our Total Sleep Solution program, we are also developing partnerships with various business partners whose products or services are complimentary to ours. For example, we have entered into agreements with Philips U.S., an affiliate of Philips Japan, under which (1) we were granted non-exclusive rights to distribute Philips U.S. sleep apnea treatment devices, such as CPAP devices, to DMEs that participate in our Total Sleep Solution program to cardiovascular centers in the United States, and (2) Philips U.S. allowed us to use its cloud-based CPAP data as part of our SleePath platform.
While we view our partnerships with Medtronic, Philips Respironics and other business partners as strategic, our direct sales represented more than 75% of our total revenues in each of the past two fiscal years.
Marketing. Our marketing efforts are focused on developing a strong reputation with physicians and hospitals that we have identified as key opinion leaders in cardiology, sleep, and internal medicine based on their knowledge of our technology, clinical expertise and reputation. We do so by various marketing channels, including hosting clinical education programs and symposium and participating in professional conferences to promote our products and increase awareness amongst physicians, primarily cardiologists, to the linkage between sleep apnea and CVD and to the advantages of shifting the point of care for sleep apnea from sleep centers to the cardiology care point.
Third Party Reimbursement
General. In the United States and elsewhere, demand for our products is dependent to a large extent on availability of reimbursement from third-party payors, including governmental payors, such as Medicare and Medicaid, and private payors, such as medical insurance providers. The manner in which reimbursement is sought and obtained varies based upon the type of payor involved and the setting in which the product is furnished and utilized. In general, third-party payors will provide coverage and reimbursement for medically reasonable and necessary procedures and tests that utilize medical devices and may provide separate reimbursement codes and payments for HSATs, such as our WatchPAT device and related professional and technical services. However, our Endo PAT product has not obtained, and we do not expect it will obtain, coverage or reimbursement from third-party payors. In determining payment rates, third-party payors are continuously scrutinizing the costs of medical products and services.
United States. The American Medical Association, or AMA, has developed a coding system known as the Current Procedural Terminology, or CPT, codes, which have been adopted by the Medicare program to describe and develop payment amounts for certain physician services. The Medicare Physician Fee Schedule uses CPT and other codes as part of the determination of allowable payment amounts to physicians. In determining appropriate payment amounts, the Medicare and Medicaid Services, or CMS, receive guidance from the AMA and CPT codes are assigned by either the AMA (for CPT codes) or CMS (for Medicare specific codes).
| 53 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In November 2010, the AMA granted the WatchPAT a CPT code category I - 95800, or CPT 95800, thereby confirming the WatchPAT device as a commercial clinical product for test administration, which encouraged commercial third-party payors, such as Aetna, Signa, United and others, to update their coverage policies to include CPT 95800 and, thereby, the WatchPAT. In March 2017, AASM published guidelines establishing updated clinical practice recommendations for the diagnosis of OSA in adults, pursuant to which devices that measure (1) a minimum of the following sensors: nasal pressure, chest and abdominal respiratory inductance plethysmography, and oximetry, or (2) PAT with oximetry and actigraphy, such as our WatchPAT device, are technically adequate to diagnose OSA, and therefore recommended for OSA diagnosis. These AASM guidelines facilitated a positive coverage decision by AIM Specialty Health, or AIM, a specialty benefit management company who advises many U.S. health insurance plans on coverage policies, and, in November 2017, AIM updated its guidelines to include CPT 95800. This resulted in expanded coverage to multiple BCBS payors across the country.
To our knowledge, currently only a few domestic health insurance plans do not offer coverage of CPT 95800, with Blue Cross Blue Shield of California being the largest one. Nevertheless, in general, most Medicaid payors currently do not cover HSATs, such as our WatchPAT. In addition, while private healthcare insurers often follow reimbursement policies adopted by Medicare, this is not always the case and the reimbursement terms of different private insurers vary. We invest, and plan to continue to invest, resources in efforts to have our WatchPAT device reimbursement code adopted by private healthcare insurers.
International. In other markets outside the U.S., HSAT has been endorsed to different degrees. For example, in Sweden, which is characterized with scattered population, HSATs have been promoted as the only means of diagnosis, and, in Germany, an HSAT is the first-line diagnosis tool and PSG is only allowed if multiple HSAT attempts failed to deliver conclusive diagnosis. On the other hand, in Japan, local authorities have limited HSAT clearance to diagnose OSA for the purpose of prescribing therapy to those patients who are categorized as severe and, to our knowledge, PSG remains the dominant means of sleep apnea diagnosis. In addition, in many foreign markets, including the countries in the European Union, pricing of medical devices is subject to governmental control. To our knowledge, WatchPAT is covered by medical insurance to different degrees in Japan, the UK, The Netherlands, Sweden, Germany, Switzerland, Italy, Israel and few smaller countries.
Outlook. In the United States, there have been, and we expect that there will continue to be, a number of federal and state proposals to limit payments by governmental payors for medical devices, and the procedures in which medical devices are used. For example, in March 2010, comprehensive healthcare reform legislation was enacted through the passage of The Patient Protection and Affordable Care Act, or PPACA, which entails significant initiatives to revise Medicare payment methodologies. The PPACA also includes taxes impacting certain health-related industries, including medical device manufacturers. We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or internationally, or the effect any future legislation or regulation will have on us. Such legislation and regulation of healthcare costs may, however, result in decreased lower reimbursements by governmental and private payors for our products, which may adversely affect our business, financial condition and results of operations.
Seasonality
We have not identified seasonal effects in relation to a specific quarter or quarters in our business. However, in the past several years, the results of our first quarter were typically weaker than other quarters, which may be due to some of our customers capital expenditures cycles, which are not in our control.
| 54 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Competition
Our industry is subject to rapid change from the introduction of new products and technologies and other activities of industry participants. In particular, the sleep tests’ marketplace is highly competitive and has relatively few barriers to entry. We believe that the primary competitive factors affecting sales of our products and related services are:
| · | market acceptance by physicians and key opinion leaders, especially within the cardiology market; |
| · | obtaining required local regulatory approvals or licenses for the sale of our products in the pertinent territories; |
| · | obtaining insurance reimbursement status from the relevant third party payors, especially within the United States; |
| · | company, product and brand recognition; |
| · | product efficacy, safety, reliability and durability; |
| · | product ease of use and patient comfort; and |
| · | technological innovation, product enhancements and speed of innovation. |
We compete primarily with international and local vendors of sleep tests, including in the following main categories:
| · | PSG tests: PSG systems are provided by several companies, including Philips U.S. (part of Philips Medical), Embla, Nihon Kohden, Viasys Healthcare, Puritan Bennett, Cadwell Laboratories, Cleavemed, Stellate Healthcare, Grass Technologies (a subsidiary of Astro-Med Inc.). As more fully described under “Our Products and Services - The WatchPAT - Key Benefits of WatchPAT” above, we believe that HSATs in general, and our WatchPAT products in particular, are competitive in price and features and have certain advantages as compared to PSG tests; |
| · | HSATs (PSG): Suppliers of home sleep testing for diagnostic purposes that offer devices that perform full PSG tests at home, such as Embla and Aura-Grass, which, consequently, typically do not provide a significant cost benefit relative to in-lab PSG tests; |
| · | HSATs: Suppliers of home sleep testing for diagnostic purposes that offer ambulatory systems, such as Embletta, ARES, Philips U.S., Resmed and Nox, which devices typically measure four to five physiological parameters, but lack measurement of sleep stages and TSS and requires nasal cannula. As more fully described under “Our Products and Services - The WatchPAT - Key Benefits of WatchPAT” above, we believe that, while our WatchPAT products may be more expensive than such HSATs, they offer various features and advantages as compared to such HSATs; and |
| · | Pulse oximetry devices: Suppliers of pulse oximetry devices, such as Nonin and Masimo. In contrast to diagnostic devices, pulse oximetry devices that only measure one or two physiological parameters (oxygen saturation and motion) participate in the sleep space mostly as a screening tool. |
| 55 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Many of these competitors and potential competitors have significantly greater financial, human and other resources than we do, and have established relationships with healthcare professionals, customers and third-party payors. In addition, many of our competitors are more established globally and better positioned with sales and distribution networks, greater resources for product development, additional lines of products and the ability to offer financial incentives that we cannot provide. Our products and services could also be rendered obsolete or uneconomical by technological advances developed by one or more of our competitors.
Intellectual Property
General. Our intellectual property and proprietary technology are important to the development, manufacturing, and sale of our current and future pipeline products. We seek to protect our intellectual property, core technologies and other know-how through a combination of patents, copyrights, trademarks, trade secret laws, non-disclosure and confidentiality agreements and other contractual arrangements with our employees, consultants, partners, suppliers, customers and others. We primarily rely on our own research and development efforts to enhance and develop our technology and products although, in some instances that do not involve our core competencies, such as with CloudPAT, we choose to license customized platforms from third parties.
Patents. To date, we had been granted a total of 109 patents and have seven pending national phase applications. The family of patents that specifically covers WatchPAT consists of 56 granted patents worldwide and five pending patent applications, while Endo PAT is covered by 28 granted patents worldwide and one pending patent application. In addition, we have 25 granted patents and one pending patent application, which relate to features which are common to both WatchPAT and Endo PAT.
We submit applications under the Patent Cooperation Treaty, or PCT, an international patent law treaty that provides a unified procedure for filing a single initial patent application to seek patent protection for an invention simultaneously in each of the member states. Although a PCT application cannot be issued as a patent, it allows for the applicant to seek protection in any of the member states through national-phase applications. National phase applications are examined by the allocable authorities in each member state in which we elect to file an application. In addition, during the national phase under the PCT, we can also elect to file an application in Europe, in which case we will not be required to file a separate state specific application for each member state in Europe, until such time as the European application is granted a patent, whereupon state specific validations may subsequently be selected.
The main patents of both our WatchPAT and Endo PAT technology have been issued or are currently pending, in the United States, Japan, Europe and other international markets. Most of our patents and patent applications cover our technology around possible methods of measuring the PAT signal and the application thereof.
Absent patent-term extensions, several key patents and pending patent applications for (1) the WatchPAT are nominally set to expire between 2021 and 2029 in Europe, Japan, and other foreign jurisdictions and between 2022 and 2029 in the U.S. and (2) the Endo PAT are nominally set to expire between 2021 and 2025 in Europe, Japan and other foreign jurisdictions and between 2022 and 2030 in the United States. While the original patent covering the PAT signal and certain embodiments thereof expired in June 2017, we believe that since our products have undergone substantial development and changes since our original products were first introduced, our current products should be protected in the U.S., Japan, and many other countries by newer supplemental patents that we obtained and which are scheduled to expire between 2022 and 2030. However, we cannot be sure that any of our patents will be commercially useful in protecting our technology. Moreover, while our policy is to obtain patents by application, license or otherwise, to maintain trade secrets and to seek to operate without infringing on the intellectual property rights of third parties, technologies related to our business have been rapidly developing in recent years. Additionally, patent applications that we may file or license from third parties may not result in the issuance of patents, and our issued patents and any issued patents that we may receive in the future may be challenged, invalidated or circumvented. If third parties prepare and file patent applications that also claim technology to which we have rights, we may have to partake in proceedings to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable to us. Moreover, because of the extensive time required for clinical development and regulatory review of a product we may develop, it is possible that, before our products can be fully commercialized or commercialized in additional jurisdictions, related patents will have expired or will expire a short period following commercialization, thereby reducing the advantage of such patent. For a more comprehensive discussion of the risks related to our intellectual property, see “Risk Factors – Risks Related to Our Intellectual Property”.
| 56 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Other. In addition to patent protection, we also rely on trade secrets, including unpatented know-how, technology innovation, technical specifications and other proprietary information, as well as trade names, trademarks and service marks and non-disclosure and confidentiality agreements and other contractual arrangements with our employees, consultants, partners, suppliers, customers and others in attempting to develop and maintain our competitive position. We have obtained trademark registrations in the U.S. for, among others, PAT, Endo PAT, WatchPAT, EndoScore, ITAMAR, CloudPAT and SleePath and some of them are also registered in additional jurisdictions, including Europe, Japan, Canada, China, India, Russia, Mexico, Korea and Singapore.
However, our trade secrets may become known or be independently discovered by competitors, our confidentiality agreements may be breached, and our tradenames may not achieve the brand recognition that we pursue. For a more comprehensive discussion of the risks related to our intellectual property, see “Risk Factors – Risks Related to Our Intellectual Property”.
Research and Development
We devote a significant amount of our resources towards research and development in order to introduce new products and continuously enhance existing products and to support our growth strategy. We have assembled a core team of experienced research and development professionals as well as an advisory board comprised of experts in their respective fields. These professionals are involved in advancing our core technologies, as well as in applying these core technologies to our product development activities.
In order to carry our research and development activities, which take place primarily in Israel, we maintain teams in the following areas: physiology, hardware development, software, algorithms, data processing, clinical application development and clinical trials. As of December 31, 2017, we had 14 full-time employees engaged in research and development.
Since our incorporation, we have been engaged in the research of the PAT signal, as well as in the continuous research and development of our PAT-based technology and additional products and applications based on this technology, including in conjunction with additional technologies.
As part of our research and development activities, we also initiate or monitor, from time to time, clinical and research collaborations, such as with academic centers, in order to, among other things, achieve scientific backing of our products; promote recognition of our products within the medical community; support, where necessary, regulatory authorizations required to market and sell our products in applicable territories, and to examine the applicability of our products in various clinical markets, particular population groups and various comorbidities. During 2017, we conducted clinical trials in connection with our WatchPAT and Endo PAT products, the main purpose of which was to develop new indications for such products and obtain results comparing the use to such products to other products, in order to support applications for authorizations to market and sell our products in additional territories.
| 57 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
See also Item 5.C “Research and Development, Patents and Licenses.”
Manufacturing and Supply
Our products consist of off-the-shelf and custom made components. Our manufacturing, quality assurance testing, final integration, packaging and shipping operations as well as our final assembly activities are primarily performed at our facility in Caesarea, Israel, where we employed, as of December 31, 2017, 59 full-time employees.
We engage various suppliers and subcontractors who deliver us materials and components used in our products, including plastic and electronic components, as well as development services and database management services. We also engage subcontractors, on an as needed basis, to manufacture finished products, based on our product specifications and requirements. We are not bound by any minimum purchase volume undertakings with such subcontractor. Engaging subcontractors to manufacture our finished products on an as needed basis, in addition to our own manufacturing work force, allows us flexibility to manage and meet our manufacturing goals and we believe that our manufacturing capacity, comprised of our own manufacturing work force and of our suppliers and subcontractors, is suitable and adequate for our operations as currently conducted and as currently foreseen.
We typically engage our subcontractors by means of a renewable frame work agreement or by a particular purchase order. We aim to engage different subcontractors in various locations, to reduce any potential dependency in any particular subcontractor. In addition, we believe that there are sufficient alternative subcontractors in the market, which would allow us to replace any subcontractor, if necessary, though such replacement may be a lengthy process. In addition, if needed, we may transfer some to the final assembly stages, to our own manufacturing facility.
As of the date of this registration statement, several of our subcontractors are single source subcontractors. Depending on the type of such subcontractors and the alternative we choose (such as using alternative subcontractors and manufacturing the component ourselves), we estimate that replacement of such single source subcontractors may range between six and twelve months. In addition, while we were not able to identify an alternative supplier for a component incorporated in one of our older models of the WatchPAT, which model we only sell in China, we plan to obtain regulatory approval to sell our more advanced WatchPAT product model in China. Nevertheless, there is no assurance if and when we will obtain such approval. Due to their nature, certain components must be ordered from such single source subcontractors a few months in advance, resulting in substantial lead time. In the event that such limited source suppliers are unable to meet our requirements in a timely manner, we may experience a limited interruption in production until we can obtain an alternate source of supply. See “Risk Factors–Risks Related to Our Business– We are dependent upon third-party manufacturers and suppliers, which make us vulnerable to supply disruptions” and “Risk Factors–Risks Related to Our Business– Long lead times required by certain suppliers could prevent us from meeting the demand for our products. If we do not accurately forecast such demand, our operating results could be adversely affected. However, as explained above, we take various steps in order to mitigate this risk, including by (1) providing our relevant suppliers with a purchasing forecast and estimate of future orders, (2) requiring such single source subcontractors to provide us long prior notice should the subcontractor wish to terminate our agreement, and (3) we constantly hold safety inventory stock sufficient to meet our estimated manufacturing forecasts, aligned with the lead time of each component.
| 58 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We believe that our manufacturing processes and our subcontractors’ manufacturing processes are in compliance with pertinent U.S. and international quality and safety standards, such as ISO 9001:2000 and the FDA’s quality system regulations.
Government Regulations
Overview
We must comply with the laws, regulations and standards applicable to our activities in the countries in which we operate. In particular, we are subject to laws, regulations and standards applicable to our manufacturing activities as well as to laws and regulatory requirements in each country in which we seek to market and sell our products, including the United States, Europe, Japan, Canada and Israel. In each country where we seek to market and sell our products, we typically need to first obtain a local approval or clearance allowing us to market and sell our products in the pertinent territory. The requirements, length of time and costs associated with obtaining such local approvals differ from country to country. Depending on the pertinent territory, we either hold such approvals independently or through a local subsidiary or through a local partner with whom we maintain a contractual arrangement securing our rights in such marketing and sales approvals, such as in Japan where our partner, Philips Japan, is the one holding the marketing approval. Except as described below, the WatchPAT related services and accessories (see under the section titled “Our Products and Services - WatchPAT Related Services and Accessories”) that we currently offer, do not require any separate sale or marketing regulatory approval or clearance beyond the ones we obtained for (or included in) the WatchPAT as described below.
We are also subject to announced and unannounced audits by such regulatory bodies, primarily of our manufacturing facility in Israel. Our products and operations are also often subject to the rules or norms of industrial standards bodies, such as the International Standards Organization (ISO) or the rules of associations of healthcare professionals. For example, in the U.S. we maintain certifications of a Nationally Recognized Testing Laboratory, or NRTL, which is a third-party organization that certifies products for the North American market. NRTLs are recognized by the Occupational Safety and Health Administration (OSHA) under U.S. deferral regulations to provide product safety testing and certification for products to be used in the U.S. workplace. Future products, or components thereof, may also be subject to regulation by the Federal Communications Commission, or the FCC, formed by the Communications Act of 1934 due to inclusion of digital or communication components.
In addition, we are subject to medical device reporting regulations under the FDA regulations as well as under applicable foreign regulations in the countries in which we market and sell our products. While the specifics of the reporting regulations in each country in which we market and sell our products may vary, we are generally required to report adverse events or incidents about which we received or become aware of information that reasonably suggests that one of our marketed devices or a malfunction of such device has caused or may have caused or contributed to a death or serious injury, or of a recurring malfunction likely to contribute to death or a serious injury. The decision of whether an adverse event or incident is reportable under the applicable regulations requires our management’s judgment. Any adverse event or incident involving our products could result in regulatory actions, such as inspection and mandatory product recalls, as well as voluntary corrective actions that we may initiate for various reasons, such as product recalls or customer notifications.
In Israel, the U.S., Europe and other territories we are also subject to environmental regulations governing the use of certain hazardous materials, such as RoHS and RoHS II, EU directives that require products sold in Europe to meet certain design specifications, which exclude the use of hazardous substances; REACH, an EU regulation covering the registration, evaluation, authorizations and restriction of chemicals; and EU Directive 2002/96/EC on Waste Electrical and Electronic Equipment (known as the “WEEE” Directive), which requires producers of electrical and electronic equipment to register in different European countries and to provide collection and recycling facilities for used products.
We invest resources in order to maintain our regulatory compliance, successfully pass audits and maintain our certifications and marketing and sales approvals.
The United States Market
The development, manufacturing, marketing and sales and post sales of medical devices, including our products, is subject to the regulation of the U.S. Food and Drug Administration, or the FDA, pursuant to the Federal Food, Drug and Cosmetic Act of 1938, as amended, or the FDCA, and regulations promulgated thereunder. The FDA requirements include, among others, manufacturing quality control requirements in accordance with current good manufacturing practices (cGMP) regulation, compilation of scientific reports with respect to our products, appointment of the U.S. agent, and adhering to auditing and supervision by the FDA of our manufacturing facility. We are required to accommodate our manufacturing facility to the FDA requirements, such as the requirements of the Registrars Quality Systems, or QRS, which may be reviewed by the FDA periodically. We and the third-party manufacturers on which we rely for the manufacture of our products and their respective components are subject to requirements that our products be manufactured, packaged and labeled in conformity with cGMP. To comply with cGMP requirements, manufacturers must continue to spend time, money and effort to meet requirements relating to personnel, facilities, equipment, production and process, labeling and packaging, quality control, recordkeeping and other requirements. We are also required to comply with regulatory requirements which may be set forth by particular states.
| 59 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Generally, unless an exemption applies, each medical device that we propose to market in the U.S. must first receive one of the following types of FDA authorizations:
| · | premarket notification under Section 510(k) of the FDCA, to which we refer as a 510(k) clearance; or |
| · | premarket approval under Section 515 of the FDCA, or PMA. The applicant may also submit a De novo application, in which case the regulator shall determine whether the device shall be classified from class III to class II or class I, with new classification or regulation. |
Under the FDCA, medical devices are classified into three classes - Class I, Class II or Class III, depending on the level of risk associated with the medical devices. While the requirements in respect of Class I devices are less burdensome and such devices are generally exempt from the submission of an application for authorization (although are still required to comply with FDA controls), Class II devices require submission to the FDA of a 510(k) clearance, requesting permission to commercially distribute the device, whereas Class III devices, which pose the greatest risks to patients, require submission of a PMA.
To date, all our devices (excluding our CloudPAT, which is exempt and listed as a medical device data system) were classified as Class II devices. A 510(k) clearance, submitted in connection with our Class II medical devices, must demonstrate that our device is “substantially equivalent” to another commercially available device that was cleared under the 510(k) process or previous legislation. Class II devices such as our devices are subject to the FDAs General Controls as well as to special controls determined by the FDA, to ensure the safety and effectiveness of the device. Such special controls may include performance standards, post-market surveillance, patient registries and FDA guidance documents.
We currently have FDA clearance, through the 510(k) clearance path, for our WatchPAT200/U, WatchPAT300 and Endo PAT 2000. Our CloudPAT is listed separately as a medical device data system that is exempt from such FDA clearance.
The European Market
In the European market, our devices are regulated by the European Union National Competent Authorities, or the NCAs, of the Member States of the European Economic Area, or the EEA. Generally, in order to market and sell our devices in the EEA, we must submit an application to a Notified Body, an entity that has been accredited by an EU member state to assess whether medical devices (or other products) conform to predefined standards. In the case of medical devices, the Notified Body assess whether medical devices, such as ours, confirm with the applicable E.U. Medical Devices Directive, which defines the standards for medical devices. Such conformity assessment may include inspection and examination of a product, its design, and the manufacturing environment and processes associated with it. With this Declaration of Conformity, the manufacturer can label the medical device with the CE Mark, which is required for distribution and sale in the EU. Additionally, the EU member state accrediting the Notified Body will then inform the European Commission that the product complies with set standards. The CE marking is a certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the EEA. Obtaining a CE mark allows us to market and sell our products in the European Union as well as in the EFTA states (Iceland, Lichtenstein, Switzerland and Norway) and allows the enforcements agencies in such states not to approve the marketing and sale of similar products which do not bear the CE mark. While the CE mark allows us to market and sell our devices in most EEA states, certain states, such as Italy and Spain, also set forth their own local specific requirements, which differ from state to state, with which we must comply in addition to the CE mark requirements.
| 60 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Pursuant to the European conformity directive regarding medical devices (Medical Devices Directive 93/42/EEC, as amended by amended by Directive 2007/47/EC), or the EEC directive, as manufactures of medical devices, we are also required to comply with the European Conformity requirements and are also subject to auditing by Notified Bodies once a year, and to an unannounced audit once every three years. The new European Medical Devices Regulation, or MDR, which was published in May 2017 with a transition period of three years, replaces the Medical Devices Directive (93/42/EEC). Starting May 2020, the new MDR will apply and no new applications under the previous directives will be permitted. During the said three-year transition period, we will need to update our technical documentation and other quality management system processes to meet the new MDR requirements.
During 2017, we decided to transition to a new Notified Body. As part of the transition, a quality audit was performed and the technical dossier of one product was reviewed. In January 2018, we received a new CE certificate from our new Notified Body, BSI Group, bearing an expiration date of October 10, 2019, the same expiration date as the previous certificate issued by our former Notified Body, for our WatchPAT200/U (including one of its probes) and Endo PAT 2000, to which we refer as the main certificate. In addition, we have obtained a CE certificate for our accessories, such as probes and sensors that we sell for use with such products, to which we refer as the accessory certificate, which certificate does not bear any expiration date (and is not required to be issued by a Notified Body like the main certificate). It should be noted that under the MDR requirements, CE certificates issued under the previous directives prior to May 2020 shall remain valid in accordance with their term, beyond the expiration of the transition period, however certain limitations set forth in the MDR, such as the need to use classifications that are different from the previous directives, would apply. We do not expect such limitations to have any material impact on our ability to maintain our accessory certificate (or obtain a new one if such new classifications shall apply) beyond May 2020.
We currently have a CE mark for our WatchPAT200/U and Endo PAT 2000.
The Japanese Market
In Japan, the Pharmaceutical Medical Devices Authority, or PMDA, is the regulatory body supervising and regulating the marketing and sale of medical devices such as our products, similarly to the FDA. In order to market and sell medical devices, such as ours, in Japan, we must comply with Japans Pharmaceuticals and Medical Devices Act, or the PMD Act. Among other requirements, as part of the approval process, medical device manufacturers must comply with the MHLW Ordinance No. 169 related to quality management systems, register design and manufacturing facilities, and appoint an in-country representative, also known as MAH/D-MAH.
We currently hold PMDA authorizations to market and sell our WatchPAT200/U and Endo PAT 2000 in Japan. Such authorizations are held by the local MAH/D-MAH with whom we maintain a contractual engagement.
The Canadian Market
Health Canada is the Canadian authority supervising and regulating the marketing and sale of medical devices such as our products, similarly to the U.S. FDA.
We currently have authorizations of Health Canada to market and sell our WatchPAT200/U and Endo PAT 2000. However, in August 2018 we announced that we will not renew such authorizations and therefore will not offer our products for sale in Canada starting January 2019.
| 61 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The Israeli Market
General. All of our products are approved for sale and distribution in Israel by the Israeli Ministry of Health. Our manufacturing activities in Israel are also subject to regulation by the Israeli Ministry of Health. In addition to marketing and selling our products, we or our partners also must obtain pertinent approvals or permits to perform our clinical trials in the countries in which we perform such trials, such as in compliance with an international guideline for the ethical conduct of clinical research known as the Declaration of Helsinki. In Israel, our clinical trials require a permit for a research plan (protocol) by the Helsinki Committee, operating under the Israeli People’s Health Regulations (Human Subject Research), 1980.
Israeli Innovation Authority. From time to time, eligible participants may receive grants under programs of the IIA. Grants received are generally repaid through a mandatory royalty based on revenues from the sale of products (and ancillary services) incorporating know-how developed, in whole or in part, with the grants. This governmental support is conditioned upon the participant’s ability to comply with certain applicable requirements and conditions specified in the IIA’s programs and the R&D Law.
Under the R&D Law, research and development programs that meet specified criteria and are approved by the Research Committee of the IIA are eligible for grants of, usually, up to 66% of certain approved expenditures of such programs, as determined by said committee. In exchange, the recipient of such grants is required to pay the IIA royalties from the revenues derived from products incorporating know-how developed within the framework of each such program or derived therefrom (including ancillary services in connection therewith), up to an aggregate of 100% of the dollar-linked value of the total grants received in respect of such program, plus interest.
The R&D Law also provides that know-how developed under an approved research and development program or rights associated with such know-how (1) may not be transferred to third parties in Israel without the approval of the IIA (such approval is not required for the sale or export of any products resulting from such research or development); and (2) may not be transferred to any third parties outside Israel, except in certain special circumstances and subject to the IIA’s prior approval, which approval, if any, may generally be obtained, in the following cases: (a) the grant recipient pays to the IIA a portion of the sale price paid in consideration for such IIA-funded know-how (according to certain formulas, which may result in repayment of up to 600% of the grant amounts plus interest), or (b) the grant recipient receives know-how from a third party in exchange for its IIA-funded know-how. Such approval is not required for the export of any products resulting from such research or development.
The R&D Law imposes reporting requirements with respect to certain changes in the ownership of a grant recipient. The law requires the grant recipient and its controlling shareholders and foreign interested parties to notify the IIA of any change in control of the recipient or a change in the holdings of the means of control of the recipient and requires a non-Israel interested party to undertake to the IIA to comply with the R&D Law. In addition, the rules of the IIA may require additional information or representations in respect of certain of such events. For this purpose, “control” is defined as the ability to direct the activities of a company other than any ability arising solely from serving as an officer or director of the company. A person is presumed to have control if such person holds 50% or more of the means of control of a company. “Means of control” refers to voting rights or the right to appoint directors or the chief executive officer. An “interested party” of a company includes a holder of 5% or more of its outstanding share capital or voting rights, its chief executive officer and directors, someone who has the right to appoint its chief executive officer or at least one director, and a company with respect to which any of the foregoing interested parties owns 25% or more of the outstanding share capital or voting rights or has the right to appoint 25% or more of the directors. Accordingly, any non-Israeli who acquires 5% or more of our ordinary shares will be required to notify us that it has become an interested party and to sign an undertaking to comply with the R&D Law.
| 62 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The Israeli authorities have indicated in the past that the government may further reduce or abolish the IIA grants in the future. Even if these grants are maintained, we cannot presently predict what would be the amounts of future grants, if any, that we might receive. In addition, an amendment to the R&D Law that became effective on January 1, 2016, provides the IIA with authority to establish new guidelines regarding the R&D Law, which may affect our existing and/or future IIA programs and incentives for which we may be eligible. We cannot predict what changes, if any, the IIA may make.
Our research and development efforts for the development of Endo PAT 3000, a new generation of our Endo PAT product, during the period between 2003 and 2005, were financed in part through royalty-bearing grants from the IIA, in a total amount of approximately NIS 3.8 million (equivalent to $1.03 million). Since we have ceased our development efforts of Endo PAT 3000 and do not intend to use the know-how developed with the support of these grants in any of our other products in the near future, we believe that the terms of these IIA royalty-bearing grants mean that we are not required to repay these grants to the IIA. However, in 2009 the IIA informed us that we must pay royalties on the sale of all of our products since 2012 and, since then, we have been in discussions with the IIA in an attempt to resolve this disagreement. While we disagree with the IIA demand, there is no assurance that we will necessarily prevail in opposing this demand. Since we made a full accrual in our financial statements for such possible liability, even if we do not prevail, the primary effect will be on our cash flows and financial condition.
| C. | Organizational Structure |
Our wholly owned subsidiaries act primarily as sales, marketing and customer service organizations in the countries where they are incorporated and in most instances for neighboring countries. The following table sets forth the legal name, location and country of incorporation and percentage ownership of each of our current principal operating subsidiaries*:
| Subsidiary Name | Country of Incorporation | Ownership Percentage | ||||
| Itamar Medical, Inc. | Delaware, United States | 100 | % | |||
| Itamar Medical Japan Co. Ltd.* | Japan | 100 | % | |||
| I.M.E. 2016 B.V. | The Netherlands | 100 | % | |||
* Currently in the process of dissolution.
| D. | Property, Plants and Equipment |
General. Other than the leased properties described below, we do not own or lease any material tangible fixed assets. We believe that these offices and facilities are suitable and adequate for our operations as currently conducted and as currently foreseen. In the event additional or substitute offices and facilities are required, we believe that we could obtain such offices and facilities at commercially reasonable rates.
Israel. Our headquarters, manufacturing and research and development facilities as well as sales offices are located in the Northern Caesarea Business Park, Caesarea, Israel, where we lease approximately 14,000 square feet of office space pursuant to a lease that is currently scheduled to expire in January 2019. The aggregate annual rent for our Israeli office facility was approximately $309,000 in 2017, compared with $296,000 in 2016. We provided our Israeli office lessor with a bank guarantee in the amount of approximately $160,000 to secure our obligations under the lease.
| 63 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In addition to the above, we lease storage facilities in the Northern Caesarea Business Park, Caesarea, Israel, where we lease approximately 1,900 square feet of storage space pursuant to a lease that expires in December 2018 with an option for us to extend the term of the lease until June 2021. The aggregate annual rent for our Israeli storage facility was approximately $28,500 in 2017, compared with $15,400 in 2016. We provided our Israeli storage space lessor with a bank guarantee in the amount of approximately $15,000 to secure our obligations under the lease.
Other Locations. We lease approximately 10,900 square feet of office space in Atlanta, Georgia, pursuant to a lease that expires in March 2022, with an option for us to extend the term of the lease until March 2025. The aggregate annual rent of such facility was approximately $186,000 in 2017, compared with $31,000 in 2016 (the lease commenced in November 2016). We provided our U.S. office lessor with a bank deposit in the amount of approximately $18,000 and a bank guarantee in the amount of approximately $107,000 (which will be reduced by approximately $27,000 each year) to secure our obligations under the lease.
| ITEM 4A. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| ITEM 5. | OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and the related notes included elsewhere in this registration statement. The discussion below contains forward-looking statements that are based upon our current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from these expectations due to inaccurate assumptions and known or unknown risks and uncertainties, including those identified under “Cautionary Note Regarding Forward-Looking Statements” and under “Risk Factors” elsewhere in this registration statement.
| A. | Operating Results |
Overview
Introduction
We are a medical technology company that designs, develops, manufactures and sells sleep apnea diagnostic ambulatory products and related services.
We believe a key competitive differentiator for us is the use of the PAT biological signal along with other measurements, such as actigraphy, heartrate, chest motion, body position and snoring. All of these inputs are analyzed by our proprietary technology and algorithms.
Our PAT-based technology is implemented in a simple to use non-invasive watch-like wrist worn device called WatchPAT that uses a finger mount bio-sensor to measure and record the PAT signal, which is then transferred to either a local (zzzPAT) or cloud-based (CloudPAT) software for analysis and reporting of sleep apnea diagnosis. The results of our proprietary analysis are automatically populated into an easy to read report that allows physicians to make accurate diagnosis of sleep apnea.
| 64 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Our Total Sleep Solution (TSS) is a comprehensive marketing program we offer to physicians that combines products and services, including our proprietary diagnostic test and data analytics as well as access to resale of third party sleep apnea treatment devices and a network of IDTFs and DMEs. TSS is designed to allow any medical practice or physician that is not a sleep physician by specialty, easy access to a comprehensive suite of products and services for the diagnosis, treatment and management of patients they suspect suffer from sleep apnea. We believe the combination of our proprietary test combined with the ease of single point of contact management of the diagnosis and treatment of sleep apnea provided by TSS has been a driver of the increased usage of our tests. Specific products and services included in the TSS program include CloudPAT and SleePath for cloud-based data and information mobilization solutions, access to the resale of sleep apnea therapeutic products such as CPAP devices, PAMS and MADs, related services and logistical solutions such as WatchPAT Direct.
Since 2015, we have focused on offering TSS to the cardiology market through various business models, however the Test as a Service (TaaS), also known CPT model, is the primary model we utilized to date. In the TaaS model, the medical practice or physician ordering the TaaS pays a fixed fee per HSAT that includes all the components associated with the test, including the disposable biosensor, hardware rental fees and access to our CloudPAT platform.
Since our incorporation in 1997, we have incurred operating and net losses in most of our years of operation and, as of December 31, 2017, we had an accumulated deficit of $105 million. We expect to continue to incur operating and net losses for the foreseeable future, as we continue to invest in research and development and marketing and sales operations aimed at growing our business.
In the years ended December 31, 2017, 2016 and 2015, we have generated revenues of $20.7 million, $18.4 million, and $16.8 million, respectively. We have grown our WatchPAT related product revenue from approximately $12.4 million for the year ended December 31, 2015 to $18.1 million for the year ended December 31, 2017, reflecting a growth rate each year of at least 15%.
For recent business events and key milestones, see under Item 4.A “Information on the Company - History and Development of the Company - Recent Major Business Developments.”
Trend Information and Outlook
We identified the following significant trends and uncertainties that we believe will continue to materially influence our market, financial condition and the demand for our products:
| · | We expect to continue to generate revenues mainly from the sales of our WatchPAT product in the United States, Japan Europe and China, which is consistent with our strategy to expand our sales of the WatchPAT in general and in those markets in particular. The level of our future revenues, however, is hard to predict and depends on many factors which are outside of our control. |
| · | We expect that the sales of our Endo PAT product will remain at the same level or even continue to decrease primarily due to (i) our strategic decision to reduce our sales and marketing efforts for such product, (ii) the reduction of research funds available to research institutions, which represent the vast majority of customers who purchase this product from us, and (iii) the ongoing difficulties associated with obtaining coverage or reimbursement from third party payors for the use of such product for clinical use in the U.S. |
| 65 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | We market our products, directly or through our sales channels, primarily to health facilities, physicians and research institutions, many of whom rely on coverage or reimbursement for the healthcare services they receive or provide to their patients, from third-party payors, such as private insurance plans offered by medical insurance companies. Currently, many medical insurers cover or allow only partial reimbursement of expenses associated with medical tests that use our products. However, we believe that the changes in the guidelines issued by AIM and AASM in the past two years (see under Item 4.B “Information on the Company - Business Overview –Third-Party Reimbursement”) may lead to the inclusion by more medical insurers of the WatchPAT test in the basket of medical examinations and procedures covered by additional states in the U.S. |
| · | We estimate that the costs for our selling and marketing expenses will increase in future years, as we continue to build our business, including by expanding our footprint and the territories in which we operate. |
| · | We also estimate that the costs for developing our products will increase in future years, as we execute our plan to develop new products and services, including new applications that are based on our PAT-based technology, to accelerate adoption by cardiologists. |
| · | We estimate that our general and administrative expenses will slightly increase, primarily due to the continued expansion of our management team as well as compliance costs associated with becoming subject to reporting and other requirements under applicable U.S. securities laws and Nasdaq rules. |
In 2018, we intend to continue to invest in selling and marketing, in developing new products and services and to otherwise implement our strategy (see under Item 4.B “Information on the Company - Business Overview – Our Strategy” above). We believe that this strategy will enable us to support continued sales growth and enhance market acceptance for our offerings. However, we expect to continue to incur operating losses in the near future as we increase our sales and marketing activities associated with implementing our strategy, mainly in the United States, Japan, Europe and China, and otherwise continue to invest capital in the development and expansion of our products and our business generally, including commitment of substantial resources toward reimbursements and clinical studies.
Our ability to continue our growth and achieve profitability depends, in part, on the global economy and the growth rates and changes in trends in industries in which we operate, including the availability of reimbursement for the use of our products by medical insurance companies as described elsewhere in this registration statement, as well as the level of market acceptance of our products and services. As such, our results may be adversely affected if, among other things, there is an economic slowdown or a failure of our products to achieve market recognition or demand.
While we believe that some of the trends and plans described above will present significant opportunities for us, they also pose significant challenges, uncertainties and risks, including those described under Item 3.D “Risk Factors” above.
For additional details regarding our capital resources and contractual obligations, see Item 5.B “Operating and Financial Review and Prospects– Liquidity and Capital Resources” and Item 5.F “Operating and Financial Review and Prospects– Tabular Disclosure of Contractual Obligations.”
| 66 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Financial Overview
Revenues. Our revenues consist primarily of sales of our WatchPAT product and, to a lesser extent, our Endo PAT product and related services to hospitals, clinics and physicians practices, including health management organizations, or HMOs, directly as well as through distribution channels. These products are offered mainly as a combination of TaaS or CPT (as part of our TSS program in the cardiology field in the U.S.), capital equipment (which can be used for several years) and one-time disposable probes. For additional details regarding the manner in which we recognize revenues, see the discussion under the caption “Critical Accounting Policies and Significant Judgments and Estimates - Revenue Recognition” below.
Cost of Revenues. Our cost of revenues consists of costs of raw materials and subcontractors, as well as labor, utility and maintenance costs associated with the operation of our manufacturing facility, depreciation and shipping and handling.
Operating Expenses. Our current operating expenses consist of three components:
| · | Selling and Marketing. Our selling and marketing expenses consist primarily of salaries, including share-based compensation and related personnel expenses to selling, marketing and business development personnel, sales commission and related personnel expenses, sales offices maintenance and administrative costs conferences and trade shows, advertising and marketing, cost of third party consultants (including in respect of our efforts to increase the number of insurers entitled to reimbursement for use of our products) and travel expenses. |
| · | Research and Development. Our research and development expenses consist primarily of salaries, including share-based compensation and related personnel expenses, cost of third party consultants, advisory board, raw materials, costs related to conducting clinical studies, patent costs, regulatory costs and travel expenses. Some development costs that relate to development of products or processes that are technically and commercially feasible and for which we have sufficient resources to complete development and intent to use or sell them are capitalized and subsequently amortized. |
| · | General and Administrative. General and administrative expenses consist primarily of salaries, including share-based compensation and related personnel expenses, professional service fees for accounting, legal, bookkeeping, directors’ fees and associate costs, and doubtful debts. |
Financial Expenses and Income. Financial expenses and income consist primarily of changes in the fair value of warrants and embedded warrants of our convertible notes that were fully repaid in February 2018, interest expenses and exchange rate differences on such convertible notes and other loans, interest income and exchange rate differences on bank deposits and marketable securities, change in the fair value of marketable securities and foreign currencies gains or losses. The warrants, including the embedded warrants in our convertible notes, are measured on each reporting date and the results from the changes in their fair value which is being impacted, among other things, by the changes in our share price are included in financial expenses or income, net. Typically, when share price increases, the fair value of the embedded warrants increases, which results in higher financial expenses, and when share price decreases, the fair value of the embedded warrants decreases, which results in lower financial expenses or financial income. For additional details regarding the manner in which we record financial expenses and income, see the discussion under the caption “Critical Accounting Policies and Significant Judgments and Estimates - Investments in Debt Securities, Derivatives and Non-Derivative Financial Liabilities” below.
| 67 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Taxes on Income. We are subject to income taxes in Israel, the United States, Japan and the Netherlands. For additional details regarding our income taxes, see Note 13 to our audited consolidated financial statements included elsewhere in this registration statement, and the discussion in “Item 10E – Taxation” below.
Critical Accounting Policies and Significant Judgments and Estimates
The preparation of financial statements in conformity with IFRS requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent liabilities at the date of the financial statements and the reported amounts of assets, liabilities, revenues and expenses during the reporting period.
The accounting estimates used in the preparation of our financial statements require management to make assumptions regarding circumstances and events that involve considerable uncertainty. Management prepares the estimates based on past experience, various facts, external circumstances, and reasonable assumptions according to the pertinent circumstances of each estimate. Actual results may differ from these estimates under different assumptions or conditions. Estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimates are revised and in any affected future periods.
While our significant accounting policies are more fully described in Note 2 to our audited consolidated financial statements included elsewhere in this registration statement, we believe the following accounting policies are most critical to understanding and evaluating our reported financial results.
Revenue Recognition. Revenue is measured at the fair value of the consideration received or receivable, net of returns and discounts. We recognize revenue from the sale of our products, net of provision for returns, when persuasive evidence exists (usually in the form of an executed sales agreement) that the significant risks and rewards of ownership of the products have been transferred to the buyer, recovery of the consideration is probable, the associated costs and possible return of products can be estimated reliably, there is no continuing management involvement with the products, and the amount of revenue can be measured reliably. Revenue is recognized provided there are no material remaining performance obligations required of us or matters requiring customer acceptance. The timing of the transfer of risks and rewards may be upon shipment or upon delivery to the customer site, based on the contract terms or legal requirements.
We recognize estimated sales discounts as a reduction of sales in the same period at which revenue is recognized. We adjust reserves to reflect differences between estimated and actual. There were no material differences between the estimated and actual amounts. We estimate our sales returns reserve based on historical return rates and analysis of specific accounts.
Revenues from sales agreements consisting of multiple elements, such as devices, consumables, access to our CloudPAT platform, WatchPAT Direct logistic services and support and other service agreements, are separated into different components and are separately recognized for each component. A component constitutes a separate accounting unit if, and only if, it has value, separately, for the customer. Components that are not separated are grouped together. The revenue from each such component is recognized upon fulfillment of the conditions for recognition of revenue, based on the nature of the component, i.e., as products or as services. In general, we determine the fair value for each element based on selling prices when the product or service is sold separately. In cases where the components are not sold separately, for example, in the case of installations or training, we establish the value assigned to this element, based on estimated costs plus a reasonable margin.
We recognize revenue from leasing our products over the lease term, in conformity with the agreement with the customer. In some cases, we handle sale transactions in these devices as a finance lease and recognize revenues in respect of the products supplied based on their relative fair value compared to all the components in the transaction.
| 68 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
When we sell our products through distributors, revenue is being recognized upon delivery of the product to the distributor, as the distributor does not have the right to return the products and the material risks and rewards inherent to the ownership of the products are transferred at this time.
Inventory Valuation. Inventories are valued using the lower of cost and net realizable value. The cost of inventories is based on the “moving-average” method, including expenditures incurred in acquiring the inventories and the costs incurred in bringing it to its existing location and condition. We analyze our inventory balances to determine if, as a result of internal events, such as physical damage, or external events, such as technological changes or market conditions, certain portions of such balances have become obsolete or impaired. When an impairment situation arises, the inventory balance is adjusted to its net realizable value, whereas, if an obsolescence situation occurs, the inventory obsolescence reserve is increased. In both cases, these adjustments are recognized against the results of the period. Net realizable value is the estimated selling price in the ordinary course of business less the estimated costs to complete and sell the inventories.
Share-Based Compensation. The grant-date fair value of share-based payment awards granted to our employees and directors is recognized as an expense, with a corresponding increase in equity, over the period that the employees and directors become unconditionally entitled to the awards. The amount recognized as an expense in respect of share-based payment awards that are conditional upon meeting service and non-market performance conditions, is adjusted to reflect the number of awards that are expected to vest. For share-based payment awards with non-vesting conditions or with market performance vesting conditions, the grant date fair value of the share-based payment awards is measured to reflect such conditions, and therefore we recognize an expense in respect of the awards whether or not the conditions have been met.
| 69 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The fair value at the time of granting of share-based payment awards to consultants and service providers are recognized over the consultants’ and the service providers’ period of service against an increase in equity. The fair value of the services is calculated on the basis of the fair value of the awards and not on the basis of the fair value of the services, since it is not possible to reliably estimate the fair value of the services rendered.
The fair value of our option grants is computed as of the grant date based on various economic models, using the standard parameters established in that model including estimates relating to the share price on the measurement date, exercise price of the instrument, expected volatility (based on the historical volatility), the expected life span of the options, and the risk-free interest rate (based on Israeli government bonds). As our ordinary shares are publicly traded on the TASE, we do not need to estimate the fair market value of our shares as we use the actual closing market price of our ordinary shares on the date of grant, as reported by the TASE.
We elected to record the increase in equity against salary expense directly to retained earnings.
Derivatives. We recognize all derivative instruments as assets or liabilities in the statements of financial position at their estimated fair values, and the changes in such fair values are recognized in the statements of operations within “financial income (expenses), net” for the period in which they occur. During the reported periods, we did not have derivatives designated as hedges. We review our contracts to identify the existence of embedded derivatives. Identified embedded derivatives are analyzed to determine if they need to be separated from the host contract and recognized in the statements of financial position as assets or liabilities, applying the same valuation rules used for other derivative instruments.
Derivatives with either a conversion price or an exercise price that are denominated in NIS, a currency different than our functional currency, are accounted for as a derivative financial instrument measured at fair value through the statements of operations on each reporting date and constitute a liability.
The fair value of derivatives which are embedded in our formerly outstanding convertible notes is measured based on direct or indirect observed market data, using the binomial model, based on relevant parameters of the conditions of the convertible notes which have been identified for determining the fair value of the warrant component.
