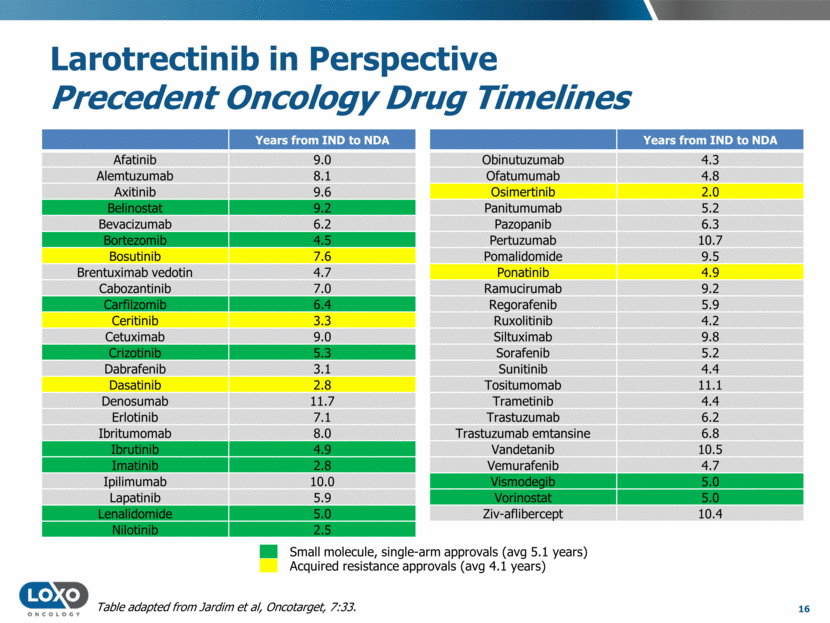
GP,",VW$,#94B&1
M$MP(#5(.&UYMB)MT6D&:UCB.F-!P]'H7\M3!$'CE8'&P>TC2^I:#L[%D6?A
MWV1\1 @>I&:?=RIC2GG''WP%*(04;WGUR=42&>O!LYZ0DCA!2/=<\1FLU32
M4@6RFEWTE&=%*V3:!=;(L/(SSTQ(NPX@*CQ54!X0/0KRF&K^N$PDH0!?.X% +I0\)"NA0PZ"#':TFXE0GAUS,H$D$,8F:MK%XF4V,8&
MTX3 RCL,^:U2M': B180M_CH&PY6"-*'C#5XZ$%.WYH9,-0FQ)60N4ZB3(Y:M[ #BTD7'
MO%2*CC$NY0*0 W2.9SIM&RP^$X*KM"3$OPU6IR77UE51(@_%11D'#VH;%U\2
MEB^5L%L9#S _3: GI=N-[-)2.A2,].^=#_9P8OD"#/S*Y2$B8$<]3DM<"RN+
M+HZ:*T/JNM>%],+)972A?V?FLA0]+GH5\BP ,I%GT((0K$0,WOL*%:E)ANJY
M\"HQ\1X"SIV\[XA]LRYW.3*_R/(%'4TH+V+Z6!0\"4!/?.J%-/>JV<3Z&6OE
M2$)M!W,L)TT9(K[II*L!P.BA4J6H0.[.3;5X,=44$9B*=N1P/XH/U3X$G1,2
M325PPK;'+ U1"XF4=,FJ4%*NV-IGQ>+'&,(_ B@55.4[Q5L5V&/;QA( =L4K
M^]B\$]#^< 0'6.(-@/(L5*@ (PE)WD@F!?4G O" $)@,JQN4-IC#*,;@ 5@4
M1IK2K9?&"$^1YJ23![ #0;3P9J1Z7$_7.4&MJAEYM_R@DPFP@T%8'%FK?5-A
MN\E/B_@N4DU FSN%C35 CVO17-4P9.X*-YL[MB@P9(@[WF"1Q9+P2CE?(C6 8HYDD!8=+EN+W9FD
MM3/&Z@[?28KY$CSEH$4[]UC0R[%"??*V7&WK&@X<]:D/&C0A-5>(0.-E30!(
M+X.T/"C&*)HSALB9;]4U^J3#2F P?H4'O5B']>"PJ#.W ]LRM<,'4\N:N%""
MT$.'KF.(:" IP_*,JEH1W,,]+7L,VJ&ZF*H$OZJM_7"=MLDS;1,> #@B^RL_
M8_N@V'D&!W 1N80XA6+IF:+K$%*A
MM-F#B@VJ$'* NO/B/<@@M:LC+M>I$1#:+.*HC4H0,CW*P^ +ES_RF@Z$Q$ I
M!TW M$DI1#W^&S.V,J[8BZP,FYE>$$1*V*DJ6T6%H!V<<"%<2HV6B;9 ":(E9*CE
M "6 *8?5NL.\$Z,Q44<'&ZBP&JL@=!&+T#)A$@XM\[-C@32C,"Z[T46H<,>(
MDK\V^PHMN@<[;(R8[! X[$/^B[_C"L7N@IS DHPT^K#DZYE*4[\G0H>),8#'
MP;ZY\91D3((DK!W'N( )/="IH9$\*D*\:(K+R/
M&YSGN8\)(0#?0(=<:9]<:29YF) B*SVC2*-0"DCS>*GTZ)#^U("RV8.LNY"1
M>0"-B'RI?9('AKE*4H23U/B8:$ /<\(ZQI%#0[&(6A"S Y),E]%-E>E-4,&B
M)B".5?DU_WJ-VFP3XR;DSHR]8F,J'PO\P.-1"M'*DJGGTE&]+"/
MI2&,/?0: H0* _-%><@5'/ =T)@D>$('$7DAY,D0,%$B*0N$QLB$ VFPHVJQ
MR(@4+M&]U9K+[?G+P(11Y$H]TJ(<3%E,0Z2QV9L\4C+"\K1$:)2)E, OZ-C!
M0:@F)8B+FHJPJ#*<[A 00"&:,C^A.YH'_\R !K*!"=+M-9\1O&,ETK0!"2=
MO4YR0AYHKOL;# <---D8!X3<" R"K_FI*D:1LFX3A!TH+0QZ*!R!,4WX"P"P
MQA.,ER8P#/Z\2@)(@AV0"!TZI#S[T17L#+CSB1W8 2WRKP'YL3U]U"C,2;M4
M$QZ(F;AH2J!<"E'^D!N$JRV9XQ]9VP^N
MPJSJ)->B$*:F9,08K*6-8+35:UC)N,HF(L;@&
MG+(!F!,)32D\G:W\C<)_.==DF7E/0GY/(_
M%%/ C&L[6K#)70N\Z1+HS377$.O0%@A4I4TMJ)'A$DP' $-6GO5AG0,IMPF
M\^.)E#( >@R6*<.!Q#+.E,*;RH#0I00[X6B9C66:E^C))XL4@$G?,A*(VDT_
MP)I:FW0"55,H+9N\CPU<&/73VKJ40&*+B^V-N+S8 %"BD#D,9J,DQ0B ] Q
MBL*>Z $ WC90#BD3'!=
M D@0(2MA##Z,+HJ&0O6) W!5VZ#^$F.HPXU8XXM,1 $.8]# *0
M4_)"N ;KA3]5X=:]6&0U"C>C$A_&U<30MGD8!,,: .>$Q,'T##BZ6 .XSB+*
MX+)-0-(2@#2<(,""N'_44[0UNYF)UL-(S[P#UXBEA.F*UL1PRV< 9)TUC(ME
M0Q<34[G(@6? XP4SO!%5KS$4S0'HXE(CK:DYEG*X1;G@ 2F-X1G^&W*BG$O1
M0'D(!,,2XKQ]4 &;FJK5C8%0L!V8G\I+LHB4$,10@JG48KI#!R7X"@$(421-
M#%?-0YW-($)XLSF^)4*(X==YX''&0UTB5*=[AA^S!U 6D@IIDE SEUP"AE[H
M%A\)M?G^>=9V7I)>" 0E4 (GR 20DX=,:()_/H46<8?;LY-[R.?[B8P7ZV3>6=IHIY>$ 1_!FBO+(=_4P(H_=ONU#S**.F"AI*1
M.$PA>0IYF&B0 >C:8+6^PTAZ85TAHR6/NEG:)&8CE(@@YF4
MP.@?F\J"#@1-> F:%A*B-M?;VZM[$.K0:[-^=H(!@D3T0#L_NCW?J:9Y[L!G
M^+=>B#&$22:WWK/UZ&K#LV>;%EK<6R&K)NR=
MN)(GI&K OF=2.IQ^3HD5(1JDF&QAQD.[]N7,_M:4$(2E>M#^?OYI-?WN
M\2;O\@:7F'XZ6#7O]2;#>>YD[]AL]I;O^7[7:,CM0*@G!Z;O_>9OZ.:?VM+.
M_B;O5"NM81/P T?P%9MDRL'A!'?P!Q]G<90)\8;PY$9= .#;"M?P#?>69M%=
M#@?Q$+>VT_2:2!9QZ&9)'-CM$V=Q_B8CTVCQ&)=Q<9$0 [!Q)=BT&>_M3 @,
M \@!02A7'1?R^>8.&W^W%1_R=V7^DMP&ANWSHGOX(KZ \BAW,5 >ZB"O#&A!
MAQ>E#?
MKDBG#$#_[2GW;2UGJDBO-E"7C$)/[VB>=6/&
M03]G'"70]=N^-GF(F5FG]6[G=EW/@1WHP4 ]V[7=8\@A$"ZAUS/@9HD!VOG
M=CKF:6O^I_5F9L7#.(AZ.)IR]_:3[@5WI0]69HBQ_-R"FSA8GPR$G/73"9=*
MZ/9-MDEZWW62V'>*:2L>H'4>&$,L1[Q*X':B[.URU'6L62Q:/^ /POA:W^X9
MIS#@F8P1N5_5,*%=W8BR]E(1KC]IGC(!D'=P&22(ZXOWY@CL?8R^:7#O!B57
M3;%+TZ(Y!*OL$.R$B3[(!Z5\OP>,54E6G]
MC3@ \JRK (3[Z9#[XKVG3?U*904CDPA\<(S[Y:[$XS:NZ$=>F?,,P^9
MJ*?7QJLNAG&/Q,U\\!SD $"QH\""!:/@6RCM0T,G"B+T(&.0A+R)&? T/&D"7,:- @I0T
M&CAHTB"AC_B4$ P445Z2DR8MJLQ8J: !=35W8A14< ?/B.@H$M3$L"3!2D$7
M/A- 4,"SB)F(0EQJ]2K/0#^Q!3D*#"S;F.90Q,)(X
M@R1)PF-'=8,X!K_L#D#I0LC>^SI9S!@_3YH 5P!-;FE%T [V+"5/#@44(!Q#
M@ $PTE+1&.#@ 6SA$Y-9Q,%%B8,%*.$AB1_15E1$@1#@('9! 7/: %&5.".-
M7>D& %#^N+CHSW1 9":@H'.,XX31OHHSV)5:536:.GM=.!!DJKTHI_@05J0
M2Q'=<\\S9JZ546$ 0/7C8@$$0M\X=/UD)VYTHK.D5YCFN)2MZ#RZ&(5!V:..
MK1A1>=:="]J*:[)PG4<0D1HM:Q6G )S:++8TVG.C717F-&=ONNA 8,-D^D$1$R
M7@#!JB2/J3AY5!,E!.6@X*4402" *R4/^"/."*-0+\X%0HC7RS LM3R4\7(A#$[9S
M-:Z?KF-DZ.0?44K S:(!4-5$!@6B-49T!W+:Q"KM" "R/0.0.4;2@IZ,$ )!
