Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K/A
(Amendment No. 1)
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of the Securities Exchange Act of 1934
Date of Report (Date of earliest event reported): July 23, 2015
VIEWRAY, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 333-193498 | 42-1777485 | ||
| (State or Other Jurisdiction of Incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification Number) |
2 Thermo Fisher Way
Oakwood Village, Ohio 44146
(Address of principal executive offices, including zip code)
(440) 703-3210
(Registrant’s telephone number, including area code)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Table of Contents
| 1 | ||||||
| 3 | ||||||
| ITEM 1.01 |
5 | |||||
| ITEM 2.01 |
6 | |||||
| 6 | ||||||
| 12 | ||||||
| 36 | ||||||
| MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
76 | |||||
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT |
91 | |||||
| DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS |
94 | |||||
| 103 | ||||||
| 118 | ||||||
| MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY AND RELATED STOCKHOLDER MATTERS |
126 | |||||
| 129 | ||||||
| 133 | ||||||
| ITEM 3.02 |
134 | |||||
| ITEM 3.03 |
136 | |||||
| ITEM 5.01 |
137 | |||||
| ITEM 5.02 |
137 | |||||
| ITEM 5.03 |
AMENDMENTS TO ARTICLES OF INCORPORATION OR BYLAWS; CHANGE IN FISCAL YEAR |
137 | ||||
| ITEM 5.06 |
137 | |||||
| ITEM 5.07 |
138 | |||||
| ITEM 9.01 |
138 | |||||
i
Table of Contents
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Current Report on Form 8-K, or this Report, contains forward-looking statements, including, without limitation, in the sections captioned “Description of Business,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Plan of Operations,” and elsewhere. Any and all statements contained in this Report that are not statements of historical fact may be deemed forward-looking statements. Terms such as “may,” “might,” “would,” “should,” “could,” “project,” “estimate,” “pro-forma,” “predict,” “potential,” “strategy,” “anticipate,” “attempt,” “develop,” “plan,” “help,” “believe,” “continue,” “intend,” “expect,” “future” and terms of similar import (including the negative of any of the foregoing) may be intended to identify forward-looking statements. However, not all forward-looking statements may contain one or more of these identifying terms. Forward-looking statements in this Report may include, without limitation, statements regarding (i) the plans and objectives of management for future operations, including plans or objectives relating to the development of commercially viable pharmaceuticals, (ii) a projection of income (including income/loss), earnings (including earnings/loss) per share, capital expenditures, dividends, capital structure or other financial items, (iii) our future financial performance, including any such statement contained in a discussion and analysis of financial condition by management or in the results of operations included pursuant to the rules and regulations of the SEC and (iv) the assumptions underlying or relating to any statement described in points (i), (ii) or (iii) above.
The forward-looking statements are not meant to predict or guarantee actual results, performance, events or circumstances and may not be realized because they are based upon our current projections, plans, objectives, beliefs, expectations, estimates and assumptions and are subject to a number of risks and uncertainties and other influences, many of which we have no control over. Actual results and the timing of certain events and circumstances may differ materially from those described by the forward-looking statements as a result of these risks and uncertainties. Factors that may influence or contribute to the inaccuracy of the forward-looking statements or cause actual results to differ materially from expected or desired results may include, without limitation:
| • | market acceptance of MRI-guided radiation therapy; |
| • | the benefits of MRI-guided radiation therapy; |
| • | our ability to successfully sell and market MRIdian in our existing and expanded geographies; |
| • | the performance of MRIdian in clinical settings; |
| • | competition from existing technologies or products or new technologies and products that may emerge; |
| • | the pricing and reimbursement of MRI-guided radiation therapy; |
| • | the implementation of our business model and strategic plans for our business and MRIdian; |
| • | the scope of protection we are able to establish and maintain for intellectual property rights covering MRIdian; |
| • | our ability to obtain regulatory approval in targeted markets for MRIdian; |
| • | estimates of our future revenue, expenses, capital requirements and our need for additional financing; |
| • | our financial performance; |
| • | our expectations related to the use of proceeds from the Offering (as defined below); |
| • | developments relating to our competitors and the healthcare industry; and |
| • | other risks and uncertainties, including those listed under the section titled “Risk Factors.” |
Readers are cautioned not to place undue reliance on forward-looking statements because of the risks and uncertainties related to them and to the risk factors. We disclaim any obligation to update the forward-looking
1
Table of Contents
statements contained in this Report to reflect any new information or future events or circumstances or otherwise, except as required by law.
Readers should read this Report in conjunction with the discussion under the caption “Risk Factors,” our financial statements and the related notes thereto in this Report, and other documents which we may file from time to time with the Securities and Exchange Commission, or the SEC.
2
Table of Contents
ViewRay, Inc., a Delaware Corporation formerly known as Mirax Corp., previously filed a Current Report on Form 8-K dated July 29, 2015, or the Report, in connection with the closing of the Merger and the Offering, each as defined below. This Amendment Number 1 to the Report, or the Amended Report, is being filed for the purpose of including the financial statements of ViewRay, as defined below, for the three and six months ended June 30, 2015, and correcting certain other disclosures. As a result, the following sections under Item 2.01 of this Amended Report contain revisions from the disclosure provided in the Report:
| • | The Merger and Related Transactions; |
| • | Business; |
| • | Risk Factors; |
| • | Management’s Discussion and Analysis of Financial Condition and Results of Operations; |
| • | Security Ownership of Certain Beneficial Owners and Management; and |
| • | Executive Compensation. |
In addition, Item 9.01 of this Amended Report has been revised in order to file the following exhibits in place of the original filed versions thereof:
| • | Exhibit 99.1—Unaudited financial statements of ViewRay as of, and for the six months ended, June 30, 2015 and 2014, and the accompanying notes; |
| • | Exhibit 99.2—Audited financial statements of ViewRay as of, and for the fiscal years ended, December 31, 2014 and 2013; |
| • | Exhibit 99.3—Pro Forma Financial Information. |
We were incorporated as Mirax Corp. in Nevada on September 6, 2013. Prior to the Merger and Split-Off (each as defined below), we were in the business of selling cell phone cases to retailers.
As previously reported, on July 8, 2015, we completed a 1.185763-for-1 forward stock split of our common stock in the form of a dividend with the result that 4,343,339 shares of common stock, par value $0.001 per share, outstanding immediately prior to the stock split became 5,150,176 shares of common stock, par value $0.001 per share, outstanding immediately thereafter. Also as previously reported, on July 15, 2015, we changed our name to ViewRay, Inc. by filing the Certificate of Amendment to our Articles of Incorporation. Additionally, on July 21, 2015, we changed our domicile from the State of Nevada to the State of Delaware by reincorporation, or the Conversion, and as a result of the Conversion, increased our authorized capital stock from 75,000,000 shares of common stock, par value $0.001 per share, to 300,000,000 shares of common stock, par value $0.01 per share, or the Common Stock, and 10,000,000 shares of “blank check” preferred stock, par value $0.01 per share. Upon effectiveness of the Conversion, our corporate matters and affairs ceased to be governed by the Nevada Revised Statutes and became subject to the Delaware General Corporation Law. All share and per share numbers in this Report relating to our Common Stock have been adjusted to give effect to this forward stock split and this Conversion, unless otherwise stated. On July 23, 2015, we adopted the Amended and Restated Certificate of Incorporation by filing the Amended and Restated Certificate of Incorporation with the Secretary of State of the State of Nevada and adopted the Amended and Restated Bylaws.
On July 23, 2015, our wholly-owned subsidiary, Vesuvius Acquisition Corp., a corporation formed in the State of Delaware on July 16, 2015, or the Acquisition Sub, merged with and into ViewRay Technologies, Inc., a corporation incorporated in 2004 in the State of Florida originally under the name of ViewRay Incorporated, subsequently reincorporated in the State of Delaware in 2007, referred to herein as ViewRay. Pursuant to this transaction, or the Merger, ViewRay was the surviving corporation and became our wholly-owned subsidiary. All of the outstanding capital stock of ViewRay was converted into shares of our Common Stock, as described in more detail below.
In connection with the Merger and pursuant to the Split-Off Agreement (as defined below), we transferred our pre-Merger assets and liabilities to our pre-Merger majority stockholder, in exchange for the surrender by her and cancellation of 4,150,171 shares of our Common Stock. See Item 2.01, “Split-Off,” below.
As a result of the Merger and Split-Off, we discontinued our pre-Merger business, acquired the business of ViewRay and will continue the existing business operations of ViewRay as a publicly-traded company under the name ViewRay, Inc.
Also on July 23, 2015, we closed a private placement offering, or the Offering, of 5,340,704 shares of our Common Stock, at a purchase price of $5.00 per share. Additional information concerning the Offering is presented below under Item 2.01, “Merger and Related Transactions—the Offering” and “Description of Securities,” and Item 3.02, “Unregistered Sales of Equity Securities.”
In accordance with “reverse merger” or “reverse acquisition” accounting treatment, our historical financial statements as of period ends, and for periods ended, prior to the Merger will be replaced with the historical financial statements of ViewRay, prior to the Merger, in all future filings with the SEC.
Also on July 23, 2015, we changed our fiscal year from a fiscal year ending on November 30 of each year, which was used in our most recent quarterly report filed with the SEC, to one ending on December 31 of each year, which is the fiscal year end of ViewRay.
As used in this Report henceforward, unless otherwise stated or the context clearly indicates otherwise, the terms the “Company,” the “Registrant,” “we,” “us” and “our” refer to ViewRay, Inc., incorporated in Delaware, after giving effect to the Merger and the Split-Off.
3
Table of Contents
This Report contains summaries of the material terms of various agreements executed in connection with the transactions described herein. The summaries of these agreements are subject to, and are qualified in their entirety by, reference to these agreements, which are filed as exhibits hereto and incorporated herein by reference.
This Report is being filed in connection with a series of transactions consummated by the Company and certain related events and actions taken by the Company.
This Report responds to the following Items in Form 8-K:
| Item 1.01 | Entry into a Material Definitive Agreement | |
| Item 2.01 | Completion of Acquisition or Disposition of Assets | |
| Item 3.02 | Unregistered Sales of Equity Securities | |
| Item 3.03 | Material Modification to Rights of Security Holders | |
| Item 5.01 | Changes in Control of Registrant | |
| Item 5.02 | Departure of Directors or Certain Officers; Election of Directors; Appointment of Certain Officers; Compensatory Arrangements of Certain Officers | |
| Item 5.03 | Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year. | |
| Item 5.06 | Change in Shell Company Status | |
| Item 5.07 | Submission of Matters to a Vote of Security Holders | |
| Item 9.01 | Financial Statements and Exhibits | |
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, or the Exchange Act). As a result of the Merger, we have ceased to be a “shell company”. The information contained in this Report, together with the information contained in our Annual Report on Form 10-K for the fiscal year ended November 30, 2014, and our subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as filed with the SEC, constitute the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act of 1933, as amended, or the Securities Act.
4
Table of Contents
| ITEM 1.01 | ENTRY INTO A MATERIAL DEFINITIVE AGREEMENT |
The information contained in Item 2.01 below relating to the various agreements described therein is incorporated herein by reference.
5
Table of Contents
| ITEM 2.01 | COMPLETION OF ACQUISITION OR DISPOSITION OF ASSETS |
THE MERGER AND RELATED TRANSACTIONS
Merger Agreement
On July 23, 2015, or the Closing Date, the Company, Acquisition Sub and ViewRay entered into an Agreement and Plan of Merger and Reorganization, or the Merger Agreement, which closed on the same date. Pursuant to the terms of the Merger Agreement, Acquisition Sub merged with and into ViewRay, which was the surviving corporation and thus became our wholly-owned subsidiary.
Pursuant to the Merger, we discontinued our prior business of selling cell phone cases to retailers and acquired the business of ViewRay, which designs, manufactures and markets MRIdian®, the first and only MRI-guided radiation therapy system that images and treats cancer patients simultaneously. See “Description of Business” below.
At the Closing Date, each of the 313,821 shares of ViewRay’s common stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.975 shares of our Common Stock, and each of the 10,212,447 shares of ViewRay’s preferred stock issued and outstanding immediately prior to the closing of the Merger was converted into 2.975 shares of our Common Stock. As a result, an aggregate of 31,315,579 shares of our Common Stock were issued to the holders of ViewRay’s capital stock.
In addition, pursuant to the Merger Agreement:
| • | warrants to purchase 43,103 shares of ViewRay’s Series C Preferred Stock issued and outstanding immediately prior to the closing of the Merger were converted into warrants to purchase 128,231 shares of our Common Stock; and |
| • | options to purchase 1,464,630 shares of ViewRay’s common stock issued and outstanding immediately prior to the closing of the Merger were assumed and converted into options to purchase 4,357,180 shares of our Common Stock. |
See “Description of Securities—Warrants” and “—Options” below for more information.
The pre-Merger stockholders of the Company, other than our former sole officer and director, retained an aggregate of 1,000,000 shares of Common Stock.
The Merger Agreement contained customary representations and warranties and pre- and post-closing covenants of each party and customary closing conditions. Breaches of the representations and warranties will be subject to indemnification provisions.
The Merger was treated as a recapitalization and reverse acquisition of the Company for financial accounting purposes. ViewRay is considered the acquirer for accounting purposes, and our historical financial statements before the Merger will be replaced with the historical financial statements of ViewRay before the Merger in future filings with the SEC.
The Merger is intended to be treated as a tax-free reorganization under Section 368(a) of the Internal Revenue Code of 1986, as amended.
The issuance of shares of our Common Stock, and warrants and options to purchase our Common Stock, to holders of ViewRay’s capital stock, options and warrants in connection with the Merger was not registered under the Securities Act, in reliance upon the exemption from registration provided by Section 4(a)(2) of the Securities Act, which exempts transactions by an issuer not involving any public offering, and Regulation D promulgated by the Securities and Exchange Commission, or the SEC, under that section. These securities may not be offered or sold in the United States absent registration or an applicable exemption from the registration requirement, and are subject to further contractual restrictions on transfer as described below.
6
Table of Contents
The form of the Merger Agreement is filed as an exhibit to this Report. All descriptions of the Merger Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
Split-Off
Immediately prior to the closing of the Merger, under the terms of a split-off agreement, or the Split-Off Agreement, and a general release agreement, the Company transferred all of its pre-Merger operating assets and liabilities to its wholly-owned special-purpose subsidiary, Mirax Enterprise Corp., a Nevada corporation, or the Split-Off Subsidiary, formed on July 16, 2015. Thereafter, pursuant to the Split-Off Agreement, the Company transferred all of the outstanding shares of capital stock of the Split-Off Subsidiary to Dinara Akzhigitova, the pre-Merger majority stockholder of the Company, and the former sole officer and director of the Company, in consideration of and in exchange for (i) the surrender and cancellation of an aggregate of 4,150,171 shares of our Common Stock held by Ms. Akzhigitova (which were cancelled and will resume the status of authorized but unissued shares of our Common Stock) and (ii) certain representations, covenants and indemnities, together referred to as the Split-Off. All descriptions of the Split-Off Agreement and the general release agreement herein are qualified in their entirety by reference to the text thereof filed as exhibits hereto, which are incorporated herein by reference.
The Offering
Concurrently with the closing of the Merger, we held a closing of our Offering in which we sold 5,340,704 shares of our Common Stock, at a purchase price of $5.00 per share, or the Offering Price.
Investors in the Offering will have anti-dilution protection with respect to the shares of Common Stock sold in the Offering such that if within six months after the initial closing of the Offering the Company issues additional shares of Common Stock or Common Stock equivalents (subject to customary exceptions, including but not limited to shares of Common Stock issued or issuable pursuant to an acquisition, joint venture or technology license agreement; securities issued to financial institutions or lessors in connection with credit arrangements, equipment financings or lease arrangements, in the aggregate not exceeding 5% of the Common Stock outstanding; and issuances of awards under our 2015 Equity Incentive Plan, or the 2015 Plan) for consideration per share less than the Offering Price, or the Lower Price, each such investor will be entitled to receive from the Company additional shares of Common Stock in an amount such that, when added to the number of shares of Common Stock initially purchased by such investor and still held of record and beneficially owned by such investor at the time of the dilutive issuance, or the Held Shares, will equal the number of shares of Common Stock that such investor’s Offering subscription amount for the Held Shares would have purchased at the Lower Price. Holders of a majority of the then-held Held Shares may waive the anti-dilution rights of all Offering investors with respect to a particular issuance by the Company.
The aggregate gross proceeds from the Offering were $26,703,526 (before deducting placement agent fees and expenses of the Offering, which are estimated at $1,836,282).
The Offering was exempt from registration under Section 4(a)(2) of the Securities Act and Rule 506 of Regulation D promulgated by the SEC thereunder. The Common Stock in the Offering was sold to “accredited investors,” as defined in Regulation D, and was conducted on a “reasonable best efforts” basis.
The closing of the Offering and the closing of the Merger were conditioned upon each other.
In connection with the Offering, we agreed to pay Northland Securities, Inc., Katalyst Securities LLC, Trout Capital LLC and MLV & Co. LLC, each a U.S. registered broker-dealer, or collectively, the Placement Agents, a cash commission of 8% of the gross proceeds raised from investors in the Offering, and to issue to the Placement Agents warrants to purchase a number of shares of Common Stock equal to 8% of the number of shares of Common Stock sold in the Offering, with a term of five years and an exercise price of $5.00 per share, or the Placement Agent Warrants; however, no commission was payable and no Placement Agent Warrants will be issued in connection with the sale of 3,400,003 shares of Common Stock in the Offering that were purchased by existing ViewRay stockholders.
7
Table of Contents
As a result of the foregoing, the Placement Agents and their sub-agents were paid an aggregate commission of $776,280 and were issued Placement Agent Warrants to purchase an aggregate of 155,256 shares of our Common Stock. We have also agreed to reimburse the Placement Agents for up to $175,000 of expenses incurred in connection with the Offering.
We have agreed to indemnify the Placement Agents to the fullest extent permitted by law, against certain liabilities that may be incurred in connection with the Offering, including certain civil liabilities under the Securities Act, and, where such indemnification is not available, to contribute to the payments the Placement Agents and their sub-agents may be required to make in respect of such liabilities.
All descriptions of the Placement Agent Warrants herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
Registration Rights
In connection with the Offering, we entered into a Registration Rights Agreement, pursuant to which we have agreed that promptly, but no later than 90 calendar days from the final closing of the Offering, the Company will file a registration statement with the SEC, or the Registration Statement, covering (a) the shares of Common Stock issued in the Offering, (b) the shares of Common Stock issuable upon exercise of the Placement Agent Warrants, (c) the shares of Common Stock issued in exchange for the equity securities of ViewRay outstanding prior to the Merger and (d) shares of Common Stock held by certain pre-Merger security holders of the Company, or collectively, the Registrable Shares. The Company will use its commercially reasonable efforts to ensure that such Registration Statement is declared effective within 180 calendar days after the final closing of the Offering. If the Company is late in filing the Registration Statement, if the Registration Statement is not declared effective within 180 days after the final closing of the Offering, the Company fails to maintain the Registration Statement continuously effective as to all Registrable Shares included in such Registration Statement or the Company fails to satisfy the current public information as required under Rule 144(c), the Company will make payments to each holder of Registrable Shares as monetary penalties at a rate equal to 12% of the Offering Price per annum for each share affected during the period; provided, however, that in no event will the aggregate of any such penalties exceed 5% of the Offering Price per share. No monetary penalties will accrue with respect to any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of Common Stock which may be included in the Registration Statement, or Cutback Comment.
The Company must keep the Registration Statement effective for two years from the date it is declared effective by the SEC or until (i) the Registrable Shares have been sold in accordance with such effective Registration Statement or (ii) the Registrable Shares have been previously sold in accordance with Rule 144.
The holders of Registrable Shares (including any shares of Common Stock removed from the Registration Statement as a result of a Cutback Comment) and the stockholders of the Company prior to the Merger (but not holders of the shares issued to the stockholders of ViewRay in consideration for the Merger) will have “piggyback” registration rights for such Registrable Shares with respect to any registration statement filed by the Company following the effectiveness of the Registration Statement that would permit the inclusion of such shares, subject to customary cutback in an underwritten offering, which would be pro rata.
We will pay all expenses in connection with any registration obligation provided in the Registration Rights Agreement, including, without limitation, all registration, filing, stock exchange fees, printing expenses, all fees and expenses of complying with applicable securities laws, and the fees and disbursements of our counsel and of our independent accountants and reasonable fees and disbursements of counsel to the investors, in an amount not to exceed $35,000. Each investor will be responsible for its own sales commissions, if any, transfer taxes and the expenses of any attorney or other advisor such investor decides to employ.
All descriptions of the Registration Rights Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
8
Table of Contents
2008 Stock Option and Incentive Plan and Outstanding Options Thereunder
Pursuant to the Merger Agreement and upon the closing of the Merger, we assumed each option to purchase ViewRay common stock that remained outstanding under the ViewRay Incorporated 2008 Stock Option and Incentive Plan, or the 2008 Plan, whether vested or unvested, and converted it into an option to purchase such number of shares of our Common Stock equal to the number of shares of ViewRay common stock subject to the option immediately prior to the Merger multiplied by the applicable Merger exchange rate (which was equal to 2.975) (with any fraction rounded down to the nearest whole number). The exercise price per share of each such assumed option is equal to the exercise price of the ViewRay option prior to the assumption divided by the applicable Merger exchange rate (which was equal to 2.975) (rounded down to the nearest whole cent). Otherwise, each assumed option continues to have, and will be subject to, the same terms and conditions as applied to the ViewRay option immediately prior to the Merger, including, without limitation, the same vesting schedule. The terms of the 2008 Plan continue to govern the options covering an aggregate of 4,357,180 shares of our Common Stock assumed by us except that all references in the 2008 Plan to ViewRay will now be deemed to be us. See “Market Price of and Dividends on Common Equity and Related Stockholder Matters—Stock Plans” and “Executive Compensation—Equity Compensation Plans” below for more information about the 2008 Plan and the outstanding stock options thereunder.
2015 Equity Incentive Plan
Before the Merger, our Board of Directors adopted, and our stockholders approved, the 2015 Plan that became effective immediately prior to the consummation of the Merger, which provides for the issuance of incentive awards of up to 4,708,343 shares of our Common Stock to officers, employees, consultants and directors. Upon the consummation of the Merger, we granted options to purchase 1,507,147 shares of our Common Stock to certain of our employees under the 2015 Plan. The 2015 Plan also provides that the number of shares reserved for issuance thereunder will be increased annually on the first day of each year beginning in 2017 and ending in 2025 by four percent (4%) of the shares of our Common Stock outstanding (on an as-converted basis) on the last day of the immediately preceding year or such smaller number of shares of our Common Stock as determined by the our Board of Directors. See “Market Price of and Dividends on Common Equity and Related Stockholder Matters—Stock Plans” and “Executive Compensation—Equity Compensation Plans” below for more information about the 2015 Plan and the outstanding stock options granted thereunder.
Employee Stock Purchase Plan
Before the Merger, our Board of Directors adopted, and our stockholders approved, the Employee Stock Purchase Plan, or the ESPP, that became effective immediately prior to the consummation of the Merger. The ESPP provides for the issuance of up to 285,621 shares of our Common Stock for purchases made under the ESPP. The ESPP also provides that the number of shares reserved for issuance thereunder will be increased annually on the first day of each year beginning in 2017 and ending in 2025 by one percent (1%) of the shares of our Common Stock outstanding (on an as-converted basis) on the last day of the immediately preceding year or such smaller number of shares of stock as determined by the our Board of Directors. Our board of directors has not yet determined the timing for the offering periods under the ESPP. See “Market Price of and Dividends on Common Equity and Related Stockholder Matters—Stock Plans” and “Executive Compensation—Equity Compensation Plans” below for more information about the ESPP.
Departure and Appointment of Directors and Officers
Our Board of Directors is authorized to consist of, and currently consists of, seven members. On the Closing Date, Dinara Akzhigitova, our sole director before the Merger, resigned her position as a director, and Chris A. Raanes (as Chairman), James F. Dempsey, Ph.D., Joshua Bilenker, M.D., David Bonita, M.D., Caley Castelein, M.D., Mark S. Gold, M.D. and Aditya Puri were appointed to the Board of Directors.
Also on the Closing Date, Ms. Akzhigitova, our President, Secretary and Treasurer, and our principal executive, financial and accounting officer for SEC reporting purposes before the Merger, resigned from these positions, and Chris A. Raanes was appointed as our Chief Executive Officer and President and Chairman of the Board of Directors, David Chandler was appointed as our Chief Financial Officer, James F. Dempsey, Ph.D. was appointed as our Chief Scientific Officer and Michael Brandt was appointed as our Senior Vice President of Sales by our Board
9
Table of Contents
of Directors. Mr. Raanes will be our principal executive officer and Mr. Chandler will be our principal financial and accounting officer for SEC reporting purposes.
See “Management – Directors and Executive Officers” below for information about our new directors and executive officers.
Lock-up Agreements and Other Restrictions
In connection with the Merger, each of our executive officers; directors named above; stockholders holding 10% or more of our Common Stock after giving effect to the Merger, the Split-Off and the Offering; Montrose Capital Limited and its affiliates, or Montrose; and certain key employees, or the Restricted Holders, holding at the Closing Date an aggregate of 25,871,046 shares of our Common Stock, entered into lock-up agreements, or the Lock-Up Agreements, whereby they are restricted for a period of six months after the Merger, or the Restricted Period, from certain sales or dispositions (including pledge) of all (or 80% in case of Montrose and its affiliates) of our Common Stock held by (or issuable to) them, such restrictions together referred to as the Lock-Up. The foregoing restrictions will not apply to the resale of shares of Common Stock by any Restricted Holder in any registered secondary offering of equity securities by the Company (and, if such offering is underwritten, with the written consent of the lead or managing underwriter), or to certain other transfers customarily excepted.
In addition, each Restricted Holder agreed, for a period of 12 months following the Closing Date, that it will not, directly or indirectly, effect or agree to effect any short sale (as defined in Rule 200 under Regulation SHO of the Exchange Act), whether or not against the box, establish any “put equivalent position” (as defined in Rule 16a-1(h) under the Exchange Act) with respect to the Common Stock, borrow or pre-borrow any shares of Common Stock, or grant any other right (including, without limitation, any put or call option) with respect to the Common Stock or with respect to any security that includes, relates to or derives any significant part of its value from the Common Stock or otherwise seek to hedge its position in the Common Stock.
Pro Forma Ownership
Immediately after giving effect to (i) the Merger, (ii) the cancellation of 4,150,171 shares in the Split-Off and (iii) the closing of the Offering, there were 37,656,288 shares of our Common Stock issued and outstanding as of the Closing Date, as follows:
| • | the stockholders of ViewRay prior to the Merger hold 34,715,582 shares of our Common Stock; |
| • | the stockholders of the Company prior to the Merger hold 1,000,005 shares of our Common Stock; and |
| • | investors in the Offering hold 1,940,702 shares of our Common Stock, excluding shares held by stockholders of ViewRay prior to the Merger; |
In addition,
| • | 155,256 shares of Common Stock issuable upon the exercise of the Placement Agent Warrants; |
| • | warrants to purchase an additional 128,231 shares of our Common Stock are held by former ViewRay warrant holders; |
| • | options to purchase an aggregate of 4,357,180 shares of our Common Stock were issued under the 2008 Plan to former ViewRay option holders that have been assumed by the Company in connection with the Merger; |
| • | 285,621 shares of our Common Stock are reserved under the ESPP, as of the Closing Date; and |
| • | 4,708,343 shares of our Common Stock are reserved for issuance under the 2015 Plan as future incentive awards to executive officers, employees, consultants and directors, as of the Closing Date: |
10
Table of Contents
| • | options to purchase 1,507,147 shares of our Common Stock were issued under the 2015 Plan to our officers and employees upon the closing of the Merger. |
No other securities convertible into or exercisable or exchangeable for our Common Stock are outstanding.
Our Common Stock is quoted on the QB tier of OTC Markets under the symbol “VRAY,” which changed from “MRXC” on July 20, 2015.
Accounting Treatment; Change of Control
The Merger is being accounted for as a “reverse merger” or “reverse acquisition,” and ViewRay is deemed to be the acquirer in the reverse merger. Consequently, the assets and liabilities and the historical operations that will be reflected in the financial statements prior to the Merger will be those of ViewRay, and will be recorded at the historical cost basis of ViewRay, and the consolidated financial statements after completion of the Merger will include the assets and liabilities of ViewRay, historical operations of ViewRay, and operations of the Company and its subsidiaries from the closing date of the Merger. As a result of the issuance of the shares of our Common Stock pursuant to the Merger, a change in control of the Company occurred as of the date of consummation of the Merger. Except as described in this Report, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our Board of Directors and, to our knowledge, no other arrangements exist that might result in a change of control of the Company.
We continue to be a “smaller reporting company,” as defined under the Exchange Act, and an “emerging growth company” under the Jumpstart Our Business Startups Act, or the JOBS Act, following the Merger. We believe that as a result of the Merger we have ceased to be a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act).
11
Table of Contents
Immediately following the Merger, the business of ViewRay became our business.
Corporate Information
As described above, we were incorporated in Nevada as Mirax Corp. on September 6, 2013, and reincorporated in Delaware as ViewRay, Inc. on July 21, 2015. Our original business was sale of the cell phone cases to retailers. Prior to the Merger, our Board of Directors determined to discontinue operations in this area seek a new business opportunity. As a result of the Merger, we have acquired the business of ViewRay. ViewRay commenced operations as a Florida corporation in 2004, subsequently reincorporated in Delaware in in 2007, and changed its name to ViewRay Technologies, Inc. in July 2015.
Our authorized capital stock currently consists of 300,000,000 shares of Common Stock, and 10,000,000 shares of the preferred stock. Our Common Stock is quoted on the OTC Markets (OTCQB) under the symbol “VRAY,” which changed from “MRXC” on July 20, 2015.
Our principal executive offices are located at 2 Thermo Fisher Way, Oakwood Village, Ohio 44146. Our telephone number is (440) 703-3210. Our website address is www.viewray.com. (The information contained on, or that can be accessed through, our website is not a part of this Report.)
Company Overview
We design, manufacture and market MRIdian, the first and only MRI-guided radiation therapy system that images and treats cancer patients simultaneously. MRI is a broadly used imaging tool which has the ability to differentiate between types of soft tissue clearly, unlike X-ray or computed tomography, or CT, the most commonly used imaging technologies in radiation therapy today. MRIdian integrates MRI technology, radiation delivery and our proprietary software to locate, target and track the position and shape of soft-tissue tumors while radiation is delivered. These capabilities allow MRIdian to deliver radiation to the tumor accurately while delivering less radiation to healthy tissue than existing radiation therapy treatments. We believe this innovation leads to improved patient outcomes and reduced side effects from off-target radiation delivery. We received 510(k) marketing clearance from the U.S. Food and Drug Administration, or FDA, for MRIdian in May 2012 and received permission to affix the CE mark in November 2014. Patients are actively receiving treatment on MRIdian systems at three cancer centers.
Cancer is a leading cause of death globally and the second leading cause of death in the United States. Radiation therapy is a common method used to treat cancer that uses lethal doses of ionizing energy to damage genetic material in cells. Nearly two-thirds of all treated cancer patients in the United States will receive some form of radiation therapy during the course of their illness, according to estimates by the American Society for Radiation Oncology, or ASTRO. In 2013, IMV Medical Information Division, Inc., or IMV, reported that 93% of patients receiving radiation therapy in the United States were treated by a linear accelerator, or linac. The global linac market was $2.8 billion in 2011 and was expected to grow to $3.7 billion by 2016 according to a 2012 Markets and Markets report. IAEA Human Health Campus reported that there are over 11,000 linacs installed at over 7,500 centers worldwide. We believe the addressable market for MRIdian is the annual market for linacs due to MRIdian’s ability to treat a broad spectrum of disease sites. However, we believe that MRIdian may be used more frequently for complex cancer cases that may be difficult to treat on a standard linac due to the location of the tumor in relation to the surrounding soft tissues. We currently estimate the annual market for linacs to be 1,100 units per year globally, the majority of which are replacement units.
Despite the prevalence of MRI for diagnostic purposes and its ability to image soft tissue clearly, the radiation therapy industry has been unable to integrate MRI into external-beam radiation therapy systems. Existing radiation therapy systems use X-ray-based imaging technologies, such as CT, which cannot differentiate between types of soft tissue or provide an accurate visualization of a tumor and its position in relation to critical organs. In addition, existing systems that offer imaging during the course of a treatment are limited by the rate at which they can image due to the level of radiation to which they expose the patient. These constraints make it difficult for a clinician to locate a tumor accurately, track its motion in real-time or adapt treatment as anatomy changes. It is very difficult to irradiate a tumor while minimizing the amount of radiation hitting critical organs without the ability to see the
12
Table of Contents
tumor’s exact location and shape. If a tumor is insufficiently irradiated, it may not respond to treatment, resulting in a lower probability of survival for the patient. If organs and other healthy soft tissues are irradiated, side effects can be severe, including organ failure and secondary cancers.
MRIdian is a next-generation, radiation therapy solution that enables treatment and real-time imaging of a patient’s anatomy simultaneously. The high-quality images that it generates clearly differentiate the targeted tumor, surrounding soft tissue and nearby critical organs. MRIdian also records the level of radiation dose that the treatment area has received, enabling physicians to adapt the prescription between treatments as needed. We believe this improved visualization and accurate dose recording will enable better treatment, improve patient outcomes and reduce side effects. Key benefits to users and patients include improved imaging and patient alignment, on-table adaptive treatment planning, motion management and an accurate recording of the delivered radiation dose. Physicians have already used MRIdian to treat a broad spectrum of radiation therapy patients with more than 20 different types of cancer, as well as patients for whom radiation therapy was previously not an option.
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. At June 30, 2015, we had three MRIdian systems installed and had 13 signed orders for new systems for a backlog value of $71.9 million, of which we expect to recognize approximately 15% to 29% as revenue in the remainder of 2015 representing two to four MRIdian systems. We generated revenue of $0.5 million and $3.3 million during the six months ended June 30, 2015 and 2014 and had net losses of $20.6 million and $16.6 million during the six months ended June 30, 2015 and 2014. We generated revenue of $3.2 million in 2013 and $6.4 million in 2014 and had net losses of $27.2 million in 2013 and $33.8 million in 2014.
Cancer and Radiation Therapy Market
Incidence of Cancer
Cancer is a leading cause of death globally and the second leading cause of death in the United States behind cardiovascular disease. According to the American Cancer Society, approximately 1.6 million people were expected to be diagnosed with cancer in the United States during 2014 and approximately 0.6 million were expected to die from cancer, accounting for nearly one of every four deaths. As a result of a growing and aging population, The World Health Organization’s (the “WHO”), Global Initiative for Cancer Registry Development estimates that the number of new cancer cases worldwide will grow from 14.1 million in 2008 to 19.3 million in 2025.
Cancer Therapy
The primary goal of cancer therapy is to kill cancerous tissues while minimizing damage to healthy tissues. There are three main ways to treat cancer: surgery, chemotherapy and radiation. Surgery attempts to physically remove the tumor from the body, while minimizing trauma to healthy tissue and preventing the spread or translocation of the disease to other parts of the body. Surgery is particularly effective because the surgeon can directly observe the tumor and surrounding healthy tissue throughout the course of the procedure and adapt his or her plan mid-procedure accordingly. Chemotherapy uses drugs to kill cancer cells. Unlike surgery, most forms of chemotherapy circulate systemically to reach cancer cells almost anywhere in the body. Chemotherapy is most effective at destroying microscopic levels of disease. Radiation is used to damage genetic material in cells with a lethal dose of ionizing energy. Effective radiation therapy balances destroying cancer cells with minimizing damage to normal cells. It can be used at high doses to ablate a tumor, an effect similar to surgery, or at moderate doses to target local microscopic disease, as is done with chemotherapy. Other, more recently developed ways of treating cancer include hormone therapy and targeted therapy, such as immunotherapy.
Radiation Therapy
Radiation therapy has become widespread, with nearly two-thirds of all treated cancer patients in the United States receiving some form of radiation therapy during the course of their treatment, according to estimates by ASTRO. For most cancer types treated with radiation therapy, at least 75% of the patients are treated with the intent to cure the cancer. For lung and brain cancers, that number is somewhat lower, with 59% of lung cancer patients and 50% of brain cancer patients being treated with the goal of curing cancer. The remainder of cases are treated with palliative intent to relieve pain. Radiation therapy is a non-invasive outpatient procedure with little or no recovery
13
Table of Contents
time and can be used on patients who are inoperable. According to IMV, 93% of patients receiving radiation therapy in the United States are treated using a linac.
Radiation is used to kill cancer cells primarily by damaging their DNA, but can also kill healthy cells in the same way or cause them to become cancerous themselves. As a result, the goal of curative radiation therapy is to balance delivery of a sufficiently high dose of radiation to a tumor to kill the cancer cells while, at the same time, minimizing damage to healthy cells, particularly those in critical organs. Normal cells are better able to repair themselves after radiation than tumor cells, so doses of radiation are often fractionated, or delivered in separate sessions with rest periods in between. As a result, standard radiation therapy is often given once a day, five times a week, for one to seven weeks. In 2012, patients made an estimated 20.9 million radiation therapy treatment visits in the United States.
Radiation Therapy Equipment Market
The global linac market was $2.8 billion in 2011 and was expected to grow to $3.7 billion by 2016 according to a 2012 Markets and Markets report. According to IAEA Human Health Campus, there are 11,000 linacs installed at over 7,500 centers worldwide. In the United States, there are 3,800 linacs installed at over 2,500 centers. The annual market for linacs is estimated to be 1,100 units per year globally, the majority of which are replacement units.
In the radiation therapy market, new technologies have historically been adopted at a rapid rate. According to IMV, the percentage of centers performing intensity modulated radiation therapy, or IMRT, grew from 30% in 2002 to 96% in 2012. The percentage of sites utilizing image guided radiation therapy, or IGRT, grew more quickly: from 15% in 2004 to 83% in 2012. The majority of IGRT uses on-board X-ray systems. As leading cancer centers adopt and study MRI-guided radiation therapy, we believe that our next-generation system will also follow a rapid adoption curve among the broader linac replacement market.
Radiation Therapy Treatment Process
Following diagnosis of the disease state, radiation treatment generally consists of the following steps.
| • | Imaging and tumor contouring. To design the treatment plan, physicians obtain initial images of the tumor. This is done most commonly using a CT scan, often supplemented by an MRI, a positron emission tomography, or PET, scan, or both. These images, also known as simulation scans, are then imported into a treatment planning software system and aligned to each other. Based on clinical experience, a physician will manually draw, or contour, specific areas on the aligned images to characterize the location and extent of the tumor highlighting the following: |
| • | Gross tumor volume, or GTV, a volumetric region encompassing the visible tumor. |
| • | Clinical target volume, or CTV, a larger area encompassing the GTV, where the cancer may have already or may be likely to spread. |
| • | Planning target volume, or PTV, a further enlarged area to allow for inexact imaging, patient movement during treatment or tumor movement between planning and treatment. The PTV may be sized multiple times larger than the CTV, risking radiation damage to healthy tissue, including in many cases critical organs. |
| • | Treatment planning and dose prescription. Once the clinician has a three-dimensional map of the tumor, surrounding healthy tissues and nearby critical organs, a physician determines a treatment plan using one of the methods below. Creation of these plans typically takes one to two weeks. A typical curative radiation therapy treatment dose will be delivered over the course of several weeks with 10 to 35 radiation therapy sessions, referred to as fractions, lasting from a few minutes to an hour or more depending on the treatment plan. |
| • | 3D-CRT planning. Using a method called three-dimensional conformal radiation therapy, or 3D-CRT, a clinician will decide what beam angles and shapes to use to target a tumor and how long each beam |
14
Table of Contents
| will irradiate it. A computer will then calculate the potential dose delivered, and a clinician will manually adjust the plan to arrive at an acceptable dose. |
| • | IMRT planning. Using a method called intensity modulated radiation therapy, or IMRT, a physician will use computer software to optimize a treatment plan to achieve a more precise dose distribution than 3D-CRT by using thousands of beamlets, IMRT has been shown to result in better patient outcomes than 3D-CRT. |
| • | SRS and SBRT planning. Stereotactic radiosurgery, or SRS, and stereotactic body radiation therapy, or SBRT, are methods of delivery using 3D-CRT or IMRT designed to deliver high doses of precisely targeted radiation in a reduced number of sessions, usually one to five fractions. SRS is used in brain and spine applications, and has been shown to be particularly effective in those areas, while SBRT is used in the rest of the body, and has been shown to be particularly effective in early-stage lung cancer. |
| • | Alignment. Prior to radiation delivery, clinicians typically take images to assist with patient alignment. Most systems use a form of on-board CT called cone-beam CT to image, which delivers inferior contrast and a higher radiation dose than diagnostic CT. A less commonly used imaging technology is fluoroscopy, a real-time 2D X-ray system that can expose a patient to even higher doses of radiation than cone-beam CT. Because of the limited soft tissue contrast of X-ray-based imaging, clinicians often use registration markers such as nearby bone structures or surgically implanted fiducial markers to align patients with the treatment beams. Patients may also be immobilized by restraining devices, or techniques such as respiratory control or abdominal compression, which are employed to minimize motion due to breathing. To track breathing and other body motions during treatment, specific trackers may be used, also known as 4D radiation therapy. Use of any image or registration marker to help with alignment is called image-guided radiation therapy, or IGRT. |
| • | Delivery. Based on alignment with these images, markers or other radiation therapy trackers, treatment begins and radiation is delivered to the patient. In some cases, additional 2D X-ray images are taken intermittently or registration makers are monitored during treatment to try to account for tumor movement. |
| • | Review. After a treatment session, a physician will review the delivered treatment to ensure that it is proceeding according to plan. Currently, there are no methods to record the actual dose that was delivered to the tumor and nearby critical structures. In those rare occasions when a physician is able to observe changes in the size or shape of a tumor, he or she may decide to adjust the treatment plan. However, revising a treatment plan may take several days and delays treatment. |
Limitations of Radiation Therapy
Limitations with radiation therapy arise as a result of imaging technologies that make accurate visualization of a tumor and its relation to critical organs difficult or impossible during treatment. As a result, we believe treatments are not as effective or safe as they could be.
| • | Inability to accurately locate a tumor for treatment alignment. To locate a tumor, current radiation therapy systems rely on on-table CT scans which are unable to differentiate between types of soft tissue. Therefore, surrogate registration markers, including existing bone structures, external marks and surgically implanted fiducials, are frequently used to align a patient to the treatment beams prior to commencing treatment. |
15
Table of Contents
Comparison of On-Table CT Images to On-Table MRIdian Images
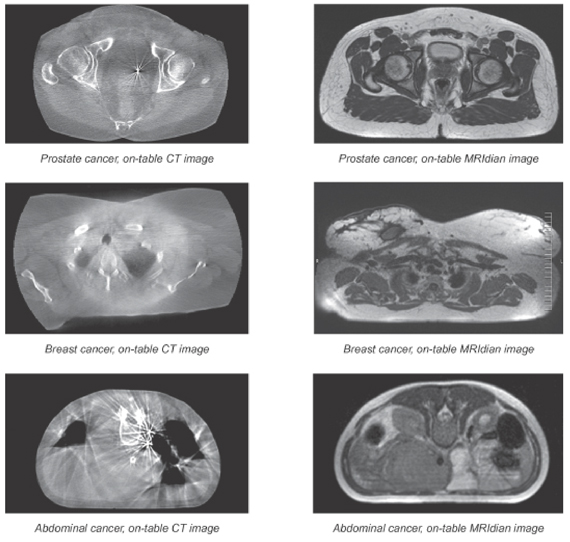
The spatial relationship between the tumors, particularly those in soft tissue, and registration markers is likely to change between initial imaging and the first treatment session. By relying on a proxy for tumor location rather than the tumor itself, clinicians risk missing the tumor when they deliver treatment beams into a patient’s body. Furthermore, fiducial markers can migrate inside the body, are unable to track changes in the tumor shape, may interfere with imaging, are invasive, require time to heal and have a high incidence of side effects and complications.
| • | Inability to adapt treatment on table. A physician designs a treatment plan and dose prescription based on images that are captured at the beginning of therapy. Creating a treatment plan can take one to two weeks, and treatment itself can take up to seven weeks. However, during the course of therapy, tumors often change size, orientation or shape and patient anatomy can change for reasons such as weight loss or gain. Adjusting for these changes would require replanning which may take several days and is resource intensive. In addition, due to limitations in imaging technologies, physicians may be unaware of changes in the tumor and surrounding anatomy and continue to dose according to the original treatment plan. As a result of these limitations, replanning is infrequently performed. |
| • | Inability to track tumor and organ motion accurately. In addition to difficulty locating a tumor accurately in a patient’s body, a further challenge is accounting for ongoing tumor movement during treatment. Tumors have been shown to move multiple centimeters relative to surrogate registration markers over the course of only a few seconds. Although physicians use internal markers and external cameras and blocks to track respiratory and other motion, they are unable to track the tumor itself and its location relative to other |
16
Table of Contents
| soft tissues. This limitation increases the probability of missing the targeted treatment area. As a result, physicians usually enlarge the total region to be irradiated, causing an additional risk of side effects. |
| • | Inability to record cumulative radiation delivered. In order to determine treatment effectiveness, it is important to track how much radiation has been delivered to a tumor or surrounding healthy tissue. Currently, there are no methods to record the actual dose of radiation that was delivered to the tumor and nearby critical structures. Therefore, physicians must assume that the radiation is delivered according to plan, rather than making decisions based on actual dose delivered. |
Each of these limitations increases the risk of missing a tumor and hitting healthy tissue during treatment. If a tumor is insufficiently irradiated, it may not respond to treatment, resulting in a lower probability of survival for the patient. The ability to avoid irradiating healthy tissue has been shown to reduce side effects. If healthy tissues, particularly critical organs, are irradiated, the side effects can be severe, including scarring of lung tissue, fibrosis and cardiotoxicity in lung and breast cancers, incontinence and sexual dysfunction in pelvic and prostate cancers, infertility in pediatric cancers, memory loss, seizures and necrosis in brain cancer and secondary cancers.
Although MR technology is an imaging tool broadly used to differentiate between types of soft tissue in diagnostic settings, to date such technology has not been used with radiation therapy because the magnetic field generated by an MRI interferes with the linac’s ability to accelerate electrons and the linac produces radio frequencies that distort the MR images. Current forms of CT have improved over time, but issues with radiation dose and image quality limit the utility of these technologies. Fluoroscopy and cone-beam CT involve the use of X-rays, a form of ionizing radiation, and pose an increased risk of radiation-induced cancer to the patient.
Our Solution
We have developed MRIdian to address the key limitations of existing external-beam radiation therapy technologies. MRIdian employs MRI-based technology to provide real-time imaging that clearly defines the targeted tumor from the surrounding soft tissue and other critical organs during radiation treatment. MRIdian allows physicians to record the level of radiation exposure that the tumor has received and adapt the prescription between fractions as needed. We believe this combination of enhanced visualization and accurate dose recording will significantly improve the safety and efficacy of radiation therapy, leading to better outcomes for patients.
We believe that MRIdian provides the following clinical and commercial benefits to physicians, hospitals and patients:
| • | Improved tumor visibility and patient alignment. The soft-tissue contrast of MRidian’s on-board MRI enables clinicians to locate, target and track the tumor and healthy tissues and accurately align a patient to the treatment beams without the use of X-ray, CT or surrogate registration markers. If the clinician prefers, the software has the ability to automatically map the patient’s soft tissue anatomy each treatment session in less than one minute, and MRIdian can use that information to automatically align the patient. |
| • | On-table adaptive planning. Due to changing anatomy the clinician may be unable to obtain an optimal match between the patient on the table and the treatment plan. Using an MR image captured at the beginning of each therapy session, MRIdian automatically maps the patients’ soft tissue anatomy in 3D and calculates the dose that would be delivered using the current treatment plan. If the prescribed treatment is not clinically acceptable to the physician, MRIdian has the ability to automatically recalculate and adapt the plan to changing anatomy at the time of treatment. Utilizing our proprietary Monte Carlo algorithm and software, replanning can be done in less than two minutes while the patient is on the table. We believe hospitals will be able to bill incrementally for this replanning. |
| • | Ability to track tumors and manage patient motion. MRIdian can capture multiple soft-tissue imaging planes concurrently during treatment, refreshing the image multiple times per second. This real-time imaging enables the physician to track the movement of the tumor and the surrounding healthy tissue directly, rather than relying on registration markers such as existing bones or implanted fiducials. If a tumor or critical organ moves beyond a physician-defined boundary, the treatment beam automatically pauses. This beam control becomes especially important in the situations where a tumor may be in close proximity to a critical organ, such as the heart during lung and breast cancer treatments or the rectum during prostate |
17
Table of Contents
| cancer treatments. This knowledge of the tumor location has enabled physicians to treat patients who would not previously have been considered radiation therapy candidates. |
| • | Record and evaluate the delivered dose. Using our proprietary algorithm and advanced MR imaging, MRIdian calculates the dose delivered after each treatment, enabling the physician to review and re-optimize the patient’s treatment session if needed. In addition, MRIdian can utilize diagnostic CT images that are fused with the MR images at each treatment in order to more accurately calculate dose. MRIdian also captures and records a video, known as a MRIdian Movie™, of the delivered treatments which can be evaluated by the physician or shared with patients. |
| • | Fits into existing treatment paradigms and workflow. MRIdian can be used for 3D-CRT, IMRT, IGRT, SBRT and SRS and can also be used to treat a broad spectrum of disease sites. In addition, we believe MRIdian’s increased target accuracy will allow physicians to treat patients with higher doses over fewer treatment fractions and potentially improve patient throughput and efficiency. MRIdian fits inside most standard radiation therapy vaults without significant modifications and is supported by existing codes that are available for linac reimbursement. |
We believe the ability to image with MRI and treat cancer patients simultaneously will lead to improved patient outcomes and reduced side effects from off-target radiation delivery.