The fair value of the Viola Warrants and the Warrants (Series 4) (see Note 14 to our audited consolidated financial statements included elsewhere in this registration statement) as of December 31, 2015 and during the nine month period ended September 30, 2016 was measured at quoted market value of the Warrants (Series 4), due to the fact that the Viola Warrants and the Warrants (Series 4) are essentially identical in their conditions. Starting with the fourth quarter of 2016 and until December 31, 2017, we believed that there was no active market for the traded Warrants (Series 4), primarily due to an ongoing gradual decline in the frequency and volume of trading in such warrants with significant variance in the transactions prices of the warrants without a corresponding material change in the share price, and often with a negative correlation between the change in the share price and the change in the warrants price. Consequently, we estimated the fair value of the Viola Warrants and the Warrants (Series 4) as of December 31, 2016 and for periods thereafter based on observable market data, directly or indirectly, based on the binomial model and based on relevant parameters of the terms of the Viola Warrants and the Warrants (Series 4).
| 70 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The following parameters were used in the calculation of the fair value of the above derivatives, using the binomial model: discount rate for notes (yield to maturity of the notes), the discount rate of the Viola Warrants and Warrants (Series 4) (risk free interest), the share price and standard deviation of the share price.
Recently Issued Accounting Pronouncements
For information with respect to recent accounting pronouncements, see Note 2(r) to our audited consolidated financial statements included elsewhere in this registration statement.
Results of Operations
The following discussion of our results of operations for the years ended December 31, 2017, 2016 and 2015 and for the six-month periods ended June 30, 2018 and 2017, including the following tables, which present selected financial information data in dollars and as a percentage of total revenues, is based upon our consolidated statements of operations contained in our consolidated financial statements for those periods, and the related notes, included in this registration statement.
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||
| 2018 | 2017 | 2017 | 2016 | 2015 | ||||||||||||||||
| (in thousands, except per share and share data) | ||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Revenues | $ | 11,546 | $ | 9,403 | $ | 20,701 | $ | 18,440 | $ | 16,807 | ||||||||||
| Cost of revenues | 2,680 | 2,286 | 5,002 | 4,979 | 4,401 | |||||||||||||||
| Gross profit | 8,866 | 7,117 | 15,699 | 13,461 | 12,406 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Selling and marketing expenses | 6,078 | 6,091 | 12,140 | 14,035 | 10,684 | |||||||||||||||
| Research and development expenses | 1,856 | 1,962 | 4,129 | 3,225 | 2,831 | |||||||||||||||
| General and administrative expenses | 2,724 | 2,710 | 5,278 | 6,213 | 4,350 | |||||||||||||||
| Total operating expenses | 10,658 | 10,763 | 21,547 | 23,473 | 17,865 | |||||||||||||||
| Operating loss | (1,792 | ) | (3,646 | ) | (5,848 | ) | (10,012 | ) | (5,459 | ) | ||||||||||
| Financial income (expenses) from cash and investments | 182 | 1,454 | 1,591 | 716 | (354 | ) | ||||||||||||||
| Financial expenses from notes and loans | (761 | ) | (3,291 | ) | (4,884 | ) | (4,760 | ) | (4,229 | ) | ||||||||||
| Gain (loss) from derivatives instruments, net | 2,110 | 3,835 | 3,925 | (216 | ) | 7,930 | ||||||||||||||
| Financial income (expenses), net | 1,531 | 1,998 | 632 | (4,260 | ) | 3,347 | ||||||||||||||
| Loss before taxes on income | (261 | ) | (1,648 | ) | (5,216 | ) | (14,272 | ) | (2,112 | ) | ||||||||||
| Taxes on income | (51 | ) | (42 | ) | (85 | ) | (131 | ) | (135 | ) | ||||||||||
| Net loss | $ | (312 | ) | $ | (1,690 | ) | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||||
| Loss per share: | ||||||||||||||||||||
| Basic | $ | (0.00 | ) | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.01 | ) | |||||
| Diluted | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.02 | ) | |||||
| 71 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||
| 2018 | 2017 | 2017 | 2016 | 2015 | ||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Revenues | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | ||||||||||
| Cost of revenues | 23.2 | 24.3 | 24.2 | 27.0 | 26.2 | |||||||||||||||
| Gross profit | 76.8 | 75.7 | 75.8 | 73.0 | 73.8 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Selling and marketing expenses | 52.6 | 64.8 | 58.6 | 76.1 | 63.6 | |||||||||||||||
| Research and development expenses | 16.1 | 20.9 | 19.9 | 17.5 | 16.8 | |||||||||||||||
| General and administrative expenses | 23.6 | 28.8 | 25.5 | 33.7 | 25.9 | |||||||||||||||
| Total operating expenses | 92.3 | 114.5 | 104.1 | 127.3 | 106.3 | |||||||||||||||
| Operating loss | (15.5 | ) | (38.8 | ) | (28.2 | ) | (54.3 | ) | (32.5 | ) | ||||||||||
| Financial income (expenses) from cash and investments | 1.6 | 15.5 | 7.7 | 3.9 | (2.1 | ) | ||||||||||||||
| Financial expenses from notes and loans | (6.6 | ) | (35.0 | ) | (23.6 | ) | (25.8 | ) | (25.2 | ) | ||||||||||
| Gain (loss) from derivatives instruments, net | 18.2 | 40.8 | 19.0 | (1.2 | ) | 47.2 | ||||||||||||||
| Financial income (expenses), net | 13.2 | 21.3 | 3.1 | (23.1 | ) | 19.9 | ||||||||||||||
| Loss before taxes on income | (2.3 | ) | (17.5 | ) | (25.2 | ) | (77.4 | ) | (12.6 | ) | ||||||||||
| Taxes on Income | (0.4 | ) | (0.5 | ) | (0.4 | ) | (0.7 | ) | (0.8 | ) | ||||||||||
| Net loss | (2.7 | )% | (18.0 | )% | (25.6 | )% | (78.1 | )% | (13.4 | )% | ||||||||||
Comparison of the Year Ended December 31, 2017 to the Year Ended December 31, 2016
Revenues. The following tables provide a breakdown of our revenues, by line of product and by geographic area, during the years ended December 31, 2017 and 2016, as well as the percentage change between such years:
| Year Ended December 31, | Increase | |||||||||||
| 2017 | 2016 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| WatchPAT and other related services | $ | 18,105 | $ | 15,697 | 15.3 | |||||||
| Endo PAT and other related services | 2,596 | 2,743 | (5.4 | ) | ||||||||
| Total | $ | 20,701 | $ | 18,440 | 12.3 | |||||||
| Year Ended December 31, | Increase | |||||||||||
| 2017 | 2016 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| United States and Canada | $ | 14,764 | $ | 13,343 | 10.6 | |||||||
| Japan | 2,965 | 2,161 | 37.2 | |||||||||
| Europe | 1,746 | 1,542 | 13.2 | |||||||||
| Asia Pacific (excluding Japan) | 759 | 1,017 | (25.4 | ) | ||||||||
| Other | 467 | 377 | 23.9 | |||||||||
| Total | $ | 20,701 | $ | 18,440 | 12.3 | |||||||
Our revenues in 2017 increased by 12.3% to $20.7 million, compared with $18.4 million in 2016. The increase is mainly attributable to an increase of 15.3% in revenues from sales of our WatchPAT product that was partially offset by a decrease of $0.1 million, or 5.4%, in the revenues from sales of our Endo PAT product in 2017, compared with 2016.
The increase in revenues from sales of our WatchPAT product in 2017 is mainly associated with (i) an increase in the volume of sales of disposables being used with each WatchPAT test sold in the U.S.; and (ii) an increase in the volume of sales of WatchPAT units in Japan.
| 72 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The decrease in revenues from sales of Endo PAT in 2017 is primarily due to (i) a continued decrease in our sales and marketing efforts of this product, which is consistent with the trend of decreased volume of sales of the Endo PAT product in recent years; and (ii) the decrease in the volume of sales to research institutions which purchase this product, associated with the reduction of research funds available to such customers.
The ratio of revenues from the sale of disposables being used with each test out of total revenues in 2017 increased to 54.8%, from 49.5% in 2016 (such ratio in the U.S. increased to 61.7% in 2017, from 53.6% in 2016), while the ratio of revenues from the sale of devices out of total revenues in 2017 decreased to 36.3%, from 40.0% in 2016. Revenues from sales of CPAP device were immaterial in each of 2017 and 2016, and represented less than 4% of our total revenues in each of these years. Revenues from other services such as access to our CloudPAT platform, WatchPAT Direct logistic services and support and other service agreements were also immaterial in each of 2017 and 2016 and represented less than 5% of our total revenues in each of these years.
Cost of Revenues and Gross Profit. Our cost of revenues for 2017 was $5.0 million, similar to 2016, whereas our gross profit for 2017 increased by 16.6% to $15.7 million, compared with $13.5 million in 2016. The increase in absolute gross profit is primarily due to our increased volume of sales. The increase in our gross profit margin, to 75.8% in 2017 from 73.0% in 2016, is primarily attributable to: (i) allocation of fixed costs and overhead expenses on a higher volume of sales; (ii) increased efficiency and cost reduction in the production process; and (iii) in 2016, a mixture of products with a lower gross margin, such as the CPAP devices sold during 2016 (which, although comprising less than 4% of our total revenues in each of 2017 and 2016, had a negative impact of nearly 1.5% on our gross margin in 2016).
Operating Expenses. The following table sets forth a breakdown of our operating expenses (excluding cost of revenues) for the years ended December 31, 2017 and 2016 as well as the percentage change between such years:
| Year Ended December 31, | Increase | |||||||||||
| 2017 | 2016 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| Selling and marketing | $ | 12,140 | $ | 14,035 | (13.5 | ) | ||||||
| Research and development | 4,129 | 3,225 | 28.0 | |||||||||
| General and administrative | 5,278 | 6,213 | (15.0 | ) | ||||||||
| Total | $ | 21,547 | $ | 23,473 | (8.2 | ) | ||||||
Selling and Marketing. Selling and marketing expenses for 2017 decreased by 13.5% to $12.1 million, compared with $14.0 million in 2016. This decrease is primarily attributable to a decrease of $1.8 million in employee related costs (including payroll, share-based compensation and travel expenses), mostly related to the reduction in the mid-management team of the U.S. Subsidiary as part of our new strategy and the reduction in the operations (including personnel) of our Japanese subsidiary. The headcount of selling and marketing personnel decreased from 55 as of December 31, 2016 to 42 as of December 31, 2017.
Research and Development. Research and development, or R&D, expenses increased by 28.0% to $4.1 million in 2017, compared with $3.2 million in 2016. This increase is primarily due to the following: (i) a clinical study in the U.S. carried out in 2017 in order to expand the acquaintance of the medical community with our PAT signal technology; and (ii) an increase in employee related costs associated with new R&D projects. The headcount of R&D personnel increased from 13 as of December 31, 2016 to 14 as of December 31, 2017.
General and Administrative Expenses. General and administrative, or G&A, expenses decreased by 15.0% to $5.3 million in 2017, compared with $6.2 million in 2016. This decrease is primarily attributable to a decrease of $0.7 million in allowance for doubtful debts and a decrease in share-based compensation expenses that was partially offset by an increase in employee related costs. The headcount of G&A personnel increased from 21 as of December 31, 2016 to 23 as of December 31, 2017.
Operating Loss. Based on the foregoing, our operating loss decreased from $10.0 million in 2016 to $5.8 million in 2017.
Financial Income (Expenses), net. Financial income, net for 2017 was $0.6 million, compared to financial expenses, net of $4.3 million in 2016. The change is primarily because in 2017, we incurred a net gain from change in fair value of derivative instruments, which amounted to $4.0 million, compared to a net loss from derivative instruments of $0.2 million in 2016. This gain was partially offset by financial expenses from notes and loans in the amount of $4.9 million in 2017, compared with $4.8 million in 2016.
| 73 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The transition from loss to gain with respect to derivatives is due to change in the fair value of the Viola Warrants we issued in November 2015 and January 2016, Warrants (Series 4) we issued to our shareholders as part of a rights offering in December 2015 and the warrants embedded in our formerly outstanding convertible notes. According to IFRS, a valuation at each reporting date of such derivative instruments is required since they are denominated in NIS. Fair value is primary impacted by our share price and the reduction of the maturity period.
Net Loss. Net loss for 2017 decreased by $9.1 million, or 63.2%, to $5.3 million, compared with a net loss of $14.4 million in 2016. This decrease is primarily attributable to the decrease in our operating loss and the transition from net financial expenses to net financial income, as described above.
Comparison of the Year Ended December 31, 2016 to the Year Ended December 31, 2015
Revenues. The following tables provide a breakdown of our revenues, by line of product and by geographical area, during the years ended December 31, 2016 and 2015, as well as the percentage change between such years:
| Year Ended December 31, | Increase | |||||||||||
| 2016 | 2015 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| WatchPAT and other related services | $ | 15,697 | $ | 12,414 | 26.4 | |||||||
| Endo PAT and other related services | 2,743 | 4,393 | (37.6 | ) | ||||||||
| Total | $ | 18,440 | $ | 16,807 | 9.7 | |||||||
| Year Ended December 31, | Increase | |||||||||||
| 2016 | 2015 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| United States and Canada | $ | 13,343 | $ | 10,485 | 27.3 | |||||||
| Japan | 2,161 | 2,045 | 5.7 | |||||||||
| Europe | 1,542 | 2,155 | (28.4 | ) | ||||||||
| Asia Pacific (excluding Japan) | 1,017 | 1,511 | (32.7 | ) | ||||||||
| Other | 377 | 611 | (38.3 | ) | ||||||||
| Total | $ | 18,440 | $ | 16,807 | 9.7 | |||||||
Our revenues in 2016 increased by 9.7% to $18.4 million, compared with $16.8 million in 2015. The increase is attributable to an increase of 26.4% in revenues from sales of our WatchPAT product, which was partially offset by a decrease of $1.7 million, or 37.6%, in the revenues from sales of our Endo PAT product in 2016, compared with 2015.
The increase in revenues from sale of our WatchPAT product in 2016 is mainly associated with the following: (i) an increase in the volume of sales of disposables being used with each test sold in the U.S.; and (ii) an increase in the volume of sales of WatchPAT units in Japan.
The decrease in revenues from sale of our Endo PAT product in 2016 is primarily due to (i) a continued decrease in our sales and marketing efforts of this product, which is consistent with the trend of decreased revenues from the Endo PAT product in recent years; and (ii) the decrease in the volume of sales to research institutions which purchase this product, associated with the reduction of research funds available to such customers. It should be noted that in 2015, revenues from the sales of our Endo PAT product derived from distribution agreements with distributors in China and in Japan, were $0.8 million. These distribution agreements were terminated during 2016 and 2017, respectively.
The ratio of revenues from the sale of disposables being used in each test out of total revenues in 2016 increased to 49.5%, from 46.9% in 2015 (such ratio in the U.S. increased to 53.6% in 2016, from 44.0% in 2015), while the ratio of revenues from the sale of devices out of total revenues in 2016 decreased to 40.0%, from 44.5% in 2016. Revenues from sales of CPAP device were immaterial in each of 2016 and 2015 and represented less than 4% of our total revenues in each of these years. Revenues from other services, such as access to our CloudPAT platform, WatchPAT Direct logistic services and support and other service agreements were also immaterial in each of 2016 and 2015 and represented less than 5% of our total revenues in each of these years.
| 74 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Cost of Revenues and Gross Profit. Our cost of revenues for 2016 increased by 13.1% to $5.0 million, compared with $4.4 million in 2015, whereas our gross profit for 2016 increased by 8.5% to $13.5 million, compared with $12.4 million in 2015. The increase in absolute gross profit reflects primarily our increased volume of sales. The decrease in our gross profit margin to 73.0% in 2016 from 73.8% in 2015, is primarily attributable to a mixture of products with a lower gross margin, such as the CPAP devices sold during 2016 (which, although comprising less than 4% of our total revenues in 2016, had a negative impact of nearly 1.5% on our gross margin in 2016). This decrease was partially offset by: (i) allocation of fixed costs and overhead expenses on a higher volume of sales; (ii) an increase in the average selling prices of our products in 2016; and (iii) increased efficiency and cost reduction in the production process, including an increase in sales of the improved version of the WatchPAT product, the cost of manufacture of which is lower than that of the previous version.
Operating Expenses. The following table sets forth a breakdown of our operating expenses (excluding cost of revenues) for the years ended December 31, 2016 and 2015, as well as the percentage change between such years:
| Year Ended December 31, | Increase | |||||||||||
| 2016 | 2015 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| Selling and marketing | $ | 14,035 | $ | 10,684 | 31.4 | |||||||
| Research and development | 3,225 | 2,831 | 13.9 | |||||||||
| General and administrative | 6,213 | 4,350 | 42.8 | |||||||||
| Total | $ | 23,473 | $ | 17,865 | 31.4 | |||||||
Selling and Marketing. Selling and marketing expenses for 2016 increased by 31.4% to $14.0 million, compared with $10.7 million in 2015. This increase is primarily due to the following: (i) an increase of $2.9 million in employee related costs (including payroll, share-based compensation, sales commissions and travel expenses), mostly related to recruitment of personnel in the U.S. and in Japan; and (ii) an increase in advertising, public relations and sales promotion expenses, including expenses relating to marketing campaigns and trade shows. The headcount of selling and marketing personnel increased from 52 as of December 31, 2015 to 55 as of December 31, 2016.
Research and Development. R&D expenses increased by 13.9% to $3.2 million in 2016, compared with $2.8 million in 2015. This increase is primarily due to the following: (i) an increase in employee related costs associated with increase in salaries; and (ii) an increase in payments to consultants and subcontractors for new developments. This increase was partially offset by a decrease in expenses pertaining to clinical tests. The headcount of R&D personnel was 13 as of December 31, 2015 and 2016.
General and Administrative Expenses. G&A expenses increased by 42.8% to $6.2 million in 2016, compared with $4.4 million in 2015. This increase is primarily attributable to the following: (i) an increase of $0.8 million in allowance for doubtful debts; (ii) an increase of $0.9 million in share-based compensation expenses; and (iii) an increase in employee-related costs associated with increase in salaries. This increase was partially offset by a decrease in expenses relating to a public offering that was not consummated. The headcount of G&A personnel decreased from 24 as of December 31, 2015 to 21 as of December 31, 2016.
Operating Loss. Based on the foregoing, our operating loss increased from $5.5 million in 2015 to $10.0 million in 2016.
| 75 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Financial Income (Expenses), net. Financial expenses, net, for 2016 was $4.3 million, compared with financial income, net of $3.3 million in 2015. The change is primarily due to the following factors: (i) in 2016, we incurred a net loss of $0.2 million from derivative instruments, compared with a net gain from derivative instruments of $7.9 million in 2015; and (ii) financial expenses from notes and loans in the amount of $4.8 million in 2016, compared with $4.2 million in 2015. This loss was partially offset by financial income from cash and investments in the amount of $0.7 million, compared to financial expenses from cash and investments in the amount of $0.4 million in 2015.
The transition from gain to loss with respect to derivatives is due to change in the fair value of the Viola Warrants we issued in November 2015 and February 2016, Warrants (Series 4) issued to the public as part of our rights offering in December 2015 and the warrants embedded in our formerly outstanding convertible notes. According to IFRS, a valuation at each reporting date of such derivative instruments is required since they are denominated in NIS. The fair value is primary impacted by our share price and the reduction of the maturity period.
Net Loss. Net loss for 2016 increased by $12.2 million, or 541.0%, to $14.4 million, compared with a net loss of $2.2 million in 2015. This increase is primarily due to the transition from net financial income to net financial expenses and to the increase in the operating loss, as described above.
Comparison of the Six Months ended June 30, 2018 to the Six Months ended June 30, 2017
Revenues. The following tables provide a breakdown of our revenues, by line of product and by geographical area, during the six-month periods ended June 30, 2018 and 2017, as well as the percentage change between such periods:
| Six Months Ended June 30, | Increase | |||||||||||
| 2018 | 2017 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| WatchPAT and other related services | $ | 10,651 | $ | 8,210 | 29.7 | |||||||
| Endo PAT and other related services | 895 | 1,193 | (25.0 | ) | ||||||||
| Total | $ | 11,546 | $ | 9,403 | 22.8 | |||||||
| Six Months Ended June 30, | Increase | |||||||||||
| 2018 | 2017 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| United States and Canada | $ | 7,963 | $ | 6,522 | 22.1 | |||||||
| Japan | 2,257 | 1,428 | 93.1 | |||||||||
| Europe | 801 | 645 | 24.2 | |||||||||
| Asia Pacific (excluding Japan) | 316 | 531 | (40.5 | ) | ||||||||
| Other | 209 | 277 | (24.5 | ) | ||||||||
| Total | $ | 11,546 | $ | 9,403 | 22.8 | |||||||
Our revenues in the six months ended June 30, 2018 increased by 22.8% to $11.5 million, compared with $9.4 million in the six months ended June 30, 2017. The increase is mainly attributable to an increase of 29.7% in revenues from sales of our WatchPAT product, which was partially offset by a decrease of $0.3 million, or 25.0%, in the revenues from sale of our Endo PAT product in the six months ended June 30, 2018, compared with the six months ended June 30, 2017.
| 76 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The increase in revenues from sale of our WatchPAT product in the six-month period ended June 30, 2018, is mainly associated with the following: (i) an increase in the volume of sales of disposables being used with each WatchPAT test sold in the U.S.; and (ii) an increase of 85% in sales in Japan, which is attributable to an increase in the volume of sales.
The decrease in revenues from sale of our Endo PAT product in the six-month period ended June 30, 2018, is primarily due to a continued decrease in our sales and marketing efforts of this product, which is consistent with the trend of decreased revenues from the Endo PAT product in recent years.
The ratio of revenues from the sale of disposables being used with each test, out of total revenues in the six months ended June 30, 2018 increased to 54.7%, from 53.5% in the six-month period ended June 30, 2017 (such ratio in the U.S. increased to 66.4% in the six-month period ended June 30, 2018, from 62.7% in the six-month period ended June 30, 2017), while the ratio of revenues from the sale of devices out of total revenues in the six-month period ended June 30, 2018 decreased to 36.4%, from 38.3% in the six-month period ended June 30, 2017. Revenues from sales of CPAP devices were immaterial in each of the six-month periods ended June 30, 2018 and 2017 and represented less than 1% of our total revenues during such periods. Revenues from other services, such as access to our CloudPAT platform, WatchPAT Direct logistic services and support and other service agreements were also immaterial in each of the six-month periods ended June 30, 2018 and 2017 and represented less than 5% of our total revenues during such periods.
Cost of Revenues and Gross Profit. Our cost of revenues for the six months ended June 30, 2018 increased by 17.2% to $2.7 million, compared with $2.3 million in the six months ended June 30, 2017, whereas our gross profit for the six months ended June 30, 2018 increased by 24.6% to $8.9 million, compared with $7.1 million in the six months ended June 30, 2018. The increase in absolute gross profit is primarily due to our increased volume of sales. The increase in our gross profit margin to 76.8% in the six months ended June 30, 2018, from 75.7% in six months ended June 30, 2017, is primarily attributable to: (i) allocation of fixed costs and overhead expenses on a higher volume of sales; and (ii) increased efficiency and cost reduction in the production process.
Operating Expenses. The following table sets forth a breakdown of our operating expenses (excluding cost of revenues) for the six-month periods ended June 30, 2018 and 2017, as well as the percentage change between such periods:
| Six Months Ended June 30, | Increase | |||||||||||
| 2018 | 2017 | (decrease) | ||||||||||
| (in thousands) | % | |||||||||||
| Selling and marketing | $ | 6,078 | $ | 6,091 | (0.2 | ) | ||||||
| Research and development | 1,856 | 1,962 | (5.4 | ) | ||||||||
| General and administrative | 2,724 | 2,710 | 0.5 | |||||||||
| Total | $ | 10,658 | $ | 10,763 | (1.0 | ) | ||||||
Selling and Marketing. Selling and marketing expenses for the six months ended June 30, 2018 were $6.1 million, similar to the level thereof in the six months ended June 30, 2017, but there was a change in composition. There was an increase in the expenses primarily due to the following: (i) an increase in employee related costs (including payroll, share-based compensation, sales commissions and travel expenses), mostly related to recruitment of personnel in the U.S.; and (ii) an increase in consulting and legal expenses, mainly due to our efforts to increase the insurance coverage for reimbursement for use of our products. This increase was offset by (i) a decrease of employee related costs (including payroll, share-based compensation, sales commissions and travel expenses), related to our Japanese subsidiary; and (ii) a decrease in advertising, public relations and sales promotion expenses, including expenses relating to marketing campaigns and trade shows.
Research and Development. R&D expenses decreased by 5.4% to $1.9 million in the six months ended June 30, 2018, compared with $2.0 million in the six months ended June 30, 2017. This decrease is primarily due to the following: (i) a decrease in expenses associated with a clinical study in the U.S. carried out in 2017 and 2018 in order to expand the acquaintance of the medical community with our PAT signal technology; and (ii) a decrease in expenses related to consultants and subcontractors.
General and Administrative Expenses. G&A expenses for the six months ended June 30, 2018 were $2.7 million, similar to the level thereof in the six months ended June 30, 2017.
| 77 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Operating Loss. Based on the foregoing, our operating loss decreased from $3.6 million in the six months ended June 30, 2017 to $1.8 million in the six months ended June 30, 2018.
Financial Income, net. Financial income, net for the six months ended June 30, 2018 was $1.5 million, compared to $2.0 million in the six months ended June 30, 2018. The change is primarily because in the six months ended June 30, 2018 we incurred net gain from changes in the fair value of derivative instruments, which amounted to $2.1 million, compared with $3.8 million in the six months ended June 30, 2017. This gain was partially offset by (i) financial expenses from notes and loans in the amount of $0.8 million in the six months ended June 30, 2018, compared with $3.3 million in the six months ended June 30, 2017, mainly attributable to the full repayment of the remainder of the principal amount of the convertible notes in February 2018; and (ii) financial income from cash and investments in the amount of $0.2 million in the six months ended June 30, 2018, compared with $1.5 million in the six months ended June 30, 2017, mainly due to decrease in cash and investments balances as a result of the full repayment of the remainder of the principal amount of the convertible notes in February 2018.
The decrease in gain with respect to derivatives is due to change in the fair value of the Viola Warrants we issued in November 2015 and January 2016, Warrants (Series 4) we issued to our shareholders as part of a rights offering in December 2015 and the warrants embedded in our formerly outstanding convertible notes. According to IFRS, a valuation at each reporting date of such derivative instruments is required since they are denominated in NIS. The fair value is primary impacted by our share price and the reduction of the maturity period.
Net Loss. Net loss for the six months ended June 30, 2018 decreased by $1.4 million, or 81.5%, to $0.3 million, compared with a net loss of $1.7 million in the six months ended June 30, 2017. This decrease is primarily attributable to the decrease in our operating loss, offset by the decrease in net financial income, as described above.
Impact of Currency Fluctuations and of Inflation
Our financial results may be impacted by foreign currency fluctuations and inflation although, except as set forth below, foreign currency fluctuations and the rate of inflation did not have a material impact on our financial results in the past three years.
Since the majority of our revenues are paid in or linked to the dollar, we believe that inflation and fluctuations in the NIS/dollar exchange rate have no material effect on our revenues. However, a significant portion of the cost of our Israeli operations, mainly personnel and facility-related, is incurred in NIS and, consequently, inflation in Israel and fluctuations in the dollar/NIS exchange rate may have an impact on our expenses and, as a result, on our net income or loss. Our NIS costs, as expressed in dollars, are influenced by the extent to which any increase in the rate of inflation in Israel is not offset (or is offset on a lagging basis) by a devaluation of the NIS in relation to the dollar. To protect against the changes in value of forecasted foreign currency cash flows resulting from payments in NIS, we maintain liquid means on hand in NIS and dollar and we execute, from time to time, hedging transactions in accordance with our needs. As of June 30, 2018 and December 31, 2017, we did not enter into any hedge transaction. Even if we enter into such hedging transactions, these measures may not adequately protect us from material adverse effects due to the impact of currency fluctuations or inflation.
For additional details, see Item 11 “Quantitative and Qualitative Disclosures about Market Risk” below.
| 78 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| B. | Liquidity and Capital Resources |
General
Since our incorporation in 1997, we have incurred operating and net losses in most of our years of operation. As of December 31, 2017, we had an accumulated deficit of approximately $105.0 million. We expect to continue to incur operating and net losses for the foreseeable future, as we continue to invest in research and development and marketing and sales operations aimed at growing our business.
In the past several years, we financed our operations primarily through issuance of equity and debt to the public, private placements of our ordinary shares to institutional and other investors and loans from our major shareholders and commercial banks.
Our funding and treasury activities are conducted within corporate practices to maximize investment returns while maintaining appropriate liquidity for both our short and long-term needs. Cash and cash equivalents are held primarily in dollars and NIS. Marketable securities are currently held mainly in NIS.
Principal Financing Activities
Since January 1, 2015, we have engaged in the following principal financing activities:
| · | 2015 Viola Investment. On August 26, 2015, we entered into a share purchase agreement with Viola Growth II A.V. LP, Viola Growth II (A) LP and Viola Growth II (B) LP, or collectively, Viola (the “Viola Investment Agreement” or the “Viola Transaction”): |
| o | On November 5, 2015 (and, as a second stage of the transaction, on February 1, 2016), following approval by our shareholders of the Viola Transaction, we completed the transaction and issued to Viola, in the aggregate for these two closings, 66,876,907 ordinary shares (representing, as of February 1, 2016, approximately 25.47% of our issued and outstanding shares on a post-issuance basis) at a purchase price of NIS 1.449 per share (equivalent to $0.38, based on the exchange rate as of that date) (the “Viola PPS”), resulting in aggregate proceeds (before expenses) of NIS 96.9 million (equivalent to approximately $25.2 million, based on the exchange rate as of that date). As a result, Viola became and, to our knowledge, still is, our largest shareholder. |
| o | In addition, we issued to Viola, for no additional consideration, warrants (the “Viola Warrants”) exercisable into up to 33,438,454 ordinary shares (i.e., a ratio of one warrant for every two shares). The Viola Warrants have an exercise price of NIS 1.642 per share (equivalent to $0.45) for the first 21 months of the term thereof and an exercise price of NIS 1.745 (equivalent to $0.47) for the remainder of the term, subject to adjustments. The Viola Warrants expire on the earlier of: (i) the passage of 42 months following their issuance (i.e., on May 4, 2019); (ii) in the event of a public offering with a pre-money valuation of our Company of at least at $250 million; or (c) in the event of a merger or sale of our Company which reflects a company value of at least $250 million and the result of which will be that the shareholders in our Company before such event will hold less than the majority of voting rights in the surviving entity. |
| o | The Viola Investment Agreement contained customary terms and conditions, including (i) representations and warranties of the parties which survived the completion of the transaction and, in general, expired in late 2017; and (ii) we agreed to grant customary registration rights to Viola, subject to obtaining the applicable regulatory approvals to the extent required under applicable law, and that such registration rights will include, at a minimum, two demand registration rights and unlimited piggyback and Form F-3 registrations. |
| 79 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | 2015 Rights Offering. In connection with the Viola Transaction, we conducted a rights offering to our shareholders pursuant to a shelf offering report that we published on December 2, 2015: |
| o | On December 29, 2015, we completed the rights offering and issued to the subscribing shareholders a total of 12,876,603 ordinary shares (representing as of such date approximately 4.95% of our issued and outstanding shares on a post-issuance basis) at a price per share equal to the Viola PPS, resulting in aggregate proceeds (before expenses) of NIS 18.7 million (equivalent to approximately $4.7 million, based on the exchange rate as of that date). |
| o | In addition, we issued to the subscribing shareholders, for no additional consideration, Warrants (Series 4) exercisable into up to 6,438,152 ordinary shares (i.e., a ratio of one warrant for every two shares). The Warrants (Series 4), which are listed for trading on the TASE, have an exercise price equal to the exercise price of the Viola Warrants and expire on May 4, 2019. |
| o | We used the proceeds from the rights offering for various general and corporate purposes, including repayment of $1.8 million of outstanding loans from some of our major shareholders. |
| · | 2017 Credit Line. On March 29, 2017, we entered into a credit line agreement with an Israeli commercial bank (as amended on January 30, 2018 and May 28, 2018, the “Credit Agreement”), whereby we secured a credit line in a total amount of up to $10 million comprised of (i) up to $6 million in long-term loan (the “Loan”); and (ii) up to $4 million of revolving credit line against our trade accounts receivable (the “Revolving Credit Line”): |
| o | The Loan may be drawn at any time through February 28, 2019 and bears interest at the annual interest rate of the quarterly dollar LIBOR rate plus 5.5%. The principal amount of the Loan and the interest accrued thereon is repayable in equal quarterly installments over three years from the date of the draw. |
| o | The Revolving Credit Loan may be drawn at any time through January 12, 2019 and is renewable annually. It bears interest at the annual interest rate of the monthly dollar LIBOR rate plus 4.25%. |
| o | The right to make any draws, whether under the Loan or the Revolving Credit Loan, is conditioned upon us having cash balances of not less than 40% of the total amount drawn in our account with the lending bank. |
| o | In addition, we issued the bank warrants exercisable into up to 798,088 ordinary shares at an exercise price of NIS 1.36 per share (equivalent to $0.37 per share), subject to adjustments. The warrants are exercisable until May 14, 2022. |
| 80 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| o | In order to secure our obligations to the bank, we pledged and granted to the bank a first priority floating charge on all of our assets and a first priority fixed charge on (i) our intellectual property, goodwill, holdings in our subsidiaries and certain other, immaterial, assets and (ii) all of the assets of the U.S. Subsidiary. We refer to the agreements relating to such charges and other security interests as the Security Agreements. The Security Agreements contain a number of customary restrictive terms and covenants that limit our operating flexibility, such as (i) limitations on the creation of additional liens, on the incurrence of indebtedness, on the provision of loans and guarantees and on distribution of dividends; and (ii) the ability of the bank to accelerate repayment in certain events, such as breach of covenants, liquidation, and a change of control of our Company. Such provisions may hinder our future operations or the manner in which we operate our business, which could have a material adverse effect on our business, financial condition or results of operations. |
| o | As of June 30, 2018, we had a total outstanding principal amount of $5.0 million under the Credit Agreement, of which (i) $2.9 million were drawn in February 2018 as a Loan, currently repayable on November 20, 2018; and (ii) $2.1 million were drawn under the Revolving Credit Loan. |
| · | 2018 Repayment of Series L Convertible Notes. In February 2018, we repaid all of the outstanding principal amount and accrued interest of our then outstanding Series L Convertible Notes, or the convertible notes, which were issued as part of a public offering we conducted in 2013 and had a conversion price of NIS 1.92 per share (equivalent to $0.54, based on the exchange rate on the last exercise date, i.e., on February 12, 2018). The full repayment, which totaled in a sum of NIS 32.1 million (equivalent to approximately $9.2 million, based on the exchange rate as of the repayment date), does not include (i) repayment to Dr. Giora Yaron (through a company wholly owned by him), our Chairman of the Board of Directors and a major shareholder, and Medtronic, our major shareholder, both of whom held convertible notes and agreed to waive such repayment and used the funds otherwise owed to them to make the investment described under “2018 Private Placement” below; and (ii) repayment of NIS 1.6 million (equivalent to approximately $0.5 million, based on the exchange rate as February 28, 2018) to Mr. Martin Gerstel, a member of our Board of Directors and a major shareholder, who held convertible notes and agreed to postpone such repayment from February 2018 to June 2018. |
| · | 2018 Shareholders’ Loan. As described under “2018 Repayment of Series L Convertible Notes” above, the repayment of the convertible notes does not include (i) the repayment to Dr. Giora Yaron (through a company wholly owned by him) and Medtronic, who agreed to waive such repayment and used the funds otherwise owed to them to make the investment described under “2018 Private Placement” below; and (ii) repayment of NIS 1.6 million (equivalent to approximately $0.5 million, based on the exchange rate as February 28, 2018) to Mr. Martin Gerstel who held convertible notes and agreed to postpone such repayment from February 2018 to June 2018. Such amounts were treated as shareholders’ loan until repaid or converted to investment in shares as part of the 2018 private placement described in the next paragraph. |
| · | 2018 Private Placement. On March 22, 2018, we entered into separate securities purchase agreements with Dr. Giora Yaron (through a company wholly owned by him), our Chairman of the Board of Directors and a major shareholder; Viola, our largest shareholder; and Medtronic, a major shareholder, and various funds affiliated with three Israeli institutional investors, Yelin Lapidot, Meitav Dash and Phoenix: |
| o | On May 27, 2018, following approval by our shareholders of the private placement contemplated by these securities purchase agreements, we completed the transaction and issued to the investors a total of 22,013,893 ordinary shares (representing as of such date approximately 7.7% of our issued and outstanding shares on a post-issuance basis) at a purchase price of NIS 0.947 per share (equivalent to $0.27, based on the exchange rate as of that date), resulting in aggregate proceeds (before expenses) of NIS 20.8 million (equivalent to approximately $6.0 million, based on the exchange rate as of that date). Out of the total NIS 20.8 million investment, Dr. Yaron, Viola and Medtronic invested NIS 2.1 million, NIS 5.2 million and NIS 2.4 million, respectively. |
| o | The ordinary shares issued to the investors are subject to resale restrictions under Israeli law as applicable to private placements, including an initial six month full lockup resale restriction that will expire in late November 2018. |
| 81 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| o | The securities purchase agreement contained customary terms and conditions, including limited representations and warranties of the parties which survived the completion of the transaction and, in general, expire on May 27, 2019. |
Working Capital
As of June 30, 2018, we had $8.5 million in cash, cash equivalents and marketable securities, compared with $10.8 million as of December 31, 2017, $26.1 million as of December 31, 2016. The decrease in the six months ended June 30, 2018, compared to the year ended December 31, 2017 derives primarily from the repayment of the second and last installment of our convertible notes of $9.4 million, offset by the $6.0 million of gross proceeds from the private placement in May 2018, a draw of $5.0 million out of our bank credit line and from the cash flows used in operating activities in an amount of $2.0 million (which includes interest payment on our convertible notes and our bank credit line). The decrease in the year ended December 31, 2017, compared to the year ended December 31, 2016 derives primarily from the repayment of the first installment of our convertible notes in the amount of $10.4 million during 2017 and from the cash flows used in operating activities in an amount of $6.2 million (which includes interest payment on our convertible notes). This decrease was partially offset because the value of our cash balances, most of which held in NIS deposits and marketable securities, increased due to the approximately 9.8% devaluation in the dollar/NIS exchange rate in the year ended December 31, 2017.
As of June 30, 2018, we did not have any debt to a third party, other than the short-term loans from a bank under the Credit Agreement. As of December 31, 2017 and 2016, we did not have any debt to a third party, other than the convertible notes in the amount of $10.7 million and $17.8 million, respectively, which, as described above, were fully paid during 2018.
As of June 30, 2018, our working capital amounted to $7.4 million, compared with $3.4 million as of December 31, 2017 and $18.8 million as of December 31, 2016. The increase in the six months ended June 30, 2018, compared to the year ended December 31, 2017 is primarily due to the proceeds from the private placement in May 2018 and the draw out of our bank credit line, offset by (i) the repayment of the second half of the principal amount of our convertible; and (ii) the decrease in cash, cash equivalents and marketable securities resulting from the financing of our operating activities. The decrease in the year ended December 31, 2017 compared to the year ended December 31, 2016 is primarily due to: (i) the reclassification of the second half of the principal amount of our convertible notes from long-term liabilities to short-term liabilities; and (ii) the decrease in cash, cash equivalents and marketable securities resulting from the financing of our operating activities.
Cash Flows
The following table presents the major components of net cash flows used in and provided by operating, investing and financing activities for the periods presented:
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||||||
| 2018 | 2017 | 2017 | 2016 | 2015 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||
| Net cash used in operating activities* | $ | (2,016 | ) | $ | (4,031 | ) | (6,182 | ) | $ | (10,630 | ) | $ | (8,610 | ) | ||||||
| Net Cash provided by (used in) investing activities | 3,017 | (145 | ) | (318 | ) | (568 | ) | 5,474 | ||||||||||||
| Net Cash provided by (used in) financing activities | (140 | ) | (10,324 | ) | (10,324 | ) | 1,100 | 26,840 | ||||||||||||
| Increase (decrease) in cash and cash equivalents | $ | 861 | $ | (14,500 | ) | $ | (16,824 | ) | $ | (10,098 | ) | $ | 23,704 | |||||||
* Including interest on our convertible notes.
| 82 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Operating Activities
Cash flows from operating activities consist primarily of loss adjusted for various non-cash items, including depreciation and amortization, share-based compensation expenses and gain or loss from reevaluation of derivatives. In addition, cash flows from operating activities are impacted by changes in operating assets and liabilities, which include inventories, accounts receivable and other assets and accounts payable.
Net cash used in operating activities for the year ended December 31, 2017 was $6.2 million. This net cash used in operating activities primarily reflects a net loss of $5.3 million, net of net non-cash expenses of $1.2 million, an increase of $0.8 million in trade receivables due to an increase in revenues in 2017 and an increase of $0.7 million in inventories due to the aforesaid increase in revenues and the desire to hold inventories levels for one additional quarter, offset by an increase of $0.6 million in accounts payable due to the increase in our level of operating expenses, and interest of $1.4 million paid on our convertible notes. Net non-cash expenses of $1.2 million consisted primarily of depreciation and amortization of $0.5 million, net financial expenses of $3.1 million, and share-based compensation of $1.3 million, offset by a net gain from changes in fair value of derivative instruments of $3.9 million relating to warrants, including warrants embedded in our convertible notes, mainly attributable to share price decrease.
Net cash used in operating activities for the year ended December 31, 2016 was $10.6 million. This net cash used in operating activities primarily reflects a loss of $14.4 million, net of net non-cash expenses of $7.4 million, an increase of $1.5 million in trade receivables due to an increase in revenues in 2016, an increase of $0.4 million in inventories, offset by an increase of $0.5 million in accounts payable. Net non-cash expenses of $7.4 million consisted primarily of depreciation and amortization of $0.4 million, net financial expenses of $4.1 million, net loss from changes in fair value of derivative instruments of $0.2 million, and a net loss from changes in fair value of derivative instruments of $0.2 million relating to warrants, including warrants embedded in our convertible notes, and share-based compensation of $1.8 million.
Net cash used in operating activities for the year ended December 31, 2015 was $8.6 million. This net cash used in operating activities primarily reflects a loss of $2.2 million and net non-cash gain of $2.5 million, an increase of $1.3 million in trade receivables due to increase in revenues in 2015, an increase of $0.3 million in inventories, and a decrease of $0.4 million in accounts payable. Net non-cash gain of $2.5 million consisted primarily of offset by depreciation and amortization of $0.4 million, net financial expenses of $4.6 million, and share-based compensation of $0.4 million, offset by a net gain from changes in fair value of derivative instruments of $8.0 million relating to warrants, including warrants embedded in our convertible notes, mainly due to share price decrease.
Net cash used in operating activities for the six months ended June 30, 2018 was $2.0 million. This net cash used in operating activities primarily reflects a net loss of $0.3 million, net of net non-cash income of $(0.7) million and an increase of $0.7 million in inventories due to the aforesaid increase in revenues and the desire to hold inventory levels for one additional quarter, offset by increase of $0.2 million in accounts payable and interest of $0.6 million paid on our convertible notes and bank credit line. Net non-cash expenses of $(0.7) million consisted primarily of depreciation and amortization of $0.2 million, net financial expenses of $0.6 million, and share-based compensation of $0.5 million, offset by a net gain from changes in fair value of derivative instruments of $2.1 million relating to warrants, including warrants embedded in our convertible notes, mainly attributable to share price decrease.
| 83 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Net cash used in operating activities for the six months ended June 30, 2017 was $4.0 million. This net cash used in operating activities primarily reflects a net loss of $1.7 million, net of net non-cash expenses of $(1.2) million, and an increase of $0.6 million in accounts payable due to the increase in our level of operating, and interest of $0.9 million paid on our convertible notes. Net non-cash expenses of $(1.2) million consisted primarily of depreciation and amortization of $0.2 million, net financial expenses of $1.6 million, and share-based compensation of $0.7 million, offset by a net gain from changes in fair value of derivative instruments of $3.8 million relating to warrants, including warrants embedded in our convertible notes, mainly attributable to share price decrease.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2017 was $0.3 million. This net cash used in investing activities is primarily attributable to capital expenditures and capitalized development costs of $0.3 million.
Net cash used in investing activities for the year ended December 31, 2016 was $0.6 million. This net cash used in investing activities is primarily attributable to capital expenditure and capitalized development costs of $0.5 million.
Net cash provided by investing activities for the year ended December 31, 2015 was $5.5 million. This net cash provided by investing activities is primarily attributable to realization of marketable securities in the amount of $6.1 million, offset by capital expenditure and capitalized development costs of $0.6 million.
Net cash provided by investing activities for the six months ended June 30, 2018 was $3.0 million. This net cash provided by investing activities is primarily attributable to realization of marketable securities in the amount of $3.1 million.
Net cash used in investing activities for the six months ended June 30, 2017 was $0.1 million. This net cash used in investing activities is primarily attributable to capital expenditure and capitalized development costs of $0.1 million
Financing Activities
Net cash used in financing activities for the year ended December 31, 2017 was $10.3 million. This net cash used in financing activities is primarily due to first repayment of our convertible notes in the amount of $10.4 million, offset by exercises of stock options in the amount of $0.1 million.
Net cash provided by financing activities for the year ended December 31, 2016 was $1.1 million. This net cash provided by financing activities is primarily due to net proceeds generated from the issuance of additional shares and warrants to Viola in the second stage of the Viola Transaction in the net amount of $1.1 million.
Net cash provided by financing activities for the year ended December 31, 2015 was $26.8 million. This net cash provided by financing activities is primarily due to net proceeds from issuance of shares and warrants to Viola in the first stage of the Viola Transaction in the net amount of $23.2 million, net proceeds from issuance of shares and warrants in a rights offering to our shareholders in the net amount of $5.3 million, and to exercise of stock options in the amount of $0.2 million, offset by repayment of shareholders’ loans in the amount of $1.8 million.
| 84 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Net cash used in financing activities for the six months ended June 30, 2018 was $0.1 million. This net cash used in financing activities is primarily due to the second and final repayment of our convertible notes in the amount of $9.9 million, offset by the gross proceeds of $5.2 million from the private placement in May 2018 and a draw of $5.0 million out of our bank credit line.
Net cash used in financing activities for the six months ended June 30, 2017 was $10.3 million. This net cash used in financing activities is primarily due to first repayment of our convertible notes in the amount of $10.4 million.
Principal Capital Expenditure and Divestitures
During the year ended December 31, 2017, our capital expenditures and capitalized development costs totaled $0.3 million, compared to $0.5 million during the year ended December 31, 2016 and $0.6 million during the year ended December 31, 2015, most of which were used for the purchase of production and research and development equipment, office furniture and equipment and computers and self-manufactured equipment (WatchPAT devices that are used by our customers). We have no significant capital expenditures in progress.
We did not affect any principal divestitures in the past three years.
Outlook
Currently, our principal commitments consist mainly of our lease obligations and bank credit line. See also Item 5.F “Tabular Disclosure of Contractual Obligations.”
In light of our cash balances and other factors, including our ability to use our bank credit line, we believe that our existing capital resources will be adequate to satisfy our working capital and capital expenditure requirements for a period of no less than 12 months from the effective date of this registration statement. However, from time to time, we intend to seek additional financing sources to maintain and grow our business. See also under Item 3.D “Risk Factors – Risks Related to Our Business and Operations - We will require additional funds to support our strategy and long-term operational plans, and, if additional funds are not available, we may need to significantly scale back or even cease our planned operations”.
| C. | Research and Development, Patents and Licenses, etc. |
We view sleep medicine in general and in particular, sleep in cardiology, as our main business. Therefore, our research and development efforts in recent years were focused on (i) enhancing and improving the technology underlying our main platform, the WatchPAT200, primarily in order to address market needs (such as by adding the ability to identify central sleep apnea and Cheyne-Stokes respiration that is typical to cardiac patients); (ii) evolution of our product lines by introducing a new generation of products (such as the WatchPAT300, for which we obtained FDA clearance on August 17, 2018); and (iii) improving our solutions in collaboration with other companies in the sleep arena, with the goal of introducing a superior solution to our customers.
| 85 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
We also invest in clinical research to support the expansion of our sleep apnea solutions in the cardiology market and in the sleep medicine market in general, as well as in order to substantiate and support the data which is at the basis of the products we are developing or enhancing. We also use such research to gain recognition in the medical community and for scientific publications.
