
Corporate Update January 2024 Developing Therapeutic Antibodies Targeting Allergic, Inflammatory and Proliferative Disease Exhibit 99.1

Disclaimer This presentation contains forward-looking statements. All statements other than statements of historical fact contained in this presentation, including statements regarding the financial position of Allakos Inc. (“Allakos,” the “Company,” “we” or “our”); estimated lirentelimab closeout, severance and other costs; the timing of payment of restructuring expenditures; estimated ending 2023 and 2024 cash, cash equivalents and investments; estimated cash runway; business strategy; plans and objectives for future operations; our expectations regarding the potential benefits, activity, effectiveness and safety of our product candidates; our expectations with regard to the initiation, design, timing and results of our clinical studies, preclinical studies and research and development programs, including the timing and availability of data from such studies; our preclinical, clinical and regulatory development plans for our product candidates; and our anticipated milestones are forward-looking statements. Allakos has based these forward-looking statements on its estimates and assumptions and its current expectations and projections about future events. The words “anticipate,” “believe,” “continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “target,” “should,” “would,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. The forward-looking statements included in this presentation speak only as of the date of this presentation and are subject to a number of risks, uncertainties, and assumptions, including, but not limited to: the Company’s stages of clinical drug development; the Company’s ability to timely initiate and complete clinical trials for AK006; the Company’s ability to obtain required regulatory approvals for its clinical trials; uncertainties related to the enrollment of patients in its clinical trials; the Company’s ability to demonstrate sufficient safety and efficacy of its product candidates in its clinical trials; uncertainties related to the success of clinical trials, regardless of the outcomes of preclinical testing and prior clinical trials; the Company’s ability to advance additional product candidates beyond AK006; the Company’s ability to obtain additional capital to finance its operations; general economic and market conditions; and other risks described in the “Risk Factors” section included in our periodic filings that we have made and will make with the Securities and Exchange Commission (“SEC”). In addition, Allakos operates in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for Allakos’ management to predict all risks, nor can Allakos assess the impact of all factors on its business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements that Allakos may make. In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this presentation are inherently uncertain and may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Accordingly, you should not rely upon forward-looking statements as predictions of future events. Allakos does not undertake any obligation to update or revise any forward-looking statements, to conform these statements to actual results or to make changes in Allakos’ expectations, except as required by law. Additional Information: The Company has filed and will file Current Reports on Form 8-K, Quarterly Reports on Form 10-Q, and Annual Reports on Form 10-K, and other documents with the SEC. You should read these documents for more complete information about the Company. You may get these documents for free by visiting EDGAR on the SEC website at www.sec.gov. This presentation concerns products that are under clinical investigation, and which have not yet been approved for marketing by the U.S. Food and Drug Administration. It is currently limited by federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated.

Agenda Robert Alexander, CEO Introduction Craig Paterson, CMO Results of ATLAS and MAVERICK Phase 2 Studies Baird Radford, CFO Restructuring and Financial Implications Robert Alexander, CEO AK006 Program Update Q&A

