UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended: October 31, 2019
[_] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from: _____________________
Commission file number: 000-55008
ORGANICELL REGENERATIVE MEDICINE, INC.
(Exact name of registrant as specified in its charter)
| Nevada | 47-4180540 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification No.) |
4045 Sheridan Ave, Suite 239
Miami, FL 33140
(Address of principal executive offices)
(888) 963-7881
(Issuer’s telephone number)
Securities registered under Section 12(b) of the Act:
| Title of each class | Trading Symbol(s) | Name of each exchange on which registered |
| None | N/A | N/A |
Securities registered under Section 12(g) of the Act:
Common Stock, $0.001 par value
(Title of class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes ☐ No ☒
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Yes ☐ No ☒
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “non-accelerated filer”, “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ☐ | Accelerated filer | ☐ | ||
| Non-accelerated filer | ☒ | Smaller reporting company | ☒ | ||
| Emerging growth company | ☐ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes ☐ No ☒
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. $4,154,116 based on the closing price of $0.052 per share of common stock and 79,886,847 shares of common stock of the Registrant held by non-affiliates on April 30, 2019, the last business day of the Registrant’s mostly recently completed second fiscal quarter.
As of September 30, 2020, there were 875,194,450 shares of common stock, $0.001 par value per share, issued and outstanding.
DOCUMENTS INCORPORATED BY REFERENCE: None
| i |
This Annual Report on Form 10-K and certain information incorporated herein by reference contain forward-looking statements and information within the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995, Section 27A of the Securities Act of 1933, and Section 21E of the Securities Exchange Act of 1934. This information includes assumptions made by, and information currently available to management, including statements regarding future economic performance and financial condition, liquidity and capital resources, acceptance of our products by the market, and management’s plans and objectives. In addition, certain statements included in this and our future filings with the Securities and Exchange Commission (“SEC”), in press releases, and in oral and written statements made by us or with our approval, which are not statements of historical fact, are forward-looking statements. Words such as “may,” “could,” “should,” “would,” “believe,” “expect,” “expectation,” “anticipate,” “estimate,” “intend,” “seeks,” “plan,” “project,” “continue,” “predict,” “will,” “should,” and other words or expressions of similar meaning are intended by us to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements are found at various places throughout this report and in the documents incorporated herein by reference. These statements are based on our current expectations about future events or results and information that is currently available to us, involve assumptions, risks, and uncertainties, and speak only as of the date on which such statements are made.
Forward-looking statements include, but are not limited to, the following:
| n | Our products’ advantages; | |
| n | Expectations regarding our future growth; | |
| n | Expectations regarding available cash resources to fund current operations and future growth; | |
| n | Our ability to comply with regulations governing the production and sale of our products; | |
| n | Our ability to receive regulatory approvals; | |
| n | Market opportunities for our services and products; | |
| n | Our ability to compete effectively; | |
| n | Our ability to respond to market forces; and | |
| n | Our ability to protect our intellectual property. |
Actual results and outcomes may differ materially from those expressed or implied in these forward-looking statements. Factors that may cause such a difference include, but are not limited to, those discussed in Part I, Item 1A, “Risk Factors,” below. Except as expressly required by the federal securities laws, we undertake no obligation to update any such factors, or to publicly announce the results of, or changes to any of the forward-looking statements contained herein to reflect future events, developments, changed circumstances, or for any other reason.
Unless otherwise noted, as used herein, the terms “Organicell Regenerative Medicine”, “Organicell”, the “Company”, “we”, “our” and “us” refer to Organicell Regenerative Medicine, Inc., a Nevada corporation formerly known as Biotech Product Services and Research, Inc., and its subsidiaries consolidated as a combined entity.
| 1 |
Overview
We are engaged in the health care industry, principally focusing on supplying products and services related to the growing field of regenerative anti-aging medicine (“RAAM”). Our focus is the processing, distribution and supply of biologically processed cellular and tissue-based products developed from internally-based research and development activities and/or from other state-of-the-art RAAM-related products developed by third parties under exclusive and/or favorable supply arrangements and to provide other related services used in the regenerative medicine field (“RAAM Products”). Organicell distributes and supplies the RAAM Products and market RAAM-related services to the health care industry through a doctors and clinics (collectively, the “Providers”).
From November 2016 to February 2018, we operated our own laboratory facilities to process and distribute RAAM Products developed through trade secrets acquired in connection with the employment of newly hired executives during November 2016 and March 2017. During this time, we also implemented an in-house sales force and made arrangements with newly identified independent distributors to sell our RAAM Products.
In February 2018, we sold or transferred our laboratory facilities and all related assets (“Sale”), including intellectual property rights, to Vera Acquisition LLC, a Utah limited liability company (“Vera”). From the date of the Sale until the Company’s new laboratory facility became operational, as described below, the Company relied on short-term supply agreements with third party manufacturers to provide it with the products it sold and distributed to its customers.
Commencing in February 2019, the Company began taking steps to once again operate a placental tissue bank processing laboratory in Miami, Florida for the purpose of performing research and development and the manufacturing and processing of anti-aging and cellular therapy derived products. This new laboratory facility became operational in May 2019 and thereupon, the Company began producing products that are now being sold and distributed to its customers.
The Company has actively taken steps to meet compliance with current and anticipated United States Food and Drug Administration (“FDA”) regulations expected to be enforced beginning in May 2021 requiring that the sale of products that fall under Section 351 of the Public Health Services Act pertaining to marketing traditional biologics and human cells, tissues and cellular and tissue based products (“HCT/Ps”) can only be sold pursuant to an approved biologics license application (“BLA”). To date, the Company has obtained approximately 14 Investigation New Drug (“IND”), emergency IND (“eIND”) and/or non-emergency IND (“non-eIND”) approvals from the FDA, including applicable Institutional Review Board (“IRB”) approvals which authorized the Company to commence clinical trials or treatments in connection with the use of the Company’s products and related treatment protocols. The Company is aggressively pursuing efforts to commence and complete the clinical studies as well as obtaining approval to commence additional studies for other specific indications it has identified that the use of its products will provide more favorable and desired health related benefits for patients seeking alternative treatment options than are currently available.
COVID-19 Impact To Economy And Business Environment
The current outbreak of the novel coronavirus (“COVID-19”) and resulting impact to the United States economic environments began to take hold during March 2020. The adverse public health developments and economic effects of the COVID-19 outbreak in the United States, have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. These restrictions have adversely affected the Company’s sales, results of operations and financial condition. In response to the COVID-19 outbreak, the Company (a) has accelerated its research and development activities, particularly in regards to potential health benefits of the Company’s products in addressing various health concerns associated with COVID-19 and (b) is aggressively seeking to raise additional debt and/or equity financing to support working capital requirements until sale for its products to providers resumes to levels pre COVID-19.
There is no assurance as to when the adverse impact to the United States and worldwide economies resulting from the COVID-19 outbreak will be eliminated, if at all, and whether any new or recurring pandemic outbreaks will occur again in the future causing similar or worse devastating impact to the United States and worldwide economies and to our business.
| 2 |
Developments During Fiscal 2018 and 2019:
After the completion of the Sale of our laboratory facilities and all related assets, including intellectual property rights, to Vera in February 2018, the Company remained in the business of selling and distributing regenerative biologic therapies based on amnion placental tissue derived products to doctors and hospitals but was required to depend on third party supply agreements, rather than from products manufactured internally by ANU, for the supply of these advanced biologically processed cellular and tissue based products.
Since the Sale was completed, including the departure of several key executives in connection therewith, the Company had difficulty in generating sufficient revenues and, as a result, continued to have a lack of working capital to meet current operating costs, hiring of additional sales personnel, pay past due accounts payable obligations to its vendors, pay past due and/or current salaries to its remaining management or fund potential growth opportunities.
On April 23, 2018, the Company and Management and Business Associates, LLC, a Florida limited liability company (“MBA”), executed a Plan and Agreement of Reorganization (“Reorganization”) whereby the Company agreed to issue to MBA an aggregate of 222,425,073 shares of its common stock of the Company, representing at the time 51% of the outstanding shares of common stock of the Company on fully-diluted basis, for $0.001 per share (or an aggregate of $222,425), in consideration for Mr. Manuel Iglesias’ agreement to serve as the Company’s Chief Executive Officer and a member of the Board of the Company. Manuel E. Iglesias is the sole Manager of MBA and thus may be deemed to control MBA. The Reorganization was effective as of April 13, 2018 (“Effective Date”).
On May 21, 2018, the Company filed a Certificate of Amendment with the Secretary of State of Nevada to change the Company’s name from Biotech Products Services and Research, Inc. to Organicell Regenerative Medicine, Inc., effective June 20, 2018 in order to express more clearly the Company’s focus in the stem cell business (the “Name Change”). However, due to the Company’s failing to have the required Exchange Act reports filed with the SEC at the time of the filing, FINRA did not announce or effectuate the Name Change in the marketplace. If the Company intends to proceed with the Name Change, the Company will be required to submit a new Issuer Company-Related Notification Form for approval upon the Company becoming current in its Exchange Act filings.
On June 14, 2018, the Company filed a Certificate of Withdrawal with the Secretary of State of Nevada thereby withdrawing and terminating all previously issued designations of the Company’s Series A Preferred Stock and Series B Preferred Stock. On June 1, 2018, the Company submitted an Issuer Company-Related Notification Form (“June 1 Notification Form”) with the Financial Industry Regulatory Agency (“FINRA”) pursuant to Rule 10b-17 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), regarding the Name Change and Reverse Split.
During February 2019, the Company began arranging to once again operate a new laboratory facility in Miami, Florida for the purpose of performing research and development, production and manufacturing of anti-aging and cellular therapy products. This new laboratory facility became operational in May 2019 and during the same period, the Company began producing and distributing the products that are being sold and distributed to its customers. The Company believes that this strategy will provide the Company with competitive advantages and greater assurances that it can continue to comply with expected future FDA regulations.
On September 24, 2019, due to the Company’s limited success since the Reorganization in stabilizing revenues and the growing urgency for the Company to remain compliant and meet the anticipated new and more stringent regulatory deadlines to be imposed by the FDA in connection with the Company’s products and operations that were previously announced to go into effect in May 2021, the Board determined that it would require the services of a full-time CEO with the requisite expertise and experience to lead the Company as it (a) moves forward with its strategy to expand its research and development efforts and submit IND applications for FDA approval to commence clinical trials for its products to assure that the Company, its operations and its products remain compliant with FDA regulations and (b) implements additional strategies to minimize the potential impact in the future on sales of its products as a result of future changes in FDA regulations and/or restrictions associated with clinical trials that are utilizing the products that are currently being sold by the Company. Accordingly, the Board voted to remove Manuel Iglesias from his position as CEO of the Company. The Board has since appointed Albert Mitrani to serve as the Company’s CEO.
| 3 |
In connection with the Company’s ongoing research and development efforts and the Company’s efforts to meet compliance with current and anticipated United States Food and Drug Administration (“FDA”) regulations expected to be enforced beginning in May 2021 requiring that the sale of products that fall under Section 351 of the Public Health Services Act pertaining to marketing traditional biologics and human cells, tissues and cellular and tissue based products (“HCT/Ps”) can only be sold pursuant to an approved biologics license application (“BLA”), the Company has obtained certain Investigation New Drug (“IND”), emergency IND (“eIND”) and/or non-emergency IND (“non-eIND”) approvals from the FDA, including applicable Institutional Review Board (“IRB”) approvals which authorized the Company to commence clinical trials or treatments in connection with the use of the Company’s products and related treatment protocols. The status of the Company’s current IND’s, eIND’s, non-eIND’s submitted and approved for past or planned treatments and/or clinical trials are described below:
Company’s FDA approved phase I/II IND, eIND’s and non-eIND’s:
| 1. | IND # 19881 approved on 04/30/2020 - A Phase I/II Randomized, Double Blinded, Placebo Trial to Evaluate the Safety and Potential Efficacy of Intravenous Infusion of OrganicellTM Flow for the Treatment of Moderate to Severe Acute Respiratory Syndrome (SARS) Related to COVID-19 Infection vs Placebo. IRB was approved by the Institute of Regenerative and Cellular Medicine (“IRCM”) on 06/04/2020 (approval number: IRCM-2020-254). Clinical trial is currently in process. | |
| 2. | eIND#22370 approved on 05/11/2020 - Treatment for Acute hypoxic respiratory failure with ARDS secondary to COVID-19 infection for single patient. | |
| 3. | eIND#22371 approved on 05/11/2020 - Treatment for Acute hypoxic respiratory secondary to bilateral pneumonia secondary to COVID-19 with ARDS for single patient. | |
| 4. | eIND#22897 approved on 05/29/2020 – Treatment for Acute respiratory failure with hypoxia, secondary to COVID-19 with ARDS for single patient. | |
| 5. | eIND#25426 approved on 07/24/2020 - Treatment of COVID-19 positive for single patient. | |
| 6. | eIND#25888 approved on 8/01/2020 - Treatment of post COVID-19 complication for single patient | |
| 7. | eIND#26560 approved on 8/17/2020 - Treatment of post-COVID-19 complications for single patient. | |
| 8. | eIND#26561 approved on 8/17/2020 - Treatment of post-COVID-19 complications for single patient. | |
| 9. | eIND#26676 approved on 8/20/2020 - Treatment of respiratory failure due to COVID-19 infection for single patient. | |
| 10. | eIND#26700 approved on 8/21/2020 - Treatment for ARDS associated with COVID-19 for single patient. | |
| 11. | eIND#26776 approved on 8/25/2020 - Treatment of COVID-19 positive for single patient. | |
| 12. | eIND#26777 approved on 8/25/2020 - Treatment of COVID-19 positive for single patient. | |
| 13. | eIND#26864 approved on 9/05/2020 - Treatment of COVID-19 positive for single patient. | |
| 14. | Non-eIND#26821 approved on 9/22/2020 - Treatment of post COVID-19 complications for single patient. | |
| 15. | Expanded Access to ZofinTM (OrganicellTM Flow) approved on 09/24/2020 - Treatment of Patients with COVID-19 Outpatient and Inpatient Population. IRB pending. | |
| 16. | A Phase II Multicenter, Randomized, Double Blinded, Placebo Trial to Evaluate the Efficacy and Safety of Intramuscular Injections of ZofinTM Comparing with Intravenous Infusions for the Treatment of Post COVID-19 Complications and Severe Sequelae vs Placebo. IND submitted on 09/28/2020. Pending IND and IRB approval. |
The Company is aggressively pursuing efforts to commence and complete the above described clinical studies as well as obtaining approval to commence additional studies for other specific indications it has identified that the use of its products will provide more favorable and desired health related benefits for patients seeking alternative treatment options than are currently available. The ability of the Company to succeed in these efforts is subject to among other things, the Company having sufficient available working capital to fund the substantial costs of completing clinical trials, which the Company currently does not have, and ultimately the approval from the FDA.
| 4 |
Industry Overview
Health Care Industry Overview:
The traditional health care industry in the United States is predominantly controlled by the rules of the Centers for Medicare & Medicaid Services (“CMS”) (wwws.cms.gov) and commercial health insurance companies. This control limits patients’ access to alternative medical therapies, that recent medical literature demonstrates highly beneficial outcomes in the field of anti-aging and regenerative medicine. Traditional allopathic medicine of health care provided to patients in the United States relies on government and commercial health insurance for payment of the costs associated with their day-to-day health care. Because of this close relationship, physicians must follow government and commercial insurers guidelines in order to stay in the plans and receive reimbursement. Physicians are restricted in their ability to expand the nature of the treatments provided beyond industry practices because of legal ramifications and/or lack of knowledge concerning protocol of cutting-edge anti-aging and regenerative medical treatments.
Despite the above, anecdotal and medical literature has shown an increased demand by patients for access to alternative medical therapies and treatments. Patients are seeking these alternatives to traditional allopathic medicine, due to the adverse events associated with traditional pharmaceuticals, risks associated with surgeries, and that traditional medicine and insurers are not addressing wellness or preventive medicine sufficiently. To address a wide variety of aging issues, safe alternatives to pathologies, including access to other treatments and pharmaceuticals and to achieve beneficial “elective” health treatments, we intend utilize the latest regenerative technologies. These alternative pathways to date have had significant restrictions because of regulations imposed by the FDA, other regulatory bodies and insurers due to lack of randomized controlled studies, yet many published case series demonstrate safety and efficacy. Patients and consumers are looking to safe alternatives compared more traditional medicine, including the following:
| · | Cellular/ Tissue based therapies |
| o | Adipose-derived stromal vascular fraction |
| o | Bone marrow-derived stem cell therapies |
| o | Peripheral blood derived therapies (i.e., platelet rich plasma); |
| o | Placental-based therapies |
| Ø | Technology documented since 1910 for safety and efficacy, tissue processed from human amniotic membrane and fluid, donated by consenting mothers delivering a full-term healthy baby by scheduled Caesarean section, avoiding any ethical or moral concerns, proven safety record, case series documented success in a multitude of systemic and local pathologies |
| o | Growth factor, cytokine therapies |
| · | Anti-Aging |
| o | Supplements |
| Ø | Vitamin |
| Ø | Mineral |
| Ø | Medical foods |
| o | Weight control |
| o | Topical lotions and creams for the largest organ the skin |
| · | Nontraditional medical alternatives |
| o | Acupuncture |
| o | Naturopathic |
| o | Chiropractic |
| · | Self-directed |
| o | Meditation |
| o | Yoga |
| o | Tai Chi |
| 5 |
Currently, patients who desire alternative treatments rely on the following options:
| n | Medical Tourism |
| o | In United States |
| o | Off-shore United States |
| Ø | Central and South America |
| Ø | Caribbean |
| Ø | Europe |
| n | Consulting directly with physicians knowledgeable in providing regenerative medical services |
| n | Unlicensed life coaches |
Business Strategy
Current Business Strategy:
Our current business strategy is to achieve the following goals and milestones:
Develop and expand operations to provide for growth of our revenues for the sales and distribution of RAAM related products;
| o | Increase revenues for RAAM related products; |
| · | Hiring of additional in-house sales personnel |
| · | Selectively engaging independent distributors |
| · | Marketing private label products to distributors |
| · | Increasing market recognition for our Organicell brand from: |
| Ø | marketing and participating in industry trade shows |
| o | Expand our sales market outside of the United States |
| o | Increase the number of RAAM product offerings for various modalities using proprietary processing, formulas and administration techniques |
| o | Extending our referral network of Providers based on: |
| · | Superior product offerings |
| · | Demonstrating a realistic and executable regulatory roadmap to assure Company and product compliance with current and anticipated FDA regulations |
| · | Developing and providing educational support to Providers regarding our products and regulatory concerns |
| 6 |
Execute on current strategy to assure the Company’s ability to maintain compliance with existing and the anticipated changes to FDA regulations regarding the use and sale of our current products published in November 2017 and expected to take effect by May 2021, as well as readiness to respond to ongoing future changes to those regulations:
| o | Perform clinical based studies associated with the use of our products (independently and/or in conjunction with Providers and/or Manufacturers) and seek accelerated approval for each product application in accordance with the 21st Century Cures Act (“Cures Act”) and/or through the granting of an FDA-approved biologics application (BLA) to allow products to be lawfully marketed and/or sold in the United States in accordance with newly established FDA guidelines outlined in November 2017 expected to take effect by May 2021; and | |
| o | Continue to build out our lab facilities to meet expected production and research requirements; and | |
| o | Engage high profile and industry recognized medical advisors and scientists to help identify new and emerging technologies concerning biologics and to assure our Products remain cutting edge and competitive to products offered by other companies; and | |
| o | Identify alternative products and services to (a) offset any potential decline in revenues resulting from FDA limitations on the sales and distribution of our existing products currently being sold and distributed as a result of our commencement of clinical trials using such products and/or future expected FDA restrictions on RAAM products and (b) provide our Providers with alternative product and treatment options to remain competitive with the market and our Providers to meet the needs and demands of their patients; and | |
| o | Expand our sales market and network of Providers outside of the United States | |
| o | Identify sources of exclusive and superior suppliers of RAAM products; and | |
| o | Identify strategic relationships to acquire existing Providers and/or suppliers or owners of IP associated with additional desired RAAM products; and |
| o | Engage new researchers that bring additional expertise and capacity to develop ongoing research and development and growth opportunities for additional RAAM-related products. |
Secure additional working capital;
| o | Fund shortfalls in working capital to fund ongoing expenses and required payments to vendors and creditors until revenues are stabilized; and | |
| o | Fund ongoing costs to pursue clinical trials; and | |
| o | Fund capital expenditures associated with maintaining compliance of our facilities and products; and | |
| o | Fund our strategy to develop and expand our revenues for the sales and distribution of RAAM related products described above; and | |
| o | Hire additional personnel to support our growth and planned expansion; and | |
| o | Enhance our CRM, e-commerce and ERP capabilities to facilitate marketing, sales and distribution functionality and accounting for our operations. |
Enhance Company Corporate Governance;
| o | Revisit previously announced plans to complete a reverse split, and a reduction in the authorized shares outstanding. The Company believes a reverse split will bring value to the issued and outstanding shares of the Company by limiting dilution of operating results by an excessive number of shares overhanging the market; | |
| o | Appoint additional independent members to the Board of Directors that will provide overall industry expertise and fulfill audit committee and independent director requirements to meet listing requirements for the national stock exchanges; and | |
| o | Continue to develop and expand the Company’s internal control policies |
Potential Effects of COVID-19 Pandemic
The current outbreak of the novel coronavirus (“COVID-19”) and resulting impact to the United States economic environments began to take hold during March 2020. The adverse public health developments and economic effects of the COVID-19 outbreak in the United States, have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. These restrictions have adversely affected the Company’s sales, results of operations and financial condition. In response to the COVID-19 outbreak, the Company (a) has accelerated its research and development activities, particularly in regards to potential health benefits of the Company’s products in addressing various health concerns associated with COVID-19 and (b) is aggressively seeking to raise additional debt and/or equity financing to support working capital requirements until sale for its products to providers resumes to levels pre COVID-19.
| 7 |
There is no assurance as to when the adverse impact to the United States and worldwide economies resulting from the COVID-19 outbreak will be eliminated, if at all, and whether any new or recurring pandemic outbreaks will occur again in the future causing similar or worse devastating impact to the United States and worldwide economies.
Market Overview
The population of the United States and the developed world is getting older and living longer. According to a United States Consensus Bureau’s report, “An Aging World: 2015,” America’s 65-and-over population is projected to nearly double over the next three decades, ballooning from 48 million to 88 million by 2050 and that worldwide, the 65-and-over population will more than double to 1.6 billion by 2050. According to the report, in 2015, 14.9% of the U.S. population was 65 or over and the United States was the 48th oldest country out of 228 countries and areas in the world in 2015. Baby boomers began reaching age 65 in 2011 and by 2050 the older share of the U.S. population will increase to 22.1%.
The world average age of death has increased by 35 years since 1970, with declines in death rates in all age groups, including those aged 60 and older (Source: Institute for Health Metrics and Evaluation, 2013; Mathers et al., 2015). The leading causes of death are shifting, in part because of increasing longevity. Between 1990 and 2013, the number of deaths from non-communicable diseases (“NCDs”) has increased by 42%; and the largest increases in the proportion of global deaths took place among the population aged 80 and over. An estimated 42.8% of deaths worldwide occur in the population aged 70 and over, with 22.9% in the population aged 80 and over.
Also, according to the Center for Disease Control (“CDC”), “Medical Tourism” (a term commonly used to describe people traveling outside their home country for medical treatment) is a worldwide, multibillion-dollar phenomenon that is expected to grow substantially in the next 5–10 years. Studies have estimated that hundreds of thousands of medical tourists travel from the United States annually and that patients pursue medical care abroad for a variety of reasons, including a desire to receive a procedure or therapy not available in their country of residence. Common categories of procedures that US travelers pursue during medical tourism trips include orthopedic surgery, cosmetic surgery, cardiology (cardiac surgery), oncologic care, and dentistry. Common destinations include Thailand, Mexico, Singapore, India, Malaysia, Cuba, Brazil, Argentina, and Costa Rica.
If we are able to implement our intended business plan, we believe that we will be well situated to address this increased consumer demand for alternative medical treatments.
Marketing and Sales
Currently, we market our RAAM products and services to a network of Providers through in-house, contracted sales personnel and/or from independent distributors. As of October 31, 2019, we had two salespeople who marketed our RAAM products and services by using social media outlets, medical conferences and seminars and from development of prior and newly identified Providers and related professional relationships. In addition, we had arrangements with several independent distributors that were marketing and distributing our products. We intend in the future to expand our in-house sales force and independent distributors as our working capital improves, our product line expands and as volumes increase. We also intend to develop and offer ongoing training seminars to provide the best possible information on the latest advances on anti-aging, and regenerative medicine to Providers.
Sources and Availability of Raw Materials and the Names of Principal Suppliers
From the completion of the Sale in February 2018 through April 2019, we purchased all of our RAAM Products through supply arrangements directly with third-party manufacturers or indirectly from distributors of other third-party manufacturers.
Beginning May 2019, we once again began to manufacture our own RAAM Products in our newly developed Miami, Florida laboratory facilities and acquired the required raw materials and supplies for our RAAM research and development and the manufacturing of our RAAM placental-related products from unaffiliated third-party laboratories pursuant Supply Arrangements.
| 8 |
In the event any one or more of our current suppliers are unwilling or unable to sell us required raw materials and/or products, for any reason, we may not be able to provide replacement products to our customers, or if other supply arrangements can be made, the replacement products and terms may not be as favorable.
Dependence on One or a Few Major Customers
During the year ended October 31, 2019, one customer accounted for approximately 12.2% of our revenues. Our RAAM business is not expected to be dependent on any one or more customers, especially as our customer and distribution network expands. We expect that our customer and consumers will be broad based and throughout the United States and worldwide.
Patents, Trademarks, Licenses, Franchises, Concessions, Royalty Agreements or Labor Contracts
The table below sets forth a summary of our intellectual property rights.
| Patents: | None |
| Patent Applications: |
OrganicellTM has a U.S. Provisional Patent Application on file for its OrganicellTM line of products and the proprietary techniques used in during processing perinatal fluid. U.S. Provisional Patent Application No. 63/008,355 Titled: COMPOSITIONS COMPRISING NANOPARTICLES, METHOD OF MAKING AND USES THEREOF Filed: April 10, 2020 Inventor: Maria Ines Mitrani Applicant: Organicell Regenerative Medicine, Inc. Conversion Filing Deadline: April 10, 2021 Assignment: MARIA INES MITRANI (Assignor), ORGANICELL REGENERATIVE MEDICINE, INC. (Assignee) Recorded: April 15, 2020 Real/Frame: 052403 / 0365 |
| Trademarks: |
Word Mark: ZOFIN Goods/Services: Biologically derived products developed from perinatal tissue material for medical, regenerative and aesthetic purposes Serial Number: 90050511 Filing Date: July 13, 2020 Owner: Organicell Regenerative Medicine, Inc. Status: Pending, awaiting examination
Word Mark: Organicell Goods/Services: Biologically derived products developed from perinatal tissue material for medical, regenerative and aesthetic purposes Serial Number: 88903989 Filing Date: May 6, 2020 Owner: Organicell Regenerative Medicine, Inc. Status: Office Action issued August 8, 2020
|
| 9 |
Word Mark: Organicell Goods/Services: Non-medicated anti-aging serum; non-medicated skin serums; all of the aforementioned goods are made in whole or in substantial part of organic ingredients Serial Number: 87311045 Filing Date: January 23, 2017 Owner: Organicell Regenerative Medicine, Inc. Registration Number: 5289671 Registration Date: September 19, 2017 Status: Live
Word Mark: PATIENT PURE X - PPX Goods/Services: plasma extracts for medical use, namely, plasma extract containing purified and concentrated exosomes derived from whole human blood Serial Number: 88771931 Filing Date: January 24, 2020 Owner: Organicell Regenerative Medicine, Inc. Status: Notice of Allowance issued July 28, 2020
Word Mark: PATIENT PURE X - PPX Goods/Services: plasma processing services for others, namely, extracting purified and concentrated exosomes based on whole blood harvested from patients for use by hospitals, clinics, or other organizations or persons involved in delivering healthcare services to patients Serial Number: 88771934 Filing Date: January 24, 2020 Owner: Organicell Regenerative Medicine, Inc. Status: Notice of Allowance issued August 18, 2020 | |
| Registered Copyrights: | None |
| Domain Names: | www.organicell.com |
| IP Licenses: |
None |
| 10 |
The status of the Company’s current IND’s, eIND’s, non-eIND’s submitted and approved for past or planned treatments and/or clinical trials are described below:
Company’s FDA approved phase I/II IND, eIND’s and non-eIND’s:
| 1. | IND # 19881 approved on 04/30/2020 - A Phase I/II Randomized, Double Blinded, Placebo Trial to Evaluate the Safety and Potential Efficacy of Intravenous Infusion of OrganicellTM Flow for the Treatment of Moderate to Severe Acute Respiratory Syndrome (SARS) Related to COVID-19 Infection vs Placebo. IRB was approved by the Institute of Regenerative and Cellular Medicine (“IRCM”) on 06/04/2020 (approval number: IRCM-2020-254). Clinical trial is currently in process. | |
| 2. | eIND#22370 approved on 05/11/2020 - Treatment for Acute hypoxic respiratory failure with ARDS secondary to COVID-19 infection for single patient. | |
| 3. | eIND#22371 approved on 05/11/2020 - Treatment for Acute hypoxic respiratory secondary to bilateral pneumonia secondary to COVID-19 with ARDS for single patient. | |
| 4. | eIND#22897 approved on 05/29/2020 – Treatment for Acute respiratory failure with hypoxia, secondary to COVID-19 with ARDS for single patient. | |
| 5. | eIND#25426 approved on 07/24/2020 - Treatment of COVID-19 positive for single patient. | |
| 6. | eIND#25888 approved on 8/01/2020 - Treatment of post COVID-19 complication for single patient. | |
| 7. | eIND#26560 approved on 8/17/2020 - Treatment of post-COVID-19 complications for single patient. | |
| 8. | eIND#26561 approved on 8/17/2020 - Treatment of post-COVID-19 complications for single patient. | |
| 9. | eIND#26676 approved on 8/20/2020 - Treatment of respiratory failure due to COVID-19 infection for single patient. | |
| 10. | eIND#26700 approved on 8/21/2020 - Treatment for ARDS associated with COVID-19 for single patient. | |
| 11. | eIND#26776 approved on 8/25/2020 - Treatment of COVID-19 positive for single patient. | |
| 12. | eIND#26777 approved on 8/25/2020 - Treatment of COVID-19 positive for single patient. | |
| 13. | eIND#26864 approved on 9/05/2020 - Treatment of COVID-19 positive for single patient. | |
| 14. | Non-eIND#26821 approved on 9/22/2020 - Treatment of post COVID-19 complications for single patient. | |
| 15. | Expanded Access to ZofinTM (OrganicellTM Flow) approved on 09/24/2020 - Treatment of Patients with COVID-19 Outpatient and Inpatient Population. IRB pending. | |
| 16. | A Phase II Multicenter, Randomized, Double Blinded, Placebo Trial to Evaluate the Efficacy and Safety of Intramuscular Injections of ZofinTM Comparing with Intravenous Infusions for the Treatment of Post COVID-19 Complications and Severe Sequelae vs Placebo. IND submitted on 09/28/2020. Pending IND and IRB approval. |
Pursuant to our employment agreements with our executives, all work product that is created, prepared, produced, authored, edited, amended, conceived or reduced to practice by each executive individually or jointly with others during the period of their employment by the Company and relating in any way to the business or contemplated business, research or development of the Company (regardless of when or where the Work Product is prepared or whose equipment or other resources is used in preparing the same), as well as any and all rights in and to copyrights, trade secrets, trademarks (and related goodwill), patents and other intellectual property rights therein arising in any jurisdiction throughout the world and all related rights of priority under international conventions with respect thereto, including all pending and future applications and registrations thereof, and continuations, divisions, continuations-in-part, reissues, extensions and renewals thereof (collectively, "Intellectual Property Rights"), the sole and exclusive property of the Company. All of the Work Product consisting of copyrightable subject matter shall be deemed "work made for hire" as defined in 17 U.S.C. § 101 and such copyrights are therefore owned by the Company or if not applicable, deemed to be irrevocably assigned to the Company, for no additional consideration. The Intellectual Property Rights in any “Pre-existing Materials” included contained in the Work Product shall be retained by the executive but the executive shall be deemed to have granted to the Company an irrevocable, worldwide, unlimited, royalty-free license to use, publish, reproduce, display, distribute copies of, and prepare derivative works based upon, such Pre-Existing Materials and derivative works thereof. The Company may not assign, transfer and sublicense such rights to others without executive’s consent, other than to a wholly-owned subsidiary of the Company. The executive shall provide written notice to the Company’s Chief Executive Officer therein notifying the Company new intellectual property including the Pre-Existing Materials.
Competition
The regenerative medicine field is highly competitive and subject to rapid technological change and regulation. Companies compete on the basis of product efficacy, pricing, and ease of handling/logistics. A critically important factor for growth in the US market is third-party reimbursement, which is difficult to obtain, and the process can be time-consuming and expensive. We expect that it will take some time before RAAM products will be widely accepted under health insurance coverage. In addition, growth of this industry is expected to expand as additional research and development into the benefits of regenerative products and specific products becomes more widely accepted as a result of FDA mandated or optional clinical trials are performed by industry stakeholders.
| 11 |
As stated previously, there is a growing urgency in the industry for companies to meet the anticipated new and more stringent regulatory deadlines to be imposed by the FDA in connection with regulation of RAAM products that were previously announced to go into effect in May 2021. As a result of these concerns, the Company and our competitors are expected to need to pursue research and development efforts and submit IND applications for FDA approval to commence clinical trials for RAAM products being sold to assure that their respective operations and products remain compliant with FDA regulations and there is no adverse impact to future operations. In addition, the Company believes that the ability to demonstrate that products and operations comply with regulations are important factors for companies in the industry to be successful in the future.
We intend to perform clinical trials for our RAAM Products for the purpose of obtaining biologics license status from the FDA to provide us with advantages over our competitors, including acceleration for acceptance of our products in traditional insurance plans, compliance with FDA regulations and to provide our customers with superior education and support of the benefits of our products. Initially we are positioning ourselves as a cash-based health care alternative for consumers that can provide higher levels of improvement, that is not available from traditional allopathic medicine at this time.
The Company competes in multiple areas of clinical treatment where regenerative biomaterials may be employed to modulate inflammation, enhance healing and reduce scar tissue formation: advanced wound care treatment, spine, orthopedic, surgery and sports medicine.
The primary competitive products in this space include autologous serums derived from blood, bone marrow, and adipose tissue (Regenexx) and allograft products derived from amniotic fluid or amniotic membrane, umbilical cord blood or umbilical cord tissue matrix, or from culture-expanded perinatal cells. Our competitors are primarily producer-distributor companies which include Predictive Biotech, Kimera Labs, MiMedix Group, Inc., Invitrx Therapeutics, Liveyon, BioD (“dermaSciences”), and Direct Biologics, as well as a number of distributors who sell white-labeled products from those producer-distributor entities. Additionally, there are a variety of accredited blood, bone, and soft tissue banks that we will be competing against, including Utah Cord Bank and Cord for Life.
As stated previously, the demand for RAAM products is very high and expected to grow with the growing baby boomer generation getting older, the increase in patients desiring to seek health care options outside of traditional therapies, the growing trend in the desire of individuals to remain active longer in life and the ongoing rise in health care costs which RAAM products may provide a more efficient and economical alternative for certain conditions.
Government Regulation
General
The Company’s operations are subject to FDA regulations in connection with the sales and distribution of its RAAM products. In addition, the Company relies on supply agreements with birth tissue recovery companies, supply manufacturers and/or third party distributors for the supply of RAAM products and/or the Company’s intended objectives to conduct research and development and clinical trials of RAAM products, all of whom are required to comply with FDA regulations. We anticipate these regulations will be heavily enforced and subject to more restrictive regulations by the FDA in the future. A summary of the current FDA regulations is set forth below:
FDA Premarket Clearance and Approval Requirements
Tissue Products
Currently the products that are sold by the Company are derived from human tissue that is purchased by the Company and processed directly in the Company’s laboratory facilities. At times when the Company did not manufacture its own products, the products sold were manufactured and processed by third party manufacturers. As discussed below, some tissue-based products are regulated solely under Section 361 of the Public Health Service Act as human cells, tissues and cellular and tissue-based products, or HCT/Ps, which do not require premarket clearance or approval by the FDA. Other tissue products are regulated as biologics and, in order to be lawfully marketed in the United States, require an FDA-approved BLA.
| 12 |
The FDA is continually changing and formulating new guidelines for this industry. In addition, the FDA has published some additional draft guidelines related to this industry and the ultimate form of the regulations are not yet known.