Our research and development activities for all our products principally take place in Israel with the exception of clinical trials that are also conducted outside of Israel. As of December 31, 2017, we employed 14 persons in research and development, compared to 13 persons as of December 31, 2016 and 2015.
We have committed substantial financial resources to our research and development efforts. During the years ended December 31, 2017, 2016 and 2015, our research and development expenditures were $4.2 million, $3.3 million and $2.9 million, respectively (including development costs of $0.1 million in each of those years, which were capitalized).
As described in Item 4.B “Information on the Company - Business Overview - Government Regulations,” we participated in the past in programs sponsored by the IIA.
| D. | Trend Information |
See Item 5.A “Operating Results – Overview - Trend Information and Outlook.”
| E. | Off-Balance Sheet Arrangements |
We do not have any off-balance sheet arrangements, as such term is defined under Item 5.E of the instructions to Form 20-F, that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
| F. | Tabular Disclosure of Contractual Obligations |
The following table summarizes our significant contractual obligations and commercial commitments, as of December 31, 2017:
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating leases (1) | $ | 1,917 | $ | 931 | $ | 722 | $ | 264 | $ | — | ||||||||||
| Long-term debt obligations (2) | 11,473 | 11,473 | — | — | — | |||||||||||||||
| (1) | Includes lease payments for our facilities, offices and motor vehicles. |
| (2) | Includes principal and interest payments on our convertible notes. However, in February 2018, we fully repaid such convertible notes. |
Severance payments of $2.3 million are payable only upon termination, retirement or death of the respective employee. Of this amount, $0.3 million is unfunded. Since we are unable to reasonably estimate the timing of settlement, the timing of such payments is not specified in the table. See also Note 11 to our audited consolidated financial statements included elsewhere in this registration statement.
| 86 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
As required by IFRS, our obligation to pay royalties to the IIA is presented in our consolidated financial statements as part of our long-term liabilities and accrued expenses in respect of future sales of our products. However, since these obligations are contingent upon the volume and timing of sales of our products, we are unable to reasonably estimate the timing and scope of such payments and they are not specified in the table. See also Item 4.B “Information on the Company – Business Overview – Government Regulations – The Israeli Market - Grants from the IIA” above and Note 12 to our audited consolidated financial statements included elsewhere in this registration statement.
| ITEM 6. | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
| A. | Directors and Senior Management |
The following lists the name, age, principal position and a biographical description of each of our current directors and senior management.
| Name | Age | Director Since | Position with the Company | |||
| Giora Yaron, PhD | 70 | 1997 | Chairman of the Board of Directors | |||
| Gilad Glick | 45 | — | President and Chief Executive Officer, Acting Vice President Marketing and Sales, Acting President of the U.S. Subsidiary | |||
| Shy Basson | 46 | — | Chief Financial Officer | |||
| Shlomo Ayanot | 62 | — | Vice President, Engineering and Operations | |||
| Jacob (Koby) Sheffy, PhD | 66 | — | Senior Vice President of Research and Chief Technology Officer | |||
| Itay Kariv | 60 | — | Vice President of Research and Development | |||
| Efrat Litman | 45 | — | Vice President of Advanced Research and Development | |||
| Eilon Livne | 48 | — | Vice President Sales and Channels Development EMEA | |||
| Martin Gerstel (1) | 77 | 1997 | Director | |||
| Ilan Biran (2) | 72 | 2013 | Director | |||
| Jonathan Kolber | 56 | 2015 | Director | |||
| Sami Totah | 60 | 2015 | Director | |||
| Christopher M. Cleary | 58 | 2017 | Director | |||
| Yaffa Krindel Sieradzki (1) (2) | 64 | 2016 | External Director | |||
| Zipora (Tzipi) Ozer-Armon (1) (2) | 53 | 2016 | External Director |
| (1) | Member of our Compensation Committee of the Board of Directors (the “Compensation Committee”). |
| (2) | Member of our Audit Committee of the Board of Directors (the “Audit Committee”). |
| 87 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Giora Yaron, PhD, is a co-founder of our Company and has served as Chairman of our Board of Directors since 2016. Between 1997 and 2016, Dr. Yaron served as Co-Chairman of our Board of Directors. Dr. Yaron also serves as a member of the Board of Directors of Amdocs Limited (NASDAQ:DOX), as Chairman of the Board of Directors of Excelero (ExpressIO), a provider of ultra-fast block storage solutions and as the Chairman of the Board of Directors of Equalum, a provider of a real-time Data Beaming for Big Data Analytics. Dr. Yaron co-founded several privately-held technology companies, sold to multinational corporations, including, P-cube, Pentacom, Qumranet, Exanet, Comsys and Hyperwise Security. Since 2010, Dr. Yaron serves as Chairman of the Executive Council of the Tel Aviv University, and previously served as Chairman of Ramot, the Tel Aviv University technology transfer company from 2010 until 2015. In 2009, Dr. Yaron also co-founded Qwilt, Inc., a privately-held video technology provider and serves on its Board of Directors. Between 1996 and 2006, Dr. Yaron served as a member of the Board of Directors of Mercury Interactive, a publicly-traded IT optimization software provider, acquired by Hewlett-Packard, including as its Chairman of the Board of Directors between 2004 and 2006. Between 1992 and 1995, Dr. Yaron served as President of Indigo NV. Prior to joining Indigo, Dr. Yaron served as Corporate Vice President of National Semiconductor. Dr. Yaron has previously served on the advisory board of Rafael Advanced Defense Systems, Ltd., a developer of high-tech defense systems, and on the advisory board of the Israeli Ministry of Defense. Dr. Yaron holds a PhD in device physics, and a Bachelor’s degree in physics and mathematics from the Hebrew University of Jerusalem.
Gilad Glick has served as our Chief Executive Officer and President since July 2013. Mr. Glick also serves as a director and as acting president of the U.S. Subsidiary, Itamar Medical Inc. Prior to joining Itamar Medical, Mr. Glick served in various positions in the medical devices industry, spanning across multiple countries in Europe and the U.S. in a variety of functional areas including sales, marketing, service and research & development. Between June 2008 and July 2013, Mr. Glick held the position of worldwide vice president of sales and marketing of Biosense Webster, a Johnson & Johnson company, overseeing all strategic and commercial activities. Mr. Glick earned an M.B.A from the Maastricht School of Management, majoring in general and strategic management. He is also a graduate of the Strategic Marketing Management Executive Program at the Stanford Graduate School of Business.
Shy Basson has served as our Chief Financial Officer since May 2017. Mr. Basson also serves as a director of the U.S. Subsidiary, Itamar Medical Inc. Prior to joining Itamar Medical, between January 2008 and October 2016, Mr. Basson served as Chief Financial Officer, Business and Strategy of WeFi, Inc., a provider of mobile data collection and Wi-Fi connectivity solutions. Prior thereto Mr. Basson served as Director of Business Development at AOL (a Time Warner Company). Prior thereto, Mr. Basson served as the CFO of ICQ. Mr. Basson holds a B.A. degree in business administration and accounting from the College of Management in Rishon Lezion and an M.B.A from the Kellogg-Recanati Business School of the Tel Aviv University and is a Certified Public Accountant in Israel.
Shlomo Ayanot has served as our vice president of engineering and operations since 1999. Prior to joining Itamar Medical, between 1997 and 1999, Mr. Ayanot served as vice president of operations at TADIN – Tal Advanced Instruments Ltd., a provider of computerized control systems for production in the microelectronics field. Between 1993 and 1997 Mr. Ayanot served as Vice President of Engineering and Operations of Tamar Electronics Systems Ltd. Between 1983 and 1993, Mr. Ayanot served as production & engineering manager in K&S Industries Ltd. Mr. Ayanot holds a B.Sc degree in industrial management, economic track, from the Technion - Israel Institute of Technology.
Jacob (Koby) Sheffy, PhD, has served as our Senior Vice President, Chief Technology Officer and Chief Scientist, since 1997. Prior to joining Itamar Medical, Dr. Sheffy held senior positions at the Israeli Navy, the Division of Missiles at Rafael - Advanced Defense Systems Ltd. (the Armament Development Authority) and was a research fellow in the School of Engineering at Oxford, UK. Prior to joining Itamar Medical, Dr. Sheffy held a position as the research and development director of the Sleep medicine center at the Technion - Israel Institute of Technology, and also acted as the manager of one of its sleep labs. Dr. Sheffy holds a PhD in biomedical engineering and physiology from Oxford and a B.Sc in electrical engineering from the Technion - Israel Institute of Technology.
| 88 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Itay Kariv has served as our Vice President of Research and Development since October 2018. Between January 2015 and October 2018, Mr. Kariv served as our Vice President of Advanced Research and Development. Mr. Kariv has more than 25 years of experience in research and development managerial roles. Prior to joining Itamar Medical, Mr. Kariv held several managerial positions, including as a Program Director at St. Jude Medical between 2008 and 2014 and as Research and Development Director and subsequently as Program Director at Biosense Webster, a Johnson & Johnson company, between 2001 and 2008. Prior to that, between 1999 and 2001, Mr. Kariv served as Vice President of Research and Development at MeetU.com, Ltd., and as Vice President of Research and Development at Lognet Systems Ltd. between 1997 and 1999. Mr. Kariv holds a Landscape Architect degree and a B.Sc and M.Sc in Computer Science, all from the Technion - Israel Institute of Technology.
Efrat Litman has served as our Vice President of Advanced Research and Development since October 2018. Between April 2017 and October 2018, Ms. Litman served as our Vice President of Research, Development and Technology and from March 2011 to April 2017 as Vice President of Research and Development. Ms. Litman has 25 years of experience in research and development work. Prior to joining Itamar Medical, Ms. Litman held several positions as a project and product manager and algorithm team leader in high-tech and bio-tech industries and the Israel Defense Force, including over eight years at Orbotech Ltd. Ms. Litman holds a B.Sc degree in Physics and Mathematics from the Talpiot program of the Hebrew University of Jerusalem.
Eilon Livne has served as our Vice President of Sales and Channels Development, EMEA Region since 2015. Between 2014 and 2015, Mr. Livne served as our Head of Wellness Activity, USA. Between 2013 and 2014, Mr. Livne served as the VP Sales and Marketing of Adhestick Ltd., leading the international sales and marketing of its consumer goods products line. Between 2002 and 2013, Mr. Livne served as the CEO of Silverline Jewellery Ltd. Between 2001 and 2002, Mr. Livne served as Product Manager of Giteko Technologies, and prior to that, he served as Senior Consultant at the Governmental Incentives Department at Ernst & Young Israel. Mr. Livne holds a B.A. degree in Economy and Accountancy from the Rupin Academic Institute and is a Certified Public Accountant in Israel.
Martin S. Gerstel has served as a director on our Board of Directors since 1997. Between 1997 and, 2016, Mr. Gerstel also served as the Co-Chairman of our Board of Directors. Mr. Gerstel also serves as the Chairman of the Board of Directors of Evogene Ltd. (NASDAQ:EVGN, TASE:EVGN), a developer of novel products for life science markets since 2004. In addition, between 1997 and 2017, Mr. Gerstel served as the Chairman of the Board of Directors of Compugen Ltd., (NASDAQ:CGEN, TASE:CGEN), a predictive drug discovery and development company. Between 2009 and 2010, Mr. Gerstel also served as Compugen’s Chief Executive Officer (and Co-Chief Executive Officer). Between 2004 and 2006, Mr. Gerstel served as chairman of Keddem Bioscience Ltd., a drug discovery company. In addition, Mr. Gerstel currently serves as a director of YEDA Research and Development Company Ltd., the technology transfer company for the Weizmann Institute of Science. Mr. Gerstel also served as a director of Yissum Ltd., the technology transfer company of the Hebrew University of Jerusalem, between 2003 and 2015. Mr. Gerstel is also a member of the Board of Governors and the executive committee of the Weizmann Institute of Science and the Board of Governors of the Hebrew University of Jerusalem. Prior to relocating to Israel in 1994, Mr. Gerstel was the Co-Chairman and CEO of ALZA Corporation, a U.S. pharmaceutical company specializing in advanced drug delivery (sold to Johnson & Johnson). Mr. Gerstel holds an M.B.A. degree from Stanford Graduate School of Business and a B.Sc from Yale University.
Ilan Biran has served as a director on our Board of Directors since 2013. Mr. Biran also serves as a director of Bezeq - The Israel Communications Company Ltd. and certain of its subsidiaries since April 2018 and has previously served as Bezeq’s Chief Executive Officer. Mr. Biran serves as a director of Kinneret College on the Sea of Galilee (R.A). Mr. Biran has previously served as the Chairman of the Board of Directors of Rafael - Advanced Defense Systems Ltd. and D.B.S. Satellite Services (1998) Ltd and as an external director on the Board of Directors of Israel Discount Bank Ltd. Mr. Biran served in the Israel Defense Forces for 32 years, most notably as the former General Director of the Ministry of Defense, and in various staff and command positions, including commanding general, central command, head of the technology and logistics branch, and head of the operations division at the general staff. Mr. Biran has received an honorary degree from the Technion Israel Institute of Technology in 2013. Mr. Biran holds an Associate Diploma in Strategy and Political Economic Research from Georgetown University and the U.S. Marine Corps Command and Staff College. Mr. Biran also holds a B.A. in Economics and Business Administration from the Bar Ilan University.
Jonathan Kolber has served as a director on our Board of Directors since 2015. Mr. Kolber is a general partner of Viola Growth, a technology buyout and growth capital fund that is an affiliate of the Viola Group. Between 1986 and 1997, Mr. Kolber was a founder and manager of Claridge Israel, which invested in Teva Pharmaceuticals, ECI Telecom, Osem and Optrotech. In 1998, Mr. Kolber became the Chief Executive Officer of Koor Industries, one of Israel’s largest conglomerates, which was sold to the IDB Group in 2006. Mr. Kolber has served as chairman, chief executive officer and director in over 40 public and private companies in Israel and North America. He is a director of the Peres Center for Peace and the Chairman of the Friends of the Tel Aviv Medical Center. Mr. Kolber also serves as a member of the Board of Directors of Optimax Ltd., Aeronautics Systems Ltd., Anfield Ltd., Isrex (94) Ltd., and Koortrade Ltd. He holds a Bachelor’s degree in Near Eastern Language and Literature from Harvard University and a Certificate of Advanced Arabic Language from the American University of Cairo.
| 89 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Sami Totah has served as a director on our Board of Directors since 2015. Mr. Totah is a partner of Viola Growth, a technology buyout and growth capital fund that is an affiliate of the Viola Group. Mr. Totah has served as chairman of the board of directors of several Israeli start-up companies since 2003, including Pilat Media, Sheer Networks, Red Bend, and Flash Networks. Between 1984 and 2002, Mr. Totah served in various positions at Amdocs, including the position of Chief Operating Officer. Mr. Totah is a practical software engineer and participated in professional courses over the years, including courses of the Executive M.B.A. program of the Hebrew University of Jerusalem Business School.
Christopher M. Cleary has served as a director on our Board of Directors since 2017. Since 2014, Mr. Cleary has served as the Vice President of Corporate Development for Medtronic plc. Prior to 2014, Mr. Cleary was the CEO for Alesia Capital Services LLC, providing advisory and financial analysis services to Fortune 500 companies, including Medtronic. Prior to that Mr. Cleary served in a multitude of managerial roles at GE Capital. Mr. Cleary holds a B.A. from Colorado College.
Yaffa Krindel Sieradzki has served as an external director on our Board of Directors since 2016. Since February 2018, Ms. Krindel has also served as a director of Sol-Gel Technologies Ltd. (NASDAQ:SLGL), a pharmaceutical company, BGN Technologies Ltd., the technology transfer company of Ben Gurion University, and two medical device start-up companies. Between 1997 and 2007, Ms. Krindel served as Partner and Managing Partner of Star Ventures, a private venture capital fund headquartered in Munich, Germany. Between 1993 and 1997, Ms. Krindel served as CFO and later as director of BreezeCOM Ltd., an Israeli telecommunications company, which was traded on Nasdaq and the TASE. Between 1992 and 1996, Ms. Krindel served as CFO and VP Finance of Lannet Data Communications Ltd., an Israeli telecommunications company, publicly traded on Nasdaq which is now part of Avaya Inc. Ms. Krindel also served on the board of directors of Fundtech Ltd., which was traded on Nasdaq until its acquisition by GTCR, Voltaire Ltd. until its acquisition by Mellanox Technologies Ltd. and Syneron Medical until its acquisition by Apax. Ms. Krindel holds an M.B.A. degree from the Tel Aviv University and a B.A. in Economics and Japanese Studies from the Hebrew University of Jerusalem.
Zipora (Tzipi) Ozer-Armon has served as an external director on our Board of Directors since 2016. She currently serves as the Chief Executive Officer of Lumenis, a position she has held since joining Lumenis in May 2012. Prior to joining Lumenis, Ms. Ozer-Armon held various management positions at Teva Pharmaceutical Industries Ltd. since October 2009, most recently serving as head of Teva’s Japanese market activities. Previously, Ms. Ozer-Armon held various management positions at SanDisk Corporation, following its acquisition of M-Systems Ltd., between 2006 and 2008, including Senior Vice President, Retail Sales and Marketing. Prior thereto, between 2004 and 2006, Ms. Ozer-Armon served as Corporate Vice President, General Manager of the DiskOnKey division at M-Systems Ltd. and between 1999 and 2004 as Vice President of Corporate Development at Comverse Inc. Between 1995 and 1999, Ms. Ozer-Armon served as Vice President at Shaldor Ltd., a management consulting firm based in Israel and between 1991 and 1995, Ms. Ozer-Armon served as Vice President at the London office of A.T. Kearney, a global management consulting firm. In addition, Ms. Ozer- Armon served as an external director on the Board of Directors of Cargal Ltd., which was a TASE-listed company and was a member of its audit committee between February 2012 and December 2013. Ms. Ozer-Armon holds a B.A. degree in economics, magna cum laude, and an M.B.A. degree, majoring in finance and marketing, both from the Tel Aviv University.
| 90 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Additional Information
There are no family relationships between any of the directors or members of senior management named above.
Our articles of association provide for a Board of Directors of not less than five (5) and not more than nine (9) members, including two external directors as required by the Companies Law. Our Board of Directors is currently composed of eight (8) directors (including the two (2) external directors). Officers serve at the pleasure of the Board of Directors, subject to the terms of any agreement between the officer and us.
Dr. Yaron and Messrs. Gerstel, Biran, Kolber, Cleary and Totah will serve as directors until our annual general meeting of shareholders to be held in 2019. Ms. Krindel Sieradzki and Ms. Ozer-Armon were elected as external directors in June 2016 for a three-year term.
We are not aware of any arrangements or understandings with major shareholders, customers, suppliers or others, pursuant to which any person referred to above was selected as a director or member of senior management.
| B. | Compensation |
Aggregate Executive Compensation
Our objective is to attract, motivate and retain highly skilled personnel who will assist Itamar Medical to reach its business objectives, performance and the creation of shareholder value and otherwise contribute to its long-term success. In March 2016, our shareholders approved an amended compensation policy for our executive officers and directors, or the Compensation Policy. The Compensation Policy was designed to correlate executive compensation with Itamar Medical’s objectives and goals and otherwise embrace a performance culture that is based on merit, and differentiates and rewards excellent performance in the long term.
On October 9, 2018, we held a special general meeting of our shareholders. At the meeting, our shareholders approved amendments to the Compensation Policy relating to the criteria for our purchase of directors and officers liability insurance, primarily in order to (1) increase the maximum annual premium we may pay for such insurance from $100,000 to $150,000 (or $350,000 for as long as we are subject to the SEC reporting requirements), and (2) allow us to purchase “run-off” directors and officers liability insurance in special events, such as public offerings or sale of our Company, provided such premium shall not exceed $600,000.
The following table sets forth all compensation we paid with respect to all of our directors and executive officers as a group for the periods indicated:
| Salaries, fees, commissions and bonuses | Pension, retirement and similar benefits | Share-based compensation | ||||||||||
| (dollars in thousands) | ||||||||||||
| 2017 - All directors and executive officers as a group, consisting of 16 persons(1) for the year ended December 31, 2017 | $ | 1,579 | $ | 105 | $ | 1,175 | ||||||
| 2016 - All directors and executive officers as a group, consisting of 15 persons(2) for the year ended December 31, 2016 | $ | 1,791 | $ | 121 | $ | 1,242 | ||||||
| (1) | Includes three persons who are no longer serving as one of our directors or executive officers and excludes two executive officers which were appointed during 2018. |
| (2) | Includes three persons who are no longer serving as one of our directors or executive officers and excludes one executive officer who joined us in May 2017. |
We provide leased cars or reimbursement of car expenses to our executive officers in Israel and reimbursement of other expenses pursuant to our standard policies and procedures.
| 91 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
During 2017, we granted to our directors and officers listed in Item 6A above:
| · | options to purchase, in the aggregate, 2,447,412 ordinary shares at a weighted average exercise price per share of NIS 1.25 (equivalent to $0.34), of which (i) 1,529,864 are performance-based options that will vest in December 2021 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such options will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 per share (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the options will vest, and (ii) 917,548 options will vest over a period of four years following the grant date. Of the 2,447,412 options, 550,000 options will expire five years from the grant date (i.e., in May 2022) and the balance of 1,897,412 options will expire in January 2026. The weighted average fair value of these options as of the grant date was $0.17 per option; and |
| · | 337,542 ordinary shares issuable upon the vesting of outstanding performance-based RSUs, which will vest in December 2020 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such RSUs will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 per share (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the RSUs will vest. The weighted average fair value of these RSUs as of the grant date was $0.11 per RSU. |
For a discussion of the accounting method and assumptions used in valuation of such options and RSUs, see Note 15 to our audited consolidated financial statements included elsewhere in this registration statement. See also “Item 6.E. - Directors, Senior Management and Employee – Share Ownership –– Equity Incentive Plans” below.
In addition, since January 1, 2018, we also granted to our directors and officers listed in Item 6A above:
| · | options to purchase, in the aggregate, 1,165,256 ordinary shares at a weighted average exercise price per share of NIS 1.09 (equivalent to $0.30), of which (i) 496,882 are performance-based options that will vest in December 2021 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such options will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 per share (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the options will vest, and (ii) 668,374 options will vest over a period of four years following the grant date. Of the 1,165,256 options, 550,000 options will expire five years from the grant date (i.e., in May 2023) and the balance of 615,256 options will expire in January 2026. The weighted average fair value of these options as of the grant date was $0.17 per option; and |
| · | 115,036 ordinary shares issuable upon the vesting of outstanding performance-based RSUs, which will vest in December 2020 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such RSUs will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 per share (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the RSUs will vest. The weighted average fair value of these RSUs as of the grant date was $0.11 per RSU. |
| 92 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Individual Compensation of Covered Executives
The table and summary below outline the compensation granted, and which we have previously publicly disclosed, to our most highly compensated “office holders” during or with respect to the year ended December 31, 2017. The Companies Law defines the term “office holder” of a company to include a director, the chief executive officer, the chief business manager, a vice president and any officer that reports directly to the chief executive officer. We refer to the individuals for whom disclosure is provided herein as our “Covered Executives.” We note that under the Companies Law, once our ADSs will become listed on the Nasdaq Capital Market, we will be required to disclose the compensation granted to our five most highly compensated office holders in the proxy statements we publish for our annual shareholders meetings.
For purposes of the table and the summary below, “compensation” includes base salary, bonuses (including sales commissions), equity-based compensation, retirement or termination payments, benefits and perquisites such as car, and social benefits and any undertaking to provide such compensation. All amounts reported in the table are in terms of cost to the Company, as recognized in our financial statements for the year ended December 31, 2017.
| Name and Principal Position (1) | Annual Base Salary (2) | Bonus (3) | Equity-Based Compensation (4) | All Other Compensation (5) | Total | |||||||||||||||
| (dollars in thousands) | ||||||||||||||||||||
| Gilad Glick, President and Chief Executive Officer (6) (7) | 321 | 69 | 613 | 20 | 1,023 | |||||||||||||||
| Shy Basson, Chief Financial Officer | 125 | - | 61 | 43 | 229 | |||||||||||||||
| Jacob Sheffy, PhD, Senior Vice President of Research and Chief Technology Officer | 178 | - | 57 | 57 | 292 | |||||||||||||||
| * | Since all or part of the compensation may be denominated in currencies other than the dollar, fluctuations in dollar amounts may be attributed to exchange rate fluctuations. In particular, for purposes of this table, cash compensation amounts denominated in currencies other than the dollar were converted into dollars at an exchange rate of NIS 3.5997 per $1.00, which reflects the average applicable conversion rate for 2017. |
| (1) | Unless otherwise indicated herein, all Covered Executives are engaged on a full-time (100%) basis. |
| (2) | Reflects the annual gross salary of the Covered Executives, other than (i) Mr. Glick, who is engaged through a consultancy agreement, where such figure reflects the annual fixed compensation and (ii) Mr. Basson, whose employment period started on May 1, 2017. |
| (3) | Amounts reported in this column represent annual bonuses granted to the Covered Executives. Consistent with our Compensation Policy, such bonuses are based upon (i) for the CEO – see footnote 6 below; and (ii) for the other executive officers - achievement of targets of revenues generated by the individual and/or his/her team or division and/or the Company, as well as, in appropriate circumstances, other measurable criteria, which, in general, may not exceed six monthly salaries. |
| (4) | Amounts reported in this column represent the accounting expense recognized by the Company associated with stock-based compensation in accordance with accounting guidance for stock-based compensation. For a discussion of the assumptions used in reaching this valuation, see Note 15 to our audited consolidated financial statements. All of the awards were in the form of stock options or RSUs, and were made pursuant to one of our equity incentive plans. Vesting of the options and RSUs will accelerate upon certain change of control events. |
| 93 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| (5) | Amounts reported in this column include benefits and perquisites, including those mandated by applicable law. Such benefits and perquisites may include, to the extent applicable to the Covered Executive, payments, contributions and/or allocations for savings funds (e.g., Managers Life Insurance Policy), education funds (“Keren Hishtalmut”), pension, severance, vacation, car or car allowance, medical insurances and benefits, risk insurances (e.g., life, or work disability insurance), convalescence or recreation pay, relocation, employers payments for social security, tax gross-up payments and other benefits and perquisites consistent with Itamar Medical’s guidelines. |
| (6) | Consistent with our Compensation Policy, and as approved by our shareholders in May 2018, Mr. Glick is entitled to an annual bonus, subject to Mr. Glick achieving certain criteria and milestones set by our Compensation Committee and Board of Directors. The milestone for the annual bonus for the years 2018 through 2022 is based upon our annual revenue in such years, which is tied to our annual budget for the applicable year. The annual bonus payable to Mr. Glick for each year may not exceed an amount equal to 7.7 monthly salaries of Mr. Glick in such year, which currently equates to a maximum annual bonus of approximately $187,500. |
| (7) | As approved by our shareholders, Mr. Glick received in March 2016 (as amended in May 2018) a grant of (i) options to purchase up to 2,043,111 ordinary shares, at an exercise price of NIS 1.55 (equivalent to $0.42), of which 510,778 options vest one year after the grant date, with the balance vesting in 12 equal quarterly installments; and (ii) 10,080,824 ordinary shares issuable upon the vesting of outstanding performance-based options and RSUs (consisting of 8,388,512 options with an exercise price of NIS1.40 (equivalent to $0.38) and 1,692,512 RSUs with an exercise price of NIS 0.30 (equivalent to $0.08)), which will vest on December 20, 2020, if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such options and RSUs will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the RSUs will vest. These options and RSUs expire in 2026. Vesting of the options and RSUs will accelerate upon certain change of control events. |
We have entered into written employment or services agreements with each of our executive officers. All of these agreements contain customary provisions regarding confidentiality, intellectual property assignment and non-solicitation provisions as well as an undertaking not to compete with us or in our field of business. However, the enforceability of the noncompetition provisions may be limited under applicable law. Members of our senior management may also be eligible for bonuses in accordance with our Compensation Policy and as set forth by our Compensation Committee and Board of Directors.
On May 23, 2018, we held our annual meeting of shareholders for 2018, at which our shareholders approved, among other matters, the following changes to the compensation payable to Mr. Glick, our President and Chief Executive Officer:
base salary - effective April 1, 2018, the monthly payment shall be denominated in NIS (rather than in dollars) and such payment shall increase by 10% (at the time of the shareholder approval), from a monthly payment of $26,176 plus VAT to NIS 102,450 (equivalent to approximately $27,900) plus VAT; This amount includes the total cost of social benefits payable to Mr. Glick;
| · | modification of the performance criteria related to the vesting of stock options and RSUs previously granted to our President and Chief Executive Officer – Mr. Glick received in March 2016 a grant of (i) options to purchase up to 2,043,111 ordinary shares, at an exercise price of $0.42, of which 510,778 options vest one year after the grant date, with the balance vesting in 12 equal quarterly installments; and (ii) 10,080,824 ordinary shares issuable upon the exercise of 8,388,512 outstanding performance-based stock options and the vesting of 1,692,312 outstanding performance-based RSUs, which will vest on January 21, 2020 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such options and RSUs will vest if the price of our ordinary shares is, at such time, at least NIS 2.13 per share (equivalent to $0.49). At the annual meeting, our shareholders approved that (i) the above minimum trading price will be reduced from NIS 2.13 (equivalent to $0.49) to NIS 1.70 (equivalent to $0.46) and (ii) a change of the January 21, 2020 vesting date to December 20, 2020; and |
| · | an annual cash bonus for the years 2018 through 2022 – At the annual meeting, our shareholders approved that Mr. Glick will be entitled to an annual cash bonus in each of the years 2018 to 2022 (inclusive), or the bonus years, as follows: |
| 94 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| o | a maximum bonus of up to 7.7 monthly base salaries (excluding the social benefits component) per year (which, based on his current monthly base salary, equates to $187,500). |
| o | The bonus is payable subject to meeting sales revenue goals that reflect growth in our revenues at a rate to be determined by the Compensation Committee and the Board of Directors by the end of the first quarter of each bonus year as part of the annual budget approval. The sales revenue goal for each bonus year is divided into three levels of sales revenues: the minimum goal, the target goal and the maximum entitlement goal. |
| o | In the event that the actual sales revenues in any bonus year are within the range between two goals (the minimum goal and the target goal or between the target goal and the maximum entitlement goal), the amount of the bonus shall be calculated linearly based on the increase in sales revenue in that bonus year. |
| o | Payment of the bonus is also contingent on meeting a minimum operating income or a maximum operating loss goal. Such operating income or operating loss is on an adjusted, non-IFRS basis, which neutralizes certain non-cash and non-recurring components. |
| o | For the purpose of examining compliance with the said goals at the end of each relevant year, the effects of the following events (relative to that bonus year’s budget) will be neutralized: (1) an increase our expenses for clinical trials (both in view of the entry into a new clinical trial and in light of the expansion of existing clinical trial); (2) increase or decrease in our costs in respect of payments to sales personnel (including costs of recruiting new sales personnel); (3) expenses associated with changing the reimbursement policy of medical insurers during the budget year and/or changes in the standard requirements applicable to our products; (4) expenses related to the process of listing on Nasdaq; (5) expenses incurred by our Company in respect of listing of securities for trading or sale in the United States solely for sales by our shareholders that exercise their registration rights or expenses in respect of unsuccessful capital raising; and (6) expenses related to the annual bonus to our chief executive officer or to any other officer in that year. |
| o | The annual bonus is payable once a year, following the approval of our annual financial statements for the preceding year. |
Compensation of Non-Employee Directors
All of our directors are entitled to reimbursement of expenses. In addition, other than Mr. Cleary (who is entitled only to reimbursement of expenses), our non-employee directors, including external directors, receive the following compensation:
| · | Dr. Yaron, the chairman of our Board of Directors, is entitled, pursuant to the consultancy agreement we entered into with a company wholly owned by Dr. Yaron in March 2001 (as amended), to a monthly payment of $6,250, plus VAT. Under the agreement, Dr. Yaron is required to provide us with consulting services, including service as a chairman of our Board of Directors, on a part-time basis of 20% of the work week. |
| · | Mr. Biran, Ms. Krindel Sieradzki and Ms. Ozer-Armon are each entitled to an annual fee of NIS 48,825 (equivalent to approximately $13,300) and attendance fees of NIS 3,265 (equivalent to approximately $900) per meeting attended, linked to the Israeli CPI. |
| · | Messrs. Gerstel, Kolber and Totah are each entitled (in the case of Messrs. Kolber and Totah, by payment to an affiliate of Viola) to an annual fee of NIS 36,675 (equivalent to approximately $10,000) and attendance fees of NIS 2,455 (equivalent to approximately $700) per meeting attended, linked to the Israeli CPI. |
| 95 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
According to the Compensation Policy, directors and officers may be granted equity based compensation subject to certain criteria and limitations set forth therein, including the following:
| · | grants may be made not more than twice a year for officers and not more than once a year for directors; |
| · | equity-based awards shall vest as determined by us at the time of grant. However, other than in the event of acceleration, no portion of any grant may vest prior to the end of the one year anniversary of the date of grant or from the commencement date of the directors’ or officers engagement with us; |
| · | the equity-based award shall have a fair value that will not exceed, with respect to each year of vesting (measured on a linear basis), the equivalent of (i) the value of 18 months’ salary with respect to the chief executive officer, (ii) six months’ salary with respect to each other officer, and (iii) NIS 300,000 (equivalent to approximately $81,600) for each director; and |
| · | the exercise price of options whose vesting is subject to the passage of time (and not subject to meeting milestones) will be no less than the average fair market value of the ordinary shares for the thirty (30) trading days prior to the date such grant was approved by our Board of Directors multiplied by 105%. |
Consistent with the Compensation Policy and as further approved by our shareholders, we made the following grants of equity-based awards to our non-employee directors since January 1, 2017:
| · | 550,000 options to three directors (namely, to Mr. Biran and to Viola Growth Management Fund 2 Ltd., or Viola 2, in respect of the services of Messrs. Kolber and Totah) in May 2017 at an exercise price of NIS 1.45 (equivalent to $0.39) per share. These options will vest over a period of four years following the grant date and will expire five years from the grant date (i.e., in May 2022); and |
| · | 550,000 options to three directors (namely, to Mr. Biran and to Viola 2 in respect of the services of Messrs. Kolber and Totah) in May 2018 at an exercise price of NIS 1.14 (equivalent to $0.31) per share. These options will vest over a period of four years following the grant date and will expire five years from the grant date (i.e., in May 2023). |
The aforesaid stock options granted to the non-employee directors are part of a grant that was divided into three equal tranches. The allotment and the vesting period for the first tranche begins on the date of grant (550,000 options granted in May 2017); the allotment and the vesting period for the second tranche begins on first anniversary of the date of grant (550,000 options granted in May 2018); and the allotment and the vesting period for the third tranche begins on the third anniversary of the date grant (550,000 options that will be granted in May 2019). Each tranche vests in four equal portions annually over four years. The exercise price for each tranche is set on the date of allotment and is based on the average market price of our ordinary shares on the TASE prior to such allotment date, plus 10%.
For additional details regarding the stock options granted to non-employee directors, see Note 15a to our audited consolidated financial statements included elsewhere in this registration statement.
Other than the foregoing fees, reimbursement for expenses and the award of stock options, we do not compensate our directors for serving on our Board of Directors.
Change of Control Arrangements
All of our executive officers as well as certain additional key employees are entitled to accelerated vesting of the ordinary shares subject to outstanding options and RSUs granted to them in connection with a sale of the Company or similar change of control events.
| 96 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| C. | Board Practices |
Introduction
According to the Companies Law and our articles of association, the management of our business is vested in our Board of Directors. The Board of Directors may exercise all powers and may take all actions that are not specifically granted to our shareholders and, according to the Companies Law and our articles of association is primarily responsible for outlining our policies and supervising our chief executive officer.
Election of Directors; Board Meetings
Under our articles of association, our Board of Directors must consist of not less than five (5) and not more than nine (9) members, including two external directors as required by the Companies Law. Our Board of Directors is currently composed of eight (8) directors (including two (2) external directors). Pursuant to applicable Nasdaq rules, following our listing on the Nasdaq Capital Market, director nominees will be recommended for the Board of Directors’ selection by a majority of our “independent directors” within the meaning of the Nasdaq rules.
Pursuant to our articles of association, other than the external directors, for whom special election and removal requirements apply under the Companies Law (as described below), the vote required to appoint a director is a simple majority vote of holders of our ordinary shares participating and voting at the relevant shareholders meeting. Our articles of association provide that, unless otherwise provided by law, our directors (other than external directors and “independent directors” as such term is defined by the Companies Law) may be elected solely at our shareholders annual general meetings, which are required to be held at least once during every calendar year and not more than fifteen months after the last preceding annual general meeting. However, our articles of association allow our Board of Directors to appoint directors to fill vacancies on our Board of Directors, which occurred for any reason, or as additional directors, provided that the number of board members shall not exceed the maximum number of directors, as mentioned above. The appointment of a director by the Board of Directors shall remain in effect until the annual general meeting of our shareholders following the appointment or until the end of his tenure, in accordance with our articles of association.
Except for our external directors (as described below), our directors hold office until the next annual meeting of shareholders following the annual meeting at which they were appointed.
Under our articles of association and the Companies Law, (i) directors (other than external directors and “independent directors” as such term is defined by the Companies Law) may be removed by our shareholders before the expiration of their term by a special majority vote of at least 75% of the votes of shareholders present and voting at the meeting, not taking into account abstentions; (ii) external directors may be removed by our shareholders before the expiration of their term only in limited circumstances as described under the section titled “External Directors” below; and (iii) “independent directors” (as such term is defined by the Companies Law) may be removed before the expiration of their term only by a simple majority of the shareholders, or by a court, and then only if the independent directors cease to meet the statutory qualifications with respect to their appointment or if they violate their duty of loyalty to the Company. In addition, under the Companies Law, directors may be removed upon the occurrence of disqualifying events, such as bankruptcy or conviction of the director in certain criminal offenses.
Under the Companies Law, our Board of Directors is required to determine the minimum number of directors who must have “accounting and financial expertise” (as such term is defined in regulations promulgated under the Companies Law). Our Board of Directors determined that the Board of Directors should consist of at least two directors who have “accounting and financial expertise”. In this respect, our Board of Directors has determined that each of Ms. Krindel Sieradzki, Ms. Ozer-Armon and Mr. Ilan Biran have the requisite “accounting and financial expertise”.
Meetings of the Board of Directors are generally held at least once each quarter, with additional special meetings scheduled when required.
| 97 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Alternate directors
Our articles of association provide, as allowed by the Companies Law, that any director may, by written notice to us, appoint another person who is qualified to serve as a director to serve as an alternate director. The alternate director will be regarded as a director. However, the appointment of an alternate director does not negate the responsibilities of the appointing director and such responsibilities prior to the appointment will continue to be the responsibilities of the appointing director, giving consideration to the circumstances of the appointment. Under the Companies Law, a person who is not qualified to be appointed as a director, a person who is already serving as a director or a person who is already serving as an alternate director for another director, may not be appointed as an alternate director. Nevertheless, a director who is already serving as a director may be appointed as an alternate director for a member of a committee of the Board of Directors so long as he or she is not already serving as a member of such committee, and if the alternate director is to replace an external director, he or she is required to be an external director and to have either “accounting and financial expertise” or “professional qualifications,” depending on the qualifications of the external director he or she is replacing. The term of appointment of an alternate director may be for one meeting of the Board of Directors or until notice is given of the cancellation of the appointment.
External Directors
The Companies Law requires Israeli companies with shares that have been offered to the public, such as Itamar Medical, to appoint at least two external directors. Effective from April 2016, companies whose shares are traded on specified U.S. stock exchanges, including Nasdaq, and which do not have a controlling shareholder, may (but are not required to) elect to opt out of the requirement to maintain external directors or retain external directors but opt out of the composition requirements under the Companies Law with respect to either or both of the audit and compensation committees. We currently do not qualify for such exemption because, under the Companies Law, Viola is considered a controlling shareholder of our Company.
To qualify as an external director, an individual (or the individual’s relative, partner, employer or any entity under the individual’s control) may not have, and may not have had at any time during the previous two years, (i) in a company such as Itamar Medical (where Viola is considered a controlling shareholder according to the Companies Law), any “affiliation” with the company, the company’s controlling shareholder or its relative, or another entity affiliated with the company or its controlling shareholder, or (ii) in a company without a controlling shareholder (or a shareholder that owns more than 25% of its voting power), any “affiliation” with any person who, at the time of appointment, is the chairman, the chief executive officer, the chief financial officer or a 5% shareholder of the company. The term affiliation includes:
| · | an employment relationship; |
| · | a business or professional relationship; |
| · | control; and |
| · | service as an “office holder,” excluding service as a director that was appointed to serve as an external director of a company that is about to make its initial public offering. |
In addition, pursuant to the Companies Law, (i) an external director must have either “accounting and financial expertise” or “professional qualifications” (as such terms are defined in regulations promulgated under the Companies Law; and (ii) at least one of the external directors must have “accounting and financial expertise”. Our external directors are Ms. Krindel Sieradzki and Ms. Ozer-Armon. We have determined that both Ms. Krindel Sieradzki and Ms. Ozer-Armon have the requisite “accounting and financial expertise”.
| 98 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
No person may serve as an external director if the person’s position or other activities create, or may create a conflict of interest with the person’s responsibilities as an external director or may otherwise interfere with the person’s ability to serve as an external director. If, at the time an external director is to be appointed, all current members of the Board of Directors who are not controlling shareholders or their relatives are of the same gender, then the external director must be of the other gender.
External directors are elected by shareholders. The shareholders voting in favor of their election must include at least a majority of the shares of the non-controlling shareholders of the company who voted on the matter. This minority approval requirement need not be met if the total shareholdings of those non-controlling shareholders who vote against their election represent 2% or less of all of the voting rights in the company.
The initial term of an external director is three years and he or she may be reelected for up to two additional three-year terms. Thereafter, in a company whose shares are listed for trading on, among others, the Nasdaq Capital Market, such as Itamar Medical, he or she may be reelected by our shareholders for additional periods of up to three years each, if our Audit Committee and the Board of Directors confirm that, in light of the external director’s expertise and special contribution to the work of the Board of Directors and its committees, the reelection for such additional period is beneficial to the Company. Reelection of an external director may be effected through one of the following mechanisms: (i) the Board of Directors proposed the reelection of the nominee and the election was approved by the shareholders by the majority required to appoint external directors for their initial term as described above; or (ii) a shareholder holding 1% or more of the voting rights proposed the reelection of the nominee or the external director himself or herself proposed their own reelection, and the reelection is approved by a majority of the votes cast by the shareholders of the company, excluding the votes of controlling shareholders and those who have a personal interest in the matter as a result of their relations with the controlling shareholders; provided that the aggregate votes cast in favor of the reelection by such non-excluded shareholders constitute more than 2% of the voting rights in the company.
External directors can be removed from office only by the same special percentage of shareholders as can elect them, or by a court, and then only if the external directors cease to meet the statutory qualifications with respect to their appointment or if they violate their duty of loyalty to the company.
Any committee of the Board of Directors must include at least one external director, except that the audit and compensation committees must include all of the external directors. An external director is entitled to compensation as provided in regulations adopted under the Companies Law and is otherwise prohibited from receiving any other compensation, directly or indirectly, in connection with such service.
Independent Directors
Under the Nasdaq rules, a majority of our Board of Directors must qualify as independent directors within the meaning of Nasdaq Listing Rule 5605(a)(2). Our Board of Directors has determined that all of our directors qualify as “independent directors” within the meaning of such rule.
Committees of the Board of Directors
Subject to the provisions of the Companies Law, our Board of Directors may delegate its powers to committees consisting of board members. Our Board of Directors has established an audit committee and a compensation committee, and, from time to time, establishes other “ad-hoc” committees of members of the Board of Directors for specific duties or assignments and limited duration.
| 99 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Audit Committee
Pursuant to applicable SEC and Nasdaq rules, we are required to have an audit committee of at least three members, each of whom must satisfy the independence requirements of the SEC and Nasdaq. In addition, pursuant to Nasdaq rules, all of the members of the audit committee must be financially literate and at least one member must possess accounting or related financial management expertise. The audit committee must also have a written charter specifying the committee’s duties and responsibilities, which include, among other things, the selection and evaluation of our independent auditors.
Under the Companies Law, our Board of Directors is required to appoint an audit committee, which must be comprised of at least three directors, include all of the external directors, a majority of its members must satisfy the independence standards under the Companies Law, and the chairman is required to be an external director. The duties of the audit committee under the Companies Law include, among others, examining flaws in the business management of the company and suggesting remedial measures to the Board, assessing the Company’s internal audit system and the performance of its internal auditor, and, as more fully described under Item 10.B. below, approval of certain interested party transactions.
Our Audit Committee adopted a written charter (to be effective upon the listing of our ADSs on the Nasdaq Capital Market) specifying the committee’s duties and responsibilities, which include, among other things, assisting our Board of Directors in overseeing the accounting and financial reporting processes of our Company and audits of our financial statements, including the integrity of our financial statements; compliance with legal and regulatory requirements; our independent public accountants’ appointment, qualifications and independence; the performance of our internal audit function and independent public accountants; finding any defects in the business management of our Company for which purpose the Audit Committee may consult with our independent auditors and internal auditor and proposing to the Board of Directors ways to correct such defects; approving related-party transactions; and such other duties as may be directed by our Board of Directors or required by applicable law.
Under the Companies Law, the board of directors of a public company in Israel must appoint a financial statement examination committee, which consists of members with accounting and financial expertise or the ability to read and understand financial statements. According to a resolution of our Board of Directors, our Audit Committee has been assigned the responsibilities and duties of a financial statements examination committee, as permitted under relevant regulations promulgated under the Companies Law. However, the requirement to appoint such financial statement examination committee does not apply to public companies that choose to comply with the dual reporting regime available for companies whose shares are listed abroad, like Itamar Medical once our ADSs are listed on the Nasdaq Capital Market.
In addition, pursuant to the audit committee charter, our Audit Committee functions as our Qualified Legal Compliance Committee, or the QLCC. In its capacity as the QLCC, the Audit Committee is also responsible for investigating reports made by attorneys appearing and practicing before the SEC in representing us of perceived material violations of U.S. federal or state securities laws, breaches of fiduciary duty or similar violations by us or any of our agents.
Our Audit Committee is currently composed of Ms. Krindel Sieradzki, the chairperson of our Audit Committee, Ms. Ozer-Armon and Mr. Ilan Biran, all of whom satisfy the respective “independence” requirements of the Companies Law, SEC and Nasdaq rules for audit committee members.
| 100 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Compensation Committee
Pursuant to applicable Nasdaq rules, the compensation payable to a company’s chief executive officer and other executive officers must generally be approved by a compensation committee comprised solely of independent directors.
Under the Companies Law, our Board of Directors is required to appoint a compensation committee, which must be comprised of at least three directors, include all of the external directors, its other members must satisfy certain independence standards under the Companies Law, and the chairman is required to be an external director. Under the Companies Law, the role of the compensation committee is to recommend to the Board of Directors, for ultimate shareholder approval by a special majority, a policy governing the compensation of office holders based on specified criteria; to review, from time to time, modifications to the compensation policy and examine its implementation; to approve, as more fully described under “Approval of Related Party Transactions Under Israeli Law” below, the actual compensation terms of office holders prior to approval thereof by the Board of Directors; and to resolve whether to exempt the compensation terms of a candidate for chief executive officer from shareholder approval.
Our Compensation Committee adopted a written charter (to be effective upon the listing of our ADSs on the Nasdaq Capital Market) specifying the committee’s duties and responsibilities, which include, among other things, the duties and roles assigned to it pursuant to the Companies Law and applicable Nasdaq rules described above; and oversight and administration of our equity based plans.
Our Compensation Committee is currently composed of Ms. Ozer-Armon, the chairperson of our Compensation Committee, Ms. Krindel Sieradzki and Mr. Gerstel, all of whom satisfy the respective “independence” requirements of the Companies Law, SEC and Nasdaq rules for compensation committee members. The committee meets at least once each quarter, with additional special meetings scheduled when required.
