UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 20-F
| (Mark One) | ||
| [ ] | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| OR | ||
| [X] |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
for the fiscal year ended December 31, 2013
|
||
| OR | ||
| [ ] |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
For the transition period from ________________ to ________________
|
||
| OR | ||
| [ ] |
SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
|
Date of event requiring this shell company report
|
||
Commission file number: 001-35990
PROSENSA HOLDING N.V.
(Exact name of Registrant as specified in its charter)
The Netherlands
(Jurisdiction of incorporation)
J.H. Oortweg 21
2333 CH Leiden, the Netherlands,
+31 (0)71 33 22 100
(Address of principal executive offices)
Berndt Modig
Tel: +31 61 47 90 201
J.H. Oortweg 21
2333 CH Leiden, the Netherlands
(Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person)
Copies to:
Richard D. Truesdell Jr.
Davis Polk & Wardwell LLP
450 Lexington Avenue
New York, NY 10017
Phone: (212) 450 4000
Fax: (212) 701 5800
Securities registered or to be registered pursuant to Section 12(b) of the Act:
|
Title of each class
|
Name of each exchange on which registered
|
|
Ordinary Shares, nominal value €0.01 per share
|
The NASDAQ Stock Market LLC
|
Securities registered or to be registered pursuant to Section 12(g) of the Act:
None
(Title of Class)
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act:
None
(Title of Class)
Indicate the number of outstanding shares of each of the issuer’s classes of capital stock or common stock as of the close of business covered by the annual report.
Common shares: 35,932,792
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
[ ] Yes [ x ] No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
[ ] Yes [ x ] No
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
[ x ] Yes [ ] No
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
[ ] Yes [ x ] No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer [ ]
|
Accelerated filer [ ]
|
Non-accelerated filer [x]
|
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
|
US GAAP [ ]
|
International Financial Reporting Standards as issued
by the International Accounting Standards Board [x]
|
Other [ ]
|
If “Other” has been checked in response to the previous question indicate by check mark which financial statement item the registrant has elected to follow.
Item 17 [ ] Item 18 [ ]
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes [ ] No [x]
PROSENSA HOLDING N.V.
TABLE OF CONTENTS
____________________
Page
|
FORWARD-LOOKING STATEMENTS
|
iii | |
|
PART I
|
4
|
|
|
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
|
4
|
|
|
A.
|
Directors and senior management
|
4
|
|
B.
|
Advisers
|
4
|
|
C.
|
Auditors
|
4
|
|
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
|
4
|
|
|
A.
|
Offer statistics
|
4
|
|
B.
|
Method and expected timetable
|
4
|
|
ITEM 3. KEY INFORMATION
|
5
|
|
|
A.
|
Selected Financial Data
|
5
|
|
B.
|
Capitalization and indebtedness
|
7
|
|
C.
|
Reasons for the offer and use of proceeds
|
7
|
|
D.
|
Risk factors
|
7
|
|
ITEM 4. INFORMATION ON THE COMPANY
|
33
|
|
|
A.
|
History and development of the company
|
33
|
|
B.
|
Business overview
|
34
|
|
C.
|
Organizational structure
|
62
|
|
D.
|
Property, plant and equipment
|
62
|
|
ITEM 4A. UNRESOLVED STAFF COMMENTS
|
62
|
|
|
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
|
62
|
|
|
A.
|
Operating results
|
62
|
|
B.
|
Liquidity and capital resources
|
76
|
|
C.
|
Research and development, patents and licenses, etc.
|
78
|
|
D.
|
Trend information
|
79
|
|
E.
|
Off-balance sheet arrangements
|
79
|
|
F.
|
Tabular disclosure of contractual obligations
|
79
|
|
G.
|
Safe harbor
|
79
|
|
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
|
79 | |
|
A.
|
Directors and senior management
|
79
|
|
B.
|
Compensation
|
82
|
|
C.
|
Board practices
|
86
|
|
D.
|
Employees
|
88
|
|
E.
|
Share ownership
|
88
|
|
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
|
89 | |
|
A.
|
Major shareholders
|
89
|
|
B.
|
Related party transactions
|
91
|
|
C.
|
Interests of Experts and Counsel
|
92
|
|
ITEM 8. FINANCIAL INFORMATION
|
93 | |
|
A.
|
Consolidated statements and other financial information
|
93
|
|
B.
|
Significant changes
|
93
|
|
ITEM 9. THE OFFER AND LISTING
|
93 | |
|
A.
|
Offering and listing details
|
93
|
|
B.
|
Plan of distribution
|
93
|
|
C.
|
Markets
|
93
|
|
D.
|
Selling shareholders
|
93
|
|
E.
|
Dilution
|
93
|
|
F.
|
Expenses of the issue
|
94
|
|
ITEM 10. ADDITIONAL INFORMATION
|
94 | |
|
A.
|
Share capital
|
94
|
|
B.
|
Memorandum and articles of association
|
94
|
i
|
C.
|
Material contracts
|
94
|
|
D.
|
Exchange controls
|
94
|
|
E.
|
Taxation
|
94
|
|
F.
|
Dividends and paying agents
|
101
|
|
G.
|
Statement by experts
|
101
|
|
H.
|
Documents on display
|
101
|
|
I.
|
Subsidiary information
|
101
|
|
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT RISK
|
101 | |
|
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
|
102 | |
|
A.
|
Debt securities
|
102
|
|
B.
|
Warrants and rights
|
102
|
|
C.
|
Other securities
|
102
|
|
D.
|
American Depositary Shares
|
102
|
|
PART II
|
103 | |
|
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
|
103 | |
|
A.
|
Defaults
|
103
|
|
B.
|
Arrears and delinquencies
|
103
|
|
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
|
103 | |
|
ITEM 15. CONTROLS AND PROCEDURES
|
103 | |
|
A.
|
Disclosure Controls and Procedures
|
103
|
|
B.
|
Management’s Annual Report on Internal Control over Financial Reporting
|
103
|
|
C.
|
Attestation Report of the Registered Public Accounting Firm
|
103
|
|
D.
|
Changes in Internal Control over Financial Reporting
|
103
|
|
ITEM 16. [RESERVED]
|
103 | |
|
ITEM 16A. Audit committee financial expert
|
103 | |
|
ITEM 16B. Code of ethics
|
103 | |
|
ITEM 16C. Principal Accountant Fees and Services
|
103 | |
|
ITEM 16D. Exemptions from the listing standards for audit committees
|
104 | |
|
ITEM 16E. Purchases of equity securities by the issuer and affiliated purchasers
|
104 | |
|
ITEM 16F. Change in registrant’s certifying accountant
|
104 | |
|
ITEM 16G. Corporate governance
|
104 | |
|
ITEM 16H. Mine safety disclosure
|
104 | |
|
PART III
|
105 | |
|
ITEM 17. Financial statements
|
105 | |
|
ITEM 18. Financial statements
|
105 | |
|
ITEM 19. Exhibits
|
105 | |
Unless otherwise indicated or the context otherwise requires, all references in this Annual Report on Form 20-F (the “Annual Report”) to “Prosensa Holding N.V.” or “Prosensa,” the “Company,” “we,” “our,” “ours,” “us” or similar terms refer to Prosensa Holding N.V., together with its subsidiaries. The trademarks, trade names and service marks appearing in this Annual Report are the property of their respective owners.
____________________
ii
FORWARD-LOOKING STATEMENTS
This Annual Report contains statements that constitute forward-looking statements. Many of the forward-looking statements contained in this Annual Report can be identified by the use of forward-looking words such as “anticipate,” “believe,” “could,” “expect,” “should,” “plan,” “intend,” “will,” “estimate” and “potential,” among others.
Forward-looking statements appear in a number of places in this Annual Report and include, but are not limited to, statements regarding our intent, belief or current expectations. Forward-looking statements are based on our management’s beliefs and assumptions and on information currently available to our management. Such statements are subject to risks and uncertainties, and actual results may differ materially from those expressed or implied in the forward-looking statements due to various factors, including, but not limited to, those identified under the section “Item 3. Key Information—D. Risk factors” in this Annual Report.
Forward-looking statements speak only as of the date they are made, and we do not undertake any obligation to update them in light of new information or future developments or to release publicly any revisions to these statements in order to reflect later events or circumstances or to reflect the occurrence of unanticipated events.
iii
PART I
ITEM 1. IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS
A. Directors and senior management
Not applicable.
B. Advisers
Not applicable.
C. Auditors
Not applicable.
ITEM 2. OFFER STATISTICS AND EXPECTED TIMETABLE
A. Offer statistics
Not applicable.
B. Method and expected timetable
Not applicable.
4
ITEM 3. KEY INFORMATION
A. Selected Financial Data
The summary income statement data for the years ended December 31, 2011, 2012 and 2013 of Prosensa Holding N.V. are derived from the consolidated financial statements included in this Annual Report. We maintain our books and records in euros, and we prepare our financial statements under International Financial Reporting Standars (“IFRS”) as issued by the International Accounting Standards Board (“IASB”).
This financial information should be read in conjunction with “Item 5—Operating and Financial Review and Prospects” and our consolidated audited financial statements, including the notes thereto, included in this Annual Report.
|
For the years ended December 31,
|
|||||||||||
|
2011
|
2012
|
2013
|
|||||||||
| (€ in thousands) | |||||||||||
|
License revenue
|
6,510 | 5,726 | 5,626 | ||||||||
|
Collaboration revenue
|
2,179 | 2,127 | 3,312 | ||||||||
|
Total revenue
|
8,689 | 7,853 | 8,938 | ||||||||
|
Other income
|
36 | 174 | 560 | ||||||||
|
Research and development expense
|
(15,348 | ) | (14,393 | ) | (18,460 | ) | |||||
|
General and administrative expense
|
(5,203 | ) | (4,023 | ) | (7,734 | ) | |||||
|
Other gains/(losses) - net
|
22 | 49 | 112 | ||||||||
|
Operating (loss)/gain
|
(11,804 | ) | (10,340 | ) | (16,584 | ) | |||||
|
Finance income
|
434 | 796 | 645 | ||||||||
|
Finance costs
|
(209 | ) | (348 | ) | (665 | ) | |||||
|
Finance income – net
|
225 | 448 | (20 | ) | |||||||
|
Net Loss
|
(11,579 | ) | (9,892 | ) | (16,604 | ) | |||||
|
Other comprehensive income
|
– | – | – | ||||||||
|
Total comprehensive loss(1)
|
(11,579 | ) | (9,892 | ) | (16,604 | ) | |||||
|
Loss per share from operations attributable to the equity holders of the company during the year (in EUR per share)
|
|||||||||||
|
Basic and diluted loss per share (2)
|
(0.60 | ) | (0.37 | ) | (0.51 | ) | |||||
(1) Total comprehensive loss is fully attributable to equity holders of the Company
(2) Share count at December 31, 2011, 2012 and 2013 was 19,463,777, 29,002,298 and 35,932,792, respectively. Basic and diluted figures are the same because of loss position.
5
|
|
Exchange Rate Information
|
Our business is primarily conducted in the European Union, and we maintain our books and records in euros. We have presented results of operations in euros. In this Annual Report, translations from euros to U.S. dollars were made at the rate of €0.725 to $1.00, the official exchange rate quoted as of December 31, 2013 by the European Central Bank. Such U.S. dollar amounts are not necessarily indicative of the amounts of U.S. dollars that could actually have been purchased upon exchange of euros at the dates indicated. All amounts paid to us under the agreement with GlaxoSmithKline (GSK) are paid in British pounds. Translation of British pounds to euros in this annual report were made at the rate in effect at the time of the relevant payment.
The following table presents information on the exchange rates between the euro and the U.S. dollar for the periods indicated:
|
Period-end
|
Average for
period
|
Low
|
High
|
|||||||||||||
|
(€ per U.S. dollar)
|
||||||||||||||||
|
Year Ended December 31:
|
||||||||||||||||
|
2009
|
0.694 | 0.717 | 0.661 | 0.796 | ||||||||||||
|
2010
|
0.748 | 0.754 | 0.687 | 0.837 | ||||||||||||
|
2011
|
0.773 | 0.718 | 0.672 | 0.776 | ||||||||||||
|
2012
|
0.758 | 0.778 | 0.743 | 0.827 | ||||||||||||
|
2013
|
0.725 | 0.753 | 0.724 | 0.783 | ||||||||||||
|
Month Ended:
|
||||||||||||||||
|
January 31, 2013
|
0.738 | 0.753 | 0.738 | 0.769 | ||||||||||||
|
February 28, 2013
|
0.762 | 0.749 | 0.738 | 0.765 | ||||||||||||
|
March 31, 2013
|
0.781 | 0.771 | 0.764 | 0.783 | ||||||||||||
|
April 30, 2013
|
0.765 | 0.768 | 0.762 | 0.780 | ||||||||||||
|
May 31, 2013
|
0.769 | 0.770 | 0.761 | 0.778 | ||||||||||||
|
June 30, 2013
|
0.765 | 0.758 | 0.746 | 0.768 | ||||||||||||
|
July 31, 2013
|
0.753 | 0.765 | 0.753 | 0.780 | ||||||||||||
|
August 31, 2013
|
0.756 | 0.751 | 0.747 | 0.757 | ||||||||||||
|
September 30, 2013
|
0.740 | 0.749 | 0.738 | 0.762 | ||||||||||||
|
October 31, 2013
|
0.733 | 0.733 | 0.724 | 0.741 | ||||||||||||
|
November 30, 2013
|
0.735 | 0.741 | 0.735 | 0.748 | ||||||||||||
|
December 31, 2013
|
0.725 | 0.730 | 0.724 | 0.739 | ||||||||||||
6
B. Capitalization and indebtedness
Not applicable.
C. Reasons for the offer and use of proceeds
Not applicable.
D. Risk factors
Our business, financial condition or results of operations could be materially and adversely affected if any of these risks occurs, and as a result, the market price of our ordinary shares could decline. This Annual Report also contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially and adversely from those anticipated in these forward-looking statements as a result of certain factors.
Risks related to our business and industry
We depend heavily on the success of drisapersen. Drisapersen and all of our other product candidates are still in preclinical and clinical development and clinical trials of our product candidates may not be successful. If we are unable to commercialize drisapersen and our other product candidates, or experience significant delays in doing so, our business, financial condition and results of operations will be materially adversely affected.
We have invested a significant portion of our efforts and financial resources in the development of drisapersen and our other product candidates targeting patients with Duchenne muscular dystrophy, or DMD. All of our product candidates are still in preclinical and clinical development. Our ability to generate royalty and product revenues, which we do not expect will occur for at least the next several years, if ever, will depend heavily on the successful development and eventual commercialization of these product candidates, particularly drisapersen.
In connection with obtaining marketing approval from regulatory authorities for the sale of any product candidate, we must complete preclinical development and conduct extensive clinical trials to demonstrate the safety and efficacy of our product candidates in humans. Clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of testing. The outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results. In September 2013, we announced that the Phase III clinical trial of drisapersen did not meet its primary endpoint. Although we believe that the collective data from our various Phase II and Phase III clinical trials of drisapersen, including retrospective and subgroup analyses, provide strong support for concluding that drisapersen showed clinically meaningful improvements over placebo in these trials, we cannot be sure that our data will be sufficient to satisfy the European Medicines Agency, or EMA, or the U.S. Food and Drug Administration, or FDA. We may need to conduct additional clinical trials at significant delay and cost or abandon development of drisapersen altogether.
Even if we receive regulatory approval for and are able to commercialize drisapersen and our other product candidates, our success will be subject to the following risks:
|
·
|
we may not achieve market acceptance of drisapersen by physicians, patients and third-party payors;
|
|
·
|
drisapersen and our other product candidates may not have an acceptable safety profile following approval;
|
|
·
|
we may not be able to manufacture drisapersen in compliance with requirements of the EMA, the FDA and similar regulatory agencies in commercial quantities sufficient to meet market demand;
|
|
·
|
we may not achieve sufficient pricing for drisapersen to compensate for development, post-licensing and commercialization costs;
|
|
·
|
we may not compete successfully with any alternative therapies for DMD; and
|
|
·
|
we may not successfully enforce and defend our intellectual property rights and claims.
|
The occurrence of any of these events could materially adversely affect our business, financial condition and results of operations.
7
Our conclusions regarding the efficacy of drisapersen are based on retrospective analyses of the results of our clinical trials, these analyses may be considered less reliable indicators of efficacy than pre-specified analyses.
After determining that we did not achieve the primary efficacy endpoint in the completed Phase III clinical trial of drisapersen, we performed retrospective and subgroup analyses of the Phase III clinical trial and prior Phase II clinical trials of drisapersen that we believe provide strong support for concluding that drisapersen showed clinically meaningful improvements over placebo in these trials. Although we believe that these additional analyses were warranted, a retrospective analysis performed after unblinding trial results can result in the introduction of bias if the analysis is inappropriately tailored or influenced by knowledge of the data and actual results. Because of these limitations, regulatory authorities typically give greatest weight to results from pre-specified analyses and less weight to results from post-hoc, retrospective analyses. Thus, this increases the likelihood that we will have to conduct an additional clinical trial or trials of drisapersen before we can apply for marketing approval.
Because we are developing product candidates for the treatment of diseases in which there is little clinical experience and, in some cases, using new endpoints or methodologies, there is more risk that the outcome of our clinical trials will not be favorable.
There is currently no approved disease-modifying therapy for DMD. In addition, there has been limited historical clinical trial experience generally for the development of drugs to treat the underlying cause of DMD. As a result, the design and conduct of clinical trials for this disease, particularly for drugs to address the underlying cause of this disease, is subject to increased risk. In particular, regulatory authorities in the United States and European Union have not issued definitive guidance as to how to measure and achieve efficacy.
In the last several years, the six minute walk test, or 6MWT, has been used in several trials of product candidates for patients with DMD, and is accepted by U.S. and European regulators to be an appropriate primary outcome measure for DMD trials. Because of the limited clinical experience in this indication however, regulators have not yet established what difference in the distance walked in the 6MWT, or 6MWD, is required to be demonstrated in a clinical trial of a DMD therapy in order to signify a clinically meaningful result and/or obtain regulatory approvals. As a result, it is not clear what is required in terms of 6MWD or other end points to obtain regulatory approval for drisapersen and our other product candidates. If we are required to conduct additional clinical trials of drisapersen, the design of such trials could be subject to such uncertainties.
We could also face similar challenges in designing clinical trials and obtaining regulatory approval for future product candidates, including any that we may develop for myotonic dystrophy (DM1) or Huntington’s disease (HD) because there is also limited historical clinical trial experience for the development of drugs to treat these diseases.
The termination of our collaboration with GSK requires that we quickly expand our development, regulatory and potentially sales and marketing capabilities and take over sponsorship of clinical trials, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
In January 2014, we and GSK mutually terminated our collaboration for the development of drisapersen and our other DMD product candidates. Under the termination agreement, GSK will transfer to us data and other intellectual property, inventory, regulatory filings and clinical trial sponsorships, clinical trial reports, material agreements and biological materials as soon as practicable and within 120 days of the effective date of the agreement. We will have to quickly and greatly expand our capabilities to achieve a smooth transition of the development of drisapersen from GSK to us, and we may be unable to do so effectively or at all. In the future we will be entirely responsible for managing our clinical trials. We may not have the resources or expertise to support the clinical trials needed to develop our product candidates, and we may not be able obtain those resources and expertise efficiently or at all. We may also be the subject to litigation in connection with our clinical sponsorship. Although GSK has agreed to indemnify us in certain circumstances, we may not be able to recoup any such expenses in a timely matter, or at all.
We will also be fully responsible for obtaining regulatory approvals for drisapersen and our other product candidates. We have never successfully obtained regulatory approvals for a product candidate and we may not have the expertise to do so. If drisapersen or another product candidate receives marketing approval, we will be solely responsible for commercializing it. We have never commercialized a product and may not be successful in doing so. We may enter into future collaborations with respect to these operations, and any future collaboration would be subject to numerous execution risks.
8
We expect to experience significant growth in the number of our employees and the scope of our operations, particularly in the areas of drug development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plans or disrupt our operations.
We have a history of operating losses, and we may not achieve or sustain profitability. We anticipate that we will continue to incur losses for the foreseeable future. If we fail to obtain additional funding to conduct our planned research and development effort, we could be forced to delay, reduce or eliminate our product development programs or commercial development efforts.
We incurred net losses of €16.6 million for the year ended December 31, 2013 (2012: €9.9 million, 2011: €11.6 million). Our losses have resulted principally from expenses incurred in research and development of our product candidates and from general and administrative expenses that we have incurred while building our business infrastructure. We expect to continue to incur significant operating losses in the future as we continue our research and development efforts and seek to obtain regulatory approval and commercialization of our product candidates. We are currently conducting a full evaluation of the benefit-to-risk profile of drisapersen; and until we conclude our analysis, we will not be able to estimate our near-to-medium term research and development budget.
To date, we have financed our operations through offerings of equity securities, upfront, milestone and expense reimbursement payments received from GSK under our collaboration and funding from patient organizations, governmental bodies and bank loans. Funding from GSK under our collaboration agreement comprised a significant portion of our revenue, and we will not receive any such additional funds now that the collaboration has terminated. To date we have not generated any revenues from product sales. Based on our current plans, we do not expect to generate significant product revenues unless we obtain marketing approval for, and commercialize, drisapersen or any of our other product candidates. We believe that our existing cash and cash equivalents will enable us to fund our operating expenses and capital expenditure requirements for at least the next 12 months. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect.
We will need to seek additional funding. Additional funds may not be available on a timely basis, on favorable terms, or at all, and such funds, if raised, may not be sufficient to enable us to continue to implement our long-term business strategy.
Even if we do generate product revenues, we may never achieve or sustain profitability on a quarterly or annual basis. Our failure to sustain profitability would depress the market price of our ordinary shares and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. A decline in the market price of our ordinary shares also could cause you to lose all or a part of your investment.
We will need substantial additional funding. If we are unable to raise capital when needed, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
Until recently we collaborated with GSK on the development of the products in our DMD portfolio, and the funding we received from GSK covered a substantial portion of our development costs. Now that the GSK collaboration has been terminated, we will be responsible for all of the research and development costs for our product candidates going forward. In addition, the Phase III trial of drisapersen that was completed in September 2013 did not meet its primary endpoint; and consequently regulators may require that we conduct additional trials of drisapersen in order to apply for regulatory approvals. Such trials would be costly. As a result, we expect our research and development expenses to increase significantly. We are currently conducting a full evaluation of the benefit-to-risk profile of drisapersen; and until we conclude our analysis, we will not be able to estimate our near-to-medium term research and development budget. If we obtain regulatory approval for drisapersen or any of our other product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, distribution and manufacturing. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our product development programs or commercialization efforts.
9
Raising additional capital may cause dilution to our shareholders, restrict our operations or require us to relinquish rights to our technologies or product candidates.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, grants and clinical trial support from governmental and philanthropic organizations and patient advocacy groups, or marketing, distribution or licensing arrangements with third parties. In the event we need to seek additional funds through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a holder of our ordinary shares. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends.
If we raise additional funds through collaborations, strategic alliances or marketing, distribution or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams or product candidates or to grant licenses on terms that may not be favorable to us. If we are unable to raise additional funds when needed, we may be required to delay, limit, reduce or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
Exchange rate fluctuations or abandonment of the euro currency may materially affect our results of operations and financial condition.
Due to the international scope of our operations, fluctuations in exchange rates, particularly between the euro, and the U.S. dollar, may adversely affect us. Although we are based in the Netherlands, we source research and development, manufacturing, consulting and other services from several countries. Further, potential future revenue may be derived from abroad, particularly from the United States. As a result, our business and share price may be affected by fluctuations in foreign exchange rates between the euro and these other currencies, which may also have a significant impact on our reported results of operations and cash flows from period to period. Currently, we do not have any exchange rate hedging arrangements in place.
In addition, the possible abandonment of the euro by one or more members of the European Union could materially affect our business in the future. Despite measures taken by the European Union to provide funding to certain E.U. member states in financial difficulties and by a number of European countries to stabilize their economies and reduce their debt burdens, it is possible that the euro could be abandoned in the future as a currency by countries that have adopted its use. This could lead to the re-introduction of individual currencies in one or more E.U. member states, or in more extreme circumstances, the dissolution of the European Union. The effects on our business of a potential dissolution of the European Union, the exit of one or more E.U. member states from the European Union or the abandonment of the euro as a currency, are impossible to predict with certainty, and any such events could have a material adverse effect on our business, financial condition and results of operations.
Risks related to the development and clinical testing of our product candidates
Clinical drug development involves a lengthy and expensive process with uncertain timelines and uncertain outcomes. If clinical trials of our product candidates are prolonged or delayed or unsuccessful, we may be unable to obtain required regulatory approvals, and therefore be unable to commercialize our product candidates on a timely basis or at all.
To obtain the requisite regulatory approvals to market and sell any of our product candidates, we must demonstrate through extensive preclinical and clinical trials that our products are safe and effective in humans. The process for obtaining governmental approval to market our products is rigorous, time-consuming and costly. It is impossible to predict the extent to which this process may be affected by legislative and regulatory developments. Due to these and other factors, such as the fact that no product using antisense oligonucleotides, or AONs, for systemic use has been approved for sale in the European Union and only one AON is approved for systemic use in the United States, our current product candidates or any of our other future product candidates could take a significantly longer time to gain regulatory approval than expected or may never gain regulatory approval. This could delay or eliminate any potential product revenue by delaying or terminating the potential commercialization of our product candidates.
Clinical trials must be conducted in accordance with EMA, FDA and other applicable regulatory authorities’ legal requirements, regulations or guidelines, and are subject to oversight by these governmental agencies and Institutional Review Boards, or IRBs, at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with supplies of our product candidates produced under
10
current good manufacturing practices, or cGMP, and other requirements. We depend on medical institutions and clinical research organizations, or CROs , to conduct our clinical trials in compliance with Good Clinical Practice, or GCP. To the extent the CROs fail to enroll participants for our clinical trials, fail to conduct the studies to GCP standards or are delayed for a significant time in the execution of trials, including achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business. In addition, clinical trials are conducted in countries outside the European Union and the United States, which may subject us to further delays and expenses as a result of increased shipment costs, additional regulatory requirements and the engagement of non-E.U. and non-U.S. CROs, as well as expose us to risks associated with clinical investigators who are unknown to the EMA or the FDA, and different standards of diagnosis, screening and medical care.
Our lead compound drisapersen completed a Phase III clinical trial in September 2013 but failed to meet its primary endpoint. The trials of our other compounds are less advanced or have not yet started. The commencement and completion of clinical trials for our products may be delayed, suspended or terminated as a result of many factors, including but not limited to:
|
·
|
negative or inconclusive results, such as the Phase III clinical trial of drisapersen, which may require us to conduct additional preclinical or clinical trials or to abandon projects that we expect to be promising;
|
|
·
|
safety or tolerability concerns could cause us to suspend or terminate a trial if we find that the participants are being exposed to unacceptable health risks;
|
|
·
|
the delay or refusal of regulators or IRBs to authorize us to commence a clinical trial at a prospective trial site and changes in regulatory requirements, policies and guidelines;
|
|
·
|
regulators or IRBs requiring that we or our investigators suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements;
|
|
·
|
delays or failure to reach agreement on acceptable clinical trial contracts or clinical trial protocols with prospective trial sites;
|
|
·
|
delays in patient enrollment and variability in the number and types of patients available for clinical trials;
|
|
·
|
the inability to enroll a sufficient number of patients in trials to ensure adequate statistical power to detect statistically significant treatment effects;
|
|
·
|
lower than anticipated retention rates of patients and volunteers in clinical trials;
|
|
·
|
our third-party research contractors failing to comply with regulatory requirements or meet their contractual obligations to us in a timely manner, or at all;
|
|
·
|
difficulty in maintaining contact with patients after treatment, resulting in incomplete data;
|
|
·
|
delays in establishing the appropriate dosage levels;
|
|
·
|
the difficulty in certain countries in identifying the sub-populations that we are trying to treat in a particular trial, which may delay enrollment and reduce the power of a clinical trial to detect statistically significant results;
|
|
·
|
the quality or stability of the product candidate falling below acceptable standards;
|
|
·
|
the inability to produce or obtain sufficient quantities of the product candidate to complete clinical trials; and
|
|
·
|
exceeding budgeted costs due to difficulty in predicting accurately costs associated with clinical trials.
|
Since we have adopted a platform technology approach and all of our current clinical development candidates use similar process technology and are similarly applied to DMD, any of the above unforeseen difficulties that arise with one of our clinical development candidates may negatively affect all of our product candidates.
11
The EMA, the FDA and other regulatory authorities have substantial discretion in the approval process, and determining when or whether regulatory approval will be obtained for any of our product candidates. Even if we believe the data collected from clinical trials of our product candidates are promising, such data may not be sufficient to support approval by the EMA, the FDA or any other regulatory authority. In general, the FDA has usually suggested two adequately designed and powered and well-controlled trials to demonstrate effectiveness, and the FDA may require additional clinical trials, possibly using a different design, before it will consider granting approval to drisapersen.
Any delay in commencing or completing clinical trials for our products could increase our product development costs and delay marketing approval and commercialization of our products. For instance, in 2009 we held a pre-IND meeting with the FDA to discuss PRO044 clinical studies, and the FDA determined that we had insufficient data at that time to support the initiation of a clinical trial of PRO044 in the United States. PRO044 continues to be on clinical hold in the United States pending the results of long-term preclinical safety studies which are expected to be completed in the first half of 2014. In addition, in 2010, the FDA determined that inadequate data had been presented to support long-term studies of drisapersen and requested additional safety data. Upon submission of additional data, the FDA lifted the clinical hold on drisapersen in 2011.
It is also possible that none of our products will complete clinical trials in any of the markets in which we intend to sell those products. If this were to happen, we would not receive the regulatory approvals needed and we would not be able to market our products. Significant clinical trial delays could also allow our competitors to bring products to market before we do or shorten any periods during which we have the exclusive right to commercialize our product candidates and impair our ability to commercialize our product candidates and may harm our business and results of operations.
Failure to obtain marketing authorization for our product candidates will result in our being unable to market and sell such products, which would materially adversely affect our business, financial conditional and results of operation. Approval by one regulatory authority does not ensure approval by regulatory authorities in other jurisdictions. If we fail to obtain approval in any jurisdiction, the geographic market for our product candidates could be limited. Similarly, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates.
If serious adverse, undesirable or unacceptable side effects are identified during the development of our product candidates, we may need to abandon our development of such product candidates; and will not have a complete picture of the benefit-to-risk profile of drisapersen until GSK completes the transfer to us of drisapersen safety data.
If our product candidates are associated with serious adverse, undesirable or unacceptable side effects, we may need to abandon their development or limit development to certain uses or sub-populations in which such side effects are less prevalent, less severe or more acceptable from a risk-benefit perspective. Many compounds that initially showed promise in early-stage or clinical testing have later been found to cause side effects that prevented further development of the compound.
Transient proteinuria (protein in the urine) was observed in all twelve patients in our Phase I/II study of drisapersen, and eleven patients demonstrated signs of proteinuria at week twelve of the extension phase of this trial. Other reported adverse events from this study included injection site reactions, which were seen to varying degrees of severity in all subjects, raised cystatin C (a protein indicative of kidney function), decreased C3 (a protein indicating immunological activity), low platelet counts and raised liver enzymes, including GLDH and gamma GT, which may indicate abnormal liver function. In addition, clinical trial experience to date with drisapersen indicates adverse events that include proteinuria, local injection site reactions (pain, bruising, erythema, induration, pigmentation), thrombocytopenia (decrease in the amount of platelets in the blood) and increases in certain liver enzymes. In addition to the adverse events noted above, single reports have been received of the following clinical conditions: intracranial venous sinus thrombosis (blood clot), extramembranous glomerulonephritis (inflammation affecting part of the kidney) and nephrotic-linked proteinuria. Such side effects could be raised by the FDA, the EMA and other regulatory authorities and could be an impediment to receipt of marketing approval or physician or patient acceptance of drisapersen or our other product candidates because of concerns related to safety.
In January 2014, we and GSK mutually terminated our collaboration for the development of drisapersen and our other DMD product candidates. Under the termination agreement, GSK agreed to transfer to us, inter alia, data and clinical trial reports as soon as practicable and within 120 days of the effective date of the agreement. As of the date of this Annual Report, the transition is still in process. In particular, GSK has not yet transferred to us certain data and safety data for various clinical trials conducted by GSK for which dosing has
12
been suspended but safety data is still being collected. Therefore, as of the date of this Annual Report we may not have complete information regarding the safety profile of drisapersen. Information we receive from GSK in the future may affect our evaluation of the benefit-to-risk profile of drisapersen and our future development plans for it.
We depend on enrollment of patients in our clinical trials for our product candidates. If we are unable to enroll patients in our clinical trials, our research and development efforts and business, financial condition and results of operations could be materially adversely affected.
Successful and timely completion of clinical trials will require that we enroll a sufficient number of patient candidates. Trials may be subject to delays as a result of patient enrollment taking longer than anticipated or patient withdrawal. Patient enrollment depends on many factors, including the size of the patient population, eligibility criteria for the trial, the proximity of patients to clinical sites, the nature of the trial protocol, competing clinical trials and the availability of new drugs approved for the indication the clinical trial is investigating.
The successful completion of our clinical trials for our DMD product candidates is dependent upon our ability to enroll a sufficient number of patients in the sub-populations of DMD patients that our particular product candidates target. Our product candidates focus on the treatment of DMD, which is a rare disease with a small patient population. As our products target sub-populations of DMD patients and trial enrollment is limited to boys in a certain age range only, the number of patients eligible for our trials is even smaller. Further, there are only a limited number of specialist physicians and major clinical centers are concentrated in a few geographic regions. In addition, other companies are conducting clinical trials and have announced plans for future clinical trials that are seeking, or are likely to seek, to enroll patients with the same conditions that we are studying and patients are generally only able to enroll in one single trial at a time. The small population of patients, competition for these patients. Limited trial sites and past clinical trial results may make it difficult for us to enroll enough patients to complete our clinical trials in a timely and cost-effective manner.
We may become exposed to costly and damaging liability claims, either when testing our product candidates in the clinic or at the commercial stage; and our product liability insurance may not cover all damages from such claims.
We are exposed to potential product liability and professional indemnity risks that are inherent in the research, development, manufacturing, marketing and use of pharmaceutical products. Currently we have no products that have been approved for commercial sale; however, the current and future use of product candidates by us in clinical trials, and the sale of any approved products in the future, may expose us to liability claims. These claims might be made by patients that use the product, healthcare providers, pharmaceutical companies, our collaborators or others selling such products. Any claims against us, regardless of their merit, could be difficult and costly to defend and could materially adversely affect the market for our product candidates or any prospects for commercialization of our product candidates.
Although the clinical trial process is designed to identify and assess potential side effects, it is always possible that a drug, even after regulatory approval, may exhibit unforeseen side effects. If any of our product candidates were to cause adverse side effects during clinical trials or after approval of the product candidate, we may be exposed to substantial liabilities. Physicians and patients may not comply with any warnings that identify known potential adverse effects and patients who should not use our product candidates.
Although we maintain limited product liability insurance for our product candidates, it is possible that our liabilities could exceed our insurance coverage. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for any of our product candidates. However, we may not be able to maintain insurance coverage at a reasonable cost or obtain insurance coverage that will be adequate to satisfy any liability that may arise. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business operations could be impaired.
Should any of the events described above occur, this could have a material adverse effect on our business, financial condition and results of operations.
Even if our product candidates obtain regulatory approval, they will be subject to continual regulatory review.
If marketing authorization is obtained for any of our product candidates, the product will remain subject to continual review and therefore authorization could be subsequently withdrawn or restricted. We will be subject to ongoing obligations and oversight by regulatory authorities, including adverse event reporting requirements, marketing restrictions and, potentially, other post-marketing obligations, all of which may result in significant expense and limit our ability to commercialize such products.
13
If there are changes in the application of legislation or regulatory policies, or if problems are discovered with a product or our manufacture of a product, or if we or one of our distributors, licensees or co-marketers fails to comply with regulatory requirements, the regulators could take various actions. These include imposing fines on us, imposing restrictions on the product or its manufacture and requiring us to recall or remove the product from the market. The regulators could also suspend or withdraw our marketing authorizations, requiring us to conduct additional clinical trials, change our product labeling or submit additional applications for marketing authorization. If any of these events occurs, our ability to sell such product may be impaired, and we may incur substantial additional expense to comply with regulatory requirements, which could materially adversely affect our business, financial condition and results of operations.
Due to our limited resources and access to capital, we must and have in the past decided to prioritize development of certain product candidates; these decisions may prove to have been wrong and may adversely affect our revenues.
Because we have limited resources and access to capital to fund our operations, we must decide which product candidates to pursue and the amount of resources to allocate to each. Our decisions concerning the allocation of research, collaboration, management and financial resources toward particular compounds, product candidates or therapeutic areas may not lead to the development of viable commercial products and may divert resources away from better opportunities. Similarly, our decisions to delay, terminate or collaborate with third parties in respect of certain product development programs may also prove not to be optimal and could cause us to miss valuable opportunities. If we make incorrect determinations regarding the market potential of our product candidates or misread trends in the biopharmaceutical industry, in particular for DMD therapies, our business, financial condition and results of operations could be materially adversely affected.
Because we are subject to environmental, health and safety laws and regulations, we may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities which may adversely affect our business and financial condition.
Our operations, including our research, development, testing and manufacturing activities, are subject to numerous environmental, health and safety laws and regulations. These laws and regulations govern, among other things, the controlled use, handling, release and disposal of, and the maintenance of a registry for, hazardous materials and biological materials, such as chemical solvents, human cells, carcinogenic compounds, mutagenic compounds and compounds that have a toxic effect on reproduction, laboratory procedures and exposure to blood-borne pathogens. If we fail to comply with such laws and regulations, we could be subject to fines or other sanctions.
As with other companies engaged in activities similar to ours, we face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of or exposure to hazardous or biological materials. Environmental, health and safety laws and regulations are becoming more stringent. We may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, our production and development efforts may be interrupted or delayed and our financial condition and results of operations may be materially adversely affected.
Our research and development activities could be affected or delayed as a result of possible restrictions on animal testing.
Certain laws and regulations require us to test our product candidates on animals before initiating clinical trials involving humans. Animal testing activities have been the subject of controversy and adverse publicity. Animal rights groups and other organizations and individuals have attempted to stop animal testing activities by pressing for legislation and regulation in these areas and by disrupting these activities through protests and other means. To the extent the activities of these groups are successful, our research and development activities may be interrupted, delayed or become more expensive.
14
Risks related to regulatory approval of our product candidates
Our ability to obtain marketing approval for the product candidates for which we have retained commercialization rights depends on obtaining marketing approval for drisapersen and the success of our regulatory strategy for our candidates that target ultra-orphan sub-populations of DMD patients. If either is not successful, we may not obtain marketing approvals for our product candidates.
If we are not successful in obtaining marketing approval for drisapersen, or we do so in a way that is limiting with respect to our other DMD product candidates, our ability to obtain regulatory approval for such product candidates, and consequently our business and results of operations, could be adversely affected.
Our regulatory strategy for obtaining approval for the product candidates to which we have retained commercialization rights depends on the acceptance of our proposed extrapolation principle that if exon skipping works for one AON compound that is shown to be safe and effective, then the principle should to a certain extent also apply to subsequent compounds for rarer sub-populations. Because DMD is a rare disease and our product candidates after drisapersen target smaller sub-populations for which it is less feasible to conduct placebo-controlled studies, our ability to obtain marketing approval may be dependent on regulators’ acceptance of this extrapolation principle. If regulators do not accept this principle, we may be delayed in or prevented from obtaining marketing approval.
No exon-skipping therapies using AONs for systemic use have yet been approved or marketed in the European Union, and only one AON for systemic use has been approved in the United States.
Our exon-skipping therapy using AONs is intended to correct genetic defects that cause disease in humans, and our current product candidates target DMD. Our compounds have not yet been incorporated into a commercial product and are still in development. To date, no product using AONs for systemic use has been approved for sale in the European Union. In the United States currently only one AON is approved by the FDA for systemic use. Therefore, we are not certain that our technology will meet the applicable safety and efficacy standards of the regulatory authorities. In addition, any regulatory setbacks faced by third parties developing similar compounds could affect the receptiveness of regulators to our compounds.
Any failures or setbacks involving our therapy, including adverse effects resulting from the use of this therapy in humans, could have a detrimental impact on our internal product candidate pipeline and our ability to maintain and/or enter into new corporate collaborations regarding these technologies, which would materially adversely affect our business, financial position and results of operations.
The breakthrough therapy designation for drisapersen may not actually lead to a faster development or regulatory review or approval process.
In June 2013, drisapersen was granted breakthrough therapy designation by the FDA. The breakthrough therapy designation is a program designed to expedite the development and review of drugs for serious or life-threatening conditions. However, we may not experience a faster development or review process compared to conventional FDA procedures. In addition, there is no guarantee of approval, and the FDA may withdraw our breakthrough therapy designation if the FDA believes that the designation is no longer supported by clinical data.
Enacted and future legislation may increase the difficulty and cost for us to obtain marketing approval of and commercialize our product candidates and may affect the prices we may set.
In the United States, the European Union and some other foreign jurisdictions, there have been a number of legislative and regulatory changes and proposed changes regarding the healthcare system. These changes could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to profitably sell any products for which we obtain marketing approval.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the Medicare Modernization Act, changed the way Medicare covers and pays for pharmaceutical products. The legislation expanded Medicare coverage for drug purchases by the elderly and introduced a new reimbursement methodology based on average sale prices for physician-administered drugs. In addition, this legislation provided authority for limiting the number of drugs that will be covered in any therapeutic class. Cost-reduction initiatives and other provisions of this legislation could decrease the coverage and price that we receive for any approved products. While the Medicare Modernization Act applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own reimbursement rates. Therefore, any reduction in reimbursement that results from the Medicare Modernization Act may result in a similar reduction in payments from private payors.
15
More recently, in March 2010, President Obama signed into law the Health Care Reform Law, a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for health care and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Effective October 1, 2010, the Health Care Reform Law revises the definition of “average manufacturer price” for reporting purposes, which could increase the amount of Medicaid drug rebates to states. Further, the new law imposes a significant annual fee on companies that manufacture or import branded prescription drug products. Substantial new provisions affecting compliance have also been enacted, which may affect our business practices with health care practitioners. We will not know the full effects of the Health Care Reform Law until applicable federal and state agencies issue regulations or guidance under the new law. Although it is too early to determine the effect of the Health Care Reform Law, the new law appears likely to continue the pressure on pharmaceutical pricing, especially under the Medicare program, and may also increase our regulatory burdens and operating costs.
Both in the US and in the EU, legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional activities for pharmaceutical products. We are not sure whether additional legislative changes will be enacted, or whether the regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals of our product candidates, if any, may be.
In the area of companion diagnostics, FDA officials indicated in 2010 that the agency planned to issue two guidances in this area. The FDA issued a first draft guidance in July 2011, and the FDA plans to finalize this guidance in 2014. The FDA has yet to issue a second draft guidance, and may decide not to issue a second draft guidance or finalize the existing draft guidance. The FDA’s issuance of a final guidance, or issuance of additional draft guidance, could affect our development of in vitro companion diagnostics and the applicable regulatory requirements. In addition, increased scrutiny by the U.S. Congress of the FDA’s approval process may significantly delay or prevent marketing approval, as well as subject us to more stringent product labeling and post-marketing testing and other requirements.
In the EU, Regulation (EC) 1901/2006, the so called Paediatric Regulation, came into force in January 2007 and introduced considerable changes into the regulatory environment for pediatric medicines. The effects of this regulation are still not fully known. Additionally, an official guideline is being developed which addresses the development of medicinal products for the treatment of Duchenne and Becker muscular dystrophy. This guideline is planned to be adopted during the development of our compounds and may have an effect on the regulatory process for gaining market access in the EU.
Our relationships with customers and payors will be subject to applicable anti-kickback, fraud and abuse and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm and diminished profits and future earnings.
Healthcare providers, physicians and others play a primary role in the recommendation and prescription of any products for which we obtain marketing approval. Our future arrangements with third-party payors and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, primarily in the United States, that may constrain the business or financial arrangements and relationships through which we market, sell and distribute our products for which we obtain marketing approval. Restrictions under applicable healthcare laws and regulations, include the following:
|
·
|
the U.S. healthcare anti-kickback statute prohibits, among other things, persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under U.S. healthcare programs such as Medicare and Medicaid;
|
|
·
|
the U.S. False Claims Act imposes criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the U.S. government, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government;
|
|
·
|
the U.S. Health Insurance Portability and Accountability Act of 1996, as amended by the Health Information Technology for Economic and Clinical Health Act, imposes criminal and civil liability for executing a scheme to defraud any healthcare benefit program and also imposes obligations, including mandatory contractual terms, with respect to safeguarding the privacy, security and transmission of individually identifiable health information;
|
16
|
·
|
the U.S. false statements statute prohibits knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of or payment for healthcare benefits items or services;
|
|
·
|
the transparency requirements under the Health Care Reform Law require manufacturers of drugs, devices, biologics and medical supplies to report to the U.S. Department of Health and Human Services information related to physician payments and other transfers of value and physician ownership and investment interests; and
|
|
·
|
analogous laws and regulations, such as state anti-kickback and false claims laws, may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers, and some state laws require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government in addition to requiring manufacturers to report information related to payments to physicians and other health care providers or marketing expenditures.
|
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative penalties, damages, fines, exclusion from U.S. government funded healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations. If any of the physicians or other providers or entities with whom we expect to do business with are found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs.
Risks related to commercialization of our product candidates
We operate in highly competitive and rapidly changing industries, which may result in others discovering, developing or commercializing competing products before or more successfully than we do.
The biopharmaceutical and pharmaceutical industries are highly competitive and subject to significant and rapid technological change. Our success is highly dependent on our ability to discover, develop and obtain marketing approval for new and innovative products on a cost-effective basis and to market them successfully. In doing so, we face and will continue to face intense competition from a variety of businesses, including large, fully integrated pharmaceutical companies, specialty pharmaceutical companies and biopharmaceutical companies, academic institutions, government agencies and other private and public research institutions in Europe, the United States and other jurisdictions. These organizations may have significantly greater resources than we do and conduct similar research, seek patent protection and establish collaborative arrangements for research, development, manufacturing and marketing of products that compete with our product candidates.
We believe that our key competitor in DMD is Sarepta Therapeutics, Inc., or Sarepta, a U.S. Company focused on the development of their lead product candidate eteplirsen. Eteplirsen is currently in Phase II trials in DMD and employs the same exon 51 skipping approach as drisapersen. Sarepta may be in discussions with the FDA for accelerated approval of eteplirsen, which if granted, could place us at a competitive disadvantage. Even without accelerated approval, depending on the overall clinical profile, efficacy and commercialization of eteplirsen, Sarepta may render our development and discovery efforts in the area of DMD uncompetitive. Other companies are also developing alternative therapeutic approaches to the treatment of DMD. These approaches may be used as complementary to our products targeting DMD, but they could also be competitive.
The highly competitive nature of and rapid technological changes in the biotechnology and pharmaceutical industries could render our product candidates or our technology obsolete or non-competitive. Our competitors may, among other things:
|
·
|
develop and commercialize products that are safer, more effective, less expensive, or more convenient or easier to administer;
|
17
|
·
|
obtain quicker regulatory approval;
|
|
·
|
establish superior proprietary positions;
|
|
·
|
have access to more manufacturing capacity;
|
|
·
|
implement more effective approaches to sales and marketing; or
|
|
·
|
form more advantageous strategic alliances.
|
Should any of these factors occur, our business, financial condition and results of operations could be materially adversely affected.
We rely on obtaining and maintaining orphan drug status for market exclusivity. Orphan drug status may not ensure that we have market exclusivity in a particular market, and we could lose orphan market exclusivity if another drug is approved first using the same method of action or demonstrates clinical superiority.
All of our DMD compounds have been granted orphan drug status by the FDA and EMA. If drisapersen or our other product candidates were to lose orphan drug status or the marketing exclusivity that it provides, our business and results of operations could be materially adversely affected. In the United States, a product candidate with orphan drug status qualifies for market exclusivity for seven years after FDA approval, unless a chemically identical competing product for the same indication is proved to be “clinically superior,” that is, safer, more effective or significantly more convenient. Thus, if drisapersen is granted regulatory approval in the United States, the FDA may not approve a competing generic product during the market exclusivity period; however, a chemically dissimilar product such as Sarepta’s eteplirsen would not be affected by drisapersen’s U.S. market exclusivity and could similarly obtain market exclusivity in the United States if it were to receive FDA approval.
In Europe, EMA regulations provide ten-year marketing exclusivity in Europe for orphan drugs, subject to certain exceptions, including the demonstration of “clinically relevant superiority” by a similar medicinal product. EMA orphan marketing exclusivity applies to drug products for the same indication that use the same method of action but can be chemically dissimilar. Eteplirsen has been granted orphan drug designation in the European Union. If Sarepta were to obtain marketing approval from the EMA for eteplirsen before drisapersen is approved by the EMA, Sarepta could have the benefit of orphan drug marketing exclusivity to our detriment because both products use the same method of action (exon skipping) for patients with DMD. Drisapersen would have to demonstrate a clinically relevant advantage over eteplirsen (in efficacy, safety and/or pharmacokinetics) in order to defeat such market exclusivity in Europe. If drisapersen is approved by the EMA before eteplirsen, eteplirsen could defeat drisapersen’s market exclusivity in Europe by demonstrating a clinically relevant advantage.
The successful commercialization of our product candidates will depend in part on the extent to which governmental authorities and health insurers establish adequate reimbursement levels and pricing policies.
The successful commercialization of our product candidates will depend, in part, on the extent to which third-party coverage and reimbursement for our products will be available from government and health administration authorities, private health insurers and other third-party payors.
These bodies may deny or revoke the reimbursement status of a given drug product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in product development. Obtaining and maintaining reimbursement status is time-consuming and costly. Significant uncertainty exists as to the reimbursement status of newly approved medical products. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely. In addition, many governments and health insurers are increasingly attempting to manage healthcare costs by limiting both coverage and the level of reimbursement of new products. As a result, they may not cover or provide adequate payment for our future products.
The unavailability or inadequacy of third-party coverage and reimbursement could have a material adverse effect on the market acceptance of our product candidates and the future revenues we may expect to receive from those products. In addition, we are unable to predict what additional legislation or regulation relating to the healthcare industry or third-party coverage and reimbursement may be enacted in the future, or what effect such legislation or regulation would have on our business.
18
Our products may not gain market acceptance, in which case we may not be able to generate product revenues, which will materially adversely affect our business, financial condition and results of operations.
Even if the EMA, the FDA or any other regulatory authority approves the marketing of any product candidates that we develop on our own or with a collaboration partner, physicians, healthcare providers, patients or the medical community may not accept or use them. If these products do not achieve an adequate level of acceptance, we may not generate significant product revenues or any profits from operations. The degree of market acceptance of any of our product candidates will depend on a variety of factors, including:
|
·
|
the timing of market introduction;
|
|
·
|
the number and clinical profile of competing products;
|
|
·
|
our ability to provide acceptable evidence of safety and efficacy;
|
|
·
|
the prevalence and severity of any side effects;
|
|
·
|
relative convenience and ease of administration;
|
|
·
|
cost-effectiveness;
|
|
·
|
patient diagnostics and screening infrastructure in each market;
|
|
·
|
marketing and distribution support;
|
|
·
|
availability of coverage, reimbursement and adequate payment from health maintenance organizations and other insurers, both public and private; and
|
|
·
|
other potential advantages over alternative treatment methods.
|
If our product candidates fail to gain market acceptance, this will have a material adverse impact on our ability to generate revenues to provide a satisfactory, or any, return on our investments. Even if some products achieve market acceptance, the market may prove not to be large enough to allow us to generate significant revenues.
Adverse events in the field of RNA modulation therapy could damage public perception of our product candidates and negatively affect our business.
The commercial success of our products will depend in part on public acceptance of the use of RNA modulation therapy for the treatment of human diseases. Adverse events in clinical trials of our product candidates or in clinical trials of others developing RNA-modulating products and the resulting publicity, as well as any other adverse events in the field of RNA modulation therapy that may occur in the future, could result in a decrease in demand for any products that we may develop. For example, a recently published report described a case of renal damage in a healthy volunteer enrolled in a clinical trial sponsored by Santaris Pharma A/S. The trial used a type of so-called locked-acid AON compound that primarily targets the kidney, intestines and liver and works by blocking messenger RNA for the enzyme PCSK9. If public perception is influenced by claims that RNA modulation therapy is unsafe, our products may not be accepted by the general public or the medical community.
Future adverse events in RNA modulation therapy or the biopharmaceutical industry could also result in greater governmental regulation, stricter labeling requirements and potential regulatory delays in the testing or approvals of our products. Any increased scrutiny could delay or increase the costs of obtaining regulatory approval for our product candidates.
We have never commercialized a product candidate before and may lack the necessary expertise, personnel and resources to successfully commercialize our products on our own or together with suitable partners.
We have never commercialized a product candidate, and we currently have no sales force, marketing or distribution capabilities. We will have to develop our own sales, marketing and supply organization or outsource these activities to a third party.
19
Factors that may affect our ability to commercialize our product candidates on our own include recruiting and retaining adequate numbers of effective sales and marketing personnel, obtaining access to or persuading adequate numbers of physicians to prescribe our drug candidates and other unforeseen costs associated with creating an independent sales and marketing organization. Developing a sales and marketing organization will be expensive and time-consuming and could delay the launch of our product candidates. We may not be able to build an effective sales and marketing organization. If we are unable to build our own distribution and marketing capabilities or to find suitable partners for the commercialization of our product candidates, we may not generate revenues from them or be able to reach or sustain profitability.
Risks related to our dependence on third parties
GSK may not fulfill its obligations under the termination agreement, which could delay or terminate the development and commercialization of our product candidates and substantially harm our business .
The termination of our collaboration with GSK is governed by a termination agreement. Among other things, the termination agreement requires GSK to transfer to us certain data, inventory, regulatory filings, sponsorships, clinical study reports and material agreements relating to the development of our products. The termination agreement also requires GSK to indemnify us for certain breaches of its obligations under the agreement.
If GSK were to breach the termination agreement, we cannot be certain that:
|
·
|
we would have the resources to pursue our claims against GSK;
|
|
·
|
arbitrators would find in our favor and grant us an arbitration award;
|
|
·
|
delays in recovery would not arise during the arbitration process that could irreparably harm the commercialization and development of our products;
|
|
·
|
we would be able to prove the extent of the damages we incurred or will incur as a result of GSK’s breach;
|
|
·
|
any recovery we received from GSK would be sufficient to compensate us for the loss their breach caused us; and
|
|
·
|
GSK would pay any arbitration award rendered against it without further litigation or that we would have the resources to pursue enforcement of any arbitration award.
|
The data, inventory, regulatory filings, clinical trial sponsorships, clinical study reports and material agreements that the termination agreement obligates GSK to provide to us are critical to the development and commercialization of our product candidates. Therefore, if GSK does not transfer these items to us per the terms of the termination agreement or we are unable to recover from GSK any of the losses any breach would cause us, our business could be substantially harmed.
If we fail to maintain our current strategic relationship with LUMC our business, commercialization prospects and financial condition may be materially adversely affected.
We have an exclusive worldwide license from Leiden University Medical Center, or LUMC, for the application of LUMC’s proprietary RNA modulation exon-skipping technology to develop treatments for DMD, other neuromuscular disorders and indications outside the field of neuromuscular disorders. These intellectual property rights have been the basis of our research so far and the development of treatments targeting DMD. The continuation of a good relationship with LUMC is essential for our business prospects. If our relationship with LUMC were to deteriorate or LUMC were to challenge our use of its intellectual property or our calculations of the payments we owe under our agreement, our business, financial condition, commercialization prospects and results of operations could be materially adversely affected.
If we fail to enter into new strategic relationships our business, financial condition, commercialization prospects and results of operations may be materially adversely affected.
Our product development programs and the potential commercialization of our product candidates will require substantial additional cash to fund expenses. Therefore, in addition to our relationship with LUMC, for some of our product candidates, we may decide to enter into new collaborations with pharmaceutical or biopharmaceutical companies for the development and potential commercialization of those product candidates.
