Exhibit 99.1
Corium Announces Positive Topline Results from Phase 2a Study of Transdermal MicroCor® PTH in Post-Menopausal Women
Demonstrates Rapid Drug Uptake, Significant Increases in Bone Formation Biomarkers and Excellent Skin Tolerability
Company to Webcast Third Quarter Fiscal 2015 Results and Discussion of Topline Clinical Results on Wednesday, July 29th
MENLO PARK, Calif., July 28, 2015 — Corium International, Inc. (Nasdaq:CORI), a commercial-stage biopharmaceutical company focused on the development, manufacture and commercialization of specialty transdermal products, today announced positive topline interim results from its Phase 2a study designed to determine the pharmacokinetics (PK), pharmacodynamics (PD) and safety and tolerability of its MicroCor transdermal system for the rapid delivery of a treatment for osteoporosis. The product delivers human parathyroid hormone, or hPTH(1-34) (known as teriparatide), a peptide that has been clinically proven to stimulate formation of new bone and reduce the risk of fractures. Teriparatide is approved for the treatment of osteoporosis by the U.S. Food and Drug Administration (FDA) as a daily injection. The MicroCor transdermal system uses a novel biodegradable microstructure technology capable of delivering a wide range of drugs as an alternative to daily injections.
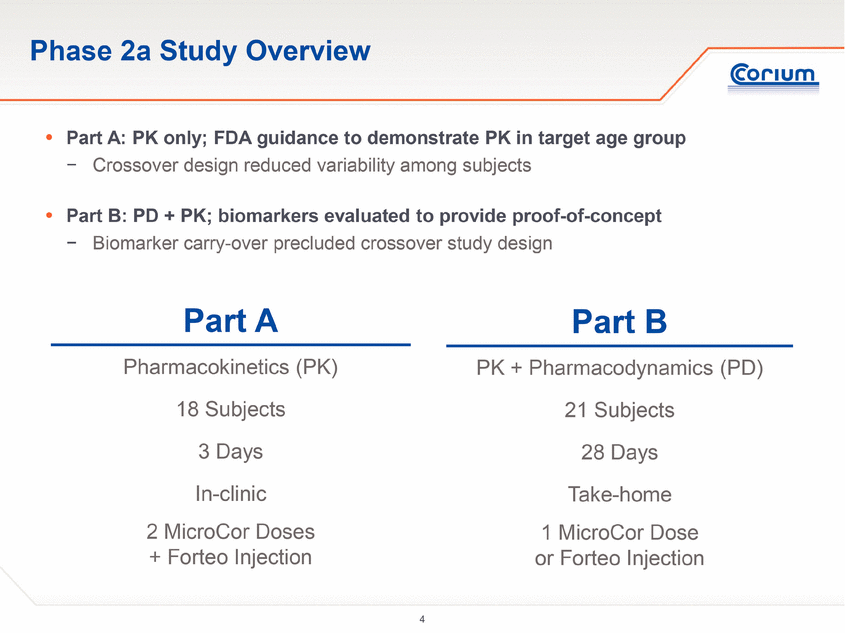
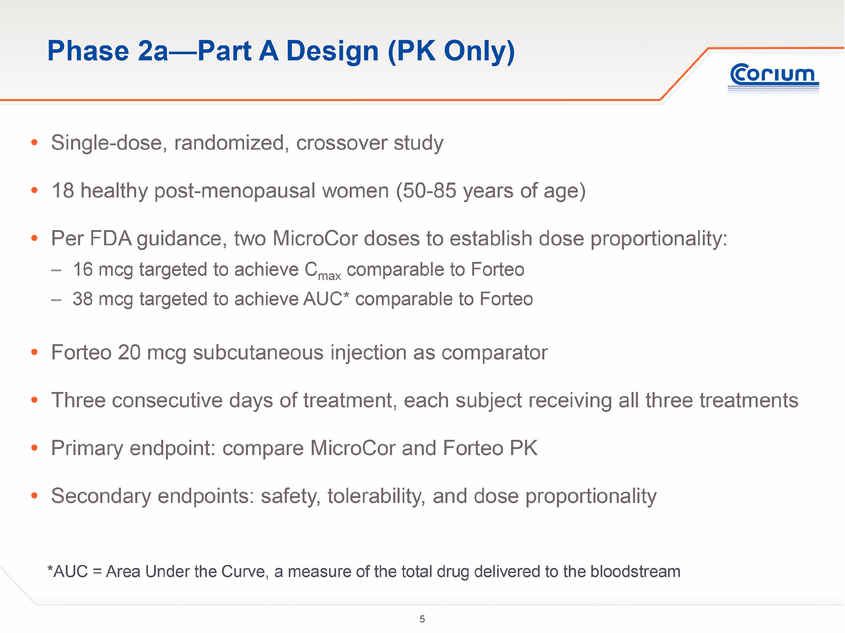
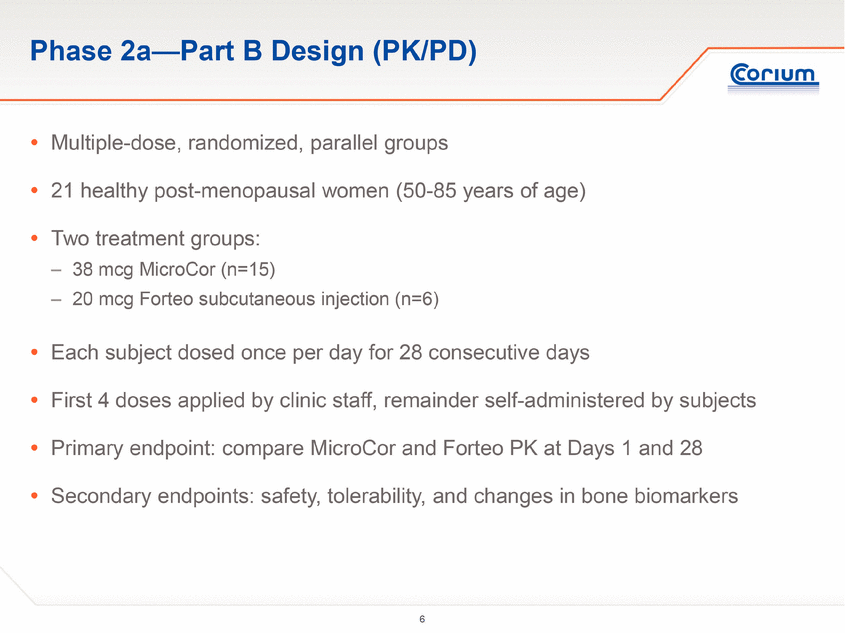
The Phase 2a study was conducted as a two-part clinical trial:
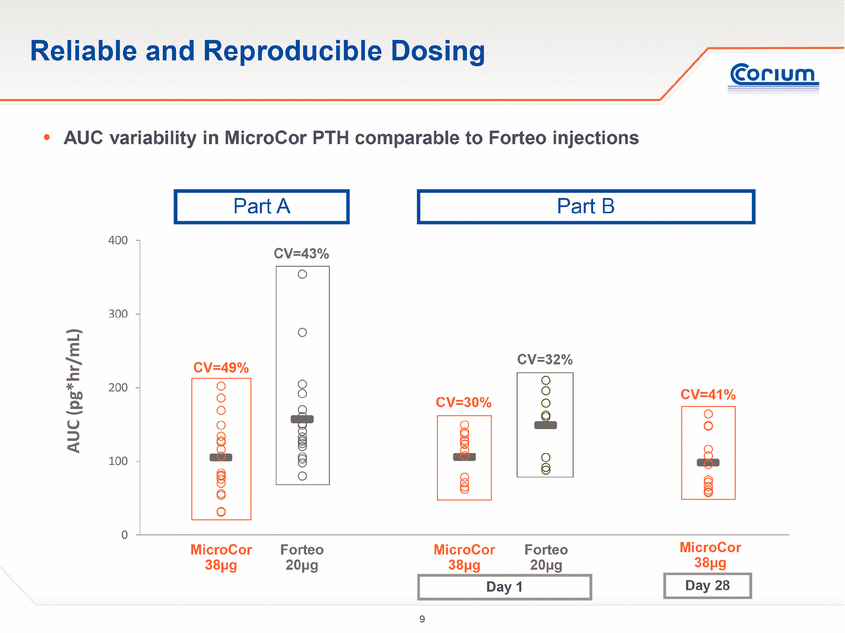
· Part A was designed to evaluate single dose PK and the safety and tolerability of MicroCor PTH in healthy post-menopausal women, compared to the only approved hPTH(1-34) therapy, Eli Lilly’s Forteo® (teriparatide (rDNA origin) injection)
· Part B of the study was designed to evaluate the PK and the safety and tolerability of PTH delivered for 28 consecutive days via either the MicroCor needle-free system or Forteo subcutaneous injection. Doses were initially administered at the investigational site followed by self-administration at home or in the clinic for a total of 28 days per subject. PD was evaluated by measuring changes in levels of well-characterized bone biomarkers.