ATLAS: Phase 2 Study of Lirentelimab in Atopic Dermatitis
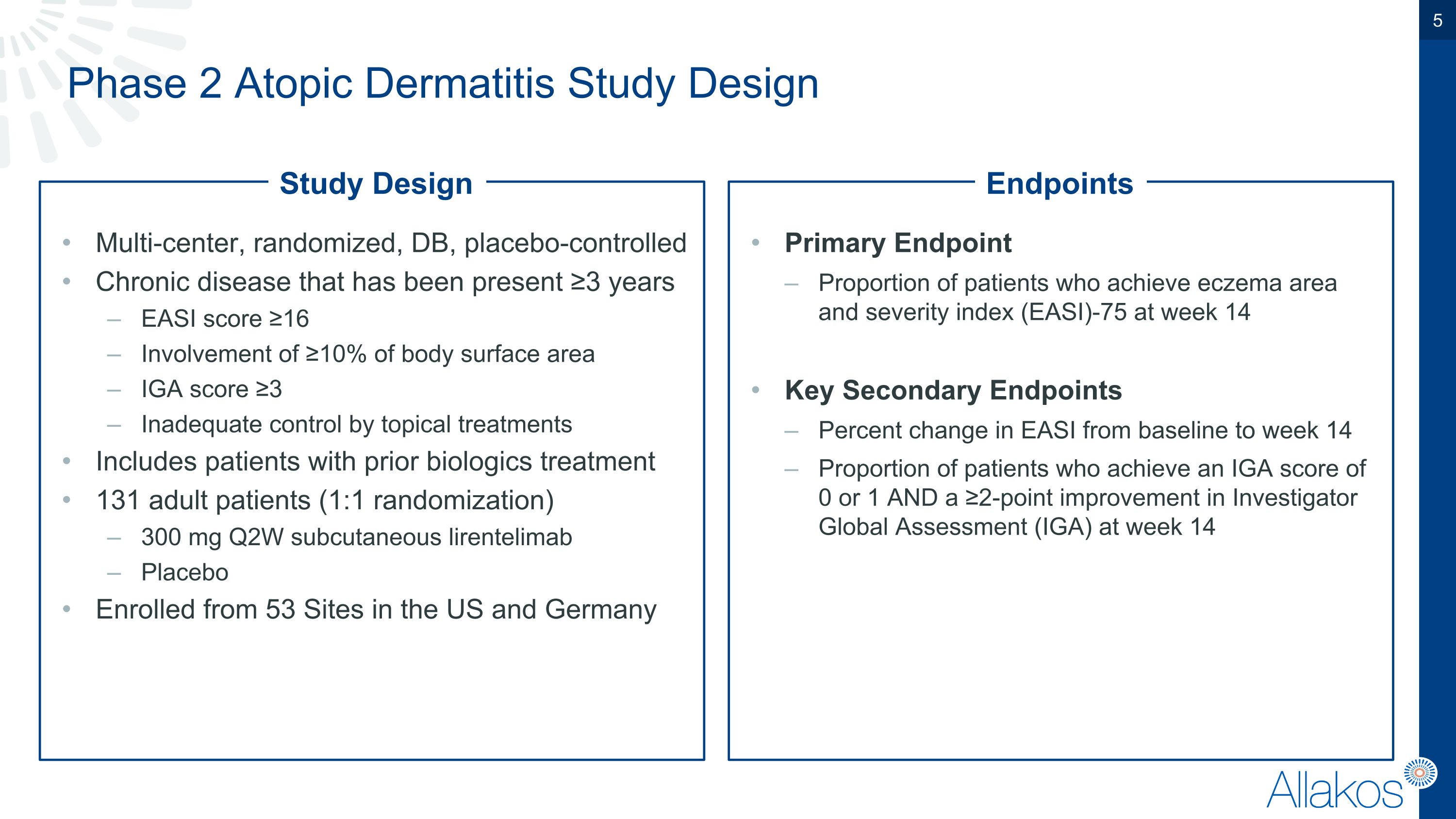
Phase 2 Atopic Dermatitis Study Design Multi-center, randomized, DB, placebo-controlled Chronic disease that has been present ≥3 years EASI score ≥16 Involvement of ≥10% of body surface area IGA score ≥3 Inadequate control by topical treatments Includes patients with prior biologics treatment 131 adult patients (1:1 randomization) 300 mg Q2W subcutaneous lirentelimab Placebo Enrolled from 53 Sites in the US and Germany Primary Endpoint Proportion of patients who achieve eczema area and severity index (EASI)-75 at week 14 Key Secondary Endpoints Percent change in EASI from baseline to week 14 Proportion of patients who achieve an IGA score of 0 or 1 AND a ≥2-point improvement in Investigator Global Assessment (IGA) at week 14 Study Design Endpoints

ATLAS: Baseline Demographics & Patient Characteristics Baseline Characteristics (mITT population) Phase 2 ATLAS (AD) Patient Characteristics Lirentelimab n=61 Placebo n=61 All n=122 Age (years), median (IQR) 39 (31-54) 35 (26-49) 37 (26-53) Female sex, n (%) 32 (52.5%) 34 (55.7%) 66 (54.1%) White, n (%) 39 (63.9%) 33 (54.1%) 72 (59.0%) From US Sites, n (%) 49 (80.3%) 49 (80.3%) 98 (80.3%) BMI (kg/m2), median (IQR) 27.5 (24.1-34.3) 29.3 (24.0-31.9) 28.1 (24.0-33.3) Duration of AD diagnosis (years), median (IQR) 22.4 (7.7-32.3) 21.9 (11.9-29.4) 22.0 (10.2-29.6) Prior biologic use for AD, n (%) 10 (16.4%) 12 (19.7%) 22 (18.0%) Peripheral blood eosinophils (cells/µL), median 180 290 230 IgE (kU/L), median 250.0 391.0 355.8 Baseline EASI [0-72], median (IQR) 26.8 (23.6-30.6) 26.9 (23.7-33.4) 26.9 (23.6-32.1) Baseline IGA=4, n (%) 32 (52.5%) 31 (50.8%) 63 (51.6%) Baseline BSA [0-100%], median (IQR) 42.0 (31.0-57.3) 46.6 (39.0-58.9) 46.0 (33.5-58.9) Baseline SCORAD [0-103], median (IQR) 66.2 (59.7-73.2) 69.6 (59.4-75.5) 68.7 (59.5-74.3) Baseline ppNRS [0-10], median (IQR) 7.8 (6.9-8.6) 7.2 (6.3-8.1) 7.5 (6.3-8.3) Baseline DLQI [0-30], median (IQR) 15 (10-21) 14 (10-20) 14 (10-21)