20
We face significant competition in seeking appropriate collaborators. Collaborations are complex and time-consuming to negotiate and document. We may also be restricted under existing and future collaboration agreements from entering into agreements on certain terms with other potential collaborators. We may not be able to negotiate collaborations on acceptable terms, or at all. If that were to occur, we may have to curtail the development of a particular product candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of our sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we will not be able to bring our product candidates to market and generate product revenue. If we do enter into a new collaboration agreement, we could be subject to the following risks, each of which may materially harm our business, commercialization prospects and financial condition:
|
·
|
we may not be able to control the amount and timing of resources that the collaboration partner devotes to the product development program;
|
|
·
|
the collaboration partner may experience financial difficulties;
|
|
·
|
we may be required to relinquish important rights such as marketing, distribution and intellectual property rights;
|
|
·
|
a collaboration partner could move forward with a competing product developed either independently or in collaboration with third parties, including our competitors; or
|
|
·
|
business combinations or significant changes in a collaboration partner’s business strategy may adversely affect our willingness to complete our obligations under any arrangement.
|
We rely on third parties to provide services in connection with our preclinical and clinical development programs. The inadequate performance by or loss of any of these service providers could affect our product candidate development.
In the past we relied on GSK to conduct clinical trials for the DMD compounds it had licensed from us under our collaboration. Going forward we will rely on other third parties, including CROs, medical institutions, clinical investigators and contract laboratories, to conduct significant portions of our preclinical and clinical trials. Although we will oversee these activities to ensure compliance with our quality standards, budgets and timelines, we have had and will continue to have less control over the conduct of the clinical trials, the timing and completion of the trials, the required reporting of adverse events and the management of data developed through the trials than potentially would be the case if we relied entirely upon our own staff. Such third parties may have staffing difficulties, may undergo changes in priorities or may become financially distressed, adversely affecting the management of the clinical trials.
Problems with the timeliness or quality of the work of third parties may lead us to seek to terminate the relationship and use an alternative service provider. However, making this change may be costly and may delay the trials, and contractual restrictions may make such a change difficult or even impossible. In addition, it may be very challenging, and in some cases impossible, to find a replacement service provider that can conduct our trials in an acceptable manner and at an acceptable cost. In addition, if such third parties fail to comply with regulations regarding clinical trials and laboratory practices and other regulations of the EMA, FDA or other regulatory authorities, we may experience serious delays to our clinical trials, have to suspend clinical trials or lose the regulatory approval for a specific product. Any such failure could give rise to a material interruption in the development of the product and have a material adverse effect on our commercial potential.
We currently rely on third-party suppliers and other third parties for production of our product candidates and our dependence on these third parties may impair the advancement of our research and development programs and the development of our product candidates.
Currently, we develop and manufacture the drug product for our initial preclinical studies using standardized manufacturing processes in our own laboratories. We currently rely on and expect to continue to rely on third parties for the supply of raw materials and to manufacture drug supplies for clinical trials and commercial quantities of our products and drug candidates. For the foreseeable future, we expect to continue to rely on such third parties.
21
Reliance on third-party providers may expose us to more risk than if we were to manufacture product candidates ourselves. We are obliged to work with manufacturing companies and third party suppliers that are licensed by the EMA, the FDA or other regulatory authorities and that comply with EMA, FDA or other regulatory authorities’ laws and regulations on an ongoing basis. We do not have control over a third-party manufacturer’s compliance with these laws, regulations and applicable cGMP standards and other laws and regulations, such as those related to environmental health and safety matters. Any failure to achieve and maintain compliance with these laws, regulations and standards could subject us to the risk that we may have to suspend the manufacturing of our product candidates or that obtained approvals could be revoked, which would adversely affect our business and reputation. Furthermore, third-party providers may breach existing agreements they have with us because of factors beyond our control. They may also terminate or refuse to renew their agreement because of their own financial difficulties or business priorities, at a time that is costly or otherwise inconvenient for us. If we were unable to find adequate replacement or another acceptable solution in time, our clinical trials could be delayed or our commercial activities could be harmed.
While we generally contract with multiple suppliers of raw materials, drug substances, drug product formulation and filling, for each specific AON there is only one contractor for drug substance manufacture and one contractor for drug product manufacture. In addition, we have only a single contractor for the packaging and distribution of product for clinical trials. While we believe we have limited the risk of interruption of production delays because our manufacturing process is highly standardized and therefore we believe that a contractor for one AON product candidate could be brought online to manufacture another AON product candidate, we may incur delays in qualifying an alternative manufacturer for a new compound. Any such delay could delay our clinical trials or have an adverse impact on any commercial activities.
In addition, the fact that we are dependent on our suppliers and other third parties for the manufacture, filling, storage and distribution of our product candidates means that we are subject to the risk that the products may have manufacturing defects that we have limited ability to prevent or control. The sale of products containing such defects could adversely affect our business, financial condition and results of operations.
Growth in the costs and expenses of components or raw materials may also adversely influence our business, financial condition and results of operations. Supply sources could be interrupted from time to time and, if interrupted, that supplies could be resumed (whether in part or in whole) within a reasonable timeframe and at an acceptable cost or at all.
Risks related to intellectual property and information technology
Our business will be adversely affected if we are unable to gain access to relevant intellectual property rights of third parties, or if our licensing partners terminate our rights to license in relevant intellectual property rights.
We currently rely, and may in the future rely, on certain intellectual property rights licensed from third parties to protect our technology. In particular, we have entered into a collaboration arrangement with LUMC for the reciprocal licensing of our and LUMC’s individual and joint intellectual property rights in relation to certain patent rights and know-how rights, which have been the basis of our research and development of treatment targeting DMD. Pursuant to this agreement with LUMC, we have an exclusive worldwide license for the exploitation of key intellectual property rights in this respect. Our intellectual property portfolio for DMD consists of patents and patent applications licensed from LUMC, patents and patent applications for which we and LUMC are joint owners and patents and patent applications that we own. LUMC retains full ownership of certain patents and patent applications which we have licensed under the agreement.
We have the responsibility to maintain and control the intellectual property portfolio licensed from LUMC, however, the licensed rights may not be adequately maintained by LUMC. In addition, our licensed rights may be suspended, terminated or otherwise lost in consequence of a breach of the agreement with LUMC or due to other relevant facts and circumstances such as insolvency of the licensor. Our ability to comply with our contractual obligations towards LUMC may be affected by factors that we can only partially influence or control.
If LUMC were to terminate the license, we would be prevented from continuing our use of this technology in clinical trials or, if our products are approved for marketing, from using this technology in products that could be sold commercially. The loss of rights under this license could preclude us from further developing, commercializing and marketing our products, which would have a material adverse effect on our business, financial condition, results of operations and prospects.
22
We rely on patents and other intellectual property rights to protect our product candidates and technologies, the enforcement, defense and maintenance of which may be challenging and costly. Failure to enforce or protect these rights adequately could harm our ability to compete and impair our business.
Our commercial success depends in part on obtaining and maintaining patents and other forms of intellectual property rights for our product candidates, methods used to manufacture those products and the methods for treating patients using those products, or on licensing in such rights. Failure to protect or to obtain, maintain or extend adequate patent and other intellectual property rights could materially adversely affect our ability to develop and market our products and product candidates.
Issued patents covering one or more of our products could be found invalid or unenforceable if challenged in court.
To protect our competitive position, we may from time to time need to engage in litigation or participate in adversary administrative proceedings in order to enforce or defend any patents or other intellectual property rights owned by or licensed to us, or to determine or challenge the scope or validity of patents or other intellectual property rights of third parties. For example, in 2009, AVI Biopharma Inc. (now Sarepta) filed an opposition against EP ’249, one of our patents, with the European Patent Office, or EPO, requesting revocation of the patent as granted, alleging, among other things, lack of novelty, inventive step and sufficiency of disclosure. The EPO Opposition Division, presiding in oral proceedings on November 16, 2011 in Munich, Germany, maintained the EP ’249 patent in an amended form. We believe that the patent as maintained in amended form by the EPO Opposition Division still provides protection for our lead product candidate drisapersen. We and Sarepta both had appealed the written decision of the EPO. Written submissions were filed by each party on August 23rd, 2013 and on January 8th 2014. In a next step the Board of Appeal of the EPO should provide its preliminary opinion on the Appeal and summon parties to oral proceedings in Munich. For additional information please see “Business—Intellectual property—Opposition proceedings against EP ’249.”
In addition, we are facing possible administrative challenges involving our patents in the form of interference proceedings in the United States Patent and Trademark Office (PTO). The interferences, if formally declared by the PTO, will determine who was first to invent these compounds and therefore who is entitled to the patent claiming them. In the course of interference proceedings, all parties are called upon to submit evidence of the date when they conceived of their respective inventions. Patent interferences are typically complex, highly-contested legal proceedings that can be expensive and prolonged and can cause significant delay in the issuance of patents.
In one instance we have taken steps to precipitate an interference proceeding by filing a suggestion for interference and a patent application with claims identical to those of a patent that is owned by Royal Holloway and Bedford New College, University of London. Our reason for taking this step is that we believe that our rights to the invention are superior to those of Royal Holloway and Bedford New College, University of London because we believe that the inventors identified in our patent application conceived of the invention before Linda Popplewell, the inventor named in U.S. patent no. 8,324,37. The patent and patent application in question involve exon 45. Another potential interference proceeding, involving exon 53, is one that is likely to arise because the University of Western Australia (which has licensed U.S. patent no. 8,455,636 to Sarepta) has taken steps to initiate a proceeding involving our patent application U.S.S.N. 11/233,495, as well as a patent owned by Royal Holloway and Bedford New College, University of London. Here too we believe that we have superior rights because of an earlier invention date. In addition, there may be a basis for an interference proceeding involving one of our patent applications with exon 51.
If the administrative law judge conducting an interference proceeding determines that another party is entitled to the contested patent claims, we may be required to obtain a license to proceed with the US marketing of the product utilizing the technology that is claimed (which may the case with our PRO045 or PRO053 products). There can be no assurance that such a license will be available, in which case we could be prevented from marketing the relevant product in the United States. Any determination of the judge can be challenged by either party in U.S. Federal court. We could incur substantial cost in defending our patents in these proceedings. If the outcome of either proceeding is unfavorable, our business prospects could be materially and adversely affected.
As enforcement of intellectual property rights is difficult, unpredictable and expensive, we may fail in enforcing our rights—in which case our competitors may be permitted to use our technology without being required to pay us any license fees. In addition, however, litigation involving our patents carries the risk that one or more of our patents will be held invalid (in whole or in part, on a claim-by-claim basis) or held unenforceable. Such an adverse court ruling could allow third parties to commercialize our products or our platform technology, and then compete directly with us, without payment to us.
23
If we were to initiate legal proceedings against a third party to enforce a patent covering one of our products, the defendant could counterclaim that our patent is invalid and/or unenforceable. In patent litigation in the United States or in Europe, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the U.S. Patent and Trademark Office, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, we would lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without infringing our patents or other intellectual property rights.
Intellectual property rights of third parties could adversely affect our ability to commercialize our product candidates, such that we could be required to litigate or obtain licenses from third parties in order to develop or market our product candidates. Such litigation or licenses could be costly or not available on commercially reasonable terms.
Our competitive position may suffer if patents issued to third parties or other third party intellectual property rights cover our products or elements thereof, our manufacture or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize products or drug candidates unless we successfully pursue litigation to nullify or invalidate the third party intellectual property right concerned, or enter into a license agreement with the intellectual property right holder, if available on commercially reasonable terms. In addition, we are aware of issued patents and pending patent applications held by third parties that may be construed as covering some of our product candidates, in particular with respect to PRO045 or PRO053, and, as described earlier, we currently expect interference proceeding to arise with respect to the patents covering these products, and believe it is possible that an interference proceeding involving a patent applicable to PRO051 may also occur. We believe that if such patents or patent applications (if issued as currently pending) were asserted against us, we would have defenses against such claims, including defenses of patent invalidity and unenforceability. However, if such defenses were not successful and such patents were successfully asserted against us such that they are found to be valid and enforceable, and infringed by our product candidates, unless we obtain a license to such patents, which may not be available on commercially reasonable terms or at all, we could be prevented from continuing to develop or commercialize our products. We could also be required to pay substantial damages.
It is also possible that we failed to identify relevant patents or applications. For example, U.S. applications filed before November 29, 2000 and certain U.S. applications filed after that date that will not be filed outside the United States remain confidential until patents issue. Patent applications in the United States and elsewhere are published approximately 18 months after the earliest filing for which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Therefore, patent applications covering our products or platform technology could have been filed by others without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our platform technologies, our products or the use of our products.
Third party intellectual property right holders, including our competitors, may actively bring infringement claims against us. The granting of orphan drug status in respect of any of our product candidates does not guarantee our freedom to operate and is separate from our risk of possible infringement of third parties’ intellectual property rights. We may not be able to successfully settle or otherwise resolve such infringement claims. If we are unable to successfully settle future claims on terms acceptable to us, we may be required to engage or continue costly, unpredictable and time-consuming litigation and may be prevented from or experience substantial delays in marketing our products.
If we fail in any such dispute, in addition to being forced to pay damages, we or our licensees may be temporarily or permanently prohibited from commercializing any of our product candidates that are held to be infringing. We might, if possible, also be forced to redesign drug candidates so that we no longer infringe the third party intellectual property rights. Any of these events, even if we were ultimately to prevail, could require us to divert substantial financial and management resources that we would otherwise be able to devote to our business.
24
Intellectual property litigation could cause us to spend substantial resources and distract our personnel from their normal responsibilities.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments and if securities analysts or investors perceive these results to be negative, we could have a substantial adverse effect on the price of our ordinary shares. Such litigation or proceedings could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
We enjoy only limited geographical protection with respect to certain patents and may face difficulties in certain jurisdictions, which may diminish the value of intellectual property rights in those jurisdictions.
We generally file our first patent application (i.e. priority filing) at the European Patent Office (EPO). International applications under the Patent Cooperation Treaty, or PCT, are usually filed within twelve months after the priority filing. Based on the PCT filing, national and regional patent applications may be filed in the United States, Australia, New Zealand, Japan, and Canada and all European Patent Convention, or EPC, member states by filing at the EPO and, depending on the individual case, also in any or all of, inter alia, China, India, Singapore and Israel. We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. In addition, we may decide to abandon national and regional patent applications before grant. Finally, the grant proceeding of each national/regional patent is an independent proceeding which may lead to situations in which applications might in some jurisdictions be refused by the relevant registration authorities, while granted by others. It is also quite common that depending on the country, the scope of patent protection may vary for the same drug candidate and/or technology.
The laws of some jurisdictions do not protect intellectual property rights to the same extent as the laws in the United States and the European Union, and many companies have encountered significant difficulties in protecting and defending such rights in such jurisdictions. If we or our licensors encounter difficulties in protecting, or are otherwise precluded from effectively protecting, the intellectual property rights important for our business in such jurisdictions, the value of these rights may be diminished and we may face additional competition from others in those jurisdictions.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or any of our licensors is forced to grant a license to third parties with respect to any patents relevant to our business, our competitive position may be impaired and our business and results of operations may be adversely affected.
Intellectual property rights do not necessarily address all potential threats to our competitive advantage.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations, and may not adequately protect our business, or permit us to maintain our competitive advantage. The following examples are illustrative:
|
·
|
Others may be able to make compounds that are the same as or similar to our product candidates but that are not covered by the claims of the patents that we own or have exclusively licensed.
|
|
·
|
We or our licensors or any future strategic partners might not have been the first to conceive or reduce to practice the inventions covered by the issued patent or pending patent application that we own or have exclusively licensed.
|
|
·
|
We or our licensors or any future strategic partners might not have been the first to file patent applications covering certain of our inventions.
|
25
|
·
|
Others may independently develop similar or alternative technologies or duplicate any of our technologies without infringing our intellectual property rights.
|
|
·
|
It is possible that our pending patent applications will not lead to issued patents.
|
|
·
|
Issued patents that we own or have exclusively licensed may not provide us with any competitive advantage, or may be held invalid or unenforceable, as a result of legal challenges by our competitors.
|
|
·
|
Our competitors might conduct research and development activities in countries where we do not have patent rights and then use the information learned from such activities to develop competitive products for sale in our major commercial markets.
|
|
·
|
We may not develop additional proprietary technologies that are patentable.
|
|
·
|
The patents of third parties may have an adverse effect on our business.
|
Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biopharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological complexity and legal complexity. Therefore, obtaining and enforcing biopharmaceutical patents is costly, time-consuming and inherently uncertain. In addition, the America Invents Act (AIA) has been recently enacted in the United States, resulting in significant changes to the U.S. patent system. The U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the U.S. Patent and Trademark Office, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. Similarly, the complexity and uncertainty of European patent laws has also increased in recent years. For example, the EPC was amended in April 2010 by limiting the time permitted for filing divisional applications. In addition, the EP patent system is relatively stringent in the type of amendments that are allowed during prosecution. These changes could limit our ability to obtain new patents in the future that may be important for our business.
Confidentiality agreements with employees and others may not adequately prevent disclosure of trade secrets and protect other proprietary information.
We consider proprietary trade secrets and/or confidential know-how and unpatented know-how to be important to our business. We may rely on trade secrets and/or confidential know-how to protect our technology, especially where patent protection is believed to be of limited value. However, trade secrets and/or confidential know-how are difficult to maintain as confidential.
To protect this type of information against disclosure or appropriation by competitors, our policy is to require our employees, consultants, contractors and advisors to enter into confidentiality agreements with us. However, current or former employees, consultants, contractors and advisers may unintentionally or willfully disclose our confidential information to competitors, and confidentiality agreements may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. Enforcing a claim that a third party obtained illegally and is using trade secrets and/or confidential know-how is expensive, time consuming and unpredictable. The enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction.
Failure to obtain or maintain trade secrets and/or confidential know-how trade protection could adversely affect our competitive position. Moreover, our competitors may independently develop substantially equivalent proprietary information and may even apply for patent protection in respect of the same. If successful in obtaining such patent protection, our competitors could limit our use of our trade secrets and/or confidential know-how.
Under certain circumstances and to guarantee our freedom to operate, we may also decide to publish some know-how to prevent others from obtaining patent rights covering such know-how.
26
Our information technology systems could face serious disruptions that could adversely affect our business.
Our information technology and other internal infrastructure systems, including corporate firewalls, servers, leased lines and connection to the Internet, face the risk of systemic failure that could disrupt our operations. A significant disruption in the availability of our information technology and other internal infrastructure systems could cause interruptions in our collaborations with our partners and delays in our research and development work.
Risks related to employee matters and managing growth
Our future growth and ability to compete depends on retaining our key personnel and recruiting additional qualified personnel.
Our success depends upon the continued contributions of our key management, scientific and technical personnel, many of whom have substantial experience with or been instrumental for us and our unique RNA modulation therapy and related technologies. These key management individuals include the members of our management board consisting of Hans Schikan, our Chief Executive Officer; Berndt Modig, our Chief Financial Officer; Giles Campion, our Chief Medical Officer and Senior Vice President Research and Development; and Luc Dochez, our Chief Business Officer and Senior Vice President Business Development. The key members of our management team include Larry Bell, our Vice President of Regulatory Affairs; Judith van Deutekom, our Vice President Drug Discovery; Tina Flatau, our Vice President Alliances and Project Management; Paul van Hagen, our Senior Director Finance and Control; Richard Holslag, our Vice President Manufacturing; Sjef de Kimpe, our Vice President Early Drug Development; and Allison Morgan, our Vice President Clinical Development.
The loss of key managers and senior scientists could delay our research and development activities. In addition, the competition for qualified personnel in the biopharmaceutical and pharmaceutical field is intense, and our future success depends upon our ability to attract, retain and motivate highly-skilled scientific, technical and managerial employees. We face competition for personnel from other companies, universities, public and private research institutions and other organizations. If our recruitment and retention efforts are unsuccessful in the future, it may be difficult for us to implement business strategy, which could have a material adverse effect on our business.
Risks related to our ordinary shares
The price of our ordinary shares may be volatile and may fluctuate due to factors beyond our control.
The share price of publicly traded emerging biopharmaceutical and drug discovery and development companies has been highly volatile and is likely to remain highly volatile in the future. The market price of our ordinary shares may fluctuate significantly due to a variety of factors, including:
|
·
|
positive or negative results of testing and clinical trials by us, strategic partners, or competitors;
|
|
·
|
delays in entering into strategic relationships with respect to development and/or commercialization of our product candidates or entry into strategic relationships on terms that are not deemed to be favorable to us;
|
|
·
|
technological innovations or commercial product introductions by us or competitors;
|
|
·
|
changes in government regulations;
|
|
·
|
developments concerning proprietary rights, including patents and litigation matters;
|
|
·
|
public concern relating to the commercial value or safety of any of our product candidates;
|
|
·
|
financing or other corporate transactions;
|
|
·
|
publication of research reports or comments by securities or industry analysts;
|
|
·
|
general market conditions in the pharmaceutical industry or in the economy as a whole; or
|
|
·
|
other events and factors beyond our control.
|
27
For example, on September 20, 2013, our share price dropped 70% following the announcement that the Phase III clinical trial of drisapersen failed to meet the primary endpoint of a statistically significant improvement in 6MWD compared to a placebo. In addition, the stock market in general has recently experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of individual companies. Broad market and industry factors may materially affect the market price of companies’ stock, including our, regardless of actual operating performance.
Certain of our principal shareholders and members of our management board own a majority of our ordinary shares and as a result will be able to exercise significant control over us, and your interests may conflict with the interests of such shareholders.
Our principal shareholders, executive officers and directors in the aggregate hold 86% of our ordinary shares. Depending on the level of attendance at our general meetings of shareholders, these shareholders may be in a position to determine the outcome of decisions taken at any such general meeting. Any shareholder or group of shareholders controlling more than 50% of the capital present and voting at our general meetings of shareholders may control any shareholder resolution requiring a simple majority, including the appointment of supervisory board members, certain decisions relating to our capital structure, the approval of certain significant corporate transactions and amendments to our Articles of Association. Among other consequences, this concentration of ownership may have the effect of delaying or preventing a change in control and might therefore negatively affect the market price of our ordinary shares.
Future sales, or the possibility of future sales, of a substantial number of our ordinary shares could adversely affect the price of the shares and dilute shareholders.
Future sales of a substantial number of our ordinary shares, or the perception that such sales will occur, could cause a decline in the market price of our ordinary shares
Provisions of our Articles of Association or Dutch corporate law might deter acquisition bids for us that might be considered favorable and prevent or frustrate any attempt to replace or remove the then management board and supervisory board.
Certain provisions of our Articles of Association may make it more difficult for a third party to acquire control of us or effect a change in our management board or supervisory board. These provisions include: the authorization of a class of preference shares that may be issued to a friendly party; staggered four-year terms of our supervisory board members; a provision that our management board and supervisory board members may only be removed by the general meeting of shareholders by a two-thirds majority of votes cast representing more than 50% of our outstanding share capital (unless the removal was proposed by the supervisory board); and a requirement that certain matters, including an amendment of our Articles of Association, may only be brought to our shareholders for a vote upon a proposal by our management board that has been approved by our supervisory board.
Our anti-takeover provision may prevent a beneficial change of control.
We have adopted an anti-takeover measure pursuant to which our management board may, subject to supervisory board approval but without shareholder approval, issue (or grant the right to acquire) cumulative preferred shares. We may issue an amount of cumulative preferred shares up to 100% of our issued capital immediately prior to the issuance of such cumulative preferred shares. In such event, the cumulative preferred shares (or right to acquire cumulative preferred shares) will be issued to a separate, newly established foundation.
The cumulative preferred shares will be issued to the foundation for their nominal value, of which only 25% will be due upon issuance. The voting rights of our shares are based on nominal value and as we expect our shares to trade substantially in excess of nominal value, cumulative preferred shares issued at nominal value can obtain significant voting power for a substantially reduced price and thus be used as a defensive measure. These cumulative preferred shares will have both a liquidation and dividend preference over our ordinary shares and will accrue cash dividends at a fixed rate. The management board may issue these cumulative preferred shares to protect us from influences that do not serve our best interests and threaten to undermine our continuity, independence and identity. These influences may include a third-party acquiring a significant percentage of our ordinary shares, the announcement of a public offer for our ordinary shares, other concentration of control over our ordinary shares or any other form of pressure on us to alter our strategic policies. If the management board determines to issue the cumulative preferred shares to such a foundation, the foundation’s articles of association will provide that it will act to serve the best interests of us, our associated business and all parties connected to us, by opposing any influences that conflict with these interests and threaten to undermine our continuity, independence and identity. This foundation will be structured to operate independently of us.
28
We have broad discretion in the use of our cash on hand and may not use it effectively.
As of December 31, 2013 we had €82.2 million cash on hand. Our management will have broad discretion in the use of such cash and could spend it in ways that do not improve our results of operations or enhance the value of our ordinary shares. The failure by our management to apply these funds effectively could result in financial losses that could have a material adverse effect on our business, cause the price of our ordinary shares to decline and delay the development of our product candidates. Pending their use, we may invest our cash in a manner that does not produce income or that loses value.
We do not expect to pay dividends in the foreseeable future.
We have not paid any dividends since our incorporation. Even if future operations lead to significant levels of distributable profits, we currently intend that any earnings will be reinvested in our business and that dividends will not be paid until we have an established revenue stream to support continuing dividends. Payment of future dividends to shareholders will in addition effectively be at the discretion of the management board, subject to the approval of the supervisory board after taking into account various factors including our business prospects, cash requirements, financial performance and new product development. In addition, payment of future dividends may be made only if our shareholders’ equity exceeds the sum of our paid-in and called-up share capital plus the reserves required to be maintained by Dutch law or by our Articles of Association. Accordingly, investors cannot rely on dividend income from our ordinary shares and any returns on an investment in our ordinary shares will likely depend entirely upon any future appreciation in the price of our ordinary shares.
Holders of our ordinary shares outside the Netherlands may not be able to exercise preemptive rights.
In the event of an increase in our share capital, holders of our ordinary shares are generally entitled under Dutch law to full preemptive rights, unless these rights are excluded either by a resolution of the general meeting of shareholders, or by a resolution of the management board (if the management board has been designated by the general meeting of shareholders for this purpose). Certain holders of our ordinary shares outside the Netherlands, in particular U.S. holders of our ordinary shares, may not be able to exercise preemptive rights unless a registration statement under the Securities Act of 1933, as amended (Securities Act) is declared effective with respect to our ordinary shares issuable upon exercise of such rights or an exemption from the registration requirements is available.
We are a Dutch public company with limited liability. The rights of our shareholders may be different from the rights of shareholders in companies governed by the laws of U.S. jurisdictions.
We are a Dutch public company with limited liability (naamloze vennootschap). Our corporate affairs are governed by our Articles of Association and by the laws governing companies incorporated in the Netherlands. The rights of shareholders and the responsibilities of members of our management board and supervisory board may be different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. In the performance of its duties, our management board and supervisory board are required by Dutch law to consider the interests of our company, its shareholders, its employees and other stakeholders, in all cases with due observation of the principles of reasonableness and fairness. It is possible that some of these parties will have interests that are different from, or in addition to, your interests as a shareholder.
We are not obligated to and do not comply with all the best practice provisions of the Dutch Corporate Governance Code. This may affect your rights as a shareholder.
As a Dutch company we are subject to the Dutch Corporate Governance Code, or DCGC. The DCGC contains both principles and best practice provisions for management boards, supervisory boards, shareholders and general meetings of shareholders, financial reporting, auditors, disclosure, compliance and enforcement standards. The DCGC applies to all Dutch companies listed on a government-recognized stock exchange, whether in the Netherlands or elsewhere, including the Nasdaq Global Select Stock Market, or Nasdaq. The principles and best practice provisions apply to our management board and our supervisory board (in relation to role and composition, conflicts of interest and independency requirements, board committees and remuneration), shareholders and the general meeting of shareholders (for example, regarding anti-takeover protection and our obligations to provide information to its shareholders) and financial reporting (such as external auditor and internal audit requirements). We do not comply with all the best practice provisions of the DCGC. This may affect your rights as a shareholder and you may not have the same level of protection as a shareholder in a Dutch company that fully complies with the DCGC.
29
Claims of U.S. civil liabilities may not be enforceable against us.
We are incorporated under the laws of the Netherlands. Substantially all of our assets are located outside the United States. The majority of our managing directors and supervisory directors reside outside the United States. As a result, it may not be possible for investors to effect service of process within the United States upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the United States.
The United States and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the United States, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent jurisdiction in the Netherlands. Such party may submit to the Dutch court the final judgment rendered by the U.S. court. If and to the extent that the Dutch court finds that the jurisdiction of the U.S. court has been based on grounds which are internationally acceptable and that proper legal procedures have been observed, the court of the Netherlands will, in principle, give binding effect to the judgment of the U.S. court, unless such judgment contravenes principles of public policy of the Netherlands. Dutch courts may deny the recognition and enforcement of punitive damages or other awards. Moreover, a Dutch court may reduce the amount of damages granted by a U.S. court and recognize damages only to the extent that they are necessary to compensate actual losses or damages. Enforcement and recognition of judgments of U.S. courts in the Netherlands are solely governed by the provisions of the Dutch Civil Procedure Code.
Based on the lack of a treaty as described above, U.S. investors may not be able to enforce against us or members of our management board or supervisory board, officers or certain experts named herein who are residents of the Netherlands or countries other than the United States any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws.
We are a foreign private issuer and, as a result, we are not subject to U.S. proxy rules and are subject to Exchange Act reporting obligations that, to some extent, are more lenient and less frequent than those of a U.S. domestic public company.
We report under the Securities Exchange Act of 1934, as amended, or the Exchange Act, as a non-U.S. company with foreign private issuer status. Because we qualify as a foreign private issuer under the Exchange Act and although we are subject to Dutch laws and regulations with regard to such matters and intend to furnish quarterly financial information to the SEC, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies, including (i) the sections of the Exchange Act regulating the solicitation of proxies, consents or authorizations in respect of a security registered under the Exchange Act; (ii) the sections of the Exchange Act requiring insiders to file public reports of their stock ownership and trading activities and liability for insiders who profit from trades made in a short period of time; and (iii) the rules under the Exchange Act requiring the filing with the Securities and Exchange Commission (SEC) of quarterly reports on Form 10-Q containing unaudited financial and other specified information, or current reports on Form 8-K, upon the occurrence of specified significant events. In addition, foreign private issuers are not required to file their annual report on Form 20-F until 120 days after the end of each fiscal year, while U.S. domestic issuers that are accelerated filers are required to file their annual report on Form 10-K within 75 days after the end of each fiscal year. Foreign private issuers are also exempt from the Regulation Fair Disclosure, aimed at preventing issuers from making selective disclosures of material information. As a result of the above, you may not have the same protections afforded to shareholders of companies that are not foreign private issuers.
As a foreign private issuer and as permitted by the listing requirements of Nasdaq, we rely on certain home country governance practices rather than the corporate governance requirements of Nasdaq.
We are a foreign private issuer. As a result, in accordance with the listing requirements of Nasdaq, we rely on home country governance requirements and certain exemptions thereunder rather than relying on the corporate governance requirements of Nasdaq. In accordance with Dutch law and generally accepted business practices, our articles of association do not provide quorum requirements generally applicable to general meetings of shareholders. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. Although we must provide shareholders
30
with an agenda and other relevant documents for the general meeting of shareholders, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands, thus our practice will vary from the requirement of Nasdaq Listing Rule 5620(b). In addition, we have opted out of shareholder approval requirements for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of us and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. Accordingly, you may not have the same protections afforded to shareholders of companies that are subject to these Nasdaq requirements.
We may lose our foreign private issuer status which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses.
We are a foreign private issuer and therefore we are not required to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers. We may no longer be a foreign private issuer as of June 30, 2014 which would require us to comply with all of the periodic disclosure and current reporting requirements of the Exchange Act applicable to U.S. domestic issuers as of January 1, 2015. In order to maintain our current status as a foreign private issuer, either (a) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the United States or (b)(i) a majority of our executive officers or directors may not be United States citizens or residents, (ii) more than 50 percent of our assets cannot be located in the United States and (iii) our business must be administered principally outside the United States. If we lost this status, we would be required to comply with the Exchange Act reporting and other requirements applicable to U.S. domestic issuers, which are more detailed and extensive than the requirements for foreign private issuers. We may also be required to make changes in our corporate governance practices in accordance with various SEC and Nasdaq rules. The regulatory and compliance costs to us under U.S. securities laws if we are required to comply with the reporting requirements applicable to a U.S. domestic issuer may be significantly higher than the cost we would incur as a foreign private issuer. As a result, we expect that a loss of foreign private issuer status would increase our legal and financial compliance costs and would make some activities highly time consuming and costly. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our supervisory board.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to “emerging growth companies” will make our ordinary shares less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act. For as long as we continue to be an “emerging growth company,” we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not “emerging growth companies,” including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. As an “emerging growth company” we are required to report only two years of financial results and selected financial data compared to three and five years, respectively, for comparable data reported by other public companies. We may take advantage of these exemptions until we are no longer an “emerging growth company.” We could be an “emerging growth company” for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our ordinary shares held by non-affiliates exceeds $700 million as of any June 30 (the end of our second fiscal quarter) before that time, in which case we would no longer be an “emerging growth company” as of the following December 31 (our fiscal year end). We cannot predict if investors will find our ordinary shares less attractive because we may rely on these exemptions. If some investors find our ordinary shares less attractive as a result, there may be a less active trading market for our ordinary shares and the price of our ordinary shares may be more volatile.
If we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud. As a result, shareholders could lose confidence in our financial and other public reporting, which would harm our business and the trading price of our ordinary shares.
31
Effective internal controls over financial reporting are necessary for us to provide reliable financial reports and, together with adequate disclosure controls and procedures, are designed to prevent fraud. Any failure to implement required new or improved controls, or difficulties encountered in their implementation could cause us to fail to meet our reporting obligations. In addition, any testing by us conducted in connection with Section 404 of the Sarbanes-Oxley Act of 2002, or any subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses or that may require prospective or retroactive changes to our financial statements or identify other areas for further attention or improvement. Inferior internal controls could also cause investors to lose confidence in our reported financial information, which could have a negative effect on the trading price of our ordinary shares.
We will be required to disclose changes made in our internal controls and procedures on a quarterly basis and our management will be required to assess the effectiveness of these controls annually. However, for as long as we are an “emerging growth company” under the JOBS Act, our independent registered public accounting firm will not be required to attest to the effectiveness of our internal controls over financial reporting pursuant to Section 404. We could be an “emerging growth company” for up to five years. An independent assessment of the effectiveness of our internal controls could detect problems that our management’s assessment might not. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation.
If securities or industry analysts cease to publish research, or publish inaccurate or unfavorable research, about our business, the price of our ordinary shares and our trading volume could decline.
The trading market for our ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who cover us downgrade our ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ordinary shares would likely decline. If one or more of these analysts cease coverage of our company or fail to publish reports on us regularly, demand for our ordinary shares could decrease, which might cause the price of our ordinary shares and trading volume to decline.
We may be classified as a passive foreign investment company (a “PFIC”) in 2014 or any future taxable years. If we are a PFIC for any taxable year, this could result in adverse U.S. federal income tax consequences to U.S. investors.
Under the Internal Revenue Code of 1986, as amended (the Code), we will be a PFIC for any taxable year in which, after the application of certain “look-through” rules with respect to subsidiaries, either (i) 75% or more of our gross income consists of “passive income,” or (ii) 50% or more of the average quarterly value of our assets consist of assets that produce, or are held for the production of, “passive income.” Passive income generally includes interest, dividends, rents, certain non-active royalties and capital gains. Whether we will be a PFIC in any year depends on the composition of our income and assets, and the relative fair market value of our assets from time to time, which has varied, and we expect will continue to vary, substantially over time. While we believe that we were not a “passive foreign investment company” for U.S. federal income tax purposes for our 2013 taxable year, because (i) we currently own a substantial amount of passive assets, including cash, and (ii) the value of our assets, including our intangible assets, that generate non-passive income for PFIC purposes, is uncertain and has varied, and we expect will continue to vary, substantially over time, there can be no assurance that we will not be a PFIC in 2014 or any future taxable years. Based on the current value of our intangible assets that is implied by the recent sales prices for our ordinary shares reported by NASDAQ, U.S. investors should be aware that we may be a PFIC in 2014, although a determination of whether we are a PFIC for 2014 will depend on the composition of our income and the composition and relative values of our assets, including our intangible assets, over the entire year.
If we are a PFIC for any taxable year during which a U.S. investor holds ordinary shares, the U.S. investor may be subject to adverse tax consequences, including (i) the treatment of all or a portion of any gain on disposition as ordinary income, (ii) the application of a deferred interest charge on such gain and the receipt of certain dividends and (iii) compliance with certain reporting requirements.
32
ITEM 4. INFORMATION ON THE COMPANY
A. History and development of the company
We are an innovative biotechnology company engaged in the discovery and development of ribonucleic acid-modulating, or RNA-modulating, therapeutics for the treatment of genetic disorders. Our primary focus is on rare neuromuscular and neurodegenerative disorders with a large unmet medical need, including Duchenne muscular dystrophy, myotonic dystrophy and Huntington’s disease. Our clinical portfolio of RNA-based product candidates is focused on the treatment of Duchenne muscular dystrophy, or DMD. Each of our DMD compounds has been granted orphan drug status in the United States and the European Union.
DMD is one of the most prevalent rare genetic diseases globally affecting up to 1 in 3,500 boys and is invariably fatal. There is currently no approved disease-modifying therapy for DMD. The progressive muscle-wasting that characterizes this disease is caused by inadequate production of dystrophin, a protein necessary for muscle function, as a result of mutations in the dystrophin gene. The different mutations, which are mostly deletions of one or more exons, found in the dystrophin gene result in distinct sub-populations of DMD patients. We are designing product candidates to address several sub-populations using our platform technology. Our first product candidate, drisapersen, aims to address a variety of mutations in the dystrophin gene, such as a deletion of exon 50 or exons 48 to 50.
Prosensa’s lead candidate for DMD, drisapersen, which has previously been referred to as PRO051 or GSK2402968, was taken through clinical development as part of a collaboration with GlaxoSmithKline (GSK) dating back to 2009. This collaboration was mutually ended in January 2014, and all licenses and rights to drisapersen and the DMD portfolio are fully retained by Prosensa.
Each of our DMD candidates aims to restore dystrophin expression and improve muscle condition and function in specific sub-populations of DMD, and drisapersen aims to address up to 13% of all DMD patients. In clinical trials, drisapersen has been shown to induce dystrophin expression and has shown a treatment effect on DMD patients. To date, over 300 patients have participated in clinical studies of drisapersen at more than 50 trial sites in 25 countries.
Results of a randomized Phase II placebo-controlled study of drisapersen (DMD114117) in 53 DMD patients were presented in April 2013 and demonstrated a statistically significant and clinically meaningful difference in the primary endpoint, which was the distance walked in the six minute walk test, or 6MWD, between the placebo group and the continuous active-treatment group at a dose of 6 mg/kg/week after 24 weeks. This clinically meaningful benefit was maintained after 48 weeks of treatment, and drisapersen was generally well tolerated throughout the study.
Drisapersen successfully completed a twelve-patient Phase I/II study, and all patients were enrolled in an open-label extension study, (DMD114673), which was initiated in August 2009 with results available up to 177 weeks. The results indicate that drisapersen treatment may lead to stabilization of the disease, as shown by an improvement (median) or a slower than expected decline (mean) in the 6MWD.
A Phase III study of drisapersen was initiated in December 2010, and results were announced on September 20, 2013. This study was a randomized, double-blind and placebo-controlled trial, assessing drisapersen at a dose of 6 mg/kg/week in 186 boys. The study did not meet its primary endpoint of statistically significant difference in the 6MWD at 48 weeks. As a result, dosing in all studies was put on hold by GSK pending a full evaluation of the benefit-to-risk profile of drisapersen treatment across all studies. Discussions regarding a possible recommencement of treatment are ongoing.
Results from DMD114876 (or DEMAND V), a U.S.-based Phase II placebo controlled exploratory study of 24 week dosing of drisapersen 3mg/kg/week and 6mg/kg/week were presented on September 25, 2013. The results of this study indicate that, compared to placebo, boys in the higher-dose drisapersen group (6 mg/kg once weekly) experienced stabilization and even improvements in their muscle function and physical activity as measured by the six-minute walk test (6MWT) for the 24-week treatment phase. Boys in the 6mg/kg/week treatment arm showed a 27.1 meter improvement (including a 16.1 meter increase from baseline) over the boys in the placebo group at the end of the treatment period (p=0.069), ), indicating a clinically meaningful outcome for the primary endpoint. The study was not statistically powered to show a significant difference between the arms.
The 48 week data from this study were presented on March 17, 2014 by Craig McDonald at a poster session at the Muscular Dystrophy Association 2014 Clinical Conference in Chicago, IL. A clinically meaningful treatment difference of 27.9 m over placebo (p=0.177) was maintained for 24 weeks after drisapersen administration ceased. This includes an overall mean increase from baseline of 14.7 meters. In the drisapersen 6 mg/kg/week group, an improvement was seen in the percent-predicted 6MWD of 5.2% (p=0.051) and 4.8% (p=0.154) when compared to placebo at weeks 24 and 48, respectively.
In January 2014, Prosensa announced findings from subjects who had reached 48 weeks treatment in study DMD114349, an open-label continuation study to follow the placebo-controlled drisapersen studies DMD114117 and DMD114044. At 48 weeks in study DMD114349, the subjects enrolled from study DMD114117 showed a treatment difference of 52 meters (n=30) when comparing subjects treated with drisapersen at 6mg/kg/week (n=17) throughout both studies, versus subjects who had received placebo (n=13) prior to initiating the open-label continuation treatment period. For subjects enrolled in study DMD114349 from study DMD114044, the treatment difference at 48 weeks in study DMD114349 was 49 meters (n=83) for those dosed at 6mg/kg/week throughout (n=52) versus subjects previously allocated to placebo treatment (n=31) in study DMD114044.
33
Prosensa continues to work to fully complete an evaluation of all data and patient groups across all DMD studies with drisapersen, and to engage collaboratively with patient groups, clinical experts and regulators to understand the benefit-to-risk ratio of drisapersen therapy. The outcome of this evaluation may have a material impact on the further development of drisapersen and other DMD compounds by us. A negative outcome of the evaluation could alter our development plans and costs.
On June 27, 2013, GSK announced that the U.S. Food and Drug Administration, or FDA, had notified it that drisapersen has been granted breakthrough therapy designation for the treatment of DMD. Breakthrough therapy designation is an FDA program intended to facilitate and expedite development and review of new drugs to address unmet medical need in the treatment of serious or life-threatening conditions.
PRO044, our next most advanced product candidate, addresses a separate sub-population of DMD patients. We developed PRO044 using our exon-skipping technology to generate a product candidate with the same mechanism of action that is applicable for drisapersen. PRO044 has completed a Phase I/II study in Europe, and results were presented in October 2013. We are currently evaluating the steps forward in the PRO044 clinical program, also in view of the ongoing analysis of the drisapersen results. We have four additional earlier-stage compounds that address other distinct sub-populations of DMD patients. Of these, PRO045 entered clinical trials in the first quarter of 2013, and PRO053 entered clinical trials in mid-2013. PRO052 and PRO055 are in advanced preclinical development. We have also started a research program, PROSPECT, which includes a new and innovative application of our exon-skipping technology platform to specifically target rarer mutations in the dystrophin gene.
From October 2009 to January 2014, Prosensa operated under a collaboration agreement with GSK for the development and commercialization of RNA-based therapeutics for DMD, with GSK exclusively licensing worldwide rights to develop and commercialize drisapersen and having an option to exclusively license PRO044 and two other compounds in our DMD portfolio. This collaboration was mutually terminated as of January 12, 2014, and Prosensa has regained all rights for the development and commercialization of drisapersen, PRO044 and the DMD portfolio. Upon entering into the agreement with GSK, we received a £16.0 million (€17.2 million) nonrefundable upfront payment from GSK and received £41.5 million (€47.4 million) in total under the agreement.
Our DMD programs are part of our broader RNA modulation platform technology, which in addition to exon skipping, is focused on reducing mutant RNA associated with trinucleotide repeat expansion diseases. Our current programs in this area are in myotonic dystrophy (DM1) and in Huntington’s disease (HD). Both DM1 and HD are rare diseases with a severe impact on patients, and currently no disease-modifying therapies exist for either.
We started operations in 2002 and are located in Leiden, the Netherlands. We work closely with Leiden University Medical Center, or LUMC, with whom we entered into an exclusive licensing agreement in 2003 for LUMC’s proprietary RNA modulation exon-skipping technology to develop treatments for DMD, other neuromuscular disorders and indications outside the field of neuromuscular disorders. In 2013, we raised €64.0 million (before offering expenses) from our initial public offering of our ordinary shares on Nasdaq. Since 2002, we have raised €56.4 million from private placements of equity securities, including from a number of venture capital firms. In addition, we have received grants and loans from several DMD-focused patient advocacy organizations to support our research of therapies for DMD. As of December 31, 2013 we had €82.2 million in cash and cash equivalents.
B. Business overview
Our research and development pipeline
The following table summarizes our product development pipeline in DMD as well as DM1 and HD:
34
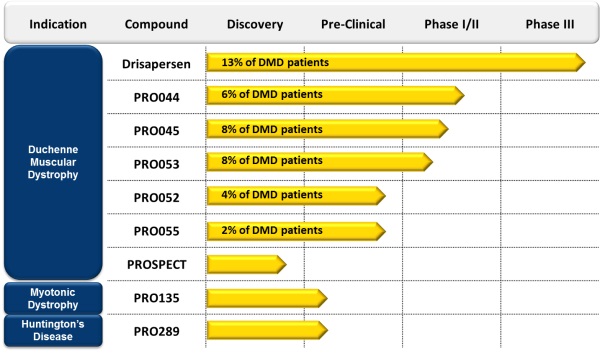
|
Note:
|
Percentages are based on the sub-population of DMD patients that could potentially be addressed by each product candidate. The product label may be limited to a subset of this population based on ambulation, age and other factors. “Theoretic applicability of antisense-mediated exon skipping for Duchenne muscular dystrophy mutations,” Annemieke Aartsma-Rus et al. (Human Mutation, March 2009).
|
Our strategy
Our goal is to become a leader in the rare genetic disease field, with a particular focus on DMD. We intend to mature into a fully integrated biopharmaceutical company focused on the discovery, development and commercialization of our products.
The key components of our business objectives are:
|
·
|
Develop potential treatments for significant subsets of boys with DMD, a rare genetic disease with no disease modifying therapies.
|
|
·
|
Leverage our know-how from drisapersen to accelerate and reduce development risk of our earlier-stage DMD products
|
Given similarities in technology and targets, we expect there to be development and commercial synergies between drisapersen and our product candidates, PRO044, PRO045, PRO053, PRO052 and PRO055, which each address distinct sub-populations of DMD patients. We are leveraging the know-how from clinical development and regulatory advice for drisapersen to optimize strategies, reduce development risk and accelerate speed to market for these products, thereby expanding our commercial DMD portfolio and maximizing the patient population addressed by our product candidates.
|
·
|
Independently commercialize our proprietary products in DMD
|
We believe that the depth of our internal scientific capabilities, strength of our clinical pipeline and relationships with key stakeholders, such as clinical experts, academia and patient advocacy groups place us in a strong position to commercialize our own products. Since we are developing DMD products for different but closely related patient sub-populations, we believe that the additional infrastructure required to commercialize additional proprietary DMD products will be relatively limited. We therefore believe that it would be cost-effective for us to establish our own scalable marketing effort and sales force.
35
|
·
|
Leverage our proprietary RNA modulation and exon-skipping drug discovery platform
|
We intend to leverage our proprietary technology platform and expertise in RNA modulation to further progress our technology to develop other product candidates for rare genetic diseases such as myotonic dystrophy and Huntington’s disease.
|
·
|
Continue to invest in and strengthen our intellectual property portfolio
|
We own or exclusively license 8 U.S. patents and 16 U.S. patent applications and 4 European patents and 22 European patent applications covering our RNA modulation technology platform and our exon-skipping product candidates. We plan to continue to leverage this portfolio to create value and to file new patent applications, in-license other intellectual property and take other steps to expand and strengthen our intellectual property position.
We may also enter into strategic collaborations that deliver additional expertise and enhance or accelerate implementation of our strategy.
Our RNA-modulating therapeutics
Human genes contain the DNA blueprint instructions for protein synthesis and gene regulation in the body. Within the DNA strand, multiple series of three-letter nucleotide bases (known as “codons”) together make up the active instructions called “exons,” which are read by cellular machinery to make proteins. Interspersed among exons are sections of DNA known as “introns,” which are assumed to have no direct role in protein synthesis.
To make a particular protein, the relevant section of DNA is copied (in a process known as transcription) to make pre-mRNA (pre-messenger ribonucleic acid), which contains both exons and introns and therefore cannot be read by the cellular machinery as an instruction to make a protein. The introns are then removed (in a process known as splicing) so that the instructions in the active mRNA (messenger RNA) can be read by the cellular machinery to make a particular protein.
Schematic overview of RNA Synthesis
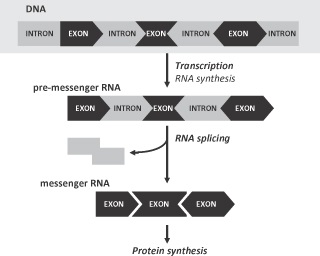
The dystrophin gene, which is central to the pathology of DMD, is comprised of 79 exons, which are interspersed with non-coding introns. In patients with DMD, synthesis of the dystrophin protein is disrupted because of mutations in the DNA that contains the dystrophin gene. The extent and nature of the mutations are different in different sub-populations of DMD patients and may be due to, among other things, deleted or duplicated exons or small mutations that result in disrupted coding of the mRNA. This defective mRNA is said to be “out of the transcription reading frame” and results in a premature halt of the protein synthesis and a truncated, non-functional dystrophin. Consequently, patients with DMD have a lack of functional dystrophin protein in their muscles. Our aim is to restore the coding reading frame of mRNA so that it can be read to produce functional dystrophin protein.
36
Our technology enables us to design sequences of nucleotide bases that bind to specified regions of pre-mRNA involved in the splicing of exons. As a result, when the pre-mRNA is spliced to mRNA, the disrupted coding section is skipped or passed over completely, and the transcription reading frame is corrected. The single stranded oligonucleotides (short nucleotide sequences) that we design are known as antisense oligonucleotides, or AONs; and our preferred chemical structure is a backbone of 2’ O-methyl phosphorothioate (or 2’O-Me) groups that protect the AONs from rapid enzyme degradation once they are inside the body’s cells. In DMD, we apply this technology to address particular mutations occurring at different sites in the dystrophin gene.
Our RNA modulation platform technology can also be applied to treat genetic disorders that are caused by mutations where trinucleotide bases, such as CAG or CUG, are expanded in certain genes, generating a longer series of trinucleotide repeats than is normal. These extended repeated sequences can cause the resulting RNA transcript or protein to be unstable, dysfunctional or even toxic, and lead to various diseases. We currently have AON programs targeting two such disorders, HD and DM1.
Overview of DMD
DMD is a rare, severe muscle wasting disease that occurs in up to 1 in 3,500 male births. It is commonly diagnosed between the ages of three to five, when boys begin to show signs of impaired motor development. Boys with DMD continue to develop in strength and function, albeit at a lower rate than healthy boys, up until about 7 years. They then begin to progressively lose muscle function, generally becoming wheelchair-bound around the age of 12, with loss of respiratory and cardiac function in the ensuing years. DMD patients typically die in their twenties of either cardiac or respiratory failure.
Currently, there is no approved disease-modifying treatment for DMD. Corticosteroid therapy is the current standard of care in most developed countries and may be administered as early as 3 years, although administration commonly starts at 4-6 years. Steroids have been demonstrated to temporarily preserve muscle function and delay disease progression milestones, such as time to wheelchair dependency, by approximately 2 years, but the ultimate course of the disease is unaffected. In addition, steroids have potentially serious side effects, negatively affecting growth, bone mineral density and the immune system.
DMD is caused by various mutations in the dystrophin gene, such as deletions, duplications or small mutations of one or more exons, which result in a lack of functional dystrophin in the muscle fiber. Dystrophin is a protein with a central role in linking the contractile apparatus of a muscle fiber to the cell membrane and surrounding extracellular matrix. An absence or a low level of dystrophin also leads to reduced levels of various other proteins. This leads to progressive muscle fiber degeneration characteristic of DMD, with fatty and fibrous tissue replacement of muscle.
Deletions of any number of exons in the dystrophin gene may occur. The different mutations result in distinct sub-populations of DMD patients. The figure below shows the effect of the deletion of exons 48—50 in the dystrophin gene.
Effect of a deletion of exons 48-50 in the dystrophin gene
In this example, the deletion of exons 48—50 results in an mRNA that is out of the transcription reading frame since exons 47 and 51 do not fit together. Consequently, functional dystrophin will not be produced.
Exon skipping technology applied to DMD
Our therapeutic strategy for DMD is to use AON-induced exon skipping to allow a shorter but still functional dystrophin protein to be produced so that muscle performance in patients with DMD may be maintained or even improved. Our AONs bind highly specifically to a selected exon in the pre-mRNA coded by the dystrophin gene. The exon is selected because it is, for instance, adjacent to a region where one or more exons have been deleted. As a result, the exon to which the AON binds is skipped over in the splicing process, allowing two non-consecutive exons to be joined across the gap. This results in what is termed “restoration of the transcription reading frame”—in other words, the genetic instructions or code become legible again, and a shorter but still functional dystrophin protein is produced. The figure below shows how an AON binding with exon 51 of the gene’s pre-mRNA can result in an in-frame mRNA transcript and production of a shorter but still functional dystrophin protein.
37
Example of effect of drisapersen
In this example, there is a deletion of exons 48 to 50; drisapersen binds with exon 51 in the pre-mRNA, and after splicing, this results in exon 47 and exon 52 becoming consecutive exons in the mRNA. Since exon 47 and exon 52 connect properly, an in-frame mRNA results and a shorter but still functional dystrophin protein may be produced, which is expected to result in improved muscle physiology and hence clinical outcome.
Natural history of DMD and outcome measures
Natural history data can illustrate expected rates of physical decline, which helps to put into context the effects of novel drug treatments. The six minute walk test (6MWT) is a test that measures the distance a subject can walk in 6 minutes using a standardized corridor length and turning point at each end (the six minute walk distance or 6MWD). This test has been used in observational research studies to follow the natural history of DMD disease progression over time as subjects gradually lose the ability to walk.
Several key studies have demonstrated the effect of DMD on 6MWD. One study reported an average 57 meter decrease at 52 weeks from baseline in average 6MWD by boys with DMD, whereas comparable healthy boys showed an average increase in 6MWD of 13 meters. A more recent study of 113 boys reported an average decrease in 6MWD of 23 meters in the first year of observation and 65 meters in the second year. In the latter study, when grouped by age, boys below 7 years remained stable with a slight increase in average 6MWD in the first and second years, but the average 6MWD of boys over 7 declined by about 42 meters and 80 meters, respectively.
Reports conclude that both age and baseline walking ability are important variables that determine decline in 6MWT performance. Patients with lower initial 6MWD values have shown greater decline over the course of 48 week evaluations. Baseline 6MWD performance has been correlated to time to 10% worsening in function, which is taken to be a clinically meaningful disease progression measure.
The diagram below is a conceptual representation created by analyzing patient data from various studies to contrast 6MWD performance by DMD patients and healthy control subjects and to illustrate the typical decline of 40-60 meters per year in 6MWD performance by boys with DMD over age 7.
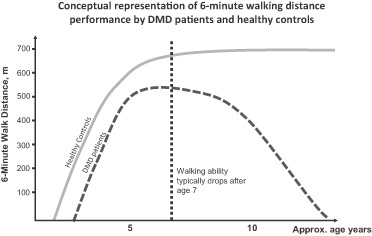
The 6MWD has been confirmed in studies as a safe and feasible end point in DMD, and is accepted by U.S. and European regulators to be an appropriate primary outcome measure for DMD trials. It is believed that the test is a sensitive measure to show effects of treatment within a timeframe of one year. Other measures of treatment effectiveness include the North Star Ambulatory Assessment, or NSAA, which has been validated in DMD at various ages, and timed tests such as rise from the floor, stair climbing and 10 meter walk/run. Muscle strength in upper and lower limbs (knees, elbows and shoulders) is also assessed, as is breathing function.
38
The 6MWD has successfully been used as a primary endpoint to assess ambulatory function in studies leading to regulatory approval of therapies designed to treat the underlying cause of disorders with neuromuscular and pulmonary manifestations that are associated with deterioration in 6MWD over time, such as Pompe’s disease, mucopolysaccharidosis I, Hunter syndrome, pulmonary arterial hypertension and Morquio's syndrome (referred to as mucopolysaccharidosis IV or Morquio's). Individual results for 6MWD compare each subject’s baseline result against a result at a study time point, and individual and averaged differences in 6MWD from baseline are further compared between treatment and non-treatment groups. The data comparing treatment and non-treatment groups may be presented in a number of ways, such as absolute differences (meters) and percent change from baseline. Studies of therapies for the diseases mentioned above have reported differences from baseline between placebo and treatment groups of 23 to 44 meters, which has been considered clinically meaningful in these contexts and used in support of applications for regulatory approval. There is not yet an agreed regulatory position on the clinical relevance of absolute differences in meters or percent change from baseline in DMD, however recent published data support the clinically meaningful change in 6MWD to be in the range of 20-30 meters as a targeted treatment effect in 12 month clinical trials of DMD therapies in ambulant patients. In the drisapersen program, we supplement the 6MWD primary endpoint with secondary outcome functional measures, imaging and biomarkers to demonstrate the multidimensional response to therapy, which we believe is likely to be important for applications for regulatory approval.
