
J.P. Morgan Healthcare Conference January 2022 Exhibit 99.1

This presentation contains certain forward-looking statements regarding the business of Mirati Therapeutics, Inc. (“Mirati”). Any statement describing Mirati’s goals, expectations, financial or other projections, intentions or beliefs, development plans and the commercial potential of Mirati’s drug development pipeline, including without limitation adagrasib (MRTX849), sitravatinib, MRTX1133 and MRTX1719 is a forward-looking statement and should be considered an at-risk statement. Such statements are subject to risks and uncertainties, particularly those challenges inherent in the process of discovering, developing and commercialization of new drug products that are safe and effective for use as human therapeutics, and in the endeavor of building a business around such drugs. Mirati’s forward-looking statements also involve assumptions that, if they never materialize or prove correct, could cause its results to differ materially from those expressed or implied by such forward-looking statements. Although Mirati’s forward-looking statements reflect the good faith judgment of its management, these statements are based only on facts and factors currently known by Mirati. As a result, you are cautioned not to rely on these forward-looking statements. These and other risks concerning Mirati’s programs are described in additional detail in Mirati’s quarterly reports on Form 10-Q and annual reports on Form 10-K, which are on file with the U.S. Securities and Exchange Commission (the “SEC”) available at the SEC’s Internet site (www.sec.gov). Mirati assumes no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. January 10, 2022 Safe Harbor Statement

Our vision Unified for patients, our vision is to unlock the science behind the promise of a life beyond cancer. Our mission To discover, design and deliver breakthrough therapies to transform the lives of patients with cancer and their loved ones.
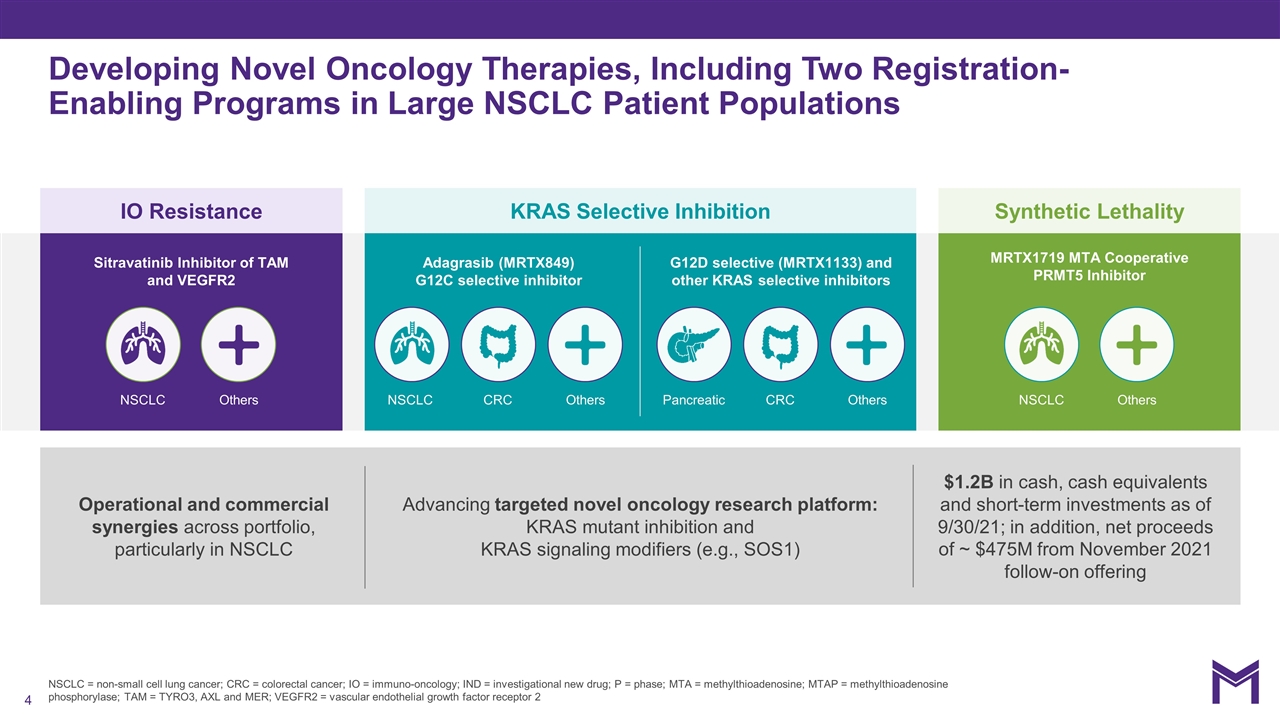
NSCLC = non-small cell lung cancer; CRC = colorectal cancer; IO = immuno-oncology; IND = investigational new drug; P = phase; MTA = methylthioadenosine; MTAP = methylthioadenosine phosphorylase; TAM = TYRO3, AXL and MER; VEGFR2 = vascular endothelial growth factor receptor 2 Developing Novel Oncology Therapies, Including Two Registration-Enabling Programs in Large NSCLC Patient Populations IO Resistance KRAS Selective Inhibition Synthetic Lethality Operational and commercial synergies across portfolio, particularly in NSCLC Advancing targeted novel oncology research platform: KRAS mutant inhibition and KRAS signaling modifiers (e.g., SOS1) $1.2B in cash, cash equivalents and short-term investments as of 9/30/21; in addition, net proceeds of ~ $475M from November 2021 follow-on offering Adagrasib (MRTX849) G12C selective inhibitor G12D selective (MRTX1133) and other KRAS selective inhibitors Sitravatinib Inhibitor of TAM and VEGFR2 MRTX1719 MTA Cooperative PRMT5 Inhibitor NSCLC NSCLC CRC Pancreatic CRC NSCLC Others Others Others Others