ATLAS Primary Efficacy Endpoint Proportion of EASI-75 Responders at Week 14 (mITT) % of Subjects (11/61) (14/61) Lirentelimab Placebo *ns

Secondary Endpoint: Change in EASI Score Change in EASI Score from Baseline to Week 14 (mITT) Percent Change EASI LS Mean Change from BL Absolute Change Lirentelimab (n=61) Placebo (n= 61) 26.9 26.8 Median BL = Lirentelimab (n=61) Placebo (n= 61) EASI LS Mean % Change from BL *ns *ns

Significant Change in EASI Scores in Patients with Higher Inflammatory Disease Burden Percent Change in EASI Score from Baseline to Week 14 (mITT) Baseline IGA-4 Difference from placebo p-values derived using MMRM Baseline IGA-3 Lirentelimab (n=29) Placebo (n= 30) 24.0 Median BL = Lirentelimab (n=32) Placebo (n= 31) EASI LS Mean % Change from BL 31.9 29.7 Median BL = *ns p=0.0476 24.2 EASI LS Mean % Change from BL

Exploratory Endpoint: Improvement in ppNRS (Itch) Proportion of ppNRS Responders at Week 14 (mITT) Difference from placebo p-values derived using Cochran-Mantel-Haenszel (CMH) Includes all subjects regardless of baseline ppNRS. No minimum ppNRS required for study entry. % of Subjects (14/61) (7/61) Lirentelimab Placebo (12/61) (5/61) Lirentelimab Placebo % of Subjects p=0.0903 p=0.0696 Achieving ≥3-Point Improvement Achieving ≥4-Point Improvement

MAVERICK: Phase 2b Study of Lirentelimab in Chronic Spontaneous Urticaria

Phase 2b Chronic Spontaneous Urticaria Study Multi-center, randomized, DB, placebo-controlled Active moderate-to-severe symptoms CSU diagnosis with onset ≥6 months prior to screening Diagnosis of CSU refractory to antihistamines Presence of itch and hives despite current use of antihistamines UAS7 score ≥16 and HSS7 score ≥8 at baseline Includes patients with prior biologics treatment 127 adult patients (1:1 randomization) 300 mg Q2W subcutaneous lirentelimab Placebo Enrolled from 56 Sites in the US, Germany, and Poland Primary Endpoint Change from baseline in UAS7 at week 12 Key Secondary Endpoints Absolute change in ISS7 Absolute change in HSS7 Proportion of patients who achieve UAS7=0 Study Design Endpoints