Some of the mutations that we are targeting for treatment are very rare, and numbers of eligible patients for clinical trials are in the low double digits. In these sub-populations, it is not feasible to design clinical studies involving statistically meaningful numbers of placebo-controlled patients. For this reason we have started a natural history study for which we intend to recruit up to 250 DMD patients from the ages of 3-18 at clinics in ten countries. As of March 2014, more than 190 patients have been enrolled in this study, the goal of which is to characterize DMD at various stages of progression using the same measures used in the clinical studies, such as 6MWD, and use the data to help interpret clinical trial results for our product candidates that are targeted at ultra-rare mutations that cause DMD, for which we anticipate that it will be difficult to recruit placebo groups.
Drisapersen
Our lead compound, drisapersen, is an AON with highly specific binding to exon 51 of the dystrophin gene’s pre-mRNA. The binding of drisapersen to exon 51 specifically induces exon 51 to be skipped when the mRNA for the dystrophin protein is created from the pre-mRNA. Given the frequencies reported in various international DMD mutation databases, the skipping of exon 51 could in principle correct the transcription reading frame and produce shortened but functional dystrophin expression in approximately 13% of all DMD patients.
Drisapersen has been granted orphan drug status in the United States, the European Union, Japan and Australia. In the United States, if a product with orphan status receives FDA approval, the FDA will not approve a later product for the same indication that uses the same active ingredient for seven years, unless the later product is shown to be clinically superior. In the European Union, the EMA will not approve a later product for the same indication and with the same method of action for ten years after the earlier product receives EMA approval, subject to certain exceptions, including the demonstration of clinical superiority.
We demonstrated clinical proof of concept in four DMD patients receiving a single intramuscular 0.8 mg dose of drisapersen in a trial initiated in May 2006. In this study, drisapersen was safe and effective in specifically inducing exon 51 skipping and restoring dystrophin expression to 35% of normal levels in up to 97% of muscle fibers in the treated muscle area.
We conducted a subsequent Phase I/II dose-ranging safety study in which drisapersen was administered to 12 patients subcutaneously, once weekly for 5 weeks. The study, initiated in March 2008 and conducted at two European clinical centers, demonstrated that drisapersen was well-tolerated in all patients and novel dystrophin expression was detected in each treated patient. An open-label extension study (DMD114673) of this Phase I/II study was initiated in August 2009 and patients have continued in this study for over four years, with efficacy results available up to 177 weeks.
From October 2009 to January 2014, Prosensa operated under a collaboration agreement with GSK for the clinical development of drisapersen, and studies operated in this period were sponsored by, and under the sole budgetary decision-making of GSK. This collaboration was mutually terminated on January 12, 2014, and Prosensa has regained all rights for development and commercialization of drisapersen, including relevant intellectual property, and Prosensa’s DMD portfolio.
Ongoing clinical development
The following table sets forth information regarding clinical trials of drisapersen:
39
|
Study
|
Phase; study design
|
Total patients
enrolled
|
Status
|
Dates (1)(2)
|
|
PRO051-02 (DMD114673)
|
Phase I/II; open label, repeat dose escalation (extension phase ongoing 6 mg/kg/wk intermittent)
|
12
|
Complete; extension study
ongoing, dosing on hold
|
March 2008 – ongoing (2)
|
|
DMD114118
|
Phase I; placebo-controlled, pharmacokinetics /safety
|
20
|
Complete
|
July 2010 – August
2012
|
|
DMD114117
|
Phase II; placebo-controlled, dose regimen comparison
|
53
|
Complete
|
September 2010 –
April 2013
|
|
DMD114876
|
Phase II; placebo-controlled, dose-comparison
|
51
|
Report in progress
|
November 2011 –April 2014
(expected)
|
|
DMD114044
|
Phase III; placebo-controlled
|
186
|
Complete
|
December 2010 –
December 2013
|
|
DMD114349
|
Extension study for DMD114117 and DMD114044
|
233
(patients previously in
DMD114117 and
DMD114044)
|
Report in progress
|
October 2011 –
Mid 2014
|
|
DMD115501
|
Extension study for DMD114876
|
21 (patients previously in
DMD114876)
|
Dosing on hold
|
May 2013-Ongoing (2)
|
|
(1)
|
The earlier date is the date the trial was initiated (first patient in), and the later date is the date the clinical trial report was available (formal report or posting to Clinical Trial Register) or is currently expected to be available.
|
|
(2)
|
Following the phase III results dosing of drisapersen in the open-label extension studies PRO051-02 (DMD114673), DMD114349, DMD115501 was put on hold in September 2013; a decision to close study 114349 was taken in February 2014.
|
Phase I/II—PRO051-02. A Phase I/II study was initiated in March 2008 to evaluate systemic administration of drisapersen. Twelve patients were given weekly subcutaneous injections for five weeks at two European clinical centers at four dosing cohorts (0.5, 2.0, 4.0 and 6.0 mg/kg). The primary endpoint was safety, and secondary endpoints included pharmacokinetics and molecular and clinical effects. Muscle biopsies were taken, and changes in RNA splicing and dystrophin protein levels assessed, at baseline and two weeks after the last dose in the 0.5 mg/kg cohort and at two and seven weeks in the other three cohorts. Dose-dependent dystrophin expression was detected in each patient, and specific exon 51 skipping was confirmed in the majority of patients.
Drisapersen Phase I/II—PRO051-02 study design and ongoing extension phase
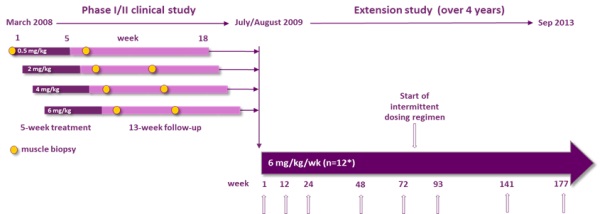
An open-label extension phase (DMD114673) followed the initial five-week study after an interval of six to fifteen months. Improvement in 6MWD versus baseline was observed at the 12-week point in the extension phase. The extension phase has continued for more than four years with all patients participating, including two boys who were not able to complete the 6MWT at the start of the extension study (one because he had lost his ability to
40
walk (non-ambulant) before the dose escalation phase started, and one because he could not complete the 6MWT at the start of the extension phase). At the 177-week time-point, 8 of the 10 subjects able to complete the 6MWT at the extension baseline were still able to perform the walking evaluation. Although the number of patients is small, the results appear to show delay of disease progression in these eight patients. This is particularly meaningful because the subjects’ ages ranged from 9-15 years at 177 weeks, which is when their 6MWD would otherwise be expected to decline significantly based on natural history studies. Dosing of drisapersen is currently on hold pending a full risk-benefit assessment across all studies. Discussions regarding a possible recommencement of treatment are ongoing. The chart below shows the 6MWD versus extension baseline for the ten subjects who were able to perform the 6MWT at the extension baseline and at several points in time up to 177 weeks, which is the latest assessment date for which data are available.
Drisapersen Phase I/II—PRO051-02 extension phase 6MWD (0 = extension baseline)
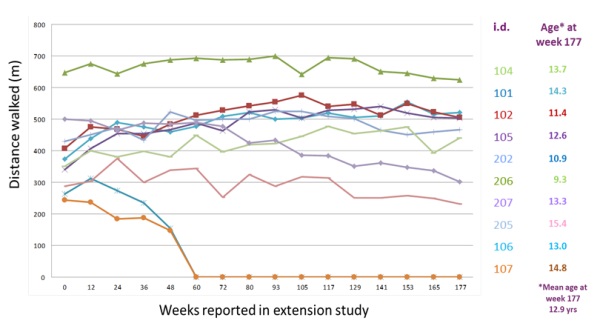
Drisapersen has been generally well tolerated throughout the Phase I/II study and open-label extension phase. Transient proteinuria (protein in the urine) was observed in all twelve patients, and eleven patients demonstrated signs of proteinuria at week 12 of the extension phase. After 72 weeks, all patients were assigned to an intermittent dosing regimen (eight weeks on, four weeks off). Up to week 177, nine subjects have incurred treatment interruptions primarily due to increases in kidney and liver safety biomarkers, injection site skin reactions, lowered blood cell counts or inflammatory reactions. Also seen in varying degrees in all subjects were raised cystatin C (a protein indicative of kidney function); decreased C3 levels, (a protein indicating immunological activity); low platelet counts, and raised liver enzymes (including GLDH and gamma GT), which may indicate abnormal liver function. Biomarkers such as protein in the urine returned to normal levels during the off-treatment period indicating that this effect is reversible, whilst inflammatory reactions appeared to be manageable with non-prescription treatments such as anti-inflammatories and/or antihistamines. Injection site reactions were seen to varying degrees of severity in all subjects. The majority of these adverse effects were transient in nature, or resolved during the four week off-treatment periods.
Phase I—DMD114118. This double-blind, escalating dose, randomized, placebo-controlled study in non-ambulant DMD subjects was initiated in July 2010, to assess the safety, tolerability and pharmacokinetics of single subcutaneous injections of drisapersen. It was planned that 32 non-ambulant DMD subjects would receive a single dose of 3, 6, 9, or 12 mg/kg. Twenty subjects received treatment, and the study completed after cohort 3 (9 mg/kg), because the maximum tolerated dose was identified, in line with the objectives of the study. Conclusions from the study were that the pharmacokinetics of drisapersen was similar for non-ambulant DMD subjects compared to ambulant DMD subjects for single doses. Single doses of drisapersen at 3 mg/kg and 6 mg/kg had no significant safety or tolerability concerns in the exposed subjects. Pharmacokinetics and safety results of this study together with data from the Phase I/II—PRO051-02 study suggested that drisapersen 6 mg/kg is the appropriate subcutaneous dose for further characterization in multiple dose studies in the non-ambulant DMD population.
Phase II—DMD114117. This exploratory placebo-controlled study of drisapersen at a 6 mg/kg (subcutaneous) dose was initiated in September 2010. The study consisted of three arms: (1) a continuous dosing regimen of 6 mg/kg/week, including 18 subjects; (2) an intermittent dosing regimen of 9 doses of 6 mg/kg spread over 6 weeks, followed by 4 weeks off drug, including 17 subjects; and (3) a matched placebo
41
arm, including 18 subjects. A total of 53 DMD subjects aged 5 and above with a rise from floor of ≤ 7 seconds were recruited. The primary endpoint was 24-week efficacy. Secondary endpoints included safety and tolerability over 48 weeks and efficacy (including 6MWD, NSAA, timed tests and muscle strength) after 48 weeks. Muscle biopsies were taken pre- and post-treatment (after 24 weeks). Although this was an exploratory study, and not statistically powered to evaluate efficacy, the study reported that drisapersen demonstrated a statistically significant difference in 6MWD between the 6 mg/kg week continuous dosing arm and the placebo arm at 24 weeks. The mean difference in 6MWD was 35.09 meters (p=0.014), which is seen as a clinically meaningful benefit. P-value is a measure of how likely data is to have occurred by chance. The clinically meaningful benefit was maintained after 48 weeks of treatment, with a mean difference in 6MWD of 35.84 meters (p=0.051). Intermittent dosing did not result in a statistically significant difference from the placebo group at 24 weeks; however, a clinically meaningful difference was reported at 48 weeks, with a mean difference in 6MWD of 27.08 meters (p=0.147). The NSAA and timed tests showed trends that supported the 6MWD findings. Little change in muscle strength was observed at either time point for either treatment arm. Preliminary results suggest that treatment with drisapersen was in general associated with increased levels of dystrophin expression when compared with pre-treatment levels.
Drisapersen, administered weekly or intermittently, was generally well tolerated. Injection site reactions and renal adverse events (including subclinical proteinuria) were observed in all treatment arms, including the placebo arm, but occurred more frequently in the continuous and intermittent treatment arms. There were no clinically meaningful adverse events related to inflammation, coagulation or hepatotoxicity. There were no reports of thrombocytopenia, and no subject discontinued treatment. There were no significant safety concerns related to drisapersen identified in this study.
Phase II—DMD114876. This is a study conducted in centres in the United States comparing two doses of drisapersen (3 mg/kg/week and 6 mg/kg/week) in 51 subjects over 48 weeks. The study began in November 2011, and the primary outcome measure was 6MWT at 24 weeks. Secondary outcome measures included the NSAA, timed tests and muscle strength tests. In addition, muscle biopsies were collected and analyzed for dystrophin production and muscle imaging was investigated as an exploratory outcome measure in collaboration with the National Institutes of Health. The population of this study was similar to the subjects enrolled in the Phase II study (DMD114117) described above. After the 24 week timepoint, all boys were withdrawn from therapy to assess the pharmacokinetics and clinical effect of treatment withdrawal. The study was not designed to have sufficient power to show a statistically significant slowing of disease progression as measured by the 6MWD, however at the 24 week time point, the 6 mg/kg/wk arm (n=18) showed a clinically meaningful (but not statistically significant) difference from placebo (n=16), with a mean change of +27.1m versus placebo (p=0.069), including a 16.1 meter increase from baseline. The 3 mg/kg/wk arm (n=17) showed no clinically relevant difference from placebo at 24 weeks.
Enrollment of DMD114876 was completed in November 2012 and the 48 week data from this study were presented on March 17, 2014 by Craig McDonald during a poster session at the Muscular Dystrophy Association 2014 Clinical Conference in Chicago, IL. A clinically meaningful treatment difference of 27.9 m over placebo (p=0.177) was maintained for 24 weeks after drisapersen administration ceased. This includes an overall mean increase from baseline of 14.7 meters. In the drisapersen 6 mg/kg/week group, an improvement was seen in the percent-predicted 6MWD of 5.2% (p=0.051) and 4.8% (p=0.154) when compared to placebo at weeks 24 and 48, respectively.
Phase III—DMD114044. This randomized, double-blind, placebo-controlled study was initiated in December 2010 and preliminary results were reported in September 2013. The study assessed once-weekly subcutaneous administration of drisapersen at 6 mg/kg dosing in 186 boys over five years of age and with a minimum 6MWD of 75 meters at enrollment. The goal of the study was to demonstrate a mean improvement of 30 meters in 6MWD at 48 weeks compared with placebo. Secondary outcome measures included the NSAA, timed tests, muscle strength tests and quality of life measures. No baseline muscle biopsies were taken, but selected biopsy samples were analyzed for dystrophin production. Enrollment was completed in July 2012 and preliminary results of analyses were available in September 2013. Preliminary results indicated that the study did not meet its primary endpoint of a statistically significant or clinically meaningful treatment difference (10.3m; p=0.42) over placebo in 6MWD after 48 weeks of treatment, and there was no statistically significant or clinically meaningful treatment differences between drisapersen and placebo on the majority of secondary endpoints over the same timeframe. There was a statistically significant (p<0.001) decline in CK, a potential marker of muscle cell integrity, observed at week 48 for the drisapersen group compared with placebo. The pre-planned subgroup ≤ 7 years showed a non-statistically significant treatment difference of 21m over 48 weeks.
Phase II/III continuation—DMD114349. This open-label continuation study initiated in September 2011, dosing continued up to September 2013 and data up to 96 weeks have been recorded. The study enrolled 233 subjects completing the DMD114117 or DMD114044 clinical studies on to a subcutaneous dosing regimen of 6mg/kg week. For information on safety issues relating to this study, see “—Drisaperson safety reports” below.
We are currently working to complete the full evaluation of the benefit-to-risk profile of drisapersen treatment across all studies including data from the continuation study DMD114349, as well as DMD114117, DMD114044 and DMD114876. The outcome of the evaluation may have a material impact on the further development of drisapersen and other DMD compounds by us. A negative outcome of the evaluation could alter our development plans and costs.
42
Drisapersen safety reports
The most commonly reported adverse events (side effects) from clinical trial experience with drisapersen to date include injection site reactions in the skin and proteinuria (protein in the urine).
The injection site reactions reported included: erythema (redness), pain, discoloration and less commonly induration (sclerosis or hardening) and lipoatrophy (local loss of fat in the skin).
Mild, reversible proteinuria, often associated with elevations in biomarkers of proximal tubule dysfunction (these are measured characteristics which indicate changes in the way the kidney is working), have been commonly reported. In addition, two cases of severe proteinuria have been reported; both of these events resolved following symptomatic treatment and withdrawal of drisapersen; one of these two cases was associated with renal (related to the kidney) and pulmonary vein thrombosis (clot) (this case was reported as nephrotic syndrome; a kidney disorder generally associated with proteinuria, low levels of albumin in blood serum and edema - swelling).
In connection with the Phase II/III continuation of DMD114349, there have been a number of significant adverse events reported. These reports are mainly similar to previous long-term drisapersen trials (such as proteinuria or protein in the urine), except for the occurrence of moderate to severe thrombocytopenia (a decrease in the number of platelets that help blood to clot).
Nine reports were received of moderate to severe (as defined by the reporting study investigator) thrombocytopenia (a relative decrease in platelet [blood clotting cell] counts) in which the counts fell to as low as 3 x109 L in one subject; four of these nine subjects reported typical clinical signs of low platelet counts (e.g. bruising), however no severe bleeding was reported in any of the nine cases. The onset of thrombocytopenia occurred between 9 and 24 months after the start of treatment with drisapersen. All nine subjects recovered (i.e. their platelet counts counts normalized) after treatment with drisapersen had stopped; four of the nine subjects also received a platelet transfusion and/or treatment with intravenous immunoglobulin.
These adverse events can generally be monitored in the clinical setting. Defined stopping criteria are included in the study protocols for both proteinuria and thrombocytopenia.
PRO044
Our second exon skipping product candidate for DMD is PRO044, which, like drisapersen, is a highly sequence-specific AON that, in this case, induces exon 44 to be skipped when the mRNA for the dystrophin protein is created from the pre-mRNA. Given the frequencies reported in various international DMD mutation databases, the skipping of exon 44 could in principle correct the transcription reading frame and produce shortened but functional dystrophin expression in approximately 6% of all DMD patients. PRO044 has obtained orphan drug designation in the United States and the European Union.
We initiated a dose-escalation trial, assessing six doses (0.5, 1.5, 5, 8, 10 and 12 mg/kg/week) in 18 DMD patients in December 2009. Subjects received PRO044 subcutaneously at 0.5, 1.5, 5, 8, 10 or 12 mg/kg/week (3 subjects per dose). Additionally, nine of the 18 subjects received an additional five administrations intravenously at 1.5, 5 or 12 mg/kg/week. Key endpoints included dystrophin expression, safety and tolerability and pharmacokinetic assessments. Enrollment was completed in the first quarter of 2013. PRO044 was generally well tolerated up to dose levels of 12mg/kg for five weeks by subcutaneous or intravenous administration. Liver, inflammatory and blood coagulation findings have not raised any clinical safety concerns. Safety findings of the study are consistent with the known class safety profile and no drug related serious adverse events were reported. Follow-on program design and timing will be confirmed pending the technical review of these and other emerging data. PRO044 is currently on clinical hold in the United States pending the results of long-term preclinical safety studies which are expected to be completed in the first half of 2014.
Follow-on compounds
PRO045 has a similar chemical structure and the same mechanism of action as drisapersen and PRO044 and induces exon 45 skipping when the mRNA for the dystrophin protein is created from the pre-mRNA. Given the frequencies reported in various international DMD mutation databases, the skipping of exon 45 could in principle correct the transcription reading frame and produce shortened but functional dystrophin expression in
43
approximately 8% of all DMD patients. PRO045 has been granted orphan drug designation in the United States and the European Union, and we commenced a Phase I/II study of PRO045 in the first quarter of 2013 in Europe, results of which are expected in the second half of 2014. PRO053 induces exon 53 skipping and could in principle correct the transcription reading frame and produce functional dystrophin expression in approximately 8% of all DMD patients. PRO053 has been granted orphan drug designation in the United States and the European Union. We commenced a Phase I/II study for PRO053 in September 2013 which is ongoing. The safety findings reported to date from both studies are consistent with published safety profile of this class of molecule.
PRO052 and PRO055 induce exon 52 skipping and exon 55 skipping, respectively. Both have been granted orphan drug designation in the United States and the European Union and are currently in advanced preclinical development. Finally, our research program, PROSPECT, which includes a new and innovative application of our exon-skipping technology platform, applies multiple exon skipping, to specifically target rarer mutations (initially in the exon 10-30 region) in the dystrophin gene. This approach could have applicability between 5-13% of the DMD population. This program is still in the discovery phase.
Our regulatory strategy for DMD
DMD is a rare disease, and our DMD product candidates target discrete sub-populations of DMD patients that are distinguished by different mutations in the dystrophin gene. After drisapersen, our subsequent product candidates target mutations of decreasing prevalence or increasing rarity, affecting such small populations of patients that they are sometimes referred to as “ultra-orphan.” With increased rarity of mutations and affected patients, it is not feasible to conduct fully statistically powered pivotal studies. Regulatory authorities acknowledge that placebo study arms are less statistically robust and scientifically meaningful as patient population size decreases. For this reason, we are working closely with the FDA, EMA and several national competent authorities in Europe on the design of alternative pathways for the development and approval for our therapeutic candidates. Our proposed extrapolation principle is that if exon skipping works for one AON compound that is shown to be safe and effective, then the principle should to a certain extent also apply to subsequent compounds for rarer sub-populations, so that the numerical burden of proof is partly cumulative. We believe our natural history study will enable such alternative approval pathways by facilitating direct comparison of natural history data and interventional study data without needing to recruit large placebo groups for every exon skipping product candidate.
PRO135 in myotonic dystrophy
Myotonic dystrophy type 1 (DM1) is the most common neuromuscular dystrophy, affecting 1 in 8,000 individuals worldwide. DM1 is characterized by progressive, multi-system symptoms that typically include muscle weakness and wasting, myotonia (temporary rigidity or slow relaxation of muscles), cardiac conduction defects/arrhythmia and hormonal changes. Life expectancy is reduced due to increased mortality resulting from pulmonary and cardiac complications. The disease affects males and females equally, and the severity of symptoms increases in successive family generations. There is currently no disease modifying treatment for DM1. DM1 is caused by expansion of an unstable (CUG) trinucleotide repeat in the non-coding region of the DMPK gene. This leads to DMPK mRNA transcripts bearing long sequences of CUG nucleotide repeats that can form hairpin-like structures that bind proteins involved in RNA splicing. As a result, the splicing of certain downstream pre-mRNA transcripts that code for other proteins is affected and the resulting proteins are less or non-functional.
Our AON program for DM1, PRO135, selectively targets the toxic RNA transcripts resulting from expansion of (CUG) trinucleotide repeats in the transcription of the DMPK gene. PRO135 candidates reduce the levels of toxic transcripts in myoblasts (precursors of muscle fibers) derived from various DM1 patients. The PRO135 lead candidates are currently being tested in preclinical mouse models of DM1. Preliminary results have shown DMPK mRNA reduction in muscle as well as correction of processing of several RNA transcripts that are normally affected by the repeat expansion. Preclinical studies to identify reversal of myotonic phenotype are currently ongoing, and progression into Phase I trials is planned for early 2017. We have exclusive worldwide rights to the PRO135 program, and we are fully responsible for its research and development.
PRO289 in Huntington’s disease
Huntington’s disease (HD) is a devastating progressive neurodegenerative disorder characterized by chorea (abnormal involuntary movement) spreading to all muscles, progressive dementia and psychiatric manifestations such as depression and psychosis. In western countries, prevalence is estimated to be 5-10 in 100,000 individuals. HD symptoms usually begin to manifest between the ages of 30 to 50; death commonly follows 15
44
to 20 years after onset of neurological impairment. No effective disease modifying treatment for HD currently exists. HD is caused by expansion of an unstable (CAG) trinucleotide repeat in the coding region of the HTT gene, giving rise to an expanded stretch of CAG nucleotides that become repeatedly expanded on transcription. During protein synthesis, these CAG repeats are translated into a series of uninterrupted glutamine residues, which alter the function of the HD protein and cause it to accumulate in brain cells, leading to the symptoms of HD. AON candidates in our PRO289 program target these repeats and have been shown to reduce levels of expanded HTT transcripts and mutant huntingtin protein in fibroblast cultures (precursors of connective tissue cells) derived from HD patients. We are currently testing PRO289 candidates in various preclinical models of HD. We have exclusive worldwide rights to PRO289, and we are fully responsible for its research and development.
Technology platform
Our technology platform enables us to modulate gene expression through the binding of specifically designed AONs to target RNA, which may induce exon skipping or exon inclusion, reduce mutated toxic RNA or protein, remove specific protein domains or block RNA expression. We focus on genetic disorders representing market opportunities with significant unmet medical needs. Our current focus is on the design, selection and preclinical development of molecules with favorable bioactivity, safety and biodistribution profiles for use in DMD (exon skipping), DM1 (reduction of mutated toxic RNA) and HD (reduction of mutated toxic protein). However, our platform may be applied to other genetic diseases for which RNA modulation would be corrective, or to other trinucleotide repeat expansion diseases, such as several types of spinocerebellar ataxia (SCA), amyotrophic lateral sclerosis (ALS) or X-linked spinal and bulbar muscular atrophy (SBMA).
Collaboration, license and funding arrangements
GlaxoSmithKline
From October 2009 to January 2014, we operated under an exclusive worldwide collaboration with GSK for the development and commercialization of RNA-based therapeutics for DMD, with GSK exclusively licensing worldwide rights to develop and commercialize drisapersen and obtaining an option to exclusively license PRO044 and other specified assets in our DMD portfolio. Under the collaboration agreement, GSK paid us a total of £41.5 million (€47.4 million) in upfront and milestone payments. Under the collaboration agreement, GSK was responsible for all costs of clinical development of drisapersen.
In January 2014, we and GSK mutually terminated the collaboration pursuant to a termination agreement, which terminates all intellectual property license grants as well as any rights arising under the collaboration agreement (other than rights to payments that accrued prior to termination of the collaboration). In addition, the termination agreement requires GSK to transfer to us certain data and know how, inventory, regulatory filings, clinical trial sponsorships, clinical study reports and material agreements relating to the development of our products as soon as reasonably practicable, but in no case later than 120 days after the effective date of the termination agreement. We and GSK each agreed to indemnify the other party with respect to certain acts and omissions. Going forward, we will be solely responsible for the cost of developing drisapersen and our other product candidates.
Leiden University Medical Center
In September 2003, we obtained an exclusive worldwide license from LUMC for the application of its proprietary RNA modulation exon-skipping technology to develop treatments for selected neuromuscular disorders. This technology was developed at the Human Genetics department of Professor Dr. Gert-Jan van Ommen. The license agreement gives us the rights to apply the RNA modulation technology for the development of treatments for neuromuscular disorders as well as, after an amendment of the agreement in March 2008, indications outside the field of neuromuscular disorders. We now use this intellectual property related to RNA modulation technology for all of the compounds that we are currently developing. LUMC reserves the right to use its technology for research and educational purposes and any other purpose not subject to its agreement with us. We have also granted LUMC a non-exclusive, royalty-free license to certain intellectual property we own for the purpose of enabling LUMC to perform internal, non-commercial research.
In consideration for the expansion of the licensed field to include all applications pursuant to the 2008 amendment and for the occurrence of certain milestones and events, we paid LUMC €890,000. We are also obliged to make milestone payments upon the occurrence of events with respect to each subfield in which we pursue a drug indication using the LUMC patent rights, including commencing the first toxicity study, successfully completing the first Phase I study, filing for regulatory approval and satisfying certain sales thresholds. The aggregate milestone payments could total approximately €1.4 million, if we achieve such
45
milestones in relation to an initial orphan drug indication. If we achieve such milestones in relation to a non-orphan drug indication, the aggregate milestone payments could total €5.5 million. We also may be obligated to pay LUMC additional milestone payments of €1.25 million per product in relation to products approved for additional orphan drug indications. In addition, we are obligated to pay LUMC a mid-single digit percentage royalty on net sales on a country-by-country basis if and when any of our products is brought to market. If we sublicense our rights under the LUMC agreement to a third party, as we have to GSK, we are obligated to pay LUMC a tiered royalty percentage (ranging from the low-to-mid-twenties) on the net licensing income and a low single-digit percentage royalty on the net sales generated by the sublicensees. To date we have paid LUMC €3.6 million in milestone payments under the agreement.
If we and LUMC file for a joint patent, such patent will remain jointly owned by us and LUMC. We control any enforcement rights in case of infringement related to any of the joint patent rights and we are required to pay all patent prosecution and maintenance costs during the term of the agreement. If the agreement is terminated, we are jointly responsible with LUMC for patent prosecution and maintenance costs for any patents jointly owned by LUMC and us.
The agreement will expire on a country-by-country basis upon the latest of (a) the expiration date of the last to expire of LUMC’s patent rights issued in such country, (b) the expiration date of the last to expire grant of orphan drug status issued in such country or (c) 15 years from the first commercial sale of a product in such country. The agreement may be terminated by LUMC for (i) our failure to perform any of our material duties under the agreement that is not cured within six months’ written notice, (ii) our material breach of the agreement that is not cured within 90 days’ written notice thereof, or (iii) our insolvency. We may terminate the agreement without cause upon six months written notice.
Other funding arrangements
We have obtained the following funding from patient organizations and governmental bodies: €3,390,000 from Association Française contre les Myopathies, or AFM, €740,000 from Agentschap NL, €500,000 from the Stichting Duchenne Parents Project, €387,000 from Charley’s Fund, €502,000 from Everest International Pte Ltd, €500,000 from Duchenne Children’s Trust, €250,000 from Cure Duchenne, €163,000 from Aktion Benni & Co. and €65,000 from Villa Joep. These arrangements are generally in the form of below-market interest rate loans that become due upon the meeting of certain milestones in product development, generally a minimum threshold of commercialization success or successful completion of certain clinical trials. The AFM agreement gives us the option to terminate the agreement if we undergo a change of control. If we exercise our option to terminate the agreement, we are required to repay the loan, together with interest. In the event that we abandon the program for reasons not justified by a scientific or medical reason, we are similarly required to repay the loan, together with interest. The AFM agreement also requires us to grant AFM a non-exclusive royalty-free license to the intellectual property covered by the agreement in the event that we abandon the research funded by the agreement. Furthermore, we have received a number of other loans from patient organizations and governmental bodies in smaller amounts. We have also received grant funding. We are part of two pan-European consortia, each consortium has been awarded a Framework Programme 7 (“FP7”) research grant of €6 million from the European Commission. In August 2013 one consortium received a €6 million grant from the European Commission to support the ongoing clinical study of PRO045, and in November 2013 the other received a €6 million grant from the European Commission for a separate collaborative bioimaging project to support our PRO053 and natural history programs. Such grants are typically for research, and typically the grant program lasts 3 to 4 years.
Competition
We are engaged in segments of the biopharmaceutical and pharmaceutical industries that are highly competitive and rapidly changing. Large pharmaceutical, specialty pharmaceutical and biotechnology companies, academic institutions, governmental agencies and other public and private research organizations are commercializing or pursuing the development of products that target genetic disorders, including the same diseases we are targeting. If approved, we expect our product candidates to face intense and increasing competition as new products enter the DMD markets and advanced technologies become available. Our product candidates will face competition based on their safety and effectiveness, the timing and scope of regulatory approvals, the availability and cost of supply, marketing and sales capabilities, reimbursement coverage, price, patent position and other factors. Our competitors may succeed in developing competing products before we do, obtaining regulatory approval for products or gaining acceptance for the same markets that we are targeting. If we are not “first to market” with one of our product candidates for a given disease indication or a given product profile, our competitive position could be compromised because it may be more difficult for us to obtain marketing approval for that product candidate and/or successfully market that product candidate as a second product to market.
46
We believe that our key competitor in DMD is Sarepta Therapeutics, Inc., or Sarepta, a U.S. company focused on the development of their lead product candidate eteplirsen, which employs the same exon 51 skipping approach as drisapersen. In January 2014, Sarepta announced data from their open label phase IIb continuation study, with 12 patients, of whom 8 have received eteplirsen for 120 weeks. In November 2013 Sarepta reported that in discussions with the FDA on accelerated approval for eteplirsen, FDA stated that they "currently consider an NDA filing for eteplirsen as premature". Depending on the overall clinical profile, efficacy and commercialization of eteplirsen, Sarepta may render our development and discovery efforts in the area of DMD uncompetitive. If, however, drisapersen obtains marketing approval from the EMA prior to eteplirsen, drisapersen could have the benefit of orphan drug marketing exclusivity in Europe for ten years because both products use the same method of action (exon skipping) for patients with DMD. Eteplirsen (or any other DMD product that uses exon skipping) would have to demonstrate a clinically relevant advantage over drisapersen (in efficacy, safety and/or pharmacokinetics) in order to defeat such market exclusivity in Europe. Because orphan drug marketing exclusivity in the United States is tied to the active ingredient rather than method of action, and drisapersen and eteplirsen use different active ingredients, if FDA marketing approval is obtained, we believe both products could be marketed simultaneously in the United States.
Other companies are also developing alternative therapeutic approaches to the treatment of DMD. For example, PTC Therapeutics, Inc. is developing ataluren, currently in late Phase III development, a small molecule that enables formation of functional dystrophin in approximately 13% of DMD patients that have point mutations that create premature termination of RNA transcription and cannot be treated by exon skipping. In addition to the development programs above, Asklepios Biopharmaceuticals, Inc. is developing biostrophin, a gene-therapy approach currently in Phase I development and Summit plc is developing a utrophin (dystrophin-like protein) modulation program that uses orally administered small molecules to increase the production of utrophin. Summit’s most advanced molecule, SMT C1100, is currently in Phase I clinical trials.
Different therapeutic approaches aiming at muscle tissue growth through myostatin inhibition are being investigated in clinical studies. These include PF-06252616 by Pfizer Inc. and ACE-031 by Acceleron Pharma (in collaboration with Shire). A Phase I clinical trial to test PF-06252616, a myostatin antibody, is ongoing in healthy volunteers. A Phase II, dose-escalation study of ACE-031 in boys with DMD has been terminated based on preliminary safety data. There has been no investigation of potential complementarity of exon skipping with alternative approaches such as these. In theory exon skipping and, for example, utrophin upregulation could be mutually enhancing; however experts hypothesize that there could be an issue of competition for binding at the cell membranes of the muscle cells.
In addition, corticosteroids are the current standard of care for DMD patients, although they do not address the underlying cause of DMD and have serious side effects. We believe that corticosteroids will continue to be prescribed in conjunction with any of our product candidates that obtain regulatory approval.
Manufacturing
We develop and manufacture the drug product for our initial preclinical studies using standardized manufacturing processes in our own laboratories. A selected number of Contract Manufacturing Organizations (CMOs) manufacture scaled-up quantities of the drug, based on our advice and know-how. We and the CMOs use multiple qualified suppliers for the raw materials, and the raw materials are widely commercially available from catalog.
We expect to continue our reliance on third-party CMOs to manufacture drug supplies for clinical trials and commercial quantities of our products and drug candidates. Our policy is to contract with multiple suppliers of raw materials, drug substances, drug product formulation and filling; the exception is that the packaging and distribution of product for clinical trials is allocated to a single contractor. To avoid capacity issues, for each specific AON there is only one CMO for drug substance and one CMO for drug product, although the manufacturing process for our product candidates is highly standardized across all CMOs. By maintaining agreements with multiple parties for the same stage of the manufacturing process, we believe we limit the risk of interruption of production and delays in clinical development and trials. If any CMOs should become unavailable to us for any reason, we believe that with the standardization of the manufacturing platform, there will be a number of potential replacement CMOs. Nevertheless, we may incur some delay in activating an alternative CMO for a new compound. In the future, we may decide to perform some parts of the drug substance production, drug product formulation, filling and packaging in-house.
47
Intellectual property
We actively seek to protect the intellectual property and proprietary technology that we believe is important to our business, including by seeking and maintaining patents intended to cover our product candidates, product platform, proprietary processes and any other inventions that are commercially important to the development of our business. We also rely on trade secrets that may be important to the development of our business and actively seek to protect the confidentiality of such trade secrets.
Our success will depend on our ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and proprietary rights of third parties. For more information, please see “Item 3. Key Information—D. Risk Factors—Risks related to our intellectual property and information technology.”
We own or exclusively license 4 U.S. patents, 13 U.S. patent applications, 2 European patents, 17 European patent applications (including 1 that is allowable), 2 international patent applications, and 76 national patent applications or granted patents in other jurisdictions relating to our DMD program. As of such date we also owned or exclusively licensed 4 U.S. patents, 3 U.S. patent applications, 2 European patents, 5 European patent applications, 1 international application and 29 national patent applications or granted patents in other jurisdictions relating to our other discovery programs.
Included among the patent and patent applications described above are four families of patents and patent applications that we have exclusively licensed from LUMC. These patents and patent applications claim, among other things, compositions of matter, methods of production and methods of use related to our exon skipping technology. We own or co-own 19 additional families of patents and patent applications encompassing several aspects of the same technology, for the treatment of DMD as well as distinct technologies for the treatment of other diseases for which we are developing drug candidates. Each patent family consists of patents and patent applications in the United States, as well as broadly equivalent patents in several other jurisdictions, including members of the European Patent Convention, Canada and Australia.
Our patents and patent applications directed to drisapersen include one U.S. patent and two U.S. patent applications and one European patent and one European Patent application. The issued U.S. patent expires in 2023, and the U.S. patent applications, if issued, would expire in 2023. The European issued patent expires in 2021, and the European patent application, if issued, would also expire in 2021. Such patents and patent applications include claims to compositions of matter and second medical use. For each of our other DMD candidate compounds, we are seeking to obtain patents that include claims to compositions of matter and second medical use. Our other issued patents and patent applications, if issued, that are directed to our DMD and other discovery programs are scheduled to expire between 2021 and 2033.
The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent’s term may be shortened if a patent is terminally disclaimed over another patent or as a result of delays in patent prosecution by the patentee, and a patent’s term maybe lengthened by patent term adjustment, which compensates a patentee for administrative delays by the U.S. Patent and Trademark Office in granting a patent. The patent term of a European patent is 20 years from its filing date, which, unlike in the U.S., is not subject to adjustment.
The term of a patent that covers an FDA-approved drug or biologic may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the drug or biologic is under regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug may be extended. Similar provisions are available in Europe and other jurisdictions to extend the term of a patent that covers an approved drug. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products. We anticipate that some of our issued patents may be eligible for patent term extensions. See “Item 4. Information on the Company—B. Business Overview—Government Regulation—United States—Hatch-Waxman Act.”
48
Opposition proceedings against EP ‘249
In August 2008, EP 1619249 (‘249) was granted to LUMC. This patent relates to exon skipping using an oligonucleotide with a certain length, directed to the interior of a broad range of exons (including exons 43 through 46 and 50 through 53), as well as a number of method claims comprising the use of such an oligonucleotide.
In 2009, AVI Biopharma, Inc. (now Sarepta) filed an opposition against EP ‘249 with the European Patent Office, or EPO, requesting revocation of the patent as granted, alleging, inter alia, lack of novelty, inventive step and sufficiency of disclosure. We on behalf of LUMC in turn requested maintenance of the patent on the basis as granted.
The EPO Opposition Division, presiding in oral proceedings on November 16, 2011, in Munich, Germany, maintained the EP ‘249 patent in an amended form. The allowed patent as maintained protects, among other things, the skipping of exon 51 in the dystrophin gene using a 14- to 40-mer AON as a potential therapy to treat DMD. We believe that the patent as maintained in amended form by the EPO Opposition Division still provides protection for our lead product candidate drisapersen. The appeals process is ongoing: we and Sarepta both had the right to appeal the written decision of the EPO. Each party filed an appeal against the decision of the Opposition Division on August 23, 2013, and on January 8, 2014, each party filed its reply to the appeal filed by the other party. For a discussion of the risks associated with these proceedings, see “Item 3. Key Information—D. Risk factors—Risks related to intellectual property and information technology.”
For additional information regarding potential challenges to our intellectual property, please see “Item 3. Key Information—D. Risk Factors—Issued patents covering one or more of our products could be found invalid or unenforceable if challenged in court.”
Commercialization strategy and organization
Given our current stage of product development, we currently do not have a commercial infrastructure or distribution capability. We have, as a part of strategic planning toward and eventual commercialization of one of our compounds, created several sales and marketing build out scenarios that can be efficiently implemented as we reach certain regulatory milestones. We have in place a pre-launch plan that focuses on drisapersen with key medical education events to occur in 2014 and will be updating a drisapersen launch plan given returned rights and new assumptions.
Based on available population data and birth rates, a reported incidence of one in 3,500 births and an expected average life span of 25 years, we estimate the number of DMD patients in selected developed countries with a sustainable reimbursement infrastructure is approximately 75,000, of which about 15,000 are in the United States and 20,000 are in Europe. We believe that drisapersen could potentially address up to approximately 13% of DMD patients.
If the Prosensa product candidates are granted marketing approval, we intend to market them with our own commercial team. We are operating in the rare disease area in which patients typically form local, national and global patient groups that are well-informed concerning the latest treatment possibilities. A relatively small number of medical centers of expertise tend to offer specialized treatment for these diseases. As a result, physicians can be located and targeted efficiently by a small and highly educated in-house team.
We currently intend to focus our initial commercial efforts for the Prosensa product candidates in the U.S. and European markets, which we believe represent the largest market opportunities for us in the short term. In addition, we believe that Japan and key countries in Latin America represent significant opportunities. Prosensa may explore various strategic opportunities, including potential partnering, licensing or collaboration arrangements with industry partners whose past experience in rare diseases and orphan drugs in key Asia Pacific and Latin American countries is considered a strength.
Government regulation
Our business is subject to extensive government regulation. Regulation by governmental authorities in the United States, the European Union and other jurisdictions is a significant factor in the development, manufacture and marketing of any drugs and in ongoing research and development activities. All of our products are subject to rigorous preclinical and clinical trials and other pre-marketing approval requirements by the FDA, the EMA and other regulatory authorities in the United States, the European Union and in other jurisdictions.
49
United States
In the United States, the FDA regulates drugs and medical devices under the Federal Food, Drug, and Cosmetic Act, or the FDCA, and regulations implemented by the agency. If we fail to comply with the applicable United States requirements at any time during the product development process, including non-clinical testing, clinical testing, the approval process or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include, but are not limited to, the FDA’s refusal to allow us to proceed with clinical testing, refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, adverse publicity, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency enforcement action could have a material adverse effect on us.
Approval or clearance of drugs
The process required by the FDA before a drug may be marketed in the United States generally involves satisfactorily completing each of the following:
|
·
|
preclinical laboratory tests, animal studies and formulation studies all performed in accordance with the FDA’s Good Laboratory and Good Manufacturing Practice regulations, as applicable;
|
|
·
|
submission to the FDA of an investigational new drug, or IND, application for human clinical testing, which must become effective before human clinical trials may begin;
|
|
·
|
performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication;
|
|
·
|
submission of data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labelling;
|
|
·
|
submission to the FDA of a New Drug Application, or NDA;
|
|
·
|
satisfactory completion of an FDA inspection of the manufacturing facility or facilities, including those of third parties, at which the product is produced to assess compliance with strictly enforced current Good Manufacturing Practices;
|
|
·
|
potential FDA audit of the non-clinical and clinical trial sites that generated the data in support of the NDA; and
|
|
·
|
FDA review and approval of the NDA before any commercial marketing, sale or shipment of the product.
|
The testing, collection and submission of data and the preparation of necessary applications are expensive and time-consuming. The FDA may not act quickly or favorably in reviewing these applications, and we may encounter significant difficulties or costs in our efforts to obtain FDA approvals that could delay or preclude us from marketing our products.
Preclinical studies and Investigational New Drug application
Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animal studies, in order to assess the potential safety and efficacy of the product. The conduct of the preclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an Investigational New Drug, or IND, application. The IND becomes effective 30 days after receipt by the FDA, unless the FDA raises concerns or questions about the conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns before the clinical trials can begin. Submission of the IND may result in the FDA not allowing the trials to commence or not allowing the trial to commence on the terms originally specified in the IND. If the FDA raises concerns or questions either during this initial 30 day period, or at any time during the IND process, they may choose to impose a partial or complete clinical hold. This order issued by the FDA would delay either a proposed clinical study or cause suspension of an ongoing study, until all outstanding concerns have been adequately addressed and the FDA have notified the Company that investigations may proceed. This could cause significant delays or difficulties in completing planned clinical studies in a timely manner.
50
Clinical trials
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND. An independent Institutional Review Board, or IRB, must also review and approve the clinical trial before it can begin and monitor the study until it is completed. The IRB will consider, among other things, clinical trial design, patient informed consent, ethical factors, the safety of human subjects and the possible liability of the institution. The FDA, the IRB or the sponsor may suspend or discontinue a clinical trial at any time or impose sanctions for various reasons, including a finding that the clinical trial is not being conducted in accordance with FDA requirements or the subjects are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive Good Clinical Practice rules and the requirements for informed consent.
Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Additional studies may be required after approval.
Phase I clinical trials are initially conducted in a limited population to test the product candidate for safety, dose tolerance, absorption, metabolism, distribution and excretion in healthy humans or, on occasion, in patients, such as cancer patients.
Phase II clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, determine the efficacy of the product candidate for specific targeted indications and determine dose tolerance and optimal dosage. Multiple Phase II clinical trials may be conducted by the sponsor to obtain information prior to beginning larger and more costly Phase III clinical trial.
Phase III clinical trials proceed if the Phase II clinical trials demonstrate that a dose range of the product candidate is effective and has an acceptable safety profile. Phase III clinical trials are undertaken in large patient populations to further evaluate dosage, provide substantial evidence of clinical efficacy and further test for safety in an expanded and diverse patient population at multiple, geographically dispersed clinical trial sites. A well-controlled, statistically relevant Phase III trial may be designed to deliver the data that the regulatory authorities will use to decide whether or not to approve a drug: such Phase III studies are referred to as ‘pivotal’.
In some cases, the FDA may approve an NDA for a product candidate with the sponsor’s agreement to conduct additional clinical trials to further assess the drug’s safety and effectiveness after NDA approval. Such post-approval trials are typically referred to as Phase IV clinical trials. These studies are used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of drugs approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase IV clinical trial requirement. Failure to promptly conduct Phase IV clinical trials could result in withdrawal of approval for products.
New Drug Application
The results of product candidate development, preclinical testing and clinical trials are submitted to the FDA as part of an NDA. The NDA also must contain extensive manufacturing information and detailed information on the composition of the product and proposed labeling as well as payment of a user fee. Once the submission has been accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or the PDUFA, the FDA has ten months in which to complete its initial review of a standard NDA and respond to the applicant, and six months for a priority NDA. The FDA does not always meet its PDUFA goal dates for standard and priority NDAs. The review process is often significantly extended by FDA requests for additional information or clarification. The review process and the PDUFA goal date may be extended by three months if the FDA requests, or the NDA sponsor otherwise provides additional information or clarification regarding information already provided in the submission within the last three months before the PDUFA goal date.
At the conclusion of the FDA’s review they will issue an action letter. If the FDA’s evaluations of the NDA and the clinical and manufacturing procedures and facilities are favorable and there are no outstanding issues, the FDA will issue an approval letter. If the application is not approved, the FDA will issue a complete response letter, which will contain the conditions that must be met in order to secure final approval of the NDA, and when possible will outline recommended actions the sponsor might take to obtain approval of the application.
51
Sponsors that receive a complete response letter may submit to the FDA information that represents a complete response to the issues identified by the FDA. Such resubmissions are classified under PDUFA as either Class 1 or Class 2. The classification of a resubmission is based on the information submitted by an applicant in response to an action letter. Under the goals and policies agreed to by the FDA under PDUFA, the FDA has two months to review a Class 1 resubmission and six months to review a Class 2 resubmission. The FDA will not approve an application until issues identified in the complete response letter have been addressed.
The FDA may also refer the NDA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved. The FDA is not bound by the recommendation of the advisory committee, but it generally follows such recommendations. The FDA may deny approval of an NDA if the applicable regulatory criteria are not satisfied, or it may require additional clinical data or an additional pivotal Phase III clinical trial. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. Data from clinical trials are not always conclusive and the FDA may interpret data differently than we or our collaborators do. Once issued, the FDA may withdraw a drug approval if ongoing regulatory requirements are not met or if safety problems occur after the drug reaches the market. In addition, the FDA may require further testing, including Phase IV clinical trials, and surveillance programs to monitor the effect of approved drugs which have been commercialized. The FDA has the power to prevent or limit further marketing of a drug based on the results of these post-marketing programs. Drugs may be marketed only for the approved indications and in accordance with the provisions of the approved label. Further, if there are any modifications to a drug, including changes in indications, labeling or manufacturing processes or facilities, we may be required to submit and obtain FDA approval of a new NDA or NDA supplement, which may require us to develop additional data or conduct additional preclinical studies and clinical trials. We cannot be sure that any additional approval for new indications for any product will be approved on a timely basis, if at all.
The FDA has several programs that are intended to facilitate and expedite development and review of new drugs to address unmet medical need in the treatment of serious or life-threatening conditions. These programs are intended to help ensure that therapies for serous conditions are available as soon as it can be concluded that the therapies benefits justify their risks. These programs include breakthrough therapy designation, fast track designation, priority review and accelerated approval.
Breakthrough therapy designation
Breakthrough therapy designation is intended to expedite the development and review of products for serious and life-threatening conditions. Preliminary clinical evidence must demonstrate the drug may have substantial improvement over other available therapy. This designation conveys all of the same features of fast track designation, as well as more intensive FDA guidance throughout the development program. This guidance can include meetings throughout the development cycle, providing timely advice to ensure both the nonclinical and clinical programs are as efficient as practicable, and includes involvement of senior managers and experienced staff in cross-disciplinary and collaborative reviews. In general, sponsors must apply for breakthrough therapy designation, although the FDA may suggest to the sponsor that they consider submitting a request for the designation.
Fast track designation
The FDA’s fast track program is intended to facilitate the development and expedite the review of drugs that are intended for the treatment of a serious or life-threatening condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the fast track program, the sponsor of a new product candidate may request the FDA to designate the product candidate for a specific indication as a fast track drug concurrent with or after the filing of the IND for the product candidate. The FDA must determine if the product candidate qualifies for fast track designation within 60 days of receipt of the sponsor’s request.
If fast track designation is obtained, the FDA may initiate review of sections of an NDA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the time period specified in the PDUFA, which governs the time period goals the FDA has committed to reviewing an application, does not begin until the complete application is submitted. Additionally, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
In some cases, either a breakthrough therapy or a fast-track-designated product candidate may also qualify for one or more of the following programs:
52
Priority review. Under FDA policies, a product candidate is eligible for priority review, or review within six months from the time a complete NDA is accepted for filing, if the product candidate provides a significant improvement compared to marketed drugs in the treatment, diagnosis or prevention of a disease. Under PDUFA, the FDA is further entitled to take 60 days to determine that an application is sufficiently complete to allow the filing, therefore a priority review timetable may be 60 days before acceptance plus review. We cannot guarantee any of our product candidates will receive a priority review designation, or, if such a priority designation is received, that review or approval will be faster than conventional FDA procedures, or that the FDA will ultimately grant approval.
Accelerated approval. Under the FDA’s accelerated approval regulations, the FDA is authorized to approve product candidates that have been studied for their safety and effectiveness in treating serious or life-threatening illnesses, and that provide meaningful therapeutic benefit to patients over existing treatments based upon either a surrogate endpoint that is reasonably likely to predict clinical benefit or on the basis of an effect on a clinical endpoint other than patient survival. In clinical trials, surrogate endpoints are alternative measurements of the symptoms of a disease or condition that are substituted for measurements of observable clinical symptoms. A product candidate approved on this basis is subject to rigorous post-marketing compliance requirements, including the completion of Phase IV or post-approval clinical trials to validate the surrogate endpoint or confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or to validate a surrogate endpoint or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
When appropriate, we intend to seek either breakthrough therapy, fast track designation, priority review or accelerated approval for our products. We cannot predict whether any of our products will obtain a breakthrough therapy, fast track, priority review or accelerated approval designation, or the ultimate impact, if any, of these designations on the approval process, on the timing, or the likelihood of FDA approval of any of our product candidates.
Orphan drug designation
Orphan drug designation in the United States is designed to encourage sponsors to develop drugs intended for rare diseases or conditions. In the United States, a rare disease or condition is statutorily defined as a condition that affects fewer than 200,000 individuals in the United States or that affects more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available the drug for the disease or condition will be recovered from sales of the drug in the United States.
Orphan drug designation qualifies a company for tax credits and market exclusivity for seven years following the date of the drug’s marketing approval if granted by the FDA. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. A drug becomes an orphan when it receives orphan drug designation from the Office of Orphan Products Development, or OOPD, at the FDA based on acceptable confidential requests made under the regulatory provisions. The drug must then go through the new drug approval process like any other drug. Orphan drug designations are decided solely by the OOPD staff, but the OOPD occasionally will request opinions from the Center for Drug Evaluation and Research, especially when dealing with issues such as the appropriateness of the requested indication or the scientific rationale described by the sponsor.
A sponsor may request orphan drug designation of a previously unapproved drug or new orphan indication for an already marketed drug. In addition, a sponsor of a drug that is otherwise the same drug as an already approved orphan drug may seek and obtain orphan drug designation for the subsequent drug for the same rare disease or condition if it can present a plausible hypothesis that its drug may be clinically superior to the first drug. More than one sponsor may receive orphan drug designation for the same drug for the same rare disease or condition, but each sponsor seeking orphan drug designation must file a complete request for designation.
Drisapersen was granted orphan drug designation in the United States in 2005. All of our other DMD development compounds (PRO044, PRO045, PRO053, PRO052 and PRO055) have also been granted orphan drug designation.
The period of exclusivity begins on the date that the marketing application is approved by the FDA and applies only to the indication for which the drug has been designated. The FDA could approve a second application for the same drug for a different use or a second application for a clinically superior version of the drug for the same use. The FDA cannot, however, approve the same drug made by another manufacturer for the same indication during the market exclusivity period unless it has the consent of the sponsor or the sponsor is unable to provide sufficient quantities.
53
Hatch-Waxman Act
In addition, under the FDCA, as amended by the Hatch-Waxman Act of 1984, or the Hatch-Waxman Act, a drug can be classified as a new chemical entity if the FDA has not previously approved any other new drug containing the same active agent. Under sections 505(c)(3)(D)(ii) and 505(j)(5)(D)(ii) of the FDCA, as amended by the Hatch-Waxman Act, the first applicant to gain approval of an NDA for a new chemical entity may, in the absence of patent protections, be eligible for five years of market exclusivity in the United States following regulatory approval.
During the five-year exclusivity period, the FDA may not accept for review an abbreviated new drug application, or a 505(b)(2) NDA submitted by a competitor for another version of such drug, where the applicant does not own or have a legal right of reference to all the data required for approval. Protection under the Hatch-Waxman Act will not prevent the filing or approval of another full NDA, but the applicant would be required to conduct its own adequate and well-controlled clinical trials to demonstrate safety and effectiveness. The Hatch-Waxman Act also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplements to existing NDAs if new clinical investigations are essential to the approval of the applications, for example for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving abbreviated NDAs filed by competitors for drugs containing the original active agent.
The Hatch-Waxman Act also permits a patent restoration term of up to five years as compensation for the portion of a drug’s patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and it must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may consider applying for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond the current expiration date, depending on the expected length of clinical trials and other factors involved in the filing of the relevant NDA.
Post-approval regulation
If regulatory approval for marketing of a product or new indication for an existing product is obtained, we will be required to comply with all regular post-approval regulatory requirements as well as any post-approval requirements that the FDA have imposed as part of the approval process. We will be required to report certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling requirements. Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including current Good Manufacturing Practices regulations, which impose certain procedural and documentation requirements upon drug manufacturers. Accordingly, we and our third-party manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with current Good Manufacturing Practices regulations and other regulatory requirements. Discovery of problems with a product after approval for marketing may result in restrictions on a product, manufacturer, or holder of an approved NDA, including withdrawal of the product from the market.
European Union
The process regarding approval of medicinal products in the EU follows roughly the same lines as in the United States and likewise generally involves satisfactorily completing each of the following:
|
·
|
preclinical laboratory tests, animal studies and formulation studies all performed in accordance with the applicable EU Good Laboratory Practice regulations;
|
|
·
|
submission to the relevant national authorities of a clinical trial application or CTA, which must be approved before human clinical trials may begin;
|
54
|
·
|
performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication;
|
|
·
|
submission to the relevant competent authorities of a marketing authorization application or MAA, which includes the data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labelling;
|
|
·
|
satisfactory completion of an inspection by the relevant national authorities of the manufacturing facility or facilities, including those of third parties, at which the product is produced to assess compliance with strictly enforced current Good Manufacturing Practices;
|
|
·
|
potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and
|
|
·
|
review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product.
|
Preclinical studies
Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animal studies, in order to assess the potential safety and efficacy of the product. The conduct of the preclinical tests and formulation of the compounds for testing must comply with the relevant EU regulations and requirements. The results of the preclinical tests, together with relevant manufacturing information and analytical data, are submitted as part of the CTA.