M/J(2X#Y20&M!*2C1"(0@K#<8= R">=>;1_,"1_S
M@!X$V>*_2MPC$]XS0!)ZE!$@,6\0'J'>8@[ O-+AHQR#B%T!48@FQ'8L"31Q-2*0 GK,-K@VB<00;P&BT99"S/ H!1;B*FC)"#3<%"1Q)D
M*0@K\H1\1JL)^FI"J6>JIT/C""0/S"(:.*R%(5=Z&
MKXQ\0PF4>,;C%B), *A,2HT303E$:1!7+0E3/.C%O0I@.]PQQC'NHT1G#"*
M5P7P-*MJU, PDHE[,28))%L(IOQBD*X%96KD1$TTXO440C 3(Y6PCEHHH;B(
M3"F5]%SCB>*VD&-AI%ZX) @/-.'0$MJQIUW95ET6)"%$!D_^2./$!]!.XH:/
M"/!_//%C (;!2&L)@C/K9!*P, +1)\TM,@7(A#W,M,FN5,*3".$I2/8G$QRX
M#EXR"<"\(D(.5XIJJR:9G[A.-859U[T
M?%>-4$$0Z3XGW(-P!D#K%PUU%K5V9#84"8"EQ!D:*O& 5C0;+#Z>@F6>
M]<1'-!HW@26<) XA-.*/HD^IP(F-DAB 'T"D !"D A=CW30C)QTX-D$R.Z
M$E+^K.+!H7F&.2<1@!>Y9%:%KK$*W!2HD:Y+;^7!J/S-QV@!P,9ZN0A"TN;XNI PP&& 10
M@O/$"A?W68L HY33IG#YQO@41+$+(9^J!C">!#)).?E#+WDL7! !G (CO?"D
M $9I / @]CXEUHMCXI4:K08U/^,1@(@'F5V>B&PS[+0!XD(E,5&++A+B1 -:8JKIEPB!K=(MK!Q-;O40F $%6'GTP
M-GF*=>Z+
MFQL- =:-$@.Z%/9@Z2RLS\R<;1HRJI]JCB7F'4OJE6"QR?C\JA?--.N,.)H
M@V X/^3^H7)&UDP0#^]$'L"@CUH/K&L 1 NWP+T9.M9U#PF_I7PTDFQM$+,Y5V(?:@TD*! 8Q8
M=ABIEXEL$YH0E9@Z9I[4Q,JK%]%NY&(+\J\NMBK-Q-*29GO*1Q/13I>&^J1*: MB
M/)+B=\@)0(EQ/(-P2F.*0Z.2B2:PR0#Y3L\]U H '% BC;CD,T8YPH/)=&5J
M/IHU3@U."5P>0"<[T75X+P*D2ZY+IPH/A"8HX:3,]=8VY8W^B."6;G!-.*FX
MJ;X[3_*&HR<+B=<:4UZW02+212IPQ4->R#A:[14#9P3*Q7:F-DW(;+=H8@<6
M-J\?0;Z3K2I60;W \"T0L?"8[$9@? =WY$5\TYU#:#5HN,AITY>2
MTR\=(]C\O=BPA?73NGK?J?$5I "-74A(1'X0 -Y#MRO&=4UKCX]XO9;'_%NR
M\C_C=8PL< <^\F/*X>+:FPW[LG W?(KDAQ%N"06P6!8 @&A?_)L15T-4TM3K
M=48R%*,S_3\Q\CB<6DU,%SX0CS3 SYY "M
M4 I^))6?C$DEK(X@#(?>R!,L
MA,]@R .N$%3'R9J0$ G.S10'?AJH?.(@L1<)WIT]K%I0-)7?942A*(_6Z(=!
M8!P!H=_^U^$2;SQ0]50/2:F$&?99+6($Y*G$LO7@;<@1(9A)]7D<0K#.,RC:
M:8D$+ZIC#YF88]!6_VECWV!*)$9$Q8#$[6'$7$VBK0'7121? &B&WOD=IA0C
MD2G<\4%']DU&%8$$18QB)ZH%""JB4(3%E$7$.OA' '1D1YI$#HP-@Z 6
M/L2@+Y(5(K(<27Z0OXRAIWW8$";&&5&B4V%$XA4$?OA91(C:0JB5YD7$K&'7
M0O3) 'S#6^B*CQ@@)E;2)9TD2RH4#HB>-AG@80!2WXP76>0B;.6@3?2B1E30
M-$)00OXBMN@=M:F$[/%$.NZ=(9E$578E/UY1WYW72;PD1MS^8 Q8K'UR3A&
M1%AY8UR0P]!9W$$P&$_DWT$,P [(D"X.SL,IP6*$RN[-@D("UV.B3H_A)4\<
MXV[HHSL2!E$(I6I1))8)T6,B'%:L8>3A@Z%,AO<8A &P%YX1P#-8X6XE4F1]
MC,&8B6%6F9\$H?\H@;S)6R]HPB#X1XO@CE#VHZH<'E>L'(+%Y5_I1;3T23W"
M9"4VYITMAN<0' !H!G%%"4\"%4%(&N+A$I%@BEUV!0$:(!T1EW.&86T9@ V9
M%$)^!&EIYP9V2 $V94:XIT(@)GDX)UG>B0GR2EX<6$;(HGF6)!TJS"GRQ._9
MF+-Q!,1H5X\#<*(!D 2D ._H-1)((LLGH(]
MR,,+9)_.J :5-"B>Y./MS&1$Q MO^)'W-6;V*6@SS1_#'<%],3B>*=&F N8]BU=].:J4)88X9%02%8 H.!%Y;>)0IEM.8Z)H,,A6;
MF 2$+(0?K2<^0**>M%U6\B> +@Z,1 7'W5J!VE$PUH6BA8:A]&7)Q0FJL,E'
M2F=JX9)Y?02F"&6%PN:%7I4LRHRDJL1?>NA;S(/2J8H!&$H+YEJ^TFGX&1:O+Q&;RG?619IE:@HGD030Q!.2*(,
MK;F,?&@3F68? !V(>8F62KPJ,_1CD&;BKMEA#C1!+\034J5D= X0Y%!G1!#J
M^67GC%#*I90M9R*@.H%]'7JG9AE?.�J6AC/5=DI1E#*MDH>8+;%A!J 5%9HL-;$. Q"#FR74'+<#C2!$VAM
MOFVM$^#'$QK%2.H+USK!UG)M-:I5'>Y$\EU';_ HXA%%>"G^ARIBU4]T[=F:
M;1/XUZ*-1A!F!+A*"69@F/\"9Z:2K*;<2*L^)6HAY5L(
M[*RQ(<8^Q>;^R.ZP:*A4Z)5E!-(**7EI)2NJ"@]1I:8NE[^ZK-VPWU*0Z8[-
M8%[@[JH:GG=.R#KJZD=,:+L]@R,YDA(X4E41+6I\1+R\KC=9)?=ZA7PBR/[%
MJ57XV@_=5$H<;%#DWD+$"^36+$'T)N(Y 2$87)[<" #=(L,&0!.A;TD9&'JZ7!H[-#@HX900%*M'[-F=0
MC)]*+N?(NBE;FBO!WJ+_= W'Y63056K)]N3$EBH+XP/M&D@IFJQG$)Z4.!2!
MYEHE0":FJ095BL]:IA.HD2RRJ.D:S9/=H4,@A._#.<'?.F^RS$6/Y2^J;I(\
M^)SP+J-J^!$]N0[6$8 7C8-_\*U58.A";-6\]I8=XT.N?N=;^$^Q^5'4B@HY
M ,E!$(0WH.#[2(95VYR:4(TR,.>G*X,)XB+9<(DLPMIHI 42
M=ZFKN=7^YTXG#,O3> A&1IR>LP8%FGIN$,MR=19QZ=8P*;L/ )\7V=&KW2T$
MI4B:X-2M#'N:/\&%U5GL;^:E[JKE,V2"( /,J6D+(Y3M4AIP?H6.&NE=XZ3
M6AFB&/N4"?;*K:##.KB#.MB)G&'2V$S)Q+F8D%PPE!H$KZTE/67"8-Q#+SB
M=XQE4&@F)UN+I5@A.=?4 P^-( C"(+2L))+D"A" I?26F]#E,;.*FQD D2"FI,!$G*;O=E0Q1JQL29=>E R:3A\ 2ZN$:AZI
M@L8=U'4I1UDPR:Q/0GKGO@C33W(UQF!,-"14E7@$*_Z;+//^S\W8@X!0=/3R
MZVA.=3)_A!,$60OV]: HTKH8\_ 5K$R=4\-2R0D@1$//*1F]UNU6?54E()U([8 F/,-R>Z(,\\!*">Q.V.X1_VTT[' ?D0=#
MP9:AW)=MZMPS:()N[PFEXF+#DBD.]$(T2-L+JC:JZ5WVF==O*Y\!^/:(C8EV
MCW"D-BL ,,$$W_%C"?$^[5,?._#2^O LJX26@$_'2L9J*O.*Y4 25&U!'$!<
M*QT!<)A95#(I.^@@X4!P_01]"!^;28:&V%5DS,L^%M1'Q!T %*,]V%6A+RJ.
M/B/C;
M^M4ET\K^B8&VF[]IB4O[I12Q 59VQCIG.;3*
MZM@VP#*$2*G**'VX:!=OPZ)#(.FT696TEMM1N(ML%FZ91YK4TH.:-G.
