UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
|
¨
|
REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934
|
OR
|
þ
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2015
|
OR
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
OR
|
¨
|
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
for the transition period from __________ to ___________
|
|
Commission file number 001-36848
Check-Cap Ltd.
(Exact name of the Registrant as specified in its charter)
Israel
(Jurisdiction of incorporation or organization)
Check-Cap Building
Abba Hushi Avenue
P.O. Box 1271
Isfiya, 30090
Mount Carmel, Israel
(Address of principal executive offices)
Securities registered or to be registered pursuant to Section 12(b) of the Act:
Title of Each Class
Ordinary Shares, par value NIS 0.20
Name of each exchange on which registered
NASDAQ Capital Market
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act: None
As of December 31, 2015, the registrant had 11,811,709 ordinary shares outstanding, NIS 0.2 par value per share.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes o No x
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer or a non-accelerated filer.
|
o Large Accelerated filer
|
o Accelerated filer
|
x Non-accelerated filer
|
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
x US GAAP
|
o International Financial Reporting Standards as issued by the International Accounting Standards Board
|
o Other
|
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
o Item 17 o Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of the securities under a plan confirmed by a court.
Yes o No o
ii
TABLE OF CONTENTS
|
PART I
|
Page
|
|
| 3 | ||
| 3 | ||
| 3 | ||
|
A.
|
Selected financial data
|
3 |
|
B.
|
Capitalization and indebtedness
|
4 |
|
C.
|
Reasons for the offer and use of proceeds
|
4 |
|
D.
|
Risk factors
|
4 |
| 34 | ||
|
A.
|
History and Development of the Company
|
34 |
|
B.
|
Business Overview
|
35 |
|
C.
|
Organizational Structure
|
61 |
|
D.
|
Property, Plants and Equipment
|
61 |
| 61 | ||
| 62 | ||
|
A.
|
Operating Results
|
65 |
|
B.
|
Liquidity and Capital Resources
|
69 |
|
C.
|
Research and development, patents and licenses, etc.
|
78 |
|
D.
|
Trend Information
|
78 |
|
E.
|
Off-balance Sheet Arrangements
|
78 |
|
F.
|
Tabular Disclosure of Contractual Obligations.
|
78 |
| 79 | ||
|
A.
|
Directors and senior management
|
79 |
|
B.
|
Compensation
|
84 |
|
C.
|
Board Practices
|
88 |
|
D.
|
Employees
|
101 |
|
E.
|
Share Ownership
|
101 |
| 105 | ||
|
A.
|
Major shareholders
|
105 |
|
B.
|
Related party transactions
|
107 |
|
C.
|
Interests of experts and counsel
|
111 |
| 111 | ||
|
A.
|
Statements and Other Financial Information
|
111 |
|
B.
|
Significant Changes
|
111 |
| 112 | ||
|
A.
|
Offer and listing details
|
112 |
|
B.
|
Plan of distribution
|
112 |
|
C.
|
Markets
|
112 |
|
D.
|
Selling shareholders
|
112 |
|
E.
|
Dilution
|
113 |
|
F.
|
Expenses of the issue
|
113 |
iii
| 113 | ||
|
A.
|
Share capital
|
113 |
|
B.
|
Memorandum and articles of association
|
113 |
|
C.
|
Material contracts
|
118 |
|
D.
|
Exchange controls
|
118 |
|
E.
|
Taxation
|
118 |
|
F.
|
Dividends and paying agents
|
130 |
|
G.
|
Statement by experts
|
130 |
|
H.
|
Documents on display
|
130 |
|
I.
|
Subsidiary Information
|
130 |
| 131 | ||
| 131 | ||
|
PART II
|
||
| 131 | ||
| 131 | ||
| 131 | ||
| 132 | ||
| 132 | ||
| 132 | ||
| 133 | ||
| 133 | ||
| 133 | ||
| 133 | ||
| 133 | ||
| 134 | ||
|
PART III
|
||
| 135 | ||
| 135 | ||
| 135 | ||
| 137 |
iv
CERTAIN INFORMATION
In this Annual Report on Form 20-F, or the Annual Report, unless the context indicates otherwise, references to “NIS” are to the legal currency of Israel, “U.S. dollars,” “$” or “dollars” are to United States dollars, and the terms “we,” “us,” “our company,” “our,” and “Check-Cap” refer to Check-Cap Ltd. Unless otherwise indicated, U.S. dollar translation of NIS amounts presented in this Annual Report are translated using the rate of $1.00 = NIS 3.902, the exchange rate published by the Bank of Israel on December 31, 2015, and U.S. dollar translation of Euro amounts presented in this Annual Report are translated using the rate of $1.00 = Euro 0.9208, the exchange rates published by the Wall Street Journal on December 31, 2015.
We are a clinical stage medical diagnostics company engaged in the development of an ingestible capsule system that utilizes ultra low-dose X-rays for the detection and imaging of colonic polyps and colorectal cancers, or CRC. While CRC is the second leading cause of death from cancer for both sexes combined in the United States and is largely preventable with early detection, according to 2013 National Health Interview Survey, only 58% of Americans between the ages of 50 to 75 reported being current with CRC screening recommendations. Unlike other screening modalities that are designed to generate structural information of the internal colon for the detection of colonic polyps and CRC, such as optical colonoscopy, computed tomographic colonography, or CTC, and other capsule-based technologies, our system is designed to be ingested without any cathartic preparation of the colon, and to travel through the gastrointestinal tract naturally while the patient continues his or her normal daily routine. Furthermore, unlike existing CRC imaging modalities currently on the market, all of which require the patient to fast for several hours prior to administration, the procedure for the Check-Cap system is designed to enable patients to continue eating normally. Our system is comprised of three main components: (1) ingestible scanning capsule; (2) Capsule Positioning System, or CPS, a recorder worn on the patient’s back; and (3) a PC-based work station for data reconstruction and image processing. We believe that this solution will be attractive to both physicians and patients, with the potential to increase the number of people undergoing CRC screening.
Beginning with the fourth quarter of 2015, we prepared our consolidated financial statements in accordance with accounting principles generally accepted in the United States of America, or U.S. GAAP. We recast the comparative amounts included in our financial statements and in this report to U.S. GAAP. Prior to the fourth quarter of 2015, we prepared our financial reports in accordance with International Financial Reporting Standards as issued by International Accounting Standard Board, or IFRS. We elected to use U.S. GAAP to increase transparency and comparability of our financial reports and facilitate research and analysis by shareholders, analysts and other participants in the U.S. capital markets.
We effected a 1-for-20 reverse stock split of our ordinary shares effective immediately prior to the consummation of our initial public offering on February 24, 2015, in accordance with the approval of our shareholders at a meeting held on January 15, 2015. All share numbers in this Annual Report are reflected on a post- reverse stock split basis.
FORWARD-LOOKING STATEMENTS
This Annual Report contains statements that may be deemed to be “forward-looking statements” within the meaning of the federal securities laws. These statements relate to anticipated future events, future results of operations and/or future financial performance. In some cases, you can identify forward-looking statements by their use of terminology such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “future,” “intend,” “may,” “ought to,” “plan,” “possible,” “potentially,” “predicts,” “project,” “should,” “will,” “would,” negatives of such terms or other similar terms. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. The forward-looking statements in this Annual Report include, without limitation, statements relating to:
|
•
|
our goals, targets and strategies;
|
|
|
•
|
the timing and conduct of the clinical trials for our scanning system, including statements regarding the timing, progress and results of current and future preclinical studies and clinical trials, and our research and development programs;
|
|
|
•
|
the clinical utility, potential advantages and timing or likelihood of regulatory filings and approvals of our system;
|
|
•
|
our future business development, results of operations and financial condition;
|
|
|
•
|
our ability to protect our intellectual property rights;
|
|
|
•
|
our plans to develop, launch and commercialize our system and any future products;
|
|
|
•
|
the timing, cost or other aspects of the commercial launch of our system;
|
|
|
•
|
market acceptance of our product;
|
|
|
•
|
our estimates regarding expenses, future revenues, capital requirements and our need for additional financing and strategic partnerships;
|
|
|
•
|
our estimates regarding the market opportunity for our system;
|
|
|
•
|
the impact of government laws and regulations;
|
|
|
•
|
our ability to recruit and retain qualified clinical, regulatory and research and development personnel;
|
|
|
•
|
unforeseen changes in healthcare reimbursement for any of our approved product;
|
|
|
•
|
difficulties in maintaining commercial scale manufacturing capacity and capability; our ability to generate growth;
|
|
|
•
|
our failure to comply with regulatory guidelines;
|
|
|
•
|
uncertainty in industry demand and patient wellness behavior;
|
|
|
•
|
general economic conditions and market conditions in the medical device industry;
|
|
|
•
|
future sales of large blocks or our securities, which may adversely impact our share price;
|
|
|
•
|
depth of the trading market in our securities; and
|
|
|
•
|
our expectations regarding the use of proceeds of our initial public offering and the concurrent private placement.
|
The preceding list is not intended to be an exhaustive list of all of our forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including those described in Item 3D “Key Information - Risk factors.”
You should not unduly rely on any forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Annual Report, to conform these statements to actual results or to changes in our expectations.
2
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
|
A.
|
Directors and Senior Management
|
Not required.
|
B.
|
Advisers
|
Not required.
|
C.
|
Auditors
|
Not required.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
Not required.
ITEM 3. KEY INFORMATION
|
A.
|
Selected financial data
|
The following selected consolidated financial data should be read in conjunction with Item 5 “Operating and Financial Review and Prospects” and the consolidated Financial Statements and Notes thereto included elsewhere in this Annual Report.
The following table summarizes our historical consolidated financial data. We have derived the selected consolidated statements of operations data for the years ended December 31, 2015, 2014 and 2013 and the selected consolidated balance sheet data as of December 31, 2015 and 2014 from our audited consolidated financial statements included elsewhere in this Annual Report. We have derived the selected consolidated financial data as of December 31, 2013 and 2012 from our audited consolidated financial statements not included in this Annual Report. Selected financial data as of, and for the year ended, December 31, 2011 have been omitted from this Annual Report because of our status as an emerging growth company under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, and as per related guidance provided by the SEC. Our Consolidated Financial Statements have been prepared in accordance with U.S. GAAP.
Certain factors that affect the comparability of the information set forth in the following table are described in Item 5 “Operating and Financial Review and Prospects” and the Consoliated Financial Statements and related notes thereto included elsewhere in this Annual Report.
Consolidated Statements of Operations Data
|
Year Ended December 31,
|
||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
|||||||||||||
|
(US$ in thousands, except per share data)
|
||||||||||||||||
|
Operating expenses(1)
|
||||||||||||||||
|
Research and development expenses, net(2)
|
$ | 5,837 | $ | 2,832 | $ | 2,893 | $ | 2,377 | ||||||||
|
General and administrative expenses
|
6,626 | 1,703 | 1,090 | 1,118 | ||||||||||||
|
Other income (expenses)
|
- | - | 11 | (14 | ) | |||||||||||
|
Operating loss
|
12,463 | 4,535 | 3,972 | 3,509 | ||||||||||||
|
Finance income (expenses), net
|
173 | 3,925 | 604 | (841 | ) | |||||||||||
|
Net loss
|
$ | 12,290 | $ | 610 | $ | 3,368 | $ | 4,350 | ||||||||
|
Net loss per ordinary share of NIS 0.20 par value, basic and diluted(3)
|
$ | 1.06 | $ | 1.18 | $ | 3.27 | $ | 3.88 | ||||||||
|
Weighted average number of ordinary shares outstanding – basic and diluted (in thousands)(3)
|
11,918 | 2,181 | 1,627 | 1,627 | ||||||||||||
3
Consolidated Balance Sheet Data
|
As of December 31,
|
||||||||||||||||
|
2015
|
2014
|
2013
|
2012
|
|||||||||||||
|
(US$ in thousands, except per share data)
|
||||||||||||||||
|
Cash and cash equivalents
|
$ | 9,392 | $ | 1,075 | $ | 4,975 | 4,579 | |||||||||
|
Working capital(4)
|
12,856 | (1,622 | ) | 4,134 | 7,931 | |||||||||||
|
Total assets
|
15,298 | 2,985 | 5,374 | 8,975 | ||||||||||||
|
Capital stock
|
46,763 | 20,999 | 20,687 | 20,631 | ||||||||||||
|
Total shareholders’ equity (deficiency)
|
$ | 12,648 | $ | (826 | ) | $ | (528 | ) | 2,782 | |||||||
_____________________
|
(1)
|
Includes share-based compensation expense in the total amount of $3.7 million, $312,000 and $56,000 for the years ended December 31, 2015, 2014 and 2013, respectively. For additional information, see Item 5B “Operating and Financial Review and Prospects—Liquidity and Capital Resources—Application of Critical Accounting Policies and Estimates-Share-based compensation.”
|
|
(2)
|
Research and development expenses, net is presented net of amount of grants received from the Office of the Chief Scientist of the Ministry of Economy and Industry (formerly named the Ministry of Economy), or the OCS, and Israel-United States Binational Industrial Research and Development Foundation, or the BIRD Foundation. The effect of the participation by the OCS and BIRD totaled $354,000, $643,000 and $148,000 for the years ended December 31, 2015, 2014 and 2013, respectively. See Item 5A “Operating and Financial Review and Prospects—Operating Results - Financial Operations Overview—Research and Development, Expenses, Net” for more information.
|
|
(3)
|
Basic and diluted loss per ordinary share is computed based on the basic and diluted weighted average number of ordinary shares outstanding during each period. For purposes of these calculations, the following ordinary shares were deemed to be outstanding: (i) 99,774 ordinary shares that were issuable to Mr. Guy Neev upon exercise of options, referred to as the Neev Options, which options were exercised immediately prior to the consummation of our initial public offering on February 24, 2015; (ii) 375,204 ordinary shares issuable under warrants that are automatically exercised, for no consideration (unless the holder thereof objects to such exercise), upon the exercise by Mr. Guy Neev of the Neev Options, of which warrants to purchase 195,012 ordinary shares were exercised during the year ended December 31, 2015; (iii) 2,658,463 ordinary shares issuable upon the exercise of outstanding warrants with an exercise price of NIS 0.20 per share, of which warrants to purchase 1,557,507 ordinary shares were exercised during the year ended December 31, 2015; and (iv) 210,964 ordinary shares issuable upon the exercise of certain outstanding options and warrants with an exercise price of NIS 0.20 per share. For additional information, see Note 15 to our Consoliated Financial Statements for the year ended December 31, 2015 included elsewhere in this Annual Report.
|
|
(4)
|
Working capital is defined as total current assets minus total current liabilities.
|
|
B.
|
Capitalization and Indebtedness
|
Not required.
|
C.
|
Reasons for the Offer and Use of Proceeds
|
Not required.
|
D.
|
Risk factors
|
In conducting our business, we face many risks that may interfere with our business objectives. Some of these risks could materially and adversely affect our business, financial condition and results of operations. In particular, we are subject to various risks resulting from changing economic, political, industry, business and financial conditions. The risks and uncertainties described below are not the only ones we face. Additional risks and uncertainties not currently known to us or that we currently deem to be immaterial may also materially adversely affect our business operations.
You should carefully consider the following factors and other information in this Annual Report before you decide to invest in our ordinary shares. If any of the risks referred to below occur, our business, financial condition and results of operations could suffer. In any such case, the trading price of our ordinary shares could decline, and you may lose all or part of your investment.
4
Risks Related to Our Business
We have a history of losses, may incur future losses and may not achieve profitability.
We are a clinical and development-stage medical diagnostics company with a limited operating history. We have incurred net losses in each fiscal year since we commenced operations in 2009. We incurred net losses of $3.4 million in 2013, $610,000 in 2014 and $12.3 million in 2015. As of December 31, 2015, our accumulated deficit was $34.1 million. Our losses could continue for the foreseeable future as we continue our investment in research and development and clinical trials to complete the development of our technology and to attain regulatory approvals, begin the commercialization efforts for our system, increase our marketing and selling expenses, and incur additional costs as a result of being a public company in the United States. As discussed in Note 1B to the financial statements presented elsewhere in this Annual Report, successful completion of our development program and, ultimately, the attainment of profitable operations is dependent upon future events, including obtaining adequate financing to fulfill its development activities and achieving a level of sales adequate to support our cost structure. The extent of our future operating losses and the timing of becoming profitable are highly uncertain, and we may never achieve or sustain profitability.
We may not succeed in completing the development of our product, commercializing our product and generating significant revenues.
Since commencing our operations, we have focused on the research and development and limited clinical trials of our capsule. Our product is not approved for commercialization and has never generated any revenues. Our ability to generate revenues and achieve profitability depends on our ability to successfully complete the development of our product, obtain market approval and generate significant revenues. The future success of our business cannot be determined at this time, and we do not anticipate generating revenues from product sales for the foreseeable future. In addition, we have no experience in commercializing our capsule and face a number of challenges with respect to our commercialization efforts, including, among others, that:
|
•
|
we may not have adequate financial or other resources to complete the development of our product;
|
|
•
|
we may not be able to manufacture our products in commercial quantities, at an adequate quality or at an acceptable cost;
|
|
•
|
we may not be able to establish adequate sales, marketing and distribution channels;
|
|
•
|
healthcare professionals and patients may not accept our system;
|
|
•
|
we may not be aware of possible complications from the continued use of our system since we have limited clinical experience with respect to the actual use of our system;
|
|
•
|
other technological breakthroughs in colorectal cancer, or CRC screening, treatment and prevention may reduce the demand for our system;
|
|
•
|
changes in the market for CRC screening, new alliances between existing market participants and the entrance of new market participants may interfere with our market penetration efforts;
|
|
•
|
third-party payors may not agree to reimburse patients for any or all of the purchase price of our capsule, which may adversely affect patients’ willingness to purchase our capsule;
|
|
•
|
uncertainty as to market demand may result in inefficient pricing of our system;
|
|
•
|
we may face third-party claims of intellectual property infringement;
|
|
•
|
we may fail to obtain or maintain regulatory approvals for our system in our target markets or may face adverse regulatory or legal actions relating to our system even if regulatory approval is obtained; and
|
|
•
|
we are dependent upon the results of ongoing clinical studies relating to our system and the products of our competitors.
|
5
If we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our system could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
Clinical failure can occur at any stage of clinical development. Our clinical experience to date does not necessarily predict future results and may not have revealed certain potential limitations of the technology and potential complications from our system and may require further clinical validation. Any product version we advance through clinical trials may not have favorable results in later clinical trials or receive regulatory approval
Clinical failure can occur at any stage of clinical development. To date, we have performed clinical studies with several versions of both scanning and non-scanning capsules, in conjunction with iterative versions of the tracking and recording systems (CPS). Our clinical trials have been conducted using prior versions of our scanning capsules and were conducted under differing protocols and using limited groups of patients. Therefore, we have a limited ability to identify potential problems and/or inefficiencies concerning current and future versions of our system in advance of its use in expanded groups of patients and we cannot assure you that its actual clinical performances will be satisfactory to support proposed indications and regulatory approvals, or that its use will not result in unanticipated complications. Furthermore, the results from laboratory, pre-clinical, and completed clinical studies, as well as preliminary analyses of the currently ongoing clinical studies, may not be indicative of final clinical results obtained from our current system or future versions of our system on expanded screening populations. In addition, the results of our clinical trials are subject to human analyses and interpretation of the data accumulated, which could be affected by various errors due to, among others, lack of sufficient clinical experience with our system, interpretation errors in the analysis of the clinical trials results, including the reconstructed images by our system, or due to uncertainty in the actual efficacy of our system in its current clinical stage. Therefore, the safety and efficacy of our system and the clinical results to date will require further independent professional validation and require further clinical study. If our system does not function as expected over time, we may not be able to develop our system at the rate or to the stage we desire, we could be subject to liability claims, our reputation may be harmed, our system may not achieve regulatory clearances, and our system may not be widely adopted.
We expect to derive most of our revenues from sales of one product or product line. Our inability to successfully commercialize this product, or any subsequent decline in demand for this product, could severely harm our ability to generate revenues.
We are currently dependent on the successful commercialization of our system to generate revenues. As a result, factors adversely affecting our ability to successfully commercialize, or the pricing of or demand for, this product could have a material adverse effect on our financial condition and results of operations. If we are unable to successfully commercialize or create market demand for our system, we will have limited ability to generate revenues.
Furthermore, and consequently, we are vulnerable to fluctuations in demand for our system. Such fluctuations in demand may be due to many factors, including, among others:
|
•
|
market acceptance of a new product, including healthcare professionals’ and patients’ preferences;
|
|
•
|
development of similarly cost-effective products by our competitors;
|
|
•
|
development delays of our system;
|
|
•
|
technological innovations in CRC screening, treatment and prevention;
|
|
•
|
adverse medical side effects suffered by patients using our system, whether actually resulting from the use of our system or not;
|
|
•
|
changes in regulatory policies toward CRC screening or imaging technologies;
|
|
•
|
changes in regulatory approval or clearance requirements for our product;
|
|
•
|
third-party claims of intellectual property infringement;
|
|
•
|
budget constraints and the availability of reimbursement or insurance coverage from third-party payors for our system;
|
|
•
|
increases in market acceptance of other technologies; and
|
6
|
•
|
adverse responses from certain of our competitors to the offering of our system.
|
If healthcare professionals do not recommend our product to their patients, our system may not achieve market acceptance and we may not become profitable.
CRC screening candidates are generally referred by their healthcare professional to a specified device and screening technologies are purchased by prescription. If healthcare professionals, including physicians, do not recommend or prescribe our product to their patients, our system may not achieve market acceptance and we may not become profitable. In addition, physicians have historically been slow to change their medical diagnostic and treatment practices because of perceived liability risks arising from the use of new products. Delayed adoption of our system by healthcare professionals could lead to a delayed adoption by patients and third-party payors. Healthcare professionals may not recommend or prescribe our system until certain conditions have been satisfied including, among others:
|
•
|
there is sufficient long-term clinical and health-economic evidence to convince them to alter their existing screening methods and device recommendations;
|
|
•
|
there are recommendations from other prominent physicians, educators and/or associations that our system is safe and effective;
|
|
•
|
we obtain favorable data from clinical and health-economic studies for our system;
|
|
•
|
reimbursement or insurance coverage from third-party payors is available; and
|
|
•
|
they become familiar with the complexities of our system.
|
We cannot predict when, if ever, healthcare professionals and patients may adopt the use of our system. Even if favorable data is obtained from clinical and health-economic studies for our system, there can be no assurance that prominent physicians would endorse it or that future clinical studies will continue to produce favorable data regarding our system. In addition, prolonged market exposure may also be a pre-requisite to reimbursement or insurance coverage from third-party payors. If our system does not achieve an adequate level of acceptance by patients, healthcare professionals and third-party payors, we may not generate significant product revenues and we may not become profitable.
If we are unable to market and sell our system, we may not become profitable.
We have not had any sales of our system to date. There can be no assurance that we will be able to receive regulatory clearance for our system in the foreseeable future or ever or that our system will be accepted as comparable or superior to existing technologies for the visualization, imaging or screening of the colon. Our ability to market and sell our system successfully depends on one or more of the following:
|
•
|
the existence of clinical and health-economic data sufficient to support the use of our system for the visualization, imaging, or screening of the colon as compared to other colon visualization, imaging or screening methods (if clinical trials indicate that our system is not as clinically effective as other current methods, or if our technology causes unexpected complications or other unforeseen negative effects, we may not obtain regulatory clearance or approval to market and sell our system or physicians may be reluctant to use it);
|
|
•
|
the availability of sufficient clinical and health-economic data for physicians to use our system in their practice and for private third-party payors to make an adequate reimbursement decision to provide coverage for our system; or
|
|
•
|
the availability of a reliable contrast agent for our system that is accepted by and health-economic physicians and patients.
|
If one or more of the above conditions is not satisfied, we may not be able to market and sell our system or the demand for our system may be lower than expected and sales of our system may not contribute to our growth at the rate we expect or at all.
7
We expect to face competition from large, well-established manufacturers of traditional technologies for detecting gastrointestinal disorders, as well as from other manufacturers of optical capsule endoscopy systems or new competitive technologies.
Competition for our system comes from traditional well-entrenched manufacturers of equipment for detecting gastrointestinal disorders and diseases, such as colonoscopy, sigmoidoscopy, optical capsule endoscopy and CTC. The principal manufacturers of equipment for optical colonoscopy, sigmoidoscopy and optical capsule endoscopy are Olympus, Pentax, Richo Company Ltd., Hoya, Covidien plc and Fuji Film. The principal manufacturers of equipment for CTC are General Electric Healthcare Systems, Siemens Medical Solutions, Philips Medical Systems Ltd. and Toshiba Corporation. All of these companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide distribution channels for providing medical instruments to physicians.
In addition, several companies have developed or are developing technologies based on molecular diagnostics (from blood and other bodily fluids), or MDx, tests that investigate the link between genes and the function of metabolic pathways, drug metabolism and disease development with a primary focus on the study of DNA, RNA and proteins. Genetic markers can be traced within stool samples in minute quantities. A U.S. based company, Exact Sciences, has developed a special collecting kit for stool samples and an analyzer to detect neoplasia associated DNA markers and the presence of occult hemoglobin in human stool. The method of screening is included in colorectal cancer screening guidelines, including those of the American Cancer Society.
To our knowledge, certain companies are developing or commercializing optics-based capsule endoscopy systems. The existing capsule technology requires intense bowel cleansing, more so than is required for optical colonoscopy, and the potential ingestion of agents to boost capsule transit through the colon. Given Imaging, an Israeli-based company that was acquired by Covidien plc (NYSE: COV) in February 2014 (subsequently acquired by Medtronic), has developed visualization capsules for the detection of disorders of the esophagus, small bowel and colon. It received the CE Mark to market PillCam™ COLON throughout the European Union in 2006. In early 2014, the FDA cleared PillCam COLON 2 system with an indication to provide visualization of the colon. It may be used for detection of colon polyps in patients after an incomplete optical colonoscopy with adequate preparation, and when a complete evaluation of the colon was not technically possible. In Early 2016, the FDA cleared PillCam COLON2 system with an expended indication for use: High risk population to undergo colonoscopy. Patients with major risks for colonoscopy or moderate sedation, but who tolerate colonoscopy and moderate sedation in the event a clinically significant colon abnormality was identified on capsule endoscopy. Other companies, including Olympus, CapsoVision, Intromedic and RF System, are developing similar approaches for optical capsule endoscopy.
Procedures for bowel cleansing that are less onerous are constantly being developed, which could make our entry into the market more difficult. For instance, bowel cleansing initiated by the ingestion of pills rather than through drinking large amounts of distasteful liquids may be viewed as an improvement to the cleansing process, but other screening methods may be even more palatable to patients.
If we are unable to convince physicians to adopt our system over the current technologies marketed by our competitors, our results of operations may suffer.
We are planning to rely on local distributors and/or strategic partners to market and distribute our system in those countries where we intend to market and distribute our system.
We are planning to rely on local distributors and/or strategic partners for the marketing and distribution of our system. Our success in generating sales in countries or regions where we will engage local distributors will depend in part on the efforts of third parties over whom we have limited control. If we are unable to identify suitable local distributors in the countries where we intend to market and distribute our system, our business, financial condition and results of operations could be negatively affected.
We have limited manufacturing capabilities and if we are unable to scale our manufacturing operations to meet anticipated market demand, our growth could be limited and our business, financial condition and results of operations could be materially adversely affected.
We currently have limited resources, facilities and experience in commercially manufacturing sufficient quantities of our system, external receiver and software application to meet the demand we expect from our expanded commercialization efforts. We expect to face certain technical challenges as we increase manufacturing capacity, including, among others, logistics associated with the handling of radioactive materials, equipment design and automation, material procurement, lower than expected yields and increased scrap costs, as well as challenges related to maintaining quality control and assurance standards. Furthermore, we may encounter similar or unforeseen challenges initiating and later expanding production of any new products. If we are unable to scale our manufacturing capabilities to meet market demand, our growth could be limited and our business, financial condition and results of operations could be materially adversely affected.
8
In addition, we have received and may receive in the future grants from the Government of the State of Israel through the OCS (for more information, see “Risks Related to Our Operations in Israel”), the terms of which require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless prior approval is received from the OCS, which we may not receive. We are currently considering whether it would be possible to assemble the capsule without the X-ray source in Israel, and have the X-ray source subsequently inserted into our system at a reactor or cyclotron site or at a distribution center outside Israel. Even following the full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Encouragement of Industrial Research and Development Law 5744-1984, or the Research Law. The foregoing restrictions may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel.
Our reliance on single source suppliers could harm our ability to meet demand for our product in a timely manner or within budget.
We currently depend on single source supplier for some of the components necessary for the production of our system. For example, for the current version of the system used in clinical trials, we currently have a single supplier for the motor that we are using to rotate the collimated X-ray source in our system and for the X-ray detectors used in our system. There are a limited number of manufacturers worldwide who are capable of manufacturing the motor and the specially designed X-ray detectors that we currently use in our system. In addition, the application-specific integrated circuit, or ASIC, residing in our system is currently manufactured for us by a single semiconductor fabrication plant, or FAB. There are many alternative FABs worldwide and the design of our current ASIC could be adapted in the event it became necessary to use an alternative FAB. Our X-ray source manufacturer is considered a single source supplier. Our X-ray source is custom made and therefore, would require us to qualify an alternative supplier, if required. Our current suppliers have been able to supply the required quantities of such components to date. However, if the supply of these components is disrupted or terminated or if our current suppliers are unable to supply required quantities of components, we may not be able to find alternative sources for these key components in a timely manner. Although we are planning to maintain strategic inventory of key components, the inventory may not be sufficient to satisfy the demand for our system if such supply is interrupted or otherwise affected by catastrophic events such as a fire at our storage facility. As a result, we may be unable to meet the demand for our system, which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to change the manufacturer of any of these key components, there may be a significant delay in locating a suitable alternative manufacturer. In addition, we may be required to verify that the new manufacturer maintains facilities and procedures that comply with FDA and other applicable quality standards and with all applicable regulations and guidelines. The delays associated with the identification of a new manufacturer could delay our ability to manufacture our system in a timely manner or within budget. Furthermore, in the event that the manufacturer of a key component of our system ceases operations or otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture the component. The occurrence of any of these events could harm our ability to meet demand for our system in a timely manner or within budget.
The use of any of our scanning capsule, cps or workstation could result in product liability or similar claims that could be expensive damage our reputation and harm our business.
Our business exposes us to an inherent risk of potential product liability or similar claims related to the manufacturing, marketing and sale of medical devices. The medical device industry has historically been litigious, and we face financial exposure to product liability or similar claims if the use of any of our scanning capsule, cps or workstation were to cause or contribute to injury or death, including, without limitation, harm to the body caused by the procedure or inaccurate diagnoses from the procedure that could affect treatment options. There is also the possibility that defects in the design or manufacture of any of these products might necessitate a product recall. Although we plan to maintain product liability insurance, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration, and a substantial diversion of management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations.
Our system is a complex medical device that requires training for qualified personal and care for data analysis.
Our system is a complex medical device that requires training for qualified personal, including physicians, and care for data analysis. Although our distributors will be required to ensure that our system is only prescribed by trained clinicians, the potential for misuse of our system still exists due to its complexity. Such misuse could result in adverse medical consequences for patients that could damage our reputation, subject us to costly product liability litigation and otherwise have a material adverse effect on our business, financial condition and results of operations.
9
We depend on third parties to manage our clinical studies and trials and to perform related data collection and analysis and to enroll patients for our clinical trials, and, as a result, we may face costs and delays that are beyond our control.
We rely on third parties, including clinical investigators and clinical sites, to manage our clinical trials and to perform data collection and analysis and to enroll patients for our clinical trials. Although we have and expect to continue to have contractual arrangements with these third parties, we may not be able to control the amount and timing of resources that these parties devote to our studies and trials or the quality of these resources. If these third parties fail to properly manage our studies and trials or enroll patients for our clinical trials, we will be unable to complete them at all or in a satisfactory or timely manner, which could prevent us from obtaining regulatory approvals for, or achieving market acceptance of, our product.
In addition, termination of relationships with third parties may result in delays, inability to enter into arrangements with alternative third parties or do so on commercially reasonable terms. Switching or adding additional clinical sites involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new clinical site commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
We intend to sell our products in the United States, Europe and Japan and, if we are unable to manage our operations in these territories, our business, financial condition and results of operations could be materially adversely affected.
Our headquarters and substantially all of our operations and employees are presently located in Israel, but we intend to market our products in the United States, Europe and Japan. Accordingly, we are subject to risks associated with international operations, and our international sales and operations will require significant management attention and financial resources. In addition, our international sales and operations will subject us to risks inherent in international business activities, many of which are beyond our control and include, among others:
|
•
|
foreign certification, registration and other regulatory requirements;
|
|
•
|
customs clearance and shipping delays;
|
|
•
|
import and export controls;
|
|
•
|
trade restrictions;
|
|
•
|
multiple and possibly overlapping tax structures;
|
|
•
|
difficulty forecasting the results of our international operations and managing our inventory due to our reliance on third-party distributors;
|
|
•
|
differing laws and regulations, business and clinical practices, third-party payor reimbursement policies and patient preferences;
|
|
•
|
differing standards of intellectual property protection among countries;
|
|
•
|
difficulties in staffing and managing our international operations;
|
|
•
|
difficulties in penetrating markets in which our competitors’ products are more established;
|
|
•
|
currency exchange rate fluctuations; and
|
|
•
|
political and economic instability, war or acts of terrorism.
|
If we are unable to manage our international operations effectively, our business, financial condition and results of operations could be materially adversely affected.
10
We will require additional funding and may license certain rights to third parties in order to complete the development and commercialization of our system and the development and commercialization of any future products.
Our operations have consumed substantial amounts of cash. We expect that we will need to continue to spend substantial amounts in order to complete the development, clinical development, regulation and commercialization of our system. Although we intend to use the proceeds of our initial public offering and the concurrent private placement to finance these efforts, we will need to raise additional funds and may license certain rights to third parties to obtain the required capital prior to completing the development and commercialization of our product. Additional financing may not be available to us on a timely basis on terms acceptable to us, or at all. In addition, any additional financing may be dilutive to our shareholders or may require us to grant a lender a security interest in our assets.
Furthermore, if adequate additional financing on acceptable terms is not available, we may not be able to develop our system at the rate or to the stage we desire and we may have to delay or abandon the commercialization of our system. Alternatively, we may be required to prematurely license to third parties the rights to further develop or to commercialize our system on terms that are not favorable to us. Any of these factors could materially adversely affect our business, financial condition and results of operations.
If we lose our key personnel or are unable to attract and retain additional personnel, our business and ability to compete will be harmed.
Our success relies upon the continued service and performance of the principal members of our management and research and development team. In order to implement our business strategy, we will need to retain our key personnel with expertise in the areas of research and development, clinical testing, government regulation, manufacturing, finance, marketing and sales. Our product development plans depend in part on our ability to retain engineers with expertise in a variety of technical fields. The loss of a number of these persons or our inability to attract and retain qualified personnel could harm our business and our ability to compete.
Substantially all of our operations are currently conducted at a single location near Haifa, Israel, and any disruption at our facility could materially adversely affect our business, financial condition and results of operations.
Substantially all of our operations are conducted at a single location near Haifa, Israel. We take precautions to safeguard our facility, including obtaining insurance coverage and implementing health and safety protocols. However, a natural or other disaster, such as a fire, flood or an armed conflict involving Israel, as detailed further below, could damage or destroy our facility and our manufacturing equipment or inventory, cause substantial delays in our operations and otherwise cause us to incur additional unanticipated expenses. In addition, the insurance we maintain against fires, floods and other natural disasters may not be adequate to cover our losses in any particular case and it does not cover losses resulting from armed conflicts or terrorist attacks in Israel. Damage to our facility, our other property or to any of our suppliers, whether located in Israel or elsewhere, due to fire, a natural disaster or casualty event or an armed conflict, could materially adversely affect our business, financial condition and results of operations, with or without insurance.
We have and will incur significant increased costs as a result of operating as a public company in the United States, and our management will be required to devote substantial time to compliance initiatives.
As a public company whose securities are traded in the United States, we have and will continue to incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules and regulations implemented by the U.S. Securities and Exchange Commission and the NASDAQ Stock Market, impose various requirements on public companies, including requiring the establishment and maintenance of effective disclosure and financial controls. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations have and will increase our legal and financial compliance costs and will make some activities more time consuming and costly. These rules and regulations make it more difficult and more expensive for us to obtain certain types of insurance including director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantial costs to maintain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We cannot predict or estimate the amount or timing of additional costs we may incur in order to comply with such requirements.
11
We are required to develop and maintain proper and effective internal controls over financial reporting. We may not complete our analysis of our internal controls over financial reporting in a timely manner, or these internal controls may have one or more material weaknesses, which may adversely affect investor confidence in our company and, as a result, the value of our securities.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis will be a costly and time-consuming effort that will need to be evaluated frequently. Section 404 of the Sarbanes-Oxley Act requires the management of public companies to conduct an annual review and evaluation of their internal controls and to obtain an attestation report from their registered public accounting firm regarding the effectiveness of internal controls. We are required to perform the annual review and evaluation of our internal controls no later than in connection with the second annual report on Form 20-F filed after closing of our initial public offering, which occurred on February 24, 2015. However, if we qualify as a smaller reporting company and/or emerging growth company, which we expect to, we will be exempt from the auditors’ attestation requirement until such time as we no longer qualify as a smaller reporting company and/or emerging growth company. We would no longer qualify as a smaller reporting company if the market value of our public float exceeded $75 million as of the last day of our second fiscal quarter in any fiscal year following the date of our initial public offering. We would no longer qualify as an emerging growth company at such time as described in the risk factor immediately below.
We are in the early stages of the costly and challenging process of compiling the system and processing documentation necessary to evaluate and correct a material weakness in internal controls needed to comply with Section 404. The material weakness relates to our being a small company with a limited number of employees which limits our ability to assert the controls related to the segregation of duties. During the evaluation and testing process, if we identify one or more additional material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective. If we are unable to assert that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our securities to decline.
While we currently qualify as an “emerging growth company” under the JOBS Act, we will cease to be an emerging growth company on or before the end of 2020, and at such time our costs and the demands placed upon our management will increase.
We will continue to be deemed an emerging growth company until the earliest of (i) the last day of the fiscal year in which our annual gross revenues exceed $1 billion (as indexed for inflation); (ii) the last day of the fiscal year in which the fifth anniversary of the date of the first sale of securities under this registration statement; (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt; or (iv) the date on which we are deemed to be a ‘large accelerated filer,’ as defined by the U.S. Securities and Exchange Commission, which would generally occur upon our attaining a public float of at least $700 million. Once we lose emerging growth company status, we expect the costs and demands placed upon our management to increase, as we will be required to comply with additional disclosure and accounting requirements, particularly if we also no longer qualify as a smaller reporting company.
Risks Related to Regulations
If we are unable to obtain, or experience significant delays in obtaining, FDA clearances or approvals, CE Certificates of Conformity, or equivalent third country approvals for our system or future products or product enhancements, our ability to commercially distribute and market our products could suffer.
Our products are subject to rigorous regulation by FDA and numerous other federal, state and foreign governmental authorities and notified bodies. The process of obtaining regulatory clearances or approvals, CE Certificates of Conformity, or equivalent third country approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals, CE Certificates of Conformity, or equivalent third country approvals on a timely basis, if at all. In particular, we expect to eventually generate a portion of our revenues from sales of our system and future products in the United States, the European Union, or third countries. Before a new medical device, or a new use of, or claim for, an existing product can be marketed in the United States, it must first receive clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FDA approval of a premarket approval application, or PMA, unless an exemption applies. FDA will clear marketing of a low to moderate risk medical device through the 510(k) process if sufficiently similar predicate devices have previously been cleared via this pathway. In the 510(k) clearance process, FDA must only determine that the proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use/indications for use, technological characteristics and principles of operation in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence.
High risk devices deemed to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices, or devices not deemed substantially equivalent to a previously cleared device, require approval of a PMA. The PMA process is more costly, lengthy and uncertain than the 510(k) clearance process. The PMA pathway requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on the data obtained in clinical trials. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to FDA’s satisfaction the safety and efficacy of the device for its intended use.
12
In instances where a device is novel and there is no suitable predicate device, but that device is deemed to be of low to moderate risk, FDA can reclassify the device to class I or class II via de novo reclassification. This process involves the submission of a reclassification petition, and FDA accepting that “special controls” are adequate to ensure the product’s performance and safety. FDA now allows “direct” de novo reclassification petitions, a mechanism by which a sponsor can directly submit a detailed de novo reclassification petition as the device’s initial submission without having to first receive a not substantially equivalent, or NSE, decision on a 510(k) submission.
These processes can be expensive and lengthy. FDA’s 510(k) clearance process usually takes from 6 to 9 months, but it can last longer. Direct de novo reclassification typically takes at least 9 to 12 months from filing to clearance. The PMA pathway is much more costly and uncertain than the 510(k) clearance process or de novo reclassification, and generally takes at least 12 to 18 months, or even longer, from the time the application is filed with FDA to ultimate approval.
We are not aware of any legally marketed predicate device upon which FDA could base a determination of substantial equivalence under a 510(k) clearance process. Our strategy therefore is to submit a direct de novo reclassification petition for our system. To support this petition, our objective is to demonstrate that the device poses a low to moderate risk to patients. We cannot assure you that FDA will not demand that we obtain PMA approval of our system.
FDA can delay, limit or deny clearance or approval of an application for many reasons, including, among others:
|
•
|
we may not be able to demonstrate to FDA’s satisfaction that our products are safe and effective for their intended use;
|
|
•
|
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval;
|
|
•
|
in the case of a PMA submission, that the manufacturing process or facilities we use may not meet applicable requirements; and
|
|
•
|
changes in FDA’s 510(k) clearance, de novo reclassification, or PMA approval processes and policies, or the adoption of new regulations may require additional data.
|
We may not obtain the necessary regulatory clearances, approvals, CE Certificates of Conformity or equivalent third country approvals to market our system or future products in the United States or elsewhere. Any delay in, or failure to receive or maintain, clearance, approval or CE Certificates of Conformity for our system or other products under development could prevent us from generating revenue from these products or achieving profitability.
There is no guarantee that the FDA will grant de novo reclassification or PMA approval of our system and failure to obtain necessary 510(k) clearances or approvals for our future products would adversely affect our ability to grow our business.
Our system and some of our future products will require FDA clearance of a 510(k), de novo reclassification, or may require FDA approval of a PMA. The FDA may not approve or clear our system or our future products for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance, de novo reclassification or PMA for our system or any other future product, new intended uses or modifications to these products once they are cleared or approved for marketing.
Our strategy is to submit a direct de novo reclassification petition for our system. A de novo reclassification generally applies where there is no predicate device and the FDA believes the device poses a low to moderate risk. De novo reclassifications can either be submitted in lieu of a 510(k) notice, such as in our case, or after a 510(k) notice has been filed and found NSE. If a 510(k) notice is found NSE, a de novo petition must be submitted within 30 days from the receipt of the NSE determination.
To support our direct de novo reclassification petition, our objective is to demonstrate that the device poses a low to moderate risk to patients. If the FDA determines that our system is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the device for its intended use. By statute, the FDA has 180 days to review the “accepted application,” although, generally, review of the application can take between one and three years. During this review period, the FDA may request additional information or clarification of information already provided or even request new data that may require us to conduct additional tests. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations. The FDA’s review of a PMA could significantly delay our plans to get to market. There is also no guarantee that the FDA would approve a PMA. Failure to receive clearance or approval for our system or future products would have an adverse effect on our ability to expand our business.
13
If we or our future distributors do not obtain and maintain the necessary regulatory clearances or approvals, or CE Certificates of Conformity, or equivalent third country approvals in a specific country or region, we will not be able to market and sell our system or future products in that country or region.
We intend to market our system in a number of international markets. To be able to market and sell our system in a specific country or region, we and/or our distributors must comply with the regulations of that country or region. While the regulations of some countries do not impose barriers to marketing and selling part or all of our products or only require notification, others require that we and/or our distributors obtain the approval of a specified regulatory authorities or that we obtain CE Certificates of Conformity from a Notified Body. We are engaged with Dekra Certification as our Notified Body for such purposes. These regulations, including the requirements for approvals or CE Certificates of Conformity, and the time required for regulatory review, vary from country to country. Obtaining regulatory approvals or CE Certificates of Conformity is expensive and time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals or CE Certificates of Conformity for our system or any future products in each country or region in which we plan to market such products. If we modify our system or any future products, we or our distributors may need to apply for new regulatory approvals or our Notify Body may need to review the planned changes before we are permitted to sell them. We may not meet the quality and safety standards required to maintain the authorizations or CE Certificates of Conformity that we or our distributors have received. If we or our distributors are unable to maintain our authorizations or CE Certificates of Conformity in a particular country or region, we will no longer be able to sell our system or any future products in that country or region, and our ability to generate revenues will be materially and adversely affected.
Our system may be considered a drug-device combination product because of the preparatory use of Iodine or barium sulfate to provide a coating for colonic imaging. We cannot be sure how the FDA or the competent regulatory authorities of foreign countries will regulate this product. The review of combination products is often more complex and more time consuming than the review of products under the jurisdiction of only one center within the FDA.
Our system may be considered a combination product because of the preparatory use of barium sulfate or Iodine to provide a coating for colonic imaging. A combination product is the combination of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are combined or mixed and produced as a single entity; packaged together in a single package or as a unit; or a drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication or effect. For a combination product, the FDA must determine which center or centers within the FDA will review the product and under what legal authority the product candidate will be reviewed. The combination product’s primary mode of action is used to determine which center within the FDA has primary regulatory jurisdiction over the product. The other centers within the agency also may provide consulting or collaborative reviews of the product as necessary. We believe that we have put forth a reasonable argument to the FDA that our system should be regulated as a device and or a combination product with a device primary mode of action. However, we cannot be sure as to whether the FDA will treat our system as a device or a combination product. The review of combination products is often more complex and more time consuming than the review of a product under the jurisdiction of only one center within the FDA. In the case of the system, should the FDA determine that the barium sulfate is not being used in accordance with its approved labeling, the Center for Drug Evaluation and Research may take a prominent role it its regulation. If the FDA does not approve or clear our system, or any future products, in a timely fashion, or at all, our business and financial condition will be adversely affected.
Similar obstacles may be encountered in foreign countries should our system be considered as a combination product.
14
If the indications for use or instructions for use for which the Iodine-based contrast agent or the barium sulfate- based contrast agent is approved are not sufficiently broad to support its use prior to the ingestion of our capsules, the FDA or the competent regulatory authorities in the EU Member States and other foreign countries may consider that contrast agent is being used off-label.
Ingestion of our system requires the preparatory use of Iodine or barium sulfate to provide a coating for colonic imaging. We cannot be sure that the indications for which Iodine-based contrast agent or the barium sulfate-based contrast agent are approved in the United States, the EU Member States or in other countries is sufficiently broad to cover such use. If the FDA or the competent regulatory authorities in the EU Member States and in other countries consider that Iodine and/or barium sulfate is not approved for the purpose for which it is used with the system, we may be considered to promote the off-label use of the Iodine and/or barium sulfate. Because the promotion of off-label use of drugs or medicinal products is prohibited in the United States, the EU Member States and in other countries, we could face both related issues with the FDA and/or the competent authorities of the EU Member States and/or other countries. In these circumstances, the FDA and/or the competent regulatory authorities in the EU Member States and/or other countries may require us to obtain appropriate regulatory approvals for the Iodine-based contrast agent or the barium sulfate-based contrast agent prior to marketing our system with such substances. Under such circumstances, should we fail to obtain approval of the contrast agent for use with our system, in a timely fashion, or at all, our business and financial condition will be adversely affected.
If we are unable to successfully complete clinical trials with respect to our system, we may be unable to receive regulatory approvals or clearances, CE Certificates of Conformity or equivalent third country approvals for our system and/or our ability to achieve market acceptance of our system will be harmed.
The development of medical devices typically includes pre-clinical studies. Certain other devices require the submission of data generated from clinical trials, which can be long, expensive and uncertain processes, subject to delays and failure at any stage. The data obtained from the studies and trials may be inadequate to support regulatory clearances or approvals, or to obtain CE Certificates of Conformity or equivalent third country approval, or to allow market acceptance of the products being studied. Our system technology is currently undergoing clinical development and clinical trials. To date, we have performed clinical studies with several versions of our system and with several versions of our non-scanning capsules.
The development of sufficient and appropriate clinical protocols to demonstrate safety and efficacy are required, and we may not adequately develop such protocols to support clearance, approval, or to obtain CE Certificates of Conformity or equivalent third country approval. The clinical trials that were conducted using prior versions of our system, were conducted under differing protocols and used groups of patients different from those we intend to study in future clinical trials. Further, FDA, the competent regulatory authorities of other countries, or our Notified Body in the EU may require us to submit data on a greater number of patients than we originally anticipated and/or for a longer follow-up period or they may change the data collection requirements or data analysis applicable to our clinical trials.
The commencement or completion of any of our clinical studies or trials may be delayed or halted, or be inadequate to support regulatory clearance, approval or product acceptance, or to obtain CE Certificates of Conformity or equivalent third country approval, for numerous reasons, including, among others:
|
•
|
patients do not enroll in the clinical trial at the rate we expect;
|
|
•
|
patients do not comply with trial protocols;
|
|
•
|
patient follow-up is not at the rate we expect;
|
|
•
|
undetected capsule retention in patients
|
|
|
•
|
patients experience adverse side effects, including related to excessive radiation exposure as a result of capsule malfunction or break down;
|
|
•
|
patients die during a clinical trial, even though their death may be unrelated to our product;
|
|
•
|
FDA, institutional review boards, or IRBs, or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold;
|
|
•
|
IRBs, Ethics Committees and third-party clinical investigators may delay or reject our trial protocol and Informed Consent Form;
|
15
|
•
|
third-party clinical investigators decline to participate in a study or trial or do not perform a study or trial on our anticipated schedule or consistent with the investigator agreements, study or trial protocol, good clinical practices or other FDA or IRBs, Ethics Committees, or any other applicable requirements;
|
|
•
|
third-party organizations do not perform data collection, monitoring and analysis in a timely or accurate manner or consistent with the study or trial protocol or investigational or statistical plans;
|
|
•
|
regulatory inspections of our studies, trials or manufacturing facilities may require us to, among other things, undertake corrective action or suspend or terminate our studies or clinical trials;
|
|
•
•
|
changes in governmental regulations or administrative actions;
we may not be able to develop our system at the rate or to the stage we desire:
|
|
•
|
the interim or final results of the study or clinical trial are inconclusive or unfavorable as to safety or efficacy;
|
|
•
•
|
a regulatory agency or our Notified Body concludes that our trial design is or was inadequate to demonstrate safety and efficacy; and
If we not continue to obtain a permit to employ Jewish employees on Saturdays and Jewish holidays to conduct our clinical trials, as required under the Israeli Hours of Work and Rest Law, 1951, and we are unsuccessful in employing only non-Jewish employees on Jewish rest days and holidays, we may be compelled to cease or halt our clinical trials during Saturdays and Jewish holidays, which could decrease our clinical capacity.
|
The results of pre-clinical and clinical studies do not necessarily predict future clinical trial results, and predecessor clinical trial results may not be repeated in subsequent clinical trials. Additionally, FDA, the competent regulatory authorities of EU Member States, other third country regulatory entities, or our Notified Body may disagree with our interpretation of the data from our pre-clinical studies and clinical trials, or may find the clinical trial design, conduct or results inadequate to demonstrate safety or efficacy, and may require us to pursue additional pre-clinical studies or clinical trials, which could further delay the clearance, approval, or CE Certificate of Conformity of our products. The data we collect from our non-clinical testing, our pre-clinical studies and other clinical trials may not be sufficient to support regulatory clearance, approval or to obtain CE Certificates of Conformity.
If the third parties on which we rely to conduct our clinical trials and clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval, CE Certificates of Conformity, or equivalent third country approval for, or commercialize, our system or future products.
We do not have the ability to independently conduct our clinical trials for our system and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct such trials. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain CE Certificates of Conformity, regulatory clearance, approval for, or successfully commercialize, our system or future products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
The results of our current or future clinical trials may not support our product candidate requirements or intended use claims or may result in the discovery of adverse side effects.
Even if our current or future clinical trials are completed as planned, we cannot be certain that their results will support our product requirements or intended use claims, which could inhibit our marketing strategies, or that the FDA, foreign authorities or our Notified Body will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that clinical trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our system, or any future products, are safe and effective for the desired or proposed indicated uses, which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our product submissions and, ultimately, our ability to commercialize our system, or any future products, and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
16
Even if our system or future products are cleared or approved by regulatory authorities or if we obtain CE Certificates of Conformity from our Notified Body, modifications to our system or future products may require new regulatory clearances or approvals, new CE Certificates of Conformity, or may require us to recall or cease marketing it until the necessary clearances, approvals or CE Certificates of Conformity are obtained.
Once marketed, modifications to our system or future products may require new regulatory approvals, clearances, including 510(k) clearances or premarket approvals, or require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. Any modification to a 510(k)-cleared device that could significantly affect its safety or efficacy, or that would constitute a major change in its intended use, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance. A manufacturer may determine that a modification could not significantly affect safety or efficacy and does not represent a major change in its intended use, so that no new 510(k) clearance is necessary. However, the FDA can review a manufacturer’s decision and may disagree. The FDA may also on its own initiative determine that a new clearance or approval is required. We may make modifications to our system in the future that we believe do not or will not require additional clearances or approvals. Further, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective. Any recall or FDA requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
If a manufacturer determines that a modification to an FDA-cleared device could significantly affect its safety or efficacy, or would constitute a major change in its intended use, then the manufacturer must file for a new 510(k) clearance or possibly a premarket approval application. Where we determine that modifications to our products require a new 510(k) clearance or premarket approval application, we may not be able to obtain those additional clearances or approvals for the modifications or additional indications in a timely manner, or at all.
Any modification to a PMA-approved device must either be approved in a PMA Supplement, or if the modification does not impact the device’s safety or effectiveness, described in a 30-Day Notice or in the device’s Annual Report. The FDA may not approve a modification described in a PMA Supplement, in which case the modified device cannot be marketed. The FDA can also disagree that a change described in a 30-Day Notice or Annual Report is appropriately described in either filing, and request that the company file a PMA Supplement and/or request that the company cease marketing the modified device until the PMA Supplement is approved.
Similar rules also apply in foreign jurisdictions. In the European Union, or EU, we must inform the Notified Body that carried out the conformity assessment of the medical devices we market or sell in the EU of any planned substantial changes to our quality system or changes to our devices which could affect compliance with the Essential Requirements laid down in Annex I to the Council Directive 93/42/EEC concerning medical devices, or Medical Devices Directive, or the devices’ intended purpose. The Notified Body will then assess the changes and verify whether they affect the products’ conformity with the Essential Requirements laid down in Annex I to the Medical Devices Directive or the conditions for the use of the device. If the assessment is favorable, the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive.
If the Notified Body or relevant regulatory authorities disagree with our assessments and require modifications to an existing CE Certificate of Conformity, the preparation of a new CE Certificates of Conformity or new regulatory clearances or approvals for modifications, we may be required to recall and to stop marketing the modified devices.
Obtaining clearances and approvals, or new or amended CE Certificates of Conformity for device modifications can be a time consuming process, and delays in obtaining required future clearances, approvals, or CE Certificates of Conformity would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
17
Even if our system and future products are cleared or approved by regulatory authorities or if we obtain CE Certificates of Conformity from our Notified Body, if we or our suppliers fail to comply with ongoing FDA or other foreign regulatory authority requirements, or if we experience unanticipated problems with our products, our products could be subject to restrictions or withdrawal from the market.
The manufacturing processes, reporting requirements, post-approval clinical data and promotional activities associated with any product for which we obtain clearance or approval CE Certificates of Conformity, or equivalent third country approval will be subject to continuous regulatory review, oversight and periodic inspections by FDA other domestic and foreign regulatory authorities and our Notified Body. In particular, we and certain of our suppliers are required to comply with FDA’s Quality System Regulations, or QSR, as well as current good manufacturing practices, or cGMP. In the EU, we will also be subject to the quality system requirements laid down in the Annexes to the Medical Devices Directive. Such compliance can be facilitated by, among other things, a certificate of compliance with ISO 13485:2003. Through compliance with the ISO 13485:2003 standard, we will benefit from a presumption of conformity with the relevant quality system requirements laid down in the Annexes to Medical Devices Directive. These regulations and standards govern the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval, CE Certificates of Conformity, or equivalent third country approval. Regulatory authorities, such as FDA, and our Notified Body enforce the QSR and other regulations through periodic inspections. The failure by us or one of our suppliers to comply with applicable statutes and regulations falling within the competence of FDA and other regulatory authorities or our Notified Body, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, any of the following enforcement actions:
|
•
|
untitled letters, warning letters, fines, injunctions, corporate integrity agreements, consent decrees and civil penalties;
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
•
|
customer notifications for repair, replacement or refunds;
|
|
•
|
recall, detention or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products;
|
|
•
|
operating restrictions;
|
|
•
|
withdrawing 510(k) clearances on PMA approvals that have already been granted;
|
|
•
|
suspension or withdrawal of our CE Certificates of Conformity;
|
|
•
|
refusal to grant export approval for our products; or
|
|
•
|
criminal prosecution.
|
If any of these actions were to occur, it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, or if we obtain CE Certificates of Conformity, such clearance or approval, or CE Certificates of Conformity may be subject to limitations on the intended uses for which the product may be marketed and reduce our potential to successfully commercialize the product and generate revenue from the product. If FDA or the competent regulatory authorities of foreign countries determines that our promotional materials, labeling, training or other marketing or educational activities constitute the promotion of an unapproved use or the promotion of an intended purpose not covered by our CE mark, they could request that we cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with medical device reporting requirements, including the reporting of adverse events and malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse side effects or adverse side effects of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements such as QSR, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension or withdrawal of regulatory approvals or CE Certificates of Conformity, product seizures, injunctions or the imposition of civil or criminal penalties, all of which would adversely affect our business, financial condition and operating results and prospects.
18
If we fail to maintain necessary regulatory clearances or CE Certificates of Conformity for our system and indications in our target foreign markets, if clearances or approvals, or CE Certificates of Conformity for future products and indications are delayed or not issued, or if there are regulatory changes in our existing or future target markets, our commercial operations could be harmed.
Our system is a medical device that is subject to extensive regulations that are intended to assure its safety, effectiveness and compliance with applicable consumer laws. If we fail to obtain and maintain these regulatory approvals or clearances, or CE Certificates of Conformity, our ability to sell our system and generate revenues will be materially harmed.
These laws and regulations relate to the design, development, testing, manufacturing, storage, labeling, packaging, content and language of the instructions for use of the device, sale, promotion, distribution, importing and exporting, shipping, post-sale surveillance and recall from our system’s markets, and all countries in which we intend to sell our system apply some form of regulations of this kind. Most notably, we must comply with the Medical Devices Directive and are subject to extensive regulation in the United States by FDA and other federal, state and local authorities. In the EU, compliance with the requirements laid down in the Medical Devices Directive, including the Essential Requirements laid down its Annex I thereto, is a prerequisite to be able to affix the CE mark of conformity to our medical devices. Without such CE mark, our products cannot be marketed or sold in the EU. To demonstrate compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Apart from low risk medical devices (Class I with no measuring function and which are not sterile), in relation to which the manufacturer can make an EC Declaration of Conformity based on self-assessment of the conformity of its products with the Essential Requirements laid down in Annex I to the Medical Devices Directive, a conformity assessment procedure requires the intervention a Notified Body. The Notified Body would typically audit and examine products’ Technical File, which we must create, and the quality system for manufacture, design and final inspection of our devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements laid down in Annex I to the Medical Devices Directive or the quality system requirements laid down in the other Annexes to the Directive. Following the issuance of this CE Certificate of Conformity, we can draw up an EC Declaration of Conformity and affix the CE mark to the products covered by this CE Certificate of Conformity and by the EC Declaration of Conformity Other countries outside the EU also accept the CE mark as a certification of quality, efficacy and safety of medical devices and an element of related authorization of the products in their territory.
We will be subject to annual audits by a Notified Body under the Medical Devices Directive. During this audit, the third-party assessor or Notified Body will examine the maintenance and implementation of our quality control system, device post-marketing vigilance system and any changes or modifications made to our products.
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the EU. These proposals are intended to strengthen the medical devices rules in the EU. On October 22, 2013, the European Parliament voted in favor of an amended draft of the Regulation. The proposed text is currently being discussed by the Council of the European Union. These adopted or expected regulatory changes may adversely affect our business, financial condition and results of operations or restrict our operations.
Our failure to comply with radiation safety or radio frequency regulations in a specific country or region could impair our ability to commercially distribute and market our system in that country or region.
Our system includes a small X-ray source and wireless radio frequency transmitter and receiver, and is therefore subject to equipment authorization requirements in a number of countries and regions. In the United States, the EU and Japan, authorities often require advance clearance of all radiation and radio frequency devices before they can be sold or marketed in these jurisdictions, subject to limited exceptions. Modifications to the approved system design and specifications may require new or further regulatory clearances or approvals before we are permitted to market and sell a modified system. If we are unable to obtain any required clearances or approvals from the authorities responsible for the radiation as well as the radio frequency regulations in these and other jurisdictions, the sale or use of our system could be prevented in these countries. Any such action could negatively affect our business, financial condition and results of operations.
19
Our system may in the future be subject to product recalls that could harm our reputation, business and financial results.
FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture or a public health/safety issue. In the case of FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Once marketed, recalls of any of our products, including our system, would divert managerial and financial resources and have an adverse effect on our business, financial condition and results of operations. FDA requires that certain classifications of recalls be reported to FDA within 10 working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to FDA. We may initiate voluntary recalls involving our products in the future that we determine do not require us to notify FDA. If FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, FDA could take enforcement action against us based on our failure to report the recalls when they were conducted.
If our system or future products cause or contribute to a death or a serious injury, or malfunction in such a way that causes or contributes to a death or serious injury, we will be subject to medical device reporting regulations, which can result in corrective actions or enforcement actions from regulatory authorities.
Under FDA medical device reporting regulations, medical device manufacturers are required to report to FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of our device (or any similar future product) were to recur. If we fail to investigate and report these events to FDA within the required timeframes, or at all, FDA could take enforcement action against us. Any such adverse event involving our products also could result in future corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, including any legal action taken against us, will require us to devote sufficient time and capital to the matter, distract management from operating our business, and may harm our reputation and financial results.
In addition, we must also comply with the EU Medical Device Vigilance System (MEDDEV 2.12/1 rev.8), which is intended to protect the health and safety of patients, users and others by establishing reporting procedures and reducing the likelihood of reoccurrence of incidents related to the use of a medical device. Under this system, incidents (which are defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, may lead to or may have led to the death of a patient, or user or other persons or to a serious deterioration in such person’s state of health) must be reported by manufacturers through a Manufacturer’s Incident Reports to competent authorities within periods of time specified in the MEDDEV 2.12/1 rev. 8. Such incidents are evaluated and, where appropriate, information is disseminated between the competent authorities of the EU Member States. The MEDDEV 2.12/1 rev. 8 is also intended to facilitate a direct, early and harmonized establishment of Field Safety Corrective Actions, or FSCAs, across the EU Member States in which the device is being marketed. An FSCA is an action taken by a manufacturer to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An FSCA may include device recall, modification, exchange, or destruction. FSCAs must be reported by the manufacturer or the manufacturer’s European Authorized Representative, to its customers and/or the end users of the device through a Field Safety Notice. FSCAs must also be reported to the competent authorities of the EU Member States.
Our business is subject to complex environmental legislation that may increase our costs and our risk of noncompliance.
Our research and development and manufacturing processes involve the handling of potentially harmful radioactive and other hazardous materials. We are subject to local laws and regulations governing the use, shipping, handling, storage and disposal of these materials, and we incur expenses related to compliance with these laws and regulations. If we are found to have violated environmental, health and safety laws, whether as a result of human error, equipment failure or other causes, we could be held liable for damages, penalties and costs of remedial actions which could materially adversely affect our business, financial condition and results of operations. In the future, we could be subject to additional environmental requirements or existing environmental laws could become more stringent, which could lead to greater compliance costs and increasing risks and penalties associated with violations. For example, changes to, or restrictions on, permitting requirements or processes, hazardous or radioactive material storage or handling might require an unplanned capital investment or relocation. If we fail to comply with existing or new environmental laws or regulations, our business, financial condition and results of operations could be materially adversely affected.
20
If we are unable to achieve reimbursement and coverage from third-party payors for procedures using our system, or if reimbursement is insufficient to create an economic benefit for purchasing or using our system when compared to alternative procedures, demand for our products may not grow at the rate we expect.
