Confidentially submitted to the United States Securities and Exchange Commission on November 21, 2014.
This draft registration statement has not been publicly filed with the Securities and Exchange Commission
and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
AMENDMENT NO. 1
ToFORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
CHECK-CAP LTD.
(Exact name of Registrant as specified in its charter)
|
Israel
(State or other jurisdiction of
incorporation or organization)
|
3844
(Primary Standard Industrial
Classification Code Number)
|
Not Applicable
(I.R.S. Employer
Identification Number)
|
Check-Cap Building
Abba Hushi Avenue
P.O. Box 1271
Isfiya, 30090
Mount Carmel, Israel
+972-4-8303400
(Address, including zip code, and telephone number,
including area code, of Registrant’s principal executive offices)
Puglisi & Associates
850 Library Avenue, Suite 204
Newark, Delaware 19711
302-738-6680
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
|
Mitchell S. Nussbaum, Esq.
Angela M. Dowd, Esq.
Loeb & Loeb LLP
345 Park Avenue
New York, New York 10154
(212) 407-4000 - Telephone
(212) 407-4990 - Facsimile
|
Eran Yaniv, Adv.
Sharon Rosen, Adv.
Fischer Behar Chen Well
Orion & Co.
3 Daniel Frisch Street
Tel Aviv, 6473104, Israel
+972 3 6944111 - Telephone
+972 3 6091116 - Facsimile
|
Yvan-Claude Pierre, Esq.
Daniel I. Goldberg, Esq.
Reed Smith LLP
599 Lexington Avenue
New York, New York 10022
(212) 521-5400 - Telephone
(212) 521-5450 - Facsimile
|
Shlomo Landress, Adv.
Amit, Pollak, Matalon & Co.
Nitsba Tower,
17 Yitzhak Sadeh St.,
Tel Aviv 67775, Israel
+972 3 568 9000
Telephone
+972 3 568 9001
Facsimile
|
Approximate date of commencement of proposed sale to the public: As soon as practicable after the effective date of this Registration Statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, as amended, or the Securities Act, check the following box. T
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. £
__________________________
Calculation of Registration Fee
|
Title of Each Class of Securities to be Registered
|
Proposed Maximum Aggregate Offering
Price (1)
|
Amount of
Registration Fee (1)
|
||||||
|
Ordinary shares, par value NIS 0.01 (2)(3)
|
$ | $ | ||||||
|
Underwriter warrants(4)(6)
|
||||||||
|
Ordinary shares, par value NIS 0.01 underlying the underwriter warrants(3)(5)
|
$ | $ | ||||||
|
TOTAL
|
||||||||
|
|
(1)
|
Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(o) under the Securities Act.
|
|
|
(2)
|
Includes ordinary shares initially offered and sold outside the United States that may be resold from time to time in the United States either as part of their distribution or within 40 days after the later of the effective date of this registration statement and the date the ordinary shares are first bona fide offered to the public, and also includes ordinary shares that may be purchased by the underwriters pursuant to an option to purchase additional ordinary shares to cover over-allotments, if any. The ordinary shares are not being registered for the purpose of sales outside the United States.
|
|
|
(3)
|
Pursuant to Rule 416 of the Securities Act, the securities being registered hereunder include such additional securities as may be issued after the date hereof as a result of share splits, share dividends or similar transactions.
|
|
|
(4)
|
We have agreed to issue, upon closing of this offering, warrants exercisable for a period of four years following the effective date of this registration statement representing 5% of the aggregate number of ordinary shares issued in the offering, but not including the over-allotment option, or the “underwriter warrants,” to Chardan Capital Markets, LLC. Resales of the underwriter warrants on a delayed or continuous basis pursuant to Rule 415 under the Securities Act are registered hereby. Resales of ordinary shares issuable upon exercise of the underwriter warrants are also being similarly registered on a delayed or continuous basis hereby. See “Underwriting.”
|
|
|
(5) |
No fee required pursuant to Rule 457(g) under the Securities Act.
|
|
|
(6)
|
Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(g) under the Securities Act. We have calculated the proposed maximum aggregate offering price of the ordinary shares underlying the underwriters’ warrants by assuming that such warrants are exercisable to purchase ordinary shares at a price per ordinary share equal to 100% of the price per ordinary share sold in this offering.
|
The Registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this registration statement shall hereafter become effective in accordance with Section 8(a) of the Securities Act or until the registration statement shall become effective on such date as the Commission acting pursuant to said section 8(a), may determine.
The information contained herein is subject to completion or amendment. A registration statement relating to these securities has been filed with the U.S. Securities and Exchange Commission. These securities may not be sold until the registration statement becomes effective. This prospectus is not an offer to sell and is not a solicitation of an offer to buy in any jurisdiction in which an offer, solicitation, or sale is not permitted.
|
PRELIMINARY PROSPECTUS
|
SUBJECT TO COMPLETION, DATED NOVEMBER 21, 2014
|

Check-Cap Ltd.
Ordinary Shares
Check-Cap Ltd. is offering ordinary shares in its initial public offering. We currently expect the initial public offering price to be between $ and $ per ordinary share.
Prior to this offering, there has been no public market for our ordinary shares. We have applied for the listing of our ordinary shares on the NASDAQ Capital Market under the symbol “CHEK”. There is no assurance that this application will be approved.
Concurrently with this offering, we expect to complete a private placement of ordinary shares or the “Private Placement” at a purchase price per ordinary share equal to the public offering price in accordance with Regulation S under the Securities Act of 1933, as amended, or the “Securities Act” or Regulation D under the Securities Act, to certain investors including certain of our affiliates. The sale of such ordinary shares will not be registered under the Securities Act. We expect to receive $ in aggregate net proceeds from the Private Placement. See “Summary-Recent Developments—Credit Line Agreement; Private Placement”. The closing of the Private Placement is conditioned upon the completion of the offering to which this prospectus relates. However, the completion of the offering to which this prospectus relates is not conditioned upon the closing of the Private Placement.
We are an “emerging growth company” under applicable U.S. federal securities laws and may elect to comply with reduced public company reporting requirements. See “Implications of Being an Emerging Growth Company” on page 6 of this prospectus.
Investing in our ordinary shares involves a high degree of risk. You should read carefully the “Risk Factors” beginning on page 14 of this prospectus before investing in our ordinary shares that are the subject of this offering.
Neither the U.S. Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of the disclosures in this prospectus. Any representation to the contrary is a criminal offense.
|
Per Ordinary Share
|
Total
|
|||||||
|
Public offering price
|
$ | $ | ||||||
|
Underwriting discount and commissions (1)
|
$ | $ | ||||||
|
Proceeds, before expenses, to us
|
$ | $ | ||||||
|
|
(1)
|
We have also agreed to issue, upon closing of this offering, compensation warrants to Chardan Capital Markets, LLC as representative of the underwriters, entitling it to purchase up to ordinary shares. For a description of other terms of the compensation warrants and a description of the additional compensation to be received by the underwriters see “Underwriting.”
|
The underwriters have an option exercisable within 45 days from the date of this prospectus to purchase up to of additional ordinary shares from us at the public offering price, less the underwriting discount, solely to cover over-allotments. The ordinary shares issuable upon exercise of the underwriters’ over-allotment option have been registered under the registration statement of which this prospectus forms a part. In addition to the underwriting discount, we have agreed to pay certain of the expenses of underwriters incurred in connection with this offering, see “Underwriting” beginning on page 156 of this prospectus.
The underwriters expect to deliver the ordinary shares to purchasers on or about , 2015.
|
Chardan Capital Markets, LLC
|
Maxim Group LLC
|
, 2015
TABLE OF CONTENTS
Page
|
1
|
|
|
14
|
|
|
47
|
|
|
48
|
|
|
49
|
|
|
50
|
|
|
53
|
|
|
55
|
|
|
56
|
|
|
58
|
|
|
71
|
|
|
101
|
|
|
110
|
|
|
130
|
|
|
132
|
|
|
136
|
|
|
144
|
|
|
150
|
|
|
156
|
|
|
163
|
|
|
163
|
|
|
163
|
|
|
164
|
|
|
F-1
|
i
You should rely only on the information contained in this prospectus, any amendment or supplement to this prospectus or any free writing prospectus prepared by us or on our behalf. We have not, and the underwriters have not, authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and the underwriters are not, making an offer of these securities, or soliciting any offers to buy these securities, in any jurisdiction where the offer or solicitation is not permitted. You should not assume that the information contained in this prospectus is accurate as of any date other than the date on the front of this prospectus, regardless of the time of delivery of this prospectus or of any sale of our ordinary shares.
Neither we nor any of the underwriters has done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required other than the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of our ordinary shares set forth in, and the possession and distribution of, this prospectus outside of the United States.
We obtained statistical data, market data and other industry data and forecasts used throughout this prospectus from market research, publicly available information and industry publications. While we believe that the statistical data, industry data and forecasts and market research are reliable, we have not independently verified the data.
ii
|
PROSPECTUS SUMMARY
The following summary does not contain all of the information you should consider before investing in our ordinary shares. You should read the following summary together with the entire prospectus carefully, including the “Risk Factors” section beginning on page 14 and the financial statements and the accompanying notes to those financial statements beginning on page F-1 before making an investment decision. Unless otherwise indicated, all information in this prospectus assumes no exercise of the underwriters’ over-allotment option and no exercise of the underwriter warrants. Unless the context otherwise requires, references to “we,” “our,” “us,” “our company,” and “Check-Cap” refer to Check-Cap Ltd., an Israeli company. The terms “dollar,” “US$” or “$” refer to U.S. dollars, the lawful currency of the United States, and the term “NIS” refers to New Israeli Shekels, the lawful currency of the State of Israel. Unless otherwise indicated, U.S. dollar translation of NIS amounts presented in this prospectus are translated using the rate of $1.00 = NIS 3.4380, the exchange rate published by the Bank of Israel on June 30, 2014, and U.S. dollar translation of Euro amounts presented in this prospectus are translated using the rate of $1.00 = Euro 1.3693, the exchange rates published by the Wall Street Journal on June 30, 2014.
Our Company
We are a clinical stage medical diagnostics company engaged in the development of an ingestible imaging capsule that utilizes low-dose X-rays for the screening for colorectal cancer, or CRC. While CRC is the second leading cause of death from cancer in the United States and is largely preventable with early detection, about one-half of Americans over the age of 50 do not undergo any form of CRC screening due in large part to the pain, discomfort and embarrassment related to current screening methods. Unlike other structural screening methods that are designed to generate structural information of the colon for the detection of pre-cancerous polyps, such as optical colonoscopy, computed tomographic colonography, or CTC, and other capsule-based technology, our imaging capsule is designed to be ingested without any cleansing of the colon and to travel through the gastrointestinal tract naturally while the patient continues his or her normal daily routine. Furthermore, unlike all other CRC imaging devices currently on the market, which require the patient to fast for several hours prior to the procedure, the Check-Cap procedure enables patients to continue eating normally. We believe that this solution will be attractive to both physicians and patients, thereby increasing the number of people willing to undergo screening for CRC.
Our imaging capsule is being designed to create a reconstructed three-dimensional image of the colon and to detect clinically significant polyps with a high degree of sensitivity. Colon polyps are fleshy growths that occur on the lining of the colon. Polyps in the colon are extremely common, and when certain types of polyps grow large enough they can become cancerous.
Our imaging capsule will be swallowed by the patient and propelled by natural motility through the gastrointestinal tract and excreted naturally with no need for retrieval for data collection. Unlike other CRC screening methods, this process should not disrupt a patient’s normal activities or require fasting. Our imaging capsule employs X-rays which are considered low dose by the Radiation Safety Division of the Radiological Protection Branch of the Soreq Nuclear Research Center, which allows it to image the lining of the colon even when surrounded by intestinal content. As such, we believe that patients using our imaging capsule will not be required to undergo any prior bowel cleansing.
Our imaging capsule is being designed to transmit the data it collects to an external data recorder that will be worn by the patient. The external data recorder is being designed to transmit the data to physicians, who will then utilize our data viewer software application to analyze the data collected by our imaging capsule. We intend for physicians to be able to review the colon’s inner images at any location at any time, in less time than is required to perform an optical colonoscopy.
In order to enable a complete view of capsule positioning and motility, we have designed an external receiver and electromagnetic localization system, or RELS, which is mounted on the patient’s back throughout the entire screening procedure. The RELS is being designed to provide the physician with accurate localization data aligned with a reconstructed image.
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Medical Center in Israel and used a prior version of our imaging capsule, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our imaging technology to the human colon, generating images taken in the colon without any prior bowel-cleansing. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent.
In the event that polyps are identified by our imaging capsule, the patient would be required to undergo a subsequent traditional colonoscopy procedure to examine, remove and biopsy the polyps. For those patients who require a subsequent polypectomy, concerns regarding pain, discomfort and embarrassment may still remain with respect to the subsequent polypectomy. We do not, however, believe that these concerns will make the use of our imaging capsule any less attractive to doctors and patients. Although patients who are initially screened utilizing a traditional colonoscopy could avoid the need for a second procedure if polyps are discovered because they could undergo a polypectomy during the initial screening, if necessary, we believe that our imaging capsule will still be attractive to doctors and patients since a large majority of patients who are screened will not require a subsequent polypectomy. According to a review published by the Agency for Healthcare Research and Quality in October 2008, out of 100 adults aged 50-75, only 25-30 persons have one or more polyps and only 15 persons have significant (10+mm) polyps.
|
1
|
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Medical Center in Israel and used a prior version of our imaging capsule, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our imaging technology to the human colon, generating images taken in the colon without any prior bowel-cleansing. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted.
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of the Check-Cap imaging system. The 10-case study is the first part of a multi-center, prospective clinical feasibility study to establish the safety, functionality and preliminary efficacy of the Check-Cap imaging system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity fecal occult blood tests, or FOBTs, and fecal immunochemical tests, or FITs, as well as from optical colonoscopies. The feasibility study is designed to include a total of 60 subjects. The study is being conducted in Israel at the Tel Aviv Medical Center and Laniado Hospital and is planned to also be conducted at the Erasmus University Medical Center in the Netherlands. The clinical feasibility study will also evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified by our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
Another objective of the 10-case study was to estimate total radiation exposure for each case study. This was calculated using standard established factors for calculating effective radiation exposure, such as the duration of the capsule inside the body, and was based on the activity of the radiation source inside the imaging capsule and radiation energy, both of which were measured for each case study. The average calculated exposure for the entire procedure in the 10-case study, from ingestion of the capsule to excretion, was 0.03 mSv (STD 0.007 mSv). This level of radiation exposure is similar to a single chest X-ray (approximately 0.06mSv) and two orders of magnitude less than a CTC.
Following the successful completion of the broader multi-center, prospective clinical feasibility study, we plan to submit a request for CE marking for the marketing and sale of our capsule in the European Union during 2015. We expect to perform post-marketing studies in Europe following CE marking. We anticipate launching our product commercially in Europe during 2016.
We plan to submit to the U.S. Food and Drug Administration, or FDA, a request to conduct a pivotal study in the United States in late 2015 to (i) demonstrate device safety as evidenced by a lack of device-related serious adverse events; and (ii) provide efficacy data concerning our imaging capsule’s sensitivity and specificity. We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study. Pivotal studies are expected, among other things, to compare the images of polyps identified by our imaging system with the same polyps detected by traditional optical colonoscopy and CTC in instances where patients were referred after positive exam results. These clinical findings will be analyzed in comparison to results obtained from FOBTs and FITs. Subject to successful completion of our clinical trials, we anticipate launching our product commercially in the United States during 2017. We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have been granted patents for our core patent by the U.S. Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan. We also filed patent applications describing the use of our imaging technology in several other medical applications.
|
2
|
Since our formation, we have not generated any revenues. We do not anticipate generating any revenues for the foreseeable future and we do not yet have any specific launch dates for our product. For the six months ended June 30, 2014, we had a total comprehensive loss of $2.2 million. For the year ended December 31, 2013, we had a total comprehensive loss of $4.0 million. As of June 30, 2014, we had an accumulated deficit of $24.7 million and a total shareholders’ deficit of $998,000.
Industry Background
According to the American Cancer Society, or the ACS, CRC is the third most common cancer diagnosed and the second leading cause of death from cancer in the United States. The ACS estimates that in 2014, in the United States approximately 136,830 people are expected to be diagnosed with CRC and approximately 50,310 people will die from CRC. According to the World Health Organization, or the WHO, in 2012, in Europe there were an estimated 471,000 cases of CRC and approximately 228,000 died from the disease, and in Japan there were an estimated 112,675 cases of CRC and approximately 49,345 died from the disease. According to the WHO, in 2020 the expected numbers of cases of CRC are estimated to be 159,972 in the United States, 528,481 in Europe, 128,346 in Japan and 1,678,127 worldwide.
CRC screening can reduce death rates from CRC by detecting polyps at an earlier, more treatable stage. CRC is one of the few cancers that can be prevented through screening because pre-cancerous polyps, from which colon cancers often develop, can be identified and removed. Moreover, the five-year survival rate is greater than 90% for CRC patients diagnosed at an early, localized stage. However, less than 40% of cases are currently diagnosed at that stage. According to the Centers for Disease Control and Prevention, or the CDC, at least 6 out of every 10 deaths from CRC could be prevented if every adult age 50 years or older was screened regularly and approximately 30,000 lives could be saved each year in the United States if the screening recommendations were followed. The ACS’ goal is to have 80% of those 50 years and older who are covered by the program screened by 2018.
Today, there is a range of options for CRC screening in the average risk population, with current technology falling into two general categories: (i) structural exams, such as optical colonoscopy, sigmoidoscopy, CTC and optical capsules (all of which require aggressive bowel preparation), which are invasive exams that enable physicians to visualize the colon for abnormalities, and (ii) stool tests, such as FOBTs, FITs and stool DNA tests, which test for blood and irregularities in DNA. Notwithstanding the many CRC screening alternatives, the fact that the tests are encouraged by clinicians and insurers and the clinical value of screening for CRC, a large portion of the population are still reticent to undergo CRC screening and are not satisfied with the currently available alternatives.
The ACS recommends that men and women over the age of 50 undergo an optical colonoscopy every 10 years or other structural tests, such as sigmoidoscopy or virtual colonoscopy, every five years or alternatively, a FOBT should be performed every year. According to the U.S. Census Bureau, as of mid-2014, there were projected to be approximately 91 million Americans aged 50-75 years. Assuming the longest screening interval of 10 years, the addressable annual U.S. patient population is at least 9.1 million.
Optical colonoscopy is currently considered the most reliable method for detecting disorders of the colon and is the standard screening tool for early detection of colon cancer. Optical colonoscopy demonstrates a high degree (approximately 95%) of sensitivity (i.e., detection of individuals with cancer) and specificity (i.e., avoiding false negative results). Optical colonoscopy involves the insertion of a flexible colonoscope, which is an approximately160 centimeters long endoscope, by a physician into a patient’s colon through the anus in order to visually inspect the interior of the colon. Air must be pumped in through the rectum in a process called “insufflation.” Sigmoidoscopy, or FSIG, is an endoscopic procedure that examines the lower part of the colon lumen. The exam may be performed with a variety of endoscopic instruments, including a standard 60 centimeter sigmoidoscope. FSIG is typically performed without sedation and with a more limited bowel preparation than a standard optical colonoscopy. An optical colonoscopy and sigmoidoscopy can perform both diagnostic and limited treatment functions, by allowing for the removal of polyps and adenomas during the course of the procedure; however, both of these procedures carry some risks of bowel perforations and bleeding and related limitations as they require prior cleansing of the bowel, insufflation and sedation, involve potential complications and may cause patient anxiety, discomfort and, in some cases, pain. In addition, a patient’s normal daily routine is disrupted for one or two days. |
3
|
CTC, or virtual colonoscopy, is an imaging procedure that results in cross-sectional, two- or three-dimensional views of the entire colon with the use of a special X-ray machine linked to a computer. Here, as well, a flexible tube is inserted into the rectum in order to allow air or carbon dioxide to open the colon. The patient then passes through the CT scanner, which creates multiple images of the colon interior. This method does not allow for treatment and the subject is exposed to a high dose of radiation. A full bowel cleansing is currently necessary for a successful examination by CTC.
FOBT is based on an analysis of stool samples and is currently the most widely used non-invasive screening test. It has a lower sensitivity in detecting polyps (measured by the percentage of polyps being found). According to the CDC, in 2012, only approximately 10.6% of men and 10.2% of women in the United States underwent the procedure due to its inconvenience and unreliable performance. FOBT is being replaced by a more sensitive blood stool technology FIT, but it is also not designed to detect the majority of non-bleeding polyps.
In 2009, optical capsule endoscopy became commercially available in Europe for CRC screening. In early 2014, the FDA granted approval for optical capsule endoscopy procedure to be used for CRC screening for use in patients who have had an incomplete optical colonoscopy. However, this technology requires bowel cleansing to a greater degree than is required for a regular optical colonoscopy, which can result in dehydration and in turn can lead to cancellation of the procedure in certain cases. Moreover, because this procedure must be completed within several hours in order to maintain a clean colon and to accommodate the capsule’s limited battery life, patients are required to drink large amounts of liquid so that the capsule can flow through the gastrointestinal tract during the time allotted. Furthermore, camera-based optical capsule endoscopy procedures generate a large number of images, often requiring more physician time to analyze the images than to conduct an optical colonoscopy.
Several companies are developing technologies based on molecular diagnostics (from blood and other bodily fluids), or MDx, tests that investigate the link between genes and the function of metabolic pathways, drug metabolism and disease development with a primary focus on the study of DNA, RNA and proteins. Genetic markers can be traced within stool samples in minute quantities. For example, a special collecting kit for stool samples and an analyzer to diagnose CRC based on these stool-based markers has been developed and recently approved by the FDA. While the method of screening for CRC using stool DNA testing has been endorsed by several societies, this test does not generate structural information on the colon and therefore, does not detect most pre-cancerous polyps.
Our Solution
We believe that our imaging capsule could represent a potential breakthrough in CRC screening by providing a structural exam without the pain, discomfort and embarrassment experienced by some patients undergoing a traditional optical colonoscopy and other currently available screening methods, by offering the following benefits:
|
|
·
|
eliminating the need for fasting and prior bowel cleansing, which would differentiate our imaging capsule from every other currently available structural screening exam;
|
|
|
·
|
providing patients with a procedure that requires them to swallow our capsule and small amounts of a contrast agent, thereby minimizing any disruption to their normal activities;
|
|
|
·
|
eliminating need to sedate patients;
|
|
|
·
|
obviating the requirement for the insufflation (the forcing of air into the gastrointestinal tract) of patients;
|
|
|
·
|
administering our technology on an outpatient basis;
|
|
|
·
|
providing digital reporting, storage and remote consulting capabilities; and
|
|
|
·
|
enabling a physician to analyze the results in approximately 10 minutes, which would be less time than is required to conduct an optical colonoscopy.
|
|
|
4
|
Although our imaging capsule utilizes radiation that is considered low dose, we believe that the risks associated with such radiation exposure are low compared to risks associated with other procedures such as perforation, bleeding or sedation related effects (optical colonoscopy and sigmoidoscopy) and dehydration and damage to kidneys (optical capsules). Furthermore, stool test methods (FOBTs, FITs and stool DNA) require a follow-up optical colonoscopy if abnormalities are detected. Unlike FOBTs, FITs and stool DNA tests, our capsule-based imaging modality generates structural information on the colon, which could assist in the detection of pre-cancerous polyps. We, therefore, do not believe that the low dose radiation in our imaging capsule will make our imaging capsule less attractive to physicians and patients than other less effective products that do not employ any radiation.
We believe that gastroenterologists will embrace our technology and encourage the use of our imaging capsule. This may increase the number of people undergoing CRC screening and may cause more people with polyps to obtain polypectomy – a therapeutic procedure during which polyps are removed and which currently receives different reimbursement coverage.
Our imaging capsule and RELS are intended to be prescribed to patients by physicians. One or two days prior to swallowing our capsule, a patient will begin drinking small amounts of a radio opaque contrast agent (such as barium sulfate or iodine) with his or her meals, which enhances the contrast of the colon surface. The capsule is propelled by natural motility through the gastrointestinal tract. As it makes its way through the gastrointestinal tract, information is transmitted to a receiving device worn by the patient that stores the information for offline analysis. After our imaging capsule is expelled from a patient’s body, the RELS will transmit the data to physicians, who will then utilize our data reviewer software application to analyze the data collected by our imaging capsule. Our proprietary software is being designed to process the data and produce a two and three-dimensional visualization of the colon. A physician will then analyze the visualization to determine whether any anatomical anomalies are present on the surface of the colon.
Our imaging capsule consists of an X-ray source and several X-ray detectors. The X-ray source is contained in a rotating radiation shield, enabling the generation of 360-degree angular scans. The collection of successive angular scans enables the virtual reconstruction of a portion of the colon. During movement of our imaging capsule longitudinally through the colon, successive images of portions of the colon are collected to enable the three-dimensional reconstruction of the colon. Our imaging capsule is also intended to identify polyps, which protrude inward into the colon, through the detection of irregularities in the topography of the colon.
 Image for illustration purpose only
|
5
|
Our Strategy
Our goal is to become a leading supplier of CRC screening technology and, subject to the successful completion of the development of our technology and the receipt of the requisite regulatory approvals, to establish our technology as a leading CRC screening method. Key elements of our strategy include:
|
|
·
|
obtaining CE marking for the marketing and sale of our imaging capsule in the European Union, followed by obtaining regulatory approvals for the use of our imaging capsule initially in the United States and Japan. In Europe and Japan, we intend to offer our imaging capsule as an imaging and screening tool for the general population. In the United States, we will first seek to obtain regulatory approvals for our imaging capsule as an adjunct tool to FOBTs and FITs, and after we have conducted more extensive clinical studies, we anticipate applying to the FDA for the use of our imaging capsule as an initial screening tool;
|
|
|
·
|
obtaining third-party reimbursement for our technology;
|
|
|
·
|
enhancing our existing technology portfolio and developing new technologies; and
|
|
|
·
|
successfully marketing our product to establish a large customer base.
|
|
Our Challenges
Because we are still in the clinical and development stage, we are subject to certain challenges, including, among others, that:
|
|
·
|
our technology has been tested on a limited basis and therefore we cannot assure the product’s clinical value;
|
|
|
·
|
we need to CE mark the devices in the European Union and obtain the requisite regulatory approvals in the United States, Japan and other markets where we plan to focus our commercialization efforts;
|
|
·
|
we need to raise an amount of capital sufficient to complete the development of our technology, obtain the requisite regulatory approvals and commercialize our current and future products;
|
|
|
·
|
we need to obtain reimbursement coverage from third-party payors for procedures using our imaging capsule;
|
|
|
·
|
we need to increase our manufacturing capabilities; and
|
|
|
·
|
we need to establish and expand our customer base while competing against other sellers of capsule endoscopes as well as other current and future CRC screening technologies and methods.
|
|
Our ability to operate our business and achieve our goals and strategies is subject to numerous risks as described more fully in “Risk Factors.”
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” pursuant to the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may take advantage of certain exemptions from specified disclosure and other requirements that are otherwise generally applicable to public companies. These exemptions include:
|
|
·
|
being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure;
|
|
|
·
|
not being required to comply with the auditor attestation requirements for the assessment of our internal control over financial reporting provided by Section 404 of the Sarbanes-Oxley Act of 2002;
|
|
|
·
|
not being required to comply with any requirements adopted by the Public Company Accounting Oversight Board requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and our financial statements;
|
|
|
·
|
reduced disclosure obligations regarding executive compensation; and
|
|
|
·
|
not being required to hold a nonbinding advisory vote on executive compensation or seek shareholder approval of any golden parachute payments not previously approved.
|
|
|
6
|
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. However, we have elected to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted for public companies. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
We will remain an emerging growth company until the earliest of (i) the last day of the fiscal year in which our total annual gross revenues exceed $1.0 billion; (ii) the last day of the fiscal year in which the fifth anniversary of the date of the first sale of ordinary shares under this registration statement occurs; (iii) the date on which we have, during the previous three-year period, issued more than $1.0 billion in non-convertible debt; or (iv) the date on which we are deemed to be a “large accelerated filer” under the Securities Exchange Act of 1934, as amended, or the Exchange Act. When we are no longer deemed to be an emerging growth company, we will not be entitled to rely on the exemptions provided in the JOBS Act discussed above. We may choose to take advantage of some, but not all, of the exemptions available to emerging growth companies. We have taken advantage of some of the reduced reporting exemptions in this prospectus. Accordingly, the information contained herein and in future filings with the U.S. Securities and Exchange Commission may be different from the information provided by other public companies in similar filings.
Concurrent Private Placement
Concurrently with this offering, we expect to complete a Private Placement of ordinary shares at a purchase price per ordinary share equal to the public offering price in accordance with Regulation S under the Securities Act or Regulation D under the Securities Act, to certain investors including certain of our affiliates. The sale of such ordinary shares will not be registered under the Securities Act. We expect to receive $ in aggregate net proceeds from the Private Placement. The closing of the Private Placement is conditioned upon the completion of the offering to which this prospectus relates. However, the completion of the offering to which this prospectus relates is not conditioned upon the closing of the Private Placement. Corporate Information
We were incorporated as a limited liability private company under the laws of the State of Israel on April 5, 2009, and on May 31, 2009, we acquired all of the business operations and substantially all of the assets of Check-Cap LLC, a Delaware limited liability company formed in December 2004. Our principal executive offices are located at Check-Cap Building, Abba Hushi Avenue, P.O. Box 1271, Isfiya, 30090, Mount Carmel, Israel. Our telephone number is +972-4-8303400. Our website address is www.check-cap.com. Information contained on, or accessible through, our website does not constitute part of this prospectus and is not incorporated by reference herein.
Throughout this prospectus we refer to the trademark “CHECK-CAP” that we use in our business. Furthermore, we received a notice of allowance for the “CHECK-CAP” mark and design logo in the United States and hold a registered trademark for the “CHECK-CAP” design logo in Europe. Other trademarks and service marks appearing in this prospectus are the property of their respective holders.
Recent Developments
Credit Line Agreement; Private Placement
On August 20, 2014, we entered into a certain credit line agreement, pursuant to which we obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders, or the Lenders. The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014.
|
7
|
We issued to each Lender at closing a warrant, collectively referred to as the Credit Line Warrants, to purchase a number of our ordinary shares constituting 2% of our share capital on a fully diluted basis (assuming conversion of all of our convertible securities into ordinary shares at a 1:1 conversion rate) as of the closing for each $1 million (or portion thereof) extended by such Lender. The Credit Line Warrants are exercisable for a period of ten years at an exercise price of NIS 0.01 per share, and may be exercised on a net issuance basis.
Under the terms of the agreement, if we intend to consummate an initial public offering, or an IPO, and such IPO is expected to be consummated on or prior to February 18, 2015, or if we consummate an IPO on or prior to February 18, 2015, we will be entitled to call all or any part of the credit line amount and direct that such called amount be invested in ordinary shares in a private placement transaction that is exempt from the registration requirements of the Securities Act at a price per ordinary share equal to the public offering price per share in the IPO. In the event that we call less than the full credit line amount, we will call amounts on a pro rata basis among the Lenders based on their pro rata share of the total credit line amount. If we consummate an IPO on or prior to February 18, 2015, any part of the credit line amount not called by us will be released to the Lenders.
If we do not consummate an IPO on or prior to February 18, 2015, we may call the credit line amount at any time thereafter until April 14, 2016, subject to certain conditions. Any part of the credit line amount not called by us on or prior to such date will be released to the Lenders. If we call the credit line amount on or prior to April 14, 2016, the amount called will bear interest at the annual rate of 7%; provided that the aggregate interest rate will not be less than 5%. The called credit line amount will automatically convert into shares of our company upon the earlier of a qualified financing round (which includes a public offering, including an IPO), an M&A. Event (as defined in the agreement) and April 14, 2016, and the Lenders may elect to convert the entire called credit line upon a non-qualified financing round, all under the terms and conditions set forth in the credit line agreement. In the event that the qualified financing round is an IPO, in lieu of automatic conversion, we are entitled to deposit in trust an amount equal to 125% of the called credit line amount and irrevocably instruct the trustee to submit an offer, on behalf of each Lender, for the purchase of the IPO shares at the IPO price determined by our lead underwriters.
Israel-United States Binational Industrial Research and Development Foundation Grant
On July 13, 2014, we entered into a Cooperation and Project Funding Agreement with the Israel-United States Binational Industrial Research and Development Foundation, or the BIRD Foundation, and Synergy Research Inc., or Synergy, pursuant to which the BIRD Foundation has agreed to award a grant in the maximum amount of the lesser of (i) $900,000 and (ii) 50% of the actual expenditures for the funding of a project entitled “Collection & Analysis of Gastrointestinal Images for Diagnostic Adenomatic Polyps and Colorectal Cancer.” The development work is expected to be performed over a 24 month period by Synergy (or a subcontractor on its behalf) and us. Of the total grant amount, we are expected to receive an aggregate of $567,000 to fund our expenditures for the project, in five installments. We received our first advance payment from the BIRD Foundation of $68,000 in July 2014. Our research and development expenses, net is presented net of the differences between the fair value of the liability and the grant amount received from the BIRD Foundation.
Under the terms of the grant agreement, we are required to repay the total grant in an amount equal to 5% of gross revenues derived from the product funded by the project, up to 100%, 113%, 125%, 138% and 150% of the grant amount, linked to the U.S. Consumer Price Index, if repaid within one year, two years, three years, four years and five or more years, respectively, of the original project completion date in accordance with the project proposal. Under the terms of the agreement, if any portion of the product funded by the project is sold outright to a third party prior to full repayment of the grant to the BIRD Foundation, one-half of the sale proceeds will be applied to the repayment of the grant. If the funded product is licensed to a third party, 30% of all payments received under the respective license agreement must be paid to the BIRD Foundation in repayment of the grant.
|
8
|
The Offering
|
|
|
Issuer
|
Check-Cap Ltd.
|
|
Ordinary shares offered by us in this offering
|
ordinary shares
|
|
Over-allotment option
|
The underwriters have an option for a period of 45 days to purchase up to additional ordinary shares to cover over-allotments, if any.
|
|
Ordinary shares outstanding immediately prior to the offering
|
ordinary shares
|
|
Ordinary shares to be issued in the concurrent Private Placement
|
|
|
Ordinary shares to be outstanding immediately after the offering and the concurrent Private Placement(1)
|
ordinary shares (or ordinary shares if the underwriters exercise in full their option to purchase additional shares).
|
|
Use of Proceeds
|
We estimate that the net proceeds from our issuance and sale of ordinary shares in this offering will be approximately $ million, based on the offering price of $ per share, and after deducting underwriting discounts and commissions and offering expenses payable by us. If the representative of the underwriters exercises the over-allotment option in full, we estimate that the net proceeds from this offering will be approximately $ million, based on the offering price of $ per share, and after deducting underwriting discounts and commissions and offering expenses payable by us. We will also expect to receive net proceeds of approximately $ million from the sale of ordinary shares in the concurrent Private Placement after deducting commissions payable by us. We currently expect to use the net proceeds from this offering and the concurrent Private Placement as follows:
· approximately $ million on research and development;
· approximately $ million on obtaining regulatory approvals of our product including approximately
$ million on clinical trials in Europe and the United States;
· approximately $ million to launch our product in selected countries in Europe;
· approximately $ million to build our manufacturing capabilities; and
· the balance, if any, for other general corporate purposes.
See “Use of Proceeds” beginning on page 48 of this prospectus.
|
| Private Placement |
Concurrently with this offering, we expect to complete a Private Placement of ordinary shares at a purchase price per ordinary share equal to the public offering price in accordance with Regulation S under the Securities Act or Regulation D under the Securities Act, to certain investors including certain of our affiliates. The sale of such ordinary shares will not be registered under the Securities Act. We expect to receive $ in aggregate net proceeds from the Private Placement. The closing of the Private Placement is conditioned upon the completion of the offering to which this prospectus relates. However, the completion of the offering to which this prospectus relates is not conditioned upon the closing of the Private Placement. See “Summary-Recent Developments—Credit Line Agreement; Private Placement”
|
9
|
Underwriter Compensation Warrants
|
We will issue to Chardan Capital Markets, LLC as representative of the underwriters, upon closing of this offering, compensation warrants entitling the underwriter to purchase 5% of the aggregate number of ordinary shares issued in this offering, but not including the over-allotment option. The underwriter warrants may be exercised for a period of four years following the date of effectiveness of the Registration Statement on Form F-1 of which this prospectus forms a part.
|
|
Dividend Policy
|
We do not anticipate declaring or paying any cash dividends on our ordinary shares following this offering.
|
|
Transfer Agent and the Registrar
|
|
|
Risk Factors
|
Investing in our securities involves a high degree of risk. See “Risk Factors” beginning on page 14 of this prospectus. See “Risk Factors” and other information included in this prospectus for a discussion of factors you should carefully consider before deciding to invest in our ordinary shares.
|
|
Proposed Symbol and Listing
|
We have applied for the listing our ordinary shares on the NASDAQ Capital Market under the symbol “CHEK.”
|
|
(1) The number of ordinary shares to be outstanding after our initial public offering and the concurrent Private Placement is based on ordinary shares issued and outstanding as of , 2014, and excludes:
|
|
·
|
19,498,426 ordinary shares issuable upon the exercise of outstanding warrants to purchase preferred shares (comprised of (i) warrants to purchase 836,412 Series C-1 preferred shares, (ii) warrants to purchase 1,546,996 Series C-2 preferred shares, (iii) warrants to purchase 503,872 Series D-1 preferred shares and (iv) warrants to purchase 16,611,146 Series D-2 preferred shares, following their conversion into warrants to purchase ordinary shares immediately prior to the closing of this offering) with a weighted average exercise price of $0.44 per ordinary share;
|
|
|
·
|
273,592 ordinary shares issuable upon the exercise of outstanding warrants, which will be automatically exercised, without consideration, if and when Guy Neev exercises any part of his options to purchase 1,995,475 ordinary shares, or the Neev Options, in proportion to (i) the portion of the Neev Options exercised by Guy Neev and (ii) the number of warrants held by the optionee with respect to which such warrants were granted that were exercised prior to the exercise of the Neev Options;
|
|
|
·
|
57,599,850 ordinary shares issuable upon the exercise of outstanding warrants with an exercise price of NIS 0.01 per ordinary share, of which (i) warrants to purchase 53,169,092 ordinary shares are fully vested; and (ii) warrants to purchase 4,430,758 ordinary shares will become fully vested upon the closing of this offering;
|
|
|
·
|
12,415,436 ordinary shares issuable upon the exercise of outstanding options with a weighted average exercise price of $0.17 per ordinary share, granted under our 2006 Unit Option Plan;
|
10
|
·
|
11,630,739 ordinary shares issuable upon the exercise of outstanding options with an exercise price of NIS 0.01 per ordinary share granted under our 2006 Unit Option Plan, which will become fully vested upon the closing of this offering;
|
|
| · | 769,453 ordinary shares issuable upon the exercise of options with an exercise price of $0.2478 per ordinary share, under our 2006 Unit Option Plan, which we have agreed that certain executive officers will be entitled to upon completion of an equity financing, which includes this offering;
|
|
| · | the ordinary shares issuable upon the exercise of warrants to be issued to certain finders in connection with the credit line agreement if either (i) the credit line amount extended to us is invested in ordinary shares in a private placement on or prior to February 18, 2015 or (ii) if we do not consummate an IPO on or prior to February 18, 2015 and we call the credit line amount, upon conversion of the credit line amount into ordinary shares in accordance with the terms of the credit line agreement; | |
|
·
|
150,000 ordinary shares issuable upon the exercise of warrants with an exercise price per ordinary shares equal to the initial public offering price to be issued to our U.S. legal counsel as partial compensation for services rendered in connection with the offering; and
|
|
|
·
|
the ordinary shares issuable upon exercise of the underwriter’s compensation warrants to be issued in connection with this offering.
|
|
|
Except as otherwise indicated, information in this prospectus reflects or assumes:
|
||
|
·
|
the adoption of our amended and restated articles of association prior to the closing of this offering, which will replace our articles of association currently in effect;.
|
|
|
·
|
a one-for- reverse split of our ordinary shares, which will occur prior to the pricing of this offering;
|
|
|
·
|
the conversion of all outstanding preferred shares into an aggregate of ordinary shares immediately prior to the closing of this offering;
|
|
|
·
|
the issuance of 1,995,475 ordinary shares to Mr. Guy Neev upon the exercise prior to the closing of this offering of the Neev Options;
|
|
|
·
|
the issuance of 7,503,521 ordinary shares issuable under warrants that will be automatically exercised, without consideration, upon the exercise by Guy Neev of the Neev Options;
|
|
| · |
the issuance of ordinary shares in the Private Placement at the initial public offering price per ordinary share;
|
|
|
·
|
an initial public offering price of $ , which is the mid-point of the range set forth of the front cover of this prospectus; and
|
|
|
·
|
that the underwriters do not exercise their over-allotment option.
|
|
11
|
Summary Financial Data
You should read the following summary financial information in conjunction with our financial statements and related notes, “Selected Financial Information” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
The following tables set forth our summary financial data. You should read the following summary financial data in conjunction with, and it is qualified in its entirety by reference to, our historical financial information and other information provided in this prospectus, including “Selected Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes appearing elsewhere in this prospectus.
The summary statements of comprehensive loss data for the years ended December 31, 2012 and 2013, and the statements of financial position data as of December 31, 2013 are derived from our audited financial statements appearing elsewhere in this prospectus. The summary statements of comprehensive loss data for the six-month periods ended June 30, 2013 and 2014, and the statements of financial position data as of June 30, 2014 are derived from our unaudited financial statements appearing elsewhere in this prospectus. The unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, reflect all adjustments necessary to present fairly our financial position as of June 30, 2014 and our results of operations for the six months ended June 30, 2013 and 2014. The historical results set forth below are not necessarily indicative of the results to be expected in future periods. Our financial statements have been prepared in accordance with International Financial Reporting Standards, or IFRS, as issued by the International Accounting Standards Board. Statements of Comprehensive Loss Data
|
|
Year Ended December 31,
|
Six Months Ended June 30,
|
|||||||||||||||
|
2013
|
2012
|
2014
|
2013
|
|||||||||||||
|
(US$ in thousands, except per share data)
|
||||||||||||||||
|
(Unaudited)
|
||||||||||||||||
|
Research and development expenses, net(1)
|
2,662 | 2,692 | 1,640 | 1,364 | ||||||||||||
|
General and administrative expenses
|
1,090 | 1,203 | 564 | 520 | ||||||||||||
|
Other expenses (income)
|
(10 | ) | 13 | -- | -- | |||||||||||
|
Operating loss
|
3,742 | 3,908 | 2,204 | 1,884 | ||||||||||||
|
Finance income
|
(63 | ) | (416 | ) | (60 | ) | (45 | ) | ||||||||
|
Finance expenses
|
316 | 229 | 85 | 230 | ||||||||||||
|
Finance expenses (income), net
|
253 | (187 | ) | 25 | 185 | |||||||||||
|
Loss and total comprehensive loss for the period
|
3,995 | 3,721 | 2,229 | 2,069 | ||||||||||||
|
Loss per ordinary share of NIS 0.01 par value, basic and diluted(2)
|
0.18 | 0.17 | 0.10 | 0.09 | ||||||||||||
|
Weighted average number of ordinary shares outstanding – basic and diluted (in thousands)(2)
|
32,542 | 32,530 | 32,542 | 32,542 | ||||||||||||
|
Pro forma loss per ordinary share of NIS 0.01 par value(3)
|
||||||||||||||||
|
Basic and diluted (unaudited)(2)
|
||||||||||||||||
|
Pro forma weighted average number of ordinary shares outstanding - basic and diluted (in thousands) (unaudited)(2)
|
||||||||||||||||
12
| Statements of Financial Position Data |
As of December 31, 2013
|
As of June 30, 2014
|
|||||||||||
|
Actual
|
Pro forma(3)
|
Pro forma as
adjusted(4)
|
|||||||||||
|
(US$ in thousands)
Unaudited
|
|||||||||||||
|
Cash and cash equivalents
|
$ | 4,975 | $ | 2,794 | $ | ||||||||
|
Working capital(5)
|
4,131 | 1,990 | |||||||||||
|
Total assets
|
5,375 | 3,276 | |||||||||||
|
Capital stock
|
23,676 | 23,716 | |||||||||||
|
Total shareholders’ equity (deficit)
|
$ | 1,191 | $ | (998 | ) | ||||||||
|
|
(1)
|
Research and development expenses, net is presented net of the differences between the amount of grants received from the Office of the Chief Scientist of the Ministry of Economy (formerly named the Ministry of Industry, Trade and Labor), or the OCS, and the fair value of their financial liability. The effect of the participation by the OCS totaled $0.4 million and $0.2 million for the years ended December 31, 2013 and 2012, respectively. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview—Research and Development, Expenses, Net” for more information.
|
|
(2)
|
Basic and diluted loss per ordinary share is computed based on the basic and diluted weighted average number of ordinary shares outstanding during each period. For purposes of these calculations, the following ordinary shares are deemed to be outstanding: (i) the 1,995,475 ordinary shares issuable to Mr. Guy Neev upon exercise of the Neev Options; and (ii) the 7,503,521 ordinary shares issuable under warrants that will be automatically exercised without consideration upon the exercise by Mr. Guy Neev of the Neev Options. For additional information, see Note 17 to our financial statements for the year ended December 31, 2013 included elsewhere in this prospectus.
|
|
|
(3)
|
On a pro forma basis to give effect to the conversion immediately prior to the completion of this offering of all of our outstanding preferred shares into ordinary shares.
|
|
|
(4)
|
On a pro forma as adjusted basis to give further effect to (i) the issuance and sale of ordinary shares by us in this offering at an assumed initial public offering price of $ per share, the midpoint of the estimated initial public offering price range set forth on the cover of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us and (ii) the issuance and sale of ordinary shares by us in the concurrent Private Placement at an assumed price of $_______ per ordinary share, the estimated public offering price, after deducting commissions and estimated expenses payable by us in connection with the concurrent Private Placement.
|
|
|
(5)
|
Working capital is defined as total current assets minus total current liabilities.
|
13
Investing in our securities involves a high degree of risk. You should carefully consider the following risk factors and all other information contained in this prospectus, including the financial statements and the related notes appearing at the end of this prospectus, before purchasing our securities. If any of the following risks actually occur, they may materially harm our business and our financial condition and results of operations. In any such event, the market price of our securities could decline and you could lose all or part of your investment
Risks Related to Our Business
We have a history of losses, may incur future losses and may not achieve profitability.
We are a clinical and development-stage medical diagnostics company with a limited operating history. We have incurred net losses in each fiscal year since we commenced operations in 2009. We incurred net losses of $3.7 million in 2012, $4.0 million in 2013 and $2.2 million in the six months ended June 30, 2014. As of June 30, 2014, our accumulated deficit was $24.7 million. Our losses could continue for the foreseeable future as we continue our investment in research and development and clinical trials to complete the development of our technology and to attain regulatory approvals, begin the commercialization efforts for our imaging capsule, increase our marketing and selling expenses, and incur additional costs as a result of being a public company in the United States. The extent of our future operating losses and the timing of becoming profitable are highly uncertain, and we may never achieve or sustain profitability.
We may not succeed in completing the development of our product, commercializing our product and generating significant revenues.
Since commencing our operations, we have focused on the research and development and limited clinical trials of our imaging capsule. Our product is not approved for commercialization and has never generated any revenues. Our ability to generate revenues and achieve profitability depends on our ability to successfully complete the development of our product, obtain market approval and generate significant revenues. The future success of our business cannot be determined at this time, and we do not anticipate generating revenues from product sales for the foreseeable future. In addition, we have no experience in commercializing our imaging capsule and face a number of challenges with respect to our commercialization efforts, including, among others, that:
|
|
·
|
we may not have adequate financial or other resources to complete the development of our product;
|
|
|
·
|
we may not be able to manufacture our products in commercial quantities, at an adequate quality or at an acceptable cost;
|
|
|
·
|
we may not be able to establish adequate sales, marketing and distribution channels;
|
|
|
·
|
healthcare professionals and patients may not accept our imaging capsule;
|
|
|
·
|
we may not be aware of possible complications from the continued use of our imaging capsule since we have limited clinical experience with respect to the actual use of our imaging capsule;
|
|
|
·
|
technological breakthroughs in CRC screening, treatment and prevention may reduce the demand for our imaging capsule;
|
|
|
·
|
changes in the market for CRC screening, new alliances between existing market participants and the entrance of new market participants may interfere with our market penetration efforts;
|
|
|
·
|
third-party payors may not agree to reimburse patients for any or all of the purchase price of our imaging capsule, which may adversely affect patients’ willingness to purchase our imaging capsule;
|
|
|
·
|
uncertainty as to market demand may result in inefficient pricing of our imaging capsule;
|
|
|
·
|
we may face third-party claims of intellectual property infringement;
|
14
|
|
·
|
we may fail to obtain or maintain regulatory approvals for our imaging capsule in our target markets or may face adverse regulatory or legal actions relating to our imaging capsule even if regulatory approval is obtained; and
|
|
|
·
|
we are dependent upon the results of ongoing clinical studies relating to our imaging capsule and the products of our competitors.
|
If we are unable to meet any one or more of these challenges successfully, our ability to effectively commercialize our imaging capsule could be limited, which in turn could have a material adverse effect on our business, financial condition and results of operations.
Our early clinical experience to date may not have revealed certain potential limitations of the technology and potential complications from our imaging capsule.
To date, we have performed clinical studies with a prior version of our imaging capsule and with several versions of non-imaging capsules. The clinical trial that was conducted using the prior version of our imaging capsule was conducted under a different protocol and used a different group of patients. Therefore, we will have a limited ability to identify potential problems and/or inefficiencies concerning our imaging capsule in advance of its use in patients and we cannot assure you that its actual clinical performances will be satisfactory, or that its use will not result in unanticipated complications. Furthermore, the results from our first clinical studies and the previous pre-clinical studies may not be indicative of the clinical results obtained when we examine our final imaging capsule on real screening population. If our imaging capsule does not function as expected over time, we could be subject to liability claims, our reputation may be harmed and our imaging capsule would not be widely adopted.
We expect to derive most of our revenues from sales of one product or product line. Our inability to successfully commercialize this product, or any subsequent decline in demand for this product, could severely harm our ability to generate revenues.
We are currently dependent on the successful commercialization of our imaging capsule to generate revenues. As a result, factors adversely affecting our ability to successfully commercialize, or the pricing of or demand for, this product could have a material adverse effect on our financial condition and results of operations. If we are unable to successfully commercialize or create market demand for our imaging capsule, we will have limited ability to generate revenues.
Furthermore, and consequently, we are vulnerable to fluctuations in demand for our imaging capsule. Such fluctuations in demand may be due to many factors, including, among others:
|
|
·
|
market acceptance of a new product, including healthcare professionals’ and patients’ preferences;
|
|
|
·
|
development of similarly cost-effective products by our competitors;
|
|
|
·
|
development delays of our imaging capsule;
|
|
|
·
|
technological innovations in CRC screening, treatment and prevention;
|
|
|
·
|
adverse medical side effects suffered by patients using our imaging capsule, whether actually resulting from the use of our imaging capsule or not;
|
|
|
·
|
changes in regulatory policies toward CRC screening or imaging technologies;
|
|
|
·
|
changes in regulatory approval or clearance requirements for our product;
|
|
|
·
|
third-party claims of intellectual property infringement;
|
|
|
·
|
budget constraints and the availability of reimbursement or insurance coverage from third-party payors for our imaging capsule;
|
|
|
·
|
increases in market acceptance of other technologies; and
|
|
|
·
|
adverse responses from certain of our competitors to the offering of our imaging capsule.
|
15
If healthcare professionals do not recommend our product to their patients, our imaging capsule may not achieve market acceptance and we may not become profitable.
CRC screening candidates are generally referred by their healthcare professional to a specified device and screening technologies are purchased by prescription. If healthcare professionals, including physicians, do not recommend or prescribe our product to their patients, our imaging capsule may not achieve market acceptance and we may not become profitable. In addition, physicians have historically been slow to change their medical diagnostic and treatment practices because of perceived liability risks arising from the use of new products. Delayed adoption of our imaging capsule by healthcare professionals could lead to a delayed adoption by patients and third-party payors. Healthcare professionals may not recommend or prescribe our imaging capsule until certain conditions have been satisfied including, among others:
|
|
·
|
there is sufficient long-term clinical evidence to convince them to alter their existing screening methods and device recommendations;
|
|
|
·
|
there are recommendations from other prominent physicians, educators and/or associations that our imaging capsule is safe and effective;
|
|
|
·
|
we obtain favorable data from clinical studies for our imaging capsule;
|
|
|
·
|
reimbursement or insurance coverage from third-party payors is available; and
|
|
|
·
|
they become familiar with the complexities of our imaging capsule.
|
We cannot predict when, if ever, healthcare professionals and patients may adopt the use of our imaging capsule. Even if favorable data is obtained from clinical studies for our imaging capsule, there can be no assurance that prominent physicians would endorse it or that future clinical studies will continue to produce favorable data regarding our imaging capsule. In addition, prolonged market exposure may also be a pre-requisite to reimbursement or insurance coverage from third-party payors. If our imaging capsule does not achieve an adequate level of acceptance by patients, healthcare professionals and third-party payors, we may not generate significant product revenues and we may not become profitable.
If we are unable to market and sell our imaging capsule, we may not become profitable.
We have not had any sales of our imaging capsule to date. There can be no assurance that we will be able to receive regulatory clearance for our imaging capsule in the foreseeable future or ever or that our imaging capsule will be accepted as comparable or superior to existing technologies for the visualization, imaging or screening of the colon. Our ability to market and sell our imaging capsule successfully depends on one or more of the following:
|
|
·
|
the existence of clinical data sufficient to support the use of our imaging capsule for the visualization, imaging, or screening of the colon as compared to other colon visualization, imaging or screening methods (if clinical trials indicate that our imaging capsule is not as clinically effective as other current methods, or if our technology causes unexpected complications or other unforeseen negative effects, we may not obtain regulatory clearance or approval to market and sell our imaging capsule or physicians may be reluctant to use it);
|
|
|
·
|
the availability of sufficient clinical data for physicians to use our imaging capsule in their practice and for private third-party payors to make an adequate reimbursement decision to provide coverage for our imaging capsule; and
|
|
|
·
|
the availability of a reliable contrast agent for our imaging capsule that is accepted by physicians and patients.
|
If one or more of the above conditions is not satisfied, we may not be able to market and sell our imaging capsule or the demand for our imaging capsule may be lower than expected and sales of our imaging capsule may not contribute to our growth at the rate we expect or at all.
16
We expect to face competition from large, well-established manufacturers of traditional technologies for detecting gastrointestinal disorders, as well as from other manufacturers of optical capsule endoscopy systems.
Competition for our imaging capsule comes from traditional well-entrenched manufacturers of equipment for detecting gastrointestinal disorders and diseases, such as colonoscopy, sigmoidoscopy, optical capsule endoscopy and CTC. The principal manufacturers of equipment for optical colonoscopy, sigmoidoscopy and optical capsule endoscopy are Olympus, Richo Company Ltd., Hoya, Covidien plc and Fuji Film. The principal manufacturers of equipment for CTC are General Electric Healthcare Systems, Siemens Medical Solutions, Philips Medical Systems Ltd. and Toshiba Corporation. All of these companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide distribution channels for providing medical instruments to physicians.
In addition, several companies are developing technologies based on molecular diagnostics (from blood and other bodily fluids), or MDx, tests that investigate the link between genes and the function of metabolic pathways, drug metabolism and disease development with a primary focus on the study of DNA, RNA and proteins. Genetic markers can be traced within stool samples in minute quantities. A U.S. based company, Exact Sciences, is developing a special collecting kit for stool samples and an analyzer to diagnose CRC based on these stool-based markers. The method of screening for colon cancer using stool DNA testing has been endorsed by the ACS, the U.S. Multi-Society Task Force on Colorectal Cancer and the American College of Radiology, but not by the U.S. Preventive Services Task Force.
Certain companies are developing or commercializing optics-based optical capsule endoscopy systems. The existing capsule technology requires intense bowel cleansing, even more so than is required for colonoscopy. Given Imaging, an Israeli-based company that was acquired by Covidien plc (NYSE: COV) in February 2014, has developed visualization capsules for the detection of disorders of the esophagus, small bowel and colon. It launched its PillCamColon capsule in Europe in 2007 and has limited sales. In early 2014, the FDA granted approval for optical capsule endoscopy for colon cancer screening only for patients after incomplete colonoscopy. However, this technology requires bowel cleansing to an even greater degree than that required for regular optical colonoscopy, which can result in dehydration and, in turn, can lead to cancellation of the procedure in certain cases. Other companies, including Olympus, Intromedic and RF System, are developing similar approaches for optical capsule endoscopy.
Procedures for bowel cleansing that are less onerous are constantly being developed, which could make our entry into the market more difficult. For instance, bowel cleansing initiated by the ingestion of pills rather than through drinking large amounts of distasteful liquids may be viewed as an improvement to the cleansing process, but other screening methods may be even more palatable to patients.
If we are unable to convince physicians to adopt our imaging capsule over the current technologies marketed by our competitors, our results of operations may suffer.
We are planning to rely on local distributors to market and distribute our imaging capsule.
We are planning to rely on distributors for the marketing and distribution of our imaging capsule. Our success in generating sales in countries or regions where we will engage local distributors will depend in part on the efforts of third parties over whom we have limited control. If we are unable to identify suitable local distributors in the countries where we intend to market and distribute our imaging capsule, our business, financial condition and results of operations could be negatively affected.
We have limited manufacturing capabilities and if we are unable to scale our manufacturing operations to meet anticipated market demand, our growth could be limited and our business, financial condition and results of operations could be materially adversely affected.
We currently have limited resources, facilities and experience in commercially manufacturing sufficient quantities of our imaging capsule, external receiver and software application to meet the demand we expect from our expanded commercialization efforts. We expect to face certain technical challenges as we increase manufacturing capacity, including, among others, logistics associated with the handling of radioactive materials, equipment design and automation, material procurement, lower than expected yields and increased scrap costs, as well as challenges related to maintaining quality control and assurance standards. Furthermore, we may encounter similar or unforeseen challenges initiating and later expanding production of any new products. If we are unable to scale our manufacturing capabilities to meet market demand, our growth could be limited and our business, financial condition and results of operations could be materially adversely affected.
In addition, we have received grants from Government of the State of Israel through the OCS (see “Risk Factors – Risks Related to Our Operations in Israel”), the terms of which require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless prior approval is received from the OCS, which we may not receive. We are currently considering whether it would be possible to assemble the capsule without the X-ray source in Israel, and have the X-ray source subsequently inserted into our imaging capsule at a reactor or cyclotron site or at a distribution center outside Israel. Even following the full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Encouragement of Industrial Research and Development Law 5744-1984, or the Research Law. The foregoing restrictions may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel.
17
Our reliance on limited source suppliers could harm our ability to meet demand for our product in a timely manner or within budget.
We currently depend on limited source suppliers for some of the components necessary for the production of our product. For example, for the current version of the imaging capsule used in clinical trials, we currently have one leading supplier for the motor that we are using to rotate the collimated X-ray source in our imaging capsule and we currently have one leading supplier for the X-ray detectors used in our imaging capsule. There are a limited number of manufacturers worldwide who are capable of manufacturing the motor and the specially designed X-ray detectors that we currently use in our imaging capsule. In addition, the application-specific integrated circuit, or ASIC, residing in our imaging capsule is currently manufactured for us by a single semiconductor fabrication plant, or FAB. There are many alternative FABs worldwide and the design of our current ASIC could be adapted in the event it became necessary to use an alternative FAB. Our current suppliers have been able to supply the required quantities of such components to date. However, if the supply of these components is disrupted or terminated or if our current suppliers are unable to supply required quantities of components, we may not be able to find alternative sources for these key components in a timely manner. Although we are planning to maintain strategic inventory of key components, the inventory may not be sufficient to satisfy the demand for our imaging system if such supply is interrupted or otherwise affected by catastrophic events such as a fire at our storage facility. As a result, we may be unable to meet the demand for our imaging system, which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to change the manufacturer of any of these key components, there may be a significant delay in locating a suitable alternative manufacturer. In addition, we may be required to verify that the new manufacturer maintains facilities and procedures that comply with FDA and other applicable quality standards and with all applicable regulations and guidelines. The delays associated with the identification of a new manufacturer could delay our ability to manufacture our imaging system in a timely manner or within budget. Furthermore, in the event that the manufacturer of a key component of our imaging system ceases operations or otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture the component. The occurrence of any of these events could harm our ability to meet demand for our imaging system in a timely manner or within budget.
The use of any of our imaging capsule, external receiver or software application could result in product liability or similar claims that could be expensive damage our reputation and harm our business.
Our business exposes us to an inherent risk of potential product liability or similar claims related to the manufacturing, marketing and sale of medical devices. The medical device industry has historically been litigious, and we face financial exposure to product liability or similar claims if the use of any of our imaging capsule, external receiver or software application were to cause or contribute to injury or death, including, without limitation, harm to the body caused by the procedure or inaccurate diagnoses from the procedure that could affect treatment options. There is also the possibility that defects in the design or manufacture of any of these products might necessitate a product recall. Although we plan to maintain product liability insurance, the coverage limits of these policies may not be adequate to cover future claims. In the future, we may be unable to maintain product liability insurance on acceptable terms or at reasonable costs and such insurance may not provide us with adequate coverage against potential liabilities. A product liability claim, regardless of merit or ultimate outcome, or any product recall could result in substantial costs to us, damage to our reputation, customer dissatisfaction and frustration, and a substantial diversion of management attention. A successful claim brought against us in excess of, or outside of, our insurance coverage could have a material adverse effect on our business, financial condition and results of operations.
Our imaging capsule is a complex medical device that requires intensive training and care for data analysis.
Our imaging capsule is a complex medical device that requires intensive training and care for data analysis. Although our distributors will be required to ensure that our imaging capsule is only prescribed by trained clinicians, the potential for misuse of our imaging capsule still exists due to its complexity. Such misuse could result in adverse medical consequences for patients that could damage our reputation, subject us to costly product liability litigation and otherwise have a material adverse effect on our business, financial condition and results of operations.
18
We depend on third parties to manage our clinical studies and trials and to perform related data collection and analysis and, as a result, we may face costs and delays that are beyond our control.
We rely on third parties, including clinical investigators and clinical sites, to manage our clinical trials and to perform data collection and analysis. Although we have and expect to continue to have contractual arrangements with these third parties, we may not be able to control the amount and timing of resources that these parties devote to our studies and trials or the quality of these resources. If these third parties fail to properly manage our studies and trials, we will be unable to complete them at all or in a satisfactory manner, which could prevent us from obtaining regulatory approvals for, or achieving market acceptance of, our product.
In addition, termination of relationships with third parties may result in delays, inability to enter into arrangements with alternative third parties or do so on commercially reasonable terms. Switching or adding additional clinical sites involves additional cost and requires management time and focus. In addition, there is a natural transition period when a new clinical site commences work. As a result, delays occur, which can materially impact our ability to meet our desired clinical development timelines.
We intend to sell our products in the United States, Europe and Japan and, if we are unable to manage our operations in these territories, our business, financial condition and results of operations could be materially adversely affected.
Our headquarters and all of our operations and employees are presently located in Israel, but we intend to market our products in the United States, Europe and Japan. Accordingly, we are subject to risks associated with international operations, and our international sales and operations will require significant management attention and financial resources. In addition, our international sales and operations will subject us to risks inherent in international business activities, many of which are beyond our control and include, among others:
|
|
•
|
foreign certification, registration and other regulatory requirements;
|
|
|
•
|
customs clearance and shipping delays;
|
|
|
•
|
import and export controls;
|
|
|
•
|
trade restrictions;
|
|
|
•
|
multiple and possibly overlapping tax structures;
|
|
|
•
|
difficulty forecasting the results of our international operations and managing our inventory due to our reliance on third-party distributors;
|
|
|
•
|
differing laws and regulations, business and clinical practices, third-party payor reimbursement policies and patient preferences;
|
|
|
•
|
differing standards of intellectual property protection among countries;
|
|
|
•
|
difficulties in staffing and managing our international operations;
|
|
|
•
|
difficulties in penetrating markets in which our competitors’ products are more established;
|
|
|
•
|
currency exchange rate fluctuations; and
|
|
|
•
|
political and economic instability, war or acts of terrorism.
|
If we are unable to manage our international operations effectively, our business, financial condition and results of operations could be materially adversely affected.
19
We will require additional funding in order to complete the commercialization of our imaging capsule and the development and commercialization of any future products.
Our operations have consumed substantial amounts of cash. We expect that we will need to continue to spend substantial amounts in order to complete the development, clinical development, regulation and commercialization of our imaging capsule. Although we intend to use the proceeds of this offering and the concurrent Private Placement to finance these efforts, we will need to raise additional funds prior to commercialization of our product. Additional financing may not be available to us on a timely basis on terms acceptable to us, or at all. In addition, any additional financing may be dilutive to our shareholders or may require us to grant a lender a security interest in our assets.
Furthermore, if adequate additional financing on acceptable terms is not available, we may not be able to develop our imaging capsule at the rate or to the stage we desire and we may have to delay or abandon the commercialization of our imaging capsule. Alternatively, we may be required to prematurely license to third parties the rights to further develop or to commercialize our imaging capsule on terms that are not favorable to us. Any of these factors could materially adversely affect our business, financial condition and results of operations.
If we lose our key personnel or are unable to attract and retain additional personnel, our business and ability to compete will be harmed.
We are dependent on the principal members of our management, research and development team and scientific staff. In order to implement our business strategy, we will need to retain our key personnel with expertise in the areas of research and development, clinical testing, government regulation, manufacturing, finance, marketing and sales. Our product development plans depend in part on our ability to retain engineers with expertise in a variety of technical fields. The loss of a number of these persons or our inability to attract and retain qualified personnel could harm our business and our ability to compete.
Substantially all of our operations are currently conducted at a single location near Haifa, Israel, and any disruption at our facility could materially adversely affect our business, financial condition and results of operations.
Substantially all of our operations are conducted at a single location near Haifa, Israel. We take precautions to safeguard our facility, including obtaining insurance coverage and implementing health and safety protocols and off-site storage of computer data. However, a natural or other disaster, such as a fire, flood or an armed conflict involving Israel, as detailed further below, could damage or destroy our facility and our manufacturing equipment or inventory, cause substantial delays in our operations and otherwise cause us to incur additional unanticipated expenses. In addition, the insurance we maintain against fires, floods and other natural disasters may not be adequate to cover our losses in any particular case and it does not cover losses resulting from armed conflicts or terrorist attacks in Israel. Damage to our facility, our other property or to any of our suppliers, whether located in Israel or elsewhere, due to fire, a natural disaster or casualty event or an armed conflict, could materially adversely affect our business, financial condition and results of operations, with or without insurance.
We will incur significant increased costs as a result of operating as a public company in the United States, and our management will be required to devote substantial time to new compliance initiatives.
As a public company in the United States, we will incur significant legal, accounting and other expenses that we did not incur as a private company. The Sarbanes-Oxley Act of 2002, as well as rules and regulations implemented by the U.S. Securities and Exchange Commission and the NASDAQ Stock Market, impose various requirements on public companies, including requiring the establishment and maintenance of effective disclosure and financial controls. Our management and other personnel will need to devote a substantial amount of time to these compliance initiatives. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time consuming and costly. We cannot predict or estimate the amount of additional costs we may incur as a result of becoming a public company or the timing of such costs. These rules and regulations could make it more difficult and more expensive for us to obtain certain types of insurance including director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantial costs to maintain the same or similar coverage. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees or as executive officers. We cannot predict or estimate the amount or timing of additional costs we may incur in order to comply with such requirements.
20
We will be required to develop and maintain proper and effective internal controls over financial reporting. We may not complete our analysis of our internal controls over financial reporting in a timely manner, or these internal controls may have one or more material weaknesses, which may adversely affect investor confidence in our company and, as a result, the value of our ordinary shares.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis will be a costly and time-consuming effort that will need to be evaluated frequently. Section 404 of the Sarbanes-Oxley Act requires the management of public companies to conduct an annual review and evaluation of their internal controls and to obtain an attestation report from their registered public accounting firm regarding the effectiveness of internal controls. We would be required to perform the annual review and evaluation of our internal controls no later than in connection with the second annual report on Form 20-F filed after the offering to which this prospectus relates. However, if we qualify as a smaller reporting company and/or emerging growth company, which we expect to, we will be exempt from the auditors’ attestation requirement until such time as we no longer qualify as a smaller reporting company and/or emerging growth company. We would no longer qualify as a smaller reporting company if the market value of our public float exceeded $75 million as of the last day of our second fiscal quarter in any fiscal year following this offering. We would no longer qualify as an emerging growth company at such time as described in the risk factor immediately below.
We are in the early stages of the costly and challenging process of compiling the system and processing documentation necessary to evaluate and correct a material weakness in internal controls needed to comply with Section 404. The material weakness relates to our being a small company with a limited number of employees which limits our ability to assert the controls related to the segregation of duties. During the evaluation and testing process, if we identify one or more additional material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal controls are effective. If we are unable to assert that our internal control over financial reporting is effective, we could lose investor confidence in the accuracy and completeness of our financial reports, which would cause the price of our ordinary shares to decline.
While we currently qualify as an “emerging growth company” under the JOBS Act, we will cease to be an emerging growth company on or before the end of 2019, and at such time our costs and the demands placed upon our management will increase.
We will continue to be deemed an emerging growth company until the earliest of (i) the last day of the fiscal year in which our annual gross revenues exceed $1 billion (as indexed for inflation); (ii) the last day of the fiscal year in which the fifth anniversary of the date of the first sale of ordinary shares under this registration statement; (iii) the date on which we have, during the previous three-year period, issued more than $1 billion in non-convertible debt; or (iv) the date on which we are deemed to be a ‘large accelerated filer,’ as defined by the U.S. Securities and Exchange Commission, which would generally occur upon our attaining a public float of at least $700 million. Once we lose emerging growth company status, we expect the costs and demands placed upon our management to increase, as we will be required to comply with additional disclosure and accounting requirements, particularly if we also no longer qualify as a smaller reporting company.
Risks Related to Regulations
If we are unable to obtain, or experience significant delays in obtaining, FDA clearances or approvals, CE Certificates of Conformity, or equivalent third country approvals for our imaging capsule or future products or product enhancements, our ability to commercially distribute and market our products could suffer.
Our products are subject to rigorous regulation by FDA and numerous other federal, state and foreign governmental authorities and notified bodies. The process of obtaining regulatory clearances or approvals, CE Certificates of Conformity, or equivalent third country approvals to market a medical device can be costly and time consuming, and we may not be able to obtain these clearances or approvals, CE Certificates of Conformity, or equivalent third country approvals on a timely basis, if at all. In particular, we expect to eventually generate a portion of our revenues from sales of our imaging capsule and future products in the United States, the European Union, or third countries. Before a new medical device, or a new use of, or claim for, an existing product can be marketed in the United States, it must first receive clearance under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FDA approval of a premarket approval application, or PMA, unless an exemption applies. FDA will clear marketing of a low to moderate risk medical device through the 510(k) process if sufficiently similar predicate devices have previously been cleared via this pathway. In the 510(k) clearance process, FDA must only determine that the proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use/indications for use, technological characteristics and principles of operation in order to clear the proposed device for marketing. Clinical data is sometimes required to support substantial equivalence.
21
High risk devices deemed to pose the greatest risk, such as life-sustaining, life-supporting, or implantable devices, or devices not deemed substantially equivalent to a previously cleared device, require approval of a PMA. The PMA process is more costly, lengthy and uncertain than the 510(k) clearance process. The PMA pathway requires an applicant to demonstrate the safety and effectiveness of the device based, in part, on the data obtained in clinical trials. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to FDA’s satisfaction the safety and efficacy of the device for its intended use.
In instances where a device is novel and there is no suitable predicate device, but that device is deemed to be of low to moderate risk, FDA can reclassify the device to class I or class II via de novo reclassification. This process involves the submission of a reclassification petition, and FDA accepting that “special controls” are adequate to ensure the product’s performance and safety. FDA now allows “direct” de novo reclassification petitions, a mechanism by which a sponsor can directly submit a detailed de novo reclassification petition as the device’s initial submission without having to first receive a not substantially equivalent, or NSE, decision on a 510(k) submission.
These processes can be expensive and lengthy. FDA’s 510(k) clearance process usually takes from 6 to 9 months, but it can last longer. Direct de novo reclassification typically takes at least 9 to 12 months from filing to clearance. The PMA pathway is much more costly and uncertain than the 510(k) clearance process or de novo reclassification, and generally takes at least 12 to 18 months, or even longer, from the time the application is filed with FDA to ultimate approval.
We are not aware of any legally marketed predicate device upon which FDA could base a determination of substantial equivalence under a 510(k) clearance process. Our strategy therefore is to submit a direct de novo reclassification petition for our imaging capsule. To support this petition, our objective is to demonstrate that the device poses a low to moderate risk to patients. We cannot assure you that FDA will not demand that we obtain PMA approval of our imaging capsule.
FDA can delay, limit or deny clearance or approval of an application for many reasons, including, among others:
|
|
•
|
we may not be able to demonstrate to FDA’s satisfaction that our products are safe and effective for their intended use;
|
|
|
•
|
the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval;
|
|
|
•
|
in the case of a PMA submission, that the manufacturing process or facilities we use may not meet applicable requirements; and
|
|
|
•
|
changes in FDA’s 510(k) clearance, de novo reclassification, or PMA approval processes and policies, or the adoption of new regulations may require additional data.
|
We may not obtain the necessary regulatory clearances, approvals, CE Certificates of Conformity or equivalent third country approvals to market our imaging capsule or future products in the United States or elsewhere. Any delay in, or failure to receive or maintain, clearance, approval or CE Certificates of Conformity for our imaging capsule or other products under development could prevent us from generating revenue from these products or achieving profitability.
22
There is no guarantee that the FDA will grant de novo reclassification or PMA approval of our imaging capsule and failure to obtain necessary 510(k) clearances or approvals for our future products would adversely affect our ability to grow our business.
Our imaging capsule and some of our future products will require FDA clearance of a 510(k), de novo reclassification, or may require FDA approval of a PMA. The FDA may not approve or clear our imaging capsule or our future products for the indications that are necessary or desirable for successful commercialization. Indeed, the FDA may refuse our requests for 510(k) clearance, de novo reclassification or premarket approval for our imaging capsule or any other future product, new intended uses or modifications to these products once they are cleared or approved for marketing.
Our strategy is to submit a direct de novo reclassification petition for our imaging capsule. A de novo reclassification generally applies where there is no predicate device and the FDA believes the device poses a low to moderate risk. De novo reclassifications can either be submitted in lieu of a 510(k) notice, such as in our case, or after a 510(k) notice has been filed and found NSE. If a 510(k) notice is found NSE, a de novo petition must be submitted within 30 days from the receipt of the NSE determination.
To support our direct de novo reclassification petition, our objective is to demonstrate that the device poses a low to moderate risk to patients. If the FDA determines that our imaging capsule is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process. A PMA application must be supported by extensive data, including, but not limited to, technical, preclinical, clinical trial, manufacturing and labeling data, to demonstrate to the FDA’s satisfaction the safety and efficacy of the device for its intended use. By statute, the FDA has 180 days to review the “accepted application,” although, generally, review of the application can take between one and three years. During this review period, the FDA may request additional information or clarification of information already provided or even request new data that may require us to conduct additional tests. Also during the review period, an advisory panel of experts from outside the FDA may be convened to review and evaluate the application and provide recommendations to the FDA as to the approvability of the device. In addition, the FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations. The FDA’s review of a PMA could significantly delay our plans to get to market. There is also no guarantee that the FDA would approve a PMA. Failure to receive clearance or approval for our imaging capsule or future products would have an adverse effect on our ability to expand our business.