Clinical trial approval
Pursuant to the Clinical Trials Directive 2001/20/EC, as amended, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, approval must be obtained from the competent national authority of a European Union member state in which a study is planned to be conducted. To this end, a CTA is submitted, which must be supported by an investigational medicinal product dossier, or IMPD, and further supporting information prescribed by the Clinical Trials Directive and other applicable guidance documents. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the clinical trial application in that country.
Clinical drug development is often described as consisting of four temporal phases (Phase I- IV), see for example EMA’s note for guidance on general considerations for clinical trials (CPMP/ICH/291/95).
|
·
|
Phase I (Most typical kind of study: Human Pharmacology);
|
|
·
|
Phase II (Most typical kind of study: Therapeutic Exploratory);
|
|
·
|
Phase III (Most typical kind of study: Therapeutic Confirmatory); and
|
|
·
|
Phase IV (Variety of Studies: Therapeutic Use).
|
Studies in Phase IV are all studies (other than routine surveillance) performed after drug approval and related to the approved indication.
The phase of development provides an inadequate basis for classification of clinical trials because one type of trial may occur in several phases. The phase concept is a description, not a set of requirements. The temporal phases do not imply a fixed order of studies since for some drugs in a development plan the typical sequence will not be appropriate or necessary.
Manufacturing of investigational products is subject to the holding of authorization and must be carried out in accordance with current Good Manufacturing Practices.
Health authority interactions
During the development of a medicinal product, frequent interactions with the EU regulators are vital to make sure all relevant input and guidelines/regulations are taken into account in the overall program. Prosensa has established an ongoing dialogue with EMA and certain national authorities by making use of the mechanisms that exist for interaction and input.
55
|
·
|
Informal interactions: we have had several informal meetings with interested parties from EMA. These interactions have provided important input into the development programs.
|
|
·
|
Formal CHMP scientific advice: the development plans for PRO045 and PRO053 were reviewed with the EU regulators through a scientific advice procedure with the scientific advice working party, or SAWP, of The Committee for Medicinal Products for Human Use, or CHMP. As per the procedure, a letter of intent was submitted and subsequently a briefing document including the questions was submitted. After assessment of the documentation supplied, EMA provided a list of issues for clarification, which were addressed by Prosensa in a face-to-face meeting with the SAWP and subsequently a formal advice letter was issued. Of note, for orphan medicinal products, one can apply for protocol assistance, which results in the same outcome, but is free of charge.
|
|
·
|
Formal national feedback: in order to increase success of the CTA applications, we have held meetings on the proposed CTAs for the PRO045 compound with the UK and Dutch regulators. Their input has been incorporated into the protocols.
|
|
·
|
Business pipeline meetings: in addition to the scientific input outlined above, procedural advice has also been obtained through business pipeline meetings with EMA. In these meetings, we were able to present our entire pipeline for DMD, where we received input from key EMA personnel on the appropriate procedures to be followed (scientific advice/protocol assistance, PIPs and MAA) and how best to proceed.
|
|
·
|
Paediatric Investigation Plans: Regulation (EC) 1901/2006 came into force on 26 January 2007 and has as its primary purpose the improvement of the health of children without subjecting children to unnecessary trials, or delaying the authorization of medicinal products for use in adults.
|
The regulation established the Paediatric Committee, or PDCO, which is responsible for coordinating the EMA’s activities regarding medicines for children. The PDCO’s main role is to determine all the studies that applicants need to do in the pediatric population as part of the so-called Paediatric Investigation Plans, or PIPs.
All applications for marketing authorization for new medicines that were not authorized in the EU before 26 January 2007 have to include the results of studies carried out in children of different ages. As indicated, the PDCO determines what these studies entail and describes them in a PIP. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized.
|
·
|
The PDCO can grant deferrals for some medicines, allowing a company to delay development of the medicine in children until there is enough information to demonstrate its effectiveness and safety in adults.
|
|
·
|
The PDCO can also grant waivers when development of a medicine in children is not needed or is not appropriate, such as for diseases that only affect the elderly population.
|
Before a marketing authorization application can be filed, or an existing marketing authorization can be amended, the EMA checks that companies actually comply with the agreed studies and measures listed in each relevant PIP.
With the regulation coming into force, several incentives for the development of medicines for children also became available in the EU:
|
·
|
medicines that have been authorized across the EU with the results of PIP studies included in the product information are eligible for an extension of their patent protection by six months. This is the case even when the studies’ results are negative;
|
|
·
|
for orphan paediatric medicines, the incentive is an additional two years of market exclusivity in addition to the ten years market exclusivity that is granted on authorisation of any orphan medicine;
|
|
·
|
scientific advice and protocol assistance at the EMA are free of charge for questions relating to the development of medicines for children; and
|
56
|
·
|
medicines developed specifically for children that are already authorized but are not protected by a patent or supplementary protection certificate, can apply for a paediatric use marketing authorization, or PUMA. If a PUMA is granted, the product will benefit from 10 years of market protection as an incentive.
|
Since our compounds in the DMD portfolio are all intended for the use in children by the very nature of the disease, a PIP has been agreed for drisapersen and we have completed PIPs for PRO045 and PRO053. Additional PIP procedures are ongoing with PDCO for the other programs in clinical development.
Marketing authorization application
Available authorization procedures
Authorization to market a product in the European Union member states proceeds under one of four procedures: a centralized authorization procedure, a mutual recognition procedure, a decentralized procedure or a national procedure.
|
·
|
Centralized authorization procedure. Certain drugs defined as medicinal products developed by means of biotechnological processes must undergo the centralized authorization procedure for marketing authorization, which, if granted, is automatically valid in all European Union member states. The EMA and the European Commission administer the centralized authorization procedure.
|
Pursuant to Regulation 726/2004, this procedure is mandatory for:
a) medicinal products developed by means of one of the following biotechnological processes:
|
·
|
recombinant DNA technology;
|
|
·
|
controlled expression of genes coding for biologically active proteins in prokaryotes and eukaryotes including transformed mammalian cells; and
|
|
·
|
hybridoma and monoclonal antibody methods;
|
b) advanced therapy medicinal products as defined in Article 2 of Regulation 1394/2007 on advanced therapy medicinal products;
c) medicinal products for human use containing a new active substance which, on the date of entry into force of this Regulation, was not authorized in the European Union, for which the therapeutic indication is the treatment of any of the following diseases:
|
·
|
acquired immune deficiency syndrome;
|
|
·
|
cancer;
|
|
·
|
neurodegenerative disorder;
|
|
·
|
diabetes;
|
|
·
|
auto-immune diseases and other immune dysfunctions; and
|
|
·
|
viral diseases; and
|
d) medicinal products that are designated as orphan medicinal products pursuant to Regulation 141/2000.
All of Prosensa’s products in development for DMD have orphan status in the EU and therefore the Centralized Procedure will be used for any Marketing Authorisation Applications.
The centralized authorization procedure is optional for other medicinal products if they contain a new active substance or if the applicant shows that the medicinal product concerned constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization is in the interest of patients at a European Community level.
57
Under the centralized authorization procedure, the CHMP serves as the scientific committee that renders opinions about the safety, efficacy and quality of human products on behalf of the EMA. The CHMP is composed of experts nominated by each member state’s national drug authority, with one of them appointed to act as Rapporteur for the co-ordination of the evaluation with the possible assistance of a further member of the Committee acting as a Co-Rapporteur. After approval, the Rapporteur(s) continue to monitor the product throughout its life cycle. The CHMP has 210 days, to adopt an opinion as to whether a marketing authorization should be granted. The process usually takes longer as additional information is requested, which triggers clock-stops in the procedural timelines. The process is complex and involves extensive consultation with the regulatory authorities of member states and a number of experts. Once the procedure is completed, a European Public Assessment Report, or EPAR, is produced. If the opinion is negative, information is given as to the grounds on which this conclusion was reached. The opinion produced by the CHMP is sent to the European Commission and used in reaching the final decision.
In general, if the centralized procedure is not followed, there are three alternative procedures. If marketing authorization in only one member state is preferred, an application can be filed with the national competent authority of a member state. The other two options are a mutual recognition by European Union member states and the decentralized procedure, both under Directive 2001/83. A marketing authorization may be granted only to an applicant established in the European Union.
|
·
|
Mutual recognition procedure. If an authorization has been granted by one member state, or the Reference Member State, an application may be made for mutual recognition in one or more other member states, or the Concerned Member State(s).
|
|
·
|
Decentralized procedure. The third option is the decentralized procedure. The decentralized procedure may be used to obtain a marketing authorization in several European member states when the applicant does not yet have a marketing authorization in any country.
|
|
·
|
National procedure. Applicants following the national procedure will be granted a marketing authorization that is valid only in a single member state. Furthermore, this marketing authorization is not based on recognition of another marketing authorization for the same product awarded by an assessment authority of another member state. The national procedure can also serve as the first phase of a mutual recognition procedure.
|
It is not always possible for applicants to follow the national procedure. In the case of medicinal products in the category for which the centralized authorization procedure is compulsory, that procedure must be followed. In addition, the national procedure is not available in the case of medicinal product dossiers where the same applicant has already obtained marketing authorization in one of the other European Union member states or has already submitted an application for marketing authorization in one of the other member states and the application is under consideration. In the latter case, applicants must follow a mutual recognition procedure.
After a drug has been authorized and launched, it is a condition of maintaining the marketing authorization that all aspects relating to its quality, safety and efficacy must be kept under review. Sanctions may be imposed for failure to adhere to the conditions of the marketing authorization. In extreme cases, the authorization may be revoked, resulting in withdrawal of the product from sale.
Accelerated assessment procedure
When appropriate, we may seek accelerated assessment for our products. When an application is submitted for a marketing authorization in respect of a drug for human use which is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation, the applicant may request an accelerated assessment procedure pursuant to article 14, paragraph 9 of Regulation 726/2004. If the CHMP accepts the request, the review period would be shortened from 210 to 150 days.
Conditional approval
As per Regulation EC 726/2004, Art. 14(7), a medicine that would fulfill an unmet medical need may, if its immediate availability is in the interest of public health, be granted a conditional marketing authorization on the basis of less complete clinical data than are normally required, subject to specific obligations being imposed on the authorization holder. These specific obligations are to be reviewed annually by the EMA. The list of these obligations shall be made publicly accessible. Such an authorization shall be valid for one year, on a renewable basis.
58
Exceptional circumstances
As per Regulation EC 726/2004, Art. 14(8), products for which the applicant can demonstrate that comprehensive data (in line with the requirements laid down in Annex I of Directive 2001/83/EC, as amended) cannot be provided (due to specific reasons foreseen in the legislation) might be eligible for marketing authorization under exceptional circumstances. This type of authorization is reviewed annually to reassess the risk-benefit balance. The fulfillment of any specific procedures/obligations imposed as part of the marketing authorization under exceptional circumstances is aimed at the provision of information on the safe and effective use of the product and will normally not lead to the completion of a full dossier/approval.
We cannot predict whether any of our products will obtain any of such designations or predict the ultimate impact, if any, of such designations on the timing, conditions or likelihood of EMA authorization.
Period of authorization and renewals
Marketing authorization shall be valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the EMA or by the competent authority of the authorizing member state. To this end, the marketing authorization holder shall provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization shall be valid for an unlimited period, unless the Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the EU market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization shall cease to be valid (the so-called sunset clause).
Orphan drug designation
Regulation 141/2000 states that a drug shall be designated as an orphan drug if its sponsor can establish:
|
·
|
(a)(i) that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in ten thousand persons in the European Community when the application is made, or;
|
|
·
|
(a)(ii) that it is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the European Community and that without incentives it is unlikely that the marketing of the drug in the European Community would generate sufficient return to justify the necessary investment; and
|
|
·
|
(b) that there exists no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the European Community or, if such method exists, the drug will be of significant benefit to those affected by that condition.
|
Regulation 847/2000 holds criteria for the designation of orphan drugs.
An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. Marketing authorization for an orphan drug leads to a ten-year period of market exclusivity. This period may, however, be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan drug designation, for example because the product is sufficiently profitable not to justify market exclusivity. Market exclusivity can be revoked only in very selected cases, such as consent from the marketing authorization holder, inability to supply sufficient quantities of the product, demonstration of “clinically relevant superiority” by a similar medicinal product, or, after a review by the Committee for Orphan Medicinal Products, requested by a member state in the fifth year of the marketing exclusivity period (if the designation criteria are believed to no longer apply). Medicinal products designated as orphan drugs pursuant to Regulation 141/2000 shall be eligible for incentives made available by the European Community and by the member states to support research into, and the development and availability of, orphan drugs.
We have applied for and been granted orphan status in the EU for all compounds in our portfolio which are intended for the treatment of DMD.
59
Regulatory data protection
Without prejudice to the law on the protection of industrial and commercial property, all applications for marketing authorization receive an 8+2+1 protection regime.
This regime consists of a regulatory data protection period of eight years plus a concurrent market exclusivity of ten years plus an additional market exclusivity of one further year if, during the first eight years of those ten years, the marketing approval holder obtains an approval for one or more new therapeutic indications which, during the scientific evaluation prior to their approval, are determined to bring a significant clinical benefit in comparison with existing therapies. Under the current rules, a third party may reference the preclinical and clinical data of the original sponsor beginning eight years after first approval, but the third party may market a generic version after only ten (or eleven) years have lapsed.
As indicated, additional data protection can be applied for when an applicant has complied with all requirements as set forth in an approved PIP.
Manufacturing
The manufacturing of authorized drugs, for which a separate manufacturer’s license is mandatory, must be conducted in strict compliance with the EMA’s current Good Manufacturing Practices requirements and comparable requirements of other regulatory bodies, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. The EMA enforces its current Good Manufacturing Practices requirements through mandatory registration of facilities and inspections of those facilities. The EMA may have a coordinating role for these inspections while the responsibility for carrying them out rests with the member states competent authority under whose responsibility the manufacturer falls. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and could subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil and criminal penalties.
Marketing and promotion
The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Community notably under Directive 2001/83 in the European Community code relating to medicinal products for human use as amended by Directive 2004/27. The applicable regulation aims to ensure that information provided by holders of marketing authorizations regarding their products is truthful, balanced and accurately reflects the safety and efficacy claims authorized by the EMA or by the competent authority of the authorizing member state. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
Environmental, health and safety
We and the operations of our CMOs are further subject to various foreign, national, federal, state and local laws and regulations relating to environmental, health and safety matters, including the handling, disposal, release, and use of and maintenance of a registry for hazardous materials, among others. Although we do not believe that we will be required to make material operating or capital expenditures in connection with such laws and regulations, we may be required to incur significant costs to comply with these laws and regulations in the future, and complying with these laws and regulations may result in a material adverse effect upon our business, financial condition and results of operations. Further, our failure or the failure of our CMOs to comply with such laws and regulations could have a material adverse effect on our business and reputation, result in an interruption or delay in the development or manufacture of our products, or increase the costs for the development or manufacture of our products.
Pharmaceutical pricing and reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any drug products for which we obtain regulatory approval. Sales of any of our product candidates, if approved, will depend, in part, on the extent to which the costs of the products will be covered by third-party payors, including government health programs such as Medicare and Medicaid, commercial health insurers and managed care organizations.
The process for determining whether a payor will provide coverage for a drug product may be separate from the process for setting the price or reimbursement rate that the payor will pay for the drug product once coverage is approved. Third party payors may limit coverage to specific drug products on an approved list, or formulary, which might not include all of the approved drugs for a particular indication.
60
In order to secure coverage and reimbursement for any product that might be approved for sale, we may need to conduct pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, in addition to the costs required to obtain FDA or other comparable regulatory approvals.
Our product candidates may not be considered medically necessary or cost-effective. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Third party reimbursement may not be sufficient to enable us to maintain price levels high enough to realize an appropriate return on our investment in product development.
The containment of healthcare costs has become a priority of governments, and the prices of drugs have been a focus in this effort. Third party payors are increasingly challenging the prices charged for medical products and services and examining the medical necessity and cost-effectiveness of medical products and services, in addition to their safety and efficacy. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
The U.S. government, state legislatures and non-U.S. governments have shown significant interest in implementing cost containment programs to limit the growth of government-paid health care costs, including price controls, restrictions on reimbursement and requirements for substitution of generic products for branded prescription drugs. Adoption of such controls and measures, and tightening of restrictive policies in jurisdictions with existing controls and measures, could limit payments for pharmaceuticals such as the product candidates that we are developing and could adversely affect our net revenue and results.
Pricing and reimbursement schemes vary widely from country to country. Some countries provide that drug products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies. For example, the European Union provides options for its member states to restrict the range of drug products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. European Union member states may approve a specific price for a drug product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the drug product on the market. Other member states allow companies to fix their own prices for drug products, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert competitive pressure that may reduce pricing within a country. There can be no assurance that any country that has price controls or reimbursement limitations for drug products will allow favorable reimbursement and pricing arrangements for any of our products.
The marketability of any products for which we receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on drug pricing. Coverage policies, third-party reimbursement rates and drug pricing regulation may change at any time. In particular, the Patient Protection and Affordable Care Act was enacted in the United States in March 2010 and contains provisions that may reduce the profitability of drug products, including, for example, increased rebates for drugs sold to Medicaid programs, extension of Medicaid rebates to Medicaid managed care plans, mandatory discounts for certain Medicare Part D beneficiaries and annual fees based on pharmaceutical companies’ share of sales to federal health care programs. Even if favorable coverage and reimbursement status is attained for one or more products for which we receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
C. Organizational structure
The registrant corporation, Prosensa Holding N.V. has four wholly owned subsidiaries which are each listed in Exhibit 8.1 filed hereto. We primarily operate our business out of our operating subsidiary, Prosensa Therapeutics B.V.
61
D. Property, plant and equipment
We lease approximately 20,000 square feet of office and laboratory space at J.H. Oortweg 21 in Leiden, The Netherlands under non-cancellable operating lease agreements. The lease terms are between 1 and 5 years. This facility serves as the corporate headquarters and central laboratory facility. We believe that our existing facilities are adequate to meet current needs and that suitable additional alternative spaces will be available in the future on commercially reasonable terms.
ITEM 4A. UNRESOLVED STAFF COMMENTS
As of December 31, 2013 there were no unresolved staff comments.
ITEM 5. OPERATING AND FINANCIAL REVIEW AND PROSPECTS
You should read the following discussion and analysis of our financial condition and results of operations together with the information under “Selected Financial Data” and our consolidated audited financial statements, including the notes thereto, included in this Annual Report. The following discussion is based on our financial information prepared in accordance with IFRS as issued by the IASB, which might differ in material respects from generally accepted accounting principles in other jurisdictions. The following discussion includes forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those described under “Risk Factors” and elsewhere in this Annual Report.
A. Operating results
Overview
We are an innovative biotechnology company engaged in the discovery and development of ribonucleic acid-modulating, or RNA-modulating, therapeutics for the treatment of genetic disorders. Our primary focus is on rare neuromuscular and neurodegenerative disorders with a large unmet medical need, including Duchenne muscular dystrophy, myotonic dystrophy and Huntington’s disease. Our clinical portfolio of RNA-based product candidates is focused on the treatment of Duchenne muscular dystrophy, or DMD. Each of our DMD compounds has been granted orphan drug status in the United States and the European Union.
We have financed our operations through offerings of equity securities, upfront, milestone and expense reimbursement payments received from GlaxoSmithKline, or GSK, under our research and development collaboration and license agreement, or the GSK Agreement, as well as funding from patient organizations, governmental bodies and bank loans. From January 1, 2002 until December 31, 2013, we raised gross proceeds of €56.4 million from private placements of equity securities and received £41.5 million (€47.4 million) in payments from GSK and €7.3 million in loans from patient organizations and governmental bodies. In July 2013, we raised proceeds of €64.0 million (before offering expenses) from our initial public offering (“IPO”) of ordinary shares on Nasdaq. As of December 31, 2013, we had cash and cash equivalents of €82.2 million. To date, we have not generated any revenues from royalties or product sales. Based on our current plans, we do not expect to generate royalty or product revenues unless and until we obtain marketing approval for, and we commercialize, drisapersen or any of our other product candidates.
We have generated losses since we began our drug development operations in 2002. For the years ended December 31, 2013, 2012 and 2011, we incurred net losses of €16.6 million, €9.9 million and €11.6 million, respectively. As of December 31, 2013, we had an accumulated deficit of €56.4 million. We expect to continue incurring losses as we continue our clinical and preclinical development programs, apply for marketing approval for our product candidates, and subject to obtaining regulatory approval of our product candidates, build a sales and marketing force in preparation for the potential commercialization of our product candidates.
Collaboration and license agreements
GlaxoSmithKline
From October 2009 to January 2014, we operated under an exclusive worldwide collaboration with GSK for the development and commercialization of RNA-based therapeutics for DMD, with GSK exclusively licensing worldwide rights to develop and commercialize drisapersen and obtaining an option to exclusively license PRO044 and other specified assets in our DMD portfolio. Under the collaboration agreement, GSK paid us a total of £41.5 million (€47.4 million) in upfront and milestone payments. Under the collaboration agreement, GSK was responsible for all costs of clinical development of drisapersen.
62
In January 2014, we and GSK mutually terminated the collaboration pursuant to a termination agreement, which terminates all intellectual property license grants as well as any rights arising under the collaboration agreement (other than rights to payments that accrued prior to termination of the collaboration). In addition, the termination agreement requires GSK to transfer to us certain data and know how, inventory, regulatory filings, clinical trial sponsorships, clinical study reports and material agreements relating to the development of our products as soon as reasonably practicable, but in no case later than 120 days after the effective date of the termination agreement. We and GSK each agreed to indemnify the other party with respect to certain acts and omissions. Going forward, we will be solely responsible for the cost of developing and commercializing drisapersen and our other product candidates which will have significant financial and operational implications.
Leiden University Medical Center
We have entered into an exclusive worldwide license agreement with Leiden University Medical Center, or LUMC, for the rights to apply LUMC’s proprietary RNA modulation exon-skipping technology to develop treatments for DMD, other neuromuscular disorders and indications outside the field of neuromuscular disorders. We are obligated to make milestone payments upon the occurrence of events with respect to each subfield in which we pursue a drug indication using the LUMC patent rights, including commencing the first toxicity study, successfully completing the first Phase I study, filing for regulatory approval and satisfying certain sales thresholds. The aggregate milestone payments could total approximately €1.4 million, if we achieve such milestones in relation to an initial orphan drug indication. If we achieve such milestones in relation to a non-orphan drug indication, the aggregate milestone payments could total €5.5 million. We may also be obligated to pay LUMC additional milestone payments of €1.25 million per product in relation to products approved for additional orphan drug indications. In addition, we are obligated to pay LUMC a mid-single digit percentage royalty on net sales on a country-by-country basis if and when any of our products is brought to market. If we sublicense our rights under the LUMC agreement to a third party, we are obligated to pay LUMC a tiered royalty percentage (ranging from the low-to-mid-twenties) on the net licensing income and a low single-digit percentage royalty on the net sales generated by the sublicensee. See “Item 4. Information on the Company—B. Business Overview—Collaboration, license and funding arrangements—Leiden University Medical Center.”
To date, we have paid LUMC €3.6 million in milestone payments under the agreement.
Critical accounting policies and significant judgments and estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which we have prepared in accordance with IFRS as issued by the IASB. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenues and expenses during the reporting periods. Actual results may differ from these estimates under different assumptions or conditions.
Subsequent to the announcement in September 2013 that the Phase III trial of drisapersen did not meet its primary endpoint, we initiated an evaluation of all data and patient groups across all DMD studies with drisapersen. The outcome of the evaluation will have a material impact on the further development of drisapersen and other DMD compounds and potentially impact the following accounts in the Company’s consolidated financial statements on an ongoing basis.
Other loans & Finance costs
Certain loans from patient organizations have no fixed redemption schemes and repayment is due when certain pre-determined milestones are met. A change in the estimated date the pre-defined milestones will be reached impacts the carrying value of the loans. The final outcome of the evaluation may result in deferral of the redemption dates of our loans. The effect of a one year delay of the redemption dates of these loans would decrease our borrowing balance by €0.5 million as of December 31, 2013.
Intangible assets
As of December 31, 2013 we recorded patents and licenses with a net book value of €0.5 million. We assess that the asset’s recoverable amount (determined based on value in use calculations) still exceeds the carrying value of €0.5 million in case of a negative outcome of the evaluation and no impairment is required.
63
While our significant accounting policies are more fully described in the notes to our consolidated financial statements appearing elsewhere in this Annual Report, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating our financial condition and results of operations.
Deferred revenue & License income
As of December 31, 2013 our deferred revenue totaled €14.6 million, comprised of deferred license revenue (€14.5 million) and deferred other income of €0.1 million. Effective January 12, 2014, we and GSK mutually terminated our collaboration agreement. In January 2014, the deferred license revenue balance will be fully released as we do not have any remaining performance obligations and it is not impacted by the final outcome of the evaluation.
Revenue recognition and cost of license revenue
Nonrefundable upfront licensing fees and certain guaranteed, time-based payments that require continuing involvement in the form of research and development, manufacturing or other efforts are recognized in the period of expected performance. Other upfront payments are recognized by reference to the stage of completion of the underlying agreement at the balance sheet date when the rendering of services can be estimated reliably. The rendering of services can be estimated reliably when the stage of completion of the transaction at the balance sheet date can be measured reliably and costs incurred for the transaction and the costs to complete the transaction can be measured reliably. When these criteria are not met, revenue arising from the rendering of services is recognized only to the extent of the expenses recognized that are recoverable. Milestone payments are recognized when substantive services have been performed in relation to the payment and contractual milestones having been fully achieved.
Collaboration revenue, which may or may not be related to a licensing agreement, is recognized on the basis of labor hours delivered at the contractual full-time employee rates.
The related cost of license revenue is based on the determination of net license revenue as defined in our license agreements after deducting allocable expenses and is subject to a certain amount of judgment.
Other income
We are part of two pan-European consortia, each consortium has been awarded a Framework Programme 7 (“FP7”) research grant of €6 million from the European Commission to support respectively ongoing clinical study PRO045 and the development of imaging biomarkers for Duchenne muscular dystrophy (DMD). Grant proceeds are deferred and other income is recognized based on the percentage of completion method.
To encourage research and development we obtained certain loans that generally bear interest at a rate below the market interest rate, considered by the company to be 12% over the last five years. The difference between fair value of the loans and the notional amount of the loans at inception is treated as a donation received for certain research performed by the Company for an amount of €109 thousand in the year ended December 31, 2013 (2012: €170 thousand; 2011: nil). As of December 31, 2013 €104 thousand is deferred and will be recognized in Other income over the periods when expenses are incurred.
Research and development expense
Research expenses are recognized as expenses when incurred. Costs incurred on development projects are recognized as intangible assets as of the date as of which it can be established that it is probable that future economic benefits attributable to the asset will flow to us considering its technological and commercial feasibility. This is generally the case when regulatory approval for commercialization is achieved and costs can be measured reliably. Given the current stage of the development of our products, no development expenditures have yet been capitalized. Intellectual property-related costs for patents are part of the expenditure for the research and development projects. Therefore, registration costs for patents are expensed when incurred as long as the research and development project concerned does not meet the criteria for capitalization.
As part of the process of preparing our financial statements we are required to estimate our accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on our behalf, estimating the level of service performed and the associated cost incurred for the service when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the
64
service providers and make adjustments if necessary. The significant estimates in our accrued research and development expenses are related to fees paid to clinical research organizations, or CROs, in connection with research and development activities for which we have not yet been invoiced. We base our expenses related to CROs on our estimates of the services received and efforts expended pursuant to quotes and contracts with CROs that conduct research and development on our behalf.
The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepayment expense accordingly. Although we do not expect our estimates to be materially different from amounts actually incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and could result in us reporting amounts that are too high or too low in any particular period.
Share-based compensation
Share options
We have adopted share-based compensation plans pursuant to which certain participants are granted the right to acquire ordinary shares. We refer to these rights as “options.” Upon the exercise or award or vesting of a non-cash-settled award under the plans, ordinary shares are issued.
The vesting of options is generally conditional on the participant completing a four year period of service. Each option grant vests based on the following schedule: (a) 25% on the first anniversary of the date of the grant, provided that on that date the relevant participant is still employed by us, and (b) the remaining 75% in 36 equal monthly installments over a three year period following the first anniversary of the date of grant subject to continued employment of the relevant participant. The Company may decide to accelerate the vesting of the options as a result of a change of control.
On December 5, 2012, we granted options to members of our management board subject to vesting only upon a liquidity event such as a change of control or an initial public offering and are further subject to continued employment until the date such an event occurs. The number of options that vest will depend on our share price following an initial public offering.
The outstanding options granted can be exercised up to 10 years after the grant date and then expire.
Following the completion of our IPO, option pricing and values are determined based on the quoted market price of our ordinary shares.
We value options granted to participants at fair value on the date of grant and recognize the corresponding compensation expense of those grants, net of estimated forfeitures, over the requisite service period, which is generally the vesting period of the respective grant. Market performance conditions attached to option grants are included when determining the grant date fair value of such options.
We issue options with only service-based vesting conditions or with service-based vesting conditions combined with vesting conditions subject to a liquidity event and record the expense for these options using the straight-line method for each separate vesting tranche.
Prior to our IPO, participants were not granted the right to acquire ordinary shares but (non-voting) depository receipts (“Depositary Receipts”) in respect of our ordinary shares. Upon the exercise or award or vesting of a non-cash-settled award under the plans, ordinary shares were issued to a Dutch foundation called Stichting Administratiekantoor Prosensa Holding (the “Foundation”), whose purpose was to facilitate administration of options and pool the voting interests of the underlying shares. The Foundation thereupon granted a Depository Receipt for each issued ordinary share to the person entitled to such ordinary share under an award. Legal title to the shares issued under the share-based compensation plan is held by the Foundation, and the voting rights attached to the shares are exercised by the Foundation at its own discretion.
On October 10, 2013 the Board of the Foundation resolved to terminate the administration of the ordinary shares and to transfer the ordinary shares to the respective Depository Receipt holder. After the completion of the transfers, the Foundation will be dissolved. Going forward participants will receive ordinary shares upon the exercise or award or vesting of a non-cash-settled award under the plans instead of depository receipts.
65
The fair value of options under the various grants is based on a Monte-Carlo simulation using the following assumptions:
|
2011
|
2012
|
2013
|
|||||||||
|
Weighted average fair value of options granted:
|
€ | € | $ | ||||||||
|
Options with only service-based vesting conditions
|
0.99 | 0.69 | 5.19 | ||||||||
|
Options with change of control and service-based vesting conditions
|
— | 0.01 | — | ||||||||
|
Options with IPO and service-based vesting conditions, €0.70 exercise price
|
— | 1.89 | — | ||||||||
|
Options with IPO and service-based vesting conditions, €0.01 exercise price
|
— | 1.97 | — | ||||||||
|
Exercise price
|
0.01 - 1.00 | 0.01 - 0.70 | 4.26 - 19.25 | ||||||||
|
Risk-free interest rate
|
1.90 | % | 1.5 | % | 1.1 - 2.0 | % | |||||
|
Volatility
|
80 | % | 80 | % | 95 - 100 | % | |||||
|
Dividend yield
|
0.0 | % | 0.0 | % | 0.0 | % | |||||
|
Expected life (in years)
|
2-3 years
|
1-2 years
|
6 years
|
||||||||
Prior to July 2013, we were a private company and lacked company-specific historical and implied volatility information. Therefore, we estimated our expected volatility based on the historical volatility of publicly traded peer companies and expect to continue to do so until such time as we have adequate historical data regarding the volatility of our traded share price. The publicly traded peer companies we use for this analysis include companies active in the development of drugs for unmet medical needs, including DMD, and/or applying RNA-modulating or genetics-based technology and therapies and include our main competitor, Sarepta Therapeutics Inc., as well as Santhera Pharmaceuticals Holding AG and Summit plc. Most of the selected guideline companies only have a pipeline with compounds in development and have not yet commercialized any products. The estimated volatility that we applied is based on the median of the selected guideline companies and measured based on their observed daily share price returns over a historic period equal to the period for which expected volatility is estimated. Volatility is defined as the annualized standard deviation of share price returns.
We expect all vested options to be exercised. Prior to our IPO, we considered the expected life of the options to be in line with the vesting period of the options. The expected life for all options granted subsequent to the completion of our IPO is estimated at 6 years.
As of December 31, 2013, the outstanding options had the following exercise prices:
|
Exercise price in per share
|
Options
|
||
|
€ 0.01
|
1,489,681 | ||
|
€ 0.70
|
133,333 | ||
|
€ 1.00
|
140,000 | ||
|
€ 1.85
|
83,250 | ||
|
€ 2.54
|
130,950 | ||
|
$ 4.26
|
167,920 | ||
|
$19.25
|
40,000 | ||
| 2,185,134 | |||
During 2011, the exercise price of 519,350 options was changed to €0.01 to align the exercise price of the options granted under our 2007 Employee Stock Option Plan and our 2010 Stock Option Plan. The incremental fair value granted amounted to €410,000 and was the difference between the fair value of the options with revised exercise price (€0.01) and the fair value of the options with the original exercise price, both estimated as of the date of the modification. From the modification date this incremental fair value is recognized as an expense over the remainder of the applicable vesting period, in addition to the expense recognized for the original fair value of the options.
These assumptions represent our best estimates, but the estimates involve inherent uncertainties and the application of our judgment. As a result, if factors change and we use significantly different assumptions or estimates, our share-based compensation expense could be materially different. We recognize compensation expense for only the portion of equity instruments that are expected to vest. In developing a forfeiture rate estimate, we have considered our historical experience to estimate pre-vesting forfeitures. If our actual forfeiture rate is materially different from the estimate, our share-based compensation expense could be different from what we have recorded in the current period.
66
The following table summarizes by grant date the number of options granted between January 1, 2011 and December 31, 2013:
|
Grant date
|
Options
granted
|
Exercise price (€)
|
Fair value of
ordinary
share (€)
|
Per share
estimated fair
value of
options (€)
|
|
March 22, 2011
|
707,500
|
0.01 - 1.00
|
1.00
|
0.99
|
|
September—November, 2011
|
8,500
|
0.01
|
1.00
|
0.99
|
|
January 1, 2012
|
10,000
|
0.01
|
0.70
|
0.69
|
|
March 31, 2012
|
15,000
|
0.01
|
0.70
|
0.69
|
|
July 17, 2012
|
61,600
|
0.01
|
0.70
|
0.69
|
|
October 22, 2012
|
31,250
|
0.01
|
0.70
|
0.69
|
|
December 5, 2012
|
1,000,000
|
0.01 - 0.70
|
0.70
|
0.01 - 1.97
|
|
July 3, 2013
|
40,000
|
$ 19.25
|
$ 19.25
|
$ 12.81
|
|
December 10, 2013
|
167,920
|
$ 4.26
|
$ 4.26
|
$ 3.38
|
The fair value of all 2,185,134 vested and unvested outstanding options as of December 31, 2013, of which 923,528 are vested and 1,261,606 are unvested, was approximately $10.2 million, or $4.3 million for the vested options and $5.9 million for the unvested options, in each case based on the fair value for our ordinary shares of $4.67 per share, as of December 31, 2013.
On December 5, 2012, we granted to the members of our management board 1.0 million options that vest only upon a liquidity event such as a change of control or our IPO, subject to the continued employment of the members of the management board. Following our IPO, the maximum number of options eligible to vest automatically reduced from 1.0 million to 800,000, of which Giles Campion, Luc Dochez and Berndt Modig are each eligible to vest in up to 133,333 options and Hans Schikan is eligible to vest in up to 400,000 options. The vesting of the threshold number of 12.5% of the total number of options granted is subject to the sustained closing market price for our ordinary shares exceeding €8.90 per share during any 20 consecutive trading days outside a lock-up or blackout period. The number of options subject to vesting increases for every €1.00 increment in the sustained closing market price for our ordinary shares, with 12.5% of the total number of the options granted subject to vesting on the day immediately following a sustained closing market price of at least €8.90 per share and 100% subject to vesting on the day immediately following a sustained closing market price of at least €15.90 per share. Upon each subsequent achievement of a sustained, higher closing market price (in €1.00 increments), an additional number of options will be eligible to vest. Upon achievement of such sustained closing market price levels, 25% of the options vest on the day immediately following attainment of such pricing levels, and an additional 2.083% will vest at the end of each successive one-month period following the initial vesting date until the third anniversary of the initial vesting date. As of December 31st, 2013 none of these options are vested. The options may not be exercised unless the grantee continues to be an employee, officer or director of, or consultant or advisor to, us as of the date of exercise.
The Management Board determined that May 24, 2013, the date which Prosensa first publicly filed its offering prospectus with the Securities and Exchange Commission, is considered to be the date at which the IPO became probable. Therefore from May 24, 2013 onwards we recognize expenses over the estimated vesting period. Expenses of €624 thousand related to the December 5, 2012 grant were recognized during the year ended December 31, 2013. The total expense to be recognized is approximately €1.6 million, which will be recognized over the estimated vesting period.
Restricted shares
We have made, as part of our share-based compensation plan, awards entitling recipients to acquire Depositary Receipts (“Restricted Shares”) against payment of the nominal value of the underlying ordinary shares (the “Purchase Price”), subject to our right to repurchase all or part of such Depositary Receipts at the lower of the Purchase Price and the fair value of the ordinary shares during the four year vesting period if the participant ceases to perform services to us.
On October 10, 2013 the Board of the Foundation resolved to terminate the administration of the ordinary shares and to transfer the ordinary shares to the respective Depository Receipt holder. After the completion of the transfers, the Foundation will be dissolved. Going forward participants will be entitled to acquire ordinary shares instead of Depositary Receipts as Restricted Shares against payment of the nominal value of the underlying ordinary shares.
67
We have the right to repurchase 100% of the Restricted Shares awarded, and this right to repurchase shares will be reduced based on the following schedule: (a) 25% on a date of the year following the vesting commencement date no later than the anniversary of the grant, and (b) the remaining 75% in 36 equal monthly installments over a three year period following the first anniversary of the first vesting date.
The following table summarizes by grant date the number of Restricted Shares awarded between January 1, 2011 and December 31, 2013:
|
Grant date
|
Restricted
shares
awarded
|
Purchase price (€)
|
Per share
estimated fair value of
restricted share (€)
|
||||||||
|
March 22, 2011
|
78,500 | 0.01 | 1.00 | ||||||||
|
October 17, 2011
|
11,250 | 0.01 | 1.00 | ||||||||
|
December 5, 2012
|
115,000 | 0.01 | 0.70 | ||||||||
The fair value of the Restricted Share awards was measured based on the estimated fair value of the ordinary shares at the moment of purchase, which was €0.70 in 2012 and €1.00 in 2011, in line with the valuations of our ordinary shares. We recognize the corresponding compensation expense of those awards, net of estimated forfeitures, similar to the approach taken for option grants as indicated above, over the requisite service period, which is generally the vesting period of the respective award. We do not plan to pay dividends in the foreseeable future; therefore, dividend payments have not been considered in the valuation of the Restricted Share awards.
The fair value of all the 204,750 vested and unvested outstanding restricted shares as of December 31, 2013, of which 163,939 are vested and 40,812 are unvested, was approximately $1 million, or $0.8 million for the vested restricted shares and $0.2 million for the unvested restricted shares, in each case based on the fair value of our ordinary shares of $4.67 per share as of December 31, 2013.
Income taxes
We are subject to income taxes in the Netherlands and in the United States. Significant judgment is required in determining the use of net operating loss carry forwards and taxation of upfront and milestone payments for income tax purposes. There are many transactions and calculations for which the ultimate tax determination is uncertain. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the current and deferred income tax assets and liabilities in the period in which such determination is made.
No tax charge or income was recognized during the reporting periods since we are in a loss-making position and have a history of losses. We have tax loss carry-forwards of €58.7 million as of December 31, 2013. As a result of the Dutch income tax law, tax loss carry-forwards are subject to a time limitation of nine years. Deferred income tax assets are recognized for tax losses and other temporary differences to the extent that the realization of the related tax benefit through future taxable profits is probable. We recognize deferred tax assets arising from unused tax losses or tax credits only to the extent the relevant fiscal unity has sufficient taxable temporary differences or if there is convincing other evidence that sufficient taxable profit will be available against which the unused tax losses or unused tax credits can be utilized by the fiscal unity. Our judgment is that sufficient convincing other evidence is not available and a deferred tax asset is therefore not recognized.
In order to promote innovative technology development activities and investments in new technologies, a corporate income tax incentive has been introduced in Dutch tax law called the Innovation Box. For the qualifying profits, we effectively owe only 5% income tax, instead of the general tax rate of 25% which results in an estimated effective tax rate of 10%. The agreement with the tax authorities is currently signed for the years 2011 to 2015 but is expected to be extended.
Borrowings
From January 1, 2002 through December 31, 2013, we received the following loans from patient organizations and governmental bodies: €3,390,000 from Association Française contre les Myopathies (AFM), €739,000 from Agentschap NL, €500,000 from the Stichting Duchenne Parents Project, €387,000 from Charley’s Fund, €502,000 from Everest International Pte Ltd, €250,000 from Cure Duchenne, €163,000 from Aktion Benni & Co., €500,000 from Duchenne Children’s Trust and €65,000 from Villa Joep. These
68
arrangements are generally in the form of below-market interest rate loans that become due upon the meeting of certain milestones in product development, generally a minimum threshold of commercialization success or successful completion of certain clinical trials. Significant judgment is required in determining when these pre-determined milestones are met and may vary over time, implying a change in the value of the loans. The AFM agreement gives us the option to terminate the agreement if we undergo a change of control. If we exercise our option to terminate the agreement, we are required to repay the loan, together with interest. In the event that we abandon the program for reasons not justified by a scientific or medical reason, we are similarly required to repay the loan, together with interest. The AFM agreement also requires us to grant to AFM a non-exclusive royalty-free license to the intellectual property covered by the agreement in the event that we abandon the research funded by the agreement.
Recent accounting pronouncements
There are no IFRS standards as issued by the IASB or interpretations issued by the IFRS interpretations committee (e.g. IFRS 10, 11, 12, 13 and IAS 19) that are effective for the first time for the financial year beginning on or after January 1, 2013 that would be expected to have a material impact on our financial position.
JOBS Act exemptions
On April 5, 2012, the JOBS Act was signed into law. The JOBS Act contains provisions that, among other things, reduce certain reporting requirements for an “emerging growth company.” As an emerging growth company, we are electing to take advantage of the following exemptions:
|
·
|
not providing an auditor attestation report on our system of internal controls over financial reporting;
|
|
·
|
not providing all of the compensation disclosure that may be required of non-emerging growth public companies under the U.S. Dodd-Frank Wall Street Reform and Consumer Protection Act;
|
|
·
|
not disclosing certain executive compensation-related items such as the correlation between executive compensation and performance and comparisons of the Chief Executive Officer’s compensation to median employee compensation; and
|
|
·
|
not complying with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements (auditor discussion and analysis).
|
The JOBS Act permits an “emerging growth company” such as us to take advantage of an extended transition period to comply with new or revised accounting standards applicable to public companies. We are choosing to “opt out” of this provision and, as a result, we will comply with new or revised accounting standards as required when they are adopted. This decision to opt out of the extended transition period under the JOBS Act is irrevocable.
These exemptions will apply for a period of five years following the completion of our IPO or until we no longer meet the requirements of being an “emerging growth company,” whichever is earlier. We would cease to be an emerging growth company if we have more than $1.0 billion in annual revenue, have more than $700 million in market value of our ordinary shares held by non-affiliates or issue more than $1.0 billion of non-convertible debt over a three-year period.
Financial operations overview
Total revenue
Our revenues to date have consisted principally of license revenue and collaboration revenue. For 2013, 2012 and 2011, all of our license revenue and collaboration revenue was generated under the GSK Agreement.
|
·
|
License revenue. License revenue generally includes upfront payments and milestone payments. In 2009, we received a £16.0 million (€17.2 million) nonrefundable upfront payment from GSK in connection with our entry into the GSK Agreement. Until December 31, 2013 we recognized this payment ratably over five years, and in each year we recognized revenue of €3.4 million. In addition, we recognized revenue of €2.2 million (2012: €2.3 million, 2011: €3.1 million) related to unconditional milestone payments for research under the GSK Agreement.
|
69
|
·
|
Collaboration revenue. Collaboration revenue is revenue from contracts, typically for research and development activities under the GSK Agreement. In 2013 we recognized €3.3 million (2012: €2.1 million, 2011: €2.2 million) of collaboration revenues. In each period the revenues were mainly for services provided under the GSK Agreement related to the research and development of drisapersen and PRO044.
|
Our collaboration with GSK was mutually terminated in January 2014. The remaining license revenue balance (€14.5 million) deferred as of December 31, 2013 will be fully released in the three months period ended March 31, 2014.
The timing of our operating cash flows may vary significantly from the recognition of the related cash flows, as the revenue from some upfront or initiation payments is deferred and recognized as revenue when earned, while other revenue is earned when received, such as milestone payments or service fees. Our revenue has varied, and varies substantially from quarter to quarter and year to year, depending upon, among other things, the number of milestones achieved, the level of revenues earned for ongoing development efforts. Pursuant the termination of our collaboration with GSK, we do not expect any future license or collaboration revenue under the collaboration other than payments that had accrued as of the date of the termination. Any new collaboration arrangements we may enter into and the terms we are able to negotiate with our partners will impact our revenue for future periods. We therefore believe that period to period comparisons should not be relied upon as indicative of our future revenues.
Other income
Other income for the year ended December 31, 2013 amounts to €0.6 million (2012: €0.2 million; 2011: €0.04 million). We are part of two pan-European consortia. During 2013, each consortium has been awarded Framework Programme 7 (“FP7”) research grants from the European Commission. In August 2013 one consortium received a €6.0 million grant to support the ongoing clinical study PRO045 and in November 2013 the other received a €6.0 million grant for a separate collaborative bioimaging project to support our PRO053 and Natural History programs. We have also received governmental research subsidies. Grant proceeds are deferred and other income is recognized based on the percentage of completion method for an amount €0.5 million in the year ended December 31, 2013 (2012: nil; 2011: €0.04 million).
To encourage research and development we obtained certain loans that generally bear interest at a rate below the market interest rate, considered by the company to be 12% over the last five years. The difference between the fair value of the loans and the notional amount of the loans at inception is treated as a donation received for certain research performed by the company, which totaled €0.1 million during the year ended December 31, 2013 (2012: €0.2 million; 2011: nil). As of December 31, 2013 an amount of €0.1 million is deferred and will be recognized in Other income over the periods when expenses are incurred.
Cost of license revenue
We are obligated to make payments to LUMC upon the achievement of specified milestones, if any of our product candidates become successfully commercialized and if we generate certain revenues from sublicensing to a third party our rights under the agreement, as we historically have to GSK. For the years ended December 31, 2013, 2012 and 2011, we did not incur any costs associated with license revenue as we did not generate any applicable revenue.
Research and development expense
Research and development expense consists principally of:
|
·
|
salaries for research and development staff and related expenses, including social security costs;
|
|
·
|
costs for production of preclinical compounds and drug substances by contract manufacturers;
|
|
·
|
fees and other costs paid to contract research organizations in connection with additional preclinical testing and the performance of clinical trials;
|
|
·
|
costs of related facilities, materials and equipment;
|
|
·
|
costs associated with obtaining and maintaining patents and other intellectual property; and
|
70
|
·
|
amortization and depreciation of tangible and intangible fixed assets used to develop our product candidates.
|
Our research and development expense during the years ended December 31, 2013, 2012 and 2011 primarily related to the following key programs:
|
·
|
PRO044. In 2011, we and GSK agreed to amend the development plan for PRO044 to extend the period of GSK’s option to obtain an exclusive worldwide license for PRO044 and provide additional funding for further development activities for PRO044. However, we continued to incur expenses for the clinical study for PRO044 that we initiated in December 2009 and for which the final clinical study report which will be completed in 2014.
|
|
·
|
PRO045 & PRO053. We commenced a Phase I/II study of PRO045 and of PRO053. Our research and development expenses increased substantially in connection with these clinical trials.
|
|
·
|
Other development programs (DMD and Non-DMD projects). Other research and development expenses mainly relate to preclinical studies of PRO052 and PRO055, as well as our PROSPECT program and DM1 and HD programs. The expenses mainly consist of salaries, costs for production of the preclinical compounds and costs paid to contract research organizations in conjunction with preclinical testing.
|
Since January 1, 2009, we have cumulatively spent €70.5 million on research and development.
Our future research and development expense may vary substantially from the periods presented in this report. Under our historical collaboration agreement with GSK, GSK reimbursed us or paid directly for the cost of the clinical trials of drisapersen, as well as a substantial portion of the costs of the clinical trials of PRO044 and the natural history study and paid us milestone payments upon successful compound development. Following the mutual termination of the collaboration in January 2014, we will bear the full cost of any additional clinical trials of drisapersen and our other DMD product candidates. Subsequent to the announcement in September 2013 that the Phase III trial of drisapersen did not meet its primary endpoint, we initiated an evaluation of all data and patient groups across all DMD studies with drisapersen and have engaged with patient representatives, academic experts and regulators to understand the benefit-to-risk ratio of drisapersen therapy. The outcome of this evaluation will have a material impact on the further development of drisapersen and other DMD compounds. If the FDA or EMA were to require us to conduct additional studies of drisapersen in order to support an application for marketing approval or require us to conduct additional preclinical and clinical studies beyond those which we currently anticipate will be required to complete clinical development of our other DMD product candidates, we could be required to expend significant additional financial resources and time on the completion of the clinical development of drisapersen and the rest of our DMD portfolio. Depending on the outcome of our evaluation of drisapersen, our research and development expense may increase substantially. In addition, we expect that our research and development expense in relation to our non-DMD product candidates will increase as we further advance that portfolio.
The successful development of our product candidates is highly uncertain. At this time we cannot reasonably estimate the nature, timing and estimated costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from, any of our product candidates. This is due to numerous risks and uncertainties associated with developing drugs, including the uncertainty of:
|
·
|
the scope, rate of progress and expense of our research and development activities;
|
|
·
|
clinical trial and early-stage results;
|
|
·
|
the terms and timing of regulatory approvals;
|
|
·
|
the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights; and
|
|
·
|
the ability to market, commercialize and achieve market acceptance for drisapersen or any other product candidate that we may develop in the future.
|
A change in the outcome of any of these variables with respect to the development of drisapersen or any other product candidate that we may develop could mean a significant change in the costs and timing associated with the development of drisapersen or such product candidate.
71
General and administrative expense
Our general and administrative expense consists principally of:
|
·
|
salaries for employees other than research and development staff, as well as expenses related to share-based compensation awards granted to all of our employees;
|
|
·
|
professional fees for auditors and other consulting expenses not related to research and development activities;
|
|
·
|
professional fees for lawyers not related to the protection and maintenance of our intellectual property;
|
|
·
|
cost of facilities, communication and office expenses;
|
|
·
|
IT expenses; and
|
|
·
|
amortization and depreciation of tangible and intangible fixed assets not related to research and development activities.
|
Since our IPO in July 2013 we have incurred additional costs associated with operating as a public company. These public company-related expenses include costs of additional personnel, additional legal fees, accounting and audit fees, managing directors’ and supervisory directors’ liability insurance premiums and costs related to investor relations.
Finance income
Our cash and cash equivalents have been deposited primarily in saving and deposit accounts with original maturities of 3 months or less. Saving and deposit accounts generate a small amount of interest income. We expect to continue this investment philosophy.
Comparison of the year ended December 31, 2012 and 2013
|
Year ended December 31,
|
|||||||||||
|
2012
|
2013
|
Change
|
|||||||||
|
(€ in thousands)
|
%
|
||||||||||
|
License revenue
|
5,726 | 5,626 | (1.7 | ) | |||||||
|
Collaboration revenue
|
2,127 | 3,312 | 55.7 | ||||||||
|
Total revenue
|
7,853 | 8,938 | 13.8 | ||||||||
|
Other income
|
174 | 560 | 221.8 | ||||||||
|
Research and development expense
|
(14,393 | ) | (18,460 | ) | 28.3 | ||||||
|
General and administrative expense
|
(4,023 | ) | (7,734 | ) | 92.2 | ||||||
|
Other gains -net
|
49 | 112 | 128.6 | ||||||||
|
Operating loss
|
(10,340 | ) | (16,584 | ) | 60.4 | ||||||
|
Finance income
|
796 | 645 | (19.0 | ) | |||||||
|
Finance costs
|
(348 | ) | (665 | ) | 91.1 | ||||||
|
Finance income/(expense) – net
|
448 | (20 | ) | (104.5 | ) | ||||||
|
Other comprehensive income
|
– | – | – | ||||||||
|
Net loss
|
(9,892 | ) | (16,604 | ) | 67.9 | ||||||
License revenue
License revenue decreased from €5.7 million in 2012 to €5.6 million in 2013. In 2009, we received a £16.0 million (€17.2 million) nonrefundable upfront payment from GSK in connection with our entry into the GSK Agreement. We are recognizing this payment ratably over five years, and in each of 2013 and 2012, we recognized revenue of €3.4 million related to this agreement. In addition, we recognized revenue of €2.2 million and €2.3 million related to unconditional milestone payments received under the GSK Agreement in the years 2013 and 2012, respectively.
Collaboration revenue
Collaboration revenue increased from €2.1 million in 2012 to €3.3 million in 2013. This revenue was generated from services provided under the GSK Agreement and increased due to additional research and development activities on drisapersen and PRO044.
72
Other income
Other income increased from €0.2 million in 2012 to €0.6 million in 2013. During 2013 we were awarded research grants from the European Commission as well as other research subsidies. Grant proceeds are deferred and other income is recognized based on the percentage of completion method which totaled €0.5 million during the year ended December 31, 2013.
To encourage research and development we obtained certain loans that generally bear interest at a rate below the market interest rate, considered by the company to be 12% over the last five years. The difference between fair value of the loans and the notional amount of the loans at inception is treated as a donation received for certain research performed by the company and totaled €0.1 million for the year ended December 31, 2013 (2012: €0.2 million). As of December 31, 2013 €0.1 million is deferred and will be recognized in Other income over the periods during which expenses are incurred.
Research and development expense
|
Project expenses by project
|
2012
|
2013
|
Change
|
||||||||
|
(€ in thousands)
|
%
|
||||||||||
|
DMD Projects
|
|||||||||||
|
PRO044
|
1,438 | 1,168 | (18.8 | ) | |||||||
|
PRO045 and PRO053
|
3,283 | 4,850 | 47.7 | ||||||||
|
Other DMD projects
|
3,723 | 5,545 | 48.9 | ||||||||
|
Non-DMD projects
|
1,444 | 1,183 | (18.1 | ) | |||||||
|
Infrastructure costs
|
4,505 | 5,714 | 26.8 | ||||||||
|
Total
|
14,393 | 18,460 | 28.3 | ||||||||
Research and development expense increased from €14.4 million in 2012 to €18.5 million in 2013. Our research and development expense is highly dependent on the development phases of our projects and therefore fluctuates highly from year to year. The variances in expense between 2012 and 2013 are mainly due to the following projects:
|
·
|
DMD projects. While we incurred expenses for preclinical safety studies for PRO045 and PRO053 in 2012, our research and development expenses in 2013 mainly related to the ongoing phase I/II study of PRO045 and PRO053. In 2013 we also incurred expenses for the 3-months preclinical safety studies for PRO052, the Natural History and PROSPECT programs.
|
|
·
|
Non-DMD projects. The expenses for our non-DMD projects DM1 and HD mainly consist of outsourced in vivo proof-of-concept studies and the corresponding manufacturing of the preclinical compounds in both years.
|
|
·
|
Infrastructure costs. We incur a significant amount of costs associated with our research and development that are non-project specific, including intellectual property-related expenses, depreciation expenses and facility costs. Because these are less dependent on individual ongoing programs, they are not allocated to specific projects. These costs were higher in 2013 versus 2012 mainly due to intellectual property expense. We expect these costs to be in this range in 2014.
|
General and administrative expense
General and administrative expense increased substantially from €4.0 million in 2012 to €7.7 million in 2013. The increase was primarily related to costs associated with our IPO in the amount of €1.4 million, additional costs incurred for operating as a public company and non-cash share-based compensation charges. We expect that general and administrative expense will increase when our business expands.
Other gains - net
Other gains mainly related to foreign currency results on outstanding receivable balances in 2012 and 2013. Other gains were insignificant in 2012.