MAVERICK: Baseline Demographics & Patient Characteristics Baseline Characteristics (mITT population) Phase 2 MAVERICK (CSU) Patient Characteristics Lirentelimab n=64 Placebo n=59 All n=123 Age, mean ± SD 42 ± 14 46 ± 16 44 ± 15 Female sex, n (%) 48 (75.0%) 55 (93.2%) 103 (83.7%) White, n (%) 52 (81.3%) 42 (71.2%) 94 (76.4%) From US Sites, n (%) 55 (85.9%) 53 (89.8%) 108 (87.8%) BMI (kg/m2), mean ± SD 29.7 ± 7.1 30.8 ± 6.3 30.2 ± 6.8 Duration of CSU diagnosis (years), median (range) 4.8 (0.5-47.3) 4.4 (0.5-53.3) 4.5 (0.5-53.3) History of CSU-associated Angioedema, n (%) 23 (35.9%) 25 (42.4%) 48 (39.0%) Prior omalizumab use, n (%) 22 (34.4%) 18 (30.5%) 40 (32.5%) Peripheral blood eosinophils (cells/µL), median 130 120 120 IgE (kU/L), median 105 58.1 77.3 Baseline UAS7 [0-42], mean ± SD 31.4 ± 7.2 32.4 ± 7.4 31.9 ± 7.3 Baseline UAS7 28-42, n (%) 40 (62.5%) 43 (72.9%) 83 (67.5%) Baseline HSS7 [0-21], mean ± SD 15.1 ± 4.1 16.0 ± 3.9 15.6 ± 4.0 Baseline ISS7 [0-21], mean ± SD 16.3 ± 4.4 16.3 ± 4.2 16.3 ± 4.3 Baseline UCT [0-16], mean ± SD 3 ± 2 3 ± 3 3 ± 3 Baseline DLQI [0-30], mean ± SD 16 ± 8 15 ± 8 16 ± 8
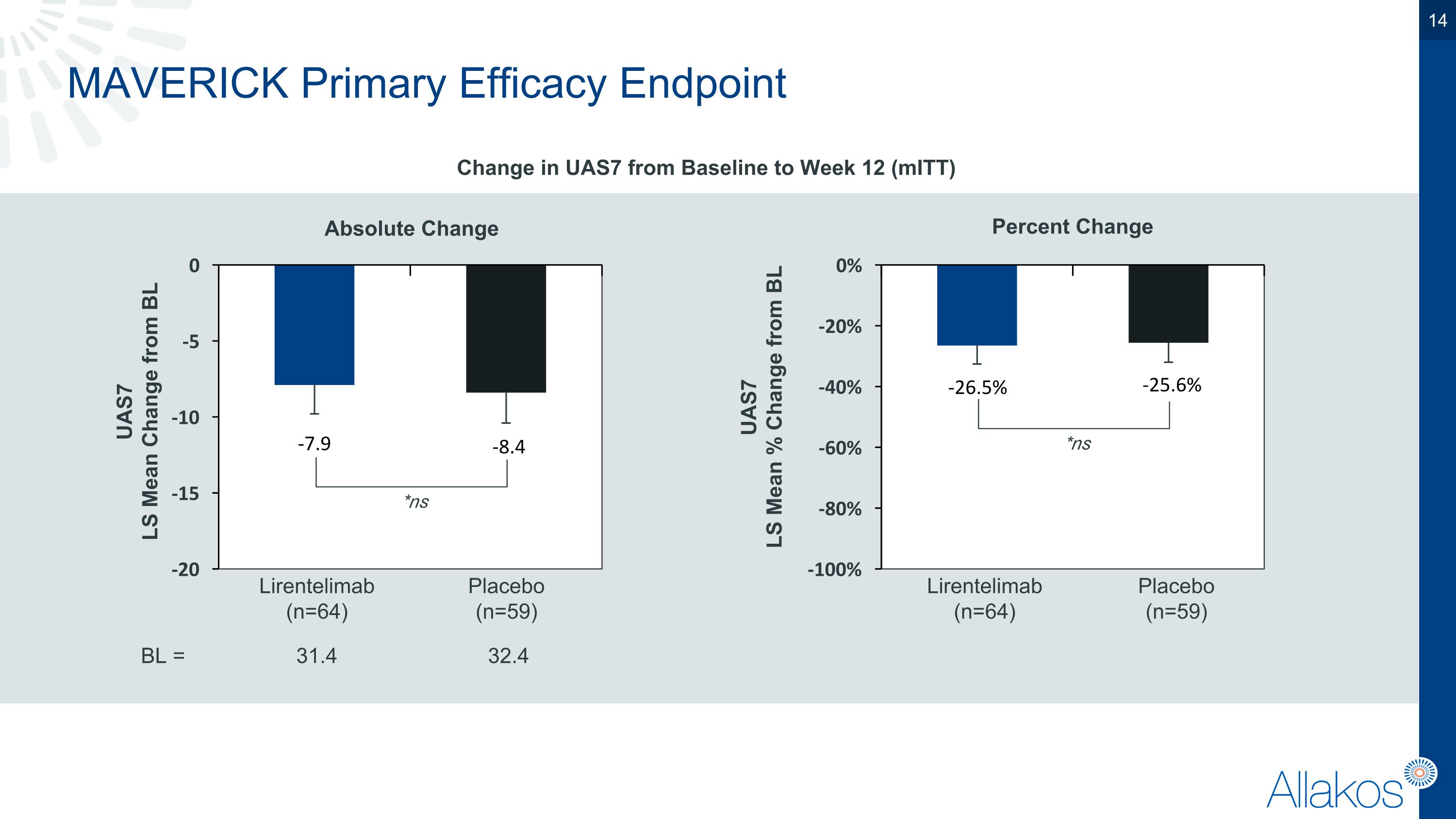
MAVERICK Primary Efficacy Endpoint Change in UAS7 from Baseline to Week 12 (mITT) Percent Change Absolute Change 32.4 UAS7 LS Mean % Change from BL UAS7 LS Mean Change from BL Lirentelimab (n=64) Placebo (n=59) Lirentelimab (n=64) Placebo (n=59) 31.4 BL = *ns *ns
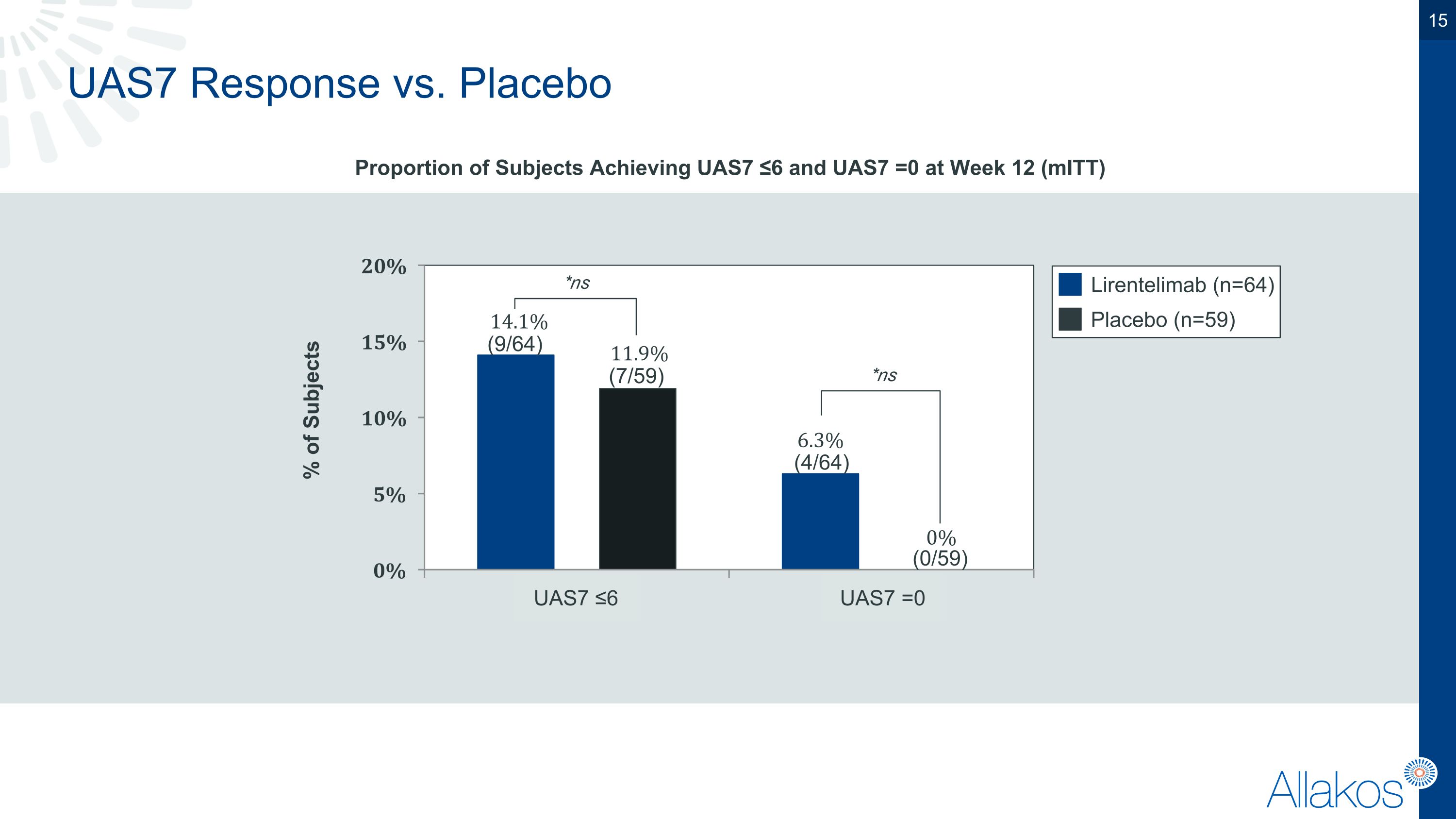
UAS7 Response vs. Placebo Proportion of Subjects Achieving UAS7 ≤6 and UAS7 =0 at Week 12 (mITT) Lirentelimab (n=64) Placebo (n=59) % of Subjects (9/64) (7/59) (0/59) UAS7 ≤6 UAS7 =0 *ns (4/64) *ns