M/<@9IQ%,NCK5%\YI4QL&>W&'R)'[58A,0K8MD2(UN=SPE!I$$W2Z5[H-7 V14%/'!5=4V(
M3.2U;=)N3D;Z3^SF,7=]V^/ &R5861AG;\17PIX4$U[/T (G?/D"O5WCSXBW
MY"6OU+!G,U)@%[V11QS6CY=$B?P%!O>A5 #P0!@W=I^L[0!:N\+]K=,'18Z;
M1 H@38]@QM6R2C^TGY*-S[L_D'LP-BWL]O[%"&TA___/T D$2BP$CZ#!_&A
MH\3# ($!! XTT20/(;Y[@P8FR53^\2"PC$W0<7PF*$>! 0]S!'K&D67+@Y4&
M$G(Y+E!# C@&A71I4-# 7AQA$@#@9*?!>Y5P.#3@)!J^3 ,I%;6H*4G#AP><
MK&19;E!2 3<#Z3PHSXE )5K'$MKA<,#2IBR/\C#9-LE/D4D<$MBA"1]9LV@1
M4B6X$]V@''D/!!HGE:7@)$[XX,)!J=**3*%D\MG42M$\I
M5RPM4- ]@WZ3:V4M$*0\2CA0TXT\LY8KZIE DCK) !XRL8XE[]8Z*;$$QRHKNX,H
M&4TZO-H*"Y_60!+.Q!-13%'%$R5&*-!I[X:490GQAUK
MO(<' 01SL<<>70GFFC& 7+"&)M<\1YREG22)726U&VK<7CHSRRS')
MK%&>+,D\DTDCH;2RS#=['$?,$^W!LAPWCR2G112; $ ).,V,$4FIIF3231<+
M/13011EMU-%'(7W3GG(* &"C2#'-5---.>W4TT]!!50>' "H+-1344U5U559
MY?$X HXK]59::W5UEMQ]92U 8 +-=?@0U6V%GO20* $S^'5;999EMUEE&
MG0 @$">K=;::[&MR)Y !"@NVV_!#5=<50D1H E%QTU7W749'<1=?GN)N)*W
M%(2XDHE3!(82B#6QAZ5G(*8D.'S&L5CCCTM>N1*^$FI99HA[B<;*E"'.Y&:5
M*?$5W@5WV($'E2"M^&.,&TXZ8&!@5IHQ[W0C1%H <$@84I)9O2>'J0-$2*$=
M YG:/Q?[E': DRL*6]H_\:DD@*GACGMJ RC2Y&VYI^85!TH2IF3J 9!^<0>X
M">"8W4J$@KN)1^W96MJNG8X\7WG^""& *,EW>N8PI*66MNI,CRJ !Y%5U9IK
MZ7 P0#>U^ZM1";C9KBC:M0VJ!._; 5 ='TUPCSN 0/;TV^S MX4[@*CFC29Q
MN(MTM/'3,8\>WFB,G59ZCNZA1"@",.R<:JL9/4Z UE=]_O&Q!AD@]]6GWL%U
MN 5 ^Z#9_31(^-[AIGOWN_$_]M+:_Q:X2HQO:DX G[KN)RT!!.!_C#+=^:X7
M07&]3EJ7DR ^@$% [B'$>Y^+E"#$EC7' :!K=I.6[@["NK&MJ&Q34T+"6,>V
M 0J A@LT7@UIF(.ZW7"! >#?U'!0G_L!SC;+ \ .M 0OUA' 9)I((IP>2,(+
M3A%;0Y+^%K6FV(N[$0!I#_M8TR!%OR0<\%.]^!@E,):)NZ'0("I\'_SDAP_Z
ML4T>SP#&'9^AB>4UX8Y]?(L)CV6R9_1"$[W(1!/X%S\.!? @Z!CEG<(H8\Y<9<96)N-&IC"%TT21?N:8XM0] / S'+#*ZA).+T^0Q&X8.S;GP&<^@R#V6] R;561)
MT7#F0XV $TP29_P-"?^/AXFB-E$LB_P
MU,DX*"%1?S9R0%/+@31U@TZ!LK.@8')H1;+93'4@!)K:M,XXM!F2,PUB:@4
M1CQ/*2WWX>,9XR1$+5VB2EHB)(:NK)2T8JF?NP4 J,#@7]=V63L"'BM9)A(G
M.=\"TRK5JYN!^*F3[J'5?/:EJYATB<<&6E*76/.1?K)F.>QQ)FV>LYG2)-U8
MS!B(@3X4ILU\(CK5RY@T"7;0> [$(B;J0 P9;Y&L/
M'E!7)O=][FVMHT)TG&9JW<(7/F[I0H30SYCU.BJ16C++8P7S&5.%*B-OH[B[
M"J<<2I@J 0@ACQULUE?J3>0.XBB/'#@('<#( ?\*T![T*&&UQRH (8:9$/"8
M=P!$T01UY8N0^UJHH+W(P7,!H-T=Z9>_0,%!9;6K)70V(E&L+!)X!D .=T!1O
M6!S@[7XW3'0(5Y$O6MZ?#^(]_T0Q/UK$6PY$QKH^=YHQDOXEVEIYI0J7:)CA$>TYS^+/5P&#?4EB$ZRFH5+ ,3)3:$5&4>RA^L&41-Z:I6L
M-*QT T@"R$J/N&M"BUC-4D$XNS^Z>25KIQVWLR$DM[C#@6+'?,P$3\W,:%;S
M\IIFQ5_>+LYQDVZJ,0TW W:$@.6%&^0J,FH%^' @@F0CB92U>?4"R]F-JX1=9;EANO@3E'"$)[5W2#E"-Q7[G=&!=[
M2Q_&3>3YEFP@")!( A!@(V<>+A@E[K^#T)FXH<&S 1#[8 &@17GYXT&6%=@T
MI\9M@;]&>MYP4);GB@>$"E2"-W4K+;XTU\N+YP%%0!Y$]RRO .N,?:^6'C?+
M&09NJ-;>5 .P^/S8CK42ZT4@:ES)G7@O=MZ#[LGD0>K@I!L8!7CNXI40Z[CA
M0**QEV)1)EF6OYWLZ&=/>H272HC^7LS?D!WB7Z,7:39NY>_8+S.(#++\UZLSK#)B ( /P1P>%2P[YQ $SSF -YNL QOBM2A
M'-JJ"+^+':"R@Y[@>AZ3EH:#*5V+<)8SH<&SWAR29?@1VX@3BI8YWB:K0;@8:"=N1."4 !UT1.U8ZP,%
M;3E KO#<1@ *X%R,(@5'1'%DBKP\2C7( ;QP3 K3S"!F;J<$)/'T@J)Z,),$
MSH**\/&.<+B,L1KS[XH::7F0)_*.:$=:#[JT A+%CC$"[833QU!,-4\Z@15;")#\&YT;W\TTA7UT>C$YJY:B <21!TJ 1CZ[Q?-9B5&
M)>7LI]NZ$7HZD.:("NH08I*P\!,K"!\X29':;SP>K/!ZK7_^IG!/F ]WJ(XQ
MHF@GB\46#<)[&+$O="L=\4$=XLP":Y#?MC(*AU'!BH*3Z/';V*XHD^P\6&<'
M,$8>,D$ET2T%7W*W$ +D7.Y%'NS@(*K&.$/<>J%%"HD(XU&RGK(GT<,(&PD)
M#8*3=I+IFB;[IJ8R1L\KN[)4#,(@8]$+"?,@9LXJX>(98D_D9HX L(&#@(@B
M^([9[#(DH=#T:B&A,$:4-'$;"8I/IJ8@HL'+,LTZ)@EYTFWDUHA]IO$FI076
MBJ*%J"44%2PH#_,CS1)W L 0JPB$NAOX(8H"6-YJBHI2RW]9.7!16F0L(<]OY?AQ.:4%
M>0QR ':@9W(L,*E(X JO\R;L'KW2&L1
M/SFB' YKQ:I3,[>ZG J)
M_CXMR7@P,4M
M!P8!'@W^]$#[M"LY,R$0TR?=E$1I4$ZY$A] 3G\J8I)&)PO)5#@2H]*RP+HX45)'D 4Z/I5?3+B$F%2%2$.5ZU5<7M274TT_7+MRVZ#R@U'<,
MH#TF,4"\!U 1HH50[54;Z%Y+%1\*\78(P+TR-;(XJ? <;T']$U9!-3-W==S&
M(O8J@Q>=1%6'58$@ZTKB;,L LC)8 \E\%32WZ!M.E&I.$ _^>P=Y)$QE&6Y4
MY>A&W0/;"HQ#)R1(H\&*/FJU/B94)=$;DQ);83,JQQ']-O4GT[0E^--*.<+M
MA@MM!C)W?"8I TE :FS>I$)%?65D%0D@>V>GOBYC+2(%6:=W F!FC:)@1XX]
M155/L2E9X:=K'I9K3\A)Z(', [-99PD'@8LD]?_9S9F&I%F_Q
M"H @K< "@!8MVC/D%:,ZF408@MO# "NBF)VCD>XFF >QK,)6NA_%M:Z>'(HCE9NXE4%A[)/F7 P 6
M,!X,XD;64OBU?8!!-3@I=[>((F0SD""D$8EQI16@X@VC#F2+,6.,G'(H;S' &0$
M1LH!&M_^4$_"4G2_S6@'5]#4"IT" 0.0S%12L V)QT'Q(7(]5SQ1EZ6>P3 (
M3?I(>(H$=U*6IX$&]7],F"(;&$U+M7ZWS(\!M$81\L%@69?7%UQ=UR!PET:1
MUE2+>%J;CI'1P1Y.T&"#LWR3.2W##$M40QZ P0GX1^DX0D%]+3B\YV[P) V._9-L]2-OX@BDD04ADO*&
M#=)D6P)*X1:#EJ?4>#&I>> 7N\M_G>A4Q":&X@2#& ' H%968*B 2F:E9E"
MY4$0=D"S9"P(9Q*",'8X=8,NM0*5-SCVD \6$@/>BE(T@G;T2FX#3(
M,*C@:KH]CW=E5[EO2\0[IFI5676K717C=F Q/'BJI._;^%B7^$?D(-%S?<0Z
MO"<'KFN)L(^34&B!A8R*#V(@G_?^!\?6?550H64Q;GC .B(7A4L[3@'I;'Q$
M"M][=YU4=#$(-\_74^<81X\ECC(C?V0ECQF5SO"R)?"P84FFX$K-((6Q;8I1
MD64L!>^AK43 &DX0#:WXDE%V4,]-$@_-LV./MW6,)KTQ;96@-N1!#-M'-4A:
MOJFR5!L2,�MB6(?@+@ %HK@3TG$)P !G%9=,5-5CCX(+3NA'8 ]*(4FX4Z
MH>.F )2 !Y#O4@SR #+A^9ZKP4#Z)J2K@U3CR\E)"?> CB-1R8E5C4_( *C%
MEP94$RAAA&Y8??LSPPFGH(XT,I/L 'QZ:]^ZI8N3O\,8=C 3KP,#*JT-XRZL
MF8W90PG^P_P,46Y*31X$+@;':7FT$B O7"/=9FHF8! (881$N"B@E![U,P9[
MXOO. ^5R@!+DO*U*39VC >,*H,DKJVE8VZ0E%*T+@)TJ808E5,>OQ_KZ$PI)
M+\Z:1K-Q-4*3Q1)#KD5:-\H;"1C_KIN+S5=OU&X--PXMHF_EQ@"J@72"5:L7
M%9!DLKE\!R89([X!H&GZ%J"/=(<;-UO=^:T7G"6\U3:\S*X-0H[)]9Y;E2-0
M[GGY6;G/20GW.<*<;0?F58^=,Y>(M-0/ E$AJ"\0M_CPA:+)+.-6RR'+ 5^/
M)6Z4H)J/E,)[!Q'Q >!36;A/6A*%"L\6GM@QQX])$5DI?1#^)JG(4?:F-=G9
M66K_Y"8 N!'*EUO9N T'D.;'Y$:["$ABK4X FB+W[DH>Z(QP0H;(!'%TO@%A4/(4Z=Z5KX%B,MM[6(XYK0[^&6_/V4T_=*Z[A>\%40
M1 ;!FW/87:)#V7@!!W;(KF)4A["LR"RB$)U#4J3N,AFJ8)J*OP
M>H$M]EG$J(M#[2$3G/PY*:&VYL( ]E3*_0<88D\ >""2+!_U[L6SW!6;3 N2
MINLD>*":O1KC!" '.$:/JLP\V0+^DMOSP6)PS+O,>'J/[%.-NGR:$S>+P/?K
M))(E&DC] &I#$*@+CLD+-<*,(P#"R8"!@O 9/(CO68&! YP@1(@.!T-"#P_V
M8DC@646#E1@.R($.7\>!!J)ME,?#(\B-#S7Q($#@P"!YT08 "! (\),. 3<
M!!"@ "%Y".7E&$@@$T)[*0=21!BMB)."F7+V#]YH.>+HS!]BP!H8^C(BT5]$=#"M!#$1@[\VJ.K$Z
M?@PYLN3)E"M;OHPYL^;-G!]&(R2(4B^B='N!KE3.8+EGSZ*1QH>N]3-U1:/9
MCF8/X6K^VZ^+ @,]2%/:A_.BM8YV3S*Z C^5HLLTB-!HEN4J"0I.>ISM9[W=
M01^4"3D^FL;';0P'/339BC1;FT=X[[;KA^,H1=?4^]ZS2H.N:TI=67O/E).;
M6KA<]N#CE'8VG">W:;AAAFR1!YO+,6WW7PABK<1
MA24B"!DZXY#VC$\ #' 51*9=Y^!&"BYXT&[1=(A/?/R%UAAD]I2C(&T5/==FQ5H9YY_M7634GHN]6>@@@Y*:*'X$-($>,^PB _^,'L1 *:ADDZ&
MYZ267HIIIIINRNF;Z!@0EI^=CDIJJ:8>U 15HN(3R$\XF'EJK++.2FNMMMXJ
MYZ>AXLIKK[XZ1HA;!V0"C"8"_<35K\HNRVRSSCY;F:Y]0DMMM98^,U58 BB&
M$S#6?@MNN.*.:Z>T *Q*;KKJ3G:/(-RZ=5.RZ\Y+;[WV,BMM .C>RV^Z]PS"
M)[P#! )KOP8?C'#"==ZC224.6ZAPQ- ^,X@2.^"00Q*">"MQQQY_#'+((G]K
MSSV,CHQRRBJOS'+++K\,<\PRSTQSS3;?C'/..N_,<\\^_PQTT$(/37311A^-
M=-)*+\UTTTX_#7744D]-==567XW^==9:;\UUUUY_#7;88H]-=MEFGXUVVG2>
M?.DX^U+&]K/R5!)()G$'_4P@?BD,C& LU7.EIG=/6DY_&Z>\37\2WORO$TXT
MX<0@-5(FSR!^[T0(V\ TD<,.@BR^$269[S23ISPD 9FQD^-#S!([G+(1(?M&
M$PCH+#WCN..!5%*P9/?(_A@ZR55"R:&>YV#82>#!1TCR?_[^=B:!O'=A((XW
ML7OOD/52.KO+XT,));E!)T\Y.03"@[S5W3*A &2D 1-/"83_:N3^QX'./'-#0'DHH@#IZP15@Y*"(SPB '@OEJ.*Q1!,!D.!F
M*$$ A["D%V#1I$'&T19/8J8)!R@B;'80% ,(H(6.J>0LNH0 5WQ2'QFB2CUE@@?C"00A45@ 'FRG$@88 /PB$P24D)J_J$0$(MI%[Y"".NJG$,PV2
MB0!0PC; "$0 >"!/QY2%GQMI@@'>@8]>].)(1!F$.?'(4'D<@)F!LF,5"6"
M'22T)O.+1D$MB9EGV(TRGXIC#G*0G$:&Y!Z52T)"$R* 2Q*JDJ:LB"8$D,S+
MC",)CL,*,'QRT8#J2S/%JA16!F%)='#) &C!2HP$029@.$&@TR,IJ>=:+Q(1PU1ZVW&RAE!N(%X_W192Q'2"P&8\QYDDM Z6&0/>=BC
MK$B:AQ+J>1!4JH\'K63)-VDHQ*K^XN-(SQ@.36%3V03)X[(&T01#8]>7 5P.
