Confidentially submitted to the United States Securities and Exchange Commission on August 30, 2019. This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential.
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM F-1
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
BRIACELL THERAPEUTICS CORP.
(Exact name of Registrant as specified in its charter)
| British Columbia | 2834 | 47-1099599 | ||
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation or organization) | Classification Code Number) | Identification Number) |
Suite 300 – 235 West 15th Street
West Vancouver, BC V7T 2X1
Telephone: (604) 921-1810
(Address, including zip code, and telephone number, including area code, of Registrant’s principal executive offices)
Paracorp Incorporated
2804 Gateway Oaks Drive #100,
Sacramento, CA 95833
Telephone: (888) 280-6563
(Name, address, including zip code, and telephone number, including area code, of agent of service)
Copies to:
Gregory Sichenzia, Esq. Avital Perlman, Esq. Sichenzia Ross Ference LLP 1185 Avenue of Americas 37th Floor New York, NY 10036 Telephone: (212) 930-9700 Facsimile: (212) 930-9725 |
Aaron Sonshine
Bennett Jones LLP 3400 One First Canadian Place P.O. Box 130, Toronto, ON M5X 1A4 Telephone:
(416) 777-6448
|
Virgil
Z. Hlus |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. [ ]
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. [ ]
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933. Emerging growth company [X]
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards† provided pursuant to Section 7(a)(2)(B) of the Securities Act. [ ]
† The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
CALCULATION OF REGISTRATION FEE
| Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee | ||||||
| Units (3) | $ | (4) | $ | |||||
| Common shares, no par value, included in the Units | — | (5) | — | |||||
| Warrants included in the Units (6) | — | (5) | — | |||||
| Common shares underlying the warrants included in the Units | $ | $ | ||||||
Warrants to be issued to the Underwriters | (7) | |||||||
| Common shares underlying warrants to be issued to the Underwriters | (8) | |||||||
| Total | $ | $ | ||||||
| (1) | Calculated pursuant to Rule 457(o) on the basis of the maximum aggregate offering price of all of the securities to be registered. |
| (2) | Pursuant to Rule 416, the securities being registered hereunder include such indeterminate number of additional securities as may be issued after the date hereof as a result of stock splits, stock dividends or similar transactions. |
| (3) | Each Unit consists of one common share and one warrant, each whole warrant exercisable for one share of Common Stock. |
| (4) | Includes common shares and/or warrants representing 15% of the number of common shares and warrants included in the Units offered to the public that the underwriters have the option to purchase to cover over-allotments, if any. |
| (5) | Included in the price of the Units. No separate registration fee required pursuant to Rule 457(g) under the Securities Act of 1933, as amended. |
| (6) | The warrants are exercisable at a price per common share equal to 125% of the Unit offering price. |
| (7) | No fee pursuant to Rule 457(g) under the Securities Act of 1933, as amended. |
| (8) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457(g) under the Securities Act of 1933, as amended. We have agreed to issue to the underwriters warrants to purchase a number of common shares equal to 5% of the aggregate number of Units sold in the offering (including the Units issuable upon exercise of the over-allotment option). The warrants are exercisable at a per share exercise price equal to 125% of the per unit public offering price for five years after the effective date of this registration statement. |
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED AUGUST 30, 2019 |
_________ Units

BriaCell Therapeutics Corp.
We are offering _____________ units, each unit consisting of one common share, no par value per share, and one warrant, at a price of $ per unit, in a firm commitment underwritten offering. Each warrant will entitle the holder to purchase one common share at an exercise price of $ , equal to 125% of the public offering price of one unit, and expire five years from the date of issuance. The common shares and warrants that are part of the units are immediately separable and will be issued separately in this offering. The offering also includes the common shares issuable from time to time upon exercise of the warrants.
Our common shares are currently quoted on the U.S. OTCQB marketplace of OTC Markets Group, or OTCQB, under the symbol “BCTXF” and on the TSX Venture Exchange, or TSXV, under the symbol “BCT.”
On August 28, 2019, the closing price of our common shares was $0.06 per share, as reported on the OTCQB. We are in the process of applying to list our common shares and our warrants on the Nasdaq Capital Market under the symbols “ ” and “ ”, respectively. No assurance can be given that our application will be approved.
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Start-ups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings.
Investing in our securities involves a high degree of risk. See “Risk Factors” on page 11 to read about factors you should consider before buying our securities.
| Per Share | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions(1) | $ | $ | ||||||
| Proceeds to BriaCell Therapeutics Corp., before expenses | $ | $ |
| (1) | Underwriting discounts and commissions do not include a non-accountable expense allowance equal to 1% of the initial public offering price payable to the underwriters. We refer you to “Underwriting” beginning on page 106 for additional information regarding underwriters’ compensation. |
We have granted the underwriters a 45-day option to purchase up to an aggregate of additional common shares and/or warrants to purchase up to additional shares (equal to 15% of the shares and warrants included within the units sold in the offering) in any combination thereof, solely to cover over-allotments, if any. The purchase price to be paid per additional share by the underwriters shall be equal to the public offering price of one unit, less the underwriting discount, and the purchase price to be paid per additional warrant by the underwriters shall be $0.00001. If the underwriters exercise the option in full, the total underwriting discounts and commissions payable by us will be $ and the total proceeds to us, before expenses, will be $ .
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the units to investors on or about , 2019.
ThinkEquity
a division of Fordham Financial Management, Inc.
The date of this prospectus is , 2019
TABLE OF CONTENTS
| 3 |
You should rely only on the information contained in this prospectus, any amendment or supplement to this prospectus or any free writing prospectus prepared by or on our behalf. Neither we, nor the Underwriters, have authorized any other person to provide you with different or additional information. Neither we, nor the Underwriters, take responsibility for, nor can we provide assurance as to the reliability of, any other information that others may provide. The Underwriters are not making an offer to sell these securities in any jurisdiction where the offer or sale is not permitted. The information contained in this prospectus is accurate only as of the date of this prospectus or such other date stated in this prospectus, and our business, financial condition, results of operations and/or prospects may have changed since those dates.
Except as otherwise set forth in this prospectus, neither we nor the Underwriters have taken any action to permit a public offering of these securities outside the United States and Canada or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of these securities and the distribution of this prospectus outside the United States.
Unless the context otherwise requires, in this prospectus, the term(s) “we”, “us”, “our”, “Company”, “our company”, “BriaCell,” and “our business” refer to BriaCell Therapeutics Corp. and our subsidiaries.
MARKET, INDUSTRY AND OTHER DATA
This prospectus contains estimates, projections and other information concerning our industry, our business, and the markets for our products. Information that is based on estimates, forecasts, projections, market research or similar methodologies is inherently subject to uncertainties, and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. Unless otherwise expressly stated, we obtained this industry, business, market and other data from our own internal estimates and research as well as from reports, research surveys, studies and similar data prepared by market research firms and other third parties, industry, medical and general publications, government data, and similar sources.
In addition, assumptions and estimates of our and our industry’s future performance are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors.” These and other factors could cause our future performance to differ materially from our assumptions and estimates. See “Cautionary Statement Regarding Forward-Looking Statements.”
CURRENCY AND EXCHANGE RATES
All dollar amounts in this prospectus are expressed in Canadian dollars unless otherwise indicated. The Company’s accounts are maintained in Canadian dollars and the Company’s financial statements are prepared in accordance with International Financial Reporting Standards as issued by the International Accounting Standards Board. All reference to “U.S. dollars”, “USD”, or to “US$” are to United States dollars.
The following table sets forth the rate of exchange for the Canadian dollar, expressed in United States dollars in effect at the end of the periods indicated, the average of exchange rates in effect during such periods, and the high and low exchange rates during such periods based on the noon rate of exchange as reported by the Bank of Canada for conversion of Canadian dollars into United States dollars.
On August 28, 2019, the noon buying rate was US$1.00 = C$1.3301.
| Canada Dollar per U.S. Dollar Noon Buying Rate | ||||||||||||||||
| Average | High | Low | Period-End | |||||||||||||
| Year ended July, 31, | 1.2738 | 1.3310 | 1.2128 | 1.3148 | ||||||||||||
| 2018 | ||||||||||||||||
| Most recent six months | ||||||||||||||||
| February 2019 | 1.3206 | 1.3298 | 1.3095 | 1.3169 | ||||||||||||
| March 2019 | 1.3368 | 1.3438 | 1.3260 | 1.3363 | ||||||||||||
| April 2019 | 1.3378 | 1.3493 | 1.3316 | 1.3423 | ||||||||||||
| May 2019 | 1.3459 | 1.3527 | 1.3410 | 1.3527 | ||||||||||||
| June 2019 | 1.3287 | 1.3470 | 1.3087 | 1.3087 | ||||||||||||
| July 2019 | 1.3101 | 1.3182 | 1.3038 | 1.3148 | ||||||||||||
| 4 |
This summary highlights selected information contained elsewhere in this prospectus and does not contain all of the information that you should consider in making your investment decision. Before deciding to invest in our common shares, you should read this entire prospectus carefully, including the sections of this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and the related notes included elsewhere in this prospectus.
Overview of the Company
BriaCell is an immuno-oncology focused biotechnology company developing targeted and safe approaches for the management of cancer, with a focus on advanced breast cancer. BriaCell has completed positive proof-of-concept studies of Bria-IMT™, a whole cell targeted immunotherapy, in patients with advanced breast cancer.
BriaCell is currently conducting a Phase I/IIa clinical trial of Bria-IMT™, BriaCell’s lead candidate, in a Combination Study with immune checkpoint inhibitors such as Keytruda® (manufactured by Merck & Co., Inc. (NYSE: MRK)]) and is launching combination therapy with the Incyte drugs INCMGA00012 (an anti-PD-1 antibody similar to pembrolizumab [KEYTRUDA®) and epacadostat, and orally bioavailable small-molecule inhibitor of indoleamine 2,3-dioxygenase 1 (IDO1). The Combination Study is listed in ClinicalTrials.gov as NCT03328026.
BriaCell and Incyte Corporation (NASDAQ: INCY) have formed a non-exclusive clinical trial collaboration to evaluate the effects of combinations of novel clinical candidates. Under the agreement, Incyte and BriaCell will be evaluating novel combinations of compounds from Incyte’s development portfolio with Bria-IMT™ in advanced breast cancer patients.
BriaCell is also developing Bria-OTS™, an off-the-shelf personalized immunotherapy, for advanced breast cancer. Bria-OTS™ immunotherapy treatments are personalized to match the patient without the need for personalized manufacturing. Bria-OTS™, which is expected to cover over 99 percent of the patient population, is designed to produce a potent and selective immune response against the cancer of each patient while eliminating the time, expense and complex manufacturing logistics associated with other personalized immunotherapies.
BriaCell’s pipeline also includes other immunotherapy cell lines in development for other cancers (including lung, prostate and melanoma), the development of other immunotherapy approaches, and small molecule inhibitors of protein kinase C delta which are postulated to be effective in cancers caused by mutations in the RAS oncogene.
Products/Pipeline
Bria-IMT™
BriaCell is currently conducting a Phase I/IIa clinical trial of Bria-IMT™ BriaCell’s lead candidate, in combination with pembrolizumab (KEYTRUDA®; manufactured by Merck & Co., Inc.). The combination study is listed in ClinicalTrials.gov as NCT03328026.
Positive Proof of Concept
| ● | BriaCell has achieved positive proof of concept based on data from a Phase I/IIa study of Bria-IMT™ in advanced breast cancer patients. |
| ● | The data shows promising anti-tumor activity of Bria-IMT™ in heavily pre-treated advanced breast cancer patients. |
| ● | Impressive Phase IIa efficacy data is similar to, or superior to, those of other approved breast cancer drugs when they were at a similar clinical-stage of development. |
| ● | The data shows an outstanding safety and tolerability profile for Bria-IMT™ in advanced breast cancer patients. |
| ● | The data confirms the “HLA Matching Hypothesis” and supports BriaCell’s strategy for the development of Bria-OTS™, BriaCell’s first personalized off-the-shelf immunotherapy for advanced breast cancer. |
About Bria-IMT™
Developed and characterized by a team of dedicated scientists and clinicians, Bria-IMT™ (SV-BR-1-GM) is a targeted immunotherapy being developed for the treatment of breast cancer. Bria-IMT™ is a genetically engineered human breast cancer cell line with features of immune cells and clinically applied as a targeted immunotherapy.
In short, Bria-IMT™ immunotherapy is a genetically engineered human breast cancer cell line which activates the immune cells to attack and destroy breast cancer tumors.
Mechanism of Action of Bria-IMT™: The mechanism of action of Bria-IMT™ is currently under investigation. It is likely that the expression of certain breast cancer antigens (proteins expressed in breast cancer cells) in Bria-IMT™ generates strong antibody and T-cell responses – which results in recognition and destruction of cancerous cells.63
63 Lacher M.D., Bauer G. Fury B., Graeve S., Fledderman E.L., Petrie T.D., Coleal-Bergum D.P., Hackett T., Perotti N.H., Kong Y.Y., Kwok W.W., Wagner J.P., Wiseman C.L., and Williams W.V. SV-BR-1-GM, a Clinically Effective GM-CSF- Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4+ T Lymphocytes. Frontiers in Immunology 2018; 9: Article 776.
| 5 |
Bria-IMT™ is designed to secrete granulocyte/macrophage-colony stimulating factor (GM-CSF), a factor that stimulates components of the immune system. Specifically, GM-CSF activates dendritic cells, the cells that start immune responses. These activated dendritic cells then activate T cells, a key component of the immune system, to recognize the tumor cells as foreign, and eliminate them. To amplify this action, we have combined Bria-IMT™ with other immune system activators including cyclophosphamide (used in low doses to reduce immune suppression), and interferon-α, a cytokine. We believe this approach of simultaneous activation of the immune system via different pathways will improve the immune system response to attack and destroy cancer cells.
Using BriaCell’s novel technology platform and our strong R&D capabilities, we plan to develop immunotherapies for other cancer indications Bria-OTS™
| ● | Bria-OTS™ is under development as an off-the-shelf personalized immunotherapy for advanced breast cancer. |
| ● | The concept for Bria-OTS™ comes from BriaCell’s work with Bria-IMT™, where we noted that if a patient “matches” Bria-IMT™ in their HLA type, they were more likely to respond. |
| ● | HLA molecules are the molecules that start immune responses, but are polymorphic – i.e. they are different in different in different people, although some people will share HLA types. |
| 6 |
| ● | Bria-OTS™ is made from cell lines that are genetically engineered to expresses the immune boosters GM-CSF and interferon-α, as well as specific HLA types (a.k.a. alleles). |
| ● | Different cell lines are being pre-manufactured to express different HLA types covering >99% of the overall breast cancer patient population. |
| ● | Using the BriaDX™, a companion diagnostic test performed on the patient’s saliva, the suitable personalized treatment will be selected for each patient for administration. |
| ● | This approach allows personalized treatment without the need for personalized manufacturing. Additionally, it saves time, and skips expensive and complicated manufacturing procedures associated with other personalized treatments. |
| ● | Bria-OTS™ cell lines are being engineered with the goal of transferring them to production in 2019 and commencing clinical evaluation in 2020. |
Bria-OTS™ Immunotherapy Covers ~99% of the Breast Cancer Population

BriaDx™
BriaDX™ is a diagnostic test that BriaCell is developing to detect the patients most likely to respond to Bria-IMT™. Currently, BriaDx™ includes HLKA typing of the patients. Additional diagnostics are being developed based on the expression of specific biomarkers in the responder (i.e. the patients for which Bria-IMT™ immunotherapy was highly effective) vs the non-responder patients from clinical studies of Bria-IMT™ in advanced breast cancer patients.
| 7 |
Blood and tumor samples from the patients are analyzed using cutting-edge technologies including gene expression analysis, and proteomics (i.e., defined as the large-scale study of the structure and function of proteins).
We have been characterizing the molecular fingerprint of the responder patients, and have developed a diagnostic test, BriaDX™, to identify these patients.
The insights gained from biomarker studies conducted to date have provided us with a solid basis for the development of Bria-OTS™, an off-the-shelf personalized immunotherapy which would treat over 99% of patients with advanced breast cancer.
BriaDx™ is being developed to help understand which patients are most likely to respond to Bria-IMT™ targeted immunotherapy. Based on the proposed mechanism of action of Bria-IMT™ (see Figure) HLA molecules play a key role inducing cellular immune responses to Bria-IMT™ which boosts the patient’s immune response to their cancer.
proposed mechanism of action of Bria-IMT™

HLA molecules are polymorphic, in that they are different in different individuals, but shared by some individuals (similar to eye color). We have noted that matching of Bria-IMT™ HLA types with the patients may predict those more likely to respond. This is supported by our clinical data to date.
| 8 |
Available Clinical Data for Treatment with the Bria-IMT™ Regimen
BriaCell conducted three Proof of Concept clinical trials, one using parental SV-BR-1 cells and the other two using Bria-IMT™ (i.e., genetically engineered SV-BR-1 cells – producing GM-CSF also called SV-BR-1-GM), in metastatic (i.e., Stage IV) breast cancer patients who had failed prior treatments. The patients were treated according to the following schedule, and the results are summarized below.

First Proof of Concept Trial1
| ● | Used unmodified cell line (parental SV-BR-1 cells) + GM-CSF + cyclophosphamide | |
| ● | N = 14 late stage, treatment-refractory breast cancer patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 12.1 months |
Second Proof of Concept Trial2
| ● | Used Bria-IMT™ (genetically engineered SV-BR-1 cells – producing GM-CSF) + cyclophosphamide + interferon-α | |
| ● | N = 4 late stage, treatment-refractory (3 breast cancer, and 1 ovarian cancer) patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 35 months | |
| ● | One robust responder with >90% regression during treatment, subsequent relapse (upon halting treatment) responded to re-treatment | |
| ● | This patient matched Bria-IMT™ at a key HLA type (HLA-DRB3) |
Third Proof of Concept Trial3
Thirty patients were screened, 24 enrolled and 23 dosed in the Phase I/IIa study (2017-2018)
| ● | Bria-IMT™ was very well tolerated (over 100 doses given) | |
| ● | The majority of adverse events (AEs) were limited to expected minor local irritation at the injection sites | |
| ● | No related grade >3 or unexpected AEs | |
| ● | No related serious AEs | |
| ● | No serious, unexpected, related AEs |
1 Wiseman, C. L. & Kharazi, A. Phase I Study with SV-BR-1 Breast Cancer Cell Line Vaccine and GMCSF: Clinical Experience in 14 Patients. Open Breast Cancer J. 2, 4–11 (2010).
2 Wiseman, C. L. & Kharazi, A. Objective Clinical Regression of Metastatic Breast Cancer in Disparate Sites after Use of Whole-Cell Vaccine Genetically Modified to Release Sargramostim. Breast J. 12, 475–480 (2006).
3 Listed on ClinicalTrials.gov as NCT03066947 https://clinicaltrials.gov/ct2/show/NCT03066947?term=BriaCell&rank=2
| 9 |
Most patients who have dropped out did so due to worsening of their underlying disease.
| ● | Bria-IMT™ appears to be most effective in patients who match with Bria-IMT ™ at 2 HLA loci (types) further supporting our “HLA Matching Hypothesis”, and the development of Bria-OTS ™ to cover 90% of the patient population. | |
| ● | Effectiveness also depends on the ability of the patient to develop an immune response to Bria-IMT™ | |
| ● | Results are shown in the tables here, combining the second and third proof of concept studies which both used Bria-IMT™ in an identical regimen. |
| Patients (n) | HLA match | Tumor Shrinkage | Biological Response* | |||||||||
| 5 | >2 | 40 | % | 60 | % | |||||||
| 19 | >1 | 21 | % | 37 | % | |||||||
| 8 | 0 | 0 | % | 0 | % | |||||||
*Biological response includes tumor shrinkage or lower circulating cancer associated cells
BriaCell also evaluated the ability of patients to mount a delayed-type hypersensitivity (DTH) response to the injected Bria-IMT™. A positive response was noted in 22 patients while 5 were not responsive. The results, in terms of those who experienced tumor shrinkage, are shown here.
| Patients (n) | All
Patients (n=27) | No
HLA match (n=7) | 1+
HLA Match (n=20) | 2+
HLA matches (n=5) | ||||
| Negative DTH (n=5) | 0% (0/5) | 0% (0/0) | 0% (0/5) | 0% (0/2) | ||||
| Positive DTH (n=22) | 18% (4/22) | 0% (0/7) | 27% (4/15) | 67% (2/3) |
| ● | Bria-IMT™ was dosed in 27 patients (4 in 2004-2006, 23 in 2017-2018) as the Bria-IMT™ regimen alone. | |
| ● | Bria-IMT™ has been very well tolerated (over 100 doses given to date). | |
| ● | Tumor regression was seen in patients who were able to mount an immune response and matched Bria-IMT™ at HLA types confirming our main hypothesis and supporting using HLA typing as a marker to predict who is most likely to respond. | |
| ● | BriaCell continues to monitor their clinical trials proposing that BriaDx™ would include HLA typing as well as other potential biomarkers (such as the ability to mount a DTH response) to identify the patients most likely to respond to the Bria-IMT™ regimen. |
PKCδ
Protein Kinase C Delta (PKCδ) Inhibitors

Activated PKCδ inhibits RAS degradation which in turn stimulates tumor growth.
| 10 |
Early-Stage Preclinical Program
| ● | 30% of all human malignancies display activating RAS mutations with another 60% showing over-activity of Ras-signaling pathways.64 |
| ● | BriaCell’s novel, proprietary PKCδ inhibitors have shown activity against multiple RAS transformed tumors.65 |
| ● | This target has an attractive safety profile based on in vivo studies and knock out mouse studies.66 |
| ● | PKCδ also has potential activity as an immunotherapeutic by blocking TGFβ signaling.67 |
| ● | PKCδ inhibitors are applicable to specific niche tumor types which provide an accelerated clinical development plan. |
| ● | Could be in clinical testing within 24 months |
64 Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012 May 15; 72(10): 2457–2467
65 Xia, S., Forman, L. W. & Faller, D. V. Protein Kinase Cδ Is Required for Survival of Cells Expressing Activated p21RAS. J. Biol. Chem. 282, 13199–13210 (2007); Chen, Z. et al. Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer 18, 759–71 (2011); Xia, S., Chen, Z., Forman, L. W. & Faller, D. V. PKCδ survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell. Signal. 21, 502–508 (2009); Liou, J. S., Chen, C.-Y., Chen, J. S. & Faller, D. V. Oncogenic Ras Mediates Apoptosis in Response to Protein Kinase C Inhibition through the Generation of Reactive Oxygen Species. J. Biol. Chem. 275, 39001–39011 (2000); Liou, J. S., Chen, J. S. & Faller, D. V. Characterization of p21Ras-mediated apoptosis induced by protein kinase C inhibition and application to human tumor cell lines. J. Cell. Physiol. 198, 277–294 (2004); Chen, C. Y., Liou, J., Forman, L. W. & Faller, D. V. Differential regulation of discrete apoptotic pathways by Ras. J. Biol. Chem. 273, 16700–9 (1998); Chen, C. Y. & Faller, D. V. Direction of p21ras-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene 11, 1487–98 (1995); Chen, C. Y. & Faller, D. V. Phosphorylation of Bcl-2 protein and association with p21Ras in Ras-induced apoptosis. J. Biol. Chem. 271, 2376–9 (1996); Chen, C.-Y., Liou, J., Forman, L. W. & Faller, D. V. Correlation of genetic instability and apoptosis in the presence of oncogenic Ki-Ras. Cell Death Differ. 5, 984–995 (1998); Chen, C. Y. et al. The recruitment of Fas-associated death domain/caspase-8 in Ras-induced apoptosis. Cell Growth Differ. 12, 297–306 (2001).
66 Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002 Apr 25;416(6883):865-9.
67 Wermuth PJ, Addya S, Jimenez SA. Effect of Protein Kinase C delta (PKC-δ) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts In Vitro. PLoS ONE, November 2011, Volume 6, Issue 11, e27110; PMCID: PMC3214051; Li Z, Jimenez SA. Protein Kinase C δ and c-Abl Kinase Are Required for Transforming Growth Factor β Induction of Endothelial–Mesenchymal Transition In Vitro. Arthritis and Rheumatism, Vol. 63, No. 8, August 2011, pp 2473–2483 PMCID: PMC3134600; Bujor AM, Asano Y, Haines P, Lafyatis R, Trojanowska M. The c-Abl Tyrosine Kinase Controls Protein Kinase C δ –Induced Fli-1 Phosphorylation in Human Dermal Fibroblasts. Arthritis & Rheumatism, Vol. 63, No. 6, June 2011, pp 1729–1737. PMCID: PMC3381734
| 11 |

| ● | Structural aspects of first generation inhibitor rottlerin and staurosporine (pan-PKC inhibitor) were combined to create second generation inhibitor KAM1 |
| ● | Third generation inhibitors such as BC-106 have improved potency and selectivity. |
| ● | Fourth generation inhibitors are under development to optimize their drug-like characteristics. |
| ● | PKCδ inhibitors lack endothelial cell cytotoxicity & PKCδ deficient mice develop normally and are fertile → No marked intrinsic toxicity by inhibiting PKCδ |
PKCδ Inhibitors Block Growth in Various Cancers

PKCδ inhibitor reduces tumor burden in a human lung cancer model (lower is better)68
68 Chen, Z. et al. Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer 18, 759–71 (2011).
| 12 |

PKCδ inhibitors block growth of melanoma cells (lower is better)69

PKCδ inhibitors inhibit growth of neuroendocrine tumor cell lines (lower is better).70
69 Chen, C. Y., Liou, J., Forman, L. W. & Faller, D. V. Differential regulation of discrete apoptotic pathways by Ras. J. Biol. Chem. 273, 16700–9 (1998).
70 Chen, Z. et al. Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer 18, 759–71 (2011).
| 13 |

PKCδ
inhibitors decrease tumor size and improve survival in pancreatic cancer model.71
(A)
lower is better
(B) higher is better
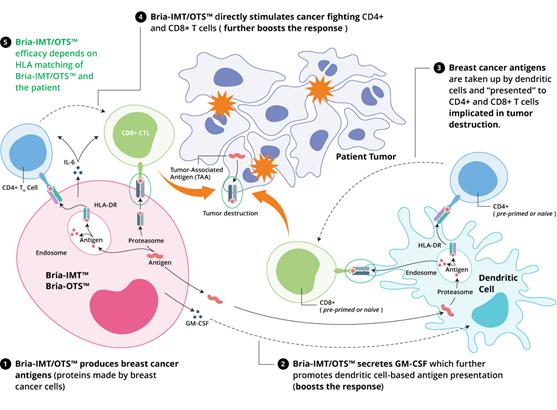
Mechanism of Action of BRIA-IMT™ and BRIA-OTS™
The mechanism of action of Bria-IMT™/Bria-OTS™ is currently under investigation.
We believe that Bria-IMT™/Bria-OTS™, activates the patient’s immune system to recognize tumor cells and destroy them. We hypothesize that Bria-IMT™/Bria-OTS™, exerts its action via changing the tumor’s antigen-presentation system {i.e. the system that presents antigen material on the surface of the tumor cell – to be recognized by the T cells of the immune system as either self (i.e., safe) or foreign (i.e., to be destroyed)}. Specifically, Bria-IMT™/Bria-OTS™, is thought to stimulate dendritic cells, a key component of the antigen-presenting system, to display certain immunogenic (i.e., immune response-generating) protein fragments to T cells, which activates the T cells to destroy the tumor cells either directly, or indirectly by inducing a humoral (antibody-generating) immune response. In addition, we also have shown that Bria-IMT™ is capable of directly stimulating cancer-fighting T cells. This unique property of Bria-IMT™ further boosts the immune response against the tumor cells and enhances anti-cancer activity.72
Our preliminary analyses have shown several up-regulated genes in Bria-IMT™ that encode proteins known to be immunogenic (i.e. immune response-generating), suggesting that Bria-IMT™ can stimulate the immune system against the cancer cells.
71 Chen, Z., Forman, L. W., Williams, R. M. & Faller, D. V. Protein kinase C-delta inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer 14, 90 (2014).
72 Lacher M.D., Bauer G. Fury B., Graeve S., Fledderman E.L., Petrie T.D., Coleal-Bergum D.P., Hackett T., Perotti N.H., Kong Y.Y., Kwok W.W., Wagner J.P., Wiseman C.L., and Williams W.V. SV-BR-1-GM, a Clinically Effective GM-CSF- Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4+ T Lymphocytes. Frontiers in Immunology 2018; 9: Article 776.
| 14 |
Bria-IMT™ is a human breast cancer cell line which expresses Her2/neu (a protein well known for its overexpression in breast cancer but also associated other epithelial malignancies including ovarian, pancreatic, colon, bladder and prostate cancers). Bria-IMT™ has been engineered to produce and secrete granulocyte/macrophage-colony stimulating factor (GM-CSF), a protein that promotes dendritic cell function, a key component of the immune system, and hence activates the immune system.
BRIA-IMT™ & BRIA-OTS™
Potential Mechanisms of Specific Immune Activation in Advanced Breast Cancer

| 1. | Bria-IMT/OTS™ produces breast cancer antigens (proteins made by breast cancer cells) |
| 2. | Bria-IMT/OTS™ secretes GM-CSF which further promotes dendritic cell-based antigen presentation (boosts the response) |
| 3. | Breast cancer antigens are taken up by dendritic cells and “presented” to CD4+ and CD8+ T cells implicated in tumor destruction. |
| 4. | Bria-IMT/OTS™ directly stimulates cancer fighting CD4+ and CD8+ T cells (further boosts the response) |
| 5. | Bria-IMT/OTS™ efficacy depends on HLA matching of Bria-IMT/OTS™ and the patient |
Clinical Trials
Phase I/IIA Combination Study of BRIA-IMT™ with KEYTRUDA® in Advanced Breast Cancer
The FDA has approved the combination study of Bria-IMT™ with pembrolizumab (KEYTRUDA®; manufactured by Merck & Co., Inc.). The Company is currently enrolling patients for this study.
KEYTRUDA® Combination: Patients will be treated with the combination of Bria-IMT™ and the anti-PD-1 antibody KEYTRUDA®.
| 15 |

Rationale for the Combination Study of BRIA-IMT™ with KEYTRUDA®
The immune checkpoint inhibitors such as pembrolizumab (KEYTRUDA®; anti-PD-1) have come to the forefront in the fight against cancer with substantial benefits for some patients. Most recently, the significance of immune checkpoint inhibitors was recognized by the Nobel committee by awarding Dr. Tasuku Honjo and Dr. James P. Allison with the 2018 Nobel Prize in Physiology or Medicine (Scientists behind game-changing cancer immunotherapies win Nobel medicine prize), validating the Company’s decision to launch a combination therapy with the immune checkpoint inhibitors.
| 16 |
Drs. Alison and Honjo independently, using different strategies, showed a new approach of treating patients by awakening certain cells of the immune system (T cells) to attack tumors. This new approach of treating patients with immune checkpoint inhibitors (such as KEYTRUDA®), designed to overcome immune suppression in cancer patients, is revolutionizing the fight against cancer.
In 2010 an important pre-clinical study by Dr. Allison’s group showed that combination with anti-PD-1 antibodies potentiated the tumor-destroying effect of melanoma cells engineered to produce granulocyte-macrophage colony-stimulating factor (GM-CSF), a substance that activates the immune system, compared to the treatment with the GM-CSF producing cells alone. Bria-IMT™ similarly uses a breast cancer cell line which produces GM-CSF. Bria-IMT™ has also been shown to indirectly and directly stimulate T cells, and hence boost the immune system. BriaCell has published these findings in a leading immunology journal. It is important to note that pembrolizumab has not been shown to work on its own in breast cancer but is approved for other indications.
KEYTRUDA® (pembrolizumab)
Manufactured by Merck & Co., Inc., KEYTRUDA® (pembrolizumab) is a prescription medicine that may treat certain cancers by working with the immune system. It has been approved for the treatment of a number of cancer indications excluding breast cancer.
BriaCell & Incyte Collaboration and Supply Agreement
Non-exclusive clinical trial collaboration to evaluate the effects of combinations of novel clinical candidates
| ● | The clinical study will focus on (but not limited to) BriaCell’s lead candidate, Bria-IMT™, in combination with Incyte’s selected compounds for advanced breast cancer. |
| ● | Incyte to provide compounds from its development portfolio, including INCMGA0012, an anti-PD-1 monoclonal antibody, and epacadostat, an IDO1 inhibitor, for use in combination studies with BriaCell’s lead candidate, Bria-IMT™. |
| ● | Incyte (INCY-NASDAQ; ~US$20Bn market capitalization) is a global biopharmaceutical company focused on discovering and developing novel therapeutics in oncology and other serious diseases. |
| ● | Incyte has a deep and rich pipeline in immuno-oncology with numerous molecular targets including PD-1, IDO, GITR, OX40, TIM-3, LAG-3, ARG, AXL/MER and PD-L1xCD137 |
| ● | The first 6 patients will receive the Bria-IMT™ regimen in combination with INCMGA00012. Once safety of the combination has been established, subsequent cohorts will receive a triple combination of the Bria-IMT™ regimen with INCMGA00012 and epacadostat. |
| ● | The design of the clinical study is shown below. Dosing of the novel combinations will commence in September 2019. |
| 17 |

Completed Proof of Concept Trials
BriaCell conducted three Proof of Concept clinical trials, one using parental SV-BR-1 cells and the other two using Bria-IMT™ (i.e., genetically engineered SV-BR-1 cells – producing GM-CSF also called SV-BR-1-GM), in metastatic (i.e., Stage IV) breast cancer patients who had failed prior treatments. The patients were treated according to the following schedule, and the results are summarized below.
| 18 |