Strong Execution and Progress in 2021 Positioning for a Transformational 2022 Q2 2021 Received Breakthrough Therapy Designation for adagrasib in advanced 2L+ NSCLC Initiated K-10, a P3 trial of adagrasib plus cetuximab in 2L CRC Initiated K-14, a P1/1b trial of adagrasib plus BI's SOS1 inhibitor Announced collaboration with Zai Lab in China for adagrasib and Stand Up to Cancer $4M grant to support research on KRAS mutant cancers Q3 2021 Announced positive P1b and top-line P2 monotherapy data in 2L+ NSCLC; also presented encouraging CRC monotherapy and combination data with cetuximab Presented preliminary results from P1b cohort of K-1 in adagrasib plus pembrolizumab in 1L NSCLC, supporting go forward dose of 400 mg BID adagrasib Announced long-term survival data from P2 study of sitravatinib plus nivolumab in patients who progressed following CPI therapy, further supporting P3 SAPPHIRE study design in 2L or 3L non-squamous NSCLC Presented POC data for next generation spectrum-selective mutant KRAS inhibitors Announced clinical collaboration with Sanofi to evaluate adagrasib in combination with SAR442720, in KRASG12C+ NSCLC Q1 2021 Initiated K-1, a P2 adagrasib monotherapy single-arm cohort, in 1L NSCLC patients with STK11 co-mutations Initiated K-7, a P2 trial of adagrasib plus pembrolizumab in 1L NSCLC Initiated K-12, a P3 trial of monotherapy adagrasib versus docetaxel in 2L NSCLC Announced strategic R&D collaboration with MD Anderson Cancer Center and participation in McKesson's MYLUNG consortium Q4 2021 Submitted the NDA for adagrasib in 2L+ NSCLC as a part of the real time oncology review program with the FDA Presented encouraging preliminary adagrasib monotherapy data from K-1 in previously-treated patients with pancreatic cancer Submitted IND for MRTX1719, a MTA-cooperative PRMT5 inhibitor, in MTAP-deleted cancer Announced clinical collaboration with Verastem Oncology to evaluate adagrasib in combination with VS-6766 in KRASG12C+ NSCLC Strengthened capital position with secondary public offering resulting in net proceeds of approximately $475M K= KRYSTAL; L = Line; P = Phase; BID = Twice Daily Dosing; POC = Proof-of-Concept; NSCLC = Non-Small Cell Lung Cancer; CRC = colorectal cancer; NDA = New Drug Application; PRMT5 = Protein Arginine Methyltransferase 5; SAM - S-adenosylmethionine; MTA: methylthioadenosine; MTAP: methylthioadenosine phosphorylase; IND: investigational new drug

Key Achievements in 2021 Initiated K-1, a P2 adagrasib monotherapy single-arm cohort, in 1L NSCLC patients with STK11 co-mutations Received Breakthrough Therapy Designation for adagrasib in advanced 2L+ NSCLC Announced positive P1b and top-line P2 monotherapy data in 2L+ NSCLC; also presented encouraging CRC monotherapy and combination data with cetuximab Presented preliminary results from P1b cohort of K-1 in adagrasib plus pembrolizumab in 1L NSCLC, supporting go forward dose of 400 mg BID adagrasib Submitted the NDA for adagrasib in 2L+ NSCLC as a part of the real time oncology review program with the FDA Submitted IND for MRTX1719, a MTA-cooperative PRMT5 inhibitor, in MTAP-deleted cancer Strengthened capital position with secondary public offering K= KRYSTAL; L = Line; P = Phase; BID = Twice Daily Dosing; POC = Proof-of-Concept; NSCLC = Non-Small Cell Lung Cancer; CRC = colorectal cancer; NDA = New Drug Application; PRMT5 = Protein Arginine Methyltransferase 5; SAM - S-adenosylmethionine; MTA: methylthioadenosine; MTAP: methylthioadenosine phosphorylase; IND: investigational new drug

Adagrasib (MRTX849): KRASG12C Selective Inhibitor
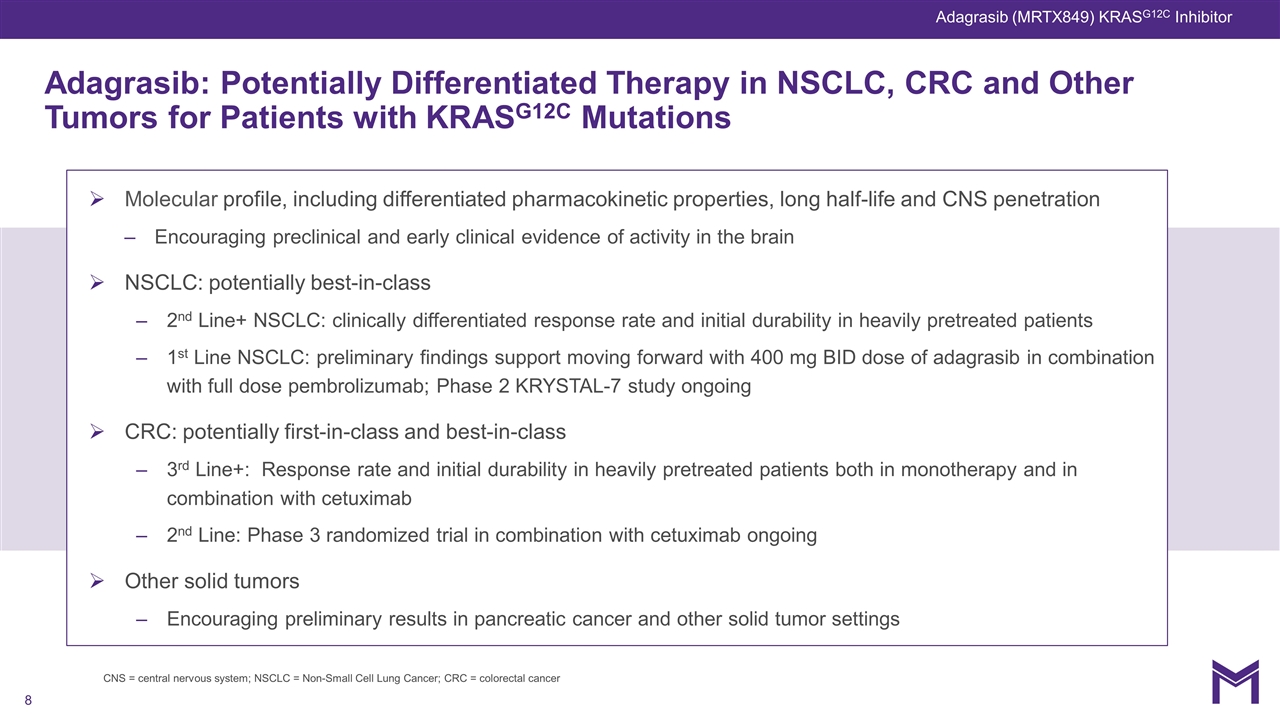