Key topline results from the Phase 2a study:
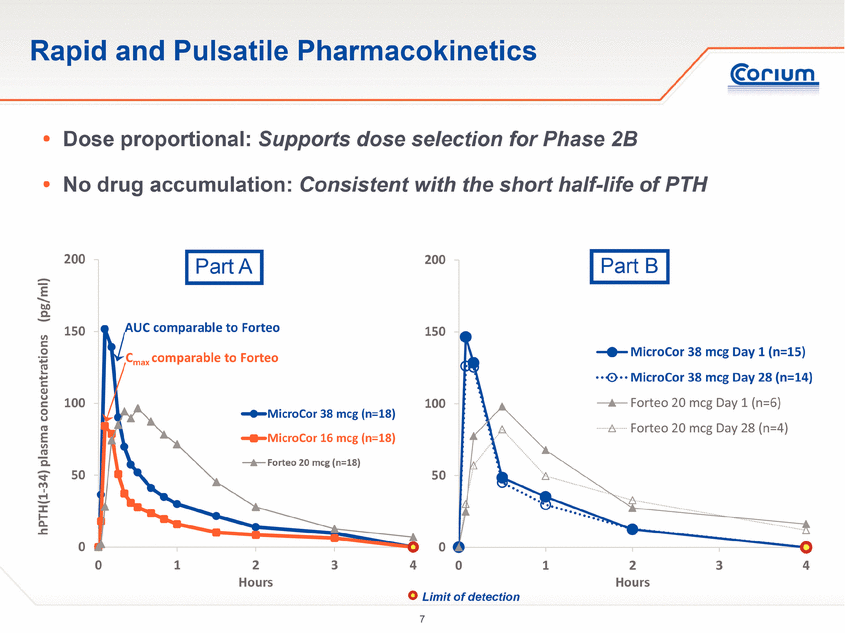
· Rapid uptake and clearance of hPTH(1-34) in a pulsatile delivery profile was demonstrated in the blood concentrations of all subjects treated with the MicroCor PTH system. Pulsatile delivery of hPTH(1-34) is known to be an important factor in stimulating bone formation.
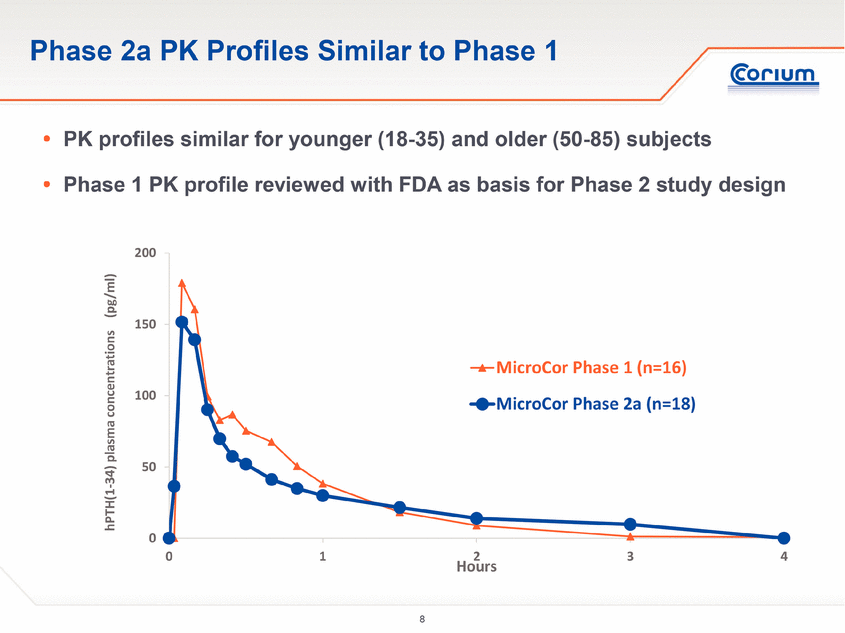
· The PK profiles in the 33 subjects aged 50-85 treated with MicroCor in the Phase 2a study were consistent with those of the 16 subjects aged 18-35 treated in the Phase 1 study, demonstrating the reproducibility of the MicroCor system’s rapid-onset capability across a wide range of ages.