The demand for our system will depend significantly on the eligibility of the procedures performed using our system for reimbursement through government-sponsored healthcare payment systems and private third-party payors. Reimbursement practices vary significantly from country to country and within some countries, by region, and we must obtain reimbursement approvals on a country-by-country and/or region-by-region basis. In general, the process of obtaining reimbursement and coverage approvals has been longer outside of the United States. We may not be able to obtain reimbursement approvals in a timely manner or at all and existing reimbursement and coverage policies may be revised from time to time by third-party payors. If physicians, hospitals and other healthcare providers are unable to obtain sufficient coverage and reimbursement from third-party payors for procedures using our system, if reimbursement is, or is perceived by our customers to be, insufficient to create an economic incentive for purchasing or using our system, or if such reimbursement does not adequately compensate physicians and health care providers compared to the other procedures they offer, demand for our products may not grow at the rate we expect.
Federal and state privacy laws, and equivalent laws of third countries, may increase our costs of operation and expose us to civil and criminal sanctions.
The Health Insurance Portability and Accountability Act of 1996, as amended, and the regulations that have been issued under it, to which we refer collectively as HIPAA, and similar laws outside the United States, contain substantial restrictions and requirements with respect to the use and disclosure of individuals’ protected health information. The HIPAA privacy rules prohibit “covered entities,” such as healthcare providers and health plans, from using or disclosing an individual’s protected health information, unless the use or disclosure is authorized by the individual or is specifically required or permitted under the privacy rules. Under the HIPAA security rules, covered entities must establish administrative, physical and technical safeguards to protect the confidentiality, integrity and availability of electronic protected health information maintained or transmitted by them or by others on their behalf. While we do not believe that we are a covered entity under HIPAA, many of our customers are covered entities subject to HIPAA. Such customers may require us to enter into business associate agreements, which will obligate us to safeguard certain health information we obtain in the course of our relationship with them, restrict the manner in which we use and disclose such information and impose liability on us for failure to meet our contractual obligations.
In addition, under The Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, which was signed into law as part of the U.S. stimulus package in February 2009, certain of HIPAA’s privacy and security requirements are now also directly applicable to “business associates” of covered entities and subject them to direct governmental enforcement for failure to comply with these requirements. We may be deemed as a “business associate” of some of our customers. As a result, we may be subject as a “business associate” to civil and criminal penalties for failure to comply with applicable privacy and security rule requirements. Moreover, HITECH created a new requirement obligating “business associates” to report any breach of unsecured, individually identifiable health information to their covered entity customers and imposes penalties for failing to do so.
In addition to HIPAA, most U.S. states have enacted patient confidentiality laws that protect against the disclosure of confidential medical information, and many U.S. states have adopted or are considering adopting further legislation in this area, including privacy safeguards, security standards, and data security breach notification requirements. These U.S. state laws, which may be even more stringent than the HIPAA requirements, are not preempted by the federal requirements, and we are therefore required to comply with them to the extent they are applicable to our operations.
These and other possible changes to HIPAA or other U.S. federal or state laws or regulations, or comparable laws and regulations in countries where we conduct business, could affect our business and the costs of compliance could be significant. Failure by us to comply with any of the standards regarding patient privacy, identity theft prevention and detection, and data security may subject us to penalties, including civil monetary penalties and in some circumstances, criminal penalties. In addition, such failure may damage our reputation and adversely affect our ability to retain customers and attract new customers.
The protection of personal data, particularly patient data, is subject to strict laws and regulations in many countries. The collection and use of personal health data in the EU is governed by the provisions of Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data, commonly known as the Data Protection Directive. The Directive imposes a number of requirements including an obligation to seek the consent of individuals to whom the personal data relates, the information that must be provided to the individuals, notification of data processing obligations to the competent national data protection authorities of individual EU Member States and the security and confidentiality of the personal data. The Data Protection Directive also imposes strict rules on the transfer of personal data out of the EU to the US. Failure to comply with the requirements of the Data Protection Directive and the related national data protection laws of the EU Member States may result in fines and other administrative penalties and harm our business. We may incur extensive costs in ensuring compliance with these laws and regulations, particularly if we are considered to be a data controller within the meaning of the Data Protection Directive.
21
The adoption of healthcare reform and deficit reduction measures in the United States may adversely affect our business and financial results.
On March 23, 2010, President Obama signed into law major healthcare reform legislation under the Patient Protection and Affordable Care Act of 2010, or the PPACA, which was modified on March 30, 2010 by the enactment of the Health Care and Education Reconciliation Act of 2010. This law substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the device industry. The PPACA is intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, and impose additional health policy reforms. Under the PPACA, it is expected that expanded healthcare coverage will be made available to millions of Americans. The increased costs to the U.S. government from the PPACA are expected to be funded through a combination of payment reductions for providers over time and several new taxes. The PPACA imposes, among other things, an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States beginning in 2013, resulting in an anticipated cost to the medical device industry of up to $20 billion over the next decade. We likely will be subject to the excise tax with respect to our system if it is approved for sale in the United States. The PPACA also limits the rate of growth in Medicare payments to providers and authorizes certain voluntary demonstration projects beginning no later than 2013 around development of bundling payments for acute, inpatient hospital services, physician services, and post acute services for episodes of hospital care. In addition, the PPACA provides for the establishment of an Independent Payment Advisory Board, or IPAB, that, beginning in 2014, could recommend changes in Medicare payments to physicians and other providers that would take effect unless Congress passes an alternative measure to achieve the same amount of savings. The IPAB has not yet been created. The PPACA also increases fraud and abuse penalties and expands the scope and reach of the Federal Civil False Claims Act and government enforcement tools, which may adversely impact healthcare companies.
The U.S. Supreme Court heard a constitutional challenge to the PPACA and in June 2012 held that the PPACA is constitutional. However, states are allowed to opt out of the expansion of eligibility criteria for Medicaid under the PPACA and many states have chosen to do so, causing many uninsured patients to remain without coverage. In addition to the PPACA, the effect of which cannot presently be quantified given its recent enactment, various healthcare reform proposals have also emerged at the state level. We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or the effect any future legislation or regulation will have on us. However, we anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for any approved product, and could adversely affect our business. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Insurers may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals, all of which may adversely affect our business, financial condition and results of operations, possibly materially.
In addition to health reform, other deficit reduction measures could affect reimbursement for our device and related procedures. For example, beginning April 1, 2013, Medicare payments for all items and services have been reduced by 2% under the sequestration (i.e., automatic spending reductions) required by the Budget Control Act of 2011, as amended by the American Taxpayer Relief Act of 2012. These cuts will remain in effect until 2024 unless Congress enacts legislation to cancel or delay the cuts. These payment reductions, or similar efforts to reduce Medicare spending to control the federal deficit, could adversely affect our business by reducing reimbursement to the providers who purchase and use our devices and perform related procedures.
The implementation of the reporting and disclosure obligations of the Physician Payment Sunshine Act provisions of health care reform could adversely affect our business. A health care reform provision, generally referred to as the Physician Payment Sunshine Act or Open Payments Program, has imposed new reporting and disclosure requirements for drug and device manufacturers with regard to payments or other transfers of value made to certain practitioners (including physicians, dentists and teaching hospitals), and for such manufacturers and for group purchasing organizations, with regard to certain ownership interests held by physicians in the reporting entity. On February 1, 2013, the Centers for Medicare and Medicaid Services, or CMS, released the final rule to implement the Physician Payment Sunshine Act. As required under the Physician Payment Sunshine Act, CMS will publish information from these reports on a publicly available website, including amounts transferred and physician, dentist and teaching hospital identities.
22
The final rule implementing the Physician Payment Sunshine Act is complex, ambiguous, and broad in scope. If we participate in federal healthcare programs, meaning our product is reimbursed by a federal healthcare program such as Medicare, Medicaid, or Children’s Health Insurance Program, our product would be considered a “covered device.” Within 180 days of becoming “covered,” we would be required to collect and report detailed information regarding certain financial relationships we have with physicians and teaching hospitals. The Physician Payment Sunshine Act preempts similar state reporting laws, although we may be required to report under certain of such state laws. Our compliance with the new final rule imposes additional costs on us and requires additional resources including dedicated personnel with experience and expertise in this area. Failure to comply may expose us to federal and/or state enforcement action and fines.
If we fail to comply with the U.S. federal Anti-Kickback Statute and similar state and third country laws, we could be subject to criminal and civil penalties and exclusion from federally funded healthcare programs including the Medicare and Medicaid programs and equivalent third country programs, which would have a material adverse effect on our business and results of operations.
A provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration, directly or indirectly, in cash or in kind, to induce or reward the referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable, in whole or in part, by Medicare, Medicaid or any other federal healthcare program. PPACA, among other things, clarified that a person or entity needs not to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor may be subject to scrutiny. The federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states have adopted laws similar to the federal Anti-Kickback Statute, and some of these laws are even broader than the federal Anti-Kickback Statute in that their prohibitions may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
All of our financial relationships with healthcare providers, purchasers, formulary managers, and others who provide products or services to federal healthcare program beneficiaries are potentially governed by the federal Anti-Kickback Statute and similar state laws. We believe our operations are in compliance with the federal Anti-Kickback Statute and similar state laws. However, we cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
There are other federal and state laws that may affect our ability to operate, including the federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. PPACA amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Moreover, we may be subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs. Moreover, there are analogous state laws. Violations of these laws can result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
Similar restrictions are imposed by the national legislation of many third countries in which our medical devices will be marketed. Moreover, the provisions of the Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more aggressive and frequent investigations and enforcement by both the U.S. Securities and Exchange Commission and the Department of Justice. A determination that our operations or activities violated United States or foreign laws or regulations could result in imposition of substantial fines, interruption of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. In addition, lawsuits brought by private litigants may also follow as a consequence.
23
If the U.S. Nuclear Regulatory Commission, or NRC, or other nuclear regulatory commissions around the world, would take the position that a system containing radioactive material cannot be passed in excreta into the sanitary sewer system without limitation, we may be subject to further regulations and patients may be required to retrieve our system after use.
As our system includes an ingestible capsule with a radioactive source, we must address NRC regulations in addition to FDA requirements as well as regulations of other nuclear regulatory commissions in jurisdictions in which we intend to commercialize our system. Our system is loaded with the X-ray source, sealed and then ingested by the patient. Although the NRC places conditions and limitations on the disposal of radioactive material in the sanitary sewer, such conditions and limitations do not apply to radioactive material contained in the excreta of individuals that are undergoing medical diagnosis or therapy with radioactive material. However, there is no assurance that the NRC or other regulatory commissions worldwide will take a similar position in relation to our system and we may face limitations by the NRC or other nuclear regulatory commissions in jurisdictions in which we intend to commercialize our system in relation to the disposal of our capsule in the sanitary system, such as requiring patients to retrieve our system after use, which could make our system less attractive.
Our failure to comply with the necessary regulatory approval regarding the use of radioactive materials could significantly impair our ability to develop, manufacture and/or sell our system.
The manufacture of our system requires the use and storage of radioactive materials. In order to use such materials in the development and manufacture of our system in Israel, we are required to obtain a permit from the Israeli Commissioner for Environmental Radiation, or the Commissioner, pursuant to the Israeli Pharmaceutical Regulations (Radioactive Elements and By-Products) – 1980. Should we fail to comply with the conditions of our currently existing permit, the Commissioner would have authority to cancel our permit. Should the Commissioner determine that our activities or facilities constitute a danger to the health and well-being of a person, the public or the environment, the cancellation of our permit could be immediate and without prior notice. Furthermore, we cannot guarantee the annual renewal of our permit and/or annual renewal subject to identical conditions, as the approval of an annual application and the conditions thereof are at the discretion of the Commissioner. Similar requirements and regulations may apply to the manufacture of our system in other countries. Cancellation of or failure to renew our permit could have materially adverse consequences on our ability to manufacture and sell our products and therefore on our ability to continue our business and operations.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights, our competitive position could be harmed.
Our success and ability to compete depends in large part upon our ability to protect our intellectual property. Although we have patents issued in Israel, Europe, United States, Japan, China, India, Hong Kong, and Australia, we continue to file and prosecute in many of the same countries and additional countries such as Canada and South Korea. We face several risks and uncertainties in connection with our intellectual property rights, including, among others:
|
•
|
pending and future patent applications may not result in the issuance of patents or, if issued, may not be issued in a form that will be advantageous to us;
|
|
•
|
our issued patents may be challenged, invalidated or legally circumvented by third parties;
|
|
•
|
our patents may not be upheld as valid and enforceable or prevent the development of competitive products;
|
|
•
|
the eligibility of certain inventions related to diagnostic medicine, more specifically diagnostic methods and processes, for patent protection in the United States has been limited recently which may affect our ability to enforce our issued patents in the United States or may make it difficult to obtain broad patent protection going forward in the United States;
|
|
•
|
for a variety of reasons, we may decide not to file for patent protection on various improvements or additional features; and
|
|
•
|
intellectual property protection and/or enforcement may be unavailable or limited in some countries where laws or law enforcement practices may not protect our proprietary rights to the same extent as the laws of the United States, the European Union, Canada or Israel.
|
24
Consequently, our competitors could develop, manufacture and sell products that directly compete with our products, which could decrease our sales and diminish our ability to compete. In addition, competitors could attempt to develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property does not adequately protect us from our competitors’ products and methods, our competitive position could be materially adversely affected.
Because the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our system.
There is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. We are presently unaware of any other parties’ valid patents and proprietary rights which our evolving product designs would infringe. Searches typically performed to identify potentially infringed patents of third parties are often not conclusive and, because patent applications can take many years to issue, there may be applications now pending, which may later result in issued patents which our current or future products may infringe. In addition, our competitors or other parties may assert that our system and the methods it employs may be covered by patents held by them. If our system infringes a valid patent, we could be prevented from manufacturing or selling it unless we can obtain a license or redesign the product to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid infringement. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and could divert our management’s attention from operating our business.
The steps we have taken to protect our intellectual property may not be adequate, which could have a material adverse effect on our ability to compete in the market.
In addition to patents, we rely on confidentiality, non-compete, non-disclosure and assignment of inventions provisions, as appropriate, with our employees, consultants and, to some extent, our distributors, to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not be adequate to protect our intellectual property from unauthorized disclosure, third-party infringement or misappropriation, for the following reasons:
|
•
|
the agreements may be breached, may not provide the scope of protection we believe they provide or may be determined to be unenforceable;
|
|
•
|
we may have inadequate remedies for any breach;
|
|
•
|
proprietary information could be disclosed to our competitors; or
|
|
•
|
others may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technologies.
|
Specifically with respect to non-compete agreements, under current United States and Israeli law, we may be unable to enforce these agreements, in whole or in part, and it may be difficult for us to restrict our competitors from gaining the expertise that our former employees gained while working for us. For example, Israeli courts have recently required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, we may be unable to prevent our competitors from benefiting from the expertise of our former employees. In addition, some states in the United States, such as California, have laws which severely restrict the use of non-compete undertakings.
If, for any of the above reasons, our intellectual property is disclosed or misappropriated, it could harm our ability to protect our rights and could have a material adverse effect on our business, financial condition and results of operations.
Furthermore, although our employees and consultants have agreed to assign to us all rights to any intellectual property created in the scope of their employment or engagement with us and most of our current employees and consultants, have agreed to waive their economic rights with respect to our intellectual property, we cannot assure you that such claims will not be brought against us by current or former employees or consultants, despite their contractual representations and obligations toward us, or by any of the medical and/or governmental institutions that employ or engage such consultants, claiming alleged rights to our intellectual property or demanding remuneration in consideration for assigned intellectual property rights, which could result in litigation and adversely affect our business, financial condition and results of operations.
25
Third-party claims of infringement or other claims against us could require us to redesign our system, seek licenses, or engage in future costly intellectual property litigation, which could negatively affect our future business and financial performance.
Substantial litigation over intellectual property rights exists in the medical device industry in general and in the medical imaging or screening sectors in particular. We expect that we may be subject to third-party infringement claims as our revenues increase, the number of competitors grows and the functionality of products and technology in different industry segments converges. Third parties may currently have, or may eventually be issued, patents on which our current or future products or technologies may infringe.
In addition, litigation in which we are accused of infringement may cause negative publicity, adversely impact prospective customers, cause product shipment delays, prohibit us from manufacturing, marketing or selling our current or future products, require us to develop non-infringing technology, make substantial payments to third parties or enter into royalty or license agreements, which may not be available on acceptable terms, or at all. If a successful claim of infringement were made against us and we could not develop non-infringing technology or license the infringed or similar technology in a timely and cost-effective manner, our ability to generate significant revenues may be substantially harmed and we could be exposed to significant liability. A court could enter orders that temporarily, preliminarily or permanently enjoin us or our customers from making, using, selling, offering to sell or importing our current or future products, or could enter an order mandating that we undertake certain remedial activities. Claims that we have misappropriated the confidential information or trade secrets of third parties can have a similar negative impact on our reputation, business, financial condition or results of operations.
We may also become involved in litigation in connection with our brand name rights. We do not know whether others will assert that our brand name infringes their trademark rights. In addition, names we choose for our products may be claimed to infringe names held by others. If we have to change the names we use, we may experience a loss in goodwill associated with our brand name, customer confusion and a loss of sales.
Third parties may challenge the validity of our issued patents or challenge patent applications in administrative proceedings before various patent offices which, if successful, could negatively affect our future business and financial performance.
Various patent offices, including in the United States and Europe, provide administrative proceedings by which a third party can challenge the validity of an issued patent or challenge an application that is being examined absent any threat of litigation. In some instances, including in the United States, the administrative proceedings provide a more efficient and favorable forum to challenge our patents which may lead to more opportunities for competitors to do so, particularly smaller competitors with limited resources. Moreover, the standards utilized in these administrative proceedings, at least in the United States, provide certain legal advantages versus challenging the validity of a patent in a district court. If a third party is successful in one of these administrative proceedings, the patent will no longer be enforceable in the corresponding jurisdiction. With this loss in patent rights, we will not be able to prevent third parties from offering identical or similar competing products which may result in lower profits and a less substantial market share.
We may need to initiate lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual property rights, which would harm our ability to compete in the market.
We rely on patents to protect a portion of our intellectual property and our competitive position. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in the medical device industry are generally uncertain. In order to protect or enforce our patent rights, we may initiate patent and related litigation against third parties, such as infringement suits or interference proceedings. Any lawsuits that we initiate could be expensive, take significant time and divert our management’s attention from other business concerns and the outcome of litigation to enforce our intellectual property rights in patents, copyrights, trade secrets or trademarks is highly unpredictable. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing. In addition, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, including attorney fees, if any, may not be commercially valuable. The occurrence of any of these events could have a material adverse effect on our business, financial condition and results of operations.
26
We may not be able to enforce covenants not to compete at all or, we may be unable to enforce them for the duration contemplated in our employment contracts and may, therefore, be unable to prevent competitors from benefiting from the expertise of some of our former employees involved in research and development activities.
We currently have non-competition agreements with substantially all of our employees who are involved in research and development, all of whom are located in Israel. These agreements prohibit our employees, if they cease working for us, from directly competing with us or working for our competitors for a limited period of time following termination of employment. In many jurisdictions, courts are increasingly refusing to enforce restrictions on competition by former employees or have interpreted them narrowly. For example, in Israel, where all of our employees reside, courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, an Israeli court may refuse to enforce our non-compete restrictions or reduce the contemplated period of non-competition such that we may be unable to prevent our competitors from benefiting from the expertise of our former employees.
Risks Related to Our Operations in Israel
Our principal offices, research and development facilities and some of our suppliers are located in Israel and, therefore, our business, financial condition and results of operation may be adversely affected by political, economic and military instability in Israel.
Our principal offices, research and development facilities are located in northern Israel. In addition, substantially all of our employees and officers, and certain of our directors, are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations.
During the Second Lebanon War of 2006, between Israel and Hezbollah, a militant Islamic movement, rockets were fired from Lebanon into Israel, including into the Haifa area, where our facility is located, causing casualties and major disruption of economic activities in northern Israel. An escalation in tension and violence between Israel and the militant Hamas movement (which controls the Gaza Strip) and other Palestinian Arab groups, culminated with Israel’s military campaign in Gaza in December 2008, in November 2012 and again in July and August 2014 in an endeavor to prevent continued rocket attacks against Israel’s southern towns, as well as other tension and violence between Israel and Palestinian Arab groups and individuals. It is unclear whether any negotiations that may occur between Israel and the Palestinian Authority will result in an agreement. In addition, Israel faces threats from more distant neighbors, in particular, Iran, an ally of Hezbollah and Hamas.
Popular uprisings in various countries in the Middle East and North Africa are affecting the political stability of those countries. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and these countries. Furthermore, several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in the region continue or intensify. Such restrictions may seriously limit our ability to sell our products to customers in those countries. Parties with whom we may do business could decline to travel to Israel during periods of heightened unrest or tension. In addition, the political and security situation in Israel may result in parties with whom we may have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements. In addition, any hostilities involving Israel could have a material adverse effect on our facilities including our corporate office or on the facilities of our local suppliers, in which event all or a portion of our inventory may be damaged, and our ability to deliver products to customers could be materially adversely affected. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or significant downturns in the economic or financial condition of Israel, could adversely affect our operations and product development, cause our revenues to decrease and adversely affect our share price. Similarly, Israeli corporations are limited in conducting business with entities from several countries.
27
Our commercial insurance policy does not cover losses associated with terrorist attacks. Although the Israeli government in the past covered the reinstatement value of certain damages that were caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts, terrorist activities or political instability in the region would likely negatively affect business conditions generally and could harm our results of operations.
Our operations could also be disrupted by the obligations of personnel to perform military service. As of February 15, 2016, we had 59 employees and independent contractors, all of whom were based in Israel. Some of these employees may be called upon to perform up to 54 days in each three year period (and in the case of military officers, up to 84 days in each three year period) of military reserve duty until they reach the age of 40 (and in some cases, depending on their specific military profession up to 45 or even 49 years of age) and, in certain emergency circumstances, may be called to immediate and unlimited active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists and it is possible that there will be similar large-scale military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of employees related to military service, which could materially adversely affect our business and results of operations.
Pursuant to the terms of the Israeli government grants we received for research and development expenditures, we are obligated to pay certain royalties on our revenues to the Israeli government. The terms of the grants require us to satisfy specified conditions and to make additional payments in addition to repayment of the grants upon certain events
We have received grants from the Government of the State of Israel through the OCS for the financing of a portion of our research and development expenditures pursuant to the Research Law and related regulations. As of December 31, 2015, we had received funding from the OCS in the aggregate amount of $3.6 million. As of December 31, 2015, we had not paid any royalties to the OCS and had a contingent obligation to the OCS in the amount of $3.8 million. In December 2015, we received approval for an additional grant from the OCS in the amount of up to $340,000. In January 2016, we applied for an additional grant from the OCS in the amount of up to $6.9 million. We may apply for additional OCS grants in the future. However, as the funds available for OCS grants out of the annual budget of the State of Israel have been reduced in the past and may be further reduced in the future, we cannot predict whether we will be entitled to any future grants, or the amounts of any such grants.
The terms of the Israeli government participation require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless prior approval is received from the OCS, which we may not receive. In addition, payment of additional amounts would be required if manufacturing is moved outside of Israel, in which case the royalty repayment rate is increased and the royalty ceiling can reach up to three times the amount of the grants received, and if OCS developed know-how is transferred outside of Israel, the royalty ceiling can reach up to six times the amount of grants received (plus interest). We are currently considering whether it would be possible to assemble the capsule without the X-ray source in Israel, and have the X-ray source subsequently inserted into our system at a reactor or cyclotron site or at a distribution center outside Israel. Even following the full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions and requirements for payment may impair our ability to sell our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction involving the transfer outside of Israel of technology or know-how developed with OCS funding (such as a merger or similar transaction) may be reduced by any amounts that we are required to pay to the OCS.
If we fail to comply with any of the conditions and restrictions imposed by the Research Law, or by the specific terms under which we received the grants, we may be required to refund any grants previously received together with interest and penalties, and, in certain circumstances, may be subject to criminal charges.
Your rights and responsibilities as a shareholder are governed by Israeli law which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our ordinary shares are governed by our amended articles of association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in U.S. based corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain from abusing its power in the company, including, among other things, in voting at a general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company or prevent any other power granted to a shareholder under the company's articles of association, has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders of U.S. corporations.
28
It may be difficult to enforce a judgment of a U.S. court against us, certain of our officers and directors or the Israeli experts named in this Annual Report in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on certain of our officers and directors and these experts.
We are incorporated in Israel. All but one of our executive officers, the Israeli experts and two of our directors listed in this Annual Report reside in Israel, and substantially all of our assets and a substantial portion of the assets of these persons are located in Israel. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws on the grounds that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court.
Provisions of Israeli law and our amended articles of association may delay, prevent or otherwise impede a merger with, or an acquisition of, us, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a full tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital and the approval of a majority of the offerees that do not have a personal interest in the tender offer, unless at least 98% of the company’s outstanding shares are tendered. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer (unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek appraisal rights), may, at any time within six months following the completion of the tender offer, petition an Israeli court for an appraisal right, to alter the consideration for the acquisition. In addition, a statutory merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
Under the Israeli Patents Law, 5727-1967
We may become subject to claims for payment of compensation for assigned service inventions by our current or former employees or consultants, , which could result in litigation and adversely affect our business.
Under the Israeli Patents Law, 5727-1967, or the Patents Law, inventions conceived by an employee during the scope of his or her employment are regarded as “service inventions” and are owned by the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patents Law also provides that if no such agreement between an employer and an employee exists, which prescribes whether, to what extent, and on what conditions the employee is entitled to remuneration for his or her service inventions, then such matters may, upon application by the employee, be decided by a government-appointed compensation and royalties committee established under the Patents Law, or the Committee. A significant portion of our intellectual property has been developed by our employees in the course of their employment. our employees, consultants and third party with whom we are engaged, such as certain clinical investigators, have agreed to waive and assign to us all rights to any intellectual property created in the scope of their employment or engagement with us, and most of our current employees and consultants, including all those involved in the development of our intellectual property, have agreed to waive their economic rights with respect to service inventions. However, despite such contractual obligations, we cannot assure you that claims will not be brought against us by current or former employees or consultants, or by any of the medical institutions in which our clinical trials are being performed among else, in light of certain provisions and regulations of government medical institutions which provide for certain limitations on intellectual property rights arise during medical research performed in government institutions, demanding remuneration in consideration for assigned alleged service inventions or any other intellectual rights. If any such claims were filed, we could potentially be required to pay remuneration to our current or former employees, consultants or any of the medical institutions or their investigators, for such assigned service inventions, or for any intellectual property rights allegedly incurred during the engagement with us, or be forced to litigate such claims, which could negatively affect our business.
29
Risks Related to the Company
For as long as we are an “emerging growth company,” we will not be required to comply with certain reporting requirements that apply to other public companies. We cannot predict whether the reduced disclosure requirements applicable to emerging growth companies will make our securities less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may choose to take advantage of certain exemptions from reporting requirements applicable to other public companies that are not emerging growth companies. These include: (i) not being required to comply with the auditor attestation requirements for the assessment of our internal controls over financial reporting provided by Section 404 of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act; (ii) not being required to comply with any requirements adopted by the Public Company Accounting Oversight Board, or PCAOB, requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer; (iii) not being required to comply with any new audit rules adopted by the PCAOB after April 5, 2012 unless the U.S. Securities and Exchange Commission determines otherwise, (iv) not being required to provide certain disclosure regarding executive compensation required of larger public companies; and (v) not being required to hold a non-binding advisory vote on executive compensation or seek shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years from the end of our current fiscal year, although, if the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of any June 30 before the end of that five-year period, we would cease to be an emerging growth company as of the following December 31. We cannot predict if investors will find our securities less attractive if we choose to rely on these exemptions. If some investors find our securities less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our securities and the price for our securities may be more volatile. Further, as a result of these scaled regulatory requirements, our disclosure may be more limited than that of other public companies and you may not have the same protections afforded to security holders of such companies.
We are a foreign private issuer and, as a result, we are not be subject to U.S. proxy rules and are subject to the Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those applicable to a U.S. issuer.
We report under the Exchange Act as a foreign private issuer. Because we qualify as a foreign private issuer under the Exchange Act, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. We intend to furnish quarterly reports to the SEC on Form 6-K for so long as we are subject to the reporting requirements of Section 13(g) or 15(d) of the Exchange Act, although the information we furnish may not be the same as the information that is required in quarterly reports on Form 10-Q for U.S. domestic issuers. In addition, while U.S. domestic issuers that are not large accelerated filers or accelerated filers are required to file their annual reports on Form 10-K within 90 days after the end of each fiscal year, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. Although we intend to make interim reports available to our shareholders in a timely manner, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
As a foreign private issuer, we are permitted, to follow, and follow certain home country corporate governance practices instead of otherwise applicable NASDAQ requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.
As a foreign private issuer, we are permitted to follow certain home country corporate governance practices instead of those otherwise required under the Listing Rules of the NASDAQ Stock Market for domestic U.S. issuers. For instance, we follow home country practice in Israel with regard to, among other things, director nomination procedures, the approval of compensation of officers, and quorum requirements at general meetings of our shareholders. In addition, we intend to follow our home country law instead of the Listing Rules of the NASDAQ Stock Market that require us to obtain shareholder approval for certain dilutive events, such as the establishment or amendment of certain equity based compensation plans, an issuance that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or greater interest in the company, and certain acquisitions of the stock or assets of another company. Following our home country governance practices as opposed to the requirements that would otherwise apply to a United States company listed on NASDAQ may provide less protection to you than what is accorded to investors under the Listing Rules of the NASDAQ Stock Market applicable to domestic U.S. issuers.
30
Exchange rate fluctuations between the U.S. dollar and the NIS and the Euro and inflation may negatively affect our earnings and we may not be able to hedge our currency exchange risks successfully.
The dollar is our functional and reporting currency. However, a significant portion of our operating expenses, including personnel and facilities related expenses, are incurred in NIS. As a result, we are exposed to the risks that the NIS may appreciate relative to the U.S. dollar, or, if the NIS instead devalues relative to the U.S. dollar, that the inflation rate in Israel may exceed such rate of devaluation of the NIS, or that the timing of such devaluation may lag behind inflation in Israel. In any such event, the dollar cost of our operations in Israel would increase and our dollar-denominated results of operations would be adversely affected. The Israeli rate of inflation has not had a material adverse effect on our financial condition during the years 2013, 2014 and 2015. In addition, we expect to incur operating expenses denominated in Euros, and therefore, our operating results are also subject to fluctuations due to changes in the U.S. dollar/Euro exchange rate. Given our general lack of currency hedging arrangements to protect us from fluctuations in the exchange rates of the NIS, the Euro and other foreign currencies in relation to the U.S. dollar (and/or from inflation of such foreign currencies), we may be exposed to material adverse effects from such movements. We cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the NIS against the U.S. dollar.
Our management team’s lack of experience as officers of publicly-traded companies may hinder our ability to comply with the Sarbanes-Oxley Act.
It may be time-consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance staff or consultants in order to develop and implement appropriate internal controls and reporting procedures. If we are unable to comply with the Sarbanes-Oxley Act’s internal controls requirements, we may not be able to obtain the independent auditor certifications that the Sarbanes-Oxley Act requires publicly-traded companies to obtain.
Additional financing may result in dilution to our shareholders.
We may need to raise additional funds in the future to finance internal growth, to make acquisitions or for other reasons. Any required additional financing may not be available on terms acceptable to us, or at all. If we raise additional funds by issuing equity securities, you may experience significant dilution of your ownership interest and the newly issued securities may have rights senior to those of the holders of our ordinary shares. Alternatively, if we raise additional funds by obtaining loans from third parties, the terms of those financing arrangements may include negative covenants or other restrictions on our business that could impair our operational flexibility, and would also require us to fund additional interest expense. If additional financing is not available when required or is not available on acceptable terms, we may be unable to successfully commercialize our product or continue our research and development.
We have never declared or paid a dividend and currently do not intend to pay cash dividends in the foreseeable future. Any return on investment may be limited to the value of our securities.
We have never declared and do not anticipate paying cash dividends on our ordinary shares in the foreseeable future. Our board of directors has discretion to declare and pay dividends on our ordinary shares and will make any determination to do so based on a number of factors, such as our operating results, financial condition, current and anticipated cash needs and other business and economic factors that our board of directors may deem relevant. In addition, we are only permitted to pay dividends out of “profits” (as defined by the Israeli Companies Law, 1999, or the Israeli Companies Law), provided that there is no reasonable concern that the dividend distribution will prevent us from meeting our existing and foreseeable obligations, as they become due. If we do not pay dividends, our ordinary shares may be less valuable because a return on your investment will only occur if the trading price of our securities appreciates. Further, you should not rely on an investment in us if you require dividend income from your investments.
If securities or industry analysts do not publish research or reports about us or our business or publish unfavorable research about us or our business, the price of our securities and their trading volume could decline.
The trading market for our securities will depend in part on the research and reports that securities or industry analysts publish about us or our business. We currently have limited research coverage by securities and industry analysts. If one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. We do not have control over these analysts and we do not have commitments from them to continue to write research reports about us or our business. The price of our ordinary shares could decline if one or more equity research analysts downgrade our ordinary shares or if those analysts issue other unfavorable commentary or cease publishing reports about us or our business.
31
Our stock price has and may be subject to fluctuation, and purchasers of our securities could incur substantial losses.
Our stock price has been and may be subject to fluctuation. The stock market in general has experienced extreme volatility that has often been unrelated to the operating performance of particular companies. As a result of this volatility, investors may not be able to sell their securities at or above the purchase price. The market price for our ordinary shares on the NASDAQ Capital Market may be may fluctuate as a result of a number of factors, some of which are beyond our control, including the following:
|
•
•
|
we may not be able to develop our system at the rate or to the stage we desire;
inability to obtain the approvals necessary to commence further clinical trials;
|
|
•
|
unsatisfactory results of clinical trials;
|
|
•
|
announcements of regulatory approval or the failure to obtain it, or specific label indications or patient populations for its use, or changes or delays in the regulatory review process;
|
|
•
|
any intellectual property infringement actions in which we may become involved;
|
|
•
|
announcements concerning our competitors or the medical device industry in general;
|
|
•
|
achievement of expected product sales and profitability or our failure to meet expectations;
|
|
•
|
our commencement of, or involvement in, litigation;
|
|
•
|
any major changes in our board of directors or management;
|
|
•
|
legislation in the United States relating to the sale or pricing of medical device; or
|
|
Ÿ
|
future substantial sales of our ordinary shares.
|
In addition, the stock market in general, and NASDAQ Stock Market in particular, have experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of small companies. Broad market and industry factors may negatively affect the market price of our ordinary shares, regardless of our actual operating performance. Further, a systemic decline in the financial markets and related factors beyond our control may cause our share price to decline rapidly and unexpectedly.
We have broad discretion in how we use the net proceeds from our recent initial public offering and concurrent private placement, and we may not use these proceeds effectively.
Our management has broad discretion as to the application of the net proceeds of our recent initial public offering and concurrent private placement. Our shareholders may not agree with the manner in which our management chooses to allocate and spend the net proceeds. Moreover, our management may use the net proceeds for corporate purposes that may not increase our profitability or our market value.
32
Risks Related to Taxation
There is a risk that we could be treated as a domestic (U.S.) corporation for U.S. federal income tax purposes by reason of the transactions related to our acquisition of all of the business operations and substantially all of the assets of Check-Cap LLC on May 31, 2009 (hereinafter sometimes referred to as the “reorganization”).
Section 7874(b) of the Internal Revenue Code of 1986, as amended, or the Code, generally provides that a foreign corporation (i.e., a corporation created or organization under the laws of a jurisdiction outside of the United States) would be treated as a domestic (U.S.) corporation for U.S. federal income tax purposes if, pursuant to a plan or a series of related transactions, (1) the foreign corporation acquires, directly or indirectly, substantially all of the assets of a domestic corporation (or substantially all of the properties constituting a trade or business of a domestic partnership), (2) after the acquisition, the former shareholders of the acquired corporation by reason of holding shares of the acquired corporation (or, in the case of an acquisition with respect to a domestic partnership, the former partners of the domestic partnership by reason of holding a capital or profits interest in the domestic partnership) own at least 80% of the stock (by vote or value) of the acquiring corporation, and (3) after the acquisition, the expanded affiliated group that includes the acquiring corporation does not have substantial business activities in the foreign country in which, or under the laws of which, the acquiring corporation is created or organized when compared to the total business activities of such expanded affiliated group. On the basis of analysis of the relevant facts and circumstances and the relevant law (including the temporary regulations under Section 7874 applicable at the time of the reorganization), it was determined that the third condition described in the preceding sentence was not met with respect to the reorganization and, therefore, that the inversion tax rules of Section 7874(b) would not apply to treat us as a domestic corporation for U.S. federal income tax purposes. However, since this determination was made on the basis of all of the relevant facts and circumstances, and it is not clear which facts and circumstances the Internal Revenue Service, or the IRS, may consider more important than others, this conclusion is not free from doubt.
If Section 7874(b) were to apply to the reorganization (and we were to be treated as a domestic corporation for U.S. federal income tax purposes), then, among other things, (i) we would be subject to U.S. federal income tax on our worldwide taxable income (if and when we have taxable income); (ii) certain payments (e.g., interest and dividends) that we make (or have made) to our foreign investors may be (or may have been) subject to U.S. withholding taxes; (iii) we may be subject to significant penalties for the failure to file certain tax returns and reports, including reports with respect to our foreign bank accounts; and (iv) the U.S. unitholders of Check-Cap LLC would not have been subject to U.S. federal income tax on royalties that are deemed to be paid to them under Section 367(d) of the Code as a result of the reorganization. As discussed under “Management’s Discussion and Analysis of Financial Condition and Results of Operations- Liquidity and Capital Resources – Application of Critical Accounting Policies and Estimates – Royalties provision – Reimbursement liability to Check-Cap LLC unitholders,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations-Tabular Disclosure of Contractual Obligations,” as part of the reorganization, we committed to reimburse the unitholders of Check-Cap LLC for any tax burdens that may be imposed on them due to the reorganization, including royalties that are deemed to be paid to the U.S. unitholders under Section 367(d) of the Code.
Prospective investors are urged to consult their own advisors on these issues. The balance of this discussion, including the discussion under Item 10E “Additional Information - Taxation – U.S. Federal Income Taxation,” assumes that we will be and have been treated as a foreign corporation for U.S. federal income tax purposes.
We may be eligible for tax benefits from government programs, which require us to meet certain conditions, including regarding the location of our property, plant and equipment and manufacturing in Israel. We can provide no assurance that we would continue to be eligible for such benefits and/or that any such benefits will not be terminated in the future.
Our manufacturing facilities in Israel may qualify as a “Benefited Enterprise” under the Israeli Law for Encouragement of Capital Investments, 1959, or the Investment Law, which would entitle us to receive certain tax benefits. In order to be eligible for such benefits, we would be required to meet certain conditions, including the making of a minimum capital investment in our productive assets and the carrying on of a required portion of our manufacturing in Israel. The amount of the benefit will be determined in accordance with various conditions, including the location of our property, plant and equipment and the location of certain of our sub-contractors. If we cease to meet the required conditions for eligibility, the tax benefits could be cancelled and we could be required to pay increased taxes or to refund the amounts of the benefits received with interest and penalties. We can provide no assurance as to the amount of future capital investment in our productive assets, our future manufacturing location and the future location of our property, plant and equipment and certain of our sub-contractors, and therefore, we cannot provide assurance that we will be eligible for such tax benefits or assurance as to the amount of such tax benefits. Even if we continue to meet the relevant requirements, the tax benefits that Benefited Enterprises receive may not be continued in the future at their current levels or at all. If these tax benefits were reduced or eliminated, the amount of taxes that we would be required to pay would likely increase, as all of our operations would consequently be subject to corporate tax at the standard rate, which could adversely affect our results of operations. See Item 10E “Additional Information - Taxation— Israeli Tax Considerations and Government Programs —Law for the Encouragement of Capital Investments, 5719-1959” for additional information concerning these tax benefits.
33
There is a risk that we may be classified as a passive foreign investment company, or PFIC, which could result in adverse U.S. federal income tax consequences to U.S. investors.
In general, we will be treated as a PFIC for any taxable year in which either (1) at least 75% of our gross income (including our pro rata share of the gross income of our 25% or more-owned corporate subsidiaries) is passive income or (2) at least 50% of the average value of our assets (including our pro rata share of the assets of our 25% or more-owned corporate subsidiaries) is attributable to assets that produce, or are held for the production of, passive income. Passive income generally includes dividends, interest, rents, royalties, and gains from the disposition of passive assets. If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder (as defined in Item 10E “Additional Information - Taxation—U.S. Federal Income Taxation—General”) of our securities, the U.S. Holder may be subject to increased U.S. federal income tax liability upon a sale or other disposition of our securities or the receipt of certain excess distributions from us and may be subject to additional reporting requirements.
Our actual PFIC status for our current taxable year or any subsequent taxable year is uncertain and will not be determinable until after the end of such taxable year. Accordingly, there can be no assurance with respect to our status as a PFIC for our current taxable year or any subsequent taxable year.
U.S. investors are urged to consult their own tax advisors regarding the possible application of the PFIC rules. For more information, see Item 10E “Additional Information - Taxation—U.S. Federal Income Taxation—U.S. Holders—Passive Foreign Investment Company Rules.”
There is a risk that a holder of Long Term Incentive Warrants will recognize ordinary compensation income on the exercise of the Long Term Incentive Warrants, which may result in U.S. federal and Israeli income tax liability to such holder without the receipt of cash.
While not free from doubt, the Long Term Incentive Warrants may be treated for U.S. federal and Israeli income tax purposes as compensatory warrants (i.e., issued to compensate an original purchaser of units in our initial public offering for holding the ordinary shares underlying the units for a certain period of time after the closing date of our initial public offering). Based on this characterization, a holder may recognize ordinary compensation income for U.S. federal and Israeli income tax purposes on the exercise of the Long Term Incentive Warrants, as described under Item 10E “Additional Information - Taxation - Israeli Tax Considerations and Government Programs - Taxation of our Shareholders - Taxation of Non-Israeli Shareholders upon Exercise of Long Term Incentive Warrants” and “Item 10E “Additional Information - Taxation - U.S. Federal Income Taxation - U.S. Holders - Exercise of Long Term Incentive Warrants” and “Non-U.S. Holders.” Such compensation income may result in U.S. federal or Israeli income tax liability to such holder without the receipt of cash. Holders of Long Term Incentive Warrants are urged to consult their own tax advisors with respect to the U.S. federal and Israeli income tax consequences that may arise with respect to the Long Term Incentive Warrants.
ITEM 4. INFORMATION ON OUR COMPANY
A. History and Development of the Company
Our History
|
Our legal and commercial name is Check-Cap Ltd. We were formed as a company in Israel on April 5, 2009. On May 31, 2009, we acquired all of the business operations and substantially all of the assets of Check-Cap LLC, a Delaware limited liability company formed in December 2004. On May 15, 2015, we formed our wholly-owned subsidiary Check-Cap US, Inc., a Delaware corporation.
|
On February 24, 2015, we successfully completed an initial public offering in the United States and the listing of our securities on the NASDAQ Capital Market.
We are subject to the provisions of the Israeli Companies Law. Our principal executive offices are located at Check-Cap Building, Abba Hushi Avenue, P.O. Box 1271, Isfiya, 30090, Mount Carmel, Israel. Our telephone number is +972-4-8303400 and our website is located at www.check-cap.com (the information contained therein or linked thereto shall not be considered incorporated by reference in this annual report). Our U.S. agent is Puglisi & Associates, located at 850 Library Avenue, Suite 204, Newark, Delaware 19711.
Principal Capital Expenditures
For a discussion of our capital expenditures, see Item 5. “Operating and Financial Review and Prospects—Liquidity and Capital Resources.”
34
B. Business Overview
Our Company
We are a clinical stage medical diagnostics company engaged in the development of an ingestible capsule system that utilizes ultra low-dose X-rays for the detection and imaging of colonic polyps and CRC. While CRC is the second leading cause of death from cancer for both sexes combined in the United States and is largely preventable with early detection, according to 2013 National Health Interview Survey, only 58% of Americans between the ages of 50 to 75 reported being current with CRC screening recommendations. Unlike other screening modalities that are designed to generate structural information of the internal colon for the detection of colonic polyps and CRC, such as optical colonoscopy, CTC, and other capsule-based technologies, our system is designed to be ingested without any cathartic preparation of the colon, and to travel through the gastrointestinal tract naturally while the patient continues his or her normal daily routine. Furthermore, unlike existing CRC imaging modalities currently on the market, all of which require the patient to fast for several hours prior to administration, the procedure for our system is designed to enable patients to continue eating normally. Our system is comprised of three main components: (1) ingestible scanning capsule; (2) CPS, a recorder worn on the patient’s back; and (3) a PC-based work station for data reconstruction and image processing. We believe that this solution will be attractive to both physicians and patients, with the potential to increase the number of people undergoing CRC screening.
Our scanning capsule will be swallowed and propelled by natural motility through the gastrointestinal tract and excreted naturally with no need for retrieval for data collection. Unlike other CRC screening methods, this process should not disrupt a patient’s normal activities or require fasting. Our scanning capsule employs ultra low-dose X-rays, which allow the system to image the interior lining of the colon even when surrounded by intestinal content. As such, we believe that patients using our system will not be required to undergo any prior bowel preparation. The Radiation Safety Division of the Soreq Nuclear Research Center found, as set forth in its report of November 2010 that was prepared at our request and based on the information provided by us and the relevant methods and principles known at such time, or the Report, that the radiation dose to the patient in the proposed screening procedure utilizing the scanning device developed by us at that time in routine operation and normal conditions is low relative to the radiation dose involved in conventional imaging procedures using X-rays (such as fluoroscopy and CT) and is also low when compared to the radiation dose involved in established screening procedures such as mammography, all as more fully described in the Report.
Our scanning capsule is being designed to transmit position, motility, and the data it collects to an external data recorder and capsule positioning system (CPS) that will be worn by the patient. The external data recorder is being designed to enable the transfer of the data to our PC-based work station with viewer software application to allow physicians to analyze the data collected by our scanning capsule. The CPS is being designed to provide the physician with accurate localization data aligned with a reconstructed image. We intend for physicians to be able to review the colon’s inner images at any location at any time, in less time than is required to perform an optical colonoscopy.
Colonic polyps are tissue growths that occur on the lining of the colon. Polyps in the colon are extremely common, and certain types of polyps can become cancerous over time. In the event that polyps are identified through our system, the patient may be advised to undergo a subsequent traditional colonoscopy procedure to examine, remove and biopsy the polyps. For those patients who require a subsequent polypectomy, concerns regarding pain, discomfort and embarrassment may still remain with respect to the subsequent polypectomy. We do not, however, believe that these concerns will make the use of our system any less attractive to physicians and patients. Although patients who are initially screened utilizing a traditional colonoscopy could avoid the need for a second procedure if polyps are discovered because they could undergo a polypectomy during the initial screening, if necessary, we believe that our system will still be attractive to physicians and patients as a majority of patients who are screened will not require a subsequent polypectomy. Published data from a multi-center CT colonography screening study of 2,531 asymptomatic adults showed that if all patients with a lesion measuring 5mm or more on CT colonography were referred for colonoscopy, the colonoscopy-referral rate would have been 17%.
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Sourasky Medical Center in Israel and used a prior version of our system, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our system to the human colon, generating images taken in the colon without any prior bowel preparation. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted.
Another objective of the 10-case study was to estimate total radiation exposure for each case study. This was calculated using standard established factors for calculating effective radiation exposure, such as the duration of the capsule inside the body, and was based on the activity of the radiation source inside the scanning capsule and radiation energy, both of which were measured for each case study. The average calculated exposure for the entire procedure in the 10-case study, from ingestion of the capsule to excretion, was 0.03 mSv (STD 0.007 mSv). This level of radiation exposure is similar to a single chest X-ray (approximately 0.06mSv) and two orders of magnitude less than a CTC.
35
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of the our system. The 10-case study was the first phase of a multi-center, prospective clinical feasibility study to establish the safety, functionality and preliminary efficacy of our system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity FOBTs and FITs, as well as from optical colonoscopies. The feasibility study is designed allow for recruitment of 100 subjects. The study is being conducted at multiple centers in Israel, with the potential to be conducted at a single site in the Netherlands. The clinical feasibility study will evaluate the image resolution generated by the capsule in an a human colon without cathartic preparation, will assess polyp imaging in various shapes and in different segments of the colon and will evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified through our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
A preliminary analysis conducted on the first 49 capsules swallowed by participants enrolled in the multi-center, prospective clinical feasibility study showed 48 of 49 capsules swallowed and naturally eliminated without major or minor side effects after 73.2±45.4 hours. Image reconstructions allowed 3D views of the colonic wall and lumen with the typical contour of different segments (hepatic flexure, triangular shape of the transverse colon). Both pedunculated and sessile polyps were detected in several patients and validated later by colonoscopy.
To date, we have achieved key product development milestones, including the demonstrated ability of our system to reconstruct the human colon and to identify polyps, and design freeze of the current version of our system. Following the successful completion of the multi-center, prospective clinical feasibility study and design release and transfer to manufacturing phases, we plan to submit during the second half of 2016, a request for CE marking for the marketing and sale of our capsule in the European Union. We expect to perform post-marketing studies in Europe following CE marking for the purpose of collecting additional clinical data to support market adoption. Subject to regulatory approvals, available capital, and engagement with strategic partners, we anticipate launching our system commercially in Europe during 2017.
We plan to conduct a pre-submission meeting with the FDA, during 2016. Subject to this meeting, we plan subsequently to submit a request for the approval of an investigational device exemption, or IDE, for a pilot study in the United States. Subject to successful completion of the pilot study and receipt of required approvals, we plan to initiate during 2017, a pivotal study in the United States to (i) demonstrate device safety as evidenced by a lack of device-related serious adverse events; and (ii) provide efficacy data concerning our system’s performance. We anticipate that FDA approval for the pivotal study will be subject to our providing sufficient clinical data from previous clinical studies, which may include the multi-center, prospective clinical feasibility study and U.S. pilot study. However, there can be no assurance that the FDA will grant approval for the pilot and/or pivotal studies to be conducted in the United States.
We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study, subject to available capital and engagement with strategic partners. Pivotal studies are expected, among other things, to compare polyps identified by our system with the polyps identified by traditional optical colonoscopy. These clinical findings may be analyzed in comparison with results obtained from FOBTs and FITs.
Following and subject to the successful completion of our pivotal trial, our current strategy is to submit a direct de novo reclassification petition, which we anticipate submitting in 2018, for initial FDA clearance for the marketing of our system in the United States. Direct de novo reclassification typically takes at least 9 to 12 months from filing to clearance. If the FDA determines that our system is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process. The PMA pathway is much more costly and uncertain than the 510(k) clearance process or de novo reclassification, and generally takes at least 12 to 18 months, or even longer, from the time the application is filed with FDA to ultimate approval.
Timelines expectations are based on our current estimations and expectations, which may continue to be updated along with our progress, which is subject to the occurrence of various factors and future events, among others, the satisfactory completion of system’s development process, testing, and integration, which may require more time than currently expected, as well as the success of our clinical trials and the completion of our required regulatory approvals, all of which are uncertain as of the date of this Annual Report.
36
We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have been granted patents for our core patent by the U.S. Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan. We also filed patent applications describing the use of our technology in several other medical applications.
Since our formation, we have not generated any revenue. We do not anticipate generating any revenue for the foreseeable future and we do not yet have any specific launch dates for our product. We incurred net losses of $3.4 million in 2013, $610,000 in 2014 and $12.3 million in 2015. As of December 31, 2015, we had an accumulated deficit of $34.1 million and a total shareholders’ equity of $12.6 million.
Our Solution
CRC screening can reduce the incidence of and mortality from the disease by detecting polyps at an earlier, more treatable stage. CRC is one of the few cancers that can be prevented through screening because pre-cancerous polyps, from which colon cancers often develop, can be identified and removed. Today, there is a range of options for CRC screening in the average-risk population, with current technology falling into two general categories: (i) structural exams, such as optical colonoscopy (which is currently regarded as the “gold standard” for CRC screening), sigmoidoscopy, CTC and optical capsules (all of which require aggressive bowel preparation), which are invasive exams that enable physicians to visualize the colon for abnormalities; and (ii) stool-based tests, such as FOBTs, FITs and stool DNA tests, which test for blood and irregularities in DNA. Notwithstanding the many CRC screening alternatives, the fact that the tests are encouraged by clinicians and insurers and the clinical value of screening for CRC, a large portion of the population are still reticent to go for CRC screening and are not satisfied with the currently available alternatives.
We believe that our system could represent a potential breakthrough in CRC screening by providing a structural exam without the pain, discomfort and embarrassment experienced by some patients undergoing a traditional optical colonoscopy and other currently available screening methods by offering the following benefits:
|
•
|
eliminating the need for fasting and prior bowel preparation, which would differentiate our system from every other currently available structural screening exam;
|
|
•
|
providing patients with a procedure that requires them to swallow our capsule and small amounts of a contrast agent, thereby minimizing any disruption to their normal activities;
|
|
•
|
eliminating the need to sedate patients;
|
|
•
|
obviating the requirement for the insufflation (the forcing of air into the gastrointestinal tract) of patients;
|
|
•
|
administering our technology on an outpatient basis;
|
|
•
|
providing digital reporting, storage and remote consulting capabilities; and
|
|
•
|
enabling a physician to analyze the results in approximately 10 minutes, which would be less time than is required to conduct an optical colonoscopy.
|
Although our system utilizes radiation that is considered ultra low-dose, we believe that the risks associated with such radiation exposure are low compared to risks associated with other procedures such as perforation, bleeding or sedation related effects (optical colonoscopy and sigmoidoscopy) and dehydration and damage to kidneys (optical capsules). Unlike FOBTs, FITs and stool DNA tests, our capsule-based imaging modality generates structural information on the colon, which could assist in the detection of pre-cancerous polyps. We therefore do not believe that the ultra low-dose radiation in our capsule will make our system less attractive to physicians and patients than other less-effective products that do not employ any radiation.
We believe that gastroenterologists will adopt our technology and encourage the use of our capsule. This may increase the number of people undergoing CRC screening and may cause more people with polyps to obtain a polypectomy – a therapeutic procedure during which polyps are removed and which currently receives higher reimbursement coverage than both screening and diagnostic colonoscopies.
37
Our goal is to become a leading supplier of CRC screening technology and to establish our technology as a leading CRC screening method. Key elements of our strategy include:
|
•
|
obtaining CE marking for the marketing and sale of our system in the European Union, followed by obtaining regulatory approvals for the sale of our system initially in the United States and Japan;
|
|
•
|
In Europe and Japan, we intend to offer our system as an imaging and screening tool for the general population. In the United States, we may choose to first obtain regulatory clearance/approval for our system in a screening sub-population or as an adjunct tool to FOBTs and FITs, and after we have conducted more extensive clinical studies in the United States, we would anticipate applying to the FDA for the use of our system as an primary screening tool;
|
|
•
|
obtaining third-party reimbursement for our technology;
|
|
•
|
improving and enhancing our existing technology portfolio and developing new technologies; and
|
|
•
|
successfully marketing our product to establish a large customer base.
|
Our Technology
Our technology is based on an ingestible capsule, which is swallowed by the patient and propelled by natural motility through the gastrointestinal tract. Our capsule transmits information to a receiving device worn on the patient’s body that stores the information for off-line analysis. Our capsule consists of an X-ray source and several X-ray detectors. The X-ray source is contained in a rotating radiation shield, enabling the generation of 360-degree angular scans. The collection of successive angular scans is intended to enable the virtual reconstruction of a portion of the colon's inner surface. During movement of our capsule longitudinally through the colon, successive images of portions of the colon enable the three-dimensional reconstruction of the colon. Our system is also intended to enable identification of polyps, which protrude inward into the colon, through the detection of irregularities in the topography of the colon's inner surface.
Our system is intended to be prescribed to patients by physicians. Immediately prior to capsule ingestion, patients will drink a small amount of a contrast agent (such as barium sulfate or iodine) combined with oral fiber, and continue to do so with normal daily meals in order to enhance the contrast of the colon surface. The capsule is propelled by natural motility through the gastrointestinal tract. As it makes its way through the gastrointestinal tract, information is transmitted to a receiving device worn by the patient that stores the information for off-line analysis. After our capsule is expelled from a patient’s body, the CPS data will be downloaded into our workstation through which physicians will utilize our data viewer software application to analyze the data collected by our system. Our proprietary software is being designed to process the data and produce a two and three-dimensional visualization of the colon's inner surface. A physician will then analyze the visualization to determine whether any anatomical anomalies are present on the inner surface of the colon.
Our system consists of the following three main subsystems that together enable the generation of high resolution 3D imaging of the colon's inner surface, further described below: (i) ultra-low-dose X-ray based colon scanning capsule; (ii) CPS; and (iii) PC-based 3D imaging workstation.
Scanning Capsule
The scanning capsule, which is designed to measure, collect and transmit the clinical data, is comprised of the following components:
|
•
|
X-ray Source – including radioactive material sealed in a cylindrical housing.
|
|
•
|
Collimator – Radiation shield around the source, which absorbs most of the radiation. Several radial holes enable emission of radiation in defined directions.
|
|
•
|
X-ray Sensor – Comprised of several solid state X-ray detectors for measuring the scattered radiation intensity.
|
|
•
|
Tilt Sensor – Indication of capsule motion (3D acceleration).
|
|
|
|
•
•
|
Rotation Motor – For rotating the collimator and X-ray Source.
Compass sensor – indication of true north (reference coordinate system).
|
38
|
•
|
Source Concealment Mechanism – Conceals the source inside the radiation shield.
|
|
•
|
R-T – Radio frequency transceiver device to communicate with the receiver.
|
|
•
|
Batteries – Electrical power supply for the capsule.
|
|
•
|
Memory – Data storage. The capsule should be able to store up to an hour of measured data.
|
|
•
|
CPS coil – Transmits a continuous electromagnetic filed utilized by an external localization system to track 3D position.
|
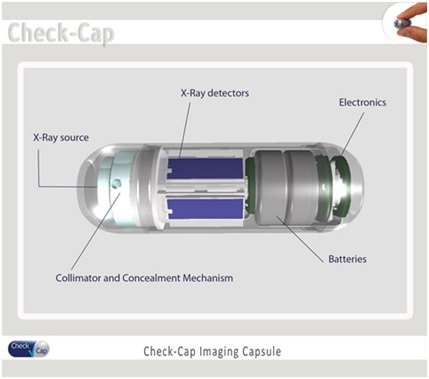
Image for illustration purpose only
Capsule Positioning System
The Capsule Positioning System, or CPS, is a small, disposable system of biocompatible stickers which are deigned to automatically track the imaging capsule’s positioning and orientation throughout the gastrointestinal tract transition, control the capsule scan mechanism through an embedded scan control algorithm or SCA, capture imaging data from the capsule through radio frequency communication to a non-volatile memory device and enable data retrieval through either wired or wireless communication to an external processor.
The CPS is comprised of the following components:
|
•
|
Sticker Housings – Biocompatible and water-resistant stickers and housing integrating all functional components, attached to the patient’s back, enabling five days of continuous operation.
|
|
•
|
Recorder–Consists of receiver electronics embedded software and nonvolatile memory.
|
|
•
|
Antennas – Radio frequency antennas are embedded into the sticker housings and used to communicate with the capsule.
|
|
|
•
|
Activation/Deactivation Circuit – Used to activate/deactivate the CPS through a specialized protocol.
|
39
|
•
|
UI Indicators - Provides user with vocal, light or vibration indication as required.
|
|
•
|
PCB – Electronics’ printed circuit boards.
|
|
•
|
Microcontroller – Runs embedded software, logic that manages the CPS and SCA.
|
|
•
|
RF Transceivers – Several transceivers used to communicate with the capsule.
|
|
•
|
TILT/Compass Sensors – To determinate patient’s body movements.
|
|
•
|
Batteries – Electrical power supply for the CPS.
|
|
•
|
Memory – Non-volatile data storage to store data acquired by the system.
|
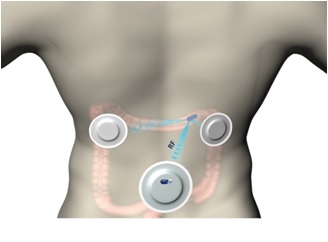
Image for illustration purpose only
Workstation
The workstation is a specialized, user friendly, personal computer-based software package designed to retrieve and process clinical data from the CPS and to reconstruct and produce 3D visualization of the colon's inner surface. The workstation is comprised of the following software components:
|
•
|
Communication Driver Software – to communicate with the CPS and retrieve collected data following procedure completion.
|
|
•
|
Data Processing Software – to process and reconstruct clinical data into a 3D structure.
|
|
•
|
Data Display and Management Software – includes the following functions:
|
|
○
|
3D visualization of the reconstructed colon surface.
|
|
○
|
Annotation tools.
|
|
○
○
|
Registration of patient and capsule data and management of the patient database.
Report – to enable generation of clinical results report out.
|
40
Image for illustration purpose only
Our System – Non-Clinical, Pre-Clinical and Clinical History
We have developed and validated our capsule-based imaging modality for providing structural information on colonic polypoid lesions and masses for CRC screening. Below is a summary of the validation tests carried out by us in the laboratory, in phantoms, animals and humans, which were designed to evaluate this new imaging modality’s performance and potential clinical value.
Non-Clinical and Pre-Clinical Testing
Imaging Performance Testing
The Check-Cap ingestible capsule transmits data as the capsule progresses through the colon. This data consists of imaged slices perpendicular to the capsule’s longitudinal axis; slices are then reconstructed by the workstation to produce 2D and 3D images of the inner surface of the colon. Following are performance measurements of the capsule imaging.
• Modulation Transfer Function, or MTF. The capsule was moved along a longitudinal-edge phantom setup in 3mm steps. The figure below shows a typical raw signal after filtering for peak detection. The same test was carried out using an angular-edge phantom setup, which demonstrated similar results to those shown below. These tests do not take into account noise characteristics.
41
Image for illustration purpose only
For each position of the capsule in the phantom, the mean signal intensity (peak) was measured, the result of which is shown in the right figure below. Resulting line spread function, or LSF, which is the differential of the curve in the left figure below.
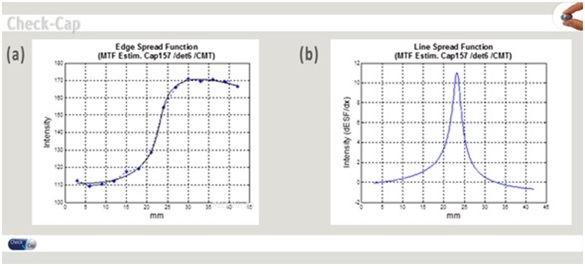
Image for illustration purpose only
The graphs above demonstrate that the existing design of our system can detect objects of approximately 2-3mm when noise is not taken into account.
● Resolution Limit: Estimation of the Smallest Visible Object Size. In order to estimate the size of the smallest visible object, both spatial resolution and noise characteristics must be taken into account. The graph below presents the estimated MTF of our system. Noise analysis indicates MTF 1/3 for minimum visibility, which demonstrates that the smallest visible object that can be detected with the existing design of our system (in the conditions used, which included a colon diameter of 30mm) is of approximately 5-6 mm (see graph below).
42
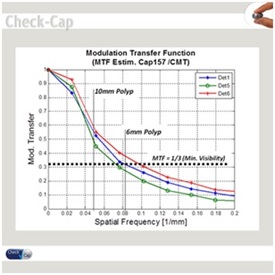
Image for illustration purpose only
Image Reconstruction
Two main characteristics of our system contribute to the image reconstruction performance:
|
•
|
The number of photons hitting the detector per time frame.
|
|
•
|
The angular spread of the photon beam coming out of the capsule collimator.
|
Based on the laboratory tests performed with the current version of our system, polyps of 6 mm and larger should be visible and 10 mm polyps and larger are expected to be detected at high sensitivity. To further enhance the visibility of 6 mm - 9 mm polyps, a new design of the collimator was successfully tested in a prototype version of our capsule, which is expected to enable 2.5 times the number of photons to be detected by the detectors, allowing the implementation of an image enhancement algorithm, which is expected to improve the imaging performance.
Animal Testing and Tissue Equivalent Phantom Image Reconstruction
The physics of our imaging modality was tested in the laboratory on phantoms with tissue equivalent material and in animals to ensure that laboratory conditions mimic real life clinical scenarios.
Following the initial proof of concept, we performed a series of studies in order to evaluate the feasibility and preliminary safety of our technology. All of the studies were performed in pigs ranging from 60 to 90 kg. Pigs, which are commonly used in gastrointestinal studies, were selected as the animal model for preliminary evaluation of our system based on the resemblance of the porcine colon size and morphology to the human colon. However, there are marked differences between the colon of pigs and that of humans. The pig colon is much longer, has a larger diameter in addition to other anatomical differences. In the pig model, the pressure waves of peristalsis are believed to be more frequent and shorter than in humans. As a result, we believe that colon content movement is substantially slower and more frequent in pigs than in humans. In these studies, we did not intend to collect statistically significant data; hence, the tests were repeated a limited number of times until adequate data was collected.