Our Strategy
Our objective is to make MRI-guided radiation delivery the standard of care for radiation therapy. To achieve this goal, we intend to do the following:
| • | Target top-tier hospitals in initial global sales efforts to influence and increase market adoption. We intend to market MRIdian to a broad range of customers worldwide, including university research and teaching hospitals, private practices, community hospitals, government institutions and freestanding cancer centers. We are initially focusing on the leading hospitals worldwide which are typically early adopters of best-in-class technology and are able to influence and promote adoption by other centers both locally and globally. We plan to continue to work with these institutions to position MRIdian as a marketing tool they can use to differentiate their offerings from their peers and promote broader market awareness on the benefits of MRI-guided radiation therapy. |
| • | Commercialize MRIdian with a targeted sales force in the United States and through distributors in international markets. We intend to market MRIdian through a combination of direct sales and distributors. We are building a small, specialty sales force for the United States and Canada and are using distributors in international markets. At June 30, 2015, we had five signed orders with U.S. customers and eight signed orders with customers outside the United States for new MRIdian systems, and we intend to continue to expand our presence in key markets to capitalize on the growing international opportunity for MRIdian. We are engaging distributors and seeking government approval where needed to market MRIdian in China, Japan, Canada, Russia, Hong Kong, Turkey and Korea, and we intend to work with distributors and regulators in other countries in the future. |
| • | Increase broader awareness of MRIdian’s capabilities to expand our share of the radiation therapy market. We intend to educate radiation oncologists about the capabilities and resulting benefits of MRIdian over traditional radiation therapy systems. In order to drive awareness and adoption, we also intend to support the publication of clinical and scientific data and analysis, work with key opinion leaders, present at leading academic conferences and engage in outreach at leading hospitals worldwide. We also plan to leverage our existing customer network as a reference for new potential users to experience our technology in use in the clinical setting. |
| • | Maintain our competitive lead in MRI-guided radiation therapy through continued innovation. We plan to continue to invest in our technology to maintain our leadership position in the emerging MRI-guided radiation therapy market. We intend to develop and introduce enhancements to the system and software to provide improved capabilities for MRIdian users and patients. In addition, we plan to explore potential benefits of integrating our MRI technology with alternative beam technologies. We believe we have a |
18
Table of Contents
| strong intellectual property portfolio that covers the MRIdian system as well as critical design elements and key aspects of its subsystem and components. We will continue to enhance this portfolio as we develop new features and technologies. |
| • | Continue to work with leading hospitals to optimize efficiency and patient throughput. We strive to maximize the efficiency and effectiveness of the MRIdian system for our customers. We plan to continue to work closely with key opinion leaders, clinicians and hospitals in a proactive manner to determine how best to refine and improve MRIdian’s features, optimize workflow and maximize patient throughput. We utilize this customer feedback to guide product development and increase MRIdian’s reliability and efficiency, which we believe will result in positive experiences for our customers and ensure their continued usage and recommendation of MRIdian. |
| • | Drive cost reductions in the design and manufacture of MRIdian and improve our margins. We plan to continue to explore ways to bring down our cost of goods to improve margins for MRIdian. We believe we can reduce costs in the design and manufacture of MRIdian. |
MRIdian
MRIdian is a next-generation, MRI-guided radiation therapy system that is comprised of four major components, (i) the MRI system, (ii) the radiation delivery system, (iii) integrated treatment planning and delivery software and (iv) a safety and control system.
MRIdian

MRI System
The MRI system is the component of MRIdian that captures soft tissue images of the patient’s body. To address the technical complications that arise from combining an MRI with a linac, we have designed a proprietary split superconducting magnet that allows treatment through a central gap, eliminating MRI components in the path of the beam. Our MRI system captures and displays live, high-quality images in three planes at two frames per second or in one plane at four frames per second. The images are used to track tissues and control radiation treatment beam delivery.
While other MRI systems in development utilize a high field strength magnet to provide a clearer image, use of higher field strength magnets results in distortions of soft tissue images by up to approximately six millimeters at 1.5 tesla and unfavorable dose distribution distortions, which are unacceptable for delivering accurate radiation therapy.
19
Table of Contents
We have engineered our MRI system to be able to produce clear images using a low field strength 0.35 tesla magnet which enables us to avoid the image and dose distortions that are a result of using a higher field strength magnet. In addition, MRIdian’s 0.35 tesla field strength prevents heating of the patient during uninterrupted imaging, which could occur in a higher field strength magnet requiring the imaging to be discontinued or interrupted.
MRI System

Radiation Delivery System
Radiation is delivered from three Cobalt-60 radiation therapy heads symmetrically mounted on a rotating ring gantry, providing full 360 degree coverage and simultaneous dose delivery, as opposed to prior Cobalt-60 systems that have historically been limited by imprecise radiation dose applications. Each head is equipped with a double focused multi-leaf collimator, designed to overcome the wide-beam edge of previous-generation Cobalt-60 systems and shape the beam for precision radiation therapy treatments. It allows the delivery of treatment plans for 3D-CRT, IMRT and SBRT that are clinically equivalent to those produced on the most advanced linear accelerators available today. Stereotactic procedures are possible with a positioning accuracy of less than one millimeter. Cobalt-60 is used because it does not create any radio frequency which interferes with the MRI.
Comparison of Previous-Generation Cobalt Beam to ViewRay Cobalt Beam

20
Table of Contents
Integrated Treatment Planning and Delivery Software
Our treatment planning and delivery software can create treatment plans and manage the treatment delivery process. It is designed to create optimized 3D-CRT, IMRT and SBRT plans for delivery on MRIdian. Using this software, the on-table adaptive planning process typically takes less than two minutes, and includes: auto-contouring, dose prediction and treatment plan optimization. For contouring, the software will automatically draw the outline of the tumor and nearby organs. The clinician can then make refinements before treatment, if necessary. Dose prediction can be calculated immediately before treatment, allowing the current state of the patient’s anatomy to be taken into account. The software can generate an optimal treatment plan solution in less than one minute, allowing it to re-plan while the patient is on the treatment couch.
MRIdian has soft-tissue tracking beam control capability. While the radiation dose is being delivered, the software analyzes the acquired images and can determine tumor or organ location relative to set tolerances. If the targeted tumor or a critical organ moves beyond a physician-defined boundary, the treatment beams will automatically pause. When the tumor moves back into the target zone, the treatment will automatically resume. Physicians can set both spatial and time thresholds for pausing treatment delivery. This enables the system to account for tumor and patient motion during treatment.
The software archives all the information generated during treatment and builds a database of patient-specific planning, delivery and imaging data. It also includes a review tool which provides clinicians with a visual comparison of the delivered versus planned treatment. At the end of each treatment, the software determines the delivered dose by combining the recorded actions of the radiation delivery system with the daily image and auto-contouring of the patient. With this information, clinicians can fine-tune prescriptions based on the actual dose delivered. In addition, it provides a MRIdian Movie™ of each delivered treatment which can be evaluated by the physician or exported and shared with the patient.
Safety and Control System
In addition to complying with the applicable FDA and Nuclear Regulatory Commission, or NRC, requirements, the radiation delivery subsystem also meets a double fault tolerant design standard and has redundant safety systems. If any two components in the radiation delivery subsystem fail simultaneously, such as power and pneumatics, the system reverts to a safe state. MRIdian also contains redundant computer control for safety and system logging and double encoders on all axes of motion for safety. The control system continuously monitors performance to ensure all systems are performing and communicating appropriately.
Installed Base and Clinical Use
We received initial 510(k) marketing clearance from the FDA for our treatment planning and delivery software in January 2011 and for MRIdian in May 2012. We also received permission to affix the CE mark in November 2014, allowing MRIdian to be sold within the EEA. We received a license and permission to import MRIdian into the United Arab Emirates in December 2014. We received regulatory approval in Italy in January 2015, which is required in addition to the CE mark. We are currently seeking government approval to market MRIdian in China, Japan, Canada, Russia, Hong Kong, Turkey and Korea. Other countries where we will be seeking approval in the near term are Canada, Egypt and Australia. We may also seek required approvals in other countries in the future.
We have three units installed at three leading cancer centers in the United States including Washington University and Siteman Cancer Center at Barnes-Jewish Hospital, or Washington University in St. Louis; University of California, Los Angeles Health System and Jonsson Comprehensive Cancer Center, or University of California, Los Angeles; and The University of Wisconsin Carbone Cancer Center, or the University of Wisconsin–Madison. In January 2014, Washington University in St. Louis, a National Cancer Institute Designated Comprehensive Cancer Center, became the first center to treat patients with MRIdian. Washington University in St. Louis is scaling up its use of MRIdian in its clinical practice, and is now treating as many as 15 patients per day. In September 2014, Washington University in St. Louis used MRIdian to perform the first on-table adaptive treatments as part of an ongoing clinical service. Also in September 2014, the University of Wisconsin–Madison treated its first patients with MRIdian and became the first center to employ the soft-tissue tracking beam control capability unique to MRIdian. In October 2014, University of California, Los Angeles, became the third center to use MRIdian in
21
Table of Contents
clinical practice. We are working with each of these centers to determine how best to refine and improve MRIdian’s features, optimize workflow and maximize patient throughput.
At June 30, 2015, over 234 patients have received over 3,494 treatment sessions at these centers using MRIdian in over 34 different disease sites. These included cancers of the prostate, breast, lung, colorectal and bladder, which are among the most prevalent types of cancer in the United States according to the Centers for Disease Control and Prevention, or CDC, as well as the liver, stomach, esophagus and pancreas, which are among the most prevalent types of cancer outside of the United States according to the WHO.
Backlog
In 2013, we executed new sales contracts with a total value of $17.4 million, and in 2014 we executed new sales contracts with a total value of $37.6 million. At June 30, 2015, we had three MRIdian systems installed and had 13 signed orders for new systems for a backlog value of $71.9 million, of which we expect to recognize approximately 15% to 29% as revenue in the remainder of 2015 representing two to four MRIdian systems.
We define backlog as the accumulation of all orders for which revenue has not been recognized and we consider valid. Backlog includes customer deposits received which are recorded as a liability on the balance sheet. Orders may be revised or cancelled according to their terms or upon mutual agreement between the parties. Therefore, it is difficult to predict with certainty the amount of backlog that will ultimately result in revenue. The determination of backlog includes objective and subjective judgment about the likelihood of an order contract becoming revenue. We perform a quarterly review of backlog to verify that outstanding orders in backlog remain valid, and based upon this review, orders that are no longer expected to result in revenue are removed from backlog.
Among other criteria, to consider a sale to be in backlog we must possess an outstanding and effective written agreement for the delivery of a MRIdian signed by the customer, as well as receipt of a minimum customer deposit or letter of credit. For removal of an order from our backlog, the following criteria are considered: changes in customer or distributor plans or financial conditions; the customer’s or distributor’s continued intent and ability to fulfil the order contract; changes to regulatory requirements; the status of regulatory approval required in the customer’s jurisdiction, if any; and other reasons for potential cancellation of order contracts.
Installation Process
Following execution of a contract, it generally takes nine to 12 months for a customer to prepare an existing facility or construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures. MRIdian is designed to fit into a typical radiation therapy vault, similar to other replacement linear accelerators. MRIdian’s components all fit through standard hospital vault entrances for assembly. On-site training takes approximately one week and can be conducted concurrent with installation and acceptance testing.
Our customers are responsible for removing any outgoing linear accelerator and preparing the mounting pad, power and support system connections. Additional room modifications required are consistent with those generally required for MRI systems such as radio frequency shielding of the room and additional power.
Clinical Development
To date, we have primarily relied on clinical symposia and case studies presented at ASTRO and ESTRO to raise awareness of MRI-guided radiation therapy and to market MRIdian to leading cancer centers. In order to promote broader adoption rates at other cancer centers and hospitals, we plan to work with our customers to collect and publish data on clinical efficacy, treatment times and clinical results for patients who have been treated on a MRIdian. While we do not currently have statistically significant, objective evidence that MRIdian improves patient outcomes or decreases healthcare costs, we are currently sponsoring two studies at Washington University in St. Louis to compare MRIdian to other IGRT systems. We plan to continue to support further studies to demonstrate the benefits of MRI-guided radiation therapy and adaptive treatment planning. As data accumulate from the use of MRIdian, we plan to work with professional healthcare organizations to further support global marketing efforts, additional product clearances, approvals and/or registrations and potential improvements in reimbursement.
22
Table of Contents
Selling and Marketing
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. We market MRIdian to a broad range of worldwide customers, including university research and teaching hospitals, community hospitals, private practices, government institutions and freestanding cancer centers. As with the traditional linac market, our sales and revenue cycle varies based on the customer and can be lengthy, sometimes lasting up to 18 to 24 months or more from initial customer contact to contract execution.
To sell MRIdian globally, we use a combination of sales executives, sales directors and a network of international third-party distributors with internal support from sales operations, product management and application specialists. A targeted group of three sales directors and one vice president are responsible for selling MRIdian within the United States. Our product management function helps market MRIdian and works with our engineering group to identify and develop upgrades and enhancements. We also have a team of application specialists who provide post-sales support.
Although we do not currently engage in advertising efforts, our selling and marketing practices include participating in trade shows and symposia.
Competition
We compete directly with companies marketing IGRT devices for the treatment of cancer using CT, ultrasound, optical tracking and X-ray imaging. We also compete with companies developing next-generation IGRT devices, specifically those developing MRI-guided devices, amongst others. We expect technological advances, including the ability to provide real-time imaging, clinical outcomes, size, price, operational complexity and operational efficiency to drive competitive market dynamics.
Our major competitors with devices approved for distribution in the United States or globally include Varian Medical Systems, Inc., or Varian, Elekta AB, or Elekta, and Accuray Incorporated. Many of our direct competitors have greater financial, sales and marketing, service infrastructure and research and development capabilities than we do, as well as more established reputations and current market share. The main limitations of currently approved devices are the lack of real-time, clear images before and during the treatment, as well as the ability to perform on-table adaptive planning.
We are also aware of one commercial and two academic ongoing research efforts to develop radiation therapy systems incorporating MRI. Elekta and Royal Philips have formed a consortium to develop a commercial Elekta-Philips MRI-linac. The University of Sydney, Ingham Institute and the University of Queensland have formed a partnership to develop an MRI-linac and the University of Alberta’s Cross Cancer Institute is working on an MRI-linac as well. Although these academic research efforts may not compete directly with us commercially, if one of our competitors were to form a partnership with one of these institutions to commercialize their system, it could impact our sales negatively. Of these three, we believe the Elekta-Philips MRI-linac is the most advanced in development, although we believe this system is still years away from approval. MRIdian is the first and only commercially available MRI-guided radiation therapy device to image and treat cancer patients simultaneously.
The limited capital expenditure budgets of our customers results in all suppliers to these entities competing for a limited pool of funds. Our customers may be required to select between two items of capital equipment. For example, some of our potential customers are considering expensive proton therapy systems which could consume a significant portion of their capital expenditure budgets.
Manufacturing
We have adopted a model in which we rely on subsystem manufacturing, assembly and testing by our key suppliers. The MRIdian system is integrated at the customer site. Through this approach, we avoid the majority of the fixed cost structure of manufacturing facilities. We purchase major components and subsystems for MRIdian from national and international third-party OEM suppliers and contract manufacturers. These major components include the magnet, MRI electronics, ring gantry, radiation therapy heads, Cobalt-60 sources, multi-leaf collimators, patient-
23
Table of Contents
treatment table and computers. We also directly purchase minor components and parts. At the customer site, we assemble and integrate these components with our proprietary software and perform multiple levels of testing and qualification. The system undergoes a final acceptance test, which is performed in conjunction with the customer.
Many of the major subsystems and components of MRIdian are currently procured through single and sole source suppliers. Among these are the magnet, MRI electronics, MRI coils, ring gantry, radiation-therapy heads, Cobalt-60 sources, multi-leaf collimators and the patient-treatment table. We have entered into multi-year supply agreements for all major components and subsystems. Our restated joint development and supply agreement with Euromechanics Medical GmbH, as amended, has expired by its terms. We do not believe that that the expiration will have a negative impact on our manufacturing operations or business because we are currently negotiating an extension of the agreement and we are continuing to operate under the agreement as if the agreement had not expired during these negotiations. Except for the MRI power, control and image reconstruction subsystem, we own the design of all other major subsystems and components.
We manage our supplier relationships with scheduled business reviews and periodic program updates. We closely monitor supplier quality and delivery performance to ensure compliance with all MRIdian system specifications. We believe our supply chain has adequate capacity to meet our projected sales over the next several years.
Intellectual Property
The proprietary nature of, and protection for, MRIdian components, new technologies, processes and know-how are important to our business. Our policy is to seek patent protection in the United States and in certain foreign jurisdictions for our MRIdian systems and other technology where available and when appropriate. We also in-license technology, inventions and improvements we consider important to the development of our business.
We hold the exclusive worldwide license for certain patents and patent applications covering our combination of MRI and radiation therapy technologies. Specifically, we hold a license to two issued U.S. patents, one allowed U.S. patent application, 14 issued foreign patents (eight of which were issued in Great Britain, Germany, France and the Netherlands as a result of two patent applications filed and allowed through the European Patent Office) and four pending foreign patent applications at July 13, 2015. We own an additional eight issued U.S. patents, seven issued foreign patents, 16 pending U.S. patent applications and 48 pending foreign patent applications, and, at July 13, 2015, one of our foreign patent applications is allowed. Assuming all required fees are paid, individual patents or patent applications owned or licensed by us will expire between 2025 and 2034. We also have a joint ownership interest with Case Western Reserve University in one issued patent and one U.S. patent application.
Our portfolio includes patents and patent applications directed to system-wide aspects of MRIdian and to key aspects of its subsystems and components. The initial licensed patents for our core technology broadly cover the simultaneous use of MR imaging and isotopic external-beam radiation therapy. Such patents have been granted in the United States, Europe, Hong Kong, Australia and Japan, and additional related patent applications remain pending in China, Canada, the United States, Australia and Japan. In the United States we have pending continuation applications of the licensed patents that extend this core technology to alternate beam technologies. Additionally, we have patents and patent applications that cover critical design elements including, among others, our approach to Cobalt IMRT, our methods for integrating MRI and the radiation delivery system, and the design of our disassemblable (“pop apart”) magnet which enables the MRI sub-system to fit into most standard radiation therapy vaults. The U.S. patent application on our approach to Cobalt IMRT has been issued, the patent application on our split gradient coil has been issued in the United States, Japan, Australia and China and numerous applications on other design elements are pending in the United States and foreign jurisdictions. In addition, we have a U.S. patent and U.S. and foreign patent applications that cover the use of MRI imaging at a frequency sufficient to account for real-time organ motion to provide video rate tissue tracking in disciplines outside of radiation therapy.
We continue to review new technological developments in our system and in the field as a whole in order to make decisions about what filings would be most appropriate for us. An additional key component of our intellectual property is our proprietary software used in planning and delivering MRIdian’s therapeutic radiation dose.
In December 2004, we entered into a licensing agreement with the University of Florida Research Foundation, Inc., or UFRF, whereby UFRF granted us a worldwide exclusive license to certain of UFRF’s patents in exchange for 33.653 shares of Common Stock and a royalty from sales of products developed and sold by us utilizing the licensed
24
Table of Contents
patents. We were obligated to meet certain product development and commercialization milestones by various dates through December 31, 2014. The significant milestones met prior to December 31, 2013 included: (i) completion of a business plan and Small Business Technology Transfer grant application; (ii) securing a minimum of $20.0 million venture financing; (iii) successful relocation and build out of the company’s headquarters; (iv) receipt of the first magnet from an OEM partner; (v) hiring of a chief executive officer with industry experience in developing and commercializing similar products; and (vi) filing for FDA approval. The final milestone, which required us to recognize the first commercial sale of the MRIdian system to retail customers by December 31, 2014, was met during the year ended December 31, 2013. If these milestones had not been accomplished, UFRF would have had the right to terminate the licensing agreement. Royalty payments are based on 1% of net sales, defined as the amount collected on sales of licensed products and/or licensed processes after deducting trade and/or quantity discounts, credits on returns and allowances, outbound transportation costs paid and sales tax. Minimum quarterly royalty payments of $50,000 commenced with the quarter ended March 31, 2014 and are payable in advance. Minimum royalties paid in any calendar year will be credited against earned royalties for such calendar year. The royalty payments continue until the earlier of (i) the date that no licensed patents remain enforceable or (ii) the payment of earned royalties, once begun in 2014, cease for more than four consecutive calendars quarters.
In addition to our patents, we also rely upon trade secrets, know-how, trademarks, copyright protection and continuing technological and licensing opportunities to develop and maintain our competitive position. We have periodically monitored and continue to monitor the activities of our competitors and other third parties with respect to their use of intellectual property. We require our employees, consultants and outside scientific collaborators to execute confidentiality and invention assignment agreements upon commencing employment or consulting relationships with us. Despite these safeguards, any of our know-how or trade secrets not protected by a patent could be disclosed to, or independently developed by, a competitor.
Coverage and Reimbursement
Reimbursement rates in the United States have generally supported a favorable return on investment for the purchase of new radiotherapy equipment, including MRIdian. Payments for standard radiation therapy treatments using MRIdian, including 3D-CRT, IMRT and SBRT, are generally covered and reimbursed under existing Current Procedural Terminology, or CPT, codes and policies currently in place. User experience to date indicates that our initial customers have treated a wide spectrum of different patients and treatment modalities using MRIdian. Physicians use the MRIdian system’s on-board MRI to perform a complex simulation weekly for IMRT or daily for SBRT, special physics consult and adaptive re-planning. Each of these are distinct procedures which can be billed by physicians using existing CPT codes, so long as such procedures meet medical necessity and other coverage criteria as established by government and other third-party payors.
Third-party payors, including governmental healthcare programs such as Medicare and Medicaid, establish coverage policies and reimbursement rates for diagnostic examinations and therapeutic procedures performed by physicians in hospitals and free-standing clinics. Private insurers often model their payment rates and coverage policies based on those established by the government. The U.S. Congress from time to time considers various Medicare and other healthcare reform proposals that could affect both private and public third-party payor coverage and reimbursement for healthcare services provided in hospitals and clinics. In addition, third-party payors regularly update reimbursement amounts, including annual updates to payments to physicians, hospitals and clinics for medical procedures, including radiation treatments using MRIdian.
The changes to simplify the billing for conventional radiation therapy and IMRT by CMS in its final rule for the Hospital Outpatient Prospective Payment System, or HOPPS, effective January 1, 2015, are further indicative of an overall U.S. health policy trend towards cost containment. Whether this trend results in capitated payments per patient or case payment per procedure, we believe MRIdian’s capability of enabling physicians to visualize the
25
Table of Contents
treatment area and adapt therapy in real-time to maximize the clinical benefit to the patient will result in cost savings to both providers and third-party payors.
We plan to work with our customers to collect and publish data on clinical results for patients who have undergone procedures on MRIdian. We are currently sponsoring a study that is estimated to be completed in 2017 at Washington University in St. Louis to compare MRIdian to other IGRT systems, and we plan to continue to support further studies to demonstrate the benefits of MRI-guided radiation therapy and adaptive treatment planning. As data accumulate from the use of our system, we plan to work with professional healthcare organizations to further support global marketing efforts, additional product clearances, approvals and/or registrations and potential improvements in reimbursement. Additionally, we currently provide reimbursement support to our customers through a third-party vendor.
Foreign Reimbursement Regulations
Internationally, reimbursement and healthcare payment systems vary from country to country and include single-payor, government managed systems as well as systems in which private payors and government-managed systems exist side-by-side. In general, the process of obtaining coverage approvals is slower outside of the United States. Our ability to achieve adoption of MRIdian as well as significant sales volume in international markets we enter will depend in part on the availability of reimbursement for procedures performed using MRIdian.
Research and Development
Continued innovation and development of advanced technologies is critical to our goal of making MRI-guided radiation therapy the standard of care for cancer treatment. Our current development activities include improvements in and expansion of product capabilities, continued clinical workflow refinements, design improvements to reduce system costs and improvements in reliability.
The modular design of MRIdian enables the development of new capabilities and performance enhancements by generally allowing each subsystem to evolve within the overall platform design. Access to regular MRIdian upgrades protects customer investment in MRIdian, and facilitates the adoption of new features and capabilities among existing installed base customers. We expect these upgrades will generally consist of new software features and MRI imaging enhancements designed to leverage the capabilities of MRIdian’s on-table adaptive planning, real-time tumor tracking, and the other inherent capabilities of MRI imaging technologies, as well as hardware enhancements as they become available. Ultimately we have a vision of expanding the system’s on-table adaptive feature into a continuously-adaptive capability, which adjusts and corrects for any changes as they happen, on the table, in real-time.
In addition, we believe our existing and expanding IP portfolio will enable us to continuously develop innovative technologies to further strengthen the differentiation of MRIdian in the marketplace. Magnetic resonance imaging is a powerful and versatile measurement technique and is widely used throughout radiology and medicine because of its ability to generate information about tissues and disease states.
At June 30, 2015, we had a total of 32 employees in our research and development departments. Research and development expenses were $4.5 million and $5.2 million during the six months ended June 30, 2015 and 2014, and $9.4 million and $8.8 million for the years ended December 31, 2014 and 2013. We plan to continue to increase our investment in research and development in future periods.
Government Regulation
U.S. Medical Device Regulation and Nuclear Materials Regulation
As a manufacturer and seller of medical devices and devices that deliver radiation, we and some of our suppliers and distributors are subject to extensive and rigorous regulation by the FDA, the NRC, other federal, state and local authorities in the United States and foreign regulatory authorities. Regulations promulgated by the FDA relating to medical devices and radiation-producing devices govern, among other things, the following activities that we perform or that are performed on our behalf, and that we will continue to perform or have performed on our behalf:
26
Table of Contents
| • | product design, development and testing; |
| • | manufacturing; |
| • | approval or clearance; |
| • | packaging, labeling and storage; |
| • | marketing, advertising and promotion; |
| • | distribution, including importing and exporting; |
| • | installation; |
| • | possession and disposal; |
| • | record keeping; |
| • | service and surveillance, including post-approval monitoring and reporting; |
| • | complaint handling; and |
| • | repair or recall of products and issuance of field safety corrective actions. |
FDA Clearance and Approval of Medical Devices
The FDA regulates the research, testing, manufacturing, safety, labeling, storage, recordkeeping, promotion, distribution, and production of medical devices in the United States to ensure that medical products distributed domestically are safe and effective for their intended uses. Unless an exemption applies, the FDA requires that all new medical devices and all marketed medical devices that have been significantly changed, or that will be marketed with a new indication for use, obtain either clearance via a 510(k) pre-market notification or approval via a Premarket Approval, or PMA, application before the manufacturer may commercially distribute the product in the United States. The type of marketing authorization necessary is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes. Devices deemed to pose the lowest risk are placed in Class I, and most Class I devices are exempt from premarket notification requirements. Class I devices are those for which safety and effectiveness can be reasonably assured by adherence to a set of regulations referred to as General Controls, which require compliance with the applicable portions of the FDA’s Quality System Regulation, or QSR, and regulations regarding facility registration and product listing, reporting of adverse events and malfunctions, and appropriate, truthful and non-misleading labeling and promotional materials. However, some Class I devices, called Class I reserved devices, also require premarket clearance by the FDA through the 510(k) premarket notification process described below.
Moderate risk devices are placed in Class II and are subject to General Controls as well as Special Controls, which can include performance standards, guidelines and post-market surveillance. Most Class II devices are subject to premarket review and clearance by the FDA pursuant to Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or FDCA.
Devices deemed by the FDA to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices, or devices deemed not substantially equivalent to a previously cleared 510(k) device are placed in Class III. Class III devices require FDA approval of a PMA prior to marketing.
MRIdian has been classified as a Class II medical device subject to the 510(k) clearance process.
510(k) clearance process. Most Class II devices are subject to premarket review and clearance by the FDA. Premarket review and clearance by the FDA for Class II devices is accomplished through the 510(k) premarket notification process. Under the 510(k) process, the manufacturer must submit to the FDA a premarket notification, demonstrating that the device is “substantially equivalent” to either:
27
Table of Contents
| • | a device that was legally marketed prior to May 28, 1976, the date upon which the Medical Device Amendments of 1976 were enacted; or |
| • | another commercially available, similar device that was cleared through the 510(k) process. |
To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence.
The process of obtaining 510(k) clearance usually takes from three to 12 months from the date the application is filed and generally requires submitting supporting design and test data, which can be extensive and can prolong the process for a considerable period of time. If the FDA agrees that the device is substantially equivalent, it will grant clearance to commercially market the device. We received our 510(k) clearances for the treatment planning and delivery software system in January 2011 and for MRIdian in May 2012.
After a device receives 510(k) clearance, any modification that could significantly affect its safety or effectiveness, or that would constitute a new or major change in the intended use of the device, may require a new 510(k) clearance or, depending on the modification, could require approval of a PMA. The FDA requires each manufacturer to make this determination in the first instance, but the FDA can review any such decision. If the FDA disagrees with the manufacturer’s decision, it may retroactively require the manufacturer to submit a request for 510(k) clearance or PMA approval and can require the manufacturer to cease marketing and/or recall the product until 510(k) clearance or PMA approval is obtained. Since obtaining 510(k) clearances in 2011 and 2012, we have made changes to MRIdian that we believe do not require new 510(k) clearance.
Premarket application approval process. Submission and approval of a PMA is required before marketing of a Class III product may proceed. Under the PMA application process, the applicant must generally conduct at least one clinical investigation and submit extensive data and clinical information demonstrating reasonable assurance of the safety and effectiveness of the device for its intended use to the FDA’s satisfaction. Accordingly, a PMA application typically includes, but is not limited to, extensive technical information regarding device design and development, pre-clinical and clinical trial data, manufacturing information, labeling and financial disclosure information for the clinical investigators in device studies. The PMA process is much more demanding than the 510(k) premarket notification process.
Following receipt of a PMA application, the FDA conducts an administrative review to determine whether the application is sufficiently complete to permit a substantive review. If it is not, the agency will refuse to file the PMA. If it is, the FDA will accept the application for filing and begin the review. The FDA, by statute and by regulation, has 180 days to review a filed PMA application, although the review of an application more often occurs over a significantly longer period of time. During this review period, the FDA may request additional information or clarification of information already provided, and the FDA may issue a major deficiency letter to the applicant, requesting the applicant’s response to deficiencies communicated by the FDA. Before approving or denying a PMA, an FDA advisory committee may review the PMA at a public meeting and provide the FDA with the committee’s recommendation on whether the FDA should approve the submission, approve it with specific conditions, or not approve it. Overall, the PMA application process typically takes between one to three years, but may take significantly longer. The FDA may approve a PMA application with post-approval conditions intended to ensure the safety and effectiveness of the device including, among other things, restrictions on labeling, user training requirements, restrictions on promotion, sale and distribution, and requirements for the collection of long-term follow-up data.
MRIdian is not currently approved under a PMA approval, and we have no plans for any indication or system improvements or extensions that we believe would require a PMA.
Clinical trials. Clinical trials are generally required to support a PMA application and are sometimes required for 510(k) clearance. Such trials require submission of an investigational device exemption, or IDE, application to the FDA for a specified number of patients and study sites, unless the product is deemed a non-significant risk device eligible for more abbreviated IDE requirements. If an IDE is required, the FDA and the appropriate institutional review boards, or IRBs, at the clinical sites must approve the study before clinical trials may begin. If the device is
28
Table of Contents
considered a non-significant risk device, IDE submission to FDA is not required. Instead, only approval from the IRB overseeing the clinical trial is required. Clinical trials are subject to extensive monitoring, record keeping and reporting requirements. Clinical trials must be conducted under the oversight of an IRB for the relevant clinical trial sites and must comply with FDA regulations, including but not limited to those relating to good clinical practices. To conduct a clinical trial, the patient’s informed consent must be obtained in form and substance that complies with both FDA requirements and state and federal privacy and human subject protection regulations. The clinical trial sponsor, the FDA or the IRB could suspend or terminate a clinical trial at any time for various reasons, including a belief that the subjects are being exposed to an unacceptable health risk. Even if a trial is completed, the results of clinical testing may not adequately demonstrate the safety and effectiveness of the device or may otherwise not be sufficient to obtain FDA clearance or approval to market the product.
Continuing FDA regulation. Any devices we manufacture or distribute pursuant to 510(k) clearance or PMA approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions.
In addition, our manufacturing operations for medical devices and those of our suppliers must comply with the FDA’s QSR. The QSR requires that each manufacturer, including third party manufacturers, establish and implement a quality system by which the manufacturer monitors the manufacturing process and maintains records that show compliance with FDA regulations and the manufacturer’s written specifications and procedures. Among other things, the QSR requires that manufacturers establish performance requirements before production and follow stringent requirements applicable to the device design, testing, production, control, record keeping, documentation, labeling and installation, as well as supplier/contractor selection, complaint handling and other quality assurance procedures during all aspects of the manufacturing process. Compliance with the QSR is necessary to be able to continue to market medical devices that have received FDA approval or clearance, and to receive FDA clearance or approval to market new or significantly modified medical devices. The FDA makes announced and unannounced inspections of medical device manufacturers, and these inspections may include the manufacturing facilities of subcontractors. Following an inspection, the FDA may issue reports, known as FDA Form 483 reports, listing the investigator’s observations of conditions or practices which indicate the possibility that an FDA-regulated product may be in violation of FDA’s requirements. FDA may also issue warning letters documenting regulatory violations observed during an inspection. The manufacturer’s failure to adequately respond to such reports or warning letters may result in FDA enforcement action against the manufacturer and related consequences, including, among other things, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, FDA refusal to grant 510(k) clearance or PMA approval to new devices, withdrawal of existing clearances or approvals, and criminal prosecution.
Manufacturers must also comply with post-market surveillance regulations, including medical device reporting regulations, which require that manufacturers review and report to the FDA any incident in which their device may have caused or contributed to a death or serious injury, or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur. In addition, corrections and removal reporting regulations require that manufacturers report to the FDA field corrections and product recalls or removals if undertaken to reduce a risk to health posed by the device or to remedy a violation of the Federal Food, Drug, and Cosmetic Act that may present a risk to health. The FDA may also order a mandatory recall if there is a reasonable probability that the device would cause serious adverse health consequences or death.
The FDA and the Federal Trade Commission, or FTC, also regulate the promotion and advertising of MRIdian. In general, we may not promote or advertise MRIdian for uses not within the scope of our clearances or approvals or make unsupported safety and effectiveness claims.
Failure to comply with applicable FDA requirements, including delays in or failures to report incidents to the FDA or off-label promotion, can result in enforcement action by the FDA, which can include any of the following sanctions:
| • | warning letters, untitled letters, fines, injunctions, consent decrees and civil penalties; |
| • | customer notifications or repair, replacement, refunds, recall, administrative detention or seizure of our MRIdian systems; |
29
Table of Contents
| • | operating restrictions or partial suspension or total shutdown of production; |
| • | refusing or delaying requests for 510(k) clearance or PMA approval of new or modified products; |
| • | withdrawing 510(k) clearances or PMA approvals that have already been granted; |
| • | refusal to grant export approval for products; or |
| • | criminal prosecution. |
Radiological health. We are also regulated by the FDA under the Electronic Product Radiation Control provisions of the FDCA because MRIdian contains gamma radiation producing components, and because we assemble these components during manufacturing and service activities. The Electronic Product Radiation Control provisions require gamma radiation producing products to comply with certain regulations and applicable performance standards. Manufacturers are required to certify in product labeling and reports to the FDA that their products comply with all necessary standards as well as maintain manufacturing, testing and sales records for their products. The Electronic Product Radiation Control provisions also require manufacturers to report product defects and affix appropriate labeling to covered products. Failure to comply with these requirements could result in enforcement action by the FDA, which can include any of the sanctions described above.
Nuclear Regulatory Commission and U.S. State Agencies
In the United States, as a manufacturer of medical devices and devices utilizing radioactive byproduct material (i.e. depleted uranium shielding and Cobalt-60 sources) we are subject to extensive regulation by not only federal governmental authorities, such as the NRC, but also by state and local governmental authorities, such as the Ohio Department of Health, to ensure such devices are safe and effective. In Ohio, the Department of Health, by agreement with the NRC, regulates the possession, use, and disposal of radioactive byproduct material as well as the manufacture of devices containing radioactive sealed sources to ensure compliance with state and federal laws and regulations. We have received sealed source device approval from the Ohio Department of Health for MRIdian and have entered into a standby letter of credit with PNC for $103,000 to provide certification of financial assurance for decommissioning Cobalt-60 radioactive materials in accordance with Ohio Department of Health regulations. The company and/or its supplier of radiation sources must also comply with NRC and U.S. Department of Transportation regulations on the labeling and packaging requirements for shipment of radiation sources to hospitals or other users of MRIdian. Compliance with NRC, state and local requirements is required for distribution, installation, use and service within each state that we intend to install MRIdian systems.
Existing radiation therapy facilities practicing nuclear medicine, brachytherapy or Gamma Knife therapy are already required to have necessary NRC and/or state licenses and a radiation safety program requiring compliance to various provisions under 10 CFR 35 “Medical uses of byproduct material.” Use of MRIdian is regulated under 10 CFR 35.1000 “Other medical uses of byproduct material or radiation from byproduct material.” In 2013, the NRC released a licensing guidance under 10 CFR 35.1000 to guide our customers in the NRC requirements applicable for the use of MRIdian. We believe that this guidance is favorable in that it is consistent with clinical use of existing image-guided radiation therapy devices.
Moreover, our use, management, and disposal of certain radioactive substances and wastes are subject to regulation by several federal and state agencies depending on the nature of the substance or waste material. We believe that we are in compliance with all federal and state regulations for this purpose.
Outside the United States, various laws apply to the import, distribution, installation and use of MRIdian, in consideration of the nuclear materials within MRIdian.
U.S. Privacy and Security Laws
We may also be subject to data privacy and security regulation by both the federal government and the states in which we conduct our business. The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and their respective implementing regulations, including the final omnibus rule published on January 25, 2013, imposes
30
Table of Contents
specified requirements relating to the privacy, security and transmission of individually identifiable health information. Further, “business associates,” defined as independent contractors or agents of covered entities that create, receive, maintain or transmit protected health information in connection with providing a service for or on behalf of a covered entity are also subject to certain HIPAA privacy and security standards. HITECH also increased the civil and criminal penalties that may be imposed against covered entities, business associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions. In addition, state laws govern the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
U.S. Fraud and Abuse Laws and Regulations
The healthcare industry is also subject to a number of fraud and abuse laws and regulations, including physician anti-kickback, false claims and physician payment transparency laws. Violations of these laws can lead to civil and criminal penalties, including exclusion from participation in federal healthcare programs and significant monetary penalties, among others. These laws, among other things, constrain the sales, marketing and other promotional activities of manufacturers of medical products, such as us, by limiting the kinds of financial arrangements we may have with hospitals, physicians and other potential purchasers of medical products who may seek reimbursement from a federal or state health care program such as Medicare or Medicaid.
Anti-kickback laws. The federal Anti-Kickback Statute makes it a criminal offense to knowingly and willfully solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase, order, lease of any good, facility, item or service, that are reimbursable by a state or federal health care program, such as Medicare or Medicaid. The term “remuneration” has been broadly interpreted to include anything of value. The Anti-Kickback Statute has been interpreted to apply to the purchase of medical devices from a particular manufacturer or the referral of patients to a particular supplier of diagnostic services utilizing such devices. Although, there are established statutory exceptions and regulatory safe harbors that define certain financial transactions and practices that are not subject to the Anti-Kickback Statute, the exceptions and safe harbors are drawn narrowly. Failure to meet all of the requirements of a particular applicable statutory exception or regulatory safe harbor does not make the conduct per se illegal under the Anti-Kickback Statute. Instead, the legality of the arrangement will be evaluated on a case-by-case basis based on a cumulative review of all its facts and circumstances.
Generally, courts have taken a broad interpretation of the scope of the Anti-Kickback Statute, holding that the statute may be violated if merely one purpose of a payment arrangement is to induce referrals or purchases. Further, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Violations of this law are punishable by up to five years in prison, and can also result in criminal fines, administrative civil money penalties and exclusion from participation in federal healthcare programs. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal False Claims Act. Many states have also adopted statutes similar to the federal Anti-Kickback Statute, some of which apply to payments in connection with the referral of patients for healthcare items or services reimbursed by any source, not only governmental payor programs.
False Claims Act. The federal civil False Claims Act prohibits anyone from knowingly and willfully presenting, or causing to be presented, claims for payment, that are false or fraudulent, for services not provided as claimed. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud. Because the complaint is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government is ultimately successful in obtaining redress in the matter or if the plaintiff succeeds in obtaining redress without the government’s involvement, then the plaintiff will receive a percentage of the recovery. When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties ranging from $5,500 to $11,000 for each separate false claim, and may be excluded from participation in federal health care programs, and, although the federal False Claims Act is a
31
Table of Contents
civil statute, violations may also implicate various federal criminal statutes. Several states have also adopted comparable state false claims act, some of which apply to all payors.
Civil monetary penalties laws. The civil monetary penalties statute imposes penalties against any person or entity that, among other things, is determined to have presented or caused to be presented a claim to a federal health program that the person knows or should know is for an item or service that was not provided as claimed or is false or fraudulent.
Other fraud and abuse laws. HIPAA also created new federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the federal Anti-Kickback Statute, the intent standard for certain healthcare fraud statutes under HIPAA was amended by the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, the Affordable Care Act, such that a person or entity no longer needs to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation.
Physician payment transparency laws. There has been a recent trend of increased federal and state regulation of payments made to physicians and other healthcare providers and entities. The Affordable Care Act, among other things, imposed new reporting requirements on certain manufacturers, including certain device manufacturers, for payments provided to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit timely, accurately, and completely the required information may result in civil monetary penalties of up to an aggregate of $150,000 per year and up to an aggregate of $1 million per year for “knowing failures.” Device manufacturers were required to begin collecting data on August 1, 2013 and submit reports on aggregate payment data to the government for the first reporting period (August 1, 2013 – December 31, 2013) by March 31, 2014, and to report detailed payment data for the first reporting period and submit legal attestation to the accuracy of such data by June 30, 2014. Thereafter, device manufacturers must submit reports by the 90th day of each subsequent calendar year. CMS made all reported data publicly available on September 30, 2014.
Certain states also mandate implementation of compliance programs, impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare providers and entities.
The laws and regulations and their enforcement are constantly undergoing change, and we cannot predict what effect, if any, changes may have on our business. In addition, new laws and regulations may be adopted which adversely affect our business. There has been a trend in recent years, both in the United States and internationally, toward more stringent regulation and enforcement of requirements applicable to medical device manufacturers and requirements regarding protection and confidentiality of personal data.
State Certificate of Need Laws
In some states, a certificate of need, or CON, or similar regulatory approval is required by hospitals and other healthcare providers prior to the acquisition of high-cost capital items, including MRIdian, or the provision of new services. These laws generally require appropriate state agency determination of public need and approval prior to the acquisition of such capital items or addition of new services. CON requirements may preclude our customers from acquiring, or significantly delay acquisition of, MRIdian and/or from performing treatments using MRIdian. CON laws are the subject of ongoing legislative activity, and a significant increase in the number of states regulating the offering and use of MRIdian through CON or similar requirements could adversely affect us.
Healthcare Reform
In the United States and foreign jurisdictions, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system seeking, among other things, to reduce healthcare costs that could affect our results of operations.
32
Table of Contents
By way of example, in the United States, the Affordable Care Act was signed into law in March 2010, which is expected to substantially change the way healthcare is delivered and financed by both governmental and private insurers. Among other things, the Affordable Care Act:
| • | imposed an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States; |
| • | established a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| • | implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| • | created an independent payment advisory board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This included reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and will stay in effect through 2024 unless additional Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals and imaging centers.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for MRIdian or additional pricing pressure.
Foreign Regulation of Medical Devices
Our activities outside the United States are subject to regulatory requirements that vary from country to country and frequently differ significantly from those in the United States. Failure to obtain and maintain regulatory approval or clearance in any foreign country in which we market or plan to market MRIdian may have a negative effect on our ability to generate revenue and harm our business.
In general, MRIdian is regulated outside the United States as medical devices by foreign governmental agencies similar to the FDA and the FTC. In addition, in foreign countries where we have operations or sell MRIdian, we are subject to laws and regulations applicable to manufacturers of medical devices, radiation producing devices and to the healthcare industry, and laws and regulation of general applicability relating to environmental protection, safe working conditions, manufacturing practices and other matters. These laws and regulations are often comparable to, or more stringent than U.S. laws and regulations. Our sales of MRIdian in foreign countries are also subject to regulation of matters such as product standards, packaging requirements, labeling requirements, import restrictions, tariff regulations, duties and tax requirements. We rely in some countries on our foreign distributors to assist us in complying with applicable regulatory requirements.
Regulation in the EU
In the European Union, or EU, we are required under the European Medical Device Directive (Council Directive 93/42/EEC) to affix the CE mark to our MRIdian systems in order to sell the MRIdian systems in member countries of the EU. The CE mark is an international symbol that represents adherence to certain essential principles of safety and effectiveness mandated in the European Medical Device Directive (the so-called “essential requirements”). Once affixed, the CE mark enables a product to be sold within the European Economic Area, which is composed of the 28 Member States of the EU plus Norway, Iceland and Liechtenstein.
33
Table of Contents
To demonstrate compliance with the essential requirements, we must undergo a conformity assessment procedure which varies according to the type of medical device and its classification. Except for low risk medical devices (Class I with no measuring function and which are not sterile) where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a Member State of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This Certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
We received the CE Certificate of Conformity from our Notified Body in November 2014, allowing us to affix the CE mark to MRIdian in order to sell it throughout the EEA.
If we modify MRIdian we may need to apply for permission to affix the CE mark to the modified product. Additionally, we will need to apply for a CE mark for any new products that we may develop in the future. We cannot be certain that we will be able to obtain permission to affix the CE mark for modified or new products or that we will continue to meet the quality and safety standards required to maintain the permissions that we have already received or may receive in the future. In addition, if we are unable to obtain permission to affix the CE mark to our future products, we would be unable to sell them in EU member countries.
Regulation in Other Countries
We will be subject to additional regulations in foreign countries in which we intend to market, sell and import MRIdian. We or our distributors must receive all necessary approvals or clearance prior to marketing and importing MRIdian in those international markets. We received a license and permission to import MRIdian into the United Arab Emirates in December 2014. We received regulatory approval in Italy in January 2015, which is required in addition to the CE mark. We are currently seeking government approval to market MRIdian in China, Japan, Canada, Korea, Russia, Hong Kong and Turkey. Other countries where we will be seeking approval in the near term are Canada, Egypt, and Australia. We will seek approvals in other countries as may be required in the future.
The International Standards Organization promulgates internationally recognized standards, including those for the requirements of quality systems. We are certified to the ISO 13485:2003 standard, which specify the quality system requirements for medical device manufacturers. To support our ISO certifications, we are subject to surveillance audits by a Notified Body yearly and recertification audits every three years that assess our continued compliance with the relevant ISO standards. Our most recent recertification audit occurred in March 2015.
Employees
At June 30, 2015, we had 88 full-time employees. Within our workforce at June 30, 2015, 32 employees are engaged in research and development and 56 employees in business development, finance, human resources, facilities, information technology and general management and administration. We have no collective bargaining agreements with our employees, and we have not experienced any work stoppages. We consider our relations with our employees to be good.
Facilities
Our corporate headquarters are located in Oakwood Village, Ohio, where we lease and occupy approximately 41,000 square feet of office space. The current term of our Oakwood Village lease expires on October 31, 2017, with an option to extend the term through October 31, 2027. We also maintain an office in Mountain View, California, where we lease and occupy approximately 25,500 square feet of office space. The current term of our Mountain View lease expires on November 30, 2019. In connection with our Mountain View, California lease, we entered into a standby letter of credit with PNC Bank, National Association for $0.5 million, which is still outstanding at June 30, 2015.
34
Table of Contents
We believe that our existing facilities are adequate for our current needs. When our leases expire, we may exercise our renewal option or look for additional or alternate space for our operations and we believe that suitable additional or alternative space will be available in the future on commercially reasonable terms.
35
Table of Contents
You should consider carefully the risks and uncertainties described below, together with all of the other information in this Current Report on Form 8-K. If any of the following risks are realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. The risks described below are not the only risks facing the Company. Risks and uncertainties not currently known to us or that we currently deem to be immaterial also may materially adversely affect our business, financial condition, results of operations and/or prospects.
Risks Related to Our Business and Strategy
We have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future. These factors raise substantial doubt about our ability to continue as a going concern.
We have historically incurred substantial net losses, including net losses of $20.6 million and $16.6 million during the six months ended June 30, 2015 and 2014, and $33.8 million and $27.2 million during the years ended December 31, 2014 and 2013, respectively. We expect our net losses to continue as a result of ongoing expansion of our commercial operations, including increased manufacturing, sales and marketing costs. These net losses have had, and will continue to have, a negative impact on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability could harm our business, financial condition, results of operations and cash flows.
Further, the net losses we incur may fluctuate significantly from quarter-to-quarter and year-to-year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance quarter-to-quarter and year-to-year, due to factors including the timing of clinical trials, any litigation that we may file or that may be filed against us, the execution of collaboration, licensing or other agreements and the timing of any payments we make or receive thereunder. These factors raise substantial doubt about our ability to continue as a going concern.
Our independent registered public accounting firm has issued an opinion on our 2014 financial statements that included an explanatory paragraph referring to our ability to continue as a going concern. Our financial statements do not include any adjustments that may result from the outcome of this uncertainty. We believe the initial closing of the Offering enables us to continue as a going concern.