· The PK profile of MicroCor PTH was dose proportional across the two doses administered in the study.
· Subjects exhibited similar PK profiles at Day 1 of treatment compared to Day 28, indicating the absence of drug accumulation from sequential daily treatments.
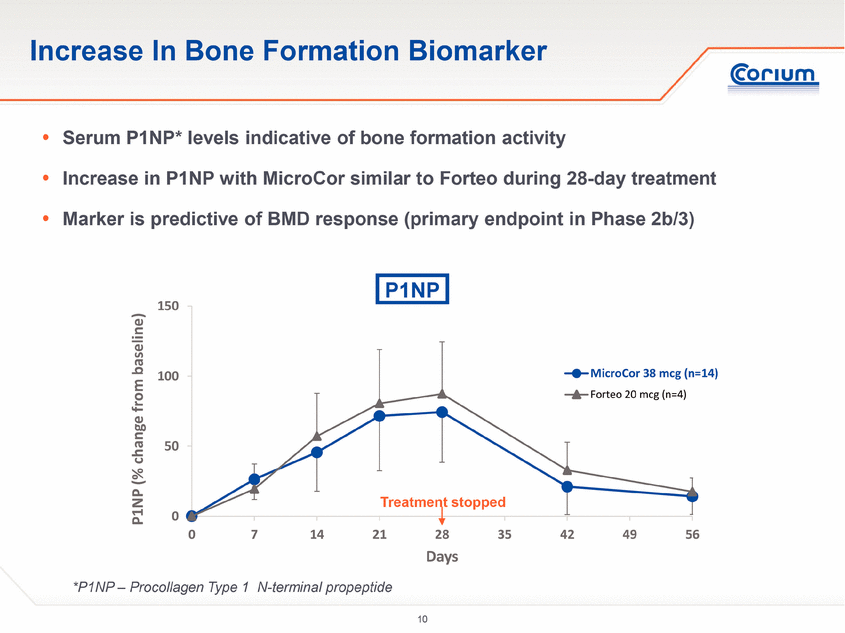
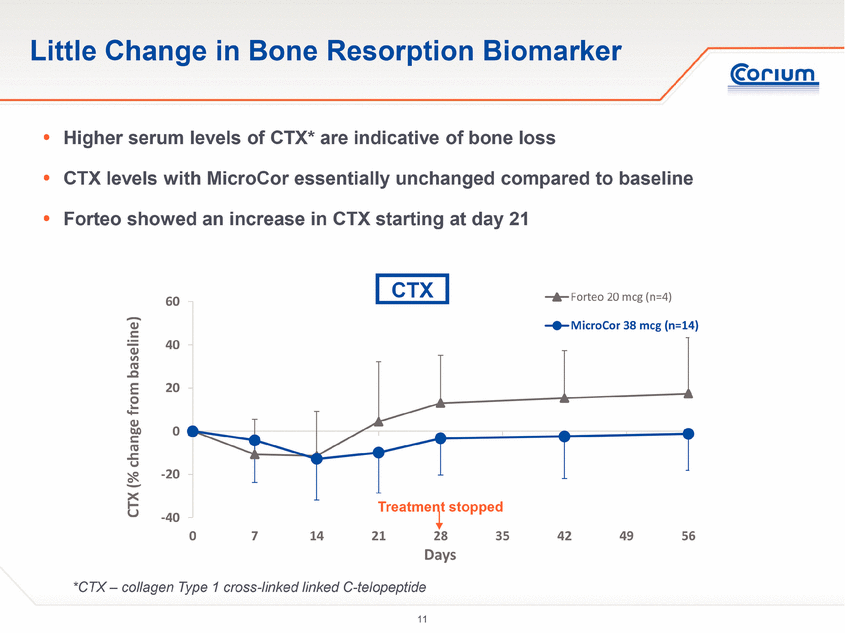
· Increases in serum concentrations of bone-formation markers that are widely understood to be consistent with bone-building activity were comparable among subjects treated with MicroCor PTH and those treated with Forteo injections.