Complete Response in Hives and Itch % of Subjects (2/59) (4/64) Lirentelimab Placebo Lirentelimab (1/59) (6/64) Placebo % of Subjects *ns *ns Proportion of Complete Responders in Hives and Itch at Week 12 (mITT) Achieving HSS7=0 Achieving ISS7=0
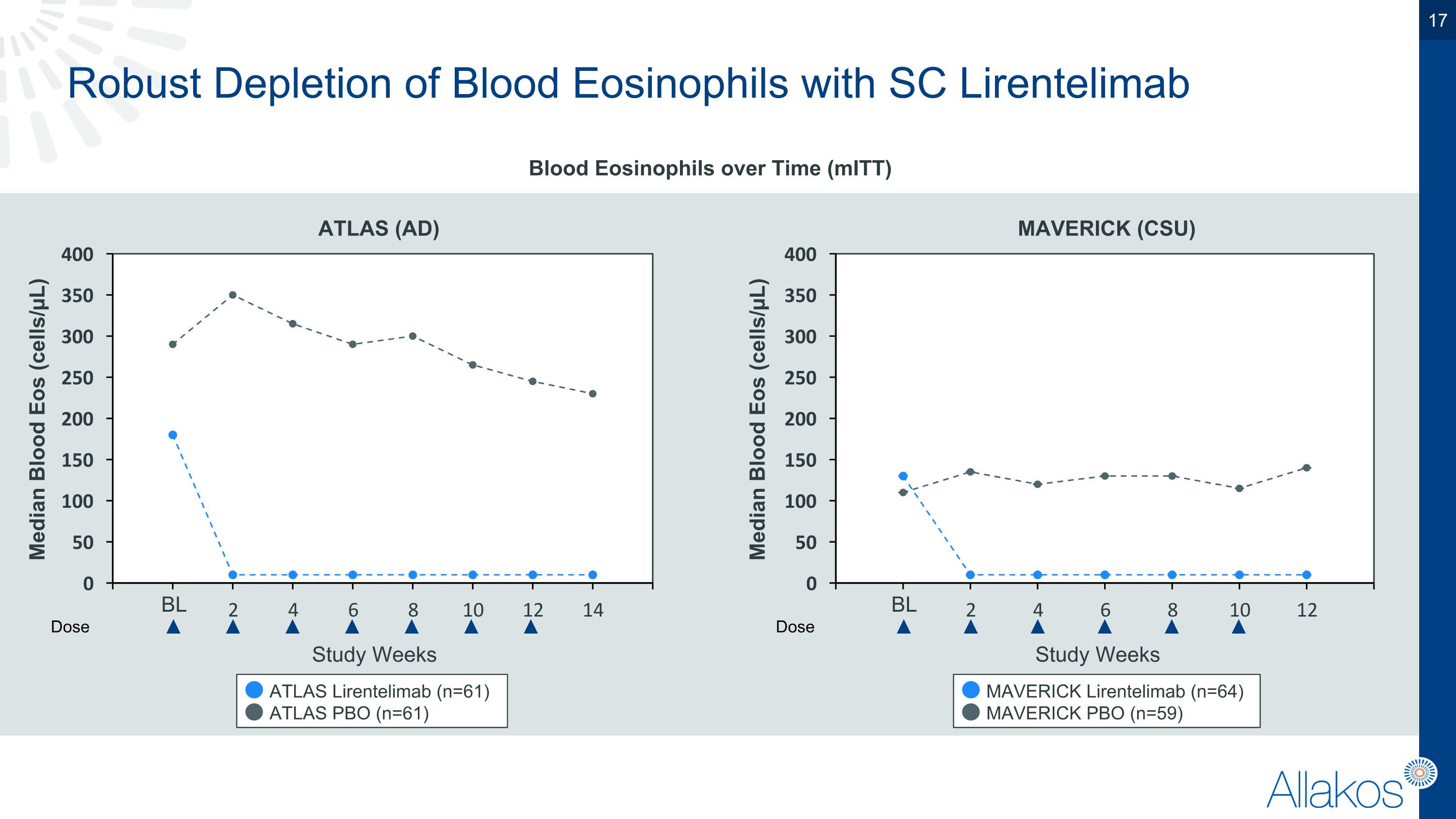
Robust Depletion of Blood Eosinophils with SC Lirentelimab Blood Eosinophils over Time (mITT) Study Weeks Dose Median Blood Eos (cells/µL) BL ATLAS Lirentelimab (n=61) ATLAS PBO (n=61) Dose Median Blood Eos (cells/µL) BL Study Weeks ATLAS (AD) MAVERICK (CSU) MAVERICK Lirentelimab (n=64) MAVERICK PBO (n=59)