Products Regulated as HCT/Ps
The FDA has specific regulations governing human cells, tissues and cellular and tissue-based products, or HCT/Ps. An HCT/P is a product containing or consisting of human cells or tissue intended for transplantation into a human patient. HCT/Ps that meet the criteria for regulation solely under Section 361 of the Public Health Service Act (so-called “361 HCT/Ps”) are not subject to approval requirements and they are subject to post-market regulatory requirements.
To be a 361 HCT/P, a product generally should meet following criteria:
| · | Be minimally manipulated, no structural change, or be mixed with anything; |
| · | Be intended for homologous use, essentially used for the same purpose that it was used in the donor; |
| · | Its manufacture must not involve combination with another article, except for water, crystalloids or a sterilizing, preserving or storage agent; and |
| · | It must not be dependent upon the metabolic activity of living cells for its primary function. |
Products Regulated as Biologics- The BLA Pathway
The typical steps for obtaining FDA approval of a BLA to market a biologic product in the U.S. include:
| · | Completion of preclinical laboratory tests, animal studies and formulations studies under the FDA’s good laboratory practices regulations; |
| · | Submission to the FDA of an Investigational New Drug Application (“IND”) for human clinical testing, which must become effective before human clinical trials may begin and which must include independent Institutional Review Board (“IRB”) approval at each clinical site before the trials may be initiated; |
| · | Performance of adequate and well-controlled clinical trials in accordance with Good Clinical Practices to establish the safety and efficacy of the product for each indication; |
| · | Submission to the FDA of a Biologics License Application for marketing the product, which includes, among other things, reports of the outcomes and full data sets of the clinical trials, and proposed labeling and packaging for the product; |
| · | Satisfactory completion of an FDA Advisory Committee review; and |
| · | Satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with Current Good Manufacturing Practices (“cGMP”) regulations. |
| 13 |
Generally, clinical trials are conducted in three phases:
| · | Phase I trials typically involve a small number of healthy volunteers and are designed to provide information about the product safety. |
| · | Phase II trials are conducted in a larger but limited group of patients afflicted with a specific diagnosis in order to determine preliminary efficacy, and to identify possible adverse effects. |
| o | Dosage studies are designated as Phase IIA and efficacy studies are designated as Phase IIB. |
| · | Phase III clinical trials are generally large-scale, multi-center, comparative trials conducted with patients who have a specific condition in order to provide statistically valid proof of efficacy, as well as safety and potency. |
| · | In some cases, the FDA will require Phase IV, or post-marketing trials, to collect additional data after a product is on the market. |
The process of obtaining an approved BLA requires the expenditure of substantial time, effort and financial resources and may take years to complete.
FDA Post-Market Regulation
Tissue processors are required to register as an establishment with the FDA. We intend on becoming a registered establishment, accredited by the American Association of Tissue Banks (“AATB”) for the storage and distribution of tissue products that we purchase directly or indirectly from third party manufacturers. Once we are registered, we will be required to comply with regulations, including those regulations regarding storage, controls, access, labeling, record keeping, security, processes, compliance with established Good Tissue Practices, and documentation associated with the sale of our products by our customers to their patients. Our facilities will be subject to periodic inspections to assess our records and determination of our compliance with the regulations.
Products covered by a BLA, 510(k) clearance, or a PMA are subject to numerous additional regulatory requirements, which include, among others, compliance with cGMP, which imposes certain procedural, substantive and record keeping requirements, labeling regulations, the FDA’s general prohibition against promoting products for unapproved or “off-label” uses, and additional adverse event reporting.
Other Regulation Specific to Tissue Products
The AATB, has issued operating standards for tissue banking, whether manufacturing and/or storing products as a distributor of manufactured products by third parties. Compliance with these standards is a requirement in order to become a licensed tissue bank.
21st Century Cures Act
In December 2016, President Obama signed the 21st Century Cures Act (the “Act”) into law. The Act includes many provisions that aim to speed up the process of bringing new drugs and devices to market. One of the Act’s most significant amendments to the Federal Food, Drug and Cosmetic Act will allow the FDA to grant accelerated approval to regenerative medicine products, while also providing the agency with wide discretion on creating new approaches to regenerative medicine. This legislative development is the result of increased pressure from patients and other stakeholders to move regenerative medicine advancements more quickly from the lab into the clinic.
| 14 |
Specifically, the new accelerated approval pathway authorized by the Act allows certain regenerative medicine products to be designated as “regenerative advanced therapy” and become eligible for priority review by FDA. To qualify for this pathway, the product must be aimed at a serious disease and have the potential to deal with currently unmet medical needs. It must also meet the Act’s new definition of a regenerative advanced therapy, which is defined as “cell therapy, therapeutic tissue engineering products, human cell and tissue products, and combination products using any such therapies or products, except for those regulated solely under section 361 of the Public Health Service Act.” This broad definition would seem to encompass the majority of regenerative medicine products known to be currently in the development stages.
As with the existing accelerated approval pathway for drugs and biologics, this new regulatory pathway would allow a regenerative medicine product to be approved for marketing based on surrogate or intermediate clinical trial endpoints rather than longer term clinical outcomes. The use of such endpoints can decrease the number, duration, and complexity of clinical trials that are needed to prove a longer-term outcome. Subsequently, a sponsor would have to conduct confirmatory clinical trials to ensure that the surrogate or intermediate endpoint was in fact predictive of patients’ clinical response to the product, otherwise the accelerated approval could be withdrawn.
The Act also requires the FDA to work with the National Institute of Standards and Technology (“NIST”) and other stakeholders to develop standards and consensus definitions for regenerative medicine products. Such standards are expected to play a large role in advancing this nascent industry by allowing companies to rely on FDA-recognized standards, rather than creating and validating their own as is the case today.
The Act attempts to create a research network and a public-private partnership to assist developers in generating definitive evidence about whether their proposed therapies indeed provide clinical benefits that are hoped for. The Act also requires the FDA to track and report the number and type of applications filed for regenerative medicine products, including the number of products approved through the new accelerated approval pathway. The law also includes provisions that require the FDA to publish guidance on how it will design and implement an approval process for regenerative medicine devices.
November 2017 FDA Guidelines
In November 2017, the FDA released four guidance documents (two final, two draft) in an effort to implement a “comprehensive policy framework” for existing laws and regulations governing regenerative medicine products, including human cells, tissues, and cellular and tissue-based products (“HCT/Ps”). These guidance documents build upon the previous regulatory framework for these products, which was completed in 2005. A guidance document cannot alter a regulation, but can clarify how the FDA intends to enforce the regulation. The Comprehensive regenerative medicine policy framework intends to spur innovation, efficient access to potentially transformative products, while ensuring safety & efficacy.
The framework builds upon the FDA’s existing risk-based regulatory approach to more clearly describe what products are regulated as drugs, devices, and/or biological products. Further, two of the guidance documents propose an efficient, science-based process for helping to ensure the safety and effectiveness of these therapies, while supporting development in this area. The suite of guidance documents also defines a risk-based framework for how the FDA intends to focus its enforcement actions against those products that raise potential significant safety concerns. This modern framework is intended to balance the agency’s commitment to safety with mechanisms to drive further advances in regenerative medicine so innovators can bring new, effective therapies to patients as quickly and safely as possible. The policy also delivers on important provisions of the Act.
Final Guidance Documents
The two final guidance documents clarify the FDA’s interpretation of the risk-based criteria manufacturers use to determine whether a product is subject to the FDA’s premarket review.
The first guidance provides greater clarity around when cell and tissue-based products would be exempted from the established regulations if they are removed from and implanted into the same individual within the same surgical procedure and remain in their original form. The second final guidance helps stakeholders better understand how existing regulatory criteria apply to their products by clarifying how the agency interprets the existing regulatory definitions “minimal manipulation” and “homologous use.” As this field advances, the FDA has noted that there are a growing number of regenerative medicine products subject to FDA premarket authorization. These guidance documents will help explain how the FDA will provide a risk-based framework for its oversight. The policy framework defines how the FDA intends to take action against unsafe products while facilitating continued innovation of promising technologies.
| 15 |
To accomplish this goal, the guidance document has clarified the FDA’s view of “minimal manipulation” and “homologous use.” These are two concepts that are defined in current regulation to establish the legal threshold for when a product is subject to the FDA’s premarket approval requirements. By further clarifying these terms in the final guidance, the FDA is applying a modern framework for its oversight.
FDA regulations at 21 C.F.R. Part 1271, previous draft guidance documents, and untitled letters establish the agency’s approach to regulating HCT/Ps. Some HCT/Ps are exempt from premarket approval and are subject to regulation solely under section 361 of the Public Health Service Act (“PHS Act”) (so-called “361 HCT/Ps”) whereas others require premarket approval (i.e., as a drug, device, or biologic) (so-called “351 HCT/Ps”). Both 361 HCT/Ps and 351 HCT/Ps are subject to FDA requirements (at Part 1271) for registration and listing, donor-eligibility, current good tissue practices, and other requirements intended to prevent transmission of communicable diseases. Those that are the subject of the “same surgical procedure” exception – are exempt from both premarket approval requirements and the requirements of Part 1271. This regime is outlined in a flow chart, which is one of the few new features of the final guidance documents and is presented below:
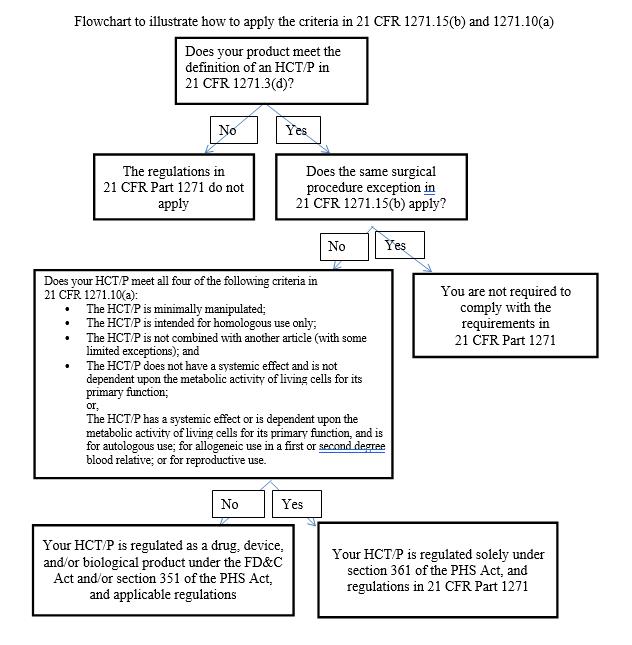
| 16 |
Enforcement Discretion
In order to allow manufacturers of products time to comply with the requirements, the FDA announced that it intended (originally through November 2020) to exercise enforcement discretion for certain products that are subject to the FDA’s premarket review under the existing regulations, but are not currently meeting these requirements. The FDA does not intend to exercise such enforcement discretion for those products that pose a potential significant safety concern. Going forward, the FDA will apply a risk-based approach to enforcement, taking into account how products are being administered as well as the diseases and conditions for which they are being used. This risk-based approach allows product manufacturers time to engage with the FDA, as to determine if they need to submit a marketing authorization application and, if so, submit their application to the FDA for approval.
On July 20, 2020, the FDA announced it was extending the enforcement discretion policy an additional six months through May 2021 as a result of the challenges presented by the COVID-19 pandemic.
The FDA’s enforcement discretion policy for IND and premarket approval requirements does not apply to products that have been associated with reported safety concerns or have the potential to cause significant safety concerns to patients. The FDA has stepped up its oversight of cellular and related products in recent years and has issued compliance actions, including numerous warning and untitled letters, and pursued litigation for serious violations of the law, including some involving patient harm.
Although the FDA has not changed its basic approach to regulating HCT/Ps, the FDA intends to exercise enforcement discretion up through May 2021 with regard to 351 HCT/Ps requiring premarket approval. The guidance states that, in order to “give manufacturers time to determine if they need to submit an IND or marketing application in light of this guidance,” the FDA intends to exercise enforcement discretion (i.e., the Agency may permit marketing without an approved marketing application) if the HCT/P “is intended for autologous use and its use does not raise reported safety concerns or potential significant safety concerns.”
The FDA has indicated it intends to focus enforcement actions on “products with higher risk,” taking into account factors such as non-autologous (allogeneic) use, the route of administration, the site of administration, and whether the product is intended for homologous or non-homologous use. For example, HCT/Ps administered via intravenous injection or infusion, aerosol inhalation, intraocular injection, or injection or infusion into the central nervous system, will be prioritized over HCT/Ps administered by intradermal, subcutaneous, or intra-articular injection. Similarly, HCT/Ps intended for non-homologous use, particularly those intended to treat serious or life-threatening conditions, “are more likely to raise significant safety concerns than HCT/Ps intended for homologous use”.
The Company believes that the new regulatory restrictions being implemented by the FDA are intended to assure that all parties involved in the chain of gathering, processing, distributing and/or administrating RAAM related products have met the required standards to assure that the manufacturing, marketing the administration of the RAAM regulated products are not misleading and are performed in a safe and ethical manner and in accordance with the “objective intent” of the manufacturer.
New Draft Guidance Documents
The two draft guidances provide important information to help spur development and access to innovative regenerative therapies. The first draft guidance, which builds off the regenerative medicine provisions in the Act, addresses how the FDA intends to simplify and streamline its application of the regulatory requirements for devices used in the recovery, isolation, and delivery of regenerative medicine advanced therapies, including combination products. The guidance specifies that devices intended for use with a specific RMAT may, together with the RMAT, be considered to comprise a combination product.
| 17 |
The second draft guidance describes the expedited programs that may be available to sponsors of regenerative medicine therapies, including the new Regenerative Medicine Advanced Therapy (“RMAT”) designation created by the 21st Century Cures Act, Priority Review, and Accelerated Approval. In addition, the guidance describes the regenerative medicine therapies that may be eligible for RMAT designation – including cell therapies, therapeutic tissue engineering products, human cell and tissue products, and combination products using any such therapies or products, as well as gene therapies that lead to a durable modification of cells or tissues (including genetically modified cells).
Fraud, Abuse and False Claims
We are directly and indirectly subject to various federal and state laws governing relationships with healthcare providers and pertaining to healthcare fraud and abuse, including anti-kickback laws. In particular, the federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing, arranging for or recommending a good or service for which payment may be made in whole or part under federal healthcare programs, such as the Medicare and Medicaid programs. (See 42 U.S.C. § 1320a-7b). Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. In implementing the statute, the Office of Inspector General of the U.S. Department of Health and Human Services (“OIG”) has issued a series of regulations, known as the “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute.
AdvaMed has established guidelines and protocols for medical device manufacturers in their relationships with healthcare professionals on matters including research and development, product training and education, grants and charitable contributions, support of third-party educational conferences, and consulting arrangements. Adoption of the AdvaMed Code by a medical device manufacturer is voluntary, and while the OIG and other federal and state healthcare regulatory agencies encourage its adoption and may look to the AdvaMed Code, they do not view adoption of the AdvaMed Code as proof of compliance with applicable laws. We have incorporated the principles of the AdvaMed Code in our standard operating procedures, sales force training programs, and relationships with health care professionals.
Manufacturing (Processing)
From February 2018, when we sold our manufacturing assets to a third party in connection with the Sale through April 2019, we relied upon third party manufacturers and processors. In May 2019, we opened our new placental tissue bank processing laboratory in Miami, Florida and resumed operations of a placental tissue bank processing laboratory in Miami, Florida.
During the period that we were not manufacturing our own products, the products we sold to our customers were delivered directly to them by the manufacturer of the products. Now that we are once again are operating a laboratory facility, we intend on becoming a registered establishment, accredited by the American Association of Tissue Banks (“AATB”) for the storage and distribution of tissue products that we purchase directly or indirectly from third party manufacturers.
Our laboratory and distribution facilities are subject to periodic unannounced inspections by regulatory authorities based on the activities we may be engaged, and may undergo compliance inspections conducted by the FDA and corresponding state and foreign agencies based on our operations. We intend to seek American Association Blood Banks (“AABB”) or AATB accreditation in connection with the storage of products we intend to distribute.
| 18 |
Placental Donation Program
During the times that we operated our laboratory facilities, we purchased placental tissue that was used in our minimally manipulated 361 compliant process to produce allografts to be used in regenerative therapy specialties from several birth tissue recovery companies. During this time, we were able to procure an adequate supply of tissue to meet our anticipated demand. We do not expect there will be any shortages of placental tissue and/or birth tissue supply companies for our future processing requirements.
Environmental Laws
From the date of the Sale in February 2018 through April 2019, we did not process or directly handle biomedical materials. Beginning in May 2019, we operated laboratory facilities that process or directly handled biomedical materials whereby we receive and/or generate wastes that are required to be disposed. We contract with third parties for the transport, treatment, and disposal of the waste that we obtain and at all times plan on being compliant with applicable laws and regulations promulgated by the Resource Conservation and Recovery Act, the U.S. Environmental Protection Agency and similar state agencies.
During the period from the Sale through May 2019, we sold products that were purchased from third party manufacturers. All of our shipments prior to December 2018, were delivered directly from the product manufacturers to our customers and accordingly we did not take possession of any product at any time.
Employees
At October 31, 2019, we had six full-time employees and no part-time employees. We also engaged two other persons as consultants that assisted with various administrative activities. From time to time, the Company engages independent contractors for sales and administration activities. There are no collective bargaining agreements.
Corporate History and Change in Control
The Company was incorporated in the state of Nevada on August 9, 2011 as Bespoke Tricycles Inc. for the purpose of designing, manufacturing, and selling vending tricycles for commercial customers. On June 24, 2015, Albert Mitrani, our Chairman, Chief Executive Officer and President, purchased an aggregate of 135,000,000 shares of common stock of Bespoke Tricycles, Inc. from John Goodhew, representing approximately 87.8% of the then issued and outstanding shares of the Company on a fully-diluted basis and constituting a change in control of the Company. The transaction was in accordance with the terms and provisions of the stock purchase agreement, dated May 29, 2015 (“Mitrani Purchase Agreement”), by and among the Company, Mr. Mitrani and Mr. Goodhew. The purchase price of $40,000 for the shares was paid by Mr. Mitrani to Mr. Goodhew on June 24, 2016. In connection with the execution and delivery of the Mitrani Purchase Agreement, as of May 29, 2015, Mr. Goodhew resigned as the sole officer of the Company and appointed Albert Mitrani to the Board of Directors and as the sole officer of the Company. Mr. Goodhew remained on the Board of Directors of the Company.
On August 6, 2015, Mr. Mitrani returned 60,120,000 shares of common stock of the Company to the Company for cancellation. As a result, Mr. Mitrani’s ownership was 74,880,000 shares of common stock of the Company, representing approximately 80% of the 93,600,000 shares of common stock issued and outstanding on such date.
On September 1, 2015, the Company filed a Certificate of Amendment with the Secretary of State of Nevada therein changing its name to Biotech Products Services and Research, Inc. and increasing the amount of authorized common stock from 90 million (90,000,000) shares to 250 million (250,000,000) shares. The amount authorized “blank check” preferred stock remained 10 million (10,000,000) and the par value of the common stock and preferred stock remained $0.001 per share.
| 19 |
On September 17, 2015, the Company completed an eighteen-for-one (18:1) forward split of the Company’s issued and outstanding common stock. Unless otherwise noted, the disclosure in this Annual Report on Form 10-K, including the consolidated audited financial statements contained herein, reflect a retroactive adjustment for the forward stock split. The forward stock split had no effect on the authorized capital stock of the Company.
On November 1, 2016, the Company filed a Certificate of Designation with the Secretary of State of Nevada therein designating out of the 10,000,000 authorized shares of Preferred Stock, a class of Preferred Stock as “Series A Non-Convertible Preferred Stock” consisting of 100 shares (the “Series A Certificate of Designation “). On March 2, 2017, the Company filed with the Secretary of State of Nevada an amendment to increase the number of shares provided for in the Series A Certificate of Designation from 100 shares to 400 shares. Generally, the outstanding shares of Series A Non-Convertible Preferred Stock shall vote together with the shares of common stock and other voting securities of the Company as a single class and, regardless of the number of shares of Series A Non-Convertible Preferred Stock outstanding, and as long as at least one share of Series A Non-Convertible Preferred Stock is outstanding, such shares shall represent eighty percent (80%) of all votes entitled to be voted at any annual or special meeting of stockholders of the Company or action by written consent of stockholders. Each outstanding share of the Series A Non-Convertible Preferred Stock shall represent its proportionate share of the 80% which is allocated to the outstanding shares of Series A Non-Convertible Preferred Stock.
On November 1, 2016, the Company filed a Certificate of Designation with the Secretary of State of Nevada therein designating out of the 10,000,000 authorized shares of Preferred Stock, a class of Preferred Stock as “Series B Convertible Preferred Stock” consisting of 1,000,000 shares (“Series B Certificate of Designation”). Each holder of Series B Preferred Stock shall have the right, at such holder’s option, at any time or from time to time from and after the day immediately following the date the Series B Preferred Stock is first issued, to convert each share of Series B Preferred Stock into 20 fully-paid and non-assessable shares of common stock.
On June 6, 2017, pursuant to the Nevada Revised Statutes and the Bylaws of the Company, the Board of Directors of the Company and the stockholders holding the Company’s outstanding Series A Preferred Stock, having the voting equivalency of 80% of the outstanding capital stock, approved the filing of an amendment to the Articles of Incorporation of the Company to increase the authorized amount of common stock from 250,000,000 to 750,000,000, without changing the par value of the common stock or authorized number and par value of “blank check” Preferred Stock. On June 19, 2017, the Company filed a Definitive 14C with the SEC regarding the corporate action. On June 22, 2017, the Company filed a Certificate of Amendment to the Company’s Articles of Incorporation with the Secretary of State of Nevada to effectuate the corporate action on July 10, 2017.
On April 23, 2018, in connection with the Reorganization, the Company issued MBA an aggregate of 222,425,073 shares of common stock of the Company, representing at the time a 51% fully diluted equity interest in the Company at a price of $0.001 per share (an aggregate value of $222,425). The foregoing issuance resulted in a change in control of the Company.
On May 8, 2018, the Company received the written consent of the Board of Directors of the Company (“Board”) and, on May 9, 2018, the written consent of the shareholders holding a majority in interest of the voting power of the Company (86.9%) adopting resolutions which authorized the Company to amend its Articles of Incorporation to change the name of the Company from "Biotech Products Services and Research, Inc." to “Organicell Regenerative Medicine, Inc.” The Company filed a Certificate of Amendment with the Nevada Secretary of State and, effective June 20, 2018, the Company’s name has been changed to Organicell Regenerative Medicine, Inc.
On May 8, 2018, the Board adopted resolutions to (i) amend its Articles of Incorporation to reduce the number of authorized shares of common stock from 750,000,000 to 250,000,000 and (ii) reverse split the issued and outstanding shares of the Company’s common stock on a ratio of seventeen (17) current shares for one (1) share of new shares. On May 9, 2018, shareholders holding a majority in interest of the voting power of the Company (86.9%) approved the amendment and the reverse stock split.
| 20 |
On June 1, 2018, the Company filed a Company-Related Action Notification with FINRA (“Notification Form”) to provide notice of certain proposed actions by the Company, including the amendment and reverse stock split. However, due to the Company’s failing to have the required Exchange Act reports filed with the SEC at the time of the filing, FINRA did not announce or effectuate the Name Change or Reverse Split in the marketplace. On June 18, 2018, the Company filed a Certificate of Correction with the Secretary of State of Nevada to reverse the amendments related to the Reverse Split. If the Company intends in the future to proceed with the Name Change and/or Reverse Split with FINRA, the Company will be required to submit a new Issuer Company-Related Notification Form for approval upon the Company becoming current in its Exchange Act filings. Should the Company proceed with the Reverse Split, then at such time as FINRA processes the announcement, the Company would then effect the reverse split of its common stock and amend its Articles to reduce its authorized common stock.
On June 6, 2018, the Company approved resolutions to cancel and terminate the Series A Preferred Stock designation and filed a certificate of withdrawal with the State of Nevada on June 14, 2018 thereby withdrawing and terminating the designation of the Series A Preferred Stock. As a result of the aforementioned actions, as of June 14, 2018, there were no shares of Series A Preferred Stock authorized or outstanding.
On June 6, 2018, the Company approved resolutions to cancel and terminate the Series B Preferred Stock designations and filed a certificate of withdrawal with the State of Nevada on June 14, 2018 thereby withdrawing and terminating the designation of the Series B Preferred Stock. As a result of the aforementioned actions, as of June 14, 2018, there were no shares of Series B Preferred Stock authorized or outstanding.
On May 18, 2020 and May 19, 2020, pursuant to the Nevada Revised Statutes and the Bylaws of the Company, the Board of Directors of the Company and the stockholders having the voting equivalency of 50.30% of the outstanding capital stock, respectively, approved the filing of an amendment to the Articles of Incorporation of the Company to increase the authorized amount of common stock from 750,000,000 to 1,500,000,000, without changing the par value of the common stock or authorized number and par value of “blank check” Preferred Stock. On June 2, 2020, the Company filed a Definitive 14C with the SEC regarding the corporate action. On June 24, 2020, the Company filed a Certificate of Amendment to the Company’s Articles of Incorporation with the Secretary of State of Nevada to effectuate the corporate action on June 24, 2020.
AN INVESTMENT IN OUR SECURITIES IS HIGHLY SPECULATIVE AND INVOLVES A HIGH DEGREE OF RISK. WE FACE A VARIETY OF RISKS THAT MAY AFFECT OUR OPERATIONS OR FINANCIAL RESULTS AND MANY OF THOSE RISKS ARE DRIVEN BY FACTORS THAT WE CANNOT CONTROL OR PREDICT. BEFORE INVESTING IN THE SECURITIES YOU SHOULD CAREFULLY CONSIDER THE FOLLOWING RISKS, TOGETHER WITH THE FINANCIAL AND OTHER INFORMATION CONTAINED IN THIS REPORT. IF ANY OF THE FOLLOWING RISKS ACTUALLY OCCURS, OUR BUSINESS, PROSPECTS, FINANCIAL CONDITION AND RESULTS OF OPERATIONS COULD BE MATERIALLY ADVERSELY AFFECTED. IN THAT CASE, THE TRADING PRICE OF OUR COMMON STOCK WOULD LIKELY DECLINE AND YOU MAY LOSE ALL OR A PART OF YOUR INVESTMENT. ONLY THOSE INVESTORS WHO CAN BEAR THE RISK OF LOSS OF THEIR ENTIRE INVESTMENT SHOULD CONSIDER AN INVESTMENT IN OUR SECURITIES.
This Annual Report contains certain statements relating to future events or the future financial performance of our Company. Prospective investors are cautioned that such statements are only predictions and involve risks and uncertainties, and that actual events or results may differ materially. In evaluating such statements, prospective investors should specifically consider the various factors identified in this Annual Report, including the matters set forth below, which could cause actual results to differ materially from those indicated by such forward-looking statements.
If any of the following or other risks materialize, the Company’s business, financial condition, and results of operations could be materially adversely affected which, in turn, could adversely impact the value of our securities. In such a case, investors in our securities could lose all or part of their investment.
| 21 |
Prospective investors should consider carefully whether an investment in the Company is suitable for them in light of the information contained in this Report and the financial resources available to them. The risks described below do not purport to be all the risks to which the Company could be exposed. This section is a summary of certain risks and is not set out in any particular order of priority. They are the risks that we presently believe are material to the operations of the Company. Additional risks of which we are not presently aware or which we presently deem immaterial may also impair the Company’s business, financial condition or results of operations.
Risks Related to Our Business
The ongoing COVID-19 outbreak and economic crisis has caused a significant disruption to the overall economy and there is no certainty as to when or how the situation will evolve, including whether or not the virus will be controlled and/or the state of our economy and business environment upon emerging from the crisis.
The current outbreak of the novel coronavirus (“COVID-19”) and resulting impact to the United States economic environments began to take hold during March 2020. The adverse public health developments and economic effects of the COVID-19 outbreak in the United States, have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. These restrictions have adversely affected the Company’s sales, results of operations and financial condition. In response to the COVID-19 outbreak, the Company (a) has accelerated its research and development activities, particularly in regards to potential health benefits of the Company’s products in addressing various health concerns associated with COVID-19 and (b) is aggressively seeking to raise additional debt and/or equity financing to support working capital requirements until sale for its products to providers resumes to levels pre COVID-19.
There is no assurance as to when the adverse impact to the United States and worldwide economies resulting from the COVID-19 outbreak will be eliminated, if at all, and whether any new or recurring pandemic outbreaks will occur again in the future causing similar or worse devastating impact to the United States and worldwide economies and our business.
There is no assurance that the COVID-19 crisis will be fully resolved or if resolved, that the overall economy will resume in a manner that allows the Company to resume operations as planned. We may not be able to generate revenues or achieve profitability in the future. Our failure to achieve or maintain profitability could negatively impact the value of our common stock.
We have limited cash on hand and there is substantial doubt as to our ability to continue as a going concern.
The Company incurred operating losses of $1,776,490 for the year ended October 31, 2019. In addition, the Company had an accumulated deficit of $16,285,222 at October 31, 2019. The Company had a negative working capital position of $1,677,684 at October 31, 2019. In their report for the fiscal year ended October 31, 2019, our auditors have expressed that there is substantial doubt as to our ability to continue as a going concern. We have incurred operating losses since our formation and expect to incur substantial losses and negative operating cash flows for the foreseeable future and may never become profitable. We also expect to continue to incur significant operating and capital expenditures for the next several years and anticipate that our expenses will increase substantially in the foreseeable future. We also expect to experience negative cash flow for the foreseeable future as we fund our operating losses and capital expenditures. As a result, we will need to generate significant revenues in order to achieve and maintain profitability. We may not be able to generate these revenues or achieve profitability in the future. Our failure to achieve or maintain profitability could negatively impact the value of our common stock.
| 22 |
We have a limited operating history upon which investors can evaluate our future prospects.
In connection with the change in control of our Company in June 2015, there was a change in the Company’s management, board of directors and line of business. Our current processing facility only began operations in May 2019. Therefore, we have limited operating history upon which an evaluation of our current business plan or performance and prospects can be made. The business and prospects of the Company must be considered in the light of the potential problems, delays, uncertainties and complications encountered in connection with a newly established business. The risks include, but are not limited to, the possibility that we will not be able to develop or identify functional and scalable products and services, or that although functional and scalable, our products and services will not be economical to market; that our competitors hold proprietary rights that preclude us from marketing such products; that our competitors market a superior or equivalent product; that we are not able to upgrade and enhance our technologies and products to accommodate new features and expanded service offerings; or the failure to receive necessary regulatory clearances for our products. To successfully introduce and market our products at a profit, we must establish brand name recognition and competitive advantages for our products. There are no assurances that the Company can successfully address these challenges. If it is unsuccessful, the Company and its business, financial condition and operating results could be materially and adversely affected.
Given the limited operating history, management has little basis on which to forecast future demand for our products from our existing customer base, much less new customers. The current and future expense levels of the Company are based largely on estimates of planned operations and future revenues rather than experience. It is difficult to accurately forecast future revenues because the business of the Company is new and its market has not been developed. If the forecasts for the Company prove incorrect, the business, operating results and financial condition of the Company will be materially and adversely affected. Moreover, the Company may be unable to adjust its spending in a timely manner to compensate for any unanticipated reduction in revenue. As a result, any significant reduction in revenues would immediately and adversely affect the business, financial condition and operating results of the Company.
We depend upon our officers and key personnel, the loss of which could seriously harm our business.
Our operating performance is substantially dependent on the continued services of our executive officers and key employees, in particular, Albert Mitrani, our Chief Executive Officer and President; and Ian T. Bothwell, our Chief Financial Officer. The unexpected loss of the services of any of them could have a material adverse effect on our business, operations, financial condition and operating results, as well as the value of our common stock.
We may not be able to compete successfully with current and future competitors.
We have many potential competitors in the regenerative medicine industry. We will compete, in our current and proposed businesses, with other established companies, most of which have far greater marketing and financial resources and experience than we do. We cannot guarantee that we will be able to penetrate our intended markets and be able to compete profitably, if at all. In addition to established competitors, there are moderate obstacles for competitors to enter this market, but they are not insurmountable if they have the financial resources and intellectual team. Effective competition could result in price reductions, reduced margins or have other negative implications, any of which could adversely affect our business and chances for success. Competition is likely to increase significantly as new companies enter the market and current competitors expand their services. Many of these potential competitors are likely to enjoy substantial competitive advantages, including, but not limited to, larger staffs, greater name recognition, larger and established customer bases and substantially greater financial, marketing, technical and other resources. To be competitive, we must respond promptly and effectively to industry dynamics, evolving standards and competitors’ innovations by continuing to enhance our services and sales and marketing channels. Any pricing pressures, reduced margins or loss of market share resulting from increased competition, or our failure to compete effectively, could fatally damage our business and chances for success.
| 23 |
We currently rely on non-exclusive supply arrangements with birth tissue recovery companies for obtaining the raw material used in manufacturing the products we sell. Also, during the periods that we did not operate our own manufacturing facility, we have relied on non-exclusive supply arrangements from other third-party manufacturers or distributors of products from third party manufacturers to obtain the supply of products we sold.
If our current supply arrangements under supply agreements with birth tissue recovery companies or third party manufacturers or distributors of products from third party manufacturers are disrupted for any reason, we may not be able to provide products to our customers, or if other supply arrangements can be made, the products and terms may not be as favorable, and that will adversely impact our operations and profitability.
If we do not continually update our products and/or services, they may become obsolete and we may not be able to compete with other companies.
We cannot assure you that we will be able to keep pace with technological advances, or that our current suppliers will be able to keep pace with technological advances and as such, our products and/or services may become obsolete. We cannot assure you that competitors will not develop related or similar services and offer them before we do, or do so more successfully, or that they will not develop services and products more effective than any that we and/or our suppliers have or are intending to develop. In addition, although we may be able to identify new suppliers that can provide more effective services and products to be more competitive, we may not be able to arrange satisfactory arrangements in a timely manner, if at all. If that happens, our business, prospects, results of operations and financial condition will be materially adversely affected.
We enter into supply agreements for the raw materials and/or products we sell, which make us vulnerable to the ability of such suppliers to remain current and innovative in their product offerings, to timely process and supply the products we desire to purchase, and to remain compliant with the current and changing regulatory environment. If our raw material and/or product suppliers are not successful in managing these responsibilities, it will have an adverse effect on our operations and profitability.
Our current birth tissue supply arrangements for manufacturing the products we sell and our third-party supply arrangements for the supply of products we sell provide for the supply and pricing for those products. There can be no assurance that our suppliers will continue to produce the products that we currently purchase under our existing arrangements, that our suppliers will be able to comply with the required FDA regulations for the manufacturing of such products, that our suppliers will continue to develop technology associated with their manufactured products to remain competitive with other companies, or that our suppliers will remain a going concern in the future. If any of our suppliers were to cause a disruption in our ability to obtain products as desired and expected and/or we are not provided advance notice of such potential disruption, we may not be able to timely identify and replace our current suppliers, if at all, and as a result, we may not be able to provide products to our customers, which will have an adverse impact to our operations.
In the event of default under our outstanding indebtedness, or we are unable to pay other obligations and accounts payable when due, our creditors may file a creditors petition or force us into involuntary bankruptcy which may have an adverse impact on our business.
The Company had a negative working capital position of $1,677,684 at October 31, 2019. In addition, the outbreak of the novel coronavirus (“COVID-19”) during March 2020 and the resulting adverse public health developments and economic effects to the United States business environments have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. The Company’s efforts to establish a stabilized source of sufficient revenues to cover operating costs has yet to be achieved and ultimately may prove to be unsuccessful unless additional sources of working capital through operations or debt and/or equity financings are realized. The Company has not repaid its outstanding indebtedness on the required due dates and the loans remain still outstanding. Management anticipates that the Company will remain dependent, for the near future, on additional investment capital to fund ongoing operating expenses. The Company does not have significant fixed and/or intangible assets to pledge for the purpose of borrowing additional capital. In addition, the Company relies on short term supply agreements to obtain the supply of raw materials used in manufacturing the products it currently sells and distributes to its customers. The Company’s current market capitalization and common stock liquidity will hinder its ability to raise equity proceeds to implement its business plan and could adversely affect the value of our securities, including the common stock.
| 24 |
We may be required to borrow funds in the future.