M&'?)3F;'@X,FQ&<<>!+L>,9CFXU40@"7L\=6+T<>!,GC-;LU2& 7.B$1C<>A
MY;C'/%CTC1P$=4)P1,@\YD@7,I$FL >9!V])0Q[>W@,=V$ 0;U);D4#(A$:/
MX2->Y'%(@_16O3U )+%1Q-N*"H5L6BSY2!'
M;OC*'8/<(W#M 9,2#/":V1;S(#$RY7+N1]UQ/ C%O;$'F3HDW+JD[P NK(@@
M0MN8RVK^9SB;K<* !
MG4HS]+K1(#PH0$BB\9(!W).OR#L()78 Y^+9 V $X($BRXH. MB4QKT8W,4J
M]>*0R ,Q(*5$7#1,>$"%> )TS5':@33O:1!@@$K0Z #8 HP
MB!7C8 =)(, FA"29^2 .T$81$KNJ:5, !4A9S5(+W $R6D)A":C@8.L(R/
M3.Q@'&+DR $&,I9#^=$ >7-";LB1OK)"I+ETV2A]V3( '&C"'KV8=9":P .B
ME&,'$G0@M 5S#R4$XBB9<,>'">#0BX[G $VX0<:@70T+'JWBWR8
M8^L0XP"4\J^%#,#DZQ9$ 0I06;80( ?>JEAR3O$"5Q.Y(A^NT7,"6>,)&>!^
MV+8ZULL!;ASX;1T?'L#887-ON3\I)(1( IK5F[%H@-H@;32UN8]H:SF[@32:
M +;=T774_U:$C\CV3*MA$HCQ+7P 1IC;L\/M-W3<6PG8JWD.8&+R)HBT'#C8
MYLSNBI! T @EJ*[$#@1P"GWRX,3^G5M.073=<%AF_"",#$ 2,L':Q]P#G*PQ
M3LR)B ]!Z-7A E#*3T,Y\JI28OIZ@6RRE?#W)Q& (CR ,3[D)P"$9Z(AE4@"
M4!/9Q4$D@:N:&$ PWE%-'@P"ENC2-0)3R$R"9P"4@!@(!3#FD45TI'$YX Y3
M%%$#@'""L'5< FR#, YH-!XCU#OHQ0.R85(AE8$#0PG UAH#4#R!EA/X4'_/
M PE9Q\$\"IE(0!SU O2=V&@HF^DI12K%GY/,C#CH!T (T&"IPBJ00!81@@!
M4'+7$12>8W7H0%I)4 E;]6#;YE+ZI7%H@20&P6Z0466SU01T4T[X('B")H$Q
M83T!P!7^Y%"!A-!GFU5^A."&D\02QL!5H[1K#T%.1D )2E!]XR%S@B!I.* .
MA% .5 !%"%H6:"!@!,HD8( R!!VQ>&!G4_WW!STJ0$ S.(HF< ]P .!8 #
MHI-#Y/ 0 74 A/ ,459+ F"!VB$(HD6&KH8#TI0$(DB"T3< @M!Q!& 23B
M3C""!8 G, Q@<;.22',+AH
M#O%-,R8SZ/59A" P! -!Z!)-4$1$F@>@C<4CH@/S'@F_/C^$.L&%H(&D+ZU
M S,B +<4 #!6#MP6$3E@#Z>@AH17D/4@ @B%#^$@B@62"9^EAP.@$=OG-S51
M/(%@?NAP?(E$ .:Q#@)G#[E@3>]0 .:G6 ]1">Q(%QC#*AEA$ 39"Z0E08&
M0^<0#PX0"/?0;(. ^^!&*GA! *KU
M O=#6G\H7O) " ?P'@P91KYX)@5P1WFE;V&IBZY'8QW)$ "@!$2!#>-GA$@(
M !3Q.Y9D#WM& /) "0-@'O?0!)(W'MSV4OM%1.WG8ZWGA2BE!+O'9CC@8R67
M'(( %AV#QBP _:P?;"3;+05:,C^5@E*$"DZMTE,N24.L /Q$"0O90^ZECP7
MX4\LZ 1)$)S5EP2WB0\J5W]*L9JD8003P%WHYA;! "J=7[@:0^15W.A%)$*^ P=2%+B1S!=:#U8X88F,0C6
M9!!0E3P?A@Z?8@"ID9248 #80'CDE9AG054#.(X:2E44<9!/@4I81@FS.5F#
M@ '@,%F%0 #EL!RB=%2H8Y, )0"5H EI% U>E#.?PFD$^0P^MEGDL'X%H8/@
MXXN?(@B!)B^'Q3;RD E-H$,FZ!@R!XU52E7R$&$'(8'G, MY:$4X>1 SQ'B:
MMA'V$ 3^KZ(? R-^Q9.$!T0:]5"+OI4#S)0*UN11X[%VITA;SC5'1F&=YT<
M@I!"J;8#.U QH").TA%ZV"*\O ,9>=A5<4#@'0 HH@#,:1VSUBE!2AM-9($
M#G /3D!#>WD F;<#5.0.(4$A9<>5.<"(?G*G*C:GEYE7H$F&=@=!Y2>EJ*1)
MY(!JTJ83"1JL;L9)$:0./9B/5)1(="A3Y7 Z.> $-U5E#XH/ZH ,.^ ]B!X
MP+29.F$$M_EB3Q8-O1"H*32 7Q1J K"(OI:'-'9/XO -X^ $+8H8!DDF@FEG
MF&H10 5*J?&.1R5!U!H;/S0/22D2-*EBW-$$,9@:-"%]O@#^8/.V+68DGV*4
M!.5' (8A>,D",#7" S2TB8*08B*A+T X#B]U*!F#R?ERQ?1P3CN,!#9LX
MK@)0"RK6;$K06'C:!$?D9_HZ,P+Y$+)G$O+C> %0$!I%14DP24@Z#E+:7SC
MBDM2"8GX%@@%X Z$:P#QD D_J02DMP/EP*'X
M< I!< W1"/V,+FZQCX[<%$QLG6+^ZE,X11&XN0,+X$15& %.P)&T-["B+ 8(U%QFL./%G,AA8JW%]NABH&H [/
M< XD0-V,P@*3,$?*5B]0 F(.8M;=S%B45$^?"@_F4+)$@\'$$>]\&(Q7"15
M!ADU47G^"$&VKG:2H%0CF9 $SS9+V+!^Q' 0D]J?Q#>G')<)<*4R//H0Y?>@
M$J%_PPM,.AD-O^I[;6M*@8!8O@9K"#%;RW40$AFF,*9KC0' =_*:4,EQSY&BSU6H
M#C$(PT*,U*MZJ$5%L\5O= .8#A42:-3)]"5P?$I'$B@A3F \(")E8 #A$"A
MP_NCPMP$!.B+7'E'/S6]%Y6$H>QX3JR0"$&0P% .D'E^X@N[UUF^U^G#\J )
M4%J?6)%"B/2^&D?%D$$FR5%E5HD#>Z!P(" /X0"^X(.A:KO^?$ 7/N&C$]$@
M"%]!C&1\%9GTP$%%2MO\T+S#%M;\T!GY$ !;%DZ0"0< A4]'CSA@TFNL8EJL@F?1<,)ZP[9*8P,PQO3EQ^ 1J%7\'K.U
M=I6P!P2P#7H;QZA3J 6B>S^S#KI[J\$< ,ECL.0J@=Z"I,NQI'#+R$MU$/Z'
M%3L I6"1%RBTOT%*?5WR:*USH-P)1][4XR$%H)5#T?%%6#Z##=Y *\"7I3[
M@*,!RS>KIP@D?8*QS[.(92W(I.+^5:JHM03ZA-(Q"[L.112L[$YZK5$BU:V^
MI=E<.43 S1A*;@9(K7^;R36Z2HM5?,>%SSP$F@>Y*P%8#O*3$48EQL;!QF<\V@U&F@4Z]*\2GB R4TB]6*DP3!H*1SC
M04Z5L$XBU2@UNG/M6LMX,:[V@!C/70^]@*YXM6NBZE4'+AR"1AIK_)J:)+W?
MI@EL< "&I=^*;4\,Y0[X*I]!G1/XW:F/#%.!.UFD%88\&VI.O!1(A ['16[U
M&'V21;-:'20@D+:IBY+P \;EY8H5L93)L\Y<,;Z)%*$+.@#8( R:G,]92!KJ
M<,L\XP[^#[1;Z& ,M\=&-AXLP/0I\^A[W#3E@N=]XB<"/WI^AIH:Y#"R*K8#
M+ARFOKL<:70F!L!,*^AK U +=6$ VQ#A I#(6W):L"': $P.K$$!@".QJ D6Y)>^*!([]W/=#$@84N3
M>T9;Y<#KV#CB@J=) ,QF=$1.FC1;R)?6![XJ\ZH1TN*W$P[,H2IS+!@XP#VNL2T<4 %?D
M?I1 %'K9U^,Q[?;+\,L- NN [XR8>;V^E9V3'(;4/P10;/B&9G;.(NN<87A*
M0U8WWC\)0RKF />S XTE#["$Y!6O7C,OQA71M-K.M+#+E0H8$<@N#PZP$DN)
M.K.5//\>)0.2S"G#Y3B40]LB"(_V$8@B2^:4*LFRYBUXJ3UAV.C,EIZF0X20
M&Q*X.MC:&T-$% 29/A(A093-KK!#KH!.BV9"6J844$5F4!(NI1>1 X+@AYDS
MN1')3*?PRIJN;Y4 $=I (E1GJK^D4.!T 0"0*9)L.U\1 "-*@)+$+O!J'*N
MUG5=1,,&_A!N"#^!EE$@Z)FVKY,EP1$ 0)(1"0#)8Y6?>7H%R97\M'U)H)-C
M2'@K_Q!^V!H!KQ:S1%4'T)&:Y(S &48,8$0*!ERT#X,B4,5%.G/(1*M!1P
MN9/C@74P@0G(A&\< 0'/8@K0=&\'@4""(A) =_6B0))* A'8(4_)A'J$ B@A
MQ*- @$K^.Y_=/< #1P >-Y\-&*17@,^6 @3DK:@3W[TF*@,QW2B7AZ"&O>P%
M ASH %@3O$)(K!18*4 FG8Z"0!,4(&7^ 81&"?/0%!\MI7(GDMH1P&N4@6<
M'=AK0%Z8SP0(_7OX\>7/IU^_O5L<^7C#)@0 "_ !A@?3GMF!&8&&
MZ:BN'%K<*1H#B*.$A]@&>D;#5@B@ <_Q^'A+$H\% B=)N($Q@C5FB .10,*")&X09(XL[@5
MQ]G!L8&*]#(');H2:!PR<:BDB:HR2<(=@60],Q,ETM/D0@.VN(D8 I ;"%"T
M)BSQIIW0P:$)"A4Q@"T%G['K@$$T@1&=)%3#Q]IQ!))0H&C@-$ )T\J)[E _
M!R+DLQVT$G-%O;KL,C\HDR3@ $(.)">Z JILSXD#/A4S0;2:$(Q@:0N@5A,/
ML=4V-T.3Z!:Z>PK-5 E@DA!62"7^GKVM6WRBR:$JF*+9X=0FM"4GVV$-]1>?