Internal Audit
Under the Companies Law, our Board of Directors is also required to appoint an internal auditor proposed by the audit committee. The role of the internal auditor is to examine, among other things, whether our activities comply with the law and orderly business procedure. The internal auditor may not be an interested party or office holder, or a relative of any interested party or office holder, and may not be a member of our independent accounting firm. The Companies Law defines the term “interested party” to include a person who holds 5% or more of a company’s outstanding share capital or voting rights, a person who has the right to appoint one or more directors or the general manager, or any person who serves as a director or as the general manager. Ms. Irena Ben-Yakar of Brightman Almagor & Zohar (Deloitte Israel), an Israeli accounting firm, serves as our internal auditor.
Directors’ Service Contracts
Our Chairman of the Board. We entered into a services agreement with a company wholly owned by Dr. Giora Yaron, the Chairman of our Board of Directors. See Item 6.B “Directors, Senior Management and Employees – Compensation – Individual Compensation of Covered Executives.”
Other. Except as set forth above and in Item 6.B “Directors, Senior Management and Employees – Compensation,” there are no arrangements or understandings between us and any of our current directors or executive officers for benefits upon termination of service.
| 101 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Fiduciary Duties of Office Holders
The Companies Law imposes a duty of care and a duty of loyalty on all office holders of a company.
The duty of care requires an office holder to act with the level of skill with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care of an office holder includes a duty to use reasonable means to obtain:
| · | information on the advisability of a given action brought for his approval or performed by him by virtue of his position; and |
| · | all other important information pertaining to these actions. |
The duty of loyalty of an office holder requires an office holder to act in good faith and for the benefit of the company, and includes a duty to:
| · | refrain from any conflict of interest between the performance of his duties in the company and his performance of his other duties or personal affairs; |
| · | refrain from any action that constitutes competition with the company’s business; |
| · | refrain from exploiting any business opportunity of the company to receive a personal gain for himself or others; and |
| · | disclose to the company any information or documents relating to the company’s affairs which the office holder has received due to his position as an office holder. |
Each person listed in the table under Item 6.A “Directors and Senior Management” above is considered an office holder under the Companies Law.
Approval of Related Party Transactions Under Israeli Law
General. Under the Companies Law, a company may approve an action by an office holder from which the office holder would otherwise have to refrain, as described above, if:
| · | the office holder acts in good faith and the act or its approval does not cause harm to the company; and |
| · | the office holder disclosed the nature of his or her interest in the transaction (including any significant fact or document) to the company at a reasonable time before the company’s approval of such matter. |
Disclosure of Personal Interests of an Office Holder. The Companies Law requires that an office holder disclose to the company, promptly, and, in any event, not later than the board meeting at which the transaction is first discussed, any direct or indirect personal interest that he or she may have and all related material information known to him or her relating to any existing or proposed transaction by the company. If the transaction is an extraordinary transaction, the office holder must also disclose any personal interest held by:
| 102 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | the office holder’s relatives. Relatives are defined to include the spouse, siblings, parents, grandparents, descendants, spouse’s descendants and the spouses of any of these people; or |
| · | any corporation in which the office holder or his or her relatives holds 5% or more of the shares or voting rights, serves as a director or general manager or has the right to appoint at least one director or the general manager. |
Under the Companies Law, an extraordinary transaction is a transaction:
| · | not in the ordinary course of business; |
| · | not on market terms; or |
| · | that is likely to have a material impact on the company’s profitability, assets or liabilities. |
The Companies Law does not specify to whom within the company nor the manner in which required disclosures are to be made. We require our office holders to make such disclosures to our Board of Directors.
Under the Companies Law, once an office holder complies with the above disclosure requirement, the Board of Directors may approve a transaction between the company and an office holder, or a third party in which an office holder has a personal interest, unless the articles of association provide otherwise and provided that the transaction is not detrimental to the company’s interest. If the transaction is an extraordinary transaction, first the audit committee and then the board of directors, in that order, must approve the transaction. Under specific circumstances, shareholder approval may also be required. A director who has a personal interest in an extraordinary transaction, which is considered at a meeting of the board of directors or the audit committee, may not be present at this meeting or vote on this matter, unless a majority of the Board of Directors or the audit committee, as the case may be, has a personal interest. If a majority of the Board of Directors has a personal interest, then shareholder approval is generally also required.
Approval of Office Holder Compensation. Pursuant to the Companies Law, every Israeli public company, such as Itamar Medical, must adopt a compensation policy, recommended by the compensation committee, and approved by the Board of Directors and the shareholders, in that order. The shareholder approval requires a majority of the votes cast by shareholders, excluding any controlling shareholder and those who have a personal interest in the matter. In general, all office holders’ terms of compensation – including fixed remuneration, bonuses, equity compensation, retirement or termination payments, indemnification, liability insurance and the grant of an exemption from liability – must comply with the company’s compensation policy. In March 2016, our shareholders approved the Compensation Policy.
In addition, the compensation terms of directors, the chief executive officer, and any employee or service provider who is considered a controlling shareholder, must be approved separately by the compensation committee, the Board of Directors and, subject to certain exceptions, the shareholders of the company (by the same majority noted above), in that order. The compensation terms of other officers require the approval of the compensation committee and the board of directors.
| 103 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Exculpation, Indemnification and Insurance of Directors and Officers
Exculpation of Office Holders. Under the Companies Law, an Israeli company may not exempt an office holder from his or her liability for a breach of the duty of loyalty to the company, but may exempt an office holder, in advance, from his or her liability, in whole or in part, for a breach of his or her duty of care to the company (except with regard to distributions), if the articles of association so provide. Our articles of association permit us to exempt our office holders, retroactively or in advance, from his or her liability, in whole or in part, for a breach of his or her duty of care to the company, up to the highest amount permitted by law.
Office Holders’ Insurance. As permitted by the Companies Law, our articles of association provide that, subject to the provisions of the Companies Law, we may enter into a contract for the insurance of the liability of any of our office holders concerning an act performed by him or her in his or her capacity as an office holder for:
| · | a breach of his or her duty of care to us or to another person; |
| · | a breach of his or her duty of loyalty to us, provided that the office holder acted in good faith and had reasonable cause to assume that his or her act would not prejudice our interests; |
| · | a financial liability imposed upon him or her in favor of another person; |
| · | expenses he or she incurs as a result of administrative proceedings that may be instituted against him or her under Israeli securities laws, if applicable, and payments made to injured persons under specific circumstances thereunder; |
| · | expenses he or she incurs as a result of administrative proceedings that may be instituted against him or her, including reasonable litigation expenses; and |
| · | any other matter in respect of which it is permitted or will be permitted under applicable law to insure the liability of an office holder in the Company. |
Indemnification of Office Holders. As permitted by the Companies Law, our articles of association provide that we may indemnify any of our office holders for an act performed in his or her capacity as an office holder, retroactively (after the liability has been incurred) or in advance against the following:
| · | a financial liability incurred by, or imposed on, him or her in favor of another person by any judgment, including a settlement or an arbitration award approved by a court; provided that our undertaking to indemnify with respect to such events on a prospective basis is, according to the Companies Law, limited to events that our Board of Directors believes are foreseeable in light of our actual operations at the time of providing the undertaking and to a sum or standard that our Board of Directors determines to be reasonable under the circumstances, and further provided that such events and amount or criteria are set forth in the undertaking to indemnify; |
| · | reasonable litigation expenses, including attorney’s fees, incurred by the office holder as a result of an investigation or proceeding instituted against him by a competent authority, provided that such investigation or proceeding concluded without the filing of an indictment against him or concluded with the imposition of a financial liability in lieu of criminal proceedings with respect to a criminal offense that does not require proof of criminal intent, all according to the law, or in connection with a financial sanction; |
| 104 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or charged to him or her by a court, resulting from the following: proceedings we institute against him or her or instituted on our behalf or by another person; a criminal indictment from which he or she was acquitted; or a criminal indictment in which he or she was convicted for a criminal offense that does not require proof of intent; |
| · | expenses he or she incurs as a result of administrative proceedings that may be instituted against him or her under Israeli securities laws, if applicable, and payments made to injured persons under specific circumstances thereunder; |
| · | expenses paid in connection with the administrative proceeding which was instituted against him or her, including reasonable litigation expenses, such as attorneys fees; and |
| · | any other matter in respect of which it is permitted or will be permitted under applicable law to indemnify an office holder in the Company. |
Limitations on Exculpation, Insurance and Indemnification. The Companies Law provides that a company may not indemnify an office holder nor exculpate an office holder nor enter into an insurance contract which would provide coverage for any monetary liability incurred as a result of any of the following:
| · | a breach by the office holder of his or her duty of loyalty, unless with respect to indemnification and insurance, the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company; |
| · | a breach by the office holder of his or her duty of care if the breach was committed intentionally or recklessly, unless it was committed only negligently; |
| · | any act or omission committed with the intent to derive an illegal personal benefit; or |
| · | any fine levied against the office holder. |
In addition, under the Companies Law, exculpation of, an undertaking to indemnify or indemnification of, and procurement of insurance coverage for, our office holders must be approved by our Compensation Committee and our Board of Directors and, in specified circumstances, such as if the office holder is a director, is generally required to be approved by our shareholders.
We have entered into agreements with each of our current directors and executive officers to indemnify them to the fullest extent permitted by law, subject to limited exceptions. The maximum aggregate amount of indemnification that we may pay to our directors and executive officers based on such indemnification agreements is, generally, NIS 15.0 million (equivalent to approximately $4.1 million) (linked to the Israeli CPI) for all office holders.
We also currently maintain directors and officers’ liability insurance with an aggregate coverage limit of $20 million for an annual premium of approximately $25,000. We intend to seek appropriate additional coverage, so it is in line with standard insurance policies for companies of similar size whose shares or ADSs are traded on the Nasdaq Capital Market.
| 105 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| D. | Employees |
The following table details certain data on the workforce of Itamar Medical and its consolidated subsidiaries as of the dates indicated:
| As of December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Numbers of employees by geographic location | ||||||||||||
| United States | 43 | 46 | 39 | |||||||||
| Israel | 94 | 88 | 82 | |||||||||
| Japan | 1 | 9 | 11 | |||||||||
| Total workforce | 138 | 143 | 132 | |||||||||
| Numbers of employees by category of activity | ||||||||||||
| Sales and marketing | 28 | 35 | 31 | |||||||||
| Support in sales and marketing | 14 | 20 | 21 | |||||||||
| Management and administrative | 23 | 21 | 24 | |||||||||
| Operations, engineering and manufacturing | 59 | 54 | 43 | |||||||||
| Research, development and technologies | 14 | 13 | 13 | |||||||||
| Total workforce | 138 | 143 | 132 | |||||||||
The overall decrease in our workforce, from 143 employees in 2016 to 138 employees in 2017, was primarily due to the reduction of mid-level management personnel in the U.S. Subsidiary and the reduction in the operations of our Japanese subsidiary. The overall increase in our workforce, from 132 employees in 2015 to 143 employees in 2016, was primarily due to recruitment of sales and marketing personnel in the U.S. and Japan and an increase in manufacturing and operations personnel in Israel.
We consider our relations with our employees to be good and we have never experienced a strike or work stoppage.
Our employees are not represented by labor unions. Nevertheless, with respect to our employees in Israel, certain provisions of the collective bargaining agreements between the ‘Histadrut’ (General Federation of Labor in Israel) and the Coordination Bureau of Economic Organizations (including the Industrialists’ Association) may be applicable to our employees by virtue of an order of the Israeli Ministry of Labor. These provisions concern mainly the length of the workday, minimum daily wages, insurance for work-related accidents, determination of severance pay and other conditions of employment. We generally provide our employees with benefits and working conditions beyond the required minimums.
Pursuant to Israeli law, we are legally required to pay severance benefits upon certain circumstances, including the retirement or death of an employee or the termination of employment of an employee without due cause. Israeli employers and employees are required to pay predetermined amounts to the National Insurance Institute, which is substantially similar to the United States Social Security Administration. In 2017, payments to the National Insurance Institute contributed by us, as the employer, amounted to approximately 6.4% of wages.
| 106 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| E. | Share Ownership |
Beneficial Ownership of Executive Officers and Directors
The following table lists, as of August 1, 2018, the number of our ordinary shares beneficially owned by each of our directors and executive officers and our directors and executive officers as a group:
| Number
of Ordinary Shares Beneficially Owned (1) |
Percentage
of Outstanding Ordinary Shares (2) |
|||||||
| Giora Yaron, PhD | 30,844,425 | (3) | 10.72 | % | ||||
| Gilad Glick | 3,186,419 | (4) | 1.10 | % | ||||
| Shy Basson | 137,831 | (5) | * | |||||
| Shlomo Ayanot | 1,606,185 | (6) | * | |||||
| Jacob (Koby) Sheffy, PhD | 1,927,682 | (7) | * | |||||
| Itay Kariv | 115,697 | (8) | * | |||||
| Efrat Litman | 605,448 | (9) | * | |||||
| Eilon Livne | 61,705 | (10) | * | |||||
| Martin Gerstel | 15,515,157 | (11) | 5.40 | % | ||||
| Ilan Biran | 380,417 | (12) | * | |||||
| Jonathan Kolber | — | (13) | * | |||||
| Sami Totah | — | (14) | * | |||||
| Christopher M. Cleary | — | (15) | * | |||||
| Yaffa Krindel Sieradzki | 82,500 | (16) | * | |||||
| Zipora (Tzipi) Ozer-Armon | 82,500 | (17) | * | |||||
| Directors and officers as a group (consisting of 15 persons)* | 54,545,966 | (18) | 18.5 | % | ||||
| * | Less than 1% of our outstanding shares. |
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Ordinary shares relating to convertible securities (such as warrants and options) currently exercisable or exercisable within 60 days of the date of this table are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. Except as indicated by footnote, and subject to community property laws where applicable, the persons named in the table above have sole voting and investment power with respect to all shares shown as beneficially owned by them. |
| (2) | The percentages shown are based on 286,723,094 shares issued and outstanding as of August 1, 2018. This figure of outstanding ordinary shares excludes (i) 39,876,606 ordinary shares issuable upon the exercise of outstanding warrants, exercisable at an exercise price of NIS 1.745 (equivalent to $0.47) per share, with the latest expiration date of these warrants being May 4, 2019; (ii) 798,088 ordinary shares issuable upon the exercise of outstanding warrants issued to a bank, exercisable at an exercise price of NIS 1.36 (equivalent to $0.37) per share, with the latest expiration date of these warrants being May 14, 2022; (iii) 3,448,425 ordinary shares issuable upon the vesting of RSUs; and (iv) employee stock options to purchase an aggregate of 33,539,448 ordinary shares at a weighted average exercise price of approximately NIS 1.34 (equivalent to $0.37) per share, with the latest expiration date of these options being January 2026 (of which, options to purchase 10,783,127 of our ordinary shares were exercisable as of August 1, 2018). |
| 107 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| (3) | Includes (i) 29,722,133 ordinary shares; (ii) 741,875 ordinary shares issuable upon exercise of Warrants (Series 4) at an exercise price of NIS 1.745 (equivalent to $0.47) per share. The warrants expire on May 4, 2019; and (iii) 380,417 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 1.14 to NIS 1.79 (equivalent to between ($0.31 and $0.49) per share. These options expire between 2021 and 2024. Some of these securities are held through a company wholly owned by Dr. Yaron. |
| (4) | Includes (i) 310,948 ordinary shares; and (ii) 2,875,471 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 1.55 to NIS 1.71 (equivalent to between ($0.42 and $0.47) per share. These options expire between 2023 and 2026. |
| (5) | Includes 137,381 ordinary shares issuable upon exercise of stock options at an exercise price of NIS1.28 (equivalent to $0.35) per share. These options expire in 2026. |
| (6) | Includes (i) 871,986 ordinary shares; and (ii) 734,199 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 0.23 to NIS 1.73 (equivalent to between $0.06 and $0.47) per share. These options expire between 2019 and 2026. |
| (7) | Includes (i) 1,015,673 ordinary shares; and (ii) 912,009 ordinary shares issuable exercise of stock options at exercise prices ranging between NIS 0.23 to NIS 1.73 (equivalent to between $0.06 and $0.47) per share. These options expire between 2019 and 2026. |
| (8) | Includes 115,697 ordinary shares issuable upon exercise of stock options at an exercise price of NIS 1.55 (equivalent to $0.42) per share. These options expire in 2026. |
| (9) | Includes 605,448 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 0.48 to NIS 2.19 (equivalent to between $0.13 and $0.60) per share. These options expire between 2019 and 2026. |
| (10) | Includes 61,705 ordinary shares issuable upon exercise of stock options at an exercise price of NIS 1.55 (equivalent to $0.42) per share. These options expire in 2026. |
| (11) | Includes (i) 14,838,891 ordinary shares; (ii) 584,599 ordinary shares issuable upon exercise of Warrants (Series 4) at an exercise price of NIS 1.745 (equivalent to $0.47) per share. The warrants expire on May 4, 2019; and (iii) 91,667 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 1.39 to NIS 1.79 (equivalent to between $0.38 and $0.49) per share. These options expire between 2021 and 2022. |
| (12) | Includes 380,417 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 1.39 to NIS 1.58 (equivalent to between $0.38 and $0.43) per share. These options expire between 2021 and 2024. |
| (13) | Mr. Kolber is a partner in Viola Growth, which is an affiliate of Viola, one of our major shareholders (See Item 7A under “Security Ownership of Certain Beneficial Owners and Management”). |
| (14) | Mr. Totah is a partner in Viola Growth, which is an affiliate of Viola, one of our major shareholders (See Item 7A under “Security Ownership of Certain Beneficial Owners and Management”). |
| (15) | Mr. Cleary is a Vice President of Corporate Development for Medtronic plc, which is an affiliate of MS Pace, one of our major shareholders (See Item 7A under “Security Ownership of Certain Beneficial Owners and Management”). |
| (16) | Includes 82,500 ordinary shares issuable upon exercise of stock options at an exercise price ranging between NIS 1.29 to NIS 1.48 (equivalent to between $0.35 and $0.40) per share. These options expire between June 2021 and June 2022. |
| (17) | Includes 82,500 ordinary shares issuable upon exercise of stock options at an exercise price ranging between NIS 1.29 to NIS 1.48 (equivalent to between $0.35 and $0.40) per share. These options expire between June 2021 and June 2022. |
| (18) | Includes (i) 46,759,631 ordinary shares; (ii) 1,326,474 ordinary shares issuable upon the exercise of outstanding warrants, exercisable at an exercise price of NIS 1.745 (equivalent to $0.47) per share, with the latest expiration date of these warrants being May 4, 2019; and (iii) employee stock options to purchase an aggregate of 6,459,861 ordinary shares at a weighted average exercise price of approximately NIS 1.36 (equivalent to approximately $0.37) per share, with the latest expiration date of these options being January 20, 2026. |
| 108 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Equity Incentive Plans
Our Equity Incentive Plans
In February 2007, we adopted the 2007 Israeli Share Option Plan, or the 2007 Option Plan, under which stock options may be granted to employees employed by us or by our affiliates, to permit our Israeli employees to benefit from tax advantages that became available at that time under Section 102 of the Israeli Tax Ordinance. The 2007 Option Plan had a term of 10 years and expired in February 2017, although we still have outstanding options under the 2007 Option Plan.
In February 2007, we also adopted the 2007 Equity Incentive Plan, or the 2007 Incentive Plan, under which stock options may be granted to employees, officers, directors and consultants of our Company and our subsidiaries that are non-Israeli residents. The 2007 Incentive Plan had a term of 10 years and expired in February 2017, although we still have outstanding options under the 2007 Incentive Plan.
In January 2016, we adopted the Israeli Equity Incentive Plan for Israeli directors, officers, employees and consultants, or the 2016 Israeli Plan, and the 2016 U.S. Equity Incentive Plan for non-Israeli directors, officers, employees and consultants, or the 2016 Non-Israeli Plan. We refer to these two plans together as the 2016 Plans. Under such plans, we may grant stock options, RSUs and other equity-based awards to employees, officers, directors and consultants of our Company and our subsidiaries. The 2016 Plans have a term of ten years and will terminate in January 2026.
Each of the aforesaid equity incentive plans, to which we refer together as the Equity Plans, is administered by our Board of Directors (although our Board of Directors may delegate such authority to any committee thereof). Subject to the Equity Plans and applicable law, our Board of Directors has the authority to make all determinations deemed necessary or advisable for the administration of such plans, including to whom equity awards may be granted, the time and the extent to which these awards may be exercised, the exercise or purchase price of shares covered by each option or other award, the type of awards and how to interpret such plans. Among others, the Board has the authority to provide for, or, where applicable, recommend for approval by the Board of Directors, accelerated vesting of the ordinary shares subject to outstanding awards. See also Item 6.B – “Change of Control Arrangements.”
As of August 1, 2018, we did not have any remaining ordinary shares reserved for grant of awards under the Equity Plans. However, our Board of Directors may, from time to time, determine to reserve additional ordinary shares under the Equity Plans, based upon, among other things, our retention and hiring needs. Such increase does not require shareholder approval. In this respect, see under Item 3.D Risk Factors – “As a foreign private issuer whose shares are listed on the Nasdaq Capital Market we intend to follow certain home country corporate governance practices instead of certain Nasdaq requirements.”
Grants in 2017
In 2017, we granted under the Equity Plans (i) options exercisable into up to 3,742,218 ordinary shares (compared with options exercisable into up to 24,641,766 ordinary shares that we granted in 2016); and (ii) up to 362,858 ordinary shares issuable upon the vesting of performance-based RSUs (compared with up to 3,538,306 ordinary shares issuable upon the vesting of performance-based RSUs that were granted during 2016).
| 109 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Total Outstanding Options and RSUs
The following table sets forth, as of December 31, 2017, the number of options outstanding under our Equity Plans and their respective exercise prices and expiration dates:
| Number of Outstanding Options |
Range
of exercise prices |
Weighted average remaining contractual life (in years) | ||
| 2,982,200 | $0.06 - $0.13 | 1.23 | ||
| 243,750 | $0.14 - $0.21 | 1.54 | ||
| 18,940,463 | $0.32 - $0.40 | 7.46 | ||
| 9,371,726 | $0.41 - $0.54 | 6.28 | ||
| 891,713 | $0.55 - $0.68 | 3.77 | ||
| 220,000 | $0.31 | 5.50 | ||
| Total: (*) (**) 32,649,852 | 6.39 |
| (*) | Includes 9,716,559 options that are vested and exercisable as of December 31, 2017. |
| (**) | Includes 15,896,403 performance-based options, at exercise prices that range between of NIS 1.17 to NIS1.40 (equivalent to between $0.32 and $0.38), which performance criteria is that they will vest in December 2020 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such options will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 per share (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the options will vest. |
The following table sets forth, as of December 31, 2017, the number of RSUs outstanding under our Equity Plans and their respective weighted average grant date fair value:
| RSUs | Number | Weighted
average grant date fair value | ||||||
| Outstanding at beginning of the year | 3,398,889 | $ | 0.17 | |||||
| Granted | 362,858 | $ | 0.11 | |||||
| Vested | — | — | ||||||
| Forfeited | (519,115 | ) | $ | 0.17 | ||||
| Outstanding at end of the year (*) | 3,242,632 | $ | 0.16 | |||||
| (*) | All of the RSUs are performance-based RSUs, which performance criteria is that they will vest in December 2020 if the price of our ordinary shares is, at such time, at least NIS 4.24 per share (equivalent to $1.15), or 50% of such options will vest if the price of our ordinary shares is, at such time, at least NIS 1.70 per share (equivalent to $0.46). In case that, on the applicable measurement date, the price of our ordinary shares is within the range between these two vesting-trigger share prices, the relative quantity of the options will vest. Out of these performance-based RSUs, 1,741,344 RSUs which were granted to one employee and one consultant (of which 1,692,312 RSUs were granted in March 2016 to our President and Chief Executive Officer) require the payment of an exercise price of NIS 0.30 per share (equivalent to $0.08). |
For additional details and a discussion of the accounting method and assumptions used in valuation of such options and RSUs, see Note 15 to our audited consolidated financial statements included elsewhere in this registration statement.
In addition, between January 1, 2018 and June 30, 2018, we granted under the Equity Plans (i) options exercisable into 2,516,193 ordinary shares at exercise prices ranging between NIS 1.02 and NIS 1.14 (equivalent to between $0.28 and $0.31) per share. These options expire between 2023 and 2026; and (ii) 278,566 performance-based RSUs. Out of these RSUs, 49,032 RSUs are with an exercise price of NIS 0.30 (equivalent to $0.08).
| 110 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| ITEM 7. | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
| A. | Major Shareholders |
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth certain information regarding the beneficial ownership by all shareholders who, to our knowledge, own beneficially more than 5% of our ordinary shares (to whom we sometime refer in this registration statement as our major shareholders) as of August 1, 2018:
| Number of Ordinary Shares Beneficially Owned (1) | Percentage of Outstanding Ordinary Shares (2) | |||||||
| Viola | 105,950,372 | (3) | 33.08 | % | ||||
| MS Pace | 41,154,813 | (4) | 14.31 | % | ||||
| Giora Yaron, PhD | 30,844,425 | (5) | 10.72 | % | ||||
| Yelin Lapidot | 25,660,532 | (6) | 8.95 | % | ||||
| Migdal | 20,827,689 | (7) | 7.27 | % | ||||
| Martin Gerstel | 15,515,175 | (8) | 5.40 | % | ||||
| Meitav Dash | 14,420,652 | (9) | 5.02 | % | ||||
| (1) | Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Ordinary shares relating to convertible securities (such as warrants and options) currently exercisable or exercisable within 60 days of the date of this table are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. Except as indicated by footnote, and subject to community property laws where applicable, the persons named in the table above have sole voting and investment power with respect to all shares shown as beneficially owned by them. |
| (2) | The percentages shown are based on 286,723,094 shares issued and outstanding as of August 1, 2018. This figure of outstanding ordinary shares excludes (i) 39,876,606 ordinary shares issuable upon the exercise of outstanding warrants, exercisable at an exercise price of NIS 1.745 (equivalent to $0.47) per share, with the latest expiration date of these warrants being May 4, 2019; (ii) 798,088 ordinary shares issuable upon the exercise of outstanding warrants issued to a bank, exercisable at an exercise price of NIS 1.36 (equivalent to $0.37) per share, with the latest expiration date of these warrants being May 14, 2022; (iii) 3,448,425 ordinary shares issuable upon the vesting of RSUs; and (iv) employee stock options to purchase an aggregate of 33,539,448 ordinary shares at a weighted average exercise price of approximately NIS1.34 (equivalent to $0.37) per share, with the latest expiration date of these options being January 20, 2026 (of which, options to purchase 10,783,127 of our ordinary shares were exercisable as of August 1, 2018). |
| (3) | The following is based on information provided to the Company by Viola Growth II GP Ltd., a Cayman Islands company. The number of ordinary shares reported in the table consists of (i) 72,346,918 ordinary shares held by Viola Growth 2 A.V. Limited Partnership, or Viola, an Israeli limited partnership; (ii) 33,438,454 ordinary shares issuable upon exercise of the Viola Warrants held by Viola, at an exercise price of NIS 1.745 (equivalent to $0.47) per share. The warrants expire on May 4, 2019; and (iii) 165,000 ordinary shares issuable upon exercise of stock options held by Viola Growth Management Fund 2 Ltd., or Viola 2, an Israeli company, at an exercise price of NIS 1.54 (equivalent to $0.42) per share. The general partner of Viola is Viola Growth II Limited Partnership, a Cayman Island limited partnership. The general partner of Viola Growth II Limited Partnership is Viola Growth II GP Ltd., a Cayman Islands company, which is wholly owned by Viola 2. Messrs. Shlomo Dovrat, Harel Beit-On and Avi Zeevi, all of whom are Israeli citizens, hold indirect interests in, and are the controlling shareholders of, Viola 2 and, consequently, may be deemed to be the beneficial owners of the ordinary shares held by Viola and Viola 2. However, each of Messrs. Dovrat, Beit-On and Zeevi disclaims beneficial ownership of all of the foregoing shares, except to the extent of their respective pecuniary interest therein. The business address of Viola is Ackerstein Towers, Building D, 12 Abba Eban Avenue, Herzeliya 4672530, Israel. |
| 111 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| (4) | The following is based on information provided to the Company by MS Pace LP, or MS Pace, a Delaware limited partnership. The general partner of MS Pace is MS Pace Management, LLC, which is 51% held by an affiliate of Medtronic International Technology, Inc., or Medtronic, and the remaining 49% interest therein is held by Sightline MS GP, LLC, a third party unrelated to Medtronic. Medtronic also holds 20% of the limited partnership interests in MS Pace. Medtronic is an indirect wholly owned subsidiary of Medtronic plc, an Irish corporation whose shares are traded on the NYSE. The number of ordinary shares reported in the table consists of (i) 40,307,413 ordinary shares held by MS Pace and (ii) 847,400 ordinary shares issuable upon exercise of Warrants (Series 4) held by Medtronic, which warrants are to be transferred to MS Pace. These warrants are exercisable at an exercise price of NIS 1.745 (equivalent to $0.47) per share and expire on May 4, 2019. The business address of MS Pace is 8500 Normandale Lake Boulevard, Suite 1070, Bloomington, Minnesota 55437. |
| (5) | Dr. Yaron is the Chairman of our Board of Directors. Includes (i) 29,722,133 ordinary shares; (ii) 741,875 ordinary shares issuable upon exercise of Warrants (Series 4) at an exercise price of NIS 1.745 (equivalent to $0.47) per share. The warrants expire on May 4, 2019; and (iii) 380,417 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 1.14 to NIS 1.79 (equivalent to between ($0.31 and $0.49) per share. These options expire between 2021 and 2024. Some of these securities are held through a company wholly owned by Dr. Yaron. The business address of Dr. Yaron is c/o Itamar Medical Ltd., 9 Halamish Street, Caesarea 3088900, Israel. |
| (6) | The following is based on information provided to the Company by Yelin Lapidot Mutual Funds Management Ltd., or Yelin Lapidot. Yelin Lapidot is a wholly-owned subsidiary of Yelin Lapidot Holdings Management Ltd. (“Yelin Lapidot Holdings”). Yelin Lapidot operates under independent management and makes its own independent voting and investment decisions. Messrs. Dov Yelin and Yair Lapidot, who are the principal shareholders and directors of Yelin Lapidot Holdings, may be deemed to be the beneficial owners of the shares held by Yelin Lapidot. However, each of Messrs. Yelin and Lapidot disclaims beneficial ownership of all of the foregoing shares except to the extent of their respective pecuniary interest therein. The business address of Yelin Lapidot is 50 Dizengoff St., Dizengoff Center, Gate 3, Top Tower, 13th floor, Tel Aviv 6433222, Israel. |
| (7) | The following is based on information provided to the Company by Migdal Insurance & Financial Holdings Ltd, or Migdal. The ordinary shares shown as beneficially owned by Migdal consist of shares held for members of the public through, among others, provident funds, mutual funds, pension funds and insurance policies, which are managed by subsidiaries of Migdal, each of which subsidiaries operates under independent management and makes independent voting and investment decisions. Consequently, Migdal disclaims beneficial ownership of all of the foregoing shares except to the extent of its pecuniary interest therein. The business address of Migdal is 4 Efal Street; P.O. Box 3063; Petah Tikva 4951104, Israel. |
| (8) | Mr. Gerstel is a director of the Company. Includes (i) 14,838,891ordinary shares; (ii) 584,599 ordinary shares issuable upon exercise of Warrants (Series 4) at an exercise price of NIS 1.745 (equivalent to $0.47) per share. The warrants expire on May 4, 2019; and (iii) 91,667 ordinary shares issuable upon exercise of stock options at exercise prices ranging between NIS 1.39 to NIS 1.79 (equivalent to between $0.38 and $0.49) per share. These options expire between 2021 and 2022. The business address of Mr. Gerstel is c/o Itamar Medical Ltd., 9 Halamish Street, Caesarea 3088900, Israel. |
| (9) | The following is based on information provided to the Company by Meitav Dash Investments Ltd., or Meitav Dash. The ordinary shares shown as beneficially owned by Meitav Dash consist of shares held for members of the public through, among others, provident funds, mutual funds, pension funds and insurance policies, which are managed by various direct or indirect, majority or wholly-owned subsidiaries of Meitav Dash, each of which subsidiaries operates under independent management and makes independent voting and investment decisions. Consequently, Meitav Dash disclaims beneficial ownership of all of the foregoing shares except to the extent of its pecuniary interest therein. The business address of Meitav Dash is 30 Derekh Sheshet HaYamim, Bnei Brak 5120261, Israel. |
| 112 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
To our knowledge, (i) we are not directly or indirectly owned or controlled by another corporation, by any foreign government or by any other natural or legal person severally or jointly, except as disclosed in the above table regarding our major shareholders, and (ii) there are no arrangements which would result in our change in control at a subsequent date.
Significant Changes in the Ownership of Major Shareholders
During the past three years, the significant changes in the percentage ownership of our major shareholders were, to our knowledge, as follows:
| · | On July 5, 2018, Meitav Dash became the beneficial owner of more than 5% of our outstanding ordinary shares as shown in the table above. |
| · | On May 27, 2018, we completed a private placement and issued to the investors, which included several of our major shareholders (namely, Viola, Medtronic, Dr. Yaron, Yelin Lapidot and Meitav Dash), a total of 22,013,893 ordinary shares (representing as of such date approximately 7.7% of our issued and outstanding shares on a post-issuance basis). For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| · | On February 1, 2016, in connection with the issuances of our ordinary shares under the Viola Transaction on November 5, 2015 and February 1, 2016, Viola became the beneficial owner of approximately 25.5% of our issued and outstanding shares as of February 1, 2016. |
Major Shareholders Voting Rights
Our major shareholders do not have different voting rights.
Record Holders
Based on a review of the information provided to us by the TASE, as of August 1, 2018, there were 613 holders of record of our ordinary shares, of which 11 record holders, holding approximately 5.5% of our outstanding ordinary shares, had registered addresses in the United States. These numbers are not representative of the number of beneficial holders of our ordinary shares nor is it representative of where such beneficial holders reside primarily because many of these ordinary shares may be held of record by brokers or other nominees.
| B. | Related Party Transactions |
Financings
See Item 5.B “Operating and Financial Review and Prospects– Liquidity and Capital Resources – Principal Financing Activities” with respect to certain investments and loans made by our major shareholders and members of our Board of Directors.
| 113 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Directors and Officers Compensation
See Item 6.B “Directors, Senior Management and Employees - Compensation” and 6.C “Directors, Senior Management and Employees – Board Practices” with respect to compensation payable, and insurance and indemnification granted, to our senior management and directors.
Medtronic Co-Marketing Agreement
In March 2014, we entered into a co-marketing agreement with Medtronic, Inc., whereby Medtronic was granted exclusive rights to co-market our WatchPAT product to electrophysiologists (physicians specializing in cardiology arrhythmias) in the United States and undertook to make specified investments in marketing of the product as well as meet minimum sales quotas. In April 2015, as part of several amendments to the marketing agreement, the aforesaid obligation to make specified investments and meet minimum sales quotas was canceled. Pursuant to this agreement, Medtronic markets WatchPAT as part of a comprehensive solution offered by Medtronic to physicians. Since June 30, 2017, the term of this agreement (as amended) is automatically renewed for 30 day intervals, unless earlier terminated by either party upon 14 days prior notice.
Medtronic is entitled to a portion of the net sales made under this agreement. However, the total net sales under this agreement (and, consequently, the consideration payable to Medtronic) have been immaterial to us in the past three years (net sales under this agreement were approximately $0.31 million in 2017, compared to $0.18 million in 2016 and $0.22 million in 2015).
| ITEM 8. | FINANCIAL INFORMATION |
| A. | Consolidated Statements and Other Financial Information |
Financial Statements
See the consolidated financial statements, including the notes thereto, included in Item 18 “Financial Statements” of this registration statement.
Export Sales
In the year ended December 31, 2017, the amount of our export sales (i.e., sales outside of Israel) was approximately $20.4 million, which represents 98.7% of our total sales.
Legal Proceedings
We are currently not, and have not been in the recent past, a party to any legal proceedings which may have or have had in the recent past significant effects on our financial position or profitability. However, we have been in the past, and may be from time to time in the future, named as a defendant in certain routine litigation incidental to our business.
Dividend Distribution Policy
We have never declared or paid on our ordinary shares and do not intend to pay cash dividends on our ordinary shares or ADSs in the foreseeable future. Our earnings and other cash resources will be used to continue the development and expansion of our business. Any future dividend policy will be determined by our Board of Directors and will be based upon conditions then existing, including our results of operations, financial condition, current and anticipated cash needs, contractual restrictions and other conditions.
| 114 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
According to the Companies Law, a company may distribute dividends only out of its “profits,” as such term is defined in the Companies Law, as of the end of the most recent fiscal year or as accrued over a period of two years, whichever is higher. Our Board of Directors is authorized to declare dividends, provided that there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due. Notwithstanding the foregoing, dividends may be paid with the approval of a court, provided that there is no reasonable concern that payment of the dividend will prevent us from satisfying our existing and foreseeable obligations as they become due. Profits, for purposes of the Companies Law, means the greater of retained earnings or earnings accumulated during the preceding two years, after deduction of previous distributions that were not already deducted from the surpluses, as evidenced by financial statements prepared no more than six months prior to the date of distribution.
| B. | Significant Changes |
Except as otherwise disclosed in this registration statement, no significant change has occurred since December 31, 2017.
| ITEM 9. | THE OFFER AND LISTING |
| A. | Offer and Listing Details |
Trading in the Ordinary Shares
Our ordinary shares have been trading on the TASE under the symbol “ITMR” since March 13, 2007. No trading market currently exists for our ADSs or ordinary shares in the United States. We have applied to have our ADSs listed on the Nasdaq Capital Market.
The table below sets forth the high and low reported market (sale) prices of our ordinary shares (in NIS and dollars) on the TASE for the periods indicated below. The translation into dollars is based on the daily representative rate of exchange published by the Bank of Israel.
| High | Low | |||||||||||||||
| NIS | $ | NIS | $ | |||||||||||||
| Annual | ||||||||||||||||
| 2013 | 2.195 | 0.629 | 1.280 | 0.353 | ||||||||||||
| 2014 | 2.680 | 0.772 | 1.480 | 0.396 | ||||||||||||
| 2015 | 1.997 | 0.506 | 1.360 | 0.351 | ||||||||||||
| 2016 | 1.499 | 0.389 | 1.040 | 0.269 | ||||||||||||
| 2017 | 1.718 | 0.450 | 1.031 | 0.293 | ||||||||||||
| Quarterly 2016 | ||||||||||||||||
| First Quarter | 1.467 | 0.376 | 1.240 | 0.314 | ||||||||||||
| Second Quarter | 1.396 | 0.367 | 1.040 | 0.269 | ||||||||||||
| Third Quarter | 1.470 | 0.385 | 1.164 | 0.311 | ||||||||||||
| Fourth Quarter | 1.499 | 0.389 | 1.126 | 0.296 | ||||||||||||
| Quarterly 2017 | ||||||||||||||||
| First Quarter | 1.718 | 0.450 | 1.270 | 0.351 | ||||||||||||
| Second Quarter | 1.380 | 0.392 | 1.100 | 0.310 | ||||||||||||
| Third Quarter | 1.250 | 0.358 | 1.031 | 0.293 | ||||||||||||
| Fourth Quarter | 1.470 | 0.417 | 1.130 | 0.322 | ||||||||||||
| Quarterly 2018 | ||||||||||||||||
| First Quarter | 1.336 | 0.385 | 0.931 | 0.268 | ||||||||||||
| Second Quarter | 1.170 | 0.333 | 0.966 | 0.269 | ||||||||||||
| Third Quarter | 1.319 | 0.368 | 1.060 | 0.291 | ||||||||||||
| Fourth Quarter (through October 7, 2018) | 1.389 | 0.382 | 1.290 | 0.353 | ||||||||||||
| Monthly | ||||||||||||||||
| April 2018 | 1.170 | 0.333 | 0.966 | 0.269 | ||||||||||||
| May 2018 | 1.090 | 0.305 | 0.987 | 0.272 | ||||||||||||
| June 2018 | 1.120 | 0.307 | 1.000 | 0.279 | ||||||||||||
| July 2018 | 1.189 | 0.324 | 1.060 | 0.291 | ||||||||||||
| August 2018 | 1.227 | 0.339 | 1.114 | 0.300 | ||||||||||||
| September 2018 | 1.319 | 0.368 | 1.175 | 0.325 | ||||||||||||
| October 2018 (through October 7, 2018) | 1.389 | 0.382 | 1.290 | 0.353 | ||||||||||||
| 115 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
For a description of the rights of our ADSs, see “Item 12. Description of Securities Other Than Equity Securities – D. American Depositary Shares.”
| B. | Plan of Distribution |
Not applicable.
| C. | Markets |
Our ordinary shares are listed and traded on the TASE. No trading market currently exists for our ADSs or ordinary shares in the United States. We have applied to the Nasdaq to have our ordinary shares in the form of ADSs commence trading on the Nasdaq Capital Market.
| D. | Selling Shareholders |
Not applicable.
| E. | Dilution |
Not applicable.
| F. | Expense of the Issue |
Not applicable.
| 116 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| ITEM 10. | ADDITIONAL INFORMATION |
| A. | Share Capital |
As of August 1, 2018, our authorized share capital consisted of 750,000,000 ordinary shares, par value NIS 0.01 per share, of which 286,723,094 ordinary shares were issued and outstanding as of such date. As of such date, (i) an additional 39,876,606 of our ordinary shares were issuable upon the exercise of outstanding warrants to purchase our ordinary shares at an exercise price of NIS 1.745 (equivalent to $0.47) per share; (ii) an additional 798,088 of our ordinary shares were issuable upon the exercise of outstanding warrants, issued to a bank, to purchase our ordinary shares at an exercise price of NIS 1.36 (equivalent to $0.37) per share; (iii) an additional 32,989,448 of our ordinary shares were issuable upon the exercise of outstanding options to purchase our ordinary shares. The exercise price of the options outstanding ranges between NIS 0.10 and NIS 2.50 (equivalent to between $0.03 to $0.68) per share; and (iv) an additional 3,448,425 of our ordinary shares were issuable upon the vesting of RSUs.
All of our outstanding ordinary shares have been validly issued, fully paid and non-assessable.
As of December 31, 2017, our authorized share capital consisted of 750,000,000 ordinary shares, par value NIS 0.01 per share, of which 264,495,375 ordinary shares were issued and outstanding as of such date. As of December 31, 2017, (i) an additional 39,876,606 of our ordinary shares were issuable upon the exercise of outstanding warrants to purchase our ordinary shares at an exercise price of NIS 1.745 (equivalent to $0.47) per share; (ii) an additional 98,088 of our ordinary shares were issuable upon the exercise of outstanding warrants, issued to a bank, to purchase our ordinary shares at an exercise price of NIS 1.36 (equivalent to $0.37) per share; (iii) an additional 20,037,659 of our ordinary shares were issuable upon the conversion of outstanding convertible notes at a conversion price of NIS 1.92 (equivalent to $0.52) per share; (iv) an additional 32,649,852 of our ordinary shares were issuable upon the exercise of outstanding options to purchase our ordinary shares. The exercise price of the options outstanding ranges between NIS 0.10 and NIS 2.50 (equivalent to between $0.03 and $0.68) per share; and (v) an additional 3,242,632 of our ordinary shares were issuable upon the vesting of RSUs.
As of January 1, 2017, our authorized share capital consisted of 750,000,000 ordinary shares, par value NIS 0.01 per share, of which 262,917,624 ordinary shares were issued and outstanding as of such date. As of January 1, 2017, (i) an additional 39,876,606 of our ordinary shares were issuable upon the exercise of outstanding warrants to purchase our ordinary shares at an exercise price of NIS 1.642 (equivalent to $0.45) per share; (ii) an additional 40,075,317 of our ordinary shares were issuable upon the conversion of outstanding convertible notes at a conversion price of NIS 1.92 (equivalent to $0.52) per share; (iii) an additional 36,639,843 of our ordinary shares were issuable upon the exercise of outstanding options to purchase our ordinary shares. The exercise price of the options outstanding ranges between NIS 0.10 and NIS 2.50 (equivalent to between $0.03 and $0.68) per share; and (iv) an additional 3,538,305 of our ordinary shares were issuable upon the vesting of RSUs.
As of January 1, 2016, our authorized share capital consisted of 750,000,000 ordinary shares, par value NIS 0.01 per share, of which 259,582,951 ordinary shares were issued and outstanding as of such date. As of January 1, 2015, (i) an additional 38,388,982 of our ordinary shares were issuable upon the exercise of outstanding warrants to purchase our ordinary shares at an exercise price of NIS 1.642 (equivalent to $0.45) per share; (ii) an additional 40,075,317 of our ordinary shares were issuable upon the conversion of outstanding convertible notes at a conversion price of NIS 1.92 (equivalent to $0.52) per share; and (iii) an additional 36,639,843 of our ordinary shares were issuable upon the exercise of outstanding options to purchase our ordinary shares. The exercise price of the options outstanding ranges between NIS 0.10 and NIS 2.50 (equivalent to between $0.03 to $0.68) per share.
| 117 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Below is information regarding changes in our ordinary share capital since January 1, 2015 through August 14, 2018:
| · | During 2015, we issued (i) an aggregate of 2,043,851 ordinary shares in connection with the exercise of stock options. Total aggregate consideration received in consideration for these issuances was approximately $154,000; and (ii) stock options exercisable into an aggregate of up to 1,602,250 of our ordinary shares, with exercise prices ranging between NIS 1.77 and NIS 1.98 (equivalent to between $0.48 to $0.54) per share. |
| · | On November 5, 2015, we issued to Viola 63,900,759 ordinary shares at a purchase price of NIS 1.449 per share (equivalent to $0.38 based on the exchange rate as of that date), resulting in aggregate proceeds (before expenses) of NIS 92.6 million (equivalent to approximately $24.1 million, based on the exchange rate as of that date). In addition, we issued to Viola, for no additional consideration, the Viola Warrants exercisable into up to 31,950,380 ordinary shares. The Viola Warrants have an exercise price of NIS 1.642 per share (equivalent to $0.45) for the first 21 months of the term thereof and an exercise price of NIS 1.745 (equivalent to $0.47) for the remainder of the term, subject to adjustments. For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| · | On December 29, 2015, we completed a rights offering and issued to the subscribing shareholders a total of 12,876,303 ordinary shares at a price per share equal to NIS 1.449 per share (equivalent to $0.37, based on the exchange rate as of that date), resulting in aggregate proceeds (before expenses) of NIS 18.7 million (equivalent to approximately $4.7 million, based on the exchange rate as of that date). In addition, we issued to the subscribing shareholders, for no additional consideration, Warrants (Series 4) exercisable into up to 6,438,152 ordinary shares (i.e., a ratio of one warrant for every two shares). For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| · | During 2016, we issued (i) an aggregate of 358,525 ordinary shares in connection with the exercise of stock options. Total aggregate consideration received in consideration for these issuances was approximately $17,000; (ii) stock options exercisable into an aggregate of up to 24,641,766 of our ordinary shares, with exercise prices ranging between NIS 1.33 and NIS 1.55 (equivalent to between $0.36 to $0.42) per share. Stock options exercisable into an aggregate of up to 7,376,681 of our ordinary shares were cancelled as part of this issuance; and (iii) RSUs exercisable upon vesting into an additional 3,538,306 ordinary shares. Out of those RSUs, 1,692,312 RSUs have an exercise price of NIS 0.30 (equate to $0.08). |
| · | On February 1, 2016, we issued to Viola 2,976,148 ordinary shares at a purchase price of NIS 1.449 per share (equivalent to $0.37, based on the exchange rate as of that date), resulting in aggregate proceeds (before expenses) of NIS 4.3 million (equivalent to approximately $1.1 million, based on the exchange rate as of that date). In addition, we issued to Viola, for no additional consideration, the Viola Warrants exercisable into up to 1,488,074 ordinary shares. The Viola Warrants have an exercise price of NIS 1.642 per share (equivalent to $0.45) for the first 21 months of the term thereof and an exercise price of NIS 1.745 (equivalent to $0.47) for the remainder of the term, subject to adjustments. For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| · | During 2017, we issued (i) an aggregate of 1,577,751 ordinary shares in connection with the exercise of stock options. Total aggregate consideration received in consideration for these issuances was approximately $97,000; (ii) stock options exercisable into an aggregate of up to 3,742,218 of our ordinary shares, with exercise prices ranging between NIS 1.17 and NIS 1.68 (equivalent to between $0.32 to $0.46) per share; and (iii) RSUs exercisable upon vesting into an additional 362,858 ordinary shares. |
| 118 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | On June 15, 2017 in connection with a bank credit line we secured, we issued the bank warrants exercisable into up to 798,088 ordinary shares at an exercise price of NIS 1.36 per share (equivalent to $0.37 per share), subject to adjustments. For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| · | During 2018 (through August 14, 2018), we issued (i) an aggregate of 213,826 ordinary shares in connection with the exercise of stock options. Total aggregate consideration received in consideration for these issuances was approximately $25,000; (ii) stock options exercisable into an aggregate of up to 2,516,193 of our ordinary shares, with exercise prices ranging between NIS 1.02 and NIS 1.14 (equivalent to between $0.28 to $0.31) per share; and (iii) RSUs exercisable upon vesting into an additional 278,566 ordinary shares. Out of those RSUs, 49,032 RSUs have an exercise price of NIS 0.30 (equate to $0.08). |
| · | On May 27, 2018, we completed a private placement and issued to the investors a total of 22,013,893 ordinary shares at a purchase price of NIS 0.947 per share (equivalent to $0.27), resulting in aggregate proceeds (before expenses) of NIS 20.8 million (equivalent to approximately $6.0 million, based on the exchange rate as of that date). For additional details, see Item 5.B “Liquidity and Capital Resources – Principal Financing Activities.” |
| B. | Memorandum and Articles of Association |
Set out below is a description of certain provisions of our memorandum of association and articles of association and of the Companies Law (as currently in effect) related to such provisions, unless otherwise specified. This description is only a summary and does not purport to be complete and is qualified by reference to the full text of the memorandum and articles, which are incorporated by reference as exhibits to this registration statement.
