SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN PRIVATE ISSUER
Pursuant to Rule 13a-16 or 15d-16
of the Securities Exchange Act of 1934
For the month of June 2016
Commission File Number: 001-36581
Vascular Biogenics Ltd.
(Translation of registrant’s name into English)
6 Jonathan Netanyahu St.
Or Yehuda
Israel 60376
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F x Form 40-F ¨
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1):
Yes ¨ No x
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7):
Yes ¨ No x
Indicate by check mark whether by furnishing the information contained in this Form, the registrant is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes ¨ No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82-
Other Events
On June 3, 2016, Vascular Biogenics Ltd. issued the press release “Clinical Data Presented at ASCO Demonstrate Significant Overall Survival Benefit in rGBM Patients Receiving VB-111 Compared with Historical Avastin® Meta-analysis Data.” A copy of the press release is attached hereto as Exhibit 99.1 and is incorporated herein by reference.
Exhibits
| 99.1 | Vascular Biogenics Ltd. Press Release: Clinical Data Presented at ASCO Demonstrate Significant Overall Survival Benefit in rGBM Patients Receiving VB-111 Compared with Historical Avastin® Meta-analysis Data. | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| VASCULAR BIOGENICS LTD. | ||||||
| Date: June 3, 2016 | By: | /s/ Dror Harats | ||||
| Name: | Dror Harats | |||||
| Title: | Chief Executive Officer | |||||
EXHIBIT INDEX
| 99.1 | Clinical Data Presented at ASCO Demonstrate Significant Overall Survival Benefit in rGBM Patients Receiving VB-111 Compared with Historical Avastin® Meta-analysis Data. | |
Exhibit 99.1
Clinical Data Presented at ASCO Demonstrate Significant Overall Survival Benefit in rGBM Patients
Receiving VB-111 Compared with Historical Avastin® Meta-analysis Data
| • | VBL’s Phase 2 study in recurrent glioblastoma (rGBM) met the primary endpoint of statistically-significant increase in median overall survival, with 59 weeks in patients treated with continuous exposure of VB-111, compared to 32 weeks in patients with limited VB-111 exposure (p=0.048). |
| • | Median overall survival for patients on continuous exposure of VB-111 was 59 weeks, compared with 32 weeks in historical pooled Avastin trials (p= 0.0295). |
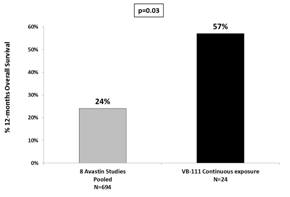
| • | 12-Month overall survival was 57% in patients on continuous exposure of VB-111, compared with 24% in historical pooled Avastin trials (p=0.03). |
TEL AVIV, Israel, June 3, 2016 – VBL Therapeutics (Nasdaq: VBLT), today announced the presentation of new data comparing clinical outcomes with VB-111 with pooled data from 8 studies that investigated Avastin® (bevacizumab) in recurrent glioblastoma (rGBM). The data are presented at the 2016 American Society of Clinical Oncology (ASCO) annual meeting, taking place in Chicago.
The study, “VB-111, An Anti-Cancer Gene Therapy in Combination with Avastin Significantly Improves Overall Survival Compared to Avastin Monotherapy in Patients with rGBM: A Phase 2 Historically Controlled Trial,” was based on a literature search of studies investigating Avastin in GBM patients, published during the period January 2005 to November 2015. Out of 662 abstracts and 53 full text articles assessed for eligibility, the independent academic investigators identified 8 studies for inclusion in a meta-analysis, outcomes were then compared with the VB-111 Phase 2 data in rGBM. Survival data from these studies was extracted based on tables giving individual patient data or directly from Kaplan-Meier curves using software which converted curves into numerical data.
In the Phase 2 VB-111 trial, the median overall survival of patients who received continuous exposure of VB-111 in combination with Avastin was 59 weeks. This is compared to 32 weeks in the pooled data from the 8 studies in the meta-analysis (p= 0.0295; Hazard Ratio 0.62, 95% CI: 0.40-0.96). Median survival ranged from 26.0 weeks to 45.7 weeks in the meta-analysis. Overall survival at 12 months for patients on continuous exposure of VB-111 was 57%, compared with 24% overall survival (range 16% - 38%) for the pooled Avastin® treated rGBM data (p= 0.03).