Finance income
Finance income decreased from €0.8 million in 2012 to €0.6 million in 2013 due to lower interest rates on cash deposits in 2013 offset by higher cash balances in 2013. Finance income in these years consisted primarily of interest income recognized on short-term deposits.
73
Finance cost
Finance cost increased from €0.3 million in 2012 to €0.7 million in 2013. Higher finance costs are mainly due to new borrowings from patient organizations and governmental bodies totaling €2.6 million received in December 2012 and during 2013.
Comparison of the year ended December 31, 2011 and 2012
|
Year ended December 31,
|
|||||||||||
|
2011
|
2012
|
Change
|
|||||||||
|
(€ in thousands)
|
%
|
||||||||||
|
License revenue
|
6,510 | 5,726 | (12.0 | ) | |||||||
|
Collaboration revenue
|
2,179 | 2,127 | (2.4 | ) | |||||||
|
Total revenue
|
8,689 | 7,853 | (9.6 | ) | |||||||
|
Other income
|
36 | 174 | 383.3 | ||||||||
|
Research and development expense
|
(15,348 | ) | (14,393 | ) | (6.2 | ) | |||||
|
General and administrative expense
|
(5,203 | ) | (4,023 | ) | (22.7 | ) | |||||
|
Other gains -net
|
22 | 49 | 122.7 | ||||||||
|
Operating loss
|
(11,804 | ) | (10,340 | ) | (12.4 | ) | |||||
|
Finance income
|
434 | 796 | 83.4 | ||||||||
|
Finance costs
|
(209 | ) | (348 | ) | 66.5 | ||||||
|
Finance income/(expense) – net
|
225 | 448 | 99.1 | ||||||||
|
Other comprehensive income
|
– | – | – | ||||||||
|
Net loss
|
(11,579 | ) | (9,892 | ) | (14.6 | ) | |||||
License revenue
License revenue decreased from €6.5 million in 2011 to €5.7 million in 2012. In 2009, we received a £16.0 million (€17.2 million) nonrefundable upfront payment from GSK in connection with our entry into the GSK Agreement. We are recognizing this payment ratably over five years, and in each of 2012 and 2011, we recognized revenue of €3.4 million. In addition, we recognized revenue of €2.3 million and €3.1 million related to unconditional milestone payments received under the GSK Agreement during the years ended December 31, 2012 and 2011, respectively. With respect to the year ended December 31, 2012, the unconditional payments received under the GSK Agreement related to the clinical candidate selection of PRO052 and PRO055, as well as an advanced option payment for PRO044. With respect to the year ended December 31, 2011, unconditional milestone payments received under the GSK Agreement related to PRO045 and PRO053.
Collaboration revenue
Collaboration revenue decreased slightly from €2.2 million in 2011 to €2.1 million in 2012. This revenue was generated mainly from services provided under the GSK Agreement related to the research and development of drisapersen and PRO044.
Other income
Other income is incidental by nature. In 2012, we received a loan from a patient organization bearing an interest rate below the market interest rate. The difference between fair value of the loan and the notional amount of the loan at inception was considered a donation and recorded as other income. In 2011, we received a grant that was recorded as other income.
74
Research and development expense
|
Project expenses by project
|
2011
|
2012
|
Change
|
||||||||
|
(€ in thousands)
|
%
|
||||||||||
|
DMD Projects
|
|||||||||||
|
PRO044
|
1,699 | 1,438 | (15.4 | ) | |||||||
|
PRO045 and PRO053
|
4,745 | 3,283 | (30.8 | ) | |||||||
|
Other DMD projects
|
3,128 | 3,723 | 19.0 | ||||||||
|
Non-DMD projects
|
1,214 | 1,444 | 18.9 | ||||||||
|
Infrastructure costs
|
4,562 | 4,505 | (1.2 | ) | |||||||
|
Total
|
15,348 | 14,393 | (6.2 | ) | |||||||
Research and development expense decreased from €15.3 million in 2011 to €14.4 million in 2012. Our research and development expense is highly dependent on the development phases of our research projects and therefore fluctuates highly from year to year.
The variances in expense between 2011 and 2012 are mainly due to the following projects:
|
·
|
DMD projects. In 2011 and 2012, we incurred clinical costs and costs related to the in-house analyses of drug substance and drug product for PRO044. We incurred expenses for non-clinical safety work and manufacturing for PRO045 and PRO053 in 2011 and 2012.
|
|
·
|
Non-DMD projects. The expenses for our non-DMD projects for DM1 and HD mainly consist of outsourced in vivo proof-of-concept studies and the corresponding manufacture of the preclinical compounds.
|
|
·
|
Infrastructure costs. We incur a significant amount of costs associated with our research and development that are non-project specific, including intellectual property-related expenses, depreciation expenses and facility costs. Because these are less dependent on individual ongoing programs, they are not allocated to specific projects. These costs remained stable over the years 2011 and 2012.
|
General and administrative expense
General and administrative expense decreased from €5.2 million in 2011 to €4.0 million in 2012. The decrease was primarily related to legal fees related to financing activities, non-cash share-based compensation charges and the move to larger facilities in Leiden during 2011.
Other gains/(losses)
Other gains/(losses) were insignificant in 2011 and 2012.
Finance income
Finance income increased from €0.4 million in 2011 to €0.8 million in 2012. Finance income in these periods consisted primarily of interest income recognized on short-term deposits. Finance income increased in 2012 as a result of an increase in average cash and cash equivalents following our private placement of preferred equity securities to new and existing investors with total net proceeds of €22.7 million in January, 2012.
Finance cost
Finance cost increased from €0.2 million in 2011 to €0.3 million in 2012. Higher finance costs are mainly due to new borrowings from patient organizations and governmental bodies of €3.9 million in 2012.
B. Liquidity and capital resources
To date, we have financed our operations through our IPO, private placements of our equity securities, upfront, milestone and expense reimbursement payments from GSK and funding from patient organizations, governmental bodies and bank loans.
75
Cash flows
Comparison of the year ended December 31, 2012 and 2013
Our cash and cash equivalents as of December 31, 2013 were €82.2 million. The table below summarizes our statement of cash flows for the years ended December 31, 2012 and 2013:
|
Year ended
December 31,
|
||||||||
|
2012
|
2013
|
|||||||
|
(€ in thousands)
|
||||||||
|
Net cash (used in)/generated from operating activities
|
(3,725 | ) | (21,968 | ) | ||||
|
Net cash (used in)/generated from investing activities
|
(327 | ) | (426 | ) | ||||
|
Net cash (used in)/generated from financing activities
|
25,977 | 63,877 | ||||||
|
Net increase (decrease) in cash and cash equivalents
|
21,925 | 41,483 | ||||||
|
Currency effect cash and cash equivalents
|
70 | 11 | ||||||
|
Cash, cash equivalents and bank overdrafts at the beginning of the period
|
18,743 | 40,738 | ||||||
|
Cash, cash equivalents and bank overdrafts at the end of the period
|
40,738 | 82,232 | ||||||
The net cash used in operating activities of €3.7 million in the year ended December 31, 2012 increased to net cash used in operating activities of €22.0 million in 2013 due to higher collection of milestone payments in 2012 and a higher operating loss of €6.2 million in 2013. For an explanation of the higher operating loss of €6.2 million, please see “Item 5. Operating and Financial Review and Prospects—A. Operating Results”.
The net cash used in investing activities increased from €0.3 million in the year ended December 31, 2012 to €0.4 million in the year ended December 31, 2013 due to higher investments in tangible fixed assets.
The increase in net cash generated from financing activities from €26.0 million in the year ended December 31, 2012 to €63.9 million in the year ended December 31, 2013 is primarily attributable to the net proceeds of the share issuance in 2012 for an amount of €22.7 million compared to the net proceeds of €63.2 million received from our IPO in July 2013 offset by a decrease of €2.8 million in borrowings received.
Comparison of the year ended December 31, 2011 and 2012
The table below summarizes our statement of cash flows for the years ended December 31, 2012 and 2011:
|
Year ended
December 31,
|
||||||||
|
2011
|
2012
|
|||||||
|
(€ in thousands)
|
||||||||
|
Net cash (used in)/generated from operating activities
|
(7,291 | ) | (3,725 | ) | ||||
|
Net cash (used in)/generated from investing activities
|
(1,651 | ) | (327 | ) | ||||
|
Net cash (used in)/generated from financing activities
|
(102 | ) | 25,977 | |||||
|
Net increase (decrease) in cash and cash equivalents
|
(9,044 | ) | 21,925 | |||||
|
Currency effect cash and cash equivalents
|
- | 70 | ||||||
|
Cash, cash equivalents and bank overdrafts at the beginning of the period
|
27,787 | 18,743 | ||||||
|
Cash, cash equivalents and bank overdrafts at the end of the period
|
18,743 | 40,738 | ||||||
The decrease in net cash used in operating activities from €7.3 million in 2011 to €3.7 million in 2012 was mainly due to a lower operating loss of €1.5 million and the receipt of milestone payments that were €6.6 million higher in 2012 compared to 2011, offset by changes in the working capital balances in 2012 as compared to 2011 in the amount of €5.1 million. For an explanation of the lower operating loss of €1.5 million, please see “Item 5. Operating and Financial Review and Prospects—A. Operating Results”.
The decrease in net cash used in investing activities from €1.7 million in 2011 to €0.3 million in 2012 was due to our move to a larger facility in Leiden in 2011, which resulted in relatively higher investments in laboratory and office equipment in that year.
76
The decrease in net cash used in financing activities from €0.1 million in 2011 to net cash generated from financing activities of €26.0 million in 2012 is mainly due to our private placements of 9,107,144 preferred (Class B) shares to new and existing investors for total net proceeds of €22.7 million in January 2012. In addition, in 2012 we received funding from patient organizations and governmental bodies totaling €3.9 million, offset by the repayment of finance leases in the amount of €0.4 million.
Funding requirements
Our funding requirements may vary substantially from the periods presented in this report. Under our historical collaboration with GSK, GSK reimbursed us for the cost of the clinical trials of drisapersen, as well as a substantial portion of the costs of the clinical trials of PRO044 and the natural history study, and paid us milestone payments upon successful compound development. Following the mutual termination of the collaboration in January 2014, we will bear the full cost of any additional clinical trials of drisapersen and our other DMD product candidates and will receive no future payments under the collaboration other than those accrued on the date of the termination. Furthermore, subsequent to the announcement in September 2013 that the Phase III trial of drisapersen did not meet its primary endpoint, we initiated an evaluation of all data and patient groups across all DMD studies with drisapersen and have engaged with patient representatives, academic experts and regulators to understand the benefit-to-risk ratio of drisapersen therapy. The outcome of this evaluation will have a material impact on the further development of drisapersen and other DMD compounds. If the FDA or EMA were to require us to conduct additional studies of drisapersen in order to support an application for marketing approval or require us to conduct additional preclinical and clinical studies beyond those which we currently anticipate will be required to complete clinical development of our other DMD product candidates, we could be required to expend significant additional financial resources and time on the completion of the clinical development of drisapersen and the rest of our DMD portfolio. Depending on the outcome of our evaluation of drisapersen, our research and development expense may increase substantially. In addition, we expect that our research and development expense in relation to our non-DMD product candidates will increase as we further advance that portfolio.
We believe that our existing cash and cash equivalents and research funding that we expect to receive will be sufficient to fund our operating expenses, debt service obligations and capital expenditure requirements for at least the next 12 months. We have based this estimate on assumptions that may prove to be wrong, and we could use our capital resources sooner than we currently expect.
Our present and future funding requirements will depend on many factors, including, among other things:
|
·
|
the progress, timing and completion of preclinical testing and clinical trials for any current or future compounds, including our DMD compounds;
|
|
·
|
the number of potential new compounds we identify and decide to develop;
|
|
·
|
the costs involved in filing patent applications and maintaining and enforcing patents or defending against claims or infringements raised by third parties;
|
|
·
|
the time and costs involved in obtaining regulatory approval for our compounds and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of these compounds;
|
|
·
|
selling and marketing activities undertaken in connection with the anticipated commercialization of our DMD compounds and any other current or future compounds and costs involved in the creation of an effective sales and marketing organization; and
|
|
·
|
the amount of revenues, if any, we may derive either directly or in the form of royalty payments from future sales of our products.
|
For more information as to the risks associated with our future funding needs, see “Item 3. Key Information—D. Risk Factors.”
Capital Expenditures
The following table sets forth our capital expenditures for the years ended December 31, 2013, 2012 and 2011.
77
|
Year ended December 31,
|
||||||||||||
|
2011
|
2012
|
2013
|
||||||||||
|
Investments in tangible fixed assets
|
1,446 | 182 | 605 | |||||||||
|
Investments in intangible assets
|
158 | 198 | 121 | |||||||||
|
Total
|
1,604 | 380 | 726 | |||||||||
During 2011, we moved to larger facilities, which resulted in additional investments in laboratory and office equipment. For the year ended December 31, 2013, we invested €0.7 million in tangible and intangible fixed assets, including €57 thousand in tangible assets not paid for at December 31, 2013. We also lease various laboratory and office equipment under non-cancellable finance lease agreements. In 2011, 2012 and 2013 we recorded non-cash increases to our property and equipment related to assets acquired under finance leases of €560 thousand, €52 thousand and €66 thousand respectively. Our investments were made primarily in the Netherlands.
Also in 2014 we plan to make investments to enhance our research and development capacity. We anticipate that they will be funded from existing cash balances.
C. Research and development, patents and licenses, etc.
See “Item 4. Information on the Company—A. History and Development of the Company” and “Item 4. Information on the Company—B. Business Overview.”
D. Trend information
See “Item 5. Operating and Financial Review and Prospects.”
E. Off-balance sheet arrangements
As of the date of this Annual Report, we do not have any, and during the periods presented we did not have any, off-balance sheet arrangements.
F. Tabular disclosure of contractual obligations
The table below sets forth our contractual obligations and commercial commitments as of December 31, 2013 that are expected to have an impact on liquidity and cash flow in future periods.
|
Payments due by period
|
||||||||||||||||||||
|
Less than
1 year
|
Between 1
and 2 years
|
Between 2
and 5 years
|
More than
5 years
|
Total
|
||||||||||||||||
|
(€ in thousands)
|
||||||||||||||||||||
|
Debt obligations (1)(2)
|
100 | 100 | 200 | 7,330 | 7,730 | |||||||||||||||
|
Operating lease obligations (3)
|
798 | 782 | 533 | - | 2,113 | |||||||||||||||
|
Finance lease obligations (4)
|
91 | - | - | - | 91 | |||||||||||||||
|
Total
|
989 | 882 | 733 | 7,330 | 9,934 | |||||||||||||||
|
(1)
|
Debt obligations consist of bank borrowings and loans from patient organizations and governmental bodies. Most loans from patient organizations and governmental bodies have no fixed repayment scheme, but repayment (including incurred interest) is due when certain pre-determined milestones are met. See “Item 5. Operating and Financial Review and Prospects—A. Operating results—Critical accounting policies and significant judgments and estimates—Borrowings.” Because we cannot predict what portion will be due in less than five years, we have classified these repayments in the category “More than 5 years.”
|
|
(2)
|
As disclosed in our consolidated financial statements, certain loan agreements include additional payments due after we reach a predefined commercial sales milestone. These conditional payments total a maximum of approximately €5.0 million if all such commercial sales milestones are exceeded. These amounts do not include conditional future interest payable.
|
|
(3)
|
Operating lease obligations consist of payments pursuant to lease agreements for office and laboratory facilities, which terms will expire before the third quarter of 2016, as well as lease agreements for office furniture, which will expire between March 2014 and January 2016.
|
|
(4)
|
Finance lease obligations consist of payments to lessors of laboratory equipment and software. Finance lease liabilities are secured on the assets held under lease, which revert to the lessor in the event of default.
|
We enter into contracts in the normal course of business with CROs for clinical trials and clinical supply manufacturing and with vendors for safety and research studies, research supplies and other services and products for operating purposes. These contracts generally provide for termination on notice, and therefore are cancelable contracts and not included in the table of contractual obligations and commitments.
78
In 2006, we received a bank loan of €900,000 from ABN Amro N.V. that matures in 2017. The loan bears interest equal to Euribor plus 1.75% per year. We repay an amount of €25,000 per quarter, and €400,000 was outstanding under this agreement as of December 31, 2013. We also have an undrawn revolving facility with €208 thousand of availability as of December 31, 2013. The amount available to us under the credit facility decreases by €46 thousand per year. The security for the loan and the credit facility consists of all cash and cash equivalents that are deposited with ABN Amro, including a minimum of €200,000 (2012 and 2011: €500,000) that must be maintained at all times in an ABN Amro bank account.
G. Safe harbor
See “Forward Looking Statements.”
ITEM 6. DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES
A. Directors and senior management
We have a two-tier board structure consisting of our supervisory board (raad van commissarissen) and a separate management board (raad van bestuur).
Our supervisory board supervises the policies of the management board and the general course of the affairs of our business. The supervisory board gives advice to the management board and is guided by the interests of the business when performing its duties. The management board is in charge of managing us under the supervision of the supervisory board. The management board provides the supervisory board with such necessary information as the supervisory board requires to perform its duties.
The following table presents the supervisory directors appointed by the general meeting of shareholders on June 14, 2013 as well as the supervisory director, Dr. Georges Gemayel, appointed by the extraordinary general meeting of shareholders on January 23, 2014. Rémi Droller resigned per March 12, 2014. David Mott is the chairman of our supervisory board. The term of each of our supervisory directors will terminate on the date of the annual general meeting of shareholders in the year indicated below.
|
Name
|
Age
|
Term
|
|
Rémi Droller
|
38
|
2014
|
|
Daan Ellens
|
65
|
2015
|
|
Peter Goodfellow
|
63
|
2015
|
|
Georges Gemayel
|
53
|
2016
|
|
Martijn Kleijwegt
|
59
|
2016
|
|
David Mott
|
49
|
2016
|
|
Patrick Van Beneden
|
52
|
2014
|
The following is a brief summary of the business experience of our supervisory directors. Unless otherwise indicated, the current business address for each of our supervisory directors is J.H. Oortweg 21, 2333 CH Leiden, the Netherlands.
Rémi Droller served on our supervisory board since 2008 until March 12, 2014 (effective date of his resignation). Mr. Droller is a managing partner at Kurma Life Sciences Partners, which he joined in September 2010. Mr. Droller was previously with CDC Innovation from 2000 to 2003 and with AGF Private Equity SA (now Idinvest Partners) from 2003 to 2010 where he was in charge of the development of the life sciences investment activity. During this period, Mr. Droller served on the board of several investee companies such as Adocia S.A.S., AM Pharma Holding B.V., BioAlliance Pharma S.A., Domain Therapeutics S.A., IntegraGen S.A., Novagali Pharma S.A., STAT Diagnostica S.L. and Zealand Pharma A.V. Mr. Droller holds a master’s degree in molecular biology from Paris VI University and a master’s degree in finance and innovation management from Masternova – AgroParisTech. Mr. Droller was nominated to serve on our board by Idinvest Partners, one of our shareholders.
Daan Ellens has served on the board since 2007. Dr. Ellens also serves as chairman of the supervisory board of Hybrigenics SA and Zealand Pharma A/S. Prior to these positions, Dr. Ellens was Chief Executive Officer of Rhein Biotech N.V. from 1994 to 2002. Dr. Ellens holds a Ph.D. degree in molecular biology from the University of Utrecht in the Netherlands and a M.B.A. degree from the University Eindhoven, the Netherlands. Dr. Ellens’ extensive and successful track record as one of the most successful European biotechnology entrepreneurs adds significant value to our team. We believe that Dr. Ellens is qualified to serve on our supervisory board because of his career in the pharmaceutical industry, including his extensive experience as an entrepreneur and executive and his service on the boards of directors of other biopharmaceutical companies.
79
Georges Gemayel has served on the board since 2014. Dr. Gemayel is a member of the boards of Orphazyme, EpiTherapeutics, the International Institute of New England, and OxThera, as well as the Executive Chairman of the board of Vascular Magnetics Inc., USA. Dr. Gemayel also sits on the board of directors for NPS Pharmaceuticals, where he serves on the audit committee. Previously, Dr. Gemayel served as the Executive Chairman of the Board of Directors of FoldRX, as President and CEO of Altus Pharmaceuticals, and as Executive Vice President of Genzyme Corporation. Dr. Gemayel started his career in pharma at Hoffman-La Roche after graduating with a PhD and a DEA in pharmacology from Université Paris Sud (Paris XI) in 1987. Dr. Gemayel also holds a Doctorat d’Exercice in pharmacy from Universite St. Joseph. We believe that Dr. Gemayel is qualified to serve on our supervisory board because of his experience as a board member and executive officer of other pharmaceutical companies.
Peter Goodfellow has served on our supervisory board since 2008 and is also a member of our scientific advisory board. Dr. Goodfellow was formerly Senior Vice President for Discovery Research at GlaxoSmithKline plc from 1998 to 2006. In addition, Dr. Goodfellow serves as a director of the Institute of Cancer Research and as a member of several advisory boards, including the Scientific Advisory Board of the Institute of Molecular and Cell Biology, the Gates Foundation Discovery Board and Sanofi’s Strategic Development and Scientific Advisory Council. He has also held the Balfour chair in genetics at Cambridge University and research positions at the Imperial Cancer Research Fund. Dr. Goodfellow holds bachelor’s and doctorate degrees from the University of Bristol and a doctorate degree from Oxford University. We believe that Dr. Goodfellow is qualified to serve on our supervisory board because of his career in the pharmaceutical industry, including his extensive experience as an executive and service on the boards of directors of other biopharmaceutical companies.
Martijn Kleijwegt has served on our supervisory board since 2008. Mr. Kleijwegt founded Life Sciences Partners in 1998 and has been managing partner of Life Sciences Partners since then. Mr. Kleijwegt has served on the boards of a number of companies including Qiagen N.V., Rhein Biotech N.V., Crucell N.V., Movetis N.V. and Pronota N.V. Mr. Kleijwegt graduated from the University of Amsterdam with a degree in economics. Mr. Kleijwegt was nominated to serve on our board by Life Sciences Partners, one of our shareholders. We believe that Mr. Kleijwegt is qualified to serve on our supervisory board because of his experience in the biopharmaceutical industry as a venture capital investor, a founder of Life Sciences Partners and a member of the boards of directors of other biopharmaceutical companies.
David Mott has served on our supervisory board since 2012 and is the chairman of our supervisory board Mr. Mott has served as a general partner of New Enterprise Associates, an investment firm focused on venture capital and growth equity investments, since September 2008, where he leads the healthcare investing practice. Prior to joining NEA, Mr. Mott was President and Chief Executive Officer of MedImmune LLC, subsidiary of AstraZeneca plc, and Executive Vice President of AstraZeneca plc. From 1992 to 2008, Mr. Mott worked at MedImmune Limited and served in numerous roles during his tenure including Chief Operating Officer, Chief Financial Officer, President and Chief Executive Officer. Prior to joining MedImmune, Mr. Mott was a Vice President in the Health Care Investment Banking Group at Smith Barney, Harris Upham & Co. Inc. Mr. Mott is currently Chairman of 3-V Biosciences, Inc., Mersana Theraputics (acting CEO and Chairman), Tesaro, Inc. (NASDAQ: TSRO), and Zyngenia, Inc. and is a director of Ardelyx, Inc., Ediner Pharmaceuticals,Inc. and Epizyme, Inc. (NASDAQ: EPZM). Mr. Mott also serves on the governing board of St. Albans School. Mr. Mott received a bachelor of arts degree from Dartmouth College. Mr. Mott was nominated to serve on our board by New Enterprise Associates, one of our shareholders. We believe that Mr. Mott is qualified to serve on our supervisory board because of his experience as a venture capital investor, his experience in the pharmaceutical industry and his service on the boards of directors of other biopharmaceutical companies.
Patrick Van Beneden was appointed to our supervisory board on June 14, 2013. He has built a track record in life sciences, both in early and late stage investments and successful exits. Mr. Van Beneden joined Gimv in 1985. He is a Director of ActoGeniX, JenaValve, Complix, AgroSavfe, Biotech Fonds Vlaanderen, and Gimv Agri+. Mr. Van Beneden also serves as a member of the Advisory Board of Oxford BioScience Partners and Sofinnova SA. His former board seats include Innogenetics, Astex, Ablynx, Crucell, Avalon, Hypnion, Endosense, Devgen and Crop Design. He holds a degree in Financial Sciences from Vlekho, Brussels. Mr. Van Beneden was nominated to serve on our board by Gimv N.V., one of our shareholders. We believe that Mr. Van Beneden is qualified to serve on our supervisory board because of his experience as a venture capital investor, his experience in the pharmaceutical industry and his service on the boards of directors of other biopharmaceutical companies.
80
The following table lists the members of our current management board:
|
Name
|
Age
|
Position
|
|
Hans Schikan
|
55
|
Chief Executive Officer
|
|
Berndt Modig
|
55
|
Chief Financial Officer
|
|
Giles Campion
|
59
|
Chief Medical Officer and Senior Vice President R&D
|
|
Luc Dochez
|
39
|
Chief Business Officer and Senior Vice President Business Development
|
The following is a brief summary of the business experience of the members of our management board. Unless otherwise indicated, the current business addresses for the members of our management board is J.H. Oortweg 21, 2333 CH Leiden, the Netherlands.
Hans Schikan has served as our Chief Executive Officer since January 2009. Mr. Schikan has more than 25 years of senior managerial experience in the pharmaceutical and biotechnology industries. From May 2004 until January 2009, Mr. Schikan worked at Genzyme Corporation in various executive roles, including Vice President of Global Marketing and Strategic Development for its rare genetic disease franchise. In this position he oversaw the launch of various orphan drugs globally. Prior to Genzyme, Mr. Schikan worked at Organon International from 1986 to 2004 in various senior business roles across different geographies, including managing director positions in multiple countries. Mr. Schikan also serves as Non-executive Director of Swedish Orphan Biovitrum A.B., Executive Board Member of the Dutch Top Institute Pharma, and Member of the Core Team of the Dutch Top Sector Life Sciences & Health. He is also past Chairman of Nefarma, the Dutch Association of Research Based Pharmaceutical Industry. Mr. Schikan holds a Pharm.D. degree from the University of Utrecht, the Netherlands.
Giles Campion has served as our Chief Medical Officer and Senior Vice-President of Research and Development since May 2009. Board certified in rheumatology, Dr. Campion has more than twenty years of experience in the pharmaceutical and biotechnology industries and is an expert in translational medicine. From 2005 to 2008, Dr. Campion was head of global medicine, GE Healthcare. Dr. Campion has held posts of increasing seniority in both large pharmaceutical and biotechnology companies working in Europe and the United States, covering many different therapeutic areas. Dr. Campion holds a bachelor’s and doctorate degree in medicine from the University of Bristol.
Luc Dochez has served as our Chief Business Officer and Senior Vice-President of Business Development since November 2008. Mr. Dochez has over 15 years of experience in the biotechnology industry. Before joining Prosensa, Mr. Dochez was a consultant within Arthur D. Little’s biotechnology practice from 1998 to 2001, Director of Business Development at Methexis Genomics N.V. from 2001 to 2002, Vice President of Business Development at TiGenix N.V. from 2002 to 2008 and President of TiGenix Inc. from 2007 to 2008. Mr. Dochez holds a Pharm.D. degree from the University of Leuven, a postgraduate degree in business economics from the same university and a M.B.A. degree from Vlerick Management School. Mr. Dochez is currently an independent supervisory board member at Ovizio S.A.
Berndt Modig has served as our Chief Financial Officer since March 2010. Mr. Modig has more than twenty-five years of international experience in finance and operations, private equity and mergers and acquisitions. Before joining Prosensa, Mr. Modig was Chief Financial Officer at Jerini AG from October 2003 to November 2008, where he directed private financing rounds, its initial public offering in 2005 and its acquisition by Shire plc in 2008. Prior to Jerini, Mr. Modig served as Chief Financial Officer at Surplex AG from 2001 to 2003 and as Finance Director Europe of U.S.-based Hayward Industrial Products Inc. from 1999 to 2001. In previous positions, Mr. Modig was a partner in the Brussels-based private equity firm Agra Industria from 1994 to 1999 and a Senior Manager in the Financial Services Industry Group of Price Waterhouse LLP in New York from 1991 to 1994. Mr. Modig served as a director of Mobile Loyalty plc from 2012 to 2013. Mr. Modig has a bachelor’s degree in business administration, economics and German from the University of Lund, Sweden and a M.B.A. degree from INSEAD, France and is a Certified Public Accountant.
81
B. Compensation
Management services agreements
There are management services agreements with each of our managing directors. The management services agreements contain a termination notice period of three months for the managing director and six months for us. All of the management services agreements provide that the managing director may be terminated in the event of an urgent reason (dringende reden) at any time, without advance notice. Each management services agreement provides for severance pay upon (i) a termination of the managing director’s employment without cause, (ii) the resignation of the managing director within 12 months following a change in control event or (iii) or at the end of their term of appointment, unless the managing director is re-appointed. For termination without cause, this severance pay is calculated based on that managing director’s annual remuneration. The management services agreements for Giles Campion, Luc Dochez and Berndt Modig provide for severance pay upon a termination of the managing director’s employment by us without cause in the amount of 50% of their annual remuneration. The management services agreement for Hans Schikan provides for severance pay upon termination of his employment by us without cause in the amount of 100% of his annual remuneration. Each management services agreement provides for a lump-sum payment following a change in control subject to certain conditions. That payment is currently equal to 130% of the individual’s annual gross fixed salary in effect at the time of the change in control for Giles Campion, Luc Dochez and Berndt Modig and 150% for Hans Schikan. In the event of a disability of a managing director, we will continue to pay that managing director’s salary for an extended period up to 104 weeks. This obligation will survive a termination of the management service agreement. The management services agreements contain post-termination restrictive covenants, including post-termination non-competition and non-solicitation covenants. These covenants typically last for a period of at least six months post-termination.
Long-term incentive plans
Share incentive plans
Pursuant to each of the 2004 Employee Stock Option Plan, the 2006 Employee Stock Option Plan, the 2007 Employee Stock Option Plan and the 2010 Equity Incentive Plan (collectively, the “Incentive Plans”), certain participants were granted the right to acquire (non-voting) depositary receipts (“Depositary Receipts”) issued in respect of our ordinary shares and/or cash settled instruments the value of which is linked to our ordinary shares (the “Awards”).
Upon the exercise or award or vesting of a non-cash-settled Award under the Incentive Plans, ordinary shares were issued to a Dutch foundation called Stichting Administratiekantoor Prosensa Holding (the “Foundation”), whose purpose was to facilitate administration of share-based compensation awards and pool the voting interests of the underlying shares. The Foundation thereupon granted a Depository Receipt for each issued ordinary share to the person entitled to such ordinary share under an Award. On October 10, 2013 the Board of the Foundation resolved to terminate the administration of the ordinary shares and to transfer the ordinary shares to the respective Depository Receipt holder. After the completion of the transfers, the Foundation will be dissolved. Going forward participants will receive ordinary shares upon the exercise or award or vesting of a non-cash-settled award under the Incentive Plans.
2010 Equity Incentive Plan
In 2010 we established the 2010 Equity Incentive Plan (“the 2010 Plan”) with the purpose of advancing the interests of our shareholders by enhancing our ability to attract, retain and motivate individuals who are expected to make important contributions to us and by providing those persons with performance-based incentives that are intended to better align their interests with those of our shareholders.
The outstanding Awards under the 2010 Plan cover up to 4,080,276 awards and the 2010 Plan permits grants of Awards covering up to 4% of our outstanding ordinary shares each year from the date of the annual general meeting of shareholders until the date of the subsequent annual general meeting of shareholders.
Plan Administration. The 2010 Plan is administered by our management board. Approval of the supervisory board is required for all aggregate and significant individual grants of Awards under the 2010 Plan and the terms and conditions of exercise, vesting, purchase or repurchase in relation thereto.
Eligibility. All of our employees, managing directors and supervisory directors, as well as consultants, advisors and members of advisory committees are eligible to be granted Awards.
82
Awards. Awards include Options, SARs, Restricted Shares, Restricted Stock Units and Other Stock-Based Awards (each as defined below).
Share Options
Subject to the approval of the supervisory board, the management board may grant options to purchase ordinary shares (“Options”) and determine the number of shares to be covered by each Option, the exercise price of each Option and the conditions and limitations applicable to the exercise of each Option. We grant Options with only service-based vesting conditions or with service-based vesting conditions combined with vesting conditions subject to a liquidity event. With respect to Options with only service-based vesting conditions, 25% of the Options vest on the first anniversary of the grant date, and the remaining 75% vest in 36 equal monthly installments over a three year period following the first anniversary of the date of grant subject to continued employment of the relevant participant. With respect to Options with service-based vesting conditions combined with vesting conditions subject to our IPO, 25% of the Options vest upon specified price thresholds of our ordinary shares, and 2.0833% vest monthly thereafter subject to the continued employment of the relevant participant.
The exercise price is specified in the applicable Option agreement and may be less than, equal to, or greater than the fair market value per ordinary share on the date the Option is granted. The fair market value per ordinary share is based on the quoted market price of our ordinary shares. Each Option is exercisable at such times and subject to such terms and conditions as the management board may specify in the applicable Option agreement with a maximum term of ten years.
Stock Appreciation Rights
Subject to the approval of the supervisory board, the management board may grant Awards consisting of Stock Appreciation Rights (“SARs”) entitling the holder, upon exercise, to receive an amount of Depositary Receipts or cash or a combination thereof determined by reference to appreciation, from and after the date of grant, in the fair market value of an ordinary share over the measurement price established by the management board. The management board establishes the measurement price of each SAR and specifies it in the applicable SAR agreement pursuant to an applicable formula. The measurement price may not be less than 100% of the fair market value on the date the SAR is granted. Each SAR is exercisable at such times and subject to such terms and conditions as the management board may specify in the applicable SAR agreement with a maximum term of ten years. We have never issued SARs.
Restricted Shares
Subject to the approval of the supervisory board, the management board may grant Awards entitling the holder to acquire Depositary Receipts (“Restricted Shares”), subject to our right to repurchase all or part of such Depositary Receipts at their issue price or other stated or formula price from the holder in the event that conditions specified by the management board in the applicable Award agreement are not satisfied prior to the end of the applicable restriction period or periods established by the management board for such Award. The management board determines the terms and conditions of a Restricted Share.
Restricted Stock Units
Subject to the approval of the supervisory board, the management board may grant Awards entitling the holder to receive ordinary shares or cash to be delivered at vesting (“Restricted Stock Units”). The management board determines the terms and conditions of a Restricted Stock Unit. Holders of Restricted Stock Units may be entitled to dividend equivalents. We have never issued Restricted Stock Units.
Other Stock-Based Awards
Other Awards of Depositary Receipts, and other Awards that are valued in whole or in part by reference to, or are otherwise based on, Depositary Receipts or other property, may be granted to participants (“Other Stock-Based Awards”). Such Other Stock-Based Awards are also available as a form of payment in the settlement of other Awards granted under the 2010 Plan or as payment in lieu of compensation to which a participant is otherwise entitled. Other Stock-Based Awards may be paid in Depositary Receipts or cash, as the management board determines. The management board determines the terms and conditions of each Other Stock-Based Award, including the conditions for vesting and repurchase and the issue price, if any. We have never issued Other Stock-Based Awards.
83
Amendment. Subject to the approval of the supervisory board, the management board may amend, suspend or terminate the 2010 Plan or any portion thereof at any time. Unless otherwise specified in the amendment, any amendment to the 2010 Plan shall apply to, and be binding on the holders of, all Awards outstanding under the 2010 Plan at the time the amendment is adopted, provided the supervisory board determines that such amendment, taking into account any related action, does not materially and adversely affect the rights of participants under the 2010 Plan. In addition, we may from time to time establish one or more sub-plans under the 2010 Plan for purposes of satisfying applicable securities, tax or other laws of various jurisdictions.
Prior stock option plans
In 2007 we established our 2007 Employee Stock Option Plan (as amended and restated in 2010, the “2007 Plan”), in 2006 we established our 2006 Employee Stock Option Plan (the “2006 Plan”) and in 2004 we established our 2004 Employee Stock Option Plan (the “2004 Plan,” and together with the 2007 Plan and the 2006 Plan, the “Prior Plans”). Each of the Prior Plans permits the grant of options (“Options”) to purchase Depositary Receipts. The Options are subject to transfer restrictions. Each of the Prior Plans was terminated at our annual general meeting of shareholders on June 14, 2013.
Plan Administration. Under each of the Prior Plans, an Option will be granted with the approval of the supervisory board and is evidenced by an Option agreement signed by the participant to indicate his acceptance of its terms and conditions. We do not intend to issue any future Options under any of the Prior Plans.
Eligibility. Under the 2006 Plan, Options may only be granted to an employee or a supervisory board member. Under the 2007 Plan and 2004 Plan, Options may be granted to an employee, a supervisory board member or a scientific advisory board member.
Option Exercise Price. The exercise price to acquire one Depositary Receipt upon exercise of an Option is determined by the management board in its sole discretion upon granting the Options. The exercise prices for currently granted and un-exercised Options under the Prior Plans range from €0.01 to €2.54.
Vesting Period. Under each of the Prior Plans, the option period commences at the date of grant and lasts ten years. Under the 2007 Plan, generally 25% of the Depositary Receipts underlying Options vest on the first anniversary of the grant date, and 2.0833% vest monthly thereafter. Under each of the 2006 Plan and the 2004 Plan, generally all the Depositary Receipts underlying Options vested immediately, with us having the right to repurchase a portion of the underlying Depositary Receipts if the employee or supervisory board member left within four years of the grant date.
Amendment. Subject to the approval of our shareholders and the supervisory board, we are authorized to amend each of the Prior Plans at any time and in any manner we deem fit. Amendments will in no event prejudice the existing rights of a participant, unless that participant consents to the amendments in writing.
Compensation of managing directors and supervisory directors
Dutch law provides that we must establish a policy in respect of the remuneration of our managing directors. The remuneration policy was adopted by the general meeting of shareholders on June 14, 2013 on the proposal of the supervisory board. The supervisory board determines the remuneration of the management directors in accordance with the remuneration policy. A proposal by the supervisory board with respect to remuneration schemes in the form of shares or rights to shares is submitted by the supervisory board to the general meeting of shareholders for its approval. This proposal must set out at least the maximum number of shares or rights to shares to be granted to members of the management board and the criteria for granting or amendment. At our annual general meeting of shareholders on June 14, 2013, a remuneration policy was approved that includes the following elements: the chairman of the management board may be eligible to receive a bonus of up to 50% of the annual base gross compensation and the other managing directors may be eligible to receive a bonus of up to 30% of the annual base gross compensation, each based upon achieving the set financial and operating goals for the period. The bonus payments may be increased by the supervisory board at its sole discretion. Our remuneration policy allows for termination payments of up to 100% of the managing director’s base salary and bonus. This policy also allows for a lump-sum payment to our managing directors following a change of control of up to 200% of base salary and bonus, although none of our management services agreements with our managing directors currently are at this threshold. The maximum number of shares or rights to shares that may be granted to managing directors is up to 4% of our issued shares.
For the year ended December 31, 2013, we paid our managing directors an aggregate of €1.0 million in compensation and benefits in kind (excluding bonuses).The managing directors received no bonuses during 2013. The bonuses to the managing directors are discretionary and are set by our supervisory board. For the year ended December 31, 2013, the managing directors were granted nil Options. See “Item 5. Operating and Financial Review and Prospects—A. Operating Results—Critical accounting policies and significant judgments and estimates—Share-based compensation—Share options.”
84
The general meeting of shareholders determines the compensation of the supervisory directors. Our Compensation Committee is in place to aid in such determination. At our annual general meeting of shareholders on June 14, 2013, the following remuneration policy for our supervisory directors was established: each supervisory director will be entitled to an annual retainer of $40,000, provided that the chairman of the supervisory board will be entitled to an annual retainer of $50,000. Each member of the audit committee will be entitled to an additional annual retainer of $5,000, provided that the chairman of the audit committee will be entitled to an additional annual retainer of $7,500. Each member of the compensation committee and nominating and corporate governance committee will be entitled to an additional annual retainer of $2,500, provided that the chairmen of such committees will be entitled to additional annual retainers of $5,000. Such amounts will be prorated if a supervisory director resigns his relevant office in the course of a financial year. In addition, each supervisory director is entitled to an annual grant of 10,000 Options under the 2010 Plan; and new supervisory directors will be entitled to an additional initial grant of 20,000 Options under the 2010 Plan.
For the year ended December 31, 2013, we paid in addition an aggregate of €34 thousand in compensation to Daan Ellens and Peter Goodfellow for their service as supervisory directors prior to the IPO. Dr. Goodfellow also received compensation of €4 thousand in connection with consulting services provided to us in 2013. The supervisory directors who were nominated by our venture capital investors did not receive any remuneration. Supervisory directors are, however, entitled to be reimbursed for their reasonable expenses incurred in attending meetings of the supervisory board or in otherwise acting for us. In addition, supervisory directors are entitled to receive share-based compensation subject to service-based vesting. For the year ended December 31, 2013, supervisory directors were granted 40,000 Options.
At the Extraordinary General Meeting of Shareholders of Prosensa Holding N.V. on January 23, 2014 Dr Georges Gemayel was appointed in the position of Supervisory Board director for a term ending at the end of the 2016 AGM. The shareholders approved an initial grant of 30,000 options and an additional compensation fee of US$ 2,000 per supervisory board meeting to be held outside the US to Dr Georges Gemayel.
The total amount set aside or accrued by us to provide pension, retirement or similar benefits for our managing directors and supervisory directors for the year ended December 31, 2013 was €0.
Insurance and indemnification
Managing directors and supervisory directors have the benefit of indemnification provisions in our Articles of Association. These provisions give managing directors and supervisory directors the right, to the fullest extent permitted by law, to recover from us amounts, including but not limited to litigation expenses, and any damages they are ordered to pay, in relation to acts or omissions in the performance of their duties. However, there is generally no entitlement to indemnification for acts or omissions that amount to willful (opzettelijk), intentionally reckless (bewust roekeloos) or seriously culpable (ernstig verwijtbaar) conduct. In addition, we have entered into agreements with our managing directors and supervisory directors to indemnify them against expenses and liabilities to the fullest extent permitted by law. These agreements also provide, subject to certain exceptions, for indemnification for related expenses including, among others, attorneys’ fees, judgments, penalties, fines and settlement amounts incurred by any of these individuals in any action or proceeding. In addition to such indemnification, we provide our managing directors and supervisory directors with directors’ and officers’ liability insurance.
Insofar as indemnification of liabilities arising under the Securities Act may be permitted to supervisory directors, managing directors or persons controlling us pursuant to the foregoing provisions, we have been informed that, in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
C. Board practices
Supervisory board
Supervisory directors are appointed by the general meeting of shareholders upon a binding nomination of the supervisory board for a term of up to four years. The binding nomination will be prepared by the nominating and corporate governance committee.
Our Articles of Association provide for a term of appointment of supervisory directors of up to four years. Our supervisory directors have been appointed for different terms as a result of which only approximately one-third of our supervisory board members will be subject to election in any one year. Such an appointment has the effect of creating a staggered board and may deter a takeover attempt.
85
The supervisory board meets as often as a supervisory board member deems necessary. In a meeting of the supervisory board, each supervisory director has a right to cast one vote. All resolutions by the supervisory board are adopted by an absolute majority of the votes cast. In the event the votes are equally divided, the chairman has a decisive vote. A supervisory director may grant another supervisory director a written proxy to represent him at the meeting, but a supervisory director cannot represent more than one supervisory director.
Our supervisory board can pass resolutions outside of meetings, provided that the resolution is adopted in writing and all supervisory directors have consented to adopting the resolution outside of a meeting. The chairman shall prepare and sign a report of the resolutions adopted in this manner.
Our supervisory directors do not have a retirement age requirement under our Articles of Association.
Management board
The supervisory board determines the number of managing directors. Managing directors are appointed by the general meeting of shareholders upon a binding nomination of the supervisory board for a term of up to four years.
At least once per year the management board informs the supervisory board in writing of the main lines of our strategic policy, the general and financial risks and the management and control system.
We have a strong centralized management board led by Hans Schikan, our Chief Executive Officer, with broad experience in general management, strategy, corporate development, operations, in particular the commercialization of orphan drugs for rare diseases, information technology, finance, sales, communications and training. Our managing directors have worked together as a team for many years.
Supervisory Board Committees
Audit committee
The audit committee, consists of Daan Ellens, Patrick Van Beneden and Georges Gemayel and assists the supervisory board in overseeing our accounting and financial reporting processes and the audits of our financial statements. Mr. Ellens serves as Chairman of the committee. The audit committee consists exclusively of members of our supervisory board who are financially literate, and Daan Ellens is considered an “audit committee financial expert” as defined by the SEC. In addition, under SEC and Nasdaq rules, there are heightened independence standards for members of the audit committee, including a prohibition against the receipt of any compensation from us other than standard supervisory director fees. Mr. Van Beneden and Dr. Gemayel meet the Nasdaq independence standard, but Mr. Ellens does not. We rely on the phase-in rules of the SEC and Nasdaq with respect to the independence of our audit committee. These rules require that all members of our audit committee must meet the general Nasdaq independence standard within one year of our IPO. The audit committee is governed by a charter that complies with Nasdaq rules.
The audit committee is responsible for:
|
·
|
recommending the appointment of the independent auditor to the general meeting of shareholders;
|
|
·
|
the appointment, compensation, retention and oversight of any accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit services;
|
|
·
|
pre-approving the audit services and non-audit services to be provided by our independent auditor before the auditor is engaged to render such services;
|
|
·
|
evaluating the independent auditor’s qualifications, performance and independence, and presenting its conclusions to the full supervisory board on at least an annual basis;
|
|
·
|
reviewing and discussing with the management board and the independent auditor our annual audited financial statements and quarterly financial statements prior to the filing of the respective annual and quarterly reports;
|
|
·
|
reviewing our compliance with laws and regulations, including major legal and regulatory initiatives and also reviewing any major litigation or investigations against us that may have a material impact on our financial statements; and
|
86
|
·
|
approving or ratifying any related person transaction (as defined in our related person transaction policy) in accordance with our related person transaction policy.
|
The audit committee meets as often as one or more members of the audit committee deem necessary, but in any event at least four times per year. The audit committee meets at least once per year with our independent accountant, without our management board being present.
Compensation committee
The compensation committee which consists of David Mott, Daan Ellens and Georges Gemayel, assists the supervisory board in determining management board compensation. Mr. Mott serves as Chairman of the committee. The committee recommends to the supervisory board for determination the compensation of each of our managing directors. Under Nasdaq rules, there are heightened independence standards for members of the compensation committee, including a prohibition against the receipt of any compensation from us other than standard supervisory director fees. All of our compensation committee members will meet this heightened standard.
The compensation committee is responsible for:
|
·
|
identifying, reviewing and approving corporate goals and objectives relevant to management board compensation;
|
|
·
|
analyzing the possible outcomes of the variable remuneration components and how they may affect the remuneration of the managing directors;
|
|
·
|
evaluating each managing director’s performance in light of such goals and objectives and determining each managing director’s compensation based on such evaluation;
|
|
·
|
determining any long-term incentive component of each managing director’s compensation in line with the remuneration policy and reviewing our management board compensation and benefits policies generally;
|
|
·
|
periodically reviewing, in consultation with our Chief Executive Officer, our management succession planning; and
|
|
·
|
reviewing and assessing risks arising from our compensation policies and practices for our employees and whether any such risks are reasonably likely to have a material adverse effect on us.
|
Nominating and corporate governance committee
The nominating and corporate governance committee, which consists of Peter Goodfellow, Martijn Kleijwegt and David Mott, assists our supervisory board in identifying individuals qualified to become members of our supervisory board and management board consistent with criteria established by our supervisory board and in developing our corporate governance principles. Mr. Mott serves as Chairman of the committee.
The nominating and corporate governance committee is responsible for:
|
·
|
reviewing and evaluating the composition, function and duties of our supervisory board and management board;
|
|
·
|
recommending nominees for selection to our supervisory board, its corresponding committees and our management board;
|
|
·
|
making recommendations to the supervisory board as to determinations of supervisory board member independence;
|
|
·
|
leading the supervisory board in a self-evaluation, at least annually, to determine whether it and its committees are functioning effectively;
|
|
·
|
overseeing and recommending for adoption by the general meeting of shareholders the compensation for our supervisory board members; and
|
87
|
·
|
developing and recommending to the supervisory board our rules governing the supervisory board, rules governing the management board and code of business conduct and ethics and reviewing and reassessing the adequacy of such rules governing the supervisory board, rules governing the management board and Code of Business Conduct and Ethics and recommending any proposed changes to the supervisory board.
|
D. Employees
As of December 31, 2013, we had 89 employees, 27 of whom hold M.D. or Ph.D. degrees. None of our employees is subject to a collective bargaining agreement or represented by a trade or labor union. We have established a workers’ council for our employees effective as of February 2014. We consider our relations with our employees to be good.
E. Share ownership
See “Item 7. Major Shareholders and Related Party Transactions—A. Major shareholders.”
ITEM 7. MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS
A. Major shareholders
The following table sets forth information relating to the beneficial ownership of our ordinary shares as of February 28, 2014, by:
|
·
|
each person, or group of affiliated persons, known by us to beneficially own more than 5% of our outstanding ordinary shares;
|
|
·
|
each of our managing directors and supervisory directors; and
|
|
·
|
all managing directors and supervisory directors as a group.
|
The number of ordinary shares beneficially owned by each entity, person, managing director or supervisory director is determined in accordance with the rules of the SEC, and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares over which the individual has sole or shared voting power or investment power as well as any shares that the individual has the right to acquire within 60 days of February 28, 2014 through the exercise of any option, warrant or other right. Except as otherwise indicated, and subject to applicable community property laws, the persons named in the table have sole voting and investment power with respect to all ordinary shares held by that person.
The percentage of shares beneficially owned is computed on the basis of 35,932,792 of our ordinary shares as of February 28, 2014. Ordinary shares that a person has the right to acquire within 60 days of February 28, 2014 are deemed outstanding for purposes of computing the percentage ownership of the person holding such rights, but are not deemed outstanding for purposes of computing the percentage ownership of any other person, except with respect to the percentage ownership of all managing directors and supervisory directors as a group. Unless otherwise indicated below, the address for each beneficial owner listed is c/o Prosensa Holding B.V., at J.H. Oortweg 21, 2333 CH Leiden, the Netherlands.
|
Shares beneficially
owned
|
||||
|
Name and address of beneficial owner
|
Number
|
Percent
|
||
|
5% Shareholders
|
||||
|
New Enterprise Associates 13, L.P.(3)
|
6,480,171
|
18.03
|
||
|
ABV IV Holdings N.V.(1)
|
6,213,924
|
17.29
|
||
|
LSP Prosensa Pooling B.V.(2)
|
6,213,924
|
17.29
|
||
|
Gimv N.V.(4)
|
2,620,248
|
7.29
|
||
|
Idinvest Partners(5)
|
2,620,248
|
7.29
|
||
|
MedSciences Prosensa Holding B.V.(6)
|
1,996,253
|
5.56
|
||
|
Managing Directors and Supervisory Directors
|
||||
|
Hans Schikan(7)
|
619,344
|
1.72
|
||
|
Berndt Modig(8)
|
189,360
|
0.53
|
||
88
|
Shares beneficially
owned
|
||||||||
|
Name and address of beneficial owner
|
Number
|
Percent
|
||||||
|
5% Shareholders
|
||||||||
|
New Enterprise Associates 13, L.P.(3)
|
6,480,171 | 18.03 | ||||||
|
ABV IV Holdings N.V.(1)
|
6,213,924 | 17.29 | ||||||
|
LSP Prosensa Pooling B.V.(2)
|
6,213,924 | 17.29 | ||||||
|
Deerfield Mgmt, L.P.(4)
|
3,224,524 | 8.97 | ||||||
|
Gimv N.V.(5)
|
2,620,248 | 7.29 | ||||||
|
Idinvest Partners(6)
|
2,620,248 | 7.29 | ||||||
|
MedSciences Prosensa Holding B.V.(7)
|
1,996,253 | 5.56 | ||||||
|
Managing Directors and Supervisory Directors
|
||||||||
|
Hans Schikan(8)
|
619,344 | 1.72 | ||||||
|
Berndt Modig(9)
|
189,360 | 0.53 | ||||||
|
Giles Campion(10)
|
190,767 | 0.53 | ||||||
|
Luc Dochez(11)
|
197,455 | 0.55 | ||||||
|
Rémi Droller(12)
|
— | — | ||||||
|
Daan Ellens(13)
|
251,244 | 0.70 | ||||||
|
Georges Gemayel
|
— | — | ||||||
|
Peter Goodfellow(14)
|
55,301 | 0.15 | ||||||
|
Martijn Kleijwegt(15)
|
6,213,924 | 17.29 | ||||||
|
David Mott(16)
|
6,480,171 | 18.03 | ||||||
|
Patrick Van Beneden(17)
|
2,620,248 | 7.29 | ||||||
|
All managing directors and supervisory directors as a group (11 persons)
|
16,817,814 | 46.80 | ||||||
(1) The shares directly held by ABV IV Holdings N.V. (“ABV IV”) are indirectly held by Abingworth Bioventures IV LP and Abingworth Bioventures IV Executives LP (the “Abingworth Funds”) and by Abingworth Management Limited (“Abingworth Management”), the investment manager for the Abingworth Funds. Voting and investment power of Abingworth Management Limited is exercised by an investment committee consisting of 4 people. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The address of each of ABV IV, the Abingworth Funds and Abingworth Management is 38 Jermyn Street, London, SW1Y 6DN.
(2) The shares directly held by LSP Prosensa Pooling B.V. (“LSP Pooling”) are indirectly held by LSP III Omni Investment Coöperatief UA (“LSP III”) and Coöperatief LSP IV UA (“LSP IV”), the sole shareholders of LSP Pooling. LSP III Management B.V. is the sole director of LSP III and LSP IV Management B.V. is the sole director of LSP IV. LSP III Management B.V. and LSP IV Management B.V. are the directors of LSP Pooling and have the ultimate voting and investment power over the shares held by LSP Pooling. The individual directors of LSP III Management B.V. and LSP IV Management B.V. are Martijn Kleijwegt, Rene Kuijten and Joachim Rothe. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The principal business address of each of LSP Pooling, LSP III, LSP IV, LSP III Management B.V., LSP IV Management B.V. and each director is Johannes Vermeerplein 9, 1071 DV Amsterdam, the Netherlands.
(3) The shares directly held by New Enterprise Associates 13, L.P., or NEA 13 are indirectly held by NEA Partners 13, L.P., or NEA Partners 13, the sole general partner of NEA 13, NEA 13 GP, LTD, or NEA 13 LTD, the sole general partner of NEA Partners 13 and each of the individual Directors of NEA 13 LTD. The individual Directors, or collectively, the Directors, of NEA 13 LTD are M. James Barrett, Peter J. Barris, Forest Baskett, Ryan D. Drant, Patrick J. Kerins, Krishna Kolluri, C. Richard Kramlich, David M. Mott (a member of our board of directors), Scott D. Sandell, Ravi Viswanathan and Harry R. Weller. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The principal business address of New Enterprise Associates, Inc. is 1954 Greenspring Drive, Suite 600, Timonium, MD 21093.
(4) The address of Deerfield Mgmt, L.P. is 780 Third Avenue, 37th Floor, New York, NY 10017. Based on information reported in Schedule 13G filed with the SEC on September 30, 2013, by Deerfield Mgmt, L.P., consists of (a) 1,101,482 shares held by Deerfield Partners, L.P., (b) 1,418,235 shares held by Deerfield International Master Fund, L.P., (c) 376,537 shares held by Deerfield Special Situations Fund, L.P. and (d) 328,270 shares held by Deerfield Special Situations International Master Fund, L.P. Deerfield Mgmt, L.P. is the general partner of such funds.
(5) Consists of 262,025 ordinary shares held by Adviesbeheer Gimv Life Sciences 2007 NV and 2,358,223 ordinary shares held by Gimv NV. Gimv NV is a company listed on NYSE Euronext Brussels. Adviesbeheer Gimv Life Sciences 2007 NV is a subsidiary of Gimv NV. Investment and voting control over the shares are exercised by the board of directors of Gimv NV which is comprised of the following twelve members: Urbain Vandeurzen, Koen Dejonckheere, Dirk Boogmans, Christ’l Joris, Sophie Manigart, Martine Reynaers, Eric Spiessens, Emile van der Burg, Bart Van Hooland, Christine Van Broeckhoven, Francis Vanderhoydonck and Johan Van den Driessche. Each of these individuals disclaims any beneficial ownership of the shares owned by Gimv NV and Adviesbeheer Gimv Life Sciences 2007 NV except to the extent of his or her pecuniary interest in such entity. The address for Gimv NV and Adviesbeheer Gimv Life Sciences 2007 NV is Karel Oomsstraat 37, B-2018, Antwerpen, Belgium.