Purposes and Objects of the Company
We are a public company registered under the Companies Law as Itamar Medical Ltd. Our registration number with the Israeli Registrar of Companies is 51-243421-8. Pursuant to our memorandum and articles of association, our objectives are to engage in any lawful activity as determined from time to time by our Board of Directors.
The Powers of the Directors
Under the provisions of the Companies Law and our articles of association, a director generally cannot participate in a meeting nor vote on a proposal, arrangement or contract in which he or she is personally interested. In addition, our directors generally cannot vote compensation to themselves or any members of their body without the approval of our Compensation Committee and our shareholders at a general meeting. See Item 6.C “Directors, Senior Management and Employees – Board Practices – Approval of Related Party Transactions Under Israeli Law.”
The authority of our directors to enter into borrowing arrangements on our behalf is not limited, except in the same manner as any other transaction by us.
| 119 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Under our articles of association, retirement of directors from office is not subject to any age limitation and our directors are not required to own shares in our Company in order to qualify to serve as directors.
Rights Attached to Shares
Our authorized share capital consists of 750,000,000 ordinary shares of a nominal value of NIS 0.01 each. The shares do not entitle their holders to preemptive rights.
Dividend rights. Subject to any preferential, deferred or other rights or restrictions attached to any special class of shares with regard to dividends, the profits of the Company available for dividend and resolved to be distributed shall be applied in payment of dividends upon the shares of the Company in the same manner with respect to all of the shares granting a right to receive dividends on the date that resolution is adopted (or on later date, as determined by the Board of Directors). Our Board of Directors may declare dividends only out of profits legally available for distribution, in accordance with the provisions of the Companies Law. In this respect, see Item 8.A “Financial Information – Consolidated and Other Financial Information – Dividend Distribution Policy.”
Our Board of Directors is entitled to invest or utilize any unclaimed amount of dividend in any manner to our benefit until it is claimed. We are not obligated to pay interest or linkage on an unclaimed dividend.
Voting rights. Holders of ordinary shares have one vote for each ordinary share held on all matters submitted to a vote of shareholders. Such voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Rights to share in profits. Our shareholders have the right to share in our profits distributed as a dividend and any other permitted distribution. See this Item 10.B “Additional Information – Memorandum and Articles of Association – Rights Attached to Shares – Dividend Rights” above.
Rights to share in surplus in the event of liquidation. In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of ordinary shares in proportion to the nominal value of their holdings. This right may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Liability to capital calls by the Company. Under our memorandum and articles of association as well as the Companies Law, the liability of our shareholders is limited to the unpaid amount of the purchase price (i.e., the par value of the shares and the premium thereon, if any) that such shareholder (or its predecessor) initially undertook to pay for the shares issued thereto.
Limitations on any existing or prospective major shareholder. See Item 6.C “Directors, Senior Management and Employees – Board Practices – Approval of Related Party Transactions Under Israeli Law.”
Changing Rights Attached to Shares
The rights attached to any class of shares (unless otherwise provided by the terms of issuance of the shares of that class) may be varied with the consent in writing of the holders of all the issued shares of that class, or with the sanction of a vote at a meeting of the shareholders passed at a separate meeting of the holders of the shares of the class by a majority of the voting rights of such class represented at the meeting in person or by proxy and voting thereon.
| 120 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Under our articles of association, unless otherwise provided by the conditions of issuance, the enlargement of an existing class of shares, or the issuance of additional shares thereof, shall not be deemed to modify or abrogate the rights attached to the previously issued shares of such class or of any other class.
Shareholders Meetings
The Board of Directors must convene an annual meeting of shareholders at least once every calendar year, within fifteen months of the last annual meeting. A special meeting of shareholders may be convened by the Board of Directors, as it decides.
The Companies Law generally allows shareholders (1) who hold at least 1% of the outstanding shares of a public company to submit a proposal for inclusion on the agenda of a general meeting of the company’s shareholders and (2) who hold at least 5% of the outstanding ordinary shares of a public company to convene a special meeting of shareholders upon request in accordance with the Companies Law. Our articles of association contain procedural guidelines and disclosure items with respect to the submission of shareholder proposals for shareholders meetings.
In accordance with our articles of association, shareholders meetings require notice in the manner prescribed by the Companies Law. Under the Companies Law, shareholders meetings generally require prior notice of not less than 21 days or, with respect to certain matters, such as election of directors and affiliated party transactions, not less than 35 days.
The quorum required at any meeting of shareholders consists of at least two shareholders present in person or represented by proxy, within half an hour from the time appointed for holding the meeting, who hold or represent, in the aggregate, at least 33 and 1/3% of the total voting rights in the Company. A meeting adjourned for lack of a quorum generally is adjourned to the same day in the following week at the same time and place or any time and place as the directors designate in a notice to the shareholders. If, at such adjourned meeting, a quorum is not present within half an hour from the time appointed for holding the meeting, any two shareholders present in person or by proxy who hold or represent, in the aggregate, at least 10% of the outstanding share capital of the Company, shall constitute a quorum.
Under our articles of association, all shareholder resolutions require approval of no less than a majority of the voting rights represented at the meeting in person or by proxy and voting thereon, except that (1) amendments to our articles of association (including any change to provisions relating to the composition of our Board of Directors) and (2) removal of directors (who are not external directors or “independent directors” as such term is defined by the Companies Law) by our shareholders, require a special majority of 75% or more of the voting power represented at the meeting in person or by proxy and voting thereon .
Pursuant to our articles of association, our directors (except outside directors) are elected at our annual general meeting of shareholders by a vote of the holders of a majority of the voting power represented and voting at such meeting. For additional details regarding the election of our directors, see Item 6.C “Directors, Senior Management and Employees – Board Practices – Election of Directors; Board Meetings.”
| 121 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Limitations on the Rights to Own Securities in Our Company
Neither our memorandum of association or our articles of association nor the laws of the State of Israel restrict in any way the ownership or voting of shares by non-residents, except with respect to subjects of countries which are in a state of war with Israel. See also this Item 10.B “Additional Information – Memorandum and Articles of Association –Provisions Restricting Change in Control of Our Company” below.
Duties of Shareholders
Disclosure by Controlling Shareholders. Under the Companies Law, the disclosure requirements that apply to an office holder also apply to a controlling shareholder of a public company. A controlling shareholder is a shareholder who has the ability to direct the activities of a company, including a shareholder that owns 25% or more of the voting rights if no other shareholder owns more than 50% of the voting rights, but excluding a shareholder whose power derives solely from his or her position on the Board of Directors or any other position with the company. We consider Viola to be a controlling shareholder of our Company under the Companies Law.
Approval of Certain Transactions. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, and the engagement of a controlling shareholder as an office holder or employee (including compensation therefor), generally require the approval of the audit committee (or compensation committee with respect to engagement as an office holder or employee), the Board of Directors and the shareholders, in that order. The shareholder approval must include at least a majority of the shares of non-interested shareholders voted on the matter. However, the transaction can be approved by shareholders without this special approval if the total shares of non-interested shareholders that voted against the transaction do not represent more than 2% of the voting rights in the company. In addition, any such extraordinary transaction whose term is longer than three years may require further shareholder approval every three years, unless, where permissible under the Companies Law, the audit committee approves that a longer term is reasonable under the circumstances. With respect to approval of compensation to directors and executive officers, see also Item 6.C “Directors, Senior Management and Employees – Board Practices – Approval of Related Party Transactions Under Israeli Law.”
General Duties of Shareholders. In addition, under the Companies Law, each shareholder has a duty to act in good faith toward the company and other shareholders and to refrain from abusing his or her power in the company, such as in shareholder votes. In addition, specified shareholders have a duty of fairness toward the company. These shareholders include any controlling shareholder, any shareholder who knows that it possesses the power to determine the outcome of a shareholder vote and any shareholder who, pursuant to the provisions of the articles of association, has the power to appoint or prevent the appointment of an office holder or any other power with respect to the company. However, the Companies Law does not define the substance of this duty of fairness.
Provisions Restricting Change in Control of Our Company
Except for requiring a special majority voting in order to amend our articles of association, there are no specific provisions of our memorandum, articles of association or other constituent documents that would have an effect of delaying, deferring or preventing a change in control of Itamar Medical or that would operate only with respect to a merger, acquisition or corporate restructuring involving us (or any of our subsidiaries). However, as described below, certain provisions of the Companies Law may have such effect.
| 122 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The Companies Law includes provisions that allow a merger transaction and requires that each company that is a party to the merger have the transaction approved by its Board of Directors and a vote of the majority of its shares. For purposes of the shareholder vote of each party, unless a court rules otherwise, the merger will not be deemed approved if shares representing a majority of the voting power present at the shareholders meeting and which are not held by the other party to the merger (or by any person who holds 25% or more of the voting power or the right to appoint 25% or more of the directors of the other party) vote against the merger. Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that as a result of the merger the surviving company will be unable to satisfy the obligations of any of the parties to the merger. In addition, a merger may not be completed unless at least (1) 50 days have passed from the time that the requisite proposals for approval of the merger were filed with the Israeli Registrar of Companies by each merging company and (2) 30 days have passed since the merger was approved by the shareholders of each merging company.
The Companies Law also provides that an acquisition of shares in a public company must be made by means of a “special” tender offer if as a result of the acquisition (1) the purchaser would become a 25% or greater shareholder of the company, unless there is already another 25% or greater shareholder of the company or (2) the purchaser would become a 45% or greater shareholder of the company, unless there is already a 45% or greater shareholder of the company. These requirements do not apply if, in general, the acquisition (1) was made in a private placement that received shareholder approval, (2) was from a 25% or greater shareholder of the company which resulted in the acquirer becoming a 25% or greater shareholder of the company, or (3) was from a 45% or greater shareholder of the company which resulted in the acquirer becoming a 45% or greater shareholder of the company. A “special” tender offer must be extended to all shareholders, but the offeror is not required to purchase more than 5% of the company’s outstanding shares, regardless of how many shares are tendered by shareholders. In general, the tender offer may be consummated only if (1) at least 5% of the company’s outstanding shares will be acquired by the offeror and (2) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer.
If, as a result of an acquisition of shares, the acquirer will hold more than 90% of a company’s outstanding shares, the acquisition must be made by means of a tender offer for all of the outstanding shares. In general, if less than 5% of the outstanding shares are not tendered in the tender offer and more than half of the offerees who have no personal interest in the offer tendered their shares, all the shares that the acquirer offered to purchase will be transferred to it. Shareholders may request appraisal rights in connection with a full tender offer for a period of six months following the consummation of the tender offer, but the acquirer is entitled to stipulate that tendering shareholders will forfeit such appraisal rights.
Lastly, Israeli tax law treats some acquisitions, such as stock-for-stock exchanges between an Israeli company and a foreign company, less favorably than U.S. tax laws. For example, Israeli tax law may, under certain circumstances, subject a shareholder who exchanges his ordinary shares for shares in another corporation to taxation prior to the sale of the shares received in such stock-for-stock swap.
Changes in Our Capital
Changes in our capital, such as increase of authorized share capital or creation of another class of shares, are subject to the approval of the shareholders by a simple majority. See Item 10.B “Additional Information – Memorandum and Articles of Association –Shareholders Meetings” above.
| 123 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Private Placements
Under the Companies Law, if (i) as a result of a private placement a person would become a controlling shareholder (as defined under the Companies Law) or (ii) a private placement will entitle investors to receive 20% or more of the voting rights of a company as calculated before the private placement, and all or part of the private placement consideration is not in cash or in public traded securities or is not in market terms and if as a result of the private placement the holdings of a substantial shareholder shall increase or as a result of it a person shall become a substantial shareholder, then in either case, the issuance of shares must be approved by the board of directors and by the shareholders of the company. A “substantial shareholder” is defined as a shareholder who holds five percent or more of the company’s outstanding share capital, assuming the exercise of all of the securities convertible into shares held by that person. In order for the private placement to be considered on “market terms,” the board of directors has to determine, on the base of detailed explanation, that the private placement is on market terms, unless proven otherwise.
| C. | Material Contracts |
Agreement with Kaiser
On August 16, 2007, our U.S. Subsidiary entered into a master products and services agreement with Kaiser Foundation Health Plan, Inc., or Kaiser, one of the largest medical insurers and hospital systems in the U.S. (as amended, the “Kaiser Framework Agreement”).
Under the Kaiser Framework Agreement, we undertook to supply Kaiser and its affiliates with our WatchPAT and Endo PAT products and ancillary accessories as well as offer maintenance services for the products supplied by us under the agreement. The agreement also contains provisions regarding (1) the pricing terms of the products and services offered by us to Kaiser, (2) payment terms, (3) the warranty we provide with respect to the supply of our products, including in case of product recalls, and (4) our undertaking to indemnify the customer in case that our products infringe upon the intellectual property rights of third parties.
The current term of the Kaiser Framework Agreement is until October 31, 2018 with Kaiser having the right to extend the term of the agreement for two additional one (1) year periods upon notice to us prior to the extension of the current term.
Philips Japan Distribution Agreement
On February 24, 2014, we entered into a distribution agreement with Philips Respironics GK, a subsidiary of Koninklijke Philips NV (also known as Royal Philips), or Philips Japan (as amended, the “Distribution Agreement”).
Under the Distribution Agreement, Philips Japan was granted exclusive rights to distribute our WatchPAT products and ancillary accessories in Japan. The agreement contains customary provisions, including the terms of warranty for defective products and our undertaking to indemnify Philips in case that our products infringe upon the intellectual property rights of third parties. Philips Japan does not have the right to return the products that we deliver them pursuant to the Distribution Agreement.
We may terminate the agreement if, among other things, Philips Japan does not meet certain minimum purchase requirements of our products specified in the Distribution Agreement.
The term of the Distribution Agreement is scheduled to expire on December 31, 2018. Upon termination of the agreement, Philips Japan, which holds the Japanese’s regulatory approval for marketing the WatchPAT in Japan, is required to transfer the regulatory approval to us.
| 124 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Agreement with VA
On October 12, 2011 and March 12, 2014, our U.S. Subsidiary entered into a Solicitation/Contract/Order for Commercial Items agreement with the Department of Veterans Affairs, or VA, one of the largest U.S. hospital and clinics chains (as amended, the “VA Framework Agreement”).
Under the VA Framework Agreement, we undertook to supply VA and its affiliates with our WatchPAT and Endo PAT products and ancillary accessories as well as offer maintenance services for the products supplied by us under the agreement. The agreement also contains provisions regarding (1) the pricing terms of the products and services offered by us to VA, (2) payment terms, (3) the warranty we provide with respect to the supply of our products, including in case of product recalls, and (4) our undertaking to indemnify VA in case that our products infringe upon the intellectual property rights of third parties.
The current term of the VA Framework Agreement is until June 14, 2023.
2017 Credit Line
See the summary under Item 5.B “Operating and Financial Review and Prospects– Liquidity and Capital Resources – Principal Financing Activities - 2017 Credit Line”.
2018 Private Placement
See the summary under Item 5.B “Operating and Financial Review and Prospects– Liquidity and Capital Resources – Principal Financing Activities – 2018 Private Placement”.
| D. | Exchange Controls |
Israeli law and regulations do not impose any material foreign exchange restrictions on non-Israeli holders of our ordinary shares. There are currently no Israeli currency control restrictions on payments of dividends or other distributions with respect to our ordinary shares or the proceeds from the sale of the shares, except for the obligation of Israeli residents to file reports with the Bank of Israel regarding certain transactions. However, legislation remains in effect pursuant to which currency controls can be imposed by administrative action at any time.
The ownership or voting of our ordinary shares by non-residents of Israel, except with respect to citizens of countries which are in a state of war with Israel, is not restricted in any way by our memorandum of association or articles of association or by the laws of the State of Israel.
| 125 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| E. | Taxation |
The following is a discussion of Israeli and United States tax consequences material to our shareholders. The discussion is not intended, and should not be construed, as legal or professional tax advice and does not exhaust all possible tax considerations.
Holders of our ADSs should consult their own tax advisors as to the United States, Israeli or other tax consequences of the purchase, ownership and disposition of our ADSs, including, in particular, the effect of any foreign, state or local taxes.
Israeli Tax Considerations
The following is a summary of the principal tax laws applicable to companies in Israel, with special reference to their effect on us. The following also contains a discussion of the material Israeli tax consequences to purchasers of our ordinary shares or ADSs. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. To the extent that the discussion is based on new tax legislation which has not been subject to judicial or administrative interpretation, we cannot assure you that the views expressed in the discussion will be accepted by the appropriate tax authorities or the courts. The discussion is not intended, and should not be construed, as a legal or professional tax advice and is not exhaustive of all possible tax considerations.
General Corporate Tax Structure
Most of our production facilities have been granted “Approved Enterprise” and “Benefited Enterprise” status under the Law for the Encouragement of Capital Investments, 1959, or the Investment Law. We are a “Foreign Investors’ Company” as defined by the Investment Law, which means we are entitled to tax benefits for taxable income arising from our Approved or Benefited Enterprise status.
A company having an Approved Enterprise, like us, that distributes a dividend from income that was tax exempt, will be required in the tax year of the dividend distribution to pay corporate tax on the amount of the dividend distributed (including the company tax required as a result of the distribution) at the corporate tax rate that would have been applicable to it in the year the income was generated if it had not been exempt from tax.
Generally, Israeli companies are subject to corporate tax on taxable income at the rate of 25% for the 2016 tax year, 24% for the 2017 tax year and 23% for the 2018 tax year and thereafter. However, the effective tax rate payable by a company that generates income qualifying for benefits under the Investment Law may be considerably less. Israeli companies are generally subject to capital gains tax at the corporate tax rate.
We are permitted to measure our Israeli taxable income in dollars pursuant to regulations published by the Israeli Minister of Finance, which provide the conditions for doing so. We believe that we meet, and will continue to meet, the necessary conditions and as such, starting with our 2016 tax year, we measure our results for tax purposes based on the U.S. dollar/NIS exchange rate on December 31 of the relevant tax year.
Tax Benefits under the Law for the Encouragement of Capital Investments, 1959, as amended.
Most of our production facilities have been granted “Approved Enterprise” and “Benefited Enterprise” status under the Investment Law, including its various amendments. We are a “Foreign Investors’ Company” as defined by the Investment Law, which means we are entitled to tax benefits for taxable income arising from our Approved or Benefited Enterprise status. Since our incorporation we incurred significant losses and therefore we did not start benefiting from such status. To be eligible for these tax benefits, one must continue to meet certain conditions stipulated in the Investment Law and its regulations and the criteria set out in the specific certificate of approval. In the event we are considered as having failed to comply with these conditions, in whole or in part, the eligibility for the benefits may be canceled and we may be required to refund the relevant amount, including inflation adjustments and interest. Since we have accumulated tax losses, we did not benefit from such tax benefits. Once we utilize all of our accumulated tax losses, we expect to derive tax benefits in Israel relating to our Benefited Enterprise and Preferred Enterprise programs for which we are eligible.
| 126 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
A company having an Approved Enterprise, like us, that distributes a dividend from income that was tax exempt, will be required in the tax year of the dividend distribution to pay corporate tax on the amount of the dividend distributed (including the company tax required as a result of the distribution) at the corporate tax rate that would have been applicable to it in the year the income was generated if it had not been exempt from tax. Since we have accumulated losses, we did not benefit from such status.
Income from sources other than the “Approved Enterprise” and “Benefited Enterprise” status are taxable at regular corporate tax rates.
See also Note 19 to our audited consolidated financial statements included elsewhere in this registration statement.
Tax Benefits and Grants for Research and Development
Israeli tax law allows, under specified conditions, a tax deduction for expenditures, including capital expenditures, for the year in which they are incurred. These expenses must relate to scientific research and development projects and must be approved by the relevant Israeli government ministry, determined by the field of research, and the research and development must be conducted for the promotion of the company and carried out by or on behalf of the company seeking such deduction. However, the amount of such deductible expenses shall be reduced by the sum of any funds received through government grants for the financing of such scientific research and development projects. Expenditures not so approved are deductible over a three-year period.
Tax Benefits under the Law for the Encouragement of Industry (Taxes), 1969
Under the Law for the Encouragement of Industry (Taxes), 1969, or the Industry Encouragement Law, Industrial Companies (as defined below) are entitled to the following tax benefits, among others:
| · | deductions over an eight-year period for purchases of know-how and patents; |
| · | deductions over a three-year period of expenses involved with the issuance and listing of shares on a stock market; |
| · | the right to elect, under specified conditions, to file a consolidated tax return with other related Israeli Industrial Companies; and |
| · | accelerated depreciation rates on equipment and buildings. |
Eligibility for benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority. Under the Industry Encouragement Law, an “Industrial Company” is defined as a company which is an Israeli resident for tax purposes, which at least 90% of the income of which, in any tax year, determined in Israeli currency, exclusive of income from government loans, capital gains, interest and dividends, is derived from an “Industrial Enterprise” owned by it.
| 127 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
An “Industrial Enterprise” is defined as an enterprise whose major activity in a given tax year is industrial production activity. We believe that we currently qualify as an Industrial Company within the definition of the Industry Encouragement Law. No assurance can be given that we will continue to qualify as an Industrial Company or that the benefits described above will be available in the future.
Capital Gains Tax on Sales of our Ordinary Shares
Israeli law generally imposes a capital gains tax on the sale of any capital assets by residents of Israel, as defined for Israeli tax purposes, and on the sale of assets located in Israel, including shares in Israeli companies, by both residents and non-residents of Israel, unless a specific exemption is available or unless a tax treaty between Israel and the shareholder’s country of residence provides otherwise. The Tax Ordinance distinguishes between real gain and inflationary surplus. The inflationary surplus is a portion of the total capital gain equivalent to the increase of the relevant asset’s purchase price attributable to an increase in the Israeli consumer price index, or a foreign currency exchange rate, between the date of purchase and the date of sale. The real gain is the excess of the total capital gain over the inflationary surplus.
The following discussion refers to the sale of our ordinary shares. However, the same tax treatment would apply to the sale of our ADSs.
Taxation of Israeli Residents
The tax rate generally applicable to the capital gains derived from the sale of shares, whether listed on a stock market or not, is 25% for Israeli individuals, unless such shareholder is considered a “significant shareholder” at any time during the 12-month period preceding such sale (i.e., such shareholder holds directly or indirectly, including jointly with others, at least 10% of any means of control in the company) in which case the tax rate will be 30%. Israeli companies are subject to the corporate tax rate on capital gains derived from the sale of listed shares. However, different tax rates may apply to dealers in securities and shareholders who acquired their shares prior to an initial public offering.
As of January 1, 2017, shareholders that are individuals who have taxable income that exceeds NIS 640,000 in a tax year (linked to the Israeli CPI each year), will be subject to an additional tax, referred to as Income Surtax, at the rate of 3% on their taxable income for such tax year which is in excess of such threshold. For this purpose taxable income will include taxable capital gains from the sale of our shares and taxable income from dividend distributions.
Taxation of Non-Israeli Residents
Non-Israeli residents are generally exempt from Israeli capital gains tax on any gains derived from the sale of shares publicly traded on the TASE provided such gains did not derive from a permanent establishment of such shareholders in Israel. If non-Israeli resident shareholders acquired their shares prior to the issuer’s initial public offering, a partial or full exemption may be available under certain requirements. However, non-Israeli corporations will not be entitled to such exemption if Israeli residents (i) have a controlling interest of more than 25% in such non-Israeli corporation, or (ii) are the beneficiaries of or are entitled to 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly.
| 128 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In addition, the sale, exchange or disposition of our ordinary shares by a shareholder who is a U.S. resident (for purposes of the U.S.-Israel Tax Treaty), and who holds ordinary shares as a capital asset, is also exempt from Israeli capital gains tax under the U.S.-Israel Tax Treaty unless either (i) the shareholder holds, directly or indirectly, shares representing 10% or more of our voting power during any part of the 12-month period preceding such sale, (ii) the capital gains arising from such sale are attributable to a permanent establishment of the shareholder located in Israel, (iii) the gain is from sale of shares of a real estate association (as defined in the Israeli Land Appreciation Tax Law), or (iv) the U.S. shareholder, being an individual, is present in Israel for a period or periods aggregating 183 days or more during the taxable year in which the sale was made. If the above conditions are not met, the U.S. resident would be subject to Israeli tax, unless exempt under the Israeli domestic law as described above. Under the U.S.-Israel Tax Treaty, the gain would be treated as foreign source income for United States foreign tax credit purposes and such U.S. resident would generally be permitted to claim a credit for such taxes against the United States federal income tax imposed on such sale, exchange or disposition, subject to the limitations under the United States federal income tax laws applicable to foreign tax credits.
Taxation of Dividends Paid on our Ordinary Shares
The following discussion refers to dividends paid on our ordinary shares. However, the same tax treatment would apply to dividends paid on our ADSs.
Taxation of Israeli Residents
Israeli resident individuals are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares, other than bonus shares (share dividends) or stock dividends. The tax rate generally applicable to such dividends is 25% or 30% for a shareholder that is considered a significant shareholder at any time during the 12-month period preceding such distribution. Dividends paid out of profits sourced from ordinary income are subject to withholding tax at the rate of 25% or 30%. Dividends paid from income derived from our Approved and Benefited Enterprises are subject to withholding tax at the rate of 15%. Dividends paid as of January 1, 2014 from income derived from Preferred Enterprise and Preferred Technology Enterprise will be subject to withholding tax at the rate of 20%. Currently we do not have Preferred Enterprise and Preferred Technology Enterprise. We cannot assure you that we will designate the profits that are being distributed in a way that will reduce shareholders’ tax liability. All dividend distributions to Israeli resident corporations are not subject to a withholding tax.
For information with respect to the applicability of Income Surtax on distribution of dividends, please see “Capital Gains Tax on Sales of Our Ordinary Shares” and “Taxation of Israeli Residents” above in this Item 10.
Taxation of Non-Israeli Residents
Non-residents of Israel, both companies and individuals, are generally subject to Israeli income tax on the receipt of dividends paid on our ordinary shares, at the aforementioned rates applicable to Israeli residents, which tax will be withheld at source, unless a different rate is provided in a treaty between Israel and the shareholder’s country of residence.
Under the U.S.-Israel Treaty, the maximum Israeli withholding tax on dividends paid by us is 25%. The U.S.-Israel Tax Treaty further provides for a 12.5% Israeli dividend withholding tax rate on dividends paid by an Israeli company to a U.S. corporation owning at least 10% or more of such Israeli company’s issued voting power for, in general, the part of the tax year which precedes the date of payment of the dividend and the entire preceding tax year. The lower 12.5% rate applies only to dividends paid from regular income (and not derived from an Approved, Benefited or Preferred Enterprise) in the applicable period and does not apply if the company has more than 25% of its gross income derived from certain types of passive income. If the conditions above in this paragraph are met, dividends from income of an Approved, Benefited or Preferred Enterprise are subject to a 15% withholding tax rate under the U.S.-Israel Tax Treaty.
| 129 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Residents of the United States generally will have withholding tax in Israel deducted at source. They may be entitled to a credit or deduction for United States federal income tax purposes in the amount of the taxes withheld, subject to detailed rules contained in United States tax legislation.
A non-resident of Israel who has dividend income derived from or accrued in Israel, from which tax was withheld at source, is generally exempt from the duty to file tax returns in Israel with respect to such income, provided such income was not derived from a business conducted in Israel by the taxpayer.
United States Federal Income Tax Consequences
THE FOLLOWING SUMMARY IS INCLUDED HEREIN FOR GENERAL INFORMATION AND IS NOT INTENDED TO BE, AND SHOULD NOT BE CONSIDERED TO BE, LEGAL OR TAX ADVICE. EACH U.S. HOLDER SHOULD CONSULT WITH HIS OR HER OWN TAX ADVISOR AS TO THE PARTICULAR U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND SALE OF ORDINARY SHARES AND AMERICAN DEPOSITORY SHARES, INCLUDING THE EFFECTS OF APPLICABLE STATE, LOCAL, FOREIGN OR OTHER TAX LAWS AND POSSIBLE CHANGES IN THE TAX LAWS.
Subject to the limitations described in the next paragraph, the following discussion summarizes the material U.S. federal income tax consequences to a “U.S. Holder” arising from the purchase, ownership and sale of our ordinary shares and ADSs. For this purpose, a “U.S. Holder” is a holder of ordinary shares or ADSs that is: (i) an individual citizen or resident of the United States, including an alien individual who is a lawful permanent resident of the United States or meets the substantial presence residency test under U.S. federal income tax laws; (ii) a corporation (or entity treated as a corporation for U.S. federal income tax purposes) or a partnership (other than a partnership that is not treated as a U.S. person under any applicable U.S. Treasury regulations) created or organized under the laws of the United States or the District of Columbia or any political subdivision thereof; (iii) an estate, the income of which is includable in gross income for U.S. federal income tax purposes regardless of source; (iv) a trust if a court within the United States is able to exercise primary supervision over the administration of the trust and one or more U.S. persons have authority to control all substantial decisions of the trust; or (v) a trust that has a valid election in effect to be treated as a U.S. person to the extent provided in U.S. Treasury regulations.
This summary is for general information purposes only and does not purport to be a comprehensive description of all of the U.S. federal income tax considerations that may be relevant to a decision to purchase our ordinary shares or ADSs. This summary generally considers only U.S. Holders that will own our ordinary shares or ADSs as capital assets. Except to the limited extent discussed below, this summary does not consider the U.S. federal tax consequences to a person that is not a U.S. Holder, nor does it describe the rules applicable to determine a taxpayer’s status as a U.S. Holder. This summary is based on the provisions of the Internal Revenue Code of 1986, as amended, or the Code, final, temporary and proposed U.S. Treasury regulations promulgated thereunder, administrative and judicial interpretations thereof, and the Convention between the Government of the United States of America and the Government of Israel With Respect to Taxes on Income, or the U.S./Israel Income Tax Treaty, all as in effect as of the date hereof and all of which are subject to change, possibly on a retroactive basis, and all of which are open to differing interpretations. We will not seek a ruling from the U.S. Internal Revenue Service, or IRS, with regard to the U.S. federal income tax treatment of an investment in our ordinary shares or ADSs by U.S. Holders and, therefore, can provide no assurances that the IRS will agree with the conclusions set forth below.
| 130 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
This discussion does not address all of the aspects of U.S. federal income taxation that may be relevant to a particular U.S. holder based on such holder’s particular circumstances and in particular does not discuss any estate, gift, generation-skipping, transfer, state, local, excise or foreign tax considerations. In addition, this discussion does not address the U.S. federal income tax treatment of a U.S. Holder who is: (i) a bank, life insurance company, regulated investment company, or other financial institution or “financial services entity”; (ii) a broker or dealer in securities or foreign currency; (iii) a person who acquired our ordinary shares or ADSs in connection with employment or other performance of services; (iv) a U.S. Holder that is subject to the U.S. alternative minimum tax, or ATM; (v) a U.S. Holder that holds our ordinary shares or ADSs as a hedge or as part of a hedging, straddle, conversion or constructive sale transaction or other risk-reduction transaction for U.S. federal income tax purposes; (vi) a tax-exempt entity; (vii) real estate investment trusts or grantor trusts; (viii) a U.S. Holder that expatriates out of the United States or a former long-term resident of the United States; or (ix) a person having a functional currency other than the dollar. This discussion also does not address the U.S. federal income tax treatment of a U.S. Holder that owns, directly or constructively, at any time, ordinary shares or ADSs representing 10% or more of our voting power. Additionally, the U.S. federal income tax treatment of persons who hold ordinary shares or ADSs through a partnership or other pass-through entity are not considered.
Each prospective investor is advised to consult his or her own tax adviser for the specific tax consequences to that investor of purchasing, holding or disposing of our ordinary shares or ADSs, including the effects of applicable state, local, foreign or other tax laws and possible changes in the tax laws.
Taxation of Dividends Paid on Ordinary Shares or ADSs
We do not intend to pay dividends in the foreseeable future. In the event that we do pay dividends, and subject to the discussion under the heading “Passive Foreign Investment Companies”, or PFIC below, a U.S. Holder, other than certain U.S. Holder’s that are U.S. corporations, will be required to include in gross income as ordinary income the amount of any distribution paid on ordinary shares or ADSs (including the amount of any Israeli tax withheld on the date of the distribution), to the extent that such distribution does not exceed our current and accumulated earnings and profits, as determined for U.S. federal income tax purposes. The amount of a distribution which exceeds our earnings and profits will be treated first as a non-taxable return of capital, reducing the U.S. Holder’s tax basis for the ordinary shares or ADSs to the extent thereof, and then capital gain. We do not expect to maintain calculations of our earnings and profits under U.S. federal income tax principles and, therefore, U.S. Holders should expect that the entire amount of any distribution generally will be reported as dividend income.
On December 22, 2017, President Trump signed into law the Tax Cuts and Jobs Act, or the TCJA. The TCJA provides a 100% deduction for the foreign-source portion of dividends received from “specified 10-percent owned foreign corporations” by U.S. corporate holders, subject to a one-year holding period. No foreign tax credit, including Israeli withholding tax (or deduction for foreign taxes paid with respect to qualifying dividends), would be permitted for foreign taxes paid or accrued with respect to a qualifying dividend. Deduction would be unavailable for “hybrid dividends”. The dividend received deduction enacted under the TCJA may not apply to dividends from a PFIC, as discussed below.
In general, preferential tax rates for “qualified dividend income” and long-term capital gains are applicable for U.S. Holders that are individuals, estates or trusts. For this purpose, “qualified dividend income” means, among other things, dividends received from a “qualified foreign corporation”. A “qualified foreign corporation” is a corporation that is entitled to the benefits of a comprehensive tax treaty with the United States which includes an exchange of information program. The IRS has stated that the Israel/U.S. Tax Treaty satisfies this requirement and we believe we are eligible for the benefits of that treaty.
| 131 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In addition, our dividends, if any, will be qualified dividend income if our ordinary shares or ADSs are readily tradable on the Nasdaq or another established securities market in the United States. Dividends will not qualify for the preferential rate if we are treated, in the year the dividend is paid or in the prior year, as a PFIC, as described below under PFIC. A U.S. Holder will not be entitled to the preferential rate: (i) if the U.S. Holder has not held our ordinary shares or ADSs for at least 61 days of the 121 day period beginning on the date which is 60 days before the ex-dividend date; or (ii) to the extent the U.S. Holder is under an obligation to make related payments on substantially similar property. Any days during which the U.S. Holder has diminished its risk of loss on our ordinary shares or ADSs are not counted towards meeting the 61-day holding period. Finally, U.S. Holders who elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code will not be eligible for the preferential rate of taxation.
The amount of a distribution with respect to our ordinary shares or ADSs will be measured by the amount of the fair market value of any property distributed, and for U.S. federal income tax purposes, the amount of any Israeli taxes withheld therefrom. Cash distributions paid by us in NIS, if any, will be included in the income of U.S. Holders at a dollar amount based upon the spot rate of exchange in effect on the date the dividend is includible in the income of the U.S. Holder, and U.S. Holders will have a tax basis in such NIS for U.S. federal income tax purposes equal to such dollar value. If the U.S. Holder subsequently converts the NIS into U.S. dollars or otherwise disposes of it, any subsequent gain or loss in respect of such NIS arising from exchange rate fluctuations will be U.S. source ordinary exchange gain or loss.
Distributions paid by us will generally be foreign source income for U.S. foreign tax credit purposes and will generally be considered passive category income for such purposes. Subject to the limitations set forth in the Code and the TCJA, U.S. Holders may elect to claim a foreign tax credit against their U.S. federal income tax liability for Israeli income tax withheld from distributions received in respect of the ordinary shares or ADSs. The rules relating to the determination of the U.S. foreign tax credit are complex, and U.S. Holders should consult with their own tax advisors to determine whether, and to what extent, they are entitled to such credit. U.S. Holders that do not elect to claim a foreign tax credit may instead claim a deduction for Israeli income taxes withheld, provided such U.S. Holders itemize their deductions.
Taxation of the Disposition of Ordinary Shares or ADSs
Except as provided under the PFIC rules described below under “Passive Foreign Investment Companies,” upon the sale, exchange or other disposition of our ordinary shares or ADSs, a U.S. Holder will recognize capital gain or loss in an amount equal to the difference between such U.S. Holder’s tax basis for the ordinary shares or ADSs in dollars and the amount realized on the disposition in dollar (or its dollar equivalent determined by reference to the spot rate of exchange on the date of disposition, if the amount realized is denominated in a foreign currency). The gain or loss realized on the sale, exchange or other disposition of ordinary shares or ADSs will be long-term capital gain or loss if the U.S. Holder has a holding period of more than one year at the time of the disposition.
Gain realized by a U.S. Holder on a sale, exchange or other disposition of ordinary shares or ADSs will generally be treated as U.S. source income for U.S. foreign tax credit purposes. A loss realized by a U.S. Holder on the sale, exchange or other disposition of our ordinary shares or ADSs is generally allocated to U.S. source income. The deductibility of a loss realized on the sale, exchange or other disposition of ordinary shares or ADSs is subject to limitations. An additional 3.8% net investment income tax (described below) may apply to gains recognized upon the sale, exchange or other taxable disposition of our ordinary shares or ADS by certain U.S. Holders who meet certain income thresholds.
| 132 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Passive Foreign Investment Companies
Special U.S. federal income tax laws apply to U.S. taxpayers who own shares of a corporation that is a PFIC. We will be treated as a PFIC for U.S. federal income tax purposes for any taxable year that either:
| · | 75% or more of our gross income (including our pro rata share of gross income for any company, in which we are considered to own 25% or more of the shares by value), in a taxable year is passive; or |
| · | At least 50% of our assets, averaged over the year and generally determined based upon fair market value (including our pro rata share of the assets of any company in which we are considered to own 25% or more of the shares by value) are held for the production of, or produce, passive income. |
For this purpose, passive income generally consists of dividends, interest, rents, royalties, annuities and income from certain commodities transactions and from notional principal contracts. Cash is treated as generating passive income.
We believe that we will not be a PFIC for the current taxable year and do not expect to become a PFIC in the foreseeable future. The tests for determining PFIC status are applied annually, and it is difficult to make accurate projections of future income and assets which are relevant to this determination. In addition, our PFIC status may depend in part on the market value of our ordinary shares. Accordingly, there can be no assurance that we currently are not or will not become a PFIC.
If we currently are or become a PFIC, each U.S. Holder who has not elected to treat us as a qualified electing fund by making a “QEF election,” or who has not elected to mark the shares to market (as discussed below), would, upon receipt of certain distributions by us and upon disposition of our ordinary shares or ADSs at a gain: (I) have such distribution or gain allocated ratably over the U.S. Holder’s holding period for the ordinary shares or ADSs, as the case may be; (ii) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (iii) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, when shares of a PFIC are acquired by reason of death from a decedent that was a U.S. Holder, the tax basis of such shares would not receive a step-up to fair market value as of the date of the decedent’s death, but instead would be equal to the decedent’s basis if lower, unless all gain were recognized by the decedent. Indirect investments in a PFIC may also be subject to these special U.S. federal income tax rules.
The PFIC rules described above would not apply to a U.S. Holder who makes a QEF election for all taxable years that such U.S. Holder has held the ordinary shares or ADSs while we are a PFIC, provided that we comply with specified reporting requirements. Instead, each U.S. Holder who has made such a QEF election is required for each taxable year that we are a PFIC to include in income such U.S. Holder’s pro rata share of our ordinary earnings as ordinary income and such U.S. Holder’s pro rata share of our net capital gains as long-term capital gain, regardless of whether we make any distributions of such earnings or gain. In general, a QEF election is effective only if we make available certain required information. The QEF election is made on a shareholder-by-shareholder basis and generally may be revoked only with the consent of the IRS. Although we have no obligation to do so, we currently intend to notify U.S. Holders if we believe we will be treated as a PFIC for any tax year in order to enable U.S. Holders to consider whether to make a QEF election. In addition, we intend to furnish U.S. Holders annually with information needed in order to complete IRS Form 8621 and to make and maintain a valid QEF election for any year in which we or any of our subsidiaries are a PFIC. U.S. Holders should consult with their own tax advisors regarding eligibility, manner and advisability of making a QEF election if we are treated as a PFIC.
| 133 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
In addition, the PFIC rules described above would not apply if we were a PFIC and a U.S. Holder made a mark-to-market election. A U.S. Holder of our ordinary shares or ADSs which are regularly traded on a qualifying exchange, including Nasdaq, can elect to mark the ordinary shares or ADSs to market annually, recognizing as ordinary income or loss each year an amount equal to the difference as of the close of the taxable year between the fair market value of the ordinary shares or ADSs and the U.S. Holder’s adjusted tax basis in the ordinary shares or ADSs. Losses are allowed only to the extent of net mark-to-market gain previously included income by the U.S. Holder under the election for prior taxable years.
U.S. Holders who hold our ordinary shares or ADSs during a period, if any, in which we are a PFIC will be subject to the foregoing rules, even if we cease to be a PFIC.
U.S. Holders are strongly urged to consult their tax advisors about the PFIC rules, including tax return filing requirements and the eligibility, manner, and consequences to them of making a QEF or mark-to-market election with respect to our ordinary shares or ADSs in the event that we are a PFIC.
Tax on Net Investment Income
For taxable years beginning after December 31, 2013, U.S. Holders who are individuals, estates or trusts will generally be required to pay a 3.8% Medicare tax on their net investment income (including dividends on and gains from the sale or other disposition of our ordinary shares or ADSs), or in the case of estates and trusts on their net investment income that is not distributed. In each case, the 3.8% Medicare tax applies only to the extent the U.S. Holder’s total adjusted income exceeds applicable thresholds.
Tax Consequences for Non-U.S. Holders of Ordinary Shares or ADSs
Except as provided below, an individual, corporation, estate or trust that is not a U.S. Holder referred to below as a non-U.S. Holder, generally will not be subject to U.S. federal income or withholding tax on the payment of dividends on, and the proceeds from the disposition of, our ordinary shares or ADSs.
A non-U.S. Holder may be subject to U.S. federal income tax on a dividend paid on our ordinary shares or ADSs or gain from the disposition of our ordinary shares or ADSs if: (1) such item is effectively connected with the conduct by the non-U.S. Holder of a trade or business in the United States and, if required by an applicable income tax treaty is attributable to a permanent establishment or fixed place of business in the United States; (2) in the case of a disposition of our ordinary shares or ADSs, the individual non-U.S. Holder is present in the United States for 183 days or more in the taxable year of the disposition and other specified conditions are met.
In general, non-U.S. Holders will not be subject to backup withholding with respect to the payment of dividends on our ordinary shares or ADSs if payment is made through a paying agent, or office of a foreign broker outside the United States. However, if payment is made in the United States or by a U.S. related person, non-U.S. Holders may be subject to backup withholding, unless the non-U.S. Holder provides an applicable IRS Form W-8 (or a substantially similar form) certifying its foreign status, or otherwise establishes an exemption.
| 134 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The amount of any backup withholding from a payment to a non-U.S. Holder will be allowed as a credit against such holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that the required information is timely furnished to the IRS.
Information Reporting and Withholding
A U.S. Holder may be subject to backup withholding with respect to cash dividends and proceeds from a disposition of our ordinary shares or ADSs. The rate of backup withholding was decreased from 28% to 24% effective January 1, 2018, within the framework of TCJA. In general, backup withholding will apply only if a U.S. Holder fails to comply with specified identification procedures. Backup withholding will not apply with respect to payments made to designated exempt recipients, such as corporations and tax-exempt organizations. Backup withholding is not an additional tax and may be claimed as a credit against the U.S. federal income tax liability of a U.S. Holder, provided that the required information is timely furnished to the IRS.
Pursuant to recently enacted legislation, a U.S. Holder with interests in “specified foreign financial assets” (including, among other assets, our ordinary shares or ADSs, unless such shares or ADSs are held on such U.S. Holder’s behalf through a financial institution) may be required to file an information report with the IRS if the aggregate value of all such assets exceeds $50,000 on the last day of the taxable year or $75,000 at any time during the taxable year (or such higher dollar amount as may be prescribed by applicable IRS guidance); and may be required to file a Report of Foreign Bank and Financial Accounts, or FBAR, if the aggregate value of the foreign financial accounts exceeds $10,000 at any time during the calendar year. You should consult your own tax advisor as to the possible obligation to file such information report.
Tax Cuts and Jobs Act
On December 22, 2017, President Trump signed into law the TCJA. Although this is the most extensive overhaul of the United States tax regime in over thirty years, except for the aspects mentioned above in this section, the provisions of the TCJA are expected to materially impact U.S. Holder’s with respect to such holder’s ownership of our ordinary shares or the ADSs.
Medical Devices Excise Tax
The Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010 imposes significant new taxes on medical device makers in the form of a 2.3% excise tax on U.S. medical device sales, with certain exemptions, beginning in January 2013. The Consolidated Appropriations Act, 2016, signed into law on December 18, 2015, includes a two-year suspension on the medical device excise tax. Thus, the medical device excise tax did not apply to the sale of a taxable medical device by the manufacturer, producer, or importer of the device during the period beginning on January 1, 2016, and ending on December 31, 2017. On January 22, 2018, H.R. 195 was signed into law and extended the suspension for an additional two years until December 31, 2019. On July 24, 2018, the U.S. House of Representatives passed H.R. 184, a bill that would permanently repeal the tax on medical devices.
| F. | Dividends and Paying Agents |
We have never declared or paid cash dividends to our shareholders. Currently, we do not intend to pay cash dividends. We intend to reinvest any earnings in developing and expanding our business. Any future determination relating to our dividend policy will be at the discretion of our Board of Directors and will depend on a number of factors, including future earnings, our financial condition, operating results, contractual restrictions, capital requirements, business prospects, applicable Israeli law and other factors our Board of Directors may deem relevant. Accordingly, we have not appointed any paying agent.
| 135 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| G. | Statement by Experts |
The consolidated financial statements of Itamar Medical Ltd. as of December 31, 2017 and 2016, and for each of the years in the three-year period ended December 31, 2017, have been included herein and in the registration statement in reliance upon the report of Somekh Chaikin, a member firm of KPMG International, independent registered public accounting firm, appearing elsewhere herein, and upon the authority of said firm as experts in accounting and auditing.
| H. | Documents on Display |
When this registration statement becomes effective, we will be subject to the reporting requirements of the Exchange Act, as applicable to “foreign private issuers” as defined in Rule 3b-4 under the Exchange Act, and in accordance therewith, we will file annual and interim reports and other information with the SEC.
As a foreign private issuer, we will be exempt from certain provisions of the Exchange Act. Accordingly, our proxy solicitations will not be subject to the disclosure and procedural requirements of Regulation 14A under the Exchange Act and transactions in our equity securities by our officers and directors will be exempt from reporting and the “short-swing” profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we will not be required under the Exchange Act to file periodic reports and financial statements as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act.