If clinicians do not widely adopt MRI-guided radiation therapy or MRIdian fails to achieve and sustain sufficient market acceptance, we will not generate sufficient revenue and our growth prospects, financial condition and results of operations could be harmed.
Our MRI-guided radiation therapy system, MRIdian, may never gain significant acceptance in the marketplace and, therefore, may never generate substantial revenue or allow us to achieve or maintain profitability. Widespread adoption of MRI-guided radiation therapy depends on many factors, including acceptance by clinicians that MRI-guided radiation therapy is clinically-effective and cost-effective in treating a wide range of cancers, demand by patients for such treatment, successful education of clinicians on the various aspects of this therapeutic approach and coverage and adequate reimbursement for procedures performed using MRI-guided radiation therapy. If we are not successful in conveying to hospitals that MRI-guided radiation therapy provides equivalent or superior radiation therapy compared to existing technologies, we may experience reluctance or refusal on the part of hospitals to order, and third-party payors to pay for performing, a treatment in which MRIdian is utilized. Our ability to achieve commercial market acceptance for MRIdian or any other future products also depends on the strength of our sales, marketing and distribution organizations. In addition, our expectations regarding cost savings from using MRIdian may not be accurate. These hurdles may make it difficult to demonstrate to physicians, hospitals and other healthcare providers that MRIdian is an appropriate option for radiation therapy, may be superior to available radiation therapy systems and may be more cost-effective than alternative technologies.
36
Table of Contents
Furthermore, we may encounter difficulty in gaining inclusion in cancer treatment guidelines and gaining broad market acceptance by healthcare providers, third-party payors and patients. Healthcare providers may have difficulty in obtaining adequate reimbursement from government and/or third-party payors for cancer treatment, which may negatively impact adoption of MRIdian.
We may not be able to generate sufficient revenue from the commercialization of MRIdian to achieve and maintain profitability.
We rely solely on the commercialization of MRIdian to generate revenue, and we expect to generate substantially all of our revenue in the future from sales of MRIdian. Through June 30, 2015, we have installed only three systems that are currently treating patients. During the six months ended June 30, 2015 and the year ended December 31, 2014, we recognized revenue of $462,000 and $6.4 million, respectively, from installed MRIdian systems at Washington University and Siteman Cancer Center at Barnes Jewish Hospital, or Washington University in St. Louis, University of California, Los Angeles and Health System and Jonsson Comprehensive Cancer Center, or UCLA, and The University of Wisconsin Carbone Cancer Center, or the University of Wisconsin—Madison. In order to successfully commercialize MRIdian, we will need to continue to expand our marketing efforts to develop new relationships and expand existing relationships with customers, to receive clearance or approval for MRIdian in additional countries, to achieve and maintain compliance with all applicable regulatory requirements and to develop and commercialize new features for MRIdian. We cannot assure you that we will be able to achieve or maintain profitability. If we fail to successfully commercialize MRIdian, we may never receive a return on the substantial investments in product development, sales and marketing, regulatory compliance, manufacturing and quality assurance we have made, as well as further investments we intend to make, which may cause us to fail to generate revenue and gain economies of scale from such investments.
In addition, potential customers may decide not to purchase MRIdian, or our customers may decide to cancel orders due to changes in treatment offerings, research and product development plans, difficulties in obtaining coverage or reimbursement for MRI-guided radiation therapy treatment, complications with facility build-outs, utilization of MRI-guided radiation therapy or other cancer treatment methods developed by other parties, lack of financing or the inability to obtain or delay in obtaining a certificate of need from state regulatory agencies or zoning restrictions, all of which are circumstances outside of our control.
In addition, demand for MRIdian systems may not increase as quickly as we predict, and we may be unable to increase our revenue levels as we expect. Even if we succeed in increasing adoption of MRIdian systems by hospitals and other healthcare providers, maintaining and creating relationships with our existing and new customers and developing and commercializing new features for MRIdian, we may not be able to generate sufficient revenue to achieve or maintain profitability.
We are an early, commercial-stage company and have a limited history commercializing MRIdian, which may make it difficult to evaluate our current business and predict our future performance.
We are an early, commercial-stage company and have a limited operating history. We commenced operations as a Florida corporation in 2004 and subsequently reincorporated in Delaware in 2007. However, we did not begin commercial operations until 2013. Our limited history commercializing MRIdian may make it difficult to evaluate our current business and predict our future performance. Any assessment of our profitability or prediction about our future success or viability is subject to significant uncertainty. We have encountered and will continue to encounter risks and difficulties frequently experienced by early, commercial-stage companies in rapidly evolving industries. If we do not address these risks successfully, our business could be harmed.
If MRIdian does not perform as expected, or if we are unable to satisfy customers’ demands for additional product features, our reputation, business and results of operations will suffer.
Our success depends on the market’s confidence that MRIdian can provide reliable, high-quality MRI-guided radiation therapy. There are only three MRIdian systems being used in commercial practice, and therefore we have very few statistics regarding the efficacy or reliability of MRIdian. We believe that our customers are likely to be particularly sensitive to product defects and errors, including functional downtime that limits the number of patients that can be treated using the system or a failure that is costly to repair. For example, in January 2014, we initiated a correction of the system at Washington University in St. Louis due to a defect we identified in an advanced software
37
Table of Contents
feature in the treatment planning system of MRIdian. We promptly updated our software to resolve this defect and notified the U.S. Food and Drug Administration, or FDA, of this correction. We cannot assure that similar product defects or other errors will not occur in the future. This could also include the mistreatment of a patient with MRIdian caused by human error on the part of MRIdian’s operators or prescribing physicians or as a result of a machine malfunction. We may be subject to regulatory enforcement action or legal claims arising from any defects or errors that may occur. Any failure of MRIdian to perform as expected could harm our reputation, business and results of operations.
Furthermore, the Cobalt-60 radioactive materials used in MRIdian systems decay over time, which eventually leads to longer treatment times and may have a negative impact on the number of patients a hospital can treat during a day. U.S. regulations require inspection of Cobalt-60 every five years, at which time customers may consider replacing the Cobalt-60 source. This natural decay or a customer’s failure to replace the Cobalt-60 may have a negative impact on MRIdian performance.
In addition, our customers are technologically well informed and at times have specific demands or requests for additional functionality. If we are unable to meet those demands through the development of new features for MRIdian or future products, those new features or products do not function at the level that our customers expect, we are unable to increase throughput as expected or we are unable to obtain regulatory clearance or approval of those new features or products, where applicable, our reputation, business and results of operations could be harmed.
The safety and efficacy of MRIdian for certain uses is not currently supported by long-term clinical data, and MRIdian may therefore be less safe and effective than initially anticipated.
MRIdian has received premarket clearance by the FDA under Section 510(k) of the Federal Food, Drug and Cosmetic Act, or FDCA. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, with respect to intended use, technology and safety and effectiveness, in order to clear the proposed device for marketing. This process is typically shorter and generally requires the submission of less supporting documentation than the FDA’s premarket approval process and does not always require long-term clinical studies. Additionally, to date, we have not been required to complete long-term clinical studies in connection with the sale of MRIdian outside the United States. As a result, we currently lack the breadth of published long-term clinical data supporting the efficacy of MRIdian and the benefits it offers that might have been generated in connection with other approval processes. In addition, because MRIdian has only been on the market since 2013, we have limited complication or patient survival rate data with respect to treatment using the system. If future patient studies or clinical testing do not support our belief that MRIdian offers a more advantageous treatment for a wide variety of cancer types, market acceptance of the system could fail to increase or could decrease and our business could be harmed.
If we choose to, or are required to, conduct additional studies, such studies or experience could reduce the rate of coverage and reimbursement by both public and private third-party payors for procedures that are performed with MRIdian, slow the market adoption of our product by physicians, significantly reduce our ability to achieve expected revenues and prevent us from becoming profitable. In addition, if future studies and experience indicate that MRIdian causes unexpected or serious complications or other unforeseen negative effects, we could be subject to mandatory product recalls or suspension or withdrawal of FDA clearance, and our reputation with physicians, patients and healthcare providers may suffer.
There have been instances of patients’ severe injury or death due to either operator misuse or system malfunction with other radiation therapy systems. If our redundant safety systems do not operate as we expect, MRIdian could severely injure or kill a patient. This could result in lawsuits, fines or damage to our reputation.
We may be delayed or prevented from implementing our long-term sales strategy if we fail to educate clinicians and patients about the benefits of MRIdian.
In order to increase revenue, we must increase awareness of the range of benefits that we believe MRIdian offers to both existing and potential customers, primarily cancer clinicians. An important part of our sales strategy involves educating and training clinicians to utilize the entire functionality of MRIdian. In addition, we must further educate clinicians about the ability of MRIdian to treat a wide range of cancer types effectively and efficiently. If clinicians are not properly educated about the use of MRIdian for radiation therapy, they may be unwilling or unable to take
38
Table of Contents
advantage of the full range of functionality that we believe MRIdian offers, which could have a negative impact on MRIdian sales. Clinicians may decide that certain tumors can be adequately treated using traditional radiation therapy systems, notwithstanding the benefits of MRIdian. Cobalt-60 systems have historically had certain limitations which have resulted in an increased use of linacs and a decreased use of Cobalt-60 systems. These historical limitations included imprecise radiation dose applications and an unsharp, wide-beam edge. If we do not adequately educate physicians about the functionality of our Cobalt-60 system to address some of the limitations that have affected Cobalt-60 systems, we may be delayed or prevented from implementing our long-term sales strategy. We must also succeed in educating clinicians about the potential for reimbursement for procedures performed using MRIdian. In addition, we need to increase awareness of MRIdian among potential patients, who are increasingly educated about cancer treatment options and therefore impact adoption of new technologies by clinicians. If our efforts to expand sales of MRIdian in the long-term are not successful, our business and results of operations will be harmed.
We may not be able to gain the support of leading hospitals and key opinion leaders, or to publish the results of our clinical trials in peer-reviewed journals, which may make it difficult to establish MRIdian as a standard of care and achieve market acceptance.
Our strategy includes developing relationships with leading hospitals and key opinion leaders in our industry. If these hospitals and key industry thought leaders determine that MRIdian is not clinically effective or that alternative technologies are more effective, or if we encounter difficulty promoting adoption or establishing MRIdian as a standard of care, our ability to achieve market acceptance of MRIdian could be significantly limited.
We believe that publication of scientific and medical results in peer-reviewed journals and presentation of data at leading conferences are critical to the broad adoption of MRIdian. Publication in leading medical journals is subject to a peer-review process, and peer reviewers may not consider the results of studies involving MRIdian sufficiently novel or worthy of publication.
We have a limited history of manufacturing, assembling and installing MRIdian in commercial quantities and may encounter related problems or delays that could result in lost revenue.
The pre-installation manufacturing processes for MRIdian include sourcing components from various third-party suppliers, subassembly, assembly, system integration and testing. We must manufacture and assemble MRIdian in compliance with regulatory requirements and at an acceptable cost in order to achieve and maintain profitability. We have only a limited history of manufacturing, assembling and installing MRIdian and, as a result, we may have difficulty manufacturing, assembling and installing MRIdian in sufficient quantities in a timely manner. To manage our manufacturing and operations with our suppliers, we forecast anticipated product orders and material requirements to predict our inventory needs up to a year in advance and enter into purchase orders on the basis of these requirements. Our limited manufacturing history may not provide us with sufficient data to accurately predict future component demand and to anticipate our costs effectively.
Further, we have experienced and may in the future experience delays in obtaining components from suppliers and installing our systems at customer sites associated with contractor timing delays, which could impede our ability to manufacture, assemble and install MRIdian on our expected timeline. Alternatively, delays or postponements of scheduled customer installations could lead to excess inventory due to our limited flexibility to postpone or delay component shipments from suppliers. Accordingly, we may encounter difficulties in production of MRIdian, including problems with quality control and assurance, component supply shortages or surpluses, increased costs, shortages of qualified personnel and difficulties associated with compliance with local, state, federal and foreign regulatory requirements. In addition, if we are unable to maintain larger-scale manufacturing capabilities, our ability to generate revenue will also be limited and our reputation could be harmed. If we cannot achieve the required level and quality of production, we may need to make changes in our supply chain or enter into licensing and other arrangements with third parties who possess sufficient manufacturing facilities and capabilities in compliance with regulatory requirements. Even if we outsource necessary production or enter into licensing or other third-party arrangements, the associated cost could reduce our gross margin and harm our financial condition and results of operations.
39
Table of Contents
We have limited experience in marketing and selling MRIdian, and if we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of MRIdian and we may never generate sufficient revenue to achieve or sustain profitability.
We have limited experience in marketing and selling MRIdian. We have only been selling MRIdian since 2013 and our three MRIdian systems installed have only been used for treating patients since early 2014. In addition, MRIdian is a new technology in the radiation therapy systems sector and our future sales will largely depend on our ability to increase our marketing efforts and adequately address our customers’ needs. We believe it is necessary to maintain a sales force that includes sales representatives with specific technical backgrounds that can support our customers’ needs. We will also need to attract and develop sales and marketing personnel with industry expertise. Competition for such employees is intense and we may not be able to attract and retain sufficient personnel to maintain an effective sales and marketing force. If we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of MRIdian and we may never generate sufficient revenue to achieve or sustain profitability.
The long sales cycle and low unit volume sales of MRIdian, as well as other factors, may contribute to substantial fluctuations in our operating results and stock price and make it difficult to compare our results of operations to prior periods and predict future financial results.
Because of the relatively small number of systems we expect to install in any period, each installation of a MRIdian will represent a significant percentage of our revenue for a particular period. Additionally, customer site construction, certificate of need and additional zoning and licensing permits are often required in connection with the sale of a MRIdian, any of which may further delay the installation process. When we are responsible for installing a system, we only recognize revenue from the sale of a MRIdian after the system has been installed and accepted by the customer. When a qualified third party is responsible for the installation, we only recognize revenue when title is transferred. Therefore, if we do not install a MRIdian or transfer title when anticipated, our operating results will vary significantly from our expectations. We have had experiences with customers postponing installation of MRIdian systems due to delays in facility build-outs, which are often lengthy and costly processes for our existing and potential customers. In addition, if our customers delay or cancel purchases, we may be required to modify or terminate contractual arrangements with our suppliers, which may result in the loss of deposits. Due to future fluctuations in revenue and costs, as well as other potential fluctuations, you should not rely upon our operating results in any particular period as an indication of future performance. In addition to the other risks described herein, the following factors may also contribute to these fluctuations:
| • | timing of when we are able to recognize revenue associated with sales of MRIdian; |
| • | actions relating to regulatory matters, including regulatory requirements in some states for a certificate of need prior to the installation of a MRIdian; |
| • | delays in shipment due to, for example, unanticipated construction delays at customer locations where MRIdian is to be installed, labor disturbances or natural disasters; |
| • | delays in our manufacturing processes or unexpected manufacturing difficulties; |
| • | timing of the announcements of contract executions or other customer and commercial developments; |
| • | timing of the announcement, introduction and delivery of new products or product features by us and by our competitors; |
| • | timing and level of expenditures associated with expansion of sales and marketing activities and our overall operations; |
| • | fluctuations in our gross margins and the factors that contribute to such fluctuations, as described in “Management’s Discussion and Analysis of Financial Condition and Results of Operations” elsewhere in this Report; |
| • | our ability to effectively execute on our strategic and operating plans; |
40
Table of Contents
| • | the extent to which MRIdian gains market acceptance and the timing of customer demand for MRIdian; |
| • | our ability to protect our proprietary rights and defend against third-party challenges; |
| • | disruptions in the supply or changes in the costs of raw materials, labor, product components or transportation services; and |
| • | changes in third-party coverage and reimbursement, government regulation or in a customer’s ability to obtain financing. |
These factors are difficult to forecast and may contribute to fluctuations in our reported revenue and results of operations and variation from our expectations, particularly during the periods in which our sales volume is low. Any such fluctuations in our financial results may cause volatility in our stock price.
Each MRIdian is a major capital equipment item and is subject to a lengthy sales cycle. The time from initial customer contact to execution of a contract can take 18 to 24 months or more. Following execution of a contract, it generally takes nine to 12 months for a customer to customize an existing facility or construct a new vault, which is inclusive of the time from when a customer places the order to when the system is delivered. During this time, facilities support and transitioning, as well as permitting, are typically required, which can take several months. The time required to customize an existing facility, including modifications of a standard vault to accommodate an MRI, is currently three months. If a customer does not have an existing vault available, it may take longer to construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures. If a small number of customers defer installation of a MRIdian for even a short period, recognition of a significant amount of revenue may be deferred to a subsequent period. Because our operating costs are relatively fixed, our inability to recognize revenue in a particular period may impact our profitability in that period. As a result, the inability to recognize revenue in a particular period may make it difficult to compare our operating results with prior periods. The price of a MRIdian requires a portion of our target customers to obtain outside financing before committing to purchase a MRIdian system. Such financing may be difficult for our customers to obtain in any given period, if at all. The requirement of site-specific modifications or construction may also delay adoption or overall demand. In addition, while we believe that our backlog of orders provides a better measure at any particular point in time of the long-term performance prospects of our business than our operating results for a particular period, investors may attribute significant weight to our operating results for a particular period, which may be volatile and as a result cause fluctuations in our stock price.
A large portion of our revenue in any given reporting period will be derived from a small number of contracts.
Given a significant portion of the purchase price for MRIdian will generally be recognized as revenue in a single reporting period, we expect a small number of contracts in any given reporting period to account for a substantial part of our revenue in any such period, and we expect this trend to continue. Any decrease in revenue from these contracts could harm our operating results. Accordingly, our revenue and results of operations may vary from period to period. We are also subject to credit risk associated with the concentration of our accounts receivable from our customers. If one or more of our customers at any given time were either to terminate their contracts with us, cease doing business with us or to fail to pay us on a timely basis, our business, financial condition and results of operations could be harmed.
The payment structure we use in our customer arrangements may lead to fluctuations in operating cash flows in a given period.
While our customers typically provide a deposit upon entering into a sales contract with us, the substantial majority of the payment owed for a MRIdian is not due until the time of shipment of a MRIdian or following final acceptance by the customer upon installation. If we miss targeted shipments or our customers do not actively work towards completing installation, our receipt of payments and our operating cash flows could be impacted. In addition, if customers do not adhere to our payments terms, our operating cash flows could be impacted in any given period. Due to these fluctuations in operating cash flows and other potential fluctuations, you should not rely upon our operating results in any particular period as an indication of future performance.
41
Table of Contents
Amounts included in backlog may not result in actual revenue and are an uncertain indicator of our future earnings.
We define backlog as the accumulation of all orders for which revenue has not been recognized and we consider valid. The determination of backlog includes, among other factors, our subjective judgment about the likelihood of an order becoming revenue and the regulatory approval required in the customer’s jurisdiction, if any. Our judgments in this area have been, and in the future may be, incorrect and we cannot assure you that, for any order included in backlog, we will recognize revenue with respect to such order. In addition, orders can be delayed for a number of reasons, many of which are beyond our control, including supplier delays which may cause delays in our manufacturing process, customer delays in commencing or completing construction of its facility, delays in obtaining zoning or other approvals and delays in obtaining financing. We may not be aware of these delays affecting our suppliers and customers and as a result may not consider them when evaluating the contemporaneous effect on backlog. Moreover, orders generally do not have firm dates by when a customer must take delivery, which could allow a customer to delay the order without cancelling the contract. Further, our backlog could be reduced due to cancellation of orders by customers. Should a cancellation occur, our backlog and anticipated revenue would be reduced unless we were able to replace such order. Reported reductions in our backlog could negatively impact our future results of operations or the price of our Common Stock.
We evaluate our backlog at least quarterly to determine if the orders continue to meet our criteria for inclusion in backlog. Our criteria include an outstanding and effective written agreement for the delivery of a MRIdian signed by customers, receipt of a minimum customer deposit or a letter of credit, any changes in customer or distributor plans or financial conditions, the customer’s or distributor’s continued intent and ability to fulfill the order contract, changes to regulatory requirements, the status of regulatory approval required in the customer’s jurisdiction, if any, or reasons for cancellation of order contracts. We may adjust our reported backlog as a result of these factors and due to changes in our judgment about the timing of shipment of a system for particular projects or the status of our regulatory approval in a particular jurisdiction, where applicable. Projects we once categorized as included within our backlog may be removed if we determine, based on the aforementioned criteria, that a particular order or orders no longer constitute valid backlog. In addition, one or more of our contracts have in the past and may in the future contribute to a material portion of our backlog in any one year. Because revenue will not be recognized until we have fulfilled our obligations to a customer, there may be a significant amount of time from signing a contract with a customer or shipping a system and revenue recognition. We cannot assure you that our backlog will result in revenue on a timely basis or at all, or that any cancelled contracts will be replaced.
Our ability to achieve profitability depends substantially on increasing our gross margins by reducing costs of MRIdian and improving our economies of scale, which we may not be able to achieve.
We are not, and never have been, profitable. The MRIdian purchase contracts we have entered into to date have been at a range of selling prices. Generally, earlier contracts have been at lower prices and more recent contracts have been at higher prices. Our earlier contracts resulted in negative gross margins. In order to become profitable we will need to be able to enter into contracts at increased prices. Our intention is to enter into purchase contracts for MRIdian systems with selling prices that are increasingly closer to our list price of a MRIdian. Our ability to enter into contracts at higher selling prices depends on a number of factors including:
| • | our ability to achieve commercial market acceptance for our system; |
| • | the pricing of competitors’ cancer therapy systems; |
| • | availability of coverage and adequate reimbursement by commercial and government payors; and |
| • | our ability to manufacture and install our systems in a timely and cost-effective manner. |
We bear the risk of warranty claims on all products we supply, including equipment and component parts manufactured by third parties. We cannot assure you that we will be successful in claiming recovery under any warranty or indemnity provided to us by our suppliers or vendors in the event of a successful warranty claim against us by a customer or that any recovery from such vendor or supplier would be adequate. In addition, warranty claims brought by our customers related to third-party components may arise after our ability to bring corresponding warranty claims against such suppliers expires, which could result in additional costs to us. There is a risk that
42
Table of Contents
warranty claims made against us will exceed our warranty reserve and our business, financial condition and results of operations could be harmed.
Our customer contracts provide that our customers commit to purchase a MRIdian for a fixed price, and a MRIdian will generally not be delivered for 11 to 15 months. In some circumstances, delivery can be postponed several months due to customer delays related to construction, vault preparation or concurrent facility expansion, and the cost of product supplies may increase significantly in the intervening time period. In addition, inflation may generally reduce the real value of the purchase price payable upon the achievement of future progress payment milestones. Either of these occurrences could cause our gross margins to decline or cause us to lose money on the sale of a MRIdian.
Moreover, our gross margins may decline in a given period due in part to significant replacement rates for components, resulting in increased warranty expense, negative profit margins on service contracts and customer dissatisfaction. If we are unable to reduce our expenses and improve or maintain quality and reliability, our profitability may be negatively impacted. Additionally, we may face increased demands for compensation from customers who are not satisfied with the quality and reliability of MRIdian, which could increase our service costs or require us to issue credits against future service payments and negatively impact future product sales. For example, we may have to extend a warranty period due to our failure to meet up-time requirements. Even if we are able to implement cost reduction and quality improvement efforts successfully, our service operations may remain unprofitable given the relatively small size and geographic dispersion of our installed base, which prevents us from achieving significant economies of scale for the provision of services. If we are unable to continue to sell MRIdian at increasingly higher prices that result in higher gross margins, we may never become profitable.
We may not be able to develop new products or enhance the capabilities of MRIdian to keep pace with our industry’s rapidly changing technology and customer requirements.
Our industry is characterized by rapid technological changes, new product introductions and enhancements and evolving industry standards. Our business prospects depend on our ability to develop new products and applications for our technology in new markets that develop as a result of technological and scientific advances, while improving the performance and cost-effectiveness of MRIdian. New technologies, techniques or products could emerge that might offer better combinations of price and performance than MRIdian systems. The market for radiation therapy treatment products is characterized by rapid innovation and advancement in technology. It is important that we anticipate changes in technology and market demand, as well as physician, hospital and healthcare provider practices to successfully develop, obtain clearance or approval, if required, and successfully introduce new, enhanced and competitive technologies to meet our prospective customers’ needs on a timely and cost-effective basis. Nevertheless, we must carefully manage our introduction of new products. If potential customers believe that such products will offer enhanced features or be sold for a more attractive price, they may delay purchases until such products are available. We may also have excess or obsolete inventory as we transition to new products, and we have no experience in managing product transitions. If we do not successfully innovate and introduce new technology into our anticipated product lines or effectively manage the transitions of our technology to new product offerings, our business, financial condition and results of operations could be harmed.
We face competition from numerous companies, many of whom have greater resources than we do or offer alternative technologies at lower prices than our MRIdian systems, which may make it more difficult for us to achieve significant market penetration and profitability.
The market for radiation therapy equipment is characterized by intense competition and pricing pressure. In particular, we compete with a number of existing therapy equipment companies, including Elekta AB, Varian Medical Systems, Inc. and Accuray Incorporated. Many of these competitors are large, well-capitalized companies with significantly greater market share and resources than we have. As a result, these companies may be better positioned than we are to spend more aggressively on marketing, sales, intellectual property and other product initiatives and research and development activities. In addition, we may compete with certain MRI-linear accelerator research projects that are currently in development and may be commercialized, including projects by the University of Alberta’s Cross Cancer Institute and a partnership of the University of Sydney, Ingham Institute and the University of Queensland.
43
Table of Contents
Existing technologies may offer certain advantages compared to the MRI technology used by our MRidian system. For example, computed tomography, or CT, is known to hold certain potential advantages over MRI technology for use in radiation therapy. Diagnostic CT is currently the most widely adopted imaging modality for treatment planning, and can be used to directly measure the electron density of patient tissues, which enables more accurate dose computation. In addition, CT imaging provides superior imaging of bones and boney anatomy than MRI, which is advantageous when imaging those structures for planning and alignment for treatment. Finally, CT is a less expensive technology than MRI and might be preferred by customers seeking a lower cost solution.
Our current competitors or other potential competitors may develop new products for the treatment of cancer at any time. In addition, competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards or customer requirements. If we are unable to develop products that compete effectively against the products of existing or future competitors, our future revenue could be negatively impacted. Some of our competitors may compete by changing their pricing model or by lowering the price of their therapy systems. If these competitors’ pricing techniques are effective, it could result in downward pressure on the price of all therapy systems. If we are unable to maintain or increase our selling prices in the face of competition, we may not improve our gross margins.
In addition to the competition that we face from technologies performing similar functions to MRIdian, competition also exists for the limited capital expenditure budgets of our customers. A potential purchaser may be forced to choose between two items of capital equipment. Our ability to compete may also be negatively impacted when purchase decisions are based largely upon price, because MRIdian is a premium-priced system relative to other capital expenditures and alternative radiation therapy technologies. In certain circumstances, a purchaser may decide that an alternative radiation therapy system priced below MRIdian may be sufficient for its patient population given the relative upfront cost savings. In addition to the cost of the MRIdian system, U.S. customers are required to inspect the Cobalt-60 every five years, and our customers may incur significant costs associated with the inspection, replacement and disposal of Cobalt-60.
Negative press regarding MRI-guided radiation therapy for the treatment of cancer could harm our business.
The comparative efficacy and overall benefits of MRI-guided radiation therapy are not yet well understood, particularly with respect to certain types of cancer. These types of reports could negatively impact the market’s acceptance of MRI-guided radiation therapy, and therefore our ability to generate revenue could be negatively impacted.
We may acquire other businesses, form joint ventures or make investments in other companies or technologies that could negatively affect our operating results, dilute our stockholders’ ownership, increase our debt or cause us to incur significant expense.
We may pursue acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our proprietary technology and industry experience to expand our offerings or distribution. We have no experience with acquiring other companies and limited experience with forming strategic partnerships. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in the incurrence of debt, contingent liabilities or future write-offs of intangible assets or goodwill, any of which could have a negative impact on our cash flows, financial condition and results of operations. Integration of an acquired company also may disrupt ongoing operations and require management resources that we would otherwise focus on developing our existing business. We may experience losses related to investments in other companies, which could harm our financial condition and results of operations. We may not realize the anticipated benefits of any acquisition, strategic alliance or joint venture.
Foreign acquisitions involve unique risks in addition to those mentioned above, including those related to integration of operations across different cultures and languages, currency risks and the particular economic, political and regulatory risks associated with specific countries.
To finance any acquisitions or joint ventures, we may choose to issue shares of common stock as consideration, which could dilute the ownership of our stockholders. Additional funds may not be available on terms that are
44
Table of Contents
favorable to us, or at all. If the price of our Common Stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration.
Risks Related to Our Reliance on Third Parties
We rely on a limited number of third-party suppliers and, in some cases, sole suppliers, for the majority of our components, subassemblies and materials and may not be able to find replacements or immediately transition to alternative suppliers.
We rely on several sole suppliers, including Japan Superconductor Technology, Inc., Siemens AG, Best Theratronics Ltd., Manufacturing Sciences Corporation, Tesla Engineering Limited, PEKO Precision Products, Inc., Euromechanics GmbH and Quality Electrodynamics, LLC, for certain components of MRIdian. These sole suppliers, and any of our other suppliers, may be unwilling or unable to supply components of MRIdian to us reliably and at the levels we anticipate or are required by the market. For us to be successful, our suppliers must be able to provide us with products and components in substantial quantities, in compliance with regulatory requirements, in accordance with agreed upon specifications, at acceptable costs and on a timely basis. An interruption in our commercial operations could occur if we encounter delays or difficulties in securing these components, and if we cannot then obtain an acceptable substitute. Any such interruption could harm our reputation, business, financial condition and results of operations.
If we are required to transition to new third-party suppliers for certain components of MRIdian, we believe that there are only a few other manufacturers that are currently capable of supplying the necessary components. In addition, the use of components or materials furnished by these alternative suppliers could require us to alter our operations. Furthermore, if we are required to change the manufacturer of a critical component of MRIdian, we will be required to verify that the new manufacturer maintains facilities, procedures and operations that comply with our quality and applicable regulatory requirements, which could further impede our ability to manufacture MRIdian in a timely manner. We currently do not carry inventory for components for more than two or three systems or have open purchase orders for components for more than four or six systems at any given time. Transitioning to a new supplier could be time-consuming and expensive, may result in interruptions in our operations and product delivery, could affect the performance specifications of MRIdian or could require that we modify the design of MRIdian. If the change in manufacturer results in a significant change to MRIdian, a new 510(k) clearance from the FDA or similar international regulatory authorization may be necessary, which could cause substantial delays. The occurrence of any of these events could harm our ability to meet the demand for MRIdian in a timely manner or cost-effectively.
We cannot assure you that we will be able to secure alternative equipment and materials and utilize such equipment and materials without experiencing interruptions in our workflow. If we should encounter delays or difficulties in securing, reconfiguring or revalidating the equipment and components we require for MRIdian, our reputation, business, financial condition and results of operations could be negatively impacted.
We depend on third-party distributors to market and distribute MRIdian in international markets.
A significant portion of our backlog is composed of international sales, and we expect a significant amount of our revenue to come from international sales. We depend on a number of distributors for sales in these international markets, and we have not to date completed the installation of any MRIdian systems internationally. We cannot control the efforts and resources our third-party distributors will devote to marketing MRIdian. Our distributors may not be able to successfully market and sell MRIdian and may not devote sufficient time and resources to support the marketing and selling efforts that enable the product to develop, achieve or sustain market acceptance. In some jurisdictions, we rely on our distributors to manage the regulatory process, and we are dependent on their ability to do so effectively. In addition, if a dispute arises with a distributor or if a distributor is terminated by us or goes out of business, it may take time to locate an alternative distributor, to seek appropriate regulatory approvals and to train such distributor’s personnel to market MRIdian, and our ability to sell and service MRIdian in the region formerly serviced by such terminated distributor could be harmed. Any of our distributors could become insolvent or otherwise become unable to pay amounts owed to us when due. Any of these factors could reduce our revenue from affected international markets, increase our costs in those markets or damage our reputation. In addition, if we are unable to attract additional international distributors, our international revenue may not grow.
45
Table of Contents
Failures by our third-party distributors to timely deliver or properly install MRIdian could harm our reputation.
We rely on arrangements with third-party distributors for sales and installation of MRIdian in international markets. To date, our third-party distributors have not completed the installation of any MRIdian systems internationally. As a result of our reliance on third-party distributors, we may be subject to disruptions and increased costs due to factors beyond our control, including labor strikes, third-party error and other issues. If the services of any of these distributors become unsatisfactory, including the failure of such distributors to properly install MRIdian, we may experience delays in meeting our customers’ product demands and we may not be able to find a suitable replacement on a timely basis or on commercially reasonable terms. Any failure to deliver, install or service products in a timely manner may damage our reputation and could cause us to lose current or potential customers.
We rely on third parties to store our inventory and to perform spare parts shipping and other logistics functions on our behalf. A failure or disruption with our logistics providers could harm our business.
Customer service is a critical element of our sales strategy. Third-party logistics providers store most of our spare parts inventory in depots around the world and perform a significant portion of our spare parts logistics and shipping activities. If any of our logistics providers suffers an interruption in its business or experiences delays, disruptions or quality control problems in its operations or we have to change and qualify alternative logistics providers for our spare parts, shipments of spare parts to our customers may be delayed and our reputation, business, financial condition and results of operations could be negatively harmed.
If third-party payors do not provide coverage and adequate reimbursement to our customers, it could negatively impact sales of MRIdian.
In the United States, hospitals and other healthcare providers who purchase MRIdian generally rely on third-party payors to reimburse all or part of the costs and fees associated with the treatments performed with our system, including the cost of MRIdian. Accordingly, sales of MRIdian depend, in part, on whether coverage and adequate reimbursement for standard planning methodologies are available to our customers from third-party payors, such as government healthcare insurance programs, including the Medicare and Medicaid programs, private insurance plans, health maintenance organizations and preferred provider organizations. In general, third-party payors in the United States have become increasingly cost-conscious, which has limited coverage for, and reimbursement of, certain procedures such as MRI-guided radiation therapy. Third-party payors have also increased utilization controls related to the use of products such as ours by healthcare providers.
Furthermore, there is no uniform policy on coverage and reimbursement for MRI-guided radiation therapy among third-party payors. Payors continue to review their coverage policies carefully for existing and new therapies and can, without notice, deny coverage for treatments that include the use of MRIdian.
The Medicare program is increasingly used as a model for how private payors and other governmental payors develop their coverage and reimbursement policies for medical services and procedures. Medicare coverage of advanced and conventional radiation therapies using MRIdian currently varies depending upon the geographic location in which the services are provided. The Centers for Medicare & Medicaid Services, or CMS, has not adopted national coverage determination for such therapies that would determine coverage nationally. In the absence of such a national coverage determination, Medicare Administrative Contractors, or MACs, with jurisdiction over specific geographic regions have the discretion to determine whether and when the use of MRI-guided radiation therapy will be considered medically necessary and covered in their respective regions. A number of MACs have adopted or proposed local coverage determinations covering MRI-guided radiation therapy. However, these local coverage determinations do not ensure that coverage will be available for MRI-guided radiation therapy for all types of cancer as the coverage policies may limit coverage to only certain types of cancer.
Even if MRI-guided radiation therapy is covered and reimbursed by third-party payors, adverse changes in payors’ coverage and reimbursement policies that affect MRIdian could harm our ability to market and sell MRIdian. We cannot be sure that third-party payors will reimburse our customers for procedures using MRIdian at a level that will enable us to achieve or maintain adequate sales and price levels for MRIdian. Without coverage and adequate reimbursement from third-party payors, the market for MRIdian may be limited.
46
Table of Contents
Third-party payors regularly update reimbursement amounts and also, from time to time, revise the methodologies used to determine reimbursement amounts. This includes annual updates to payments to physicians, hospitals and ambulatory surgery centers for the radiation treatments performed with MRIdian. Because the cost of MRIdian generally is recovered by the healthcare provider as part of the payment for performing the treatment and not separately reimbursed, these updates could directly impact the demand for MRIdian. An example of payment updates is the Medicare program’s updates to hospital and physician payments, which are done on an annual basis using a prescribed statutory formula.
Under the Medicare Physician Fee Schedule, or MPFS, rule for 2015, in the past, when the application of the formula resulted in lower payment, Congress has passed interim legislation to prevent the reductions. In April 2015, however, the Medicare Access and CHIP Reauthorization Act of 2015, or MACRA, was signed into law, which repealed and replaced the statutory formula for Medicare payment adjustments to physicians. MACRA provides a permanent end to the annual interim legislative updates that had previously been necessary to delay or prevent significant reductions to payments under the Medicare Physician Fee Schedule. MACRA extended existing payment rates through June 30, 2015, with a 0.5% update for July 1, 2015 through December 31, 2015, and for each calendar year through 2019, after which there will be a 0% annual update each year through 2025. In addition, MACRA requires the establishment of the Merit-Based Incentive Payment System, beginning in 2019, under which physicians may receive performance-based payment incentives or payment reductions based on their performance with respect to clinical quality, resource use, clinical improvement activities and meaningful use of electronic health records. MACRA also requires the Centers for Medicare & Medicaid Services, or CMS, beginning in 2019, to provide incentive payments for physicians and other eligible professionals that participate in alternative payment models, such as accountable care organizations, that emphasize quality and value over the traditional volume-based fee-for-service model. It is unclear what impact, if any, MACRA will have on our business and operating results, but any resulting decrease in payment may result in reduced demand for our services.
With respect to hospital payments, in its final rule for the Hospital Outpatient Prospective Payment System, or HOPPS, effective January 1, 2015, CMS will implement changes in the coding system to simplify the billing of conventional radiation therapy performed in the hospital outpatient setting into three levels—simple, intermediate or complex—and to simplify the billing of intensity modulated radiation therapy, or IMRT, into two levels, simple or complex. Simple IMRT treatments (e.g., breast and prostate) may be paid at a lower rate than complex IMRT, which includes all other disease sites. We believe that MRIdian will tend to be used for a higher proportion of complex treatments than other radiation therapy systems, and accordingly, we believe that the new HOPPS rules will have a minimal impact on our customers.
Any significant cuts in reimbursement rates or changes in reimbursement methodology or administration for MRI-guided radiation therapy, or concerns or proposals regarding further cuts or changes in methodology or administration, could further increase uncertainty, influence our customers’ decisions, reduce demand for MRIdian, cause customers to cancel orders and impact our revenue and harm our business.
Foreign governments also have their own healthcare reimbursement systems, which vary significantly by country and region, and we cannot be sure that adequate reimbursement will be made available with respect to MRIdian under any foreign reimbursement system.
Our employees, consultants and commercial partners may engage in misconduct or other improper activities, including insider trading and non-compliance with regulatory standards and requirements.
We are exposed to the risk that our employees, consultants and commercial partners may engage in fraudulent or illegal activity. Misconduct by these parties could include intentional, reckless or negligent conduct or disclosure of unauthorized activities to us that violates the regulations of the FDA and non-U.S. regulators, including those laws requiring the reporting of true, complete and accurate information to such regulators, manufacturing standards, healthcare fraud and abuse laws and regulations in the United States and abroad or laws that require the true, complete and accurate reporting of financial information or data. In particular, sales, marketing and business arrangements in the healthcare industry, including the sale of medical devices, are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. It is not always possible to identify and deter misconduct by our employees and other third parties, and the precautions we take to detect and prevent this
47
Table of Contents
activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us and we are not successful in defending ourselves or asserting our rights, those actions could result in the imposition of significant fines or other sanctions, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings and curtailment of operations, any of which could adversely affect our ability to operate our business and our results of operations. Whether or not we are successful in defending against such actions or investigations, we could incur substantial costs, including legal fees, and divert the attention of management in defending ourselves against any of these claims or investigations.
Risks Related to Our Financial Condition and Capital Requirements
We may need to raise additional capital to fund our existing commercial operations, develop and commercialize new features for MRIdian and new products and expand our operations.
Based on our current business plan, we believe the net proceeds from the Offering, together with our current cash and cash equivalents and cash receipts from sales of MRIdian systems, will enable us to conduct our planned operations for at least the next 12 months. If our available cash balances, net proceeds from the Offering and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements including because of lower demand for MRIdian as a result of lower than currently expected rates of reimbursement from commercial third-party payors and government payors or due to other risks described herein, we may seek to sell common or preferred equity or convertible debt securities, enter into an additional credit facility or another form of third-party funding or seek other debt financing.
We may consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities or for other reasons, including to:
| • | increase our sales and marketing efforts to increase market adoption of MRIdian and address competitive developments; |
| • | provide for supply and inventory costs associated with plans to accommodate potential increases in demand for MRIdian systems; |
| • | fund development and marketing efforts of any future products or additional features to then-current products; |
| • | acquire, license or invest in new technologies; |
| • | acquire or invest in complementary businesses or assets; and |
| • | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| • | our ability to achieve revenue growth and improve gross margins; |
| • | our rate of progress in establishing coverage and reimbursement arrangements with domestic and international commercial third-party payors and government payors; |
| • | the cost of expanding our operations and offerings, including our sales and marketing efforts; |
| • | our rate of progress in, and cost of the sales and marketing activities associated with, establishing adoption of MRIdian; |
| • | the cost of research and development activities; |
48
Table of Contents
| • | the effect of competing technological and market developments; |
| • | costs related to international expansion; and |
| • | the potential cost of and delays in product development as a result of any regulatory oversight applicable to MRIdian. |
The various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, dilution to our stockholders could result. Any equity securities issued also could provide for rights, preferences or privileges senior to those of holders of our Common Stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences and privileges senior to those of holders of our Common Stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to certain components contained within MRIdian, or grant licenses on terms that are not favorable to us.
We will incur significant costs as a result of operating as a public company and our management expects to devote substantial time to public company compliance programs.
As a public company, we will incur significant legal, accounting and other expenses due to our compliance with regulations and disclosure obligations applicable to us, including compliance with the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, as well as rules implemented by the Securities and Exchange Commission, or SEC, and the OTC Markets. Stockholder activism, the current political environment and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact, in ways we cannot currently anticipate, the manner in which we operate our business. Our management and other personnel will devote a substantial amount of time to these compliance programs and monitoring of public company reporting obligations and as a result of the new corporate governance and executive compensation related rules, regulations and guidelines prompted by the Dodd-Frank Wall Street Reform and Consumer Protection Act, or the Dodd-Frank Act, and further regulations and disclosure obligations expected in the future, we will likely need to devote additional time and costs to comply with such compliance programs and rules. These rules and regulations will cause us to incur significant legal and financial compliance costs and will make some activities more time-consuming and costly.
To comply with the requirements of being a public company, we may need to undertake various actions, including implementing new internal controls and procedures and hiring new accounting or internal audit staff. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal control over financial reporting. We are continuing to develop and refine our disclosure controls and other procedures that are designed to ensure that information required to be disclosed by us in the reports that we file with the SEC is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that information required to be disclosed in reports under the Securities Exchange Act of 1934, or the Exchange Act, is accumulated and communicated to our principal executive and financial officers. Our current controls and any new controls that we develop may become inadequate and weaknesses in our internal control over financial reporting may be discovered in the future. Any failure to develop or maintain effective controls could negatively impact the results of periodic management evaluations and annual independent registered public accounting firm attestation reports regarding the effectiveness of our internal control over financial reporting that we may be required to include in our periodic reports we will file with the SEC under Section 404 of the Sarbanes-Oxley Act, harm our operating results, cause us to fail to meet our reporting obligations or result in a restatement of our prior period financial statements. In the event that we are not able to demonstrate compliance with the Sarbanes-Oxley Act, that our internal control over financial reporting is perceived as inadequate or that we are unable to produce timely or accurate financial statements, investors may lose confidence in our operating results and the price of our Common Stock could decline. In addition, if we are unable to continue to meet these requirements, our Common Stock may not be able to remain eligible for quotation on the OTC Markets or meet the eligibility requirements for the NASDAQ Stock Market, or NASDAQ.
We are not currently required to comply with the SEC rules that implement Section 404 of the Sarbanes-Oxley Act and are therefore not yet required to make a formal assessment of the effectiveness of our internal control over financial reporting for that purpose. Upon becoming a public company, we will be required to comply with certain
49
Table of Contents
of these rules, which will require management to certify financial and other information in our quarterly and annual reports and provide an annual management report on the effectiveness of our internal control over financial reporting commencing with our second annual report. We are just beginning the costly and challenging process of compiling the system and processing documentation needed to comply with such requirements. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, we will be unable to assert that our internal control over financial reporting is effective.
Our independent registered public accounting firm will not be required to formally attest to the effectiveness of our internal control over financial reporting until the later of our second annual report or the first annual report required to be filed with the SEC following the date we are no longer an “emerging growth company” as defined in the JOBS Act depending on whether we choose to rely on certain exemptions set forth in the JOBS Act. If we are unable to assert that our internal control over financial reporting is effective, or if our independent registered public accounting firm is unable to express an opinion on the effectiveness of our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, which could harm our business.
Compliance with recently adopted rules of the SEC relating to “conflict minerals” may require us and our suppliers to incur substantial expense and may result in disclosure by us that certain minerals used in products we manufacture or contract to manufacture are not “DRC conflict free.”
Section 1502 of the Dodd-Frank Act required the SEC to promulgate rules requiring disclosure by a public company of any “conflict minerals” (tin, tungsten, tantalum and gold) necessary to the functionality or production of a product manufactured or contracted to be manufactured by the public company. The SEC adopted final rules in 2012 that took effect at the end of January 2013. Because we manufacture or contract to manufacture a product that contains tin, tungsten, tantalum or gold, we will be required under these rules to determine whether those minerals are necessary to the functionality or production of MRIdian and, if so, conduct a country of origin inquiry with respect to all such minerals. If any such minerals may have originated in the Democratic Republic of the Congo, or DRC, or any of its adjoining countries, or covered countries, then we must conduct diligence on the source and chain of custody of those conflict minerals to determine if they originated in one of the covered countries and, if so, whether they financed or benefited armed groups in the covered countries. Disclosures relating to the products that may contain conflict minerals, the country of origin of those minerals and whether they are “DRC conflict free” must be provided in a Form SD (and accompanying conflict minerals report, if required, to disclose the diligence undertaken by us in sourcing the minerals and our conclusions relating to such diligence). If we are required to submit a conflict minerals report, after 2015 that report must be audited by an independent auditor pursuant to existing government auditing standards. Compliance with this new disclosure rule may be very time-consuming for management and our supply chain personnel (as well as time-consuming for our suppliers) and could involve the expenditure of significant amounts of money by us and them. Disclosures, mandated by this new rule, which can be perceived by the market to be “negative,” may cause customers to refuse to purchase MRIdian. We cannot assure you that the cost of compliance with the rule will not harm our business, financial condition or results of operations.
Our loan and security agreement with Capital Royalty Partners II L.P., Capital Royalty Partners II - Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Parallel Investment Opportunities Partners II L.P., or together, CRG, contains operating and financial covenants that may restrict our business and financing activities.
At June 26, 2015, we had $30 million in outstanding debt to CRG. Borrowings under our loan and security agreement with CRG are secured by substantially all of our personal property, including our intellectual property. Our loan and security agreement restricts our ability to, among other things:
| • | dispose of or sell our assets; |
| • | make material changes in our business; |
| • | merge with or acquire other entities or assets; |
| • | incur additional indebtedness; |
50
Table of Contents
| • | create liens on our assets; |
| • | pay dividends; |
| • | make investments; and |
| • | pay off subordinated indebtedness. |
The operating and financial restrictions and covenants in our loan and security agreement, as well as any future financing agreements into which we may enter, may restrict our ability to finance our operations and engage in, expand or otherwise pursue our business activities and strategies. Our ability to comply with these covenants may be affected by events beyond our control, and future breaches of any of these covenants could result in a default under our loan and security agreement. If not waived, future defaults could cause all of the outstanding indebtedness under our loan and security agreement to become immediately due and payable and terminate all commitments to extend further credit.
If we do not have or are unable to generate sufficient cash available to repay our debt obligations when they become due and payable, either upon maturity or in the event of a default, we may not be able to obtain additional debt or equity financing on favorable terms, if at all, which may negatively impact our ability to operate and continue our business as a going concern.
Our ability to use net operating losses to offset future taxable income may be subject to certain limitations.
At December 31, 2014, we had federal net operating loss carryforwards, or NOLs, of $125.3 million, which begin to expire in the year ended December 31, 2024, and state NOLs of approximately $1.5 million, which begin to expire in the year ended December 31, 2019. We also had federal research and development tax credit carryforwards of $2.4 million for the year ended December 31, 2014. These credits expire at various dates through the year ending December 31, 2024. Under Section 382 of the Internal Revenue Code of 1986, as amended, or the Code, a corporation that undergoes an “ownership change” is subject to limitations on its ability to utilize its NOLs to offset future taxable income. We believe we have experienced at least one ownership change in the past. We are currently analyzing the tax impacts of such ownership change on our federal NOLs and credit carryforwards. Future changes in our stock ownership, including this or future offerings, as well as other changes that may be outside of our control, could result in additional ownership changes under Section 382 of the Code. Our NOLs may also be limited under similar provisions of state law. We have recorded a full valuation allowance related to our NOLs and other deferred tax assets due to the uncertainty of the ultimate realization of the future tax benefits of such assets.
We face risks related to the current global economic environment, which could delay or prevent our customers from obtaining financing to purchase MRIdian and implement the required facilities, which could harm our business, financial condition and results of operations.
The state of the global economy continues to be uncertain. The current global economic conditions and uncertain credit markets and concerns regarding the availability of credit pose a risk that could impact customer demand for MRIdian, as well as our ability to manage normal commercial relationships with our customers, suppliers and creditors, including financial institutions. If the current global economic environment deteriorates, our business could be negatively affected.