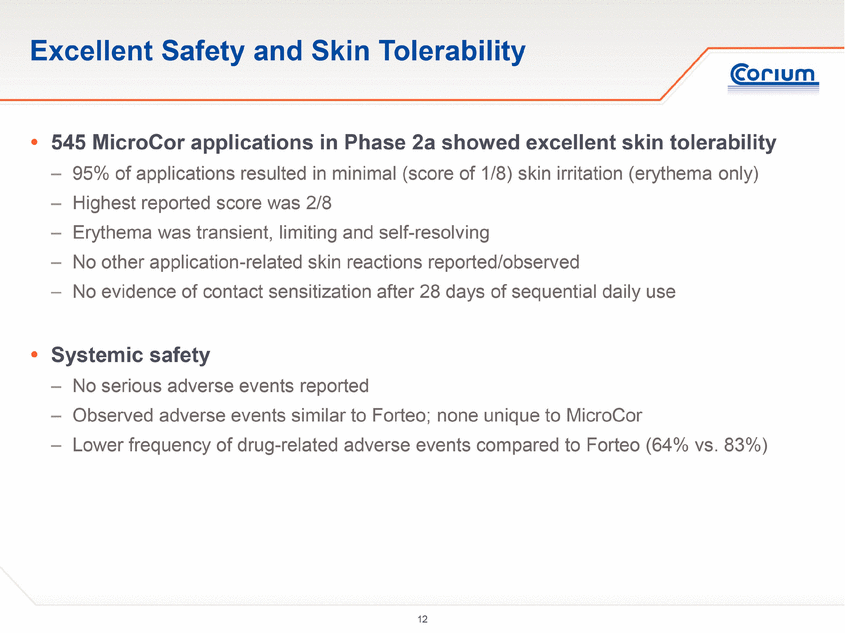
· Subjects treated with MicroCor PTH experienced excellent skin tolerability and no systemic adverse events beyond those observed in subjects treated with Forteo injections. All subjects treated with MicroCor PTH completed the trial.
· Results support advancing the MicroCor PTH transdermal system into late-stage clinical development in osteoporotic patients.
“These Phase 2a results met each of the trial’s objectives, and provide a solid basis for advancing the MicroCor PTH product into late-stage clinical development,” said Parminder “Bobby” Singh, Ph.D., Chief Technology Officer and Vice President, Research and Development at Corium. “The data demonstrate a rapid uptake of hPTH(1-34), a pharmacokinetic profile that is consistent with our earlier studies in younger subjects, and pharmacodynamics in post-menopausal women consistent with the known activity of hPTH(1-34) in promoting bone growth. With more than 600 applications of MicroCor in our Phase 1 and Phase 2a studies combined, we have now validated that our MicroCor platform provides robust and reproducible drug delivery with excellent skin safety across a wide range of age groups.”
Study Design
The study was performed at a single trial site in Australia and enrolled 39 postmenopausal women aged 50 to 85 (mean age of 63 years).
Part A of the study was a single-dose, crossover study of 18 subjects. Subjects were randomized to one of the three initial treatment arms: MicroCor PTH 16 mcg, MicroCor PTH 38 mcg or Forteo® 20 mcg injection. The primary objective was to assess the single dose PK of MicroCor PTH compared to Forteo injection. The secondary objectives were assessment of safety and tolerability and dose proportionality between the 16 mcg and 38 mcg MicroCor PTH systems.
Part B of the study was a 28-day, multiple dose, parallel group study of 21 subjects. All study participants were taught self-administration techniques in a clinical setting, after which they self-administered their medication at home or in the clinic. Subjects were randomized to two treatment groups: MicroCor PTH 38 mcg, or Forteo 20 mcg injection. The MicroCor PTH patch was applied to a naïve skin site on the lateral abdomen for five minutes on a daily basis for 28 days. The Forteo 20 mcg/daily injection was administered in the abdomen wall. Study participants were assessed periodically at the clinic after randomization. The primary objective was to assess the multiple dose PK of MicroCor PTH compared to Forteo injections. The secondary objectives were assessment of safety and tolerability and changes in serum levels of bone biomarkers.
Corium plans to submit the full results of this Phase 2a study for presentation at a future scientific meeting following completion of ongoing analysis.