Molecular profile, including differentiated pharmacokinetic properties, long half-life and CNS penetration Encouraging preclinical and early clinical evidence of activity in the brain NSCLC: potentially best-in-class 2nd Line+ NSCLC: clinically differentiated response rate and initial durability in heavily pretreated patients 1st Line NSCLC: preliminary findings support moving forward with 400 mg BID dose of adagrasib in combination with full dose pembrolizumab; Phase 2 KRYSTAL-7 study ongoing CRC: potentially first-in-class and best-in-class 3rd Line+: Response rate and initial durability in heavily pretreated patients both in monotherapy and in combination with cetuximab 2nd Line: Phase 3 randomized trial in combination with cetuximab ongoing Other solid tumors Encouraging preliminary results in pancreatic cancer and other solid tumor settings Adagrasib: Potentially Differentiated Therapy in NSCLC, CRC and Other Tumors for Patients with KRASG12C Mutations Adagrasib (MRTX849) KRASG12C Inhibitor CNS = central nervous system; NSCLC = Non-Small Cell Lung Cancer; CRC = colorectal cancer
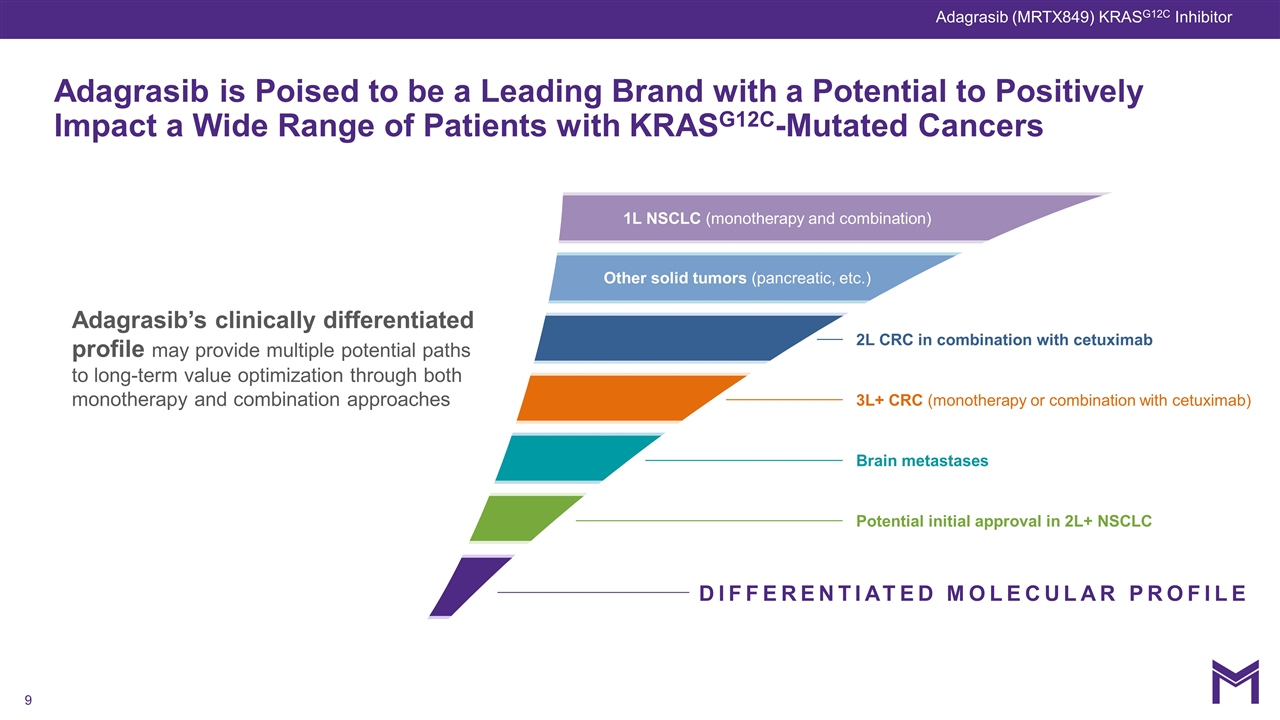
Adagrasib’s clinically differentiated profile may provide multiple potential paths to long-term value optimization through both monotherapy and combination approaches DIFFERENTIATED MOLECULAR PROFILE Potential initial approval in 2L+ NSCLC Brain metastases 3L+ CRC (monotherapy or combination with cetuximab) 2L CRC in combination with cetuximab Other solid tumors (pancreatic, etc.) Adagrasib is Poised to be a Leading Brand with a Potential to Positively Impact a Wide Range of Patients with KRASG12C-Mutated Cancers 1L NSCLC (monotherapy and combination) Adagrasib (MRTX849) KRASG12C Inhibitor
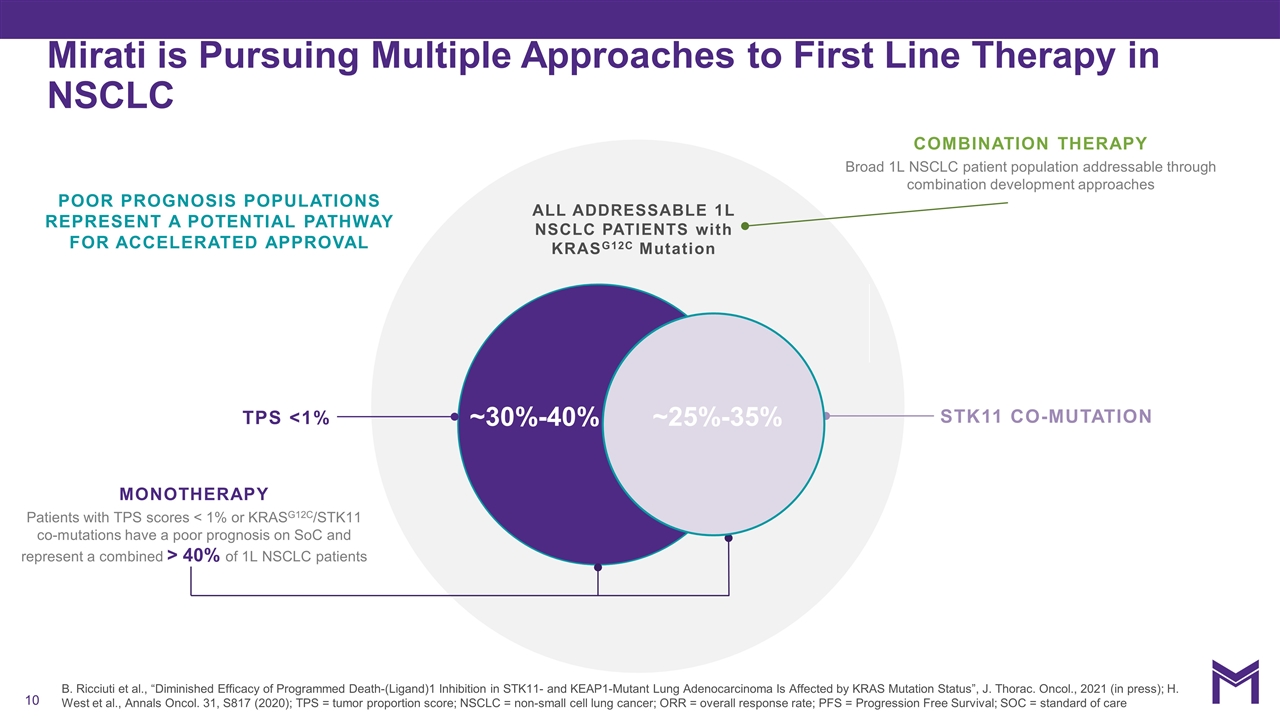
Mirati is Pursuing Multiple Approaches to First Line Therapy in NSCLC B. Ricciuti et al., “Diminished Efficacy of Programmed Death-(Ligand)1 Inhibition in STK11- and KEAP1-Mutant Lung Adenocarcinoma Is Affected by KRAS Mutation Status”, J. Thorac. Oncol., 2021 (in press); H. West et al., Annals Oncol. 31, S817 (2020); TPS = tumor proportion score; NSCLC = non-small cell lung cancer; ORR = overall response rate; PFS = Progression Free Survival; SOC = standard of care STK11 CO-MUTATION COMBINATION THERAPY Broad 1L NSCLC patient population addressable through combination development approaches ALL ADDRESSABLE 1L NSCLC PATIENTS with KRASG12C Mutation ~30%-40% POOR PROGNOSIS POPULATIONS REPRESENT A POTENTIAL PATHWAY FOR ACCELERATED APPROVAL TPS <1% MONOTHERAPY Patients with TPS scores < 1% or KRASG12C/STK11 co-mutations have a poor prognosis on SoC and represent a combined > 40% of 1L NSCLC patients ~25%-35%

Adagrasib: Highly Experienced Commercial Oncology Team Preparing for Successful Launch in 2022 Proven Differentiated Profile Clinically shown differentiated efficacy enabled by a 24-hour half-life that covers the target through the dosing cycle Robust early clinical activity in colorectal and pancreatic cancers Encouraging early data in patients with brain metastases Top Biotech and Pharma Talent Ability to recruit and retain top talent across biotech and pharma given overwhelming interest in commercialization roles Experienced management team with significant oncology launch experience: Integrated Execution and Relentless Mindset Cross functional and integrated teams in place, including Medical Affairs, Sales Management, Market Access and R&D The Covid-19 pandemic has changed the rules of engagement of prescriber access, leveling the playing field between biotech and big pharma from "repetition" to "relevance" Well Capitalized Available capital that provides sufficient runway to support commercialization in 2022 and continued development of clinical and preclinical portfolio Commercial team progressively built over previous 2 years OPDIVO®, TAGRISSO®, IBRANCE®, XALCORI® and SUTENT® are registered trademarks of their respective companies. Adagrasib (MRTX849) KRASG12C Inhibitor