Risks Related to Administrative, Organizational and Commercial Operations and Growth
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
We anticipate growth in our business operations. This future growth could create a strain on our organizational, administrative and operational infrastructure, including manufacturing operations, quality control, technical support and customer service, sales force management and general and financial administration. We may not be able to maintain the quality of or installation timelines of MRIdian or satisfy customer demand as it grows. Our ability to manage our growth properly will require us to continue to improve our operational, financial and management controls, as well as our reporting systems and procedures. We may implement new enterprise software systems in a
51
Table of Contents
number of areas affecting a broad range of business processes and functional areas. The time and resources required to implement these new systems is uncertain and failure to complete this in a timely and efficient manner could harm our business.
If we are unable to support demand for MRIdian and our future products, including ensuring that we have adequate resources to meet increased demand, or we are unable to successfully manage the evolution of our MRI-guided radiation technology, our business could be harmed.
As our commercial operations and sales volume grow, we will need to continue to increase our workflow capacity for manufacturing, customer service, billing and general process improvements and expand our internal quality assurance program, among other things. We will also need to purchase additional equipment, some of which can take several months or more to procure, set up and validate, and increase our manufacturing, maintenance, software and computing capacity to meet increased demand. We cannot assure you that any of these increases in scale, expansion of personnel, purchase of equipment or process enhancements will be successfully implemented.
The loss of our President and Chief Executive Officer or Chief Scientific Officer or our inability to attract and retain highly skilled scientists and salespeople could negatively impact our business.
Our success depends on the skills, experience and performance of our President and Chief Executive Officer, Chris A. Raanes, and our Chief Scientific Officer and founder, James F. Dempsey, Ph.D. The individual and collective efforts of these employees will be important as we continue to develop MRIdian and as we expand our commercial activities. The loss or incapacity of existing members of our executive management team could negatively impact our operations if we experience difficulties in hiring qualified successors. Our executive officers have employment agreements; however, the existence of an employment agreement does not guarantee the retention of the executive officer for any period of time.
Our commercial, manufacturing and research and development programs and operations depend on our ability to attract and retain highly skilled engineers, scientists and technicians. We may not be able to attract or retain qualified managers, engineers, scientists and technicians in the future due to the competition for qualified personnel among medical device businesses, particularly in California and Ohio. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. Recruiting and retention difficulties can limit our ability to support our commercial, manufacturing and research and development programs. All of our employees are at-will, which means that either we or the employee may terminate his or her employment at any time.
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of MRIdian could lead to the filing of product liability claims were someone to allege that MRIdian did not effectively treat the conditions its users were intending to target, caused other serious medical conditions or otherwise failed to perform as designed. We may also be subject to liability for errors in, a misunderstanding of or inappropriate reliance upon the information we provide in the ordinary course of our business activities, such as customer support or operating instructions. A product liability claim could result in substantial damages and be costly and time-consuming for us to defend.
We maintain product liability insurance in the amount of $9.0 million per occurrence and $9.0 million in the aggregate, but this insurance may not fully protect us from the financial impact of defending against product liability claims. Any product liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could lead to regulatory investigations, product recalls or withdrawals, damage our reputation or cause current vendors, suppliers and customers to terminate existing agreements and potential customers and partners to seek other suppliers of radiation therapy systems, any of which could negatively impact our results of operations.
52
Table of Contents
Sanctions against Russia, and Russia’s response to those sanctions, could harm our business, financial condition and results of operations.
Due to Russia’s recent military intervention in Ukraine and annexation of Crimea, the United States and the European Union, or EU, have imposed sanctions on certain individuals and one financial institution in Russia and have proposed the use of broader economic sanctions. In response, Russia has imposed entry bans on certain U.S. lawmakers and officials. We have engaged a third-party distributor and are currently in discussions with potential customers in Russia. If the United States or the EU were to impose sanctions on Russian businesses, or if Russia were to take retaliatory action against U.S. companies operating in Russia, our sales and marketing efforts in Russia could be harmed.
We face risks associated with our international business.
In addition to our marketing and sales of MRIdian in the United States, we also market MRIdian in North America, Europe and the Pacific Rim, with contracts signed with customers and distributors in Taiwan, Turkey, Korea, China, the United Arab Emirates, Hong Kong, Japan, Italy and Russia. Our international business operations are subject to a variety of risks, including:
| • | difficulties in staffing and managing foreign and geographically dispersed operations; |
| • | having to comply with various U.S. and international laws, including export control laws and the U.S. Foreign Corrupt Practices Act of 1977, or the FCPA, and anti-money laundering laws; |
| • | differing regulatory requirements for obtaining clearances or approvals to market MRIdian; |
| • | changes in uncertainties relating to foreign rules and regulations that may impact our ability to sell MRIdian, perform services or repatriate profits to the United States; |
| • | tariffs, export or import restrictions, restrictions on remittances abroad, imposition of duties or taxes that limit our ability to move MRIdian out of these countries or interfere with the import of essential materials into these countries; |
| • | limitations on our ability to enter into cost-effective arrangements with distributors of MRIdian, or at all; |
| • | fluctuations in foreign currency exchange rates; |
| • | imposition of limitations on production, sale or export of MRI-guided radiation therapy systems in foreign countries; |
| • | imposition of limitations on or increase of withholding and other taxes on remittances and other payments by foreign subsidiaries or joint ventures; |
| • | differing multiple payor reimbursement regimes, government payors or patient self-pay systems; |
| • | imposition of differing labor laws and standards; |
| • | economic, political or social instability in foreign countries and regions; |
| • | an inability, or reduced ability, to protect our intellectual property, including any effect of compulsory licensing imposed by government action; and |
| • | availability of government subsidies or other incentives that benefit competitors in their local markets that are not available to us. |
We expect that we will begin expanding into other target markets; however, we cannot assure you that our expansion plans will be realized, or if realized, be successful. We expect each market to have particular regulatory and funding hurdles to overcome and future developments in these markets, including the uncertainty relating to
53
Table of Contents
governmental policies and regulations, could harm our business. If we expend significant time and resources on expansion plans that fail or are delayed, our reputation, business and financial condition may be harmed.
Our results may be impacted by changes in foreign currency exchange rates.
Currently, the majority of our international sales contracts are denominated in U.S. dollars. We pay certain of our suppliers in a foreign currency under the terms of their supply agreements, and we may pay other suppliers in the future in foreign currency. As a result, an increase in the value of the U.S. dollar relative to foreign currencies could require us to reduce our selling price or risk making MRIdian less competitive in international markets or our costs could increase. Also, if our international sales increase, we may enter into a greater number of transactions denominated in non-U.S. dollars, which could expose us to foreign currency risks, including changes in currency exchange rates. We do not currently engage in any hedging transactions. If we are unable to address these risks and challenges effectively, our international operations may not be successful and our business could be harmed.
We could be negatively impacted by violations of applicable anti-corruption laws or violations of our internal policies designed to ensure ethical business practices.
We operate in a number of countries throughout the world, including in countries that do not have as strong a commitment to anti-corruption and ethical behavior that is required by U.S. laws or by corporate policies. We are subject to the risk that we, our U.S. employees or our employees located in other jurisdictions or any third parties such as our sales agents and distributors that we engage to do work on our behalf in foreign countries may take action determined to be in violation of anti-corruption laws in any jurisdiction in which we conduct business, including the FCPA and the Bribery Act of 2010, or the U.K. Anti-Bribery Act. In addition, we operate in certain countries in which the government may take an ownership stake in an enterprise and such government ownership may not be readily apparent, thereby increasing potential anti-corruption law violations. Any violation of the FCPA and U.K. Anti-Bribery Act or any similar anti-corruption law or regulation could result in substantial fines, sanctions, civil and/or criminal penalties and curtailment of operations in certain jurisdictions and might harm our business, financial condition or results of operations. In addition, we have internal ethics policies with which we require our employees to comply in order to ensure that our business is conducted in a manner that our management deems appropriate. If these anti-corruption laws or internal policies were to be violated, our reputation and operations could also be substantially harmed. Further, detecting, investigating and resolving actual or alleged violations is expensive and can consume significant time and attention of our senior management.
We are subject to export restrictions and laws affecting trade and investments, and the future sale of our MRIdian system may be further limited or prohibited in the future by a government agency or authority.
As a global company headquartered in the United States, our MRIdian system is subject to U.S. laws and regulations that may limit, restrict or require a license to export (and re-export from other countries) our MRIdian system and related product and technical information due to its use of hazardous materials, including Cobalt-60, lead and depleted uranium. We are also subject to the export and import laws of those foreign jurisdictions to which we sell or from which we re-export our MRIdian system. Compliance with these laws and regulations could significantly limit our operations and our sales in the future and failure to comply, even indirectly, could result in a range of penalties, including restrictions on exports of our MRIdian system for a specified time period, or forever, and severe monetary penalties. In certain circumstances, these restrictions may affect our ability to interact with any of our future foreign subsidiaries and otherwise limit our trade with third parties, including suppliers and customers, operating inside and outside the United States. In addition, if we introduce new products, we may need to obtain licenses or approvals from the United States and other governments to ship them into foreign countries. Failure to receive the appropriate approvals may mean that our commercial efforts and expenses related to such efforts may not result in any revenue, which could harm our business.
We depend on our information technology systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant elements of our operations. We have developed propriety software for the management and operation of MRIdian by our customers. We have installed, and expect to expand, a number of enterprise software systems that affect a broad range of business processes and functional areas, including for example, systems handling human resources, financial controls and reporting, contract management, regulatory compliance and other infrastructure operations. In addition to the
54
Table of Contents
aforementioned business systems, we intend to extend the capabilities of both our preventative and detective security controls by augmenting the monitoring and alerting functions, the network design and the automatic countermeasure operations of our technical systems. These information technology and telecommunications systems support a variety of functions, including sales and marketing, manufacturing operations, customer service support, billing and reimbursement, research and development activities and general administrative activities.
Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems or those used by our third-party service providers could prevent us from providing maintenance and support services to our customers, conducting research and development activities and managing the administrative aspects of our business. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could harm our business.
Our operations are vulnerable to interruption or loss due to natural or other disasters, power loss, strikes and other events beyond our control.
We conduct a significant portion of our activities, including administration and data processing, at facilities located in California, Ohio and other areas that have experienced major earthquakes, tornadoes and other natural disasters. A major earthquake, tornado or other disaster (such as a major fire, hurricane, flood, tsunami, volcanic eruption or terrorist attack) affecting our facilities, or those of our suppliers, could significantly disrupt our operations, and delay or prevent product shipment or installation during the time required to repair, rebuild or replace our suppliers’ damaged manufacturing facilities; these delays could be lengthy and costly. If any of our customers’ facilities are negatively impacted by a disaster, shipments of MRIdian could be delayed. Additionally, customers may delay purchases of MRIdian until operations return to normal. Even if we are able to quickly respond to a disaster, the ongoing effects of the disaster could create some uncertainty in the operations of our business. In addition, our facilities may be subject to a shortage of available electrical power and other energy supplies. Any shortages may increase our costs for power and energy supplies or could result in blackouts, which could disrupt the operations of our affected facilities and harm our business. Further, MRIdian is typically shipped from a limited number of ports, and any disaster, strike or other event blocking shipment from these ports could delay or prevent shipments and harm our business. In addition, concerns about terrorism, the effects of a terrorist attack, political turmoil or an outbreak of epidemic diseases, such as ebola or influenza, could have a negative effect on our operations, those of our suppliers and customers and the ability to travel, which could harm our business, financial condition and results of operations.
Risks Related to Intellectual Property
Litigation or other proceedings or third-party claims of intellectual property infringement could require us to spend significant time and money and could prevent us from selling MRIdian or impact our stock price.
There is considerable intellectual property litigation and contested patent disputes in the medical device area. Third parties may, in the future, assert claims that we are employing their proprietary technology without authorization, including claims from competitors or from non-practicing entities that have no relevant product revenue and against whom our own patent portfolio may have no deterrent effect. As we continue to commercialize MRIdian in its current or an updated form, launch new products and enter new markets, we expect that competitors may claim that MRIdian infringes their intellectual property rights as part of business strategies designed to impede our successful commercialization and entry into new markets. Although we are presently unaware of any basis by which a third party would be justified in making such claims, in the future, we may receive letters or other threats or claims from third parties inviting us to take licenses under, or alleging that we infringe, their patents. Third parties may have obtained, and may in the future obtain, patents under which such third parties may claim that the use of our technologies constitutes patent infringement.
Moreover, we may become party to future adversarial proceedings regarding our patent portfolio or the patents of third parties. Such proceedings could include contested post-grant proceedings such as oppositions, inter partes
55
Table of Contents
review, reexamination, interference or derivation proceedings before the U.S. Patent and Trademark Office or foreign patent offices. The legal threshold for initiating litigation or contested proceedings is low, so that even lawsuits or proceedings with a low probability of success might be initiated. Litigation and contested proceedings can also be expensive and time-consuming, and our adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we can.
We could incur substantial costs and divert the attention of our management and technical personnel in defending ourselves against any of these claims or in any of such proceedings. Any adverse ruling or perception of an adverse ruling in defending ourselves against these claims could have a negative impact on our cash position and stock price. Furthermore, parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement or misappropriation against us, we may be required to pay damages, obtain one or more licenses from third parties or be prohibited from selling certain products, all of which could have a negative impact on our cash position, business and financial condition.
In addition, we may be unable to obtain these licenses at a reasonable cost, if at all. We could therefore incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our gross margins. Moreover, we could encounter delays in product introductions while we attempt to develop alternative methods or products. Defense of any lawsuit or adversarial proceeding or failure to obtain any of these licenses on favorable terms could prevent us from commercializing products, and the prohibition of sale of MRIdian or future products could impact our ability to grow and maintain profitability and harm our business.
If we are unable to adequately protect our proprietary technology or maintain issued patents that are sufficient to protect MRIdian, others could compete against us more directly, which could harm our business, financial condition and results of operations.
Our commercial success will depend in part on our success in obtaining and maintaining issued patents and other intellectual property rights in the United States and elsewhere and protecting our proprietary technology. If we do not adequately protect our intellectual property and proprietary technology, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
We hold the exclusive worldwide license for certain patents and applications covering our combination of MRI and radiation therapy technologies. Specifically, we hold a license to two issued U.S. patents, one allowed U.S. patent application, 14 issued foreign patents (eight of which were issued in Great Britain, Germany, France and the Netherlands as a result of two patent applications filed and allowed through the European Patent Office) and four pending foreign patent applications at July 13, 2015. We own an additional eight issued U.S. patents, seven issued foreign patents, 16 pending U.S. patent applications and 48 pending foreign patent applications, and, at July 13, 2015, one of our foreign patent applications is allowed. Assuming all required fees are paid, individual patents or patent applications owned or licensed by us will expire between 2025 and 2034. We also have a joint ownership interest with Case Western Reserve University in one issued patent and one U.S. patent application.
We cannot provide any assurances that any of our patents have, or that any of our pending patent applications that mature into issued patents will include, claims with a scope sufficient to protect MRIdian, any additional features we develop for MRIdian or any new products. Other parties may have developed technologies that may be related or competitive to our platform, may have filed or may file patent applications and may have received or may receive patents that overlap or conflict with our patent applications, either by claiming the same methods or devices or by claiming subject matter that could dominate our patent position. The patent positions of medical device companies, including our patent position, involve complex legal and factual questions, and, therefore, the issuance, scope, validity and enforceability of any patent claims that we may obtain cannot be predicted with certainty. Patents, if issued, may be challenged, deemed unenforceable, invalidated or circumvented. U.S. patents and patent applications may also be subject to supplemental examination or contested post-grant proceedings such as inter partes review, reexamination, interference or derivation proceedings before the U.S. Patent and Trademark Office and challenges in district court. Patents may be subjected to opposition, post-grant review or comparable proceedings lodged in various foreign, both national and regional, patent offices. These proceedings could result in either loss of the patent or denial of the patent application or loss or reduction in the scope of one or more of the claims of the patent or patent application. In addition, such proceedings may be costly. Thus, any patents that we may own or exclusively
56
Table of Contents
license may not provide any protection against competitors. Furthermore, an adverse decision in an interference proceeding can result in a third party receiving the patent right sought by us, which in turn could affect our ability to commercialize MRIdian.
Furthermore, though an issued patent is presumed valid and enforceable, its issuance is not conclusive as to its validity or its enforceability and it may not provide us with adequate proprietary protection or competitive advantages against competitors with similar products. Competitors may also be able to design around our patents. Other parties may develop and obtain patent protection for more effective technologies, designs or methods. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or trade secrets by consultants, suppliers, vendors, former employees and current employees. The laws of some foreign countries do not protect our proprietary rights to the same extent as the laws of the United States, and we may encounter significant problems in protecting our proprietary rights in these countries. If any of these developments were to occur, they each could have a negative impact on our sales.
Our ability to enforce our patent rights depends on our ability to detect infringement. It is difficult to detect infringers who do not advertise the components that are used in their products. Moreover, it may be difficult or impossible to obtain evidence of infringement in a competitor’s or potential competitor’s product. Any litigation to enforce or defend our patent rights, even if we were to prevail, could be costly and time-consuming and would divert the attention of our management and key personnel from our business operations. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded if we were to prevail may not be commercially meaningful.
In addition, proceedings to enforce or defend our patents could put our patents at risk of being invalidated, held unenforceable or interpreted narrowly. Such proceedings could also provoke third parties to assert claims against us, including that some or all of the claims in one or more of our patents are invalid or otherwise unenforceable. If any of our patents covering MRIdian are invalidated or found unenforceable, our financial position and results of operations could be negatively impacted. In addition, if a court found that valid, enforceable patents held by third parties covered MRIdian, our financial position and results of operations could be harmed.
The degree of future protection for our proprietary rights is uncertain, and we cannot ensure that:
| • | any of our patents, or any of our pending patent applications, if issued, will include claims having a scope sufficient to protect MRIdian or any other products; |
| • | any of our pending patent applications will issue as patents; |
| • | we will be able to successfully commercialize MRIdian on a substantial scale before our relevant patents expire; |
| • | we were the first to make the inventions covered by each of our patents and pending patent applications; |
| • | we were the first to file patent applications for these inventions; |
| • | others will not develop similar or alternative technologies that do not infringe our patents; |
| • | any of our patents will be found to ultimately be valid and enforceable; |
| • | any patents issued to us will provide a basis for an exclusive market for our commercially viable products, will provide us with any competitive advantages or will not be challenged by third parties; |
| • | we will develop additional proprietary technologies or products that are separately patentable; or |
| • | our commercial activities or products will not infringe upon the patents of others. |
We rely upon unpatented trade secrets, unpatented know-how and continuing technological innovation to develop and maintain our competitive position, which we seek to protect, in part, by confidentiality agreements with our employees and our collaborators and consultants. We also have agreements with our employees and selected
57
Table of Contents
consultants that obligate them to assign their inventions to us and have non-compete agreements with some, but not all, of our consultants. It is possible that technology relevant to our business will be independently developed by a person that is not a party to such an agreement. Furthermore, if the employees and consultants who are parties to these agreements breach or violate the terms of these agreements, we may not have adequate remedies for any such breach or violation, and we could lose our trade secrets through such breaches or violations. Further, our trade secrets could otherwise become known or be independently discovered by our competitors.
If we are not able to meet the requirements of our license agreement with the University of Florida Research Foundation, Inc., we could lose access to the technologies licensed thereunder and be unable to manufacture, market or sell MRIdian.
We license patents and patent applications from the University of Florida Research Foundation, Inc., or UFRF, covering our combination of MRI and radiation therapy, and other key technologies, incorporated into MRIdian under a license agreement that requires us to pay royalties to UFRF. In addition, the license agreement obligates us to pursue an agreed development plan and to submit periodic reports and restricts our ability to take actions to defend the licensed patents. The license agreement terminates when the underlying patents expire in 2025, although UFRF has the right to unilaterally terminate the agreement if we do not meet our royalty payment obligations, including minimum royalty payments of $50,000 per quarter, or if we fail to satisfy other development and commercialization obligations related to our utilization of the technology. If UFRF were to terminate the agreement or if we were to otherwise lose the ability to exploit the licensed patents, our competitive advantage could be reduced, we may not be able to find a source to replace the licensed technology and we may be prevented from selling MRIdian. The license agreement reserves to UFRF the initial right to defend or prosecute any claim arising with respect to the licensed technology. If UFRF does not vigorously defend the patents, we may be required to engage in expensive patent litigation to enforce our rights and any competitive advantage we have based on the licensed technology may be hampered. Any of these events could harm our business, financial condition and results of operations.
Recent changes in U.S. patent laws may limit our ability to obtain, defend or enforce our patents.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents. The Leahy-Smith America Invents Act, or the Leahy-Smith Act, includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and also affect patent litigation. The United States Patent Office recently developed new regulations and procedures to govern administration of the Leahy-Smith Act, and many of the substantive changes to patent law associated with the Leahy-Smith Act, and in particular, the first to file provisions, only became effective on March 16, 2013. The first to file provisions limit the rights of an inventor to patent an invention if not the first to file an application for patenting that invention, even if such invention was the first invention. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business.
However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the enforcement and defense of our issued patents. For example, the Leahy-Smith Act provides that an administrative tribunal known as the Patent Trial and Appeals Board, or PTAB, provides a venue for companies to challenge the validity of competitor patents at a cost that is much lower than district court litigation and on timelines that are much faster. Although it is not clear what, if any, long-term impact the PTAB proceedings will have on the operation of our business, the initial results of patent challenge proceedings before the PTAB since its inception in 2013 have resulted in the invalidation of many U.S. patent claims. The availability of the PTAB as a lower-cost, faster and potentially more potent tribunal for challenging patents could therefore increase the likelihood that our own patents will be challenged, thereby increasing the uncertainties and costs of maintaining and enforcing them. Moreover, if such challenges occur with regard to our UFRF-licensed patents, as indicated above, we have only limited rights to control the defense.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position could be harmed.
In addition to patent protection, we also rely upon copyright and trade secret protection, as well as non-disclosure agreements and invention assignment agreements with our employees, consultants and third parties, to protect our
58
Table of Contents
confidential and proprietary information. For example, significant elements of MRIdian are based on unpatented trade secrets and know-how that are not publicly disclosed. In addition to contractual measures, we try to protect the confidential nature of our proprietary information using physical and technological security measures. Such measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and recourse we take against such misconduct may not provide an adequate remedy to protect our interests fully. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret can be difficult, expensive and time-consuming, and the outcome is unpredictable. In addition, trade secrets may be independently developed by others in a manner that could prevent legal recourse by us. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any such information was independently developed by a competitor, our competitive position could be harmed.
We may not be able to enforce our intellectual property rights throughout the world.
The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States. Many companies have encountered significant problems in protecting and defending intellectual property rights in certain foreign jurisdictions. This could make it difficult for us to stop the infringement of our patents, if obtained, or the misappropriation of our other intellectual property rights. For example, many foreign countries have compulsory licensing laws under which a patent owner must grant licenses to third parties. In addition, many countries limit the enforceability of patents against third parties, including government agencies or government contractors. In these countries, patents may provide limited or no benefit. Patent protection must ultimately be sought on a country-by-country basis, which is an expensive and time-consuming process with uncertain outcomes. Accordingly, we may choose not to seek patent protection in certain countries, and we will not have the benefit of patent protection in such countries.
Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to protect our intellectual property rights in such countries may be inadequate. In addition, changes in the law and legal decisions by courts in the United States and foreign countries may affect our ability to obtain adequate protection for our technology and the enforcement of intellectual property.
Third parties may assert ownership or commercial rights to inventions we develop.
Third parties may in the future make claims challenging the inventorship or ownership of our intellectual property. We have written agreements with collaborators that provide for the ownership of intellectual property arising from our collaborations. These agreements provide that we must negotiate certain commercial rights with collaborators with respect to joint inventions or inventions made by our collaborators that arise from the results of the collaboration. In some instances, there may not be adequate written provisions to address clearly the resolution of intellectual property rights that may arise from a collaboration. If we cannot successfully negotiate sufficient ownership and commercial rights to the inventions that result from our use of a third-party collaborator’s materials where required, or if disputes otherwise arise with respect to the intellectual property developed with the use of a collaborator’s technology, we may be limited in our ability to capitalize on the market potential of these intellectual property rights. In addition, we may face claims by third parties that our agreements with employees, contractors or consultants obligating them to assign intellectual property to us are ineffective or in conflict with prior or competing contractual obligations of assignment, which could result in ownership disputes regarding intellectual property we have developed or will develop and interfere with our ability to capture the commercial value of such intellectual property. Litigation may be necessary to resolve an ownership dispute, and if we are not successful, we may be precluded from using certain intellectual property or may lose our exclusive rights in that intellectual property. Either outcome could harm our business.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
We employ individuals who were previously employed at universities or other medical device companies, including our competitors or potential competitors. Although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our
59
Table of Contents
employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Risks Related to Regulatory Matters
MRIdian and our operations are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
MRIdian is a medical device that is subject to extensive regulation in the United States and elsewhere, including by the FDA and its foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices:
| • | design, development and manufacturing; |
| • | testing, labeling, content and language of instructions for use and storage; |
| • | clinical trials; |
| • | product safety; |
| • | marketing, sales and distribution; |
| • | premarket clearance and approval; |
| • | record keeping procedures; |
| • | advertising and promotion; |
| • | recalls and field safety corrective actions; |
| • | post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; |
| • | post-market approval studies; and |
| • | product import and export. |
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales.
In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the FDCA or approval of a premarket approval, or PMA, application from the FDA, unless an exemption applies. In the 510(k) clearance process, the FDA must determine that a proposed device is “substantially equivalent” to a device legally on the market, known as a “predicate” device, in order to clear the proposed device for marketing. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence. In the PMA process, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices.
60
Table of Contents
Modifications to products that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made to products cleared through a 510(k) may require a new 510(k) clearance. Both the PMA approval and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months, but can last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is filed with the FDA. In addition, PMA generally requires the performance of one or more clinical trials. Despite the time, effort and cost, we cannot assure you that any particular device will be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business.
In the United States, we have obtained 510(k) premarket clearance from the FDA to market MRIdian for the provision of stereotactic radiosurgery and precision radiotherapy for lesions, tumors and conditions anywhere in the body where radiation treatment is indicated. An element of our strategy is to continue to upgrade MRIdian to incorporate new software and hardware enhancements. We expect that such upgrades, as well as other future modifications to MRIdian, may require new 510(k) clearance; however, future upgrades may be subject to the substantially more costly, time-consuming and uncertain PMA process. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, product introductions or modifications could be delayed or canceled, which could cause our sales to decline.
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
| • | we may not be able to demonstrate to the FDA’s satisfaction that MRIdian is substantially equivalent to the proposed predicate device or safe and effective for its intended use; |
| • | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; and |
| • | the manufacturing process or facilities we use may not meet applicable requirements. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared product on a timely basis. For example, in response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) clearance process, the FDA initiated an evaluation, and in January 2011, announced several proposed actions intended to reform the clearance process. The FDA intends these reform actions to improve the efficiency and transparency of the clearance process, as well as bolster patient safety. In addition, as part of the Food and Drug Administration Safety and Innovation Act, or FDASIA, enacted in 2012, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance and approval. Some of these proposals and reforms could impose additional regulatory requirements upon us that could delay our ability to obtain new 510(k) clearances, increase the costs of compliance or restrict our ability to maintain our current clearances.
Even after we have obtained the proper regulatory clearance or approval to market a product, we have ongoing responsibilities under FDA regulations. The failure to comply with applicable regulations could jeopardize our ability to sell MRIdian and result in enforcement actions such as:
| • | warning letters; |
| • | fines; |
| • | injunctions; |
| • | civil penalties; |
| • | termination of distribution; |
61
Table of Contents
| • | recalls or seizures of products; |
| • | delays in the introduction of products into the market; |
| • | total or partial suspension of production; |
| • | refusal to grant future clearances or approvals; |
| • | withdrawals or suspensions of current clearances or approvals, resulting in prohibitions on sales of MRIdian; and |
| • | in the most serious cases, criminal penalties. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and harm our reputation, business, financial condition and results of operations.
In order to sell MRIdian in member countries of the European Economic Area, or EEA, MRIdian must comply with the essential requirements of the EU Medical Devices Directive (Council Directive 93/42/EEC). Compliance with these requirements is a prerequisite to be able to affix the Conformité Européene, or CE, mark to MRIdian, without which they cannot be sold or marketed in the EEA. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I with no measuring function and which are not sterile), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the EU Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a Member State of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a CE Certificate of Conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device (e.g., product labeling and instructions for use) are supported by suitable evidence. We received the CE mark for MRIdian in November 2014. If we fail to remain in compliance with applicable European laws and directives, we would not be able to continue to affix the CE mark to MRIdian, which would prevent us from selling MRIdian within the EEA. We will also need to obtain regulatory approval in other foreign jurisdictions in which we plan to market and sell MRIdian.
Modifications to MRIdian and our future products may require new 510(k) clearances or PMA approvals, or may require us to cease marketing or recall the modified products until clearances are obtained.
In the United States, we have obtained 510(k) premarket clearance from the FDA to market MRIdian for the provision of stereotactic radiosurgery and precision radiotherapy for lesions, tumors and conditions anywhere in the body where radiation treatment is indicated. Any modification to a 510(k)-cleared device that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, design or manufacture, requires a new 510(k) clearance or, possibly, approval of a PMA. The FDA requires every manufacturer to make this determination in the first instance, but the FDA may review any manufacturer’s decision. The FDA may not agree with our decisions regarding whether new clearances or approvals are necessary. We have made modifications to MRIdian in the past and have determined based on our review of the applicable FDA regulations and guidance that in certain instances new 510(k) clearances or PMA approvals were not required. We may make similar modifications or add additional features in the future that we believe do not require a new 510(k) clearance or approval of a PMA. If the FDA disagrees with our determination and requires us to submit new 510(k) notifications
62
Table of Contents
or PMA applications for modifications to our previously cleared products for which we have concluded that new clearances or approvals are unnecessary, we may be required to cease marketing or to recall the modified product until we obtain clearance or approval, and we may be subject to significant regulatory fines or penalties.
Furthermore, the FDA’s ongoing review of the 510(k) clearance process may make it more difficult for us to make modifications to our previously cleared products, either by imposing more strict requirements on when a new 510(k) notification for a modification to a previously cleared product must be submitted, or applying more onerous review criteria to such submissions. For example, the FDA is currently reviewing its guidance describing when it believes a manufacturer is obligated to submit a new 510(k) for modifications or changes to a previously cleared device. The FDA is expected to issue revised guidance to assist device manufacturers in making this determination. It is unclear whether the FDA’s approach in this new guidance will result in substantive changes to existing policy and practice regarding the assessment of whether a new 510(k) is required for changes or modifications to existing devices. The FDA continues to review its 510(k) clearance process, which could result in additional changes to regulatory requirements or guidance documents, which could increase the costs of compliance or restrict our ability to maintain current clearances.
If treatment guidelines for cancer radiation therapies change or the standard of care evolves, we may need to redesign and seek new marketing authorization from the FDA for MRIdian.
If treatment guidelines for cancer radiation therapies or the standard of care evolves, we may need to redesign MRIdian and seek new clearances or approvals from the FDA for MRIdian. Our 510(k) clearance from the FDA is based on current treatment guidelines. If treatment guidelines change so that different treatments become desirable, the clinical utility of MRIdian could be diminished and our business could suffer. For example, competition by other forms of cancer treatment, in particular personalized medicine approaches in targeting drugs and biologics, could reduce the use of radiation therapy as a standard of care in certain indications.
The misuse or off-label use of MRIdian may harm our reputation in the marketplace, result in injuries that lead to product liability suits or result in costly investigations, fines or sanctions by regulatory bodies if we are deemed to have engaged in the promotion of these uses, any of which could be costly to our business.
Clinicians or physicians may misuse MRIdian or use improper techniques if they are not adequately trained, potentially leading to injury and an increased risk of product liability. If MRIdian is misused or used with improper technique, we may become subject to costly litigation by our customers or their patients. Product liability claims could divert management’s attention from our core business, be expensive to defend and result in sizeable damage awards against us that may not be covered by insurance. In addition, any of the events described above could harm our business.
In addition, MRIdian has been cleared by the FDA for specific treatments. We train our marketing and direct sales force to not promote MRIdian for uses outside of the FDA-cleared indications for use, known as “off-label uses.” For example, MRIdian has not been indicated for diagnostic use. We cannot, however, prevent a physician from using MRIdian off-label, when in the physician’s independent professional medical judgment he or she deems it appropriate. There may be increased risk of injury to patients if physicians attempt to use MRIdian off-label. Furthermore, the use of MRIdian for indications other than those cleared by the FDA or approved by any foreign regulatory body may not effectively treat such conditions, which could harm our reputation in the marketplace among physicians and patients.
If the FDA or any foreign regulatory body determines that our promotional materials or training constitute promotion of an off-label use, it could request that we modify our training or promotional materials or subject us to regulatory or enforcement actions, including the issuance or imposition of an untitled letter, which is used for violators that do not necessitate a warning letter, injunction, seizure, civil fine or criminal penalties. It is also possible that other federal, state or foreign enforcement authorities might take action under other regulatory authority, such as false claims laws, if they consider our business activities to constitute promotion of an off-label use, which could result in significant penalties, including, but not limited to, criminal, civil and administrative penalties, damages, fines, disgorgement, exclusion from participation in government healthcare programs and the curtailment of our operations.
63
Table of Contents
Our MRIdian systems may cause or contribute to adverse medical events that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our MRIdian systems, or a recall of our MRIdian systems either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
We are subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that MRIdian may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of MRIdian. If we fail to comply with our reporting obligations, the FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device clearance, seizure of MRIdian or delay in clearance of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. For example, in January 2014, we initiated a correction of the system at Washington University in St. Louis due to a defect we identified in an advanced software feature in the treatment planning system of MRIdian. We promptly updated our software to resolve this defect and notified the FDA of this correction, but the FDA has not formally classified this correction as a recall. We cannot assure you that similar product defects or other errors will not occur in the future. Recalls involving MRIdian could be particularly harmful to our business, financial condition and results of operations because it is currently our only product.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for MRIdian in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales.
If we or our distributors do not obtain and maintain international regulatory registrations or approvals for MRIdian, we will not be able to market and sell MRIdian outside of the United States.
Sales of our devices outside the United States are subject to foreign regulatory requirements that vary widely from country to country. In addition, the FDA regulates exports of medical devices from the United States. While the regulations of some countries may not impose barriers to marketing and selling MRIdian or only require notification, others require that we or our distributors obtain the approval of a specified regulatory body. We have applied for and received regulatory approval in Europe, the United Arab Emirates, Taiwan and Italy, where regulatory approval is required in addition to the CE mark, and are seeking regulatory approval to market MRIdian in China, Japan, Canada, Russia, Hong Kong, Turkey and Korea. We currently have orders to deliver MRIdian to customers in the United States, Taiwan, Korea, Italy and the United Arab Emirates, which we include in our backlog due to the status of each sales order and our regulatory approval processes in these countries. Complying with foreign regulatory requirements, including obtaining registrations or approvals, can be expensive and time-consuming, and we cannot be certain that we or our distributors will receive regulatory approvals in each country in which we plan to market MRIdian or that we will be able to do so on a timely basis. The time required to obtain registrations or approvals, if required by other countries, may be longer than that required for FDA clearance, and requirements for such registrations or approvals may significantly differ from FDA requirements. If we modify MRIdian, we or our distributors may need to apply for additional regulatory approvals before we are permitted to sell the modified product. In addition, we may not continue to meet the quality and safety standards required to
64
Table of Contents
maintain the authorizations that we or our distributors have received. If we or our distributors are unable to maintain our authorizations in a particular country, we will no longer be able to sell MRIdian in that country, which could harm our business.
Regulatory approval by the FDA does not ensure approval by regulatory authorities in other countries, and approval by one or more foreign regulatory authorities does not ensure approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others.
We must manufacture MRIdian in accordance with federal and state regulations, and we could be forced to recall our installed systems or terminate production if we fail to comply with these regulations.
The methods used in, and the facilities used for, the manufacture of MRIdian must comply with the FDA’s Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of MRIdian. Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. MRIdian is also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing.
We cannot guarantee that we or any subcontractors will take the necessary steps to comply with applicable regulations, which could cause delays in the delivery of MRIdian. In addition, failure to comply with applicable FDA requirements or later discovery of previously unknown problems with MRIdian or manufacturing processes could result in, among other things:
| • | warning letters or untitled letters; |
| • | fines, injunctions or civil penalties; |
| • | suspension or withdrawal of approvals or clearances; |
| • | seizures or recalls of MRIdian; |
| • | total or partial suspension of production or distribution; |
| • | administrative or judicially imposed sanctions; |
| • | FDA’s refusal to grant pending or future clearances or approvals for MRIdian; |
| • | clinical holds; |
| • | refusal to permit the import or export of MRIdian; and |
| • | criminal prosecution of us or our employees. |
Any of these actions could significantly and negatively impact supply of MRIdian. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we could lose customers and suffer reduced revenue and increased costs.
Legislative or regulatory reforms in the United States or the EU may make it more difficult and costly for us to obtain regulatory clearances or approvals for MRIdian or to produce, market or distribute MRIdian after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in Congress that could significantly change the statutory provisions governing the regulation of medical devices or the reimbursement thereof. In addition, the FDA or Nuclear Regulatory Commission, or NRC, regulations and guidance are often revised or reinterpreted by the FDA or
65
Table of Contents
NRC in ways that may significantly affect our business and our MRIdian systems. For example, in response to industry and healthcare provider concerns regarding the predictability, consistency and rigor of the 510(k) clearance process, the FDA initiated an evaluation, and in January 2011, announced several proposed actions intended to reform the clearance process. In addition, as part of FDASIA, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance or approval. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to manufacture, market or distribute MRIdian or future products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require:
| • | additional testing prior to obtaining clearance or approval; |
| • | changes to manufacturing methods; |
| • | recall, replacement or discontinuance of MRIdian or future products; or |
| • | additional record keeping. |
Any of these changes could require substantial time and cost and could harm our business and our financial results.
In September 2012, the European Commission published proposals for the revision of the EU regulatory framework for medical devices. The proposal would replace the EU Medical Devices Directive and the Active Implantable Medical Devices Directive with a new regulation (the Medical Devices Regulation). Unlike the Directives that must be implemented into national laws, the Regulation would be directly applicable in all EEA Member States and so is intended to eliminate current national differences in regulation of medical devices.
In October 2013, the European Parliament approved a package of reforms to the European Commission’s proposals. Under the revised proposals, only designated “special notified bodies” would be entitled to conduct conformity assessments of high-risk devices. These special notified bodies will need to notify the European Commission when they receive an application for a conformity assessment for a new high-risk device. The European Commission will then forward the notification and the accompanying documents on the device to the Medical Devices Coordination Group, or MDCG (a new, yet to be created, body chaired by the European Commission, and representatives of EEA Member States), for an opinion. These new procedures may result in a longer or more burdensome assessment of our new products.
If finally adopted, the Medical Devices Regulation is expected to enter into force sometime in 2016 and become applicable three years thereafter. In its current form it would, among other things, also impose additional reporting requirements on manufacturers of high-risk medical devices, impose an obligation on manufacturers to appoint a “qualified person” responsible for regulatory compliance and provide for more strict clinical evidence requirements.
Our business involves the use of hazardous materials and we and our third-party manufacturers must comply with environmental laws and regulations, which may be expensive and restrict how we do business.
Our third-party manufacturers’ activities and our own activities involve the controlled storage, use and disposal of hazardous materials, including Cobalt-60, lead and depleted uranium. We and our manufacturers are subject to federal, state, local and foreign laws and regulations governing the use, generation, manufacture, storage, handling and disposal of these hazardous materials. We currently carry no insurance specifically covering environmental claims relating to the use of hazardous materials, but we do reserve funds to address these claims at both the federal and state levels. Although we believe that our safety procedures for handling and disposing of these materials and waste products comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental injury or contamination from the use, storage, handling or disposal of hazardous materials. In the event of an accident, state or federal or other applicable authorities may curtail our use of these materials and interrupt our business operations. In addition, if an accident or environmental discharge occurs, or if we discover contamination caused by prior operations, including by prior owners and operators of properties we acquire, we could be liable for
66
Table of Contents
cleanup obligations, damages and fines. If such unexpected costs are substantial, this could significantly harm our financial condition and results of operations.
If we are found to have violated laws protecting the confidentiality of patient health information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
There are a number of federal and state laws protecting the confidentiality of certain patient health information, including patient records, and restricting the use and disclosure of that protected information.
In particular, the U.S. Department of Health and Human Services has promulgated rules governing the privacy and security of individually identifiable health information under the Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, or HIPAA. These privacy and security rules protect medical records and other personal health information by limiting their use and disclosure, giving individuals the right to access, amend and seek accounting of their own health information, limiting most uses and disclosures of health information to the minimum amount reasonably necessary to accomplish the intended purpose, and requiring administrative, technical and physical safeguards. Although we are not a covered entity under HIPAA, we have entered into agreements with certain covered entity customers, such as health care providers, under which we are considered to be a “business associate” under HIPAA. As a business associate, we are contractually bound and may also be directly responsible under HIPAA, as amended by HITECH, to implement policies, procedures and reasonable and appropriate security measures to protect any individually identifiable health information we may create, receive, maintain or transmit on behalf of covered entities. We may also be subject to state laws protecting the confidentiality of medical records where those state laws have stricter provisions than HIPAA. Our failure to protect or secure any individually identifiable health information received on behalf of customers could subject us to civil and criminal liability, including the imposition of monetary fines, and adverse publicity, and could harm our business and impair our ability to attract new customers.
We are subject to federal and state fraud and abuse laws and health information privacy and security laws, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
There are numerous U.S. federal and state laws pertaining to healthcare fraud and abuse, including anti-kickback, false claims and physician transparency laws. Our relationships with providers and hospitals are subject to scrutiny under these laws. We may also be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual or furnishing or arranging for a good or service, for which payment may be made, in whole or in part, under federal healthcare programs, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act; |
| • | federal civil and criminal false claims laws and civil monetary penalty laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other third-party payors that are false or fraudulent; |
| • | HIPAA, which created federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| • | the federal physician sunshine requirements under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the Affordable |
67
Table of Contents
| Care Act, which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to the U.S. Department of Health and Human Services information related to payments and other transfers of value to physicians, which is defined broadly to include other healthcare providers and teaching hospitals and ownership and investment interests held by physicians and their immediate family members; |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; |
| • | state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; and |
| • | state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures. |
These laws, among other things, constrain our business, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers of medical devices. We have a variety of arrangements with our customers that could implicate these laws. Due to the breadth of these laws, the narrowness of statutory exceptions and safe harbors available, and the range of interpretations to which they are subject to, it is possible that some of our current or future practices might be challenged under one or more of these laws. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to, and thus could harm our business, financial condition and results of operations.
If our operations are found to be in violation of any of the laws described above or any other governmental regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment and the curtailment or restructuring of our operations, any of which could negatively impact our ability to operate our business and our results of operations.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, could harm our cash flows, financial condition and results of operations.
In March 2010, the Affordable Care Act was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other things, the Affordable Care Act:
| • | requires each medical device manufacturer to pay a sales tax equal to 2.3% of the price for which such manufacturer sells its medical devices; |
| • | establishes a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| • | implements payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| • | establishes an Independent Payment Advisory Board that will submit recommendations to reduce Medicare spending if projected Medicare spending exceeds a specified growth rate. |
In addition, other legislative changes have been proposed and adopted since the Affordable Care Act was enacted. On August 2, 2011, the Budget Control Act of 2011 was signed into law, which, among other things, created the Joint Select Committee on Deficit Reduction to recommend to Congress proposals in spending reductions. The Joint Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes reductions to Medicare payments to providers of 2% per fiscal year, which went into effect on April 1, 2013 and, due to subsequent legislative amendments to the statute, will remain in effect through 2024 unless additional
68
Table of Contents
Congressional action is taken. On January 2, 2013, the American Taxpayer Relief Act of 2012 was signed into law, which, among other things, reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
We expect that additional state and federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that federal and state governments will pay for healthcare products and services, which could result in reduced demand for MRIdian or additional pricing pressure.
Risks Related to Ownership of Our Common Stock
The price of our Common Stock may be volatile and may be influenced by numerous factors, some of which are beyond our control.
Factors that could cause volatility in the market price of our Common Stock include, but are not limited to:
| • | actual or anticipated fluctuations in our financial condition and operating results; |
| • | actual or anticipated changes in our growth rate relative to our competitors; |
| • | commercial success and market acceptance of MRIdian; |
| • | success of our competitors in discovering, developing or commercializing products; |
| • | ability to commercialize or obtain regulatory approval for MRIdian, or delays in commercializing or obtaining regulatory approval; |
| • | strategic transactions undertaken by us; |
| • | additions or departures of key personnel; |
| • | product liability claims; |
| • | prevailing economic conditions; |
| • | disputes concerning our intellectual property or other proprietary rights; |
| • | FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry; |
| • | healthcare reform measures in the United States; |
| • | sales of our Common Stock by our officers, directors or significant stockholders; |
| • | future sales or issuances of equity or debt securities by us; |
| • | business disruptions caused by earthquakes, tornadoes or other natural disasters; and |
| • | issuance of new or changed securities analysts’ reports or recommendations regarding us. |
In addition, the stock markets in general, and the markets for medical device companies in particular, have experienced extreme volatility that have been often unrelated to the operating performance of the issuer. These broad market fluctuations may negatively impact the price or liquidity of our Common Stock. In the past, when the price of a stock has been volatile, holders of that stock have sometimes instituted securities class action litigation against the issuer. If any of our stockholders were to bring such a lawsuit against us, we could incur substantial costs defending the lawsuit and the attention of our management would be diverted from the operation of our business.
69
Table of Contents
We are an “emerging growth company” and we cannot be certain if the reduced disclosure requirements applicable to emerging growth companies will make our Common Stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act, and may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We cannot predict if investors will find our Common Stock less attractive because we may rely on these exemptions. If some investors find our Common Stock less attractive as a result, there may be a less active trading market for our Common Stock and our stock price may be more volatile.
In addition, Section 102 of the JOBS Act also provides that an “emerging growth company” can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act of 1933, as amended, or the Securities Act, for complying with new or revised accounting standards. An “emerging growth company” can therefore delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. However, we are choosing to “opt out” of such extended transition period, and as a result, we will comply with new or revised accounting standards on the relevant dates on which adoption of such standards is required for non-emerging growth companies. Section 107 of the JOBS Act provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
Future sales of our Common Stock or securities convertible or exchangeable for our Common Stock may cause our stock price to decline.
If our existing stockholders or optionholders sell, or indicate an intention to sell, substantial amounts of our Common Stock in the public market after the lock-up and legal restrictions on resale lapse, the price of our Common Stock could decline. The perception in the market that these sales may occur could also cause the price of our Common Stock to decline. Based on shares of ViewRay common stock outstanding at June 30, 2015, we have outstanding a total of 37,656,288 shares of Common Stock.
In addition, based on the number of shares subject to outstanding awards under our 2008 Stock Option and Incentive Plan, or 2008 Plan, or available for issuance thereunder, at June 30, 2015, and including the initial reserves under our 2015 Equity Incentive Award Plan, or 2015 Plan, 9,238,026 shares of common stock that are either subject to outstanding options, outstanding but subject to vesting or reserved for future issuance under the 2008 Plan or 2015 Plan will become eligible for sale in the public market to the extent permitted by the provisions of various vesting schedules, the lock-up agreements and Rule 144 and Rule 701 under the Securities Act. We also plan to file a registration statement permitting shares of common stock issued in the future pursuant to the 2008 Plan and 2015 Plan to be freely resold by plan participants in the public market, subject to the lock-up agreements, applicable vesting schedules and, for shares held by directors, executive officers and other affiliates, volume limitations under Rule 144 under the Securities Act. The 2015 Plan contains provisions for the annual increase of the number of shares reserved for issuance under such plan, as described elsewhere in this report, which shares we also intend to register. If the shares we may issue from time to time under the 2008 Plan or 2015 Plan are sold, or if it is perceived that they will be sold, by the award recipients in the public market, the price of our Common Stock could decline.
Holders of our Common Stock, including shares issuable upon exercise of our Common Stock warrants, are entitled to rights with respect to the registration of their shares under the Securities Act. Registration of these shares under the Securities Act would result in the shares becoming freely tradable without restriction under the Securities Act, except for shares purchased by affiliates, subject to the lock-up agreements described above in the section entitled “The Merger and Related Transactions—Lock-up Agreements and Other Restrictions”. Sales of such shares could also cause the price of our Common Stock to decline.
70
Table of Contents
You may experience dilution of your ownership interests because of the future issuance of additional shares of our common or preferred stock or other securities that are convertible into or exercisable for our common or preferred stock.
In the future, we may issue our authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our present stockholders and the purchasers of our Common Stock offered hereby. We are authorized to issue an aggregate of 300,000,000 shares of common stock and 10,000,000 shares of “blank check” preferred stock. We may issue additional shares of our Common Stock or other securities that are convertible into or exercisable for our Common Stock in connection with hiring or retaining employees, future acquisitions, future sales of our securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of our Common Stock may create downward pressure on the trading price of the common stock. We may need to raise additional capital in the near future to meet our working capital needs, and there can be no assurance that we will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with these capital raising efforts, including at a price (or exercise prices) below the price you paid for your stock.
There may never be an active, liquid and orderly trading market for our Common Stock, which may make it difficult for you to sell your shares of our Common Stock.