The first test was performed to demonstrate imaging proof-of-concept using a wired system. This technology included all the basic features intended to be included in the clinical system, but on a larger scale due to the use of off-the-shelf components. The subsequent studies used versions of the system that integrated most of the imaging components, software and electronics of the system that we used with humans. Since off-the-shelf components were used, the animal capsules were larger and heavier than the version of our system that are used clinically.
Raw data from an animal colon showing a decrease in X-ray florescence, or XRF photon signals and an increase in Compton backscattering, or CMT, signals corresponding to the position of a polyp that was detected when our capsule passed over the polyp is shown in the image below. These two signals are combined in order to form a three dimensional image below.
43
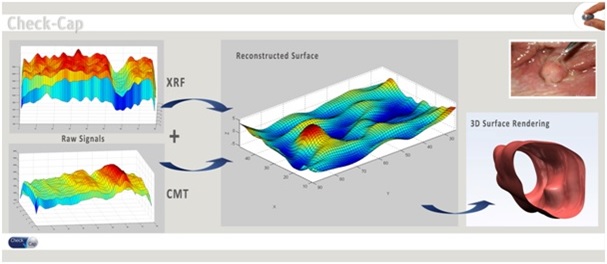
Image for illustration purpose only
The animal studies conducted to date demonstrate that our technology provides sufficient resolution, in these studies, for the detection of 10 mm polyps which is the size of clinically significant polyps. The animal studies also demonstrated that 5 mm polyps can be detected, though with lower resolution than 10 mm polyps in the first animal capsule. Animal health was maintained throughout the studies. No adverse effects related to passage of our capsule were noted.
The capsules evaluated in the animal studies were significantly larger than the capsules that we are using with humans. The differences in anatomy, physiology, and capsules may have several effects on the data compared to use in the human population. Motility of the capsules through the digestive system was slow due to the specific shape of the porcine gastrointestinal tract. In addition, because of the size of the capsule, it was retained in the stomach for many hours and even days. Accordingly, the animal model required that normal ingestion be replaced by direct insertion of the capsule into the small bowel. In order to simplify the development and animal testing, we used Tungsten radiation source with long half-life (120 days).
Following the success of the animal testing, a series of in-vitro tests were conducted to simulate different clinical scenarios in the laboratory using a miniaturized human capsule. Polyps were created and reconstruction of the laboratory phantoms with a human capsule was generated to assess the ability to detect polyps as the capsule advances in the colon. The in-vitro tests demonstrated the imaging capabilities of our imaging technology. Below is the reconstruction of a laboratory phantom image.
Image for illustration purpose only
44
Polyp Detection Analysis
Laboratory tests were carried out to estimate the capsule’s ability to detect polyps in phantoms and demonstrate sensitivity and specificity of such detection. Below is an example of the reconstruction of a scan composed of three slices: XRF, CMT and a fused (combined) image.
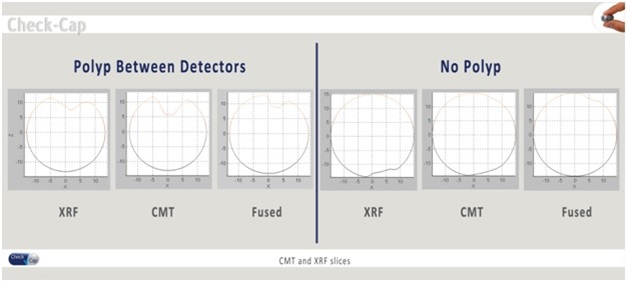
Receiver Operating Characteristics
Standard receiver operating characteristics, or ROC, curves were generated from phantom data with 8 mm polyp in a 30 mm barrel phantom with 3% iodine concentration mimicking the colon contents. CMT, XRF and fused (combined) data were analyzed based on 2D slices that were generated and standard deviation indicator. There were a few cases where the noise in the phantoms was high enough to generate polyp false positive condition separately for each data type, especially in CMT. However, fusion of CMT and XRF data contributed to noise reduction and enabled to demonstrate 100% true positive and 0% false positive.

Clinical Trials
We initiated our first human clinical study in Germany in January 2010. The purpose of the study was to collect pressure and motility information from the digestive system of human volunteers. This study was conducted with a passive capsule that contained no X-ray source or detectors. It included several electronic components of the system and had similar dimensions to the current capsule.
45
We concluded a limited, single-center, feasibility study at Rambam Medical Center in Haifa to assess the motility of our capsule in healthy subjects, with the objective of optimizing the daily routine of the subjects in order to shorten the transit time of our capsule. The study, in which 15 healthy subjects participated and used a dummy capsule with the same weight and dimensions as our current capsule, did not identify any safety issues and all capsules were retrieved. A structured daily routine determined the timing of the following: capsule ingestion, the subjects’ daily meals, the contrast agent ingestion and one daily dose of 10 mg of Bisacodyl, and all subjects continued their regular active lifestyles (such as work and sports). The average transit time of the dummy capsule in the 15 subjects was approximately 24 hours less than the average transit time of our capsule in subjects participating in the multi-center feasibility study in which the participants do not ingest a daily dose of Bisacodyl, are less active and are continuously monitored in a designated apartment until the capsule is excreted.
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Sourasky Medical Center in Israel and used a prior version of our system, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our system to the human colon, generating images taken in the colon without any prior bowel preparation. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted.
Another objective of the 10-case study was to estimate total radiation exposure for each case study. This was calculated using standard established factors for calculating effective radiation exposure, such as the duration of the capsule inside the body, and was based on the activity of the radiation source inside the scanning capsule and radiation energy, both of which were measured for each case study. The average calculated exposure for the entire procedure in the 10-case study, from ingestion of the capsule to excretion, was 0.03 mSv (STD 0.007 mSv). This level of radiation exposure is similar to a single chest X-ray (approximately 0.06mSv) and two orders of magnitude less than a CTC.
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of our system. The 10-case study was the first phase of a multi-center, prospective clinical feasibility study to establish the safety, functionality and preliminary efficacy of our system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity FOBTs and FITs, as well as from optical colonoscopies. The feasibility study is designed allow for recruitment of 100 subjects. The study is being conducted at multiple centers in Israel, with the potential to be conducted at a single site in the Netherlands. The clinical feasibility study will evaluate the image resolution generated by the capsule in an a human colon without cathartic preparation, will assess polyp imaging in various shapes and in different segments of the colon and will evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified through our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
A preliminary analysis conducted on the first 49 capsules swallowed by participants enrolled in the multi-center, prospective clinical feasibility study showed 48 of 49 capsules swallowed and naturally eliminated without major or minor side effects after 73.2±45.4 hours. Image reconstructions allowed 3D views of the colonic wall and lumen with the typical contour of different segments (hepatic flexure, triangular shape of the transverse colon). Both pedunculated and sessile polyps were detected in several patients and validated later by colonoscopy.
To date, we have achieved key product development milestones, including the demonstrated ability of our system to reconstruct the human colon and to identify polyps, and design freeze of the current version of our system. Following the successful completion of the multi-center, prospective clinical feasibility study and design release and transfer to manufacturing phases, we plan to submit during the second half of 2016, a request for CE marking for the marketing and sale of our capsule in the European Union. We expect to perform post-marketing studies in Europe following CE marking for the purpose of collecting additional clinical data to support market adoption. Subject to regulatory approvals, available capital, and engagement with strategic partners, we anticipate launching our system commercially in Europe during 2017.
We plan to conduct a pre-submission meeting with the FDA, during 2016. Subject to this meeting, we plan subsequently to submit a request for the approval of an IDE, for a pilot study in the United States. Subject to successful completion of the pilot study and receipt of required approvals, we plan to initiate during 2017, a pivotal study in the United States to (i) demonstrate device safety as evidenced by a lack of device-related serious adverse events; and (ii) provide efficacy data concerning our system’s performance. We anticipate that FDA approval for the pivotal study will be subject to our providing sufficient clinical data from previous clinical studies, which may include the multi-center, prospective clinical feasibility study and U.S. pilot study. However, there can be no assurance that the FDA will grant approval for the pilot and/or pivotal studies to be conducted in the United States.
46
We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study, subject to available capital and engagement with strategic partners. Pivotal studies are expected, among other things, to compare polyps identified by our system with the polyps identified by traditional optical colonoscopy. These clinical findings may be analyzed in comparison with results obtained from FOBTs and FITs.
Following and subject to the successful completion of our pivotal trial, our current strategy is to submit a direct de novo reclassification petition, which we anticipate submitting in 2018, for initial FDA clearance for the marketing of our system in the United States. Direct de novo reclassification typically takes at least 9 to 12 months from filing to clearance. If the FDA determines that our system is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process The PMA pathway is much more costly and uncertain than the 510(k) clearance process or de novo reclassification, and generally takes at least 12 to 18 months, or even longer, from the time the application is filed with FDA to ultimate approval.
Timelines expectations are based on our current estimations and expectations, which may continue to be updated along with our progress, which is subject to the occurrence of various factors and future events, among others, the satisfactory completion of system’s development process, testing, and integration, which may require more time than currently expected, as well as the success of our clinical trials and the completion of our required regulatory approvals, all of which are uncertain as of the date of this Annual report.
We concluded a limited, single-center, feasibility study at Rambam Medical Center in Haifa to assess the motility of our capsule in healthy subjects, with the objective of optimizing the daily routine of the subjects in order to shorten the transit time of our capsule. The study, in which 15 healthy subjects participated and used a dummy capsule with the same weight and dimensions as our current capsule, did not identify any safety issues and all capsules were retrieved. A structured daily routine determined the timing of the following: capsule ingestion, the subjects’ daily meals, the contrast agent ingestion and one daily dose of 10 mg of Bisacodyl, and all subjects continued their regular active lifestyles (such as work and sports). The average transit time of the dummy capsule in the 15 subjects was approximately 24 hours less than the average transit time of our capsule in subjects participating in the multi-center feasibility study in which the participants do not ingest a daily dose of Bisacodyl, are less active and are continuously monitored in a designated apartment until the capsule is excreted.
We recently also initiated an in-house study, approved through Bnei-Zion Medical Center to assess the CPS CE version standalone functionality (no capsule ingestion required). Healthy subjects selected for this study will externally carry the CPS and a non-scanning capsule, through which we can assess the effect of human routine behavior on system performance. The study is conducted in-house and does not require any medical surveillance.
Research and Development
Our research and development strategy is centered on developing our system. Our research and development team is located at our facilities in Isfiya, Israel, and consists of 50 employees and independent contractors as of February 15, 2016 and is supported by highly experienced consultants.
We have received grants from the Government of the State of Israel through the OCS for the financing of a portion of our research and development expenditures pursuant to the Research Law and related regulations. As of December 31, 2015, we had received funding from the OCS in the aggregate amount of $3.6 million and had a contingent obligation to the OCS in the amount of $3.8 million. As of December 31, 2015, we had not paid any royalties to the OCS. In December 2015, we received approval for an additional grant from the OCS in the amount of up to $340,000, and in January 2016, we applied for an additional grant from the OCS in the amount of up to $6.9 million. We may apply for additional OCS grants in the future. However, as the funds available for OCS grants out of the annual budget of the State of Israel have been reduced in the past and may be further reduced in the future, we cannot predict whether we will be entitled to any future grants, or the amounts of any such grants.
We incurred approximately $5.8 million, $2.8 million and $2.9 million in research and development expenses, net (after deducting participation by others and government grants) for the years ended December 31, 2015, 2014 and 2013, respectively. For additional information, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview— Research and Development Expenses, Net.”
47
Intellectual Property
An important part of our competitive strategy is to seek, when appropriate, protection for our products and proprietary technology through a combination of U.S. and foreign patents, trademarks, trade secrets and contractual arrangements with our employees, consultants and suppliers. These measures, however, may not be adequate to protect our technology from unauthorized disclosure, third-party infringement or misappropriation as these parties may breach these agreements, and we may not have adequate remedies for any such breach. We intend to prosecute and defend our proprietary technology. The primary test for patent protection eligibility includes novelty, non-obviousness and usefulness.
We submit applications under the Patent Cooperation Treaty, or PCT, which is an international patent law treaty that provides a unified procedure for filing a single initial patent application to seek patent protection for an invention simultaneously in each of the member states. Although a PCT application is not itself examined and cannot issue as a patent, it allows the applicant to seek protection in any of the member states through national-phase applications.
As of February 1, 2016, we had 20 granted patents (not including separate validations in Europe), 1 “allowed” patent application and 34 pending patent applications (not including “allowed”) worldwide relating to various elements and functions of our products and related enhancements. We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have received patent grants for our core patent by the United States Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan.
Our registered U.S. Patent Number 7,787,926 discloses an ingestible capsule with a radiation source and radiation detectors that, when used in conjunction with a radio opaque contrast agent, is adapted to detect clinically relevant findings in the colon. Utilizing X-ray fluorescence and Compton back scatterings, the capsule is able to measure the distance between the capsule and the colon wall and to distinguish between gas, intestinal contents, and clinically significant findings in the gastrointestinal tract.
A second PCT patent application (PCT/IL2008/000163), which is pending in several countries in the national-phase and granted in Europe and Hong Kong, discloses additional features such as a rotating collimator and improved scanning mechanisms, the capability to determine tissue density to differentiate between different types of polyps, as well as the capability to determine capsule movement in the colon. Another PCT application (PCT/IL2011/000462), which is pending in several countries in the PCT national-phase, discloses a number of alternate fail safe concealment mechanisms that can be utilized in the capsule to ensure that the X-ray source is blocked when the capsule is not scanning and is open when it is scanning, allowing the capsule to image the colon. The fail safe feature ensures that in the event of power failure, the radiation source is blocked and X-rays do not escape. Recently the application filed in Australia has been granted and the application filed in Europe has been allowed.
In another PCT patent application (PCT/IL2008/000765), which was granted in the United States, Europe, Israel and Japan, we disclose an imaging catheter that utilizes X-ray fluorescence, Compton back scattering and electron back scattering. The imaging catheter is designed for use in cardiac applications as well as intra-operative imaging applications such as imaging inside blood vessels where optical imaging cannot be performed because of obscuring circumstances.
While our policy is to obtain patents by application, to maintain trade secrets and to seek to operate without infringing on the intellectual property rights of third parties, technologies related to our business have been rapidly developing in recent years. Additionally, patent applications that we may file may not result in the issuance of patents, and our issued patents and any issued patents that we may receive in the future may be challenged, invalidated or circumvented. For example, we cannot predict the extent of claims that may be allowed or enforced in our patents nor be certain of the priority of inventions covered by pending third-party patent applications. If third parties prepare and file patent applications that also claim technology or therapeutics to which we have rights, we may have to partake in proceedings to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable to us. Moreover, because of the extensive time required for clinical development and regulatory review of a product we may develop, it is possible that, before our system can be commercialized, related patents will have expired or will expire a short period following commercialization, thereby reducing the advantage of such patent. Loss or invalidation of certain of our patents, or a finding of unenforceability or limited scope of certain of our intellectual property, could have a material adverse effect on us. See “Item 3D “Key Information - Risk Factors— Risks Related to Our Intellectual Property.”
48
In addition to patent protection, we rely on trade secrets, including unpatented know-how, technology innovation, drawings, technical specifications and other proprietary. We also rely on protection available under trademark laws, and received a notice of allowance for the “CHECK-CAP” mark and design logo in the United States and hold a registered trademark for the “CHECK-CAP” design logo in Europe. Applications for additional marks and design logos are pending in the United States and Europe.
Competition
Competition for our system comes primarily from traditional well-entrenched technologies for detecting gastrointestinal disorders and diseases, such as optical colonoscopy, sigmoidoscopy and CTC. The principal manufacturers of equipment for optical colonoscopy and sigmoidoscopy are Olympus, Richo Company Ltd., Hoya and Fuji Film. The principal manufacturers of equipment for CTC are General Electric Healthcare Systems, Siemens Medical Solutions, Philips Medical Systems Ltd. and Toshiba Corporation. All of these companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide channels for distribution medical instruments to physicians.
Tests that investigate the link between genes and the function of metabolic pathways, drug metabolism and disease development with a primary focus on the study of DNA, RNA and proteins. Genetic markers can be traced within stool samples in minute quantities.
Olympus is a Japanese company that manufactures optics and imaging products in China. Olympus’ medical division develops and sells products in the endoscopy, endosurgery and endotherapy fields. The FDA approved Olympus’ Endo-Capsule product for the visualization of the small bowel in late 2007 and the company is currently developing a capsule-based technology for CRC screening.
IntroMedic is a private Korean medical device manufacturer specializing in the development of technologies in the field of optical capsule endoscopy. IntroMedic’s MiroCam product, which is currently undergoing FDA trials in the United States, uses an oxide silicone chip for imaging and human body communication technology for the transmission of images. The company is developing a capsule-based technology for CRC screening.
RF System is a Japanese manufacturer of medical device equipment and cameras for industrial use. Its Sayaka product is a new, “battery free” capsule endoscope that uses induction charging to draw power. The company is developing a capsule technology for CRC screening.
All of the abovementioned CRC optical capsule endoscopy products under development rely on similar optics (camera) and complementary metal oxide semiconductor or CMOS, based technology, thus requiring a significant bowel cleansing prior to performing the procedure.
In addition, several companies have developed or are developing technologies based on molecular diagnostics (from blood and other bodily fluids), or MDx, tests that investigate the link between genes and the function of metabolic pathways, drug metabolism and disease development with a primary focus on the study of DNA, RNA and proteins. Genetic markers can be traced within stool samples in minute quantities. A U.S. based company, Exact Sciences, has developed a special collecting kit for stool samples and an analyzer to detect neoplasia associated DNA markers and the presence of occult hemoglobin in human stool. The method of screening is included in the colorectal cancer screening guidelines, including the American Cancer Society.
To our knowledge, certain companies are developing or commercializing optics-based capsule endoscopy systems. The existing capsule technology requires intense bowel cleansing, more so than is required for optical colonoscopy, and the potential ingestion of agents to boost capsule transit through the colon. Given Imaging, an Israeli-based company that was acquired by Covidien plc (NYSE: COV) in February 2014 (subsequently acquired by Medtronic), has developed visualization capsules for the detection of disorders of the esophagus, small bowel and colon. It received the CE Mark to market PillCam™ COLON throughout the European in 2006. In early 2014, the FDA cleared PillCam COLON 2 system with an indication to provide visualization of the colon. It may be used for detection of colon polyps in patients after an incomplete optical colonoscopy with adequate preparation, and when a complete evaluation of the colon was not technically possible. In Early 2016, the FDA cleared PillCam COLON2 system with an expended indication for use: High risk population to undergo colonoscopy. Patients with major risks for colonoscopy or moderate sedation, but who tolerate colonoscopy and moderate sedation in the event a clinically significant colon abnormality was identified on capsule endoscopy. Other companies, including Olympus, CapsoVision, Intromedic and RF System, are developing similar approaches for optical capsule endoscopy.
49
Sales and Marketing
Our goal is to establish our position as a leading player in the CRC screening market. Although we do not currently generate revenues, we expect to generate revenues after we obtain regulatory clearance or approval through sales of our system.
Because we are still in the clinical and development stage, we are subject to certain challenges, including, among others, that:
|
•
|
our technology has been tested on a limited basis and therefore we cannot assure the product’s clinical value;
|
|
•
|
we need to receive CE Mark of conformity for the system in the European Union and obtain the requisite regulatory approvals in the United States, Japan and other markets where we plan to focus our commercialization efforts;
|
|
•
|
we need to raise an amount of capital sufficient to complete the development of our technology, obtain the requisite regulatory approvals and commercialize our current and future products;
|
|
•
|
we need to obtain reimbursement coverage from third-party payors for procedures using our system;
|
|
•
|
we need to increase our manufacturing capabilities; and
|
|
•
|
we need to establish and expand our user base while competing against other sellers of capsule endoscopy systems as well as other current and future CRC screening technologies and methods.
|
Our ability to operate our business and achieve our goals and strategies is subject to numerous risks as described more fully in “Risk Factors.”
Subject to our receipt of regulatory approvals, available capital, and engagement with strategic partners, we expect to commercialize our system during 2017 in selected markets in Europe, where regulatory hurdles are generally lower and in which current penetration of optical colonoscopy as a screening tool is low relative to the U.S. market. We intend to target major markets in Europe. In these markets, we are planning to work with strategic partners and/or local distributors who are active in the gastroenterology field and who have already demonstrated excellent performance in introducing new and innovative technologies.
In the European Union and Japan, we intend to offer our system as a primary screening tool for the general population. In the United States, we may target the population in a two-staged approach: first, we may seek to obtain the requisite regulatory clearance/approval for our system in a screening sub-population or as an adjunct tool to FOBTs and FITs, and after we have generated additional clinical data to support general screening indication, we would seek FDA clearance/approval for the use of our system as a primary screening tool for the general population.
Subject to the successful completion of our clinical trials and the receipt of our initial FDA clearance/approval, we expect to launch the product in the U.S. market, where we will consider setting up our own sales force or aligning with a strategic partner. Initially, we are planning to sell our system to the private sector. Simultaneously, we will work intensively to obtain reimbursement by Medicare and private insurers within the shortest possible time frame.
Subject to local regulatory approval, we also intend to market our system in key markets in Asia. Initial efforts will focus in Japan in view of its developed CRC screening market.
Manufacturing and Suppliers
Our manufacturing operations are conducted at a facility located in Isfiya, Mount Carmel, Israel. We lease approximately 610 square meters at this facility under a lease agreement providing for a term of 10 years, commencing on June 1, 2012 and ending on May 31, 2022, and in December 2015 we increased the leased area at our Isfia facility by an additional 290 square meters for clinical and development purposes, to a total of 900 square meters, under an addendum to the lease agreement with similar terms as in our current lease agreement. We have the right to terminate the agreement at any time, upon as least 60 days prior written notice. We currently have sufficient space to manufacture our system for the clinical studies but have limited resources, facilities and experience in commercially manufacturing large quantities of our system, external receiver and software application to meet the demand we expect from our expanded commercialization efforts. We expect to face certain technical challenges as we increase manufacturing capacity, including, among others, logistics associated with the handling of radioactive materials, equipment design and automation, material procurement, lower than expected yields and increased scrap costs, as well as challenges related to maintaining quality control and assurance standards. Our production objective is to establish a scalable capacity in order to meet such expanded demand for our system and market expansion. If we are unable to scale our manufacturing capabilities to meet market demand, our growth could be limited and our business, financial condition and results of operations could be materially adversely affected.
50
We are currently upgrading and expanding our production system and capacity and developing supply chain systems to support production for clinical trials and to meet standards for CE marking. Our current capacity was built to accommodate our clinical phase. We have integrated a product life management system to enable overall life cycle tracking and documentation including full configuration management control and manufacturing documentation.
During the clinical testing phase in Europe, we are planning to conduct both the assembling of our system and the insertion of the X-ray source at our facilities. As we proceed to the commercial phase in Europe and the U.S. clinical trial, we expect to collaborate with a third-party in the manufacturing and integration of the X-ray source into our system, in order to increase the time and cost efficiency of the process. We are currently in discussions with several potential partners for manufacturing and distribution purposes.
We do not currently have any sales. We are planning to develop a scale-up plan to meet our expected commercial supply needs. We are also working on a plan to expand our manufacturing capacity to support the expected larger clinical volume and subsequent higher volumes expected in the early commercialization stage. We are considering, among other options, the expansion of our assembly line in Israel, the buildup of new assembly lines in the United States and Europe, and alternative sources for the key capsule components (such as the motor, X-ray detectors, electrical components and PCBs). All of the facilities in which manufacturing and assembly of our products will be conducted will need to comply with applicable regulations and standards for medical devices.
We have received grants from Government of the State of Israel through the OCS, the terms of which require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless prior approval is received from the OCS, which we may not receive.
We are currently considering whether it would be possible to assemble the capsule without the X-ray source in Israel, and have the X-ray source subsequently inserted into our capsule at a reactor or cyclotron site or at a distribution center outside Israel. Even following the full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. See “Risk Factors – Risks Related to Our Operations in Israel.”
We currently depend on single source suppliers for some of the components necessary for the production of our system. For example, for the current version of the capsule used in clinical trials, we currently have one leading supplier for the motor that we are using to rotate the collimated X-ray source in our capsule and we currently have one leading supplier for the X-ray detectors used in our capsule. There are a limited number of manufacturers worldwide who are capable of manufacturing the motor and the specially designed X-ray detectors that we currently use in our capsule. In addition, the ASIC residing in our capsule is currently manufactured for us by a single FAB. Our X-ray source manufacturer is considered a single source supplier. Since our X-ray source is custom made and not off-the-shelf product, we will be required to qualify an alternative supplier, should we need to engage with an alternative manufacturer. There are many alternative FABs worldwide and the design of our current ASIC could be adapted in the event it became necessary to use an alternative FAB. Our current suppliers have been able to supply the required quantities of such components to date. However, if the supply of these components is disrupted or terminated or if our current suppliers are unable to supply required quantities of components, we may not be able to find alternative sources for these key components in a timely manner. Although we are planning to maintain strategic inventory of key components, the inventory may not be sufficient to satisfy the demand for our system if such supply is interrupted, or subject to risk of loss due to catastrophic events such as a fire at our storage facility. As a result, we may be unable to meet the demand for our system, which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to change the manufacturer of any of these key components, there may be a significant delay in locating a suitable alternative manufacturer. In addition, we may be required to verify that the new manufacturer maintains facilities and procedures that comply with FDA and other applicable quality standards and with all applicable regulations and guidelines. The delays associated with the identification of a new manufacturer could delay our ability to manufacture our system in a timely manner or within budget. Furthermore, in the event that the manufacturer of a key component of our system ceases operations or otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture the component. The occurrence of any of these events could harm our ability to meet demand for our system in a timely manner or within budget.
51
Environmental Health and Safety Matters
We are subject to environmental health and safety laws and regulations in Israel, governing, among other things, the use of radioactive materials, including the Israeli Work Safety Regulations (Occupational Safety and Health of Ionizing Radiation Practitioners) 1992-5753 and Women Employment Regulations (Work with Ionizing Radiation), 1979-5739. Our current research and development activities require, and our currently contemplated commercial activities will require, permits from various governmental authorities including, Israel’s Ministry of Environmental Protection, Israel’s Ministry of Health and local municipal authorities. Failure to obtain or maintain any such permits could have a material adverse effect on our business, financial condition and results of operations. The Ministry of Environmental Protection and the Ministry of Health conduct periodic inspections in order to review and ensure our compliance with the various regulations.
These laws, regulations and permits could potentially require expenditure by us for compliance or remediation. If we fail to comply with such laws, regulations or permits, we may be subject to fines and other civil, administrative or criminal sanctions, including the revocation of permits and licenses necessary to continue our business activities. In addition, we may be required to pay damages or civil judgments in respect of third-party claims, including those relating to personal injury (including exposure to radioactive materials) or contribution claims. Some environmental, health and safety laws allow for strict, joint and several liability for remediation costs, regardless of comparative fault. We may be identified as a responsible party under such laws. Such developments could have a material adverse effect on our business, financial condition and results of operations.
In addition, laws and regulations relating to environmental, health and safety matters are often subject to change. In the event of any changes or new laws or regulations, we could be subject to new compliance measures or to penalties for activities which were previously permitted.
U.S. Government Regulation
Our system is a medical device subject to extensive regulation by FDA and other U.S. federal and state regulatory bodies. To ensure that medical products distributed in the United States are safe and effective for their intended use, FDA has imposed regulations that govern, among other things, the following activities that we or our partners perform and will continue to perform:
|
•
|
product design and development;
|
|
•
|
product testing;
|
|
•
|
validation and verifications;
|
|
•
|
product manufacturing;
|
|
•
|
product labeling;
|
|
•
|
product storage, shipping and handling;
|
|
•
|
premarket clearance or approval;
|
|
•
|
advertising and promotion;
|
|
•
|
product marketing, sales and distribution; and
|
|
•
|
post-market surveillance reporting death or serious injuries and medical device reporting.
|
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, before we can commercially distribute medical devices in the United States, we must obtain, depending on the type of device, either prior 510(k) clearance or PMA approval from FDA. FDA classifies medical devices into one of three classes:
|
•
|
Class I devices, which are subject to only general controls (e.g., labeling, medical devices reporting, and prohibitions against adulteration and misbranding) and, in some cases, to the 510(k) premarket clearance requirements;
|
52
|
•
|
Class II devices, generally requiring 510(k) premarket clearance before they may be commercially marketed in the United States; and
|
|
•
|
Class III devices, consisting of devices deemed by FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, generally requiring submission of a PMA supported by clinical trial data.
|
510(k) Clearance Pathway
To obtain 510(k) clearance, we must submit a premarket notification, or 510(k) notice, demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which FDA has not yet called for the submission of premarket approval applications. FDA’s 510(k) clearance pathway takes approximately from 6 to 9 months, but it can take significantly longer. FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, require premarket approval. FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), or a premarket approval, but FDA can review any such decision and can disagree with a manufacturer’s determination. If FDA disagrees with a manufacturer’s determination, FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket approval is obtained. If FDA requires us to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
De Novo Reclassification
If FDA finds that there is no suitable predicate device for our system, it will automatically be considered a class III device. However, in instances where a device is novel and there is no suitable predicate device, but that device is deemed to be of low to moderate risk, FDA can reclassify the device to class I or class II via de novo reclassification. This process involves the submission of a reclassification petition, and FDA accepting that “special controls” are adequate to ensure the product’s performance and safety.
FDA now allows “direct” de novo reclassification petitions, a mechanism by which a sponsor can directly submit a detailed de novo reclassification petition as the device’s initial submission without having to first receive a not substantially equivalent, or NSE, decision on a 510(k) submission. The direct de novo pathway takes at least 9 to 12 months from submission of the petition to device clearance.
Our current strategy is to submit a direct de novo reclassification petition for our system. To support a direct de novo reclassification petition, our objective is to demonstrate that the device poses a low to moderate risk to patients. If FDA determines that our system is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process.
Alternatively, if we seek 510(k) clearance and our device is found not substantially equivalent, or NSE, a de novo petition must be filed within 30 days from the receipt of the NSE determination. The request should include a description of the device, labeling for the device, reasons for the recommended classification and information to support the recommendation. The de novo process following an NSE determination has a 60-day review period, although it is typical for the review to take far longer. If FDA classifies the device into class II, the company will then receive an approval order to market the device. This device type can then be used as a predicate device for future 510(k) submissions. However, if FDA subsequently determines that the device will remain in the class III category, then the device may not be marketed until the company has obtained an approved PMA.
Premarket Approval Pathway
A premarket approval application must be submitted if the device cannot be cleared through the 510(k) process or is found ineligible for de novo reclassification. The premarket approval application process is generally more costly and time consuming than the 510(k) process. A premarket approval application must be supported by extensive data including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to FDA’s satisfaction the safety and effectiveness of the device for its intended use.
53
After a premarket approval application is sufficiently complete, FDA will accept the application and begin an in-depth review of the submitted information. By statute, FDA has 180 days to review the “accepted application,” although, generally, review of the application can take at least 12 to 18 months, but it may take significantly longer. During this review period, FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside FDA may be convened to review and evaluate the application and provide recommendations to FDA as to the approvability of the device. In addition, FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations. New premarket approval applications or premarket approval application supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. Premarket approval supplements often require submission of the same type of information as a premarket approval application, except that the supplement is limited to information needed to support any changes from the device covered by the original premarket approval application, and may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials
Clinical trials are almost always required to support a premarket approval application or de novo reclassification petition and are sometimes required for a 510(k) premarket notification. If the device presents a “significant risk,” as defined by FDA, to human health, FDA requires the device sponsor to file an IDE application with FDA and obtain IDE approval prior to commencing the human clinical trials. The investigational device exemption application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The investigational device exemption application must be approved in advance by FDA for a specified number of patients, unless the product is deemed a “non-significant risk” device and eligible for more abbreviated investigational device exemption requirements. Clinical trials for a significant risk device may begin once the investigational device exemption application is approved by FDA and the appropriate institutional review boards at the clinical trial sites. Future clinical trials of our motion preservation designs will require that we obtain an investigational device exemption from FDA prior to commencing clinical trials and that the trial be conducted under the oversight of an institutional review board at the clinical trial site. Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy. A clinical trial may be suspended by FDA or the investigational review board at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the study. Even if a study is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device, or may be equivocal or otherwise not be sufficient to obtain approval of our product. Similarly, in Europe the clinical study must be approved by the local ethics committee and in some cases, including studies of high-risk devices, by the Ministry of Health in the applicable country.
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
|
•
|
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
|
|
•
|
Quality System Regulation, or QSR, and current good manufacturing practices, or cGMP, which require manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;
|
|
•
|
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication;
|
|
•
|
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices;
|
|
•
|
approval of product modifications that affect the safety or effectiveness of one of our approved devices;
|
54
|
•
|
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
|
|
•
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
•
|
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
|
|
•
|
FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
|
|
•
|
regulations pertaining to voluntary recalls; and
|
|
•
|
notices of corrections or removals.
|
We and our third-party manufacturers must register with FDA as medical device manufacturers and must obtain all necessary state permits or licenses to operate our business. As manufacturers, we and our third-party manufacturers are subject to announced and unannounced inspections by FDA to determine our compliance with quality system regulation and other regulations. We have not yet been inspected by the FDA.
Failure to comply with applicable regulatory requirements can result in enforcement action by FDA, which may include any of the following sanctions:
|
•
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
•
|
customer notifications for repair, replacement, refunds;
|
|
•
|
recall, detention or seizure of our products;
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products;
|
|
•
|
operating restrictions;
|
|
•
|
withdrawing 510(k) clearances on PMA approvals that have already been granted;
|
|
•
|
refusal to grant export approval for our products; or
|
|
•
|
criminal prosecution.
|
Regulatory Pathway for our System
We have established a clinical and regulatory strategy with our advisors and have conducted a pre-IDE meeting, now referred to as a pre-submission meeting, with FDA (an informal interaction to facilitate a clearer understanding of FDA’s expectations). During this process, we received FDA’s feedback on our submission and our questions and we are planning to continue the dialogue with FDA before the submission of a formal request for the IDE that is necessary for us to conduct the U.S. pivotal clinical trial.
Our current strategy is to submit a direct de novo reclassification petition for our system. Although FDA could require us to submit a PMA, we believe that the device could be considered for evaluation under FDA’s de novo reclassification provisions, which allow a novel device to be reclassified into class I or class II. To support this, our objective is to demonstrate that device poses a low to moderate risk to patients.
We believe that important potential benefits of our system for CRC screening are the elimination of the need for bowel preparation, the elimination of the need for conscious sedation, the minimally invasive, painless nature of the examination, and the ability to pursue normal daily activities immediately following the procedure. Furthermore, the system is being designed to generate information from segments of the colon (e.g., cecum and ascending colon) that are difficult to access by conventional optical colonoscopy (i.e., incomplete colonoscopies) without the risks and discomforts of operative examination or other invasive methods. We believe these benefits will be attractive to a large number of patients from the target populations that so far refrained from any screening tests. Thus, we anticipate that our capsule will increase the public compliance to screening recommendation.
55
If FDA determines that our system is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process. Because of the technological characteristics of this device, the non-clinical tests (including lab and animals) and clinical data required may not be significantly different between a de novo and PMA regulatory processes. We believe that under both scenarios, we will be required to conduct a multi-center clinical study to establish the safety and efficacy and to demonstrate sensitivity and specificity of our system in several hundreds of patients.
FCC Clearance and Regulation
Because our system includes a wireless radio frequency transmitter and receiver, it is subject to equipment authorization requirements in the United States. The U.S. Federal Communications Commission, or FCC, requires authorization of radio frequency devices before they can be sold or marketed in the United States, subject to limited exceptions. The authorization requirements are intended to confirm that the proposed products comply with FCC radio frequency emission, power level standards, and other technical requirements and will not cause interference. Our capsule is using the same frequency band as other approved capsules, and we expect that it will comply with the FCC’s technical requirements, so it is expected that it will be authorized by the FCC as well.
Third-Party Coverage and Reimbursement
Coverage of and reimbursement for our system and related procedures, after approval, will be subject to the requirements of various third-party payors, including government-sponsored healthcare payment systems and private third-party payors. Coverage policies and reimbursement methodologies vary significantly from program-to-program, and may be subject to federal and state regulations. For example, the Medicare statute requires all items and services to be “reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member” in order to be covered. Medicare currently does not provide separate reimbursement for many devices, but may include payment for the device in the payment for the related procedure. Third-party payors’ coverage and reimbursement policies, including their interpretations of whether an item or service is “reasonable and necessary” or experimental and their payment methodologies, are subject to change pursuant to legislation, regulation, or, in the case of private payors, negotiations with providers.
Fraud and Abuse Laws
In the United States, the healthcare industry is subject to extensive federal, state, and local regulation. Both federal and state governmental agencies subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices, by limiting the kinds of financial arrangements (including sales programs) we may have with hospitals, physicians and other potential purchasers of the medical devices. The laws and regulations that may affect our ability to operate include, but are not limited to:
|
•
|
The federal Anti-Kickback Statute, which prohibits, among other things, knowingly or willingly offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any health care items or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between medical device manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Further, PPACA, among other things, clarified that a person or entity needs not to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor may be subject to scrutiny;
|
56
|
•
|
The federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. In addition, PPACA amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Many medical device manufacturers and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under the civil False Claims Act for a variety of alleged improper marketing activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to use the company’s products. In addition, in recent years the government has pursued civil False Claims Act cases against a number of manufacturers for causing false claims to be submitted as a result of the marketing of their products for unapproved, and thus non-reimbursable, uses. Device manufacturers also are subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs;
|
|
•
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the payor. Several states now require medical device manufacturers to report expenses relating to the marketing and promotion or require them to implement compliance programs or marketing codes. For example, California, Connecticut and Nevada mandate implementation of corporate compliance programs, while Massachusetts and Vermont impose more detailed restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to health care providers;
|
|
•
|
The federal Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the U.S. Securities and Exchange Commission. Violations of these laws can result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence; and
|
|
•
|
The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires manufacturers of “covered products” (drugs, devices, biologics, or medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program) to track and publicly report payments and other transfers of value that they provide to U.S. physicians and teaching hospitals, as well as any ownership interests that U.S. physicians hold in applicable manufacturer. Applicable manufacturers must submit a report to the Centers for Medicare & Medicaid Services, or CMS, by the 90th day of each calendar year disclosing payments and transfers of value made in the preceding calendar year.
|
Violations of any of the laws described above or any other governmental regulations that apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government-funded healthcare programs, like Medicare and Medicaid, and the curtailment or restructuring of our operations.
Privacy Laws
HIPAA/HITECH and related U.S. federal and state laws protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the HIPAA.
These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their protected health information and limiting most use and disclosures of health information to the minimum degree reasonably necessary to accomplish the intended purpose. Because we intend to sell products, once approved, to persons and entities subject to HIPAA and are exposed to personally-identifiable health information in the course of our operations, we also may be subject to certain elements of HIPAA, particularly as a business associate to covered entities, as well as similar state laws. HIPAA imposes civil and criminal penalties for violations of its provisions, which could be substantial. State privacy laws have their own penalty provisions which may be applicable.
57
NRC Regulatory Issues
As our system includes an ingestible capsule with a radioactive source, the company must address NRC regulations, or relevant Agreement State regulations, in addition to FDA requirements. An Agreement State is a state that has signed an agreement with the NRC authorizing the state to regulate certain uses of radioactive materials within the state. Agreement State regulations are substantially similar to the NRC’s regulations.
The capsule is loaded with the X-ray source, sealed and then ingested by the patient. The capsule is excreted naturally through the patient’s system and is not recovered and therefore is disposed of in the sanitary sewer system. Although the NRC regulations in 10 CFR Part 20 place certain conditions and limitations on the disposal of radioactive material in the sanitary sewer, such conditions and limitations do not apply to radioactive material contained in excreta of individuals that are undergoing medical diagnosis or therapy with radioactive material. Our regulatory advisors have advised us that the NRC staff likely would take the position that a capsule containing radioactive material can be passed in excreta into the sanitary sewer system without limitation. If, however, the NRC were to find that our capsule system could not be passed in excreta into the sanitary sewer system without limitation pursuant to 10 CFR 20.2003(b) the NRC may place restrictions on the disposal of the capsule system in the sanitary sewer system.
An entity which manufactures, prepares, or transfers a medical capsule containing radioactive byproduct material needs to be licensed or covered by a license issued by the NRC or an Agreement State. An NRC or Agreement State licensee authorized to possess and/or distribute byproduct material can transfer the byproduct material only to another NRC, or Agreement State, approved entity or licensee. The NRC’s regulations permit only individuals who are authorized users (e.g., individuals who meet certain training and experience criteria regarding the safe use of radioactive drugs) or persons working under the supervision of an authorized user to administer radioactive drugs for medical use.
The NRC regulations do exempt certain products from the NRC’s regulations. Existing exemptions from licensing requirements for the use of byproduct material include exemptions for specific products (e.g., time pieces), exemptions for classes of products (e.g., gas or aerosol detectors), and broader materials exemptions for “exempt concentrations” and “exempt quantities” of radioactive material. These two broad materials exemptions specifically exclude the transfer of byproduct material contained in any food, drug, or product designed for ingestion by a human being. Capsules containing our X-ray source would not qualify as an “exempt quantity” because of their intended use (i.e., for ingestion) even though they may contain a smaller quantity of the source than the exempt quantities set forth in the regulations.
Accordingly, we will need to obtain the appropriate licenses from the NRC or an Agreement State prior to the clinical investigation and/or marketing of the device. We intend to engage a radiopharmaceutical company to manufacture our system. The fact that another company will be manufacturing the capsule, however, does not exempt us from also obtaining radioactive materials licenses from the NRC or an Agreement State. Distribution activities are generally classified by the NRC as either “distribution” or “redistribution”, and both types of activities require a specific license. “Distribution” refers to the initial transfer from the manufacturing radiopharmacy, while “redistribution” refers to a subsequent transfer of the drug by an NRC licensee to an authorized user. In order to distribute the capsule commercially, we will need to obtain an NRC or Agreement State “medical distribution” radioactive materials license and may also need to obtain a radioactive materials license authorizing the possession of the radioactive material. Both types of licenses may be obtained by submitting a license application request to the NRC or an Agreement State. In the event that we develop the capsule outside the United States, we would be required to have one of our U.S. offices apply for and receive both the possession and medical distribution radioactive materials licenses. If we do not have an office in the United States, then we can contract with a company with a U.S. office to apply for and obtain these licenses, and that company would be the licensed U.S. distributor of the capsule.
We may be able to petition the NRC to carve out an exemption for the distribution licensing requirement to permit distribution to all health care professionals and not just those licensed by the NRC. This has been done successfully by other medical device companies. For example, Tri-Med, Inc. manufactures an ingestible capsule containing radioactive material for testing of H. Pylori. The company petitioned the NRC in 1994 for an exemption from the distribution licensing regulation. The NRC evaluated the petition and issued a proposed ruling for comments. After receiving comments on the proposed ruling, the NRC issued a final ruling, in 1997, providing for the exemption. This exemption is narrowly drawn, and specific to the distribution of a “radioactive drug containing one microcurie of carbon-14 urea to any person for ‘in vivo’ diagnostic use.” In creating the exemption, the NRC noted the importance of bringing an inexpensive and effective diagnostic tool to a large number of people, along with the minimum radiation contained in the capsule.
58
We may consider petitioning the NRC in a similar manner to make the device more widely available. As our system imparts comparable radiation exposure to the Tri-Med device described above, and has the potential to be used widely for diagnosis, our system may be a candidate for such an exemption.
Regulation in Europe, Japan and Other Countries
In the EU, a company that wishes to bring a medical device to market must CE mark the device following demonstration of conformity with the Essential Requirements laid down in Annex I to the Medical Devices Directive.
Compliance with these requirements entitles manufacturers to affix the CE mark of conformity to their medical devices, without which the medical devices cannot be commercialized in the EU. To demonstrate compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive and obtain the right to affix the CE mark of conformity to our medical devices, we and our products must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Apart from low risk medical devices (Class I with no measuring function and which are not sterile), in relation to which the manufacturer can issue an EC Declaration of Conformity based on self-assessment of the conformity of its products with the Essential Requirements laid down in the Medical Devices Directive, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization designated by the competent authorities of a EU Member States to conduct conformity assessments. The Notified Body will typically audit and examine the Technical File that the manufacturer has created for the medical devices and the quality system for the manufacture, design and final inspection of the devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements laid down in Annex I to the Medical Devices Directive or the quality system requirements laid down in the other Annexes to the Directive. Following the issuance of this CE Certificate of Conformity, the manufacturer is required to draw up an EC Declaration of Conformity and to affix the CE mark to the products covered by this CE Certificate of Conformity and the EC Declaration of Conformity.
We will be required to demonstrate that our products comply with the Essential Requirements laid down in Annex I to the Medical Devices Directive through a conformity assessment procedure. We cannot be certain that we will be able to fulfill the quality and performance requirements laid down in Annex I to the Medical Devices Directive and to obtain or maintain CE Certificates of Conformity for our products. If we are unable to obtain or maintain these CE Certificates of Conformity for our products, we will not be able to sell our products in any EU Member States nor in various other third countries where CE marking is accepted as evidence of compliance with relevant national laws.
In Japan, manufacturing and marketing medical devices are regulated by the Pharmaceutical Affairs Law, or PAL. In accordance with the PAL, manufacturers must obtain a license for manufacturing medical devices from the Ministry of Health, Labour and Welfare, or MHLW. A license is required for each manufacturing plant specified by an MHLW Ministerial Ordinance.
A licensed manufacturer is responsible only for manufacturing medical devices. In regard to the marketing of medical devices, the PAL specifies that a Marketing Authorization Holder, or MAH, licensed by MHLW is responsible for putting medical devices into marketplace. Licenses for marketing medical devices are divided into the following 3 types, which correspond to the classifications below:
|
•
|
No. 1 type license for marketing – Specially controlled medical devices (Class III, IV)
|
|
•
|
No. 2 type license for marketing – Controlled medical devices (Class II)
|
|
•
|
No. 3 type license for marketing – General medical devices (Class I)
|
Generally, the process for obtaining marketing clearance for medical devices in Japan ranges from twelve months for products with only very minor modifications from previously cleared product versions, to a few years in the case of a completely new device.
In order for us to market our products in countries other than the United States, the EU and Japan (which were described above), we must obtain regulatory approvals and comply with extensive safety and quality regulations in these countries. These regulations, including the requirements for approvals or clearance and the time required for regulatory review varies from country to country. It is customary that once a product has been cleared for sales in the US and is CE marked in the EU, many other countries will follow. Failure to obtain regulatory approval or clearance in any foreign country in which we plan to market our product may harm our ability to generate revenue and harm our business.
59
Third-Party Reimbursement
Reimbursement in the United States
In the United States, healthcare providers that purchase medical devices generally rely on third-party payors, such as Medicare, Medicaid, private health insurance plans and health maintenance organizations, to reimburse all or a portion of the cost of the devices, as well as any related healthcare services utilizing the devices.
Coverage is not guaranteed simply because a product has received FDA clearance or approval. Medicare’s general definition of a medically necessary service is one that is reasonable and necessary for the diagnosis or treatment of an illness or injury, or that improves the functioning of a malformed body member. In order to be eligible for reimbursement, a device must be proven to be cost-effective, demonstrating potential decrease in spending to the U.S. health economy.
According to various studies and publications, a key criterion for to reimbursement for colon cancer screening is patient adherence (for instance, “Cost-Effectiveness of Colonoscopy in Screening for CRC,” Annals of Internal Medicine, October 17, 2000 vol. 133 no. 8 573-584). Adherence is strongly affected by patients’ willingness to use the device as a screening tool for CRC. Several models have been designed to demonstrate the cost effectiveness of optical colonoscopy, CTC, fecal testing and optical capsule endoscopy. Today, several technologies achieved Medicare coverage for CRC Screening, including: FOBT / FIT, Flexible Sigmoidoscopy, Optical Colonoscopy and Barium Enema, and stool DNA.
In 2009, the Centers for Medicare and Medicaid Services issued a decision memorandum rejecting federal reimbursement for CTC screening for CRC. Their main argument for the decision was that based on available evidence, screening with CTC would not necessarily result in cost saving, at least at current screening compliance rates. CTC was not seen as a tool which could potentially increase patients’ adherence. This procedure involves bowel preparation, as well as insufflations of the colon and the exposure of patients to very significant amount of radiation.
An important European study (C. Hassan et al, “Cost Effectiveness of Optical capsule endoscopy,” Endoscopy 2008, 40, 414-421) assessed the potential cost effectiveness of screening with optical capsule endoscopy and compared the cost-effectiveness with that of optical colonoscopy. Effectiveness of screening was measured in terms of life-years saved through prevention or down staging of CRC. The conclusion was that adherence to optical capsule endoscopy may be presumed to be substantially higher than that of optical colonoscopy.
Third-party payors in the United States began issuing coverage policies for optical capsule endoscopy in early 2002. Initially, all reimbursement policies provided coverage for optical capsule endoscopy of the small bowel only for the diagnosis of obscure gastrointestinal bleeding. Subsequently, reimbursement coverage has been expanded to include other diagnoses and as of December 31, 2012, approximately 220 million people in the United States are covered with most reimbursement policies providing coverage for a number of small bowel indications, including obscure gastrointestinal bleeding, suspected Crohn’s disease, suspected small bowel tumors and other small bowel pathologies.
In 2013, for procedures performed in a physician’s office, the national average global fee paid by Medicare under the CPT code for optical capsule endoscopy of the small bowel was $971. For procedures performed in an outpatient hospital setting, the national average physician fee paid by Medicare was $197 and the national average payment rate to the hospital for the technical component was $751. For optical capsule endoscopy of the esophagus, in 2013, the national average global fee paid by Medicare for a procedure in a physician’s office was $812. The national average physician fee paid by Medicare in an outpatient hospital setting was $54 and the national average payment rate to the hospital for the technical component was $927.
Below are the fees paid by Medicare in 2015 under the CPT code for screening optical colonoscopy for individuals not at high risk:
Payment for Physician’s Services:
|
Code
|
Description
|
Payment (1)
|
|||
|
G0121
|
Colorectal cancer screening; colonoscopy on individual not meeting criteria for high risk
|
$
|
208.83
|
||
Payment for the Hospital:
|
Code
|
Short Description
|
APC
|
Payment (2)
|
||||
|
G0105
|
Colorectal scrn; not high risk individual
|
0158 (CRC
|
$
|
646.73
|
|||
60
On December 2, 2015, Exact Sciences Corp. announced that it expects to be reimbursed 483.35 for Cologuard tests provided to Medicare beneficiaries.
Private Payers
The national average cost of an optical colonoscopy may range from $2,000 to $3,000. The cost also depends on the state or city in which the procedure takes place. Currently, there is no optical capsule endoscopy reimbursement available for the colon in the United States, nor is there a CPT code for such capsule or related method of screening. There can be no assurance that coverage will be obtained in the near future or at all. Third-party payors may deny coverage if they determine that a procedure was not reasonable or necessary as determined by the payor, was experimental or was used for an unapproved indication. During the past several years, the major third-party payors have substantially revised their reimbursement methodologies in an attempt to contain or reduce their healthcare reimbursement costs.
Reimbursement rates vary depending on the third-party payor and individual insurance plan involved, the procedure performed and other factors. Medicare reimbursement for in-patient hospital services is based on a fixed amount per admission based on the patient’s specific diagnosis and the procedure performed during the hospital stay. As a result, any illness to be treated or procedure to be performed in an in-patient setting will be reimbursed only at a prescribed rate set by the government.
Coverage Outside the United States
In countries outside the United States, coverage for CRC screening is obtained from various sources, including governmental authorities, private health insurance plans, and labor unions. In some countries, private insurance systems may also offer payments for some therapies. Although not as prevalent as in the United States, health maintenance organizations are emerging in certain European countries. Coverage systems in international markets vary significantly by country and, within some countries, by region. Coverage approvals must be obtained on a country-by-country or region-by-region basis.
C. Organizational Structure
On May 15, 2015, we formed our wholly-owned subsidiary Check-Cap US, Inc., a Delaware corporation.
D. Property, Plants and Equipment
Our principal executive offices and operations are conducted at a facility located in Isfiya, Mount Carmel, Israel since June 1, 2009. We lease approximately 610 square meters at this facility under a lease agreement providing for a term of 10 years, commencing on June 1, 2012 and ending on May 31, 2022, unless earlier terminated by us upon at least 60 days prior written notice, and in December 2015 we increased the leased premises at our Isfia facility by an additional 290 square meters for clinical and development purposes, to a total of 900 square meters, under an addendum to the lease agreement with similar terms as in our current lease agreement.. We believe that our current facilities are adequate to meet our current needs for the clinical phase of our development.
ITEM 4A. UNRESOLVED STAFF COMMENTS
None.
61
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our consolidated financial statements and related notes included in this Annual Report beginning on page F-1. The following discussion and analysis contain forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under Item 3D “Key Information - Risk factors” and elsewhere in this Annual Report.
Overview
We are a clinical stage medical diagnostics company engaged in the development of an ingestible capsule system that utilizes ultra low-dose X-rays for the detection and imaging of colonic polyps and colorectal cancers, or CRC. While CRC is the second leading cause of death from cancer for both sexes combined in the United States and is largely preventable with early detection, according to 2013 National Health Interview Survey, only 58% of Americans between the ages of 50 to 75 reported being current with CRC screening recommendations. Unlike other screening modalities that are designed to generate structural information of the internal colon for the detection of colonic polyps and CRC, such as optical colonoscopy, computed tomographic colonography, or CTC, and other capsule-based technologies, our system is designed to be ingested without any cathartic preparation of the colon, and to travel through the gastrointestinal tract naturally while the patient continues his or her normal daily routine. Furthermore, unlike existing CRC imaging modalities currently on the market, all of which require the patient to fast for several hours prior to administration, the procedure for the Check-Cap system is designed to enable patients to continue eating normally. Our system is comprised of three main components: (1) ingestible scanning capsule; (2) Capsule Positioning System, or CPS, a recorder worn on the patient’s back; and (3) a PC-based work station for data reconstruction and image processing. We believe that this solution will be attractive to both physicians and patients, with the potential to increase the number of people undergoing CRC screening.
Check-Cap LLC was formed in 2004 as a Delaware limited liability company to develop a novel and superior solution for colon cancer screening. In 2009, all of the business operations and substantially all of the assets of Check-Cap LLC were transferred to Check Cap Ltd., a newly-incorporated Israeli company. Our offices are located near Haifa, in the northern part of Israel. Our management team includes an experienced group of executives in the business, research, clinical and regulatory fields. As of February 15, 2016, our research and development team consists of 50 experienced professionals (including employees and independent contractors) in the fields of physics, electronics, software and mechanical engineering.
For the past several years, we have focused on the research and development of our imaging technology. We have successfully imaged sections of the colon's inner surfaces and detected polyps of various sizes in pigs without any prior bowel preparation through the insertion of a larger version of our system in their colons. We also conducted a clinical study in Europe with a passive (non-imaging) version of our capsule that has similar dimensions to our first prototype capsule, in order to collect pressure and motility data from humans. This information assisted in the development of the initial operational algorithm utilized by our capsule software or the version of our system used in humans.
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Sourasky Medical Center in Israel and used a prior version of our system, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our system to the human colon, generating images taken in the colon without any prior bowel preparation. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted.
Another objective of the 10-case study was to estimate total radiation exposure for each case study. This was calculated using standard established factors for calculating effective radiation exposure, such as the duration of the capsule inside the body, and was based on the activity of the radiation source inside the scanning capsule and radiation energy, both of which were measured for each case study. The average calculated exposure for the entire procedure in the 10-case study, from ingestion of the capsule to excretion, was 0.03 mSv (STD 0.007 mSv). This level of radiation exposure is similar to a single chest X-ray (approximately 0.06mSv) and two orders of magnitude less than a CTC.
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of our system. The 10-case study was the first phase of a multi-center, prospective clinical feasibility study to establish the safety, functionality and preliminary efficacy of our system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity FOBTs and FITs, as well as from optical colonoscopies. The feasibility study is designed allow for recruitment of 100 subjects. The study is being conducted at multiple centers in Israel, with the potential to be conducted at a single site in the Netherlands. The clinical feasibility study will evaluate the image resolution generated by the capsule in an a human colon without cathartic preparation, will assess polyp imaging in various shapes and in different segments of the colon and will evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified through our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
62
A preliminary analysis conducted on the first 49 capsules swallowed by participants enrolled in the multi-center, prospective clinical feasibility study showed 48 of 49 capsules swallowed and naturally eliminated without major or minor side effects after 73.2±45.4 hours. Image reconstructions allowed 3D views of the colonic wall and lumen with the typical contour of different segments (hepatic flexure, triangular shape of the transverse colon). Both pedunculated and sessile polyps were detected in several patients and validated later by colonoscopy.
To date, we have achieved key product development milestones, including the demonstrated ability of our system to reconstruct the human colon and to identify polyps, and design freeze of the current version of our system. Following the successful completion of the multi-center, prospective clinical feasibility study and design release and transfer to manufacturing phases, we plan to submit during the second half of 2016, a request for CE marking for the marketing and sale of our capsule in the European Union. We expect to perform post-marketing studies in Europe following CE marking for the purpose of collecting additional clinical data to support market adoption. Subject to regulatory approvals, available capital, and engagement with strategic partners, we anticipate launching our system commercially in Europe during 2017.
We plan to conduct a pre-submission meeting with the U.S. Food and Drug Administration, or FDA, during 2016. Subject to this meeting, we plan subsequently to submit a request for the approval of an investigational device exemption, or IDE, for a pilot study in the United States. Subject to successful completion of the pilot study and receipt of required approvals, we plan to initiate during 2017, a pivotal study in the United States to (i) demonstrate device safety as evidenced by a lack of device-related serious adverse events; and (ii) provide efficacy data concerning our system’s performance. We anticipate that FDA approval for the pivotal study will be subject to our providing sufficient clinical data from previous clinical studies, which may include the multi-center, prospective clinical feasibility study and U.S. pilot study. However, there can be no assurance that the FDA will grant approval for the pilot and/or pivotal studies to be conducted in the United States.
We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study, subject to available capital and engagement with strategic partners. Pivotal studies are expected, among other things, to compare polyps identified by our system with the polyps identified by traditional optical colonoscopy. These clinical findings may be analyzed in comparison with results obtained from FOBTs and FITs.
Following and subject to the successful completion of our pivotal trial, our current strategy is to submit a direct de novo reclassification petition, which we anticipate submitting in 2018, for initial FDA clearance for the marketing of our system in the United States. Direct de novo reclassification typically takes at least 9 to 12 months from filing to clearance. If the FDA determines that our system is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process The PMA pathway is much more costly and uncertain than the 510(k) clearance process or de novo reclassification, and generally takes at least 12 to 18 months, or even longer, from the time the application is filed with FDA to ultimate approval.
Timelines expectations are based on our current estimations and expectations, which may continue to be updated along with our progress, which is subject to the occurrence of various factors and future events, among others, the satisfactory completion of system’s development process, testing, and integration, which may require more time than currently expected, as well as the success of our clinical trials and the completion of our required regulatory approvals, all of which are uncertain as of the date of this Annual report.
Our system is being designed to create a reconstructed three-dimensional image of the interior surface of the colon and to enable detection of clinically significant polyps with a high degree of sensitivity. Colonic polyps are tissue growths that occur on the lining of the colon. Polyps in the colon are extremely common, and certain types of polyps can become cancerous over time.
Our scanning capsule will be swallowed by the patient and propelled by natural motility through the gastrointestinal tract and excreted naturally with no need for retrieval for data collection. Unlike other CRC screening methods, this process should not disrupt a patient’s normal activities or require fasting. Our scanning capsule employs low-dose X-rays, which allow the system to image the inner surface of the colon even when surrounded by intestinal content. As such, we believe that patients using our system will not be required to undergo any prior bowel preparation. The Radiation Safety Division of the Soreq Nuclear Research Center found, as set forth in its report of November 2010 that was prepared at our request and based on the information provided by us and the relevant methods and principles known at such time, or the Report, that the radiation dose to the patient in the proposed screening procedure utilizing the scanning device developed by us at that time in routine operation and normal conditions is low relative to the radiation dose involved in conventional imaging procedures using X-rays (such as fluoroscopy and CT) and is also low when compared to the radiation dose involved in established screening procedures such as mammography, all as more fully described in the Report.
63
Our scanning capsule is being designed to transmit position, motility, and the data it collects to an external data recorder and capsule positioning system (CPS) that will be worn by the patient. The external data recorder is being designed to enable the transfer of the data to our PC-based work station with viewer software application to allow physicians to analyze the data collected by our scanning capsule. The CPS is being designed to provide the physician with accurate localization data aligned with a reconstructed image. We intend for physicians to be able to review the colon’s inner images at any location at any time, in less time than is required to perform an optical colonoscopy.
In order to enable a complete view of capsule positioning and motility, we have designed a data recorder and Capsule Positioning System, or CPS, which is worn on the patient’s back throughout the entire procedure. The CPS is being designed to provide the physician with accurate localization data aligned with a reconstructed image.
In the event that polyps are identified through our system, the patient may be advised to undergo a subsequent traditional colonoscopy procedure to examine, remove and biopsy the polyps. For those patients who require a subsequent polypectomy, concerns regarding pain, discomfort and embarrassment may still remain with respect to the subsequent polypectomy. We do not, however, believe that these concerns will make the use of our system any less attractive to physicians and patients. Although patients who are initially screened utilizing a traditional colonoscopy could avoid the need for a second procedure if polyps are discovered because they could undergo a polypectomy during the initial screening, if necessary, we believe that our system will still be attractive to physicians and patients as a large majority of patients who are screened will not require a subsequent polypectomy. Published data from a multi-center CT colonography screening study of 2,531 asymptomatic adults showed that if all patients with a lesion measuring 5mm or more on CT colonography were referred for colonoscopy, the colonoscopy-referral rate would have been 17%.
We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have been granted patents for our core patent by the U.S. Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan. We also filed patent applications describing the use of our technology in several other medical applications.
We have generated significant losses to date, and we expect to continue to generate losses for at least the next several years as we continue our investment in research and development and clinical trials in order to complete the development of our system and to attain regulatory approvals, begin the commercialization efforts for our products, increase our marketing and selling expenses, and incur additional costs as a result of becoming a public company in the United States. The extent of our future operating losses and the timing of becoming profitable are highly uncertain, and we may never achieve or sustain profitability. As of December 31, 2015, we had accumulated losses of approximately $34.1 million.
On February 24, 2015, we consummated an initial public offering of 2,000,000 units, each consisting of one ordinary share and one half of a Series A Warrant to purchase one ordinary share. The price per unit sold in the initial public offering was $6.00. Each unit in the initial public offering was issued with one and one half Long Term Incentive Warrants. We granted the underwriters in the initial public offering a 45-day over-allotment option to purchase up to 300,000 additional units (together with an accompanying 450,000 Long Term Incentive Warrants) from us to cover over-allotments. On March 6, 2015, the option to purchase additional 100,000 units was partially exercised. On March 18, 2015, the units were separated into one ordinary share and one-half of a Series A Warrant to purchase one ordinary share and the units ceased to exist. On April 6, 2015, the option to purchase additional 150,000 ordinary shares and 75,000 Series A Warrant was partially exercised. The aggregate offering price of the securities sold in the initial public offering (including the over-allotment option) was approximately $13.5 million. The total expenses of the offering, including underwriting discounts and commissions, were approximately $2.9 million (including certain warrants with value of $196,000 issued in connection with the IPO). The net proceeds we received from the initial public offering (including the over-allotment option) was approximately $10.8 million. Concurrently with our initial public offering, we consummated the private placement of 2,000,000 units in a private placement and received aggregate gross proceeds of $12,000,000 from the private placement. We believe that the net proceeds from our initial public offering and concurrent private placement, together with our existing cash, will be sufficient to fund our currently anticipated cash requirements at least until March 31, 2017. Nevertheless, we will require significant additional financing in the future to fund our operations if and when we progress with our clinical trials in Europe and the United States, and other territories.
For a more detailed description of our business and plans, see Item 4B “Information on Our Company – Business Overview.”
64
|
A.
|
Operating Results
|
Financial Operations Overview
Revenue
We have not generated any revenue since our inception. To date, we have funded our operations primarily through equity financings, as well as from grants that we received from the OCS. If our product development efforts result in clinical success, regulatory approval and the successful commercialization of our imaging system, we expect to generate revenue from sales of our imaging system.
Operating Cost and Expenses
Our operating costs and expenses are classified into two categories: research and development expenses and general and administrative expenses. For each category, the largest component is personnel costs, which consists of salaries, employee benefits and share-based compensation. Operating costs and expenses also include allocated overhead costs for depreciation of equipment. Operating costs and expenses are generally recognized as incurred. We expect personnel costs to continue to increase as we hire new employees to continue to grow our business.
Research and Development Expenses, Net
Research and development activities are central to our business model. We intend to increase our research and development operations in order to complete the development of our imaging system and to attain regulatory approvals.
Since 2013, we have spent approximately $11.6 million on research and development expenses as of December 31, 2015, of which $1.1 million was funded by government grants. Our total research and development expenses, net of participations in the years ended December 31, 2015, 2014 and 2013 were approximately $5.8 million, $2.8 million and $2.9 million, respectively. All research and development expenses are expensed as incurred. We expect research and development expenses to increase in absolute terms in the near term.