Notwithstanding the foregoing, we will furnish reports with the SEC on Form 6-K containing unaudited financial information for the first three quarters of each fiscal year and we will solicit proxies and furnish proxy statements for all meetings of shareholders, a copy of which proxy statement is furnished promptly thereafter with the SEC under the cover of a Current Report on Form 6-K.
In addition, since our ordinary shares are traded on the TASE, we file Hebrew language periodic and immediate reports with, and furnish information to, the TASE and the Israel Securities Authority, or the ISA, as required under Chapter Six of the Israeli Securities Law, 1968. Copies of our filings with the ISA can be retrieved electronically through the MAGNA distribution site of the ISA (www.magna.isa.gov.il) and the TASE website (www.maya.tase.co.il). As permitted under the ISL and approved by our shareholders in May 2018, once our ADSs will become listed on the Nasdaq Capital Market, we intend to comply with the Israeli regime for dual listed companies under Chapter E3 of the ISL, which will allow us to use in Israel the same periodic reports, financial and other relevant disclosure information (in English) that we submit to the SEC and Nasdaq.
This registration statement and the exhibits thereto and any other document we file pursuant to the Exchange Act may be inspected without charge and copied at prescribed rates at the following SEC public reference rooms: 100 F Street, N.E., Washington, D.C. 20549; and on the SEC Internet site (http://www.sec.gov) and on our website www.itamar-medical.com. However, the content of our website is not incorporated by reference into this registration statement.
You may obtain information on the operation of the SEC’s public reference room in Washington, D.C. by calling the SEC at 1-800-SEC-0330 or by visiting the SEC’s website at http://www.sec.gov.
| 136 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The documents concerning our Company which are referred to in this registration statement may also be inspected at our offices located at 9 Halamish Street, Caesarea 3088900, Israel.
| I. | Subsidiary Information |
Not applicable.
| ITEM 11. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISKS |
General
Market risk is the risk of loss related to changes in market prices, including interest rates and foreign exchange rates, of financial instruments that may adversely impact our consolidated financial position, results of operations or cash flows.
We are exposed to a variety of these market risks, primarily changes in interest rates and foreign currency fluctuations. To manage the volatility related to the foreign currency exposure, we may enter from time to time into various derivative transactions. However, we do not use financial instruments for trading purposes and are not a party to any leveraged derivative.
As of December 31, 2017 and June 30, 2018, we had cash and cash equivalents and marketable securities of approximately $10.8 million and $8.5 million, respectively. As of those dates, most of such cash and cash equivalents were held in dollars and NIS. The majority of our cash and cash equivalents are invested in banks in Israel and, to a smaller extent, in banks in the United States. The Israeli bank deposits are not insured, while the deposits made in the United States are in excess of insured limits and are not otherwise insured.
Interest Rate Risk
We are subject to market risk from exposure to changes in interest rates relating to borrowings under our bank credit line, which carries interest at a rate that is based on the LIBOR. As of December 31, 2017, we had borrowings of approximately $10.7 million under our convertible notes, which were fully repaid in February 2018. As of June 30, 2018, we had borrowings of approximately $5.0 million under the bank credit line. Based on the scheduled amount of the borrowings expected to be outstanding under such credit line in 2018, we estimate that each 10% increase in our borrowing rates would result in additional interest expense to us of approximately $0.5 million.
We follow an investment policy that was set by our Board of Directors whose primary objectives are to preserve principal while maximizing the income that we receive from our investments without significantly increasing risk and loss. Currently, we invest our free cash in bank deposits which are exposed to market risk due to fluctuation in interest rates, which may affect our interest, except that given the low levels of interest rates worldwide, our interest income is not material and a reduction in interest rates would not cause us a significant reduction in the absolute amounts of interest income to us. Our investment balances are comprised mainly of bank deposits. Because of their short-term nature, the carrying value of the bank deposits usually approximates their fair value.
| 137 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Foreign Currency Exchange Risk
Our functional and reporting currency is the U.S. dollar. Although the dollar is our functional currency, a significant portion of our expenses are denominated in NIS and a relatively small portion of our expenses is denominated in Euros, and currently most of our revenues are denominated in dollars. Therefore, our foreign currency exposures give rise to market risk associated with exchange rate movements of the dollar, mainly against the NIS and the Euro. In addition, while our convertible notes (which have been fully repaid in February 2018) were denominated in NIS, our bank credit line is denominated in dollars. For example, in the past, we kept a substantial part of our cash positions in NIS to hedge against our liability related to our convertible notes. Our NIS and Euro expenses consist principally of payroll to our employees in Israel, payments made to subcontractors for purchasing components to our products, research and development activities and marketing and sales activities. We anticipate that a significant portion of our expenses will continue to be denominated in currencies other than the dollar. If the dollar fluctuates significantly against either the NIS or the Euro, it may have a negative impact on our results of operations. To date, fluctuations in the exchange rates have not materially affected our results of operations or financial condition.
Due to the fact that exchange rates between the dollar and the NIS (as well as between the dollar and other currencies) fluctuate continuously, such fluctuations have an impact on our results and period-to-period comparisons of our results. The effects of foreign currency remeasurements are reported in our consolidated statements of operations. In order to reduce some of this currency exposure, we keep cash balances in NIS. These measures, however, may not adequately protect us from the material adverse effects of such fluctuations.
As of December 31, 2017 and June 30, 2018, we did not enter into any hedge transaction but we may do so in the future. Even if we do enter into such hedge transactions in the future, we cannot guarantee that such measures will effectively protect us from adverse effects due to the impact of fluctuations in currency exchange rates.
In addition, we have balance sheet exposure arising from assets and liabilities denominated in currencies other than the dollar, mainly in NIS and Euros. Any change of the conversion rates between the U.S. dollar and these currencies may create financial gain or loss.
The tables below provide information as of the dates indicated regarding our foreign currency-denominated monetary assets and liabilities (U.S. dollars in thousands).
| As of December 31, 2017 | As of June 30, 2018 | |||||||
| Assets: | ||||||||
| New Israeli Shekels | $ | 8,741 | $ | 2,409 | ||||
| Euros | 852 | 491 | ||||||
| Other currencies | 22 | 6 | ||||||
| Total | 9,615 | 2,906 | ||||||
| Liabilities: | ||||||||
| New Israeli Shekels | 15,564 | 2,177 | ||||||
| Euros | 117 | 75 | ||||||
| Total | 15,681 | 2,252 | ||||||
| Net assets (liabilities) | $ | (6,066 | ) | $ | 654 | |||
| 138 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| ITEM 12. | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
| A. | Debt Securities. |
Not applicable.
| B. | Warrants and rights. |
Not applicable.
| C. | Other Securities. |
Not applicable.
| D. | American Depositary Shares |
General
The Bank of New York Mellon, as depositary, will register and deliver American Depositary Shares, also referred to as ADSs. Each ADS will represent ● ordinary shares (or a right to receive ● ordinary shares) deposited with Bank Leumi or Bank Hapoalim, as custodian for the depositary in Israel. Each ADS will also represent any other securities, cash or other property which may be held by the depositary. The deposited shares together with any other securities, cash or other property held by the depositary are referred to as the deposited securities. The depositary’s office at which the ADSs will be administered as well as its principal executive office are located at 240 Greenwich Street, New York, New York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having uncertificated ADSs registered in your name, or (B) indirectly by holding a security entitlement in ADSs through your broker or other financial institution that is a direct or indirect participant in The Depository Trust Company, also called DTC. If you hold ADSs directly, you are a registered ADS holder, also referred to as an ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
Registered holders of uncertificated ADSs will receive statements from the depositary confirming their holdings.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. Israeli law governs shareholder rights. The depositary will be the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. For more complete information, you should read the entire deposit agreement and the form of ADR.
| 139 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay or distribute to ADS holders the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, upon payment or deduction of its fees and expenses. You will receive these distributions in proportion to the number of shares your ADSs represent.
Cash. The depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and cannot be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest.
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. See “Item 10. Additional Information – E. Taxation”. The depositary will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some of the value of the distribution.
Shares. The depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution. The depositary will only distribute whole ADSs. It will sell shares which would require it to deliver a fraction of an ADS (or ADSs representing those shares) and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares (or ADSs representing those shares) sufficient to pay its fees and expenses in connection with that distribution.
Rights to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may (i) exercise those rights on behalf of ADS holders, (ii) distribute those rights to ADS holders or (iii) sell those rights and distribute the net proceeds to ADS holders, in each case after deduction or upon payment of its fees and expenses. To the extent the depositary does not do any of those things, it will allow the rights to lapse. In that case, you will receive no value for them. The depositary will exercise or distribute rights only if we ask it to and provide satisfactory assurances to the depositary that it is legal to do so. If the depositary will exercise rights, it will purchase the securities to which the rights relate and distribute those securities or, in the case of shares, new ADSs representing the new shares, to subscribing ADS holders, but only if ADS holders have paid the exercise price to the depositary. U.S. securities laws may restrict the ability of the depositary to distribute rights or ADSs or other securities issued on exercise of rights to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
Other Distributions. The depositary will send to ADS holders anything else we distribute on deposited securities by any means it thinks is legal, fair and practical. If it cannot make the distribution in that way, the depositary has a choice. It may decide to sell what we distributed and distribute the net proceeds, in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with that distribution. U.S. securities laws may restrict the ability of the depositary to distribute securities to all or certain ADS holders, and the securities distributed may be subject to restrictions on transfer.
| 140 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or anything else to ADS holders. This means that you may not receive the distributions we make on our shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposits shares or evidence of rights to receive shares with the custodian in accordance with the deposit agreement. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs for the purpose of withdrawal at the depositary’s office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or stock transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person the ADS holder designates at the office of the custodian. Or, at your request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible. However, the depositary is not required to accept surrender of ADSs to the extent it would require delivery of a fraction of a deposited share or other security. The depositary may charge you a fee and its expenses for instructing the custodian regarding delivery of deposited securities.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to the ADS holder a statement confirming that the ADS holder is the registered holder of uncertificated ADSs. Upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to the ADS holder an ADR evidencing those ADSs.
Voting Rights
How do you vote?
ADS holders may instruct the depositary how to vote the number of deposited shares their ADSs represent. If we request the depositary to solicit your voting instructions (and we are not required to do so), the depositary will notify you of a shareholders’ meeting and send or make voting materials available to you. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date set by the depositary. The depositary will try, as far as practical, subject to the laws of Israel and the provisions of our articles of association or similar documents, to vote or to have its agents vote the shares or other deposited securities as instructed by ADS holders. If we do not request the depositary to solicit your voting instructions, you can still send voting instructions, and, in that case, the depositary may try to vote as you instruct, but it is not required to do so.
| 141 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Except by instructing the depositary as described above, you will not be able to exercise voting rights unless you surrender your ADSs and withdraw the shares. However, you may not know about the meeting enough in advance to withdraw the shares. In any event, the depositary will not exercise any discretion in voting deposited securities and it will only vote or attempt to vote as instructed, except that, if a holder of our ADSs does not provide the depositary with voting instructions for an agenda item in our shareholders meeting in a timely manner, we may instruct the depositary, if we reasonably do not know of any substantial opposition to such agenda item and the matter is not materially adverse to the interests of shareholders, to treat the holder as giving a discretionary proxy to a person designated by us as to that matter.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise voting rights and there may be nothing you can do if your shares are not voted as you requested.
In order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to Deposited Securities, if we request the Depositary to act, we agree to give the depositary notice of any such meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date.
Fees and Expenses
| Persons depositing or withdrawing shares or ADS holders must pay: |
For: | |
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property
Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates | |
| $.05 (or less) per ADS | Any cash distribution to ADS holders | |
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders | |
| $.05 (or less) per ADS per calendar year | Depositary services | |
| Registration or transfer fees that depend on the applicable bank or custodian through which you hold our ordinary shares | Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares | |
| Expenses of the depositary | Cable and facsimile transmissions (when expressly provided in the deposit agreement)
Converting foreign currency to U.S. dollars | |
| Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes | As necessary | |
| Any charges incurred by the depositary or its agents for servicing the deposited securities | As necessary |
| 142 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may collect any of its fees by deduction from any cash distribution payable (or by selling a portion of securities or other property distributable) to ADS holders that are obligated to pay those fees. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid.
From time to time, the depositary may make payments to us to reimburse us for costs and expenses generally arising out of establishment and maintenance of the ADS program, waive fees and expenses for services provided to us by the depositary or share revenue from the fees collected from ADS holders. In performing its duties under the deposit agreement, the depositary may use brokers, dealers, foreign currency dealers or other service providers that are owned by or affiliated with the depositary and that may earn or share fees, spreads or commissions.
The depositary may convert currency itself or through any of its affiliates and, in those cases, acts as principal for its own account and not as agent, advisor, broker or fiduciary on behalf of any other person and earns revenue, including, without limitation, transaction spreads, that it will retain for its own account. The revenue is based on, among other things, the difference between the exchange rate assigned to the currency conversion made under the deposit agreement and the rate that the depositary or its affiliate receives when buying or selling foreign currency for its own account. The depositary makes no representation that the exchange rate used or obtained in any currency conversion under the deposit agreement will be the most favorable rate that could be obtained at the time or that the method by which that rate will be determined will be the most favorable to ADS holders, subject to the depositary’s obligations under the deposit agreement. The methodology used to determine exchange rates used in currency conversions is available upon request.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until those taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your American Depositary Shares to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to ADS holders any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
| 143 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
Tender and Exchange Offers; Redemption, Replacement or Cancellation of Deposited Securities
The depositary will not tender deposited securities in any voluntary tender or exchange offer unless instructed to do by an ADS holder surrendering ADSs and subject to any conditions or procedures the depositary may establish.
If deposited securities are redeemed for cash in a transaction that is mandatory for the depositary as a holder of deposited securities, the depositary will call for surrender of a corresponding number of ADSs and distribute the net redemption money to the holders of called ADSs upon surrender of those ADSs.
If there is any change in the deposited securities such as a sub-division, combination or other reclassification, or any merger, consolidation, recapitalization or reorganization affecting the issuer of deposited securities in which the depositary receives new securities in exchange for or in lieu of the old deposited securities, the depositary will hold those replacement securities as deposited securities under the deposit agreement. However, if the depositary decides it would not be lawful and to hold the replacement securities because those securities could not be distributed to ADS holders or for any other reason, the depositary may instead sell the replacement securities and distribute the net proceeds upon surrender of the ADSs.
If there is a replacement of the deposited securities and the depositary will continue to hold the replacement securities, the depositary may distribute new ADSs representing the new deposited securities or ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities.
If there are no deposited securities underlying ADSs, including if the deposited securities are cancelled, or if the deposited securities underlying ADSs have become apparently worthless, the depositary may call for surrender or of those ADSs or cancel those ADSs upon notice to the ADS holders.
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
| 144 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
How may the deposit agreement be terminated?
The depositary will initiate termination of the deposit agreement if we instruct it to do so. The depositary may initiate termination of the deposit agreement if
| · | 90 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and accepted its appointment; |
| · | we delist our shares from an exchange on which they were listed and do not list the shares on another exchange; |
| · | we appear to be insolvent or enter insolvency proceedings |
| · | all or substantially all the value of the deposited securities has been distributed either in cash or in the form of securities; |
| · | there are no deposited securities underlying the ADSs or the underlying deposited securities have become apparently worthless; or |
| · | there has been a replacement of deposited securities. |
If the deposit agreement will terminate, the depositary will notify ADS holders at least 90 days before the termination date. At any time after the termination date, the depositary may sell the deposited securities. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement, unsegregated and without liability for interest, for the pro rata benefit of the ADS holders that have not surrendered their ADSs. Normally, the depositary will sell as soon as practicable after the termination date.
After the termination date and before the depositary sells, ADS holders can still surrender their ADSs and receive delivery of deposited securities, except that the depositary may refuse to accept a surrender for the purpose of withdrawing deposited securities or reverse previously accepted surrenders of that kind if it would interfere with the selling process. The depositary may refuse to accept a surrender for the purpose of withdrawing sale proceeds until all the deposited securities have been sold. The depositary will continue to collect distributions on deposited securities, but, after the termination date, the depositary is not required to register any transfer of ADSs or distribute any dividends or other distributions on deposited securities to the ADSs holder (until they surrender their ADSs) or give any notices or perform any other duties under the deposit agreement except as described in this paragraph.
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary as well as of our respective directors, officers and other affiliates. It also limits our liability and the liability of the depositary. We and the depositary:
| · | are only obligated to take the actions specifically set forth in the deposit agreement without negligence or bad faith, and the depositary will not be a fiduciary or have any fiduciary duty to holders of ADSs; |
| · | are not liable if we are or it is prevented or delayed by law or by events or circumstances beyond our or its ability to prevent or counteract with reasonable care or effort from performing our or its obligations under the deposit agreement; |
| · | are not liable if we or it exercises discretion permitted under the deposit agreement; |
| 145 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement, or for any; |
| · | have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; |
| · | may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person;. |
| · | are not liable for the acts or omissions of any securities depository, clearing agency or settlement system; and |
| · | the depositary has no duty to make any determination or provide any information as to our tax status, or any liability for any tax consequences that may be incurred by ADS holders as a result of owning or holding ADSs or be liable for the inability or failure of an ADS holder to obtain the benefit of a foreign tax credit, reduced rate of withholding or refund of amounts withheld in respect of tax or any other tax benefit. |
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of ADSs, make a distribution on ADSs, or permit withdrawal of shares, the depositary may require:
| · | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
| · | satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
| · | compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
ADS holders have the right to cancel their ADSs and withdraw the underlying shares at any time except:
| · | when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii) the transfer of shares is blocked to permit voting at a shareholders meeting; or (iii) we are paying a dividend on our shares; |
| · | when you owe money to pay fees, taxes and similar charges; or |
| 146 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| · | when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of shares or other deposited securities. |
This right of withdrawal may not be limited by any other provision of the deposit agreement.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the Direct Registration System, also referred to as DRS, and Profile Modification System, also referred to as Profile, will apply to the ADSs. DRS is a system administered by DTC that facilitates interchange between registered holding of uncertificated ADSs and holding of security entitlements in ADSs through DTC and a DTC participant. Profile is a feature of DRS that allows a DTC participant, claiming to act on behalf of a registered holder of uncertificated ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery as described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile system and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder communications; Inspection of register of holders of ADSs; Disclosure of information
The depositary will make available for your inspection at its office all communications, including any proxy solicitation material, that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities, such as Form 6-K that we intend to furnish to the SEC with respect to our unaudited financial information for the first three quarters of each fiscal year and whenever we solicit proxies and furnish proxy statements for meetings of our shareholders. The depositary will also send you copies of those communications or otherwise make those communications available to you in accordance with the deposit agreement, including by mailing notices to registered holders (at our expense). You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs, by way of submitting a request to the depositary’s office located at 240 Greenwich Street, New York, New York 10286.
When required in order to comply with applicable laws and regulations or the articles of association or similar document of our Company, we may from time to time request each holder of ADSs to provide to the depositary information relating to, among other things, the capacity in which it holds ADSs, its identity and any other matter where disclosure of such matter is, in our reasonable opinion, required for that compliance. For example, as required by the Companies Law, we may request that you indicate, when instructing the depositary how to vote, whether or not you have a “personal interest” (as defined under the Companies Law) in certain matters on the agenda of the general meeting of our shareholders.
| 147 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| ITEM 13. | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
Not applicable.
| ITEM 14. | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
None.
| ITEM 15. | CONTROLS AND PROCEDURES |
Not applicable.
| ITEM 16A. | AUDIT COMMITTEE FINANCIAL EXPERT |
Not applicable.
| ITEM 16B. | CODE OF ETHICS |
Not applicable.
| ITEM 16C. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
Not applicable.
| ITEM 16D. | EXEMPTIONS FROM THE LISTING REQUIREMENTS AND STANDARDS FOR AUDIT COMMITTEES |
Not applicable.
| ITEM 16E. | PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS |
Not applicable.
| Item 16F. | CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT |
Not applicable.
| Item 16G. | CORPORATE GOVERNANCE |
Not applicable.
| Item 16H. | MINE SAFETY DISCLOSURE |
Not applicable.
| 148 |
| This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential |
| ITEM 17. | FINANCIAL STATEMENTS |
The Company has elected to furnish financial statements and related information specified in Item 18.
| ITEM 18. | FINANCIAL STATEMENTS |
The consolidated financial statements and the related notes required by this Item are included in this registration statement beginning on page F-1.
AUDITED CONSOLIDATED FINANCIAL STATEMENTS
UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
| 149 |
Itamar Medical Ltd.
Consolidated Financial Statements
As of December 31, 2017
| F-1 |
Consolidated Financial Statements
As of December 31, 2017
Table of Contents
| F-2 |

Report of Independent Registered Public Accounting Firm
To the Shareholders and Board of Directors
Itamar Medical Ltd.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated statements of financial position of Itamar Medical Ltd. and its subsidiaries (the “Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive income (loss), changes in equity, and cash flows, for each of the years in the three-year period ended December 31, 2017, and the related notes (collectively, the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2017, in conformity with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Somekh Chaikin |
|
| Certified Public Accountants (Isr.) | |
| Member firm of KPMG International | |
We have served as the Company’s auditor since 1997.
Tel-Aviv, Israel
August 9, 2018
| F-3 |
CONSOLIDATED STATEMENTS OF FINANCIAL POSITION
| December 31, | ||||||||||
| Note | 2017 | 2016 | ||||||||
| U.S. dollars in thousands | ||||||||||
| Assets | ||||||||||
| Current assets | ||||||||||
| Cash and cash equivalents | $ | 7,643 | $ | 23,358 | ||||||
| Investments in marketable securities | 20 | 3,173 | 2,781 | |||||||
| Trade receivables | 3 | 5,362 | 4,490 | |||||||
| Other receivables | 3 | 685 | 750 | |||||||
| Inventories | 4 | 2,260 | 1,784 | |||||||
| Total current assets | 19,123 | 33,163 | ||||||||
| Non-current assets | ||||||||||
| Long-term restricted deposits and prepaid expenses | 382 | 460 | ||||||||
| Long-term trade receivables | 3 | 473 | 659 | |||||||
| Property and equipment | 5 | 1,022 | 1,008 | |||||||
| Intangible assets | 6 | 277 | 257 | |||||||
| Total non-current assets | 2,154 | 2,384 | ||||||||
| Total assets | $ | 21,277 | $ | 35,547 | ||||||
| Liabilities | ||||||||||
| Current liabilities | ||||||||||
| Trade payables | $ | 1,262 | $ | 1,324 | ||||||
| Short-term employee benefits | 7 | 223 | 198 | |||||||
| Current maturities of convertible notes | 8 | 10,696 | 9,621 | |||||||
| Provisions | 9 | 183 | 167 | |||||||
| Accrued expenses | 1,405 | 939 | ||||||||
| Other accounts payable | 10 | 1,998 | 2,071 | |||||||
| Total current liabilities | 15,767 | 14,320 | ||||||||
| Non-current liabilities | ||||||||||
| Convertible notes, net of current maturities | 8 | - | 8,170 | |||||||
| Derivative instruments | 11 | 2,875 | 6,800 | |||||||
| Long-term employee benefits | 7 | 310 | 156 | |||||||
| Other long-term liabilities | 12 | 948 | 860 | |||||||
| Total non-current liabilities | 4,133 | 15,986 | ||||||||
| Total liabilities | 19,900 | 30,306 | ||||||||
| Commitments | 12 | |||||||||
| Equity | 14 | |||||||||
| Ordinary share capital | 683 | 679 | ||||||||
| Additional paid-in capital | 105,585 | 105,492 | ||||||||
| Capital reserve from marketable securities available-for-sale | 113 | (45 | ) | |||||||
| Accumulated deficit | (105,004 | ) | (100,885 | ) | ||||||
| Total equity | 1,377 | 5,241 | ||||||||
| Total liabilities and equity | $ | 21,277 | $ | 35,547 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year Ended December 31, | ||||||||||||||
| Note | 2017 | 2016 | 2015 | |||||||||||
| U.S. dollars in thousands (except per share data) | ||||||||||||||
| Revenues | 16 | $ | 20,701 | $ | 18,440 | $ | 16,807 | |||||||
| Cost of revenues | 17 | 5,002 | 4,979 | 4,401 | ||||||||||
| Gross profit | 15,699 | 13,461 | 12,406 | |||||||||||
| Selling and marketing expenses | 12,140 | 14,035 | 10,684 | |||||||||||
| Research and development expenses | 4,129 | 3,225 | 2,831 | |||||||||||
| General and administrative expenses | 5,278 | 6,213 | 4,350 | |||||||||||
| Total operating expenses | 21,547 | 23,473 | 17,865 | |||||||||||
| Operating loss | (5,848 | ) | (10,012 | ) | (5,459 | ) | ||||||||
| Financial income (expenses) from cash and investments | 18 | 1,591 | 716 | (354 | ) | |||||||||
| Financial expenses from notes and loans | 18 | (4,884 | ) | (4,760 | ) | (4,229 | ) | |||||||
| Gain (loss) from derivatives instruments, net | 18 | 3,925 | (216 | ) | 7,930 | |||||||||
| Financial income (expenses), net | 632 | (4,260 | ) | 3,347 | ||||||||||
| Loss before taxes on income | (5,216 | ) | (14,272 | ) | (2,112 | ) | ||||||||
| Taxes on income | 13 | (85 | ) | (131 | ) | (135 | ) | |||||||
| Net loss | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||||
| Loss per share (in U.S. dollars): | 19 | |||||||||||||
| Basic | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.01 | ) | |||||
| Diluted | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.02 | ) | |||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME (LOSS)
| Year Ended December 31, | ||||||||||||||
| Note | 2017 | 2016 | 2015 | |||||||||||
| U.S. dollars in thousands | ||||||||||||||
| Net loss | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||||
| Other comprehensive loss items that will not be carried to the statements of operations | ||||||||||||||
| Actuarial losses of defined benefit plan, net of tax | 7 | (112 | ) | (107 | ) | (72 | ) | |||||||
| Total other comprehensive loss for the year that will not be carried to the statements of operations, net of tax | (112 | ) | (107 | ) | (72 | ) | ||||||||
| Other comprehensive income (loss) items that after preliminary recognition in comprehensive income (loss), were or will be carried to the statements of operations | ||||||||||||||
| Net change in fair value of marketable securities available-for-sale, net of tax | 158 | 9 | (123 | ) | ||||||||||
| Net change in fair value of marketable securities available-for-sale, net of tax that was carried to the statements of operations | - | - | 523 | |||||||||||
| Total other comprehensive income items that after preliminary recognition in comprehensive income, were or will be carried to the statements of operations, net of tax | 158 | 9 | 400 | |||||||||||
| Other total comprehensive income (loss) | 158 | (98 | ) | 328 | ||||||||||
| Total comprehensive loss | $ | (5,255 | ) | $ | (14,501 | ) | $ | (1,919 | ) | |||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-6 |
CONSOLIDATED STATEMENTS OF CHANGES IN EQUITY
| Ordinary share capital | Additional paid-in capital | Capital reserve from marketable securities available- for-sale | Accumulated deficit | Total | ||||||||||||||||
| U.S. dollars in thousands | ||||||||||||||||||||
| For the year ended December 31, 2015 | ||||||||||||||||||||
| Balance as of January 1, 2015 | $ | 467 | $ | 81,384 | $ | (454 | ) | $ | (86,167 | ) | $ | (4,770 | ) | |||||||
| Total comprehensive loss: | ||||||||||||||||||||
| Net loss | - | - | - | (2,247 | ) | (2,247 | ) | |||||||||||||
| Other comprehensive income, net of tax | - | - | 400 | (72 | ) | 328 | ||||||||||||||
| Total comprehensive loss | - | - | 400 | (2,319 | ) | (1,919 | ) | |||||||||||||
| Transactions carried directly to equity: | ||||||||||||||||||||
| Issuance of shares due to the exercise of options | 5 | 149 | - | - | 154 | |||||||||||||||
| Issuance of shares and warrants, net | 198 | 22,953 | - | - | 23,151 | |||||||||||||||
| Share-based payment | - | - | - | 428 | 428 | |||||||||||||||
| Early repayment of loan from shareholders | - | - | - | (93 | ) | (93 | ) | |||||||||||||
| Balance as of December 31, 2015 | $ | 670 | $ | 104,486 | $ | (54 | ) | $ | (88,151 | ) | $ | 16,951 | ||||||||
| For the year ended December 31, 2016 | ||||||||||||||||||||
| Balance as of January 1, 2016 | $ | 670 | $ | 104,486 | $ | (54 | ) | $ | (88,151 | ) | $ | 16,951 | ||||||||
| Total comprehensive loss: | ||||||||||||||||||||
| Net loss | - | - | - | (14,403 | ) | (14,403 | ) | |||||||||||||
| Other comprehensive income, net of tax | - | - | 9 | (107 | ) | (98 | ) | |||||||||||||
| Total comprehensive loss | - | - | 9 | (14,510 | ) | (14,501 | ) | |||||||||||||
| Transactions carried directly to equity: | ||||||||||||||||||||
| Issuance of shares due to the exercise of options | 1 | 16 | - | - | 17 | |||||||||||||||
| Issuance of shares and warrants, net | 8 | 990 | - | - | 998 | |||||||||||||||
| Share-based payment | - | - | - | 1,776 | 1,776 | |||||||||||||||
| Balance as of December 31, 2016 | $ | 679 | $ | 105,492 | $ | (45 | ) | $ | (100,885 | ) | $ | 5,241 | ||||||||
| For the year ended December 31, 2017 | ||||||||||||||||||||
| Balance as of January 1, 2017 | $ | 679 | $ | 105,492 | $ | (45 | ) | $ | (100,885 | ) | $ | 5,241 | ||||||||
| Total comprehensive loss: | ||||||||||||||||||||
| Net loss | - | - | - | (5,301 | ) | (5,301 | ) | |||||||||||||
| Other comprehensive income, net of tax | - | - | 158 | (112 | ) | 46 | ||||||||||||||
| Total comprehensive loss | - | - | 158 | (5,413 | ) | (5,255 | ) | |||||||||||||
| Transactions carried directly to equity: | ||||||||||||||||||||
| Issuance of shares due to the exercise of options | 4 | 93 | - | - | 97 | |||||||||||||||
| Share-based payment | - | - | - | 1,294 | 1,294 | |||||||||||||||
| Balance as of December 31, 2017 | $ | 683 | $ | 105,585 | $ | 113 | $ | (105,004 | ) | $ | 1,377 | |||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-7 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Net loss | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||
| Adjustments for: | ||||||||||||
| Depreciation and amortization | 509 | 434 | 367 | |||||||||
| Share-based payment | 1,294 | 1,776 | 428 | |||||||||
| Capital gain from sale of property and equipment | (8 | ) | - | - | ||||||||
| Change in provision for doubtful and bad debt | 147 | 849 | 52 | |||||||||
| Net financial expenses | 3,133 | 4,110 | 4,591 | |||||||||
| Loss (gain) from reevaluation of derivatives | (3,925 | ) | 216 | (7,962 | ) | |||||||
| Increase in trade receivables | (833 | ) | (1,548 | ) | (1,307 | ) | ||||||
| Decrease (increase) in other accounts receivable | 169 | (157 | ) | (51 | ) | |||||||
| Increase in inventories | (711 | ) | (430 | ) | (268 | ) | ||||||
| Increase (decrease) in trade payables | (66 | ) | 289 | 5 | ||||||||
| Increase (decrease) in other accounts payable | 669 | 188 | (412 | ) | ||||||||
| Increase (decrease) in employee benefits | 67 | (111 | ) | 61 | ||||||||
| Increase (decrease) in provisions | 16 | (71 | ) | (112 | ) | |||||||
| Income tax expenses | 85 | 131 | 179 | |||||||||
| Taxes paid during the year | (83 | ) | (228 | ) | (44 | ) | ||||||
| Interest received during the year | 18 | 41 | 11 | |||||||||
| Interest paid during the year | (1,362 | ) | (1,716 | ) | (1,901 | ) | ||||||
| Net cash used in operating activities | (6,182 | ) | (10,630 | ) | (8,610 | ) | ||||||
| Cash flows for investing activities | ||||||||||||
| Sale of marketable securities available-for-sale | - | - | 6,080 | |||||||||
| Purchase of property and equipment, intangible assets and capitalization of development expenditure | (296 | ) | (455 | ) | (562 | ) | ||||||
| Investment in restricted long-term deposits | (22 | ) | (113 | ) | (44 | ) | ||||||
| Net cash provided by (used in) investing activities | (318 | ) | (568 | ) | 5,474 | |||||||
| Cash flow for financing activities | ||||||||||||
| Issuance of shares and warrants | - | 998 | 23,151 | |||||||||
| Repayment of convertible notes | (10,421 | ) | - | - | ||||||||
| Issuance of warrants | - | 85 | 5,300 | |||||||||
| Repayment of shareholders’ loans | - | - | (1,765 | ) | ||||||||
| Issuance of shares due to the exercise of stock options | 97 | 17 | 154 | |||||||||
| Net cash provided by (used in) financing activities | (10,324 | ) | 1,100 | 26,840 | ||||||||
| Increase (decrease) in cash and cash equivalents | (16,824 | ) | (10,098 | ) | 23,704 | |||||||
| Cash and cash equivalents at beginning of year | 23,358 | 33,019 | 9,417 | |||||||||
| Effect of exchange rate fluctuations on balances of cash and cash equivalents | 1,109 | 437 | (102 | ) | ||||||||
| Cash and cash equivalent balance at end of year | $ | 7,643 | $ | 23,358 | $ | 33,019 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-8 |
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – GENERAL
| a. | Reporting entity |
Itamar Medical Ltd. (the “Company”) is a company incorporated in Israel, with registered office at 9 Halamish Street, North Industrial Zone, Caesarea, Israel. The consolidated financial statements of the Company as of December 31, 2017 and 2016 and for each of the three years in the period ended December 31, 2017 comprise the Company and its subsidiaries (together referred to as the “Group”). The core business of the Group is to design, develop, manufacture and sell sleep apnea diagnostic ambulatory products and related services, using the Peripheral Arterial Tone (“PAT”) biological signal along with other measurements such as actigraphy, heartrate, chest motion, body position and snoring and analyzed by the Group’s proprietary technology and algorithms. The ordinary shares, par value of New Israeli Shekel (“NIS”) 0.01 per share, of the Company are listed on the Tel Aviv Stock Exchange Ltd. (“TASE”).
| b. | Material events subsequent to December 31, 2017 |
Subsequent to December 31, 2017, in February 2018, the Company repaid the total outstanding principal and interest of its convertible notes (see Note 8(b)), other than $1.7 million owed to three shareholders who held convertible notes, of which $0.5 million was repaid to one shareholder in June 2018 and the balance owed to the other two shareholders was invested by them in the private placement that was completed in May 2018, and under which the Company raised approximately $6.0 million in a private placement of its ordinary shares to certain existing shareholders and various funds affiliated with three Israeli institutional investors.
| c. | The Company’s financial position |
The Company’s management and Board of Directors are in the opinion that, based on the positive trend of its operating results, the bank credit facility (see Note 8a) and the private placement described in b. above, and the Company’s ability to adjust its budget to business developments, the Company has enough financial resources in order to continue its business activities in the foreseeable future. In addition, the management continuously assesses its actual results, compared its approved budget and its financial covenants is able to respond by reducing its operating expenses in case it does not meet its targets.
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES
| a. | International financial reporting standards |
These consolidated financial statements have been prepared in accordance with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”). These consolidated financial statements were approved by the Company’s Board of Directors on August 9, 2018.
| b. | Reporting and functional currency |
These consolidated financial statements are presented in U.S. dollars (“dollar” or “$”), which is the Company’s functional currency representing the principal economic environment in which the Company operates, and have been rounded to the nearest thousand unless otherwise indicated.
| c. | Basis of measurement |
These consolidated financial statements have been prepared on the historical cost basis, except for certain investments and derivatives and other financial instruments measured at fair value through profit or loss, financial instruments classified as available-for-sale, inventories (measured at the lower of cost or net realizable value), provisions, assets and liabilities for of employee benefits, and deferred tax assets and liabilities.
| F-9 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| d. | Principles of consolidation |
Subsidiaries are entities controlled by the Company. The financial statements of the subsidiaries, which are wholly-owned, are included in the consolidated financial statements of the Company from the date of their incorporation. Intercompany balances and transactions between Group companies are eliminated in consolidation.
| e. | Use of estimates and critical assumptions |
The preparation of financial statements in accordance with IFRS requires management to make estimates and assumptions that affect reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements as well as affect the reported amounts of revenues and expenses during the period. These estimates and assumptions are reviewed on an ongoing basis using available information. Actual results could differ from these estimates and assumptions. The items subject to significant estimates and assumptions by management include impairment tests of long-lived assets; share-based compensation; recognition of deferred income tax assets; the measurement of financial instruments at fair value, the fair value of the embedded warrant component of convertible notes, the fair value of warrants where there is no active market; and the assets and liabilities related to employee benefits.
| f. | Foreign currency transactions and balances |
Transactions in foreign currency are translated to the respective functional currency of the Group entities at exchange rates as of the transaction dates.
Monetary assets and liabilities denominated in foreign currencies at the reporting date are translated to the functional currency at the exchange rate at that date. The foreign currency gain or loss on monetary items is the difference between the amortized cost in the functional currency at the beginning of the year, adjusted for effective interest and payments during the year, and the amortized cost in foreign currency, translated at the exchange rate at the end of the year. Non-monetary assets and liabilities denominated in foreign currency that are measured in terms of historical cost, are translated using the exchange rate at the date of the transaction.
Foreign currency differences arising from translation into the functional currency are recognized in the statements of operations, except for differences arising from the translation of financial equity instruments classified as available-for-sale (except in case of impairment when the translation differences recognized in other comprehensive income are reclassified to profit or loss) recognized in other comprehensive income.
| g. | Cash and Cash Equivalents |
Cash and cash equivalents are comprised of available amounts of cash and cash equivalents, mainly represented by highly-liquid short-term investments (with original maturities of three months or less), which are readily convertible into known amounts of cash, and which are not subject to significant risks of changes in their values.
| h. | Financial instruments: |
Trade accounts receivable and other accounts receivable
Trade accounts receivable and other accounts receivable are classified as loans and receivables and are recorded at their amortized cost representing the net present value of the consideration receivable or payable as of the transaction date.
Due to their short-term nature, the Group initially recognizes these receivables at the original invoiced amount. Allowances for doubtful accounts were recognized based on incurred loss estimates against general and administrative expenses.
Long-term trade receivables and other investments
Long-term trade receivables are initially recognized at their amortized cost. Subsequent changes in net present value are recognized in the statements of operations as part of “Financial income (expenses), net”.
| F-10 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Investments in financial instruments held for trading as well as those investments available-for- sale, are recognized at their estimated fair value, in the first case through the statements of operations as part of “Financial income (expenses), net” and in the second case, changes in valuations are recognized as part of “Other comprehensive income (loss)” for the year within “Capital reserve” until their time of disposition, when all valuation effects accrued in equity are reclassified to “Financial income (expenses), net” in the statements of operations. These investments are tested for impairment upon the occurrence of a significant adverse change or at least once a year during the last quarter.
Debt and other financial obligations
Bank loans and notes payable, are recognized at their amortized cost. Interest accrued on financial instruments is recognized within “Other accounts payable and accrued expenses” against financial expenses. Direct costs incurred in debt issuances or borrowings, adjust the carrying amount of the related debt and are amortized as interest expense as part of the effective interest rate of each instrument over its maturity. These costs include commissions and professional fees.
In the statements of cash flows, interest received and interest paid on bank loans and notes payable are presented in cash flows from operating activities.
Liabilities, which are convertible into shares, denominated in a currency different than the functional currency of the Company or linked to the Israeli Consumer Price Index (the “Israeli CPI”), constitute a hybrid instrument presented in full as a financial liability. For measurement, the instrument is separated into two components: (i) a liability component with no conversion feature, which is measured at amortized cost according to the effective interest method, and (ii) a conversion option, which constitutes an embedded derivative accounted for as a derivative financial instrument at fair value and is measured through the statements of operations as part of “Financial income (expenses), net”.
Issuance of bundle of securities
The consideration received from the issuance of a bundle of securities is attributed initially to financial liabilities measured each period at fair value, and then to financial liabilities measured only upon initial recognition at fair value. The remaining amount is the value of the equity component. Direct issuance costs are attributed to the specific securities in respect of which they were incurred, whereas joint issuance costs are attributed to the securities on a proportionate basis according to the grant of the consideration from the issuance of the bundle, as described above.
Derivative financial instruments
The Group recognizes all derivative instruments as assets or liabilities in the statements of financial position at their estimated fair values, and the changes in such fair values are recognized in the statements of operations within “Financial income (expenses), net” for the period in which they occur. During the reported years, the Group did not have derivatives designated as hedges. The Group reviews its contracts to identify the existence of embedded derivatives. Identified embedded derivatives are analyzed to determine if they need to be separated from the host contract and recognized in the statements of financial position as assets or liabilities, applying the same valuation rules used for other derivative instruments.
Fair value measurements
Under IFRS, fair value represents an “Exit Value”, which is the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date, considering the counterparty’s credit risk in the valuation. The concept of Exit Value is premised on the existence of a market and market participants for the specific asset or liability. When there is no market and/or market participants willing to make a market, IFRS establishes a fair value hierarchy that gives the highest priority to unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to measurements involving significant unobservable inputs (Level 3 measurements).
| F-11 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The three levels of the fair value hierarchy are as follows:
| · | Level 1— Quoted prices (unadjusted) in active markets for identical assets or liabilities that the Group has the ability to access at the measurement date. A quote price in an active market provides the most reliable evidence of fair value and is used without adjustment to measure fair value whenever available. |
| · | Level 2— Inputs, other than quoted prices in active markets, that are observable for the asset or liability, either directly or indirectly, and are used mainly to determine the fair value of securities, investments or loans that are not actively traded. |
| · | Level 3— Unobservable inputs for the asset or liability are used when little or no market data is available. The Group used unobservable inputs to determine fair values, to the extent there are no Level 1 or Level 2 inputs, in valuation models such as Black-Scholes, binomial, discounted cash flows or multiples, including risk assumptions consistent with what market participants would use to arrive at fair value. |
| i. | Inventories |
Inventories are valued using the lower of cost and net realizable value. The cost of inventories is based on the “moving-average” method, including expenditures incurred in acquiring the inventories and the costs incurred in bringing it to its existing location and condition. The Group analyzes its inventory balances to determine if, as a result of internal events, such as physical damage, or external events, such as technological changes or market conditions, certain portions of such balances have become obsolete or impaired. When an impairment situation arises, the inventory balance is adjusted to its net realizable value, whereas, if an obsolescence situation occurs, the inventory obsolescence reserve is increased. In both cases, these adjustments are recognized against the results of the period. Net realizable value is the estimated selling price in the ordinary course of business less the estimated costs to complete and sell the inventories.
| j. | Property and equipment |
Property and equipment are recognized at their acquisition or construction cost, as applicable, less accumulated depreciation and accumulated impairment losses. Depreciation of property and equipment is recognized as part of operating expenses, and is calculated using the straight-line method over the estimated useful lives of the assets. As of December 31, 2017, the average useful lives by category of property and equipment were as follows:
| % | |
| Office furniture and equipment | 10 |
| Equipment and devices for leasing and for internal use | 15 |
| Computers | 33 |
Leasehold improvements are amortized over the shorter of the lease term and their useful lives.
Depreciation methods and useful lives are reviewed at the end of each reporting year and adjusted if appropriate.
| k. | Intangible assets |
The Group capitalizes intangible assets acquired, as well as costs incurred in the development of certain intangible assets for internal use, when future economic benefits associated are identified and there is evidence of control over such benefits.
| F-12 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Intangible assets are recognized at their acquisition or development cost, as applicable. All of the Group’s intangible assets are definite life intangible assets, and are amortized on straight-line basis over the useful life of the asset, which on average is approximately three years.
Expenditure on research activities undertaken with the prospect of gaining new scientific or technical knowledge and understanding, are recognized in the statements of operations when incurred. Development activities are related to a plan to produce new products or processes, or to significantly improve existing products or processes. Development expenditure is capitalized only if: (i) the expenditure can be measured reliably; (ii) the product or process is technically and commercially feasible; and (iii) future economic benefits are probable, and the Group intends to and has sufficient resources to complete development and to use or sell the asset. The expenditure capitalized in respect of development activities includes the cost of materials, direct labor and overhead costs that are directly attributable to preparing the asset for its intended use. Other development expenditure is recognized in the statements of operations as incurred.
In subsequent periods, capitalized development expenditure is measured at cost less accumulated amortization and any accumulated impairment losses.
Amortization methods and useful lives are reviewed at the end of each reporting year and adjusted if appropriate.
| l. | Impairment of property and equipment, intangible assets of definite life and other investments |
These assets are tested for impairment upon the occurrence of factors such as the occurrence of a significant adverse event, changes in the Group’s operating environment or in technology, as well as expectations of lower operating results, in order to determine whether their carrying amounts may not be recovered. An impairment loss is recorded in the statements of operations for the period within “Other expenses, net”, for the excess of the asset’s carrying amount over its recoverable amount, corresponding to the higher of the fair value less costs to sell the asset, and the asset’s value in use, the latter represented by the net present value of estimated cash flows related to the use and eventual disposal of the asset.
No impairment loss was recorded during the reported years.
| m. | Provisions |
The Group recognizes provisions when it has a legal or constructive obligation resulting from past events, whose resolution would imply cash outflows or the delivery of other resources owned by the Group.
Obligations or losses related to contingencies are recognized as liabilities in the statements of financial position only when present obligations exist resulting from past events and it is probable to result in an outflow of resources and the amount can be measured reliably. Otherwise, a qualitative disclosure is included in the notes to the financial statements. The provisions are determined by discounting the future cash flows at a pre-tax interest rate, reflecting the current market estimates of the time value of the money and the specific risks of the liability without weighting the Group’s credit risk. The carrying value of the provision is then adjusted in every period so as to reflect the passage of time and the adjustment amount is credited to financial expenses.
| n. | Post-employment benefits |
The costs of defined contribution plans are recognized in the operating results as they are incurred. Liabilities arising from such plans are settled through cash transfers to the employees’ retirement accounts with insurance companies or with funds managed by others, without generating future obligations. The majority of the Israeli employees are under defined contribution plans.
The rest of the Israeli employees are under defined benefit plans. The costs associated with defined benefit plans are recognized as services are rendered, based on actuarial estimations of the benefits’ present value with the advice of external actuaries.
| F-13 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Termination benefits, not associated with a restructuring event, which mainly represent severance payments mandated by law, are recognized in the operating results for the period in which they are incurred.
| o. | Share-based payment transactions |
The grant-date fair value of share-based payment awards granted to employees and directors is recognized as an expense, with a corresponding increase in equity, over the period that the employees and directors become unconditionally entitled to the awards. The amount recognized as an expense in respect of share-based payment awards that are conditional upon meeting service and non-market performance conditions, is adjusted to reflect the number of awards that are expected to vest.
For share-based payment awards with non-vesting conditions or with market performance vesting conditions, the grant date fair value of the share-based payment awards is measured to reflect such conditions, and therefore the Group recognizes an expense in respect of the awards whether or not the conditions have been met.
The fair value at the time of grant of share-based payment awards to consultants and service providers are recognized over the consultants’ and the service providers’ period of service against an increase in equity. The fair value of the services is calculated on the basis of the fair value of the awards and not on the basis of the fair value of the services, since it is not possible to reliably estimate the fair value of the services rendered.
The Group elected to record the increase in equity against salary expense directly to retained earnings.
| p. | Revenue recognition |
Revenue is measured at the fair value of the consideration received or receivable, net of returns and discounts. The Group recognizes revenue from the sale of its products, net of provision for returns, when persuasive evidence exists (usually in the form of an executed sales agreement) that the significant risks and rewards of ownership of the products have been transferred to the buyer, recovery of the consideration is probable, the associated costs and possible return of products can be estimated reliably, there is no continuing management involvement with the products, and the amount of revenue can be measured reliably. Revenue is recognized when title to the products and risk of loss transfers to customers, provided there are no material remaining performance obligations required of the Group or any matters requiring customer acceptance. The timing of the transfer of risks and rewards may be upon shipment or upon delivery to the customer site, based on the contract terms or legal requirements.