If we or our future distributors do not obtain and maintain the necessary regulatory clearances or approvals, or CE Certificates of Conformity, or equivalent third country approvals in a specific country or region, we will not be able to market and sell our imaging capsule or future products in that country or region.
We intend to market our imaging capsule in a number of international markets. To be able to market and sell our imaging capsule in a specific country or region, we or our distributors must comply with the regulations of that country or region. While the regulations of some countries do not impose barriers to marketing and selling part or all of our products or only require notification, others require that we or our distributors obtain the approval of a specified regulatory authorities or that we obtain CE Certificates of Conformity from a Notified Body. These regulations, including the requirements for approvals or CE Certificates of Conformity, and the time required for regulatory review, vary from country to country. Obtaining regulatory approvals or CE Certificates of Conformity is expensive and time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals or CE Certificates of Conformity for our imaging capsule or any future products in each country or region in which we plan to market such products. If we modify our imaging capsule or any future products, we or our distributors may need to apply for new regulatory approvals or our Notify Body may need to review the planned changes before we are permitted to sell them. We may not meet the quality and safety standards required to maintain the authorizations or CE Certificates of Conformity that we or our distributors have received. If we or our distributors are unable to maintain our authorizations or CE Certificates of Conformity in a particular country or region, we will no longer be able to sell our imaging capsule or any future products in that country or region, and our ability to generate revenues will be materially and adversely affected.
23
Our imaging capsule may be considered a drug-device combination product because of the preparatory use of Iodine or barium sulfate to provide a coating for colonic imaging. We cannot be sure how the FDA or the competent regulatory authorities of foreign countries will regulate this product. The review of combination products is often more complex and more time consuming than the review of products under the jurisdiction of only one center within the FDA.
Our imaging capsule may be considered a combination product because of the preparatory use of barium sulfate or Iodine to provide a coating for colonic imaging. A combination product is the combination of two or more regulated components, i.e., drug/device, biologic/device, drug/biologic, or drug/device/biologic, that are combined or mixed and produced as a single entity; packaged together in a single package or as a unit; or a drug, device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended for use only with an approved individually specified drug, device, or biological product where both are required to achieve the intended use, indication or effect. For a combination product, the FDA must determine which center or centers within the FDA will review the product and under what legal authority the product candidate will be reviewed. The combination product’s primary mode of action is used to determine which center within the FDA has primary regulatory jurisdiction over the product. The other centers within the agency also may provide consulting or collaborative reviews of the product as necessary. We believe that we have put forth a reasonable argument to the FDA that our imaging capsule should be regulated as a device and or a combination product with a device primary mode of action. However, we cannot be sure as to whether the FDA will treat our imaging capsule as a device or a combination product. The review of combination products is often more complex and more time consuming than the review of a product under the jurisdiction of only one center within the FDA. In the case of the imaging capsule, should the FDA determine that the barium sulfate is not being used in accordance with its approved labeling, the Center for Drug Evaluation and Research may take a prominent role it its regulation. If the FDA does not approve or clear our imaging capsule, or any future products, in a timely fashion, or at all, our business and financial condition will be adversely affected.
Similar obstacles may be encountered in foreign countries should our imaging capsule be considered as a combination product.
If the indications for use or instructions for use for which the Iodine-based contrast agent or the barium sulfate- based contrast agent is approved are not sufficiently broad to support its use prior to the ingestion of our imaging capsules, the FDA or the competent regulatory authorities in the EU Member States and other foreign countries may consider that contrast agent is being used off-label.
Ingestion of our imaging capsule requires the preparatory use of Iodine or barium sulfate to provide a coating for colonic imaging. We cannot be sure that the indications for which Iodine-based contrast agent or the barium sulfate-based contrast agent are approved in the United States, the EU Member States or in other countries is sufficiently broad to cover such use. If the FDA or the competent regulatory authorities in the EU Member States and in other countries consider that Iodine and/or barium sulfate is not approved for the purpose for which it is used with the imaging capsules, we may be considered to promote the off-label use of the Iodine and/or barium sulfate. Because the off-label use of drugs or medicinal products is generally prohibited in the United States, the EU Member States and in other countries, we could face both related issues with the FDA and/or the competent authorities of the EU Member States and/or other countries. In these circumstances, the FDA and/or the competent regulatory authorities in the EU Member States and/or other countries may require us to obtain appropriate regulatory approvals for the Iodine-based contrast agent or the barium sulfate-based contrast agent prior to marketing our imaging capsules with such substances. Under such circumstances, should we fail to obtain approval of the contrast agent for use with our imaging capsule, in a timely fashion, or at all, our business and financial condition will be adversely affected.
If we are unable to successfully complete clinical trials with respect to our imaging capsule, we may be unable to receive regulatory approvals or clearances, CE Certificates of Conformity or equivalent third country approvals for our imaging capsule and/or our ability to achieve market acceptance of our imaging capsule will be harmed.
The development of medical devices typically includes pre-clinical studies. Certain other devices require the submission of data generated from clinical trials, which can be long, expensive and uncertain processes, subject to delays and failure at any stage. The data obtained from the studies and trials may be inadequate to support regulatory clearances or approvals, or to obtain CE Certificates of Conformity or equivalent third country approval, or to allow market acceptance of the products being studied. Our imaging capsule technology is currently undergoing clinical development and clinical trials. To date, we have performed clinical studies with a prior version of our imaging capsule and with several versions of non-imaging capsules.
24
The development of sufficient and appropriate clinical protocols to demonstrate safety and efficacy are required, and we may not adequately develop such protocols to support clearance, approval, or to obtain CE Certificates of Conformity or equivalent third country approval. The clinical trial that was conducted using the prior version of our imaging capsule, was conducted under a different protocol and used a group of patients different from those we intend to study in future clinical trials. Further, FDA, the competent regulatory authorities of other countries, or our Notified Body in the EU may require us to submit data on a greater number of patients than we originally anticipated and/or for a longer follow-up period or they may change the data collection requirements or data analysis applicable to our clinical trials.
The commencement or completion of any of our clinical studies or trials may be delayed or halted, or be inadequate to support regulatory clearance, approval or product acceptance, or to obtain CE Certificates of Conformity or equivalent third country approval, for numerous reasons, including, among others:
|
|
•
|
patients do not enroll in the clinical trial at the rate we expect;
|
|
|
•
|
patients do not comply with trial protocols;
|
|
|
•
|
patient follow-up is not at the rate we expect;
|
|
|
•
|
patients experience adverse side effects;
|
|
|
•
|
patients die during a clinical trial, even though their death may be unrelated to our product;
|
|
|
•
|
FDA or other regulatory authorities do not approve a clinical trial protocol or a clinical trial, or place a clinical trial on hold;
|
|
|
•
|
institutional review boards, or IRBs, Ethics Committees and third-party clinical investigators may delay or reject our trial protocol and Informed Consent Form;
|
|
|
•
|
third-party clinical investigators decline to participate in a study or trial or do not perform a study or trial on our anticipated schedule or consistent with the investigator agreements, study or trial protocol, good clinical practices or other FDA or IRBs, Ethics Committees, or any other applicable requirements;
|
|
|
•
|
third-party organizations do not perform data collection, monitoring and analysis in a timely or accurate manner or consistent with the study or trial protocol or investigational or statistical plans;
|
|
|
•
|
regulatory inspections of our studies, trials or manufacturing facilities may require us to, among other things, undertake corrective action or suspend or terminate our studies or clinical trials;
|
|
|
•
|
changes in governmental regulations or administrative actions;
|
|
|
•
|
the interim or final results of the study or clinical trial are inconclusive or unfavorable as to safety or efficacy; and
|
|
|
•
|
a regulatory agency or our Notified Body concludes that our trial design is or was inadequate to demonstrate safety and efficacy.
|
The results of pre-clinical and clinical studies do not necessarily predict future clinical trial results, and predecessor clinical trial results may not be repeated in subsequent clinical trials. Additionally, FDA, the competent regulatory authorities of EEU Member States, other third country regulatory entities, or our Notified Body may disagree with our interpretation of the data from our pre-clinical studies and clinical trials, or may find the clinical trial design, conduct or results inadequate to demonstrate safety or efficacy, and may require us to pursue additional pre-clinical studies or clinical trials, which could further delay the clearance, approval, or CE marking of our products. The data we collect from our non-clinical testing, our pre-clinical studies and other clinical trials may not be sufficient to support regulatory clearance, approval or to obtain CE Certificates of Conformity.
25
If the third parties on which we rely to conduct our clinical trials and clinical development do not perform as contractually required or expected, we may not be able to obtain regulatory clearance or approval, CE Certificates of Conformity, or equivalent third country approval for, or commercialize, our imaging capsule or future products.
We do not have the ability to independently conduct our clinical trials for our imaging capsule and we must rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct such trials. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if these third parties need to be replaced, or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain CE Certificates of Conformity, regulatory clearance, approval for, or successfully commercialize, our imaging capsule or future products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected. Furthermore, our third-party clinical trial investigators may be delayed in conducting our clinical trials for reasons outside of their control.
The results of our clinical trials may not support our product candidate claims or may result in the discovery of adverse side effects.
Even if our clinical trials are completed as planned, we cannot be certain that their results will support our product claims or that the FDA, foreign authorities or our Notified Body will agree with our conclusions regarding them. Success in pre-clinical studies and early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that clinical trials will replicate the results of prior trials and pre-clinical studies. The clinical trial process may fail to demonstrate that our imaging capsule, or any future products, are safe and effective for the proposed indicated uses, which could cause us to abandon a product and may delay development of others. Any delay or termination of our clinical trials will delay the filing of our product submissions and, ultimately, our ability to commercialize our imaging capsule, or any future products, and generate revenues. It is also possible that patients enrolled in clinical trials will experience adverse side effects that are not currently part of the product candidate’s profile.
Even if our imaging capsule or future products are cleared or approved by regulatory authorities or if we obtain CE Certificates of Conformity from our Notified Body, modifications to our imaging capsule or future products may require new regulatory clearances or approvals, new CE Certificates of Conformity, or may require us to recall or cease marketing it until the necessary clearances, approvals or CE Certificates of Conformity are obtained.
Once marketed, modifications to our imaging capsule or future products may require new regulatory approvals, clearances, including 510(k) clearances or premarket approvals, or require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. Any modification to a 510(k)-cleared device that could significantly affect its safety or efficacy, or that would constitute a major change in its intended use, requires a new 510(k) clearance or, possibly, a PMA. The FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new approval, supplement or clearance. A manufacturer may determine that a modification could not significantly affect safety or efficacy and does not represent a major change in its intended use, so that no new 510(k) clearance is necessary. However, the FDA can review a manufacturer’s decision and may disagree. The FDA may also on its own initiative determine that a new clearance or approval is required. We may make modifications to our imaging capsule in the future that we believe do not or will not require additional clearances or approvals. Further, our products could be subject to recall if the FDA determines, for any reason, that our products are not safe or effective. Any recall or FDA requirement that we seek additional approvals or clearances could result in significant delays, fines, increased costs associated with modification of a product, loss of revenue and potential operating restrictions imposed by the FDA.
If a manufacturer determines that a modification to an FDA-cleared device could significantly affect its safety or efficacy, or would constitute a major change in its intended use, then the manufacturer must file for a new 510(k) clearance or possibly a premarket approval application. Where we determine that modifications to our products require a new 510(k) clearance or premarket approval application, we may not be able to obtain those additional clearances or approvals for the modifications or additional indications in a timely manner, or at all.
26
Any modification to a PMA-approved device must either be approved in a PMA Supplement, or if the modification does not impact the device’s safety or effectiveness, described in a 30-Day Notice or in the device’s Annual Report. The FDA may not approve a modification described in a PMA Supplement, in which case the modified device cannot be marketed. The FDA can also disagree that a change described in a 30-Day Notice or Annual Report is appropriately described in either filing, and request that the company file a PMA Supplement and/or request that the company cease marketing the modified device until the PMA Supplement is approved.
Similar rules also apply in foreign jurisdictions. In the European Union, or EU, we must inform the Notified Body that carried out the conformity assessment of the medical devices we market or sell in the EU of any planned substantial changes to our quality system or changes to our devices which could affect compliance with the Essential Requirements laid down in Annex I to the Council Directive 93/42/EEC concerning medical devices (“Medical Devices Directive”) or the devices’ intended purpose. The Notified Body will then assess the changes and verify whether they affect the products’ conformity with the Essential Requirements laid down in Annex I to the Medical Devices Directive or the conditions for the use of the device. If the assessment is favorable, the Notified Body will issue a new CE Certificate of Conformity or an addendum to the existing CE Certificate of Conformity attesting compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive.
If the Notified Body or relevant regulatory authorities disagree with our assessments and require modifications to an existing CE Certificate of Conformity, the preparation of a new CE Certificates of Conformity or new regulatory clearances or approvals for modifications, we may be required to recall and to stop marketing the modified devices.
Obtaining clearances and approvals, or new or amended CE Certificates of Conformity for device modifications can be a time consuming process, and delays in obtaining required future clearances, approvals, or CE Certificates of Conformity would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Even if our imaging capsule and future products are cleared or approved by regulatory authorities or if we obtain CE Certificates of Conformity from our Notified Body, if we or our suppliers fail to comply with ongoing FDA or other foreign regulatory authority requirements, or if we experience unanticipated problems with our products, our products could be subject to restrictions or withdrawal from the market.
The manufacturing processes, reporting requirements, post-approval clinical data and promotional activities associated with any product for which we obtain clearance or approval CE Certificates of Conformity, or equivalent third country approval will be subject to continuous regulatory review, oversight and periodic inspections by FDA other domestic and foreign regulatory authorities and our Notified Body. In particular, we and certain of our suppliers are required to comply with FDA’s Quality System Regulations, or QSR. In the EU, we will also be subject to the quality system requirements laid down in the Annexes to the Medical Devices Directive. Such compliance can be facilitated by, among other things, a certificate of compliance with ISO 13485:2003. Through compliance with the ISO 13485:2003 standard, we will benefit from a presumption of conformity with the relevant quality system requirements laid down in the Annexes to Medical Devices Directive. These regulations and standards govern the methods and documentation of the design, testing, production, control, quality assurance, labeling, packaging, storage and shipping of any product for which we obtain clearance or approval, CE Certificates of Conformity, or equivalent third country approval. Regulatory authorities, such as FDA, and our Notified Body enforce the QSR and other regulations through periodic inspections. The failure by us or one of our suppliers to comply with applicable statutes and regulations falling within the competence of FDA and other regulatory authorities or our Notified Body, or the failure to timely and adequately respond to any adverse inspectional observations or product safety issues, could result in, among other things, any of the following enforcement actions:
|
|
•
|
untitled letters, warning letters, fines, injunctions, corporate integrity agreements, consent decrees and civil penalties;
|
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
|
|
•
|
customer notifications for repair, replacement or refunds;
|
27
|
|
•
|
recall, detention or seizure of our products;
|
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products;
|
|
|
•
|
operating restrictions;
|
|
|
•
|
withdrawing 510(k) clearances on PMA approvals that have already been granted;
|
|
|
•
|
suspension or withdrawal of our CE Certificates of Conformity;
|
|
|
•
|
refusal to grant export approval for our products; or
|
|
|
•
|
criminal prosecution.
|
If any of these actions were to occur, it would harm our reputation and cause our product sales and profitability to suffer and may prevent us from generating revenue. Furthermore, our key suppliers may not currently be or may not continue to be in compliance with all applicable regulatory requirements which could result in our failure to produce our products on a timely basis and in the required quantities, if at all.
Even if regulatory clearance or approval of a product is granted, or if we obtain CE Certificates of Conformity, such clearance or approval, or CE Certificates of Conformity may be subject to limitations on the intended uses for which the product may be marketed and reduce our potential to successfully commercialize the product and generate revenue from the product. If FDA or the competent regulatory authorities of foreign countries determines that our promotional materials, labeling, training or other marketing or educational activities constitute the promotion of an unapproved use or the promotion of an intended purpose not covered by our CE mark, they could request that we cease or modify our training or promotional materials or subject us to regulatory enforcement actions. It is also possible that other federal, state or foreign enforcement authorities might take action if they consider our training or other promotional materials to constitute promotion of an unapproved use, which could result in significant fines or penalties under other statutory authorities, such as laws prohibiting false claims for reimbursement.
In addition, we may be required to conduct costly post-market testing and surveillance to monitor the safety or effectiveness of our products, and we must comply with medical device reporting requirements, including the reporting of adverse events and malfunctions related to our products. Later discovery of previously unknown problems with our products, including unanticipated adverse side effects or adverse side effects of unanticipated severity or frequency, manufacturing problems, or failure to comply with regulatory requirements such as QSR, may result in changes to labeling, restrictions on such products or manufacturing processes, withdrawal of the products from the market, voluntary or mandatory recalls, a requirement to repair, replace or refund the cost of any medical device we manufacture or distribute, fines, suspension or withdrawal of regulatory approvals or CE Certificates of Conformity, product seizures, injunctions or the imposition of civil or criminal penalties, all of which would adversely affect our business, financial condition and operating results and prospects.
If we fail to maintain necessary regulatory clearances or CE Certificates of Conformity for our imaging capsule and indications in our target foreign markets, if clearances or approvals, or CE Certificates of Conformity for future products and indications are delayed or not issued, or if there are regulatory changes in our existing or future target markets, our commercial operations could be harmed.
Our imaging capsule is a medical device that is subject to extensive regulations that are intended to assure its safety, effectiveness and compliance with applicable consumer laws. If we fail to obtain and maintain these regulatory approvals or clearances, or CE Certificates of Conformity, our ability to sell our imaging capsule and generate revenues will be materially harmed.
28
These laws and regulations relate to the design, development, testing, manufacturing, storage, labeling, packaging, content and language of the instructions for use of the device, sale, promotion, distribution, importing and exporting, shipping, post-sale surveillance and recall from our imaging capsule’s markets, and all countries in which we intend to sell our imaging capsule apply some form of regulations of this kind. Most notably, we must comply with the Medical Devices Directive and are subject to extensive regulation in the United States by FDA and other federal, state and local authorities. In the EU, compliance with the requirements laid down in the Medical Devices Directive, including the Essential Requirements laid down its Annex I thereto, is a prerequisite to be able to affix the CE mark of conformity to our medical devices. Without such CE mark, our products cannot be marketed or sold in the EU. To demonstrate compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Apart from low risk medical devices (Class I with no measuring function and which are not sterile), in relation to which the manufacturer can make an EC Declaration of Conformity based on self-assessment of the conformity of its products with the Essential Requirements laid down in Annex I to the Medical Devices Directive, a conformity assessment procedure requires the intervention a Notified Body. The Notified Body would typically audit and examine products’ Technical File, which we must create, and the quality system for manufacture, design and final inspection of our devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements laid down in Annex I to the Medical Devices Directive or the quality system requirements laid down in the other Annexes to the Directive. Following the issuance of this CE Certificate of Conformity, we can draw up an EC Declaration of Conformity and affix the CE mark to the products covered by this CE Certificate of Conformity and by the EC Declaration of Conformity Other countries outside the EU also accept the CE mark as a certification of quality, efficacy and safety of medical devices and an element of related authorization of the products in their territory.
We will be subject to annual audits by a Notified Body under the Medical Devices Directive. During this audit, the third-party assessor or Notified Body will examine the maintenance and implementation of our quality control system, device post-marketing vigilance system and any changes or modifications made to our products.
On September 26, 2012, the European Commission adopted a package of legislative proposals designed to replace the existing regulatory framework for medical devices in the EU. These proposals are intended to strengthen the medical devices rules in the EU. On October 22, 2013, the European Parliament voted in favor of an amended draft of the Regulation. The proposed text is currently being discussed by the Council of the European Union. These adopted or expected regulatory changes may adversely affect our business, financial condition and results of operations or restrict our operations.
Our imaging capsule may in the future be subject to product recalls that could harm our reputation, business and financial results.
FDA and similar foreign governmental authorities have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture. In the case of FDA, the authority to require a recall must be based on an FDA finding that there is a reasonable probability that the device would cause serious injury or death. In addition, foreign governmental bodies have the authority to require the recall of our products in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us or one of our distributors could occur as a result of component failures, manufacturing errors, design or labeling defects or other deficiencies and issues. Once marketed, recalls of any of our products, including our imaging capsule, would divert managerial and financial resources and have an adverse effect on our business, financial condition and results of operations. FDA requires that certain classifications of recalls be reported to FDA within 10 working days after the recall is initiated. Companies are required to maintain certain records of recalls, even if they are not reportable to FDA. We may initiate voluntary recalls involving our products in the future that we determine do not require us to notify FDA. If FDA disagrees with our determinations, they could require us to report those actions as recalls. A future recall announcement could harm our reputation with customers and negatively affect our sales. In addition, FDA could take enforcement action against us based on our failure to report the recalls when they were conducted.
If our imaging capsule or future products cause or contribute to a death or a serious injury, or malfunction in such a way that causes or contributes to a death or serious injury, we will be subject to medical device reporting regulations, which can result in corrective actions or enforcement actions from regulatory authorities.
Under FDA medical device reporting regulations, medical device manufacturers are required to report to FDA information that a device has or may have caused or contributed to a death or serious injury or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of our device (or any similar future product) were to recur. If we fail to investigate and report these events to FDA within the required timeframes, or at all, FDA could take enforcement action against us. Any such adverse event involving our products also could result in future corrective actions, such as recalls or customer notifications, or agency action, such as inspection or enforcement action. Any corrective action, whether voluntary or involuntary, including any legal action taken against us, will require us to devote sufficient time and capital to the matter, distract management from operating our business, and may harm our reputation and financial results.
29
In addition, we must also comply with the EU Medical Device Vigilance System (MEDDEV 2.12/1 rev.8), which is intended to protect the health and safety of patients, users and others by establishing reporting procedures and reducing the likelihood of reoccurrence of incidents related to the use of a medical device. Under this system, incidents (which are defined as any malfunction or deterioration in the characteristics and/or performance of a device, as well as any inadequacy in the labeling or the instructions for use which, directly or indirectly, may lead to or may have led to the death of a patient, or user or other persons or to a serious deterioration in such person’s state of health) must be reported by manufacturers through a Manufacturer’s Incident Reports to competent authorities within periods of time specified in the MEDDEV 2.12/1 rev. 8. Such incidents are evaluated and, where appropriate, information is disseminated between the competent authorities of the EU Member States. The MEDDEV 2.12/1 rev. 8 is also intended to facilitate a direct, early and harmonized establishment of Field Safety Corrective Actions, or FSCAs, across the EU Member States in which the device is being marketed. An FSCA is an action taken by a manufacturer to reduce a risk of death or serious deterioration in the state of health associated with the use of a medical device that is already placed on the market. An FSCA may include device recall, modification, exchange, or destruction. FSCAs must be reported by the manufacturer or the manufacturer’s European Authorized Representative, to its customers and/or the end users of the device through a Field Safety Notice. FSCAs must also be reported to the competent authorities of the EU Member States.
Our failure to comply with radiation safety or radio frequency regulations in a specific country or region could impair our ability to commercially distribute and market our imaging capsule in that country or region.
Our imaging capsule includes a tiny X-ray source and wireless radio frequency transmitter and receiver, and is therefore subject to equipment authorization requirements in a number of countries and regions. In the United States, the EU and Japan, authorities often require advance clearance of all radiation and radio frequency devices before they can be sold or marketed in these jurisdictions, subject to limited exceptions. Modifications to the approved system design and specifications may require new or further regulatory clearances or approvals before we are permitted to market and sell a modified system. If we are unable to obtain any required clearances or approvals from the authorities responsible for the radiation as well as the radio frequency regulations in these and other jurisdictions, the sale or use of our imaging capsule could be prevented in these countries. Any such action could negatively affect our business, financial condition and results of operations.
Our business is subject to complex environmental legislation that may increase our costs and our risk of noncompliance.
Our research and development and manufacturing processes involve the handling of potentially harmful radioactive and other hazardous materials. We are subject to local laws and regulations governing the use, shipping, handling, storage and disposal of these materials, and we incur expenses related to compliance with these laws and regulations. If we are found to have violated environmental, health and safety laws, whether as a result of human error, equipment failure or other causes, we could be held liable for damages, penalties and costs of remedial actions which could materially adversely affect our business, financial condition and results of operations. In the future, we could be subject to additional environmental requirements or existing environmental laws could become more stringent, which could lead to greater compliance costs and increasing risks and penalties associated with violations. For example, changes to, or restrictions on, permitting requirements or processes, hazardous or radioactive material storage or handling might require an unplanned capital investment or relocation. If we fail to comply with existing or new environmental laws or regulations, our business, financial condition and results of operations could be materially adversely affected.
If we are unable to achieve reimbursement and coverage from third-party payors for procedures using our imaging capsule, or if reimbursement is insufficient to create an economic benefit for purchasing or using our imaging capsule when compared to alternative procedures, demand for our products may not grow at the rate we expect.
The demand for our imaging capsule will depend significantly on the eligibility of the procedures performed using our imaging capsule for reimbursement through government-sponsored healthcare payment systems and private third-party payors. Reimbursement practices vary significantly from country to country and within some countries, by region, and we must obtain reimbursement approvals on a country-by-country and/or region-by-region basis. In general, the process of obtaining reimbursement and coverage approvals has been longer outside of the United States. We may not be able to obtain reimbursement approvals in a timely manner or at all and existing reimbursement and coverage policies may be revised from time to time by third-party payors. If physicians, hospitals and other healthcare providers are unable to obtain sufficient coverage and reimbursement from third-party payors for procedures using our imaging capsule, if reimbursement is, or is perceived by our customers to be, insufficient to create an economic incentive for purchasing or using our imaging capsule, or if such reimbursement does not adequately compensate physicians and health care providers compared to the other procedures they offer, demand for our products may not grow at the rate we expect.
30
Federal and state privacy laws, and equivalent laws of third countries, may increase our costs of operation and expose us to civil and criminal sanctions.
The Health Insurance Portability and Accountability Act of 1996, as amended, and the regulations that have been issued under it, to which we refer collectively as HIPAA, and similar laws outside the United States, contain substantial restrictions and requirements with respect to the use and disclosure of individuals’ protected health information. The HIPAA privacy rules prohibit “covered entities,” such as healthcare providers and health plans, from using or disclosing an individual’s protected health information, unless the use or disclosure is authorized by the individual or is specifically required or permitted under the privacy rules. Under the HIPAA security rules, covered entities must establish administrative, physical and technical safeguards to protect the confidentiality, integrity and availability of electronic protected health information maintained or transmitted by them or by others on their behalf. While we do not believe that we are a covered entity under HIPAA, many of our customers are covered entities subject to HIPAA. Such customers may require us to enter into business associate agreements, which will obligate us to safeguard certain health information we obtain in the course of our relationship with them, restrict the manner in which we use and disclose such information and impose liability on us for failure to meet our contractual obligations.
In addition, under The Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, which was signed into law as part of the U.S. stimulus package in February 2009, certain of HIPAA’s privacy and security requirements are now also directly applicable to “business associates” of covered entities and subject them to direct governmental enforcement for failure to comply with these requirements. We may be deemed as a “business associate” of some of our customers. As a result, we may be subject as a “business associate” to civil and criminal penalties for failure to comply with applicable privacy and security rule requirements. Moreover, HITECH created a new requirement obligating “business associates” to report any breach of unsecured, individually identifiable health information to their covered entity customers and imposes penalties for failing to do so.
In addition to HIPAA, most U.S. states have enacted patient confidentiality laws that protect against the disclosure of confidential medical information, and many U.S. states have adopted or are considering adopting further legislation in this area, including privacy safeguards, security standards, and data security breach notification requirements. These U.S. state laws, which may be even more stringent than the HIPAA requirements, are not preempted by the federal requirements, and we are therefore required to comply with them to the extent they are applicable to our operations.
These and other possible changes to HIPAA or other U.S. federal or state laws or regulations, or comparable laws and regulations in countries where we conduct business, could affect our business and the costs of compliance could be significant. Failure by us to comply with any of the standards regarding patient privacy, identity theft prevention and detection, and data security may subject us to penalties, including civil monetary penalties and in some circumstances, criminal penalties. In addition, such failure may damage our reputation and adversely affect our ability to retain customers and attract new customers.
The protection of personal data, particularly patient data, is subject to strict laws and regulations in many countries. The collection and use of personal health data in the EU is governed by the provisions of Directive 95/46/EC of the European Parliament and of the Council of 24 October 1995 on the protection of individuals with regard to the processing of personal data and on the free movement of such data, commonly known as the Data Protection Directive. The Directive imposes a number of requirements including an obligation to seek the consent of individuals to whom the personal data relates, the information that must be provided to the individuals, notification of data processing obligations to the competent national data protection authorities of individual EU Member States and the security and confidentiality of the personal data. The Data Protection Directive also imposes strict rules on the transfer of personal data out of the EU to the US. Failure to comply with the requirements of the Data Protection Directive and the related national data protection laws of the EU Member States may result in fines and other administrative penalties and harm our business. We may incur extensive costs in ensuring compliance with these laws and regulations, particularly if we are considered to be a data controller within the meaning of the Data Protection Directive.
31
The adoption of healthcare reform and deficit reduction measures in the United States may adversely affect our business and financial results.
On March 23, 2010, President Obama signed into law major healthcare reform legislation under the Patient Protection and Affordable Care Act of 2010, or the PPACA, which was modified on March 30, 2010 by the enactment of the Health Care and Education Reconciliation Act of 2010. This law substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts the device industry. The PPACA is intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for healthcare and health insurance industries, impose new taxes and fees on pharmaceutical and medical device manufacturers, and impose additional health policy reforms. Under the PPACA, it is expected that expanded healthcare coverage will be made available to millions of Americans. The increased costs to the U.S. government from the PPACA are expected to be funded through a combination of payment reductions for providers over time and several new taxes. The PPACA imposes, among other things, an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States beginning in 2013, resulting in an anticipated cost to the medical device industry of up to $20 billion over the next decade. We likely will be subject to the excise tax with respect to our imaging capsule if it is approved for sale in the United States. The PPACA also limits the rate of growth in Medicare payments to providers and authorizes certain voluntary demonstration projects beginning no later than 2013 around development of bundling payments for acute, inpatient hospital services, physician services, and post acute services for episodes of hospital care. In addition, the PPACA provides for the establishment of an Independent Payment Advisory Board, or IPAB, that, beginning in 2014, could recommend changes in Medicare payments to physicians and other providers that would take effect unless Congress passes an alternative measure to achieve the same amount of savings. The IPAB has not yet been created. The PPACA also increases fraud and abuse penalties and expands the scope and reach of the Federal Civil False Claims Act and government enforcement tools, which may adversely impact healthcare companies.
The U.S. Supreme Court heard a constitutional challenge to the PPACA and in June 2012 held that the PPACA is constitutional. However, states are allowed to opt out of the expansion of eligibility criteria for Medicaid under the PPACA and many states have chosen to do so, causing many uninsured patients to remain without coverage. In addition to the PPACA, the effect of which cannot presently be quantified given its recent enactment, various healthcare reform proposals have also emerged at the state level. We cannot predict whether future healthcare initiatives will be implemented at the federal or state level or the effect any future legislation or regulation will have on us. However, we anticipate that the PPACA, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage criteria and an additional downward pressure on the price that we receive for any approved product, and could adversely affect our business. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Insurers may also refuse to provide any coverage of uses of approved products for medical indications other than those for which the FDA has granted market approvals, all of which may adversely affect our business, financial condition and results of operations, possibly materially.
In addition to health reform, other deficit reduction measures could affect reimbursement for our device and related procedures. For example, beginning April 1, 2013, Medicare payments for all items and services have been reduced by 2% under the sequestration (i.e., automatic spending reductions) required by the Budget Control Act of 2011, as amended by the American Taxpayer Relief Act of 2012. These cuts will remain in effect until 2024 unless Congress enacts legislation to cancel or delay the cuts. These payment reductions, or similar efforts to reduce Medicare spending to control the federal deficit, could adversely affect our business by reducing reimbursement to the providers who purchase and use our devices and perform related procedures.
32
If we fail to comply with the U.S. federal Anti-Kickback Statute and similar state and third country laws, we could be subject to criminal and civil penalties and exclusion from federally funded healthcare programs including the Medicare and Medicaid programs and equivalent third country programs, which would have a material adverse effect on our business and results of operations.
A provision of the Social Security Act, commonly referred to as the federal Anti-Kickback Statute, prohibits the knowing and willful offer, payment, solicitation or receipt of any form of remuneration, directly or indirectly, in cash or in kind, to induce or reward the referring, ordering, leasing, purchasing or arranging for, or recommending the ordering, purchasing or leasing of, items or services payable, in whole or in part, by Medicare, Medicaid or any other federal healthcare program. PPACA, among other things, clarified that a person or entity needs not to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor may be subject to scrutiny. The federal Anti-Kickback Statute is very broad in scope and many of its provisions have not been uniformly or definitively interpreted by existing case law or regulations. In addition, most of the states have adopted laws similar to the federal Anti-Kickback Statute, and some of these laws are even broader than the federal Anti-Kickback Statute in that their prohibitions may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the source of payment. Violations of the federal Anti-Kickback Statute may result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
All of our financial relationships with healthcare providers, purchasers, formulary managers, and others who provide products or services to federal healthcare program beneficiaries are potentially governed by the federal Anti-Kickback Statute and similar state laws. We believe our operations are in compliance with the federal Anti-Kickback Statute and similar state laws. However, we cannot be certain that we will not be subject to investigations or litigation alleging violations of these laws, which could be time-consuming and costly to us and could divert management’s attention from operating our business, which in turn could have a material adverse effect on our business. In addition, if our arrangements were found to violate the federal Anti-Kickback Statute or similar state laws, the consequences of such violations would likely have a material adverse effect on our business, results of operations and financial condition.
There are other federal and state laws that may affect our ability to operate, including the federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. PPACA amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Moreover, we may be subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs. Moreover, there are analogous state laws. Violations of these laws can result in substantial criminal, civil or administrative penalties, damages, fines and exclusion from participation in federal healthcare programs.
Similar restrictions are imposed by the national legislation of many third countries in which our medical devices will be marketed. Moreover, the provisions of the Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more aggressive and frequent investigations and enforcement by both the U.S. Securities and Exchange Commission and the Department of Justice. A determination that our operations or activities violated United States or foreign laws or regulations could result in imposition of substantial fines, interruption of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. In addition, lawsuits brought by private litigants may also follow as a consequence.
33
If the U.S. Nuclear Regulatory Commission, or NRC, or other nuclear regulatory commissions around the world, would take the position that an imaging capsule containing radioactive material cannot be passed in excreta into the sanitary sewer system without limitation, we may be subject to further regulations and patients may be required to retrieve our imaging capsule after use.
As our imaging capsule includes an ingestible capsule with a radioactive source, we must address NRC regulations in addition to FDA requirements. Our imaging capsule is loaded with the X-ray source, sealed and then ingested by the patient. Although the NRC places conditions and limitations on the disposal of radioactive material in the sanitary sewer, such conditions and limitations do not apply to radioactive material contained in the excreta of individuals that are undergoing medical diagnosis or therapy with radioactive material. However, there is no assurance that the NRC or other regulatory commissions worldwide will take a similar position in relation to our imaging capsule and we may face limitations by the NRC in relation to the disposal of our imaging capsule in the sanitary system, such as requiring patients to retrieve our imaging capsule after use, which could make our imaging capsule less attractive.
Our failure to comply with the necessary regulatory approval regarding the use of radioactive materials could significantly impair our ability to develop, manufacture and/or sell our imaging capsule.
The manufacture of our imaging capsule requires the use of radioactive materials. In order to use such materials in the development and manufacture of our imaging capsule in Israel, we are required to obtain a permit from the Israeli Commissioner for Environmental Radiation, or the Commissioner, pursuant to the Israeli Pharmaceutical Regulations (Radioactive Elements and By-Products) – 1980. Should we fail to comply with the conditions of our currently existing permit, the Commissioner would have authority to cancel our permit. Should the Commissioner determine that our activities or facilities constitute a danger to the health and well-being of a person, the public or the environment, the cancellation of our permit could be immediate and without prior notice. Furthermore, we cannot guarantee the annual renewal of our permit and/or annual renewal subject to identical conditions, as the approval of an annual application and the conditions thereof are at the discretion of the Commissioner. Similar requirements and regulations may apply to the manufacture of our imaging capsule in other countries. Cancellation of or failure to renew our permit could have materially adverse consequences on our ability to manufacture and sell our products and therefore on our ability to continue our business and operations.
Risks Related to Our Intellectual Property
If we are unable to protect our intellectual property rights, our competitive position could be harmed.
Our success and ability to compete depends in large part upon our ability to protect our intellectual property. Although we have patents issued in Israel, Europe, United States, Japan, China, India, Hong Kong, and Australia, we continue to file and prosecute in many of the same countries and additional countries such as Canada and Korea. We face several risks and uncertainties in connection with our intellectual property rights, including, among others:
|
|
•
|
pending and future patent applications may not result in the issuance of patents or, if issued, may not be issued in a form that will be advantageous to us;
|
|
|
•
|
our issued patents may be challenged, invalidated or legally circumvented by third parties;
|
|
|
•
|
our patents may not be upheld as valid and enforceable or prevent the development of competitive products;
|
|
|
•
|
the eligibility of certain inventions related to diagnostic medicine, more specifically diagnostic methods and processes, for patent protection in the United States has been limited recently which may affect our ability to enforce our issued patents in the United States or may make it difficult to obtain broad patent protection going forward in the United States;
|
|
|
•
|
for a variety of reasons, we may decide not to file for patent protection on various improvements or additional features; and
|
|
|
•
|
intellectual property protection and/or enforcement may be unavailable or limited in some countries where laws or law enforcement practices may not protect our proprietary rights to the same extent as the laws of the United States, the European Union, Canada or Israel.
|
34
Consequently, our competitors could develop, manufacture and sell products that directly compete with our products, which could decrease our sales and diminish our ability to compete. In addition, competitors could attempt to develop their own competitive technologies that fall outside of our intellectual property rights. If our intellectual property does not adequately protect us from our competitors’ products and methods, our competitive position could be materially adversely affected.
Because the medical device industry is litigious, we are susceptible to intellectual property suits that could cause us to incur substantial costs or pay substantial damages or prohibit us from selling our imaging capsule.
There is a substantial amount of litigation over patent and other intellectual property rights in the medical device industry. Whether a product infringes a patent involves complex legal and factual issues, the determination of which is often uncertain. We are presently unaware of any other parties’ valid patents and proprietary rights which our evolving product designs would infringe. Searches typically performed to identify potentially infringed patents of third parties are often not conclusive and, because patent applications can take many years to issue, there may be applications now pending, which may later result in issued patents which our current or future products may infringe. In addition, our competitors or other parties may assert that our imaging capsule and the methods it employs may be covered by patents held by them. If our imaging capsule infringes a valid patent, we could be prevented from manufacturing or selling it unless we can obtain a license or redesign the product to avoid infringement. A license may not always be available or may require us to pay substantial royalties. We also may not be successful in any attempt to redesign our product to avoid infringement. Infringement and other intellectual property claims, with or without merit, can be expensive and time-consuming to litigate and could divert our management’s attention from operating our business.
The steps we have taken to protect our intellectual property may not be adequate, which could have a material adverse effect on our ability to compete in the market.
In addition to patents, we rely on confidentiality, non-compete, non-disclosure and assignment of inventions provisions, as appropriate, with our employees, consultants and, to some extent, our distributors, to protect and otherwise seek to control access to, and distribution of, our proprietary information. These measures may not be adequate to protect our intellectual property from unauthorized disclosure, third-party infringement or misappropriation, for the following reasons:
|
|
•
|
the agreements may be breached, may not provide the scope of protection we believe they provide or may be determined to be unenforceable;
|
|
|
•
|
we may have inadequate remedies for any breach;
|
|
|
•
|
proprietary information could be disclosed to our competitors; or
|
|
|
•
|
others may independently develop substantially equivalent or superior proprietary information and techniques or otherwise gain access to our trade secrets or disclose such technologies.
|
Specifically with respect to non-compete agreements, under current U.S. and Israeli law, we may be unable to enforce these agreements, in whole or in part, and it may be difficult for us to restrict our competitors from gaining the expertise that our former employees gained while working for us. For example, Israeli courts have recently required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, we may be unable to prevent our competitors from benefiting from the expertise of our former employees. In addition, some states in the United States, such as California, have laws which severely restrict the use of non-compete undertakings.
If, for any of the above reasons, our intellectual property is disclosed or misappropriated, it could harm our ability to protect our rights and could have a material adverse effect on our business, financial condition and results of operations.
35
Third-party claims of infringement or other claims against us could require us to redesign our imaging capsule, seek licenses, or engage in future costly intellectual property litigation, which could negatively affect our future business and financial performance.
Substantial litigation over intellectual property rights exists in the medical device industry in general and in the medical imaging sector in particular. We expect that we may be subject to third-party infringement claims as our revenues increase, the number of competitors grows and the functionality of products and technology in different industry segments converges. Third parties may currently have, or may eventually be issued, patents on which our current or future products or technologies may infringe.
In addition, litigation in which we are accused of infringement may cause negative publicity, adversely impact prospective customers, cause product shipment delays, prohibit us from manufacturing, marketing or selling our current or future products, require us to develop non-infringing technology, make substantial payments to third parties or enter into royalty or license agreements, which may not be available on acceptable terms, or at all. If a successful claim of infringement were made against us and we could not develop non-infringing technology or license the infringed or similar technology in a timely and cost-effective manner, our ability to generate significant revenues may be substantially harmed and we could be exposed to significant liability. A court could enter orders that temporarily, preliminarily or permanently enjoin us or our customers from making, using, selling, offering to sell or importing our current or future products, or could enter an order mandating that we undertake certain remedial activities. Claims that we have misappropriated the confidential information or trade secrets of third parties can have a similar negative impact on our reputation, business, financial condition or results of operations.
We may also become involved in litigation in connection with our brand name rights. We do not know whether others will assert that our brand name infringes their trademark rights. In addition, names we choose for our products may be claimed to infringe names held by others. If we have to change the names we use, we may experience a loss in goodwill associated with our brand name, customer confusion and a loss of sales.
Third parties may challenge the validity of our issued patents or challenge patent applications in administrative proceedings before various patent offices which, if successful, could negatively affect our future business and financial performance.
Various patent offices, including in the United States and Europe, provide administrative proceedings by which a third party can challenge the validity of an issued patent or challenge an application that is being examined absent any threat of litigation. In some instances, including in the United States, the administrative proceedings provide a more efficient and favorable forum to challenge our patents which may lead to more opportunities for competitors to do so, particularly smaller competitors with limited resources. Moreover, the standards utilized in these administrative proceedings, at least in the United States, provide certain legal advantages versus challenging the validity of a patent in a district court. If a third party is successful in one of these administrative proceedings, the patent will no longer be enforceable in the corresponding jurisdiction. With this loss in patent rights, we will not be able to prevent third parties from offering identical or similar competing products which may result in lower profits and a less substantial market share.
We may need to initiate lawsuits to protect or enforce our patents and other intellectual property rights, which could be expensive and, if we lose, could cause us to lose some of our intellectual property rights, which would harm our ability to compete in the market.
We rely on patents to protect a portion of our intellectual property and our competitive position. Patent law relating to the scope of claims in the technology fields in which we operate is still evolving and, consequently, patent positions in the medical device industry are generally uncertain. In order to protect or enforce our patent rights, we may initiate patent and related litigation against third parties, such as infringement suits or interference proceedings. Any lawsuits that we initiate could be expensive, take significant time and divert our management’s attention from other business concerns and the outcome of litigation to enforce our intellectual property rights in patents, copyrights, trade secrets or trademarks is highly unpredictable. Litigation also puts our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing. In addition, we may provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, including attorney fees, if any, may not be commercially valuable. The occurrence of any of these events could have a material adverse effect on our business, financial condition and results of operations.
36
We may not be able to enforce covenants not to compete at all or, we may be unable to enforce them for the duration contemplated in our employment contracts and may, therefore, be unable to prevent competitors from benefiting from the expertise of some of our former employees involved in research and development activities.
We currently have non-competition agreements with substantially all of our employees who are involved in research and development, all of whom are located in Israel. These agreements prohibit our employees, if they cease working for us, from directly competing with us or working for our competitors for a limited period of time following termination of employment. In many jurisdictions, courts are increasingly refusing to enforce restrictions on competition by former employees or have interpreted them narrowly. For example, in Israel, where all of our employees reside, courts have required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property. If we cannot demonstrate that harm would be caused to us, an Israeli court may refuse to enforce our non-compete restrictions or reduce the contemplated period of non-competition such that we may be unable to prevent our competitors from benefiting from the expertise of our former employees.
Risks Related to Our Operations in Israel
Our principal offices, research and development facilities and some of our suppliers are located in Israel and, therefore, our business, financial condition and results of operation may be adversely affected by political, economic and military instability in Israel.
Our principal offices, research and development facilities are located in northern Israel. In addition, all of our employees and officers, and most of our directors, are residents of Israel. Accordingly, political, economic and military conditions in Israel may directly affect our business. Since the establishment of the State of Israel in 1948, a number of armed conflicts have taken place between Israel and its neighboring countries. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its trading partners could adversely affect our operations and results of operations. In addition, our operations may be adversely affected by the call-up of certain of our employees, including members of our senior management, to active military services in the case of such hostilities.
During the Second Lebanon War of 2006, between Israel and Hezbollah, a militant Islamic movement, rockets were fired from Lebanon into Israel, including into the Haifa area, where our facility is located, causing casualties and major disruption of economic activities in northern Israel. An escalation in tension and violence between Israel and the militant Hamas movement (which controls the Gaza Strip) and other Palestinian Arab groups, culminated with Israel’s military campaign in Gaza in December 2008, in November 2012 and again in July and August 2014 in an endeavor to prevent continued rocket attacks against Israel’s southern towns. It is unclear whether any negotiations that may occur between Israel and the Palestinian Authority will result in an agreement. In addition, Israel faces threats from more distant neighbors, in particular, Iran, an ally of Hezbollah and Hamas.
Popular uprisings in various countries in the Middle East and North Africa are affecting the political stability of those countries. Such instability may lead to deterioration in the political and trade relationships that exist between the State of Israel and these countries. Furthermore, several countries, principally in the Middle East, restrict doing business with Israel and Israeli companies, and additional countries may impose restrictions on doing business with Israel and Israeli companies if hostilities in the region continue or intensify. Such restrictions may seriously limit our ability to sell our products to customers in those countries. Parties with whom we may do business could decline to travel to Israel during periods of heightened unrest or tension. In addition, the political and security situation in Israel may result in parties with whom we may have agreements involving performance in Israel claiming that they are not obligated to perform their commitments under those agreements pursuant to force majeure provisions in such agreements. In addition, any hostilities involving Israel could have a material adverse effect on our facilities including our corporate office or on the facilities of our local suppliers, in which event all or a portion of our inventory may be damaged, and our ability to deliver products to customers could be materially adversely affected. Any hostilities involving Israel or the interruption or curtailment of trade between Israel and its present trading partners, or significant downturns in the economic or financial condition of Israel, could adversely affect our operations and product development, cause our revenues to decrease and adversely affect our share price following this offering. Similarly, Israeli corporations are limited in conducting business with entities from several countries.
37
Our commercial insurance policy does not cover losses associated with terrorist attacks. Although the Israeli government in the past covered the reinstatement value of certain damages that were caused by terrorist attacks or acts of war, we cannot assure you that this government coverage will be maintained, or if maintained, will be sufficient to compensate us fully for damages incurred. Any losses or damages incurred by us could have a material adverse effect on our business. Any armed conflicts, terrorist activities or political instability in the region would likely negatively affect business conditions generally and could harm our results of operations.
Our operations could also be disrupted by the obligations of personnel to perform military service. As of November 21, 2014, we had 37 employees and independent contractors, all of whom were based in Israel. Some of these employees may be called upon to perform up to 54 days in each three year period (and in the case of officers, up to 84 days in each three year period) of military reserve duty until they reach the age of 40 (and in some cases, depending on their specific military profession up to 45 or even 49 years of age) and, in certain emergency circumstances, may be called to immediate and unlimited active duty. In response to increases in terrorist activity, there have been periods of significant call-ups of military reservists and it is possible that there will be similar large-scale military reserve duty call-ups in the future. Our operations could be disrupted by the absence of a significant number of employees related to military service, which could materially adversely affect our business and results of operations.
Pursuant to the terms of the Israeli government grants we received for research and development expenditures, we are obligated to pay certain royalties on our revenues to the Israeli government. The terms of the grants require us to satisfy specified conditions and to make additional payments in addition to repayment of the grants upon certain events.
We have received grants from the Government of the State of Israel through the OCS for the financing of a portion of our research and development expenditures pursuant to the Research Law and related regulations. As of June 30, 2014, we had received funding from the OCS in the aggregate amount of $3.1 million. Since June 30, 2014, we have received additional funding from the OCS in the aggregate amount of $476,000 under an approved OCS grant in the total amount of $702,000 for a research and development program for the 12 month period commencing March 1, 2014. As of June 30, 2014, we had not paid any royalties to the OCS and had a contingent obligation to the OCS in the amount of $1.6 million. We may apply for additional OCS grants in the future. However, as the funds available for OCS grants out of the annual budget of the State of Israel have been reduced in the past and may be further reduced in the future, we cannot predict whether we will be entitled to any future grants, or the amounts of any such grants.
The terms of the Israeli government participation require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless prior approval is received from the OCS, which we may not receive. In addition, payment of additional amounts would be required if manufacturing is moved outside of Israel, in which case the royalty repayment rate is increased and the royalty ceiling can reach up to three times the amount of the grants received, and if OCS developed know-how is transferred outside of Israel, the royalty ceiling can reach up to six times the amount of grants received (plus interest). We are currently considering whether it would be possible to assemble the capsule without the X-ray source in Israel, and have the X-ray source subsequently inserted into our imaging capsule at a reactor or cyclotron site or at a distribution center outside Israel. Even following the full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions and requirements for payment may impair our ability to sell our technology assets outside of Israel or to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. Furthermore, the consideration available to our shareholders in a transaction involving the transfer outside of Israel of technology or know-how developed with OCS funding (such as a merger or similar transaction) may be reduced by any amounts that we are required to pay to the OCS.
If we fail to comply with any of the conditions and restrictions imposed by the Research Law, or by the specific terms under which we received the grants, we may be required to refund any grants previously received together with interest and penalties, and, in certain circumstances, may be subject to criminal charges.
38
Your rights and responsibilities as a shareholder will be governed by Israeli law which differs in some material respects from the rights and responsibilities of shareholders of U.S. companies.
The rights and responsibilities of the holders of our ordinary shares are governed by our amended articles of association and by Israeli law. These rights and responsibilities differ in some material respects from the rights and responsibilities of shareholders in U.S.-based corporations. In particular, a shareholder of an Israeli company has a duty to act in good faith and in a customary manner in exercising its rights and performing its obligations towards the company and other shareholders, and to refrain from abusing its power in the company, including, among other things, in voting at a general meeting of shareholders on matters such as amendments to a company’s articles of association, increases in a company’s authorized share capital, mergers and acquisitions and related party transactions requiring shareholder approval. In addition, a shareholder who is aware that it possesses the power to determine the outcome of a shareholder vote or to appoint or prevent the appointment of a director or executive officer in the company has a duty of fairness toward the company. There is limited case law available to assist us in understanding the nature of this duty or the implications of these provisions. These provisions may be interpreted to impose additional obligations and liabilities on holders of our ordinary shares that are not typically imposed on shareholders of U.S. corporations.
It may be difficult to enforce a judgment of a U.S. court against us, our officers and directors or the Israeli experts named in this prospectus in Israel or the United States, to assert U.S. securities laws claims in Israel or to serve process on our officers and directors and these experts.
We are incorporated in Israel. All of our executive officers and the Israeli experts and all but two of our directors listed in this prospectus reside in Israel, and substantially all of our assets and most of the assets of these persons are located in Israel. Therefore, a judgment obtained against us, or any of these persons, including a judgment based on the civil liability provisions of the U.S. federal securities laws, may not be collectible in the United States and may not be enforced by an Israeli court. It also may be difficult for you to effect service of process on these persons in the United States or to assert U.S. securities law claims in original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws on the grounds that Israel is not the most appropriate forum in which to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proven as a fact by expert witnesses, which can be a time consuming and costly process. Certain matters of procedure will also be governed by Israeli law. There is little binding case law in Israel that addresses the matters described above. As a result of the difficulty associated with enforcing a judgment against us in Israel, you may not be able to collect any damages awarded by either a U.S. or foreign court. See “Enforceability of Civil Liabilities” for additional information on your ability to enforce a civil claim against us and our executive officers or directors, or some of the experts named in this prospectus.
Provisions of Israeli law and our amended articles of association may delay, prevent or otherwise impede a merger with, or an acquisition of, us, even when the terms of such a transaction are favorable to us and our shareholders.
Israeli corporate law regulates mergers, requires tender offers for acquisitions of shares above specified thresholds, requires special approvals for transactions involving directors, officers or significant shareholders and regulates other matters that may be relevant to such types of transactions. For example, a tender offer for all of a company’s issued and outstanding shares can only be completed if the acquirer receives positive responses from the holders of at least 95% of the issued share capital and the approval of a majority of the offerees that do not have a personal interest in the tender offer, unless at least 98% of the company’s outstanding shares are tendered. Furthermore, the shareholders, including those who indicated their acceptance of the tender offer (unless the acquirer stipulated in its tender offer that a shareholder that accepts the offer may not seek appraisal rights), may, at any time within six months following the completion of the tender offer, petition an Israeli court to alter the consideration for the acquisition. In addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party. See “Description of Share Capital — Acquisitions under Israeli Law” for additional information.
Furthermore, Israeli tax considerations may make potential transactions unappealing to us or to our shareholders whose country of residence does not have a tax treaty with Israel exempting such shareholders from Israeli tax. For example, Israeli tax law does not recognize tax-free share exchanges to the same extent as U.S. tax law. With respect to mergers, Israeli tax law allows for tax deferral in certain circumstances but makes the deferral contingent on the fulfillment of a number of conditions, including, in some cases, a holding period of two years from the date of the transaction during which sales and dispositions of shares of the participating companies are subject to certain restrictions. Moreover, with respect to certain share swap transactions, the tax deferral is limited in time, and when such time expires, the tax becomes payable even if no disposition of the shares has occurred.
39
We may become subject to claims for payment of compensation for assigned service inventions by our current or former employees, which could result in litigation and adversely affect our business.
Under the Israeli Patents Law, 5727-1967, or the Patents Law, inventions conceived by an employee during the scope of his or her employment are regarded as “service inventions” and are owned by the employer, absent a specific agreement between the employee and employer giving the employee service invention rights. The Patents Law also provides that if no such agreement between an employer and an employee exists, which prescribes whether, to what extent, and on what conditions the employee is entitled to remuneration for his or her service inventions, then such matters may, upon application by the employee, be decided by a government-appointed compensation and royalties committee established under the Patents Law, or the Committee. Recent conflicting decisions of the Committee and the Israeli Supreme Court have created uncertainty with respect to an employee’s right to receive remuneration for service inventions. In an August 2012 decision, the Israeli Supreme Court held that an employee’s contractual waiver of rights to compensation for service inventions does not necessarily preclude the employee’s claim to such compensation, and as a result an employee who executed a waiver may still bring a claim for compensation for service inventions before the Committee. However, in a decision issued in May 2014, the Committee held that employees may waive their right to remuneration for service inventions. We understand that a petition was recently filed and is currently pending with the Israeli High Court of Justice claiming that the Committee did not have authority to render such decision. A significant portion of our intellectual property has been developed by our employees in the course of their employment. Although our employees have agreed to assign to us all rights to any intellectual property created in the scope of their employment and most of our current employees, including all those involved in the development of our intellectual property, have agreed to waive their economic rights with respect to service inventions, we cannot assure you that claims will not be brought against us by current or former employees demanding remuneration in consideration for assigned service inventions. If any such claims were filed, we could be required to pay remuneration to our current or former employees for such assigned service inventions, or be forced to litigate such claims, which could negatively affect our business.
Risks Related to the Company
For as long as we are an “emerging growth company,” we will not be required to comply with certain reporting requirements that apply to other public companies. We cannot predict whether the reduced disclosure requirements applicable to emerging growth companies will make our ordinary shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may choose to take advantage of certain exemptions from reporting requirements applicable to other public companies that are not emerging growth companies. These include: (i) not being required to comply with the auditor attestation requirements for the assessment of our internal controls over financial reporting provided by Section 404 of the Sarbanes-Oxley Act of 2002, or Sarbanes-Oxley Act, (ii) not being required to comply with any requirements adopted by the Public Company Accounting Oversight Board, or PCAOB, requiring mandatory audit firm rotation or a supplement to the auditor’s report in which the auditor would be required to provide additional information about the audit and the financial statements of the issuer, (iii) not being required to comply with any new audit rules adopted by the PCAOB after April 5, 2012 unless the U.S. Securities and Exchange Commission determines otherwise, (iv) not being required to provide certain disclosure regarding executive compensation required of larger public companies, and (v) not being required to hold a non-binding advisory vote on executive compensation or seek shareholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years from the end of our current fiscal year, although, if the market value of our ordinary shares that is held by non-affiliates exceeds $700 million as of any June 30 before the end of that five-year period, we would cease to be an emerging growth company as of the following December 31. We cannot predict if investors will find our ordinary shares less attractive if we choose to rely on these exemptions. If some investors find our ordinary shares less attractive as a result of any choices to reduce future disclosure, there may be a less active trading market for our ordinary shares and our share price may be more volatile. Further, as a result of these scaled regulatory requirements, our disclosure may be more limited than that of other public companies and you may not have the same protections afforded to shareholders of such companies.
40
We will be a foreign private issuer and, as a result, we will not be subject to U.S. proxy rules and will be subject to the Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those applicable to a U.S. issuer.
Upon the completion of this offering, we will report under the Exchange Act as a foreign private issuer. Because we qualify as a foreign private issuer under the Exchange Act, we will be exempt from certain provisions of the Exchange Act that are applicable to U.S. public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act, (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time, and (iii) the rules under the Exchange Act requiring the filing with the SEC of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. We intend to furnish quarterly reports to the SEC on Form 6-K for so long as we are subject to the reporting requirements of Section 13(g) or 15(d) of the Exchange Act, although the information we furnish may not be the same as the information that is required in quarterly reports on Form 10-Q for U.S. domestic issuers. In addition, while U.S. domestic issuers that are not large accelerated filers or accelerated filers are required to file their annual reports on Form 10-K within 90 days after the end of each fiscal year, in the fiscal years ending on or after December 15, 2011, foreign private issuers will not be required to file their annual report on Form 20-F until 120 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. Although we intend to make interim reports available to our shareholders in a timely manner, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of otherwise applicable NASDAQ requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.
As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of those otherwise required under the Listing Rules of the NASDAQ Stock Market for domestic U.S. issuers. For instance, we intend to follow home country practice in Israel with regard to, among other things, the composition of our board of directors, director nomination procedures, the approval of compensation of officers, and quorum requirements at general meetings of our shareholders. In addition, we intend to follow our home country law instead of the Listing Rules of the NASDAQ Stock Market that require us to obtain shareholder approval for certain dilutive events, such as the establishment or amendment of certain equity based compensation plans, an issuance that will result in a change of control of the company, certain transactions other than a public offering involving issuances of a 20% or greater interest in the company, and certain acquisitions of the stock or assets of another company. Following our home country governance practices as opposed to the requirements that would otherwise apply to a United States company listed on NASDAQ may provide less protection to you than what is accorded to investors under the Listing Rules of the NASDAQ Stock Market applicable to domestic U.S. issuers.
Exchange rate fluctuations between the U.S. dollar and the NIS and the Euro and inflation may negatively affect our earnings and we may not be able to hedge our currency exchange risks successfully.
The dollar is our functional and reporting currency. However, a significant portion of our operating expenses, including personnel and facilities related expenses, are incurred in NIS. As a result, we are exposed to the risks that the NIS may appreciate relative to the U.S. dollar, or, if the NIS instead devalues relative to the U.S. dollar, that the inflation rate in Israel may exceed such rate of devaluation of the NIS, or that the timing of such devaluation may lag behind inflation in Israel. In any such event, the dollar cost of our operations in Israel would increase and our dollar-denominated results of operations would be adversely affected. The Israeli rate of inflation has not had a material adverse effect on our financial condition during 2012 and 2013 and during the six months ended June 30, 2014. In addition, we expect to incur operating expenses denominated in Euros, and therefore, our operating results are also subject to fluctuations due to changes in the U.S. dollar/Euro exchange rate. Given our general lack of currency hedging arrangements to protect us from fluctuations in the exchange rates of the NIS, the Euro and other foreign currencies in relation to the U.S. dollar (and/or from inflation of such foreign currencies), we may be exposed to material adverse effects from such movements. We cannot predict any future trends in the rate of inflation in Israel or the rate of devaluation (if any) of the NIS against the U.S. dollar.
41
Our management team’s lack of experience as officers of publicly-traded companies may hinder our ability to comply with the Sarbanes-Oxley Act.
It may be time-consuming, difficult and costly for us to develop and implement the internal controls and reporting procedures required by the Sarbanes-Oxley Act. We may need to hire additional financial reporting, internal controls and other finance staff or consultants in order to develop and implement appropriate internal controls and reporting procedures. If we are unable to comply with the Sarbanes-Oxley Act’s internal controls requirements, we may not be able to obtain the independent auditor certifications that the Sarbanes-Oxley Act requires publicly-traded companies to obtain.
Risks Related to this Offering and Our Ordinary Shares
The price of our ordinary shares may be volatile, and the market price of our ordinary shares after this offering and the concurrent Private Placement may drop below the price you pay.
The initial public offering price per share may vary from the market price of our ordinary shares that prevails after the offering. If an active market for our ordinary shares develops and continues, the price of our ordinary shares nevertheless may be volatile. Market prices for securities of early-stage medical device companies have historically been particularly volatile. As a result of this volatility, you may not be able to sell your ordinary shares at or above the initial public offering price paid per ordinary share. The factors that may cause the market price of our ordinary shares to fluctuate include, but are not limited to:
|
|
•
|
progress, or lack of progress, in developing and commercializing our products;
|
|
|
•
|
favorable or unfavorable decisions about our products or intellectual property from government regulators, insurance companies or other third-party payors;
|
|
|
•
|
our ability to recruit and retain qualified regulatory and research and development personnel;
|
|
|
•
|
changes in investors’ and securities analysts’ perception of the business risks and conditions of our business;
|
|
|
•
|
changes in our relationship with key collaborators;
|
|
|
•
|
changes in the market valuation or earnings of our competitors or companies viewed as similar to us;
|
|
|
•
|
changes in key personnel;
|
|
|
•
|
depth of the trading market in our ordinary shares;
|
|
|
•
|
termination of the lock-up agreement or other restrictions on the ability of us or any of our existing shareholders to sell ordinary shares after this offering and the concurrent Private Placement;
|
|
|
•
|
changes in our capital structure, such as future issuances of securities or the incurrence of additional debt;
|
|
|
•
|
the granting or exercise of employee stock options or other equity awards;
|
|
|
•
|
realization of any of the risks described under this section entitled “Risk Factors;” and
|
|
|
•
|
general market and economic conditions.
|
In addition, the equity markets have experienced significant price and volume fluctuations that have affected the market prices for the securities of newly public companies for a number of reasons, including reasons that may be unrelated to our business or operating performance. These broad market fluctuations may result in a material decline in the market price of our ordinary shares and you may not be able to sell your ordinary shares at prices you deem acceptable. In the past, following periods of volatility in the equity markets, securities class action lawsuits have been instituted against public companies. Such litigation, if instituted against us, could result in substantial cost and the diversion of management attention.
42
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
If you purchase ordinary shares in this offering, the public offering price that you pay per ordinary share will be substantially higher than the net tangible book value per ordinary share immediately after this offering. Therefore, you will incur an immediate dilution of $ (or %) in net tangible book value per ordinary share from the price you paid, based on the public offering price of $ per ordinary share. The exercise of outstanding warrants and options may result in further dilution of your investment, but only if the public offering price is greater than $ per ordinary share. In addition, if we raise funds by issuing additional shares or convertible securities in the future, the newly issued shares may further dilute your ownership interest.
Future sales of our ordinary shares, or the perception that future sales may occur, may cause the market price of our ordinary shares to decline, even if our business is doing well.
Sales of substantial amounts of our ordinary shares in the public market after this offering and the concurrent Private Placement, or the perception that these sales may occur, could materially and adversely affect the price of our ordinary shares and could impair our ability to raise capital in the future, particularly through the sale of additional equity securities. The ordinary shares sold in this offering will be freely tradable, without restriction, in the public market, except for any ordinary shares sold to our affiliates.
In connection with this offering, we, our officers and directors and the holders of 1% or more of our ordinary shares have agreed prior to the commencement of this offering, subject to limited exceptions, not to sell or transfer any of our ordinary shares for 180 days after the date of this prospectus without the consent of Chardan Capital Markets, LLC. However, Chardan Capital Markets, LLC may release these shares from any restrictions at any time. We cannot predict what effect, if any, market sales of shares held by any shareholder or the availability of shares for future sale will have on the market price of our ordinary shares.
Approximately ordinary shares may be sold in the public market by existing shareholders after the date of this prospectus and an additional ordinary shares may be sold in the public market by existing shareholders on or about 181 days after the date of this prospectus, subject to volume and other limitations imposed under the federal securities laws. See “Shares Eligible for Future Sale” for a more detailed description of the restrictions on selling our ordinary shares after this offering.
In addition, we are issuing a warrant to Chardan Capital Markets, LLC in this offering to purchase an additional ordinary shares. As of November 21, 2014, we had outstanding options to purchase 26,041,650 ordinary shares (including the Neev Options, which will be exercised prior to the closing of this offering), outstanding warrants to purchase 19,498,426 preferred shares (all of which will convert into warrants to purchase ordinary shares immediately prior to the closing of this offering) and outstanding warrants to purchase an aggregate of 65,376,963 ordinary shares. We plan to register for offer and sale the ordinary shares that are reserved for issuance pursuant to outstanding options. Shares covered by such registration statements upon the exercise of stock options generally will be eligible for sale in the public market, except that affiliates will continue to be subject to volume limitations and other requirements of Rule 144 under the Securities Act of 1933, as amended, or the Securities Act. After this offering and the concurrent Private Placement, the holders of approximately ordinary shares will be entitled to registration rights.
The market price of our ordinary shares may drop significantly when the restrictions on resale by our existing shareholders lapse and these shareholders are able to sell our ordinary shares into the market. In addition, a sale by the company of additional ordinary shares or similar securities in order to raise capital might have a similar negative impact on the share price of our ordinary shares. A decline in the price of our ordinary shares might impede our ability to raise capital through the issuance of additional ordinary shares or other equity securities, and may cause you to lose part or all of your investment in our ordinary shares.
An active trading market may not develop for our ordinary shares, and you may not be able to sell your ordinary shares at or above the initial public offering price per share.
There is no established trading market for our ordinary shares, and the market for our ordinary shares may be highly volatile or may decline regardless of our operating performance. Prior to this offering, you could not buy or sell our ordinary shares publicly. An active public market for our ordinary shares may not develop or be sustained after this offering. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market in our ordinary shares or how liquid that market might become. If a market does not develop or is not sustained, it may be difficult for you to sell your ordinary shares at the time you wish to sell them, at a price that is attractive to you, or at all.
43
The initial public offering price per ordinary share has been determined through negotiation between us and representatives of the underwriter, and may not be indicative of the market prices that prevail after this offering. You may not be able to sell your ordinary shares at or above the initial public offering price per share.
Our management will have broad discretion over the use of proceeds from this offering and the concurrent Private Placement and may not obtain a favorable return on the use of these proceeds.
Our management will have broad discretion in determining how to apply the net proceeds from this offering and the concurrent Private Placement and may spend the proceeds in a manner that our shareholders may not deem desirable. We currently intend to use the net proceeds that we will receive from this offering and the concurrent Private Placement to finance the continuation of our product development and clinical studies in Europe and in the United States. We cannot assure you that these uses or any other use of the net proceeds of this offering and the concurrent Private Placement will yield favorable returns or results.
Additional financing may result in dilution to our shareholders.
We may need to raise additional funds in the future to finance internal growth, to make acquisitions or for other reasons. Any required additional financing may not be available on terms acceptable to us, or at all. If we raise additional funds by issuing equity securities, you may experience significant dilution of your ownership interest and the newly issued securities may have rights senior to those of the holders of our ordinary shares. Alternatively, if we raise additional funds by obtaining loans from third parties, the terms of those financing arrangements may include negative covenants or other restrictions on our business that could impair our operational flexibility, and would also require us to fund additional interest expense. If additional financing is not available when required or is not available on acceptable terms, we may be unable to successfully commercialize our product or continue our research and development.
We have never declared or paid a dividend and currently do not intend to pay cash dividends in the foreseeable future. Any return on investment may be limited to the value of our ordinary shares.
We have never declared and do not anticipate paying cash dividends on our ordinary shares in the foreseeable future. Our board of directors has discretion to declare and pay dividends on our ordinary shares and will make any determination to do so based on a number of factors, such as our operating results, financial condition, current and anticipated cash needs and other business and economic factors that our board of directors may deem relevant. In addition, we are only permitted to pay dividends out of “profits” (as defined by the Israeli Companies Law, 5759-1999, or the Israeli Companies Law), provided that there is no reasonable concern that the dividend distribution will prevent us from meeting our existing and foreseeable obligations, as they become due. If we do not pay dividends, our ordinary shares may be less valuable because a return on your investment will only occur if the trading price of our ordinary shares appreciates. Further, you should not rely on an investment in us if you require dividend income from your investments.
If securities or industry analysts do not publish research or reports about us or our business or publish unfavorable research about us or our business, the price of our ordinary shares and their trading volume could decline.
The trading market for our ordinary shares will depend in part on the research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us the trading price for our securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our securities could decrease, which could cause the price of our securities and their trading volume to decline.
44
Risks Related to Taxation
There is a risk that we could be treated as a domestic (U.S.) corporation for U.S. federal income tax purposes by reason of the transactions related to our acquisition of all of the business operations and substantially all of the assets of Check-Cap LLC on May 31, 2009 (hereinafter sometimes referred to as the “reorganization”).
Section 7874(b) of the Internal Revenue Code of 1986, as amended, or the Code, generally provides that a foreign corporation (i.e., a corporation created or organization under the laws of a jurisdiction outside of the United States) would be treated as a domestic (U.S.) corporation for U.S. federal income tax purposes if, pursuant to a plan or a series of related transactions, (1) the foreign corporation acquires, directly or indirectly, substantially all of the assets of a domestic corporation (or substantially all of the properties constituting a trade or business of a domestic partnership), (2) after the acquisition, the former shareholders of the acquired corporation by reason of holding shares of the acquired corporation (or, in the case of an acquisition with respect to a domestic partnership, the former partners of the domestic partnership by reason of holding a capital or profits interest in the domestic partnership) own at least 80% of the stock (by vote or value) of the acquiring corporation, and (3) after the acquisition, the expanded affiliated group that includes the acquiring corporation does not have substantial business activities in the foreign country in which, or under the laws of which, the acquiring corporation is created or organized when compared to the total business activities of such expanded affiliated group. On the basis of an analysis by Deloitte Touche Tohmatsu, or Deloitte, of the relevant facts and circumstances and the relevant law (including the temporary regulations under Section 7874 applicable at the time of the reorganization), it was determined that the third condition described in the preceding sentence was not met with respect to the reorganization and, therefore, that the inversion tax rules of Section 7874(b) would not apply to treat us as a domestic corporation for U.S. federal income tax purposes. However, since this determination was made on the basis of all of the relevant facts and circumstances, and it is not clear which facts and circumstances the Internal Revenue Service, or the IRS, may consider more important than others, this conclusion is not free from doubt.
If Section 7874(b) were to apply to the reorganization (and we were to be treated as a domestic corporation for U.S. federal income tax purposes), then, among other things, (i) we would be subject to U.S. federal income tax on our worldwide taxable income (if and when we have taxable income), (ii) certain payments (e.g., interest and dividends) that we make (or have made) to our foreign investors may be (or may have been) subject to U.S. withholding taxes, (iii) we may be subject to significant penalties for the failure to file certain tax returns and reports, including reports with respect to our foreign bank accounts, and (iv) the U.S. unitholders of Check-Cap LLC would not have been subject to U.S. federal income tax on royalties that are deemed to be paid to them under Section 367(d) of the Code as a result of the reorganization. (As discussed under “Management’s Discussion and Analysis of Financial Condition and Results of Operations – Liquidity and Capital Resources – Contractual Obligations” and “– Application of Critical Accounting Policies and Estimates – Royalties provision – Reimbursement liability to Check-Cap LLC unitholders,” as part of the reorganization, we committed to reimburse the unitholders of Check-Cap LLC for any tax burdens that may be imposed on them due to the reorganization, including royalties that are deemed to be paid to the U.S. unitholders under Section 367(d) of the Code.)
Prospective investors are urged to consult their own advisors on these issues. The balance of this discussion, including the discussion under “Taxation – U.S. Federal Income Taxation,” assumes that we will be and have been treated as a foreign corporation for U.S. federal income tax purposes.
We may be eligible for tax benefits from government programs, which require us to meet certain conditions, including regarding the location of our property, plant and equipment and manufacturing in Israel. We can provide no assurance that we would continue to be eligible for such benefits and/or that any such benefits will not be terminated in the future.
Our manufacturing facilities in Israel may qualify as a “Benefited Enterprise” under the Israeli Law for Encouragement of Capital Investments, 1959, or the Investment Law, which would entitle us to receive certain tax benefits. In order to be eligible for such benefits, we would be required to meet certain conditions, including the making of a minimum capital investment in our productive assets and the carrying on of a required portion of our manufacturing in Israel. The amount of the benefit will be determined in accordance with various conditions, including the location of our property, plant and equipment and the location of certain of our sub-contractors. If we cease to meet the required conditions for eligibility, the tax benefits could be cancelled and we could be required to pay increased taxes or to refund the amounts of the benefits received with interest and penalties. We can provide no assurance as to the amount of future capital investment in our productive assets, our future manufacturing location and the future location of our property, plant and equipment and certain of our sub-contractors, and therefore, we cannot provide assurance that we will be eligible for such tax benefits or assurance as to the amount of such tax benefits. Even if we continue to meet the relevant requirements, the tax benefits that Benefited Enterprises receive may not be continued in the future at their current levels or at all. If these tax benefits were reduced or eliminated, the amount of taxes that we would be required to pay would likely increase, as all of our operations would consequently be subject to corporate tax at the standard rate, which could adversely affect our results of operations. See “Taxation— Israeli Tax Considerations and Government Programs —Law for the Encouragement of Capital Investments, 5719-1959” for additional information concerning these tax benefits.
45
There is a risk that we may be classified as a passive foreign investment company, or PFIC, which could result in adverse U.S. federal income tax consequences to U.S. investors.
In general, we will be treated as a PFIC for any taxable year in which either (1) at least 75% of our gross income (including our pro rata share of the gross income of our 25% or more-owned corporate subsidiaries) is passive income or (2) at least 50% of the average value of our assets (including our pro rata share of the assets of our 25% or more-owned corporate subsidiaries) is attributable to assets that produce, or are held for the production of, passive income. Passive income generally includes dividends, interest, rents, royalties, and gains from the disposition of passive assets. If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder (as defined in the section titled “Taxation—U.S. Federal Income Taxation—General”) of the ordinary shares, the U.S. Holder may be subject to increased U.S. federal income tax liability upon a sale or other disposition of the ordinary shares or the receipt of certain excess distributions from us and may be subject to additional reporting requirements.