If the Company incurs indebtedness, a portion of its cash flow will have to be dedicated to the payment of principal and interest on such indebtedness. Typical loan agreements also might contain restrictive covenants, which may impair the Company’s operating flexibility. Such loan agreements would also provide for default under certain circumstances, such as failure to meet certain financial covenants. A default under a loan agreement could result in the loan becoming immediately due and payable and, if unpaid, a judgment in favor of such lender which would be senior to the rights of the Company’s stockholders. A judgment creditor would have the right to foreclose on any of the Company’s assets resulting in a material adverse effect on the Company’s business, operating results or financial condition.
Currently the Company has limited assets which could be used as collateral in obtaining future borrowings. Because of the Company’s inability to provide lenders with collateral and a limited history of successful operations, the Company may not be successful in its efforts to obtain additional funds though borrowings and as a result may not be able to fund required costs of operations.
Our growth depends on external sources of capital, which may not be available on favorable terms or at all.
Our access to capital will depend upon a number of factors over which we have little or no control, including general market conditions, government regulations and the market’s perception of our current and potential future earnings. If general economic instability or downturn leads to an inability to borrow at attractive rates or at all, our ability to obtain capital to finance working capital requirements could be negatively impacted.
If we are unable to obtain capital on terms and conditions that we find acceptable, we likely will have to scale back our business operations. In addition, our ability to refinance all or any debt we may incur in the future, on acceptable terms or at all, is subject to all of the above factors, and will also be affected by our future financial position, results of operations and cash flows, which additional factors are also subject to significant uncertainties, and therefore we may be unable to refinance any debt we may incur in the future, as it matures, on acceptable terms or at all. All of these events would have a material adverse effect on our business, financial condition, liquidity and results of operations.
Failure to establish or enhance our brand recognition could have a material adverse effect on our business and results of operations.
We believe we will need to expend significant time, effort and resources to enhance the recognition of our brands. We believe developing our brand will be important to our sales and marketing efforts. If we fail to establish or enhance the recognition of our brands, it could have a material adverse effect on our ability to sell our products and adversely affect our business and results of operations. If we fail to develop a positive public image and reputation, our business with our existing customers could decline and we may fail to develop additional business, which could adversely affect our results of operations.
Defects in the products we sell or failures in quality control related to our distribution of products could impair our ability to sell our products or could result in product liability claims, litigation and other significant events involving substantial costs.
Detection of any significant defects in our products that we sell or failure in our quality control procedures or the quality control procedures of our suppliers may result in, among other things, delay in time-to-market, loss of sales and market acceptance of our products, diversion of development resources, injury to our reputation and restrictions imposed by governmental agencies. The costs we may incur in correcting any product defects may be substantial and we may not be able to identify adequate remedies, if required. Additionally, errors, defects or other performance problems could result in financial or other damages to our customers, which could result in litigation. Product liability litigation, even if we prevail and/or our suppliers, would be time consuming and costly to defend, and if we and/or our product suppliers do not prevail, could result in the imposition of a damages award. We presently maintain product liability insurance and we are named insured on our suppliers’ insurance policy; however, it may not be adequate to cover any claims.
| 25 |
There can be no assurances of protection for proprietary rights or reliance on trade secrets.
In certain cases, the Company may rely on trade secrets to protect intellectual property, proprietary technology and processes, which the Company has acquired, developed or may develop in the future. There can be no assurances that secrecy obligations will be honored or that others will not independently develop similar or superior products or technology. The protection of intellectual property and/or proprietary technology through claims of trade secret status has been the subject of increasing claims and litigation by various companies both in order to protect proprietary rights as well as for competitive reasons even where proprietary claims are unsubstantiated. The prosecution of proprietary claims or the defense of such claims is costly and uncertain given the uncertainty and rapid development of the principles of law pertaining to this area. The Company, in common with other firms, may also be subject to claims by other parties with regard to the use of intellectual property, technology information and data, which may be deemed proprietary to others.
Our ability to become profitable and continue as a going concern will be dependent on our ability to attract, employ and retain highly skilled individuals to serve our clients.
The nature of our business requires that we employ skilled persons to perform highly skilled and specialized tasks for our Company. Our failure to retain such personnel could have a material adverse effect on our ability to offer services to clientele, and could potentially have a negative effect on our business. There is no guarantee that skilled persons will be available and willing to work for us in the future, nor is there any guarantee that we could afford to retain them if they are available at a future time.
Our ability to commence and complete clinical studies and other research and development objectives that are required by the FDA, including possible deadlines for certain products as early as May 2021 will require that we are properly funded to assure that we can commence and proceed with the required research activities promptly and that the results are favorable.
The Company is aggressively pursuing efforts to commence and complete clinical studies as well as obtaining approval to commence additional studies for other specific indications it has identified that the use of its products will provide more favorable and desired health related benefits for patients seeking alternative treatment options than are currently available. The ability of the Company to succeed in these efforts is subject to among other things, the Company having timely and sufficient available working capital to fund the substantial costs of completing clinical trials, which the Company currently does not have, and ultimately the approval from the FDA.
Our projections and forward-looking information may prove to be incorrect.
Management has prepared projections regarding the Company’s anticipated financial performance. The Company’s projections are hypothetical and based upon a presumed financial performance of the Company, the addition of a sophisticated and well-funded marketing plan, and other factors influencing the business of the Company. The projections are based on Management’s best estimate of the probable results of operations of the Company, based on present circumstances, and have not been reviewed by the Company’s independent accountants. These projections are based on several assumptions, set forth therein, which Management believes are reasonable. Some assumptions upon which the projections are based, however, invariably will not materialize due to the inevitable occurrence of unanticipated events and circumstances beyond Management’s control. Therefore, actual results of operations will vary from the projections, and such variances may be material. Assumptions regarding future changes in sales and revenues are necessarily speculative in nature. In addition, projections do not and cannot take into account such factors as general economic conditions, unforeseen regulatory changes, the entry into the Company’s market of additional competitors, the terms and conditions of future capitalization, and other risks inherent to the Company’s business. While Management believes that the projections accurately reflect possible future results of the Company’s operations, those results cannot be guaranteed.
| 26 |
We may not be able to manage our growth effectively.
We must continually implement and improve our products and/or services, operations, operating procedures and quality controls on a timely basis, as well as expand, train, motivate and manage our work force in order to accommodate anticipated growth and compete effectively in our market segment. Successful implementation of our strategy also requires that we establish and manage a competent, dedicated work force and employ additional key employees in corporate management, product development, client service and sales. We can give no assurance that our personnel, systems, procedures and controls will be adequate to support our existing and future operations. If we fail to implement and improve these operations, there could be a material, adverse effect on our business, operating results and financial condition.
If we make any acquisitions or enter into a merger or similar transaction, our business may be negatively impacted.
We have no present plans for any specific acquisition. However, in the event that we make acquisitions in the future, we could have difficulty integrating the acquired companies’ personnel and operations with our own. In addition, the key personnel of the acquired business may not be willing to work for us. We cannot predict the effect expansion may have on our core business. Regardless of whether we are successful in making an acquisition, the negotiations could disrupt our ongoing business, distract our management and employees and increase our expenses. In addition to the risks described above, acquisitions, mergers and other similar transactions are accompanied by a number of inherent risks, including, without limitation, the following:
| ● | the difficulty of integrating acquired products, services or operations; | |
| ● | the potential disruption of the ongoing businesses and distraction of our Management and the management of acquired companies; | |
| ● | the difficulty of incorporating acquired rights or products into our existing business; | |
| ● | difficulties in disposing of the excess or idle facilities of an acquired company or business and expenses in maintaining such facilities; | |
| ● | difficulties in maintaining uniform standards, controls, procedures and policies; | |
| ● | the potential impairment of relationships with employees and customers as a result of any integration of new management personnel; | |
| ● | the potential inability or failure to achieve additional sales and enhance our customer base through cross-marketing of the products to new and existing customers; | |
| ● | the effect of any government regulations which relate to the business acquired; and | |
| ● | potential unknown liabilities associated with acquired businesses or product lines, or the need to spend significant amounts to retool, reposition or modify the marketing and sales of acquired products or the defense of any litigation, whether or not successful, resulting from actions of the acquired company prior to our acquisition. |
Our business could be severely impaired if and to the extent that we are unable to succeed in addressing any of these risks or other problems encountered in connection with these acquisitions, many of which cannot be presently identified, these risks and problems could disrupt our ongoing business, distract our management and employees, increase our expenses and adversely affect our results of operations.
| 27 |
There might be unanticipated obstacles to the execution of our business plan.
The Company’s business plans may change significantly. The Company’s potential business endeavors are capital intensive. Management believes that the Company’s chosen activities and strategies are achievable in light of current economic and legal conditions with the skills, background, and knowledge of the Company’s principals and advisors. Management reserves the right to make significant modifications to the Company’s stated strategies depending on future events.
We may engage in transactions that present conflicts of interest.
The Company’s officers and directors may enter into agreements with the Company from time to time which may not be equivalent to similar transactions entered into with an independent third party. A conflict of interest arises whenever a person has an interest on both sides of a transaction. While we believe that it will take prudent steps to ensure that all transactions between the Company and any officer or director is fair, reasonable, and no more than the amount it would otherwise pay to a third party in an “arms-length” transaction, there can be no assurance that any transaction will meet these requirements in every instance.
We have agreed to indemnify our officers and directors against lawsuits to the fullest extent of the law.
Organicell is a Nevada corporation. Nevada law permits the indemnification of officers and directors against expenses incurred in successfully defending against a claim. Nevada law also authorizes Nevada corporations to indemnify their officers and directors against expenses and liabilities incurred because of their being or having been an officer or director. Our organizational documents provide for this indemnification to the fullest extent permitted by law.
We currently do not maintain any directors & officers insurance coverage. The commercial insurance policies we do have in place contain policy limits and exclusions for certain coverages and losses. In the event that we are found liable for damage or other losses, and such amounts are not covered under our existing insurance policies, we would incur substantial and protracted losses in paying any such claims or judgments. Although we intend to acquire coverage immediately upon resources becoming available, there is no guarantee that we can secure such coverage or that any insurance coverage would protect us from any damages or loss claims filed against it.
Our operating results may fluctuate significantly as a result of a variety of factors, many of which are outside of our control.
We are subject to the following factors, among others, that may negatively affect our operating results:
| · | The announcement or introduction of new products by our competitors; |
| · | Failure of Government and private health plans to adequately and timely reimburse the users of our products; |
| · | Our ability to upgrade and develop our systems and infrastructure to accommodate growth; |
| · | Our ability to attract and retain key personnel in a timely and cost effective manner; |
| · | The amount and timing of operating costs and capital expenditures relating to the expansion of our business, operations and infrastructure; |
| · | Regulation by Federal, State or Local Governments; and |
| · | General economic conditions (including fallout from current and future pandemics) as well as economic conditions specific to the healthcare industry. |
| 28 |
We have based our current and future expense levels largely on our investment plans and estimates of future events, although certain of our expense levels are, to a large extent, fixed. We may be unable to adjust spending in a timely manner to compensate for any unexpected revenue shortfall. Accordingly, any significant shortfall in revenue relative to our planned expenditures would have an immediate adverse effect on our business, results of operations and financial condition. Further, as a strategic response to changes in the competitive environment, we may from time to time make certain pricing, service or marketing decisions that could have a material and adverse effect on our business, results of operations and financial condition. Due to the foregoing factors, our revenue and operating results are and will remain difficult to forecast.
We are in a highly competitive and evolving field and face competition from well-established tissue processors and medical device manufacturers, as well as new market entrants.
Our business is in a very competitive and evolving field. Competition from other tissue processors, medical device companies and from research and academic institutions is intense, expected to increase, subject to rapid change, and could be significantly affected by new product introductions. The presence of this competition in our market may lead to pricing pressure, which would make it more difficult to sell our products at a price that will make us profitable or prevent us from selling our products at all. Our success will depend on our ability and/or the ability of our suppliers to perfect and protect their intellectual property rights related to their technologies as well as to develop new technologies and new applications for our technologies. Our failure to compete effectively would have a material and adverse effect on our business, results of operations and financial condition.
Rapid technological change could cause our products to become obsolete.
The technologies underlying the products we sell and intend to sell are subject to rapid and profound technological change. Competition intensifies as technical advances in each field are made and become more widely known. We can give no assurance that our suppliers will be able to develop services, products, or processes with significant advantages over the competing products, services, and processes. Any such occurrence could have a material and adverse effect on our business, results of operations and financial condition.
Our products are dependent on the availability of sufficient quantities of tissue from human donors, and any disruption in supply could adversely affect our business.
The success of the human tissue products we sell depends upon, among other factors, the availability of sufficient quantities of tissue from human donors. The availability of donated tissue could be adversely impacted by regulatory changes, public opinion of the donor process as well as our and our suppliers’ reputations in the industry. Any disruption in the supply of donated human tissue could restrict our growth and could have a material adverse impact on our business and financial condition. We cannot be sure that the supply of human tissue will continue to be available at current levels or will be sufficient to meet our future needs.
The products we offer are derived from human tissue and therefore have the potential for disease transmission.
The utilization of human tissue creates the potential for transmission of communicable disease, including, but not limited to, HIV, viral hepatitis, syphilis and other viral, fungal or bacterial pathogens. Our suppliers are required to comply with federal and state regulations intended to prevent communicable disease transmission.
Although we believe that our suppliers maintain strict quality controls over the procurement and processing of the human tissue used to make the products we sell, there is no assurance that these quality controls are or will continue to be adequate. In addition, negative publicity concerning disease transmission from other companies improperly processed donated tissue could have a negative impact on the demand for our products.
| 29 |
In order to grow revenues from certain of our products, we must expand our relationships with distributors and independent sales representatives.
We derive significant revenues through our relationships with distributors and independent sales representatives. During the year ended October 31, 2019, one distributor was affiliated with revenues received from customers comprising approximately 18.2% of our revenues. If such relationships were terminated for any reason, it could materially and adversely affect our ability to generate revenues and profits. We intend to obtain the assistance of additional distributors and independent sales representatives to continue our sales growth with respect to certain of our products. We may not be able to find additional distributors and independent sales representatives who will agree to market and/or distribute those products on commercially reasonable terms, if at all. In addition, adding new distributors and independent sales representatives require additional administrative and accounting efforts for which the Company may not have sufficient resources to manage effectively. If we are unable to establish new distribution and independent sales representative relationships or renew current distribution and sales agency agreements on commercially acceptable terms or manage the growth effectively, our business, financial condition and results of operations could be materially and adversely affected.
We continue to invest significant capital in expanding our internal sales force, and there can be no assurance that these efforts will continue to result in significant increases in sales.
We are engaged in a major initiative to build and further expand our internal sales and marketing capabilities which has contributed to our increased sales. As a result, we continue to invest in a direct sales force for certain of our products to allow us to reach new customers. These expenses impact our operating results, and there can be no assurance that we will continue to be successful in significantly expanding the sales of our products.
Our revenues may need to depend on adequate reimbursement from public and private insurers and health systems.
Currently, a significant number of public and private insurers and health systems currently do not provide reimbursement for our products. Our success and extent of our growth depends on the extent to which reimbursement for the costs of our products and related treatments will be available from third party payers, such as public and private insurers and health systems. Government and other third-party payers attempt to contain healthcare costs by limiting both coverage and the level of reimbursement of new products. Therefore, significant uncertainty usually exists as to the reimbursement status of new healthcare products. If we are not successful in obtaining adequate reimbursement for our products from these third-party payers, the market's acceptance of our products could be adversely affected. Inadequate reimbursement levels also likely would create downward price pressure on our products. Even if we do succeed in obtaining widespread reimbursement for our products, future changes in reimbursement policies could have a negative impact on our business, financial condition and results of operations.
To be commercially successful, we must convince physicians that our products are compliant with regulations, safe and effective alternatives to existing treatments and that our products should be used in their procedures.
We believe physicians will only adopt our products if they determine, based on experience, clinical data and published peer reviewed journal articles, that the use of our products in a particular procedure is a favorable alternative to conventional methods. Physicians may be slow to change their medical treatment practices for the following reasons, among others:
| · | Their lack of experience with prior procedures in the field using our products; |
| · | Lack of evidence supporting additional patient benefits and our products over conventional methods; |
| · | Perceived liability risks generally associated with the use of new products and procedures; |
| · | Perceived exposure from regulatory agencies that monitor the use of our products; |
| · | Limited availability of reimbursement from third party payers; and |
| · | The time that must be dedicated to training. |
| 30 |
In addition, we believe recommendations for and support of our products by influential physicians are essential for market acceptance and adoption. If we do not receive this support or if we are unable to demonstrate favorable long-term clinical data, physicians and hospitals may not use our products, which would significantly reduce our ability to achieve expected revenue and would prevent us from sustaining profitability.
We will need to expand our organization and managing growth may be more difficult than expected.
Managing our growth may be more difficult than we expect. We anticipate that a period of significant expansion will be required to penetrate and service the market for our existing and anticipated future products and to continue to develop new products. This expansion will place a significant strain on management, operational and financial resources. To manage the expected growth of our operations and personnel, we must both modify our existing operational and financial systems, procedures and controls and implement new systems, procedures and controls. We must also expand our finance, administrative, and operations staff. Management may be unable to hire, train, retain, motivate and manage necessary personnel or to identify, manage and exploit existing and potential strategic relationships and market opportunities.
We face the risk of product liability claims and may not be able to obtain or maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the manufacturing, processing and marketing of human tissue products. We may be subject to such claims if the products we sell cause, or appear to have caused, an injury. Claims may be made by patients, healthcare providers or others selling our products. We currently maintain product liability insurance that contain limits of coverage for the insured. Defending a lawsuit, regardless of merit, could be costly, divert management attention and result in adverse publicity, which could result in the withdrawal of, or reduced acceptance of, our products in the market. There can be no assurance that adequate insurance will be available in the event of a lawsuit, if at all. A product liability claim could result in significant costs and significant harm to our business.
We may implement a product recall or voluntary market withdrawal, which could significantly increase our costs, damage our reputation and disrupt our business.
The manufacturing, marketing and processing of the tissue products we sell or intend to sell involve an inherent risk that they do not meet applicable quality standards and requirements. In that event, there may be recall or market withdrawal required by a regulatory authority. A recall or market withdrawal of one of our products would be costly and would divert management resources. A recall or withdrawal of one of the products we sell, or a similar product processed, also could impair sales of our products as a result of confusion concerning the scope of the recall or withdrawal, or as a result of the damage to our reputation for quality and safety.
Significant disruptions of information technology systems or breaches of information security could adversely affect our business.
We rely to a large extent upon sophisticated information technology systems to operate our business. In the ordinary course of business, we collect, store and transmit large amounts of confidential information (including, but not limited to, personal information and intellectual property). We also have outsourced significant elements of our operations to third parties, including significant elements of our information technology infrastructure and, as a result, we are managing many independent vendor relationships with third parties who may or could have access to our confidential information. The size and complexity of our information technology and information security systems, and those of our third-party vendors with whom we contract (and the large amounts of confidential information that is present on them), make such systems potentially vulnerable to service interruptions or to security breaches from inadvertent or intentional actions by our employees or vendors, or from malicious attacks by third parties. Such attacks are of ever-increasing levels of sophistication and are made by groups and individuals with a wide range of motives (including, but not limited to, industrial espionage and market manipulation) and expertise. While we have invested significantly in the protection of data and information technology, there can be no assurance that our efforts will prevent service interruptions or security breaches. Although we may obtain cyber-insurance coverage that may cover certain events described above, this insurance is subject to deductibles and coverage limitations and we may not be able to maintain this insurance. Also, it is possible that claims could exceed the limits of our coverage. Any interruption or breach in our systems could adversely affect our business operations and/or result in the loss of critical or sensitive confidential information or intellectual property, and could result in financial, legal, business and reputational harm to us or allow third parties to gain material, inside information that they use to trade in our securities.
| 31 |
New lines of business or new products and services may subject us to additional risks.
From time to time, we may implement or may acquire new lines of business or offer new products and services within existing lines of business. There are risks and uncertainties associated with these efforts, particularly in instances where the markets are not fully developed or are evolving. In developing and marketing new lines of business and new products and services, we may invest significant time and resources. External factors, such as regulatory compliance obligations, competitive alternatives, and shifting market preferences, may also impact the successful implementation of a new line of business or a new product or service. Failure to successfully manage these risks in the development and implementation of new lines of business or new products or services could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Our Intellectual Property
There can be no assurances of protection for proprietary rights or reliance on trade secrets.
In certain cases, the Company may rely on trade secrets to protect intellectual property, proprietary technology and processes, which the Company has acquired, developed or may develop in the future. There can be no assurances that secrecy obligations will be honored or that others will not independently develop similar or superior products or technology. The protection of intellectual property and/or proprietary technology through claims of trade secret status has been the subject of increasing claims and litigation by various companies both in order to protect proprietary rights as well as for competitive reasons even where proprietary claims are unsubstantiated. The prosecution of proprietary claims or the defense of such claims is costly and uncertain given the uncertainty and rapid development of the principles of law pertaining to this area. The Company, in common with other firms, may also be subject to claims by other parties with regard to the use of intellectual property, technology information and data, which may be deemed proprietary to others.
Our suppliers’ ability to protect their intellectual property and proprietary technology through patents and other means is uncertain and may be inadequate, which could have a material and adverse effect on us.
We depend significantly on our suppliers’ ability to protect their proprietary rights to the technologies used in the products we purchase from them and resell. Traditional legal means afford only limited protection and may not adequately protect their rights or permit them to gain or keep any competitive advantage. To the extent that they are unable to protect their intellectual property against infringement by others or by claims of infringement by such suppliers, our business could be materially adversely affected.
We may be subject to damages resulting from claims that we, our employees, or our independent contractors have wrongfully used or disclosed alleged trade secrets of others.
Some of our employees were previously employed at other medical device or tissue companies. We may also hire additional employees who are currently employed at other medical device or tissue companies, including our competitors. Additionally, consultants or other independent agents with which we may contract may be or have been in a contractual arrangement with one or more of our competitors. Although no claims against us are currently pending, we may be subject to claims that these employees or independent contractors have used or disclosed any party's trade secrets or other proprietary information. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management. If we fail to defend such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key personnel or their work product could hamper or prevent our ability to market existing or new products, which could severely harm our business.
| 32 |
If we are unable to protect our trademarks from infringement, our business prospects may be harmed.
We currently have applied for a registered trademark for the use of Organicell in association with our family of biologic products offered in the United States. Although we may take steps to monitor the possible infringement or misuse of our Organicell or other trademarks once they are obtained, it is possible that third parties may infringe, dilute or otherwise violate our trademark rights. Any unauthorized use of our trademarks could harm our reputation or commercial interests. In addition, our enforcement against third-party infringers or violators may be unduly expensive and time-consuming, and any remedy obtained may constitute insufficient redress relative to the damages we may suffer. Our business may be materially adversely affected in the event we are unable to protect our trademarks.
Risks Related to Regulatory Approval of Our Products and Other Government Regulations
To the extent our products do not qualify for regulation as human cells, tissues and cellular and tissue-based products under Section 361 of the Public Health Service Act, this could result in removal of the applicable products from the market, would make the introduction of new tissue products more expensive and significantly delay the expansion of our tissue product offerings and subject us to additional post-market regulatory requirements.
The products we offer are derived from human tissue. The FDA has specific regulations governing human cells, tissues and cellular and tissue-based products, or HCT/Ps. An HCT/P is a product containing or consisting of human cells or tissue intended for transplantation into a human patient. HCT/Ps that meet the criteria for regulation solely under Section 361 of the Public Health Service Act (so-called “361 HCT/Ps”) are not subject to any premarket clearance or approval requirements and are subject to less stringent post-market regulatory requirements.
If a product is deemed not to be a 361 HCT/P, FDA regulations will require premarket clearance or approval requirements that will involve significant time and cost investments by the Company. Further, there can be no assurance that the FDA will not, at some future point, change its position on current or future products' 361 HCT/P status, and any regulatory reclassification could have adverse consequences for us and make it more difficult or expensive for us to conduct our business by requiring premarket clearance or approval and compliance with additional post-market regulatory requirements with respect to those products. Moreover, increased regulatory scrutiny within the industry in which we operate could lead to increased regulation of HCT/Ps, including 361 HCT/Ps. We also cannot assure you that the FDA will not impose more stringent definitions with respect to products that qualify as 361 HCT/Ps.
See “Government Regulation” in Item 1 for a discussion of 361 HCT/Ps and the FDA's position on our products. If the FDA does allow the Company to continue to market those products that fall under the proposed regulations without a biologics license either prior to or after finalization of the draft guidance documents, it may impose conditions, such as labeling restrictions and compliance with cGMP. Although the Company is preparing for these requirements in connection with its pursuit of a BLA for certain of its products, earlier compliance with these conditions would require significant additional time and cost investments by the Company. It is also possible that the FDA will not allow the Company to market any form of it’s products without a biologics license even prior to finalization of the draft guidance documents and could even require the Company to recall it’s products.
The FDA has recently announced that it intends to begin enforcement of regulations to manufacturers of certain biologics tissue products, including the products that we may purchase through supply agreements with those identified manufacturers. If the FDA were to take enforcement action against those suppliers, it would have a material adverse impact to our operations.
In November 2017, the FDA issued guidance documents to clarify the FDA’s interpretation of the risk-based criteria manufacturers used to determine which manufactured tissue products are subject to the FDA’s premarket review and in order to be lawfully marketed in the United States, require an FDA-approved BLA.
| 33 |
The FDA intends to exercise enforcement discretion through May 2021 with regard to allowing manufacturers for certain products that are subject to the FDA’s premarket review under the existing regulations, but are not currently meeting these requirements.
The Company believes that the current products it distributes are not specifically identified within the scope of these regulations and that the new regulatory restrictions being implemented by the FDA are intended to assure that all parties involved in the chain of gathering, processing, distributing and/or administrating RAAM related products have met the required standards to assure that the manufacturing, marketing the administration of the RAAM regulated products are not misleading and are performed in a safe and ethical manner and in accordance with the “objective intent” of the manufacturer.
There is no assurance that the FDA will not take enforcement action against us or our suppliers in connection with the products we manufacture and/or purchase from suppliers and sell to our customers. Furthermore, our supply agreements provide that we comply with all FDA requirements for in the use of the products we purchase from our suppliers, including the way we market the products to our customers, including our representatives and sub-distributors, and any activities that we take that might be inconsistent with the “manufacturers objective intent”, including potential significant safety concerns on how the products are being administered as well as the diseases and conditions for which they are being used. If the FDA were to take any adverse action against ourselves and/or our suppliers and/or representatives and distributors and/or it is determined that any of our activities are the basis for FDA enforcement, it will have a significant adverse effect on our operations.
Our business is subject to continuing regulatory compliance by the FDA and other authorities, which is costly and our failure to comply could result in negative effects on our business.
As discussed above, the FDA has specific regulations governing our tissue-based products, or HCT/Ps. The FDA has broad post-market and regulatory and enforcement powers. The FDA's regulation of HCT/Ps includes requirements for registration and listing of products, donor screening and testing, processing and distribution (“Current Good Tissue Practices”), labeling, record keeping and adverse-reaction reporting, and inspection and enforcement.
Biologics and medical devices are subject to even more stringent regulation by the FDA. Even if pre-market clearance or approval is obtained, the approval or clearance may place substantial restrictions on the indications for which the product may be marketed or to whom it may be marketed, may require warnings to accompany the product or impose additional restrictions on the sale and/or use of the product. In addition, regulatory approval is subject to continuing compliance with regulatory standards, including the FDA's quality system regulations.
If we fail to comply with the FDA regulations regarding our tissue products or medical devices, the FDA could take enforcement action, including, without limitation, any of the following sanctions and the manufacture of our products or processing of our tissue could be delayed or terminated:
| · | Untitled letters, warning letters, fines, injunctions, and civil penalties; |
| · | Recall or seizure of our products; |
| · | Operating restrictions, partial suspension or total shutdown of production; |
| · | Refusing our requests for clearance or approval of new products; |
| · | Withdrawing or suspending current applications for approval or approvals already granted; |
| · | Refusal to grant export approval for our products; and |
| · | Criminal prosecution. |
| 34 |
It is likely that the FDA's regulation of HCT/Ps will continue to evolve in the future. Complying with any such new regulatory requirements may entail significant time delays and expense, which could have a material adverse effect on our business. The AATB has issued operating standards for tissue banking. Compliance with these standards is a requirement in order to become an accredited tissue bank. In addition, some states have their own tissue banking regulations.
In November 2017, the FDA released four guidance documents (two final, two draft) in an effort to implement a “comprehensive policy framework” for existing laws and regulations governing regenerative medicine products, including human cells, tissues, and cellular and tissue-based products (“HCT/Ps”). These guidance documents build upon the previous regulatory framework for these products, which was completed in 2005. The Comprehensive regenerative medicine policy framework intends to spur innovation, efficient access to potentially transformative products, while ensuring safety & efficacy.
The framework builds upon the FDA’s existing risk-based regulatory approach to more clearly describe what products are regulated as drugs, devices, and/or biological products. Further, two of the guidance documents propose an efficient, science-based process for helping to ensure the safety and effectiveness of these therapies, while supporting development in this area. The suite of guidance documents also defines a risk-based framework for how the FDA intends to focus its enforcement actions against those products that raise potential significant safety concerns. This modern framework is intended to balance the agency’s commitment to safety with mechanisms to drive further advances in regenerative medicine so innovators can bring new, effective therapies to patients as quickly and safely as possible. The policy also delivers on important provisions of the Act.
Although the FDA has not changed its basic approach to regulating HCT/Ps, the agency intends to exercise enforcement discretion until May 2021 with regard to 351 HCT/Ps requiring premarket approval. The guidance states that, in order to “give manufacturers time to determine if they need to submit an IND or marketing application in light of this guidance,” the FDA intends to exercise enforcement discretion (i.e., the agency may permit marketing without an approved marketing application) if the HCT/P “is intended for autologous use and its use does not raise reported safety concerns or potential significant safety concerns.”
The Company believes that the new regulatory restrictions being implemented by the FDA are intended to assure that all parties involved in the chain of gathering, processing, distributing and/or administrating RAAM related products have met the required standards to assure that the manufacturing, marketing the administration of the RAAM regulated products are not misleading and are performed in a safe, ethical and in accordance with “objective intent”.
In addition, procurement of certain human organs and tissue for transplantation is subject to the restrictions of the National Organ Transplant Act (“NOTA”), which prohibits the transfer of certain human organs, including skin and related tissue for valuable consideration, but permits the reasonable payment associated with the removal, transportation, implantation, processing, preservation, quality control and storage of human tissue and skin. We reimburse tissue banks, hospitals and physicians for their services associated with the recovery, storage and transportation of donated human tissue. Although we have independent third party appraisals that confirm that reasonableness of the service fees we pay, if we were to be found to have violated NOTA's prohibition on the sale or transfer of human tissue for valuable consideration, we would potentially be subject to criminal enforcement sanctions, which could materially and adversely affect our results of operations.
Finally, as discussed above, we and other manufacturers of skin substitutes are required to provide ASP information to CMS on a quarterly basis. The Medicare payment rates are updated quarterly based on this ASP information. If a manufacturer is found to have made a misrepresentation in the reporting of ASP, such manufacturer is subject to civil monetary penalties of up to $10,000 for each misrepresentation for each day in which the misrepresentation was applied.
| 35 |
We and our sales representatives, whether employees or independent contractors, must comply with various federal and state anti-kickback, self-referral, false claims and similar laws, any breach of which could cause a material adverse effect on our business, financial condition and results of operations.
Our relationships with physicians, hospitals and other healthcare providers are subject to scrutiny under various federal anti-kickback, self-referral, false claims and similar laws, often referred to collectively as healthcare fraud and abuse laws. Healthcare fraud and abuse laws are complex, and even minor, inadvertent violations can give rise to claims that the relevant law has been violated. Possible sanctions for violation of these fraud and abuse laws include monetary fines, civil and criminal penalties, exclusion from federal and state healthcare programs, including Medicare, Medicaid, Veterans Administration health programs, workers' compensation programs and TRICARE (the healthcare system administered by or on behalf of the U.S. Department of Defense for uniformed services beneficiaries, including active duty and their dependents, retirees and their dependents), and forfeiture of amounts collected in violation of such prohibitions. Certain states have similar fraud and abuse laws, imposing substantial penalties for violations. Any Government investigation or a finding of a violation of these laws would likely result in a material adverse effect on the market price of our common stock, as well as our business, financial condition and results of operations.
Anti-kickback laws and regulations prohibit any knowing and willful offer, payment, solicitation or receipt of any form of remuneration in return for the referral of an individual or the ordering or recommending of the use of a product or service for which payment may be made by Medicare, Medicaid or other Government-sponsored healthcare programs. We will enter into consulting agreements, speaker agreements, research agreements and product development agreements with physicians, including some who may order our products or make decisions to use them. In addition, some of these physicians own our stock, which they purchased in arm's length transactions on terms identical to those offered to non-physicians, or received stock awards from us as consideration for services performed by them. While these transactions were structured with the intention of complying with all applicable laws, including state anti-referral laws and other applicable anti-kickback laws, it is possible that regulatory or enforcement agencies or courts may in the future view these transactions as prohibited arrangements that must be restructured or for which we would be subject to other significant civil or criminal penalties. As discussed above, we have incorporated the AdvaMed code principles into our relationships with healthcare professionals under our consulting agreements, and our policies regarding payment of travel and lodging expenses, research and educational grant procedures and sponsorship of third-party conferences. In addition, we have conducted training sessions on these principles. However, there can be no assurance that regulatory or enforcement authorities will view these arrangements as being in compliance with applicable laws or that one or more of our employees or agents will not disregard the rules we have established. Because our strategy relies on the involvement of physicians who consult with us on the design of our products, perform clinical research on our behalf or educate the market about the efficacy and uses of our products, we could be materially impacted if regulatory or enforcement agencies or courts interpret our financial relationships with physicians who refer or order our products to be in violation of applicable laws and determine that we would be unable to achieve compliance with such applicable laws. This could harm our reputation and the reputations of the physicians we engage to provide services on our behalf. In addition, the cost of noncompliance with these laws could be substantial since we could be subject to monetary fines and civil or criminal penalties, and we could also be excluded from federally-funded healthcare programs, including Medicare and Medicaid, for non-compliance.
The Federal False Claims Act (“FCA”) imposes civil liability on any person or entity that submits, or causes the submission of, a false or fraudulent claim to the U.S. Government. Damages under the FCA can be significant and consist of the imposition of fines and penalties. The FCA also allows a private individual or entity with knowledge of past or present fraud against the Federal Government to sue on behalf of the Government to recover the civil penalties and treble damages. The U.S. Department of Justice (“DOJ”) on behalf of the Government has previously alleged that the marketing and promotional practices of pharmaceutical and medical device manufacturers, including the off-label promotion of products or the payment of prohibited kickbacks to doctors, violated the FCA, resulting in the submission of improper claims to federal and state healthcare entitlement programs such as Medicaid. In certain cases, manufacturers have entered into criminal and civil settlements with the federal government under which they entered into plea agreements, paid substantial monetary amounts and entered into corporate integrity agreements that require, among other things, substantial reporting and remedial actions going forward.
The scope and enforcement of all of these laws is uncertain and subject to rapid change, especially in light of the lack of applicable precedent and regulations. There can be no assurance that federal or state regulatory or enforcement authorities will not investigate or challenge our current or future activities under these laws. Any investigation or challenge could have a material adverse effect on our business, financial condition and results of operations. Any state or federal regulatory or enforcement review of us, regardless of the outcome, would be costly and time consuming. Additionally, we cannot predict the impact of any changes in these laws, whether these changes are retroactive or will have effect on a going-forward basis only.
| 36 |
We face significant uncertainty in the industry due to Government healthcare reform.
There have been and continue to be proposals by the Federal Government, State Governments, regulators and third-party payers to control healthcare costs, and generally, to reform the healthcare system in the United States. There are many programs and requirements for which the details have not yet been fully established or the consequences are not fully understood. These proposals may affect aspects of our business. We also cannot predict what further reform proposals, if any, will be adopted, when they will be adopted, or what impact they may have on us.
Risks Relating to Ownership of Our Common Stock
Our articles of incorporation allow for our board to create a new series of preferred stock without further approval by our stockholders, which could adversely affect the rights of the holders of our common stock.