First Proof of Concept Trial73
| ● | Used unmodified cell line (parental SV-BR-1 cells) + GM-CSF + cyclophosphamide | |
| ● | N = 14 late stage, treatment-refractory breast cancer patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 12.1 months |
Second Proof of Concept Trial74
| ● | Used Bria-IMT™ (genetically engineered SV-BR-1 cells – producing GM-CSF) + cyclophosphamide + interferon-α | |
| ● | N = 4 late stage, treatment-refractory (3 breast cancer, and 1 ovarian cancer) patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 35 months | |
| ● | One robust responder with >90% regression during treatment, subsequent relapse (upon halting treatment) responded to re-treatment |
Third Proof of Concept Trial75
Thirty patients were screened, 24 enrolled and 23 dosed in the Phase I/IIa study (2017-2018)
| ● | Bria-IMT™ was very well tolerated (over 100 doses given) | |
| ● | The majority of adverse events (AEs) were limited to expected minor local irritation at the injection sites | |
| ● | No related grade >3 or unexpected AEs | |
| ● | No related serious AEs | |
| ● | No serious, unexpected, related AEs |
73 Wiseman, C. L. & Kharazi, A. Phase I Study with SV-BR-1 Breast Cancer Cell Line Vaccine and GMCSF: Clinical Experience in 14 Patients. Open Breast Cancer J. 2, 4–11 (2010).
74 Wiseman, C. L. & Kharazi, A. Objective Clinical Regression of Metastatic Breast Cancer in Disparate Sites after Use of Whole-Cell Vaccine Genetically Modified to Release Sargramostim. Breast J. 12, 475–480 (2006).
75 Listed on ClinicalTrials.gov as NCT03066947 https://clinicaltrials.gov/ct2/show/NCT03066947?term=BriaCell&rank=2
| 19 |
Most patients who have dropped out did so due to worsening of their underlying disease.
| ● | Bria-IMT™ appears to be most effective in patients who match with Bria-IMT ™ at 2 HLA loci (types) further supporting our “HLA Matching Hypothesis”, and the development of Bria-OTS ™ to cover 90% of the patient population. | |
| ● | Effectiveness also depends on the ability of the patient to develop an immune response to Bria-IMT™ | |
| ● | Results are shown in the tables here, combining the second and third proof of concept studies which both used Bria-IMT™ in an identical regimen. |
| Patients (n) | HLA match | Tumor Shrinkage | Biological Response* | |||||||||
| 5 | >2 | 40 | % | 60 | % | |||||||
| 19 | >1 | 21 | % | 37 | % | |||||||
| 8 | 0 | 0 | % | 0 | % | |||||||
*Biological response includes tumor shrinkage or lower circulating cancer associated cells
| Patients (n) | All Patients (n=27) | No HLA match (n=7) | 1+ HLA Match (n=20) | 2+ HLA matches (n=5) | ||||
| Negative DTH (n=7) | 0% (0/1) | 0% | 0% (0/7) | 0% (0/1) | ||||
| Positive DTH (n=20) | 20% (4/20) | 0% (0/7) | 31% (4/13) | 50% (2/4) |
| ● | Initial safety data appears superior to that of the other advanced or approved drugs for breast cancer when they were at a similar stage of clinical development. | |
| ● | Initial efficacy data is similar or superior to those of other advanced or approved drugs for breast cancer when they were at a similar clinical stage of development. | |
| ● | Bria-IMT™ was dosed in 27 patients (4 in 2004-2006, 23 in 2017-2018) as the Bria-IMT™ regimen alone. | |
| ● | Bria-IMT™ has been very well tolerated (over 100 doses given to date). | |
| ● | The majority of adverse events (AEs) were limited to expected minor local irritation at the injection sites. | |
| ● | Tumor regression was seen in patents who were able to mount an immune response and matched Bria-IMT™ at HLA types confirming our main hypothesis. |
Market
It is estimated that in 2019, approximately 268,600 women will be diagnosed with breast cancer in the United States.1 According to the National Breast Cancer Foundation, on every two minutes an American woman is diagnosed with breast cancer and more than 40,500 die each year.2 Although about 100 times less common than in women, breast cancer also affects men.3 It is estimated that the lifetime risk of men getting breast cancer is about 1 in 1,0004, and the ACS estimates that approximately 2,670 new cases of invasive male breast cancer will be diagnosed and approximately 500 men will die from breast cancer in 2019.5
1 https://www.breastcancer.org/symptoms/understand_bc/statistics
2 https://www.nationalbreastcancer.org/breast-cancer-facts
3 https://www.medicinenet.com/breast_cancer_in_males_and_females/ask.htm
4 https://www.medicinenet.com/breast_cancer_in_males_and_females/ask.htm
5 https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
| 20 |
According to the May 2019 “Global Oncology Trends 2019” report by the IQVIA Institute, the global market for cancer drugs (including immunotherapy drugs) is expected to reach nearly $240 billion by the end of 2023, growing at a compound annual growth rate, or CAGR of 9-12% between 2019 and 2023.6
Marketing and Sales Strategy
The product will initially be marketed to oncologists who are well versed in the use of immunotherapy for cancer. Partnering with other pharma companies in order to market combinations with a number of drugs is also an option that we intend to pursue. This study will utilize a frozen formulation which consists of irradiated SV-BR-1-GM cells in viable freezing media. This formulation will permit stockpiling of the immunotherapy so that it can be sent on demand to clinical sites. The eventual goal is to reach all oncologists who treat late stage breast cancer either by direct outreach or by partnering with another company that has an established presence in the oncology space.
Other Commercial Considerations
There is a high unmet medical need in late stage breast cancer, providing potential for accelerated approval of Bria-IMT™. The FDA is interested in facilitating the availability of novel therapies of patients with unmet medical needs, especially those that can target the population most likely to respond. In addition, Bria-IMT™ may fit the description of an orphan drug, especially if HLA matching is required. These two facts may help facilitate accelerated approval of Bria-IMT™.
Production and Marketing Plan
Bria-IMT™ cells grow in simple tissue culture media and are irradiated prior to inoculation. Bria-IMT™ manufacturing will be performed by Contract Manufacturing Organizations (CMOs). Recently we have been working with KBI Biopharma who have developed a frozen formulation, where the cells are grown, harvested and irradiated followed by cryopreservation in a viable state. The cells are stockpiled and shipped directly to clinical sites for inoculation. Each lot of Bria-IMT™ is tested for potency (GM-CSF production), identity (HER2+ and ER/PR-) and adventitious agents to assure that each patient receives a safe and effective treatment. To date, there have been no issues with these tests. Additional manufacturing facilities have been evaluated and may be enlisted as demand grows.
Corporate Background
We were incorporated in the province of British Columbia on July 26, 2006. Our common shares have been quoted on the OTCQB under the symbol BCTXF and on the TSX Venture Exchange under the symbol “BCT.V”.
Our principal executive office is located at Suite 300 – 235 West 15th Street, West Vancouver, British Columbia, V7T 2X1 and our telephone number in Canada is (604) 921-1810. Our web address is https://briacell.com/. The information contained on our website or available through our website is not incorporated by reference into and should not be considered a part of this prospectus, and the reference to our website in this prospectus is an inactive textual reference only. Any website references (URL’s) in this prospectus are inactive textual references only and are not active hyperlinks. The contents of our website is not part of this prospectus, and you should not consider the contents of our website in making an investment decision with respect to our common shares. Paracorp Incorporated is our agent in the United States, and its address is 2804 Gateway Oaks Drive #100, Sacramento, CA 95833, Tel: (888) 280-6563, Fax: (800) 603-5868; Attn: Katelyn Bean (kbean@myparacorp.com).
The Company’s corporate offices in the United States are located at 820 Heinz Avenue, Berkley, California 94710. The Company’s two wholly owned subsidiaries BriaCell Therapeutics Corp., a Delaware corporation, and Sapientia Pharmaceuticals Inc., a Delaware corporation, were formed on April 3, 2014 and September 20, 2012 respectively.
6 IQVIA Institute. Global Oncology Trends 2019: Therapeutics, Clinical Development and Health System Implications. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trends-2019.pdf?&_=1565186166041.
| 21 |
Implications of Being an “Emerging Growth Company” and a Foreign Private Issuer
As a company with less than U.S. $1.07 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
| ● | reduced executive compensation disclosure; | |
| ● | exemptions from the requirement to hold a non-binding advisory vote on executive compensation, including golden parachute compensation; and | |
| ● | an exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting pursuant to the Sarbanes-Oxley Act of 2002. |
We may take advantage of these provisions until we are no longer an emerging growth company. We would cease to be an emerging growth company upon the earlier to occur of: (1) the last day of our fiscal year following the fifth anniversary of the completion of this offering; (2) the last day of the fiscal year in which we have total annual gross revenue of U.S.$1.07 billion or more; (3) the date on which we have issued more than U.S.$1.0 billion in nonconvertible debt during the previous three years; or (4) the date on which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission, or the SEC.
We currently report and will continue to report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. Even after we no longer qualify as an emerging growth company, as long as we continue to qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including:
| ● | the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations with respect to a security registered under the Exchange Act; | |
| ● | the sections of the Exchange Act requiring insiders to file public reports of their share ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and | |
| ● | the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial statements and other specified information, and current reports on Form 8-K upon the occurrence of specified significant events, although we report our results of operations on a quarterly basis under the Canadian securities laws. |
Both foreign private issuers and emerging growth companies are also exempt from certain more stringent executive compensation disclosure rules. Thus, even if we no longer qualify as an emerging growth company, but remain a foreign private issuer, we will continue to be exempt from the more stringent compensation disclosures required of companies that are neither an emerging growth company nor a foreign private issuer.
We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents, and any one of the following three circumstances applies: (i) the majority of our executive officers or directors are U.S. citizens or residents, (ii) more than 50% of our assets are located in the United States or (iii) our business is administered principally in the United States.
In this prospectus, we have taken advantage of certain of the reduced reporting requirements as a result of being an emerging growth company and a foreign private issuer. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold equity securities.
| 22 |
THE OFFERING
The information below is only a summary of more detailed information included elsewhere in this prospectus. This summary may not contain all the information that is important to you or that you should consider before making a decision to invest in our securities. Please read this entire prospectus, including the risk factors, carefully.
| Securities offered by us | ______________ units, each consisting of one common share and one warrant, each whole warrant exercisable for one common share. The warrants included within the units are exercisable immediately, have an exercise price of $ per share, equal to 125% of the public offering price of one unit, and expire five years from the date of issuance. The common shares and warrants that are part of the units are immediately separable and will be issued separately in this offering. | |
| Public offering price | $ per unit. | |
| Over-allotment option | We have granted the underwriters a 45-day option to purchase up to an aggregate of __________ additional common shares and/or warrants to purchase up to __________ additional common shares (equal to 15% of the common shares and warrants underlying the units sold in the offering) in any combination thereof, solely to cover over-allotments, if any. The purchase price to be paid per additional common share by the underwriters shall be equal to the public offering price of one unit, less the underwriting discount, and the purchase price to be paid per additional warrant by the underwriters shall be $0.00001. | |
| Common shares outstanding prior to this offering | 196,378,450 shares of common stock. | |
| Common shares outstanding after this offering | ____________ shares (or ___________ shares if the underwriters exercise their over-allotment option in full).(1)(2) | |
| Use of proceeds | We expect to receive approximately $_______ in net proceeds from the sale of units offered by us in this offering (approximately $_________ if the underwriters exercise their over-allotment option in full), after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us of $________, based on an assumed offering price of $_______ per unit. We intend to use the net proceeds from this offering to advance our clinical trials and as further set forth in the “Use of Proceeds” section. | |
| Risk factors | An investment in our securities involves significant risks. See the section entitled “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our securities. | |
| Lock-up | We and our directors and executive officers have agreed with the underwriters not to offer, issue, sell, contract to sell, encumber, grant any option for the sale of or otherwise dispose of any of our securities for a period of six months commencing on the date of this prospectus. See “Underwriting” beginning on page 106. | |
| Market and trading symbol for our common shares and warrants | Our common shares are currently quoted on the OTCQB under the symbol “BCTXF” and on the TSXV under the symbol “BCT.V”. We have applied to list the common shares and warrants included within the units on the Nasdaq Capital Market under the symbols “_________” and “_________”, respectively. No assurance can be given that such listings will be approved. |
| 23 |
| (1) | The number of common shares to be outstanding after this offering is based on 196,378,450 common shares outstanding as of August 28, 2019 and excludes the following: |
| ● | 54,579,759 shares of common stock issuable upon the exercise of outstanding warrants, at a weighted average exercise price of $0.128; | |
| ● | 4,732,035 shares of common stock issuable upon the exercise of outstanding warrants, at a weighted average exercise price of $0.151; | |
| ● | 6,972,600 shares of common stock issuable upon the exercise of outstanding options, at a weighted average exercise price of $0.174; and | |
| ● | an estimated 4,485,234 shares of common stock and warrants exercisable into an additional 4,480,030 shares of common stock, both underlying convertible debentures in the aggregate amount of $448,803; and | |
| ● | an estimated ____________ common shares issuable upon exercise of the warrants included in the units (or ____ common shares if the underwriters exercise their over-allotment option in full with respect to the warrants contained in the units). |
| (2) | Except as otherwise indicated herein, all information in this prospectus assumes no exercise of the underwriters’ over-allotment option. |
| 24 |
Summary Financial Data
The summary financial information set forth below has been derived from our audited financial statements for the fiscal years ended July 31, 2018, 2017, 2016, 2015 and 2014 and from our unaudited financial statements for the nine months ended April 30, 2019. You should read the following summary financial data for the years ended July 31, 2018 and 2017 and the nine months ended April 30, 2019 together with our historical financial statements and the notes thereto included elsewhere in this prospectus and with the information set forth in the section titled “Management’s Discussion and Analysis of Financial Conditions and Results of Operations”. The audited financial statements for the years ended July 31, 2016, 2015 and 2014 are not included in this prospectus.
| As of April 30, | As of July 31, | |||||||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||||||
| Balance Sheet Data | ||||||||||||||||||||||||
| Cash and cash equivalents | 938,932 | 938,448 | 1,264,429 | 171,865 | 464,732 | 26,070 | ||||||||||||||||||
| Total Assets | 1,613,299 | 2,977,140 | 2,039,199 | 1,091,587 | 1,660,288 | 26,082 | ||||||||||||||||||
| Total Liabilities | 747,414 | 1,745,850 | 1,104,147 | 63,470 | 152,425 | 27,312 | ||||||||||||||||||
| Total Shareholders’ Equity (deficit) | 865,885 | 1,231,290 | 935,052 | 1,028,117 | 1,507,863 | (1,230 | ) | |||||||||||||||||
| Nine months ended April 30, | Year ended July 31, | |||||||||||||||||||||||
| 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||||||
| ($ thousands) | ||||||||||||||||||||||||
| Operating Data | ||||||||||||||||||||||||
| Revenues and other income | - | - | - | - | - | - | ||||||||||||||||||
| Expenses: | ||||||||||||||||||||||||
| Research costs | 3,605,429 | 3,112,579 | 2,125,941 | 944,942 | 390,036 | - | ||||||||||||||||||
| General and administrative costs | 847,266 | 1,387,713 | 820,281 | 584,105 | 892,611 | 1,240 | ||||||||||||||||||
| Share-based compensation | 60,570 | 476,211 | 272,014 | 648,149 | 516,288 | - | ||||||||||||||||||
| Listing costs | - | - | - | - | 1,599,488 | - | ||||||||||||||||||
| Surrender of royalty rights | - | - | - | - | 150,000 | - | ||||||||||||||||||
| Total expenses | 4,513,265 | 4,976,503 | 3,218,236 | 2.177,196 | 3,548,423 | 1,240 | ||||||||||||||||||
| Operating loss | (4,513,265 | ) | (4,976,503 | ) | (3,218,236 | ) | (2.177,196 | ) | (3,548,423 | ) | (1,240 | ) | ||||||||||||
| Interest income | 12,000 | 15,991 | 6,428 | 4,738 | 9,227 | - | ||||||||||||||||||
| Interest expenses | (26,352 | ) | (20,364 | ) | - | - | - | - | ||||||||||||||||
| Change in fair value of convertible debt | 392,721 | (407,709 | ) | - | - | - | - | |||||||||||||||||
| Loss on available for sale investments | - | - | - | (27,763 | ) | - | - | |||||||||||||||||
| Foreign exchange gain (loss) | 39,902 | (24,078 | ) | (8,913 | ) | (14,561 | ) | 50,385 | (2 | ) | ||||||||||||||
| 418,271 | (436,160 | ) | (2,485 | ) | (37,586 | ) | 59,612 | (2 | ) | |||||||||||||||
| Loss For The Period | (4,094,994 | ) | (5,412,663 | ) | (3,220,721 | ) | (2,214,782 | ) | (3,488,811 | ) | (1,242 | ) | ||||||||||||
| Items That Will Subsequently Be Reclassified To Profit Or Loss | ||||||||||||||||||||||||
| Foreign currency translation adjustment | 8,589 | (33,340 | ) | 41,828 | 18,575 | (48,921 | ) | - | ||||||||||||||||
| Unrealized loss on available for sale investments | - | - | - | (6,892 | ) | (20,871 | ) | - | ||||||||||||||||
| Items Reclassified To Profit Or Loss | ||||||||||||||||||||||||
| Reclass of unrealized losses on available for sale investments | - | - | - | 27,763 | - | - | ||||||||||||||||||
| Comprehensive loss for the period | (4,086,405 | ) | (5,446,003 | ) | (3,178,893 | ) | (2,175,336 | ) | (3,558,603 | ) | (1,242 | ) | ||||||||||||
| Basic and Fully Diluted Loss Per Share | (0.02 | ) | (0.04 | ) | (0.03 | ) | (0.03 | ) | (0.05 | ) | (0.00 | ) | ||||||||||||
| Weighted Average Number Of Shares Outstanding | 166,323,680 | 128,344,435 | 101,912,205 | 86,541,678 | 74,761,026 | 53,111,568 | ||||||||||||||||||
| 25 |
An investment in our common shares involves a high degree of risk. You should carefully consider the following factors and other information in this prospectus before deciding to invest in us. If any of the following risks actually occur, our business, financial condition, results of operations and prospects for growth would likely suffer. As a result, you could lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may materially and adversely affect our business, financial condition and results of operations. See also “Cautionary Statement Regarding Forward-Looking Statements.”
Risks Related to Our Business
We have a history of losses, may incur future losses and may not achieve profitability
BriaCell is a development stage immune-oncology biotechnology corporation that to date has not recorded any revenues from the sale of diagnostic or therapeutic products. Since incorporation, BriaCell has accumulated net losses and expects such losses to continue as it commences product and pre-clinical development and eventually enters into license agreements for its technology. We incurred net losses of $2,175,336, $3,178,893 and $5,446,003 in the fiscal years ended July 31, 2016, 2017, and 2018, respectively and have incurred a net loss of $4,094,994 (unaudited) through the first three quarters of our fiscal year ended July 31, 2019. Management expects to continue to incur substantial operating losses unless and until such time as product sales generate sufficient revenues to fund continuing operations. BriaCell has neither a history of earnings nor has it paid any dividends and it is unlikely to pay dividends or enjoy earnings in the immediate or foreseeable future.
We are an early stage development company
The Company expects to spend a significant amount of capital to fund research and development. As a result, the Company expects that its operating expenses will increase significantly and, consequently, it will need to generate significant revenues to become profitable. Even if the Company does become profitable, it may not be able to sustain or increase profitability on a quarterly or annual basis. The Company cannot predict when, if ever, it will be profitable. There can be no assurances that the Intellectual Property of BriaCell, or other technologies it may acquire, will meet applicable regulatory standards, obtain required regulatory approvals, be capable of being produced in commercial quantities at reasonable costs, or be successfully marketed. The Company will be undertaking additional laboratory studies or trials with respect to the Intellectual Property of BriaCell, and there can be no assurance that the results from such studies or trials will result in a commercially viable product or will not identify unwanted side effects.
We have an unproven market for our product candidates
The Company believes that the anticipated market for its potential products and technologies if successfully developed will continue to exist and expand. These assumptions may prove to be incorrect for a variety of reasons, including competition from other products and the degree of commercial viability of the potential product.
We may not succeed in adapting to and meeting the business needs associated with our anticipated growth
Anticipated growth in all areas of BriaCell’s business is expected to continue to place a significant strain on its managerial, operational and technical resources. The Company expects operating expenses and staffing levels to increase in the future. To manage such growth, the Company must expand its operational and technical capabilities and manage its employee base while effectively administering multiple relationships with various third parties. There can be no assurance that the Company will be able to manage its expanding operations effectively. Any failure to implement cohesive management and operating systems, to add resources on a cost-effective basis or to properly manage the Company’s expansion could have a material adverse effect on its business and results of operations.
| 26 |
We are heavily reliant on third-parties to carry out a large portion of our business
The Company does not expect to have any in-house manufacturing, pharmaceutical development or marketing capability. To be successful, a product must be manufactured and packaged in commercial quantities in compliance with regulatory requirements and in reasonable time frames and at accepted costs. The Company intends to contract with third parties to develop its products. No assurance can be given that the Company or its suppliers will be able to meet the supply requirements in respect of the product development or commercial sales.
Production of therapeutic products may require raw materials for which the sources and amount of supply are limited, or may be hindered by quality or scheduling issues in respect of the third party suppliers over which the Company has limited control. An inability to obtain adequate supplies of raw materials could significantly delay the development, regulatory approval and marketing of a product. The Company has limited in-house personnel to internally manage all aspects of product development, including the management of multi-center clinical trials. The Company is significantly reliant on third-party consultants and contractors to provide the requisite advice and management. There can be no assurance that the clinical trials and product development will not encounter delays which could adversely affect prospects for the Company’s success.
To be successful, an approved product must also be successfully marketed. The market for the Company’s product being developed by the Company may be large and will require substantial sales and marketing capability. At the present time, the Company does not have any internal capability to market pharmaceutical products. The Company intends to enter into one or more strategic partnerships or collaborative arrangements with pharmaceutical companies or other companies with marketing and distribution expertise to address this need. If necessary, the Company will establish arrangements with various partners for geographical areas. There can be no assurance that the Company can market, or can enter into a satisfactory arrangement with a third party to market a product in a manner that would assure its acceptance in the marketplace. However, if a satisfactory arrangement with a third party to market and/or distribute a product is obtained; the Company will be dependent on the corporate collaborator(s) who may not devote sufficient time, resources and attention to the Company’s programs, which may hinder efforts to market the products.
Should the Company not establish marketing and distribution strategic partnerships and collaborative arrangements on acceptable terms, and undertake some or all of those functions, the Company will require significant additional human and financial resources and expertise to undertake these activities, the availability of which is not guaranteed. The Company will rely on third parties for the timely supply of raw materials, equipment, contract manufacturing, and formulation or packaging services. Although the Company intends to manage these third-party relationships to ensure continuity and quality, some events beyond the Company’s control could result in complete or partial failure of these goods and services. Any such failure could have a material adverse effect on the financial conditions and result of operation of the Company
Due to the complexity of the process of developing pharmaceutical products, the Company’s business may depend on arrangements with pharmaceutical and biotechnology companies, corporate and academic collaborators, licensors, licensees and others for the research, development, clinical testing, technology rights, manufacturing, marketing and commercialization of its products. Such agreements could obligate the Company to diligently bring potential products to market, make milestone payments and royalties that, in some instances, could be substantial, and incur the costs of filing and prosecuting patent applications. There can be no assurance that the Company will be able to establish or maintain collaborations that are important to its business on favorable terms, or at all.
A number of risks arise from the Company’s potential dependence on collaborative agreements with third parties. Product development and commercialization efforts could be adversely affected if any collaborative partner terminates or suspends its agreement with the Company, causes delays, fails to on a timely basis develop or manufacture in adequate quantities a substance needed in order to conduct clinical trials, fails to adequately perform clinical trials, determines not to develop, manufacture or commercialize a product to which it has rights, or otherwise fails to meet its contractual obligations. The Company’s collaborative partners could pursue other technologies or develop alternative products that could compete with the products the Company is developing.
The Company has signed Non-Disclosure Agreements (“NDA”) with many different third parties as is customary in the industry. There is no guarantee that, despite the terms of the NDA which bind third parties, the Company will ultimately be able to prevent from such third parties from breaching their obligations under the NDA. Use of the Company’s confidential information in an unauthorized manner is likely to negatively affect the Company.
| 27 |
Pre-clinical studies and initial clinical trials are not necessarily predictive of future results
Pre-clinical tests and Phase I/II clinical trials are primarily designed to test safety, to study pharmacokinetics and pharmacodynamics and to understand the side effects of product candidates at various doses and schedules. Success in pre-clinical and early clinical trials does not ensure that later large-scale efficacy trials will be successful nor does it predict final results. Favorable results in early trials may not be repeated in later trials.
A number of companies in the life sciences industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated. Any pre-clinical data and the clinical results obtained for BriaCell’s technology may not predict results from studies in larger numbers of subjects drawn from more diverse populations or in the commercial setting, and also may not predict the ability of our products to achieve their intended goals, or to do so safely.
An inability to obtain raw materials or product supply could have a material adverse impact on the Company’s business, financial condition and results of operations
Raw materials and supplies are generally available in quantities to meet the needs of the Company’s business. The Company will be dependent on third-party manufacturers for the pharmaceutical products that it markets. An inability to obtain raw materials or product supply could have a material adverse impact on the Company’s business, financial condition and results of operations.
We must obtain additional capital to continue our operations
The Company anticipates that additional capital will be required to complete its current research and development programs. It is anticipated that future research, additional pre-clinical and toxicology studies and manufacturing initiatives, including that to prepare for market approval and successful product market launch will require additional funds. Further financing may dilute the current holdings of shareholders and may thereby result in a loss for the shareholders. There can be no assurance that the Company will be able to obtain adequate financing, or financing on terms that are reasonable or acceptable for these or other purposes, or to fulfill the Company’s obligations under various license agreements. Failure to obtain such additional financing could result in delay or indefinite postponement of further research and development of the Company’s technologies with the possible loss of license rights to these technologies.
Although the Company’s common shares are quoted or listed for trading on the OTCQB and TSXV, there can be no assurance that a liquid market for our common shares will develop, which may have an adverse effect on the market price of the Company’s common shares.
We are highly dependent on our key personnel
Although the Company is expected to have experienced senior management and personnel, the Company will be substantially dependent upon the services of a few key personnel, particularly Dr. Charles Wiseman, Dr. Markus Lacher and Dr. William V. Williams and other professionals for the successful operation of its business. Phase I of the Company’s research and development is planned to be completed by qualified professionals and is expected to concentrate on treatment of advanced breast cancer. The loss of the services of any of these personnel could have a material adverse effect on the business of the Company. The Company may not be able to attract and retain personnel on acceptable terms given the intense competition for such personnel among high technology enterprises, including biotechnology, and healthcare companies, universities and non-profit research institutions. If we lose any of these persons, or are unable to attract and retain qualified personnel, our business, financial condition and results of operations may be materially and adversely affected.
| 28 |
If the Company experiences a data security breach and confidential information is disclosed, the Company may be subject to penalties and experience negative publicity
The Company and its customers could suffer harm if personal and health information were accessed by third parties due to a system security failure. The collection of data requires the Company to receive and store a large amount of personally identifiable data. Recently, data security breaches suffered by well-known companies and institutions have attracted a substantial amount of media attention, prompting legislative proposals addressing data privacy and security. The Company may become exposed to potential liabilities with respect to the data that it collects, manages and processes, and may incur legal costs if information security policies and procedures are not effective or if the Company is required to defend its methods of collection, processing and storage of personal data. Future investigations, lawsuits or adverse publicity relating to its methods of handling such information could have a material adverse effect on the Company’s business, financial condition and results of operations due to the costs and negative market reaction relating to such developments.
The report of our independent registered public accounting firm expresses substantial doubt about our ability to continue as a going concern
Our independent registered public accounting firm indicated in its report on our financial statements for the year ended July 31, 2018, that conditions exist that raise substantial doubt about our ability to continue as a “going concern.” A going concern paragraph included in our independent registered public accounting firm’s report on our consolidated financial statements could impair investor perceptions and our ability to finance our operations through the sale of equity, incurring debt, or other financing alternatives. Our ability to continue as a going concern will depend upon many factors beyond our control including the availability and terms of future funding. If we are unable to achieve our goals and raise the necessary funds to finance our operations, our business would be jeopardized, and we may not be able to continue. If we ceased operations, it is likely that all of our investors would lose their investment.
We may not succeed in completing the development of our products, commercializing our products or generating significant revenues
Since commencing our operations, we have focused on the research and development and limited clinical trials of our product candidates. Our ability to generate revenues and achieve profitability depends on our ability to successfully complete the development of our products, obtain market approval and generate significant revenues. The future success of our business cannot be determined at this time, and we do not anticipate generating revenues from product sales for the foreseeable future. In addition, we face a number of challenges with respect to our future commercialization efforts, including, among others, that:
| ● | we may not have adequate financial or other resources to complete the development of our product, including two stages of clinical development that are necessary in order to commercialize our products; | |
| ● | we may not be able to manufacture our products in commercial quantities, at an adequate quality or at an acceptable cost; | |
| ● | we may not be able to maintain our CE mark due to the regulatory changes; | |
| ● | we may never receive FDA approval for our intended development plans; | |
| ● | we may not be able to establish adequate sales, marketing and distribution channels; | |
| ● | healthcare professionals and patients may not accept our product candidates; | |
| ● | technological breakthroughs in cancer detection, treatment and prevention may reduce the demand for our product candidates; | |
| ● | changes in the market for cancer treatment, new alliances between existing market participants and the entrance of new market participants may interfere with our market penetration efforts; |
| 29 |
| ● | third-party payors may not agree to reimburse patients for any or all of the purchase price of our products, which may adversely affect patients’ willingness to purchase our product candidates; | |
| ● | uncertainty as to market demand may result in inefficient pricing of our product candidates; | |
| ● | we may face third-party claims of intellectual property infringement; | |
| ● | we may fail to obtain or maintain regulatory approvals for our products candidates in our target markets or may face adverse regulatory or legal actions relating to our product candidates even if regulatory approval is obtained; and | |
| ● | we are dependent upon the results of ongoing clinical studies relating to our product candidates and the products of our competitors. We may fail in obtaining positive results. |
If we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our product candidates could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
If product liability lawsuits are brought against us, we may incur substantial liabilities and the commercialization of our drug candidates may be affected
As our drug candidates enter clinical trials, we will face an inherent risk of product liability suits and will face an even greater risk if we obtain approval to commercialize any drugs. For example, we may be sued if our drug candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent in the drug, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection acts. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our drug candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| ● | decreased demand for our drugs; | |
| ● | injury to our reputation; | |
| ● | withdrawal of clinical trial participants and inability to continue clinical trials; | |
| ● | initiation of investigations by regulators; | |
| ● | costs to defend the related litigation; | |
| ● | a diversion of management’s time and our resources; | |
| ● | substantial monetary awards to trial participants or patients; | |
| ● | product recalls, withdrawals or labeling, marketing or promotional restrictions; | |
| ● | loss of revenue; | |
| ● | exhaustion of any available insurance and our capital resources; | |
| ● | the inability to commercialize any drug candidate; and | |
| ● | a decline in the price of our common shares. |
| 30 |
We shall seek to obtain the appropriate insurance once our candidates are ready for clinical trial. However, our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of drugs we develop, alone or with collaborators. We currently do not have in place product liability insurance and although we plan to have in place such insurance as and when the products are ready for commercialization, as well as insurance covering clinical trials, the amount of such insurance coverage may not be adequate, we may be unable to maintain such insurance, or we may not be able to obtain additional or replacement insurance at a reasonable cost, if at all. Our insurance policies may also have various exclusions, and we may be subject to a product liability claim for which we have no coverage. We may have to pay any amounts awarded by a court or negotiated in a settlement that exceed our coverage limitations or that are not covered by our insurance, and we may not have, or be able to obtain, sufficient capital to pay such amounts. Even if our agreements with any future corporate collaborators entitle us to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
Additionally, we may be sued if the products that we commercialize, market or sell cause or are perceived to cause injury or are found to be otherwise unsuitable, and may result in:
| ● | decreased demand for those products; | |
| ● | damage to our reputation; | |
| ● | costs incurred related to product recalls; | |
| ● | limiting our opportunities to enter into future commercial partnership; and | |
| ● | a decline in the price of our common shares. |
Risks Related to Our Intellectual Property
We may not successfully develop, maintain and protect our proprietary products and technologies
BriaCell’s success depends to a significant degree upon its ability to develop, maintain and protect proprietary products and technologies. BriaCell files patent applications in the United States and other countries as part of its global strategy to protect its Intellectual Property and maintains certain US and Non-US patents in its IP portfolio. However, patents provide only limited protection of BriaCell’s Intellectual Property. The assertion of patent protection involves complex legal and factual determinations and is therefore uncertain and can be expensive. BriaCell cannot provide assurances that patents will be granted with respect to any of its pending patent applications, or that the scope of any of its granted patents, or any patents granted in the future, will be sufficiently broad to offer meaningful protection, or that it will develop and file patent applications on additional proprietary technologies that are patentable, or, if patentable, that any patents will be granted from such patent applications. BriaCell’s current or future patents could be successfully challenged, invalidated or circumvented. This could result in BriaCell’s patent rights failing to create an effective competitive barrier. Losing a significant patent or failing to get a patent to issue from a pending patent application that BriaCell considers significant could have a material adverse effect on BriaCell’s business. The laws governing the scope of patent coverage in various countries continue to evolve. The laws of some foreign countries may not protect BriaCell’s Intellectual Property rights to the same extent as the laws of the United States. BriaCell has applied for patent protection only in selected countries. Therefore, third parties may be able to replicate BriaCell technologies covered by BriaCell’s patent portfolio in countries in which it does not have patent protection.
BriaCell’s future success and competitive position depends in part upon its ability to maintain its Intellectual Property portfolio. There can be no assurance that any patents will be issued on any existing or future patent applications.
| 31 |
We are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our product candidates
There is a substantial amount of litigation over patent and other Intellectual Property rights in the biotechnology industry. Whether or not a product infringes a patent involves complex legal and factual considerations, the determination of which is often uncertain. Our management is presently unaware of any other parties’ patents and proprietary rights which our products under development would infringe. Searches typically performed to identify potentially infringed patents of third parties are often not conclusive and, because patent applications can take many years to issue, there may be applications now pending, which may later result in issued patents which our current or future products may infringe or be alleged to infringe. In addition, our competitors or other parties may assert that our product candidates and the methods employed may be covered by patents held by them. If any of our products infringes a valid patent, we could be prevented from manufacturing or selling such product unless we are able to obtain a license or able to redesign the product in such a manner as to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid infringement, nor does a later redesign protect BriaCell from prior infringement. Infringement and other Intellectual Property claims, with or without merit, can be expensive and time-consuming to litigate and can divert our management’s attention from operating our business.
The steps we have taken to protect our Intellectual Property may not be adequate, which could have a material adverse effect on our ability to compete in the market
BriaCell’s ability to establish and maintain a competitive position may be achieved in part by prosecuting claims against others who it believes to be infringing its rights. In addition, enforcement of BriaCell’s patents in foreign jurisdictions will depend on the legal procedures in those jurisdictions. In addition to filing patent applications, we rely on confidentiality, non-compete, non-disclosure and assignment of inventions provisions, as appropriate, in our agreements with our employees, consultants, and service providers, to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not be adequate to protect our Intellectual Property from unauthorized disclosure, third-party infringement or misappropriation, for the following reasons:
| ● | the agreements may be breached, may not provide the scope of protection we believe they provide or may be determined to be unenforceable; | |
| ● | we may have inadequate remedies for any breach; | |
| ● | proprietary information could be disclosed to our competitors; or | |
| ● | others may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technologies. |
Specifically, with respect to non-compete agreements, both state law and precedent varies greatly from state to state and we may be unable to enforce these agreements, in whole or in part, and it may be difficult for us to restrict our competitors from gaining the expertise that our former employees gained while working for us. If our Intellectual Property is disclosed or misappropriated, it could harm our ability to protect our rights and could have a material adverse effect on our business, financial condition and results of operations.
We may need to initiate lawsuits to protect or enforce our patents and other Intellectual Property rights, which could be expensive and, if we lose, could cause us to lose some of our Intellectual Property rights, which would harm our ability to compete in the market
We rely on patents, confidentiality and trade secrets to protect a portion of our Intellectual Property and our competitive position. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in the biotechnology/pharmaceutical industry can be uncertain. In order to protect or enforce our patent rights, we may initiate patent and related litigation against third parties, such as infringement suits or requests for injunctive relief. BriaCell’s ability to establish and maintain a competitive position may be achieved in part by prosecuting claims against others who it believes to be infringing its rights. In addition, enforcement of BriaCell’s patents in foreign jurisdictions will depend on the legal procedures in those jurisdictions. Any lawsuits that we initiate could be expensive, take significant time and divert our management’s attention from other business concerns and the outcome of litigation to enforce our Intellectual Property rights in patents, copyrights, trade secrets or trademarks is highly unpredictable. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, or adversely affect its ability to distribute any products that are subject to such litigation. In addition, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, including attorney fees, if any, may not be commercially valuable. The occurrence of any of these events could have a material adverse effect on our business, financial condition and results of operations.
| 32 |
We may be subject to damages resulting from claims that we or our employees or contractors have wrongfully used or disclosed alleged trade secrets of their former employers
Many of our employees and contractors were previously employed at universities or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, we may be subject to claims that we or any employee or contractor has inadvertently or otherwise used or disclosed trade secrets or other proprietary information of his or her former employers. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable Intellectual Property rights or personnel. A loss of key research personnel or their work product could hamper or prevent our ability to commercialize certain therapeutic candidates, which could severely harm our business, financial condition and results of operations. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Risks Related to Regulations
Changes in legislation and regulations may affect our revenue and profitability
Existing and proposed changes in the laws and regulations affecting public companies may cause the Company to incur increased costs as the Company evaluates the implications of new rules and responds to new requirements. Failure to comply with new rules and regulations could result in enforcement actions or the assessment of other penalties. New laws and regulations could make it more difficult to obtain certain types of insurance, including director’s and officer’s liability insurance, and the Company may be forced to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage, to the extent that such coverage remains available.
The impact of these events could also make it more difficult for the Company to attract and retain qualified persons to serve on the Company’s board of directors, or as executive officers. The Company may be required to hire additional personnel and utilize additional outside legal, accounting and advisory services, all of which could cause the Company’s general and administrative costs to increase beyond what the Company currently has planned. Although the Company evaluates and monitors developments with respect to new rules and laws, the Company cannot predict or estimate the amount of the additional costs the Company may incur or the timing of such costs with respect to such evaluations and/or compliance and cannot provide assurances that such additional costs will render the Company compliant with such new rules and laws.
If we or our licensees are unable to obtain U.S., Canadian and/or foreign regulatory approval for our product candidates, we will be unable to commercialize our therapeutic candidates
To date, we have not marketed, distributed or sold an approved product. Our therapeutic candidates are subject to extensive governmental regulations relating to development, clinical trials, manufacturing and commercialization of drugs. We may not obtain marketing approval for any of our therapeutic candidates in a timely manner or at all. In connection with the clinical trials for our product candidates and other therapeutic candidates that we may seek to develop in the future, either on our own or throughout licensing arrangements, we face the risk that:
| ● | a product candidate may not prove safe or efficacious; | |
| ● | the results with respect to any product candidate may not confirm the positive results from earlier preclinical studies or clinical trials; | |
| ● | the results may not meet the level of statistical significance required by the FDA, Health Canada or other regulatory authorities; and | |
| ● | the results will justify only limited and/or restrictive uses, including the inclusion of warnings and contraindications, which could significantly limit the marketability and profitability of the therapeutic candidate. |
| 33 |
Any delay in obtaining, or the failure to obtain, required regulatory approvals will materially and adversely affect our ability to generate future revenues from a particular product candidate. Any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product or may impose restrictive conditions of use, including cautionary information, thereby limiting the size of the market for the product. We and our licensees, as applicable, also are, and will be, subject to numerous foreign regulatory requirements that govern the conduct of clinical trials, manufacturing and marketing authorization, pricing and third-party reimbursement. The foreign regulatory approval process includes all of the risks associated with the FDA approval process that we describe above, as well as risks attributable to the satisfaction of foreign requirements. Approval by the FDA does not ensure approval by regulatory authorities outside the United States. Foreign jurisdictions may have different approval processes than those required by the FDA and may impose additional testing requirements for our therapeutic candidates.
If the third parties on which we rely to conduct our clinical trials and clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval for, or commercialize, our product candidates
We do not have the ability to independently conduct our clinical trials for our product candidates and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct such trials. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance for, or successfully commercialize, our product candidates on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
Modifications to our product candidates, or to any other product candidates that we may develop in the future, may require new regulatory clearances or approvals or may require us or our licensees, as applicable, to recall or cease marketing these therapeutic candidates until clearances are obtained
Modifications to our product candidates, after they have been approved for marketing, if at all, or to any other pharmaceutical product that we may develop in the future, may require new regulatory clearance, or approvals, and, if necessitated by a problem with a marketed product, may result in the recall or suspension of marketing of the previously approved and marketed product until clearances or approvals of the modified product are obtained. The FDA requires pharmaceutical products manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance. A manufacturer may determine in conformity with applicable regulations and guidelines that a modification may be implemented without pre-clearance by the FDA; however, the FDA can review a manufacturer’s decision and may disagree. The FDA may also on its own initiative determine that a new clearance or approval is required. If the FDA requires new clearances or approvals of any pharmaceutical product or medical device for which we or our licensees receive marketing approval, if any, we or our licensees may be required to recall such product and to stop marketing the product as modified, which could require us or our licensees to redesign the product and will have a material adverse effect on our business, financial condition and results of operations. In these circumstances, we may be subject to significant enforcement actions.
| 34 |
The results of our clinical trials may not support our product claims or may result in the discovery of adverse side effects
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our product claims or that any regulatory authority whose approval we will require in order to market and sell our products in any territory will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that clinical trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our product candidates are safe and effective for the proposed indicated uses, which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our regulatory submissions and, ultimately, our ability to commercialize our product candidates and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
Clinical trials involve a lengthy and expensive process with an uncertain outcome, and results of earlier studies and trials may not be predictive of future trial results
We have limited experience in conducting and managing the clinical trials necessary to obtain regulatory approvals, including FDA approval. Clinical trials are expensive and complex, can take many years and have uncertain outcomes. We cannot predict whether we or our licensees will encounter problems with any of the completed, ongoing or planned clinical trials that will cause us, our licensees or regulatory authorities to delay or suspend clinical trials, or delay the analysis of data from completed or ongoing clinical trials. We estimate that clinical trials of our most advanced therapeutic candidates will continue for several years, but they may take significantly longer to complete. Failure can occur at any stage of the testing and we may experience numerous unforeseen events during, or as a result of, the clinical trial process that could delay or prevent commercialization of our current or future therapeutic candidates, including but not limited to:
| ● | delays in securing clinical investigators or trial sites for the clinical trials; | |
| ● | delays in obtaining institutional review board and other regulatory approvals to commence a clinical trial; | |
| ● | slower than anticipated patient recruitment and enrollment; | |
| ● | negative or inconclusive results from clinical trials; | |
| ● | unforeseen safety issues; | |
| ● | uncertain dosing issues; | |
| ● | an inability to monitor patients adequately during or after treatment; and | |
| ● | problems with investigator or patient compliance with the trial protocols. |
A number of companies in the pharmaceutical and biotechnology industries, including those with greater resources and experience than us, have suffered significant setbacks in advanced clinical trials, even after seeing promising results in earlier clinical trials. Despite the results reported in earlier clinical trials for our therapeutic candidates, we do not know whether any phase 3 or other clinical trials we or our licensees may conduct will demonstrate adequate efficacy and safety to result in regulatory approval to market our therapeutic candidates. If later-stage clinical trials of any therapeutic candidate do not produce favorable results, our ability to obtain regulatory approval for the therapeutic candidate may be adversely impacted, which will have a material adverse effect on our business, financial condition and results of operations.
| 35 |
The pharmaceutical business is subject to increasing government price controls and other restrictions on pricing, reimbursement and access to drugs, which could adversely affect our future revenues and profitability
To the extent our products are developed, commercialized, and successfully introduced to market, they may not be considered cost-effective and third-party or government reimbursement might not be available or sufficient. Globally, governmental and other third-party payors are becoming increasingly aggressive in attempting to contain health care costs by strictly controlling, directly or indirectly, pricing and reimbursement and, in some cases, limiting or denying coverage altogether on the basis of a variety of justifications, and we expect pressures on pricing and reimbursement from both governments and private payors inside and outside the U.S. to continue.
In the U.S., we are subject to substantial pricing, reimbursement, and access pressures from state Medicaid programs, private insurance programs and pharmacy benefit managers, and implementation of U.S. health care reform legislation is increasing these pricing pressures. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the “PPACA” or the “Affordable Care Act”), instituted comprehensive health care reform, and includes provisions that, among other things, reduce and/or limit Medicare reimbursement, require all individuals to have health insurance (with limited exceptions), and impose new and/or increased taxes. The future of the Affordable Care Act and its constituent parts are uncertain at this time.
In almost all markets, pricing and choice of prescription pharmaceuticals are subject to governmental control. Therefore, the price of our products and their reimbursement in Europe and in other countries is and will be determined by national regulatory authorities. Reimbursement decisions from one or more of the European markets may impact reimbursement decisions in other European markets. A variety of factors are considered in making reimbursement decisions, including whether there is sufficient evidence to show that treatment with the product is more effective than current treatments, that the product represents good value for money for the health service it provides, and that treatment with the product works at least as well as currently available treatments.
The continuing efforts of government and insurance companies, health maintenance organizations, and other payors of health care costs to contain or reduce costs of health care may affect our future revenues and profitability or those of our potential customers, suppliers, and collaborative partners, as well as the availability of capital.
United States federal and state privacy laws, and equivalent laws of other nations, may increase our costs of operation and expose us to civil and criminal sanctions
The Health Insurance Portability and Accountability Act of 1996, as amended, and the regulations that have been issued under it, or collectively HIPAA, and similar laws outside the United States, contain substantial restrictions and requirements with respect to the use and disclosure of individuals’ protected health information. The HIPAA privacy rules prohibit “covered entities,” such as healthcare providers and health plans, from using or disclosing an individual’s protected health information, unless the use or disclosure is authorized by the individual or is specifically required or permitted under the privacy rules. Under the HIPAA security rules, covered entities must establish administrative, physical and technical safeguards to protect the confidentiality, integrity and availability of electronic protected health information maintained or transmitted by them or by others on their behalf. While we do not believe that we will be a covered entity under HIPAA, we believe many of our customers will be covered entities subject to HIPAA. Such customers may require us to enter into business associate agreements, which will obligate us to safeguard certain health information we obtain in the course of our relationship with them, restrict the manner in which we use and disclose such information and impose liability on us for failure to meet our contractual obligations.
In addition, under The Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, which was signed into law as part of the U.S. stimulus package in February 2009, certain of HIPAA’s privacy and security requirements are now also directly applicable to “business associates” of covered entities and subject them to direct governmental enforcement for failure to comply with these requirements. We may be deemed as a “business associate” of some of our customers. As a result, we may be subject as a “business associate” to civil and criminal penalties for failure to comply with applicable privacy and security rule requirements. Moreover, HITECH created a new requirement obligating “business associates” to report any breach of unsecured, individually identifiable health information to their covered entity customers and imposes penalties for failing to do so.
| 36 |
In addition to HIPAA, most U.S. states have enacted patient confidentiality laws that protect against the disclosure of confidential medical information, and many U.S. states have adopted or are considering adopting further legislation in this area, including privacy safeguards, security standards, and data security breach notification requirements. These U.S. state laws, which may be even more stringent than the HIPAA requirements, are not preempted by the federal requirements, and we are therefore required to comply with them to the extent they are applicable to our operations.
These and other possible changes to HIPAA or other U.S. federal or state laws or regulations, or comparable laws and regulations in countries where we conduct business, could affect our business and the costs of compliance could be significant. Failure by us to comply with any of the standards regarding patient privacy, identity theft prevention and detection, and data security may subject us to penalties, including civil monetary penalties and in some circumstances, criminal penalties. In addition, such failure may damage our reputation and adversely affect our ability to retain customers and attract new customers.
The protection of personal data, particularly patient data, is subject to strict laws and regulations in many countries. The collection and use of personal health data in the EU is governed by the provisions of Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data, commonly known as the Data Protection Directive. The Directive imposes a number of requirements including an obligation to seek the consent of individuals to whom the personal data relates, the information that must be provided to the individuals, notification of data processing obligations to the competent national data protection authorities of individual EU Member States and the security and confidentiality of the personal data. The Data Protection Directive also imposes strict rules on the transfer of personal data out of the EU to the U.S. Failure to comply with the requirements of the Data Protection Directive and the related national data protection laws of the EU Member States may result in fines and other administrative penalties and harm our business. We may incur extensive costs in ensuring compliance with these laws and regulations, particularly if we are considered to be a data controller within the meaning of the Data Protection Directive.
If we fail to comply with the U.S. federal Anti-Kickback Statute and similar state and foreign country laws, we could be subject to criminal and civil penalties and exclusion from federally funded healthcare programs including the Medicare and Medicaid programs and equivalent third country programs, which would have a material adverse effect on our business and results of operations
A provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration, directly or indirectly, in cash or in kind, to induce or reward the referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable, in whole or in part, by Medicare, Medicaid or any other federal healthcare program. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor may be subject to scrutiny. The federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states have adopted laws similar to the federal Anti-Kickback Statute, and some of these laws are even broader than the federal Anti-Kickback Statute in that their prohibitions may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
All of our future financial relationships with U.S. healthcare providers, purchasers, formulary managers, and others who provide products or services to federal healthcare program beneficiaries will potentially be governed by the federal Anti-Kickback Statute and similar state laws. We believe our operations will be in compliance with the federal Anti-Kickback Statute and similar state laws. However, we cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
| 37 |
There are other federal and state laws that may affect our ability to operate, including the federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. Moreover, we may be subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs. Moreover, there are analogous state laws. Violations of these laws can result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
Moreover, the provisions of the Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more aggressive and frequent investigations and enforcement by both the SEC and the Department of Justice. A determination that our operations or activities violated U.S. or foreign laws or regulations could result in imposition of substantial fines, interruption of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. In addition, lawsuits brought by private litigants may also follow as a consequence.
Risks Related to Our Securities and this Offering
An active trading market for our securities may not develop and our securityholders may not be able to resell their common shares or warrants
Although our common shares are quoted on the OTCQB and listed on the TSXV, an active trading market for our common shares has not developed. We are in the process of applying to have our common shares and warrants listed on the Nasdaq Capital Market but an active trading market for our shares or warrants may never develop or be sustained following this offering. We cannot predict the extent to which an active market for our common shares or warrants will develop or be sustained after the listing of such securities on Nasdaq. If an active trading market for our common shares or warrants does not develop after this offering, the market price and liquidity of our common shares or warrants may be materially and adversely affected.
The warrants may not have any value
The warrants will be exercisable for five years from the date of initial issuance at an initial exercise price equal to 125% of the public offering price per unit set forth on the cover page of this prospectus. There can be no assurance that the market price of the common shares will ever equal or exceed the exercise price of the warrants. In the event that the share price of our common shares does not exceed the exercise price of the warrants during the period when the warrants are exercisable, the warrants may not have any value.
A warrant does not entitle the holder to any rights as common stockholders until the holder exercises the warrant for one common share
Until you acquire shares upon exercise of your warrants, the warrants will not provide you any rights as a common stockholder. Upon exercise of your warrants, you will be entitled to exercise the rights of a common stockholder only as to matters for which the record date occurs after the exercise date.
| 38 |
Future issuance of our common shares could dilute the interests of existing shareholders
We may issue additional common shares in the future. The issuance of a substantial number of common shares could have the effect of substantially diluting the interests of our shareholders. In addition, the sale of a substantial amount of common shares in the public market, in the initial issuance, in a situation in which we acquire a company and the acquired company receives common shares as consideration and the acquired company subsequently sells its common shares, or by investors who acquired such common shares in a private placement, could have an adverse effect on the market price of our Common shares.
We have a significant number of options and warrants outstanding, and while these options and warrants are outstanding, it may be more difficult to raise additional equity capital
As of July 31, 2019, we had outstanding options and warrants to purchase 8,722,600 and 72,543,829 common shares, respectively. In addition, we had convertible debentures in the amount of $448,803 that convert into 4,480,030 common shares and an additional 4,480,030 warrants. The holders of these options and warrants are given the opportunity to profit from a rise in the market price of our common shares. We may find it more difficult to raise additional equity capital while these options and warrants are outstanding. At any time during which these warrants are likely to be exercised, we may be unable to obtain additional equity capital on more favorable terms from other sources. Additionally, the exercise of these options and warrants will cause the increase of our outstanding Common shares, which could have the effect of substantially diluting the interests of our current shareholders.
Sales of a substantial number of shares of our common shares in the public market by our existing shareholders could cause our share price to fall
Sales of a substantial number of shares of our common shares in the public market, or the perception that these sales might occur, could depress the market price of our common shares and could impair our ability to raise capital through the sale of additional equity securities. We are unable to predict the effect that sales may have on the prevailing market price of our common shares. All of the shares owned by our directors and officers are subject to lock-up agreements with the underwriters of this offering that restrict such shareholders’ ability to transfer our common shares for at least six months from the date of this offering. All of our outstanding shares held by our directors and officers will become eligible for unrestricted sale upon expiration of the lockup period. In addition, shares issued or issuable upon exercise of options and warrants vested as of the expiration of the lock-up period will be eligible for sale at that time. Sales of shares by these shareholders could have a material adverse effect on the trading price of our common shares. We intend to register the offering, issuance, and sale of all common shares that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements.
We are an Emerging Growth Company, which may reduce the amount of information available to investors
The Jumpstart Our Business Start-ups Act, or the JOBS Act, and our status as a foreign private issuer will allow us to postpone the date by which we must comply with some of the laws and regulations intended to protect investors and to reduce the amount of information we provide in our reports filed with the SEC, which could undermine investor confidence in our company and adversely affect the market price of our Common shares.
For as long as we remain an “emerging growth company” as defined in the JOBS Act, we intend to take advantage of certain exemptions from various requirements that are applicable to public companies that are not emerging growth companies including:
| ● | the provisions of the Sarbanes-Oxley Act requiring that our independent registered public accounting firm provide an attestation report on the effectiveness of our internal control over financial reporting; | |
| ● | Section 107 of the JOBS Act, which provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. This means that an “emerging growth company” can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We may elect to delay such adoption of new or revised accounting standards. As a result of this adoption, our financial statements may not be comparable to companies that comply with the public company effective date; and | |
| ● | any rules that may be adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report on the financial statements. |
| 39 |
We intend to take advantage of these exemptions until we are no longer an “emerging growth company.” We will remain an emerging growth company until the earlier of (1) the last day of the fiscal year of the fifth anniversary of the consummation of this offering, (b) in which we have total annual gross revenue of at least $US 1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our Common shares that is held by non-affiliates exceeds $US 700 million as of the prior June 30, and (2) the date on which we have issued more than $US 1.0 billion in non-convertible debt during the prior three-year period.
We cannot predict if investors will find our common shares or warrants less attractive because we may rely on these exemptions. If some investors find our common shares or warrants less attractive as a result, there may be a less active trading market for our common shares or warrants, and our common share or warrant price may be more volatile and may decline.
We are a foreign private issuer and, as a result, we are not subject to U.S. proxy rules and are subject to reporting obligations that, to some extent, are more lenient and less frequent than those applicable to a U.S. issuer
Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. publicly reporting companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act, (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time, and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. In addition, while U.S. domestic issuers that are not large accelerated filers or accelerated filers are required to file their annual reports on Form 10-K within 90 days after the end of each fiscal year, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information.
We have never paid cash dividends on our capital stock and we do not anticipate paying any dividends in the foreseeable future. Consequently, any gains from an investment in our common shares will likely depend on whether the price of our Common shares increases, which may not occur
We have not paid cash dividends on any capital stock to date and we currently intend to retain our future earnings, if any, to fund the development and growth of our business. Consequently, in the foreseeable future, you will likely only experience a gain from your investment in our Common shares if the price of our Common shares increases beyond the price in which you originally acquired the Common shares.
In the event a market develops for our common shares or warrants, the market price of our common shares or warrants may be volatile
In the event a market develops for our common shares or warrants, the market price of our common shares or warrants may be highly volatile. Some of the factors that may materially affect the market price of our common shares or warrants are beyond our control, such as changes in financial estimates by industry and securities analysts, conditions or trends in the industry in which we operate or sales of our common shares or warrants. These factors may materially adversely affect the market price of our common shares or warrants, regardless of our performance. In addition, the public stock markets have experienced extreme price and trading volume volatility. This volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the operating performance of the specific companies. These broad market fluctuations may adversely affect the market price of our Common shares.
| 40 |
Our executive officers, directors and principal shareholders will maintain the ability to exert significant control over matters submitted to our shareholders for approval
Assuming the sale by us of ________ common shares in this offering (or _______ shares if the underwriters exercise their option to purchase additional shares in full)], our executive officers, directors and principal shareholders who owned more than 5% of our outstanding common shares before this offering will, in the aggregate, beneficially own shares representing approximately __% (or __% if the underwriters exercise their option to purchase additional shares in full)] of our share capital following the completion of this offering. As a result, if these shareholders were to act together, they would be able to control all matters submitted to our shareholders for approval, as well as our management and affairs. For example, these persons, if they act together, would control the election of directors and approval of any merger, consolidation or sale of all or substantially all of our assets. This concentration of voting power could delay or prevent an acquisition of our company on terms that other shareholders may desire or result in management of our company that our public shareholders disagree with.
If you purchase our common shares in this offering, you will incur immediate and substantial dilution in the book value of your shares
The public offering price of our common shares will be substantially higher than the net tangible book value per share of our common shares. Therefore, if you purchase common shares in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. To the extent outstanding options and warrants are exercised, you will incur further dilution. Based on the public offering price of $_____ per share, you will experience immediate dilution of $_____ per share, representing the difference between our as adjusted net tangible book value per share after giving effect to this offering at the assumed initial public offering price. In addition, purchasers of common shares in this offering will have contributed approximately __% of the aggregate price paid by all purchasers of our shares but will own only approximately __% of our common shares outstanding after this offering.
Our management will have broad discretion in the use of the net proceeds from this offering and may allocate the net proceeds from this offering in ways that you and other shareholders may not approve
Our management will have broad discretion in the use of the net proceeds, including for any of the purposes described in the section titled “Use of Proceeds,” and you will not have the opportunity as part of your investment decision to assess whether the net proceeds are being used appropriately. Because of the number and variability of factors that will determine our use of the net proceeds from this offering, their ultimate use may vary substantially from their currently intended use. The failure of our management to use these funds effectively could harm our business. Pending their use, we may invest the net proceeds from this offering in short-term, investment-grade, interest-bearing securities and depositary institutions. These investments may not yield a favorable return to our shareholders.
If we are or become classified as a passive foreign investment company, our U.S. shareholders may suffer adverse tax consequences as a result
Generally, for any taxable year, if at least 75% of our gross income is passive income, or at least 50% of the value of our assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income (including amounts derived by reason of the temporary investment of funds raised in offerings of our shares) and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. If we are characterized as a PFIC, our U.S. shareholders may suffer adverse tax consequences, including having gains realized on the sale of our common shares treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends received on our common shares by individuals who are U.S. holders, and having interest charges apply to distributions by us and gains from the sales of our shares.
| 41 |
Our status as a PFIC will depend on the nature and composition of our income and the nature, composition and value of our assets (which, assuming we are not a “controlled foreign corporation,” or a CFC, under Section 957(a) of the Internal Revenue Code of 1986, as amended, or the Code, for the year being tested, may be determined based on the fair market value of each asset, with the value of goodwill and going concern value determined in large part by reference to the market value of our common shares, which may be volatile). Our status may also depend, in part, on how quickly we utilize the cash proceeds from this offering in our business. Based upon the value of our assets, including any goodwill, and the nature and composition of our income and assets, we do not believe that we were classified as a PFIC for the taxable year ended July 31, 2018 and we do not believe that we will be classified as a PFIC for the taxable year ending July 31, 2019 or in the immediately foreseeable future. Because the determination of whether we are a PFIC for any taxable year is a factual determination made annually after the end of each taxable year, there can be no assurance that we will not be considered a PFIC in any taxable year. [Accordingly, our legal counsel expresses no opinion with respect to our PFIC status for our taxable year ended July 31, 2018, and also expresses no opinion with regard to our expectations regarding our PFIC status in the future.]
The tax consequences that would apply if we are classified as a PFIC would also be different from those described above if a U.S. shareholder were able to make a valid qualified electing fund, or QEF, election. At this time, we do not expect to provide U.S. shareholders with the information necessary for a U.S. shareholder to make a QEF election. Prospective investors should assume that a QEF election will not be available.
If securities or industry analysts do not publish or cease publishing research or reports about us, our business or our market, or if they adversely change their recommendations or publish negative reports regarding our business or our shares, our share price and trading volume could decline
The trading market for our securities will be influenced by the research and reports that industry or securities analysts may publish about us, our business, our market or our competitors. We do not have any control over these analysts and we cannot provide any assurance that analysts will cover us or provide favorable coverage. If any of the analysts who may cover us adversely change their recommendation regarding our shares, or provide more favorable relative recommendations about our competitors, the market value of our securities would likely decline. If any analyst who may cover us were to cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which in turn could cause the price of our common shares and warrants and our trading volume to decline.
Certain Canadian legislation contain provisions that may have the effect of delaying or preventing a change in control
Canadian legislation could discourage potential acquisition proposals, delay or prevent a change in control and limit the price that certain investors may be willing to pay for our subordinate voting shares. For instance, a non-Canadian must file an application for review with the Minister responsible for the Investment Canada Act and obtain approval of the Minister prior to acquiring control of a “Canadian business” within the meaning of the Investment Canada Act, where prescribed financial thresholds are exceeded. Furthermore, limitations on the ability to acquire and hold our subordinate voting shares and multiple voting shares may be imposed by the Competition Act (Canada). This legislation permits the Commissioner of Competition, or Commissioner, to review any acquisition or establishment, directly or indirectly, including through the acquisition of shares, of control over or of a significant interest in us. Otherwise, there are no limitations either under the laws of Canada or British Columbia, or in our articles on the rights of non-Canadians to hold or vote our subordinate voting shares and multiple voting shares. Any of these provisions may discourage a potential acquirer from proposing or completing a transaction that may have otherwise presented a premium to our shareholders. See “Description of Securities—Certain Important Provisions of Our Articles and the BCBCA.”
Because we are a corporation incorporated in British Columbia and some of our directors and officers are resident in Canada, it may be difficult for investors in the United States to enforce civil liabilities against us based solely upon the federal securities laws of the United States. Similarly, it may be difficult for Canadian investors to enforce civil liabilities against our directors and officers residing outside of Canada
We are a corporation incorporated under the laws of British Columbia with our principal place of business in West Vancouver. Some of our directors and officers and the auditors or other experts named herein are residents of Canada and all or a substantial portion of our assets and those of such persons are located outside the United States. Consequently, it may be difficult for U.S. investors to effect service of process within the United States upon us or our directors or officers or such auditors who are not residents of the United States, or to realize in the United States upon judgments of courts of the United States predicated upon civil liabilities under the Securities Act. Investors should not assume that Canadian courts: (1) would enforce judgments of U.S. courts obtained in actions against us or such persons predicated upon the civil liability provisions of the U.S. federal securities laws or the securities or blue sky laws of any state within the United States or (2) would enforce, in original actions, liabilities against us or such persons predicated upon the U.S. federal securities laws or any such state securities or blue sky laws.
| 42 |
Similarly, some of our directors and officers are residents of countries other than Canada and all or a substantial portion of the assets of such persons are located outside Canada. As a result, it may be difficult for Canadian investors to initiate a lawsuit within Canada against these non-Canadian residents. In addition, it may not be possible for Canadian investors to collect from these non-Canadian residents judgments obtained in courts in Canada predicated on the civil liability provisions of securities legislation of certain of the provinces and territories of Canada. It may also be difficult for Canadian investors to succeed in a lawsuit in the United States, based solely on violations of Canadian securities laws.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Some discussions in this prospectus may contain forward-looking statements that involve risks and uncertainties. These statements relate to future events or future financial performance. A number of important factors could cause our actual results to differ materially from those expressed in or implied by any forward-looking statements made by us in this prospectus. Forward-looking statements are often identified by words like: “believe,” “expect,” “estimate,” “anticipate,” “intend,” “project,” “may,” “will,” “should,” “plans,” “predicts,” “potential” or “continue” or the negative of these terms or other comparable terminology. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the section titled “Risk Factors” beginning on page 11, that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. In addition, you are directed to factors discussed in the “Description of Business” section beginning on page 39, the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section beginning on page 34, as well as those discussed elsewhere in this prospectus.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, or achievements. Except as required by applicable law, including the securities laws of the United States and Canada, we do not intend to update any of the forward-looking statements to conform these statements to actual results.
| 43 |
We estimate that the net proceeds from the sale of common shares and warrants in this offering will be approximately $_____ million, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, based on the public offering price of $_____ per common share. If the underwriters exercise their overallotment option in full, we estimate that the net proceeds to us from this offering will be approximately $ ____ million, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
Assuming net proceeds of $8,750,000, we intend to use $4,000,000 of the net proceeds from this offering for clinical trials of Bria-IMT™, $1,200,000 for initial clinical trials of Bria-OTS™, with the remainder for working capital and general corporate purposes.
We would receive additional gross proceeds of _____________ if all of the warrants included in the units are exercised, assuming no exercise of the underwriters’ over-allotment option. We intend to use any such proceeds for working capital and general corporate purposes. General corporate purposes may include capital expenditures.
We may also use a portion of the net proceeds from this offering to acquire or invest in complementary products, technologies or businesses, although we have no present agreements or commitments to do so.
Although we currently anticipate that we will use the net proceeds from this offering as described above, there may be circumstances where a reallocation of funds is necessary. Due to the uncertainties inherent in the clinical development and regulatory approval process, it is difficult to estimate with certainty the exact amounts of the net proceeds from this offering that may be used for any of the above purposes on a stand-alone basis. Amounts and timing of our actual expenditures will depend upon a number of factors, including our sales, marketing and commercialization efforts, regulatory approval and demand for our product candidates, operating costs and other factors described under “Risk Factors” in this prospectus. Accordingly, our management will have flexibility in applying the net proceeds from this offering. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions on how to use the proceeds.
| 44 |
If you invest in our units in this offering, your interest will be immediately diluted to the extent of the difference between the public offering price per common share in this offering and the as further adjusted net tangible book value per common share after this offering. Dilution results from the fact that the public offering price per common share is substantially in excess of the net tangible book value per common share. As of April 30, 2019, we had a historical net tangible book value of $0.521984 million, or $0.003 per common share. Our net tangible book value per share represents total tangible assets less total liabilities, divided by the number of common shares outstanding on April 30, 2019.
After giving effect to the sale of units in this offering at the public offering price of $___ per common share in each unit, after deducting the estimated underwriting discounts and commissions and estimated offering expenses, and assuming no value is attributed to the warrants, our as adjusted net tangible book value at April 30, 2019 would have been $ ____ per share. This represents an immediate increase in as adjusted net tangible book value of $ ____ per share to existing shareholders and immediate dilution of $____ per share to new investors.
The following table illustrates this dilution per common share:
| Public offering price per common share | $ | |||||||
| Historical net tangible book value per common share as of April 30, 2019 | $ | 0.003 | ||||||
| Increase in as adjusted net tangible book value per common share attributable to new investors | $ | |||||||
| As adjusted net tangible book value per common share after this offering | $ | |||||||
| Dilution per common share to new investors participating in this offering | $ |
If the underwriters exercise in full their option to purchase additional common shares, the as adjusted net tangible book value will increase to $____per common share, representing an immediate increase in as adjusted net tangible book value to existing shareholders of $____ per common share and an immediate dilution of $____ per common share to new investors participating in this offering.
We may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our equity holders.
The above discussion and tables are based on 196,378,450 shares of common stock outstanding as of April 30, 2019 and excludes the following:
| ● | 63,079,759 shares of common stock issuable upon the exercise of outstanding warrants, at a weighted average exercise price of $0.175; | |
| ● | 4,732,035 shares of common stock issuable upon the exercise of outstanding warrants, at a weighted average exercise price of $0.151; | |
| ● | 8,722,600 shares of common stock issuable upon the exercise of outstanding options, at a weighted average exercise price of $0.178; | |
| ● | 4,480,030 shares of common stock and warrants exercisable into an additional 4,480,030 shares of common stock, both underlying convertible debentures in the aggregate amount of $448,803; and | |
| ● | an estimated ____________ common shares issuable upon exercise of the warrants included in the units (or ____ common shares if the underwriters exercise their over-allotment option in full with respect to the warrants contained in the units). |
We do not anticipate that we will declare or pay dividends in the foreseeable future on our common shares. Instead, we anticipate that all of our earnings will be used for the operation and growth of our business. Any future determination to declare cash dividends would be subject to the discretion of our board of directors and would depend upon various factors, including our results of operations, financial condition and liquidity requirements, restrictions that may be imposed by applicable law and our contracts and other factors deemed relevant by our board of directors.
| 45 |
The following table sets forth our consolidated capitalization as of April 30, 2019:
| - | on an actual basis, as determined in accordance with IFRS; and | |
| - | on an as-adjusted basis to reflect the net proceeds from our sale of ____units in this offering at the public offering price of $ ____per unit, after deducting the underwriting discounts and commissions and the estimated offering expenses. |
This table should be read in conjunction with the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Use of Proceeds” sections, as well as our audited financial statements, included elsewhere in this prospectus. The following table assumes no exercise by the underwriters of the overallotment option to purchase additional common shares in this offering.
| As of April 30, 2019 | ||||||||
| Actual | Pro Forma(1) | |||||||
| Cash, cash equivalents, and short term investments | $ | 938,932 | $ | ● | ||||
| Unsecured convertible loan | $ | 419,123 | ● | |||||
| Stockholders’ equity: | ||||||||
| common stock, no par value; unlimited number of shares authorized, 196,378,450 shares issued and outstanding, actual; unlimited number of shares authorized, ● shares issued and outstanding, pro forma; | 13,867,219 | ● | ||||||
| Share based payment reserve | 1,117,260 | ● | ||||||
| Warrant reserve | 2,913,722 | |||||||
| Accumulated other comprehensive loss | (96,925 | ) | ● | |||||
| Retained deficit | (16,935,391 | ) | ● | |||||
| Total stockholders’ equity | 865,885 | ● | ||||||
| Total capitalization | $ | 865,885 | $ | ● | ||||
| (1) | The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual initial public offering price and other terms of our initial public offering determined at pricing. |
Holders
We had 54 holders of record for our common shares as of July 31, 2019.
| 46 |
ENFORCEMENT OF CIVIL LIABILITIES
We are incorporated under the laws of British Columbia. Some of our directors and officers, and some of the experts named in this prospectus, are residents of Canada or otherwise reside outside of the United States, and all or a substantial portion of their assets, and all or a substantial portion of our assets, are located outside of the United States. We have appointed an agent for service of process in the United States, but it may be difficult for shareholders who reside in the United States to effect service within the United States upon those directors, officers and experts who are not residents of the United States. It may also be difficult for shareholders who reside in the United States to realize in the United States upon judgments of courts of the United States predicated upon our civil liability and the civil liability of our directors, officers and experts under the United States federal securities laws. There can be no assurance that U.S. investors will be able to enforce against us, members of our board of directors, officers or certain experts named herein who are residents of Canada or other countries outside the United States, any judgments in civil and commercial matters, including judgments under the federal securities laws.
| 47 |
SELECTED CONSOLIDATED FINANCIAL DATA
The selected financial information set forth below has been derived from our audited financial statements for the fiscal years ended July 31, 2018, 2017, 2016, 2015 and 2014 and from our unaudited financial statements for the nine months ended April 30, 2019. You should read the following summary financial data for the years ended July 31, 2018 and 2017 and the nine months ended April 30, 2019 together with our historical financial statements and the notes thereto included elsewhere in this prospectus and with the information set forth in the section titled “Management’s Discussion and Analysis of Financial Conditions and Results of Operations”. The audited financial statements for the years ended July 31, 2016, 2015 and 2014 are not included in this prospectus.
| As of April 30, | As of July 31, | |||||||||||||||
| 2019 | 2018 | 2017 | 2016 | |||||||||||||
| Balance Sheet Data | ||||||||||||||||
| Cash and cash equivalents | 938,932 | 938,448 | 1,264,429 | 171,865 | ||||||||||||
| Total Assets | 1,613,299 | 2,977,140 | 2,039,199 | 1,091,587 | ||||||||||||
| Total Liabilities | 747,414 | 1,745,850 | 1,104,147 | 63,470 | ||||||||||||
| Total Shareholders’ Equity (deficit) | 865,885 | 1,231,290 | 935,052 | 1,028,117 | ||||||||||||
| 48 |
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our financial statements and related notes included elsewhere in this prospectus. This discussion and other parts of this prospectus contain forward-looking statements based upon current expectations that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of several factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
The preparation of financial statements in conformity with these accounting principles requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of contingent liabilities at the financial statement date and reported amounts of revenue and expenses during the reporting period. On an on-going basis, we review our estimates and assumptions. The estimates were based on historical experience and other assumptions that we believe to be reasonable under the circumstances. Actual results are likely to differ from those estimates or other forward-looking statements under different assumptions or conditions, but we do not believe such differences will materially affect our financial position or results of operations. Our actual results may differ materially as a result of many factors, including those set forth under the headings entitled “Special Note Regarding Forward-Looking Statements” and “Risk Factors”.
Recent Developments
Conversion of Certain Convertible Notes
During the nine months ended April 30, 2019, 6,746,458 shares were issued at $0.10 per share in respect of the partial conversion of certain Convertible Notes. Upon exercise of these Convertible Notes, Noteholders received 6,746,458 warrants with an exercise price of $0.14, expiring within three years. On April 23, 2019, the Company revised the exercise price of these warrants from $0.14 to $0.12, and all future warrants to be issued in respect of the conversion of the balance of the Convertible Notes.
Exercise of Warrants
During the nine months ended April 30, 2019, 1,000,000 shares were issued in respect of 1,000,000 warrants that were exercised at an exercise price of $0.14 for gross proceeds of $140,000.
Private Placement
On February 26, 2019, BriaCell announced a non-brokered private placement financing of 5,000,000 common shares of the Company at a price of C$0.10 per common share for gross proceeds of C$500,000. Recently-appointed Director of the Company, Jamieson Bondarenko, purchased the 5,000,000 common shares. Upon closing of the offering, Mr. Bondarenko had a beneficial ownership of an aggregate of 23,070,500 common shares, representing approximately 13.7% of BriaCell’s issued and outstanding common shares.
On April 1, 2019, BriaCell announced that it completed a non-brokered private placement of 29,735,240 common shares of the Company at a price of C$0.10 per common share for gross proceeds of C$2,973,524 (the “Private Placement”) which includes Mr. Bondarenko’s C$500,000 equity investment.
In August 2019, the Company entered into agreements for a private placement of 5,714,730shares of common stock at C$0.07 per share to several of its board members for gross proceeds of approximately C$400,000. The financing is expected to close in September 2019 subject to obtaining consent of the Company’s note holders.
New Board Composition
In February and March 2019, the Company’s Board of Directors was substantially restructured with the appointment of Jamieson Bondarenko, Dr. Rebecca Taub and Vaughn C. Embro-Pantalony to replace three resigning directors. Additionally, on August 12, 2019, Richard Berman was appointed to our Board of Directors. After these restructuring events, the current Board of Directors consists of:
| ● | Dr. William V. Williams, Director and Chief Executive Officer; | |
| ● | Jamieson Bondarenko, Director and Chairman of the Board; | |
| ● | Dr. Charles Wiseman, Director; | |
| ● | Dr. Rebecca Taub, Director | |
| ● | Vaughn C. Embro-Pantalony, Director; and | |
| ● | Richard Berman, Director |
Overview
Critical Accounting Policies and Estimates
| 1. | Critical Estimates and Judgements |
The preparation of these consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and reported amounts of expenses during the reporting period. Actual outcomes could differ from these estimates. The financial statements include estimates which, by their nature, are uncertain. The impacts of such estimates are pervasive throughout the financial statements, and may require accounting adjustments based on future occurrences. Revisions to accounting estimates are recognized in the period in which the estimate is revised and also in future periods when the revision affects both current and future periods.
The critical judgments and significant estimates in applying accounting policies that have the most significant effect on the amounts recognized in the consolidated financial statements are:
| ● | The series of loans made to the subsidiary company are considered part of the parent company’s net investment in a foreign operation as the Company does not plan to settle these balances in the foreseeable future. As a result of this assessment, the unrealized foreign exchange gains and losses on the intercompany loans are recorded through comprehensive loss. If the Company determined that settlement of these amounts was planned or likely in the foreseeable future, the resultant foreign exchange gains and losses would be recorded through profit or loss. | |
| ● | The determination that the unrealized decrease in the fair value of available for sale investments is other than temporary. | |
| ● | The fair value of the share consideration deemed issued to acquire BriaCell. |
| 2. | New Accounting Policies Adopted |
During the nine month period ended April 30, 2019, the following new accounting policies were adopted.
IFRS 9 – Financial instruments (“IFRS 9”) was issued by the IASB its final form in July 2014 and will replace IAS 39 Financial Instruments: Recognition and Measurement (“IAS 39”). IFRS 9 uses a single approach to determine whether a financial asset is measured at amortized cost or fair value, replacing the multiple rules in IAS 39. The approach in IFRS 9 is based on how an entity manages its financial instruments in the context of its business model and the contractual cash flow characteristics of the financial assets. Most of the requirements in IAS 39 for classification and measurement of financial liabilities were carried forward unchanged to IFRS 9. The new standard also requires a single impairment method to be used, replacing the multiple impairment methods in IAS39. The standard is effective for annual periods beginning on or after January 1, 2018. Management adopted the standard as August 1, 2018 and assed that the adoption of IFRS 9 did not have a significant impact to the consolidated financial statements.
| 49 |
IFRS 15 - Revenue from contracts with customers (“IFRS 15”) proposes to replace IAS 18 – Revenue, IAS 11 – Construction contracts and some revenue-related interpretations. The standard contains a single model that applies to contracts with customers and two approaches to recognizing revenue: at a point in time or over time. The model features a contract-based five step analysis of transaction to determine whether, how much and when revenue is recognized. New estimates and judgemental thresholds have been introduced, which may affect the amount and/or timing of revenue recognized. IFRS 15 is effective for annual periods beginning on or after January 1, 2018. Earlier adoption is permitted. The Company adopted the standard as of August 1, 2019. Since the has not yet generated any revenues, the Company was no impact to the consolidated financial statements as a result of the adoption of this standard.
| 3. | Accounting Standards Issued but Not Yet Effective |
Certain pronouncements were issued by the IASB or the IFRIC that are mandatory for future accounting periods. Many are not applicable to or do not have a significant impact on BriaCell and have been excluded from the list below. The following have not yet been adopted and are being evaluated to determine their impact on BriaCell.
IFRS 16 - Leases (“IFRS 16”) replaces IAS 17, Leases (“IAS 17”). The new model requires the recognition of almost all lease contracts on a lessee’s statement of financial position as a lease liability reflecting future lease payments and a ‘right-of-use asset’ with exceptions for certain short-term leases and leases of low-value assets. In addition, the lease payments are required to be presented on the statement of cash flow within operating and financing activities for the interest and principal portions, respectively. IFRS 16 is effective for annual periods beginning on or after January 1, 2019, with early adoption permitted if IFRS 15, Revenue from Contracts with Customers, is also applied. The Company has determined there will not be a significant impact to the consolidated financial statements as a result of the adoption of this standard.
IFRS 17 – Insurance Contract (“IFRS 17”) was issued by the IASB in May 2017, which replaces IFRS 4 Insurance Contracts. IFRS 17 requires entities to measure insurance contract liabilities at their current fulfillment values using one of three measurement models, depending on the nature of the contract. IFRS 17 is effective for annual periods beginning on or after January 1, 2021. IFRS 17 will affect how the Company’s accounts for its insurance contracts and how it reports its financial performance in our consolidated statements of operations. The Company has determined there will not be a significant impact to the consolidated financial statements as a result of the adoption of this standard.
Results of Operations
Comparison of the Nine month period ended April 30, 2019, compared to the nine month period ended April 30, 2018
Research Cost
For the nine month period ended April 30, 2019, research costs amounted to $3,605,429 as compared to $1,889,597 for nine month period ended April 30, 2018. The increase in research costs is as a result of supporting the Company’s ongoing Phase I/IIa clinical trial and relates primarily to increased clinical trial expenses, including the development of new Bria-IMT™ cell banks. BriaCell also has contracted with a second supplier of Bria-IMT™ and there is ongoing formulation work to develop a more user-friendly formulation that does not require culturing cells and same day irradiation.
Work also has begun on the development of second generation Bria-IMT™ and BriaCell has submitted five grant applications, applying for non-dilutive funding to support our research efforts, using our grant consultant, the FreeMind Group.
| 50 |
General and Administrative Expenses
For the nine month period ended April 30, 2019, general and administrative expenses amounted to $847,266 as compared to $908,902 for the nine month period ended April 30, 2018.
Share based Compensation
For the nine month period ended April 30, 2019, share based compensation amounted to $60,570 as compared to $256,080 for the nine month period ended April 30, 2018. No options were issued during the current period. The current charge relates to the tail end of the fair value of the options issued in the prior periods.
Interest Income
For the nine month period ended April 30, 2019, interest income amounted to $12,000 as compared to $8,912 for the nine month period ended April 30, 2018. Interest income earned during each quarter is a function of the amount of funds held in interest bearing accounts.
Interest expense
For the nine month period ended April 30, 2019, interest expense amounted to $26,352 as compared to $228,887 expense for the nine month period ended April 30, 2018. Interest expense is incurred as a result of the issuance of interest bearing convertible notes in March 2018. The prior period includes the issuance costs of the convertible debt.
Change in fair value of convertible debt
For the nine month period ended April 30, 2019, the increase in fair value of convertible debt amounted to $392,721 as compared to nil for the nine month period ended April 30, 2018.
Foreign Exchange Gain
For the nine month period ended April 30, 2019, the foreign exchange gain amounted to $39,902 as compared to a gain of $5,836 for the nine month period ended April 30, 2018. The Company is exposed to financial risk related to the fluctuation of foreign exchange rates. The Company operates in the United States and Canada, most of its monetary assets are held in Canadian dollars and most of its expenditures are made in US dollars. The Company has not hedged its exposure to currency fluctuations.
Loss for the period
The Company reported a loss for the nine month period ended April 30, 2019 of $4,094,994 as compared to a loss of $3,268,718 for the nine month period ended April 30, 2018. The primary reason for increase in the loss during the current period due to the increased research activities compared to the prior period.
Comprehensive loss for the period
The Company reported a comprehensive loss for the nine month period ended April 30, 2019 of $4,086,405 as compared to a comprehensive loss of $3,336,184 for the nine month period ended April 30, 2018. The primary reason for the increase in the loss during the current period is due to the increased research activities compared to the prior period.
The difference between net loss and comprehensive loss results from the foreign currency translation adjustment that arises upon the translation of the accounting records of the Company’s US subsidiary, whose functional currency is the US dollar into Canadian dollars for financial statement presentation purposes.
| 51 |
Comparison of the year ended July 31, 2018 to the year ended July 31, 2017
Research Costs
For the year ended July 31, 2018, research costs amounted to $3,112,579 as compared to $2,125,941 for the year ended July 31, 2017. The increase in research costs is as a result of supporting the Company’s ongoing Phase I/IIa clinical trial and relates primarily to increased clinical trial expenses, including the development of new BriaVax™ cell banks. BriaCell also has contracted with a second supplier of BriaVax™ and there is ongoing formulation work to develop a more user-friendly formulation that does not require culturing cells and same day irradiation. Work also has begun on the development of second generation BriaVax™ and BriaCell has submitted five grant applications, applying for non-dilutive funding to support our research efforts, using our grant consultant, the FreeMind Group.
General and Administrative Expenses
For the year ended July 31, 2018, general and administrative expenses amounted to $1,387,713 as compared to $820,281 for the year ended July 31, 2017. The increase is primarily to an increase in consulting, professional fees and Shareholder communications incurred in 2018 as compared to 2017 and is in line with the Companies increased research activities and increase investor relations activities. In addition, we incurred certain expenses in connection with the debt financing.
Share-based Compensation
For the year ended July 31, 2018, share-based compensation amounted to $476,211 as compared to $272,014 for the year ended July 31, 2017. The increase in share-based compensation in the current period is as a result of increased number of stock options granted in the year ended July 31, 2018 as compared to the prior year.
Interest Income
For the year ended July 31, 2018, interest income amounted to $15,991 as compared to $6,428 for the year ended July 31, 2017. Interest income earned during each quarter is a function of the amount of funds held in interest bearing accounts.
Interest expense
For the year ended July 31, 2018, interest expense amounted to $20,364 as compared to $nil for the year ended July 31, 2017. Interest expense was incurred from the convertible notes.
Change in fair value of convertible debt
For the year ended July 31, 2018, change in fair value of convertible debt amounted to $407,709 as compared to $nil for the year ended July 31, 2017.
Foreign Exchange Gain
For the year ended July 31, 2018, the foreign exchange loss amounted to $24,078 as compared to a loss of $8,913 for the year ended July 31, 2017. The Company is exposed to financial risk related to the fluctuation of foreign exchange rates. The Company operates in the United States and Canada, most of its monetary assets are held in Canadian dollars and most of its expenditures are made in US dollars. The Company has not hedged its exposure to currency fluctuations.
Loss for the period
The Company reported a loss for the year ended July 31, 2018 of $5,412,663 as compared to a loss of $3,220,721 for the year ended July 31, 2017. The primary reason for increase in the loss in 2018 is due to the increase in research activities, general and administrative expenses and share-based compensation.
Comprehensive loss for the period
The Company reported a comprehensive loss for the year ended July 31, 2018 of $5,446,003 as compared to a comprehensive loss of $3,178,893 for the year ended July 31, 2017. The primary reason for increase in the loss in 2018 is due to the increase in research activities, General and Administrative Expenses and Share-based Compensation.
| 52 |
The difference between net loss and comprehensive loss results from Foreign currency translation adjustment that arises upon the translation of the accounting records of BTC who’s functional currency is the US dollar into Canadian dollars for financial statement presentation purposes.
Going Concern Uncertainty
The financial statements have been prepared on a going concern basis which assumes that the Company will be able to realize its assets and discharge its liabilities in the normal course of business for the foreseeable future. The continuing operations of the Company are dependent upon its ability to continue to raise adequate financing and to commence profitable operations in the future.
As at April 30, 2019, the Company has total assets of $1,613,299 (July 31, 2018 - $2,977,140) and working capital of $343,606 (July 31, 2018 - $700,350).
It is management’s opinion that the Company will require additional funding, either through debt or equity issuances, in order to maintain its research and developmental activities. To this end, the company is currently raising funds to continue to funds its operation. These uncertainties may cast significant doubt on the Company’s ability to continue as a going concern.
Liquidity and Capital Resources
The Company’s approach to managing liquidity risk is to ensure that it will have sufficient liquidity to meet liabilities as they come due. As at April 30, 2019, the Company had a working capital balance of $343,606 (July 31, 2018 - $700,350). As a result, the Company currently has little exposure to liquidity risk. However, the Company has not yet achieved profitable operations and expects to incur further losses in the development of its products; which will require that the Company continues to raise capital to meet its objectives.
Off-balance Sheet Arrangements
None.
Tabular Disclosure of Contractual Obligations
The following table summarizes our contractual obligations as of July 31, 2018 and the effect those commitments are expected to have on our liquidity and cash flow.
| Payments due by periods | ||||||||||||||||||||
| Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
| Unsecured Convertible Loans(1) | 1,460,138 | 1,460,138 | - | - | - | |||||||||||||||
| Office lease | 65,085 | 59,661 | 5,424 | - | - | |||||||||||||||
| Total | 1,525,223 | 1,519,799 | 5,424 | - | - | |||||||||||||||
(1) The unsecured convertible loans may be converted into common stock of the company or repaid in cash.
| 53 |
Overview of the Company
BriaCell is an immuno-oncology biotechnology company with a strong focus on cancer immunotherapy. Immunotherapies have come to the forefront in the fight against cancer. They harness the body’s own immune system to recognize and destroy cancer cells. BriaCell owns the US patent to SV-BR-1-GM (Bria-IMT™), a whole-cell targeted immunotherapy for cancer (U.S. Patent No. 7,674,456), as well as patents related to PKCδ inhibitors (U.S. Patent Nos. 9,364,460 and 9,572,793). The Company is currently advancing its targeted immunotherapy program by prioritizing a Phase I/IIa clinical trial with Bria-IMT™ in combination with an immune checkpoint inhibitor and a companion diagnostic test, BriaDX™, to identify patients most likely to benefit from Bria-IMT™. The Bria-IMT™ regimen was evaluated in 4 patients in a prior study in 2004 – 2006 by Dr. Charles Wiseman, the scientific founder and member of the Board of Directors. Encouraging results were obtained, especially in a patient who matched Bria-IMT™ at the HLA-DRB3 allele. In 2017-2018 BriaCell evaluated 23 patients with advanced breast cancer with the Bria-IMT™ regimen and obtained confirmation of the ability of the Bria-IMT™ regimen to induce regression of metastatic breast cancer in patients who match Bria-IMT™ at least at one HLA allele. A combination study with the immune checkpoint inhibitor Keytruda® was initiated and the first patient dosing in the “combination therapy” clinical trial occurred in September 2018. As of August 13, 2019, 6 patients had been dosed in the combination therapy trial with Bria-IMT™ and the immune checkpoint inhibitor Keytruda (Merck) and the study is ongoing with additional patients enrolling.
The Company was incorporated under the Business Corporations Act (British Columbia) (“BCBCA”) on July 26, 2006 as Ansell Capital Corp. and is listed on the TSX Venture Exchange (“TSXV”). The Company is developing a new therapy for advanced breast cancer. The address for the Company’s headquarters is Suite 300 – 235 West 15th Street, West Vancouver, British Columbia, V7T 2X1. The Company’s corporate offices in the United states are located at 820 Heinz Avenue, Berkley, California 94710. The Company’s two wholly owned subsidiaries BriaCell Therapeutics Corp., a Delaware corporation (“BTC”) and Sapientia Pharmaceuticals Inc., a Delaware corporation (“Sapientia”), were formed on April 3, 2014 and September 20, 2012 in Delaware, USA respectively. The Company’s registered agent in the United States is Paracorp Incorporated located at 2804 Gateway Oaks Drive #100, Sacramento, CA 95833.
On July 24, 2017, the Company entered into a definitive share exchange agreement (the “Share Exchange Agreement”) between BTC, Sapientia and all the shareholders of Sapientia. Sapientia is a biotechnology company based in Havertown, PA, that is developing novel targeted therapeutics for multiple indications including several cancers and fibrotic diseases. Pursuant to the terms of the Share Exchange Agreement, BTC acquired from the Sapientia Shareholders all of the issued and outstanding shares in the capital of Sapientia. As consideration, the Sapientia Shareholders, received an aggregate of 2,500,002 common shares in the capital of BriaCell on a pro-rata basis, which were issued on September 5, 2017. As part of the share exchange, BriaCell acquired all rights, including composition of matter patents, and preclinical study data to a novel therapeutic technology platform, known as protein kinase C delta (PKCδ) inhibitors, which represents a unique, highly-targeted approach to treat cancer and to boost the immune system.
Market
It is estimated that in 2019, approximately 268,600 women will be diagnosed with breast cancer in the United States.7 According to the National Breast Cancer Foundation, on every two minutes an American woman is diagnosed with breast cancer and more than 40,500 die each year.8 Although about 100 times less common than in women, breast cancer also affects men.9 It is estimated that the lifetime risk of men getting breast cancer is about 1 in 1,00010, and the ACS estimates that approximately 2,670 new cases of invasive male breast cancer will be diagnosed and approximately 500 men will die from breast cancer in 2019.11
According to the May 2019 “Global Oncology Trends 2019” report by the IQVIA Institute, the global market for cancer drugs (including immunotherapy drugs) is expected to reach nearly $240 billion by the end of 2023, growing at a compound annual growth rate, or CAGR of 9-12% between 2019 and 2023.12
According to breastcancer.org, about 12.8% percent of women will be diagnosed with breast cancer at some point during their lifetime.13 According to cancer.net, as of January 2019, there are over 3 million U.S. women who have been diagnosed with breast cancer.14 Approximately 80% of cases present as invasive breast cancer.15 6-10% of new breast cancer diagnoses are Stage IV (metastatic or MBC, cancer which has already spread to other organs).16 20-30% of all women diagnosed with breast cancer will develop MBC.17 Breast cancer can be subdivided based on receptor status – the hormone receptors for estrogen (ER) and progesterone (PR), collectively referred to as hormone receptors (HR), and the Her2/neu growth factor receptor (HER2). In one large study of breast cancer, 72.7% were found to be HR+/HER2−, 12.2% were triple-negative (HR−/HER2−), 10.3% were HR+/HER2+, and 4.6% were HR−/HER2+.18
7 https://www.breastcancer.org/symptoms/understand_bc/statistics
8 https://www.nationalbreastcancer.org/breast-cancer-facts
9 https://www.medicinenet.com/breast_cancer_in_males_and_females/ask.htm
10 https://www.medicinenet.com/breast_cancer_in_males_and_females/ask.htm
11 https://www.cancer.org/cancer/breast-cancer-in-men/about/key-statistics.html
12 IQVIA Institute. Global Oncology Trends 2019: Therapeutics, Clinical Development and Health System Implications. https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/global-oncology-trends-2019.pdf?&_=1565186166041.
13 https://www.breastcancer.org/symptoms/understand_bc/statistics
14 https://www.cancer.net/cancer-types/breast-cancer/statistics
15https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-
figures/breast-cancer-facts-and-figures-2017-2018.pdf
16 http://mbcn.org/incidence-and-incidence-rates/
17 http://mbcn.org/incidence-and-incidence-rates/
18 Howlader, N.; Altekruse, S. F.; Li, C. I.; Chen, V. W.; Clarke, C. A.; Ries, L. A.; Cronin, K. A., US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014, 106 (5).
| 54 |
It is estimated that over 150,000 women in the US are living with metastatic breast cancer19. For those with metastatic disease at diagnosis, their 5-year survival is 27%.20 For patients who develop MBC after initially having localized disease, if they had a good response to treatment (disease-free interval of >24 months), their survival is similar to that of patients with MBC at initial diagnosis, but if their disease-free interval is <24 months, their prognosis is worse.21 We currently propose that Brias-IMT’s indication will be for the treatment of patients with metastatic breast cancer (MBC) who have failed at least two lines of therapy. Similarly, another study showed that the median overall survival among patients with de novo stage IV MBC was 39.2 months while for patients with and relapsed disease it was 27.2 months.22 Median progression free survival after first-line therapy is only 9 months and the survival benefit decreases with subsequent lines of therapy.23 A recent study showed that of 386 patients with MBC, 374 (97%) received first-line therapy, 254 (66%) received second-line therapy, 175 (45%) received third-line therapy, and 105 (27%) received therapy beyond third-line.24
Figure: Overview of current drugs for breast cancer, demonstrating the pattern of novel therapeutic introductions and significant market uptake. These precedents demonstrate a strong market pull for Bria-IMT™.
| Drug | Technology | Company | Indication | 2018 Sales US (Mil $US) | 2018 Sales Ex-US (Mil $US) | 2018 Sales WW (Mil $US) | ||||||||||||
| HERCEPTIN® (trastuzumab) | Monoclonal antibody | Roche | HER2+BC & HER2+ metastatic gastric cancer | 2,955 | 4,140 | 7,096 | ||||||||||||
| IBRANCE® (palbociclib) in combination with fluvestrant or aromatase inhibitor | CDK 4/6 inhibitor | Pfizer | HR+/HER2- MBC | 2,922 | 1,196 | 4,118 | ||||||||||||
| PERJETA® (pertuzumab) in combination with Herceptin® (trastuzumab) and chemotherapy | HER2/neu receptor antagonist | Roche | HER2+ early BC that has a high likelihood of recurrence | 1,347 | 1,499 | 2,846 | ||||||||||||
| FASLODEX® (fulvestrant) | Estrogen receptor antagonist | AstraZeneca | HR+/HER2- MBC | 537 | 491 | 1,028 | ||||||||||||
| KADCYLA® (ado-trastuzumab emtansine) | HER2 targeted antibody & microtubule inhibitor conjugate | Roche | HER2+BC | 365 | 630 | 995 | ||||||||||||
| LYNPARZA® (olaparib) | Poly (ADP-ribose) polymerase (PARP) inhibitor | AstraZeneca | BC & Ovarian cancer | 345 | 302 | 647 | ||||||||||||
| Verzenio® (abemaciclib) monotherapy or in combination with fulvestrant or aromatase inhibitor | CDK 4/6 inhibitor | Eli Lilly | HR+/HER2- MBC | 255 | - | 255 | ||||||||||||
| KISQALI® (ribociclib) in combination with fluvestrant or aromatase inhibitor | CDK 4/6 inhibitor | Novartis | HR+/HER2- MBC | 235 | - | 235 | ||||||||||||
19 Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017 Jun;26(6):809-815. doi: 10.1158/1055-9965.EPI-16-0889. Epub 2017 May 18.
20 SEER Cancer Statistics Factsheets: Female Breast Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/breast.html; American Cancer Society. Breast Cancer Facts & Figures 2017-2018. Atlanta: American Cancer Society, Inc. 2017.
21 Lobbezoo, D. J. A. et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res. Treat. 141, 507–514 (2013).
22 Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010 Nov; 21(11):2169–74.
23 Bonotto M, Gerratana L, Iacono D, Minisini AM, Rihawi K, Fasola G, Puglisi F. Treatment of Metastatic Breast Cancer in a Real-World Scenario: Is Progression-Free Survival With First Line Predictive of Benefit From Second and Later Lines? Oncologist. 2015 Jul;20(7):719-24. doi: 10.1634/theoncologist.2015-0002. Epub 2015 May 27.
24 Kotsakis A, Ardavanis A, Koumakis G, Samantas E, Psyrri A, Papadimitriou C. Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: Results from the EMERGE multicenter retrospective chart review study. BMC Cancer. 2019 Jan 18;19(1):88. doi: 10.1186/s12885-019-5301-5.
| 55 |
The best response to Bria-IMT™ to date is in patients who matched Bria-IMT™ at 1 or more HLA alleles, with higher response rates for patients with 2+ HLA allele matches. If one HLA allele match is found to be sufficient, we will be able to treat ~50-60% of the patient population, while patients with 2+ HLA matches constitutes ~15-35% of cases.25. The market for breast cancer drugs is a multibillion-dollar market with new drugs being approved on an ongoing basis, indicating the shortage of safe and effective treatments for this deadly disease. The Figure summarizes current drugs on the market utilized in combination therapy along with their reported market sales, which further supports market potential for Bria-IMTTM to be used for combination therapy for breast cancer patients.26
We propose the following calculation in order to show the rationale behind the number of patients that we anticipate can be currently treated by SV-BR-1-GM:
| ● | There are ~150,000 women with metastatic breast cancer in the US27 | |
| ● | 45% will receive third line therapy28 = 68,000 patients available | |
| ● | 68,000 x ~50% (matched for 1 HLA allele group)29 = ~34,000 patients available for treatment30 |
These assumptions above are limited to third or later lines of therapy. There is also potential to move into second-line and first-line treatment, which would markedly expand the population to be treated.
Treatment with a combination therapy comprised of Bria-IMT™ + checkpoint inhibitor is expected to provide a new therapeutic option in patients who currently have no effective therapeutic options. The parallel development of BriaDx (companion diagnostic) by BriaCell, as a strategy to identify those patients most likely to respond to Bria-IMT™, may eventually lead to even higher response rates — potentially substantially higher than currently achievable by other treatments for breast cancer.
BriaCell is in contact with several large pharmaceutical companies for potential collaborations for the development of a combination therapy with Bria-IMT™ and immune checkpoint inhibitors, and already has in place a collaboration with Incyte Corporation. We will continue to pursue these discussions with the goal of using Bria-IMT™ in combination with checkpoint inhibitors. This will also help increase the visibility of our therapy and may lead to additional funding sources for future clinical trials.
Competition
Currently available therapeutic options for breast cancer offer some hope for patients, but there is much room for improvement. Comparable studies looking primarily at second line or later treatment are shown in the Table. Evaluating response rates (partial and complete responses = ORR), progression free survival (PFS) and overall survival (OS) from clinical trials in similar subjects with metastatic or recurrent breast cancer indicate that response rates range from 6.9% up to 59%, depending on the population studied and the intervention (median 24%). PFS ranges from 8 weeks to 12 months (median 5 months) and OS from 6 months to 31 months (median 13 months).
25 Gragert, Loren, Abeer Madbouly, John Freeman, and Martin Maiers. 2013. “Six-Locus High Resolution HLA Haplotype Frequencies Derived from Mixed-Resolution DNA Typing for the Entire US Donor Registry.” Human Immunology. https://doi.org/10.1016/j.humimm.2013.06.025
26 References for the Figure:
Ibrance: https://s21.q4cdn.com/317678438/files/doc_financials/Quarterly/2018/q4/Q4-2018-PFE-Earnings-Release.pdf
Kisqali: https://www.novartis.com/sites/www.novartis.com/files/q4-2018-media-release-en.pdf
Verzenio: https://www.prnewswire.com/news-releases/lilly-reports-strong-fourth-quarter-and-full-year-2018-financial-results-
lowers-2019-eps-guidance-to-reflect-the-pending-acquisition-of-loxo-oncology-300790727.html
Lynparza: https://www.astrazeneca.com/content/dam/az/PDF/2018/full-year/Full-Year_2018_Results_announcement.pdf
Halaven: https://www.eisai.com/ir/library/settlement/pdf/e2018Q4_52.pdf (Note that sales figures are in billions of yes)
Balixafortide: https://www.biospace.com/article/polyphor-announces-financial-results-for-the-full-year-2018/
https://www.polyphor.com/wp-content/uploads/2019/03/Polyphor-FY-2018-Results-Presentation-March-2019.pdf
27 Mariotto AB, Etzioni R, Hurlbert M, Penberthy L, Mayer M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol Biomarkers Prev. 2017 Jun;26(6):809-815. doi: 10.1158/1055-9965.EPI-16-0889. Epub 2017 May 18
28 Kotsakis A, Ardavanis A, Koumakis G, Samantas E, Psyrri A, Papadimitriou C. Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: Results from the EMERGE multicenter retrospective chart review study. BMC Cancer. 2019 Jan 18;19(1):88. doi: 10.1186/s12885-019-5301-5.
29 Gragert, Loren, Abeer Madbouly, John Freeman, and Martin Maiers. 2013. “Six-Locus High Resolution HLA Haplotype Frequencies Derived from Mixed-Resolution DNA Typing for the Entire US Donor Registry.” Human Immunology. https://doi.org/10.1016/j.humimm.2013.06.025
30 Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). 2019 Apr 10;11:151-164. doi: 10.2147/BCTT.S176070. eCollection 2019; SEER Cancer Statistics Factsheets: Female Breast Cancer. National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/statfacts/html/breast.html; American Cancer Society. Breast Cancer Facts & Figures 2017-2018. Atlanta: American Cancer Society, Inc. 2017.
| 56 |
| Table: Studies evaluating second-line or later treatment options. Data depict an unpredictable response rate to treatment ranging from 6.9-59%, therefore establishing and confirming the opportunity for Bria-IMT™. | ||||||||||||||||||
| Study | Treatment & Design | # of Pts | ORR | PFS/TTP | OS | |||||||||||||
| Perez31 | Paclitaxel Monotherapy | 212 | 21.5 | % | 4.7 mo | 12.8 mo | ||||||||||||
| Seidman32 | Gemcitabine Monotherapy | 160 | 26 | % | ||||||||||||||
| Zelek33 | Vinorelbine Monotherapy | 40 | 25 | % | 6 mo | |||||||||||||
| Licchetta34 | Cyclophosphamide and megestrol acetate | 29 | 31 | % | 7.4 mo | 13.4 mo | ||||||||||||
| Harvey35 | Docetaxel Monotherapy 60 mg/m2 | 122 | 22.1 | % | 12.7 wk | 10.6 mo | ||||||||||||
| Docetaxel Monotherapy 75 mg/m2 | 146 | 23.3 | % | 15.0 wk | 10.3 mo | |||||||||||||
| Docetaxel Monotherapy 100 mg/m2 | 139 | 36.0 | % | 16.6 wk | 12.3 mo | |||||||||||||
| Rivera36 | Docetaxel Monotherapy q3wk | 59 | 35.6 | % | 5.7 mo | 18.3 mo | ||||||||||||
| Docetaxel Monotherapy qwk | 59 | 20.3 | % | 5.5 mo | 18.6 mo | |||||||||||||
| Gradishar37 | ABI-007 (Nab paclitaxel) | 229 | 33 | % | 23.0 wk | 65.0 wk | ||||||||||||
| Paclitaxel Monotherapy | 225 | 19 | % | 16.9 wk | 55.7 wk | |||||||||||||
| ABI-007 (Nab paclitaxel) 2nd line | 132 | 27 | % | 20.9 wk | 56.4 wk | |||||||||||||
| Paclitaxel Monotherapy 2nd line | 136 | 13 | % | 16.1 wk | 46.7 wk | |||||||||||||
| Perez38 | Ixabepilone Monotherapy | 126 | 11.5 | % | 3.1 mo | 8.6 mo | ||||||||||||
| Leyland-Jones39 | Trastuzumab with paclitaxel | 32 | 59 | % | 12.2 mo | |||||||||||||
| von Minckwitz40 | Trastuzumab with capecitabine | 78 | 48.1 | % | 8.2 mo | 25.5 mo | ||||||||||||
| Capecitabine Monotherapy | 78 | 27.0 | % | 5.6 mo | 20.4 mo | |||||||||||||
| Verma41 | Trastuzumab emtansine | 495 | 43.6 | % | 9.6 mo | 30.9 mo | ||||||||||||
| lapatinib plus capecitabine | 496 | 30.8 | % | 6.4 mo | 25.1 mo | |||||||||||||
| Geyer42 | Lapatinib plus capecitabine | 163 | 22 | % | 8.4 mo | |||||||||||||
| Capecitabine Monotherapy | 161 | 14 | % | 4.4 mo | ||||||||||||||
| Bartsch43 | Capecitabine and trastuzumab | 40 | 20 | % | 8 mo | 24 mo | ||||||||||||
| Blackwell44 | Lapatinib Monotherapy | 148 | 6.9 | % | 8.1 wk | 39.0 wk | ||||||||||||
| Lapatinib with trastuzumab | 148 | 10.3 | % | 12.0 wk | 51.6 wk | |||||||||||||
31 Perez, E. A., Vogel, C. L., Irwin, D. H., Kirshner, J. J. & Patel, R. Multicenter Phase II Trial of Weekly Paclitaxel in Women With Metastatic Breast Cancer. J. Clin. Oncol. 19, 4216–4223 (2001).
32 Seidman, A. D. Gemcitabine as single-agent therapy in the management of advanced breast cancer. Oncology (Williston Park). 15, 11–4 (2001).
33 Zelek, L. et al. Weekly vinorelbine is an effective palliative regimen after failure with anthracyclines and taxanes in metastatic breast carcinoma. Cancer 92, 2267–72 (2001)
34 Licchetta A, Correale P, Migali C, Remondo C, Francini E, Pascucci A, Magliocca A, Guarnieri A, Savelli V, Piccolomini A, Carli AF, Francini G. Oral metronomic chemo-hormonal-therapy of metastatic breast cancer with cyclophosphamide and megestrol acetate. J Chemother. 2010 Jun;22(3):201-4.
35 Harvey, V. et al. Phase III Trial Comparing Three Doses of Docetaxel for Second-Line Treatment of Advanced Breast Cancer. J. Clin. Oncol. 24, 4963–4970 (2006).
36 Rivera, E. et al. Phase 3 study comparing the use of docetaxel on an every-3-week versus weekly schedule in the treatment of metastatic breast cancer. Cancer 112, 1455–1461 (2008).
37 Gradishar WJ. Taxanes for the treatment of metastatic breast cancer. Breast Cancer (Auckl). 2012;6:159-71. doi: 10.4137/BCBCR.S8205. Epub 2012 Oct 25.
38 Perez, E. A. et al. Efficacy and Safety of Ixabepilone (BMS-247550) in a Phase II Study of Patients With Advanced Breast Cancer Resistant to an Anthracycline, a Taxane, and Capecitabine. J. Clin. Oncol. 25, 3407–3414 (2007).
39 Leyland-Jones, B. et al. Pharmacokinetics, Safety, and Efficacy of Trastuzumab Administered Every Three Weeks in Combination With Paclitaxel. J. Clin. Oncol. 21, 3965–3971 (2003). Only 41% of patients had prior systemic chemotherapy
40 von Minckwitz G et el. Trastuzumab beyond progression: overall survival analysis of the GBG 26/BIG 3-05 phase III study in HER2-positive breast cancer. Eur J Cancer. 2011 Oct;47(15):2273-81. doi: 10.1016/j.ejca.2011.06.021. Epub 2011 Jul 7. Prior therapy limited to trastuzamab alone or in combination with a taxane.
41 Verma, S. et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 367, 1783–1791 (2012).
42 Geyer, C. E. et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 355, 2733–2743 (2006).
43 Bartsch, R. et al. Capecitabine and Trastuzumab in Heavily Pretreated Metastatic Breast Cancer. J. Clin. Oncol. 25, 3853–3858 (2007).
44 Blackwell, K. L. et al. Randomized Study of Lapatinib Alone or in Combination With Trastuzumab in Women With ErbB2-Positive, Trastuzumab-Refractory Metastatic Breast Cancer. J. Clin. Oncol. 28, 1124–1130 (2010).
| 57 |
MBC treated with second or higher lines of therapy has a very poor prognosis and few effective therapies that consistently induce long-term remission,45 which indicates the market demand and clinical need for new and improved therapeutic drugs and treatment options in order to improve these response outcomes and patient survival rates. Thus, Bria-IMT™ has the potential to induce long-term remission, especially in combination with immunotherapies. Current treatment of MBC is outlined in the Figure, which illustrates different therapeutic treatment options and drugs used upon diagnoses from biopsy and identification of breast cancer biomarkers.46