Our actual PFIC status for our current taxable year or any subsequent taxable year is uncertain and will not be determinable until after the end of such taxable year. Accordingly, there can be no assurance with respect to our status as a PFIC for our current taxable year or any subsequent taxable year.
U.S. investors are urged to consult their own tax advisors regarding the possible application of the PFIC rules. For more information, see “Taxation—U.S. Federal Income Taxation—U.S. Holders—Passive Foreign Investment Company Rules.”
46
SPECIAL NOTE ON FORWARD-LOOKING STATEMENTS
This prospectus contains statements that may be deemed to be “forward-looking statements” within the meaning of the federal securities laws. These statements relate to anticipated future events, future results of operations and/or future financial performance. In some cases, you can identify forward-looking statements by their use of terminology such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “future,” “intend,” “may,” “ought to,” “plan,” “possible,” “potentially,” “predicts,” “project,” “should,” “will,” “would,” negatives of such terms or other similar terms. These forward-looking statements involve known and unknown risks, uncertainties and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. The forward-looking statements in this prospectus include, without limitation, statements relating to:
|
|
•
|
our goals and strategies;
|
|
|
•
|
the timing and conduct of the clinical trials for our ingestible imaging capsule, including statements regarding the timing, progress and results of current and future preclinical studies and clinical trials, and our research and development programs;
|
|
|
•
|
the clinical utility, potential advantages and timing or likelihood of regulatory filings and approvals of our ingestible imaging capsule;
|
|
|
•
|
our future business development, results of operations and financial condition;
|
|
|
•
|
our ability to protect our intellectual property rights;
|
|
|
•
|
our plans to develop, launch and commercialize our imaging capsule and any future products;
|
|
|
•
|
the timing, cost or other aspects of the commercial launch of our imaging capsule;
|
|
|
•
|
market acceptance of our product;
|
|
|
•
|
our estimates regarding expenses, future revenues, capital requirements and our need for additional financing;
|
|
|
•
|
our estimates regarding the market opportunity for our imaging capsule;
|
|
|
•
|
the impact of government laws and regulations;
|
|
|
•
|
our ability to recruit and retain qualified regulatory and research and development personnel;
|
|
|
•
|
unforeseen changes in healthcare reimbursement for any of our approved product;
|
|
|
•
|
difficulties in maintaining commercial scale manufacturing capacity and capability; our ability to generate growth;
|
|
|
•
|
our failure to comply with regulatory guidelines;
|
|
|
•
|
uncertainty in industry demand and patient wellness behavior;
|
|
|
•
|
general economic conditions and market conditions in the medical device industry;
|
|
|
•
|
future sales of large blocks or our ordinary shares, which may adversely impact our share price;
|
|
|
•
|
depth of the trading market in our ordinary shares; and
|
|
|
•
|
our intended use of proceeds of this offering and the concurrent Private Placement.
|
The preceding list is not intended to be an exhaustive list of all of our forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties, including those described in “Risk Factors.”
You should not unduly rely on any forward-looking statements. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that future results, levels of activity, performance and events and circumstances reflected in the forward-looking statements will be achieved or will occur. Except as required by law, we undertake no obligation to update publicly any forward-looking statements for any reason after the date of this prospectus, to conform these statements to actual results or to changes in our expectations.
47
We estimate that we will receive net proceeds from this offering of approximately $ million, or $ million if the underwriters exercise in full their option to purchase additional ordinary shares, based on an assumed initial public offering price of $ , the midpoint of the price range set forth on the cover page of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses. We will also expect to receive net proceeds of approximately $____ million from the sale of ___________ ordinary shares in the concurrent Private Placement after deducting commissions payable by us.
A $1.00 increase (decrease) in the assumed initial public offering price of $ would increase (decrease) the net proceeds that we receive from the offering by approximately $ million, assuming that the number of ordinary shares offered, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting and other discounts and commissions and estimated offering expenses. Similarly, each increase (decrease) of 100,000 shares in the number of ordinary shares offered by us would increase (decrease) the net proceeds to us from this offering by approximately $ million, assuming that the assumed initial public offering price remains the same, and after deducting the underwriting and other discounts and commissions and estimated offering expenses.
We currently intend to use the net proceeds we receive from this offering and the concurrent Private Placement as follows:
|
|
•
|
approximately $ million on research and development;
|
|
|
•
|
approximately $ million on obtaining regulatory approvals of our product, including approximately $ million on clinical trials in Europe and the United States;
|
|
|
•
|
approximately $ million to launch our product in selected countries in Europe;
|
|
|
•
|
approximately $ million to build our manufacturing capabilities; and
|
|
|
•
|
the balance, if any, for working capital and other general corporate purposes.
|
The expected use of net proceeds of this offering and the concurrent Private Placement represents our current intentions based upon our present plans and business conditions. Investors are cautioned, however, that expenditures may vary substantially from these estimates. Investors will be relying on the judgment of our management, who will have broad discretion regarding the application of the proceeds of this offering and the concurrent Private Placement. The amounts and timing of our actual expenditures will depend upon numerous factors, including the amount of cash generated by our operations, the amount of competition and other operational factors. From time to time, we will evaluate these and other factors to determine if our allocation of resources, including the proceeds of this offering and the concurrent Private Placement, is being optimized.
Circumstances that may give rise to changes in our use of net proceeds from this offering and the concurrent Private Placement include:
|
|
•
|
the timing of clinical studies and eventual FDA or other regulatory approvals of our imaging capsule;
|
|
|
•
|
the need or desire on our part to accelerate, increase or eliminate existing initiatives due to, among other things, changing market conditions and competitive developments; and
|
|
|
•
|
the availability of other sources of cash including cash flow from operations and new bank debt financing arrangements, if any.
|
Pending any use as described above, we intend to invest the net proceeds to us from this offering and the concurrent Private Placement in bank deposits, U.S. government securities and Israeli government securities. We cannot predict whether the net proceeds from such investments will produce a favorable return.
48
DIVIDEND POLICY
We have never declared or paid dividends on our ordinary shares and currently do not intend to pay cash dividends on our ordinary shares in the foreseeable future. We currently intend to retain all of our future earnings, if any, to finance the growth and development of our business.
Our ability to distribute dividends also may be limited by future contractual obligations and by Israeli law. The Israeli Companies Law restricts our ability to declare dividends. Unless otherwise approved by a court, we can distribute dividends only from “profits” (as defined by the Israeli Companies Law), and only if there is no reasonable concern that the dividend distribution will prevent us from meeting our existing and foreseeable obligations as they become due. Subject to the foregoing, payment of future dividends, if any, will be at the discretion of our board of directors and will depend on various factors, such as our financial condition, operating results, current and anticipated cash needs and other business and economic factors that our board of directors may deem relevant. See “Description of Share Capital—Dividend and Liquidation Rights.” In addition, the payment of dividends may be subject to Israeli withholding taxes. See “Taxation—Israeli Tax Considerations and Government Programs—Taxation of Our Shareholders—Taxation of Non-Israeli Shareholders on Receipt of Dividends.” Furthermore, if we pay a dividend out of income attributed to our Benefited Enterprise that was generated during the tax exemption period, we may be subject to tax on the grossed-up amount of such distributed income at the corporate tax rate which would have been applied to our Benefited Enterprise’s income had we not enjoyed the exemption. See “Taxation – Israeli Tax Considerations and Government Programs — Law for the Encouragement of Capital Investments, 5719-1959 — Tax Benefits Subsequent to the 2005 Amendment.”
49
CAPITALIZATION
The following table sets forth our capitalization as of June 30, 2014:
|
|
•
|
on an actual basis;
|
|
|
•
|
on a pro forma basis to give effect to: (i) the conversion of all of our outstanding preferred shares into an aggregate of ordinary shares immediately prior to the completion of this offering; (ii) the issuance of 1,995,475 ordinary shares to Mr. Guy Neev upon the exercise of the Neev Options, which will occur, prior to the closing of this offering; and (iii) the issuance of 7,503,521 ordinary shares issuable under warrants that will be automatically exercised without consideration upon the exercise of the Neev Options;
|
|
|
•
|
on a pro forma as adjusted basis to give further effect to (i) the issuance and sale of ordinary shares by us in this offering at an assumed initial public offering price of $ per ordinary share, the midpoint of the estimated initial public offering price range set forth on the cover of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us and (ii) the issuance and sale of ordinary shares by us in the Private Placement at an assumed price of $_______ per ordinary share, the midpoint of the estimated public offering price range, after deducting commissions and estimated expenses payable by us in connection with the Private Placement.
|
You should read this table in conjunction with our audited financial statements and related notes and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus.
|
As of June 30, 2014
|
||||||||||||
|
Actual
|
Pro forma
|
Pro forma as adjusted
|
||||||||||
|
(in thousands of US$)
(Unaudited)
|
||||||||||||
|
Liabilities:
|
||||||||||||
|
Other financial liabilities
|
$ | 657 | $ | $ | ||||||||
|
Shareholders’ equity:
|
||||||||||||
|
Preferred share capital, 242,845,819 shares authorized, 86,778,910 shares issued, actual, no shares issued, pro forma, no shares issued, pro forma, as adjusted
|
226 | - | - | |||||||||
|
Ordinary share capital, 907,154,181 shares authorized, shares issued, actual, shares issued, pro forma, shares issued, pro forma, as adjusted
|
53 | |||||||||||
|
Premium on preferred shares
|
21,167 | - | - | |||||||||
|
Share options
|
1,793 | |||||||||||
|
Premium on ordinary shares
|
6 | |||||||||||
|
Capital reserve for ordinary share-based payment
|
471 | |||||||||||
|
Accumulated deficit
|
(24,714 | ) | ||||||||||
|
Total shareholders’ deficit
|
$ | (998 | ) | $ | $ | |||||||
The number of issued and outstanding shares as of June 30, 2014 on a pro forma as adjusted basis in the table excludes:
|
|
•
|
19,498,426 ordinary shares issuable upon the exercise of outstanding warrants to purchase preferred shares (comprised of (i) warrants to purchase 836,412 Series C-1 preferred shares, (ii) warrants to purchase 1,546,996 Series C-2 preferred shares, (iii) warrants to purchase 503,872 Series D-1 preferred shares; and (iv) warrants to purchase 16,611,146 Series D-2 preferred shares, following their conversion into warrants to purchase ordinary shares immediately prior to the closing of this offering) with a weighted average exercise price of $0.44 per ordinary share;
|
50
|
|
•
|
273,592 ordinary shares issuable upon the exercise of outstanding warrants, which will be automatically exercised, without consideration, if and when Guy Neev exercises any part of the Neev Options, in proportion to (i) the portion of the Neev Options exercised by Guy Neev and (ii) the number of warrants held by the optionee with respect to which such warrants were granted that were exercised prior to the exercise of the Neev Options;
|
|
|
•
|
57,599,850 ordinary shares issuable upon the exercise of outstanding warrants with an exercise price of NIS 0.01 per ordinary share, of which (i) warrants to purchase 53,169,092 ordinary shares are fully vested; and (ii) warrants to purchase 4,430,758 ordinary shares will become fully vested upon the closing of this offering;
|
|
|
•
|
12,415,436 ordinary shares issuable upon the exercise of outstanding options with a weighted average exercise price of $0.17 per ordinary share, granted under our 2006 Unit Option Plan;
|
|
|
•
|
11,630,739 ordinary shares issuable upon the exercise of outstanding options with an exercise price of NIS 0.01 per ordinary share granted under our 2006 Unit Option Plan, which will become fully vested upon the closing of this offering;
|
|
|
•
|
769,453 ordinary shares issuable upon the exercise of options with an exercise price of $0.2478 per ordinary share, under our 2006 Unit Option Plan, which we have agreed that certain executive officers will be entitled to upon completion of an equity financing, which includes this offering;
|
|
|
•
|
the ordinary shares issuable upon the exercise of warrants to be issued to certain finders in connection with the credit line agreement if either (i) the credit line amount extended to us is invested in ordinary shares in a private placement on or prior to February 18, 2015 or (ii) if we do not consummate an IPO on or prior to February 18, 2015 and we call the credit line amount, upon conversion of the credit line amount into ordinary shares;
|
|
|
•
|
150,000 ordinary shares issuable upon the exercise of warrants with an exercise price per ordinary shares equal to the initial public offering price to be issued to our U.S. legal counsel as partial compensation for services rendered in connection with the offering; and
|
|
|
•
|
the ordinary shares issuable upon exercise of the underwriter’s compensation warrants to be issued in connection with this offering.
|
51
DILUTION
If you invest in our ordinary shares, your ownership interest will be diluted to the extent of the difference between the initial public offering price per ordinary share and the pro forma as adjusted net tangible book value per ordinary share immediately after this offering. Dilution results from the fact that the initial public offering price per share is substantially in excess of the book value per share attributable to the existing shareholders for the presently outstanding ordinary shares.
As of June 30, 2014, our historical net tangible book value (deficit) was $(1.0) million, or $(0.031) per ordinary share. Net tangible book value (deficit) per ordinary share represents the amount of our total tangible assets less total liabilities, divided by 32,541,630, the number of ordinary shares outstanding on June 30, 2014, which number includes: (i) the 23,042,634 ordinary shares that were actually issued and outstanding on June 30, 2014; (ii) the 1,995,475 ordinary shares issuable to Mr. Guy Neev upon exercise of the Neev Options, which shares were deemed to be outstanding on June 30, 2014; and (iii) the 7,503,521 ordinary shares issuable under warrants that will be automatically exercised, without consideration, upon the exercise by Mr. Guy Neev of the Neev Options, which were also deemed to be outstanding on June 30, 2014.
Our pro forma net tangible book value (deficit) as of June 30, 2014 was $ , or $ per ordinary share. Pro forma net tangible book value (deficit) per ordinary share represents the amount of our total tangible assets less our total liabilities, divided by , the number of our ordinary shares outstanding and deemed to be outstanding as described above, as of June 30, 2014, after giving effect to the conversion of all of our outstanding preferred shares into ordinary shares immediately prior to the completion of this offering.
Our pro forma as adjusted net tangible book value as of June 30, 2014, was $ , or $ per ordinary share. The pro forma as adjusted net tangible book value gives further effect to (i) the sale of ordinary shares in this offering at the initial public offering price of $ per share (the midpoint of the range set forth on the cover page of this prospectus), after deducting underwriting discounts and commissions and estimated offering expenses payable by us; and (ii) the sale of ordinary shares in the Private Placement at the initial public offering price of $ per share , after deducting commissions and estimated expenses payable by us in connection with the Private Placement. The difference between the initial public offering price and the pro forma as adjusted net tangible book value per share represents an immediate dilution of $ per share to new investors purchasing ordinary shares in this offering.
The following table illustrates this dilution on a per share basis to new investors:
|
Assumed initial public offering price per share
|
$
|
||||||
|
Pro forma net tangible book value (deficit) per share before this offering, as of June 30, 2014
|
$
|
||||||
|
Increase in pro forma net tangible book value per share attributable to new investors in this offering and new investors in the Private Placement
|
|||||||
|
Pro forma net tangible book value per share after the offering and the Private Placement
|
|||||||
|
Dilution in pro forma tangible book value per share to new investors in this offering
|
$
|
If the underwriters exercise their option to purchase additional ordinary shares in full, the pro forma as adjusted net tangible book value per share after giving effect to the offering and the Private Placement would be $ per ordinary share. This represents an increase in pro forma as adjusted net tangible book value of $ per share to existing shareholders and dilution in pro forma as adjusted net tangible book value of $ per ordinary share to new investors.
A $1.00 increase (decrease) in the assumed initial public offering price of $ , the mid-point of the price range set forth on the cover page of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value after this offering and the Private Placement by $ and the pro forma as adjusted net tangible book value per ordinary share after this offering and the Private Placement by $ per share and would increase (decrease) the dilution per share to new investors in this offering by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same. The information discussed above is illustrative only and may change based on the actual initial public offering price and other terms of the offering determined at pricing.
52
The following table summarizes, on a pro forma as adjusted basis as of June 30, 2014, the total number of ordinary shares purchased from us, the total consideration paid, or to be paid, and the average price per ordinary share paid, or to be paid, by existing shareholders and by new investors in this offering and the concurrent Private Placement at an assumed initial public offering price of $ per ordinary share, which is the midpoint of the price range listed on the cover page of this prospectus, before deducting underwriting discounts and commissions and estimated offering expenses payable by us. As the table shows, new investors purchasing shares in this offering will pay an average price per share substantially higher than our existing shareholders paid.
|
Average Price
Per
Ordinary
Share
|
|||||||||
|
Shares Purchased
|
Total Consideration
|
||||||||
|
Number
|
Percentage
|
Amount
|
Percentage
|
||||||
|
Existing shareholders
|
|||||||||
|
New Investors
|
|||||||||
|
Total
|
|||||||||
The table above is based on ordinary shares outstanding on June 30, 2014 and includes:
|
|
•
|
the 1,995,475 ordinary shares issuable to Mr. Guy Neev upon exercise of the Neev Options, which shares were deemed to be outstanding on June 30, 2014;
|
|
|
•
|
the 7,503,521 ordinary shares issuable under warrants that will be automatically exercised without consideration upon the exercise by Mr. Guy Neev of the Neev Options, which shares were deemed to be outstanding on June 30, 2014;
|
|
|
•
|
the issuance of ordinary shares in the Private Placement at the initial public offering price per ordinary share; and
|
|
|
•
|
the conversion of all of our outstanding preferred shares into an aggregate of ordinary shares immediately prior to the completion of this offering.
|
The table above does not include:
|
|
•
|
19,498,426 ordinary shares issuable upon the exercise of outstanding warrants to purchase preferred shares (comprised of (i) warrants to purchase 836,412 Series C-1 preferred shares, (ii) warrants to purchase 1,546,996 Series C-2 preferred shares, (iii) warrants to purchase 503,872 Series D-1 preferred shares and (iv) warrants to purchase 16,611,146 Series D-2 preferred shares, following their conversion into warrants to purchase ordinary shares immediately prior to the closing of this offering) with a weighted average exercise price of $0.44 per ordinary share;
|
|
|
•
|
273,592 ordinary shares issuable upon the exercise of outstanding warrants, which will be automatically exercised, without consideration, if and when Guy Neev exercises any part of the Neev Options, in proportion to (i) the portion of the Neev Options exercised by Guy Neev and (ii) the number of warrants held by the optionee with respect to which such warrants were granted that were exercised prior to the exercise of the Neev Options;
|
|
|
•
|
57,599,850 ordinary shares issuable upon the exercise of outstanding warrants with an exercise price of NIS 0.01 per ordinary share, of which (i) warrants to purchase 53,169,092 ordinary shares are fully vested; and (ii) warrants to purchase 4,430,758 ordinary shares will become fully vested upon the closing of this offering;
|
|
|
•
|
12,415,436 ordinary shares issuable upon the exercise of outstanding options with a weighted average exercise price of $0.17 per ordinary share, granted under our 2006 Unit Option Plan;
|
|
|
•
|
11,630,739 ordinary shares issuable upon the exercise of outstanding options with an exercise price of NIS 0.01 per ordinary share granted under our 2006 Unit Option Plan, which will become fully vested upon the closing of this offering;
|
53
|
|
•
|
769,453 ordinary shares issuable upon the exercise of options with an exercise price of $0.2478 per ordinary share, under our 2006 Unit Option Plan, which we have agreed that certain executive officers will be entitled to upon completion of an equity financing, which includes this offering;
|
|
|
•
|
the ordinary shares issuable upon the exercise of warrants to be issued to certain finders in connection with the credit line agreement if either (i) the credit line amount extended to us is[invested in] [converted into] ordinary shares in a private placement on or prior to February 18, 2015 or (ii) if we do not consummate an IPO on or prior to February 18, 2015 and we call the credit line amount, upon conversion of the credit line amount into ordinary shares; and
|
|
|
•
|
150,000 ordinary shares issuable upon the exercise of warrants with an exercise price per ordinary shares equal to the initial public offering price to be issued to our U.S. legal counsel as partial compensation for services rendered in connection with the offering; and
|
|
|
•
|
ordinary shares issuable upon exercise of the underwriter’s compensation warrants to be issued in connection with this offering.
|
If the underwriters exercise their over-allotment option to purchase additional shares in full, the following will occur:
|
|
•
|
the percentage of ordinary shares held by existing shareholders will decrease to approximately % of the total number of ordinary shares outstanding after this offering; and
|
|
|
•
|
the number of ordinary shares held by new investors will increase to , or approximately % of the total number of ordinary shares outstanding after this offering.
|
To the extent that outstanding options and warrants are exercised, you will experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities may result in further dilution to our shareholders.
54
ENFORCEABILITY OF CIVIL LIABILITIES
We are incorporated under the laws of the State of Israel. Service of process upon us and upon our directors and officers and the Israeli experts named in this prospectus, substantially all of whom reside outside the United States, may be difficult to obtain within the United States. Furthermore, because substantially all of our assets, and those of our directors and officers and the Israeli experts named herein, are located outside the United States, any judgment obtained in the United States against us or any of these persons may not be collectible within the United States.
We have irrevocably appointed Puglisi & Associates as our agent to receive service of process in any action against us in any United States federal or state court arising out of this offering or any purchase or sale of securities in connection with this offering. The address of Puglisi & Associates is 850 Library Avenue, Suite 204, Newark, Delaware 19711.
We have been informed by our legal counsel in Israel, Fischer Behar Chen Well Orion & Co., that there is doubt as to the enforceability of civil liabilities under U.S. securities laws pursuant to original actions instituted in Israel. Israeli courts may refuse to hear a claim based on an alleged violation of U.S. securities laws on the grounds that Israel is not the most appropriate forum to bring such a claim. In addition, even if an Israeli court agrees to hear a claim, it may determine that Israeli law and not U.S. law is applicable to the claim. If U.S. law is found to be applicable, the content of applicable U.S. law must be proved as a fact by expert witnesses, which can be a time-consuming and costly process. Certain matters of procedure may also be governed by Israeli law.
Subject to certain time limitations and legal procedures, Israeli courts may enforce a U.S. judgment in a civil matter, including a judgment based upon the civil liability provisions of the Securities Act and the Exchange Act and including a monetary or compensatory judgment in a non-civil matter, provided that, among other things:
|
|
•
|
the judgment was rendered by a court which was, according to the laws of the state of the court, competent to render the judgment;
|
|
|
•
|
the judgment may no longer be appealed;
|
|
|
•
|
the obligation imposed by the judgment is enforceable according to the rules relating to the enforceability of judgments in Israel and the substance of the judgment is not contrary to public policy; and
|
|
|
•
|
the judgment is executory in the state in which it was given.
|
Even if such conditions are met, an Israeli court may not declare a foreign civil judgment enforceable if:
|
|
•
|
the judgment was given in a state whose laws do not provide for the enforcement of judgments of Israeli courts (subject to exceptional cases);
|
|
|
•
|
the enforcement of the judgment is likely to prejudice the sovereignty or security of the State of Israel;
|
|
|
•
|
the judgment was obtained by fraud;
|
|
|
•
|
the opportunity given to the defendant to bring its arguments and evidence before the court was not reasonable in the opinion of the Israeli court;
|
|
|
•
|
the judgment was rendered by a court not competent to render it according to the laws of private international law as they apply in Israel;
|
|
|
•
|
the judgment is contradictory to another judgment that was given in the same matter between the same parties and that is still valid; or
|
|
|
•
|
at the time the action was brought in the foreign court, a lawsuit in the same matter and between the same parties was pending before a court or tribunal in Israel.
|
Foreign judgments enforced by Israeli courts generally will be payable in Israeli currency, which can then be converted into non-Israeli currency and transferred out of Israel. The usual practice in an action before an Israeli court to recover an amount in a non-Israeli currency is for the Israeli court to render judgment for the equivalent amount in Israeli currency at the rate of exchange in force on the date of the judgment, but the judgment debtor may make payment in foreign currency. Pending collection, the amount of the judgment of an Israeli court stated in Israeli currency ordinarily will be linked to the Israeli consumer price index plus interest at the annual statutory rate set by Israeli regulations prevailing at that time. Judgment creditors must bear the risk of unfavorable exchange rates.
55
SELECTED FINANCIAL DATA
You should read the following selected financial information in conjunction with our audited financial statements and related notes beginning on page F-1 and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” beginning on page 58 in this prospectus.
The following tables set forth our selected financial data. You should read the following selected financial data in conjunction with, and it is qualified in its entirety by reference to, our historical financial information and other information provided in this prospectus, including “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes appearing elsewhere in this prospectus.
The selected statements of comprehensive loss data for the years ended December 31, 2012 and 2013 and the statements of financial position data as of December 31, 2012 and 2013 are derived from our audited financial statements appearing elsewhere in this prospectus. The selected statements of comprehensive loss data for the six months ended June 30, 2014 and the statements of financial position data as of June 30, 2014, are derived from our unaudited financial statements appearing elsewhere in this prospectus. The historical results set forth below are not necessarily indicative of the results to be expected in future periods. Our financial statements have been prepared in accordance with IFRS, as issued by the International Accounting Standards Board, or IASB.
Statements of Comprehensive Loss Data
|
Year Ended December 31,
|
Six Months Ended June 30,
|
|||||||||||||||
|
2013
|
2012
|
2014
|
2013
|
|||||||||||||
|
(US$ in thousands, except per share data)
|
||||||||||||||||
|
(Unaudited)
|
||||||||||||||||
|
Research and development expenses, net(1)
|
2,662 | 2,692 | 1,640 | 1,364 | ||||||||||||
|
General and administrative expenses
|
1,090 | 1,203 | 564 | 520 | ||||||||||||
|
Other expenses (income)
|
(10 | ) | 13 | -- | -- | |||||||||||
|
Operating loss
|
3,742 | 3,908 | 2,204 | 1,884 | ||||||||||||
|
Finance income
|
(63 | ) | (416 | ) | (60 | ) | (45 | ) | ||||||||
|
Finance expenses
|
316 | 229 | 85 | 230 | ||||||||||||
|
Finance expenses (income), net
|
253 | (187 | ) | 25 | 185 | |||||||||||
|
Loss and total comprehensive loss for the period
|
3,995 | 3,721 | 2,229 | 2,069 | ||||||||||||
|
Loss per ordinary share of NIS 0.01 par value, basic and diluted(2)
|
0.18 | 0.17 | 0.10 | 0.09 | ||||||||||||
|
Weighted average number of ordinary shares outstanding – basic and diluted (in thousands)(2)
|
32,542 | 32,530 | 32,542 | 32,542 | ||||||||||||
|
Pro forma loss per ordinary share of NIS 0.01 par value(3)
|
||||||||||||||||
|
Basic and diluted (unaudited)(2)
|
||||||||||||||||
|
Pro forma weighted average number of ordinary shares outstanding - basic and diluted (in thousands) (unaudited)(2)
|
||||||||||||||||
56
Statements of Financial Position Data
|
As of December 31, 2013
|
As of June 30, 2014
|
||||||||||||
|
Actual
|
Pro forma(3)
|
Pro forma as
adjusted(4)
|
|||||||||||
|
(US$ in thousands)
Unaudited
|
|||||||||||||
|
Cash and cash equivalents
|
$ | 4,975 | $ | 2,794 | $ | ||||||||
|
Working capital(5)
|
4,131 | 1,990 | |||||||||||
|
Total assets
|
5,375 | 3,276 | |||||||||||
|
Capital stock
|
23,676 | 23,716 | |||||||||||
|
Total shareholders’ equity (deficit)
|
$ | 1,191 | $ | (998 | ) | $ | |||||||
|
|
(1)
|
Research and development expenses, net is presented net of the differences between the amount of grants received from the OCS and the fair value of their financial liability. The effect of the participation by the OCS totaled $0.4 million, and $0.2 million for the years ended December 31, 2013, and 2012, respectively. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview—Research and development, net” for more information.
|
|
|
(2)
|
Basic and diluted loss per ordinary share is computed based on the basic and diluted weighted average number of ordinary shares outstanding during each period. For purposes of these calculations, the following ordinary shares are deemed to be outstanding: (i) the 1,995,475 ordinary shares issuable to Mr. Guy Neev upon exercise of the Neev Options; and (ii) the 7,503,521 ordinary shares issuable under warrants that will be automatically exercised without consideration upon the exercise by Mr. Guy Neev of the Neev Options. For additional information, see Note 17 to our financial statements for the year ended December 31, 2013 included elsewhere in this prospectus.
|
|
|
(3)
|
On a pro forma basis to give effect to the conversion immediately prior to the completion of this offering of all of our outstanding preferred shares into ordinary shares.
|
|
|
(4)
|
On a pro forma as adjusted basis to give further effect to (i) the issuance and sale of ordinary shares by us in this offering at an assumed initial public offering price of $ per share, the midpoint of the estimated initial public offering price range set forth on the cover of this prospectus, after deducting underwriting discounts and commissions and estimated offering expenses payable by us and (ii) the issuance and sale of ____________ordinary shares by us in the Private Placement at an assumed price of $_______ per share, the estimated public offering price, after deducting commissions and estimated expenses payable by us in connection with the Private Placement.
|
|
|
(5)
|
Working capital is defined as total current assets minus total current liabilities.
|
57
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with our financial statements and related notes included in this prospectus beginning on page F-1. The following discussion and analysis contain forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under “Risk Factors” and elsewhere in this prospectus.
Overview
We are a clinical stage medical diagnostics company engaged in the development of an ingestible imaging capsule that utilizes low-dose X-rays for CRC screening. While CRC is the second leading cause of death from cancer in the United States and is largely preventable with early detection, about one-half of Americans over the age of 50 do not undergo any form of CRC screening due in large part to the pain, discomfort and embarrassment related to current screening methods. Unlike other structural screening methods that are designed to generate structural information of the colon for the detection of pre-cancerous polyps, such as optical colonoscopy, CTC and other capsule-based technology, our imaging capsule is designed to be ingested without any cleansing of the colon and to travel through the gastrointestinal tract naturally while the patient continues his or her normal daily routine. Furthermore, unlike all other CRC imaging devices currently on the market, which require the patient to fast for several hours prior to the procedure, the Check-Cap procedure enables patients to continue eating normally. We believe that this solution will be attractive to both physicians and patients, thereby increasing the number of people willing to undergo screening for CRC.
Check-Cap LLC was formed in 2004 as a Delaware limited liability company to develop a novel and superior solution to colon cancer screening. In 2009, all of the business operations and substantially all of the assets of Check-Cap LLC were transferred to Check Cap Ltd., a newly-incorporated Israeli company. Our offices are located near Haifa, in the northern part of Israel. Our management team includes an experienced group of executives in the business, research, clinical and regulatory fields. As of June 30, 2014, our research and development team consists of 32 experienced professionals (including employees and independent contractors) in the fields of physics, electronics, software and mechanical engineering.
For the past several years, we have focused on the research and development of our imaging technology. We have successfully imaged sections of the colon and detected polyps of various sizes in pigs without any prior bowel cleansing through the insertion of a larger version of our imaging capsule in their colons. We also conducted a clinical study in Europe with a passive (non-imaging) version of our capsule that has similar dimensions to our first prototype imaging capsule, in order to collect pressure and motility data from humans. This information assisted in the development of the initial operational algorithm utilized by our capsule software used for the version of our imaging capsule used in humans.
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Medical Center in Israel and used a prior version of our imaging capsule, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our imaging technology to the human colon, generating images taken in the colon without any prior bowel-cleansing. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted. See “Risk Factors – Risks Related to our Business”.
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of the Check-Cap imaging system. The 10-case study is the first part of a multi-center prospective clinical feasibility study to establish the safety, functionality and preliminary efficacy of the Check-Cap imaging system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity FOBTs and FITs, as well as from optical colonoscopies. The feasibility study is designed to include a total of 60 subjects. The study is being conducted in Israel at the Tel Aviv Medical Center and Laniado Hospital and is planned to also be conducted at the Erasmus University Medical Center in the Netherlands. The clinical feasibility study will also evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified by our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
58
Another objective of the 10-case study was to estimate total radiation exposure for each case study. This was calculated using standard established factors for calculating effective radiation exposure, such as the duration of the capsule inside the body, and was based on the activity of the radiation source inside the imaging capsule and radiation energy, both of which were measured for each case study. The average calculated exposure for the entire procedure in the 10-case study, from ingestion of the capsule to excretion, was 0.03 mSv (STD 0.007 mSv). This level of radiation exposure is similar to a single chest X-ray (approximately 0.06mSv) and two orders of magnitude less than CTC.
Following the successful completion of the broader multi-center, prospective clinical feasibility study, we plan to submit a request for CE marking for the marketing and sale of our capsule in the European Union during 2015. We expect to perform post-marketing studies in Europe following CE marking. We anticipate launching our product commercially in Europe during 2016.
We plan to submit to the FDA a request to conduct a pivotal study in the United States in late 2015 to (i) demonstrate device safety as evidenced by a lack of device-related serious adverse events; and (ii) provide efficacy data concerning our imaging capsule’s sensitivity and specificity. We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study. Pivotal studies are expected, among other things, to compare the images of polyps identified by our imaging system with the same polyps detected by traditional optical colonoscopy and CTC in instances where patients were referred after positive exam results. These clinical findings will be analyzed in comparison to results obtained from FOBTs and FITs. Subject to successful completion of our clinical trials, we anticipate launching our product commercially in the United States during 2017.
Our imaging capsule is being designed to create a two and three-dimensional reconstructed image of the colon and to detect clinically significant polyps with a high degree of sensitivity. Colon polyps are fleshy growths that occur on the lining of the colon. Polyps in the colon are extremely common, and when certain types of polyps grow large enough they can become cancerous.
Our imaging capsule will be swallowed by the patient and propelled by natural motility through the gastrointestinal tract and excreted naturally with no need for retrieval for data collection. Unlike other CRC screening methods, this process should not disrupt a patient’s normal activities or require fasting. Our imaging capsule employs X-rays which are considered low dose by the Radiation Safety Division of the Radiological Protection Branch of the Soreq Nuclear Research Center, which allows it to image the lining of the colon even when surrounded by intestinal content. As such, we believe that patients using our imaging capsule will not be required to undergo any prior bowel cleansing.
Our imaging capsule is being designed to transmit the data it collects to an external data recorder that will be worn by the patient. The external data recorder is being designed to transmit the data to a physician, who will then utilize our data viewer software application to analyze the data collected by our imaging capsule. We intend for physicians to be able to review the colon’s inner images at any location at any time, in less time than is required to perform an optical colonoscopy.
In order to enable a complete view of capsule positioning and motility, we have designed the RELS, which is mounted on the patient’s back throughout the entire screening procedure. The RELS is being designed to provide the physician with accurate localization data aligned with a reconstructed image.
In the event that polyps are identified by our imaging capsule, the patient would be required to undergo a subsequent traditional colonoscopy procedure to examine, remove and biopsy the polyps. For those patients who require a subsequent polypectomy, concerns regarding pain, discomfort and embarrassment may still remain with respect to the subsequent polypectomy. We do not, however, believe that these concerns will make the use of our imaging capsule any less attractive to doctors and patients. Although patients who are initially screened utilizing a traditional colonoscopy could avoid the need for a second procedure if polyps are discovered because they could undergo a polypectomy during the initial screening, if necessary, we believe that our imaging capsule will still be attractive to doctors and patients since a large majority of patients who are screened will not require a subsequent polypectomy. According to a review published by the Agency for Healthcare Research and Quality in October 2008, out of 100 adults aged 50-75, only 25-30 persons have one or more polyps and only 15 persons have significant (10+mm) polyps.
59
We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have been granted patents for our core patent by the U.S. Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan. We also filed patent applications describing the use of our imaging technology in several other medical applications.
We have generated significant losses to date, and we expect to continue to generate losses for at least the next several years as we continue our investment in research and development and clinical trials in order to complete the development of our imaging capsule and to attain regulatory approvals, begin the commercialization efforts for our products, increase our marketing and selling expenses, and incur additional costs as a result of becoming a public company in the United States. The extent of our future operating losses and the timing of becoming profitable are highly uncertain, and we may never achieve or sustain profitability. As of June 30, 2014, we had accumulated losses of approximately $24.7 million.
We believe that the net proceeds from this offering, together with our existing cash, will be sufficient to fund our projected operating requirements until the end of 2016. Until such time as we can generate a sufficient amount of product revenue, if ever, we expect to finance our future cash needs through public or private equity offerings, debt financings and/or corporate collaboration.
For a more detailed description of our business and plans, see “Business.”
Financial Operations Overview
Revenue
We have not generated any revenue since our inception. To date, we have funded our operations primarily through equity financings, as well as from grants that we received from the OCS. If our product development efforts result in clinical success, regulatory approval and the successful commercialization of our imaging capsule, we expect to generate revenue from sales of our imaging capsule.
Operating Cost and Expenses
Our operating costs and expenses are classified into two categories: research and development expenses and general and administrative expenses. For each category, the largest component is personnel costs, which consists of salaries, employee benefits and share-based compensation. Operating costs and expenses also include allocated overhead costs for depreciation of equipment. Operating costs and expenses are generally recognized as incurred. We expect personnel costs to continue to increase as we hire new employees to continue to grow our business.
Research and Development Expenses, Net
Research and development activities are central to our business model. We intend to increase our research and development operations in order to complete the development of our imaging capsule and to attain regulatory approvals.
Since 2012, we have spent approximately $7.6 million on research and development expenses as of June 30, 2014, of which $0.6 million was funded by government grants. Our total research and development expenses, net of participations in the year ended December 31, 2013 and the six months ended June 30, 2014 were approximately $2.7 million and $1.6 million, respectively. All research and development expenses are expensed as incurred. We expect research and development expenses to increase in absolute terms in the near term.
Research and development expenses primarily consist of:
|
|
•
|
personnel-related expenses, including salaries, benefits and related expenses;
|
60
|
|
•
|
payments made to third-party contract research organizations, contract manufacturers, investigative sites and consultants;
|
|
|
•
|
manufacturing development costs;
|
|
|
•
|
costs associated with preclinical studies and clinical trials;
|
|
|
•
|
facilities, depreciation and other expenses, which include direct and allocated expenses for rent and maintenance of facilities; and
|
|
|
•
|
costs associated with obtaining patents and maintaining.
|
Our research and development expenses, net are net of grants we have received from the Government of Israel through the OCS. In exchange for these grants, we are required to pay the OCS royalties from our revenues up to an aggregate of 100% (which may be increased under certain circumstances) of the U.S. dollar-linked value of the grant, plus interest at the rate of 12-month LIBOR. Pursuant to regulations under the Research Law, the rate of repayment ranges between 3% to 5% of revenues (or 6% with respect to certain limited programs and at an increased rate under certain circumstances). As of June 30, 2014, we had received funding from the OCS in the aggregate amount of $3.1 million. Since June 30, 2014, we have received additional funding from the OCS in the aggregate amount of $476,000 under an approved OCS grant in the total amount of $702,000 for a research and development program for the 12 month period commencing March 1, 2014. As of June 30, 2014, we had not paid any royalties to the OCS and had a contingent obligation to the OCS in the amount of $1.6 million.. For additional information regarding the OCS grants, see below under “Royalties provisions - Government grants from the Office of the Chief Scientist” and “Taxation - Israeli Tax Considerations and Government Programs - The Encouragement of Industrial Research and Development Law, 5744-1984.” There can be no assurance that we will continue to receive grants from the OCS in amounts sufficient for our operations, if at all.
In July 2014, we received our first advance payment from the BIRD Foundation of $68,000 as part of a grant for the funding of a project entitled “Collection & Analysis of Gastrointestinal Images for Diagnostic Adenomatic Polyps and Colorectal Cancer.” We expect to receive an aggregate of $567,000 to fund our expenditures for the project, in five installments. See “-Liquidity and Capital Resources- Sources of Liquidity” for a description of the Cooperation and Project Funding Agreement with the BIRD Foundation and Synergy.
General and Administrative Expenses
Our general and administrative expenses consist primarily of salaries and other related costs, including share-based compensation expense, for persons serving as our executive, finance, legal, human resources and administrative personnel, professional service fees and other general corporate expenses, such as communication, office and travel expenses. We expect that our general and administrative expenses will increase in the future as we incur additional general and administrative costs associated with being a public company in the United States, including compliance under the Sarbanes-Oxley Act of 2002 and rules promulgated by the U.S. Securities and Exchange Commission. These public company-related expense increases will likely include costs of additional legal fees, accounting and audit fees, directors’ and officers’ liability insurance premiums and costs related to investor relations.
Financial Income and Finance Expenses
Our finance income and finance expenses consist primarily of fair value changes with respect to the warrants to purchase Series C-1 preferred shares and warrants to purchase Series C-2 preferred shares issued to Pontifax (investing through three affiliated funds: Pontifax (Cayman) II, L.P., Pontifax (Israel) II L.P., Pontifax (Israel) II- Individual Investors L.P. which we collectively refer to as “Pontifax”), interest earned on our cash, cash equivalents and short-term investments and changes in provision for royalties to the OCS.
Foreign currency transactions are translated into U.S. dollars using the exchange rates prevailing at the dates of the transactions or valuation where items are re-measured. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation of year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the statement of comprehensive loss to “finance expenses”/”finance income.”
61
Taxes on Income
The standard corporate tax rate in Israel for the 2014 tax year and thereafter is 26.5%, increased from 25% for the 2012 and 2013 tax years.
We do not generate taxable income in Israel, as we have historically incurred operating losses resulting in carry forward tax losses totaling approximately $18.9 million as of June 30, 2014. We anticipate that we will be able to carry forward these tax losses indefinitely to future tax years. However, a tax loss that can be utilized in a certain tax year, cannot be carried forward to future tax years. Accordingly, we do not expect to pay taxes in Israel until we have taxable income after the full utilization of our carry forward tax losses.
Under the Law for the Encouragement of Capital Investments, 5719-1959 and other Israeli legislation, we may be entitled to certain additional tax benefits, including reduced tax rates, accelerated depreciation and amortization rates for tax purposes on certain assets, deduction of public offering expenses in three equal annual installments and amortization of other intangible property rights for tax purposes. See “Taxation— Israeli Tax Considerations and Government Programs” for additional information concerning these tax benefits.
Results of Operations
Six Months Ended June 30, 2014 Compared to Six Months Ended June 30, 2013
|
Six months ended June 30,
|
||||||||
|
2014
|
2013
|
|||||||
|
(Unaudited)
(US$ in thousands)
|
||||||||
|
Research and development expenses, net
|
$ | 1,640 | $ | 1,364 | ||||
|
General and administrative expenses
|
564 | 520 | ||||||
|
Operating loss
|
2,204 | 1,884 | ||||||
|
Finance income
|
(60 | ) | (45 | ) | ||||
|
Finance expenses
|
85 | 230 | ||||||
|
Finance expenses, net
|
25 | 185 | ||||||
|
Loss for the period
|
2,229 | 2,069 | ||||||
|
Total comprehensive loss for the period
|
$ | 2,229 | $ | 2,069 | ||||
Research and Development Expenses, net. Our research and development expenses, net for the six months ended June 30, 2014 were $1.64 million, as compared with $1.36 million for the six months ended June 30, 2013, an increase of $276,000, or 20%. The increase in research and development expenses, net was primarily due to an increase in amounts payable to subcontractors (an increase of $157,000), as well as an increase in materials (an increase of $69,000) and cost of salaries and related expenses (an increase of $66,000). This increase was partially offset by a decrease in cost for registration of patents (a decrease of $46,000).
|
Six months ended June 30,
|
||||||||||||
|
2014
|
2013
|
Change
|
||||||||||
|
(Unaudited)
|
||||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$ | 1,105 | $ | 1,039 | $ | 66 | ||||||
|
Share-based payment
|
32 | 9 | 23 | |||||||||
|
Materials
|
177 | 108 | 69 | |||||||||
|
Subcontractors
|
215 | 58 | 157 | |||||||||
|
Depreciation
|
36 | 35 | 1 | |||||||||
|
Cost for registration of patents
|
28 | 74 | (46 | ) | ||||||||
|
Other research and development
|
47 | 41 | 6 | |||||||||
| 1,640 | 1,364 | 276 | ||||||||||
|
Less participation of the OCS
|
- | - | - | |||||||||
|
Total research and development expenses, net
|
$ | 1,640 | $ | 1,364 | $ | 276 | ||||||
62
General and Administrative Expenses. Our general and administrative expenses for the six months ended June 30, 2014 were $564,000, as compared to $520,000 for the six months ended June 30, 2013, an increase of $44,000, or 8.5%. The increase in general and administrative expenses was due to increases in cost of salaries and related expenses, share-based payment, professional fees and other general and administrative expenses, primarily business travel (all of which amounted to an increase of $55,000). This increase was partially offset by an $11,000 decrease in facility cost.
|
Six months ended June 30,
|
||||||||||||
|
2014
|
2013
|
Change
|
||||||||||
|
(Unaudited)
|
||||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$ | 342 | $ | 334 | $ | 8 | ||||||
|
Share-based payment
|
8 | 1 | 7 | |||||||||
|
Professional fees
|
43 | 39 | 4 | |||||||||
|
Facility cost
|
45 | 56 | (11 | ) | ||||||||
|
Depreciation
|
3 | 3 | - | |||||||||
|
Other general and administrative
|
123 | 87 | 36 | |||||||||
|
Total general and administrative expenses, net
|
$ | 564 | $ | 520 | $ | 44 | ||||||
Operating Loss. As a result of a $276,000 increase in research and development expenses, net for the six months ended June 30, 2014 compared to the corresponding period in 2013, and a $44,000 increase in general and administrative expenses in the same period, our operating loss for the six months ended June 30, 2014 was $2.2 million, as compared to $1.9 million for the six months ended June 30, 2013, an increase of $320,000, or 17.0%.
Finance Income. Our finance income for the six months ended June 30, 2014 was $60,000, as compared to $45,000 for the corresponding period in 2013, an increase of $15,000. Finance income for the six months ended June 30, 2014 consists primarily of changes in provision for royalties ($42,000) and interest income on short-term deposits ($9,000). Finance income for the six months ended June 30, 2013 consists primarily of interest income on short term deposits ($40,000).
Finance Expenses. Our finance expenses for the six months ended June 30, 2014 were $85,000, as compared to $230,000 for the corresponding period in 2013, a decrease of $145,000. Finance expenses for the six months ended June 30, 2014 consist primarily of fair value changes on the warrants to purchase Series C-1 and C-2 preferred shares issued to Pontifax ($70,000) and losses on exchange rate fluctuations ($12,000). Finance expenses for the six months ended June 30, 2013 consist primarily of fair value changes on the warrants to purchase Series C-1 and C-2 preferred shares issued to Pontifax ($122,000), and changes in provision for royalties ($99,000).
Net Loss. Our net loss for the six months ended June 30, 2014 was $2.2 million, as compared to $2.1 million for the corresponding period in 2013, an increase of $160,000, or 7.7%.
Year Ended December 31, 2013 Compared to Year Ended December 31, 2012
|
Year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
(US$ in thousands, except per
share data)
|
||||||||
|
Research and development expenses, net
|
$ | 2,662 | $ | 2,692 | ||||
|
General and administrative expenses
|
1,090 | 1,203 | ||||||
|
Other expenses (income)
|
(10 | ) | 13 | |||||
|
Operating loss
|
3,742 | 3,908 | ||||||
|
Finance income
|
(63 | ) | (416 | ) | ||||
|
Finance expenses
|
316 | 229 | ||||||
|
Finance expenses (income), net
|
253 | (187 | ) | |||||
|
Loss for the year
|
3,995 | 3,721 | ||||||
|
Total comprehensive loss for the year
|
$ | 3,995 | $ | 3,721 | ||||
63
Research and Development Expenses, net. Our total research and development expenses for the year ended December 31, 2013 were $3.03 million, as compared with $2. 92 million for the year ended December 31, 2012, an increase of $111,000 or 3.8%. The increase in research and development expenses between 2013 and 2012 was primarily due to increases in cost of salaries and related expenses, registration of patents and other research and development expenses, mainly clinical trial-related expenses (all of which amounted to an increase of $226,000), which was partially offset by a decrease in amounts payable to subcontractors (a decrease of $112,000). Our research and development expenses, net for the year ended December 31, 2013 were $2.66 million, as compared with $2.69 million for the year ended December 31, 2012, a decrease of $30,000, or 1.1%. The decrease in research and development expenses, net was primarily due to an increase in OCS grants (an increase of $141,000).
|
2013
|
2012
|
Change
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$ | 2,170 | $ | 2,091 | $ | 79 | ||||||
|
Share-based payment
|
41 | 25 | 16 | |||||||||
|
Materials
|
307 | 329 | (22 | ) | ||||||||
|
Subcontractors
|
218 | 330 | (112 | ) | ||||||||
|
Depreciation
|
70 | 67 | 3 | |||||||||
|
Cost for registration of patents
|
118 | 52 | 66 | |||||||||
|
Other research and development
|
110 | 29 | 81 | |||||||||
| 3,034 | 2,923 | 111 | ||||||||||
|
Less participation of the OCS
|
(372 | ) | (231 | ) | (141 | ) | ||||||
|
Total research and development expenses, net
|
$ | 2,662 | $ | 2,692 | $ | (30 | ) | |||||
General and Administrative Expenses. Our general and administrative expenses for the year ended December 31, 2013 were $1.1 million, as compared to $1.2 million for the year ended December 31, 2012, a decrease of $113,000, or 9.4%. The decrease in general and administrative expenses was primarily due to decreases in facility cost and other general and administrative expenses, primarily business travel, communication and insurance (all of which amounted to a decrease of $178,000). This decrease was partially offset by a $25,000 increase in professional fees and a $30,000 increase in salaries and related expenses.
|
2013
|
2012
|
Change
|
||||||||||
|
(US$ in thousands)
|
||||||||||||
|
Salaries and related expenses
|
$ | 683 | $ | 653 | $ | 30 | ||||||
|
Share-based payment
|
16 | 6 | 10 | |||||||||
|
Professional fees
|
95 | 70 | 25 | |||||||||
|
Facility cost
|
104 | 142 | (38 | ) | ||||||||
|
Depreciation
|
7 | 7 | - | |||||||||
|
Other general and administrative
|
185 | 325 | (140 | ) | ||||||||
|
Total general and administrative expenses
|
$ | 1,090 | $ | 1,203 | $ | (113 | ) | |||||
Operating Loss. As a result of a $30,000 decrease in research and development expenses, net for the year ended December 31, 2012 compared to the year ended December 31, 2013, and a $113,000 decrease in general and administrative expenses in the same period, our operating loss for the year ended December 31, 2013 was $3.7 million, as compared to $3.9 million for the year ended December 31, 2012, a decrease of $166,000, or 4.2%.
Finance Income. Our finance income for the year ended December 31, 2013 was $63,000, as compared to $416,000 for the year ended December 31, 2012, a decrease of $353,000. Finance income for the year ended December 31, 2013 consists primarily of interest income on short-term deposits. Finance income for the year ended December 31, 2012 consists primarily of interest income on short term deposits ($183,000), and changes in provision for royalties ($233,000).
Finance Expenses. Our finance expenses for the year ended December 31, 2013 were $316,000, as compared to $229,000 for the year ended December 31, 2012, an increase of $87,000. Finance expenses for the year ended December 31, 2013 consist primarily of changes in a provision for royalties ($256,000) and fair value changes on the warrants to purchase Series C-1 and C-2 preferred shares issued to Pontifax ($55,000). Finance expenses for the year ended December 31, 2012 primarily consist of fair value changes on the warrants to purchase Series C-1 and C-2 preferred shares issued to Pontifax ($116,000) and changes in provision for royalties ($85,000) and losses on exchange rate fluctuations ($22,000).
64
Net Loss. Our net loss for the year ended December 31, 2013 was $4.0 million, as compared to $3.7 million for the year ended December 31, 2012, an increase of $274,000, or 7.4%.
Liquidity and Capital Resources
Sources of Liquidity
To date, we have funded our operations primarily with the approximately $24.5 million raised through equity financings, $12.0 million through a credit line transaction and the $3.5 million in grants that we received from the OCS.
In 2012, we completed a private placement of 2,510,783 of our Series D-3 preferred shares for an aggregate consideration of $1.1 million, pursuant to a Share Purchase Agreement dated January 10, 2012.
On July 13, 2014, we entered into a Cooperation and Project Funding Agreement with the BIRD Foundation and Synergy, pursuant to which the BIRD Foundation has agreed to award a grant in the maximum amount of the lesser of (i) $900,000 and (ii) 50% of the actual expenditures for the funding of a project entitled “Collection & Analysis of Gastrointestinal Images for Diagnostic Adenomatic Polyps and Colorectal Cancer.” The development work is expected to be performed over a 24 month period by Synergy (or a subcontractor on its behalf) and us. Of the total grant amount, we are expected to receive an aggregate of $567,000 to fund our expenditures for the project, in five installments. We received our first advance payment from the BIRD Foundation of $68,000 in July 2014. Our research and development expenses, net is presented net of the differences between the fair value of the liability and the grant amount received from the BIRD Foundation.
Under the terms of the grant agreement, we are required to repay the total grant in an amount equal to 5% of gross revenues derived from the product funded by the project, up to 100%, 113%, 125%, 138% and 150% of the grant amount, linked to the U.S. Consumer Price Index, if repaid within one year, two years, three years, four years and five or more years, respectively, of the original project completion date in accordance with the project proposal. Under the terms of the agreement, if any portion of the product funded by the project is sold outright to a third party prior to full repayment of the grant to the BIRD Foundation, one-half of the sale proceeds will be applied to the repayment of the grant. If the funded product is licensed to a third party, 30% of all payments received under the respective license agreement must be paid to the BIRD Foundation in repayment of the grant.
On August 20, 2014, we entered into a certain credit line agreement, pursuant to which we obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders, or the Lenders. The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014.
We issued to each Lender at closing a warrant, collectively referred to as the Credit Line Warrants, to purchase a number of our ordinary shares constituting 2% of our share capital on a fully diluted basis (assuming conversion of all of our convertible securities into ordinary shares at a 1:1 conversion rate) as of the closing for each $1 million (or portion thereof) extended by such Lender. The Credit Line Warrants are exercisable for a period of ten years at an exercise price of NIS 0.01 per share, and may be exercised on a net issuance basis.
Under the terms of the agreement, if we intend to consummate an initial public offering, or an IPO, and such IPO is expected to be consummated on or prior to February 18, 2015, or if we consummate an IPO on or prior to February 18, 2015, we will be entitled to call all or any part of the credit line amount and direct that such called amount be invested in ordinary shares in a private placement transaction that is exempt from the registration requirements of the Securities Act at a price per ordinary share equal to the public offering price per share in the IPO. In the event that we call less than the full credit line amount, we will call amounts on a pro rata basis among the Lenders based on their pro rata share of the total credit line amount. If we consummate an IPO on or prior to February 18, 2015, any part of the credit line amount not called by us will be released to the Lenders.
If we do not consummate an IPO on or prior to February 18, 2015, we may call the credit line amount at any time until April 14, 2016, subject to certain conditions. Any part of the credit line amount not called by us on or prior to such date will be released to the Lenders. If we call the credit line amount on or prior to April 14, 2016, the amount called will bear interest at the annual rate of 7%; provided that the aggregate interest will not be less than 5%. The called credit line amount will automatically convert into shares of our company upon the earlier of a qualified financing round (which includes a public offering, including an IPO), an M&A Event (as defined in the agreement) and April 14, 2016, and the Lenders may elect to convert the entire called credit line upon a non-qualified financing round, all under the terms and conditions set forth in the credit line agreement. In the event that the qualified financing round is an IPO, in lieu of automatic conversion, we are entitled, to the extent permitted by law, to deposit in trust an amount equal to 125% of the called credit line amount and irrevocably instruct the trustee to submit an offer, on behalf of each Lender, for the purchase of the IPO shares at the IPO price determined by our lead underwriters.
65
For the years ended December 31, 2013 and 2012, we received $647,000 and $541,000, respectively, in grants from the OCS for the financing of a portion of our research and development expenditure. In July 2014 and September 2014, we received additional funding from the OCS in the aggregate amount of $476,000 for the financing of a portion of our research and development expenditure.
We believe that based on our current business plan, our existing cash resources and the net proceeds from this offering will be sufficient to fund our currently anticipated cash requirements through 2016. Nevertheless, we will require significant additional financing in the future to fund our operations if and when we progress with our clinical trials in Europe and the United States.
Historical Cash Flows
The following table summarizes our statement of cash flows for the years ended December 31, 2012 and 2013 and the six months ended June 30, 2014.
|
Year ended December 31,
|
Six Months Ended June 30,
|
|||||||||||||||
|
2013
|
2012
|
2014
|
2013
|
|||||||||||||
|
(US$ in thousands)
|
||||||||||||||||
| (Unaudited) | ||||||||||||||||
|
Net cash used in operating activities
|
(3,657 | ) | (4,160 | ) | (2,159 | ) | (1,843 | ) | ||||||||
|
Net cash generated from (used in) investing activities
|
3,402 | (3,543 | ) | (22 | ) | 3,420 | ||||||||||
|
Net cash generated from financing activities
|
647 | 1,573 | -- | -- | ||||||||||||
Operating Activities
Net cash used in operating activities for the six months ended June 30, 2014 was $2.2 million, as compared to $1.8 million for the six months ended June 30, 2013. The increase in net cash used in operating activities in 2014 was attributable primarily to an increase in operating loss.
Net cash used in operating activities for the year ended December 31, 2013 was $3.7 million, as compared to $4.2 million for the year ended December 31, 2012. The decrease in net cash used in operating activities in 2013 was primarily due to an increase in the total comprehensive loss for the year.
Investing Activities
Net cash used in investing activities for the six months ended June 30, 2014 consists of purchase of property and equipment in the amount of $22,000. Net cash generated from investing activities for the six months ended June 30, 2013 consists primarily of proceeds from short-term bank deposits of $3.4 million, which were partially offset by purchase of property and equipment in the amount of $27,000.
Net cash provided by investing activities for the year ended December 31, 2013 consists primarily of proceeds from short-term bank deposits of $3.4 million, which were partially offset by purchase of property and equipment in the amount of $45,000. Net cash used in investing activities for the year ended December 31, 2012 consists primarily of investment in short-term bank deposits of $3.4 million, which were partially offset by purchase of property and equipment in the amount of $105,000.
66
Financing Activities
We did not generate cash from, or use cash in, financing activities for the six months ended June 30, 2014 and June 30, 2013.
Net cash generated from financing activities for the year ended December 31, 2013 was $647,000, comprised of an OCS grant. Net cash generated from financing activities in 2012 was $1.6 million comprised primarily of net proceeds from the issuance of Series D-3 preferred shares ($1.0 million) and an OCS grant ($541,000).
Contractual Obligations
The following table summarizes our contractual obligations as of June 30, 2014:
|
Payments due by period
|
||||||||||||||||||||
|
(US$ in thousands)
|
||||||||||||||||||||
|
Total
|
Less than 1
year
|
1-3 years
|
4-5 years
|
More than 5
years
|
||||||||||||||||
|
Operating lease obligations(1):
|
653 | 193 | 168 | 119 | 173 | |||||||||||||||
|
Other long term liabilities reflected on the Statements of Financial Position:
|
||||||||||||||||||||
|
Royalties to ASIC designer(2)
|
170 | - | 74 | 96 | - | |||||||||||||||
|
Office of the Chief Scientist(3)
|
1,618 | - | 318 | 1,300 | - | |||||||||||||||
|
Reimbursement liability to
|
||||||||||||||||||||
|
Check-Cap LLC unitholders(4)
|
749 | - | 19 | 137 | 593 | |||||||||||||||
|
Total
|
2,537 | - | 411 | 1,533 | 593 | |||||||||||||||
|
|
(1)
|
Operating lease obligations consist of payments pursuant to a lease agreement for office facilities as well as lease agreements for vehicles, which generally run for a period of three years.
|
|
|
(2)
|
See “Provision for royalties to an ASIC designer.”
|
(3) See “Research and Development Expenses, Net.”
|
|
(4)
|
On May 31, 2009, we entered into an asset transfer agreement with Check-Cap LLC pursuant to which Check-Cap LLC transferred all of its business operations and substantially all of its assets to us, in connection with which we undertook to reimburse the unitholders of Check-Cap LLC for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unitholders of Check-Cap LLC under Section 367(d) of the Code and is based in part on our forecasted sales. The liability is calculated using the provisions of IAS 39, under which expected cash outflows were discounted using a 17.6% discount factor commensurate with our company’s risk at the date of initial recognition of the liability. Any updates in the expected cash outflows and the liability will be recorded to profit and loss each period. As of June 30, 2014, it was probable that we will be required to reimburse the U.S. unitholders of Check-Cap LLC, and accordingly, a liability for this reimbursement has been accounted for in our financial statements for such period in the amount of $749,000. See “Related Party Transactions—Transactions with Check-Cap LLC and the Members and Manager of Check-Cap LLC.” |
Funding Requirements
We expect to incur losses from operations for the foreseeable future. We expect to incur increasing research and development expenses, including expenses related to the hiring of personnel and additional clinical trials. We expect that our general and administrative expenses will also increase as we expand our finance and administrative staff, add infrastructure, and incur additional costs related to being a public company in the United States, including directors’ and officers’ insurance, investor relations programs, and increased professional fees. Our future capital requirements will depend on a number of factors, including the timing and outcome of clinical trials and regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims and other intellectual property rights, the availability of financing, and our success in developing markets for our products.
67
Our expected future expenditures related to product, clinical and regulatory clearances are as follows:
|
|
•
|
completion of the clinical development of the final product;
|
|
|
•
|
conducting clinical trials in Europe, the United States and other territories;
|
|
|
•
|
development of future generations of our imaging capsule and future products;
|
|
|
•
|
FDA and additional regulatory filing costs; and
|
|
|
•
|
patent maintenance fees.
|
We believe that the net proceeds from this offering, together with our existing cash, will be sufficient to fund our operating expenses and capital expenditure requirements at least until the end of 2016. We have based this estimate on assumptions that may prove to be wrong, and we may use our available capital resources more quickly than we currently expect. Because of the numerous risks and uncertainties associated with the development and commercialization of our imaging capsule, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
In the absence of additional funding, we expect our continuing operating losses to result in increases in our cash used in operations over the next several quarters and years.
Off-balance Sheet Arrangements
We do not have any material off-balance sheet arrangements.
Quantitative and Qualitative Disclosure of Market Risks
Exchange Rate Risk
Some of our assets and liabilities are affected by fluctuations in the exchange rate between the U.S. dollar and the NIS and between the U.S. dollar and the Euro. For example, salaries and related expenses for Israeli employees are paid in NIS and some of our suppliers are located in Europe and require payment in Euros.
As of June 30, 2014, our total assets and liabilities linked to the NIS amounted to $433,000 and $900,000, respectively. A 10% depreciation of the dollar in relation to the NIS would cause an exchange rate loss of $52,000.
As of June 30, 2014, we had no assets linked to the Euro and our total liabilities linked to the Euro amounted to 170,000. A 10% depreciation of the dollar in relation to the Euro would cause an exchange rate loss of $17,000.
During 2013, the exchange rate between the NIS and the U.S. dollar decreased by 2.3% and the exchange rate between the Euro and the U.S. dollar increased by 2.0%. During the nine months ended September 30, 2014, the exchange rate between the NIS and the U.S. dollar increased by 6.5% and the exchange rate between the Euro and the U.S. dollar decreased by 8.1%. To date, we have not hedged the risks associated with fluctuations in currency exchange rates.
Interest Rate Risk
Our obligation to pay royalties to the OCS is linked to LIBOR and we are therefore exposed to changes in LIBOR.
As of June 30, 2014, we reported a total liability of $1.6 million with respect to our obligation to pay royalties to the OCS. A 25% increase in LIBOR would cause a financing loss of $3,000, based on the LIBOR as of June 30, 2014, which was 0.53%.
In addition, we intend to invest our cash balances, including certain of the net proceeds from this offering pending their ultimate use, primarily in bank deposits and securities issued by the U.S. and Israeli governments. We are exposed to market risks resulting from changes in interest rates relating primarily to our financial investments in cash and deposits. We do not use derivative financial instruments to limit exposure to interest rate risk. Our interest income may decline in the future as a result of changes in the financial markets; however, we believe any such potential loss would be immaterial to us.
68
Application of Critical Accounting Policies and Estimates
We prepare our financial statements in accordance with IFRS as issued by the IASB.
The preparation of our financial statements in conformity with IFRS requires management to make estimates, judgments and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements as well as reported expenses during the reporting periods. On an ongoing basis, management evaluates such estimates and judgments, including those described in greater detail below. Our management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made and on various assumptions that we believe are reasonable under the circumstances. Actual results may differ from these estimates under different assumptions or conditions.
While our significant accounting policies are more fully described in Note 2 to our financial statements appearing elsewhere in this prospectus, we believe that the accounting policies discussed below are critical to fully understand and evaluate our financial condition and results of operations.
Share-based compensation
We account for our share-based compensation for employees in accordance with the provisions of IFRS 2 “Share-based Payment,” which requires us to measure the cost of share-based compensation based on the fair value of the award on the grant date. The cost is recognized as compensation expense over the requisite service period which is usually the vesting period, based upon the grant date fair value of the equity or liability instruments issued. We recognize the compensation expenses over the vesting period using the accelerated method pursuant to which each vesting tranche is treated as a separate amortization period from the grant date to the vest date, and classify these amounts in the financial statements based on the department to which the related employee reports.
We selected the Black-Scholes option pricing model as the most appropriate method for computing the fair value of our share-based awards, using the standard parameters established in that model including estimates relating to the fair value of our ordinary shares, volatility, estimated life of the instruments, risk-free interest rates and dividends yield as described below.
Option Valuations
The determination of the grant date fair value of options using an option pricing model is affected by estimates and assumptions with respect to a number of complex and subjective variables. These variables include the expected volatility of our share price over the expected term of the options, share option exercise and cancellation behaviors, risk-free interest rates and expected dividends, which are estimated as follows:
|
|
•
|
Fair Value of our Ordinary Shares. Because our shares were not publicly traded prior to this offering, we estimate the fair value of our ordinary shares, as discussed below in “—Valuation of our ordinary shares”.
|
|
|
•
|
Volatility. The expected share price volatility is based on the historical equity volatility of the ordinary shares of comparable companies that are publicly traded.
|
|
|
•
|
Expected Term. The expected term of options granted represents the estimated period of time that options granted are expected to be outstanding. Since adequate historical experience is not available to provide a reasonable estimate, the expected term is determined based on the midpoint between the available exercise dates (the end of the vesting periods) and the last available exercise date (the contracted expiration date).
|
|
|
•
|
Risk-Free Rate. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the contractual life of the options.
|
69
|
|
•
|
Expected Dividend Yield. We have never declared or paid any cash dividends and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
|
If any of the estimates and assumptions used in the Black-Scholes model will change significantly, our share-based compensation for future awards may differ materially from those projected and recorded previously.
The following table presents the weighted-average assumptions used in estimating the fair value of options granted to employees during the years 2013 and 2012.
|
Parameters
|
June 2013 Grant
|
April-August 2012
Grants
|
||||||
|
Expected volatility (in %)
|
40-60 | 64-68 | ||||||
|
Option term (in years)
|
5-10 | 5-10 | ||||||
|
Risk free interest rate (in %)
|
0.75-2.25 | 0.75-2.25 | ||||||
|
Anticipated rate of dividends (in %)
|
0 | 0 | ||||||
The following table presents the grant dates, number of underlying shares and related exercise prices of awards granted to employees and non-employees since January 1, 2012 as well as the estimated fair value of the underlying ordinary shares on the grant date.
|
Grant date
|
Number of shares
subject to awards
granted
|
Exercise price per
share
|
Estimated fair value
per ordinary share at
grant date
|
|||||||
|
04/04/2012
|
1,160,000 | $ | 0.25 | $ | 0.07 | |||||
|
28/08/2012
|
350,000 | $ | 0.25 | $ | 0.07 | |||||
|
12/06/2013
|
2,355,000 | $ | 0.25 | $ | 0.152 | |||||
Based on the assumed initial public offering price of $ per share, the midpoint of the range set forth on the cover page of this prospectus, the intrinsic value of the awards outstanding as of June 30, 2014 was $ million, of which $ related to vested options and $ related to unvested options.
Valuation of our ordinary shares
Due to the absence of an active public market for our ordinary shares prior to completion of this offering, the fair value of our ordinary shares for purposes of determining the exercise price for award grants has been determined in good faith by our management and approved by our board of directors. In connection with preparing our financial statements, our management considered the fair value of our ordinary shares based on a number of objective and subjective factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, referred to as the AICPA Practice Aid.
Royalties provision
Government grants from the Office of the Chief Scientist
We have received research and development funding from the Government of the State of Israel through the OCS in the form of grants, which we are required to return with interest under certain conditions. For additional information regarding the OCS grants, see “— Research and Development Expenses, Net” and “Taxation — Israeli Tax Considerations and Government Programs — The Encouragement of Industrial Research and Development Law, 5744-1984.” Such grants qualify as “forgivable loans” in accordance with IAS 20, “Accounting for Government Grants and Disclosure of Government Assistance,” since they are repayable only if we generate revenues related to the underlying project.
In accordance with IAS 20, the grant is accounted for as a liability unless it is more likely than not that we will meet the terms of forgiveness of the loan, in which case the forgivable loan is accounted for as a government grant and recognized as a reduction of the research and development expenses.
70
We consider it more likely than not that the project underlying our OCS grants will reach the revenue generating stage and therefore, we record a liability in respect of the OCS grants. No minimum royalties are required to be paid in connection with the OCS grants. On the date of initial recognition, the grant is presented as a financial liability at the fair value of the expected cash flows to be repaid, discounted at a discount rate commensurate with the risk level of the research and development project. The difference between the amount of the grant and its fair value is treated as a government grant. In subsequent periods, the financial liability is measured at the present value of the expected cash flows to be paid in the future, discounted each period at the original interest rate used in computing the initial liability. Changes in fair value are recorded to profit and loss each period.
In calculating the present value of future payments to the OCS, we used a discount rate of 21.8% for grants received during the years 2012 and 2013.
Provision for royalties to an ASIC designer
In December 2007, we entered into an agreement for the development of an application specific integrated circuit, or ASIC, component to be used as an amplifier for the capture of signals at low frequencies from X-ray detectors contained in our product. The ASIC developer is entitled to receive royalties from us in the amount of €0.5 (approximately $0.68) for every ASIC component that we will sell, up to €200,000 (approximately $273,000). The net present value of the royalty liability to the ASIC designer is dependent upon our management estimates and assumption as to future product shipments and interest rates used to calculate the present value of the cash payments required to repay the royalties to the ASIC designer. In calculating the present value of future royalty payments to the ASIC designer, we used a discount factor of 17.6%, commensurate with our risk at the date of initial recognition of the liability.
Reimbursement liability to Check-Cap LLC unitholders
On May 31, 2009, we entered into an asset transfer agreement with Check-Cap LLC pursuant to which Check-Cap LLC transferred all of its business operations and substantially all of its assets to us. In connection with the transaction we undertook to reimburse the unitholders of Check-Cap LLC for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unitholders of Check-Cap LLC under Section 367(d) of the Code, and is based in part on our forecasted sales. The liability is calculated using the provisions of IAS 39, under which expected cash outflows were discounted using a 17.6% discount factor commensurate with our company’s risk at the date of initial recognition of the liability. Any updates in the expected cash outflows and the liability will be recorded to profit and loss each period. As of June 30, 2014, it was probable that we will be required to reimburse the U.S. unitholders of Check-Cap LLC, and accordingly, a liability for this reimbursement has been accounted for in our financial statements in the amount of $749,000. Due to the fact that we are still in the development stage and have not generated revenues, the sales forecast is highly subjective and may vary significantly in the future. As more information is gathered to assist our management in making forecasts, the liability will be updated.
Fair value of financial instruments
On June 1, 2009, in connection with that certain Series C Preferred Share Purchase Agreement, we issued to Pontifax, as the lead investor, warrants for the purchase of 836,412 Series C-1 preferred shares and warrants for the purchase of 1,007,975 Series C-2 preferred shares. These warrants are exercisable on a cashless basis and therefore, are classified as a liability in the statements of financial position. The fair value of this financial instrument is determined based on an option pricing model using similar assumptions to those used for our share-based compensation awards to employees.
Recent Accounting Pronouncements
IFRS 13 “Fair Value Measurement”
IFRS 13 establishes a single source of guidance for fair value measurement and disclosures about fair value measurement. The standard defines fair value, establishes a framework for measuring fair value, and requires disclosures about fair value measurement. The scope of IFRS 13 is broad: it applies to both financial instruments items and non-financial instruments items for which other IFRSs require or permit fair value measurement and disclosure about fair value measurement, except in specified circumstances. In general, the disclosure requirements in IFRS 13 are more extensive than those required in the current standards.
71
IFRS 13 is effective for annual periods beginning on or after January 1, 2013, with earlier application permitted. The application of the standard has had no effect on our financial results.
JOBS Act Exemption
The JOBS Act permits an “emerging growth company,” such as us, to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
72
BUSINESS
Our Company
We are a clinical stage medical diagnostics company engaged in the development of an ingestible imaging capsule that utilizes low-dose X-rays for CRC screening. While CRC is the second leading cause of death from cancer in the United States and is largely preventable with early detection, about one-half of Americans over the age of 50 do not undergo any form of CRC screening due in large part to the pain, discomfort and embarrassment related to current screening methods. Unlike other structural screening methods that are designed to generate structural information of the colon for the detection of pre-cancerous polyps, such as optical colonoscopy, CTC and other capsule-based technology, our imaging capsule is designed to be ingested without any cleansing of the colon and to travel through the gastrointestinal tract naturally while the patient continues his or her normal daily routine. Furthermore, unlike all other CRC imaging devices currently on the market, which require the patient to fast for several hours prior to the procedure, the Check-Cap procedure enables patients to continue eating normally. We believe that this solution will be attractive to both physicians and patients, thereby increasing the number of people willing to undergo screening for CRC.
Our imaging capsule is being designed to create a reconstructed two and three-dimensional image of the colon and to detect clinically significant polyps with a high degree of sensitivity. Colon polyps are fleshy growths that occur on the lining of the colon. Polyps in the colon are extremely common, and when certain types of polyps grow large enough they can become cancerous.
Our imaging capsule will be swallowed by the patient, and propelled by natural motility through the gastrointestinal tract and excreted naturally with no need for retrieval for data collection. Unlike other CRC screening methods, this process should not disrupt a patient’s normal activities or require fasting. Our imaging capsule employs X-rays which are considered low dose according to the Radiation Safety Division of the Radiological Protection Branch of the Soreq Nuclear Research Center, which allows it to image the lining of the colon even when surrounded by intestinal content. As such, we believe that patients using our imaging capsule will not be required to undergo any prior bowel cleansing.
Our imaging capsule is being designed to transmit the data it collects to an external data recorder that will be worn by the patient. The external data recorder is being designed to transmit the data to physicians, who will then utilize our data viewer software application to analyze the data collected by our imaging capsule. We intend for physicians to be able to review the colon’s inner images at any location at any time, in less time than is required to perform an optical colonoscopy.
In order to enable a complete view of capsule positioning and motility, we have designed an external receiver and electromagnetic localization system, or RELS, which is mounted on the patient’s back throughout the entire screening procedure. The RELS is being designed to provide the physician with accurate localization data aligned with a reconstructed image.