Research and development expenses consist primarily of costs incurred for our research activities, including:
|
•
|
employee-related expenses for research and development staff, including salaries, benefits and related expenses, including share-based compensation and travel expenses;
|
|
•
|
payments made to third-party contract research organizations, contract manufacturers, investigative sites and consultants;
|
|
|
•
|
manufacturing development costs;
|
|
•
|
costs associated with preclinical and clinical activities and regulatory operations;
|
|
|
•
|
facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities; and
|
|
•
|
costs associated with obtaining and maintaining patents.
|
Our research and development expenses, net are net of grants we have received from the Government of Israel through the OCS. In exchange for these grants, we are required to pay the OCS royalties from our revenues up to an aggregate of 100% (which may be increased under certain circumstances) of the U.S. dollar-linked value of the grant, plus interest at the rate of 12-month LIBOR. Pursuant to regulations under the Research Law, the rate of repayment ranges between 3% to 5% of revenues (or 6% with respect to certain limited programs and at an increased rate under certain circumstances). As of December 31, 2015, we had received funding from the OCS in the aggregate amount of $3.6 million. As of December 31, 2015, we had not paid any royalties to the OCS and had a contingent obligation to the OCS in the amount of $3.8 million. In December 2015, we received approval for an additional grant from the OCS in the amount of up to $340,000, and in January 2016, we applied for an additional grant from the OCS in the amount of up to $6.9 million. For additional information regarding the OCS grants, see Item 5B “Operating and Financial Review and Prospects - Liquidity and Capital Resources – Application of Critical Accounting Policies and Estimates –Royalties provision - Government grants from the Office of the Chief Scientist and BIRD Foundation” and in Item 10E “Additional Information - Taxation - Israeli Tax Considerations and Government Programs - The Encouragement of Industrial Research and Development Law, 5744-1984.” There can be no assurance that we will continue to receive grants from the OCS in amounts sufficient for our operations, if at all.
65
As of December 31, 2015, we had received funding from the BIRD Foundation of $127,000 as part of a grant for the funding of a project entitled “Collection & Analysis of Gastrointestinal Images for Diagnostic Adenomatic Polyps and Colorectal Cancer.” We expect to receive an aggregate of $900,000 to fund our expenditures for the project, in five installments. As of December 31, 2015, we had not paid any royalties to the BIRD Foundation and had a contingent liability to BIRD Foundation in the amount of $127,000. See Item 5B “Operating and Financial Review and Prospects — Liquidity and Capital Resources — Sources of Liquidity” for a description of the Cooperation and Project Funding Agreement with the BIRD Foundation and Synergy Research Inc., or Synergy.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries and other related costs, including share-based compensation expense, for persons serving as our executive, finance, legal, human resources and administrative personnel, professional service fees and other general corporate expenses, such as communication, office and travel expenses. We expect that our general and administrative expenses will continue to increase in the future as we incur additional general and administrative costs associated with being a public company in the United States, including compliance under the Sarbanes-Oxley Act of 2002 and rules promulgated by the U.S. Securities and Exchange Commission. These public company-related expense increases will likely include costs of additional legal fees, accounting and audit fees, directors’ and officers’ liability insurance premiums and costs related to investor relations.
Financial Income and Finance
Our finance income and finance expenses consist primarily of fair value changes with respect to warrants to purchase Series D-1 and D-2 preferred shares issued to investors and service providers in connection with our D1 investment round, and warrants to purchase Series C-1 preferred shares and warrants to purchase Series C-2 preferred shares issued to Pontifax (investing through three affiliated funds: Pontifax (Cayman) II, L.P., Pontifax (Israel) II L.P., Pontifax (Israel) II- Individual Investors L.P. which we collectively refer to as the “Pontifax Funds”), interest earned on our cash, cash equivalents and short-term investments and changes in provision for royalties primarily to Check –Cap LLC unitholders.
Foreign currency transactions are translated into U.S. dollars using the exchange rates prevailing at the dates of the transactions or valuation where items are re-measured. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation of year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the statement of operations to “finance expenses”/“finance income.”
Taxes on Income
The standard corporate tax rate in Israel for the 2015 and 2014 tax years is 26.5%, increased from 25% for the tax year 2013 and decreased to 25% for the 2016 tax year.
We do not generate taxable income in Israel, as we have historically incurred operating losses resulting in carry forward tax losses totaling approximately $23.6 million as of December 31, 2015. We anticipate that we will be able to carry forward these tax losses indefinitely to future tax years. However, a tax loss that can be utilized in a certain tax year, cannot be carried forward to future tax years. Accordingly, we do not expect to pay taxes in Israel until we have taxable income after the full utilization of our carry forward tax losses.
Under the Law for the Encouragement of Capital Investments, 5719-1959 and other Israeli legislation, we may be entitled to certain additional tax benefits, including reduced tax rates, accelerated depreciation and amortization rates for tax purposes on certain assets, deduction of public offering expenses in three equal annual installments and amortization of other intangible property rights for tax purposes. See Item 10E “Additional Information — Taxation— Israeli Tax Considerations and Government Programs” for additional information concerning these tax benefits.
66
Results of Operations
For convenience purposes, the numbers set forth in the management's discussion and analysis below are, where applicable, rounded up and presented in millions, whereas the numbers in the tables below are presented in thousands. As result, the percentages set forth in the year-over-year comparisons below are based on numbers that have (where applicable) been rounded up to millions, which may slightly differ than the percentages that would result from the corresponding numbers set forth in the table that are presented in thousands.
Year Ended December 31, 2015 Compared to Year Ended December 31, 2014
|
Year Ended December 31,
|
||||||||
|
2015
|
2014
|
|||||||
|
(US$ in thousands, except per
share data)
|
||||||||
|
Research and development expenses, net
|
$
|
5,837
|
$
|
2,832
|
||||
|
General and administrative expenses
|
6,626
|
1,703
|
||||||
|
Operating loss
|
12,463
|
4,535
|
||||||
|
Finance income, net
|
173
|
3,925
|
||||||
|
Net loss
|
$
|
12,290
|
$
|
610
|
||||
Research and Development Expenses, net. Our total research and development expenses for the year ended December 31, 2015 were $5.8 million, as compared with $2.8 million for the year ended December 31, 2014, an increase of $3.0 million or 107%. The increase in research and development expenses between 2015 and 2014 was primarily due to the progress in the development of our imaging system, including increased expenditures due to clinical trial costs associated with the recruitment of 28 employees and independent contractors to the research and development team.
|
2015
|
2014
|
Change
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$
|
3,585
|
$
|
2,425
|
$
|
1,160
|
||||||
|
Share-based compensation
|
790
|
104
|
686
|
|||||||||
|
Materials
|
608
|
385
|
223
|
|||||||||
|
Subcontractors and consultants
|
688
|
294
|
394
|
|||||||||
|
Depreciation
|
85
|
72
|
13
|
|||||||||
|
Cost for registration of patents
|
153
|
72
|
81
|
|||||||||
|
Other research and development expenses
|
282
|
123
|
159
|
|||||||||
|
6,191
|
3,475
|
2,716
|
||||||||||
|
Less participation of the OCS and BIRD Foundation
|
(354)
|
(643
|
)
|
289
|
||||||||
|
Total research and development expenses, net
|
$
|
5,837
|
$
|
2,832
|
$
|
3,005
|
||||||
General and Administrative Expenses. Our general and administrative expenses for the year ended December 31, 2015 were $6.6 million, as compared to $1.7 million for the year ended December 31, 2014, an increase of $4.9 million, or 288%. The increase in general and administrative expenses is primarily due to the following:
|
·
|
a $2.7 million increase in share-based compensation, of which $2.0 million relates to the grant of options to purchase 581,542 ordinary shares from October 14, 2015 to our management and warrants to purchase 221,539 ordinary shares to Pontifax in consideration of their commitment to provide us, for no additional consideration, business development services and a representative designated by Pontifax to serve as the chairman of our Board of Directors, as further described in Note 11B to our financial statements appearing elsewhere in this Annual Report.
|
|
·
|
a $2.2 million increase in salaries and related expenses, professional services and other general administrative expenses incurred in connection with our initial public offering and concurrent private placement and other public company costs.
|
|
2015
|
2014
|
Change
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$
|
1,830
|
$
|
952
|
$
|
878
|
||||||
|
Share-based compensation
|
2,934
|
208
|
2,726
|
|||||||||
|
Professional services
|
609
|
114
|
495
|
|||||||||
|
Office rent and maintenance
|
108
|
105
|
3
|
|||||||||
|
Depreciation
|
7
|
7
|
-
|
|||||||||
|
Other general and administrative expenses
|
1,138
|
317
|
821
|
|||||||||
|
Total general and administrative expenses
|
$
|
6,626
|
$
|
1,703
|
$
|
4,923
|
||||||
67
Operating Loss. As a result of a $3.0 million increase in research and development expenses, net for the year ended December 31, 2015 compared to the year ended December 31, 2014, and a $4.9 million increase in general and administrative expenses in the same period, our operating loss for the year ended December 31, 2015 was $12.5 million, as compared with $4.5 million for the year ended December 31, 2014, an increase of $8.0 million, or 178%.
Finance Income, net. Our finance income, net for the year ended December 31, 2015 was $173,000, as compared to $3.9 million for the year ended December 31, 2014, a decrease of $3.8 million. The change in our finance income, net is primarily due to the following:
|
·
|
For the year ended December 31, 2014 we had finance income of $3.5 million as a result of changes in fair value of the warrants to purchase Series D-1 and D-2 preferred shares issued to investors and service providers in connection with our D1 investment round and the warrants to purchase Series C-1 and C-2 preferred shares issued to Pontifax, as compared with $174,000 for the year ended December 31, 2015.
|
|
·
|
For the year ended December 31, 2014, we had finance income of $415,000 as a result of changes in provision for royalties, as compared with a finance expense of $33,000 in the year ended December 31, 2015.
|
Net Loss. Our net loss for the year ended December 31, 2015 was $12.3 million, as compared to $610,000 for the year ended December 31, 2014, an increase of $11.7 million.
Year Ended December 31, 2014 Compared to Year Ended December 31, 2013
|
Year Ended December 31,
|
||||||||
|
2014
|
2013
|
|||||||
|
(US$ in thousands, except per share data)
|
||||||||
|
Research and development expenses, net
|
$
|
2,832
|
$
|
2,893
|
||||
|
General and administrative expenses
|
1,703
|
1,090
|
||||||
|
Other income
|
-
|
(11
|
)
|
|||||
|
Operating loss
|
4,535
|
3,972
|
||||||
|
Finance income, net
|
3,925
|
604
|
||||||
|
Net loss
|
$
|
610
|
$
|
3,368
|
||||
Research and Development Expenses, net. Our total research and development expenses, net for the year ended December 31, 2014 were $2.83 million, as compared with $2.89 million for the year ended December 31, 2013, a decrease of $61,000 or 2.0%. The decrease in research and development expenses between 2014 and 2013 was primarily due to a $495,000 increase in grants we received from OCS and BIRD Foundation. This increase, however, was partially offset primarily by increases in cost of salaries and related expenses, share-based compensation, materials, amounts payable to subcontractors and consultants, depreciation and other research and development expenses, mainly clinical trial-related expenses (all of which amounted to an increase of $478,000).
|
2014
|
2013
|
Change
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$
|
2,425
|
$
|
2,178
|
$
|
247
|
||||||
|
Share-based compensation
|
104
|
40
|
64
|
|||||||||
|
Materials
|
385
|
307
|
78
|
|||||||||
|
Subcontractors and consultants
|
294
|
218
|
76
|
|||||||||
|
Depreciation
|
72
|
70
|
2
|
|||||||||
|
Cost for registration of patents
|
72
|
118
|
(46
|
)
|
||||||||
|
Other research and development expenses
|
123
|
110
|
13
|
|||||||||
|
3,475
|
3,041
|
434
|
||||||||||
|
Less participation of the OCS and BIRD Foundation
|
(643
|
)
|
(148
|
)
|
(495
|
)
|
||||||
|
Total research and development expenses, net
|
$
|
2,832
|
$
|
2,893
|
$
|
(61
|
)
|
|||||
68
General and Administrative Expenses. Our general and administrative expenses for the year ended December 31, 2014 were $1.7 million, as compared to $1.1 million for the year ended December 31, 2013, an increase of $613,000, or 54.5%. The increase in general and administrative expenses is primarily related to expenses incurred in connection with our initial public offering and concurrent private placement.
|
2014
|
2013
|
Change
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$
|
952
|
$
|
682
|
$
|
270
|
||||||
|
Share-based compensation
|
208
|
16
|
192
|
|||||||||
|
Professional fees
|
114
|
96
|
18
|
|||||||||
|
Office rent and maintenance
|
105
|
104
|
1
|
|||||||||
|
Depreciation
|
7
|
7
|
-
|
|||||||||
|
Other general and administrative expenses
|
317
|
185
|
132
|
|||||||||
|
Total general and administrative expenses
|
$
|
1,703
|
$
|
1,090
|
$
|
613
|
||||||
Operating Loss. As a result of a $61,000 decrease in research and development expenses, net for the year ended December 31, 2014 compared to the year ended December 31, 2013, and a $613,000 increase in general and administrative expenses in the same period, our operating loss for the year ended December 31, 2014 was $4.53 million, as compared to $3.97 million for the year ended December 31, 2013, an increase of $563,000, or 14.2%.
Finance Income, net. Our finance income, net for the year ended December 31, 2014 was $3,925,000, as compared to $604,000 for the year ended December 31, 2013, an increase of $3,321,000. The change in our finance income, net is primarily due to the following:
|
·
|
For the year ended December 31, 2014 we had a finance income of $3.5 million as a result of a changes in fair value of the warrants to purchase Series D-1 and D-2 preferred shares issued to investors and service providers in connection with our D1 investment round and the warrants to purchase Series C-1 and C-2 preferred shares issued to Pontifax, as compared with $623,000 in the year ended December 31, 2013.
|
|
·
|
For the year ended December 31, 2014 we had a finance income of $415,000 as a result of changes in provision for royalties, as compared with a finance expense of $104,000 in the year ended December 31, 2013.
|
Net Loss. Our net loss for the year ended December 31, 2014 was $610,000, as compared to $3.4 million for the year ended December 31, 2013, a decrease of $2.8 million.
|
B.
|
Liquidity and Capital Resources
|
Sources of Liquidity
To date, we have funded our operations primarily with the approximately $24.5 million raised through equity financings consummated prior to our initial public offering, $13.5 million through our initial public offering (including the over-allotment exercise), $12.0 million through the private placement consummated concurrently with our initial public offering, $3.6 million through grants that we received from the OCS and the BIRD Foundation and $1.0 million drawn down under a credit line. The cash proceeds from our initial public offering and concurrent private placement were received subsequent to year end and do not form part of the cash, net asset or working capital figures noted above.
On July 13, 2014, we entered into a Cooperation and Project Funding Agreement with the BIRD Foundation and Synergy, pursuant to which the BIRD Foundation has agreed to award a grant in the maximum amount of the lesser of (i) $900,000; and (ii) 50% of the actual expenditures for the funding of a project entitled “Collection & Analysis of Gastrointestinal Images for Diagnostic Adenomatic Polyps and Colorectal Cancer.” The development work is expected to be performed over a 24 month period by Synergy (or a subcontractor on its behalf) and us. As of December 31, 2015, we had received funding from the BIRD Foundation in the aggregate amount of $127,000. Our research and development expenses, net is presented net the grant amount received from the BIRD Foundation. As of December 31, 2015 we had a contingent obligation to the BIRD foundation in the amount of $127,000.
69
We are required to repay the total sum granted to us and Synergy by the BIRD Foundation, linked to the U.S. Consumer Price Index from date of receipt of each payment, up to 100%, 113%, 125%, 138% and 150% of the linked sum granted by the BIRD Foundation if repaid within one year, two years, three years, four years and five or more years, respectively, of the original project completion date in accordance with the project proposal. Repayments are made at the rate of 5% of gross revenues derived from the product funded by the project. Under the terms of the agreement, if any portion of the product funded by the project is sold outright to a third party prior to full repayment of the grant to the BIRD Foundation, one-half of the sale proceeds will be applied to the repayment of the grant. If the funded product is licensed to a third party, 30% of all payments received under the respective license agreement must be paid to the BIRD Foundation in repayment of the grant.
On August 20, 2014, we entered into a certain credit line agreement, pursuant to which we obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders, or the Lenders. The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014.
We issued to each Lender at closing a warrant, collectively referred to as the Credit Line Warrants, to purchase a number of our ordinary shares constituting 2% of our share capital on a fully diluted basis (assuming conversion of all of our then outstanding convertible securities into ordinary shares at a 1:1 conversion rate) as of the closing for each $1 million (or portion thereof) extended by such Lender. We issued Credit Line Warrants to purchase in the aggregate 2,658,463 of our ordinary shares. The Credit Line Warrants are exercisable for a period of ten years at an exercise price of NIS 0.20 per share, and may be exercised on a net issuance basis. As of December 31, 2015, Credit Line Warrants to purchase an aggregate 1,557,507 ordinary shares have been exercised and Credit Line Warrants to purchase an aggregate 24,277 warrants expired as a result of exercise, of certain Credit Line Warrants, on a net issuance basis.
Under the terms of the credit line agreement, we directed that the full credit line amount be invested in the private placement that was consummated simultaneously with our initial public offering that was consummated on February 24, 2015. We issued to the Lenders a total of 2,000,000 units, each consisting of one ordinary share and one half of a Series A Warrant to purchase one ordinary share, together with 3,000,000 Long Term Incentive Warrants for aggregate gross proceeds of $12,000,000.
On January 4, 2015, we entered into a credit line agreement with Bank Leumi le-Israel B.M., or Bank Leumi, pursuant to which we were entitled to obtain a credit line in the principal amount of up to $1,000,000, or the Bank Leumi Credit Facility. The Bank Leumi Credit Facility was required to be repaid in full by us no later than April 1, 2015 and Bank Leumi’s consent was required for early repayment. The drawn portion of the Bank Leumi Credit Facility bore interest at an annual rate of LIBOR plus 5.25% on the basis of a 365-day year, until paid in full. We drew the entire $1,000,000 Bank Leumi Credit Facility. We paid Bank Leumi a facility fee of $20,000 in connection with the facility. To secure the repayment of the Bank Leumi Credit Facility, we granted Bank Leumi (i) a first ranking fixed charge over our goodwill; and (ii) a first ranking floating charge over all of the assets and rights of any type whatsoever, which we had or may acquire in the future, subject to the rights of the OCS and the BIRD Foundation and the rights under existing and future liens in favor of the First Intentional Bank of Israel Ltd. securing debt or indentures of up to an aggregate amount of $100,000. On March 16, 2015, we repaid all amounts outstanding under the Bank Leumi Credit Facility with the proceeds of our initial public offering and concurrent private placement.
On February 24, 2015, we consummated an initial public offering in the United States of 2,000,000 units, each consisting of one ordinary share and one half of a Series A Warrant to purchase one ordinary share. The price per unit sold in the initial public offering was $6.00. Each unit in the initial public offering was issued with one and one half Long Term Incentive Warrants. We granted the underwriters in the initial public offering a 45-day over-allotment option to purchase up to 300,000 additional units (together with an accompanying 450,000 Long Term Incentive Warrants) from us to cover over-allotments. On March 6, 2015, the option to purchase additional 100,000 units was partially exercised. On March 18, 2015, the units were separated into one ordinary share and one-half of a Series A Warrant to purchase one ordinary share and the units ceased to exist. On April 6, 2015, the option to purchase additional 150,000 ordinary shares and 75,000 Series A Warrant was partially exercised. The aggregate offering price of the securities sold in the initial public offering (including the over-allotment option) was approximately $13.5 million. The total expenses of the offering, in cash, including underwriting discounts and commissions, were approximately $2.9 million. Issuance expenses include certain warrants with value of $196,000 issued in connection with the initial public offering. The net proceeds we received from the initial public offering (including the over-allotment option) was approximately $10.8 million, (net of issuance cost of approximately $1.2 million, including certain warrants with value of $125 issued in connection with the private placement). In January, 2015, we issued a total of 2,452,376 Long Term Incentive Warrants to purchasers of securities in our initial public offering who completed the required registration process by August 23, 2015.
70
Immediately prior to the consummation of the initial public offering, certain members of the Company's management exercised 307,467 options granted to them under the 2006 Unit Option Plan.
For the years ended December 31, 2015, 2014 and 2013, we received, in cash, $11,000, $558,000 and $647,000, respectively, in grants from the OCS for the financing of a portion of our research and development expenditure.
We believe that based on our current business plan, our existing cash resources and the net proceeds from our initial public offering and concurrent private placement will be sufficient to fund our currently anticipated cash requirements at least until March 31, 2017. Nevertheless, we will require significant additional financing in the future to fund our operations if and when we progress with our clinical trials in Europe and the United States, and other territories.
Historical Cash Flows
The following table summarizes our statement of cash flows for the years ended December 31, 2015, 2014 and 2013.
|
Year Ended December 31,
|
||||||||||||
|
2015
|
2014
|
2013
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Net cash used in operating activities
|
$ | (8,628 | ) | $ | (3,855 | ) | $ | (3,010 | ) | |||
|
Net cash provided by (used in) investing activities
|
$ | (5,070 | ) | $ | (46 | ) | $ | 3,402 | ||||
|
Net cash provided by financing activities
|
$ | 22,013 | $ | - | $ | - | ||||||
Operating Activities
Net cash used in operating activities for the year ended December 31, 2015 was $8.6 million, as compared to $3.9 million for the year ended December 31, 2014. The increase in net cash used in operating activities in 2015 was attributable primarily to an increase in operating loss.
Net cash used in operating activities for the year ended December 31, 2014 was $3.9 million, as compared to $3.0 million for the year ended December 31, 2013. The increase in net cash used in operating activities in 2014 was attributable primarily to an increase in operating loss.
Investing Activities
Net cash used in investing activities for the year ended December 31, 2015 consists of investment in short-term investment in the amount of $4.8 million and purchase of property and equipment in the amount of $270,000. Net cash used in investing activities for the year ended December 31, 2014 consists of purchase of property and equipment in the amount of $46,000. Net cash provided by investing activities for the year ended December 31, 2013 consists primarily of proceeds from short-term bank deposits of $3.45 million, which were partially offset primarily by purchase of property and equipment in the amount of $45,000.
Financing Activities
Net cash provided by financing activities for the year ended December 31, 2015 was $22.0 million, comprised primarily of issuance of ordinary shares in the private placement net of issuance expenses in the amount of $11.0 million and issuance of ordinary shares in the IPO net of issuance cost in the amount of $10.9 million. We have not generated cash from financing activities for the years ended December 31, 2014 and December 31, 2013.
71
Funding Requirements
We expect to incur losses from operations for the foreseeable future. We expect to incur increasing research and development expenses, including expenses related to the hiring of personnel and conducting additional clinical trials. We expect that our general and administrative expenses will also increase as we expand our finance and administrative staff, add infrastructure, and incur additional costs related to being a public company in the United States, including directors’ and officers’ insurance, investor relations programs, and increased professional fees. Our future capital requirements will depend on a number of factors, including the timing and outcome of clinical trials and regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims and other intellectual property rights, the availability of financing, the costs involved in manufacturing our product, and our success in developing markets for our products.
Our expected future expenditures related to product, clinical and regulatory clearances includes the follows:
|
•
|
completion of the clinical development of our system;
|
|
|
•
|
conducting clinical trials in Europe, the United States and other territories for purposes of regulatory approval and post-marketing validation;
|
|
|
•
|
development of future generations of our system and future products;
|
|
|
•
|
FDA and additional regulatory filing activities; and
|
|
|
•
|
patent maintenance fees.
|
We believe that the net proceeds from our initial public offering and concurrent private placement, together with our existing cash, will be sufficient to fund our operating expenses and capital expenditure requirements at least until March 31, 2017. We have based this estimate on assumptions that may prove to be wrong, and we may use our available capital resources more quickly than we currently expect. Because of the numerous risks and uncertainties associated with the development clinical validation, and regulation of our system, we are unable to estimate the amounts of any increased capital outlays and operating expenditures associated with current and anticipated clinical development and clinical trials.
In the absence of additional funding, we expect our continuing operating losses to result in increases in our cash used in operations over the next several quarters and years.
Application of Critical Accounting Policies and Estimates
Our consolidated financial statements are prepared in accordance with United States generally accepted accounting principles. The preparation of our financial statements requires us to make estimates, judgments and assumptions that can affect the reported amounts of assets and liabilities, disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting periods. We base our estimates, judgments and assumptions on historical experience and other factors that we believe to be reasonable under the circumstances. Materially different results can occur as circumstances change and additional information becomes known. Besides the estimates identified above that are considered critical, we make many other accounting estimates in preparing our financial statements and related disclosures. See Note 2 to our audited consolidated financial statements presented elsewhere in this Annual Report for a description of the significant accounting policies that we used to prepare our consolidated financial statements. The critical accounting policies that were impacted by the estimates, judgments and assumptions used in the preparation of our consolidated financial statements are discussed below.
Share-based compensation
We account for share-based compensation in accordance with ASC No. 718, “Compensation-Stock Compensation.” ASC No. 718 requires companies to estimate the fair value of equity-based payment awards on the date of grant using an Option-Pricing Model, or OPM. The value of the portion of the award that is ultimately expected to vest is recognized as an expense over the requisite service periods in our consolidated statements of operations.
We selected the Black-Scholes-Merton option-pricing model as the most appropriate method for computing the fair value of our share-based awards, using the standard parameters established in that model including estimates relating to the fair value of our ordinary shares, volatility, estimated life of the instruments, risk-free interest rates and dividends yield as described below.
72
Option Valuations
The determination of the grant date fair value of options using an option pricing-model is affected by estimates and assumptions with respect to a number of complex and subjective variables. These variables include the expected volatility of our share price over the expected term of the options, share option exercise and cancellation behaviors, risk-free interest rates and expected dividends, which are estimated as follows:
|
•
|
Fair Value of our Ordinary Shares. Prior to our initial public offering, due to the absence of a public market for our ordinary shares, we estimated the fair value of our ordinary shares, as discussed below in “—Valuation of our ordinary shares.” Following our initial public offering, the fair value of our ordinary shares is determined based on the trading price of our ordinary shares on the Nasdaq Capital Market.
|
|
|
•
|
Expected Volatility. We estimated the expected share price volatility for our ordinary shares by considering the historic price volatility for industry peers based on price observations over a period equivalent to the expected term of the share option grants. Industry peers consist of public companies in the medical device and healthcare industries. We intend to continue to consistently apply this process using the same or similar industry peers until a sufficient amount of historical information regarding the volatility of our ordinary share price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be utilized in the calculation.
|
|
•
|
Expected Term. The expected term of options granted represents the period of time that options granted are expected to be outstanding, and is determined based on the simplified method in accordance with ASC No. 718-10-S99-1 (SAB No. 110), as adequate historical experience is not available to provide a reasonable estimate. ASC No. 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
|
|
|
•
|
Risk-Free Rate. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the contractual life of the options.
|
|
•
|
Expected Dividend Yield. We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
|
If any of the estimates and assumptions used in the Black-Scholes-Merton option-pricing model will change significantly, our share-based compensation for future awards may differ materially from those projected and recorded previously.
The fair value for options granted in 2015, 2014 and 2013 is estimated at the date of grant using a Black-Scholes-Merton option-pricing model with the following assumptions:
|
Parameters
|
February- December
2015 Grants
|
October 2014 Grant
|
June 2013 Grant
|
|||||
|
Expected volatility (in %)
|
44-62
|
50-60
|
40-60
|
|||||
|
Expected term (in years)
|
4-10
|
5-6
|
5-10
|
|||||
|
Risk free interest rate (in %)
|
1.29-2.28
|
1.45-1.72
|
0.75-2.25
|
|||||
|
Anticipated rate of dividends (in %)
|
0
|
0
|
0
|
|||||
|
Share Price
|
$3-$5.12
|
$3.01
|
$3.04
|
73
The following tables presents the grant dates, number of underlying shares and related exercise prices of awards granted to employees and non-employees during the years 2015, 2014, 2013 as well as the estimated fair value of the underlying ordinary shares on the grant date:
|
Grant date
|
No. of options
|
Expiration date
|
Exercise price
|
Fair value on
grant date
|
|||||||||
|
May 15, 2013 (1)
|
38,473 |
February 24, 2025
|
$ | 4.96 | $ | 3.04 | |||||||
|
June 6, 2013 (2)
|
117,750 |
June 12, 2023
|
$ | 4.96 | $ | 3.04 | |||||||
|
October 14, 2014 (3)
|
581,542 |
October 14, 2024
|
(* | ) | $ | 3.01 | |||||||
|
February 24, 2015 (6)
|
302,420 |
February 24, 2025
|
$ | 5.06 | $ | 2.73 | |||||||
|
March 15, 2015 (7)
|
60,484 |
March 15, 2025
|
$ | 5.08 | $ | 2.71 | |||||||
|
May 19, 2015 (8)
|
523,621 |
May 19, 2025
|
$ | 4.57 | $ | 2.50 | |||||||
|
June 1, 2015 (9)
|
48,387 |
June 1, 2025
|
$ | 5.06 | $ | 2.80 | |||||||
|
June 3, 2015 (10)
|
189,043 |
June 3, 2025
|
$ | 4.35 | $ | 2.60 | |||||||
|
August 13, 2015 (11)
|
324,750 |
August 13, 2025
|
$ | 3.68 | $ | 1.80 | |||||||
|
August 13, 2015 (12)
|
425,000 |
August 13, 2025
|
$ | 3.68 | $ | 1.85 | |||||||
|
October 20, 2015 (13)
|
38,369 |
October 20, 2025
|
$ | 2.79 | $ | 1.72 | |||||||
|
Grant date
|
No. of warrants
|
Expiration date
|
Exercise price
|
Fair value on
grant date
|
|||||||||
|
February 18, 2015
|
100,000 |
February 18, 2019
|
$ | 7.50 | $ | 1.62 | |||||||
|
February 24, 2015
|
15,000 |
February 24, 2019
|
$ | 6.00 | $ | 2.25 | |||||||
|
October 14, 2014 (4)
|
221,539 |
October 14, 2022
|
(* | ) | $ | 3.01 | |||||||
|
Various dates in 2015 (5)
|
87,127 |
Various dates in 2017
|
(** | ) | $ | 1.43 | |||||||
|
(*)
|
See Note 11B(3) to our audited consolidated financial statements presented elsewhere in this Annual Report.
|
|
(**)
|
See Note 11B(6) to our audited consolidated financial statements presented elsewhere in this Annual Report.
|
|
(1)
|
38,473 ordinary shares issuable upon the exercise of options with an exercise price of $4.96 per ordinary share which we agreed that certain executive officers will be entitled to upon completion of our initial public offering. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $116,000. This amount is charged to statement of operations over the vesting periods.
|
|
(2)
|
On June 6, 2013, our Board of Directors approved the grant of options to purchase 117,750 ordinary shares, at an exercise price of $4.96, to certain of our employees and consultants. Of such options, options to purchase 16,147 ordinary shares were vested on the grant date, options to purchase 67,583 ordinary shares will vest over two years, options to purchase 25,688 ordinary shares will vest over three years and options to purchase 8,332 ordinary shares will vest over four years. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $84,000. This amount is charged to statement of operations over the vesting periods.
|
|
(3)
|
On October 14, 2014, our Board of Directors approved the grant of options to purchase 581,542 ordinary shares, at an exercise price of NIS 0.2 per share, to our management. Management subsequently agreed that the exercise price of fifty-percent of such options will increase to equal the price at which our ordinary shares are sold to the public in the initial public offering, or if units are sold in the initial public offering, the exercise price per share will increase to be equal to the effective price per share of the ordinary shares underlying the units sold to the public in the offering, and will vest and become exercisable only upon the consummation of the initial public offering. The remaining options with an NIS 0.2 exercise price will vest on a quarterly basis in eight equal installments during a period of 24 months from issuance. Upon the closing of the initial public offering on February 24, 2015, any unvested portion of these warrants became fully vested and exercisable. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $1.7 million. This amount is charged to statement of operations over the vesting periods.
|
74
|
(4)
|
In addition, in October 2014, our Board of Directors approved the grant of warrants to purchase 221,539 ordinary shares NIS 0.2 par value of the Company at an exercise price of NIS 0.2 per share to Pontifax in consideration of their commitment to provide to us, for no consideration, business development services and a representative designated by Pontifax to serve as the chairman of our Board of Directors. Pontifax subsequently agreed that the exercise price of fifty-percent of such warrants will increase to equal the price at which our ordinary shares are sold to the public in the initial public offering or if units are sold in the initial public offering, the exercise price per share will increase to be equal to the effective price per share of the ordinary shares underlying the units sold to the public in the offering, and will vest and become exercisable only upon the consummation of the initial public offering prior to their expiration date. The remaining warrants with an NIS 0.2 exercise price will vest on a quarterly basis in eight equal installments during a period of 24 months from issuance. Upon the closing of the initial public offering on February 24, 2015, any unvested portion of these warrants became fully vested and exercisable. In addition, Pontifax agreed to reduce the term of the warrants such that these warrants will now expire after eight years (instead of ten years) following their issuance, i.e., on October 14, 2022. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $667,000. This amount is charged to statement of operations over the vesting periods.
|
|
(5)
|
Certain finders engaged by us were entitled, according to the terms of their respective engagements with us, to be issued warrants to purchase ordinary shares upon and subject to the closing of a private placement pursuant to the credit line agreement dated August 20, 2014, as amended. On April 6, 2015, our Board of Directors approved the grant of warrants to purchase 70,010 ordinary shares, at an exercise price of $ 5.06 per share. On October 20, 2015 our Board of Directors approved the grant of additional warrants to purchase 17,117 ordinary shares, at an exercise price of NIS 0.2 per share. The warrants included in the two grants were fully (100%) vested on grant date. The compensation payment was based on the fair value on the grant date, and was estimated at approximately $125,000. This amount is charged to shareholders' equity as private placement issuance cost.
|
|
(6)
|
On January 15, 2015, our shareholders approved the grant of options to certain members of our Board of Directors to purchase 302,420 ordinary shares, at an exercise price equal to the effective price per share of the ordinary shares underlying the units sold to the public in the initial public offering. Following the initial public offering the effective exercise price was determined at$5.06 per share. The options shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $826,000. This amount is charged to statement of operations over the vesting periods.
|
|
(7)
|
On April 6, 2015, our shareholders approved the grant of options to purchase 60,484 ordinary shares, at an exercise price of $5.08, to a member of our Board of Directors. The options shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $164,000. This amount is charged to statement of operations over the vesting periods.
|
|
(8)
|
On May 19, 2015 our shareholders approved the grant of options to purchase 463,137 ordinary shares, at an exercise price of $4.57, to our president. The options shall vest over a period of four years commencing on the date of grant, such that 25% of the options shall vest on the first anniversary of the date of grant and thereafter, the remaining options will vest in monthly installments.
|
In addition, our shareholders approved the grant of options to purchase 60,484 ordinary shares, at an exercise price of $4.57, to a member of our Board of Director. The options shall vest over a period of three years commencing on the date of grant in quarterly installments.
The compensation expense was based on the fair value on the grant date, and was estimated at approximately $1.3 million. This amount is charged to statement of operations over the vesting periods.
|
(9)
|
On February 12, 2015, our shareholders approved the grant of options to purchase 48,387 ordinary shares, at an exercise price of $5.06, to a member of our Board of Directors, in consideration for certain business development services in Asia under a consulting agreement. The options shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $136,000. This amount is charged to statement of operations over the vesting periods.
|
75
|
(10)
|
On June 3, 2015, our Board of directors approved the grant of options to purchase 189,043 ordinary shares, at an exercise price of $4.35, to certain of our employees and consultants. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $491,000. This amount is charged to statement of operations over the vesting periods.
|
|
(11)
|
On August 13, 2015 our Board of Directors approved the grant of options to purchase 324,750 ordinary shares to our chief executive officer at an exercise price of $3.68. The options shall vest over a period of four years commencing on the date of grant, such that 25% of the options shall vest on the first anniversary of the date of grant and thereafter, the remaining options will vest in monthly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $586,000. This amount is charged to statement of operations over the vesting periods.
|
|
(12)
|
On August 13, 2015 our Board of Directors approved the grant of options to purchase up to 425,000 ordinary shares to our chief executive officer at an exercise price of $3.68. The options shall vest over a period of four years commencing on the date of grant, such that on each of the four anniversaries after the date of grant a number of options will vest and become exercisable calculated as follows: (a) 425,000 multiplied by a quotient equal to the aggregate number of our Series A Warrants and Long Term Incentive Warrants that have been exercised prior to the applicable anniversary measurement date (to be adjusted to reflect any stock splits, reverse splits, bonus shares and the like) divided by 8,500,000, less (b) the aggregate number of such options that vested prior to such vesting measurement date, provided that in no event shall more than 106,250 of such options vest during any 12-month period of his employment (to be accumulated on a `carry-forward` basis). The compensation expense was based on the fair value on the grant date, and was estimated at approximately $700,000. No expenses were recorded during the year ended December 31, 2015.
|
|
(13)
|
On October 20, 2015 our Board of Directors approved the grant of options to purchase 38,369 ordinary shares to certain of our employees at an exercise price of $2.79. The options shall vest over a period of four years commencing on the date of grant, such that 25% of the options shall vest on the first anniversary of the date of grant and thereafter, the remaining options will vest in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $58,000. This amount is charged to statement of operations over the vesting periods.
|
Valuation of our ordinary shares
Prior to the completion of our initial public offering, due to the absence of an active public market for our ordinary shares, the fair value of our ordinary shares for purposes of determining the exercise price for awards was determined by our management and approved by our board of directors. In connection with preparing our financial statements, our management considered the fair value of our ordinary shares based on a number of objective and subjective factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, referred to as the AICPA Practice Aid.
Commencing February 24, 2015, the date our units (each consisting of one ordinary share and one half of a Series A Warrant to purchase one ordinary share) began trading on the NASDAQ Capital Market, the fair value of our ordinary shares underlying the share options for purposes of determining the exercise price for awards was derived by reference to the closing price of our unit on NASDAQ Capital Market on the relevant date, using relative fair value for each component in the unit.
After March 18, 2015, the date the units were separated into one ordinary share and one-half of a Series A Warrant to purchase one ordinary share and our ordinary shares began trading on the NASDAQ Capital Market, the grant date fair value for awards is based on the closing price of our ordinary shares on the NASDAQ Capital Market on the date of grant and fair value for all other purposes related to share-based awards is the closing price of our ordinary shares on NASDAQ Capital Market on the relevant date.
76
Royalties provision
Provision for royalties to an ASIC designer
In December 2007, we entered into an agreement for the development of an application specific integrated circuit, or ASIC, component to be used as an amplifier for the capture of signals at low frequencies from X-ray detectors contained in our product. The ASIC developer is entitled to receive royalties from us in the amount of €0.5 (approximately $0.54) for every ASIC component that we will sell, up to €200,000 (approximately $218,000). The net present value of the royalty liability to the ASIC designer is dependent upon our management estimates and assumption as to future product shipments and interest rates used to calculate the present value of the cash payments required to repay the royalties to the ASIC designer. In calculating the present value of future royalty payments to the ASIC designer, we used a discount factor of 17.6%, commensurate with our risk at the date of initial recognition of the liability. Any updates in the expected product shipments and the liability will be recorded to profit and loss each period. As of December 31, 2015, it was probable that we will be required pay the above mentioned royalties, and accordingly, a liability for this reimbursement has been accounted for in our financial statements in the amount of $159,000.
Reimbursement liability to Check-Cap LLC unitholders
On May 31, 2009, we entered into an asset transfer agreement with Check-Cap LLC pursuant to which Check-Cap LLC transferred all of its business operations and substantially all of its assets to us. In connection with the transaction we undertook to reimburse the unitholders of Check-Cap LLC for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unitholders of Check-Cap LLC under Section 367(d) of the Code, and is based in part on our forecasted sales. The reimbursement liability is calculated using the provisions of ASC 820, under which expected cash outflows were discounted using a 17.6% discount factor commensurate with our company’s risk at the date of initial recognition of the liability. Any updates in the expected cash outflows and the liability will be recorded to profit and loss each period. As of December 31, 2015, it was probable that we will be required to reimburse the U.S. unitholders of Check-Cap LLC, and accordingly, a liability for this reimbursement has been accounted for in our financial statements in the amount of $418,000. Due to the fact that we are still in the development stage and have not generated revenues, the sales forecast is highly subjective and may vary significantly in the future. As more information is gathered to assist our management in making forecasts, the liability will be updated.
Fair value of financial instruments
On June 1, 2009, in connection with that certain Series C Preferred Share Purchase Agreement, we issued to the Pontifax Funds, as the lead investor, warrants for the purchase of 41,822 Series C-1 preferred shares and warrants for the purchase of 50,399 Series C-2 preferred shares. These warrants converted into warrants to purchase 92,221 ordinary shares immediately prior to the consummation of our initial public offering (the "Conversion"). These warrants included anti-dilution protection provision requiring a reduction in original exercise price as a result of subsequent issuance below the original exercise price, and therefore were classified as a liability in the balance sheet. Prior to the Conversion, the fair value of this financial instrument was determined based on an option-pricing model using similar assumptions to those used for our share-based compensation awards to employees.
In consideration for brokerage services in connection with the Series C preferred share investment: (i) on December 15, 2009, the Company issued warrants to purchase 18,586 preferred C2 shares, with an exercise price of $5.38 per share, which expired on November 22, 2014.; and (ii) on April 27, 2010, the Company issued warrants to purchase 8,366 preferred C2 shares, with an exercise price of $5.38 per share, which expired on January 21, 2015. The warrants were classified as a liability in accordance with ASC No. 480. Immediately prior to our initial public offering upon the conversion of the warrants to purchase preferred shares into warrants to purchase ordinary shares, these warrants were converted into equity.
On March 17, 2011, in connection with Series D Preferred Share Purchase Agreement, we issued warrants for the purchase of 810,013 Series D-2 preferred shares to Series D1 investors. In addition, as consideration for brokerage services and in connection with Series D Preferred Share Purchase Agreement, we issued warrants for the purchase of 25,196 Series D-1 preferred shares and warrants for the purchase of 20,570 Series D-2 preferred shares in consideration for brokerage services. These warrants included price protection provision requiring a reduction in original exercise price as a result of subsequent issuance below the original exercise price, and therefore were classified as a liability in the balance sheet. Prior to the Conversion, the fair value of this financial instrument was determined based on an option-pricing model using similar assumptions to those used for our share-based compensation awards to employees. Series D-1 and Series D-2 warrants expired on March 17, 2015.
77
Recent Accounting Pronouncements
In November, 2015, the Financial Accounting Standards Board, or FASB, issued Accounting Standards Update ("ASU") 2015-17, Topic 740 - Balance Sheet Classification of Deferred Taxes. The ASU requires entities to present deferred tax assets and deferred tax liabilities as noncurrent in a classified balance sheet. Netting of deferred tax assets and deferred tax liabilities by tax jurisdiction is still required under the new guidance. We will apply the ASU in its future financial statements.
In 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. We are currently evaluating the effect, if any, that the adoption of this guidance will have on our financial statements.
In June 2014, the FASB issued Accounting Standards Update ("ASU") 2014-10 regarding development stage entities. The ASU removes the definition of development stage entity, as was previously defined under U.S. GAAP, from the accounting standards codification, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, the ASU eliminates the requirements for development stage entities to (i) present inception-to-date information in the statement of income, cash flow and stockholders' equity, (ii) label the financial statements as those of a development stage entity, (iii) disclose a description of the development stage activities in which the entity is engaged, and (iv) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. The amendments' to ASU 2014-10 are effective for annual reporting periods beginning after December 15, 2014. We have applied the ASU in our financial statements.
JOBS Act Exemption
The JOBS Act permits an “emerging growth company,” such as us, to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
C. Research and development, patents and licenses, etc.
For a description of our research and development programs and the amounts that we have incurred over the last three years pursuant to those programs, see Item 4B “Information on Our Company—Business Overview—Research and Development.”
D. Trend Information
Our results of operations and financial condition may be affected by various trends and factors discussed in Item 3D “Key Information - Risk factors,” Item 4 “Information on Our Company” and elsewhere in this Item 5 “Operating and Financial Review and Prospects”
E. Off-balance Sheet Arrangements
We do not have any material off-balance sheet arrangements.
F. Tabular Disclosure of Contractual Obligations
The following table summarizes our contractual obligations as of December 31, 2015:
|
Payments due by period
|
||||||||||||||||||||
|
(US$ in thousands)
|
||||||||||||||||||||
|
Total
|
Less than 1
year
|
1-3 years
|
4-5 years
|
More than 5
years
|
||||||||||||||||
|
Operating lease obligations(1):
|
$
|
739
|
$
|
208
|
$
|
268
|
$
|
154
|
$
|
109
|
||||||||||
|
Other long term liabilities reflected on the Statements of Financial Position:
|
||||||||||||||||||||
|
Royalties to ASIC designer(2)
|
$
|
159
|
$
|
4
|
$
|
155
|
$
|
-
|
$
|
-
|
||||||||||
|
Reimbursement liability to
|
||||||||||||||||||||
|
Check-Cap LLC unitholders(3)
|
418
|
1
|
72
|
144
|
201
|
|||||||||||||||
|
Total
|
$
|
577
|
$
|
5
|
$
|
227
|
$
|
144
|
$
|
201
|
||||||||||
78
|
(1)
|
Operating lease obligations consist of payments pursuant to a lease agreement for office facilities as well as lease agreements for vehicles, which generally run for a period of three years.
|
|
|
(2)
|
See Item 5B “Operating and Financial Review and Prospects—Liquidity and Capital Resources—Application of Critical Accounting Policies and Estimates—Royalties provision - Provision for royalties to an ASIC designer.”
|
|
|
(3)
|
On May 31, 2009, we entered into an asset transfer agreement with Check-Cap LLC pursuant to which Check-Cap LLC transferred all of its business operations and substantially all of its assets to us, in connection with which we undertook to reimburse the unitholders of Check-Cap LLC for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unitholders of Check-Cap LLC under Section 367(d) of the Code and is based in part on our forecasted sales. The liability is calculated based on expected cash outflows discounted using a 17.6% discount factor commensurate with our risk at the date of initial recognition of the liability. Any updates in the expected cash outflows and the liability will be recorded to profit and loss each period. As of December 31, 2015, it was probable that we will be required to reimburse the U.S. unitholders of Check-Cap LLC, and accordingly, a liability for this reimbursement has been accounted for in our financial statements for such period in the amount of $418,000. See Item 7B “Major Shareholders and Related Party Transactions—Related Party Transactions—Transactions with Check-Cap LLC and the Members and Manager of Check-Cap LLC.”
|
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
|
A.
|
Directors and senior management
|
The following table sets forth information for our executive officers and directors as of the date of this Annual Report. Unless otherwise stated, the address for our directors and executive officers is c/o Check-Cap Ltd., Abba Hushi Avenue, P.O. Box 1271, Isfiya, 30090, Mount Carmel, Israel.
|
Name
|
Age
|
Position(s)
|
||
|
William Densel
|
48
|
Chief Executive Officer and Director
|
||
|
Lior Torem
|
46
|
Chief Financial Officer
|
||
|
Yoav Kimchy
|
54
|
Chief Technology Officer
|
||
|
Alex Ovadia
|
53
|
Vice President, Research and Development
|
||
|
Tomer Kariv(1)
|
55
|
Chairman of the Board of Directors
|
||
|
Walter L. Robb
|
87
|
Director
|
||
|
Steven Hanley(1)(2)
|
48
|
Director
|
||
|
Yuval Yanai(1)(2)
|
63
|
External Director
|
||
|
XiangQian (XQ) Lin
|
32
|
Director
|
||
|
Mary Jo Gorman (2)
|
56
|
External Director
|
|
(1)
|
Member of our Nominating Committee.
|
|
(2)
|
Member of our Audit Committee and Compensation Committee.
|
79
Executive Officers and Directors
William (Bill) Densel has served as our Chief Executive Officer and a member of our Board of Directors since August 2015. Mr. Densel served as our President of U.S. Operations from May 2015 until August 2015 and has served as the President and Chief Executive Officer and member of the Board of Directors of our U.S. subsidiary, Check-Cap US, Inc., since July 2015. Mr. Densel has a 25-year leadership career, including in his recent roles as Chief Executive Officer of Beacon Endoscopic, Inc. (2013), which was acquired by Covidien plc. in less than one year after he began to serve in such capacity; as General Manager, CardioSCORE for BG Medicine Inc. (2012-2013); and in his roles at Dune Medical Devices, Inc. (2009-2011), lastly as Chief Executive Officer. Mr. Densel has effectively led teams and utilized his influence across key decision makers in larger organizations such as Cytyc Corporation (2006-2009), which was acquired by Hologic in 2007, where he served in several positions, including Vice President, Marketing – GYN Surgical, Vice President, Emerging Surgical Technologies and Senior Director, Neuroscience; Boston Scientific Corporation (2004-2006), where he served as Director, New Market Development & Strategic Planning – Endosurgery; and Genzyme Biosurgery, where he served in various positions, lastly as Senior Director, Marketing and Business Development - General and GYN Surgery (1993-2004). Mr. Densel has launched several new medical devices during his career, affording him extensive FDA and clinical trial experience. Mr. Densel served in the U.S. Navy from 1989 to 1993, lastly as Lieutenant, Special Operations. Mr. Densel has a BA degree in Economics from Duke University.
Lior Torem has served as our chief financial officer since May 2010, on a part-time basis until March 2015 and on a full-time basis since March 2015. Mr. Torem has served as a member of the Board of Directors of our U.S. subsidiary, Check-Cap US, Inc., since July 2015. Beginning in January 2010, Mr. Torem served as an executive consultant to our company. From November 2008 to March 2015, Mr. Torem served as the chief financial officer, on a part-time basis, of Superfish, Inc., a developer of visual search technology for searching images. From 2003 to 2009, Mr. Torem served as vice president of finance at Actelis Networks Inc., a provider of high performance, scalable broadband over copper solutions, and from 2001 to 2003, served as vice president of finance at its subsidiary Actelis Networks Ltd. Prior to that, from 1997 to 2000, Mr. Torem served as corporate controller at Mentergy Ltd., a publicly-traded company that provides e-learning solutions and satellite communications services. Mr. Torem holds a B.A. degree in accounting and economics from Bar Ilan University, an MBA degree from Haifa University and he is a Certified Public Accountant in Israel.
Yoav Kimchy has served as our chief technology officer since our inception in April 2009 served as and a member of our board of directors since from 2009 and until December 1, 2015. Dr. Kimchy founded Check-Cap LLC in December 2004, serving as its president and chief executive officer until March 2008, and as its president and chief technical officer since March 2008. Dr. Kimchy has also served as a member of the board of directors of Check-Cap Ltd. (Delaware), the manager of Check-Cap LLC, since December 2004. Between 2005 and 2012, Dr. Kimchy also served as our vice president of research and development. Between 2000 and 2003, Dr. Kimchy served as the vice president of research and development of V-Target Ltd. (Israel), a medical device company developing gamma imaging applications. Prior to that, from 1998 to 2000, Dr. Kimchy served as director of cardiovascular research at Impulse Dynamics Ltd. (Israel), a medical device company developing a unique therapeutic pulsing technology for chronic heart failure. From 1994 to 1998, Dr. Kimchy served as a systems engineer and algorithm specialist for an Israeli government contract project with the Israeli Navy. Dr. Kimchy also served as a lieutenant in the Israeli Navy. Dr. Kimchy holds a B.Sc. degree in physics and mathematics from the Hebrew University of Jerusalem, an M.Sc. degree in biomedical engineering from Tel Aviv University and a PhD from the Technion-Israel Institute of Technology.
Alex Ovadia has served as our vice president of research and development since January 2013 and as our Israeli Site Manager and Chief Operating Officer since July 1, 2015. Prior to that, between 2001 and 2012, Mr. Ovadia served in various positions as global director and senior manager for CT research and development at Philips Healthcare. In these capacities he led global organizations including systems engineering, physics, system verification and hardware development. Mr. Ovadia was also appointed as a member of Global CT R&D staff and member of management of Philips Medical System Technologies Ltd. Prior to that, from 1990 to 2001, Mr. Ovadia served as project/systems engineering manager for large scale military projects, mainly aircraft upgrades for U.S. and European governments performed by Elbit Systems Ltd. Mr. Ovadia holds a BSc degree in electrical engineering from the Technion-Israel Institute of Technology.
Tomer Kariv has served as a member of our Board of Directors since June 2009 and as the chairman of our Board of Directors since July 2010, and has served as a member of our Nominating Committee since October 2015. Mr. Kariv has served as a member of the board of directors of Check Cap Ltd. (Delaware) and a manager of Check-Cap LLC since March 2008. Mr. Kariv is the co-founder and since December 2004, has served as Chief Executive Officer of Pontifax, a group of Israeli-based life sciences venture funds focusing on investments in bio-pharmaceutical and med-tech technologies. Mr. Kariv has also served as an active board member of many of the funds’ portfolio companies, assuming a special responsibility for strategic planning. Among others, since March 2008, Mr. Kariv has served as a board member of Macrocure Ltd. and Arno Therapeutics Inc. In addition, Mr. Kariv serves as a board member of Otic Pharma Ltd. and EyeYon Medical Ltd. During the 10 years prior to establishing Pontifax, Mr. Kariv played a key role in investing, managing and nurturing technology driven companies and startups and has held senior management positions at top Israeli financial institutions. Mr. Kariv practiced law with Sullivan & Cromwell, a leading law firm in New York, and holds a B.A. degree in Economics from Harvard University and a J.D. from Harvard Law School.
80
Dr. Walter L. Robb has served as a member of our Board of Directors since May 2009. Dr. Robb has served as a member of the Board of Directors of Check Cap Ltd. (Delaware) and a manager of Check-Cap LLC since February 2005. Dr. Robb is the general partner of each of Counterpoint Ventures Fund LP, established in 2004, and Counterpoint Ventures Fund II LP, established in 2011. Since 2009, Dr. Robb has served as the interim Chief Executive Officer of Cyclics Corporation, which manufactures and supplies resins and thermoplastic tooling products. Since 1993, Dr. Robb has served as a management consultant and president of Vantage Management, Inc. a private investment and consulting firm. Dr. Robb served as a member of the boards of directors of Celgene Corporation from 1992 to 2011 and has served as a member of the board of directors of Mechanical Technology, Inc. since 2000. Dr. Robb has also served as a director in several start-up companies over the last 20 years. Until 1993, Dr. Robb served as senior vice president and director for corporate research and development for General Electric, and served on General Electric’s Corporate Executive Council from 1986 to 1993. Dr. Robb was the director of GE Medical Systems from 1973 to 1986. In September 1994, Dr. Robb received the National Medal of Technology from President Clinton for leadership in the CT and MR Imaging Industry. Dr. Robb holds a BS degree in chemical engineering from Penn State University and an MS degree and PhD in chemical engineering, both from the University of Illinois.
Steven Hanley has served as a member of our Board of Directors since February 2015 and has served as a member of our Nominating Committee since October 2015 and as a member of our Audit Committee and Compensation Committee since December 2015. Mr. Hanley provided consultancy services to us on behalf of Neem LLC from November 2009 until December 31, 2014 and served as a Scientific Advisor to our company from June 2011 until his election to our Board of Directors in February 2015. Mr. Hanley is currently the Co-Founder, board member and Chief Executive Officer of MediBeacon Inc., an optical diagnostic company based in St. Louis, Missouri formed upon acquiring assets and intellectual property from Covidien in 2012. Mr. Hanley is an experienced global business leader who has managed highly complex pharmaceutical and medical device operations with annual global revenue exceeding $1 billion. As the President of Covidien plc’s Imaging Solutions business unit, he led a multifunctional organization that included sales, marketing, logistics, manufacturing, as well as research and development. Internationally, his track record includes numerous new drug and device product introductions and sales force expansion in Eastern Europe, China and Latin America. Mr. Hanley is experienced working in different cultures and successfully navigating dynamic regulatory environments. Over his nearly 18 years with the Covidien family of companies, Mr. Hanley developed a large network of business leaders and clinicians to help determine market needs, commercial potential and product positioning. As a sales leader, Mr. Hanley called on radiologists, nuclear medicine physicians, cardiologists, as well as surgeons in specialties including general, orthopedic, and OB/GYN. Mr. Hanley is Principal and Founder of Neem LLC, which was founded in 2009 to focus on startup and entrepreneurial medical device and other life science companies with whom the firm works to bridge the gap between breakthrough technology and commercialization. Mr. Hanley is the Chairman of the Board of Managers for Daya CNS LLC, based in St Louis Missouri. In addition, Mr. Hanley is currently on the Advisory Board for Kogent Surgical LLC, based in St Louis Missouri. Mr. Hanley has his bachelor’s and master’s degrees in business administration from Marquette University.
Yuval Yanai has served as a member of our board of directors as an external director (within the meaning of the Israeli Companies Law) and as the Chairman of our Audit Committee and Compensation Committee since March 15, 2015, and has served as the Chairman of our Nominating Committee since October 2015. Mr. Yanai served as Senior Vice President and Chief Financial Officer of Given Imaging Ltd. from September 2005 through March 2014. From October 2000 through August 2005, Mr. Yanai served as Senior Vice President and Chief Financial Officer of Koor Industries Ltd., one of Israel’s largest holding companies. Prior to that, from April 1998 to September 2000, Mr. Yanai served as Vice President and Chief Financial Officer of NICE Systems Ltd., an Israeli global provider of Insight from Interactions, and, from 1991 to April 1998, he served as the Vice President, Finance and Chief Financial Officer of Elscint Ltd., a former Israeli company engaged in the developing and manufacturing of medical imaging devices that was acquired by larger companies in this field. Mr. Yanai joined Elscint in 1985 and served as Corporate Controller and Corporate Treasurer through 1991. Mr. Yanai serves as an external director (within the meaning of the Israeli Companies Law) of Macrocure Ltd., an Israeli company whose shares are listed on the NASDAQ Global Market and serves as the Chairman of its audit committee. Mr. Yanai also serves as an external director (within the meaning of the Israeli Companies Law) of Medical Compression Systems (D.B.N) Ltd., an Israeli company whose shares are listed on the Tel Aviv Stock Exchange, and serves as the Chairman of its audit committee, compensation committee and financial reporting committee. Mr. Yanai also serves as an external director (within the meaning of the Israeli Companies Law) of Haddasah Medical Center, and serves as the Chairman of its finance and Information Technologies committees. Mr. Yanai also serves as an external director (within the meaning of the Israeli Companies Law) of Clal Biotechnology Industries and serves as the Chairman of its audit and financial reporting committees, and as a member of its compensation committee. Mr. Yanai also serves as the chairman of the board of directors of Endobetix Ltd., and as a member of the board of directors of Efrant Ltd, and CompuLab Ltd. Previously, Mr. Yanai served as a director of Citycon Oj, Starplast Industries Ltd., Adama Ltd. (formerly Makteshim-Agan Industries Ltd.), ECI Telecom Ltd., Equity One, Inc., BVR Systems Ltd., Tadiran Communication Ltd., The Elisra Group and Telrad Networks Ltd. Mr. Yanai holds a B.Sc. degree in Accounting and Economics from Tel-Aviv University.
81
XiangQian (XQ) Lin has served as a member of our Board of Directors, since February 2015. In addition, we have engaged Mr. Lin to provide to us certain business development services in Asia since June 1, 2015. Mr. Lin has served as the Group President and Chief Executive Officer of the Esco Group of Companies, a leading global life sciences tools provider active in laboratory, medical and pharmaceutical equipment based in Singapore, since February 2011. Since 2014, Mr. Lin has also served as the Managing Partner of Esco Ventures, a strategic investment arm of the Esco Group of Companies, focused on early stage investment in life sciences tools and med-tech start-ups, since he founded it in August 2014. From January 1997 until February 2011, Mr. Lin held various positions, lastly as Vice President, of Esco Micro Pte Ltd., a family business which he re-engineered into a successful life sciences tools company when he founded the Esco Biotech unit in 2000. From January 2007 to December 2010, Mr. Lin also served as the President of Esco Technologies, Inc., a wholly-owned U.S. subsidiary of Esco Micro Pte Ltd., which he established following a buy-out of a joint venture partner. Mr. Lin is the only non-U.S. member of the US NSF Standard 49 Joint Committee and is also a member of the Young Presidents Organization and the Singapore Venture Capital and Private Equity Association. Mr. Lin holds a BSc degree in Economics from the Wharton School, University of Pennsylvania.
Dr. Mary Jo Gorman has served as a member of our board of directors as an external director (within the meaning of the Israeli Companies Law) and as a member of our Audit Committee and Compensation Committee since May 2015. Dr. Gorman serves as Managing Director at Prosper Capital, an early stage investment fund focused on women led businesses. From 2006 to present, Dr. Gorman also serves as Founder of Advanced ICU Care, the largest telemedicine ICU services provider in the United States, in which she also served as Chairman and Chief Executive Officer from 2006 and 2014. From 1999 to 2006, Dr. Gorman served at IPC-The Hospitalist Company (NASDAQ:IPCM), a leading national physician group practice, as Chief Medical Officer (2003-2006), Vice President of Medical Affairs (2001-2003) and Regional Medical Director (1999-2001). From 1996 to 1998, Dr. Gorman served as President of Inpatient Care Group, a hospitalist group in St. Louis, Missouri, which she had founded to provide hospitalist services to primary care physicians and hospitals and which was subsequently sold to IPC. From 1991 to 2008, Dr. Gorman served as President of Critical Care Services, Inc., a privately held corporation which she had founded and which was later sold to Advanced ICU Care. Dr. Gorman was awarded with the following awards: 2015 Distinguished Alumni Award, Southern Illinois University School of Medicine; 2013 Distinguished Alumni Award, Olin School of Business, Washington University; 2011 EY Entrepreneurial Winning WomenTM Class of 2011; 2009 Top 25 Influential Women in Healthcare by Modern HealthCare Magazine. Dr. Gorman holds a B.A. degree in Chemistry and Biology (Cum Laude) from St. Louis University, an M.B.A degree from Olin School of Business, Washington University and an M.D. from Southern Illinois University School of Medicine.
Guy Neev served as our chief executive officer and a member of our board of directors from our inception in April 2009 until August 13, 2015 his employment with us ended on January 31, 2016.
Arrangements Concerning Election of Directors; Family Relationships
We are not a party to, and are not aware of, any voting agreements among our shareholders. In addition, there are no family relationships among our executive officers and directors.
Scientific Advisors
Certain of our officers and employees including our chief executive officer, our chief technology officer, our vice president, Research & Development and our clinical director have consulted on an individual, as needed basis with the individuals listed below with respect to matters of scientific relevance. We refer to these individuals as our Scientific Advisors. The amount of consulting services provided by our Scientific Advisors ranges from several hours a month to several hours a year. The names and biographies of the individuals who act as our Scientific Advisors are set forth below:
Dr. Douglas K. Rex is a Distinguished Professor of Medicine at Indiana University School of Medicine, Chancellor’s Professor at Indiana University Purdue University Indianapolis, and Director of Endoscopy at Indiana University Hospital in Indianapolis. He graduated from Harvard College, Summa Cum Laude in 1976 and with highest distinction from Indiana University School of Medicine in 1980. He served as Chief Medical Resident at Indiana University Hospital and joined the faculty at Indiana University in 1985. He received the Outstanding Teacher Award in the Introduction to Medicine course five times and has been awarded the Indiana University School of Medicine Outstanding Teacher Award as well as Department of Medicine’s Excellence in Teaching Award. He is a full-time clinical gastroenterologist at Indiana University Hospital. His major research interests have been in colorectal disease and, in particular, colorectal cancer screening and the technical performance of colonoscopy. He co-authored the colorectal cancer screening recommendations of the American College of Gastroenterology and the U.S. Multi-Society Task Force on Colorectal Cancer. He also authored the recommendations on quality in colonoscopy of the U.S. Multi-Society Task Force on Colorectal Cancer and the American College of Gastroenterology/American Society of Gastrointestinal Endoscopy. He has authored more than 200 original research papers, 55 book chapters, 200 invited papers, 40 editorials, and 40 guideline papers. He is an Associate Editor of Journal Watch Gastroenterology and serves on the editorial boards of 11 gastroenterology journals. He is the current chair of the U.S. Multi-Society (ACG, ASGE, AGA, ACP-ASIM) Task Force on Colorectal Cancer. Dr. Rex has also served in the American College of Gastroenterology as Chairman of the Board of Governors and is a Past President of the ACG. He is a member of the Governing Board of ASGE and a recipient of the Rudolph V. Schindler Award from ASGE.