Sitravatinib + Checkpoint Inhibitors

Pircher et al., Synergies of Targeting Tumor Angiogenesis and Immune Checkpoints. Int J Mol Sci, 2017. 18(11). Garton et al., Anti-KIT Monoclonal Antibody Treatment Enhances the Antitumor Activity of Immune Checkpoint Inhibitors by Reversing Tumor-Induced Immunosuppression. Mol Cancer Ther, 2017. 16(4) Akalu, Y.T., C.V. Rothlin, and S. Ghosh, TAM receptor tyrosine kinases as emerging targets of innate immune checkpoint blockade for cancer therapy. Immunol Rev, 2017. 276(1) Graham, D.K., D. DeRyckere, K.D. Davies, and H.S. Earp, The TAM family. Nat Rev Cancer, 2014. 14(12) Du, W., Huang, H., Sorrelle, N., & Brekken, R. A. (2018). Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight, 3(21). Sitravatinib + Checkpoint Inhibitors Sitravatinib Inhibits TAM (TYRO3, AXL and MER), VEGFR2, and KIT Receptors and May Restore Immune Response Increase dendritic cell maturity & antigen presentation capacity Increase NK cell response Increase T cell expansion & trafficking into tumors Rationale for Targeting TAM & Split RTKs to Enhance Immune Response to Checkpoint Inhibitors Targeting VEGFR2 reduces Tregs & MDSCs Targeting KIT also depletes MDSCs Releases brakes for expansion of CD8+ T cells via PD-1 inhibition Both TAM & Split RTKs cooperate to: Targeting MERTK & AXL shifts tumor associated macrophage (TAM) type to M1 M1 macrophages secrete cytokines that enhance immune response (IL-12, TNF) CD8+ CD4+ M1 TAM M2 TAM iDC mDC MDSC Treg Targeting TAM: Targeting Split RTKs:
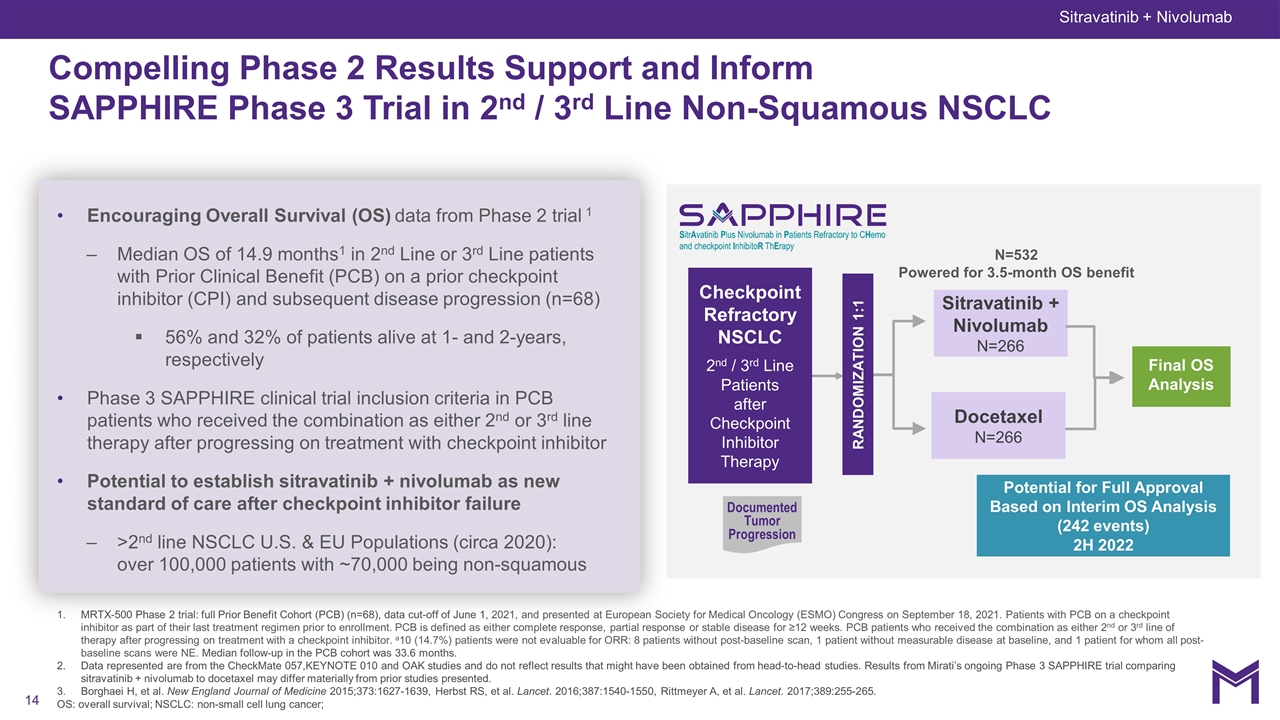
MRTX-500 Phase 2 trial: full Prior Benefit Cohort (PCB) (n=68), data cut-off of June 1, 2021, and presented at European Society for Medical Oncology (ESMO) Congress on September 18, 2021. Patients with PCB on a checkpoint inhibitor as part of their last treatment regimen prior to enrollment. PCB is defined as either complete response, partial response or stable disease for ≥12 weeks. PCB patients who received the combination as either 2nd or 3rd line of therapy after progressing on treatment with a checkpoint inhibitor. a10 (14.7%) patients were not evaluable for ORR: 8 patients without post-baseline scan, 1 patient without measurable disease at baseline, and 1 patient for whom all post-baseline scans were NE. Median follow-up in the PCB cohort was 33.6 months. Data represented are from the CheckMate 057,KEYNOTE 010 and OAK studies and do not reflect results that might have been obtained from head-to-head studies. Results from Mirati’s ongoing Phase 3 SAPPHIRE trial comparing sitravatinib + nivolumab to docetaxel may differ materially from prior studies presented. Borghaei H, et al. New England Journal of Medicine 2015;373:1627-1639, Herbst RS, et al. Lancet. 2016;387:1540-1550, Rittmeyer A, et al. Lancet. 