In the event that polyps are identified by our imaging capsule, the patient would be required to undergo a subsequent traditional colonoscopy procedure to examine, remove and biopsy the polyps. For those patients who require a subsequent polypectomy, concerns regarding pain, discomfort and embarrassment may still remain with respect to the subsequent polypectomy. We do not, however, believe that these concerns will make the use of our imaging capsule any less attractive to doctors and patients. Although patients who are initially screened utilizing a traditional colonoscopy could avoid the need for a second procedure if polyps are discovered because they could undergo a polypectomy during the initial screening, if necessary, we believe that our imaging capsule will still be attractive to doctors and patients since a large majority of patients who are screened will not require a subsequent polypectomy. According to a review published by the Agency for Healthcare Research and Quality in October 2008, out of 100 adults aged 50-75, only 25-30 persons have one or more polyps and only 15 persons have significant (10+mm) polyps.
A clinical proof-of-concept study, which was based on a 10-case study conducted at Tel Aviv Medical Center in Israel and used a prior version of our imaging capsule, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our imaging technology to the human colon, generating images taken in the colon without any prior bowel-cleansing. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted.
73
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of the Check-Cap imaging system. The 10-case clinical proof-of-concept study is the first part of a multi-center, prospective clinical feasibility study to establish the safety and functionality of the Check-Cap imaging system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity FOBTs and FITs, as well as from optical colonoscopies. The feasibility study is designed to include a total of 60 subjects. The study is being conducted in Israel at the Tel Aviv Medical Center and Laniado Hospital and is planned to also be conducted at the Erasmus University Medical Center in the Netherlands. The clinical feasibility study will also evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified by our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
Another objective of the 10-case study was to estimate total radiation exposure for each case study. This was calculated using standard established factors for calculating effective radiation exposure, such as the duration of the capsule inside the body, and was based on the activity of the radiation source inside the imaging capsule and radiation energy, both of which were measured for each case study. The average calculated exposure for the entire procedure in the 10-case study, from ingestion of the capsule to excretion, was 0.03 mSv (STD 0.007 mSv). This level of radiation exposure is similar to a single chest X-ray (approximately 0.06mSv) and two orders of magnitude less than CTC.
Following the successful completion of the broader multi-center, prospective clinical feasibility study, we plan to submit a request for CE marking for the marketing and sale of our capsule in the European Union during 2015. We expect to perform post-marketing studies in Europe following CE marking. We anticipate launching our product commercially in Europe during 2016.
We plan to submit to the FDA a request to conduct a pivotal study in the United States to establish clinical efficacy in late 2015. We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study. Pivotal studies are expected, among other things, to compare the images of polyps identified by our imaging system with the same polyps detected by traditional optical colonoscopy and CTC in instances where patients were referred after positive exam results. These clinical findings will be analyzed in comparison to results obtained from FOBTs and FITs. Subject to successful completion of our clinical trials, we anticipate launching our product commercially in the United States during 2017.
We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have been granted patents for our core patent by the U.S. Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan. We also filed patent applications describing the use of our imaging technology in several other medical applications.
Since our formation, we have not generated any revenues. We do not anticipate generating any revenues for the foreseeable future and we do not yet have any specific launch dates for our product. For the six months ended June 30, 2014, we had a total comprehensive loss of $2.2 million. For the year ended December 31, 2013, we had a total comprehensive loss of $4.0 million. As of June 30, 2014, we had an accumulated deficit of $24.7 million and a total shareholders’ deficit of $998,000.
Our Solution
CRC screening can reduce death rates from CRC by detecting polyps at an earlier, more treatable stage. CRC is one of the few cancers that can be prevented through screening because pre-cancerous polyps, from which colon cancers often develop, can be identified and removed. Today, there is a range of options for CRC screening in the average-risk population, with current technology falling into two general categories: (i) structural exams, such as optical colonoscopy (which is currently regarded as the “gold standard” for CRC screening), sigmoidoscopy, CTC and optical capsules (all of which require aggressive bowel preparation), which are invasive exams that enable physicians to visualize the colon for abnormalities; and (ii) stool tests, such as FOBTs, FITs and stool DNA tests, which test for blood and irregularities in DNA. Notwithstanding the many CRC screening alternatives, the fact that the tests are encouraged by clinicians and insurers and the clinical value of screening for CRC, a large portion of the population are still reticent to go for CRC screening and are not satisfied with the currently available alternatives.
74
We believe that our imaging capsule could represent a potential breakthrough in CRC screening by providing a structural exam without the pain, discomfort and embarrassment experienced by some patients undergoing a traditional optical colonoscopy and other currently available screening methods by offering the following benefits:
|
|
•
|
eliminating the need for fasting and prior bowel cleansing, which would differentiate our imaging capsule from every other currently available structural screening exam;
|
|
|
•
|
providing patients with a procedure that requires them to swallow our capsule and small amounts of a contrast agent, thereby minimizing any disruption to their normal activities;
|
|
|
•
|
eliminating need to sedate patients;
|
|
|
•
|
obviating the requirement for the insufflation (the forcing of air into the gastrointestinal tract) of patients;
|
|
|
•
|
administering our technology on an outpatient basis;
|
|
|
•
|
providing digital reporting, storage and remote consulting capabilities; and
|
|
|
•
|
enabling a physician to analyze the results in approximately 10 minutes, which would be less time than is required to conduct an optical colonoscopy.
|
Although our imaging capsule utilizes radiation that is considered low dose, we believe that the risks associated with such radiation exposure are low compared to risks associated with other procedures such as perforation, bleeding or sedation related effects (optical colonoscopy and sigmoidoscopy) and dehydration and damage to kidneys (optical capsules). Furthermore, stool test methods (FOBTs, FITs and stool DNA) require a follow-up optical colonoscopy if abnormalities are detected. Unlike FOBTs, FITs and stool DNA tests, our capsule-based imaging modality generates structural information on the colon, which could assist in the detection of pre-cancerous polyps. We, therefore, do not believe that the low dose radiation in our imaging capsule will make our imaging capsule less attractive to physicians and patients than other products that do not employ any radiation.
We believe that gastroenterologists will embrace our technology and encourage the use of our imaging capsule. This may increase the number of people undergoing CRC screening and may cause more people with polyps to obtain a polypectomy – a therapeutic procedure during which polyps are removed and which currently receives higher reimbursement coverage than both screening and diagnostic colonoscopies.
Our goal is to become a leading supplier of CRC screening technology and to establish our technology as a leading CRC screening method. Key elements of our strategy include:
|
|
•
|
obtaining CE marking for the marketing and sale of our imaging capsule in the European Union, followed by obtaining regulatory approvals for the sale of our imaging capsule initially in the United States and Japan.
|
|
|
•
|
In Europe and Japan, we intend to offer our imaging capsule as an imaging and screening tool for the general population. In the United States, we currently plan to first obtain regulatory approvals for our imaging capsule as an adjunct tool to FOBTs and FITs, and after we have conducted more extensive clinical studies in the United States, we anticipate applying to the FDA for the use of our imaging capsule as an initial screening tool;
|
|
|
•
|
obtaining third-party reimbursement for our technology;
|
|
|
•
|
enhancing our existing technology portfolio and developing new technologies; and
|
|
|
•
|
successfully marketing our product to establish a large customer base.
|
75
Our Technology
Our technology is based on an ingestible imaging capsule, which is swallowed by the patient and propelled by natural motility through the gastrointestinal tract. Our imaging capsule transmits information to a receiving device worn on the patient’s body that stores the information for off-line analysis. Our imaging capsule consists of an X-ray source and several X-ray detectors. The X-ray source is contained in a rotating radiation shield, enabling the generation of 360-degree angular scans. The collection of successive angular scans is intended to enable the virtual reconstruction of a portion of the colon. During movement of our imaging capsule longitudinally through the colon, successive images of portions of the colon enable the three-dimensional reconstruction of the colon. Our imaging capsule is also intended to identify polyps, which protrude inward into the colon, through the detection of irregularities in the topography of the colon.
Our imaging system and RELS are intended to be prescribed to patients by physicians. One or two days prior to swallowing our capsule, a patient will begin drinking small amounts of a radio opaque contrast agent (such as barium sulfate or iodine) with his or her meals, which enhances the contrast of the colon surface. The capsule is propelled by natural motility through the gastrointestinal tract. As it makes its way through the gastrointestinal tract, information is transmitted to a receiving device worn by the patient that stores the information for offline analysis. After our imaging capsule is expelled from a patient’s body, the RELS will transmit the data to physicians, who will then utilize our data reviewer software application to analyze the data collected by our imaging capsule. Our proprietary software is being designed to process the data and produce a two and three-dimensional visualization of the colon. A physician will then analyze the visualization to determine whether any anatomical anomalies are present on the surface of the colon. In Europe and Japan, we intend to offer our imaging capsule as an imaging and screening tool for the general population. In the United States, we currently plan for the technology to initially be an adjunct to FOBT or FIT to help detect polyps, masses, cancers and other lesions, and after we have conducted more extensive clinical studies in the United States, we anticipate applying to the FDA for the use of our imaging capsule as an initial screening tool.
Our imaging capsule consists of the following three main subsystems that together enable the generation of high resolution 3D imaging of the colon, further described below: (i) X-ray radar-based colon imaging capsule; (ii) RELS; and (iii) PC-based 3D imaging workstation.
Imaging Capsule
The imaging capsule, which is designed to measure, collect and transmit the clinical data, is comprised of the following components:
|
|
•
|
X-ray Source – including radioactive material sealed in a cylindrical housing.
|
|
|
•
|
Collimator – Radiation shield around the source, which absorbs most of the radiation. Several radial holes enable distribution of radiation in defined directions.
|
|
|
•
|
X-ray Sensor – Comprised of several solid state X-ray detectors for measuring the scattered radiation intensity.
|
|
|
•
|
Activation Circuit – Activates and/or deactivates the capsule. The circuit shall operate either by illumination of light or magnetic induction. The activation shall be coded to avoid false activation.
|
|
|
•
|
Tilt Sensor – Indication of capsule motion (3D acceleration).
|
|
|
•
|
Rotation Motor – For rotating the collimator and X-ray Source.
|
|
|
•
|
Source Concealment Mechanism – Conceals the source inside the radiation shield.
|
|
|
•
|
R-T – Radio frequency transceiver device to communicate with the receiver.
|
|
|
•
|
Batteries – Electrical power supply for the capsule.
|
|
|
•
|
Memory – Data storage. The imaging capsule should be able to store up to an hour of measured data.
|
|
|
•
|
ELS coil – Transmits a continuous electromagnetic wave utilized by an external localization system to track 3D position.
|
76

Image for illustration purpose only
External Receiver and Electromagnetic Localization System
The external receiver and electromagnetic localization system, or RELS, is a miniaturized, disposable and biocompatible sticker which is being designed to automatically track the imaging capsule’s positioning and orientation throughout the gastrointestinal tract transition, control the capsule scan mechanism through an embedded activation algorithm, capture imaging data from the capsule through radio frequency communication to a non-volatile memory device and enable data retrieval through wireless communication to an external processor.
The RELS is comprised of the following components:
|
|
•
|
Sticker Housing – Biocompatible and water-resistant sticker and housing integrating all functional components, attached to the patient’s back, enabling five days of continuous operation.
|
|
|
•
|
Recorder–Consists of receiver electronics embedded software and nonvolatile memory.
|
|
|
•
|
Antennas – Two radio frequency antennas are embedded into the sticker housing and used to communicate with the capsule.
|
|
|
•
|
Activation/Deactivation Circuit – Used to activate/deactivate the RELS through a specialized protocol.
|
|
|
•
|
UI Indicators - Provides user with vocal indication as required.
|
|
|
•
|
Vibro – Provides user with vibration indication as required.
|
|
|
•
|
PCB – Electronics’ printed circuit board.
|
|
|
•
|
Microcontroller – Runs embedded software and logic that manages the RELS.
|
|
|
•
|
RF Transceivers – Several transceivers used to communicate with the imaging capsule.
|
|
|
•
|
TILT/Compass Sensors – To determinate patient’s body movements.
|
77
|
|
•
|
Batteries – Electrical power supply for the RELS.
|
|
|
•
|
Memory – Non-volatile data storage to store data acquired by the imaging capsule.
|

Image for illustration purpose only
Workstation
The workstation is a specialized, user friendly, personal computer-based software package designed to retrieve and process clinical data from the RELS and to reconstruct and produce 3D visualization of the colon surface. The workstation is comprised of the following software components:
|
|
•
|
Communication Driver Software – to communicate with the RELS and retrieve collected data following procedure completion.
|
|
|
•
|
Data Processing Software – to process and reconstruct clinical data into a 3D structure.
|
|
|
•
|
Data Display and Management Software – includes the following functions:
|
|
|
○
|
3D visualization of the reconstructed colon surface.
|
|
|
○
|
Measurement and annotation tools.
|
|
|
○
|
Registration of patient and capsule data and management of the patient database.
|
78

Image for illustration purpose only
Our Imaging Capsule – Non-Clinical, Pre-Clinical and Clinical History
For the past nine years, we have developed and validated our new capsule-based imaging modality for CRC screening. Below is a summary of the validation tests carried out by us in the laboratory, in phantoms, animals and humans, which were designed to evaluate this new imaging modality’s performance and potential clinical value.
Non-Clinical and Pre-Clinical Testing
Imaging Performance Testing
The Check-Cap ingestible capsule transmits colon surface data as the capsule progresses through the colon. This data consists of imaged slices perpendicular to the capsule’s longitudinal axis; slices are then reconstructed by the workstation to produce 2D and 3D images of the colon. Following are performance measurements of the capsule imaging.
• Modulation Transfer Function, or MTF. The existing design of our imaging capsule was moved along a longitudinal-edge phantom setup in 3mm steps. The figure below shows a typical raw signal after filtering for peak detection. The same test was carried out using an angular-edge phantom setup, which demonstrated similar results to those shown below. These tests do not take into account noise characteristics.

Image for illustration purpose only
79
For each position of the capsule in the phantom, the mean signal intensity (peak) was measured, the result of which is shown in the left figure below. Resulting line spread function, or LSF, which is the differential of the curve in the left figure below, is shown in the right figure below.

Image for illustration purpose only
The graphs above demonstrate that the existing design of our imaging capsule can detect objects of approximately 2-3mm when noise is not taken into account.
● Resolution Limit: Estimation of the Smallest Visible Object Size. In order to estimate the size of the smallest visible object, both spatial resolution and noise characteristics must be taken into account. The graph below presents the estimated MTF of our imaging capsule. Noise analysis indicates MTF 1/3 for minimum visibility, which demonstrates that the smallest visible object that can be detected with the existing design of our imaging capsule (in the conditions used, which included a colon diameter of 30mm) is of approximately 5-6 mm (see graph below).

Image for illustration purpose only
80
Image Reconstruction
Two main characteristics of our imaging capsule contribute to the image reconstruction performance:
|
|
•
|
The number of photons hitting the detector per time frame.
|
|
|
•
|
The angular spread of the photon beam coming out of the capsule collimator.
|
Based on the laboratory tests performed with the current version of our imaging capsule, polyps of 6 mm and larger should be visible and 10 mm polyps and larger are expected to be detected at high sensitivity. To further enhance the visibility of 6 mm - 9 mm polyps, a new design of the collimator was successfully tested in a future version of our imaging capsule, which enables 2.5 times the number of photons to be detected on the detectors, allowing the implementation of image enhancement algorithm, which is expected to improve the imaging performance.
Animal Testing and Tissue Equivalent Phantom Image Reconstruction
The physics of the Check-Cap imaging modality was tested in the laboratory on phantoms with tissue equivalent material and in animals to ensure that laboratory conditions mimic real life clinical scenarios.
Following the initial proof of concept, we performed a series of studies in order to evaluate the feasibility and preliminary safety of our technology. All of the studies were performed in pigs ranging from 60 to 90 kg. Pigs, which are commonly used in gastrointestinal studies, were selected as the animal model for preliminary evaluation of our imaging capsule based on the resemblance of the porcine colon size and morphology to the human colon. However, there are marked differences between the colon of pigs and that of humans. The pig colon is much longer, has a larger diameter in addition to other anatomical differences. In the pig model, the pressure waves of peristalsis are believed to be more frequent and shorter than in humans. As a result, we believe that colon content movement is substantially slower and more frequent in pigs than in humans. In these studies, we did not intend to collect statistically significant data; hence, the tests were repeated a limited number of times until adequate data was collected.
The first test was performed to demonstrate imaging proof-of-concept using a wired imaging capsule. This technology included all the basic features intended to be included in the clinical system, but on a larger scale due to the use of off-the-shelf components. The subsequent studies used versions of the imaging capsule that integrated most of the imaging components, software and electronics of the imaging capsule that we used with humans. Since off-the-shelf components were used, the animal capsules were larger and heavier than the version of our imaging capsule that are used clinically.
Raw data from an animal colon showing a decrease in X-ray florescence, or XRF, photon signals and an increase in Compton backscattering, or CMT, signals corresponding to the position of a polyp that was detected when our imaging capsule passed over the polyp is shown in the image below. These two signals are combined in order to form a three dimensional image below.
81

Image for illustration purpose only
The animal studies conducted to date demonstrate that our technology provides sufficient resolution for the detection of 10 mm polyps, which is the size of clinically significant polyps. The animal studies also demonstrated that 5 mm polyps can be detected, though with lower resolution than 10 mm polyps in the first animal capsule. Animal health was maintained throughout the studies. No adverse effects related to passage of our imaging capsule were noted.
The imaging capsules evaluated in the animal studies were significantly larger than the imaging capsules that we are using with humans. The differences in anatomy, physiology, and imaging capsules may have several effects on the data compared to use in the human population. Motility of the imaging capsules through the digestive system was slow due to the specific shape of the porcine gastrointestinal tract. In addition, because of the size of the imaging capsule, it was retained in the stomach for many hours and even days. Accordingly, the animal model required that normal ingestion be replaced by direct insertion of the imaging capsule into the small bowel. In order to simplify the development and animal testing, we used Tungsten radiation source with long half-life (120 days).
Following the success of the animal testing, a series of in-vitro tests were conducted to simulate different clinical scenarios in the laboratory using a miniaturized human imaging capsule. Polyps were created and reconstruction of the laboratory phantoms with a human-imaging capsule was generated to assess the ability to detect polyps as the capsule advances in the colon. The in-vitro tests demonstrated the imaging capabilities of our imaging technology. Below is the reconstruction of a laboratory phantom image.

Image for illustration purpose only
82
Polyp Detection Analysis
Laboratory tests were carried out to estimate the capsule’s ability to detect polyps in phantoms and demonstrate sensitivity and specificity of such detection. Below is an example of the reconstruction of a scan composed of three slices: XRF, CMT and a fused (combined) image.
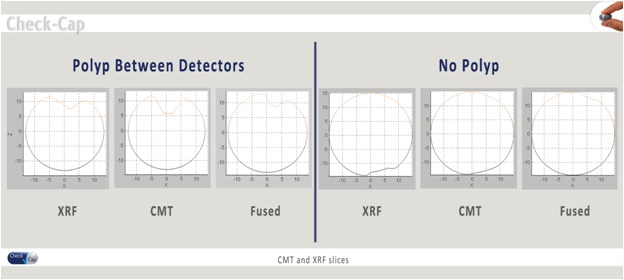
Receiver Operating Characteristics
Standard receiver operating characteristics, or ROC, curves were generated from phantom data with 8 mm polyp in a 30 mm barrel phantom with 3% iodine concentration mimicking the colon contents. CMT, XRF and fused (combined) data were analyzed based on 2D slices that were generated and standard deviation indicator. There were a few cases where the noise in the phantoms was high enough to generate polyp false positive condition separately for each data type, especially in CMT. However, fusion of CMT and XRF data contributed to noise reduction and enabled to demonstrate 100% true positive and 0% false positive.
83

Clinical Trials
We initiated our first human clinical study in Germany in January 2010. The purpose of the study was to collect pressure and motility information from the digestive system of human volunteers. This study was conducted with a passive capsule that contained no X-ray source or detectors. It included several electronic components of the imaging capsule and had similar dimensions to the current imaging capsule.
The information we obtained from the first clinical study was used to develop the initial operational algorithm for the imaging capsule that was used in our next clinical study, which was a clinical proof-of-concept study conducted at the Tel-Aviv Medical Center in Israel. The study was designed to assess the safety and feasibility of our imaging capsule with respect to total and segmental transit time, and the effect of variable colon dimensions on these parameters. The clinical proof-of-concept study, which was based on a 10-case study and used a prior version of our imaging capsule, did not identify any material safety or feasibility issues. The study demonstrated the applicability of our imaging technology to the human colon, generating images taken in the colon without any prior bowel-cleansing. All subjects ingested the capsule easily with smooth passage within the designated transit time, on average, within two to three days. There were no reported device-related adverse events. Mild effects on bowel movements were noted, which were determined to be related to the contrast agent and passed within one to two days after the capsule was excreted.
The 10-case clinical proof-of-concept study focused on assessing the safety and feasibility of the Check-Cap imaging system. The 10-case study is the first part of a multi-center, prospective clinical feasibility study to establish the safety, functionality and preliminary efficacy of the Check-Cap imaging system in patients eligible for CRC screening, by comparing results from the clinical feasibility study with those from non-invasive, low-sensitivity FOBTs and FITs, as well as from optical colonoscopies. The feasibility study is designed to include a total of 60 subjects. The study is being conducted in Israel at the Tel Aviv Medical Center and Laniado Hospital and is planned to also be conducted at the Erasmus University Medical Center in the Netherlands. The clinical feasibility study will also evaluate the safety of the device in terms of total and segmental transit time and analyze the effects of the presence of polyps and variable colon dimensions on these parameters. The study will seek to create a clinical atlas of images that will enable comparisons between images acquired by different CRC screening modalities. During the feasibility study we will collect data about the overall imaging of the colon’s internal surfaces during the passage of the capsule to support the development of a correlation map of polyps identified by our imaging system with polyps imaged by optical colonoscopy and CTC. Additionally, the feasibility study will measure total radiation exposure and the distribution of contrast material within the colon.
84
Following the successful completion of the broader multi-center, prospective clinical feasibility study, we plan to submit a request for CE marking for the marketing and sale of our capsule in the European Union during 2015.
We plan to submit to the FDA a request to conduct a pivotal study in the United States in late 2015. The data from the multi-center, prospective clinical feasibility study will be submitted to the FDA with our request for the approval of an investigational device exemption, or IDE, for a pivotal study in the United States to: (i) demonstrate device safety as evidenced by a lack of device-related serious adverse events; and (ii) provide efficacy data concerning our imaging capsule’s sensitivity and specificity. Subject to the FDA’s approval, we intend to conduct a multi-center, prospective screening study in the United States to assess the performance of our imaging capsule in selected groups of patients. We will first seek to obtain regulatory approvals for the use of our imaging capsule in the United States for screening of the colon as an adjunct to negative FOBTs or FITs to detect polyps, masses, cancers and other lesions. The proposed pivotal study will be designed to establish that our imaging capsule is a tool to detect significant polyps that are not detected via the current FIT or FOBT screening paradigm. We have been in discussions with the FDA regarding the design of the proposed pivotal study and end points, as well as the sample size. After we have conducted more extensive clinical studies in the United States, we anticipate applying to the FDA for the use of our imaging capsule as an initial screening tool for the general population. Subject to successful completion of our clinical trials, we anticipate launching our product commercially in the United States during 2017.
We also intend to pursue clinical trials for regulatory approvals in Japan and China in parallel to the U.S. pivotal study.
We recently concluded a limited, single-center, feasibility study at Rambam Medical Center in Haifa to assess the motility of our capsule in healthy subjects, with the objective of optimizing the daily routine of the subjects in order to shorten the transit time of our imaging capsule. The study, in which 15 healthy subjects participated and used a dummy capsule with the same weight and dimensions as our current imaging capsule, did not identify any safety issues and all capsules were retrieved. A structured daily routine determined the timing of the following: capsule ingestion, the subjects’ daily meals, the contrast agent ingestion and one daily dose of 10 mg of Bisacodyl, and all subjects continued their regular active lifestyles (such as work and sports). The average transit time of the dummy capsule in the 15 subjects was approximately 24 hours less than the average transit time of our imaging capsule in subjects participating in the multi-center feasibility study in which the participants do not ingest a daily dose of Bisacodyl, are less active and are continuously monitored in a designated apartment until the capsule is excreted.
Research and Development
Our research and development strategy is centered on developing our imaging capsule. Our research and development team is located at our facilities in Isfiya, Israel, and consists of 32 employees and independent contractors as of June 30, 2014 and is supported by highly experienced consultants.
We have received government grants as part of our research and development programs approved by the OCS. The total gross amount of grants actually received by us from the OCS, including accrued LIBOR interest, as of June 30, 2014, totaled approximately $3.1 million. Since June 30, 2014, we have received additional funding from the OCS in the aggregate amount of $476,000 under an approved OCS grant in the total amount of $702,000 for a research and development program for the 12 month period commencing March 1, 2014. The financial liability recorded by us with respect to such OCS grants totaled approximately $1.2 million as of December 31, 2012 and $1.6 million as of December 31, 2013 and June 30, 2014. Because the repayment of the OCS grants will be in the form of future royalties, commitments to the OCS are presented on our balance sheet as a financial liability at the fair value of the expected cash flows to be repaid, discounted at a discount rate commensurate with the risk level of the research and development project. As of June 30, 2014, we had not paid any royalties to the OCS.
We incurred approximately $1.6 million in research and development expenses, net (after deducting participation by others and government grants) for the six months ended June 30, 2014 and $2.7 million in research and development expenses, net (after deducting participation by others and government grants) for each of the years ended December 31, 2013 and 2012. For additional information, see “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Financial Operations Overview— Research and Development Expenses, Net.”
85
Intellectual Property
An important part of our competitive strategy is to seek, when appropriate, protection for our products and proprietary technology through a combination of U.S. and foreign patents, trademarks, trade secrets and contractual arrangements with our employees, consultants and suppliers. These measures, however, may not be adequate to protect our technology from unauthorized disclosure, third-party infringement or misappropriation as these parties may breach these agreements, and we may not have adequate remedies for any such breach. We intend to prosecute and defend our proprietary technology. The primary test for patent protection eligibility includes novelty, non-obviousness and usefulness.
We submit applications under the Patent Cooperation Treaty, or PCT, which is an international patent law treaty that provides a unified procedure for filing a single initial patent application to seek patent protection for an invention simultaneously in each of the member states. Although a PCT application is not itself examined and cannot issue as a patent, it allows the applicant to seek protection in any of the member states through national-phase applications.
As of October 5, 2014, we had 16 granted patents (not including separate validations in Europe), 1 “allowed” patent application and 22 pending patent applications (not including “allowed”) worldwide relating to various elements and functions of our products and related enhancements. We have submitted patent applications covering our technology in the United States, member states of the European Patent Organisation, Australia, Brazil, Canada, China, Hong Kong, India, Israel, Japan and South Korea. We have received patent grants for our core patent by the United States Patent and Trademark Office as well as from the European Patent Office, Australia, China, Hong Kong, Israel, India and Japan.
Our registered U.S. Patent Number 7,787,926 discloses an ingestible capsule with a radiation source and radiation detectors that, when used in conjunction with a radio opaque contrast agent, is adapted to detect clinically relevant findings in the colon. Utilizing X-ray fluorescence and Compton back scatterings, the capsule is able to measure the distance between the capsule and the colon wall and to distinguish between gas, intestinal contents, and clinically significant findings in the gastrointestinal tract.
A second PCT patent application (PCT/IL2008/000163), which is pending in several countries in the national-phase and granted in Europe and Hong Kong, discloses additional features such as a rotating collimator and improved scanning mechanisms, the capability to determine tissue density to differentiate between different types of polyps, as well as the capability to determine capsule movement in the colon. Another PCT application (PCT/IL2011/000462), which is pending in several countries in the PCT national-phase, discloses a number of alternate fail safe concealment mechanisms that can be utilized in the capsule to ensure that the X-ray source is blocked when the capsule is not scanning and is open when it is scanning, allowing the capsule to image the colon. The fail safe feature ensures that in the event of power failure, the radiation source is blocked and X-rays do not escape.
In another PCT patent application (PCT/IL2008/000765), which was granted in the United States, Europe, Israel and Japan, we disclose an imaging catheter that utilizes X-ray fluorescence, Compton back scattering and electron back scattering. The imaging catheter is designed for use in cardiac applications as well as intra-operative imaging applications such as imaging inside blood vessels where optical imaging cannot be performed because of obscuring circumstances.
While our policy is to obtain patents by application, to maintain trade secrets and to seek to operate without infringing on the intellectual property rights of third parties, technologies related to our business have been rapidly developing in recent years. Additionally, patent applications that we may file may not result in the issuance of patents, and our issued patents and any issued patents that we may receive in the future may be challenged, invalidated or circumvented. For example, we cannot predict the extent of claims that may be allowed or enforced in our patents nor be certain of the priority of inventions covered by pending third-party patent applications. If third parties prepare and file patent applications that also claim technology or therapeutics to which we have rights, we may have to partake in proceedings to determine priority of invention, which could result in substantial costs to us, even if the eventual outcome is favorable to us. Moreover, because of the extensive time required for clinical development and regulatory review of a product we may develop, it is possible that, before our imaging capsule can be commercialized, related patents will have expired or will expire a short period following commercialization, thereby reducing the advantage of such patent. Loss or invalidation of certain of our patents, or a finding of unenforceability or limited scope of certain of our intellectual property, could have a material adverse effect on us. See “Risk Factors— Risks Related to Our Intellectual Property.”
86
In addition to patent protection, we rely on trade secrets, including unpatented know-how, technology innovation, drawings, technical specifications and other proprietary. We also rely on protection available under trademark laws, and received a notice of allowance for the “CHECK-CAP” mark and design logo in the United States and hold a registered trademark for the “CHECK-CAP” design logo in Europe.
Competition
Competition for our optical capsule endoscopy products comes primarily from traditional well-entrenched technologies for detecting gastrointestinal disorders and diseases, such as optical colonoscopy, sigmoidoscopy and CTC. The principal manufacturers of equipment for optical colonoscopy and sigmoidoscopy are Olympus, Richo Company Ltd., Hoya and Fuji Film. The principal manufacturers of equipment for CTC are General Electric Healthcare Systems, Siemens Medical Solutions, Philips Medical Systems Ltd. and Toshiba Corporation. All of these companies have substantially greater financial resources than we do, and they have established reputations as well as worldwide channels for distribution medical instruments to physicians.
In addition, several companies are developing technologies based on molecular diagnostics (MDx) (from blood and other bodily fluids), tests that investigate the link between genes and the function of metabolic pathways, drug metabolism and disease development with a primary focus on the study of DNA, RNA and proteins. Genetic markers can be traced within stool samples in minute quantities. A U.S.-based company, Exact Sciences, is developing a special collecting kit and associated analyzer to diagnose CRC using stool-based markers. The method of screening for colon cancer using stool DNA testing has been endorsed by the ADS, the U.S. Multi-Society Task Force on Colorectal Cancer and the American College of Radiology, but not by the U.S. Preventive Services Task Force.
In addition, we have competitors who offer or are developing optics-based optical capsule endoscopy systems. Given Imaging, an Israeli-based company that was acquired by Covidein plc (NYSE: COV) in February 2014, has developed visualization capsules for the detection of disorders of the esophagus, small bowel and colon. It launched the PillCam Colon Capsule in Europe in 2007 and has limited sales. In early 2014, the FDA granted approval for optical capsule endoscopy for CRC screening for use in patients after incomplete colonoscopy. However, this technology requires bowel cleansing to a greater degree than is required for a regular optical colonoscopy, which can result in dehydration and in turn can lead to cancellation of the procedure in certain cases. Moreover, because this procedure must be completed within several hours in order to maintain a clean colon and to accommodate the capsule’s limited battery life, patients are required to drink large amounts of liquid so that the capsule can flow through the gastrointestinal tract during the time allotted. Furthermore, camera-based optical capsule endoscopy procedures generate a large number of images, often requiring more physician time to analyze the images than to conduct a colonoscopy.
Olympus is a Japanese company that manufactures optics and imaging products in China. Olympus’ medical division of develops and sells products in the endoscopy, endosurgery and endotherapy fields. The FDA approved Olympus’ Endo-Capsule product for the visualization of the small bowel in late 2007 and the company is currently developing a capsule-based technology for CRC screening.
IntroMedic is a private Korean medical device manufacturer specializing in the development of technologies in the field of optical capsule endoscopy. IntroMedic’s MiroCam product, which is currently undergoing FDA trials in the United States, uses an oxide silicone chip for imaging and human body communication technology for the transmission of images. The company is developing a capsule-based technology for CRC screening.
RF System is a Japanese manufacturer of medical device equipment and cameras for industrial use. Its Sayaka product is a new, “battery free” capsule endoscope that uses induction charging to draw power. The company is developing a capsule technology for CRC screening.
All of the abovementioned CRC optical capsule endoscopy products under development rely on similar optics (camera) and complementary metal oxide semiconductor or CMOS, based technology, thus requiring a significant bowel cleansing prior to performing the procedure.
87
Sales and Marketing
Our goal is to establish our position as a leading player in the CRC screening market. Although we do not currently generate revenues, we expect to generate revenues after we obtain regulatory clearance or approval through sales of our imaging capsule system.
Because we are still in the clinical and development stage, we are subject to certain challenges, including, among others, that:
|
|
•
|
our technology has been tested on a limited basis and therefore we cannot assure the product’s clinical value;
|
|
|
•
|
we need to CE mark the devices in the European Union and obtain the requisite regulatory approvals in the United States, Japan and other markets where we plan to focus our commercialization efforts;
|
|
|
•
|
we need to raise an amount of capital sufficient to complete the development of our technology, obtain the requisite regulatory approvals and commercialize our current and future products;
|
|
|
•
|
we need to obtain reimbursement coverage from third-party payors for procedures using our imaging capsule;
|
|
|
•
|
we need to increase our manufacturing capabilities; and
|
|
|
•
|
we need to establish and expand our customer base while competing against other sellers of capsule endoscopes as well as other current and future CRC screening technologies and methods.
|
Our ability to operate our business and achieve our goals and strategies is subject to numerous risks as described more fully in “Risk Factors.”
We expect to initially launch our imaging capsule in 2016 in selected markets in Europe, where regulatory hurdles are generally lower and in which current penetration of optical colonoscopy as a screening tool is low relative to the U.S. market. We intend to target major markets in Europe, including Germany, Spain, Italy, France and the United Kingdom. In these markets, we are planning to work with local distributors who are active in the gastroenterology field and who have already demonstrated excellent performance in introducing new and innovative technologies.
In the European Union and Japan, we intend to offer our imaging capsule as a primary screening tool for the general population. In the United States, we intend to target the population in a two-staged approach: first, we plan to obtain the requisite regulatory approvals to offer our imaging capsule as an adjunct tool to FOBTs and FITs, and after we have generated additional clinical data to support general screening indication, we plan to seek FDA approval for the use of our imaging capsule as a primary screening tool for the general population.
After receipt of the initial FDA approval, we expect to launch the product in 2017 in the U.S. market, where we will consider setting up our own sales force or joining forces with a strategic partner. Initially, we are planning to sell our imaging capsule to the private sector. Simultaneously, we will work intensively to obtain reimbursement by Medicare and private insurers within the shortest possible time frame.
Subject to local regulatory approval, we also intend to market our imaging capsule in key markets in Asia. Initial efforts will focus in Japan in view of its well-developed CRC screening market.
Manufacturing and Suppliers
Our manufacturing operations are conducted at a facility located in Isfiya, Mount Carmel, Israel. We lease approximately 610 square meters at this facility under a lease agreement providing for a term of 10 years, commencing on June 1, 2012 and ending on May 31, 2022. We have the right to terminate the agreement at any time. We currently have sufficient space to manufacture our imaging capsule for the clinical studies but have limited resources, facilities and experience in commercially manufacturing large quantities of our imaging capsule, external receiver and software application to meet the demand we expect from our expanded commercialization efforts. We expect to face certain technical challenges as we increase manufacturing capacity, including, among others, logistics associated with the handling of radioactive materials, equipment design and automation, material procurement, lower than expected yields and increased scrap costs, as well as challenges related to maintaining quality control and assurance standards. Our production objective is to establish a scalable capacity in order to meet such expanded demand for our imaging capsule and market expansion. If we are unable to scale our manufacturing capabilities to meet market demand, our growth could be limited and our business, financial condition and results of operations could be materially adversely affected.
88
We are currently building our production capacity and developing supply chain systems to support production for clinical trials. Our current capacity was built to accommodate our clinical phase. We have integrated a product life management system to enable overall life cycle tracking and documentation including full configuration management control and manufacturing documentation.
During the clinical testing phase in Europe, we are planning to conduct both the assembling of our imaging capsule and the insertion of the X-ray source at our facilities. As we proceed to the commercial phase in Europe and the U.S. clinical trial, we expect to collaborate with a third-party in the manufacturing and integration of the X-ray source into our imaging capsule, in order to increase the time and cost efficiency of the process. We are currently in discussions with several potential partners.
We do not currently have any sales. We are planning to develop a scale-up plan to meet our expected commercial supply needs. We are also working on a plan to expand our manufacturing capacity to support the expected larger clinical volume and subsequent higher volumes expected in the early commercialization stage. We are considering, among other options, the expansion of our assembly line in Israel, the buildup of new assembly lines in the United States and Europe, and alternative sources for the key capsule components (such as the motor, X-ray detectors, electrical components and PCBs). All of the facilities in which manufacturing and assembly of our products will be conducted will need to comply with applicable regulations and standards for medical devices.
We have received grants from Government of the State of Israel through the OCS, the terms of which require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless prior approval is received from the OCS, which we may not receive. We are currently considering whether it would be possible to assemble the capsule without the X-ray source in Israel, and have the X-ray source subsequently inserted into our imaging capsule at a reactor or cyclotron site or at a distribution center outside Israel. Even following the full repayment of any OCS grants, we must nevertheless continue to comply with the requirements of the Research Law. The foregoing restrictions may impair our ability to outsource or transfer development or manufacturing activities with respect to any product or technology outside of Israel. See “Risk Factors – Risks Related to Our Operations in Israel.”
We currently depend on limited source suppliers for some of the components necessary for the production of our imaging capsule. For example, for the current version of the imaging capsule used in clinical trials, we currently have one leading supplier for the motor that we are using to rotate the collimated X-ray source in our imaging capsule and we currently have one leading supplier for the X-ray detectors used in our imaging capsule. There are a limited number of manufacturers worldwide who are capable of manufacturing the motor and the specially designed X-ray detectors that we currently use in our imaging capsule. In addition, the ASIC residing in our imaging capsule is currently manufactured for us by a single FAB. There are many alternative FABs worldwide and the design of our current ASIC could be adapted in the event it became necessary to use an alternative FAB. Our current suppliers have been able to supply the required quantities of such components to date. However, if the supply of these components is disrupted or terminated or if our current suppliers are unable to supply required quantities of components, we may not be able to find alternative sources for these key components in a timely manner. Although we are planning to maintain strategic inventory of key components, the inventory may not be sufficient to satisfy the demand for our imaging system if such supply is interrupted, or subject to risk of loss due to catastrophic events such as a fire at our storage facility. As a result, we may be unable to meet the demand for our imaging system, which could harm our ability to generate revenues, lead to customer dissatisfaction and damage our reputation. If we are required to change the manufacturer of any of these key components, there may be a significant delay in locating a suitable alternative manufacturer. In addition, we may be required to verify that the new manufacturer maintains facilities and procedures that comply with FDA and other applicable quality standards and with all applicable regulations and guidelines. The delays associated with the identification of a new manufacturer could delay our ability to manufacture our imaging system in a timely manner or within budget. Furthermore, in the event that the manufacturer of a key component of our imaging system ceases operations or otherwise ceases to do business with us, we may not have access to the information necessary to enable another supplier to manufacture the component. The occurrence of any of these events could harm our ability to meet demand for our imaging system in a timely manner or within budget.
89
Employees
As of November 21, 2014, we had 37 employees and independent contractors, of whom 7 persons were in administrative, accounting and human resources and 30 persons were in research and development. As of December 31, 2013, we had 34 employees and independent contractors, of whom 6 persons were in administrative, accounting and human resources and 28 persons were in research and development. As of December 31, 2012, we had 36 employees and independent contractors, of whom 6 persons were in administrative, accounting and human resources and 30 persons were in research and development. As of December 31, 2011, we had 30 employees and independent contractors, of whom 5 persons were in administrative, accounting and human resources and 25 persons were in research and development. All of our employees are based in Israel.
Under Israeli law, we and our employees are subject to protective labor provisions, including the length of the workday, minimum wages for employees, annual leave, sick pay, determination of severance pay and advance notice of termination of employment, as well as procedures for hiring and dismissing employees and equal opportunity and anti-discrimination laws. While none of our employees is party to any collective bargaining agreements, orders issued by the Israeli Ministry of Economy (formerly named the Ministry of Industry, Trade and Labor) may make certain industry-wide collective bargaining agreements applicable to us. These agreements affect matters such as the length of the workday and week, recuperation pay, travel expenses and pension rights. We have never experienced labor-related work stoppages and believe that our relationships with our employees are good.
Israeli law generally requires the payment of severance compensation by employers upon the retirement, death or dismissal of an employee. We fund our ongoing severance obligations by making monthly payments to insurance policies. All of our current employees have agreed that upon termination of their employment, they will be entitled to receive only the amounts accrued in the insurance policies with respect to severance pay. For information regarding the severance pay to which our chief executive officer would be entitled upon termination of his employment, see “—Employment Agreements with Executive Officers.” Furthermore, Israeli employees and employers are required to pay predetermined sums to the National Insurance Institute, which is similar to the U.S. Social Security Administration. These amounts also include payments for national health insurance.
In addition, we have various consulting arrangements with experts in regulatory, research and clinical matters.
Scientific Advisors
Certain of our officers and employees including our Chief Executive Officer, our Chief Technology Officer, our Vice President, Research & Development and our clinical director have consulted on an individual, as needed basis with the individuals listed below with respect to matters of scientific relevance. We refer to these individuals as our Scientific Advisors. The amount of consulting services provided by our Scientific Advisors ranges from several hours a month to several hours a year. We intend to establish a routine of group meetings of these individuals as additional clinical data is generated. The names and biographies of the individuals who act as our Scientific Advisors are set forth below:
Dr. Douglas K. Rex is the Chancellor’s Professor and Professor of Medicine at Indiana University School of Medicine and Director of Endoscopy at Indiana University Hospital. His major research interests have been in colorectal disease and, in particular, CRC screening and the technical performance of optical colonoscopy. He co-authored the CRC screening recommendations of the American College of Gastroenterology and those of the Gastroenterology Consortium. He has authored more than 110 original research papers, 50 book chapters, 100 invited papers and editorials, and 15 guideline papers. He is an Associate Editor of Journal Watch Gastroenterology and Reviews on Gastroenterological Disorders, and is a member of the Editorial Boards of Clinical Gastroenterology and Hepatology, Journal of Clinical Gastroenterology, World Journal of Gastroenterology and Gastroenterology and Hepatology. He has served as the ACG representative to the National Colorectal Cancer Round Table, and from 2000-2006 was the chairman of the U.S. Multi-Society (ACG, ASGE, AGA, ACP-ASIM) Task Force on Colorectal Cancer. He has also served in the American College of Gastroenterology as Chairman of the Board of Governors, Secretary, and Treasurer and is a Past President of the ACG. He was awarded Distinguished Professor in 2009. He graduated from Harvard College, summa cum laude, in 1976, and with highest distinction from Indiana University School of Medicine in 1980. Mr. Rex has served as a Scientific Advisor to the Company since July 2009.
90
Dr. Walter L. Robb is a mentor, director and angel investor to many Tech Valley start-ups. Dr. Robb joined General Electric in 1951 and served in various capacities at GE in management, research and development, retiring in 1992 as Senior Vice President for Corporate Research and Development and a member of the company’s Corporate Executive Council. For a time he directed GE’s Schenectady R&D Center, one of the world’s largest and most diversified industrial laboratories, with about 1,650 scientists, engineers and technicians concentrating on the company’s longer-range research and development needs. Dr. Robb headed GE Medical Systems (headquartered in Milwaukee) from 1973 to 1986, building it into the world’s leading producer of medical diagnostic imaging equipment. He holds 12 patents and authored more than a score of articles in professional journals. He is a member of the National Academy of Engineering. In 1993, he received the National Medal of Technology from then President Clinton for his leadership in the CT and MR imaging industry. Dr. Robb serves on the board of directors of Celgene Corporation and Mechanical Technology, Inc., which are publicly traded. Dr. Robb received a BS degree in chemical engineering from Penn State University in 1948 and an MS degree and PhD in chemical engineering both from the University of Illinois in 1951. Dr. Robb has served as a Scientific Advisor to the Company since May 2009.
Dr. Perry J. Pickhardt is a professor in the University of Wisconsin Medical School. He served in the U.S. Navy, spending one year as the Department Head of Radiology, U.S. Naval Hospital Guantanamo Bay, Cuba and three years as the head of GI-GU Imaging at the National Naval Medical Center in Bethesda, MD. He also served as an Assistant Professor of Radiology at the Uniformed Services University of the Health Sciences in Bethesda. Among other projects at NNMC, Dr. Pickhardt organized a large multi-center screening trial evaluating CTC and served as the PI. Among other awards and honors, Dr. Pickhardt completed a Figley Fellowship at the AJR Editorial Office in Winston- Salem, NC in 2002. CTC and CRC screening continue to be Dr. Pickhardt’s primary clinical and research interests. His work has resulted in over 250 scientific publications and book chapters, and 3 textbooks including a textbook on body CT. He graduated first in his class from the University of Wisconsin with a B.S. in Physics in 1991 and from the University of Michigan Medical School in 1995. Dr. Pickhardt has served as a Scientific Advisor to the Company since August 2009.
Steve Hanley is currently the Chief Executive Officer of MediBeacon LLC. Mr. Hanley is an experienced global business leader who has managed highly complex pharmaceutical and medical device operations with annual global revenue exceeding $1 billion. As the President of Covidien plc’s Imaging Solutions business unit, he led a multifunctional organization that included sales, marketing, logistics, manufacturing, as well as research and development. Internationally, his track record includes numerous new drug and device product introductions and sales force expansion in Eastern Europe, China and Latin America. Mr. Hanley is experienced working in different cultures and successfully navigating dynamic regulatory environments. Over his nearly 18 years with the Covidien family of companies, Steve developed a large network of business leaders and clinicians to help determine market needs, commercial potential and product positioning. As a sales leader, Mr. Hanley called on radiologists, nuclear medicine physicians, cardiologists, as well as surgeons in specialties including general, orthopedic, and OB/GYN. Mr. Hanley is Principal and Founder of Neem LLC, which was founded in 2009 to focus on startup and entrepreneurial medical device and other life science companies with whom the firm works to bridge the gap between breakthrough technology and commercialization. Mr. Hanley is the Chairman of the Board of Managers for Daya CNS LLC based in St Louis Missouri. In addition, Mr. Hanley currently is on the Advisory Board for Kogent Surgical LLC based in St Louis Missouri. Mr. Hanley received his bachelor’s and master’s degrees in business administration from Marquette University in 1989 and 1995, respectively. Mr. Hanley has served as a Scientific Advisor to the Company since June 2011.
Prof. Nadir Arber is a full Professor of Medicine and Gastroenterology. He is a holder of the Yechiel and Helen Lieber chair for Cancer Research at Tel Aviv Sourasky Medical Center and Tel Aviv University, Sackler School of Medicine. Prof. Arber serves as the Director of the Integrated Cancer Prevention Center (ICPC) at Tel Aviv Sourasky Medical Center in Tel Aviv. He also chairs the grants committee of the Sackler School of Medicine at Tel Aviv University. Prof. Arber is the Head of Cancer Research Center, Head of Djerassi Oncology Center, and President of the Israeli Association of Gastroenterology. Prof. Arber, a noted expert in the field of early detection and prevention, has been serving as the principal investigator (PI) of several international, multicenter clinical trials in the field of early detection, prevention and therapy of gastrointestinal malignancies using NSAIDs and in particular, CRC. Prof. Arber has published more than 330 publications. Prof. Arber received a MD from the Hadassah School of Medicine of the Hebrew University of Jerusalem in 1987, an MSc degree from Sackler School of Medicine, Tel Aviv University in 1991 and a MHA degree from Rekanaty School of Management, Tel Aviv University in 1991. Prof. Arber has served as a Scientific Advisor to the Company since [January] 2012.
91
Dr. Jonathan A. Leighton is Professor of Medicine and Chair of the Division of Gastroenterology and Vice Chair of the Department of Medicine at the Mayo Clinic in Arizona. In addition, Dr. Leighton is a member of the Board of Trustees of the American College of Gastroenterology. Dr. Leighton is an active member of, the American Gastroenterology Association, the American Society of Gastrointestinal Endoscopy, and the American College of Gastroenterology. His research interests include inflammatory bowel disease, biomarker discovery, as well as small bowel imaging including optical capsule endoscopy and deep enteroscopy. Dr. Leighton received an MD degree from the University of Arizona in 1981 and completed his internship and residency at the University of Texas Health Science Center in San Antonio, Texas in 1984. Dr. Leighton has served as a Scientific Advisor to the Company since July 2014.
Property
Our principal executive offices and operations are conducted at a facility located in Isfiya, Mount Carmel, Israel since June 1, 2009. We currently lease approximately 610 square meters at this facility under a lease agreement providing for a term of 10 years, commencing on June 1, 2012 and ending on May 31, 2022, unless earlier terminated by us upon at least 60 days prior written notice. We believe that our current facilities are adequate to meet our needs for the clinical phase of our development.
Environmental Health and Safety Matters
We are subject to environmental health and safety laws and regulations in Israel, governing, among other things, the use of radioactive materials, including the Israeli Work Safety Regulations (Occupational Safety and Health of Ionizing Radiation Practitioners) 1992-5753 and Women Employment Regulations (Work with Ionizing Radiation), 1979-5739. Our activities require permits from various governmental authorities including, Israel’s Ministry of Environmental Protection, Israel’s Ministry of Health and local municipal authorities. The Ministry of Environmental Protection and the Ministry of Health conduct periodic inspections in order to review and ensure our compliance with the various regulations.
These laws, regulations and permits could potentially require expenditure by us for compliance or remediation. If we fail to comply with such laws, regulations or permits, we may be subject to fines and other civil, administrative or criminal sanctions, including the revocation of permits and licenses necessary to continue our business activities. In addition, we may be required to pay damages or civil judgments in respect of third-party claims, including those relating to personal injury (including exposure to radioactive materials) or contribution claims. Some environmental, health and safety laws allow for strict, joint and several liability for remediation costs, regardless of comparative fault. We may be identified as a responsible party under such laws. Such developments could have a material adverse effect on our business, financial condition and results of operations.
In addition, laws and regulations relating to environmental, health and safety matters are often subject to change. In the event of any changes or new laws or regulations, we could be subject to new compliance measures or to penalties for activities which were previously permitted.
Legal Proceedings
From time to time, we may be involved in litigation that arises through the normal course of business. As of the date of this filing, we are not a party to any material litigation nor are we aware of any such threatened or pending litigation.
There are no material proceedings in which any of our directors, officers or affiliates or any registered or beneficial shareholder of more than 5% of our ordinary shares, or any associate of any of the foregoing is an adverse party or has a material interest adverse to our interest.
92
U.S. Government Regulation
Our imaging capsule is a medical device subject to extensive regulation by FDA and other U.S. federal and state regulatory bodies. To ensure that medical products distributed in the United States are safe and effective for their intended use, FDA has imposed regulations that govern, among other things, the following activities that we or our partners perform and will continue to perform:
|
|
•
|
product design and development;
|
|
|
•
|
product testing;
|
|
|
•
|
validation and verifications;
|
|
|
•
|
product manufacturing;
|
|
|
•
|
product labeling;
|
|
|
•
|
product storage, shipping and handling;
|
|
|
•
|
premarket clearance or approval;
|
|
|
•
|
advertising and promotion;
|
|
|
•
|
product marketing, sales and distribution; and
|
|
|
•
|
post-market surveillance reporting death or serious injuries and medical device reporting.
|
FDA’s Premarket Clearance and Approval Requirements
Unless an exemption applies, before we can commercially distribute medical devices in the United States, we must obtain, depending on the type of device, either prior 510(k) clearance or PMA approval from FDA. FDA classifies medical devices into one of three classes:
|
|
•
|
Class I devices, which are subject to only general controls (e.g., labeling, medical devices reporting, and prohibitions against adulteration and misbranding) and, in some cases, to the 510(k) premarket clearance requirements;
|
|
|
•
|
Class II devices, generally requiring 510(k) premarket clearance before they may be commercially marketed in the United States; and
|
|
|
•
|
Class III devices, consisting of devices deemed by FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a predicate device, generally requiring submission of a PMA supported by clinical trial data.
|
510(k) Clearance Pathway
To obtain 510(k) clearance, we must submit a premarket notification, or 510(k) notice, demonstrating that the proposed device is substantially equivalent to a previously cleared 510(k) device or a device that was in commercial distribution before May 28, 1976 for which FDA has not yet called for the submission of premarket approval applications. FDA’s 510(k) clearance pathway takes approximately from 6 to 9 months, but it can take significantly longer. FDA may require additional information, including clinical data, to make a determination regarding substantial equivalence.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in its intended use, will require a new 510(k) clearance or, depending on the modification, require premarket approval. FDA requires each manufacturer to determine whether the proposed change requires submission of a 510(k), or a premarket approval, but FDA can review any such decision and can disagree with a manufacturer’s determination. If FDA disagrees with a manufacturer’s determination, FDA can require the manufacturer to cease marketing and/or recall the modified device until 510(k) clearance or premarket approval is obtained. If FDA requires us to seek 510(k) clearance or premarket approval for any modifications to a previously cleared product, we may be required to cease marketing or recall the modified device until we obtain this clearance or approval. Also, in these circumstances, we may be subject to significant regulatory fines or penalties.
93
De Novo Reclassification
If FDA finds that there is no suitable predicate device for our imaging capsule, it will automatically be considered a class III device. However, in instances where a device is novel and there is no suitable predicate device, but that device is deemed to be of low to moderate risk, FDA can reclassify the device to class I or class II via de novo reclassification. This process involves the submission of a reclassification petition, and FDA accepting that “special controls” are adequate to ensure the product’s performance and safety.
FDA now allows “direct” de novo reclassification petitions, a mechanism by which a sponsor can directly submit a detailed de novo reclassification petition as the device’s initial submission without having to first receive a not substantially equivalent, or NSE, decision on a 510(k) submission. The direct de novo pathway takes at least 9 to 12 months from submission of the petition to device clearance.
Our current strategy is to submit a direct de novo reclassification petition for our imaging capsule. To support a direct de novo reclassification petition, our objective is to demonstrate that the device poses a low to moderate risk to patients. If FDA determines that our imaging capsule is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process.
Alternatively, if we seek 510(k) clearance and our device is found not substantially equivalent, or NSE, a de novo petition must be filed within 30 days from the receipt of the NSE determination. The request should include a description of the device, labeling for the device, reasons for the recommended classification and information to support the recommendation. The de novo process following an NSE determination has a 60-day review period, although it is typical for the review to take far longer. If FDA classifies the device into class II, the company will then receive an approval order to market the device. This device type can then be used as a predicate device for future 510(k) submissions. However, if FDA subsequently determines that the device will remain in the class III category, then the device may not be marketed until the company has obtained an approved PMA.
Premarket Approval Pathway
A premarket approval application must be submitted if the device cannot be cleared through the 510(k) process or is found ineligible for de novo reclassification. The premarket approval application process is generally more costly and time consuming than the 510(k) process. A premarket approval application must be supported by extensive data including, but not limited to, technical, preclinical, clinical trials, manufacturing and labeling to demonstrate to FDA’s satisfaction the safety and effectiveness of the device for its intended use.
After a premarket approval application is sufficiently complete, FDA will accept the application and begin an in-depth review of the submitted information. By statute, FDA has 180 days to review the “accepted application,” although, generally, review of the application can take at least 12 to 18 months, but it may take significantly longer. During this review period, FDA may request additional information or clarification of information already provided. Also during the review period, an advisory panel of experts from outside FDA may be convened to review and evaluate the application and provide recommendations to FDA as to the approvability of the device. In addition, FDA will conduct a preapproval inspection of the manufacturing facility to ensure compliance with quality system regulations. New premarket approval applications or premarket approval application supplements are required for modifications that affect the safety or effectiveness of the device, including, for example, certain types of modifications to the device’s indication for use, manufacturing process, labeling and design. Premarket approval supplements often require submission of the same type of information as a premarket approval application, except that the supplement is limited to information needed to support any changes from the device covered by the original premarket approval application, and may not require as extensive clinical data or the convening of an advisory panel.
Clinical Trials
Clinical trials are almost always required to support a premarket approval application or de novo reclassification petition and are sometimes required for a 510(k) premarket notification. If the device presents a “significant risk,” as defined by FDA, to human health, FDA requires the device sponsor to file an IDE application with FDA and obtain IDE approval prior to commencing the human clinical trials. The investigational device exemption application must be supported by appropriate data, such as animal and laboratory testing results, showing that it is safe to test the device in humans and that the testing protocol is scientifically sound. The investigational device exemption application must be approved in advance by FDA for a specified number of patients, unless the product is deemed a “non-significant risk” device and eligible for more abbreviated investigational device exemption requirements. Clinical trials for a significant risk device may begin once the investigational device exemption application is approved by FDA and the appropriate institutional review boards at the clinical trial sites. Future clinical trials of our motion preservation designs will require that we obtain an investigational device exemption from FDA prior to commencing clinical trials and that the trial be conducted under the oversight of an institutional review board at the clinical trial site. Our clinical trials must be conducted in accordance with FDA regulations and federal and state regulations concerning human subject protection, including informed consent and healthcare privacy. A clinical trial may be suspended by FDA or the investigational review board at any time for various reasons, including a belief that the risks to the study participants outweigh the benefits of participation in the study. Even if a study is completed, the results of our clinical testing may not demonstrate the safety and efficacy of the device, or may be equivocal or otherwise not be sufficient to obtain approval of our product. Similarly, in Europe the clinical study must be approved by the local ethics committee and in some cases, including studies of high-risk devices, by the Ministry of Health in the applicable country.
94
Pervasive and Continuing FDA Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
|
|
•
|
product listing and establishment registration, which helps facilitate FDA inspections and other regulatory action;
|
|
|
•
|
Quality System Regulation, or QSR, which requires manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the manufacturing process;
|
|
|
•
|
labeling regulations and FDA prohibitions against the promotion of products for uncleared, unapproved or off-label use or indication;
|
|
|
•
|
clearance of product modifications that could significantly affect safety or efficacy or that would constitute a major change in intended use of one of our cleared devices;
|
|
|
•
|
approval of product modifications that affect the safety or effectiveness of one of our approved devices;
|
|
|
•
|
medical device reporting regulations, which require that manufacturers comply with FDA requirements to report if their device may have caused or contributed to a death or serious injury, or has malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction of the device or a similar device were to recur;
|
|
|
•
|
post-approval restrictions or conditions, including post-approval study commitments;
|
|
|
•
|
post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device;
|
|
|
•
|
FDA’s recall authority, whereby it can ask, or under certain conditions order, device manufacturers to recall from the market a product that is in violation of governing laws and regulations;
|
|
|
•
|
regulations pertaining to voluntary recalls; and
|
|
|
•
|
notices of corrections or removals.
|
We and our third-party manufacturers must register with FDA as medical device manufacturers and must obtain all necessary state permits or licenses to operate our business. As manufacturers, we and our third-party manufacturers are subject to announced and unannounced inspections by FDA to determine our compliance with quality system regulation and other regulations. We have not yet been inspected by the FDA.
Failure to comply with applicable regulatory requirements can result in enforcement action by FDA, which may include any of the following sanctions:
|
|
•
|
untitled letters, warning letters, fines, injunctions, consent decrees and civil penalties;
|
|
|
•
|
unanticipated expenditures to address or defend such actions;
|
95
|
|
•
|
customer notifications for repair, replacement, refunds;
|
|
|
•
|
recall, detention or seizure of our products;
|
|
|
•
|
operating restrictions or partial suspension or total shutdown of production;
|
|
|
•
|
refusing or delaying our requests for 510(k) clearance or premarket approval of new products or modified products;
|
|
|
•
|
operating restrictions;
|
|
|
•
|
withdrawing 510(k) clearances on PMA approvals that have already been granted;
|
|
|
•
|
refusal to grant export approval for our products; or
|
|
|
•
|
criminal prosecution.
|
Regulatory Pathway for our Imaging Capsule
We have established a clinical and regulatory strategy with our advisors and have conducted a pre-IDE meeting with FDA (an informal interaction to facilitate a clearer understanding of FDA’s expectations). During this process, we received FDA’s feedback on our submission and our questions and we are planning to continue the dialogue with FDA before the submission of a formal request for the IDE that is necessary for us to conduct the U.S. pivotal clinical trial.
Our current strategy is to submit a direct de novo reclassification petition for our imaging capsule. Although FDA could require us to submit a PMA, we believe that the device could be considered for evaluation under FDA’s de novo reclassification provisions, which allow a novel device to be reclassified into class I or class II. To support this, our objective is to demonstrate that device poses a low to moderate risk to patients.
We believe that important potential benefits of our system for CRC screening are the elimination of the need for bowel cleansing, the elimination of the need for conscious sedation, the minimally invasive, painless nature of the examination, and the ability to pursue normal daily activities immediately following the procedure. Furthermore, the system is being designed to generate information from segments of the colon (e.g., cecum and ascending colon) that are difficult to access by conventional optical colonoscopy (i.e., incomplete colonoscopies) without the risks and discomforts of operative examination or other invasive methods. We believe these benefits will be attractive to a large number of patients from the target populations that so far refrained from any screening tests. Thus, we anticipate that our capsule will increase the public compliance to screening recommendation.
If FDA determines that our imaging capsule is not a candidate for de novo reclassification, it will require approval of the device for market through the PMA process. Because of the technological characteristics of this device, the non-clinical tests (including lab and animals) and clinical data required may not be significantly different between a de novo and PMA regulatory processes. We believe that under both scenarios, we will be required to conduct a multi-center clinical study to establish the safety and efficacy and to demonstrate sensitivity and specificity of our imaging capsule in several hundreds of patients.
FCC Clearance and Regulation
Because our imaging capsule includes a wireless radio frequency transmitter and receiver, it is subject to equipment authorization requirements in the United States. The U.S. Federal Communications Commission, or FCC, requires authorization of radio frequency devices before they can be sold or marketed in the United States, subject to limited exceptions. The authorization requirements are intended to confirm that the proposed products comply with FCC radio frequency emission, power level standards, and other technical requirements and will not cause interference. Our capsule is using the same frequency band as other approved capsules, and we expect that it will comply with the FCC’s technical requirements, so it is expected that it will be authorized by the FCC as well.
Third-Party Coverage and Reimbursement
Coverage of and reimbursement for our imaging capsule and related procedures, after approval, will be subject to the requirements of various third-party payors, including government-sponsored healthcare payment systems and private third-party payors. Coverage policies and reimbursement methodologies vary significantly from program-to-program, and may be subject to federal and state regulations. For example, the Medicare statute requires all items and services to be “reasonable and necessary for the diagnosis or treatment of illness or injury or to improve the functioning of a malformed body member” in order to be covered. Medicare currently does not provide separate reimbursement for many devices, but may include payment for the device in the payment for the related procedure. Third-party payors’ coverage and reimbursement policies, including their interpretations of whether an item or service is “reasonable and necessary” or experimental and their payment methodologies, are subject to change pursuant to legislation, regulation, or, in the case of private payors, negotiations with providers.
96
Fraud and Abuse Laws
In the United States, the healthcare industry is subject to extensive federal, state, and local regulation. Both federal and state governmental agencies subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. These laws constrain the sales, marketing and other promotional activities of manufacturers of medical devices, by limiting the kinds of financial arrangements (including sales programs) we may have with hospitals, physicians and other potential purchasers of the medical devices. The laws and regulations that may affect our ability to operate include, but are not limited to:
|
|
•
|
The federal Anti-Kickback Statute, which prohibits, among other things, knowingly or willingly offering, paying, soliciting or receiving remuneration, directly or indirectly, in cash or in kind, to induce or reward the purchasing, leasing, ordering or arranging for or recommending the purchase, lease or order of any health care items or service for which payment may be made, in whole or in part, by federal healthcare programs such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between medical device manufacturers on one hand and prescribers, purchasers and formulary managers on the other. Further, PPACA, among other things, clarified that a person or entity needs not to have actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. Although there are a number of statutory exemptions and regulatory safe harbors to the federal Anti-Kickback Statute protecting certain common business arrangements and activities from prosecution or regulatory sanctions, the exemptions and safe harbors are drawn narrowly, and practices that do not fit squarely within an exemption or safe harbor may be subject to scrutiny; |
|
|
•
|
The federal civil False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment of government funds or knowingly making, using or causing to be made or used, a false record or statement material to an obligation to pay money to the government or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. In addition, PPACA amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Many medical device manufacturers and other healthcare companies have been investigated and have reached substantial financial settlements with the federal government under the civil False Claims Act for a variety of alleged improper marketing activities, including providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees, grants, free travel, and other benefits to physicians to induce them to use the company’s products. In addition, in recent years the government has pursued civil False Claims Act cases against a number of manufacturers for causing false claims to be submitted as a result of the marketing of their products for unapproved, and thus non-reimbursable, uses. Device manufacturers also are subject to other federal false claim laws, including, among others, federal criminal healthcare fraud and false statement statutes that extend to non-government health benefit programs; |
|
|
•
|
Analogous state laws and regulations, such as state anti-kickback and false claims laws, may apply to items or services reimbursed under Medicaid and other state programs or, in several states, apply regardless of the payor. Several states now require medical device manufacturers to report expenses relating to the marketing and promotion or require them to implement compliance programs or marketing codes. For example, California, Connecticut and Nevada mandate implementation of corporate compliance programs, while Massachusetts and Vermont impose more detailed restrictions on device manufacturer marketing practices and tracking and reporting of gifts, compensation and other remuneration to health care providers;
|
97
|
|
•
|
The federal Foreign Corrupt Practices Act of 1997 and other similar anti-bribery laws in other jurisdictions generally prohibit companies and their intermediaries from providing money or anything of value to officials of foreign governments, foreign political parties, or international organizations with the intent to obtain or retain business or seek a business advantage. Recently, there has been a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the Department of Justice and the U.S. Securities and Exchange Commission. Violations of these laws can result in the imposition of substantial fines, interruptions of business, loss of supplier, vendor or other third-party relationships, termination of necessary licenses and permits, and other legal or equitable sanctions. Other internal or government investigations or legal or regulatory proceedings, including lawsuits brought by private litigants, may also follow as a consequence; and |
|
|
•
|
The federal Physician Payment Sunshine Act, being implemented as the Open Payments Program, requires certain medical device manufacturers to engage in extensive tracking of payments or transfers of value to physicians and teaching hospitals, maintenance of a payments database, and public reporting of the payment data. Device manufacturers with products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program are required to track and report such payments. Applicable manufacturers should have begun tracking on August 1, 2013 and must report payment data to CMS by June 30, 2014.
|
Violations of any of the laws described above or any other governmental regulations that apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from government-funded healthcare programs, like Medicare and Medicaid, and the curtailment or restructuring of our operations.
Privacy Laws
HIPAA/HITECH and related U.S. federal and state laws protect the confidentiality of certain patient health information, including patient records, and restrict the use and disclosure of that protected information. In particular, the U.S. Department of Health and Human Services promulgated patient privacy rules under the HIPAA.
These privacy rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their protected health information and limiting most use and disclosures of health information to the minimum degree reasonably necessary to accomplish the intended purpose. Because we intend to sell products, once approved, to persons and entities subject to HIPAA and are exposed to personally-identifiable health information in the course of our operations, we also may be subject to certain elements of HIPAA, particularly as a business associate to covered entities, as well as similar state laws. HIPAA imposes civil and criminal penalties for violations of its provisions, which could be substantial. State privacy laws have their own penalty provisions which may be applicable.
NRC Regulatory Issues
As our imaging capsule includes an ingestible capsule with a radioactive source, the company must address NRC regulations, or relevant Agreement State regulations, in addition to FDA requirements. An Agreement State is a state that has signed an agreement with the NRC authorizing the state to regulate certain uses of radioactive materials within the state. Agreement State regulations are substantially similar to the NRC’s regulations.
The capsule is loaded with the X-ray source, sealed and then ingested by the patient. The capsule is excreted naturally through the patient’s system and is not recovered and therefore is disposed of in the sanitary sewer system. Although the NRC regulations in 10 CFR Part 20 place certain conditions and limitations on the disposal of radioactive material in the sanitary sewer, such conditions and limitations do not apply to radioactive material contained in excreta of individuals that are undergoing medical diagnosis or therapy with radioactive material. Our regulatory advisors have advised us that the NRC staff likely would take the position that a capsule containing radioactive material can be passed in excreta into the sanitary sewer system without limitation. If, however, the NRC were to find that our capsule system could not be passed in excreta into the sanitary sewer system without limitation pursuant to 10 CFR 20.2003(b) the NRC may place restrictions on the disposal of the capsule system in the sanitary sewer system.
98
An entity which manufactures, prepares, or transfers a medical capsule containing radioactive byproduct material needs to be licensed or covered by a license issued by the NRC or an Agreement State. An NRC or Agreement State licensee authorized to possess and/or distribute byproduct material can transfer the byproduct material only to another NRC, or Agreement State, approved entity or licensee. The NRC’s regulations permit only individuals who are authorized users (e.g., individuals who meet certain training and experience criteria regarding the safe use of radioactive drugs) or persons working under the supervision of an authorized user to administer radioactive drugs for medical use.
The NRC regulations do exempt certain products from the NRC’s regulations. Existing exemptions from licensing requirements for the use of byproduct material include exemptions for specific products (e.g., time pieces), exemptions for classes of products (e.g., gas or aerosol detectors), and broader materials exemptions for “exempt concentrations” and “exempt quantities” of radioactive material. These two broad materials exemptions specifically exclude the transfer of byproduct material contained in any food, drug, or product designed for ingestion by a human being. Capsules containing our X-ray source would not qualify as an “exempt quantity” because of their intended use (i.e., for ingestion) even though they may contain a smaller quantity of the source than the exempt quantities set forth in the regulations.
Accordingly, we will need to obtain the appropriate licenses from the NRC or an Agreement State prior to the clinical investigation and/or marketing of the device. We intend to engage a radiopharmaceutical company to manufacture our imaging capsule. The fact that another company will be manufacturing the capsule, however, does not exempt us from also obtaining radioactive materials licenses from the NRC or an Agreement State. Distribution activities are generally classified by the NRC as either “distribution” or “redistribution”, and both types of activities require a specific license. “Distribution” refers to the initial transfer from the manufacturing radiopharmacy, while “redistribution” refers to a subsequent transfer of the drug by an NRC licensee to an authorized user. In order to distribute the capsule commercially, we will need to obtain an NRC or Agreement State “medical distribution” radioactive materials license and may also need to obtain a radioactive materials license authorizing the possession of the radioactive material. Both types of licenses may be obtained by submitting a license application request to the NRC or an Agreement State. In the event that we develop the capsule outside the United States, we would be required to have one of our U.S. offices apply for and receive both the possession and medical distribution radioactive materials licenses. If we do not have an office in the United States, then we can contract with a company with a U.S. office to apply for and obtain these licenses, and that company would be the licensed U.S. distributor of the capsule.
We may be able to petition the NRC to carve out an exemption for the distribution licensing requirement to permit distribution to all health care professionals and not just those licensed by the NRC. This has been done successfully by other medical device companies. For example, Tri-Med, Inc. manufactures an ingestible capsule containing radioactive material for testing of H. Pylori. The company petitioned the NRC in 1994 for an exemption from the distribution licensing regulation. The NRC evaluated the petition and issued a proposed ruling for comments. After receiving comments on the proposed ruling, the NRC issued a final ruling, in 1997, providing for the exemption. This exemption is narrowly drawn, and specific to the distribution of a “radioactive drug containing one microcurie of carbon-14 urea to any person for ‘in vivo’ diagnostic use.” In creating the exemption, the NRC noted the importance of bringing an inexpensive and effective diagnostic tool to a large number of people, along with the minimum radiation contained in the capsule.
We may consider petitioning the NRC in a similar manner to make the device more widely available. As our imaging capsule imparts comparable radiation exposure to the Tri-Med device described above, and has the potential to be used widely for diagnosis, our imaging capsule may be a candidate for such an exemption.
Regulation in Europe, Japan and Other Countries
In the EU, a company that wishes to bring a medical device to market must CE mark the device following demonstration of conformity with the Essential Requirements laid down in Annex I to the Medical Devices Directive.
99
Compliance with these requirements entitles manufacturers to affix the CE mark of conformity to their medical devices, without which the medical devices cannot be commercialized in the EU. To demonstrate compliance with the Essential Requirements laid down in Annex I to the Medical Devices Directive and obtain the right to affix the CE mark of conformity to our medical devices, we and our products must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Apart from low risk medical devices (Class I with no measuring function and which are not sterile), in relation to which the manufacturer can issue an EC Declaration of Conformity based on self-assessment of the conformity of its products with the Essential Requirements laid down in the Medical Devices Directive, a conformity assessment procedure requires the intervention of a Notified Body, which is an organization designated by the competent authorities of a EU Member States to conduct conformity assessments. The Notified Body will typically audit and examine the Technical File that the manufacturer has created for the medical devices and the quality system for the manufacture, design and final inspection of the devices before issuing a CE Certificate of Conformity demonstrating compliance with the relevant Essential Requirements laid down in Annex I to the Medical Devices Directive or the quality system requirements laid down in the other Annexes to the Directive. Following the issuance of this CE Certificate of Conformity, the manufacturer is required to draw up an EC Declaration of Conformity and to affix the CE mark to the products covered by this CE Certificate of Conformity and the EC Declaration of Conformity.
We will be required to demonstrate that our products comply with the Essential Requirements laid down in Annex I to the Medical Devices Directive through a conformity assessment procedure. We cannot be certain that we will be able to fulfill the quality and performance requirements laid down in Annex I to the Medical Devices Directive and to obtain or maintain CE Certificates of Conformity for our products. If we are unable to obtain or maintain these CE Certificates of Conformity for our products, we will not be able to sell our products in any EU Member States nor in various other third countries where CE marking is accepted as evidence of compliance with relevant national laws.
In Japan, manufacturing and marketing medical devices are regulated by the Pharmaceutical Affairs Law, or PAL. In accordance with the PAL, manufacturers must obtain a license for manufacturing medical devices from the Ministry of Health, Labour and Welfare, or MHLW. A license is required for each manufacturing plant specified by an MHLW Ministerial Ordinance.
A licensed manufacturer is responsible only for manufacturing medical devices. In regard to the marketing of medical devices, the PAL specifies that a Marketing Authorization Holder, or MAH, licensed by MHLW is responsible for putting medical devices into marketplace. Licenses for marketing medical devices are divided into the following 3 types, which correspond to the classifications below:
|
|
•
|
No. 1 type license for marketing – Specially controlled medical devices (Class III, IV)
|
|
|
•
|
No. 2 type license for marketing – Controlled medical devices (Class II)
|
|
|
•
|
No. 3 type license for marketing – General medical devices (Class I)
|
Generally, the process for obtaining marketing clearance for medical devices in Japan ranges from twelve months for products with only very minor modifications from previously cleared product versions, to a few years in the case of a completely new device.
