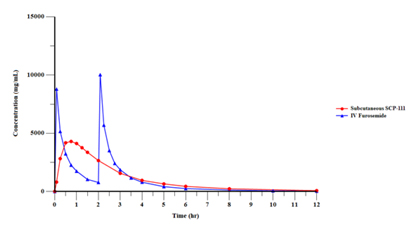
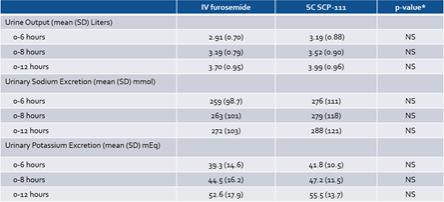
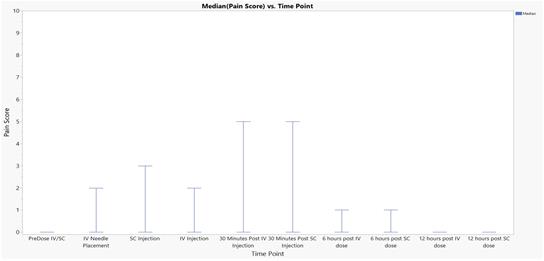
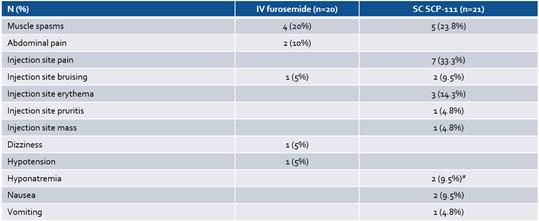
In addition, the Credit Agreement contains customary events of default that entitle the Agent to cause the Company’s indebtedness under the Credit Agreement to become immediately due and payable, and to exercise remedies against the Loan Parties and the collateral securing the Term Loan, including cash. Under the Credit Agreement, an event of default will occur if, among other things, the Company fails to make payments under the Credit Agreement (subject to specified periods), the Company or its subsidiaries breach any of the covenants under the Credit Agreement (subject to specified cure periods with respect to certain breaches), a material adverse change occurs, the Company, its subsidiaries or their respective assets become subject to certain legal proceedings, such as bankruptcy proceedings, the Company and/or its subsidiaries are unable to pay their debts as they become due or default on contracts with third parties which would permit the holder of indebtedness in excess of a certain threshold to accelerate the maturity of such indebtedness or that could cause a material adverse change. Upon the occurrence and for the duration of an event of default, an additional default interest rate equal to 3.0% per annum may apply to all obligations owed under the Credit Agreement.
The Credit Agreement is filed as Exhibit 10.1 to this Current Report and is incorporated herein by reference. The description of the Credit Agreement in this Current Report is a summary only, does not purport to be complete, and is qualified in its entirety by the terms of the Credit Agreement.
Revenue Participation Right Purchase and Sale Agreement
On the Closing Date, the Company entered into a revenue participation right purchase and sale agreement (the “Revenue Purchase and Sale Agreement”) with Perceptive Credit Holdings IV, LP (the “Purchaser”). Under the terms of the Revenue Purchase and Sale Agreement, in exchange for the Purchaser’s payment to the Company of a purchase price of $50.0 million, in the aggregate subject to certain conditions at closing (the “Purchase Price”), the Company has agreed to sell to the Purchaser its right to receive payment in full of a tiered single digit percentage of net sales of FUROSCIX (the “Revenue Payment”) for each calendar quarter commencing on the effective date of the Revenue Purchase and Sale Agreement, subject to adjustments on June 30, 2028 and June 30, 2030 depending on the amount of Revenue Payments received by such dates. The Purchaser’s right to receive the Revenue Payment terminates and the Company no longer has the obligation to pay Purchaser Revenue Payments once the Purchaser receives 200.0% (subject to reductions on September 30, 2027 and September 30, 2029 depending on the amount of Revenue Payments received by such dates) of the Purchase Price. The Company may also buy-out the Purchaser’s rights to receive the Revenue Payments by paying Purchaser a tiered multiple on the Purchaser Price.
The Revenue Purchase and Sale Agreement contains various representations and warranties, including with respect to organization, authorization, and certain other matters, certain covenants with respect to payment, reporting, intellectual property, in-licenses, out-licenses, and certain other actions, indemnification obligations and other provisions customary for transactions of this nature.
The foregoing description of the material terms of the Revenue Purchase and Sale Agreement is qualified in its entirety by the full terms and conditions of the Revenue Purchase and Sale Agreement.
The Revenue Purchase and Sale Agreement is filed as Exhibit 10.2 to this Current Report and is incorporated herein by reference. The description of the Revenue Purchase and Sale Agreement in this Current Report is a summary only, does not purport to be complete, and is qualified in its entirety by the terms of the Revenue Purchase and Sale Agreement.
| Item 1.02. |
Termination of a Material Definitive Agreement. |
The information included in Item 1.01 of this Current Report on Form 8-K concerning the prepayment and termination of the Existing Credit Agreement is hereby incorporated by reference in this Item 1.02.
| Item 2.02. |
Results of Operations and Financial Condition. |
The Company is currently finalizing its financial quarterly closing process for the three months ended June 30, 2024. While complete financial information and operating data are not yet available, set forth below are certain preliminary estimates of the results of operations that the Company expects to report for its second quarter of 2024. However, the Company’s actual results may differ materially from these estimates due to the completion of its financial closing procedures, final adjustments and other developments that may arise between now and the time the financial results for the three months ended June 30, 2024 are finalized.