2017;389:255-265. OS: overall survival; NSCLC: non-small cell lung cancer; Sitravatinib + Nivolumab Compelling Phase 2 Results Support and Inform SAPPHIRE Phase 3 Trial in 2nd / 3rd Line Non-Squamous NSCLC Sitravatinib + Nivolumab N=266 Docetaxel N=266 Checkpoint Refractory NSCLC 2nd / 3rd Line Patients after Checkpoint Inhibitor Therapy Documented Tumor Progression RANDOMIZATION 1:1 Final OS Analysis Potential for Full Approval Based on Interim OS Analysis (242 events) 2H 2022 N=532 Powered for 3.5-month OS benefit Encouraging Overall Survival (OS) data from Phase 2 trial 1 Median OS of 14.9 months1 in 2nd Line or 3rd Line patients with Prior Clinical Benefit (PCB) on a prior checkpoint inhibitor (CPI) and subsequent disease progression (n=68) 56% and 32% of patients alive at 1- and 2-years, respectively Phase 3 SAPPHIRE clinical trial inclusion criteria in PCB patients who received the combination as either 2nd or 3rd line therapy after progressing on treatment with checkpoint inhibitor Potential to establish sitravatinib + nivolumab as new standard of care after checkpoint inhibitor failure >2nd line NSCLC U.S. & EU Populations (circa 2020): over 100,000 patients with ~70,000 being non-squamous

MRTX1719: Novel PRMT5 Inhibitor in MTAP-deleted Cancers
1 cBioPortal PRMT5 = Protein Arginine Methyltransferase 5; SAM - S-adenosylmethionine; MTA: methylthioadenosine; MTAP: methylthioadenosine phosphorylase; IND: investigational new drug MRTX1719 PRMT5 Inhibitor MRTX1719: Novel PRMT5 Inhibitor Selective for MTAP-deleted Cancers MTAP deletions occur in approximately 10%1 of all human cancers including pancreatic, lung, and bladder Patients have a poor prognosis, representing a significant unmet medical need Internally discovered PRMT5 inhibitor represent a potential precision medicine for MTAP-deleted cancers Program leverages a synthetic lethal approach and selectively targets the PRMT5/MTA complex in MTAP-deleted cancer cells Designed to spare normal human cells and demonstrates improved therapeutic index in preclinical studies relative to first generation approaches IND cleared and Phase 1/2 clinical trial to initiate in Q1:2022
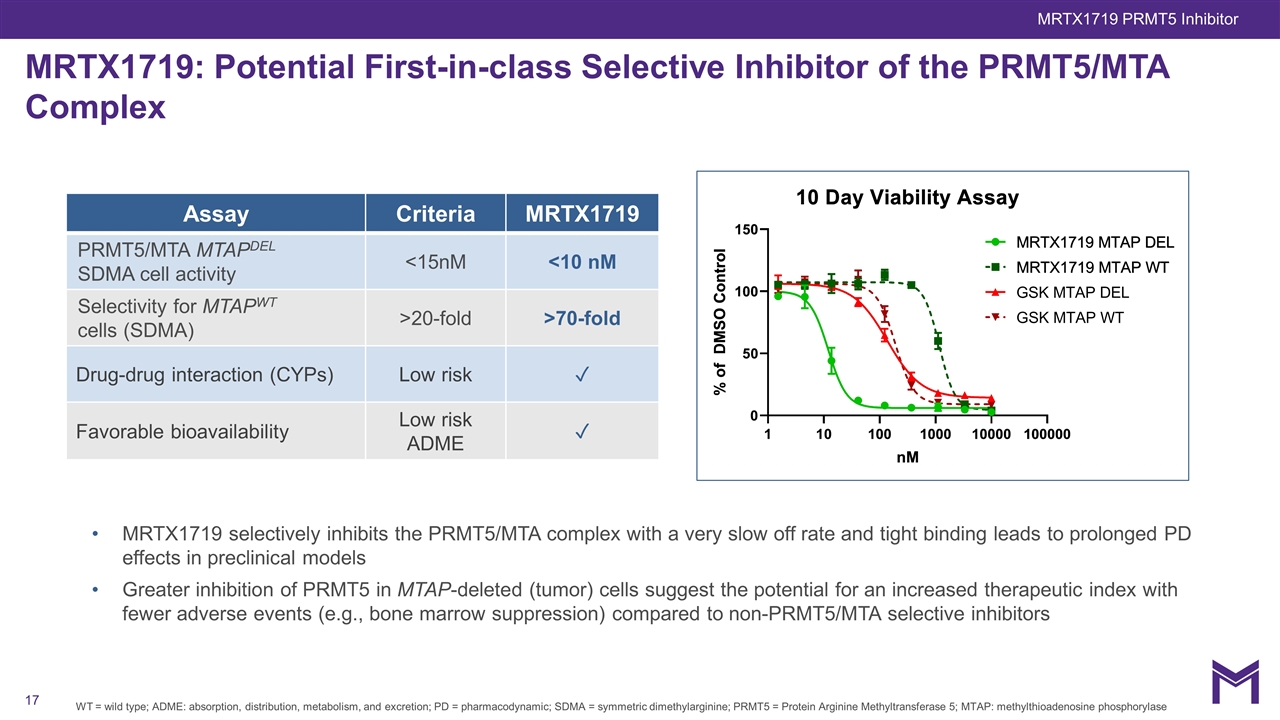
MRTX1719: Potential First-in-class Selective Inhibitor of the PRMT5/MTA Complex MRTX1719 selectively inhibits the PRMT5/MTA complex with a very slow off rate and tight binding leads to prolonged PD effects in preclinical models Greater inhibition of PRMT5 in MTAP-deleted (tumor) cells suggest the potential for an increased therapeutic index with fewer adverse events (e.g., bone marrow suppression) compared to non-PRMT5/MTA selective inhibitors WT = wild type; ADME: absorption, distribution, metabolism, and excretion; PD = pharmacodynamic; SDMA = symmetric dimethylarginine; PRMT5 = Protein Arginine Methyltransferase 5; MTAP: methylthioadenosine phosphorylase Assay Criteria MRTX1719 PRMT5/MTA MTAPDEL SDMA cell activity <15nM <10 nM Selectivity for MTAPWT cells (SDMA) >20-fold >70-fold Drug-drug interaction (CYPs) Low risk ✓ Favorable bioavailability Low risk ADME ✓ MRTX1719 PRMT5 Inhibitor
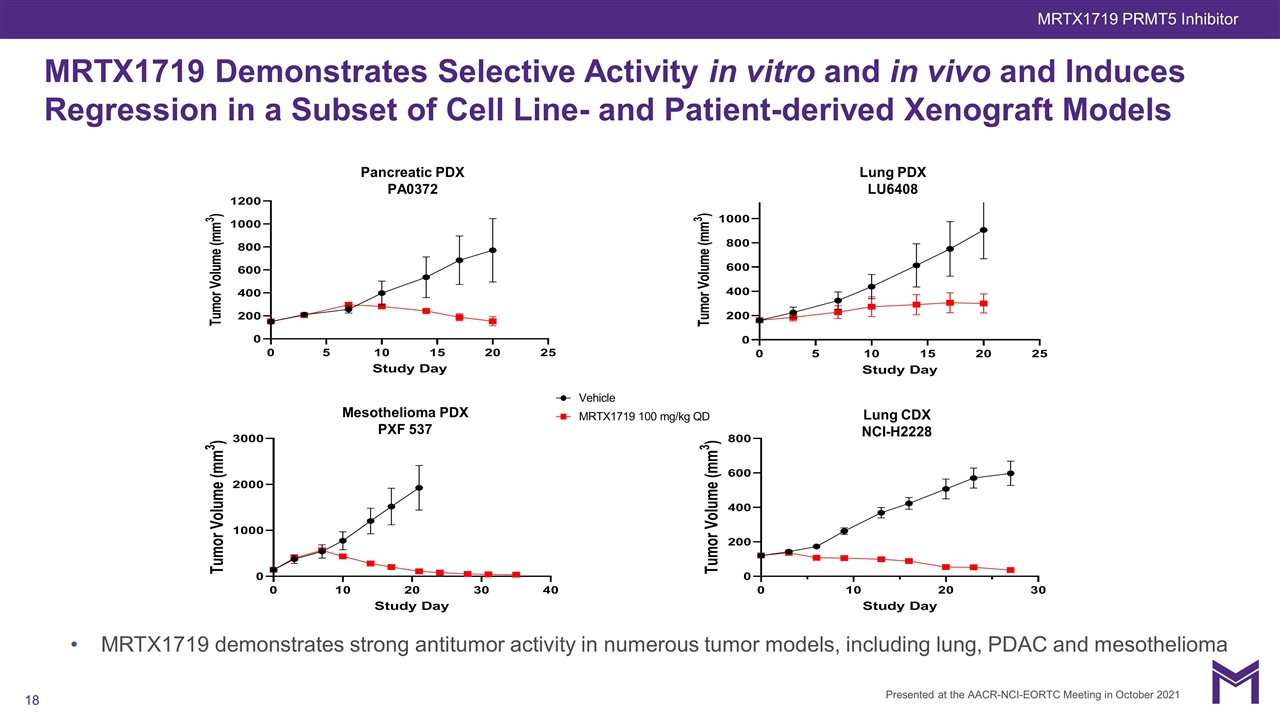
MRTX1719 Demonstrates Selective Activity in vitro and in vivo and Induces Regression in a Subset of Cell Line- and Patient-derived Xenograft Models Mesothelioma PDX PXF 537 Lung CDX NCI-H2228 Pancreatic PDX PA0372 Lung PDX LU6408 MRTX1719 demonstrates strong antitumor activity in numerous tumor models, including lung, PDAC and mesothelioma Presented at the AACR-NCI-EORTC Meeting in October 2021 MRTX1719 PRMT5 Inhibitor