M>@;YS-4_!&4
MGH](QI0$&8U\?11<=T8)HA<'IN%AQV2',\J. M0(DB8E'7"/KKKMOMN
MO-V[9^\1=Y(''63[%ARFOUOL&Z9YRBEGGIT.?TQ'F/J6AQS'!_)/GDP^S(&'
M?TEM4K0#)2^'UJ-ALD?'T",'?6_(&T?GY\<&:KWUUTL_^L36AX6Q\<,/_[MW
MU>,^\_/8A;>]>*<^K[Q)>XA/-O#CD4?^GG'.@0G6Y"V'O')\*-%SH->1%11P
MVYT?T?G^CTY*%AWN7V?<3HWZ-B
M@S]TE . \$O>WNJ1O?OMQ!WEV%]N*.@>=.Q.4,OSS^"DU[?:22]Z#&R<>_YV
MD_G!;'U'LX<[*I$>>]AF;N"#'4P(P9R\Y5"'.^1A#WWXP]S@ #WWT,0 <+@3
M184)B$MDXD[LH0D3-5&*4Y3/F.!6-[<\C(I;S.$@OL=%,$HQ$TW(71C-B ][
MH(, !I,')19#'WDD@4YGI&,=[3C%C!@ !P/ 3E$(P\EWE&0HAG'%0=Y2/I0
M)(?UX!DB!QF-!3I2DO IY"2W&(@!'&"/@8%C(RWY25#2D8C^34C"( P92E2F
M4I6K9&4K7?E*NV&.E(2H(2QM>4M!3Z,\R<5#XO*]XM0!HWMJ(SWP. I\==<\_YP.,'>0@!V\T
M83,:FGD
MWH!XSB8H01 (!5TE%.14F5;0'DK^X $/#-:>:&"4HMV2*?=F036ZK1-N\"1J
M%6?:'F#@0 DXF&-[GB&(),"JG_'1:#^)Z 2FU@=)V7S4-^,CSE/6)ZLP*5)'
MCJ@>0O S\E%" #P+@ (( "T@<\]-N>4@F M&@( '$^
M8@ ;&Q'HO6L9Z,(Q$#D14IV92VI7AO;]Q#(/\!H0GIXD(.@ZG ,I&#Q()K %D9U@&8-9NVMF<>*_UL;162,]%N-P"!\(]QZHJW
M3/#@'N:JI3R:$-H!C!8Y[I#3#]%AA- 28+0D_>,!YH8.)VB%$J0M&7;'D0.$
M$@( 21C^42]2@X^UU/*HU!5-( PPUH)8*+VFI]U)0;2HG E+I;FR+=D Y-0+7 7
MMST$G%M,H0 Y8,0YB %@U[M5AGFIQ"M*)1A,!
M:$(TQJ&)3+W$. 3&&R76:^M,$."\\4&1:HH8F@6;&!_ED*C^U!)+"0 $AR#!
MR71[T%8W#P^ 9T,9<7U(D\Y!%-<)7_P3#@R@B7%$X[YWCH\]GMPB)QC@9_?@
MC!.B00Y<6T<^*+JR0)X1K!\3H!9Y(\1$I)*#<<2WGYEHKIZ@+6)\5"+' U''
ME/%V;"S/A\EW#B'ATK,#'D .<*E-CD'D/1#2"&#.)1L.^ BPF_KMSX-_4A_\
MY'&3#!H H8-IR9GLD7)AW:/C]Y@N/JA@\" 8>1VPGO^DG \HH- %9CBS
MO[Y%S9M'$C*UHI3_6!%+'?&7?*[0<2KC@=
MD!) (?,X.H8#01@ +6*R\ILC=^.!G'"/U<*]M4&P3,$!2O /7$&67G@;-$J2
M4ILC8*"+@[BY"B0W,1F JR[S0&XB,L!RU.";@G ;@, 4L3QVH))R =>1"$
M $F"C?#^FMC0 B=@'%:9J$&8NL>@A R)27J2D4Q!F,H[]-(XQ'N9 0:4'&&0H>
M8 G > P$!*S\.C/"L#@>F0WPTZY!N+ES KA[(,EQ.P"(^S:!D+R($X ="(2(
M"(TJ8PH8&:XA((!LDCK3<*/^R^"! &".('27+LN8'4BCB/A)3FH(IJB$
M>A@0^*@$;6,R')('N1J-B<@2 5 "-EC'>^B%>PD$A)@X@3C#]\ &RSNHOI&)
M =@/GTB/+AO*)%@O?,B!'? /#V,.TER)0(BA'>@W>SB% !B9M0K.JW&'AC /
M$;2'86"IBHC*AA@) G@QF/"U ;F^R'!('$B(9;D7=[ T>W/,(- $7PM/)?#*
M>Z $F$N"IY2ZN H8%Z$*TZ0(MA"$V,2'X'3('0B PI"'"[E'?0,1T&"+FQ@*
MK1B*'<"K )B;J\B0>,O":& *S!BMEP@$ 5@"+LB! #'Y@2:J' *!C6B,),=
M$E6+#)'^!V @ ! @4 $HC 4#"P"Q2Y@P@@*PG@X3@#B)-V29#,#AL7\[@*+@
M"B;;D"800'QH @(8MQJ=BMVTTJ:P4 QU WP(!PS)@>C(PNH@A^7"@7+8+7E8
M4=$8B@7S1[9T,B-#A\\ K!VUC>]H @>P,K9S4I(XKRK#@4 ("6-#/9U4P %@
M% "E#JZ8!QPXR__D')EP#"< *Q1#OS, ?\ $..(-.TRN;MXL>[TMT65#;+8
M2MJPM,V3LMV8-1-AQJKHD,I1CDH8M.XY@%SPL2;P3WPP L3#AZ2#-P.@E92@
M$WG ",;#-RH5-L("ID0 %O L&#=B5LH=!,+@$'0^!K(0@L =F Z?3X8R>T)CW,;.N'-?S@U28 \UH
M& KD<*,@CD6$8C[J@J>FQ@1NP<#VY8!, 9[@#8<<@("*(?X:A(>D$WJ
MT C4N BT&PUY2[;@*V$$ 1 9#NQ \T-+("-J$K2NC9\
M0-*=V $'Z*=!:*V; S1DL9G7X;'^\M,*=0 !M@&/DJ/2J4.NYW)C0@W\L LR"/3*+D:/^TJ1SV2), !4KM7
M?& &A^@>Q/06C1BOKK53^9*_1'T/^S/00'L4L/@&BSB:T^J[_U3*;K(.)5""
MP7C*D#D,E3J 2@ U6 D;75-R#0XZJ"-M&PZ2"D >9@UDI%#L 15YAH
MKJ_:-7R DW$(X,$H#"75W"HM#<"IB"^:!UDI -!BKRM.#U?=NO.+7(QS(\D4
M@&C(H*CRF?;(B&A@4 ,@AZE0C=>0!]1(L)MC'#OI7QZVT79-WP2$B0R3LB"&
MN!Q>O9G<6@S^"$H;3LZ!F=>[AV>P'D.#D!,L5'8KF4%8*0 P@-! QMSH!1X1
MNZU0#=_;6P-H4>;EX?8(0--P@P,@G=< ' ,0!)B%.3%YAG\KQ@DAS0/(!/:Y
MB:E CL%(A9#IEGHHD@E@9!Q2H\*8C930EB%Q@KQ:.!%CB7;^S8%!0$:00XD7
M>)]Q (9R&!9!8
M-\QH623D5/6U>.*NF$#I $"\LX62N!8$>W#(_Z#&W*B$N1(P9KF'4Q"$L8Z<
M9R#1LUS@@3"+^TO I_9GCTS;?W8*THB-+?AGF[FY6U:CO5NMY@(Q-S)%>W.1
M 4#FM4:CDT;! 'B!=Q"'Y75DYI PSWJ89QB$H7(/"G5-8/A08 #)T.BJ _0;
MBX-7,0D$'8@ &].RT?H)K(B&>);G'>%)2!X(CQ7,@>AG):C6J\A4E3,MIM&Y
MZE7 EE/^%Q&SSH2P% ((7XHF 'QS,2F1DJC,
MFOQ5Z1T(JB80@7D@T7'+\;RX44V8 $H8! P(-G!:8(6=B20@!)5J+P$TW+3=
M@?&RYQ]/@JRU9\XRQ4P!CK.$5_":C$A:K:_,<4$8[51PX8^\!(VJ2O15ZQN6
MC9E8E).1AZREBQGV:1T 4_F0!T)XKY$\Y>'4E!P_[=?Z:-#(8+# P89 A-#SDFU] @5VET0]_.[^ >;A 38G- S7$I.%V&CVK^ Q&-:'4T$E/B
M8#*="#U5KB"<52)\^KF3PX.TQ0'&D:$$GJZ/?Y3+!CD.>Q2W>9W7231FR.6L5@>I"X='T73V
MD-?*V?.THTBXP5ET$'0!C&NMMV#^V7 'AB^'*#(YR:)JP"QW,#D)G#EBG-TX\CL51^ [BBX;K&'8'MU21/"@L!*
M.YZ(=$:CN#( =5AZ4I\'E:7 J!C 0@$
M;35G^1 $W<8(;4/P/PD$(0:QP,M@*.-;F*@RW[H*SMEJ##MWI4$'RQ:$>B#N
M6AF$?ZN$JZ"P>0BS=9:S@IBC&Y8' 5&?RK\*Q_CM\24'@*@D@!*^@@8/(LPD
M !@^'CR4*,&7:0!#A_B2X)A7\%F@: 8"%;QWH,E% L_NX:,DX%FT 80,HGN&
M3Q-%A#;^;^+,J7,GSYX^?^)L.:C@H &]"BK,= _'#HW1"/"8*: 2T0":#CX;
M0% 3@0(@LQ*\-TX>0J'X[(G%00"=2IGX*@40E'(A/GD["(Q#5X 'RGDY+P%R\47S
M6C ) 0/KL!$0X 1GT6BH!YQ^BH,L/B<$"&0JR",'/D("CKX5$'Q'5)SDRAW4
M%""XP5YQ[SD@*:B N]($)0K0E./ 97Q-#)"[69HZOG+E#5(*X(:WQQWX>A%
MGC53N0$@\8U# ADY4* 2^8)4 V-]E3CCL'V:/$5(?1!!+^,.'A,XA5!=%$
M72:_S387=;T,0)5!]^PPP%7XW",004WLAH\Z!6A1$(G R'/ ;*<851Q>2 W@
M%D+HO%<0)0/85E 3 !PE2 &N/,D=.9!R<,.ARDAPCV!_)@A "8:%$@!Y,.#I:A\]%%D-Z3
MPP[/$$"5(,?IE9M+P R02T$M\>=?0>44<( \3WT'IUR$$%"./#E BM ]Y61Z
MD$(,(5L 5=/BPUR&-?6&IZIUX4#^TE\HW9-$ ,_(8T .9)6Y&TW( 16OO//2
M6V]9 PS5G@$&4()L#I>IU$0E.03 @SUVQ3D( : B-M"< 1R5&$$>Y6M0- *H
MJT,.!<1UGP \9#*( 0',EA4.A"0QP(\:*E&)$@%L1==-'PZVD6)I0HS/A978
MUH2P90H0""4Y<$L( 8O!0![" TB@&WH.&#MP ((
MBU0 !4!GSW -[MLO ?]FR]=-*"9ACT%-" V3 3B4,U*&:]W# P&#(+N#/ KM