Conference Call and Webcast Details
Corium will host a conference call and live webcast to report financial results for the Third Quarter Fiscal 2015, and Corium’s Chief Technical Officer Dr. Bobby Singh will join to discuss topline results from the Phase 2a study of MicroCor PTH on Wednesday, July 29, 2015 at 4:30 p.m. Eastern time, 1:30 p.m. Pacific time.
Investors and analysts can access the call toll-free by dialing 844-831-3024 (United States) or +1 315-625-6887 (international). The conference ID # is 97011877. The conference call will also be available via a live audio webcast on the Investors section of Corium’s website at http://ir.coriumgroup.com/events.cfm. Please access the website 10 minutes prior to the start of the call to ensure adequate time for any software downloads that may be necessary. A replay of the conference call will be available for two weeks and may be accessed by dialing toll-free 855-859-2056 (United States) or +1 404-537-3406 (international) and entering the conference ID # 97011877 or by visiting Corium’s website.
About MicroCor®
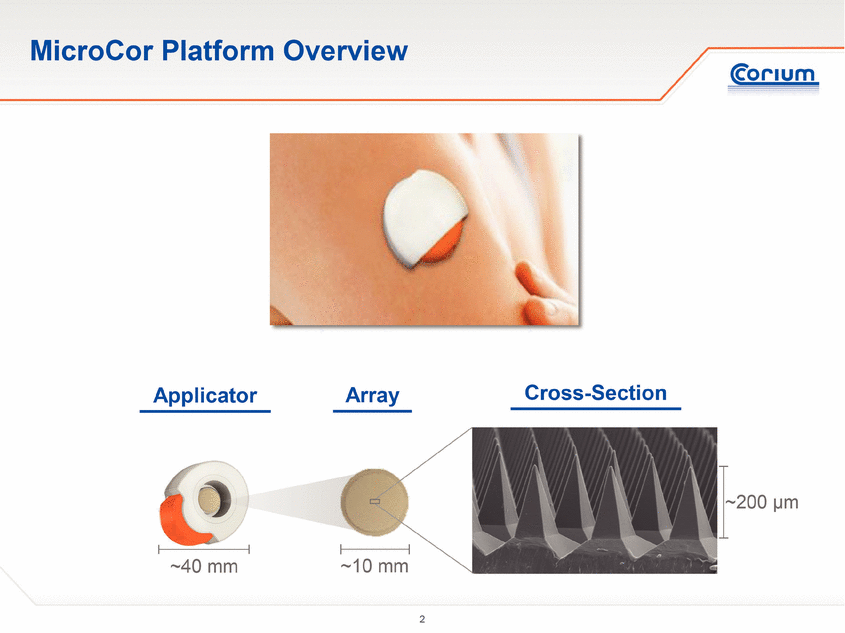
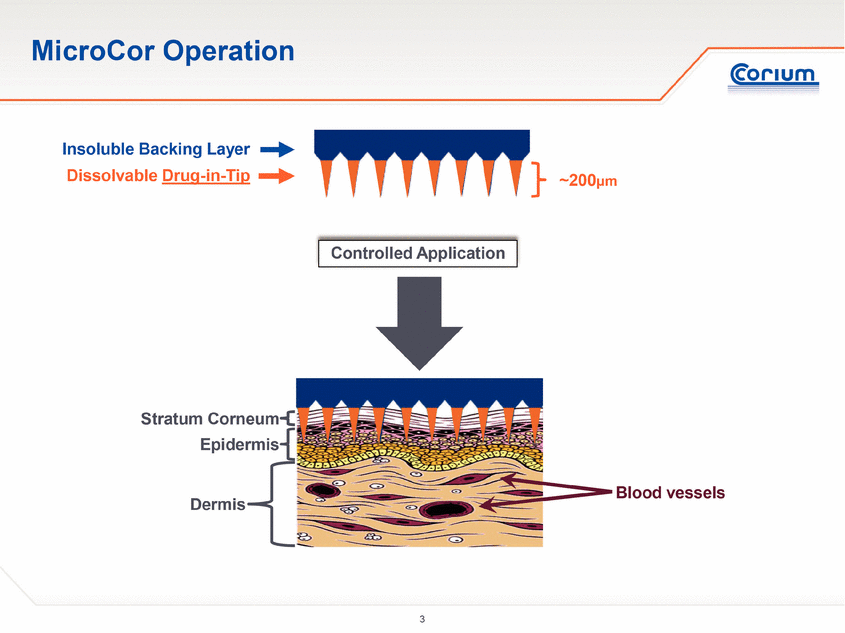
Corium’s MicroCor system is a clinical-stage platform technology utilizing dissolving microstructures for the safe, effective and convenient transdermal delivery of small molecules and biologics, including vaccines, peptides and proteins. Corium’s “drug-in-tip” technology directly integrates active therapeutic agents with proprietary polymer combinations to create arrays of solid-state biodegradable microstructures, or “microneedles,” to optimally deliver therapeutic or prophylactic agents either locally or systemically. MicroCor is designed to penetrate the superficial layers of the skin, eliminating bleeding and the discomfort associated with traditional injections. Unlike liquid injectable formulations, the solid-state nature of the MicroCor system enables room-temperature stability, simplifying handling, storage and reducing spoilage. In addition, there are no needles or sharps left behind after use, providing a safer delivery system for healthcare workers and caregivers. Corium has established GMP manufacturing facilities and quality systems for scale-up, and has developed cost-effective manufacturing processes to support early-stage through clinical development programs.