Figure Current treatment paradigm for metastatic breast cancer and comparison between different treatment strategies and combination therapies dependent upon biomarker identification and activity within the breast cancer signaling pathway. Information from the NCCN Guidelines available at https://www.nccn.org/professionals/physician_gls/default.aspx
Of patients treated with trastuzumab for MBC, one study showed that 241/331 (72%) progressed within 27 months (32% per year) with median survival of 13-14 months (CI 10-15 months).47 This indicates the high unmet need in this patient population which should facilitate regulatory review of effective novel therapies such as Bria-IMT™.
While there are approximately 36 different biotech companies working to create an effective breast cancer vaccine, a significant gap remains in the effectiveness and safety of second or higher lines of therapy. The most studied targeted immunotherapy, Neuvax (Galena), a HER2 peptide vaccine, failed a Phase III trial, but there is encouraging data to support at least three ongoing clinical trials combining trastuzumab with HER2 epitope immunogens.48 The NCI randomized trial adding PANVAC (a poxviral-based immunogen) to docetaxel increased the median PFS from 3.9 months to 7.9 months and is to be used as a basis for larger, more sophisticated clinical trials.49 An immunogen targeting a carbohydrate antigen, globo-H, was associated with improved PFS, but only in the subset able to mount antibody responses.50 A Johns Hopkins breast cancer trial using a breast cancer cell line transfected with the gene for GM-CSF has not been positive but, using the same cell line with trastuzumab, 40% of patients enjoyed clinical benefit (CR+PR+stable) at one year.51 Finally, the study of targeted cancer immunotherapies in combination with other therapies is receiving much attention, particularly combination with checkpoint inhibitors.52
45 Dawood S, Broglio K, Ensor J, Hortobagyi GN, Giordano SH. Survival differences among women with de novo stage IV and relapsed breast cancer. Ann Oncol. 2010 Nov; 21(11):2169–74; Bonotto M, Gerratana L, Iacono D, Minisini AM, Rihawi K, Fasola G, Puglisi F. Treatment of Metastatic Breast Cancer in a Real-World Scenario: Is Progression-Free Survival With First Line Predictive of Benefit From Second and Later Lines? Oncologist. 2015 Jul;20(7):719-24. doi: 10.1634/theoncologist.2015-0002. Epub 2015 May 27; Kotsakis A, Ardavanis A, Koumakis G, Samantas E, Psyrri A, Papadimitriou C. Epidemiological characteristics, clinical outcomes and management patterns of metastatic breast cancer patients in routine clinical care settings of Greece: Results from the EMERGE multicenter retrospective chart review study. BMC Cancer. 2019 Jan 18;19(1):88. doi: 10.1186/s12885-019-5301-5.
46 NCCN Guidelines Version 2.2019, 07/02/2019 © 2019 National Comprehensive Cancer Network (NCCN®), available at https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
47 Rossi, V.; Nole, F.; Redana, S.; Adamoli, L.; Martinello, R.; Aurilio, G.; Verri, E.; Sapino, A.; Viale, G.; Aglietta, M.; Montemurro, F., Clinical outcome in women with HER2-positive de novo or recurring stage IV breast cancer receiving trastuzumab-based therapy. Breast 2014, 23 (1), 44-9.
48 Mittendorf, E. A.; Peoples, G. E., Injecting Hope--A Review of Breast Cancer Vaccines. Oncology (Williston Park) 2016, 30 (5), 475-81, 485.
49 Heery, C. R.; Ibrahim, N. K.; Arlen, P. M.; Mohebtash, M.; Murray, J. L.; Koenig, K.; Madan, R. A.; McMahon, S.; Marte, J. L.; Steinberg, S. M.; Donahue, R. N.; Grenga, I.; Jochems, C.; Farsaci, B.; Folio, L. R.; Schlom, J.; Gulley, J. L., Docetaxel Alone or in Combination With a Therapeutic Cancer Vaccine (PANVAC) in Patients With Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol 2015, 1 (8), 1087-95.
50 Huang, C.; Yu, A.; Tseng, L., Randomized phase II/III trial of active immunotherapy with OPT-822/OPT-821 in patients with metastatic breast cancer. J Clin Oncol 2016, 34 (15).
51 Chen, G.; Gupta, R.; Petrik, S.; Laiko, M.; Leatherman, J. M.; Asquith, J. M.; Daphtary, M. M.; Garrett-Mayer, E.; Davidson, N. E.; Hirt, K.; Berg, M.; Uram, J. N.; Dauses, T.; Fetting, J.; Duus, E. M.; Atay-Rosenthal, S.; Ye, X.; Wolff, A. C.; Stearns, V.; Jaffee, E. M.; Emens, L. A., A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol Res 2014, 2 (10), 949-61.
52 McArthur, H. L.; Page, D. B., Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy. Clin Adv Hematol Oncol 2016, 14 (11), 922-933.
| 58 |
There are a large number of therapies with other mechanisms of action in development for this indication which could limit uptake as other therapies are being rolled out, even if they could be used in combination with Bria-IMT™. We plan to develop the clinical data for Bria-IMT™, envisioned to show excellent efficacy and safety, and use this information to reach out to oncologists seeking additional therapeutic options for their patients. We will include in this effort a physician education campaign targeting the oncologists most likely to treat metastatic breast cancer. As these physicians become more aware of the data regarding Bria-IMT™ in breast cancer, we will make sure they also understand how best to use Bria-IMT™ in combination with other therapies that have complementary of synergistic mechanisms of action. This will also come from future clinical studies focusing on combination therapy.
There are several other approaches to developing targeted breast cancer immunotherapies. These include using peptide cocktails,53 a triple peptide regimen,54 recombinant HER2,55 antigen-pulsed dendritic cells,56 DNA immunogens,57 whole cell allogeneic GM-CSF secreting SKBR3 or T47D cells,58 an (HLA)-A2/A3-restricted immunogenic peptide derived from the HER2 protein,59 oxidized mannan-MUC1,60 and personalized peptide immunogens.61
Among the most promising results in patients with advanced disease have been using whole cell preparations, particularly if the cells are engineered to express GM-CSF.62 We are taking this approach and capitalizing on positive initial results with Bria-IMT monotherapy in difficult to treat patients using a regimen that both limits regulatory T cell activity (using low dose cyclophosphamide pre-treatment) and boosts the immune response (using post-dose alpha interferon in the inoculation sites). The combination with pembrolizumab is a logical extension of our findings where 21 of 23 MBC patients had demonstrable PD-L1 expression on the CTCs/CAMLs. The overall strategy to include an adaptive design, once the initial milestones have been met, to enroll additional patients for product registratioin, will allow rapid progression of the best therapeutic option to a biological licensing application (BLA).
Products/Pipeline
Bria-IMT™
BriaCell is currently conducting a Phase I/IIa clinical trial of Bria-IMT™ BriaCell’s lead candidate, in combination with pembrolizumab (KEYTRUDA®; manufactured by Merck & Co., Inc.). The combination study is listed in ClinicalTrials.gov as NCT03328026.
Positive Proof of Concept
| ● | BriaCell has achieved positive proof of concept based on data from a Phase I/IIa study of Bria-IMT™ in advanced breast cancer patients. |
| ● | The data shows promising anti-tumor activity of Bria-IMT™ in heavily pre-treated advanced breast cancer patients. |
| ● | Impressive Phase IIa efficacy data is similar to, or superior to, those of other approved breast cancer drugs when they were at a similar clinical-stage of development. |
| ● | The data shows an outstanding safety and tolerability profile for Bria-IMT™ in advanced breast cancer patients. |
| ● | The data confirms the “HLA Matching Hypothesis” and supports BriaCell’s strategy for the development of Bria-OTS™, BriaCell’s first personalized off-the-shelf immunotherapy for advanced breast cancer. |
About Bria-IMT™
Developed and characterized by a team of dedicated scientists and clinicians, Bria-IMT™ (SV-BR-1-GM) is a targeted immunotherapy being developed for the treatment of breast cancer. Bria-IMT™ is a genetically engineered human breast cancer cell line with features of immune cells and clinically applied as a targeted immunotherapy.
In short, Bria-IMT™ immunotherapy is a genetically engineered human breast cancer cell line which activates the immune cells to attack and destroy breast cancer tumors.
Mechanism of Action of Bria-IMT™: The mechanism of action of Bria-IMT™ is currently under investigation. It is likely that the expression of certain breast cancer antigens (proteins expressed in breast cancer cells) in Bria-IMT™ generates strong antibody and T-cell responses – which results in recognition and destruction of cancerous cells.63
63 Lacher M.D., Bauer G. Fury B., Graeve S., Fledderman E.L., Petrie T.D., Coleal-Bergum D.P., Hackett T., Perotti N.H., Kong Y.Y., Kwok W.W., Wagner J.P., Wiseman C.L., and Williams W.V. SV-BR-1-GM, a Clinically Effective GM-CSF- Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4+ T Lymphocytes. Frontiers in Immunology 2018; 9:Article 776.
| 59 |

Bria-IMT™ is designed to secrete granulocyte/macrophage-colony stimulating factor (GM-CSF), a factor that stimulates components of the immune system. Specifically, GM-CSF activates dendritic cells, the cells that start immune responses. These activated dendritic cells then activate T cells, a key component of the immune system, to recognize the tumor cells as foreign, and eliminate them. To amplify this action, we have combined Bria-IMT™ with other immune system activators including cyclophosphamide (used in low doses to reduce immune suppression), and interferon-α, a cytokine. We believe this approach of simultaneous activation of the immune system via different pathways will improve the immune system response to attack and destroy cancer cells.
Using BriaCell’s novel technology platform and our strong R&D capabilities, we plan to develop immunotherapies for other cancer indications Bria-OTS™
| ● | Bria-OTS™ is under development as an off-the-shelf personalized immunotherapy for advanced breast cancer. |
| ● | The concept for Bria-OTS™ comes from BriaCell’s work with Bria-IMT™, where we noted that if a patient “matches” Bria-IMT™ in their HLA type, they were more likely to respond. |
| ● | HLA molecules are the molecules that start immune responses, but are polymorphic – i.e. they are different in different in different people, although some people will share HLA types. |
| 60 |
| ● | Bria-OTS™ is made from cell lines that are genetically engineered to expresses the immune boosters GM-CSF and interferon-α, as well as specific HLA types (a.k.a. alleles). |
| ● | Different cell lines are being pre-manufactured to express different HLA types covering >99% of the overall breast cancer patient population. |
| ● | Using the BriaDX™, a companion diagnostic test performed on the patient’s saliva, the suitable personalized treatment will be selected for each patient for administration. |
| ● | This approach allows personalized treatment without the need for personalized manufacturing. Additionally, it saves time, and skips expensive and complicated manufacturing procedures associated with other personalized treatments. |
| ● | Bria-OTS™ cell lines are being engineered with the goal of transferring them to production in 2019 and commencing clinical evaluation in 2020. |
Bria-OTS™ Immunotherapy Covers ~99% of the Breast Cancer Population

BriaDx™
BriaDX™ is a diagnostic test that BriaCell is developing to detect the patients most likely to respond to Bria-IMT™. Currently, BriaDx™ includes HLKA typing of the patients. Additional diagnostics are being developed based on the expression of specific biomarkers in the responder (i.e. the patients for which Bria-IMT™ immunotherapy was highly effective) vs the non-responder patients from clinical studies of Bria-IMT™ in advanced breast cancer patients.
| 61 |
Blood and tumor samples from the patients are analyzed using cutting-edge technologies including gene expression analysis, and proteomics (i.e., defined as the large-scale study of the structure and function of proteins).
We have been characterizing the molecular fingerprint of the responder patients, and have developed a diagnostic test, BriaDX™, to identify these patients.
The insights gained from biomarker studies conducted to date have provided us with a solid basis for the development of Bria-OTS™, an off-the-shelf personalized immunotherapy which would treat over 99% of patients with advanced breast cancer.
BriaDx™ is being developed to help understand which patients are most likely to respond to Bria-IMT™ targeted immunotherapy. Based on the proposed mechanism of action of Bria-IMT™ (see Figure) HLA molecules play a key role inducing cellular immune responses to Bria-IMT™ which boosts the patient’s immune response to their cancer.
proposed mechanism of action of Bria-IMT™

HLA molecules are polymorphic, in that they are different in different individuals, but shared by some individuals (similar to eye color). We have noted that matching of Bria-IMT™ HLA types with the patients may predict those more likely to respond. This is supported by our clinical data to date.
| 62 |
Available Clinical Data for Treatment with the Bria-IMT™ Regimen
BriaCell conducted three Proof of Concept clinical trials, one using parental SV-BR-1 cells and the other two using Bria-IMT™ (i.e., genetically engineered SV-BR-1 cells – producing GM-CSF also called SV-BR-1-GM), in metastatic (i.e., Stage IV) breast cancer patients who had failed prior treatments. The patients were treated according to the following schedule, and the results are summarized below.

First Proof of Concept Trial1
| ● | Used unmodified cell line (parental SV-BR-1 cells) + GM-CSF + cyclophosphamide | |
| ● | N = 14 late stage, treatment-refractory breast cancer patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 12.1 months |
Second Proof of Concept Trial2
| ● | Used Bria-IMT™ (genetically engineered SV-BR-1 cells – producing GM-CSF) + cyclophosphamide + interferon-α | |
| ● | N = 4 late stage, treatment-refractory (3 breast cancer, and 1 ovarian cancer) patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 35 months | |
| ● | One robust responder with >90% regression during treatment, subsequent relapse (upon halting treatment) responded to re-treatment | |
| ● | This patient matched Bria-IMT™ at a key HLA type (HLA-DRB3) |
Third Proof of Concept Trial3
Thirty patients were screened, 24 enrolled and 23 dosed in the Phase I/IIa study (2017-2018)
| ● | Bria-IMT™ was very well tolerated (over 100 doses given) | |
| ● | The majority of adverse events (AEs) were limited to expected minor local irritation at the injection sites | |
| ● | No related grade >3 or unexpected AEs | |
| ● | No related serious AEs | |
| ● | No serious, unexpected, related AEs |
1 Wiseman, C. L. & Kharazi, A. Phase I Study with SV-BR-1 Breast Cancer Cell Line Vaccine and GMCSF: Clinical Experience in 14 Patients. Open Breast Cancer J. 2, 4–11 (2010).
2 Wiseman, C. L. & Kharazi, A. Objective Clinical Regression of Metastatic Breast Cancer in Disparate Sites after Use of Whole-Cell Vaccine Genetically Modified to Release Sargramostim. Breast J. 12, 475–480 (2006).
3 Listed on ClinicalTrials.gov as NCT03066947 https://clinicaltrials.gov/ct2/show/NCT03066947?term=BriaCell&rank=2
| 63 |
Most patients who have dropped out did so due to worsening of their underlying disease.
| ● | Bria-IMT™ appears to be most effective in patients who match with Bria-IMT ™ at 2 HLA loci (types) further supporting our “HLA Matching Hypothesis”, and the development of Bria-OTS ™ to cover 90% of the patient population. | |
| ● | Effectiveness also depends on the ability of the patient to develop an immune response to Bria-IMT™ | |
| ● | Results are shown in the tables here, combining the second and third proof of concept studies which both used Bria-IMT™ in an identical regimen. |
| Patients (n) | HLA match | Tumor Shrinkage | Biological Response* | |||||||||
| 5 | >2 | 40 | % | 60 | % | |||||||
| 19 | >1 | 21 | % | 37 | % | |||||||
| 8 | 0 | 0 | % | 0 | % | |||||||
*Biological response includes tumor shrinkage or lower circulating cancer associated cells
BriaCell also evaluated the ability of patients to mount a delayed-type hypersensitivity (DTH) response to the injected Bria-IMT™. A positive response was noted in 22 patients while 5 were not responsive. The results, in terms of those who experienced tumor shrinmkage, are shown here.
| Patients (n) | All Patients (n=27) | No HLA match (n=7) | 1+ HLA Match (n=20) | 2+ HLA matches (n=5) | ||||
| Negative DTH (n=5) | 0% (0/5) | 0% (0/0) | 0% (0/5) | 0% (0/2) | ||||
| Positive DTH (n=22) | 18% (4/22) | 0% (0/7) | 27% (4/15) | 67% (2/3) |
| ● | Bria-IMT™ was dosed in 27 patients (4 in 2004-2006, 23 in 2017-2018) as the Bria-IMT™ regimen alone. | |
| ● | Bria-IMT™ has been very well tolerated (over 100 doses given to date). | |
| ● | Tumor regression was seen in patients who were able to mount an immune response and matched Bria-IMT™ at HLA types confirming our main hypothesis and supporting using HLA typing as a marker to predict who is most likely to respond. | |
| ● | BriaCell continues to monitor their clinical trials proposing that BriaDx™ would include HLA typing as well as other potential biomarkers (such as the ability to mount a DTH response) to identify the patients most likely to respond to the Bria-IMT™ regimen. |
PKCδ
Protein Kinase C Delta (PKCδ) Inhibitors

Activated PKCδ inhibits RAS degradation which in turn stimulates tumor growth.
| 64 |
Early-Stage Preclinical Program
| ● | 30% of all human malignancies display activating RAS mutations with another 60% showing over-activity of Ras-signaling pathways.64 |
| ● | BriaCell’s novel, proprietary PKCδ inhibitors have shown activity against multiple RAS transformed tumors.65 |
| ● | This target has an attractive safety profile based on in vivo studies and knock out mouse studies.66 |
| ● | PKCδ also has potential activity as an immunotherapeutic by blocking TGFβ signaling.67 |
| ● | PKCδ inhibitors are applicable to specific niche tumor types which provide an accelerated clinical development plan. |
| ● | Could be in clinical testing within 24 months |
64 Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012 May 15; 72(10): 2457–2467
65 Xia, S., Forman, L. W. & Faller, D. V. Protein Kinase Cδ Is Required for Survival of Cells Expressing Activated p21RAS. J. Biol. Chem. 282, 13199–13210 (2007); Chen, Z. et al. Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer 18, 759–71 (2011); Xia, S., Chen, Z., Forman, L. W. & Faller, D. V. PKCδ survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell. Signal. 21, 502–508 (2009); Liou, J. S., Chen, C.-Y., Chen, J. S. & Faller, D. V. Oncogenic Ras Mediates Apoptosis in Response to Protein Kinase C Inhibition through the Generation of Reactive Oxygen Species. J. Biol. Chem. 275, 39001–39011 (2000); Liou, J. S., Chen, J. S. & Faller, D. V. Characterization of p21Ras-mediated apoptosis induced by protein kinase C inhibition and application to human tumor cell lines. J. Cell. Physiol. 198, 277–294 (2004); Chen, C. Y., Liou, J., Forman, L. W. & Faller, D. V. Differential regulation of discrete apoptotic pathways by Ras. J. Biol. Chem. 273, 16700–9 (1998); Chen, C. Y. & Faller, D. V. Direction of p21ras-generated signals towards cell growth or apoptosis is determined by protein kinase C and Bcl-2. Oncogene 11, 1487–98 (1995); Chen, C. Y. & Faller, D. V. Phosphorylation of Bcl-2 protein and association with p21Ras in Ras-induced apoptosis. J. Biol. Chem. 271, 2376–9 (1996); Chen, C.-Y., Liou, J., Forman, L. W. & Faller, D. V. Correlation of genetic instability and apoptosis in the presence of oncogenic Ki-Ras. Cell Death Differ. 5, 984–995 (1998); Chen, C. Y. et al. The recruitment of Fas-associated death domain/caspase-8 in Ras-induced apoptosis. Cell Growth Differ. 12, 297–306 (2001).
66 Miyamoto A, Nakayama K, Imaki H, Hirose S, Jiang Y, Abe M, Tsukiyama T, Nagahama H, Ohno S, Hatakeyama S, Nakayama KI. Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cdelta. Nature. 2002 Apr 25;416(6883):865-9.
67 Wermuth PJ, Addya S, Jimenez SA. Effect of Protein Kinase C delta (PKC-δ) Inhibition on the Transcriptome of Normal and Systemic Sclerosis Human Dermal Fibroblasts In Vitro. PLoS ONE, November 2011, Volume 6, Issue 11, e27110; PMCID: PMC3214051; Li Z, Jimenez SA. Protein Kinase C δ and c-Abl Kinase Are Required for Transforming Growth Factor β Induction of Endothelial–Mesenchymal Transition In Vitro. Arthritis and Rheumatism, Vol. 63, No. 8, August 2011, pp 2473–2483 PMCID: PMC3134600; Bujor AM, Asano Y, Haines P, Lafyatis R, Trojanowska M. The c-Abl Tyrosine Kinase Controls Protein Kinase C δ –Induced Fli-1 Phosphorylation in Human Dermal Fibroblasts. Arthritis & Rheumatism, Vol. 63, No. 6, June 2011, pp 1729–1737. PMCID: PMC3381734
| 65 |

| ● | Structural aspects of first generation inhibitor rottlerin and staurosporine (pan-PKC inhibitor) were combined to create second generation inhibitor KAM1 |
| ● | Third generation inhibitors such as BC-106 have improved potency and selectivity. |
| ● | Fourth generation inhibitors are under development to optimize their drug-like characteristics. |
| ● | PKCδ inhibitors lack endothelial cell cytotoxicity & PKCδ deficient mice develop normally and are fertile → No marked intrinsic toxicity by inhibiting PKCδ |
PKCδ Inhibitors Block Growth in Various Cancers

PKCδ inhibitor reduces tumor burden in a human lung cancer model (lower is better)68
68 Chen, Z. et al. Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer 18, 759–71 (2011).
| 66 |

PKCδ inhibitors block growth of melanoma cells (lower is better)69

PKCδ inhibitors inhibit growth of neuroendocrine tumor cell lines (lower is better).70
69 Chen, C. Y., Liou, J., Forman, L. W. & Faller, D. V. Differential regulation of discrete apoptotic pathways by Ras. J. Biol. Chem. 273, 16700–9 (1998).
70 Chen, Z. et al. Protein kinase Cδ inactivation inhibits cellular proliferation and decreases survival in human neuroendocrine tumors. Endocr. Relat. Cancer 18, 759–71 (2011).
| 67 |