The Group recognizes estimated sales discounts as a reduction of sales in the same period revenue is recognized. The Group adjusts reserves to reflect differences between estimated and actual. The Group estimates its sales returns reserve based on historical return rates and analysis of specific accounts.
Revenues from sales agreements consisting of multiple elements, such as devices, consumables, access to the CloudPAT application, WatchPAT Direct logistic services and support and other service agreements, are separated into different components and are separately recognized for each component. A component constitutes a separate accounting unit if and only if it has value, separately, for the customer. Components not separated, are grouped together. The revenue from each such component is recognized upon fulfillment of the conditions for recognition of revenue, based on the nature of the component, i.e., as products or as services. In general, the Group determines the fair value for each element, based on selling prices when the product or service is sold separately. In cases where the components are not sold separately, for example, in the case of installations or training, the Group establishes the value assigned to this element, based on estimated costs plus a reasonable margin.
| F-14 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The Group recognizes revenue from leasing its products over the lease term, in conformity with the agreement with the customer. In some cases, the Group handles sale transactions in these devices as finance lease and recognizes revenues in respect of the products supplied, based on their relative fair value compared to all the components in the transaction.
When the Group sells its products through distributors, revenue is being recognized upon delivery of the product to the distributor, as the distributors does not have the right to return and the material risks and rewards inherent to the ownership of the products are transferred at this time.
| q. | Income taxes |
The effects reflected in the statements of operations for income taxes include the amounts incurred during the period and the amounts of deferred income taxes, determined according to the income tax law applicable to each Group company. Consolidated deferred income taxes represent the addition of the amounts determined in each Group company by applying the enacted statutory income tax rate to the total temporary differences resulting from comparing the book and taxable values of assets and liabilities, considering tax assets such as loss carryforwards and other recoverable taxes, to the extent that it is probable that future taxable profits will be available against which they can be utilized. The measurement of deferred income taxes at the reporting period reflects the tax consequences that follow the manner in which the Group expects to recover or settle the carrying amount of its assets and liabilities. Deferred income taxes for the period represent the difference between balances of deferred income taxes at the beginning and the end of the period. Deferred income tax assets and liabilities relating to different tax jurisdictions are not offset. According to IFRS, all items charged or credited directly in shareholders’ equity or as part of other comprehensive income or loss for the period are recognized net of their current and deferred income tax effects. The effect of a change in enacted statutory tax rates is recognized in the period in which the change is officially enacted.
Deferred tax assets that were not recognized are reevaluated at each reporting date and recognized if it has become probable that future taxable income will be available against which they can be utilized.
| r. | New standards and interpretations not yet adopted: |
There are a number of IFRS standards issued as of the date of issuance of these consolidated financial statements which have not yet been adopted by the Company, described as follow:
IFRS 9, Financial Instruments: Classification and Measurement (“IFRS 9”)
IFRS 9 sets forth the guidance relating to the classification and measurement of financial assets and liabilities, the accounting for expected credit losses of financial assets and commitments to extend credits, as well as the requirements for hedge accounting. IFRS 9 is effective beginning January 1, 2018. Among other aspects, IFRS 9 changes the classification categories for financial assets under International Accounting Standard 39 (“IAS 39”), Financial Instruments: Recognition and Measurement, of: (i) held to maturity; (ii) loans and receivables; (iii) fair value through the statements of operations; and (iv) available for sale; and replaces them with categories that reflect the measurement method, the contractual cash flow characteristics and the entity’s business model for managing the financial asset: (i) amortized cost, that will significantly comprise IAS 39 held to maturity and loans and receivables categories; (ii) fair value through other comprehensive income, similar to IAS 39 held to maturity category; and (iii) fair value through the statements of operations with the same IAS 39 definitions.
In addition, under the new impairment model in IFRS 9 that is based on expected credit losses, impairment losses for the entire lifetime of financial assets, including trade accounts receivable, are recognized on initial recognition, and at each subsequent reporting period, even in the absence of a credit event or if the loss has not yet been incurred, considering for their measurement past events and current conditions, as well as reasonable and supportable forecasts affecting collectability. The adoption of IFRS 9, starting January 1, 2018, did not have a material effect on the Group’s operating results and financial condition.
| F-15 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
IFRS 15, Revenues from Contracts with Customers (“IFRS 15”)
Under IFRS 15, an entity recognizes revenue to depict the transfer of promised products or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services, following a five step model: Step 1: Identify the contract(s) with a customer (agreement that creates enforceable rights and obligations); Step 2: Identify the different performance obligations (promises) in the contract and account for those separately; Step 3: Determine the transaction price (amount of consideration an entity expects to be entitled in exchange for transferring promised goods or services); Step 4: Allocate the transaction price to each performance obligation based on the relative stand-alone selling prices of each distinct good or service; and Step 5: Recognize revenue when (or as) the entity satisfies a performance obligation by transferring control of a promised good or service to the customer. A performance obligation may be satisfied at a point in time (typically for the sale of products) or over time (typically for the sale of services and construction contracts). IFRS 15 also includes disclosure requirements to provide comprehensive information about the nature, amount, timing and uncertainty of revenue and cash flows arising from the entity’s contracts with customers. IFRS 15 is effective on January 1, 2018 and will supersede all existing guidance on revenue recognition.
Beginning January 1, 2018, the Group adopted IFRS 15, with the cumulative impact approach, while adjusting retained earnings as of January 1, 2018, based on the analysis performed by the Group, there was no effect on retained earnings as of January 1, 2018. As part of the initial adoption of IFRS 15, the Group elected to implement the following exemptions:
| (a) | Application of the cumulative impact approach only for contracts that have not been concluded at the date of transition; and |
| (b) | Examining the aggregate impact of changes in the contract that occurred before the date of initial application, instead of an examination of each change separately. |
The Group examined the expected effects of the implementation of IFRS 15 and in its assessment of the implementation of IFRS 15, the Group expects that it will have an immaterial effect on its consolidated financial statements as a result of recognizing receivables in respect of contract assets that the rights in their respect are unconditional, together with corresponding deferred revenue.
IFRS 16, Leases (“IFRS 16”)
IFRS 16 supersedes IAS 17, Leases (in this Section “IAS 17") and its related interpretations. The provisions of IFRS 16 abrogate the existing requirement from lessees to classify the lease as operating or finance. Instead, with respect to lessees, IFRS 16 presents one model for the accounting treatment of all leases, according to which the lessee must recognize a right to use asset and a lease liability in its financial statements. However, IFRS 16 includes two exceptions to the general model, according to which a lessee may choose not to implement the recognition requirements for an asset, a right of use and a liability for short-term lease of up to one year and/or leases in which the underlying asset is of low value.
In addition, IFRS 16 allows the lessee to apply the definition of a lease in one of the following two alternatives consistently to all leases: retrospective application for all lease agreements, i.e., a reevaluation of the existence of a lease for each contract separately or alternatively the application of a practical relief. The provisions of IAS 17 and International Financial Reporting Interpretations Committee (“IFRIC”) 4, “Determining Whether an Arrangement Contains a Lease” (IFRIC 4), defines criteria with respect to the existing agreements as at the date of initial application of IFRS 16. In addition, IFRS 16 provides new and broader disclosure requirements than those existing today.
| F-16 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
IFRS 16 is effective for annual periods beginning on or after January 1, 2019, with the possibility of early adoption.
IFRS 16 includes various alternatives for the implementation of the transitional provisions, so that one of the following alternatives can be chosen consistently for all leases at initial application: full retrospective application, or application of the cumulative effect, i.e., implementation of IFRS 16 (with the possibility of several concessions) for the first time with adjustments to the opening balance of retained earnings as of that date.
The manner of implementation of IFRS 16 and expected effects
The Group intends to adopt IFRS 16 as of January 1, 2019 in the cumulative effect approach, while adjusting the retained earnings as at January 1, 2019.The Group elected to adopt the relief, whereby one discount rate will be used for lease contracts having similar characteristics in a reasonable manner.
The Group has not yet decided whether to adopt the exemptions that IFRS 16 allows not to apply the recognition of the asset as a right of use and a liability for short-term leases of up to one year and not to implement the recognition requirements for the asset and the lease period ends within 12 months from the date of initial application.
Expected effect
The Group intends to choose to apply the transitional provision according to which it will recognize the IFRS 16 implementation date of the lease liability according to the present value of the balance of the future lease payments, discounted at the lessee’s incremental interest rate on that date and simultaneously recognize the same amount as the lease asset, which were recognized as an asset or liability prior to the IFRS 16 implementation date. As a result, implementation of IFRS 16 is not expected to have an effect on retained earnings as at the date of initial application
The Group is required to recognize at the initial implementation date a right to use asset and lease liability for all leases in which it is found that it has the right to control the use of identified assets for a specified period of time. Under the assumption that the Group will not implement the relief with regard to leases whose lease period ends within 12 months from the date of the initial implementation, these changes are expected to result in an increase of $0.9 million in the balance of the right-of-use assets and approximately $0.4 million in the balance of other receivables and an increase of approximately $1.3 million in the balance of the lease liability as of June 30, 2018. Accordingly, depreciation and amortization expenses in respect of an asset will be recognized, and the need to record impairment in respect of a right-of-use asset will be examined in accordance with the provisions of IAS 36, Impairment of Assets. In addition, financial expenses in respect of a lease liability will be recognized. Therefore, as from January 1, 2019, rental expenses relating to assets leased under operating leases, which were presented under general and administrative expenses in the statements of operations, will be capitalized as assets and will be amortized under depreciation and amortization expenses in subsequent periods. If the Group chooses to implement the aforementioned relief, the changes are expected to result in an increase of approximately $0.7 million in the balance of the right-of-use assets and an identical increase in the balance of the lease liability as of June 30, 2018. In addition, the range of nominal discount rates used for measuring lease liabilities ranges from 9% in respect of NIS-denominated leases to 12.8% in respect of dollar-denominated leases. This range is affected by differences in the length of the lease period, differences in the various asset groups, and a change between the discount rates of the Group companies and the like.
| F-17 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 3 - TRADE AND OTHER RECEIVABLES
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Trade receivables: | ||||||||
| Open accounts | $ | 6,048 | $ | 5,417 | ||||
| Checks receivable | 327 | 182 | ||||||
| 6,375 | 5,599 | |||||||
| Less - allowance for doubtful accounts | 540 | 450 | ||||||
| 5,835 | 5,149 | |||||||
| Is presented in the statements of financial position as follows: | ||||||||
| Under current assets | 5,362 | 4,490 | ||||||
| Under non-current assets | 473 | 659 | ||||||
| $ | 5,835 | $ | 5,149 | |||||
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Other receivables: | ||||||||
| Institutions | $ | 330 | $ | 325 | ||||
| Advances to suppliers | 51 | 74 | ||||||
| Employees | 121 | 154 | ||||||
| Prepaid expenses | 170 | 185 | ||||||
| Miscellaneous | 13 | 12 | ||||||
| $ | 685 | $ | 750 | |||||
The Group’s exposure to credit risk, currency risk and impairment loss in respect of trade and other receivables is described in Note 20.
NOTE 4 – INVENTORIES
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Raw materials and auxiliary materials | $ | 969 | $ | 753 | ||||
| Work in process | 214 | 227 | ||||||
| Finished products | 1,077 | 804 | ||||||
| $ | 2,260 | $ | 1,784 | |||||
| F-18 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 5 – PROPERTY AND EQUIPMENT
| Computers and equipment | Equipment and devices for leasing and for internal use | Office furniture and equipment | Leasehold improvements | Total | ||||||||||||||||
| U.S. dollars in thousands | ||||||||||||||||||||
| Cost: | ||||||||||||||||||||
| Balance as of January 1, 2017 | $ | 1,883 | $ | 750 | $ | 459 | $ | 317 | $ | 3,409 | ||||||||||
| Additions | 64 | 349 | 21 | 4 | 438 | |||||||||||||||
| Disposals | - | (6 | ) | (13 | ) | (4 | ) | (23 | ) | |||||||||||
| Balance as of December 31, 2017 | 1,947 | 1,093 | 467 | 317 | 3,824 | |||||||||||||||
| Accumulated depreciation: | ||||||||||||||||||||
| Balance as of January 1, 2017 | 1,570 | 409 | 255 | 167 | 2,401 | |||||||||||||||
| Depreciation | 62 | 240 | 71 | 37 | 410 | |||||||||||||||
| Disposals | - | - | (7 | ) | (2 | ) | (9 | ) | ||||||||||||
| Balance as of December 31, 2017 | 1,632 | 649 | 319 | 202 | 2,802 | |||||||||||||||
| Depreciated balance as of December 31, 2017 | 315 | 444 | 148 | 115 | 1,022 | |||||||||||||||
| Cost: | ||||||||||||||||||||
| Balance as of January 1, 2016 | 1,745 | 641 | 366 | 259 | 3,011 | |||||||||||||||
| Additions | 138 | 317 | 93 | 58 | 606 | |||||||||||||||
| Disposals | - | (208 | ) | - | - | (208 | ) | |||||||||||||
| Balance as of December 31, 2016 | 1,883 | 750 | 459 | 317 | 3,409 | |||||||||||||||
| Accumulated depreciation: | ||||||||||||||||||||
| Balance as of January 1, 2016 | 1,499 | 414 | 200 | 143 | 2,256 | |||||||||||||||
| Depreciation | 71 | 113 | 55 | 24 | 263 | |||||||||||||||
| Disposals | - | (118 | ) | - | - | (118 | ) | |||||||||||||
| Balance as of December 31, 2016 | 1,570 | 409 | 255 | 167 | 2,401 | |||||||||||||||
| Depreciated balance as of December 31, 2016 | $ | 313 | $ | 341 | $ | 204 | $ | 150 | $ | 1,008 | ||||||||||
The Group has assets that have been fully depreciated and are still in use. As of December 31, 2017 and 2016, the original cost of such assets is $2,614 thousand and $2,115 thousand, respectively.
| F-19 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – INTANGIBLE ASSETS
| Computer software | Capitalized development cost | Marketing rights for a medical product | Total | |||||||||||||
| U.S. dollars in thousands | ||||||||||||||||
| Cost: | ||||||||||||||||
| Balance as of January 1, 2017 | $ | 704 | $ | 606 | $ | 375 | $ | 1,685 | ||||||||
| Additions | 42 | 110 | - | 152 | ||||||||||||
| Balance as of December 31, 2017 | 746 | 716 | 375 | 1,837 | ||||||||||||
| Accumulated amortization: | ||||||||||||||||
| Balance as of January 1, 2017 | 602 | 471 | 355 | 1,428 | ||||||||||||
| Amortization for the year | 76 | 36 | 20 | 132 | ||||||||||||
| Balance as of December 31, 2017 | 678 | 507 | 375 | 1,560 | ||||||||||||
| Amortized balance as of December 31, 2017 | $ | 68 | $ | 209 | $ | - | $ | 277 | ||||||||
| Cost: | ||||||||||||||||
| Balance as of January 1, 2016 | $ | 659 | $ | 506 | $ | 375 | $ | 1,540 | ||||||||
| Additions | 45 | 100 | - | 145 | ||||||||||||
| Balance as of December 31, 2016 | 704 | 606 | 375 | 1,685 | ||||||||||||
| Accumulated amortization: | ||||||||||||||||
| Balance as of January 1, 2016 | 529 | 447 | 281 | 1,257 | ||||||||||||
| Amortization for the year | 73 | 24 | 74 | 171 | ||||||||||||
| Balance as of December 31, 2016 | 602 | 471 | 355 | 1,428 | ||||||||||||
| Amortized balance as of December 31, 2017 | $ | 102 | $ | 135 | $ | 20 | $ | 257 | ||||||||
The capitalized development costs are in respect of the Group’s CloudPAT, a cloud-based information technology platform designed to allow customers to transfer the sleep apnea test results of the Group’s products.
NOTE 7 – EMPLOYEE BENEFITS
Employee benefits include retirement benefit obligations, short-term benefits and share-based payments. As for retirement benefit obligations, the Group has defined benefit plans for which it contributes to insurance policies.
As for share-based payments, see Note 15 and as for benefits to key executives, see Note 21.
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Presented as part of current liabilities – accounts payable: | ||||||||
| Short-term employee benefits | $ | 223 | $ | 198 | ||||
| Presented as part of non-current liabilities: | ||||||||
| Long-term employee benefits | $ | 310 | $ | 156 | ||||
| F-20 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Retirement benefit plans - defined benefit plan
| 1) | Movement in net liabilities for defined benefit plans: |
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Balance at beginning of year | $ | 156 | $ | 168 | ||||
| Expense recognized on the statements of operations: | ||||||||
| Current service costs and interest costs | 61 | 156 | ||||||
| Recognized loss including other: | ||||||||
| Actuarial losses carried to other comprehensive income | 112 | 107 | ||||||
| Other movements: | ||||||||
| Benefits paid | - | (134 | ) | |||||
| Deposits made by the Group | (19 | ) | (141 | ) | ||||
| Balance at end of year | $ | 310 | $ | 156 | ||||
| 2) | Expenses recognized in the statements of operations: |
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Current service costs | $ | 21 | $ | 138 | ||||
| Interest costs | 6 | 1 | ||||||
| Transfer of profits to benefits | 18 | 16 | ||||||
| Total | $ | 45 | $ | 155 | ||||
| 3) | The principal actuarial assumptions as of the report date (based on weighted average): |
| December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| % | % | % | ||||||||||
| Discount rate at the end of the year | 2.74 | 3.35 | 3.58 | |||||||||
| Future salary growth | 3.27 | 3.32 | 3.47 | |||||||||
NOTE 8 – CREDIT FACILITY WITH ABANK AND CONVERTIBLE NOTES
| a. | Credit facility with a bank |
In March 2017, the Company received a bank credit in a total amount of up to $10 million. The credit is comprised of a $6 million long-term loan and a $4 million credit facility against trade accounts receivable, based on specific customer invoices.
The long-term loan is repayable in equal quarterly installments over three years from the date of the draw and bears annual interest of the quarterly dollar LIBOR rate plus 5.5%. The credit facility bears annual interest of the monthly dollar LIBOR rate plus 4.25%.
On January 30, 2018, the terms of the bank credit were amended such that the exercise period of the loan and the credit facility were extended until February 28, 2019 and January 12, 2019, respectively. In addition, the Company undertook that upon the withdrawal of credit, the balance of the cash in the Company’s account with the bank will not be less than 40% of the amount of the outstanding credit actually provided to the Company.
| F-21 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
As part of the bank credit, the Company issued to the bank warrants exercisable into 798,088 of the Company’s ordinary shares at an exercise price of NIS 1.36 per share. The fair value of the warrants was measured using a Black-Scholes valuation model and the cost of $137 thousand was accounted for as an integral part of the effective interest rate of the bank credit.
On February 20, 2018, the Company withdrew $5.0 million from the credit facility for a period of three months. The loans were renewed until November 20, 2018.
| b. | Convertible notes |
In March 2013, the Company issued, NIS 72,256 thousand par value convertible notes listed for trading on the TASE for total net proceeds of $19.5 million. The notes matured in two principal repayments on February 28, 2017 and on February 28, 2018, and bore fixed interest at 8.65% per annum, and were payable semi-annually: on August 28 and on February 28, through February 2018.
The net proceeds from the issuance of the convertible notes were split into two components for measurement purposes: (i) a liability component without a conversion feature that is measured at amortized cost according to the effective interest method; and (ii) a conversion option that is an embedded derivative and is measured at fair value at each reporting date.
The effective interest rate as of the date of the issuance was 27.7%. The attributed transaction costs were allocated to the different components pro-rata to the amounts of their initial recognition before allocation of the said costs.
The notes were convertible, so that each NIS 1.92 par value notes could have been converted into one ordinary share (which, as a result of a rights offering conducted by the Company in December 2015 right, was adjusted such that every 1.92 NIS par value of the notes could be converted to 1.00904 ordinary shares).
On February 28, 2017, the first installment of the notes in a total amount of NIS 38,128 thousand par value (approximately $10,421 thousand) was repaid and on February 28, 2018, the second and last installment of the notes in a total amount of NIS 38,128 thousand par value (approximately $10,940 thousand) was repaid, other than NIS 6.0 million (approximately $1,700 thousand) owed to three shareholders who held notes, of which $500 thousand was repaid to one shareholder in June 2018 and the balance owed to the other two shareholders was invested by them in the private placement described in the following sentence. In May 2018, the Company raised approximately $6.0 million in a private placement of its ordinary shares to certain existing shareholders and various funds affiliated with three Israeli institutional investors. None of the notes were converted.
NOTE 9 – PROVISIONS
| Warranties | Returns | Total | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Balance as of January 1, 2016 | $ | 104 | $ | 134 | $ | 238 | ||||||
| Provisions made during the year | 58 | 2 | 60 | |||||||||
| Provisions reversed during the year | (26 | ) | - | (26 | ) | |||||||
| Provisions realized during the year | (43 | ) | (62 | ) | (105 | ) | ||||||
| Balance as of December 31, 2016 | 93 | 74 | 167 | |||||||||
| Provisions made during the year | 135 | 91 | 226 | |||||||||
| Provisions reversed during the year | (27 | ) | - | (27 | ) | |||||||
| Provisions realized during the year | (101 | ) | (82 | ) | (183 | ) | ||||||
| Balance as of December 31, 2017 | $ | 100 | $ | 83 | $ | 183 | ||||||
| F-22 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – OTHER ACCOUNTS PAYABLE
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Employees | $ | 1,117 | $ | 963 | ||||
| Institutions | 339 | 306 | ||||||
| Interest payable | 326 | 588 | ||||||
| Advance payments from customers | 193 | 158 | ||||||
| Other | 23 | 56 | ||||||
| $ | 1,998 | $ | 2,071 | |||||
For information about the Group’s exposure to currency and liquidity risks in respect of the payables balances, see Note 20.
NOTE 11 – DERIVATIVES
Composition
| December 31 | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Liabilities | ||||||||
| The conversion component in the convertible notes | $ | 96 | $ | 2,237 | ||||
| Viola Warrants (non-traded)* | 2,315 | 3,827 | ||||||
| Warrants (Series 4) (traded) issued in the 2015 rights offering* | 464 | 736 | ||||||
| $ | 2,875 | $ | 6,800 | |||||
* See Note 14.
All of the above derivatives have either a conversion price or an exercise price that are denominated in NIS, a currency different than the functional currency of the Company and as a result are accounted for as a derivative financial instrument measured at fair value through the statements of operations on each reporting date and constitute a liability.
The following parameters were used in the calculation of the fair value of the above derivatives, using the binomial model:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Discount rate for notes (yield to maturity of the notes) | 104.18 | % | 46.62 | % | ||||
| The discount rate of the Viola Warrants and Warrants (Series 4) (risk free interest) | 0.11 | % | 0.43 | % | ||||
| Share price (in NIS) | 1.340 | 1.487 | ||||||
| Standard deviation of the share price | 56.13 | % | 57.90 | % | ||||
| F-23 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The fair value of the Viola Warrants and the Warrants (Series 4) (see Note 14) as of December 31, 2015 and during the nine month period ended September 30, 2016 was measured at quoted market value of the Warrants (Series 4), due to the fact that the Viola Warrants and the Warrants (Series 4) are essentially identical in their conditions. Starting with the fourth quarter of 2016 and until December 31, 2017, the Company believed that there was no active market for the traded Warrants (Series 4) primarily due to an ongoing gradual decline in the frequency and volume of trading in such warrants with significant variance in the transactions prices of the warrants without a corresponding material change in the share price, and often with a negative correlation between the change in the share price and the change in the warrants price. Consequently, the Company estimated the fair value of the Viola Warrants and the Warrants (Series 4) as of December 31, 2016 and for periods thereafter based on observable market data, directly or indirectly, based on the binomial model and based on relevant parameters of the terms of the Viola Warrants and the Warrants (Series 4).
NOTE 12 – COMMITMENETS
Obligation to pay royalties to the Israeli Government’s Innovation Authority (“IIA”)
The Company has received royalty-bearing grants sponsored by the IIA for the support of research and development activities of the Endo PAT3000 product (the development of which was discontinued before its completion with no sales to date). However, according to the IIA, the Company must pay royalties on all sales of all of the Company’s cardiology products, and not only for sales of the Endo PAT3000 and/or its technology, up to the total amount of $1,060 thousands.
The Company accrued for the royalties’ obligation once the grants became repayable, although the Company is in discussions with the IIA regarding the Company’s obligation to pay royalties on products other than the supported Endo PAT3000.
Lease Commitments
The Group has non-cancelable lease agreements for buildings and vehicles. Minimum lease commitments expected under these operating leases are as follows:
| Year Ending December 31, | U.S. dollars in thousands | |||
| 2018 | $ | 931 | ||
| 2019 | 452 | |||
| 2020 | 270 | |||
| 2021 | 210 | |||
| 2022 | 54 | |||
NOTE 13 – INCOME TAXES
| a. | Corporate tax rates in Israel |
The tax rates relevant to corporates in Israel in the years 2015 – 2017 were as follows: 2015 – 26.5%; 2016 – 25%; 2017 – 24%.
On December 22, 2016, the Knesset (the Israeli parliament) approved the Economic Efficiency Law (Legislative Amendments to Achieve Budget Targets for the 2017 and 2018 Budget Years), 2016, which stipulates, inter alia, the reduction of corporate tax rates from 25% to 23% in two phases. The first phase is to a rate of 24%, starting on January 2017 and the second phase is to a rate of 23% starting on January 2018 and thereafter.
| b. | Benefits under the Investment Encouragement Law |
Approved enterprise, benefited enterprise and preferred enterprise
Most of the production facilities of the Company have been granted “Approved Enterprise” and “Benefited Enterprise” status under the Law for the Encouragement of Capital Investments, 1959 (the “Investment Law”). The Company is a “Foreign Investors’ Company” as defined by the Investment Law, which means it is entitled to tax benefits for taxable income arising from its Approved or Benefited Enterprise status.
| F-24 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
A company having an Approved Enterprise, like the Company, that distributes a dividend from income that was tax exempt, will be required in the tax year of the dividend distribution to pay corporate tax on the amount of the dividend distributed (including the company tax required as a result of the distribution) at the corporate tax rate that would have been applicable to it in the year the income was generated if it had not been exempt from tax.
| c. | Taxation of Non-Israeli subsidiaries |
Subsidiaries incorporated outside of Israel are assessed for tax under the tax in their countries of residence. The primary tax rates applicable to the non-Israeli subsidiaries in the Group are:
Japan – tax rate of 25.5% during 2015, 2016 and 2017; U.S. – federal tax rate of 35% during 2015, 2016 and 2017 and, effective 1 January 2018, 21%. The reduction did not have a material impact on the tax expenses of the U.S. subsidiary in the year ended December 31, 2017.
Tax expenses in the statements of operations mainly refer to operations of the subsidiaries in the U.S. and Japan. The Company does not pay taxes in Israel, as it has tax losses carryforward to future years. No deferred tax asset was recognized in respect of those carryforward tax losses, in the absence of expected utilization thereof in the foreseeable future.
The Company did not include a calculation of the theoretical tax due to the fact that the total tax expenses in the statements of operations are not material.
| d. | Carryforward tax losses |
The Company has carryforward tax losses (including carryforward research and development expenses) as of December 31, 2017, amounting to $112 million.
| e. | Tax assessment |
The Company has not received final tax assessments since its incorporation. The Company has self-assessments deemed to be final through the 2012 tax year.
NOTE 14 – EQUITY
| a. | Ordinary shares and additional paid-in capital |
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Number of shares in thousands | ||||||||||||
| Issued and outstanding share capital (ordinary shares): | ||||||||||||
| Outstanding shares at the beginning of the year | 262,917 | 259,581 | 180,762 | |||||||||
| Shares issued in private placements during the year | - | 2,976 | 76,777 | |||||||||
| Shares issued in exercise of stock options during the year | 1,578 | 360 | 2,042 | |||||||||
| Outstanding at the end of the year | 264,495 | 262,917 | 259,581 | |||||||||
| Authorized | 750,000 | 750,000 | 750,000 | |||||||||
The rights of the ordinary shares include voting rights at the general meeting of shareholders, the rights to receive dividends and rights to participate in the distribution of the surplus assets of the Company in the event of liquidation.
| F-25 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| b. | Investment agreement with Viola and the rights offering to the Company’s shareholders |
On August 26, 2015, the Company entered into a share purchase agreement with Viola Growth II A.V. LP, Viola Growth II (A) LP and Viola Growth II (B) LP (collectively, “Viola”). On November 5, 2015 (and, as a second stage of the transaction, on February 1, 2016), following approval by the Company’s shareholders of the transactions contemplated by the share purchase agreement with Viola (the “Viola Transaction”), the Company issued to Viola, in the aggregate for these two closings, 66,876,907 ordinary shares at a purchase price of NIS 1.449 per share (equates to $0.38), resulting in aggregate proceeds (before expenses) of NIS 96.9 million (equates to approximately $25.2 million). In addition, the Company issued to Viola warrants exercisable into up to 33,438,454 ordinary shares (the “Viola Warrants”) for no additional consideration. The Viola Warrants are not listed for trading.
The Viola Warrants are exercisable at an exercise price of (i) for the first 21 months following their issuance, NIS 1.642 per share (equates to $0.47); and (ii) for the remainder of their term, NIS 1.745 per share (equates to $0.50), in each case, subject to adjustments.
The Viola Warrants expire on the earlier of: (i) the passage of 42 months following their issuance (i.e., on May 4, 2019); (ii) in the event of a public offering with a pre-money valuation of the Company of at least at $250 million; or (c) in the event of a merger or sale of the Company which reflects a company value of at least $250 million and the result of which will be that the shareholders in the Company before said event will hold less than the majority of voting rights in the surviving company.
In December 2015, as part of a rights offering to its shareholders (other than Viola), the Company issued 12,876,303 ordinary shares at a price of NIS 1.449 per share (equates to $0.37), resulting in aggregate proceeds (before expenses) of NIS 18.7 million (equates to approximately $4.7 million). In addition, the Company issued to the subscribing shareholders warrants exercisable into up to 6,438,152 ordinary shares (“Warrants (Series 4)”) for no additional consideration. The Warrants (Series 4) were listed on the TASE.
Each Warrant (Series 4) is exercisable at an exercise price of (i) for the first 21 months following their issuance, NIS 1.642 per share (equates to $0.47) and (ii) for the remainder of their term, NIS 1.745 per share (equates to $0.50), in each case, subject to adjustments.
The net considerations of the issuance of shares and warrants as part of the Viola Transaction and the rights offering were attributed first to the liability component (warrants) and the remaining amount was attributed to the equity component (shares). The issuance costs, in both transactions, are attributed to the shares, the Viola Warrants and the Warrants (Series 4) according to the consideration attributed to each of the components. The issuance costs were deducted from the consideration attributed to the shares. The issuance costs attributed to the Viola Warrants and the Warrants (Series 4) were immediately credited to the statements of operations as financial expenses.
NOTE 15 – SHARE-BASED PAYMENTS
| a. | Description of share-based payment arrangements and grants |
| 1) | Performance-based options |
The vesting of options granted to employees, officers and consultants as of January 1, 2015 was partially (28.5%) contingent upon the continued employment (vesting period over four years) and partially (71.5%) on the following performance criteria: meeting overall Company objectives, the employee meeting business and professional goals of the organization unit and on the personal level (in respect of the year ended December 31, 2014 only) and contingent upon the continued employment (vesting period over four years). The overall Company’s objectives included two cumulative threshold conditions which include target revenues and minimum operating income or loss, in line with the work plan approved by the Company’s Board of Directors. In the year ended December 31, 2015, the Company did not meet the overall Company objectives. Unvested options as of December 31, 2015 were cancelled or replaced on January 21, 2016 with new grants of options and restricted share units (“RSUs”) that are either contingent upon the continued employment only or are also contingent on meeting performance criteria, as detailed in (2) below.
| F-26 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| 2) | Options and RSUs with service conditions and market conditions |
On January 21, 2016, the Company’s Board of Directors approved a new share-based plan for options and RSUs for key employees that will vest on January 21, 2020 (or earlier in case of an acceleration event), if the share price is at least NIS 2.13 (equates to $0.61) (the “First Trigger Price”), at which time 50% of the RSUs will vest and if the share price is NIS 4.24 (equates to $1.22), 100% will vest. In the range between these two share prices, a relative quantity will vest. An acceleration event is defined as an event in which all the issued and outstanding share capital of the Company (including by way of a merger in which the Company’s shareholders prior to the merger will hold less than 10% of the issued and outstanding share capital and voting rights in the company surviving the merger) is sold for consideration reflecting a price per share that is not lower than NIS 2.13 (equates to $0.61). The above vesting is also contingent upon continued employment.
On March 14, 2018, the Company’s Board of Directors approved a change of the First Trigger Price from NIS 2.13 to NIS 1.70 (equates to $0.49) and a change of the January 21, 2020 vesting date to December 20, 2020. On May 23, 2018 the Company’s shareholders approved such changes with respect to the portion of such options and RSUs granted to the Company’s President and Chief Executive Officer (the “CEO”).
| 3) | Options to key employees, employees and directors with only service condition |
On January 21, 2016, the Company’s Board of Directors, as part of the share-based plan described in (2) above, also approved a grant of options that will vest as followed: 25% will vest and become exercisable one year following the date of grant and the remaining 75% will vest and become exercisable in 12 equal quarterly portions, beginning on the first anniversary of the date of grant.
Grants to other employees not participating in the key employees share-based plan, usually vest over four years, as follows: 2/3 will vest and be exercisable two years following the date of grant, and the remaining 1/3 will vest and become exercisable in four equal quarterly portions, at the end of each calendar quarter commencing on the second anniversary of the date of grant.
Options granted to directors during the years ended December 31, 2015, 2016 and 2017, were usually divided into three tranches, each equal to 33% of the amount of options granted. The allotment and the vesting period for the first tranche began on the date of grant; the allotment and the vesting period for the second tranche will begin on first anniversary of the date of grant; and the allotment and the vesting period for the third tranche will begin on the third anniversary of the date grant. Each tranche vests in four equal portions annually over four years. The exercise price for each tranche is set on the date of allotment and is based on the average price of the market price of the ordinary share prior to such allotment date plus 10%. The grants to directors were measured on the grant date for all three tranches using the binomial model. The option data in d. below include only options already allotted.
| 4) | Grants of options and RSUs under the January 2016 plan |
In March 2016, the Company granted to (i) the CEO options exercisable into 3,620,834 ordinary shares in lieu of all the options granted in the past that have not yet vested, and (ii) 16 other employees options exercisable into 3,755,847 ordinary shares in lieu of all the options granted in the past that have not yet vested. In addition, the terms of options exercisable into 741,314 ordinary shares granted in the years ended December 31, 2014 and 2015 to other employees was modified, such that the performance conditions of their exercise were cancelled and their vesting period has been extended. The said replacement was handled as a change in the conditions in accordance with IFRS 2, Share-based Payment. The incremental value measured at the date of replacement was not significant.
| F-27 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
In addition, in March 2016, the Company granted to employees and officers of the Company 15,270,957 options and 3,465,761 RSUs.
In May 2016, the Company granted 1,759,999 options to directors.
In September 2016, the Company granted to employees 1,114,129 options and 72,545 RSUs.
In February 2017, the Company granted 711,000 options to employees. In May 2017, the Company granted 440,000 options to directors and 100,000 options were granted to a consultant.
In September 2017, the Company granted 2,281,218 options and 362,858 RSUs to employees. In addition, 100,000 options were granted to a consultant.
On March 14, 2018, the Company’s Board of Directors approved a grant of 2,066,193 options and 278,566 RSUs to employees, officers and consultants of the Company.
| b. | Measurement of fair value of share-based payments |
The fair value of the options granted to the CEO, employees, directors and consultants is measured according to the Black-Scholes pricing model. The fair value of stock options and performance-based RSUs granted to officers and key employees where vesting is made on the basis of the increase in the Company’s share price is measured by implementing the Monte Carlo Simulation. The options granted to directors but which have not yet been allocated, nor set an exercise price, were priced using the binomial model.
Following are the parameters used to measure the fair value on the date of grant of share-based awards:
| Options
with service conditions only | Options
with service conditions and market conditions | RSUs | ||||||||||
| The number of shares arising from the exercise of the options (in thousands) | 2,097 | 1,645 | 363 | |||||||||
| The parameters included when calculating fair value: | ||||||||||||
| The share price (at the grant date) (in NIS) | 1.13 – 1.61 | 1.13 | 1.13 | |||||||||
| The exercise price (in NIS) | 1.28 – 1.68 | 1.17 | 0.00 | |||||||||
| Expected volatility (weighted average) | 56.9% - 58.9% | 56.9% | 56.9% | |||||||||
| Expected lifetime (weighted average) | 3– 6 years | 5.5 years | N/A | |||||||||
| Risk-free interest rate | 0.4% - 1.43% | 0.9% | 0.9% | |||||||||
| Expected dividend rate | 0% | 0% | 0% | |||||||||
The expected volatility was determined based on the historical volatility of the share price. The expected lifetime of the options is determined in accordance with management’s estimation of the duration of the employees’ holdings of such awards, given their position in the Company and the Company’s past experience with respect to employee attrition. The risk-free interest rate is based on interest rates of Israeli government bonds denominated in NIS, whose remaining period is equal to the expected lifetime of the options.
As to grant of stock options and RSUs subsequent to the reporting date and to the change subsequent to the reporting date of the terms of the options and RSUs having service conditions and market conditions, see d. below.
| F-28 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| c. | Extension of the exercise period of options granted to the CEO and to officers and key employees of the Company and its subsidiaries |
On March 21, 2017, the Company’s Board of Directors resolved to extend by five years, till January 20, 2026, the exercise period of a total of 18,890,695 options, consisting of 3,699,208 options with service conditions and 15,191,487 options with service conditions and market conditions, granted to officers and key employees of the Company and its subsidiaries. There was no change in the other terms of the options, including the exercise price and the vesting terms. The new exercise period is in line with the Company’s compensation policy which allows an exercise period of up to ten years. On May 14, 2017 the Company’s shareholders approved such extension with respect to the portion of such options granted to the CEO.
The fair value of the extension of the exercise period of the options was $475 thousand. The assessment of the fair value of change on the service-based options has been executed using Black-Scholes pricing model. The assessment of the fair value of change on the options with service conditions and market conditions has been executed using a Monte-Carlo Simulation. The assumptions used are detailed below:
| Service options | Performance options | |||||||
| Expected volatility | 57.6% | 57.6% | ||||||
| Average lifetime (in years) | 4.8 – 5.9 | 8.8 | ||||||
| Risk free interest rate | 0.91% - 1.36% | 2.0% | ||||||
| Expected dividends rate | 0% | 0% | ||||||
| d. | Reconciliation of outstanding options and RSU’s |
The number of options and RSUs and the weighted average exercise price for every option or RSU:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2017 | 2016 | 2015 | ||||||||||||||||||||||
| Number of awards | Range of exercise price (NIS) | Number of Awards | Range of exercise price (NIS) | Number of awards | Range of exercise price (NIS) | |||||||||||||||||||
| Outstanding at beginning of year | 40,178,148 | 0.00 – 2.50 | 24,316,648 | 0.10 – 2.50 | 26,935,899 | 0.10 – 2.50 | ||||||||||||||||||
| Granted during the year | 4,105, 076 | 0.00 – 1.68 | 28,180,067 | 0.00 – 1.55 | 1,602,250 | 1.77 - 1.98 | ||||||||||||||||||
| Forfeited and expired during the year | (6,825,690 | ) | - | (11,962,761 | ) | - | (2,179,232 | ) | - | |||||||||||||||
| Exercised during the year | (1,565,050 | ) | 0.23 | (355,806 | ) | 0.10 - 0.48 | (2,042,269 | ) | 0.23 – 1.73 | |||||||||||||||
| Outstanding at end of year | * 35,892,484 | 0.00 - 2.50 | 40,178,148 | 0.00 - 2.50 | 24,316,648 | 0.10 - 2.50 | ||||||||||||||||||
| Exercisable at end of year | 9,716,559 | 0.23 - 2.50 | 12,319,881 | 0.23 - 2.50 | 12,078,958 | 0.10 - 2.50 | ||||||||||||||||||
| * Including: | ||||||||||||||||||||||||
| Options with service conditions only | 16,753,449 | |||||||||||||||||||||||
| Options with service conditions and market conditions | 15,896,403 | |||||||||||||||||||||||
| RSUs | 3,242,632 | |||||||||||||||||||||||
| Total | 35,892,484 | |||||||||||||||||||||||
As a result of the grant of options and RSUs, the Company recorded for the years ended December 31, 2017, 2016 and 2015, a non-cash expense of $1,294 thousand, $1,776 thousand and $428 thousand, respectively. The balance of expenditure amounting to $1,865 thousand will be recorded by the Company over the remaining vesting period of the options and RSUs.
The weighted average share price upon exercise of the options, for options exercised in the year ended December 31, 2017 and 2016 and 2015 was $0.35, $0.34 and $0.42, respectively. The weighted average remaining contractual life of the options outstanding as of December 31, 2017, 2016 and 2015 was 6.23 years, 4.22 years and 6.47 years, respectively.
| F-29 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 16 – REVENUES
The Company operates in one business sector.
The following is a breakdown of revenues according to product groups:
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| WatchPAT and other related services | $ | 18,105 | $ | 15,697 | $ | 12,414 | ||||||
| Endo PAT and other related services | 2,596 | 2,743 | 4,393 | |||||||||
| $ | 20,701 | $ | 18,440 | $ | 16,807 | |||||||
The following is a breakdown of revenues on the basis of geographical regions (based on the geographical location of the customer).
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| United States and Canada | $ | 14,764 | $ | 13,343 | $ | 10,485 | ||||||
| Japan | 2,965 | 2,161 | 2,045 | |||||||||
| Europe | 1,746 | 1,542 | 2,155 | |||||||||
| Asia Pacific (excluding Japan) | 759 | 1,017 | 1,511 | |||||||||
| Israel | 260 | 268 | 301 | |||||||||
| Others | 207 | 109 | 310 | |||||||||
| $ | 20,701 | $ | 18,440 | $ | 16,807 | |||||||
The majority of the Company’s long lived assets are in Israel.
Revenue from major customers
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Customer A | $ | 3,622 | $ | 3,549 | $ | 2,927 | ||||||
| Customer B | 2,621 | 2,119 | 1,567 | |||||||||
| Customer C | 2,510 | 2,096 | 924 | |||||||||
| $ | 8,753 | $ | 7,764 | $ | 5,418 | |||||||
| F-30 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
NOTE 17 – COST OF REVENUES
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Raw materials, auxiliary materials, subcontractors (including changes in inventories) | $ | 1,767 | $ | 2,173 | $ | 1,803 | ||||||
| Payroll and related expenses (including share-based payment) | 1,956 | 1,749 | 1,698 | |||||||||
| Shipping | 500 | 386 | 377 | |||||||||
| Depreciation and amortization | 190 | 124 | 134 | |||||||||
| Other | 589 | 547 | 389 | |||||||||
| $ | 5,002 | $ | 4,979 | $ | 4,401 | |||||||
NOTE 18 – FINANCIAL INCOME AND EXPENSES
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Financial income (expenses) from cash and investments: | ||||||||||||
| In respect of investments in bank deposits and marketable securities * | $ | 1,389 | $ | 547 | $ | (530 | ) | |||||
| Other financial income | 202 | 169 | 176 | |||||||||
| 1,591 | 716 | (354 | ) | |||||||||
| Financial expenses from notes and loans: | ||||||||||||
| Convertible notes* | 4,427 | 4,610 | 3,657 | |||||||||
| Long-term loans from shareholders* | - | - | 199 | |||||||||
| Other financial expenses | 427 | 185 | 282 | |||||||||
| Exchange rate differences | 30 | (35 | ) | 91 | ||||||||
| 4,884 | 4,760 | 4,229 | ||||||||||
| Gain (loss) on derivative financial instruments: | ||||||||||||
| Gain on revaluation to fair value of the warrants embedded in the convertible notes | 2,141 | 1,567 | 5,358 | |||||||||
| Gain (loss) on revaluation to fair value of warrants | 1,784 | (1,783 | ) | 2,572 | ||||||||
| $ | 3,925 | $ | (216 | ) | $ | 7,930 | ||||||
* Including the effect of changes in the exchange rate of the NIS against the dollar.
NOTE 19 – LOSS PER SHARE
| a. | Basic loss per share |
The computation of basic loss per share was based on the net loss attributable to ordinary shares divided by the weighted average number of ordinary shares outstanding.
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Net loss attributed to the ordinary shares | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||
| F-31 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Weighted average number of ordinary shares
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Number of shares in thousands | ||||||||||||
| Balance at the beginning of the year | 262,917 | 259,581 | 181,626 | |||||||||
| The effect of private placement and issue of rights | - | 2,716 | 9,915 | |||||||||
| The effect of exercise of options into shares | 1,192 | 245 | 268 | |||||||||
| Weighted average number of ordinary shares used in computation of basic loss per share | 264,109 | 262,542 | 191,809 | |||||||||
| b. | Diluted loss per share |
The computation of diluted loss per share was based on the net loss attributed to the ordinary shares divided by the weighted average number of ordinary shares outstanding, after adjustment for all potentially dilutive ordinary shares, as follows:
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Net loss used in computation of basic earnings per share | $ | (5,301 | ) | $ | (14,403 | ) | $ | (2,247 | ) | |||
| Changes in the fair value of the Viola warrants and Warrants (Series 4), which are classified as a liability | (1,785 | ) | - | - | ||||||||
| Financial expenses in respect of convertible notes | - | - | (1,711 | ) | ||||||||
| Net loss attributed to the ordinary shares (diluted) | $ | (7,086 | ) | $ | (14,403 | ) | $ | (3,958 | ) | |||
Weighted average number of ordinary shares (diluted)
| Year Ended December 31, | ||||||||||||
| 2017 | 2016 | 2015 | ||||||||||
| Number of shares in thousands | ||||||||||||
| Weighted average number of ordinary shares used in computation of basic loss per share | 264,109 | 262,542 | 191,809 | |||||||||
| Effect of conversion of convertible notes | - | - | 40,075 | |||||||||
| Effect of the exercise of the Viola warrants and Warrants (Series 4) | 39,877 | - | - | |||||||||
| Weighted average number of ordinary shares used in computation of diluted loss per share per share | 303,986 | 262,542 | 231,884 | |||||||||
In the calculation of the weighted average number of ordinary shares (diluted) for the year ended December 31, 2017, 23,588,582 shares in respect of convertible notes, 32,719,056 shares in respect of options and 3,242,632 shares in respect of RSUs granted to employees, directors and consultants were not included, due to their anti-dilutive effect.
| F-32 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
In the calculation of the weighted average number of ordinary shares (diluted) for the year ended December 31, 2016, 40,075,289 shares in respect of convertible notes, 33,438,454 shares in respect of the Viola Warrants, 6,438,152 shares in respect of Warrants (Series 4) and 36,779,259 shares in respect of options and 3,398,889 shares in respect of RSUs granted to employees, directors and consultants were not included, due to their anti-dilutive effect.
In the calculation of the weighted average number of ordinary shares (diluted) for the year ended December 31, 2015, 31,950,380 shares in respect of the Viola Warrants, 6,438,152 shares in respect of Warrants (Series 4) and 24,316,648 shares in respect of options granted to employees, directors and consultants were not included, due to their anti-dilutive effect.
NOTE 20 –FINANCIAL INSTRUMENTS
This note provides qualitative information regarding the exposure to each of the following risks, and the Group’s objectives, policy and processes relating to measurement of such risks. Quantitative disclosure is provided throughout these consolidated financial statements.
Credit risk
As of December 31, 2017 and 2016, the maximum exposure to credit risk is represented by the balance of financial assets. Management has developed policies for the authorization of credit to customers. The accounting exposure to credit risk is monitored constantly according to the behavior of payment of the debtors. Credit is assigned on a customer-by-customer basis and is subject to assessments which consider the customers’ payment capacity, as well as past behavior regarding due dates, balances past due and delinquent accounts. Approximately 42%, 42% and 27%, respectively, of the Group’s revenues in the years ended December 31, 2017, 2016 and 2015, arise from sales to single customers. Other than this, there are no other concentrations of credit risk.
The Group’s revenues are primarily derived from sales to customers in the U.S., Japan and Europe. Management regularly monitors trade receivables and the financial statements include specific provisions for doubtful debt, which properly reflect, in the opinion of management, the inherent loss in debt whose collection is in doubtful.