(6) Consists of 601,802 ordinary shares held by FCPI Allianz Innovation 8, 765,479 ordinary shares held by FCPI Capital Croissance, 678,821 ordinary shares held by FCPI Objectif Innovation Patrimoine, 298,556 ordinary shares held by FCPI Capital Croissance 3 and 275,590 ordinary shares held by FCPI Objectif Innovation Patrimoine 3 (the “Idinvest Funds”). Idinvest Partners is the investment management company to each of the Idinvest Funds. Christophe Baviere and Benoist Grossmann are respectively CEO and Managing Partner of Idinvest Partners and as such represent the interests of the Idinvest Funds over the ordinary shares held by them. Each of Christophe Baviere and Benoist Grossmann disclaim beneficial ownership of all applicable shares except to the extent of any pecuniary interest therein. The address for each of the Idinvest Funds is c/o Idinvest Partners, 117, avenue des Champs Elysées, 75008 Paris, France.
(7) The shares directly held by MedSciences Prosensa Holding B.V. are indirectly held by MedSciences Capital B.V. and MedSciences Capital II B.V., the sole shareholders of MedSciences Prosensa Holding B.V. The Managing Director of both MedSciences Capital B.V. and MedSciences Capital II B.V. is MedSciences Capital Management B.V. The Managing Director of MedSciences Capital Management B.V. is Kempen Capital Management N.V. The individual Directors, or collectively, the Directors, of Kempen Capital Management N.V. are Paul Gerla and Erik Luttenberg. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The principal business address of MedSciences Prosensa Holding B.V, MedSciences Capital B.V., MedSciences Capital II B.V., MedSciences Capital Management B.V. and Kempen Capital Management N.V. is Beethovenstraat 300, 1077 WZ Amsterdam, The Netherlands.
(8) Consists of (a) 400,000 ordinary shares and (b) 193,112 options and 26,232 restricted ordinary shares that vest within 60 days of February 28, 2014.
(9) Consists of (a) 20,000 ordinary shares and (b) 160,616options and 8,744 restricted ordinary shares that vest within 60 days of February 28, 2014.
(10) Consists of (a) 20,000 ordinary shares, and (b) 170,767 options that vest within 60 days of February 28, 2014.
(11) Consists of (a) 61,635 ordinary shares and (b) 105,109 options and 30,711 restricted shares that vest within 60 days of February 28, 2014.
(12) Rémi Droller resigned effective March 12, 2014.
(13) Consists of (a) 217,031 ordinary shares and (b) 469 options and 33,744 restricted shares that vest within 60 days of February 28, 2014.
89
(14) Consists of 55,301 options to purchase ordinary shares that vest within 60 days of February 28, 2014.
(15) Consists of 6,213,924 shares held by LSP Prosensa Pooling B.V. Mr. Kleijwegt is a director of LSP III Management B.V. and LSP IV Management B.V., which has ultimate voting and investment power of shares held of record by LSP Prosensa Pooling B.V. He disclaims beneficial ownership except to the extent of any pecuniary interest therein.
(16) Consists of 6,480,171 shares held by New Enterprise Associates 13, L.P. Mr. Mott is a member of the board of directors of NEA 13 LTD, which has ultimate voting and investment power over shares held of record by New Enterprise Associates 13, L.P. He disclaims beneficial ownership of such shares except to the extent of any pecuniary interest therein.
(17) Consists of 262,025 ordinary shares held by Adviesbeheer Gimv Life Sciences 2007 N.V. and 2,358,223 ordinary shares held by Gimv N.V. Mr. Van Beneden is a partner and executive vice-president of Gimv. N.V., which has ultimate voting and investment power of shares held of record by Adviesbeheer Gimv Life Sciences 2007 N.V. He disclaims beneficial ownership except to the extent of any pecuniary interest therein.
Holders
As of February 28, 2014, we had 58 shareholders of record of our common stock.
B. Related party transactions
The following is a description of related party transactions we have entered into since January 1, 2010 with any of our members of our supervisory board or management board and the holders of more than 5% of our ordinary shares.
Series B preferred share financing
In January 2012, we entered into a subscription agreement pursuant to which we issued and sold an aggregate of 5,000,004 of our Class B2 shares at a price per share of €2.30 for an aggregate purchase price of €11,500,009 and (ii) an aggregate of 4,107,140 of our Class B3 shares at a price per share of €2.80 for an aggregate purchase price of €11,499,992. The following table sets forth the number of our Class B2 and Class B3 shares purchased by our managing directors, supervisory directors and 5% shareholders and their affiliates:
|
Name and address of beneficial owner
|
Class B2 shares | Class B3 shares | ||
|
5% Shareholders
|
||||
|
New Enterprise Associates 13, L.P.(1)
|
3,304,348
|
2,714,285
|
||
|
ABV IV Holdings N.V.(2)
|
478,262
|
392,857
|
||
|
LSP Prosensa Pooling B.V.(3)
|
478,262
|
392,857
|
||
|
Gimv N.V.(4).
|
315,218
|
258,928
|
||
|
Idinvest Partners(5)
|
315,218
|
258,928
|
||
|
MedSciences Prosensa Holding B.V.(6)
|
108,696
|
89,285
|
||
(1) The shares directly held by New Enterprise Associates 13, L.P., or NEA 13 are indirectly held by NEA Partners 13, L.P., or NEA Partners 13, the sole general partner of NEA 13, NEA 13 GP, LTD, or NEA 13 LTD, the sole general partner of NEA Partners 13 and each of the individual Directors of NEA 13 LTD. The individual Directors, or collectively, the Directors, of NEA 13 LTD are M. James Barrett, Peter J. Barris, Forest Baskett, Ryan D. Drant, Patrick J. Kerins, Krishna Kolluri, C. Richard Kramlich, David M. Mott (a member of our board of directors), Scott D. Sandell, Ravi Viswanathan and Harry R. Weller. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The principal business address of New Enterprise Associates, Inc. is 1954 Greenspring Drive, Suite 600, Timonium, MD 21093.
(2) The shares directly held by ABV IV Holdings N.V. (“ABV IV”) are indirectly held by Abingworth Bioventures IV LP and Abingworth Bioventures IV Executives LP (the “Abingworth Funds”) and by Abingworth Management Limited (“Abingworth Management”), the investment manager for the Abingworth Funds. Voting and investment power of Abingworth Management Limited is exercised by an investment committee consisting of 4 people. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The address of each of ABV IV, the Abingworth Funds and Abingworth Management is 38 Jermyn Street, London, SW1Y 6DN.
(3) The shares directly held by LSP Prosensa Pooling B.V. (“LSP Pooling”) are indirectly held by LSP III Omni Investment Coöperatief UA (“LSP III”) and Coöperatief LSP IV UA (“LSP IV”), the sole shareholders of LSP Pooling. LSP III Management B.V. is the sole director of LSP III and LSP IV Management B.V. is the sole director of LSP IV. LSP III Management B.V. and LSP IV Management B.V. are the directors of LSP Pooling and have the ultimate voting and investment power over the shares held by LSP Pooling. The individual directors of LSP III Management B.V. are Martijn Kleijwegt, Rene Kuijten and Joachim Rothe and the individual directors of LSP IV Management B.V. are Martijn Kleijwegt, Rene Kuijten and Joachim Rothe. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The principal business address of each of LSP Pooling, LSP III, LSP IV, LSP III Management B.V., LSP IV Management B.V. and each director is Johannes Vermeerplein 9, 1071 DV Amsterdam, the Netherlands.
(4) Consists of 31,522 Class B2 shares and 25,893 Class B3 shares purchased by Adviesbeheer Gimv Life Sciences 2007 NV and 283,696 ordinary shares Class B2 shares and 233,035 Class B3 shares purchased by Gimv NV. Gimv NV is a company listed on NYSE Euronext Brussels. Adviesbeheer Gimv Life Sciences 2007 NV is a subsidiary of Gimv NV. Investment and voting control over the shares are exercised by the board of directors of Gimv NV which is comprised of the following twelve members: Urbain Vandeurzen, Koen Dejonckheere, Dirk Boogmans, Christ’l Joris, Sophie Manigart, Martine Reynaers, Eric Spiessens, Emile van der Burg, Bart Van Hooland, Christine Van Broeckhoven, Francis Vanderhoydonck and Johan Van den Driessche. Each of these individuals disclaims any beneficial ownership of the shares owned by Gimv NV and Adviesbeheer Gimv Life Sciences 2007 NV except to the extent of his or her pecuniary interest in such entity. The address for Gimv NV and Adviesbeheer Gimv Life Sciences 2007 NV is Karel Oomsstraat 37, B-2018, Antwerpen, Belgium.
90
(5) Consists of 163,914 Class B2 shares and 134,642 Class B3 shares purchased by FCPI Capital Croissance 3 and 151,304 Class B2 shares and 124,286 Class B3 shares purchased by FCPI Objectif Innovation Patrimoine 3 (the “Idinvest Funds”). Idinvest Partners is the investment management company to each of the Idinvest Funds. Christophe Baviere and Benoist Grossmann are respectively CEO and Managing Partner of Idinvest Partners and as such represent the interests of the Idinvest Funds over the ordinary shares held by them. Each of Christophe Baviere and Benoist Grossmann disclaim beneficial ownership of all applicable shares except to the extent of any pecuniary interest therein. The address for each of the Idinvest Funds is c/o Idinvest Partners, 117, avenue des Champs Elysées, 75008 Paris, France.
(6) The shares directly held by MedSciences Prosensa Holding B.V. are indirectly held by MedSciences Capital B.V. and MedSciences Capital II B.V., the sole shareholders of MedSciences Prosensa Holding B.V. The Managing Director of both MedSciences Capital B.V. and MedSciences Capital II B.V. is MedSciences Capital Management B.V. The Managing Director of MedSciences Capital Management B.V. is Kempen Capital Management N.V. The individual Directors, or collectively, the Directors, of Kempen Capital Management N.V. are Paul Gerla and Erik Luttenberg. All indirect holders of the above referenced shares disclaim beneficial ownership of all applicable shares except to the extent of their actual pecuniary interest therein. The principal business address of MedSciences Prosensa Holding B.V, MedSciences Capital B.V., MedSciences Capital II B.V., MedSciences Capital Management B.V. and Kempen Capital Management N.V. is Beethovenstraat 300, 1077 WZ Amsterdam, The Netherlands.
Shareholders’ agreement
On December 8, 2008, all of our then existing shareholders entered into a shareholders agreement, as amended on June 29, 2010 and January 16, 2012 (as amended, the “Shareholders’ Agreement”). The Shareholders' Agreement governed the composition of our board of directors and provided anti-dilution and preemptive rights. The Shareholders' Agreement was terminated in connection with our initial public offering in July 2013.
Equity Incentive Plans and Foundation
See "Item 6--B.Compensation--Long-term incentive plans."
Registration rights agreement
Following the consummation of our IPO, we entered into a registration rights agreement with certain of our existing shareholders pursuant to which we granted them the rights set forth below.
Demand registration rights. Certain of our shareholders that are party to the Registration Rights Agreement (the “RRA Shareholders”) are entitled to request that we effect up to an aggregate of four demand registrations under the Registration Rights Agreement, and no more than one demand registration within any six-month period, covering the RRA Shareholders’ ordinary shares that are subject to transfer restrictions under Rule 144 (“registrable securities”). The demand registration rights are subject to certain customary conditions and limitations, including customary underwriter cutback rights and deferral rights. No demand registration rights exist while a shelf registration is in effect.
Piggyback registration rights. If we propose to register any ordinary shares (other than in a shelf registration or on a registration statement on Form S-4 or S-8), the RRA Shareholders are entitled to notice of such registration and to include their registrable securities in that registration. The registration of RRA Shareholders’ registrable securities pursuant to a piggyback registration does not relieve us of the obligation to effect a demand registration. The managing underwriter has the right to limit the number of registrable securities included in a piggyback registration if the managing underwriter believes it would interfere with the successful marketing of the ordinary shares.
Form F-3 registration rights. When we are eligible to use Form F-3, one or more RRA Shareholders have the right to request that we file a registration statement on Form F-3. RRA Shareholders will have the right to cause us to undertake underwritten offerings from the shelf registration, but no more than one underwritten offering in a six-month period. Each underwritten takedown constitutes a demand registration for purposes of the maximum number of demand registrations we are obligated to effectuate.
Subject to limited exceptions, the Registration Rights Agreement provides that we must pay all registration expenses in connection with a demand, piggyback or shelf registration. The Registration Rights Agreement contains customary indemnification and contribution provisions.
Indemnification Agreements
We have entered into indemnification agreements with our managing directors and supervisory directors. The indemnification agreements and our Articles of Association require us to indemnify our managing directors and supervisory directors to the fullest extent permitted by law. See “Item 6B. Compensation – Insurance and Indemnification” for a description of these indemnification agreements..
C. Interests of Experts and Counsel
Not applicable.
91
ITEM 8. FINANCIAL INFORMATION
A. Consolidated statements and other financial information
Financial statements
See “Item 18. Financial Statements,” which contains our financial statements prepared in accordance with IFRS.
Legal Proceedings
From time to time we are involved in legal proceedings that arise in the ordinary course of business. We believe that the outcome of these proceedings, if determined adversely, will not have a material adverse effect on our financial position. During the period covered by the audited and approved financial statements contained herein, we have not been a party to or paid any damages in connection with litigation that has had a material adverse effect on our financial position. Any future litigation may result in substantial costs and be a distraction to management and employees. No assurance can be given that future litigation will not have a material adverse effect on our financial position. See “Item 3. Key Information—D. Risk factors.”
Dividends and Dividend Policy
We have not declared cash dividends on our common stock in the years 2013, 2012 or 2011. We currently expect to retain future earnings, if any, to finance the operation and expansion of our business, and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to our dividend policy will be made at the discretion of our board of directors.
B. Significant changes
A discussion of the significant changes in our business can be found under “Item 4. Information on the Company—A. History and development of the company.”
ITEM 9. THE OFFER AND LISTING
A. Offering and listing details
Not applicable.
B. Plan of distribution
Not applicable.
C. Markets
Our ordinary shares began trading on the Nasdaq Global Select Market on June 28, 2013 under the symbol RNA. The following table sets forth the high and low sales prices as reported by NASDAQ for each quarter:
|
High
|
Low
|
|||||||
|
Year Ended December 31, 2013
|
||||||||
|
June 28, 2013
|
$ | 20.00 | $ | 16.97 | ||||
|
Third Quarter
|
34.55 | 5.65 | ||||||
|
Fourth Quarter
|
7.30 | 3.43 | ||||||
D. Selling shareholders
Not applicable.
E. Dilution
Not applicable.
92
F. Expenses of the issue
Not applicable.
ITEM 10. ADDITIONAL INFORMATION
A. Share capital
Not applicable.
B. Memorandum and articles of association
Our shareholders adopted the Articles of Association filed as Exhibit 3.2 to our registration statement on Form F-1 (file no. 333-188855) with the SEC on May 24, 2013.
We incorporate by reference into this annual report on Form 20-F the description of our Articles of Association effective upon the closing of our IPO contained in our F-1 registration statement (File No. 333-188855) originally filed with the SEC on May 24, 2013, as amended. Such description sets forth a summary of certain provisions of our articles of association as currently in effect.
C. Material contracts
Except as otherwise disclosed in this annual report on Form 20-F (including the Exhibits), we are not currently, and have not been in the last two years, party to any material contract, other than contracts entered into in the ordinary course of business.
D. Exchange controls
Cash dividends payable on our ordinary shares and cash interest payments to holders of our debt securities may be remitted from the Netherlands to non-residents without legal restrictions imposed by the laws of the Netherlands, except that (i) such payments must be reported to the Dutch Central Bank for statistical purposes only and (ii) the transfer of funds to jurisdictions subject to general economic sanctions adopted in connection with policies of the United Nations, European Commission or similar measures imposed directly by the Government of the Netherlands may be restricted.
E. Taxation
The following summary contains a description of certain Dutch and U.S. federal income tax consequences of the acquisition, ownership and disposition of ordinary shares, but it does not purport to be a comprehensive description of all the tax considerations that may be relevant to a decision to purchase ordinary shares. The summary is based upon the tax laws of the Netherlands and regulations thereunder and on the tax laws of the United States and regulations thereunder as of the date hereof, which are subject to change.
Dutch tax considerations
The following discussion is a summary of the material Dutch tax considerations relating to the purchase, ownership and disposition of our ordinary shares.
Taxation in the Netherlands
The following is intended as general information only and it does not purport to present any comprehensive or complete description of all aspects of Dutch tax law which could be of relevance to a holder of ordinary shares (a “Shareholder”). For Dutch tax purposes, a Shareholder may include an individual or entity who does not have the legal title of the shares, but to whom nevertheless the shares are attributed based either on such individual or entity holding a beneficial interest in the shares or based on specific statutory provisions, including statutory provisions pursuant to which shares are attributed to an individual who is, or who has directly or indirectly inherited from a person who was, the settlor, grantor or similar originator of a trust, foundation or similar entity that holds the shares.
Prospective Shareholders should therefore consult their tax adviser regarding the tax consequences of any purchase, ownership or disposal of ordinary shares.
93
The following summary is based on the Dutch tax law as applied and interpreted by Dutch tax courts and as published and in effect on the date hereof, without prejudice to any amendments introduced at a later date and implemented with or without retroactive effect.
For the purpose of this paragraph, “Dutch Taxes” shall mean taxes of whatever nature levied by or on behalf of the Netherlands or any of its subdivisions or taxing authorities. The Netherlands means the part of the Kingdom of the Netherlands located in Europe.
Withholding tax
A Shareholder is generally subject to Dutch dividend withholding tax at a rate of 15% on dividends distributed by the Company. Generally, a Company is responsible for the withholding of such dividend withholding tax at source; the dividend withholding tax is for the account of the Shareholder.
Dividends distributed by the Company include, but are not limited to:
|
·
|
distributions of profits in cash or in kind, whatever they be named or in whatever form
|
|
·
|
proceeds from the liquidation of the Company, or proceeds from the repurchase of ordinary shares by the Company, in excess of the average paid-in capital recognized for Dutch dividend withholding tax purposes
|
|
·
|
the nominal value of ordinary shares issued to a Shareholder or an increase in the nominal value of ordinary shares, to the extent that no contribution, recognized for Dutch dividend withholding tax purposes, has been made or will be made; and
|
|
·
|
partial repayment of paid-in capital, that is
|
|
·
|
not recognized for Dutch dividend withholding tax purposes, or
|
|
·
|
recognized for Dutch dividend withholding tax purposes, to the extent that the Company has “net profits” (zuivere winst), unless
|
(a) the general meeting of Shareholders has resolved in advance to make such repayment, and
(b) the nominal value of the ordinary shares concerned has been reduced with an equal amount by way of an amendment to the Articles of Association of the Company.
The term “net profits” includes anticipated profits that have yet to be realized.
Notwithstanding the above, no withholding is required in the event of a repurchase of ordinary shares, if certain conditions are fulfilled.
If a Shareholder is resident or deemed to be resident in the Netherlands, other than an individual who has opted to be treated as if resident in Netherlands, such Shareholder is generally entitled to an exemption or a full credit for any Dutch dividend withholding tax against his Dutch (corporate) income tax liability and to a refund of any residual Dutch dividend withholding tax.
If a Shareholder is resident in a country other than the Netherlands under circumstances exemptions from, reduction in or refunds of, dividend withholding tax may be available pursuant to Dutch domestic law or treaties or regulations for the avoidance of double taxation.
According to Dutch domestic anti-dividend stripping rules, no credit against Dutch (corporate) income tax, exemption from, reduction in or refund of, Dutch dividend withholding tax will be granted if the recipient of the dividend paid by the Company is not considered to be the beneficial owner (uiteindelijk gerechtigde) of such dividends as meant in these rules.
Taxes on income and capital gains
This section does not purport to describe the possible Dutch tax considerations or consequences that may be relevant to a Shareholder:
|
·
|
who is an individual and for whom the income or capital gains derived from the ordinary shares are attributable to employment activities, the income from which is taxable in the Netherlands
|
94
|
·
|
that is an entity that is not subject to Dutch corporate income tax or is in full or in part exempt from Dutch corporate income tax (such as pension funds)
|
|
·
|
that is an investment institution (beleggingsinstelling) as defined in article 6a or 28 of the Dutch 1969 Corporate income tax act (Wet op de vennootschapsbelasting 1969 “CITA”); or
|
|
·
|
which is entitled to the participation exemption (deelnemingsvrijstelling) with respect to the ordinary shares as defined in article 13, CITA.
|
Residents in the Netherlands
The description of certain Dutch tax consequences in this paragraph is only intended for the following Shareholders:
(a) individuals who are resident or deemed to be resident in the Netherlands for Dutch income tax purposes
(b) individuals who opt to be treated as if resident in the Netherlands for Dutch income tax purposes ((a) and (b) jointly “Dutch Individuals”); and
(c) entities that are subject to the CITA and are resident or deemed to be resident in the Netherlands for corporate income tax purposes “Dutch Corporate Entities”).
Dutch Individuals engaged or deemed to be engaged in an enterprise or in miscellaneous activities
Dutch Individuals are generally subject to income tax at statutory progressive rates with a maximum of 52% with respect to any benefits derived or deemed to be derived from Dutch Enterprise Shares (as defined below), including any capital gains realized on the disposal thereof.
“Dutch Enterprise Shares” are shares or any right to derive benefits from shares:
|
·
|
which are attributable to an enterprise from which a Dutch Individual derives profits, whether as an entrepreneur or pursuant to a co-entitlement to the net worth of such enterprise (other than as an entrepreneur or a shareholder); or
|
|
·
|
of which the benefits are taxable in the hands of a Dutch Individual as benefits from miscellaneous activities (resultaat uit overige werkzaamheden) including, without limitation, activities which are beyond the scope of active portfolio investment activities.
|
Dutch Individuals having a (fictitious) substantial interest
Dutch Individuals are generally subject to income tax at statutory rate of 25% with respect to any benefits derived or deemed to be derived from shares, excluding Dutch Enterprise Shares, (including any capital gains realized on the disposal thereof) that are attributable to a (fictitious) substantial interest (such shares being “Substantial Interest Shares”).
Generally, a Shareholder has a substantial interest (aanmerkelijk belang) in the Company if such Shareholder, alone or together with his partner, directly or indirectly:
|
·
|
owns, or holds certain rights on, shares representing 5% or more of the total issued and outstanding capital of the Company, or of the issued and outstanding capital of any class of shares of the Company;
|
|
·
|
holds rights to acquire shares, whether or not already issued, representing 5% or more of the total issued and outstanding capital of the Company, or of the issued and outstanding capital of any class of shares of the Company; or
|
|
·
|
owns, or holds certain rights on, profit participating certificates that relate to 5% or more of the annual profit of the Company or to 5% or more of the liquidation proceeds of the Company.
|
A Shareholder will also have a substantial interest if his partner or one of certain relatives of the Shareholder or of his partner has a substantial interest.
Generally, a Shareholder has a fictitious substantial interest (fictief aanmerkelijk belang) in the Company if, without having an actual substantial interest in the Company:
95
|
·
|
an enterprise has been contributed to the Company in exchange for shares on an elective non-recognition basis;
|
|
·
|
the shares have been obtained under gift law, inheritance law or matrimonial law, on a non-recognition basis, while the previous Shareholder had a substantial interest in the Company;
|
|
·
|
the shares have been acquired pursuant to a share merger, legal merger or legal demerger, on an elective non-recognition basis, while the Shareholder prior to this transaction had a substantial interest in an entity that was party thereto; or
|
|
·
|
the shares held by the Shareholder, prior to dilution, qualified as a substantial interest and, by election, no gain was recognized upon disqualification of these shares.
|
Dutch Individuals not engaged or deemed to be engaged in an enterprise or in miscellaneous activities or having a (fictitious) substantial interest
Generally, a Dutch Individual who owns shares, excluding Dutch Enterprise Shares and Substantial Interest Shares, will be subject annually to an income tax imposed on a fictitious yield on such shares under the regime for savings and investments (inkomen uit sparen en beleggen). Irrespective of the actual income or capital gains realized, the annual taxable benefit of all the assets and liabilities of a Dutch Individual that are taxed under this regime, including the shares, is set at a fixed amount. The fixed amount equals 4% of the fair market value of the assets reduced by the liabilities and measured, in general, exclusively at the beginning of every calendar year. The tax rate under the regime for savings and investments is a flat rate of 30%.
Dutch Corporate Entities
Dutch Corporate Entities are generally subject to corporate income tax at statutory rates up to 25% with respect to any benefits derived or deemed to be derived (including any capital gains realized on the disposal) of shares.
Non-residents in the Netherlands
A Shareholder other than a Dutch Individual or Dutch Corporate Entity, will not be subject to any Dutch Taxes on income or capital gains with respect to the ownership and disposal of the shares, other than dividend withholding tax as described above, except if:
|
·
|
the Shareholder derives profits from an enterprise, whether as entrepreneur (ondernemer) or pursuant to a co-entitlement to the net worth of such enterprise other than as an entrepreneur or a Shareholder, which enterprise is, in whole or in part, carried on through a permanent establishment (vaste inrichting) or a permanent representative (vaste vertegenwoordiger) in the Netherlands, to which the shares are attributable;
|
|
·
|
the Shareholder is an individual and derives benefits from miscellaneous activities (resultaat uit overige werkzaamheden) carried out in the Netherlands in respect of the shares, including, without limitation, activities which are beyond the scope of active portfolio investment activities;
|
|
·
|
the Shareholder is an individual and has a substantial interest or a fictitious substantial interest in the Company, which (fictitious) substantial interest is not attributable to the assets of an enterprise;
|
|
·
|
the Shareholder is not an individual and has a substantial interest or a fictitious substantial interest in the Company, which (fictitious) substantial interest is not attributable to the assets of an enterprise and (one of) the main purposes of the chosen ownership structure is the evasion of Dutch income tax or dividend withholding tax;
|
|
·
|
the Shareholder is an individual and is entitled to a share in the profits of an enterprise, other than by way of the holding securities, which enterprise is effectively managed in the Netherlands and to which enterprise the shares are attributable; or
|
|
·
|
the Shareholder is not an individual and is entitled to a share in the profits of an enterprise or a co-entitlement to the net worth of an enterprise, other than by way of the holding of securities, which enterprise is effectively managed in the Netherlands and to which enterprise the shares are attributable.
|
96
Gift tax and inheritance tax
No Dutch gift or inheritance tax is due in respect of any gift of the shares by, or inheritance of the shares on the death of, a Shareholder, except if:
|
·
|
at the time of the gift or death of the Shareholder, the Shareholder is resident, or is deemed to be resident, in the Netherlands
|
|
·
|
the Shareholder passes away within 180 days after the date of the gift of the shares and is not, or not deemed to be, at the time of the gift, but is, or deemed to be, at the time of his death, resident in the Netherlands; or
|
|
·
|
the gift of the shares is made under a condition precedent and the Shareholder is resident, or is deemed to be resident, in the Netherlands at the time the condition is fulfilled.
|
For purposes of Dutch gift or inheritance tax, an individual who is of Dutch nationality will be deemed to be resident in the Netherlands if he has been resident in the Netherlands at any time during the ten years preceding the date of the gift or his death. For purposes of Dutch gift tax, any individual, irrespective of his nationality, will be deemed to be resident in the Netherlands if he has been resident in the Netherlands at any time during the 12 months preceding the date of the gift.
Other Taxes and Duties
No other Dutch Taxes, including turnover tax and taxes of a documentary nature, such as capital tax, stamp or registration tax or duty, are payable by or on behalf of a Shareholder by reason only of the purchase, ownership and disposal of the ordinary shares.
Residency
A Shareholder will not become resident, or deemed resident in the Netherlands for tax purposes by reason only of holding the ordinary shares.
U.S. federal income tax considerations for U.S. holders
The following is a description of the material U.S. federal income tax consequences to the U.S. Holders described below of owning and disposing of ordinary shares. It is not a comprehensive description of all tax considerations that may be relevant to a particular person’s decision to hold the securities. This discussion applies only to a U.S. Holder that holds ordinary shares as capital assets for tax purposes. In addition, it does not describe all of the tax consequences that may be relevant in light of a U.S. Holder’s particular circumstances, including alternative minimum tax consequences, the potential application of the provisions of the Code known as the Medicare contribution tax, and tax consequences applicable to U.S. Holders subject to special rules, such as:
|
·
|
certain financial institutions;
|
|
·
|
dealers or traders in securities who use a mark-to-market method of tax accounting;
|
|
·
|
persons holding ordinary shares as part of a hedging transaction, “straddle,” wash sale, conversion transaction or integrated transaction or persons entering into a constructive sale with respect to the ordinary shares;
|
|
·
|
persons whose “functional currency” for U.S. federal income tax purposes is not the U.S. dollar;
|
|
·
|
tax exempt entities, including “individual retirement accounts” and “Roth IRAs”;
|
|
·
|
entities classified as partnerships for U.S. federal income tax purposes;
|
|
·
|
persons that own or are deemed to own ten percent or more of our voting shares;
|
|
·
|
persons who acquired our ordinary shares pursuant to the exercise of an employee stock option or otherwise as compensation; and
|
|
·
|
persons holding ordinary shares in connection with a trade or business conducted outside the United States.
|
If an entity that is classified as a partnership for U.S. federal income tax purposes holds ordinary shares, the U.S. federal income tax treatment of a partner will generally depend on the status of the partner and the activities of the partnership. Partnerships holding ordinary shares and partners in such partnerships are encouraged to consult their own tax advisers as to the particular U.S. federal income tax consequences of holding and disposing of ordinary shares.
97
The discussion is based on the Code, administrative pronouncements, judicial decisions, final, temporary and proposed Treasury regulations, and the income tax treaty between the Netherlands and the United States (the “Treaty”) all as of the date hereof, changes to any of which may affect the tax consequences described herein—possibly with retroactive effect.
A “U.S. Holder” is a holder who, for U.S. federal income tax purposes, is a beneficial owner of ordinary shares who is eligible for the benefits of the Treaty and is:
|
·
|
a citizen or individual resident of the United States;
|
|
·
|
a corporation, or other entity taxable as a corporation, created or organized in or under the laws of the United States, any state therein or the District of Columbia; or
|
|
·
|
an estate or trust the income of which is subject to U.S. federal income taxation regardless of its source.
|
U.S. Holders are encouraged to consult their own tax advisers concerning the U.S. federal, state, local and foreign tax consequences of owning and disposing of ordinary shares in their particular circumstances.
Taxation of distributions
Subject to the passive foreign investment company rules described below, distributions paid on ordinary shares, other than certain pro rata distributions of ordinary shares, will generally be treated as dividends to the extent paid out of our current or accumulated earnings and profits (as determined under U.S. federal income tax principles). Because we do not maintain calculations of our earnings and profits under U.S. federal income tax principles, we expect that distributions generally will be reported to U.S. Holders as dividends. Subject to applicable limitations, dividends paid to certain non-corporate U.S. Holders may be taxable at preferential rates applicable to long-term capital gain. The amount of a dividend will include any amounts withheld by us in respect of Dutch withholding taxes. The amount of the dividend will be treated as foreign-source dividend income to U.S. Holders and will not be eligible for the dividends-received deduction generally available to U.S. corporations under the Code. Dividends will be included in a U.S. Holder’s income on the date of the U.S. Holder’s receipt of the dividend. The amount of any dividend income paid in euros will be the U.S. dollar amount calculated by reference to the exchange rate in effect on the date of actual or constructive receipt, regardless of whether the payment is in fact converted into U.S. dollars. If the dividend is converted into U.S. dollars on the date of receipt, a U.S. Holder should not be required to recognize foreign currency gain or loss in respect of the dividend income. A U.S. Holder may have foreign currency gain or loss if the dividend is converted into U.S. dollars after the date of receipt.
Subject to applicable limitations, some of which vary depending upon the U.S. Holder’s particular circumstances, Dutch income taxes withheld from dividends on ordinary shares at a rate not exceeding the rate provided by the Treaty will be creditable against the U.S. Holder’s U.S. federal income tax liability. The rules governing foreign tax credits are complex and U.S. Holders should consult their tax advisers regarding the creditability of foreign taxes in their particular circumstances. In lieu of claiming a foreign tax credit, U.S. Holders may, at their election, deduct foreign taxes, including any Dutch income tax, in computing their taxable income, subject to generally applicable limitations under U.S. law. An election to deduct foreign taxes instead of claiming foreign tax credits applies to all foreign taxes paid or accrued in the taxable year.
Sale or other taxable disposition of ordinary shares
Subject to the passive foreign investment company rules described below, gain or loss realized on the sale or other taxable disposition of ordinary shares will be capital gain or loss, and will be long-term capital gain or loss if the U.S. Holder held the ordinary shares for more than one year. The amount of the gain or loss will equal the difference between the U.S. Holder’s tax basis in the ordinary shares disposed of and the amount realized on the disposition, in each case as determined in U.S. dollars. This gain or loss will generally be U.S.-source gain or loss for foreign tax credit purposes. The deductibility of capital losses is subject to limitations.
Passive foreign investment company rules
Under the Code, we will be a PFIC for any taxable year in which, after the application of certain “look-through” rules with respect to subsidiaries, either (i) 75% or more of our gross income consists of “passive income,” or (ii) 50% or more of the average quarterly value of our assets consist of assets that produce, or are held for the production of, “passive income.” Passive income generally includes interest, dividends, rents, certain non-active royalties and capital gains. Whether we will be a PFIC in any year depends on the composition of our income and assets, and the relative fair market value of our assets from time to time, which has varied, and we expect will continue to vary, substantially over time. While we believe that we were not a
98
“passive foreign investment company” for U.S. federal income tax purposes for our 2013 taxable year, because (i) we currently own a substantial amount of passive assets, including cash, and (ii) the value of our assets, including our intangible assets, that generate non-passive income for PFIC purposes, is uncertain and has varied, and we expect will continue to vary, substantially over time, it is uncertain whether we will be, and there can be no assurance that we will not be a PFIC in 2014 or any future taxable years. Based on the current value of our intangible assets that is implied by the recent sales prices for our ordinary shares reported by NASDAQ, U.S. investors should be aware that we may be a PFIC in 2014, although a determination of whether we are a PFIC for 2014 will depend on the composition of our income and the composition and relative values of our assets, including our intangible assets, over the entire year. If we are a PFIC for any year during which a U.S. Holder holds ordinary shares, we generally would continue to be treated as a PFIC with respect to that U.S. Holder for all succeeding years during which the U.S. Holder holds ordinary shares, even if we ceased to meet the threshold requirements for PFIC status.
If we are a PFIC for any taxable year during which a U.S. Holder holds ordinary shares, the U.S. Holder may be subject to adverse tax consequences. Generally, gain recognized upon a disposition (including, under certain circumstances, a pledge) of ordinary shares by the U.S. Holder would be allocated ratably over the U.S. Holder’s holding period for such shares. The amounts allocated to the taxable year of disposition and to years before we became a PFIC would be taxed as ordinary income. The amount allocated to each other taxable year would be subject to tax at the highest rate in effect for that taxable year for individuals or corporations, as appropriate, and would be increased by an additional tax equal to interest on the resulting tax deemed deferred with respect to each such other taxable year. Further, to the extent that any distribution received by a U.S. Holder on its ordinary shares exceeds 125% of the average of the annual distributions on such ordinary shares received during the preceding three years or the U.S. Holder’s holding period, whichever is shorter, that distribution would be subject to taxation in the same manner described immediately above with respect to gain on disposition
Alternatively, if we are a PFIC and if our ordinary shares are “regularly traded” on a “qualified exchange,” a U.S. Holder could make a mark-to-market election that would result in tax treatment different from the general tax treatment described in the preceding paragraph. Our ordinary shares would be treated as “regularly traded” in any calendar year in which more than a de minimis quantity of the ordinary shares, are traded on a qualified exchange on at least 15 days during each calendar quarter. The Nasdaq Global Select Market is a qualified exchange for this purpose. If a U.S. Holder makes the mark-to-market election, the U.S. Holder generally will recognize as ordinary income any excess of the fair market value of the ordinary shares at the end of each taxable year over their adjusted tax basis, and will recognize an ordinary loss in respect of any excess of the adjusted tax basis of the ordinary shares over their fair market value at the end of the taxable year (but only to the extent of the net amount of income previously included as a result of the mark-to-market election). If a U.S. Holder makes the election, the U.S. Holder’s tax basis in the ordinary shares will be adjusted to reflect these income or loss amounts. Any gain recognized on the sale or other disposition of ordinary shares in a year when we are a PFIC will be treated as ordinary income and any loss will be treated as an ordinary loss (but only to the extent of the net amount of income previously included as a result of the mark-to-market election).
A timely election to treat a PFIC as a qualified electing fund under Section 1295 of the Code would result in alternative treatment. U.S. Holders should be aware, however, that we do not intend to satisfy the record-keeping and other requirements that would permit U.S. Holders to make qualified electing fund elections if we were a PFIC.
In addition, if we are a PFIC or, with respect to particular U.S. Holders, are treated as a PFIC for the taxable year in which we paid a dividend or for the prior taxable year, the preferential rates discussed above with respect to dividends paid to certain non-corporate U.S. Holders would not apply.
If a U.S. Holder owns ordinary shares during any year in which we are a PFIC, the holder generally must file an IRS Form 8621, generally with the holder’s federal income tax return for that year.
U.S. Holders should consult their tax advisers regarding whether we are or or may become a PFIC and the potential application of the PFIC rules.
Information reporting and backup withholding
Payments of dividends and sales proceeds that are made within the United States or through certain U.S.-related financial intermediaries generally are subject to information reporting, and may be subject to backup withholding, unless (i) the U.S. Holder is a corporation or other exempt recipient or (ii) in the case of backup withholding, the U.S. Holder provides a correct taxpayer identification number and certifies that it is not subject to backup withholding.
99
The amount of any backup withholding from a payment to a U.S. Holder will be allowed as a credit against the holder’s U.S. federal income tax liability and may entitle it to a refund, provided that the required information is timely furnished to the IRS.
Certain U.S. Holders who are individuals may be required to report information relating to their ownership of an interest in certain foreign financial assets, including stock of a non-U.S. person, generally on Form 8938, subject to exceptions (including an exception for stock held through a U.S. financial institution). U.S. Holders should consult their tax advisers regarding their reporting obligations with respect to ordinary shares.
F. Dividends and paying agents
Not applicable.
G. Statement by experts
Not applicable.
H. Documents on display
We are subject to the informational requirements of the Exchange Act. Accordingly, we are required to file reports and other information with the SEC, including annual reports on Form 20-F and reports on Form 6-K. You may inspect and copy reports and other information filed with the SEC at the Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Information on the operation of the Public Reference Room may be obtained by calling the SEC at 1-800-SEC-0330. In addition, the SEC maintains an Internet website that contains reports and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
I. Subsidiary information
Not applicable.
ITEM 11. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT RISK
We are exposed to a variety of financial risks: market risk (including foreign exchange risk and interest rate risk), credit risk and liquidity risk. Our overall risk management program focuses on the unpredictability of financial markets.
Market risk
We are exposed to foreign exchange risk arising from various currency exposures, primarily with respect to the British pound and the U.S. dollar. Our functional currency is the euro, but we receive payments from our main business partner GSK in British pounds and acquire materials in U.S. dollars. The exposure to British pounds is expected to be lower in future periods as a result of the termination of the GSK collaboration agreement. No formal practice has been established to manage the foreign exchange risk against our functional currency. As of December 31, 2013, there was outstanding a net amount of trade receivables denominated in British pounds of €3.1 million and trade payables denominated in US dollars of €0.4 million.
Proceeds from our IPO in U.S. dollars were converted to our functional currency, the euro.
Our interest rate risk arises from long-term borrowings that are issued at variable rates. This risk is partially offset by cash held at variable rates. Borrowings issued at fixed rates expose us to interest rate risk. During 2013, 2012 and 2011, our borrowings were denominated in euros. We manage our cash flow interest rate risk by using floating-to-fixed interest rate swaps.
If interest rates on borrowings had been 0.1% higher/lower with all other variables held constant, after-tax total comprehensive loss for the years ended December 31, 2013, 2012 and 2011 would have been respectively €0.8 million, €0.6 million and €0.4 million lower/higher as a result of changes in the fair value of the borrowings. The effect of a change in interest rates of 0.1% on borrowings would have had an insignificant effect on after-tax total comprehensive loss for the year as a result of changes in the fair value of the floating to fixed interest rate swap.
100
Credit Risk
GSK funding (milestone payments, collaboration revenue and reimbursable research expenses) has been critical for our product development programs and until the termination of our agreement with GSK, we considered it the main credit risk. We have a limited group of external counterparties of which the most significant was GSK. We manage credit risk on a group basis.
Our cash and cash equivalents are invested primarily in saving and deposit accounts with original maturities of three months or less. Saving and deposit accounts generate interest income. For banks and financial institutions, only independently rated parties with a minimum rating of ‘A’ are accepted at the beginning of the term.
We do business with a limited group of external parties. If external parties are independently rated, these ratings are used. If there is no independent rating, the credit quality of these parties is assessed, taking into account their financial position, past experience and other factors. As of December 31, 2013 no credit limits had been exceeded since the beginning of 2011. We have not incurred any losses over the reporting period, and management does not expect losses from non-performance by counterparties.
Liquidity Risk
We believe that our existing cash and cash equivalents and research funding that we expect to receive will be sufficient to fund our operating expenses, debt service obligations and capital expenditure requirements for at least the next 12 months.
ITEM 12. DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES
A. Debt securities
Not applicable.
B. Warrants and rights
Not applicable.
C. Other securities
Not applicable.
D. American Depositary Shares
Not applicable.
101
PART II
ITEM 13. DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES
A. Defaults
No matters to report.
B. Arrears and delinquencies
No matters to report.
ITEM 14. MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS
Not applicable.
ITEM 15. CONTROLS AND PROCEDURES
A. Disclosure Controls and Procedures
Our managing board, including our chief executive officer and chief financial officer, after evaluating the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of December 31, 2013, have concluded that based on the evaluation of these controls and procedures required by Rule 13a-15(b) of the Exchange Act, our disclosure controls and procedures were effective .
B. Management’s Annual Report on Internal Control over Financial Reporting
This Annual Report does not include a report of management's assessment regarding internal control over financial reporting due to a transition period established by rules of the SEC for newly public companies.
C. Attestation Report of the Registered Public Accounting Firm
This Annual Report does not include an attestation report of our registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
D. Changes in Internal Control over Financial Reporting
There has been no change in our internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act) that occurred during the period covered by this Annual Report that has materially affected, or is reasonably likely to materially affect, internal control over financial reporting.
ITEM 16. [RESERVED]
ITEM 16A. Audit committee financial expert
Our supervisory board has determined that Daan Ellens is an audit committee financial expert, as that term is defined by the SEC, and will be independent within the one year transition period measured from the date of our IPO, in accordance with Nasdaq rules.
ITEM 16B. Code of ethics
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics which covers a broad range of matters including the handling of conflicts of interest, compliance issues and other corporate policies such as insider trading and equal opportunity and non-discrimination standards. Our Code of Business Conduct applies to all of our supervisory directors, managing directors and employees. We have published our Code of Business Conduct and Ethics on our website, www.prosensa.eu.
ITEM 16C. Principal Accountant Fees and Services
The information required under by item 16C is included in note 32 of the consolidated financial statements in Item 18 of Part III.
102
ITEM 16D. Exemptions from the listing standards for audit committees
Not applicable.
ITEM 16E. Purchases of equity securities by the issuer and affiliated purchasers
In 2013, no purchases of our equity securities were made by or on behalf of Prosensa or any affiliated purchaser.
ITEM 16F. Change in registrant’s certifying accountant
Not applicable.
ITEM 16G. Corporate governance
Summary of Significant Corporate Governance Differences From Nasdaq Listing Standards
Our ordinary shares are listed on the Nasdaq Global Select Market, or Nasdaq. We are therefore required to comply with certain of the Nasdaq’s corporate governance listing standards, or the Nasdaq Standards. As a foreign private issuer, we may follow our home country’s corporate governance practices in lieu of certain of the Nasdaq Standards. Our corporate governance practices differ in certain respects from those that U.S. companies must adopt in order to maintain a Nasdaq listing. A brief, general summary of those differences is provided as follows.
Quorum requirements
In accordance with Dutch law and generally accepted business practices, our articles of association do not provide quorum requirements generally applicable to general meetings of shareholders. To this extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum that may not be less than one-third of the outstanding voting stock.
Solicitation of proxies
Although we must provide shareholders with an agenda and other relevant documents for the general meeting of shareholders, Dutch law does not have a regulatory regime for the solicitation of proxies and the solicitation of proxies is not a generally accepted business practice in the Netherlands. Thus our practice will vary from the requirement of Nasdaq Listing Rule 5620(b), which requires an issuer that is not a limited partnership to solicit proxies and provide proxy statements for all meetings of shareholders and shall provide copies of such proxy solicitation to Nasdaq.
Shareholder approval
We have exercised our option as a foreign private issuer to opt out of Nasdaq shareholder approval requirements for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of us and certain private placements. To this extent, our practice may vary from the requirements of Nasdaq Listing Rule 5635.
ITEM 16H. Mine safety disclosure
Not applicable.
103
PART III
ITEM 17. Financial statements
We have responded to Item 18 in lieu of this item.
ITEM 18. Financial statements
Financial Statements are filed as part of this annual report, see page F-1.
ITEM 19. Exhibits
(a) The following documents are filed as part of this registration statement:
| Exhibit No. |
Exhibit
|
|
1.1*
|
Articles of Association of Prosensa Holding N.V.
|
|
2.1
|
Form of Registration Rights Agreement between Prosensa Holding N.V. and the shareholders listed therein (incorporated by reference to exhibit 4.1 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 18, 2013).
|
|
4.1
|
Second Amended and Restated Shareholders’ Agreement dated January 16, 2012 between Prosensa Holding B.V. and certain of its shareholders (incorporated by reference to exhibit 10.1 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 10, 2013).
|
|
4.2
|
Classes B2 Shares and B3 Shares Subscription Agreement dated January 16, 2012 between Prosensa Holding B.V. and certain of its shareholders (incorporated by reference to exhibit 10.2 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 10, 2013).
|
|
4.3†
|
Amended Research and Licensing Agreement, dated March 1, 2008 between Prosensa Holding B.V. and Leiden University Medical Center (incorporated by reference to exhibit 10.3 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 10, 2013).
|
|
4.4†
|
Research and Development Collaboration and License Agreement, dated October 6, 2009 between Prosensa Holding B.V. and Glaxo Group Limited (incorporated by reference to exhibit 10.4 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 10, 2013).
|
|
4.5†
|
Amendment Agreement #1, dated July 1, 2011 between Prosensa Holding B.V. and Glaxo Group Limited (incorporated by reference to exhibit 10.5 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on May 24, 2013).
|
|
4.6*
|
Termination Agreement, dated January 12, 2014, between Prosensa Holding NV and Glaxo Group Limited.
|
|
4.7†
|
Research and Development Collaboration Agreement, dated January 1, 2010 between Prosensa Holding B.V. and L’Association Française Contre les Myopathies (incorporated by reference to exhibit 10.6 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on May 24, 2013).
|
|
4.8
|
Form of Supervisory Director and Managing Director Indemnification Agreement (incorporated by reference to exhibit 10.7 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 10, 2013).
|
|
4.9
|
English language summary of Lease Agreement between Prosensa Therapeutics B.V. and Stichting Biopartner Academisch Bedrijven Centrum Leiden (incorporated by reference to exhibit 10.8 of the Prosensa Holding N.V. registration statement on Form F-1 (Registration no. 333-188855) filed with the Commission on June 10, 2013).
|
|
8.1*
|
List of subsidiaries.
|
|
12.1*
|
Certification of Hans Schikan pursuant to 17 CFR 240.13a-14(a).
|
|
12.2*
|
Certification of Berndt Modig pursuant to 17 CFR 240.13a-14(a).
|
|
13.1*
|
Certification of Hans Schikan pursuant to 17 CFR 240.13a-14(b) and 18 U.S.C.1350
|
|
13.2*
|
Certification of Berndt Modig pursuant to 17 CFR 240.13a-14(b) and 18 U.S.C.1350
|
|
*
|
Filed herewith
|
|
†
|
Confidential treatment requested as to portions of the exhibit. Confidential materials omitted and filed separately with the Securities and Exchange Commission.
|
104
(b) Financial Statement Schedules
None.
105
Signatures
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 20-F and has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Leiden, the Netherlands on March 17, 2014.
|
PROSENSA HOLDING N.V.
|
|||
|
By:
|
/s/Hans G.C.P. Schikan
|
||
|
Name:
|
Hans G.C.P. Schikan
|
||
|
Title:
|
Chief Executive Officer
|
||
|
By:
|
/s/Berndt A.E. Modig
|
||
|
Name:
|
Berndt A.E. Modig
|
||
|
Title:
|
Chief Financial Officer
|
||
Index to consolidated financial statements
|
Report of independent, registered public accounting firm
|
F-2
|
|
Consolidated statement of comprehensive income
|
F-3
|
|
Consolidated balance sheet
|
F-4
|
|
Consolidated statement of changes in equity
|
F-5
|
|
Consolidated statement of cash flows
|
F-6
|
|
Notes to the consolidated financial statements
|
F-7
|
F - 1
Report of Independent Registered Public Accounting Firm
To Management Board and shareholders of Prosensa Holding N.V.:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statement of comprehensive income, changes in equity and cash flows present fairly, in all material respects, the financial position of Prosensa Holding N.V. and its subsidiaries at December 31, 2013 and December 31, 2012, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2013 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
PricewaterhouseCoopers Accountants N.V.
Utrecht, The Netherlands
March 17, 2014
/s/ drs. A.CM. van der Linden RA
F - 2
Consolidated statement of comprehensive income
|
€ (‘000)
|
Year ended 31 December
|
|||||||||||
|
Note
|
2011
|
2012
|
2013
|
|||||||||
|
License revenue
|
19 | 6,510 | 5,726 | 5,626 | ||||||||
|
Collaboration revenue
|
19 | 2,179 | 2,127 | 3,312 | ||||||||
|
Total revenue
|
8,689 | 7,853 | 8,938 | |||||||||
|
Other income
|
20 | 36 | 174 | 560 | ||||||||
|
Research and development expense
|
22 | (15,348 | ) | (14,393 | ) | (18,460 | ) | |||||
|
General and administrative expense
|
23 | (5,203 | ) | (4,023 | ) | (7,734 | ) | |||||
|
Other gains/(losses) - net
|
25 | 22 | 49 | 112 | ||||||||
|
Operating (loss)/gain
|
(11,804 | ) | (10,340 | ) | (16,584 | ) | ||||||
|
Finance income
|
26 | 434 | 796 | 645 | ||||||||
|
Finance costs
|
26 | (209 | ) | (348 | ) | (665 | ) | |||||
|
Finance income – net
|
225 | 448 | (20 | ) | ||||||||
|
Net Loss
|
(11,579 | ) | (9,892 | ) | (16,604 | ) | ||||||
|
Other comprehensive income
|
- | - | - | |||||||||
|
Total comprehensive loss
|
(11,579 | ) | (9,892 | ) | (16,604 | ) | ||||||
|
Loss per share from operations attributable to the equity holders of the company during the year (in € per share)
|
||||||||||||
|
Basic and diluted loss per share
|
28 | (0.60 | ) | (0.37 | ) | (0.51 | ) | |||||
* Total comprehensive loss is fully attributable to equity holders of the Company
The notes are an integral part of these consolidated financial statements.
F - 3
Consolidated balance sheet
|
€ (‘000)
|
As of December 31
|
||||||||
|
Note
|
2012
|
2013
|
|||||||
|
Assets
|
|||||||||
|
Non-current assets
|
|||||||||
|
Leasehold improvements and equipment
|
6 | 2,421 | 2,177 | ||||||
|
Intangible assets
|
7 | 869 | 758 | ||||||
|
Other financial assets
|
8,11 | 589 | 289 | ||||||
|
Total non-current assets
|
3,879 | 3,224 | |||||||
|
Current assets
|
|||||||||
|
Trade and other receivables
|
13 | 2,422 | 4,403 | ||||||
|
Prepayments
|
290 | 931 | |||||||
|
Cash and cash equivalents
|
14 | 40,738 | 82,232 | ||||||
|
Total current assets
|
43,450 | 87,566 | |||||||
|
Total assets
|
47,329 | 90,790 | |||||||
|
Equity and liabilities
|
|||||||||
|
Equity attributable to owners of the parent
|
|||||||||
|
Share capital
|
290 | 359 | |||||||
|
Share premium
|
56,118 | 119,222 | |||||||
|
Other reserves
|
1,056 | 2,123 | |||||||
|
Accumulated deficit
|
(31,998 | ) | (41,890 | ) | |||||
|
Unappropriated earnings
|
(9,892 | ) | (16,604 | ) | |||||
|
Total equity
|
15 | 15,574 | 63,210 | ||||||
|
Liabilities
|
|||||||||
|
Non-current liabilities
|
|||||||||
|
Borrowings – non-current portion
|
18 | 6,198 | 7,630 | ||||||
|
Derivative financial instruments
|
38 | 22 | |||||||
|
Deferred revenue
|
19,20 | 13,329 | 10,852 | ||||||
|
Total non-current liabilities
|
19,565 | 18,504 | |||||||
|
Current liabilities
|
|||||||||
|
Borrowings – current portion
|
18 | 343 | 191 | ||||||
|
Derivative financial instruments
|
10 | 8 | |||||||
|
Trade and other payables
|
17 | 5,065 | 5,150 | ||||||
|
Deferred revenue
|
19,20 | 6,772 | 3,727 | ||||||
|
Total current liabilities
|
12,190 | 9,076 | |||||||
|
Total liabilities
|
31,755 | 27,580 | |||||||
|
Total equity and liabilities
|
47,329 | 90,790 | |||||||
The notes are an integral part of these consolidated financial statements.
F - 4
Consolidated statement of changes in equity
|
€ (‘000)
|
Common Share
capital
|
Class O Share
capital
|
Class A Share
capital
|
Class B Share
capital
|
Total Share
capital
|
Share
premium
|
Other
reserves
|
Retained
earnings
|
Unappropriated
earnings
|
Total
equity
|
||||||||||||||||||||
|
Balance at 1 January 2011
|
26 | 7 | 74 | 84 | 190 | 33,557 | 207 | (19,778 | ) | (641 | ) | 13,535 | ||||||||||||||||||
|
Net loss
|
(11,579 | ) | (11,579 | ) | ||||||||||||||||||||||||||
|
Appropriation of result
|
(641 | ) | 641 | - | ||||||||||||||||||||||||||
|
Share - based payments
|
669 | 669 | ||||||||||||||||||||||||||||
|
Proceeds from shares issued
|
5 | 5 | 5 | |||||||||||||||||||||||||||
|
Balance at 31 December 2011
|
31 | 7 | 74 | 84 | 195 | 33,557 | 876 | (20,419 | ) | (11,579 | ) | 2,630 | ||||||||||||||||||
|
Balance at 1 January 2012
|
31 | 7 | 74 | 84 | 195 | 33,557 | 876 | (20,419 | ) | (11,579 | ) | 2,630 | ||||||||||||||||||
|
Net loss
|
(9,892 | ) | (9,892 | ) | ||||||||||||||||||||||||||
|
Appropriation of result
|
(11,579 | ) | 11,579 | - | ||||||||||||||||||||||||||
|
Share - based payments
|
180 | 180 | ||||||||||||||||||||||||||||
|
Proceeds from shares issued
|
4 | 91 | 95 | 22,937 | 23,032 | |||||||||||||||||||||||||
|
Share issuance cost
|
(376 | ) | (376 | ) | ||||||||||||||||||||||||||
|
Balance at 31 December 2012
|
35 | 7 | 74 | 175 | 290 | 56,118 | 1,056 | (31,998 | ) | (9,892 | ) | 15,574 | ||||||||||||||||||
|
Balance at January 1, 2013
|
35 | 7 | 74 | 175 | 290 | 56,118 | 1,056 | (31,998 | ) | (9,892 | ) | 15,574 | ||||||||||||||||||
|
Net loss
|
(16,604 | ) | (16,604 | ) | ||||||||||||||||||||||||||
|
Appropriation of result
|
(9,892 | ) | 9,892 | - | ||||||||||||||||||||||||||
|
Share-based payments
|
1,067 | 1,067 | ||||||||||||||||||||||||||||
|
Proceeds from shares issued
|
69 | 69 | 63,958 | 64,027 | ||||||||||||||||||||||||||
|
Share issuance cost
|
(854 | ) | (854 | ) | ||||||||||||||||||||||||||
|
Conversion preference shares
|
256 | (7 | ) | (74 | ) | (175 | ) | - | - | |||||||||||||||||||||
|
Balance at December 31, 2013
|
359 | - | - | - | 359 | 119,222 | 2,123 | (41,890 | ) | (16,604 | ) | 63,210 | ||||||||||||||||||
The notes are an integral part of these consolidated financial statements.