M0(D3LLEM4RK*%33.MS#M .3C11 (8DZC= )O+@X'4.&),%60 ,50( :&49
MW^X$3*!E!J
M=5F2 2CGP)?8HPFXH<3=U)$+ 9"/"K))GP":4+1"=8]#*G-"( BF!+*(J3RE
MV0$A># SA(-23B@'^!)$)#1ZG.(6B
MQ 'EHHDXVBN/>MQC'L>!@^_H#&H#X$&2ZJ$PJ"@A1-'P&P%:*,%HX( Z3F ;
M)(/SC0G(R2#8Z%P!VI0#2L2#18,H '*V(0FW,,>E#!0#FH('7EP:0!T0@DP
M<)!"::GH()"\2@9W<)E=>E$M!1 8#AC2B]H9 Y+ H9Q!!)$$E(Q#"08Z 'N>
M80 Y!<*6.*D$#JIWBP/(TFDY6%![") $@SA!>\ 0)"'/TH00V:1OZRI(F91P
MF&:"X!E) DE? D@U@Q@![:Y!P=59D* ALXT2LQHH",')D+4=S0!3AP$
M8@>4 MX #D"(0/!@4%/-Y4%@:3F,$>(PZ)"F31EBCTJ 4TTR/$ !6**6%44'
M:@AT F_Z69Y[*&R0@^"!.LHDITH80&6#N$4')&*8NAID'?P4;#70=I%.\R6Y..G40!U/*'@]J7*P,PN*"S".^)@6!L-($\F&M7+R3KN>6,"/P/%-K[G>V!,SPN'1!T QIKJ
M4GSB:-R31>.(UK ,["S!UD7'.1D6AYV\8Q8-^,8-*FV19VR3-P9JP"="![G6
M 1.68)F>@\+&,Q[T9$&'^1X9IB>'A8L2/=>%RL_@<[0F\PS^Z) XS"&A\$UB
M,FEGC4/*PF4&.(848D$1YL1%H2R+OQER91MS!M[Q/?1@XI)>O;;WFMCVUZDZ84F>L%M
M3:2B%MX>-[=[D0MR=]O;X1YWM]/=[G+#>]RG<#>\4S&+4^3BWNCNA;B]G>YQ
MUX(5]$YW*E@1[G_S.Q7EC@ERL^UPG]S#'0I;LKWL$0B1/CSC>91'$BJL\8_S
M9!Y. "3(2QZOHPW;Y"I?N3R64(N5PSSF($>'ZY20A)LOX>8ZO[G-=\[SG?<\
M"3T/^L^+KO.@(_WH2L>YSXTN]*7^)]WI2@#&.&HF\VQ;4S::KEYRM]>'YVYWBSU$>'??.]_[[O>_ S[P
M@A\\X0OO][<;/O&*7SSC&^_XQT,^\I*?/.4K;_G+8S[SFM\\YSOO^<^#/O2B
M'_U&ND[ZC_/IJ:=?/>M;[_K7KW=P@QA$+Q#O+&#\-1->WPDW=V"HG$0C$\(7
MONEU(@]-[+Y>T?@K)2A.F%X,7Q.BCA=I5(\38*2W+%'-@<=A[_WO@S_\JZ]E
M1"/*V9VXXW#E)VJ\RK2Y'6#6)DX( /T%$-$#6-TFCJ$7, :!9M 2C_T90/S9
MQ.X 0 #^G,FKS$NJ_ =/<([VW(0@H%(E/)/X6> %8F &1I[CQ,DS]$(2 #&
MA<3\-0'V9:> 44F$56B':'0YF<0[9\%@)
MY90@?$EAV5Y!H!8.!%9.^$:1'$YZ(=G^G1T-RLUP($2 [("=M4\#V@1DP%.&
MR$^*O=U_V0U(O!U:V-@+-.$]W%,3R,4P%(#U7>$C0F(D2B*]I$IS3)432.%!
M:,'U&$0Y- %R (/Z\,"*:$(3N$4F.$$T*$$3$ +WY03=%(G^2@P&,& $#]2>
M%^$+C#1!DMC#.,#(#BA%701")BC!#M!-9"Q9?%Q-6($)0C2(FA26NLC-/#S-
MY"S9.#@!4U0"KPU3-B5!S:!#"[422KP##F@/.61CX4A8+X! $SQ*?4UB/,KC
M/-*CS8C@3=2##H@ #3Y#)RD!.%%%2YP3.>S%.EW&(.QA"ZZ%C265;0!#5_RC
M#6;% .! RK -0PT #FK%9-3.;P!4$BR9;V2B3N@A0D@3=,Q*#J2,$4X&U."@
MPZ3*#2D!*=D&QWW,\03"?)EC2V8DU R%/9Q"( #-2-9C41KE4<*>=32C QZ
M)3JA2;0DI 2"47 )2["-("3D01S^3B#B5NT"EW^Q3R@!$UH@CP4P&A>A+!4#I_=QF**0$3P@ 2L
M0SV01GB(QUDM96>JYWJRI^,I)5"D2V;JTD925(F0(0 T2SD$0A,08 MJ#C!4
M0@'@0+7^)0:8E(;TV>!J_ LI_44..,"7N /SK"9='D02N E00 :@1,JDQ-#<
MG*4!K,U?' 4(([;-%7K87_-"@. ( 6V,,$O) (V-1?L.AJ&1 EC&%[ZNB.
M\JCHL8@.-]!\$#8G CMM00@[=];C U1XD0:(@3_U!U"I$E"VN5FO(<3&(!'7.?1]<*,9)+" )\EQ_]FB@"NJ@?AWGK(]>\LY-9(W5)08F3E%U8 AE^$Y6Z@3=
M",_+!,=39-(= <,/A82KI L$W@,YW$.JJ,YPA-D=-:->Y.#^0<#)'J9)1$R<
M0?# ;FPIKI5#J3Z.$Q1 F6! 3I&#/.BF$MB#J[AJCA)JLBKKLJZ7ABBI@![8
MV+5$P]6%N8C=7_"&;U!*UO3"5&:?H,G?0M;@NN3(E2C)6C3JKCPJ:TP)!TW:
MB6)&F*%F]PA" �H0%(::+#IP3''7U'FI"$-"4):DU: 62&.S1EJ,A,+Q1
M)MC#3FI0DF1"!#%KQ5KLQ>81.N! "57"DS!.AD2A3=0K#5$"#[@/2@1,+Q@'
M*CU%1 1(W-A$7B67MN+:_%$%(9 ,,,Q*R0P OO3"7=C&'<%?):R-L-&)0235
M(!2)0AC ("C"R^1 IL15L4&& 33^@1#0D@?T% &V%B5@&BB L3 #T
M97G,0RQEY&5LAEL@B\?!(D($P@%4VZX8@.# 4FP,@!)&1"R.MZ6+7 $2_ $KYWT4? %8W &
M:_ &_ '@W (B_ (DW )F_ )HW *J_ *LW +N_ +PW ,R_ ,TW -V_ -
MXW .Z_ .JR<$\W )RXT/__ ,EX/_V0.1O-AE%(8XI"]A'"$]/7$>D4.G:EPY
M4'&VE>JL'3![R<,V6*_U*@MW0O$( \/5*D&I30;V;?'&O4>I^L0Z%%GRG=C;
MW0,2>1V^YI$;AZF0W80["+%/X#%./,LXC .I_@2MQ0LBXX0\1%FEH4/W6N\?
MWZULK4LEKAASV$E7M,EJ.4LQ;K$!.1P'UX1EL #JQ:V7)IQA>XBN,RH!+/\$(% >O:$+V2&).!_]U4-!P -*/((-@5.:'333V#TF0B
M.:L7-_&&X^0?4CAB3SS#522!12/^1$-G0@R"JDX(0D3<=+QL-8&98Y2=ATSP
M@"D^0R: +$N\+X6QKZ8B$0#AA)009C$"'P\Q'_$%P,E"3I4 B6,%D4-PN8
MR#.\X#SD@D_SQC,,0B70I@<.@M6A"#Q1X'D!#J58P!MA(( L,*0BW5=GBS2"L!PTXCA%#A@W?9
M!G 3 G3^,)OL#88Q9,,0'HIWH\9N+ZV1.LZD 0,Q# (K2%AL]<*41!D^; ,A
M*$)Z#+?8,K*!HP9+6&T RXW;18>1LBUTU'9U=C@E: (.TN)0Z+5FZTPT"/BN
M$((V9Y)5X<",]YJ)/8\B%^JW.;#2X@OFC@ 0AJ,NIR+A-/<.3/ I5Z&X!O+,Y4UV!38"L
MA/E+-,%120\DU:)L#4 JKF@TD,,$N$H4X422&T0MZTT)9X+^7' .R;D..%3&
M 3R#$S3E 5P%!^T "#2B']'H.'A$I]]X7A%R.8 YD0Q'#K"?$V! 6(+$31L'
M_ 7+7=QX<0CH-#O!!&A"ILQ#@PB[J^QK(-R#JS^*)IB- R0*7R *#OQ%3#"%
M&0Y*)B"X 429NMS%-@)C$P@B#RC"-C2H+QFT])BA&=X#(7PS 6! -EU&_YRI
M7?&&715-F _%9@RG[U#"3<\E:Q!$D2;*,T&2&!V(3? 'G)4F9Q&3.V 2%]L
M@[R03C_-(QL5<20!/'E$S8S#"S0#[1S%D^3% 52"1AL8<]C%4.AT#YH$Q]F1
M_^W 5QB +Q#M,^B)ZEC3+5D<03C^CC&<0ES*@Q%H 650!1UIM$R2L!O=-YH71&NFAM+V JMGB"_]T5O@
M25YEU\8'"$$<_CV=42>1TI@\S8,D$CHDP3,\UKHHP2"HPPMTZOY#XWG"_:2O26A*4(K'&%9@@=>/ %JQ&\PP2<@GD *7[\C)\K@4X[+)B/@Q:\
MZ=]K]2#^Z*WBQDT.O- QE8,@ 0/>=$*$,)W$*&\' 8/UJMEH!P/ TF2Y"B0
M"6%&C1LY=O3X$61(D2-)EC1Y\B Z0O7&'>BU UBF)I5RV%/B!&$T \\05C(P
MJ]>!0/@$)3G(@Q(A'@5B;/#M0#M\]=)0*#"+DI, X'H,R:C* 3F,T E '$!INN/-C.5P"*B$$GERYS"0Z]""#J!3@H.0TASUR &CE$0$=I881!X1
M>OG& /(RV>$JI@P@I !*!-D.(7OLT<0) UX81XFSW)&G"4'X\@NP:'@@Q)YS
MSFL")WSD00>')/+#H47VY#IHNF>> >$;?)J:9X=*S(NF$AZ '&R0')1X)D%\
M8),-L4&2L >ILMA0J!>->A&@ ,,4**<6B:@S1T<3ME"B8,$25,R?,KII2H>
M**.D/!'0(PP?&3=JC:&##(N(1AP(J.ZY0@T]%%'G[,&GMVA"#(02FNX1[J!>
M#!@'(24(<." N02I(F\D)+RH$K^DE",!R54]<\H%P=Q@(! ["(/'R4$*?(J
M)VI$ASJ$RJ%$'DH,4+( 8#1AD"Y?'$#L($((4/4F=*I$B$E:&HL$ TP7?HXPP
M00@H(,N#Y,GD/7 ,^&_=L@1Q-@E5G8B(3G$<>,;6A(;-6HGC#LKD@/?2 L8!