Safety
Overall Safety Summary Safety profile of SC lirentelimab was consistent with previously reported lirentelimab studies Most common adverse events were injection-related reactions (IRRs) ATLAS (AD): 18.5% lirentelimab experienced IRRs vs. 6.2% placebo MAVERICK (CSU): 18.2% lirentelimab experienced IRRs vs 8.2% placebo Common IRR symptoms included Headache, Chills, Nausea, Dizziness, and Flushing

Financial Update

Expected Cash Runway into Middle of 2026 Estimated 2024 Net Cash Used in Operating Activities Estimated net cash used in operating activities (GAAP) $85 to $90 million Less: estimated lirentelimab closeout, severance and other costs ($30 million) Adjusted net cash used in operating activities (non-GAAP) $55 to $60 million The Company will halt lirentelimab-related activities across clinical, manufacturing, research and administrative functions Reduce our workforce by almost 50% The Company anticipates that the significant majority of the restructuring expenditures will be paid in the first half of 2024 Restructuring Actions

AK006 Development Plans and Update

Siglec-6 is a more potent inhibitory receptor than Siglec-8 Siglec-6 regulates more cellular processes than Siglec-8: Signal Transduction Transcription Translation Cellular metabolism Degranulation AK006 has two key attributes Long residence time on the cell surface which correlates to increased inhibitory activity Antibody Dependent Cellular Phagocytosis (ADCP) AK006 Targets a Different Receptor with Different Underlying Biology Siglec-6 Biology and AK006