82
Dr. Walter L. Robb has served as a member of our Board of Directors since May 2009. Dr. Robb has served as a member of the board of directors of Check Cap Ltd. (Delaware) and a manager of Check-Cap LLC since February 2005. Dr. Robb is the general partner of each of Counterpoint Ventures Fund LP, established in 2004, and Counterpoint Ventures Fund II LP, established in 2011. Since 2009, Dr. Robb has served as the interim chief executive officer of Cyclics Corporation, which manufactures and supplies resins and thermoplastic tooling products. Since 1993, Dr. Robb has served as a management consultant and president of Vantage Management, Inc. a private investment and consulting firm. Dr. Robb served as a member of the boards of directors of Celgene Corporation from 1992 to 2011 and has served as a member of the board of directors of Mechanical Technology, Inc. since 2000. Dr. Robb has also served as a director in several start-up companies over the last 20 years. Until 1993, Dr. Robb served as senior vice president and director for corporate research and development for General Electric, and served on General Electric’s Corporate Executive Council from 1986 to 1993. Dr. Robb was the director of GE Medical Systems from 1973 to 1986. In September 1994, Dr. Robb received the National Medal of Technology from President Clinton for leadership in the CT and MR Imaging Industry. Dr. Robb holds a BS degree in chemical engineering from Penn State University and an MS degree and PhD in chemical engineering, both from the University of Illinois.
Dr. Perry J. Pickhardt is a Professor of Radiology at the University of Wisconsin School of Medicine & Public Health. He served in the U.S. Navy, spending one year as the Department Head of Radiology, U.S. Naval Hospital Guantanamo Bay, Cuba and three years as the head of GI-GU Imaging at the National Naval Medical Center in Bethesda, MD. He also served as an Assistant Professor of Radiology at the Uniformed Services University of the Health Sciences in Bethesda. Among other projects at NNMC, Dr. Pickhardt organized a large multi-center screening trial evaluating CTC and served as the PI. Among other awards and honors, Dr. Pickhardt completed a Figley Fellowship at the AJR Editorial Office in Winston- Salem, NC in 2002. CTC and CRC screening continue to be among Dr. Pickhardt’s primary clinical and research interests. His work has resulted in over 300 scientific publications and book chapters, as well as several textbooks, including works on body CT and CTC. He graduated first in his class from the University of Wisconsin with a B.S. in Physics in 1991 and from the University of Michigan Medical School in 1995. Dr. Pickhardt has served as a Scientific Advisor to the Company since August 2009.
Prof. Nadir Arberis a full Professor of Medicine and Gastroenterology. He is a holder of the Yechiel and Helen Lieber chair for Cancer Research at Tel Aviv University, Sackler School of Medicine. Prof. Arber serves as the Director of the Integrated Cancer Prevention Center (ICPC) at Tel Aviv Sourasky Medical Center in Tel Aviv. He chaired the grants committee of the Sackler School of Medicine at Tel Aviv University. Prof. Arber is the Head of Cancer Research Center, Head of Djerassi Oncology Center, and President of the Israeli Association of Gastroenterology. Prof. Arber, a noted expert in the field of early detection and prevention, has been serving as the principal investigator (PI) of several international, multicenter clinical trials in the field of early detection, prevention and therapy of gastrointestinal malignancies using NSAIDs and in particular, CRC. Prof. Arber has published more than 330 publications. Prof. Arber received a MD from the Hadassah School of Medicine of the Hebrew University of Jerusalem in 1987, an MSc degree from Sackler School of Medicine, Tel Aviv University in 1991 and a MHA degree from Rekanaty School of Management, Tel Aviv University in 1991. Prof. Arber has served as a Scientific Advisor to the Company since January 2012. We have entered into a consultation service agreement with Prof. Arber who is a leading investigator in our multicenter clinical study which is held at Tel Aviv Sourasky Medical Center and other sites.
Dr. Jonathan A. Leighton is Professor of Medicine and Vice Chair of the Department of Medicine at the Mayo Clinic in Arizona. In addition, Dr. Leighton is a member of the Board of Trustees of the American College of Gastroenterology. Dr. Leighton is an active member of, the American Gastroenterology Association, the American Society of Gastrointestinal Endoscopy, and the American College of Gastroenterology. His research interests include inflammatory bowel disease, biomarker discovery, as well as small bowel imaging including optical capsule endoscopy and deep enteroscopy. Dr. Leighton received an MD degree from the University of Arizona in 1981 and completed his internship and residency at the University of Texas Health Science Center in San Antonio, Texas in 1984. Dr. Leighton has served as a Scientific Advisor to the Company since July 2014.
Dr. Seth A. Gross is an Associate Professor of Medicine at NYU School of Medicine. Dr. Gross is the Gastroenterology Section Chief at Tisch Hospital and Director of Endoscopy at NYU Langone Medical Center. His areas of interest include CRC screening and gastrointestinal malignancies. Dr. Gross is an associate editor for Gastrointestinal Endoscopy. Dr. Gross is active in both the ACG and ASGE and serves on several committees. Dr. Gross is currently the secretary at the NYSGE.
83
|
B.
|
Compensation of Directors and Executive Officers
|
At our shareholders 2015 Annual General Meeting which was held on December 1, 2015, our shareholders approved, following the approval of our Compensation Committee and Board of Directors the payment to each director who is not an employee (other than the Chairman of our Board of Directors and our external directors, within the meaning of the Israeli Companies Law) an annual fee of US$45,000, paid on a quarterly basis. As approved by our shareholders at an extraordinary general meeting held on May 19, 2015, our external directors (within the meaning of the Israeli Companies Law) are entitled to the same annual fee for the duration of their respective initial three-year terms. In addition, our directors benefit from the directors’ and officers’ indemnification agreement as well as from our directors’ and officers’ liability insurance policy. The directors are also entitled to reimbursement of expenses (including travel, stay and lodging), subject to the Israeli Companies Law and the regulations promulgated thereunder, and in accordance with our company practices and our Compensation Policy for Executive Officers and Directors as approved by our shareholders on August 13, 2015 (the “Compensation Policy”).
The aggregate compensation paid and share-based compensation and other payments expensed by us to our directors and executive officers with respect to the year ended December 31, 2015 was $4.8 million. This amount includes approximately $272,000 set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include business travel, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in our industry. As of December 31, 2015, options to purchase 707,814 ordinary shares granted to our directors and executive officers were outstanding under our 2006 Unit Option Plan at a weighted average exercise price of approximately $4.24 per share, and options to purchase 1,147,968 ordinary shares granted to our chief executive officer were outstanding under the United States Sub-Plan to our 2015 Equity Incentive Plan at a weighted average exercise price of approximately $4.04 per share.
Effective as of January 1, 2013, our former chief executive officer, our chief financial officer and our chief technology officer agreed to a temporary 20% reduction in their salaries, as part of cost cutting measures. To compensate such executives for the salary reduction, we agreed that upon completion of an equity financing, which included our initial public offering, each such executive will be entitled to the following: (i) a cash payment equal to the cost of such executive’s salary reduction plus 8% annual interest; (ii) options to purchase a number of ordinary shares equal to such executive’s cash payment divided by $4.96 with an exercise price per share equal to $4.96 and (iii) a one-time bonus equal to one and a half monthly salaries of such executive, provided that the aggregate bonuses for all such executives may not exceed $60,000 and the aggregate compensation for all such executives may not exceed $250,000. In connection with the consummation of our initial public offering and concurrent private placement, we granted to such executive options to purchase an aggregate 38,473 ordinary shares. Such options were granted under our 2006 Unit Option Plan and were fully vested upon grant.
Effective as of the closing of the credit line agreement that we entered into on August 20, 2014, the employment terms of each of our executive officers were amended as follows: (i) the respective base salaries of each of our executive officers increased effective as of November 1, 2014 by an aggregate annual amount of $348,000 for all executive officers (based on pre-reduced salaries); (ii) each of our chief executive officer and chief financial officer is entitled to a one-time bonus of specified amounts in the aggregate amount of $200,000 for both executive officers; (iii) each of our executive officers shall be entitled to an annual bonus up to certain specified amounts, at the discretion of our board of directors and subject to milestones determined by the board of directors; and (iv) we granted our executive officers options to purchase, in the aggregate, 581,542 of our ordinary shares, constituting, in the aggregate, 5.25% of our fully diluted share capital as of the closing, with an exercise price of NIS 0.20 per share. Each of the executive officers subsequently agreed that the exercise price of fifty-percent of their respective options will increase to equal the price at which our ordinary shares are sold to the public in the initial public offering of our securities, or if units are sold in the initial public offering of our securities, the exercise price per share will increase to be equal to the effective price per share of the ordinary shares underlying the units sold to the public in the offering. The executive officers also agreed that such portion of their options will vest and become exercisable only upon the consummation of the initial public offering of our securities prior to their expiration date. The remaining options with an NIS 0.20 exercise price will vest on a quarterly basis in eight equal installments during a period of 24 months from grant. In addition, the executive officers agreed to reduce the term of their respective options such that these options will now expire after eight years (instead of ten years) following their issuance, i.e., on October 14, 2022. Upon the closing of our initial public offering any unvested portion of the options became fully vested and exercisable.
84
Other than under our employment agreement with Mr. Densel, our Chief Executive who also serves as a director (see below “Employment Agreement with Executive Officers”), we do not have any written agreements with any director providing for benefits upon the termination of such director’s relationship with us.
The table below sets forth the compensation paid to our five most highly compensated senior office holders (as defined in the Israeli Companies Law) during or with respect to the year ended December 31, 2015, in the disclosure format based on Regulation 21 of the Israeli Securities Regulations (Periodic and Immediate Reports), 1970. We refer to the five individuals for whom disclosure is provided herein as our “Covered Executives.”
For purposes of the table and the summary below, and in accordance with the above mentioned securities regulations, “compensation” includes salary cost, consultancy fees, bonuses, equity-based compensation, retirement or termination payments, benefits and perquisites such as car, phone and social benefits and any undertaking to provide such compensation. All amounts reported in the table are in terms of cost to us, as recognized in our financial statements for the year ended December 31, 2015 plus compensation paid to such Covered Executive following the end of the year in respect of services provided during the year. Each of the Covered Employees was covered by our D&O liability insurance policy and was entitled to indemnification and exculpation in accordance with applicable law and our articles of association.
The exchange rate that we used to calculate the "Salary Cost" of our chief financial officer, chief technology officer and chief operations officer and Israeli site manager, as presented in the following table, was NIS 3.8869 to US$1.00, and is provided herein for convenience (such exchange rate is based on the average of 12 months last-day exchange rate, during year 2015, as published by the Bank of Israel).
|
Name and Principal Position(1)
|
Salary Cost(2)
|
Bonus(3)
|
Other(4)
|
Share-Based
Compensation(5)
|
Total
|
|||||||||||||||
|
US$
|
||||||||||||||||||||
|
William Densel - chief executive officer and director
|
247,840 | 43,750 | (6) | - | 538,239 | 829,829 | ||||||||||||||
|
Guy Neev - former chief executive officer
|
389,303 | 84,000 | 140,000 | 896,436 | 1,509,739 | |||||||||||||||
|
Lior Torem - chief financial officer
|
275,729 | 27,500 | - | 224,109 | 527,338 | |||||||||||||||
|
Yoav Kimchy - chief technology officer and former director
|
291,093 | 36,000 | (6) | - | 224,109 | 551,202 | ||||||||||||||
|
Alex Ovadia - chief operations officer and Israeli site manager
|
350,889 | 36,000 | - | 224,109 | 610,998 | |||||||||||||||
|
(1)
|
Unless otherwise indicated herein, all Covered Executives are employed on a full-time (100%) basis.
|
|
(2)
|
Salary cost includes the Covered Executive’s gross salary plus payment of social benefits made by us on behalf of such Covered Executive. Such benefits may include, to the extent applicable to the Covered Executive, payments, contributions and/or allocations for savings funds, education funds, pension, severance, risk insurances, payments for social security and tax gross-up payments, vacation, car, medical insurances and benefits, convalescence or recreation pay and other benefits and perquisites consistent with our policies.
|
|
(3)
|
Represents annual bonuses granted to the Covered Executives based on formulas set forth in their respective employment agreements.
|
|
(4)
|
Represents a one-time severance fee made by us to Mr. Guy Neev, our former chief executive officer, who served as our chief executive officer and a director from April 2009 until August 2015 and has served as an employee with us until January 31, 2016.
|
|
(5)
|
Represents the share-based compensation expenses recorded in our consolidated financial statements for the year ended December 31, 2015 based on the fair value of the grant date of the options, in accordance with accounting guidance for equity-based compensation.
|
|
(6)
|
The Bonus includes amounts that are subject to our shareholders approval, in accordance with the Israeli Companies Law.
|
|
(7)
|
The table does not include information regarding warrants to the Pontifax Funds to purchase an aggregate of 221,539 of our ordinary shares at an exercise price of NIS 0.20 per share, or the Pontifax Warrants, in consideration of their commitment to provide to us business development services and a representative designated by Pontifax to serve as the chairman of our board of directors. Upon the closing of our initial public offering any unvested portion of the warrants became fully vested and exercisable For additional information regarding the Pontifax Warrants, see Item 7.B. “Major Shareholders and Related Party Transactions—Related Party Transactions —Pontifax Warrants”. For information regarding the fair value of the Pontifax Warrants, see Note 16A to our audited consolidated financial statements presented elsewhere in this Annual Report.
|
85
Employment Agreements with Executive Officers
We have entered into written employment agreements with each of our executive officers. These agreements contain standard provisions for a company in our industry regarding non-competition, confidentiality of information and assignment of inventions. The enforceability of covenants not to compete in Israel and the United States is subject to limitations. For example, Israeli courts have recently required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property.
We entered into an Employment Agreement dated as of July 1, 2015, as amended, with William Densel, or Mr. Densel’s Employment Agreement, pursuant to which Mr. Densel serves as President and Chief Executive Officer of our company and our subsidiary Check-Cap U.S., Inc. Mr. Densel’s Employment Agreement may be terminated by either us or Mr. Densel upon not less than 90 days’ prior written notice to the other party. Pursuant to Mr. Densel’s Employment Agreement, we also have the right to terminate Mr. Densel’s employment for “Cause” (as defined in Mr. Densel’s Employment Agreement) or if Mr. Densel is unable to perform his duties for an aggregate period of at least 90 days occurring in any 180 day period by reason of illness, injury or physical or mental impairment. Pursuant to Mr. Densel’s Employment Agreement, Mr. Densel is entitled to a salary of $350,000 per annum. In addition, Mr. Densel’s Employment Agreement provides that our Board of Directors shall be entitled to grant to Mr. Densel an annual bonus of up to 50% of his annual salary. Payment of the annual bonus shall be subject to the achievement of certain milestones determined and approved by our Compensation Committee and Board of Directors. Pursuant to Mr. Densel’s Employment Agreement, we granted Mr. Densel the following options:
|
(i)
|
Options (the “Initial Grant Options”) to purchase 463,137 ordinary shares at an exercise price equal to the closing price of our ordinary shares on May 19, 2015 (the date on which Mr. Densel’s initial engagement with us was approved by our shareholders). The Initial Grant Options shall vest over a period of four years commencing on the date of grant (i.e., May 19, 2015), such that 25% of the options shall vest on the first anniversary of the date of Mr. Densel’s employment and thereafter, the remaining Initial Grant Options will vest in monthly installments, subject to Mr. Densel’s continuing employment with us or Check-Cap U.S., Inc. on each applicable vesting date. In the event of consummation of an M&A Transaction (as defined in Mr. Densel’s Employment Agreement), subject to Mr. Densel’s continuing employment through the effective date of such M&A Transaction, any unvested Initial Grant Options shall automatically vest and become exercisable.
|
|
(ii)
|
Options (“Second Grant Options”) to purchase 324,750 ordinary shares at an exercise price equal to the higher of: (a) the average of the closing prices of our ordinary shares over the 30 trading days immediately prior to the date of grant (i.e., August 13, 2015); and (b) the closing price of our ordinary shares on the trading day immediately prior to the date of grant. The Second Grant Options shall vest over a period of four years commencing on their date of grant, such that 25% of the Second Grant Options shall vest on the first anniversary of the date of grant and thereafter, the remaining Second Grant Options will vest in monthly installments, subject to Mr. Densel’s continuing employment as our Chief Executive Officer on each applicable vesting date. In the event of consummation of an M&A Transaction, subject to Mr. Densel’s continuing employment with us or Check-Cap U.S., Inc. through the effective date of such M&A Transaction, any unvested Second Grant Options shall automatically vest and become exercisable.
|
|
(iii)
|
Options (“Third Grant Options”) to purchase 425,000 ordinary shares at an exercise price equal to the higher of: (a) the average of the closing prices of our ordinary shares over the 30 trading days immediately prior to the date of grant (i.e., August 13, 2015); and (ii) the closing price of our ordinary shares on the trading day immediately prior to the date of grant. The Third Grant Options shall vest over a period of four years commencing on their date of grant pursuant to a formula set forth in Mr. Densel’s Employment Agreement and subject to the attainment of certain 12-month milestones to be set by our Board of Directors. In the event of consummation of an M&A Transaction, subject to Mr. Densel’s continuing employment with us or Check-Cap U.S., Inc. through the effective date of such M&A Transaction, any unvested Third Grant Options shall automatically vest and become exercisable.
|
86
Mr. Densel’s Employment Agreement also contains confidentiality, intellectual property, non-competition and non-solicitation provisions. Mr. Densel’s Employment Agreement provides that in the event that his engagement with us is terminated by us without “Cause” (other than due to death or disability), he shall be entitled to receive, during the six month period following the termination date, his annual salary of $350,000, payable monthly and medical and dental insurance.
We entered into an Employment Agreement dated May 1, 2010, as amended, with Lior Torem, or Mr. Torem’s Employment Agreement, and also a Consulting Agreement dated as of September 9, 2012 with L.T. Factor Ltd., or the Consultant, of which Lior Torem is manager and sole shareholder, or the Consulting Agreement. We refer to Mr. Torem’s Employment Agreement and the Consulting Agreement collectively as the “Torem Agreements.” Mr. Torem serves as our Chief Financial Officer pursuant to the Torem Agreements. The Torem Agreements may be terminated by either us, the Consultant or Mr. Torem upon 90 days’ prior notice to the other party. Pursuant to the Torem Agreements, Mr. Torem is entitled to a salary of $220,000 per annum. In addition, pursuant to the Torem Agreements, we paid Mr. Torem a one-time bonus of $50,000 in March, 2015 and our Board of Directors is entitled to grant to Mr. Torem an annual bonus of up to 25% of his annual salary. Payment of the annual bonus shall be subject to the achievement of certain milestones determined by the Board of Directors. The Torem Agreements also contain confidentiality, intellectual property, non-competition and non-solicitation provisions.
We entered into an Employment Agreement dated as of September 17, 2014, as amended, with Yoav Kimchy, or Mr. Kimchy’s Employment Agreement, pursuant to which Mr. Kimchy serves our Chief Technology Officer. Mr. Kimchy’s Employment Agreement may be terminated by either us or Mr. Kimchy upon 120 business days’ prior notice to the other party. Pursuant to Mr. Kimchy’s Employment Agreement, we also have the right to terminate Mr. Kinchy’s employment for “Cause” (as defined in Mr. Kimchy’s Employment Agreement) or if Mr. Kimchy is unable to perform his duties for a period of two consecutive months by reason of illness or accident. Pursuant to Mr. Kimchy’s Employment Agreement, Mr. Kimchy is entitled to a salary of $240,000 per annum. In addition, Mr. Kimchy’s Employment Agreement provides that our Board of Directors is entitled to grant to Mr. Kimchy an annual bonus of up to 30% of his annual salary. Payment of the annual bonus shall be subject to the achievement of certain milestones determined by the Board of Directors. Mr. Kimchy’s Employment Agreement also contains confidentiality, intellectual property, non-competition and non-solicitation provisions.
We entered into an Employment Agreement dated as of December 27, 2012, as amended, with Alex Ovadia, or Mr. Ovadia’s Employment Agreement, pursuant to which Mr. Ovadia serves as our Vice President of Research and Development, Israeli Site Manager and Chief Operations Officer. Mr. Ovadia’s Employment Agreement may be terminated by either us or Mr. Ovadia upon 60 days’ prior notice to the other party. Pursuant to Mr. Ovadia’s Employment Agreement, we also have the right to terminate Mr. Ovadia’s employment for “Cause” (as defined in Mr. Ovadia’s Employment Agreement). Pursuant to Mr. Ovadia’s Employment Agreement, Mr. Ovadia is entitled to a salary of $240,000 per annum. In addition, Mr. Ovadia’s Employment Agreement provides that our Board of Directors is entitled to grant to Mr. Ovadia an annual bonus of up to 30% of his annual salary. Payment of the annual bonus shall be subject to the achievement of certain milestones determined by the Board of Directors. Mr. Ovadia’s Employment Agreement also contains confidentiality, intellectual property, non-competition and non-solicitation provisions.
We entered into an Employment Agreement dated as of May 1, 2007, as amended, with Guy Neev, or Mr. Neev’s Employment Agreement, pursuant to which he served as our Chief Executive Officer. Mr. Neev’s Employment Agreement was able to be terminated by either us or Mr. Neev upon 120 business days’ prior notice to the other party. Pursuant to Mr. Neev’s Employment Agreement, we also had the right to terminate Mr. Neev’s employment for “Cause” (as defined in Mr. Neev’s Employment Agreement). Pursuant to Mr. Neev’s Employment Agreement, he was entitled to a salary of $280,000 per annum. In addition, pursuant to Mr. Neev’s Employment Agreement, we paid Mr. Neev a one-time bonus of $150,000 in March, 2015 and our Board of Directors was entitled to grant to Mr. Neev an annual bonus of up to 30% of his annual salary. Payment of the annual bonus was subject to the achievement of certain milestones determined by the Board of Directors. Mr. Neev’s Employment Agreement also contained confidentiality, intellectual property, non-competition and non-solicitation provisions. On August 13, 2015, we and Mr. Neev entered into a Separation and Release Agreement, or the Separation Agreement, pursuant to which Mr. Neev ceased to serve as our Chief Executive Officer effective upon the approval by our shareholders of the engagement of William Densel as our Chief Executive Officer on August 13, 2015, and his employment with us terminated on January 31, 2016. In accordance with the terms of Mr. Neev’s Employment Agreement and the Separation Agreement, we paid Mr. Neev his salary and benefits through the termination date of his employment, as well as a one-time severance payment of $140,000 and his annual bonus of $84,000 for the 12-month period ended August 18, 2015.
87
Other than the foregoing, our agreements with our executive officers do not provide for benefits upon the termination of their respective employment with us, other than payment of salary and benefits during the required notice period for termination of these agreements, which varies under these individual agreements.
|
C.
|
Board Practices
|
Board of Directors
Under the Israeli Companies Law, the management of our business, including strategy and policies, is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors. Our chief executive officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are appointed by our chief executive officer, and are subject to the terms of any applicable employment agreements that we may enter into with them.
Under our amended articles of association, which became effective upon the closing of our initial public offering, our board of directors must consist of at least four and not more than eleven directors, including at least two external directors required to be appointed under the Israeli Companies Law.
Our Board of Directors currently consists of seven members, four of whom satisfy the independence requirements of the NASDAQ Listing Rules, including two external directors (as defined under the Israeli Companies Law) such that we comply with the NASDAQ Listing Rule that requires that a majority of our board of directors be comprised of independent directors, within the meaning of NASDAQ Listing Rules. The definition of independent director under the NASDAQ Listing Rules and external director under the Israeli Companies Law overlap to a significant degree, such that we would generally expect our two directors serving as external directors under the Israeli Companies Law to satisfy the requirement to be independent under NASDAQ Listing Rule. The definition of external director under the Israeli Companies Law includes a set of statutory criteria that must be satisfied, including criteria whose aim is to ensure that there is no factor which would impair the ability of the external director to exercise independent judgment. The definition of independent director under NASDAQ Listing Rule specifies similar, if slightly less stringent, requirements in addition to the requirement that the board consider any factor which would impair the ability of the independent director to exercise independent judgment. However, external directors must be elected by a special majority of shareholders while independent directors may be elected by an ordinary majority. See “— External Directors” for a description of the requirements under the Israeli Companies Law for a director to serve as an external director.
Other than external directors, who are subject to special election requirements under the Israeli Companies Law (as detailed below), our amended and restated articles of association require that our directors be elected by the general meeting of our shareholders by the vote of a majority of the ordinary shares present, in person or by proxy, and voting at that meeting. Each director (other than the external directors) will hold office until the first annual general meeting of shareholders following his or her appointment, unless the tenure of such director expires earlier pursuant to the Israeli Companies Law or unless he or she is removed from office as described below. In addition, our amended articles of association allow our board of directors to appoint directors (other than the external directors) to fill vacancies on our board of directors, for a term of office equal to the remaining period of the term of office of the director(s) whose office(s) have been vacated. See “— External Directors” for a description of the procedure for the election of external directors.
In accordance with the exemption available to foreign private issuers under the NASDAQ Listing Rules, we do not follow the requirements of the NASDAQ Listing Rules with regard to the process of nominating directors, and instead, follow Israeli law and practice, in accordance with which our board of directors (or a committee thereof) is authorized to recommend to our shareholders director nominees for election. However, in October 2015, our Board of Directors voluntarily established a Nominating Committee, whose role is to select and recommend to the Board of Directors for selection, director nominees, while considering the appropriate size and composition of the Board of Directors, the requirements applicable to all members of the Board of Directors and the criteria for the selection of new members of the Board of Directors.
Under the Israeli Companies Law and our amended and restated articles of association, nominations for directors may also be added to the agenda of a future general meeting of shareholders, at the request of any one or more shareholders holding at least 1% of our outstanding voting power. Any director nominated by a shareholder is required to certify to us, as required by all director nominees, that he or she meets all the requirements of the Israeli Companies Law for election as a director of a public company, and possesses the necessary qualifications and is able to dedicate sufficient time, to fulfill his or her duties as a director of our company, taking into consideration our company’s size and special needs.
88
Under the Israeli Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial expertise. See “— External Directors.” In determining the number of directors required to have such expertise, our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the minimum number of directors of our company who are required to have accounting and financial expertise is one.
External Directors
Under the Israeli Companies Law, our board of directors is required to include at least two members who qualify, and were elected as, external directors (within the meaning of the Israeli Companies Law). We elected one external director, Mr. Yuval Yanai, prior to our initial public offering, whose election was ratified at a meeting of our shareholders held on May 19, 2015. A second external director, Ms. Mary Jo Gorman, was elected by our shareholders at a shareholder meeting held on May 19, 2015.
The provisions of the Israeli Companies Law set forth special approval requirements for the election of external directors. External directors must be elected by a majority vote of the shares present and voting on the matter at a shareholders meeting, provided that either:
|
•
|
such majority includes a majority of the shares held by all shareholders who are non-controlling shareholders and shareholders who do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding abstentions; or
|
|
•
|
the total number of shares held by shareholders who are non-controlling shareholders and shareholders who do not have a personal interest in the election of the external director (other than a personal interest not derived from a relationship with a controlling shareholder) voted against the election of the external director does not exceed 2% of the aggregate voting rights in the company.
|
The term “controllingshareholder” is defined in the Israeli Companies Law as a shareholder with the ability to direct the activities of a company, other than by virtue of being an office holder. A shareholder is deemed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in the company or has the right to appoint 50% or more of the directors of a company or its general manager. For purposes of shareholder approval of certain extraordinary and interested party transactions, as well as corporate approval of executive compensation, a controlling shareholder is deemed to include any shareholder (or two or more shareholders having a personal interest in the same matter being brought for approval) who hold(s) in the aggregate 25% or more of the voting rights in a public company if no other shareholder holds more than 50% of the voting rights in the company.
The term “personalinterest” is defined in the Israeli Companies Law as a person’s or entity’s personal interest in an act or a transaction of a company, (i) including the personal interest of (a) any spouse, sibling, parent, grandparent or descendant of the persons, any descendant, sibling or parent of a spouse of the person and the spouse of any of the foregoing; and (b) an entity in which the person or entity or any of the foregoing relatives of the person serves as a director or the chief executive officer, owns at least 5% of its issued share capital or voting rights or has the right to appoint one or more directors or the chief executive officer; but (ii) excluding a personal interest arising solely from the ownership of shares. In the case of a person voting by proxy, “personal interest” includes the personal interest of the proxy holder or the shareholder granting the proxy (even if the proxy holder has no personal interest in the matter), whether or not the proxy holder has discretion how to vote.
The initial term of an external director is three years. Thereafter, an external director may be reelected by shareholders to serve in that capacity for up to two additional three-year terms, provided that either:
|
•
|
his or her service for each such additional term is recommended by one or more shareholders holding at least 1% of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate voting rights in the company, and provided further that the external director is not an affiliated or competing shareholder, as defined in the Israeli Companies Law, or a relative of such a shareholder at the time of the appointment, and is not affiliated with such a shareholder at the time of appointment or within the two years preceding the date of appointment;
|
89
|
•
|
his or her service for each such additional term is recommended by the board of directors and is approved at a shareholders meeting by the same majority required for the initial election of an external director (as described above); or
|
|
•
|
such external director nominates himself or herself for each such additional term and his or her election is approved at a shareholders meeting by the same disinterested majority as required for the election of an external director nominated by a 1% or more shareholder (as described above).
|
However, the term of office for external directors for Israeli companies traded on certain foreign stock exchanges, including the NASDAQ Capital Market, may be extended indefinitely in increments of additional three-year terms, in each case provided that the audit committee and the board of directors of the company confirm that, in light of the external director’s expertise and special contribution to the work of the board of directors and its committees, the reelection for such additional period(s) is beneficial to the company, and provided that the external director is reelected subject to the same shareholder vote requirements as if elected for an additional term (as described above). Prior to the approval of the reelection of the external director at a general shareholders meeting, the company’s shareholders must be informed of the term previously served by him or her and of the reasons why the board of directors and audit committee recommended the extension of his or her term.
If the board of directors has determined that an external director ceases to meet the statutory qualifications for appointment or if he or she violates his or her duty of loyalty to the company, the board of directors is required to call a special general meeting of shareholders for the removal of the external director. In such circumstances, the removal of the external director by the shareholders requires the same special shareholder majority that is required for the election of an external director, as described above. An external director may also be removed by order of an Israeli court, at the request of a director or shareholder, if the court finds that the external director has ceased to meet the statutory qualifications for his or her appointment or has violated his or her duty of loyalty to the company. If an external directorship becomes vacant and there are fewer than two external directors on the board of directors at the time, then the board of directors is required under the Israeli Companies Law to call a shareholders’ meeting as soon as practicable to appoint a replacement external director.
Each committee of the board of directors that exercises the powers of the board of directors must include at least one external director, except that the audit committee and the compensation committee must include all external directors then serving on the board of directors. Under the Israeli Companies Law, external directors of a company are prohibited from receiving, directly or indirectly, any compensation for their services as external directors other than pursuant to the Israeli Companies Law and the regulations promulgated thereunder. Compensation of an external director is determined prior to his or her appointment and may not be changed during any three-year term subject to certain exceptions.
The Israeli Companies Law provides that a person is not qualified to serve as an external director if (i) the person is a relative of a controlling shareholder of the company; or (ii) if that person or his or her relative, partner, employer, another person to whom he or she was directly or indirectly subordinate, or any entity under the person’s control, has or had, during the two years preceding the date of appointment as an external director: (a) any affiliation with the company, with any person or entity controlling the company or a relative of such person at the time of appointment, or with any entity controlled by or under common control with the company at the time of appointment or during the two years preceding the appointment; or (b) in the case of a company with no controlling shareholder or a shareholder holding 25% or more of its voting rights, had at the date of appointment as an external director, any affiliation with a person then serving as chairman of the board or chief executive officer, a holder of 5% or more of the issued share capital or voting power in the company or the most senior financial officer.
The term “relative” is defined as a spouse, sibling, parent, grandparent or descendant; spouse’s sibling, parent or descendant; and the spouse of each of the foregoing persons.
The term “affiliation” includes (subject to certain exceptions): an employment relationship; a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships); control; and service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director was appointed as a director of the private company in order to serve as an external director following the initial public offering.
The term “office holder” is defined under the Israeli Companies Law as a general manager, chief business manager, deputy general manager, vice general manager, any other person assuming the responsibilities of any of these positions regardless of that person’s title, a director and any other manager directly subordinate to the general manager.
90
In addition, no person may serve as an external director if that person’s positions or professional or other activities create, or may create, a conflict of interest with that person’s responsibilities as a director or otherwise interfere with that person’s ability to serve as a director or if the person is an employee of the Israel Securities Authority or of an Israeli stock exchange. A person may furthermore not continue to serve as an external director if he or she received direct or indirect compensation other than as permitted by the Israeli Companies Law and the regulations promulgated thereunder.
Following the termination of an external director’s service on a board of directors, such former external director and his or her spouse and children and other relatives may not be provided a direct or indirect benefit by the company, its controlling shareholder or any entity under its controlling shareholder’s control. This includes engagement as an officer or director of the company or a company controlled by its controlling shareholder or employment by, or provision of services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by such person. This restriction extends for a period of two years with regard to the former external director and his or her spouse or child and for one year with respect to other relatives of the former external director.
If at the time at which an external director is appointed all members of the board of directors who are not controlling shareholders or relatives of controlling shareholders of the company are of the same gender, the external director to be appointed must be of the other gender. A director of one company may not be appointed as an external director of another company if a director of the other company is acting as an external director of the first company at such time.
According to the Israeli Companies Law and regulations promulgated under the Israeli Companies Law, a person may be appointed as an external director only if he or she has professional qualifications or if he or she has accounting and financial expertise (each, as defined below). At least one of the external directors must be determined by our board of directors to have accounting and financial expertise. However, as a company listed on the NASDAQ Capital Market, neither of our external directors is required to possess accounting and financial expertise as long as each possesses the requisite professional qualifications, and at least one of our other directors (i) meets the independence requirements under the Exchange Act and the NASDAQ Listing Rules for membership on the audit committee; and (ii) has accounting and financial expertise as defined under Israeli Companies Law.
A director with accounting and financial expertise is a director who, due to his or her education, experience and skills, possesses an expertise in, and an understanding of, financial and accounting matters and financial statements, such that he or she is able to understand the financial statements of the company and initiate a discussion about the presentation of financial data. A director is deemed to have professional qualifications if he or she has any of (i) an academic degree in economics, business management, accounting, law or public administration; (ii) an academic degree or has completed another form of higher education in the primary field of business of the company or in a field which is relevant to his/her position in the company; or (iii) at least five years of experience serving in one of the following capacities, or at least five years of cumulative experience serving in two or more of the following capacities: (a) a senior business management position in a company with a significant volume of business; (b) a senior position in the company’s primary field of business; or (c) a senior position in public administration or service. The board of directors is charged with determining whether a director possesses financial and accounting expertise or professional qualifications.
Our board of directors has determined that Mr. Yuval Yanai has accounting and financial expertise, as required under the Israeli Companies Law and that Ms. Mary Jo Gorman has the requisite professional qualifications as required under the Israeli Companies Law.
Audit Committee
Our audit committee currently consists of Steven Hanley and our two external directors, Yuval Yanai and May Jo Gorman. Yuval Yanai serves as the Chairman of the audit committee.
Israeli Companies Law Requirements
Under the Israeli Companies Law, we are required to appoint an audit committee. The audit committee must be comprised of at least three directors, including all of the external directors, one of whom must serve as chairman of the committee. The audit committee may not include the chairman of the board, a controlling shareholder of the company or a relative of a controlling shareholder, a director employed by or providing services on a regular basis to the company, to a controlling shareholder or to an entity controlled by a controlling shareholder or a director who derives most of his or her income from a controlling shareholder.
91
In addition, under the Israeli Companies Law, the audit committee of a publicly traded company must consist of a majority of independent directors, within the meaning of the Israeli Companies Law. In general, an “independent director” under the Israeli Companies Law is defined as either an external director or a director who meets the following criteria:
|
•
|
the audit committee has determined that he or she meets the qualifications for being appointed as an external director, except for (i) the requirement that the director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed outside of Israel); and (ii) the requirement for accounting and financial expertise or professional qualifications; and
|
|
•
|
he or she has not served as a director of the company for a period exceeding nine consecutive years. For this purpose, a break of less than two years in the service shall not be deemed to interrupt the continuation of the service.
|
NASDAQ Listing Requirements
Under the NASDAQ corporate governance rules, we are required to maintain an audit committee consisting of at least three independent directors, within the meaning of the Exchange Act and NASDAQ Listing Rules, each of whom must be able to read and understand fundamental financial statements, including the company’s balance sheet, income statement and cash flow statement (and one of whom has past employment experience in finance or accounting, requisite professional certification in accounting or other comparable experience or background that leads to financial sophistication) and none of whom has participated in the preparation of our or any of our subsidiary’s financial statements at any time during the prior three years.
All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the U.S. Securities and Exchange Commission and the NASDAQ Listing Rules. Our board of directors has determined that Mr. Yanai is an audit committee financial expert as defined by the U.S. Securities and Exchange Commission rules and has the requisite financial sophistication required by the NASDAQ Listing Rules.
Each of the members of the audit committee qualifies as an “independent director” within the meaning of NASDAQ Listing Rules and is “independent” as such term is defined in Rule 10A-3(b)(1) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which is different from the general NASDAQ test for independence of board and committee members.
Audit Committee Role
Our board of directors adopted an audit committee charter, which became effective upon the listing of our securities on the NASDAQ Capital Market, which sets forth the responsibilities of the audit committee consistent with the rules of the U.S. Securities and Exchange Commission and the NASDAQ Listing Rules, as well as the requirements for audit committees under the Israeli Companies Law, including the following:
|
•
|
oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors or shareholders for their approval, as applicable, in accordance with the requirements of the Israeli Companies Law;
|
|
•
|
recommending the engagement or termination of the person filling the office of our internal auditor; and
|
|
•
|
recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors or shareholders for their approval, as applicable, in accordance with the requirements of the Israeli Companies Law.
|
Our audit committee provides assistance to our board of directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent of management.
92
Under the Israeli Companies Law, our audit committee is responsible for:
|
•
|
determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices;
|
|
•
|
determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest) and whether such transaction is extraordinary or material under Israeli Companies Law (see “— Approval of Related Party Transactions under Israeli Law”);
|
|
•
|
determining whether a competitive process must be implemented for the approval of certain transactions with controlling shareholders or its relative or in which a controlling shareholder has a personal interest (whether or not the transaction is an extraordinary transaction), under the supervision of the audit committee or other party determined by the audit committee and in accordance with standards determined by the audit committee, or whether a different process determined by the audit committee should be implemented for the approval of such transactions;
|
|
•
|
determining the process for the approval of certain transactions with controlling shareholders or in which a controlling shareholder has a personal interest that the audit committee has determined are not extraordinary transactions but are not immaterial transactions;
|
|
•
|
where the board of directors approves the working plan of the internal auditor, to examine such working plan before its submission to the board of directors and proposing amendments thereto;
|
|
•
|
examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities;
|
|
•
|
examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the compensation of our auditor; and
|
|
•
|
establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
|
Our audit committee may not approve any actions requiring its approval (see “— Approval of Related Party Transactions under Israeli Law”), unless at the time of the approval a majority of the committee’s members are present, which majority consists of independent directors within the meaning of the Israeli Companies Law, including at least one external director.
Compensation Committee and Compensation Policy
Our compensation committee currently consists of Steven Hanley and our two external directors, Yuval Yanai and Mary Jo Gorman. Yuval Yanai serves as the Chairman of the compensation committee.
Israeli Companies Law Requirements
Under the Israeli Companies Law, the board of directors of a public company must appoint a compensation committee. The compensation committee must be comprised of at least three directors, including all of the external directors, who must constitute a majority of the members of the compensation committee. However, subject to certain exceptions, Israeli companies whose securities are traded on certain U.S. stock exchanges, such as the NASDAQ Capital Market, that do not have a controlling shareholder, do not have to meet such majority requirement, provided that the compensation committee meets other Israeli Companies Law composition requirements and the requirements of the jurisdiction where the company’s securities are traded. Each compensation committee member that is not an external director must be a director whose compensation does not exceed an amount that may be paid to an external director under regulations promulgated under the Israeli Companies Law. The compensation committee is subject to the same Israeli Companies Law restrictions as the audit committee as to who may not be a member of the committee. See “— Audit Committee — Israeli Companies Law Requirements.”
Under a recent amendment to the Israeli Companies Law, the audit committee may act as the compensation committee if it complies with the requirements regarding the composition of the compensation committee.
93
NASDAQ Listing Requirements
Under the NASDAQ corporate governance rules, we are required to maintain a compensation committee consisting of at least two directors, each of whom is an independent director within the meaning of the NASDAQ Listing Rules.
Compensation Committee Role
Our board of directors adopted a compensation committee charter which became effective upon the listing of our shares on the NASDAQ Capital Market, which sets forth the responsibilities of the compensation committee consistent with the NASDAQ Listing Rules and the requirements for compensation committees under the Israeli Companies Law, including the following:
|
•
|
recommending to the board of directors for its approval (i) a compensation policy; (ii) whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years); and (iii) periodic updates to the compensation policy. See “— Compensation Committee and Compensation Policy.” In addition, the compensation committee is required to periodically examine the implementation of the compensation policy;
|
|
•
|
the approval of the terms of employment and service of office holders (including determining whether the compensation terms of a candidate for chief executive officer of the company need not be brought to approval of the shareholders); and
|
|
•
|
reviewing and approving grants of options and other incentive awards to persons other than office holders to the extent such authority is delegated by our board of directors, subject to the limitations on such delegation as provided in the Israeli Companies Law.
|
Compensation Policy
Under the Israeli Companies Law, the duties of the compensation committee include the recommendation to the company’s board of directors of a policy regarding the terms of engagement of office holders, as such term is defined in the Israeli Companies Law, to which we refer to as a compensation policy, and any extensions and updates thereto. The compensation policy must be adopted by the company’s board of directors, after considering the recommendations of the compensation committee, and will need to be brought for approval by the company’s shareholders, which approval requires a Special Approval for Compensation (as defined below under “— Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions”).
We were required to adopt a compensation policy within nine months following the listing of our securities on the NASDAQ Capital Market. Accordingly, our shareholders approved the adoption of our Compensation Policy for Executive Officers and Directors at our Extraordinary General Meeting held on August 13, 2015, following the recommendation of our Compensation Committee and Board of Directors, in accordance with the provisions of the Israeli Companies Law.
Our Compensation Policy for Executive Officers and Directors serves as the basis for decisions concerning the financial terms of employment or engagement of our office holders, including exculpation, insurance, indemnification and any benefit, monetary payment or obligation of payment in respect of employment or engagement, including and any severance payment or benefit.
The Compensation Policy was determined, and must later reevaluated, according to certain factors, including: (i) the advancement of a company’s objectives, business plan and its long-term strategy; (ii) the creation of appropriate incentives for executives, while considering (among other things) the company’s risk management policy; (iii) the size and the nature of the company’s operations; and (iv) with respect to variable compensation, the contribution of the office holder towards the achievement of the company’s long-term goals and the maximization of its profits, all with a long-term objective and in accordance with the position of the office holder.
94
In accordance with the Israeli Companies law, our Compensation Policy refers to the following factors:
|
·
|
the knowledge, skills, expertise, professional experience and accomplishments of the relevant office holder;
|
|
·
|
the office holder’s roles and responsibilities and prior compensation agreements with him or her;
|
|
·
|
the ratio of the cost of the offered terms to the cost of compensation of the other employees of the company (including any employees employed through manpower companies), specifically to the cost of the average and median salaries of such employees and the impact of the disparities between them upon work relationships in the company;
|
|
·
|
with respect to variable compensation - the possibility of reducing variable compensation at the discretion of the board of directors, and the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and
|
|
·
|
with respect to severance compensation, the period of employment or service of the office holder, the terms of his or her compensation during such period, the company’s performance during such period, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
|
In addition, in according with the Israeli Companies Law, our Compensation Policy includes the following principles:
|
·
|
the link between variable compensation (e.g., bonuses) and long-term performance and measurable criteria (i.e., variable compensation must be determined based on long-term performance and measurable criteria). Only “non-material” portion of variable compensation may be determined based on criteria that is not measurable, taking into account office holders contribution to the company;
|
|
·
|
the ratio of variable to fixed compensation, and the ceiling for the value of variable compensation, which is determined at the time of payment, except that the ceiling for equity-based compensation is determined at the time of grant;
|
|
·
|
the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements;
|
|
·
|
the minimum holding or vesting period for variable, equity-based compensation, while taking into account long-term objectives; and
|
|
·
|
maximum limits for severance compensation.
|
For further information regarding our Compensation Policy, please see Appendix A included in Exhibit 99.3 attached to our proxy statement, which was filed in connection with the Company's Extraordinary General Meeting that was held on August 13, 2015, filed on Form 6-k with the SEC on July 6, 2015.
Nominating Committee
In accordance with the exemption available to foreign private issuers under the NASDAQ Listing Rules, we do not follow the requirements of the NASDAQ Listing Rules with regard to the process of nominating directors, and instead, follow Israeli law and practice, in accordance with which our Board of Directors (or a committee thereof) is authorized to recommend to our shareholders director nominees for election. However, in October 2015, our Board of Directors voluntarily established a Nominating Committee, whose role is to select and recommend to the Board of Directors for selection, director nominees, while considering the appropriate size and composition of the Board of Directors, the requirements applicable to all members of the Board of Directors and the criteria for the selection of new members of the Board of Directors. The Nominating Committee is currently comprised of the following directors: Yuval Yanai (an external director within the meaning of the Israeli Companies Law and an independent director within the meaning of the NASDAQ Listing Rules), who serves as the Chairman of the Nominating Committee, Tomer Kariv (the Chairman of our Board of Directors) and Steven Hanley (an independent director within the meaning of the NASDAQ Listing Rules).
95
Internal Auditor
Under the Israeli Companies Law, the board of directors of an Israeli public company must appoint an internal auditor recommended by the audit committee. An internal auditor may not be:
|
•
|
a person (or a relative of a person) who holds more than 5% of the company’s outstanding shares or voting rights;
|
|
•
|
a person (or a relative of a person) who has the power to appoint a director or the general manager of the company;
|
|
•
|
an office holder, within the meaning of the Israeli Companies Law (including a director and the general manager) of the company (or a relative thereof); or
|
|
•
|
a member of the company’s independent accounting firm, or anyone on his or her behalf.
|
The role of the internal auditor is to examine, among other things, our compliance with applicable law and orderly business procedures. The audit committee is required to oversee the activities and to assess the performance of the internal auditor as well as to review the internal auditor’s work plan. We have appointed Ms. Dana Gottesman Erlich of BDO as our internal auditor.
Approval of Related Party Transactions under Israeli Law
Fiduciary Duties of Directors and Executive Officers
The Israeli Companies Law codifies the fiduciary duties that office holders owe to a company. Each person listed in the table under “Management—Executive Officers and Directors” is an office holder under the Israeli Companies Law.
An office holder’s fiduciary duties consist of a duty of care and a duty of loyalty. The duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care includes a duty to use reasonable means to obtain:
|
•
|
information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and
|
|
•
|
all other important information pertaining to any such action.
|
The duty of loyalty requires an office holder to act in good faith and in the best interests of the company, and includes, among other things, the duty to:
|
•
|
refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or personal affairs;
|
|
•
|
refrain from any activity that is competitive with the company;
|
|
•
|
refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and
|
|
•
|
disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder.
|
We may approve an act specified above which would otherwise constitute a breach of the office holder’s duty of loyalty, provided that the office holder acted in good faith, the act or its approval does not harm the company and the office holder discloses his or her personal interest a sufficient amount of time before the date for discussion of approval of such act.
96
Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions
Disclosure of Personal Interests of an Office Holder
The Israeli Companies Law requires that an office holder promptly disclose to the company any “personal interest” that he or she may be aware of and all related material information or documents concerning any existing or proposed transaction with the company. An interested office holder’s disclosure must be made promptly and in any event no later than the first meeting of the board of directors at which the transaction is considered. A personal interest includes an interest of any person in an act or transaction of a company, including a personal interest of such person’s relative or of a corporate entity in which such person or a relative of such person holds 5% or more of the outstanding shares or voting rights, is a director or general manager or in which he or she has the right to appoint at least one director or the general manager, but excluding a personal interest arising from one’s ownership of shares in the company. A personal interest includes the personal interest of a person for whom the office holder holds a voting proxy or the personal interest of the office holder with respect to his or her vote on behalf of a person for whom he or she holds a proxy even if such shareholder has no personal interest in the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction. Under the Israeli Companies Law, an extraordinary transaction is defined as any of the following: a transaction other than in the ordinary course of business; a transaction that is not on market terms; or a transaction that may have a material impact on a company’s profitability, assets or liabilities.
Generally, a person who has a personal interest in a matter which is considered at a meeting of the board of directors or the audit committee shall not be present at such a meeting or vote on that matter unless, with respect to an office holder, the chairman of the audit committee or board of directors (as applicable) determines that the office holder should be present during the discussions in order to present the transaction that is subject to approval (provided that the office holder may not vote on the matter). If a majority of the members of the audit committee or the board of directors (as applicable) has a personal interest in the approval of a transaction, then all directors may participate in discussions of the audit committee or the board of directors (as applicable) on such transaction and the voting on approval thereof. If a majority of the members of the board of directors has a personal interest in the approval of a transaction, shareholder approval is also required for such transaction.
Approval of Transactions with Officer Holders
If it is determined that an office holder has a personal interest in a transaction that is not an extraordinary transaction, approval by the board of directors is required for the transaction, unless the company’s articles of association provide for a different method of approval. Further, so long as an office holder has disclosed his or her personal interest in a transaction, the board of directors may approve an act by the office holder that would otherwise be deemed a breach of his or her duty of loyalty, provided that the transaction is in the company’s best interest and the office holder acted in good faith. An extraordinary transaction in which an office holder has a personal interest requires approval first by the company’s audit committee and subsequently by the board of directors.
Compensation of Officers Other than the Chief Executive Officer
The compensation of an office holder (other than the chief executive officer) who is not a director requires approval first by the company’s compensation committee, then by the company’s board of directors, according to the company’s compensation policy. In special circumstances the compensation committee and board of directors may approve a compensation arrangement that is inconsistent with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and such arrangement must be approved by a majority vote of the shares present and voting at a shareholders meeting on the matter, provided that either: (i) such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and shareholders who do not have a personal interest in such compensation arrangement present and voting on the matter, excluding abstentions; or (ii) the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in the matter and who vote against the matter does not exceed 2% of the company’s aggregate voting rights. We refer to this as the Special Approval for Compensation. However, if the shareholders of the company do not approve a compensation arrangement with an executive officer that is inconsistent with the company’s compensation policy, the compensation committee and board of directors may, in special circumstances, override the shareholders’ decision if each of the compensation committee and the board of directors discuss the arrangement again, analyze the shareholders’ objection and provide detailed reasons for their decision.
97
Compensation of Chief Executive Officer
The compensation of a public company’s chief executive officer requires the approval of first, the company’s compensation committee; second, the company’s board of directors and third (except for a number of exceptions), the company’s shareholders by the Special Approval for Compensation. However, if the shareholders of the company do not approve a compensation arrangement with a chief executive officer, the compensation committee and board of directors may, in special circumstances, override the shareholders’ decision if each of the compensation committee and the board of directors discuss the arrangement again, analyze the shareholders’ objection and provide detailed reasons for their decision. The compensation committee and board of directors approval should be in accordance with the company’s compensation policy; however, in special circumstances, they may approve compensation terms of a chief executive officer that are inconsistent with such policy provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by the Special Approval for Compensation.
Compensation of Directors
Arrangements regarding the compensation of a director require the approval of the compensation committee, board of directors and (except for a number of exceptions) shareholders by ordinary majority, in that order. The approval of the compensation committee and board of directors must be in accordance with the compensation policy. In special circumstances the compensation committee and board of directors may approve a compensation arrangement that is inconsistent with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by the Special Approval for Compensation.
With respect to compensation of an officer (including chief executive officer) or director who is also a controlling shareholder, see “— Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions.”
Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions
Pursuant to Israeli law, the disclosure requirements regarding personal interests that apply to directors and executive officers also apply to a controlling shareholder of a public company. In the context of a transaction involving a shareholder of the company, a controlling shareholder also includes a shareholder who holds 25% or more of the voting rights in the company if no other shareholder holds more than 50% of the voting rights in the company. For this purpose, the holdings of all shareholders who have a personal interest in the same transaction will be aggregated. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, and the terms of engagement with a controlling shareholder or a relative thereof, directly or indirectly (including through a corporation controlled by a controlling shareholder), for the provision of services to the company and his or her terms of employment or service as an office holder or employment as other than an office holder, require the approval of each of (i) the audit committee or the compensation committee with respect to the terms of service or employment by the company as an office holder, an employee or service provider; (ii) the board of directors; and (iii) the shareholders, in that order. The shareholder approval requires one of the following, which we refer to as a Special Majority:
|
•
|
a majority of the shares held by all shareholders who do not have a personal interest in the transaction and who are present and voting on the matter approves the transaction, excluding abstentions; or
|
|
•
|
the shares voted against the transaction by shareholders who have no personal interest in the transaction and who are present and voting at the meeting do not exceed 2% of the voting rights in the company.
|
Each shareholder voting on the approval of an extraordinary transaction with a controlling shareholder must inform the company prior to voting whether or not he or she has a personal interest in the approval of the transaction, otherwise, the shareholder is not eligible to vote on the proposal and his or her vote will not be counted for purposes of the proposal.
To the extent that any such transaction with a controlling shareholder is for a period of more than three years, approval is required once every three years, unless, with respect to any such extraordinary transactions, the audit committee determines that the duration of the transaction is reasonable given the related circumstances.
98
The compensation committee and board approval for arrangements regarding the terms of service or employment of a controlling shareholder must be in accordance with the company’s compensation policy. In special circumstances the compensation committee and board of directors may approve a compensation arrangement that is inconsistent with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by the Special Majority.
Pursuant to regulations promulgated under the Israeli Companies Law, certain transactions with a controlling shareholder or his or her relative, or with directors, relating to terms of service or employment that would otherwise require approval of a company’s shareholders may be exempt from shareholder approval upon certain determinations of the audit committee and board of directors. Under these regulations, a shareholder holding at least 1% of the issued share capital or voting power of the company may require, within 14 days of the publication or announcement of such determinations, that despite such determinations by the audit committee and the board of directors, such transaction will require shareholder approval under the same majority requirements that would otherwise apply to such transactions.
In addition, disclosure of a personal interest in a private placement of a public company (including disclosure of any material fact or document) is required by (i) a shareholder holding 5% or more of the company’s issued and outstanding capital or its voting rights whose holdings will increase as result of the private placement and a shareholder who will hold 5% or more of the company’s issued and outstanding capital or its voting rights as a result of the private placement, if 20% or more of the company’s outstanding share capital prior to the private placement is issued in the private placement and the payment for which is not only in cash or listed securities or the transaction is not on market terms; and (ii) a person or entity that will become a controlling shareholder as a result of the private placement.
Shareholder Duties
Pursuant to the Israeli Companies Law, a shareholder has a duty to act in good faith and in a customary manner toward the company and other shareholders and to refrain from abusing his or her power in the company, including, among other things, in voting at a meeting of shareholder with respect to the following matters:
|
•
|
an amendment to the company’s articles of association;
|
|
•
|
an increase of the company’s authorized share capital;
|
|
•
|
a merger; and
|
|
•
|
the approval of related party transactions and acts of office holders that require shareholder approval.
|
In addition, a shareholder has a general duty to refrain from discriminating against other shareholders.
Certain shareholders have a duty of fairness toward the company. These shareholders include any controlling shareholder, any shareholder who knows that he or she has the power to determine the outcome of a shareholder vote and any shareholder who has the power to appoint or to prevent the appointment of an office holder of the company or other power towards the company. The Israeli Companies Law does not define the substance of the duty of fairness, except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Israeli Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care, but only if a provision authorizing such exculpation is included in its articles of association. Our amended articles of association include such a provision, to the fullest extent permitted by law. The company may not exculpate in advance a director from liability arising out of a prohibited dividend or other distribution to shareholders.
99
Under the Israeli Companies Law and the Israeli Securities Law, 5728-1968, or the Israeli Securities Law, a company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking made in advance of any such event or following an event, provided its articles of association include a provision authorizing such indemnification:
|
•
|
a financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria;
|
|
•
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction;
|
|
•
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; and
|
|
•
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder, or certain compensation payments made to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Israeli Securities Law.
|
Under the Israeli Companies Law and the Israeli Securities Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder if and to the extent provided in the company’s articles of association:
|
•
|
a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
|
•
|
a breach of the duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder;
|
|
•
|
a financial liability imposed on the office holder in favor of a third party; and
|
|
•
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder or certain compensation payments to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Securities Law.
|
Under the Israeli Companies Law, a company may not indemnify, exculpate or enter into an insurance contract for office holder liability, for any of the following:
|
•
|
a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company;
|
|
•
|
a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder;
|
|
•
|
an act or omission committed with intent to derive illegal personal benefit; or
|
|
•
|
a fine, monetary sanction or forfeit levied against the office holder.
|
Under the Israeli Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to the chief executive officer or a director or under certain circumstances, also by the shareholders. See “— Approval of Related Party Transactions under Israeli Law.”
100
Our amended articles of association permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted under the Israeli Companies Law and the Israeli Securities Law. We have obtained directors’ and officers’ liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by the Israeli Companies Law.
We have entered into indemnification and exculpation agreements with each of our current officers and directors exculpating them from a breach of their duty of care to us to the fullest extent permitted by the Israeli Companies Law and undertaking to indemnify them to the fullest extent permitted by the Israeli Companies Law and the Israeli Securities Law, to the extent that these liabilities are not covered by insurance. This indemnification is limited to events determined as foreseeable by our board of directors based on our activities, as set forth in the indemnification agreements. Under such indemnification agreements, the maximum aggregate amount of indemnification that we may pay to any and all of our currently serving or future officers and directors together may not exceed the higher of $5 million and 25% of our shareholders equity according to our most recent financial statements at the time of payment. In the opinion of the SEC, however, indemnification of directors and office holders for liabilities arising under the Securities Act is against public policy and therefore unenforceable.
|
D.
|
Employees
|
As of December 31, 2015, we had 59 employees and independent contractors based in Israel and 1 employee based in the U.S of whom 9 persons were in administrative, accounting and human resources and 50 persons were in research and development. As of December 31, 2014, we had 36 employees and independent contractors, all based in Israel, of whom 6 persons were in administrative, accounting and human resources and 30 persons were in research and development. As of December 31, 2013, we had 34 employees and independent contractors, all based in Israel, of whom 6 persons were in administrative, accounting and human resources and 28 persons were in research and development.
Under Israeli law, we and our employees are subject to protective labor provisions, including the length of the workday, minimum wages for employees, annual leave, sick pay, determination of severance pay and advance notice of termination of employment, as well as procedures for hiring and dismissing employees and equal opportunity and anti-discrimination laws. While none of our employees is party to any collective bargaining agreements, orders issued by the Israeli Ministry of Economy and Industry (formerly named the Ministry of IEconomy) may make certain industry-wide collective bargaining agreements applicable to us. These agreements affect matters such as the length of the workday and week, recuperation pay, travel expenses and pension rights. We have never experienced labor-related work stoppages and believe that our relationships with our employees are a significant part of our operations and that we maintain a good and positive relationship with our employees.
Israeli law generally requires the payment of severance compensation by employers upon the retirement, death or dismissal of an employee. We fund our ongoing severance obligations by making monthly payments to insurance policies. All of our current employees have agreed that upon termination of their employment, they will be entitled to receive only the amounts accrued in the insurance policies with respect to severance pay. For information regarding the severance pay to which our chief executive officer would be entitled upon termination of his employment, see Item 6B “Directors, Senior Management and Employees—Compensation—“—Employment Agreements with Executive Officers.” Furthermore, Israeli employees and employers are required to pay predetermined sums to the National Insurance Institute, which is similar to the U.S. Social Security Administration. These amounts also include payments for national health insurance.
In addition, we have various consulting arrangements with experts in regulatory, research and clinical matters, including with physicians.
E. Share Ownership
Share Ownership of Executive Officers and Directors
For information concerning the beneficial ownership of our ordinary shares by our executive officers and directors, see the table in Item 7A. “Major Shareholders and Related Party Transactions—Major shareholders.” As of December 31, 2015, options to purchase 707,814 ordinary shares granted to our directors and executive officers were outstanding under our 2006 Unit Option Plan at a weighted average exercise price of approximately $4.24 per share, and options to purchase 1,147,968 ordinary shares granted to our chief executive officer were outstanding under the United States Sub-Plan to our 2015 Equity Incentive Plan at a weighted average exercise price of approximately $4.04 per share.
101
Option and Incentive Plans
2006 Unit Option Plan
In connection with the transfer of all of the business operations and substantially all of the assets of Check-Cap LLC to us in 2009, we assumed the Check-Cap LLC 2006 Unit Option Plan, or the 2006 Unit Option Plan. Under the 2006 Unit Option Plan, we have been permitted to grant options to purchase our ordinary shares to our employees, consultants and service providers. For the purpose of the 2006 Unit Option Plan: (i) an “employee” means any person, including officers, directors or affiliates who are employed by us or by our affiliates; (ii) a “consultant” means any person who is engaged by us to render consulting or advisory services to us or to any of our entities provided that such services are provided in good faith and are (a) not in connection with the offer or sale of our securities in a capital raising transaction and (b) not directly or indirectly promoting or maintaining a market for our securities; (iii) a “service provider” means an employee, director, supplier or officer holder as defined in the Israeli Companies Law; and (iv) an “affiliate” means any entity which is directly or indirectly our parent or subsidiary.
Our board of directors has had the authority to administer the 2006 Unit Option Plan and to grant options under the 2006 Unit Option Plan, including, without limitation, the authority to determine the persons to whom options shall be granted, the number of shares subject to each option, the time or times at which the options will be granted, restrictions on the transferability of the options, and the schedule and conditions on which such options may be exercised.
The 2006 Unit Option Plan provides for the grant of options pursuant to Sections 102 and 3(i) of the Israeli Income Tax Ordinance [New Version], 5721-1961, which we refer to as the Tax Ordinance. The 2006 Unit Option Plan provides that Section 102 options may be granted only to employees who are Israeli residents and who do not own interests possessing more than 10% of the total combined voting power of all classes of our equity or the equity of our affiliates immediately before such option is granted. Options granted to optionees who are Israeli residents that are not intended to qualify as Section 102 Options are granted under Section 3(i) of the Ordinance, which does not provide for similar tax benefits, and are referred to as Section 3(i) options. The 2006 Unit Option Plan was submitted for the approval of the Israeli Tax Authority, which we refer to as the ITA, as required by applicable law.
Section 102 of the Tax Ordinance allows employees, directors and officers, who are not controlling shareholders and who are Israeli residents, to receive favorable tax treatment for compensation granted in the form of shares or options. Section 102 of the Ordinance includes two alternatives for tax treatment involving the issuance of options or shares to a trustee for the benefit of the grantees, which are referred to as the capital gains track and the ordinary income track, and also includes an additional alternative for the issuance of options or shares issued directly to the grantee. Under the 2006 Unit Option Plan, Section 102 options may be designated as options granted under any one of the foregoing alternatives and such designation shall be included in the option agreement for the option award.
Options granted to date to employees under the 2006 Unit Option Plan were granted under Section 102(b)(2) of the Tax Ordinance, which permits the issuance to a trustee under the “capital gains track.” In order to comply with the terms of the capital gains track, all options granted under a specific plan and subject to the provisions of Section 102 of the Tax Ordinance, as well as the shares issued upon exercise of such options and other shares received subsequently following any realization of rights with respect to such options, such as share dividends and share splits, must be registered in the name of a trustee selected by the board of directors and held in trust for the benefit of the relevant employee, director or officer for a period of two years from the date of the grant. However, under this track, we are not allowed to deduct an expense with respect to the issuance of the options or shares.
The exercise price of an option granted under the 2006 Unit Option Plan has been determined by the board of directors or a committee appointed by it. The first option grant to an employee generally vests over a period of three years and nine months commencing on the date of grant, such that 8.33% vest on the first anniversary of the date of grant and an additional 8.33% vest on each subsequent three-month period thereafter, for 33 months. Additional option grants to an employee generally vest over a period of three years commencing on the three months anniversary of the date of grant, such that 8.33% is fully vested on the date of grant and an additional 8.33% vest on each subsequent three-month period thereafter, for 33 months. Options granted under the 2006 Unit Option Plan generally expire within ten years of the grant date or upon the earlier termination of employment of, or services provided by, the optionee, as applicable, subject to the extended period of exercisability upon termination of employment or services, as applicable. Our board of directors may determine a shorter exercisability period for an option at the time of grant of such option. Upon termination of the employment of or services rendered by an optionee, as applicable (other than for cause, disability or death), generally vested options may be exercised within three months after the date of such termination or within such shorter time period (not to be less than 30 days) or such longer time period (not to exceed five years) as our board of directors or a committee appointed by it shall determine, but in any event no later than the expiration date of the options. If the employment or services of the optionee are terminated because of death or disability (or if the optionee dies within three months after termination of employment or services other than for cause), the optionee’s options may be exercised by the optionee or the optionee’s legal representative or authorized assignee to the extent exercisable on the date of such termination or within 12 months thereafter or as otherwise determined by the board of directors or a committee appointed by it. If the employment or services of the optionee are terminated for cause, all outstanding options will, to the extent not previously exercised, be of no force and effect as of the date of termination, unless otherwise determined by the board of directors or a committee appointed by it.