Our Board of Directors has the authority to fix and determine the relative rights and preferences of preferred stock. Our Board of Directors have the authority to issue up to 10,000,000 shares of our preferred stock terms of which may be determined by the Board without further stockholder approval. As a result, our Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of common stock and the right to the redemption of the shares, together with a premium, prior to the redemption of our common stock. In addition, our Board of Directors could authorize the issuance of a series of preferred stock that has greater voting power than our common stock or that is convertible into our common stock, which could decrease the relative voting power of our common stock or result in dilution to our existing stockholders. Although we have no present intention to issue any additional shares of preferred stock or to create any additional series of preferred stock, we may issue such shares in the future.
You may experience dilution of your ownership interests because of the future issuance of additional shares of common stock.
In the future, we may issue additional authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of our shareholders. We may also issue additional shares of our securities that are convertible into or exercisable for common stock, as the case may be, in connection with hiring or retaining employees, future acquisitions, future sales of its securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of common stock may create downward pressure on the value of our securities. There can be no assurance that we will not be required to issue additional shares of common stock, warrants or other convertible securities in the future in conjunction with any capital raising efforts, including at a price (or exercise prices) below the price at which our shares may be valued or are trading in a public market.
Offers or availability for sale of a substantial number of shares of our common stock may cause the price of our common stock to decline.
If our stockholders sell substantial amounts of their shares of our common stock, or shares of our common stock underlying any outstanding securities held by them, in the public market under Rule 144 or upon registration of such shares pursuant to an effective registration statement, it could create a circumstance commonly referred to as an “overhang” and in anticipation of which the market price of our common stock could fall. The existence of an overhang, whether or not sales have occurred or are occurring, also could make more difficult our ability to raise additional financing through the sale of equity or equity-related securities in the future at a time and price that we deem reasonable or appropriate.
| 37 |
There can be no assurances that an active trading market may develop for our common stock, or if developed, be maintained.
The average trading volume in our stock has been historically low, with little or no trading at all on some days. As a result, an investor may find it difficult to dispose of, or to obtain accurate quotations of the price of, our common stock. Accordingly, investors must assume they may have to bear the economic risk of an investment in our common stock for an indefinite period of time. There can be no assurance that a more active market for the common stock will develop, or if one should develop, there is no assurance that it will be maintained. This severely limits the liquidity of our common stock, and would likely have a material adverse effect on the market price of our common stock and on our ability to raise additional capital.
Our common stock is subject to the “penny stock” rules of the SEC and the trading market in the securities is limited, which makes transactions in the stock cumbersome and may reduce the value of an investment in the stock.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to us, as any equity security that has a market price of less than $5.00 per share or with an exercise price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require:
| ● | that a broker or dealer approve a person’s account for transactions in penny stocks; and |
| ● | the broker or dealer receives from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased. |
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must:
| ● | obtain financial information and investment experience objectives of the person; and |
| ● | make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks. |
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth:
| ● | the basis on which the broker or dealer made the suitability determination; and |
| ● | that the broker or dealer received a signed, written agreement from the investor prior to the transaction. |
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for investors to dispose of common stock and cause a decline in the market value of stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
| 38 |
The price of our common stock may become volatile, which could lead to losses by investors and costly securities litigation.
The trading price of our common stock is likely to be highly volatile and could fluctuate in response to factors such as:
| ● | actual or anticipated variations in our operating results; |
| ● | announcements of developments by us or our competitors; |
| ● | announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
| ● | adoption of new accounting standards affecting our Company’s industry; |
| ● | additions or departures of key personnel; |
| ● | sales of our common stock or other securities in the open market; and |
| ● | other events or factors, many of which are beyond our control. |
The stock market is subject to significant price and volume fluctuations. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been initiated against the company. Litigation initiated against us, whether or not successful, could result in substantial costs and diversion of our management’s attention and resources, which could harm our business and financial condition.
We do not anticipate dividends to be paid on our common stock, and investors may lose the entire amount of their investment.
Cash dividends have never been declared or paid on the common stock, and we do not anticipate such a declaration or payment for the foreseeable future. We expect to use future earnings, if any, to fund business growth. Therefore, stockholders will not receive any funds absent a sale of their shares. We cannot assure stockholders of a positive return on their investment when they sell their shares, nor can we assure that stockholders will not lose the entire amount of their investment.
We must obtain approval from FINRA if we wish to reduce our authorized shares of common stock and/or to effectuate a reverse split of the issued and outstanding shares of the common stock, of which the impact to the trading price of our common stock and/or the liquidity for trading our common stock may be adverse to current stockholders and may not result in desired benefits to the Company.
The Company currently has 1,500,000,000 authorized shares of common stock and 875,194,450 shares issued and outstanding. The Company expects that it will continue to issue common stock in the future in connection with debt and/or equity financings, transactions with third parties, performance incentives and as compensation to its employees. The Company believes that a reverse split would bring value to the issued and outstanding shares of the Company by limiting dilution of operating results by an excessive number of shares overhanging the market.
| 39 |
The Company’s ability to effectuate a reverse split will require approval from FINRA. FINRA has previously informed the Company that it will not approve and process announcements for company-related actions such as a reverse split, until the Company’s delinquencies in its Exchange Act reports with the SEC have been fully resolved and a Notification Form is submitted.
If completed, and the reverse split does not bring value to the current shareholders and/or our ability to attract prospective investors, including possible adverse impact to the trading price of our common stock and/or the liquidity for trading our common stock, it would likely have a material adverse effect on the market price of our common stock and on our ability to raise additional capital.
If securities analysts do not initiate coverage or continue to cover our common stock or publish unfavorable research or reports about our business, this may have a negative impact on the market price of our common stock.
The trading market for the common stock will depend on the research and reports that securities analysts publish about our business and the Company. We do not have any control over these analysts. There is no guarantee that securities analysts will cover the common stock. If securities analysts do not cover the common stock, the lack of research coverage may adversely affect its market price. If we are covered by securities analysts, and our stock is the subject of an unfavorable report, our stock price and trading volume would likely decline. If one or more of these analysts ceases to cover the Company or fails to publish regular reports on the Company, we could lose visibility in the financial markets, which could cause our stock price or trading volume to decline.
Approximately 48.41% of the outstanding shares of common stock is currently owned and/or controlled by our Board members and executive management of the Company. Our Board members and executive management currently have significant ability to influence the election of our directors and the outcome of matters submitted to our stockholders.
As of September 30, 2020, there are 875,194,450 shares of common stock outstanding, of which 423,719,370 shares of common stock (approximately 48.41% of the outstanding shares of common stock) are owned and/or controlled by our Board and executive officers, Albert Mitrani, Ian T. Bothwell, Dr. Maria Mitrani, Dr. George Shapiro, Michael Carbonara and Dr. Allen Meglin, and two of the members of management are spouses, Albert Mitrani and Dr. Maria Mitrani. In addition, all four of our executive officers are also members of the Board of Directors, which currently consists of six members. In addition, our executive officers may receive additional stock grants in the future based on the achievement of certain performance milestones and from the conversion of unpaid compensation into common stock, which if fully issued would provide our Board and executive officers with over 51% of the outstanding shares of common stock outstanding. As a result, the foregoing persons have the ability to significantly influence the outcome of issues submitted to our stockholders. Although our officers and directors have a fiduciary obligation to the Company stockholders, their interests may not always coincide with our interests or the interests of other stockholders. As a consequence, it may be difficult for the other stockholders to remove our management. The ownership of these officers/directors could also deter unsolicited takeovers, including transactions in which stockholders might otherwise receive a premium for their shares over then current market prices.
We identified material weaknesses in our internal controls over financial reporting that existed at October 31, 2019. If we fail to properly identify or remediate any future weaknesses or deficiencies, or fail to achieve and maintain effective internal control, our ability to produce accurate and timely financial statements could be impaired and investors could lose confidence in our financial statements.
Internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements in accordance with GAAP. At October 31, 2019, our management determined that our internal controls over financial reports were ineffective. Although management intends to implement remedial actions to correct these inefficiencies, there can be no assurance that our remedial measures will be sufficient to address the material weaknesses or that our internal control over financial reporting will not be subject to additional material weaknesses in the future. If the remedial measures that we take are insufficient to address the material weaknesses or if additional material weaknesses or significant deficiencies in our internal control are discovered or occur in the future, our consolidated financial statements may contain material misstatements, and we could be required to restate our financial results. Additionally, we may encounter problems or delays in implementing any changes necessary for management to make a favorable assessment of our internal control over financial reporting. If we cannot favorably assess the effectiveness of our internal control over financial reporting, investors could lose confidence in our financial information and the price of our common stock could decline.
| 40 |
The Financial Industry Regulatory Authority (“FINRA”) sales practice requirements may also limit a stockholder’s ability to buy and sell our common stock.
In addition to the “penny stock” rules described above, the Financial Industry Regulatory Authority, which we refer to as FINRA, has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our common stock and have an adverse effect on the market for shares of our common stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
Not applicable.
The Company’s corporate administrative offices were moved to office space located at 515 North Shore Drive, Miami Beach, Florida 33141. The office space is leased from MariLuna, LLC, a Florida limited liability company which is owned by Dr. Maria Mitrani, the Chief Science Officer and director of the Company. The term of the lease runs through June 2023 and the monthly rent was$2,900 through July 2020, at which time it increased to $3,500 per month. The Company paid a security deposit of $5,000.
Since February 2019, we have rented laboratory and general office space located at 1951 NW 7th Ave., Suite 300, Miami, Florida 33136 pursuant to a Services Agreement, dated February 2019, between Organicell Regenerative Medicine Inc., as licensee, and CIC Miami, LLC, as licensor, for approximately $7,000 per month.
We also maintain websites located at www.organicell.com, the contents of which are not incorporated into this Report. Our telephone number is (888) 963-7881.
From time to time, we may become involved in various lawsuits and legal proceedings which arise in the ordinary course of business. However, litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
We are currently not aware of any pending legal proceedings to which we are a party or of which any of our property is the subject, nor are we aware of any such proceedings that are contemplated by any governmental authority.
ITEM 4. MINE SAFETY DISCLOSURES.
Not applicable.
| 41 |
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
Market Information
The symbol for our common stock is BPSR. Due to the late filing of this Form 10-K and other Exchange Act Reports, our common stock is currently quoted on the OTCPink tier of the over-the counter market operated by OTC Markets Group, Inc.
Common Stock
As of September 30, 2020, 875,194,450 shares of our common stock were outstanding.
Holders of Our Common Stock
As of September 30, 2020, we had approximately 191 record holders of our common stock. One of these holders is CEDE and Company which is the mechanism used for brokerage firms to hold securities in book entry form on behalf of their clients and as of September 30, 2020, they held 33,033,743 shares of common stock for these shareholders. Accordingly, we believe that we have significantly in excess of 1,000 beneficial shareholders as of the date of this report.
Dividend Policy
We have never paid or declared dividends on our securities. The payment of cash dividends, if any, in the future is within the discretion of our Board and will depend upon our earnings, our capital requirements, financial condition and other relevant factors. We do not expect to pay dividends for the foreseeable future, and intend to retain future earnings, if any, towards the use in our business and growth strategies.
Securities Authorized for Issuance under Equity Compensation Plans
2020 Plan
On February 26, 2020, the Company established the 2020 Stock Incentive Plan (“2020 Plan”). The 2020 Plan permits the grant of options, appreciation rights, dividend equivalent right and restricted common stock of the Company (“Award”) to any person who is an employee or director of, or consultant to the Company. The maximum aggregate number of shares that may be issued pursuant to all Awards is 50,000,000 shares, plus an annual increase to be added on the first day of the calendar year beginning January 1, 2021 equal to (i) the greater of such number of shares as (A) will set the maximum number of shares that may be issued pursuant to all Awards equal to 15% of the number of Shares outstanding as of such date; or (B) 2% of the number of shares outstanding as of such date; or (ii) a lesser number of shares determined by the administrator of the 2020 Plan (“Administrator”) in good faith. The maximum aggregate number of shares available for grant of shares and/or incentive stock options shall be 25,000,000 shares, increased on the first day of the calendar year beginning January 1, 2021, in a number of Shares proportionate to the increase in the total number of shares that may be issued pursuant to all Awards under the Plan.
The Plan shall be administered by (A) the board of the directors of the Company (“Board”) or (B) a committee (“Committee”) designated by the Board, which Committee shall be constituted in such a manner as to satisfy the applicable laws and to permit such grants and related transactions under the Plan to be exempt from Section 16(b) of the Exchange Act in accordance with Rule 16b-3. Once appointed, such Committee shall continue to serve in its designated capacity until otherwise directed by the Board. The Board may at any time amend, suspend or terminate the Plan; provided, however, that no such amendment shall be made without the approval of the Company’s shareholders to the extent such approval is required by applicable laws.
The Company has yet to appoint the Administrator for the Plan and no Awards have yet to be granted under the Plan.
| 42 |
Board Stock Compensation Plan
On February 26, 2020, the Company established the Board Stock Compensation Plan (“Board Plan”) which provides compensation for non-executive Board members for participation in Board meetings retroactive to November 1, 2019. The Board Plan provides for a grant of $7,500 in equivalent shares of common stock (based on trading price at the end of the applicable current quarter) on the last day of each respective fiscal quarter that a member attends at least 75% of all meetings held during such quarter and in which a minimum of 1 meeting is held, for a maximum annual compensation amount of $30,000 per year per member. In addition, Board members that participate on future board committees will also be eligible to receive additional compensation for serving on such committees, in amounts to be determined by the Board. The maximum aggregate number of shares that are currently authorized to be issued pursuant to the Board Plan is 5,000,000 shares.
On April 15, 2020, the Company issued 236,808 shares of common stock to a non-executive Board member in accordance with the Board Plan.
Management and Consultants Performance Stock Plan
On April 25, 2020, the Company approved the adoption of the Management and Consultants Performance Stock Plan (“MCPP”) providing for the grant to current senior executive members of management and third-party consultants of an aggregate of approximately 205,000,000 shares of common stock of the Company (“Shares”) based on the achievement of certain defined operational performance milestones (“Milestones”).
On June 29, 2020, the Board amended the MCPP, providing for the additional grant of common stock of the Company to the current senior executive members of management and the current non-executive members of the Board based on the Company completing any transaction occurring while employed and/or serving as a member of the Board, respectively, that results in a change in control of the Company or any sale of substantially all the assets of the Company (“Transaction”) which upon after giving effect to such issuance of shares below, corresponds to a minimum pre-Transaction fully diluted price per share of the Company’s common stock in the amounts indicated below.
| Pre-Transaction Price Per Share Valuation (a) | Executive Bonus Shares Issued (b) | Non-executive Board Bonus Shares Issued (c) | ||||||||
| $ | 0.22 | 40,000,000 | 2,000,000 | |||||||
| $ | 0.34 | 60,000,000 | 3,000,000 | |||||||
| $ | 0.45 | 80,000,000 | 4,000,000 | |||||||
| $ | 0.54 | 100,000,000 | 5,000,000 | |||||||
| (a) | proforma for issuance of all shares to be issued pursuant to the MCPP and other in the money contingent share issuances |
| (b) | per each executive consisting of Albert Mitrani, Dr. Mari Mitrani, Ian Bothwell, and Dr. George Shapiro |
| (c) | per each non-executive Board member consisting of Dr. Allen Meglin and Michael Carbonara |
| 43 |
On August 14, 2020, the Board amended the MCPP, providing for the additional grant of common stock of the Company to each Dr. Maria I. Mitrani and Ian Bothwell based on the Company obtaining aggregate gross fundings (grants for research and development and clinical trials, purchase contracts for Company products, debt and/or equity financings) or other financial awards during the term of employment with the Company based on the amounts indicated below:
Aggregate Funding Amount | Shares | |||||||||
| From | To | |||||||||
| $ | 2,500,000 | $ | 5,000,000 | 5,000,000 | ||||||
| $ | 5,000,001 | $ | 10,000,000 | 10,000,000 | ||||||
| $ | 10,000,001 | $ | 30,000,000 | 30,000,000 | ||||||
On September 23, 2020, the Board amended the MCPP, providing for the grant of common stock of the Company of 15.0 million, 7.5 million and 15.0 million shares of common stock of the Company, respectively, to each Albert Mitrani, Dr. Maria I. Mitrani and Ian Bothwell upon such time that the Company’s common stock trades above $0.25 per share, $0.50 per share and $0.75 per share, respectively, for 30 consecutive trading days subsequent to March 31, 2021 and provided such milestone occurs during the term of employment with the Company.
In addition, each of the current executives were entitled to receive an additional 7 million shares, which when combined with all previous IND and/or eIND’s Milestones previously issued under the MCPP of 43 million shares, represents the total of all incentive shares to be issued to each executive in connection with the combined thirteen IND’s and/or eIND’s Milestones achieved through September 23, 2020. In the future, each of the current executives shall be entitled to receive 5 million shares as a performance incentive for each IND and/or “Expanded Access” approval (and excluding all eIND’s) received by the Company that involve more than 15 patients and provided such milestone occurs during the term of employment with the Company.
Pursuant to the MCPP, as of September 23, 2020, a total of 233,000,000 shares have been issued and approximately 582,500,000 shares are authorized to be issued under the MCPP subject to the achievement of the defined contingent performance based milestones described above and provided the milestones are achieved while the individual is employed and/or serving as a member of the Board:
| MCPP | MCPP | |||||||||||
| MCPP | Remaining | Total | ||||||||||
| Shares | Shares | Shares | ||||||||||
| Name | Awarded | Available | Approved | |||||||||
| Albert Mitrani | 50,000,000 | 137,500,000 | 187,500,000 | |||||||||
| Ian Bothwell | 50,000,000 | 167,500,000 | 217,500,000 | |||||||||
| Dr. Maria I. Mitrani | 50,000,000 | 167,500,000 | 217,500,000 | |||||||||
| Dr. George Shapiro | 50,000,000 | 100,000,000 | 150,000,000 | |||||||||
| Dr. Allen Meglin | – | 5,000,000 | 5,000,000 | |||||||||
| Michael Carbonara | – | 5,000,000 | 5,000,000 | |||||||||
| Consultants | 33,000,000 | – | 33,000,000 | |||||||||
| Total | 233,000,000 | 582,500,000 | 815,500,000 | |||||||||
| 44 |
| Plan category | Number
of securities to | Weighted-average | Number
of securities remaining | |||||||||
| 2020 Plan | -0- | -0- | 50,000,000 | |||||||||
| Board Stock Compensation Plan | -0- | -0- | 4,763,192 | |||||||||
| Management And Consultants Performance Stock Plan | -0- | -0- | 582,500,000 | |||||||||
Recent Sales of Unregistered Securities
| 1. | On February 5, 2019, the Company entered into an unsecured loan agreement with a third party with a principal balance of $25,000. The outstanding principal was due March 8, 2019. The loan was not repaid on the maturity date as required. The third party agreed to accept payment in kind consisting of certain products of the Company in lieu of cash interest. |
| 2. | On March 7, 2019, the Company sold an aggregate of 7,500,000 shares of common stock and granted warrants to purchase an aggregate 2,000,000 common shares to three “accredited investors” investors. The warrants had exercise prices of $0.08 and had a one -year term. The aggregate grant date fair value of the warrants issued in connection with these issuances were $6,600. The warrants expired on March 7, 2020. The proceeds were used for working capital. |
| 3. | During March 2019, the Company issued a $30,000 of convertible 6% debentures (“30,000 Debenture”) to one accredited investor. The principal amount of the $30,000 Debenture, plus accrued and unpaid interest through June 30, 2020 were payable on the 10th business day subsequent to June 30, 2020, unless the payment of the $30,000 Debenture was prepaid at the sole option of the Company, or was converted as provided for under the terms of the $30,000 Debenture, and/or accelerated due to an event of default in accordance with the terms of the $30,000 Debenture. |
During June 2019, the Company and the holder of the $30,000 Debenture agreed to convert the principal amount of the $30,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $30,478 into 1,111,111 shares of common stock of the Company (approximately $0.0274 per share representing a premium to the trading price of $0.0253 as of the effective date of the transaction).
| 4. | During April 2019, the Company sold 5,102,000 shares of common stock to seven “accredited investors” at $0.03 per share for an aggregate purchase price of $154,500. The proceeds were used for working capital. |
| 5. | During May 2019, the Company and holders of the $100,000 Debentures agreed to convert the principal amount of the $100,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $100,622 into 3,773,584 shares of common stock of the Company (approximately $0.0267 per share representing a discount to the trading price of $0.0285 as of the effective date of the transaction). |
| 6. | On May 1, 2019, the Company, Mint Organics and the holder of a promissory note issued by Mint Organics agreed to a settlement of the outstanding loan whereby the Company agreed to issue the holder of the note 2,735,000 shares of newly issued common stock of the Company. At the time of the settlement, the outstanding obligation under the note, including late fees and penalties was approximately $72,568. The common stock issued was priced at $0.0265 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction. |
| 45 |
| 7. | On May 1, 2019, the Company and Mint Organics entered into an exchange agreement whereby the Company agreed to acquire the 150 shares of Mint Series A Preferred Stock and the 150,000 warrants to purchase shares of common stock of the Company originally issued to Mr. Wayne Rohrbaugh in connection with the initial capitalization of Mint Organics (see note 15) in exchange for 4,400,000 shares of common stock of the Company (approximately $0.034 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction). |
| 8. | On May 1, 2019, the Company and Mint Organics Florida entered into an exchange agreement whereby the Company agreed to acquire the 21.25 units from the minority equity holder of Mint Organics Florida (see note 15) in exchange for 2,400,000 shares of common stock of the Company (approximately $0.042 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction). |
| 9. | During July 2019, the Company sold 2,500,000 shares of common stock to one “accredited investor” at $0.02 per share for an aggregate purchase price of $50,000. The proceeds were used for working capital. |
| 10. | During August 2019 through September 2019, the Company sold 5,250,000 shares of common stock to four “accredited investors” at $0.02 per share for an aggregate purchase price of $105,000. The proceeds were used for working capital. |
| 11. | On October 10, 2019, the Company and an investor (“Noteholder”) agreed to a funding facility arrangement (“Funding Facility”) whereby the Noteholder was required to fund the Company an initial tranche of $100,000 on October 15, 2019 (“Initial Funding Date”) and had the option to fund the Company up to an aggregate of $500,000 (“Funding Facility Limit”) in minimum $100,000 monthly tranches by no later than February 15, 2020 (“Funding Expiration Date”). The Funding Facility matures on February 15, 2021 (“Maturity Date”) and accrues interest at 6.0% per annum. The Funding Facility, plus all accrued interest, automatically converts into 40,000,000 shares of newly issued common stock of the Company if the Noteholder funds the full $500,000 by the Funding Expiration Date. The Noteholder fully funded the Funding Facility as prescribed on February 12, 2020 and the Company converted the Funding Facility into 40,000,000 shares of common stock of the Company that were issued to the Noteholders designated entity, Republic Asset Holdings LLC. |
On April 27, 2020, the Company sold 5,000,000 shares of common stock to Republic Asset Holdings LLC., a Company controlled by Michael Carbonara, a director of the Company, at $0.02 per share for an aggregate purchase price of $100,000. The proceeds were used for working capital.
| 12. | During November 2019 through January 2020, the Company sold 3,250,000 shares of common stock to three “accredited investors” at $0.02 per share for an aggregate purchase price of $65,000. The proceeds were used for working capital. |
| 13. | During February 2020 through April 2020, the Company sold 11,050,000 shares of common stock to five “accredited investors” at $0.02 per share for an aggregate purchase price of $221,000. The proceeds were used for working capital. |
| 14. | During April 2020 through May 2020, the Company sold 11,000,000 shares of common stock to Dr. Allen Meglin, a director of the Company at $0.02 per share for an aggregate purchase price of $220,000. During July and August 2020, the Company sold an additional 1,166,666 shares and 422,514 shares of common stock to Dr. Allen Meglin at $0.03 per share and $0.10 per share, respectively, for an aggregate purchase price of $77,251. The proceeds from all of the above sales were used for working capital. |
| 15. | During May 2020, the Company sold 3,000,000 shares of common stock to two “accredited investors” at $0.02 per share for an aggregate purchase price of $60,000. The proceeds were used for working capital. |
| 46 |
| 16. | During July and August 2020, the Company completed the private placement to 19 accredited investors for the sale of 13,499,992 shares of Common stock of the Company at a selling price of $0.03 per share for an aggregate amount of $405,000 (“Sale”). The proceeds are being used to fund the Company’s public company financial reporting requirements. |
| 17. | During the period July 2020, the Company sold 1,000,000 shares of common stock to two “accredited investors”, at $0.02 per share and $0.03 per share, respectively for an aggregate purchase price of $25,000. The proceeds were used for working capital. |
| 18. | During the period August 2020, the Company sold 8,606,665 shares of common stock to nine “accredited investors”, at prices ranging from $0.03 per share and $0.06 per share, for an aggregate purchase price of $392,100. The proceeds were used for working capital. |
| 19. | During the period September 2020, the Company sold 4,800,000 shares of common stock to five “accredited investors”, at prices ranging from $0.06 per share and $0.10 per share, for an aggregate purchase price of $410,000. The proceeds were used for working capital. |
None of the above issuances involved any underwriters, underwriting discounts or commissions, or any public offering and we believe were exempt from the registration requirements of the Securities Act of 1933, as amended (the “Securities Act”) by virtue of Section 4(a)(2) and Regulation D promulgated thereunder due to the fact that there was no solicitation or advertising and the did not involve a public offering of securities.
ITEM 6. SELECTED FINANCIAL DATA.
As a “smaller reporting company,” as defined by Item 10 of Regulation S-K, we are not required to provide the information required by this item of Form 10-K.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
You should read the following discussion together with our consolidated financial statements and the related notes included elsewhere in this report. This discussion contains forward-looking statements, which involve risks and uncertainties. Our actual results may differ materially from those we currently anticipate as a result of many factors, including the factors we describe under “Risk Factors” and elsewhere in this report.
Forward Looking Statements
Some of the information in this section contains forward-looking statements that involve substantial risks and uncertainties. You can identify these statements by forward-looking words such as “may,” “will,” “expect,” “anticipate,” “believe,” “estimate” and “continue,” or similar words. You should read statements that contain these words carefully because they:
| ● | discuss our future expectations; | |
| ● | contain projections of our future results of operations or of our financial condition; and | |
| ● | state other “forward-looking” information. |
We believe it is important to communicate our expectations. However, there may be events in the future that we are not able to accurately predict or over which we have no control. Our actual results and the timing of certain events could differ materially from those anticipated in these forward-looking statements as a result of certain factors, including those set forth under “Item 1. Business,” “Item 1A Risk Factors,” “Business” and elsewhere in this report.
| 47 |
COVID-19 Impact To Economy And Business Environment
The current outbreak of the novel coronavirus (“COVID-19”) and resulting impact to the United States economic environments began to take hold during March 2020. The adverse public health developments and economic effects of the COVID-19 outbreak in the United States, have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. These restrictions have adversely affected the Company’s sales, results of operations and financial condition.
There is no assurance as to when the adverse impact to the United States and worldwide economies resulting from the COVID-19 outbreak will be eliminated, if at all, and whether any new or recurring pandemic outbreaks will occur again in the future causing a similar or worse devastating impact to the United States and worldwide economies or our business.
Results of Operations
Fiscal year ended October 31, 2019 as compared to fiscal year ended October 31, 2018
Revenues
Our revenues for the year ended October 31, 2019 were $1,702,271, compared with revenues of $964,530 for the year ended October 31, 2018. The increase in revenues during the year ended October 31, 2019 of $737,741 (76.5%) was primarily the result of the Company being able to realize an increase of approximately 38.7% (approximately $373,166) in the average sales prices for the products sold during the year ended October 31, 2019 compared with the average sales prices realized on products sold during the year ended October 31, 2018 and the Company’s ability to increase unit sales of its products by 27.3% (approximately $364,575) during the year ended October 31, 2019 compared with the year ended October 31, 2018. The increase in the prices realized on product sold and units sold was partly attributable to favorable responses to the Company’s sales and marketing efforts establishing greater market awareness, less discounting of product prices to new customers, the introduction of new and more advanced product offerings and increased research and development efforts which provided customers with greater comfort in the company’s products and ability to better address potential market uncertainty regarding anticipated FDA regulations.
Cost of Revenues
Our cost of revenues for the year ended October 31, 2019 were $300,837, compared with cost of revenues of $209,298 for the year ended October 31, 2018 and increase of $91,539 (43.7%). The increase in cost of revenues during the year ended October 31, 2019 was primarily the result of the increase in the number of units sold amounting to $64,430 and increases in the average cost per unit sold amounting to $27,109 during the year ended October 31, 2019 compared with October 31, 2018. The costs per unit sold were significantly higher during the periods February 6, 2018 through April 2019 as those units sold were acquired through third party manufacturers. During the other periods, the Company’s costs of revenues were significantly lower as a result of manufacturing those products in-house.
Gross Profit
Our gross profit for the year ended October 31, 2019 was $1,401,434, compared with gross profit of $755,232 for the year ended October 31, 2018 an increase of $646,202 (85.6%). The increase in gross profit during the year ended October 31, 2019 was the result of higher sales prices received for products sold to its customers partially offset by higher costs of revenues for those products sold. The increase in the prices realized on product sold and units sold was partly attributable to favorable responses to the Company’s sales and marketing efforts establishing greater market awareness, less discounting of product prices to new customers, the introduction of new and more advanced product offerings and increased research and development efforts which provided customers with greater comfort in the company’s products and ability to better address potential market uncertainty regarding anticipated FDA regulations.
| 48 |
General and Administrative Expenses
General and administrative expenses for the year ended October 31, 2019 were $3,177,924, compared with $4,245,349 for the year ended October 31, 2018, a decrease of $1,067,425 (25.1%). The decrease in the general and administrative expenses for the year ended October 31, 2019 was primarily the result of reduced stock-based compensation costs to executives during the year ended October 31, 2019 totaling $3,296,580, decreases in bad debt reserves and escrow receivable reserves of $99,105 and reduced salaries of approximately $142,000 attributable to the resignation of certain executives in connection with the Sale and Taddeo settlement and reduced salaries under current executives employment agreements, partially offset from non-recurring reductions in payroll costs totaling approximately $1,063,083 resulting from the termination and/or restructuring of executive employments agreements in connection with the Sale and the Taddeo settlement and gains realized on the sale of the Anu assets of $824,798 during the year ended October 31, 2018 and increased commissions paid on sales of products of $108,598, increased marketing related costs of $288,937, $138,438 of increase laboratory related expenses and $56,313 of increased professional fees and office expenses during the year ended October 31, 2019 compared with the year ended October 31, 2018.
Other Income (Expense)
Other income, net, for the year ended October 31, 2019 was $38,191, compared with other income, net, of $58,694 for the year ended October 31, 2018, a decrease of $20,503. The net decrease in the other income was the result of reduced income realized on the reduction of derivative liabilities of $265,597, partially offset by increased income from the settlement of obligations of $63,367 and reduced interest costs and amortization of discounts associated with the SPA and other interest bearing obligations totaling $181,727.
Liquidity and Capital Resources
During the fiscal year ended October 31, 2019 and through the date of the filing of this Form 10-K, the Company has relied on the sale of debt or equity securities, the restructuring of debt obligations and/or the issuance and/or exchange of equity securities to meet the shortfall in cash to fund its operations.
| 1. | On February 5, 2019, the Company entered into an unsecured loan agreement with a third party with a principal balance of $25,000. The outstanding principal was due March 8, 2019. The loan was not repaid on the maturity date as required. The third party agreed to accept payment in kind consisting of certain products of the Company in lieu of cash interest. |
| 2. | On March 7, 2019, the Company sold an aggregate of 7,500,000 shares of common stock and granted warrants to purchase an aggregate 2,000,000 common shares to three “accredited investors” investors. The warrants had exercise prices of $0.08 and had a one -year term. The aggregate grant date fair value of the warrants issued in connection with these issuances were $6,600. The warrants expired on March 7, 2020. The proceeds were used for working capital. |
| 3. | During March 2019, the Company issued a $30,000 of convertible 6% debentures (“30,000 Debenture”) to one accredited investor. The principal amount of the $30,000 Debenture, plus accrued and unpaid interest through June 30, 2020 were payable on the 10th business day subsequent to June 30, 2020, unless the payment of the $30,000 Debenture was prepaid at the sole option of the Company, or was converted as provided for under the terms of the $30,000 Debenture, and/or accelerated due to an event of default in accordance with the terms of the $30,000 Debenture. |
During June 2019, the Company and the holder of the $30,000 Debenture agreed to convert the principal amount of the $30,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $30,478 into 1,111,111 shares of common stock of the Company (approximately $0.0274 per share representing a premium to the trading price of $0.0253 as of the effective date of the transaction).
| 4. | During April 2019, the Company sold 5,102,000 shares of common stock to seven “accredited investors” at $0.03 per share for an aggregate purchase price of $154,500. The proceeds were used for working capital. |
| 49 |
| 5. | During May 2019, the Company and holders of the $100,000 Debentures agreed to convert the principal amount of the $100,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $100,622 into 3,773,584 shares of common stock of the Company (approximately $0.0267 per share representing a discount to the trading price of $0.0285 as of the effective date of the transaction). |
| 6. | On May 1, 2019, the Company, Mint Organics and the holder of a promissory note issued by Mint Organics agreed to a settlement of the outstanding loan whereby the Company agreed to issue the holder of the note 2,735,000 shares of newly issued common stock of the Company. At the time of the settlement, the outstanding obligation under the note, including late fees and penalties was approximately $72,568. The common stock issued was priced at $0.0265 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction. |
| 7. | On May 1, 2019, the Company and Mint Organics entered into an exchange agreement whereby the Company agreed to acquire the 150 shares of Mint Series A Preferred Stock and the 150,000 warrants to purchase shares of common stock of the Company originally issued to Mr. Wayne Rohrbaugh in connection with the initial capitalization of Mint Organics (see note 15) in exchange for 4,400,000 shares of common stock of the Company (approximately $0.034 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction). |
| 8. | On May 1, 2019, the Company and Mint Organics Florida entered into an exchange agreement whereby the Company agreed to acquire the 21.25 units from the minority equity holder of Mint Organics Florida (see note 15) in exchange for 2,400,000 shares of common stock of the Company (approximately $0.042 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction). |
| 9. | During July 2019, the Company sold 2,500,000 shares of common stock to one “accredited investor” at $0.02 per share for an aggregate purchase price of $50,000. The proceeds were used for working capital. |
| 10. | During August 2019 through September 2019, the Company sold 5,250,000 shares of common stock to four “accredited investors” at $0.02 per share for an aggregate purchase price of $105,000. The proceeds were used for working capital. |
| 11. | On September 19, 2019, the Company’s wholly owned subsidiary, General Surgical Florida, received $100,000 in connection with an unsecured line of credit (“Credit Facility”). The Credit Facility matures in one-year and the Company is required to make 52 weekly payments of $2,403 (payments totaling $125,000). The Credit Facility can be prepaid at any time by the Company. The effective annual interest rate of the facility based on 52 equal monthly payments is 45.67% Proceeds received from the Credit Facility were used for working capital. |
| 12. | On October 10, 2019, the Company and an investor (“Noteholder”) agreed to a funding facility arrangement (“Funding Facility”) whereby the Noteholder was required to fund the Company an initial tranche of $100,000 on October 15, 2019 (“Initial Funding Date”) and had the option to fund the Company up to an aggregate of $500,000 (“Funding Facility Limit”) in minimum $100,000 monthly tranches by no later than February 15, 2020 (“Funding Expiration Date”). The Funding Facility matures on February 15, 2021 (“Maturity Date”) and accrues interest at 6.0% per annum. The Funding Facility, plus all accrued interest, automatically converts into 40,000,000 shares of newly issued common stock of the Company if the Noteholder funds the full $500,000 by the Funding Expiration Date. The Noteholder fully funded the Funding Facility as prescribed on February 12, 2020 and the Company converted the Funding Facility into 40,000,000 shares of common stock of the Company that were issued to the Noteholders designated entity, Republic Asset Holdings LLC. |
On April 27, 2020, the Company sold 5,000,000 shares of common stock to Republic Asset Holdings LLC., a Company controlled by Michael Carbonara, a director of the Company, at $0.02 per share for an aggregate purchase price of $100,000. The proceeds were used for working capital.
| 50 |
| 13. | During November 2019 through January 2020, the Company sold 3,250,000 shares of common stock to three “accredited investors” at $0.02 per share for an aggregate purchase price of $65,000. The proceeds were used for working capital. |
| 14. | During February 2020 through April 2020, the Company sold 11,050,000 shares of common stock to five “accredited investors” at $0.02 per share for an aggregate purchase price of $221,000. The proceeds were used for working capital. |
| 15. | During April 2020 through May 2020, the Company sold 11,000,000 shares of common stock to Dr. Allen Meglin, a director of the Company at $0.02 per share for an aggregate purchase price of $220,000. During July and August 2020, the Company sold an additional 1,166,666 shares and 422,514 shares of common stock to Dr. Allen Meglin at $0.03 per share and $0.10 per share, respectively, for an aggregate purchase price of $77,251. The proceeds from all of the above sales were used for working capital. |
| 16. | During May 2020, the Company sold 3,000,000 shares of common stock to two “accredited investors” at $0.02 per share for an aggregate purchase price of $60,000. The proceeds were used for working capital. |
| 17. | During July and August 2020, the Company completed the private placement to 19 accredited investors for the sale of 13,499,992 shares of Common stock of the Company at a selling price of $0.03 per share for an aggregate amount of $405,000 (“Sale”). The proceeds are being used to fund the Company’s public company financial reporting requirements. |
| 18. | During the period July 2020, the Company sold 1,000,000 shares of common stock to two “accredited investors”, at $0.02 per share and $0.03 per share, respectively for an aggregate purchase price of $25,000. The proceeds were used for working capital. |
| 19. | During the period August 2020, the Company sold 8,606,665 shares of common stock to nine “accredited investors”, at prices ranging from $0.03 per share and $0.06 per share, for an aggregate purchase price of $392,100. The proceeds were used for working capital. |
| 20. | During the period September 2020, the Company sold 4,800,000 shares of common stock to five “accredited investors”, at prices ranging from $0.06 per share and $0.10 per share, for an aggregate purchase price of $410,000. The proceeds were used for working capital. |
The Company issued the foregoing securities pursuant to the exemption from the registration requirements of the Securities Act afforded by Section 4(a)(2) of the Securities Act and/or Regulation D promulgated thereunder.