MRTX1719 Profile and Preclinical Results Shape Clinical Development Strategy in MTAP-deleted Cancers DL: dose level; NSCLC: non-small cell lung cancer; MPNST: malignant peripheral nerve sheet tumor Key Eligibility Criteria Solid tumor with homozygous MTAP deletion Unresectable or metastatic disease No prior PRMT5 inhibitor (Phase 2 only) No prior MAT2A inhibitor (Phase 2 only) Study Endpoints Safety PK/PD Clinical Activity Expansion Criteria Dose expansion to be based on PK, PD, and safety DL 1 Screening Phase 1 Dose Escalation DL 2 DL X Phase 1b Expansion Phase 2 Cohorts Dose Expansion Mesothelioma Pancreatic NSCLC MPNST Other Tumors with MTAP-Del Potential Sub-Studies Combination Formulation Food Effect DDI MRTX1719 clinical development will include broad range of MTAP-deleted cancers as both single agent and in combination MRTX1719 PRMT5 Inhibitor

MRTX1133: KRASG12D Selective Inhibitor
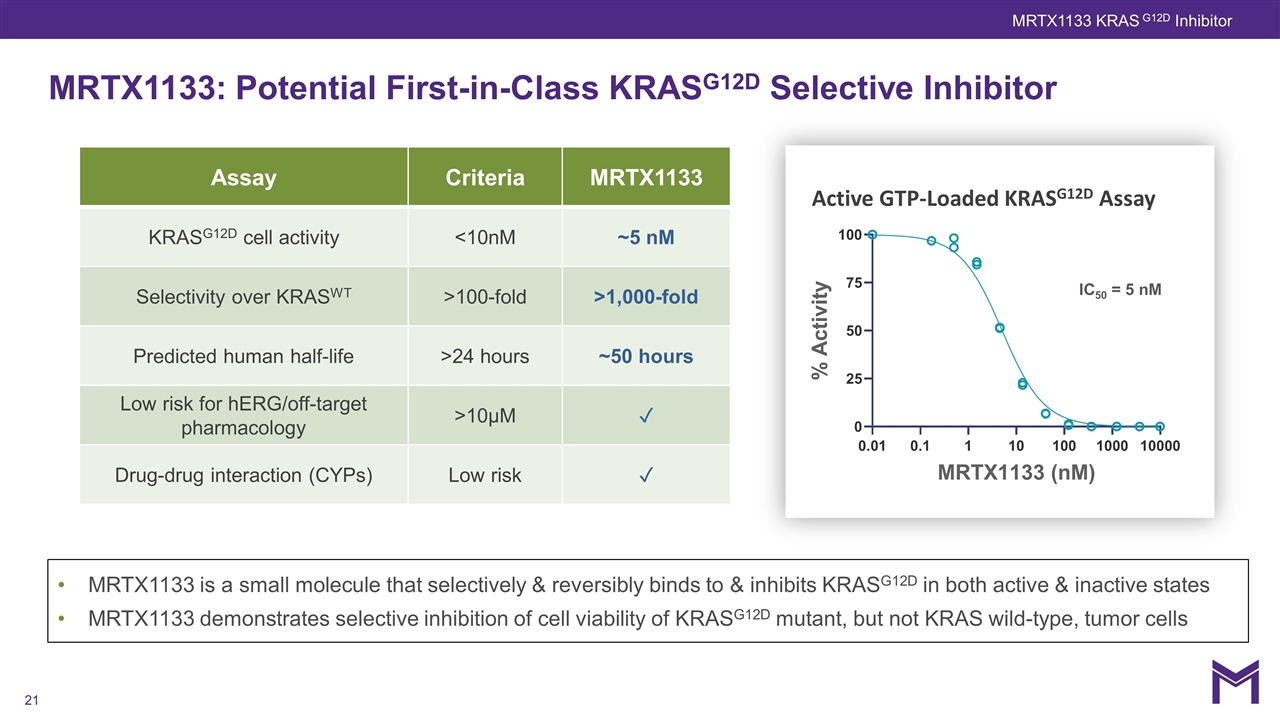
MRTX1133 is a small molecule that selectively & reversibly binds to & inhibits KRASG12D in both active & inactive states MRTX1133 demonstrates selective inhibition of cell viability of KRASG12D mutant, but not KRAS wild-type, tumor cells MRTX1133: Potential First-in-Class KRASG12D Selective Inhibitor IC50 = 5 nM Active GTP-Loaded KRASG12D Assay MRTX1133 KRAS G12D Inhibitor Assay Criteria MRTX1133 KRASG12D cell activity <10nM ~5 nM Selectivity over KRASWT >100-fold >1,000-fold Predicted human half-life >24 hours ~50 hours Low risk for hERG/off-target pharmacology >10µM ✓ Drug-drug interaction (CYPs) Low risk ✓
MRTX1133: Clinical Development Path and Design Principles Optimizing target coverage throughout the dosing interval is important for maximizing antitumor activity in KRAS mutated cancers Evaluation of long-acting IV formulations, including liposomal strategies, to maximize the extent and duration of target coverage is ongoing IV injectable route of administration commercially attractive and compatible with development as a monotherapy or in combination with standard of care regimens IND filing planned for 2H 2022 MRTX1133 KRASG12D Inhibitor Multi-cohort Phase 1 monotherapy trial comparable to adagrasib Rapid dose escalation strategies to define a tolerated and active dose Multiple expansion cohorts for pancreatic, colorectal, lung and other G12D patients Rational combination approaches are similar to G12C and enabled in first-in-human clinical trials PATH TO CLINICAL DEVELOPMENT CLINICAL TRIAL DESIGN PRINCIPLES IND: investigational new drug; IV = intravenous

Differentiated Discovery Capabilities Enhance Potential for Long-Term Growth and Sustainability
Highly Productive Discovery Capability Advancing Additional Best-in-Class / First-in-Class Opportunities Mirati has built a highly productive in-house discovery and preclinical development capability Our research has resulted in 25 published patent applications for 8 portfolio projects across both clinical or preclinical compounds Adagrasib (KRASG12C), MRTX1133 (KRASG12D), MRTX1719 (PRMT5), ORIC-944 (EED)*, and our IND-track program for SOS1 Our discovery efforts are focused on the advancement of novel targeted cancer therapies that: Further complement existing pipeline Offer potential practice-changing opportunities for cancer patients Near-term focus on advancing our in-house SOS1 and next-generation KRAS programs *In August 2020, Mirati and Oric entered into an exclusive worldwide license for development and commercialization rights for PRC2 inhibitor, ORIC-944.