Our Common Stock is quoted on OTC Markets, OTCQB tier of OTC Markets Group Inc., an over-the-counter quotation system. In that venue, our stockholders may find it difficult to obtain accurate quotations as to the market value of their shares of our Common Stock, and may find few buyers to purchase their stock and few market makers to support its price. As a result of these and other factors, you may be unable to resell your shares of our Common Stock at or above the price for which you purchased them, at or near quoted bid prices, or at all. Further, an inactive market may also impair our ability to raise capital by selling additional equity in the future, and may impair our ability to enter into strategic partnerships or acquire companies or products by using shares of our Common Stock as consideration.
We intend to list shares of our Common Stock on a national securities exchange in the future, but we do not now, and may not in the future, meet the initial listing standards of any national securities exchange, which is often a more widely-traded and liquid market. Some, but not all, of the factors which may delay or prevent the listing of our Common Stock on a more widely-traded and liquid market include the following: our stockholders’ equity may be insufficient; the market value of our outstanding securities may be too low; our net income from operations may be too low; our Common Stock may not be sufficiently widely held; we may not be able to secure market makers for our Common Stock; and we may fail to meet the rules and requirements mandated by the several exchanges and markets to have our Common Stock listed. Should we fail to satisfy the initial listing standards of the national exchanges, or our Common Stock is otherwise rejected for listing, and remains listed on the OTC Markets or is suspended from the OTC Markets, the trading price of our Common Stock could suffer and the trading market for our Common Stock may be less liquid and our Common Stock price may be subject to increased volatility.
Our Common Stock is or may become subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
Rule 15g-9 under the Exchange Act establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require: (a) that a broker or dealer approve a person’s account for transactions in penny stocks; and (b) the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
71
Table of Contents
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must: (a) obtain financial information and investment experience objectives of the person and (b) make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form: (a) sets forth the basis on which the broker or dealer made the suitability determination; and (b) confirms that the broker or dealer received a signed, written agreement from the investor prior to the transaction. Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. If our Common Stock is or becomes subject to the “penny stock” rules, it may be more difficult for investors to dispose of our Common Stock and cause a decline in the market value of our Common Stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker or dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Our operating results for a particular period may fluctuate significantly or may fall below the expectations of investors or securities analysts, each of which may cause our stock price to fluctuate or decline.
We expect our operating results to be subject to fluctuations. Our operating results will be affected by numerous factors, including:
| • | variations in the level of expenses related to MRIdian or future development programs; |
| • | level of underlying demand for MRIdian and any other products we develop; |
| • | addition or termination of clinical trials or funding support; |
| • | receipt, modification or termination of government contracts or grants, and the timing of payments we receive under these arrangements; |
| • | our execution of any collaborative, licensing or similar arrangements, and the timing of payments we may make or receive under these arrangements; |
| • | any intellectual property infringement lawsuit or opposition, interference or cancellation proceeding in which we may become involved; and |
| • | regulatory developments affecting MRIdian or our competitors. |
If our operating results for a particular period fall below the expectations of investors or securities analysts, the price of our Common Stock could decline substantially. Furthermore, any fluctuations in our operating results may, in turn, cause the price of our Common Stock to fluctuate substantially. We believe that comparisons of our financial results from various reporting periods are not necessarily meaningful and should not be relied upon as an indication of our future performance.
72
Table of Contents
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
Based on the beneficial ownership of our Common Stock at July 16, 2015, after giving effect to the purchase of common stock in the initial closing of the Offering, our officers and directors, together with holders of 5% or more of our outstanding common stock before the Offering and their respective affiliates, will beneficially own approximately 66.4% of our Common Stock. Accordingly, these stockholders will continue to have significant influence over the outcome of corporate actions requiring stockholder approval, including the election of directors, merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. The interests of these stockholders may not be the same as or may even conflict with your interests. For example, these stockholders could delay or prevent a change in control of the company, even if such a change in control would benefit our other stockholders, which could deprive our stockholders of an opportunity to receive a premium for their common stock as part of a sale of the company or our assets and might affect the prevailing price of our Common Stock. The significant concentration of stock ownership may negatively impact the price of our Common Stock due to investors’ perception that conflicts of interest may exist or arise.
Shares of our Common Stock that have not been registered under federal securities laws are subject to resale restrictions imposed by Rule 144, including those set forth in Rule 144(i) which apply to a former “shell company.”
Prior to the closing of the Merger, we were deemed a “shell company” under applicable SEC rules and regulations because we had no or nominal operations and either no or nominal assets, assets consisting solely of cash and cash equivalents, or assets consisting of any amount of cash and cash equivalents and nominal other assets. Pursuant to Rule 144 promulgated under the Securities Act of 1933, as amended, or the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from the date on which this Report, reflecting our status as a non-shell company, is filed with the SEC and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Form 8-K reports. We intend to register such shares for sale under the Securities Act, but are currently a “voluntary filer” and are not subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act. As a result, unless we register such shares for sale under the Securities Act, most of our stockholders will be forced to hold their shares of our Common Stock for at least that 12-month period before they are eligible to sell those shares, and even after that 12-month period, sales may not be made under Rule 144 unless we and the selling stockholders are in compliance with other requirements of Rule 144. Further, it will be more difficult for us to raise funding to support our operations through the sale of debt or equity securities unless we agree to register such securities under the Securities Act, which could cause us to expend significant time and cash resources. Additionally, our previous status as a shell company could also limit our use of our securities to pay for any Mergers we may seek to pursue in the future (although none are currently planned). The lack of liquidity of our securities as a result of the inability to sell under Rule 144 for a longer period of time than a non-former shell company could cause the market price of our securities to decline.
Provisions of our charter documents or Delaware law could delay or prevent an acquisition of the company, even if such an acquisition would be beneficial to our stockholders, which could make it more difficult for you to change management.
Provisions in our certificate of incorporation and our bylaws may discourage, delay or prevent a merger, acquisition or other change in control that stockholders may consider favorable, including transactions in which stockholders might otherwise receive a premium for their shares. In addition, these provisions may frustrate or prevent any attempt by our stockholders to replace or remove our current management by making it more difficult to replace or remove our board of directors. These provisions include:
| • | a classified board of directors so that not all directors are elected at one time; |
| • | a prohibition on stockholder action through written consent; |
| • | no cumulative voting in the election of directors; |
73
Table of Contents
| • | the exclusive right of our board of directors to elect a director to fill a vacancy created by the expansion of the board of directors or the resignation, death or removal of a director; |
| • | a requirement that special meetings of stockholders be called only by the board of directors, the chairman of the board of directors, the chief executive officer or, in the absence of a chief executive officer, the president; |
| • | an advance notice requirement for stockholder proposals and nominations; |
| • | the authority of our board of directors to issue preferred stock with such terms as our board of directors may determine; and |
| • | a requirement of approval of not less than 66 2/3% of all outstanding shares of our capital stock entitled to vote to amend any bylaws by stockholder action, or to amend specific provisions of our certificate of incorporation. |
In addition, Delaware law prohibits a publicly held Delaware corporation from engaging in a business combination with an interested stockholder, generally a person who, together with its affiliates, owns, or within the last three years has owned, 15% or more of our voting stock, for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. Accordingly, Delaware law may discourage, delay or prevent a change in control of the company. Furthermore, our certificate of incorporation will specify that the Court of Chancery of the State of Delaware will be the sole and exclusive forum for most legal actions involving actions brought against us by stockholders. We believe this provision benefits us by providing increased consistency in the application of Delaware law by chancellors particularly experienced in resolving corporate disputes, efficient administration of cases on a more expedited schedule relative to other forums and protection against the burdens of multi-forum litigation. However, the provision may have the effect of discouraging lawsuits against our directors and officers. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any applicable action brought against us, a court could find the choice of forum provisions contained in our certificate of incorporation to be inapplicable or unenforceable in such action.
Provisions in our charter documents and other provisions of Delaware law could limit the price that investors are willing to pay in the future for shares of our Common Stock.
We do not anticipate paying any cash dividends on our Common Stock in the foreseeable future; therefore, capital appreciation, if any, of our Common Stock will be your sole source of gain for the foreseeable future.
We have never declared or paid cash dividends on our Common Stock. We do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We currently intend to retain all available funds and any future earnings to fund the development and growth of our business. In addition, our current loan and security agreement with CRG contains, and our future loan arrangements may contain, terms prohibiting or limiting the amount of dividends that may be declared or paid on our Common Stock. As a result, capital appreciation, if any, of our Common Stock will be your sole source of gain for the foreseeable future.
If securities or industry analysts do not publish research, or publish inaccurate or unfavorable research, about our business, our stock price and trading volume could decline.
The trading market for our Common Stock will depend, in part, on the research and reports that securities or industry analysts publish about us or our business. Securities and industry analysts do not currently, and may never, publish research on the company. If no securities or industry analysts commence coverage of the company, the price for our Common Stock could be negatively impacted. In the event securities or industry analysts initiate coverage, if one or more of the analysts who cover us downgrade our Common Stock or publish inaccurate or unfavorable research about our business, our stock price could decline. In addition, if our operating results fail to meet the forecast of analysts, our stock price could decline. If one or more of these analysts cease coverage of the company or fail to publish reports on us regularly, demand for our Common Stock could decrease, which might cause our stock price and trading volume to decline.
74
Table of Contents
* * *
The risks above do not necessarily comprise all of those associated with an investment in the Company. This Report contains forward looking statements that involve unknown risks, uncertainties and other factors that may cause the actual results, financial condition, performance or achievements of the Company to be materially different from any future results, performance or achievements expressed or implied by such forward looking statements. Factors that might cause such a difference include, but are not limited to, those set out above.
75
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following management’s discussion and analysis should be read in conjunction with the historical financial statements and the related notes thereto contained in this report. The management’s discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect” and the like, and/or future tense or conditional constructions (“will,” “may,” “could,” “should,” etc.), or similar expressions, identify certain of these forward-looking statements. These forward-looking statements are subject to risks and uncertainties, including those under “Risk Factors” in this Report, that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. The Company’s actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors. The Company does not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this report.
On July 23, 2015, our wholly-owned subsidiary, Vesuvius Acquisition Corp., a corporation formed in the State of Delaware on July 16, 2015, or the Acquisition Sub, merged with and into ViewRay Technologies, Inc., a corporation incorporated in 2004 in the state of Florida originally under the name of ViewRay Incorporated, subsequently reincorporated in Delaware in 2007, referred to as ViewRay. Pursuant to this transaction, ViewRay was the surviving corporation and became our wholly-owned subsidiary. All of the outstanding stock of ViewRay was converted into shares of our Common Stock.
In connection with the Merger and pursuant to the Split-Off Agreement, we transferred our pre-Merger assets and liabilities to our pre-Merger majority stockholder, in exchange for the surrender by her and cancellation of 4,150,171 shares of our Common Stock.
As a result of the Merger and Split-Off, we discontinued our pre-Merger business and acquired the business of ViewRay and will continue the existing business operations of ViewRay as a publicly-traded company under the name ViewRay, Inc.
As the result of the Merger and the change in business and operations of the Company, a discussion of the past financial results of the Company is not pertinent, and under applicable accounting principles the historical financial results of ViewRay, the accounting acquirer, prior to the Merger are considered the historical financial results of the Company.
The following discussion highlights ViewRay’s results of operations and the principal factors that have affected our financial condition as well as our liquidity and capital resources for the periods described, and provides information that management believes is relevant for an assessment and understanding of the statements of financial condition and results of operations presented herein. The following discussion and analysis are based on ViewRay’s audited and unaudited financial statements contained in this Report, which we have prepared in accordance with United States generally accepted accounting principles. You should read the discussion and analysis together with such financial statements and the related notes thereto.
Basis of Presentation
The audited financial statements of ViewRay for the fiscal years ended December 31, 2014 and 2013, and the unaudited condensed financial statements of ViewRay for the three months ended March 31, 2015, contained herein include a summary of our significant accounting policies and should be read in conjunction with the discussion below. In the opinion of management, all material adjustments necessary to present fairly the results of operations for such periods have been included in these audited and unaudited financial statements. All such adjustments are of a normal recurring nature.
Company Overview
We design, manufacture and market MRIdian, the first and only MRI-guided radiation therapy system to image and treat cancer patients simultaneously. MRI is a broadly used imaging tool that has the ability to differentiate between
76
Table of Contents
types of soft tissue clearly, unlike X-ray or computed tomography, or CT, which are the most commonly used imaging technologies in radiation therapy today. MRIdian integrates MRI technology, radiation delivery and our proprietary software to locate, target and track the location and shape of soft-tissue tumors while radiation is delivered. These capabilities allow MRIdian to accurately deliver radiation to the tumor while reducing the amount delivered to healthy tissue, as compared to other radiation therapy treatments today. We believe this leads to improved patient outcomes and reduced side effects from off-target radiation delivery.
We received initial 510(k) marketing clearance from the FDA for our treatment planning and delivery software in January 2011 and for MRIdian in May 2012. We also received permission to affix the CE mark in November 2014, allowing MRIdian to be sold within the European Economic Area. At June 30, 2015, over 234 patients had received radiation treatment on MRIdian systems at three cancer centers located at Washington University in St. Louis, University of California, Los Angeles and the University of Wisconsin—Madison.
We currently market MRIdian through a direct sales force in the United States and distributors in the rest of the world. We market MRIdian to a broad range of worldwide customers, including university research and teaching hospitals, community hospitals, private practices, government institutions and freestanding cancer centers. Our sales and revenue cycle varies based on the customer and can be lengthy, sometimes lasting up to 18 to 24 months or more from initial customer contact to sales contract execution. Following execution of a sales contract, it generally takes nine to 12 months for a customer to customize an existing facility or construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures.
We generated product and service revenue of $0.5 million and $3.3 million and had net losses of $20.6 million and $16.6 million during the six months ended June 30, 2015 and 2014, respectively. At June 30, 2015, we had 13 signed orders representing backlog value of $71.9 million, of which we expect to recognize approximately 15% to 29% as revenue in the remainder of 2015 representing two to four MRIdian systems.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We expect our expenses will increase substantially in connection with our ongoing activities, as we:
| • | add personnel to support our product development and commercialization efforts; |
| • | continue our research and development efforts; |
| • | seek regulatory approval for MRIdian in foreign countries; and |
| • | operate as a public company. |
Accordingly, we may seek to fund our operations through public or private equity or debt financings or other sources. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and our ability to develop enhancements to and integrate new technologies into MRI-guided radiation therapy systems.
Backlog
We define backlog as the accumulation of all orders for which revenue has not been recognized and we consider valid. Backlog includes customer deposits received which are recorded as a liability on the balance sheet. Orders may be revised or cancelled according to their terms or upon mutual agreement between the parties. Therefore, it is difficult to predict with certainty the amount of backlog that will ultimately result in revenue. The determination of backlog includes objective and subjective judgment about the likelihood of an order contract becoming revenue. We perform a quarterly review of backlog to verify that outstanding orders in backlog remain valid, and based upon this review, orders that are no longer expected to result in revenue are removed from backlog. Our criteria include an outstanding and effective written agreement for the delivery of a MRIdian signed by customers, receipt of a minimum customer deposit or a letter of credit, any changes in customer or distributor plans or financial conditions, the customer’s or distributor’s continued intent and ability to fulfill the order contract, changes to regulatory requirements, the status of regulatory approval required in the customer’s jurisdiction, if any, or reasons for cancellation of order contracts.
77
Table of Contents
During 2014 and the six months ended June 30, 2015, we executed new sales contracts with a total value of $37.6 million and $17.2 million, respectively. At December 31, 2014, we had 10 signed sales contracts for MRIdian systems in backlog with a total value of $54.7 million, of which we expected to recognize approximately 40% to 60% as revenue in the 2015 representing four to six MRIdian systems. At June 30, 2015, we had 13 signed sales contracts for MRIdian systems in backlog with a total value of $71.9 million, of which we expect to recognize approximately 15% to 29% as revenue in the remainder of 2015 representing two to four MRIdian systems.
Components of Statements of Operations
Revenue
Product Revenue. Product revenue consists of sales of MRIdian systems, as well as optional components, such as additional planning workstations and body coils.
Following execution of a sales contract, it generally takes nine to 12 months for a customer to customize an existing facility or construct a new vault. Upon the commencement of installation at a customer’s facility, it typically takes two to three months to complete the installation and on-site testing of the system, including the completion of acceptance test procedures. On-site training takes approximately one week and can be conducted concurrent with installation and acceptance testing. Sales contracts generally include customer deposits upon execution of the agreement, and in certain cases, additional amounts due at shipment or commencement of installation, and final payment due generally upon customer acceptance.
Service Revenue. We generally offer maintenance service at no cost to customers to cover parts, labor and maintenance for one to two years. In addition, we offer multi-year, post-installation maintenance and support contracts that provide various levels of service support, which enables our customers to select the level of on-going support services, including parts and labor, which they require. These post-installation contracts are for a period of one to five years and provide services ranging from 24/7 on-site parts, labor and preventative maintenance to labor only with a longer response time. We also offer technology upgrades to our MRIdian systems, when and if available, for an additional fee. Service revenue is recognized on a straight-line basis over the term during which the contracted services are provided.
Cost of Revenue
Product Cost of Revenue. Product cost of revenue primarily consists of the cost of materials, installation and services associated with the manufacture and installation of MRIdian systems, as well as medical device excise tax and royalty payments to the University of Florida Research Foundation. Product cost of revenue also includes lower of cost or market inventory, or LCM, adjustments if the carrying value of the inventory is greater than its net realizable value. For strategic reasons, we sold our initial MRIdian systems prior to the receipt of FDA marketing clearance at prices lower than our projected costs to manufacture and install. As we accumulated materials, installation and other costs for these systems, we regularly assessed the carrying value of the related inventory value and recorded charges, or LCM adjustments, to reduce inventory to the lower of cost and net realizable value. The remaining realizable value of inventory was charged to product cost of revenue as those initial sites were completed and accepted. This resulted in LCM charges of $308 thousand and $598 thousand for the six months period ended June 30, 2015 and 2014, respectively, and of $4.6 million in 2013 and $0.6 million in 2014. During 2013 and 2014, LCM charges increased as we progressed with the initial installations of three MRIdian systems, and we expect our LCM charges to decrease as we raise our selling prices and reduce our manufacturing costs.
We expect our materials, installation and service costs to decrease as we continue to scale our operations, improve product designs and work with our third-party suppliers to lower costs.
Service Cost of Revenue. Service cost of revenue is comprised primarily of personnel costs, training and travel expenses to service and maintenance of installed MRIdian systems. Service cost of revenue also includes the costs of replacement parts under maintenance and support contracts.
78
Table of Contents
Operating Expenses
Research and Development. Research and development expenses consist primarily of compensation and related costs for personnel, including stock-based compensation, employee benefits and travel. Other significant research and development costs arise from development, manufacturing and commercialization of MRIdian. These costs consist of third-party consulting services, laboratory supplies, research materials, medical equipment, computer equipment and licensed technology, and related depreciation and amortization. We expense research and development expenses as incurred. As we continue to invest in improving MRIdian and developing new technologies, we expect research and development expenses to increase in absolute dollars.
Selling and Marketing. Selling and marketing expenses consist primarily of compensation and related costs for personnel, including stock-based compensation, employee benefits and travel associated with our selling and marketing organization, including our direct sales force and sales management and our marketing and customer support personnel. Selling and marketing expenses also include costs related to trade shows and marketing programs. We expense selling and marketing costs as incurred. We expect selling and marketing expenses to increase in future periods as we expand our sales force and our marketing and customer support organization and increase our participation in trade shows and marketing programs.
General and Administrative. Our general and administrative expenses consist primarily of compensation and related costs for personnel, including stock-based compensation, employee benefits and travel, for our finance, human resources, regulatory and other administrative personnel. In addition, general and administrative expenses include third-party consulting, legal, audit, accounting services, quality and regulatory functions and facilities costs, and gain or loss on the disposal of property and equipment. We expect general and administrative expenses to increase in absolute dollars following the consummation of the Merger due to additional legal, accounting, insurance, investor relations and other costs associated with being a public company, as well as other costs associated with growing our business.
Interest Income
Interest income consists primarily of interest income received on our cash and cash equivalents.
Interest Expense
Interest expense consists primarily of interest and amortization of the debt discount related to our convertible promissory notes issued in 2014 and long-term debt with Hercules Technology III, L.P. and Hercules Technology Growth Capital, Inc., or together, Hercules.
Other Income (Expense), Net
Other income (expense), net consists primarily of foreign currency exchange gains and losses and changes in the fair value of a convertible preferred stock warrant.
The outstanding convertible stock warrant is re-measured to fair value at each balance sheet date with the corresponding gain or loss from the adjustment recorded as a component of other income (expense), net.
We recorded adjustments to the fair value of the convertible preferred stock warrant until the conversion of the underlying convertible preferred stock into common stock upon consummation of the Merger, at which time the liability was reclassified to additional paid-in capital.
79
Table of Contents
Results of Operations
The following tables set forth our results of operations for the periods presented (in thousands):
| Six Months Ended June 30, | Years Ended December 31, | |||||||||||||||
| 2015 | 2014 | 2014 | 2013 | |||||||||||||
| Revenue: |
||||||||||||||||
| Product |
$ | 99 | $ | 3,267 | $ | 5,988 | $ | 2,253 | ||||||||
| Service |
363 | 70 | 411 | 12 | ||||||||||||
| Grant |
— | — | — | 894 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
462 | 3,337 | 6,399 | 3,159 | ||||||||||||
| Cost of revenue: |
||||||||||||||||
| Product |
545 | 5,105 | 8,176 | 8,173 | ||||||||||||
| Service |
1,065 | 255 | 975 | 14 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cost of revenue |
1,610 | 5,360 | 9,151 | 8,187 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross margin |
(1,148 | ) | (2,023 | ) | (2,752 | ) | (5,028 | ) | ||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
4,506 | 5,160 | 9,404 | 8,780 | ||||||||||||
| Selling and marketing |
2,191 | 2,579 | 4,681 | 3,781 | ||||||||||||
| General and administrative |
11,497 | 5,912 | 14,742 | 9,508 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses: |
18,194 | 13,651 | 28,827 | 22,069 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(19,342 | ) | (15,674 | ) | (31,579 | ) | (27,097 | ) | ||||||||
| Interest income |
1 | — | 1 | 4 | ||||||||||||
| Interest expense |
(1,323 | ) | (980 | ) | (2,243 | ) | (97 | ) | ||||||||
| Other income (expense), net |
35 | 43 | 21 | (32 | ) | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss before provision for income taxes |
(20,629 | ) | (16,611 | ) | (33,800 | ) | (27,222 | ) | ||||||||
| Provision for income taxes |
— | — | — | — | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (20,629 | ) | $ | (16,611 | ) | $ | (33,800 | ) | $ | (27,222 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
80
Table of Contents
Comparison of the Six Months Ended June 30, 2015 and 2014
Revenue
| Six Months Ended June 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Product |
$ | 99 | $ | 3,267 | $ | (3,168 | ) | |||||
| Service |
363 | 70 | 293 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
$ | 462 | $ | 3,337 | $ | (2,875 | ) | |||||
|
|
|
|
|
|
|
|||||||
Total revenue during the six months ended June 30, 2015 decreased $2.9 million compared to the six months ended June 30, 2014, primarily due to a $3.3 million system revenue recognized in June 2014 related to University of California, Los Angeles.
Product Revenue. Product revenue during the six months ended June 30, 2015 decreased $3.2 million compared to the six months ended June 30, 2014. This decrease was due to $3.2 million system sold to University of California, Los Angeles in June 2014 compared to $99 thousand component part sold to University of Wisconsin-Madison at June 2015.
Service Revenue. Service revenue during the six months ended June 30, 2015 increased $0.3 million compared to the six months ended June 30, 2014. This increase was due to maintenance service revenue recognized for additional MRIdian system installed at each of University of California, Los Angeles and the University of Wisconsin—Madison subsequent to the quarter ended June 30, 2014, in addition to MRIdian system installed at Washington University in St. Louis in 2013.
Cost of Revenue
| Six Months Ended June 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Product |
$ | 545 | $ | 5,105 | $ | (4,560 | ) | |||||
| Service |
1,065 | 225 | 810 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cost of revenue |
$ | 1,610 | $ | 5,360 | $ | (3,750 | ) | |||||
|
|
|
|
|
|
|
|||||||
Product Cost of Revenue. Product cost of revenue decreased $4.6 million during the six months ended June 30, 2015 compared to the six months ended June 30, 2014 primarily due to MRIdian system sold to University of California, Los Angeles in June 2014 compared to component part sold to University of Wisconsin-Madison in 2015. There was a $308 thousand LCM adjustment during the six months ended June 30, 2015 compared to $598 thousand during the six months ended June 30, 2014. The LCM adjustment decrease primarily resulted from the increase in the selling prices of our MRIdian system and decrease in our product costs through a continued effort to improve product design and supply chain management.
We currently expect that margins on current orders will continue this trend and show improvements from historic margins.
Service Cost of Revenue. Service cost of revenue during the six months ended June 30, 2015 increased $0.8 million, compared to the six months ended June 30, 2014. The increase in service cost of revenue was due to the provisioning of services for additional MRIdian system installed at each of University of California, Los Angeles and the University of Wisconsin—Madison subsequent to the quarter ended June 30, 2014, in addition to MRIdian system installed at Washington University in St. Louis in 2013.
Operating Expenses
| Six Months Ended June 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Research and development |
$ | 4,506 | $ | 5,160 | $ | (654 | ) | |||||
| Selling and marketing |
2,191 | 2,579 | (388 | ) | ||||||||
| General and administrative |
11,497 | 5,912 | 5,585 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
$ | 18,194 | $ | 13,651 | $ | 4,543 | ||||||
|
|
|
|
|
|
|
|||||||
81
Table of Contents
Research and Development. Research and development expenses during the six months ended June 30, 2015 decreased $0.7 million, compared to the six months ended June 30, 2014. This decrease was primarily attributable to a $0.7 million decrease in personnel costs and travel expenses.
Selling and Marketing. Selling and marketing expenses during the six months ended June 30, 2015 decreased $0.4 million, compared to the six months ended June 30, 2014. This decrease was primarily due to a $0.3 million decrease in trade show and related activities expenses.
General and Administrative. General and administrative expenses during the six months ended June 30, 2015 increased $5.6 million, compared to the six months ended June 30, 2014. This increase was primarily attributable to a $2.9 million write-off of deferred IPO financing cost in June 2015, $0.5 million increase in accounting and legal fees related to our planned initial public offering, a $1.3 million increase in personnel and related costs as a result of higher employee headcount, and a $0.5 million increase in facility costs as we leased new office space in September 2014.
Interest Expense
| Six Months Ended June 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Interest expense |
$ | (1,323 | ) | $ | (980 | ) | $ | (343 | ) | |||
Interest expense increased $0.3 million during the six months ended June 30, 2015, which was primarily due to full amortization of unamortized discount and penalties paid upon early termination of Hercules debt in June 2015.
Other Income (Expense), Net
| Six Months Ended June 30, | ||||||||||||
| 2015 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Other income (expense), net |
$ | 35 | $ | 43 | $ | (8 | ) | |||||
Other income (expense), net during the six months ended June 30, 2015 consisted primarily of the change in fair value of our convertible preferred stock warrant liability.
Comparison of the Years Ended December 31, 2014 and 2013
Revenue
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Product |
$ | 5,988 | $ | 2,253 | $ | 3,735 | ||||||
| Service |
411 | 12 | 399 | |||||||||
| Grant |
— | 894 | (894 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
$ | 6,399 | $ | 3,159 | $ | 3,240 | ||||||
|
|
|
|
|
|
|
|||||||
Total revenue in 2014 increased $3.2 million compared to 2013, primarily due to an increase in product revenue of $3.7 million and an increase in service revenue of $0.4 million, partially offset by a $0.9 million decrease in grant revenue due to expiration of the Grant Agreement with the State of Ohio in 2013.
82
Table of Contents
Product Revenue. Product revenue in 2014 increased $3.7 million compared to 2013. This increase was due to the acceptance of a MRIdian system at each of University of California, Los Angeles and the University of Wisconsin—Madison in 2014, partially offset by the acceptance of a MRIdian system at Washington University in St. Louis in 2013.
Service Revenue. Service revenue in 2014 increased $0.4 million compared to 2013. This increase was due to maintenance service revenue recognized for the MRIdian system installed at Washington University in St. Louis in 2013 and the MRIdian system installed at each of University of California, Los Angeles and the University of Wisconsin—Madison in 2014.
Cost of Revenue
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Product cost of revenue |
$ | 8,176 | $ | 8,173 | $ | 3 | ||||||
| Service cost of revenue |
975 | 14 | 961 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cost of revenue |
$ | 9,151 | $ | 8,187 | $ | 964 | ||||||
|
|
|
|
|
|
|
|||||||
Product Cost of Revenue. The change in product cost of revenue in 2014 was insignificant compared to 2013. Product cost of revenue in 2013 consisted of $3.6 million in inventory costs related to customer acceptance of a MRIdian system at Washington University in St. Louis and LCM adjustments of $4.6 million. In 2014, product cost of revenue consisted primarily of the release of inventory costs of $5.6 million for accepted MRIdian systems at University of California, Los Angeles and University of Wisconsin—Madison, additional site preparation and installation costs incurred in 2014 of $2.0 million and LCM adjustments of $0.6 million. The LCM adjustments in 2014 decreased $4.0 million compared to 2013 as a result of increase in the selling prices of our MRIdian system and decrease in our product costs through a continued effort to improve product design and supply chain management.
We currently expect that margins on current orders will continue this trend and show improvements from historic margins. We expect to achieve cost savings of approximately $1.0 million per current system from the initial MRIdian systems installed in 2013 and 2014. We believe that the combination of higher system prices and lower projected inventory costs as we increase our sales volume and leverage our supplier relationships will enable us to continue to improve our margins.
Service Cost of Revenue. Service cost of revenue in 2014 increased $0.9 million, compared to 2013. The increase in service cost of revenue was due to the provisioning of services for the MRIdian system installed at each of Washington University in St. Louis, University of California, Los Angeles and University of Wisconsin–Madison.
Operating Expenses
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Research and development |
$ | 9,404 | $ | 8,780 | $ | 624 | ||||||
| Selling and marketing |
4,681 | 3,781 | 900 | |||||||||
| General and administrative |
14,742 | 9,508 | 5,234 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
$ | 28,827 | $ | 22,069 | $ | 6,758 | ||||||
|
|
|
|
|
|
|
|||||||
Research and Development. Research and development expenses in 2014 increased $0.6 million, or 7%, compared to 2013. This increase was primarily attributable to a $0.7 million increase in project material costs and a $0.2 million increase in travel expenses, offset by a $0.2 million decrease in grant project contractor expense due to the expiration of the State of Ohio grant in April 2013.
83
Table of Contents
Selling and Marketing. Selling and marketing expenses in 2014 increased $0.9 million, or 24%, compared to 2013. This increase was primarily attributable to a $0.3 million increase in travel-related expenses to promote international sales, a $0.2 million increase in trade show expenses, a $0.2 million increase in public relations and website redesign expenses and a $0.1 million increase in marketing consultant expenses.
General and Administrative. General and administrative expenses in 2014 increased $5.2 million, or 55%, compared to 2013. This increase was primarily attributable to a $1.3 million increase in accounting and legal fees, a $1.2 million increase in travel expenses, a $1.2 million increase in personnel and related costs as a result of higher employee headcount, and a $1.0 million increase in regulatory consulting expenses. The change was also attributable to a $0.5 million increase in rent and facility expenses due to our new office lease in Mountain View, California, which commenced during the third quarter of 2014.
Interest Expense
| Year Ended December 31, | ||||||||||||
| 2014 | 2013 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Interest expense |
$ | (2,243 | ) | $ | (97 | ) | $ | (2,146 | ) | |||
Interest expense increased $2.1 million in 2014, which was primarily due to the long-term debt we incurred in December 2013.
Other Income (Expense), Net
| Year Ended December 31, | ||||||||||||
| 2014 | 2014 | Change | ||||||||||
| (in thousands) | ||||||||||||
| Other income (expense), net |
$ | 21 | $ | (32 | ) | $ | 53 | |||||
Other income (expense), net in 2014 consisted primarily of the change in fair value of our convertible preferred stock warrant liability.
Liquidity and Capital Resources
Since ViewRay’s inception in 2004 as a Florida corporation, we have incurred significant net losses and negative cash flows from operations. During 2014 and the six months ended June 30, 2015, we had net losses of $33.8 million and $20.6 million, respectively. At December 31, 2014 and June 30, 2015, we had an accumulated deficit of $152.0 million and $172.7 million, respectively.
At December 31, 2014 and June 30, 2015, we had cash and cash equivalents of $11.1 million and $17.7 million, respectively. To date, we have financed our operations principally through private placements of ViewRay’s convertible preferred stock, issuances of convertible promissory notes and receipts of customer deposits for new orders and payments from customers for systems installed. Through June 30, 2015, we have received net proceeds of $15.7 million from the issuance of shares of ViewRay’s convertible preferred stock. We expect that our existing cash and cash equivalents, together with cash receipts from sales of MRIdian systems and the net proceeds from the Offering, will enable us to conduct our planned operations for at least the next 12 months.
We could potentially use our available financial resources sooner than we currently expect, and we may incur additional indebtedness to meet future financing needs. Adequate additional funding may not be available to us on acceptable terms or at all. In addition, although we anticipate being able to obtain additional financing through non-dilutive means, we may be unable to do so. Our failure to raise capital as and when needed could have significant negative consequences for our business, financial condition and results of operations. Our future capital requirements and the adequacy of available funds will depend on many factors, including those set forth in the section titled “Risk Factors.”
84
Table of Contents
The following table summarizes our cash flows for the periods presented:
| Six Months Ended June 30, | Year Ended December 31, | |||||||||||||||
| 2015 | 2014 | 2014 | 2013 | |||||||||||||
| (in thousands) | ||||||||||||||||
| Cash used in operating activities |
$ | (20,286 | ) | $ | (20,471 | ) | $ | (27,469 | ) | $ | (25,371 | ) | ||||
| Cash used in investing activities |
(637 | ) | (563 | ) | (2,603 | ) | (1,593 | ) | ||||||||
| Cash provided by (used in) financing activities |
27,492 | (26 | ) | 14,672 | 49,395 | |||||||||||
Operating Activities
We have historically experienced negative cash outflows as we developed MRIdian and continued to expand our business. Our net cash used in operating activities primarily results from our net loss adjusted for non-cash expenses and changes in working capital components as we have grown our business, and is influenced by the timing of cash payments for inventory purchase and cash receipts from our customers. Our primary source of cash flow from operating activities is cash receipts from customers including sales of MRIdian systems and, to a lesser extent, by up-front payments from customers. Our primary uses of cash from operating activities are amounts due to vendors for purchased components and employee-related expenditures. Our cash flows from operating activities will continue to be affected principally by our working capital requirements and the extent to which we build up our inventory balances and increase spending on personnel and other operating activities as our business grows.
During the six months ended June 30, 2015, operating activities used $20.3 million in cash, primarily as a result of our net loss of $20.6 million and $3.7 million net change in our operating assets and liabilities, offset by aggregate non-cash charges of $4.0 million. The net change in our operating assets and liabilities was primarily the result of higher customer deposits and deferred revenue balances, offset by higher deferred costs and purchases of inventory, and decreases in accounts payable, accrued expenses and other long-term liabilities. The decrease in accounts payable of $3.0 million was the result of timing of payments as a result of the growth in our business and a $1.4 million payment to one of our major vendors. These changes were offset by an increase in inventory and deposits on purchased inventory due to installations of MRIdian systems. Customer deposits and deferred revenue increased $4.5 million during the six months ended June 30, 2015 primarily due to new sales contracts. Non-cash charges primarily included $0.6 million for depreciation and amortization, $0.3 million inventory lower of cost or market charges related to installation currently in process, $0.1 million for stock-based compensation and $0.1 million for amortization of debt discount and accrued interest related to our debt incurred in December 2013, which was partially offset by a $51,000 change in the fair value of convertible preferred stock warrant liability.
During 2014, operating activities used $27.5 million in cash, primarily as a result of our net loss of $33.8 million offset by $4.0 million net change in our operating assets and liabilities, and aggregate non-cash charges of $2.3 million. The net change in our operating assets and liabilities was primarily the result of higher accounts payable, customer deposits and deferred revenue balances, offset by higher deferred costs and accounts receivable balances, increased purchases of inventory, and decreases in accrued expenses and other long-term liabilities. The increase in accounts payable of $3.2 million was due to timing of payments as a result of the growth in our business. The increase in accounts receivable of $0.7 million and the increase in customer deposits and deferred revenue of $10.1 million was primarily due to revenue and new sales order growth in 2014. These changes were offset by a $4.7 million increase in deferred costs and a $3.8 million increase in inventory and deposits on purchased inventory due to installations of MRIdian systems. The decrease of $0.2 million in accrued expenses and other long-term liabilities was mainly due to $1.5 million payment of our accrued purchase commitments, offset by a $1.3 million increase in accrued expenses and other long-term liabilities attributable to higher accrued personnel costs due to growth in headcount, higher accrued sales tax and medical device excise tax liabilities, and increase in accrued interest as a result of debt incurred in December 2013. Non-cash charges primarily included $1.0 million for depreciation and amortization, $0.3 million for stock-based compensation, $0.6 million of LCM adjustments related to the reduction of the carrying value of inventory to its net realizable value and $0.4 million for amortization of debt discount and accrued interest related to our 2014 convertible promissory notes and debt incurred in December 2013.
85
Table of Contents
During 2013, operating activities used $25.4 million in cash, primarily as a result of our net loss of $27.2 million and a $4.3 million net change in our operating assets and liabilities, which was partially offset by aggregate non-cash charges of $6.1 million. The net change in our operating assets and liabilities was primarily the result of increased purchases of inventory and lower accounts payable, offset by increase in accrued expenses and other long-term liabilities. The increase in inventory of $5.2 million and deposits on purchased components of $0.9 million was mainly due to the installation of MRIdian systems. The decrease in accounts payable of $0.7 million was due to timing differences in making payments when compared to December 31, 2012. These changes were offset by a $2.1 million increase in accrued expenses and other long-term liabilities attributable to higher accrued personnel costs due to growth in headcount. Non-cash charges primarily included $1.1 million for depreciation and amortization and $4.6 million of LCM adjustments related to the reduction of the carrying value of inventory to its net realizable value.
Investing Activities
Cash used in investing activities during the six months ended June 30, 2015 of $0.6 million primarily resulted from capital expenditures to purchase property and equipment.
Cash used in investing activities during 2014 of $2.6 million primarily resulted from capital expenditures to purchase property and equipment of $2.0 million and an increase in restricted cash of $0.6 million.
Cash used in investing activities during 2013 of $1.6 million primarily resulted from capital expenditures to purchase property and equipment of $1.2 million and to purchase an intellectual property license of $0.5 million.
Financing Activities
On June 26, 2015, we entered into a Term Loan Agreement, or the Term Loan, with Capital Royalty Partners II L.P and Parallel Investment Opportunities Partners II L.P or together, CRG, for up to $50.0 million of which $30.0 million was made available to us upon closing with the remaining $20.0 million to be available on or before June 26, 2016 at our option upon achieving certain milestones. We drew down the first $30.0 million on closing date. The Term Loan has a maturity date of June 26, 2020 (i.e. 5 years) and bears cash interest at a rate of 12.50% per annum to be paid quarterly during the first 3 years. After the first 3 years of interest only payment, we have the option to pay cash interest at a rate of 8% and deferred payment in-kind interest at 4.50% per annum. Principal payment and any deferred payment in-kind interest will be paid quarterly in equal installments following the 3rd year through maturity date.
The Term Loan is subject to a prepayment penalty of 3% on the outstanding balance during the first 12 months following the funding of the Term Loan, 2% on the outstanding balance after year 1 but on or before year 2, 1% on the outstanding balance after year 2 but on or before year 3, and 0% on the outstanding loan if prepaid after year 3 thereafter until maturity. The Term Loan is also subject to a facility fee of 5% based on the sum of the Term Loan drawn and any outstanding payment in-kind payable on maturity date or the date such Term Loan becomes due for whatever reason. All direct financing costs were accounted for as a discount on the Term Loan and will be amortized to interest expense during the life of the Term Loan using the effective interest method. The Term Loan is subject to financial covenants and is collateralized by essentially all our assets and limits the Company’s ability with respect to additional indebtedness, investments or dividends, among other things, subject to customary exceptions.
On June 26, 2015, the Company paid off in full the $13.0 million outstanding term debt with Hercules using part of the proceeds received from the CRG Term Loan.
During the six months ended June 30, 2015, financing activities provided $27.5 million in cash primarily from the net proceeds of $15.7 million related to the issuance of ViewRay’s Series C convertible preferred stock, which was partially offset by $15.0 million payments of term loan and $0.6 million payment of costs related to the initial public offering.
During 2014, financing activities provided $14.7 million in cash primarily from the net proceeds related to the issuance of convertible promissory notes of $9.9 million and net proceeds from the issuance of convertible preferred stock of $5.0 million.
86
Table of Contents
During 2013, financing activities provided $49.4 million in cash from the net proceeds related to the issuance of convertible preferred stock of $34.9 million, net of issuance costs, and net proceeds of $14.5 million related to the term loan we entered into with Hercules in December 2013.
Off-Balance Sheet Arrangements
During the six months ended June 30, 2015 and 2014, we did not have any off-balance sheet arrangements as defined by applicable SEC regulations.
Critical Accounting Policies and Estimates
Our financial statements are prepared in accordance with generally accepted accounting principles in the United States, or GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, expenses and related disclosures. We evaluate our estimates and assumptions on an ongoing basis. Our estimates are based on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Our actual results could differ from these estimates.
We believe that the assumptions and estimates have the greatest potential impact on our financial statements. Therefore, we consider these to be our critical accounting policies and estimates. For further information on all of our significant accounting policies, see the notes to our financial statements.
Revenue Recognition
Revenue recognition for systems that we install generally occurs when the customer acknowledges that the system operates in accordance with our standard product specifications, the customer accepts the installed unit and we transfer title and risk of loss to the customer. Service revenue is recognized on a straight-line basis over the term during which contracted services are provided. We use judgment to estimate revenue allocations from sales arrangements with multiple deliverables between the product and service revenue. In situations where a deliverable in a multi-element arrangement has a value to the customer on a stand-alone basis, we are required to allocate the fair value of the various elements based on the selling price of each element. The principal deliverables consist of (i) sale of MRIdian systems, which generally includes installation, site preparation and software, and (ii) product support, which includes extended service and maintenance. We determine selling prices using vendor specific objective evidence, or VSOE, if it exists, or third-party evidence, or TPE. If neither VSOE or TPE exists for a deliverable, we use best estimated selling price, or BESP. We allocate revenue to multiple elements generally using the relative fair values as determined by BESP. We regularly review VSOE, TPE and BESP for all of our products and services.
We also receive payments for cost reimbursement of allowable expenditures and payments for the achievement of certain milestones under government grants in return for qualifying property and equity purchases and research and development activities over a contractually defined period. These payments are nonrefundable. Government grants generally provide us with fixed payments and a contractually defined period of research. Grant revenues are recognized as associated expenses incurred and are billed to grantors in conjunction with the terms of the grants.
Stock-Based Compensation
Stock-based compensation expense is measured and recognized in the financial statements based on the fair value of the awards granted. The fair value of each option award is estimated on the grant date using the Black-Scholes option-pricing model. Stock-based compensation expense is recognized, net of forfeitures, over the requisite service periods of the awards, which is generally four years.
Our use of the Black-Scholes option-pricing model requires the input of highly subjective assumptions, including the fair value of the underlying Common Stock, expected term of the option, expected volatility of the price of our Common Stock, risk-free interest rates and the expected dividend yield of our Common Stock. The assumptions used in our option-pricing model represent management’s best estimates. These estimates involve inherent uncertainties and the application of management’s judgment. If factors change and different assumptions are used, our stock-based compensation expense could be materially different in the future.
87
Table of Contents
These assumptions and estimates are as follows:
Fair Value of Common Stock. As our stock is not publicly traded, we must estimate the fair value of common stock, as discussed in “Common Stock Valuations” below.
Expected Term. The expected term represents the period that our option awards are expected to be outstanding. We consider several factors in estimating the expected term of options granted, including the expected lives used by a peer group of companies within our industry that we consider to be comparable to our business and the historical option exercise behavior of our employees, which we believe is representative of future behavior.
Expected Volatility. As we do not have a trading history for our Common Stock, the expected stock price volatility for our Common Stock was estimated by taking the average historic price volatility for industry peers based on daily price observations over a period equivalent to the expected term of the stock option grants. Industry peers consist of several public companies in our industry, which were the same as the comparable companies used in the common stock valuation analysis. We intend to continue to consistently apply this process using the same or similar public companies until a sufficient amount of historical information regarding the volatility of our own share price becomes available, or unless circumstances change such that the identified companies are no longer similar to us, in which case, more suitable companies whose share prices are publicly available would be used in the calculation.
Risk-Free Interest Rate. We base the risk-free interest rate on the yields of zero coupon U.S. Treasury securities with maturities similar to the term of employee stock option awards.
Expected Dividend Yield. We have never declared or paid any cash dividends on our Common Stock and do not presently plan to pay cash dividends in the foreseeable future. Consequently, we used an expected dividend yield of zero.
During 2014 and the six months ended June 30, 2015, stock-based compensation expense was $0.3 million and $0.1 million, respectively. As of June 30, 2015, we had $639 thousand of total unrecognized stock-based compensation costs, net of estimated forfeitures, which we expect to recognize over a weighted-average period of 2.6 years.
Common Stock Valuations. The fair value of the common stock underlying our stock options was determined by our board of directors, which intended all options granted to be exercisable at a price per share not less than the per share fair value of our Common Stock underlying those options on the date of grant. The valuations of our Common Stock were determined in accordance with the guidelines outlined in the American Institute of Certified Public Accountants Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or AICPA Practice Aid. The assumptions we use in the valuation model are based on future expectations combined with management judgment. In the absence of a public trading market, our board of directors, with input from management, exercised significant judgment and considered numerous objective and subjective factors to determine the fair value of our Common Stock as of the date of each option grant, including the following factors:
| • | valuations performed by unrelated third-party specialists; |
| • | the prices, rights, preferences, and privileges of our convertible preferred stock relative to those of our Common Stock; |
| • | the prices of ViewRay’s former convertible preferred stock sold to outside investors in arm’s-length transactions; |
| • | the lack of marketability of our Common Stock; |
| • | our actual operating and financial performance; |
| • | current business conditions and projections; |
| • | our hiring of key personnel and the experience of our management; |
| • | our history and the timing of the introduction of new products and services; |
88
Table of Contents
| • | our stage of development; |
| • | the likelihood of achieving a liquidity event, such as an initial public offering or a merger or acquisition of our business given prevailing market conditions; |
| • | the illiquidity of stock-based awards involving securities in a private company; |
| • | the market performance of comparable publicly traded companies; and |
| • | the U.S. and global capital market conditions. |
For the valuations of our Common Stock, our management estimated, as of each valuation date, our enterprise value on a continuing operations basis, using the income approach and various market approaches described in the AICPA Practice Aid.
For valuations after the consummation of the Merger, our board of directors will determine the fair value of each share of underlying common stock based on a recent practicable closing price of our Common Stock from the date of grant, as reported on our principal trading market.
Common Stock Warrant
In December 2013 in connection with the Term Loan, we issued a warrant to Hercules to purchase 128,231 shares of our Common Stock with an exercise price of $5.84 per share, subject to certain adjustments. This warrant is exercisable in whole or in part at any time prior to the expiration date of the warrant, which is the later of (i) December 16, 2023 and (ii) the date that is five years following the effective date of the registration statement of an initial underwritten public offering of our Common Stock.
The common stock warrant is recorded as stockholders’ deficit and adjusted to fair value at each balance sheet date, with the change in fair value being recorded as a component of other income (expense), net in the statements of operations. We will continue to record adjustments to the fair value of the common stock warrant until the earlier of the exercise or the expiration of the warrant.
Inventory Valuation
Inventory consists primarily of purchased components for assembling MRIdian systems and other direct costs associated with MRIdian system installation. Inventory is stated at the lower of cost (on a weighted average basis) or market value. When the net realizable value of the inventory is lower than related costs, we reduce the carrying value of the inventory for the difference while recording a corresponding charge to cost of product revenues. The assumptions we used in estimating the net realizable value of the inventory primarily include the total cost to complete the applicable MRIdian system. We recorded an inventory lower of cost and market adjustment of $0.3 million and 0.6 million during 2014 and the six months ended June 30, 2015 and 2014.
Prior to January 1, 2015, our inventory cost is measured on a first-in, first-out basis through specific identification. To support the increasing MRIdian system installations and inventory purchase activities, starting January 1, 2015, we elected to change inventory cost measurement to weighted average basis. The accounting principle change does not have an impact on prior periods’ financial statements, therefore no retrospective adjustment is required. The accounting principle change does not have an impact on product cost of revenue or net loss for the three months ended March 31, 2015.
Income Taxes
We are subject to income taxes in the United States, and we use estimates in determining our provision for income taxes. We use the asset and liability method of accounting for income taxes. Under this method, we calculate deferred tax asset or liability account balances at the balance sheet date using current tax laws and rates in effect for the year in which the differences are expected to affect our taxable income.
89
Table of Contents
We estimate actual current tax exposure together with assessing temporary differences resulting from differences in accounting for reporting purposes and tax purposes for certain items, such as accruals and allowances not currently deductible for tax purposes. These temporary differences result in deferred tax assets and liabilities, which are included in our consolidated balance sheets. In general, deferred tax assets represent future tax benefits to be received when certain expenses previously recognized in our consolidated statements of operations and comprehensive loss become deductible expenses under applicable income tax laws or when net operating loss or credit carryforwards are utilized. Accordingly, realization of our deferred tax assets is dependent on future taxable income against which these deductions, losses and credit carryforwards can be utilized.