PKCδ
inhibitors decrease tumor size and improve survival in pancreatic cancer model.71
(A)
lower is better
(B) higher is better
Mechanism of Action of BRIA-IMT™ and BRIA-OTS™
The mechanism of action of Bria-IMT™/Bria-OTS™ is currently under investigation.
We believe that Bria-IMT™/Bria-OTS™, activates the patient’s immune system to recognize tumor cells and destroy them. We hypothesize that Bria-IMT™/Bria-OTS™, exerts its action via changing the tumor’s antigen-presentation system {i.e. the system that presents antigen material on the surface of the tumor cell – to be recognized by the T cells of the immune system as either self (i.e., safe) or foreign (i.e., to be destroyed)}. Specifically, Bria-IMT™/Bria-OTS™, is thought to stimulate dendritic cells, a key component of the antigen-presenting system, to display certain immunogenic (i.e., immune response-generating) protein fragments to T cells, which activates the T cells to destroy the tumor cells either directly, or indirectly by inducing a humoral (antibody-generating) immune response. In addition, we also have shown that Bria-IMT™ is capable of directly stimulating cancer-fighting T cells. This unique property of Bria-IMT™ further boosts the immune response against the tumor cells and enhances anti-cancer activity.72
Our preliminary analyses have shown several up-regulated genes in Bria-IMT™ that encode proteins known to be immunogenic (i.e. immune response-generating), suggesting that Bria-IMT™ can stimulate the immune system against the cancer cells.
71 Chen, Z., Forman, L. W., Williams, R. M. & Faller, D. V. Protein kinase C-delta inactivation inhibits the proliferation and survival of cancer stem cells in culture and in vivo. BMC Cancer 14, 90 (2014).
72 Lacher M.D., Bauer G. Fury B., Graeve S., Fledderman E.L., Petrie T.D., Coleal-Bergum D.P., Hackett T., Perotti N.H., Kong Y.Y., Kwok W.W., Wagner J.P., Wiseman C.L., and Williams W.V. SV-BR-1-GM, a Clinically Effective GM-CSF- Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4+ T Lymphocytes. Frontiers in Immunology 2018; 9:Article 776.
| 68 |
Bria-IMT™ is a human breast cancer cell line which expresses Her2/neu (a protein well known for its overexpression in breast cancer but also associated other epithelial malignancies including ovarian, pancreatic, colon, bladder and prostate cancers). Bria-IMT™ has been engineered to produce and secrete granulocyte/macrophage-colony stimulating factor (GM-CSF), a protein that promotes dendritic cell function, a key component of the immune system, and hence activates the immune system.
BRIA-IMT™ & BRIA-OTS™
Potential Mechanisms of Specific Immune Activation in Advanced Breast Cancer

| 1. | Bria-IMT/OTS™ produces breast cancer antigens (proteins made by breast cancer cells) |
| 2. | Bria-IMT/OTS™ secretes GM-CSF which further promotes dendritic cell-based antigen presentation (boosts the response) |
| 3. | Breast cancer antigens are taken up by dendritic cells and “presented” to CD4+ and CD8+ T cells implicated in tumor destruction. |
| 4. | Bria-IMT/OTS™ directly stimulates cancer fighting CD4+ and CD8+ T cells (further boosts the response) |
| 5. | Bria-IMT/OTS™ efficacy depends on HLA matching of Bria-IMT/OTS™ and the patient |
Clinical Trials
Phase I/IIA Combination Study of BRIA-IMT™ with KEYTRUDA® in Advanced Breast Cancer
The FDA has approved the combination study of Bria-IMT™ with pembrolizumab (KEYTRUDA®; manufactured by Merck & Co., Inc.). The Company is currently enrolling patients for this study.
KEYTRUDA® Combination: Patients will be treated with the combination of Bria-IMT™ and the anti-PD-1 antibody KEYTRUDA®.
| 69 |

Rationale for the Combination Study of BRIA-IMT™ with KEYTRUDA®
The immune checkpoint inhibitors such as pembrolizumab (KEYTRUDA®; anti-PD-1) have come to the forefront in the fight against cancer with substantial benefits for some patients. Most recently, the significance of immune checkpoint inhibitors was recognized by the Nobel committee by awarding Dr. Tasuku Honjo and Dr. James P. Allison with the 2018 Nobel Prize in Physiology or Medicine (Scientists behind game-changing cancer immunotherapies win Nobel medicine prize), validating the Company’s decision to launch a combination therapy with the immune checkpoint inhibitors.
| 70 |
Drs. Alison and Honjo independently, using different strategies, showed a new approach of treating patients by awakening certain cells of the immune system (T cells) to attack tumors. This new approach of treating patients with immune checkpoint inhibitors (such as KEYTRUDA®), designed to overcome immune suppression in cancer patients, is revolutionizing the fight against cancer.
In 2010 an important pre-clinical study by Dr. Allison’s group showed that combination with anti-PD-1 antibodies potentiated the tumor-destroying effect of melanoma cells engineered to produce granulocyte-macrophage colony-stimulating factor (GM-CSF), a substance that activates the immune system, compared to the treatment with the GM-CSF producing cells alone. Bria-IMT™ similarly uses a breast cancer cell line which produces GM-CSF. Bria-IMT™ has also been shown to indirectly and directly stimulate T cells, and hence boost the immune system. BriaCell has published these findings in a leading immunology journal. It is important to note that pembrolizumab has not been shown to work on its own in breast cancer but is approved for other indications.
KEYTRUDA® (pembrolizumab)
Manufactured by Merck & Co., Inc., KEYTRUDA® (pembrolizumab) is a prescription medicine that may treat certain cancers by working with the immune system. It has been approved for the treatment of a number of cancer indications excluding breast cancer.
BriaCell & Incyte Collaboration and Supply Agreement
Non-exclusive clinical trial collaboration to evaluate the effects of combinations of novel clinical candidates
| ● | The clinical study will focus on (but not limited to) BriaCell’s lead candidate, Bria-IMT™, in combination with Incyte’s selected compounds for advanced breast cancer. |
| ● | Incyte to provide compounds from its development portfolio, including INCMGA0012, an anti-PD-1 monoclonal antibody, and epacadostat, an IDO1 inhibitor, for use in combination studies with BriaCell’s lead candidate, Bria-IMT™. |
| ● | Incyte (INCY-NASDAQ; ~US$20Bn market capitalization) is a global biopharmaceutical company focused on discovering and developing novel therapeutics in oncology and other serious diseases. |
| ● | Incyte has a deep and rich pipeline in immuno-oncology with numerous molecular targets including PD-1, IDO, GITR, OX40, TIM-3, LAG-3, ARG, AXL/MER and PD-L1xCD137 |
| ● | The first 6 patients will receive the Bria-IMT™ regimen in combination with INCMGA00012. Once safety of the combination has been established, subsequent cohorts will receive a triple combination of the Bria-IMT™ regimen with INCMGA00012 and epacadostat. |
| ● | The design of the clinical study is shown below. Dosing of the novel combinations will commence in September 2019. |
| 71 |

Completed Proof of Concept Trials
BriaCell conducted three Proof of Concept clinical trials, one using parental SV-BR-1 cells and the other two using Bria-IMT™ (i.e., genetically engineered SV-BR-1 cells – producing GM-CSF also called SV-BR-1-GM), in metastatic (i.e., Stage IV) breast cancer patients who had failed prior treatments. The patients were treated according to the following schedule, and the results are summarized below.
| 72 |