MO)JZ)TC^?)!:K1X,1( V.ASPLJ<]?M'QUIZ2V=)-G!R4:G)CUUETW22&Z4%"E3O2L? 9A >OLKQ'$Y6,LJ>7
MI6\EH\Q8!W '/M8A/R7T*2/MP@N^*A$4AX7#-V;9G?ET%#/J_$L>'3H(\H9V
ML!LEK$; ZP7O.O,Z$YX0A2AQ@GMP= !"E,,!SO"15?!!#DW^M&0[3'K&$@)!
M%@ZJ;'#OP0K,+(4.)WPK&KVP%##X)A=;K0,'P(B&!+3!'MVUADZ\,@!M4G(
M0N%C!W\9'B%4$PB:S"5^\Z/3.";P#"HPH5GM,9D4$=(N6EFJ',# 6)^%#="
M[ 'PY";;O!!#!UA8!OWJ&"[-$$(HU2)')@;G9\,($0G0<1-VM$"%18U.1E1
M(F1$D5/BLR
M@[!'#@31FX*N4!YZ4LD"\?$93%FL$O!P0""B@5!!G&<0ZKS.,ZK7+CA11V8O
M%($'%4F)U1V$4NC801.N4BX*T1*F,35A;Y0 C%Y<#ATM20:C'D1/$.W@%$H8
M )$,P(IV$<*60709$0\SFDP\(P>!6&(T=E ^Q ( X,PQD@'MSHG1-%9 ^C3
M]GK7BT#L9#1*=(E9O#+/)@A$<+Q[AF%F(1Y-7-2F!L"(TW+ @W5FSBX.4PKJ
MCO.,7++#1]23671$@!="G&<8#N@%J7C0!,0H00 8"N<&')M*4%2_EH!*K&\3H"*$N0CKA.* $@>@8M8,#N/!D+0)&#@R
M XR8R 'H%,FG-#)JMU#6%[B0CSPH8AA-4$VJKD*(88R7@/L@">5^&YE\.&$
MO29D$ ;H$D.>L8/TTHD29X$449@6EP/@($MF+(]+?(^RM4/2R P^?U0!->%$2,-4+)331DEC!DR9,Z[13%A?(06[.OQ3L%#>=
M2GL"/@#!RL,#'/3V&?=P0P1B+(]RT-A)[D3(7^K( W78@S[^$T%'!B>0 S;P
MH!P3/JNI\**:BI6GJB[TUNK(H2D"D,9%9\6!(.X1B-H>8%C;:0)&I(7<0!Q@
MHGKIK0$"8"(I?Y'E&$A CDWLTH3K)Q1.(O.0$>5@C"'@Y1Q) X ^O"/"
MBB)$'.11W4A=94B_7=MI[MC&.>[QG( [#
MQSR$.!##*5+=XO[V1M;!#$PUJ!S1L,>U[\%%>:,C&D)$QS/0G>.4),ZSS[!V
MP;57CJOD>QT'4<=<^JR6@[CCS>O^:-# VQUP>5SE'K=$B.+LT96'VD,=()]+
M15OF670;SD4PG_;,:>Z1@>A[Y&\>1\!=E GIV>6AMUR47MX%';:H\'S<6=DS#77_.8YO_F]&-QU,>G\Z$GOG'ND*A-)D%/I._]F
MD,B#$ Z8Q56P)]_'_SA%__XR5]^\Y\?_>E7__K9WW[W
MOQ_^\9?__.E??_O?'__YU[_N!;]___^_=:P/ >0 &>J"92 *\9A[MRD[#1"
M'63.)%@B).;!W-YE' 3P(U[D(Q0').('&#!P),BA^'8'N3[B'?!">D*B*URO
M/'+A R4F&C0ARAH.!#LB!4N"' 0P!E_012[05VH0_>1A3:*K'+C(.<9!$W@0
M)3B0.>3!6'!O))B0(Q1)!D^B*Z+A&2J/H9 C!YW#7WH!W'RP +5O$+!%?Y*CP0"N?C9"CZQM![ZH)*:+/8B!(Z#B(WQ"P7C^0SG@A@"L!3Q&
M<",H075D(R0:S CM 93^$!M& BY,XAX$P4N>92Z4X,!*HFQ,(@GB,.VZXP_)
M8TC>14_F#QC41PFD!125([Z8S>),(A!*"#FDI0"Z9/(^(AKH1!!H,2$ XQ9[
MJ"04@@"*D0# !R62JQ518C04C,E@*1?',/K0X1Z P0G60WN2+2,<+QA8L"30
MH1;N : ZHHCL9QE#(AJ8B I7'ZT*$)_)$C7ZD7
MNF40$ ,:C$$8FJ8<""W"*$2)!B'K^&L[T"$5KLR\,F& F&>!VL7:S,M%*B$0
MY&I%"GD$0"@I'
M,B,6X2;&*@70R",:;(U6R,$O!&$ICO)O$/,@(#,0MF.\#"=K$*(7DF N(#,>
M=T>)\#+D<(!VG 125@Y.V+$UUN67YN8/&Y)@"3S-))4M$&+S&?+22;:C'"A3(U*E8GB'
M,)/0C-[#K/KR'MJB.5GD+C6A030!+W-A'$Z!5HA2(SC#:>JB/-!3'5+A-!JR
MMWK!U2J!-#,"-%T&(W[E/9T3,[80BC8Q?AYC(/@+/R\$&*[F/4*+$!!#:&(F
M/!=H,V,314MT#^EQTO!"(08!B*!2^N3!"12!I]3B]"8C!T0@"+X$S+"+AM8D
MH6*$.BS3 8I$9WP#JL:K+^02&VITB<9!'@\ (YK@ &A&H.*CMTR*O.Z#I4!%
M$W(I%9_L,D.$.%1O N(-!R9O+H)""20B&KXA1@!)<78 "0%M.+Q/604]Y 32\4MXH !T9"5W!TL]RX(JHE^ IZ\<
MXS.\R">"*U2W=&KRA##[2E/ U'*^I*$F
M8@<&(!IUXK9$9QP*X(:L- <^TOV2HL" 07"HQ0 *%0?&P:.8!!C*(0?ZBGI&
M+E/;,0FPP0!$0 @N8[DF @?&="(>T@D"X=)HM;;,E0!2<7A.$2%D4D@08ARR
MM=> 81SLQ$E,*P?RR0GXQ;M&L$S)4#F% *!X'-:P\A&I:^ZAF$;
MP-7B3&<><& +ZB$5#D =0,4\GB%P2N,NCD<@7J )XH$8FF00@"%EY &@[!-7
M*D4 O,1+,LM:3^8NPJ@60J8>5 ]N? D#'&8F*($UH"L@
M#\(K;NL@FB!4""M;LN2(K$8AZ*0WG@%"+*^.+D4C+]&:(2K(Z;$6'3J9P
MZ.<99*S 5(=&+JDOB%"Y+!)$")C<.G.O"0 E+7]VL*4&@R!#O#<=D W
M\B<)<"+^25+N /%U6^N5-PC $KAC!U!G*"9-9C#"4BKJ-+]"P,;!(YV$ 'ZA
M/4H6"J5R +1E.Y*D,*NIC99++B#CJQ2G6]_%+0:@EQIJ%<$L" :J">S!CA+B
M @ T') '@=%'A@4I=A ';P#'RQ!!$!$=:X,/2I!*?2BT00A>S/HIH:"=T!1
M'O(W(P(A309!=V+6^8I"7;"12O@L!S!B1V(R5$XD&J95*F:19K#U.,K!;B1S
M#@$J?L!&BK!#0C@$G1B(3!%2V<1D%:':U?#1(("JJ(J$#! 79;^PAZ4HA[6$4W
M3I'H,I^@BEEXI6D2PC9G\53GT&AKY9P*H$/&0&8P(@3)2X931*50480F: LS^'%E5 N$<=L"?\0$;P.,MH IJTX0<)H#'
MC($UW24OS$LL2DZBP/"Q"$ 04&=YH,N*>RFY)J()SE80ZH$8%I8C;1G!1-D
M=" +:I6W$/AU:T9=*@9>3(420&L\LA5:'#1-[,%4#I<-D'$T&(8T'M=BGDTE
MLD]R@5:MB8)*"&'SS5U
M4*@;74CIN.+ B<0D@HL[(\L!NK96F.O($.+'"8[$::$)X]RK8&8V4,'
ML$$$7@HX!HJ]_,R9=@<'!(PG4"<7:@54U' >*F&!8*0H\&3$GF%CELL][!J
MY.Q)$W@@EP2#(;J"(TDK(^!F7:!V@=/+ &A+HF_T8MQ$KRCCMGO#AALJ6PV"
M$F(;&Z"F#LLC$ZJML@(2<&Q"9^2A8I39?C+A% [@'';4=V!/;[@64R>+I7JI
M77AB-*22+#$B7].*D\BGOV_FR@A(RP:-E44HCJ8I!(:;! "DA(ZYV0$H(3*T46P%!Y>8 N(
M(0GZA6%"*WMQ((KB@Y;E9M! JJJD4A L)0EG.R>^A%P*P E.KVP)@;9. :0P
M(!.&86K&JS/F9-NO%!M\(A4>@K?^O<-2>&!HF802>F$"+"$)F" :&)X0U+JA
M"J ):H$OAD)8-,$P!IXVA#Q_3 DFNB/E6X6,"B-6)BN?Y.;V'J3!6*HLU&EW
M, "T!ATX'VNN"D @IMB_!2R+!8!.".&VB,'EX>0 5"=5[]S^!DI&\F,7J<.I
MLB.)?<-2&N@ JD8G$+!T!:)1!L<>5@@8:)1\E^2K'G]8",L>CMGBH&C6
M]\@>7G(\GP5&LD1F5,(E:#M(KB. 8H(Z# ,8XL,_*A4=VG;#DD 3?**4UP4V
M:-+9?T_^*ITQ2[:'B;YA"="A$O $$1M$U DU')2_62YL%1U%'E(F5 C"#B?-
MVI3 GVN!Q L9G( !RI#(0!I7'R$,(2%+IMI3<8A+.J!/G+-T:J*T4P4WUD$
MHP,!($ X"#2O'J$##I24P\<07Y-3^ @IP1D
M%SY%$S;*ZY4#1Q,GA*(I4=<0GZ8]:D8Z:1F@P0&L?C
M (Y*'@7AN\>#4$U\E' 8.$#IGCTMM>IE/6"1H28>'?&EPG!@!S!\@325:\I#
MD!-Y3=Z.4T).GI.V23+AJWJU5@X#!K3P+)G4S^N;+ERY@S:][,
M>?*])EL/'-#D5_2@>Y2:>(5I;Y#HNU>![4#<9.'>A;V:X&-\((=)V09\XQMD
MR-[- SP<^\VAQ(#)>SN8$*$#@ ]9[K [ S;75R-UJ.)H.L0C;9
M\!XA P0,#)J'3@ER04K0.=&$K[[CZTJPQ] S2G04S0XX)!&($^@T\0P^,LF#
MEU-0[:?.3SB<9D\@&E%B3WU.4=)?#OPU!)P!##+U($/H)%%B9S#&*..,--9H
MXXTX8D9..6E-9<]U4\W#T#WWU(2..$7B,T^2_97SHSUI28@/,#GP5%./^-23
MI#P\-B3/.!W)LX,FY%CI%4/J>%7^#D_RW*,EB^0PV= \:UXYCCU0XF//.>+\
MR% Y?5[59I! UD.GE2RBD^<\?J*ST(_JW(E/1W)>M\TZ5Y5#CI=#1ME1/>.8
MF20ADMF):(_HQ-D>EHZFM>2D8$XZZ9:/\?@J(8)=90\Y[OB9YYR5YBCLL,2N
MJHZ$2:)CY:!3I;5CL%.%ZFM:O]X3ZW77>O7CEY16$LV7.#PH)G_W!+)B0ZT:
MN9"7;%[7II!*!LMM0_9TV9$]M%YYU3T]SAMFIPV5X\YU15J+Z&Z(UFMF(-:U
M5R>05\);[,045VSQQ1@+6\E&.,I#8L8@AQSC/94<+/)E)&]Z\LHLMSQQ$SM0
MD@1:SR3^D4-'Z%2"IN44W[?334$9P
M&].$$BN.DPF9(T>C=G^C)VTQ.L"T#HRD%X^C.KK_;8;..#BR[GKK.P==3B:9
MX#[C[3?*X_HS5D9S,#JUMP=,)L^HS7R2\SS3^V3VF&Y9]O),_VUFS^S^.O/M
M/>,G9OHI 97RM%/F3O,QO!+UEH=+(?F906I_Q/,#^J 0PSC>=T9F,
M$+ R
MT;"*($9"":8UY!DBRDPO"$" 0P@/AD,FG@.L)52P8@2$[%1+VY(@ [R9P<^
M)(0)C:2$#A:@*)2I! &L,XX"*E*(&8K7 M@9BLR3VJIK,<[&0<,SL=O5Z(@PKB@X\[*8<2
M<# PBQ$"!]$H1S2RLT:*H8]CDY&(9N01C6?P0 G1T-[0
M4)DJ013@BIJ1V8V2 HQBKA(=.8@A0P:Q@YI Y@#/<,+ 5B!T_:BV. $8@F
M9()?F>B%$_)XTEZL<$6S+,FDHCK5@Q$BH;LIP%O0(8@F].)'Y6BJ)HHD#T(T
MH1(%PZG^$W3*TZX\PPF!<(P2INJ$# JBFYKH12#R.I5,H&=-3I\=4$=[88"T,/<9
MV:P)-VLRCM$TA! :-6<'!2-3G3:5$AT9AUV>2IU*=+4<0/N9Z,HA,W384[.6
MT<0!TC((Q)CD&0#53,T&41V#-F2ZP-!$:-_RNVBDMV0_B^V4-!&7?;ZU39F8
M)0$&48D5)9=%;H7K'PW0E0'-M"&!@(H\FP!>?!1R-V7-%?#^S-76)E36+$Z@
M!$?[#JQF -#S@@1,*
M<-8FN$0I$4$1@M !YC'S,0E)*"6<)WH 1/W5'H!<90YZ"YX<) $=/"#G@C1!