About Osteoporosis and Parathyroid Hormone
Osteoporosis is a disease characterized by low bone mass and structural deterioration of bone tissue, leading to bone fragility and an increased susceptibility to fractures. According to the National Osteoporosis Foundation, about 54 million Americans have osteoporosis and low bone mass, placing them at increased risk for fractures. Studies suggest that approximately one in two women and up to one in four men age 50 and older will break a bone due to osteoporosis. By 2025, experts predict that osteoporosis will be responsible for 3 million fractures resulting in an estimated $25.3 billion in costs each year.
Human parathyroid hormone, or hPTH, is a natural hormone that acts to regulate calcium and phosphate metabolism in bone. hPTH(1-34) has an identical sequence to the 34 N-terminal amino acids (the biologically active region) of the 84-amino acid human parathyroid hormone. The skeletal effects of hPTH(1-34) depend upon the pattern of systemic exposure. Once-daily administration of hPTH(1-34) stimulates new bone formation by preferential stimulation of osteoblastic (bone forming) activity over osteoclastic (bone resorption) activity. In contrast, a continuous high concentration of endogenous PTH in the blood stream, as seen in hyperparathyroidism, may be detrimental to bone health because bone resorption may be stimulated more than bone formation.
About Corium
Corium International, Inc. is a commercial-stage biopharmaceutical company focused on the development, manufacture and commercialization of specialty pharmaceutical products that leverage the company’s advanced transdermal and transmucosal delivery systems. Corium has developed and is the sole commercial manufacturer of six prescription drug and consumer products with partners Teva Pharmaceuticals, Par Pharmaceutical and Procter & Gamble. The company has two proprietary
transdermal platforms: Corplex™ for small molecules and MicroCor®, a biodegradable microstructure technology for small molecules and biologics, including vaccines, peptides and proteins. The company’s late-stage pipeline includes a contraceptive patch co-developed with Agile Therapeutics that is currently in Phase 3 trials, and additional transdermal products that are being co-developed with Teva. Corium has multiple proprietary programs in preclinical and clinical development for the treatment of osteoporosis and neurological disorders. For further information, please visit www.coriumgroup.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995, including statements regarding our business strategy, clinical trial plans and the advancement of our technologies and our proprietary and partnered products and product candidates. Forward-looking statements are based on management’s current expectations and projections and are subject to risks and uncertainties, which may cause Corium’s actual results to differ materially from the statements contained herein. Further information on potential risk factors that could affect Corium’s business and its financial results are detailed in Corium’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2015, filed with the Securities and Exchange Commission on May 8, 2015, and other reports as filed from time to time with the Securities and Exchange Commission. Undue reliance should not be placed on forward-looking statements, especially guidance on future financial performance, which speaks only as of the date they are made. Corium undertakes no obligation to update publicly any forward-looking statements to reflect new information, events or circumstances after the date they were made or to reflect the occurrence of unanticipated events.
# # #
Investor and Media Contact:
Karen L. Bergman or Susan Pietropaolo
BCC Partners
kbergman@bccpartners.com or spietropaolo@bccpartners.com
650-575-1509 or 845-638-6290