Going Concern Consideration
The accompanying consolidated financial statements have been prepared in conformity with generally accepted accounting principles, which contemplate continuation of the Company as a going concern. The Company has had limited revenues since its inception. The Company incurred operating losses of $1,776,490 for the year ended October 31, 2019. In addition, the Company had an accumulated deficit of $16,285,222 at October 31, 2019. The Company had a negative working capital position of $1,677,684 at October 31, 2019.
In addition to the above, the outbreak of the novel coronavirus (“COVID-19”) during March 2020 and the resulting adverse public health developments and economic effects to the United States business environments have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. These restrictions have adversely affected the Company’s sales, results of operations and financial condition. In response to the COVID-19 outbreak, the Company (a) has accelerated its research and development activities, particularly in regards to potential health benefits of the Company’s products in addressing various health concerns associated with COVID-19 and (b) is aggressively seeking to raise additional debt and/or equity financing to support working capital requirements until sale for its products to providers resumes to levels pre COVID-19.
| 51 |
As a result of the above, the Company’s efforts to establish a stabilized source of sufficient revenues to cover operating costs has yet to be achieved and ultimately may prove to be unsuccessful unless (a) the United States economy resumes to pre-COVID-19 conditions and (b) additional sources of working capital through operations or debt and/or equity financings are realized. These financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
Management anticipates that the Company will remain dependent, for the near future, on additional investment capital to fund ongoing operating expenses and the costs to perform required clinical studies in connection with the sale of its products. The Company does not have any assets to pledge for the purpose of borrowing additional capital. In addition, the Company relies on its ability to produce and sell products it manufactures that are subject to changing technology and regulations that it currently sells and distributes to its customers. The Company’s current market capitalization and common stock liquidity will hinder its ability to raise equity proceeds. The Company anticipates that future sources of funding, if any, will therefore be costly and dilutive, if available at all.
In view of the matters described in the preceding paragraphs, recoverability of the recorded asset amounts shown in the accompanying consolidated balance sheet assumes that (1) the effects of the COVID-19 crisis resume to pre-COVID 19 market conditions, (2) the Company will be able to establish a stabilized source of revenues, (3) obligations to the Company’s creditors are not accelerated, (4) the Company’s operating expenses remain at current levels and/or the Company is successful in restructuring and/or deferring ongoing obligations, (5) the Company is able to continue to produce products or obtain products under supply arrangements which are in compliance with current and future regulatory guidelines, (6) the Company is able to continue its research and development activities, particularly in regards to remaining compliant with the FDA and the safety and efficacy of its products, and (7) the Company obtains additional working capital to meet its contractual commitments and maintain the current level of Company operations through debt or equity sources.
There is no assurance as to when the adverse impact to the United States and worldwide economies resulting from the COVID-19 outbreak will be eliminated, if at all, and whether any new or recurring pandemic outbreaks will occur again in the future causing similar or worse devastating impact to the United States and worldwide economies and our business. In addition, there is no assurance that the Company will be able to complete its revenue growth strategy, its expected required research and development activities or otherwise obtain sufficient working capital to cover ongoing cash requirements. Without sufficient cash reserves, the Company’s ability to pursue growth objectives will be adversely impacted. Furthermore, despite significant effort since July 2015, the Company has thus far been unsuccessful in achieving a stabilized source of revenues. As described above, the COVID-19 crisis has significantly impaired the Company and the overall Unites States and World economies. If revenues do not increase and stabilize, if the COVID-19 crisis is not satisfactorily managed and/or resolved or if additional funds cannot otherwise be raised, the Company might be required to seek other alternatives which could include the sale of assets, closure of operations and/or protection under the U.S. bankruptcy laws. As of October 31, 2019, based on the factors described above, the Company concluded that there was substantial doubt about its ability to continue to operate as a going concern for the 12 months following the issuance of these financial statements.
Cash and Cash Equivalents
The following table summarizes the sources and uses of cash for the years stated. The Company held no cash equivalents for any of the periods presented.
| For the Fiscal Year Ended October 31, | ||||||||
| 2019 | 2018 | |||||||
| Cash, beginning of year | $ | 43,016 | $ | 39,560 | ||||
| Net cash used in operating activities | (565,454 | ) | (511,997 | ) | ||||
| Net cash provided by (used in) investing activities | (32,736 | ) | 95,453 | |||||
| Net cash provided by financing activities | 687,731 | 420,000 | ||||||
| Cash, end of year | $ | 132,557 | $ | 43,016 | ||||
| 52 |
During the year ended October 31, 2019, the Company used cash in operating activities of $565,454, compared to $511,997 for the year ended October 31, 2018, a reduction of in cash used of $53,457. The change in cash used in operating activities was due to a decrease in the net loss during the year ended October 31, 2019 resulting from increased revenues and gross margin combined with lower general and administrative expenses after adjusting for non-cash charges (mostly related to stock based compensation, bad debt expense, settlement of executive employment obligations, reduction in derivative liabilities and the gain from the sale of the Anu assets) and liabilities owed to executive management.
During the year ended October 31, 2019, the Company had cash used in investing activities of ($32,736), compared to cash provided by investing activities of $95,453 for the year ended October 31, 2018. The increase in the change in cash used in investing activities was due primarily to increased costs associated with acquisition of fixed assets and reduced proceeds received from the sale of the Anu assets of $140,022, and reduced expenditures associated with the purchase of minority interests in Mint Organics of $40,000.
During the year ended October 31, 2019, the Company had cash provided by financing activities of $687,731, compared to cash provided by financing activities of $420,000 for the year ended October 31, 2017, an overall increase of $267,731. The increase in cash provided by financing activities was due to increases in proceeds received in connection with the sale of equity securities of $359,500, partially offset from the decreases in the issuances of notes payable of $65,000 and payments on notes payable of $12,562 and capital lease obligations of $14,207.
Off-Balance Sheet Arrangements
Our liquidity is not dependent on the use of off-balance sheet financing arrangements (as that term is defined in Item 303(a) (4) (ii) of Regulation S-K) and as of October 31, 2019 and through the date of this report, we had no such arrangements.
New Accounting Pronouncements
In February 2016, a pronouncement was issued by the FASB that creates new accounting and reporting guidelines for leasing arrangements. The new guidance requires organizations that lease assets to recognize assets and liabilities on the balance sheet related to the rights and obligations created by those leases, regardless of whether they are classified as finance or operating leases. Consistent with current guidance, the recognition, measurement, and presentation of expenses and cash flows arising from a lease primarily will depend on its classification as a finance or operating lease. The guidance also requires new disclosures to help financial statement users better understand the amount, timing, and uncertainty of cash flows arising from leases. The new standard is effective for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period, with early application permitted. The new standard is to be applied using a modified retrospective approach. The Company does not expect that implementation of the new pronouncement will have a material impact to its financial statements.
Critical Accounting Policies
Our audited consolidated financial statements reflect the selection and application of accounting policies which require us to make significant estimates and judgments. See Note 2 to our audited consolidated financial statements included in this Annual Report on Form 10-K, “Summary of Significant Accounting Policies”.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
As a “smaller reporting company,” as defined by Item 10 of Regulation S-K, we are not required to provide the information required by this item of Form 10-K.
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
| 53 |
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| 54 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors of
Organicell Regenerative Medicine, Inc.
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of Organicell Regenerative Medicine, Inc. (the “Company”) as of October 31, 2019 and 2018, the related consolidated statements of operations, changes in stockholders’ deficit and cash flows for each of the two years in the period ended October 31, 2019, and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of October 31, 2019 and 2018, and the results of its operations and its cash flows for each of the two years in the period ended October 31, 2019, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph – Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As more fully described in Note 3, the Company has a significant working capital deficiency, has incurred significant losses and needs to raise additional funds to meet its obligations and sustain its operations. These conditions raise substantial doubt about the Company's ability to continue as a going concern. Management's plans in regard to these matters are also described in Note 3. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) ("PCAOB") and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company's internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provides a reasonable basis for our opinion.
/s/ Marcum llp
Marcum llp
We have served as the Company’s auditor since 2015
Fort Lauderdale, FL
October 15, 2020
| 55 |
Organicell Regenerative Medicine, Inc.
As of October 31, 2019 and 2018
| October 31, | October 31, | |||||||
| ASSETS | 2019 | 2018 | ||||||
| Current Assets | ||||||||
| Cash | $ | 132,557 | $ | 43,016 | ||||
| Accounts receivable, net of allowance for bad debts | 26,031 | 48,025 | ||||||
| Prepaid expenses | 121,394 | 15,221 | ||||||
| Inventories | 77,963 | – | ||||||
| Total Current Assets | 357,945 | 106,262 | ||||||
| Property and equipment, net | 263,315 | 5,778 | ||||||
| Security deposits | 5,000 | 5,000 | ||||||
| TOTAL ASSETS | $ | 626,260 | $ | 117,040 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current Liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 552,426 | $ | 476,833 | ||||
| Accrued liabilities to management | 852,706 | 327,662 | ||||||
| Notes payable | 212,438 | 60,000 | ||||||
| Capital lease obligations | 72,208 | – | ||||||
| Convertible debentures | 220,000 | 320,000 | ||||||
| Deferred revenue | – | 21,520 | ||||||
| Liabilities attributable to discontinued operations | 125,851 | 125,851 | ||||||
| Total Current Liabilities | 2,035,629 | 1,331,866 | ||||||
Long term capital lease obligations
| 153,180 | – | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ Deficit | ||||||||
| Common stock, $0.001 par value, 1,500,000,000 shares authorized; 502,936,805 and 436,490,110 shares issued and outstanding, respectively | 502,937 | 436,490 | ||||||
| Additional paid-in capital | 14,219,736 | 12,853,608 | ||||||
| Accumulated deficit | (16,285,222 | ) | (14,547,901 | ) | ||||
| Total stockholders’ deficit attributable to Organicell Regenerative Medicine, Inc. | (1,562,549 | ) | (1,257,803 | ) | ||||
| Non-controlling interest | – | 42,977 | ||||||
| Total Stockholders’ Deficit | (1,562,549 | ) | (1,214,826 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 626,260 | $ | 117,040 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 56 |
Organicell Regenerative Medicine, Inc.
CONSOLIDATED STATEMENTS OF OPERATIONS
For the Years Ended October 31, 2019 and 2018
| Year Ended October 31, | ||||||||
| 2019 | 2018 | |||||||
| Revenues | $ | 1,702,271 | $ | 964,530 | ||||
| Cost of revenues | 300,837 | 209,298 | ||||||
| Gross profit | 1,401,434 | 755,232 | ||||||
| General and administrative expenses | 3,177,924 | 4,245,349 | ||||||
| Loss from operations | (1,776,490 | ) | (3,490,117 | ) | ||||
| Other income (expense) | ||||||||
| Interest expense | (46,600 | ) | (228,327 | ) | ||||
| Reduction of derivative liabilities | – | 265,597 | ||||||
| Other | 84,791 | 21,434 | ||||||
| Loss before income taxes | (1,738,299 | ) | (3,431,413 | ) | ||||
| Provision for income taxes | – | – | ||||||
| Net loss | (1,738,299 | ) | (3,431,413 | ) | ||||
| Net income (loss) attributable to the non-controlling interest | (978 | ) | 30,745 | |||||
| Net loss attributable to Organicell Regenerative Medicine, Inc. | $ | (1,737,321 | ) | $ | (3,462,158 | ) | ||
| Net loss per common share - basic and diluted | $ | (0.00 | ) | $ | (0.01 | ) | ||
| Weighted average number of common shares outstanding - basic and diluted | 466,984,320 | 271,809,401 | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 57 |
Organicell Regenerative Medicine, Inc.
CONSOLIDATED CHANGES TO STOCKHOLDERS’ DEFICIT
For the Years Ended October 31, 2019 and 2018
| Preferred Stock | Additional | Total Stockholders’ Deficit Attributable | Non- | Total | |||||||||||||||||||||||||||||
| Series A | Series B | Common Stock | Paid In | Accumulated | To | Controlling | Stockholders’ | ||||||||||||||||||||||||||
| Shares | Par Value | Shares | Par Value | Shares | Par Value | Capital | Deficit | Organicell | Interest | Deficit | |||||||||||||||||||||||
| Balance October 31, 2017 | 400 | $ | – | – | $ | – | 111,464,987 | $ | 111,465 | $ | 7,417,321 | $ | (11,085,743 | ) | $ | (3,556,957 | ) | $ | 52,744 | $ | (3,504,213 | ) | |||||||||||
| Cancellation of preferred stock in connection with Reorganization | (400 | ) | – | – | – | – | – | – | – | – | – | – | |||||||||||||||||||||
| Acquisition of non-controlling interest | – | – | – | – | – | – | 3,658 | – | 3,658 | (43,658 | ) | (40,000 | ) | ||||||||||||||||||||
| Proceeds from sale of common stock | – | – | – | – | 6,250,050 | 6,250 | 93,750 | – | 100,000 | – | 100,000 | ||||||||||||||||||||||
| Exercise of cashless warrants | – | – | – | – | 70,382,456 | 70,382 | (70,382 | ) | – | – | – | – | |||||||||||||||||||||
| Stock-based compensation | – | – | – | – | 248,392,617 | 248,393 | 3,772,453 | – | 4,020,846 | 3,146 | 4,023,992 | ||||||||||||||||||||||
| Executive forgiveness of employment obligations in connection with Reorganization | – | – | – | – | – | – | 1,636,808 | – | 1,636,808 | – | 1,636,808 | ||||||||||||||||||||||
| Net income (loss) | – | – | – | – | – | – | – | (3,462,158 | ) | (3,462,158 | ) | 30,745 | (3,431,413 | ) | |||||||||||||||||||
| Balance October 31, 2018 | – | – | – | – | 436,490,110 | 436,490 | 12,853,608 | (14,547,901 | ) | (1,257,803 | ) | 42,977 | (1,214,826 | ) | |||||||||||||||||||
| Proceeds from sale of common stock | – | – | – | – | 20,352,000 | 20,352 | 439,148 | – | 459,500 | – | 459,500 | ||||||||||||||||||||||
| Exchange of debt obligations | – | – | – | – | 7,619,695 | 7,620 | 196,044 | – | 203,664 | – | 203,664 | ||||||||||||||||||||||
| Stock-based compensation | – | – | – | – | 31,675,000 | 31,675 | 695,737 | – | 727,412 | – | 727,412 | ||||||||||||||||||||||
| Acquisition of non-controlling interests | – | – | – | – | 6,800,000 | 6,800 | 35,199 | – | 41,999 | (41,999 | ) | – | |||||||||||||||||||||
| Net income (loss) | – | – | – | – | – | – | – | (1,737,321 | ) | (1,737,321 | ) | (978 | ) | (7,738,299 | ) | ||||||||||||||||||
| Balance October 31, 2019 | – | $ | – | – | $ | – | 502,936,805 | $ | 502,937 | $ | 14,219,736 | $ | (16,285,222 | ) | $ | (1,562,549 | ) | $ | – | $ | (1,562,549 | ) | |||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 58 |
Organicell Regenerative Medicine, Inc.
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended October 31, 2019 and 2018
| Year Ended October 31, | ||||||||
| 2019 | 2018 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net loss | $ | (1,738,299 | ) | $ | (3,431,413 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation expense | 14,794 | 7,835 | ||||||
| Bad debt expense | 10,635 | 62,240 | ||||||
| Allowance for escrow receivable reserve | – | 47,500 | ||||||
| Stock-based compensation | 727,412 | 4,023,992 | ||||||
| Interest expense paid in kind | 13,668 | – | ||||||
| Amortization of debt discount | – | 179,968 | ||||||
| Settlement of executive employment obligations | – | (1,063,083 | ) | |||||
| Gain on sale of Anu assets | – | (821,070 | ) | |||||
| Reduction of derivative liabilities | – | (265,597 | ) | |||||
| Changes in operating assets and liabilities: | ||||||||
| Accounts receivable, net of allowance for bad debts | 11,359 | 4,695 | ||||||
| Prepaid expenses | (106,173 | ) | (8,217 | ) | ||||
| Inventories | (77,963 | ) | 60,319 | |||||
| Accounts payable and accrued expenses | 75,589 | 64,789 | ||||||
| Accrued liabilities to management | 525,044 | 686,578 | ||||||
| Deferred rent | – | 1,948 | ||||||
| Deferred revenue | (21,520 | ) | (62,480 | ) | ||||
| Net cash used in operating activities | (565,454 | ) | (511,997 | ) | ||||
| CASH FLOWS FROM INVESTING | ||||||||
| Purchase of fixed assets | (32,736 | ) | (4,569 | ) | ||||
| Purchase of non-controlling interests in Mint Organics | – | (40,000 | ) | |||||
| Proceeds from the sale of Anu assets | – | 140,022 | ||||||
| Net cash provided by (used in) investing activities | (32,736 | ) | 95,453 | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Proceeds from issuance of notes payable & debentures | 255,000 | 320,000 | ||||||
| Payments on notes payables | (12,562 | ) | – | |||||
| Payments on capital leases | (14,207 | ) | – | |||||
| Proceeds from sale of common stock and warrants | 459,500 | 100,000 | ||||||
| Net cash provided by financing activities | 687,731 | 420,000 | ||||||
| Increase in cash | 89,541 | 3,456 | ||||||
| Cash at beginning of year | 43,016 | 39,560 | ||||||
| Cash at end of year | $ | 132,557 | $ | 43,016 | ||||
SUPPLEMENTAL CASH FLOW INFORMATION: | ||||||||
| Cash paid for taxes | $ | – | $ | – | ||||
| Cash paid for interest | $ | 20,165 | $ | 32,534 | ||||
| NON-CASH INVESTING AND FINANCING TRANSACTIONS: | ||||||||
| Executive forgiveness of employment obligations in connection with Reorganization | $ | – | $ | 1,636,808 | ||||
| Outstanding SPA and other obligations satisfied in connection with the Sale | $ | – | $ | 809,978 | ||||
| Capital lease obligations | $ | 239,595 | $ | – | ||||
| Conversion of debt into common stock | $ | 203,668 | $ | – | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 59 |
ORGANICELL REGENERATIVE MEDICINE, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – ORGANIZATION AND DESCRIPTION OF BUSINESS
Organicell Regenerative Medicine, Inc. (formerly Biotech Products Services and Research, Inc.) (“Organicell” or the “Company”) was incorporated on August 9, 2011 in the State of Nevada. Until October 30, 2015, the Company’s business included the designing, manufacturing, and selling vending tricycles for commercial customers. Since June 2015, the Company has been engaged in the health care industry, principally focusing on supplying products and services related to the growing field of regenerative anti-aging medicine.
On April 23, 2018, the Company and Management and Business Associates, LLC, a Florida limited liability company (“MBA”), executed a Plan and Agreement of Reorganization (“Reorganization”), whereby the Company issued to MBA an aggregate of 222,425,073 shares of its common stock of the Company, representing at the time 51% of the outstanding shares of common stock of the Company on fully-diluted basis, for $0.001 per share (or an aggregate of $222,425), in consideration for Mr. Manuel Iglesias’ agreement to serve as the Company’s Chief Executive Officer (“CEO”) and a member of the Board of the Company. The Reorganization was effective as of April 13, 2018 (“Effective Date”). The Reorganization also provided for the cancelation and termination of the Company’s previously issued and outstanding Series A Preferred Stock and Series B Preferred Stock. As a result of the above Reorganization, MBA acquired at the time a controlling interest of the Company (see Note 5).
On May 21, 2018, the Company filed a Certificate of Amendment with the Secretary of State of Nevada to change the Company’s name from Biotech Products Services and Research, Inc. to Organicell Regenerative Medicine, Inc., effective June 20, 2018 (the “Name Change”). As discussed in Note 12, the Name Change has not yet been effectuated in the marketplace by the Financial Industry Regulatory Agency (“FINRA”).
For the year ended October 31, 2019, the Company principally operated through General Surgical of Florida, Inc., a Florida corporation (“General Surgical”) and wholly owned subsidiary, with a business purpose to sell cellular therapy products to doctors and hospitals.
During the year ended October 31, 2019, the Company revenues were principally derived from the sale and distribution of regenerative biologic therapies based on amnion placental tissue derived products to doctors and hospitals. For the period November 1, 2018 through April 2019, the Company sold products produced and supplied through third party supply agreements. During February 2019, the Company began arranging to operate a new laboratory facility for the purpose of performing research and development, production and manufacturing of anti-aging and cellular therapy products. This new laboratory facility became operational in May 2019 and during the same period, the Company began producing and distributing the products that are being sold to its customers.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The consolidated financial statements include the accounts of the Company and its wholly-owned and majority owned subsidiaries. All significant intercompany accounts and transactions have been eliminated.
| 60 |
Concentrations of Credit Risk
The balance sheet items that potentially subject us to concentrations of credit risk are primarily cash and cash equivalents and accounts receivable. Balances in accounts are insured up to Federal Deposit Insurance Corporation (“FDIC”) limits of $250,000 per institution. At October 31, 2019, the Company did not have any cash balances in financial institutions in excess of FDIC insurance coverage.
During the fiscal year ended October 31, 2019, the Company had one customer that accounted for approximately $206,400 of revenues (12.2%). No other customer accounted for more than 10% of the total revenues for the year ended October 31, 2019.
During the period November 1, 2018 through April 30, 2019, the Company purchased finished goods inventory that was sold to customers from two suppliers, of which each accounted for approximately $29,000 and $65,000 or 31.0% and 69.0%, respectively, of the total amount of finished goods inventory purchased during that period.
During the May 1, 2019 through October 31, 2019, the Company purchased the tissue raw material used in manufacturing of its products from two suppliers, of which each accounted for approximately $61,000 and $47,500 or 56.0% and 44.0%, respectively, of the total amount of tissue raw material purchased during that period.
The Company’s sales and supply agreements are non-exclusive and the Company does not believe it has any exposure based on the customers of its products and/or the availability of raw materials and/or products from other suppliers. Since May 1, 2019, the Company manufactured and distributed proprietary products that reduce exposure from the reliance on third party suppliers of inventory but increased exposure of reliance on raw materials and other supplies used in the manufacturing of its products.
Use of Estimates
The preparation of financial statements in conformity with generally accepted accounting principles of the United States requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the year. Management bases its estimates on historical experience and on other assumptions considered to be reasonable under the circumstances. However, actual results may differ from the estimates.
Cash Equivalents
The Company considers all highly liquid investments with maturities of three months or less when purchased to be cash equivalents.
Accounts Receivable
Accounts receivable are recorded at fair value on the date revenue is recognized. The Company provides allowances for doubtful accounts for estimated losses resulting from the inability of its customers to repay their obligation. If the financial condition of the Company's customers were to deteriorate, resulting in an impairment of their ability to repay, additional allowances may be required. The Company provides for potential uncollectible accounts receivable based on specific customer identification and historical collection experience adjusted for existing market conditions.
The policy for determining past due status is based on the contractual payment terms of each customer, which are generally net 30 or net 60 days. Once collection efforts by the Company and its collection agency are exhausted, the determination for charging off uncollectible receivables is made. For the year ended October 31, 2019 and 2018, the Company recorded bad debt expense of $10,635 and $62,420, respectively.
| 61 |
Inventory
Inventory is stated at the lower of cost or net realizable value using the average cost method. We provide reserves for potential excess, dated or obsolete inventories based on an analysis of forecasted demand compared to quantities on hand and any firm purchase orders, as well as product shelf life. At October 31, 2019, we determined that there were not any reserves required in connection with our finished goods.
Property and Equipment
Property and equipment are stated at cost. Depreciation and amortization are provided using the straight-line method over the estimated useful lives of the related assets. The estimated useful lives of property and equipment range from 3 to 15 years. Upon sale or retirement, the cost and related accumulated depreciation and amortization are eliminated from their respective accounts, and the resulting gain or loss is included in results of operations. Repairs and maintenance charges, which do not increase the useful lives of the assets, are charged to operations as incurred.
Revenue Recognition
Effective November 1, 2018, the Company adopted FASB Accounting Standards Update (“ASU”) Topic 606 “Revenue from Contracts with Customers” which requires the Company to recognize revenue in amounts that reflect the prorata completion of the performance obligations of the Company required under the contracts. The Company applied the new standard using a modified retrospective approach.
The Company recognizes revenue only when it transfers control of a promised good or service to a customer in an amount that reflects the consideration it expects to receive in exchange for the good or service. Our performance obligations are satisfied and control is transferred at a point-in-time, which is typically when the transfer and title to the product sold has taken place and there is evidence of our customer’s satisfactory acceptance of the product shipment or delivery. Due to the nature of the Company’s sales transactions, this adoption did not have any impact to the Company’s financial statements for the year ended October 31, 2019.
Net Income (Loss) Per Common Share
Basic income (loss) per common share is calculated by dividing the Company's net loss applicable to common shareholders by the weighted average number of common shares during the period. Diluted earnings per share is calculated by dividing the Company's net income available to common shareholders by the diluted weighted average number of shares outstanding during the year. The diluted weighted average number of shares outstanding is the basic weighted number of shares adjusted for any potentially dilutive debt or equity.
At October 31, 2019, the Company had 4,529,371 common shares issuable upon the exercise of warrants that were not included in the computation of dilutive loss per share because their inclusion is anti-dilutive for the year ended October 31, 2019. At October 31, 2018, the Company had 3,647,484 common shares issuable upon the exercise of warrants that were not included in the computation of dilutive loss per share because their inclusion is anti-dilutive for the year ended October 31, 2018.
Stock-Based Compensation
All stock-based payments to employees, including grants of employee stock options, are recognized in the financial statements based on their fair values.
Stock options and warrants issued to consultants and other non-employees as compensation for services provided to the Company are accounted for based upon the estimated fair value of the option or warrant.
| 62 |
Income Taxes
The Company is required to file a consolidated tax return that includes all of its subsidiaries.
Provisions for income taxes are based on taxes payable or refundable for the current year taxable income for federal and state income tax reporting purposes and deferred income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax basis and operating loss carryforwards. Deferred income tax expense represents the change during the period in the deferred tax assets and deferred tax liabilities. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of the operations in the period that includes the enactment date. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some or all of the deferred tax assets will not be realized.
The Company accounts for uncertain tax positions in accordance with FASB Topic 740 – Income Taxes. This pronouncement prescribes a recognition threshold and measurement process for financial statement recognition of uncertain tax positions taken or expected to be taken in a tax return. The interpretation also provides guidance on recognition, derecognition, classification, interest and penalties, accounting in interim period, disclosure and transition.
For the year ended October 31, 2019 the Company incurred operating losses, and therefore, there was not any income tax expense amount recorded during that period. During the year ended October 31, 2018 there was a change in ownership which caused a change in control under IRC Section 382 (“Section 382 event”). Prior to the Section 382 event, the Company utilized a portion of its available net operating loss carryforwards to offset income through that date mainly resulting from the sale of ANU. Any remaining net operating losses which had been carried forward from years ended October 31, 2017 and before the Section 382 event were lost. There is a full valuation allowance for years ended October 31, 2019 and 2018.
Since January 1, 2018, the nominal corporate tax rate in the United States of America is 21 percent due to the passage of the "Tax Cuts and Jobs Act" on December 20, 2017 by the US Senate and House of Representatives.
Valuation of Derivatives
The Company evaluates its convertible instruments, options, warrants or other contracts to determine if those contracts or embedded components of those contracts qualify as derivatives to be separately accounted for under ASC Topic 815, “Derivatives and Hedging.” The result of this accounting treatment is that the fair value of the derivative is marked-to-market each balance sheet date and recorded as a liability. In the event that the fair value is recorded as a liability, the change in fair value is recorded in the statement of operations as other income (expense). Upon conversion or exercise of a derivative instrument, the instrument is marked to fair value at the conversion date and then that fair value is reclassified to equity. Equity instruments that are initially classified as equity that become subject to reclassification under ASC Topic 815 are reclassified to liabilities at the fair value of the instrument on the reclassification date. We analyzed the derivative financial instruments in accordance with ASC 815.
The Company utilized Monte Carlo Simulation models that value the derivative liability based on a probability weighted discounted cash flow model. The Company utilized the fair value standard set forth by the Financial Accounting Standards Board, defined as the amount at which the assets (or liability) could be bought (or incurred) or sold (or settled) in a current transaction between willing parties, that is, other than in a forced or liquidation sale.
The derivative liabilities result in a reduction of the initial carrying amount (as unamortized discount) of the Convertible Notes. This derivative liability is marked-to-market each quarter with the change in fair value recorded in the income statement. Unamortized discount is amortized to interest expense using the effective interest method over the life of the Convertible Note.
| 63 |
Fair Value of Financial Instruments
The Company includes fair value information in the notes to financial statements when the fair value of its financial instruments is different from the book value. When the book value approximates fair value, no additional disclosure is made.
The Company follows FASB ASC 820, Fair Value Measurements and Disclosures, which defines fair value, establishes a framework for measuring fair value and enhances disclosures about fair value measurements. It defines fair value as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. The Company’s financial instruments consist of cash and cash equivalents, accounts payable, accrued liabilities and convertible debt. The estimated fair value of cash, accounts payable and accrued liabilities approximate their carrying amounts due to the short-term nature of these instruments.
The Company follows the provisions of ASC 820 with respect to its financial instruments. As required by ASC 820, assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to their fair value measurement. The Company’s convertible features associated with its promissory notes (see Note 9) which were required to be measured at fair value on a recurring basis under of ASC 815 as of January 31, 2018, the date immediately prior to the event that eliminated the convertible instrument related to the derivative liability, and October 31, 2017, were all measured at fair value using Level 3 inputs. Level 3 inputs are unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities as of January 31, 2018 and October 31, 2017:
Level one — Quoted market prices in active markets for identical assets or liabilities;
Level two — Inputs other than level one inputs that are either directly or indirectly observable such as quoted prices for similar assets or liabilities, quoted prices in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; and
Level three — Unobservable inputs that are supported by little or no market activity and developed using estimates and assumptions, which are developed by the reporting entity and reflect those assumptions that a market participant would use.
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
Determining which category an asset or liability falls within the hierarchy requires significant judgment. The Company evaluates its hierarchy disclosures each quarter. The Company’s derivative liability is measured at fair value on a recurring basis. The Company classifies the fair value of the derivative liability under level three.
Based on ASC Topic 815 and related guidance, the Company concluded the common stock issuable pursuant to the conversion features of the convertible promissory notes are required to be accounted for as derivatives as of the issue date due to a reset feature on the exercise price. At the date of issuance common stock derivative liabilities were measured at fair value using either quoted market prices of financial instruments with similar characteristics or other valuation techniques. The Company records the fair value of these derivatives on its balance sheet at fair value with changes in the values of these derivatives reflected in the consolidated statements of operations as “change in fair value of derivative liabilities.” These derivative instruments are not designated as hedging instruments under ASC 815-10 and are disclosed on the balance sheet under Derivative Liabilities.
Further, and in accordance with ASC 815, the embedded derivatives are revalued using a Monte Carlo Simulation model at issuance and at each balance sheet date and marked to fair value with the corresponding adjustment as a “gain or loss on change in fair values” in the consolidated statement of operations.
| 64 |
The Company classifies the fair value of these securities under level three of the fair value hierarchy of financial instruments. Changes in the unobservable input values would likely cause material changes in the fair value of the Company’s Level 3 financial instruments.
During the year ended October 31, 2018, the Company recorded a gain of $265,597 associated with the change in fair value of the derivative liabilities from October 31, 2017.
The Company did not have any convertible instruments outstanding at October 31, 2019 and 2018 that qualify as derivatives.
Subsequent Events
The Company has evaluated subsequent events that occurred after October 31, 2019 through the financial statement issuance date for subsequent event disclosure consideration.
New Accounting Pronouncements
In February 2016, a pronouncement was issued by the FASB that creates new accounting and reporting guidelines for leasing arrangements. The new guidance requires organizations that lease assets to recognize assets and liabilities on the balance sheet related to the rights and obligations created by those leases, regardless of whether they are classified as finance or operating leases. Consistent with current guidance, the recognition, measurement, and presentation of expenses and cash flows arising from a lease primarily will depend on its classification as a finance or operating lease. The guidance also requires new disclosures to help financial statement users better understand the amount, timing, and uncertainty of cash flows arising from leases. The new standard is effective for annual reporting periods beginning after December 15, 2018, including interim periods within that reporting period, with early application permitted. The new standard is to be applied using a modified retrospective approach. The Company does not expect that implementation of the new pronouncement will have a material impact to its financial statements.
NOTE 3 – GOING CONCERN
The accompanying consolidated financial statements have been prepared in conformity with generally accepted accounting principles, which contemplate continuation of the Company as a going concern. The Company has had limited revenues since its inception. The Company incurred operating losses of $1,776,490 for the year ended October 31, 2019. In addition, the Company had an accumulated deficit of $16,285,222 at October 31, 2019. The Company had a negative working capital position of $1,677,684 at October 31, 2019.
In addition to the above, the outbreak of the novel coronavirus (“COVID-19”) during March 2020 and the resulting adverse public health developments and economic effects to the United States business environments have adversely affected the demand for our products and services by our customers and from patients of our customers as a result of quarantines, facility closures and social distancing measures put into effect in connection with the COVID-19 outbreak and which currently still continue to have a negative impact to our business and the economy. These restrictions have adversely affected the Company’s sales, results of operations and financial condition. In response to the COVID-19 outbreak, the Company (a) has accelerated its research and development activities, particularly in regards to potential health benefits of the Company’s products in addressing various health concerns associated with COVID-19 and (b) is aggressively seeking to raise additional debt and/or equity financing to support working capital requirements until sale for its products to providers resumes to levels pre COVID-19.
As a result of the above, the Company’s efforts to establish a stabilized source of sufficient revenues to cover operating costs has yet to be achieved and ultimately may prove to be unsuccessful unless (a) the United States economy resumes to pre-COVID-19 conditions and (b) additional sources of working capital through operations or debt and/or equity financings are realized. These financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern.
| 65 |
Management anticipates that the Company will remain dependent, for the near future, on additional investment capital to fund ongoing operating expenses and the costs to perform required clinical studies in connection with the sale of its products. The Company does not have any assets to pledge for the purpose of borrowing additional capital. In addition, the Company relies on its ability to produce and sell products it manufactures that are subject to changing technology and regulations that it currently sells and distributes to its customers. The Company’s current market capitalization and common stock liquidity will hinder its ability to raise equity proceeds. The Company anticipates that future sources of funding, if any, will therefore be costly and dilutive, if available at all.