In order for us to market our products in countries other than the United States, the EU and Japan (which were described above), we must obtain regulatory approvals and comply with extensive safety and quality regulations in these countries. These regulations, including the requirements for approvals or clearance and the time required for regulatory review varies from country to country. It is customary that once a product has been cleared for sales in the US and is CE marked in the EU, many other countries will follow. Failure to obtain regulatory approval or clearance in any foreign country in which we plan to market our product may harm our ability to generate revenue and harm our business.
100
INDUSTRY
Colorectal Cancer
CRC is a cancer that develops in the colon or the rectum. The colon and rectum are parts of the digestive system, which is also called the gastrointestinal tract. The digestive system processes food for energy and rids the body of solid waste in the form of fecal matter or stool. After food is chewed and swallowed, it travels through the esophagus to the stomach. There, it is partially broken down and sent to the small intestine, where digestion continues and most of the nutrients are absorbed. The small intestine joins the large intestine in the lower right abdomen. The first and longest part of the large intestine is the colon, a muscular tube approximately five feet long. Water and mineral nutrients are absorbed from the food matter in the colon. Waste (feces) left from this process passes into the rectum, the final six inches of the large intestine, and is then expelled from the anus.
CRC usually develops slowly over a period of many years, generally beginning with a noncancerous polyp, which eventually becomes cancerous. A polyp is a growth of tissue that develops in the lining of the colon or rectum. Certain kinds of polyps, called adenomatous polyps or adenomas, are most likely to become cancerous. More than half of all individuals will eventually develop one or more adenomas.
Once cancer forms in the large intestine, it can grow through the lining and into the wall of the colon or rectum. Cancers that have invaded the wall can also penetrate blood vessels or lymph vessels, which are thin channels that carry away cellular waste and fluid. Cancer cells typically spread first into nearby lymph nodes, which are bean-shaped structures that help fight infections. Cancerous cells can also be carried in blood vessels to the liver or lungs, or can spread in the abdominal cavity to other areas, such as the ovary. The process through which cancer cells travel to distant parts of the body through blood or lymphatic vessels is called metastasis.
According to the ACS, CRC is the third most common cancer diagnosed and the second leading cause of death from cancer in the United States. The ACS estimates that in 2014, in the United States approximately 136,830 people are expected to be diagnosed with CRC and approximately 50,310 people will die from CRC. According to the WHO, in 2012, in Europe there were an estimated 471,000 cases of CRC and approximately 228,000 died from the disease and in Japan there were an estimated 112,675 cases of CRC and approximately 49,345 died from the disease. According to the WHO, in 2020 the expected numbers of cases of CRC are estimated to be 159,972 in the United States, 528,481 in Europe, 128,346 in Japan and 1,678,127 worldwide.
Costs associated with CRC treatment are very high and are usually assigned to three post-diagnostic time periods:
|
|
•
|
initial treatment – during the first three months following diagnosis;
|
|
|
•
|
maintenance care – between initial and terminal treatment; and
|
|
|
•
|
terminal treatment – during the final six months prior to death.
|
A recent study published by the Journal of the National Cancer Institute found that investing in some CRC screening programs could cut future, more expensive treatment costs in half.
CRC Screening
CRC screening can reduce death rates from CRC both by preventing the disease and by detecting it at earlier more treatable stages. CRC is one of the few cancers that can be prevented through screening because pre-cancerous polyps, from which colon cancers usually develop, can be identified and removed. The five-year survival rate is greater than 90% for CRC patients diagnosed at an early, localized stage. However, less than 40% of cases are currently diagnosed at that stage. According to the CDC, at least 6 out of every 10 deaths from CRC could be prevented if every adult age 50 years or older was screened regularly and that approximately 30,000 lives could be saved each year in the United States if the screening recommendations were followed. The ACS’s goal is to have 80% of those 50 years and older who are covered by the program screened by 2018.
The ACS recommends that men and women over the age of 50 receive an optical colonoscopy every 10 years or other structural test, such as sigmoidoscopy or virtual colonoscopy, every five years or alternatively, FOBT should be performed every year. According to the U.S. Census Bureau, as of mid-2014, there were projected to be approximately 91 million Americans aged 50-75 years. Assuming the longest screening interval of 10 years, the addressable annual U.S. patient population is at least 9.1 million.
101
Despite evidence supporting the effectiveness of CRC screening and the availability of various screening tests, in 2010, approximately 58.6% of Americans 50 years and older had been screened for CRC, according to the CDC. This compares unfavorably to 72% to 83% for screening of other types of cancers, such as breast and cervical cancer. The CRC screening rates are even lower among people aged 50 to 64 according to the CDC.
In the 2008 ACS guidelines for CRC screening in average risk-adults, titled “A Joint Guideline from the American Cancer Society, the U.S. Multi-Society Task Force on CRC, and the American College of Radiology”, screening tests were grouped into those that primarily detect cancer early and those that can detect both early cancer and adenomatous polyps. The latter provide greater potential for prevention through removal of polyps, or polypectomy. The panel recommended that, when possible, clinicians should make patients aware of the full range of screening options, but at a minimum they should be prepared to offer patients a choice between a screening test that is effective at both early cancer detection and cancer prevention through the detection and removal of polyps and a screening test that is primarily effective at early cancer detection. It was the opinion of the panel that colon cancer prevention should be the primary goal of CRC screening. Tests that are designed to detect both early cancer and adenomatous polyps should be encouraged if resources are available and patients are willing to undergo an invasive test. These tests include the partial or full structural exams mentioned above. These tests require bowel preparation and an office or hospital visit and have various levels of risk to patients. These tests also have other limitations, including greater patient requirements for successful completion, and potential harm to the patients.
Structural Methods – Tests that Detect both Polyps and Cancer Tumors
Optical Colonoscopy
Optical colonoscopy, one of the most commonly performed medical procedures in the United States, allows direct photographic inspection of the entire length of the colon, as well as same-session biopsy sampling or definitive treatment by polypectomy in the case of pre-cancerous polyps and some early-stage cancers. Optical colonoscopy is capable of examining the entire colon and rectum. At present, a colonoscopy once every 10 years is an acceptable option for CRC screening in average-risk adults beginning at age 50 years, as recommended by the U.S. Preventive Services Task Force.
Patients are generally required to adopt a liquid diet one or two days before the examination, followed by ingestion of oral lavage solutions, saline laxatives, or pills to stimulate bowel movements until the bowel is clean. Proper bowel preparation is a critical element in the accuracy and cost-effectiveness of screening with optical colonoscopy. It is common, but not necessary, for a patient to receive a mild sedative prior to the procedure.
A principal benefit of optical colonoscopy is that it allows for a full structural examination of the colon and rectum in a single session and for the detection of colorectal polyps and cancers accompanied by biopsy or polypectomy. All other forms of screening, such as FOBT, FIT, CTC and stool DNA, require optical colonoscopy as a follow-on procedure to conduct a biopsy or polypectomy if a positive result is obtained. Patient surveys indicate that those willing to undergo invasive testing tend to choose optical colonoscopy as their preferred test. In addition to the dietary preparation and bowel cleansing, optical colonoscopy usually requires a day dedicated to the examination, and because of sedation, a chaperone for transportation. Surveys indicate that a significant percentage of adults prefer non-invasive options for CRC screening.
Optical colonoscopy is not an infallible procedure. Controlled studies have shown the optical colonoscopy miss rate for large adenomas (=10 mm) to be 6% to 12%, which is operator dependent. The reported optical colonoscopy miss rate for cancer is about 5%. Optical colonoscopy can result in significant side effects, most often associated with polypectomy. The most common serious complication is post-polypectomy bleeding. Another significant risk associated with optical colonoscopy is perforation of the bowel wall.
Formal quality-assurance programs do not exist, and the current reimbursement system for optical colonoscopy does not reward careful examination. Instead, it tends to reward rapidly performed examinations and examinations sometimes repeated at unnecessarily short intervals.
102
CTC – CT Colonography
Computed tomography colonography (CTC), also referred to as virtual colonoscopy, is an imaging examination of the entire colon and rectum. CTC uses computed tomography (CT) to acquire images and advanced two-dimensional and three-dimensional image display techniques for interpretation. Since its introduction in the mid-1990s, there have been rapid advancements in CTC technology.
Computer imaging graphics allow for the visualization of three-dimensional endoscopic flight paths through the inside of the colon, which are simultaneously viewed with interactive two-dimensional images. The integrated use of the three-dimensional and two-dimensional techniques allows for ease of polyp detection, as well as the characterization of lesion density and location. CTC provides a time-efficient procedure with good accuracy and minimal invasiveness. However, the required colon preparation is similar to the cleansing required prior to optical colonoscopy. No sedation or recovery time is required and a chaperone is not needed to provide transportation after the procedure.
At this time, reimbursement for screening CTC is limited, although 47 U.S. states now offer Medicare reimbursement for diagnostic CTC. However, because reimbursement for screening is still uncommon, the current professional capacity to deliver CTC is also limited. Capacity is expected to increase when third-party payers expand reimbursement for screening.
There is controversy surrounding the long-term potential harms associated with radiation dose effects from repeated CT examinations at shorter intervals, usually less than three years. One aspect of this controversy relates to risk-estimation models, and the other pertains to the long-term risk of cancer from single and repeated medical imaging exposures. While current estimates of the potential cancer risk related to low-dose radiation exposures during medical procedures derive from models based on long-term outcomes in survivors of acute radiation doses from atomic weapons, there is disagreement over whether this model truly is applicable to periodic exposures from medical imaging. Since CTC is a minimally invasive test, the risk for colonic perforation during screening is extremely low.
In addition to radiation risks, CTC requires the same full bowel preparation and restricted diet as an optical colonoscopy, which may decrease patient adherence. CTC also has the burdensome effect of requiring insufflation of the colon, which causes discomfort to patients.
CTC is similar to endoscopy and double contrast barium enema (DCBE) where effectiveness of the procedure is operator-dependent, and thus initiatives towards training and certification are important.
Sigmoidoscopy
Sigmoidoscopy, also known as flexible sigmoidoscopy or FSIG, is an endoscopic procedure that examines the lower part of the colon lumen. The exam may be performed with a variety of endoscopic instruments, including a standard 60 cm sigmoidoscope. FSIG is typically performed without sedation and with a more limited bowel preparation than standard optical colonoscopy. Since sedation is not required, it can be performed in office-based settings and by non-physicians, including properly trained nurses or physician assistants. Since FSIG examines only the distal third of the colon, not all of the polyps or tumors can be detected at the time of screening. The examination reaches the splenic flexure, which is about 40 cm from the anus. If one polyp is detected in that distal segment, the patient is referred to full optical colonoscopy since the probability for additional polyps in the rest of the colon is high.
The primary limitation of FSIG is that it does not examine the entire colon but only the rectum, sigmoid, and descending colon. Another limitation of FSIG is that there may be considerable variation both in the depth of insertion of the sigmoidoscope. Adenoma detection by FSIG depends on the skill of the examiner, which may reduce the effectiveness of FSIG for CRC screening. FSIG is sometimes performed by primary care physicians who are generally less trained and experienced than a gastrointestinal specialist who performs hundreds or thousands of examination per year. The complications of FSIG include colonic perforation, even if no biopsy or polypectomy is performed, though this rarely occurs.
Patients who had also undergone unsedated FSIG screening were more than twice as likely to say that they would not return for additional screening compared with those who had undergone optical colonoscopy with sedation.
103
Stool Test Methods – Tests that Primarily Detect Cancer
Stool tests of various types do not generate structural information of the colon but provide an indication on the presence of blood or DNA changes in the colon. As such, they are designed to detect early stage cancer rather than pre-cancerous polyps. Although early stage detection of cancer is important, there is a clear advantage for technologies that generate structural information of the colon, which enable detection of pre-cancerous polyps.
FOBT- Fecal Occult Blood Test
Fecal occult blood test (FOBT) is the most common stool blood test in use for CRC screening and the only CRC screening test for which there is evidence of efficacy from prospective, randomized controlled trials. This test involves the collection of two samples from each of three consecutive bowel movements. Collection of all six samples is important because test sensitivity improves with each additional stool sample. FOBT is generally regarded as having a high specificity for bleeding polyps but low specificity for polyps that do not bleed. The sensitivity and specificity of a FOBT has been shown to be highly variable and varies based on a number of factors. These include: the brand or variant of the test, specimen collection technique, number of samples collected per test, whether or not the stool specimen is rehydrated (i.e., adding a drop of water to the slide window before processing), and variations in interpretation, screening interval and other factors.
Annual screening with high-sensitivity FOBT has been shown in published, peer-reviewed literature to detect a majority of prevalent CRC in an asymptomatic population and is an acceptable option for colorectal screening in average-risk adults aged 50 years and older. Any positive test result should be followed up with optical colonoscopy.
FIT - Fecal Immunochemical Test
The concept of applying an immunochemical method to testing stool for occult blood was first proposed in the 1970s, and commercialization of the technology began in the 1980s. However, it is more expensive than FOBT.
Fecal immunochemical test (FIT) has several technological advantages when compared with FOBT. FIT detects human globin, a protein that along with heme constitutes human hemoglobin. Thus, FIT is more specific for human blood than standard FOBT tests. Further, unlike FOBT, FIT is not subject to false-negative results in the presence of high-dose vitamin C supplements. In addition, because globin is degraded by digestive enzymes in the upper gastrointestinal tract, FIT has higher specificity for lower gastrointestinal bleeding. Finally, the sample collection for some variants of FIT are less demanding of patients than FOBT, requiring fewer samples and less direct handling of stool.
The spectrum of benefits, limitations, and harms is similar to a FOBT. An additional advantage of FIT over FOBT appears to be fewer demands on patients undergoing FIT compared with FOBT. FIT does not require a restricted diet, and the sampling procedures for some forms of FIT are less demanding. Thus, patients’ compliance is much higher leading to improved cancer detection rate.
Stool DNA
Stool DNA, or sDNA, allows for the testing of altered DNA that may predict the presence of CRC. Adenoma and carcinoma cells that contain altered DNA are continuously shed into the large bowel lumen and passed in the feces. Because DNA is stable in stool, it can be differentiated and isolated from bacterial DNA found in the feces. No single gene mutation is present in cells shed by every adenoma or cancer.
A multi-target DNA stool assay is required to achieve adequate sensitivity. Several studies on the sensitivity and specificity of sDNA testing for CRC detection have been published utilizing a panel of DNA markers. Test sensitivity for CRC in these studies ranged from 52% to 91%.
The primary benefit of sDNA is that this methodology has acceptable sensitivity for CRC and is built upon the concept of detecting molecular markers associated with advanced colorectal neoplasia. It is not dependent on the detection of occult bleeding, which is intermittent and nonspecific, and it requires only a single stool collection. Further, newer versions may have better sensitivity as more is learned about markers that are common across all prevalent CRC, as well as advanced adenomas. sDNA sampling is noninvasive and does not cause the patient physical harm.
104
Patient and provider acceptance of this technique appears to be high, with available data indicating that sDNA is preferred over other tests by some individuals, and among others testing with sDNA, it is at least as acceptable to patients as testing with FOBT.
A limitation of sDNA testing for the detection of CRC and large adenomas is that test sensitivity is based on a panel of markers that appears to identify the majority of CRC cases, but not all. Further, it is not known what proportion of advanced adenomas is identified with the current commercial version of the sDNA test. Other potential limitations that have considerable implications for cost effectiveness are the unit cost of the current test, which is much higher than the other stool tests, and the frequency with which the test should be performed, which is uncertain. On August 11, 2014, the FDA approved a new sDNA screening test called Cologuard for colorectal cancer. The test, which was developed by Exact Sciences, identifies 11 molecular features including blood and tumor-associated DNA in the stool of patients. In a major clinical trial supporting its approval, Cologuard demonstrated greater sensitivity than FIT. The overall sensitivity for CRC of any stage was 92% for Cologuard compared to 73.8% for FIT. Sensitivity of the Cologuard for the detection of pre-cancerous polyps that are greater than 10 mm was 42%. Cologuard may be a strong alternative to older technologies due to the higher sensitivity. One potential limitation of Cologuard is the specificity, which is lower than FIT. The specificity rate determined in the clinical trial supporting its approval was 86.6% for Cologuard versus 94.9% for FIT. If used alone, Cologuard might lead to unnecessary colonoscopies as patients are referred for more extensive imaging when no cancer is present. Increasing the specificity by also performing a FIT would increase patient costs and minimize the impact of the new test.
Optics-Based CapsuleEndoscopy
Certain companies are developing or commercializing optics-based capsule endoscopy systems. Given Imaging, an Israeli-based company that was acquired by Covidien plc (NYSE: COV) in February 2014, has developed visualization capsules for the detection of disorders of the esophagus, small bowel and colon. It launched the PillCam Colon capsule in Europe in 2007 and has limited sales. In early 2014, the FDA granted approval for optical capsule endoscopy for colon cancer screening only for patients after incomplete colonoscopy. However, this technology requires bowel cleansing even to a greater degree than for regular colonoscopy, which can result in dehydration and can, in turn, lead to cancellation of the procedure in certain cases. Other companies, including Olympus and Intromedic, are developing similar approaches for optical capsule endoscopy.
The following table outlines some of the benefits and limitations of the currently available CRC screening tests.
|
Test
|
Benefits
|
Performance and Complexity*
|
Limitations
|
Test Time Interval*
|
Cost Range*
|
|
Flexible Sigmoidoscopy
|
* Fairly quick and safe
* Few complications
* Minimal bowel preparation
* Minimal discomfort
* Does not require sedation or a specialist
|
Performance High for lower one-third of the colon
Complexity Intermediate
|
* Views only lower one-third of colon
* Cannot remove large polyps
* Small risk of infection or bowel tear
* Slightly more effective when combined with annual FOB testing
* Optical colonoscopy needed if abnormalities are detected
|
5 years
|
Intermediate
|
|
Optical Colonoscopy
|
* Examines entire colon
* Can biopsy and remove polyps
* Can diagnose other diseases
* Required for abnormal results from all other tests
|
Performance
Highest
Complexity
High
|
* Can miss some polyps and cancers
* Full bowel preparation needed
* Can be expensive
* Sedation of some kind usually needed, necessitating chaperone
* Patient may miss a day of work
* Highest risk of bowel tears or infection, compared to other tests
|
10 years
|
High
|
|
Double Contrast Barium Enema
|
* Can usually view entire colon
* Few complications
* No sedation needed
|
Performance
High
Complexity
High
|
* Can miss some small polyps and cancers
* Full bowel preparation needed
* Some false positive test results
* Cannot remove polyps
* Exposure to low dose radiation
* Optical colonoscopy necessary if abnormalities are detected
|
5 years
|
Intermediate
|
105
|
Computed Tomographic Colonography
|
* Full bowel preparation
* Fairly quick
* Few complications
* No sedation needed
* Noninvasive
|
Performance
High
Complexity
Intermediate
|
* Can miss some polyps and cancers
* Full bowel preparation needed
* Cannot remove polyps
* Exposure to low dose radiation
* Optical colonoscopy necessary if abnormalities are detected
|
5 years
|
High
|
|
Fecal Occult Blood Test
|
* No bowel preparation
* Sampling is done at home
* Low cost
* Noninvasive
|
Performance
Intermediate for cancer
Complexity
Lowest
|
* Requires multiple stool samples
* Will most polyps and some cancers
* May produce false-positive test results
* Pre-test dietary limitations
* Slightly more effective when combined with a flexible sigmiodoscopy every 5 years
* Optical colonoscopy necessary if abnormalities are detected
|
Annual
|
Low
|
|
Stool DNA Test
|
* No bowel preparation
* Sampling is done at home
* Requires a single stool sample
* Noninvasive
|
Performance
Intermediate for cancer
Complexity
Low
|
* Will miss most polyps and some cancers
* High cost compared to other stool tests
* New Technology with uncertain interval between testing
* Optical colonoscopy necessary if abnormalities are detected
|
CMS proposes to cover Colo-Guard Test once every 3 years for beneficiaries who meet certain criteria
|
Intermediate
|
106
|
Fecal Immunochemical Test (FIT)
|
* No direct risk to the colon
* No bowel preparation
* No pre-test dietary restrictions
* Sampling done at home
|
Performance
Intermediate for cancer
Complexity
Low
|
* Will miss most polyps and some cancers
* May produce false-positive test results
* Optical colonoscopy necessary if abnormalities are detected
|
Annual
|
Low
|
|
Capsule Endoscopy
|
* Noninvasive
* No hospitalization
* No sedation
* No pain
|
Performance Good for medium and large size polyps
Complexity
High
|
* Rigorous bowel preparation needed
* Additional drugs and fluids during the 10 hours passage time
* Uncontrolled propulsion in the colon
* Cannot remove large polyps
* Intense preparation and additional drugs may affect kidneys and mineral balance
* Optical colonoscopy needed if abnormalities are detected
|
Uncertain
|
High
|
* Complexity involves patient preparation, inconvenience, facilities and equipment needed, and patient discomfort
New Technologies
Biomarkers
Biomarkers to detect CRC are currently under development and in clinical testing by several companies. A biomarker is a substance, usually in the blood, used as an indicator of a biologic state. It is a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. There has been increasing interest in the medical community to the entrance of biomarkers to detect cancer and other diseases.
Blood-Based Epigenetic Biomarker
Epigenetics provides an additional layer of regulation in addition to DNA sequence information, and some epigenetic changes can be inherited from one generation to the next. DNA methylation is a core epigenetic mechanism where cytosine residues on DNA are tagged with a methyl group. Clusters of cytosines sometimes occur in the upstream regulatory sequences of genes, creating ‘CpG islands’ that are hypo- or hypermethylated. Methyl-binding proteins can interact with the methylated DNA to facilitate gene repression or silencing. There are many hypermethylated genes associated with CRC that influence key signaling pathways. Adenomatous polyposis coli (APC) is the most commonly mutated gene in CRC, and is known to be silenced via hypermethylation. APC normally facilitates the destruction of transcription factor responsible for cell proliferation and migration, and the loss of APC can upregulate those and other processes.
A molecular diagnostics company, Epigenomics, is utilizing an epigenetic marker to create a blood-based test for the early detection of CRC in patients who are not compliant to existing tools such as optical colonoscopy or stool tests. The test is called Epi proColon® and detects the methylated form of Septin9 (SEPT9), a gene that is typically unmethylated in normal colorectal cells. The blood-based test eliminates the need for upfront invasive procedures or handling of stool samples. In a large clinical study, Epi proColon® demonstrated sensitivity of approximately 68%, which mean that 68 out of every 100 patients with CRC were correctly identified using Epi proColon®. Positive results from the test are followed up by traditional methods such as a optical colonoscopy to confirm the findings and remove any precancerous polyps. Epi proColon® is currently marketed in Europe and is under regulatory review in the United States.
107
On March 27, 2014, an FDA Advisory Committee provided recommendations for the approval of Epigenomics’ premarket approval (PMA) application for Epi proColon®. The committee voted 9 to 0 with one abstention in favor of the safety profile. This is consistent with the minimal requirements of the test, which include a routine blood draw to collect a sample for analysis. The panel chairperson voted unfavorably to break a 5-5 split with regard to the effectiveness of the product, citing concerns that long-term data supporting the benefits of the test are unavailable. Epigenomics has plans to conduct a post-approval study to address this issue. Finally, the Advisory Committee voted 5-4 in support of a positive risk/benefit profile for the test. The FDA will continue to evaluate Epi proColon®.
On June 2, 2014, the FDA issued a response letter to Epigenomics related to its PMA for its blood-based CRC test indicating that the PMA application does not contain sufficient evidence to support approval of its test.
Third-Party Reimbursement
Reimbursement in the United States
In the United States, healthcare providers that purchase medical devices generally rely on third-party payors, such as Medicare, Medicaid, private health insurance plans and health maintenance organizations, to reimburse all or a portion of the cost of the devices, as well as any related healthcare services utilizing the devices.
Coverage is not guaranteed simply because a product has received FDA clearance or approval. Medicare’s general definition of a medically necessary service is one that is reasonable and necessary for the diagnosis or treatment of an illness or injury, or that improves the functioning of a malformed body member. In order to be eligible for reimbursement, a device must be proven to be cost-effective, demonstrating potential decrease in spending to the U.S. health economy.
According to various studies and publications, a key criterion for to reimbursement for colon cancer screening is patient adherence (for instance, “Cost-Effectiveness of Colonoscopy in Screening for CRC,” Annals of Internal Medicine, October 17, 2000 vol. 133 no. 8 573-584). Adherence is strongly affected by patients’ willingness to use the device as a screening tool for CRC. Several models have been designed to demonstrate the cost effectiveness of optical colonoscopy, CTC, fecal testing and optical capsule endoscopy. Today, several technologies achieved Medicare coverage for CRC Screening, including: FOBT / FIT, Flexible Sigmoidoscopy, Optical Colonoscopy and Barium Enema. In August 2014, the Centers for Medicare and Medicaid Services issued a proposed decision memorandum for CRC screening using Cologuard, a multitargeted stool DNA test. CTC (virtual colonoscopy) is not yet eligible for coverage.
In 2009, the Centers for Medicare and Medicaid Services issued a decision memorandum rejecting federal reimbursement for CTC screening for CRC. Their main argument for the decision was that based on available evidence, screening with CTC would not necessarily result in cost saving, at least at current screening compliance rates. CTC was not seen as a tool which could potentially increase patients’ adherence. This procedure involves bowel preparation, as well as insufflations of the colon and the exposure of patients to very significant amount of radiation.
An important European study (C. Hassan et al, “Cost Effectiveness of Optical capsule endoscopy,” Endoscopy 2008, 40, 414-421) assessed the potential cost effectiveness of screening with optical capsule endoscopy and compared the cost-effectiveness with that of optical colonoscopy. Effectiveness of screening was measured in terms of life-years saved through prevention or down staging of CRC. The conclusion was that adherence to optical capsule endoscopy may be presumed to be substantially higher than that of optical colonoscopy.
Third-party payors in the United States began issuing coverage policies for optical capsule endoscopy in early 2002. Initially, all reimbursement policies provided coverage for optical capsule endoscopy of the small bowel only for the diagnosis of obscure gastrointestinal bleeding. Subsequently, reimbursement coverage has been expanded to include other diagnoses and as of December 31, 2012, approximately 220 million people in the United States are covered with most reimbursement policies providing coverage for a number of small bowel indications, including obscure gastrointestinal bleeding, suspected Crohn’s disease, suspected small bowel tumors and other small bowel pathologies.
In 2013, for procedures performed in a physician’s office, the national average global fee paid by Medicare under the CPT code for optical capsule endoscopy of the small bowel was $971. For procedures performed in an outpatient hospital setting, the national average physician fee paid by Medicare was $197 and the national average payment rate to the hospital for the technical component was $751. For optical capsule endoscopy of the esophagus, in 2013, the national average global fee paid by Medicare for a procedure in a physician’s office was $812. The national average physician fee paid by Medicare in an outpatient hospital setting was $54 and the national average payment rate to the hospital for the technical component was $927.
108
Below are the fees paid by Medicare in 2014 under the CPT code for screening optical colonoscopy:
Payment for Physician’s Services:
|
Code
|
Description
|
Payment (1)
|
|||
|
G0105
|
Colorectal cancer screening; colonoscopy on individual at high risk
|
$
|
208.83
|
||
|
G0121
|
Colorectal cancer screening; colonoscopy on individual not meeting criteria for high risk
|
$
|
208.83
|
||
Payment for the Hospital:
|
Code
|
Short Description
|
APC
|
Payment (2)
|
||||
|
G0105
|
Colorectal scrn; high risk ind
|
0158 (Colorectal cancer
|
$
|
646.73
|
|||
|
G0121
|
Colon ca scrn not high risk ind
|
screening; Colonoscopy)
|
|||||
Private Payers
The national average cost of an optical colonoscopy may range from $2,000 to $3,000. The cost also depends on the state or city in which the procedure takes place. Currently, there is no optical capsule endoscopy available for the colon in the United States, nor is there a CPT code for such capsule or related method of screening. There can be no assurance that coverage will be obtained in the near future or at all. Third-party payors may deny coverage if they determine that a procedure was not reasonable or necessary as determined by the payor, was experimental or was used for an unapproved indication. During the past several years, the major third-party payors have substantially revised their reimbursement methodologies in an attempt to contain or reduce their healthcare reimbursement costs.
Reimbursement rates vary depending on the third-party payor and individual insurance plan involved, the procedure performed and other factors. Medicare reimbursement for in-patient hospital services is based on a fixed amount per admission based on the patient’s specific diagnosis and the procedure performed during the hospital stay. As a result, any illness to be treated or procedure to be performed in an in-patient setting will be reimbursed only at a prescribed rate set by the government.
In countries outside the United States, coverage for CRC screening is obtained from various sources, including governmental authorities, private health insurance plans, and labor unions. Although not as prevalent as in the United States, health maintenance organizations are emerging in certain European countries. Coverage systems in international markets vary significantly by country and, within some countries, by region. Coverage approvals must be obtained on a country-by-country or region-by-region basis. In general, the process of obtaining coverage approvals has been slower outside of the United States.
Coverage Outside the United States
In countries outside the United States, coverage is obtained from various sources, including governmental authorities, private health insurance plans, and labor unions. In some countries, private insurance systems may also offer payments for some therapies. Although not as prevalent as in the United States, health maintenance organizations are emerging in certain European countries. Coverage systems in international markets vary significantly by country and, within some countries, by region. Coverage approvals must be obtained on a country-by-country or region-by-region basis.
109
Executive Officers and Directors
The following table sets forth information for our executive officers and directors as of the date of this prospectus. Unless otherwise stated, the address for our directors and executive officers is c/o Check-Cap Ltd., Abba Hushi Avenue, P.O. Box 1271, Isfiya, 30090, Mount Carmel, Israel.
|
Name
|
Age
|
Position(s)
|
||
|
Guy Neev
|
47
|
Chief Executive Officer and Director
|
||
|
Lior Torem
|
45
|
Chief Financial Officer
|
||
|
Yoav Kimchy
|
53
|
Chief Technology Officer and Director
|
||
|
Alex Ovadia
|
53
|
Vice President, Research and Development
|
||
|
Tomer Kariv
|
53
|
Chairman of the Board of Directors
|
||
|
Walter L. Robb
|
86
|
Director
|
||
|
Richard Stone
|
71
|
Director
|
||
|
Alon Dumanis
|
64
|
Director
|
Prior to the closing of this offering we will establish an Audit Committee and a Compensation Committee, each in compliance with the requirements of the Israeli Companies Law and NASDAQ Capital Market. Prior to the closing of this offering we will also make a determination as to which of our directors are “independent directors” under the rules of the NASDAQ Stock Market and the U.S. Securities and Exchange Commission.
Executive Officers and Directors
Guy Neev has served as our chief executive officer since our inception in April 2009 and has served as a member of our board of directors since April 2009. Since March 2008, Mr. Neev has served as the chief executive officer of Check-Cap LLC and as a member of the board of directors of Check Cap Ltd. (Delaware), the manager of Check-Cap LLC. Prior to assuming his position as Check-Cap LLC’s chief executive officer, Mr. Neev served as an executive consultant to Check-Cap LLC and as a part-time chief executive officer for several early stage medical devices companies in Israel. Between 2004 and 2007, Mr. Neev served as chief executive officer and vice president of business development of Cappella Inc., a medical devices company developing stents for cardiovascular applications, after serving as a cardiovascular business unit manager at Boston Scientific Corporation from 2003 to 2004. Prior to that, from 1997 to 2003, Mr. Neev served as business unit manager at Azimuth Technologies Ltd. and as chief executive officer of its subsidiary, Waycomm Wireless Solutions Ltd. Mr. Neev also served as a major in the Israeli Air Force. Mr. Neev holds a B.A. degree in economics from Bar Ilan University, Israel and a M.I.B. (Masters in International Business) degree from Norges Handelshoyskole, Bergen, Norway. Mr. Neev was appointed as a director pursuant to our articles of association in effect prior to the closing of this offering.
Lior Torem has served as our chief financial officer, on a part-time basis, since May 2010. Since November 2008, Mr. Torem has also served as the chief financial officer, on a part-time basis, of Superfish, Inc., a developer of visual search technology for searching images. Prior to that and beginning in January 2010, Mr. Torem served as an executive consultant to the company. Prior to that, from 2003 to 2009, Mr. Torem served as vice president of finance at Actelis Networks Inc., a provider of high performance, scalable broadband over copper solutions, and from 2001 to 2003, served as vice president of finance at its subsidiary Actelis Networks Ltd. Prior to that, from 1997 to 2000, Mr. Torem served as corporate controller at Mentergy Ltd., a publicly-traded company that provides e-learning solutions and satellite communications services. Mr. Torem holds a B.A. degree in accounting and economics from Bar Ilan University, an MBA degree from Haifa University and he is a Certified Public Accountant in Israel.
Yoav Kimchy has served as our chief technology officer since our inception in April 2009 and a member of our board of directors since April 2009. Dr. Kimchy founded Check-Cap LLC in December 2004, serving as its president and chief executive officer until March 2008, and as its president and chief technical officer since March 2008. Dr. Kimchy has also served as a member of the board of directors of Check-Cap Ltd. (Delaware), the manager of Check-Cap LLC, since December 2004. Between 2005 and 2012, Dr. Kimchy also served as our vice president of research and development. Between 2000 and 2003, Dr. Kimchy served as the vice president of research and development of V-Target Ltd. (Israel), a medical device company developing gamma imaging applications. Prior to that, from 1998 to 2000, Dr. Kimchy served as director of cardiovascular research at Impulse Dynamics Ltd. (Israel), a medical device company developing a unique therapeutic pulsing technology for chronic heart failure. From 1994 to 1998, Dr. Kimchy served as a systems engineer and algorithm specialist for an Israeli government contract project with the Israeli Navy. Dr. Kimchy also served as a lieutenant in the Israeli Navy. Dr. Kimchy holds a B.Sc. degree in physics and mathematics from the Hebrew University of Jerusalem, an M.Sc. degree in biomedical engineering from Tel Aviv University and a PhD from the Technion-Israel Institute of Technology. Dr. Kimchy was appointed as a director pursuant to the appointment rights of the holders of our ordinary shares under our articles of association in effect prior to the closing of this offering.
110
Alex Ovadia has served as our vice president of research and development since January 2013. Prior to that, between 2001 and 2012, Mr. Ovadia served in various positions as global director and senior manager for CT research and development at Philips Healthcare. In these capacities he led global organizations including systems engineering, physics, system verification and hardware development. Mr. Ovadia was also appointed as a member of Global CT R&D staff and member of management of Philips Medical System Technologies Ltd. Prior to that, from 1990 to 2001, Mr. Ovadia served as project/systems engineering manager for large scale military projects, mainly aircraft upgrades for U.S. and European governments performed by Elbit Systems Ltd. Mr. Ovadia holds a BSc degree in electrical engineering from the Technion-Israel Institute of Technology.
Tomer Kariv has served as a member of our board of directors since June 2009 and as the chairman of our board of directors since July 2010. Mr. Kariv has served as a member of the board of directors of Check Cap Ltd. (Delaware) and a manager of Check-Cap LLC since March 2008. Mr. Kariv is the co-founder and since December 2004, has served as Chief Executive Officer of Pontifax, a group of Israeli-based life sciences venture funds focusing on investments in bio-pharmaceutical and med-tech technologies. Mr. Kariv has also served as an active board member of many of the funds’ portfolio companies, assuming a special responsibility for strategic planning. Among others, since March 2008, Mr. Kariv has served as a board member of Macrocure Ltd, Arno Therapeutics Inc. and Medical Compression Systems Ltd. During the 10 years prior to establishing Pontifax, Mr. Kariv played a key role in investing, managing and nurturing technology driven companies and startups and has held senior management positions at top Israeli financial institutions. Mr. Kariv practiced law with Sullivan & Cromwell, a leading corporate law firm in New York, and holds a B.A. degree in Economics from Harvard University and a J.D. from Harvard Law School. Mr. Kariv was appointed as a director pursuant to the appointment rights of Pontifax under our articles of association in effect prior to the closing of this offering.
Walter L. Robb has served as a member of our board of directors since May 2009. Dr. Robb has served as a member of the board of directors of Check Cap Ltd. (Delaware) and a manager of Check-Cap LLC since February 2005. Dr. Robb is the general partner of Counter Point Ventures a venture capital firm established in 2007, and served as the interim chief executive officer of Cyclics Corporation, which manufactures and supplies resins and thermoplastic tooling products, in 2009. Since 1993, Dr. Robb has served as a management consultant and president of Vantage Management, Inc. a private investment and consulting firm, and has been a member of the boards of directors of Celgene Corporation since 1992 and Mechanical Technology, Inc. since 2000. Dr. Robb has also served as a director in several start-up companies over the last 20 years. Until 1993, Dr. Robb served as senior vice president and director for corporate research and development for General Electric, and served on General Electric’s Corporate Executive Council from 1986 to 1993. Dr. Robb was the director of GE Medical Systems from 1973 to 1986. In September 1994, Dr. Robb received the National Medal of Technology from President Clinton for leadership in the CT and MR Imaging Industry. Dr. Robb holds a BS degree in chemical engineering from Penn State University and an MS degree and PhD in chemical engineering, both from the University of Illinois. Dr. Robb was appointed as a director pursuant to the appointment rights of the holders of our former Series A preferred shares under our articles of association in effect prior to the closing of this offering.
Richard Stone has served as a member of our board of directors since May 2009. Professor Stone has served as a member of the board of directors of Check Cap LLC since August 2005. Professor Stone began his legal career in private practice and in 1969, was appointed to the position of Assistant Solicitor General of the United States, an office which he held until 1973. In 1974, Professor Stone was appointed to the faculty of Columbia Law School, where he still teaches today primarily in the area of taxation. Professor Stone holds an A.B. from Harvard College and an LL.B. degree from Harvard Law School. Professor Stone was appointed as a director pursuant to the appointment rights of the holders of our former Series B preferred shares under our articles of association in effect prior to the closing of this offering.
111
Alon Dumanis has served as a member of our board of directors since 2010. Dr. Dumanis has over 30 years of experience in international business development, entrepreneurial, and technological management of high-technology companies, the domains of which, include information, aviation, electronics, life science and emerging technology. Dr. Dumanis has managed multibillion dollar research and development programs and many large scale projects in engineering, security and information. Since 2001, Dr. Dumanis has served as the chief executive officer of Docor International Management Ltd., a Dutch investment company and since 2010, has served as the chief executive officer of its parent company, Crecor B.V. Dr. Dumanis is currently the chairman of the boards of directors of Xsight Systems Ltd., Softlib Ltd. and DNR Imaging Systems Ltd. and is a member of the board of directors of Spectronix Ltd. (TASE-SPCT), Nova Measuring Instruments Ltd. (NASDAQ-NVMI), Aposense Ltd. (TASE –APOS), Collplant Holdings Ltd. (TASE-CLPT) and other hi-technology companies of Docor’s investment portfolio. Dr. Dumanis is a former member of the board of directors of El Al Israel Airlines Ltd. (TASE –ELAL), Tadiran Communications Ltd. (TASE-TDCM) and Inventech Investments Co. Ltd. (TASE-IVTC). From 1996 to 2000, Dr. Dumanis was Head of Material Command for the Israel Air Force, where he held the rank of Brigadier General. Dr. Dumanis holds a BS degree and MS degree in Aeronautical Engineering both from the Technion-Israel Institute of Technology, and a PhD in Aeronautical Engineering from Purdue University in Indiana USA. Dr. Dumanis was appointed as a director pursuant to the appointment rights of Docor International B.V. under our articles of association in effect prior to the closing of this offering.
Arrangements Concerning Election of Directors; Family Relationships
Our current board of directors consists of six directors. Pursuant to our articles of association in effect prior to this offering, certain of our shareholders had rights to appoint members of our board of directors. See “Management — Executive Officers and Directors.” All rights to appoint directors and observers will terminate upon the closing of this offering, although currently-serving directors that were appointed prior to this offering will continue to serve pursuant to their appointment until the next annual meeting of shareholders.
We are not a party to, and are not aware of, any voting agreements among our shareholders. In addition, there are no family relationships among our executive officers and directors.
Corporate Governance Practices
Under the Israeli Companies Law, companies incorporated under the laws of the State of Israel whose shares are publicly traded, including companies with shares listed on the NASDAQ Capital Market, are considered public companies under Israeli law and are required to comply with various corporate governance requirements relating to such matters as external directors, the audit committee, the compensation committee and an internal auditor. This is the case even if our shares are not listed on the Tel Aviv Stock Exchange. These requirements are in addition to the corporate governance requirements imposed by the Listing Rules of the NASDAQ Stock Market and other applicable provisions of U.S. securities laws to which we will become subject (as a foreign private issuer) upon the closing of this offering and the listing of our ordinary shares on the NASDAQ Capital Market. Under the Listing Rules of the NASDAQ Stock Market, a foreign private issuer, such as us, may generally follow its home country rules of corporate governance in lieu of the comparable requirements of the Listing Rules of the NASDAQ Stock Market, except for certain matters including (among others) the composition and responsibilities of the audit committee and the independence of its members within the meaning of the rules and regulations of the U.S. Securities and Exchange Commission. We intend to rely on this “home country practice exemption” solely with respect to the following items:
|
|
•
|
Independent directors. Israeli law does not require that a majority of the directors serving on our board of directors be “independent,” as defined under NASDAQ Listing Rule 5605(a)(2), and rather requires we have at least two external directors who meet the requirements of the Israeli Companies Law, as described above under “Management—Board Practices—External Directors.” We are required, however, to ensure that all members of our audit committee are “independent” under the applicable NASDAQ and SEC criteria for independence (as we cannot exempt ourselves from compliance with that SEC independence requirement, despite our status as a foreign private issuer), and we must also ensure that a majority of the members of our audit committee are “unaffiliated directors” as defined in the Israeli Companies Law. Furthermore, Israeli law does not require, nor do our independent directors conduct, regularly scheduled meetings at which only they are present, which the NASDAQ Listing Rules otherwise require. |
112
|
|
•
|
Nomination of our directors. Israeli law and our amended and restated articles of association do not require director nominations to be made by a nominating committee of our board of directors consisting solely of independent directors, as required under the Listing Rules of the NASDAQ Stock Market. In accordance with Israeli law and practice, directors are recommended by our board of directors for election by our shareholders (other than directors elected by our board of directors to fill a vacancy).
|
|
|
•
|
Compensation of officers. Israeli law and our amended and restated articles of association do not require that the independent members of our board of directors (or a compensation committee composed solely of independent members of our board of directors) determine an executive officer’s compensation, as is generally required under the Listing Rules of the NASDAQ Stock Market with respect to the chief executive officer and all other executive officers. For details regarding the approvals required under the Israeli Companies Law for the approval of compensation of the chief executive officer, all other executive officers and directors, see below under “Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions— Compensation of Chief Executive Officer,” “Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions— Compensation of Officers Other than the Chief Executive Officer” and “Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions— Compensation of Directors,” respectively. |
|
|
•
|
Shareholder approval. We will seek shareholder approval for all corporate actions requiring such approval under the requirements of the Israeli Companies Law, rather than seeking approval for corporate actions in accordance with NASDAQ Listing Rule 5635. In particular, under this NASDAQ rule, shareholder approval is generally required for: (i) an acquisition of shares/assets of another company that involves the issuance of 20% or more of the acquirer’s shares or voting rights or if a director, officer or 5% shareholder has greater than a 5% interest (or such persons collectively have a 10% or greater interest) in the target company or the assets to be acquired or the consideration to be received and the present or potential issuance of ordinary shares, or securities convertible into or exercisable for ordinary shares, could result in an increase in outstanding common shares or voting power of 5% or more; (ii) the issuance of shares leading to a change of control; (iii) adoption/amendment of a stock option or purchase plan or other equity compensation arrangements, pursuant to which stock may be acquired by officers, directors, employees or consultants (with certain limited exception); and (iv) issuances of 20% or more of the shares or voting rights (including securities convertible into, or exercisable for, equity) of a listed company via a private placement (and/or via sales by directors/officers/5% shareholders) if such equity is issued (or sold) at below the greater of the book or market value of shares. By contrast, under the Israeli Companies Law, the adoption of, and material changes to, equity-based compensation plans generally require the approval of the board of directors (for details regarding the approvals required under the Israeli Companies Law for the approval of compensation of the chief executive officer, all other executive officers and directors, see below under “Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions— Compensation of Chief Executive Officer,” “Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions— Compensation of Officers Other than the Chief Executive Officer” and “Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions— Compensation of Directors,” respectively). |
|
|
•
|
Quorum requirement. Under our amended and restated articles of association and as permitted under the Israeli Companies Law, a quorum for any meeting of shareholders shall be the presence of at least two shareholders present in person, by proxy or by a voting instrument, who hold at least 25% of the voting power of our shares instead of 33 1/3% of the issued share capital required under the NASDAQ Listing Rules. At an adjourned meeting, any number of shareholders shall constitute a quorum, unless the meeting of shareholders was convened at the demand of shareholders, in which case, the quorum shall be the presence of one or more shareholders holding at least 5% of our issued share capital and at least one percent of the voting power of our shares, or one or more shareholders with at least 5% of the voting power of our shares. |
113
Except as stated above, we intend to comply with the rules generally applicable to U.S. domestic companies listed on NASDAQ. We may in the future decide to use the foreign private issuer exemption with respect to some or all of the other NASDAQ corporate governance rules. Following our home country governance practices, as opposed to the requirements that would otherwise apply to a company listed on NASDAQ, may provide less protection than is accorded to investors under NASDAQ listing requirements applicable to domestic issuers. For more information, see “Risk Factors —As a foreign private issuer, we are permitted, and intend, to follow certain home country corporate governance practices instead of otherwise applicable NASDAQ requirements, which may result in less protection than is accorded to investors under rules applicable to domestic U.S. issuers.”
Board Practices
Board of Directors
Under the Israeli Companies Law, the management of our business, including strategy and policies, is vested in our board of directors. Our board of directors may exercise all powers and may take all actions that are not specifically granted to our shareholders or to management. Our executive officers are responsible for our day-to-day management and have individual responsibilities established by our board of directors. Our chief executive officer is appointed by, and serves at the discretion of, our board of directors, subject to the employment agreement that we have entered into with him. All other executive officers are appointed by our chief executive officer, and are subject to the terms of any applicable employment agreements that we may enter into with them.
Under our amended articles of association, which will be effective upon the closing of this offering, our board of directors must consist of at least and not more than directors, including at least two external directors required to be appointed under the Israeli Companies Law. Our board of directors will consist of directors upon the closing of this offering, including two new directors, who are our external directors whose service will commence upon the completion of this offering. The appointment of the external directors is subject to ratification at a meeting of our shareholders to be held no later than three months following the closing of this offering.
In accordance with the exemption available to foreign private issuers under NASDAQ Listing Rules, we do not intend to follow the requirements of the NASDAQ Listing Rules with regard to having a majority of independent directors on our board of directors, and instead, will follow Israeli law and practice, in accordance with which our board of directors will consist of at least two external directors. The definition of independent director under NASDAQ rules and external director under the Israeli Companies Law overlap to a significant degree, such that we would generally expect our two directors serving as external directors under the Israeli Companies Law to satisfy the requirement to be independent under NASDAQ rules. The definition of external director under the Israeli Companies Law includes a set of statutory criteria that must be satisfied, including criteria whose aim is to ensure that there is no factor which would impair the ability of the external director to exercise independent judgment. The definition of independent director under NASDAQ rules specifies similar, if slightly less stringent, requirements in addition to the requirement that the board consider any factor which would impair the ability of the independent director to exercise independent judgment. However, external directors must be elected by a special majority of shareholders while independent directors may be elected by an ordinary majority. See “— External Directors” for a description of the requirements under the Israeli Companies Law for a director to serve as an external director.
Other than external directors, who are subject to special election requirements under the Israeli Companies Law (as detailed below), our amended and restated articles of association require that our directors be elected by the general meeting of our shareholders by the vote of a majority of the ordinary shares present, in person or by proxy, and voting at that meeting. Each director (other than the external directors) will hold office until the first annual general meeting of shareholders following his or her appointment, unless the tenure of such director expires earlier pursuant to the Israeli Companies Law or unless he or she is removed from office as described below. In addition, our amended articles of association allow our board of directors to appoint directors (other than the external directors) to fill vacancies on our board of directors, for a term of office equal to the remaining period of the term of office of the director(s) whose office(s) have been vacated. See “— External Directors” for a description of the procedure for the election of external directors.
In accordance with the exemption available to foreign private issuers under the NASDAQ Listing Rules, we do not intend to follow the requirements of the NASDAQ Listing Rules with regard to the process of nominating directors, and instead, will follow Israeli law and practice, in accordance with which our board of directors (or a committee thereof) is authorized to recommend to our shareholders director nominees for election.
114
Under the Israeli Companies Law and our amended and restated articles of association, nominations for directors may also be added to the agenda of a future general meeting of shareholders, at the request of any one or more shareholders holding at least 1% of our outstanding voting power. Any director nominated by a shareholder is required to certify to us, as required by all director nominees, that he or she meets all the requirements of the Israeli Companies Law for election as a director of a public company, and possesses the necessary qualifications and is able to dedicate sufficient time, to fulfill his or her duties as a director of our company, taking into consideration our company’s size and special needs.
Under the Israeli Companies Law, our board of directors must determine the minimum number of directors who are required to have accounting and financial expertise. See “— External directors.” In determining the number of directors required to have such expertise, our board of directors must consider, among other things, the type and size of the company and the scope and complexity of its operations. Our board of directors has determined that the minimum number of directors of our company who are required to have accounting and financial expertise is one.
External Directors
Under the Israeli Companies Law, our board of directors is required to include at least two members who qualify, and were elected as, external directors (within the meaning of the Israeli Companies Law). and have agreed to serve as our external directors following the closing of this offering, subject to ratification at a meeting of our shareholders to be held no later than three months following the closing of this offering.
The provisions of the Israeli Companies Law set forth special approval requirements for the election of external directors. External directors must be elected by a majority vote of the shares present and voting on the matter at a shareholders meeting, provided that either:
|
|
•
|
such majority includes at least a majority of the shares held by all shareholders who are non-controlling shareholders and shareholders who do not have a personal interest in the election of the external director (other than a personal interest not deriving from a relationship with a controlling shareholder) that are voted at the meeting, excluding abstentions, which we refer to as a disinterested majority; or
|
|
|
•
|
the total number of shares held by shareholders who are non-controlling shareholders and shareholders who do not have a personal interest in the election of the external director (other than a personal interest not derived from a relationship with a controlling shareholder) voted against the election of the external director does not exceed 2% of the aggregate voting rights in the company.
|
The term “controlling shareholder” is defined in the Israeli Companies Law as a shareholder with the ability to direct the activities of a company, other than by virtue of being an office holder. A shareholder is deemed to be a controlling shareholder if the shareholder holds 50% or more of the voting rights in the company or has the right to appoint 50% or more of the directors of a company or its general manager. For purposes of shareholder approval of certain extraordinary and interested party transactions, as well as corporate approval of executive compensation, a controlling shareholder is deemed to include any shareholder (or two or more shareholders having a personal interest in the same matter being brought for approval) who hold(s) in the aggregate 25% or more of the voting rights in a public company if no other shareholder holds more than 50% of the voting rights in the company.
The term “personal interest” is defined in the Israeli Companies Law as a person’s or entity’s personal interest in an act or a transaction of a company, (i) including the personal interest of (a) any spouse, sibling, parent, grandparent or descendant of the persons, any descendant, sibling or parent of a spouse of the person and the spouse of any of the foregoing; and (b) an entity in which the person or entity or any of the foregoing relatives of the person serves as a director or the chief executive officer, owns at least 5% of its issued share capital or voting rights or has the right to appoint one or more directors or the chief executive officer, but (ii) excluding a personal interest arising solely from the ownership of shares. In the case of a person voting by proxy, “personal interest” includes the personal interest of the proxy holder or the shareholder granting the proxy (even if the proxy holder has no personal interest in the matter), whether or not the proxy holder has discretion how to vote.
115
The initial term of an external director is three years. Thereafter, an external director may be reelected by shareholders to serve in that capacity for up to two additional three-year terms, provided that either:
|
|
•
|
his or her service for each such additional term is recommended by one or more shareholders holding at least 1% of the company’s voting rights and is approved at a shareholders meeting by a disinterested majority, where the total number of shares held by non-controlling, disinterested shareholders voting for such reelection exceeds 2% of the aggregate voting rights in the company, and provided further that the external director is not an affiliated or competing shareholder, as defined in the Israeli Companies Law, or a relative of such a shareholder at the time of the appointment, and is not affiliated with such a shareholder at the time of appointment or within the two years preceding the date of appointment; or
|
|
|
•
|
his or her service for each such additional term is recommended by the board of directors and is approved at a shareholders meeting by the same majority required for the initial election of an external director (as described above).
|
However, the term of office for external directors for Israeli companies traded on certain foreign stock exchanges, including the NASDAQ Capital Market, may be extended indefinitely in increments of additional three-year terms, in each case provided that the audit committee and the board of directors of the company confirm that, in light of the external director’s expertise and special contribution to the work of the board of directors and its committees, the reelection for such additional period(s) is beneficial to the company, and provided that the external director is reelected subject to the same shareholder vote requirements as if elected for an additional term (as described above). Prior to the approval of the reelection of the external director at a general shareholders meeting, the company’s shareholders must be informed of the term previously served by him or her and of the reasons why the board of directors and audit committee recommended the extension of his or her term.
If the board of directors has determined that an external director ceases to meet the statutory qualifications for appointment or if he or she violates his or her duty of loyalty to the company, the board of directors is required to call a special general meeting of shareholders for the removal of the external director. In such circumstances, the removal of the external director by the shareholders requires the same special shareholder majority that is required for the election of an external director, as described above. An external director may also be removed by order of an Israeli court, at the request of a director or shareholder, if the court finds that the external director has ceased to meet the statutory qualifications for his or her appointment or has violated his or her duty of loyalty to the company. If an external directorship becomes vacant and there are fewer than two external directors on the board of directors at the time, then the board of directors is required under the Israeli Companies Law to call a shareholders’ meeting as soon as practicable to appoint a replacement external director.
Each committee of the board of directors that exercises the powers of the board of directors must include at least one external director, except that the audit committee and the compensation committee must include all external directors then serving on the board of directors. Under the Israeli Companies Law, external directors of a company are prohibited from receiving, directly or indirectly, any compensation for their services as external directors other than pursuant to the Israeli Companies Law and the regulations promulgated thereunder. Compensation of an external director is determined prior to his or her appointment and may not be changed during any three-year term subject to certain exceptions.
The Israeli Companies Law provides that a person is not qualified to serve as an external director if (i) the person is a relative of a controlling shareholder of the company, or (ii) if that person or his or her relative, partner, employer, another person to whom he or she was directly or indirectly subordinate, or any entity under the person’s control, has or had, during the two years preceding the date of appointment as an external director: (a) any affiliation with the company, with any person or entity controlling the company or a relative of such person at the time of appointment, or with any entity controlled by or under common control with the company at the time of appointment or during the two years preceding the appointment; or (b) in the case of a company with no controlling shareholder or a shareholder holding 25% or more of its voting rights, had at the date of appointment as an external director, any affiliation with a person then serving as chairman of the board or chief executive officer, a holder of 5% or more of the issued share capital or voting power in the company or the most senior financial officer.
The term “relative” is defined as a spouse, sibling, parent, grandparent or descendant; spouse’s sibling, parent or descendant; and the spouse of each of the foregoing persons.
116
The term “affiliation” includes (subject to certain exceptions): an employment relationship; a business or professional relationship even if not maintained on a regular basis (excluding insignificant relationships); control; and service as an office holder, excluding service as a director in a private company prior to the initial public offering of its shares if such director was appointed as a director of the private company in order to serve as an external director following the initial public offering.
The term “office holder” is defined under the Israeli Companies Law as a general manager, chief business manager, deputy general manager, vice general manager, any other person assuming the responsibilities of any of these positions regardless of that person’s title, a director and any other manager directly subordinate to the general manager.
In addition, no person may serve as an external director if that person’s positions or professional or other activities create, or may create, a conflict of interest with that person’s responsibilities as a director or otherwise interfere with that person’s ability to serve as a director or if the person is an employee of the Israel Securities Authority or of an Israeli stock exchange. A person may furthermore not continue to serve as an external director if he or she received direct or indirect compensation other than as permitted by the Israeli Companies Law and the regulations promulgated thereunder.
Following the termination of an external director’s service on a board of directors, such former external director and his or her spouse and children and other relatives may not be provided a direct or indirect benefit by the company, its controlling shareholder or any entity under its controlling shareholder’s control. This includes engagement as an officer or director of the company or a company controlled by its controlling shareholder or employment by, or provision of services to, any such company for consideration, either directly or indirectly, including through a corporation controlled by such person. This restriction extends for a period of two years with regard to the former external director and his or her spouse or child and for one year with respect to other relatives of the former external director.
If at the time at which an external director is appointed all members of the board of directors who are not controlling shareholders or relatives of controlling shareholders of the company are of the same gender, the external director to be appointed must be of the other gender. A director of one company may not be appointed as an external director of another company if a director of the other company is acting as an external director of the first company at such time.
According to the Israeli Companies Law and regulations promulgated under the Israeli Companies Law, a person may be appointed as an external director only if he or she has professional qualifications or if he or she has accounting and financial expertise (each, as defined below). At least one of the external directors must be determined by our board of directors to have accounting and financial expertise. However, as a company listed on the NASDAQ Capital Market, neither of our external directors is required to possess accounting and financial expertise as long as each possesses the requisite professional qualifications, and at least one of our other directors (i) meets the independence requirements under the Exchange Act and the NASDAQ Listing Rules for membership on the audit committee and (ii) has accounting and financial expertise as defined under Israeli Companies Law.
A director with accounting and financial expertise is a director who, due to his or her education, experience and skills, possesses an expertise in, and an understanding of, financial and accounting matters and financial statements, such that he or she is able to understand the financial statements of the company and initiate a discussion about the presentation of financial data. A director is deemed to have professional qualifications if he or she has any of (i) an academic degree in economics, business management, accounting, law or public administration, (ii) an academic degree or has completed another form of higher education in the primary field of business of the company or in a field which is relevant to his/her position in the company, or (iii) at least five years of experience serving in one of the following capacities, or at least five years of cumulative experience serving in two or more of the following capacities: (a) a senior business management position in a company with a significant volume of business; (b) a senior position in the company’s primary field of business; or (c) a senior position in public administration or service. The board of directors is charged with determining whether a director possesses financial and accounting expertise or professional qualifications.
Our board of directors has determined that has accounting and financial expertise and possesses professional qualifications, as required under the Israeli Companies Law.
117
Audit Committee
Following the listing of our ordinary shares on the NASDAQ Capital Market, our audit committee will consist of , along with our two external director nominees, and . will serve as the Chairman of the audit committee.
Israeli Companies Law Requirements
Under the Israeli Companies Law, we are required to appoint an audit committee. The audit committee must be comprised of at least three directors, including all of the external directors, one of whom must serve as chairman of the committee. The audit committee may not include the chairman of the board, a controlling shareholder of the company or a relative of a controlling shareholder, a director employed by or providing services on a regular basis to the company, to a controlling shareholder or to an entity controlled by a controlling shareholder or a director who derives most of his or her income from a controlling shareholder.
In addition, under the Israeli Companies Law, the audit committee of a publicly traded company must consist of a majority of unaffiliated directors, within the meaning of the Israeli Companies Law. In general, an “unaffiliated director” under the Israeli Companies Law is defined as either an external director or a director who meets the following criteria:
|
|
•
|
the audit committee has determined that he or she meets the qualifications for being appointed as an external director, except for (i) the requirement that the director be an Israeli resident (which does not apply to companies such as ours whose securities have been offered outside of Israel or are listed outside of Israel) and (ii) the requirement for accounting and financial expertise or professional qualifications; and
|
|
|
•
|
he or she has not served as a director of the company for a period exceeding nine consecutive years. For this purpose, a break of less than two years in the service shall not be deemed to interrupt the continuation of the service.
|
NASDAQ Listing Requirements
Under the NASDAQ corporate governance rules, we are required to maintain an audit committee consisting of at least three independent directors, within the meaning of the Exchange Act and NASDAQ Listing Rules, each of whom must be able to read and understand fundamental financial statements, including the company’s balance sheet, income statement and cash flow statement (and one of whom has past employment experience in finance or accounting, requisite professional certification in accounting or other comparable experience or background that leads to financial sophistication) and none of whom has participated in the preparation of our or any of our subsidiary’s financial statements at any time during the prior three years.
All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the U.S. Securities and Exchange Commission and the NASDAQ Listing Rules. Our board of directors has determined that is an audit committee financial expert as defined by the U.S. Securities and Exchange Commission rules and has the requisite financial sophistication required by the NASDAQ Listing Rules.
Each of the members of the audit committee is “independent” as such term is defined in Rule 10A-3(b)(1) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, which is different from the general NASDAQ test for independence of board and committee members.
Audit Committee Role
Our board of directors has adopted an audit committee charter to be effective upon the listing of our shares on the NASDAQ Capital Market that will set forth the responsibilities of the audit committee consistent with the rules of the U.S. Securities and Exchange Commission and the NASDAQ Listing Rules, as well as the requirements for audit committees under the Israeli Companies Law, including the following:
|
|
•
|
oversight of our independent registered public accounting firm and recommending the engagement, compensation or termination of engagement of our independent registered public accounting firm to the board of directors or shareholders for their approval, as applicable, in accordance with the requirements of the Israeli Companies Law;
|
118
|
|
•
|
recommending the engagement or termination of the person filling the office of our internal auditor; and
|
|
|
•
|
recommending the terms of audit and non-audit services provided by the independent registered public accounting firm for pre-approval by our board of directors or shareholders for their approval, as applicable, in accordance with the requirements of the Israeli Companies Law.
|
Our audit committee provides assistance to our board of directors in fulfilling its legal and fiduciary obligations in matters involving our accounting, auditing, financial reporting, internal control and legal compliance functions by pre-approving the services performed by our independent accountants and reviewing their reports regarding our accounting practices and systems of internal control over financial reporting. Our audit committee also oversees the audit efforts of our independent accountants and takes those actions that it deems necessary to satisfy itself that the accountants are independent of management.
Under the Israeli Companies Law, our audit committee is responsible for:
|
|
•
|
determining whether there are deficiencies in the business management practices of our company, including in consultation with our internal auditor or the independent auditor, and making recommendations to the board of directors to improve such practices;
|
|
|
•
|
determining whether to approve certain related party transactions (including transactions in which an office holder has a personal interest) and whether such transaction is extraordinary or material under Israeli Companies Law (see “— Approval of Related Party Transactions under Israeli Law”);
|
|
|
•
|
determining whether a competitive process must be implemented for the approval of certain transactions with controlling shareholders or its relative or in which a controlling shareholder has a personal interest (whether or not the transaction is an extraordinary transaction), under the supervision of the audit committee or other party determined by the audit committee and in accordance with standards determined by the audit committee, or whether a different process determined by the audit committee should be implemented for the approval of such transactions;
|
|
|
•
|
determining the process for the approval of certain transactions with controlling shareholders or in which a controlling shareholder has a personal interest that the audit committee has determined are not extraordinary transactions but are not immaterial transactions;
|
|
|
•
|
where the board of directors approves the working plan of the internal auditor, to examine such working plan before its submission to the board of directors and proposing amendments thereto;
|
|
|
•
|
examining our internal controls and internal auditor’s performance, including whether the internal auditor has sufficient resources and tools to dispose of its responsibilities;
|
|
|
•
|
examining the scope of our auditor’s work and compensation and submitting a recommendation with respect thereto to our board of directors or shareholders, depending on which of them is considering the compensation of our auditor; and
|
|
|
•
|
establishing procedures for the handling of employees’ complaints as to the management of our business and the protection to be provided to such employees.
|
Our audit committee may not approve any actions requiring its approval (see “— Approval of Related Party Transactions under Israeli Law”), unless at the time of the approval a majority of the committee’s members are present, which majority consists of unaffiliated directors including at least one external director.
Compensation Committee and Compensation Policy
Following the listing of our ordinary shares on the NASDAQ Capital Market, our compensation committee will consist of , and . will serve as the Chairman of the compensation committee.
119
Israeli Companies Law Requirements
Under a recently enacted amendment to the Israeli Companies Law, which became effective in December 2012, the board of directors of a public company must appoint a compensation committee. The compensation committee must be comprised of at least three directors, including all of the external directors, who must constitute a majority of the members of the compensation committee. However, subject to certain exceptions, Israeli companies whose securities are traded on certain U.S. stock exchanges, such as NASDAQ, that do not have a controlling shareholder, do not have to meet such majority requirement, provided that the compensation committee meets other Israeli Companies Law composition requirements and the requirements of the jurisdiction where the company’s securities are traded. Each compensation committee member that is not an external director must be a director whose compensation does not exceed an amount that may be paid to an external director under regulations promulgated under the Israeli Companies Law. The compensation committee is subject to the same Israeli Companies Law restrictions as the audit committee as to who may not be a member of the committee. See “— Audit Committee — Companies Law Requirements.”
NASDAQ Listing Requirements
Under the NASDAQ corporate governance rules, we are required to maintain a compensation committee consisting of at least two directors, each of whom is an independent director within the meaning of the NASDAQ Listing Rules.
Compensation Committee Role
Our board of directors has adopted a compensation committee charter to be effective upon the listing of our shares on the NASDAQ Capital Market that will set forth the responsibilities of the compensation committee consistent with the NASDAQ Listing Rules and the requirements for compensation committees under the Israeli Companies Law, including the following:
|
|
•
|
recommending to the board of directors for its approval (i) a compensation policy, (ii) whether a compensation policy should continue in effect, if the then-current policy has a term of greater than three years (approval of either a new compensation policy or the continuation of an existing compensation policy must in any case occur every three years); and (iii) periodic updates to the compensation policy. See “— Compensation Policy.” In addition, the compensation committee is required to periodically examine the implementation of the compensation policy;
|
|
|
•
|
the approval of the terms of employment and service of office holders (including determining whether the compensation terms of a candidate for chief executive officer of the company need not be brought to approval of the shareholders); and
|
|
|
•
|
reviewing and approving grants of options and other incentive awards to persons other than office holders to the extent such authority is delegated by our board of directors, subject to the limitations on such delegation as provided in the Israeli Companies Law.
|
Compensation Policy
Under the Israeli Companies Law, the duties of the compensation committee include the recommendation to the company’s board of directors of a policy regarding the terms of engagement of office holders, as such term is defined in the Israeli Companies Law, to which we refer to as a compensation policy, and any extensions and updates thereto. The compensation policy must be adopted by the company’s board of directors, after considering the recommendations of the compensation committee, and will need to be brought for approval by the company’s shareholders, which approval requires a Special Approval for Compensation (as defined below under “— Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions”).
We will be required to adopt a compensation policy within nine months following our listing on the NASDAQ Capital Market.
120
The compensation policy must serve as the basis for decisions concerning the financial terms of employment or engagement of office holders, including exculpation, insurance, indemnification or any monetary payment or obligation of payment in respect of employment or engagement. The compensation policy must relate to certain factors, including advancement of the company’s objectives, the company’s business plan and its long-term strategy, and creation of appropriate incentives for office holders, and must consider (among other things) the company’s risk management, size and the nature of its operations. The compensation policy must also consider the following additional factors:
|
|
•
|
the knowledge, skills, expertise and accomplishments of the relevant office holder;
|
|
|
•
|
the office holder’s roles and responsibilities and prior compensation agreements with him or her;
|
|
|
•
|
the relationship between the terms offered and the average compensation of the other employees of the company (including any employees employed through manpower companies);
|
|
|
•
|
the impact of disparities in salary upon work relationships in the company;
|
|
|
•
|
the possibility of reducing variable compensation at the discretion of the board of directors, and the possibility of setting a limit on the exercise value of non-cash variable equity-based compensation; and
|
|
|
•
|
as to severance compensation, the period of employment or service of the office holder, the terms of his or her compensation during such period, the company’s performance during such period, the person’s contribution towards the company’s achievement of its goals and the maximization of its profits, and the circumstances under which the person is leaving the company.
|
The compensation policy must also include the following principles:
|
|
•
|
the link between variable compensation and long-term performance and measurable criteria;
|
|
|
•
|
the relationship between variable and fixed compensation, and the ceiling for the value of variable compensation;
|
|
|
•
|
the conditions under which an office holder would be required to repay compensation paid to him or her if it was later shown that the data upon which such compensation was based was inaccurate and was required to be restated in the company’s financial statements;
|
|
|
•
|
the minimum holding or vesting period for variable, equity-based compensation; and
|
|
|
•
|
maximum limits for severance compensation.
|
Internal Auditor
Under the Israeli Companies Law, the board of directors of an Israeli public company must appoint an internal auditor recommended by the audit committee. An internal auditor may not be:
|
|
•
|
a person (or a relative of a person) who holds more than 5% of the company’s outstanding shares or voting rights;
|
|
|
•
|
a person (or a relative of a person) who has the power to appoint a director or the general manager of the company;
|
|
|
•
|
an office holder, within the meaning of the Israeli Companies Law (including a director and the general manager) of the company (or a relative thereof); or
|
|
|
•
|
a member of the company’s independent accounting firm, or anyone on his or her behalf.
|
The role of the internal auditor is to examine, among other things, our compliance with applicable law and orderly business procedures. The audit committee is required to oversee the activities and to assess the performance of the internal auditor as well as to review the internal auditor’s work plan. We intend to appoint an internal auditor following the closing of this offering.
Approval of Related Party Transactions under Israeli Law
Fiduciary Duties of Directors and Executive Officers
The Israeli Companies Law codifies the fiduciary duties that office holders owe to a company. Each person listed in the table under “Management — Executive Officers and Directors” is an office holder under the Israeli Companies Law.
121
An office holder’s fiduciary duties consist of a duty of care and a duty of loyalty. The duty of care requires an office holder to act with the level of care with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care includes a duty to use reasonable means to obtain:
|
|
•
|
information on the advisability of a given action brought for his or her approval or performed by virtue of his or her position; and
|
|
|
•
|
all other important information pertaining to any such action.
|
The duty of loyalty requires an office holder to act in good faith and in the best interests of the company, and includes, among other things, the duty to:
|
|
•
|
refrain from any conflict of interest between the performance of his or her duties to the company and his or her other duties or personal affairs;
|
|
|
•
|
refrain from any activity that is competitive with the company;
|
|
|
•
|
refrain from exploiting any business opportunity of the company to receive a personal gain for himself or herself or others; and
|
|
|
•
|
disclose to the company any information or documents relating to the company’s affairs which the office holder received as a result of his or her position as an office holder.
|
We may approve an act specified above which would otherwise constitute a breach of the office holder’s duty of loyalty, provided that the office holder acted in good faith, the act or its approval does not harm the company and the office holder discloses his or her personal interest a sufficient amount of time before the date for discussion of approval of such act.
Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions
Disclosure of Personal Interests of an Office Holder
The Israeli Companies Law requires that an office holder promptly disclose to the company any “personal interest” that he or she may be aware of and all related material information or documents concerning any existing or proposed transaction with the company. An interested office holder’s disclosure must be made promptly and in any event no later than the first meeting of the board of directors at which the transaction is considered. A personal interest includes an interest of any person in an act or transaction of a company, including a personal interest of such person’s relative or of a corporate entity in which such person or a relative of such person holds 5% or more of the outstanding shares or voting rights, is a director or general manager or in which he or she has the right to appoint at least one director or the general manager, but excluding a personal interest arising from one’s ownership of shares in the company. A personal interest includes the personal interest of a person for whom the office holder holds a voting proxy or the personal interest of the office holder with respect to his or her vote on behalf of a person for whom he or she holds a proxy even if such shareholder has no personal interest in the matter. An office holder is not, however, obliged to disclose a personal interest if it derives solely from the personal interest of his or her relative in a transaction that is not considered an extraordinary transaction. Under the Israeli Companies Law, an extraordinary transaction is defined as any of the following: a transaction other than in the ordinary course of business; a transaction that is not on market terms; or a transaction that may have a material impact on a company’s profitability, assets or liabilities.
Generally, a person who has a personal interest in a matter which is considered at a meeting of the board of directors or the audit committee may not be present at such a meeting or vote on that matter unless, with respect to an office holder, the chairman of the audit committee or board of directors (as applicable) determines that the office holder should be present in order to present the transaction that is subject to approval. If a majority of the members of the audit committee or the board of directors (as applicable) has a personal interest in the approval of a transaction, then all directors may participate in discussions of the audit committee or the board of directors (as applicable) on such transaction and the voting on approval thereof. If a majority of the members of the board of directors has a personal interest in the approval of a transaction, shareholder approval is also required for such transaction.
122
Approval of Transactions with Officer Holders
If it is determined that an office holder has a personal interest in a transaction that is not an extraordinary transaction, approval by the board of directors is required for the transaction, unless the company’s articles of association provide for a different method of approval. Further, so long as an office holder has disclosed his or her personal interest in a transaction, the board of directors may approve an act by the office holder that would otherwise be deemed a breach of his or her duty of loyalty, provided that the transaction is in the company’s best interest and the office holder acted in good faith. An extraordinary transaction in which an office holder has a personal interest requires approval first by the company’s audit committee and subsequently by the board of directors.
Compensation of Officers Other than the Chief Executive Officer
The compensation of an office holder (other than the chief executive officer) who is not a director requires approval first by the company’s compensation committee, then by the company’s board of directors, according to the company’s compensation policy. In special circumstances the compensation committee and board of directors may approve a compensation arrangement that is inconsistent with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and such arrangement must be approved by a majority vote of the shares present and voting at a shareholders meeting on the matter, provided that either: (i) such majority includes at least a majority of the shares held by all shareholders who are not controlling shareholders and shareholders who do not have a personal interest in such compensation arrangement present and voting on the matter, excluding abstentions; or (ii) the total number of shares of non-controlling shareholders and shareholders who do not have a personal interest in the matter and who vote against the matter does not exceed 2% of the company’s aggregate voting rights. We refer to this as the Special Approval for Compensation. However, if the shareholders of the company do not approve a compensation arrangement with an executive officer that is inconsistent with the company’s compensation policy, the compensation committee and board of directors may, in special circumstances, override the shareholders’ decision if each of the compensation committee and the board of directors discuss the arrangement again, analyze the shareholders’ objection and provide detailed reasons for their decision.
Compensation of Chief Executive Officer
The compensation of a public company’s chief executive officer requires the approval of first, the company’s compensation committee; second, the company’s board of directors and third (except for a number of exceptions), the company’s shareholders by the Special Approval for Compensation. However, if the shareholders of the company do not approve a compensation arrangement with a chief executive officer, the compensation committee and board of directors may, in special circumstances, override the shareholders’ decision if each of the compensation committee and the board of directors discuss the arrangement again, analyze the shareholders’ objection and provide detailed reasons for their decision. The compensation committee and board of directors approval should be in accordance with the company’s compensation policy; however, in special circumstances, they may approve compensation terms of a chief executive officer that are inconsistent with such policy provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by the Special Approval for Compensation.
Compensation of Directors
Arrangements regarding the compensation of a director require the approval of the compensation committee, board of directors and (except for a number of exceptions) shareholders by ordinary majority, in that order. The approval of the compensation committee and board of directors must be in accordance with the compensation policy. In special circumstances the compensation committee and board of directors may approve a compensation arrangement that is inconsistent with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by the Special Approval for Compensation.
123
With respect to compensation of an officer (including chief executive officer) or director who is also a controlling shareholder, see “— Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions.”
Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions
Pursuant to Israeli law, the disclosure requirements regarding personal interests that apply to directors and executive officers also apply to a controlling shareholder of a public company. In the context of a transaction involving a shareholder of the company, a controlling shareholder also includes a shareholder who holds 25% or more of the voting rights in the company if no other shareholder holds more than 50% of the voting rights in the company. For this purpose, the holdings of all shareholders who have a personal interest in the same transaction will be aggregated. Extraordinary transactions with a controlling shareholder or in which a controlling shareholder has a personal interest, including a private placement in which a controlling shareholder has a personal interest, and the terms of engagement with a controlling shareholder or a relative thereof, directly or indirectly (including through a corporation controlled by a controlling shareholder), for the provision of services to the company and his or her terms of employment or service as an office holder or employment as other than an office holder, require the approval of each of (i) the audit committee or the compensation committee with respect to the terms of service or employment by the company as an office holder, an employee or service provider, (ii) the board of directors and (iii) the shareholders, in that order. The shareholder approval requires one of the following, which we refer to as a Special Majority:
|
|
•
|
at least a majority of the shares held by all shareholders who do not have a personal interest in the transaction and who are present and voting on the matter approves the transaction, excluding abstentions; or
|
|
|
•
|
the shares voted against the transaction by shareholders who have no personal interest in the transaction and who are present and voting at the meeting do not exceed 2% of the voting rights in the company.
|
Each shareholder voting on the approval of an extraordinary transaction with a controlling shareholder must inform the company prior to voting whether or not he or she has a personal interest in the approval of the transaction, otherwise, the shareholder is not eligible to vote on the proposal and his or her vote will not be counted for purposes of the proposal.
To the extent that any such transaction with a controlling shareholder is for a period of more than three years, approval is required once every three years, unless, with respect to any such extraordinary transactions, the audit committee determines that the duration of the transaction is reasonable given the related circumstances.
The compensation committee and board approval for arrangements regarding the terms of service or employment of a controlling shareholder must be in accordance with the company’s compensation policy. In special circumstances the compensation committee and board of directors may approve a compensation arrangement that is inconsistent with the company’s compensation policy, provided that they have considered the same considerations and matters required for the approval of a compensation policy in accordance with the Israeli Companies Law and that shareholder approval was obtained by the Special Majority.
Pursuant to regulations promulgated under the Israeli Companies Law, certain transactions with a controlling shareholder or his or her relative, or with directors, relating to terms of service or employment that would otherwise require approval of a company’s shareholders may be exempt from shareholder approval upon certain determinations of the audit committee and board of directors. Under these regulations, a shareholder holding at least 1% of the issued share capital or voting power of the company may require, within 14 days of the publication or announcement of such determinations, that despite such determinations by the audit committee and the board of directors, such transaction will require shareholder approval under the same majority requirements that would otherwise apply to such transactions.
In addition, disclosure of a personal interest in a private placement of a public company (including disclosure of any material fact or document) is required by (i) a shareholder holding 5% or more of the company’s issued and outstanding capital or its voting rights whose holdings will increase as result of the private placement and a shareholder who will hold 5% or more of the company’s issued and outstanding capital or its voting rights as a result of the private placement, if 20% or more of the company’s outstanding share capital prior to the private placement is issued in the private placement and the payment for which is not only in cash or listed securities or the transaction is not on market terms; and (ii) a person or entity that will become a controlling shareholder as a result of the private placement.
124
Shareholder Duties
Pursuant to the Israeli Companies Law, a shareholder has a duty to act in good faith and in a customary manner toward the company and other shareholders and to refrain from abusing his or her power in the company, including, among other things, in voting at a meeting of shareholder with respect to the following matters:
|
|
•
|
an amendment to the company’s articles of association;
|
|
|
•
|
an increase of the company’s authorized share capital;
|
|
|
•
|
a merger; and
|
|
|
•
|
the approval of related party transactions and acts of office holders that require shareholder approval.
|
In addition, a shareholder has a general duty to refrain from discriminating against other shareholders.
Certain shareholders have a duty of fairness toward the company. These shareholders include any controlling shareholder, any shareholder who knows that he or she has the power to determine the outcome of a shareholder vote and any shareholder who has the power to appoint or to prevent the appointment of an office holder of the company or other power towards the company. The Israeli Companies Law does not define the substance of the duty of fairness, except to state that the remedies generally available upon a breach of contract will also apply in the event of a breach of the duty to act with fairness.
Exculpation, Insurance and Indemnification of Directors and Officers
Under the Israeli Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care, but only if a provision authorizing such exculpation is included in its articles of association. Our amended articles of association to be effective upon the closing of this offering include such a provision, to the fullest extent permitted by law. The company may not exculpate in advance a director from liability arising out of a prohibited dividend or other distribution to shareholders.
Under the Israeli Companies Law and the Israeli Securities Law, 5728-1968, or the Israeli Securities Law, a company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking made in advance of any such event or following an event, provided its articles of association include a provision authorizing such indemnification:
|
|
•
|
a financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria;
|
|
|
•
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction;
|
|
|
•
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; and
|
125
|
|
•
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder, or certain compensation payments made to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Israeli Securities Law.
|
Under the Israeli Companies Law and the Israeli Securities Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder if and to the extent provided in the company’s articles of association:
|
|
•
|
a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
|
|
•
|
a breach of the duty of care to the company or to a third party, to the extent such a breach does not arise out of the negligent conduct of the office holder;
|
|
|
•
|
a financial liability imposed on the office holder in favor of a third party; and
|
|
|
•
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder or certain compensation payments to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Securities Law.
|
Under the Israeli Companies Law, a company may not indemnify, exculpate or enter into an insurance contract for office holder liability, for any of the following:
|
|
•
|
a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company;
|
|
|
•
|
a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder;
|
|
|
•
|
an act or omission committed with intent to derive illegal personal benefit; or
|
|
|
•
|
a fine, monetary sanction or forfeit levied against the office holder.
|
Under the Israeli Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to the chief executive officer or a director or under certain circumstances, also by the shareholders. See “— Approval of Related Party Transactions under Israeli Law.”
Our amended articles of association, which will be effective upon the closing of this offering, permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted under the Israeli Companies Law and the Israeli Securities Law. We have obtained directors’ and officers’ liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by the Israeli Companies Law. In accordance with our articles of association, in the event that our insurance also covers our liability, our office holders shall have precedence over us in collecting insurance payments.
We have previously entered into indemnification and exculpation agreements with each of our current officers and directors (other than Alex Ovadia) exculpating them from a breach of their duty of care to us to the fullest extent permitted by the Israeli Companies Law and undertaking to indemnify them to the fullest extent permitted by the Israeli Companies Law (other than indemnification for litigation expenses in connection with a monetary sanction), to the extent that these liabilities are not covered by insurance. This indemnification is limited to events determined as foreseeable by our board of directors based on our activities, as set forth in the indemnification agreements. Under such indemnification agreements, the maximum aggregate amount of indemnification that we may pay to any and all of our currently serving or future officers and directors together may not exceed 25% of our share capital according to our most recent annual financial statements at the time of payment. We intend to enter into a new form of indemnification and exculpation agreement with each of our current officers and directors exculpating them from a breach of their duty of care to us to the fullest extent permitted by the Israeli Companies Law and undertaking to indemnify them to the fullest extent permitted by the Israeli Companies Law and the Israeli Securities Law prior to the closing of this offering.
126
Code of Business Conduct and Ethics
We intend to adopt a Code of Business Conduct and Ethics applicable to all of our directors and employees, including our chief executive officer, chief financial officer, controller or principal accounting officer, or other persons performing similar functions, which complies with the “code of ethics” contemplated by Item 16B of Form 20-F promulgated by the U.S. Securities and Exchange Commission. Upon the effectiveness of the registration statement of which this prospectus forms a part, the full text of the Code of Business Conduct and Ethics will be posted on our website at www.check-cap.com. Information contained on, or that can be accessed through, our website does not constitute a part of this prospectus and is not incorporated by reference herein. If we make any amendment to the Code of Business Conduct and Ethics or grant any waivers, including any implicit waiver, from a provision of the code of ethics, we will disclose the nature of such amendment or waiver on our website to the extent required by the rules and regulations of the U.S. Securities and Exchange Commission. Under Item 16B of the U.S. Securities and Exchange Commission’s Form 20-F, if a waiver or amendment of the Code of Business Conduct and Ethics applies to our principal executive officer, principal financial officer, principal accounting officer or controller and relates to standards promoting any of the values described in Item 16B(b) of Form 20-F, we are required to disclose such waiver or amendment on our website in accordance with the requirements of Instruction 4 to such Item 16B.
Compensation of Executive Officers and Directors
The aggregate compensation paid and share-based compensation and other payments expensed by us and our subsidiaries to our directors and executive officers with respect to the year ended December 31, 2013 was $0.6 million. This amount includes approximately $71,000 set aside or accrued to provide pension, severance, retirement or similar benefits or expenses, but does not include business travel, relocation, professional and business association dues and expenses reimbursed to office holders, and other benefits commonly reimbursed or paid by companies in our industry. As of December 31, 2013, options to purchase 8,733,816 ordinary shares granted to our directors and executive officers were outstanding under our 2006 Unit Option Plan at a weighted average exercise price of approximately $0.13 per share.
Effective as of January 1, 2013, our chief executive officer, chief financial officer and chief technology officer agreed to a temporary 20% reduction in their salaries, as part of cost cutting measures. To compensate such executives for the salary reduction, we agreed that upon completion of an equity financing, which includes this offering, each such executive will be entitled the following: (i) a cash payment equal to the cost of such executive’s salary reduction plus 8% annual interest; (ii) options to purchase a number of ordinary shares equal to such executive’s cash payment divided by $0.2748 with an exercise price per share equal to $0.2748. We anticipate that in total we will grant options to purchase 769,453 ordinary shares. Such options will be granted under our 2006 Unit Option Plan and will be fully vested upon grant; and (iii) a one-time bonus equal to one and a half monthly salaries of such executive, provided that the aggregate bonuses for all such executives may not exceed $60,000 and the aggregate compensation for all such executives may not exceed $250,000.
Effective as of the closing of the credit line agreement that we entered into on August 20, 2014 (See “Prospectus Summary—Recent Developments— Credit Line Agreement”), the employment terms of each of our executive officers were amended as follows: (i) the respective salaries of each of our executive officers increased effective as of November 1, 2014; (ii) each of our chief executive officer and chief financial officer is entitled to a one-time bonus of specified amounts; (iii) each of our executive officers shall be entitled to an annual bonus up to certain specified amounts, at the discretion of our board of directors and subject to milestones determined by the board of directors; and (iv) we granted our executive officers options to purchase, in the aggregate, 11,630,739 of our ordinary shares, constituting, in the aggregate, 5.25% of our fully diluted share capital as of the closing, with an exercise price of NIS 0.01 per share. The options vest on a quarterly basis in eight equal installments during a period of 24 months from grant and expire ten years following their issuance, i.e., on October 14, 2024. Upon the closing of this offering or a reverse merger, private placement, PIPE (private investment in public equity) transaction or any other form of equity financing in our company, any unvested portion of the options shall become fully vested and exercisable.
127
We do not have any written agreements with any director providing for benefits upon the termination of such director’s relationship with us.
Employment Agreements with Executive Officers
We have entered into written employment agreements with each of our executive officers. These agreements contain provisions standard for a company in our industry regarding non-competition, confidentiality of information and assignment of inventions. The enforceability of covenants not to compete in Israel and the United States is subject to limitations. For example, Israeli courts have recently required employers seeking to enforce non-compete undertakings of a former employee to demonstrate that the competitive activities of the former employee will harm one of a limited number of material interests of the employer which have been recognized by the courts, such as the secrecy of a company’s confidential commercial information or its intellectual property.
In the event our chief executive officer’s employment is terminated other than for cause, he will be entitled to a one-time severance fee in an amount equal to 50% of his annual salary prior to the voluntary 20% reduction in his salary effective as of January 1, 2013. If his employment is terminated prior to the consummation of an initial public offering of our shares, we may elect, at our discretion, in lieu of payment of the foregoing severance fee, to grant to him fully vested options to purchase 4,000,000 of our ordinary shares, at an exercise price of NIS 0.01, exercisable for a period of ten years from the issuance thereof. If his employment is terminated after the consummation of an initial public offering of our shares, the severance fee will be amended to 50% of his amended annual salary following the initial public offering (and we will not be entitled to grant options in lieu of payment of the severance fee). Other than the foregoing, our agreements with our executive officers do not provide for benefits upon the termination of their respective employment with us, other than payment of salary and benefits during the required notice period for termination of these agreements, which varies under these individual agreements.
2006 Unit Option Plan
In connection with the transfer of all of the business operations and substantially all of the assets of Check-Cap LLC to us in 2009, we assumed the Check-Cap LLC 2006 Unit Option Plan, or the 2006 Unit Option Plan. The 2006 Unit Option Plan is our sole equity incentive plan. Under the 2006 Unit Option Plan, we are permitted to grant options to purchase our ordinary shares to our employees, consultants and service providers. For the purpose of the 2006 Unit Option Plan: (i) an “employee” means any person, including officers, directors or affiliates who are employed by us or by our affiliates; (ii) a “consultant” means any person who is engaged by us to render consulting or advisory services to us or to any of our entities provided that such services are provided in good faith and are (a) not in connection with the offer or sale of our securities in a capital raising transaction and (b) not directly or indirectly promoting or maintaining a market for our securities; (iii) a “service provider” means an employee, director, supplier or officer holder as defined in the Israeli Companies Law; and (iv) an “affiliate” means any entity which is directly or indirectly our parent or subsidiary.
Our board of directors has the authority to administer the 2006 Unit Option Plan and to grant options under the 2006 Unit Option Plan, including, without limitation, the authority to determine the persons to whom options shall be granted, the number of shares subject to each option, the time or times at which the options will be granted, restrictions on the transferability of the options, and the schedule and conditions on which such options may be exercised.
The 2006 Unit Option Plan provides for the grant of options pursuant to Sections 102 and 3(i) of the Israeli Income Tax Ordinance [New Version], 5721-1961, which we refer to as the Tax Ordinance. The 2006 Unit Option Plan provides that Section 102 options may be granted only to employees who are Israeli residents and who do not own interests possessing more than 10% of the total combined voting power of all classes of our equity or the equity of our affiliates immediately before such option is granted. Options granted to optionees who are Israeli residents that are not intended to qualify as Section 102 Options are granted under Section 3(i) of the Ordinance, which does not provide for similar tax benefits, and are referred to as Section 3(i) options. The 2006 Unit Option Plan was submitted for the approval of the Israeli Tax Authority, which we refer to as the ITA, as required by applicable law.
Section 102 of the Tax Ordinance allows employees, directors and officers, who are not controlling shareholders and who are Israeli residents, to receive favorable tax treatment for compensation granted in the form of shares or options. Section 102 of the Ordinance includes two alternatives for tax treatment involving the issuance of options or shares to a trustee for the benefit of the grantees, which are referred to as the capital gains track and the ordinary income track, and also includes an additional alternative for the issuance of options or shares issued directly to the grantee. Under the 2006 Unit Option Plan, Section 102 options may be designated as options granted under any one of the foregoing alternatives and such designation shall be included in the option agreement for the option award.
128
Options granted to date to employees under the 2006 Unit Option Plan were granted under Section 102(b)(2) of the Tax Ordinance, which permits the issuance to a trustee under the “capital gains track.” In order to comply with the terms of the capital gains track, all options granted under a specific plan and subject to the provisions of Section 102 of the Tax Ordinance, as well as the shares issued upon exercise of such options and other shares received subsequently following any realization of rights with respect to such options, such as share dividends and share splits, must be registered in the name of a trustee selected by the board of directors and held in trust for the benefit of the relevant employee, director or officer for a period of two years from the date of the grant. However, under this track, we are not allowed to deduct an expense with respect to the issuance of the options or shares.
The exercise price of an option granted under the 2006 Unit Option Plan is determined by the board of directors or a committee appointed by it. The first option grant to an employee generally vests over a period of three years and nine months commencing on the date of grant, such that 8.33% vest on the first anniversary of the date of grant and an additional 8.33% vest on each subsequent three-month period thereafter, for 33 months. Additional option grants to an employee generally vest over a period of two years and nine months commencing on the date of grant, such that 8.33% is fully vested on the date of grant and an additional 8.33% vest on each subsequent three-month period thereafter, for 33 months. Options granted under the 2006 Unit Option Plan generally expire within ten years of the grant date or upon the earlier termination of employment of, or services provided by, the optionee, as applicable, subject to the extended period of exercisability upon termination of employment or services, as applicable. Our board of directors may determine a shorter exercisability period for an option at the time of grant of such option. Upon termination of the employment of or services rendered by an optionee, as applicable (other than for cause, disability or death), generally vested options may be exercised within three months after the date of such termination or within such shorter time period (not to be less than 30 days) or such longer time period (not to exceed five years) as our board of directors or a committee appointed by it shall determine, but in any event no later than the expiration date of the options. If the employment or services of the optionee are terminated because of death or disability (or if the optionee dies within three months after termination of employment or services other than for cause), the optionee’s options may be exercised by the optionee or the optionee’s legal representative or authorized assignee to the extent exercisable on the date of such termination or within 12 months thereafter or as otherwise determined by the board of directors or a committee appointed by it. If the employment or services of the optionee are terminated for cause, all outstanding options will, to the extent not previously exercised, be of no force and effect as of the date of termination, unless otherwise determined by the board of directors or a committee appointed by it.
In the event of a merger or consolidation of our company in which our company is not the surviving entity, an acquisition of all or substantially all of the outstanding capital of our company or the sale of all or substantially all of our assets, the optionee shall be provided the opportunity to (i) exercise his or her options in connection with the transaction and to receive in the transaction such consideration as the holder of ordinary shares shall receive in the transaction or (ii) retain his or her options or receive a substitute option from the surviving company, if any.
As of June 30, 2014, we had granted options to purchase an aggregate 16,990,075 ordinary shares under the 2006 Unit Option Plan, of which options to purchase an aggregate 34,882 ordinary shares had been exercised into our ordinary shares, options to purchase an aggregate 2,514,434 ordinary shares had been forfeited without having been exercised and options to purchase an aggregate 14,430,911 ordinary shares were outstanding at a weighted average exercise price of approximately $0.14 per share. As of such date, 1,790,091 ordinary shares were available for future grants under the 2006 Unit Option Plan.
129
RELATED PARTY TRANSACTIONS
Other than the executive and director compensation and indemnification and exculpation arrangements discussed in “Management,” and the transaction described below, we have not entered into any transactions since January 1, 2011 to which we have been or are a party to and in which any of our directors, executive officers or holders of more than 5% of our share capital, or any immediate family member of, or person sharing the household with, any of these individuals or entities, had or will have a direct or indirect material interest.
Credit Line Agreement; Private Placement
On August 20, 2014, we entered into a certain credit line agreement, pursuant to which we obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders, including Pontifax, Docor International B.V. and Counterpoint Ventures Fund II LP. The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014, and may be released or called by us under certain terms and conditions. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations-Liquidity and Capital Resources-Sources of Liquidity.” Assuming that we call the full credit line amount for investment in our ordinary shares in the Private Placement and assuming that the purchase price per ordinary share sold in the Private Placement is $ , the midpoint of the range set forth on the cover page of this prospectus, we anticipate that Pontifax, Docor International, B.V and Counterpoint Ventures Fund II LP will acquire , and ordinary shares in the Private Placement for an aggregate purchase price of $ , $ and $ respectively.
Pontifax Warrant
On October 14, 2014, we issued a warrant to purchase 4,430,758 of our ordinary shares to Pontifax in consideration of its commitment to provide us, for no consideration, the following services, if and to the extent requested by us: (i) business development services, in such scope and substance as shall be agreed between us and Pontifax; and (ii) a representative designated by Pontifax to serve as the chairman of our board of directors. The warrant has an exercise price per share equal to NIS 0.01. The warrant vests on a quarterly basis in eight equal installments during a period of 24 months from issuance and expire ten years following their issuance, i.e., on October 14, 2024. Upon the closing of this offering or a reverse merger, private placement, PIPE (private investment in public equity) transaction or any other form of equity financing in our company, any unvested portion of the warrant will become fully vested and exercisable.
Share Purchase Agreement
We entered into a Share Purchase Agreement, dated as of March 4, 2011, with Pontifax (Cayman) II, L.P., Pontifax (Israel) II L.P., Pontifax (Israel) II- Individual Investors L.P., BXR Portfolio Limited (formerly named Eastern Petroleum Investment Company Limited), Docor International B.V., Jacobs Investment Company LLC and certain additional investors for the private placement of 24,545,195 of our Series D-1 preferred shares and warrants for the purchase of 16,199,826 of our Series D-2 preferred shares, for an aggregate consideration of $9.3 million. All such warrants are exercisable no later than March 16, 2015. All of such Series D-1 preferred shares will convert into ordinary shares immediately prior to the closing of this offering.
Shareholders Agreement
Contemporaneously with the closing of the Share Purchase Agreement described above, we entered into a shareholders agreement, dated as of March 17, 2011, or the Shareholders Agreement, to which each of the holders of our preferred shares, including Pontifax (Cayman) II, L.P., Pontifax (Israel) II L.P., Pontifax (Israel) II- Individual Investors L.P., BXR Portfolio Limited (formerly named Eastern Petroleum Investment Company Limited), Docor International B.V., Spearhead Investments (Bio) Ltd., Jacobs Investment Company LLC and Emigrant Alternative Portfolios LLC, is a party, either as an original signatory or by virtue of a joinder thereto, and certain of the holders of our ordinary shares, including Yoav Kimchy and Sigalit Kimchy are parties. Prior to the closing of this offering, we will amend and restate the Shareholders Agreement such that it will consist primarily of the provisions regarding registration rights described below, and the undertaking discussed below under “—Transactions with Check-Cap LLC and the Members and Manager of Check-Cap LLC.”
130
Demand Registration Rights
At any time after the closing of this offering, but subject to the terms of the 180-day lock-up agreements entered into between certain of the parties to the Shareholders Agreement and the underwriters of this offering, at the request of, either (i) holders of a majority of our former Series D preferred shares (all of which will convert into ordinary shares immediately prior to the closing of this offering), or (ii) holders of a majority of our former Series C preferred shares (all of which will convert into ordinary shares immediately prior to the closing of this offering), or (iii) persons holding at least 20% of the ordinary shares issued upon conversion of the former preferred shares or in respect thereof, we must register any or all of such shareholders’ ordinary shares. We are required to effect up to two such registrations for each former holder of our preferred shares, with respect to all of such person’s or entities’ shares. We will be required to give notice of a demand registration to the other holders of registrable securities that will be entitled to registration rights and include their shares in such registration if they so timely request.
Piggyback Registration Rights
Following our initial public offering, all of the former holders of our preferred shares that are a party to the Shareholders Agreement will have the right to request that we include their registrable securities in certain registration statements that we file in connection with the public offering of our shares. We will be required to give notice of our intention to effect such a registration to all holders of our registrable securities that will be entitled to registration rights and include their shares in such registration if they so timely request.
Registration Priority
To the extent it is not in our best interest for all of the former holders of our preferred shares to participate in any demand or piggyback registration, the shares to be included in the registration statement on behalf of the former preferred shareholders shall be allocated as follows: first, shares sought to be registered by the former holders of our Series D preferred shares; second, shares sought to be registered by the former holders of Series C preferred shares; third, shares sought to be registered by the former holders of our Series A preferred shares; and fourth, shares sought to be registered by the former holders of our Series B preferred shares.
Expenses
We have agreed to pay all expenses incurred in carrying out the above registrations, including the fees of one counsel chosen by the selling shareholders that are a party to the Shareholders Agreement. However, we shall not be required to pay any registration expenses in connection with any initiated registration that is subsequently withdrawn, other than a withdrawal due to a material adverse change not known to the holder of registrable securities at the time of such demand or request by us or the underwriters to reduce the size of the offering as a result of which the holders of at least a majority of the registrable securities elect to withdraw. A registration shall not count as a permitted registration until it has remained effective for a period of at least 120 days.
Transactions with Check-Cap LLC and the Members and Manager of Check-Cap LLC
On May 31, 2009, we entered into an asset transfer agreement with Check-Cap LLC pursuant to which Check-Cap LLC transferred all of its business operations and substantially all of its assets to us. Our shareholders’ holdings on the date of the asset transfer transaction reflected their interests as members of Check-Cap LLC. In the framework of the asset transfer agreement and under the Shareholders Agreement, we undertook to use commercially reasonable efforts to procure that distributions or advance funds are made to our shareholders holding (at the date of the transaction) ordinary shares, Series A preferred shares and/or Series B preferred shares (i.e., the shareholders who are also members of Check-Cap LLC), as would be necessary to eliminate the tax impact on such shareholders of the reorganization and the transfer of all of the business operations and substantially all of the assets from Check-Cap LLC to us. Notwithstanding the foregoing, we will not advance payments to such shareholders to address the fact that they will no longer receive a “pass through” of losses generated by us as they previously received while owning units of Check-Cap LLC. These advances, if and to the extent made, will be deducted from any distributions such shareholders are entitled to receive from us. We have reserved $749,000 in our financial statements for the six months ended June 30, 2014 on account of such advances.
In connection with the asset transfer agreement, we assumed the former obligation of Check-Cap LLC to distribute any proceeds it collects on the $1 million key man life insurance policy with respect to Yoav Kimchy to the former holders of the Series A preferred units in an amount equal to their respective capital contributed to Check-Cap LLC, less any amounts previously distributed to them, plus any accrued and unpaid dividends due to them as of the date of distribution.
Check-Cap Ltd. (Delaware), which is the manager of Check-Cap LLC and is wholly-owned by Mr. Kimchy, our chief technology officer and a director, handles from time to time certain logistical, administrative and investor relations matters for us with our U.S. suppliers and investors under a Services Agreement dated October 1, 2010, which expires on December 31, 2014. Under the agreement, we agreed to pay Check-Cap Ltd. (Delaware) in consideration of its services a quarterly payment of $20,000, which amount essentially covers its costs of performing such functions. We last utilized such services as of December 31, 2012; however, we may utilize such services again in the future. For the year ended December 31, 2012, we paid Check-Cap Ltd. (Delaware) an aggregate of $80,000 for such services.
131
PRINCIPAL SHAREHOLDERS
The following table sets forth information regarding beneficial ownership of our ordinary shares as of (i) immediately prior to this offering and (ii) as adjusted to give effect to this offering and the concurrent Private Placement, by:
|
|
•
|
each person, or group of affiliated persons, known to us to be the beneficial owner of 5% or more of our outstanding shares;
|
|
|
•
|
each of our directors and executive officers; and
|
|
|
•
|
all of our directors and executive officers as a group.
|
Beneficial ownership is determined in accordance with the rules of the U.S. Securities and Exchange Commission. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting or investment power with respect to those securities, and include shares subject to options and warrants that are exercisable within 60 days following November 21, 2014. Such shares are also deemed outstanding for purposes of computing the percentage ownership of the person holding the option or warrant, but not the percentage ownership of any other person. The percentage of beneficial ownership of our ordinary shares before the offering is based on [ ] ordinary shares issued and outstanding as of November 21, 2014. The percentage of beneficial ownership of our ordinary shares after the offering is based on ordinary shares outstanding after the offering and the concurrent Private Placement, which includes the ordinary shares isssuable upon the conversion of our preferred shares into ordinary shares immediately prior to the consummation of this offering plus the ordinary shares to be sold by us in the offering and the Private Placement, assuming the underwriter does not exercise its over-allotment option.
As of November 21, 2014, to our knowledge there were 98 record holders of our ordinary shares. To our knowledge, as of November 21, 2014, 46 of the record holders of our ordinary shares were in the United States, which held, in the aggregate 30.6 of our outstanding ordinary shares as of such date. Following the adoption of our amended and restated articles of association, none of our shareholders will have voting rights different from the voting rights of other shareholders. To the best of our knowledge, we are not owned or controlled, directly or indirectly, by another corporation or by any government. We are not aware of any arrangement that may, at a subsequent date, result in a change of control of our company.
Except as indicated in the footnotes below, we believe that the persons named in the table below have sole voting and investment power with respect to the ordinary shares indicated in the table as being beneficially owned by them. Unless otherwise noted below, each shareholder’s address is c/o Check-Cap Ltd., Check-Cap Building, Abba Hushi Ave., P.O. Box 1271, Isfiya, 30090, Mount Carmel, Israel.
|
Ordinary Shares Beneficially Owned Prior to this Offering
|
Ordinary Shares Beneficially Owned After this Offering and the Private Placement (Assuming No Exercise of the Over-Allotment Option)
|
Ordinary Shares Beneficially Owned After this Offering and the Private Placement (Assuming Full Exercise of the Over-Allotment Option)
|
||||||||||||||||||||||
|
Name and Address of Beneficial Owner
|
Number
|
Percent
|
Number
|
Percent
|
Number
|
Percent
|
||||||||||||||||||
|
Pontifax(1)(11)
|
28,493,491 | 25.59 | % | |||||||||||||||||||||
|
Shanghai Fosun Pharmaceutical Group Co. Ltd.(2)(11)
|
17,723,031 | 13.90 | % | |||||||||||||||||||||
|
Yoav and Sigalit Kimchy(3)(11)
|
13,209,776 | 12.03 | % | |||||||||||||||||||||
|
BXR Portfolio Limited(4)
|
11,135,453 | 9.75 | % | |||||||||||||||||||||
|
Docor International B.V.(5)(12)
|
9,420,292 | 8.50 | % | |||||||||||||||||||||
|
Spearhead Investments (Bio) Ltd.(6)(12)
|
6,740,191 | 6.14 | % | |||||||||||||||||||||
|
Jacobs Investment Company LLC(7)(12)
|
5,954,704 | 5.40 | % | |||||||||||||||||||||
|
Emigrant Alternative Investments LLC(8)(12)
|
5,800,389 | 5.28 | % | |||||||||||||||||||||
|
Counterpoint Ventures Fund L.P.(9)(12)
|
5,742,015 | 5.22 | % | |||||||||||||||||||||
|
Directors and Executive Officers:
|
||||||||||||||||||||||||
|
Guy Neev(10)
|
7,197,203 | 6.23 | % | |||||||||||||||||||||
|
Lior Torem
|
* | * | ||||||||||||||||||||||
|
Yoav Kimchy(11)
|
13,209,776 | 12.03 | % | |||||||||||||||||||||
|
Alex Ovadia
|
* | * | ||||||||||||||||||||||
|
Tomer Kariv(1)(12)
|
28,493,491 | 25.59 | % | |||||||||||||||||||||
|
Walter L. Robb(9)(12)
|
5,884,498 | 5.35 | % | |||||||||||||||||||||
|
Richard Stone
|
* | * | ||||||||||||||||||||||
|
Alon Dumanis
|
-- | -- | ||||||||||||||||||||||
|
Directors and executive officers as a group (8 persons)(12)
|
56,621,918 | 50.86 | % | |||||||||||||||||||||
132
|
___________________
|
|
*
|
Less than 1% of our outstanding ordinary shares.
|
|
(1)
|
Includes (i) 8,865,752 preferred shares directly held by Pontifax (Cayman) II L.P. that are convertible (at an assumed 1 for 1 conversion rate) into 8,865,752 ordinary shares within 60 days of November 21, 2014, warrants to purchase 1,543,311 preferred shares (which are convertible into 1,543,311 ordinary shares at an assumed 1 for 1 conversion rate) directly held by Pontifax (Cayman) II L.P. that are exercisable within 60 days of November 21, 2014, warrants to purchase 3,248,880 ordinary shares directly held by Pontifax (Cayman) II L.P. that are exercisable within 60 days of November 21, 2014 and warrants to purchase 270,740 ordinary shares directly held by Pontifax (Cayman) II L.P. that are exercisable within 60 days of November 21, 2014; (ii) 6,678,235 preferred shares directly held by Pontifax (Israel) II L.P. that are convertible (at an assumed 1 for 1 conversion rate) into 6,678,235 ordinary shares within 60 days of July 3, 2014, warrants to purchase 1,162,518 preferred shares (which are convertible into 1,162, 518 ordinary shares at an assumed 1 for 1 conversion rate) directly held by Pontifax (Israel) II L.P. that are exercisable within 60 days of November 21, 2014, warrants to purchase 2,447,258 ordinary shares directly held by Pontifax (Israel) II L.P. that are exercisable within 60 days of November 21, 2014 and warrants to purchase 203,938 ordinary shares directly held by Pontifax (Israel) II L.P. that are exercisable within 60 days of November 21, 2014; and (iii) 2,592,417 preferred shares directly held by Pontifax (Israel) II – Individual Investors LLP that are convertible (at an assumed 1 for 1 conversion rate) into 2,592,417 ordinary shares within 60 days of November 21, 2014, warrants to purchase 451,277 preferred shares (which are convertible into 451,277 ordinary shares at an assumed 1 for 1 conversion rate) directly held by Pontifax (Israel) II – Individual Investors LLP that are exercisable within 60 days of November 21, 2014, warrants to purchase 949,999 ordinary shares directly held by Pontifax (Israel) II – Individual Investors LLP that are exercisable within 60 days of November 21, 2014 and warrants to purchase 79,167 ordinary shares directly held by Pontifax (Israel) II – Individual Investors LLP that are exercisable within 60 days of November 21, 2014. Does not include (i) warrants held by the foregoing entities to purchase an aggregate of 2,065,289 preferred shares that are not exercisable within 60 days of November 21, 2014; and (ii) warrants held by the foregoing entities to purchase an aggregate of 3,876,913 ordinary shares that are not exercisable within 60 days of November 21, 2014, which will become fully vested (to the extent not previously vested) upon completion of this offering. Pontifax Management II L.P., or Pontifax Management, is the general partner of Pontifax (Cayman) II, L.P., Pontifax (Israel) II, L.P. and Pontifax (Israel) II – Individual Investors, L.P., and Pontifax Management 2 G.P. (2007) Ltd., or Pontifax Management GP, is the general partner of Pontifax Management. Pontifax has further advised us that Mr. Tomer Kariv, the chairman of our board of directors, and Mr. Ran Nussbaum are directors of Pontifax Management GP and, as such, hold voting and dispositive power over the shares held by the Pontifax entities. |
133
|
(2)
|
Includes warrants to purchase 17,723,031 ordinary shares held by Shanghai Fosun Pharmaceutical Group Co. Ltd. that are exercisable within 60 days of November 21, 2014.
|
|
(3)
|
Includes (i) 6,391,042 ordinary shares held directly by Yoav Kimchy; (ii) options to purchase 427,692 ordinary shares held by Yoav Kimchy that are exercisable within 60 days of November 21, 2014; and (iii) 6,391,042 ordinary shares held directly by Sigalit Kimchy. Does not include (i) options to purchase 266,124 ordinary shares held by Yoav Kimchy that will become exercisable upon completion of this offering; and (ii) options to purchase 1,453,842 ordinary shares held by Yoav Kimchy that are not exercisable within 60 days of November 21, 2014, which will become fully vested (to the extent not previously vested) upon completion of this offering. Yoav Kimchy, our chief technology officer and a director, and Sigalit Kimchy are husband and wife.
|
|
|
(4)
|
Includes (i) 6,759,724 preferred shares that are convertible (at an assumed 1 for 1 conversion rate) into 6,759,724 ordinary shares within 60 days of November 21, 2014; and (ii) warrants to purchase 4,375,729 preferred shares (which are convertible into 4,375,729 ordinary shares at an assumed 1 for 1 conversion rate) that are exercisable within 60 days of November 21, 2014. BXR Portfolio Limited (formerly named Eastern Petroleum Investment Company Limited) has advised us that it is a wholly-owned subsidiary of BXR Group Limited. BXR Group Limited has advised us that the board of directors of BXR Group Limited possesses the ultimate voting and investment power over the shares held by BXR Portfolio Limited.
|
|
(5)
|
Includes (i) 6,557,305 preferred shares that are convertible (at an assumed 1 for 1 conversion rate) into 6,557,305 ordinary shares within 60 days of November 21, 2014; (ii) warrants to purchase 647,608 preferred shares (which are convertible into 647,608 ordinary shares at an assumed 1 for 1 conversion rate) that are exercisable within 60 days of November 21, 2014; and (iii) warrants to purchase 2,215,379 ordinary shares that are exercisable within 60 days of November 21, 2014. Does not include warrants to purchase 640,081 ordinary shares that are not exercisable within 60 days of November 21, 2014. Docor International B.V. has advised us that it is a wholly-owned subsidiary of Crecor B.V., which is a wholly-owned subsidiary of the Van Leer Group Foundation. Docor International B.V. has advised us that the supervisory board and managing board of Crecor B.V. are responsible for the investments of Crecor B.V. and as such, possess the ultimate voting and investment power over the shares held by Docor International B.V. |
|
|
(6)
|
Includes 6,740,191 preferred shares that are convertible (at an assumed 1 for 1 conversion rate) into 6,740,191 ordinary shares within 60 days of November 21, 2014. Does not include warrants to purchase 773,709 ordinary shares that are not exercisable within 60 days of November 21, 2014. Spearhead Investments (Bio) Ltd. has advised us that it is wholly-owned by Biomedix Incubator Ltd., an Israeli company whose shares are publicly traded on the Tel Aviv Stock Exchange.
|
|
|
(7)
|
Includes (i) 5,517,131 preferred shares that are convertible (at an assumed 1 for 1 conversion rate) into 5,517,131 ordinary shares within 60 days of November 21, 2014; and (ii) warrants to purchase 437,573 preferred shares (which are convertible into 437,573 ordinary shares at an assumed 1 for 1 conversion rate) that are exercisable within 60 days of November 21, 2014. Does not include warrants to purchase 557,209 ordinary shares that are not exercisable within 60 days of November 21, 2014. Mr. Gary Jacobs has advised us that he serves as the Managing Director of Jacobs Investment Company, LLC, and as such, possesses the ultimate voting and investment power over the shares held by Jacobs Investment Company LLC.
|
134
|
|
(8)
|
Includes 5,800,389 preferred shares that are convertible (at an assumed 1 for 1 conversion rate) into 5,800,389 ordinary shares within 60 days of November 21, 2014. Does not include warrants to purchase 665,829 ordinary shares that are not exercisable within 60 days of November 21, 2014. Emigrant Alternative Investments LLC has advised us that it is a wholly-owned subsidiary of Emigrant Bank, which is a wholly-owned subsidiary of Emigrant Bancorp, Inc., which is a wholly-owned subsidiary of New York Private Bank & Trust Corporation (“NYPBTC”), which is wholly owned by trusts benefitting the family of Paul Milstein (the “Trusts”) and by some members of that family. Each of the foregoing may be deemed to be a beneficial owner of the shares held by Emigrant Alternative Investments LLC. Emigrant Alternative Investments LLC has advised us that Howard Milstein, through control of the Trusts, which own 100% of the voting stock of NYPBTC, and in his capacity as an officer of NYPBTC, may be deemed to have sole power to vote and dispose of the shares held by Emigrant Alternative Investments LLC. |
|
|
(9)
|
Includes: (i) 4,612,172 preferred shares that are convertible (at an assumed 1 for 1 conversion rate) into 4,612,172 ordinary shares within 60 days, held by Counterpoint Ventures Fund LP and Counterpoint Ventures Fund II LP; and (ii) warrants to purchase 1,129,843 ordinary shares that are exercisable within 60 days of November 21, 2014 held by Counterpoint Ventures Fund II LP. Mr. Robb has advised us that the general partner of each of Counterpoint Ventures Fund LP and Counterpoint Ventures Fund II LP is Lion Development LLC, which is 99% controlled by Mr. Walter Robb, and as such, Walter Robb possesses the ultimate voting and investment power over the shares held by the Counterpoint Ventures entities. Does not include warrants to purchase 529,434 ordinary shares held in the aggregate by the Counterpoint entities that are not exercisable within 60 days of November 21, 2014. In addition, Mr. Walter Robb directly holds options to purchase 142,483 ordinary shares that are exercisable within 60 days of November 21, 2014 and options to purchase 16,356 ordinary shares that are not exercisable within 60 days of November 21, 2014. |
|
|
(10)
|
Includes options to purchase 7,197,203 ordinary shares exercisable within 60 days of November 21, 2014. Does not include (i) options to purchase 266,100 ordinary shares that will become exercisable upon completion of this offering; and (ii) options to purchase 5,815,369 ordinary shares that are not exercisable within 60 days of November 21, 2014, which will become fully vested (to the extent not previously vested) upon completion of this offering.
|
|
|
(11)
|
See footnote (2).
|
|
|
(12)
|
Does not include warrants to purchase 2,065,289 ordinary shares held by the Pontifax entities, warrants to purchase 640,081 ordinary shares held by Docor International B.V., warrants to purchase 773,709 ordinary shares held by Spearhead Investments (Bio) Ltd., warrants to purchase 557,209 ordinary shares held by Jacobs Investment Company LLC, warrants to purchase 665,829 ordinary shares held by Emigrant Alternative Investments LLC and warrants to purchase 529,434 ordinary shares held by the Counterpoint Ventures entities (collectively referred to as the “Anti-Dilution Warrants”). The Anti-Dilution Warrants were issued as anti-dilution protection in connection with the grant to Guy Neev of the Neev Options. The Anti-Dilution Warrants will be automatically exercised, without consideration, if and when Guy Neev exercises any part of the Neev Options, in proportion to the portion of the Neev Options exercised by Guy Neev (and with respect to Anti- Dilution Warrants to purchase 211,718 ordinary shares held by the Pontifax entities, in proportion to the number of warrants held by the Pontifax entities with respect to which such Anti-Dilution Warrants were granted that were exercised prior to the exercise of the Neev Options). |
135
DESCRIPTION OF SHARE CAPITAL
The following description of our share capital and provisions of our amended articles of association is a summary. This summary is subject to the Israeli Companies Law and to the complete text of our amended and restated articles of association (which we will adopt substantially in the form attached as Exhibit 3.2 to the registration statement, of which this prospectus is a part, prior to the completion of this offering).
General
Immediately prior and subject to the consummation of this offering, our authorized share capital will consist solely of ordinary shares, par value NIS 0.01 per share, of which shares will be issued and outstanding (assuming that the underwriters do not exercise their option to purchase additional ordinary shares).
All of our outstanding ordinary shares will be validly issued, fully paid and non-assessable. Our ordinary shares are not redeemable and do not have any preemptive rights.
Warrants
As of November 21, 2014, the following warrants were outstanding:
|
|
•
|
the Anti-Dilution Warrants to purchase 7,777,113 ordinary shares, which will be automatically exercised, without consideration, unless the holder thereof objects to such exercise, if and when Guy Neev exercises any part of the Neev Options, in proportion to the portion of the Neev Options exercised by Guy Neev (and with respect to 273,592 of such warrants, in proportion to the number of warrants with respect to which such 273,592 warrants were granted that were exercised prior to the exercise of the Neev Options);
|
|
|
•
|
warrants to purchase 836,412 Series C-1 preferred shares with an exercise price of $0.2495 per share. The warrants are exercisable on a cashless basis;
|
|
|
•
|
warrants to purchase 1,546,996 Series C-2 preferred shares with an exercise price of $0.269 per share. Of such warrants, warrants to purchase 1,007,975 Series C-2 preferred shares are exercisable on a cashless basis; 1,007,975 C-2 warrants (with cashless exercise) were issued to Pontifax and 539,021 C2 warrants (without cashless exercise) were issued to placement agents;
|
|
|
•
|
warrants to purchase 503,872 Series D-1 preferred shares with an exercise price of $0.37708 per share;
|
|
|
•
|
warrants to purchase 16,611,146 Series D-2 preferred shares with an exercise price of $0.47135 per share; and
|
|
|
•
|
warrants to purchase 57,599,850 ordinary shares with an exercise price of NIS 0.01 per share, of which (i) warrants to purchase 53,169,092 ordinary shares are fully vested; and (ii) warrants to purchase 4,430,758 ordinary shares will become fully vested upon the closing of this offering.
|
All of the warrants to purchase preferred shares will convert into warrants to purchase ordinary shares immediately prior to and subject to the consummation of this offering.
Options
As of November 21, 2014, the following options were outstanding:
|
|
•
|
options to purchase 12,415,436 of our ordinary shares (which include options to purchase 936,936 ordinary shares issued as anti-dilution protection in connection with the grant to Guy Neev of the Neev Options), with a weighted average exercise price of $0.17 per share, were outstanding under our 2006 Unit Option Plan. Of such outstanding options, options to purchase 10,360,213 of our ordinary shares, with a weighted average exercise price of $0.17 per share, were vested as of such date;
|
|
|
•
|
options to purchase 11,630,739 of our ordinary shares, with an exercise price of NIS 0.01 per share, were outstanding under our 2006 Unit Option Plan, which will become fully vested upon the closing of this offering;
|
136
|
|
•
|
the Neev Options for 1,995,475 shares; and
|
|
|
•
|
769,453 ordinary shares issuable upon the exercise of options with an exercise price of $0.2478 per ordinary share, under our 2006 Unit Option Plan, which we have agreed that certain executive officers will be entitled to upon completion of an equity financing, which includes this offering.
|
Share History
The following is a summary of our security issuances since January 1, 2011:
|
|
•
|
Since January 1, 2011, we granted options to purchase an aggregate of 28,620,814 ordinary shares, in each case having an exercise price per share ranging from $0.00270 to $0.24780, to certain of our employees, officers and consultants under our 2006 Unit Option Plan. Of such options, options to purchase an aggregate of 1,606,562 ordinary shares have been forfeited and cancelled without being exercised as of the date of this prospectus.
|
|
|
•
|
In October 2012, we issued and sold 14,780 ordinary shares pursuant to the exercise of options held by an employee, having an exercise price per share of $0.22270.
|
|
|
•
|
Pursuant to a Share Purchase Agreement dated March 4, 2011 between us and the investors identified therein, we issued an aggregate 24,545,195 of our Series D-1 preferred shares and warrants for the purchase of 16,199,826 of our Series D-2 preferred shares, for aggregate consideration of $9.3 million. In addition, as partial consideration for brokerage services in connection with such financing, we issued warrants to purchase 503,872 Series D-1 preferred shares and warrants to purchase 411,320 Series D-2 preferred shares.
|
|
|
•
|
Pursuant to a Share Purchase Agreement dated January 10, 2012 between us and the investors identified therein, we issued a total of 2,510,783 Series D-3 preferred shares, for aggregate consideration of $1.1 million.
|
|
|
•
|
Pursuant to a Credit Line Agreement dated August 20, 2014 between us and the lenders identified therein, we issued warrants for the purchase of an aggregate 53,169,092 ordinary shares.
|
|
|
•
|
On October 14, 2014, we issued a warrant for the purchase of 4,430,758 ordinary shares to an existing shareholder as consideration for certain services it may provide at our request.
|
Registration Number and Purposes of the Company
Our registration number with the Israeli Registrar of Companies is 51-425981-1. Our purpose as set forth in our amended articles of association is to engage in any lawful activity. Our amended and restated articles of association state that the liability of our shareholders is limited, subject to the provisions of the Israeli Companies Law.
Voting Rights and Conversion
All ordinary shares have identical voting and other rights in all respects.
Transfer of Shares
Our fully paid ordinary shares are issued in registered form and may be freely transferred under our amended articles of association, unless the transfer is restricted or prohibited by another instrument, applicable law or the rules of a stock exchange on which the shares are listed for trade. The ownership or voting of our ordinary shares by non-residents of Israel is not restricted in any way by our amended articles of association or the laws of the State of Israel, except for ownership by nationals of some countries that are, or have been, in a state of war with Israel.
Election of Directors
Our ordinary shares do not have cumulative voting rights for the election of directors. As a result, the holders of a majority of the voting power represented at a shareholders meeting have the power to elect all of our directors, subject to the special approval requirements for external directors described under “Management — External Directors.”
137
Under our amended articles of association to be effective upon the closing of this offering, our board of directors must consist of not less than but no more than directors, including at least two external directors as required by the Israeli Companies Law. Pursuant to our amended articles of association, other than the external directors, for whom special election requirements apply under the Israeli Companies Law, each of our directors will be appointed by a simple majority vote of holders of our voting shares, participating and voting at an annual general meeting of our shareholders. Each director (other than external directors) will hold office until the next annual general meeting following the annual general meeting at which they were elected and until his or her successor is elected and qualified, or until his or her earlier resignation, death or removal by a vote of the majority of the voting power of our shareholders at a general meeting of until his or her office expires by operation of law, in accordance with the Israeli Companies Law. In addition, our amended articles of association allow our board of directors to appoint directors (other than external directors) to fill vacancies on the board of directors to serve for a term of office equal to the remaining period of the term of office of the directors(s) whose office(s) have been vacated. External directors are elected for an initial term of three years, may be elected for additional terms of three years each under certain circumstances, and may be removed from office pursuant to the terms of the Israeli Companies Law. See “Management — Board Practices — External Directors.”
Dividend and Liquidation Rights
We may declare a dividend to be paid to the holders of our ordinary shares in proportion to their respective shareholdings. Under the Israeli Companies Law, dividend distributions are determined by the board of directors and do not require the approval of the shareholders of a company unless the company’s articles of association provide otherwise. Our amended articles of association do not require shareholder approval of a dividend distribution and provide that dividend distributions may be determined by our board of directors.
Pursuant to the Israeli Companies Law, we may declare and pay dividends only if, upon the determination of our board of directors, there is no reasonable concern that the distribution will prevent us from being able to meet the terms of our existing and foreseeable obligations as they become due. Under the Israeli Companies Law, the distribution amount is further limited to the greater of retained earnings or earnings generated over the two most recent years legally available for distribution according to our then last reviewed or audited financial statements, provided that the date of the financial statements is not more than six months prior to the date of distribution. In the event that we do not have retained earnings or earnings generated over the two most recent years legally available for distribution, we must seek the approval of the court in order to distribute a dividend. The court may approve our request if it is convinced that there is no reasonable concern that the payment of a dividend will prevent us from satisfying our existing and foreseeable obligations as they become due.
In the event of our liquidation, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of our ordinary shares in proportion to their shareholdings. This right, as well as the right to receive dividends, may be affected by the grant of preferential dividend or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Exchange Controls
There are currently no Israeli currency control restrictions on remittances of dividends on our ordinary shares, proceeds from the sale of the shares or interest or other payments to non-residents of Israel, except for shareholders who are subjects of countries that are, or have been, in a state of war with Israel.
138
Shareholder Meetings
Under Israeli law, we are required to hold an annual general meeting of our shareholders once every calendar year that must be held no later than 15 months after the date of the previous annual general meeting. All meetings other than the annual general meeting of shareholders are referred to in our amended articles of association as extraordinary general meetings. Our board of directors may call extraordinary general meetings whenever it sees fit, at such time and place, within or outside of Israel, as it may determine. In addition, the Israeli Companies Law provides that our board of directors is required to convene an extraordinary general meeting upon the written request of (i) any two of our directors or one-quarter of the serving members of our board of directors or (ii) one or more shareholders holding, in the aggregate, either (a) 5% or more of our outstanding shares and 1% of our outstanding voting power or (b) 5% or more of our outstanding voting power.
Furthermore, the Israeli Companies Law requires that resolutions regarding the following matters be approved by our shareholders at a general meeting:
|
|
•
|
amendments to our articles of association;
|
|
|
•
|
appointment, terms of service and termination of service of our auditors;
|
|
|
•
|
appointment of external directors;
|
|
|
•
|
approval of certain related party transactions;
|
|
|
•
|
increases or reductions of our authorized share capital;
|
|
|
•
|
mergers; and
|
|
|
•
|
the exercise of our board of director’s powers by a general meeting, if our board of directors is unable to exercise its powers and the exercise of any of its powers is essential for our proper management.
|
Subject to the provisions of the Israeli Companies Law and regulations promulgated thereunder, shareholders entitled to participate and vote at general meetings are the shareholders of record on a date to be decided by the board of directors, which, as a company listed on an exchange outside Israel, may be between four and 40 days prior to the date of the meeting.
The Israeli Companies Law and our amended articles of association require that a notice of any annual general meeting or extraordinary general meeting be provided to shareholders at least 21 days prior to the meeting and if the agenda of the meeting includes, among other things, the appointment or removal of directors, the approval of transactions with office holders or interested or related parties, an approval of a merger or the approval of the compensation policy, notice must be provided at least 35 days prior to the meeting.
Under the Israeli Companies Law and under our amended articles of association, our shareholders are not permitted to take action via written consent in lieu of a meeting.
Voting rights
Quorum Requirements
Pursuant to our amended articles of association, holders of our ordinary shares have one vote for each ordinary share held on all matters submitted to a vote before the shareholders at a general meeting. The quorum required for general meetings of our shareholders is at least two shareholders present in person, by proxy or written ballot, who hold or represent between them at least 25% of the total outstanding voting rights, within half an hour of the time fixed for the commencement of the meeting. A meeting adjourned for lack of a quorum is generally adjourned to the same day in the following week at the same time and place or to a later time or date if so specified in the notice of the meeting. At the reconvened meeting, any number of shareholders present in person or by proxy shall constitute a lawful quorum.
139
Vote Requirements
Our amended articles of association provide that all resolutions of our shareholders require a simple majority vote, unless otherwise required by the Israeli Companies Law or by our amended articles of association. Under the Israeli Companies Law, certain actions require a special majority, including: (i) appointment of external directors, requiring the approval described above under “Management—Board Practices—External Directors”; (ii) approval of an extraordinary transaction with a controlling shareholder or in which the controlling shareholder has a personal interest and the terms of employment or other engagement of the controlling shareholder or a relative of the controlling shareholder (even if not extraordinary), requiring the approval described above under “Management —Approval of Related Party Transactions under Israeli Law— Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”; (iii) approval of a compensation policy, requiring the approval described under Management—Board Practices——Compensation Committee and Compensation Policy”; and (iv) approval of executive officer compensation inconsistent with our office holder compensation policy or the compensation of our chief executive officer (subject to limited exceptions), requiring the approval described above under “Management— Approval of Related Party Transactions under Israeli Law— Disclosure of Personal Interests of an Office Holder and Approval of Certain Transactions.
In addition, under the Israeli Companies Law the authorization of the chairman of the board to assume the role or responsibilities of the chief executive officer, or the authorization of the chief executive officer or his or her relative thereof to assume the role or responsibilities of the chairman of the board, for periods of no longer than three years each, is subject to receipt of the approval of a majority of the shares voting on the matter, provided that either (i) included in such majority are at least two-thirds of the shares of shareholders who are non-controlling shareholders and shareholders who do not have a personal interest in the resolution that are voted at the meeting on the matter (excluding any abstentions); or (ii) the total number of shares of shareholders specified in clause (i) who voted against the resolution does not 2% of the voting rights in the company.
Another exception to the simple majority vote requirement is a resolution for the voluntary winding up, or an approval of a scheme of arrangement or reorganization, of the company pursuant to Section 350 of the Israeli Companies Law, which requires the approval of holders of 75% of the voting rights represented at the meeting, in person, by proxy or by voting deed and voting on the resolution. Under our amended articles of association, the alteration of the rights, privileges, preferences or obligations of any class of our shares requires a simple majority of the class so affected (or such other percentage of the relevant class that may be set forth in the governing documents relevant to such class), in addition to the ordinary majority vote of all classes of shares voting together as a single class at a shareholder meeting.
Access to Corporate Records
Under the Israeli Companies Law, shareholders are provided access to: minutes of the general meetings of our shareholders; our shareholders register and principal shareholders register, articles of association and financial statements; and any document that we are required by law to file publicly with the Israeli Companies Registrar or the Israel Securities Authority. In addition, shareholders may request to be provided with any document in the company’s possession related to an action or transaction requiring shareholder approval under the related party transaction provisions of the Israeli Companies Law. We may deny this request if we believe it has not been made in good faith or if such denial is necessary to protect our interest or protect a trade secret or patent.
Modification of Class Rights
Under the Israeli Companies Law and our amended articles of association, the rights attached to any class of share, such as voting, liquidation and dividend rights, may be amended by adoption of a resolution by the holders of a majority of the shares of that class present at a separate class meeting, or otherwise in accordance with the rights attached to such class of shares, as set forth in our amended articles of association.
Registration Rights
For a discussion of registration rights we have granted to our existing shareholders prior to this offering, please see “Certain Relationships and Related Party Transactions — Shareholders Agreement.”
140
Acquisitions under Israeli Law
Full Tender Offer
A person wishing to acquire shares of an Israeli public company, and who would as a result hold over 90% of the target company’s issued and outstanding share capital, is required by the Israeli Companies Law to make a tender offer to all of the company’s shareholders for the purchase of all of the issued and outstanding shares of the company. A person wishing to acquire shares of a Israeli public company, and who would as a result hold over 90% of the issued and outstanding share capital of a certain class of shares of the company, is required to make a tender offer to all of the shareholders who hold shares of the relevant class for the purchase of all of the issued and outstanding shares of that class. If the shareholders who do not accept the offer hold less than 5% of the issued and outstanding share capital of the company or of the applicable class, and more than half of the shareholders who do not have a personal interest in the offer accept the offer, all of the shares that the acquirer offered to purchase will be transferred to the acquirer by operation of law. However, a tender offer will also be accepted if the shareholders who do not accept the offer hold less than 2% of the issued and outstanding share capital of the company or of the applicable class of shares.
Upon a successful completion of such a full tender offer, any shareholder that was an offeree in such tender offer, whether such shareholder accepted the tender offer or not, may, within six months from the date of acceptance of the tender offer, petition an Israeli court to determine whether the tender offer was for less than fair value and that the fair value should be paid as determined by the court. However, under certain conditions, the offeror may include in the terms of the tender offer that an offeree who accepted the offer will not be entitled to petition the Israeli court as described above.
If (a) the shareholders who did not respond or accept the tender offer hold at least 5% of the issued and outstanding share capital of the company, or of the applicable class, or the shareholders who accept the offer constitute less than a majority of the offerees that do not have a personal interest in the acceptance of the tender offer, or (b) the shareholders who did not accept the tender offer hold 2% or more of the issued and outstanding share capital of the company (or of the applicable class), the acquirer may not acquire shares of the company that will increase its holdings to more than 90% of the company’s issued and outstanding share capital or of the applicable class from shareholders who accepted the tender offer.
Special Tender Offer
The Israeli Companies Law provides that an acquisition of shares of an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of 25% or more of the voting rights in the company, if there is no other shareholder that holds 25% or more of the voting rights in the company, subject to exceptions. Similarly, the Israeli Companies Law provides that an acquisition of shares in an Israeli public company must be made by means of a special tender offer if as a result of the acquisition the purchaser would become a holder of more than 45% of the voting rights in the company, if there is no other shareholder of the company who holds more than 45% of the voting rights in the company, subject to certain exceptions. No tender offer is required if the acquisition of shares: (i) occurs in the context of a private placement, that was approved by the company’s shareholders and whose purpose is to give the acquirer at least 25% of the voting rights in the company if there is no person who holds 25% or more of the voting rights in the company, or as a private placement whose purpose is to give the acquirer 45% of the voting rights in the company, if there is no person who holds 45% of the voting rights in the company (ii) was from a holder of 25% or more of the voting rights in the company following which the purchaser will hold 25% or more of the voting rights in the company, or (iii) was from a holder of more than 45% of the voting rights in the company following which the purchaser will hold more than 45% of the voting rights in the company.
A special tender offer must be extended to all shareholders of a company but the offeror is not required to purchase shares representing more than 5% of the voting power attached to the company’s outstanding shares, regardless of how many shares are tendered by shareholders. A special tender offer may be consummated only if (i) at least 5% of the voting power attached to the company’s outstanding shares will be acquired by the offeror and (ii) the number of shares tendered in the offer exceeds the number of shares whose holders objected to the offer (excluding the purchaser, its controlling shareholders, holders of 25% or more of the voting rights in the company or any person having a personal interest in the acceptance of the tender offer, or anyone on their behalf, including any such person’s relatives and entities under their control). If a special tender offer is accepted, then the purchaser or any person or entity controlling it, at the time of the offer, and any person or entity under common control with the purchaser or such controlling person or entity may not make a subsequent tender offer for the purchase of shares of the target company and may not enter into a merger with the target company for a period of one year from the date of the offer, unless the purchaser or such person or entity undertook to effect such an offer or merger in the initial special tender offer.
141
Merger
The Israeli Companies Law permits merger transactions if approved by each party’s board of directors and, unless certain requirements described under the Israeli Companies Law are met, by a majority vote of each party’s shares, and, in the case of the target company, a majority vote of each class of its shares, voted on the proposed merger at a shareholders meeting. The board of directors of a merging company may not approve the merger if it determines that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities.
For purposes of the shareholder vote of a merging company whose shares are held by the other merging company or a person or entity holding 25% or more of any of the means of control of the other merging entity, unless a court rules otherwise, the merger will not be deemed approved if a majority of the votes of shares voting on the matter at the shareholders meeting (excluding abstentions) that are held by parties other than the other party to the merger, or by any other person or entity who holds 25% or more of the voting rights or the right to appoint 25% or more of the directors of the other party, or any one on their behalf including their relatives or corporations controlled by any of them, vote against the merger. If, however, the merger involves a merger with a company’s own controlling shareholder or if the controlling shareholder has a personal interest in the merger, then the merger is instead subject to the same Special Majority approval that governs all extraordinary transactions with controlling shareholders (as described under “Management — Approval of Related Party Transactions under Israeli Law — Disclosure of Personal Interests of Controlling Shareholders and Approval of Certain Transactions”).
If the transaction would have been approved by the shareholders of a merging company but for the separate approval of each class or the exclusion of the votes of certain shareholders as provided above, a court may still approve the merger upon the request of holders of at least 25% of the voting rights of a company, if the court holds that the merger is fair and reasonable, taking into account the valuation of the merging companies and the consideration offered to the shareholders.
Upon the request of a creditor of either party to the proposed merger, the court may delay or prevent the merger if it concludes that there exists a reasonable concern that, as a result of the merger, the surviving company will be unable to satisfy the obligations of the merging entities, and may further give instructions to secure the rights of creditors.
In addition, a merger may not be consummated unless at least 50 days have passed from the date on which a proposal for approval of the merger was filed by each party with the Israeli Registrar of Companies and at least 30 days have passed from the date on which the merger was approved by the shareholders of each party.
Anti-Takeover Measures under Israeli Law
The Israeli Companies Law allow us to create and issue shares having rights different from those attached to our ordinary shares, including shares providing certain preferred rights with respect to voting, distributions or other matters and shares having preemptive rights. As of the closing of this offering, no preferred shares will be authorized under our amended articles of association. In the future, if we do authorize, create and issue a specific class of preferred shares, such class of shares, depending on the specific rights that may be attached to it, may have the ability to frustrate or prevent a takeover or otherwise prevent our shareholders from realizing a potential premium over the market value of their ordinary shares. The authorization and designation of a class of preferred shares will require an amendment to our amended articles of association, which requires the prior approval of the holders of a majority of the voting power attached to our issued and outstanding shares at a general meeting. The convening of the meeting, the shareholders entitled to participate and the majority vote required to be obtained at such a meeting will be subject to the requirements set forth in the Israeli Companies Law as described above in “— Voting Rights.”
Borrowing Powers
Pursuant to the Israeli Companies Law and our amended articles of association, our board of directors may exercise all powers and take all actions that are not required under law or under our amended articles of association to be exercised or taken by our shareholders, including the power to borrow money for company purposes.
142
Changes in Capital
Our amended articles of association enable us to increase or reduce our share capital. Any such changes are subject to the provisions of the Israeli Companies Law and must be approved by a resolution duly passed by our shareholders at a general meeting by voting on such change in the capital. In addition, transactions that have the effect of reducing capital, such as the declaration and payment of dividends in the absence of sufficient retained earnings or profits, require the approval of both our board of directors and an Israeli court.
Transfer Agent and Registrar
The transfer agent and registrar for our ordinary shares is . Its address is , and its telephone number is .
Listing
We have applied for the listing of our ordinary shares on the NASDAQ Capital Market under the symbol “CHEK.”
143
Prior to this offering, no public market existed for our ordinary shares. Sales of substantial amounts of our ordinary shares following this offering, or the perception that these sales could occur, could adversely affect prevailing market prices of our ordinary shares and could impair our future ability to obtain capital, especially through an offering of equity securities. Assuming that the underwriters do not exercise their over-allotment option with respect to this offering, assuming the consummation of the concurrent Private Placement of ________ ordinary shares and assuming no exercise of options or warrants outstanding following the offering, we will have an aggregate of ordinary shares outstanding upon completion of this offering and the concurrent Private Placement. Of these shares, the ordinary shares sold in this offering will be freely tradable without restriction or further registration under the Securities Act, unless purchased by “affiliates” (as that term is defined under Rule 144 of the Securities Act), who may sell only the volume of shares described below and whose sales would be subject to additional restrictions as described below.
The remaining outstanding ordinary shares will be deemed “restricted securities” as defined in Rule 144. Restricted securities may be sold in the public market only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rule 144 or Rule 701 promulgated under the Securities Act of 1933, as amended, which rules are summarized below. In addition, our executive officers, directors and greater than 1% shareholders have entered into lock-up agreements with the representative of the underwriters under which they have agreed, subject to specific exceptions, not to sell any of our shares for at least 180 days following the date of this prospectus, as described below under “Underwriting.” As a result of these agreements, subject to the provisions of Rule 144 or Rule 701, based on an assumed offering date of , 2015, ordinary shares will be available for sale in the public market as follows:
|
|
•
|
Beginning on the date of this prospectus, all of the ordinary shares sold in this offering will be immediately available for sale in the public market;
|
|
|
•
|
Beginning 181 days after the date of this prospectus, subject to possible extension as described in “Underwriting” below, additional ordinary shares will become eligible for sale in the public market, all of which will be held by affiliates and subject to the volume and other restrictions of Rule 144, as described below; and
|
|
|
•
|
The remainder of the ordinary shares will be eligible for sale in the public market from time to time beginning on the date of this prospectus, as described below.
|
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to public company reporting requirements for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale, volume limitation or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares upon expiration of the lock-up agreements described below, without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell upon expiration of the lock-up agreements described below, within any three-month period, a number of shares that does not exceed the greater of:
|
|
•
|
1% of the number of ordinary shares then outstanding, which will equal approximately shares immediately after this offering and the concurrent Private Placement; or
|
|
|
•
|
the average weekly trading volume of our ordinary shares on the NASDAQ Capital Market during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale.
|
144
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a shareholder who purchased ordinary shares pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required by that rule to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701, subject to the lock-up agreements described below.
Form S-8 Registration Statements
Following the completion of this offering, we may file one or more registration statements on Form S-8 under the Securities Act to register the ordinary shares issued or reserved for issuance under our 2006 Unit Option Plan. The registration statement on Form S-8 will become effective automatically upon filing. Ordinary shares issued upon exercise of a share option and registered under the Form S-8 registration statement will, subject to vesting and Rule 144 volume limitations applicable to our affiliates, be available for sale in the open market immediately unless they are otherwise subject to the lock-up restrictions described under “Underwriting” below, in which case, they will be available for sale after the expiration of such lock-up.
Registration Rights
Upon the closing of this offering, but subject to the terms of any lock-up agreement, holders of a total of of our ordinary shares will have the right to require us to register these shares under the Securities Act under specified circumstances and will have incidental registration rights. After registration pursuant to these rights, these shares will become freely tradable without restriction under the Securities Act. For more information on these registration rights, see “Related Party Transactions—Shareholders Agreement.”
Lock-up Agreements
For a description of the lock-up agreements with the representative of the underwriters that restrict sales of shares by us and our executive officers and directors, see the information under the heading “Underwriting.”
145
TAXATION
The following description is not intended to constitute a complete analysis of all tax consequences relating to the ownership and disposition of our ordinary shares. You should consult your own tax advisor concerning the tax consequences of your particular situation, as well as any tax consequences that may arise under the laws of any state, local, foreign or other taxing jurisdiction.
Israeli Tax Considerations and Government Programs
The following is a brief summary of the material Israeli tax laws applicable to us, and certain Israeli Government programs that benefit us. This section also contains a discussion of material Israeli tax consequences concerning the ownership and disposition of our ordinary shares purchased by investors in this offering. This summary does not discuss all the aspects of Israeli tax law that may be relevant to a particular investor in light of his or her personal investment circumstances or to some types of investors subject to special treatment under Israeli law. Examples of such investors include residents of Israel or traders in securities who are subject to special tax regimes not covered in this discussion. Because parts of this discussion are based on new tax legislation that has not yet been subject to judicial or administrative interpretation, we cannot assure you that the appropriate tax authorities or the courts will accept the views expressed in this discussion. The discussion below is subject to change, including due to amendments under Israeli law or changes to the applicable judicial or administrative interpretations of Israeli law, which change could affect the tax consequences described below.
General Corporate Tax Structure in Israel
Israeli resident companies are generally subject to corporate tax, currently at the rate of 26.5% of a company’s taxable income. However, the effective tax rate payable by a company that derives income from an Approved Enterprise, a Benefited Enterprise or a Preferred Enterprise (as discussed below) may be considerably less. Capital gains derived by an Israeli resident company are subject to tax at the prevailing corporate tax rate.
Law for the Encouragement of Industry (Taxes), 5729-1969
The Law for the Encouragement of Industry (Taxes), 5729-1969, generally referred to as the Industry Encouragement Law, provides several tax benefits for “Industrial Companies.” The Industry Encouragement Law defines an “Industrial Company” as a company resident in Israel, of which 90% or more of its income in any tax year, other than income from defense loans, is derived from an “Industrial Enterprise” owned by it. An “Industrial Enterprise” is defined as an enterprise whose principal activity in a given tax year is industrial production.
The following corporate tax benefits, among others, are available to Industrial Companies:
|
|
•
|
amortization over an eight-year period of the cost of purchased know-how and patents and rights to use a patent and know-how which are used for the development or advancement of the Industrial Enterprise;
|
|
|
•
|
under limited conditions, an election to file consolidated tax returns with related Israeli Industrial Companies; and
|
|
|
•
|
expenses related to a public offering are deductible in equal amounts over three years.
|
Eligibility for the benefits under the Industry Encouragement Law is not subject to receipt of prior approval from any governmental authority. We believe that we may qualify as an “Industrial Company” within the meaning of the Industry Encouragement Law; however, there can be no assurance that we will qualify as an Industrial Company or that the benefits described above will be available in the future.
Law for the Encouragement of Capital Investments, 5719-1959
The Law for the Encouragement of Capital Investments, 5719-1959, generally referred to as the Investment Law, provides certain incentives for capital investments in production facilities (or other eligible assets) by “Industrial Enterprises” (as defined under the Investment Law).
The Investment Law was significantly amended effective April 1, 2005, referred to as the 2005 Amendment, and further amended as of January 1, 2011, referred to as the 2011 Amendment. Pursuant to the foregoing amendments, generally tax benefits that were granted in accordance with the provisions of the Investment Law prior to each such amendment remain in force; however, any benefits granted subsequent to the respective amendment are subject to the provisions of the Investment Law as amended.
146
Tax Benefits Prior to the 2005 Amendment
Prior to the 2005 Amendment, a capital investment in eligible production facilities (or other eligible assets) could, upon application to the Investment Center of the Israeli Ministry of Economy (formerly named the Ministry of Industry, Trade and Labor), or the Investment Center, be designated as an “Approved Enterprise” and accordingly, entitled to certain tax benefits under the Investment Law. Each certificate of approval for an Approved Enterprise relates to a specific investment program in the Approved Enterprise, delineated both by the financial scope of the investment and by the physical characteristics of the facility or the asset. We do not have any Approved Enterprises.
Tax Benefits Subsequent to the 2005 Amendment
Pursuant to the 2005 Amendment, a company whose facilities meet certain criteria set forth in the 2005 Amendment, may claim certain tax benefits offered by the Investment Law (as further described below) directly in its tax returns, without the need to obtain prior approval. In order to receive the tax benefits, a company must make an investment which meets all of the conditions, including exceeding a minimum entitling investment amount, set forth in the Investment Law. Such investment allows a company to receive “Benefited Enterprise” status, and may be made over a period of no more than three years ending at the end of the year in which the company chose to have the tax benefits apply to its Benefited Enterprise, referred to as the “Year of Election.”
The extent of the tax benefits available under the 2005 Amendment to qualifying income of a Benefited Enterprise depends on, among other things, the geographic location in Israel of the Benefited Enterprise. The location will also determine the period for which tax benefits are available. Under the “Exemption Track” the tax benefits include an exemption from corporate tax on undistributed income generated by the Benefited Enterprise for a period of two to ten years, depending on the geographic location of the Benefited Enterprise in Israel, and a reduced corporate tax rate of 10% to 25% for the remainder of the benefits period, depending on the level of foreign investment in the company in each year. . The benefits period is for a duration of seven or ten years, depending on the location of the Benefited Enterprise, from the later of the first year in which the company generated taxable income from its Benefited Enterprise and the Year of Election, but in any event not more than 12 or 14 years from the Year of Election, depending on the location of the company. A company qualifying for tax benefits under the Exemption Track which pays a dividend out of income derived by its Benefited Enterprise during the tax exemption period will be subject to corporate tax in respect of the amount of the dividend (grossed-up to reflect the pre-tax income that it would have had to earn in order to distribute the dividend) at the corporate tax rate which would have otherwise been applicable. Dividends paid out of income attributed to a Benefited Enterprise are generally subject to withholding tax at source at the rate of 15%-20% or such lower rate as may be provided in an applicable tax treaty.
The benefits available to a Benefited Enterprise are subject to the fulfillment of conditions stipulated in the Investment Law and its regulations. If a company does not meet these conditions, it may be required to refund the amount of tax benefits, as adjusted by the Israeli consumer price index, and interest, or other monetary penalties.
We currently have one Benefited Enterprise program under the Investments Law, which, we believe, entitle us to certain tax benefits with respect to income to be derived from our Benefited Enterprise. During the benefits period, taxable income from our Benefited Enterprise program (once generated) will be tax exempt for a period of ten years commencing with the year we will first earn taxable income relating to such enterprise. We chose 2010 as the Year of Election. Due to the location of our company, we believe we are entitled to a 10 year benefit period, subject to a 14 year limitation from the Year of Election, and therefore, the tax benefit period will in any event end in 2023.
Tax Benefits under the 2011 Amendment
The 2011 Amendment canceled the availability of the benefits granted to companies under the Investment Law prior to 2011 and, instead, introduced new benefits for income generated by a “Preferred Company” through its “Preferred Enterprise” (as such terms are defined in the Investment Law) as of January 1, 2011. The definition of a Preferred Company includes a company incorporated in Israel that is not wholly-owned by a governmental entity, and that has, among other things, Preferred Enterprise status and is controlled and managed from Israel. Effective January 1, 2014, a Preferred Company is entitled to a reduced corporate tax rate of 16% with respect to its income derived by its Preferred Enterprise in 2014 and thereafter, unless the Preferred Enterprise is located in a specified development zone, in which case the rate will be 9%. Our facilities are located in a specified development zone.
147
Dividends paid out of income attributed to a Preferred Enterprise are generally subject to withholding tax at source at the rate of 20% or such lower rate as may be provided in an applicable tax treaty. However, if such dividends are paid to an Israeli company, no tax is required to be withheld (although, if such dividends are subsequently distributed to individuals or a non-Israeli company, withholding tax at a rate of 20% or such lower rate as may be provided in an applicable tax treaty will apply).
The 2011 Amendment also provides transitional provisions to address companies already enjoying existing tax benefits under the Investment Law. These transitional provisions provide, among other things, that unless an irrevocable request is made to apply the provisions of the Investment Law as amended in 2011 with respect to income to be derived as of January 1, 2011, a Benefited Enterprise can elect to continue to benefit from the benefits provided to it before the 2011 Amendment came into effect, provided that certain conditions are met.
We have examined the possible effect, if any, of the provisions of the 2011 Amendment on our financial statements and have decided not to apply the new benefits under the 2011 Amendment.
The termination or substantial reduction of any of the benefits available under the Investment Law could materially increase our future tax liabilities.