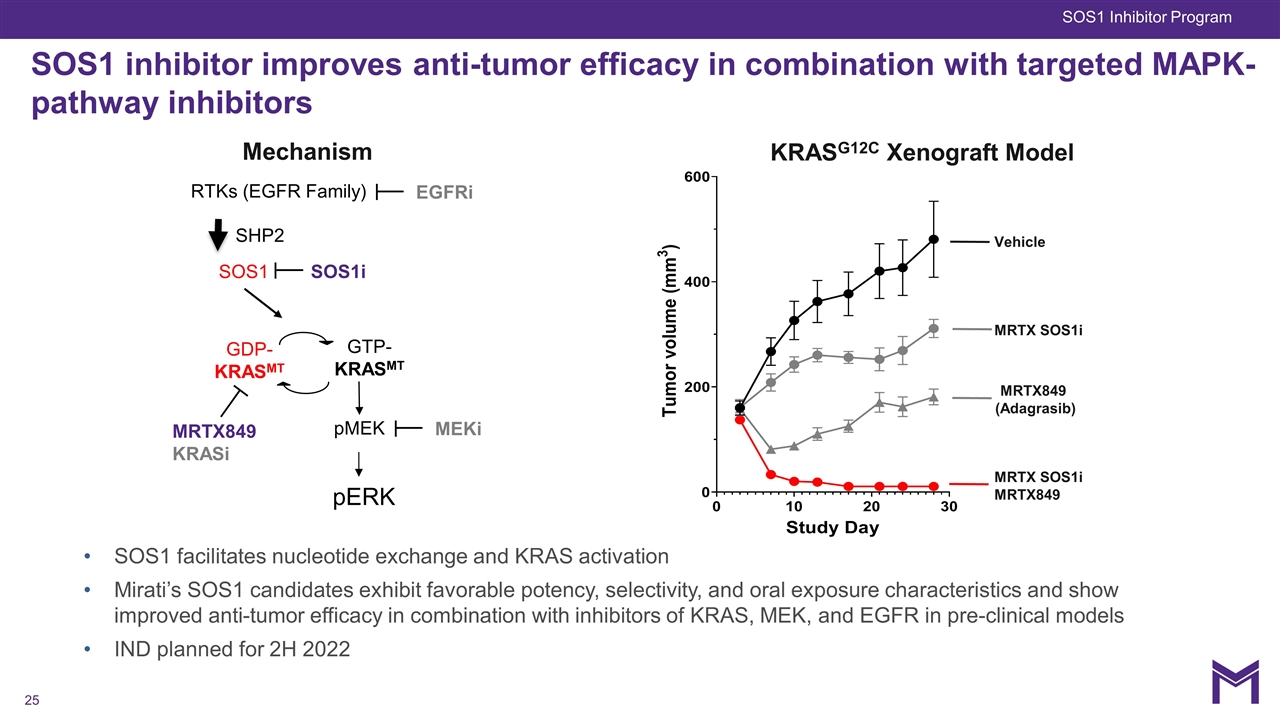
SOS1 inhibitor improves anti-tumor efficacy in combination with targeted MAPK-pathway inhibitors KRASG12C Xenograft Model Vehicle MRTX849 (Adagrasib) MRTX SOS1i MRTX SOS1i MRTX849 MRTX849 KRASi GTP-KRASMT SOS1 SHP2 RTKs (EGFR Family) GDP-KRASMT pERK SOS1i Mechanism pMEK MEKi SOS1 facilitates nucleotide exchange and KRAS activation Mirati’s SOS1 candidates exhibit favorable potency, selectivity, and oral exposure characteristics and show improved anti-tumor efficacy in combination with inhibitors of KRAS, MEK, and EGFR in pre-clinical models IND planned for 2H 2022 EGFRi SOS1 Inhibitor Program

Next Generation KRAS Program Growing our Leadership Position in the Development of Next Generation KRAS Therapies Demonstrated initial preclinical proof-of-concept data, including the meaningful tumor regression, targeting other oncogenic forms of KRAS with spectrum-selective mutant KRAS inhibitors Our next generation KRAS targeting strategy represents a potential platform approach Potential to yield development candidates targeting various KRAS mutations with distinct selectivity profiles Spectrum-selective KRAS inhibitor programs are in the lead optimization stage Specific compounds and multiple lead series under evaluation in various stages of preclinical development
Robust Pipeline with Numerous Near-Term Catalysts
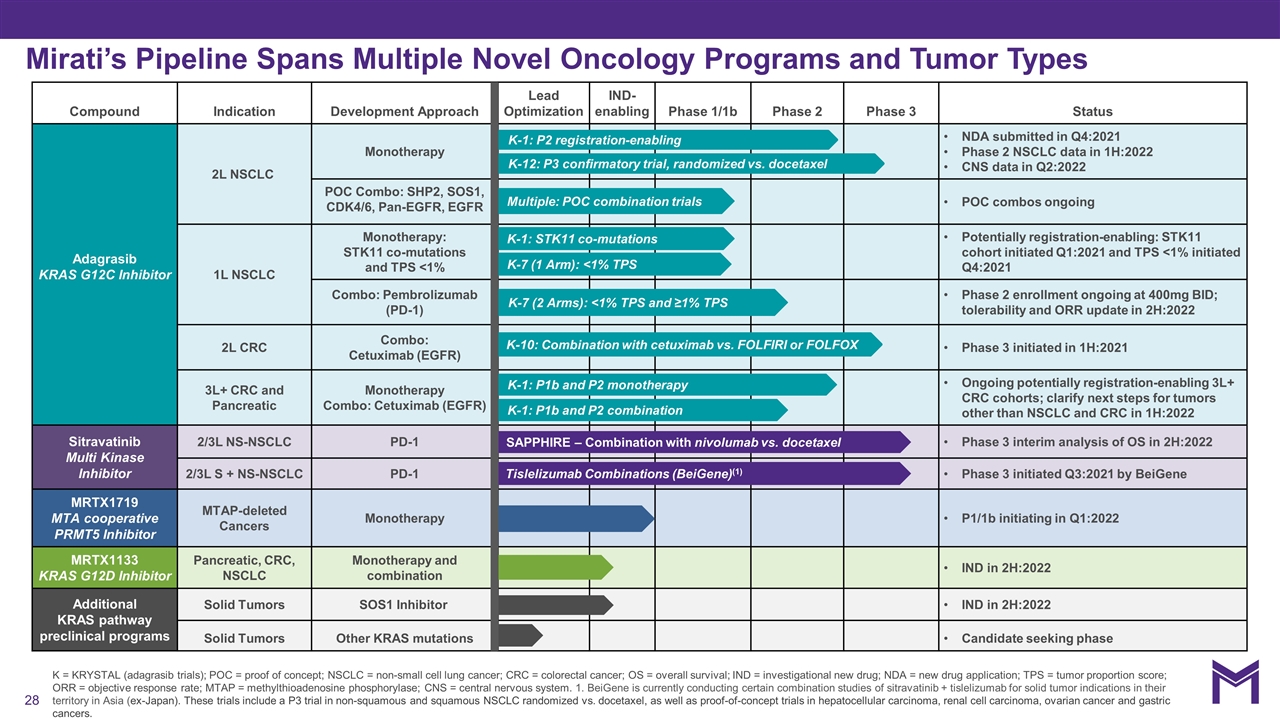
K = KRYSTAL (adagrasib trials); POC = proof of concept; NSCLC = non-small cell lung cancer; CRC = colorectal cancer; OS = overall survival; IND = investigational new drug; NDA = new drug application; TPS = tumor proportion score; ORR = objective response rate; MTAP = methylthioadenosine phosphorylase; CNS = central nervous system. 1. BeiGene is currently conducting certain combination studies of sitravatinib + tislelizumab for solid tumor indications in their territory in Asia (ex-Japan). These trials include a P3 trial in non-squamous and squamous NSCLC randomized vs. docetaxel, as well as proof-of-concept trials in hepatocellular carcinoma, renal cell carcinoma, ovarian cancer and gastric cancers. Compound Indication Development Approach Lead Optimization IND-enabling Phase 1/1b Phase 2 Phase 3 Status Adagrasib KRAS G12C Inhibitor 2L NSCLC Monotherapy NDA submitted in Q4:2021 Phase 2 NSCLC data in 1H:2022 CNS data in Q2:2022 2L NSCLC POC Combo: SHP2, SOS1, CDK4/6, Pan-EGFR, EGFR POC combos ongoing 1L NSCLC Monotherapy: STK11 co-mutations and TPS <1% Potentially registration-enabling: STK11 