Siglec-6 and Siglec-8 Differentially Interact with Proteins that Regulate Mast Cell Activity Gray lines represent direct interaction Cytokine Signaling Protein Processing (Translation) TGFb Signaling Interferon-Related Cytoplasmic Transport Mitochondrial Translocation Ubiquitin Activity (Protein Degradation) Pyrophosphate Metabolism (Cellular Metabolism/ATP) Serine/Threonine Phosphatases (Signal Transduction) Pyruvate Metabolism (Cellular Metabolism) SOURCE: Korver, W. et al Allergy 2024.
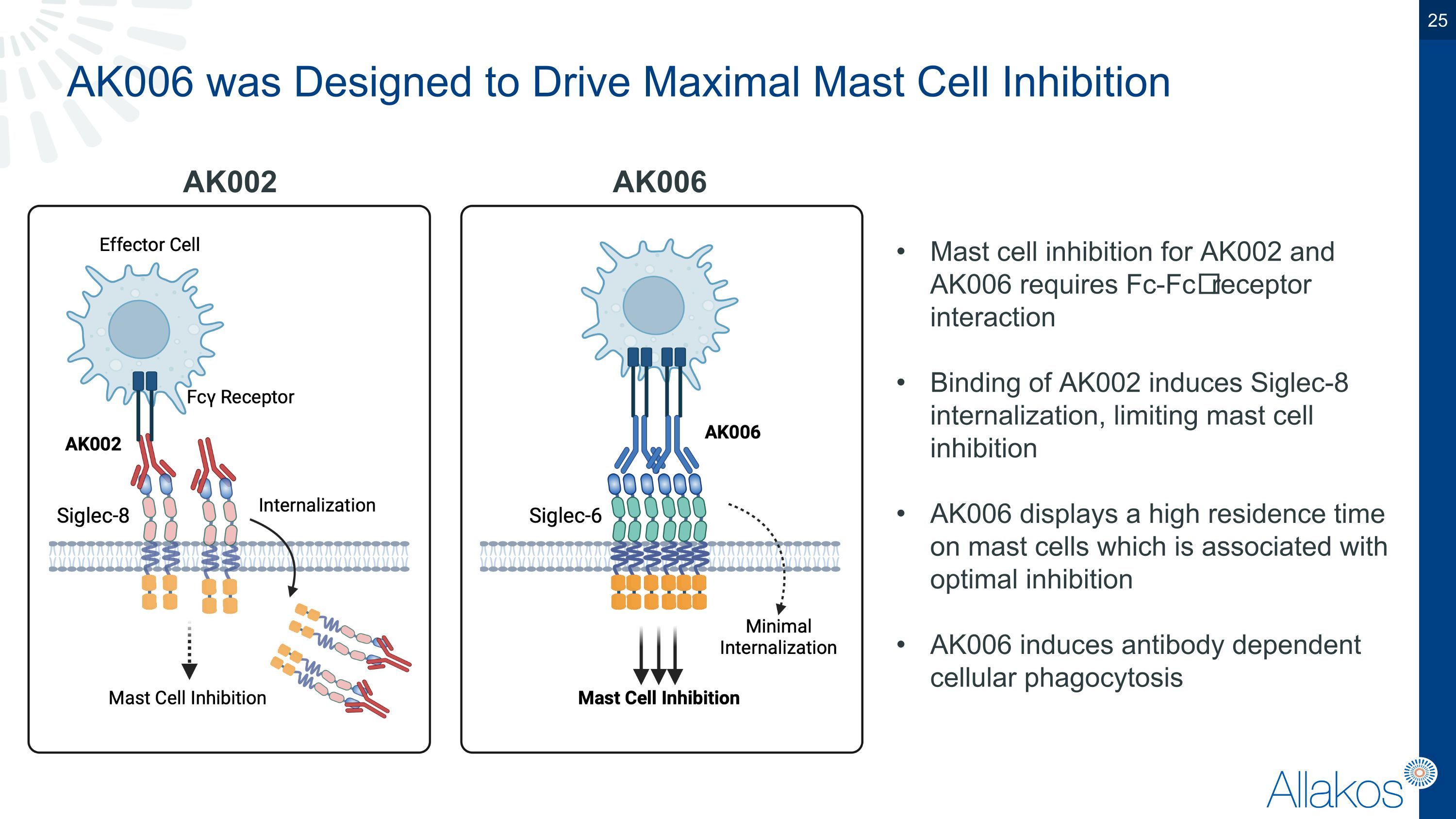
AK006 was Designed to Drive Maximal Mast Cell Inhibition AK002 AK006 Mast cell inhibition for AK002 and AK006 requires Fc-Fc𝛾 receptor interaction Binding of AK002 induces Siglec-8 internalization, limiting mast cell inhibition AK006 displays a high residence time on mast cells which is associated with optimal inhibition AK006 induces antibody dependent cellular phagocytosis