First Proof of Concept Trial73
| ● | Used unmodified cell line (parental SV-BR-1 cells) + GM-CSF + cyclophosphamide | |
| ● | N = 14 late stage, treatment-refractory breast cancer patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 12.1 months |
Second Proof of Concept Trial74
| ● | Used Bria-IMT™ (genetically engineered SV-BR-1 cells – producing GM-CSF) + cyclophosphamide + interferon-α | |
| ● | N = 4 late stage, treatment-refractory (3 breast cancer, and 1 ovarian cancer) patients | |
| ● | No significant adverse treatment-associated events, well tolerated | |
| ● | Median Overall Survival = 35 months | |
| ● | One robust responder with >90% regression during treatment, subsequent relapse (upon halting treatment) responded to re-treatment |
Third Proof of Concept Trial75
Thirty patients were screened, 24 enrolled and 23 dosed in the Phase I/IIa study (2017-2018)
| ● | Bria-IMT™ was very well tolerated (over 100 doses given) | |
| ● | The majority of adverse events (AEs) were limited to expected minor local irritation at the injection sites | |
| ● | No related grade >3 or unexpected AEs | |
| ● | No related serious AEs | |
| ● | No serious, unexpected, related AEs |
73 Wiseman, C. L. & Kharazi, A. Phase I Study with SV-BR-1 Breast Cancer Cell Line Vaccine and GMCSF: Clinical Experience in 14 Patients. Open Breast Cancer J. 2, 4–11 (2010).
74 Wiseman, C. L. & Kharazi, A. Objective Clinical Regression of Metastatic Breast Cancer in Disparate Sites after Use of Whole-Cell Vaccine Genetically Modified to Release Sargramostim. Breast J. 12, 475–480 (2006).
75 Listed on ClinicalTrials.gov as NCT03066947 https://clinicaltrials.gov/ct2/show/NCT03066947?term=BriaCell&rank=2
| 73 |
Most patients who have dropped out did so due to worsening of their underlying disease.
| ● | Bria-IMT™ appears to be most effective in patients who match with Bria-IMT ™ at 2 HLA loci (types) further supporting our “HLA Matching Hypothesis”, and the development of Bria-OTS ™ to cover 90% of the patient population. | |
| ● | Effectiveness also depends on the ability of the patient to develop an immune response to Bria-IMT™ | |
| ● | Results are shown in the tables here, combining the second and third proof of concept studies which both used Bria-IMT™ in an identical regimen. |
| Patients (n) | HLA match | Tumor Shrinkage | Biological Response* | |||||||||
| 5 | >2 | 40 | % | 60 | % | |||||||
| 19 | >1 | 21 | % | 37 | % | |||||||
| 8 | 0 | 0 | % | 0 | % | |||||||
*Biological response includes tumor shrinkage or lower circulating cancer associated cells
| Patients (n) | All Patients (n=27) | No HLA match (n=7) | 1+ HLA Match (n=20) | 2+ HLA matches (n=5) | ||||
| Negative DTH (n=7) | 0% (0/1) | 0% | 0% (0/7) | 0% (0/1) | ||||
| Positive DTH (n=20) | 20% (4/20) | 0% (0/7) | 31% (4/13) | 50% (2/4) |
| ● | Initial safety data appears superior to that of the other advanced or approved drugs for breast cancer when they were at a similar stage of clinical development. | |
| ● | Initial efficacy data is similar or superior to those of other advanced or approved drugs for breast cancer when they were at a similar clinical stage of development. | |
| ● | Bria-IMT™ was dosed in 27 patients (4 in 2004-2006, 23 in 2017-2018) as the Bria-IMT™ regimen alone. | |
| ● | Bria-IMT™ has been very well tolerated (over 100 doses given to date). | |
| ● | The majority of adverse events (AEs) were limited to expected minor local irritation at the injection sites. | |
| ● | Tumor regression was seen in patents who were able to mount an immune response and matched Bria-IMT™ at HLA types confirming our main hypothesis. |
| 74 |
Marketing and Sales Strategy
The product will initially be marketed to oncologists who are well versed in the use of immunotherapy for cancer. Partnering with other pharma companies in order to market combinations with a number of drugs is also an option that we intend to pursue. This study will utilize a frozen formulation which consists of irradiated SV-BR-1-GM cells in viable freezing media. This formulation will permit stockpiling of the immunotherapy so that it can be sent on demand to clinical sites. The eventual goal is to reach all oncologists who treat late stage breast cancer either by direct outreach or by partnering with another company that has an established presence in the oncology space.
Other Commercial Considerations
There is a high unmet medical need in late stage breast cancer, providing potential for accelerated approval of Bria-IMT™. The FDA is interested in facilitating the availability of novel therapies of patients with unmet medical needs, especially those that can target the population most likely to respond. In addition, Bria-IMT™ may fit the description of an orphan drug, especially if HLA matching is required. These two facts may help facilitate accelerated approval of Bria-IMT™.
53 Suzuki N, Hazama S, Iguchi H, Uesugi K, Tanaka H, Hirakawa K, Aruga A, Hatori T, Ishizaki H, Umeda Y, Fujiwara T, Ikemoto T, Shimada M, Yoshimatsu K, Shimizu R, Hayashi H, Sakata K, Takenouchi H, Matsui H, Shindo Y, Iida M, Koki Y, Arima H, Furukawa H, Ueno T, Yoshino S, Nakamura Y, Oka M, Nagano H. Phase II clinical trial of peptide cocktail therapy for patients with advanced pancreatic cancer: VENUS-PC study. Cancer Sci. 2017 Jan;108(1):73-80.
54 Antonilli, M.; Rahimi, H.; Visconti, V.; Napoletano, C.; Ruscito, I.; Zizzari, I. G.; Caponnetto, S.; Barchiesi, G.; Iadarola, R.; Pierelli, L.; Rughetti, A.; Bellati, F.; Panici, P. B.; Nuti, M., Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial. Int J Oncol 2016, 48 (4), 1369-78.
55 Limentani, S. A.; Campone, M.; Dorval, T.; Curigliano, G.; de Boer, R.; Vogel, C.; White, S.; Bachelot, T.; Canon, J. L.; Disis, M.; Awada, A.; Berliere, M.; Amant, F.; Levine, E.; Burny, W.; Callegaro, A.; de Sousa Alves, P. M.; Louahed, J.; Brichard, V.; Lehmann, F. F., A non-randomized dose-escalation Phase I trial of a protein-based immunotherapeutic for the treatment of breast cancer patients with HER2-overexpressing tumors. Breast Cancer Res Treat 2016, 156 (2), 319-30; Curigliano, G.; Romieu, G.; Campone, M.; Dorval, T.; Duck, L.; Canon, J. L.; Roemer-Becuwe, C.; Roselli, M.; Neciosup, S.; Burny, W.; Callegaro, A.; de Sousa Alves, P. M.; Louahed, J.; Brichard, V.; Lehmann, F. F., A phase I/II trial of the safety and clinical activity of a HER2-protein based immunotherapeutic for treating women with HER2-positive metastatic breast cancer. Breast Cancer Res Treat 2016, 156 (2), 301-10.
56 Wang, X.; Ren, J.; Zhang, J.; Yan, Y.; Jiang, N.; Yu, J.; Di, L.; Song, G.; Che, L.; Jia, J.; Zhou, X.; Yang, H.; Lyerly, H. K., Prospective study of cyclophosphamide, thiotepa, carboplatin combined with adoptive DC-CIK followed by metronomic cyclophosphamide therapy as salvage treatment for triple negative metastatic breast cancers patients (aged <45). Clin Transl Oncol 2016, 18 (1), 82-7; Qi, C. J.; Ning, Y. L.; Han, Y. S.; Min, H. Y.; Ye, H.; Zhu, Y. L.; Qian, K. Q., Autologous dendritic cell vaccine for estrogen receptor (ER)/progestin receptor (PR) double-negative breast cancer. Cancer Immunol Immunother 2012, 61 (9), 1415-24.
57 Tiriveedhi, V.; Tucker, N.; Herndon, J.; Li, L.; Sturmoski, M.; Ellis, M.; Ma, C.; Naughton, M.; Lockhart, A. C.; Gao, F.; Fleming, T.; Goedegebuure, P.; Mohanakumar, T.; Gillanders, W. E., Safety and preliminary evidence of biologic efficacy of a mammaglobin-a DNA vaccine in patients with stable metastatic breast cancer. Clin Cancer Res 2014, 20 (23), 5964-75.
58 Chen, G.; Gupta, R.; Petrik, S.; Laiko, M.; Leatherman, J. M.; Asquith, J. M.; Daphtary, M. M.; Garrett-Mayer, E.; Davidson, N. E.; Hirt, K.; Berg, M.; Uram, J. N.; Dauses, T.; Fetting, J.; Duus, E. M.; Atay-Rosenthal, S.; Ye, X.; Wolff, A. C.; Stearns, V.; Jaffee, E. M.; Emens, L. A., A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol Res 2014, 2 (10), 949-61.
59 Mittendorf, E. A.; Clifton, G. T.; Holmes, J. P.; Schneble, E.; van Echo, D.; Ponniah, S.; Peoples, G. E., Final report of the phase I/II clinical trial of the E75 (nelipepimut-S) vaccine with booster inoculations to prevent disease recurrence in high-risk breast cancer patients. Ann Oncol 2014, 25 (9), 1735-42.
60 Vassilaros, S.; Tsibanis, A.; Tsikkinis, A.; Pietersz, G. A.; McKenzie, I. F.; Apostolopoulos, V., Up to 15-year clinical follow-up of a pilot Phase III immunotherapy study in stage II breast cancer patients using oxidized mannan-MUC1. Immunotherapy 2013, 5 (11), 1177-82.
61 Takahashi, R.; Toh, U.; Iwakuma, N.; Takenaka, M.; Otsuka, H.; Furukawa, M.; Fujii, T.; Seki, N.; Kawahara, A.; Kage, M.; Matsueda, S.; Akagi, Y.; Yamada, A.; Itoh, K.; Sasada, T., Feasibility study of personalized peptide vaccination for metastatic recurrent triple-negative breast cancer patients. Breast Cancer Res 2014, 16 (4), R70.
62 Chen, G.; Gupta, R.; Petrik, S.; Laiko, M.; Leatherman, J. M.; Asquith, J. M.; Daphtary, M. M.; Garrett-Mayer, E.; Davidson, N. E.; Hirt, K.; Berg, M.; Uram, J. N.; Dauses, T.; Fetting, J.; Duus, E. M.; Atay-Rosenthal, S.; Ye, X.; Wolff, A. C.; Stearns, V.; Jaffee, E. M.; Emens, L. A., A feasibility study of cyclophosphamide, trastuzumab, and an allogeneic GM-CSF-secreting breast tumor vaccine for HER2+ metastatic breast cancer. Cancer Immunol Res 2014, 2 (10), 949-61; Lacher M.D., Bauer G. Fury B., Graeve S., Fledderman E.L., Petrie T.D., Coleal-Bergum D.P., Hackett T., Perotti N.H., Kong Y.Y., Kwok W.W., Wagner J.P., Wiseman C.L., and Williams W.V. SV-BR-1-GM, a Clinically Effective GM-CSF- Secreting Breast Cancer Cell Line, Expresses an Immune Signature and Directly Activates CD4+ T Lymphocytes. Frontiers in Immunology 2018; 9:Article 776; Wiseman, C. L. & Kharazi, A. Objective Clinical Regression of Metastatic Breast Cancer in Disparate Sites after Use of Whole-Cell Vaccine Genetically Modified to Release Sargramostim. Breast J. 12, 475–480 (2006).
| 75 |
Production and Marketing Plan
Bria-IMT™ cells grow in simple tissue culture media and are irradiated prior to inoculation. Bria-IMT™ manufacturing will be performed by Contract Manufacturing Organizations (CMOs). Recently we have been working with KBI Biopharma who have developed a frozen formulation, where the cells are grown, harvested and irradiated followed by cryopreservation in a viable state. The cells are stockpiled and shipped directly to clinical sites for inoculation. Each lot of Bria-IMT™ is tested for potency (GM-CSF production), identity (HER2+ and ER/PR-) and adventitious agents to assure that each patient receives a safe and effective treatment. To date, there have been no issues with these tests. Additional manufacturing facilities have been evaluated and may be enlisted as demand grows.
Marketing will target oncologists who are well versed in the use of immunotherapy and cancer vaccines and especially breast cancer treatment centers. We plan to develop the clinical data for Bria-IMT™ and use this information to reach out to oncologists seeking additional therapeutic options for their patients. We will include in this effort a physician education campaign targeting the oncologists most likely to treat metastatic breast cancer. As these physicians become more aware of the data regarding Bria-IMT™ in breast cancer, we will make sure they also understand how best to use Bria-IMT™ in combination with other therapies that have complementary of synergistic mechanisms of action. This will also come from the clinical studies described above focusing on combination therapy. Partnering with other pharma companies in order to market a number of drugs is also an option that we intend to pursue. Our eventual goal is to reach all oncologists who treat late stage breast cancer either by direct outreach or by partnering with another company that has an established presence in the oncology space.
License Agreements
On July 24, 2017, the Company entered into a definitive share exchange agreement (the “Share Exchange Agreement”) with its wholly-owned subsidiary, BriaCell Therapeutics Corp., and Sapientia Pharmaceuticals, Inc. including all the shareholders of Sapientia. Sapientia, a biotechnology company based in Havertown, PA, is developing novel targeted therapeutics for multiple indications including several cancers and fibrotic diseases.
Pursuant to the terms of the Share Exchange Agreement, BriaCell Therapeutics Corp agreed to acquire from the Sapientia Shareholders all of the issued and outstanding shares in the capital of Sapientia in consideration to the Sapientia Shareholders, pro rata, of an aggregate of 2,500,002 common shares in the capital of BriaCell (the “Transaction”), which were issued on September 5, 2017. As part of the Transaction, BriaCell acquired the license agreement Sapientia had with Faller-Williams Technology, pursuant to which BriaCell acquired all rights, including composition of matter patents, and preclinical study data to a novel therapeutic technology platform, known as protein kinase C delta (PKCδ) inhibitors, which represents a unique, highly-targeted approach to treat cancer and to boost the immune system.
Intellectual Property
The proprietary nature of, and protection for, our current and/or any future product candidates, processes and know-how are important to our business as is our ability to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. We seek patent protection in the United States and internationally for our current and future product candidates we may develop and other technology. In order to protect our proprietary technologies, we rely on combinations of application for patent and trade secret protection, as well as confidentiality agreements with employees, consultants, and third parties.
We have filed and own all rights in the following pending patent applications and issued patents:
Filed with the United States Patent and Trademark Office (USPTO) on June 14, 2004, U.S. Patent No. 7,674,456 B2, includes claims to the following:
| 1. | Compositions comprising SV-BR cells | |
| 2. | Therapeutic methods of using said compositions |
On February 27, 2017, BriaCell™ filed an international patent application under the Patent Cooperation Treaty (PCT) to further expand its intellectual property portfolio underlying the Company’s current and anticipated pipeline of whole-cell cancer immunotherapeutics including Bria-IMT™ and Bria-OTS™. The PCT application (PCT/US2017/019757) claims priority to two provisional patent applications filed by the Company with the USPTO in 2016. It, in essence, provides the framework for additional whole-cell cancer immunotherapeutics beyond Bria-IMT™ and strategies for patient-specific selection of the most likely effective whole-cell immunotherapeutic (BriaDx™). The PCT application entered the National Phase in the second half of 2018.
On July 24, 2017 BriaCell obtained the exclusive license to certain patents related to protein kinase C delta (PKCδ) inhibitor technology that includes patents to specific compounds, methods of using the compounds, and methods of assessing patients regarding the compounds. These patents include U.S. Patent No. 9,364,460 which issued June 14, 2016, U.S. Patent No. 9,572,793 which issued February 21, 2017, U.S. Patent No. 9,844,534 which issued December 19, 2017, and EP Patent No. 2897610 which issued January 10, 2018 that has been validated in Austria, Belgium, Switzerland, Germany, Denmark, Spain, Finland, France, Great Britain, Ireland, Italy, the Netherlands, Norway, Sweden and Turkey.
To the knowledge of the Company’s management, there are no contested proceedings or third-party claims over any of our patent applications. Our success depends upon our ability to protect our technologies through intellectual property agreements including patents, trademarks, know-how, and confidentiality agreements. However, there can be no assurance that the above-mentioned patent applications will be approved by the appropriate agencies.
All of the technology for which the patents are sought is owned by the Company. Our patents are entirely owned by the Company.
The Company has also filed applications in the United States and Canada to register the BriaCell name as a trademark.
| 76 |
Competition
Cancer immunotherapy has become a significant growth area for the biopharmaceutical industry, attracting large pharmaceutical companies as well as small niche players. Generally, our principal competitors in the cancer immunotherapy market comprise both companies with currently approved products for various indications, such as manufacturers of approved bispecific antibodies, CAR-T cells, and checkpoint inhibitors, as well as companies currently engaged in cancer immunotherapy clinical development. The large and medium-size players who have successfully obtained approval for cancer immunotherapy products include Bristol-Myers Squib Company, Merck & Co., Inc., Genentech, Inc. (a subsidiary of Roche Holding AG), AstraZeneca PLC, Celgene Corporation, Johnson & Johnson/Janssen Pharmaceuticals, Amgen, Novartis, Acerta Pharmaceuticals (a subsidiary of AstraZeneca), Juno Therapeutics, Inc. (a subsidiary of Celgene), Kite Pharma, Inc., a wholly-owned subsidiary of Gilead Sciences, Inc. and Pfizer, Inc./EMD Serono, Inc. Most of these companies, either alone or together with their collaborative partners, have substantially greater financial resources than we do.
Companies developing novel products with similar indications to those we are pursuing are expected to influence our ability to penetrate and maintain market share. For patients with early stage breast cancer, adjuvant therapy is often given to prevent recurrence and increase the chance of long-term DFS. Adjuvant therapy for breast cancer can include chemotherapy, hormonal therapy, radiation therapy, or combinations thereof. In addition, the HER2 targeted drug trastuzumab (HERCEPTIN) - alone or in combination with pertuzumab (PERJETA), both manufactured and marketed by Roche/Genentech may be given to patients with tumors with high expression of HER2 (IHC 3+), as well as other novel targets such as MUC1, which may be useful in treating breast cancer. In addition, the FDA recently approved the first ever immunotherapy regimen for breast cancer to the Roche/Genentech PD-L1 checkpoint inhibitor atezolizumab (TECENTRIQ), combined with Celgene’s nab-paclitaxel (ABRAXANE) for TNBC that cannot be removed with surgery and is locally advanced or metastatic.
There are a number of cancer vaccines in development for breast cancer, including but not limited toTPIV200 (Marker Therapeutics, Inc.), AE-37 (Antigen Express), and Stimuvax (Merck KgA). While these development candidates are aimed at a number of different targets, and AE-37 has published data in the HER2 breast cancer patient population, there is no guarantee that any of these compounds will not in the future be indicated for treatment of low-to-intermediate HER2 breast cancer patients and become directly competitive with NPS.
Many of our competitors, either alone or with their strategic partners, have substantially greater financial, technical and human resources than we do, and also have greater experience in obtaining FDA and other regulatory approvals of treatments and commercializing those treatments. Accordingly, our competitors may be more successful than us in obtaining approval for cancer immunotherapy products and achieving widespread market acceptance. Our competitors’ treatments may be more effectively marketed and sold than any products we may commercialize, thus causing limited market share before we can recover the expenses of developing and commercializing of our cancer immunotherapy product candidate.
Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These activities may lead to consolidated efforts that allow for more rapid development of cancer immunotherapy product candidates.
These competitors also compete with us in recruiting and retaining qualified scientific and management personnel, the ability to work with specific clinical contract organizations due to conflict of interest, and also the conduct of trials in the ability to recruit clinical trial sites and subjects for our clinical trials.
We expect any products that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, price and the availability of reimbursement from government and other third-party payors. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are viewed as safer, more convenient or less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for our current product candidates or any other future product candidate, which could result in our competitors establishing a strong market position before we are able to enter the market.
| 77 |
Employees
As of July 31, 2019, we had five full-time employees and one part-time employee, located in Berkeley, CA, Los Angeles, CA, Havertown, PA and Tel Aviv, Israel.
In each of the years ended July 31, 2017, 2018 and 2019, the average number of employees, including executives, has been four, of whom two were executive management and two were engaged in research and development. Of these four employees, three were located in California and one in Pennsylvania.
Research and Development Activities and Costs
For information regarding our clinical studies, please see above under the caption “– Clinical Studies in Process.”
For the years ended July 31, 2018, 2017 and 2016, we incurred $3,112,579, $2,127,941, and $944,942, respectively, of net research and development expense. For the nine months ended April 30, 2019, we incurred $ 1,056,154 of research and development expenses.
Manufacturing
We do not own or operate manufacturing facilities for the production of our product candidates, nor do we have plans to develop our own manufacturing operations in the foreseeable future. We currently depend on third-party contract manufacturers for all of our required raw materials, active pharmaceutical ingredients, and finished product candidate for our clinical trials. We currently employ internal resources and third-party consultants to manage our manufacturing contractors.
Bria-IMT™ is currently manufactured under cGMP pursuant to agreements with the University of California, Davis Health System and with KBI Biopharma, Inc., which is located in The Woodlands, Texas.
Sales and Marketing
We have not yet defined our sales, marketing or product distribution strategy for our product candidates or any future product candidates. Our future commercial strategy may include the use of strategic partners, distributors, a contract sale force, or the establishment of our own commercial and specialty sales force, as well as similar strategies for regions and territories outside the United States. We plan to further evaluate these alternatives as we approach approval for the use of our product candidates for one or more indications.
Property, Plant and Equipment
We do not own any real property. Our corporate offices in Canada are located at Suite 300, Bellevue Centre, 235-15th Street, West Vancouver, BC V7T 2XI. Our corporate and research offices in the United States are located at 820 Heinz Avenue, Berkeley, California, 94710.
We consider our current office space sufficient to meet our anticipated needs for the foreseeable future and is suitable for the conduct of our business.
Government Regulation
The Food and Drug Administration (FDA) and other regulatory authorities at federal, state, and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring, and post-approval reporting of biologics such as those we are developing. Along with third-party contractors, we will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of its current or future product candidates. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label.
| 78 |
The process required by the FDA before biologic product candidates may be marketed in the United States generally involves the following:
| ● | completion of preclinical laboratory tests and animal studies performed in accordance with the FDA’s current Good Laboratory Practices, or GLP, regulations76; |
| ● | submission to the FDA of an Investigational New Drug Application (IND), which must become effective before clinical trials may begin and must be updated annually or when significant changes are made; |
| ● | approval by an independent Institutional Review Board, or IRB, or ethics committee at each clinical site before the trial is begun; |
| ● | performance of adequate and well-controlled human clinical trials to establish the safety, purity and potency of the proposed biologic product candidate for its intended purpose; |
| ● | preparation of and submission to the FDA of a Biological Licensing Agreement (BLA), after completion of all pivotal clinical trials; |
| ● | satisfactory completion of an FDA Advisory Committee review, if applicable; |
| ● | a determination by the FDA within 60 days of its receipt of a BLA to file the application for review; |
| ● | satisfactory completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed product is produced to assess compliance with current Good Manufacturing Practices, or cGMP, and to assure that the facilities, methods and controls are adequate to preserve the biological product’s continued safety, purity and potency, and of selected clinical investigations to assess compliance with current Good Clinical Practices, or GCP; and |
| ● | FDA review and approval of the BLA to permit commercial marketing of the product for particular indications for use in the United States, which must be updated annually when significant changes are made. |
76 Note that Bria-IMT™ and Bria-OTS™ are comprised of human cell lines and as such are not evaluable in non-human animal studies. This has been made known to FDA who have not raised any objections.
| 79 |
The testing and approval process requires substantial time, effort and financial resources, and we cannot be certain that any approvals for our current or future product candidates will be granted on a timely basis, if at all. Prior to beginning the first clinical trial with a product candidate, we must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug to humans. The central focus of an IND submission is on the general investigational plan and the protocol(s) for clinical studies. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology, and pharmacodynamic characteristics of the product; chemistry, manufacturing, and controls information; and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators in accordance with GCP, which include the requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins at that site and must monitor the clinical trial until completed. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by a Data & Safety Monitoring Board (DSMB) organized by the clinical trial sponsor, which provides authorization for whether or not a clinical trial may move forward at designated check points based on access to certain data from the clinical trial and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy. There are also requirements governing the reporting of ongoing clinical studies and clinical trial results to public registries.
For purposes of BLA approval, human clinical trials are typically conducted in three sequential phases that may overlap.
| ● | Phase 1-The investigational product is initially introduced into healthy human subjects or patients with the target disease or condition. These studies are designed to test the safety, dosage tolerance, absorption, metabolism and distribution of the investigational product in humans, the side effects associated with increasing doses, and, if possible, to gain early evidence on effectiveness. |
| ● | Phase 2-The investigational product is administered to a limited patient population with a specified disease or condition to evaluate the preliminary efficacy, optimal dosages and dosing schedule and to identify possible adverse side effects and safety risks. Multiple Phase 2 clinical trials may be conducted to obtain information prior to beginning larger and more expensive Phase 3 clinical trials. |
| 80 |
| ● | Phase 3-The investigational product is administered to an expanded patient population to further evaluate dosage, to provide statistically significant evidence of clinical efficacy and to further test for safety, generally at multiple geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the investigational product and to provide an adequate basis for product approval. |
| ● | Phase 4-In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product. These so-called Phase 4 studies may be made a condition to approval of the BLA. |
Phase 1, Phase 2 and Phase 3 testing may not be completed successfully within a specified period, if at all, and there can be no assurance that the data collected will support FDA approval or licensure of the product. Concurrent with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics of the product candidate and must finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product, or for biologics, the safety, purity and potency. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
BLA Submission and Review by the FDA
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, nonclinical studies and clinical trials are submitted to the FDA as part of a BLA requesting approval to market the product for one or more indications. The BLA must include all relevant data available from pertinent preclinical and clinical studies, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product’s chemistry, manufacturing, controls, and proposed labeling, among other things. Data can come from company-sponsored clinical studies intended to test the safety and effectiveness of a use of the product, or from a number of alternative sources, including studies initiated by investigators. The submission of a BLA requires payment of a substantial user fee to FDA, and the sponsor of an approved BLA is also subject to annual product and establishment user fees. These fees are typically increased annually. A waiver of user fees may be obtained under certain limited circumstances.
Once a BLA has been submitted, the FDA’s goal is to review the application within ten months after it accepts the application for filing, or, if the application relates to an unmet medical need in a serious or life-threatening indication, six months after the FDA accepts the application for filing. The review process is often significantly extended by FDA requests for additional information or clarification. The FDA reviews a BLA to determine, among other things, whether a product is safe, pure and potent and the facility in which it is manufactured, processed, packed, or held meets standards designed to assure the product’s continued safety, purity and potency. The FDA may convene an advisory committee to provide clinical insight on application review questions. Before approving a BLA, the FDA will typically inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA, the FDA will typically inspect one or more clinical sites to assure compliance with GCP. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
| 81 |
The testing and approval process requires substantial time, effort and financial resources, and each may take several years to complete. The FDA may not grant approval on a timely basis, or at all, and we may encounter difficulties or unanticipated costs in its efforts to secure necessary governmental approvals, which could delay or preclude us from marketing its products. After the FDA evaluates a BLA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be produced, the FDA may issue an approval letter or a Complete Response Letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A Complete Response Letter indicates that the review cycle of the application is complete and the application is not ready for approval. A Complete Response Letter may request additional information or clarification. The FDA may delay or refuse approval of a BLA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval may entail limitations on the indicated uses for which such product may be marketed. For example, the FDA may approve the BLA with a Risk Evaluation and Mitigation Strategy, or REMS, plan to mitigate risks, which could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing regulatory standards is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4 post-market studies and surveillance to further assess and monitor the product’s safety and effectiveness after commercialization and may limit further marketing of the product based on the results of these post-marketing studies. In addition, new government requirements, including those resulting from new legislation, may be established, or the FDA’s policies may change, which could delay or prevent regulatory approval of our products under development.
A sponsor may seek approval of its product candidate under programs designed to accelerate FDA’s review and approval of new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for Fast Track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. For a product candidate with Fast Track designation, the FDA may consider sections of the BLA for review on a rolling basis before the complete application is submitted if relevant criteria are met. A Fast Track designated product candidate may also qualify for priority review, under which the FDA sets the target date for FDA action on the BLA at six months after the FDA accepts the application for filing. Priority review is granted when there is evidence that the proposed product would be a significant improvement in the safety or effectiveness of the treatment, diagnosis, or prevention of a serious condition. If criteria are not met for priority review, the application is subject to the standard FDA review period of 10 months after FDA accepts the application for filing. Priority review designation does not change the scientific/medical standard for approval or the quality of evidence necessary to support approval.
Under the Accelerated Approval program, the FDA may approve a BLA on the basis of either a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. Post-marketing studies or completion of ongoing studies after marketing approval are generally required to verify the biologic’s clinical benefit in relationship to the surrogate endpoint or ultimate outcome in relationship to the clinical benefit.
In addition, a sponsor may seek FDA designation of its product candidate as a Breakthrough Therapy, if the product candidate is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the therapy may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. If the FDA designates a breakthrough therapy, it may take actions appropriate to expedite the development and review of the application. Breakthrough designation also allows the sponsor to file sections of the BLA for review on a rolling basis.
Fast Track, Priority Review and Breakthrough Therapy designations do not change the standards for approval but may expedite the development or approval process.
| 82 |
Orphan Drugs
Under the Orphan Drug Act, the FDA may grant Orphan Drug Product Designation to a drug or biologic intended to treat a rare disease or condition, defined as a disease or condition with a patient population of fewer than 200,000 individuals in the United States, or a patient population greater than 200,000 individuals in the United States and when there is no reasonable expectation that the cost of developing and making available the drug or biologic in the United States will be recovered from sales in the United States for that drug or biologic. Orphan Drug Product Designation must be requested before submitting a BLA. After the FDA grants Orphan Drug Product Designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA.
If a product that has Orphan Drug Product Designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a full BLA, to market the same biologic for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan drug exclusivity does not prevent the FDA from approving a different drug or biologic for the same disease or condition, or the same drug or biologic for a different disease or condition. Among the other benefits of Orphan Drug Product Designation are tax credits for certain research and a waiver of the BLA application user fee.
A drug with Orphan Drug Product Designation may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which it received Orphan Drug Product Designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Other Healthcare Laws and Compliance Requirements
Our sales, promotion, medical education and other activities following product approval will be subject to regulation by numerous regulatory and law enforcement authorities in the United States in addition to FDA, including potentially the Federal Trade Commission, the Department of Justice, the Centers for Medicare and Medicaid Services, other divisions of the Department of Health and Human Services and state and local governments. Our promotional and scientific/educational programs must comply with the federal Anti-Kickback Statute, the Foreign Corrupt Practices Act, the False Claims Act, or FCA, the Veterans Health Care Act, physician payment transparency laws, privacy laws, security laws, and additional state laws similar to the foregoing.
The federal Anti-Kickback Statute prohibits, among other things, the offer, receipt, or payment of remuneration in exchange for or to induce the referral of patients or the use of products or services that would be paid for in whole or part by Medicare, Medicaid or other federal health care programs. Remuneration has been broadly defined to include anything of value, including cash, improper discounts, and free or reduced price items and services. The government has enforced the Anti-Kickback Statute to reach large settlements with healthcare companies based on sham research or consulting and other financial arrangements with physicians. Further, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. In addition, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the FCA. Many states have similar laws that apply to their state health care programs as well as private payors.
| 83 |
The FCA, imposes liability on persons who, among other things, present or cause to be presented false or fraudulent claims for payment by a federal health care program. The FCA has been used to prosecute persons submitting claims for payment that are inaccurate or fraudulent, that are for services not provided as claimed, or for services that are not medically necessary. Actions under the FCA may be brought by the Attorney General or as a qui tam action by a private individual in the name of the government. Violations of the FCA can result in significant monetary penalties and treble damages. The federal government is using the FCA, and the accompanying threat of significant liability, in its investigation and prosecution of pharmaceutical and biotechnology companies throughout the country, for example, in connection with the promotion of products for unapproved uses and other sales and marketing practices. The government has obtained multi-million and multibillion dollar settlements under the FCA in addition to individual criminal convictions under applicable criminal statutes. In addition, companies have been forced to implement extensive corrective action plans, and have often become subject to consent decrees or corporate integrity agreements, restricting the manner in which they conduct their business. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, also created federal criminal statutes that prohibit, among other things, knowingly and willfully executing a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Given the significant size of actual and potential settlements, it is expected that the government will continue to devote substantial resources to investigating healthcare providers’ and manufacturers’ compliance with applicable fraud and abuse laws.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, among other things, imposed new reporting requirements on drug manufacturers for payments or other transfers of value made by them to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit required information may result in civil monetary penalties. Certain states also mandate implementation of commercial compliance programs, impose restrictions on drug manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to physicians and other healthcare professionals.
We may also be subject to data privacy and security regulation by both the federal government and the states in which it conducts its business. HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and their respective implementing regulations, imposes specified requirements relating to the privacy, security and transmission of individually identifiable health information. Among other things, HITECH makes HIPAA’s privacy and security standards directly applicable to “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect.
If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to it, we may be subject to penalties, including, without limitation, civil and criminal penalties, damages, fines, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results. Also, the U.S. Foreign Corrupt Practices Act and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from making improper payments to foreign officials for the purpose of obtaining or retaining business. We cannot assure you that our internal control policies and procedures will protect us from reckless or negligent acts committed by our employees, future distributors, partners, collaborators or agents. Violations of these laws, or allegations of such violations, could result in fines, penalties or prosecution and have a negative impact on our business, results of operations and reputation.
| 84 |
Coverage and Reimbursement
Sales of pharmaceutical products depend significantly on the availability of third-party coverage and reimbursement. Third-party payors include government health administrative authorities, managed care providers, private health insurers and other organizations. Although we currently believe that third-party payors will provide coverage and reimbursement for our product candidates, if approved, these third-party payors are increasingly challenging the price and examining the cost-effectiveness of medical products and services. In addition, significant uncertainty exists as to the reimbursement status of newly approved healthcare products. We may need to conduct expensive clinical studies to demonstrate the comparative cost-effectiveness of our product candidates. Seeking coverage and reimbursement from third-party payors can be time consuming and expensive. Moreover, a payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Reimbursement may not be available or sufficient to allow us to sell our products on a competitive and profitable basis.
Foreign Regulation
In addition to regulations in the United States, we are and will be subject, either directly or through our distribution partners, to a variety of regulations in other jurisdictions governing, among other things, clinical trials and commercial sales and distribution of our products, if approved.
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in non-U.S. countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have processes that require the submission of a clinical trial application much like an IND prior to the commencement of human clinical trials. In Europe, for example, a clinical trial application, or CTA, must be submitted to the competent national health authority and to independent ethics committees in each country in which a company plans to conduct clinical trials. Once the CTA is approved in accordance with a country’s requirements, clinical trials may proceed in that country.
The requirements and process governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country, even though there is already some degree of legal harmonization in the European Union member states resulting from the national implementation of underlying E.U. legislation. In all cases, the clinical trials are conducted in accordance with GCP and other applicable regulatory requirements.
To obtain regulatory approval of a new drug or medicinal product in the European Union, a sponsor must obtain approval of a marketing authorization application. The way in which a medicinal product can be approved in the European Union depends on the nature of the medicinal product.
The centralized procedure results in a single marketing authorization granted by the European Commission that is valid across the European Union, as well as in Iceland, Liechtenstein and Norway. The centralized procedure is compulsory for human drugs that are: (i) derived from biotechnology processes, such as genetic engineering, (ii) contain a new active substance indicated for the treatment of certain diseases, such as HIV/AIDS, cancer, diabetes, neurodegenerative diseases, autoimmune and other immune dysfunctions and viral diseases, (iii) officially designated as “orphan drugs” and (iv) advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines. The centralized procedure may at the request of the applicant also be used for human drugs which do not fall within the above mentioned categories if the human drug (a) contains a new active substance which was not authorized in the European Community; or (b) the applicant shows that the medicinal product constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization in the centralized procedure is in the interests of patients or animal health at the European Community level.
Under the centralized procedure in the European Union, the maximum timeframe for the evaluation of a marketing authorization application by the EMA is 210 days (excluding clock stops, when additional written or oral information is to be provided by the applicant in response to questions asked by the Committee for Medicinal Products for Human Use, or CHMP), with adoption of the actual marketing authorization by the European Commission thereafter. Accelerated evaluation might be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of a major public health interest from the point of view of therapeutic innovation, defined by three cumulative criteria: the seriousness of the disease to be treated; the absence of an appropriate alternative therapeutic approach, and anticipation of exceptional high therapeutic benefit. In this circumstance, EMA ensures that the evaluation for the opinion of the CHMP is completed within 150 days and the opinion issued thereafter.
| 85 |
The mutual recognition procedure, or MRP, for the approval of human drugs is an alternative approach to facilitate individual national marketing authorizations within the European Union. The MRP may be applied for all human drugs for which the centralized procedure is not obligatory. The MRP is applicable to the majority of conventional medicinal products, and is based on the principle of recognition of an already existing national marketing authorization by one or more member states.
The characteristic of the MRP is that the procedure builds on an already existing marketing authorization in a member state of the E.U. that is used as reference in order to obtain marketing authorizations in other E.U. member states. In the MRP, a marketing authorization for a drug already exists in one or more member states of the E.U. and subsequently marketing authorization applications are made in other European Union member states by referring to the initial marketing authorization. The member state in which the marketing authorization was first granted will then act as the reference member state. The member states where the marketing authorization is subsequently applied for act as concerned member states.
The MRP is based on the principle of the mutual recognition by European Union member states of their respective national marketing authorizations. Based on a marketing authorization in the reference member state, the applicant may apply for marketing authorizations in other member states. In such case, the reference member state shall update its existing assessment report about the drug in 90 days. After the assessment is completed, copies of the report are sent to all member states, together with the approved summary of product characteristics, labeling and package leaflet. The concerned member states then have 90 days to recognize the decision of the reference member state and the summary of product characteristics, labeling and package leaflet. National marketing authorizations shall be granted within 30 days after acknowledgement of the agreement.
Should any Member State refuse to recognize the marketing authorization by the reference member state, on the grounds of potential serious risk to public health, the issue will be referred to a coordination group. Within a timeframe of 60 days, member states shall, within the coordination group, make all efforts to reach a consensus. If this fails, the procedure is submitted to an EMA scientific committee for arbitration. The opinion of this EMA Committee is then forwarded to the Commission, for the start of the decision-making process. As in the centralized procedure, this process entails consulting various European Commission Directorates General and the Standing Committee on Human Medicinal Products or Veterinary Medicinal Products, as appropriate.
For other countries outside of the European Union, such as countries in Eastern Europe, Latin America or Asia, the requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary from country to country. In all cases, again, the clinical trials are conducted in accordance with GCP and the other applicable regulatory requirements.
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension of clinical trials, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
| 86 |
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1 covering the securities in this offering. This prospectus, which forms part of the registration statement, does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. Some items are omitted in accordance with the rules and regulations of the SEC. For further information regarding both our Company and the securities in this offering, we refer you to the registration statement and the exhibits to the registration statement filed as part of the registration statement. The SEC maintains an internet site at www.sec.gov, from which you can electronically access the registration statement, including the exhibits to the registration statement. We also maintain a website at http://www.briacell.com. You may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
We are not a party to existing or pending legal proceedings against us, and we have no knowledge of any threatened litigation, nor are we involved as a plaintiff in any proceeding or pending litigation. There are no proceedings in which any of our directors, officers or any of their respective affiliates, or any beneficial stockholder, is an adverse party or has a material interest adverse to our interest.
| 87 |
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
Our directors hold office until the next annual general meeting of the stockholders or until their successors are elected and qualified. Our officers are appointed by our Board of Directors and hold office until the earlier of their death, retirement, resignation, or removal. Unless otherwise stated, the address of each director and officer is c/o BriaCell Therapeutics Corp., Suite 300 - Bellevue Centre, 235 - 15th Street, West Vancouver, BC V7T 2X1.
Our officers, directors and significant employees and their ages and positions are as follows:
| Name | Age | Position(s) | ||
| William V. Williams, M.D. | 64 | President, Chief Executive Officer and Director | ||
| Gadi Levin | 47 | Chief Financial Officer and Secretary | ||
| Markus Lacher, Ph.D. | 45 | Senior Director of Research and Development | ||
| Jamieson Bondarenko | 35 | Chairman of the Board of Directors | ||
| Vaughn C. Embro-Pantalony | 63 | Director | ||
| Rebecca Taub, M.D. | 68 | Director | ||
| Charles Wiseman, M.D. | 75 | Director | ||
| Richard Berman | 77 | Director |
William V. Williams, MD, President, Chief Executive Officer and Director, is a seasoned biopharmaceutical executive with over 35 years of industry and academic expertise, including significant clinical management in multinational pharmaceutical companies. Dr. Williams has served as President, Chief Executive Officer and Director of the Company since November 1, 2016. Dr. Williams served as Vice President of Exploratory Development at Incyte Corporation from March 2005 through November 2016. There he facilitated entry of over 20 compounds into the clinic, including ruxolitinib (Jakafi), baricitinib (Olumiant), and epacadostat. Dr. Williams held several positions at GlaxoSmithKline Pharmaceuticals, including Head of Experimental Medicine and Vice President of Clinical Pharmacology from December 2000 through March 2002, Director and Head of Clinical Pharmacology, Oncology, Musculoskeletal and Inflammation from March 2002 through December 2004 and Director and Head of Clinical Pharmacology, Musculoskeletal, Inflammation, Gastrointestinal and Urology from December 2004 through March 2005. He has also served as Assistant Professor of Medicine and the Director of Rheumatology Research at the University of Pennsylvania from July 1991 through January 1998. Dr. Williams earned is BSc in Chemistry and Biotechnology from Massachusetts Institute of Technology and Medical Doctorate from Tufts University School of Medicine.
Gadi Levin, CA, MBA, Chief Financial Officer and Secretary, was appointed Chief Financial Officer and Secretary of the Company on February 1, 2016. Mr. Levin has also served as Chief Financial Officer and Director of Vaxil Bio Ltd since March 1, 2016 and as Chief Financial Officer of Enthusiast Gaming Holdings Inc. since September 21, 2018. Mr. Levin has also serves as the Finance Director of Eco (Atlantic) Oil & Gas Ltd. since December 1, 2016. Mr. Levin has over 15 years of experience working with public US, Canadian and multi-jurisdictional public companies. Previously, Mr. Levin served as Chief Financial Officer of DarioHeath Corp from November 2013 through January 2015. Mr. Levin also served as the Vice President of Finance and Chief Financial Officer for two Israeli investment firms specializing in private equity, hedge funds and real estate. Mr. Levin began his CPA career at the accounting firm Arthur Andersen, where he worked for nine years, specializing in U.S. listed companies involved in IPOs. Mr. Levin has a Bachelor of Commerce degree in Accounting and Information Systems from the University of the Cape Town, South Africa, and a post graduate diploma in Accounting from the University of South Africa. He received his Chartered Accountant designation in South Africa and has an MBA from Bar Ilan University in Israel.
Markus Lacher, PhD, Senior Director of Research and Development, has served in his current role since July of 2015. Mr. Lacher previously served as a Senior Clinical Scientist for Cesca Therapeutics, Inc. from August 2014 through July 2015. He also founded T cell Therapeutics, Inc. a biotechnology company dedicated to developing and commercializing immunotherapies for the selective ablation of cancer cells resistant to standard treatments, in November 2012 and served as its Chief Executive Officer and President from inception through November 2015. From 2009 to 2014, he worked at BioTime, Inc. and its subsidiary OncoCyte Corporation where he developed key components of OncoCyte’s therapeutic and diagnostic technology. Dr. Lacher received his Ph.D. from the University of Bern (Switzerland) in 2001 and thereafter spent approximately eight years as a researcher at the University of California, San Francisco (UCSF).
| 88 |
Jamieson Bondarenko, CFA, CMT, Chairman of the Board of Directors, was appointed as a Director of the Company on February 12, 2019 and elected as Chairman in March 2019. Mr. Bondarenko provides strategic capital markets & corporate development advice to early-stage life sciences companies through his merchant capital company, JGRNT Capital Corp., a company he founded in November 2016. From December 2016 through October 2017, He served as Principal and Managing Director of the Equity Capital Markets group of Eight Capital. He also held several positions in the Capital Markets division of Dundee Securities Ltd., including Managing Director from July 2016 through December 2016, Director from October 2015 through July 2016, Vice President from December 2012 through October 2015 and Associate from February 2010 through December 2012.
Vaughn C. Embro-Pantalony, MBA, FCPA, FCMA, CDIR, ACC, Director, has been a Director of the Company since his appointment on March 18, 2019. In February 2018, he joined the Board of Directors of Soricimed Biopharma Inc., a private clinical-stage biopharma company developing targeted cancer therapies, and in August 2018 he was appointed Chairman of the Board of Soricimed and he continues to serve in this capacity. He is also a Director of Microbix Biosystems Inc., a public company and leading manufacturer of viral and bacterial antigens and reagents for the global diagnostics industry. He originally joined the Microbix Board in February 2007, and he also served as its President and Chief Executive Officer from November 2012 to July 2017. He is President of Stratpath Management Inc., consulting on strategy and governance to the life sciences sector. He has held other executive positions in life sciences with responsibility for finance, business development, strategic planning and information technology including Vice President, Finance, and Chief Financial Officer of Novopharm Limited from May 2003 through April 2006; Vice President, Information Technology, and Chief Information Officer of Bayer Inc. from July 1999 through April 2003; Vice President, Finance and Administration of Bayer Healthcare from October 1996 through June 1999; and Director, Finance and Administration and Chief Financial Officer of Zeneca Pharma Inc. from March 1995 through August 1996. He received his bachelor’s degree from Wilfrid Laurier University and his master of business administration degree from University of Windsor. He is a Fellow Chartered Professional Accountant and a Chartered Director (C. Dir.) and is Audit Committee Certified (A.C.C.) through the Directors College, McMaster University. We believe that Mr. Embro-Pantalony is qualified to serve as a member of our board of directors due to his extensive experience as a pharmaceutical and life sciences executive.
Rebecca Taub, MD, Director, has been a Director of the Company since her appointment on March 18, 2019. Dr. Taub currently serves as the President of Research and Development for Madrigal Pharmaceuticals, a clinical-stage biopharmaceutical company. She previously served as Vice President of Research and Development from July 2016 through her recent promotion to President of Research and Development on June 27, 2019. She has also served as Madrigal’s Chief Medical Officer since July 2016. Dr. Taub served as the CEO and a Director of Madrigal from September 2011 through Madrigal’s merger with Synta Pharmaceuticals Corp. in July 2016. Prior to joining Madrigal, Dr. Taub served as Senior Vice President, Research and Development of VIA Pharmaceuticals from 2008 to 2011 and as Vice President, Research, Metabolic Diseases at Hoffmann-LaRoche from 2004 to 2008. In those positions, Dr. Taub oversaw clinical development and drug discovery programs in cardiovascular and metabolic diseases including the conduct of a series of Phase I and II proof of conduct clinical trials. Dr. Taub led drug discovery including target identification, lead optimization and advancement of preclinical candidates into clinical development. From 2000 through 2003, Dr. Taub worked at Bristol-Myers Squibb Co. and DuPont Pharmaceutical Company, in a variety of positions, including Executive Director of CNS and metabolic diseases research. Before becoming a pharmaceutical executive, Dr. Taub was a tenured Professor of Genetics and Medicine at the University of Pennsylvania, and remains an adjunct professor. Dr. Taub is the author of more than 120 research articles. Before joining the faculty of the University of Pennsylvania, Dr. Taub served as an Assistant Professor at the Joslin Diabetes Center of Harvard Medical School, Harvard University and an associate investigator with the Howard Hughes Medical Institute. Dr. Taub received her M.D. from Yale University School of Medicine and B.A. from Yale College. We believe that Dr. Taub is qualified to serve as a member of our board of directors due to her extensive experience as a pharmaceutical executive heading up major development programs in non-alcoholic steatohepatitis, or NASH.
Charles Wiseman, MD, Director, is a Co-Founder of BriaCell Therapeutics Corp. and brings more than 40 years of academic and clinical experience. He is the inventor for most of the Company’s intellectual property and actively participates in its ongoing technology development. During his career, Dr. Wiseman has managed numerous clinical development teams and programs, with a focus in oncology, tumor immunology, vaccine development, and genetics. Dr. Wiseman has served as a Clinical Professor of Medicine at the Division of Medical Oncology at the Keck-USC School of Medicine since 1980. He previously served as Acting Chief of the Division of Oncology/Hematology at the White Memorial Medical Center from 1989 through 1991, as well as the principal investigator for immunotherapy treatment protocols at the St. Vincent Cancer Treatment Center and the Los Angeles Oncologic Institute from 1980 through 2006. Dr. Wiseman received his B.S. and MD at UCLA, where he also served as the President of the Student American Medical Association.
| 89 |
Richard Berman, Director, is a director of four public healthcare companies (in addition to the Company): Advaxis, Inc., since September 2005, Catasys, Inc., since February 2014, Cyroport Inc., since January 2015, and Immuron Ltd. since July 2018. He has also served as a Director of Cuentas Inc. since September 2018. From October 2014 through May 2017, Mr. Berman served as a Director of MetaStat, Inc. In 2016, he joined the Advisory Board of Medifirst. From 2006 to 2011 he was Chairman of National Investment Manager, a company with $12 billion in pension administration assets.
From 2002 to 2010 he was a director of Nexmed Inc where he also served as Chairman/CEO in 2008 and 2009 (now called Apricus Biosciences, Inc.). From 1990 to 200 he was employed by Internet Commerce Corporation (now Easylink Services) as Chairman and CEO and was a director from 1998 to 2012.
Previously, Mr Berman worked at Goldman Sachs; was Senior Vice President of Bankers Trust Company, where he stated the M&A and Leveraged Buyout Departments, created the largest battery company in the world in the 1980’s by merging Prestolite, General Battery and Exide to form Exide Technologies (XIDE); helped to create what is now Soho (NYC) by developing five buildings; and advised on over $4 billion of M&A transactions in over 300 deals.
He is a past Director of the Stern School of Business of NYU where he obtained his BS and MBA. He also has US and foreign law degrees from Boston College and The Hague Academy of International Law, respectively.
No Family Relationships
There is no family relationship between any director, executive officer and significant employee.
| 90 |
Foreign Private Issuer Status
The Nasdaq Rules include certain accommodations in the corporate governance requirements that allow foreign private issuers, such as us, to follow “home country” corporate governance practices in lieu of the otherwise applicable corporate governance standards of the Nasdaq. The application of such exceptions requires that we disclose any significant ways in which our corporate governance practices differ from the Nasdaq Rules that we do not follow. When our shares are listed on the Nasdaq, we intend to continue to follow Canadian corporate governance practices in lieu of the requirement under Rule 5620(c) of the Nasdaq Rules that a company’s articles of incorporation provide for a quorum for any meeting of the holders of the company’s common shares that is not less than 33 1/3% of the outstanding common shares of the company. Our articles of incorporation provide that a quorum of shareholders is constituted by the holders of at least 5% of the shares entitled to vote at the meeting, present in person or represented by proxy, and at least two persons entitled to vote at the meeting, present in person or represented by proxy. In addition, we do not intend to follow Rule 5635 of the Nasdaq Rules that requires that shareholder approval be required for the Company to issue securities in connection with certain events, such as the acquisition of shares or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, rights issues at or below market price, certain private placements, directed issues at or above market price and issuance of convertible notes. Neither Canadian securities laws nor British Columbia corporate law require shareholder approval for such transactions, except where such transactions constitute a “related party transaction” or “business combination” under Canadian securities laws or where such transaction is structured in a way that requires shareholder approval under the BCBCA, or where the TSX Venture Exchange requires the shareholder approval for the establishment of or amendments to equity-based compensation plans, in which case, we intend to follow our home country requirements.
Corporate Governance
Except as stated above, we intend to comply with the rules generally applicable to U.S. domestic companies listed on the Nasdaq. We may in the future decide to use other foreign private issuer exemptions with respect to some of the other Nasdaq listing requirements. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on the Nasdaq, may provide less protection than is accorded to investors under the Nasdaq Rules applicable to U.S. domestic issuers.
The Canadian securities regulatory authorities have issued corporate governance guidelines pursuant to National Policy 58-201—Corporate Governance Guidelines, or the Corporate Governance Guidelines, together with certain related disclosure requirements pursuant to NI 58-101. The Corporate Governance Guidelines are recommended as “best practices” for issuers to follow. We recognize that good corporate governance plays an important role in our overall success and in enhancing shareholder value and, accordingly, we have adopted, or in connection with the closing of this offering will adopt, certain corporate governance policies and practices which reflect our consideration of the recommended Corporate Governance Guidelines.
The disclosure set out below includes disclosure required by NI 58-101 describing our approach to corporate governance in relation to the Corporate Governance Guidelines.
Composition of our Board of Directors
Under our amended articles of incorporation that will be in place at the closing of this offering, our board of directors is to consist of three directors and up to that number which was last set by ordinary resolution of the shareholders. As of the closing of this offering, our board of directors will be comprised of six directors, and under the BCBCA, as a reporting issuer, we must have no fewer than three directors. Under the BCBCA, a director may be removed with or without cause by a resolution passed by at least two-thirds of the votes cast by shareholders present in person or by proxy at a meeting and who are entitled to vote. The directors are appointed at the annual general meeting of shareholders and the term of office for each of the directors will expire at the time of our next annual shareholders meeting. Our amended articles of incorporation will provide that, between annual general meetings of our shareholders, the directors may appoint one or more additional directors, but the number of additional directors may not at any time exceed one-third of the number of directors who held office at the expiration of the last meeting of our shareholders. Under the BCBCA, there is no minimum number of directors required to be resident Canadians as defined in the BCBCA.
| 91 |
Majority Voting Policy
We will adopt a majority voting policy to the effect that a nominee for election as a director of the Company who does not receive a greater number of votes “for” than votes “withheld” with respect to the election of directors by shareholders will be expected to offer to tender his or her resignation to the chairman of our board of directors promptly following the meeting of shareholders at which the director was elected. The nominating and corporate governance committee will consider such offer and make a recommendation to our board of directors about whether to accept it or not. Our board of directors will promptly accept the resignation unless it determines, in consultation with the nominating and corporate governance committee, that there are exceptional circumstances that should delay the acceptance of the resignation or justify rejecting it. Our board of directors will make its decision and announce it in a press release within 90 days following the meeting of shareholders. A director who tenders a resignation pursuant to our majority voting policy will not participate in any meeting of our board of directors or the nominating and corporate governance committee at which the resignation is considered. Our majority voting policy will not apply for contested meetings at which the number of directors nominated for election is greater than the number of seats available on the board.
Director Term Limits and Other Mechanisms of Board Renewal
Our board of directors has not adopted director term limits or other automatic mechanisms of board renewal. Rather than adopting formal term limits, mandatory age-related retirement policies and other mechanisms of board renewal, the nominating and corporate governance committee of our board of directors will develop a skills and competencies matrix for our board of directors as a whole and for individual directors. The nominating and corporate governance committee will also conduct a process for the assessment of our board of directors, each committee and each director regarding his or her effectiveness and contribution, and will report evaluation results to our board of directors on a regular basis.
Director Independence
Under the Nasdaq Rules, independent directors must comprise a majority of a listed company’s board of directors within a specified period after the closing of this offering. For purposes of the Nasdaq Rules, an independent director means a person other than an executive officer or employee of the company who, in the opinion of the board of directors, has no relationship with the company that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. Under NI 58-101, a director is considered to be independent if he or she is independent within the meaning of Section 1.4 of National Instrument 52-110—Audit Committees. Section 1.4 of NI 52-110 generally provides that a director is independent if he or she has no direct or indirect relationship with the issuer which could, in the view of the issuer’s board of directors, be reasonably expected to interfere with the exercise of the director’s independent judgment.
Our board of directors has undertaken a review of the independence of each director. Based on information provided by each director concerning his or her background, employment and affiliations, our board of directors has determined that Mr. Embro-Pantalony, Dr. Taub and Dr. Berman, representing three of the six members of our board of directors, are “independent” as that term is defined under the Nasdaq Rules and NI 58-101. In making this determination, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our shares by each non-employee director. Dr. Williams is not independent by reason of the fact that he is our Chief Executive Officer.
Certain members of our board of directors are also members of the boards of other public companies. See “—Directors, Executive Officers and Significant Employees”. Our board of directors has not adopted a director interlock policy, but is keeping informed of other public directorships held by its members.
Mandate of the Board of Directors
Our board of directors is responsible for supervising the management of our business and affairs, including providing guidance and strategic oversight to management. Our board will adopt a formal mandate that will include the following:
| ● | appointing our Chief Executive Officer; |
| 92 |
| ● | developing the corporate goals and objectives that our Chief Executive Officer is responsible for meeting and reviewing the performance of our Chief Executive Officer against such corporate goals and objectives; | |
| ● | taking steps to satisfy itself as to the integrity of our Chief Executive Officer and other executive officers and that our Chief Executive Officer and other executive officers create a culture of integrity throughout the organization; | |
| ● | reviewing and approving our code of conduct and reviewing and monitoring compliance with the code of conduct and our enterprise risk management processes; | |
| ● | reviewing and approving management’s strategic and business plans and our financial objectives, plans and actions, including significant capital allocations and expenditures; and | |
| ● | reviewing and approving material transactions not in the ordinary course of business. |
Meetings of Independent Directors
Our board of directors will hold regularly-scheduled quarterly meetings as well as ad hoc meetings from time to time. The independent members of our board of directors will also meet, as required, without the non-independent directors and members of management before or after each regularly scheduled board meeting.
A director who has a material interest in a matter before our board of directors or any committee on which he or she serves is required to disclose such interest as soon as the director becomes aware of it. In situations where a director has a material interest in a matter to be considered by our board of directors or any committee on which he or she serves, such director may be required to absent himself or herself from the meeting while discussions and voting with respect to the matter are taking place. Directors will also be required to comply with the relevant provisions of the BCBCA regarding conflicts of interest.
Position Descriptions
Prior to the closing of this offering, our board of directors will adopt written terms of reference for the chairman which will set out his or her key responsibilities, including duties relating to determining the frequency, dates and locations of meetings and setting board of directors meeting agendas, chairing board of directors and shareholder meetings and carrying out any other or special assignments or any functions as may be requested by our board of directors or management, as appropriate.
Prior to the closing of this offering, our board of directors will also adopt written terms of reference for each of the committee chairs which will set out each of the committee chair’s key responsibilities, including duties relating to determining the frequency, dates and locations of meetings and setting committee meeting agendas, chairing committee meetings, reporting to our board of directors and carrying out any other special assignments or any functions as may be requested by our board of directors.
In addition, prior to the closing of this offering, our board of directors, in conjunction with our Chief Executive Officer, will develop and implement a written position description for the role of our Chief Executive Officer.
Orientation and Continuing Education
Following the closing of this offering, we will implement an orientation program for new directors under which a new director will meet separately with the chairman of our board of directors, members of the senior executive team and the secretary.
The nominating and corporate governance committee will be responsible for coordinating orientation and continuing director development programs relating to the committee’s mandate. The chairman of our board of directors will be responsible for overseeing director continuing education designed to maintain or enhance the skills and abilities of our directors and to ensure that their knowledge and understanding of our business remains current.
| 93 |
Code of Conduct
The Board has not adopted a formal written Code of Business Conduct and Ethics. However, the small size of the Board and number of officers and employees allows the Board to monitor on an ongoing basis the activities of management and to ensure that the highest standard of ethical conduct is maintained. The Board views good corporate governance as an integral component to its success and to meet its responsibilities to shareholders. As the Company grows in size and scope, the Board anticipates that it will formulate and implement a formal Code of Business Conduct and Ethics.
Monitoring Compliance with the Code of Conduct
Our nominating and corporate governance committee will be responsible for reviewing and evaluating the code of conduct at least annually and will recommend any necessary or appropriate changes to our board of directors for consideration. The nominating and corporate governance committee will assist our board of directors with the monitoring of compliance with the code of conduct, and will be responsible for considering any waivers therefrom (other than waivers applicable to members of the nominating and corporate governance committee, which shall be considered by the audit committee, or waivers applicable to our directors or executive officers, which shall be subject to review by our board of directors as a whole).
Requirement for Directors and Officers to Disclose Interest in a Contract or Transaction
In accordance with the BCBCA, each director and officer must disclose the nature and extent of any interest that he or she has in a material contract or material transaction whether made or proposed with us, if the director or officer is a party to the contract or transaction, is a director or an officer or an individual acting in a similar capacity of a party to the contract or transaction, or has a material interest in a party to the contract or transaction. Subject to certain limited exceptions under the BCBCA, no director may vote on a resolution to approve a material contract or material transaction which is subject to such disclosure requirement.
As of the date hereof, except as otherwise disclosed in this prospectus, to the knowledge of the Board or the management of the Company, there are no material interests, whether direct or indirect, of any informed person of the Company, any proposed director of the Company, or any associate or affiliate of any informed person or proposed director, in any transaction since the commencement of the Company’s most recently completed financial year or in any proposed transaction which has materially affected or would materially affect the Company of any of its subsidiaries.
Benefits upon Termination of Employment
The service contracts with our directors do not provide for any benefits upon termination of employment, other than a “tail” directors and officers insurance policy.
Complaint Reporting
In order to foster a climate of openness and honesty in which any concern or complaint pertaining to a suspected violation of the law, our code of conduct or any of our policies, or any unethical or questionable act or behavior, our code of conduct will require that our employees promptly report the violation or suspected violation. In order to ensure that violations or suspected violations can be reported without fear of retaliation, harassment or an adverse employment consequence, we will adopt a whistleblowing policy which will contain procedures that are aimed to facilitate confidential, anonymous submissions of complaints by our directors, officers, employees and others.
Committees of the Board of Directors
Upon completion of this offering we will have an audit committee, a compensation committee and a nominating and corporate governance committee, with each committee having a written charter.
| 94 |
Audit Committee
Our Audit Committee is currently comprised of Vaughn C. Embro-Pantalony, Richard Berman and Dr. Rebecca Taub, and chaired by Mr. Embro-Pantalony. Our board of directors has determined that each of Mr. Berman and Mr. Embro-Pantalony is financially literate and meets the independence requirements for directors, including the heightened independence standards for members of the audit committee under Rule 10A-3 under the Exchange Act and NI 52-110. Our board of directors has determined that each of Mr. Berman and Mr. Embro-Pantalony is “financially sophisticated” within the meaning of the Nasdaq Rules, “financially literate” within the meaning of NI 52-110, and a “financial expert” as defined by Rule 10A-3 under the Exchange Act. For a description of the education and experience of each member of the audit committee, see “—Directors, Executive Officers and Significant Employees”.
We have adopted an Audit Committee Charter setting forth the purpose, composition, authority and responsibility of the audit committee. The primary function of the audit committee is to assist the Board of Directors in fulfilling its financial oversight responsibilities by reviewing the financial reports and other financial information provided by the company to regulatory authorities and the Company’s shareholders, the Company’s systems of internal controls regarding finance and accounting and the Company auditing, accounting and financial reporting processes. Consistent with this function, the Committee will encourage continuous improvement of, and should foster adherence to, Company’s policies, procedures and practices at all levels. The Committee’s primary duties and responsibilities are to:
| ● | Serve as an independent and objective party to monitor the Company’s financial reporting and internal control system and review Company’s financial statements; | |
| ● | Review and appraise the performance of the Company’s external auditors; and | |
| ● | Provide an open avenue of communication among the Company’s auditors, financial and senior management and the Board of Directors. |
The Audit Committee shall meet at least annually, or more frequently as circumstances dictate. As part of its job to foster open communication, the Audit Committee will meet at least annually with the external auditors.
To fulfill its responsibilities and duties, the Audit Committee shall:
| ● | Review and update the Audit Committee’s charter annually; | |
| ● | Review the Company’s financial statements, Management Discussion & Analysis and any annual and interim earnings, press releases before the Company publicly discloses this information and any reports or other financial information (including quarterly financial statements), which are submitted to any governmental body, or to the public, including any certification, report, opinion, or review rendered by the external auditors; | |
| ● | Review annually, the performance of the external auditors who shall be ultimately accountable to the Board of Directors and the Committee as representatives of the shareholders of the Company; | |
| ● | Obtain annually, a formal written statement of external auditors setting forth all relationships between the extern auditors and the Company, consistent with Independence Standards Board Standard I; | |
| ● | Review and discuss with the external auditors any disclosed relationships or services that may impact the objectivity and independence of the external auditors; | |
| ● | Take, or recommend that the full Board of Directors take, appropriate action to oversee the independence of the external auditors; | |
| ● | Recommend to the Board of Directors the selection and, where applicable, the replacement of the external auditors nominated annually for shareholder approval; | |
| ● | Review and approve the Company’s hiring policies regarding partners, employees and former partners and employees of the present and former external auditors of the Company; | |
| ● | Review and pre-approve all audit and audit-related services and the fees and other compensation related thereto; | |
| ● | In consultation with the external auditors, review with management the integrity of the Company’s financial reporting process, both internal and external; | |
| ● | Consider the external auditors’ judgments about the quality and appropriateness of the Company’s accounting principles as applied in its financial reporting; | |
| ● | Consider and approve, if appropriate, changes to the Company’s auditing and accounting principles and practices as suggested by the external auditors and management; |
| 95 |
| ● | Review significant judgments made by management in the preparation of the financial statements and the view of the external auditors as to appropriateness of such judgments; | |
| ● | Following completion of the annual audit, review separately with management and the external auditors any significant difficulties encountered during the course of the audit, including any restrictions on the scope of work or access to required information; | |
| ● | Review any significant disagreement among management and the external auditors in connection with the preparation of the financial statements; | |
| ● | Review with the external auditors and management the extent to which changes and improvements in financial or accounting practices have been implemented; | |
| ● | Review any complaints or concerns about any questionable accounting, internal accounting controls or auditing matters; | |
| ● | Review certification process; and | |
| ● | Review any related-party transactions. |
Principal Accountant’s Fees
Aggregate fees billed by MNP LLP, our independent auditor, in the fiscal years ended July 31, 2018 and 2017 were approximately $56,443 and $40,125, respectively, as detailed below.
| Fees billed for the fiscal year ended July 31 | ||||||||
| Service Retained | 2018 | 2017 | ||||||
| Audit fees(1) | $ | 40,000 | $ | 30,000 | ||||
| Audit-related fees | - | - | ||||||
| Tax fees(2) | $ | 12,750 | $ | 7,500 | ||||
| All other fees(3) | $ | 3,693 | $ | 2,625 | ||||
| Total | $ | 56,443 | $ | 40,125 | ||||
| (1) | Aggregate fees billed by the auditor (or accrued) for assurance and related services that are reasonably related to the performance of the audit or review of the Company’s financial statements. |
| (2) | Aggregate fees billed by the auditor (or accrued) for professional services rendered for tax compliance, tax advice and tax planning. |
| (3) | Aggregate fees billed by the auditor (or accrued) and not included above. |
Compensation Committee
Compensation committees are not mandatory in Canada. However, National Policy 58-201 - Corporate Governance Guidelines recommends that a board appoint a compensation committee composed entirely of independent directors with responsibilities for oversight of the compensation payable to senior executives. The members of the compensation committee are not required to be independent or to have any particular expertise.
Our compensation committee is comprised of Mr. Embro-Pantalony and Mr. Berman and will be chaired by Mr. Berman. The Compensation Committee is appointed by the Board to assist in promoting a culture of integrity throughout the Company, to assist the Board in setting director and senior executive compensation, and to develop and submit to the Board recommendations with respect to other employee benefits as the Compensation Committee sees fit. In the performance of its duties, the Compensation Committee is guided by the following principles:
| ● | offering competitive compensation to attract, retain and motivate highly qualified executives in order for the Company to meet its goals; and |
| ● | acting in the interests of the Company and the shareholders by being fiscally responsible. |
| 96 |
The Board relies on the knowledge and experience of the members of the Compensation Committee to set appropriate levels of compensation for senior officers. Neither the Company nor the Compensation Committee currently has, or has had at any time since incorporation, any contractual arrangement with any executive compensation consultant who has a role in determining or recommending the amount or form of senior officer compensation.
When determining compensation payable, the Compensation Committee considers both external and internal data. External data includes general markets conditions and well as information regarding compensation paid to directors, CEOs and CFOs of companies of similar size and at a similar stage of development in the industry. Internal data includes annual reviews of the performance of the directors, CEO and CFO in light of the Company’s corporate objectives and considers other factors that may have impacted the Company’s success in achieving its objectives.
Corporate Governance Committee
Corporate Governance Committees are not mandatory in Canada. However, NP 58-201 recommends that a board appoint a corporate governance committee composed entirely of independent directors with responsibility for overseeing the process for nominating directors for election by shareholders. The members of the corporate governance committee are not required to be independent or to have any particular expertise.
The Corporate Governance Committee is appointed by the Board to assist in fulfilling its corporate governance responsibilities under applicable laws. the Corporate Governance Committee is responsible for, among other things, developing the Company’s approach to governance issues and establishing sound corporate governance practices that are in the interests of shareholders and that contribute to effective and efficient decision-making.
Our Corporate Governance Committee is currently comprised of Mr. Embro-Pantalony and Dr. Taub and will be chaired by Mr. Embro-Pantalony.
Nomination Committee
Nomination Committees are not mandatory in Canada. However, NP 58-201 recommends that a board appoint a corporate governance committee composed entirely of independent directors with responsibility for overseeing the process for nominating directors for election by shareholders. The members of the nominating committee are not required to be independent or to have any particular expertise.
The Company’s Nomination Committee considers the Board’s size each year when it considers the number of directors to recommend to the shareholders for election at the annual meeting of shareholders. Further, the Nomination Committee assumes responsibility for assessing current members and nominating new members to the Board and ensuring that all Board members are informed of and are aware of their duties and responsibilities as directors.
The Nomination Committee is currently comprised of Dr. Taub and Mr. Berman and will be chaired by Dr. Taub.
Our board of directors will establish a written charter setting forth the purpose, composition, authority and responsibility of our nominating and corporate governance committee. The nomination committee’s purpose will be to assist our board of directors in:
| ● | identifying individuals qualified to become members of our board of directors; |
| ● | selecting, or recommending that our board of directors select, director nominees for the next annual meeting of shareholders and determining the composition of our board of directors and its committees; |
| ● | ensuring that our board directors are informed of and aware of their duties and responsibilities as directors |
| 97 |
| ● | developing and overseeing a process to assess our board of directors, the chairman of the board of directors, the committees of the board of directors, the chairs of the committees, individual directors and management; and |
| ● | developing and implementing our corporate governance guidelines that are in the interests of the shareholders and that contribute to effective and efficient decisions-making. |
In identifying new candidates for our board of directors, the nomination committee will consider what competencies and skills our board of directors, as a whole, should possess and assess what competencies and skills each existing director possesses, considering our board of directors as a group, and the personality and other qualities of each director, as these may ultimately determine the boardroom dynamic.
It will be the responsibility of the nomination committee to regularly evaluate the overall efficiency of our board of directors and our chairman and all board committees and their chairs. As part of its mandate, the nomination committee will conduct the process for the assessment of our board of directors, each committee and each director regarding his, her or its effectiveness and contribution, and report evaluation results to our board of directors on a regular basis.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the BCBCA, a company may indemnify: (i) a current or former director or officer of that company; (ii) a current or former director or officer of another corporation if, at the time such individual held such office, the corporation was an affiliate of the company, or if such individual held such office at the company’s request; or (iii) an individual who, at the request of the company, held, or holds, an equivalent position in another entity (an “indemnifiable person”) against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by him or her in respect of any civil, criminal, administrative or other legal proceeding or investigative action (whether current, threatened, pending or completed) in which he or she is involved because of that person’s position as an indemnifiable person, unless: (i) the individual did not act honestly and in good faith with a view to the best interests of such company or the other entity, as the case may be; or (ii) in the case of a proceeding other than a civil proceeding, the individual did not have reasonable grounds for believing that the individual’s conduct was lawful. A company cannot indemnify an indemnifiable person if it is prohibited from doing so under its articles or by applicable law. A company may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an indemnifiable person in respect of that proceeding only if the indemnifiable person has provided an undertaking that, if it is ultimately determined that the payment of expenses was prohibited, the indemnifiable person will repay any amounts advanced. Subject to the aforementioned prohibitions on indemnification, a company must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by an indemnifiable person in respect of such eligible proceeding if such indemnifiable person has not been reimbursed for such expenses, and was wholly successful, on the merits or otherwise, in the outcome of such eligible proceeding or was substantially successful on the merits in the outcome of such eligible proceeding. On application from an indemnifiable person, a court may make any order the court considers appropriate in respect of an eligible proceeding, including the indemnification of penalties imposed or expenses incurred in any such proceedings and the enforcement of an indemnification agreement. As permitted by the BCBCA, under Article 21.1, we are required to indemnify our directors and former directors (and such individual’s respective heirs and legal representatives) and permit us to indemnify any person to the extent permitted by the BCBCA.
The BCBCA provides certain protections under Part 5 – Management, Division 5 - Indemnification of Directors and Officers and Payment of Expenses, to our current and former directors and officers, as well as other eligible parties defined in Section 159 of the BCBCA (the “Eligible Parties”, each an “Eligible Party”). The Company will indemnify the Eligible Parties, to the fullest extent permitted by law and subject to certain limitations listed in Section 163 of the BCBCA, against any proceeding in which an Eligible Party or any of the heirs and personal or other legal representatives of the Eligible Party, by reason of the Eligible Party being or having been a director or officer of, or holding or having held a position equivalent to that of a director or officer of, the Company or an associated corporation (a) is or may be joined as a party, or (b) is or may be liable for or in respect of a judgment, penalty or fine in, or expenses related tom, the proceeding.
We maintain insurance policies relating to certain liabilities that our directors and officers may incur in such capacity.
| 98 |
The following table provides a summary of compensation paid, directly or indirectly, for the year ended July 31, 2018 to the directors, officers and senior management:
| Table of Compensation Excluding Compensation Securities | ||||||||||||||
| Name and position | Year | Salary, consulting fee, retainer or commission ($) | Value of all other compensation ($)(1) | Total compensation ($) | ||||||||||
| William
V. Williams(2) President, Chief Executive Officer, Director | 2018 | 175,008 | 52,747 | 227,755 | ||||||||||
| Gadi
Levin(3) Chief Financial Officer and Secretary | 2018 | 42,000 | 14,066 | 56,066 | ||||||||||
| Rahoul
Sharan(4) Former Director | 2018 | 48,000 | 35,165 | 83,165 | ||||||||||
| Saeid
Babaei(5) Former Chairman and Former Director | 2018 | 78,500 | 35,165 | 113,665 | ||||||||||
| Martin
Schmieg(6) Former Director | 2018 | 38,205 | 35,165 | 73,370 | ||||||||||
| Charles
Wiseman Director | 2018 | 48,396 | 35,165 | 83,561 | ||||||||||
| Jamieson
Bondarenko Chairman of the Board of Directors | 2018 | 3,000 | 89,951 | 92,951 | ||||||||||
| Rebecca
Taub Director | 2018 | - | - | - | ||||||||||
| Vaughn
C. Embro-Pantalony Director | 2018 | - | - | - | ||||||||||
| Richard
Berman Director | 2018 | |||||||||||||
(1) Options based awards calculated using the Black-Scholes Option Pricing Model.
(2) On November 1, 2016, Dr. William V. Williams was appointed as President and Chief Executive Officer of the Company.
(3) On February 11, 2016, Gadi Levin was appointed as Chief Financial Officer and Corporate Secretary of the Company.
(4) Mr. Sharan resigned from the Board effective March 18, 2019.
(5) Mr. Babaei resigned from the Board effective March 18, 2019.
(6) Mr. Schmieg resigned from the Board effective March 18, 2019.
| 99 |
Stock Options and Other Compensation Securities
The Company’s Stock Option Plan was previously approved by the shareholders at the Company’s annual and special meeting on November 25, 2014.
The following table provides a summary of all compensation securities granted or issued to each director, officer and senior management as of July 31, 2019.
| Compensation Securities | ||||||||||||||
| Name and position | Type of compensation security | Number of compensation securities, number of underlying securities, and percentage of class | Expiry date | exercise price ($) | ||||||||||
| William
V. Williams President & Chief Executive | Stock Options | 750,000 | March 1, 2021 | $ | 0.15 | |||||||||
| Officer, Director | Stock Options | 632,000 | November 1, 2019 | $ | 0.21 | |||||||||
| Gadi
Levin, Chief Financial Officer and Secretary | Stock Options | 200,000 | March 1, 2021 | $ | 0.15 | |||||||||
| Charles
Wiseman Director | Stock Options | 500,000 | March 1, 2021 | $ | 0.15 | |||||||||
| Jamieson
Bondarenko Chairman of the Board of Directors | Stock Options | 2,000,000 | March 15, 2021 | $ | 0.15 | |||||||||
| Rebecca
Taub Director | - | - | - | - | ||||||||||
| Vaughn
C. Embro-Pantalo ny Director | - | - | - | - | ||||||||||
| Richard
Berman Director | ||||||||||||||
| 100 |
Oversight and Description of Compensation
Compensation of Directors
The Company provides a modest cash retainer to its non-executive directors. Executive directors do not receive any cash compensation in their capacity as directors. Long term incentives (stock options) are granted from time to time, based on an existing complement of long term incentives, corporate performance and to be competitive with other companies of similar size and scope.
Compensation of Executive Officers
The Company’s Compensation Committee is responsible for, among other things, evaluating the performance of the Company’s executive officers, determining or making recommendations to the Board with respect to the compensation of the Company’s executive officers, making recommendations to the Board with respect to director compensation, incentive compensation plans and equity-based plans, making recommendations to the Board with respect to the compensation policy for the employees of the Company and ensuring that the Company is in compliance with all legal requirements with respect to compensation disclosure. In performing its duties, the Compensation Committee has the authority to engage such advisors, including executive compensation consultants, as it considers necessary.
Philosophy and Objectives
The compensation program for senior management of the Company is designed to ensure that the level and form of compensation achieves certain objectives, including:
| a) | attracting and retaining talented and highly-qualified executives; |
| b) | motivating the short and long term performances of executives; and |
| c) | creating a corporate environment which aligns their interests with those of the shareholders. |
The compensation program is designed to provide competitive levels of compensation. The Company recognizes the need to provide a total compensation package that will attract and retain qualified and experienced executives as well as align the compensation level of each executive to that executive’s level of responsibility. In general, the Company’s executive officers may receive compensation that is comprised of three components: (a) a base salary; (b) bonus compensation; and (c) equity participation through the Company’s Stock Option Plan or all such forms of compensation.
| 101 |
Base Salary
In the view of Company, paying base salaries which are competitive in the markets in which the Company operates is a first step to attracting and retaining talented, qualified and effective executives. Competitive salary information on companies earning comparative revenues in a similar industry is compiled from a variety of sources, including surveys conducted by independent consultants and national and international publications.
Bonus Compensation
The Company’s primary objective is to achieve certain strategic objectives and milestones. The Company may approve executive bonus compensation dependent upon the Company meeting those strategic objectives and milestones and sufficient cash resources being available for the granting of bonuses. Bonuses paid to the executive officers are allocated on an individual basis. Bonuses are paid to reward work done above the base level of expectations set by the base salary, wages or contractor payments. There were no bonuses paid to any of NEOs during the most recently completed financial year.
Equity Participation through Stock Option Plan
The Company has, as a part of its long-term incentive, adopted a Stock Option Plan. The Stock Option Plan is designed to encourage share ownership and entrepreneurship on the part of the senior management and other employees. The Compensation Committee believes that the Stock Option Plan aligns the interests of the NEOswith shareholders by linking a component of executive compensation to the longer term performance of the Company’s common shares.
In monitoring or adjusting the option allotments, the Compensation Committee takes into account the level of options granted for similar levels of responsibility and considers each executive officer or employee based on reports received from management, its own observations on individual performance (where possible) and its assessment of individual contribution to shareholder value, previous options grants and the objectives set for the executive officers. The scale of options will generally be commensurate to the appropriate level of base compensation for each level of responsibility.
In addition to determining the number of options to be granted pursuant to the methodology outlined above, the Compensation Committee also makes the following determinations:
| ● | The executive officers and others who are entitled to participate in the Company’s Stock Option Plan; |
| ● | The exercise price for each stock option granted, subject to the provision that the exercise price cannot be lower than the market price on the date of grant; |
| ● | The date on which each option is granted; |
| ● | The vesting period, if any, for each stock option; and |
| ● | The other material terms and conditions of each stock option grant |
Stock option grants are designed to reward the executive officers for success on a similar basis as the shareholders of the Company, although the level of reward provided by a particular stock option grant is dependent upon the volatility of the stock market. The Company believes that encouraging its executives and employees to become shareholders is the best way of aligning their interests with those of its shareholders. Equity participation is accomplished through our Stock Option Plan. Stock options are granted to senior executives taking into account a number of factors, including the amount and term of options previously granted, base salary and bonuses and competitive factors. Options are generally granted to senior executives which vest immediately.
All of the executive officers are entitled to participate in our Stock Option Plan.
| 102 |
Pension Plan Benefits
The Company does not have a pension plan in place and therefore there were no pension plan benefit awards made to a director or Executive Officer.
Employment Agreements with Executive Officers and Significant Employees
On October 12, 2016, the Company entered into an employment agreement with Dr. William V. Williams, effective October 31, 2016, to serve as Chief Executive Officer and President. Dr. William V. Williams is also a member of our board of directors. His agreement automatically renews for one (1) year terms every October 31st unless cancelled by either Dr. Williams or the Company. Pursuant to the agreement, Dr. William V. Williams is paid a base fee of USD $175,000 and received 632,000 stock options on the date of his appointment and exercisable at $0.20 per share. The agreement also provides for milestone incentive bonuses which may increase the remuneration by an additional $50,000 USD. If the agreement is terminated by the Company, all amounts accrued under the agreement shall become due and a lump sum termination fee equal to the pro rata monthly salary times the number of months employed divided by 2 (subject to a maximum of 12) shall become payable. The agreement may be terminated by Dr. William V. Williams upon delivery of 30 days written notice.
The amounts payable pursuant to the employment agreement with Dr. William V. Williams is incurred in US dollars. The Company converts the US dollar amounts in Canadian equivalents using an exchange rate for the date on which Dr. William V. Williams is paid pursuant to Dr. Williams’ employment agreement.
On February 11, 2016, the Company entered into a consulting agreement with Gadi Levin (the “Levin Agreement”). Under the Levin Agreement, Mr. Gadi Levin agreed to serve as Chief Financial Officer of the Company for a one (1) year term, renewable upon written agreement. Pursuant to the Levin Agreement, Mr. Gadi Levin receives a fee of $3,500 per month. Either party may terminate the Levin Agreement for reason of default on fifteen (15) days’ written notice (unless the default is cured within that period), or without cause on thirty (30) days’ notice. Upon termination, Mr. Gadi Levin is entitled to payment for work performed and accepted.
On July 3, 2015, the Company entered into an employment agreement, effective July 13, 2015, with Markus Lacher, Ph.D. (the “Lacher Agreement”), to serve as the Company’s Senior Director of Research and Development. Mr. Lacher’s employment is an at “at-will” employment with no specific term. Pursuant to the Lacher Agreement, Mr. Lacher is paid an annual salary of $105,000 and received 500,000 stock options on the date of his appointment that are exercisable at C$0.25 per share. The Lacher Agreement also provides for milestone incentive bonuses that are developed by the Company on an annual basis. Upon the termination of the Lacher Agreement, Mr. Lacher shall be entitled to receive payment for all unpaid salary, accrued but unpaid bonuses, if any, and vacation accrued as of the date of termination.
| 103 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information with respect to the beneficial ownership of our common shares as of July 31, 2019 by:
| ● | each person, or group of affiliated persons, known to us to be the beneficial owner of more than 5% of our outstanding common shares; |
| ● | each of our directors and executive officers; and |
| ● | all of our directors and executive officers as a group. |
The beneficial ownership of our common shares is determined in accordance with the rules of the SEC and generally includes any shares over which a person exercises sole or shared voting or investment power, or the right to receive the economic benefit of ownership. For purposes of the table below, we deem common shares issuable pursuant to options and warrants that are currently exercisable or exercisable within 60 days of July 31, 2019 to be outstanding and to be beneficially owned by the person holding the options for the purposes of computing the percentage ownership of that person, but we do not treat them as outstanding for the purpose of computing the percentage ownership of any other person.
The percentage of shares beneficially owned has been computed on the basis of 196,378,450 common shares outstanding as of July 31, 2019.
Unless otherwise noted below, the address of each shareholder, director and executive officer is c/o BriaCell Therapeutics Corp. Suite 300 – Bellevue Centre, 235- 15th Street, West Vancouver, BC V7T 2X1.
Except as indicated in footnotes to this table, we believe that the shareholders named in this table have sole voting and investment power with respect to all shares shown to be beneficially owned by them, based on information provided to us by such shareholders. The shareholders listed below do not have any different voting rights from any of our other shareholders.
| No. of Shares Beneficially Owned | Percentage Owned(6) | |||||||
| Directors and executive officers: | ||||||||
| William V. Williams (1) | 13,475,449 | 6.79 | % | |||||
| Gadi Levin(2) | 400,000 | * | % | |||||
| Markus Lacher (3) | 600,000 | * | % | |||||
| Jamieson Bondarenko(4) | 20,220,500 | 10.19 | % | |||||
| Vaughn C. Embro-Pantalony | - | - | ||||||
| Rebecca Taub | - | - | ||||||
| Charles Wiseman(5) | 13,881,287 | 7.05 | % | |||||
| Richard Berman | - | - | ||||||
| All directors and executive officers as a group (8 persons) | 48,577,236 | 24.07 | % | |||||
| * | Indicates beneficial ownership of less than 1% of the total common shares outstanding |
Notes:
| 1. | Includes 750,000 options with an exercise price of $0.15, expiring on March 1, 2021, 632,000 options with an exercise price of $0.21, expiring on November 1, 2019 and 645,600 warrants to purchase common shares with an exercise price of $0.14, expiring on March 27, 2021. | |
| 2. | Includes 200,000 with an exercise price of $0.15, expiring on March 1, 2021 options and 100,000 warrants to purchase common shares with an exercise price of $0.14, expiring on March 27, 2021. | |
| 3. | Includes 100,000 options with an exercise price of $0.15, expiring on March 1, 2021, 500,000 options with an exercise price of $0.255, expiring on November 4, 2020. | |
| 4. | Includes 2,000,000 options with an exercise price of $0.15, expiring on March 1, 2021 and 2,200,000 warrants to purchase common shares . | |
| 5. | Includes 500,000 options to purchase common shares with an exercise price of $0.15, expiring on March 1, 2021. | |
| 6. | The percentages are calculated based on 196,378,450 common shares outstanding as at July 31, 2019. |
Record Holders
Based upon a review of the information provided to us by our transfer agent, as of July 31, 2019, there were a total of 54 holders of record of our shares, of which approximately 70% are located in Canada and the remainder are located in the United States.
| 104 |
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following is a description of material transactions, or series of related material transactions since August 1, 2016, to which we are a party and in which the other parties include our directors, executive officers, holders of more than 5% of our voting securities, or any member of the immediate family of any of the foregoing persons.
On March 9, 2017 the Company and the Company’s President and CEO, completed a non-brokered private placement financing (the “Offering”) of 5,612,083 units (the “Units”) for aggregate gross proceeds to the Company in the amount of $1,346,900. Under the Offering, each Unit consisted of one common share in the capital of the Company (a “Common Share”) and one-half of one Common Share purchase warrant (a “Warrant”). On November 30, 2016, 116,963 Compensation Warrants were exercised into 116,963 common shares and 116,963 warrants for a total consideration of $21,055.
On August 2, 2017, the Company and the Company’s President and CEO completed a non-brokered private placement resulting in gross proceeds of $631,785. The non-brokered private placement involved the sale of 4,058,441 units at a price of $0.16 per unit. Each Unit consisted of one common share in the capital of the Company.
On July 24, 2017, the Company entered into a definitive share exchange agreement (the “Share Exchange Agreement”) between BriaCell Therapeutics Corp., or BTC, the Company’s US subsidiary, Sapientia and all the shareholders of Sapientia. Sapientia is a biotechnology company based in Havertown, PA, that is developing novel targeted therapeutics for multiple indications including several cancers and fibrotic diseases.
Pursuant to the terms of the Share Exchange Agreement, BTC acquired from the Sapientia Shareholders all of the issued and outstanding shares in the capital of Sapientia. As consideration, the Sapientia Shareholders, received an aggregate of 2,500,002 common shares in the capital of BriaCell on a pro-rata basis (the “Transaction”), which were issued on September 5, 2017. As part of the Transaction, BriaCell acquired all rights, including composition of matter patents, and preclinical study data to a novel therapeutic technology platform, known as protein kinase C delta (PKCδ) inhibitors, which represents a unique, highly-targeted approach to treat cancer and to boost the immune system.
Sapeintia’s Shareholders included Dr. Williams and Martin Schmieg, each of whom were directors of the Company at the time of the Share Exchange Agreement.
On March 27, 2018, the Company completed a non-brokered private placement (the “March 2018 Non-Brokered Unit Offering”) of 43,322,322 units of the Company (the “Units”) at a price of $0.10 per Unit for aggregate gross proceeds of $4,332,232. Under the March 2018 Non-Brokered Unit Offering, each Unit consists of one common share (each, a “March Common Share”) and one common share purchase warrant (each, a “March Warrant”). The March Warrants are valid for 36 months following the closing of the Non-Brokered Unit Offering and each March Warrant is exercisable for one March Common Share at an exercise price of $0.14.
Concurrent with the March 2018 Non-Brokered Unit Offering, the Company also completed a brokered private placement for the purchase of 5.0% unsecured convertible notes (each, a “March Note”) in the principal amount of US$885,000 (the “March Note Offering”). Under the terms of securities purchase agreements dated March 8, 2018 between the Company and the purchasers of March Notes, each March Note is convertible at the option of the holder into (i) common shares of BriaCell for so long as the March Note is outstanding, at a fixed conversion price of $0.10 per March Common Share, for a period of nine months from the date of issuance, which may be extended by the applicable holder for up to six additional months at the holder’s sole option, and (ii) for each March Common Share resulting from the conversion, one March Warrant. The March Warrants are valid for 36 months from their issuance date and each March Warrant is exercisable for one March Common Share at an exercise price of $0.14.
In connection with the Non-Brokered Unit Offering and the Note Offering (together, the “March Offerings”), the Company paid commissions to certain participating dealers on a portion of funds raised. In respect of the March Note Offering, an aggregate cash commissions of $235,215 and an aggregate 2,613,350 broker warrants (the “Broker Warrants”) were paid. The compensation warrants issued in connection with the March Offerings are exercisable for one March Common Share at an exercise price of $0.14 for a period of 36 months from the issue date.
The following directors and officers participated in the March 2018 Non-Brokered Unit Offering.
| Name | Amount invested | Number of shares | ||||||
| Saeid Babaei (1) | $ | 15,000.00 | 150,000 | |||||
| Rahoul Sharan(2) | $ | 50,000.00 | 500,000 | |||||
| William V. Williams | $ | 64,560.00 | 640,560 | |||||
| Martin E. Schmieg(3) | $ | 10,000.00 | 100,000 | |||||
| Gadi Levin | $ | 10,000.00 | 100,000 | |||||
| (1) | Resigned on March 18, 2019 | |
| (2) | Resigned on March 7, 2019 | |
| (3) | Resigned on March 14, 2019 |
| 105 |
On March 25, 2019 and April 1, 2019, the Company completed a non-brokered private placement (the “March 2019 Private Placement”) on of 29,735,240 shares of the Company at a price of $0.10 per share for aggregate gross proceeds of $2,973,524 (net proceeds: $2,845,784). As part of the March 2019 Private Placement, Mr. Bondarenko invested $500,000 and received 5,000,000 common shares.
On February 26, 2019, BriaCell sold $500,000 of common shares to Mr. Bondarenko.
As at April 30, 2019, included in accounts payable and accrued liabilities are amounts owing to a company controlled by an officer in the amount of $3,500 for consulting fees and an amount owing to a director of $3,750 for director’s fees. During the year ended July 31, 2018 and nine month periods ended April 30, 2019 the Company also incurred the following expenses charged by directors and key management personnel or companies controlled by these individuals:
| Issuance date | Year Ended July 31, 2018 | Nine Months Ended April 30, 2019 | ||||||
| Paid or accrued professional fees to a company controlled by an on officer of the Company | $ | 42,000 | 1 | $ | 10,500 | (1) | ||
| Paid or accrued consulting fees to companies controlled by individual directors | $ | 126,000 | 2 | $ | 7,250 | 2 | ||
| Paid or accrued wages and consulting fees to directors | $ | 263,365 | 3 | $ | 61,724 | 3 | ||
| Paid or accrued directors fees | $ | 207,471 | 4 | $ | 18,750 | 5 | ||
| 1. | Paid or accrued consulting fees to a company, controlled by Mr. Levin, the Company’s Chief Financial Officer. | |
| 2. | Paid or accrued consulting fees to a company controlled by Saeid Babaei, and to a company controlled by Rahoul Sharan, directors of the Company at the time of the payments. | |
| 3. | Paid or accrued wages to current directors Dr. Charles Wiseman and Dr. Willam V. Williams, and at the time director Martin Schmieg. | |
| 4. | Share-based compensation in respect of stock options issues during the period to the Company’s five directors for the year end July 31, 2018: Dr. Saeid Babaei, Rahoul Sharan, Isaac Maresky, Dr. William V. Williams and Dr. Joseph Wagner. | |
| 5. | Paid or accrued directors’ fees to directors Jamieson Bondarenko and Vaugh C. Embro-Pantalony. |
These transactions were in the normal course of operations and were measured at the exchange value which represented the amount of consideration established and agreed to by the related parties.
| 106 |
The following description of securities offered in this offering and provisions of our Articles of Incorporation is a summary and does not purport to be complete.
Units
Each unit being offered in this offering consists of one common share and one warrant, each warrant exercisable for one common share. The common shares and warrants that are part of the units are immediately separable and will be issued separately in this offering, although they will have been purchased together in this offering.
Common Shares
As of August 28, 2019, our authorized share capital, as provided for by our articles, consists of an unlimited number of common shares, without par value, of which approximately 196,378,450 shares are issued and outstanding. Upon the closing of this offering, our authorized share capital to consist of an unlimited amount of common shares, without par value, of which we expect 196,378,450 will be issued and outstanding (assuming that the underwriters do not exercise their option to purchase additional common shares). All of our outstanding common shares are validly issued, fully paid and non-assessable.
Our common shares are the only securities with respect to which a voting right may be exercised at a meeting of shareholders.
Dividends. Our Shareholders are entitled to receive dividends, as may be declared from time to time and in the sole discretion of the Board. Dividends shall be paid according to the number of Common Shares owned. Dividends may take the form of specific assets or of fully paid shares or of bonds, debentures or other securities of the Company, or in any one or more of those ways. Shareholders are not entitled to notice of any dividend. We have never paid cash dividends on our capital stock and we do not anticipate paying any dividends in the foreseeable future.
Voting Rights. Each common share is entitled to one vote at a meeting of shareholders.
Warrants Included in the Units
The warrants included within the units are exercisable immediately, have an exercise price of $ per share, equal to 125% of the public offering price of one unit, and expire five years from the date of issuance.
No fractional shares or scrip representing fractional shares shall be issued upon the exercise of the warrants. As to any fraction of a share which the holder would otherwise be entitled to purchase upon such exercise, we shall, at our election, either pay a cash adjustment in respect of such fraction (in an amount equal to such fraction multiplied by the exercise price) or round the number of shares to be received by the holder up to the next whole number.
Registration Number
Our registration number under the BCBCA is BC0764547.
Certain Important Provisions of our Articles and the BCBCA
The following is a summary of certain important provisions of our articles and certain related sections of the BCBCA. Please note that this is only a summary and is not intended to be exhaustive. This summary is subject to, and is qualified in its entirety by reference to, the provisions of our articles and the BCBCA.
Directors
Power to vote on matters in which a director is materially interested. Under the BCBCA a director who has a material interest in a contract or transaction, whether made or proposed, that is material to us, must disclose such interest to us, subject to certain exceptions such as if the contract or transaction: (i) is an arrangement by way of security granted by us for money loaned to, or obligations undertaken by, the director for our benefit or for one of our affiliates’ benefit; (ii) relates to an indemnity or insurance permitted under the BCBCA; (iii) relates to the remuneration of the director in his or her capacity as director, officer, employee or agent of our company or of one of our affiliates; (iv) relates to a loan to our company while the director is the guarantor of some or all of the loan; or (v) is with a corporation that is affiliated to us while the director is also a director or senior officer of that corporation or an affiliate of that corporation.
A director who holds such disclosable interest in respect of any material contract or transaction into which we have entered or propose to enter may be required to absent himself or herself from the meeting while discussions and voting with respect to the matter are taking place. Directors are also required to comply with certain other relevant provisions of the BCBCA regarding conflicts of interest.
| 107 |
Directors’ power to determine the remuneration of directors. The remuneration of our directors is determined by our directors subject to our Articles. If the directors so decide, the remuneration of the directors, if any, will be determined by the shareholders. The remuneration may be in addition to any salary or other remuneration paid to any of our employees (including executive officers) who are also directors.
Director’s power to vote on a proposal, arrangement or contract in which the director is materially interested. A director or senior officer who holds any office or possesses any property, right or interest that could result, directly or indirectly, in the creation of a duty or interest that materially conflicts with that individual’s duty or interest as a director or senior officer, must disclose the nature and extent of the conflict as required by the BCBA. A director who holds a disclosable interest in a contract or transaction into which the Company has entered or proposes to enter is not entitled to vote on any directors’ resolution to approve that contract or transaction, unless all the directors have a disclosable interest in that contract or transaction, in which case any or all of those directors may vote on such resolution. A director who holds a disclosable interest in a contract or transaction into which the Company has entered or proposes to enter and who is present at the meeting of directors at which the contract or transaction is considered for approval may be counted in the quorum at the meeting whether or not the director votes on any or all of the resolutions considered at the meeting.
Number of shares required for director’s qualification. Directors do not need to own stock of the Company to qualify to be a Director.
Shareholder Meetings
Subject to applicable stock exchange requirements, we must hold a general meeting of our shareholders at least once every year at a time and place determined by our board of directors, provided that the meeting must not be held later than 15 months after the preceding annual general meeting. A meeting of our shareholders may be held anywhere in or outside British Columbia.
A notice to convene a meeting, specifying the date, time and location of the meeting, and, where a meeting is to consider special business, the general nature of the special business must be sent to each shareholder entitled to attend the meeting and to each director not less than 21 days and no more than 60 days prior to the meeting, although, as a result of applicable securities laws, the minimum time for notice is effectively longer in most circumstances. Under the BCBCA, shareholders entitled to notice of a meeting may waive or reduce the period of notice for that meeting, provided applicable securities laws are met. The accidental omission to send notice of any meeting of shareholders to, or the non-receipt of any notice by, any person entitled to notice does not invalidate any proceedings at that meeting.
Our Articles provide that the holders of a majority of our Common Shares, issued and outstanding and entitled to vote at a meeting of the stockholders, present in person or represented by proxy, shall constitute a quorum at all meetings of the stockholders for the transaction of business except as otherwise provided by statute or by our Articles of Incorporation. If, however, such quorum shall not be present or represented at any meeting of the stockholders, the stockholders entitled to vote thereat, present in person or represented by proxy, shall have power to adjourn the meeting from time to time, without notice other than announcement at the meeting, until a quorum shall be present or represented. At such adjourned meeting at which a quorum shall be present or represented, any business may be transacted which might have been transacted at the meeting as originally notified.
Pursuant to our articles, a quorum for meetings of shareholders is present if two shareholders who, in the aggregate, hold at least 5% of the issued shares entitled to be voted at the meeting are present in person or represented by proxy. If a quorum is not present at the opening of any meeting of shareholders, the meeting stands adjourned to the same day in the next week at the same time and place, unless the meeting is requisitioned by shareholders, in which case the meeting is dissolved. When a quorum is present or represented at any meeting, the vote of the holders of a majority of the stock having voting power present in person or represented by proxy shall be sufficient to elect directors or to decide any question brought before such meeting, unless the question is one upon which by express provision of the statutes or of the Articles of Incorporation, a different vote is required in which case such express provision shall govern and control the decision of such question.
| 108 |
Each stockholder of record of the corporation shall be entitled at each meeting of stockholders to one vote for each share of stock standing in his name on the books of the corporation. Upon the demand of any stockholder, the vote for directors and the vote upon any question before the meeting shall be by ballot.
At any meeting of the stockholders any stockholder may be represented and vote by a proxy or proxies appointed by an instrument in writing. In the event that any such instrument in writing shall designate two or more persons to act as proxies, a majority of such persons present at the meeting, or, if only one shall be present, then that one shall have and may exercise all of the powers conferred by such written instrument upon all of the persons so designated unless the instrument shall otherwise provide. No proxy or power of attorney to vote shall be used to vote at a meeting of the stockholders unless it shall have been filed with the secretary of the meeting when required by the inspectors of election. All questions regarding the qualification of voters, the validity of proxies and the acceptance or rejection of votes shall be decided by the inspectors of election who shall be appointed by the Board of Directors, or if not so appointed, then by the presiding officer of the meeting.
Any action which may be taken by the vote of the stockholders at a meeting may be taken without a meeting if authorized by the written consent of stockholders holding at least a majority of the voting power, unless the provisions of the statutes or of the Articles of Incorporation require a greater proportion of voting power to authorize such action in which case such greater proportion of written consents shall be required.
Shareholder Proposals
Under the BCBCA, qualified shareholders holding at least one percent (1%) of our issued voting shares may make proposals for matters to be considered at the annual general meeting of shareholders. Such proposals must be sent to us in advance of any proposed meeting by delivering a timely written notice in proper form to our registered office in accordance with the requirements of the BCBCA and be accompanied by one written statement in support of the proposal. The notice must include information on the business the shareholder intends to bring before the meeting.
Forum Selection
We have not included a forum selection provision in our articles.
Ownership Limitation and Transfer of Shares
Our fully paid common shares in registered form and may be freely transferred under our articles of incorporation, unless the transfer is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trade. The ownership or voting of our common shares by non-residents of Canada is not restricted in any way by our articles of incorporation or the laws of Canada.
Share Transfers
Pursuant to our Articles, a transfer of a share must not be registered unless:
| (a) | Except as exempted by the BCBCA, a duly signed proper instrument of transfer in respect of the share has been received by the Company; |
| (b) | If a share certificate has been issued by the Company in respect of the share to be transferred, that share certificate has been surrendered to the Company; and |
| (c) | if a non-transferable written acknowledgment of the shareholder’s right to obtain a share certificate has been issued by the Company in respect of the share to be transferred, that acknowledgment has been surrendered to the Company. |
Change in Control
Our articles of incorporation do not contain restrictions on change in control.
| 109 |
Election of Directors
Our common shares do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect all of our directors.
The directors shall be elected at the annual meeting of the stockholders by a simple majority vote of holders of our voting shares, participating and voting at such meeting, and each director elected shall hold office until his successor is elected and qualified. However, in the event of any vacancy in the Board, including those caused by an increase in the number of Directors, such vacancy may be filled by a majority of the remaining directors, though less than a quorum, or by a sole remaining director, and each director so elected shall hold office until his successor is elected at an annual or a special meeting of the stockholders. The holders of a two-thirds of the outstanding shares of stock entitled to vote may at any time peremptorily terminate the term of office of all or any of the directors by vote at a meeting called for such purpose or by a written statement filed with the secretary or, in his absence, with any other officer. Such removal shall be effective immediately, even if successors are not elected simultaneously and the vacancies on the Board of Directors resulting therefrom shall be filled only by the stockholders.
A vacancy or vacancies in the Board of Directors shall be deemed to exist in case of the death, resignation or removal of any directors, or if the authorized number of directors be increased, or if the stockholders fail at any annual or special meeting of stockholders at which any director or directors are elected to elect the full authorized number of directors to be voted for at that meeting.
The stockholders may elect a director or directors at any time to fill any vacancy or vacancies not filled by the directors. If the Board of Directors accepts the resignation of a director tendered to take effect at a future time, the Board or the stockholders shall have power to elect a successor to take office when the resignation is to become effective.
No reduction of the authorized number of directors shall have the effect of removing any director prior to the expiration of his term of office.
Anti-Takeover Measures
Our articles of incorporation do not provide for any anti-takeover measures.
Changes in Capital
Our articles of incorporation enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the BCBCA.
We have had no change in share capital in the prior three years other than increasing the number of issued and outstanding common shares as described elsewhere in this prospectus.
Exchange Controls
The BCBCA and our articles of incorporation do not provide for any restriction in connection with the following:
| (1) | the import or export of capital, including the availability of cash and cash equivalents for use by the company’s group; and |
| (2) | the remittance of dividends, interest or other payments to nonresident holders of the company’s securities. |
Transfer Agent and Registrar
Our transfer agent is Computershare Investor Services Inc., 3rd Floor, 510 Burrard Street, Vancouver, British Columbia V6C 3B9, telephone: (604) 661-9474, facsimile: (604) 661-9401.
Listing
We are in the process of applying to have our common shares and warrants listed on The Nasdaq Capital Market under the symbols “ ” and “ ”, respectively. Our common shares are currently quoted on the OTCQB marketplace under the symbol “BCTXF” and listed on the TSX Venture Exchange under the symbol “BCT”.
| 110 |
Derivative Securities
Options and Incentive Plans and Awards
The Company’s Stock Option Plan (the “Plan”) was previously approved by the shareholders at the Company’s annual and special meeting on November 25, 2014. Pursuant to the Plan, the Company is authorized to grant options to officers, directors, employees and consultants enabling them to acquire up to 10% of the issued and outstanding Common Shares of the Company. The options can be granted for a maximum of 5 years and vest as determined by the Board. The exercise price of each option granted may not be less than the fair market value of the Common Shares at the time of grant.
Pursuant to the policies of the TSX Venture Exchange (the “TSXV”), the Company is required to obtain.
shareholder approval of the Stock Option Plan each year because the Stock Option Plan is a rolling maximum option plan whereby the maximum number of Common Shares that may be reserved for issuance and which can be purchased upon the exercise of all options granted under the Stock Option Plan is fixed at 10% of the outstanding Common Shares from time to time.
Summary of the Plan
| ● | The term of any options granted under the Plan will be fixed by the Board at the time such options are granted, provided that options will not be permitted to exceed a term of five years (or ten years if the Company is reclassified by the TSXV as a Tier 1 Issuer). |
| ● | The exercise price of any options granted under the Plan will be determined by the Board, in its sole discretion, but shall not be less than the closing price of the Company’s Common Shares on the day preceding the day on which the directors grant such options, less any discount permitted by the TSXV. |
| ● | No vesting requirements will apply to options granted hereunder, however a four month hold period will apply to all Common Shares issued under each option, commencing from the date of grant. |
| ● | All options are non-transferable. |
| ● | Options will be adjusted and/or reclassified (as applicable) in the event of any consolidation, subdivision, conversion or exchange of the Company’s Common Shares. |
| ● | No more than 5% of the issued Common Shares may be granted to any one individual in any 12 month period. |
| ● | No more than 2% of the issued Common Shares may be granted to a consultant, or any employee performing investor relations activities, in any 12 month period |
| ● | Disinterested shareholder approval must obtain if: |
| i. | A stock option plan, together with all of the Company’s previously established and outstanding stock option plans or grants, could result at any time in: |
| a) | The number of shares reserved for issuance under stock options granted to Insiders (as such term is defined in the policies of the TSXV) exceeding 10% of the issued Common Shares; |
| b) | the grant to Insiders, within a 12 month period, of a number of options exceeding 10% of the issued Common Shares; or |
| c) | the issuance to any one optionee, within a 12 month period, of a number of shares |
| d) | exceeding 5% of the issued Common Shares; or |
| ii. | the Company is decreasing the exercise price of stock options previously granted to Insiders. |
The following table sets forth certain information pertaining to the Company’s equity compensation plan as at the end of the Company’s financial year on July 31, 2019.
| Plan Category | Number of securities issuable upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities to be issued upon exercise of outstanding options, warrants and rights)(1) | |||||||||
| Equity compensation plans approved by security holders | 74,784,394 | $ | 0.174 | 12,665,245 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| 1. | Based on a total of 19,637,845 options issuable pursuant to our Stock Option Plan, representing 10% of the Company’s issued and outstanding share capital of 196,378,450 common shares as at July 31, 2019, less the total number of options already issued. |
| 111 |
Warrants
As of August 28, 2019, warrants outstanding were as follows:
Number of Warrants Outstanding | Exercise Price | Exercisable
At | Expiry Date | |||||||||
| 3,421,053 | $ | 0.30 | 3,421,053 | April 26, 2021 | ||||||||
| 1,021,500 | $ | 0.20 | 1,021,500 | December 21, 2019 | ||||||||
| 42,322,322 | $ | 0.14 | 42,322,322 | March 27, 2021 | ||||||||
| 7,814,884 | $ | 0.12 | 7,814,884 | October 2021 - July 2022 | ||||||||
| 54,579,759 | 54,579,759 | |||||||||||
Compensation Warrants
As at August 28, 2019, compensation warrants outstanding were as follows:
| Number Of | ||||||||||||
| Compensation | Exercise | Exercisable at | ||||||||||
| Warrants Outstanding | Price | August 28, 2019 | Expiry Date | |||||||||
| 273,685 | $ | 0.30 | 273,685 | April 26, 2021 (i) | ||||||||
| 1,250,000 | $ | 0.14 | 1,250,000 | March 27, 2021 (ii) | ||||||||
| 2,613,350 | $ | 0.14 | 2,613,350 | March 27, 2021 (ii) | ||||||||
| 4,137,035 | 4,137,035 | |||||||||||
| i. | Each compensation warrant can be exercised at $0.30 into one unit of BriaCell comprising of one common share and one share purchase warrant. Each resultant share purchase warrant acquired can be exercised into an additional common share of BriaCell at $0.35 if exercised by April 26, 2021. |
| ii. | Each compensation warrant can be exercised at $0.14 into one common share of BriaCell for a period of 36 months. |
SHARES ELIGIBLE FOR FUTURE SALE
Upon completion of this offering, we will have _____ common shares outstanding, or any shares that may be sold pursuant to the underwriter’s over-allotment option. All of the common shares sold in this offering will be freely transferable by persons other than by our “affiliates” without restriction or further registration under the Securities Act. Sales of substantial amounts of our common share in the public market could adversely affect prevailing market prices of our common share. Prior to this offering, there has been a limited public market for our common share. We have applied to list the common shares and warrants on the Nasdaq Capital Market, but we cannot assure you that our application will be approved or a regular trading market will develop in the common shares or warrants. We might consummate and close this offering without a listing approval letter from the Nasdaq Capital Market.
Additionally, we have approximately 6,690,102 vested options and 59,996,910 warrants outstanding as of August 28, 2019. The exercise price of the majority of these options and warrants is significantly above our current market price.
| 112 |
Rule 144
Certain of our common shares that will be outstanding upon the completion of this offering, other than those common shares sold in this offering, are “restricted securities” as that term is defined in Rule 144 under the Securities Act and may be sold publicly in the United States only if they are subject to an effective registration statement under the Securities Act or pursuant to an exemption from the registration requirement such as those provided by Rule 144 and Rule 701 promulgated under the Securities Act. In general, beginning 90 days after the date of this prospectus, a person (or persons whose shares are aggregated) who at the time of a sale is not, and has not been during the three months preceding the sale, an affiliate of ours and has beneficially owned our restricted securities for at least six months will be entitled to sell the restricted securities without registration under the Securities Act, subject only to the availability of current public information about us, and will be entitled to sell restricted securities beneficially owned for at least one year without restriction. Persons who are our affiliates and have beneficially owned our restricted securities for at least six months may sell a number of restricted securities within any three-month period that does not exceed the greater of the following:
| ● | 1% of the then outstanding common shares of the same class, which immediately after this offering will equal approximately common shares assuming the over-allotment option is not exercised; or |
| ● | If our common shares are listed on a national securities exchange, the average weekly trading volume of our common share, during the four calendar weeks preceding the date on which notice of the sale is filed with the SEC |
Sales by our affiliates under Rule 144 are also subject to certain requirements relating to manner of sale, notice and the availability of current public information about us.
Rule 701
In general, under Rule 701 of the Securities Act as currently in effect, each of our employees, consultants or advisors who purchases our common share from us in connection with a compensatory stock plan or other written agreement executed prior to the completion of this offering is eligible to resell those common shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144. However, the Rule 701 shares would remain subject to lock-up arrangements and would only become eligible for sale when the lock-up period expires.
Lock-Up Agreements
All of our directors and executive officers have signed lock-up agreements. Pursuant to such lock-up agreements, such persons have agreed, subject to certain exceptions, not to sell or otherwise dispose of common shares or any securities convertible into or exchangeable for common shares for a period of six months after the date of this prospectus without the prior written consent of the underwriters, in their sole discretion, at any time, release all or any portion of the common shares from the restrictions in any such agreement.
The following description is not intended to constitute a complete analysis of all tax consequences relating to the acquisition, ownership and disposition of our common shares. You should consult your own tax advisor concerning the tax consequences in your particular situation, as well as any tax consequences that may arise under the laws of any taxing jurisdiction.
| 113 |
Certain Canadian Federal Income Tax Considerations For United States Residents
The following is, at the date of this prospectus, a summary of certain Canadian federal income tax considerations generally applicable to the holding and disposition of common shares of the Company (referred to in this summary as “Common Shares”) acquired by a holder who, at all relevant times, (a) for the purposes of the Income Tax Act (Canada) (the “Tax Act”) (i) is not resident, or deemed to be resident, in Canada, (ii) deals at “arm’s length” with the Company and the underwriters, and is not “affiliated” with either the Company or the underwriters, (iii) holds Common Shares as capital property, (iv) does not use or hold Common Shares in the course of carrying on, or otherwise in connection with, a business carried on or deemed to be carried on in Canada and (v) is not a “registered non-resident insurer” or “authorized foreign bank” (each as defined in the Tax Act), or other holder of special status, and (b) for the purposes of the Canada-U.S. Tax Convention (1980) (the “Tax Treaty”), is a resident of the United States, has never been a resident of Canada, does not have and has not had, at any time, a permanent establishment or fixed base in Canada, and who otherwise qualifies for the full benefits of the Tax Treaty. Holders who meet all the criteria in clauses (a) and (b) above are referred to herein as “U.S. Holders”, and this summary only addresses such U.S. Holders.
This summary does not deal with special situations, such as the particular circumstances of traders or dealers, tax exempt entities, insurers or financial institutions, or other holders of special status or in special circumstances. Such holders, and all other holders who do not meet the criteria in clauses (a) and (b) above, should consult their own tax advisors.
This summary is based on the current provisions of the Tax Act in force as of the date of this prospectus, the regulations thereunder in force at the date hereof (the “Regulations”), the current provisions of the Tax Treaty, in force as of the date of this prospectus, and the Company’s understanding of the administrative policies and assessing practices of the Canada Revenue Agency published in writing prior to the date hereof. This summary takes into account all specific proposals to amend the Tax Act and Regulations publicly announced by or on behalf of the Minister of Finance (Canada) prior to the date hereof (the “Proposed Amendments”) and assumes that such Proposed Amendments will be enacted in the form proposed. However, such Proposed Amendments might not be enacted in the form proposed, or at all. This summary does not otherwise take into account or anticipate any changes in law or administrative or assessing practices, whether by legislative, governmental or judicial decision or action, nor does it take into account tax laws of any province or territory of Canada or of any other jurisdiction outside Canada, which may differ significantly from those discussed in this summary.
For the purposes of the Tax Act, all amounts relating to the acquisition, holding or disposition of Common Shares must generally be expressed in Canadian dollars. Amounts denominated in United States currency generally must be converted into Canadian dollars using the rate of exchange that is acceptable to the Canada Revenue Agency.
This summary is of a general nature only and is not intended to be, nor should it be construed to be, legal or tax advice to any particular U.S. Holder, and no representation with respect to the Canadian federal income tax consequences to any particular U.S. Holder or prospective U.S. Holder is made. This summary is not exhaustive of all Canadian federal income tax considerations. Accordingly, all prospective purchasers (including U.S. Holders as defined above) should consult with their own tax advisors for advice with respect to their own particular circumstances.
Withholding Tax on Dividends
Amounts paid or credited or deemed to be paid or credited as, on account or in lieu of payment of, or in satisfaction of, dividends on Common Shares to a U.S. Holder will be subject to Canadian withholding tax. Under the Tax Act, the rate of withholding is 25% of the gross amount of the dividend. Under the Tax Treaty, the withholding rate on any such dividend beneficially owned by a U.S. Holder that b is a resident of the United States for purposes of the Tax Treaty are fully entitled to the benefits of the Tax Treaty in respect of the receipt of such dividend is generally reduced to 15%.
Dispositions of Common Shares
A U.S. Holder generally will not be subject to tax under the Tax Act in respect of a capital gain realized on the disposition or deemed disposition of Common Share, nor will capital losses arising therefrom be recognized under the Tax Act, unless such Common Share constitutes “taxable Canadian property” (as defined in the Tax Act) of the U.S. Holder and the gain is not exempt from tax pursuant to the terms of the Tax Treaty.
| 114 |
Provided the Common Shares are listed on a “designated stock exchange” (which currently includes the NASDAQ and Tiers 1 and 2 of the TSXV) as defined in the Tax Act and are so listed at the time of disposition, the Common Shares generally will not constitute “taxable Canadian property” of a U.S. Holder at that time unless, at any time during the 60 month period immediately preceding the disposition, the following two conditions are met concurrently: (i) 25% or more of the issued shares of any class or series of shares of the Company were owned by or belonged to one or any combination of: (a) the U.S. Holder, (b) persons with whom the U.S. Holder did not deal at “arm’s length” (within the meaning of the Tax Act), and (c) partnerships in which the U.S. Holder or a person described in (b) holds a membership interest directly or indirectly through one or more partnerships,; and (ii) more than 50% of the fair market value of the Common Shares was derived directly or indirectly from one or any combination of; (a) real or immovable property situated in Canada, (b) Canadian resource properties (as defined in the Tax Act), (c) timber resource properties (as defined in the Tax Act) or (d) options in respect of, interests in, or, for civil purposes, a right in, the foregoing property, whether or not such property exists. Notwithstanding the foregoing, a Common Share may be deemed to be “taxable Canadian property” in certain other circumstances. U.S. Holders should consult their own tax advisors as to whether their Common Shares will constitute “taxable Canadian property”.
U.S. Holders who may hold common shares as “taxable Canadian property” should consult their own tax advisors with respect to the application of Canadian capital gains taxation, any potential relief under the Tax Treaty, and special compliance procedures under the Tax Act, none of which is described in this summary.
SHAREHOLDERS ARE URGED TO CONSULT THEIR OWN TAX ADVISORS AS TO THE CANADIAN OR OTHER TAX CONSEQUENCES OF THE PURCHASE, OWNERSHIP AND DISPOSITION OF OUR COMMON SHARES, INCLUDING, IN PARTICULAR, THE EFFECT OF ANY NON-U.S., STATE OR LOCAL TAXES.
Material U.S. Federal Income Tax Consequences to U.S. Holders
The following discussion describes the material United States federal income tax consequences to a United States Holder (as defined herein) of the purchase, ownership and disposition of our common shares as of the date hereof. This discussion deals only with common shares that are held as capital assets by a United States Holder. In addition, the discussion set forth below is applicable only to United States Holders (i) who are residents of the United States for purposes of the current United States—Canada Income Tax Convention (the “Treaty”), (ii) whose common shares are not, for purposes of the Treaty, effectively connected with a permanent establishment in Canada and (iii) who otherwise qualify for the full benefits of the Treaty.
As used herein, the term “United States Holder” means a beneficial owner of our common shares that is, for United States federal income tax purposes, any of the following:
| ● | an individual citizen or resident of the United States; | |
| ● | a corporation (or other entity treated as a corporation for United States federal income tax purposes) created or organized in or under the laws of the United States, any state thereof or the District of Columbia; | |
| ● | an estate the income of which is subject to United States federal income taxation regardless of its source; or | |
| ● | a trust if it (1) is subject to the primary supervision of a court within the United States and one or more United States persons have the authority to control all substantial decisions of the trust or (2) has a valid election in effect under applicable United States Treasury regulations to be treated as a United States person. |
This discussion is based upon provisions of the Internal Revenue Code of 1986, as amended (the “Code”), and regulations, rulings and judicial decisions thereunder as of the date hereof. Those authorities may be changed, perhaps retroactively, so as to result in United States federal income tax consequences different from those summarized below.
| 115 |
This discussion does not represent a detailed description of the United States federal income tax consequences applicable to you if you are subject to special treatment under the United States federal income tax laws, including if you are:
| ● | a dealer in securities or currencies; | |
| ● | a financial institution; | |
| ● | a regulated investment company; | |
| ● | a real estate investment trust; | |
| ● | an insurance company; | |
| ● | a tax-exempt organization; | |
| ● | a person holding our common shares as part of a hedging, integrated or conversion transaction, a constructive sale or a straddle; | |
| ● | a trader in securities that has elected the mark-to-market method of tax accounting for your securities; | |
| ● | a person liable for alternative minimum tax; | |
| ● | a person who owns or is deemed to own 10% or more of our stock (by vote or value); | |
| ● | a partnership or other pass-through entity for United States federal income tax purposes; | |
| ● | a person required to accelerate the recognition of any item of gross income with respect to our common shares as a result of such income being recognized on an applicable financial statement; or | |
| ● | a person whose “functional currency” is not the United States dollar. |
If a partnership (or other entity treated as a partnership for United States federal income tax purposes) holds our common shares, the tax treatment of a partner will generally depend upon the status of the partner and the activities of the partnership. If you are a partner of a partnership holding our common shares, you should consult your tax advisors.
This discussion does not contain a detailed description of all the United States federal income tax consequences to you in light of your particular circumstances and does not address the Medicare tax on net investment income or the effects of any state, local or non-United States tax laws. If you are considering the purchase of our common shares, you should consult your own tax advisors concerning the particular United States federal income tax consequences to you of the purchase, ownership and disposition of our common shares, as well as the consequences to you arising under other United States federal tax laws and the laws of any other taxing jurisdiction.
This discussion assumes that we are not, and will not become, a passive foreign investment company, as described below.
Taxation of Dividends
The gross amount of distributions on the common shares (including any amounts withheld to reflect Canadian withholding taxes) will be taxable as dividends to the extent paid out of our current or accumulated earnings and profits, as determined under United States federal income tax principles. To the extent that the amount of any distribution exceeds our current and accumulated earnings and profits for a taxable year, the distribution will first be treated as a tax-free return of capital, causing a reduction in the tax basis of the common shares, and to the extent the amount of the distribution exceeds your tax basis, the excess will be taxed as capital gain recognized on a sale or exchange. We do not, however, expect to determine earnings and profits in accordance with United States federal income tax principles. Therefore, you should expect that a distribution will generally be treated as a dividend.
Any dividends that you receive (including any withheld taxes) will be includable in your gross income as ordinary income on the day actually or constructively received by you. Such dividends will not be eligible for the dividends received deduction allowed to corporations under the Code.
| 116 |
With respect to non-corporate United States Holders, certain dividends received from a qualified foreign corporation may be subject to reduced rates of taxation. A qualified foreign corporation includes a non-U.S. corporation that is eligible for the benefits of a comprehensive income tax treaty with the United States which the United States Treasury Department determines to be satisfactory for these purposes and which includes an exchange of information provision. The United States Treasury Department has determined that the Treaty meets these requirements, but we may not be eligible for the benefits of the Treaty. However, a non-U.S. corporation is also treated as a qualified foreign corporation with respect to dividends paid by that corporation on shares that are readily tradable on an established securities market in the United States. United States Treasury Department guidance indicates that our subordinate voting shares, which will be listed on , will be readily tradable on an established securities market in the United States. There can be no assurance, however, that our subordinate voting shares will be considered readily tradable on an established securities market in later years. Non-corporate holders that do not meet a minimum holding period requirement during which they are not protected from the risk of loss or that elect to treat the dividend income as “investment income” pursuant to Section 163(d)(4) of the Code will not be eligible for the reduced rates of taxation regardless of our status as a qualified foreign corporation. In addition, the rate reduction will not apply to dividends if the recipient of a dividend is obligated to make related payments with respect to positions in substantially similar or related property. This disallowance applies even if the minimum holding period has been met. You should consult your own tax advisors regarding the application of these rules to your particular circumstances.
The amount of any dividend paid in Canadian dollars will equal the United States dollar value of the Canadian dollars received calculated by reference to the exchange rate in effect on the date the dividend is received by you, regardless of whether the Canadian dollars are converted into United States dollars. If the Canadian dollars received as a dividend are converted into United States dollars on the date they are received, you generally will not be required to recognize foreign currency gain or loss in respect of the dividend income. If the Canadian dollars received as a dividend are not converted into United States dollars on the date of receipt, you will have a basis in the Canadian dollars equal to their United States dollar value on the date of receipt. Any gain or loss realized on a subsequent conversion or other disposition of the Canadian dollars will be treated as United States source ordinary income or loss.
Subject to certain conditions and limitations, Canadian withholding taxes on dividends may be treated as foreign taxes eligible for credit against your United States federal income tax liability. For purposes of calculating the foreign tax credit, dividends paid on the subordinate voting shares will be treated as income from sources outside the United States and will generally constitute passive category income. However, in certain circumstances, if you have held the subordinate voting shares for less than a specified minimum period during which you are not protected from risk of loss, or are obligated to make payments related to the dividends, you will not be allowed a foreign tax credit for Canadian withholding taxes imposed on dividends paid on the subordinate voting shares. If you do not elect to claim a United States foreign tax credit, you may instead claim a deduction for Canadian income tax withheld, but only for a taxable year in which you elect to do so with respect to all foreign income taxes paid or accrued in such taxable year. The rules governing the foreign tax credit are complex. You are urged to consult your tax advisors regarding the availability of the foreign tax credit under your particular circumstances.
Passive Foreign Investment Company Consequences
In general, a corporation organized outside the United States will be treated as a passive foreign investment company, or PFIC, for any taxable year in which either (1) at least 75% of its gross income is “passive income”, the PFIC income test, or (2) on average at least 50% of its assets, determined on a quarterly basis, are assets that produce passive income or are held for the production of passive income, the PFIC asset test. Passive income for this purpose generally includes, among other things, dividends, interest, royalties, rents, and gains from the sale or exchange of property that gives rise to passive income. Assets that produce or are held for the production of passive income generally include cash, even if held as working capital or raised in a public offering, marketable securities, and other assets that may produce passive income. Generally, in determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
We do not believe that we are, for United States federal income tax purposes, a PFIC, and we expect to operate in such a manner so as not to become a PFIC. If, however, we are or become a PFIC, you could be subject to additional United States federal income taxes on gain recognized with respect to the common shares and on certain distributions, plus an interest charge on certain taxes treated as having been deferred under the PFIC rules.
| 117 |
Even if we determine that we are not a PFIC for a taxable year, there can be no assurance that the IRS will agree with our conclusion and that the IRS would not successfully challenge our position. Our status as a PFIC is a fact-intensive determination made on an annual basis after the end of each taxable year.
If we are a PFIC in any taxable year during which a U.S. Holder owns common shares, the U.S. Holder could be liable for additional taxes and interest charges under the “PFIC excess distribution regime” upon (1) a distribution paid during a taxable year that is greater than 125% of the average annual distributions paid in the three preceding taxable years, or, if shorter, the U.S. Holder’s holding period for the common shares, and (2) any gain recognized on a sale, exchange or other disposition, including a pledge, of the common shares, whether or not we continue to be a PFIC. Under the PFIC excess distribution regime, the tax on such distribution or gain would be determined by allocating the distribution or gain ratably over the U.S. Holder’s holding period for common shares. The amount allocated to the current taxable year (i.e., the year in which the distribution occurs or the gain is recognized) and any year prior to the first taxable year in which we are a PFIC will be taxed as ordinary income earned in the current taxable year. The amount allocated to other taxable years will be taxed at the highest marginal rates in effect for individuals or corporations, as applicable, to ordinary income for each such taxable year, and an interest charge, generally applicable to underpayments of tax, will be added to the tax.
If we are a PFIC for any year during which a U.S. Holder holds common shares, we must generally continue to be treated as a PFIC by that holder for all succeeding years during which the U.S. Holder holds the common shares, unless we cease to meet the requirements for PFIC status and the U.S. Holder makes a “deemed sale” election with respect to the common shares. If the election is made, the U.S. Holder will be deemed to sell the common shares it holds at their fair market value on the last day of the last taxable year in which we qualified as a PFIC, and any gain recognized from such deemed sale would be taxed under the PFIC excess distribution regime. After the deemed sale election, the U.S. Holder’s common shares would not be treated as shares of a PFIC unless we subsequently become a PFIC.
If we are a PFIC for any taxable year during which a U.S. Holder holds common shares and one of our non-U.S. corporate subsidiaries is also a PFIC (i.e., a lower-tier PFIC), such U.S. Holder would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC and would be taxed under the PFIC excess distribution regime on distributions by the lower-tier PFIC and on gain from the disposition of shares of the lower-tier PFIC even though such U.S. Holder would not receive the proceeds of those distributions or dispositions. Each U.S. Holder is advised to consult its tax advisors regarding the application of the PFIC rules to our non-U.S. subsidiaries.
If we are a PFIC, a U.S. Holder will not be subject to tax under the PFIC excess distribution regime on distributions or gain recognized on common shares if such U.S. Holder makes a valid “mark-to-market” election for our common shares. A mark-to-market election is available to a U.S. Holder only for “marketable stock.” Our common shares will be marketable stock as long as they remain listed on The Nasdaq Capital Market and are regularly traded, other than in de minimis quantities, on at least 15 days during each calendar quarter. If a mark-to-market election is in effect, a U.S. Holder generally would take into account, as ordinary income for each taxable year of the U.S. holder, the excess of the fair market value of common shares held at the end of such taxable year over the adjusted tax basis of such common shares. The U.S. Holder would also take into account, as an ordinary loss each year, the excess of the adjusted tax basis of such common shares over their fair market value at the end of the taxable year, but only to the extent of the excess of amounts previously included in income over ordinary losses deducted as a result of the mark-to-market election. The U.S. Holder’s tax basis in common shares would be adjusted to reflect any income or loss recognized as a result of the mark-to-market election. Any gain from a sale, exchange or other disposition of common shares in any taxable year in which we are a PFIC would be treated as ordinary income and any loss from such sale, exchange or other disposition would be treated first as ordinary loss (to the extent of any net mark-to-market gains previously included in income) and thereafter as capital loss.
A mark-to-market election will not apply to common shares for any taxable year during which we are not a PFIC, but will remain in effect with respect to any subsequent taxable year in which we become a PFIC. Such election will not apply to any non-U.S. subsidiaries that we may organize or acquire in the future. Accordingly, a U.S. Holder may continue to be subject to tax under the PFIC excess distribution regime with respect to any lower-tier PFICs that we may organize or acquire in the future notwithstanding the U.S. Holder’s mark-to-market election for the common shares.
| 118 |
The tax consequences that would apply if we are a PFIC would also be different from those described above if a U.S. Holder were able to make a valid qualified electing fund, or QEF, election. At this time, we do not expect to provide U.S. Holders with the information necessary for a U.S. Holder to make a QEF election. Prospective investors should assume that a QEF election will not be available.
Each U.S. person that is an investor of a PFIC is generally required to file an annual information return on IRS Form 8621 containing such information as the U.S. Treasury Department may require. The failure to file IRS Form 8621 could result in the imposition of penalties and the extension of the statute of limitations with respect to U.S. federal income tax.
The U.S. federal income tax rules relating to PFICs are very complex. Prospective U.S. investors are strongly urged to consult their own tax advisors with respect to the impact of PFIC status on the purchase, ownership and disposition of common shares, the consequences to them of an investment in a PFIC, any elections available with respect to the common shares and the IRS information reporting obligations with respect to the purchase, ownership and disposition of common shares of a PFIC.
Taxation of Capital Gains
For United States federal income tax purposes, you will recognize taxable gain or loss on any sale or exchange of the subordinate voting shares in an amount equal to the difference between the amount realized for the subordinate voting shares and your tax basis in the subordinate voting shares. Such gain or loss will generally be capital gain or loss and will generally be long-term capital gain or loss if you have held the subordinate voting shares for more than one year. Long-term capital gains of non-corporate United States Holders (including individuals) are eligible for reduced rates of taxation. The deductibility of capital losses is subject to limitations. Any gain or loss recognized by you will generally be treated as United States source gain or loss. Consequently, you may not be able to use the foreign tax credit arising from any Canadian tax imposed on the disposition of subordinate voting shares unless such credit can be applied (subject to applicable limitations) against tax due on other income treated as derived from foreign sources.
Sale, Exchange or Other Disposition of Common shares
Subject to the discussion above under “— Passive Foreign Investment Company Consequences,” a U.S. Holder generally will recognize capital gain or loss for U.S. federal income tax purposes upon the sale, exchange or other disposition of common shares in an amount equal to the difference, if any, between the amount realized (i.e., the amount of cash plus the fair market value of any property received) on the sale, exchange or other disposition and such U.S. Holder’s adjusted tax basis in the common shares . Such capital gain or loss generally will be long-term capital gain taxable at a reduced rate for non-corporate U.S. Holders or long-term capital loss if, on the date of sale, exchange or other disposition, the common shares were held by the U.S. Holder for more than one year. Any capital gain of a non-corporate U.S. Holder that is not long-term capital gain is taxed at ordinary income rates. The deductibility of capital losses is subject to limitations. Any gain or loss recognized from the sale or other disposition of common shares will generally be gain or loss from sources within the United States for U.S. foreign tax credit purposes.
Medicare Tax
Certain U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally are subject to a 3.8% tax on all or a portion of their net investment income, which may include their gross dividend income and net gains from the disposition of common shares. If you are a United States person that is an individual, estate or trust, you are encouraged to consult your tax advisors regarding the applicability of this Medicare tax to your income and gains in respect of your investment in common shares.
| 119 |
Information Reporting and Backup Withholding
U.S. Holders may be required to file certain U.S. information reporting returns with the IRS with respect to an investment in common shares , including, among others, IRS Form 8938 (Statement of Specified Foreign Financial Assets). As described above under “Passive Foreign Investment Company Consequences”, each U.S. Holder who is a shareholder of a PFIC must file an annual report containing certain information. U.S. Holders paying more than US$100,000 for common shares may be required to file IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) reporting this payment. Substantial penalties may be imposed upon a U.S. Holder that fails to comply with the required information reporting.
Dividends on and proceeds from the sale or other disposition of common shares may be reported to the IRS unless the U.S. Holder establishes a basis for exemption. Backup withholding may apply to amounts subject to reporting if the holder (1) fails to provide an accurate United States taxpayer identification number or otherwise establish a basis for exemption (usually on IRS Form W-9), or (2) is described in certain other categories of persons. However, U.S. Holders that are corporations generally are excluded from these information reporting and backup withholding tax rules. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules generally will be allowed as a refund or a credit against a U.S. Holder’s U.S. federal income tax liability if the required information is furnished by the U.S. Holder on a timely basis to the IRS.
U.S. Holders should consult their own tax advisors regarding the backup withholding tax and information reporting rules.
EACH PROSPECTIVE INVESTOR IS URGED TO CONSULT ITS OWN TAX ADVISOR ABOUT THE TAX CONSEQUENCES TO IT OF AN INVESTMENT IN COMMON SHARES IN LIGHT OF THE INVESTOR’S OWN CIRCUMSTANCES.
| 120 |
ThinkEquity, a division of Fordham Financial Management, Inc. is acting as representative of the underwriters of the offering. We have entered into an underwriting agreement dated , 2019 with the representative. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below, and each underwriter named below has severally and not jointly agreed to purchase from us, at the public offering price less the underwriting discounts and commissions set forth on the cover page of this prospectus, the number of units listed next to its name in the following table:
| Underwriter | Number of Units | |||
| ThinkEquity, a division of Fordham Financial Management, Inc. | ||||
| Total | ||||
The underwriters are offering the units subject to their acceptance of the units from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the units offered by this prospectus are subject to the approval of certain legal matters by their legal counsel and to certain other conditions. The underwriters are obligated, severally and not jointly, to take and pay for all of the units offered by this prospectus if any such units are taken, other than the units covered by the over-allotment option to purchase additional common shares and/or warrants described below. The underwriting agreement also provides that if an underwriter defaults, the purchase commitments of non-defaulting underwriters may be increased or this offering may be terminated.
The underwriters initially propose to offer the units to the public at the public offering price set forth on the cover page of this prospectus. In addition, the underwriters may offer some of the units to other securities dealers at the public offering price less a concession not in excess of $ per unit. If all of the units offered by us are not sold at the public offering price, the representative may change the offering price and other selling terms.
Over-Allotment Option
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 45 days after the date of this prospectus, permits the underwriters to purchase up to an aggregate of __________ additional common shares and/or warrants to purchase up to _________ additional common shares (equal to 15% of the common shares and warrants underlying the units sold in the offering) in any combination thereof, at the public offering price per share and per warrant, respectively, less underwriting discounts and commissions, solely to cover over-allotments, if any. The purchase price to be paid per additional share of common shares shall be equal to the public offering price of one unit, less the underwriting discount, and the purchase price to be paid per additional warrant shall be $0.00001. If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
Discounts, Commissions and Expenses
The following table shows the public offering price, underwriting discounts and commissions and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Unit | Total
with no Over-Allotment | Total
with Over-Allotment | ||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discounts and commissions (7.0%) | $ | $ | $ | |||||||||
| Proceeds, before expenses, to us | $ | $ | $ | |||||||||
We have agreed to pay a non-accountable expense allowance equal to 1.0% of the public offering price payable to the underwriters. We have also agreed to pay certain expenses of the representative in connection with this offering, including: (a) all filing fees and communication expenses associated with the review of this offering by the Financial Industry Regulatory Authority, Inc. (“FINRA”); (b) fees, expenses and disbursements relating to background checks of our officers and directors, in an amount not to exceed $15,000; (c) fees, expenses and disbursements relating to the registration, qualification or exemption of securities offered under the securities laws of such states and foreign jurisdictions designated by the representative; (d) fees and expenses of the representative’s legal counsel; not to exceed $125,000 (e) $29,500 for fees and expenses for the underwriters’ use of book-building, prospectus tracking and compliance software for this offering; (g) fees and expenses for data services and communications expenses; (f) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and lucite tombstones, each of which the Company or its designee will provide within a reasonable time after the closing in such quantities as the representative may reasonably request, in an amount not to exceed $3,000; and (g) up to $15,000 of the representative’s actual accountable road show expenses for the offering.
| 121 |
We estimate that the total expenses of the offering payable by us, not including underwriting discounts and commissions, will be approximately $___________.
Underwriters’ Warrants
Upon closing of this offering, we have agreed to issue to the representative or its designees as compensation warrants to purchase a number of common shares equal to 5% of the aggregate number of units sold in this offering (including the over-allotment option). The underwriters’ warrants will be exercisable at a per share exercise price equal to 125% of the public offering price per units sold in this offering. The underwriters’ warrants are exercisable at any time and from time to time, in whole or in part, during the four and one-half year period commencing six months following the effective date of the registration statement related to this offering. We have registered the warrants and common shares issuable upon the exercise of the underwriters’ warrants in the registration statement of which this prospectus is a part.
Right of First Refusal
Until eighteen months from the closing date of this offering, the representative will have, subject to certain exceptions, an irrevocable right of first refusal to act as sole investment banker, sole book-runner and/or sole placement agent, at the representative’s discretion, for each and every future U.S. public and private equity and debt offering, including all equity linked financings, during such eighteen month period for us, or any successor to or any subsidiary of us, on terms customary for the representative. The representative will have the sole right to determine whether or not any other broker-dealer shall have the right to participate in any such offering and the economic terms of any such participation. We also granted the representative a right of first refusal, for a period of eighteen months from the consummation of this offering, to act as the Company’s exclusive financial advisor, if the Company retains a financial advisor, in connection with (a) the acquisition or disposition of business units or assets, (b) the acquisition of any of its outstanding securities, (c) an exchange or tender offer, or (d) a merger, consolidation or other business combination or any recapitalization, reorganization, restructuring or other similar transaction, including, without limitation, an extraordinary dividend or distributions or a spin-off or split-off.
Lock-Up Agreements
Each of our directors and officers have agreed for a period of six months after the date of this prospectus, and we have agreed for a period of at least three months after the date of this prospectus, without the prior written consent of the representative, not to directly or indirectly (subject to limited exceptions):
| ● | issue (in the case of us), offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell, grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of our capital stock, including, but not limited to our common shares and warrants, or any securities convertible into or exercisable or exchangeable for shares of our capital stock; or | |
| ● | file or cause the filing of any registration statement under the Securities Act with respect to any shares of our capital stock, including, but not limited to our common shares and warrants, or any securities convertible into or exercisable or exchangeable for shares of our capital stock; or | |
| ● | in the case of us, complete any offering of our debt securities, other than entering into a line of credit with a traditional bank; or | |
| ● | enter into any swap or other agreement, arrangement, hedge or derivatives transaction that transfers to another, in whole or in part, directly or indirectly, any of the economic consequences of ownership of our common shares or warrants or other capital stock or any securities convertible into or exercisable or exchangeable for our common shares or other capital stock, whether any transaction described in any of the foregoing bullet points is to be settled by delivery of our common shares, warrants or other capital stock, other securities, in cash or otherwise, or publicly announce an intention to do any of the foregoing. |
| 122 |
Stabilization
In connection with this offering, the underwriters may purchase and sell our common shares or warrants in the open market. These transactions may include short sales in accordance with Regulation M under the Exchange Act, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriters of a greater number of common shares or warrants than they are required to purchase in this offering. “Covered” short sales are sales made in an amount not greater than the underwriters’ option to purchase additional common shares or warrants in this offering.
The underwriters may close out any covered short position by either exercising their over-allotment option to purchase additional common shares or warrants or purchasing common share or warrants in the open market. In determining the source of common shares or warrants to close out the covered short position, the underwriters will consider, among other things, the price of common shares or warrants available for purchase in the open market as compared to the price at which they may purchase additional common shares or warrants pursuant to the option granted to them. “Naked” short sales are any sales in excess of such option. The underwriters must close out any naked short position by purchasing common shares or warrants in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common shares or warrants in the open market after pricing that could adversely affect investors who purchase in this offering. Stabilizing transactions consist of various bids for, or purchases of, common shares or warrants made by the underwriters in the open market prior to the completion of this offering.
The underwriters may also impose a penalty bid. This occurs when a particular underwriter repays to the underwriters a portion of the underwriting discount received by it because the representatives have repurchased common shares or warrants sold by, or for the account of, such underwriter in stabilizing or short covering transactions.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriters for their own accounts, may have the effect of preventing or retarding a decline in the market price of the common shares or warrants, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of the common shares or warrants. As a result, the price of the common shares or warrants may be higher than the price that otherwise might exist in the open market. If these activities are commenced, they are required to be conducted in accordance with applicable laws and regulations, and they may be discontinued at any time. These transactions may be effected on the Nasdaq, the over-the-counter market or otherwise.
Passive Market Making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in our common shares or warrants on the Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares or warrants and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Indemnification
We have agreed to indemnify the underwriters against liabilities relating to this offering arising under the Securities Act and the Exchange Act, liabilities arising from breaches of some or all of the representations and warranties contained in the underwriting agreement, and to contribute to payments that the underwriters may be required to make for these liabilities.
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
| 123 |
Listing
Our common shares are currently quoted on the OTCQB marketplace under the symbol “BCTXF” and listed on the TSX Venture Exchange under the symbol “BCT”.
We have applied to list our common shares and the warrants included within the units on the Nasdaq Capital Market under the symbols “_____” and “__________” respectively. No assurance can be given that such listings will be approved or that a trading market will develop for common shares and warrants.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members. The representative may agree to allocate a number of securities to underwriters and selling group members for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should not be relied upon by investors.
Other Relationships
From time to time, certain of the underwriters and/or their affiliates have provided, and may in the future provide, various investment banking and other financial services for us for which services they have received and, may in the future receive, customary fees. In the course of their businesses, the underwriters and their affiliates may actively trade our securities or loans for their own account or for the accounts of customers, and, accordingly, the underwriters and their affiliates may at any time hold long or short positions in such securities or loans.
Offer restrictions outside the United States and [Canada]
Other than in the United States [and Canada], no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer to the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
| 124 |
European Economic Area—Belgium, Germany, Luxembourg and Netherlands
The information in this document has been prepared on the basis that all offers of securities will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of securities has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
| ● | to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; | |
| ● | to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43,000,000 (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50,000,000 (as shown on its last annual unconsolidated or consolidated financial statements); | |
| ● | to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)(e) of the Prospectus Directive) subject to obtaining the prior consent of the Company or any underwriter for any such offer; or | |
| ● | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by the Company of a prospectus pursuant to Article 3 of the Prospectus Directive. |
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code Monétaire et Financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the securities have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D.744-1, D.754-1 ;and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1; and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the securities cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The securities have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
| 125 |
Israel
The securities offered by this prospectus have not been approved or disapproved by the Israeli Securities Authority (the ISA), nor have such securities been registered for sale in Israel. The shares may not be offered or sold, directly or indirectly, to the public in Israel, absent the publication of a prospectus. The ISA has not issued permits, approvals or licenses in connection with the offering or publishing the prospectus; nor has it authenticated the details included herein, confirmed their reliability or completeness, or rendered an opinion as to the quality of the securities being offered. Any resale in Israel, directly or indirectly, to the public of the securities offered by this prospectus is subject to restrictions on transferability and must be effected only in compliance with the Israeli securities laws and regulations.
Italy
The offering of the securities in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, or “CONSOB”) pursuant to the Italian securities legislation and, accordingly, no offering material relating to the securities may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
| ● | to Italian qualified investors, as defined in Article 100 of Decree no.58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and | |
| ● | in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. |
Any offer, sale or delivery of the securities or distribution of any offer document relating to the securities in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
| ● | made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and | |
| ● | in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws. |
Any subsequent distribution of the securities in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such securities being declared null and void and in the liability of the entity transferring the securities for any damages suffered by the investors.
Japan
The securities have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the securities may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires securities may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of securities is conditional upon the execution of an agreement to that effect.
| 126 |
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The securities have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the securities have not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissăo do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of securities in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the securities be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of securities in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The securities may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the securities may be publicly distributed or otherwise made publicly available in Switzerland.
Neither this document nor any other offering material relating to the securities have been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of securities will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the securities have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor has the Company received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the securities within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the securities, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by the Company.
No offer or invitation to subscribe for securities is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”) has been published or is intended to be published in respect of the securities. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the securities may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances which do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the securities has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to the Company.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
| 127 |
We have not entered into any material agreements other than in the ordinary course of business and other than those described below or in this prospectus.
On September 29, 2017, the Company entered into a certain Clinical Study Agreement with Cancer Insight, LLC (the “CRO”), a cancer vaccine-focused contract/clinical research organization, pursuant to which the CRO conducted a phase I/IIA study of BriaVaxTM and provided regulatory affairs management services. As consideration, the Company paid the CRO eight equal quarterly payments of $112,848.14. On October 19, 2018 the parties amended the Clinical Study Agreement to increase the budget by a total of $332,817.66.
On October 16, 2017, the Company entered into a certain Service Agreement with Colorado State University (“CSU”), pursuant to which CSU provided certain clinical research services to the Company concerning its PKCδ inhibitors. As consideration, the Company paid CSU a fixed price amount of $191,719. On April 2, 2019, the parties amended the Service Agreement to extend the termination date to March 1, 2020 and increase CSU’s compensation from $191,719 to $219,056.
On January 26, 2018, the CRO, Jarrod Holmes, M.D., and St. Joseph Heritage Healthcare (“SJ”), a California nonprofit public benefit corporation, entered into a Clinical Trial Agreement, amended as of May 7, 2019, pursuant to which SJ agreed to participate in conducting the Company’s phase I/IIA study of BriaVaxTM .
On April 23, 2018, the CRO and the Cancer Center of Kansas, P.A. (“CCK”) entered into a Clinical Trial Agreement, amended as of August 28, 2018, pursuant to which CCK agreed to participate in conducting the Company’s phase I/IIA study of BriaVaxTM .
On August 27, 2018, the Company entered into an Amendment No. 2 to the University Agreement with the University of California, Davis Health, pursuant to which the termination date of the original University Agreement, dated June 11, 2015, was extended July 1, 2020.
On September 4, 2018, the CRO and the University of Miami (“UM”) entered into a Clinical Trial Agreement, pursuant to which UM agreed to participate in conducting the Company’s phase I/IIA study of BriaVaxTM .
On September 29, 2018, the CRO, Providence Health & Services - Washington, dba Providence Regional Medical Center Everett (“PHS”), the Everett Clinic, PLLC (the “Clinic”) and Jason Lukas, M.D., an employee of the Clinic, entered into a Clinical Trial Agreement, pursuant to which PHS and the Clinic agreed to participate in conducting the Company’s phase I/IIA study of BriaVaxTM .
On May 3, 2019, the Company entered into that certain UC Davis Stem Cell Program Services Agreement (the “UCD Agreement”) with the University of California, Davis Health (“UCD”), pursuant to which UCD shall provide the Company with certain services related to stem cells and the Company shall pay UCD a total of $35,855. The UCD agreement terminates on May 1, 2021.
On June 3, 2019, the Company entered into that certain HLA Typing Services Agreement with HistoGenetics, LLC, a New York limited liability company, effective June 1, 2019, pursuant to which HLA shall provide the Company with certain HLA typing by DNA sequencing services.
On June 3, 2019, the Company entered into a certain procurement agreement with Catalent Pharma Solutions, LLC (“Catalent”), pursuant to which Catalent shall procure certain biopharmaceutical products for the Company. As consideration, the Company paid Catalent a total of $442,982.
On June 3, 2019, the Company entered into an agreement with Catalent, pursuant to which Catalent shall provide certain clinical supply services to the Company (the “Catalent Supply Agreement”). As consideration, and upon achievement of certain milestones as set forth in the Catalent Supply Agreement, the Company shall pay Catalent up to $149,167. In connection with the Catalent Supply Agreement, on June 25, 2019, the parties entered into that certain Quality Agreement to outline the certain drug delivery and clinical supply services to be provided by Catalent.
| 128 |
No expert or counsel named in this prospectus as having prepared or certified any part of this prospectus or having given an opinion upon the validity of the securities being registered or upon other legal matters in connection with the registration or offering of the securities was employed on a contingency basis or had, or is to receive, in connection with the offering, a substantial interest, directly or indirectly, in the Company or its subsidiaries. Nor was any such person connected with the Company or any of its subsidiaries as a promoter, managing or principal underwriter, voting trustee, director, officer or employee.
The financial statements included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of MNP LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
Certain legal matters in connection with this offering will be passed upon for us by Sichenzia Ross Ference LLP, New York, New York. The validity of the issuance of our common shares offered in this prospectus and certain other legal matters as to Canadian law will be passed upon for us by Bennett Jones LLP, Toronto, Canada. The underwriters are being represented by Clark Wilson LLP, Vancouver, BC, Canada.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND
FINANCIAL DISCLOSURE
MNP LLP are our independent auditors. There have not been any disagreements with our auditors on accounting and financial disclosure or any other matter.
The estimated expenses payable by us in connection with the offering described in this prospectus (other than the underwriting discounts and commissions) will be as set forth in the table below. With the exception of the U.S. Securities and Exchange Commission registration fee, the FINRA filing fee, and the Nasdaq Capital Market listing fee, all amounts are estimates. All such expenses will be borne by the Registrant.
| Item | Amount to be Paid | |||
| SEC registration fee | $ | |||
| FINRA filing fee | ||||
| The Nasdaq Capital Market listing fee | ||||
| Printing and engraving expenses | ||||
| Legal fees and expenses | ||||
| Accounting fees and expenses | ||||
| Miscellaneous expenses | ||||
| Total | $ | |||
| 129 |
FINANCIAL STATEMENTS