The Encouragement of Industrial Research and Development Law, 5744-1984
Under the Encouragement of Industrial Research and Development Law, 5744-1984, generally referred to as the Research Law, research and development programs which meet specified criteria and are approved by a committee of the Office of the Chief Scientist, or the OCS, of the Israeli Ministry of Economy are eligible for grants. The grants awarded are typically up to 50% of the project’s expenditures, as determined by the research committee. The grantee is required to pay royalties to the State of Israel from the sale of products developed under the program. Regulations under the Research Law generally provide for the payment of royalties of 3% to 5% (or 6% with respect to certain limited programs and at an increased rate under certain circumstances) on sales of products and services based on technology developed using grants, until 100% of the grant, linked to the dollar and bearing interest at the LIBOR rate, is repaid. The terms of the Israeli government participation also require that products developed with OCS grants be manufactured in Israel and that the technology developed thereunder may not be transferred outside of Israel, unless approval is received from the OCS. However, this does not restrict the export of products that incorporate the funded technology. In addition, payment of additional amounts is required if manufacturing is moved outside of Israel, in which case the royalty repayment rate is increased and the royalty ceiling can reach up to three times the amount of the grants received, and if OCS developed know-how is transferred outside of Israel, the royalty ceiling can reach up to six times the amount of grants (plus interest).
As of June 30, 2014, we have received funding from the OCS for the financing of a portion of our research and development expenditures in an aggregate amount of $3.1 million. Since June 30, 2014, we have received additional funding from the OCS in the aggregate amount of $476,000 under an approved OCS grant in the total amount of $702,000 for a research and development program for the 12 month period commencing March 1, 2014. As of June 30, 2014, we had a contingent obligation to the OCS in the amount of $1.6 million.
Taxation of our Shareholders
Capital Gains Taxes Applicable to Non-Israeli Resident Shareholders. A non-Israeli resident who derives capital gains from the sale of shares in an Israeli resident company that were purchased after the company was listed for trading on a stock exchange outside of Israel will be exempt from Israeli tax so long as the shares were not held through a permanent establishment that the non-resident maintains in Israel. However, non-Israeli corporations will not be entitled to the foregoing exemption if Israeli residents: (i) have a controlling interest of 25% or more in such non-Israeli corporation or (ii) are the beneficiaries of, or are entitled to, 25% or more of the revenues or profits of such non-Israeli corporation, whether directly or indirectly. In addition, such exemption is not applicable to a person whose gains from selling or otherwise disposing of the shares are deemed to be business income.
148
Additionally, a sale of securities by a non-Israeli resident may be exempt from Israeli capital gains tax under the provisions of an applicable tax treaty. For example, under the Convention Between the Government of the United States of America and the Government of the State of Israel with respect to Taxes on Income, as amended, or the United States-Israel Tax Treaty, the sale, exchange or other disposition of shares by a shareholder who (i) is a U.S. resident (for purposes of the treaty); (ii) holds the shares as a capital asset; and (iii) is entitled to claim the benefits afforded to such person by the treaty, is generally exempt from Israeli capital gains tax. Such exemption will not apply if, among other things: (i) the capital gain arising from such sale, exchange or other disposition is treated as industrial or commercial profits attributed to a permanent establishment in Israel, subject to certain conditions; (ii) the shareholder holds, directly or indirectly, shares representing 10% or more of the voting capital of the corporation during any part of the 12-month period preceding the disposition, subject to certain conditions; (iii) the capital gain arising from such sale, exchange or disposition is treated as royalties; or (iv) such U.S. resident is an individual and was present in Israel for 183 days or more during the relevant taxable year. In such case, the sale, exchange or disposition of our ordinary shares would be subject to Israeli tax, to the extent applicable; however, under the United States-Israel Tax Treaty, the taxpayer would be permitted to claim a credit for such taxes against the U.S. federal income tax imposed with respect to such sale, exchange or disposition, subject to the limitations under U.S. law applicable to foreign tax credits. The United States-Israel Tax Treaty does not relate to U.S. state or local taxes.
In some instances where our shareholders may be liable for Israeli tax on the sale of their ordinary shares, the payment of the consideration may be subject to the withholding of Israeli tax at source. Shareholders may be required to demonstrate that they are exempt from tax on their capital gains in order to avoid withholding at source at the time of sale.
Taxation of Non-Israeli Shareholders on Receipt of Dividends. Non-Israeli residents are generally subject to Israeli withholding tax on the receipt of dividends paid on our ordinary shares at the rate of 25%, unless relief is provided in a treaty between Israel and the shareholder’s country of residence (subject to the receipt of a valid certificate from the Israeli Tax Authority allowing for a reduced tax rate). With respect to a person who is a “substantial shareholder” at the time of receiving the dividend or at any time during the preceding 12 months, the applicable withholding tax rate is 30%, unless such “substantial shareholder” holds such shares through a nominee company, in which case the rate is 25%. A “substantial shareholder” is generally a person who alone or together with such person’s relative or another person who collaborates with such person on a permanent basis, holds, directly or indirectly, at least 10% of any of the “means of control” of the corporation. “Means of control” generally include the right to vote, receive profits, nominate a director or an executive officer, receive assets upon liquidation, or order someone who holds any of the aforesaid rights how to act, regardless of the source of such right.
However, a distribution of dividends to non-Israeli residents is subject to withholding tax at source at a rate of 15-20% if the dividend is distributed from income attributed to an Approved Enterprise, Benefited Enterprise or a Preferred Enterprise, unless a reduced tax rate is provided under an applicable tax treaty. With respect to a Benefited Enterprise whose Year of Election is before 2014, such as ours, distribution of dividends attributed to income of such Benefited Enterprise is subject to 15% tax rate. We cannot assure you that in the event we declare a dividend we will designate the income out of which the dividend is paid in a manner that will reduce shareholders’ tax liability.
Under the United States-Israel Tax Treaty, the maximum rate of tax withheld at source in Israel on dividends paid to a holder of our ordinary shares who is a U.S. resident (for purposes of the United States-Israel Tax Treaty) is 25%. With respect to dividends paid to a U.S. corporation that held 10% or more of our outstanding voting capital throughout the tax year in which the dividend is distributed and the preceding tax year and provided that not more than 25% of the gross income of the paying corporation for such prior taxable year (if any) consists of certain interest or dividends, the maximum rate of tax withheld at source is 12.5%; provided, however, that if the paying corporation is an Approved Enterprise, the applicable withholding tax rate under such circumstances is reduced to 15%. We believe that the reference in the United States-Israel Tax Treaty to an Approved Enterprise under the Investment Law is deemed to include also a Benefitted Enterprise and Preferred Enterprise under the Investment Law.
U.S. residents who are subject to Israeli withholding tax on a dividend may be entitled to a credit or deduction for U.S. federal income tax purposes in the amount of the taxes withheld, subject to detailed rules contained in U.S. tax legislation.
149
Surtax
Subject to the provisions of an applicable tax treaty, individuals who are subject to tax in Israel are also subject to an additional tax at a rate of 2% on annual income (including, but not limited to, dividends, interest and capital gain) exceeding NIS 811,560 for 2014, which amount is linked to the annual change in the Israeli consumer price index.
Estate and Gift Tax
Israeli law presently does not impose estate or gift taxes.
U.S. Federal Income Taxation
General
The following are the material U.S. federal income tax consequences of the acquisition, ownership and disposition of our ordinary shares covered by this prospectus.
The discussion below of the U.S. federal income tax consequences to “U.S. Holders” will apply to a beneficial owner of our ordinary shares that is for U.S. federal income tax purposes:
|
|
•
|
an individual citizen or resident of the United States;
|
|
|
•
|
a corporation (or other entity treated as a corporation) that is created or organized (or treated as created or organized) in or under the laws of the United States, any state thereof or the District of Columbia;
|
|
|
•
|
an estate whose income is includible in gross income for U.S. federal income tax purposes regardless of its source; or
|
|
|
•
|
a trust if (i) a U.S. court can exercise primary supervision over the trust’s administration and one or more U.S. persons are authorized to control all substantial decisions of the trust, or (ii) it has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
|
A beneficial owner of our ordinary shares that is described above is referred to herein as a “U.S. Holder.” If a beneficial owner of our ordinary shares is not described as a U.S. Holder and is not an entity treated as a partnership or other pass-through entity for U.S. federal income tax purposes, such owner will be considered a “Non-U.S. Holder.” The material U.S. federal income tax consequences applicable specifically to Non-U.S. Holders are described below under the heading “Non-U.S. Holders.”
This discussion is based on the Internal Revenue Code of 1986, as amended (the “Code”), its legislative history, Treasury regulations promulgated thereunder, published rulings and court decisions, all as currently in effect. These authorities are subject to change or differing interpretations, possibly on a retroactive basis.
This discussion does not address all aspects of U.S. federal income taxation that may be relevant to any particular holder based on such holder’s individual circumstances. In particular, this discussion considers only holders that purchase our ordinary shares pursuant to this offering and own and hold the ordinary shares as capital assets within the meaning of Section 1221 of the Code, and does not address the potential application of the alternative minimum tax or the U.S. federal income tax consequences to holders that are subject to special rules, including:
|
|
•
|
financial institutions or financial services entities;
|
|
|
•
|
broker-dealers;
|
|
|
•
|
persons that are subject to the mark-to-market accounting rules under Section 475 of the Code;
|
|
|
•
|
tax-exempt entities;
|
|
|
•
|
governments or agencies or instrumentalities thereof;
|
|
|
•
|
insurance companies;
|
150
|
|
•
|
regulated investment companies;
|
|
|
•
|
real estate investment trusts;
|
|
|
•
|
certain expatriates or former long term residents of the United States;
|
|
|
•
|
persons that actually or constructively own 5% or more of our voting shares;
|
|
|
•
|
persons that acquired the ordinary shares pursuant to an exercise of employee options, in connection with employee incentive plans or otherwise as compensation;
|
|
|
•
|
persons that hold the ordinary shares as part of a straddle, constructive sale, hedging, conversion or other integrated transaction;
|
|
|
•
|
persons whose functional currency is not the U.S. dollar;
|
|
|
•
|
passive foreign investment companies; or
|
|
|
•
|
controlled foreign corporations.
|
This discussion does not address any aspect of U.S. federal non-income tax laws, such as gift or estate tax laws, or state, local or non-U.S. tax laws or, except as discussed herein, any tax reporting obligations applicable to a holder of our ordinary shares. Additionally, this discussion does not consider the tax treatment of partnerships or other pass-through entities or persons who hold our ordinary shares through such entities. If a partnership (or other entity classified as a partnership for U.S. federal income tax purposes) is the beneficial owner of our ordinary shares, the U.S. federal income tax treatment of a partner in the partnership generally will depend on the status of the partner and the activities of the partnership. This discussion also assumes that any distribution made (or deemed made) to a holder in respect of our ordinary shares and any consideration received (or deemed received) by a holder in connection with the sale or other disposition of our ordinary shares will be in U.S. dollars. In addition, as described in “Risk Factors – Risks Related to Taxation – There is a risk that we could be treated as a domestic (U.S.) corporation for U.S. federal income tax purposes by reason of the transactions related to our acquisition of all of the business operations and substantially all of the assets of Check-Cap LLC on May 31, 2009 (hereinafter sometimes referred to as the “reorganization”),” this discussion also assumes that we will be and have been treated as a foreign corporation for U.S. federal income tax purposes.
We have not sought, and will not seek, a ruling from the Internal Revenue Service (the “IRS”) or an opinion of counsel as to any U.S. federal income tax consequence described herein. The IRS may disagree with the description herein, and its determination may be upheld by a court. Moreover, there can be no assurance that future legislation, regulations, administrative rulings or court decisions will not adversely affect the accuracy of the statements in this discussion.
EACH PROSPECTIVE INVESTOR IN OUR ORDINARY SHARES IS URGED TO CONSULT ITS OWN TAX ADVISOR WITH RESPECT TO THE PARTICULAR TAX CONSEQUENCES TO SUCH INVESTOR OF THE ACQUISITION, OWNERSHIP AND DISPOSITION OF OUR ORDINARY SHARES, INCLUDING THE APPLICABILITY AND EFFECT OF ANY STATE, LOCAL, AND NON-U.S. TAX LAWS, AS WELL AS U.S. FEDERAL TAX LAWS AND ANY APPLICABLE TAX TREATIES.
U.S. Holders
Taxation of Cash Distributions
Subject to the passive foreign investment company, or PFIC, rules discussed below, a U.S. Holder generally will be required to include in gross income as ordinary income the amount of any cash dividend paid in respect of our ordinary shares. A cash distribution on our ordinary shares generally will be treated as a dividend for U.S. federal income tax purposes to the extent the distribution is paid out of our current or accumulated earnings and profits (as determined for U.S. federal income tax purposes). Such dividend generally will not be eligible for the dividends-received deduction generally allowed to U.S. corporations in respect of dividends received from other U.S. corporations. The portion of such cash distribution, if any, in excess of such earnings and profits will be applied against and reduce (but not below zero) the U.S. Holder’s adjusted tax basis in the ordinary shares. Any remaining excess generally will be treated as gain from the sale or other taxable disposition of such ordinary shares.
151
With respect to non-corporate U.S. Holders, any such cash dividends may be subject to U.S. federal income tax at the lower applicable regular long term capital gains tax rate (see “— Taxation on the Disposition of Ordinary Shares” below) provided that (a) our ordinary shares are readily tradable on an established securities market in the United States or we are eligible for the benefits of the United States-Israel Tax Treaty, (b) we are not a PFIC, as discussed below, for either the taxable year in which the dividend was paid or the preceding taxable year, and (c) certain holding period requirements are met. Therefore, if our ordinary shares are not readily tradable on an established securities market, and we are not eligible for the benefits of the United States-Israel Tax Treaty, then cash dividends paid by us to non-corporate U.S. Holders will not be subject to U.S. federal income tax at the lower regular long term capital gains tax rate. Under published IRS authority, shares are considered for purposes of clause (a) above to be readily tradable on an established securities market in the United States only if they are listed on certain exchanges, which presently include the NASDAQ Capital Market. Although we have applied to have our ordinary shares listed and traded on the NASDAQ Capital Market, we cannot guarantee that our application will be approved or, if approved, that our ordinary shares will continue to be listed and traded on the NASDAQ Capital Market. U.S. Holders should consult their own tax advisors regarding the availability of the lower rate for any cash dividends paid with respect to our ordinary shares.
Dividends paid to a U.S. Holder with respect to our ordinary shares generally will be foreign source income, which may be relevant in calculating such U.S. Holder’s foreign tax credit limitations. Subject to certain conditions and limitations, Israeli tax withheld on dividends may be deducted from such U.S. Holder’s taxable income or credited against such U.S. Holder’s U.S. federal income tax liability. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends that we distribute generally should constitute “passive category income,” or, in the case of certain U.S. Holders, “general category income.” A foreign tax credit for foreign taxes imposed on distributions may be denied if a U.S. Holder does not satisfy certain minimum holding period requirements. The rules relating to the determination of the foreign tax credit are complex, and U.S. Holders should consult their tax advisors to determine whether and to what extent they will be entitled to this credit.
Taxation on the Disposition of Ordinary Shares
Upon a sale or other taxable disposition of our ordinary shares, and subject to the PFIC rules discussed below, a U.S. Holder generally will recognize capital gain or loss in an amount equal to the difference between the amount realized and the U.S. Holder’s adjusted tax basis in the ordinary shares.
The regular U.S. federal income tax rate on capital gains recognized by U.S. Holders generally is the same as the regular U.S. federal income tax rate on ordinary income, except that long term capital gains recognized by non-corporate U.S. Holders generally are subject to U.S. federal income tax at a maximum regular rate of 20%. Capital gain or loss will constitute long term capital gain or loss if the U.S. Holder’s holding period for the ordinary shares exceeds one year. The deductibility of capital losses is subject to various limitations. Any such gain or loss that a U.S. Holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes.
If an Israeli capital gains tax applies to any gains from the disposition of our ordinary shares by a U.S. Holder, as discussed in “Israeli Tax Considerations and Government Programs – Taxation of our Shareholders” above, such tax may be treated as a foreign tax eligible for a deduction from such holder’s U.S. federal taxable income or a foreign tax credit against such holder’s U.S. federal income tax liability (subject to certain conditions and limitations). In addition, if such Israeli tax applies to any such gain, a U.S. Holder may be entitled to certain benefits under the United States-Israel Tax Treaty, if such holder is considered a resident of the United States for purposes of, and otherwise meets the requirements of, the United States-Israel Tax Treaty. U.S. Holders should consult their own tax advisors regarding the deduction or credit for any such Israeli tax and their eligibility for the benefits of the United States-Israel Tax Treaty.
Additional Taxes
U.S. Holders that are individuals, estates or trusts and whose income exceeds certain thresholds generally will be subject to a 3.8% Medicare contribution tax on unearned income, including, without limitation, dividends on, and gains from the sale or other taxable disposition of, our ordinary shares, subject to certain limitations and exceptions. U.S. Holders should consult their own tax advisors regarding the effect, if any, of such tax on their ownership and disposition of our ordinary shares.
152
Passive Foreign Investment Company Rules
A foreign (i.e., non-U.S.) corporation will be a PFIC if either (a) at least 75% of its gross income in a taxable year of the foreign corporation, including its pro rata share of the gross income of any corporation in which it is considered to own at least 25% of the shares by value, is passive income, or (b) at least 50% of its assets in a taxable year of the foreign corporation, ordinarily determined based on fair market value and averaged quarterly over the year, including its pro rata share of the assets of any corporation in which it is considered to own at least 25% of the shares by value, are held for the production of, or produce, passive income. Passive income generally includes dividends, interest, rents and royalties (other than certain rents or royalties derived from the active conduct of a trade or business), and gains from the disposition of passive assets.
Our actual PFIC status for our current taxable year or any subsequent taxable year is uncertain and will not be determinable until after the end of such taxable year. Accordingly, there can be no assurance with respect to our status as a PFIC for our current taxable year or any subsequent taxable year.
If we are determined to be a PFIC for any taxable year (or portion thereof) that is included in the holding period of a U.S. Holder of the ordinary shares, and the U.S. Holder did not make either a timely QEF election for our first taxable year as a PFIC in which the U.S. Holder held (or was deemed to hold) the ordinary shares, a QEF election along with a purging election, or a mark-to-market election, each as described below, such holder generally will be subject to special rules for regular U.S. federal income tax purposes with respect to:
|
|
•
|
any gain recognized by the U.S. Holder on the sale or other disposition of its ordinary shares; and
|
|
|
•
|
any “excess distribution” made to the U.S. Holder (generally, any distributions to such U.S. Holder during a taxable year of the U.S. Holder that are greater than 125% of the average annual distributions received by such U.S. Holder in respect of the ordinary shares during the three preceding taxable years of such U.S. Holder or, if shorter, such U.S. Holder’s holding period for the ordinary shares).
|
Under these rules,
|
|
•
|
the U.S. Holder’s gain or excess distribution will be allocated ratably over the U.S. Holder’s holding period for the ordinary shares;
|
|
|
•
|
the amount allocated to the U.S. Holder’s taxable year in which the U.S. Holder recognized the gain or received the excess distribution or to the period in the U.S. Holder’s holding period before the first day of our first taxable year in which we qualified as a PFIC will be taxed as ordinary income;
|
|
|
•
|
the amount allocated to other taxable years (or portions thereof) of the U.S. Holder and included in its holding period will be taxed at the highest ordinary tax rate in effect for that year and applicable to the U.S. Holder; and
|
|
|
•
|
the interest charge generally applicable to underpayments of tax will be imposed in respect of the tax attributable to each such other taxable year of the U.S. Holder.
|
In general, if we are determined to be a PFIC, a U.S. Holder may avoid the PFIC tax consequences described above with respect to the ordinary shares by making a timely QEF election (or a QEF election along with a purging election). Pursuant to the QEF election, a U.S. Holder will be required to include in income its pro rata share of our net capital gains (as long term capital gain) and other earnings and profits (as ordinary income), on a current basis, in each case whether or not distributed, in the taxable year of the U.S. Holder in which or with which our taxable year ends. However, a U.S. Holder may make a QEF election only if we agree to provide certain tax information to such holder annually. At this time, we do not intend to provide U.S. Holders with such information as may be required to make a QEF election effective.
Alternatively, if a U.S. Holder, at the close of its taxable year, owns ordinary shares in a PFIC that are treated as marketable stock, the U.S. Holder may make a mark-to-market election with respect to such ordinary shares for such taxable year. If the U.S. Holder makes a valid mark-to-market election for the first taxable year of the U.S. Holder in which the U.S. Holder holds (or is deemed to hold) the ordinary shares and for which we are determined to be a PFIC, such holder generally will not be subject to the PFIC rules described above with respect to its ordinary shares. Instead, in general, the U.S. Holder will include as ordinary income for each year that we are treated as a PFIC the excess, if any, of the fair market value of its ordinary shares at the end of its taxable year over the adjusted tax basis in its ordinary shares. The U.S. Holder also will be allowed to take an ordinary loss in respect of the excess, if any, of the adjusted tax basis of its ordinary shares over the fair market value of its ordinary shares at the end of its taxable year (but only to the extent of the net amount of previously included income as a result of the mark-to-market election). The U.S. Holder’s adjusted tax basis in its ordinary shares will be adjusted to reflect any such income or loss amounts, and any further gain recognized on a sale or other taxable disposition of the ordinary shares will be treated as ordinary income. In the case of a U.S. Holder that has held our ordinary shares during any taxable year in respect of which we were classified as a PFIC and continues to hold such ordinary shares (or any portion thereof) and has not previously determined to make a mark-to-market election, and that is now considering making a mark-to-market election, special tax rules may apply relating to purging the PFIC taint of such ordinary shares.
153
The mark-to-market election is available only for stock that is regularly traded on a national securities exchange that is registered with the U.S. Securities and Exchange Commission, including the NASDAQ Capital Market, or on a foreign exchange or market that the IRS determines has rules sufficient to ensure that the market price represents a legitimate and sound fair market value. Although we have applied to have our ordinary shares listed and traded on the NASDAQ Capital Market, we cannot guarantee that our application will be approved or, if approved, that the ordinary shares will continue to be listed and traded on the NASDAQ Capital Market. U.S. Holders should consult their own tax advisors regarding the availability and tax consequences of a mark-to-market election with respect to our ordinary shares under their particular circumstances.
If we are a PFIC and, at any time, have a foreign subsidiary that is classified as a PFIC, a U.S. Holder of our ordinary shares generally should be deemed to own a portion of the shares of such lower-tier PFIC, and generally could incur liability for the deferred tax and interest charge described above if we receive a distribution from, or dispose of all or part of our interest in, or the U.S. Holder were otherwise deemed to have disposed of an interest in, the lower-tier PFIC. A mark-to-market election generally would not be available with respect to such a lower-tier PFIC. U.S. Holders are urged to consult their own tax advisors regarding the tax issues raised by lower-tier PFICs.
A U.S. Holder that owns (or is deemed to own) ordinary shares in a PFIC during any taxable year of the U.S. Holder may have to file an IRS Form 8621 (whether or not a mark-to-market election is or has been made) with such U.S. Holder’s U.S. federal income tax return and provide such other information as may be required by the U.S. Treasury Department.
The rules dealing with PFICs and mark-to-market elections are very complex and are affected by various factors in addition to those described above. Accordingly, U.S. Holders of our ordinary shares should consult their own tax advisors concerning the application of the PFIC rules to our ordinary shares under their particular circumstances.
Non-U.S. Holders
Cash dividends paid or deemed paid to a Non-U.S. Holder with respect to our ordinary shares generally will not be subject to U.S. federal income tax unless such dividends are effectively connected with the Non-U.S. Holder’s conduct of a trade or business within the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that such holder maintains or maintained in the United States).
In addition, a Non-U.S. Holder generally will not be subject to U.S. federal income tax on any gain attributable to a sale or other taxable disposition of the ordinary shares unless such gain is effectively connected with its conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, is attributable to a permanent establishment or fixed base that such holder maintains or maintained in the United States) or the Non-U.S. Holder is an individual who is present in the United States for 183 days or more in the taxable year of such sale or other disposition and certain other conditions are met (in which case, such gain from U.S. sources generally is subject to U.S. federal income tax at a 30% rate or a lower applicable tax treaty rate).
Cash dividends and gains that are effectively connected with the Non-U.S. Holder’s conduct of a trade or business in the United States (and, if required by an applicable income tax treaty, are attributable to a permanent establishment or fixed base that such holder maintains or maintained in the United States) generally will be subject to regular U.S. federal income tax at the same regular U.S. federal income tax rates as applicable to a comparable U.S. Holder and, in the case of a Non-U.S. Holder that is a corporation for U.S. federal income tax purposes, may also be subject to an additional branch profits tax at a 30% rate or a lower applicable tax treaty rate.
154
Backup Withholding and Information Reporting
In general, information reporting for U.S. federal income tax purposes should apply to cash distributions made on our ordinary shares within the United States to a U.S. Holder (other than an exempt recipient) and to the proceeds from sales and other dispositions of the ordinary shares by a U.S. Holder (other than an exempt recipient) to or through a U.S. office of a broker. Payments made (and sales and other dispositions effected at an office) outside the United States will be subject to information reporting in limited circumstances. In addition, certain information concerning a U.S. Holder’s adjusted tax basis in its ordinary shares and adjustments to that tax basis and whether any gain or loss with respect to such ordinary shares is long term or short term also may be required to be reported to the IRS, and certain holders may be required to file an IRS Form 8938 (Statement of Specified Foreign Financial Assets) to report their interest in our ordinary shares.
Moreover, backup withholding of U.S. federal income tax at a rate of 28% generally will apply to cash dividends paid on the ordinary shares to a U.S. Holder (other than an exempt recipient) and the proceeds from sales and other dispositions of the ordinary shares by a U.S. Holder (other than an exempt recipient), in each case who:
|
|
•
|
fails to provide an accurate taxpayer identification number;
|
|
|
•
|
is notified by the IRS that backup withholding is required; or
|
|
|
•
|
in certain circumstances, fails to comply with applicable certification requirements.
|
A Non-U.S. Holder generally may eliminate the requirement for information reporting and backup withholding by providing certification of its foreign status, under penalties of perjury, on a duly executed applicable IRS Form W-8 or by otherwise establishing an exemption.
Backup withholding is not an additional tax. Rather, the amount of any backup withholding will be allowed as a credit against a U.S. Holder’s or a Non-U.S. Holder’s U.S. federal income tax liability and may entitle such holder to a refund, provided that certain required information is timely furnished to the IRS.
Holders are urged to consult their own tax advisors regarding the application of backup withholding and the availability of and procedures for obtaining an exemption from backup withholding in their particular circumstances.
155
UNDERWRITING
Under the terms and subject to the conditions contained in an underwriting agreement, we have agreed to sell to the underwriters named below, for which Chardan Capital Markets, LLC, or “Chardan”, is acting as representative, and the underwriters named below have agreed to purchase from us, the number of ordinary shares set forth opposite their respective names below.
|
Underwriter
|
Number of Ordinary
Shares
|
|
|
Chardan Capital Markets, LLC
|
||
|
Maxim Group LLC
|
||
|
Total
|
The underwriting agreement provides that the obligation of the underwriters to purchase the shares offered hereby is subject to certain conditions and that the underwriters are obligated to purchase all of the shares offered hereby if any of the shares are purchased.
If the underwriters sell more shares than the above number, the underwriters have an option for 45 days to buy up to an aggregate of additional shares from us at the public offering price less the underwriting commissions and discounts to cover these sales.
Commissions, Discounts and Other Compensation
The underwriters have advised us that they propose to offer the shares to the public at the public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per share. After this offering, the public offering price and concession may be changed by the underwriters. No such change shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The shares are offered by the underwriters as stated herein, subject to receipt and acceptance by the underwriters and subject to their right to reject any order in whole or in part.
We have agreed to pay to the underwriters a fee equal to 7% of the aggregate gross proceeds of the shares sold in this offering. This fee is to be paid by means of a discount from the offering price to purchasers in the offering. We have also agreed to pay the underwriter a structuring fee of $100,000 if the aggregate gross proceeds of this offering are less than or equal to $15 million, or $250,000 if the aggregate gross proceeds of this offering are in excess of $15 million. In addition, we have agreed to reimburse the underwriter for its reasonable out-of-pocket expenses incurred in connection with this offering in an aggregate amount not to exceed $250,000 for all such expenses. We estimate that expenses payable by us in connection with this offering, other than the underwriting discounts and commissions referred to above, will be approximately $ .
The following table summarizes the public offering price, underwriting discounts and commissions and proceeds before expenses to us assuming both no exercise and full exercise of the underwriters’ option to purchase additional shares:
|
Total
|
||||||
|
Per
Share
|
Without
Over-
Allotment
|
With
Over-
Allotment
|
||||
|
Public offering price
|
||||||
|
Underwriting discounts and commissions
|
||||||
|
Proceeds, before expenses, to us
|
||||||
Determination of Offering Price
Prior to this offering, there has not been a public market for our ordinary shares in the United States. The public offering price for our ordinary shares will be determined through negotiations between us and the representative. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and the underwriters believe to be comparable to us, estimates of our business potential, the present state of our development and other factors deemed relevant.
156
We offer no assurances that the initial public offering price will correspond to the price at which our ordinary shares will trade in the public market subsequent to this offering or that an active trading market for our ordinary shares will develop and continue after this offering.
Underwriter Warrants
We shall issue to Chardan as representative of the underwriters, upon the closing of the offering, underwriter warrants entitling the underwriter to purchase 5% of the aggregate number of ordinary shares sold in the offering. The underwriter warrants may be exercised for a period of four years following the date of effectiveness of the Registration Statement on Form F-1 of which this prospectus forms a part. The underwriter warrants will have an exercise price equal to 100% of the per share price of the shares sold in this offering but not including the over-allotment option. The underwriter warrants are not redeemable by us. We can demand that Chardan exercise the underwriter warrants if our ordinary shares trade at a price of more than 100% above the exercise price of the warrants for more than 90 consecutive days.
The underwriter warrants and the securities underlying such warrants are deemed to be underwriting compensation by FINRA and are therefore subject to a 180-day lock-up pursuant to FINRA Rule 5110(g)(1). The representative (or permitted assignee under the rule) may not sell, transfer, assign, pledge or hypothecate the underwriter warrants or the securities underlying the underwriter warrants, nor will it engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the underwriter warrants or the underlying securities for a period of 180 days from the date on which the Registration Statement on Form F-1 of which this prospectus forms a part is declared effective by the U.S. Securities and Exchange Commission, except to any FINRA member participating in the offering and their bona fide officers or partners.
Lock-up Agreements
We have agreed not to offer, sell, contract to sell, pledge, grant options to purchase, or otherwise dispose of any of our ordinary shares or securities exchangeable for or convertible into our ordinary shares for a period of 180 days after the date of this prospectus without the prior written consent of Chardan. This agreement does not apply to the issuance of shares upon the exercise of rights to acquire ordinary shares pursuant to any existing stock option or similar equity incentive or compensation plan. Our directors, executive officers and greater than 1% shareholders have agreed, subject to certain exceptions, not to, directly or indirectly, sell, hedge, or otherwise dispose of any ordinary shares, options to acquire ordinary shares or securities exchangeable for or convertible into ordinary shares, for a period of 180 days from the date on which the Registration Statement on Form F-1 of which this prospectus forms a part is declared effective by the U.S. Securities and Exchange Commission without the prior written consent of Chardan.
Right of First Refusal
Subject to certain terms and exceptions, for a period of twenty-four (24) months after the date of effectiveness of the registration statement of which this prospectus is a part, Chardan as representative of the underwriters has a right of first refusal to act as lead underwriter or book-running manager or placement agent for each and every future public and private equity and debt offerings we do, or any successor to or any subsidiary of us, on any U.S. stock exchange during such twenty-four (24) month period.
Indemnification and Contribution
The underwriting agreement provides for indemnification between us and the underwriters against specified liabilities, including liabilities under the Securities Act, and for contribution by us and the underwriters to payments that may be required to be made with respect to those liabilities. We have been advised that, in the opinion of the U.S. Securities and Exchange Commission, indemnification of liabilities under the Securities Act is against public policy as expressed in the Securities Act, and is therefore, unenforceable.
157
Short Sales, Stabilizing Transactions and Penalty Bids
In order to facilitate this offering, persons participating in this offering may engage in transactions that stabilize, maintain, or otherwise affect the price of ordinary shares during and after this offering. Specifically, the underwriters may engage in the following activities in accordance with the rules of the U.S. Securities and Exchange Commission.
Short sales. Short sales involve the sales by the underwriters of a greater number of shares than they are required to purchase in the offering. Covered short sales are short sales made in an amount not greater than the underwriters’ over-allotment option to purchase additional shares from us in this offering. The underwriters may close out any covered short position by either exercising their over-allotment option to purchase shares or purchasing shares in the open market. In determining the source of shares to close out the covered short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which they may purchase shares through the over-allotment option. Naked short sales are any short sales in excess of such over-allotment option. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the ordinary shares in the open market after pricing that could adversely affect investors who purchase in this offering.
Stabilizing transactions. The underwriters may make bids for or purchases of the shares for the purpose of pegging, fixing, or maintaining the price of the shares, so long as stabilizing bids do not exceed a specified maximum.
Penalty bids. If the underwriters purchase shares in the open market in a stabilizing transaction or syndicate covering transaction, they may reclaim a selling concession from the underwriters and selling group members who sold those shares as part of this offering. Stabilization and syndicate covering transactions may cause the price of the shares to be higher than it would be in the absence of these transactions. The imposition of a penalty bid might also have an effect on the price of the shares if it discourages re-sales of the shares.
The transactions above may occur on The NASDAQ Capital Market or otherwise. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of the shares. If these transactions are commenced, they may be discontinued without notice at any time.
Discretionary Sales
The underwriters have informed us that they do not expect to confirm sales of ordinary shares offered by this prospectus to accounts over which they exercise discretionary authority without obtaining the specific approval of the account holder.
Electronic Distribution
A prospectus in electronic format may be made available on the internet sites or through other online services maintained by one or more of the underwriters participating in this offering, or by their affiliates. Other than the prospectus in electronic format, the information on any underwriters’ web site and any information contained in any other web site maintained by an underwriter is not part of the prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or any underwriter in its capacity as underwriter and should not be relied upon by investors.
Affiliations
The underwriters and their affiliates may in the future provide various investment banking and other financial services for us for which services they may in the future receive, customary fees. Except for services provided in connection with this offering and the concurrent Private Placement, none of the underwriters has provided any investment banking or other financial services to us during the past 180 days and we do not expect to retain any of the underwriters to perform any investment banking or other financial services to us for at least 90 days after the date of this prospectus.
158
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Australia
This prospectus is not a disclosure document under Chapter 6D of the Australian Corporations Act, has not been lodged with the Australian Securities and Investments Commission and does not purport to include the information required of a disclosure document under Chapter 6D of the Australian Corporations Act. Accordingly, (i) the offer of the securities under this prospectus is only made to persons to whom it is lawful to offer the securities without disclosure under Chapter 6D of the Australian Corporations Act under one or more exemptions set out in section 708 of the Australian Corporations Act, (ii) this prospectus is made available in Australia only to those persons as set forth in clause (i) above, and (iii) the offeree must be sent a notice stating in substance that by accepting this offer, the offeree represents that the offeree is such a person as set forth in clause (i) above, and, unless permitted under the Australian Corporations Act, agrees not to sell or offer for sale within Australia any of the securities sold to the offeree within 12 months after its transfer for the offeree under this prospectus.
China
The information in this document does not constitute a public offer of the securities, whether by way of sale or subscription, in the People’s Republic of China (excluding, for purposes of this paragraph, Hong Kong Special Administrative Region, Macau Special Administrative Region and Taiwan). The securities may not be offered or sold directly or indirectly in the PRC to legal or natural persons other than directly to “qualified domestic institutional investors.”
European Economic Area — Belgium, Germany, Luxembourg and the Netherlands
The information in this document has been prepared on the basis that all offers of ordinary shares will be made pursuant to an exemption under the Directive 2003/71/EC (“Prospectus Directive”), as implemented in Member States of the European Economic Area (each, a “Relevant Member State”), from the requirement to produce a prospectus for offers of securities.
An offer to the public of ordinary shares has not been made, and may not be made, in a Relevant Member State except pursuant to one of the following exemptions under the Prospectus Directive as implemented in that Relevant Member State:
|
|
•
|
to legal entities that are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
|
|
|
•
|
to any legal entity that has two or more of (i) an average of at least 250 employees during its last fiscal year; (ii) a total balance sheet of more than €43 million (as shown on its last annual unconsolidated or consolidated financial statements) and (iii) an annual net turnover of more than €50 million (as shown on its last annual unconsolidated or consolidated financial statement);
|
|
|
•
|
to fewer than 100 natural or legal persons (other than qualified investors within the meaning of Article 2(1)I of the Prospectus Directive) subject to obtaining the prior consent of the company or any underwriter for any such offer; or
|
|
|
•
|
in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of ordinary shares shall result in a requirement for the publication by the company of a prospectus pursuant to Article 3 of the Prospectus Directive.
|
159
France
This document is not being distributed in the context of a public offering of financial securities (offre au public de titres financiers) in France within the meaning of Article L.411-1 of the French Monetary and Financial Code (Code monétaire et financier) and Articles 211-1 et seq. of the General Regulation of the French Autorité des marchés financiers (“AMF”). The ordinary shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France.
This document and any other offering material relating to the ordinary shares have not been, and will not be, submitted to the AMF for approval in France and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in France.
Such offers, sales and distributions have been and shall only be made in France to (i) qualified investors (investisseurs qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-1 to D.411-3, D. 744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation and/or (ii) a restricted number of non-qualified investors (cercle restreint d’investisseurs non-qualifiés) acting for their own account, as defined in and in accordance with Articles L.411-2-II-2° and D.411-4, D.744-1, D.754-1 and D.764-1 of the French Monetary and Financial Code and any implementing regulation.
Pursuant to Article 211-3 of the General Regulation of the AMF, investors in France are informed that the ordinary shares cannot be distributed (directly or indirectly) to the public by the investors otherwise than in accordance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 to L.621-8-3 of the French Monetary and Financial Code.
Ireland
The information in this document does not constitute a prospectus under any Irish laws or regulations and this document has not been filed with or approved by any Irish regulatory authority as the information has not been prepared in the context of a public offering of securities in Ireland within the meaning of the Irish Prospectus (Directive 2003/71/EC) Regulations 2005 (the “Prospectus Regulations”). The ordinary shares have not been offered or sold, and will not be offered, sold or delivered directly or indirectly in Ireland by way of a public offering, except to (i) qualified investors as defined in Regulation 2(l) of the Prospectus Regulations and (ii) fewer than 100 natural or legal persons who are not qualified investors.
Israel
The ordinary shares offered by this prospectus may not be offered or sold to any person resident in Israel or entity organized or formed in Israel, unless it is an ‘‘institutional investor,’’ as set forth in Section 15A(b)(1) of the Israeli Securities Law 5728-1968, or the Israeli Securities Law, and has provided the requisite certification under the First Addendum of the Israeli Securities Law, or pursuant to other exemptions available under the Israeli Securities Law.
Italy
The offering of the ordinary shares in the Republic of Italy has not been authorized by the Italian Securities and Exchange Commission (Commissione Nazionale per le Società e la Borsa, “CONSOB” pursuant to the Italian securities legislation and, accordingly, no offering material relating to the ordinary shares may be distributed in Italy and such securities may not be offered or sold in Italy in a public offer within the meaning of Article 1.1(t) of Legislative Decree No. 58 of 24 February 1998 (“Decree No. 58”), other than:
|
|
•
|
to Italian qualified investors, as defined in Article 100 of Decree no. 58 by reference to Article 34-ter of CONSOB Regulation no. 11971 of 14 May 1999 (“Regulation no. 1197l”) as amended (“Qualified Investors”); and
|
|
|
•
|
in other circumstances that are exempt from the rules on public offer pursuant to Article 100 of Decree No. 58 and Article 34-ter of Regulation No. 11971 as amended. Any offer, sale or delivery of the ordinary shares or distribution of any offer document relating to the ordinary shares in Italy (excluding placements where a Qualified Investor solicits an offer from the issuer) under the paragraphs above must be:
|
160
|
|
•
|
made by investment firms, banks or financial intermediaries permitted to conduct such activities in Italy in accordance with Legislative Decree No. 385 of 1 September 1993 (as amended), Decree No. 58, CONSOB Regulation No. 16190 of 29 October 2007 and any other applicable laws; and
|
|
|
•
|
in compliance with all relevant Italian securities, tax and exchange controls and any other applicable laws.
|
Any subsequent distribution of the ordinary shares in Italy must be made in compliance with the public offer and prospectus requirement rules provided under Decree No. 58 and the Regulation No. 11971 as amended, unless an exception from those rules applies. Failure to comply with such rules may result in the sale of such ordinary shares being declared null and void and in the liability of the entity transferring the ordinary shares for any damages suffered by the investors.
Japan
The ordinary shares have not been and will not be registered under Article 4, paragraph 1 of the Financial Instruments and Exchange Law of Japan (Law No. 25 of 1948), as amended (the “FIEL”) pursuant to an exemption from the registration requirements applicable to a private placement of securities to Qualified Institutional Investors (as defined in and in accordance with Article 2, paragraph 3 of the FIEL and the regulations promulgated thereunder). Accordingly, the ordinary shares may not be offered or sold, directly or indirectly, in Japan or to, or for the benefit of, any resident of Japan other than Qualified Institutional Investors. Any Qualified Institutional Investor who acquires ordinary shares may not resell them to any person in Japan that is not a Qualified Institutional Investor, and acquisition by any such person of ordinary shares is conditional upon the execution of an agreement to that effect.
Portugal
This document is not being distributed in the context of a public offer of financial securities (oferta pública de valores mobiliários) in Portugal, within the meaning of Article 109 of the Portuguese Securities Code (Código dos Valores Mobiliários). The ordinary shares have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in Portugal. This document and any other offering material relating to the ordinary shares has not been, and will not be, submitted to the Portuguese Securities Market Commission (Comissão do Mercado de Valores Mobiliários) for approval in Portugal and, accordingly, may not be distributed or caused to distributed, directly or indirectly, to the public in Portugal, other than under circumstances that are deemed not to qualify as a public offer under the Portuguese Securities Code. Such offers, sales and distributions of ordinary shares in Portugal are limited to persons who are “qualified investors” (as defined in the Portuguese Securities Code). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Sweden
This document has not been, and will not be, registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this document may not be made available, nor may the ordinary shares be offered for sale in Sweden, other than under circumstances that are deemed not to require a prospectus under the Swedish Financial Instruments Trading Act (1991:980) (Sw. lag (1991:980) om handel med finansiella instrument). Any offering of ordinary shares in Sweden is limited to persons who are “qualified investors” (as defined in the Financial Instruments Trading Act). Only such investors may receive this document and they may not distribute it or the information contained in it to any other person.
Switzerland
The ordinary shares may not be publicly offered in Switzerland and will not be listed on the SIX Swiss Exchange (“SIX”) or on any other stock exchange or regulated trading facility in Switzerland. This document has been prepared without regard to the disclosure standards for issuance prospectuses under art. 652a or art. 1156 of the Swiss Code of Obligations or the disclosure standards for listing prospectuses under art. 27 ff. of the SIX Listing Rules or the listing rules of any other stock exchange or regulated trading facility in Switzerland. Neither this document nor any other offering material relating to the ordinary shares may be publicly distributed or otherwise made publicly available in Switzerland.
161
Neither this document nor any other offering material relating to the ordinary shares has been or will be filed with or approved by any Swiss regulatory authority. In particular, this document will not be filed with, and the offer of ordinary shares will not be supervised by, the Swiss Financial Market Supervisory Authority (FINMA).
This document is personal to the recipient only and not for general circulation in Switzerland.
United Arab Emirates
Neither this document nor the ordinary shares have been approved, disapproved or passed on in any way by the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates, nor have we received authorization or licensing from the Central Bank of the United Arab Emirates or any other governmental authority in the United Arab Emirates to market or sell the ordinary shares within the United Arab Emirates. This document does not constitute and may not be used for the purpose of an offer or invitation. No services relating to the ordinary shares, including the receipt of applications and/or the allotment or redemption of such shares, may be rendered within the United Arab Emirates by us.
No offer or invitation to subscribe for ordinary shares is valid or permitted in the Dubai International Financial Centre.
United Kingdom
Neither the information in this document nor any other document relating to the offer has been delivered for approval to the Financial Services Authority in the United Kingdom and no prospectus (within the meaning of section 85 of the Financial Services and Markets Act 2000, as amended (“FSMA”)) has been published or is intended to be published in respect of the ordinary shares. This document is issued on a confidential basis to “qualified investors” (within the meaning of section 86(7) of FSMA) in the United Kingdom, and the ordinary shares may not be offered or sold in the United Kingdom by means of this document, any accompanying letter or any other document, except in circumstances that do not require the publication of a prospectus pursuant to section 86(1) FSMA. This document should not be distributed, published or reproduced, in whole or in part, nor may its contents be disclosed by recipients to any other person in the United Kingdom.
Any invitation or inducement to engage in investment activity (within the meaning of section 21 of FSMA) received in connection with the issue or sale of the ordinary shares has only been communicated or caused to be communicated and will only be communicated or caused to be communicated in the United Kingdom in circumstances in which section 21(1) of FSMA does not apply to us.
In the United Kingdom, this document is being distributed only to, and is directed at, persons (i) who have professional experience in matters relating to investments falling within Article 19(5) (investment professionals) of the Financial Services and Markets Act 2000 (Financial Promotions) Order 2005 (“FPO”), (ii) who fall within the categories of persons referred to in Article 49(2)(a) to (d) (high net worth companies, unincorporated associations, etc.) of the FPO or (iii) to whom it may otherwise be lawfully communicated (together “relevant persons”). The investments to which this document relates are available only to, and any invitation, offer or agreement to purchase will be engaged in only with, relevant persons. Any person who is not a relevant person should not act or rely on this document or any of its contents.
162
LEGAL MATTERS
Certain legal matters concerning this offering will be passed upon for us by Loeb & Loeb LLP, New York, New York. Certain legal matters with respect to matters of Israeli law including the validity of the ordinary shares offered by this prospectus will be passed upon for us by Fischer Behar Chen Well Orion & Co., Tel Aviv, Israel. Certain legal matters related to the offering will be passed upon for the underwriters by Reed Smith LLP, New York, New York and Amit, Pollak, Matalon & Co., Tel Aviv, Israel.
The financial statements included in this prospectus, have been audited by Brightman Almagor Zohar & Co., a member firm of Deloitte Touche Tohmatsu, or Deloitte, an independent registered public accounting firm, as stated in their report appearing herein, which report express an unqualified opinion and includes an explanatory paragraph referring to the fact that we are in a development stage. Such financial statements have been so included in reliance upon the report of such firm given upon their authority as experts in accounting and auditing. The address of Brightman Almagor Zohar & Co., a member firm of Deloitte, is 1 Azrieli Center, Tel Aviv, 67021, Israel.
We have filed with the SEC a registration statement on Form F-1 under the Securities Act with respect to the ordinary shares offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules that are part of the registration statement. For further information about us and about the ordinary shares, you should refer to our registration statement and its exhibits. Statements made in this prospectus concerning the contents of any contract, agreement or other document are summaries of all material information about the documents summarized, but are not complete descriptions of all terms of these documents. If we filed any of these documents as an exhibit to the registration statement, you may read the document itself for a complete description of its terms.
Upon the completion of this offering, we will become subject to periodic reporting and other information requirements of the Exchange Act as applicable to foreign private issuers and will file reports, including annual reports on Form 20-F, and other information with the SEC. As we are a foreign private issuer, we are exempt from some of the Exchange Act reporting requirements, namely, the rules prescribing the furnishing and content of proxy statements to shareholders and Section 16 short swing profit reporting for our officers and directors and for holders of more than 10% of our shares. In addition, we will not be required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. You may read and copy any document we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information on the public reference rooms and their copy charges. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding issuers, such as us, who file electronically with the SEC. The address of that website is http://www.sec.gov.
163
EXPENSES RELATING TO THIS OFFERING
The following table sets forth all expenses to be paid by the registrant, other than estimated underwriting discounts and commissions, in connection with this initial public offering. All amounts shown are estimates except for the SEC registration fee, the FINRA filing fee and the NASDAQ listing fee:
|
SEC registration fee*
|
|||
|
FINRA filing fee*
|
|||
|
NASDAQ listing fee*
|
|||
|
Legal fees and expenses*
|
|||
|
Accounting fees and expenses*
|
|||
|
Transfer agent and registrar’s fees and expenses*
|
|||
|
Printing expenses*
|
|||
|
Miscellaneous fees and expenses*
|
|||
|
Total*
|
* To be filed by amendment
164
FINANCIAL STATEMENTS AS OF DECEMBER 31, 2013
|
Page
|
|
|
F - 2
|
|
|
Financial statements:
|
|
|
F - 3
|
|
|
F - 4
|
|
|
F - 5
|
|
|
F - 6
|
|
|
F - 7 - F - 36
|
F - 1

To the Shareholders of
Check-Cap Ltd.
Isfiya, Israel
We have audited the accompanying statements of financial position of Check-Cap Ltd. (hereafter- "the Company") as of December 31, 2013 and 2012, and the statements of profit or loss and other comprehensive income, statements of changes in equity, and statements of cash flows for each of the two years in the period ended December 31, 2013. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2013 and 2012, and the results of operations, and cash flows for each of the two years in the period ended December 31, 2013, in conformity with International Financial Reporting Standards as issued by International Accounting Standards Board (IFRS).
The Company is in the development stage as of December 31, 2013. As discussed in Note 1B to the financial statements, successful completion of the Company's development program and, ultimately, the attainment of profitable operations is dependent upon future events, including obtaining adequate financing to fulfill its development activities and achieving a level of sales adequate to support the Company's cost structure.
/s/ Brightman Almagor Zohar & Co.
Certified Public Accountants
A Member Firm of Deloitte Touche Tohmatsu
Tel Aviv, Israel
July 3, 2014

F - 2
STATEMENTS OF FINANCIAL POSITION
|
December 31,
|
|||||||||||
|
2013
|
2012
|
||||||||||
|
Note
|
in USD thousands
|
||||||||||
|
Assets
|
|||||||||||
|
Current assets
|
|||||||||||
|
Cash and cash equivalents
|
5 | 4,975 | 4,583 | ||||||||
|
Restricted deposit
|
46 | 43 | |||||||||
|
Short-term investment
|
5 | - | 3,450 | ||||||||
|
Other current assets
|
6 | 130 | 164 | ||||||||
|
Total current assets
|
5,151 | 8,240 | |||||||||
|
Property and equipment, net
|
7 | 224 | 256 | ||||||||
|
Total assets
|
5,375 | 8,496 | |||||||||
|
Liabilities and shareholders’ equity
|
|||||||||||
|
Current liabilities
|
|||||||||||
|
Trade accounts payable
|
221 | 140 | |||||||||
|
Other current liabilities
|
141 | 140 | |||||||||
|
Employee benefits
|
8A | 658 | 509 | ||||||||
|
Total current liabilities
|
1,020 | 789 | |||||||||
|
Non-current liabilities
|
|||||||||||
|
Royalties provision
|
10A | 2,577 | 2,046 | ||||||||
|
Other financial liabilities
|
11D1 | 587 | 532 | ||||||||
|
Total non-current liabilities
|
3,164 | 2,578 | |||||||||
|
Shareholders’ equity
|
1D | ||||||||||
|
Preferred share capital
|
226 | 226 | |||||||||
|
Ordinary share capital
|
53 | 53 | |||||||||
|
Premium on preferred shares
|
21,167 | 21,167 | |||||||||
|
Share options
|
1,793 | 1,793 | |||||||||
|
Premium on ordinary shares
|
6 | 6 | |||||||||
|
Capital reserve for ordinary share-based payment
|
431 | 374 | |||||||||
|
Accumulated deficit
|
(22,485 | ) | (18,490 | ) | |||||||
|
Total shareholders’ equity
|
1,191 | 5,129 | |||||||||
|
Total liabilities and shareholders’ equity
|
5,375 | 8,496 | |||||||||
|
July 3, 2014
|
||||||
|
Date of approval of
financial statements
|
Tomer Kariv- Chairman of the Board of Directors
|
Guy Neev-CEO
|
Lior Torem-CFO
|
The accompanying notes to the financial statements are an integral part of them.
F - 3
STATEMENTS OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME
|
Year ended December 31,
|
|||||||||||
|
Note
|
2013
|
2012
|
|||||||||
|
in USD thousands
|
|||||||||||
|
Research and development expenses, net
|
13 | 2,662 | 2,692 | ||||||||
|
General and administrative expenses
|
14 | 1,090 | 1,203 | ||||||||
|
Other expenses (income)
|
(10 | ) | 13 | ||||||||
|
Operating loss
|
3,742 | 3,908 | |||||||||
|
Finance income
|
15 | (63 | ) | (416 | ) | ||||||
|
Finance expenses
|
16 | 316 | 229 | ||||||||
|
Finance expenses (income), net
|
253 | (187 | ) | ||||||||
|
Loss for the year
|
3,995 | 3,721 | |||||||||
|
Total comprehensive loss for year
|
3,995 | 3,721 | |||||||||
|
Loss per ordinary share (in USD) Basic and diluted
|
0.18 | 0.17 | |||||||||
|
Weighted average number of ordinary shares outstanding - basic and diluted
(in thousands)
|
17 | 32,542 | 32,530 | ||||||||
|
Pro forma loss per ordinary share (in USD) Basic and diluted (unaudited)
|
1D |
X.XX
|
X.XX
|
||||||||
|
Pro forma weighted average number of ordinary shares outstanding - basic and diluted (in thousands) (unaudited)
|
XXX
|
XXX
|
|||||||||
The accompanying notes to the financial statements are an integral part of them.
F - 4
CHECK-CAP LTD.
|
Preferred share capital
|
Ordinary share capital
|
Premium on preferred shares
|
Share options
|
Premium on ordinary shares
|
Capital reserve for ordinary share based payment
|
Accumulated deficit
|
Total
|
|||||||||||||||||||||||||
|
In USD thousands
|
||||||||||||||||||||||||||||||||
|
For the year ended
December 31, 2013
|
||||||||||||||||||||||||||||||||
|
Balance - January 1, 2013
|
226 | 53 | 21,167 | 1,793 | 6 | 374 | (18,490 | ) | 5,129 | |||||||||||||||||||||||
|
Ordinary share based payment
|
- | - | - | - | - | 57 | - | 57 | ||||||||||||||||||||||||
|
Comprehensive loss for the year
|
- | - | - | - | - | - | (3,995 | ) | (3,995 | ) | ||||||||||||||||||||||
|
Total shareholders’ equity as of December 31, 2013
|
226 | 53 | 21,167 | 1,793 | 6 | 431 | (22,485 | ) | 1,191 | |||||||||||||||||||||||
|
For the year ended
December 31, 2012
|
||||||||||||||||||||||||||||||||
|
Balance - January 1, 2012
|
219 | 53 | 20,145 | 1,793 | 3 | 343 | (14,769 | ) | 7,787 | |||||||||||||||||||||||
|
Issue of preferred D3 shares, net
|
7 | - | 1,022 | - | - | - | - | 1,029 | ||||||||||||||||||||||||
|
Issue of ordinary share under
employee share option plan
|
- | (* | ) | - | - | 3 | - | - | 3 | |||||||||||||||||||||||
|
Recognition of share based payment
|
- | - | - | - | 31 | - | 31 | |||||||||||||||||||||||||
|
Comprehensive loss for the year
|
- | - | - | - | - | - | (3,721 | ) | (3,721 | ) | ||||||||||||||||||||||
|
Total shareholders’ equity as of December 31, 2012
|
226 | 53 | 21,167 | 1,793 | 6 | 374 | (18,490 | ) | 5,129 | |||||||||||||||||||||||
|
(*)
|
Less than NIS 1 thousands
|
The accompanying notes to the financial statements are an integral part of them.
F - 5
CHECK-CAP LTD.
|
Year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Loss for the year
|
(3,995 | ) | (3,721 | ) | ||||
|
Net gain arising on financial assets and liabilities designated as at fair value
through profit or loss
|
55 | 116 | ||||||
|
Depreciation and amortization
|
77 | 73 | ||||||
|
Ordinary share-based compensation
|
57 | 31 | ||||||
|
Royalties provision
|
(116 | ) | (464 | ) | ||||
|
Changes in assets and liabilities items:
|
||||||||
|
Decrease in other current assets
|
34 | 25 | ||||||
|
Increase (decrease) in trade accounts payable and other current liabilities
|
82 | (385 | ) | |||||
|
Increase in employees benefits
|
149 | 165 | ||||||
|
Net cash used in operating activities
|
(3,657 | ) | (4,160 | ) | ||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Purchase of property and equipment
|
(45 | ) | (105 | ) | ||||
|
Proceeds from disposal of property and equipment
|
- | 16 | ||||||
|
Increase in restricted deposit
|
(3 | ) | (4 | ) | ||||
|
Decrease (increase) short-term investment
|
3,450 | (3,450 | ) | |||||
|
Net cash generated from (used in) investing activities
|
3,402 | (3,543 | ) | |||||
|
CASH FLOWS FROM FINANCING ACTIVITIES
|
||||||||
|
Proceeds from issuance of preferred shares, net
|
- | 1,029 | ||||||
|
Receipt of loan from the Office of Chief Scientist
|
647 | 541 | ||||||
|
Proceeds from issue of ordinary shares
|
- | 3 | ||||||
|
Net cash generated from financing activities
|
647 | 1,573 | ||||||
|
Effect of exchange rate changes on cash and cash equivalents
|
- | (7 | ) | |||||
|
Net increase (decrease) in cash and cash equivalents
|
392 | (6,137 | ) | |||||
|
Cash and cash equivalents at the beginning of the year
|
4,583 | 10,720 | ||||||
|
Cash and cash equivalents at the end of the year
|
4,975 | 4,583 | ||||||
The accompanying notes to the financial statements are an integral part of them.
F - 6
|
NOTE 1
|
-
|
GENERAL INFORMATION
|
|
|
A.
|
General
|
Check-Cap Ltd. (hereafter-"the Company") is a limited liability private company incorporated in Israel. The registered address of its offices is Abba Hushi Blvd., Isfiya.
The Company is engaged in the development of an ingestible imaging capsule that utilizes low-dose X-rays for the screening for precancerous polyps and colorectal cancer. The capsule is designed to enable an early detection of the disease without the need for a prior burdensome and uncomfortable bowel cleansing, in a patient-friendly and non-invasive screening procedure.
|
|
B.
|
Financial Position
|
Since its inception, the Company has devoted substantially all of its efforts to research and development, clinical trials, recruiting management and technical staff, acquiring assets and raising capital. The Company is still in its development and clinical stage and has not yet generated revenues. The extent of the Company's future operating losses and the timing of becoming profitable are uncertain. The Company has incurred losses of $22,485 and $18,490 thousands for the years ended December 31, 2013 and 2012, respectively. The Company funds its operations primarily through equity financings.
Management expects that the Company will continue to generate losses from its development and clinical activities which will result in a negative cash flow from operating activity. Management expects that the Company's existing cash along with its plans to raise additional funds in the future will be sufficient to fund the Company's projected operating requirements for at least another 18 months following December 31, 2013. The Company's ability to continue its projected plans beyond such date through profitability is dependent upon its ability to secure additional funds.
|
|
C.
|
Agreement for transfer of assets
|
On May 31, 2009, the Company entered into an asset transfer agreement with Check-Cap LLC (hereafter-”the Predecessor Entity”), a company with the same shareholders as the Company at the time of transfer. According to the agreement, the Predecessor Entity transferred all of its business operations and substantially all of its assets to the Company, including development and consulting agreements, cash, property and equipment and intangible ownership rights, free of any debt. The above agreement did not have any effect on the Company's operations and was accounted for using historical amounts.
In addition, losses for tax purposes accumulated by the Predecessor Entity were not transferred to the Company.
|
|
D.
|
Pro Forma Information (unaudited)
|
The pro forma information gives effect to the conversion of all of the Company’s Preferred shares into XX,XXX,XXX ordinary shares.
Because the Company is considering an IPO, the financial statements include the effect of the conversion of all of the preferred shares into ordinary shares in a pro forma shareholders’ equity presentation, and retroactively reflect the effect of the conversion in a pro forma loss per share presentation.
F - 7
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
|
|
A.
|
Declaration regarding the implementation of International Financial Reporting Standards as issued by the International Accounting Standard Board (IFRS)
|
The financial statements of the Company have been prepared in conformity with the IFRS.
The significant accounting policies detailed below were applied consistently for all of the reporting periods presented in these financial statements, except for the changes in accounting policies that were due to the application of standards, amendments to standards and interpretations that took effect on the date of the financial statements, as specified in Note 3.
|
|
B.
|
Presentation of statements of financial position
|
The Company presents assets and liabilities in the statement of financial position by allocating items to current and non-current.
|
|
C.
|
Format for analysis of expenses in the statement of comprehensive Income
|
The format for analysis of expenses recognized in the statement of profit or loss and comprehensive income is a classification method based on the operating characteristic of the expense. The Company believes that such way of allocation provides more reliable and relevant information.
|
|
D.
|
Foreign currency:
|
|
|
1.
|
Functional currency and presentation currency
The Company has not yet generated revenues, and the majority of its expenses is in U.S. Dollar (Dollar or USD) or NIS, while none of these currencies is significantly material compared to the other. After considering the factors to determine the functional currency in IAS 21, "the Effects of Changes in Foreign Exchange Rates", management determined that the Dollar is the Company's functional currency.
Management judgment, in setting the Dollar as the Company's functional currency, is based mainly on the following criteria: The Company's budget and other Company internal reports, including reports to the Company's Board of Directors and investors, are presented in Dollars. Management is using these reports in order to make decisions for the Company. All of the Company's equity and debt financings were in Dollars; and it is expected that a significant portion of the Company's future revenue will be in Dollars. The financial statements are presented in Dollars, which is the functional currency of the Company. See Note 2Q regarding the rates of exchange and changes in them during the presented periods.
|
|
|
2.
|
Translation of transactions that are not in the functional currency
In preparing the financial statements of the Company, transactions in currencies other than the functional currency of the Company (hereafter – “foreign currency”) are recorded at the rates of exchange prevailing at the date of the transactions. At each statement of financial position date, monetary items denominated in foreign currencies are remeasured at the rates prevailing at the statement of financial position date. (Non-monetary items carried at fair value that are denominated in foreign currencies are remeasured at the rates prevailing at the date when the fair value was determined). Non-monetary items that are measured in terms of historical cost are translated at the historical exchange rates that were in effect on the date of the execution of the transactions related to them.
|
F - 8
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
- SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT.)
|
|
D.
|
Foreign currency (Cont.)
|
|
|
3.
|
Recognition of exchange differences
Exchange differences are recognized in profit and loss in the period in which they arise.
|
|
|
E.
|
Cash and cash equivalents
|
Cash and cash equivalents includes cash in hand and term deposits in banks with an original maturity of up to three months, with a high level of liquidity that may be easily converted to known amounts of cash, and that are exposed to insignificant risk of change in value.
|
|
F.
|
Property and equipment
|
|
|
(1)
|
General
|
Property and equipment are tangible items, which are held for use in the production or supply of goods or services, expected to be used for more than one period.
The Company presents its property and equipment items under the cost method.
Under the cost method, property and equipment are presented in the statement of financial position at cost less accumulated depreciation and accumulated impairment losses. The cost includes the cost of the asset acquisition, as well as the costs that can be directly attributed to bring the asset to the location and condition that are necessary for it to be capable to operate in the manner intended by management.
|
|
(2)
|
Depreciation of property and equipment
|
Depreciation of property and equipment is calculated using the straight-line method at rates considered adequate to depreciate the assets over their estimated useful lives as follows:
|
Length of useful life
|
Depreciation rate
|
|||||
|
Years
|
%
|
|||||
|
Office furniture and equipment
|
10-14 | 7-10 | ||||
|
Laboratory equipment
|
3-7 | 15-33 | ||||
|
Computers and auxiliary equipment
|
3 | 33 |
Gain or loss arising on disposal or retirement of an item of property and equipment is determined as the difference between the disposal proceeds and the net carrying amount of the asset and are recognized in the statement of profit or loss and other comprehensive income.
|
|
G.
|
Research and development costs
|
Expenditures related to research activities, for the purpose of acquiring new scientific or technical know-how, are expensed when incurred.
Development activities are related to a plan to produce new products or processes, or to significantly improve existing products or processes. Expenditures for development activities are capitalized as an intangible asset only if: the development costs may be reliably measured;
F - 9
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT.)
|
|
|
G.
|
Research and development costs (Cont.)
|
the product or process is technically and commercially feasible; future economic benefit is expected from the product and the Company intends and has adequate resources to complete the development and use or sell the asset.
None of the Company's development costs meet the above conditions. Therefore, costs were expensed as incurred.
See Note 2K below regarding the offset of grants received for participation in research and development expenses.
|
|
H.
|
Financial assets
|
|
|
(1)
|
General
|
All financial assets are recognized and derecognized in the statement of financial position on the date where the purchase or sale of the financial asset is under a contract whose terms require the transfer of the financial asset within the timeframe established by the relevant market.
The financial assets are initially recognized at their fair value, with the addition of transaction costs, except for those financial assets classified as at fair value through profit and loss, which are initially recognized at their fair values.
Financial assets are classified into loans and receivables as specified below. The classification depends on the nature and purpose of the financial asset, and is determined at the time of initial recognition.
|
|
(2)
|
Loans and receivables
|
Loans and other current assets are non-derivative financial assets, with fixed or determinate payments, that are not traded in an active market. Subsequent to initial recognition, the loans and other current assets are measured at net book value, which is amortized using the effective interest method, while considering transaction costs and after deducting impairment provisions.
(3) Impairment of financial assets
Financial assets, other than those classified as financial assets at fair value through profit and loss, are assessed for indications of impairment at the end of each reporting period. Objective evidence of impairment exists when one or more events that have occurred after the initial recognition of the financial asset, having negative impact on the estimated future cash flows.
Indications of impairment may include:
|
|
§
|
Significant financial difficulties of the issuer or debtor;
|
|
|
§
|
Probability that the debtor will enter bankruptcy or financial reorganization.
|
The Company examines indications of impairment on a group basis for specific financial assets, for which no impairment indications have been assessed, based on past experience with groups of receivables with similar characteristics and changes in the level of delinquent payments and also economic changes related to the sector and the economic environment in which they operate.
F - 10
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT.)
|
|
|
H.
|
Financial assets (Cont.)
|
(3) Impairment of financial assets (Cont.)
For financial assets carried at amortized cost, impairment loss recognized is the difference between the carrying amount of the financial asset and the present value of estimated future cash flows, discounted at the financial assets original effective interest rate.
Other than the exception of equity investments classified as available for sale, if the amount of loss due to impairment of a financial asset decreased in the following period and that decrease is objectively related to an event that occurred after the impairment was recognized, then in this case, the loss from impairment recognized in the past is fully or partially reversed through profit and loss. Such reversal is limited in amount so that the reversed financial asset value does not exceed what the amortized cost would have been at that date had no impairment have been recognized in the past.
The book value of a financial asset is directly reduced by the loss from impairment, with the exception of trade receivables, where the book value is reduced through the use of a bad debt provision. When trade receivables are considered uncollectible, they are written off against the provision account. Collections in subsequent periods of amounts previously written off are credited to the provision account. Changes in the book value of the provision are recognized in profit and loss.
|
I.
|
IFRS 13 "Fair Value Measurement"
|
IFRS 13 establishes a single source of guidance for fair value measurement and disclosures about fair value measurement. The standard defines fair value, establishes a framework for measuring fair value, and requires disclosures about fair value measurement. The scope of IFRS 13 is broad: It applies to both financial instruments items and non-financial instruments items for which other IFRSs require or permit fair value measurement and disclosure about fair value measurement, except in specified circumstances. In general, the disclosure requirements in IFRS 13 are more extensive than those required in the current standards.
IFRS 13 is effective for annual periods beginning on or after 1 January 2013, with earlier application permitted.
|
J.
|
Financial liabilities and equity instruments issued by the Company
|
(1) Classification as a financial liability or an equity instrument
Debt and equity instruments are classified as a financial liability or as an equity instrument, in accordance with the substance of the contractual arrangements.
An equity instrument is any contract that evidences a residual interest in the assets of an entity after deducting all of its' liabilities. Equity instruments issued by the Company are recorded at the proceeds received, net of direct issue costs.
Financial liabilities are classified as:
§ Financial liabilities at fair value through profit and loss; or,
§ Other financial liabilities.
F - 11
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT.)
|
|
|
J.
|
Financial liabilities and equity instruments issued by the Company (Cont.)
|
|
|
(2)
|
Warrants for the acquisition of the Company's preferred and ordinary shares
|
|
|
a.
|
Warrants to acquire shares of the Company which provide the holder with the right to acquire a fixed number of preferred shares in consideration of a fixed amount of cash are presented in equity as “receipts on account of issued preferred shares” section. For this purpose, an exercise amount varying according to the exercise date, where at the date of the issuance, the exercise price at any possible exercise date can already be determined, is treated as a fixed amount.
|
|
|
b.
|
Warrants to acquire shares of the Company shares which provide the holder with the right to acquire a fixed number of ordinary or preferred shares in consideration of a variable amount of cash or cashless right are presented in current liabilities, measured at fair value through profit and loss.
|
|
|
(3)
|
Derecognition of financial liabilities
|
A financial liability is derecognized if, and only if, it is settled. Meaning that the obligation that is specified in the contract is paid, cancelled or expired.
|
|
K.
|
Grants from the Office of the Chief Scientist of the Ministry of Economy (formerly named the Ministry of Industry, Trade and Labor) (hereafter-"OCS")
|
Grants received from the OCS, which the Company is required to return with interest under certain conditions, represent loans that may be forgiven and are treated as follows:
On the date of initial recognition, the grant is presented as a financial liability at the fair value of the expected cash flows to be repaid, discounted at a discount rate commensurate with the risk level of the research and development project. The difference between the amount of the grant and its fair value at initial recognition, is treated as a government grant (see also Note 10A and 4B2)).
In subsequent periods, the financial liability is measured at the present value of the expected cash flows to be paid in the future, discounted each period at the original interest rate of the liability, with the changes in the liability being recorded in profit and loss for each period.
|
|
L.
|
Provisions
|
Provisions are recognized when the Company has a present obligation (legal or constructive) as a result of a past event, as to which it is probable that the Company will be required to settle the obligation , and such obligation can be reliably estimated.
The amount recognized as a provision reflects the best estimate of the Company's management as to the amount required to settle the present obligation as of the statement of financial position date, taking into account the risks and uncertainties surrounding the obligation. Where a provision is measured by using the cash flows estimated to settle the obligation, its carrying amount is the present value of those anticipated cash flows.
When all or part of the present amount to settle an obligation is expected to be recovered from a third-party, the Company recognizes an asset with respect to the recovery, to the extent of the provision that was recognized, only if it is virtually certain that the reimbursement will be received and the amount can be reliably estimated. (See Note 10).
F - 12
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT.)
|
|
|
M.
|
Share-based payments
|
Share-based payments to employees and to others who provide similar services, which are settled by equity instruments of the Company, are measured at their fair value on the grant date. The Company measures the fair value of the equity instruments granted using the Black Scholes Merton formula. (See Note 12 with regard to inputs used in measurement of fair value of the share-based payments). When the equity instruments granted do not vest until such employees complete a defined period of service, comply with the conditions for exercise or defined market conditions are present, the Company recognizes the share-based payment arrangements in the financial statements over the vesting period against an increase in shareholders’ equity. As of the date of the financial statements, the Company approximates the number of equity instruments expected to vest. Any change in estimate with relation to previous periods is recognized in profit and loss over the term of the vesting period.
|
|
N.
|
Taxes on income
|
In view of the Company’s losses for tax purposes, and due to the lack of expectation of taxable income in the foreseeable future, the Company does not record deferred taxes for losses carried forward and for temporary differences between financial reporting and tax purposes.
|
|
O.
|
Employee benefits
|
Post-employment benefits
The Company has a defined contribution plan in accordance with Section 14 of the Severance Pay Law. See Note 8.
Short-term employee benefits
Short-term employee benefits are benefits which are anticipated to be utilized or paid during a period that does not exceed 12 months from the end of the period in which the service that creates the entitlement to the benefit was rendered.
Short-term employee benefits include the Company’s liabilities for salaries, vacation, recreation pay, and deposits for National Insurance. These benefits are recorded to profit and loss, when incurred. The benefits are measured on a non-capitalized basis, which the Company anticipates paying. The difference between the amount of short-term benefits to which the employee is entitled and the amount paid is therefore recognized as an asset or liability.
|
|
P.
|
Loss per share
|
The Company presents basic and fully diluted loss per share for its ordinary shares. The basic loss per share, which is the same as the fully diluted loss, is calculated by dividing the loss attributable to holders of ordinary shares of the Company, by the weighted average number of ordinary shares outstanding during the reporting period.
In calculating the loss attributable to the ordinary shareholders, the Company deducted the 8% annual preference dividends amounts for the preferred shareholders even though dividends have never been declared.
F - 13
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT.)
|
|
|
Q.
|
Exchange rates and linkage bases
|
Balances in foreign currency or linked thereto are included in the financial statements at the representative exchange rates, as published by the Bank of Israel, that were prevailing as of the statement of financial position date.
Data on exchange rates are as follows:
|
Representative
|
Representative
|
|||||||
|
exchange rate of $
|
exchange rate of ˆ
|
|||||||
|
(NIS/$ 1)
|
ˆ$(/ 1) | |||||||
|
Date of financial statements:
|
||||||||
|
December 31, 2013
|
3.471 | 0.7259 | ||||||
|
December 31, 2012
|
3.733 | 0.7586 | ||||||
|
%
|
%
|
|||||||
|
Changes in exchange rates for the period:
|
||||||||
|
Year ended:
|
||||||||
|
December 31, 2013
|
(7.02 | ) | 4.31 | |||||
|
December 31, 2012
|
(2.30 | ) | 1.96 | |||||
|
NOTE 3
|
-
|
NEW STANDARDS AND INTERPRETATIONS NOT YET ADOPTED
|
IFRS 9 Financial Instruments
IFRS 9, issued in November 2009, introduced new requirements for the classification and measurement of financial assets. IFRS 9 was amended in October 2010 to include requirements for the classification and measurement of financial liabilities and for derecognition.
Key requirements of IFRS 9:
All recognized financial assets that are within the scope of IAS 39 Financial Instruments: Recognition and Measurement are required to be subsequently measured at amortized cost or fair value. Specifically, debt investments that are held within a business model whose objective is to collect the contractual cash flows, and that have contractual cash flows that are solely payments of principal and interest on the principal outstanding are generally measured at amortized cost at the end of subsequent accounting periods. All other debt investments and equity investments are measured at their fair value at the end of subsequent accounting periods. In addition, under IFRS 9, entities may make an irrevocable election to present subsequent changes in the fair value of an equity investment (that is not held for trading) in other comprehensive income, with only dividend income generally recognized in profit or loss.
F - 14
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 3
|
-
|
NEW STANDARDS AND INTERPRETATIONS NOT YET ADOPTED (CONT.)
|
IFRS 9 Financial Instruments (Cont.)
With regard to the measurement of financial liabilities designated as at fair value through profit or loss, IFRS 9 requires that the amount of change in the fair value of the financial liability that is attributable to changes in the credit risk of that liability is presented in other comprehensive income, unless the recognition of the effects of changes in the liability's credit risk in other comprehensive income would create or enlarge an accounting mismatch in profit or loss. Changes in fair value attributable to a financial liability's credit risk are not subsequently reclassified to profit or loss. Under IAS 39, the entire amount of the change in the fair value of the financial liability designated as fair value through profit or loss is presented in profit or loss.