In view of the matters described in the preceding paragraphs, recoverability of the recorded asset amounts shown in the accompanying consolidated balance sheet assumes that (1) the effects of the COVID-19 crisis resume to pre-COVID 19 market conditions, (2) the Company will be able to establish a stabilized source of revenues, (3) obligations to the Company’s creditors are not accelerated, (4) the Company’s operating expenses remain at current levels and/or the Company is successful in restructuring and/or deferring ongoing obligations, (5) the Company is able to continue to produce products or obtain products under supply arrangements which are in compliance with current and future regulatory guidelines, (6) the Company is able to continue its research and development activities, particularly in regards to remaining compliant with the FDA and the safety and efficacy of its products, and (7) the Company obtains additional working capital to meet its contractual commitments and maintain the current level of Company operations through debt or equity sources.
There is no assurance as to when the adverse impact to the United States and worldwide economies resulting from the COVID-19 outbreak will be eliminated, if at all, and whether any new or recurring pandemic outbreaks will occur again in the future causing similar or worse devastating impact to the United States and worldwide economies and our business. In addition, there is no assurance that the Company will be able to complete its revenue growth strategy, its expected required research and development activities or otherwise obtain sufficient working capital to cover ongoing cash requirements. Without sufficient cash reserves, the Company’s ability to pursue growth objectives will be adversely impacted. Furthermore, despite significant effort since July 2015, the Company has thus far been unsuccessful in achieving a stabilized source of revenues. As described above, the COVID-19 crisis has significantly impaired the Company and the overall Unites States and World economies. If revenues do not increase and stabilize, if the COVID-19 crisis is not satisfactorily managed and/or resolved or if additional funds cannot otherwise be raised, the Company might be required to seek other alternatives which could include the sale of assets, closure of operations and/or protection under the U.S. bankruptcy laws. As of October 31, 2019, based on the factors described above, the Company concluded that there was substantial doubt about its ability to continue to operate as a going concern for the 12 months following the issuance of these financial statements.
NOTE 4 – SALE AND TRANSFER OF ANU MANUFACTURING ASSETS
Effective February 5, 2018 (“Closing Date”), Vera Acquisition LLC, a Utah limited liability company ("Vera"), Organicell, ANU and General Surgical, executed an Asset Purchase Agreement ("Purchase Agreement") pursuant to which ANU sold to Vera (“Sale”) their right, title and interest in certain tangible and other assets associated with its manufacturing operations, including prepaid expenses, raw and finished goods inventory, a long term lease for ANU’s laboratory facility in Sunrise, Florida (including associated security deposits), furniture and equipment, and certain intellectual property rights. General Surgical transferred its rights to certain third-party distribution agreements between General Surgical and distributors of products manufactured by ANU (“Sold Assets”) in exchange for a cash payment of $950,000 and the execution of a long-term distribution agreement with Organicell (“Organicell Distribution Agreement”) described below. In connection with the Sale, Vera received credit for $100,000 previously paid to ANU for prepaid product supply that was not yet delivered to Vera as of the Closing Date.
In connection with the Sale, the Company was required to use cash proceeds from the Sale to satisfy and extinguish all of the Notes outstanding related to the SPA as of the date of the Sale, totaling approximately $762,477 (comprised of $527,778 of face value of the Notes outstanding, $8,589 of accrued and unpaid interest from January 1, 2018 through the date of the Sale, $211,111 of prepayment penalties and $15,000 for reimbursement of legal fees), which were secured by a first priority lien on all of the Company’s assets, and to be used to pay all of ANU’s remaining trade accounts payable outstanding as of the Closing Date. In addition, the Purchase Agreement required ANU to fund the placental donor tissue costs that were required by Vera to process additional product subsequent to the Closing to replace the shortfall of the actual inventory product amounts as of the Closing Date and the specified inventory quantities provided for in the Purchase Agreement. The Purchase Agreement also required ANU to escrow $47,500 (5%) of the cash purchase price and for General Surgical to escrow, subsequent to the Closing Date, up to $47,500 from collections of accounts receivable that were existing as of the Closing Date for a period of 90 days subsequent to the Closing Date to cover pre-closing related liabilities of ANU that were not identified as of the Closing Date, if any, and other obligations of ANU associated with the Purchase Agreement.
| 66 |
Effective upon the closing of the Sale, Dr. Werber and Mr. Suddarth each entered into a separation and general release agreement with the Company, which provided for the immediate resignation of Dr. Werber and Mr. Suddarth of all their respective executive and board of director positions held with Organicell and/or any of Organicell’s subsidiaries, and settlement of all obligations of each party to the other pursuant to the respective employment agreements, including the release of all rights the Company may have held in any intellectual property of Dr. Werber and Mr. Suddarth and any non-compete restrictions on Dr. Werber and Mr. Suddarth. In connection with such releases, Dr. Werber and Mr. Suddarth each agreed to forfeit all warrants previously granted and outstanding (a total of 77,150,000 warrants to purchase shares of common stock of the Company), forfeit any and all accrued and unpaid amounts owing under the employment agreements for past due wages, benefits, severance obligations, unreimbursed expenses and any other obligations owing to one another as of the Closing Date (totaling $906,515) in exchange for a grant of 7,500,000 newly issued shares of restricted common stock of the Company to each of Dr. Werber and Mr. Suddarth, with a fair value of $83,250, based on the closing price of the common stock of the Company on the date of the Sale.
In connection with the Sale, ANU and General Surgical retained all cash on-hand as of the Closing Date and General Surgical retained all accounts receivable existing at the Closing Date, trademarks and inventory associated with the distribution of its “Organicell” product, and certain agreements between General Surgical and distributors of the ANU products that General Surgical intends to continue to supply after the Closing Date pursuant to the Organicell Distribution Agreement. After the completion of the Sale, the Company remained in the business of selling and distributing regenerative biologic therapies based on amnion placental tissue derived products to doctors and hospitals but was required to depend on third party supply agreements, rather than from products manufactured internally by ANU, for the supply of these advanced biologically processed cellular and tissue based products. During February 2019, the Company began arranging to operate a new laboratory facility for the purpose of performing research and development, production and manufacturing of anti-aging and cellular therapy products. This new laboratory facility became operational in May 2019 and during the same period, the Company began producing and distributing the products that are being sold to its customers.
Notice Of Change In Vera Operations
During August 2018, Vera notified the Company that it had sold most of the principal assets acquired in the Sale to another entity, that it was no longer in the business originally acquired in connection with the Sale and that it was no longer able to supply products to the Company. As a result, since that date, up thru May 2019, the date Organicell began again producing products internally, the Company has entered into other short-term supply agreements with other third-party manufacturers to provide it with the products it sells to its customers.
Since Vera’s disposition of the assets originally acquired in connection with the Sale as described above, the Company has yet to receive any payments from Vera associated with the original escrow deposit of $47,500 that was withheld from the proceeds from the Sale. Due to the uncertainty of Vera’s ability and/or desire to repay the escrow receivable amount outstanding to the Company, the Company has recorded a reserve for the full amount of escrow receivable totaling $47,500 during the fourth quarter ended October 31, 2018.
NOTE 5 – REORGANIZATION
On April 23, 2018, the Company and MBA, executed a Plan and Agreement of Reorganization (“Reorganization”) whereby the Company agreed to issue to MBA an aggregate of 222,425,073 shares of its common stock, representing at the time 51% of the outstanding shares of common stock of the Company on fully-diluted basis, for $0.001 per share, in consideration for Mr. Manuel Iglesias’ agreement to serve as the Company’s Chief Executive Officer and a member of the Board of the Company. The Reorganization was effective as of April 13, 2018.
The Company has recorded $2,758,071 of stock compensation expense associated with the issuance of the shares referred to above for Mr. Iglesias’s agreement to serve as the Company’s Chief Executive Officer. As a result of the above transactions, MBA obtained a controlling interest in the voting and equity interests of the Company. Manuel E. Iglesias is the sole Manager of MBA and thus may be deemed to control MBA. Since the date of the Reorganization, MBA’s interests held in the equity of the Company have been reduced from 51.0% to approximately 24.6%.
| 67 |
Under the terms of the Reorganization, as of the Effective Date:
| 1. | Mr. Iglesias replaced Albert Mitrani as Chief Executive Officer of the Company. |
| 2. | Mr. Iglesias and Richard Fox were appointed as members to the Board of Directors of the Company. Mr. Fox resigned in May 2019 and Mr. Robert Zucker was appointed to fill his vacancy. Mr. Zucker resigned from the Board of Directors in April 2020. |
| 3. | Ian Bothwell and Maria Mitrani resigned from the Board of Directors of the Company. Ms. Mitrani was re-appointed to the Board of Directors of the Company during August 2019. Mr. Bothwell was re-appointed to the Board during September 2019. |
| 4. | Albert Mitrani, Ian Bothwell and Maria Mitrani each agreed to terminate their respective employment agreements in favor of new employment agreements. In connection with the new employment agreements, Mr. Mitrani agreed to serve as the Company’s President, Ian Bothwell agreed to remain Chief Financial Officer and Maria Mitrani agreed to remain Chief Science Officer of the Company. |
| 5. | Albert Mitrani, Ian Bothwell and Maria Mitrani each agreed to the cancellation of their 100 shares of the Company’s Series A Preferred Stock. In addition, the Company agreed that it would cancel the Certificates of Designation for the Company’s Series A Preferred Stock and Series B Preferred Stock. |
| 6. | The Company agreed to terminate Sections 4.08(c) and 4.08(d) of the Company’s Second Amended and Restated By-Laws which had required supermajority approval of the Board for certain corporate actions. |
| 7. | Ian Bothwell and Maria Mitrani exercised, on a cashless basis, all of their warrants for 48,624,561 and 21,757,895, respectively, shares of common stock of the Company based on the exercise price of $0.001 and the closing price of the Company’s common stock on the Effective Date. |
| 8. | Ian Bothwell and Maria Mitrani were granted an additional 4,675,439 and 2,092,105, respectively, shares of common stock of the Company (see Note 12). |
| 9. | Albert Mitrani, Ian Bothwell and Maria Mitrani each agreed to release the Company for all amounts owed to them for unpaid salaries through the Effective Date and advances and/or expenses incurred prior to December 31, 2017 totaling $1,636,808. |
NOTE 6 – INVENTORIES
Inventories totaled $77,963 and $0 at October 31, 2019 and October 31, 2018, respectively.
| October 31, 2019 | October 31, 2018 | |||||||
| Raw materials and supplies | $ | 5,123 | $ | – | ||||
| Finished goods | 72,840 | – | ||||||
| Total inventories | $ | 77,963 | $ | – | ||||
NOTE 7 - PROPERTY AND EQUIPMENT
| October 31, 2019 | October 31, 2018 | |||||||
| Computer equipment | $ | 8,653 | $ | 8,653 | ||||
| Manufacturing equipment | 272,331 | – | ||||||
| 280,984 | 8,653 | |||||||
| Less: accumulated depreciation and amortization | (17,669 | ) | (2,875 | ) | ||||
| Total property and equipment, net | $ | 263,315 | $ | 5,778 | ||||
| 68 |
Depreciation expense totaled $14,794 and $7,835 for the years ended October 31, 2019 and 2018, respectively.
During March 2019, the Company entered into a lease agreement for certain lab equipment in the amount of $239,595. Under the terms of the lease agreement, the Company is required to make 60 equal monthly payments of $4,513 plus applicable sales taxes. Under the Lease Agreement, the Company has the right to acquire all of the leased equipment for $1.00. As a result, the lease agreement is being accounted for as a capital lease obligation. The annual interest rate charged in connection with the lease is 4.5%. The leased equipment is being depreciated over their estimated useful lives of 15 years.
NOTE 8 – RELATED PARTY TRANSACTIONS
Effective February 5, 2018, Dr. Werber’s and Mr. Suddarth’s executive employment agreements were terminated and the parties entered into a settlement agreement providing for the release of all obligations owed to Dr. Werber and Mr. Suddarth as of the date of the Sale in exchange for each receiving a grant for 7,500,000 shares of common stock of the Company. On April 6, 2018, Dr. Mitrani’s and Mr. Bothwell’s executive employment agreements were amended to modify the exercise price of their outstanding warrants under certain conditions. Effective April 13, 2018, Mr. Mitrani’s, Dr. Mitrani’s and Mr. Bothwell’s executive employment agreements were terminated and replaced with new executive employment agreements. On February 26, 2020, April 25, 2020 and June 29, 2020, Mr. Mitrani’s, Dr. Mitrani’s and Mr. Bothwell’s employments agreement were further amended. See Note 14 for a more detailed description of the executive employment agreements and the respective amendments referred to above.
In connection with the Reorganization, Mr. Bothwell and Dr. Mitrani each agreed to exercise on a cashless basis all of their warrants to purchase 53,300,000 and 23,850,000 shares of common stock of the Company, respectively. Based on the closing price of the Company’s common stock on the Effective Date of $0.012 per share and the warrant exercise price of $0.001 per share, Mr. Bothwell and Dr. Mitrani were required to use 4,675,439 and 2,092,105 shares of common stock received from the exercise of the warrants, respectively, to pay for the exercise price for exercising all of the warrants (see Note 13).
Effective April 13, 2018, Mr. Bothwell and Dr. Mitrani were each granted 4,675,439 and 2,092,105 shares of common stock of the Company, respectively. The newly granted shares vest immediately and were valued at $57,975 and $25,942, respectively, based on the closing trading price of the common stock on the effective date of the grant.
In connection with the previous appointment of an independent member to the Board of Directors of the Company, during August 2019, the Board approved the issuance to the director of 5,000,000 shares of unregistered common stock valued at $0.028 per share, the closing price of the common stock of the Company on the grant date.
Effective February 26, 2020, Mr. Bothwell was granted cashless warrants to purchase 7,500,000 shares of common stock of the Company. The newly granted warrants vest immediately, have an exercise price of $0.028 per share and are exercisable for ten years from the effective date of the grant.
During April 2020, June 2020, August 2020 and September 2020, each of the current executives of the Company, Albert Mitrani, Dr. Mari Mitrani, Ian Bothwell and George Shapiro (“Current Executives”) were granted rights under the Management and Consultant Performance Plan (“MCPP”) to receive common stock of the Company based on the achievement of certain defined milestones. In addition, during June 2020, each of the current non-executive members of the Board were granted rights under the MCPP to receive common stock of the Company based on the achievement of certain defined milestones (see Note 12).
The Company’s corporate administrative offices are leased from MariLuna, LLC, a Florida limited liability company which is owned by Dr. Mitrani. The term of the lease has been extended through June 2023. The current monthly rent is $2,900 and beginning July 2020, the monthly rent increases to $3,500. The Company paid a security deposit of $5,000.
In connection with Mr. Bothwell’s executive employment agreements, the Company agreed to reimburse Rover Advanced Technologies, LLC, a company owned and controlled by Mr. Bothwell for office rent and other direct expenses (phone, internet, copier and direct administrative fees, etc.).
On February 5, 2018, in connection with the Sale (see Note 4), all amounts owed to the Mr. Bothwell and Dr. Werber in connection with the SPA were repaid.
| 69 |
On February 5, 2018, in connection with Dr. Werber’s resignation and termination, Dr. Werber agreed to the forfeit and the cancellation of the 100 shares of the Series A Preferred Stock previously issued. Effective April 13, 2018, in connection with the Reorganization, Mr. Mitrani, Mr. Bothwell and Dr. Mitrani each agreed to the forfeit and cancellation of their 100 shares of the Series A Preferred Stock.
On April 6, 2018, Peter Taddeo resigned as a member of the Board of Directors of the Company and as the Chief Executive Officer and member of the board of directors of the Mint Organics Entities. In connection with Mr. Taddeo’s resignation, Mr. Taddeo entered into a Separation and General Release Agreement (“Taddeo Separation Agreement”) whereby Mr. Taddeo agreed to release the Mint Organics Entities from all obligations in connection with the Taddeo Agreement and all other agreements and/or financial obligations between the parties related to the Taddeo’s employment or services performed with any of Mint Organics Entities. In consideration for Taddeo entering into the Taddeo Separation Agreement, the Mint Organics Entities paid Taddeo $5,000 and Mr. Bothwell paid $3,000 to Taddeo for the purchase of the 1,000,000 shares of common stock of the Company that were granted to Taddeo in connection with the Taddeo Agreement. Contemporaneously with the execution of the Taddeo Separation Agreement, the Company and Mr. Taddeo entered into a Share Purchase and General Release Agreement whereby the Company agreed to purchase from Mr. Taddeo his 150 shares of Mint Series A Preferred Stock for an aggregate purchase price of $40,000 (see Note 15).
On May 1, 2019, the Company and Mint Organics entered into an exchange agreement whereby the Company agreed to acquire the 150 shares of Mint Series A Preferred Stock and the 150,000 warrants to purchase shares of common stock of the Company originally issued to Mr. Wayne Rohrbaugh in connection with the initial capitalization of Mint Organics in exchange for 4,400,000 shares of common stock of the Company.
As described in Note 5, on April 23, 2018, the Company and MBA executed a Plan and Agreement of Reorganization. As a result of the Reorganization, MBA acquired at the time a controlling interest of the Company. Manuel E. Iglesias is the sole Manager of MBA and thus may be deemed to control MBA.
For the year ended October 31, 2019 and 2018, the total amount of sales to customers related to our board of director members and/or employees of the Company totaled $71,650 and $19,550, respectively.
From time to time, Mr. Iglesias and Mr. Bothwell and/or their respective affiliates have advanced funds to the Company to pay for certain expenses of the Company. As of October 31, 2019, $220,897 and $48,184 are owed to Mr. Iglesias and Mr. Bothwell and/or their respective affiliates, respectively. In addition, the Company has not provided Mr. Bothwell required salary payments since July 2018. At October 31, 2019, salary amounts owed to Albert Mitrani, Dr. Mari Mitrani and Ian Bothwell were $132,105, $129,613 and $321,907, respectively.
As described in Note 9, Mr. Iglesias has provided a personal guaranty in connection with amounts required to paid under the Credit Facility.
During April 2020 through May 2020, the Company sold 11,000,000 shares of common stock to Dr. Allen Meglin, a director of the Company at $0.02 per share for an aggregate purchase price of $220,000. During July and August 2020, the Company sold an additional 1,166,666 shares and 422,514 shares of common stock to Dr. Allen Meglin at $0.03 per share and $0.10 per share, respectively, for an aggregate purchase price of $77,251.
On April 27, 2020, the Company sold 5,000,000 shares of common stock to Republic Asset Holdings LLC., a Company controlled by Michael Carbonara, a director of the Company, at $0.02 per share for an aggregate purchase price of $100,000.
| 70 |
During September 2018, in consideration for Dr. George Shapiro agreeing to serve as the Company’s Chief Medical Officer (“CMO”) and render other medical consulting and advisory services to the Company, the Board approved the issuance to the CMO of 2,500,000 shares of common stock. In connection with the CMO’s appointment to the Board of Directors of the Company during February 2019, during February 2019 and August 2019, the Board approved the issuance to the CMO of 2,000,000 and 3,000,000 shares, respectively, of common stock. On February 26, 2020, the Company agreed to immediately grant the CMO 5,000,000 shares of common stock in recognition of past services provided to the Company through February 2020. In addition, the Company agreed to enter into a consulting agreement with the CMO to provide ongoing services to the Company. The CMO will receive compensation of $82,250 annually, commencing March 1, 2020. The term of the consulting agreement is one year, with automatic renewals for annual periods thereafter unless prior written notice is provided by either party of the desire to terminate.
In connection with Mr. Robert Zucker’s resignation as a member of the Board of Directors of the Company in April 2020, the Board approved the issuance to Mr. Zucker of 736,808 shares of unregistered common stock of the Company valued at $0.022 per share, the closing price of the common stock of the Company on the grant date ($16,210).
NOTE 9 - NOTES PAYABLE
Private Placement Of Convertible Debentures
On June 20, 2018, the Company issued a total of $150,000 of convertible 6% debentures (“150,000 Debentures”) to an accredited investor. The principal amount of the $150,000 Debentures, plus accrued and unpaid interest through June 30, 2019 are payable on the 10th business day subsequent to June 30, 2019, unless the payment of the $150,000 Debentures are prepaid at the sole option of the Company, are converted as provided for under the terms of the $150,000 Debentures (see below), and/or accelerated due to an event of default in accordance with the terms of the $150,000 Debentures. Interest on the $150,000 Debentures for each calendar quarter ended beginning with the quarter ended June 30, 2018 is payable on the 10th business day following the immediately prior calendar quarter.
On August 10, 2018, the Company issued a total of $100,000 of convertible 6% debentures (“100,000 Debentures”) to two accredited investors. The principal amount of the $100,000 Debentures, plus accrued and unpaid interest through July 31, 2019 are payable on the 10th business day subsequent to July 31, 2019, unless the payment of the $100,000 Debentures are prepaid at the sole option of the Company, are converted as provided for under the terms of the $100,000 Debentures (see below), and/or accelerated due to an event of default in accordance with the terms of the $100,000 Debentures. Interest on the $100,000 Debentures for each calendar quarter ended beginning with the quarter ended October 31, 2018 is payable on the 10th business day following the immediately prior calendar quarter.
During October 2018, the Company issued a total of $70,000 of convertible 6% debentures (“70,000 Debentures”) to two accredited investors. The principal amount of the $70,000 Debentures, plus accrued and unpaid interest through September 30, 2019 are payable on the 10th business day subsequent to September 30, 2019, unless the payment of the $70,000 Debentures are prepaid at the sole option of the Company, are converted as provided for under the terms of the $70,000 Debentures (see below), and/or accelerated due to an event of default in accordance with the terms of the $70,000 Debentures. Interest on the $70,000 Debentures for each calendar quarter ended beginning with the quarter ended December 31, 2018 is payable on the 10th business day following the immediately prior calendar quarter.
During March 2019, the Company issued a $30,000 of convertible 6% debentures (“30,000 Debenture”) to one accredited investor. The principal amount of the $30,000 Debenture, plus accrued and unpaid interest through June 30, 2020 are payable on the 10th business day subsequent to June 30, 2020, unless the payment of the $30,000 Debenture is prepaid at the sole option of the Company, is converted as provided for under the terms of the $30,000 Debenture (see below), and/or accelerated due to an event of default in accordance with the terms of the $30,000 Debenture. Interest on the $30,000 Debenture for each calendar quarter ended beginning with the quarter ended June 30, 2019 is payable on the 10th business day following the immediately prior calendar quarter.
| 71 |
Under the terms of the $150,000 Debentures, the $100,000 Debentures, the $70,000 Debentures and the $30,000 Debenture (collectively referred to as the “Convertible Debentures”), the Company is permitted to issue additional convertible 6% debentures up to a maximum aggregate principal amount of $1,000,000 of convertible 6% debentures, all of like tenor except as to the issuance date which shall be determined based on the date that additional convertible debentures are issued, if any. The Company used the proceeds from the Convertible Debentures totaling $350,000 for general working capital purposes.
The Convertible Debentures may be prepaid at any time by the Company in whole or in part without penalty upon 30 days written notice but not to exceed 60 days (“Repayment Notice”) at a price equal to the principal amount outstanding of the Convertible Debentures’ elected to be repaid by the Company, plus all unpaid and accrued interest up through the date of prepayment provided in the Repayment Notice (“Prepayment Date”).
The Convertible Debentures (the principal and all accrued but unpaid interest thereon) contained provisions that under certain conditions, provided the ability of the holders of the Convertible Debentures at their option at any time, from time to time to convert into shares of the common stock of the Company. The conversion prices were based on the Company completing a contemplated pending reverse split at the Company’s sole discretion (which the Company elected not to pursue) or at conversion prices greatly in excess of the historical prices of the Company’s common stock and reasonably expected prices of the Company’s common stock to be realized during the term of the Convertible Debentures. As a result, none of the Convertible Debentures have been or are expected to be converted in accordance with their conversion provisions. The contingent rights to convert for certain of the convertible debentures did not result in any underlying value attributable to the fair value of the embedded derivatives liabilities associated with respective Convertible Debentures.
During May 2019, the Company and holders of the $100,000 Debentures agreed to convert the principal amount of the $100,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $100,622 into 3,773,584 shares of common stock of the Company (approximately $0.0267 per share representing a discount to the trading price of $0.0285 as of the effective date of the transaction).
During June 2019, the Company and the holder of the $30,000 Debenture agreed to convert the principal amount of the $30,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $30,478 into 1,111,111 shares of common stock of the Company (approximately $0.0274 per share representing a premium to the trading price of $0.0253 as of the effective date of the transaction).
As a result of the above conversions, the principal amount of Convertible Debentures outstanding as of October 31, 2019 was $220,000. These remaining Convertible Debentures were not repaid on their required maturity dates.
On June 25, 2020, the Company entered into a settlement and general release agreement with the holder of the $50,000 Debenture whereby the Company is required to repay the balance of the $50,000 Debenture in eight monthly installments of $6,250 plus outstanding accrued interest beginning June 30, 2020 and ending on January 31, 2021.
During October 2020, the Company and the holder of the $20,000 debenture (one of the two holders that participated in the $70,000 Debentures described above), agreed to convert the principal amount of the $20,000 debenture plus interest accrued and unpaid through the date of the conversion totaling approximately $20,100 into 160,000 shares of common stock of the Company (approximately $0.125 per share representing a discount to the trading price of $0.278 as of the effective date of the transaction).
Unsecured Promissory Note
On February 5, 2019, the Company entered into an unsecured loan agreement with a third party with a principal balance of $25,000. The outstanding principal was due March 8, 2019. The loan was not repaid on the maturity date as required. The third party subsequently agreed to apply amounts due for invoices due from third party for future purchases of the Company products to the extent of the outstanding balances owed by the Company in connection with the loan (interest and principal).
| 72 |
Credit Facility
On September 19, 2019, the Company’s wholly owned subsidiary, General Surgical Florida, received $100,000 in connection with an unsecured line of credit (“Credit Facility”). The Credit Facility matures in one-year and the Company is required to make 52 weekly payments of $2,403 (payments totaling $125,000). The Credit Facility can be prepaid at any time by the Company. The effective annual interest rate of the facility based on 52 equal monthly payments is 45.67%. Proceeds received from the Credit Facility were used for working capital purposes. Mr. Iglesias provided a personal guaranty in connection with amounts required to paid under the Credit Facility.
Capital Lease Obligations
During March 2019, the Company entered into a lease agreement for certain lab equipment in the amount of $239,595. Under the terms of the lease agreement, the Company is required to make 60 equal monthly payments of $4,513 plus applicable sales taxes. Under the Lease Agreement, the Company has the right to acquire all of the leased equipment for $1.00. As a result, the lease agreement is being accounted for as a capital lease obligation. The annual interest rate charged in connection with the lease is 4.5%. The leased equipment are being depreciated over their estimated useful lives of 15 years.
Funding Facility
On October 10, 2019, the Company and an investor (“Noteholder”) agreed to a funding facility arrangement (“Funding Facility”) whereby the Noteholder was required to fund the Company an initial tranche of $100,000 on October 15, 2019 (“Initial Funding Date”) and had the option to fund the Company up to an aggregate of $500,000 (“Funding Facility Limit”) in minimum $100,000 monthly tranches by no later than February 15, 2020 (“Funding Expiration Date”). The Funding Facility matures on February 15, 2021 (“Maturity Date”) and accrues interest at 6.0% per annum. The Funding Facility, plus all accrued interest, automatically converts into 40,000,000 shares of newly issued common stock of the Company if the Noteholder funds the full $500,000 by the Funding Expiration Date. The Noteholder fully funded the Funding Facility as prescribed on February 12, 2020 and the Company converted the Funding Facility into 40,000,000 shares of common stock of the Company that were issued to the Noteholders designated entity, Republic Asset Holdings LLC.
Mint Organics Inc.
On June 22, 2017, Mint Organics entered into an unsecured loan agreement with a third party (“Third Party”) with a principal balance of $60,000, an annual interest rate of 10%, and all accrued and unpaid interest and outstanding principal were due on the one-year anniversary of the note. The loan was not repaid on the maturity date as required.
On May 1, 2019, the Company, Mint Organics and the Third party agreed to a settlement of the outstanding loan whereby the Company agreed to issue the Third Party 2,735,000 shares of newly issued common stock of the Company. At the time of the settlement, the outstanding obligation under the note, including late fees and penalties was approximately $72,568. The common stock issued was priced at $0.0265 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction. In connection with the exchange, the Third Party provided a release to the Company in connection with any claims associated with the loan agreement.
Interest expense for the years ended October 31, 2019 and 2018 was $4,349 and $6,049, respectively.
NOTE 10 – DERIVATIVE LIABILITIES
In connection with the sale of debt or equity instruments, the Company may sell options or warrants to purchase our common stock. In certain circumstances, these options or warrants may be classified as derivative liabilities, rather than as equity. Additionally, the debt or equity instruments may contain embedded derivative instruments, such as embedded derivative features which in certain circumstances may be required to be bifurcated from the associated host instrument and accounted for separately as a derivative instrument liability.
| 73 |
The Company's derivative instrument liabilities are re-valued at the end of each reporting period, with changes in the fair value of the derivative liability recorded as charges or credits to income in the period in which the changes occur. For options, warrants and bifurcated embedded derivative features that are accounted for as derivative instrument liabilities, the Company estimates fair value using either quoted market prices of financial instruments with similar characteristics or other valuation techniques. The valuation techniques require assumptions related to the remaining term of the instruments and risk-free rates of return, our current common stock price and expected dividend yield, and the expected volatility of our common stock price over the life of the instrument.
The Company classifies the fair value of these securities under level three of the fair value hierarchy of financial instruments. The fair value of the derivative liability was calculated using a Monte Carlo Simulation model that values the liability of the Convertible Notes based on a risk neutral valuation where the price of the option is its discounted expected value. The technique applied generates a large number of possible (but random) price paths for the underlying (or underlyings) via simulation, and then calculate the associated conversion value (i.e. "payoff") of the note (limited by a percentage of trading volume) for each path. These payoffs are then averaged and discounted to the date of valuation resulting in the fair value of the option.
In connection with the Sale, the underlying debt instrument associated with the derivative liability was paid in full without utilization of any of the conversion features associated with the debt instrument. During the year ended October 31, 2018, the Company recorded a gain of $265,597 associated with the change in fair value of the derivative liabilities from October 31, 2017.
NOTE 11 — INCOME TAXES
The Company files a consolidated federal income tax return that includes all of its subsidiaries. For the year ended October 31, 2019, the Company incurred operating losses, and therefore, there was not any current income tax expense amount recorded during that period. During the year ended October 31, 2018 there was a change in ownership which caused a change in control under IRC Section 382 (“Section 382 event”). Prior to the Section 382 event, the Company utilized a portion of its available net operating loss carryforwards to offset income through that date mainly resulting from the sale of ANU.
The consolidated provision for income taxes for October 31, 2019 and 2018 consists of the following:
| Year Ended October 31, | Year Ended October 31, | |||||||
| 2019 | 2018 | |||||||
| Current: | ||||||||
| Federal | $ | – | $ | – | ||||
| State | – | – | ||||||
| $ | – | $ | – | |||||
| Deferred: | ||||||||
| Federal | $ | (185,045 | ) | $ | 911,472 | |||
| State | (19,471 | ) | 164,180 | |||||
| (204,516 | ) | 1,075,652 | ||||||
| Change in Valuation Allowance | 204,516 | (1,075,652 | ) | |||||
| $ | – | $ | – | |||||
| 74 |
Effective tax rates differ from the federal statutory rate of 21% for 2019 and the blended rate of 23.17% for 2018 applied to income before income taxes. A reconciliation of the U.S. federal statutory tax amount to the Company’s effective tax amount is as follows:
| October 31, 2019 | October 31, 2018 | |||||||
| Tax at federal statutory rate | $ | (361,687 | ) | $ | (802,067 | ) | ||
| State taxes, net of federal benefit | (74,835 | ) | (68,278 | ) | ||||
| Permanent differences | 10,468 | 427,759 | ||||||
| Effect of change in income tax rate | – | 1,052,690 | ||||||
| Other | 221,538 | 46,139 | ||||||
| Section 382 limitation | – | 419,409 | ||||||
| Total income tax expense (benefit) | (204,516 | ) | 1,075,652 | |||||
| Change in valuation allowance | 204,516 | (1,075,652 | ) | |||||
| $ | – | $ | – | |||||
The Company had a federal net operating loss carryover of $1,060,732 as of October 31, 2019. On December 22, 2017, the United States enacted into law the Tax Cuts and Jobs Act (“TCJA”). This law provides for a comprehensive overhaul of the corporate income tax code, including amongst other provisions, a reduction of the statutory corporate tax rate from 34% to 21%, effective on January 1, 2018, and an indefinite carryforward of net operating losses arising from tax years ending after December 31, 2017 limited to a deduction of 80% of taxable income. FASB ASC 740, Income Taxes, requires that the effects of changes in tax rates be recognized in the period enacted. As a result, we remeasured our deferred tax assets and liabilities to reflect the new statutory federal rate of 21% which resulted in a net adjustment of approximately $1.1 million to deferred income tax expense for the year ended October 31, 2018. This adjustment was offset by a reduction in the valuation allowance.
The tax effects of temporary differences and carry-forwards that give rise to deferred tax assets and liabilities for the Company were as follows:
| October 31, 2019 | October 31, 2018 | |||||||
| Deferred Tax Assets: | ||||||||
| Stock based compensation | $ | 2,670,914 | $ | 2,686,859 | ||||
| Accrued compensation | 136,127 | – | ||||||
| Net operating loss carryforward-Federal | 222,754 | 91,501 | ||||||
| Net operating loss carryforward-State | 34,918 | 16,612 | ||||||
| Other | 177 | 137 | ||||||
| Total deferred tax assets: | 3,064,890 | 2,795,109 | ||||||
| Deferred Tax Liabilities: | ||||||||
| Property and equipment | 66,650 | 1,385 | ||||||
| Total deferred tax liabilities: | 66,650 | 1,385 | ||||||
| Valuation Allowance | (2,998,240 | ) | (2,793,724 | ) | ||||
| Net deferred tax assets | $ | – | $ | – | ||||
| 75 |
FASB ASC 740 requires a valuation allowance against deferred tax assets if, based on the weight of available evidence, it is more likely than not that some or all of the deferred tax assets will not be realized. At October 31, 2019 and October 31, 2018, the net deferred tax asset was offset by a full valuation allowance.
Pursuant to Code Sec. 382 of the Internal Revenue Code (“the Code”), the utilization of net operating loss carryforwards may be limited as a result of a cumulative change in stock ownership of more than 50% over a three-year period. The Company underwent such a change in April 2018 and consequently, the net operating loss carryforward was adjusted to write off the portion that will expire unused. At October 31, 2018, the Company had net operating losses approximating $395,000 which carry over indefinitely.
Certain of the above amounts reported for the year ended October 31, 2018 have been revised to conform with the current year presentation and to reflect the actual amounts that were reported in the Company’s tax filings.
IRS Penalties
The Company’s income tax returns for the periods since inception through the tax year ended October 31, 2015 were not filed with the Internal Revenue Service (“IRS”) until August 2017 (“Delinquent Filed Returns”). The Company’s income tax returns for the tax year ended October 31, 2016 were filed with the IRS during December 2017. In connection with the Delinquent Filed Returns, during the period September 2017 through October 2017, the Company received notices that it was being assessed approximately $90,000 of penalties, plus interest (“IRS Penalties”), in connection with the late filing certain information returns that were included as part of the Delinquent Filed Returns. In connection with the notices, the IRS indicated its intent to levy property of the Company if the IRS penalties were not paid as required. During January 2018, the Company requested from the IRS an abatement of the IRS penalties based on reasonable cause. During April 2018, the IRS notified the Company that the IRS penalties for the tax year ended 2011 of $20,000, plus interest, were abated and the request for abatement for the IRS penalties for the tax years ended 2012 – 2015 were denied. The Company is currently appealing the initial determination by the IRS to exclude the IRS penalties for the tax years 2012-2015 in its consideration of abatement. During the period that the appeal is being reviewed and a determination is made by the IRS, the IRS has agreed to put a hold on taking any levy action against the Company for the remaining amounts of the IRS Penalties that are still outstanding. In connection with the notices, the Company has accrued $70,000 of accrued tax penalties on the balance sheet as of October 31, 2019 and 2018.