M@#O"TB:PW$$!!&@ )? @HI7!;#ERX&=@E$,"&"#TI$F]ID:#!M)&: *EJ>-D
MEJK#VP_^T)R) */7OU8')9Y([+8FH1X<:H(#FF /
MYL), )7X*D-)-@'R7J2+Z:&PX,FI!._ 154+RH!.AYH
M=+I.(,!$#]Z+;H
MV+4]$'$S7^]JIH;NB!." _Y>*34UQ4 #@1B_(_PI(3C^' E>L%'Q]QDG_RI
M[O.>6:YTUB,O2D J.@X@(&[.8P=*R)X!HJ&%B8"#$/:@1"\7A \2W=CZJQ8F
MQ"<5,\M@UM!%X@17AFD;\QFK-GX,)'3X( B-(7W P%P\00C?L#$=P8#1QQ\P
M=QW<41/B$7V&)%\-,A'U0256<8% I T2D KXL P&\ Q,(!WE0$[KA WK(!PU
M,4.#E7/T1GO257 4F!F#MTZ4T'!W%8#YD6W3-PZ[AA4Y<'7^4H$?[N ;,E.#
MP,!-/_(2E:$)+]!*1Q,-DK5.,0=/@R!58G)SW>0$JZ8.(* )P%!*26 5355=
M_&$,!V!:I>0QEW!2E= ..9 *S^ [_ 1Q'1K4]*"'G%P\M>#D\%'#R)?>S$(
M\K4-$;%GY]$]3N 8J2 "]2$=9@8\R,=?O2,2)#$1^+4I?4<5]!0.#B 8C"@(
M!^<=4+$.+\ *DU$)\W45T<8"%T\$,\Y! />H! 10(NZ >64"0=P8_P!#'4XP
M3BN241+1"YJ(#^^033.&:AZ"1%1B,IHP #B0 SL0")A"C<-1<#QY/5,-1
M'>QD8"N5!/.0!/0X'>RD"3A@#[QD1UTAA4G@#C@P#/;Q9CA0@CJ!H ,LA0."80^/
M29D>UE)*1F)+4 #E0 PB('A(]8^]( $\\6V5P(#-8E5,0 "Y<$*9, $T41/D
M\&B%)HV].&4P9"(MR"$*\DR9@%3X ' \$ @B$!.E%(U,LG_7H75)D9Z>- X?
MAVO>:$CA*(IJ@0/?D -OX5#7E5"49#Y-1QY3=0]DA0,$4 F5\$3D$0CH()X<
M8@SE^2.$,14FII_ M)7V$ 1000X3 '2:@)8[H Z5$ 1PX03?)A>WQI;N04_(
M@: 7=5G281\(2@DW]B ]=R%;H"0DPB$("G_N$ @8T* 0LE+^!3 ,*S6D,[17
M>/4?T#$2(+8#V!2@(E!%DC$4^84/22 (,L.6/&$/"PE,22 B3=54DY$4Z' F
M4Z&5TP6>M=,+\!=,W31R31";^)4#3G!,_2$(##J$+X!_0XF;.1 -\T B4^@;
MH9:C67B.)80/Y' ;. X1 C"M(0@M!+Y*$GC]F&@^A\3QJA674=,D8 ;M .
M.J )SP "\&!B9:518+F:P&":9G&.'E$),E8D[M4+2D (O DA#A!Z4"$/24!N
M5# !X0!,/' 4(!5X5P%F." "&# E0 -/7F>.U //" B2#FF2%16VV0 1L A
M$'$5QK!^#4&G]91=^-B=FY<4_^'^A;EP (*AA,_33:A&=1A "-B ;BCC.=#
M25924@2B&FOW6R_:>_J) X+UEQ&1 \,FDENFFF(2!/;P53_R#) %?_?@!"Z"
M?.@PA/:*#]@P4YD FF_&$'%Q2]]VKEL)E_AP#L.J6&UF#+RTHM!1"^4P 0^2
M%1G[ESL 0C8;;-(1"!!+/_;4"QC@%WPP*20B>QP[#EWA%TG L?PG"%);8>PD
M#Y2 *(K 4G]R &8G',\@ =X0!(* )TZ0"2\P"V\Z4%?+3M75"U\Z*1PV)7G9
M43:!JW$K7;WD)1G2$#S !)0Q>$6R2&>42^X(LN1 7-C! ^'P M\PD6TR44G
M+W)93S7^!5*]4*,6) +IL$T02P@<%(:5H7D,D02ZT:E/QPH8T*8\@'YM%@U0
MDE)N@ 7UX+5M2@G%22("FII6U! @V&;?('],$GV2X3&"T NE9@_X53.$(&,T
MD0H34 Y(5(@\&G#_6$^9L*NX9C*?PR@3IP38.ICFUZT08H@(B!_XD)-6H62L
MX(Y7 FZ$.Q$B925\M'?PBD#8U*\RQ@/D$&7$T 0"D*]%H@1)4(4"H'4O@#NI
M>3[8A$3O06]6 PRF&PC@@ &-V(TG%H[G1@A),0@CUW&5AXZZ9!8$4 LGV[Q\
M.JG-FY[JYS9]3^&%8X@#>S!Q6_,7B((.".*@688.'G-?$S"R]GH0T8"&35R'\V1'
M/) ;.> .XN%6 B B]22<&](P;.I->2D(@J!U@U F"P(@B%%1=9$;Y%OA990
M'B, ,.29F3",YH !,4P%X. UM>-6!$ :M?'[54=P$!.(SP<':4)$K!RF\HB
M.# E0LC,_16<&82KLM1OI"4@Q W1%I4A6[:BHF2@ ,+H=-FN"%X*6$RTC,
M9L4#F6NKXSH(*6L WS##2I +1H#^%ADE4VYP$ZJ1O<^P:UI# (]EKX)@=^L4
M%]QVGB#D#GK'=P9 #!.@!3Q)>(0Q<7=D:=@Q$1XS5VQ@=]C!- \:RF*U&WFI
MJQB %OU+94F &$F #6Y9E7;1"TRUL3O IV Z#DW $Z=P9S4A$ULAH1#2TNB1
M1Z1W2BR*SI,R5^<)$ASA%XXQR#UR%.TQ5='X GPQD2&Q$++19P^BC)[D!+FP
MTE/Q2CD %4#M%PU3"8%@#Z;WOEC3(!C0U=/1=@1 ;$RD32@A+,. '=2$1>9=:Q?Q'IFD66#E0G7
MUDBH<1ACTA R\1JF%-*<8P_N@"64\R-,TCV/H2=X8@_T*RC^6$(?.JZA0V+D
M4[$EB)(D^.)-1,(IDX(E8=X?
M/3*&GG$E4X[D1>[C2@[FK70=4N[C4P$/:M,]7-X00?X.K$(M7?YO)C&04W&F
MVW11P4(D2Y(61'(/C$+E#9$+V87D68(N18(G4]$K1Y[IDW[DON(EUWF>_!OD
M4*[I,=(]"..SXBES05;3I*D\'CFG3K/B+GOB+!
ME-(H6N(GNCXE:58I2?(.\6#D!(0.0:[F&JIX2%;L]((. W-"-)[MVDXC-^65
M?7,01U,ET7"LN-/M%$-]1),),[[^-K)!C&&#[ACS,]Z^[?1>[_WK;I<((4H
MNO9>+,"0S?T>\ (_\ 1?\ 9_\ B?\ J_\ S?\ [_\! ?\1(_\11?\19_\1B?
M\1J_\1R?\,S2\2 ?\B(O\=YH.L#0(\]@6=.!NIAA/*)4&3?U5 P1#J>@"6S(
M)D%VY8NX[N!#1_+0SJPS0IIA1@6R30)0 -H1
M+@>R]J<4Z67/OYN!2)31"P'P9,\@ " %\SEP"L TICA!&DV*=*& \/(\X6&
M3T0L"<,*$$ "H
MCST<)0_0=$+\]E\M.(C=EAG0P;>786:]@ [AL,G?$ WN, Z#ZEY:)/R=(4V6
M\6V#( [;0&M/-0@.L&>K7__V;R/HS#!>N"*H=AJ5X'8 T20:OEZ:-&7"AR\:
M)7O@M"2IA,]>I4I*GCUKH@0A.DK1*UH5
MV$F6!G#TPG=/B8$F)($.DA>V4A)"&%+=O'=O;:"1Y 0%<3*PB:"3Z Y0.JDI
MD#U\:[6,0UF)$,1[IPKL>+;N;%I-E9HL/1G-P$!\XPR