We assess the likelihood that our deferred tax assets will be recovered from future taxable income, and to the extent we believe that recovery is not likely, establish a valuation allowance. At December 31, 2014 and March 31, 2015, we have a full valuation allowance set up for our net deferred tax assets.
Under federal and similar state tax statutes, changes in our ownership, including ownership changes resulting from the Merger, may limit our ability to use our available net operating loss and tax credit carryforwards. The annual limitation, as a result of a change of ownership, may result in the expiration of net operating losses and credits before utilization. We believe we have experienced at least one ownership change in the past. We are currently analyzing the tax impact of such ownership change on our federal NOLs and credit carryforwards. Our ability to use our remaining net operating loss carryforwards may be further limited if we experience an ownership change or as a result of future changes in our stock ownership.
JOBS Act Accounting Election
We are an “emerging growth company” within the meaning of the JOBS Act. Section 107 of the JOBS Act provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. Thus, an emerging growth company can delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. We have irrevocably elected not to avail ourselves of this extended transition period and, as a result, we will adopt new or revised accounting standards on the relevant dates on which adoption of such standards is required for other public companies that are not emerging growth companies.
Recently Issued and Adopted Accounting Pronouncements
We review new accounting standards to determine the expected financial impact, if any, that the adoption of each such standard will have. For the recently issued accounting standards that we believe may have an impact on our financial statements, see the section entitled “Notes to Financial Statements – Note 2 – Summary of Significant Accounting Policies” in the financial information attached hereto as Exhibit 99.2.
90
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information relating to the beneficial ownership of our Common Stock at July 15, 2015, by:
| • | each person, or group of affiliated persons, known by us to beneficially own more than 5% of ViewRay’s pre-Merger outstanding shares of Common Stock; |
| • | each of our directors; |
| • | each of our named executive officers; and |
| • | all current directors and executive officers as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of July 15, 2015 through the exercise of any stock option, warrants or other rights. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all shares of Common Stock held by such person.
The percentage of shares beneficially owned is computed on the basis of 37,656,288 shares of common stock outstanding July 15, 2015, giving effect to the Offering. Shares of Common Stock that a person has the right to acquire within 60 days of July 15, 2015 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all directors and executive officers as a group. Unless otherwise indicated below, the address for each beneficial owner listed in the table is c/o ViewRay, Inc., 2 Thermo Fisher Way, Oakwood Village, Ohio 44146.
| Name and Address of Beneficial Owner |
Number of Shares Beneficially Owned |
Number of Shares Exercisable Within 60 Days |
Number of Shares Beneficially Owned |
Percentage of Beneficial Ownership |
||||||||||||
| 5% and Greater Stockholders |
||||||||||||||||
| Aisling Capital II, LP(1) |
7,675,085 | — | 7,675,085 | 20.38 | % | |||||||||||
| Beacon Bioventures Fund II Limited Partnership(2) |
7,989,923 | — | 7,989,923 | 21.22 | ||||||||||||
| Entities affiliated with OrbiMed Private Investments III, LP(3) |
7,989,916 | — | 7,989,916 | 21.22 | ||||||||||||
| Entities affiliated with Kearny Venture Partners, L.P.(4) |
4,565,659 | — | 4,565,659 | 12.12 | ||||||||||||
| Harbour Tycoon Limited(5) |
3,190,786 | — | 3,190,780 | 8.47 | ||||||||||||
| Named Executive Officers and Directors |
||||||||||||||||
| Chris A. Raanes(6) |
781,182 | 53,229 | 834,411 | 2.17 | ||||||||||||
| James F. Dempsey, Ph.D.(7) |
721,315 | 16,558 | 737,873 | 1.93 | ||||||||||||
| Michael Brandt(8) |
107,852 | 5,717 | 113,569 | * | ||||||||||||
| Joshua Bilenker, M.D.(9) |
7,675,085 | — | 7,675,085 | 20.38 | ||||||||||||
| David Bonita, M.D.(10) |
7,989,916 | — | 7,989,916 | 21.11 | ||||||||||||
| Caley Castelein, M.D.(11) |
4,565,659 | — | 4,565,659 | 12.12 | ||||||||||||
| Mark S. Gold, M.D.(12) |
119,576 | 290 | 119,866 | * | ||||||||||||
| Aditya Puri(13) |
3,190,786 | — | 3,190,786 | 8.47 | ||||||||||||
| All current directors and executive officers as a group (10 persons)(14)(15) |
25,325,041 | 78,774 | 25,403,815 | 64.55 | ||||||||||||
| * | Indicates beneficial ownership of less than 1% of the total outstanding common stock. |
91
Table of Contents
| (1) | Includes 7,125,085 shares held prior to the Offering by Aisling Capital II, LP, or Aisling. Aisling Capital Partners, LP, or Aisling GP, is the general partner of Aisling. Aisling Capital Partners LLC, or Aisling Partners, is the general partner of Aisling GP. The individual managing members, or the Aisling Managers, of Aisling Partners are Dennis Purcell, Andrew Schiff, M.D. and Steve Elms. By virtue of these relationships, Aisling GP, Aisling Partners and the Aisling Managers may be deemed to have voting and investment power over the shares held by Aisling. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, an affiliate of Aisling. Voting and dispositive decisions with respect to shares held by Aisling are not made by Dr. Bilenker; he disclaims beneficial ownership of the shares held by Aisling, except to the extent of any pecuniary interest therein, if any. The address of Aisling is c/o Aisling Capital, LLC, 888 Seventh Avenue, 30th Floor, New York, New York 10106. In addition, the number of shares beneficially owned after the Offering includes 550,000 shares of our Common Stock that Aisling purchased in the Offering at a purchase price of $5.00 per share. |
| (2) | Includes 7,125,085 shares held prior to the Offering by Beacon Bioventures Fund II Limited Partnership, or Beacon. Beacon Bioventures Advisors Fund II Limited Partnership, or BBA II, is the general partner of Beacon. Impresa Management LLC, or Impresa, is the general partner of BBA II. By virtue of these relationships, BBA II and Impresa may be deemed to have voting and investment power over the shares held by Beacon. Impresa is managed on a day-to-day basis by its President, Paul L. Mucci, and as such, Mr. Mucci may be deemed to have voting and dispositive power with respect to all shares held by Beacon. Each of the individuals and entities listed above expressly disclaims beneficial ownership of the shares held by Beacon, except to the extent of any pecuniary interest therein, if any. The address of Beacon is c/o Fidelity Biosciences, One Main Street, 13th Floor, Cambridge, Massachusetts 02142. In addition, the number of shares beneficially owned after the Offering includes 864,838 shares of our Common Stock that Beacon purchased in the Offering at a purchase price of $5.00 per share. |
| (3) | Includes (i) 7,057,866 shares held prior to the Offering by OrbiMed Private Investments III, LP, or OPI III, and (ii) 67,213 shares held prior to the Offering by OrbiMed Associates III, LP, or OA III. OrbiMed Capital GP III LLC, or GP III, is the general partner of OPI III. OrbiMed Advisors LLC, OrbiMed, is the managing member of GP III and the general partner of OA III. Samuel D. Isaly is the managing member of and owner of a controlling interest in OrbiMed. By virtue of such relationships, GP III, OrbiMed and Mr. Isaly may be deemed to have voting and investment power over the shares held by OPI III and OA III. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner of OrbiMed. Each of GP III, OrbiMed, Mr. Isaly and Dr. Bonita disclaims beneficial ownership of the shares held by OPI III and OA III, except to the extent of its or his pecuniary interest therein, if any. The address of OrbiMed Investments and OrbiMed Associates is c/o OrbiMed Advisors LLC, 601 Lexington Avenue, 54th Floor, New York, New York 10022 In addition, the number of shares beneficially owned after the Offering includes 864,837 shares of our Common Stock that entities affiliated with OPI III purchased in the Offering at a purchase price of $5.00 per share. |
| (4) | Includes (i) 1,995,037 shares held prior to the Offering by Kearny Venture Partners, L.P., or KVP, (ii) 40,685 shares held prior to the Offering by Kearny Venture Partners Entrepreneurs’ Fund, L.P., or KVPE, and (iii) 2,035,731 shares held prior to the Offering by Thomas Weisel Healthcare Venture Partners, L.P., or TWHVP. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of KVP, Kearny Venture Associates, L.L.C., or KVA, and Thomas Weisel Capital Management LLC, or TWCM. KVA is the general partner of each of KVP and KVPE. TWCM is the managing member of Thomas Weisel Healthcare Venture Partners, LLC, the general partner of TWHVP. Voting and dispositive decisions with respect to shares held by KVP, KVPE and TWHVP are made by Dr. Castelein; however, he disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. The address of KVP, KVPE and TWHVP is c/o Kearny Venture Partners, 88 Kearny Street, Suite 1800, San Francisco, California 94108. In addition, the number of shares beneficially owned after the Offering includes 494,206 shares of our Common Stock that entities affiliated with KVP purchased in the Offering at a purchase price of $5.00 per share. |
| (5) | Includes 2,564,652 shares held prior to the Offering by Harbour Tycoon Limited. Aditya Puri, a member of our board of directors, is an Investments Director at Xeraya Capital, an affiliate of Harbour Tycoon Limited. The address of Harbour Tycoon Limited is c/o 2nd Floor, The Grand Pavilion Commercial Centre, 802 West Bay Road, P.O. Box 1338, Grand Cayman KY1-1003, Cayman Islands. In addition, the number of shares beneficially owned after the Offering includes 626,128 shares of our Common Stock that Harbour Tycoon Limited purchased in the Offering at a purchase price of $5.00 per share. |
| (6) | Consists of 834,411 shares that may be acquired pursuant to the exercise of stock options within 60 days of July 15, 2015 by Mr. Raanes. |
| (7) | Consists of (i) 182,602 shares held prior to the Offering and (ii) 555,271 shares that may be acquired pursuant to the exercise of stock options within 60 days of July 15, 2015 by Dr. Dempsey. |
| (8) | Consists of 113,569 shares that may be acquired pursuant to the exercise of stock options within 60 days of July 15, 2015 by Mr. Brandt. |
| (9) | Consists of the shares held by Aisling. See footnote 1. Dr. Bilenker disclaims beneficial ownership of the shares held by Aisling, except to the extent of any pecuniary interest therein, if any. |
| (10) | Consists of the shares held by OPI III and OA III. See footnote 3. |
| (11) | Consists of the shares held by KVP, KVPE and TWHVP. See footnote 4. Dr. Castelein disclaims beneficial ownership of the shares held by KVP, KVPE and TWHVP, except to the extent of any pecuniary interest therein, if any. |
| (12) | Consists of (i) 3,206 shares held prior to the Offering by Dr. Gold, (ii) 54,129 shares held prior to the Offering by MJSK, Ltd., (iii) 44,837 shares held prior to the Offering by JMSK, Ltd. and (iv) 17,694 shares that may be acquired pursuant to the exercise of stock options within 60 days of July 15, 2015 by Dr. Gold. Janice Gold, the wife of Dr. Gold, is the President of MJSK, Ltd., and Steven Gold, the son of Dr. Gold, is the General Partner of JMSK, Ltd. Voting and dispositive decisions with respect to shares held by MJSK, Ltd. and JMSK, Ltd. are not made by Dr. Gold; he disclaims beneficial ownership of the shares held by MJSK, Ltd. and JMSK, Ltd. except to the extent of any pecuniary interest therein, if any. |
| (13) | Consists of the shares held by Harbour Tycoon Limited. See footnote 5. |
92
Table of Contents
| (14) | Includes (i) 23,421,431 shares held by entities affiliated with certain of our directors and (ii) 26,268,650 shares beneficially owned by our executive officers and directors, which includes the 23,421,431 shares held by such entities and 78,774 shares that may be acquired pursuant to the exercise of stock options within 60 days of July 15, 2015. |
| (15) | The information in this table excludes 1,507,147 shares underlying option awards granted to certain executive officers and directors upon the consummation of the Merger. These option awards become exercisable as to 1/48th of the underlying shares each month following the consummation of the Merger, and 31,398 of which will be exercisable within 60 days of July 15, 2015. See “Director Compensation” and “Executive Compensation” for information regarding these awards. |
93
Table of Contents
DIRECTORS, EXECUTIVE OFFICERS, PROMOTERS AND CONTROL PERSONS
Directors and Executive Officers
Below are the names of and certain information regarding the Company’s current executive officers and directors who were appointed effective as of the closing of the Merger:
| Name |
Age |
Position(s) | ||
| Executive Officers |
||||
| Chris A. Raanes |
50 | President, Chief Executive Officer and Director | ||
| James F. Dempsey, Ph.D. |
45 | Chief Scientific Officer and Director | ||
| Michael Brandt |
52 | Senior Vice President of Sales | ||
| D. David Chandler |
58 | Chief Financial Officer | ||
| Douglas H. Keare |
51 | Chief Operating Officer | ||
| Non-Employee Directors |
||||
| Joshua Bilenker, M.D.(1)(2) |
43 | Director | ||
| David Bonita, M.D.(1)(3) |
40 | Director | ||
| Caley Castelein, M.D.(2)(3) |
44 | Director | ||
| Mark S. Gold, M.D.(3) |
66 | Director | ||
| Aditya Puri(1)(2) |
44 | Director | ||
| (1) | Member of audit committee. |
| (2) | Member of compensation committee. |
| (3) | Member of nominating and corporate governance committee. |
Executive Officers
Chris A. Raanes has served as our President and Chief Executive Officer and as a member of the board of directors since February 2013. Mr. Raanes brings over 15 years of experience in the private and public medical device field. As our President and Chief Executive Officer, Mr. Raanes has supported our growth and strategic initiatives, including our worldwide commercial expansion of MRIdian. Previously, Mr. Raanes was Executive Vice President from July 2011 to November 2012 and Chief Operating Officer and Senior Vice President from September 2002 to July 2011 at Accuray Incorporated, a medical device company. He also served as Vice President and General Manager, Digital Imaging at PerkinElmer Inc., a healthcare company, from December 1999 to March 2002. Mr. Raanes holds a B.S. and an M.S. in Electrical Engineering from the Massachusetts Institute of Technology. We believe Mr. Raanes is qualified to serve on our board of directors because of his extensive management experience and his expertise in radiation therapy device commercialization and operations.
James F. Dempsey, Ph.D. has served as our Chief Scientific Officer since founding ViewRay in March 2004. Dr. Dempsey has been a member of the board of directors since January 2008. Dr. Dempsey brings more than 17 years of experience in the field of radiotherapy medical physics to ViewRay. He previously served as a faculty member in the University of Florida Department of Radiation Oncology, as Assistant Professor from July 2001 to July 2007 and Associate Professor from July 2007 to January 2008. Dr. Dempsey holds a B.S. in Radiochemistry from San Jose State University and a Ph.D. in Nuclear Chemistry from Washington University in St. Louis. We believe Dr. Dempsey is qualified to serve on our board of directors based on his in-depth knowledge of our product, business and industry, as well as his expertise in nuclear chemistry and physics and medical physics.
Michael Brandt has served as our Senior Vice President of Sales since January 2012. He previously served as a general manager of Accuray Incorporated’s Americas division from October 2009 to December 2012, and as general manager of Accuray Incorporated’s Europe, Middle East, India and Africa division from September 2008 to October 2009. Prior to Accuray Incorporated, Mr. Brandt worked at Philips Medical Systems, a healthcare company, serving as Senior Director MRI International from September 2007 to September 2008, as Senior Director MRI, North America from 2005 to September 2007 and as Marketing Director MRI, North America from 2001 to 2005. Mr. Brandt holds an N.D. in Electrical Engineering from Vaal University of Technology in South Africa.
94
Table of Contents
D. David Chandler has served as our Chief Financial Officer since November 2010. Mr. Chandler has over 30 years of experience in finance, strategic planning, investor relations and accounting. Before joining ViewRay, Mr. Chandler served as a Practice Manager and consulting Chief Financial Officer with vcfo Holdings, Inc., a financial consulting firm, from October 2007 to November 2010. Prior to that, he served as Chief Operating Officer and Chief Financial Officer of Straitshot Communications, Inc., a network solutions company, from June 2003 to July 2007 and Chief Financial Officer of Stellar One Corporation, a technology company, from July 1998 to April 2003. Prior to Stellar One Corporation, Mr. Chandler held a variety of financing and accounting management roles, including Business Assurance Manager with PricewaterhouseCoopers LLP. Mr. Chandler received a B.A. in Business from the University of Washington, Michael G. Foster School of Business and a B.A. in German from the University of Washington.
Douglas H. Keare has served as our Chief Operating Officer since April 2015. Mr. Keare has over twenty years of technology and medical device executive experience. Before joining ViewRay, Mr. Keare founded and served as CEO of RallyOn, Inc., a software company focused on corporate health and wellness, from October 2008 to December 2013. Prior to that, Mr. Keare served as Vice President of Customer and Technical Support at Accuray Inc. from December 2002 to January 2007. Mr. Keare also served as the President and Chief Operating Officer for Pricing Dynamics from July 2000 to July 2002. He held several positions at ADAC Laboratories in Customer Support, Operations and Quality from October 1992 to March 1999. As Vice President of Quality, he led ADAC’s successful effort to win the Malcolm Baldridge National Quality Award in 1996. Mr. Keare received a B.A. in Economics from Dartmouth College and an M.B.A. from Stanford University’s Graduate School of Business.
Board Composition
Non-Employee Directors
Joshua Bilenker, M.D. has served as a member of our board of directors since January 2008. Dr. Bilenker joined Aisling Capital LLC in April 2006 and has served as an Operating Partner since November 2013. He has served as Chief Executive Officer of Loxo Oncology, Inc., an Aisling Capital LLC portfolio company, since July 2013. Previously, Dr. Bilenker served as a Medical Officer in the Office of Oncology Drug Products at the FDA from August 2004 to April 2006. Dr. Bilenker has served on the boards of directors of Loxo Oncology, Inc. since July 2008, T2 Biosystems, Inc. since August 2011 and Roka Bioscience, Inc. since January 2012, as well as on the boards of directors of several private companies. He is also a board member of the NCCN Foundation and BioEnterprise. Dr. Bilenker holds an A.B. in English from Princeton University and an M.D. from the Johns Hopkins School of Medicine. We believe Dr. Bilenker is qualified to serve on our board of directors based on his oncology background, experience at the FDA and his extensive service as a director or officer of, and as an investor in, other healthcare companies.
David Bonita, M.D. has served as a member of our board of directors since January 2008. Dr. Bonita has served as a Private Equity Partner at OrbiMed Advisors LLC, or OrbiMed, since June 2013. Dr. Bonita joined OrbiMed in June 2004 as a Private Equity Senior Associate, and was promoted to Private Equity Principal in December 2007. Prior to OrbiMed, he was a corporate finance analyst in the healthcare investment banking group of Morgan Stanley & Co. from February 1998 to July 1999, and a corporate finance analyst in the healthcare investment banking group of UBS AG from August 1997 to February 1998. Dr. Bonita has also served on the board of directors of Loxo Oncology, Inc. since October 2013, as well as on the boards of directors of several private companies. Dr. Bonita holds an A.B. in Biological Sciences from Harvard College and an M.D. and M.B.A. from Columbia University in the City of New York. We believe Dr. Bonita is qualified to serve on our board of directors due to his extensive investment experience in the healthcare industry.
Caley Castelein, M.D. has served as a member of our board of directors since January 2008. Dr. Castelein has served as a Managing Director of Kearny Venture Partners, L.P. since September 2006. Prior to that, Dr. Castelein served as a Managing Director at Thomas Weisel Partners, which was acquired by Stifel, Nicolaus & Company, Incorporated in July 2010, from March 2003 to September 2006. Dr. Castelein has served on the boards of directors of several private companies. Dr. Castelein holds an A.B. in Biological Sciences from Harvard College and an M.D. from the University of California, San Francisco. We believe Dr. Castelein is qualified to serve on our board of directors based on his extensive investment experience in the healthcare industry.
95
Table of Contents
Mark S. Gold, M.D. has served as a member of our board of directors since our founding in March 2004. Dr. Gold was a Professor, Distinguished Professor and Chairman of Psychiatry at the University of Florida from 1990 until his retirement in June 2014. Dr. Gold has worked for over 40 years in basic science and clinical research, translating research into clinical practice. He has been a consultant and senior advisor to banks and private equity and venture capital firms on medical devices, pharmaceuticals and health care services throughout his career. He was a Founding Director of the Somerset Valley Bank and Somerset Valley Financial from 1991 to 1999. Dr. Gold has served on the board of directors of Axogen, Inc. (formerly LecTec Corporation) since September 2011. Dr. Gold is currently the Chairman of RiverMend Health. We believe Dr. Gold is qualified to serve on our board of directors because of his academic expertise and his extensive research experience across medical specialties and institutions.
Aditya Puri has served as a member of our board of directors since February 2015. Mr. Puri has served as an Investments Director at Xeraya Capital, which is responsible for life sciences investments for Khazanah Nasional Berhad, since October 2012. Previously, he was a Director in Khazanah Nasional’s Life Sciences unit since November 2011, which was responsible for Khazanah’s life sciences investments. Prior to that, Mr. Puri consulted part time in the greater Boston area for various healthcare and cleantech startups affiliated with Harvard University and Massachusetts Institute of Technology, or MIT, from 2009 to 2011. Mr. Puri also served as Managing Director of global development at Salary.com from July 2007 to April 2008. Mr. Puri was at the Yankee Group, a global technology research and consulting company, from September 2000 to March 2007, finishing his tenure as a Vice-President and member of the leadership team. Between March 1997 and April 2000, he was at Boston Scientific, a Fortune 500 medical device manufacturer. Mr. Puri serves on several boards of directors of private companies in the investment and healthcare fields. Mr. Puri has a B.S. from the University of Southern Maine and received an M.B.A. from the MIT Sloan School of Management. We believe Mr. Puri is qualified to serve on our board of directors because of his extensive experience in life sciences investment and growth.
Director Independence
Our board of directors currently consists of seven members. We are not currently subject to listing requirements of any national securities exchange that has requirements that a majority of the board of directors be “independent.” Nevertheless, our board of directors has determined that all of our directors, other than Mr. Raanes and Dr. Dempsey, qualify as “independent” directors in accordance with listing requirements of The NASDAQ Stock Market, or NASDAQ. Mr. Raanes and Dr. Dempsey are not considered independent because each is an employee of ViewRay. The NASDAQ independence definition includes a series of objective tests, such as that the director is not, and has not been for at least three years, one of our employees and that neither the director nor any of his family members has engaged in various types of business dealings with us. In addition, as required by NASDAQ rules, our board of directors has made a subjective determination as to each independent director that no relationships exist, which, in the opinion of our board of directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In making these determinations, our board of directors reviewed and discussed information provided by the directors and us with regard to each director’s business and personal activities and relationships as they may relate to us and our management. There are no family relationships among any of our directors or executive officers.
Classified Board of Directors
In accordance with our certificate of incorporation, our board of directors will be divided into three classes with staggered, three-year terms. At each annual meeting of stockholders, the successors to directors whose terms then expire will be elected to serve from the time of election and qualification until the third annual meeting following election. Our directors are divided among the three classes as follows:
| • | the Class I directors will be Chris A. Raanes and Aditya Puri, and their terms will expire at the annual meeting of stockholders to be held in 2016; |
| • | the Class II directors will be Josh Bilenker, M.D., James F. Dempsey, Ph.D. and Mark S. Gold, M.D., and their terms will expire at the annual meeting of stockholders to be held in 2017; and |
| • | the Class III directors will be David Bonita, M.D. and Caley Castelein, M.D., and their terms will expire at the annual meeting of stockholders to be held in 2018. |
96
Table of Contents
Our certificate of incorporation will provide that the authorized number of directors may be changed only by resolution of the board of directors. Any additional directorships resulting from an increase in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors. The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change in control of the company.
Role of Board in Risk Oversight Process
Risk assessment and oversight are an integral part of our governance and management processes. Our board of directors encourages management to promote a culture that incorporates risk management into our corporate strategy and day-to-day business operations. Management discusses strategic and operational risks at regular management meetings and conducts specific strategic planning and review sessions during the year that include a focused discussion and analysis of the risks facing us. Throughout the year, senior management reviews these risks with the board of directors at regular board meetings as part of management presentations that focus on particular business functions, operations or strategies, and presents the steps taken by management to mitigate or eliminate such risks.
Our board of directors does not have a standing risk management committee, but rather administers this oversight function directly through our board of directors as a whole, as well as through various standing committees of our board of directors that address risks inherent in their respective areas of oversight. In particular, our board of directors is responsible for monitoring and assessing strategic risk exposure, and our audit committee is responsible for overseeing our major financial risk exposures and the steps our management has taken to monitor and control these exposures. The audit committee also monitors compliance with legal and regulatory requirements. Our nominating and governance committee monitors the effectiveness of our corporate governance guidelines and considers and approves or disapproves any related-person transactions. Our compensation committee assesses and monitors whether any of our compensation policies and programs has the potential to encourage excessive risk-taking.
Board Committees
Audit Committee
Our audit committee oversees our corporate accounting and financial reporting process. Among other matters, the audit committee:
| • | appoints our independent registered public accounting firm; |
| • | evaluates our independent registered public accounting firm’s qualifications, independence and performance; |
| • | determines the engagement of our independent registered public accounting firm; |
| • | reviews and approves the scope of the annual audit and the audit fee; |
| • | discusses with management and our independent registered public accounting firm the results of the annual audit and the review of our quarterly financial statements; |
| • | approves the retention of our independent registered public accounting firm to perform any proposed permissible non-audit services; |
| • | monitors the rotation of partners of our independent registered public accounting firm on our engagement team as required by law; |
| • | is responsible for reviewing our financial statements and our management’s discussion and analysis of financial condition and results of operations to be included in our annual and quarterly reports to be filed with the SEC; |
97
Table of Contents
| • | reviews our critical accounting policies and estimates; and |
| • | annually reviews the audit committee charter and the committee’s performance. |
The current members of our audit committee are Drs. Bilenker and Bonita and Mr. Puri. Dr. Bonita serves as the chairperson of the audit committee. All members of our audit committee meet the requirements for financial literacy under the applicable rules and regulations of the SEC and NASDAQ. Our board of directors has determined that Dr. Bonita is an audit committee financial expert as defined under the applicable rules of the SEC and has the requisite financial sophistication as defined under the applicable rules and regulations of NASDAQ. Under the rules of the SEC, members of the audit committee must also meet heightened independence standards. However, a minority of the members of the audit committee may be exempt from the heightened audit committee independence standards for one year from the date of the Merger. Our board of directors has determined that each of Drs. Bilenker and Bonita and Mr. Puri are independent under the applicable rules of NASDAQ. The audit committee operates under a written charter that satisfies the applicable standards of the SEC and NASDAQ.
Compensation Committee
Our compensation committee reviews and recommends policies relating to compensation and benefits of our officers and employees. The compensation committee reviews and recommends corporate goals and objectives relevant to compensation of our chief executive officer and other executive officers, evaluates the performance of these officers in light of those goals and objectives and recommends to our board of directors the compensation of these officers based on such evaluations. The compensation committee also recommends to our board of directors the issuance of stock options and other awards under our stock plans. The compensation committee will review and evaluate, at least annually, the performance of the compensation committee and its members, including compliance by the compensation committee with its charter. The current members of our compensation committee are Drs. Bilenker and Castelein and Mr. Puri. Dr. Castelein serves as the chairperson of the compensation committee. Each of the members of our compensation committee is independent under the applicable rules and regulations of NASDAQ, is a “non-employee director” as defined in Rule 16b-3 promulgated under the Exchange Act and is an “outside director” as that term is defined in Section 162(m) of the U.S. Internal Revenue Code of 1986, as amended, or the Code. The compensation committee operates under a written charter.
Nominating and Corporate Governance Committee
The nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of our board of directors. In addition, the nominating and corporate governance committee is responsible for overseeing our corporate governance policies and reporting and making recommendations to our board of directors concerning governance matters. The current members of our nominating and corporate governance committee are Drs. Bonita, Castelein and Gold. Dr. Castelein serves as the chairperson of the nominating and corporate governance committee. Each of the members of our nominating and corporate governance committee is an independent director under the applicable rules and regulations of NASDAQ relating to nominating and corporate governance committee independence. The nominating and corporate governance committee operates under a written charter.
Compensation Committee Interlocks and Insider Participation
None of the members of our compensation committee has at any time been one of our officers or employees. None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers on our board of directors or compensation committee.
Board Diversity
Upon consummation of the Merger, our nominating and corporate governance committee will be responsible for reviewing with the board of directors, on an annual basis, the appropriate characteristics, skills and experience required for the board of directors as a whole and its individual members. In evaluating the suitability of individual candidates (both new candidates and current members), the nominating and corporate governance committee, in
98
Table of Contents
recommending candidates for election, and the board of directors, in approving (and, in the case of vacancies, appointing) such candidates, will take into account many factors, including the following:
| • | personal and professional integrity; |
| • | ethics and values; |
| • | experience in corporate management, such as serving as an officer or former officer of a publicly held company; |
| • | experience in the industries in which we compete; |
| • | experience as a director or executive officer of another publicly held company; |
| • | diversity of expertise and experience in substantive matters pertaining to our business relative to other board members; |
| • | conflicts of interest; and |
| • | practical and mature business judgment. |
Currently, our board of directors evaluates, and following the consummation of the Merger will evaluate, each individual in the context of the board of directors as a whole, with the objective of assembling a group that can best maximize the success of the business and represent stockholder interests through the exercise of sound judgment using its diversity of experience in these various areas.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to all of our employees, officers and directors, including those officers responsible for financial reporting. The code of business conduct and ethics is available on our website at www.viewray.com. We expect that any amendments to the code, or any waivers of its requirements, will be disclosed on our website. The reference to our web address does not constitute incorporation by reference of the information contained at or available through our website.
Limitation on Liability and Indemnification Matters
Our certificate of incorporation contains provisions that limit the liability of our directors for monetary damages to the fullest extent permitted by Delaware law. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
| • | any breach of the director’s duty of loyalty to us or our stockholders; |
| • | any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; |
| • | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the Delaware General Corporation Law; or |
| • | any transaction from which the director derived an improper personal benefit. |
Our certificate of incorporation and bylaws provide that we are required to indemnify our directors and officers, in each case to the fullest extent permitted by Delaware law. Our bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his, her or its actions in that capacity regardless of whether we would otherwise be permitted to indemnify him, her or it under Delaware law.
In addition to the indemnification required in our certificate of incorporation and bylaws, we have entered or intend to enter into indemnification agreements with each of our directors, officers and certain other employees prior to the
99
Table of Contents
consummation of the Merger. These agreements will provide for the indemnification of our directors, officers and certain other employees for all reasonable expenses and liabilities incurred in connection with any action or proceeding brought against them by reason of the fact that they are or were our agents. We believe that these provisions in our certificate of incorporation, bylaws and indemnification agreements are necessary to attract and retain qualified persons as directors and officers. This description of the limitation of liability and indemnification provisions of our certificate of incorporation, of our bylaws and of our indemnification agreements is qualified in its entirety by reference to these documents, each of which is attached as an exhibit to this Report.
The limitation of liability and indemnification provisions in our certificate of incorporation and may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. A stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. There is no pending litigation or proceeding naming any of our directors, officers or employees as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director, officer or employee.
Director Compensation
From our inception to the date of this Report, no compensation was earned by or paid to Dinara Akzhigitova, who was our sole director. Ms. Akzhigitova resigned as our sole officer and director effective as of July 23, 2015 in connection with the Merger.
ViewRay became our wholly owned subsidiary upon the closing of the Merger on July 23, 2015. The following summarizes the compensation earned by ViewRay’s non-employee directors in ViewRay’s fiscal year ending December 31, 2014.
In 2014, ViewRay paid Dr. Gold, who is not affiliated with any of ViewRay’s major investors, an aggregate in cash of $14,000, which represents a quarterly retainer of $2,000 plus $2,000 per board meeting attended. Otherwise, ViewRay did not pay any cash compensation to any of the non-employee members of ViewRay’s board of directors, and ViewRay did not pay director fees to our directors who are ViewRay’s employees. However, ViewRay reimbursed ViewRay’s non-employee directors for travel and other necessary business expenses incurred in the performance of their services for ViewRay.
In addition, in April 2014, ViewRay granted each of Drs. Gold and Roemer options to purchase ViewRay common stock, which were converted into options to purchase 4,736 shares of our Common Stock, each having an exercise prices of $0.75 per share. These options vest and become exercisable in 48 monthly installments on each monthly anniversary of May 13, 2013 for Dr. Gold and November 13, 2013 for Dr. Roemer, subject to the individual continuing to provide services through each such vesting date.
In connection with the Merger, we approved a compensation policy for our non-employee directors, or the Director Compensation Program. Pursuant to the Director Compensation Program, our non-employee directors will receive cash compensation, paid quarterly in arrears, as follows:
| • | Each non-employee director will receive an annual cash retainer in the amount of $40,000 per year. |
| • | Any non-employee Chairman will receive an additional annual cash retainer in the amount of $35,000 per year, and a lead independent director, if appointed, will receive an additional annual cash retainer in the amount of $7,500 per year. |
| • | The chairperson of the audit committee will receive additional annual cash compensation in the amount of $20,000 per year for such chairperson’s service on the audit committee. Each non-chairperson member of the audit committee will receive additional annual cash compensation in the amount of $10,000 per year for such member’s service on the audit committee. |
100
Table of Contents
| • | The chairperson of the compensation committee will receive additional annual cash compensation in the amount of $15,000 per year for such chairperson’s service on the compensation committee. Each non-chairperson member of the compensation committee will receive additional annual cash compensation in the amount of $7,000 per year for such member’s service on the compensation committee. |
| • | The chairperson of the nominating and corporate governance committee will receive additional annual cash compensation in the amount of $10,000 per year for such chairperson’s service on the nominating and corporate governance committee. Each non-chairperson member of the nominating and corporate governance committee will receive additional annual cash compensation in the amount of $5,000 per year for such member’s service on the nominating and corporate governance committee. |
Under the Director Compensation Program, upon the director’s initial appointment or election to our board of directors, each non-employee director will receive an option (the Initial Grant) to purchase that number of shares of our Common Stock such that the award has an aggregate grant date fair value (as defined below) equal to $176,400, rounded down to the nearest whole share (subject to adjustment as provided in the applicable equity plan). In addition, each non-employee director who has been serving as a director and will continue to serve as a director immediately following each annual stockholder meeting, will be automatically granted, on the date of such annual stockholder meeting, an option (the Annual Grant) to purchase that number of shares of our Common Stock such that the award has an aggregate grant date fair value equal to $70,200, rounded down to the nearest whole share (subject to adjustment as provided in the applicable equity plan). For purposes of the Initial Grant and the Annual Grant, “grant date fair value” will mean the fair value of an award as of the date of grant as determined in accordance with ASC Topic 718, “Share-Based Payment”, using the Black-Scholes pricing model and the valuation assumptions used by the company in accounting for options as of such date of grant. The Initial Grant will vest as to 1/36th of the shares subject to Initial Grant on each monthly anniversary of the applicable grant date, subject to continued service through each applicable vesting date, and the Annual Grant will vest as to 1/12th of the shares subject to the Annual Grant on each month anniversary of the applicable grant date, subject to continued service through such vesting date. In addition, pursuant to the terms of the Director Compensation Program, all equity awards outstanding and held by a non-employee director will vest in full immediately prior to the occurrence of a change in control (as defined in the applicable equity plan such awards were granted under).
Upon the consummation of the Merger, we granted each of Drs. Bilenker, Bonita, Castelein, Gold and Mr. Puri an option to purchase 19,556 shares of our Common Stock at an exercise price per share equal to $5. The options vest and become exercisable in substantially equal monthly installments over the 12 months following the grant date, subject to the individual continuing to provide services to us through the applicable vesting date.
2014 Director Compensation Table
The following table sets forth information for the year ended December 31, 2014 regarding the compensation awarded to, earned by or paid to ViewRay’s non-employee directors as if ViewRay been a reporting company on December 31, 2014. We did not pay any compensation to our one director, Dinara Akzhigitova, in fiscal year 2014::
| Name |
Fees Earned or Paid in Cash($) |
Option Awards ($)(1) |
Total($) | |||||||||
| Joshua Bilenker, M.D. |
$ | — | $ | — | $ | — | ||||||
| David Bonita, M.D. |
— | — | — | |||||||||
| Caley Castelein, M.D. |
— | — | — | |||||||||
| Mark S. Gold, M.D. |
14,000 | 2,079 | 16,079 | |||||||||
| Peter Roemer, Ph.D. |
1,225 | 2,079 | 3,304 | |||||||||
| Robert Weisskoff, Ph.D. |
— | — | — | |||||||||
| Philip Yang |
— | — | — | |||||||||
| (1) | The amounts reported in the Option Awards column represent the grant date fair values of stock options granted during the year ended December 31, 2014 as calculated in accordance with ASC Topic 718, excluding the impact of estimated forfeitures related to service-based vesting provisions. See Note 13 to our audited financial statements included elsewhere in this Report for the assumptions used in calculating these amounts. As of December 31, 2014, each of Drs. Gold and Roemer held options to purchase an aggregate of 17,404 shares of Common Stock (as converted pursuant to the Merger such that the numbers reflect the shares subject to the options as if they had been granted by us and not ViewRay). None of ViewRay’s other non-employee directors held any options or stock awards. |
101
Table of Contents
Involvement in Certain Legal Proceedings
None of our directors or executive officers has been involved in any of the following events during the past 10 years:
| • | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| • | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences); |
| • | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; or |
| • | being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
102
Table of Contents
From our inception to the date of this Report, no compensation was earned by or paid to our sole named executive officer, Dinara Akzhigitova, who served as our principal executive. Ms. Akzhigitova resigned as our sole officer and director effective as of July 23, 2015 in connection with the Merger.
ViewRay became our wholly-owned subsidiary upon the closing of the Merger on July 23, 2015. The following summarizes the compensation earned in each of ViewRay’s fiscal years ended December 31, 2014 and includes a discussion of compensation arrangements by the individuals who would have been deemed its named executive officers, or NEOs, had ViewRay been a reporting company on December 31, 2014, which would have been as follows:
| • | Chris A. Raanes, President and Chief Executive Officer; |
| • | James F. Dempsey, Ph.D., Chief Scientific Officer; and |
| • | Michael Brandt, Senior Vice President of Sales. |
This discussion contains forward-looking statements that are based on our current plans, considerations, expectations and determinations regarding future compensation programs. Actual compensation programs that we adopt may differ materially from currently planned programs as summarized in this discussion.
2014 Summary Compensation Table
The following table shows information regarding the compensation of the NEOs for services performed in the years ended December 31, 2014. All amounts reflect compensation received from ViewRay.
| Name and Principal Position |
Year | Salary($) | Bonus($) | Option Awards ($)(1) |
Non-Equity Incentive Plan Compensation ($)(2) |
All Other Compensation ($)(3) |
Total($) | |||||||||||||||||||||
| Chris A. Raanes |
2014 | 415,000 | — | 236,368 | 155,625 | 826,724 | ||||||||||||||||||||||
| President and Chief Executive Officer |
||||||||||||||||||||||||||||
| James F. Dempsey, Ph.D. |
2014 | 260,000 | — | 44,999 | 68,250 | — | 373,249 | |||||||||||||||||||||
| Chief Scientific Officer |
||||||||||||||||||||||||||||
| Michael Brandt |
2014 | 250,000 | — | 14,222 | 106,625 | — | 370,847 | |||||||||||||||||||||
| Senior Vice President of Sales |
||||||||||||||||||||||||||||
| (1) | The amounts reported in the Option Awards columns represent the grant date fair values of stock options granted during 2014 as calculated in accordance with ASC Topic 718, excluding the impact of estimated forfeitures related to service-based vesting provisions. See Note 13 to our audited financial statements included elsewhere in this Report for the assumptions used in calculating these amounts. |
| (2) | The amounts reported in the Non-Equity Incentive Plan Compensation column represent the annual cash performance-based bonuses earned by the NEOs pursuant to the achievement of certain company performance objectives. These amounts for 2014 were paid to the NEOs in early 2015. For Mr. Brandt, the amount also includes $41,000 for 2014 and $34,107 for 2013 that was payable pursuant to achievement of certain sales commissions under ViewRay’s sales compensation plan. For more information on the 2014 bonuses paid to the NEOs, please see the descriptions of the annual performance bonuses paid to the NEOs in “Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End—Terms and Conditions of Annual Bonuses” below and the description of the sales commissions paid to Mr. Brandt in “Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End—Terms and Conditions of Sales Compensation Plan” below. |
| (3) | The amounts reported in the All Other Compensation column in 2014 for Mr. Raanes represents commercial air travel of $11,367, and transportation and housing in the greater Cleveland area of $8,364 reimbursed by ViewRay pursuant to the terms and conditions of the Raanes Agreement (as defined below), whereby ViewRay reimburses the costs incurred by Mr. Raanes for commuting from his residence to |
103
Table of Contents
| ViewRay’s offices in Oakwood Village, Ohio. For more information, please see the description of the Raanes Agreement in “Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End—Terms and Conditions of Employment Agreement with Chris A. Raanes” below. |
Outstanding Equity Awards at 2014 Year-End
The following table sets forth all outstanding equity awards held by each of the NEOs at December 31, 2014: These options were converted into options to purchase our Common Stock in connection with the Merger, and the table below reflects all outstanding options as of December 31, 2014 as if they had been granted by us.
| Option Awards | ||||||||||||||||||||
| Name |
Vesting Commencement Date(1) |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Option Exercise Price ($) |
Option Expiration Date |
|||||||||||||||
| Chris A. Raanes |
2/4/2013 | (2) | 400,101 | 584,760 | 0.71 | 2/7/2023 | ||||||||||||||
| 5/13/2013 | 154,494 | 235,807 | 0.76 | 4/11/2024 | ||||||||||||||||
| 11/13/2013 | 40,230 | 108,313 | 0.76 | 4/11/2024 | ||||||||||||||||
| James F. Dempsey, Ph.D. |
1/8/2008 | (2) | 75,243 | — | 0.81 | 6/17/2018 | ||||||||||||||
| 9/30/2014 | (3) | — | 51,291 | 0.81 | 9/30/2018 | |||||||||||||||
| 6/17/2010 | (4) | 61,752 | — | 0.69 | 6/29/2020 | |||||||||||||||
| 7/14/2010 | 197,635 | — | 0.69 | 6/29/2020 | ||||||||||||||||
| 3/1/2012 | 43,494 | 19,762 | 0.71 | 8/8/2022 | ||||||||||||||||
| 5/13/2013 | 38,062 | 58,086 | 0.76 | 4/11/2024 | ||||||||||||||||
| 11/13/2013 | 64,503 | 173,653 | 0.76 | 4/11/2024 | ||||||||||||||||
| Michael Brandt |
1/9/2012 | (2) | 64,328 | 23,895 | 0.69 | 12/1/2021 | ||||||||||||||
| 3/1/2012 | 11,468 | 5,209 | 0.71 | 8/8/2022 | ||||||||||||||||
| 5/13/2013 | 10,031 | 15,315 | 0.76 | 4/11/2024 | ||||||||||||||||
| 11/13/2013 | 1,915 | 5,155 | 0.76 | 2/7/2023 | ||||||||||||||||
| (1) | Except as otherwise noted, options vest and become exercisable in 48 installments on each monthly anniversary of the vesting commencement date, such that all awards will be vested on the fourth anniversary of the vesting commencement date, subject to the holder continuing to provide services to the company through such vesting date. |
| (2) | The option vests and becomes exercisable as to 25% of the shares on the first anniversary of the vesting commencement date, and in 36 installments thereafter on each monthly anniversary of the vesting commencement date, such that all awards will be vested on the fourth anniversary of the vesting commencement date, subject to the holder continuing to provide services to the company through such vesting date. For Dr. Dempsey’s option, in the event Dr. Dempsey is terminated without “cause” within 12 months following a “change of control” (each as defined below in the section titled “—Offer Letter to James F. Dempsey, Ph.D.”), the vesting and exercisability of the option shall accelerate in full as of the date of such termination. For Mr. Raanes’ option, (i) if Mr. Raanes is terminated without “cause” or resigns for “good reason” (each as defined below in the section titled “—Employment Agreement with Chris A. Raanes”), the vesting and exercisability of the option shall accelerate with respect to the number of shares that would have vested in the 12 months following Mr. Raanes’ termination; and (ii) in the event Mr. Raanes is terminated without cause or resigns for good reason within the period of time commencing three months prior to a “change of control” (as defined below in the section titled “—Employment Agreement with Chris A. Raanes”) and ending 18 months following such change of control, then the vesting and exercisability of the option shall accelerate in full as of the date of such termination. |
| (3) | The option vests and becomes exercisable in full upon the occurrence of one of the following events on or before December 31, 2014: (a) immediately prior to the closing of a “corporate reorganization” (as defined in ViewRay’s contingent equity agreement, effective January 8, 2008) in which the company and/or our stockholders receive at least $500 million; or (b) immediately prior to the closing of a firm commitment underwritten public offering of shares of common” stock to the public at a pre-money valuation of at least $500 million. The option did not vest on or before December 31, 2014. |
| (4) | The option vests and becomes exercisable in 36 installments on each monthly anniversary of the vesting commencement date, such that all awards will be vested on the third anniversary of the vesting commencement date, subject to Dr. Dempsey continuing to provide services to the company through such vesting date. |
104
Table of Contents
Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End
In connection with the Merger, each of the NEOs continues to be employed with us under the terms of their employment agreement or offer letter, as applicable, with ViewRay.
Employment Agreement with Chris A. Raanes
In January 2013, ViewRay entered into an employment agreement with Mr. Raanes, or the Raanes Agreement, to serve as our President and Chief Executive Officer and as a member of our board of directors, providing for base salary, target annual bonus opportunity and standard employee benefit plan participation. Mr. Raanes’ base salary is subject to annual increases in the sole discretion of the board of directors. Mr. Raanes’ base salary for 2014 was $415,000, and he had an annual bonus target of 50% of base salary that is earned based on the achievement of certain milestones. Please see the section below titled “Terms and Conditions of Annual Bonuses” for a further description of the annual bonuses awarded to Mr. Raanes. In 2013, Mr. Raanes was also paid a $50,000 signing bonus, 50% of such signing bonus was paid to Mr. Raanes on the date he commenced employment with ViewRay and the remaining 50% of such signing bonus was paid on the six-month anniversary of such date. In addition, ViewRay reimbursed or directly paid for the costs incurred by Mr. Raanes for housing and transportation in the greater Cleveland area and commercial air travel from his residence in California to its offices in Ohio. In 2014, ViewRay reimbursed $8,364 in the aggregate for Mr. Raanes’ housing and transportation in the greater Cleveland area and $11,367 for commercial air travel from his residence to its offices in Ohio. Mr. Raanes is now based in our headquarters in Mountain View, California. Pursuant to the Raanes Agreement, ViewRay granted Mr. Raanes a stock option to purchase 984,861 shares of Common Stock, which equaled 5% of our issued and outstanding shares as of January 18, 2013. Under the Raanes Agreement, Mr. Raanes’ employment is terminable at-will. Mr. Raanes has also executed ViewRay’s standard confidential information and invention assignment agreement, which contains certain non-competition covenants.
The Raanes Agreement also provides Mr. Raanes with certain severance and change in control benefits. Mr. Raanes’ was eligible to participate in any carveout plan that ViewRay adopted, with minimum levels of compensation at various transaction price levels as set forth in the Raanes Agreement. ViewRay never adopted any such carveout plan (and as a result Mr. Raanes was not eligible for any such carveout payments).
In addition, pursuant to the Raanes Agreement, if Mr. Raanes’ employment is terminated without cause or Mr. Raanes resigns for “good reason” (as defined below) at any time three months prior to or 18 months following a change of control, then the vesting and exercisability of Mr. Raanes’ initial option granted under the Raanes Agreement will accelerate in full.
Additionally, in the event that Mr. Raanes is terminated without cause or resigns for good reason, subject to his executing and not revoking a general release of all claims, then Mr. Raanes will become entitled to receive (i) a severance payment equal to 12 months of his annual base salary, payable in substantially equal installments, (ii) a lump sum cash payment equal to a pro-rated portion of his annual performance bonus payable on the later of (a) the annual date bonuses are made to current employees and (b) the first installment payment for the base salary severance, (iii) payment or reimbursement by us of COBRA premiums for up to 12 months, and (iv) accelerated vesting of Mr. Raanes’ option granted under the Raanes Agreement with respect to that number of shares that would have vested had he remained employed with the company for an additional 12 months.
Under the Raanes Agreement, “change of control” means (i) a sale of all or substantially all of the assets of the company and its subsidiaries taken as a whole or (ii) a merger, consolidation or other similar business combination involving the company, if, upon completion of such transaction, the beneficial owners of voting equity securities of the company immediately prior to the transaction beneficially own less than 50% of the successor entity’s voting equity securities; provided that “change of control” will not include a transaction where the consideration received or retained by the holders of the then-outstanding capital stock of the company does not consist primarily of (a) cash or cash equivalent consideration, (b) securities which are registered under the Securities Act or any successor statute thereto, or (c) securities for which the company or any other issuer thereof has agreed, including pursuant to a
105
Table of Contents
demand, to file a registration statement within 90 days of completion of the transaction for resale to the public pursuant to the Securities Act.
Under the Raanes Agreement, “cause” means Mr. Raanes’ (i) dishonesty of a material nature; (ii) theft or embezzlement of our funds or assets; (iii) conviction of, or guilty or no contest plea to, a felony charge or misdemeanor involving moral turpitude, or the entry of a consent decree with any governmental body; (iv) noncompliance in any material respect with any laws or regulations, foreign or domestic; (v) violation of any express direction or any rule, regulation or policy established by the board of directors that is consistent with the terms of the Raanes Agreement; (vi) material breach of the Raanes Agreement or material breach of Mr. Raanes’ fiduciary duties to the company; (vii) gross incompetence, gross neglect or gross misconduct in the performance of his duties; or (viii) repeated and consistent failure to perform the duties under the Raanes Agreement during normal business hours except during vacation periods or absences due to temporary illness. If the board of directors determines in good faith that cause exists, Mr. Raanes will be given written notice by the board of directors that provides the factual basis for the determination prior to that determination being final and Mr. Raanes will have 10 business days to respond and to attempt to cure the condition, although no cure period need be offered if the board of directors reasonably determines that the conditions are not subject to cure.