Immunotherapy approaches to breast cancer management
Corporate Office – US 820 Heinz Avenue Berkeley, CA, 94710 Tel: 1-888-485-6340 Fax: 424-245-3719 |
Corporate Office - Canada Suite 300 - Bellevue Centre 235 -15th Street West Vancouver, BC, V7T 2X1 Tel: 604-921-1810 Fax: 604-921-1898 |
BriaCell Therapeutics Corp.
Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
Expressed in Canadian Dollars
Independent Auditors’ Report
To the Shareholders of BriaCell Therapeutics Corp.:
We have audited the accompanying consolidated financial statements of BriaCell Therapeutics Corp., which comprise the consolidated statements of financial position as at July 31, 2018 and 2017, and the consolidated statements of operations and comprehensive loss, changes in shareholders’ equity, and cash flows for the years then ended, and a summary of significant accounting policies and other explanatory information.
Management’s Responsibility for the Consolidated Financial Statements
Management is responsible for the preparation and fair presentation of these consolidated financial statements in accordance with International Financial Reporting Standards and for such internal control as management determines is necessary to enable the preparation of consolidated financial statements that are free from material misstatement, whether due to fraud or error.
Auditors’ Responsibility
Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We conducted our audits in accordance with Canadian generally accepted auditing standards. Those standards require that we comply with ethical requirements and plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free from material misstatement.
An audit involves performing procedures to obtain audit evidence about the amounts and disclosures in the consolidated financial statements. The procedures selected depend on the auditor’s judgment, including the assessment of the risks of material misstatement of the consolidated financial statements, whether due to fraud or error. In making those risk assessments, the auditor considers internal control relevant to the entity’s preparation and fair presentation of the consolidated financial statements in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the entity’s internal control. An audit also includes evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements.
We believe that the audit evidence we have obtained is sufficient and appropriate to provide a basis for our audit opinion.
Opinion
In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of BriaCell Therapeutics Corp. as at July 31, 2018 and 2017, and its financial performance and its cash flows for the years then ended in accordance with International Financial Reporting Standards.
Emphasis of Matter
Without modifying our opinion, we draw attention to Note 1 to the consolidated financial statements which highlights the existence of a material uncertainty relating to conditions that cast significant doubt on BriaCell Therapeutics Corp.’s ability to continue as a going concern.
 | |
Mississauga, Ontario |
Chartered Professional Accountants |
| September 27, 2018 | Licensed Public Accountants |
| F-1 |
BriaCell Therapeutics Corp.
Consolidated Statements of Financial Position
(Expressed in Canadian Dollars)
| July 31, | July 31, | |||||||
| 2018 | 2017 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash | $ | 938,448 | $ | 1,264,429 | ||||
| Short-term investments | 1,341,043 | 750,000 | ||||||
| Amounts receivables | 18,975 | 6,981 | ||||||
| Prepaid expenses | 147,734 | 15,414 | ||||||
| Total current assets | 2,446,200 | 2,036,824 | ||||||
| Security deposits | 172,980 | 2,372 | ||||||
| Investments (Note 5) | 2 | 2 | ||||||
| Office equipment | - | - | ||||||
| Intellectual property (Note 6) | 357,958 | 1 | ||||||
| Total Assets | $ | 2,977,140 | $ | 2,039,199 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued liabilities (Notes 11, 16) | $ | 285,712 | $ | 472,362 | ||||
| Advance subscription (Note 8(b)(vii)) | - | 631,785 | ||||||
| Unsecured convertible loan (Note 7) | 1,460,138 | - | ||||||
| Total liabilities | 1,745,850 | 1,104,147 | ||||||
| Shareholders’ equity | ||||||||
| Share capital (Note 8(b)) | 10,213,174 | 6,609,615 | ||||||
| Share-based payment reserve (Note 9) | 905,257 | 884,763 | ||||||
| Warrant reserve (Note 8(c)(d)) | 2,907,337 | 1,841,448 | ||||||
| Accumulated other comprehensive loss | (105,514 | ) | (72,174 | ) | ||||
| Deficit | (12,688,964 | ) | (8,328,600 | ) | ||||
| Total shareholders’ equity | 1,231,290 | 935,052 | ||||||
| Total liabilities and shareholders’ equity | $ | 2,977,140 | $ | 2,039,199 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
Nature of Operations and Going Concern (Note 1)
Commitments and Contingencies (Note 16)
Events After the Reporting Period (Note 17)
Approved on behalf of the Board:
| “Rahoul Sharan” | “Saeid Babaei” | |
| Director | Director |
| F-2 |
BriaCell Therapeutics Corp.
Consolidated Statements of Operations and Comprehensive Loss
(Expressed in Canadian Dollars)
| Years ended | ||||||||
| July 31 | ||||||||
| 2018 | 2017 | |||||||
| Expenses: | ||||||||
| Research costs (Note 14) | $ | 3,112,579 | $ | 2,125,941 | ||||
| General and administration costs (Note 15) | 1,387,713 | 820,281 | ||||||
| Share-based compensation (Notes 9(b), 11) | 476,211 | 272,014 | ||||||
| Total Expenses | 4,976,503 | 3,218,236 | ||||||
| Operating Loss | (4,976,503 | ) | (3,218,236 | ) | ||||
| Interest income | 15,991 | 6,428 | ||||||
| Interest expense (Note 7) | (20,364 | ) | - | |||||
| Loss on available for sale investments | - | - | ||||||
| Change in fair value of convertible debt (Note 7) | (407,709 | ) | - | |||||
| Foreign exchange loss | (24,078 | ) | (8,913 | ) | ||||
| (436,160 | ) | (2,485 | ) | |||||
| Loss For The Year | (5,412,663 | ) | (3,220,721 | ) | ||||
| Items That Will Subsequently Be Reclassified To Profit Or Loss | ||||||||
| Foreign currency translation adjustment | (33,340 | ) | 41,828 | |||||
| Loss on available for sale investments | ||||||||
| Unrealized loss on available for sale investments | - | - | ||||||
| (33,340 | ) | 41,828 | ||||||
| Comprehensive Loss for the Year | $ | (5,446,003 | ) | $ | (3,178,893 | ) | ||
| Basic and Fully Diluted Loss Per Share | $ | (0.04 | ) | $ | (0.03 | ) | ||
| Weighted Average Number Of Shares Outstanding | 128,344,435 | 101,912,205 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
BriaCell Therapeutics Corp.
Consolidated Statements of Cash Flows
(Expressed in Canadian Dollars)
| Years ended | ||||||||
| July 31 | ||||||||
| 2018 | 2017 | |||||||
| Cash flow from operating activities | ||||||||
| Net loss for the year | $ | (5,412,663 | ) | $ | (3,220,721 | ) | ||
| Items not affecting cash: | ||||||||
| Depreciation and amortization | 16,894 | 290 | ||||||
| Share-based compensation | 476,211 | 272,014 | ||||||
| Change in fair value of convertible loan | 407,709 | - | ||||||
| Accrued interest expense | 20,364 | - | ||||||
| Unrealized loss on available for sale investments | ||||||||
| Unrealized foreign exchange gain | ||||||||
| Changes in non-cash working capital: | ||||||||
| Amounts receivable | (11,994 | ) | (3,494 | ) | ||||
| Prepaid expenses | (117,051 | ) | (2,250 | ) | ||||
| Security deposits | (151,413 | ) | - | |||||
| Accounts payable and accrued liabilities | (186,650 | ) | 1,040,677 | ) | ||||
| (4,958,593 | ) | (1,913,484 | ) | |||||
| Cash flow from investing activities | ||||||||
| Net change in short-term investments | (591,043 | ) | 150,000 | |||||
| (591,043 | ) | 150,000 | ||||||
| Cash flow from financing activities | ||||||||
| Proceeds from private placements | 4,332,232 | 3,046,900 | ||||||
| Share issuance cost | (465,849 | ) | (238,389 | ) | ||||
| Proceeds from issuance of unsecured convertible loan | 1,138,919 | - | ||||||
| Proceeds from exercise of warrants | 286,020 | 88,959 | ||||||
| 5,291,322 | 2,897,470 | |||||||
| Increase (decrease) in cash | (258,314 | ) | 1,133,986 | |||||
| Effect of changes in foreign exchange rates | (67,667 | ) | (41,422 | ) | ||||
| Cash, beginning of year | 1,264,429 | 171,865 | ||||||
| Cash, end of year | $ | 938,448 | $ | 1,264,429 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-4 |
BriaCell Therapeutics Corp.
Statement of Changes in Shareholders’ Equity
(Expressed in Canadian Dollars)
| SHARE CAPITAL | SHARE-BASED PAYMENT | WARRANT | ACCUMULATED OTHER COMPREHENSIVE | ACCUMULATED | TOTAL SHAREHOLDERS’ | |||||||||||||||||||||||
| SHARES | AMOUNT | RESERVE | RESERVE | LOSS | DEFECIT | EQUITY | ||||||||||||||||||||||
| Balance, July 31, 2016 | 91,302,369 | 4,489,797 | 1,042,207 | 1,107,863 | (30,346 | ) | (5,581,404 | ) | 1,028,117 | |||||||||||||||||||
| Private Placement (Note 8(b)(i)) | 8,500,000 | 948,258 | - | 537,503 | - | - | 1,485,761 | |||||||||||||||||||||
| Private Placement (Note 8(b)(iv)) | 5,612,083 | 1,060,961 | - | 261,788 | - | - | 1,322,749 | |||||||||||||||||||||
| Exercise of warrants (Finders’ Options) (Note 8(b)(ii)(iii)(v)(vi)) | 490,109 | 110,599 | - | (21,639 | ) | - | - | 88,960 | ||||||||||||||||||||
| Share-based compensation (Note 9) | - | - | 272,014 | - | - | - | 272,014 | |||||||||||||||||||||
| Expiration of compensation warrants (Note 8(d)) | - | - | (44,067 | ) | - | 44,067 | - | |||||||||||||||||||||
| Cancellation of stock options (Note 9) | - | - | (429,458 | ) | - | - | 429,458 | - | ||||||||||||||||||||
| Foreign exchange translation | - | - | - | - | (41,828 | ) | - | (41,828 | ) | |||||||||||||||||||
| Loss for the year | - | - | - | - | - | (3,220,721 | ) | (3,220,721 | ) | |||||||||||||||||||
| Balance, July 31, 2017 | 105,904,561 | 6,609,615 | 884,763 | 1,841,448 | (72,174 | ) | (8,328,600 | ) | 935,052 | |||||||||||||||||||
| Private Placement (Note 8(b)(vii)) | 4,058,441 | 631,785 | - | - | - | - | 631,785 | |||||||||||||||||||||
| Acquisition of Sapientia (Note 6 and 8(b)(viii)) | 2,500,002 | 375,000 | - | - | - | - | 375,000 | |||||||||||||||||||||
| Exercise of warrants under Warrant Incentive Program (Note 8(b)(ix)) | 2,043,000 | 351,557 | - | (65,537 | ) | - | - | 286,020 | ||||||||||||||||||||
| Private Placement (Note 8(b)(x)) | 43,322,322 | 2,644,659 | - | 1,687,573 | - | - | 4,332,232 | |||||||||||||||||||||
| Share issuance costs (Note 8(b)(x)) | - | (465,850 | ) | - | - | - | - | (465,850 | ) | |||||||||||||||||||
| Issuance of compensation warrants (Note 7) | - | - | - | 97,875 | - | - | 97,875 | |||||||||||||||||||||
| Issuance of shares on conversion of debt (Note 8(b)(xi)) | 1,068,426 | 66,408 | - | 40,435 | - | - | 106,843 | |||||||||||||||||||||
| Expiration of warrants and compensation warrants (Note 8(c)(d)) | - | - | - | (694,457 | ) | - | 694,457 | - | ||||||||||||||||||||
| Share-based compensation (Notes 9(b)) | - | - | 378,336 | - | - | - | 378,336 | |||||||||||||||||||||
| Expiration of stock options (Note 9) | - | - | (357,842 | ) | - | 357,842 | - | |||||||||||||||||||||
| Foreign exchange translation | - | - | - | - | (33,340 | ) | - | (33,340 | ) | |||||||||||||||||||
| Loss for the year | - | - | - | - | - | (5,412,663 | ) | (5,412,663 | ) | |||||||||||||||||||
| Balance, July 31, 2018 | 158,896,752 | $ | 10,213,174 | $ | 905,257 | $ | 2,907,337 | $ | (105,514 | ) | $ | (12,688,964 | ) | $ | 1,231,290 | |||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 1. | Nature of Operations and Going Concern |
BriaCell Therapeutics Corp. (“BriaCell” or the “Company”) was incorporated under the Business Corporations Act (British Columbia) on July 26, 2006 and is listed on the TSX Venture Exchange (“TSX Venture”). The Company trades on the TSX Venture under the symbol “BCT.V”
The Company’s head office is located at Suite 300 – 235 West 15th Street, West Vancouver, British Columbia, V7T 2X1.
BriaCell is an immuno-oncology biotechnology company. BriaCell owns the US patent to BriaVaxTM, a whole-cell cancer vaccine (US Patent No.7674456) (the “Patent”). The Company is currently advancing its vaccine program, BriaVax™, to complete a 24-subject Phase I/IIa clinical trial and by research activities in the context of BriaDx™, a companion diagnostic test to identify patients likely benefitting from BriaVax™.
During the previous year, the Company completed two private placements of units totaling gross proceeds of $3,046,900 (Notes 8(b)(i)(iv)). In August 2017, the Company completed a third non-brokered private placement of units with gross proceeds of $631,785 (Note 8(b)(vii)) and in March 2018, the Company completed a fourth brokered private placement of units with gross proceeds of $4,332,232 (Note 8(b)(x)).
In March 2018, the Company also completed a brokered private placement of unsecured convertible notes in the principal amount of US$885,000 (Note 7).
The accompanying consolidated financial statements have been prepared on the basis of a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business for the foreseeable future. The Company has incurred losses from inception of $12,688,964 (2017 - $8,328,600), is currently in the development stage, and has not commenced commercial operations. The Company’s ability to continue as a going concern is dependent upon its ability to attain future profitable operations and to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. As at July 31, 2018, the Company had not yet completed the clinical development of or achieved regulatory approval to market BriaVaxTM, its lead product candidate and expects to incur further losses; the nature of a development stage immune-oncology company requires the raising of financial capital to support its clinical development programs and administrative costs. The uncertainty of the Company’s ability to raise such financial capital casts significant doubt on the Company’s ability to continue as a going concern. These consolidated financial statements do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should the Company not be able to continue as a going concern.
These consolidated financial statements were authorized for issue by the Board of Directors on September 27, 2018.
| 2. | Basis of Presentation |
Statement of Compliance
These consolidated financial statements have been prepared in accor1dance with International Financial Reporting Standards (“IFRS”), as issued by the International Accounting Standards Board (“IASB”) and interpretations of the IFRS Interpretations Committee (“IFRIC”).
| F-6 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 2. | Basis of Presentation (continued) |
Basis of Presentation
The consolidated financial statements are prepared on a going concern basis and have been presented in Canadian dollars which is the Company’s reporting currency. A summary of the significant accounting policies is provided in Note 3. Standards and guidelines not effective for the current accounting period are described in Note 4.
Basis of Measurement
Theses consolidated financial statements have been prepared under the historical cost basis, except for financial instruments which have been measured at fair value.
Basis of Consolidation
These consolidated financial statements include the accounts of BriaCell and its wholly-owned subsidiaries, BriaCell Therapeutics Corp. (“BTC”) and Sapientia Pharmaceuticals Inc (“Sapientia”).
All inter–company balances, and transactions, have been eliminated upon consolidation.
| 3. | Summary of Significant Accounting Policies |
The significant accounting policies used in the preparation of these consolidated financial statements set out below have been applied consistently in all material respects for all periods presented.
Cash
Cash and cash equivalents include cash on hand, deposits held with banks and other short-term highly liquid investments with original maturities of three months or less. As at July 31, 2018 and 2017, the Company had no cash equivalents.
Short-term Investments
Short-term investments consist of variable rate guaranteed investment certificates (“GICs”) with original terms of one year or less but greater than three months.
Translation of Foreign Currencies
These consolidated financial statements are presented in Canadian dollars. The functional currency of BriaCell is the Canadian dollar. The functional currency of BTC and Sapientia is the United States dollar.
Translation gains or losses resulting from the translation of the financial statements of BTC and Sapientia into Canadian dollars for presentation purposes are recorded in other comprehensive (loss) income.
Within each entity, transactions in currencies other than the functional currency (“foreign currencies”) are translated to the functional currency at the rate of exchange prevailing at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are retranslated to the functional currency at the end of each reporting period at the period-end exchange rate. Exchange gains and losses on the settlement of transactions and the translation of monetary assets and liabilities to the functional currency are recorded in profit or loss.
| F-7 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Intangible assets
Separately acquired intangible assets are measured on initial recognition at cost including directly attributable costs. Intangible assets acquired in a business combination are measured at fair value at the acquisition date. Expenditures relating to internally generated intangible assets, excluding capitalized development costs, are recognized in profit or loss when incurred.
Intangible assets with finite useful lives are amortized over their useful lives and reviewed for impairment whenever there is an indication that the asset may be impaired. The amortization period and the amortization method for an intangible asset are reviewed at least at each year end.
Intangible assets with indefinite useful lives are not systematically amortized and are tested for impairment annually, or whenever there is an indication that the intangible asset may be impaired. The useful life of these assets is reviewed annually to determine whether their indefinite life assessment continues to be supportable. If the events and circumstances do not continue to support the assessment, the change in the useful life assessment from indefinite to finite life is accounted for prospectively as a change in accounting estimate and on that date the asset is tested for impairment. Commencing from that date, the asset is amortized systematically over its useful life.
The useful lives of intangible assets are as follows:
| Patents | |||
| Useful life | 20 years | ||
| Amortization method | Straight-line | ||
| In-house development or purchase | Purchase |
Impairment of non-financial assets:
The Company evaluates the need to record an impairment of non-financial assets whenever events or changes in circumstances indicate that the carrying amount is not recoverable.
If the carrying amount of non-financial assets exceeds their recoverable amount, the assets are reduced to their recoverable amount. The recoverable amount is the higher of fair value less costs of sale and value in use. In measuring value in use, the expected future cash flows are discounted using a pre-tax discount rate that reflects the risks specific to the asset. The recoverable amount of an asset that does not generate independent cash flows is determined for the cash generating unit (“CGU”) to which the asset belongs. Impairment losses are recognized in profit or loss.
An impairment loss of an asset, other than goodwill, is reversed only if there have been changes in the estimates used to determine the asset’s recoverable amount since the last impairment loss was recognized. Reversal of an impairment loss, as above, shall not be increased above the lower of the carrying amount that would have been determined (net of depreciation or amortization) had no impairment loss been recognized for the asset in prior years and its recoverable amount. The reversal of impairment loss of an asset presented at cost is recognized in profit or loss.
| F-8 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Research and Development
Research and development costs are expensed as incurred.
Financial Instruments
Financial assets
The Company classifies its financial assets into one of the following categories, depending on the purpose for which the asset was acquired. The Company’s accounting policy for each category is as follows:
Fair value through profit or loss - This category comprises derivatives, or assets acquired or incurred principally for the purpose of being sold or repurchased in the near term. They are carried in the consolidated statement of financial position at fair value with changes in fair value recognized in the consolidated statements of operations and comprehensive loss.
Loans and receivables - These assets are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. They are carried at cost less any provision for impairment. Individually significant receivables are considered for impairment when they are past due or when other objective evidence is received that a specific counterparty will default.
Held-to-maturity investments - These assets are non-derivative financial assets with fixed or determinable payments and fixed maturities that the Company’s management has the positive intention and ability to hold to maturity. These assets are measured at amortized cost using the effective interest method. If there is objective evidence that the investment is impaired, determined by reference to external credit ratings and other relevant indicators, the financial asset is measured at the present value of estimated future cash flows. Any changes to the carrying amount of the investment, including impairment losses, are recognized in the consolidated statements of operations and comprehensive loss.
Available-for-sale - Non-derivative financial assets not included in the above categories are classified as available-for-sale. They are carried at fair value with changes in fair value recognized in comprehensive income (loss). Where a decline in the fair value of an available-for-sale financial asset constitutes objective evidence of impairment, the amount of the loss is removed from accumulated other comprehensive income (loss) and recognized in the consolidated statements of operations and comprehensive loss.
All financial assets except for those at fair value through profit or loss are subject to review for impairment at least at the end of each reporting period.
Financial assets are impaired when there is any objective evidence that the cash flows related to a financial asset or group of financial assets have been negatively impacted. Different criteria to determine impairment are applied for each category of financial assets described above.
Financial liabilities
The Company classifies its financial liabilities into one of two categories, depending on the purpose for which the corresponding asset was acquired. The Company’s accounting policy for each category is as follows:
Fair value through profit or loss - This category comprises derivatives, or liabilities acquired or incurred principally for the purpose of being sold or repurchased in the near term. They are carried in the consolidated statements of financial position at fair value with changes in fair value recognized in the consolidated statements of operations and comprehensive loss.
| F-9 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Other financial liabilities - This category includes accounts payables and accrued liabilities, all of which are recognized at amortized cost.
The Company’s financial instruments consist of the following:
| Financial assets: | Classification: | |
| Cash | Loans and receivables | |
| Short-term investments | Fair value through profit or loss | |
| Amounts receivable (excluding for HST) | Loans and receivables | |
| Investments | Available for sale | |
| Security deposits | Loans and receivables |
| Financial liabilities: | Classification: | |
| Accounts payable and accrued liabilities | Other financial liabilities | |
| Advance subscription | Other financial liabilities | |
| Unsecured convertible loan | Fair value through profit and loss |
Financial instruments recorded at fair value in the consolidated statements of financial position are classified according to a three-level hierarchy that reflects the significance of the inputs used in making the fair value measurements. The three levels of fair value hierarchy are as follows:
| ● Level 1 - | Unadjusted quoted prices in active markets for identical assets or liabilities; |
| ● Level 2 - | Inputs other than quoted prices that are observable for assets or liabilities, either directly or indirectly; and |
| ● Level 3 - | Inputs for assets or liabilities that are not based on observable market data. |
Financial instruments measured at fair value on a recurring basis include the following:
| FAIR | As at | As at | As at | ||||||||||||||||||||||||
| VALUE | July 31, 2018 | July 31, 2017 | July 31, 2016 | ||||||||||||||||||||||||
| INPUT | CARRYING | ESTIMATED | CARRYING | ESTIMATED | CARRYING | ESTIMATED | |||||||||||||||||||||
| LEVEL | AMOUNT | FAIR VALUE | AMOUNT | FAIR VALUE | AMOUNT | FAIR VALUE | |||||||||||||||||||||
| Financial Assets: | |||||||||||||||||||||||||||
| Cash | 1 | $ | 938,448 | $ | 938,448 | $ | 1,264,429 | $ | 1,264,429 | $ | 171,865 | $ | 171,865 | ||||||||||||||
| Short-term investments | 2 | $ | 1,341,043 | $ | 1,341,043 | $ | 750,000 | $ | 750,000 | $ | - | $ | - | ||||||||||||||
| Financial liabilities: | |||||||||||||||||||||||||||
| Unsecured convertible loans | 3 | $ | 1,460,138 | $ | 1,460,138 | $ | - | $ | - | $ | - | $ | - | ||||||||||||||
| F-10 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Share-based Payments
Equity-settled share-based payments for directors, officers and employees are measured at fair value at the date of grant and recorded as compensation expense with a corresponding increase to share-based payment reserve in the consolidated financial statements.
The fair value determined at the grant date of equity-settled share-based payments is expensed using the graded vesting method over the vesting period based on the Company’s estimate of payments that will eventually vest. Upon exercise of the stock options, consideration paid by the option holder together with the amount previously recognized in share-based payment reserve is recorded as an increase to share capital. Upon expiry, the amounts recorded for share-based compensation are transferred to the deficit from the share-based payment reserve. Shares are issued from treasury upon the exercise of equity-settled share-based instruments.
Compensation expense on stock options granted to non-employees is measured at the earlier of the completion of performance and the date the options are vested using the fair value method and is recorded as an expense in the same period as if the Company had paid cash for the goods or services received.
When the value of goods or services received in exchange for the share-based payment cannot be reliably estimated, the fair value is measured by use of a Black-Scholes valuation model. The expected life used in the model is adjusted, based on management’s best estimate, for the effects of non-transferability, exercise restrictions, and behavioral considerations.
Share Capital
Common shares are classified as equity. Proceeds from unit placements are allocated between shares and warrants issued using the relative fair value method. Costs directly identifiable with share capital financing are charged against share capital. Share issuance costs incurred in advance of share subscriptions are recorded as non-current deferred assets. Share issuance costs related to uncompleted share subscriptions are charged to operations in the period they are incurred.
Warrant Reserve
The fair value of warrants is determined upon their issuance either as part of unit private placements or in settlement of share issuance costs and finders’ fees, using the Black-Scholes model. All such warrants are classified in a warrant reserve within equity. If the warrants are converted, the value attributable to the warrants is transferred to common share capital. Upon expiry, the amounts recorded for expired warrants is transferred to the deficit from the warrant reserve. Shares are issued from treasury upon the exercise of share purchase warrants.
Income Taxes
Income tax expense consists of current and deferred tax expense. Current and deferred taxes are recognized in profit or loss except to the extent they relate to items recognized directly in equity or other comprehensive income.
Current tax is recognized and measured at the amount expected to be recovered from or payable to the taxation authorities based on the income tax rates enacted or substantively enacted at the end of the reporting period and includes any adjustment to taxes payable in respect of previous years.
| F-11 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 3. | Summary of Significant Accounting Policies (continued) |
Income Taxes (continued)
Deferred tax is recognized on any temporary differences between the carrying amounts of assets and liabilities in the consolidated financial statements and the corresponding tax bases used in the computation of taxable earnings. Deferred tax assets and liabilities are measured at the tax rates that are expected to apply in the period when the asset is realized and the liability is settled. The effect of a change in the enacted or substantively enacted tax rates is recognized in profit or loss and comprehensive income (loss) or equity depending on the item to which the adjustment relates.
Deferred tax assets are recognized to the extent future recovery is probable. At the end of each reporting period, deferred tax assets are reduced to the extent that it is no longer probable that sufficient taxable earnings will be available to allow all of part of the asset to be recovered.
Basic and Diluted Loss per Share
Basic loss per share is computed by dividing the loss for the year by the weighted average number of common shares outstanding during the year. Diluted earnings per share reflect the potential dilution that could occur if potentially dilutive securities were exercised or converted to common stock.
The dilutive effect of options and warrants and their equivalent is computed by application of the treasury stock method. Diluted amounts are not presented when the effect of the computations is anti-dilutive. Accordingly, at present, there is no difference in the amounts presented for basic and diluted loss per share.
Significant Accounting Judgments and Estimates
The preparation of these consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. Actual outcomes could differ from these estimates. The consolidated financial statements include estimates which, by their nature, are uncertain. The impacts of such estimates are pervasive throughout the consolidated financial statements, and may require accounting adjustments based on future occurrences. Revisions to accounting estimates are recognized in the period in which the estimate is revised and also in future periods when the revision affects both current and future periods.
The critical judgments and significant estimates in applying accounting policies that have the most significant effect on the amounts recognized in the consolidated financial statements are:
| ● | The series of loans made to the subsidiary company are considered part of the parent company’s net investment in a foreign operation as the Company does not plan to settle these balances in the foreseeable future. As a result of this assessment, the unrealized foreign exchange gains and losses on the intercompany loans are recorded through compressive loss. If the Company determined that settlement of these amounts was planned or likely in the foreseeable future, the resultant foreign exchange gains and losses would be recorded through profit or loss. | |
| ● | The determination that the unrealized decrease in the fair value of available for sale investments is other than temporary. | |
| ● | The change in the fair value of the unsecured convertible loan is based on an estimate determined by the Black-Scholes Model. |
| F-12 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 4. | Standards Issued but Not Yet Effective |
Certain pronouncements were issued by the IASB or the IFRIC that are mandatory for future accounting periods. Many are not applicable to or are not expected to have a significant impact on BriaCell and have been excluded from the list below. The following have not yet been adopted and are being evaluated to determine their impact on BriaCell.
| (i) | IFRS 9 – Financial instruments (“IFRS 9”) was issued by the IASB its final form in July 2014 and will replace IAS 39 Financial Instruments: Recognition and Measurement (“IAS 39”). IFRS 9 uses a single approach to determine whether a financial asset is measured at amortized cost or fair value, replacing the multiple rules in IAS 39. The approach in IFRS 9 is based on how an entity manages its financial instruments in the context of its business model and the contractual cash flow characteristics of the financial assets. Most of the requirements in IAS 39 for classification and measurement of financial liabilities were carried forward unchanged to IFRS 9. The new standard also requires a single impairment method to be used, replacing the multiple impairment methods in IAS39. The standard is effective for annual periods beginning on or after January 1, 2018. Management assesses that the adoption of IFRS 9 will not have a significant impact to the consolidated financial statements. |
| (ii) | IFRS 15 - Revenue from contracts with customers (“IFRS 15”) proposes to replace IAS 18 – Revenue, IAS 11 – Construction contracts and some revenue-related interpretations. The standard contains a single model that applies to contracts with customers and two approaches to recognizing revenue: at a point in time or over time. The model features a contract-based five step analysis of transaction to determine whether, how much and when revenue is recognized. New estimates and judgemental thresholds have been introduced, which may affect the amount and/or timing of revenue recognized. IFRS 15 is effective for annual periods beginning on or after January 1, 2018. Earlier adoption is permitted. The Company has determined there will not be a significant impact to the consolidated financial statements as a result of the adoption of this standard. |
| (iii) | IFRS 16 - Leases (“IFRS 16”) replaces IAS 17, Leases (“IAS 17”). The new model requires the recognition of almost all lease contracts on a lessee’s statement of financial position as a lease liability reflecting future lease payments and a ‘right-of-use asset’ with exceptions for certain short-term leases and leases of low-value assets. In addition, the lease payments are required to be presented on the statement of cash flow within operating and financing activities for the interest and principal portions, respectively. IFRS 16 is effective for annual periods beginning on or after January 1, 2019, with early adoption permitted if IFRS 15, Revenue from Contracts with Customers, is also applied. The Company has yet to evaluate the impact of this new standard. |
| (iv) | IFRS 17 – Insurance Contract (“IFRS 17”) was issued by the IASB in May 2017, which replaces IFRS 4 Insurance Contracts. IFRS 17 requires entities to measure insurance contract liabilities at their current fulfillment values using one of three measurement models, depending on the nature of the contract. IFRS 17 is effective for annual periods beginning on or after January 1, 2021. IFRS 17 will affect how the Company’s accounts for it’s insurance contracts and how it reports its financial performance in our consolidated statements of operations. The Company has yet to evaluate the impact of this new standard. |
| 5. | Investments |
The Company holds 300 common shares in Prosalutis Holdings Inc. and 300,000 common shares in Entourage Mining Ltd. The fair value of these investments as of July 31, 2018 was $2 (2017 - $2).
| F-13 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 6. | Acquisition of Sapientia Pharmaceuticals Inc. |
On July 24, 2017, the Company entered into a definitive share exchange agreement (the “Share Exchange Agreement”) between BTC, Sapientia and all the shareholders of Sapientia. Sapientia is a biotechnology company based in Havertown, PA, that is developing novel targeted therapeutics for multiple indications including several cancers and fibrotic diseases.
Pursuant to the terms of the Share Exchange Agreement, BTC acquired from the Sapientia Shareholders all of the issued and outstanding shares in the capital of Sapientia. As consideration, the Sapientia Shareholders, received an aggregate of 2,500,002 common shares in the capital of BriaCell on a pro-rata basis (the “Transaction”), which were issued on September 5, 2017. As part of the Transaction, BriaCell acquired all rights, including composition of matter patents, and preclinical study data to a novel therapeutic technology platform, known as protein kinase C delta (PKCδ) inhibitors, which represents a unique, highly-targeted approach to treat cancer and to boost the immune system.
As of the acquisition date, the Company owns 100% of the outstanding shares of Sapientia. In accordance with IFRS, the Transaction does not meet the definition of a business combination as Sapientia has not yet commenced commercial operations and is in the development stage. Consequently, the transaction has been recorded as an asset acquisition. The purchase price allocation for this acquisition is shown below:
| Purchase Price Consideration | ||||
| Fair value of 2,500,002 common shares issued | $ | 375,000 | ||
| Fair value of Purchase Price Consideration | $ | 375,000 | ||
| Net Assets Acquired | ||||
| Cash | 149 | |||
| Intellectual property | 374,851 | |||
| Fair value of Total Net Assets | $ | 375,000 | ||
The attributable intellectual property relates to Sapientia’s various patents, which the Company is amortizing over 20 years, consistent with its accounting policy (Note 3). During the year ended July 31, 2018, the Company recorded $16,894 in amortization on intellectual property (2017 - $nil).
| 7. | Unsecured Convertible Loan |
On March 16, 2018, concurrent with the Brokered Unit Offering (Note 8(b)(iv)), the Company completed a brokered private placement for the purchase of 5.0% unsecured convertible notes (each, a “Convertible Note”) in the principal amount of US$885,000. Under the terms of securities purchase agreements between the Company and the purchasers of Convertible Notes (the “Noteholders”), each Convertible Note is convertible, at the option of the holder, into (i) common shares of BriaCell for so long as the Convertible Note is outstanding, at a fixed conversion price of $0.10 per common share, for a period of nine months from the date of issuance, which may be extended by the applicable holder for up to nine additional nine month terms at the holder’s sole option, and (ii) for each common share issued as a result of conversion, one warrant. The warrants are valid for 36 months from their issuance date and each warrant is exercisable for one common share at an exercise price of $0.14. The repayment date of the Convertible Notes was September 16, 2018.
| F-14 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 7. | Unsecured Convertible Loan (continued) |
During July 2018, Noteholders converted $106,843 of Convertible Notes held into 1,068,426 shares and 1,068,426 warrants (Note 8(b)(xi)). For conversions subsequent to the year end and details of the extension of the Convertible Notes, see Note 18.
The Convertible Notes are denominated in US dollars and convertible into common shares and warrants based on the principal and interest balance translated to Canadian dollars. Management determined that the Convertible Notes represent a combined instrument that contains an embedded derivative, being the conversion option. As a result of the foreign exchange impact on the conversion factor, the conversion option does not meet the fixed for fixed criteria and therefore represents a derivative liability. In accordance with IAS 39, the Company has designated the entire Unsecured Convertible Loan at fair value through profit or loss. The Unsecured Convertible Loan was initially recorded at fair value and re-valued at each reporting date with changes in fair value being charged to interest expenses in the consolidated statements of comprehensive loss.
Fair value determination
The fair value of the Convertible Notes, including the increase thereto, has been determined using a combination of the Black-Scholes option pricing model for the equity conversion portion and the discounted cash flow method for the loan portion.
The following assumptions were used to determine the fair value of the Convertible Notes:
| March 16, 2018 | July 31, 2018 | |||||||
| (at issuance date) | (at year end) | |||||||
| Risk-free interest rate | 1.81 | % | 1.88 | % | ||||
| Expected volatility | 73 | % | 88 | % | ||||
| share price | $ | 0.10 | $ | 0.14 | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
| Annual loan interest rate | 5 | % | 5 | % | ||||
| CAD/USD rate | 1.2869 | 1.3017 | ||||||
As at July 31, 2018, the fair value of the amount owed to the Noteholders, including accrued interest was $1,460,138. Total interest expenses and losses due to changes in fair value, charged to the consolidated statements of operations and comprehensive loss were $20,364 and $407,709 respectively. In relation to the offering of these Convertible notes, the Company paid commissions and advisory fees of US$64,000 and US$100,000, respectively, to certain participating dealers. In addition, 1,250,000 Compensation Warrants and were issued.
A fair value of $97,875, for the Compensation Warrants, was determined using the Black-Scholes option pricing model and the following assumptions: - share price - $0.13; exercise price - $0.14; expected life - 3 years; annualized volatility - 100.61%; dividend yield - 0%; risk free rate - 1.99%. The fair value of these Compensation warrants was recorded as a share-based compensation expense on the consolidated statements of comprehensive loss.
| F-15 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 8. | Share Capital and Warrant Reserve |
| a) | Authorized Share Capital |
The authorized share capital consists of an unlimited number of common shares with no par value.
| b) | Issued Share Capital |
| (i) | On August 19, 2016, the Company completed a non-brokered private placement resulting in gross proceeds of $1,700,000. The non-brokered private placement involved the sale of 8,500,000 units at a price of $0.20 per unit (the “August 2016 Non-Brokered Units”). Each August 2016 Non-Brokered Unit comprised one Common Share and one common share purchase warrant (the “August 2016 Non-Brokered Warrants”). Each August 2016 Non-Brokered Warrant entitles the holder thereof to acquire one additional Common Share for an initial period of 12 months from August 19, 2016 at an exercise price of $0.30 and at an exercise price of $0.35 during the subsequent 24 months. |
Certain finders received a cash commission of $115,500 plus 595,000 compensation warrants (the “August 2016 Compensation Warrants”) exercisable into one Non-Brokered Unit at any time until August 19, 2019 at an exercise price of $0.35.
The total fair value of each August 2016 Non-Brokered Warrants and August 2016 Compensation Warrants was $472,305 and $65,198, respectively and was determined using the Black-Scholes option pricing model and the following assumptions: August 2016 Non-Brokered Warrants - share price - $0.22; exercise price - $0.35; expected life - 3 years; annualized volatility - 95.43%; dividend yield - 0%; risk free rate - 0.64%. August 2016 Compensation Warrants - share price - $0.20; exercise price - $0.20; expected life - 3 years; annualized volatility - 95.43%; dividend yield - 0%; risk free rate - 0.64%.
| (ii) | On October 7, 2016, 192,140 Compensation Warrants were exercised into 192,140 common shares and 192,140 warrants for a total consideration of $34,585. The fair value of the warrants was determined using the Black-Scholes option pricing model and the following assumptions: - share price - $0.21; exercise price - $0.35; expected life – 1.15 years; annualized volatility – 90.07%; dividend yield – 0%; risk free rate – 0.64%. Gross proceeds, less issuance costs paid in cash and less the total fair value of the warrants was charged against Share Capital in the statement of changes in shareholders’ equity. | |
| (iii) | On November 30, 2016, 116,963 Compensation Warrants were exercised into 116,963 common shares and 116,963 warrants for a total consideration of $21,055. The fair value of the warrants was determined using the Black-Scholes option pricing model and the following assumptions: - share price - $0.20; exercise price - $0.35; expected life - 1.01 years; annualized volatility - 94.09%; dividend yield - 0%; risk free rate - 0.64%. Gross proceeds, less issuance costs paid in cash and less the total fair value of the warrants was charged against Share Capital in the statement of changes in shareholders’ equity. |
| F-16 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 8. | Share Capital and Warrant Reserve (continued) |
| b) | Issued Share Capital (continued) |
| (iv) | On March 9, 2017 the Company and the Company’s President and CEO, completed a non-brokered private placement financing (the “Offering”) of 5,612,083 units (the “Units”) for aggregate gross proceeds to the Company in the amount of $1,346,900. | |
| Under the Offering, each Unit consisted of one common share in the capital of the Company (a “Common Share”) and one-half of one Common Share purchase warrant (a “Warrant”). The fair value of the warrants was determined using the Black-Scholes option pricing model and the following assumptions: - share price - $0.20; annualized volatility - 120.63%; dividend yield - 0%; risk free rate - 0.78%. Each Warrant will be exercisable for one Common Share at an exercise price of $0.30 if exercised 12 months following the date of closing of the Offering and $0.35 if exercised 24 months following the date of closing of the Offering. | ||
| The Offering was considered a “related party transaction” within the meaning of TSX Venture Policy 5.9 and Multilateral Instrument 61-101—Protection of Minority Security Holders in Special Transactions (“MI 61-101”). The Company relied on the exemptions from the valuation and minority shareholder approval requirements of MI 61-101 contained in sections 5.5(a) and 5.7(a) of MI 61-101 as neither the fair market value of the Units nor the aggregate proceeds of the Offering exceeded 25% of the Company’s market capitalization. | ||
| (v) | On March 7, 2017, 144,006 Compensation Warrants were exercised into 144,006 common shares and 144,006 warrants for a total consideration of $25,921. The fair value of the warrants was determined using the Black-Scholes option pricing model and the following assumptions: - share price - $0.18; exercise price - $0.35; expected life - 11 months; annualized volatility - 152.57%; dividend yield - 0%; risk free rate - 0.64%. Gross proceeds, less issuance costs paid in cash and less the total fair value of the warrants, were charged against Share Capital in the statement of changes in shareholders’ equity. | |
| (vi) | On April 24, 2017, 37,000 Finders’ Options were exercised into 37,000 common shares and 18,500 warrants for a total consideration of $7,400. The fair value of the warrants was determined using the Black-Scholes option pricing model and the following assumptions: - share price - $0.19; exercise price - $0.35; expected life - 24 months; annualized volatility - 117.96%; dividend yield - 0%; risk free rate - 0.64%. Gross proceeds, less issuance costs paid in cash and less the total fair value of the warrants, were charged against Shares Capital in the statement of changes in shareholders’ equity. The shares were issued on May 1, 2017. | |
| (vii) | On August 2, 2017, the Company and the Company’s President and CEO completed a non-brokered private placement resulting in gross proceeds of $631,785. The non-brokered private placement involved the sale of 4,058,441 units at a price of $0.16 per unit. Each Unit consisted of one common share in the capital of the Company. | |
| (viii) | On September 5, 2017 the Company issued 2,500,002 common shares to the Sapientia shareholders. (Note 6). |
| F-17 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 8. | Share Capital and Warrant Reserve (continued) |
| b) | Issued Share Capital (continued) |
| (ix) | On October 13, 2017, the Company introduced a warrant exercise incentive program (the “Warrant Incentive Program”) designed to encourage the early exercise of up to approximately 26 million outstanding common share purchase warrants (the “Warrants”). | |
| Under the terms of the Incentive Program, the Company offered the following inducements: (i) a temporary reduction in the respective exercise prices of the Warrants to $0.14, consistent with the current trading value of BriaCell’s shares, for each Warrant that is exercised on or before November 30, 2017 (the “Early Exercise Period”); and (ii) for each Warrant exercised during the Early Exercise Period, the holder will receive, at no additional cost, one-half of one newly issued common share purchase warrant (each an “Incentive Warrant”), with each whole Incentive Warrant exercisable into one common share for a period of 24 months from the issue date at an exercise price of $0.20. | ||
| Any Warrants that are not exercised prior to the expiry of the Early Exercise Period will remain outstanding in accordance with their original terms, and in particular, will no longer be eligible for the reduced exercise price or issuance of Incentive Warrants. | ||
| In total, 2,043,000 warrants were exercised in connection with the Warrant Incentive Program at an exercise price of $0.14 for aggregate gross proceeds of $286,020. In addition, a total of 1,021,500 Incentive Warrants were granted in connection with the Warrant Incentive Program, with each Incentive Warrant entitling the holder to purchase one additional common share of the Company at an exercise price of $0.20, expiring December 21, 2019. The fair value of the warrants was $61,629. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.16; exercise price - $0.20; expected life - 24 months; annualized volatility - 114.68%; dividend yield - 0%; risk free rate - 1.66% | ||
| (x) | On March 27, 2018, the Company completed a brokered private placement (the “Brokered Unit Offering”) of 43,322,322 units of the Company (the “Units”) at a price of $0.10 per Unit for aggregate gross proceeds of $4,332,232. Under the Brokered Unit Offering, each Unit consists of one common share (each, a “Common Share”) and one common share purchase warrant (each, a “Warrant”). The Warrants are valid for 36 months following the closing of the Brokered Unit Offering and each Warrant is exercisable for one Common Share at an exercise price of $0.14. | |
| In connection with the Brokered Unit Offering and the Note Offering (together, the “Offerings”), the Company paid commissions to certain participating dealers on a portion of funds raised. In respect of the Brokered Unit Offering, aggregate cash commissions of $235,215 and an aggregate 2,613,350 broker warrants (the “Broker Warrants”) were paid. The Broker Warrants issued in connection with the Offerings are exercisable into one Common Share at an exercise price of $0.14 for a period of 36 months from the issue date. | ||
| The fair value of Warrants and Broker Warrants was $1,479,028 and $208,545, respectively, and was determined using the Black-Scholes option pricing model and the following assumptions: share price - $0.13; exercise price - $0.14; expected life - 36 months; annualized volatility - 100.61%; dividend yield - 0%; risk free rate - 1.99%. |
| F-18 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 8. | Share Capital and Warrant Reserve (continued) |
| b) | Issued Share Capital (continued) |
| Officers and members of the Company’s Board of Directors, including BriaCell’s Chief Executive Officer, Chief Financial Officer and the Board’s Chairman (the “Related Parties”), participated in the Brokered Unit Offering, which participation constitutes a “related party transaction” as defined under Multilateral Instrument 61-101 - Protection of Minority Security Holders in Special Transactions (“MI 61-101”) and TSX Venture Exchange policy 5.9. Such related party transaction is exempt from the formal valuation and minority shareholder approval requirements of MI 61-101 as neither the fair market value of securities being issued to the related parties nor the consideration being paid by the related parties exceeded 25% of the Company’s market capitalization. | ||
| (xi) | During July 2018, the Company issued 1,068,426 shares at $0.10 per share in respect of the partial conversion of certain Convertible Notes (Note 7). Upon exercise of these Convertible Notes, Noteholders received 1,068,426 warrants with an exercise price of $0.14, expiring in July 31, 2021. The fair value of the warrants was $40,435. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.14; exercise price - $0.14; expected life - 36 months; annualized volatility - 100.41%; dividend yield - 0%; risk free rate - 2.12%. |
| c) | Share Purchase Warrants |
| (i) | A summary of changes in share purchase warrants for the years ended July 31, 2018 and 2017 is presented below: |
| Number | Weighted Average Exercise Price | |||||||
| Balance, July 31, 2016 | 18,396,434 | $ | 0.27 | |||||
| Granted on brokered private placement (Note 8(b)(i)) | 8,500,000 | 0.35 | ||||||
| Granted on non-brokered private placement (Note 8(b)(iv)) | 2,806,041 | 0.35 | ||||||
| Granted from the exercise of Compensation Warrants and Finders’ Options (Note 8(b)(ii)(iii)(v)(vi) | 471,609 | 0.35 | ||||||
| Balance, July 31, 2017 | 30,174,084 | $ | 0.30 | |||||
| Exercised (Note 8(b)(ix)) | (2,043,000 | ) | 0.14 | |||||
| Granted on Warrant Incentive Program (Note 8(b)(ix)) | 1,021,500 | 0.20 | ||||||
| Granted on Brokered Unit Offering (Note 8(b)(x)) | 43,322,322 | 0.14 | ||||||
| Granted from conversion of Notes (Note 8(b)(xi)) | 1,068,426 | 0.14 | ||||||
| Expired during the year (Note 8(c)(ii)) | (13,094,887 | ) | (0.26 | ) | ||||
| Balance, July 31, 2018 | 60,448,445 | $ | 0.19 | |||||
| F-19 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 8. | Share Capital and Warrant Reserve (continued) |
| c) | Share Purchase Warrants (continued) |
| (ii) | During the year ended July 31, 2018, 13,094,887 warrants with a fair value of $679,039 expired and the Company recorded a charge to the warrant reserve with a corresponding credit to accumulated deficit. | |
| (iii) | As at July 31, 2018, share purchase warrants outstanding were as follows: |
| Number of Warrants | Exercise Price | Exercisable at July 31, 2018 | Expiry Date | |||||||||
| 3,421,053 | $ | 0.30 | 3,421,053 | April 26, 2021 | ||||||||
| 8,500,000 | $ | 0.35 | 8,500,000 | August 19, 2019 | ||||||||
| 2,806,041 | $ | 0.35 | 2,806,041 | March 9, 2019 | ||||||||
| 192,140 | $ | 0.35 | 192,140 | October 4, 2018 | ||||||||
| 116,963 | $ | 0.35 | 116,963 | October 4, 2018 | ||||||||
| 1,021,500 | $ | 0.20 | 1,021,500 | December 21, 2019 | ||||||||
| 43,322,322 | $ | 0.14 | 43,322,322 | March 27, 2021 | ||||||||
| 1,068,426 | $ | 0.14 | 1,068,426 | July 31, 2021 | ||||||||
| 60,448,445 | 60,448,445 | |||||||||||
| d) | Compensation Warrants |
| (i) | A summary of changes in compensation warrants for the years ended July 31, 2018 and 2017 is presented below: |
| Number | Weighted Average Exercise Price | |||||||
| Balance, July 31, 2016 | 1,483,813 | $ | 0.19 | |||||
| Granted on brokered private placement (Note 8(b)(i)) | 595,000 | 0.20 | ||||||
| Expiration of compensation warrants | (581,019 | ) | (0.18 | ) | ||||
| Exercised (Note 8(b)(ii)(iii)(v)(vi)) | (490,109 | ) | (0.20 | ) | ||||
| Balance, July 31, 2017 | 1,007,685 | $ | 0.20 | |||||
| Grant on brokered private placement (Note 8(b)(x)) | 2,613,350 | 0.14 | ||||||
| Grant from placement of Convertible Notes (Note 7) | 1,250,000 | 0.14 | ||||||
| Expired during the year (Note 8(d)(ii)) | (139,000 | ) | (0.20 | ) | ||||
| Balance, July 31, 2018 | 4,732,035 | $ | 0.15 | |||||
| F-20 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 8. | Share Capital and Warrant Reserve (continued) |
| d) | Compensation Warrants (continued) |
| (ii) | During the year ended July 31, 2018, 139,000 compensation warrants with a fair value of $15,418 expired and the Company recorded a charge to the warrant reserve with a corresponding credit to accumulated deficit. | |
| (iii) | As at July 31, 2018, compensation warrants outstanding were as follows: |
| Number Of Compensation Warrants | Exercise Price | Exercisable at July 31, 2018 | Expiry Date | |||||||||
| 273,685 | $ | 0.30 | 273,685 | April 26, 2021 (i) | ||||||||
| 595,000 | $ | 0.20 | 595,000 | August 19, 2019 (ii) | ||||||||
| 1,250,000 | $ | 0.14 | 1,250,000 | March 27, 2021 (iii) | ||||||||
| 2,613,350 | $ | 0.14 | 2,613,350 | March 27, 2021 (iii) | ||||||||
| 4,732,035 | 4,732,035 | |||||||||||
| i) | Each compensation warrant can be exercised at $0.30 into one unit of BriaCell comprising one common share and one share purchase warrant. Each resultant share purchase warrant acquired can be exercised into an additional common share of BriaCell at $0.35 if exercised by April 26, 2021. | |
| ii) | Each compensation warrant can be exercised at $0.20 into one unit of BriaCell comprising one common share and one share purchase warrant. Each resultant share purchase warrant acquired can be exercised into an additional common share of BriaCell an exercise price of $0.30 through to August 19, 2019 and $0.35 for the 24 months thereafter. | |
| iii) | Each compensation warrant can be exercised at $0.14 into one common share of BriaCell for a period of 36 months. |
| e) | Shares Held in Escrow |
The escrow agreement relating to the reverse takeover transaction provided for 54,282,952 shares to be held under an escrow agreement. Shares will be released from escrow equal to 10% of the initial shares subject to the agreement upon completion of the initial public offering or purchase agreement and listing on the Canadian Securities Exchange, the remaining shares will be released in 6 equal tranches (15%) every nine-months.
As of July 31, 2018, all the 54,282,952 (July 31, 2017 - 39,329,389) shares have been released and the number of shares that remain in escrow is nil (July 31, 2017 - 14,953,563).
| F-21 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 9. | Share-Based Compensation and Share-Based Payment Reserve |
The Company has adopted a stock option plan (the “Plan”) under which it is authorized to grant options to officers, directors, employees and consultants enabling them to acquire up to 10% of the issued and outstanding common stock of the Company. The options can be granted for a maximum of 5 years and vest as determined by the Board of Directors. The exercise price of each option granted may not be less than the fair market value of the common shares at the time of grant.
A summary of changes in stock options for the years ended July 31, 2018 and 2017 is presented below:
| Number of options outstanding | Weighted average exercise price | |||||||
| Balance, July 31, 2016 | 6,968,000 | $ | 0.24 | |||||
| Granted (Note 9(a)(i)(ii)(iii)(iv)(v)) | 1,882,000 | 0.25 | ||||||
| Cancelled | (2,768,000 | ) | $ | (0.24 | ) | |||
| Balance, July 31, 2017 | 6,082,000 | 0.24 | ||||||
| Granted (Note 9(a)(vi)(vii)(viii)) | 6,165,600 | 0.15 | ||||||
| Cancelled | (175,000 | ) | (0.30 | ) | ||||
| Expired | (2,650,000 | ) | (0.23 | ) | ||||
| Balance, July 31, 2018 | 9,422,600 | $ | 0.18 | |||||
| a) | During the year ended July 31, 2018, the Company issued a total of 6,165,000 options, as follows: |
| (i) | On October 3, 2016, the Company issued 800,000 stock options to consultants, of which 25% vested immediately, and 25% vest every 90 days thereafter. The fair value of the stock options was $88,061. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.20; exercise price - $0.25; expected life - 3 years; annualized volatility - 95%; dividend yield - 0%; risk free rate - 0.59% | |
| (ii) | On November 1, 2016, a total of 632,000 stock options were issued to the Company’s CEO, which vested immediately. The fair value of the stock options was $84,981. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.19; exercise price - $0.21; expected life - 3 years; annualized volatility - 124%; dividend yield - 0%; risk free rate - 0.75% | |
| (iii) | On February 14, 2017, a total of 250,000 stock options were issued to a consultant, of which 25% vested immediately, and 25% vest every 90 days thereafter. The fair value of the stock options was $34,290. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.2; exercise price - $0.2; expected life - 3 years; annualized volatility – 115%; dividend yield – 0%; risk free rate – 0.76% | |
| (iv) | On March 20, 2017, a total of 50,000 stock options were issued to a consultant of which 25% vested immediately, and 25% vest every 90 days thereafter. The fair value of the stock options was $7,041. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.22; exercise price - $0.21; expected life – 3 years; annualized volatility – 103%; dividend yield – 0%; risk free rate – 0.67% |
| F-22 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 9. | Share-Based Compensation and Share-Based Payment Reserve (continued) |
| (v) | On March 22, 2017, a total of 150,000 stock options were issued to an employee of the Company of which 25% vested immediately, and 25% vest every 90 days thereafter. The fair value of the stock options was $21,122. The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.22; exercise price - $0.21; expected life - 3 years; annualized volatility - 103%; dividend yield - 0%; risk free rate - 0.67% | |
| (vi) | On May 1, 2018, the Company issued 2,515,600 stock options to two consultants of which 25% vested immediately, and 25% vest every 90 days thereafter. | |
| The fair value of the 2,000,000 stock options was $126,579. The fair value was estimated using the Black-Scholes option pricing model with the following weighted average assumptions: share price - $0.10; exercise price - $0.14; expected life - 36 months; annualized volatility - 99.64%; dividend yield - 0%; risk free rate - 1.88%. | ||
| The fair value of the 500,000 stock options was $30,165. The fair value was estimated using the Black-Scholes option pricing model with the following weighted average assumptions: share price - $0.10; exercise price - $0.20; expected life - 45 months; annualized volatility - 99.22%; dividend yield - 0%; risk free rate - 1.88%. | ||
| The fair value of the 15,600 stock options was $988. The fair value was estimated using the Black-Scholes option pricing model with the following weighted average assumptions: share price - $0.10; exercise price - $0.14; expected life - 36 months; annualized volatility - 99.64%; dividend yield - 0%; risk free rate - 1.88%. | ||
| (vii) | On March 1, 2018, the Company issued 3,400,000 stock options to directors, officers, employees and consultants of the Company. The fair value of the stock options was $239,119. The fair value was estimated using the Black-Scholes option pricing model with the following weighted average assumptions: share price - $0.10; exercise price - $0.15; expected life - 36 months; annualized volatility - 101.08%; dividend yield - 0%; risk free rate - 1.99%. | |
| (viii) | On July 1, 2018, the Company issued 250,000 stock options to consultant of the Company. The fair value of the stock options was $18,916. The fair value was estimated using the Black-Scholes option pricing model with the following weighted average assumptions: share price - $0.15; exercise price - $0.17; expected life - 5 years; annualized volatility - 99.74%; dividend yield - 0%; risk free rate - 2.04%. |
| b) | In relation to the options issued, the Company recognized share-based compensation expense of $378,336 for the year ended July 31, 2018 (July 31, 2017 - $272,014). | |
| c) | During the year ended July 31, 2018, 2,650,000 options expired, with a fair value of $357,843 (2017 - $nil) and 175,000 options were cancelled, with a fair value of $40,172 (2017 - $429,458). The Company removed the fair value from share-based payment reserve, with a corresponding charge to accumulated deficit. |
| F-23 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 9. | Share-Based Compensation and Share-Based Payment Reserve (continued) |
| d) | As at July 31, 2018, stock options were outstanding for the purchase of common shares as follows: |
| Number of Options | Exercise Price | Exercisable at July 31, 2018 | Expiry Date | |||||||||
| 950,000 | $ | 0.26 | 950,000 | November 4, 2025 | ||||||||
| 575,000 | $ | 0.26 | 387,500 | November 4, 2020 | ||||||||
| 150,000 | $ | 0.21 | 150,000 | November 4, 2020 | ||||||||
| 500,000 | $ | 0.26 | 500,000 | November 4, 2018 | ||||||||
| 150,000 | $ | 0.25 | 150,000 | July 31, 2018 | ||||||||
| 632,000 | $ | 0.25 | 632,000 | November 1, 2019 | ||||||||
| 250,000 | $ | 0.20 | 250,000 | February 14, 2020 | ||||||||
| 50,000 | $ | 0.21 | 50,000 | March 20, 2020 | ||||||||
| 3,400,000 | $ | 0.15 | 3,400,000 | March 1, 2021 | ||||||||
| 500,000 | $ | 0.20 | 125,000 | March 10, 2022 | ||||||||
| 2,015,600 | $ | 0.14 | 1,007,800 | May 1, 2021 | ||||||||
| 250,000 | $ | 0.14 | 62,500 | July 1, 2023 | ||||||||
| 9,422,600 | 7,664,800 | |||||||||||
As at July 31, 2018, stock options outstanding have a weighted average remaining contractual life of 2.9 years (July 31, 2017 - 2.43 years).
| 10. | Income Taxes |
The provision for taxes differs from the amount obtained by applying the combined Canadian Federal and Provincial income tax rate of 26% (2017 - 26%) to loss before income taxes. The differences relate to the following items:
| Year Ended July 31, 2018 | Year Ended July 31, 2017 | |||||||
| Net loss before recovery of income taxes | $ | 5,412,663 | $ | 3,220,721 | ||||
| Expected tax recovery based on statutory Canadian combined federal and provincial tax rates | $ | (1,407,290 | ) | $ | (837,390 | ) | ||
| Differences in foreign tax rates | (212,540 | ) | (375,590 | ) | ||||
| Tax rate changes and other adjustments | 36,770 | (3,990 | ) | |||||
| Share based compensation and non-deductible expenses | 474,840 | (26,070 | ) | |||||
| Expiry of warrants | 90,280 | - | ||||||
| Change in deferred tax assets not recognized | 1,017,940 | 1,190,900 | ||||||
| Income tax (recovery) expense | $ | - | $ | - | ||||
| F-24 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 10. | Income Taxes (continued) |
Unrecognized Deferred Tax Assets
Deferred taxes are provided as a result of temporary differences that arise due to the differences between the income tax values and the carrying amount of assets and liabilities. Deferred tax assets have not been recognized in respect of the following deductible temporary differences because it is not probable that the future taxable profit will be available against which the Company can utilize the benefits:
The following table summarizes the components of the unrecognized deductible temporary differences:
| July 31, 2018 | July 31, 2017 | |||||||
| Deferred Tax Assets | ||||||||
| Non-capital losses carried forward - USA | $ | 7,221,900 | $ | 4,313,110 | ||||
| Non-capital losses carried forward - Canada | 2,602,990 | 1,809,360 | ||||||
| Share issuance costs | 737,090 | 430,810 | ||||||
| Unrealized FX gain or losses | - | 245,990 | ||||||
| Marketable securities | 107,000 | 107,000 | ||||||
| Property, plant and equipment - Canada | 3,330 | 3,330 | ||||||
| Property, plant and equipment - USA | 2,120 | 1,980 | ||||||
| $ | 10,674,430 | $ | 6,911,580 | |||||
The Company has Canadian non-capital income tax loss carry forwards which expire between 2034 and 2038 as noted in the below table.
| 2035 | $ | 767,440 | ||
| 2036 | 467,980 | |||
| 2037 | 573,930 | |||
| 2038 | 793,640 | |||
| $ | 2,602,990 |
The Company also has US tax loss carry forwards which begin to expire in fiscal 2034 as noted in the below table.
| 2034 | $ | 1,240 | ||
| 2035 | 631,660 | |||
| 2036 | 1,134,120 | |||
| 2037 | 2,546,090 | |||
| 2038 | 2,908,790 | |||
| $ | 7,221,900 |
The Canadian financing costs will be fully amortized by 2021. The remaining deductible temporary differences may be carried forward indefinitely.
| F-25 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 11. | Related Party Transactions and Balances |
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making operating and financial decisions. This would include the Company’s senior management, who are considered to be key management personnel by the Company. Parties are also related if they are subject to common control or significant influence. Related parties may be individuals or corporate entities. A transaction is considered to be a related party transaction when there is a transfer of resources or obligations between related parties.
As at July 31, 2018, included in accounts payable and accrued liabilities are amounts owing to a company controlled by an officer is $nil (2017 - $3,500) for accounting fees; amounts owing to two companies each controlled by an individual director are $nil (2017 – $14,125) for consulting fees and amounts owing to directors of $8,548 (2017 - $12,872).
During the years ended July 31, 2018, 2017 and 2016, the Company also incurred the following expenses by key management personnel or companies controlled by these individuals:
| Years ended July 31 | ||||||||
| 2018 | 2017 | |||||||
| a) Paid or accrued professional fees to a company controlled by an officer of the Company | $ | 42,000 | $ | 48,950 | ||||
| b) Paid or accrued consulting fees to Companies controlled by individual directors | 126,500 | 134,500 | ||||||
| c) Paid or accrued wages and consulting fees to directors | 263,365 | 277,621 | ||||||
| d) Share based compensation to directors and officers | 207,471 | 84,981 | ||||||
| e)Paid or accrued professional fees to a company controlled by an officer of the Company | - | - | ||||||
| 12. | Capital Management |
The Company’s capital comprises share capital, share-based payment reserve, warrant reserve, and accumulated other comprehensive loss. The Company manages its capital structure, and makes adjustments to it, based on the funds available to the Company in order to support the Company’s business activities. The Board of Directors does not establish quantitative return on capital criteria for management; it relies on the expertise of the Company’s management to sustain future development of the business.
The intellectual property in which the Company currently has an interest is in the development stage; as such, the Company is dependent on external financing to fund its activities. In order to carry out the planned research and development and pay for administrative costs, the Company intends to raise additional amounts as needed (Note 1).
Management reviews its capital management approach on an ongoing basis and believes that this approach, given the relative size of the Company, is reasonable.
| F-26 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 13. | Financial Risk Factors |
The Company’s risk exposures and the impact on the Company’s financial instruments are summarized below:
| a) | Credit risk |
The Company has no significant concentration of credit risk arising from operations. Management believes that the credit risk concentration with respect to financial instruments is remote.
| b) | Liquidity risk |
The Company’s approach to managing liquidity risk is to ensure that it will have sufficient liquidity to meet liabilities as they come due. As at July 31, 2018, the Company had a working capital balance of $700,350 (2017 - $932,677). As a result, the Company currently has little exposure to liquidity risk. However, as described in Note 1, the Company has not yet achieved profitable operations and expects to incur further losses in the development of its products; these factors cast significant doubt about the Company’s ability to continue as a going concern.
| c) | Market Risk |
| i) | Interest rate risk |
As the Company has cash and short-term investment balances and no interest-bearing debt, interest rate risk is remote.
| ii) | Price risk |
As the Company has no revenues, price risk is remote.
| iii) | Exchange risk |
The Company is exposed to foreign exchange risk as its research operations are conducted primarily in the United States of America.
| F-27 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 14. | Research and Development Costs |
| Years ended July 31 | ||||||||
| 2018 | 2017 | |||||||
| Wages and Salaries | $ | 30,908 | $ | 518,192 | ||||
| Clinical Trials | 1,346,208 | 591,777 | ||||||
| Office Rent | 69,871 | 31,051 | ||||||
| Licensing | 34,967 | 96,309 | ||||||
| Supplies | 81,915 | 19,820 | ||||||
| Insurance | 5,596 | - | ||||||
| Patent | 167,789 | - | ||||||
| Vaccine | 1,375,325 | 868,792 | ||||||
| $ | 3,112,579 | $ | 2,125,941 | |||||
| 15. | General and Administration Costs |
| Years ended July 31 | ||||||||
| 2018 | 2017 | |||||||
| Conferences | $ | 10,781 | $ | 14,256 | ||||
| Consulting (Note 11) | 515,960 | 289,005 | ||||||
| Insurance | 20,867 | 15,358 | ||||||
| Depreciation and amortization (Note 6) | 16,894 | 290 | ||||||
| General and Administrative | 32,588 | 30,448 | ||||||
| Professional fees (Note 11) | 244,131 | 198,171 | ||||||
| Regulatory, filing and transfer agent fees | 85,496 | 30,166 | ||||||
| Rent, net of recoveries (Note 16) | 15,081 | 12,171 | ||||||
| Shareholder communications | 289,208 | 119,120 | ||||||
| Travel | 46,251 | 35,057 | ||||||
| Wages and salaries, net of recoveries (Note 11) | 110,456 | 76,239 | ||||||
| $ | 1,387,713 | $ | 820,281 | |||||
| F-28 |
BriaCell Therapeutics Corp.
Notes to the Consolidated Financial Statements
For the Years Ended July 31, 2018 and 2017
(Expressed in Canadian Dollars)
| 16. | Commitments and Contingencies |
| a) | Office Leases |
The Company’s lease arrangement for office space in Berkeley, California end in August 2019 and the annual lease commitment is approximately US$50,000 plus common area maintenance charges.
| b) | Litigation |
On July 15, 2016, two lawsuits were served against BriaCell. Both plaintiffs are claiming unpaid wages. In April 2018, one lawsuit was settled for US$20,000 and subsequent to the year end, the second lawsuit was settled for US$22,500. The Company has accrued $22,500 in the current period related to these settlements (2017 - $30,000).
| 17. | Events After the Reporting Period |
| a) | During August and September 2018, an additional $117,437 of Convertible Notes were converted and as such, the Company issued 1,174,371 shares and 1,174,371 warrants. | |
| b) | On September 17, 2018, the Company and the Noteholders agreement to extend the repayment date of the Convertible notes for an additional six month, to March 20, 2019. |
| F-29 |