102
The 2006 Unit Option Plan provides that in the event of a merger or consolidation of our company in which our company is not the surviving entity, an acquisition of all or substantially all of the outstanding capital of our company or the sale of all or substantially all of our assets, the optionee shall be provided the opportunity to (i) exercise his or her options in connection with the transaction and to receive in the transaction such consideration as the holder of ordinary shares shall receive in the transaction; or (ii) retain his or her options or receive a substitute option from the surviving company, if any.
As of December 31, 2015, we had granted options to purchase an aggregate 2,130,380 ordinary shares under the 2006 Unit Option Plan, of which options to purchase an aggregate 309,356 ordinary shares had been exercised into our ordinary shares, options to purchase an aggregate 234,191 ordinary shares had been forfeited without having been exercised and options to purchase an aggregate 1,586,834 ordinary shares were outstanding at a weighted average exercise price of approximately $4.02 per share.
2015 Equity Incentive Plan
On June 23, 2015, our Board of Directors approved and adopted the Check-Cap Ltd. 2015 Equity Incentive Plan (the “2015 Israeli Plan”) and the Check-Ltd. 2015 United States Sub-Plan to Check-Cap Ltd. 2015 Equity Incentive Plan (the “2015 U.S. Sub-Plan” and together with the 2015 Israeli Plan, the “2015 Plan”). As of such date, we ceased to grant options under the 2006 Unit Option Plan. All equity-based awards made after June 23, 2015 shall be made under the 2015 Plan. Our shareholders approved the 2015 Plan at an extraordinary general meeting of shareholders held on August 13, 2015, which approval allows us to grant options under the 2015 Plan that qualify as “incentive stock options” (“ISOs”) within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986, as amended.
Awards under the 2015 Plan. Awards under the 2015 Plan may be options granted pursuant to Section 102 of the Israeli Income Tax Ordinance (New Version), 1960, as amended (the “Ordinance” and “Section 102 Options”) or Section 3(i) of the Ordinance (“Section 3(i) Options”), ISOs and options not intended to qualify as ISOs (“Non-statutory Stock Options”), stock appreciation rights (“SARs”), restricted stock awards (“RSAs”) and restricted stock units (“RSUs”), or any combination of the foregoing.
Unless the Administrator (as defined below) determines otherwise and subject to applicable law, Section 102 Options may be granted only to Israeli employees, executives and directors (excluding controlling shareholders) and Section 3(i) Options may be granted to consultants, controlling shareholders and non-Israeli employees, executives and directors, in each case of our company or any subsidiary. The Section 102 Options may be granted either pursuant to Section 102(c) of the Ordinance, which are not required to be held in trust by a trustee, or pursuant to Section 102(b), which are required to be held in trust for a specified period to qualify for certain tax benefits. Options granted pursuant to Section 102(b) shall be designated to qualify for capital gain tax treatment in accordance with Section 102(b)(2) of the Ordinance or ordinary income tax in accordance with Section 102(b)(1) of the Ordinance and thereafter, only such type of Section 102(b) options shall be granted until the Administrator has determined otherwise in accordance with applicable law (which may not be prior to one year after the first grant of such type of Section 102(b) options).
Unless the Administrator determines otherwise and subject to applicable law, ISOs may be granted only to non-Israeli employees and Non-statutory Stock Options may be granted to non-Israeli employees and consultants, in each case of our company or any subsidiary. To the extent that the aggregate fair market value of the ordinary shares with respect to which ISOs are exercisable for the first time by a participant during any calendar year under all company plans exceeds $100,000, then unless the Administrator determines otherwise at any time and subject to applicable law, such options shall be treated as Non-statutory Stock Options.
Shares Subject to the 2015 Plan. The aggregate number of our ordinary shares that may be issued under each of the 2015 Israeli Plan and 2015 U.S. Sub-Plan is equal to the sum of: (i) 2,029,268, constituting the remaining shares authorized but unissued under the 2006 Unit Option Plan as of the date of the adoption of the 2015 Plan, which are being “rolled over” to the 2015 Plan; and (ii) any ordinary shares under outstanding options under the 2006 Unit Option Plan as of the date of the adoption of the 2015 Plan) (i.e., up to 1,685,364 ordinary shares) that are cancelled, forfeited or expire without being exercised following the date of adoption of the 2015 Plan and which are being “rolled-over” to the 2015 Plan, or in the case of the 2015 Israeli Plan, such other number of ordinary shares as our Board of Directors shall determine from time to time.
103
Administration of the 2015 Plan. The Plan 2015 will be administered by the Board of Directors or, subject to applicable law, a committee appointed by the Board of Directors (“Committee” and the “Administrator”). Subject to the provisions of the 2015 Plan and, in the case of a Committee, the specific duties delegated by the Board of Directors, and subject to the approval of any relevant authorities and compliance with all applicable laws, the Administrator shall have the full power and authority at its sole discretion, from time to time and at any time, among other things:
|
·
|
To determine whether and to what extent awards are to be granted to participants under the 2015 Plan and to select the eligible recipients of awards under the 2015 Plan;
|
|
·
|
To approve forms of agreement for use under the 2015 Plan;
|
|
·
|
To determine the terms and conditions of any award under the 2015 Plan, including the exercise price, the time or times and the extent to which the awards may be exercised (which may be based on performance criteria), any vesting acceleration or waiver of forfeiture restrictions, and any restriction or limitation regarding any award or the ordinary shares relating thereto, based in each case on such factors as the Administrator, at its sole discretion, shall determine;
|
|
·
|
To determine the fair market value of the shares covered by each award;
|
|
·
|
To make an election as to the type of Section 102 Option;
|
|
·
|
To prescribe, amend and rescind rules and regulations relating to the 2015 Plan, including rules and regulations relating to sub-plans established for the purpose of qualifying for preferred tax treatment under foreign tax laws;
|
|
·
|
To authorize conversion or substitution under the 2015 Plan of any or all awards and to cancel or suspend awards, as necessary, provided the material interests of the participants are not harmed; and
|
|
·
|
To construe and interpret the terms of the 2015 Plan and awards granted pursuant to the 2015 Plan;
|
|
·
|
To alter, revise or otherwise adjust the terms of the 2015 Plan and the award agreement, as may be required pursuant to any applicable laws of local or foreign jurisdictions.
|
Term of Awards. The term of each option shall be stated in the award agreement but in no event may it be more than ten years from the date of grant. Unless the Administrator determines otherwise and subject to applicable law, no ISO may be granted under the 2015 Plan to a grantee who possess more than 10% of the total combined voting power of our company or any of our affiliate (a “10% Shareholder”) unless the option terminates on a date that is not later than the day preceding the fifth anniversary of the date of grant. Unless otherwise specified in the applicable award agreement, the term of a SAR will be ten years.
Exercise Price. The exercise price of any award under the 2015 Plan shall be determined by the Administrator, subject to applicable law. Unless the Administrator determines otherwise and subject to applicable law, in the case of ISOs and Non-Statutory Stock Options, the exercise price per share shall be no less than the fair market value per ordinary share on the date of grant and in the case of an ISO granted to a 10% Shareholder, no less than 110% of the fair market value per ordinary share on the date of grant.
Non-Transferability of Awards. Unless the Administrator determines otherwise and subject to applicable law: (i) options and SARs may not be sold, pledged, assigned, hypothecated, transferred, or disposed of in any manner other than by will or by the laws of descent or distribution and may be exercised, during the lifetime of the participant, only by the participant. For as long as options or shares purchased upon the exercise of options are held by a trustee on behalf of the participant, all rights of the participant with respect to such options and shares shall be personal, and may not be transferred, assigned, pledged or mortgaged, other than by will or laws of descent and distribution; (ii) RSUs may not be sold, pledged, transferred, assigned or encumbered; and (iii) RSAs may not be sold, transferred, pledged, assigned or otherwise disposed of during the restricted period, provided that the Administrator may provide for the lapse of such restrictions in installments and may accelerate or waive such restrictions in whole or in part.
104
Termination of Employment or Service.
Options and SARs. If a participant ceases to be an employee or consultant, in the absence of specified period in the award agreement and unless the Administrator determines otherwise, such participant may exercise his/her options (to the extent vested on the date of such termination) or SARs within three months following such termination (but in no event later than the expiration date of the option or SAR). If a participant retires, he/she may continue to enjoy such rights with respect to awards under the 2015 Plan, on such terms and conditions as the Administrator may determine. If the participant’s employment or service is terminated as a result if his/her death or permanent disability, the participant (or, if the participant died, the participant’s estate or any person who acquired the right to exercise the option by bequest or inheritance), may exercise his/her options (to the extent vested on the date of such termination) and/or SARs within such additional period of time following such termination as specified in the award agreement (which may not be less than six months), or in the absence of a specified period in the award agreement, until 12 months following such termination or any longer period determined by the Administrator, but in no event later than the expiration date of the option or SAR.
RSAs. If a participant’s service of employment is terminated prior to the restricted period, subject to the terms of the award agreement or as otherwise determined by the Administrator, the participant’s restricted stock and any associated dividends that remain subject to forfeiture will then be forfeited automatically.
RSUs. If a participant’s service of employment is terminated prior to the RSU vesting, , subject to the terms of the award agreement or as otherwise determined by the Administrator, the participant’s RSUs that remain subject to forfeiture will then be forfeited automatically.
M&A Transaction. In the event of a Transaction (as defined in the 2015 Plan, which generally includes (among others) a sale of all or substantially all of our assets or shares, a merger, consolidation or amalgamation with or into another company or a scheme of arrangement for effecting any of the foregoing or such other transaction determined as such by the Administrator, all subject to the conditions and limitations in the 2015 Plan), unless otherwise determined by the Administrator, in its sole discretion, any award granted under the 2015 shall be assumed or substituted by us or the successor company, under terms determined by the Administrator or the terms of the 2015 Plan applied by the successor company to such assumed or substituted awards, all in accordance with the terms of the 2015 Plan. Regardless of whether or not the awards are assumed or substituted, the Administrator may, at its sole discretion, among other things, provide for the exercise of any exercisable and vested awards, the cancellation of unexercised and unvested awards, the acceleration of unvested awards, or the cancellation of awards for payment in cash, shares or other property of our company or the acquiring company or other party to the Transaction, under such terms as the Administrator may determine, all in accordance with the terms of the 2015 Plan.
Term and Termination of the 2015 Plan. The 2015 Plan became effective upon its adoption by our Board of Directors and shall continue in effect for a term of ten years. Our Board of Directors may at any time amend, alter, suspend or terminate the 2015 Plan. No amendment, alteration, suspension or termination of the 2015 Plan shall impair the rights of any participant, unless mutually agreed otherwise in writing between the participant and the Administrator.
As of December 31, 2015, we had granted options to purchase an aggregate 1,251,256 ordinary shares under the 2015 Plan, of which options to purchase an aggregate 64,919 ordinary shares had been forfeited without having been exercised and options to purchase an aggregate 1,186,337 ordinary shares were outstanding as of such date, exercisable at a weighted average exercise price of approximately $4.0 per share.
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
|
A.
|
Major shareholders
|
The following table sets forth information with respect to the beneficial ownership of our ordinary shares as of March 4, 2016 by (i) each person or entity known to us to beneficially own more than 5% of our outstanding ordinary shares; (ii) each of our executive officers and directors individually; and (iii) all of our executive officers and directors as a group.
The percentage of beneficial ownership of our ordinary shares is based on 11,870,845 ordinary shares, NIS 0.2 par value per share outstanding as of March 7, 2016. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting power or investment power with respect to securities. All options and warrants currently exercisable or exercisable into ordinary shares within 60 days of March 7, 2016 are deemed to be outstanding and beneficially owned by the shareholder holding such options or warrants for the purpose of computing the number of shares beneficially owned by such shareholder. Such shares are also deemed outstanding for purposes of computing the percentage ownership of the person holding the option or warrant. They are not, however, deemed to be outstanding and beneficially owned for the purpose of computing the percentage ownership of any other shareholder.
105
Except as indicated in the footnotes below, we believe that the persons named in the table below have sole voting and investment power with respect to the ordinary shares indicated in the table as being beneficially owned by them.
As of March 7, 2016, based on information provided to us by our transfer agent in the United States and other information reasonably available to us, we had 76 holders of record of our ordinary shares in the United States. Such holders of record held, as of that date, 39.02% of our outstanding ordinary shares. The number of record holders is not representative of the number of beneficial holders of our ordinary shares, as 16.82% of our outstanding ordinary shares are recorded in the name of Cede & Co. as nominee for the Depository Trust Company, in whose name all shares held in “street name” are held in the United States.
|
Ordinary Shares
Beneficially Owned
|
||||||||
|
Name of Beneficial Owner
|
Number
|
Percent
|
||||||
|
5% or Greater Shareholders
(other than directors and executive officers)
|
||||||||
|
Pontifax Funds(1)
|
2,561,835.5 | 19.64 | % | |||||
|
Shanghai Fosun Pharmaceutical Group Co. Ltd.(2)
|
2,197,366 | 17.53 | % | |||||
|
Quant Global Capital Advisors, LC.(3)
|
1,666,666 | 13.12 | % | |||||
|
Yoav and Sigalit Kimchy(4)
|
746,490 | 6.23 | % | |||||
|
Docor International V.(5)
|
718,760 | 5.99 | % | |||||
|
Guy Neev(6)
|
663,936 | 5.41 | % | |||||
|
Directors and Executive Officers
|
||||||||
|
William Densel
|
- | - | ||||||
|
Lior Torem(7)
|
138,061 | 1.15 | % | |||||
|
Yoav Kimchy(4)
|
746,490 | 6.23 | % | |||||
|
Alex Ovadia(8)
|
124,745 | 1.04 | % | |||||
|
Tomer Kariv(1)
|
2,561,835.5 | 19.64 | % | |||||
|
Walter L. Robb(9)
|
431,722 | 3.61 | % | |||||
|
Steven Hanley
|
* | * | ||||||
|
Yuval Yanai
|
* | * | ||||||
|
XiangQian (XQ) Lin(10)
|
374,669 | 3.10 | % | |||||
|
Mary Jo Gorman
|
* | * | ||||||
|
All director and executive officers as a group (10 persons)(11)
|
4,446,823.5 | 35.40 | % | |||||
_______________
* Less than 1% of our ordinary shares.
|
(1)
|
Includes ordinary shares directly held by Pontifax (Cayman) II, L.P., Pontifax (Israel) II, L.P. and Pontifax (Israel) II—Individual Investors, L.P (collectively, the “Pontifax Funds”). Pontifax Management II L.P. is the general partner of the Pontifax Funds and Pontifax Management 2 G.P. (2007) Ltd. is the general partner of Pontifax Management II L.P. Tomer Kariv and Ran Nussbaum are Managing Partners of each of the Pontifax Funds and Pontifax Management II L.P. and are directors of Pontifax Management 2 G.P. (2007) Ltd. and share voting and dispositive power with respect to the shares. The principal business office of each of the foregoing entities and persons is 8 Hamanofim Street, Beit Ofek, Herzliya Pituach, Israel. Includes (i) 1,385,610 outstanding ordinary shares; (ii) 749,334 ordinary shares subject to warrants that are currently exercisable; (iii) 239,391.5 ordinary shares issuable upon exercise of the Series A Warrants that are currently exercisable ; and (iv) 187,500 ordinary shares issuable upon exercise of the Long Term Incentive Warrants that are currently exercisable.
|
|
(2)
|
Based on information contained in a Schedule 13G/A filed by Shanghai Fosun Pharmaceutical Group Co. Ltd. with the SEC on January 28, 2016. Includes: (i) 1,530,699 outstanding ordinary shares; (ii) 333,333.5 ordinary shares issuable upon exercise of the Series A Warrants that are currently exercisable; and (iii) 333,333.5 ordinary shares issuable upon exercise of the Long Term Incentive Warrants that are currently exercisable.
|
106
|
(3)
|
Based on our records, including information contained in a Schedule 13G filed by Quant Global Capital Advisors, LLC with the SEC on March 6, 2015. Includes: (i) 833,333 outstanding ordinary shares; (ii) 416,666.5 ordinary shares issuable upon exercise of the Series A Warrants that are currently exercisable; and (iii) 416,666.5 ordinary shares issuable upon exercise of the Long Term Incentive Warrants that are currently exercisable.
|
|
(4)
|
Includes: (i) 319,553 ordinary shares are directly held by Yoav Kimchy; (ii) 107,384 ordinary shares subject to options held by Yoav Kimchy that are currently exercisable; and (iii) 319,553 ordinary shares are directly held by Sigalit Kimchy. Yoav Kimchy, our chief technology officer, and Sigalit Kimchy are husband and wife.
|
|
(5)
|
Includes: (i) 593,761 outstanding ordinary shares directly held by Docor International B.V., or “Docor”; (ii) 62,499.5 ordinary shares issuable upon exercise of the Series A Warrants that are currently exercisable; and (iii) 62,499.5 ordinary shares issuable upon exercise of the Long Term Incentive Warrants that are currently exercisable.
|
|
(6)
|
Includes: (i) 265,928 outstanding ordinary shares; and (ii) 398,008 ordinary shares subject to options that are currently exercisable.
|
|
(7)
|
Includes: (i) 41,539 outstanding ordinary shares; and (ii) 96,522 ordinary shares subject to options that that are currently exercisable.
|
|
(8)
|
Includes 124,745 ordinary shares, subject to options that are currently exercisable.
|
|
(9)
|
Includes: (i) 356,075 outstanding ordinary shares held by Counterpoint Ventures Fund LP and Counterpoint Ventures Fund II LP (together, the “Counterpoint Funds”); (ii) 21,250 ordinary shares issuable upon exercise of the Series A Warrants, that are currently exercisable, held by the Counterpoint Funds; (iii) 21,250 ordinary shares issuable upon exercise of the Long Term Incentive Warrants, that are currently exercisable, held by the Counterpoint Funds; and (iv) 33,147 ordinary shares subject to options that are currently exercisable or exercisable within 60 days of this table, held directly by Mr. Robb. Mr. Robb has advised us that the general partner of each of the Counterpoint Funds is Lion Development LLC, which is 99% controlled by Mr. Walter Robb, and as such, Walter Robb possesses the ultimate voting and investment power over the shares beneficially owned by the Counterpoint Ventures entities.
|
|
(10)
|
Includes: (i) 166,667 outstanding ordinary shares held by Esco Ventures Pte Ltd.; (ii) 83,333.5 ordinary shares issuable upon exercise of the Series A Warrants, that are currently exercisable, held by Esco Ventures Pte Ltd.; (iii) 83,333.5 ordinary shares issuable upon exercise of the Long Term Incentive Warrants, that are currently exercisable, held by Esco Ventures Pte Ltd and (iv) 41,335 ordinary shares subject to options that are exercisable within 60 days of this table, held directly by XiangQian (XQ) Lin. Mr. Lin has advised us that Esco Ventures Pte Ltd. is wholly-owned by him and that he possesses the ultimate voting and investment power over the shares beneficially owned by Esco Ventures Pte Ltd.
|
|
(11)
|
See footnotes (1)-(10) for certain information regarding beneficial ownership.
|
None of our shareholders have voting rights different from the voting rights of other shareholders. To the best of our knowledge, we are not owned or controlled, directly or indirectly, by another corporation or by any government. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
|
B.
|
Related Party Transactions
|
Other than the executive and director compensation and indemnification and exculpation arrangements discussed in “Management,” and the transactions described below, we have not entered into any transactions since January 1, 2012 to which we have been or are a party to and in which any of our directors, executive officers or holders of more than 5% of our share capital, or any immediate family member of, or person sharing the household with, any of these individuals or entities, had or will have a direct or indirect material interest.
Participation in Our Initial Public Offering
Certain of our shareholders who held our ordinary shares prior to our initial public offering, including affiliates of certain of our directors, purchased units, in our initial public offering that was consummated on February 24, 2015, each unit consisting of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share, issued together with Long Term Incentive Warrants, at the price paid by the public ($6 per unit), in the following amounts: Pontifax Funds purchased 125,000 units (issued together with 187,500 Long Term Incentive Warrants) and Docor International B.V. purchased 41,666 units (issued together with 62,499 Long Term Incentive Warrants). In addition, XiangQian (XQ) Lin, who was elected as a director as of the consummation of our initial public offering, purchased in our initial public offering 166,667 units (issued together with 250,000.5 Long Term Incentive Warrants), at the price paid by the public ($6 per unit). The underwriters received the same underwriting discount on the units purchased by these persons and entities as they did on the other units sold to the public in our initial public offering.
107
Credit Line Agreement; Private Placement
On August 20, 2014, we entered into a certain credit line agreement, pursuant to which we obtained a credit line in an aggregate principal amount of $12.0 million from certain lenders and existing shareholders, including Pontifax, Docor International B.V. and Counterpoint Ventures Fund II LP. The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014. Under the terms of the credit line agreement, we directed that the full credit line amount be invested in the private placement that was consummated simultaneously with our initial public offering that was consummated on February 24, 2015. We issued to the lenders a total of 2,000,000 units, each consisting of one ordinary share and one half of a Series A Warrant to purchase one ordinary share, together with 3,000,000 long term incentive warrants, for aggregate gross proceeds of $12,000,000. The Pontifax Funds, Docor International, B.V and Counterpoint Ventures Fund II LP acquired 250,001, 83,334 and 42,500 units, respectively, in the private placement for an aggregate purchase price of $1,500,000, $500,000 and $255,000, respectively. For additional information regarding the private placement, see “ Item 5B “Operating and Financial Review and Prospects — Liquidity and Capital Resources-Sources of Liquidity.”
Pontifax Warrants
On October 14, 2014, we issued warrants to purchase an aggregate of 221,539 of our ordinary shares at an exercise price of NIS 0.20 per share, or the Pontifax Warrants, to the Pontifax Funds in consideration of their commitment to provide to us, for no consideration, the following services, if and to the extent requested by us: (i) business development services, in such scope and substance as shall be agreed between us and the Pontifax; and (ii) a representative designated by Pontifax to serve as the chairman of our board of directors. The Pontifax Funds subsequently agreed that the exercise price of fifty-percent of their warrants will increase to equal the price at which our ordinary shares are sold to the public in the initial public offering of our securities, or if units are sold in the initial public offering of our securities, the exercise price per share will increase to be equal to the effective price per share of the ordinary shares underlying the units sold to the public in the offering. The Pontifax Funds also agreed that such portion of their warrants will vest and become exercisable only upon the consummation of the initial public offering of our securities prior to their expiration date. The remaining warrants with an NIS 0.20 exercise price will vest on a quarterly basis in eight equal installments during a period of 24 months from issuance. In addition, the Pontifax Funds agreed to reduce the term of their respective warrants such that these warrants will now expire after eight years (instead of ten years) following their issuance, i.e., on October 14, 2022. Upon the closing of our initial public offering any unvested portion of the warrants became fully vested and exercisable.
Shareholders Agreement
We entered into a shareholders agreement, dated as of March 17, 2011, to which each of the holders of our preferred shares, including Pontifax (Cayman) II, L.P., Pontifax (Israel) II L.P., Pontifax (Israel) II- Individual Investors L.P., BXR Portfolio Limited (formerly named Eastern Petroleum Investment Company Limited), Docor International B.V., Spearhead Investments (Bio) Ltd., Jacobs Investment Company LLC and Emigrant Alternative Portfolios LLC, is a party, either as an original signatory or by virtue of a joinder thereto, and certain of the holders of our ordinary shares, including Yoav Kimchy and Sigalit Kimchy are parties. On October 14, 2014, the parties to such shareholders agreement and the lenders under the credit line agreement dated August 20, 2014, entered into an Amended and Restated Shareholders Agreement, or the Shareholders Agreement. Prior to the closing of our initial public offering, we amended the Shareholders Agreement to eliminate provisions related to information rights and matters related to the board of directors, such that the amended Shareholders Agreement consists primarily of the provisions regarding registration rights described below, and the undertaking discussed below under “—Transactions with Check-Cap LLC and the Members and Manager of Check-Cap LLC.”
Demand Registration Rights
At any time after the closing of our initial public offering, but subject to the terms of the 180-day lock-up agreements entered into between certain of the parties to the Shareholders Agreement and the underwriters of our initial public offering, at the request of, either (i) holders of a majority of our former Series D preferred shares (all of which converted into ordinary shares immediately prior to the closing of our initial public offering); or (ii) holders of a majority of our former Series C preferred shares (all of which converted into ordinary shares immediately prior to the closing of our initial public offering); or (iii) persons holding at least 20% of the ordinary shares issued upon conversion of the former preferred shares or in respect thereof, we must register any or all of such shareholders’ ordinary shares. We are required to effect up to two such registrations for each former holder of our preferred shares, with respect to all of such person’s or entities’ shares. We are required to give notice of a demand registration to the other holders of registrable securities that are entitled to registration rights and include their shares in such registration if they so timely request.
108
Piggyback Registration Rights
All of the former holders of our preferred shares that are a party to the Shareholders Agreement have the right to request that we include their registrable securities in certain registration statements that we file in connection with the public offering of our shares. We are required to give notice of our intention to effect such a registration to all holders of our registrable securities that are entitled to registration rights and include their shares in such registration if they so timely request.
Registration Priority
To the extent it is not in our best interest for all of the former holders of our preferred shares to participate in any demand or piggyback registration, the shares to be included in the registration statement on behalf of the former preferred shareholders shall be allocated as follows: first, shares sought to be registered by the former holders of our Series D preferred shares; second, shares sought to be registered by the former holders of Series C preferred shares; third, shares sought to be registered by the former holders of our Series A preferred shares; and fourth, shares sought to be registered by the former holders of our Series B preferred shares.
Expenses
We have agreed to pay all expenses incurred in carrying out the above registrations, including the fees of one counsel chosen by the selling shareholders that are a party to the Shareholders Agreement. However, we shall not be required to pay any registration expenses in connection with any initiated registration that is subsequently withdrawn, other than a withdrawal due to a material adverse change not known to the holder of registrable securities at the time of such demand or request by us or the underwriters to reduce the size of the offering as a result of which the holders of at least a majority of the registrable securities elect to withdraw. A registration shall not count as a permitted registration until it has remained effective for a period of at least 120 days.
Transactions with Check-Cap LLC and the Members and Manager of Check-Cap LLC
On May 31, 2009, we entered into an asset transfer agreement with Check-Cap LLC pursuant to which Check-Cap LLC transferred all of its business operations and substantially all of its assets to us. Our shareholders’ holdings on the date of the asset transfer transaction reflected their interests as members of Check-Cap LLC. In the framework of the asset transfer agreement and under the Shareholders Agreement, we undertook to use commercially reasonable efforts to procure that distributions or advance funds are made to our shareholders holding (at the date of the transaction) ordinary shares, Series A preferred shares and/or Series B preferred shares (i.e., the shareholders who are also members of Check-Cap LLC), as would be necessary to eliminate the tax impact on such shareholders of the reorganization and the transfer of all of the business operations and substantially all of the assets from Check-Cap LLC to us. Notwithstanding the foregoing, we will not advance payments to such shareholders to address the fact that they will no longer receive a “pass through” of losses generated by us as they previously received while owning units of Check-Cap LLC. These advances, if and to the extent made, will be deducted from any distributions such shareholders are entitled to receive from us. We have reserved in our financial statements $418,000 as of December 31, 2015 and $393,000 as of December 31, 2014 on account of such advances.
In connection with the asset transfer agreement entered into with the Predecessor Entity in May 2009, the Company assumed the former obligation of the Predecessor Entity to distribute any proceeds it collects on the $1 million key man life insurance policy with respect to Yoav Kimchy, the Company's chief technology officer and a former director, to the former holders of the Series A preferred units in an amount equal to their respective capital contributed to the Predecessor Entity, less any amounts previously distributed to them, plus any accrued and unpaid dividends due to them as of the date of distribution
Check-Cap Ltd. (Delaware), which is the manager of Check-Cap LLC and is wholly-owned by Mr. Kimchy, our chief technology officer and a former director, handles from time to time certain logistical, administrative and investor relations matters for us with our U.S. suppliers and investors under a Services Agreement dated October 1, 2010, which expired on December 31, 2014. Under the agreement, we agreed to pay Check-Cap Ltd. (Delaware) in consideration of its services a quarterly payment of $20,000, which amount essentially covers its costs of performing such functions. We last utilized such services as of December 31, 2012. For the year ended December 31, 2012, we paid Check-Cap Ltd. (Delaware) an aggregate of $80,000 for such services.
109
Engagement of Mr. XiangQian (XQ) Lin, a Director
We have engaged Mr. XiangQian (XQ) Lin, who has served as a director since February 24, 2015, to provide to us certain business development services in Asia under a consulting agreement that we entered into with him on June 1, 2015. As compensation for his services, Mr. Lin is entitled to a monthly fee of $10,000 for up to five hours per month and $300 per hour for any consultancy hour exceeding such five hours required to perform such services. In addition, we awarded Mr. Lin a one-time option grant to purchase 48,387 ordinary shares, exercisable at $5.06 per share. The options vest over a period of three years commencing on the date of grant, in 12 equal quarterly installments, such that options to purchase 4,035 ordinary shares vested on the three month anniversary of the date of grant and options to purchase 4,032 ordinary shares vest at the end of each subsequent three-month period thereafter, subject to his continuing service on each applicable vesting date. The options were granted under our 2006 Unit Option. We also agreed to reimburse Mr Lin for all reasonable expenses incurred by him in connection with the provision of the foregoing services (including reasonable expenses incurred by a third party engaged by him to provide the above services on his behalf provided that such expenses are approved by us in writing in advance), all in accordance with our internal reimbursement policies. Mr. Lin’s engagement as a service provider is for an initial period of 12 months, which term may be extended upon the written agreement of us and Mr. Lin. In year 2015, we charged expenses in an amount of $70,000 in consideration for Mr. Lin consulting services, in addition to the said one-time option grant.
Agreements with Directors and Officers
Employment Agreements:
|
1.
|
We have entered into written employment agreements with each of our executive officers. These agreements provide for notice periods of varying duration for termination of the agreement by us or by the relevant executive officer, during which time the executive officer will continue to receive base salary and benefits. We have also entered into customary non-competition, confidentiality of information and ownership of inventions arrangements with our executive officers. However, the enforceability of the noncompetition provisions may be limited under applicable law.
|
|
2.
|
Options. Since our inception we have granted options to purchase our ordinary shares to our officers and certain of our directors. Such option agreements may contain acceleration provisions upon certain merger, acquisition, or change of control transactions. We describe our option plans under Item 6B “Directors, Senior Management and Employees—Share Ownership – Option and Incentive Plans.” If the relationship between us and an executive officer or a director is terminated, except for cause (as defined in the various option plan agreements), options that are vested will generally remain exercisable for ninety days after such termination.
|
Exculpation, Indemnification and Insurance. Our articles of association permit us to exculpate, indemnify and insure certain of our office holders to the fullest extent permitted by the Israeli Companies Law. We have entered into indemnification agreements with our office holders, exculpating them from a breach of their duty of care to us to the fullest extent permitted by law and undertaking to indemnify them to the fullest extent permitted by law, subject to certain exceptions, including with respect to liabilities resulting from this offering to the extent that these liabilities are not covered by insurance. See Item 6B Directors, Senior Management and Employees “—Compensation of Directors and Executive Officers — Exculpation”.
|
3.
|
On July 1, 2005, we entered into an agreement with Hadar Kimchy according to which Hadar Kimchy provides us with marketing communication and graphical design services in consideration for a monthly retainer of NIS 10,260 ($3,000). On August 1, 2014, the monthly retainer was increased to NIS 13,680 ($4,000). The above services are provided to us by Sigalit Kimchy, who is employed by Hadar Kimchy. Sigalit Kimchy is a shareholder and is the spouse of Yoav Kimchy, our chief technology officer and a former director. Dr. Yoav Kimchy and Sigalit Kimchy beneficially owners of 746,490 shares, for beneficial ownership details, see “Item 7. Major shareholders and related party transactions”.
|
Scientific Advisories
Dr. Walter L. Robb and Mr. Steven Hanley, who serves as members of our Board of Directos, provided us with consultancy services, on a needed basis, with matters of scientific relevance, as our Scientific Advisors. The amount of consulting services provided by our Scientific Advisors ranges from several hours a month to several hours a year.
Dr. Robb has served as a Scientific Advisor to the Company since May 2009. Mr. Hanley has provided consultancy services to the Company on behalf of Neem LLC since November 2009 and has served as a Scientific Advisor to the Company since June 2011. Mr. Hanley and Neem LLC ceased to provide such consultancy services to the Company and Mr. Hanley ceased to serve as Scientific Advisor to the Company effective as of his election as our a Board member in February 2015. For Dr. Robb and Mr. Hanley's beneficial ownership details, see “Item 7. major shareholders and related party transactions”.
110
|
C.
|
Interests of Experts and Counsel
|
Not applicable.
ITEM 8. FINANCIAL INFORMATION
|
A.
|
Consolidated Statements and Other Financial Information.
|
Consolidated Financial Statements
See Item 18 – Financial Statements.
Legal Proceedings
From time to time, we may be involved in litigation that arises through the normal course of business. As of the date of this filing, we are not a party to any material litigation nor are we aware of any such threatened or pending litigation.
There are no material proceedings in which any of our directors, officers or affiliates or any registered or beneficial shareholder of more than 5% of our ordinary shares, or any associate of any of the foregoing is an adverse party or has a material interest adverse to our interest.
Dividend Policy
We have never declared and do not anticipate paying cash dividends in the foreseeable future. See Item 3D “Key Information – Risk factors - Risks Related to the Company -
We have never declared or paid dividends on our ordinary shares and currently do not intend to pay cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business.
Our ability to distribute dividends also may be limited by future contractual obligations and by Israeli law. The Israeli Companies Law restricts our ability to declare dividends. Unless otherwise approved by a court, we can distribute dividends only from “profits” (as defined by the Israeli Companies Law), and only if there is no reasonable concern that the dividend distribution will prevent us from meeting our existing and foreseeable obligations as they become due. Subject to the foregoing, payment of future dividends, if any, will be at the discretion of our board of directors and will depend on various factors, such as our financial condition, operating results, current and anticipated cash needs and other business and economic factors that our board of directors may deem relevant. See “Description of Share Capital and Securities Offered Hereby —Dividend and Liquidation Rights.” The payment of dividends may be subject to Israeli withholding taxes. See “Taxation—Israeli Tax Considerations and Government Programs—Taxation of Our Shareholders—Taxation of Non-Israeli Shareholders on Receipt of Dividends.” Furthermore, if we pay a dividend out of income attributed to our Benefited Enterprise that was generated during the tax exemption period, we may be subject to tax on the grossed-up amount of such distributed income at the corporate tax rate which would have been applied to our Benefited Enterprise’s income had we not enjoyed the exemption. See “Taxation – Israeli Tax Considerations and Government Programs — Law for the Encouragement of Capital Investments, 5719-1959 — Tax Benefits Subsequent to the 2005 Amendment.”
See Item 10.B. “Memorandum and Articles of Association—Dividend and Liquidation Rights.”
|
B.
|
Significant Changes
|
Except as disclosed elsewhere in this Annual Report, there have been no other significant changes since December 31, 2015, until the date of the filing of this Annual Report.
111
ITEM 9. THE OFFER AND LISTING
|
A.
|
Offer and Listing Details
|
Our units were listed on the NASDAQ Capital Market on February 19, 2015 under the symbol “CHEKU.” Prior to that date, there was no public trading market for our securities. Our initial public offering was priced at $6.00 per unit on February 20, 2015. Each unit consisted of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share. Each unit was issued with one and one-half non-transferrable Long Term Incentive Warrants. Each whole Series A Warrant entitles the holder to purchase one ordinary share at an exercise price of $7.50. On March 18, 2015, the units separated and ceased to exist. Since such date, our ordinary shares and Series A Warrants have been listed on the NASDAQ Capital Market under the symbols “CHEK” and “CHEKW,” respectively. The following table sets forth for the periods indicated the high and low sales prices per ordinary share as reported on the NASDAQ Capital Market:
Ordinary Shares
|
(Year Ended)
|
High
|
Low
|
||||||
|
December 31, 2015 (from March 18, 2015)
|
$
|
6.30
|
$
|
1.80
|
||||
The high and low market prices of our ordinary shares for each financial quarter over the most recent full financial year and subsequent period are as set forth below:
Ordinary Shares
|
(Quarter Ended)
|
High
|
Low
|
||||||
|
From March 18, 2015 through March 31, 2015
|
$
|
6.30
|
$
|
5.40
|
||||
|
June 30, 2015
|
$
|
5.90
|
$
|
2.45
|
||||
|
September 30, 2015
|
$
|
4.24
|
$
|
1.82
|
||||
|
December 31, 2015
|
$
|
3.80
|
$
|
1.80
|
||||
For the most recent six months, the high and low market prices of our ordinary shares are as set forth below:
|
Month Ended
|
High
|
Low
|
||||||
|
September 2015
|
$
|
3.44
|
$
|
2.06
|
||||
|
October 2015
|
$
|
3.80
|
$
|
2.42
|
||||
|
November 2015
|
$
|
2.93
|
$
|
2.09
|
||||
|
December 2015
|
$
|
2.18
|
$
|
1.80
|
||||
|
January 2016
|
$
|
2.15
|
$
|
1.86
|
||||
|
February 2016
|
$
|
3.72
|
$
|
2.00
|
||||
|
March 2016 (through March 11, 2016)
|
$
|
3.35
|
|
$
|
2.57
|
|
||
On March 11, 2016, the last reported sale price of our ordinary shares on the NASDAQ Capital Market was $3.00.
|
B.
|
Plan of Distribution
|
Not applicable.
|
C.
|
Markets for Ordinary Shares
|
See “—Offer and Listing Details” above.
|
D.
|
Selling Shareholders
|
Not applicable.
112
|
E.
|
Dilution
|
Not applicable.
|
F.
|
Expenses of the Issue
|
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
|
A.
|
Share Capital
|
Not applicable.
|
B.
|
Memorandum and Articles of Association
|
Registration Number and Purposes of the Company
Our registration number with the Israeli Registrar of Companies is 51-425981-1. Our purpose as set forth in our amended articles of association is to engage in any lawful activity.
Voting Rights and Conversion
All ordinary shares have identical voting and other rights in all respects.
Transfer of Shares
Our fully paid ordinary shares are issued in registered form and may be freely transferred under our amended articles of association, unless the transfer is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trade. The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our amended articles of association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel, according to applicable Israeli law's requirements.
Our Board of Directors may, to the extent it deems necessary in its discretion, close the Register of Shareholders of registration of transfers of shares for a period determined by the Board of Directors, and no registrations of transfers of shares shall be made by the Company during any such period during which the Register of Shareholders is so closed. The Company shall notify the shareholders with respect to such suspension of registration in such manner as shall be determined by the Board of Directors.
Election of Directors and Appointment of an Alternate Directors
Our ordinary shares do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect all of our directors, subject to the special approval requirements for external directors described under “Management — External Directors.”
Under our amended articles of association, our board of directors must consist of not less than four but no more than eleven directors, including at least two external directors as required by the Israeli Companies Law. Pursuant to our amended articles of association, other than the external directors, for whom special election requirements apply under the Israeli Companies Law, each of our directors will be appointed by a simple majority vote of holders of our voting shares, participating and voting at an annual general meeting of our shareholders. Each director (other than external directors) will hold office until the next annual general meeting following the annual general meeting at which they were elected and until his or her successor is elected and qualified, or until the occurrence of certain events, in accordance with the Israeli Companies Law and our amended articles of association, including his or her earlier resignation, death or removal by a vote of the majority of the voting power of our shareholders at a general meeting of until his or her office expires by operation of law. In addition, our amended articles of association allow our board of directors to appoint directors (other than external directors) to fill vacancies on the board of directors to serve for a term of office equal to the remaining period of the term of office of the directors(s) whose office(s) have been vacated. External directors are elected for an initial term of three years, may be elected for additional terms of three years each under certain circumstances, and may be removed from office pursuant to the terms of the Israeli Companies Law. See “Management — Board Practices — External Directors.”
113
In accordance with our amended Articles of Association, as approved by our shareholders at the 2015 Annual General Meeting held on December 1, 2015, subject to the provisions of the Israeli Companies Law, a director may, by written notice to us, appoint, remove or replace any person as an alternate for himself, referred to as an Alternate Director. Unless the appointing director, by the instrument appointing an Alternate Director or by written notice to us, limits such appointment to a specified period of time or restricts it to a specified meeting or action of the Board of Directors, or otherwise restricts its scope, the appointment shall be for all purposes, and for a period of time concurrent with the term of the appointing director.
Dividend and Liquidation Rights
We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Israeli Companies Law, dividend distributions are determined by the board of directors and do not require the approval of the shareholders of a company unless the company’s articles of association provide otherwise. Our amended articles of association do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the Israeli Companies Law, we may declare and pay dividends only if, upon the determination of our board of directors, there is no reasonable concern that the distribution will prevent us from being able to meet the terms of our existing and foreseeable obligations as they become due. Under the Israeli Companies Law, the distribution amount is further limited to the greater of retained earnings or earnings generated over the two most recent years legally available for distribution according to our then last reviewed or audited financial statements, provided that the date of the financial statements is not more than six months prior to the date of distribution. In the event that we do not have retained earnings or earnings generated over the two most recent years legally available for distribution, we must seek the approval of the court in order to distribute a dividend. The court may approve our request if it is convinced that there is no reasonable concern that the payment of a dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to the nominal value of their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Exchange Controls
There are currently no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares or interest or other payments to non-residents of Israel, except for shareholders who are subjects of countries that are, or have been, in a state of war with Israel, according to the Israeli Law provisions.
Shareholder Meetings
Under Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be held no later than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders are referred to in our amended articles of association as special general meetings. Our board of directors may call special general meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Israeli Companies Law provides that our board of directors is required to convene a special general meeting upon the written request of (i) any two of our directors or one-quarter of the serving members of our board of directors; or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of our outstanding shares and 1% of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Furthermore, the Israeli Companies Law requires that resolutions regarding the following matters be approved by our shareholders at a general meeting:
|
•
|
amendments to our articles of association;
|
|
•
|
appointment, terms of service and termination of service of our auditors;
|
|
•
|
appointment of external directors;
|
|
•
|
approval of certain related party transactions;
|
114
|
•
|
increases or reductions of our authorized share capital;
|
|
•
|
mergers; and
|
|
•
|
the exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is essential for our proper management.
|
Subject to the provisions of the Israeli Companies Law and regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which, as a company listed on an exchange outside Israel, may be between four and 40 days prior to the date of the meeting.
The Israeli Companies Law requires that a notice of any annual general meeting or special general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes, among other things, the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, an approval of a merger or the approval of the compensation policy, notice must be provided at least 35 days prior to the meeting.
Under the Israeli Companies Law, our shareholders are not permitted to take action via written consent in lieu of a meeting.
Voting rights
Quorum Requirements
Pursuant to our amended articles of association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting. The quorum required for general meetings of our shareholders is at least two shareholders present in person, by proxy or written ballot, who hold or represent between them at least 25% of the total outstanding voting rights (or if a higher percentage is required by law, such higher percentage), within half an hour of the time fixed for the commencement of the meeting. A meeting adjourned for lack of a quorum is adjourned either to the same day in the following week at the same time and place or to such day, time and place as specified in the notice of the meeting or to such day, time and place as the chairman of the general meeting shall determine. At the reconvened meeting, at least two shareholders present in person or by proxy shall constitute a lawful quorum, unless the meeting of shareholders was convened at the demand of shareholders, in which case, the quorum shall be the presence of one or more shareholders holding at least 5% of our issued share capital and at least one percent of the voting power of our shares, or one or more shareholders with at least 5% of the voting power of our shares.
Vote Requirements
Our amended articles of association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Israeli Companies Law or by our amended articles of association. Under the Israeli Companies Law, certain actions require a special majority, including: (i) appointment of external directors, requiring the approval described in Item 6C “Directors, Senior Management and Employees—Board Practices—External Directors”; (ii) approval of an extraordinary transaction with a controlling shareholder or in which the controlling shareholder has a personal interest and the terms of employment or other engagement of the controlling shareholder or a relative of the controlling shareholder (even if not extraordinary), requiring the approval described in Item 6C “Directors, Senior Management —Approval of Related Party Transactions under Israeli Law— Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”; (iii) approval of a compensation policy, requiring the approval described in Item 6C “Directors, Senior Management and Employees— Board Practices— Compensation Committee and Compensation Policy”; and (iv) approval of executive officer compensation inconsistent with our office holder compensation policy or the compensation of our chief executive officer (subject to limited exceptions), requiring the approval described in Item 6C “Directors, Senior Management and Employees— Board Practices— Approval of Related Party Transactions under Israeli Law— Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions.”
In addition, under the Israeli Companies Law the authorization of the chairman of the board to assume the role or responsibilities of the chief executive officer, or the authorization of the chief executive officer or his or her relative thereof to assume the role or responsibilities of the chairman of the board, for periods of no longer than three years each, is subject to receipt of the approval of a majority of the shares voting on the matter, provided that either (i) included in such majority are at least two-thirds of the shares of shareholders who are non-controlling shareholders and shareholders who do not have a personal interest in the resolution that are voted at the meeting on the matter (excluding any abstentions); or (ii) the total number of shares of shareholders specified in clause (i) who voted against the resolution does not 2% of the voting rights in the company.
115
Another exception to the simple majority vote requirement is a resolution for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350 of the Israeli Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting and voting on the resolution.
Access to Corporate Records
Under the Israeli Companies Law, shareholders are provided access to: minutes of the general meetings of our shareholders; our shareholders register and principal shareholders register, articles of association and financial statements; and any document that we are required by law to file publicly with the Israeli Companies Registrar or the Israel Securities Authority. In addition, shareholders may request to be provided with any document in the company’s possession related to an action or transaction requiring shareholder approval under the related party transaction provisions of the Israeli Companies Law. We may deny this request if we believe it has not been made in good faith or if such denial is necessary to protect our interest or protect a trade secret or patent.
Modification of Class Rights
Under the Israeli Companies Law and our amended articles of association, the rights attached to any class of shares, such as voting, liquidation and dividend rights, may be modified or cancelled by adoption of a resolution by the holders of a majority of all shares as one class, without any required separate resolution of any class of shares, or otherwise in accordance with the rights attached to such class of shares, as set forth in our amended articles of association.
Registration Rights
For a discussion of registration rights we have granted to our existing shareholders, please see Item 7B “Major Shareholders and Related Party Transactions—Related Party Transactions — Shareholders Agreement.”
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of an Israeli public company, and who would as a result hold over 90% of the target company’s issued and outstanding share capital, is required by the Israeli Companies Law to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a Israeli public company, and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares of the company, is required to make a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of shares.
Upon a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli court as described above.
If (a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company, or of the applicable class, or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
116
Special Tender Offer
The Israeli Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company, if there is no other shareholder that holds 25% or more of the voting rights in the company, subject to exceptions. Similarly, the Israeli Companies Law provides that an acquisition of shares in an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the company who holds more than 45% of the voting rights in the company, subject to certain exceptions. No tender offer is required if the acquisition of shares: (i) occurs in the context of a private placement, that was approved by the company’s shareholders and whose purpose is to give the acquirer at least 25% of the voting rights in the company if there is no person who holds 25% or more of the voting rights in the company, or as a private placement whose purpose is to give the acquirer 45% of the voting rights in the company, if there is no person who holds 45% of the voting rights in the company; (ii) was from a holder of 25% or more of the voting rights in the company following which the purchaser will hold 25% or more of the voting rights in the company; or (iii) was from a holder of more than 45% of the voting rights in the company following which the purchaser will hold more than 45% of the voting rights in the company.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror; and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer (excluding the purchaser, its controlling shareholders, holders of 25% or more of the voting rights in the company or any person having a personal interest in the acceptance of the tender offer, or anyone on their behalf, including any such person’s relatives and entities under their control). If a special tender offer is accepted, then the purchaser or any person or entity controlling it, at the time of the offer, and any person or entity under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
Merger
The Israeli Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described under the Israeli Companies Law are met, by a majority vote of each party’s shares, and, in the case of the target company, a majority vote of each class of its shares, voted on the proposed merger at a shareholders meeting. The board of directors of a merging company may not approve the merger if it determines that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities.
For purposes of the shareholder vote of a merging company whose shares are held by the other merging company or a person or entity holding 25% or more of any of the means of control of the other merging entity, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of shares voting on the matter at the shareholders meeting (excluding abstentions) that are held by parties other than the other party to the merger, or by any other person or entity who holds 25% or more of the voting rights or the right to appoint 25% or more of the directors of the other party, or any one on their behalf including their relatives or corporations controlled by any of them, vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same Special Majority approval that governs all extraordinary transactions with controlling shareholders (as described in Item 6C “Directors, Senior Management and Employees—Board Practices Management — Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”).
If the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the valuation of the merging companies and the consideration offered to the shareholders.
117
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
Anti-Takeover Measures under Israeli Law
The Israeli Companies Law allow us to create and issue shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights with respect to voting, distributions or other matters and shares having preemptive rights. No preferred shares are currently authorized under our amended articles of association. In the future, if we do authorize, create and issue a specific class of preferred shares, such class of shares, depending on the specific rights that may be attached to it, may have the ability to frustrate or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization and designation of a class of preferred shares will require an amendment to our amended articles of association, which requires the prior approval of the holders of a majority of the voting power attached to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled to participate and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Israeli Companies Law and our articles of association as described above in “— Voting Rights.”
Borrowing Powers
Pursuant to the Israeli Companies Law and our amended articles of association, our board of directors may exercise all powers and take all actions that are not required under law or under our amended articles of association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
Changes in Capital
Our amended articles of association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Israeli Companies Law and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits, require the approval of both our board of directors and an Israeli court.
|
C.
|
Material Contracts
|
We have not entered into any material contracts other than in the ordinary course of business and other than those described in Item 4. “Information on Our Company,” Item 7B “Major Shareholders and Related Party Transactions - Related Party Transactions” or elsewhere in this Annual Report.
|
D.
|
Exchange controls
|
There are currently no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares or interest or other payments to non-residents of Israel, except for shareholders who are subjects of countries that are, or have been, in a state of war with Israel.
|
E.
|
Taxation
|
The following description is not intended to constitute a complete analysis of all tax consequences relating to our prior units and our ordinary shares, Series A Warrants and Long Term Incentive Warrants (sometimes referred to collectively or individually as our “ securities”). You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
118
Israeli Tax Considerations and Government Programs
The following is a brief summary of the material Israeli tax laws applicable to us and certain Israeli Government programs that benefit us. This section also contains a discussion of material Israeli tax consequences concerning the ownership and disposition of our securities purchased by investors who are non-Israeli resident shareholders. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of such investors include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. Because parts of this discussion are based on new tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion below is subject to change, including due to amendments under Israeli law or changes to the applicable judicial or administrative interpretations of Israeli law, which change could affect the tax consequences described below.
General Corporate Tax Structure in Israel
Israeli resident companies are generally subject to corporate tax, currently at the rate of 25% (effective as of January 5, 2016) of a company’s taxable income. However, the effective tax rate payable by a company that derives income from an Approved Enterprise, a Benefited Enterprise or a Preferred Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli resident company are subject to tax at the prevailing corporate tax rate.
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, provides several tax benefits for “Industrial Companies.” The Industry Encouragement Law defines an “Industrial Company” as a company resident in Israel, of which 90% or more of its income in any tax year, other than income from defense loans, is derived from an “Industrial Enterprise” owned by it. An “Industrial Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
|
•
|
amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which are used for the development or advancement of the Industrial Enterprise;
|
|
•
|
under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and
|
|
•
|
expenses related to a public offering are deductible in equal amounts over three years.
|
Eligibility for the benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority. We believe that we may qualify as an “Industrial Company” within the meaning of the Industry Encouragement Law; however, there can be no assurance that we will qualify as an Industrial Company or that the benefits described above will be available in the future.
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
The Investment Law was significantly amended effective April 1, 2005, referred to as the 2005 Amendment, and further amended as of January 1, 2011, referred to as the 2011 Amendment. Pursuant to the foregoing amendments, generally tax benefits that were granted in accordance with the provisions of the Investment Law prior to each such amendment remain in force; however, any benefits granted subsequent to the respective amendment are subject to the provisions of the Investment Law as amended.
Tax Benefits Prior to the 2005 Amendment
Prior to the 2005 Amendment, a capital investment in eligible production facilities (or other eligible assets) could, upon application to the Investment Center of the Israeli Ministry of Economy (formerly named the Ministry of Industry, Trade and Labor), or the Investment Center, be designated as an “Approved Enterprise” and accordingly, entitled to certain tax benefits under the Investment Law. Each certificate of approval for an Approved Enterprise relates to a specific investment program in the Approved Enterprise, delineated both by the financial scope of the investment and by the physical characteristics of the facility or the asset. We do not have any Approved Enterprises.
119
Tax Benefits Subsequent to the 2005 Amendment
Pursuant to the 2005 Amendment, a company whose facilities meet certain criteria set forth in the 2005 Amendment, may claim certain tax benefits offered by the Investment Law (as further described below) directly in its tax returns, without the need to obtain prior approval. In order to receive the tax benefits, a company must make an investment which meets all of the conditions, including exceeding a minimum entitling investment amount, set forth in the Investment Law. Such investment allows a company to receive “Benefited Enterprise” status, and may be made over a period of no more than three years ending at the end of the year in which the company chose to have the tax benefits apply to its Benefited Enterprise, referred to as the “Year of Election.”
The extent of the tax benefits available under the 2005 Amendment to qualifying income of a Benefited Enterprise depends on, among other things, the geographic location in Israel of the Benefited Enterprise. The location will also determine the period for which tax benefits are available. Under the “Exemption Track” the tax benefits include an exemption from corporate tax on undistributed income generated by the Benefited Enterprise for a period of two to ten years, depending on the geographic location of the Benefited Enterprise in Israel, and a reduced corporate tax rate of 10% to 25% for the remainder of the benefits period, depending on the level of foreign investment in the company in each year. The benefits period is for a duration of seven or ten years, depending on the location of the Benefited Enterprise, from the later of the first year in which the company generated taxable income from its Benefited Enterprise and the Year of Election, but in any event not more than 12 or 14 years from the Year of Election, depending on the location of the company. A company qualifying for tax benefits under the Exemption Track which pays a dividend out of income derived by its Benefited Enterprise during the tax exemption period will be subject to corporate tax in respect of the amount of the dividend (grossed-up to reflect the pre-tax income that it would have had to earn in order to distribute the dividend) at the corporate tax rate which would have otherwise been applicable. Dividends paid out of income attributed to a Benefited Enterprise are generally subject to withholding tax at source at the rate of 15%-20% or such lower rate as may be provided in an applicable tax treaty.
The benefits available to a Benefited Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations. If a company does not meet these conditions, it may be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest, or other monetary penalties.
We currently have one Benefited Enterprise program under the Investments Law, which, we believe, entitle us to certain tax benefits with respect to income to be derived from our Benefited Enterprise. During the benefits period, taxable income from our Benefited Enterprise program (once generated) will be tax exempt for a period of ten years commencing with the year we will first earn taxable income relating to such enterprise. We chose 2010 as the Year of Election. Due to the location of our company, we believe we are entitled to a 10 year benefit period, subject to a 14 year limitation from the Year of Election, and therefore, the tax benefit period will in any event end in 2023.
Tax Benefits under the 2011 Amendment
The 2011 Amendment canceled the availability of the benefits granted to companies under the Investment Law prior to 2011 and, instead, introduced new benefits for income generated by a “Preferred Company” through its “Preferred Enterprise” (as such terms are defined in the Investment Law) as of January 1, 2011. The definition of a Preferred Company includes a company incorporated in Israel that is not wholly-owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled and managed from Israel. Effective January 1, 2014, a Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to its income derived by its Preferred Enterprise in 2014 and thereafter, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 9%. Our facilities are located in a specified development zone.
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at source at the rate of 20% or such lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required to be withheld (although, if such dividends are subsequently distributed to individuals or a non-Israeli company, withholding tax at a rate of 20% or such lower rate as may be provided in an applicable tax treaty will apply).
The 2011 Amendment also provides transitional provisions to address companies already enjoying existing tax benefits under the Investment Law. These transitional provisions provide, among other things, that unless an irrevocable request is made to apply the provisions of the Investment Law as amended in 2011 with respect to income to be derived as of January 1, 2011, a Benefited Enterprise can elect to continue to benefit from the benefits provided to it before the 2011 Amendment came into effect, provided that certain conditions are met.
120
We have examined the possible effect, if any, of the provisions of the 2011 Amendment on our financial statements and have decided not to apply the new benefits under the 2011 Amendment.
The termination or substantial reduction of any of the benefits available under the Investment Law could materially increase our future tax liabilities.
The Encouragement of Industrial Research and Development Law, 5744-1984
Under the Encouragement of Industrial Research and Development Law, 5744-1984, generally referred to as the Research Law, research and development programs which meet specified criteria and are approved by a committee of the OCS are eligible for grants. The grants awarded are typically up to 50% of the project’s expenditures, as determined by the research committee. The grantee is required to pay royalties to the State of Israel from the sale of products developed under the program. Regulations under the Research Law generally provide for the payment of royalties of 3% to 5% (or 6% with respect to certain limited programs and at an increased rate under certain circumstances) on sales of products and services based on technology developed using grants, until 100% of the grant, linked to the dollar and bearing interest at the LIBOR rate, is repaid. The terms of the Israeli government participation also require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless approval is received from the OCS. However, this does not restrict the export of products that incorporate the funded technology. In addition, payment of additional amounts is required if manufacturing is moved outside of Israel, in which case the royalty repayment rate is increased and the royalty ceiling can reach up to three times the amount of the grants received, and if OCS developed know-how is transferred outside of Israel, the royalty ceiling can reach up to six times the amount of grants (plus interest).
As of December 31, 2015, we have received funding from the OCS for the financing of a portion of our research and development expenditures in an aggregate amount of $3.6 million. As of December 31, 2015, we had a contingent obligation to the OCS in the amount of $3.8 million.
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. A non-Israeli resident who derives capital gains from the sale of traded securities of an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside of Israel will be exempt from Israeli tax so long as the securities were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of 25% or more in such non-Israeli corporation; or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly.
Additionally, a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, under the Convention Between the Government of the United States of America and the Government of the State of Israel with respect to Taxes on Income, as amended, or the United States-Israel Tax Treaty, the sale, exchange or other disposition of shares by a shareholder who (i) is a U.S. resident (for purposes of the treaty); (ii) holds the shares as a capital asset; and (iii) is entitled to claim the benefits afforded to such person by the treaty, is generally exempt from Israeli capital gains tax. Such exemption will not apply if, among other things: (i) the capital gain arising from such sale, exchange or other disposition is treated as industrial or commercial profits attributed to a permanent establishment in Israel, subject to certain conditions; (ii) the shareholder holds, directly or indirectly, shares representing 10% or more of the voting capital of the corporation during any part of the 12-month period preceding the disposition, subject to certain conditions; (iii) the capital gain arising from such sale, exchange or disposition is treated as royalties; or (iv) such U.S. resident is an individual and was present in Israel for 183 days or more during the relevant taxable year. In such case, the sale, exchange or disposition of our securities would be subject to Israeli tax, to the extent applicable; however, under the United States-Israel Tax Treaty, the taxpayer would be permitted to claim a credit for such taxes against the U.S. federal income tax imposed with respect to such sale, exchange or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The United States-Israel Tax Treaty does not relate to U.S. state or local taxes.
In some instances where our shareholders may be liable for Israeli tax on the sale of their securities, the payment of the consideration may be subject to the withholding of Israeli tax at source. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
121
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents are generally subject to Israeli withholding tax on the receipt of dividends paid on our ordinary shares at the rate of 25%, unless relief is provided in a treaty between Israel and the shareholder’s country of residence (subject to the receipt of a valid certificate from the Israeli Tax Authority allowing for a reduced tax rate). With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or at any time during the preceding 12 months, the applicable withholding tax rate is 30%, unless such “substantial shareholder” holds such shares through a nominee company, in which case the rate is 25%. A “substantial shareholder” is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right.
However, a distribution of dividends to non-Israeli residents is subject to withholding tax at source at a rate of 15-20% if the dividend is distributed from income attributed to an Approved Enterprise, Benefited Enterprise or a Preferred Enterprise, unless a reduced tax rate is provided under an applicable tax treaty. With respect to a Benefited Enterprise whose Year of Election is before 2014, such as ours, distribution of dividends attributed to income of such Benefited Enterprise is subject to 15% tax rate. We cannot assure you that in the event we declare a dividend we will designate the income out of which the dividend is paid in a manner that will reduce shareholders’ tax liability.
Under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the United States-Israel Tax Treaty) is 25%. With respect to dividends paid to a U.S. corporation that held 10% or more of our outstanding voting capital throughout the tax year in which the dividend is distributed and the preceding tax year and provided that not more than 25% of the gross income of the paying corporation for such prior taxable year (if any) consists of certain interest or dividends, the maximum rate of tax withheld at source is 12.5%; provided, however, that if the paying corporation is an Approved Enterprise, the applicable withholding tax rate under such circumstances is reduced to 15%. We believe that the reference in the United States-Israel Tax Treaty to an Approved Enterprise under the Investment Law is deemed to include also a Benefitted Enterprise and Preferred Enterprise under the Investment Law.
U.S. residents who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction for U.S. federal income tax purposes in the amount of the taxes withheld, subject to detailed rules contained in U.S. tax legislation.
Taxation of Non-Israeli Shareholders upon Exercise of Long Term Incentive Warrants. While not free from doubt, the Long Term Incentive Warrants may be treated for Israeli income tax purposes as compensatory warrants (i.e., issued to compensate an original purchaser of units in this offering for holding the ordinary shares underlying the units for a certain period of time after the closing date of this offering). In such case, a holder of Long Term Incentive Warrants may recognize ordinary compensation income for Israeli income tax purposes on the exercise of the Long Term Incentive Warrants.
Non-Israeli residents are subject to Israeli income tax only on income from sources in Israel, which is income accrued or derived in Israel. Therefore, to the extent that the Long Term Incentive Warrants may be treated as compensatory warrants for Israeli tax purposes, the ordinary compensation income on the exercise of the Long Term Incentive Warrants would not be subject to Israeli income tax to the extent such income is deemed to be attributable to the performance of services outside of Israel. Furthermore, according to the United States-Israel Tax Treaty, income received by an individual for his performance of labor or personal services shall be treated as income from sources within the country where the service was provided (i.e., with respect to U.S. residents (for purposes of the treaty), U.S. source income) and therefore, would be exempt from Israeli income tax.
To the extent that the Long Term Incentive Warrants may be treated as compensatory warrants, the exercise of the Long Term Incentive Warrants may be subject to the withholding of Israeli tax at source by us and the warrants may not be exercised unless the withholding obligation has been fulfilled. Shareholders may be required to demonstrate that they are exempt from tax on such income in order to avoid withholding at source at the time of exercise.
Surtax
Subject to the provisions of an applicable tax treaty, individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 2% on annual income (including, but not limited to, dividends, interest and capital gain) exceeding NIS 811,560 for 2014, which amount is linked to the annual change in the Israeli consumer price index.
122
Estate and Gift Tax
Israeli law presently does not impose estate or gift taxes.
EACH PROSPECTIVE INVESTOR IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY ISRAELI TAX LAWS AND ANY APPLICABLE TAX TREATIES.
U.S. Federal Income Taxation
The following are the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our securities. Because the components of the units sold in our initial public offering separated on March 18, 2015, the holder of a unit should have been treated, for U.S. federal income tax purposes, as the owner of the underlying ordinary share and one-half of a Series A Warrant components of the unit, as the case may be. As a result, the discussion below of the U.S. federal income tax consequences with respect to actual holders of ordinary shares and Series A Warrants also should have applied to the holders of units (as the deemed owners of the ordinary shares and Series A Warrants underlying the units).
The discussion below of the U.S. federal income tax consequences to “U.S. Holders” will apply to a beneficial owner of our securities that is for U.S. federal income tax purposes:
|
•
|
an individual citizen or resident of the United States;
|
|
•
|
a corporation (or other entity treated as a corporation) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia;
|
|
•
|
an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or
|
|
•
|
a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons are authorized to control all substantial decisions of the trust; or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
|
A beneficial owner of our securities that is described above is referred to herein as a “U.S. Holder.” If a beneficial owner of our securities is not described as a U.S. Holder and is not an entity treated as a partnership or other pass-through entity for U.S. federal income tax purposes, such owner will be considered a “Non-U.S. Holder.” The material U.S. federal income tax consequences applicable specifically to Non-U.S. Holders are described below under the heading “Non-U.S. Holders.”
This discussion is based on the Internal Revenue Code of 1986, as amended (the “Code”), its legislative history, Treasury regulations promulgated thereunder, published rulings and court decisions, all as currently in effect. These authorities are subject to change or differing interpretations, possibly on a retroactive basis.