cohort initiated Q1:2021 and TPS <1% initiated Q4:2021 1L NSCLC Combo: Pembrolizumab (PD-1) Phase 2 enrollment ongoing at 400mg BID; tolerability and ORR update in 2H:2022 2L CRC Combo: Cetuximab (EGFR) Phase 3 initiated in 1H:2021 3L+ CRC and Pancreatic Monotherapy Combo: Cetuximab (EGFR) Ongoing potentially registration-enabling 3L+ CRC cohorts; clarify next steps for tumors other than NSCLC and CRC in 1H:2022 Sitravatinib Multi Kinase Inhibitor 2/3L NS-NSCLC PD-1 Phase 3 interim analysis of OS in 2H:2022 2/3L S + NS-NSCLC PD-1 Phase 3 initiated Q3:2021 by BeiGene MRTX1719 MTA cooperative PRMT5 Inhibitor MTAP-deleted Cancers Monotherapy P1/1b initiating in Q1:2022 MRTX1133 KRAS G12D Inhibitor Pancreatic, CRC, NSCLC Monotherapy and combination IND in 2H:2022 Additional KRAS pathway preclinical programs Solid Tumors SOS1 Inhibitor IND in 2H:2022 Solid Tumors Other KRAS mutations Candidate seeking phase Mirati’s Pipeline Spans Multiple Novel Oncology Programs and Tumor Types K-10: Combination with cetuximab vs. FOLFIRI or FOLFOX Multiple: POC combination trials K-12: P3 confirmatory trial, randomized vs. docetaxel K-7 (2 Arms): <1% TPS and ≥1% TPS SAPPHIRE – Combination with nivolumab vs. docetaxel K-1: STK11 co-mutations K-1: P2 registration-enabling Tislelizumab Combinations (BeiGene)(1) K-1: P1b and P2 monotherapy K-1: P1b and P2 combination K-7 (1 Arm): <1% TPS
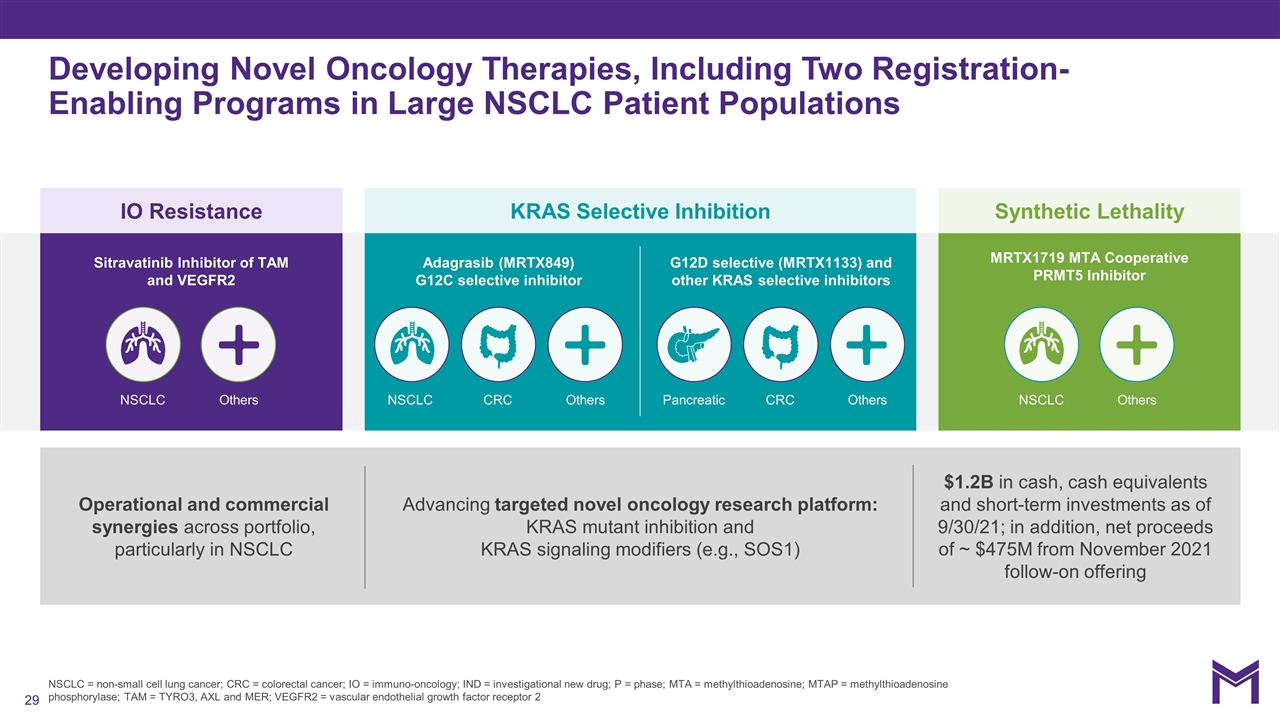
NSCLC = non-small cell lung cancer; CRC = colorectal cancer; IO = immuno-oncology; IND = investigational new drug; P = phase; MTA = methylthioadenosine; MTAP = methylthioadenosine phosphorylase; TAM = TYRO3, AXL and MER; VEGFR2 = vascular endothelial growth factor receptor 2 Developing Novel Oncology Therapies, Including Two Registration-Enabling Programs in Large NSCLC Patient Populations IO Resistance KRAS Selective Inhibition Synthetic Lethality Operational and commercial synergies across portfolio, particularly in NSCLC Advancing targeted novel oncology research platform: KRAS mutant inhibition and KRAS signaling modifiers (e.g., SOS1) $1.2B in cash, cash equivalents and short-term investments as of 9/30/21; in addition, net proceeds of ~ $475M from November 2021 follow-on offering Adagrasib (MRTX849) G12C selective inhibitor G12D selective (MRTX1133) and other KRAS selective inhibitors Sitravatinib Inhibitor of TAM and VEGFR2 MRTX1719 MTA Cooperative PRMT5 Inhibitor NSCLC NSCLC CRC Pancreatic CRC NSCLC Others Others Others Others

J.P. Morgan Healthcare Conference January 2022