Immunotherapy approaches to breast cancer management
Condensed Interim Consolidated
Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
Expressed in Canadian Dollars
(Unaudited)
Corporate Office- Canada Suite 300 - Bellevue Centre 235 -15th Street West Vancouver, BC V7T 2X1 Tel: 604-921-1810 Fax: 604-921-1898 |
Corporate Office- US 820 Heinz Avenue Berkeley, CA, 94710 Tel: 1-888-485-6340 Fax: 424-245-3719 |
| F-30 |
BriaCell Therapeutics Corp
Condensed Interim Consolidated Statements of Financial Position
For the Three and Nine Month Period Ended April 30, 2019
(Unaudited)
(Expressed in Canadian Dollars)
| April 30, | July 31, | |||||||
| 2019 | 2018 | |||||||
| Unaudited | Audited | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 938,932 | $ | 938,448 | ||||
| Short-term investments | - | 1,341,043 | ||||||
| Amounts receivables | 6,744 | 18,975 | ||||||
| Prepaid expenses | 145,344 | 147,734 | ||||||
| Total current assets | 1,091,020 | 2,446,200 | ||||||
| Security deposits | 178,376 | 172,980 | ||||||
| Investments | 2 | 2 | ||||||
| Intellectual property (Note 8) | 343,901 | 357,958 | ||||||
| Total Assets | $ | 1,613,299 | $ | 2,977,140 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued liabilities | $ | 328,291 | 285,712 | |||||
| Unsecured convertible loan (Note 7) | 419,123 | 1,460,138 | ||||||
| Total liabilities | 747,414 | 1,745,850 | ||||||
| Shareholders’ equity | ||||||||
| Share capital (Note 9b)) | 13,867,219 | 10,213,174 | ||||||
| Share-based payment reserve (Note 10) | 877,073 | 905,257 | ||||||
| Warrant reserve (Note 9(c)) | 2,913,722 | 2,907,337 | ||||||
| Accumulated other comprehensive loss | (96,925 | ) | (105,514 | ) | ||||
| Deficit | (16,695,204 | ) | (12,688,964 | ) | ||||
| Total shareholders’ equity | 865,885 | 1,231,290 | ||||||
| Total liabilities and shareholders’ equity | $ | 1,613,299 | $ | 2,977,140 | ||||
Nature of Operations (Note 1)
Commitments and Contingencies (Note 14)
These financial statements were approved and authorized for issue on behalf of the Board of Directors on June 27, 2019 by:
On behalf of the Board:
| “Jamieson Bondarenko” | “William Williams” | |
| Director | Director |
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
| F-31 |
BriaCell Therapeutics Corp
Condensed Interim Consolidated Statements of Operations and Comprehensive Loss
For the Three and Nine Month Period Ended April 30, 2019
(Unaudited)
(Expressed in Canadian Dollars)
Three months ended April 30, | Nine months ended April 30, | |||||||||||||||
| 2019 | 2018 | 2019 | 2018 | |||||||||||||
| Unaudited | Unaudited | |||||||||||||||
| Expenses: | ||||||||||||||||
| Research costs | $ | 1,056,154 | $ | 667,772 | $ | 3,605,429 | $ | 1,889,597 | ||||||||
| General and administration costs | 381,629 | 513,995 | 847,266 | 908,902 | ||||||||||||
| Share-based compensation (Note 10) | 49 | 243,223 | 60,570 | 256,080 | ||||||||||||
| Total Expenses | 1,437,832 | 1,424,990 | 4,513,265 | 3,054,579 | ||||||||||||
| Operating Loss | (1,437,832 | ) | (1,424,990 | ) | (4,513,265 | ) | (3,054,579 | ) | ||||||||
| Interest income | 673 | 2,304 | 12,000 | 8,912 | ||||||||||||
| Interest expense (Note 7) | (3,204 | ) | (228,887 | ) | (26,352 | ) | (228,887 | ) | ||||||||
| Change in fair value of convertible debt (Note 7) | (63,398 | ) | - | 392,721 | - | |||||||||||
| Foreign exchange gain (loss) | 39,902 | (4,842 | ) | 39,902 | 5,836 | |||||||||||
| (26,027 | ) | (231,425 | ) | 418,271 | (214,139 | ) | ||||||||||
| Loss For The Period | (1,463,859 | ) | (1,656,415 | ) | (4,094,994 | ) | (3,268,718 | ) | ||||||||
| Items That Will Subsequently Be Reclassified To Profit Or Loss | ||||||||||||||||
| Foreign currency translation adjustment | (14,750 | ) | (18,599 | ) | 8,589 | (67,466 | ) | |||||||||
| (14,750 | ) | (18,599 | ) | 8,589 | (67,466 | ) | ||||||||||
| Comprehensive Loss for the Period | (1,478,609 | ) | (1,675,014 | ) | (4,086,405 | ) | (3,336,184 | ) | ||||||||
| Basic and Fully Diluted Loss Per Share | (0.01 | ) | (0.01 | ) | (0.02 | ) | (0.03 | ) | ||||||||
| Weighted Average Number Of Shares Outstanding | 175,996,439 | 125,836,457 | 166,323,680 | 118,367,849 | ||||||||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
| F-32 |
BriaCell Therapeutics Corp
Condensed Interim Consolidated Statements of Cash Flows
For the Three and Nine Month Period Ended April 30, 2019
(Unaudited)
(Expressed in Canadian Dollars)
| Nine months ended | ||||||||
| April 30, | ||||||||
| 2019 | 2018 | |||||||
| Unaudited | ||||||||
| Cash flow from operating activities | ||||||||
| Net loss for the period | $ | (4,094,994 | ) | $ | (3,268,718 | ) | ||
| Items not affecting cash: | ||||||||
| Depreciation and amortization | 14,057 | - | ||||||
| Share-based compensation | 60,570 | 256,080 | ||||||
| Change in fair value of convertible loan | (392,721 | ) | - | |||||
| Changes in non-cash working capital: | ||||||||
| Amounts receivable | 12,232 | (16,849 | ) | |||||
| Prepaid expenses | 2,390 | (4,000 | ) | |||||
| Security deposits | (5,396 | ) | - | |||||
| Accounts payable and accrued liabilities | 42,579 | (624,275 | ) | |||||
| (4,361,283 | ) | (3,657,762 | ) | |||||
| Cash flow from investing activities | ||||||||
| Change in short-term investments | 1,341,043 | (3,250,000 | ) | |||||
| Acquisition of Sapientia | - | 149 | ||||||
| 1,341,043 | (3,249,851 | ) | ||||||
| Cash flow from financing activities | ||||||||
| Proceeds for private placements, net | 2,845,784 | 4,731,302 | ||||||
| Proceeds from unsecured convertible loan | - | 919,681 | ||||||
| Proceeds from exercise of warrants | 140,000 | 286,021 | ||||||
| 2,985,784 | 5,937,005 | |||||||
| Decrease in cash and cash equivalents | (34,456 | ) | (970,608 | ) | ||||
| Effect of changes in foreign exchange rates | 34,940 | 49,672 | ||||||
| Cash and cash equivalents, beginning of period | 938,448 | 1,264,429 | ||||||
| Cash and cash equivalents, end of period | $ | 938,932 | $ | 343,493 | ||||
| (a) Significant non-cash transactions | ||||||||
| Acquisition of investments in consideration of issue of shares | - | $ | 375,000 | |||||
| - | 375,000 | |||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
| F-33 |
BriaCell Therapeutics Corp
Statements of Changes in Shareholders’ Equity
(Unaudited)
(Expressed in Canadian Dollars)
| SHARE CAPITAL | SHARE-BASED PAYMENT | WARRANT | ACCUMULATED OTHER COMPREHENSIVE | ACCUMULATED | TOTAL SHAREHOLDERS’ | |||||||||||||||||||||||
| SHARES | AMOUNT | RESERVE | RESERVE | LOSS | DEFECIT | EQUITY | ||||||||||||||||||||||
| Balance, July 31, 2018 | 158,896,752 | $ | 10,213,174 | $ | 905,257 | $ | 2,907,337 | $ | (105,514 | ) | $ | (12,688,964 | ) | $ | 1,231,290 | |||||||||||||
| Issuance of shares and warrants on conversion of Convertible Notes (Note 9(b)(i)) | 6,746,458 | 397,715 | - | 276,931 | - | - | 674,646 | |||||||||||||||||||||
| Repricing of warrants (Note 9(c)(iii)) | - | (35,016 | ) | - | 35,016 | - | - | - | ||||||||||||||||||||
| Private Placement (Note 9(b)(iii)) | 29,735,240 | 2,845,784 | - | - | - | - | 2,845,784 | |||||||||||||||||||||
| Exercise of warrants (Note 9(b)(ii)) | 1,000,000 | 174,140 | - | (34,140 | ) | - | - | 140,000 | ||||||||||||||||||||
| Expiration of warrants (Note 9(c)(i)) | - | 271,422 | - | (271,422 | ) | - | - | - | ||||||||||||||||||||
| Share-based compensation (Note 10) | - | - | 60,570 | - | - | - | 60,570 | |||||||||||||||||||||
| Expiration of options | - | - | (88,754 | ) | - | - | 88,754 | - | ||||||||||||||||||||
| Foreign exchange translation | - | - | - | - | 8,589 | - | 8,589 | |||||||||||||||||||||
| Loss for the period | - | - | - | - | - | (4,094,994 | ) | (4,094,994 | ) | |||||||||||||||||||
| Balance, April 30, 2019 | 196,378,450 | $ | 13,867,219 | 877,073 | $ | 2,913,722 | $ | (96,925 | ) | $ | (16,695,204 | ) | $ | 865,885 | ||||||||||||||
| F-34 |
BriaCell Therapeutics Corp
Statements of Changes in Shareholders’ Equity
(Unaudited)
(Expressed in Canadian Dollars)
| SHARE CAPITAL | SHARE-BASED PAYMENT | WARRANT | ACCUMULATED OTHER COMPREHENSIVE | ACCUMULATED | TOTAL SHAREHOLDERS’ | |||||||||||||||||||||||
| SHARES | AMOUNT | RESERVE | RESERVE | LOSS | DEFECIT | EQUITY | ||||||||||||||||||||||
| Balance, July 31, 2017 | 105,904,561 | $ | 6,609,615 | $ | 884,763 | $ | 1,841,448 | $ | (72,174 | ) | $ | (8,328,600 | ) | $ | 935,052 | |||||||||||||
| Private Placement | 4,058,441 | 631,785 | - | - | - | - | 631,785 | |||||||||||||||||||||
| Acquisition of Sapientia | 2,500,002 | 375,000 | - | - | - | - | 375,000 | |||||||||||||||||||||
| Exercise of warrants under Warrant Incentive Program | 2,043,000 | 351,557 | - | (65,537 | ) | - | - | 286,020 | ||||||||||||||||||||
| Private Placement | 43,322,322 | 2,644,659 | - | 1,687,573 | - | - | 4,332,232 | |||||||||||||||||||||
| Share-based compensation | - | - | 256,080 | - | - | - | 256,080 | |||||||||||||||||||||
| Foreign exchange translation | - | - | - | - | (67,466 | ) | - | (67,466 | ) | |||||||||||||||||||
| Loss for the period | - | - | - | - | - | (3,268,718 | ) | (3,268,718 | ) | |||||||||||||||||||
| Balance, April 30, 2018 | 157,828,326 | $ | 10,612,616 | 1,140,843 | $ | 3,463,484 | $ | (139,640 | ) | (11,597,318 | ) | $ | 3,479,985 | |||||||||||||||
| Issuance of shares on conversion of debt | 1,068,426 | 66,408 | - | 40,435 | - | - | 106,843 | |||||||||||||||||||||
| Share-based compensation | - | - | 122,256 | - | - | - | 122,256 | |||||||||||||||||||||
| Expiration of warrants and compensation warrants | - | - | - | (694,457 | ) | - | 694,457 | - | ||||||||||||||||||||
| Expiration of stock options | - | - | (357,842 | ) | - | - | 357,842 | - | ||||||||||||||||||||
| Share issuance costs | - | (465,850 | ) | - | - | - | - | (465,850 | ) | |||||||||||||||||||
| Issuance of compensation warrants | - | - | - | 97,875 | - | - | 97,875 | |||||||||||||||||||||
| Foreign exchange translation | - | - | - | - | 34,126 | - | 34,126 | |||||||||||||||||||||
| Loss for the period | - | - | - | - | - | (2,143,945 | ) | (2,143,945 | ) | |||||||||||||||||||
| Balance, July 31, 2018 | 158,896,752 | $ | 10,213,174 | $ | 905,257 | $ | 2,907,337 | $ | (105,514 | ) | $ | (12,688,964 | ) | $ | 1,231,290 | |||||||||||||
The accompanying notes are an integral part of these condensed interim consolidated financial statements.
| F-35 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 1. | Nature of Operations and Going Concern |
BriaCell Therapeutics Corp. (“BriaCell” or the “Company”) was incorporated under the Business Corporations Act (British Columbia) on July 26, 2006 and is listed on the TSX Venture Exchange (“TSX Venture”). The Company trades on the TSX Venture under the symbol “BCT.V”
The Company’s head office is located at Suite 300 – 235 West 15th Street, West Vancouver, British Columbia, V7T 2X1.
BriaCell is an immuno-oncology biotechnology company. BriaCell owns the US patent to Bria-IMT™, a whole-cell cancer vaccine (US Patent No.7674456) (the “Patent”). The Company is currently advancing its immunotherapy program, Bria-IMT™, to complete a 24-subject Phase I/IIa clinical trial and by research activities in the context of BriaDx™, a companion diagnostic test to identify patients likely benefitting from Bria-IMT™.
The accompanying consolidated financial statements have been prepared on the basis of a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business for the foreseeable future. The Company has incurred losses from inception of $16,695,204 (July 31, 2018 - $12,688,964), is currently in the development stage, and has not commenced commercial operations. The Company’s ability to continue as a going concern is dependent upon its ability to attain future profitable operations and to obtain the necessary financing to meet its obligations and repay its liabilities arising from normal business operations when they come due. As at April 30, 2019, the Company had not yet completed the clinical development of or achieved regulatory approval to market Bria-IMT™, its lead product candidate and expects to incur further losses; the nature of a development stage immune-oncology company requires the raising of financial capital to support its clinical development programs and administrative costs. The uncertainty of the Company’s ability to raise such financial capital casts significant doubt on the Company’s ability to continue as a going concern. These consolidated financial statements do not include any adjustments to the amounts and classification of assets and liabilities that might be necessary should the Company not be able to continue as a going concern.
These consolidated financial statements were authorized for issue by the Board of Directors on June 27, 2019.
| 2. | Basis of Presentation |
Statement of Compliance
These condensed interim consolidated financial statements have been prepared in accordance with IAS 34 Interim Financial Reporting (“IAS34”) using accounting policies consistent with the International Financial Reporting Standards (“IFRS”) and interpretations of the IFRS Interpretations Committee (“IFRIC”). They do not include all information required for full annual financial statements and should be read in conjunction with the Audited Financial Statements of the Company for the year ended July 31, 2018.
| F-36 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 2. | Basis of Presentation (continued) |
Basis of Presentation
The condensed interim consolidated financial statements are prepared on a going concern basis and have been presented in Canadian dollars which is the Company’s reporting currency. A summary of the significant accounting policies is provided in Note 3. Standards and guidelines not effective for the current accounting period are described in Note 4.
Basis of Measurement
Theses condensed interim consolidated financial statements have been prepared on a going concern basis, under the historical cost basis, except for financial instruments which have been measured at fair value.
Basis of Consolidation
These condensed interim consolidated financial statements include the accounts of BriaCell and its wholly-owned US subsidiary US s BriaCell Therapeutics Corp. (“BTC”) and BTC’s wholly owned subsidiary - Sapientia. The financial statements of BriaCell are included in the consolidated financial statements from the date that control commences until the date control ceases. Control exists when the Company has the power directly or indirectly, to govern the financial and operating policies of an entity so as to obtain benefits from its activities. The Company applies the acquisition method to account for business combinations in accordance with IFRS 3.
All inter–company balances, and transactions, have been eliminated upon consolidation.
| 3. | Significant Accounting Policies |
The preparation of financial data is based on accounting principles and practices consistent with those used in the preparation of the audited financial statements as at July 31, 2018. The accompanying condensed interim consolidated financial statements should be read in conjunction with the Company’s audited financial statements for the year ended July 31, 2018.
Accounting standards implemented as at August 1, 2018
IFRS 9 – Financial instruments (“IFRS 9”) was issued by the IASB its final form in July 2014 and will replace IAS 39 Financial Instruments: Recognition and Measurement (“IAS 39”). IFRS 9 uses a single approach to determine whether a financial asset is measured at amortized cost or fair value, replacing the multiple rules in IAS 39. The approach in IFRS 9 is based on how an entity manages its financial instruments in the context of its business model and the contractual cash flow characteristics of the financial assets. Most of the requirements in IAS 39 for classification and measurement of financial liabilities were carried forward unchanged to IFRS 9. The new standard also requires a single impairment method to be used, replacing the multiple impairment methods in IAS39. The standard is effective for annual periods beginning on or after January 1, 2018. Management adopted the standard as August 1, 2018 and assessed that the adoption of IFRS 9 did not have a significant impact to the consolidated financial statements.
| F-37 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 3. | Significant Accounting Policies (continued) |
Accounting standards implemented as at August 1, 2018 (continued)
IFRS 15 - Revenue from contracts with customers (“IFRS 15”) proposes to replace IAS 18 – Revenue, IAS 11 – Construction contracts and some revenue-related interpretations. The standard contains a single model that applies to contracts with customers and two approaches to recognizing revenue: at a point in time or over time. The model features a contract-based five step analysis of transaction to determine whether, how much and when revenue is recognized. New estimates and judgemental thresholds have been introduced, which may affect the amount and/or timing of revenue recognized. IFRS 15 is effective for annual periods beginning on or after January 1, 2018. Earlier adoption is permitted. The Company adopted the standard as of August 1, 2019. Since the Company has not yet generated any revenues, the Company was no impact to the consolidated financial statements as a result of the adoption of this standard.
| 4. | Standards Issued but Not Yet Effective |
Certain pronouncements were issued by the IASB or the IFRIC that are mandatory for future accounting periods. Many are not applicable to or are not expected to have a significant impact on BriaCell and have been excluded from the list below. The following have not yet been adopted and are being evaluated to determine their impact on BriaCell.
IFRS 16 - Leases (“IFRS 16”) replaces IAS 17, Leases (“IAS 17”). The new model requires the recognition of almost all lease contracts on a lessee’s statement of financial position as a lease liability reflecting future lease payments and a ‘right-of-use asset’ with exceptions for certain short-term leases and leases of low-value assets. In addition, the lease payments are required to be presented on the statement of cash flow within operating and financing activities for the interest and principal portions, respectively. IFRS 16 is effective for annual periods beginning on or after January 1, 2019, with early adoption permitted if IFRS 15, Revenue from Contracts with Customers, is also applied. The Company has determined there will not be a significant impact to the consolidated financial statements as a result of the adoption of this standard.
IFRS 17 – Insurance Contract (“IFRS 17”) was issued by the IASB in May 2017, which replaces IFRS 4 Insurance Contracts. IFRS 17 requires entities to measure insurance contract liabilities at their current fulfilment values using one of three measurement models, depending on the nature of the contract. IFRS 17 is effective for annual periods beginning on or after January 1, 2021. IFRS 17 will affect how the Company’s accounts for its insurance contracts and how it reports its financial performance in our consolidated statements of operations. The Company has determined there will not be a significant impact to the consolidated financial statements as a result of the adoption of this standard.
| F-38 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 5. | Significant Accounting Judgements and Estimates |
The preparation of these consolidated financial statements requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the consolidated financial statements and reported amounts of expenses during the reporting period. Actual outcomes could differ from these estimates. The consolidated financial statements include estimates which, by their nature, are uncertain. The impacts of such estimates are pervasive throughout the consolidated financial statements, and may require accounting adjustments based on future occurrences. Revisions to accounting estimates are recognized in the period in which the estimate is revised and also in future periods when the revision affects both current and future periods.
The critical judgments and significant estimates in applying accounting policies that have the most significant effect on the amounts recognized in the consolidated financial statements are:
| ● | The series of loans made to the subsidiary company are considered part of the parent company’s net investment in a foreign operation as the Company does not plan to settle these balances in the foreseeable future. As a result of this assessment, the unrealized foreign exchange gains and losses on the intercompany loans are recorded through compressive loss. If the Company determined that settlement of these amounts was planned or likely in the foreseeable future, the resultant foreign exchange gains and losses would be recorded through profit or loss. | |
| ● | The determination that the unrealized decrease in the fair value of available for sale investments is other than temporary. |
| 6. | Financial Instruments |
Financial assets
The Company classifies its financial assets into one of the following categories, depending on the purpose for which the asset was acquired. The Company’s accounting policy for each category is as follows:
Fair value through profit or loss - This category comprises derivatives, or assets acquired or incurred principally for the purpose of being sold or repurchased in the near term. They are carried in the statement of financial position at fair value with changes in fair value recognized in the statement of operations.
Loans and receivables - These assets are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. They are carried at cost less any provision for impairment. Individually significant receivables are considered for impairment when they are past due or when other objective evidence is received that a specific counterparty will default.
Held-to-maturity investments - These assets are non-derivative financial assets with fixed or determinable payments and fixed maturities that the Company’s management has the positive intention and ability to hold to maturity. These assets are measured at amortized cost using the effective interest method. If there is objective evidence that the investment is impaired, determined by reference to external credit ratings and other relevant indicators, the financial asset is measured at the present value of estimated future cash flows. Any changes to the carrying amount of the investment, including impairment losses, are recognized in the statement of operations.
| F-39 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 6. | Financial Instruments (continued) |
Available-for-sale - Non-derivative financial assets not included in the above categories are classified as available-for-sale. They are carried at fair value with changes in fair value recognized in comprehensive income (loss). Where a decline in the fair value of an available-for-sale financial asset constitutes objective evidence of impairment, the amount of the loss is removed from accumulated other comprehensive income (loss) and recognized in the statement of operations.
All financial assets except for those at fair value through profit or loss are subject to review for impairment at least at the end of each reporting period.
Financial assets are impaired when there is any objective evidence that the cash flows related to a financial asset or group of financial assets have been negatively impacted. Different criteria to determine impairment are applied for each category of financial assets described above.
Financial liabilities
The Company classifies its financial liabilities into one of two categories, depending on the purpose for which the corresponding asset was acquired. The Company’s accounting policy for each category is as follows:
Fair value through profit or loss - This category comprises derivatives, or liabilities acquired or incurred principally for the purpose of being sold or repurchased in the near term. They are carried in the statement of financial position at fair value with changes in fair value recognized in the statement of operations.
Other financial liabilities - This category includes accounts payables and accrued liabilities, all of which are recognized at amortized cost.
The Company’s financial instruments consist of the following:
| Financial assets: | Classification: | |
| Cash and cash equivalents | Loans and receivables | |
| Short-term investments | Loans and receivables | |
| Amounts receivable | Loans and receivables | |
| Investments | Available for sale | |
| Security deposits | Loans and Receivables | |
| Financial liabilities: | Classification: | |
| Accounts payable and accrued liabilities | Other financial liabilities |
Financial instruments recorded at fair value in the statement of financial position are classified according to a three-level hierarchy that reflects the significance of the inputs used in making the fair value measurements. The three levels of fair value hierarchy are as follows:
| ● | Level 1 - Unadjusted quoted prices in active markets for identical assets or liabilities; | |
| ● | Level 2 - Inputs other than quoted prices that are observable for assets or liabilities, either directly or indirectly; and | |
| ● | Level 3 - Inputs for assets or liabilities that are not based on observable market data. |
| F-40 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 6. | Financial Instruments (continued) |
Financial assets measured at fair value on a recurring basis include the following:
| FAIR | As at | As at | ||||||||||||||||||
| VALUE | April 30, 2019 | July 31, 2018 | ||||||||||||||||||
| INPUT | CARRYING | ESTIMATED | CARRYING | ESTIMATED | ||||||||||||||||
| LEVEL | AMOUNT | FAIR VALUE | AMOUNT | FAIR VALUE | ||||||||||||||||
| Financial Assets: | ||||||||||||||||||||
| Cash | 1 | $ | 938,932 | $ | 938,932 | $ | 938,448 | $ | 938,448 | |||||||||||
| Short-term investments | 2 | $ | - | $ | - | $ | 1,341,043 | $ | 1,341,043 | |||||||||||
| Financial liabilities: | ||||||||||||||||||||
| Unsecured convertible loans | 3 | $ | 419,123 | $ | 419,123 | $ | 1,460,138 | $ | 1,460,138 | |||||||||||
| 7. | Unsecured convertible loan |
On March 16, 2018, concurrent with the non-brokered unit offering, the Company completed a non-brokered private placement for the purchase of 5.0% unsecured convertible notes (each, a “Convertible Note”) in the principal amount of US$885,000. Under the terms of securities purchase agreements between the Company and the purchasers of Convertible Notes (the “Noteholders”), each Convertible Note is convertible, at the option of the holder, into (i) common shares of BriaCell for so long as the Convertible Note is outstanding, at a fixed conversion price of $0.10 per common share, for a period of nine months from the date of issuance, which may be extended by the applicable holder and (ii) for each common share issued as a result of conversion, one warrant. The warrants are valid for 36 months from their issuance date and each warrant is exercisable for one common share at an exercise price of $0.14. On April 23, 2019, the Company revised the exercise price of these warrants from $0.14 to $0.12.
The original repayment date of the Convertible Notes was September 16, 2018. On September 17, 2018, the Company and the Noteholders agreed to extend the repayment date of the Convertible Notes, to March 20, 2019 and on March 8, 2019, the Company and the Noteholders agreed to extend the repayment date of the Convertible Notes, to September 7, 2019.
During the year ended July 31, 2018, Noteholders converted $106,843 of Convertible Notes into 1,068,426 shares and 1,068,426 warrants.
During the nine month period ended April 30, 2019, an additional $674,646 of Convertible Notes were converted and as such, the Company issued 6,746,458 shares and 6,746,458 warrants.
| F-41 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 7. | Unsecured convertible loan (continued) |
The Convertible Notes are denominated in US dollars and convertible into common shares and warrants based on the principal and interest balance translated to Canadian dollars. Management determined that the Convertible Notes represent a combined instrument that contains an embedded derivative, being the conversion option. As a result of the foreign exchange impact on the conversion factor, the conversion option does not meet the fixed for fixed criteria and therefore represents a derivative liability. In accordance with IAS 39, the Company has designated the entire Unsecured Convertible Loan at fair value through profit or loss. The Unsecured Convertible Loan was initially recorded at fair value and re-valued at each reporting date with changes in fair value being charged to interest expenses in the consolidated statements of comprehensive loss.
Fair value determination
The fair value of the Convertible Notes, including any adjustments thereto, has been determined using a combination of the Black-Scholes option pricing model for the equity conversion portion and the discounted cash flow method for the loan portion.
The following assumptions were used to determine the fair value of the Convertible Notes:
July 31, 2018 (at year end) | April 30, 2019 (at period end) | |||||||
| Risk-free interest rate | 1.88 | % | 1.84 | % | ||||
| Expected volatility | 88 | % | 83 | % | ||||
| Share price | $ | 0.14 | $ | 0.08 | ||||
| Expected dividend yield | 0 | % | 0 | % | ||||
| Annual loan interest rate | 5 | % | 5 | % | ||||
| CAD/USD rate | 1.3017 | 1.3423 | ||||||
As at April 30, 2019, the fair value of the amount owed to the Noteholders, including accrued interest was $419,123. Total interest expense and gain due to the change in fair value for the nine months ended April 30, 2019, charged to the consolidated statements of operations and comprehensive loss were $26,352 and $392,721 respectively (nine months ended April 30, 2018: $219,975 and $nil, respectively). Total interest expense and loss due to the change in fair value for the three months ended April 30, 2019, charged to the consolidated statements of operations and comprehensive income were $3,204 and $63,398 respectively (three months ended April 30, 2018: $226,583 and $nil, respectively).
| F-42 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 8. | Intellectual Property |
On July 24, 2017, the Company has entered into a definitive share exchange agreement (the “Share Exchange Agreement”) with its wholly-owned subsidiary, BriaCell Therapeutics Corp., Sapientia Pharmaceuticals, Inc. (“Sapientia”) and all the shareholders of Sapientia. Sapientia, a biotechnology company based in Havertown, PA, that is developing novel targeted therapeutics for multiple indications including several cancers and fibrotic diseases.
The attributable intellectual property relates to Sapientia’s various patents, which the Company is amortizing over 20 years, consistent with its accounting policy. During the nine month period ended April 30, 2019, the Company recorded $14,057 in amortization on intellectual property (2018 - $Nil).
| Sapientia | ||||
| Cost | ||||
| As at July 31, 2017 | $ | - | ||
| Additions | 374,852 | |||
| As at July 31, 2018 | 374,852 | |||
| Additions | - | |||
| As at April 30, 2019 | 374,852 | |||
| Accumulated Amortization | ||||
| As at July 31, 2017 | - | |||
| Amortization | 16,894 | |||
| As at July 31, 2018 | 16,894 | |||
| Amortization | 14,057 | |||
| As at April 30, 2019 | 30,951 | |||
| Net Book Value | ||||
| As at July 31, 2017 | - | |||
| As at July 31, 2018 | 357,958 | |||
| As at April 30, 2019 | $ | 343,901 | ||
| F-43 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 9. | Share Capital and Warrant Reserve |
| a) | Authorized share capital |
The authorized share capital consists of an unlimited number of common shares with no par value.
| b) | Issued share capital |
During the nine month period ended April 30, 2019, the Company issued shares as follows:
| i. | 6,746,458 shares were issued at $0.10 per share in respect of the partial conversion of certain Convertible Notes (Note 7). Upon exercise of these Convertible Notes, Noteholders received 6,746,458 warrants with an exercise price of $0.14, expiring within three years. The fair value of the warrants was $276,931 The fair value was estimated using the Black-Scholes option pricing model and the following weighted average assumptions: share price - $0.105-$0.135; exercise price - $0.14; expected life - 36 months; annualized volatility - 100.7%-92.2%; dividend yield - 0%; risk free rate - 1.6%.-2.2%. | ||
| ii. | 1,000,000 shares were issued in respect of 1,000,000 warrants that were exercised at an exercise price of $0.14 for gross proceeds of $140,000. The fair value of the warrants in the amount of $34,140 were released from the Warrant reserve to Share capital. | ||
| iii. | On March 25, 2019 and April 1, 2019, the Company completed a non brokered private placement (the “Private Placement”) on of 29,735,240 shares of the Company at a price of $0.10 per share for aggregate gross proceeds of $2,973,524 (net proceeds: $2,845,784). Included in the Private Placement were $500,000 from Mr. Jamieson Bondarenko, an insider of the Company, and his participation in the Private Placement is considered a “related party transaction” pursuant to Multilateral Instrument 61-101 – Protection of Minority Security Holders in Special Transactions (“MI 61-101”). The Company is exempt from the requirements to obtain a formal valuation or minority shareholder approval in connection with the insiders’ participation in the Private Placement in reliance of sections 5.5(a) and 5.7(1)(a) of MI 61-101. |
| F-44 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 9. | Share Capital and Warrant Reserve (continued) |
| c) | Share Purchase Warrants (continued) |
A summary of changes in share purchase warrants for the nine month period ending April 30, 2019 and the year ended July 31, 2018 is presented below:
| Number | Weighted Average Exercise Price | |||||||
| Balance, July 31, 2018 | 60,448,445 | $ | 0.19 | |||||
| Granted from conversion of Convertible Notes (Note 9(b)(i)) | 6,746,458 | 0.14 | ||||||
| Exercised Brokered Unit Offering (i) | (1,000,000 | ) | 0.14 | |||||
| Expired during the period (ii) | (3,115,144 | ) | 0.35 | |||||
| Balance, April 30, 2019 | 63,079,759 | $ | 0.18 | |||||
| i. | 1,000,000 warrants with a fair value of $34,140 were exercised during the period for gross proceeds of $140,000. The fair value of the warrants was released from the warrant reserve to Share Capital. | ||
| ii. | 3,115,144 warrants with a fair value of $$271,422 expired and the Company recorded a charge to the warrant reserve with a corresponding credit to accumulated deficit. | ||
| iii. | As noted in Note 7, on April 23, 2019, the Company revised the exercise price of 7,814,88,4 warrants, which were issued upon the conversion of Convertible Notes from $0.14 to $0.12. The adjustment to the revised fair value of these warrants ($35,016) was credited to the Warrant reserve with a corresponding charge to Share Capital. |
As at April 30, 2019, warrants outstanding were as follows:
| Number | Exercisable At | |||||||||||
| of | Exercise |
April 30, | Expiry | |||||||||
| Warrants | Price | 2018 | Date | |||||||||
| 3,421,053 | $ | 0.30 | 3,421,053 | April 26, 2021 | ||||||||
| 8,500,000 | $ | 0.35 | 8,500,000 | August 19, 2019 | ||||||||
| 1,021,500 | $ | 0.20 | 1,021,500 | December 21, 2019 | ||||||||
| 42,322,322 | $ | 0.14 | 42,322,322 | March 27, 2021 | ||||||||
| 7,814,884 | $ | 0.12 | 7,814,884 | October 2021 - July 2022 | ||||||||
| 63,079,759 | 63,079,759 | |||||||||||
| F-45 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 9. | Share Capital and Warrant Reserve (continued) |
| d) | Compensation Warrants |
A summary of changes in compensation warrants for the nine month period ended April 30, 2019 and the year ended July 31, 2018 is presented below:
| Balance, July 31, 2017 | 1,007,685 | $ | 0.20 | |||||
| Grant on brokered private placement | 2,613,350 | 0.14 | ||||||
| Grant from placement of Convertible Notes | 1,250,000 | 0.14 | ||||||
| Expired during the period | (139,000 | ) | (0.20 | ) | ||||
| Balance, July 31, 2018 and April 30, 2019 | 4,732,035 | $ | 0.15 |
As at April 30, 2019, compensation warrants outstanding were as follows:
| Number Of | ||||||||||||
| Compensation | Exercise | Exercisable at | ||||||||||
| Warrants | Price | April 30, 2019 | Expiry Date | |||||||||
| 273,685 | $ | 0.30 | 273,685 | April 26, 2021 (i) | ||||||||
| 595,000 | $ | 0.20 | 595,000 | August 19, 2019 (ii) | ||||||||
| 1,250,000 | $ | 0.14 | 1,250,000 | March 27, 2021 (iii) | ||||||||
| 2,613,350 | $ | 0.14 | 2,613,350 | March 27, 2021 (iii) | ||||||||
| 4,732,035 | 4,732,035 | |||||||||||
| i. | Each compensation warrant can be exercised at $0.30 into one unit of BriaCell comprising one common share and one share purchase warrant. Each resultant share purchase warrant acquired can be exercised into an additional common share of BriaCell at $0.35 if exercised by April 26, 2021. | ||
| ii. | Each compensation warrant can be exercised at $0.20 into one unit of BriaCell comprising one common share and one share purchase warrant. Each resultant share purchase warrant acquired can be exercised into an additional common share of BriaCell an exercise price of $0.30 through to August 19, 2019 and $0.35 for the 24 months thereafter. | ||
| iii. | Each compensation warrant can be exercised at $0.14 into one common share of BriaCell for a period of 36 months. |
| F-46 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 10. | Share-Based Compensation and Share-Based Payment Reserve |
The Company has adopted a stock option plan (the “Plan”) under which it is authorized to grant options to officers, directors, employees and consultants enabling them to acquire up to 10% of the issued and outstanding common stock of the Company. The options can be granted for a maximum of 5 years and vest as determined by the Board of Directors. The exercise price of each option granted may not be less than the fair market value of the common shares at the time of grant.
A summary of changes in stock options for the nine month period ended April 30, 2019 and the year ended July 31, 2018 is presented below:
| Number of options outstanding | Weighted average exercise price | |||||||
| Balance, July 31, 2017 | 6,082,000 | 0.24 | ||||||
| Granted | 6,165,600 | 0.15 | ||||||
| Cancelled | (175,000 | ) | (0.30 | ) | ||||
| Expired | (2,650,000 | ) | (0.23 | ) | ||||
| Balance, July 31, 2018 | 9,422,600 | $ | 0.18 | |||||
| Expired (i) | (700,000 | ) | (0.25 | ) | ||||
| Balance, April 30, 2019 | 8,722,600 | $ | 0.18 | |||||
| i. | 700,000 options with a fair value of $88,754 expired and the Company recorded a charge to the share based payment reserve with a corresponding credit to accumulated deficit. | |
| ii. | The Company recognized stock based compensation expense of $49 and $60,570 for the three and the nine months ended April 30, 2019, respectively ($243,223 and $256,080 for the three and nine months ended April 30, 2018, respectively) in relation to the vesting of options issued in previous periods. | |
| iii. | As at April 30, 2019, stock options were outstanding for the purchase of common shares as follows: |
| Number | Exercisable At | |||||||||||
| Of | Exercise | April 30, | Expiry | |||||||||
| Options | Price | 2019 | Date | |||||||||
| 950,000 | $ | 0.255 | 950,000 | November 4, 2025 | ||||||||
| 575,000 | $ | 0.255 | 418,750 | November 4, 2020 | ||||||||
| 150,000 | $ | 0.210 | 112,500 | March 22, 2020 | ||||||||
| 632,000 | $ | 0.250 | 632,000 | November 1, 2019 | ||||||||
| 250,000 | $ | 0.200 | 250,000 | February 14, 2020 | ||||||||
| 3,400,000 | $ | 0.150 | 3,400,000 | Mar 1, 2021 | ||||||||
| 500,000 | $ | 0.200 | 375,000 | March 10, 2022 | ||||||||
| 2,015,600 | $ | 0.140 | 1,511,700 | May 1, 2021 | ||||||||
| 250,000 | $ | 0.140 | 125,000 | July 1, 2023 | ||||||||
| 8,722,600 | 8,274,950 | |||||||||||
As at April 30, 2019, stock options outstanding have a weighted average remaining contractual life of 2.35 years. (April 30, 2018 – 3 years).
| F-47 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 11. | Related Party Transactions |
Parties are considered to be related if one party has the ability, directly or indirectly, to control the other party or exercise significant influence over the other party in making operating and financial decisions. This would include the Company’s senior management, who are considered to be key management personnel by the Company. Parties are also related if they are subject to common control or significant influence. Related parties may be individuals or corporate entities. A transaction is considered to be a related party transaction when there is a transfer of resources or obligations between related parties.
As at April 30, 2019, included in accounts payable and accrued liabilities are amounts owing to a company controlled by an officer in the amount of $3,500 (April 30, 2018 – $3,500) for consulting fees and an amount owing to a director of $3,750 (April 30, 2018– $16,000) for director’s fees.
During the three and nine month periods ended April 30, 2019 and 2018 the Company also incurred the following expenses charged by directors and key management personnel or companies controlled by these individuals:
| Three months ended | Nine months ended | |||||||||||||||
| April 30, | April 30, | |||||||||||||||
| 2019 | 2018 | 2019 | 2018 | |||||||||||||
| a) Paid or accrued professional fees to a company controlled by an officer of the Company | 10,500 | 10,500 | 38,200 | 31,500 | ||||||||||||
| b) Paid or accrued consulting fees to companies controlled by individual directors. | 7,250 | 30,750 | 73,250 | 92,250 | ||||||||||||
| c) Paid or accrued wages and consulting fees to directors | 61,724 | 66,111 | 186,359 | 195,363 | ||||||||||||
| d) Paid or accrued directors’ fees | 18,750 | - | 18,750 | - | ||||||||||||
| 12. | Capital Management |
The Company’s capital comprises share capital, share-based payment reserve, warrant reserve, and accumulated other comprehensive income (loss). The Company manages its capital structure, and makes adjustments to it, based on the funds available to the Company in order to support the Company’s business activities. The Board of Directors does not establish quantitative return on capital criteria for management; it relies on the expertise of the Company’s management to sustain future development of the business.
The intellectual property in which the Company currently has an interest is in the development stage; as such, the Company is dependent on external financing to fund its activities. In order to carry out the planned research and development and pay for administrative costs, the Company intends to raise additional amounts as needed (Note 1).
| F-48 |
BriaCell Therapeutics Corp
Notes to the Condensed Interim Consolidated Financial Statements
For the Three and Nine Months Periods Ended April 30, 2019
(Expressed in Canadian Dollars)
| 13. | Financial Risk Factors |
The Company’s risk exposures and the impact on the Company’s financial instruments are summarized below:
| a) | Credit risk |
The Company has no significant concentration of credit risk arising from operations. Management believes that the credit risk concentration with respect to financial instruments is remote.
| b) | Liquidity risk |
The Company’s approach to managing liquidity risk is to ensure that it will have sufficient liquidity to meet liabilities as they come due. As at April 30, 2019, the Company has a working capital balance of $343,606 (April 30, 2018 - working capital - $2,639,346). As a result, the Company currently has little exposure to liquidity risk. However, as described in Note 1, the Company has not yet achieved profitable operations and expects to incur further losses in the development of its products; these factors cast significant doubt about the Company’s ability to continue as a going concern.
| c) | Market Risk |
| i. | Interest rate risk | ||
| As the Company has cash and short-term investment balances and no interest-bearing debt, interest rate risk is remote. | |||
| ii. | Price risk | ||
| As the Company has no revenues, price risk is remote. | |||
| iii. | Exchange risk | ||
| The Company is exposed to foreign exchange risk as its research operations are conducted primarily in the United States of America. |
| 14. | Commitments and Contingencies |
The Company’s lease arrangement for office space in Berkeley, California end in August 2019 and the annual lease commitment is approximately US$11,000 plus common area maintenance charges.
| F-49 |