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to any particular holder based on such holder’s individual circumstances. In particular, this discussion considers only holders that own and hold our securities as capital assets within the meaning of Section 1221 of the Code, and does not address the potential application of the alternative minimum tax or the U.S. federal income tax consequences to holders that are subject to special rules, including:
|
•
|
financial institutions or financial services entities;
|
|
•
|
broker-dealers;
|
|
•
|
persons that are subject to the mark-to-market accounting rules under Section 475 of the Code;
|
|
•
|
tax-exempt entities;
|
|
•
|
governments or agencies or instrumentalities thereof;
|
123
|
•
|
insurance companies;
|
|
•
|
regulated investment companies;
|
|
•
|
real estate investment trusts;
|
|
•
|
certain expatriates or former long term residents of the United States;
|
|
•
|
persons that actually or constructively own 5% or more of our voting shares;
|
|
•
|
except as specifically discussed herein in respect of the Long Term Incentive Warrants, persons that acquired our securities pursuant to an exercise of employee options, in connection with employee incentive plans or otherwise as compensation;
|
|
•
|
persons that hold our securities as part of a straddle, constructive sale, hedging, conversion or other integrated transaction;
|
|
•
|
persons whose functional currency is not the U.S. dollar;
|
|
•
|
passive foreign investment companies; or
|
|
•
|
controlled foreign corporations.
|
This discussion does not address any aspect of U.S. federal non-income tax laws, such as gift or estate tax laws, or state, local or non-U.S. tax laws or, except as discussed herein, any tax reporting obligations applicable to a holder of our securities. Additionally, this discussion does not consider the tax treatment of partnerships or other pass-through entities or persons who hold our securities through such entities. If a partnership (or other entity classified as a partnership for U.S. federal income tax purposes) is the beneficial owner of our securities, the U.S. federal income tax treatment of a partner in the partnership generally will depend on the status of the partner and the activities of the partnership. This discussion also assumes that any distribution made (or deemed made) to a holder in respect of our securities and any consideration received (or deemed received) by a holder in connection with the sale or other disposition of our securities will be in U.S. dollars. In addition, as described “Risk Factors – Risks Related to Taxation – There is a risk that we could be treated as a domestic (U.S.) corporation for U.S. federal income tax purposes by reason of the transactions related to our acquisition of all of the business operations and substantially all of the assets of Check-Cap LLC on May 31, 2009 (hereinafter sometimes referred to as the “reorganization”),” this discussion also assumes that we will be and have been treated as a foreign corporation for U.S. federal income tax purposes. Moreover, this discussion assumes that a holder owned a number of units and will own a number of Series A Warrants and Long Term Incentive Warrants that are each divisible by two and, thus, did not have a fractional warrant at the time of the separation of a unit and will not have a fractional warrant upon the exercise of a Series A Warrant and/or Long Term Incentive Warrant, as the case may be.
We have not sought, and will not seek, a ruling from the Internal Revenue Service (the “IRS”) or an opinion of counsel as to any U.S. federal income tax consequence described herein. The IRS may disagree with the description herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
EACH HOLDER IN OUR SECURITIES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH IHOLDER OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR SECURITIES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY STATE, LOCAL, AND NON-U.S. TAX LAWS, AS WELL AS U.S. FEDERAL TAX LAWS AND ANY APPLICABLE TAX TREATIES.
Allocation of Purchase Price of a Unit and Characterization of a Unit and its Components and the Long Term Incentive Warrants
While not free from doubt, each unit should have been treated for U.S. federal income tax purposes as an investment unit consisting of one ordinary share and one-half of a Series A Warrant to acquire one ordinary share. For U.S. federal income tax purposes, each holder of a unit generally had to allocate the purchase price of a unit between the ordinary share and the one-half of a Series A Warrant that comprise the unit based on the relative fair market value of each at the time of issuance. The price allocated to the ordinary share and the one-half of a Series A Warrant generally will be the holder’s tax basis in such share or one-half of a Series A Warrant, as the case may be. While uncertain, the IRS, by analogy to the rules relating to the allocation of the purchase price to components of a unit consisting of debt and equity, may take the position that any allocation of the purchase price that we may make will be binding on a holder of a unit, unless the holder explicitly discloses in a statement attached to the holder’s timely filed U.S. federal income tax return for the taxable year that includes the acquisition date of the unit that the holder’s allocation of the purchase price between the ordinary share and the one-half of a Series A Warrant that comprise the unit is different than our allocation. Any such allocation is not, however, binding on the IRS.
124
As discussed under “U.S. Holders — Exercise of Long Term Incentive Warrants” below, while not free from doubt, the Long Term Incentive Warrants may be treated for U.S. federal income tax purposes as compensatory warrants. Based on this characterization, no portion of the purchase price of a unit generally should have been allocated to the Long Term Incentive Warrants, and a holder of the Long Term Incentive Warrants may have ordinary compensation income for U.S. federal income tax purposes on the exercise of the Long Term Incentive Warrants, as described under “U.S. Holders — Exercise of Long Term Incentive Warrants” and “Non-U.S. Holders” below.
Each holder is advised to consult its own tax advisor with respect to the risks associated with the Long Term Incentive Warrants and the investment in a unit (including alternative characterizations of the Long Term Incentive Warrants or a unit or the components thereof) and regarding the risks associated with an allocation of the purchase price of a unit between the ordinary share and the one-half of a Series A Warrant that comprised a unit that is inconsistent with any allocation of the purchase price that we may make. The balance of this discussion assumes that the characterization of the Long Term Incentive Warrants as compensatory warrants and of the units (and the components thereof) and any allocation of the purchase price of a unit, in each case as described above, are respected for U.S. federal income tax purposes.
U.S. Holders
Taxation of Cash Distributions
Subject to the passive foreign investment company, or PFIC, rules discussed below, a U.S. Holder generally will be required to include in gross income as ordinary income the amount of any cash dividend paid in respect of our ordinary shares. A cash distribution on our ordinary shares generally will be treated as a dividend for U.S. federal income tax purposes to the extent the distribution is paid out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes). Such dividend generally will not be eligible for the dividends-received deduction generally allowed to U.S. corporations in respect of dividends received from other U.S. corporations. The portion of such cash distribution, if any, in excess of such earnings and profits will be applied against and reduce (but not below zero) the U.S. Holder’s adjusted tax basis in the ordinary shares. Any remaining excess generally will be treated as gain from the sale or other taxable disposition of such ordinary shares.
With respect to non-corporate U.S. Holders, any such cash dividends may be subject to U.S. federal income tax at the lower applicable regular long term capital gains tax rate (see “— Taxation on the Disposition of Ordinary Shares or the Series A Warrants” below) provided that (a) our ordinary shares are readily tradable on an established securities market in the United States or we are eligible for the benefits of the United States-Israel Tax Treaty, (b) we are not a PFIC, as discussed below, for either the taxable year in which the dividend was paid or the preceding taxable year, and (c) certain holding period requirements are met. Therefore, if our ordinary shares are not readily tradable on an established securities market, and we are not eligible for the benefits of the United States-Israel Tax Treaty, then cash dividends paid by us to non-corporate U.S. Holders will not be subject to U.S. federal income tax at the lower regular long term capital gains tax rate. Under published IRS authority, shares are considered for purposes of clause (a) above to be readily tradable on an established securities market in the United States only if they are listed on certain exchanges, which presently include the NASDAQ Capital Market. Although our ordinary shares are currently listed and traded on the NASDAQ Capital Market, U.S. Holders nevertheless should consult their own tax advisors regarding the availability of the lower rate for any cash dividends paid with respect to our ordinary shares.
Dividends paid to a U.S. Holder with respect to our ordinary shares generally will be foreign source income, which may be relevant in calculating such U.S. Holder’s foreign tax credit limitations. Subject to certain conditions and limitations, Israeli tax withheld on dividends may be deducted from such U.S. Holder’s taxable income or credited against such U.S. Holder’s U.S. federal income tax liability. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if a U.S. Holder does not satisfy certain minimum holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and U.S. Holders should consult their tax advisors to determine whether and to what extent they will be entitled to this credit.
125
Adjustments with Respect to Warrants
The terms of each Series A Warrant and Long Term Incentive Warrant provide for an adjustment to the number of ordinary shares for which the warrant may be exercised or to the exercise price of the warrant in certain events. An adjustment that has the effect of preventing dilution generally is not taxable. However, the U.S. Holders of the Series A Warrants would be treated as receiving a constructive distribution from us if, for example, the adjustment increases the warrant holders’ proportionate interest in our assets or earnings and profits (e.g., through a decrease in the exercise price of the Series A Warrants) as a result of a distribution of cash to the holders of our ordinary shares, which is taxable to the U.S. Holders of such ordinary shares as described under “— Taxation of Cash Distributions,” above. Such constructive distribution would be subject to tax as described under that section in the same manner as if the U.S. Holders of the Series A Warrants received a cash distribution from us equal to the fair market value of such increased interest. While not clear, the U.S. Holders of the Long Term Incentive Warrants may be subject to a similar tax treatment in respect of any such constructive distribution, or, alternatively, if such warrants are treated as compensatory warrants, such holders may be deemed to have additional compensation income either at the time of the adjustment or upon the exercise of the Long Term Incentive Warrants. U.S. Holders of Long Term Incentive Warrants are urged to consult their own tax advisors on this issue.
Taxation on the Disposition of Ordinary Shares or Series A Warrants
Upon a sale or other taxable disposition of our ordinary shares or the Series A Warrants, and subject to the PFIC rules discussed below, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the securities.
The regular U.S. federal income tax rate on capital gains recognized by U.S. Holders generally is the same as the regular U.S. federal income tax rate on ordinary income, except that long term capital gains recognized by non-corporate U.S. Holders generally are subject to U.S. federal income tax at a maximum regular rate of 20%. Capital gain or loss will constitute long term capital gain or loss if the U.S. Holder’s holding period for the securities exceeds one year. The deductibility of capital losses is subject to various limitations. Any such gain or loss that a U.S. Holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
If an Israeli capital gains tax applies to any gains from the disposition of our ordinary shares or the Series A Warrants by a U.S. Holder, as discussed in “Israeli Tax Considerations and Government Programs – Taxation of our Shareholders” above, such tax may be treated as a foreign tax eligible for a deduction from such holder’s U.S. federal taxable income or a foreign tax credit against such holder’s U.S. federal income tax liability (subject to certain conditions and limitations). In addition, if such Israeli tax applies to any such gain, a U.S. Holder may be entitled to certain benefits under the United States-Israel Tax Treaty, if such holder is considered a resident of the United States for purposes of, and otherwise meets the requirements of, the United States-Israel Tax Treaty. U.S. Holders should consult their own tax advisors regarding the deduction or credit for any such Israeli tax and their eligibility for the benefits of the United States-Israel Tax Treaty.
Additional Taxes
U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally will be subject to a 3.8% Medicare contribution tax on unearned income, including, without limitation, dividends on, and gains from the sale or other taxable disposition of, our ordinary shares or the Series A Warrants, subject to certain limitations and exceptions. U.S. Holders should consult their own tax advisors regarding the effect, if any, of such tax on their ownership and disposition of our ordinary shares or the Series A Warrants.
Exercise or Lapse of a Series A Warrant
Subject to the PFIC rules discussed below, a U.S. Holder generally will not recognize gain or loss upon the exercise of a Series A Warrant for cash. An ordinary share acquired pursuant to the exercise of a Series A Warrant for cash generally will have a tax basis equal to the U.S. Holder’s tax basis in the Series A Warrant, increased by the amount paid to exercise the Series A Warrant. The holding period of such ordinary share generally would begin on the day after the date of exercise of the Series A Warrant. If a Series A Warrant is allowed to lapse unexercised, a U.S. Holder generally will recognize a capital loss equal to such holder’s tax basis in the Series A Warrant.
The tax consequences of a cashless exercise of Series A Warrants are not clear under current tax law. A cashless exercise may be tax-free, either because it is not a realization event (i.e., not a transaction in which gain or loss is realized) or because the transaction is treated as a recapitalization for U.S. federal income tax purposes. In either tax-free situation, a U.S. Holder’s tax basis in the ordinary shares received would equal the U.S. Holder’s basis in the Series A Warrants. If the cashless exercise were treated as not being a realization event, the U.S. Holder’s holding period in the ordinary shares could be treated as commencing on the date following the date of exercise of the Series A Warrants. If the cashless exercise were treated as a recapitalization, the holding period of the ordinary shares received would include the holding period of the Series A Warrants.
126
It is also possible that a cashless exercise could be treated as a taxable exchange in which gain or loss is recognized. In such event, a U.S. Holder could be deemed to have surrendered a number of Series A Warrants with a fair market value equal to the exercise price for the number of Series A Warrants deemed exercised. For this purpose, the number of Series A Warrants deemed exercised would be equal to the amount needed to receive on exercise number of ordinary shares issued pursuant to the cashless exercise of the Series A Warrants. In this situation, the U.S. Holder would recognize capital gain or loss in an amount equal to the difference between the fair market value of the Series A Warrants deemed surrendered to pay the exercise price and the U.S. Holder’s tax basis in such Series A Warrants deemed surrendered. Such gain or loss would be long-term or short-term depending on the U.S. Holder’s holding period in the Series A Warrants. In this case, a U.S. Holder’s tax basis in the ordinary shares received would equal the sum of the fair market value of the Series A Warrants deemed surrendered to pay the exercise price and the U.S. Holder’s tax basis in the Series A Warrants deemed exercised, and a U.S. Holder’s holding period for the ordinary shares should commence on the date following the date of exercise of the Series A Warrants. There also may be alternative characterizations of any such taxable exchange that would result in similar tax consequences, except that a U.S. Holder’s gain or loss would be short-term.
Due to the absence of authority on the U.S. federal income tax treatment of a cashless exercise of Series A Warrants, it is unclear which, if any, of the alternative tax consequences and holding periods described above would be adopted by the IRS or a court of law. Accordingly, U.S. Holders should consult their tax advisors regarding the tax consequences of a cashless exercise of Series A Warrants.
Exercise of Long Term Incentive Warrants
While not free from doubt, the Long Term Incentive Warrants may be treated as compensatory warrants (i.e., issued to compensate an original purchaser of units in our initial public offering for holding the ordinary shares underlying the units for a certain period of time after the closing date of our initial public offering). Based on this characterization, a U.S. Holder of the Long Term Incentive Warrants generally would recognize ordinary compensation income on the exercise of the Long Term Incentive Warrants equal to the excess of the fair market value of the ordinary shares acquired pursuant to the exercise of the Long Term Incentive Warrants over the exercise price of the Long Term Incentive Warrants. In such case, a U.S. Holder’s tax basis in the ordinary shares received should be equal to their fair market value at the time of exercise, and the holding period of such ordinary shares should begin on the date of exercise.
Passive Foreign Investment Company Rules
A foreign (i.e., non-U.S.) corporation will be a PFIC if either (a) at least 75% of its gross income in a taxable year of the foreign corporation, including its pro rata share of the gross income of any corporation in which it is considered to own at least 25% of the shares by value, is passive income, or (b) at least 50% of its assets in a taxable year of the foreign corporation, ordinarily determined based on fair market value and averaged quarterly over the year, including its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than certain rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets.
Our actual PFIC status for our current taxable year or any subsequent taxable year is uncertain and will not be determinable until after the end of such taxable year. Accordingly, there can be no assurance with respect to our status as a PFIC for our current taxable year or any subsequent taxable year.
If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder of our ordinary shares or Series A Warrants, and the U.S. Holder did not make a timely QEF election for our first taxable year as a PFIC in which the U.S. Holder held (or was deemed to hold) the ordinary shares, a purging election, a QEF election along with a purging election, or a mark-to-market election, each as described below, such holder generally will be subject to special rules for regular U.S. federal income tax purposes with respect to:
|
•
|
any gain recognized by the U.S. Holder on the sale or other disposition of its ordinary shares or Series A Warrants; and
|
|
•
|
any “excess distribution” made to the U.S. Holder (generally, any distributions to such U.S. Holder during a taxable year of the U.S. Holder that are greater than 125% of the average annual distributions received by such U.S. Holder in respect of the ordinary shares during the three preceding taxable years of such U.S. Holder or, if shorter, such U.S. Holder’s holding period for the ordinary shares).
|
127
Under these rules,
|
•
|
the U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period for the ordinary shares or Series A Warrants;
|
|
•
|
the amount allocated to the U.S. Holder’s taxable year in which the U.S. Holder recognized the gain or received the excess distribution or to the period in the U.S. Holder’s holding period before the first day of our first taxable year in which we qualified as a PFIC will be taxed as ordinary income;
|
|
•
|
the amount allocated to other taxable years (or portions thereof) of the U.S. Holder and included in its holding period will be taxed at the highest ordinary tax rate in effect for that year and applicable to the U.S. Holder; and
|
|
|
•
|
the interest charge generally applicable to underpayments of tax will be imposed in respect of the tax attributable to each such other taxable year of the U.S. Holder. |
Although a determination as to our PFIC status is made annually, an initial determination that we are a PFIC generally will apply for subsequent years to a U.S. Holder that held (or was deemed to hold) our ordinary shares or Series A Warrants while we were a PFIC, whether or not we meet the test for PFIC status in those subsequent years. If we are determined to be a PFIC in any taxable year, and then cease to meet the test for PFIC status in a subsequent taxable year, a U.S. Holder may be able to make a purging election to eliminate this continuing PFIC status with respect to its ordinary shares in certain circumstances. A purging election generally creates a deemed sale of such ordinary shares at their fair market value on the last day of our tax year during which we qualified as a PFIC (or, in the case of a purging election made in connection with a QEF election, the first day of our taxable year in which qualify as a QEF with respect to such U.S. Holder). Any gain recognized by the purging election generally will be treated as an excess distribution subject to the special tax and interest charge rules described above. As a result of the purging election, the U.S. Holder generally will increase the adjusted basis in its ordinary shares by the amount of gain recognized and will also have a new holding period in its ordinary shares for purposes of the PFIC rules.
In general, if we are determined to be a PFIC, a U.S. Holder may also avoid the PFIC tax consequences described above with respect to the ordinary shares by making a timely QEF election (or a QEF election along with a purging election). Pursuant to the QEF election, a U.S. Holder generally will be required to include in income its pro rata share of our net capital gains (as long term capital gain) and other earnings and profits (as ordinary income), on a current basis, in each case whether or not distributed, in the taxable year of the U.S. Holder in which or with which our taxable year ends if we are treated as a PFIC for that taxable year. However, a U.S. Holder may make a QEF election only if we agree to provide certain tax information to such holder annually. At this time, we do not intend to provide U.S. Holders with such information as may be required to make a QEF election effective.
Alternatively, if a U.S. Holder, at the close of its taxable year, owns ordinary shares in a PFIC that are treated as marketable stock, the U.S. Holder may make a mark-to-market election with respect to such ordinary shares for such taxable year. If the U.S. Holder makes a valid mark-to-market election for the first taxable year of the U.S. Holder in which the U.S. Holder holds (or is deemed to hold) the ordinary shares and for which we are determined to be a PFIC, such holder generally will not be subject to the PFIC rules described above with respect to its ordinary shares as long as such shares continue to be treated as marketable stock. Instead, in general, the U.S. Holder will include as ordinary income for each year that we are treated as a PFIC the excess, if any, of the fair market value of its ordinary shares at the end of its taxable year over the adjusted tax basis in its ordinary shares. The U.S. Holder also will be allowed to take an ordinary loss in respect of the excess, if any, of the adjusted tax basis of its ordinary shares over the fair market value of its ordinary shares at the end of its taxable year (but only to the extent of the net amount of previously included income as a result of the mark-to-market election). The U.S. Holder’s adjusted tax basis in its ordinary shares will be adjusted to reflect any such income or loss amounts, and any further gain recognized on a sale or other taxable disposition of the ordinary shares in a taxable year in which we are treated as a PFIC generally will be treated as ordinary income. Special tax rules may also apply if a U.S. Holder makes a mark-to-market election for a taxable year after the first taxable year in which the U.S. Holder holds (or is deemed to hold) our ordinary shares and for which we are determined to be a PFIC. Currently, a mark-to-market election may not be made with respect to warrants.
128
The mark-to-market election is available only for stock that is regularly traded on a national securities exchange that is registered with the U.S. Securities and Exchange Commission, including the NASDAQ Capital Market, or on a foreign exchange or market that the IRS determines has rules sufficient to ensure that the market price represents a legitimate and sound fair market value. Although our ordinary shares are currently listed and traded on the NASDAQ Capital Market, U.S. Holders nevertheless should consult their own tax advisors regarding the availability and tax consequences of a mark-to-market election with respect to our ordinary shares under their particular circumstances.
If we are a PFIC and, at any time, have a foreign subsidiary that is classified as a PFIC, a U.S. Holder of our ordinary shares generally should be deemed to own a portion of the shares of such lower-tier PFIC, and generally could incur liability for the deferred tax and interest charge described above if we receive a distribution from, or dispose of all or part of our interest in, or the U.S. Holder were otherwise deemed to have disposed of an interest in, the lower-tier PFIC. A mark-to-market election generally would not be available with respect to such a lower-tier PFIC. U.S. Holders are urged to consult their own tax advisors regarding the tax issues raised by lower-tier PFICs.
A U.S. Holder that owns (or is deemed to own) ordinary shares in a PFIC during any taxable year of the U.S. Holder may have to file an IRS Form 8621 (whether or not a mark-to-market election is or has been made) with such U.S. Holder’s U.S. federal income tax return and provide such other information as may be required by the U.S. Treasury Department.
The rules dealing with PFICs and purging and mark-to-market elections are very complex and are affected by various factors in addition to those described above. Accordingly, U.S. Holders of our securities should consult their own tax advisors concerning the application of the PFIC rules to our securities under their particular circumstances.
Non-U.S. Holders
Cash dividends paid or deemed paid to a Non-U.S. Holder with respect to our ordinary shares generally will not be subject to U.S. federal income tax unless such dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that such holder maintains or maintained in the United States).
In addition, a Non-U.S. Holder generally will not be subject to U.S. federal income tax on any gain attributable to a sale or other taxable disposition of our securities unless such gain is effectively connected with its conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base that such holder maintains or maintained in the United States) or the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of such sale or other disposition and certain other conditions are met (in which case, such gain from U.S. sources generally is subject to U.S. federal income tax at a 30% rate or a lower applicable tax treaty rate).
Dividends and gains that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that such holder maintains or maintained in the United States) generally will be subject to regular U.S. federal income tax at the same regular U.S. federal income tax rates as applicable to a comparable U.S. Holder and, in the case of a Non-U.S. Holder that is a corporation for U.S. federal income tax purposes, may also be subject to an additional branch profits tax at a 30% rate or a lower applicable tax treaty rate.
As in the case of a U.S. Holder, as described under “U.S. Holders — Exercise of Long Term Incentive Warrants” and “ — Adjustments with Respect to Warrants,” a Non-U.S. Holder also may have compensation income for U.S. federal income tax purposes on the exercise of (or on certain adjustments in respect of) the Long Term Incentive Warrants. A Non-U.S. Holder may be subject to U.S. federal income tax (subject to reduction or elimination by an applicable income tax treaty) with respect to such compensation income to the extent such income is deemed to be attributable to the performance (or the refraining from the performance) of services in the United States or is otherwise considered to be U.S. source income for U.S. federal income tax purposes. Non-U.S. Holders are urged to consult their own tax advisors as to the U.S. federal income tax consequences that may arise with respect to the Long Term Incentive Warrants.
Backup Withholding and Information Reporting
In general, information reporting for U.S. federal income tax purposes should apply to distributions made on our securities within the United States to a U.S. Holder (other than an exempt recipient) and to the proceeds from sales and other dispositions of our securities by a U.S. Holder (other than an exempt recipient) to or through a U.S. office of a broker. Payments made (and sales and other dispositions effected at an office) outside the United States will be subject to information reporting in limited circumstances. In addition, certain information concerning a U.S. Holder’s adjusted tax basis in its securities and adjustments to that tax basis and whether any gain or loss with respect to such securities is long term or short term also may be required to be reported to the IRS, and certain holders may be required to file an IRS Form 8938 (Statement of Specified Foreign Financial Assets) to report their interest in our securities.
129
Moreover, backup withholding of U.S. federal income tax at a rate of 28% generally will apply to dividends paid on our securities to a U.S. Holder (other than an exempt recipient) and the proceeds from sales and other dispositions of our securities by a U.S. Holder (other than an exempt recipient), in each case who:
|
•
|
fails to provide an accurate taxpayer identification number;
|
|
•
|
is notified by the IRS that backup withholding is required; or
|
|
•
|
in certain circumstances, fails to comply with applicable certification requirements.
|
A Non-U.S. Holder generally may eliminate the requirement for information reporting and backup withholding by providing certification of its foreign status, under penalties of perjury, on a duly executed applicable IRS Form W-8 or by otherwise establishing an exemption.
Backup withholding is not an additional tax. Rather, the amount of any backup withholding will be allowed as a credit against a U.S. Holder’s or a Non-U.S. Holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that certain required information is timely furnished to the IRS.
Holders are urged to consult their own tax advisors regarding the application of backup withholding and the availability of and procedures for obtaining an exemption from backup withholding in their particular circumstances.
|
F.
|
Dividends and paying agents
|
Not applicable.
|
G.
|
Statement by experts
|
Not applicable.
|
H.
|
Documents on display
|
You may inspect our securities filings, including this Annual Report and the exhibits and schedules thereto, without charge at the offices of the SEC at 100 F Street, N.E., Washington, D.C. 20549. You may obtain copies of all or any part of the Annual Report from the Public Reference Section of the SEC, 100 F Street, NE, Washington, D.C. 20549 upon the payment of the prescribed fees. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC maintains a website at www.sec.gov that contains reports, proxy and information statements and other information regarding registrants like us that file electronically with the SEC. You can also inspect the Annual Report on this website.
A copy of each document (or a translation thereof to the extent not in English) concerning our company that is referred to in this Annual Report is available for public view (subject to confidential treatment of certain agreements pursuant to applicable law) at our principal executive offices at Check-Cap Building, Abba Hushi Avenue, P.O. Box 1271, Isfiya, 30090, Mount Carmel, Israel.
|
I.
|
Subsidiary Information
|
Not applicable.
130
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURE ABOUT MARKET RISK
Exchange Rate Risk
Some of our assets and liabilities are affected by fluctuations in the exchange rate between the U.S. dollar and the NIS and between the U.S. dollar and the Euro. For example, salaries and related expenses for Israeli employees are paid in NIS and some of our suppliers are located in Europe and require payment in Euros.
As of December 31, 2015, our total assets and liabilities linked to the NIS amounted to $3.9 million and $1.4 million, respectively. A 10% evaluation of the dollar in relation to the NIS would cause an exchange rate loss of $250,000.
As of December 31, 2015, we had no assets linked to the Euro and our total liabilities linked to the Euro amounted to $159,000. A 10% depreciation of the dollar in relation to the Euro would cause an exchange rate loss of $16,000.
During the year ended December 31, 2015, the exchange rate between the U.S. dollar and the NIS increased by 0.33% and the exchange rate between the U.S. dollar and the EURO increased by 11.4%. During the year ended December 31, 2014, the exchange rate between the U.S. dollar and the NIS increased by 12.0% and the exchange rate between the U.S. dollar and the EURO increased by 13.6%. During the year ended December 31, 2013, the exchange rate between the U.S. dollar and the NIS decreased by 7.02% and the exchange rate between the U.S. dollar and the EURO increased by 4.0%. To date, we have not hedged the risks associated with fluctuations in currency exchange rate.
Interest Rate Risk
Our obligation to pay royalties to the OCS is linked to LIBOR and we are therefore exposed to changes in LIBOR.
In addition, we intend to invest our cash balances, including certain of the net proceeds from our initial public offering and concurrent private placement pending their ultimate use, primarily in bank deposits and securities issued by the U.S. and Israeli governments. We are exposed to market risks resulting from changes in interest rates relating primarily to our financial investments in cash and deposits. We do not use derivative financial instruments to limit exposure to interest rate risk. Our interest income may decline in the future as a result of changes in the financial markets; however, we believe any such potential loss would be immaterial to us.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
Not applicable.
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
Not applicable.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Use of Proceeds
Not applicable.
ITEM 15. CONTROLS AND PROCEDURES
A. Disclosure Controls and Procedures. Our management, including our Chief Executive Officer and Chief Financial Officer have evaluated the effectiveness of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) of the Exchange Act) as of the end of the period covered by this Annual Report (the “Evaluation Date”). Based on such evaluation, the Chief Executive Officer and the Chief Financial Officer have concluded that, as of the Evaluation Date, the Company’s disclosure controls and procedures are effective.
131
B. Management's Annual Report on Internal Control Over Financial Reporting. Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act. Our management, including our Chief Executive Officer and our Chief Financial Officer, conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework and criteria established in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) as of the end of the period covered by this Annual Report. Based on that evaluation, our management has concluded that our internal control over financial reporting was effective as of December 31, 2015. Notwithstanding the foregoing, there can be no assurance that our internal control over financial reporting will detect or uncover all failures of persons within the Company to comply with our internal procedures, as all internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective may not prevent or detect misstatements.
C. Attestation Report of Registered Public Accounting Firm.
This Annual Report on Form 20-F does not include an audit report on the effectiveness of our internal controls over financial reporting as of December 31, 2015 by our registered public accounting firm as permitted due to our status as an emerging growth company under the Jumpstart Our Business Startups Act of 2012.
D. Changes in Internal Control Over Financial Reporting. Based on the evaluation conducted by our Chief Executive Officer and our Chief Financial Officer pursuant to Rules 13a-15(d) and 15d-15(d) under the Exchange Act, our management has concluded that there was no change in our internal control over financial reporting that occurred during the year ended December 31, 2015 that materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
ITEM 16. [RESERVED]
ITEM 16A. AUDIT COMMITTEE FINANCIAL EXPERT.
Our Board of Directors has determined that Mr. Yuval Yanai, a member of our audit committee is an audit committee financial expert as defined by rules of the U.S. Securities and Exchange Commission and is an independent director under NASDAQ Listing Rules.
ITEM 16B. CODE OF ETHICS.
We have adopted a Code of Business Conduct and Ethics that applies to all of our directors and employees, including our chief executive officer, chief financial officer, controller or principal accounting officer, or other persons performing similar functions, which complies with the “code of ethics” contemplated by Item 16B of Form 20-F promulgated by the U.S. Securities and Exchange Commission. A copy of our Code of Business Conduct and Ethics is available on our website at http://ir.check-cap.com/corporate-governance. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report and is not incorporated by reference herein. If we make any amendment to the Code of Business Conduct and Ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the U.S. Securities and Exchange Commission. Under Item 16B of the U.S. Securities and Exchange Commission’s Form 20-F, if a waiver or amendment of the Code of Business Conduct and Ethics applies to our principal executive officer, principal financial officer, principal accounting officer or controller and relates to standards promoting any of the values described in Item 16B(b) of Form 20-F, we will disclose such waiver or amendment on our website in accordance with the requirements of Instruction 4 to such Item 16B.
132
ITEM 16C. PRINCIPAL ACCOUNTANT FEES AND SERVICES.
The following table represents aggregate fees billed to us for professional services rendered for fiscal years ended December 31, 2015 and 2014 by Brightman Almagor Zohar & Co., a member firm of Deloitte Touche Tohmatsu, an independent registered accounting firm.
|
2015
|
2014
|
|||||||
|
Audit Fees(1)
|
$ | 60,000 | $ | 60,000 | ||||
|
Audit-Related Fees(2)
|
$ | 62,000 | $ | 80,000 | ||||
|
Tax Fees(3)
|
$ | 4,500 | $ | 10,000 | ||||
|
All Other Fees(4)
|
$ | 3,000 | - | |||||
|
Total
|
$ | 129,500 | $ | 150,000 | ||||
|
(1)
|
The audit fees for the years ended December 31, 2015 and 2014 were for professional services rendered for the audits of our financial statements, consents and in connection with filings with the U.S. Securities and Exchange Commission.
|
|
(2)
|
Audit-related fees for the year ended December 31, 2014 are for services rendered by our auditors in connection with our Registration Statement on Form F-1 related to our initial public offering.
|
|
(3)
|
Tax fees for the years ended December 31, 2015 and 2014 were for services related to tax compliance, including the preparation of tax returns and tax planning and tax advice.
|
|
(4)
|
Other fees for the year ended December 31, 2015 were for services related to the application for an additional grant from the OCS.
|
Pre-Approval of Auditors' Compensation
Our audit committee has adopted a pre-approval policy for the engagement of our independent registered public accounting firm to perform certain audit and non-audit services. Pursuant to this policy, which is designed to assure that such engagements do not impair the independence of our auditors, the audit committee pre-approves annually a catalog of specific audit and non-audit services in the categories of audit services, audit-related services and tax services that may be performed by our independent registered public accounting firm. If a type of service, that is to be provided by our auditors, has not received such general pre-approval, it will require specific pre-approval by our audit committee. The policy prohibits retention of the independent registered public accounting firm to perform the prohibited non-audit functions defined in applicable SEC rules.
ITEM 16D. EXEMPTIONS FROM THE LISTING STANDARDS FOR AUDIT COMMITTEES.
Not applicable.
ITEM 16E. PURCHASES OF EQUITY SECURITIES BY THE ISSUER AND AFFILIATED PURCHASERS.
Not applicable.
ITEM 16F. CHANGE IN REGISTRANT’S CERTIFYING ACCOUNTANT.
Not applicable.
ITEM 16G. CORPORATE GOVERNANCE.
Under the Israeli Companies Law, companies incorporated under the laws of the State of Israel whose shares are publicly traded, including companies with shares listed on the NASDAQ Capital Market, are considered public companies under Israeli law and are required to comply with various corporate governance requirements relating to such matters as external directors, the audit committee, the compensation committee and an internal auditor. These requirements are in addition to the corporate governance requirements imposed by the Listing Rules of the NASDAQ Stock Market and other applicable provisions of U.S. securities laws to which we became subject (as a foreign private issuer) upon the closing of our initial public offering and the listing of our securities on the NASDAQ Capital Market. Under the Listing Rules of the NASDAQ Stock Market, a foreign private issuer, such as us, may generally follow its home country rules of corporate governance in lieu of the comparable requirements of the Listing Rules of the NASDAQ Stock Market, except for certain matters including (among others) the composition and responsibilities of the audit committee and the independence of its members within the meaning of the rules and regulations of the U.S. Securities and Exchange Commission. We currently rely on this “home country practice exemption” solely with respect to the following items:
|
•
|
Nomination of our directors. Israeli law and our amended and restated articles of association do not require director nominations to be made by a nominating committee of our board of directors consisting solely of independent directors, as required under the Listing Rules of the NASDAQ Stock Market. In accordance with Israeli law and practice, directors are recommended by our board of directors for election by our shareholders (other than directors elected by our board of directors to fill a vacancy). However, in October 2015, our Board of Directors voluntarily established a Nominating Committee, whose role is to select and recommend to the Board of Directors for selection, director nominees, while considering the appropriate size and composition of the Board of Directors, the requirements applicable to all members of the Board of Directors and the criteria for the selection of new members of the Board of Directors. The Nominating Committee is currently comprised of the following directors: Yuval Yanai (an external director within the meaning of the Israeli Companies Law and an independent director within the meaning of the NASDAQ Listing Rules), who serves as the Chairman of the Nominating Committee, Tomer Kariv (the Chairman of our Board of Directors) and Steven Hanley (an independent director within the meaning of the NASDAQ Listing Rules).
|
133
|
•
|
Compensation of officers. Israeli law and our amended and restated articles of association do not require that the independent members of our board of directors (or a compensation committee composed solely of independent members of our board of directors) determine an executive officer’s compensation, as is generally required under the Listing Rules of the NASDAQ Stock Market with respect to the chief executive officer and all other executive officers. For details regarding the approvals required under the Israeli Companies Law for the approval of compensation of the chief executive officer, all other executive officers and directors, see Item 6C “Directors, Senior Management and Employees - Board Practices - Approval of Related Party Transactions under Israeli Law - Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions”).
|
|
•
|
Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Israeli Companies Law, rather than seeking approval for corporate actions in accordance with NASDAQ Listing Rule 5635. In particular, under the NASDAQ Listing Rule, shareholder approval is generally required for: (i) an acquisition of shares/assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest (or such persons collectively have a 10% or greater interest) in the target company or the assets to be acquired or the consideration to be received and the present or potential issuance of ordinary shares, or securities convertible into or exercisable for ordinary shares, could result in an increase in outstanding common shares or voting power of 5% or more; (ii) the issuance of shares leading to a change of control; (iii) adoption/amendment of a stock option or purchase plan or other equity compensation arrangements, pursuant to which stock may be acquired by officers, directors, employees or consultants (with certain limited exception); and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Israeli Companies Law, the adoption of, and material changes to, equity-based compensation plans generally require the approval of the board of directors (for details regarding the approvals required under the Israeli Companies Law for the approval of compensation of the chief executive officer, all other executive officers and directors, see “Item 6C “Directors, Senior Management and Employees — Board Practices -Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions. ”)
|
|
•
|
Quorum requirement. Under our amended and restated articles of association and as permitted under the Israeli Companies Law, a quorum for any meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy or by a written ballot, who hold at least 25% of the voting power of our shares (or if a higher percentage is required by law, such higher percentage) instead of 33 1/3% of the issued share capital required under the NASDAQ Listing Rules. If the meeting was adjourned for lack of a quorum, at the adjourned meeting, at least two shareholders present in person or by proxy shall constitute a quorum, unless the meeting of shareholders was convened at the demand of shareholders, in which case, the quorum shall be the presence of one or more shareholders holding at least 5% of our issued share capital and at least one percent of the voting power of our shares, or one or more shareholders with at least 5% of the voting power of our shares.
|
Except as stated above, we currently intend to comply with the rules generally applicable to U.S. domestic companies listed on NASDAQ. We may in the future decide to use the foreign private issuer exemption with respect to some or all of the other NASDAQ corporate governance rules. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on NASDAQ, may provide less protection than is accorded to investors under NASDAQ listing requirements applicable to domestic issuers. For more information, see “ Item 3D “Key Information - Risk Factors Risks Related to the Company”- As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of otherwise applicable NASDAQ requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.”
ITEM 16H. MINE SAFETY DISCLOSURE.
Not applicable.
134
PART III
ITEM 17. FINANCIAL STATEMENTS
Financial Statements are set forth under Item 18.
ITEM 18. FINANCIAL STATEMENTS
Our Financial Statements beginning on pages F-1 through F-39, as set forth in the following index, are hereby incorporated herein by reference. These Financial Statements are filed as part of this Annual Report.
ITEM 19. EXHIBITS
|
No.
|
Description
|
|
|
1.1**
|
Amended and Restated Articles of Association of the Registrant
|
|
|
2.1*
|
Form of Registrant’s Ordinary Share Certificate
|
|
|
2.2*
|
Form of Unit Certificate
|
|
|
2.3*
|
Form of Series A Warrant Certificate (included in Exhibit 2.5)
|
|
|
2.4*
|
Form of Long Term Incentive Warrant Certificate (included in Exhibit 2.5)
|
|
|
2.5*
|
Form of Warrant Agreement between Check-Cap Ltd. and American Stock Transfer & Trust Company LLC, as Warrant Agent
|
|
|
2.6*
|
Form of Underwriter Warrants
|
|
|
4.2*
|
2006 Unit Option Plan and Amendments thereto
|
|
|
4.3*
|
Amended and Restated Shareholders Agreement dated as of October 14, 2014 by and among Check-Cap Ltd. and the shareholders parties thereto
|
|
|
4.4*
|
Amendment to Amended and Restated Shareholders Agreement to be dated as of , 2015 by and among Check-Cap Ltd. and the shareholders parties thereto
|
|
|
4.5*
|
Form of Series C-1 preferred shares purchase warrant
|
|
|
4.6*
|
Forms of Series C-2 preferred shares purchase warrant
|
|
|
4.7*
|
Form of Series D-1 preferred shares purchase warrant
|
|
|
4.8*
|
Form of Series D-2 preferred shares purchase warrant
|
|
|
4.9*
|
Forms of Anti-Dilution Warrants
|
|
|
4.10*
|
Asset Transfer Agreement, dated as of May 31, 2009 by and between Check-Cap Ltd. and Check-Cap LLC.
|
|
|
4.11*
|
The Agreement for ASIC Design and Development dated November 26, 2009 by and between Check-Cap Ltd. and Politechnico di Milano
|
|
|
4.12*
|
Form of Indemnification Agreement
|
|
|
4.13*
|
Credit Line Agreement, dated as of August 20, 2014 by and among Check-Cap Ltd. and certain Lenders named therein
|
|
|
4.14*
|
Form of Ordinary Shares Warrant Certificate issued pursuant to a certain Credit Line Agreement dated as of August 20, 2014
|
|
|
4.15*
|
Forms of Ordinary Shares Warrant Certificate issued to the Pontifax entities
|
|
|
4.16*
|
Addendum to Credit Line Agreement dated as of October 14, 2014 by and among Check-Cap Ltd. and certain Lenders named therein
|
|
|
4.17*
|
Second Addendum to Credit Line Agreement dated as of December 22, 2014 by and among Check-Cap Ltd. and certain Lenders named therein
|
|
|
4.18*
|
Credit Agreement dated as of January 4, 2015 by and between Check-Cap Ltd. and Bank Leumi le-Israel B.M
|
|
|
4.19*
|
English translation of the Secured Debenture issued on January 4, 2015 by Check-Cap Ltd. in favor of Bank Leumi le-Israel B.M
|
135
|
4.20***
|
2015 Equity Incentive Plan and 2015 United States Sub-Plan to 2015 Equity Incentive Plan.
|
|
|
4.21***
|
Compensation Policy for Executive Officers and Directors
|
|
|
8.1
|
List of Subsidiaries of the Registrant
|
|
|
12.1
|
Certification of Chief Executive Officer Pursuant to Rule 13a-14(a)/15d-14(a)
|
|
|
12.2
|
Certification of Chief Financial Officer Pursuant to Rule 13a-14(a)/15d-14(a)
|
|
|
13.1
|
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002
|
|
|
15.1
|
Consent of Brightman Almagor Zohar & Co., independent registered public accounting firm
|
|
101.INS****
|
XBRL Instant Document
|
|
|
101.SCH****
|
XBRL Taxonomy Extension Schema Document
|
|
|
101.CAL****
|
XBLR Taxonomy Extension Calculation Linkbase Document
|
|
|
101.DEF****
|
XBRL Taxonomy Extension Definition Linkbase
|
|
|
101.LAB****
|
XBRL Taxonomy Extension Label Linkbase
|
|
|
101.PRE****
|
XBRL Taxonomy Extension Presentation Linkbase
|
* Incorporated by reference to the Registration Statement on Form F-1 of the Registrant (File No. 333-201250).
** Incorporated by reference to the Post-Effective Amendment No. 2 to the Registration Statement on Form F-1 filed by the Registrant with the Securities Exchange Commission on December 18, 2015 (File No. 333-201250).
*** Incorporated by reference to the Form 6-K filed by the Registrant with the Securities Exchange Commission on July 6, 2015.
**** To be filed by amendment.
136
The Registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this Annual Report on its behalf.
|
CHECK-CAP LTD.
|
||
|
Date: March 15, 2016
|
By:
|
/s/ William Densel |
|
Name:
|
William Densel
|
|
|
Title:
|
Chief Executive Officer and Director
|
|
|
(Principal Executive Officer)
|
||
|
By:
|
/s/ Lior Torem | |
|
Name:
|
Lior Torem
|
|
|
Title:
|
Chief Financial Officer
|
|
|
(Principal Financial Officer
and Principal Accounting Officer)
|
||
137
CHECK CAP LTD.
CONSOLIDATED FINANCIAL STATEMENTS AS OF DECEMBER 31, 2015
CHECK CAP LTD.
CONSOLIDATED FINANCIAL STATEMENTS AS OF DECEMBER 31, 2015
Table of Contents
|
Page
|
|
|
F-3
|
|
|
F-4
|
|
|
F-5
|
|
|
F-6
|
|
|
F-7 - F-8
|
|
|
F-9 - F-40
|
F - 2

To the Shareholders and Board of Directors of
Check-Cap Ltd.
Isfiya, Israel
We have audited the accompanying Consolidated Balance Sheets of Check-Cap Ltd. and its subsidiary (the “Company”) as of December 31, 2015 and 2014, and the related Consolidated Statements of Operations, Changes in Shareholders' Equity (Deficiency) and Cash Flows for each of the three years in the period ended December 31, 2015. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Check-Cap Ltd. and its subsidiary as of December 31, 2015 and 2014, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2015, in conformity with accounting principles generally accepted in the United States of America.
The Company is in the development stage as of December 31, 2015. As discussed in Note 1B to the financial statements, successful completion of the Company's development program and, ultimately, the attainment of profitable operations is dependent upon future events, including obtaining adequate financing to fulfill its development activities and achieving a level of sales adequate to support the Company's cost structure.
Brightman Almagor Zohar & Co.
Certified Public Accountants
A Member Firm of Deloitte Touche Tohmatsu
March 15, 2016
Tel Aviv, Israel

F - 3
CHECK CAP LTD
(U.S. dollars in thousands, except share and per share data)
|
December 31,
|
|||||||||||
|
Note
|
2 0 1 5
|
2 0 1 4
|
|||||||||
|
Assets
|
|||||||||||
|
Current assets
|
|||||||||||
|
Cash and cash equivalents
|
9,392 | 1,075 | |||||||||
|
Restricted cash
|
46 | 46 | |||||||||
|
Short-term bank deposit
|
4,811 | - | |||||||||
|
Prepaid expenses and other current assets
|
3 | 680 | 117 | ||||||||
|
Total current assets
|
14,929 | 1,238 | |||||||||
|
Non-current assets
|
|||||||||||
|
Property and equipment, net
|
4 | 369 | 191 | ||||||||
|
Deferred issuance costs
|
- | 1,556 | |||||||||
|
Total non-current assets
|
369 | 1,747 | |||||||||
|
Total assets
|
15,298 | 2,985 | |||||||||
|
Liabilities and shareholders’ equity
|
|||||||||||
|
Current liabilities
|
|||||||||||
|
Accounts payable and accruals
|
|||||||||||
|
Trade
|
577 | 333 | |||||||||
|
Other
|
245 | 1,355 | |||||||||
|
Other current liabilities
|
13 | 50 | |||||||||
|
Employees and payroll accruals
|
6 | 1,238 | 1,122 | ||||||||
|
Total current liabilities
|
2,073 | 2,860 | |||||||||
|
Non-current liabilities
|
|||||||||||
|
Royalties provision
|
8A | 577 | 544 | ||||||||
|
Warrants to purchase preferred shares
|
9 | - | 407 | ||||||||
|
Total non-current liabilities
|
577 | 951 | |||||||||
|
Shareholders’ equity (deficiency)
|
10 | ||||||||||
|
Preferred shares
|
- | 226 | |||||||||
|
Preferred A, B, C1, C2, D1, D2, D3 and D4 share of NIS 0.2 par value-Authorized: 12,142,291 and 0 shares at December 31, 2014
and 2015, respectively; Issued and outstanding: 4,338,998 and 0 shares at December 31, 2014 and 2015, respectively;
|
|||||||||||
|
Share capital
|
599 | 53 | |||||||||
|
Ordinary share of NIS 0.2 par value-Authorized: 45,357,710 and 57,500,000 shares at December 31, 2014 and 2015,
respectively; Issued and outstanding: 1,152,138 and 11,811,709 shares at December 31, 2014 and 2015, respectively
|
|||||||||||
|
Additional paid-in capital
|
46,164 | 20,720 | |||||||||
|
Accumulated deficit
|
(34,115 | ) | (21,825 | ) | |||||||
|
Total shareholders’ equity (deficiency)
|
12,648 | (826 | ) | ||||||||
|
Total liabilities and shareholders’ equity (deficiency)
|
15,298 | 2,985 | |||||||||
The accompanying notes to the consolidated financial statements are an integral part of them.
F - 4
CHECK CAP LTD
(U.S. dollars in thousands, except share and per share data)
|
Year ended December 31,
|
|||||||||||||||
|
Note
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||||
|
Research and development expenses, net
|
12 | 5,837 | 2,832 | 2,893 | |||||||||||
|
General and administrative expenses
|
13 | 6,626 | 1,703 | 1,090 | |||||||||||
|
Other income
|
- | - | (11 | ) | |||||||||||
|
Operating loss
|
12,463 | 4,535 | 3,972 | ||||||||||||
|
Financial income, net
|
14 | 173 | 3,925 | 604 | |||||||||||
|
Net loss
|
12,290 | 610 | 3,368 | ||||||||||||
|
Net loss per ordinary share (in USD) basic and diluted
|
1.06 | 1.18 | 3.27 | ||||||||||||
|
Weighted average number of ordinary shares outstanding - basic and diluted (in thousands)
|
15 | 11,918 | 2,181 | 1,627 | |||||||||||
The accompanying notes to the consolidated financial statements are an integral part of them.
F - 5
CHECK CAP LTD
(U.S. dollars in thousands, except share and per share data)
|
Preferred shares (1) (2)
|
Ordinary share (1) (2)
|
|||||||||||||||||||||||||||
|
Number
|
Amount
|
Number
|
Amount
|
Additional paid-in capital
|
Accumulated deficit
|
Total shareholders' equity
|
||||||||||||||||||||||
|
Balance as of January 1, 2013
|
4,338,998 | $ | 226 | 1,152,138 | $ | 53 | $ | 20,351 | $ | (17,847 | ) | $ | 2,783 | |||||||||||||||
|
Changes during 2013:
|
||||||||||||||||||||||||||||
|
Share-based compensation
|
- | - | - | - | 57 | - | 57 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (3,368 | ) | (3,368 | ) | |||||||||||||||||||
|
Balance as of December 31, 2013
|
4,338,998 | $ | 226 | 1,152,138 | $ | 53 | $ | 20,408 | $ | (21,215 | ) | $ | (528 | ) | ||||||||||||||
|
Changes during 2014:
|
||||||||||||||||||||||||||||
|
Share-based compensation
|
- | - | - | - | 312 | - | 312 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (610 | ) | (610 | ) | |||||||||||||||||||
|
Balance as of December 31, 2014
|
4,338,998 | $ | 226 | 1,152,138 | $ | 53 | $ | 20,720 | $ | (21,825 | ) | $ | (826 | ) | ||||||||||||||
|
Changes during 2015:
|
||||||||||||||||||||||||||||
|
Conversion of preferred shares into ordinary shares
|
(4,338,998 | ) | (226 | ) | 4,338,998 | 226 | - | - | - | |||||||||||||||||||
|
Reclassification of liability warrants to equity warrants
|
- | - | - | - | 233 | - | 233 | |||||||||||||||||||||
|
Issuance of ordinary shares in the IPO, net of issuance expenses in an amount of $2,945(3)
|
- | - | 2,250,000 | 113 | 10,638 | - | 10,751 | |||||||||||||||||||||
|
Issuance of ordinary shares in the Private Placement, net of issuance expenses in an amount of $1,225(4)
|
- | - | 2,000,000 | 101 | 10,799 | - | 10,900 | |||||||||||||||||||||
|
Exercise of warrants into ordinary shares
|
- | - | 1,763,106 | 90 | (61 | ) | - | 29 | ||||||||||||||||||||
|
Share-based compensation
|
- | - | - | - | 3,724 | - | 3,724 | |||||||||||||||||||||
|
Issuance of ordinary share upon exercise of stock options by employees
|
- | - | 307,467 | 16 | - | - | 16 | |||||||||||||||||||||
|
Capital investment
|
- | - | - | - | 111 | - | 111 | |||||||||||||||||||||
|
Net loss
|
- | - | - | - | - | (12,290 | ) | (12,290 | ) | |||||||||||||||||||
|
Balance as of December 31, 2015
|
- | - | 11,811,709 | $ | 599 | $ | 46,164 | $ | (34,115 | ) | $ | 12,648 | ||||||||||||||||
(1) The preferred shares comprised of several classes of shares, see Note 10C.
(2) All shares amounts have been retroactively adjusted to reflect a 1-for-20 share reverse split, see Note 1C.
(3) Issuance expenses include certain warrants with value of $196 issued in connection with the IPO.
(4) Issuance expenses include certain warrants with value of $125 issued in connection with the Private Placement.
The accompanying notes to the consolidated financial statements are an integral part of them.
F - 6
CHECK CAP LTD
(U.S. dollars in thousands, except share and per share data)
|
Year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||||||
|
Net loss
|
(12,290 | ) | (610 | ) | (3,368 | ) | ||||||
|
Adjustments required to reconcile net loss to net cash used in operating activities:
|
||||||||||||
|
Revaluation of fair value of warrants to purchase preferred share
|
(174 | ) | (3,519 | ) | (623 | ) | ||||||
|
Depreciation and amortization
|
92 | 78 | 77 | |||||||||
|
Share-based compensation
|
3,724 | 312 | 56 | |||||||||
|
Financial expenses (income), net
|
(11 | ) | 1 | - | ||||||||
|
Changes in assets and liabilities items:
|
||||||||||||
|
Decrease (increase) in prepaid and other current assets and non-current assets
|
(563 | ) | (1,545 | ) | 514 | |||||||
|
Increase in trade accounts payable, accruals and other current liabilities
|
334 | 1,377 | 82 | |||||||||
|
Increase in employees and payroll accruals
|
227 | 466 | 148 | |||||||||
|
Increase (decrease) in royalties provision
|
33 | (415 | ) | 104 | ||||||||
|
Net cash used in operating activities
|
(8,628 | ) | (3,855 | ) | (3,010 | ) | ||||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
. | |||||||||||
|
Purchase of property and equipment
|
(270 | ) | (46 | ) | (45 | ) | ||||||
|
Restricted cash
|
- | - | (3 | ) | ||||||||
|
Investment in (proceeds from) short-term bank deposit
|
(4,800 | ) | - | 3,450 | ||||||||
|
Net cash provided by (used in) investing activities
|
(5,070 | ) | (46 | ) | 3,402 | |||||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||||||
|
Receipt of short-term loan from bank
|
1,000 | - | - | |||||||||
|
Repayment of short-term loan from bank
|
(1,000 | ) | - | - | ||||||||
|
Issuance of ordinary shares upon exercise of stock options by employees
|
16 | - | - | |||||||||
|
Exercise of warrants into ordinary shares
|
29 | - | - | |||||||||
|
Issuance of ordinary shares in the Private Placement, net of issuance expenses
|
11,021 | - | - | |||||||||
|
Issuance of ordinary shares in IPO, net of issuance expenses
|
10,947 | - | - | |||||||||
|
Net cash provided by financing activities
|
22,013 | - | - | |||||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
2 | 1 | - | |||||||||
|
Net increase (decrease) in cash and cash equivalents
|
8,317 | (3,900 | ) | 392 | ||||||||
|
Cash and cash equivalents at the beginning of the year
|
1,075 | 4,975 | 4,583 | |||||||||
|
Cash and cash equivalents at the end of the year
|
9,392 | 1,075 | 4,975 | |||||||||
The accompanying notes to the consolidated financial statements are an integral part of them.
F - 7
CHECK CAP LTD
CONSOLIDATED STATEMENTS OF CASH FLOWS
(U.S. dollars in thousands, except share and per share data)
Supplemental information for Cash Flow:
|
Year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Supplemental disclosure of non-cash flow information
|
||||||||||||
|
Reclassification of liability warrants to equity warrants
|
233 | - | - | |||||||||
|
Cashless exercise of warrants to purchase ordinary shares into ordinary shares
|
45 | - | - | |||||||||
|
Conversion of preferred shares into ordinary shares
|
226 | - | - | |||||||||
|
Supplemental disclosure of cash flow information
|
||||||||||||
|
Cash paid for interest
|
11 | - | - | |||||||||
The accompanying notes to the consolidated financial statements are an integral part of them.