Under the Raanes Agreement, “good reason” means a resignation that occurs within 30 days following Mr. Raanes’ first having knowledge of any (i) material reduction in his base salary, (ii) material breach of the Raanes Agreement by the company, or (iii) material diminution of Mr. Raanes’ title as Chief Executive Officer or responsibility as Chief Executive Officer imposed by the board of directors (other than in response to an event constituting cause). With respect to subsection (i), any reduction consistent with general reductions in the base salaries of other executives as part of a plan to avoid insolvency or manage any financial distress or hardship of the company will not be deemed to constitute a material reduction in his base salary; and with respect to subsection (ii), good reason will only exist where Mr. Raanes’ has provided the company with written notice of the breach and the company has failed to cure such breach within 10 business days of such written notice.
Offer Letter to James F. Dempsey, Ph.D.
In October 2010, ViewRay entered into an offer letter with Dr. Dempsey that provides for employment at-will and annual base salary, annual target bonus, option awards and certain other benefits. Dr. Dempsey’s base salary for 2014 was $260,000. In addition, for 2014, Dr. Dempsey has an annual target bonus of 35% of base salary awarded based on the achievement of certain milestones. Please see the section titled “Terms and Conditions of Annual Bonuses” below for a further description of the annual bonuses awarded to Dr. Dempsey. His offer letters also contain certain non-disparagement and non-competition restrictive covenants (during Dr. Dempsey’s employment and for 12 months following termination).
The offer letter also provides Dr. Dempsey with certain severance and change of control benefits.
In the event that Dr. Dempsey is terminated without cause, subject to executing and not revoking a general release of all claims, then Dr. Dempsey is entitled to receive a severance payment equal to 12 months of his base salary plus his annual bonus for the year preceding the termination date, payable in substantially equal installments over the six-month period following his termination.
“Change of control” has the same meaning as under the Raanes Agreement. “Cause” means Dr. Dempsey’s (i) willful failure to perform his material duties, other than a failure resulting from his complete or partial incapacity due to long-term physical or mental illness or impairment, (ii) willful act that constitutes gross misconduct and that is injurious to the company, (iii) willful breach of a provision of the offer letter, (iv) material or willful violation of a federal or state law or regulation applicable to the business of the company, or (v) conviction or plea of guilty or no contest to a felony.
106
Table of Contents
Offer Letter to Michael Brandt
In December 2011, ViewRay entered into an offer letter with Mr. Brandt that provides for employment at-will and other terms and conditions similar to Dr. Dempsey, except as provided below. Mr. Brandt’s base salary for 2014 was $250,000. Mr. Brandt is also eligible for additional compensation under the company’s sales compensation plan. Please see the section below titled “Terms and Conditions of Sales Compensation Plan” for a further description of Mr. Brandt’s sales commissions.
The offer letter also provides Mr. Brandt with certain severance and change of control benefits. In the event Mr. Brandt is terminated without cause or resigns for “good reason” (as defined below) at any time within 12 months following a change of control, then in addition to the severance payments described below, the vesting and exercisability of Mr. Brandt’s option granted under his offer letter will accelerate in full.
In addition, in the event that Mr. Brandt is terminated without cause or resigns for good reason, subject to executing and not revoking a general release of all claims, then Mr. Brandt is entitled to receive a severance payment equal to four months of his base salary plus one-third of his annual bonus for the year preceding the termination date payable in substantially equal installments over the four-month period following his termination. “Change of control” has the same meaning as under the Raanes Agreement. “Cause” has the same meaning as under Dr. Dempsey’s offer letter, “Good reason” means the occurrence of one or more of the following conditions, without Mr. Brandt’s consent and without remedy by the company: (i) a material reduction in his compensation, including but not limited to his level of base salary and annual bonus opportunity, other than reductions approved by the board of directors that are applicable to all employees; (ii) a material, non-voluntary, reduction in his authority, duties, position, title or responsibilities or a material, adverse change in his reporting structure; or (iii) a reduction in the kind or level of his benefits to which he was entitled immediately prior to such reduction, other than reductions approved by the board of directors that are applicable to all employees of the company.
Terms and Conditions of Annual Bonuses
For 2014, all of the NEOs were eligible for cash performance-based bonuses pursuant to the achievement of certain performance objectives. The performance targets are approved annually by ViewRay’s board of directors. When determining the 2014 performance bonus program for the NEOs, in late 2013, the board of directors set certain performance goals, using a mixture of performance objectives after receiving input from ViewRay’s Chief Executive Officer. These performance objectives included installing MRIdian systems and commencing patient treatment, obtaining new sales orders, building manufacturing capabilities, implementing cost-reduction programs and building a world class management and employee team. There was no specific weighting for each performance goal when determining the overall bonus amount, and instead the board of directors evaluated the overall achievement of all performance goals based on the importance to the success of the company. For each of these performance goals under the annual bonus program, the board of directors set general performance goals, but there was no minimum or maximum achievement for each performance target; instead, the board of directors weighed the achievement, partial achievement or non-achievement for each performance target when deciding the overall achievement level. These performance goals were not expected to be attained based on average or below-average performance. The board of directors intended for the performance targets to require significant effort on the part of the NEOs and, therefore, set these targets at levels they believed would be difficult to achieve, such that average or below-average performance would not satisfy these targets.
Each NEO’s target bonus opportunity is expressed as a percentage of base salary which can be achieved by meeting corporate goals. For each of the NEOs, his target bonus opportunity is originally set in his employment agreement or offer letter, as applicable, with the company as described above. The board of directors reviews these target percentages to ensure they are adequate, and, while reviewing these target percentages the board of directors does not follow a formula but rather uses the factors as general background information prior to determining the target bonus opportunity rates for the participating NEOs. The board of directors sets these rates based on each participating executive’s experience in his role with the company and the level of responsibility held by each executive, which the board of directors believes directly correlates to his ability to influence corporate results. For 2014, the board of directors used a guideline target bonus opportunity of 50% for Mr. Raanes and 35% for Dr. Dempsey and Mr. Brandt.
107
Table of Contents
Corporate goals and performance targets are reviewed and approved by the board of directors prior to any allocation of the annual bonuses. In early 2015, the board of directors reviewed ViewRay’s 2014 company-wide performance with respect to determining bonuses for executive officers and determined achievement of the performance goals at 75%. Following its review and determinations, the board of directors awarded cash bonuses to the NEOs at 75% of their target bonus opportunity ($155,625 for Mr. Raanes, $68,250 for Dr. Dempsey and $65,625 for Mr. Brandt). The NEOs’ 2014 annual bonuses are set forth in the “2014 Summary Compensation Table” above.
Terms and Conditions of Sales Compensation Plan
For 2014, in addition to the target performance bonus amount described above, Mr. Brandt was also eligible for performance-based cash incentives under a sales compensation plan. For 2014, under his sales compensation plan, Mr. Brandt was eligible to earn commissions based upon the achievement of certain sales targets. For 2014, each of these sales targets were set at levels we determined would require significant effort to achieve and would not be met by average or below-average performance. Commissions for the sales targets are payable monthly or semi-monthly, at our sole discretion. For 2014, Mr. Brandt was awarded $41,000 for achievement of the sales commissions goals.
Terms and Conditions of Equity Awards
In 2014, ViewRay granted two options to each of our NEOs. In April 2014, the board of directors granted the following options to purchase ViewRay’s common stock to each of the NEOs: 390,302 shares and 148,544 shares with vesting commencement dates of May 11, 2013 and November 11, 2013, respectively, for Mr. Raanes; 96,149 shares and 238,156 shares with vesting commencement dates of May 11, 2013 and November 11, 2013, respectively, for Dr. Dempsey; and 25,347 shares and 7,071 shares with vesting commencement dates of May 11, 2013 and November 11, 2013, respectively, for Mr. Brandt. These options were converted into options to purchase our common stock in connection with the Merger, so the foregoing numbers reflect the number of shares of our Common Stock as if they had been granted by us in 2014. Each of these options had an exercise price of $0.76 per share, which the board determined was the fair market value on the date of grant (as converted to reflect our Common Stock). The options vest and become exercisable in 48 monthly installments on each monthly anniversary of the applicable vesting commencement date, such that all awards will be vested on the four-year anniversary of the vesting commencement date, subject to the individual continuing to provide services to us through each such vesting date.
Upon the consummation of the Merger, Mr. Raanes, Dr. Dempsey and Mr. Brandt received an option to purchase 454,776, 273,039 and 40,987 shares of our Common Stock, respectively, at an exercise price per share equal to $5. The options will vest and become exercisable in substantially equal monthly installments over the four years following the grant date, subject to the individual continuing to provide services to us through the applicable vesting date.
Terms and Conditions of 401(k) Plan
In June 2008, ViewRay adopted our 401(k) Retirement Savings Plan for employees. The 401(k) plan is intended to qualify under Section 401(k) of the Code so that contributions to the 401(k) plan by employees or by ViewRay, and the investment earnings thereon, are not taxable to the employees until withdrawn from the 401(k) plan, and so that contributions by ViewRay, if any, will be deductible by us when made. Under the 401(k) plan, employees may elect to reduce their current compensation by up to the statutorily prescribed annual limit and to have the amount of such reduction contributed to the 401(k) plan. ViewRay does not currently make any matching contributions under our 401(k) plan. We have assumed the ViewRay 401(k) plan in connection with the Merger.
Equity Compensation Plans
The principal features of our equity incentive plans and agreements are summarized below. These summaries are qualified in their entirety by reference to the text of the plans or agreements, which are filed as exhibits to this Report.
2015 Equity Incentive Award Plan
In connection with the Merger, we have adopted the 2015 Plan, which was effective immediately prior to the consummation of the Merger. The principal purpose of the 2015 Plan is to attract, retain and motivate selected
108
Table of Contents
employees, consultants and directors through the granting of stock-based compensation awards and cash-based performance bonus awards. The material terms of the 2015 Plan are summarized below.
Share Reserve. Under the 2015 Plan, 4,708,343 shares of Common Stock will be initially reserved for issuance pursuant to a variety of stock-based compensation awards, including stock options, stock appreciation rights, or SARs, restricted stock awards, restricted stock unit awards, deferred stock awards, dividend equivalent awards, stock payment awards, performance awards and other stock-based awards. As of the date of this Current Report on Form 8-K, and in connection with the consummation of the Merger, options to purchase 1,375,786 shares of our common stock have been granted under the 2015 Plan to our executive officers and directors, and options to purchase 131,361 shares have been granted under the 2015 Plan to other employees and consultants. The number of shares initially reserved for issuance or transfer pursuant to awards under the 2015 Plan will be increased by an annual increase on the first day of each year beginning in 2017 and ending in 2026, equal to the least of (a) four percent (4%) of the shares of stock outstanding (on an as-converted basis) on the last day of the immediately preceding year and (b) such smaller number of shares of stock as determined by our board of directors; provided, however, that no more than 15,000,000 shares of common stock may be issued upon the exercise of incentive stock options, or ISOs. The following counting provisions will be in effect for the share reserve under the 2015 Plan:
| • | to the extent that an award terminates, expires or lapses for any reason or an award is settled in cash without the delivery of shares, any shares subject to the award at such time will be available for future grants under the 2015 Plan; |
| • | to the extent shares are tendered or withheld to satisfy the grant, exercise price or tax withholding obligation with respect to any award under the 2015 Plan, such tendered or withheld shares will be available for future grants under the 2015 Plan; |
| • | to the extent that shares of Common Stock are repurchased by us prior to vesting so that shares are returned to us, such shares will be available for future grants under the 2015 Plan; |
| • | the payment of dividend equivalents in cash in conjunction with any outstanding awards will not be counted against the shares available for issuance under the 2015 Plan; and |
| • | to the extent permitted by applicable law or any exchange rule, shares issued in assumption of, or in substitution for, any outstanding awards of any entity acquired in any form of combination by us or any of our subsidiaries will not be counted against the shares available for issuance under the 2015 Plan. |
Administration. The compensation committee is expected to administer the 2015 Plan unless our board of directors assumes authority for administration. The compensation committee must consist of at least three members of our board of directors, each of whom is intended to qualify as an “outside director,” within the meaning of Section 162(m) of the Code, a “non-employee director” for purposes of Rule 16b-3 under the Exchange Act and an “independent director” within the meaning of the NASDAQ rules. The 2015 Plan provides that the board of directors or compensation committee may delegate its authority to grant awards to employees other than executive officers to a committee consisting of one or more members of our board of directors or one or more of our officers, other than awards made to our non-employee directors, which must be approved by our full board of directors.
Subject to the terms and conditions of the 2015 Plan, the administrator has the authority to select the persons to whom awards are to be made, to determine the number of shares to be subject to awards and the terms and conditions of awards, and to make all other determinations and to take all other actions necessary or advisable for the administration of the 2015 Plan. The administrator is also authorized to adopt, amend or rescind rules relating to administration of the 2015 Plan. Our board of directors may at any time remove the compensation committee as the administrator and revest in itself the authority to administer the 2015 Plan. The full board of directors will administer the 2015 Plan with respect to awards to non-employee directors.
Eligibility. Options, SARs, restricted stock and all other stock-based and cash-based awards under the 2015 Plan may be granted to individuals who are then our officers, employees or consultants or are the officers, employees or consultants of certain of our subsidiaries. Such awards also may be granted to our directors. Only employees of the company or certain of our subsidiaries may be granted ISOs.
109
Table of Contents
Awards. The 2015 Plan provides that the administrator may grant or issue stock options, SARs, restricted stock awards, restricted stock unit awards, deferred stock awards, deferred stock unit awards, dividend equivalent awards, performance awards, stock payment awards and other stock-based and cash-based awards, or any combination thereof. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| • | Nonstatutory Stock Options, or NSOs, will provide for the right to purchase shares of common stock at a specified price that may not be less than the fair market value of a share of common stock on the date of grant, and usually will become exercisable (at the discretion of the administrator) in one or more installments after the grant date, subject to the participant’s continued employment or service with us and/or subject to the satisfaction of corporate performance targets and individual performance targets established by the administrator. NSOs may be granted for any term specified by the administrator that does not exceed 10 years. |
| • | Incentive Stock Options will be designed in a manner intended to comply with the provisions of Section 422 of the Code and will be subject to specified restrictions contained in the Code. Among such restrictions, ISOs must have an exercise price of not less than the fair market value of a share of our Common Stock on the date of grant, may only be granted to employees, and must not be exercisable after a period of 10 years measured from the date of grant. In the case of an ISO granted to an individual who owns (or is deemed to own) at least 10% of the total combined voting power of all classes of our capital stock, the 2015 Plan provides that the exercise price must be at least 110% of the fair market value of a share of our Common Stock on the date of grant and the ISO must not be exercisable after a period of five years measured from the date of grant. |
| • | Restricted Stock Awards may be granted to any eligible individual and made subject to such restrictions as may be determined by the administrator. Restricted stock, typically, may be forfeited for no consideration or repurchased by us at the original purchase price if the conditions or restrictions on vesting are not met. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Purchasers of restricted stock, unlike recipients of options, will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse; however, extraordinary dividends will generally be placed in escrow, and will not be released until restrictions are removed or expire. |
| • | Restricted Stock Unit Awards may be awarded to any eligible individual, typically without payment of consideration, but subject to vesting conditions based on continued employment or service or on performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| • | Deferred Stock Awards represent the right to receive shares of common stock on a future date. Deferred stock may not be sold or otherwise hypothecated or transferred until issued. Deferred stock will not be issued until the deferred stock award has vested, and recipients of deferred stock generally will have no voting or dividend rights prior to the time when the vesting conditions are satisfied and the shares are issued. Deferred stock awards generally will be forfeited, and the underlying shares of deferred stock will not be issued, if the applicable vesting conditions and other restrictions are not met. |
| • | Deferred Stock Units are denominated in unit equivalent of shares of common stock, and vest pursuant to a vesting schedule or performance criteria set by the administrator. The common stock underlying deferred stock units will not be issued until the deferred stock units have vested, and recipients of deferred stock units generally will have no voting rights prior to the time when vesting conditions are satisfied. |
| • | Stock Appreciation Rights may be granted in connection with stock options or other awards, or separately. SARs granted in connection with stock options or other awards typically will provide for payments to the holder based upon increases in the price of our Common Stock over a set exercise price. The exercise price of any SAR granted under the 2015 Plan must be at least 100% of the fair market value of a share of our |
110
Table of Contents
| Common Stock on the date of grant. Except as required by Section 162(m) of the Code with respect to a SAR intended to qualify as performance-based compensation as described in Section 162(m) of the Code, there are no restrictions specified in the 2015 Plan on the exercise of SARs or the amount of gain realizable therefrom, although restrictions may be imposed by the administrator in the SAR agreements. SARs under the 2015 Plan will be settled in cash or shares of common stock, or in a combination of both, at the election of the administrator. |
| • | Dividend Equivalent Awards represent the value of the dividends, if any, per share paid by us, calculated with reference to the number of shares covered by the award. Dividend equivalents may be settled in cash or shares and at such times as determined by our compensation committee or board of directors, as applicable. |
| • | Performance Awards may be granted by the administrator on an individual or group basis. Generally, these awards will be based upon specific performance targets and may be paid in cash or in common stock or in a combination of both. Performance awards may include “phantom” stock awards that provide for payments based upon the value of our Common Stock. Performance awards may also include bonuses that may be granted by the administrator on an individual or group basis and that may be payable in cash or in common stock or in a combination of both. |
| • | Stock Payment Awards may be authorized by the administrator in the form of common stock or an option or other right to purchase common stock as part of a deferred compensation or other arrangement in lieu of all or any part of compensation, including bonuses, that would otherwise be payable in cash to the employee, consultant or non-employee director. |
Change in Control. In the event of a change in control where the acquirer does not assume or replace awards granted prior to the consummation of such transaction, awards issued under the 2015 Plan will be subject to accelerated vesting such that 100% of such awards will become vested and exercisable or payable, as applicable. Performance awards will vest in accordance with the terms and conditions of the applicable award agreement. In the event that, within the 12 month period immediately following a change in control, a participant’s services with us are terminated by us other than for cause (as defined in the 2015 Plan) or by such participant for good reason (as defined in the 2015 Plan), then the vesting and, if applicable, exercisability of 100% of the then-unvested shares subject to the outstanding equity awards held by such participant under the 2015 Plan will accelerate effective as of the date of such termination. The administrator may also make appropriate adjustments to awards under the 2015 Plan and is authorized to provide for the acceleration, cash-out, termination, assumption, substitution or conversion of such awards in the event of a change in control or certain other unusual or nonrecurring events or transactions. Under the 2015 Plan, a change in control is generally defined as:
| • | the transfer or exchange in a single transaction or series of related transactions by our stockholders of more than 50% of our voting stock to a person or group; |
| • | a change in the composition of our board of directors over a two-year period such that the members of the board of directors who were approved by at least two-thirds of the directors who were directors at the beginning of the two-year period or whose election or nomination was so approved cease to constitute a majority of the board of directors; |
| • | a merger, consolidation, reorganization or business combination in which we are involved, directly or indirectly, other than a merger, consolidation, reorganization or business combination that results in our outstanding voting securities immediately before the transaction continuing to represent a majority of the voting power of the acquiring company’s outstanding voting securities and after which no person or group beneficially owns 50% or more of the outstanding voting securities of the surviving entity immediately after the transaction; or |
| • | stockholder approval of our liquidation or dissolution. |
Adjustments of Awards. In the event of any stock dividend, stock split, spin-off, recapitalization, distribution of our assets to stockholders (other than normal cash dividends) or any other corporate event affecting the number of outstanding shares of our Common Stock or the share price of our Common Stock other than an “equity
111
Table of Contents
restructuring” (as defined below), the administrator may make appropriate, proportionate adjustments to reflect the event giving rise to the need for such adjustments, with respect to:
| • | the aggregate number and type of shares subject to the 2015 Plan; |
| • | the number and kind of shares subject to outstanding awards and terms and conditions of outstanding awards (including, without limitation, any applicable performance targets or criteria with respect to such awards); and |
| • | the grant or exercise price per share of any outstanding awards under the 2015 Plan. |
In the event of one of the adjustments described above or other corporate transactions, in order to prevent dilution or enlargement of the potential benefits intended to be made available under the 2015 Plan, the administrator has the discretion to make such equitable adjustments and may also:
| • | provide for the termination or replacement of an award in exchange for cash or other property; |
| • | provide that any outstanding award cannot vest, be exercised or become payable after such event; |
| • | provide that awards may be exercisable, payable or fully vested as to shares of common stock covered thereby; or |
| • | provide that an award under the 2015 Plan cannot vest, be exercised or become payable after such event. |
In the event of an equity restructuring, the administrator will make appropriate, proportionate adjustments to the number and type of securities subject to each outstanding award and the exercise price or grant price thereof, if applicable. In addition, the administrator will make equitable adjustments, as the administrator in its discretion may deem appropriate to reflect such equity restructuring, with respect to the aggregate number and type of shares subject to the 2015 Plan. The adjustments upon an equity restructuring are nondiscretionary and will be final and binding on the affected holders and the Company.
For purposes of the 2015 Plan, “equity restructuring” means a nonreciprocal transaction between us and our stockholders, such as a stock dividend, stock split, spin-off, rights offering or recapitalization through a large, nonrecurring cash dividend, that affects the number or kind of shares (or other securities) or the share price of our Common Stock (or other securities) and causes a change in the per share value of the common stock underlying outstanding stock-based awards granted under the 2015 Plan. In the event of a stock split in connection with an offering, the administrator will proportionately adjust (i) the number of shares subject to any outstanding award under the 2015 Plan, (ii) the exercise or grant price of any such awards, if applicable, and (iii) the aggregate number of shares subject to the 2015 Plan.
Amendment and Termination. Our board of directors or the compensation committee (with board approval) may terminate, amend or modify the 2015 Plan at any time and from time to time. However, we must generally obtain stockholder approval:
| • | to increase the number of shares available under the 2015 Plan (other than in connection with certain corporate events, as described above); |
| • | reduce the price per share of any outstanding option or SAR granted under the 2015 Plan; |
| • | cancel any option or SAR in exchange for cash or another award when the option or SAR price per share exceeds the fair market value of the underlying shares; or |
| • | to the extent required by applicable law, rule or regulation (including any NASDAQ rule). |
Termination. Our board of directors may terminate the 2015 Plan at any time. No ISOs may be granted pursuant to the 2015 Plan after the 10th anniversary of the effective date of the 2015 Plan, and no additional annual share increases to the 2015 Plan’s aggregate share limit will occur from and after such anniversary. Any award that is
112
Table of Contents
outstanding on the termination date of the 2015 Plan will remain in force according to the terms of the 2015 Plan and the applicable award agreement.
We intend to file with the SEC a registration statement on Form S-8 covering the shares of common stock issuable under the 2015 Plan.
2008 Stock Option and Incentive Plan
We assumed the 2008 Plan in connection with the Merger, and it continues to govern the ViewRay stock options assumed and converted by us in connection with the Merger. No further awards will be granted under the 2008 Plan. The 2008 Plan was amended to increase the share reserve on June 17, 2010, July 14, 2010, September 16, 2011, August 8, 2012, February 7, 2013, May 8, 2013 and November 18, 2013. The 2008 Plan provided for the grant of ISOs, NSOs, restricted stock awards, restricted stock units and SARs. At June 30, 2015, options to purchase 4,256,303 shares of Common Stock at a weighted-average exercise price per share of $0.80 remained outstanding under the 2008 Plan. No other equity awards have been granted under the 2008 Plan.
Administration. Our board of directors, or a committee thereof appointed by our board of directors, has the authority to administer the 2008 Plan and the awards granted under it. The administrator has the authority to select the employees to whom awards will be granted under the 2008 Plan, the size and type of awards to be subject to those awards under the 2008 Plan, and the terms and conditions of the awards granted. In addition, the administrator has the authority to construe and interpret the 2008 Plan, to establish, amend or waive rules and resolutions for the 2008 Plan’s administration and to amend the terms and conditions of any outstanding award as allowed under the 2008 Plan and such awards. The administrator may make all other determinations that may be necessary or advisable for the administration of the 2008 Plan.
Awards. The 2008 Plan provides that the administrator may grant or issue stock incentive awards, including ISOs and NSOs. In addition, no more than 206,896 shares subject to equity awards can be granted to any one participant in any calendar year. Each award will be set forth in a separate agreement with the person receiving the award and will indicate the type, terms and conditions of the award.
| • | Stock Option Awards. The 2008 Plan provides for the grant of ISOs under the federal tax laws or NSOs. ISOs may be granted only to employees. NSOs may be granted to employees, directors or consultants. The exercise price of ISOs granted to employees who at the time of grant own stock representing more than 10% of the voting power of all classes of our Common Stock may not be less than 110% of the fair market value per share of our Common Stock on the date of grant, and the exercise price of ISOs granted to any other employees may not be less than 100% of the fair market value per share of our Common Stock on the date of grant. The exercise price of NSOs granted to employees, directors or consultants may not be less than (i) the minimum price required by applicable state law, (ii) the minimum price required by the company’s governing instrument or (iii) $0.01, whichever is greater. However, any option that is intended to avoid taxation under Section 409A of the Code must be granted with an exercise price equal to or greater than 100% of the fair market value per share of our Common Stock on the date of grant. Shares subject to options under the 2008 Plan generally vest in a series of installments over an optionee’s period of service. In general, the maximum term of options granted is 10 years. The maximum term of ISOs granted to an optionee who owns stock representing more than 10% of the voting power of all classes of our Common Stock is five years. Payment of the exercise price is generally made in cash, through a net share exercise election, or a promissory note. In general, options granted under the 2008 Plan will vest and become exercisable at such time as determined by the administrator. |
| • | Stock Appreciation Rights. The 2008 Plan provides that we may issue SARs. Each SAR will be governed by a SAR agreement and may be granted in connection with stock options or other awards, or separately. SARs granted in connection with stock options or other awards typically will provide for payments to the holder based upon increases in the price of our Common Stock over a set exercise price. The exercise price of any SAR granted under the 2008 Plan will not be less than the exercise price for the option if the SAR is granted in connection with an option; otherwise the exercise price must be at least 85% of the fair market value of a share of our Common Stock on the date of grant; provided that any SAR that is intended to avoid taxation under Section 409A of the Code must be granted with an exercise price equal to or greater than 100% of the fair market value per share of our Common Stock on the date of grant. There are no |
113
Table of Contents
| restrictions specified in the 2008 Plan on the exercise of SARs or the amount of gain realizable therefrom, although restrictions may be imposed by the administrator in the SAR agreements; provided that a SAR will not be exercisable for six months if the recipient of the SAR received a hardship distribution under our 401(k) plan. SARs under the 2008 Plan will be settled in cash or shares of our Common Stock, or in a combination of both, at the election of the administrator. In general, SARs may not be sold or otherwise transferred and the maximum term of SARs granted is 10 years. |
| • | Restricted Stock Awards. The 2008 Plan provides that we may issue restricted stock awards. Each restricted stock award will be governed by a restricted stock award agreement. Certain restrictions will be placed on the restricted shares of common stock that will vest as determined by the administrator. The administrator has the discretion to require a cash payment in exchange for a grant of restricted stock, but such payment is not required. In general, restricted stock may not be sold or otherwise transferred until restrictions are removed or expire. Holders of restricted stock will have voting rights and will have the right to receive dividends, if any, prior to the time when the restrictions lapse. |
| • | Restricted Stock Unit Awards. The 2008 Plan provides that we may issue restricted stock unit awards. Each restricted stock unit award will be governed by a restricted stock unit award agreement and may be awarded to any eligible individual, typically without payment of consideration although the administrator may require payment in consideration of the restricted stock units, but subject to vesting conditions based on continued employment or service or on performance criteria established by the administrator. Like restricted stock, restricted stock units may not be sold, or otherwise transferred or hypothecated, until vesting conditions are removed or expire. Unlike restricted stock, stock underlying restricted stock units will not be issued until the restricted stock units have vested, and recipients of restricted stock units generally will have no voting or dividend rights prior to the time when vesting conditions are satisfied. |
| • | Performance-Based Stock Awards. The 2008 Plan provides that we may issue performance-based stock awards. Each performance-based stock award will be governed by a performance-based stock award agreement and may be granted by the administrator as any of the above described equity awards. Generally, these awards will be based upon specific performance targets, in order to satisfy the requirements under Section 162(m) of the Code. Section 162(m) of the Code did not apply to us while we were a private company, so we did not issue any awards subject to this provision prior to termination of the 2008 Plan. |
Adjustments. In the event of certain corporate transactions or capitalization changes, the administrator of the 2008 Plan may adjust the maximum number of shares of common stock that may be delivered under the 2008 Plan, the limit on the number of shares that may be granted during a calendar year, the number and type of shares subject to outstanding wards and the exercise price of any options or SARs; provided that the administrator will be required to make such adjustments if such corporate transactions or capitalization changes constitute an “equity restructuring” as defined in ASC Topic 718.
Change of Control. If a change of control occurs, and if the agreements effectuating the change of control do not provide for the assumption or substitution of all options, SARs and restricted stock units granted under the 2008 Plan, then the administrator, may, with respect to any or all of such non-assumed options, SARs and restricted stock units, take any or all of the following actions to be effective as of the date of the change of control (or as of any other date fixed by the administrator occurring within the 30-day period ending on the date of the change of control, but only if such action remains contingent upon the effectuation of the change of control): (i) accelerate the vesting and/or exercisability of any non-assumed options, SARs and restricted stock units; (ii) cancel any such options, SARs and restricted stock units which have not vested and/or which have not become exercisable, if applicable; (iii) cancel any such options, SARs and restricted stock units in exchange for shares or a cash payment or such other property; (iv) cancel any such options and SARs after a specified date after providing the holders of such options or SARs with an opportunity to exercise such options or SARs to the extent vested and/or exercisable and reasonable notice of such opportunity to exercise; (v) require and cause the exercise of any such options and SARs by a cashless or net share exercise; and/or (vi) cancel any such options, SARs and restricted stock units and notify the holders of such options, SARs or restricted stock units of such action, but only if the fair market value of the shares subject to the awards that are options or SARs does not exceed the exercise price of the options or SARs, or in the case of restricted stock units, the fair market value of the underlying shares is zero. If a change of control occurs,
114
Table of Contents
then, each other award besides an option, SAR or restricted stock unit will be governed by applicable law and the documents effectuating the change of control.
Amendment; Termination. Our board of directors may amend, suspend or terminate the 2008 Plan or any portion thereof at any time, but no action will diminish the rights of a holder of an outstanding award without the holder’s consent, unless there is a dissolution or liquidation of the company or as required by applicable law. Our board of directors can reprice all or any portion of outstanding options and SARs as it deems appropriate without the need for any stockholder approval. In connection with the Merger, the 2008 Plan has been terminated and no further awards will be granted under the 2008 Plan. However, all outstanding ViewRay stock options assumed in connection with the Merger will continue to be governed by their existing terms.
We intend to file with the SEC a registration statement on Form S-8 covering our shares of our Common Stock issuable under the 2008 Plan to cover the ViewRay stock options assumed in the Merger.
2015 Employee Stock Purchase Plan
In connection with the Merger, we have adopted the ESPP, which became effective immediately prior to the closing of the Merger. The ESPP is designed to allow our eligible employees to purchase shares of our Common Stock, at semi-annual intervals, with their accumulated payroll deductions. The ESPP is intended to qualify under Section 423 of the Code.
Plan Administration. Subject to the terms and conditions of the ESPP, our compensation committee will administer the ESPP. Our compensation committee can delegate administrative tasks under the ESPP to the services of an agent and/or employees to assist in the administration of the ESPP. The administrator will have the discretionary authority to administer and interpret the ESPP. Interpretations and constructions of the administrator of any provision of the ESPP or of any rights thereunder will be conclusive and binding on all persons. We will bear all expenses and liabilities incurred by the ESPP administrator.
Shares Available Under ESPP. The maximum number of our shares of our Common Stock which will be authorized for sale under the ESPP is equal to the sum of (a) 285,621 shares of Common Stock and (b) an annual increase on the first day of each year beginning in 2016 and ending in 2025, equal to the lesser of (i) 1% of the shares of Common Stock outstanding (on an as converted basis) on the last day of the immediately preceding fiscal year and (ii) such number of shares of Common Stock as determined by our board of directors; provided, however, no more than 1,675,000 shares of our Common Stock may be issued under the ESPP. The shares made available for sale under the ESPP may be authorized but unissued shares or reacquired shares reserved for issuance under the ESPP.
Eligible Employees. Employees eligible to participate in the ESPP for a given offering period generally include employees who are employed by us or one of our designated subsidiaries on the first day of the offering period, or the enrollment date. Our employees and any employees of our subsidiaries who customarily work less than five months in a calendar year or are customarily scheduled to work less than 20 hours per week will not be eligible to participate in the ESPP. Finally, an employee who owns (or is deemed to own through attribution) 5% or more of the combined voting power or value of all our classes of stock or of one of our subsidiaries will not be allowed to participate in the ESPP.
Participation. Employees will enroll under the ESPP by completing a payroll deduction form permitting the deduction from their compensation of at least 1% of their compensation but not more than the lesser of 15% of their compensation and $30,000 per offering period. Such payroll deductions are expressed as a whole number percentage and the accumulated deductions will be applied to the purchase of shares on each semi-annual purchase date. However, a participant may not purchase more than 3,000 shares in each offering period, and may not subscribe for more than $25,000 in fair market value of shares our Common Stock (determined at the time the option is granted) per calendar year falling in the offering period. The ESPP administrator has the authority to change these limitations for any subsequent offering period.
Offering. Under the ESPP, participants are offered the option to purchase shares of our Common Stock at a discount during a series of successive offering periods. The offering periods will commence and end on dates as determined by the ESPP administrator. However, in no event may an offering period be longer than 27 months in length.
115
Table of Contents
The option purchase price will be the lower of 85% of the closing trading price per share of our Common Stock on the first trading date of an offering period in which a participant is enrolled or 85% of the closing trading price per share on the semi-annual purchase date, which will occur on the last trading day of each offering period.
Unless a participant has previously canceled his or her participation in the ESPP before the purchase date, the participant will be deemed to have exercised his or her option in full as of each purchase date. Upon exercise, the participant will purchase the number of whole shares that his or her accumulated payroll deductions will buy at the option purchase price, subject to the participation limitations listed above.
A participant may cancel his or her payroll deduction authorization at any time prior to the end of the offering period. Upon cancellation, the participant will have the option to either (a) receive a refund of the participant’s account balance in cash without interest or (b) exercise the participant’s option for the current offering period for the maximum number of shares of Common Stock on the applicable purchase date, with the remaining account balance refunded in cash without interest. Following at least one payroll deduction, a participant may also decrease (but not increase) his or her payroll deduction authorization once during any offering period. If a participant wants to increase or decrease the rate of payroll withholding, he or she may do so effective for the next offering period by submitting a new form before the offering period for which such change is to be effective.
A participant may not assign, transfer, pledge or otherwise dispose of (other than by will or the laws of descent and distribution) payroll deductions credited to a participant’s account or any rights to exercise an option or to receive shares of our Common Stock under the ESPP, and during a participant’s lifetime, options in the ESPP shall be exercisable only by such participant. Any such attempt at assignment, transfer, pledge or other disposition will not be given effect.
Adjustments upon Changes in Recapitalization, Dissolution, Liquidation, Merger or Asset Sale. In the event of any increase or decrease in the number of issued shares of our Common Stock resulting from a stock split, reverse stock split, stock dividend, combination or reclassification of the Common Stock, or any other increase or decrease in the number of shares of Common Stock effected without receipt of consideration by us, we will proportionately adjust the aggregate number of shares of our Common Stock offered under the ESPP, the number and price of shares which any participant has elected to purchase pursuant under the ESPP and the maximum number of shares which a participant may elect to purchase in any single offering period.
If there is a proposal to dissolve or liquidate us, then the ESPP will terminate immediately prior to the consummation of such proposed dissolution or liquidation, and any offering period then in progress will be shortened by setting a new purchase date to take place before the date of our dissolution or liquidation. We will notify each participant of such change in writing at least ten business days prior to the new exercise date. If we undergo a merger with or into another corporation or sale of all or substantially all of our assets, each outstanding option will be assumed or an equivalent option substituted by the successor corporation or the parent or subsidiary of the successor corporation. If the successor corporation refuses to assume the outstanding options or substitute equivalent options, then any offering period then in progress will be shortened by setting a new purchase date to take place before the date of our proposed sale or merger. We will notify each participant of such change in writing at least ten business days prior to the new exercise date.
Amendment and Termination. Our board of directors may amend, suspend or terminate the ESPP at any time. However, the board of directors may not amend the ESPP without obtaining stockholder approval within 12 months before or after such amendment to the extent required by applicable laws. The ESPP will terminate on the 10th anniversary of the date of its initial approval of our stockholders, unless earlier terminated.
We intend to file with the SEC a registration statement on Form S-8 covering our shares issuable under the ESPP.
Rule 10b5-1 Sales Plans
Our directors and executive officers may adopt written plans, known as Rule 10b5-1 plans, in which they will contract with a broker to buy or sell shares of Common Stock on a periodic basis. Under a Rule 10b5-1 plan, a broker executes trades pursuant to parameters established by the director or officer when entering into the plan, without further direction from the director or executive officer. The director or executive officer may amend or terminate the plan in limited circumstances. Our directors and executive officers may also buy or sell additional
116
Table of Contents
shares of Common Stock outside of a Rule 10b5-1 plan when they are not in possession of material, nonpublic information.
117
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
SEC rules require us to disclose any transaction or currently proposed transaction in which the Company is a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000 or 1% of the average of the Company’s total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s Common Stock, or an immediate family member of any of those persons. The descriptions set forth above under the captions “The Merger and Related Transactions—Merger Agreement,” “—Split-Off,” “—the Offering,” “—Registration Rights,” “—2008 Stock Option and Incentive Plan,” “—2015 Equity Incentive Plan,” “—Lock-up Agreements and Other Restrictions” and “Executive Compensation—Employment and Related Agreements” and “—Director Compensation” and below under “Description of Securities—Options” are incorporated herein by reference.
The following is a description of transactions since January 1, 2012 to which we have been a party, in which the amount involved exceeded or will exceed $120,000, and in which any of our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof, had or will have a direct or indirect material interest, other than compensation and other arrangements that are described in the section titled “Executive Compensation.” The following description is historical and has not been adjusted to give effect to the Merger or the share conversion ratio pursuant to the Merger Agreement.
Sales and Purchases of Securities
Original Series C Convertible Preferred Stock Financing, Second Tranche
In March 2012, ViewRay issued an aggregate of 1,546,472 shares of Series C convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $26.3 million to 18 accredited investors, which included 646,114 shares of Series C convertible preferred stock that were issued pursuant to a conversion of $10.3 million aggregate principal amount of convertible promissory notes issued in our 2011 bridge financing. The shares of Series C convertible preferred stock issued in March 2012 were exchanged for shares of ViewRay Series B convertible preferred stock or ViewRay common stock, depending upon whether the holders of such shares participated in ViewRay’s Series D-2 convertible preferred stock financing, in our recapitalization effected in connection with the issuance of Series D-2 convertible preferred stock in June 2013, or the Recapitalization. Participating holders of ViewRay Series C convertible preferred stock received shares of Series B convertible preferred stock on a 1:1 basis in the Recapitalization, and non-participating holders of Series C convertible preferred stock received shares of ViewRay common stock on a 10:1 basis in the Recapitalization. The table below sets forth the number of shares of Series C convertible preferred stock sold or issued to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our Common Stock in connection with the Merger.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Principal Amount Under Convertible Promissory Notes |
Accrued Interest Under Convertible Promissory Notes |
Additional Cash |
Aggregate Purchase Price($)(1) |
|||||||||||||||
| OrbiMed Private Investments III, LP(2) |
$ | 427,950 | $ | 2,529,634 | $ | 148,036 | $ | 3,794,451 | $ | 6,472,120 | ||||||||||
| Beacon Bioventures Fund II Limited(3) |
427,951 | 2,529,634 | 148,036 | 3,794,451 | 6,472,120 | |||||||||||||||
| Aisling Capital II, LP(4) |
427,951 | 2,529,634 | 148,036 | 3,794,451 | 6,472,120 | |||||||||||||||
| Kearny Venture Partners, L.P.(5) |
244,542 | 14,447 | 845 | 21,671 | 3,698,300 | |||||||||||||||
| Affiliates of Mark Gold, M.D.(6) |
18,078 | 200,000 | 8,416 | 65,000 | 273,416 | |||||||||||||||
| (1) | Includes the principal amount owed and unpaid accrued interest at a rate of 8% per annum with respect to the Series C convertible preferred stock issued pursuant to the conversion of convertible promissory notes issued in our 2011 bridge financing. |
| (2) | Includes 175,383 shares issued due to conversion of convertible promissory note principal and accrued interest and 248,530 shares purchased with additional cash issued to OrbiMed Private Investments III, LP and 1,670 shares issued due to conversion of convertible promissory note principal and accrued interest and 2,367 shares purchased with additional cash by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with |
118
Table of Contents
| OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (3) | Includes 177,053 shares issued due to conversion of convertible promissory note principal and accrued interest and 250,897 shares purchased with additional cash. Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (4) | Includes 177,053 shares issued due to conversion of convertible promissory note principal and accrued interest and 250,897 shares purchased with additional cash. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (5) | Includes 49,575 shares issued due to conversion of convertible promissory note principal and accrued interest and 70,252 shares purchased with additional cash by Kearny Venture Partners, L.P., 1,011 shares issued due to conversion of convertible promissory note principal and accrued interest and 1,432 shares purchased with additional cash by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 50,586 shares issued due to conversion of convertible promissory note principal and accrued interest and 71,684 shares purchased with additional cash by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
| (6) | Includes 10,335 shares issued due to conversion of convertible promissory note principal and accrued interest and 2,148 shares purchased with additional cash by MJSK, Ltd. and 3,445 shares issued due to conversion of convertible promissory note principal and accrued interest and 2,148 shares purchased with additional cash by Translational Research Family LP. Translational Research Family LP subsequently sold its shares to JMSK, Ltd. Janice Gold, the wife of Mark Gold, M.D., is the President of MJSK, Ltd. and a Partner of Translational Research Family LP. Steven Gold, the son of Mark Gold, M.D., is the General Partner of JMSK, Ltd. Mark Gold, M.D. is a member of our board of directors. |
Series D-1 Convertible Preferred Stock Financing, First Tranche
In November 2012, ViewRay issued an aggregate of 388,290 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.9 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in November 2012 were exchanged for shares of ViewRay Series B convertible preferred stock in the Recapitalization on a 1:1 basis. The table below sets forth the number of shares of Series D-1 convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our Common Stock in connection with the Merger.
| Name |
Number of Shares of Series D-1 Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
108,721 | $ | 1,644,260 | |||||
| Beacon Bioventures Fund II Limited(2) |
108,722 | 1,644,260 | ||||||
| Aisling Capital II, LP(3) |
108,722 | 1,644,260 | ||||||
| Kearny Venture Partners, L.P.(4) |
62,125 | 939,574 | ||||||
| (1) | Includes 107,696 shares purchased by OrbiMed Private Investments III, LP and 1,025 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 30,442 shares purchased by Kearny Venture Partners, L.P., 620 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 31,063 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
119
Table of Contents
Series D-1 Convertible Preferred Stock Financing, Second Tranche
In February 2013, ViewRay amended and restated its Series D-1 convertible preferred stock purchase agreement to issue an additional 330,608 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.0 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in February 2013 were exchanged for shares of ViewRay Series B convertible preferred stock in the Recapitalization on a 1:1 basis. The table below sets forth the number of shares of Series D-1 convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our Common Stock in connection with the Merger.
| Name |
Number of Shares of Series D-1 Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
92,570 | $ | 1,400,000 | |||||
| Beacon Bioventures Fund II Limited(2) |
92,571 | 1,400,000 | ||||||
| Aisling Capital II, LP(3) |
92,571 | 1,400,000 | ||||||
| Kearny Venture Partners, L.P.(4) |
52,896 | 799,998 | ||||||
| (1) | Includes 91,697 shares purchased by OrbiMed Private Investments III, LP and 873 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 25,920 shares purchased by Kearny Venture Partners, L.P., 528 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 26,448 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Series D-2 Convertible Preferred Stock Financing
In May and June 2013, ViewRay issued an aggregate of 996,021 shares of Series D-2 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $15.3 million to 27 accredited investors. The shares of Series D-2 convertible preferred stock issued in May and June 2013 were immediately exchanged for shares of ViewRay Series B convertible preferred stock in the Recapitalization on a 1:1 basis. The table below sets forth the number of shares of Series D-2 convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof. Each outstanding share of ViewRay Series B convertible preferred stock was converted into 2.975 shares of our Common Stock in connection with the Merger.
| Name |
Number of Shares of Series D-2 Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| OrbiMed Private Investments III, LP(1) |
277,713 | $ | 4,200,000 | |||||
| Beacon Bioventures Fund II Limited(2) |
277,713 | 4,200,000 | ||||||
| Aisling Capital II, LP(3) |
277,713 | 4,200,000 | ||||||
| Kearny Venture Partners, L.P.(4) |
158,692 | 2,399,997 | ||||||
| Mark Gold, M.D. and affiliates(5) |
4,190 | 63,404 | ||||||
| (1) | Includes 275,093 shares purchased by OrbiMed Private Investments III, LP and 2,620 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated |
120
Table of Contents
| with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes 77,760 shares purchased by Kearny Venture Partners, L.P., 1,586 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 79,346 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
| (5) | Includes 168 shares purchased by Mark Gold, M.D., 2,651 shares purchased by MJSK, Ltd. and 1,371 shares purchased by JMSK, Ltd. Janice Gold, the wife of Mark Gold, M.D., is the President of MJSK, Ltd. Steven Gold, the son of Mark Gold, M.D., is the General Partner of JMSK, Ltd. Mark Gold, M.D. is a member of our board of directors. |
Series C Convertible Preferred Stock Financing
In November 2013, ViewRay issued an aggregate of 862,064 shares of Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $15.0 million to eight accredited investors. Shares of ViewRay Series C convertible preferred stock converted into our Common Stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the number of shares of ViewRay Series C convertible preferred stock sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Aggregate Purchase Price($) |
||||||
| Royal Seal Holding Co., Limited(1) |
287,356 | $ | 4,999,999 | |||||
| OrbiMed Private Investments III, LP(2) |
160,919 | 2,800,001 | ||||||
| Beacon Bioventures Fund II Limited(3) |
160,919 | 2,800,001 | ||||||
| Aisling Capital II, LP(4) |
160,919 | 2,800,001 | ||||||
| Kearny Venture Partners, L.P.(5) |
91,951 | 1,599,991 | ||||||
| (1) | Philip Yang, who was a member of our board of directors, is Vice President of Cowealth Medical Holding Co. Ltd., which is the sole shareholder of Royal Seal Holding Co., Limited. |
| (2) | Includes 159,401 shares purchased by OrbiMed Private Investments III, LP and 1,518 shares purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (3) | Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (4) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (5) | Includes 45,057 shares purchased by Kearny Venture Partners, L.P., 918 shares purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 45,976 shares purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Convertible Promissory Note Purchase Agreement
In August and November 2014, ViewRay issued convertible promissory notes for an aggregate principal amount of $10.0 million to seven accredited investors. In December 2014, all outstanding principal and interest under the 2014 Notes were converted into 584,675 shares of ViewRay Series C convertible preferred stock at a price of $17.40 per share. See the section below titled “—Series C Convertible Preferred Stock Financing Extension” for further information. Shares of ViewRay Series C convertible preferred stock converted into our Common Stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the principal amount of the convertible
121
Table of Contents
promissory notes sold to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof.
| Name |
Aggregate Principal Price($) |
|||
| OrbiMed Private Investments III, LP(1) |
$ | 2,800,001 | ||
| Beacon Bioventures Fund II Limited(2) |
2,800,001 | |||
| Aisling Capital II, LP(3) |
2,800,001 | |||
| Kearny Venture Partners, L.P.(4) |
1,599,997 | |||
| (1) | Includes convertible promissory notes with an aggregate principal amount of $2,773,586 purchased by OrbiMed Private Investments III, LP and convertible promissory notes with an aggregate principal amount of $26,415 purchased by OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
| (2) | Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (3) | Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (4) | Includes convertible promissory notes with an aggregate principal amount of $784,007 purchased by Kearny Venture Partners, L.P., convertible promissory notes with an aggregate principal amount of $15,990 purchased by Kearny Venture Partners Entrepreneurs’ Fund, L.P. and convertible promissory notes with an aggregate principal amount of $799,999 purchased by Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
Series C Convertible Preferred Stock Financing Extension
In December 2014 and January 2015, ViewRay issued an aggregate of 935,248 shares of its Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $16.3 million to 10 accredited investors, including the conversion of all outstanding principal and interest under the 2014 Notes into shares of ViewRay Series C convertible preferred stock at a price of $17.40 per share. The aggregate gross consideration received from the sale of Series C convertible preferred stock was $6.1 million, and the aggregate gross consideration received from the conversion of all outstanding principal and interest of the 2014 Notes was $10.2 million. Shares of ViewRay Series C convertible preferred stock converted into our Common Stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the number of shares of ViewRay Series C convertible preferred stock issued to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or immediate family member thereof.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Principal Amount Under 2014 Notes |
Accrued Interest Under 2014 Notes |
Additional Cash |
Aggregate Purchase Price($)(1) |
|||||||||||||||
| OrbiMed Private Investments III, LP(2) |
163,709 | $ | 2,800,001 | $ | 48,548 | $ | — | $ | 2,848,549 | |||||||||||
| Beacon Bioventures Fund II Limited(3) |
163,709 | 2,800,001 | 48,548 | — | 2,848,549 | |||||||||||||||
| Aisling Capital II, LP(4) |
163,709 | 2,800,001 | 48,548 | — | 2,848,549 | |||||||||||||||
| Kearny Venture Partners, L.P.(5) |
93,548 | 1,599,997 | 27,742 | — | 1,627,739 | |||||||||||||||
| Affiliates of Mark Gold, M.D.(6) |
5,747 | — | — | 100,000 | 100,000 | |||||||||||||||
| (1) | Includes the outstanding principal amount owed and unpaid accrued interest at a rate of 8% per annum with respect to the Series C convertible preferred stock issued pursuant to the conversion of the 2014 Notes. |
| (2) | Includes 162,175 shares issued due to conversion of convertible promissory note principal and accrued interest to OrbiMed Private Investments III, LP and 1,544 shares issued due to conversion of convertible promissory note principal and accrued interest to OrbiMed Associates III, LP. David Bonita, M.D., a member of our board of directors, is a Private Equity Partner at OrbiMed Advisors LLC, which is an entity affiliated with OrbiMed Private Investments III, LP and OrbiMed Associates III, LP. Each of OrbiMed Advisors LLC and Dr. Bonita disclaims beneficial ownership of such shares, except to the extent of its or his pecuniary interest therein, if any. |
122
Table of Contents
| (3) | Includes 163,709 shares issued due to conversion of convertible promissory note principal and accrued interest. Robert Weisskoff, M.D., who was a member of our board of directors, is a Partner of Fidelity Biosciences, a division of FMR LLC, which is an entity affiliated with Beacon Bioventures Fund II Limited. |
| (4) | Includes 163,709 shares issued due to conversion of convertible promissory note principal and accrued interest. Joshua Bilenker, M.D., a member of our board of directors, is an Operating Partner of Aisling Capital, LLC, which is an entity affiliated with Aisling Capital II, LP. |
| (5) | Includes 45,839 shares issued due to conversion of convertible promissory note principal and accrued interest to Kearny Venture Partners, L.P., 935 shares issued due to conversion of convertible promissory note principal and accrued interest to Kearny Venture Partners Entrepreneurs’ Fund, L.P. and 46,774 shares issued due to conversion of convertible promissory note principal and accrued interest to Thomas Weisel Healthcare Venture Partners, L.P. Caley Castelein, M.D., a member of our board of directors, is a Managing Director of Kearny Venture Partners, L.P., which is an entity affiliated with Kearny Venture Partners Entrepreneurs’ Fund, L.P. and Thomas Weisel Healthcare Venture Partners, L.P. |
| (6) | Includes 5,747 shares purchased with cash by JMSK, Ltd. Steven Gold, the son of Mark Gold, M.D., is the General Partner of JMSK, Ltd. Mark Gold, M.D. is a member of our board of directors. |
In February 2015, ViewRay issued an aggregate of 862,068 shares of its Series C convertible preferred stock at a price of $17.40 per share for aggregate gross consideration of $15.0 million to one accredited investor. Shares of ViewRay Series C convertible preferred stock converted into our Common Stock on a 1:2.975 basis at the effective time of the Merger. The table below sets forth the number of shares of ViewRay Series C convertible preferred stock issued to our directors, executive officers or holders of more than 5% of ViewRay’s pre-Merger capital stock, or an affiliate or an immediate family member thereof.
| Name |
Number of Shares of Series C Convertible Preferred Stock |
Aggregate Purchase Price |
||||||
| Harbour Tycoon Limited(1) |
862,068 | $ | 15,000,000 | |||||
| (1) | Aditya Puri, a member of our board of directors, is an Investments Director at Xeraya Capital, an affiliate of Harbour Tycoon Limited. |
Participation in the Offering
Certain of our existing institutional investors, including investors affiliated with certain of our directors, have purchased an aggregate of 3,400,003 of shares of our Common Stock in the Offering, for an aggregate purchase price of $17,000,015 based on the offering price of $5.00 per share. Such purchases were made on the same terms as the shares that were sold to other investors in the Offering and not pursuant to any pre-existing contractual rights or obligations. See the footnotes to the beneficial ownership table in “Security Ownership of Certain Beneficial Owners and Management” for more details.