| F-50 |
__________ UNITS

PROSPECTUS
ThinkEquity
a division of Fordham Financial Management, Inc.
________, 2019
Through and including , 2019 (the 25th day after the date of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
INFORMATION NOT REQUIRED IN PROSPECTUS
Indemnification of Directors, Officers, Employees and Agents
Under the BCBCA, a company may indemnify: (i) a current or former director or officer of that company; (ii) a current or former director or officer of another corporation if, at the time such individual held such office, the corporation was an affiliate of the company, or if such individual held such office at the company’s request; or (iii) an individual who, at the request of the company, held, or holds, an equivalent position in another entity (an “indemnifiable person”) against all costs, charges and expenses, including an amount paid to settle an action or satisfy a judgment, reasonably incurred by him or her in respect of any civil, criminal, administrative or other legal proceeding or investigative action (whether current, threatened, pending or completed) in which he or she is involved because of that person’s position as an indemnifiable person, unless: (i) the individual did not act honestly and in good faith with a view to the best interests of such company or the other entity, as the case may be; or (ii) in the case of a proceeding other than a civil proceeding, the individual did not have reasonable grounds for believing that the individual’s conduct was lawful. A company cannot indemnify an indemnifiable person if it is prohibited from doing so under its articles or by applicable law. A company may pay, as they are incurred in advance of the final disposition of an eligible proceeding, the expenses actually and reasonably incurred by an indemnifiable person in respect of that proceeding only if the indemnifiable person has provided an undertaking that, if it is ultimately determined that the payment of expenses was prohibited, the indemnifiable person will repay any amounts advanced. Subject to the aforementioned prohibitions on indemnification, a company must, after the final disposition of an eligible proceeding, pay the expenses actually and reasonably incurred by an indemnifiable person in respect of such eligible proceeding if such indemnifiable person has not been reimbursed for such expenses, and was wholly successful, on the merits or otherwise, in the outcome of such eligible proceeding or was substantially successful on the merits in the outcome of such eligible proceeding. On application from an indemnifiable person, a court may make any order the court considers appropriate in respect of an eligible proceeding, including the indemnification of penalties imposed or expenses incurred in any such proceedings and the enforcement of an indemnification agreement. As permitted by the BCBCA, our articles require us to indemnify our directors andformer directors (and such individual’s respective heirs and legal representatives) and permit us to indemnify any person to the extent permitted by the BCBCA.
Recent Sales of Unregistered Securities
In the prior three years, we have issued and sold the securities described below without registering the securities under the Securities Act. None of these transactions involved any underwriters’ underwriting discounts or commissions, or any public offering. We believe that each of the following issuances was exempt from registration under the Securities Act in reliance on Regulation S promulgated under the Securities Act regarding sales by an issuer in offshore transactions, Regulation D under the Securities Act, Rule 701 under the Securities Act or pursuant to Section 4(a)(2) of the Securities Act regarding transactions not involving a public offering.
On March 25, 2019 and April 1, 2019, the Company completed a non-brokered private placement on of 29,735,240 shares of the Company at a price of $0.10 per share for aggregate gross proceeds of $2,973,524 (net proceeds: $2,845,784). Included in the Private Placement were $500,000 from Jamieson Bondarenko, an insider of the Company
On February 26, 2019, BriaCell announced a non-brokered private placement financing of 5,000,000 common shares of the Company to Mr. Bondarenko at a price of C$0.10 per common share for gross proceeds of C$500,000. Upon closing of the Offering, Mr. Bondarenko had a beneficial ownership of an aggregate of 23,070,500 common shares, representing approximately 13.7% of the Company’s issued and outstanding common shares.
On March 27, 2018, the Company completed a non-brokered private placement (the “Non-Brokered Unit Offering”) of 43,322,322 units of the Company (the “Units”) at a price of $0.10 per Unit for aggregate gross proceeds of $4,332,232. Under the Non-Brokered Unit Offering, each Unit consists of one common share (each, a “March Common Share”) and one common share purchase warrant (each, a “March Warrant”). The March Warrants are valid for 36 months following the closing of the Non-Brokered Unit Offering and each March Warrant is exercisable for one March Common Share at an exercise price of $0.14.
Concurrent with the Non-Brokered Unit Offering, the Company also completed a brokered private placement for the purchase of 5.0% unsecured convertible notes (each, a “March Note”) in the principal amount of US$885,000 (the “March Note Offering”). Under the terms of securities purchase agreements dated March 8, 2018 between the Company and the purchasers of March Notes, each March Note is convertible at the option of the holder into (i) common shares of BriaCell for so long as the March Note is outstanding, at a fixed conversion price of $0.10 per March Common Share, for a period of nine months from the date of issuance, which may be extended by the applicable holder for up to six additional months at the holder’s sole option, and (ii) for each March Common Share resulting from the conversion, one March Warrant. The March Warrants are valid for 36 months from their issuance date and each March Warrant is exercisable for one March Common Share at an exercise price of $0.14.
| II-1 |
In connection with the Non-Brokered Unit Offering and the Note Offering (together, the “March Offerings”), the Company paid commissions to certain participating dealers on a portion of funds raised. In respect of the March Note Offering, an aggregate cash commissions of $235,215 and an aggregate 2,613,350 broker warrants (the “Broker Warrants”) were paid. The compensation warrants issued in connection with the March Offerings are exercisable for one March Common Share at an exercise price of $0.14 for a period of 36 months from the issue date.
Officers and members of the Company’s board of directors, including BriaCell’s Chief Executive Officer, Chief Financial Officer and the board’s Chairman, participated in the NonBrokered Unit Offering.
During July 2018, certain noteholders converted $106,843 of the Notes into 1,068,426 shares and 1,068,426 warrants and during August 2018, an additional $117,437 of Notes were converted and as such, the Company issued 1,174,371 shares and 1,174,371 warrants. On September 17, 2018, the Company and the Noteholders agreement to extend the repayment date of the Convertible notes for an additional six month, to March 2019.
On March 9, 2017, the Company and the Company’s President and CEO, completed a non-brokered private placement financing of 5,612,083 units for aggregate gross proceeds to the Company in the amount of $1,346,900. Under the offering, each Unit consisted of one common share in the capital of the Company and one-half of one Common Share purchase warrant.
On August 2, 2017, the Company and the Company’s President and CEO completed a non-brokered private placement resulting in gross proceeds of $631,785. The non-brokered private placement involved the sale of 4,058,441 units at a price of $0.16 per unit. Each unit consisted of one common share in the capital of the Company.
On July 24, 2017, the Company entered into a definitive share exchange agreement between BriaCell Therapeutics Corp., or BTC, the Company’s US subsidiary, Sapienta Pharmaceuticals, or Sapientia, and all the shareholders of Sapientia. Pursuant to the terms of the share exchange agreement, BTC acquired from the Sapientia shareholders all of the issued and outstanding shares in the capital of Sapientia. As consideration, the Sapientia shareholders received an aggregate of 2,500,002 common shares in the capital of BriaCell on a pro-rata basis, which were issued on September 5, 2017. As part of the transaction, the Company acquired all rights, including composition of matter patents, and preclinical study data to a novel therapeutic technology platform, known as protein kinase C delta (PKCδ) inhibitors, which represents a unique, highly-targeted approach to treat cancer and to boost the immune system.
Exhibits and Financial Statement Schedules
(a) Exhibits:
The following exhibits are filed as part of this registration statement:
| Exhibit | Description | |
| 1.1* | Form of Underwriting Agreement | |
| 3.1 | ||
| 3.2 | ||
| 4.1* | Form of Warrant Agency Agreement by and between the Company and [Computershare] and Form of Warrant for Registered Offering | |
| 4.2* | Form of Underwriter’s Warrant | |
| 5.1* | Legal Opinion | |
| 10.1 | Stock Option Plan, dated November 25, 2014 | |
| 10.2 | Service Agreement with UC Davis, dated June 11, 2015 | |
| 10.3 | Non-Disclosure Agreement with UC Davis, dated June 12, 2015 | |
| 10.4 | Employment Agreement with Markus Lacher, dated July 3, 2015 |
| II-2 |
| * | To be filed by amendment. |
| II-3 |
Undertakings
The undersigned Registrant hereby undertakes to:
(a) file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to:
(i) include any prospectus required by section 10(a)(3) of the Securities Act;
(ii) reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or together, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and
(iii) include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in the registration statement.
(b) that, for the purpose of determining any liability under the Securities Act, each post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(c) to file a post-effective amendment to remove from registration any of the securities that remain unsold at the end of the offering.
(d) that insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Registrant, the Registrant has been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registration of expenses incurred or paid by a director, officer or controlling person to the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
| II-4 |
(e) that, for the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(f) that, for the purpose of determining liability of the Registrant under the Securities Act to any purchaser in the initial distribution of the securities, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the Registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) any preliminary prospectus or prospectus of the Registrant relating to the offering filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the Registrant or used or referred to by the Registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the Registrant or its securities provided by or on behalf of the Registrant; and
(iv) any other communication that is an offer in the offering made by the Registrant to the purchaser.
The undersigned Registrant hereby undertakes that:
1. For purposes of determining any liability under the Securities Act of 1933, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the Registrant pursuant to Rule 424(b) (1) or (4) or 497(h) under the Securities Act of 1933 shall be deemed to be part of this registration statement as of the time it was declared effective.
2. For the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
| II-5 |
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in West Vancouver, British Columbia on _____, 2019.
| BRIACELL THERAPEUTICS CORP. | ||
| (Registrant) | ||
| By: | ||
| Dr. William V. Williams | ||
| Chief Executive Officer, President and Director | ||
| (Principal Executive Officer) | ||
| By: | ||
| Gadi Levin | ||
| Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) | ||
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Dr. William V. Williams as his true and lawful attorneys-in-fact, with full power of substitution and re-substitution, for him and in his name, place and stead, in any and all capacities to sign any and all amendments (including post-effective amendments) to this registration statement and to sign a registration statement pursuant to Section 462(b) of the Securities Act of 1933, and to file the same with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
| SIGNATURE | TITLE | DATE | ||
| Chief Executive Officer (Principal | ___, 2019 | |||
| Dr. William V. Williams | Executive Officer), President and Director | |||
| Chief Financial Officer (Principal | ___, 2019 | |||
| Gadi Levin | Financial and Accounting Officer) | |||
| Director | ___, 2019 | |||
| Jamieson Bondarenko | ||||
| Director | ___, 2019 | |||
| Vaughn C. Embro-Pantalony | ||||
| Director | ___, 2019 | |||
| Rebecca Taub | ||||
| Director | ___, 2019 | |||
| Charles Wiseman | ||||
| Director | ___, 2019 | |||
| Richard Berman |
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the requirements of the Securities Act of 1933, the undersigned, the duly authorized representative in the United States of BriaCell Therapeutics Corp., has signed this registration statement on ____, 2019.
| Authorized U.S. Representative | |
| /s/ | |
| [Title] | |
| [Company] |
| II-6 |