AK006 Displays Significantly Stronger MC Inhibition than AK002 in Preclinical Studies IgE Activation AK006 inhibits IgE-dependent and –independent modes of MC activation better than AK002 n=3 human donors SOURCE: Korver, W. et al Allergy 2024. KIT Activation Peritoneal Mast Cells MRGPRX2 Activation Lesional Monocytes

AK006 Reduces Human Mast Cells via Antibody-Dependent Cellular Phagocytosis in Preclinical Studies AK006 can reduce mast cell numbers and mediate broad inhibition Ex Vivo Human Tissue Mast Cells - IFN𝞬 Activated Macrophages + IFN𝞬 Activated Macrophages Mast Cells in Tissue * p < 0.01; n=3 human donors In Vitro ADCP Assay SOURCE: Schanin, J et al. Communications Biology, 2022

AK006 Inhibits Mast Cell Activation in Human Tissues IgE-Activated Human Tissue Mast Cells AK006 inhibits IgE-mediated mast cell activation Human Mast Cell Activation Assay n=3 human donors SOURCE: Schanin J, et al. EAACI 2022 Presentation.

AK006 Protects Against Systemic Anaphylaxis in Humanized Mice Humanized Model of Anaphylactic Death Systemic Anaphylaxis AK006 inhibits IgE-mediated mast cell activation in vivo Humanized Mouse Model of Anaphylaxis Anaphylactic Death SOURCE: Schanin, J et al. Communications Biology 2022

AK006 Reduces Allergic Enteritis in Siglec-6 Transgenic Mice AK006 reduces allergen-mediated GI inflammation via mast cell inhibition in vivo SOURCE: Allakos Data on File. Stomach Mast Cells p < 0.05, n=6 mice/group Transcriptional Profiling of Stomach Mast Cells Corporate

AK006 Phase 1 Study Design Single Ascending Dose (SAD) and Multiple Ascending Dose (MAD) Cohorts in Healthy Volunteers Randomized, double-blind, placebo-controlled Intravenous AK006 SAD: 5, 20, 80, 240, 720 mg MAD: 80, 240, 720 mg monthly Subcutaneous AK006 150 and 720 mg Planned CSU Cohort Randomized, double-blind, placebo-controlled Moderate-to-severe antihistamine refractory CSU UAS7 score ≥16 and HSS7 score ≥8 at baseline Four doses of AK006 IV given monthly SAD and MAD Cohort Safety and tolerability Pharmacokinetics Pharmacodynamics Target receptor engagement in skin biopsies SC Bioavailability CSU Cohort Therapeutic activity assessed by changes in UAS7 at week 14 Trial Cohorts Endpoints

AK006 Clinical Development Plan LOW/MOD 2023 2024 2025 Q3 Q4 Q1 Q2 Q3 Q4 Q1 Q2 Q3 Q4 Part C: CSU Patients Data Availability (n=30) Part B: MAD in HVs (3 Cohorts) Part A: SAD in HVs (5 Cohorts) Ph1 First-in-Human Study Today Bioavailability Ph1b Study in TBD Indication Part D: SC in HVs (2 Cohorts) CSU, chronic spontaneous urticaria; HVs, healthy volunteers; MAD, multiple ascending dose; SAD, single ascending dose; SC, subcutaneous 1 = Phase 2 Study for timing purposes only, potential future investment for Phase 2 study not currently in budget 2026 Q1 Q2 Q3 Q4 IV SC Formulation Ph2 Study in TBD Indication1

Upcoming AK006 Milestones Q1 2024: Complete SAD and MAD dosing with Intravenous (IV) AK006 in healthy volunteers. Q1 2024: Initiate the randomized, double-blind, placebo-controlled subcutaneous (SC) AK006 cohort in healthy volunteers. Q2 2024: Report SAD and MAD safety, pharmacokinetics (PK), and pharmacodynamic (PD) results from the Phase 1 IV AK006 trial in healthy volunteers, including data to confirm Siglec-6 receptor occupancy in skin biopsy samples. Q2 2024: Initiate the randomized, double-blind, placebo-controlled Phase 1 trial of IV AK006 in patients with chronic spontaneous urticaria (CSU). Q3 2024: Report subcutaneous (SC) AK006 safety, PK, and PD results from the Phase 1 trial in healthy volunteers, including data to confirm Siglec-6 receptor occupancy in skin biopsy samples. Year End 2024: Report topline data from the Phase 1 trial of IV AK006 in patients with CSU.

Q&A

Thank You