NOTE 12 – CAPITAL STOCK
Preferred Stock
The Company is authorized to issue 10,000,000 shares of $0.001 par value preferred stock in one or more designated series, each of which shall be so designated as to distinguish the shares of each series of preferred stock from the shares of all other series and classes. The Company’s board of directors is authorized, without stockholders’ approval, within any limitations prescribed by law and the Company’s Articles of Incorporation, to fix and determine the designations, rights, qualifications, preferences, limitations and terms of the shares of any series of preferred stock.
Series A Non-Convertible Preferred Stock
On November 1, 2016, the Company filed a Certificate of Designation with the Secretary of State of Nevada therein designating out of the 10,000,000 authorized shares of Preferred Stock, a class of Preferred Stock as “Series A Non-Convertible Preferred Stock” consisting of 100 shares (the “ Series A Certificate of Designation “). On March 2, 2017, the Company filed with the Secretary of State of Nevada an amendment to increase the number of shares provided for in the Series A Certificate of Designation from 100 shares to 400 shares.
Set forth below is a summary of the Series A Certificate of Designation, as amended.
| 76 |
Voting
Generally, the outstanding shares of Series A Non-Convertible Preferred Stock shall vote together with the shares of common stock and other voting securities of the Company as a single class and, regardless of the number of shares of Series A Non-Convertible Preferred Stock outstanding, and as long as at least one share of Series A Non-Convertible Preferred Stock is outstanding, such shares shall represent 80% of all votes entitled to be voted at any annual or special meeting of stockholders of the Company or action by written consent of stockholders. Each outstanding share of the Series A Non-Convertible Preferred Stock shall represent its proportionate share of the 80% which is allocated to the outstanding shares of Series A Non-Convertible Preferred Stock.
Dividends
The holders of shares of Series A Non-Convertible Preferred Stock shall not be entitled to receive any dividends.
Ranking
The Series A Non-Convertible Preferred Stock shall, with respect to distribution rights on liquidation, winding up and dissolution, (i) rank senior to any of the shares of common stock of the Company, and any other class or series of stock of the Company which by its terms shall rank junior to the Series A Non-Convertible Preferred Stock, and (ii) rank junior to any other series or class of preferred stock of the Company and any other class or series of stock of the Company which by its term shall rank senior to the Series A Non-Convertible Preferred Stock.
So long as any shares of Series A Non-Convertible Preferred Stock are outstanding, the Company shall not alter or change any of the powers, preferences, privileges or rights of the Series A Non-Convertible Preferred Stock, without first obtaining the approval by vote or written consent, in the manner provided by law, of the holders of at least a majority of the outstanding shares of Series A Non-Convertible Preferred Stock, as to changes affecting the Series A Non-Convertible Preferred Stock.
Issued Shares
On November 1, 2016, the Company issued 100 shares of its Series A Non-Convertible Preferred Stock, par value $0.001 per share (“Series A Preferred Stock”) to the CEO. On March 8, 2017, the Company issued 100 shares of the Series A Preferred Stock, to each of the COO, CSO and CFO. In connection with an independent valuation using the “Market Approach”, the Company determined that the value attributable to the Series A Preferred Stock issued was nominal.
On February 5, 2018, in connection with the COO’s resignation and termination, the COO agreed to forfeit and the cancellation of the 100 shares of the Series A Preferred Stock previously issued.
Effective April 13, 2018, in connection with the Reorganization, the CEO, CFO and CSO each agreed to forfeit and the cancellation their 100 shares of the Series A Preferred Stock previously issued.
On June 6, 2018, the Company approved resolutions to cancel and terminate the Series A Preferred Stock designation and file a certificate of amendment with the State of Nevada, withdrawing the designation of the Series A Preferred Stock. On June 14, 2018, the Company filed a Certificate of Withdrawal with the Secretary of State of Nevada thereby withdrawing and terminating all previously issued designations of the Company’s Series A Preferred Stock. As a result of the aforementioned actions, as of June 14, 2018, there were no designations of Series A Preferred Stock authorized or outstanding.
| 77 |
Series B Convertible Preferred Stock
On November 1, 2016, the Company filed a Certificate of Designation with the Secretary of State of Nevada therein designating out of the 10,000,000 authorized shares of Preferred Stock, a class of Preferred Stock as “Series B Convertible Preferred Stock” consisting of 1,000,000 shares (“Series B Certificate of Designation”).
Set forth below is a summary of the Series B Certificate of Designation.
Conversion
Each holder of Series B Preferred Stock shall have the right, at such holder’s option, at any time or from time to time from and after the day immediately following the date the Series B Preferred Stock is first issued, to convert each share of Series B Preferred Stock into 20 fully-paid and non-assessable shares of common stock.
Rank
Except as specifically provided below, the Series B Preferred Stock shall, with respect to dividend rights, rights on liquidation, winding up and dissolution, rank junior to the Series A Non-Convertible Preferred Stock of the Company and senior to (i) all classes of common stock of the Company and (ii) any class or series of capital stock of the Company hereafter created (unless, with the consent of the holder(s) of Series B Preferred Stock).
Issued Shares
On June 6, 2018, the Company approved resolutions to cancel and terminate the Series B Preferred Stock designations and file a certificate of amendment with the State of Nevada, withdrawing the designation of the Series B Preferred Stock. On June 14, 2018, the Company filed a Certificate of Withdrawal with the Secretary of State of Nevada thereby withdrawing and terminating all previously issued designations of the Company’s Series B Preferred Stock. As a result of the aforementioned actions, as of June 14, 2018, there were no designations of Series B Preferred Stock authorized or outstanding.
Common Stock
On May 8, 2018, the Board adopted resolutions to (i) amend its Articles of Incorporation to reduce the number of authorized shares of common stock from 750,000,000 to 250,000,000 and (ii) reverse split the issued and outstanding shares of the Company’s common stock on a ratio of seventeen (17) current shares for one (1) share of new shares. On May 9, 2018, shareholders holding a majority in interest of the voting power of the Company (86.9%) approved the amendment and the reverse stock split.
On June 1, 2018, the Company filed a Company-Related Action Notification with FINRA (“Notification Form”) to provide notice of certain proposed actions by the Company, including the amendment and reverse stock split. However, due to the Company’s delinquency its Exchange Act reports with the SEC at the time of the filing (by failing to file the October 31, 2017 Annual Report and its Quarterly Reports for the quarters ended January 31, 2018 and April 30, 2018, FINRA did not announce or effectuate the Name Change or Reverse Split in the marketplace. On June 18, 2018, the Company filed a Certificate of Correction with the Secretary of State of Nevada to reverse the amendments related to the Reverse Split. FINRA has since informed the Company that a new Issuer Company-Related Notification Form will be required to be submitted should the Company desire to effectuate the Name Change and Reverse Split in the future, provided the Company is current in its Exchange Act filings. At such time as FINRA processes the announcements, the Company will effect the reverse split of its common stock and amend its Articles to reduce its authorized common stock.
| 78 |
On May 18, 2020 and May 19, 2020, pursuant to the Nevada Revised Statutes and the Bylaws of the Company, the Board of Directors of the Company and the stockholders having the voting equivalency of 50.30% of the outstanding capital stock, respectively, approved the filing of an amendment to the Articles of Incorporation of the Company to increase the authorized amount of common stock from 750,000,000 to 1,500,000,000, without changing the par value of the common stock or authorized number and par value of “blank check” Preferred Stock. On June 2, 2020, the Company filed a Definitive 14C with the SEC regarding the corporate action. On June 24, 2020, the Company filed a Certificate of Amendment to the Company’s Articles of Incorporation with the Secretary of State of Nevada to effectuate the corporate action on June 24, 2020.
Issuances of Common Stock - Sales:
On March 7, 2019, the Company sold an aggregate of 7,500,000 shares of common stock and granted warrants to purchase an aggregate 2,000,000 common shares to three “accredited investors” investors. The warrants have exercise prices of $0.08, and have a one -year term. The aggregate grant date fair value of the warrants issued in connection with these issuances were $6,600. The proceeds were used for working capital.
During April 2019, the Company sold 5,102,000 shares of common stock to seven “accredited investors” at $0.03 per share for an aggregate purchase price of $154,500. The proceeds were used for working capital.
During July 2019, the Company sold 2,500,000 shares of common stock to one “accredited investors” at $0.02 per share for an aggregate purchase price of $50,000. The proceeds were used for working capital.
During August 2019 through September 2019, the Company sold 5,250,000 shares of common stock to four “accredited investors” at $0.02 per share for an aggregate purchase price of $105,000. The proceeds were used for working capital.
During November 2019 through January 2020, the Company sold 3,250,000 shares of common stock to three “accredited investors” at $0.02 per share for an aggregate purchase price of $65,000. The proceeds were used for working capital.
During February 2020 through April 2020, the Company sold 11,050,000 shares of common stock to five “accredited investors” at $0.02 per share for an aggregate purchase price of $221,000. The proceeds were used for working capital.
During April 2020 through May 2020, the Company sold 11,000,000 shares of common stock to Dr. Allen Meglin, a director of the Company at $0.02 per share for an aggregate purchase price of $220,000. During July and August 2020, the Company sold an additional 1,166,666 shares and 422,514 shares of common stock to Dr. Allen Meglin at $0.03 per share and $0.10 per share, respectively, for an aggregate purchase price of $77,251. The proceeds from all of the above sales were used for working capital.
On April 27, 2020, the Company sold 5,000,000 shares of common stock to Republic Asset Holdings LLC., a Company controlled by Michael Carbonara, a director of the Company, at $0.02 per share for an aggregate purchase price of $100,000. The proceeds were used for working capital.
During May 2020, the Company sold 3,000,000 shares of common stock to two “accredited investors” at $0.02 per share for an aggregate purchase price of $60,000. The proceeds were used for working capital.
| 79 |
During July and August 2020, the Company completed the private placement to 19 accredited investors for the sale of 13,499,992 shares of Common stock of the Company at a selling price of $0.03 per share for an aggregate amount of $405,000 (“Sale”). In connection with the Sale, the Company agreed that all of the proceeds from the Sale are to be deposited into a separate bank account (“Sale Account”) of the Company and the proceeds are to be used exclusively to fund the costs associated with the Company’s ongoing public company filing requirements, including audit, tax, valuation and legal fees. The Company also agreed to maintain the Sale Account with a minimum cash balance of $25,000 at all times until such time that the Company has filed all required financial reports through the period ended July 31, 2021.
During the period July 2020, the Company sold 1,000,000 shares of common stock to two “accredited investors”, at $0.02 per share and $0.03 per share, respectively for an aggregate purchase price of $25,000. The proceeds were used for working capital.
During the period August 2020, the Company sold 8,606,665 shares of common stock to nine “accredited investors”, at prices ranging from $0.03 per share and $0.06 per share, for an aggregate purchase price of $392,100. The proceeds were used for working capital.
During the period September 2020, the Company sold 4,800,000 shares of common stock to five “accredited investors”, at prices ranging from $0.06 per share and $0.10 per share, for an aggregate purchase price of $410,000. The proceeds were used for working capital.
Issuances of Common Stock – Stock Compensation:
On June 5, 2018, as further amended, the Company and a third party (“Consultant”) entered into a consulting agreement whereby the Consultant agreed to provide business development and other services to the Company (“Consulting Agreement”). The Consulting Agreement terminated on May 31, 2019. Under the terms of the Consulting Agreement, the Company agreed to grant the Consultant 4,500,000 shares of common stock of the Company (“Consultant Shares”). The Consultant Shares vested as follows; 1,700,000 shares upon execution of the Consultant Agreement; 1,700,000 shares on December 5, 2018; and 1,100,000 shares on January 1, 2019. The common stock was valued at $0.017 per share based on the closing price of the common stock of the Company on the execution date of the Consulting Agreement. The Company has recorded $45,900, $18,580 and $12,020 of stock-based compensation expense on June 5, 2018, December 5, 2018 and January 1, 2019 respectively.
During September 2018, in consideration for agreeing to serve as the Company’s Chief Medical Officer (“CMO”) and render other medical consulting and advisory services to the Company, the Board approved the issuance to the CMO of 2,500,000 shares of unregistered common stock valued at $0.0138 per share, the closing price of the common stock of the Company on that date. The Company recorded $34,500 of stock-based compensation expense based on the grant date fair value of these shares during the year ended October 31, 2018. In connection with the CMO’s appointment to the Board of Directors of the Company during February 2019, during February 2019 and August 2019, the Board approved the issuance to the CMO of 2,000,000 and 3,000,000 shares, respectively, of unregistered common stock valued at $0.025 per share and $0.028 per share, respectively, the closing price of the common stock of the Company on the grant dates. The Company recorded $50,000 and $84,000, during the quarters ended April 30, 2019 and October 31, 2019, respectively, of stock-based compensation expense based fair value of these shares on the grant date.
During the period November 1, 2018 through January 31, 2019, in consideration for agreeing to render medical consulting and advisory services to the Company, the Board approved the issuance to eight individuals an aggregate of 4,200,000 shares of unregistered common stock valued between $0.0055 and $0.0244 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $28,680 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended January 31, 2019.
| 80 |
During the period February 1, 2019 through April 30, 2019, in consideration for agreeing to provide medical consulting and advisory services to the Company, the Board approved the issuance to seven individuals an aggregate of 1,750,000 shares of unregistered common stock valued between $0.024 and $0.049 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $55,500 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended April 30, 2019.
During the period February 1, 2019 through April 30, 2019, in consideration for agreeing to provide lab and administrative consulting services to the Company, the Board approved the issuance to nine individuals an aggregate of 225,000 shares of unregistered common stock valued between $0.0265 and $0.080 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $11,888 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended April 30, 2019.
During the period May 1, 2019 through July 31, 2019, in consideration for agreeing to provide medical consulting and advisory services to the Company, the Board approved the issuance to twelve individuals an aggregate of 3,475,000 shares of unregistered common stock (of which 2,000,000 of the common stock issued shall vest over 36 months beginning July 2019) valued between $0.019 and $0.040 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $46,186 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended July 31, 2019.
During the period May 1, 2019 through July 31, 2019, in consideration for agreeing to provide lab and administrative consulting services to the Company, the Board approved the issuance to six individuals an aggregate of 2,675,000 shares of unregistered common stock valued between $0.023 and $0.040 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $70,725 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended July 31, 2019.
During May 2019, the Company entered into a sales representation agreement (“Sales Rep Agreement”) with a third party (“Sales Representative”) to market, promote and sell the Company’s products. As part of the Sales Rep Agreement, the Company agreed to provide the Sales Representative 1,000,000 million shares of common stock of the Company upon execution of the Sales Rep Agreement (valued at $0.035 per share, the closing price of the common stock of the Company on that date). The Company recorded $35,000 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended July 31, 2019. The initial term of the Sales Rep Agreement was one year, subject to earlier termination as provided for in the Sales Rep Agreement. In addition, under the terms of the Sales Rep Agreement, the Sales Representative was entitled to receive performance incentives for up to 10,500,000 million additional shares of common stock of the Company based on the Sales Representative achieving certain future quarterly sales milestones. None of the performance incentive shares were earned by the Sales Representative. Effective September 30, 2019, the Sales Representative and the Company mutually agreed to terminate the Sales Rep Agreement.
During the period August 1, 2019 through October 31, 2019, in consideration for agreeing to provide medical consulting and advisory services to the Company, the Board approved the issuance to two individuals an aggregate of 200,000 shares of unregistered common stock valued between $0.035 and $0.038 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $7,300 of stock-based compensation expense during the quarter ended October 31, 2019.
During the period August 1, 2019 through October 31, 2019, in consideration for agreeing to provide lab and administrative consulting services to the Company, the Board approved the issuance to four individuals an aggregate of 5,350,000 shares of unregistered common stock valued between $0.029 and $0.038 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $162,700 of stock-based compensation expense during the quarter ended October 31, 2019.
In connection with the previous appointment of an independent member to the Board of Directors of the Company, during August 2019, the Board approved the issuance to the director of 5,000,000 shares of unregistered common stock valued at $0.028 per share, the closing price of the common stock of the Company on the grant date. The Company recorded $140,000 of stock-based compensation expense during the quarter ended October 31, 2019.
| 81 |
As described in Note 14, upon execution of the VP Agreements, each of the Sales Executives were granted 1,000,000 shares of unregistered common stock of the Company valued at $0.035 per share, the closing price of the common stock of the Company on the grant date. The Company will record $35,000 of stock-based compensation expense on the grant date for each issuance. The VP Agreements also provide each Sales Executives the right to receive an additional 750,000 shares of common stock at the end of each quarterly anniversary of the VP Agreements throughout the Initial Term (maximum 9,000,000 shares) (“Performance Shares”), provided that the VP Agreements remain in effect during the applicable quarterly period. As of September 30, 2020, each Sales Executive has vested an additional 2,250,000 Performance Shares (total 4,500,000). The Company will record stock-based compensation expense for each respective quarterly period that the Performance Shares vest of $52,500 (total $157,500).
As described in Note 14, in connection with the execution of the Consultants Agreement, the Company issued to the Consultants 12,000,000 shares of unregistered common stock (“Shares”) valued at $0.022 per share, the closing price of the common stock of the Company on the grant date. The Company will record a total of $264,000 of stock-based compensation expense based on the vesting of the Shares (50% of the Shares vest as of the Effective Date of the Consultants Agreement 50% of the Shares vest on the six-month anniversary of the Consultants Agreement). The Company recorded $132,000 of stock-based compensation expense on the grant date and $132,000 during the quarter ended October 31, 2020.
During the period November 1, 2019 through January 31, 2020, in consideration for agreeing to provide lab and administrative consulting services to the Company, the Board approved the issuance to three individuals an aggregate of 650,000 shares of unregistered common stock valued between $0.027 and $0.031 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $18,650 of stock-based compensation expense during the three months ended January 31, 2020.
During the period February 1, 2020 through April 30, 2020, in consideration for agreeing to provide lab and administrative consulting services to the Company, the Board approved the issuance to four individuals an aggregate of 2,725,000 shares of unregistered common stock valued between $0.029 and $0.034 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $89,650 of stock-based compensation expense during the three months ended April 30, 2020.
During the period May 1, 2020 through July 31, 2020, in consideration for agreeing to provide lab and administrative consulting services to the Company, the Board approved the issuance to eight individuals an aggregate of 925,000 shares of unregistered common stock valued between $0.031 and $0.048 per share, the closing price of the common stock of the Company on the respective grants dates. For certain of the issuances, the stock vests on November 1, 2020, provided the recipient remains engaged with the Company during the period. The Company recorded $17,475 and $15,500 of stock-based compensation expense during the three months ended July 31, 2020 and on November 1, 2020, respectively.
During April 2020, May 2020, and September 2020, in consideration for agreeing to provide medical consulting and advisory services to the Company, the Board approved the issuance to eight individuals an aggregate of 950,000 shares of unregistered common stock valued between $0.023 and $0.28 per share, the closing price of the common stock of the Company on the respective grants dates. The Company recorded $16,100, $6,900 and $56,600 of stock-based compensation expense based on the grant date fair value of these shares during the quarters ended April 30, 2020, July 31, 2020 and October 31, 2020, respectively.
During February 2020, in recognition of past services provided to the Company through February 2020, the Board approved the issuance to the CMO of 5,000,000 shares of unregistered common stock valued at $0.028 per share, the closing price of the common stock of the Company on the grant date. The Company recorded $140,000 of stock-based compensation expense during the quarter ended April 30, 2020 based on the fair value of these shares on the grant date.
In connection with the resignation of an independent member of the Board of Directors of the Company in April 2020, the Board approved the issuance to the director of 736,808 shares of unregistered common stock valued at $0.022 per share, the closing price of the common stock of the Company on the grant date. The Company recorded $16,210 of stock-based compensation expense during the quarter ended April 30, 2020 based on the fair value of these shares on the grant date.
| 82 |
In connection with an agreement with an independent distributor dated May 28, 2020, the Company agreed to grant the distributor 3,000,000 shares of unregistered common stock valued at $0.115 per share, the closing price of the common stock of the Company on the grant date. The Company recorded $345,000 of stock-based compensation expense during the quarter ended July 31, 2020 based on the fair value of these shares on the grant date. In addition, the distribution agreement also provides for future stock incentives based on future sales that are generated by the distributor based on a conversion price equal to 75% of the trading price of the common stock on the last day of the month in which the incentive was earned.
On May 15, 2020 (“Effective Date”), the Company entered into an advisor agreement with a third party (“Advisor”) whereby the Advisor will provide financial advisory services (see Note 14). As consideration, the Company agreed to issue the Advisor 1,000,000 shares of common stock (“Grant”), of which 250,000 shares shall be fully vested as of the Effective Date, 250,000 shares vest on the sixth month anniversary of the Effective Date, 250,000 shares vest on the ninth month anniversary of the Effective Date and 250,000 shares vest on the twelfth month anniversary of the Effective Date, provided however that the Agreement is in full effect during such vesting period(s) for the respective portion of the Grant. In addition, Company agreed to grant 3-year warrants to the Advisor to purchase 6,000,000 shares of common stock of the Company at a purchase price of $0.04 per share (“Warrants”), of which Warrants to purchase 2,000,000 unrestricted shares shall be vested upon the Effective Date of the agreement and 2,000,000 and 2,000,000 of the remaining Warrants shall vest on the eighteenth month and thirtieth month anniversary of the Effective Date of the agreement, respectively, provided however that the Agreement is renewed and in full effect during the applicable vesting period(s) for the respective portion of the grant. Notwithstanding the above, any unvested Grant or Warrants prescribed above will immediately become vested shares if (a) the Company concludes a transaction involving any of the entities introduced by Advisor based on a transaction value greater than $5MM or (b) the Company completes any transaction that results in a change in control or any financing transaction with an aggregate value of at least $25MM. The Grant shares were valued at $0.04 per share, the closing price of the common stock of the Company on the grant date. The Company will record $10,000 of stock-based compensation expense during each quarter in which the Grant shares become vested based on the fair value of these vested shares on the grant date.
During July 2020, the Company entered into a consulting agreement with a third party to provide investment banking related consulting services for a minimum period of six months. As consideration for agreeing to provide consulting services to the Company, the Company issued the consultant 5,000,000 shares of unregistered common stock valued at $0.05 per share, the closing price of the common stock of the Company on the effective date of the agreement. All of the shares granted vested immediately on the date of issuance. The Company recorded $250,000 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended July 31, 2020.
During August 2020, the Company entered into two separate consulting agreements with third parties to provide marketing and public relations services for a minimum period of six months. As consideration for agreeing to provide consulting services to the Company, the Company issued the consultants 300,000 shares and 25,000 shares, respectively, of unregistered common stock valued at $0.127 per share, the closing price of the common stock of the Company on the effective date of the agreements. The Company recorded a total of $40,790 of stock-based compensation expense based on the grant date fair value of these shares during the quarter ended October 31, 2020.
Issuances of Common Stock – Exercise of warrants, Conversion of Debt and Exchanges:
During May 2019, the Company and holders of the $100,000 Debentures agreed to convert the principal amount of $100,000 Debentures plus interest accrued and unpaid through the date of the conversion totaling $100,622 into 3,773,584 shares of common stock of the Company (approximately $0.0267 per share representing a discount to the trading price of $0.0285 as of the effective date of the transaction).
During June 2019, the Company and the holder of the $30,000 Debenture agreed to convert the principal amount of the $30,000 Debenture plus interest accrued and unpaid through the date of the conversion totaling $30,478 into 1,111,111 shares of common stock of the Company (approximately $0.0274 per share representing a premium to the trading price of $0.0253 as of the effective date of the transaction).
| 83 |
On May 1, 2019, the Company and Mint Organics entered into an exchange agreement whereby the Company agreed to acquire the 150 shares of Mint Series A Preferred Stock and the 150,000 warrants to purchase shares of common stock of the Company originally issued to Mr. Wayne Rohrbaugh in connection with the initial capitalization of Mint Organics (see note 15) in exchange for 4,400,000 shares of common stock of the Company (approximately $0.034 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction).
On May 1, 2019, the Company, Mint Organics and the holder of a promissory note issued by Mint Organics (see note 15) agreed to a settlement of the outstanding loan whereby the Company agreed to issue the holder of the note 2,735,000 shares of newly issued common stock of the Company. At the time of the settlement, the outstanding obligation under the note, including late fees and penalties was approximately $72,568. The common stock issued was priced at $0.0265 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction.
On May 1, 2019, the Company and Mint Organics Florida entered into an exchange agreement whereby the Company agreed to acquire the 21.25 units from the minority equity holder of Mint Organics Florida (see note 15) in exchange for 2,400,000 shares of common stock of the Company (approximately $0.042 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction).
As more fully described in Note 9, the Noteholder fully funded the Funding Facility as prescribed on February 12, 2020 and the Company converted the Funding Facility into 40,000,000 shares of common stock of the Company (approximately $0.013 per share representing a discount of 60.5% to the trading price of $0.032 as of the effective date of the transaction).
During October 2020, the Company and the holder of the $20,000 debenture (one of the two holders that participated in the $70,000 Debentures described above), agreed to convert the principal amount of the $20,000 debenture plus interest accrued and unpaid through the date of the conversion totaling approximately $20,100 into 160,000 shares of common stock of the Company (approximately $0.125 per share representing a discount to the trading price of $0.278 as of the effective date of the transaction).
Management and Consultants Performance Stock Plan
On April 25, 2020, the Company approved the adoption of the Management and Consultants Performance Stock Plan (“MCPP”) providing for the grant to current senior executive members of management and third-party consultants of an aggregate of approximately 205,000,000 shares of common stock of the Company (“Shares”) based on the achievement of certain defined operational performance milestones (“Milestones”).
On June 29, 2020, the Board amended the MCPP, providing for the additional grant of common stock of the Company to the current senior executive members of management and the current non-executive members of the Board based on the Company completing any transaction occurring while employed and/or serving as a member of the Board, respectively, that results in a change in control of the Company or any sale of substantially all the assets of the Company (“Transaction”) which upon after giving effect to such issuance of shares below, corresponds to a minimum pre-Transaction fully diluted price per share of the Company’s common stock in the amounts indicated below.
| Pre-Transaction Price Per Share Valuation (a) | Executive Bonus Shares Issued (b) | Non-executive Board Bonus Shares Issued (c) | ||||||||
| $ | 0.22 | 40,000,000 | 2,000,000 | |||||||
| $ | 0.34 | 60,000,000 | 3,000,000 | |||||||
| $ | 0.45 | 80,000,000 | 4,000,000 | |||||||
| $ | 0.54 | 100,000,000 | 5,000,000 | |||||||
| (a) | proforma for issuance of all shares to be issued pursuant to the MCPP and other in the money contingent share issuances |
| (b) | per each executive consisting of Albert Mitrani, Dr. Mari Mitrani, Ian Bothwell, and Dr. George Shapiro |
| (c) | per each non-executive Board member consisting of Dr. Allen Meglin and Michael Carbonara |
| 84 |
On August 14, 2020, the Board amended the MCPP, providing for the additional grant of common stock of the Company to each Dr. Maria I. Mitrani and Ian Bothwell based on the Company obtaining aggregate gross fundings (grants for research and development and clinical trials, purchase contracts for Company products, debt and/or equity financings) or other financial awards during the term of employment with the Company based on the amounts indicated below:
Aggregate Funding Amount | Shares | |||||||||
| From | To | |||||||||
| $ | 2,500,000 | $ | 5,000,000 | 5,000,000 | ||||||
| $ | 5,000,001 | $ | 10,000,000 | 10,000,000 | ||||||
| $ | 10,000,001 | $ | 30,000,000 | 30,000,000 | ||||||
On September 23, 2020, the Board amended the MCPP, providing for the grant of common stock of the Company of 15.0 million, 7.5 million and 15.0 million shares of common stock of the Company, respectively, to each Albert Mitrani, Dr. Maria I. Mitrani and Ian Bothwell upon such time that the Company’s common stock trades above $0.25 per share, $0.50 per share and $0.75 per share, respectively, for 30 consecutive trading days subsequent to March 31, 2021 and provided such milestone occurs during the term of employment with the Company.
In addition, each of the current executives were entitled to receive an additional 7 million shares, which when combined with all previous IND and/or eIND’s Milestones previously issued under the MCPP of 43 million shares, represents the total of all incentive shares to be issued to each executive in connection with the combined thirteen IND’s and/or eIND’s Milestones achieved through September 23, 2020. In the future, each of the current executives shall be entitled to receive 5 million shares as a performance incentive for each IND and/or “Expanded Access” approval (and excluding all eIND’s) received by the Company that involve more than 15 patients and provided such milestone occurs during the term of employment with the Company.
Pursuant to the MCPP, as of September 23, 2020, a total of 233,000,000 shares have been issued and approximately 582,500,000 shares are authorized to be issued under the MCPP subject to the achievement of the defined contingent performance based milestones described above and provided the milestones are achieved while the individual is employed and/or serving as a member of the Board:
| MCPP | MCPP | |||||||||||
| MCPP | Remaining | Total | ||||||||||
| Shares | Shares | Shares | ||||||||||
| Name | Awarded | Available | Approved | |||||||||
| Albert Mitrani | 50,000,000 | 137,500,000 | 187,500,000 | |||||||||
| Ian Bothwell | 50,000,000 | 167,500,000 | 217,500,000 | |||||||||
| Dr. Maria I. Mitrani | 50,000,000 | 167,500,000 | 217,500,000 | |||||||||
| Dr. George Shapiro | 50,000,000 | 100,000,000 | 150,000,000 | |||||||||
| Dr. Allen Meglin | – | 5,000,000 | 5,000,000 | |||||||||
| Michael Carbonara | – | 5,000,000 | 5,000,000 | |||||||||
| Consultants | 33,000,000 | – | 33,000,000 | |||||||||
| Total | 233,000,000 | 582,500,000 | 815,500,000 | |||||||||
| 85 |
The Company will record stock-based compensation expense in connection with any MCPP Shares that are actually awarded based on the fair value as of the initial grant date that the respective milestone for the MCPP Shares were approved. For the MCPP Shares approved on April 25, 2020, June 29, 2020, August 14, 2020 and September 23, 2020, the closing price of the common stock of the Company was $0.027, $0.056, $0.128 and $0.28, respectively.
In connection with the MCPP Shares that have been awarded to date, all such shares were issued in connection with the MCPP Shares approved on April 25, 2020 and accordingly were valued $0.027 per share, the closing price of the common stock of the Company on the date that those respective MCPP Shares were approved. The Company will record a total of $3,915,000 of stock-based compensation expense during the quarter ended July 31, 2020 and $2,376,000 during the quarter ended October 31, 2020, respectively, based on the fair value of the actual MCPP Shares awarded.
NOTE 13 – WARRANTS
A summary of warrant activity for the years ended October 31, 2019 and 2018 are presented below:
| Number of Shares | Weighted-average Exercise Price | Remaining Contractual Term (years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at October 31, 2018 | 3,687,484 | $ | 0.41 | 1.14 | $ | – | ||||||||||
| Granted | 2,000,000 | $ | 0.08 | 1.00 | $ | – | ||||||||||
| Exercised | – | $ | – | |||||||||||||
| Expired/Forfeited | (1,158,313 | ) | $ | 0.67 | 0.04 | – | ||||||||||
| Outstanding at October 31, 2019 | 4,529,371 | $ | 0.20 | 0.30 | $ | – | ||||||||||
| Exercisable at October 31, 2019 | 4,529,371 | $ | 0.20 | 0.30 | $ | – | ||||||||||
| Number of Shares | Weighted-average Exercise Price | Remaining Contractual Term (years) | Aggregate Intrinsic Value | |||||||||||||
| Outstanding at October 31, 2017 | 158,137,484 | $ | 0.05 | 9.02 | $ | – | ||||||||||
| Granted | – | $ | – | – | $ | – | ||||||||||
| Exercised | (77,300,000 | ) | $ | 0.04 | 8.20 | $ | – | |||||||||
| Expired/Forfeited | (77,150,000 | ) | $ | 0.04 | 8.17 | $ | – | |||||||||
| Outstanding and exercisable at October 31, 2018 | 3,687,484 | $ | 0.41 | 1.14 | $ | – | ||||||||||
In connection with the Sale of ANU assets on February 5, 2018, and the immediate resignation and termination of the Company’s Chief Operating Officer, the Chief Operating Officer agreed to forfeit 53,300,000 warrants to purchase shares of the Company’s common stock held at the time of the resignation and the Company agreed to grant the Chief Operating Officer 7,500,000 shares of newly issued common stock of the Company.
In connection with the Sale of ANU assets on February 5, 2018, and the immediate resignation and termination of the Company’s Chief Technology Officer, the Chief Technology Officer agreed to forfeit 23,850,000 warrants to purchase shares of the Company’s common stock held at the time of the resignation and the Company agreed to grant the Chief Technology Officer 7,500,000 shares of newly issued common stock of the Company.
| 86 |
In connection with the amendment to the Chief Financial Officer’s employment agreement on April 6, 2018, the terms of the 31,800,000 and 21,500,000 warrants to purchase common shares of the Company previously granted to the Chief Financial Officer described above were modified to provide that in the event of an occurrence of a change in control or termination of the employment (as defined in the agreement), pursuant to the terms thereof, the exercise price for all outstanding warrants granted to the Chief Financial Officer to purchase common stock of the Company during the term of his employment agreement shall be reduced to $0.001 per share. The Company valued the repricing of the warrants based on the date of the modification using the Black-Scholes option pricing model with the following weighted average assumptions: (1) risk free interest rate 2.58%, (2) remaining term of 8.6 years - 8.9 years, (3) expected stock volatility of 68%, and (4) expected dividend rate of 0%. The grant date fair value of the modified warrants originally issued during November 2016 and March 2017 was $318,000 and $215,000, respectively. The Company recorded the expense for the total amount associated with modification of $533,000 at the time of the Reorganization, the event which triggered the vesting provision associated with the modification (see Note 5).
In connection with the amendment to the Chief Science Officer’s employment agreement on April 6, 2018, the terms of the 10,000,000 and 13,850,000 warrants to purchase common shares of the Company previously granted to the Chief Science Officer described above were modified to provide that in the event of an occurrence of a change in control or termination of the employment (as defined in the agreement), pursuant to the terms thereof, the exercise price for all outstanding warrants granted to the Chief Science Officer to purchase common stock of the Company during the term of her employment agreement shall be reduced to $0.001 per share. The Company valued the repricing of the warrants based on the date of the modification using the Black-Scholes option pricing model with the following weighted average assumptions: (1) risk free interest rate 2.58%, (2) remaining term of 8.6 years - 8.9 years, (3) expected stock volatility of 68%, and (4) expected dividend rate of 0%. The grant date fair value of the modified warrants originally issued during November 2016 and March 2017 was $100,000 and $138,500, respectively. The Company recorded the expense for the total amount associated with modification of $238,500 at the time of the Reorganization, the event which triggered the vesting provision associated with the modification (see Note 5).
In connection with the Reorganization, The CFO and CSO each agreed to exercise on a cashless basis all of their warrants to purchase 53,300,000 and 23,850,000 shares of common stock of the Company, respectively. Based on the closing price of the Company’s common stock on the Effective Date of $0.012 per share and the warrant exercise price of $0.001 per share, the CFO and CSO were required to use 4,675,439 and 2,092,105 shares of common stock received from the exercise of the warrants, respectively, to pay for the exercise price for exercising all of the warrants.
On March 7, 2019, the Company issued 2,000,000 warrants in connection with common stock offerings and valued the warrants on the dates of the grant using the Black-Scholes option pricing model with the following weighted average assumptions: (1) risk free interest rate 2.44%, (2) term of 1 year, (3) expected stock volatility of 108%, and (4) expected dividend rate of 0%. All of the warrants vested immediately. The grant date fair value of the warrants issued was $6,600.
On February 26, 2020, the Company issued the CFO a cashless warrant to purchase an aggregate of 7,500,000 shares of common stock in connection with the CFO’s employment agreement. The warrant is exercisable for $0.028 per share (the closing price of the Company’s common stock on the date of grant, until the tenth anniversary date of the date of issuance. The Company valued the warrants on the dates of the grant using the Black-Scholes option pricing model with the following weighted average assumptions: (1) risk free interest rate 1.14%, (2) term of 4 years, (3) expected stock volatility of 87%, and (4) expected dividend rate of 0%. All of the warrants vested immediately. The grant date fair value of the warrants issued was $214,500. The Company will record $214,500 of stock-based compensation expense during the quarter ended April 30, 2020 based on the fair value of these warrants on the grant date.