License Agreement with University of Florida Research Foundation, Inc.
In December 2004, we entered into a Standard Exclusive License Agreement with Sublicensing Terms with University of Florida Research Foundation, Inc., or UFRF, under which we licensed certain patents from UFRF in exchange for royalty payments and an equity issuance, or the UFRF License Agreement. We entered into an amendment of the UFRF License Agreement in December 2007. Since December 2004, we have paid UFRF $226,000 in royalties and $63,000 in patent and legal fees pursuant to the terms of the UFRF License Agreement. In addition, we have issued 11,312 shares of common stock to UFRF pursuant to the terms of the UFRF License Agreement, which required us to issue UFRF a certain number of shares of common stock upon execution of the UFRF License Agreement, as well as issue UFRF additional shares of common stock to maintain UFRF’s ownership of 5% of our outstanding equity until certain financing conditions were satisfied. We have satisfied these financing conditions and have no further obligations to issue UFRF shares of our Common Stock pursuant to the terms UFRF License Agreement. Prior to the consummation of the Offering, UFRF was a beneficial owner of approximately 0.10% of our capital stock on an as-converted basis. In connection with his former employment at the University of Florida and his role in the development of the licensed patents under the UFRF License Agreement, as amended, James F. Dempsey, Ph.D., our Chief Scientific Officer and a member of our board of directors, receives a percentage of the royalty payments we pay to UFRF and is entitled to a percentage of any proceeds from the sale of Common Stock by UFRF. Specifically, under the University of Florida’s intellectual property policy, Dr. Dempsey is entitled to (i) 40% of any royalty payments we pay to UFRF or proceeds from the sale of Common Stock by
123
Table of Contents
UFRF up to $500 thousand and then (ii) 25% of any royalty payments we pay to UFRF or proceeds from the sale of Common Stock by UFRF over $500 thousand. Mark Gold, M.D., a member of our board of directors since our founding in March 2004, was a Professor, Distinguished Professor and Chairman of Psychiatry at the University of Florida from 1990 until his retirement in June 2014.
Indemnification Agreements and Directors’ and Officers’ Liability Insurance
We have entered into indemnification agreements with each of our directors and executive officers. These agreements, among other things, require us to indemnify each director and executive officer to the fullest extent permitted by Delaware law, including indemnification of expenses such as attorneys’ fees, judgments, fines and settlement amounts incurred by the director or executive officer in any action or proceeding, including any action or proceeding by or in right of us, arising out of the person’s services as a director or executive officer.
Employment Agreements and Offer Letters
In connection with the Merger, each of our new executive officers became employed with us under the terms of their employment agreement or offer letter, as applicable, with ViewRay. For more information regarding these employment agreements for Messrs. Raanes and Brandt and Dr. Dempsey, see the section titled “Executive Compensation—Narrative to 2014 Summary Compensation Table and Outstanding Equity Awards at 2014 Year End.”
Offer Letter to Doug Keare, Chief Operating Officer
In April 2015, ViewRay entered an offer letter with Mr. Keare that provides for employment at-will and annual base salary, annual target bonus (which is pro-rated for 2015), option awards and certain other benefits. Mr. Keare is also eligible for a sign-on bonus of $15,000, which is paid in 12 equal monthly installments beginning on April 30, 2015 (in connection with his commencement of employment with ViewRay). Mr. Keare will forfeit any unpaid portion of this bonus if he terminates his employment prior to April 30, 2016. His offer letter also contains certain non-disparagement and cooperation restrictive covenants (during Mr. Keare’s employment). Mr. Keare has also executed ViewRay’s standard confidential information and invention assignment agreement. Mr. Keare’s employment is terminable at-will.
The offer letter also provides Mr. Keare with certain severance and change of control benefits. In the event Mr. Keare is terminated without “cause” or resigns for “good reason” (each, as defined his employment agreement) at any time within 12 months following a change of control, then in addition to the severance payments described below, the vesting of Mr. Keare’s option granted under his offer letter will accelerate in full.
In addition, in the event that Mr. Keare is terminated without cause or resigns for good reason, subject to executing and not revoking a general release of all claims, then Mr. Keare is entitled to receive a severance payment equal to six months of his base salary plus one-half of his annual bonus for the year preceding the termination date payable in substantially equal installments over the six-month period following his termination.
Offer Letter to David Chandler, Chief Financial Officer
In November 2010, ViewRay entered into an offer letter with Mr. Chandler that provides for employment at-will and annual base salary, annual target bonus, option awards and certain other benefits. His offer letter also contains certain non-disparagement and non-competition restrictive covenants (during Mr. Chandler’s employment and for 12 months following termination of employment). Mr. Chandler has also executed ViewRay’s standard confidential information and invention assignment agreement, which contains certain non-competition covenants. In addition, ViewRay reimbursed or directly paid for the costs incurred by Mr. Chandler for housing and transportation in the greater Cleveland and San Francisco areas and commercial air travel from his residence to its offices in Ohio and California. Mr. Chandler’s employment is terminable at-will.
The offer letter also provides Mr. Chandler with certain severance and change of control benefits.
124
Table of Contents
In addition, in the event that Mr. Chandler is terminated without cause or resigns for good reason (each, as defined in his offer letter), subject to executing and not revoking a general release of all claims, then Mr. Chandler is entitled to receive a severance payment equal to 12 months of his base salary plus his annual bonus for the year preceding the termination date payable in substantially equal installments over the six-month period following his termination.
Other Transactions
We have granted stock options to our executive officers. For a description of these stock options granted to Messrs. Raanes and Brandt and Dr. Dempsey, see the section titled “Executive Compensation.” Upon the consummation of the Merger, each of Messrs. Keare and Chandler was granted an option to purchase 269,684 and 92,661 shares of our Common Stock, respectively, at an exercise price per share equal to $5. Mr. Keare’s options have a vesting commencement date of April 30, 2015 and will vest and become exercisable as to 25% of the shares on the first anniversary of the vesting commencement date, and in 36 installments thereafter on each monthly anniversary of that date, such that all shares will be vested on the fourth anniversary of the vesting commencement date, subject to his continuing to provide services to us through the applicable vesting date. Mr. Chandler’s options will vest and become exercisable in substantially equal monthly installments over the 4 years following the grant date, subject to his continuing to provide services to us through the applicable vesting date. We have also granted stock options to certain members of the board of directors. For a description of these stock options, see the section titled “Management—2013 Director Compensation Table.”
Policies and Procedures for Related-Person Transactions
Our board of directors has adopted a written related-person transaction policy, to be effective upon the consummation of the Merger, setting forth the policies and procedures for the review and approval or ratification of related-person transactions. This policy will cover, with certain exceptions set forth in Item 404 of Regulation S-K under the Securities Act, any transaction, arrangement or relationship, or any series of similar transactions, arrangements or relationships in which we were or are to be a participant, where the amount involved exceeds $120,000 and a related person had or will have a direct or indirect material interest, including, without limitation, purchases of goods or services by or from the related person or entities in which the related person has a material interest, indebtedness, guarantees of indebtedness and employment by us of a related person. In reviewing and approving any such transactions, our audit committee is tasked to consider all relevant facts and circumstances, including, but not limited to, whether the transaction is on terms comparable to those that could be obtained in an arm’s-length transaction and the extent of the related person’s interest in the transaction. All of the transactions described in this section occurred prior to the adoption of this policy.
125
Table of Contents
MARKET PRICE OF AND DIVIDENDS ON COMMON EQUITY AND
RELATED STOCKHOLDER MATTERS
Our Common Stock is quoted on the OTC Markets (OTCQB) under the symbol “VRAY,” which changed from “MRXC” on July 20, 2015.
However, there has been very limited trading to date, and an active trading market may never develop.
As of the date of this Report, we have 37,656,288 shares of Common Stock outstanding held by 200 stockholders of record.
Dividend Policy
We have never paid any cash dividends on our capital stock and do not anticipate paying any cash dividends on our Common Stock in the foreseeable future. We intend to retain future earnings to fund ongoing operations and future capital requirements. Any future determination to pay cash dividends will be at the discretion of our Board of Directors and will be dependent upon financial condition, results of operations, capital requirements and such other factors as the Board of Directors deems relevant.
Shares Eligible for Future Sale
Prior to the Merger, there has been a limited public market for our Common Stock. Future sales of our Common Stock, including shares issued upon the exercise of outstanding options or warrants, in the public market after the Merger, or the perception that those sales may occur, could cause the prevailing price for our Common Stock to fall or impair our ability to raise equity capital in the future. As described below, only a limited number of shares of our Common Stock will be available for sale in the public market for a period of several months after consummation of the Merger due to contractual and legal restrictions on resale described below. Future sales of our Common Stock in the public market either before (to the extent permitted) or after restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing price of our Common Stock at such time and our ability to raise equity capital at a time and price we deem appropriate.
Upon the completion of the Offering, we had 37,656,288 shares of common stock outstanding, of which our directors and executive officers beneficially own an aggregate of 24,571,046 shares. Of those outstanding shares, 260,000 shares of our common stock are freely tradeable, without restriction, as of the date of this Current Report on Form 8-K. No shares issued in connection with the Merger can be publicly sold under Rule 144 promulgated under the Securities Act until 12 months after the date of filing this Current Report on Form 8-K.
Sale of Restricted Shares
Of the approximately 37.7 million shares of Common Stock outstanding upon completion of the Offering, approximately 36.7 million shares of Common Stock will be “restricted securities” as such term is defined in Rule 144. These restricted securities were issued and sold by us, or will be issued and sold by us, in private transactions and are eligible for public sale only if registered under the Securities Act or if they qualify for an exemption from registration under the Securities Act, including the exemptions provided by Rule 144 or Rule 701, which rules are summarized below.
Lock-up Agreements
In connection with the Merger, holders of 25,611,046 of our Common Stock have agreed, subject to certain exceptions, not to dispose of or hedge any shares of Common Stock or securities convertible into or exchangeable for shares of Common Stock during the period from the date of the lock-up agreement continuing through the date 180 days after the date of the Merger, except with our prior written consent.
Following the lock-up periods set forth in the agreements described above, and assuming that no parties are released from these agreements and that there is no extension of the lock-up period, certain of the shares of Common Stock that are restricted securities or are held by our affiliates as of the date of the Merger will be eligible for sale in the public market in compliance with Rule 144 under the Securities Act.
126
Table of Contents
Rule 144
Pursuant to Rule 144 promulgated under the Securities Act, sales of the securities of a former shell company, such as us, under that rule are not permitted (i) until at least 12 months have elapsed from the date on which this Report, reflecting our status as a non-shell company, is filed with the SEC and (ii) unless at the time of a proposed sale, we are subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act and have filed all reports and other materials required to be filed by Section 13 or 15(d) of the Exchange Act, as applicable, during the preceding 12 months, other than Form 8-K reports. We intend to register such shares for sale under the Securities Act, but are currently a “voluntary filer” and are not subject to the reporting requirements of Section 13 or Section 15(d) of the Exchange Act. As a result, unless we register such shares for sale under the Securities Act, most of our stockholders will be forced to hold their shares of our Common Stock for at least that 12-month period before they are eligible to sell those shares, and even after that 12-month period, sales may not be made under Rule 144 unless we and the selling stockholders are in compliance with other requirements of Rule 144.
In general, Rule 144 provides that (i) any of our non-affiliates that has held restricted common stock for at least 12 months is thereafter entitled to sell its restricted stock freely and without restriction, provided that we remain compliant and current with our SEC reporting obligations, and (ii) any of our affiliates, which includes our directors, executive officers and other person in control of us, that has held restricted common stock for at least 12 months is thereafter entitled to sell its restricted stock subject to the following restrictions: (a) we are compliant and current with our SEC reporting obligations, (b) certain manner of sale provisions are satisfied, (c) a Form 144 is filed with the SEC, and (d) certain volume limitations are satisfied, which limit the sale of shares within any three-month period to a number of shares that does not exceed the greater of 1% of the total number of outstanding shares. A person who has ceased to be an affiliate at least three months immediately preceding the sale and who has owned such shares of common stock for at least one year is entitled to sell the shares under Rule 144 without regard to any of the limitations described above.
Regulation S
Regulation S under the Securities Act provides that shares owned by any person may be sold without registration in the U.S., provided that the sale is effected in an offshore transaction and no directed selling efforts are made in the U.S. (as these terms are defined in Regulation S), subject to certain other conditions. In general, this means that our shares of Common Stock may be sold in some other manner outside the U.S. without requiring registration in the U.S.
Rule 701
In general, under Rule 701 as currently in effect, any of our employees, directors, officers, consultants or advisors who acquired Common Stock from us in connection with a written compensatory stock or option plan or other written agreement, in compliance with Rule 701 under the Securities Act, before the effective date of the Merger (to the extent such Common Stock is not subject to a lock-up agreement) is entitled to rely on Rule 701 to resell such shares beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act in reliance on Rule 144, but without compliance with the holding period requirements contained in Rule 144. Accordingly, subject to any applicable lock-up agreements, beginning 90 days after we become subject to the public company reporting requirements of the Exchange Act, under Rule 701 persons who are not our “affiliates,” as defined in Rule 144, may resell those shares without complying with the minimum holding period or public information requirements of Rule 144, and persons who are our “affiliates” may resell those shares without compliance with Rule 144’s minimum holding period requirements (subject to the terms of the lock-up agreement referred to above, if applicable).
Registration Rights
In connection with the Offering, we entered into a Registration Rights Agreement, pursuant to which we have agreed that promptly, but no later than 90 calendar days from the final closing of the Offering, the Company will file a registration statement with the SEC, or the Registration Statement, covering (a) the shares of Common Stock issued in the Offering, (b) the shares of Common Stock issuable upon exercise of the Placement Agent Warrants, (c) the shares of Common Stock issued in exchange for the equity securities of ViewRay outstanding prior to the Merger, and (d) shares of Common Stock held by certain pre-Merger security holders of the Company, or
127
Table of Contents
collectively, the Registrable Shares. The Company will use its commercially reasonable efforts to ensure that such Registration Statement is declared effective within 180 calendar days after the final closing of the Offering. If the Company is late in filing the Registration Statement, if the Registration Statement is not declared effective within 180 days after the final closing of the Offering, the Company fails to maintain the Registration Statement continuously effective as to all Registrable Shares included in such Registration Statement or the Company fails to satisfy the current public information as required under Rule 144(c), the Company will make payments to each holder of Registrable Shares as monetary penalties at a rate equal to 12% of the Offering Price per annum for each share affected during the period; provided, however, that in no event will the aggregate of any such penalties exceed 5% of the Offering Price per share. No monetary penalties will accrue with respect to any Registrable Shares removed from the Registration Statement in response to a comment from the staff of the SEC limiting the number of shares of Common Stock which may be included in the Registration Statement, or Cutback Comment.
The Company must keep the Registration Statement effective for two years from the date it is declared effective by the SEC or until (i) the Registrable Shares have been sold in accordance with such effective Registration Statement, or (ii) the Registrable Shares have been previously sold in accordance with Rule 144.
The holders of Registrable Shares (including any shares of Common Stock removed from the Registration Statement as a result of a Cutback Comment) and the stockholders of the Company prior to the Merger (but not holders of the shares issued to the stockholders of ViewRay in consideration for the Merger) will have “piggyback” registration rights for such Registrable Shares with respect to any registration statement filed by the Company following the effectiveness of the Registration Statement that would permit the inclusion of such shares, subject to customary cutback in an underwritten offering, which would be pro rata.
We will pay all expenses in connection with any registration obligation provided in the Registration Rights Agreement, including, without limitation, all registration, filing, stock exchange fees, printing expenses, all fees and expenses of complying with applicable securities laws, and the fees and disbursements of our counsel and of our independent accountants and reasonable fees and disbursements of counsel to the investors, in an amount not to exceed $35,000. Each investor will be responsible for its own sales commissions, if any, transfer taxes and the expenses of any attorney or other advisor such investor decides to employ.
All descriptions of the Registration Rights Agreement herein are qualified in their entirety by reference to the text thereof filed as an exhibit hereto, which is incorporated herein by reference.
Stock Plans
We intend to file with the SEC a registration statement under the Securities Act covering the shares of Common Stock that we may issue (i) upon exercise of outstanding options under the assumed 2008 Plan, and (ii) that are outstanding or reserved for issuance under the 2015 Plan and the ESPP. Such registration statement is expected to be filed and become effective as soon as practicable after the consummation of the Merger. Accordingly, shares registered under such registration statement will be available for sale in the open market following its effective date, subject to Rule 144 volume limitations and the lock-up agreements described above, if applicable.
128
Table of Contents
We have authorized capital stock consisting of 300,000,000 shares of Common Stock and 10,000,000 shares of preferred stock. As of the date of this Report, we had 37,656,288 shares of Common Stock issued and outstanding, and no shares of preferred stock issued and outstanding.
Common Stock
The holders of outstanding shares of Common Stock are entitled to receive dividends out of assets or funds legally available for the payment of dividends of such times and in such amounts as the board from time to time may determine. Holders of Common Stock are entitled to one vote for each share held on all matters submitted to a vote of stockholders. There is no cumulative voting of the election of directors then standing for election. The Common Stock is not entitled to pre-emptive rights and is not subject to conversion or redemption. Upon liquidation, dissolution or winding up of our company, the assets legally available for distribution to stockholders are distributable ratably among the holders of the Common Stock after payment of liquidation preferences, if any, on any outstanding payment of other claims of creditors. Each outstanding share of Common Stock is duly and validly issued, fully paid and non-assessable.
Preferred Stock
Shares of preferred stock may be issued from time to time in one or more series, each of which will have such distinctive designation or title as shall be determined by our Board of Directors prior to the issuance of any shares thereof. Preferred stock will have such voting powers, full or limited, or no voting powers, and such preferences and relative, participating, optional or other special rights and such qualifications, limitations or restrictions thereof, as shall be stated in such resolution or resolutions providing for the issue of such class or series of preferred stock as may be adopted from time to time by the Board of Directors prior to the issuance of any shares thereof. The number of authorized shares of preferred stock may be increased or decreased (but not below the number of shares thereof then outstanding) by the affirmative vote of the holders of a majority of the voting power of all the then outstanding shares of our capital stock entitled to vote generally in the election of the directors, voting together as a single class, without a separate vote of the holders of the preferred stock, or any series thereof, unless a vote of any such holders is required pursuant to any preferred stock designation.
While we do not currently have any plans for the issuance of additional preferred stock, the issuance of such preferred stock could adversely affect the rights of the holders of common stock and, therefore, reduce the value of the common stock. It is not possible to state the actual effect of the issuance of any shares of preferred stock on the rights of holders of the common stock until the Board of Directors determines the specific rights of the holders of the preferred stock; however, these effects may include:
| • | Restricting dividends on the common stock; |
| • | Diluting the voting power of the common stock; |
| • | Impairing the liquidation rights of the common stock; or |
| • | Delaying or preventing a change in control of the Company without further action by the stockholders. |
Other than in connection with shares of preferred stock (as explained above), which preferred stock is not currently designated nor contemplated by us, we do not believe that any provision of our amended and restated charter or bylaws would delay, defer or prevent a change in control.
Warrants
As of the date hereof:
| • | the Placement Agent Warrants entitle their holders to purchase 155,256 shares of Common Stock, with a term of five years and an exercise price of $5.00 per share; and |
129
Table of Contents
| • | other warrants entitle their holder to purchase 128,231 shares of Common Stock, expiring on December 16, 2023 and with an exercise price of $5.85 per share. |
The Placement Agent Warrants contain “weighted average” anti-dilution protection in the event that we issue Common Stock or securities convertible into or exercisable for shares of Common Stock at a price lower than the subject warrant’s exercise price, subject to certain customary exceptions, as well as customary provisions for adjustment in the event of stock splits, subdivision or combination, mergers, etc.
See Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—Registration Rights” for a description of the registration rights granted to (among others) the holders of the Placement Agent Warrants, which description is incorporated herein by reference.
This summary descriptions of the warrants described above is qualified in their entirety by reference to the forms of such warrants filed as an exhibit to this Report.
Options
Options to purchase 4,357,180 shares of our Common Stock that were originally granted under our 2008 Plan to certain of our employees, officers and directors with a weighted average exercise price of $0.80 per share were assumed by us in connection with the Merger. Options to purchase 1,507,147 shares of our Common Stock have been granted under our 2015 Plan to certain of our employees and officers, with an exercise price of $5.00 per share.
Other Convertible Securities
As of the date hereof, other than the securities described above, the Company does not have any outstanding convertible securities.
Anti-Takeover Effects of Provisions of our Certificate of Incorporation, our Bylaws and Delaware Law
Some provisions of Delaware law, our certificate of incorporation and our bylaws that will be in effect immediately prior to the consummation of the Merger contain provisions that could make the following transactions more difficult: acquisition of us by means of a tender offer; acquisition of us by means of a proxy contest or otherwise; or removal of our incumbent officers and directors. It is possible that these provisions could make it more difficult to accomplish or could deter transactions that stockholders may otherwise consider to be in their best interest or in our best interests, including transactions that might result in a premium over the price of our Common Stock.
These provisions, summarized below, are expected to discourage coercive takeover practices and inadequate takeover bids. These provisions are also designed to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with the proponent of an unfriendly or unsolicited proposal to acquire or restructure us outweigh the disadvantages of discouraging these proposals because negotiation of these proposals could result in an improvement of their terms.
Delaware Anti-Takeover Statute
We are subject to Section 203 of the Delaware General Corporation Law, which prohibits a person deemed an “interested stockholder” from engaging in a “business combination” with a publicly held Delaware corporation for three years following the date such person becomes an interested stockholder unless the business combination is, or the transaction in which the person became an interested stockholder was, approved in a prescribed manner or another prescribed exception applies. Generally, an “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years prior to the determination of interested stockholder status did own, 15% or more of a corporation’s voting stock. Generally, a “business combination” includes a merger, asset or stock sale, or other transaction resulting in a financial benefit to the interested stockholder. The existence of this provision may have an anti-takeover effect with respect to transactions not approved in advance by the board of directors, such as discouraging takeover attempts that might result in a premium over the price of our Common Stock.
130
Table of Contents
Undesignated Preferred Stock
The ability to authorize undesignated preferred stock makes it possible for our board of directors to issue preferred stock with voting or other rights or preferences that could impede the success of any attempt to change control of the company. These and other provisions may have the effect of deterring hostile takeovers or delaying changes in control or management of the company.
Special Stockholder Meetings
Our bylaws provide that a special meeting of stockholders may be called only by our board of directors, our chairman of the board of directors, chief executive officer, or in the absence of a chief executive officer, the president.
Requirements for Advance Notification of Stockholder Nominations and Proposals
Our bylaws establish advance notice procedures with respect to stockholder proposals and the nomination of candidates for election as directors, other than nominations made by or at the direction of the board of directors or a committee of the board of directors.
Elimination of Stockholder Action by Written Consent
Our certificate of incorporation eliminates the right of stockholders to act by written consent without a meeting.
Classified Board; Election and Removal of Directors
Our board of directors is divided into three classes. The directors in each class will serve for a three-year term, one class being elected each year by our stockholders, with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Because our stockholders do not have cumulative voting rights, our stockholders holding a majority of the shares of our Common Stock outstanding will be able to elect all of our directors. In addition, our directors may not be removed without cause, and removal of our directors for cause will require a majority stockholder vote. For more information on the classified board of directors, see the section titled “Management—Board Composition.” This system of electing and removing directors may tend to discourage a third party from making a tender offer or otherwise attempting to obtain control of us, because it generally makes it more difficult for stockholders to replace a majority of the directors.
Choice of Forum
Our certificate of incorporation will provide that, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the exclusive forum for any derivative action or proceeding brought on our behalf; any action asserting a breach of fiduciary duty; any action asserting a claim against us arising pursuant to the Delaware General Corporation Law, our certificate of incorporation or our bylaws; or any action asserting a claim against us that is governed by the internal affairs doctrine.
Amendment of Charter Provisions
The amendment of any of the above provisions, except for the provision making it possible for our board of directors to issue convertible preferred stock, would require approval by holders of at least 66 2⁄3% of the voting power of our then outstanding voting stock.
The provisions of the Delaware General Corporation Law, our certificate of incorporation and our bylaws could have the effect of discouraging others from attempting hostile takeovers and, as a consequence, they may also inhibit temporary fluctuations in the price of our Common Stock that often result from actual or rumored hostile takeover attempts. These provisions may also have the effect of preventing changes in our management. It is possible that these provisions could make it more difficult to accomplish transactions that stockholders may otherwise deem to be in their best interests.
131
Table of Contents
Limitations of Liability and Indemnification Matters
For a discussion of liability and indemnification, please see the section titled “Directors, Executive Officers, Promoters and Control Persons—Limitation on Liability and Indemnification Matters.”
Transfer Agent
The transfer agent and registrar for our Common Stock is Globex Transfer, LLC. The transfer agent and registrar’s address is 780 Deltona Blvd., Suite 202, Deltona, FL 32725 and its telephone number is 813-344-4490.
132
Table of Contents
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
133
Table of Contents
| ITEM 3.02 | UNREGISTERED SALES OF EQUITY SECURITIES |
The Offering
The information regarding the Offering and the Placement Agent Warrants set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions—The Offering” and “Description of Securities” is incorporated herein by reference.
Shares Issued in Connection with the Merger
On July 23, 2015, pursuant to the terms of the Merger Agreement, all of the shares of stock of ViewRay, were exchanged for 31,315,579 restricted shares of our Common Stock. This transaction was exempt from registration under Section 4(a)(2) of the Securities Act as not involving any public offering. None of the securities were sold through an underwriter and, accordingly, there were no underwriting discounts or commissions involved.
Shares Issued to Pre-Merger Majority Stockholder
On October 28, 2013, we issued 4,150,171 shares of our Common Stock, to Dinara Akzhigitova, our initial sole officer and director, for $3,500.00. The sale of these shares was exempt from registrations pursuant to Section 4(a)(2) of the Securities Act a as not involving any public offering.
Sales of Unregistered Securities of ViewRay
The following list sets forth information as to all securities ViewRay sold from December 1, 2011 through immediately prior to the consummation of the Merger, which were not registered under the Securities Act. The following description is historical and has not been adjusted to give effect to the Merger or the share conversion ratio pursuant to the Merger Agreement.
| 1. | In March 2012, ViewRay issued an aggregate of 1,546,472 shares of Series C convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $26.3 million to 18 accredited investors, which included 646,114 shares of Series C convertible preferred stock which were issued pursuant to the conversion of $10.3 million aggregate principal amount of convertible promissory notes. |
| 2. | In November 2012, ViewRay issued an aggregate of 388,290 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.9 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in November 2012 were exchanged for shares of Series B convertible preferred stock in our recapitalization. |
| 3. | In February 2013, ViewRay amended and restated the Series D-1 convertible preferred stock purchase agreement to issue an additional 330,608 shares of Series D-1 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $5.0 million to seven accredited investors. The shares of Series D-1 convertible preferred stock issued in February 2013 were exchanged for shares of Series B convertible preferred stock in our recapitalization. |
| 4. | In May and June 2013, ViewRay issued an aggregate of 996,021 shares of Series D-2 convertible preferred stock at a price per share of $15.12 for aggregate gross consideration of $15.3 million to 27 accredited investors. The shares of Series D-2 convertible preferred stock issued in May and June 2013 were immediately exchanged for shares of Series B convertible preferred stock in our recapitalization. |
| 5. | In November 2013, ViewRay issued an aggregate of 862,064 shares of Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $15.0 million to eight accredited investors. |
| 6. | In August and November 2014, ViewRay issued convertible promissory notes for an aggregate principal amount of $10.0 million to seven accredited investors. |
| 7. | In December 2014 and January 2015, ViewRay issued an aggregate of 935,248 shares of Series C convertible preferred stock at a price per share of $17.40 for aggregate gross consideration of $16.3 million |
134
Table of Contents
| to 10 accredited investors, including the conversion of all outstanding principal and interest under the 2014 Notes into shares of Series C convertible preferred stock. |
| 8. | In February 2015, ViewRay issued 862,068 shares of Series C convertible preferred stock at a price per share of $17.40 for gross consideration of $15.0 million to one accredited investor. |
| 9. | ViewRay granted stock options and stock awards to employees, directors and consultants under the 2008 Plan covering an aggregate of 1,863,031 shares of common stock, at a weighted-average exercise price of $2.57 per share. Of these, options covering an aggregate of 329,198 shares were canceled without being exercised. |
| 10. | ViewRay sold an aggregate of 69,203 shares of common stock to employees, directors and consultants for cash consideration in the aggregate amount of $153,223 upon the exercise of stock options and stock awards. |
135
Table of Contents
| ITEM 3.03 | MATERIAL MODIFICATION TO RIGHTS OF SECURITY HOLDERS. |
The information contained in Item 5.03, “Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year” is incorporated herein by reference.
136
Table of Contents
| ITEM 5.01 | CHANGES IN CONTROL OF REGISTRANT. |
The information regarding change of control of the Company in connection with the Merger set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions” is incorporated herein by reference.
| ITEM 5.02 | DEPARTURE OF DIRECTORS OR PRINCIPAL OFFICERS; ELECTION OF DIRECTORS; APPOINTMENT OF PRINCIPAL OFFICERS; COMPENSATORY ARRANGEMENTS OF CERTAIN OFFICERS. |
The information regarding departure and election of directors and departure and appointment of principal officers of the Company in connection with the Merger set forth in Item 2.01, “Completion of Acquisition or Disposition of Assets—The Merger and Related Transactions” is incorporated herein by reference.
For information regarding the terms of employment of our newly appointed executive officers, see “Executive Compensation” and “Certain Relationships and Related Transactions—Employment Agreements and Offer Letters” in Item 2.01 of this Current Report on Form 8-K, which description is incorporated herein by reference. For certain biographical, related party and other information regarding our newly appointed executive officers, see the disclosure under the headings “Directors, Executive Officers, Promoters and Control Persons” and “Certain Relationships and Related Transactions” in Item 2.01 of this Current Report on Form 8-K, which disclosures are incorporated herein by reference.
For information about compensation to our directors, see “Directors, Executive Officers, Promoters and Control Persons—Director Compensation” in Item 2.01 of this Current Report on Form 8-K, which description is incorporated herein by reference. For information about the committees each director serves on, see “Directors, Executive Officers, Promoters and Control Persons—Board Committees” in Item 2.01 of this Current Report on Form 8-K, which description is incorporated herein by reference. There are no arrangements or understandings pursuant to which any of our current directors was appointed as a director. For certain biographical, related party and other information regarding our newly appointed directors, see the disclosure under the headings “Directors, Executive Officers, Promoters and Control Persons” and “Certain Relationships and Related Transactions” in Item 2.01 of this Current Report on Form 8-K, which disclosures are incorporated herein by reference.
Reference is made to the descriptions of the 2015 Plan, the ESPP and the assumed 2008 Plan set forth under the heading “Executive Compensation—Equity Compensation Plans” in Item 2.01 of this Current Report on Form 8-K, which descriptions are incorporated herein by reference. The descriptions of the assumed 2008 Plan, the 2015 Plan and the ESPP contained in this Report does not purport to be complete, and are qualified in their entirety by reference to the full text of applicable plans, which is attached hereto as Exhibits 10.24(a), 10.26(a) and 10.29, respectively, and are incorporated herein by reference.
| ITEM 5.03 | AMENDMENTS TO ARTICLES OF INCORPORATION OR BYLAWS; CHANGE IN FISCAL YEAR |
Amendments to Articles of Incorporation
Prior to the consummation of the Merger, we amended our Articles of Incorporation, as described more fully in our Current Report on Form 8-K, Item 3.03, “Material Modification to Rights of Security Holders,” filed on July 28, 2015. Additionally, please see the description of the Amended and Restated Articles of Incorporation in Item 2.01, “Completion of Acquisition or Disposition of Assets—Description of Securities—Anti-Takeover Effects Provisions of our Certificate of Incorporation, our Bylaws and Delaware Law.”
Our Board of Directors approved the amendment on July 21, 2015, and as described under Item 5.07, “Submission of Matters to a Vote of Security Holders,” stockholders holding 80.58% of the then outstanding shares of our common stock approved the amendment and restatement to our Articles of Incorporation on December 23, 2014. Our Amended and Restated Articles of Incorporation is filed as Exhibit 3.1 hereto and became effective on July 23, 2015.
Amendments to Bylaws
Prior to the closing of the Acquisition, we amended and restated our bylaws in their entirety. Please see the description of the Amended and Restated Bylaws in Item 2.01, “Completion of Acquisition or Disposition of Assets—Description of Securities—Anti-Takeover Effects Provisions of our Certificate of Incorporation, our Bylaws and Delaware Law.” Our Amended and Restated Bylaws are filed as Exhibit 3.2 hereto and became effective on July 23, 2015.
Change in Fiscal Year
Effective July 23, 2015, the Board approved a change to the our fiscal year end from November 30, which was used in our most recent filing with the SEC, to December 31 of each year, which is the fiscal year of ViewRay.
| ITEM 5.06 | CHANGE IN SHELL COMPANY STATUS. |
Prior to the Merger, we were a “shell company” (as such term is defined in Rule 12b-2 under the Exchange Act). As a result of the Merger, we have ceased to be a shell company. The information contained in this Report, together with the information contained in our Annual Report on Form 10-K for the fiscal year ended November 30, 2014, and our subsequent Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as filed with the SEC, constitute the current “Form 10 information” necessary to satisfy the conditions contained in Rule 144(i)(2) under the Securities Act.
137
Table of Contents
| ITEM 5.07 | SUBMISSION OF MATTERS TO A VOTE OF SECURITY HOLDERS |
On July 23, 2015, stockholders holding 80.58% of the then outstanding shares of our common stock executed a written consent in lieu of meeting to approve the following:
| • | the Merger Agreement and all transactions and agreements contemplated thereby, including the consummation of the Merger; |
| • | the Split-Off Agreement and the General Release Agreement and all transactions and agreements contemplated thereby; |
| • | the adoption of the 2015 Plan and the ESPP; |
| • | the Amended and Restated Certificate of Incorporation; |
| • | the Amended and Restated Bylaws; and |
| • | the form of indemnification agreement to be entered into by us and our executive officers and directors. |
The information regarding submission of matters to a vote of security holders set forth in Item 5.03, “Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year” is incorporated herein by reference.
| ITEM 9.01 | FINANCIAL STATEMENTS AND EXHIBITS. |
| (a) | Financial Statements of Businesses Acquired. |
In accordance with Item 9.01(a), the following are filed as exhibits to this Current Report on Form 8-K:
| • | Unaudited financial statements of ViewRay as of, and for the six months ended, June 30, 2015 and 2014, and the accompanying notes, are filed as Exhibit 99.1 hereto. |
| • | Audited financial statements of ViewRay as of, and for the fiscal years ended, December 31, 2014 and 2013 are filed as Exhibit 99.2 hereto. |
| (b) | Pro Forma Financial Information. |
In accordance with Item 9.01(b), the unaudited pro forma condensed combined financial statements as of, and for the fiscal year ended, December 31, 2014 and as of and for the three months ended March 31, 2015, and the accompanying notes, are filed as Exhibit 99.3 hereto.
| (c) | Shell Company Transactions. |
Reference is made to Items 9.01(a) and 9.01(b) and the exhibits referred to therein, which are incorporated herein by reference.
| (d) | Exhibits. |
Reference is made to the Exhibit Index following the signature page of this Current Report on Form 8-K, which is incorporated herein by reference.
In reviewing the agreements included or incorporated by reference as exhibits to this Current Report on Form 8-K, please remember that they are included to provide you with information regarding their terms and are not intended to provide any other factual or disclosure information about the Company or the other parties to the agreements. The agreements may contain representations and warranties by each of the parties to the applicable agreement. These representations and warranties have been made solely for the benefit of the parties to the applicable agreement and:
| • | should not in all instances be treated as categorical statements of fact, but rather as a way of allocating the risk to one of the parties if those statements prove to be inaccurate; |
| • | have been qualified by disclosures that were made to the other party in connection with the negotiation of the applicable agreement, which disclosures are not necessarily reflected in the agreement; |
| • | may apply standards of materiality in a way that is different from what may be viewed as material to you or other investors; and |
| • | were made only as of the date of the applicable agreement or such other date or dates as may be specified in the agreement and are subject to more recent developments. |
138
Table of Contents
Accordingly, these representations and warranties may not describe the actual state of affairs as of the date they were made or at any other time. Additional information about the Company may be found elsewhere in this Current Report on Form 8-K and the Company’s other public filings, which are available without charge through the SEC’s website at http://www.sec.gov.
139
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| VIEWRAY INCORPORATED | ||||||
| Dated: August 13, 2015 | By: | /s/ Chris A. Raanes | ||||
| Name: | Chris A. Raanes | |||||
| Title: | Chief Executive Officer | |||||
140
Table of Contents
| Exhibit |
Description | |
| 2.1* |
Agreement and Plan of Merger and Reorganization, dated as of July 23, 2015, by and among ViewRay Inc., Acquisition Sub and ViewRay Technologies, Inc. | |
| 3.1 |
Amended and Restated Certificate of Incorporation of ViewRay, Inc., filed July 23, 2015 (incorporated by reference to the Registrant’s Current Report on Form 8-K, Exhibit 3.1(b), filed on July 28, 2015). | |
| 3.2 |
Amended and Restated Bylaws of ViewRay, Inc., effective as of July 23, 2015 (incorporated by reference to the Registrant’s Current Report on Form 8-K, Exhibit 3.4(b), filed on July 28, 2015). | |
| 3.3* |
Certificate of Merger of Acquisition Sub with and into ViewRay Technologies, Inc. filed July 23, 2015. | |
| 4.1 |
Form of Common Stock Certificate (incorporated by reference to the Registrant’s Current Report on Form 8-K, Exhibit 4.2, filed on July 28, 2015). | |
| 4.2* |
Form of Registration Rights Agreement, by and among ViewRay, Inc. and certain investors named therein. | |
| 10.1* |
Split-Off Agreement, dated as of July 23, 2015, by and among ViewRay, Inc., Mirax Enterprise Corp. and Dinara Akzhigitova. | |
| 10.2* |
General Release Agreement, dated as of July 23, 2015, by and among ViewRay, Inc., Mirax Enterprise Corp. and Dinara Akzhigitova. | |
| 10.3* |
Form of Lock-Up and No Short Selling Agreement between ViewRay, Inc., and the officers, directors and shareholders party thereto. | |
| 10.4* |
Form of Securities Purchase Agreement between ViewRay, Inc., and the investors party thereto. | |
| 10.5* |
Engagement Letter, dated June 9, 2015, between ViewRay, Inc. and the Placement Agents. | |
| 10.6* |
Form of Placement Agent Warrant for Common Stock of ViewRay, Inc. | |
| 10.7(a)* |
Office Lease, effective April 17, 2008, by and between Cleveland Industrial Portfolio, LLC and ViewRay Incorporated. | |
| 10.7(b)* |
First Amendment to the Office Lease, effective April 16, 2013 by and between Cleveland Industrial Portfolio, LLC and ViewRay Incorporated. | |
| 10.7(c)* |
Second Amendment to the Office Lease, effective August 15, 2014 by and between Cleveland Industrial Portfolio, LLC and ViewRay Incorporated. | |
Table of Contents
| Exhibit |
Description | |
| 10.8* |
Office Lease, effective June 19, 2014, by and between BXP Research Park LP and ViewRay Incorporated. | |
| 10.9*† |
Employment Agreement, effective January 18, 2013, by and between ViewRay Incorporated and Chris A. Raanes. | |
| 10.10*† |
Offer Letter, effective November 11, 2010, by and between ViewRay Incorporated and D. David Chandler. | |
| 10.11*† |
First Amended and Restated Offer Letter, dated October 6, 2010, by and between ViewRay Incorporated and James F. Dempsey, Ph.D. | |
| 10.12*† |
Offer Letter, dated December 9, 2011, by and between ViewRay Incorporated and Michael Brandt. | |
| 10.13*# |
Manufacturing and Supply Agreement, effective September 18, 2013, by and between ViewRay Incorporated and Japan Superconductor Technology, Inc. | |
| 10.14(a)*# |
Development and Supply Agreement, effective May 29, 2008, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(b)*# |
Amendment No. 1 to the Development and Supply Agreement, effective December 1, 2009, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(c)*# |
Amendment No. 2 to the Development and Supply Agreement, effective May 4, 2010, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(d)*# |
Amendment No. 3 to the Development and Supply Agreement, effective February 9, 2011, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(e)*# |
Amendment No. 4 to the Development and Supply Agreement, effective May 11, 2012, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(f)*# |
Amendment No. 5 to the Development and Supply Agreement, effective May 30, 2012, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.14(g)*# |
Amendment No. 6 to the Development and Supply Agreement, effective February 21, 2014, by and between ViewRay Incorporated and Siemens Aktiengesellschaft, Healthcare Sector. | |
| 10.15*# |
Cobalt-60 Source Supply and Removal Agreement, effective December 19, 2013, by and between ViewRay Incorporated and Best Theratronics, Ltd. | |
| 10.16*# |
Development and Supply Agreement, effective June 24, 2009, by and between ViewRay Incorporated and Manufacturing Sciences Corporation. | |
| 10.17(a)*# |
Development and Supply Agreement, effective July 9, 2009, by and between ViewRay Incorporated and Tesla Engineering Limited. | |
Table of Contents
| Exhibit |
Description | |
| 10.17(b)*# |
Amendment No. 1 to the Development and Supply Agreement, effective January 20, 2015, by and between ViewRay Incorporated and Tesla Engineering Limited. | |
| 10.18*# |
Development and Supply Agreement, effective July 2, 2010, by and between ViewRay Incorporated and PEKO Precision Products, Inc. | |
| 10.19(a)*# |
Amended and Restated Joint Development and Supply Agreement, effective May 15, 2008, by and between ViewRay Incorporated and 3D Line GmbH. | |
| 10.19(b)*# |
Amendment No. 1 to the Amended and Restated Joint Development and Supply Agreement, effective August 13, 2008, by and between ViewRay Incorporated and Euromechanics Medical GmbH. | |
| 10.19(c)*# |
Amendment No. 2 to the Amended and Restated Joint Development and Supply Agreement, effective November 27, 2009, by and between ViewRay Incorporated and Euromechanics Medical GmbH. | |
| 10.20*# |
Development and Supply Agreement, effective June 1, 2010, by and between ViewRay Incorporated and Quality Electrodynamics, LLC. | |
| 10.21(a)*# |
Standard Exclusive License Agreement with Sublicensing Terms, effective December 15, 2004, by and between ViewRay Incorporated and the University of Florida Research Foundation, Inc. | |
| 10.21(b)*# |
Amendment No. 1 to the Standard Exclusive License Agreement with Sublicensing Terms, effective December 6, 2007, by and between ViewRay Incorporated and the University of Florida Research Foundation, Inc. | |
| 10.22*# |
Term Loan Agreement, effective June 26, 2015, by and among ViewRay Incorporated, the Subsidiary Guarantors (as defined therein), Capital Royalty Partners II L.P., Capital Royalty Partners II - Parallel Fund “A” L.P., Capital Royalty Partners II (Cayman) L.P. and Parallel Investment Opportunities Partners II L.P. | |
| 10.23* |
Warrant Agreement, effective December 16, 2013, by and between ViewRay Incorporated and Hercules Technology III, L.P. | |
| 10.24(a)*† |
ViewRay Incorporated 2008 Stock Incentive Plan. | |
| 10.24(b)*† |
Form of Incentive Stock Option and Reverse Vesting Agreement (Change of Control) under the 2008 Plan. | |
| 10.24(c)*† |
Form of Incentive Stock Option and Reverse Vesting Agreement under the 2008 Plan. | |
| 10.24(d)*† |
Form of Nonstatutory Stock Option and Reverse Vesting Agreement under the 2008 Plan. | |
| 10.25*† |
Contingent Equity Agreement, effective January 8, 2008, by and among ViewRay Incorporated, James F. Dempsey, Ph.D., Russell S. Donda, Jim Carnall and William Wells. | |
| 10.26(a)*† |
ViewRay, Inc. 2015 Equity Incentive Award Plan. | |
Table of Contents
| Exhibit |
Description | |
| 10.26(b)*† |
Form of Option Agreement under the 2015 Plan. | |
| 10.26(c)*† |
Form of Restricted Stock Agreement under the 2015 Plan. | |
| 10.26(d)*† |
Form of Restricted Stock Unit Agreement under the 2015 Plan. | |
| 10.27*† |
Form of Indemnification Agreement for directors and executive officers. | |
| 10.28*† |
Agreement, effective June 11, 2008, by and among ViewRay Incorporated, James F. Dempsey, Ph.D., William W. Wells, James D. Carnall and Russell S. Donda. | |
| 10.29*† |
ViewRay, Inc. 2015 Employee Stock Purchase Plan. | |
| 10.30*† |
Offer Letter, dated April 30, 2015, by and between ViewRay, Inc. and Doug Keare. | |
| 99.1 |
Unaudited financial statements of ViewRay Incorporated as of and for the three months ended March 31, 2015 and 2014. | |
| 99.2 |
Audited financial statements of ViewRay Incorporated as of and for the years ended December 31, 2014 and 2013. | |
| 99.3 |
Pro forma financial information. | |
| * | Incorporated by reference to the Registrant’s Current Report on Form 8-K filed on July 29, 2015. |
| † | Management contract or compensatory plan or arrangement |
| # | Portions of this exhibit (indicated by asterisks) have been omitted pursuant to a request for confidential treatment and this exhibit has been filed separately with the SEC |