The Company believes that the effects of adoption of IFRS 9 would not be material based on the current financial position of the Company; however, it is not practicable to provide an estimate of such effects until a detailed review has been completed.
|
NOTE 4
|
-
|
SIGNIFICANT ACCOUNTING JUDGEMENT AND KEY SOURCE OF ESTIMATIONS
|
In the application of the Company's accounting policies, which are described in Note 2, management is required to make judgments, estimations and assumptions about the carrying amounts of assets and liabilities. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates.
The estimates and underlying assumptions are reviewed on an ongoing basis and revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods.
|
|
A.
|
Share-based compensation
|
The Company accounts for its share-based compensation to employees in accordance with the provisions of IFRS 2 "Share-based Payment," which requires measuring the cost of share-based compensation based on the fair value of the award on the grant date. The cost is recognized as compensation expense over the requisite service period which is usually the vesting period, based upon the grant date fair value of the equity or liability instruments issued. The Company recognizes the compensation expenses over the vesting period using the accelerated method pursuant to which each vesting tranche is treated as a separate amortization period from the grant date to the vesting date, and classifies these amounts in the financial statements based on the department to which the related employee reports.
The Company selected the Black-Scholes Merton option pricing model as the most appropriate method for computing the fair value of its share-based awards, using the standard parameters established in that model including estimates relating to the fair value of its ordinary shares, volatility, estimated life of the instruments, risk-free interest rates and dividends yield as described below.
F - 15
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 4
|
-
|
SIGNIFICANT ACCOUNTING JUDGEMENT AND KEY SOURCE OF ESTIMATIONS (CONT.)
|
|
|
A.
|
Share-based compensation (Cont.)
|
|
|
1.
|
Option Valuations
|
The determination of the grant date fair value of options using an option pricing model is affected by estimates and assumptions with respect to a number of complex and subjective variables. These variables include the expected volatility of the Company’s share price over the expected term of the options, share option exercise and cancellation behaviors, risk-free interest rates and expected dividends, which are estimated as follows:
Fair Value of the Ordinary Shares. Since the Company's shares are not publicly traded, the Company must estimate the fair value of its ordinary shares, as discussed below in "—Valuation of the Company's ordinary shares".
Volatility. The expected share price volatility was based on the historical equity volatility of the ordinary shares of comparable companies that are publicly traded.
Expected Term. The expected term of options granted represents the estimated period of time that options granted are expected to be outstanding. Since adequate historical experience is not available to provide a reasonable estimate, the expected term is determined based on the midpoint between the available exercise dates (the end of the vesting periods) and the last available exercise date (the contracted expiration date).
Risk-Free Rate. The risk-free interest rate is based on the yield from U.S. Treasury zero-coupon bonds with a term equivalent to the expected term of the options.
Expected Dividend Yield. The Company has never declared or paid any cash dividends and does not presently plan to pay cash dividends in the foreseeable future. Consequently, the Company used an expected dividend yield of zero.
If any of the estimates and assumptions used in the Black-Scholes Merton model change significantly, the Company’s share-based compensation for future awards may differ materially from those projected and recorded previously
|
|
2.
|
Valuation of the Company's ordinary shares
|
Due to the absence of an active market for the Company's ordinary shares, the fair value of its ordinary shares for purposes of determining the exercise price for award grants was determined in good faith by the Company's management, with the assistance of a third party valuation expert, and approved by the Company's board of directors. In connection with preparing its financial statements, the Company's management considered the fair value of the Company's ordinary shares based on a number of objective and subjective factors consistent with the methodologies outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, referred to as the AICPA Practice Aid.
F - 16
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 4
|
-
|
SIGNIFICANT ACCOUNTING JUDGEMENT AND KEY SOURCE OF ESTIMATIONS (CONT.)
|
|
|
B.
|
Royalties provision
|
|
|
1.
|
Government grants from the Office of the Chief Scientist
|
The Company received research and development funding from the State of Israel through the OCS in the form of grants which the Company is required to return with interest under certain conditions. Pursuant to regulations under the Encouragement of Industrial Research and Development Law 5744- 1984 (the "Research Law"), royalties on the Company's revenues will be payable to the Israeli government, at rates ranging from 3% to 5% (or at an increased rate under certain circumstances), up to an aggregate of 100% (which may be increased under certain circumstances) of the dollar-linked value of the total grants received in respect of the approved plans, plus interest at the rate of 12-month London Interbank Offered Rate, or LIBOR. Such grants qualify as "forgivable loans" in accordance with IAS 20, "Accounting for Government Grants and Disclosure of Government Assistance", since they are repayable only if the Company generates revenues related to the underlying project.
In accordance with IAS 20, the grant is accounted for as a liability unless it is more likely than not that the Company will meet the terms of forgiveness of the loan, in which case the forgivable loan is accounted for as a government grant and recognized as a reduction of the research and development expenses. The Company considers it more likely than not that the project underlying its OCS grants will reach the revenue generating stage and therefore, it records a liability in respect of the OCS grants.
On the date of initial recognition, the grant is presented as a financial liability at the fair value of the expected cash flows to be repaid, discounted at a discount rate commensurate with the risk level of the research and development project. The difference between the amount of the grant and its fair value is treated as a government grant. In subsequent periods, the financial liability is measured at the present value of the expected cash flows to be paid in the future, discounted each period at the original interest rate used in computing the initial liability. Changes in the liability are recorded to profit and loss each period.
In calculating the present value of future payments to the OCS, the Company used a discount rate of 21.8% for grants received during 2012 and 2013.
Due to the fact that the Company is still in its development and clinical stage and has not generated revenues, the sales forecast is highly subjective and may vary significantly in the future. As more information is gathered to assist the Company’s management in making forecasts, the liability would be updated. Any updates in the expected cash outflows and the liability will be recorded to profit and loss each period.
|
|
2.
|
Provision for royalties to an ASIC designer
|
In December 2007, the Company entered into an agreement for the development of an Application Specific Integrated Circuit (hereafter –"ASIC") component to be used as an amplifier for the capture of signals at low frequencies from X-ray detectors contained in the Company's product. The ASIC developer is entitled to receive royalties from the Company in the amount of ˆ0.5 (approximately $0.69) for every ASIC component that the Company will sell, up to ˆ200 thousand (approximately $276 thousand) (hereafter-"Royalties Rights"). The net present value of the royalty liability to the ASIC designer is dependent upon the Company's management estimates and assumption as to future product shipments and interest rates used to calculate the present value of the cash payments required to repay the royalties to the ASIC designer. The liability was calculated as the present value of expected cash outflows discounted at a 17.6% discount factor, commensurate with the risk of the Company at the date of initial recognition of the liability.
F - 17
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 4
|
-
|
SIGNIFICANT ACCOUNTING JUDGEMENT AND KEY SOURCE OF ESTIMATIONS (CONT.)
|
|
|
B.
|
Royalties provision (Cont.)
|
|
|
3.
|
Reimbursement liability to Predecessor Entity unit holders
|
On May 31, 2009, the Company entered into an agreement with the Predecessor Entity pursuant to which the Predecessor Entity transferred all of its business operations and substantially all of its assets to the Company. In connection with the reorganization, the Company undertook to reimburse the unit holders of the Predecessor Entity for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unit holders of the Predecessor Entity under Section 367(d) of the U.S. Internal Revenue Code of 1986, as amended (the “Code”) and is based in part on the Company’s forecasted revenues. The liability was calculated using the provisions of IAS 39 under which expected cash outflows were discounted using a 17.6% discount factor commensurate with the risk of the Company at the date of initial recognition of the liability. Due to the fact that the Company is still in its development stage and has not generated revenues, the sales forecast is highly subjective and may vary significantly in the future. As more information gathered to assist the Company's management in making forecasts, the liability would be updated. Any updates in the expected cash outflows and the liability will be recorded to profit and loss each period.
|
C.
|
Fair value of financial instruments
|
On June 1, 2009, in connection with the certain Series C preferred share purchase agreement, the Company issued the following warrants to the lead investor: 836,712 warrants for the purchase of C1 preferred shares and 1,007,975 warrants for the purchase of C2 preferred shares. These warrants are exercisable on a cashless basis and therefore, are classified as a liability in the statement of financial position. The fair value of this financial instrument is determined based on an option pricing model using similar assumptions to those used for the Company's share-based compensation awards to employees. Changes in the inputs used in valuation of the warrants may change the fair value of liability and affect the Company's profit or loss.
|
NOTE 5
|
-
|
CASH AND CASH EQUIVALENTS AND SHORT TERM INVESTMENT
|
Composition:
|
|
Annual interest rate as
|
December 31,
|
|||||||||
|
of December 31, 2013
|
2013
|
2012
|
|||||||||
|
%
|
in USD thousands
|
||||||||||
|
Cash on hand and bank balances
|
270 | 78 | |||||||||
|
Short-term deposits
|
0-1.64 | 4,705 | 4,505 | ||||||||
|
Cash and cash equivalents
|
4,975 | 4,583 | |||||||||
|
Cash and short-term deposits in Dollar
|
4,589 | 3,476 | |||||||||
|
Short-term deposits in GBP
|
4 | - | |||||||||
|
Cash and short-term deposits in NIS
|
382 | 1,107 | |||||||||
|
Short-term investments
|
- | 3,450 | |||||||||
F - 18
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 6
|
-
|
OTHER RECEIVABLES
|
Composition:
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Government institutions
|
45 | 37 | ||||||
|
Other
|
64 | 68 | ||||||
|
Prepaid expenses
|
21 | 59 | ||||||
| 130 | 164 | |||||||
|
NOTE 7
|
-
|
PROPERTY AND EQUIPMENT, NET
|
|
Office
|
Computers
|
|||||||||||||||
|
Furniture
|
and
|
|||||||||||||||
|
And
|
Laboratory
|
auxiliary
|
||||||||||||||
|
equipment
|
equipment
|
equipment
|
Total
|
|||||||||||||
|
in USD thousands
|
||||||||||||||||
|
Cost
|
||||||||||||||||
|
Cost as of January 1, 2012
|
75 | 244 | 106 | 425 | ||||||||||||
|
Additions
|
14 | 35 | 56 | 105 | ||||||||||||
|
Disposals
|
- | (16 | ) | - | (16 | ) | ||||||||||
|
Cost as of December 31, 2012
|
89 | 263 | 162 | 514 | ||||||||||||
|
Additions
|
1 | 8 | 36 | 45 | ||||||||||||
|
Cost as of December 31, 2013
|
90 | 271 | 198 | 559 | ||||||||||||
|
Accumulated depreciation
|
||||||||||||||||
|
Accumulated depreciation as of January 1, 2012
|
18 | 114 | 53 | 185 | ||||||||||||
|
Depreciation
|
6 | 32 | 37 | 75 | ||||||||||||
|
Disposals
|
- | (2 | ) | - | (2 | ) | ||||||||||
|
Accumulated depreciation as of December 31, 2012
|
24 | 144 | 90 | 258 | ||||||||||||
|
Depreciation
|
7 | 29 | 41 | 77 | ||||||||||||
|
Accumulated depreciation as of December 31, 2013
|
31 | 173 | 131 | 335 | ||||||||||||
|
Net book value:
|
||||||||||||||||
|
December 31, 2013
|
59 | 98 | 67 | 224 | ||||||||||||
|
December 31, 2012
|
65 | 119 | 72 | 256 | ||||||||||||
F - 19
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 8
|
-
|
EMPLOYEE BENEFITS
|
|
|
A.
|
Composition
|
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
(Audited)
|
||||||||
|
in USD thousands
|
||||||||
|
Short term employee benefits:
|
||||||||
|
Benefits for vacation pay
|
222 | 230 | ||||||
|
Liability for salary, bonuses and wages
|
436 | 279 | ||||||
| 658 | 509 | |||||||
|
|
B.
|
Post-employment Benefits
|
Defined contribution plans
Severance pay plans
According to Israeli law the Company is generally required to pay severance compensation to an employee at the time of dismissal, death or retirement (including employees who leave the place of employment under other specified circumstances). The calculation of the obligation related to the termination of the employee-employer relationship is based on the employee's salary and the years of service.
Commencing June 1, 2009, the Company has defined contribution plans, in accordance with Section 14 of the Israeli Severance Pay Law, according to which the Company makes monthly payments to insurance policies for its employees. Upon termination of employment, employees will be entitled to receive only the amounts accrued in the insurance policies with respect to severance pay. Deposits to a defined contribution plan for severance pay or for pensions are recognized as an expense at the time of the deposit to the plan concurrent with obtaining the labor services from the employee, and no additional provision in the financial statements is required.
|
|
C.
|
Short-term employee benefits
|
|
|
(1)
|
Paid vacation days
|
In accordance with the Yearly Vacation Law-1951, Company employees are entitled to a number of paid vacation days for each year of employment. In accordance with the law and its appendix, and as determined in the agreement between the Company and the employees, the number of vacation days per year to which each employee is establish based on the seniority of that employee.
The employee may use vacation days based on his needs and with the Company's consent, and to accrue the remainder of unused vacation days. The vacation days utilized first are those credited for the current year and subsequently from any balance transferred from the prior year (on a LIFO basis). An employee who ceased working before utilizing the balance of vacation days accrued is entitled to payment for the balance of unutilized vacation days.
|
|
(2)
|
Related parties
|
For information regarding short term employee liabilities given to related parties see Note 20.
F - 20
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 9
|
-
|
TAXES ON INCOME
|
|
|
(1)
|
The Company received final tax assessments for the year ended December 31, 2009
|
|
|
(2)
|
Losses and deductions for tax purposes carried forward amount to approximately $16.7 thousand as of December 31, 2013. Due to the lack of expectation of taxable income in the foreseeable future, no deferred taxes were recorded for these carry forward losses and deductions.
|
|
|
(3)
|
Corporate tax rates in Israel:
|
The Israeli corporate tax rate was, 25% in 2012 and 2013.
In August 2013, the Economic Plan to change the national priorities in 2013 and 2014 ("Amended Budget Law") was officially published. The Amended Budget Law consists of fiscal changes whose main aim is to enhance the collection of taxes in those years. These changes include: (i) raising the Israeli corporate tax rate from 25% to 26.5%, (ii) cancelling the reduction of the tax rates applicable to preferred enterprises (9% in development area A and 16% in other areas), and (iii) in certain cases increasing the tax rates on dividends within the scope of the Law for the Encouragement of Capital Investments to 20% effective from January 1, 2014. Other changes introduced by the Amended Budget Law include taxing revaluation gains effective from August 1, 2013. The changes regarding the taxation of revaluation gains, however, will only become effective once regulations that define "non-corporate taxable retained earnings" are issued as well as regulations that set forth provisions for avoiding double taxation of assets outside of Israel. As of the date of publication of these financial statements, no such regulations have been issued. The change in tax rates did not have an effect on the Company's financial statements.
The Company did not record current taxes for the years ended December 31, 2012 and 2013 since it had no taxable income.
|
NOTE 10
|
-
|
COMMITMENTS AND CONTINGENT LIABILITIES
|
|
|
A.
|
Provisions
|
Royalties to the OCS
The Company has a liability to pay royalties to the Israeli government as a result of grants received from the Israeli OCS. The liability is calculated based on future sales generated by products which were developed using the OCS grants. As of December 31, 2013, it is probable that the Company will be required to pay the above mentioned royalties, and accordingly, the Company recorded, as of December 31, 2013, a provision in a total amount of $1,618 thousand, including interest (see also Note 4B1). According to the terms of the grants, the OCS is entitled to royalties equal to 3-5% (or at an increased rate under certain circumstances) of the sales of the product funded, up to the full principal amount (which may be increased under certain circumstances) of the U.S. dollar-linked value of the grants, plus interest at the rate of 12-month LIBOR.
The total amount of grants received until December 31, 2013 amounted $3,050 thousand, excluding interest.
F - 21
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 10
|
-
|
COMMITEMENTS AND CONTINGENT LIABILITIES (CONT.)
|
|
|
A.
|
Provisions (Cont.)
Royalties to the OCS (Cont.)
The movement in the provision is as follows:
|
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Balance at the beginning of the year:
|
1,190 | 990 | ||||||
|
Changes during the year:
|
||||||||
|
Amounts charged to the statement of profit and loss and other comprehensive loss
|
(219 | ) | (341 | ) | ||||
|
Amounts received during the year
|
647 | 541 | ||||||
|
Balance at year end
|
1,618 | 1,190 | ||||||
Royalties to an ASIC designer:
The Company has a liability to pay royalties for the development of an ASIC component which is used as an amplifier for the capture of signals at low frequencies from X-ray detectors contained in the capsule. The institution that developed the ASIC is entitled to a receive royalties from the Company in the amount of ˆ 0.5 ($0.69) for every ASIC component that the Company will sell, capped at ˆ 200 thousand ($276 thousand). This Royalty Right is considered a liability of the Company for the development of the ASIC component.
The royalties liability is calculated based on estimated future sales generated by products which includes the ASIC component. As of December 31, 2013 the Company believe it will be required to pay the above mentioned royalties, and accordingly, recorded, as of December 31, 2013, a provision in a total amount of $171 thousands.
Reimbursement liability to Predecessor Entity’s unit holders:
As part of the reorganization discussed in Note 1C, the Company committed to reimburse the unit holders of the Predecessor Entity for any tax burdens that may be imposed on them due to the reorganization. The reimbursement liability is calculated assuming deemed royalties are paid to the U.S. unit holders of the Predecessor Entity under Section 367(d) of the Code and is based in part on the forecasted sales of the Company. The liability was calculated using the provisions of IAS 39 under which expected cash outflows were discounted using a discount factor commensurate with the risk of the Company. Any updates in the expected cash outflows and the liability will be charged to earnings. As of December 31, 2013, the balance of the reimbursement liability totaled $788 thousands.
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Royalties to the OCS
|
1,618 | 1,190 | ||||||
|
Royalties to an ASIC designer
|
171 | 157 | ||||||
|
Reimbursement liability to Predecessor Entity’s unit holders
|
788 | 699 | ||||||
| 2,577 | 2,046 | |||||||
F - 22
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 10
|
-
|
COMMITEMENTS AND CONTINGENT LIABILITIES (CONT.)
|
|
|
B.
|
Commitments
|
|
1.
|
Rental agreement
|
In April 2009, the Company entered into a rental agreement according to which the Company rented space of approximately 550 sq. meters in a building located in Isfiya for a period of 10 years, commencing June 1, 2009. In June 2012, the Company extended the rental agreement and rented additional space of 60 sq. meters in the building's second floor. The rental period is 10 years commencing June 1, 2012, and the Company has the right to terminate the agreement at any time upon at least 60 days prior written notice. The monthly rental fee is NIS17 thousand (approximately $4.9 thousand). The rental expenses recorded by the Company for the years ended December 31, 2013 and 2012 were $56 thousands and 51 thousands respectively.
|
2.
|
Agreements for financial brokerage service
|
See Note 11Dc2.
|
3.
|
Detector development agreement
|
See Note 11Dc4.
|
|
C.
|
Liens
|
In July 2013, the Company pledged a deposit of NIS 150 thousand, respectively ($43 thousand) with a bank as a security for credit cards issued to the Company.
|
|
D.
|
Legal
|
As of the date of the financial statements, the Company has not been and is not a party to any legal proceedings.
|
NOTE 11
|
-
|
SHARE CAPITAL
|
|
|
A.
|
Authorized capital:
|
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
Thousands of shares
|
||||||||
|
Ordinary shares with par value of NIS 0.01
|
907,154 | 907,154 | ||||||
|
Preferred A shares with par value of NIS 0.01
|
6,750 | 6,750 | ||||||
|
Preferred B shares with par value of NIS 0.01
|
6,769 | 6,769 | ||||||
|
Preferred C1 shares with par value of NIS 0.01
|
17,494 | 17,494 | ||||||
|
Preferred C2 shares with par value of NIS 0.01
|
31,833 | 31,833 | ||||||
|
Preferred C3 shares with par value of NIS 0.01
|
30,000 | 30,000 | ||||||
|
Preferred D1 shares with par value of NIS 0.01
|
80,000 | 80,000 | ||||||
|
Preferred D2 shares with par value of NIS 0.01
|
60,000 | 60,000 | ||||||
|
Preferred D3 shares with par value of NIS 0.01
|
5,000 | 5,000 | ||||||
|
Preferred D3 shares with par value of NIS 0.01
|
5,000 | 5,000 | ||||||
F - 23
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 11
|
-
|
SHARE CAPITAL (CONT.)
|
|
|
B.
|
Issued capital
|
|
Number of shares
|
Share capital
|
Premium on shares
|
||||||||||||||||||||||
|
December 31,
|
December 31,
|
December 31,
|
||||||||||||||||||||||
|
2013
|
2012
|
2013
|
2012
|
2013
|
2012
|
|||||||||||||||||||
|
Thousands of shares
|
in USD thousands
|
in USD thousands
|
||||||||||||||||||||||
|
Ordinary shares with par value of NIS 0.01 fully paid up
|
23,043 | 23,043 | 53 | 53 | 6 | 6 | ||||||||||||||||||
|
Preferred A shares with par value of NIS 0.01, participating and convertible, fully paid up
|
6,750 | 6,750 | 15 | 15 | 660 | 660 | ||||||||||||||||||
|
Preferred B shares with par value of NIS 0.01, participating and convertible, fully paid up
|
6,769 | 6,769 | 15 | 15 | 1,367 | 1,367 | ||||||||||||||||||
|
Preferred C1 shares with par value of NIS 0.01, participating and convertible, fully paid up
|
16,415 | 16,415 | 42 | 42 | 3,584 | 3,584 | ||||||||||||||||||
|
Preferred C2 shares with par value of NIS 0.01, participating and convertible, fully paid up
|
29,789 | 29,789 | 79 | 79 | 7,606 | 7,606 | ||||||||||||||||||
|
Preferred D1 shares with par value of NIS 0.01, participating and convertible, fully paid up
|
24,545 | 24,545 | 68 | 68 | (*)8,721 | (*)8,721 | ||||||||||||||||||
|
Preferred D3 shares with par value of NIS 0.01, participating and convertible, fully paid up
|
2,511 | 2,511 | 7 | 7 | 1,022 | 1,022 | ||||||||||||||||||
| 279 | 279 | 22,966 | 22,966 | |||||||||||||||||||||
(*) Include proceeds allocated to D1 and D2 warrants in a total amount of $1,793
F - 24
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 11
|
-
|
SHARE CAPITAL (CONT.)
|
|
|
C.
|
Rights attached to shares
|
|
|
(1)
|
Ordinary shares
|
The ordinary shares provide their owners with rights to receive dividends in cash and shares, and rights to participate at the time of distributing liquidation dividends, subject to the preferential rights of the preferred shareholders, as described below. Additionally, the ordinary shareholders have the right to vote in shareholders’ assemblies in a manner that each share provides one voting right to its holder.
|
|
(2)
|
Liquidation preferences on Preferred shares
|
The Preferred shares provide their owners with similar rights to the ordinary shares with a liquidation preference. In the occurrence of a Liquidation Event (as such term is defined in the Company's Articles of Association), the Preferred shares confer upon their holders preference rights, such that each Preferred share entitles its holder to be paid out of the assets of the Company available for distribution to its shareholders, an amount per preferred share equal to its original issue price, plus a yield of 8% per annum, in accordance with the following order of preference:
First to the holders of Preferred D shares, second to the holders of Preferred C shares, third to the holders of preferred A shares, and forth to the holders of Preferred B shares.
After the payment in full of the above preference amount, the remaining assets of the Company available for distribution to its shareholders, if any, shall be distributed among the holders of preferred shares as a single class, pro rata in accordance with each holder's original issue price.
After the payment in full of the above preference amount, the remaining assets of the Company available for distribution to its shareholders, if any, shall be distributed among the holders of Preferred shares and Ordinary shares, pro rata based on the number of shares held by each such holder on an "as-converted" basis (i.e., treating all Preferred shares as if they had been converted to Ordinary shares)
|
|
(3)
|
Conversion
|
Each Preferred share is convertible into such number of Ordinary shares at the then applicable conversion rate of such Preferred shares. In addition, each Preferred share shall automatically be converted into such number of Ordinary shares at the then applicable conversion rate of such Preferred shares upon a an initial public offering by the Company or a corporate successor of its equity interests in which at least $50 million is raised at a pre-money Company valuation of at least $200 million or a Liquidation Event (as such term is defined in the Company’s Articles of Association). The conversion rate of each Preferred share is equal to an amount sufficient to give each Preferred shareholder an 8% annual compounded return on the purchase price paid (or deemed paid) for each such Preferred share through the conversion date (less any distributions made by the Company prior to such date with respect to such Preferred share and less any payments made to the shareholder in connection with the reorganization discussed in Note 1C) divided by the conversion price of such Preferred share in effect at the time of adjustment. The conversion price of each Preferred share is initially the purchase price paid (or deemed paid) for each such Preferred share, which is adjusted for certain shares split and combinations, distributions, mergers and reorganizations, and, with respect to the Preferred C and Preferred D shares, upon certain dilutive issuances, all as further detailed in the Company’s Articles of Association.
F - 25
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 11
|
-
|
SHARE CAPITAL (CONT.)
|
|
|
D.
|
Changes in share capital
|
|
Description
|
Amount raised, gross
in USD thousands
|
Shares issued
|
Price per share
|
Warrants issued
|
|
Series A Investment Round, February 2005
|
$675
|
6,750,000 Preferred A Shares
|
$0.10
|
None
|
|
Series B Investment Round, August 2005
|
$1,382
|
6,769,359 Preferred B Shares
|
$0.20
|
None
|
|
Conversion of April 2007 Bridge Loan, June 2009
|
$403
|
1,614,490
Preferred C1 Shares
|
$0.25
|
Expired
|
|
Series C Investment Round, June 2009
|
$7,384
|
14,800,416
Preferred C1 Shares
13,725,012
Preferred C2 Shares
|
$0.25
$0.27
|
The lead investor received 836,412 preferred C1 warrants and 1,007,975 preferred C2 warrants (1)
|
|
Joinder to Series C Investment, November 2009 to February 2010
|
$4,321
|
16,063,656
Preferred C2 Shares
|
$0.27
|
Expired
In addition, finder's received 539,021 preferred C2 warrants (2)
|
|
Anti-Dilution Warrants
|
-
|
-
|
-
|
7,804,912 ordinary share warrants (3)
|
|
Series D1 Investment Round, March 2011 (4)
|
$9,255
|
24,545,195
Preferred D1 Shares
|
$0.38
|
16,199, 826 preferred D2 warrants issued to all investors (4)
In addition, finder's received 411,320 preferred D2 warrants and 503,872 preferred D1 warrants (5)
|
|
Series D3 Investment Round, January 2012 (6)
|
$1,055
|
2,510,783 Preferred D3 Shares
|
$0.42
|
None
|
|
Subtotal
|
$24,475
|
|||
|
Less: Issuance expenses
|
($850)
|
|||
|
Less: D1 and D2 warrants classified as financial liability
|
($439)
|
|||
|
Ordinary shares
|
$59
|
|||
|
Total
|
$23,245
|
F - 26
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 11
|
-
|
SHARE CAPITAL (CONT.)
|
|
|
D.
|
Changes in share capital (Cont.)
|
|
|
(1)
|
Of the warrants issued to the lead investor, the 836,412 preferred C1warrants are exercisable at $0.2495 per share and the 1,007,975 preferred C2 warrants are exercisable at $0.2269 per share. These warrants expire on June 1, 2019 and are exercisable on a cashless basis. According to IAS 32, ‘Financial Instruments: Presentation’ (hereinafter—IAS 32), the preferred C1 and C2 warrants issued to the lead investor are classified as a financial liability, as their respective terms do not provide for fixed monetary payment in exchange for fixed number of shares. Accordingly, such warrants are presented in the statement of financial position as financial liabilities carried at fair value through profit and loss. As of December 31, 2013 and 2012, the fair value of such warrants was $587 thousand and $532 thousand, respectively. The increase in the fair value of such warrants for the year ended December 31, 2013 was $55 and was charged to the statement of profit or loss and other comprehensive income within “Changes in fair value of financial assets and liabilities designated at fair value through profit and loss”. |
|
|
(2)
|
In consideration for brokerage services in connection with the Series C preferred share investment: (i) on December 15, 2009, the Company issued warrants to purchase 371,739 preferred C2 shares, with an exercise price of $0.2690 per share, exercisable until November 22, 2014; and (ii) on April 27, 2010, the Company issued warrants to purchase 167,282 preferred C2 shares, with an exercise price of $0.2690 per share, exercisable until January 21, 2015. These grants were accounted for as a deduction of equity.
|
|
|
(3)
|
On May 11, 2010, the Company issued, free of charge, to all of its shareholders (except for certain ordinary shareholders), warrants to purchase an aggregate of 7,804,912 ordinary shares (hereafter- “Anti-dilution Warrants”). The Anti-dilution Warrants were issued to such shareholders as anti-dilution protection due to certain options granted to the Company’s CEO (hereafter- “CEO options”). The Anti-dilution Warrants will be automatically exercised, without consideration, upon the exercise by the Company’s CEO of the CEO Options. The fair value of the Anti-dilution Warrants on the grant date is immaterial.
|
|
|
(4)
|
In connection with brokerage services in connection with the Series D-1 investment, the Company issued warrants to purchase 411,320 preferred D2 shares, with an exercise price of $0.47135 per share and exercisable until March 17, 2015, and warrants to purchase 503,872 preferred D1 shares, with an exercise price of $0.37708 per share and exercisable until March 17, 2015.These grants were accounted for as a deduction of equity. In addition, the brokerage services providers received $449 thousand in cash.
|
|
|
(5)
|
On January 10, 2012, the Company entered into a share purchase agreement pursuant to which the Company issued 2,510,783 preferred D3 shares in consideration of an investment of $1,055 thousand, reflecting a per share price of $0.42 per preferred D3 share.
|
Following the issuance of the preferred D3 shares described above, the Company increased its preferred share capital by $7 thousands and recorded a premium on preferred D3 shares of $1,022 thousands, net of issuance costs.
F - 27
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 12
|
-
|
SHARE BASED PAYMENT
|
A. Details of share-based grants made by the Company
|
Month of option grant
|
No. of options
|
Grant date
|
Expiration date
|
Exercise price
|
Fair value on grant date
|
|||||||||
|
USD
|
||||||||||||||
|
April 2012
|
1,160,000 |
04/04/2012
|
04/04/2022
|
0.25 | 0.07 | |||||||||
|
August 2012
|
350,000 |
28/08/2012
|
28/08/2022
|
0.25 | 0.07 | |||||||||
|
June 2013
|
2,355,000 |
12/06/2013
|
12/06/2023
|
0.25 | 0.152 | |||||||||
|
1)
|
In connection with the transfer of all of the business operations and substantially all of the assets of Check-Cap LLC to the Company in 2009, the Company assumed the Check-Cap LLC 2006 Unit Option Plan (hereafter: “the Plan”). According to the Plan, the Company is authorized to grant options to purchase ordinary shares of the Company to employees, directors and consultants of the Company. The options granted according to the Plan are generally exercisable for 10 years from the grant date unless otherwise determined by the Company’s Board of Directors, vest over a period to be determined by the Company’s Board of Directors, and have an exercise price to be determined by the Company’s Board of Directors.
|
|
2)
|
In April 2012, the Company’s Board of Directors approved the grant of 1,160,000 options to purchase ordinary shares NIS 0.01 par value of the Company at an exercise price of $0.2478 to employees and consultants of the Company. 25,012 of these options were vested on the grant date. 551,670 of the options will vest over two years, 357,494 of the options will vest over three years and the remaining, and 188,330 options will vest over four years.
The compensation expense was based on the fair value on the grant date, and was estimated at approximately $24 thousand.
|
|
3)
|
In August 2012 the Company’s Board of Directors approved the grant of 350,000 options to purchase ordinary shares NIS 0.01 par value of the Company at an exercise price of $0.2478 to employees and consultants of the Company. 29,174 of these options were vested on the grant date. 233,328 of the options will vest over two years, 87,498 of the options will vest over three years.
The compensation expense was based on the fair value on the grant date, and was estimated at approximately $4 thousand. This amount is charged to profit and loss over the vesting periods.
|
|
4)
|
In June 2013 the Company’s Board of Directors approved the grant of 2,355,000 options to purchase ordinary shares NIS 0.01 par value of the Company at an exercise price of $0.2478 to employees and consultants of the Company. 322,928 of these options were vested on the grant date. 1,351,660 of the options will vest over two years, 513,746 of the options will vest over three years, and 166,666 options will vest over four years. The compensation expense was based on the fair value on the grant date, and was estimated at approximately $84 thousand. This amount is charged to profit and loss over the vesting periods.
|
F - 28
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 12
|
-
|
SHARE-BASED PAYMENT (CONT.)
|
B. Options Fair Value
The fair value of the options granted was estimated by applying the Black Scholes Merton model. While calculating the share based payments expenses, the Company took into account the estimated forfeiture rate of the options, based on the employees’ class.
The parameters which were used in applying the model are as follows:
|
Element
|
June 2013
|
April-August 2012
|
||||
|
Share price (in $)
|
0.152 | 0.07(**) | ||||
|
Exercise price (in $)
|
0.25 | 0.25 | ||||
|
Expected volatility (in %) (*)
|
40-60 | 64-67 | ||||
|
Option term (in years)
|
5-10 | 5-10 | ||||
|
Risk free interest rate (in %)
|
0.75-2.25 | 0.75-2.25 | ||||
|
Anticipated rate of dividends (in %)
|
0 | 0 |
|
|
(*)
|
Since the shares of the Company are not marketable, the expected volatility was determined on the basis of the historic volatility of the share price of peer companies whose shares are traded on the stock exchange.
|
|
|
(**)
|
Based on funds raising close to the date of vesting.
|
In the absence of historic data as to the expected term of the options, management determine the expected term of the options based on the midpoint between the available exercise dates (the end of the vesting periods) and the last available exercise date (the contracted expiration date).
|
|
C.
|
Effect of share based payment transactions on the Company's profit or loss and on the financial position
|
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
The part of the expense settled by equity instruments of the Company
|
53 | 31 | ||||||
F - 29
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 12
|
-
|
SHARE-BASED PAYMENT (CONT.)
|
|
|
D.
|
Additional details of options granted to employees
|
|
Number of options
|
Weighted average of exercise price
|
|||||||
|
USD
|
||||||||
|
Balance as of January 1, 2012
|
13,020,977 | 0.124 | ||||||
|
Granted
|
1,510,000 | 0.248 | ||||||
|
Exercised
|
(14,780 | ) | 0.223 | |||||
|
Forfeited
|
(508,453 | ) | 0.166 | |||||
|
Balance as of December 31, 2012
|
14,007,744 | 0.136 | ||||||
|
Granted
|
2,355,000 | 0.248 | ||||||
|
Forfeited
|
(934,485 | ) | 0.171 | |||||
|
Balance as of December 31, 2013
|
15,428,259 | 0.151 | ||||||
|
Options granted to employees and are exercisable
|
'Weighted average remaining term of the options
|
|||||||
|
Number of options
|
Years
|
|||||||
|
Balance as of December 31, 2012
|
10,652,275 | 6.686 | ||||||
|
Balance as of December 31, 2013
|
11,897,828 | 6.010 | ||||||
|
NOTE 13
|
-
|
RESEARCH AND DEVELOPMENT EXPENSES, NET
|
Composition:
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Salaries and related expenses
|
2,170 | 2,091 | ||||||
|
Share-based payment
|
41 | 25 | ||||||
|
Materials
|
307 | 329 | ||||||
|
Subcontractors
|
218 | 330 | ||||||
|
Depreciation and amortization
|
70 | 67 | ||||||
|
Cost for registration of patents
|
118 | 52 | ||||||
|
Others
|
110 | 29 | ||||||
| 3,034 | 2,923 | |||||||
|
Less participation of the OCS
|
(372 | ) | (231 | ) | ||||
|
Total research and development, net
|
2,662 | 2,692 | ||||||
F - 30
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 14
|
-
|
GENERAL AND ADMINISTRATIVE EXPENSES
|
Composition:
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Expenses for employee benefits
|
683 | 653 | ||||||
|
Share-based payment
|
16 | 6 | ||||||
|
Professional services
|
95 | 70 | ||||||
|
Office rent and maintenance
|
104 | 142 | ||||||
|
Depreciation and amortization
|
7 | 7 | ||||||
|
Others
|
185 | 325 | ||||||
|
Total general and administrative
|
1,090 | 1,203 | ||||||
|
NOTE 15
|
-
|
FINANCING INCOME
|
Composition:
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Interest income on short term deposits
|
59 | 183 | ||||||
|
Changes in provision for royalties
|
- | 233 | ||||||
|
Exchange rate differences
|
4 | - | ||||||
|
Total financing income
|
63 | 416 | ||||||
|
NOTE 16
|
-
|
FINANCING EXPENSES
|
Composition:
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Bank fees
|
5 | 6 | ||||||
|
Current changes in provision for royalties
|
256 | 85 | ||||||
|
Changes in fair value of financial assets and liabilities designated at fair value through profit and loss
|
55 | 116 | ||||||
|
Exchange rate differences
|
- | 22 | ||||||
|
Total financing expenses
|
316 | 229 | ||||||
F - 31
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 17
|
-
|
LOSS PER SHARE
|
Basic loss per share
Instruments that may potentially dilute the basic loss per share in the future, but were not included in the calculation of diluted loss per share, since their effect is anti-dilutive.
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
Thousands of shares
|
||||||||
|
Weighted average number of ordinary shares used in calculating loss per share
|
32,542 | 32,530 | ||||||
|
Adjustments:
|
||||||||
|
Warrants and share options
|
12,780 | 12,019 | ||||||
|
Preferred shares
|
86,779 | 86,779 | ||||||
| 132,101 | 131,328 | |||||||
|
NOTE 18
|
-
|
FINANCIAL INSTRUMENTS
|
|
|
A.
|
Financial instruments fair value
|
The carrying amount of the Company's financial instruments equals or approximates their fair value other than the reimbursement liability to the Predecessor Entity’s unit holders (See Note 4B3 and 10A), which its fair value as of December 31, 2013 is lower by approximately $150 thousands then its carrying amount. As of December 31, 2012, the fair value of such liability was lower by approximately $138 thousands than its carrying amount.
|
|
B.
|
Financial instruments according to category
|
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Financial assets:
|
||||||||
|
Cash and cash equivalents
|
4,975 | 4,583 | ||||||
|
Restricted deposit
|
46 | 43 | ||||||
|
Short-term investment
|
- | 3,450 | ||||||
|
Other current assets
|
130 | 164 | ||||||
| 5,151 | 8,240 | |||||||
|
Financial liabilities:
|
||||||||
|
Current liabilities:
|
||||||||
|
Trade accounts payable
|
221 | 140 | ||||||
|
Other current liabilities
|
141 | 140 | ||||||
|
Employee benefits liabilities
|
658 | 509 | ||||||
|
Non-current liabilities:
|
||||||||
|
Royalties provision
|
2,577 | 2,046 | ||||||
|
Other financial liabilities
|
587 | 532 | ||||||
| 4,184 | 3,367 | |||||||
F - 32
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 18
|
-
|
FINANCIAL INSTRUMENTS (CONT.)
|
|
|
C.
|
Purposes of financial risk management
|
The Company's finance department renders services for business operations, permits access to local and international financial markets, supervises and administers the financial risks related with the activities of the Company by means of internal reports which analyze the extent of exposure to risks according to their level and intensity. These risks include market risks (including foreign currency risk) and liquidity risk.
|
|
D.
|
Market risk
|
Foreign currency risk
The Company's functional currency is the U.S. dollar. The Company's exposures to the fluctuations occurring in the rates of exchange between the U.S. dollar and the New Israeli Shekel result mainly from salaries and related expenses that are stated in NIS.
The Company acts to reduce the currency risk by means of holding its liquid resources in short-term deposits (NIS and dollars).
During the year ended December 31, 2013, no change took place in the exposure to currency risk or in the manner in which the Company manages and measures the risk.
The book values of the financial assets and liabilities of the Company denominated in foreign currency are as follows:
|
Liabilities
|
Assets
|
|||||||||||||||
|
December 31,
|
December 31,
|
|||||||||||||||
|
2013
|
2012
|
2013
|
2012
|
|||||||||||||
|
in USD thousands
|
in USD thousands
|
|||||||||||||||
|
NIS
|
940 | 605 | 490 | 1,220 | ||||||||||||
|
Euro
|
171 | 156 | - | - | ||||||||||||
Sensitivity analysis of foreign currency
As stated above, the Company is exposed mainly to the NIS currency since salaries and related expenses are stated in N.I.S.
The following table itemizes the sensitivity to an increase or a decrease of 10% in the relevant exchange rate. 10% is the rate of sensitivity representing the assessments of management with respect to the reasonable possible change in exchange rates. The sensitivity analysis includes current balances of monetary items denominated in foreign currency and conforms their translation at the end of the period to a change of 10% in foreign currency rates.
F - 33
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 18
|
-
|
FINANCIAL INSTRUMENTS (CONT.)
|
|
|
D.
|
Market risk (Cont.)
|
Sensitivity analysis of foreign currency (Cont.)
|
Effect of NIS currency
|
||||||||
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Pre tax effect of increase of 10% in the $ currency vis-à-vis the NIS:
|
||||||||
|
Effect on profit or loss and other comprehensive income for the year
|
41 | (56 | ) | |||||
|
Effect on equity (deficiency)
|
41 | (56 | ) | |||||
|
Pre tax effect of decrease of 10% in the $ currency vis-à-vis the NIS:
|
||||||||
|
Effect on profit or loss and other comprehensive income for the year
|
(45 | ) | 62 | |||||
|
Effect on equity (deficiency)
|
(45 | ) | 62 | |||||
|
Pre tax effect of increase of 10% in the $ currency vis-à-vis the Euro:
|
||||||||
|
Effect on profit or loss and other comprehensive income for the year
|
16 | 14 | ||||||
|
Effect on equity (deficiency)
|
16 | 14 | ||||||
|
Pre tax effect of decrease of 10% in the $ currency vis-à-vis the Euro:
|
||||||||
|
Effect on profit or loss and other comprehensive income for the year
|
(17 | ) | (16 | ) | ||||
|
Effect on equity (deficiency)
|
(17 | ) | (16 | ) | ||||
|
|
E.
|
Management of credit risk
|
Most of the Company’s receivables are from government institutions that have little to no credit risk exposure.
Moreover, the Company holds cash and cash equivalents in various financial institutions. These financial institutions are located in Israel and the United States. Pursuant to the Company’s policies, evaluations of the relative financial stability of the different financial institutions are performed on a current basis.
|
F.
|
Liquidity risk
|
Carful management of liquidity risk requires a sufficient cash balance to support operating activities. Management constantly analyzes cash balances forecasts which comprised of cash and cash equivalents and assets at fair value through profit and loss. This analysis is based on forecasted cash flows, in accordance with policies and restrictions set by the Company.
The Company keeps a sufficient level of cash and cash equivalents, by taking into account the cash required for its operating activities, in order to reduce the liquidity risk which the Company is exposed to.
F - 34
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 19
|
-
|
FAIR VALUE
|
Fair value hierarchy
The table below presents an analysis of the financial instruments measured at fair value, using a valuation method:
The different levels were defined as follows:
1) Level 1: Quoted prices (unadjusted) in an active market for identical instruments.
2) Level 2: Data observed directly or indirectly that are not included in Level 1 above.
3) Level 3: Data not based on observable market data.
|
For the year ended December 31, 2013
|
||||
|
Level 3
|
||||
|
in USD thousands
|
||||
|
Other financial liabilities
|
587 | |||
|
Total
|
587 | |||
|
For the year ended December 31, 2012
|
||||
|
Level 3
|
||||
|
in USD thousands
|
||||
|
Other financial liabilities
|
532 | |||
|
Total
|
532 | |||
The fair value of the financial liability included in the level 3 categories above has been determined in accordance with generally accepted pricing models based on a Black Scholes Merton formula, with the most significant inputs being share price, exercise price, volatility, risk free rate and contractual term.
|
NOTE 20
|
-
|
TRANSACTIONS WITH INTERESTED PARTIES AND RELATED PARTIES
|
|
|
A.
|
Compensation to key management personnel and interested parties
|
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Salary and related expenses to interested parties employed by the Company
|
403 | 436 | ||||||
|
Number of personnel to which benefit applies
|
2 | 2 | ||||||
|
Salary and related expenses to key management personnel
|
381 | 195 | ||||||
|
Number of personnel to which benefit applies
|
2 | 1 | ||||||
|
Share based payment to interested parties and key management personnel
|
46 | 31 | ||||||
|
Number of personnel to which benefit applies
|
5 | 5 | ||||||
F - 35
CHECK-CAP LTD.
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 20
|
-
|
TRANSACTIONS WITH INTERESTED PARTIES AND RELATED PARTIES (CONT.)
|
|
|
B.
|
Transactions with interested and related parties
|
|
For the year ended December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Consultation (1)
|
40 | 37 | ||||||
|
Key man life insurance premium (2)
|
9 | 8 | ||||||
|
Management fees (3)
|
- | 80 | ||||||
| 49 | 125 | |||||||
|
|
(1)
|
On July 1, 2005, the Company entered into an agreement with Hadar Kimchy according to which Hadar Kimchy provides marketing communication and graphical design services to the Company in consideration for a monthly retainer of 10,260 NIS ($3 thousand). The above services are provided to the Company by Sigalit Kimchy, who is employed by Hadar Kimchy. Sigalit Kimchy is a shareholder and is the spouse of Yoav Kimchy, the Company's chief technology officer and a director.
|
|
|
(2)
|
In connection with the asset transfer agreement entered into with the Predecessor Entity in May 2009, the Company assumed the former obligation of the Predecessor Entity to distribute any proceeds it collects on the $1,000,000 key man life insurance policy with respect to Yoav Kimchy, the Company's chief technology officer and a director, to the former holders of the Series A preferred units in an amount equal to their respective capital contributed to the Predecessor Entity, less any amounts previously distributed to them, plus any accrued and unpaid dividends due to them as of the date of distribution.
|
|
|
(3)
|
Check-Cap Ltd. (Delaware), which is the manager of the Predecessor Entity and is wholly-owned by Mr. Kimchy, the Company's chief technology officer and a director, handles from time to time certain logistical, administrative and investor relations matters for us with the Company’s U.S. suppliers and investors, in consideration of a quarterly payment of $20,000, which amount essentially covers Check-Cap Ltd. (Delaware)’s costs of performing these functions. The Company last utilized such services as of December 31, 2012.
|
|
|
C.
|
Liabilities to interested parties and other related parties
|
|
December 31,
|
||||||||
|
2013
|
2012
|
|||||||
|
in USD thousands
|
||||||||
|
Current liabilities-
|
||||||||
|
Trade accounts payable
|
34 | 22 | ||||||
|
Employee benefits liabilities
|
108 | 98 | ||||||
|
Non-current liabilities-
|
||||||||
|
Reimbursement liability to Predecessor Entity's unit holders
|
788 | 699 | ||||||
F - 36
CHECK CAP LTD.
FINANCIAL STATEMENTS AS OF JUNE 30, 2014
|
Page
|
|
|
Financial statements:
|
|
|
F - 38
|
|
|
F - 39
|
|
|
F - 40
|
|
|
F - 41
|
|
|
F - 42 - F - 43
|
F - 37
CHECK CAP LTD
STATEMENTS OF FINANCIAL POSITION
|
June 30,
|
December 31,
|
||||||||||||
|
2014
|
2013
|
||||||||||||
|
unaudited
|
audited
|
||||||||||||
|
Note
|
In USD thousands
|
||||||||||||
|
Assets
|
|||||||||||||
|
Current assets
|
|||||||||||||
|
Cash and cash equivalents
|
2,794 | 4,975 | |||||||||||
|
Restricted deposit
|
46 | 46 | |||||||||||
|
Other current assets
|
230 | 130 | |||||||||||
|
Total current assets
|
3,070 | 5,151 | |||||||||||
|
Property and equipment, net
|
206 | 224 | |||||||||||
|
Total assets
|
3,276 | 5,375 | |||||||||||
|
Liabilities and shareholders’ equity (deficit)
|
|||||||||||||
|
Current liabilities
|
|||||||||||||
|
Trade accounts payable
|
278 | 221 | |||||||||||
|
Other current liabilities
|
116 | 141 | |||||||||||
|
Employee benefits
|
686 | 658 | |||||||||||
|
Total current liabilities
|
1,080 | 1,020 | |||||||||||
|
Non-current liabilities
|
|||||||||||||
|
Royalties provision
|
2,537 | 2,577 | |||||||||||
|
Other financial liabilities
|
657 | 587 | |||||||||||
|
Total non-current liabilities
|
3,194 | 3,164 | |||||||||||
|
Shareholders’ equity
|
1D
|
Pro Forma
as of
June 30, 2014
(unaudited)
|
|||||||||||
|
Preferred share capital
|
- | 226 | 226 | ||||||||||
|
Ordinary share capital
|
XXX
|
53 | 53 | ||||||||||
|
Premium on preferred shares
|
- | 21,167 | 21,167 | ||||||||||
|
Share options
|
XXX
|
1,793 | 1,793 | ||||||||||
|
Premium on ordinary shares
|
XXX
|
6 | 6 | ||||||||||
|
Capital reserve for ordinary share-based payment
|
471 | 471 | 431 | ||||||||||
|
Accumulated deficit
|
(24,714 | ) | (24,714 | ) | (22,485 | ) | |||||||
|
Total shareholders’ equity (deficit)
|
(998 | ) | (998 | ) | 1,191 | ||||||||
|
Total liabilities and shareholders’ equity (deficit)
|
- | 3,276 | 5,375 | ||||||||||
The accompanying notes to the financial statements are an integral part of them.
F - 38
CHECK CAP LTD
STATEMENTS OF PROFIT OR LOSS AND OTHER COMPREHENSIVE INCOME
|
Six months ended
June 30,
|
Three months ended June 30,
|
|||||||||||||||
|
2014
|
2013
|
2014
|
2013
|
|||||||||||||
|
unaudited
|
||||||||||||||||
|
In USD thousands
|
||||||||||||||||
|
Research and development expenses, net
|
1,640 | 1,364 | 843 | 704 | ||||||||||||
|
General and administrative expenses
|
564 | 520 | 269 | 249 | ||||||||||||
|
Operating loss
|
2,204 | 1,884 | 1,112 | 953 | ||||||||||||
|
Finance income
|
(60 | ) | (45 | ) | (20 | ) | (27 | ) | ||||||||
|
Finance expenses
|
85 | 230 | 50 | 149 | ||||||||||||
|
Finance expenses, net
|
25 | 185 | 30 | 122 | ||||||||||||
|
Loss for the period
|
2,229 | 2,069 | 1,142 | 1,075 | ||||||||||||
|
Total comprehensive loss for the period
|
2,229 | 2,069 | 1,142 | 1,075 | ||||||||||||
|
Loss per ordinary share (in USD) basic and diluted
|
0.10 | 0.09 | 0.05 | 0.05 | ||||||||||||
|
Weighted average number of ordinary shares outstanding - basic and diluted
(in thousands)
|
32,542 | 32,542 | 32,542 | 32,542 | ||||||||||||
|
Pro forma loss per ordinary share (in USD) basic and diluted
|
X.XX
|
X.XX
|
X.XX
|
X.XX
|
||||||||||||
|
Pro forma weighted average number of ordinary shares outstanding - basic and diluted (in thousands)
|
XXX
|
X.XX
|
X.XX
|
X.XX
|
||||||||||||
The accompanying notes to the financial statements are an integral part of them.
F - 39
CHECK CAP LTD
STATEMENTS OF CHANGES IN EQUITY (DEFICIT)
|
Preferred share capital
|
Ordinary share capital
|
Premium on preferred shares
|
Share options
|
Premium on ordinary shares
|
Capital reserve for ordinary share based payment
|
Accumulated deficit
|
Total
|
|||||||||||||||||||||||||
|
unaudited
|
||||||||||||||||||||||||||||||||
|
In USD thousands
|
||||||||||||||||||||||||||||||||
|
For the six months ended June 30, 2014
|
||||||||||||||||||||||||||||||||
|
Balance - January 1, 2014
|
226 | 53 | 21,167 | 1,793 | 6 | 431 | (22,485 | ) | 1,191 | |||||||||||||||||||||||
|
Ordinary share-based payment
|
- | - | - | - | - | 40 | - | 40 | ||||||||||||||||||||||||
|
Comprehensive loss for the year
|
- | - | - | - | - | - | (2,229 | ) | (2,229 | ) | ||||||||||||||||||||||
|
Total shareholders’ deficit as of June 30, 2014
|
226 | 53 | 21,167 | 1,793 | 6 | 471 | (24,714 | ) | (998 | ) | ||||||||||||||||||||||
|
For the six months ended June 30, 2013
|
||||||||||||||||||||||||||||||||
|
Balance - January 1, 2013
|
226 | 53 | 21,167 | 1,793 | 6 | 374 | (18,490 | ) | 5,129 | |||||||||||||||||||||||
|
Ordinary share-based payment
|
- | - | - | - | - | 10 | - | 10 | ||||||||||||||||||||||||
|
Comprehensive loss for the year
|
- | - | - | - | - | - | (2,069 | ) | (2,069 | ) | ||||||||||||||||||||||
|
Total shareholders’ equity as of June 30, 2013
|
226 | 53 | 21,167 | 1,793 | 6 | 384 | (20,559 | ) | 3,070 | |||||||||||||||||||||||
The accompanying notes to the financial statements are an integral part of them.
F - 40
CHECK CAP LTD
STATEMENTS OF CASH FLOWS
|
Six months ended
June 30,
|
||||||||
|
2014
|
2013
|
|||||||
|
unaudited
|
||||||||
|
in USD thousands
|
||||||||
|
CASH FLOWS FROM OPERATING ACTIVITIES
|
||||||||
|
Loss for the period
|
(2,229 | ) | (2,069 | ) | ||||
|
Net gain arising on financial assets and liabilities designated as at fair value through profit or loss
|
70 | 123 | ||||||
|
Depreciation and amortization
|
40 | 39 | ||||||
|
Ordinary share-based compensation
|
40 | 10 | ||||||
|
Changes in assets and liabilities items:
|
||||||||
|
Increase in other current assets
|
(100 | ) | (4 | ) | ||||
|
Increase in trade accounts payable and other current liabilities
|
32 | 67 | ||||||
|
Increase (decrease) in employees benefits
|
28 | (108 | ) | |||||
|
Increase (decrease) in royalties provision
|
(40 | ) | 99 | |||||
|
Net cash used in operating activities
|
(2,159 | ) | (1,843 | ) | ||||
|
CASH FLOWS FROM INVESTING ACTIVITIES
|
||||||||
|
Purchase of property and equipment
|
(22 | ) | (27 | ) | ||||
|
Increase in restricted deposit
|
- | (3 | ) | |||||
|
Decrease in short-term investment
|
- | 3,450 | ||||||
|
Net cash generated from (used in) investing activities
|
(22 | ) | 3,420 | |||||
|
Net increase (decrease) in cash and cash equivalents
|
(2,181 | ) | 1,577 | |||||
|
Cash and cash equivalents at the beginning of the period
|
4,975 | 4,583 | ||||||
|
Cash and cash equivalents at the end of the period
|
2,794 | 6,160 | ||||||
The accompanying notes to the financial statements are an integral part of them.
F - 41
|
NOTE 1
|
-
|
GENERAL INFORMATION
|
|
|
A.
|
General
|
Check Cap Ltd. (hereafter-"the Company") is a limited liability private company organized under the laws of the State of Israel. The registered address of its offices is Abba Hushi Blvd., Isfiya.
The Company is engaged in the development of an ingestible imaging capsule that utilizes low-dose X-rays for the screening for precancerous polyps and colorectal cancer. The capsule is designed to enable an early detection of the disease without the need for a prior burdensome and uncomfortable bowel cleansing, in a patient-friendly and non-invasive screening procedure.
|
|
B.
|
Financial Position
|
Since its inception, the Company has devoted substantially all of its efforts to research and development, clinical trials, recruiting management and technical staff, acquiring assets and raising capital. The Company is still in its development and clinical stage and has not yet generated revenues. The extent of the Company's future operating losses and the timing of becoming profitable are uncertain. The Company has incurred losses of $24,920, $22,485, and $20,268 thousands as of June 30, 2014 December 31, 2013 and June 30, 2013, respectively. The Company funds its operations primarily through equity financings.
|
|
C.
|
Agreement for transfer of assets
|
On May 31, 2009, the Company entered into an asset transfer agreement with Check Cap LLC (hereafter-"the Predecessor Entity"), a company with the same shareholders as the Company at the time of transfer. According to the agreement, the Predecessor Entity transferred all of its business operations and substantially all of its assets to the Company, including development and consulting agreements, cash, property and equipment and intangible ownership rights, free of any debt. The agreement did not have any effect on the Company's operations and was accounted for using historical amounts.
In addition, losses for tax purposes accumulated by the Predecessor Entity were not transferred to the Company.
|
|
D.
|
Pro Forma Information (unaudited)
|
The pro forma information gives effect to the capital restructuring concurrent with an event of an initial public offering by the Company to give effect to the conversion of all of the Company's Preferred shares into XX,XXX,XXX ordinary shares. Because the Company is considering an IPO, the financial statements include the effect of the conversion of all of the preferred shares into ordinary shares in a pro forma shareholders' equity presentation, and retroactively reflects the effect of the conversion in a pro forma loss per share presentation.
The pro forma information also gives effect to the issuance of 1,995,475 ordinary shares underlying options granted to the Company’s CEO and 7,503,521 ordinary shares underlying warrants granted to certain shareholders of the Company as anti-dilution protection upon the grant of the foregoing options to the Company’s CEO.
F - 42
CHECK CAP LTD
NOTES TO THE FINANCIAL STATEMENTS
|
NOTE 2
|
-
|
SIGNIFICANT ACCOUNTING POLICIES
|
The significant accounting policies applied in the annual financial statements of the Company as of December 31, 2013 are applied consistently in these financial statements.
|
NOTE 3
|
-
|
UNAUDITED INTERIM CONSOLIDATED FINANCIAL STATEMENTS
|
The accompanying unaudited interim financial statements have been prepared in a condensed format and include the consolidated financial operations of the Company as of June 30, 2014 and for the six months then ended, in accordance with International Financial Reporting Standards, as issued by International Accounting Standards Board (IFRS), relating to the preparation of financial statements for interim periods. Accordingly, they do not include all the information and footnotes required by generally accepted accounting principles for complete financial statements. In the opinion of management, all adjustments (consisting of normal recurring accruals) considered necessary for a fair presentation have been included. Operating results for the six months ended June 30, 2014, are not necessarily indicative of the results that may be expected for the year ended December 31, 2014.
|
NOTE 4
|
-
|
SUBSEQUENT EVENTS
|
On August 20, 2014, the Company entered into a certain credit line agreement, pursuant to which it obtained a credit line in an aggregate principal amount of $12 million from certain lenders and existing shareholders (the “Lenders”). The credit line amount was deposited in an escrow account at the closing, which was consummated on October 14, 2014.
The Company issued to each Lender at closing a warrant (collectively, the “Credit Line Warrants”), to purchase a number of the Company’s ordinary shares constituting 2% of its share capital on a fully diluted basis (assuming conversion of all of the Company’s convertible securities into ordinary shares at a 1:1 conversion rate) as of the closing for each $1 million (or portion thereof) extended by such Lender. The Credit Line Warrants are exercisable for a period of ten years at an exercise price of NIS 0.01 per share, and may be exercised on a net issuance basis.
Under the terms of the agreement, if the Company intends to consummate an initial public offering (an “IPO”), and such IPO is expected to be consummated on or prior to February 18, 2015, or if the Company consummated an IPO on or prior to February 18, 2015, the Company will be entitled to call all or any part of the credit line amount and direct that such called amount be invested in ordinary shares of the Company in a private placement transaction that is exempt from the registration requirements of the U.S. Securities Act of 1933, as amended, at a price per ordinary share equal to the public offering price per share in the IPO. In the event that the Company calls less than the full credit line amount, the Company will call amounts on a pro rata basis among the Lenders based on their pro rata share of the total credit line amount. If the Company consummates an IPO on or prior to February 18, 2015, any part of the credit line amount not called by the Company will be released to the Lenders.
If the Company does not consummate an IPO on or prior to February 18, 2015, the Company may call the credit line amount at any time thereafter until April 14, 2016, subject to certain conditions. Any part of the credit line amount not called by the Company on or prior to such date will be released to the Lenders. If the Company calls the credit line amount on or prior to April 14, 2016, the amount called will bear interest at the annual rate of 7%; provided that the aggregate interest rate will not be less than 5%. The called credit line amount will automatically convert into shares of the Company upon the earlier of a qualified financing round (which includes a public offering, including an IPO), an M&A. Event (as defined in the agreement) and April 14, 2016, and the Lenders may elect to convert the entire called credit line upon a non-qualified financing round, all under the terms and conditions set forth in the credit line agreement. In the event that the qualified financing round is an IPO, in lieu of automatic conversion, the Company is entitled to deposit in trust an amount equal to 125% of the called credit line amount and irrevocably instruct the trustee to submit an offer, on behalf of each Lender, for the purchase of the IPO shares at the IPO price determined by the lead underwriters.
F - 43
Check-Cap Ltd.
Ordinary Shares

____________________________
PROSPECTUS
____________________________
|
Chardan Capital Markets, LLC
|
Maxim Group LLC
|
, 2014
Through and including , 2014 (the 25th day after the commencement of this offering), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealers’ obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
Under the Israeli Companies Law, a company may not exculpate an office holder from liability for a breach of the duty of loyalty. An Israeli company may exculpate an office holder in advance from liability to the company, in whole or in part, for damages caused to the company as a result of a breach of duty of care, but only if a provision authorizing such exculpation is included in its articles of association. Our amended articles of association to be effective upon the closing of this offering include such a provision to the fullest extent permitted by law. The company may not exculpate in advance a director from liability arising out of a prohibited dividend or other distribution to shareholders.
Under the Israeli Companies Law and the Israeli Securities Law, a company may indemnify an office holder in respect of the following liabilities and expenses incurred for acts performed by him or her as an office holder, either pursuant to an undertaking made in advance of any such event or following an event, provided its articles of association include a provision authorizing such indemnification:
|
|
·
|
a financial liability imposed on him or her in favor of another person pursuant to a judgment, including a settlement or arbitrator’s award approved by a court. However, if an undertaking to indemnify an office holder with respect to such liability is provided in advance, then such an undertaking must be limited to events which, in the opinion of the board of directors, can be foreseen based on the company’s activities when the undertaking to indemnify is given, and to an amount or according to criteria determined by the board of directors as reasonable under the circumstances, and such undertaking shall detail the abovementioned foreseen events and amount or criteria;
|
|
|
·
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder (1) as a result of an investigation or proceeding instituted against him or her by an authority authorized to conduct such investigation or proceeding, provided that (i) no indictment was filed against such office holder as a result of such investigation or proceeding; and (ii) no financial liability was imposed upon him or her as a substitute for the criminal proceeding as a result of such investigation or proceeding or, if such financial liability was imposed, it was imposed with respect to an offense that does not require proof of criminal intent; and (2) in connection with a monetary sanction;
|
|
|
·
|
reasonable litigation expenses, including attorneys’ fees, incurred by the office holder or imposed by a court in proceedings instituted against him or her by the company, on its behalf, or by a third party, or in connection with criminal proceedings in which the office holder was acquitted, or as a result of a conviction for an offense that does not require proof of criminal intent; and
|
|
|
·
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder, or certain compensation payments made to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Israeli Securities Law.
|
Under the Israeli Companies Law and the Israeli Securities Law, a company may insure an office holder against the following liabilities incurred for acts performed by him or her as an office holder if and to the extent provided in the company’s articles of association:
|
|
·
|
a breach of the duty of loyalty to the company, provided that the office holder acted in good faith and had a reasonable basis to believe that the act would not harm the company;
|
|
|
·
|
a breach of the duty of care to the company or to a third party, to the extent such a breach arises out of the negligent conduct of the office holder;
|
|
|
·
|
a financial liability imposed on the office holder in favor of a third party; and
|
|
|
·
|
expenses, including reasonable litigation expenses and legal fees, incurred by an office holder in relation to an administrative proceeding instituted against such office holder or certain compensation payments to an injured party imposed on an office holder by an administrative proceeding, pursuant to certain provisions of the Securities Law.
|
II - 1
Under the Israeli Companies Law, a company may not indemnify, exculpate or enter into an insurance contract which would provide coverage for any monetary liability incurred as a result of any of the following:
|
|
·
|
a breach of the duty of loyalty, except for indemnification and insurance for a breach of the duty of loyalty to the company to the extent that the office holder acted in good faith and had a reasonable basis to believe that the act would not prejudice the company;
|
|
|
·
|
a breach of the duty of care committed intentionally or recklessly, excluding a breach arising out of the negligent conduct of the office holder;
|
|
|
·
|
an act or omission committed with intent to derive illegal personal benefit; or
|
|
|
·
|
a fine, monetary sanction or forfeit levied against the office holder.
|
Under the Israeli Companies Law, exculpation, indemnification and insurance of office holders in a public company must be approved by the compensation committee and the board of directors and, with respect to the chief executive officer or a director or under certain circumstances, also by the shareholders. See “— Approval of Related Party Transactions under Israeli Law.”
Our amended articles of association, which will be effective upon the closing of this offering, permit us to exculpate, indemnify and insure our office holders to the fullest extent permitted under the Israeli Companies Law and Israeli Securities Law. We have obtained directors’ and officers’ liability insurance for the benefit of our office holders and intend to continue to maintain such coverage and pay all premiums thereunder to the fullest extent permitted by the Israeli Companies Law. In accordance with our articles of association, in the event that our insurance also covers our liability, our office holders shall have precedence over us in collecting insurance payments.
We have entered into indemnification and exculpation agreements with each of our current officers and directors (other than Alex Ovadia) exculpating them from a breach of their duty of care to us to the fullest extent permitted by the Israeli Companies Law and undertaking to indemnify them to the fullest extent permitted by the Israeli Companies Law (other than indemnification for litigation expenses in connection with a monetary sanction), to the extent that these liabilities are not covered by insurance. This indemnification is limited to events determined as foreseeable by our board of directors based on our activities, as set forth in the indemnification agreements. Under such indemnification agreements, the maximum aggregate amount of indemnification that we may pay to any and all of our currently serving or future officers and directors together may not exceed 25% of our share capital according to our most recent annual financial statements at the time of payment. We intend to enter into a new form of indemnification and exculpation agreement with each of our current officers and directors exculpating them from a breach of their duty of care to us to the fullest extent permitted by the Israeli Companies Law and undertaking to indemnify them to the fullest extent permitted by the Israeli Companies Law and the Israeli Securities Law prior to the closing of this offering.
The proposed form of Underwriting Agreement filed as Exhibit 1.1 to this Registration Statement provides for indemnification of our office holders by the underwriters against certain liabilities.
Item 7. Recent Sales of Unregistered Securities
Set forth below are the sales of all securities by us since January 1, 2011.
|
|
·
|
Since January 1, 2011, we granted options to purchase an aggregate of 4,982,702 ordinary shares to certain of our employees, officers and consultants under our 2006 Unit Option Plan, of which (i) options to purchase 1,117,702 ordinary shares having an exercise price of $0.22270 were granted in June 2011; (ii) options to purchase 1,160,000 ordinary shares having an exercise price of $0.24780 were granted in April 2012; (iii) options to purchase 350,000 ordinary shares having an exercise price of $0.24780 were granted in August 2012; (iv) options to purchase 2,355,000 ordinary shares having an exercise price of $0.24780 were granted in June 2013; and (v) options to purchase 11,630,739 ordinary shares having an exercise price of NIS 0.01 were granted on August 20, 2014. Of such options, options to purchase an aggregate of 1,606,562 ordinary shares have been forfeited and cancelled without being exercised as of the date of this prospectus. We claimed exemption from registration under the Securities Act for such transactions under Section 4(a)(2) and/or Regulation S of the Securities Act. |
II - 2
|
|
·
|
In October 2012, we issued and sold 14,780 ordinary shares pursuant to the exercise of options held by an employee, having an exercise price per share of $0.22270. We claimed exemption from registration under the Securities Act for this transaction under Section 4(a)(2) and/or Regulation S of the Securities Act.
|
|
|
·
|
Pursuant to a Share Purchase Agreement dated March 4, 2011 between us and the investors identified therein, we issued an aggregate 24,545,195 of our Series D-1 preferred shares and warrants for the purchase of 16,199,826 of our Series D-2 preferred shares, for aggregate consideration of $9.3 million. In addition, as partial consideration for brokerage services in connection with such financing, we issued warrants to purchase 503,872 Series D-1 preferred shares and warrants to purchase 411,320 Series D-2 preferred shares. We claimed exemption from registration under the Securities Act for this transaction under Section 4(a)(2) and/or Regulation S of the Securities Act.
|
|
|
·
|
Pursuant to a Share Purchase Agreement dated January 10, 2012 between us and the investors identified therein, we issued a total of 2,510,783 Series D-3 preferred shares, for aggregate consideration of $1.1 million. We claimed exemption from registration under the Securities Act for this transaction under Section 4(a)(2) and/or Regulation S of the Securities Act.
|
|
|
·
|
Pursuant to a Credit Line Agreement dated August 20, 2014 between us and the lenders identified therein, we issued warrants for the purchase of an aggregate 53,169,092 ordinary shares. We claimed exemption from registration under the Securities Act for this transaction under Section 4(a)(2) and/or Regulation S of the Securities Act.
|
|
|
·
|
On October 14, 2014, we issued a warrant for the purchase of 4,430,758 ordinary shares to an existing shareholder as consideration for certain services it may provide at our request. We claimed exemption from registration under the Securities Act for this transaction under Section 4(a)(2) and/or Regulation S of the Securities Act.
|
No underwriters were employed in connection with the transactions set forth in this Item 7.
Item 8. Exhibits and Financial Statement Schedules
(a) Exhibits
The exhibits listed in the accompanying Exhibit Index are incorporated herein by reference.
(b) Financial Statement Schedules
All schedules have been omitted since they are not required or are not applicable or the required information is shown in the financial statements or related notes.
Item 9. Undertakings
The registrant hereby undertakes:
(1) for purposes of any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) for the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement.
II - 3
(4) that, for the purpose of determining any liability under the Securities Act of 1933, as amended, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(5) to remove from registration by means of a post-effective amendment any of the securities being registered that remain unsold at the termination of the offering.
(6) to file a post-effective amendment to the registration statement to include any financial statements required by Item 8.A. of Form 20-F at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(4) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Act or Rule 3-19 of this chapter if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the Form F-3.
(7) for the purposes of determining liability to any purchaser:
(i) If the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
(8) for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
(i) any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424;
(ii) any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant;
(iii) the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and
(iv) any other communication that is an offer in the offering made by the undersigned registrant to the purchaser.
The registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the U.S. Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II - 4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized in Mount Carmel, Israel, on , 2014.
|
Check-Cap Ltd.
|
|||
|
By:
|
|||
|
Name:
|
Guy Neev
|
||
|
Title:
|
Chief Executive Officer
|
||
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS, that each person whose signature appears below constitutes and appoints Guy Neev and Lior Torem, and each of them, as his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to sign any registration statement for the same offering covered by the registration statement that is to be effective upon filing pursuant to Rule 462(b) promulgated under the Securities Act of 1933 and to file the same, with all exhibits thereto, and other documents in connection therewith, with the U.S. Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated:
|
Dated: , 2014
|
By:
|
||
|
Name:
|
Guy Neev
|
||
|
Title:
|
Chief Executive Officer and Director
(Principal Executive Officer)
|
||
|
Dated: , 2014
|
By:
|
||
|
Name:
|
Lior Torem
|
||
|
Title:
|
Chief Financial Officer (Principal Financial Officer
and Principal Accounting Officer)
|
||
|
Dated: , 2014
|
By:
|
||
|
Name:
|
Tomer Kariv
|
||
|
Title:
|
Chairman of the Board of Directors
|
||
|
Dated: , 2014
|
By:
|
||
|
Name:
|
Walter L. Robb
|
||
|
Title:
|
Director
|
||
|
Dated: , 2014
|
By:
|
||
|
Title::
|
Yoav Kimchy
|
||
|
Title:
|
Director
|
||
|
Dated: , 2014
|
By:
|
||
|
Name:
|
Alon Dumanis
|
||
|
Title:
|
Director
|
||
|
Dated: , 2014
|
By:
|
||
|
Name:
|
Richard Stone
|
||
|
Title:
|
Director
|
||
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Check-Cap Ltd., has signed this registration statement or amendment thereto in New York, New York, United States of America on , 2014.
|
Authorized U.S. Representative
|
||
|
Puglisi & Associates
|
Exhibit Index
|
Exhibit
No.
|
Description
|
|
|
1.1*
|
Form of Underwriting Agreement
|
|
|
3.1
|
Fourth Amended and Restated Articles of Association of the Registrant
|
|
|
3.2
|
Fifth Amended and Restated Articles of Association of the Registrant
|
|
|
3.3
|
Sixth Amended and Restated Articles of Association of the Registrant
|
|
|
3.4*
|
Form of Amended and Restated Articles of Association of the Registrant to be effective prior to completion of this offering
|
|
|
4.1*
|
Form of Registrant’s Ordinary Share Certificate
|
|
|
4.2*
|
Form of Underwriter Warrants
|
|
|
5.1*
|
Form of opinion of Fischer Behar Chen Well Orion & Co.
|
|
|
10.1**
|
2006 Unit Option Plan, as amended
|
|
|
10.2**
|
Shareholders Agreement dated as of March 17, 2011 by and among Check-Cap Ltd. and the shareholders parties thereto
|
|
|
10.3*
|
Amended and Restated Shareholders Agreement to be dated as of , 2014 by and among Check-Cap Ltd. and the shareholders parties thereto
|
|
|
10.4**
|
Form of Series C-1 preferred shares purchase warrant
|
|
|
10.5**
|
Forms of Series C-2 preferred shares purchase warrant
|
|
|
10.6**
|
Form of Series D-1 preferred shares purchase warrant
|
|
|
10.7**
|
Form of Series D-2 preferred shares purchase warrant
|
|
|
10.8**
|
Share Purchase Agreement dated as of March 4, 2011 by and among Check-Cap Ltd. and the investors parties thereto
|
|
|
10.9**
|
Forms of Anti-Dilution Warrants
|
|
|
10.10
|
Asset Transfer Agreement, dated as of May 31, 2009 by and between Check-Cap Ltd. and Check-Cap LLC.
|
|
|
10.11
|
The Agreement for ASIC Design and Development dated November 26, 2009 by and between Check-Cap Ltd. and Politechnico di Milano
|
|
|
10.12
|
Form of Indemnification Agreement
|
|
|
10.13
|
Credit Line Agreement, dated as of August 20, 2014 by and among Check-Cap Ltd. and certain Lenders named therein
|
|
|
10.14
|
Form of Ordinary Shares Warrant Certificate issued pursuant to a certain Credit Line Agreement dated as of August 20, 2014
|
|
|
10.15*
|
Form of Ordinary Shares Warrant Certificate issued to the Pontifax entities
|
|
|
10.16
|
First Addendum to Credit Line Agreement dated as of October 14, 2014 by and among Check-Cap Ltd. and certain Lenders named therein
|
|
|
23.1*
|
Consent of Brightman Almagor Zohar & Co
|
|
|
23.2*
|
Consent of Fischer Behar Chen Well Orion & Co. (included in Exhibit 5.1)
|
|
|
24*
|
Power of Attorney (included on the signature page of this registration statement)
|
|
*
|
To be filed by amendment
|
|
**
|
Previously filed
|