F - 5
Consolidated statement of cash flows
|
€ (‘000)
|
Year ended December 31
|
|||||||||||
|
Note
|
2011
|
2012
|
2013
|
|||||||||
|
Cash flows from operating activities
|
||||||||||||
|
Net loss
|
(11,579 | ) | (9,892 | ) | (16,604 | ) | ||||||
|
Adjustments for:
|
||||||||||||
|
- Amortization/depreciation
|
6,7 | 1,060 | 1,165 | 1,203 | ||||||||
|
- Costs employee share option plan
|
16 | 669 | 180 | 1,067 | ||||||||
|
- Reversal finance income, net
|
26 | (225 | ) | (448 | ) | 20 | ||||||
|
- Changes in the fair value of derivatives
|
- | (5 | ) | (18 | ) | |||||||
|
- Changes in trade and other receivables
|
13 | 537 | (745 | ) | (2,751 | ) | ||||||
|
- Changes in trade and other payables
|
17 | 2,817 | (990 | ) | 28 | |||||||
|
- Currency effect (outstanding) receivables and payables
|
- | (141 | ) | (133 | ) | |||||||
|
- Changes in deferred revenue
|
19 | (823 | ) | 6,680 | (5,523 | ) | ||||||
| (7,544 | ) | (4,196 | ) | (22,711 | ) | |||||||
|
Interest received
|
365 | 546 | 790 | |||||||||
|
Interest paid
|
(112 | ) | (75 | ) | (47 | ) | ||||||
|
Cash (used in)/generated from operating activities
|
(7,291 | ) | (3,725 | ) | (21,968 | ) | ||||||
|
Cash flows from investing activities
|
||||||||||||
|
Purchases of tangible fixed assets
|
6 | (1,446 | ) | (182 | ) | (605 | ) | |||||
|
Purchases of intangible assets
|
7 | (158 | ) | (198 | ) | (121 | ) | |||||
|
Decrease of other financial assets
|
11 | (47 | ) | 53 | 300 | |||||||
|
Net cash (used in)/generated from investing activities
|
(1,651 | ) | (327 | ) | (426 | ) | ||||||
|
Cash flows from financing activities
|
||||||||||||
|
Proceeds from issuance of share capital
|
15 | 5 | 23,032 | 64,027 | ||||||||
|
Issuance cost deducted from share premium
|
15 | - | (376 | ) | (854 | ) | ||||||
|
Proceeds from borrowings
|
18 | 473 | 3,853 | 1,052 | ||||||||
|
Redemption financial lease
|
18 | (480 | ) | (432 | ) | (248 | ) | |||||
|
Repayments of borrowings
|
18 | (100 | ) | (100 | ) | (100 | ) | |||||
|
Net cash (used in)/generated from financing activities
|
(102 | ) | 25,977 | 63,877 | ||||||||
|
Net increase/(decrease) in cash and cash equivalents
|
(9,044 | ) | 21,925 | 41,483 | ||||||||
|
Currency effect cash and cash equivalents
|
- | 70 | 11 | |||||||||
|
Cash and cash equivalents at beginning of the period
|
27,787 | 18,743 | 40,738 | |||||||||
|
Cash and cash equivalents at end of the period
|
12 | 18,743 | 40,738 | 82,232 | ||||||||
|
Restricted cash
|
9 | 500 | 500 | 200 | ||||||||
The notes are an integral part of these consolidated financial statements.
F - 6
Notes to the consolidated financial statements
1. General information
The activities of Prosensa Holding N.V. and its subsidiaries (together “the Company”) primarily consist of developing innovative, RNA based therapeutics to fill unmet medical needs for patients with genetic diseases.
On July 3, 2013 the Company completed its initial public offering (“IPO”). The IPO process resulted in the listing of the Company’s ordinary shares, under the ticker symbol “RNA” in the United States on the NASDAQ Global Select Market.
Effective January 12, 2014, GlaxoSmithKline (“GSK”) and the Company mutually agreed to terminate the research and collaboration agreement entered into in October 6, 2009. As of the effective date, the Company received all licenses rights back and has no remaining research obligations.
The Company was previously organized as a B.V. which was incorporated and domiciled in the Netherlands. On July 3, 2013 the Company changed its legal form from a B.V. to a N.V. and remains incorporated and domiciled in the Netherlands. The address of its registered office is J.H. Oortweg 21, Leiden. Prosensa Holding N.V. is the ultimate parent of the following group of entities:
|
1.
|
Prosensa Therapeutics B.V. (100%);
|
|
2.
|
Prosensa Technologies B.V. (100%)
|
|
3.
|
Polybiotics B.V. (100%); and
|
|
4.
|
Prosensa Inc. (100%)
|
On December 10, 2013 Prosensa Inc., a 100% subsidiary of Prosensa Holding N.V. was incorporated under the laws of the state of Delaware. As of December 31, 2013 Prosensa Inc. had 2 employees in the functions of Investor Relations and Regulatory Affairs and had no other activities.
The shares of Prosensa Holding N.V. are held by multiple shareholders, none of them having a share in the Company in excess of 25%.
The Management Board and Supervisory Board approved the consolidated financial statements for issuance on March 17, 2014.
2. Summary of significant accounting policies
The principal accounting policies applied in the preparation of these consolidated financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
2.1 Basis of preparation
The consolidated financial statements of Prosensa have been prepared in accordance with International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). There are no material differences between IFRS as adopted by the European Union and IFRS as issued by the IASB. The consolidated financial statements have been prepared under the historical cost convention, as modified by derivative instruments at fair value through profit or loss.
The preparation of financial statements in conformity with IFRS requires the use of certain critical accounting estimates. It requires management to exercise its judgment in the process of applying the Company’s accounting policies. The areas involving a higher degree of judgment or complexity, or areas where assumptions and estimates are significant to the consolidated financial statements are disclosed in note 4.
The Consolidated Statements of Cashflows for the years ended December 31, 2012 and December 31, 2011, have been revised by €52 thousand and €560 thousand respectively, impacting the cash flow generated from financing activities and net cash used in investing activities with respect to finance lease additions. There were no changes to the Consolidated Balance Sheets, Net Income or Total Equity as a result of these reclassifications in the respective periods.
F - 7
2.2 Changes in accounting policy and disclosures
(a) New and amended standards adopted by the Company
The following standards and amendments to standards became effective for annual periods on January 1, 2013 and have been adopted by the Company in the preparation of the consolidated financial statements:
|
·
|
Amendment to IFRS 7 Financial instruments – disclosures
|
|
·
|
IFRS 10 Consolidated financial statements
|
|
·
|
IFRS 11 Joint arrangements
|
|
·
|
IFRS 12 Disclosures of interest in other entities
|
|
·
|
IFRS 13 Fair value measurement
|
|
·
|
Amendment to IAS 1 Presentation of financial statements
|
|
·
|
Improvements to IAS 16 Property plant and equipment
|
|
·
|
Amendment to IAS 19 Employee benefits
|
|
·
|
IAS 27 (revised 2011) Separate financial statements
|
|
·
|
IAS 28 (revised 2011) Investments in associates and joint ventures
|
|
·
|
Improvements to IAS 32 Financial statements – presentation
|
|
·
|
Improvements to IAS 34 Interim financial reporting
|
The adoption of these new standards and amendments to standards did not impact the Company’s financial position or results of operations. The adoption of IFRS 13, “Fair value measurement”, resulted in revised disclosure of the Company’s fair value measurements. Refer to note 3.3 of these consolidated financial statements for further information.
(b) New standards and interpretations not yet adopted by the Company
The standards which could have a significant effect on the consolidated financial statements of the Company are IFRS 9 “Financial Instruments”, Amendments to IAS 36 “Impairment of Assets” and IFRIC 21 “Levies”. IFRS 9 is the first step in the process of replacing IAS 39 “Financial Instruments: Recognition and Measurement”. The Company has yet to assess IFRS 9’s full impact. Amendments to IAS 36 removes certain disclosures of the recoverable amount of Cash Generating Units which had been included in IAS 36 by the issue of IFRS 13. The Company has yet to assess Amendments to IAS 36’s full impact. IFRIC 21 sets out the accounting for an obligation to pay a levy that is not income tax. The interpretation addresses what the obligating event is that gives rise to pay the levy and when a liability should be recognized. The Company has yet to assess IFRIC 21’s full impact.
2.3 Consolidation
Subsidiaries are all entities over which the Company has control. The Company controls an entity when the Company is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power over the entity.. Subsidiaries are fully consolidated from the date on which control is transferred to the Company. They are de-consolidated from the date that control ceases.
Intercompany transactions, balances and unrealized gains on transactions between group companies are eliminated. Unrealized lossess are also eliminated. When necessary amounts reported by subsidiaries have been adjusted to conform with the Company’s accounting policies.
2.4 Foreign currency translation
Functional and presentation currency
Items included in the financial statements of each of the Company’s entities are measured using the currency of the primary economic environment in which the entity operates (‘the functional currency’). The consolidated financial statements are presented in Euro (‘€’), which is the Company’s functional and presentation currency.
Transactions and balances
Foreign currency transactions are translated into the functional currency using the exchange rates prevailing at the dates of the transactions or valuation where items are re-measured. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognized in the income statement.
F - 8
Foreign exchange gains and losses that relate to borrowings and cash and cash equivalents are presented in the income statement within ‘finance income or cost.’ All other foreign exchange gains and losses are presented in the income statement within ‘other gains / (losses) – net.’
2.5 Notes to the cash flow statement
The cash flow statement has been prepared using the indirect method. The cash disclosed in the cash flow statement is comprised of cash and cash equivalents. Cash comprises cash on hand and demand deposits. Cash equivalents are short-term, highly liquid investments that are readily convertible to known amounts of cash and are subject to an insignificant risk of changes in value. Cash flows denominated in foreign currencies have been translated at the average exchange rates. Exchange differences, if any, affecting cash items are shown separately in the cash flow statement. Interest paid and received, dividends received and income tax are included in the cash from operating activities.
2.6 Leasehold improvements and equipment
Leasehold improvements and equipment comprise mainly leasehold improvements, laboratory equipment and other office equipment. Leasehold improvements and equipment are stated at historical cost less depreciation. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
Subsequent costs are included in the asset’s carrying amount or recognized as a separate asset only when it is probable that future economic benefits associated with the item will flow to the Company and the cost of the item can be measured reliably. The carrying amount of the replaced part is derecognized. All other repairs and maintenance are charged to the income statement during the financial period in which they are incurred.
Depreciation on leasehold improvements and equipment is calculated using the straight-line method to allocate their cost over their estimated useful lives, as follows:
|
Leasehold improvements
|
5-10 years
|
|
Laboratory equipment
|
3-5 years
|
|
Office equipment
|
3-5 years
|
Leasehold improvements are depreciated over the shorter of the expected lease term for the buildings the assets relate to or the estimated useful life.
Tangible fixed assets that are leased under a finance lease are depreciated over the shorter of the lease term or their estimated useful lives unless there is reasonable certainty that the Company will obtain ownership at the end of the lease term, in which case the assets are depreciated over their estimated useful lives.
The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at the end of each reporting period.
An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount (note 2.8). The impairment is recognized through operating income and loss.
Gains and losses on disposals are determined by comparing the proceeds with the carrying amount and are recognized within other gains—net in the income statement.
2.7 Intangible assets
Intangible assets comprise mainly acquired patents, licenses and software. Intangible assets are initially measured at acquisition cost or production cost, including any directly attributable costs of preparing the asset for its intended use. Amortization begins when an asset is available for use and amortization is calculated using the straight-line method to allocate their cost over their estimated useful lives, as follows:
|
Patents & licenses
|
10 years or period till patent or license expires
|
|
Software
|
3-5 years
|
The Company only owns intangible assets with a definite useful life.
F - 9
Intangible assets are carried at cost less accumulated amortization and accumulated impairment losses. The useful lives of intangible assets are reviewed at each reporting date. The effect of any adjustment to useful lives is recognized prospectively as a change of accounting estimate.
Amortization of intangible assets is recognized on the relevant line of the income statement according to the purpose for which the asset is used.
Research and development
Research expenses are recognized as expenses when incurred. Costs incurred on development projects are recognized as intangible assets as of the date as of which it can be established that it is probable that future economic benefits attributable to the asset will flow to the Company considering its technological and commercial feasibility. This is generally the case when regulatory approval for commercialization is achieved and costs can be measured reliably. Given the current stage of the development of the Company’s products, no development expenditures have yet been capitalized. Intellectual property-related costs for patents are part of the expenditure for the research and development projects. Therefore, registration costs for patents are expensed when incurred as long as the research and development project concerned does not meet the criteria for capitalization.
2.8 Impairment of non-financial assets
Assets that are subject to amortization are reviewed for impairment whenever events or changes in circumstances indicate the carrying amount may not be recoverable. An impairment loss is recognized as the amount by which the asset’s carrying amount exceeds its recoverable amount. The recoverable amount is the higher of an asset’s fair value less costs to sell and value in use. For the purpose of assessing impairment, assets are grouped at the lowest levels for which there are separately identifiable cash flows (cash-generating units). Non-financial assets that were previously impaired are reviewed for possible reversal of the impairment at each reporting date.
2.9 Other financial assets
Classification
The Company classifies its financial assets in the following categories: a) Financial assets at fair value through profit and loss, and b) Loans and receivables.
The classification depends on the purpose for which the financial assets were acquired. Management determines the classification of its financial assets at initial recognition.
a) Financial assets at fair value through profit and loss
Derivative instruments are categorized in this category as the Company currently does not apply hedge accounting (note 2.13). Assets in this category are classified as current assets if expected to be settled within 12 months, otherwise they are classified as non-current.
b) Loans and receivables
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. They are included in current assets, except for maturities greater than 12 months after the end of the reporting period. These are classified as non-current assets. The Company’s loans and receivables comprise ‘trade and other receivables’ as well as ‘financial assets non-current’ which relate to lease deposits on the balance sheet (note 2.15 and 2.16).
2.10 Recognition and measurement
Regular purchases and sales of financial assets are recognized on the trade-date—the date on which the Company commits to purchase or sell the asset. Financial assets carried at fair value through profit or loss are initially recognized at fair value, and transaction costs are expensed in the income statement. Financial assets at fair value through profit or loss are subsequently carried at fair value. Loans and receivables are subsequently carried at amortized cost using the effective interest method. Financial assets are derecognized when the rights to receive cash flows from the investments have expired or have been transferred and the Company has transferred substantially all risks and rewards of ownership.
Gains or losses arising from changes in the fair value of the ‘financial assets at fair value through profit or loss’ category are presented in the income statement within finance income-net -in the period in which they arise.
F - 10
2.11 Offsetting financial instruments
Financial assets and liabilities are offset and the net amount reported in the balance sheet when there is a legally enforceable right to offset the recognized amounts and there is an intention to settle on a net basis, or realize the asset and settle the liability simultaneously.
2.12 Impairment of financial assets
Loans and receivables
The Company assesses at the end of each reporting period whether there is objective evidence that a financial asset or group of financial assets is impaired. Impairment losses are incurred only if there is objective evidence of impairment as a result of one or more events that occurred after the initial recognition of the asset (a loss event) and that loss event (or events) has an impact on the estimated future cash flows of the financial asset or group of financial assets that can be reliably estimated.
The criteria the Company uses to determine if there is objective evidence of an impairment loss include:
|
·
|
significant financial difficulty of the obligor;
|
|
·
|
a breach of contract, such as a default or delinquency in payments;
|
|
·
|
the Company, for economic or legal reasons relating to the counterparty’s financial difficulty, granting to the counterparty a concession that the Company would not otherwise consider; or
|
|
·
|
it becomes probable that the counterparty will enter bankruptcy or other financial reorganization.
|
The amount of the loss is measured as the difference between the asset’s carrying amount and the present value of estimated future cash flows (excluding future credit losses that have not been incurred) discounted at the financial asset’s original effective interest rate. The carrying amount of the asset is reduced either directly or through use of an allowance amount. The amount of the loss is recognized in the consolidated income statement.
If, in a subsequent period, the amount of the impairment loss decreases and the decrease can be related objectively to an event occurring after the impairment was recognized (such as an improvement in the debtor’s credit rating), the reversal of the previously recognized impairment loss is recognized in the consolidated income statement.
2.13 Derivative financial instruments and hedging activities
Derivatives are initially recognized at fair value on the date a derivative contract is entered into and are subsequently re-measured at their fair value. The resulting gain or loss is recognized in the consolidated income statement as the Company currently does not apply hedge accounting.
2.14 Inventories
Inventories are stated at the lower of cost and net realizable value. Cost is determined using the first-in, first-out (FIFO) method. The cost of finished goods and work in progress comprises manufacturing design and validation costs, raw materials, direct labor, other direct costs and related production overheads (based on normal operating capacity). It excludes borrowing costs. Net realizable value is the estimated selling price in the ordinary course of business, less applicable variable selling expenses.
All inventories are used in research and development activities and have no alternative use, consequently they are valued at nil due to the uncertainty that future economic benefits will flow to the Company from their use.
2.15 Trade receivables
Trade receivables are amounts due from customers for license fee payments or services performed in the ordinary course of business. If collection is expected in one year or less (or in the normal operating cycle of the business if longer), they are classified as current assets. Trade receivables are recognized initially at fair value and subsequently measured at amortized cost using the effective interest method, less provision for impairment, if any.
2.16 Cash and cash equivalents
Cash and cash equivalents include cash on hand, deposits held at call with banks and current accounts with banks. Bank overdrafts are shown within borrowings in current liabilities on the balance sheet. Cash equivalents are short-term, liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and are subject to an insignificant risk of changes in value. Cash balances which are not freely available for use are classified as restricted cash as a component of other financial assets (note 11).
F - 11
2.17 Equity
The Company classifies an instrument, or its component parts, on initial recognition as a financial liability or an equity instrument in accordance with the substance of the contractual arrangement and the definitions of a financial liability and an equity instrument.
An instrument is classified as a financial liability when it is either (i) a contractual obligation to deliver cash or another financial asset to another entity or to exchange financial assets or financial liabilities with another entity under conditions that are potentially unfavorable to the Company; or (ii) a contract that will or may be settled in the Company’s own equity instruments and is a non-derivative for which the Company is or may be obliged to deliver a variable number of the Company’s own equity instruments or a derivative that will or may be settled other than by the exchange of a fixed amount of cash or another financial asset for a fixed number of the Company’s own equity instruments.
An equity instrument is defined as any contract that evidences a residual interest in the assets of an entity after deducting all of its liabilities. An instrument is an equity instrument only if the issuer has an unconditional right to avoid settlement in cash or another financial asset.
Ordinary shares
Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction from the proceeds, net of tax.
Preference shares
A financial instrument or its component parts are classified on initial recognition as a financial liability or a financial asset or an equity instrument in accordance with the substance of the contractual arrangement and the definitions of a financial liability or a financial asset and an equity instrument.
In previous years the Company issued three classes of preference shares, Class A, Class B and Class O shares.
a) Class A and Class B preference shares
Class A and Class B shares had a dividend preference over ordinary shares and Class O shares. In addition Class A and Class B shares had an anti-dilution protection that is not applicable for Class O and ordinary Shares. The anti-dilution protection was under the full control of the Company and did not affect the equity classification of the Class A and Class B shares.
Dividends paid on the preference shares were treated as profit appropriation.
b) Class O preference shares
Class O shares had a dividend preference over ordinary shares. Class O shares qualified as equity.
Pursuant to the Shareholders’ Agreement all of the outstanding preferred shares converted into ordinary shares upon the consummation of the IPO with a conversion rate of one-to-one.
2.18 Trade payables
Trade payables are obligations to pay for goods or services that have been acquired in the ordinary course of business from suppliers. Accounts payable are classified as current liabilities if payment is due within one year or less (or in the normal operating cycle of the business if longer). If not, they are presented as non-current liabilities.
Trade payables are recognized initially at fair value and subsequently measured at amortized cost using the effective interest method.
2.19 Borrowings
Borrowings are recognized initially at fair value, net of transaction costs incurred. Borrowings are subsequently carried at amortized cost; any difference between the proceeds (net of transaction costs) and the redemption value is recognized in the income statement over the period of the borrowings using the effective interest method.
F - 12
The Company has obtained loans from various governmental and non-profit groups. To encourage research and development , the Company obtained certain loans that generally bear an interest rate below the market interest rate, considered by the Company to be 12% over the last four years. The difference between fair value of the loans and the notional amount of the loans at inception is treated as a donation received for certain research performed by the Company and is deferred and recognized as Other income during the periods during which expenses are incurred.
Certain loans from patient organizations have no fixed redemption schemes and repayment is due when certain pre-determined milestones are met. A change in estimate on the redemption date has an impact on the borrowing value at each subsequent balance sheet date. The borrowing value at a certain date equals the discounted value of the redemption using the effective interest rate. The impact of the change in estimate is reflected as financial expenses.
2.20 Current and deferred income tax
The tax expense for the period comprises current and deferred tax. Tax effects are recognized in the income statement, except to the extent that it relates to items recognized in other comprehensive income or directly in equity. In this case the tax is also recognized in other comprehensive income or directly in equity, respectively.
The current income tax charge is calculated on the basis of the tax laws enacted or substantively enacted at the balance sheet date in the countries where the Company’s subsidiaries and associates operate and generate taxable income. Management periodically evaluates positions taken in tax returns with respect to situations in which applicable tax regulation is subject to interpretation. It establishes provisions where appropriate on the basis of amounts expected to be paid to the tax authorities.
Deferred income tax is recognized, using the liability method, on temporary differences arising between the tax basis of assets and liabilities and their carrying amounts in the consolidated financial statements. However, the deferred income tax is not accounted for if it arises from initial recognition of an asset or liability in a transaction other than a business combination that at the time of the transaction affects neither accounting nor taxable profit and loss. Deferred income tax is determined using tax rates (and laws) that have been enacted or substantially enacted by the balance sheet date and are expected to apply when the related deferred income tax asset is realized or the deferred income tax liability is settled.
Deferred income tax assets are recognized only to the extent that it is probable that future taxable profit will be available against which the temporary differences can be utilized.
Deferred income tax is provided on temporary differences arising on investments in subsidiaries and associates, except where the timing of the reversal of the temporary difference is controlled by the Company and it is probable that the temporary difference will not reverse in the foreseeable future.
Deferred income tax assets and liabilities are offset when there is a legally enforceable right to offset current tax assets against current tax liabilities and when the deferred income taxes assets and liabilities relate to income taxes levied by the same taxation authority on either the taxable entity or different taxable entities where there is an intention to settle the balances on a net basis.
2.21 Employee benefits
(a) Pension obligations
The Company operates a defined contribution pension plan for all employees funded through payments to an insurance company. The Company has no legal or constructive obligations to pay further contributions if the fund does not hold sufficient assets to pay all employees the benefits relating to employee service in the current and prior periods. The contributions are recognized as employee benefit expense when they are due. Prepaid contributions are recognized as an asset to the extent that a cash refund or a reduction in the future payments is available.
(b) Termination benefits
Termination benefits are payable when employment is terminated by the Company before the normal retirement date, or whenever an employee accepts voluntary redundancy in exchange for these benefits. The Company recognizes termination benefits when it is demonstrably committed to either: terminating the employment of current employees according to a detailed formal plan without possibility of withdrawal; or providing termination benefits as a result of an offer made to encourage voluntary redundancy. Benefits falling due more than 12 months after the end of the reporting period are discounted to their present value.
F - 13
(c) Bonus plans
The Company recognizes a liability and an expense for bonus plans if contractually obliged or if there is a past practice that has created a constructive obligation.
2.22 Share-based payments
The Company operates equity-settled share-based compensation plans, under which it receives services from managing directors, supervisory directors and selected employees as consideration for equity instruments (options or restricted shares) of the Company. The fair value of these equity instruments granted in exchange for the employee services received is recognized as an expense against a credit in equity. The total amount to be expensed is determined by reference to the grant date fair value of the equity instruments granted:
|
·
|
including any market performance conditions;
|
|
·
|
excluding the impact of any service and non-market performance vesting conditions ; and
|
|
·
|
including the impact of any non-vesting conditions.
|
Vesting conditions are included in assumptions about the number of equity instruments that are expected to vest. The total expense is recognized over the vesting period, which is the period over which all of the specified vesting conditions are to be satisfied. At the end of each reporting period, the Company revises its estimates of the number of equity instruments that are expected to vest based on the non-market vesting conditions. It recognizes the impact of the revision to previous estimates, if any, in the income statement, with a corresponding adjustment to equity.
If the terms of an equity-settled award are modified at a minimum an expense is recognized as if the terms had not been modified. An additional expense is recognized for any modification that increases the total fair value of the share-based payment arrangement or is otherwise beneficial to the employee as measured at the date of modification.
Where the Company is receiving employee services in a share-based payment transaction settled by a shareholder, it measures the services received as an equity-settled share-based payment transaction. The fair value of employee services received, measured by reference to the grant date fair value, is recognized over the vesting period with a corresponding adjustment to equity.
In 2012 the Company granted equity-settled options to members of the Management Board, subject to vesting only upon a liquidity event such as a change of control of the Company or an IPO and are further subject to continued employment conditions until the date such an event occurs. The number of options that vest will depend on the share price development following the IPO. This market performance condition is included in the fair value of the options granted.
As from the date such a liquidity event is deemed to be probable, the fair value is recognized as an expense over the estimated vesting period, taking into account the services received during the period from the grant date.
The Company’s share-based compensation plans qualify as equity-settled share-based payment transactions. When the options are exercised, the Company issues new shares. The proceeds received net of any directly attributable transaction costs are credited to share capital (nominal value) and share premium when the options are exercised.
The social security contributions payable by the Company in connection with the vesting of the share options or restricted shares is considered an integral part of the grant itself, and the charge will be treated as a cash-settled transaction. Normally, the maximum social security charge of an employee that receives option grants is already met and no additional social security charges are due.
2.23 Revenue recognition
Revenue comprises the fair value of the consideration received or receivable for the sale of licenses and services in the ordinary course of the Company’s activities. Revenue is shown net of value-added tax, returns, rebates and discounts and after eliminating sales within the Company.
The Company recognizes revenue when the amount of revenue can be reliably measured, it is probable that future economic benefits will flow to the entity and when specific criteria have been met for each of the Company’s activities as described below. The Company bases its estimates on historical results, taking into consideration the type of customer, the type of transaction and the specifics of each arrangement.
F - 14
License revenue
License revenue can comprise upfront payments and milestone payments.
a) Upfront payments
Non-refundable upfront licensing fees and certain guaranteed, time-based payments that require continuing involvement in the form of research and development, manufacturing or other efforts by the Company are recognized in the period of the expected performance.
Upon signing of the Research & Development and Collaboration & License agreement with GlaxoSmithKline (“GSK”) the Company received €17.2 million (£16.0 million) as a non-refundable upfront payment. The expected performance was deemed to occur on a straight line basis over a 5 year period. The Company was obliged, until the termination of the agreement in January 2014, to provide research and development services regarding certain pre-defined programs in the agreement. Other upfront payments received from GSK have been recognized by reference to the stage of completion of the underlying agreement at the balance sheet date (also referred to as percentage of completion method) whenever the rendering of services under a license revenue agreement could be estimated reliably.
The rendering of services can be estimated reliably when, in addition to general conditions mentioned further above, the following conditions are met:
|
·
|
stage of completion of the transaction at the balance sheet date can be measured reliably; and
|
|
·
|
costs incurred for the transaction and the costs to complete the transaction can be measured reliably.
|
When the above criteria are not met, revenue arising from the rendering of services is recognized only to the extent of the expenses recognized that are recoverable (cost-recovery approach). Costs under these types of arrangements are expensed as incurred and therefore the pattern of cost recognition may be different than revenue recognition.
b) Milestone payments
Milestone payments are contingent upon the achievement of contractually stipulated targets. The achievement of these milestones depended largely on meeting specific requirements laid out in the GSK collaboration agreement, so the related revenue was only recognized once substantive services had been performed in relation to the payment and contractual milestones had been fully achieved and confirmed by the counterparty (note 19).
Collaboration revenue
Collaboration revenue from contracts, typically from delivering research and development services, which may or may not be related to a licensing agreement, is recognized on the basis of labor hours delivered at the contractual full time employee rates.
Cost reimbursements to which the Company is entitled to under certain agreements are recognized in the income statement in the same period of the recorded cost they intend to compensate. When the reimbursable costs are not yet invoiced these amounts are included as a component of trade and other receivables on the balance sheet.
Interest income
Interest income is recognized using the effective interest method. When a loan and receivable is impaired, the Company reduces the carrying amount to its recoverable amount, being the estimated future cash flow discounted at the original effective interest rate of the instrument, and continues unwinding the discount as interest income. Interest income on impaired loan and receivables are recognized using the original effective interest rate.
2.24 Government grants
The Company receives certain government grants, which support its research effort in defined projects. These grants generally provide for reimbursement of approved costs incurred as defined in the respective grants. Income in respect of grants also includes contributions towards the costs of research and development. Income is recognized when costs under each grant are incurred in accordance with the terms and conditions of the grant and the collectability of the receivable is reasonably assured.
Government grants relating to costs are deferred and recognized in the income statement over the period necessary to match them with the costs they are intended to compensate. When the cash in relation to recognized government grants is not yet received the amount is included as a receivable on the balance sheet.
F - 15
The Company recognizes income from government grants under ‘Other income’ in the income statement.
2.25 Recognition research and development expenses
Research expenditures are recognized as expenses when incurred except when certain criteria for capitalization as intangible assets are met (note 2.7). At each balance sheet date, the Company estimates the level of service performed by the vendors and the associated cost incurred for the services performed.
2.26 Leases
Leases in which a significant portion of the risks and rewards of ownership are retained by the lessor are classified as operating leases. Payments made under operating leases (net of any incentives received from the lessor) are charged to the income statement on a straight-line basis over the period of the lease.
The Company leases certain laboratory equipment and office equipment. Leases for leasehold improvements and equipment where the Company bears substantially all the risks and rewards of ownership are classified as finance leases. Finance leases are capitalized at the lease’s commencement at the lower of the fair value of the leased property or the present value of the minimum lease payments.
Each finance lease payment is allocated between the liability and finance charges in order to achieve a constant rate on the finance balance outstanding. The finance balances, net of finance charges, are included in other long-term payables. The interest element of the finance cost is charged to the income statement over the lease period to produce a constant periodic rate of interest on the remaining balance of the liability for each period. The laboratory and office equipment acquired under finance leases are depreciated over the shorter of the useful life of the asset or the lease term.
2.27 Dividend distribution
Dividend distribution to the Company’s shareholders shall be recognized as a liability in the Company’s financial statements in the period in which the dividends are approved by the Company’s shareholders.
3. Financial risk management
3.1 Financial risk factors
The Company’s activities expose it to a variety of financial risks: market risk (including currency risk, fair value interest rate risk, cash flow interest rate risk and price risk), credit risk and liquidity risk. The Company’s overall risk management program focuses on the unpredictability of financial markets and seeks to minimize potential adverse effects on the Company’s financial performance. The Company uses derivative financial instruments on a limited scale to hedge certain risk exposures. No hedge accounting is applied.
Risk management is carried out by the financial management of the Company. The financial management identifies, evaluates and hedges financial risks in close co-operation with the Company’s operating units.
Market risk
a) Foreign exchange risk
The Company operates internationally and is exposed to foreign exchange risk arising from various currency exposures, primarily with respect to the British pound and the U.S. Dollar as the Company receives payments from its main business partner GSK in British pounds and acquires materials produced according to good manufacturing practice for medicinal products (’GMP materials’) in US dollars. From these future commercial transactions foreign exchange risk arises. The exposure to British pounds is expected to be lower in future periods as a result of the termination of the GSK collaboration agreement. No formal policy has been set up to manage the foreign exchange risk against the functional currency of the Company.
At December 31, 2013 there was a net amount of trade receivables in British pounds of €3.1 million (2012: €0.6 million; 2011: €0.8 million) and a net liability in U.S. Dollars of €0.4 million (2012: €0.2 million; 2011: €0.2 million). Foreign currency denominated trade receivables and trade payables are short term in nature (generally 30 to 45 days). As a result foreign exchange rate movements during the years presented had an immaterial effect on the financial statements.
b) Price risk
The market prices for the production of preclinical and clinical compounds and drug substances as well as external contracted research may vary over time. Currently commercial product prices of the development products are uncertain. When the development products near the regulatory approval date or potential regulatory approval date, the uncertainty of the potential sales price decreases. The Company is not exposed to commodity price risk.
F - 16
Furthermore the Company does not hold investments classified as available-for-sale or at fair value through profit or loss, therefore are not exposed to equity securities price risk.
c) Cash flow and fair value interest rate risk
The Company’s interest rate risk arises from long-term borrowings. Borrowings issued at variable rates expose the Company to cash flow interest rate risk, which is partially offset by cash held at variable rates. Borrowings issued at fixed rates expose the Company to fair value interest rate risk. During 2013, 2012 and 2011, the Company’s borrowings were mainly denominated in Euro’s.
The Company manages its cash flow interest rate risk by using floating-to-fixed interest rate swaps. Such interest rate swaps have the economic effect of converting borrowings from floating rates to fixed rates.
The Company does not enter into fixed-to-floating interest rate swaps.
At December 31, 2013 if interest rates on borrowings had been 0.1% higher/lower with all other variables held constant, post-tax results for the year would have been €8 thousand (2012: €6 thousand; 2011: €4 thousand) lower/higher as a result of changes in the fair value of the borrowings. The effect of a change in interest rates of 0.1% on borrowings would have had an insignificant effect on post-tax results for the year as a result of changes in the fair value of the floating to fixed interest rate swap.
Credit risk
Credit risk is managed on a Company basis. Credit risk arises from cash and cash equivalents as well as credit exposures to external parties, including amounts to be invoiced and outstanding receivables. For banks and financial institutions, only independently rated parties with a minimum rating of ‘A’ are accepted at the beginning of the fixed interest term.
The Company does business with a limited group of external parties of which the most significant is GSK.
If external parties are independently rated, these ratings are used. If there is no independent rating, the credit quality of these parties is assessed, taking into account its financial position, past experience and other factors.
No credit limits were exceeded during the reporting period, and management does not expect any losses from non-performance by counterparties.
Liquidity risk
Management forecasts the Company’s liquidity requirements to ensure it has sufficient cash to meet operational needs. Such forecasting takes into consideration the Company’s financing plans and expected cash flow.
As of December 31, 2013 the Company had cash and cash equivalents of €82.2 million.
The table below analyses the Company’s financial liabilities into relevant maturity groupings based on the remaining period at the balance sheet date to the contractual maturity date. Derivative financial liabilities are included in the analysis if their contractual maturities are essential for an understanding of the timing of the cash flows. The amounts disclosed in the table are the contractual undiscounted cash flows. The company revised the disclosure on contractual cash flows from discounted cash flows to undiscounted cash flows with a corresponding increase in contractual cash flows by €681 thousand for the year ended December 31, 2012. There were no changes to the Consolidated Balance Sheets, Net Income, Statement of Cashflows or Total Equity as a result of this revision in this period.
F - 17
|
€ (‘000)
|
Less than
1 year
|
Between
1 and 2 years
|
Between
2 and 5 years
|
Over 5 years
|
Undefined
|
||||||||||
|
At 31 December 2013
|
|||||||||||||||
|
Borrowings (excl. finance lease liabilities)
|
100 | 100 | 200 | - | 7,792 | ||||||||||
|
Finance lease liabilities
|
91 | - | - | - | - | ||||||||||
|
Derivative financial instruments
(interest rate swap)
|
8 | 8 | 14 | - | - | ||||||||||
|
Trade and other payables
|
5,150 | - | - | - | - | ||||||||||
|
Total
|
5,349 | 108 | 214 | - | 7,792 | ||||||||||
|
At 31 December 2012
|
|||||||||||||||
|
Borrowings (excl. finance lease liabilities)
|
100 | 100 | 300 | - | 6,450 | ||||||||||
|
Finance lease liabilities
|
243 | 29 | - | - | - | ||||||||||
|
Derivative financial instruments
(interest rate swap)
|
10 | 10 | 28 | - | - | ||||||||||
|
Trade and other payables
|
5,065 | - | - | - | - | ||||||||||
|
Total
|
5,418 | 139 | 328 | - | 6,450 | ||||||||||
From inception until December 31, 2013 the Company received €6.5 million in loans from patient organizations and government agencies. Most loans have fixed redemption criteria and repayment is due when certain pre-determined milestones have been met. The Company will reach these milestones on the successful commercialization of corresponding pipeline products. Additional conditional payments upon achievement of milestones total in the years 2013 and 2012 a maximum of approximately €5 million. All such loans have been categorized as undefined.
3.2 Capital risk management
The Company’s objectives when managing capital are to safeguard the Company’s ability to continue as a going concern in order to provide returns for shareholders, benefits for other stakeholders and to maintain an optimal capital structure to reduce the cost of capital.
In order to maintain or adjust the capital structure, the Company may adjust the amount of dividends paid to shareholders, return capital to shareholders, issue new shares or sell assets to reduce debt.
The total amount of equity as recorded on the balance sheet is seen and managed as capital by the Company.
3.3 Fair value estimation
For financial instruments that are measured in the balance sheet at fair value, IFRS 13 requires disclosure of fair value measurements by level of the following fair value measurement hierarchy:
|
·
|
Quoted prices (unadjusted) in active markets for identical assets or liabilities (level 1);
|
|
·
|
Inputs other than quoted prices included within level 1 that are observable for the asset or liability, either directly (that is, as prices) or indirectly (that is, derived from prices) (level 2);
|
|
·
|
Inputs for the asset or liability that are not based on observable market data (that is, unobservable inputs) (level 3).
|
The Company’s assets and liabilities that are measured at fair value at December 31, 2013, 2012 and 2011 are all measured as level 2 financial instruments. As of December 31, 2013, 2012 and 2011 financial instruments adjusted at fair value through profit and loss amounted to €30 thousand (2012: €48 thousand, 2011: €53 thousand), and comprised of a floating to fixed interest swap (note 10).
The fair value of financial instruments that are not traded in an active market (for example, over-the-counter derivatives) is determined by using valuation techniques. These valuation techniques maximize the use of observable market data where it is available and rely as little as possible on entity specific estimates. If all significant inputs required to fair value an instrument are observable, the instrument is included in level 2.
If one or more of the significant inputs is not based on observable market data, the instrument is included in level 3.
The carrying amount of all financial assets and financial liabilities is a reasonable approximation of the fair value and therefore information about the fair values of each class has not been disclosed.
F - 18
4. Critical accounting estimates and judgments
Estimates and judgments are continually evaluated and are based on historical experience and other factors, including expectations of future events that are believed to be reasonable under the circumstances.
4.1 Critical accounting estimates and assumptions
The preparation of financial statements in conformity with IFRS requires the Company to make estimates and assumptions that affect the reported amounts and classifications of assets and liabilities, revenues and expenses in the consolidated financial statements. The estimates that have a significant risk of causing a material adjustment to the financial statements are utilized for share-based compensation, income taxes, research and development expenditures, borrowings, intangible assets, equity and deferred revenue & licensing. Actual results could differ materially from those estimates and assumptions.
Share-based payments
The Company operates equity-settled share based compensation plans, pursuant to which certain participants are granted the right to acquire equity instruments (option or restricted shares) of the Company. The plans are accounted for in accordance with the policy as stated in note 2.22. The total amount to be expensed is determined by reference to the fair value of the options granted. The fair value of grants made under the Employee Share Option Plan is measured at the date of grant using a Monte-Carlo simulation model (note 16).
Income taxes
The Company is subject to income taxes in the Netherlands and the United States. Significant judgment is required in determining the use of net operating loss carry forwards and taxation of upfront and milestone payments for income tax purposes. There are many transactions and calculations for which the ultimate tax determination is uncertain. Where the final tax outcome of these matters is different from the amounts that were initially recorded, such differences will impact the current and deferred income tax assets and liabilities in the period in which such determination is made.
Research and development expenditures
Research expenditures are currently not capitalized but are reflected in the income statement because the criteria for capitalization are not met (note 4.2). At each balance sheet date, the Company estimates the level of service performed by the vendors and the associated costs incurred for the services performed.
Although we do not expect the estimates to be materially different from amounts actually incurred, the understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and could result in reporting amounts that are too high or too low in any particular period.
Borrowings
Certain loans from patient organizations have no fixed redemption schemes and repayment is due when certain pre-determined milestones are met. As of December 31, 2013 the Company recorded €7.3 million of such loans with no fixed redemption schemes.
Significant judgment is required in determining when the Company will achieve these pre-determined milestones and these judgments may vary over time. A change in the estimate on the redemption date has an impact on the borrowing value at the balance sheet date. Subsequent to the announcement in September 2013 that the Phase III trial of drisapersen did not meet its primary endpoint, the Company initiated an evaluation of all data and patient groups across all DMD studies with drisapersen. The final outcome of the evaluation may result in deferral of the redemption dates of the loans, however the outcome is still highly uncertain and impracticable to estimate the effect at the moment. Therefore as of December 31, 2013 no deferral of the redemption dates of the loans or adjustment to the borrowings was necessary. The effect of a one year delay in the redemption dates of these loans would decrease the Company’s borrowing balance by €0.5 million as of December 31, 2013. The borrowing value at a certain date equals the discounted value of the redemption using the effective interest rate.
Certain loans from patient organizations bear an interest rate below the market interest rate, considered by the Company to be 12% over the last four years. The difference between fair value of the loans and the notional amount of the loans at inception is treated as a donation received for certain research performed by the Company and is deferred and recognized as Other income during the periods during which expenses are incurred. The estimation of the market rate includes a certain amount of judgment. Pre-commercial financing of a biotech company has a high risk profile and might be assessed differently by each individual institution. As of December 31 2013, the effect of a 100 basis points increase in the estimated market interest rate would decrease the outstanding borrowing value for an amount of approximately €9
F - 19
Intangible assets
As of December 31, 2013 we recorded patents and licenses with a net book value of €0.5 million. Subsequent to the announcement in September 2013 that the Phase III trial of drisapersen did not meet its primary endpoint, the Company initiated an evaluation of all data and patient groups across all DMD studies with drisapersen. As of December 31, 2013 the Company assessed that the asset’s recoverable amount (determined based on value in use calculations) still exceeded the carrying value of €0.5 million in case of a negative outcome of the evaluation and no impairment is required.
Intangible assets
As of December 31, 2013 we recorded patents and licenses with a net book value of €0.5 million. Subsequent to the announcement in September 2013 that the Phase III trial of drisapersen did not meet its primary endpoint, the Company initiated an evaluation of all data and patient groups across all DMD studies with drisapersen. As of December 31, 2013 the Company assessed that the estimated future cash flows discounted at the asset’s original effective interest rate still exceeded the carrying value of €0.5 million in case of a negative outcome of the evaluation and no impairment is required.
Equity
All expenses related to the IPO were recorded in the consolidated statement of comprehensive income until the date at which it became probable that the IPO would occur. The Management Board determined that May 24, 2013, the date which Prosensa first publicly filed its offering prospectus with the Securities and Exchange Commission, is considered to be the date at which the IPO became probable. Expenses related to the IPO incurred subsequent to May 24, 2013 were deducted from the proceeds of the share issuance.
Deferred revenue & License income
Upfront license fee payments received are initially deferred and recognized based on the percentage of completion method. Use of the percentage of completion method requires the Company to estimate the work performed to date as a proportion of the total work expected to be performed. The total related costs of license revenue as well as the timing of these costs may change which impacts the recorded license income to date and the ratio long term versus short term deferred revenue.
As of December 31, 2013 the Company’s deferred license revenue balance totals €14.5 million. Effective January 12, 2014, GSK and the Company mutually terminated the collaboration agreement. In 2014, the deferred license revenue balance will be fully released as there are no remaining performance obligations.
4.2 Critical judgments in applying the entity’s accounting policies
The preparation of financial statements in conformity with IFRS also requires the Company to exercise judgment in applying the accounting policies. Critical judgments in the application of the Company’s accounting policies relate to income taxes, research and development expenditures, revenue and the cost of license revenue and other income.
Income taxes
The Dutch corporate income tax act provides for the possibility of a consolidated tax regime, referred to as a fiscal unity. A fiscal unity is a combination of a parent and subsidiaries whereby formally the parent, Prosensa Holding N.V., is the entity that is taxed for the consolidated profits of the fiscal unity.
The Company, which has a history of recent tax losses, recognizes deferred tax assets arising from unused tax losses or tax credits only to the extent the relevant fiscal unity has sufficient taxable temporary differences or there is convincing other evidence that sufficient taxable profit will be available against which the unused tax losses or unused tax credits can be utilized by the fiscal unity. Management’s judgment is that sufficient convincing other evidence is not available and a deferred tax asset is therefore not recognized.
Research and development expenditures
The project stage forms the basis for the decision whether costs incurred for the Company’s research and development projects can be capitalized or not. In general, the Company’s vision is that clinical development expenditures are not capitalized until the Company files for regulatory approval (i.e. approval to commercially use the product; for example the filing for final FDA approval in the United States or filing for market authorization with the EMA in the EU), as this is considered to be essentially the first point in time where it becomes probable that future revenues can be generated and the project becomes commercially successful.
F - 20
As of each balance sheet date, the Company estimates the level of service performed by the vendors and the associated costs incurred for the services performed. As part of the process of preparing the Company’s financial statements the Company is required to estimate its accrued expenses. This process involves reviewing quotations and contracts, identifying services that have been performed on the Company’s behalf, estimating the level of service performed and the associated cost incurred for the service when it has not yet been invoiced or otherwise notified of the actual cost. The majority of the Company’s service providers invoice it monthly in arrears for services performed or when contractual milestones are met. The Company makes estimates of its accrued expenses as of each balance sheet date in its financial statements based on facts and circumstances known to it at that time. The Company periodically confirms the accuracy of its estimates with the service providers and makes adjustments if necessary. The significant estimates in its accrued research and development expenses are related to fees paid to clinical research organizations, or CROs, in connection with research and development activities for which the Company has not yet been invoiced. The Company bases its expenses related to CROs on its estimates of the services received and efforts expended pursuant to quotes and contracts with CROs that conduct research and development on its behalf.
The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, the Company estimates the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from the Company’s estimate, it adjusts the accrual or prepayment expense accordingly. Although the Company does not expect its estimates to be materially different from amounts actually incurred, its understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and could result in reporting amounts that are too high or too low in any particular period.
Revenue and cost of license revenue
In October 2009 the Company signed a Research & Development and Collaboration & License agreement with GSK. Upon signing of the agreement the Company received €17.2 million (£ 16.0 million) as a non-refundable upfront payment. Until the termination of the agreement effective on January 12, 2014, the Company had continuing performance obligations and revenues related to the license fee payments were deferred and the related revenues were recognized in the period of the expected performance. The Company made the assumption that the expected performance occurred on a straight line basis over the 5 year period the Company was deemed obliged to provide research and development services regarding certain pre-defined programs in the agreement.
Other upfront license fee payments are deferred and revenue is recognized based on the percentage of completion method. Use of the percentage of completion method requires the Company to estimate the work performed to date as a proportion of the total work to be performed.
The related cost of license revenue is based on the determination of net license revenue as defined in our license agreements after deducting allocable expenses and is subject to a certain amount of judgment.
The total related costs of license revenue as well as the timing of these costs may change which impacts the recorded license income to date and the ratio long term versus short term deferred revenue.
Other income
The Company is part of two pan-European consortia, each of which has been awarded a Framework Programme 7 (“FP7”) research grant of €6 million from the European Commission to support respectively ongoing clinical study PRO045 and a separate collaborative bioimaging project to support our PRO053 and Natural History programs. We have also received governmental research subsidies. Grant proceeds are deferred and other income is recognized based on the percentage of completion method.
To encourage research and development the Company obtained certain loans that generally bear interest at a rate below the market interest rate, considered by the Company to be 12% over the last five years. The difference between fair value of the loans and the notional amount of the loans at inception is treated as a donation received for certain research performed by the Company and recognized in Other income over the periods during which expenses are incurred. Certain loans have no fixed redemption schemes and repayment is due when certain predetermined milestones are met. The fair value at inception is based on the initial estimation of when the milestones will be met, which requires a certain amount of judgment.
F - 21
5. Segment information
The Company operates in one reportable segment, which comprises the discovery and development of innovative, RNA based therapeutics. The Management Board is identified as the chief operating decision maker. The Management Board reviews the consolidated operating results regularly to make decisions about the resources and to assess overall performance.
As of December 31, 2013 the Company derived its revenues from a single party, GSK (based in the United Kingdom). The Company and GSK had entered into an exclusive worldwide collaboration for the development and commercialization of RNA based therapeutics for DMD. In October 2009 the Company signed a Research & Development and Collaboration & License agreement with GSK to which the 2013, 2012 and 2011 net revenues relate.
6. Leasehold improvements and equipment
|
€ (‘000)
|
Leasehold
improvements
|
Laboratory
equipment
|
Office
equipment
|
Construction
in progress
|
Total
|
||||||||||
|
Year ended 31 December 2013
|
|||||||||||||||
|
Opening net book amount
|
284 | 1,884 | 253 | - | 2,421 | ||||||||||
|
Additions
|
13 | 357 | 153 | 205 | 728 | ||||||||||
|
Depreciation charge (note 22, 23)
|
(35 | ) | (770 | ) | (167 | ) | - | (972 | ) | ||||||
|
Closing net book amount
|
262 | 1,471 | 239 | 205 | 2,177 | ||||||||||
|
At 31 December 2013
|
|||||||||||||||
|
Cost
|
353 | 4,472 | 799 | 205 | 5,829 | ||||||||||
|
Accumulated depreciation
|
(91 | ) | (3,001 | ) | (560 | ) | - | (3,652 | ) | ||||||
|
Net book amount
|
262 | 1,470 | 239 | 205 | 2,177 | ||||||||||
|
Year ended 31 December 2012
|
|||||||||||||||
|
Opening net book amount
|
315 | 2,464 | 340 | - | 3,119 | ||||||||||
|
Additions
|
4 | 167 | 63 | - | 234 | ||||||||||
|
Depreciation charge (note 22, 23)
|
(35 | ) | (747 | ) | (150 | ) | - | (932 | ) | ||||||
|
Closing net book amount
|
284 | 1,884 | 253 | - | 2,421 | ||||||||||
|
At 31 December 2012
|
|||||||||||||||
|
Cost
|
340 | 4,115 | 646 | - | 5,101 | ||||||||||
|
Accumulated depreciation
|
(56 | ) | (2,231 | ) | (393 | ) | - | (2,680 | ) | ||||||
|
Net book amount
|
284 | 1,884 | 253 | - | 2,421 | ||||||||||
|
At 1 January 2012
|
|||||||||||||||
|
Cost
|
367 | 3,948 | 631 | - | 4,946 | ||||||||||
|
Accumulated depreciation
|
(52 | ) | (1,484 | ) | (292 | ) | - | (1,827 | ) | ||||||
|
Net book amount
|
315 | 2,464 | 340 | - | 3,119 | ||||||||||
Depreciation expense of €801 thousand (2012: €757 thousand; 2011: €801 thousand) has been charged to research and development expense. Depreciation expense of €171 thousand (2012: €175 thousand; 2011: €53 thousand) has been charged to general and administrative expense.
Construction in progress mainly comprises laboratory equipment not ready for use as per December 31, 2013.
Lease rentals amounting to €750 thousand (2012: €721 thousand; 2011: €662 thousand) and €23 thousand (2012: €30 thousand; 2011: €41 thousand) relating to the lease of buildings and office equipment, respectively, are included in the income statement (note 22 and 23).
The Company leases various laboratory equipment and office equipment under non-cancellable finance lease agreements. The lease terms are between 3 and 5 years. During 2013, 2012 and 2011, the Company recorded non-cash increases to property and equipment related to assets acquired under finance leases of €66 thousand, €52 thousand and €560 thousand, respectively. At December 31, 2013 the additions in tangible assets included €57 thousand not paid for at December 31, 2013.
Laboratory equipment includes the following amounts where the Company is a lessee under a finance lease:
F - 22
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Cost
|
1,350 | 1,417 | ||||
|
Accumulated depreciation
|
(642 | ) | (922 | ) | ||
|
Net book amount
|
708 | 495 | ||||
7. Intangible assets
|
€ (‘000)
|
Patents and licenses
|
Software
|
Total
|
||||||
|
Year ended 31 December 2013
|
|||||||||
|
Opening net book amount
|
616 | 253 | 869 | ||||||
|
Additions
|
- | 121 | 121 | ||||||
|
Amortization charge
|
(94 | ) | (138 | ) | (232 | ) | |||
|
Closing net book amount
|
522 | 236 | 758 | ||||||
|
At 31 December 2013
|
|||||||||
|
Cost
|
939 | 743 | 1,682 | ||||||
|
Accumulated amortization and impairment
|
(417 | ) | (507 | ) | (924 | ) | |||
|
Net book amount
|
522 | 236 | 758 | ||||||
|
Year ended 31 December 2012
|
|||||||||
|
Opening net book amount
|
710 | 194 | 904 | ||||||
|
Additions
|
- | 198 | 198 | ||||||
|
Amortization charge
|
(94 | ) | (139 | ) | (233 | ) | |||
|
Closing net book amount
|
616 | 253 | 869 | ||||||
|
At 31 December 2012
|
|||||||||
|
Cost
|
939 | 622 | 1,561 | ||||||
|
Accumulated amortization and impairment
|
(323 | ) | (369 | ) | (692 | ) | |||
|
Net book amount
|
616 | 253 | 869 | ||||||
|
At 1 January 2012
|
|||||||||
|
Cost
|
939 | 424 | 1,363 | ||||||
|
Accumulated amortization and impairment
|
(229 | ) | (230 | ) | (459 | ) | |||
|
Net book amount
|
710 | 194 | 904 | ||||||
Amortization expense of €160 thousand (2012: €139 thousand; 2011: €111 thousand) has been charged to research and development expense. Amortization expense of €72 thousand (2012: €94 thousand; 2011: €95 thousand) has been charged to general and administrative expense.
The remaining amortization period of patent and licenses is approximately 5.5 years and 1-3 years for capitalized software.
F - 23
8. Financial instruments by category
|
€ (‘000)
|
Loans and
receivables
|
Derivatives
|
Total
|
||||||
|
31 December 2013
|
|||||||||
|
Assets as per balance sheet
|
|||||||||
|
Other financial assets
|
289 | - | 289 | ||||||
|
Trade and other receivables
|
4,403 | - | 4,403 | ||||||
|
Cash and cash equivalents
|
82,232 | - | 82,232 | ||||||
|
Total
|
86,924 | - | 86,924 | ||||||
|
Liabilities as per balance sheet
|
|||||||||
|
Borrowings (excluding finance lease liabilities)
|
7,730 | - | 7,730 | ||||||
|
Finance lease liabilities
|
91 | - | 91 | ||||||
|
Derivative financial instruments
|
- | 30 | 30 | ||||||
|
Trade and other payables excluding statutory liabilities
|
5,150 | - | 5,150 | ||||||
|
Total
|
12,971 | 30 | 13,001 | ||||||
|
31 December 2012
|
|||||||||
|
Assets as per balance sheet
|
|||||||||
|
Other financial assets
|
589 | - | 589 | ||||||
|
Trade and other receivables
|
2,422 | - | 2,422 | ||||||
|
Cash and cash equivalents
|
40,738 | - | 40,738 | ||||||
|
Total
|
43,749 | - | 43,749 | ||||||
|
Liabilities as per balance sheet
|
|||||||||
|
Borrowings (excluding finance lease liabilities)
|
6,269 | - | 6,269 | ||||||
|
Finance lease liabilities
|
272 | - | 272 | ||||||
|
Derivative financial instruments
|
- | 48 | 48 | ||||||
|
Trade and other payables excluding statutory liabilities
|
5,065 | - | 5,065 | ||||||
|
Total
|
11,606 | 48 | 11,654 | ||||||
9. Credit quality of financial assets
The credit quality of financial assets that are neither past due nor impaired can be assessed by reference to external credit ratings (if available) or by historical information about counterparty default rates:
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Receivables
|
||||||
|
Counterparties with external credit rating (Moody’s) A1
|
1,488 | 3,678 | ||||
|
Counterparties without external credit rating - note 13
|
934 | 725 | ||||
|
Total unimpaired receivables
|
2,422 | 4,403 | ||||
|
Cash at bank and short-term bank deposits (Moody’s)
|
||||||
|
Aa
|
15,459 | 10,504 | ||||
|
A
|
20,279 | 66,626 | ||||
|
Baa
|
5,000 | 5,102 | ||||
|
Total
|
40,738 | 82,232 | ||||
None of the financial assets that are fully performing have been renegotiated in the last year.