“This meta-analysis provides a large dataset from diverse sources, which is pooled together to provide a reliable historical control group; superiority of VB-111 continuous exposure over this control group supports our belief that VB-111 used in combination with Avastin can potentially prolong survival in rGBM compared with Avastin alone,” said Yael Cohen, MD, Vice President of Clinical Development at VBL Therapeutics. “The ongoing Phase 3 randomized controlled GLOBE study of VB-111 in combination with Avastin, which is being conducted in the U.S., Canada and Israel, is proceeding on track and our goal is to conduct an event-driven interim analysis according to the study protocol. While the timing of the interim analysis depends on enrollment and VB-111 activity, we expect to conduct it in the first half of 2017.”
The Phase 2 trial was a multi-center study designed to determine the safety, tolerability and efficacy of VB-111 in patients with rGBM. A total of 46 patients were enrolled. In the first stage of the study, patients were treated with VB-111 alone. Upon disease progression, patients entered the second stage of the study, in which they received either Avastin alone as standard of care (limited exposure cohort) or VB-111 in combination with Avastin (continuous exposure cohort). The primary endpoint was overall survival (OS). VBL previously announced data from this study at the 2015 ASCO meeting and at the European Cancer Congress 2015.
VBL’s ongoing pivotal Phase 3 GLOBE study in rGBM is comparing VB-111 in combination with Avastin to Avastin alone and is recruiting about 252 patients in the US, Canada and Israel. The study is proceeding under a Special Protocol Assessment (SPA) granted by the FDA, with full endorsement by the Canadian Brain Tumor Consortium (CBTC). VB-111 has received orphan drug designation in the United States and Europe and was granted Fast Track designation by the FDA for prolongation of survival in patients with glioblastoma that has recurred following treatment with standard chemotherapy and radiation.
About VBL Therapeutics
Vascular Biogenics Ltd., operating as VBL Therapeutics, is a clinical stage biopharmaceutical company focused on the discovery, development and commercialization of first-in-class treatments for cancer. The Company’s lead oncology product candidate, VB-111, is a first-in-class, targeted anti-cancer gene-therapy agent that is positioned to treat a wide range of solid tumors. VB-111 is conveniently administered as an IV infusion once every two months. It has been observed to be well-tolerated in >170 cancer patients and we have observed its efficacy signals in an “all comers” Phase 1 trial as well as in three tumor-specific Phase 2 studies. The mechanism of VB-111 combines blockade of tumor vasculature with an anti-tumor immune response. This mechanism retains activity regardless of baseline tumor mutations or the identity of the pro-angiogenic factors secreted by the tumor. VB-111 is currently being studied in a Phase 3 pivotal trial for Recurrent Glioblastoma (rGBM) and in Phase 2 trials for Ovarian Cancer and Thyroid Cancer. The GLOBE trial for rGBM is being conducted under an FDA Special Protocol Assessment (SPA), and VB-111 has obtained fast track and Orphan designations.
Forward Looking Statements:
This press release contains forward-looking statements. All statements other than statements of historical fact are forward-looking statements, which are often indicated by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “goal,” “intend,” “look forward to”, “may,” “plan,” “potential,” “predict,” “project,” “should,” “will,” “would” and similar expressions. These forward-looking statements include, but are not limited to, statements regarding the clinical development of VB-111 and its therapeutic potential and clinical results, including statements related to the Phase 3 pivotal trial for rGBM. These forward-looking statements are not promises or guarantees and involve substantial risks and uncertainties. Among the factors that could cause actual results to differ materially from those described or projected herein include uncertainties associated generally with research and development, clinical trials and related regulatory reviews and approvals, and the risk that historical clinical trial results may not be predictive of future trial results. A further list and description of these risks, uncertainties and other risks can be found in the Company’s regulatory filings with the U.S. Securities and Exchange Commission. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. VBL Therapeutics undertakes no obligation to update or revise the information contained in this press release, whether as a result of new information, future events or circumstances or otherwise.
Avastin® is a registered trademark of Genentech Inc.
INVESTOR CONTACT:
Michael Rice
LifeSci Advisors, LLC
(646) 597-6979