F - 8
CHECK CAP LTD
(U.S. dollars in thousands, except share and per share data)
|
NOTE 1 -
|
GENERAL INFORMATION
|
|
|
A.
|
General
|
|
|
(1)
|
Check Cap Ltd. (together with its wholly-owned subsidiary, the “Company") was incorporated under the laws of the state of Israel. The registered address of its offices is Abba Hushi Blvd., Isfiya.
|
|
|
(2)
|
Check-Cap Ltd has a wholly-owned subsidiary, Check-Cap U.S. Inc, incorporated under the laws of the United States (U.S.) on May 15, 2015.
|
|
|
(3)
|
The Company is a clinical stage medical diagnostics company developing the first non-invasive system for preparation-free imaging of the colon to identify precancerous polyps and cancers. The Company is developing an ingestible capsule that utilizes proprietary, ultra-low-energy X-ray-based technology to safely generate high-resolution, 3-dimensional imagery of the interior of the colon. Without requiring traditional bowel cleansing or diet and activity modifications, The Company's system is designed to increase patient acceptance and adherence to colorectal cancer screening recommendations. The Company's system is currently not cleared for marketing in any jurisdiction.
|
|
|
(4)
|
As described in Note 10E(2)(b), on February 24, 2015 the Company consummated an Initial Public Offering in the U.S. (the "IPO") concurrently with a Private Placement (the "Private Placement").
|
|
|
(5)
|
The consolidated financial statements of the Company as of and for the year ended December 31, 2015 comprise the Company and its wholly-owned U.S. subsidiary.
|
|
|
B.
|
Financial Position
|
|
|
Since its inception, the Company has devoted substantially all of its efforts to research and development, clinical trials, recruiting management and technical staff, acquiring assets and raising capital. The Company is still in its development and clinical stage and has not yet generated revenues. The extent of the Company's future operating losses and the timing of becoming profitable are uncertain. The Company has incurred losses of $12.3, $0.6 and $3.4 million for the years ended December 31, 2015, 2014 and 2013, respectively. As of December 31, 2015, the Company's accumulated deficit was $34.1 million. The Company funds its operations primarily through equity financings.
|
|
|
Management expects that the Company will continue to generate losses from its development and clinical activities which will result in a negative cash flow from operating activity. Management expects that the Company's existing cash will be sufficient to fund the Company's projected operating requirements for at least another 15 months following December 31, 2015.
|
|
|
C.
|
Reverse Share Split
|
|
|
On January 15, 2015, the Company's shareholders approved a reverse share split of 1 for 20 (i.e. 20 ordinary shares will be combined into one ordinary share), subject to and effective immediately prior to the IPO, provided that the IPO is consummated on or prior to December 31, 2015.
|
F - 9
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 1 -
|
GENERAL INFORMATION (Cont.)
|
|
|
D.
|
Conversion of Preferred Shares and Preferred Share Warrants
|
|
|
On January 15, 2015, the Company’s shareholders approved the conversion on a 1:1 basis, of each and every class and series of the Company’s authorized and outstanding preferred shares into ordinary shares, par value NIS 0.01 per share, of the Company (the “Pre- Split Ordinary Shares”) and the conversion on a 1:1 basis of all outstanding preferred share warrants into warrants to purchase Pre-Split Ordinary Shares, subject to and effective immediately prior to the IPO and provided that the IPO is consummated.
|
|
|
Immediately prior to the consummation of the IPO, on February 24, 2015, 4,338,998 preferred shares were converted into 4,338,998 Post-Split Ordinary Shares and 948,000 warrants to purchase preferred shares were converted into 948,000 warrants to purchase Pre-Split Ordinary Shares. Therefore, as of the date hereof, the Company’s share capital is comprised solely of ordinary shares and options and warrants to purchase ordinary shares.
|
|
NOTE 2 -
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
|
|
The Company’s consolidated financial statements are presented in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). The Company recasted the comparative amounts included in this financial statements to U.S. GAAP. In prior years the Company prepared its financial reports in accordance with International Financial Reporting Standards ("IFRS").
|
|
|
A.
|
Use of Estimates in Preparation of Financial Statements
|
|
|
The preparation of financial statements in conformity with GAAP requires the Company to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements, and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
|
|
|
B.
|
Principles of Consolidation
|
|
|
The Company’s consolidated financial statements include the financial statements of Check-Cap Ltd. and its wholly-owned subsidiary, Check-Cap U.S., Inc. The Company’s consolidated financial statements are presented after elimination of inter-company transactions and balances.
|
|
|
C.
|
Financial statements in U.S dollars
|
|
|
The Company has not yet generated revenues, and the majority of its expenses is in U.S. dollar (dollar or USD) or NIS, while none of these currencies is significantly material compared to the other.
|
F - 10
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 2 -
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
C.
|
Financial statements in U.S dollars (Cont.)
|
|
|
Management judgment, in setting the dollar as the Company's functional currency, is based mainly on the following criteria: The Company's budget and other Company internal reports, including reports to the Company's Board of Directors and investors, are presented in dollars. Management is using these reports in order to make decisions for the Company. All of the Company's equity and debt financings were in dollars; and it is expected that a significant portion of the Company's future revenue will be in dollars. The financial statements are presented in dollars, which is the functional currency of the Company.
|
|
|
Transactions and balances denominated in dollars are presented at their original amounts. Non-dollar transactions and balances have been re-measured to dollars in accordance with the provisions of ASC 830-10 "Foreign Currency Translation". All transaction gains and losses from re-measurement of monetary balance sheet items denominated in non-dollar currencies are reflected in the statement of operations as financial income or expenses, as appropriate.
|
|
|
D.
|
Cash and cash equivalents and restricted cash
|
|
|
Cash and cash equivalents includes cash in hand and short–term deposits in banks with an original maturity of up to three months, with a high level of liquidity that may be easily converted to known amounts of cash, and that are exposed to insignificant risk of change in value. The Company had a restricted cash balance of $46 at December 31, 2015 and 2014.
|
|
|
E.
|
Short-term bank deposit
|
|
|
Short-term bank deposits are deposits with maturities of more than three months but less than one year. The short–term bank deposits are presented at their cost, including accrued interest, which approximates fair value. As of December 31, 2015, the Company's short–term bank deposit was in dollars and bore interest rate of 0.51%.
|
|
|
F.
|
Concentration of credit risks
|
|
|
Financial instruments that potentially subject the Company to concentration of credit risk consist principally of cash, cash equivalents and short-term bank deposits. The Company deposits these instruments with highly rated financial institutions, mainly in Israeli banks. The Company has not experienced any credit losses in these accounts and does not believe it is exposed to any significant credit risk on these instruments.
|
|
|
G.
|
Property and equipment
|
|
|
Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following annual rates:
|
|
Length of useful life
|
Depreciation rate
|
|||||
|
Years
|
%
|
|||||
|
Office furniture and equipment
|
10-14 | 7-10 | ||||
|
Laboratory equipment
|
3-7 | 15-33 | ||||
|
Computers and auxiliary equipment
|
3 | 33 |
F - 11
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 2 -
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
H.
|
Impairment of Long-Lived Assets
|
|
|
The Company’s long-lived assets are reviewed for impairment in accordance with ASC 360-10 "Accounting for the Impairment or Disposal of Long-Lived Assets” whenever events or changes in circumstances indicate that the carrying amount of an asset (or asset group) may not be recoverable. Recoverability of assets (or asset group) to be held and used is measured by a comparison of the carrying amount of an asset to the future undiscounted cash flows expected to be generated by the assets. If such assets are considered to be impaired, the impairment to be recognized is measured by the amount by which the carrying amount of the assets exceeds the fair value of the assets. During the years ended December 31, 2015, 2014 and 2013, no impairment losses have been recorded.
|
|
|
I.
|
Research and development costs
|
|
|
Research and development costs are expensed as incurred and consist primarily of personnel, subcontractors and consultants (mainly in connection with clinical trials) and materials for research and development and clinical activities. Grants received by the Company from the Office of the Chief Scientist of Israel’s Ministry of Industry, Trade and Labor (the “OCS”) and from Israel-United States Binational Industrial Research and Development Foundation (the "BIRD Foundation") are recognized at the time the Company is entitled to such grants, on the basis of the costs incurred and applied as a deduction from research and development expenses. Such grants are included as a deduction of research and development costs (since at the time received the Company will generate sales from these projects and pay the royalties resulting from such sales).
|
|
|
See Note 12 below regarding the offset of grants received for participation in research and development expenses.
|
|
|
J.
|
Warrants to purchase preferred shares
|
|
|
The Company accounts for freestanding warrants to purchase its preferred shares as a liability on the balance sheets at fair value. The warrants to purchase preferred shares are recorded as a liability as they include anti-dilution protection provision requiring a reduction in original exercise price as a result of subsequent issuance below the original exercise price. The warrants are subject to re-measurement to fair value at each balance sheet date and any change in fair value is recognized as a component of financial income (expense), net, in the statements of operations. The Company must adjust the liability for changes in fair value until the earlier of the exercise or expiration of the warrants, or the conversion of convertible preferred shares into ordinary shares. Immediately prior to the consummation of the Company's IPO in February 2015, all of the remaining warrants to purchase preferred shares were converted to warrants to purchase ordinary shares, without the anti-dilution protection provision and the warrants were reclassified into equity at their current fair value (see Note 9).
|
F - 12
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 2 -
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
K.
|
Fair Value Measurement
|
|
|
Fair value is defined as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. The Company uses a three-tier fair value hierarchy to classify and disclose all assets and liabilities measured at fair value on a recurring basis, as well as assets and liabilities measured at fair value on a non-recurring basis, in periods subsequent to their initial measurement. The hierarchy requires the Company to use observable inputs when available, and to minimize the use of unobservable inputs when determining fair value. If a financial instrument uses inputs that fall in different levels of the hierarchy, the instrument will be categorized based upon the lowest level of input that is significant to the fair value calculation. The three-tiers are defined as follows:
|
|
· Level 1. Observable inputs based on unadjusted quoted prices in active markets for identical assets or liabilities;
|
|
· Level 2. Inputs, other than quoted prices in active markets, that are observable either directly or indirectly; and
|
|
· Level 3. Unobservable inputs for which there is little or no market data requiring the Company to develop its own assumptions.
|
|
|
L.
|
Contingent liabilities
|
|
|
The Company accounts for its contingent liabilities in accordance with ASC No. 450, “Contingencies”. A provision is recorded when it is both probable that a liability has been incurred and the amount of the loss can be reasonably estimated.
|
|
|
With respect to legal matters, provisions are reviewed and adjusted to reflect the impact of negotiations, estimated settlements, legal rulings, advice of legal counsel and other information and events pertaining to a particular matter. As of December 31, 2015 and 2014, the Company is not a party to any ligation that could have a material adverse effect on the Company’s business, financial position, results of operations or cash flows (see Note 8).
|
|
|
M.
|
Share-based compensation
|
|
|
In accordance with ASC 718-10 "Compensation-Stock Compensation" the Company estimates the fair value of equity-based payment awards on the date of grant using an option-pricing model. The value of the portion of the award that is ultimately expected to vest is recognized as expense over the requisite service periods in the Company's consolidated statement of operations.
|
|
|
The Company recognizes compensation expenses for the value of its awards granted based on the graded-vesting method over the requisite service period for each separately vesting portion of the award, net of estimated forfeitures. ASC No. 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. Estimated forfeitures are based on actual historical pre-vesting forfeitures.
|
F - 13
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 2 -
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
M.
|
Share-based compensation (Cont.)
|
|
|
The Company selected the Black-Scholes-Merton option-pricing model as the most appropriate fair value method for its share-option awards. The option-pricing model requires a number of assumptions, of which the most significant are the fair market value of the underlying ordinary share, expected share price volatility and the expected option term. Expected volatility was calculated based upon certain peer companies that the Company considered to be comparable. The expected option term represents the period of time that options granted are expected to be outstanding. The expected option term is determined based on the simplified method in accordance with Staff Accounting Bulletin No. 110, as adequate historical experience is not available to provide a reasonable estimate. The simplified method will continue to apply until enough historical experience is available to provide a reasonable estimate of the expected term. The risk-free interest rate is based on the yield from U.S. treasury bonds with an equivalent term. The Company has historically not paid dividends and has no foreseeable plans to pay dividends.
|
|
|
Prior to the Company's IPO, the fair value of the ordinary shares underlying the share options has been determined based on recent equity investment rounds and fair value by the Company's management and approved by the Company’s Board of Directors.
|
|
|
After the IPO and until March 18, 2015, the date the units, issued by the company in the IPO, were separated into one ordinary share and one-half of a series A warrant to purchase one ordinary share, the units ceased to exist, and the ordinary shares began trading on the NASDAQ Capital Market ("Unit Separation Date"), the fair value of the ordinary shares underlying the share options has been derived by reference to the closing price of the Company's unit on the NASDAQ Capital Market on the relevant date using relative fair value for each component in the unit.
|
|
|
After the Unit Separation Date, the grant date fair value for share-options awards is based on the closing price of the ordinary shares on the NASDAQ Capital Market on the date of grant and fair value for all other purposes related to share-options awards is the closing price of the Company's ordinary shares on the NASDAQ Capital Market on the relevant date.
|
|
|
The fair value for options granted in 2015, 2014 and 2013 is estimated at the date of grant using a Black-Scholes-Merton option-pricing model with the following assumptions:
|
|
December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Expected volatility
|
44%-62 | % | 50%-60 | % | 40%-60 | % | ||||||
|
Risk-free rate
|
1.29%-2.28 | % | 1.45%-1.72 | % | 0.75%-2.25 | % | ||||||
|
Dividend yield
|
0 | % | 0 | % | 0 | % | ||||||
|
Expected term (in years)
|
4-10 | 5-6 | 5-10 | |||||||||
|
Share price
|
$ | 3-$5.12 | $ | 3.01 | $ | 3.04 | ||||||
|
|
The Company accounts for options granted to consultants and other service providers under ASC No. 718 and ASC No. 505, “Equity-based payments to non-employees.” The fair value of these options was estimated using a Black-Scholes-Merton option-pricing model.
|
F - 14
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 2 -
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Cont.)
|
|
|
N.
|
Income taxes
|
|
|
The Company accounts for income taxes in accordance with ASC 740-10 "Accounting for Income Taxes." This Statement requires the use of the liability method of accounting for income taxes, whereby deferred tax asset and liability account balances are determined based on the differences between financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
|
|
|
In accordance with ASC 740, the Company reflects in the financial statements the benefit of positions taken in a previously filed tax return or expected to be taken in a future tax return only when it is considered ‘more-likely-than-not’ that the position taken will be sustained by a taxing authority. As of December 31, 2015 and 2014, the Company had no unrecognized income tax positions and correspondingly there is no impact on the Company’s effective income tax rate associated with these items.
|
|
|
O.
|
New Accounting Pronouncements
|
|
|
In November, 2015, the Financial Accounting Standards Board ("FASB") issued Accounting Standards Update ("ASU") 2015-17, Topic 740 - Balance Sheet Classification of Deferred Taxes. The ASU requires entities to present deferred tax assets and deferred tax liabilities as noncurrent in a classified balance sheet. Netting of deferred tax assets and deferred tax liabilities by tax jurisdiction is still required under the new guidance. The Company will apply the ASU in its future financial statements.
|
|
|
In 2014, the FASB issued Accounting Standards Update (“ASU”) 2014-15, Presentation of Financial Statements-Going Concern (Subtopic 205-40): Disclosure of Uncertainties about an Entity's Ability to Continue as a Going Concern, which defines management’s responsibility to assess an entity’s ability to continue as a going concern, and to provide related footnote disclosures if there is substantial doubt about its ability to continue as a going concern. The pronouncement is effective for annual reporting periods ending after December 15, 2016 with early adoption permitted. The Company is currently evaluating the effect, if any, that the adoption of this guidance will have on the Company’s financial statements.
|
|
|
In June 2014 the FASB issued Accounting Standards Update ("ASU") 2014-10 regarding development stage entities. The ASU removes the definition of development stage entity, as was previously defined under U.S. GAAP, from the accounting standards codification, thereby removing the financial reporting distinction between development stage entities and other reporting entities from U.S. GAAP. In addition, the ASU eliminates the requirements for development stage entities to (i) present inception-to-date information in the statement of income, cash flow and stockholders' equity, (ii) label the financial statements as those of a development stage entity, (iii) disclose a description of the development stage activities in which the entity is engaged, and (iv) disclose in the first year in which the entity is no longer a development stage entity that in prior years it had been in the development stage. The amendments' to ASU 2014-10 are effective for annual reporting periods beginning after December 15, 2014. The Company has applied the ASU in these financial statements.
|
F - 15
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 3 -
|
PREPAID EXPENSES AND OTHER CURRENT ASSETS
|
Composition:
|
December 31,
|
||||||||
| 2 0 1 5 | 2 0 1 4 | |||||||
|
Government institutions
|
400 | 46 | ||||||
|
Prepaid expenses
|
197 | 27 | ||||||
|
Deposits
|
55 | 43 | ||||||
|
Other assets
|
28 | 1 | ||||||
| 680 | 117 | |||||||
|
NOTE 4 -
|
PROPERTY AND EQUIPMENT, NET
|
|
|
Composition:
|
|
December 31,
|
||||||||
|
2 0 1 5
|
2 0 1 4
|
|||||||
|
Cost:
|
||||||||
|
Office furniture and equipment
|
100 | 92 | ||||||
|
Laboratory equipment
|
378 | 306 | ||||||
|
Computers and auxiliary equipment
|
396 | 206 | ||||||
| 874 | 604 | |||||||
|
Accumulated depreciation
|
505 | 413 | ||||||
|
Property and equipment, net
|
369 | 191 | ||||||
|
|
Depreciation expenses amounted to $92, $78 and $77 for the years ended December 31, 2015, 2014 and 2013, respectively.
|
|
NOTE 5 -
|
SHORT TERM LOANS
|
|
|
On January 4, 2015, the Company entered into a credit line agreement with Bank Leumi pursuant to which it was entitled to obtain a credit line in the principal amount of up to $1,000 (“the Bank Leumi Credit Facility”). The Bank Leumi Credit Facility was required to be repaid in full by the Company no later than April 1, 2015 and Bank Leumi’s consent was required for early repayment. The drawn portion of the Bank Leumi Credit Facility bore interest at an annual rate of LIBOR plus 5.25% on the basis of a 365-day year, until paid in full. The Company drew the entire $1,000 Bank Leumi Credit Facility. The Company paid Bank Leumi a facility fee of $20 in connection with the facility. To secure the repayment of the Bank Leumi Credit Facility, the Company granted Bank Leumi (i) a first ranking fixed charge over the Company’s goodwill; and (ii) a first ranking floating charge over all of the assets and rights of any type whatsoever, which the Company now has or may acquire in the future, subject to the rights of the OCS and the BIRD Foundation and the rights under existing and future liens in favor of the First Intentional Bank of Israel Ltd. securing debt or indentures of up to an aggregate amount of $100. On March 16, 2015, the Company repaid all amounts outstanding under the Bank Leumi Credit Facility with the proceeds of the IPO and concurrent Private Placement.
|
F - 16
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 6 -
|
EMPLOYEE BENEFITS AND PAYROLL ACCRUALS
|
|
|
A.
|
Composition:
|
|
December 31,
|
||||||||
|
2 0 1 5
|
2 0 1 4
|
|||||||
|
Short-term employee benefits:
|
||||||||
|
Benefits for vacation and recreation pay
|
275 | 332 | ||||||
|
Liability for payroll, bonuses and wages
|
963 | 790 | ||||||
| 1,238 | 1,122 | |||||||
|
|
B.
|
Post-employment Benefits
|
|
|
Pursuant to Israel’s Severance Pay Law, Israeli employees are entitled to severance pay equal to one month’s salary for each year of employment, or a portion thereof. All of the Company's employees elected to be included under section 14 of the Severance Pay Law, 1963 (“section 14”). According to this section, employees are entitled only to monthly deposits, at a rate of 8.33% of their monthly salary, made in their name with insurance companies. Payments in accordance with section 14 release the Company from any future severance payments (under the above Israeli Severance Pay Law) in respect of those employees; therefore, related assets and liabilities are not presented in the balance sheet.
|
|
|
C.
|
Short-term employee benefits
|
|
|
(1)
|
Paid vacation days
|
|
|
In accordance with the Yearly Vacation Law-1951 (the "Vacation Law"), the Company's employees are entitled to a number of paid vacation days for each year of employment. In accordance with the Vacation Law and its appendix, and as determined in the agreement between the Company and the employees, the number of vacation days per year to which each employee is entitled is based on the seniority of the employee.
|
|
|
The employee may use vacation days based on his or her needs and with the Company's consent, and to accrue the remainder of unused vacation days, subject to the provision of the Vacation Law. The vacation days utilized first are those credited for the current year and subsequently from any balance transferred from the prior year (on a “LIFO” basis). An employee who ceased working before utilizing the balance of vacation days accrued is entitled to payment for the balance of unutilized vacation days.
|
|
|
(2)
|
Related parties
|
|
|
For information regarding short-term employee liabilities given to related parties see Note 16.
|
F - 17
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 7 -
|
INCOME TAXES
|
|
|
A.
|
The Company
|
|
|
Check-Cap Ltd. is taxed according to Israeli tax laws:
|
|
|
1.
|
Corporate tax rates in Israel
|
|
|
The Israeli corporate tax rate was 26.5% in the years 2015 and 2014 and 25% from year 2016 onwards.
|
|
|
2.
|
The Law for the Encouragement of Capital Investments, 1959 (the “Investments Law”)
|
|
|
Under the Investments Law, including Amendment No. 60 to the Encouragement of Capital Investments Law as published in April 2005, by virtue of the “Approved Enterprise” or “Benefited Enterprise” status the Company is entitled to various tax benefits as follows:
|
|
|
a)
|
Reduced tax rates
|
|
|
|
The Company has one Benefited Enterprise program under the Investments Law, which entitles it to certain tax benefits with respect to income to be derived from the Company's Benefited Enterprise. During the benefits period, taxable income from its Benefited Enterprise program (once generated) will be tax exempt for a period of ten years commencing with the year the Company will first earn taxable income relating to such enterprise. The Company chose 2010 as the year of election (the "Year of Election"). Due to the location of the Company's offices, the Company believes it is entitled to a 10 year benefit period, subject to a 14 year limitation from the Year of Election, and therefore, the tax benefit period will in any event end in 2023.
|
|
|
b)
|
Conditions for entitlement to the benefits
|
|
|
|
The benefits available to a Benefited Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations.
|
|
|
c)
|
Amendment of the Law for the Encouragement of Capital Investments, 1959
|
|
|
|
The Encouragement of Capital Investments Law was amended as part of the Economic Policy Law for the years 2011-2012, which was passed by the Israeli Knesset on December 29, 2010 (the “Capital Investments Law Amendment”).
|
|
|
|
The Capital Investments Law Amendment sets alternative benefit tracks to those currently in effect under the provisions of the Encouragement of Capital Investments Law.
|
|
|
|
Thebenefits granted to the Benefited Enterprises will be unlimited in time, unlike the benefits granted to special Benefited enterprises, which will be limited for a 10-year period. The benefits shall be granted to companies that will qualify under criteria set in the law; for the most part, those criteria are similar to the criteria that were set in the Encouragement of Capital Investments Law prior to its amendment.
|
F - 18
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 7 -
|
INCOME TAXES (Cont.)
|
|
|
2.
|
The Law for the Encouragement of Capital Investments, 1959 (the “Investments Law”) (Cont.)
|
|
|
c.
|
Amendment of the Law for the Encouragement of Capital Investments, 1959 (Cont.)
|
|
|
|
Under the transitional provisions of the Encouragement of Capital Investments Law, the Company is entitled to take advantage of the tax benefits available under the Encouragement of Capital Investments Law prior to its amendment until the end of the benefits period, as defined in the Encouragement of Capital Investments Law. The Company will be allowed to set the “year of election” no later than tax year 2012, provided that the minimum qualifying investment was made not later than the end of 2010. On each year during the benefits period, the Company will be able to elect that the Capital Investments Amendment apply to the Company, thereby making the tax rates described above available to the Company. An election to have the Capital Investments Amendment apply is irrecoverable. The Company elected not to have the Capital Investments Amendment apply to the Company.
|
|
|
3.
|
In accordance with the Income Tax Ordinance, as of December 31, 2015, all of Check-Cap Ltd.’s tax assessments through tax year 2010 are considered final.
|
|
|
4.
|
The Company did not record current taxes for the years ended December 31, 2013, 2014 and 2015 since it had no taxable income.
|
|
|
5.
|
The NOL carry-forward of the Company equal to approximately $23.6 million.
|
|
|
B.
|
Check-Cap U.S. Inc.
|
|
|
1.
|
Check-Cap U.S. Inc. is taxed according to U.S. tax laws. The Company’s income is taxed in the U.S. at the rate of up to 39%.
|
|
|
2.
|
The NOL carry-forward of Check-Cap U.S. Inc equal to approximately $11.
|
|
|
C.
|
Deferred income taxes
|
|
|
In assessing the realization of deferred tax assets, the Company considers whether it is more likely than not that all or some portion of the deferred tax assets will not be realized. Based on the Company’s history of losses, the Company established a full valuation allowance.
|
|
December 31,
|
||||||||
|
2 0 1 5
|
2 0 1 4
|
|||||||
|
Carry forward tax losses
|
23,565 | 16,800 | ||||||
|
Less valuation allowance
|
(23,565 | ) | (16,800 | ) | ||||
| - | - | |||||||
F - 19
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 7 -
|
INCOME TAXES (Cont.)
|
|
|
D.
|
Reconciliation of the theoretical tax expense to actual tax expense
|
|
|
The main reconciling item between the statutory tax rate of the Company and the effective rate is the provision for full valuation allowance in respect of tax benefits from carry forward tax losses due to the uncertainty of the realization of such tax benefits (see above).
|
|
NOTE 8 -
|
COMMITMENTS AND CONTINGENT LIABILITIES
|
|
|
A.
|
Royalties provision
|
|
|
1.
|
Royalties to an ASIC designer
|
|
|
The Company has a liability to pay royalties for the development of an ASIC component which is used as an amplifier for the capture of signals at low frequencies from X-ray detectors contained in the capsule. The institution that developed the ASIC is entitled to a receive royalties from the Company in the amount of € 0.5 ($0.54) for every ASIC component that the Company will sell, capped at € 200 ($218). This royalty is considered a liability of the Company for the development of the ASIC component.
|
|
|
The royalties liability is calculated based on estimated future sales generated by products which includes the ASIC component. As of December 31, 2015, the Company believes that it will be required to pay the above mentioned royalties, and accordingly, recorded, as of December 31, 2015, a provision in a total amount of $159.
|
|
|
2.
|
Reimbursement liability to Predecessor Entity’s unit holders
|
|
|
On May 31, 2009, the Company entered into an asset transfer agreement with Check Cap LLC (the “Predecessor Entity”), a company with the same shareholders as the Company at the time of transfer. According to the agreement, the Predecessor Entity transferred all of its business operations and substantially all of its assets to the Company, including development and consulting agreements, cash, property and equipment and intangible ownership rights, free of any debt.
|
|
|
As part of the reorganization the Company committed to reimburse the unit holders of the Predecessor Entity for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unit holders of the Predecessor Entity under Section 367(d) of the Code and is based in part on the forecasted sales of the Company. The contingent liability was calculated using the expected cash outflows discounted using a discount factor commensurate with the risk of the Company. Any updates in the expected cash outflows and the liability will be charged to earnings. As of December 31, 2015, the balance of the reimbursement liability totaled $418.
|
|
December 31,
|
||||||||
|
2 0 1 5
|
2 0 1 4
|
|||||||
|
Royalties to an ASIC designer
|
159 | 151 | ||||||
|
Reimbursement liability to Predecessor Entity’s unit holders
|
418 | 393 | ||||||
| 577 | 544 | |||||||
F - 20
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 8 -
|
COMMITMENTS AND CONTINGENT LIABILITIES (Cont.)
|
|
|
B.
|
Commitments
|
|
|
(1)
|
Royalties
|
|
|
The Company’s research and development efforts are financed, in part, through funding from the OCS and BIRD Foundation. Since the Company’s inception through December 31, 2015, the Company received funding from the OCS and BIRD Foundation in the total amount of $3.6 million and $127, respectively. According to the terms of the grants, the OCS is entitled to royalties equal to 3-5% (or at an increased rate under certain circumstances) of the sales of the product funded, up to the full principal amount (which may be increased under certain circumstances) of the U.S. Dollar-linked value of the grants, plus interest at the rate of 12-month LIBOR. The obligation to pay these royalties is contingent on actual sales of the applicable products and in the absence of such sales, no payment is required.
|
|
|
As of December 31, 2015, we had a contingent obligation to the OCS in the amount of $3.8 million.
|
|
|
On July 13, 2014, the Company entered into a Cooperation and Project Funding Agreement with the BIRD Foundation and Synergy, pursuant to which the BIRD Foundation has agreed to award a grant in the maximum amount of the lesser of (i) $900; and (ii) 50% of the actual expenditures for the funding of a project entitled “Collection & Analysis of Gastrointestinal Images for Diagnostic Adenomatic Polyps and Colorectal Cancer.” The development work is expected to be performed over a 24 month period by Synergy (or a subcontractor on its behalf) and the Company.
|
|
|
According to the terms of the grant, the BIRD Foundation is entitled to royalties equal to 5% of the sales of the product funded, up to 100%, 113%, 125%, 138% and 150% of the U.S. Consumer Price Index linked sum granted by the BIRD Foundation if repaid within one year, two years, three years, four years and five or more years, respectively. Under the terms of the agreement, if any portion of the product funded by the project is sold outright to a third party prior to full repayment of the grant to the BIRD Foundation, one-half of the sale proceeds will be applied to the repayment of the grant. If the funded product is licensed to a third party, 30% of all payments received under the respective license agreement must be paid to the BIRD Foundation in repayment of the grant.
|
|
|
As of December 31, 2015, the Company had not paid any royalties to the OCS and BIRD.
|
|
|
(2)
|
Rental agreement
|
|
|
The Company leases approximately 610 square meters in Isfiya, Israel under a lease agreement providing for a term of 10 years, commenced on June 1, 2012 and ending on May 31, 2022. In December 2015 the Company increased the leased area by an additional 290 square meters for clinical and development purposes, to a total of 900 square meters, under an addendum to the lease agreement with similar terms as in the Company's current lease agreement. The Company has the right to terminate the agreement at any time, upon as least 60 days prior written notice. The rental expenses recorded by the Company for the years ended December 31, 2015, 2014 and 2013 were $67, $60 and $56, respectively.
|
|
|
(3)
|
Agreements for brokerage services
|
|
|
See Note 10E(2)(d).
|
F - 21
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 8 -
|
COMMITMENTS AND CONTINGENT LIABILITIES (Cont.)
|
|
|
B.
|
Commitments (Cont.)
|
|
|
(4)
|
Vehicle Lease and Maintenance Agreements
|
|
|
The Company entered into several 32-36 months lease and maintenance agreements for vehicles which are regularly amended as new vehicles are leased. The current monthly lease fees aggregate approximately $14. The expected lease payments for the years ending December 31, 2016, 2017 and 2018 are approximately $131, $98, $16, respectively.
|
|
Operating lease obligations as of December 31, 2015:
|
||||
|
Lease agreement for vehicles
|
29 | |||
|
Lease agreement for office facilities lease
|
13 | |||
| 42 | ||||
|
|
C.
|
Legal
|
|
|
As of the date of the financial statements, the Company has not been and is not a party to any pending litigation.
|
|
NOTE 9 -
|
FAIR VALUE MEASUREMENTS
|
|
|
Financial instruments measured at fair value on a recurring basis include warrants for preferred share. The warrants are classified as a liability in accordance with ASC No. 480 "Distinguishing Liabilities From Equity" (see Notes 10D(3) and 10D(4)). These warrants were classified as level 3 in the fair value hierarchy since some of the inputs used in the valuation were determined based on management’s assumptions. The fair value of the warrants on the issuance date and on subsequent reporting dates was determined using option-pricing-model utilizing the assumptions noted below. The fair value of the underlying preferred share price was determined by the board of directors considering, among others, third party valuations. The valuation of the Company was performed using a discounted cash flow model. The option-pricing-model method was then employed to allocate the enterprise value among the Company’s various equity classes, deriving a fully marketable value per share for the preferred share. The expected terms of the warrants were based on the remaining contractual expiration period. The expected share price volatility for the shares was determined by examining the historical volatilities of a group of the Company’s industry peers as there is no trading history of the Company’s shares. The risk-free interest rate was calculated using the average of the published interest rates for U.S. Treasury zero-coupon issues with maturities that approximate the expected term. The dividend yield assumption was zero as there is no history of dividend payments.
|
F - 22
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 9 -
|
FAIR VALUE MEASUREMENTS (Cont.)
|
The following assumptions were used to estimate the value of the warrants to purchase series C1 and C2 convertible preferred shares:
|
February 24, 2015
(conversion date)
|
December 31,
|
|||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Expected volatility
|
55.69 | % | 51.7 | % | 44 | % | ||||||
|
Risk-free rate
|
1.21 | % | 1.58 | % | 0.13 | % | ||||||
|
Dividend yield
|
0 | % | 0 | % | 0 | % | ||||||
|
Expected term (in years)
|
4.17 | 4.20 | 3.42 | |||||||||
On November 22, 2014 and January 21, 2015, 18,586 and 8,366 warrants to purchase series C2 preferred shares expired, respectively. The remaining 41,822 warrants to purchase series C1 preferred shares and 50,399 warrants to purchase series C2 preferred shares were converted to 92,221 warrants to purchase ordinary shares.
The following assumptions were used to estimate the value of the warrants to purchase series D1 and D2 preferred shares:
|
December 31,
|
||||||||
|
2 0 1 4
|
2 0 1 3
|
|||||||
|
Expected volatility
|
60.12 | % | 44 | % | ||||
|
Risk-free rate
|
0.05 | % | 0.18 | % | ||||
|
Dividend yield
|
0 | % | 0 | % | ||||
|
Expected term (in years)
|
0.21 | 1.21 | ||||||
On February 24, 2015 all of the warrants to purchase preferred shares were converted into warrants to purchase ordinary shares (See note 1D) and lost their anti-dilution protection provision. As a result, these warrants were converted into equity. Series D1 and D2 warrants expired on March 17, 2015. The Company's management estimated that the value of these warrants as of February 24. 2015 (conversion date) was immaterial.
The change in the fair value of warrants to purchase preferred shares is summarized below:
|
December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Balance at the beginning of year
|
407 | 3,926 | 4,549 | |||||||||
|
Conversion to warrants to purchase ordinary share immediately prior to the IPO
|
(233 | ) | - | - | ||||||||
|
Change in fair value
|
(174 | ) | (3,519 | ) | (623 | ) | ||||||
|
Balance at the end of year
|
- | 407 | 3,926 | |||||||||
F - 23
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 10 -
|
SHAREHOLDERS' EQUITY (DEFICIENCY)
|
|
|
A.
|
All the ordinary and preferred shares, options and warrants exercise prices, price per share and loss per share amounts have been adjusted retroactively for all periods presented in these financial statements, to reflect the 1 for 20 reverse stock split effected immediately prior to the consummation of the IPO on February 24, 2015 (See Note 1C).
|
|
|
B.
|
See Note 1D regarding the conversion on a 1:1 basis, of each and every class and series of the Company's authorized and outstanding preferred shares into the Company’s Pre-Split Ordinary Shares and the conversion on a 1:1 basis of all outstanding preferred share warrants into warrants to purchase Pre-Split Ordinary Shares, each of which occurred immediately prior to the consummation of the IPO on February 24, 2015.
|
|
|
C.
|
Composition of preferred share capital and ordinary share capital:
|
|
Authorized
|
Issued and outstanding
|
|||||||||||||||
|
December 31,
|
December 31,
|
|||||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 5
|
2 0 1 4
|
|||||||||||||
|
Number of shares of NIS 0.2 par value (in thousands)
|
||||||||||||||||
|
Ordinary shares
|
57,500 | 43,358 | 11,812 | 1,152 | ||||||||||||
|
Preferred shares:
|
||||||||||||||||
|
Series A Preferred shares
|
- | 338 | - | 338 | ||||||||||||
|
Series B Preferred shares
|
- | 338 | - | 338 | ||||||||||||
|
Series C-1 Preferred shares
|
- | 875 | - | 821 | ||||||||||||
|
Series C-2 Preferred shares
|
- | 1,592 | - | 1,489 | ||||||||||||
|
Series C-3 Preferred shares
|
- | 1,500 | - | - | ||||||||||||
|
Series D-1 Preferred shares
|
- | 4,000 | - | 1,227 | ||||||||||||
|
Series D-2 Preferred shares
|
- | 3,000 | - | - | ||||||||||||
|
Series D-3 Preferred shares
|
- | 2,250 | - | 126 | ||||||||||||
|
Series D-4 Preferred shares
|
- | 250 | - | - | ||||||||||||
|
|
D.
|
Preferred shares
|
|
|
(1)
|
The preferred shares provide their owners with similar rights to the ordinary shares with a liquidation preference. In the occurrence of a Liquidation Event (as such term was defined in the Company's Articles of Association), the preferred shares confer upon their holders preference rights, such that each preferred share entitles its holder to be paid out of the assets of the Company available for distribution to its shareholders, an amount per preferred share equal to its original issue price, plus a yield of 8% per annum.
|
|
|
(2)
|
Of the warrants issued to the lead investor, warrants to purchase 41,822 Series C1 preferred shares are exercisable at $4.99 per share and warrants to purchase 50,399 Series C2 preferred shares are exercisable at $5.38 per share. Prior to the IPO these warrants were classified as a liability in accordance with ASC 480 (see Note 9). Immediately prior to the Company's IPO upon the conversion of the warrants to purchase preferred shares into warrants to purchase ordinary shares, these warrants were converted into equity (See Note 9)
|
F - 24
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 10 -
|
SHAREHOLDERS' EQUITY (DEFICIENCY) (Cont.)
|
|
|
D.
|
Preferred shares (Cont.)
|
|
|
(3)
|
In consideration for brokerage services in connection with the series C preferred share investment: (i) on December 15, 2009, the Company issued warrants to purchase 18,586 preferred C2 shares, with an exercise price of $5.38 per share, which expired on November 22, 2014; and (ii) on April 27, 2010, the Company issued warrants to purchase 8,366 preferred C2 shares, with an exercise price of $5.38 per share, which expired on January 21, 2015. The warrants were classified as a liability in accordance with ASC No. 480 (see Note 9).Immediately prior to the Company's IPO upon the conversion of the warrants to purchase preferred shares into warrants to purchase ordinary shares, these warrants were converted into equity.
|
|
|
(4)
|
On March 17, 2011, the Company issued warrants to purchase 810,013 preferred D2 shares to series D1 investors, warrants to purchase 20,570 preferred D2 shares and warrants to purchase 25,196 preferred D1 shares in consideration for brokerage services. The warrants are classified as a liability in accordance with ASC No. 480 (see Note 9).
|
|
|
On February 24, 2015, these warrants were converted to equity and expired on March 17, 2015.
|
|
|
(5)
|
Immediately prior to the consummation of the IPO, on February 24, 2015, 4,338,998 preferred shares were converted into 4,338,998 Post-Split Ordinary Shares and warrants to purchase 948,000 preferred shares were converted into warrants to purchase 948,000 Post-Split Ordinary Shares. Therefore, as of the date hereof, the Company’s share capital is comprised solely of ordinary shares and options and warrants to purchase ordinary shares.
|
|
|
E.
|
Ordinary shares
|
|
|
(1)
|
The ordinary shares provide their owners with rights to receive dividends in cash and shares, and rights to participate at the time of distributing liquidation dividends, subject to the preferential rights of the preferred shareholders, as described below. Additionally, the ordinary shareholders have the right to vote in shareholders’ assemblies in a manner that each share provides one voting right to its holder.
|
F - 25
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 10 -
|
SHAREHOLDERS' EQUITY (DEFICIENCY) (Cont.)
|
|
|
E.
|
Ordinary shares (Cont.)
|
|
|
(2)
|
Changes in ordinary share capital:
|
|
Description
|
Amount raised, gross
|
Shares issued
|
Price per share
|
Warrants issued
|
|||||||||
|
Ordinary shares on December 31, 2014
|
$ | 59 | 1,152,138 | - | |||||||||
|
Exercise of Anti-dilution Warrants (a)
|
- | 205,599 | - |
390,276 ordinary shares warrants
|
|||||||||
|
Conversion to ordinary shares immediately prior to the IPO (Note 1D)
|
$ | 20,197 | 4,338,998 | - | |||||||||
|
Credit Line Warrants (d)
|
- | - | - |
2,658,463 ordinary shares warrants to CLA investors
|
|||||||||
|
Exercise of options (c)
|
$ | 16 | 307,467 | $ | 0.05 | ||||||||
|
Exercise of Credit Line Warrants (e)
|
$ | 29 | 1,557,507 | $ | 0.02 | ||||||||
|
IPO (b)
|
$ | 13,500 | 2,250,000 | $ | 6.00 |
1,125,000 Series A warrants, 3,375,000 Long Term Incentive Warrants (g); 100,000 warrants issued to lead underwriter and 15,000 warrants issued to legal counsel (f)
|
|||||||
|
Private Placement (d)
|
$ | 12,000 | 2,000,000 | $ | 6.00 |
1,000,000 Series A warrants and 3,000,000 Long Term Incentive Warrants issued to investors; and 87,127 warrants issued for brokerage services
|
|||||||
|
Subtotal
|
$ | 45,801 | |||||||||||
|
Less: Issuance expenses
|
$ | (3,849 | )* | ||||||||||
|
December 31, 2015
|
$ | 41,952 | |||||||||||
(*)Issuance expenses do not include certain warrants with value of $321 issued in connection with the IPO and the Private Placement.
|
|
a)
|
On May 11, 2010, the Company issued, free of charge, to all of its shareholders (except for certain ordinary shareholders), warrants to purchase an aggregate of 390,276 ordinary shares (hereafter- "Anti-dilution Warrants"). The Anti-dilution Warrants were issued in order to prevent the dilution of the holdings of such Company shareholders due to certain options granted to the Company's CEO (hereafter- "CEO options"). The Anti-dilution Warrants will be automatically exercised, without consideration (unless the holder thereof objects to such exercise), upon the exercise by the Company's CEO of the CEO Options. The fair value of the Anti-dilution Warrants on the grant date is immaterial. Immediately prior to the consummation of the IPO, the CEO exercised the CEO options and 171,715 Anti-dilution Warrants were automatically exercised and additional 33,884 were exercised following the IPO and prior to December 31, 2015. As of December 31, 2015 there are 180,192 Anti-dilution Warrants outstanding.
|
F - 26
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 10 -
|
SHAREHOLDERS' EQUITY (DEFICIENCY) (Cont.)
|
|
|
E.
|
Ordinary shares (Cont.)
|
|
|
(2)
|
Changes in ordinary share capital (Cont.):
|
|
|
b)
|
On February 24, 2015, the Company consummated an IPO in the U.S. of 2,000,000 units at a public offering price of $6 per unit, before underwriting discounts and offering expenses. Each unit consisted of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share. Each unit was issued with one and one-half non-transferrable Long Term Incentive Warrants. Each whole Series A Warrant entitles the holder to purchase one ordinary share at an exercise price of $7.50. Upon vesting, each Long Term Incentive Warrant will entitle the holder to purchase one ordinary share at an exercise price of $6.90.
|
|
|
|
The Company granted the underwriters a 45-day over-allotment option to purchase up to 300,000 additional units (together with an accompanying 450,000 Long Term Incentive Warrants). The option to purchase additional 100,000 units was partially exercised on March 6, 2015. The units were separated into one ordinary share and one-half of a Series A Warrant to purchase one ordinary share on March 18, 2015, and the units ceased to exist as of such date. On April 6, 2015, the option to purchase additional 150,000 ordinary shares and 75,000 Series A Warrants was partially exercised. The Company received net proceeds from the IPO and partial exercise of the over-allotment option of approximately $10.8 million (net of issuance cost of approximately $2.9 million, including certain warrants with value of $196 issued in connection with the IPO). The Company's ordinary shares and Series A Warrants are listed on the NASDAQ Capital Market under the symbols “CHEK,” and “CHEKW,” respectively.
|
|
|
c)
|
Immediately prior to the consummation of the IPO, certain members of the Company's management exercised 307,467 options granted to them under the 2006 Unit Option Plan.
|
|
|
d)
|
On August 20, 2014, the Company entered into a certain credit line agreement, pursuant to which it obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders (the “Lenders”). The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014. The Company issued to each Lender at closing a warrant (collectively, the “Credit Line Warrants”), to purchase a number of the Company’s ordinary shares constituting 2% of its share capital on a fully diluted basis (assuming conversion of all of the Company’s convertible securities into ordinary shares at a 1:1 conversion rate) as of the closing for each $1 million (or portion thereof) extended by such Lender. The Company issued Credit Line Warrants (“CLA Warrants”) to purchase in the aggregate 2,658,463 of its ordinary shares. The CLA Warrants are exercisable for a period of ten years at an exercise price of NIS 0.2 per share, and may be exercised on a net issuance basis. The CLA Warrants issued are considered outstanding and are exercisable at any time from the date issued with an exercise price of nominal amount.
|
|
|
|
Under the terms of the credit line agreement, the Company directed that the entire credit line amount be invested in a Private Placement, from the lenders that was deposited in escrow and consummated concurrently with the consummation of the IPO on February 24, 2015. The Company issued to the Lenders 2,000,000 units at a price of $6 per unit, before issuance cost. Each unit consisted of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share. Each unit was issued with one and one-half non-transferrable Long Term Incentive Warrants. Each whole Series A Warrant entitles the holder to purchase one ordinary share at an exercise price of $7.50. Upon vesting, each Long Term Incentive Warrant will entitle the holder to purchase one ordinary share at an exercise price of $6.90. The Company received net proceeds from the Private Placement of approximately $10.9 million, (net of issuance cost of approximately $1.2 million, including certain warrants with value of $125 issued in connection with the Private Placement).
|
F - 27
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
|
NOTE 10 -
|
SHAREHOLDERS' EQUITY (DEFICIENCY) (Cont.)
|
|
|
E.
|
Ordinary shares (Cont.)
|
|
|
2)
|
Changes in ordinary share capital (Cont.):
|
|
|
d)
|
(Cont.):
|
|
|
|
Certain finders engaged by the Company in connection with the credit line agreement and upon the closing of the Private Placement, the Company issued warrants to purchase 70,010 ordinary shares, with an exercise price of $5.06 per share and exercisable until the end of two years from grant date, and warrants to purchase 17,117 ordinary shares, with an exercise price of NIS 0.2 ($0.05) per share and exercisable until the end of two years from grant date. These grants were accounted for as a deduction of equity. In addition, the brokerage services providers received $0.9 million in cash commission.
|
|
|
e)
|
Certain Private Placement investors exercised warrants to purchase an aggregate 569,355 ordinary shares of the Company at a price per share equal to NIS 0.2 ($0.05) and warrants to purchase an aggregate 988,152 ordinary shares of the Company on a cashless basis, which resulted in the expiration of 24,277 CLA Warrants.
|
|
|
f)
|
Upon the closing of the IPO, the Company issued warrants to purchase 100,000 ordinary shares at an exercise price of $7.50 to the IPO lead underwriter and warrants to purchase 15,000 ordinary shares at an exercise price of $6 to the Company's U.S. legal counsel.
|
|
|
g)
|
On June 24, 2015, the Company entered into Amendment No. 1 to the Warrant Agreement, dated June 24, 2015, between the Company and American Stock Transfer & Trust Company LLC, as Warrant Agent, to extend the Registration Due Date to the date which is 180 days following the date of closing of the Company’s initial public offering (i.e., August 23, 2015) in order to allow the shareholders who were the original purchasers of IPO Units additional time to become the direct registered owners of the ordinary shares underlying the IPO Units. On August 23, 2015, 1,298,374 Long Term Incentive Warrants expired.
|
|
NOTE 11 -
|
SHARE-BASED COMPENSATION
|
|
|
A.
|
General
|
|
|
(1)
|
In connection with the transfer of all of the business operations and substantially all of the assets of Check-Cap LLC to the Company in 2009, the Company assumed the Check-Cap LLC 2006 Unit Option Plan (hereafter: the "2006 Plan”). According to the 2006 Plan, the Company is authorized to grant options to purchase ordinary shares of the Company to employees, directors and consultants of the Company. The options granted according to the 2006 Plan are generally exercisable for 10 years from the grant date unless otherwise determined by the Company’s Board of Directors, vest over a period to be determined by the Company’s Board of Directors, and have an exercise price to be determined by the Company’s Board of Directors.
|
|
|
(2)
|
On January 15, 2015, the Board of Directors resolved to increase the number of ordinary shares by 4% of the Company's fully-diluted share capital (including the option pool) immediately following the consummation of the IPO. As a result, the number of the Company's ordinary shares reserved for issuance under the 2006 Plan increased by 967,747.
|
F - 28
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
A.
|
General (Cont.)
|
|
|
(3)
|
On April 6, 2015, the Company’s Board of Directors resolved to increase the number of ordinary shares of the Company's reserved for issuance under the 2006 Plan by 1,718,540 shares.
|
|
|
(4)
|
On August 13, 2015, the shareholders approved and adopted the Check-Cap Ltd. 2015 Equity Incentive Plan (the “2015 Israeli Plan”) and the Check-Ltd. 2015 United States Sub-Plan to Check-Cap Ltd. 2015 Equity Incentive Plan (the “2015 U.S. Sub-Plan” and together with the 2015 Israeli Plan, the “2015 Plan”). As of such date, the Company ceased to grant options under the 2006 Plan. All of the remaining shares authorized but unissued under the 2006 Plan were rolled over to the 2015 Plan.
|
|
|
B.
|
Details of share-based grants made by the Company
|
|
|
The following tables presents the grant dates, number of underlying shares and related exercise prices of awards granted to employees and non-employees during the years 2015, 2014, 2013 as well as the estimated fair value of the underlying ordinary shares on the grant date:
|
|
Grant date
|
No. of options
|
Expiration date
|
Exercise price
|
Fair value on grant date
|
|||||||||
|
May 15, 2013 (1)
|
38,473 |
February 24, 2025
|
$ | 4.96 | $ | 3.04 | |||||||
|
June 6, 2013 (2)
|
117,750 |
June 12, 2023
|
$ | 4.96 | $ | 3.04 | |||||||
|
October 14, 2014 (3)
|
581,542 |
October 14, 2024
|
(* | ) | $ | 3.01 | |||||||
|
February 24, 2015 (6)
|
302,420 |
February 24, 2025
|
$ | 5.06 | $ | 2.73 | |||||||
|
March 15, 2015 (7)
|
60,484 |
March 15, 2025
|
$ | 5.08 | $ | 2.71 | |||||||
|
May 19, 2015 (8)
|
523,621 |
May 19, 2025
|
$ | 4.57 | $ | 2.50 | |||||||
|
June 1, 2015 (9)
|
48,387 |
June 1, 2025
|
$ | 5.06 | $ | 2.80 | |||||||
|
June 3, 2015 (10)
|
189,043 |
June 3, 2025
|
$ | 4.35 | $ | 2.60 | |||||||
|
August 13, 2015 (11)
|
324,750 |
August 13, 2025
|
$ | 3.68 | $ | 1.80 | |||||||
|
August 13, 2015 (12)
|
425,000 |
August 13, 2025
|
$ | 3.68 | $ | 1.85 | |||||||
|
October 20, 2015 (13)
|
38,369 |
October 20, 2025
|
$ | 2.79 | $ | 1.72 | |||||||
|
Grant date
|
No. of warrants
|
Expiration date
|
Exercise price
|
Fair value on grant date
|
|||||||||
|
February 18, 2015
|
100,000 |
February 18, 2019
|
$ | 7.50 | $ | 1.62 | |||||||
|
February 24, 2015
|
15,000 |
February 24, 2019
|
$ | 6.00 | $ | 2.25 | |||||||
|
October 14, 2014 (4)
|
221,539 |
October 14, 2022
|
(* | ) | $ | 3.01 | |||||||
|
Various dates in 2015 (5)
|
87,127 |
Various dates in 2017
|
(** | ) | $ | 1.43 | |||||||
(*) See Note 11B(3)
(**) See Note 11B(6)
|
|
(1)
|
38,473 ordinary shares issuable upon the exercise of options with an exercise price of $4.96 per ordinary share which the Company agreed that certain executive officers will be entitled to upon completion of the IPO. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $116. This amount is charged to statement of operations over the vesting periods.
|
F - 29
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
B.
|
Details of share-based grants made by the Company (Cont.)
|
|
|
(2)
|
On June 6, 2013, the Company’s Board of Directors approved the grant of options to purchase 117,750 ordinary shares, at an exercise price of $4.96, to employees and consultants of the Company. Of such options, options to purchase 16,147 ordinary shares were vested on the grant date, options to purchase 67,583 ordinary shares will vest over two years, options to purchase 25,688 ordinary shares will vest over three years and options to purchase 8,332 ordinary shares will vest over four years. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $84. This amount is charged to statement of operations over the vesting periods.
|
|
|
(3)
|
On October 14, 2014, the Company’s Board of Directors approved the grant of options to purchase 581,542 ordinary shares, at an exercise price of NIS 0.2 per share, to the Company's management. Management subsequently agreed that the exercise price of fifty-percent of such options will increase to equal the price at which the Company's ordinary shares are sold to the public in the IPO, or if units are sold in the IPO, the exercise price per share will increase to be equal to the effective price per share of the ordinary shares underlying the units sold to the public in the offering, and will vest and become exercisable only upon the consummation of the IPO. The remaining options with an NIS 0.2 exercise price will vest on a quarterly basis in eight equal installments during a period of 24 months from issuance. Upon the closing of the IPO on February 24, 2015, any unvested portion of these warrants became fully vested and exercisable. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $1.7 million. This amount is charged to statement of operations over the vesting periods.
|
|
|
(4)
|
In addition, in October 2014, the Company’s Board of Directors approved the grant of warrants to purchase 221,539 ordinary shares NIS 0.2 par value of the Company at an exercise price of NIS 0.2 per share to Pontifax in consideration of their commitment to provide to the Company, for no consideration, business development services and a representative designated by Pontifax to serve as the chairman of the Company's Board of Directors. Pontifax subsequently agreed that the exercise price of fifty-percent of such warrants will increase to equal the price at which the Company's ordinary shares are sold to the public in the IPO or if units are sold in the IPO, the exercise price per share will increase to be equal to the effective price per share of the ordinary shares underlying the units sold to the public in the offering, and will vest and become exercisable only upon the consummation of the IPO prior to their expiration date. The remaining warrants with an NIS 0.2 exercise price will vest on a quarterly basis in eight equal installments during a period of 24 months from issuance. Upon the closing of the IPO on February 24, 2015, any unvested portion of these warrants became fully vested and exercisable. In addition, Pontifax agreed to reduce the term of the warrants such that these warrants will now expire after eight years (instead of ten years) following their issuance, i.e., on October 14, 2022. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $667. This amount is charged to statement of operations over the vesting periods.
|
F - 30
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
B.
|
Details of share-based grants made by the Company (Cont.)
|
|
|
(5)
|
Certain finders engaged by the Company were entitled, according to the terms of their respective engagements with the Company, to be issued warrants to purchase ordinary shares upon and subject to the closing of a Private Placement pursuant to the credit line agreement dated August 20, 2014, as amended. On April 6, 2015, the Company’s Board of Directors approved the grant of warrants to purchase 70,010 ordinary shares, at an exercise price of $ 5.06 per share. On October 20, 2015 the Company’s Board of Directors approved the grant of additional warrants to purchase 17,117 ordinary shares, at an exercise price of NIS 0.2 per share. The warrants included in the two grants were fully (100%) vested on grant date. The compensation payment was based on the fair value on the grant date, and was estimated at approximately $125. This amount is charged to shareholders' equity as Private Placement issuance cost.
|
|
|
(6)
|
On January 15, 2015, the Company’s shareholders approved the grant of options to certain members of the Company's Board of Directors to purchase 302,420 ordinary shares, at an exercise price equal to the effective price per share of the ordinary shares underlying the units sold to the public in the IPO. Following the IPO the effective exercise price was determined at$5.06 per share. The options shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $826. This amount is charged to statement of operations over the vesting periods.
|
|
|
(7)
|
On April 6, 2015, the Company’s shareholders approved the grant of options to purchase 60,484 ordinary shares, at an exercise price of $5.08, to a member of the Company's Board of Directors. The options shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $164. This amount is charged to statement of operations over the vesting periods.
|
|
|
(8)
|
On May 19, 2015 the Company's shareholders approved the grant of options to purchase 463,137 ordinary shares, at an exercise price of $4.57, to the Company's president. The options shall vest over a period of four years commencing on the date of grant, such that 25% of the options shall vest on the first anniversary of the date of grant and thereafter, the remaining options will vest in monthly installments.
|
|
|
In addition, the Company’s shareholders approved the grant of options to purchase 60,484 ordinary shares, at an exercise price of $4.57, to a member of the Company's Board of Director. The options shall vest over a period of three years commencing on the date of grant in quarterly installments.
|
|
|
The compensation expense was based on the fair value on the grant date, and was estimated at approximately $1.3 million. This amount is charged to statement of operations over the vesting periods.
|
|
|
(9)
|
On February 12, 2015, the Company's shareholders approved the grant of options to purchase 48,387 ordinary shares, at an exercise price of $5.06, to a member of the Company's Board of Directors, in consideration for certain business development services in Asia under a consulting agreement. The options shall vest over a period of three years commencing on the date of grant in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $136. This amount is charged to statement of operations over the vesting periods.
|
F - 31
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
B.
|
Details of share-based grants made by the Company (Cont.)
|
|
|
(10)
|
On June 3, 2015, the Company’s Board of directors approved the grant of options to purchase 189,043 ordinary shares, at an exercise price of $4.35, to employees and consultants of the Company The compensation expense was based on the fair value on the grant date, and was estimated at approximately $491. This amount is charged to statement of operations over the vesting periods.
|
|
|
(11)
|
On August 13, 2015 the Company's Board of Directors approved the grant of options to purchase 324,750 ordinary shares to its CEO at an exercise price of $3.68. The options shall vest over a period of four years commencing on the date of grant, such that 25% of the options shall vest on the first anniversary of the date of grant and thereafter, the remaining options will vest in monthly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $586. This amount is charged to statement of operations over the vesting periods.
|
|
|
(12)
|
On August 13, 2015 the Company's Board of Directors approved the grant of options to purchase up to 425,000 ordinary shares to its CEO at an exercise price of $3.68. The options shall vest over a period of four years commencing on the date of grant, such that on each of the four anniversaries after the date of grant a number of options will vest and become exercisable calculated as follows: (a) 425,000 multiplied by a quotient equal to the aggregate number of our Series A Warrants and Long Term Incentive Warrants that have been exercised prior to the applicable anniversary measurement date (to be adjusted to reflect any stock splits, reverse splits, bonus shares and the like) divided by 8,500,000, less (b) the aggregate number of such options that vested prior to such vesting measurement date, provided that in no event shall more than 106,250 of such options vest during any 12-month period of his employment (to be accumulated on a `carry-forward` basis). The compensation expense was based on the fair value on the grant date, and was estimated at approximately $ 700. No expenses were recorded during the year ended December 31, 2015.
|
|
|
(13)
|
On October 20, 2015 the Company's Board of Directors approved the grant of options to purchase 38,369 ordinary shares to certain of the Company's employees at an exercise price of $2.79. The options shall vest over a period of four years commencing on the date of grant, such that 25% of the options shall vest on the first anniversary of the date of grant and thereafter, the remaining options will vest in quarterly installments. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $58. This amount is charged to statement of operations over the vesting periods.
|
F - 32
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
C.
|
Options Fair Value
|
|
|
|
Theparameters which were used in applying the model are as follows:
|
|
Share price (in $)
|
Exercise price (in $)
|
Expected volatility
(in %)
|
Option term
(in years)
|
Risk free interest rate
(in %)
|
Anticipated rate of dividends
(in %)
|
|||||||||||||||||||
|
May 15, 2013
|
3.04 | 4.96 | 55-62 | 5-6.5 | 1.47-1.79 | 0 | ||||||||||||||||||
|
June 6, 2013
|
3.04 | 4.96 | 40-60 | 5-10 | 0.75-2.25 | 0 | ||||||||||||||||||
|
October 14, 2014
|
3.01 |
See Note 11B (3).
|
50-60 | 5-6 | 1.45-1.72 | 0 | ||||||||||||||||||
|
February 18, 2015
|
5.06 | 7.50 | 55 | 4 | 1.36 | 0 | ||||||||||||||||||
|
February 24, 2015
|
5.12 | 6 | 56 | 4 | 1.29 | 0 | ||||||||||||||||||
|
February 24, 2015
|
5.12 | 5.06 | 55-62 | 5-6.5 | 1.47-1.79 | 0 | ||||||||||||||||||
|
March 15, 2015
|
5.08 | 5.08 | 55-61 | 5-6.5 | 1.59-1.92 | 0 | ||||||||||||||||||
|
May 19, 2015
|
4.22 | 4.57 | 44-60 | 5.5-7 | 1.62-2.03 | 0 | ||||||||||||||||||
|
June 1, 2015
|
4.18 | 5.06 | 56-61 | 5-6.5 | 1.50-2.20 | 0 | ||||||||||||||||||
|
June 3, 2015
|
4.35 | 4.35 | 56-61 | 5-10 | 1.50-2.20 | 0 | ||||||||||||||||||
|
August 13, 2015
|
3.44 | 3.68 | 55-60 | 5.5-7 | 1.58-1.94 | 0 | ||||||||||||||||||
|
August 13, 2015
|
3.44 | 3.68 | 55-60 | 5.5-7 | 1.58-1.94 | 0 | ||||||||||||||||||
|
October 20, 2015
|
3.00 | 2.79 | 57-61 | 5-10 | 1.75-2.28 | 0 | ||||||||||||||||||
|
|
D.
|
Effect of share-based compensation transactions on the Company's statements of operations
|
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Research and development, net
|
790 | 104 | 40 | |||||||||
|
General and administrative
|
2,934 | 208 | 16 | |||||||||
|
Total
|
3,724 | 312 | 56 | |||||||||
F - 33
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
E.
|
A summary of the Company's option activity related to options granted to employees, service providers and directors, and related information under the 2006 Plan and the 2015 Plan is as follows:
|
|
Year ended December 31, 2015
|
||||||||||||||||
|
Number of options
|
Weighted average of exercise price (in $)
|
Average remaining contractual life (in years)
|
Aggregate intrinsic value ($ in thousands)
|
|||||||||||||
|
Outstanding at the beginning of the year
|
1,299,514 | 2.73 | 7.36 | 1,854 | ||||||||||||
|
Granted
|
1,950,547 | 4.28 | ||||||||||||||
|
Exercised
|
(307,467 | ) | 0.05 | |||||||||||||
|
Forfeited
|
(169,423 | ) | 4.60 | |||||||||||||
|
Outstanding at the end of the year
|
2,773,171 | 4.01 | 7.99 | (5,878 | ) | |||||||||||
|
Vested and expected to vest
|
2,768,825 | 4.01 | 7.98 | (5,874 | ) | |||||||||||
|
Exercisable at the end of the year
|
1,092,544 | 3.89 | 5.94 | (2,180 | ) | |||||||||||
|
Year ended December 31, 2014
|
||||||||||||||||
|
Number of options
|
Weighted average of exercise price (in $)
|
Average remaining contractual life (in years)
|
Aggregate intrinsic value ($ in thousands)
|
|||||||||||||
|
Outstanding at the beginning of the year
|
771,446 | 3.02 | 5.55 | 510 | ||||||||||||
|
Granted
|
581,542 | 2.56 | ||||||||||||||
|
Exercised
|
- | - | ||||||||||||||
|
Forfeited
|
(53,474 | ) | 4.94 | |||||||||||||
|
Outstanding at the end of the year
|
1,299,514 | 2.73 | 7.36 | 1,854 | ||||||||||||
|
Vested and expected to vest
|
1,299,514 | 2.73 | 7.36 | 1,854 | ||||||||||||
|
Exercisable at the end of the year
|
619,057 | 2.92 | 5.15 | 1,854 | ||||||||||||
F - 34
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 11 - SHARE-BASED COMPENSATION (Cont.)
|
|
E (Cont.)
|
|
Year ended December 31, 2013
|
||||||||||||||||
|
Number of options
|
Weighted average of exercise price (in $)
|
Average remaining contractual life (in years)
|
Aggregate intrinsic value ($ in thousands)
|
|||||||||||||
|
Outstanding at the beginning of the year
|
700,428 | 2.72 | 7.08 | 665 | ||||||||||||
|
Granted
|
117,750 | 4.96 | ||||||||||||||
|
Exercised
|
- | - | ||||||||||||||
|
Forfeited
|
(46,732 | ) | 3.42 | |||||||||||||
|
Outstanding at the end of the year
|
771,446 | 3.02 | 5.55 | 510 | ||||||||||||
|
Vested and expected to vest
|
771,446 | 3.02 | 5.55 | 510 | ||||||||||||
|
Exercisable at the end of the year
|
594,916 | 2.84 | 6.01 | 500 | ||||||||||||
The weighted average grant date fair values of options granted during the years ended December 31, 2015, 2014, 2013 were $2.26, $3.01 and $3.04, respectively.
The aggregate intrinsic value in the table above represents the total intrinsic value that would have been received by the option holders had all option holders exercised their options on the last date of the exercise period. No option was exercised during the years ended December 31, 2013 and 2014. The total intrinsic value of options exercised for the year ended December 31, 2015 was $1.5 million. As of December 31, 2015 and 2014, there was $1,896 and $2,198 of total unrecognized compensation cost related to non-vested share-based compensation arrangements granted under the 2006 Plan and the 2015 Plan. This cost is expected to be recognized over a period of up to 4 years.
F - 35
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 12 - RESEARCH AND DEVELOPMENT EXPENSES, NET
Composition:
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Salaries and related expenses
|
3,585 | 2,425 | 2,178 | |||||||||
|
Share-based compensation
|
790 | 104 | 40 | |||||||||
|
Materials
|
608 | 385 | 307 | |||||||||
|
Subcontractors and consultants
|
688 | 294 | 218 | |||||||||
|
Depreciation
|
85 | 72 | 70 | |||||||||
|
Cost for registration of patents
|
153 | 72 | 118 | |||||||||
|
Others
|
282 | 123 | 110 | |||||||||
| 6,191 | 3,475 | 3,041 | ||||||||||
|
Less participation of the OCS and BIRD Foundation
|
(354 | ) | (643 | ) | (148 | ) | ||||||
|
Total research and development, net
|
5,837 | 2,832 | 2,893 | |||||||||
NOTE 13 - GENERAL AND ADMINISTRATIVE EXPENSES
Composition:
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Salaries and related expenses
|
1,830 | 952 | 682 | |||||||||
|
Share-based compensation
|
2,934 | 208 | 16 | |||||||||
|
Professional services
|
609 | 114 | 96 | |||||||||
|
Office rent and maintenance
|
108 | 105 | 104 | |||||||||
|
Depreciation
|
7 | 7 | 7 | |||||||||
|
Others
|
1,138 | 317 | 185 | |||||||||
|
Total general and administrative
|
6,626 | 1,703 | 1,090 | |||||||||
NOTE 14 - FINANCE INCOME (EXPENSES), NET
Composition:
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Interest income on short-term deposits
|
61 | 10 | 59 | |||||||||
|
Bank fees and Interest expenses
|
(47 | ) | (6 | ) | (5 | ) | ||||||
|
Changes in provision for royalties
|
(33 | ) | 415 | (104 | ) | |||||||
|
Exchange rate differences
|
18 | (13 | ) | 31 | ||||||||
|
Revaluation of fair value of warrants to purchase preferred share
|
174 | 3,519 | 623 | |||||||||
|
Total financing income
|
173 | 3,925 | 604 | |||||||||
F - 36
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 15 - LOSS PER SHARE
Basic loss per share is computed based on the weighted average number of shares outstanding during each year. Diluted net loss per share is computed based on the weighted average number of shares outstanding during each year, plus the dilutive potential of the common stock considered outstanding during the year, in accordance with ASC 260-10 "Earnings per share".
All outstanding stock options and warrants have been excluded from the calculation of the diluted loss per share for each period presented, since all such securities have an anti-dilutive effect.
The following table sets forth the computation of the Company`s basic and diluted net loss per ordinary share:
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Net loss
|
12,290 | 610 | 3,368 | |||||||||
|
Preferred shares dividend
|
295 | 1,958 | 1,958 | |||||||||
|
Net loss attributable to ordinary shares
|
12,585 | 2,568 | 5,326 | |||||||||
|
Shares used in computing net loss per ordinary shares, basic and diluted (in thousands)
|
11,918 | 2,181 | 1,627 | |||||||||
|
Net loss per ordinary share, basic and diluted
|
1.06 | 1.18 | 3.27 | |||||||||
Instruments that may potentially dilute the basic loss per share in the future, but were not included in the calculation of diluted loss per share, since their effect is anti-dilutive.
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 13
|
||||||||||
|
(number)
|
||||||||||||
|
Warrants and share options
|
9,535 | 780 | 650 | |||||||||
|
Preferred shares
|
654 | 4,339 | 4,339 | |||||||||
| 22,107 | 7,300 | 6,616 | ||||||||||
NOTE 16 - RELATED PARTIES
|
|
A.
|
Compensation to the non-executive directors:
|
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Fees, including reimbursement of expenses
|
291 | 15 | - | |||||||||
|
Share-based compensation
|
1,264 | 69 | - | |||||||||
| 1,555 | 84 | - | ||||||||||
F - 37
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 16 - RELATED PARTIES (Cont.)
|
|
B.
|
Transactions with related parties:
|
|
For the year ended December 31,
|
||||||||||||
|
2 0 1 5
|
2 0 1 4
|
2 0 1 3
|
||||||||||
|
Consulting fees, including share based compensation and reimbursement of expenses (1) (2)
|
177 | 40 | 40 | |||||||||
|
Key man life insurance premium (3)
|
11 | 11 | 9 | |||||||||
| 188 | 51 | 49 | ||||||||||
|
|
1)
|
On July 1, 2005, the Company entered into an agreement with Hadar Kimchy according to which Hadar Kimchy provides marketing communication and graphical design services to the Company in consideration for a monthly retainer of NIS 10,260 ($3).On August 1, 2014, the monthly retainer was increased to NIS 13,680 ($4). The above services are provided to the Company by Sigalit Kimchy, who is employed by Hadar Kimchy. Sigalit Kimchy is a shareholder and is the spouse of Yoav Kimchy, the Company's chief technology officer and a former director.
|
|
|
2)
|
The Company engaged Mr. XiangQian (XQ) Lin, who has served as a director since February 24, 2015, to provide certain business development services in Asia under a consulting agreement entered into with him on June 1, 2015. As compensation for his services, Mr. Lin is entitled to a monthly fee of $10 for up to five hours per month and $300 per hour for any consultancy hour exceeding such five hours required to perform such services. In addition, we awarded Mr. Lin a one-time option grant to purchase 48,387 ordinary shares, exercisable at $5.06 per share.
|
|
|
3)
|
In connection with the asset transfer agreement entered into with the Predecessor Entity in May 2009, the Company assumed the former obligation of the Predecessor Entity to distribute any proceeds it collects on the $1 million key man life insurance policy with respect to Yoav Kimchy, the Company's chief technology officer and a former director, to the former holders of the Series A preferred units in an amount equal to their respective capital contributed to the Predecessor Entity, less any amounts previously distributed to them, plus any accrued and unpaid dividends due to them as of the date of distribution.
|
F - 38
CHECK CAP LTD
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(U.S. dollars in thousands, except share and per share data)
NOTE 16 - RELATED PARTIES (Cont.)
|
|
C.
|
Participation in the Company's IPO and concurrent Private Placement
|
|
|
1)
|
IPO:
|
|
|
As discussed in Note 10E(2)(b), on February 24, 2015, the Company closed a public offering, selling 2,000,000 units at a public offering price of $6 per unit, before underwriting discounts and offering expenses. Each unit consisted of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share. Each unit was issued with one and one-half non-transferrable Long Term Incentive Warrants. The following principal shareholders, purchased units at a price of $6 per unit. The units purchased, together with the proceeds, before expenses, to the Company, are shown in the table below:
|
|
Beneficial owner
|
Shares purchased
|
Series A Warrants purchased
|
Long Term Incentive Warrants purchased
|
Proceeds, before expenses, to the Company
|
||||||||||||
|
Pontifax Funds
|
125,000 | 62,500 | 187,500 | $ | 750 | |||||||||||
|
Docor International B.V.
|
41,666 | 20,833 | 62,499 | $ | 250 | |||||||||||
|
Esco Ventures Pte Ltd
|
166,667 | 83,333.5 | 250,000.5 | $ | 1,000 | |||||||||||
|
|
2)
|
Private Placement
|
|
|
As discussed in Note 10E(2)(c), on August 20, 2014, the Company entered into a certain credit line agreement, pursuant to which it obtained a credit line in an aggregate principal amount of $12 million from the Lenders. The Company issued to the Lenders CLA Warrants to purchase in the aggregate 2,658,463 of its ordinary shares. On February 24, 2015, the Company closed a Private Placement, concurrently with the IPO, issuing 2,000,000 units to the Lenders at a public offering price of $6 per unit, before expenses. Each unit consisted of one ordinary share and one-half of a Series A Warrant to purchase one ordinary share. Each unit was issued with one and one-half non-transferrable Long Term Incentive Warrants. The following principal shareholders, purchased units at a price of $6 per unit. The units purchased, together with the proceeds, before expenses, to the Company, are shown in the table below:
|
|
Beneficial owner
|
CLA Warrants
|
Shares purchased
|
Series A Warrants purchased
|
Long Term Incentive Warrants purchased
|
Proceeds, before expenses, to the Company
|
|||||||||||||||
|
Pontifax Funds
|
332,337 | 250,001 | 125,000.5 | 375,001.5 | $ | 1,500 | ||||||||||||||
|
Docor International B.V.
|
110,769 | 83,334 | 41,667 | 125,001 | $ | 500 | ||||||||||||||
|
Counterpoint Ventures Fund II LP
|
56,493 | 42,500 | 21,250 | 63,750 | $ | 255 | ||||||||||||||
F - 39