The Group limits its exposure to credit risk by investing exclusively in money market funds, bank deposits, government and corporate bonds with rating of no less than rating A (there may be investments in corporate bonds rating BBB through portfolio managers of up to 10% of the investment portfolio managed by them).
The Group realized its investments in securities at fair value in January and February 2018, close to the date of repayment of the convertible notes. As of December 31, 2017 and 2016 the investment includes investment in corporate and Israeli government NIS-denominated bonds.
Following is the composition of investments in marketable securities:
| December 31 | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Government bonds – NIS linked to the Israeli CPI | $ | 368 | $ | 382 | ||||
| Government bonds – NIS | 838 | 720 | ||||||
| Corporate bonds- NIS linked to the Israeli CPI | 1,008 | 940 | ||||||
| Corporate bonds –NIS | 777 | 587 | ||||||
| Current account | 182 | 152 | ||||||
| $ | 3,173 | $ | 2,781 | |||||
| F-33 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
The maximum exposure to credit risk in respect of cash and cash and cash equivalents, trade receivables, other accounts receivable and other investments, as of the report date, by geographic locations was as follows:
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Israel | $ | 9,581 | $ | 25,977 | ||||
| United States and Canada | 5,815 | 4,577 | ||||||
| Asia Pacific (including Japan) | 817 | 791 | ||||||
| Europe | 909 | 426 | ||||||
| Other | 27 | 44 | ||||||
| $ | 17,149 | $ | 31,815 | |||||
Aging of receivables and impairment
| December 31, 2017 | December 31, 2016 | |||||||||||||||
| Gross Amount | Impairment | Gross amount | Impairment | |||||||||||||
| U.S. dollars in thousands | U.S. dollars in thousands | |||||||||||||||
| Not in arrears | $ | 4,503 | $ | - | $ | 4,607 | $ | 151 | ||||||||
| In arrears up to three months | 1,160 | - | 312 | - | ||||||||||||
| In arrears up to six months | 200 | 35 | 285 | 13 | ||||||||||||
| In arrears up to 12 months | 71 | 63 | 142 | 73 | ||||||||||||
| In arrears over 12 months | 442 | 442 | 253 | 213 | ||||||||||||
| $ | 6,376 | $ | 540 | $ | 5,599 | $ | 450 | |||||||||
Movements in the allowance for impairment of receivables and loans granted during the year were as follows:
| Year Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| U.S. dollars in thousands | ||||||||
| Balance at beginning of year | $ | 450 | $ | 256 | ||||
| Recognized impairment loss | 147 | 849 | ||||||
| Bad debt | (57 | ) | (655 | ) | ||||
| Balance at end of year | $ | 540 | $ | 450 | ||||
Liquidity risk
The Group’s approach to managing liquidity is to ensure, as far as possible, that it will always have sufficient liquidity to meet its liabilities when due, under both normal and stressed conditions, without incurring unacceptable losses or risking damage to the Group’s reputation.
The Group ensures sufficient cash on hand for payment of expected operating expenses, including any amounts required to fulfill financial obligations. In addition to cash flows provided by its operating activities, in order to meet the Company’s overall liquidity needs for operations, servicing debt and funding capital expenditures, the Company relies on cost-cutting and operating improvements to optimize capacity utilization and maximize profitability, as well as borrowing under credit facilities, proceeds of debt and equity offerings,
| F-34 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Below is an analysis of contractual maturities of financial liabilities, including estimated interest payments, as of December 31, 2017:
| Carrying Amount | Contractual Cash flow | Up
to 6 months | 6-12 months | 1-2 years | 2-5 years | Over
5 years | ||||||||||||||||||||||
| U.S. dollars in thousands | ||||||||||||||||||||||||||||
| December 31, 2017 | ||||||||||||||||||||||||||||
| Non-derivative financial liabilities | ||||||||||||||||||||||||||||
| Convertible notes, including current maturities and accrued interest | $ | 11,022 | $ | 11,473 | $ | 11,473 | $ | - | $ | - | $ | - | $ | - | ||||||||||||||
| Trade payables | 1,262 | 1,262 | 1,262 | - | - | - | - | |||||||||||||||||||||
| Other long-term liabilities | 948 | 948 | - | - | 948 | - | - | |||||||||||||||||||||
| Other accounts payable | 2,714 | 2,714 | 2,536 | 178 | - | - | - | |||||||||||||||||||||
| Total | $ | 15,946 | $ | 16,397 | $ | 15,271 | $ | 178 | $ | 948 | $ | - | $ | - | ||||||||||||||
| December 31, 2016 | ||||||||||||||||||||||||||||
| Non-derivative financial liabilities | ||||||||||||||||||||||||||||
| Convertible notes, including current maturities and accrued interest | $ | 18,378 | $ | 21,548 | $ | 10,774 | $ | 429 | $ | 10,345 | $ | - | $ | - | ||||||||||||||
| Trade payables | 1,324 | 1,324 | 1,324 | - | - | - | - | |||||||||||||||||||||
| Other long-term liabilities | 860 | 860 | - | - | 860 | - | - | |||||||||||||||||||||
| Other accounts payable | 2,086 | 2,086 | 1,908 | 178 | - | - | - | |||||||||||||||||||||
| Total | $ | 22,648 | $ | 25,818 | $ | 14,006 | $ | 607 | $ | 11,205 | $ | - | $ | - | ||||||||||||||
Equity Risk
Changes in the fair value of the Company’s conversion component of the convertible notes, in the fair value of the Viola Warrants and the Warrants (Series 4) that are denominated in a currency other than the Company’s functional currency affect the statements of operations. However, they do not imply any risk or variability in cash flows, considering that through their exercise, the Company will settle the aforementioned derivatives via issuance of its own shares rather than in cash.
As of December 31, 2017 and 2016, the potential change in the fair value of these derivatives that would result from a hypothetical, instantaneous, increase of 10% in the market price of the Company’s share price, with all other variables held constant, would have increased the Company’s net loss by $819 thousands in the year ended December 31, 2017 and decreased the Company’s loss by $1,682 thousands in the year ended December 31, 2016.
As of December 31, 2017 and 2016, the potential change in the fair value of these derivatives that would result from a hypothetical, instantaneous, decrease of 10% in the market price of the Company’s share price, with all other variables held constant, would have decreased the Company’s loss by $952 thousands in the year ended December 31, 2017 and decreased the Company’s net loss by $1,500 thousands in the year ended December 31, 2016.
As discussed in Note 8, the convertible notes were repaid in February 2017 and February 2018, and, together with the repayment of the notes, the conversion component expired. Accordingly, as to remaining derivatives being measured at fair value (the Viola Warrants and the Warrants (Series 4)), a hypothetical, instantaneous, increase of 10% in the market price of the Company’s share price, with all other variables held constant, is expected to increase the Company’s net loss by $800 thousands in 2018, as a result of additional negative changes in fair value associated with these warrants. A 10% hypothetical decrease in the market price of the Company’s share price would generate approximately the opposite effect.
Foreign Currency risk
The Group is exposed to foreign currency risk with respect to sales, purchases, payroll and services expenses and loans denominated in non-dollar currencies (primarily NIS, but also Euro and Japanese yen) used by the companies in the Group. The currencies in which most expenses are denominated are the dollar, NIS, Euro and Japanese yen.
| F-35 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Most of the Group’s revenues are denominated in its functional currency (the dollar) and some in Euro, whereas the Group’s payroll expenses in Israel are denominated in NIS. Therefore, the Group is exposed to fluctuations in the dollar/NIS and dollar/Euro exchange rates and strives to mitigate currency risk by maintaining liquid investments and cash positions in short-term NIS-denominated deposits, in NIS and in Euro.
The Group’s exposure to the Israeli CPI and foreign currency risk is as follows:
| December 31, 2017 | ||||||||||||||||||||||||||||
| Currency different from dollar | ||||||||||||||||||||||||||||
| Dollars | NIS | NIS
linked to the Israeli CPI | Euro | Other currencies | Non- monetary items | Total | ||||||||||||||||||||||
| U.S. dollars in thousands | ||||||||||||||||||||||||||||
| December 31, 2017 | ||||||||||||||||||||||||||||
| Assets | ||||||||||||||||||||||||||||
| Cash and cash equivalents | $ | 2,337 | $ | 5,124 | $ | - | $ | 160 | $ | 22 | $ | - | $ | 7,643 | ||||||||||||||
| Marketable securities | - | 1,797 | 1,376 | - | - | - | 3,173 | |||||||||||||||||||||
| Trade receivables (including long-term trade receivables) | 4,952 | 194 | - | 689 | - | - | 5,835 | |||||||||||||||||||||
| Accounts receivable | 137 | 45 | - | 3 | - | 500 | 685 | |||||||||||||||||||||
| Inventories | - | - | - | - | - | 2,260 | 2,260 | |||||||||||||||||||||
| Long-term restricted deposits | 108 | 205 | - | - | - | - | 313 | |||||||||||||||||||||
| Long-term prepaid expenses | - | - | - | - | - | 69 | 69 | |||||||||||||||||||||
| Property and equipment and intangible assets | - | - | - | - | - | 1,299 | 1,299 | |||||||||||||||||||||
| 7,534 | 7,365 | 1,376 | 852 | 22 | 4,128 | 21,277 | ||||||||||||||||||||||
| Liabilities | ||||||||||||||||||||||||||||
| Trade payables | 382 | 833 | - | 47 | - | - | 1262 | |||||||||||||||||||||
| Employee benefits | - | - | - | - | - | 533 | 533 | |||||||||||||||||||||
| Provisions | - | - | - | - | - | 183 | 183 | |||||||||||||||||||||
| Other accounts payable (including accrued expenses) | 1,877 | 1,117 | - | 70 | - | 339 | 3,403 | |||||||||||||||||||||
| Convertible notes | - | 10,696 | - | - | - | - | 10,696 | |||||||||||||||||||||
| Financial derivatives | - | 2,875 | - | - | - | - | 2,875 | |||||||||||||||||||||
| Other long-term accounts payable | 905 | - | 43 | - | - | - | 948 | |||||||||||||||||||||
| 3,164 | 15,521 | 43 | 117 | - | 1,055 | 19,900 | ||||||||||||||||||||||
| Total exposure in the statements of financial position in respect of financial assets and financial liabilities | $ | 4,370 | $ | (8,156 | ) | $ | 1,333 | $ | 735 | $ | 22 | $ | 3,073 | $ | 1,377 | |||||||||||||
| December 31, 2016 | ||||||||||||||||||||||||||||
| Assets | ||||||||||||||||||||||||||||
| Cash and cash equivalents | $ | 4,266 | $ | 18,371 | $ | - | $ | 680 | $ | 41 | $ | - | $ | 23,358 | ||||||||||||||
| Marketable securities | - | 1,460 | 1,321 | - | - | - | 2,781 | |||||||||||||||||||||
| Trade receivables (including long-term trade receivables) | 4,687 | 104 | - | 358 | - | - | 5,149 | |||||||||||||||||||||
| Accounts receivable | 193 | 40 | - | 2 | 5 | 510 | 750 | |||||||||||||||||||||
| Inventories | - | - | - | - | - | 1,784 | 1,784 | |||||||||||||||||||||
| Long-term restricted deposits | 108 | 179 | - | - | - | - | 287 | |||||||||||||||||||||
| Long-term prepaid expenses | - | - | - | - | - | 173 | 173 | |||||||||||||||||||||
| Property and equipment and intangible assets | - | - | - | - | - | 1,265 | 1,265 | |||||||||||||||||||||
| 9,254 | 20,154 | 1,321 | 1,040 | 46 | 3,732 | 35,547 | ||||||||||||||||||||||
| Liabilities | ||||||||||||||||||||||||||||
| Trade payables | 803 | 501 | - | 20 | - | - | 1,324 | |||||||||||||||||||||
| Employee benefits | - | - | - | - | - | 354 | 354 | |||||||||||||||||||||
| Provisions | - | - | - | - | - | 167 | 167 | |||||||||||||||||||||
| Other accounts payable (including accrued expenses) | 1,538 | 1,069 | - | 47 | 19 | 337 | 3,010 | |||||||||||||||||||||
| Convertible notes | - | 17,791 | - | - | - | - | 17,791 | |||||||||||||||||||||
| Financial derivatives | - | 6,800 | - | - | - | - | 6,800 | |||||||||||||||||||||
| Other long-term accounts payable | 836 | - | 24 | - | - | - | 860 | |||||||||||||||||||||
| 3,177 | 26,161 | 24 | 67 | 19 | 858 | 30,306 | ||||||||||||||||||||||
| Total exposure in the statements of financial position in respect of financial assets and financial liabilities | $ | 6,077 | $ | (6,007 | ) | $ | 1,297 | $ | 973 | $ | 27 | $ | 2,874 | $ | 5,241 | |||||||||||||
| F-36 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Sensitivity analysis
A stronger dollar against the following currencies at the end of each reporting period, and an increase in the Israeli CPI would have increased (decreased) equity and net income/loss by the following amounts (after-tax). The following analysis is based on changes to exchange rates and to the Israeli CPI, which the Group believes to be reasonably possible as of the end of the reported year. This analysis assumes all other variables, especially interest rates, remain constant.
| December 31 2017 | ||||||||
| Equity | Profit (loss) | |||||||
| U.S. dollars in thousands | ||||||||
| An increase in the exchange rate of the: | ||||||||
| NIS/dollar by 5% | $ | (340 | ) | $ | (340 | ) | ||
| Euro/dollar by 5% | 37 | 37 | ||||||
The weakening of these currencies against the dollar and the decrease in the Israeli CPI at a similar rate as of December 31, 2017 had a similar effect, albeit in the opposite direction, assuming that all other variables remain constant.
| December 31 2016 | ||||||||
| Equity | Profit (loss) | |||||||
| U.S. dollars in thousands | ||||||||
| An increase in the exchange rate of the: | ||||||||
| NIS/dollar by 5% | $ | (236 | ) | $ | (236 | ) | ||
| Euro/dollar by 5% | 49 | 49 | ||||||
The weakening of these currencies against the dollar and the decrease in the Israeli CPI at a similar rate as of December 31, 2016 had a similar effect, albeit in the opposite direction, assuming that all other variables remain constant.
Fair value of financial instruments measured at fair value, for disclosure purposes only
The carrying amount of the cash and cash equivalents, trade receivables, other accounts receivable, bank deposits, pledged deposits, trade payables, and other accounts payable and derivatives is identical or approximate to their fair values due to the lifetime of these items.
The fair value of other financial assets and liabilities and their carrying amounts, as presented in the statements of financial position, are as follows:
| December 31 2017 | December 31 2016 | |||||||||||||||
| Carrying amount | Fair value | Carrying amount | Fair value | |||||||||||||
| U.S. dollars in thousands | ||||||||||||||||
| Liabilities: | ||||||||||||||||
| Convertible notes ** | $ | 11,118 | $ | *11,283 | $ | 20,616 | $ | *21,062 | ||||||||
| Liability in respect of royalties to the IIA | 948 | 430 | 860 | 276 | ||||||||||||
| $ | 12,066 | $ | 11,713 | $ | 21,476 | $ | 21,338 | |||||||||
* Including interest payable, but excluding the fair value of the embedded warrants.
** Quoted market price on the TASE.
| F-37 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
Fair value hierarchy of financial instruments measured at fair value
The following table shows an analysis of the financial instruments measured at fair value using the valuation method.
| December 31, 2017 | ||||||||||||
| Level 1 | Level 3 | Total | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Financial instruments - marketable securities | $ | 3,173 | $ | - | $ | 3,173 | ||||||
| Financial instruments – derivative instruments | - | 2,875 | 2,875 | |||||||||
| December 31, 2016 | ||||||||||||
| Level 1 | Level 3 | Total | ||||||||||
| U.S. dollars in thousands | ||||||||||||
| Financial instruments - marketable securities | $ | 2,781 | $ | - | $ | 2,781 | ||||||
| Financial instruments – derivative instruments | - | 6,800 | 6,800 | |||||||||
The change from the opening balance to the closing balance of the financial instruments measured at fair value, categorized within Level 3 hierarchy, in the years ended December 31, 2017 and 2016, respectively, was caused by the revaluation to fair value of the derivatives in the amount of $3,925 thousand and $(216) thousand as described in Note 18, and from the issuance of $84 thousand of Viola Warrants in February 2016.
NOTE 21 – RELATED PARTIES
| a. | Compensation |
Compensation to key executives includes:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2017 | 2016 | 2015 | ||||||||||||||||||||||
| Number of persons | Amount | Number of persons | Amount | Number of persons | Amount | |||||||||||||||||||
| U.S.
dollars in thousands | U.S.
dollars in thousands | U.S.
dollars in thousands | ||||||||||||||||||||||
| Employee compensation | 7 | $ | 1,384 | 6 | $ | 1,590 | 7 | $ | 1,373 | |||||||||||||||
| Share-based payment | 7 | 1,083 | 6 | 1,168 | 7 | 128 | ||||||||||||||||||
| $ | 2,467 | $ | 2,758 | $ | 1,501 | |||||||||||||||||||
Compensation to directors who are not employed by the Company:
| Year Ended December 31, | ||||||||||||||||||||||||
| 2017 | 2016 | 2015 | ||||||||||||||||||||||
| Number
of persons | Amount | Number
of persons | Amount | Number
of persons | Amount | |||||||||||||||||||
| U.S.
dollars in thousands | U.S.
dollars in thousands | U.S.
dollars in thousands | ||||||||||||||||||||||
| Total benefits to directors not employed by the Company | 9 | $ | 306 | 9 | $ | 278 | 9 | $ | 263 | |||||||||||||||
| Year Ended December 31, | December 31, | |||||||||||||||||||
| 2017 | 2016 | 2015 | 2017 | 2016 | ||||||||||||||||
| Transaction amounts | Carrying amount | |||||||||||||||||||
| U.S. dollars in thousands | U.S. dollars in thousands | |||||||||||||||||||
| Key executives (including directors) of the Company | $ | 2,773 | $ | 3,035 | $ | 1,764 | $ | 1,147 | $ | 2,042 | ||||||||||
| F-38 |
ITAMAR MEDICAL LTD.
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
| b. | Capital reserve for transactions with shareholders |
Any difference between the nominal value - i.e., the cash amount received - of the loans provided by shareholders, acting in the capacity of shareholders, and their fair value on initial recognition is reflected in equity as a shareholder contribution. Upon an early extinguishment of such debt, any cost incurred as a result of the early extinguishment is reflected in equity as a shareholder distribution.
| c. | Insurance and indemnification of key management personnel |
The Company’s directors and officers are covered by a directors’ and officers’ liability insurance policy. In addition, the Company has undertaken to enter into indemnification agreements with each of its directors and officers undertaking to indemnify them to the fullest extent permitted by law.
| d. | Marketing agreement with a former controlling shareholder in the Company |
In 2014, the Company entered into a co-marketing agreement with Medtronic, Inc. (“Medtronic”) (which at the time was a controlling shareholder) that was subsequently amended in 2015. According to the agreement (as amended), Medtronic was granted exclusive rights to co-market, with the Company, the Company’s WatchPAT products within the Company’s Total Sleep Solution framework to electrophysiologists (physicians who specialize in cardiology arrhythmias) in the United States. Pursuant to this agreement, Medtronic markets WatchPAT as part of a comprehensive solution offered by Medtronic to physicians. The agreement is currently renewable automatically for 30 day-periods, unless earlier terminated by either party upon 14 days prior notice.
In the years ended on December 31, 2017, 2016 and 2015, the Company recognized revenues from sales to customers (third parties) under this agreement in the amount of approximately $307 thousand, $177 thousand and $222 thousand, respectively. The total sales commissions to Medtronic in these years under this agreement totaled approximately $61 thousand, $35 thousand and $44 thousand, respectively.
| F-39 |
Condensed Consolidated Interim
Financial Statements
As of June 30, 2018
(Unaudited)
| F-40 |
CONDENSED CONSILIDATED INTERIM Financial statements
as of June 30, 2018
(UNAUDITED)
Table of Contents
| F-41 |
Condensed consolidated interim StatementS of financial position
(Unaudited)
June 30, 2018 | December 31, 2017 | |||||||
| U.S. dollars in thousands | ||||||||
| Assets | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 8,534 | $ | 7,643 | ||||
| Investments in marketable securities | - | 3,173 | ||||||
| Trade receivables | 5,115 | 5,362 | ||||||
| Other receivables | 879 | 685 | ||||||
| Inventories | 2,807 | 2,260 | ||||||
| Total current assets | 17,335 | 19,123 | ||||||
| Non-current assets | ||||||||
| Long-term restricted deposits and prepaid expenses | 359 | 382 | ||||||
| Long-term trade receivables | 403 | 473 | ||||||
| Property and equipment | 1,034 | 1,022 | ||||||
| Intangible assets | 244 | 277 | ||||||
| Total non-current assets | 2,040 | 2,154 | ||||||
| Total assets | $ | 19,375 | $ | 21,277 | ||||
| Liabilities | ||||||||
| Current liabilities | ||||||||
| Trade payables | $ | 1,277 | $ | 1,262 | ||||
| Short-term employee benefits | 326 | 223 | ||||||
| Current maturities of convertible notes | - | 10,696 | ||||||
| Short-term bank loan | 5,000 | - | ||||||
| Provisions | 189 | 183 | ||||||
| Accrued expenses | 1,209 | 1,405 | ||||||
| Other accounts payable | 1,958 | 1,998 | ||||||
| Total current liabilities | 9,959 | 15,767 | ||||||
| Non-current liabilities | ||||||||
| Derivative instruments | 765 | 2,875 | ||||||
| Long-term employee benefits | 323 | 310 | ||||||
| Other long-term liabilities | 956 | 948 | ||||||
| Total non-current liabilities | 2,044 | 4,133 | ||||||
| Total liabilities | 12,003 | 19,900 | ||||||
| Equity | ||||||||
| Ordinary share capital | 746 | 683 | ||||||
| Additional paid-in capital | 111,433 | 105,585 | ||||||
| Capital reserve in respect of securities available-for-sale | - | 113 | ||||||
| Accumulated deficit | (104,807 | ) | (105,004 | ) | ||||
| Total equity | 7,372 | 1,377 | ||||||
| Total liabilities and equity | $ | 19,375 | $ | 21,277 | ||||
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
| F-42 |
Condensed CONSOLIDATED interim statements of OPERATIONS
(Unaudited)
Three Months Ended June 30, | Six Months Ended June 30, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| U.S. dollars in thousands (except per share data) | ||||||||||||||||
| Revenues | $ | 6,076 | $ | 5,058 | $ | 11,546 | $ | 9,403 | ||||||||
| Cost of revenues | 1,431 | 1,224 | 2,680 | 2,286 | ||||||||||||
| Gross profit | 4,645 | 3,834 | 8,866 | 7,117 | ||||||||||||
| Selling and marketing expenses | 3,269 | 2,975 | 6,078 | 6,091 | ||||||||||||
| Research and development expenses | 873 | 917 | 1,856 | 1,962 | ||||||||||||
| General and administrative expenses | 1,411 | 1,424 | 2,724 | 2,710 | ||||||||||||
| Total operating expenses | 5,553 | 5,316 | 10,658 | 10,763 | ||||||||||||
| Operating loss | (908 | ) | (1,482 | ) | (1,792 | ) | (3,646 | ) | ||||||||
| Financial income (expenses) from cash and investments | (28 | ) | 362 | 182 | 1,454 | |||||||||||
| Financial expenses from notes and loans | (183 | ) | (1,092 | ) | (761 | ) | (3,291 | ) | ||||||||
| Gain from derivatives instruments, net | 710 | 1,086 | 2,110 | 3,835 | ||||||||||||
| Financial income, net | 499 | 356 | 1,531 | 1,998 | ||||||||||||
| Loss before income taxes | (409 | ) | (1,126 | ) | (261 | ) | (1,648 | ) | ||||||||
| Taxes on Income | (15 | ) | (6 | ) | (51 | ) | (42 | ) | ||||||||
| Net loss | $ | (424 | ) | $ | (1,132 | ) | $ | (312 | ) | $ | (1,690 | ) | ||||
| Loss per share (in U.S. dollars): | ||||||||||||||||
| Basic | $ | (0.00 | ) | $ | (0.00 | ) | $ | (0.00 | ) | $ | (0.01 | ) | ||||
| Diluted | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.01 | ) | $ | (0.01 | ) | ||||
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
| F-43 |
Condensed consolidated interim statements of comprehensive INCOME (LOSS)
(Unaudited)
Six Months Ended June 30, | ||||||||
| 2018 | 2017 | |||||||
| U.S. dollars in thousands | ||||||||
| Net loss | $ | (312 | ) | $ | (1,690 | ) | ||
| Other comprehensive income (loss) items that after initial recognition in comprehensive income (loss), were or will be carried to the statement of operations | ||||||||
| Net change in fair value of marketable securities available-for-sale, net of tax | (113 | ) | 133 | |||||
| Total other comprehensive income (loss) items that after initial recognition in comprehensive income (loss), were or will be carried to the statement of operations, net of tax | (113 | ) | 133 | |||||
| Other total comprehensive income (loss) | (113 | ) | 133 | |||||
| Total comprehensive loss | $ | (425 | ) | $ | (1,557 | ) | ||
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
| F-44 |
Condensed consolidated interim statements of changes in EQUITY
(Unaudited)
| Ordinary share capital | Additional paid-in capital | Capital reserve in respect of securities available-for-sale | Accumulated deficit | Total | ||||||||||||||||
| U.S. dollars in thousands | ||||||||||||||||||||
| For the six months ended June 30, 2018 | ||||||||||||||||||||
| Balance as of January 1, 2018 | $ | 683 | $ | 105,585 | $ | 113 | $ | (105,004 | ) | $ | 1,377 | |||||||||
| Total comprehensive loss: | ||||||||||||||||||||
| Net loss | - | - | - | (312 | ) | (312 | ) | |||||||||||||
| Other comprehensive income, net of tax | - | - | (113 | ) | - | (113 | ) | |||||||||||||
| Total comprehensive loss | - | - | (113 | ) | (312 | ) | (425 | ) | ||||||||||||
| Transactions carried directly to equity: | ||||||||||||||||||||
| Issuance of shares due to the exercise of options | 1 | 24 | - | - | 25 | |||||||||||||||
| Issuance of shares in a private offering | 62 | 5,739 | 5,801 | |||||||||||||||||
| Share-based payment | - | - | - | 509 | 509 | |||||||||||||||
| Capital reserve from transactions with shareholders | - | 85 | - | - | 85 | |||||||||||||||
| Balance as of June 30, 2018 | $ | 746 | $ | 111,433 | $ | - | $ | (104,807 | ) | $ | 7,372 | |||||||||
| For the six months ended June 30, 2017 | ||||||||||||||||||||
| Balance as of January 1, 2017 | $ | 679 | $ | 105,492 | $ | (45 | ) | $ | (100,885 | ) | $ | 5,241 | ||||||||
| Total comprehensive loss: | ||||||||||||||||||||
| Net loss | - | - | - | (1,690 | ) | (1,690 | ) | |||||||||||||
| Other comprehensive income, net of tax | - | - | 133 | - | 133 | |||||||||||||||
| Total comprehensive loss | - | - | 133 | (1,690 | ) | (1,557 | ) | |||||||||||||
| Transactions carried directly to equity: | ||||||||||||||||||||
| Issuance of shares due to the exercise of options | 4 | 93 | - | - | 97 | |||||||||||||||
| Share-based payment | - | - | - | 705 | 705 | |||||||||||||||
| Balance as of June 30, 2017 | $ | 683 | $ | 105,585 | $ | 88 | $ | (101,870 | ) | $ | 4,486 | |||||||||
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
| F-45 |
Condensed consolidated interim statements of CASH FLOWS
(Unaudited)
Six Months Ended June 30, | ||||||||
| 2018 | 2017 | |||||||
| U.S. dollars in thousands | ||||||||
| Cash flows from operating activities | ||||||||
| Net loss | $ | (312 | ) | $ | (1,690 | ) | ||
| Adjustments for: | ||||||||
| Depreciation and amortization | 243 | 239 | ||||||
| Share-based payment | 509 | 705 | ||||||
| Capital gain from sale of property and equipment | - | (8 | ) | |||||
| Change in provision for doubtful and bad debt | 68 | 84 | ||||||
| Net financial cost | 571 | 1,639 | ||||||
| Gain from revaluation of derivatives | (2,110 | ) | (3,835 | ) | ||||
| Decrease in trade receivables | 249 | 179 | ||||||
| Decrease (increase) in other accounts receivable | (181 | ) | 139 | |||||
| Increase in inventories | (684 | ) | (113 | ) | ||||
| Increase (decrease) trade payables | 22 | (524 | ) | |||||
| Increase (decrease) in other accounts payable and accrued expenses | 133 | (118 | ) | |||||
| Increase in employee benefits | 116 | 144 | ||||||
| Increase in provisions | 6 | 8 | ||||||
| Income tax expenses | 51 | 42 | ||||||
| Taxes paid during the period | (105 | ) | (39 | ) | ||||
| Interest received during the period | - | 18 | ||||||
| Interest paid during the period | (592 | ) | (901 | ) | ||||
| Net cash used in operating activities | (2,016 | ) | (4,031 | ) | ||||
| Cash flow from investing activities | ||||||||
| Sale of marketable securities available-for-sale | 3,109 | - | ||||||
| Purchase of property and equipment, intangible assets and capitalization of development expenses | (92 | ) | (152 | ) | ||||
| Decrease in restricted long-term deposits | - | 7 | ||||||
| Net cash provided by (used in) investing activities | 3,017 | (145 | ) | |||||
| Cash flow for financing activities | ||||||||
| Proceeds from issuance of shares, net of share issuance costs | 5,209 | - | ||||||
| Short-term bank credit | 5,000 | - | ||||||
| Repayment of convertible notes | (9,939 | ) | (10,421 | ) | ||||
| Repayment of shareholders’ loans | (435 | ) | - | |||||
| Issuance of shares due to the exercise of stock options | 25 | 97 | ||||||
| Net cash used in financing activities | (140 | ) | (10,324 | ) | ||||
| Increase (decrease) in cash and cash equivalents | 861 | (14,500 | ) | |||||
| Cash and cash equivalents at beginning of period | 7,643 | 23,358 | ||||||
| Effect of exchange rate fluctuations on balances of cash and cash equivalents | 30 | 1,151 | ||||||
| Cash and cash equivalent balance at end of period | $ | 8,534 | $ | 10,009 | ||||
| Non-cash financing activity – repayment of notes to related parties against receipt of a loan | $ | 1,076 | $ | - | ||||
The accompanying notes are an integral part of these condensed consolidated interim financial statements.
| F-46 |
Notes to CONDENSED CONSOLIDATED INTERIM financial statements
(UNAUDITED)
Note 1 – General
| a. | Reporting entity |
Itamar Medical Ltd. (the “Company”) is a company incorporated in Israel, with registered office at 9 Halamish Street, North Industrial Zone, Caesarea, Israel. The interim condensed consolidated financial statements of the Company and its subsidiaries as of June 30, 2018 and 2017 and for each of the three and six-month periods ended June 30, 2018 and 2017 comprise the Company and its subsidiaries (together referred to as the “Group”). The core business of the Group is to design, develop, manufacture and sell sleep apnea diagnostic ambulatory products and related services, using the Peripheral Arterial Tone (“PAT”) biological signal along with other measurements such as actigraphy, heartrate, chest motion, body position and snoring and analyzed by the Group’s proprietary technology and algorithms. The ordinary shares, par value of New Israeli Shekel (“NIS”) 0.01 per share, of the Company are listed on the Tel Aviv Stock Exchange Ltd. (“TASE”).
| b. | Material events during the report period and subsequent to June 30, 2018 |
On February 20, 2018, the Company withdrew $5.0 million from a $10.0 million credit facility for a period of three months. The loans were renewed until November 20, 2018. Of the Company’s total cash, the Company is required to maintain a minimum balance of 40% of the amount of the credit, i.e., $2 million is not available for general use by the Company.
On February 28, 2018, the second and last installment of the convertible notes of the Company in a total amount of NIS 38,128 thousand par value was repaid. None of the convertible notes were converted. Out of the February 28, 2018 installment, a principal of $1.7 million (NIS 6 million) owed to three shareholders was not repaid, of which $0.5 million was repaid to one shareholder in June 2018 and the balance owed to the other two shareholders was invested by them in the private placement that was completed on May 27, 2018 (see below). On May 27, 2018, following the approval of the Company’s shareholders on May 23, 2018 of the share purchase agreements signed in March 2018, the private placement was completed for approximately $6 million (NIS 20,847 thousands) in consideration for the issuance of 22,013,893 ordinary shares at the price of NIS 0.947 per share.
| c. | The Company’s financial position |
The Company’s management and Board of Directors are in the opinion that, based on the positive trend of its operating results, the bank credit facility and the private placement described in b. above, and the Company’s ability to adjust its budget to business developments, the Company has enough financial resources in order to continue its business activities in the foreseeable future. In addition, the management continuously assesses its actual results, compared its approved budget and its financial covenants is able to respond by reducing its operating expenses in case it does not meet its targets.
NOTE 2 – SIGNIFICANT ACCOUNTING POLICIES
| a. | International Financial Reporting Standards (“IFRS”) |
These condensed consolidated interim financial statements have been prepared in accordance with IAS 34, Interim Financial Reporting. Accordingly, they do not contain all the information required in full annual financial statements. These interim financial statements should be read in conjunction with the audited consolidated financial Statements as of December 31, 2017 and for the year then ended (the “2017 Annual Financial Statements”).
These condensed consolidated interim financial statements were approved by the Company’s Board of Directors on August 9, 2018.
| F-47 |
Itamar Medical Ltd.
Notes to CONDENSED CONSOLIDATED INTERIM financial statements
(UNAUDITED)
| b. | Use of estimates, assumptions and judgments |
The preparation of interim condensed consolidated financial statements in accordance with IFRS requires management to make estimates and assumptions that affect reported amounts of assets and liabilities, and the disclosure of contingent assets and liabilities at the date of the financial statements as well as affect the reported amounts of revenues and expenses during the period. These estimates and assumptions are reviewed on an ongoing basis using available information. Actual results could differ from these estimates and assumptions. The items subject to significant estimates and assumptions by management include impairment tests of long-lived assets; share-based compensation; recognition of deferred income tax assets; the measurement of financial instruments at fair value, the fair value of the embedded warrant component of convertible notes, the fair value of warrants where there is no active market; and the assets and liabilities related to employee benefits.
| c. | New standards and interpretations adopted in the reported period |
The accounting policies applied by the Group in these condensed consolidated interim financial statements are the same as those applied by the Group in its 2017 Annual Financial Statements, except for the following new standards and amendments to the standards, that the Group adopted commencing from January 1, 2018:
| (1) | IFRS 9, Financial Instruments: Classification and Measurement (“IFRS 9") |
IFRS 9 sets forth the guidance relating to the classification and measurement of financial assets and liabilities, the accounting for expected credit losses of financial assets and commitments to extend credits, as well as the requirements for hedge accounting. The Group elected to apply the IFRS 9, effective January 1, 2018, without restating the comparative figures. The implementation of the standard did not have a material effect on the financial statements.
| (2) | IFRS 15, Revenues from Contracts with Customers (“IFRS 15") |
On January 1, 2018, the Group adopted IFRS 15, using the cumulative impact transition method applied to those contracts which were not completed as of January 1, 2018. Based on the analysis performed by the Group, there was no effect on retained earnings as of January 1, 2018.
For the three and six months ended June 30, 2018, there was no impact to revenue and to cost of revenue as result of the adoption of IFRS 15. As of January 1, 2018 and June 30, 2018, the Group had an immaterial effect on its financial statements as a result of recognizing receivables in the amount of $333 thousand and $398 thousands, respectively, in respect of contract assets that the rights in their respect are unconditional, together with a corresponding increase to deferred revenue in accordance with the guidance of IFRS 15.
Presented hereunder are the new significant accounting policies regarding revenue recognition that were applied as from January 1, 2018 following the application of IFRS 15:
The Group accounts for a contract with a customer only when the following conditions are met:
| (a) | The parties to the contract have approved the contract (in writing, orally or according to other customary business practices) and they are committed to satisfying the obligations attributable to them; |
| (b) | The Group can identify the rights of each party in relation to the products or services that will be transferred; |
| F-48 |
Itamar Medical Ltd.
Notes to CONDENSED CONSOLIDATED INTERIM financial statements
(UNAUDITED)
| (c) | The Group can identify the payment terms for the products or services that will be transferred; |
| (d) | The contract has a commercial substance (i.e. the risk, timing and amount of the entity’s future cash flows are expected to change as a result of the contract); and |
| (e) | It is probable that the consideration, to which the Group is entitled to in exchange for the products or services transferred to the customer, will be collected. |
If a contract with a customer does not meet all of the above criteria, consideration received from the customer is recognized as a liability until the criteria are met or when one of the following events occurs: (i) the Group has no remaining obligations to transfer products or services to the customer and any consideration promised by the customer has been received and cannot be returned; or (ii) the contract has been terminated and the consideration received from the customer cannot be refunded.
The Group recognizes revenue from the sale of its products, net of provision for returns and discounts, when the customer obtains control over the promised products or services, the timing of which may be upon shipment or upon delivery to the customer site, based on the contract terms or legal requirements.
Revenues from sales agreements consisting of multiple products or services, such as devices, consumables, access to the CloudPAT application, WatchPAT Direct logistic services and support and other service agreements, are separated into different performance obligations and revenue is separately recognized for each performance obligation.
The Group identifies products or services promised to the customer as being distinct performance obligations when the customer can benefit from the products or services on their own or in conjunction with other readily available resources and the Group’s promise to transfer the products or services to the customer is separately identifiable from other promises in the contract. In order to examine whether a promise to transfer products or services is separately identifiable, the Group examines whether it is providing a significant service of integrating the products or services with other products or services promised in the contract into one integrated outcome that is the purpose of the contract. Products or services that are not considered as being distinct, are grouped together as a single performance obligation. The revenue from each such performance obligation is recognized upon transfer of control over the promised products or services to customer. In general, the Group allocates the transaction price to the identified performance obligations in the contract, based on the relative stand-alone selling prices when the products or services are sold separately. In cases where the products or services are not sold separately, for example, in the case of installations or training, the Group establishes the stand-alone selling price assigned to that performance obligation, based on estimated costs plus a reasonable margin.
The revenue is measured according to the amount of the consideration to which the Group expects to be entitled in exchange for the products or services promised to the customer, other than amounts collected for third parties.
The Group recognizes estimated sales discounts as a reduction of sales in the same period revenue is recognized. The Group adjusts reserves to reflect differences between estimated and actual. The Group estimates its sales returns reserve based on historical return rates and analysis of specific accounts.
The Group recognizes revenue from leasing its products over the lease term, in conformity with the agreement with the customer. In some cases, the Group handles sale transactions of these devices as a finance lease and recognizes revenue in respect of the products supplied at the commencement date of the lease. When these transactions include multiple deliverables, revenue is recognized based on the relative stand-alone selling prices of each deliverable in the transaction when they are sold separately.
| F-49 |
Itamar Medical Ltd.
Notes to CONDENSED CONSOLIDATED INTERIM financial statements
(UNAUDITED)
When the Group sells its products through distributors, revenue is being recognized upon delivery of the product to the distributor, as the distributors does not have the right to return and the control over the products is transferred at this point in time.
Incremental costs of obtaining a contract with a customer such as sales fees to agents are recognized as an asset when the Group is likely to recover these costs. Costs to obtain a contract that would have been incurred regardless of the contract are recognized as an expense as incurred, unless the customer can be billed for those costs.
Capitalized costs are amortized in the income statement on a systematic basis that is consistent with the pattern of transfer of the products or services to which the asset relates.
A contract asset is recognized when the Group has a right to consideration for products or services it transferred to the customer that is conditional on other than the passing of time, such as future performance of the Group. Contract assets are classified as receivables when the rights in their respect become unconditional.
A contract liability is recognized when the Group has an obligation to transfer products or services to the customer for which it received consideration (or the consideration is payable) from the customer.
An asset and liability relating to the same contract are presented on a net basis in the statement of financial position. On the other hand, a contract asset and contract liability deriving from different contracts are presented on a gross basis in the statement of financial position.
Note 3 – Financial Instruments
Financial instruments that are measured at fair value for disclosure purposes only
As of June 30, 2018, the carrying amount of the cash and cash equivalents, trade receivables, other accounts receivable, bank deposits, pledged deposits, trade payables, and other accounts payable and derivatives is identical or approximate to their fair values due to the short lifetime of these items. As discussed in note 1 above, in February 2018, the Company repaid the total outstanding principal and interest of its convertible notes.
As of June 30, 2018, the Company estimated the fair value of the Viola Warrants and the Warrants (Series 4) based on the binomial model and based on relevant parameters of the terms of the Viola Warrants and the Warrants (Series 4) (Level 3 measurements).
The change from the opening balance to the closing balance of the financial instruments measured at fair value, categorized within Level 3 hierarchy, for the three and six-month periods ended June 30, 2018 and 2017 was caused by the revaluation to fair value of the derivatives in the amount of $710 thousand, $1,086 thousand, $2,110 thousand and $3,835 thousand, respectively.
Note 4 – share-based payments
On March 14, 2018, the Company’s Board of Directors (i) approved a grant of 2,066,193 options and 278,566 RSUs to 21 grantees; and (ii) resolved to change the vesting terms of the options and RSUs with service terms and market conditions granted to the President and Chief Operating Officer and certain other key employees of the Company and its subsidiaries, such that the minimum share price in respect of the market conditions will be NIS 1.70 instead of NIS 2.13 and the exercise period will be December 20, 2020 instead of January 20, 2020. There was no change in the other terms of the options and the RSUs, including the exercise price and the other vesting conditions. The change in the aforesaid terms regarding the President and Chief Operating Officer was subject to the approval of the Company’s shareholders that was obtained on May 23, 2018.
| F-50 |
Itamar Medical Ltd.
Notes to CONDENSED CONSOLIDATED INTERIM financial statements
(UNAUDITED)
Note 5 – REVENUES
The Company operates in one business sector.
The following is a breakdown of revenues according to product groups:
Three Months Ended June 30, 2018 | Six Months Ended June 30, 2018 | |||||||
| U.S. dollars in thousands | ||||||||
| WatchPAT and other related services | $ | 5,612 | $ | 10,651 | ||||
| Endo PAT and related services | 464 | 895 | ||||||
| $ | 6,076 | $ | 11,546 | |||||
The following is a breakdown of revenues on the basis of geographical regions (based on the geographical location of the customer):
Six Months Ended June 30, | ||||||||
Three Months Ended June 30, 2018 | Six Months Ended June 30, 2018 | |||||||
| U.S. dollars in thousands | ||||||||
| United States and Canada | $ | 4,505 | $ | 7,963 | ||||
| Japan | 1,049 | 2,257 | ||||||
| Europe | 423 | 801 | ||||||
| Asia Pacific (excluding Japan) | 13 | 316 | ||||||
| Israel | 52 | 162 | ||||||
| Others | 34 | 47 | ||||||
| $ | 6,076 | $ | 11,546 | |||||
The majority of the Company’s long lived assets are in Israel.
| F-51 |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
| ITEM 19. | EXHIBITS |
| Exhibit | Description | |
| 1.1#* | Memorandum of Association of the Registrant, as amended and restated. | |
| 1.2# | Amended and Restated Articles of Association of the Registrant. | |
| 2.1** | Form of Deposit Agreement between the Registrant, The Bank of New York Mellon as Depositary, and owners and holders from time to time of ADSs issued thereunder, including the Form of American Depositary Shares. | |
| 2.2* | Specimen of Ordinary Share Certificate. | |
| 2.3* | Closing Warrant Agreement by and between the Registrant and Viola P.E. 2 A.V. Limited Partnership, dated as of November 5, 2015. | |
| 2.4 | Warrant Agreement by and between the Registrant and Mizrahi Tefahot Bank Ltd., dated as of May 14, 2017, as amended on July 9, 2017, and as further amended on January 29, 2018. | |
| 2.5# | Form of Warrants (Series 4) issued to certain of the Registrant’s shareholders in connection with the Registrant’s rights offering that was completed on December 29, 2015. | |
| 4.1* | 2007 Israeli Share Option Plan. | |
| 4.2* | 2007 Equity Incentive Plan. | |
| 4.3* | Israeli Equity Incentive Plan 2016. | |
| 4.4* | 2016 U.S. Equity Incentive Plan. | |
| 4.5* | Form of Indemnification Letter. | |
| 4.6# | Compensation Policy for Executive Officers and Directors. | |
| 4.7#* | Lease Agreement by and between the Registrant and The Caesarea Edmond Benjamin De Rothschild Assets Corp. (2001) Ltd., dated July 19, 2007, as amended by Amendment No. 1 on December 25, 2008, and as further amended by Amendment No. 2 in 2013, and as further amended by Amendment No. 3 on March 23, 2015, and as further amended by Amendment No. 4 on August 9, 2015. | |
| 4.8+ | Distribution Agreement by and between the Registrant and Philips Respironics GK, dated February 24, 2014. | |
| 4.9+ | Master Products and Services Agreement by and between Kaiser Foundation Health Plan, Inc. (“Kaiser”) and Itamar Medical, Inc., a wholly owned subsidiary of the Registrant (“Itamar US”), dated August 16, 2007, as amended by the Amendment to Itamar Medical Agreement between Kaiser and Itamar US, dated August 16, 2009, and as further amended by the Amendment to Itamar Medical Agreement between Kaiser and Itamar US, dated October 16, 2009, and as further amended by the Amendment #3 to Itamar Medical Agreement between Kaiser and Itamar US, dated April 1, 2010, and as further amended by the Amendment #4 to Itamar Medical Agreement between Kaiser and Itamar US, dated February 4, 2013, and as further amended by the Amendment #5 to Itamar Medical Agreement between Kaiser and Itamar US, dated November 1, 2013, and as further amended by the Amendment #6 to Itamar Medical Agreement between Kaiser and Itamar US, dated November 1, 2015, and as further amended by the Amendment #7 to Itamar Medical Agreement between Kaiser and Itamar US, dated June 26, 2017. | |
| 4.10+ | Solicitation/Contract/Order for Commercial Items issued by Department of Veterans Affairs (“VA”) to Itamar US, dated October 12, 2011 as amended by the Solicitation/Contract/Order for Commercial Items issued by VA to Itamar US, dated March 12, 2014, and as further amended by the Amendment of the Solicitation/Contract, dated August 1, 2018, and as further amended by the Product Addition Request for Modification Form, dated September 25, 2018. | |
| 4.11## | Credit Framework Agreement by and between the Registrant and Mizrahi Tefahot Bank Ltd. (“Mizrahi”), dated March 29, 2017, as amended by Amendment No. 1 on January 29, 2018, and as further amended by Amendment No. 2 on May 28, 2018; the Secured Debenture issued by the Registrant to Mizrahi, dated May 28, 2017; the Negative Charge Irrevocable Undertaking issued by I.M.E. 2016 B.V. to Mizrahi, dated May 29, 2017; the Continuing Guarantee in an Unlimited Amount to Secure all Debts issued by Itamar US to Mizrahi, dated July 19, 2017; and the Unlimited Security Agreement by and between Itamar US and Mizrahi, dated July 19, 2017. | |
| 4.12 | Form of Securities Purchase Agreements, dated March 22, 2018, by and between the Registrant and the Purchasers signatory thereto. | |
| 8* | List of Subsidiaries of the Registrant. | |
| 15.1** | Consent of Somekh Chaikin, a member of KPMG International. | |
| ______________________________________ | ||
| # | Unofficial English translation from Hebrew original. | |
| ## | Certain parts, as indicated therein, contain unofficial English translation from Hebrew original. | |
| * | Previously filed. | |
| ** | To be subsequently filed as part of this registration statement. | |
| § | English summary of the Hebrew. | |
| + | Confidential treatment has been or will be requested with respect to certain portions of this exhibit pursuant to 17.C.F.R. §240.24b-2. Omitted portions to be filed separately with the SEC. |
| 150 |
This draft registration statement has not been publicly filed with
the Securities and Exchange Commission and all information herein remains strictly confidential
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this registration statement on its behalf.
ITAMAR MEDICAL LTD.
| By: | |||
| Name: | |||
| Title: |
Dated: ______ __, 2018
| 151 |