F - 24
10. Derivative financial instruments
|
€ (‘000)
|
Liabilities
|
|||||
|
2012
|
2013
|
|||||
|
Interest rate swaps
|
48 | 30 | ||||
|
Thereof:
|
||||||
|
non-current portion
|
38 | 22 | ||||
|
current portion
|
10 | 8 | ||||
Interest rate swap
The notional principal amount of the outstanding interest rate swap contract at December 31, 2013 was €400 thousand (2012: €500 thousand).
At December 31, 2013 the fixed interest rate was 4.15% (2012: 4.15%), and the floating rate was Euribor plus 1.75% (2012: Euribor plus 1.75%).
Gains and losses from changes in the fair value of interest rate swap contracts are recorded through Finance income - net.
Anti-dilution option
Prior to the closure of the IPO on July 3, 2013 the Company had Class A and B shareholders which were automatically converted into ordinary shares on July 3, 2013. Class A and B shareholders were equipped with an anti-dilution right, in case the Company issued additional shares, options or securities below the shareholders’ acquisition price and certain pre-defined criteria were met. The anti-dilution protection were under the full control of the Company and did not affect the equity classification of the Class A and Class B shares.
11. Other financial assets
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Deposit for rental obligations
|
89 | 89 | ||||
|
Restricted cash
|
500 | 200 | ||||
|
Total
|
589 | 289 | ||||
The restricted cash balance secures a bank loan.
12. Inventories
As of December 31, 2013 the Company valued finished goods of GMP materials to supply clinical centers at nil (2012: nil) due to the uncertainty that future economic benefit will flow to the Company from the use of the inventories.
13. Trade and other receivables
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Trade accounts receivable
|
- | 1,298 | ||||
|
Amounts to be invoiced to partners
|
1,488 | 2,380 | ||||
|
Trade receivables
|
1,488 | 3,678 | ||||
|
Value-added tax
|
457 | 351 | ||||
|
Government and other grants to be received
|
4 | 30 | ||||
|
Interest receivables on bank accounts
|
473 | 344 | ||||
|
Total
|
2,422 | 4,403 | ||||
The fair value of trade and other receivables approximates their carrying value. As of December 31, 2013 and 2012, no trade or other receivables were impaired or not performing. The carrying amount of the Company’s trade receivables are fully denominated in British pounds, while other receivables are fully denominated in Euro’s.
F - 25
The other classes within trade and other receivables do not contain impaired assets. The maximum exposure to credit risk at the reporting date is the carrying value of each class of receivable mentioned above. The Company does not hold any collateral as security.
14. Cash and cash equivalents
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Cash at bank and on hand
|
1,615 | 9,119 | ||||
|
Short-term bank deposits
|
39,123 | 73,113 | ||||
|
Total
|
40,738 | 82,232 | ||||
In 2006 the Company received a bank loan of €900 thousand from ABN Amro N.V. An amount of €200 thousand secures the bank loan (2012: €500 thousand), and is therefore considered restricted cash and recorded as a component of other financial assets. The remaining balance is at the free disposal of the Company.
15. Equity
|
Number of shares
|
||||||||||
|
Class of shares and stated value
|
2011
|
2012
|
2013
|
|||||||
|
Common shares of EUR 0.01
|
3,059,681 | 3,491,058 | 35,932,792 | |||||||
|
Class O shares of EUR 0.01
|
678,825 | 678,825 | - | |||||||
|
Class A shares of EUR 0.01
|
7,417,581 | 7,417,581 | - | |||||||
|
Class B shares of EUR 0.01
|
8,307,690 | 17,414,834 | - | |||||||
|
Total
|
19,463,777 | 29,002,298 | 35,932,792 | |||||||
The par value as of December 31, 2013 is €0.01 per share (as of December 31, 2012 and 2011: €0.01 per share). All issued shares are fully paid. Besides the minimum amount of share capital to be held under Dutch law, there are no distribution restrictions applicable to equity of the Company.
On July 3, 2013 the Company issued 6,900,000 ordinary shares at an initial public offering price of $13.00 per share. For the issuance of the 6,900,000 ordinary shares, the Company received proceeds, after deducting underwriting discounts but prior to deducting offering expenses payable by the Company, of €64.0 million ($83.4 million).
Offering expenses that were deducted from the share proceeds totaled €854 thousand. The Management Board determined that May 24, 2013, the date Prosensa publicly filed its offering prospectus with the Securities and Exchange Commission, was considered to be the date at which the IPO became probable. Expenses related to the IPO incurred subsequent to May 24, 2013 were deducted from the proceeds of the share issue.
Prior to the IPO, the Company had 3,491,058 ordinary shares, 678,825 class O shares, 7,417,581 class A shares and 17,414,834 class B shares outstanding which all had a par value of €0.01 per share. On July 3, 2013 all 25,511,240 preferred shares outstanding were automatically converted into ordinary shares totaling 29,002,298 with a par value of €0.01 per share.
As of July 3, 2013 our authorized share capital is €1,750,114.90, divided into 87,505,745 ordinary shares, each with a par value of €0.01 and 87,505,745 cumulative preferred shares, each with a par value of €0.01. Previously and for the year ended December 31, 2012 and 2011 the total authorized number of ordinary shares was 21 million shares with a par value of €0.01 per share. All issued shares are fully paid.
The Company has adopted an anti-takeover measure pursuant to which the Management Board may, subject to Supervisory Board approval but without shareholder approval, issue (or grant the right to acquire) cumulative preferred shares. The Company may issue an amount of cumulative preferred shares up to 100% of our issued capital immediately prior to the issuance of such preferred shares. In such event, the cumulative preferred shares will be issued to a separate foundation to be established for this purpose.
On January 16, 2012 the Company concluded an agreement for new equity financing led by a new investor, New Enterprise Associates (NEA), and supported by existing Prosensa investors: Abingworth, Life Sciences Partners, Gimv, Idinvest Partners and MedSciences Capital. As a result of this agreement the Company has issued 9,107,144 class B shares with total net proceeds of €22.7 million.
F - 26
Share premium
|
€ (‘000)
|
2011
|
2012
|
2013
|
||||||
|
At 1 January
|
33,557 | 33,557 | 56,118 | ||||||
|
Paid-in surplus on shares issue
|
- | 22,937 | 63,958 | ||||||
|
Issuance cost deducted from share premium
|
- | (376 | ) | (854 | ) | ||||
|
At 31 December
|
33,557 | 56,118 | 119,222 | ||||||
Other reserves
The Company has adopted a share-based compensation plan, pursuant to which the Company’s directors and selected employees are granted the right to acquire ordinary shares of the Company (option shares) (note 16). The share-based payment expenses are recorded in the income statement. The share based compensation plan is equity-settled. In case of an equity settled plan, there is no obligation to transfer economic benefits, therefore the credit entry should be recognized as an increase in equity. The Company uses Other reserves as the equity classification.
Foundation Prosensa Trust Office (Stichting Administratiekantoor Prosensa Holding B.V.)
The Foundation Prosensa Trust Office (‘Stichting Administratiekantoor Prosensa Holding B.V.’ or ‘foundation’) is a trust office with a board independent of the Company.
Prior to the IPO, upon the exercise or award or vesting of a non-cash-settled award under the plans, ordinary shares were issued to the foundation, whose purpose was to facilitate administration of options and pool the voting interests of the underlying shares. The foundation then granted a depository receipt for each issued ordinary share to the person entitled to such ordinary share under an award. The depositary receipt holder was entitled to any dividends or other distributions paid on the ordinary shares for which the depositary receipts were granted. The voting rights and other rights attached to the ordinary shares were exercised by the foundation at its own discretion. The depositary receipt holders were not entitled to attend a general meeting of shareholders or to cast a vote.
Board members of the foundation were appointed by the supervisory board. The board consisted of at least three board members. One board member was appointed on the binding nomination of the joint meeting of holders of Class A and Class B preferred shares, a second board member was appointed on the binding nomination of the general meeting of shareholders and a the third board member was an employee.
Post IPO, ordinary shares are directly issued upon the exercise or award or vesting of a non-cash-settled award under the plans instead of depository receipts. On October 10, 2013 the board of the foundation resolved to transfer the ordinary shares to the respective depository receipt holder. After the completion of the transfers the foundation will be dissolved in 2014. The Company filed a registration statement on form S-8 on March 18, 2014 that covers 3,818,193 ordinary shares to be issued by the Company pursuant to the Prosensa Holding N.V. 2010 Equity Incentive Plan (‘the plan’) as well as Ordinary Shares issuable pursuant to the Plan.
16. Share-based payments
Employee stock options
The Company has adopted share-based compensation plans, pursuant to which certain participants are granted the right to acquire ordinary shares of the Company (option shares). The share-based compensation plans are equity-settled. Upon the exercise or award or vesting of a non-cash-settled award under the plans, the Company issues ordinary shares.
Prior to our IPO, participants were not granted the right to acquire ordinary shares but (non-voting) depository receipts (“Depositary Receipts”) in respect of our ordinary shares. Upon the exercise or award or vesting of a non-cash-settled award under the plans, ordinary shares were issued to a Dutch foundation called Stichting Administratiekantoor Prosensa Holding (the “Foundation”), whose purpose was to facilitate administration of options and pool the voting interests of the underlying shares. The Foundation thereupon granted a Depository Receipt for each issued ordinary share to the person entitled to such ordinary share under an award. Legal title to the shares issued under the share-based compensation plan was held by the Foundation, and the voting rights attached to the shares were exercised by the Foundation at its own discretion.
On October 10, 2013 the board of the Foundation resolved to terminate the administration of the ordinary shares and to transfer the ordinary shares to the respective Depository Receipt holder. After the completion of the transfers, the Foundation will be dissolved.
F - 27
Going forward, participants will receive ordinary shares upon the exercise or award or vesting of a non-cash-settled award under the plans instead of depository receipts.
Options are generally conditional on the employee completing a four year period of service (the vesting conditions). Each such option award vests based on the following schedule: (a) 25% on the first anniversary of the date of the grant, provided that on that date the relevant participant is still employed by the Company, and (b) the remaining 75% in 36 equal monthly installments over a three year period following the first anniversary of the date of grant subject to continued employment of the relevant participant. The Company may decide to accelerate the vesting of the options as a result of a change of control.
The following table summarizes by grant date the number of options granted between January 1, 2011 and December 31, 2013:
|
Grant date
|
Options
granted
|
Exercise price (€)
|
Fair value of
ordinary
share (€)
|
Per share
estimated fair
value of options (€)
|
||||||||
|
March 22, 2011
|
707,500 | 0.01 - 1.00 | 1.00 | 0.99 | ||||||||
|
September—November, 2011
|
8,500 | 0.01 | 1.00 | 0.99 | ||||||||
|
January 1, 2012
|
10,000 | 0.01 | 0.70 | 0.69 | ||||||||
|
March 31, 2012
|
15,000 | 0.01 | 0.70 | 0.69 | ||||||||
|
July 17, 2012
|
61,600 | 0.01 | 0.70 | 0.69 | ||||||||
|
October 22, 2012
|
31,250 | 0.01 | 0.70 | 0.69 | ||||||||
|
December 5, 2012
|
1,000,000 | 0.01 – 0.70 | 0.70 | 0.01 - 1.97 | ||||||||
|
July 3, 2013
|
40,000 | $ | 19.25 | $ | 19.25 | $ | 12.81 | |||||
|
December 10, 2013
|
167,920 | $ | 4.26 | $ | 4.26 | $ | 3.38 | |||||
On December 5, 2012 the Company granted to the members of the Management Board 1.0 million options that vest only upon a liquidity event such as a change of control of the Company or an IPO, subject to the continued employment of the member of the Management Board. Following our IPO, the maximum number of options eligible to vest was automatically reduced from 1.0 million to 800,000. In order for any options to become subject to vesting the sustained closing market price for the ordinary shares during any 20 consecutive trading days outside a lock-up or blackout period must exceed a threshold share price. The number of options subject to vesting increases between the threshold share price and the share price entitling the participant to exercise the maximum number of options. Upon each subsequent achievement of a sustained, higher closing market price or level of proceeds from a liquidity event, an additional number of options will become subject to vesting. Each option grant subject to vesting following our IPO will vest and become exercisable based on the following schedule: 25% of the options subject to vesting will vest the day immediately following attainment of such pricing levels (the initial vesting date), and an additional 2.083% will vest at the end of each successive one month period following the initial vesting date, subject to the continued employment of the relevant member of the management board.
As of December 31, 2013 none of these options are vested.
Unless forfeited or exercised on an earlier date the options expire 10 years after the grant date.
Movements in the number of share options outstanding and their related weighted average exercise prices are as follows:
F - 28
|
2011
|
2012
|
2013
|
||||||||||||||||
|
Average
exercise price
in € per share
|
Options
|
Average
exercise price
in € per share
|
Options
|
Average
exercise price
in € per share
|
Options
|
|||||||||||||
|
At 1 January
|
1.38 | 1,090,110 | 0.38 | 1,418,232 | 0.34 | 2,207,707 | ||||||||||||
|
Granted
|
0.01 | 716,000 | 0.11 | 1,117,850 | 7.14 | 207,920 | ||||||||||||
|
Exercised
|
0.01 | 357,531 | 0.10 | 316,376 | 0.01 | 30,493 | ||||||||||||
|
Forfeited
|
0.01 | 30,347 | 0.01 | 11,999 | 0.13 | 200,000 | ||||||||||||
|
At 31 December
|
0.38 | 1,418,232 | 0.34 | 2,207,707 | 0.10 | 2,185,134 | ||||||||||||
Out of the 2,185,134 options outstanding (2012: 2,207,707; 2011:1,418,232), 923,528 options (2012: 604,252; 2011:342,872) were exercisable. The weighted average share price at the time of exercise of the options in 2013 was $4.79.
In each of 2012 and 2011 there was one opportunity to exercise options. The estimated fair value of the ordinary shares at the moment of exercise amounted to €0.70 in 2012 and €1.00 in 2011. Subsequent to our IPO the fair value of the ordinary shares at the moment of exercise of the options is the quoted share price at the time.
During 2011 the exercise price of 519,350 options was changed to €0.01 to align the exercise price to the 2010 plan. The incremental fair value granted amounts to €410 thousand and is the difference between the fair value of the options with revised exercise price (€0.01) versus the fair value of the options with the original exercise price, both estimated as at the date of the modification. From the modification date this incremental fair value is recognized as an expense over the remainder of the vesting period in addition to the expense recognized for the original fair value of the options.
Options outstanding at the end of the year have the following weighted average remaining contractual lifes and ranges of exercise prices:
|
Weighted average remaining contractual life
|
Range exercise
price in € per
share
|
Options
|
||||
|
1-5 years
|
0.01 - 2.54 | 407,144 | ||||
|
6 years
|
0.01 | 3,563 | ||||
|
7 years
|
0.01 – 1.00 | 667,742 | ||||
|
8 years
|
0.01 - 0.70 | 898,765 | ||||
|
9 years
|
$ | 4.26 – $19.25 | 207,920 | |||
|
At 31 December 2013
|
2,185,134 | |||||
|
Weighted average remaining contractual life
|
Range exercise
price in € per
share
|
Options
|
||||
|
1-5 years
|
0.01 - 2.54 | 232,091 | ||||
|
6 years
|
0.01 | 185,608 | ||||
|
7 years
|
0.01 | 13,173 | ||||
|
8 years
|
0.01 - 1.00 | 665,860 | ||||
|
9 years
|
0.01 - 0.70 | 1,110,975 | ||||
|
At 31 December 2012
|
2,207,707 | |||||
|
Weighted average remaining contractual life
|
Range exercise
price in € per share
|
Options
|
||||
|
1-5 years
|
1.85 - 2.54 | 229,950 | ||||
|
6 years
|
0.01 | 42,506 | ||||
|
7 years
|
0.01 | 420,932 | ||||
|
8 years
|
0.01 | 14,344 | ||||
|
9 years
|
0.01 - 1.00 | 710,500 | ||||
|
At 31 December 2011
|
1,418,232 | |||||
Following the completion of our IPO, option pricing and values are determined based on the quoted market price of our ordinary shares. The fair value of stock options under the various grants is based on a Monte-Carlo simulation using the following assumptions:
F - 29
|
2011
|
2012
|
2013
|
|||||||
|
Weighted average fair value of options granted:
|
€ | € | $ | ||||||
|
Options with only service-based vesting conditions
|
0.99 | 0.69 | 5.19 | ||||||
|
Options with change of control and service-based vesting conditions
|
— | 0.01 | — | ||||||
|
Options with IPO and service-based vesting conditions, €0.70 exercise price
|
— | 1.89 | — | ||||||
|
Options with IPO and service-based vesting conditions, €0.01 exercise price
|
— | 1.97 | — | ||||||
|
Exercise price
|
0.01 - 1.00 | 0.01 - 0.70 | 4.26 - 19.25 | ||||||
|
Risk-free interest rate
|
1.90 | % | 1.5 | % | 1.1 - 2.0 | % | |||
|
Volatility
|
80 | % | 80 | % | 95 - 100 | % | |||
|
Dividend yield
|
0.0 | % | 0.0 | % | 0.0 | % | |||
|
Expected life (in years)
|
2-3 years
|
1-2 years
|
6 years
|
||||||
In respect of the 1.0 million options granted to members of the Management Board in 2012, that automatically reduced to 800,000 following our IPO, the actual number of options subject to vesting will depend on the share price development following our IPO. This market performance condition is included in the fair value of the options granted. The Management Board determined that May 24, 2013, the date which Prosensa first publicly filed its offering prospectus with the Securities and Exchange Commission, is considered to be the date at which the IPO became probable. Therefore from May 24, 2013 onwards the Company recognizes expenses over the estimated vesting period. Expenses of €624 thousand related to the December 5, 2012 grant were recognized during the year ended December 31, 2013. The total expense to be recognized is approximately €1.6 million, which will be recognized over the estimated vesting period.
Valuation of ordinary shares
The methodology we used prior to our IPO in measuring share-based compensation expense is described below. After our IPO, the fair value of the ordinary shares is based on their quoted market price.
Prior to our IPO the fair value of the ordinary shares was determined by the Management Board and Supervisory Board, and took into account the most recently available valuation of ordinary shares performed by an independent valuation firm and the assessment of additional objective and subjective factors the Company believed are relevant and which may have changed from the date of the most recent valuation through the date of the grant.
The management board and supervisory board considered numerous objective and subjective factors to determine their best estimate of the fair value of the ordinary shares as of each grant date, including the following:
|
·
|
the progress of the research and development programs;
|
|
·
|
achievement of enterprise milestones, including the entering into collaboration and licensing agreements;
|
|
·
|
contemporaneous third-party valuations of the ordinary shares;
|
|
·
|
the historical and forecasted performance and operating results;
|
|
·
|
the need for future financing to fund operations;
|
|
·
|
the rights and preferences of the preferred shares and the preferred shares relative to the ordinary shares;
|
|
·
|
the likelihood of achieving a discrete liquidity event, such as a sale of the Company or an initial public offering given prevailing market conditions; and
|
|
·
|
external market and economic conditions impacting the industry sector.
|
In determining the fair values of the ordinary shares as of each award grant date, three generally accepted approaches were considered: income approach, market approach and cost approach. Based on the stage of development and information available, the Company determined that the income approach is the most appropriate method, and when applicable, the Company also employed the prior sale of company stock method to estimate the aggregate enterprise value. In addition, the Company has taken into consideration the guidance prescribed by the American Institute of Certified Public Accounts (AICPA) Audit and Accounting Practice Aid, Valuation of Privately-Held-Company Equity Securities Issued as Compensation.
Discounted cash flow (DCF), a form of the income approach, is an estimate of the present value of the future monetary benefits expected to flow to the owners of a business. It requires a projection of the cash flows that the business is expected to generate. These cash flows are converted to present value by means of discounting, using a rate of return that accounts for the time value of money and the appropriate degree of risks inherent in the business. The discount rate in the DCF analysis is based upon a weighted average cost of capital (WACC) calculated at each valuation date. The WACC is a method that market participants commonly used to price securities and is derived by using the Capital Asset Pricing Model and inputs such as the risk-free rate, beta coefficient, equity risk premiums and the size of the Company.
F - 30
The prior sale of Company stock method considers any prior arm’s length sales of the Company’s equity securities. Considerations factored into the analysis include: the type and amount of equity sold, the estimated volatility, the estimated time to liquidity, the relationship of the parties involved, the timing compared to the ordinary shares valuation date and the financial condition and structure of the Company at the time of the sale.
The indicated fair value calculated at each valuation date was allocated to the preferred shares and ordinary shares using the option pricing method (OPM), applied retrospectively. The OPM treats ordinary shares and preferred shares as call options on the total equity value of a company, with exercise prices based on the value thresholds at which the allocation among the various holders of a company’s securities changes. Under this method, the ordinary shares have value only if the funds available for distribution to shareholders exceed the value of the liquidation preference at the time of a liquidity event, such as a strategic sale, assuming the enterprise has funds available to make a liquidation preference meaningful and collectible by the holders of preferred shares. The ordinary shares are modeled as a call option on the underlying equity value at a predetermined exercise price. In the model, the exercise price is based on a comparison with the total equity value rather than, as in the case of a regular call option, a comparison with a per share price. Thus, ordinary shares are considered to be a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the preferred shares are liquidated. The OPM uses the Black Scholes option-pricing model to price the call options. This model defines the securities’ fair values as functions of the current fair value of a company and uses assumptions such as the anticipated timing of a potential liquidity event and the estimated volatility of the equity securities. Volatility has been estimated based on the observed daily share price returns of peer companies over a historic period equal to the period for which expected volatility is estimated. Volatility is defined as the annualized standard deviation of share price returns. In the allocation of equity, the Company has also considered valuation outcomes of ordinary shares through a sale of the Company versus an IPO and considered the probabilities of each at each valuation date, since the treatment of the liquidation rights is different for these two events. The aggregate value of the ordinary shares is then divided by the number of ordinary shares outstanding to arrive at the per share value.
A valuation was performed by an independent valuation firm as of December 2010 and applied to all option grants in 2011. For the grant on March 22, 2011 and the other grants made in September through November, 2011, the Company relied on the December 2010 valuation report, which the Company deemed reasonable based on its progress at that time. Of the total 716,000 options granted in 2011, 707,500 options were granted on March 22, 2011. Between the valuation of our ordinary shares performed as of December 2010 and March 2011, there were no events in our business that were deemed to have an effect on the valuation and thus we believed the December 2010 valuation to continue to be reasonable. Furthermore, there were no significant results in 2011 from any of our development programs including the Phase III-DMD114044 pivotal trial initiated in December 2010, the continuation of the dose escalation study of PRO044 and the preclinical safety studies for PRO045 and PRO053 on-going throughout 2011. Additional valuations were performed by an independent valuation firm as of September 30, 2012 and December 5, 2012 for share-based compensation grants in 2012.
The ordinary shares valuation as of December 2010 relied on a DCF to derive the total enterprise value. The cash flow projections were based on probability-weighted scenarios which considered estimates of time to market of the products, market share and pricing. The cash flow projections were estimated over a period equal to the expected product life cycle, and a terminal value period was not applied. All DMD indications in development were included in estimating cash flow projections, most importantly drisapersen. The expected sales were estimated based on a regional level, estimated patient populations and market shares. Production and research and development costs were estimated at the indication level with general and administrative costs and selling and marketing costs assessed at the overall Company level. Cash flow projections also incorporated expected royalties and milestones related to the collaboration agreement with GSK. A WACC of 16.0% was applied. Equity was allocated using the OPM, applied retrospectively, resulting in a determination by the Supervisory Board and the Management Board of a value per ordinary share of €1.00. The OPM relies on the anticipated timing and probability of a liquidity event based on then current plans and estimates of the Supervisory Board and Management Board regarding a liquidity event of 80-85% probability of a sale of the Company and 15-20% probability of an IPO. The Company assumed volatility of 80% based on historical trading volatility for the publicly traded peer companies and a time to liquidity of 1.5 years.
In December 2010, the Phase III-DMD114044 pivotal trial was initiated by GSK, the completion of this trial was later than anticipated in the projections that were used for the valuation performed in 2010. In 2011 the Company also concluded an amendment to the GSK agreement related to the development terms for PRO044. In this amendment the timing of the milestone payment for GSK licensing PRO044, which had been anticipated in the second quarter of 2011, was restructured, and the anticipated licensing to GSK of PRO044 was deferred significantly. In January 2012, the Company raised total net proceeds of €22.7 million in a private placement of the preferred (Class B) shares. The additional Class B shares were issued on a pari passu basis to the original Class B shares issued in December 2008 with a liquidation preference senior to Class A, Class O and ordinary shares. The events above in the aggregate had a downward effect on the valuation of the ordinary shares as of the end of 2011 and in 2012.
F - 31
The ordinary shares valuation as of September 30, 2012 and December 5, 2012 relied on a DCF as well as the prior sale of Company stock method in connection with the January 2012 financing round to derive the total enterprise value. There were no events between the respective valuation dates and therefore the analyses and inputs remained the same. The cash flow projections were based on probability-weighted scenarios which considered estimates of time to market of the products, market share and pricing. Projections were updated from the December 2010 valuation for anticipated timing of placebo-controlled clinical trial results, the progress since the last valuation date and the development of a competing drug for DMD that increased the risk of the losing a first-mover advantage. A WACC of 15.5% was applied. Under the prior sale of Company stock method, the Company used the implied value from the latest financing round in January 2012 and an estimated annualized return on equity over the period between the financing round and the valuation date for the valuation of the enterprise value as of September 30, 2012 considering the factors noted above. Equity was allocated using the OPM, resulting in a value per ordinary share of €0.70. The OPM relies on the anticipated timing and probability of a liquidity event based on then current plans and estimates of the supervisory board and management board regarding a liquidity event of 80-85% probability of the sale of the Company and 15-20% probability of an IPO. The Company assumed volatility of 80% based on historical trading volatility for the publicly traded peer companies and a time to liquidity of 0.50 years.
A discount for lack of marketability (DLOM) of 15% and 10%, as of December 2010 and September 30, 2012, respectively, was applied to reflect the increased risk arising from the inability to readily sell the shares. When applying the DLOM, the Black-Scholes option model was used. Under this method, the cost of the put option, which can hedge the price change before the privately held shares can be sold, was considered as the basis to determine the DLOM. The cost of the put option was the only factor considered and applied in the discount. The put option analysis reflects the potential loss from marketability over the expected time to liquidity and is a commonly applied approach to estimate this discount.
The Company expects all vested options to be exercised. Prior to our IPO, the Company considered the expected life of the options to be in line with the vesting period of the options. The expected life for all options granted subsequent to the completion of our IPO is estimated at six years.
The Company has historically been a private Company and lacks Company-specific historical and implied volatility information. Therefore, the Company estimates the expected volatility based on the historical volatility of the publicly traded peer companies as indicated above and has been set at 95% and 100% for the options granted on July 3 and December 10, 2013 respectively and at 80% for the options granted in 2011 and 2012.
See note 24 for the total expense recognized in the income statement for share options and other share-based payment granted to managing directors, supervisory directors and selected employees. Share option expenses are accounted for as a contribution to equity and separately accounted for as other reserves.
Restricted shares
In 2011 and 2012, the Company made, as part of the share-based compensation plan, awards entitling recipients to acquire Depositary Receipts (Restricted Shares) against payment of the nominal value of the underlying ordinary shares (the Purchase Price), subject to the right to repurchase all or part of such Depositary Receipts at the lower of the Purchase Price and the fair value of the ordinary shares during the four year vesting period if the participant ceases to perform services to the Company. The Company has the right to repurchase 100% of the Restricted Shares awarded, and this right to repurchase shares will be reduced based on the following schedule: (a) 25% on a date of the year following the vesting commencement date no later than the anniversary of the grant, and (b) the remaining 75% in 36 equal monthly installments over a three year period following the first anniversary of the first vesting date.
F - 32
|
2011
|
2012
|
2013
|
||||||||||||||||
|
Average
exercise price
in € per share
|
Shares
|
Average
exercise price
in € per share
|
Shares
|
Average
exercise price
in € per share
|
Shares
|
|||||||||||||
|
At 1 January
|
0.01 | - | 0.01 | 89,750 | 0.01 | 204,750 | ||||||||||||
|
Granted
|
0.01 | 89,750 | 0.01 | 115,000 | - | - | ||||||||||||
|
At 31 December
|
0.01 | 89,750 | 0.01 | 204,750 | 0.01 | 204,750 | ||||||||||||
During 2013, the Company issued nil Restricted Shares (2012: 115,000; 2011: 89,750). Restricted shares have a vesting period up to 4 years. The fair value of the Restricted Shares granted in 2012 of €0.70 (2011: €1.00) was measured based on the estimated fair value of the ordinary shares at the moment of purchase, which amounted to €0.70 in 2012 and €1.00 in 2011 in line with the valuations of the ordinary shares. The Company is not in a financial position to pay dividends in the foreseeable future. Therefore dividend payments have not been considered in the valuation of the restricted share awards.
The share-based payment expense related to the Restricted Shares amount to €57 thousand in 2013, €18 thousand in 2012 and €45 thousand in 2011, which is included in the total share-based payment expense for the year disclosed (note 24).
On October 10, 2013 the Board of the Foundation resolved to terminate the administration of the ordinary shares and to transfer the ordinary shares to the respective Depository Receipt holder. After the completion of the transfers, the Foundation will be dissolved. Going forward participants will receive ordinary shares upon the exercise or award or vesting of a non-cash-settled award under the plans instead of Depository Receipts.
Other option shares
The Company granted option rights to two employees of the Company to purchase a total of 123,750 ordinary shares owned by the shareholder at a price of €1.82 per ordinary share. The option rights are unconditional and can be exercised for ten years from the grant date. The outstanding options of 33,750 as of December 31, 2013, 2012 and 2011 are held by one single employee. The other employee exercised the options in previous years.
The weighted average remaining contractual life of the outstanding options was 4, 5 and 6 years as of December 31, 2013, 2012 and 2011, respectively.
Movements in the number of call option rights outstanding and their related weighted average exercise prices are as follows:
|
2011
|
2012
|
2013
|
||||||||||||||||
|
Average
exercise price
in € per share
|
Options
|
Average
exercise price
in € per share
|
Options
|
Average
exercise price
in € per share
|
Options
|
|||||||||||||
|
At 1 January
|
1.82 | 33,750 | 1.82 | 33,750 | 1.82 | 33,750 | ||||||||||||
|
Exercised
|
- | - | - | |||||||||||||||
|
At 31 December
|
1.82 | 33,750 | 1.82 | 33,750 | 1.82 | 33,750 | ||||||||||||
Compensation of the Management Board and the Supervisory Board including share-based payment are described in note 31.
F - 33
17. Trade and other payables
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Trade payables
|
2,390 | 1,910 | ||||
|
Holiday payments and holiday rights
|
366 | 457 | ||||
|
Social security and wage tax
|
510 | 246 | ||||
|
Other liabilities
|
1,799 | 2,537 | ||||
|
Total
|
5,065 | 5,150 | ||||
Other liabilities
Other liabilities mainly consist of accruals for services provided by vendors but not yet billed, reimbursements received from research and development partners for expenses which have yet to be incurred and miscellaneous liabilities.
Royalties LUMC
In 2008 Prosensa and Leiden Universitair Medisch Centrum (LUMC) extended and amended a research and license agreement with respect to commercialization of therapeutic products based on LUMC intellectual property rights and the joint technologies. Under this agreement the Company is obliged to make milestone payments upon the occurrence of events with respect to each subfield in which the Company pursues a drug indication using the LUMC patent rights, including commencing the first toxicity study, successfully completing the first Phase I study, filing for regulatory approval and satisfying certain sales thresholds. The aggregate milestone payments could total approximately €1.4 million if the Company achieves such milestones in relation to an initial orphan drug indication. If the Company achieves such milestones in relation to a non-orphan drug indication, the aggregate milestone payments could total €5.5 million. The Company may also be obligated to pay LUMC additional milestone payments of €1.25 million per product in relation to products approved for additional orphan drug indications. In addition, the Company is obligated to pay LUMC a mid-single digit percentage royalty on net sales on a country-by-country basis if and when any of the Company’s products is brought to market. If the Company sublicenses its rights under the LUMC agreement to a third party, the Company is obligated to pay LUMC a tiered royalty percentage (ranging from the low-to-mid-twenties) on the net licensing income and a low single-digit percentage royalty on the net sales generated by the sublicensee.
In relation to this agreement as of December 31, 2013, 2012 and 2011 no amounts were outstanding.
18. Borrowings
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Non-current
|
||||||
|
Bank borrowings
|
400 | 300 | ||||
|
Other loans
|
5,769 | 7,330 | ||||
|
Finance lease liabilities
|
29 | - | ||||
|
Total non-current
|
6,198 | 7,630 | ||||
|
Current
|
||||||
|
Bank borrowings
|
100 | 100 | ||||
|
Finance lease liabilities
|
243 | 91 | ||||
|
Total current
|
343 | 191 | ||||
|
Total
|
6,541 | 7,821 | ||||
Bank borrowings
In 2006 the Company received a bank loan of €900 thousand from ABN Amro N.V. which matures in 2017. The loan bears interest equal to Euribor plus 1.75% per year. The Company redeems an amount of €25 thousand per quarter. The current portion of €100 thousand is included in borrowings—current portion. There is no portion of the loan exceeding five years.
The Company has a credit facility with an undrawn balance of €208 thousand (2012: €254 thousand). The total amount of the credit facility available to the Company decreases by €46 thousand per annum. The securities for the bank loan and the credit facility are as follows:
F - 34
|
·
|
a collateral of all cash and cash equivalents which are deposited with ABN Amro; and
|
|
·
|
a minimum amount of €200 thousand shall be kept as security at all times at an ABN Amro Bank account.
|
The Company has entered into an interest swap agreement (note 10).
Other loans
The Company has obtained other loans from various governmental and non-profit groups. To encourage research and development the Company obtained certain loans that generally bear an interest rate below the market interest rate, considered by the Company to be 12% over the last years. The difference between fair value and the notional amount at inception is treated as a donation received for certain research performed by the Company. These borrowings were initially recognized at fair value at inception with the difference between the fair value and the notional amount recognized as other income in the statement of comprehensive income.
There is no exposure for the Company’s other borrowings to interest rate changes and contractual repricing. Interest rates are fixed until maturity. The fair values of the non-current borrowings approximate their carrying values. The fair value of current borrowings equals their carrying amount, as the impact of discounting is deemed immaterial. The carrying amounts of the Company’s borrowings are denominated in Euro’s.
The key features of the other loans are described below.
a) Villa Joep
The principal amount of the loan is €65 thousand and was received on October 10, 2005. The loan bears a below market interest rate. No fixed redemption scheme was agreed on. The loan shall be repaid in the first year after the Company reaches a pre-defined milestone. The loan is not secured.
b) Association Française contre les Myopathies
On December 22, 2005 a loan of €70 thousand was granted. In 2006 an additional loan amount of €70 thousand was received. The loans shall be repaid with fixed terms when the Company obtains regulatory approval to bring the first product candidate to the market. The loans are interest free and are not secured.
In 2012 the Company received from Association Française contre les Myopathies €3.0 million related to a funding agreement for research and development in two separate installments on April 12, 2012 and December 21, 2012. In 2013 the Company received another installment of €250 thousand. The loans shall be repaid with fixed terms when the Company meets a revenue target on the product candidates PRO044, PRO045 and PRO053.
The AFM agreement gives us the option to terminate the agreement if we undergo a change of control. If we exercise our option to terminate the agreement, we are required to repay the loan, together with interest. In the event that we abandon the program for reasons not justified by a scientific or medical reason, we are similarly required to repay the loan, together with interest.
The AFM agreement also requires us to grant to AFM a non-exclusive royalty-free license to the intellectual property covered by the agreement in the event that we abandon the research funded by the agreement.
c) Stichting Duchenne Parents Project
This loan of €500 thousand was received in two tranches, €300 thousand on July 14, 2006 and €200 thousand on October 10, 2006. The loan bears a below market interest rate that accumulates to the principal amount. No fixed redemption scheme was agreed on. The loan shall be repaid with fixed terms after the Company obtains regulatory approval to bring the first product candidate to the market. The loan is not secured.
d) Aktion Benni & Co
On May 9, 2006 a loan of €100 thousand was granted that was received in two tranches in 2006. In 2008 an additional amount of €63 thousand was received. No interest is charged and no fixed redemption scheme was agreed. The loan shall be repaid after the Company obtains regulatory approval to bring the first product candidate to the market. The loan is not secured.
e) Cure Duchenne
The (subordinated) loan of €250 thousand was received on December 22, 2006 and bears a below market interest rate. No fixed redemption scheme was agreed. The loan as well as an additional payment of €250 thousand shall be
F - 35
repaid with fixed terms after the Company obtains regulatory approval to bring the first product candidate to the market. In case of a liquidation event of the Company (e.g. change of control of the Company), the loan converts to ordinary shares with a maximum value of the loan amount, accumulated interest and an additional €250 thousand. The loan is not secured.
f) Agentschap NL
The Company received loan installments from Agentschap NL for a total amount of €739 thousand which bears an interest rate of 9.5% per annum. In 2013, 2012 and 2011 the Company received €99, €165 and €475 thousand respectively. The granted loan is an innovation credit facility (Innovatiekrediet) of the Dutch Ministry of Economic Affairs. The Company has pledged the part of its materials and immaterial fixed assets that directly relate to the project funded by the loan granted.
g) Everest International Pte Ltd
On September 27, 2012 the Company received from Everest International Pte Ltd €300 thousand as an installment of a €1.0 million funding agreement for research and development. No fixed redemption scheme was agreed on. The loan shall be repaid with fixed terms when preclinical product candidates meet commercial milestones. The loan is not secured.
On April 19, 2013 and August 2, 2013 the Company received respectively €150 thousand and €52 thousand from Everest International Pte Ltd as installments of a €1.0 million funding agreement for research and development at a below market interest rate.
h) Charley’s Fund
In 2012 the Company received from Charley’s Fund $500 thousand (€387 thousand) as installment of a $1.5 million funding agreement for research and development which bears an interest rate that approximates the market interest rate. No fixed redemption scheme was agreed on. The loan shall be repaid in installments with fixed terms when certain clinical and regulatory milestones are met. The loan is not secured.
i) Duchenne Children’s Trust
On May 15, 2013 the Company received €500 thousand from the Duchenne Children’s Trust as installment of a €1.5 million funding agreement for research and development which bears an interest rate that approximates the market interest rate. No fixed redemption scheme was agreed on. The loan shall be repaid in installments with fixed terms when certain clinical and regulatory milestones are met. The loan is not secured.
Finance lease liabilities
Finance lease liabilities are effectively secured as the rights to the leased asset revert to the lessor in the event of default. The carrying amount corresponds to the fair value as terms of the contracts were agreed at arm’s length and market conditions for such contracts have not changed since. The interest rate imposed by the lessor for all finance lease liabilities is 8% per annum.
F - 36
|
€ (‘000)
|
31 December
|
|||||
|
2012
|
2013
|
|||||
|
Gross finance lease liabilities – minimum lease payments
|
||||||
|
No later than 1 year
|
259 | 98 | ||||
|
Later than 1 year and no later than 5 years
|
30 | - | ||||
|
Later than 5 years
|
- | - | ||||
| 289 | 98 | |||||
|
Future finance charges on finance leases
|
17 | 7 | ||||
|
Total
|
272 | 91 | ||||
|
Present value of finance lease liabilities
|
||||||
|
The present value of finance lease liabilities is as follows:
|
||||||
|
No later than 1 year
|
243 | 91 | ||||
|
Later than 1 year and no later than 5 years
|
29 | - | ||||
|
Later than 5 years
|
- | - | ||||
|
Total
|
272 | 91 | ||||
19. Revenue and deferred revenue
Revenues to date have consisted principally of license revenue and collaboration revenue. The group generates both license revenue and collaboration revenue under the GSK Agreement.
License revenue
In 2009, the Company entered into the GSK Agreement for the development and commercialization of RNA-based therapeutics for DMD. Pursuant to the GSK Agreement, the Company granted GSK an exclusive worldwide license to develop and commercialize the lead compound, drisapersen. The Company also granted GSK an option to receive an exclusive worldwide license for the second lead compound, PRO044. In addition, GSK had the option to obtain an exclusive worldwide license for either PRO045 or PRO053, and an option to obtain an exclusive worldwide license for either PRO052 or PRO055. PRO045 is paired with PRO053, and PRO052 is paired with PRO055, because each of the product candidates in the respective pairings addresses a patient sub-population of a similar size and is at a comparable stage of development. Since 2009, the Company has received €47.4 million under the GSK Agreement and recognized revenue totaling €33.0 million of license revenue over the full period of the agreement until December 31, 2013. All payments by GSK are made in British pounds.
The Company recorded license revenue as follows:
|
€ (‘000)
|
Proceeds
|
Revenue
recognition
|
Revenue
|
Deferred License Revenue
|
||||||||||||||||||
| Year ended December 31, | As of December 31, | |||||||||||||||||||||
|
Type of payment
|
2011
|
2012
|
2013
|
2011
|
2012
|
2013
|
||||||||||||||||
|
Initial upfront payment
|
17,177 |
five year research term
|
3,435 | 3,435 | 3,435 | 9,447 | 6,011 | 2,576 | ||||||||||||||
|
Other upfront payments
|
24,050 |
percentage of completion
|
3,075 | 2,291 | 2,191 | 3,974 | 7,877 | 5,686 | ||||||||||||||
|
Conditional license payments
|
6,213 |
payment is unconditional
|
- | - | - | - | 6,213 | 6,213 | ||||||||||||||
|
Total
|
47,440 | 6,510 | 5,726 | 5,626 | 13,421 | 20,101 | 14,475 | |||||||||||||||
a) Initial upfront payment
In October 2009 the Company signed a Research & Development and Collaboration & License agreement with GSK. Upon signing of the agreement the Company received £16.0 million (€17.2 million) as a non-refundable upfront payment. The upfront fee relates to all compounds included in the agreement. Milestones relate to the individual projects (e.g. PRO045 / PRO053). The expected performance occurs on a straight line basis over the five year period. At initiation of the contract, the Company determined to recognize the payment ratably over a period of five years which equals the minimum research term both parties committed to under the agreement. As of December 31, 2013 the Company considers the contractual term to be the accurate term for deferring revenue.
F - 37
b) Other upfront payments
The Company receives upfront payments to fund research and development activities until GSK exercises its option for a particular compound. The upfront payments are recognized by reference to the stage of completion of the underlying agreement at the balance sheet date (also referred to as percentage of completion method) due to the fact that the rendering of services under the license revenue agreement can be estimated reliably.
Use of the percentage of completion method requires the group to estimate the work performed to date as a proportion of the total work to be performed. The Company updates the cost to complete to determine the percentage of completion at least on a quarterly basis. In 2013, the group released to revenue €2.2 million (2012: €2.3 million; 2011: €3.1 million) related to the upfront payments for the PRO045 / PRO053 program and the PRO052 / PRO055 program.
c) Advance option payments
In June 2012, the Company received a £5.0 million (€6.2 million) advance option payment under the GSK collaboration agreement. The advance option payment is deferred and will be recognized in revenue when the payment becomes unconditional.
Collaboration revenue
Collaboration revenue from contracts, typically from delivering research and development services, which may or may not be related to the licensing agreement, is recognized on the basis of labor hours delivered at the contractual full time employee rates. Under the agreement an amount of €3.3 million (2012: €2.1 million 2011: €2.2 million) was recognized as collaboration revenue from delivering research and development services for studies to GSK.
20. Other income
Other income for the year ended December 31, 2013 amounts to €560 thousand (2012: €174 thousand; 2011: €36 thousand). The Company is part of two pan-European consortia, each of which has been awarded a Framework Programme 7 (“FP7”) research grant of €6 million from the European Commission to support respectively ongoing clinical study PRO045 and the development of imaging biomarkers for Duchenne muscular dystrophy (DMD). Grant proceeds are deferred and recognized in other income based on the percentage of completion method for an amount €485 thousand in the year ended December 31, 2013.
To encourage research and development the Company obtained certain loans that generally bear interest at a rate below the market interest rate, considered by the company to be 12% over the last five years. The difference between fair value and the notional amount at inception is treated as a donation received for certain research performed by the Company for an amount of €109 thousand in the year ended December 31, 2013 (2012: €170 thousand; 2011: nil). As of December 31, 2013 an amount of €104 thousand is deferred and will be recognized in other income over the periods during which expenses are incurred.
21. Cost of license revenue
No LUMC fees were expensed during the years 2013, 2012 and 2011.
F - 38
22. Research and development expense
|
€ (‘000)
|
Year ended 31 December
|
||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Employee benefit expense (note 24)
|
4,176 | 4,507 | 5,467 | ||||||
|
Pre-clinical expenses
|
2,738 | 1,439 | 1,748 | ||||||
|
Drug manufacturing and substance
|
3,108 | 2,159 | 3,106 | ||||||
|
Laboratory and office expenses
|
840 | 597 | 619 | ||||||
|
Legal and consulting
|
1,216 | 1,204 | 1,592 | ||||||
|
Clinical expenses
|
1,001 | 1,721 | 2,333 | ||||||
|
IP maintenance fees
|
860 | 773 | 1,320 | ||||||
|
Depreciation, amortization and impairment charges (note 6 and 7)
|
912 | 896 | 961 | ||||||
|
Other operating expenses
|
497 | 1,097 | 1,314 | ||||||
|
Total research and development expense
|
15,348 | 14,393 | 18,460 | ||||||
23. General and administrative expense
|
Year ended 31 December
|
|||||||||
|
€ (‘000)
|
2011
|
2012
|
2013
|
||||||
|
Employee benefit expense (note 24)
|
2,553 | 2,191 | 3,163 | ||||||
|
Audit and consulting fees
|
663 | 462 | 2,219 | ||||||
|
Legal costs
|
622 | 340 | 1,013 | ||||||
|
IT expense
|
421 | 279 | 237 | ||||||
|
Depreciation, amortization and impairment charges (note 6 and 7)
|
148 | 269 | 243 | ||||||
|
Facilities, communication & office expenses
|
472 | 357 | 517 | ||||||
|
Other operating expenses
|
324 | 125 | 342 | ||||||
|
Total general and administrative expense
|
5,203 | 4,023 | 7,734 | ||||||
24. Employee benefit expense
|
€ (‘000)
|
Year ended 31 December
|
||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Wages and salaries
|
5,196 | 5,365 | 6,446 | ||||||
|
Social security costs
|
531 | 771 | 704 | ||||||
|
Share options granted to directors and employees
|
669 | 180 | 1,067 | ||||||
|
Pension costs – defined contribution plans
|
333 | 382 | 413 | ||||||
|
Total
|
6,729 | 6,698 | 8,630 | ||||||
The average number of employees during the years 2013, 2012 and 2011 was as follows:
|
Year ended 31 December
|
|||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Number of employees
|
75 | 82 | 89 | ||||||
F - 39
25. Other gains/(losses)—net
|
€ (‘000)
|
Year ended 31 December
|
||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Other gains
|
|||||||||
|
- Foreign exchange gains
|
22 | 94 | 143 | ||||||
|
Total other gains
|
22 | 94 | 143 | ||||||
|
Other losses
|
|||||||||
|
- other gains / (losses)
|
- | (45 | ) | (31 | ) | ||||
|
Total other losses
|
- | (45 | ) | (31 | ) | ||||
|
Other gains/(losses) - net
|
22 | 49 | 112 | ||||||
Foreign exchange gains relate largely to transactions with GSK denominated in British pounds.
26. Finance income and costs
|
€ (‘000)
|
Year ended 31 December
|
||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Finance cost:
|
|||||||||
|
Bank borrowings
|
(38 | ) | (26 | ) | (38 | ) | |||
|
Other loans
|
(90 | ) | (310 | ) | (620 | ) | |||
|
Finance lease liabilities
|
(60 | ) | (31 | ) | (16 | ) | |||
|
Fair value adjustment of long-term liabilities
|
(9 | ) | 28 | - | |||||
|
Fair value adjustment of derivative instrument
|
- | - | 18 | ||||||
|
Bank charges
|
(12 | ) | (9 | ) | (9 | ) | |||
|
Finance costs
|
(209 | ) | (348 | ) | (665 | ) | |||
|
Finance income:
|
|||||||||
|
Interest income on short-term bank deposits
|
431 | 726 | 656 | ||||||
|
Currency effect cash and cash equivalents
|
- | 70 | (11 | ) | |||||
|
Fair value adjustment long-term loans receivable
|
3 | - | - | ||||||
|
Finance income
|
434 | 796 | 645 | ||||||
|
Total finance income - net
|
225 | 448 | (20 | ) | |||||
27. Income tax expense
No tax charge or income has been recognized in the years 2013, 2012 and 2011 since the Company is in a loss-making position and has a history of losses. Thus, no deferred tax asset has been recognized for carry-forward losses (note 4).
The Company has tax loss carry-forwards of approximately €58.7 million (2012: €42.1 million, 2011: €32.2 million). An amount of €4.2 million relates to the pre-fiscal unity of the Prosensa Holding N.V. As a result of the Dutch income tax law, tax loss carry-forwards are subject to a time limitation of nine years. Tax loss carry-forwards incurred in prior years will expire as follows:
|
Fiscal year
|
Expiration year
|
Tax losses
|
||
|
2005
|
2014
|
147 | ||
|
2006
|
2015
|
1,149 | ||
|
2007
|
2016
|
2,904 | ||
|
2008
|
2017
|
3,418 | ||
|
2009
|
2018
|
11,827 | ||
|
2010
|
2019
|
407 | ||
|
2011
|
2020
|
10,797 | ||
|
2012
|
2021
|
11,399 | ||
|
2013
|
2022
|
16,604 | ||
|
Total tax loss carry - forward
|
58,652 | |||
F - 40
Deferred income tax assets are recognized for tax losses and other temporary differences to the extent that the realization of the related tax benefit through future taxable profits is probable. Deferred tax assets and liabilities are offset if they pertain to future tax effects for the same taxable entity towards the same taxation authority.
No income taxes were paid and no deferred income tax was expensed or recognized through the income statement in the years ended December 31, 2013, 2012 and 2011. Losses are attributable to operations in the Netherlands.
As the Company’s temporary differences between tax gains / losses and current gains / losses, if any, are deemed immaterial and their realization is uncertain the Company refrains from further disclosures.
In order to promote innovative technology development activities and investments in new technologies, a corporate income tax incentive has been introduced in Dutch tax law called the Innovations Box. For the qualifying profits, the Company effectively owes only 5% income tax, should available tax losses carried forward have been utilized, instead of the general tax rate of 25% which results in an estimated effective tax rate of 10%. The agreement with the tax authorities is currently signed for the years 2011 – 2015 and is expected to be extended.
28. Loss per share
Basic
Basic loss per share is calculated by dividing the loss attributable to equity holders of the Company by the weighted average number of ordinary and preferred shares in issue during the year.
|
Year ended 31 December
|
|||
|
2011
|
2012
|
2013
|
|
|
Loss attributable to equity holders of the company in EUR (‘000)
|
(11,579)
|
(9,892)
|
(16,604)
|
|
Weighted average number of Common and Preference shares in issue
|
19,240,137
|
26,574,570
|
32,424,025
|
Diluted
Diluted loss per share is calculated by adjusting the weighted average number of ordinary shares outstanding to assume conversion of all dilutive potential ordinary shares. Due to the fact that the Company is loss making all potential ordinary shares had an antidilutive effect, if converted, and thus have been excluded from the computation of loss per share.
|
Year ended 31 December
|
|||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Share options
|
1,416,622 | 1,535,973 | 2,137,943 | ||||||
|
Class B shares acquisition rights
|
- | - | - | ||||||
|
Antidilutive potential Common and Preference shares
excluded from the calculation of loss per share
|
1,416,622 | 1,535,973 | 2,137,943 | ||||||
29. Dividends per share
The Company did not declare dividends for the years ended December 31, 2013, 2012 and 2011.
30. Commitments
Operating lease commitments—Company as lessee
The Company leases office space and laboratory space under non-cancellable operating lease agreements. The lease term is between 1 and 5 years.
Furthermore the Company entered into several lease agreements for office equipment. The annual obligations amount to €23 thousand (2012: €14 thousand 2011: €41 thousand). The lease obligations term is between 1 and 3 years.
The lease expenditure charged to the income statement during the year amounted to €774 thousand (2012: €751 thousand 2011: €703 thousand). The future aggregate minimum lease payments under non-cancellable operating leases are as follows:
F - 41
|
€ (‘000)
|
31 December
|
||||||||
|
2011
|
2012
|
2013
|
|||||||
|
No later than 1 year
|
744 | 773 | 798 | ||||||
|
Later than 1 year and no later than 5 years
|
2,759 | 2,100 | 1,315 | ||||||
|
Later than 5 years
|
- | - | - | ||||||
|
Total
|
3,503 | 2,873 | 2,113 | ||||||
Guarantees:
In 2011 the Company secured the fulfillment of the terms and conditions of an operational lease agreement through a bank guarantee to the lessor for an amount of €18 thousand. The guarantee was released in 2012.
In 2012 the Company received a loan from Agentschap NL for a total amount of €640 thousand which bears an interest rate of 9.5% per annum. The Company has pledged the part of its materials and immaterial fixed assets that directly relate to the project funded by the loan granted.
In 2013 no additional guarantees were agreed.
31. Related-party transactions
The Supervisory Board members were paid €4 thousand, €7 thousand and €4 thousand for consulting services during the years ended December 31, 2013, 2012 and 2011, respectively. The services were rendered at arm’s length.
Management Board and Supervisory Board compensation
In 2013, 2012 and 2011 the Management Board was paid a total of €1.0 million, €1.6 million and €1.1 million, respectively, in salaries as well as short term benefits. During 2013 the Management Board members received no bonus payments. In 2012 the Management Board members received bonus payments in March related to 2011 and in December related to 2012. In 2013, 2012 and 2011, €764 thousand, €160 thousand and €557 thousand, respectively, was expensed for share-based payment awards. Contributions to post employment schemes amounted to €159 thousand, €161 thousand and €140 thousand during the years ended December 31, 2013, 2012 and 2011, respectively. No termination benefits or other long term benefits were paid. The Management Board held 1,559 thousand, 1,759 thousand and 969 thousand stock options as of December 31, 2013, 2012 and 2011, respectively. In addition, the Management Board held 149 thousand restricted shares and 928 thousand ordinary shares as of December 31, 2013.
On December 5, 2012 the Management Board was granted 1.0 million stock options that vest subject to a liquidity event and continued employment. As of December 31, 2012 the requirements to vest were deemed not probable under an IPO Scenario, and de minimus under a change of control scenario, and therefore no expenses were recognized in the income statement. Following our initial public offering, the number of options eligible to vest automatically reduced from 1.0 million to 800,000.
Selected members of the Supervisory Board were paid in total an amount of €34 thousand during the year ended December 31, 2013 and €50 thousand respectively during the years ended December 31, 2012 and 2011.
At the annual general meeting of shareholders on June 14, 2013 the shareholders approved, conditional upon the completion of the IPO, an individual annual grant of 10,000 options to members of the Supervisory Board. In 2013 four members of the Supervisory Board were granted 40,000 Options with an exercise price of $19.25 (2012: 15,000 Restricted Shares and 11,250 Options with an exercise price of €0.01).
Expenses of €119 thousand related to the granted options to members of the Supervisory Board were recognized during the period ended December 31, 2013. The Options and Restricted Shares were granted under the Company’s share based compensation plan (note 16).
No loans, advances and guarantees were made to the Management Board and Supervisory Board members as of December 31, 2013, 2012 and 2011.
32. Auditor fees
The auditors PricewaterhouseCoopers Accountants N.V. have performed the following services for the Company:
F - 42
|
€ (‘000)
|
Year ended 31 December
|
||||||||
|
2011
|
2012
|
2013
|
|||||||
|
Audit fees
|
56 | 36 | 123 | ||||||
|
Audit-Related fees
|
33 | - | 755 | ||||||
|
Tax Fees
|
- | - | - | ||||||
|
All Other Fees
|
- | - | - | ||||||
|
Total
|
89 | 36 | 878 | ||||||
33. Events after the balance sheet date
In January 2014 the Company and GSK mutually terminated the collaboration for the development of drisapersen and the Company’s other DMD product candidates. Under the termination agreement, GSK will transfer to us data and other intellectual property, inventory, regulatory filings and clinical trial sponsorships, clinical trial reports, material agreements and biological materials as soon as practicable and within 120 days of the effective date of the agreement. Under the agreement GSK returns to us the exclusive worldwide license to develop and commercialize drisapersen as well as the options to receive exclusive licenses for the other DMD compounds. As a result of the termination agreement the Company will not receive future milestone payments upon successful compound development and commercialization and percentage royalties in the low tens on product sales. The termination agreement releases us from any performance obligations under the upfront payments already received from GSK.
F - 43