On May 15, 2020 (“Effective Date”), the Company granted the Advisor warrants to purchase 6,000,000 shares of common stock of the Company at a purchase price of $0.04 per share (“Warrants”) and exercisable for three years from the Effective Date. Warrants to purchase 2,000,000 shares shall be vested upon the Effective Date of the agreement and 2,000,000 and 2,000,000 of the remaining Warrants shall vest on the eighteenth month and thirtieth month anniversary of the Effective Date of the agreement, respectively, provided however that the agreement is renewed and in full effect during the applicable vesting period(s) for the respective portion of the grant. Notwithstanding the above, any unvested Warrants prescribed above will immediately become vested if (a) the Company concludes a transaction involving any of the entities introduced by Advisor based on a transaction value greater than $5,000,000 or (b) the Company completes any transaction that results in a change in control or any financing transaction with an aggregate value of at least $25,000,000. The Company valued the warrants on the dates of the grant using the Black-Scholes option pricing model with the following weighted average assumptions: (1) risk free interest rate 0.31%, (2) term of 3 years, (3) expected stock volatility of 90%, and (4) expected dividend rate of 0%. The grant date fair value of the warrants issued was $121,200. The Company will record $40,400 of stock-based compensation expense during the period that the Grant shares vest based on the fair value of these warrants on the grant date (see Note 14).
| 87 |
NOTE 14 – COMMITMENTS AND CONTINGENCIES
Executive Employment Agreements
Effective November 4, 2016, the Company entered into executive employment agreements with Albert Mitrani, Dr. Maria Mitrani, Bruce Werber, and Ian Bothwell. On March 8, 2017, the Company entered into an executive employment agreement with Terrell Suddarth, and amended the employment agreements of Dr. Mitrani, Dr. Werber and Mr. Bothwell. On February 5, 2018, Dr. Werber’s and Mr. Suddarth’s’ employment agreements were terminated in connection with the Sale. On April 6, 2018, the Company amended Mr. Bothwell’s and Dr. Mitrani’s employment agreement, which provided among other things, that in the event of an occurrence of a change in control or termination of the employment (as defined in agreement) pursuant to the terms thereof, the exercise price for all outstanding warrants granted to Mr. Bothwell and Dr. Mitrani to purchase common stock of the Company during the term of the agreement shall be reduced to $0.001 per share. In addition, Mr. Bothwell’s employment agreement was amended to increase the initial term and the automatic renewal term provided for in the employment agreement from three years to five years, increased the amount of automobile expense allowance and removed the cap for the reimbursement of office related expenses. Collectively, the aforementioned executive employment agreements are referred to as the FY 2017 Executive Employment Agreements.
In connection with Sale (see Note 4), Werber and Suddarth each entered into a Separation and General Release Agreement with the Company effective upon the closing of the Sale which provided for the immediate resignation of Werber and Suddarth of all their respective executive and Board of Director positions held with the Company and/or any of the Company’s subsidiaries, and the termination and settlement of all obligations of each party to the other pursuant to the respective employment agreements, including the release of all rights the Company may have held in any intellectual property of Werber and Suddarth and any non-compete restrictions on Werber and Suddarth. In connection with such releases, Werber and Suddarth each agreed to forfeit all warrants previously granted and outstanding (a total of 77,150,000 warrants to purchase shares of common stock of the Company), forfeit any and all accrued and unpaid amounts owing under the employment agreements for past due wages, benefits, severance obligations, unreimbursed expenses and any other obligations owing to one another as of the date of the Sale totaling $906,515 in exchange for a grant of 7,500,000 shares of restricted common stock of the Company to each of Werber and Suddarth (the grant date fair value of the newly issued shares issued to each of Werber and Suddarth was $83,250).
In connection with the Reorganization, Mr. Mitrani’s, Dr. Mitrani’s and Mr. Bothwell’s FY 2017 Executive Employment Agreements were terminated in favor of newly executed employment agreements (collectively referred to as the April 2018 Executive Employment Agreements). In addition, as a condition of the Reorganization, Mr. Mitrani, Mr. Bothwell and Dr. Mitrani each agreed to release the Company for all amounts owed to them for unpaid salaries through the Effective Date and unpaid advances and/or expenses incurred prior to December 31, 2017 totaling $1,636,808. This amount is reflected in stockholders’ deficit for the year ended October 31, 2018. The significant terms provided for in the FY 2017 Executive Employment Agreements and the April 2018 Executive Employment Agreements are summarized below:
April 2018 Executive Employment Agreements
General
Pursuant to Albert Mitrani’s April 2018 Executive Employment Agreement, Mr. Mitrani serves as the Company’s President and Chief Operating Officer. Mr. Mitrani’s base annual salary is $162,500, which shall accrue commencing on the Effective Date and shall be payable in equal semi-monthly installments, commencing May 1, 2018, in arrears. The base salary shall be reviewed at least annually by the Board and the Board may, but shall not be required to, increase the base salary during the Employment Term. Mr. Mitrani is also entitled to a commission on all sales attributable to him (i.e., excluding existing customers of the Company at the time of the Reorganization) at the rate of five percent (5%) of the "Net Sales" as defined in the agreement and an expense allowance of $5,000 per month.
Pursuant to Ian Bothwell’s April 2018 Executive Employment Agreement, Mr. Bothwell continues to serve as the Company’s Chief Financial Officer. Mr. Bothwell’s base annual salary is $162,500, which shall accrue commencing on the Effective Date and shall be payable in equal semi-monthly installments, commencing May 1, 2018, in arrears. The base salary shall be reviewed at least annually by the Board and the Board may, but shall not be required to, increase the base salary during the Employment Term. Mr. Bothwell has not been paid salary since July 2018.
| 88 |
Pursuant to Dr. Maria I. Mitrani’s April 2018 Executive Employment Agreement, Dr. Mitrani continues to serve as the Company’s Chief Science Officer. Dr. Mitrani’s base annual salary is $162,500, which shall accrue commencing on the Effective Date and shall be payable in equal semi-monthly installments, commencing May 1, 2018, in arrears. The base salary shall be reviewed at least annually by the Board and the Board may, but shall not be required to, increase the base salary during the Employment Term.
Term
The term of each of the April 2018 Executive Employment Agreements commences as of the Effective Date and continues until December 31, 2020 (Mr. Bothwell) or December 31, 2023 (Mr. Mitrani and Dr. Mitrani) (“Initial Term”), unless terminated earlier pursuant to the terms of the April 2018 Executive Employment Agreement; provided that on such expiration of the Initial Term, and each annual anniversary thereafter (such date and each annual anniversary thereof, a “Renewal Date”), the agreement shall be deemed to be automatically extended, upon the same terms and conditions, for successive periods of one year, unless either party provides written notice of its intention not to extend the term of the April 2018 Executive Employment Agreement at least 90 days’ prior to the applicable renewal Date. The period during which the Executive is employed by the Company hereunder is hereinafter referred to as the “Employment Term.”
Unpaid Advances
The Company was required to repay the unpaid advances subsequent to December 31, 2017, and the unreimbursed expenses incurred subsequent to December 31, 2017, on May 15, 2018. Such payments were not made as required.
Fringe Benefits and Perquisites
During the Employment Term, each Executive shall be entitled to fringe benefits and perquisites consistent with the practices of the Company, and to the extent the Company provides similar benefits or perquisites (or both) to similarly situated executives of the Company.
Termination
The Company may terminate the April 2018 Executive Employment Agreement at any time for good cause, as defined in the April 2018 Executive Employment Agreement, including, the Executive’s death, disability, Executive’s willful and intentional failure or refusal to follow reasonable instructions of the Company’s Board of Directors, reasonable and material policies, standards and regulations of the Company’s Board of Directors or management.
Amendments To The April 2018 Executive Employment Agreements
February 26, 2020 Amendment
On February 26, 2020, the Company agreed to modify the employment agreement of Mr. Ian T. Bothwell, the Company’s Chief Financial Officer to provide Mr. Bothwell with:
| a) | an extension to his employment agreement dated April 13, 2018 from December 2020 to December 2023 consistent with other executives of the Company; and |
| b) | and a one-time bonus in the form of a fully vested cashless warrant to purchase 7,500,000 shares of common stock of the Company, exercisable for ten years at an exercise price of $0.28 per share, the closing price of the common stock on the date of the grant. |
On February 26, 2020, pursuant to the respective employment agreements with each of the Company’s executive officers, the Board granted each of Mr. Albert Mitrani, Dr. Maria Mitrani and Mr. Ian Bothwell a cash bonus of $37,500 for the calendar year ended December 31, 2019.
| 89 |
April 25, 2020 Amendment
On April 25, 2020, the Company agreed to amend and revise the each of Albert Mitrani, Ian Bothwell and Dr. Maria I. Mitrani, (individually each of A. Mitrani, Bothwell and Dr. Mitrani are referred to as an “Executive” and collectively the “Executives”) April 2018 Executive Employment Agreements. The primary amended terms associated with the agreements for each Executive were substantially similar and consisted of the following:
| Term: | An extension to the term of the employment agreements dated April 13, 2018 from December 31, 2023 to December 31, 2025. | |
| Base Salary: | An increase in base annual salary from $162,500 to $300,000. The amended salary amount of $300,000 shall be retroactively adjusted to commence as of January 1, 2019. The increased annual salary of $137,500 (“Incremental Salary”) over the prior annual salary amount of $162,500 (“Original Base Salary”) shall only be paid only upon there being sufficient available cash. Beginning July 1, 2020, at the sole option of the Executive, any portion of unpaid Original Base Salary for periods after January 1, 2020, including unpaid bonus salary, may be converted by Executive into common stock at a conversion rate equal to the average trading price during the month in which the accrued salary pertains. For any unpaid Original Base Salary that existed prior to January 1, 2020, including unpaid bonus salary, the amounts may be converted at a conversion price using the closing trading price of the stock on the last trading day in December 2019.
Beginning December 1, 2020, at the sole option of the Executive, all unpaid Incremental Salary for periods after January 1, 2020 may be converted by the Executive into common stock at a conversion rate equal to the average trading price during the month in which the accrued salary pertains. For any unpaid Incremental Salary that existed prior to January 1, 2020, the amounts may be converted at a conversion price using the closing trading price of the stock on the last trading day in December 2019.
Until such time as the Executive elects to convert, the accrued and unpaid salary, including Original Base Salary and Incremental Salary shall remain an obligation of the Company. |
Severance Provisions:
| 1. | Company termination without cause, Executive for good reason: |
| a) | All existing accrued obligations existing at time of termination shall be paid to Executive. |
| b) | Any unvested equity grants in favor of Executive shall immediately become fully vested and any pending grants pursuant to the MCPP eligible to be issued to Executive shall be granted to Executive, regardless of whether the associated milestone were achieved prior to termination, |
| c) | Executive shall be entitled to a cash payment equal to his unpaid base salary for the remaining term in effect at time of the time of the termination or an amount equal to four times (4x's) the base salary in effect at the time of termination, whichever is greater, |
| d) | Executive shall be entitled to a cash payment equal to his 200% of the prior year’s cash or stock bonus (excluding any stock grants received pursuant to the MCPP). |
| 2. | Change In Control: In the event of a Change in Control and the Executive’s employment agreement is not extended for period of five years from the date of the Change in Control with all other terms and conditions of the agreement remaining the same, then the Executive may terminate the agreement for good reason and all respective severance terms as provided for a termination by Executive for good reason described in clause 1 above shall be provided to Executive. |
| 3. | Executive termination due to disability, death, or non-renewal by Company: |
| a) | All existing accrued obligations existing at time of termination shall be paid to Executive. |
| b) | Any unvested equity grants in favor of Executive shall immediately become fully vested and any pending grants pursuant to the MCPP eligible to be issued to Executive shall be granted to Executive, regardless of whether the associated milestone were achieved prior to termination. |
| c) | Executive shall be entitled to a cash payment equal to 299% of Executive’s base salary in effect at the time of termination, plus a gross up amount to cover Executive’s tax liability associated with such payment. |
| d) | 200% of the prior years cash or stock bonus (excluding MCPP performance stock grants). |
| 90 |
June 29, 2020 Amendment
On June 29, 2020, the board of directors of the Company (“Board”) agreed to further amend and revise the April 2018 Executive Employment Agreements for each of Executives. The primary amended terms associated with the agreements for each Executive were substantially similar and consisted of the following:
| Base Salary: | An increase in the Executives annual base annual salary upon such time that the Company achieves monthly revenues in the amounts provided below, provided such monthly revenue increase occurs for four consecutive months. Upon the achievement of the defined salary milestone, the salary adjustment will be retroactive to the first month in which the salary threshold was met. Any adjustment pursuant to this provision shall not be reduced for any future reduction in revenues that may occur. |
| Monthly Revenues (in millions) | Base Salary Increase | |||||
| $ | 1.00 | $ | 130,000 | |||
| $ | 1.50 | $ | 200,000 | |||
| $ | 2.00 | $ | 275,000 | |||
| $ | 3.50 | $ | 630,000 | |||
| $ | 5.00 | $ | 900,000 | |||
Coordinator Agreement
Effective September 30, 2019, the Sales Representative and the Company mutually agreed to terminate the Sales Rep Agreement in exchange for the principal executive of the Sales Representative (“Coordinator”) agreeing to become an employee of the Company effective October 1, 2019 (“Coordinator Agreement”). The Coordinator Agreement provided that the parties would seek to negotiate in good faith a definitive employment agreement. The parties did not executive a definitive agreement and the Coordinator is no longer providing services to the Company.
Advisor Agreement
Effective May 15, 2020 (“Effective Date”), the Company entered into a one-year agreement (“Advisor Agreement”) with an individual to provide financial advisory services to the Company (“Advisor”). The Advisor Agreement is subject to successive, automatic one (1) year extensions unless either party has given the other 30- day written notice prior to the expiration of then in effect termination date, of their desire not to renew the Advisor Agreement. As the compensation for Advisor’s services and his fulfillment of all obligations under the agreement the Company agreed to issue the Advisor 1,000,000 shares of common stock (“Stock Grant”), of which 250,000 shares shall be fully vested as of the Effective Date, 250,000 shares vest on the sixth month anniversary of the Effective Date, 250,000 shares vest on the ninth month anniversary of the Effective Date and 250,000 shares vest on the twelfth month anniversary of the Effective Date, provided however that the Advisor Agreement is in full effect during such vesting period(s) for the respective portion of the Stock Grant. In addition, Company agreed to grant 3-year warrants to the Advisor to purchase 6,000,000 shares of common stock of the Company at a purchase price of $0.04 per share (“Warrants”), of which Warrants to purchase 2,000,000 unrestricted shares shall be vested upon the Effective Date of the Advisor Agreement and 2,000,000 and 2,000,000 of the remaining Warrants shall vest on the eighteenth month and thirtieth month anniversary of the Effective Date of the Advisor agreement, respectively, provided however that the Advisor Agreement is in full effect during the applicable vesting period(s) for the respective portion of the grant. Notwithstanding the above, any unvested Stock Grant or Warrants prescribed above will immediately become vested shares if (a) the Company concludes a transaction involving any of the entities introduced by Advisor based on a transaction value greater than $5,000,000 or (b) the Company completes any transaction that results in a change in control or any financing transaction with an aggregate value of at least $25,000,00. The Advisor Agreement may be terminated by the Company based on Advisor’s breach of any of the terms of the Advisor Agreement, the Company’s determination that Advisor is not meeting the desired objectives or if either party provides notice of the desire not to renew the Advisor Agreement upon expiration.
| 91 |
Sales Executives
On January 6, 2020, the Company entered into employment agreements with two individuals (“Sales Executives”), each to serve as a Vice President – Global Sales and Marketing. The terms of each Sales Executive employment agreement are identical (“VP Agreements”). The initial term of the VP agreements are for three years and provide for automatic annual renewals thereafter, unless either party provides 90-day written notice prior to expiration of the then current term. The VP Agreements may also be terminated by the Company beginning June 30, 2020 in the event the Sales Executive fails to meet certain defined minimum revenue growth milestones. The Sales Executives will receive compensation in the form of monthly salary of $18,000 and a quarterly override based on revenues earned by the Company during a quarterly period that exceed $600,000 beginning for the quarter ended June 30, 2020. In addition, upon execution of the Agreement, each of the Sales Executives were granted 1,000,000 shares of unregistered common stock of the Company valued at $0.035 per share, the closing price of the common stock of the Company on the grant date. The Company will record $35,000 of stock-based compensation expense on the grant date for each issuance. The VP Agreements also provide the Sales Executives with the right for each to receive an additional 750,000 shares of common stock at the end of each quarterly anniversary of the VP Agreements throughout the Initial Term (maximum 9,000,000 shares) (“Performance Shares”), provided that the VP Agreements remain in effect during the applicable quarterly period. The vesting of the Performance Shares may also be accelerated based on achievement of certain revenue milestones. The Company will record stock-based compensation expense for each respective quarterly period that the Performance Shares vest of $52,500.
Consultants Agreement
Effective March 30, 2020 (the “Effective Date”), the Company entered into a consulting agreement (“Agreement”) with Assure Immune L.L.C. (the “Consultant”) for an initial term of one year (the “Initial Term”) with automatic renewals for two (2) additional annual periods (each a “Renewal Term,” and together with the “Initial Term,” the “Term”), unless written notice is provided by either party at least 45 days prior to the applicable termination date. Under the Agreement, the Consultant will provide the Company during the Term with expertise, experience, advice and direction associated with the critical functional executive level roles of the Company as it relates to the oversight and management of the Company’s regulatory, research and development and laboratory operations, consistent with the Company’s corporate mission and strategies and subject to the resource limitations of the Company. In connection with the Agreement, the Consultants will receive monthly fees of $30,000 during the Initial Term and monthly consulting fees of $35,000 and $40,000 the first and second Renewal Terms, if any. In addition. the Company agreed to issue to the Consultant or its designees 12,000,000 shares of common stock of the Company (“Shares”), 50% of which Shares vest as of the Effective Date and balance of which Shares vest upon the six-month anniversary of the Effective Date. The Agreement also provides that upon the commencement of each Renewal Term, if any, the Consultant will receive up to 6,000,000 additional Shares, 50% of which Shares will vest on the commencement date of the Renewal Term and the balance of which additional Shares will vest on the six (6) month anniversary of such date. In connection with the Agreement, the Consultant (and its principals) are obligated to comply with customary confidentiality, non-compete and non-solicitation covenants and have agreed that all intellectual property developed during the term of the Agreement shall remain the property of the Company.
In addition to the Shares to be issued above, the Consultant or its designees will be entitled to participate in the Company’s Management and Consultants Performance Stock Plan (the “MCPP”), more fully described in Note 12. Pursuant to the MCPP, the Consultant or its designees may be awarded up to 33,000,000 Shares, based on the achievement of certain defined operational performance milestones (“Milestones”) during the Term of the Agreement and for a period of twelve (12) months after the expiration or earlier termination of the Agreement, provided that expiration or termination is not for “cause” or the Consultant’s non-renewal of the Agreement.
Leases
Ethan NY
On September 3, 2015, Ethan NY entered into a five-year lease agreement (“Ethan Lease”) for a store located in New York City, New York. The Ethan Lease commenced on October 1, 2015. Under the terms of the Ethan Lease, minimum monthly lease payments of $9,500 per month were to commence in December 2015 through October 2020. During June 2016, Ethan NY exited from its leased premises. Ethan NY did not make any of the required minimum monthly lease payments as required. The total amount of minimum lease payments that Ethan NY is obligated to pay pursuant to this 5-year lease is $586,242 (excluding late fees and interest provided for under the Ethan Lease).
| 92 |
All of Ethan NY’s obligations under the Ethan Lease are recourse only to the assets at Ethan NY, except for certain obligations under the Ethan Lease that were guaranteed by a former employee. Under the terms of the Ethan Lease, the obligations of Ethan NY for future rents are to be mitigated based on the amount of any future rents that are received for the rental of the leased premises to other tenants during the initial term. During August 2016, Ethan NY received confirmation that the leased premises had been leased to another tenant. In connection with the termination of the Ethan Lease, Ethan NY has made several unsuccessful attempts to contact the landlord for the purpose of obtaining a settlement and release for any amounts that the landlord may claim are owing under the Ethan Lease, if any. Ethan NY is not aware of any claim pending or threatened in connection with the Ethan Lease. At October 31, 2018 and 2019, Ethan NY has recorded in liabilities of discontinued operations the amount of rent obligations through June 30, 2016 and a reserve for estimated losses in connection with termination of the Ethan Lease of $101,905 and $101,905, respectively.
Lab Facilities:
Anu Life Sciences, Inc.
Anu Life Sciences Inc. a Florida corporation (“ANU”), entered into a five-year lease agreement (“Lab Lease”) for an approximately 3,500 square foot laboratory and administrative office facility in Sunrise, Florida. The Lab Lease was effective July 1, 2017 and was to expire on June 30, 2022.
As described in Note 4, in connection with the Sale, ANU sold or transferred to Vera its right, title and interest in the Lab Lease (including the associated security deposits of $37,275) and all leasehold improvements.
The minimum monthly lease payments under the Lab Lease, excluding applicable Florida sales tax and additional rents as may be required under the terms of the Lab Lease, were approximately $7,900 for the first 24 months and $9,000 per month, $9,200 per month and $9,400 per month for the third, fourth and fifth years, respectively. Minimum lease payments commenced July 1, 2017. The Company recorded lease expense on a straight-line basis over the life of the lease. The Company recorded lease expense in connection with the Lab Lease of $25,803 for the period November 1, 2017 through February 5, 2018.
2019 Lab Facility:
In connection with the Company’s decision to again operate a placental tissue bank processing laboratory in Miami, Florida, during February 2019, the Company entered into a renewable month to month lease agreement (“Miami Lab Lease”) for an approximately 450 square foot laboratory and a 100 square foot administrative office facility. Monthly lease payments are approximately $5,200 plus administrative fees and taxes. In connection with the Miami Lab Lease, the Company was required to post a security deposit of $6,332.
Effective March 2019, the Company entered into an agreement to lease certain manufacturing equipment (“Equipment Lease”) to be used in its lab, including a full care maintenance plan for such equipment totaling approximately $239,595. The lease agreement is for five years and requires minimum monthly lease payments of approximately $4,513 per month, plus sales taxes.
The minimum lease payments pursuant to the Equipment Lease are as follows:
| Year Ended | Minimum | ||||
| October 31, | Rent | ||||
| 2020 | $ | 72,208 | |||
| 2021 | 54,156 | ||||
| 2022 | 54,156 | ||||
| 2023 | 54,156 | ||||
| 2024 | 18,052 | ||||
| Total | $ | 252,728 | |||
| 93 |
Administrative Office:
The Company’s corporate administrative offices are leased from MariLuna, LLC, a Florida limited liability company which is owned by Dr. Mitrani. The term of the lease runs through June 2023 and the monthly rental rate through June 2020 is $2,900 and thereafter $3,500.
The minimum lease payments pursuant to the office lease are as follows:
| Year Ended | Minimum | ||||
| October 31, | Rent | ||||
| 2020 | $ | 37,200 | |||
| 2021 | 42,000 | ||||
| 2022 | 42,000 | ||||
| 2023 | 28,000 | ||||
| 2024 | – | ||||
| Total | $ | 149,200 | |||
Preparation of IRB, Pre-IND, IND Protocols for Clinical Applications and Clinical Trial Initiation and Monitoring:
In connection with the Company’s ongoing research and development efforts and the Company’s efforts to meet compliance with current and anticipated United States Food and Drug Administration (“FDA”) regulations expected to be enforced beginning in May 2021 pertaining to marketing traditional biologics and human cells, tissues and cellular and tissue based products that fall under Section 351 of the Public Health Services Act (“HCT/Ps”), the Company has applied for and received Investigation New Drug (“IND”) approval from the FDA to commence clinical trials in connection with the use of the Company’s products and related treatment protocols for specific indications. The ability to successfully complete the above efforts will be dependent on the Company’s ability to timely fund the required payments and complete the applicable clinical trials, which is subject to available working capital generated from operations, financing arrangements with the third party vendors involved in the studies and/or from additional debt and/or equity financings as well as ultimate approval from the FDA.
Contingent Convertible Obligations Into Equity Securities
Private Placement Of Convertible 6% Debentures
As more fully described in Note 9, the remaining outstanding Convertible Debentures (the principal and all accrued but unpaid interest thereon) contained provisions that under certain conditions, provided the ability of the holders of the Convertible Debentures at their option at any time, from time to time to convert into shares of the common stock of the Company. The conversion prices were based on the Company completing a contemplated pending reverse split at the Company’s sole discretion (which the Company elected not to pursue) or at conversion prices greatly in excess of the historical prices of the Company’s common stock and reasonably expected prices of the Company’s common stock to be realized during the term of the Convertible Debentures. As a result, none of the Convertible Debentures have been or are expected to be converted in accordance with their conversion provisions. The contingent rights to convert for certain of the convertible debentures did not result in any underlying value attributable to the fair value of the embedded derivatives liabilities associated with respective Convertible Debentures.
| 94 |
Obligations Due Under Executive Employment Agreements
Beginning July 1, 2020, at the sole option of the Executive, any portion of unpaid Original Base Salary for periods after January 1, 2020, including unpaid bonus salary, may be converted by Executive into common stock at a conversion rate equal to the average trading price during the month in which the accrued salary pertains. For any unpaid Original Base Salary that existed prior to January 1, 2020, including unpaid bonus salary, the amounts may be converted at a conversion price using the closing trading price of the stock on the last trading day in December 2019.
Beginning December 1, 2020, at the sole option of the Executive, all unpaid Incremental Salary for periods after January 1, 2020 may be converted by the Executive into common stock at a conversion rate equal to the average trading price during the month in which the accrued salary pertains. For any unpaid Incremental Salary that existed prior to January 1, 2020, the amounts may be converted at a conversion price using the closing trading price of the stock on the last trading day in December 2019.
None of the Executives have yet to elect to convert any portion of their unpaid Original Base Salary.
As of June 30, 2020, there was approximately $721,415 of unpaid Original Base Salary and Incremental Salary related to the period prior to December 31, 2019 and $93,110 of unpaid Original Base Salary and Incremental Salary related to the period January 1, 2020 through June 30, 2020.
NOTE 15 – MINT ORGANICS
Mint Organics Inc. (“Mint Organics”) authorized capital consists of (i) 1,000 shares of Class A voting common stock, par value $0.001 per share (“Class A Common Stock”); (ii) 1,000 shares of Class B Non-voting common stock, par value $0.001 per share (“Class B Common Stock”); and (iii) 1,000 shares of Preferred Stock, par value $0.001 per share. Organicell owns 550 shares of Class A Common Stock, representing 100% of the outstanding shares of Class A Common Stock. There are no shares of Class B Common Stock currently outstanding.
Pursuant to the Certificate Of Designation filed on February 28, 2017 and as amended on March 23, 2017, Mint Organics authorized 300 shares of Series A convertible preferred stock, par value $0.001 per share and a stated value of $1,000 per share (“Mint Series A Preferred Stock”). The Mint Series A Preferred Stock is non-voting and non-redeemable. The amount of each share of the Mint Series A Preferred Stock shall automatically convert into 1.5 shares of Class B Common Stock of Mint Organics upon the earlier of (a) the fifth anniversary of the date such share of Mint Series A Preferred Stock was issued; or (b) Mint Organics’ receipt of the necessary licenses and permits required to operate business operations in the medical cannabis industry. In addition, commencing on the first anniversary of the issuance date and for the 90-day period thereafter, each holder of the Mint Series A Preferred Stock shall have the right, but not the obligation, to convert some or all of such holder’s shares of Mint Series A Preferred Stock (or Class B Common Stock equivalent) into unregistered shares, par value $0.001 per share, of common stock of Organicell, based on the stated value divided by the average trading price of Organicell common stock for the ten trading days prior the conversion date. Notwithstanding the foregoing, the number of shares of Class B Common Stock issuable upon the conversion of the outstanding Mint Series A Preferred Stock shall be adjusted to ensure that the outstanding Class B Common Stock represents 45% of the outstanding capital stock of Mint Organics (based on conversion of 300 shares of the Mint Series A Preferred Stock or pro rata portion thereof).
Mint Organics issued to each of Taddeo and Rohrbaugh (i) 150 shares of Mint Series A Preferred Stock and (ii) a warrant exercisable for up to 150,000 shares of Organicell’s common stock for $0.15 per share exercisable from the date of issuance until the third anniversary of the date of issuance (see Note 13).
Mr. Peter Taddeo (“Taddeo) and Mr. Wayne Rohrbaugh (“Rohrbaugh”) each invested $150,000 to fund the initial operations of Mint Organics. The Company immediately established Mint Organics, , a 55%-owned subsidiary of the Company and Mint Organics Florida, Inc. (“Mint Organics Florida”), a wholly owned subsidiary of Mint Organics, each dedicated to obtain a license to dispense medical cannabis in Florida (collectively Mint Organics and Mint Organics Florida are referred to as the “Mint Organics Entities”). In connection with the investment, Mint Organics issued to each of Taddeo and Rohrbaugh (i) 150 shares of Mint Series A Preferred Stock and (ii) a warrant exercisable for up to 150,000 shares of Organicell’s common stock for $0.15 per share exercisable from the date of issuance until the third anniversary of the date of issuance (see Note 13).
| 95 |
In addition, in connection with the agreement, Taddeo was appointed as the Chief Executive Officer and as a director of the Mint Organics Entities. Rohrbaugh was appointed as the Chief Operating Officer and as a director of the Mint Organics Entities.
On March 8, 2017, Mint Organics issued warrants to purchase shares of Class A Common Stock, of Mint Organics, vesting on the date Mint Organics, through one of its subsidiaries, obtains a license from a state to dispense cannabis until the fifth anniversary thereof to the following executives of Mint Organics:
| Name: | Warrants | Exercise Price: | ||||||
| Albert Mitrani | 79 | $ | 0.001 | |||||
| Ian T. Bothwell | 79 | $ | 0.001 | |||||
| Dr. Maria I. Mitrani | 79 | $ | 0.001 | |||||
| TOTAL | 237 | |||||||
In connection with an independent valuation using a Black-Scholes option model, the fair value of the warrants issued were determined to be $34,949. At the time of issuance, the Company estimated that the warrants would be fully vested by December 31, 2017. The Company has recorded amortization expense totaling $0 and $6,889 during the year ended October 31, 2019 and 2018, respectively, as additional stock-based compensation.
Taddeo Employment Agreement
Pursuant to an employment agreement entered into effective May 1, 2017, with Mr. Taddeo (“Taddeo”) and Mint Organics (“Taddeo Employment Agreement”), Mr. Taddeo shall serve as the Chief Executive Officer of Mint Organics (“Mint CEO”) and a member of the Board of Directors of Mint Organics (“Mint Board”). The employment term shall be for three years, unless terminated earlier pursuant to the terms of the agreement, and thereafter deemed to be automatically extended, upon the same terms and conditions, for successive periods of one year, unless either party provides written notice of its intention not to extend the term at least 90 days prior to the applicable renewal date. The Mint CEO’s base annual salary is $180,000 during the period prior to Mint Organics, through one of its subsidiaries, or by other means, obtains or acquires access for a license from a state to dispense cannabis which shall accrue commencing as of the effective date and shall be payable upon Mint Organics generating sufficient net revenue or obtaining sufficient third party financing; and thereafter payable in periodic installments in accordance with Mint Organics customary payroll practices, but no less frequently than monthly. The Mint CEO’s base salary shall automatically be adjusted to an annual rate of base salary of $250,000 once the license is obtained. The base salary shall be reviewed at least annually by the Mint Board and the Mint Board may, but shall not be required to, increase the base salary during the employment term. In connection with the execution of the agreement, Mint Organics agreed to pay the Mint CEO a $25,000 signing bonus which shall be accrued and paid by Mint Organics upon Mint Organics having sufficient cash flow. The agreement also contains terms regarding eligibility for future annual bonuses, annual equity awards under Mint Organics’ equity plan, if any, fringe benefits and perquisites consistent with the practices Mint Organics (including health and dental insurance, an automobile expense allowance of $1,000 per month, and reimbursement for all reasonable and necessary out-of-pocket business, entertainment and travel expenses incurred by the Mint CEO in accordance with Mint Organics’ expense reimbursement policies. Mint Organics may terminate the agreement at any time with or without “Cause” and the Mint CEO may resign at any time with or without “Good Reason” (as defined in the agreement). The nature of the obligations owing to the Mint CEO upon termination is more fully described in the agreement. In connection with the execution of the agreement, the Company granted the Mint CEO 1,000,000 shares of unregistered common stock of Organicell, which vested on December 31, 2017 (see Note 12).
On April 6, 2018, Peter Taddeo (“Mint CEO”) resigned as a member of the Board of Directors of the Company and as the Chief Executive Officer and member of the board of directors of the Mint Organics Entities. In connection with Mr. Taddeo’s resignation, Mr. Taddeo entered into a Separation and General Release Agreement (“Taddeo Separation Agreement”) whereby Mr. Taddeo agreed to release the Mint Organics Entities from all obligations in connection with the Taddeo Agreement and all other agreements and/or financial obligations between the parties related to the Taddeo’s employment or services performed with any of Mint Organics Entities totaling $156,568. In consideration for Taddeo entering into the Taddeo Separation Agreement, the Mint Organics Entities paid Taddeo $5,000 and Mr. Bothwell paid $3,000 to Taddeo for the purchase of the 1,000,000 shares of common stock of the Company that were granted to Taddeo in connection with the Taddeo Agreement. Contemporaneously with the execution of the Taddeo Separation Agreement, the Company and Mr. Taddeo entered into a Share Purchase and General Release Agreement whereby the Company agreed to purchase from Mr. Taddeo his 150 shares of Mint Series A Preferred Stock for an aggregate purchase price of $40,000.
| 96 |
Exchange Agreement
On May 1, 2019, the Company and Mint Organics entered into an exchange agreement whereby the Company agreed to acquire the 150 shares of Mint Series A Preferred Stock and the 150,000 warrants to purchase shares of common stock of the Company originally issued to Mr. Wayne Rohrbaugh in connection with participation agreement referred to above in exchange for 4,400,000 shares of common stock of the Company (approximately $0.034 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction). In connection with the exchange, Mr. Rohrbaugh provided a release to the Company in connection with any claims associated with his original investment.
Mint Organics Florida, Inc.
Mint Organics Florida’s authorized capital structure consists of (1) 10,000 shares of Class A voting common stock (“Class A Common Stock”), par value $0.001 per share and (ii) 10,000 shares of Class B Non-voting common stock (“Class B Common Stock”), par value $0.001 per share. The Class A Common Stock shall have the sole right and power to vote on all matters on which a vote of shareholders is to be taken. In all matters, with respect to actions both by vote and by consent, each holder of shares of the Class A Common Stock shall be entitled to cast one vote in person or by proxy for each share of Class A Common Stock standing in such holder’s name on the transfer books of the Corporation. The Class B Common Stock shall not be entitled to vote on any matters.
On February 28, 2017, the Board of Mint Organics Florida issued 2,125 shares of Class A Common Stock, par value $0.001 per share, of Mint Organics Florida to Mint Organics and determined that the fair consideration for the initial issuance of the Class A Common Stock is $0.001 per share.
Offering:
On March 17, 2017, Mint Organics Florida initiated an offering to raise up to $1,000,000 in exchange for up to 212.5 shares of Class B Common Stock, representing approximately 10.0% of the outstanding equity of Mint Organics Florida as of the date of the offering. The proceeds of the offering were to be used for general working capital purposes. On April 6, 2017, Mint Organics received proceeds of $100,000 in connection with the sale of 21.25 units to an investor in connection with the offering (representing a 1% minority interest in the equity of Mint Organics Florida).
On May 1, 2019, the Company and Mint Organics Florida entered into an exchange agreement whereby the Company agreed to acquire the 21.25 units from the investor referred to above in exchange for 2,400,000 shares of common stock of the Company (approximately $0.042 per share representing a discount to the trading price of $0.049 as of the effective date of the transaction). In connection with the exchange, the investor provided a release to the Company in connection with any claims associated with the investor’s original investment.
Non-controlling interests in Mint Organics and Mint Organics Florida
The Company’s non-controlling interests in Mint Organics and Mint Organics Florida at October 31, 2019 and October 31, 2018 are determined based on the pro rata equity percentage held by the non-controlling equity holders of Mint Organics and Mint Organics Florida during each of the respective periods, provided however, that the carrying amount of non-controlling interests shall not be negative.
Effective May 1, 2019, the Company has acquired all of the minority interests issued in Mint Organics and Mint Organics Florida, and accordingly, there no longer exists any non-controlling interests in those entities as of such date.
At October 31, 2018, the non-controlling interests of Mint Organics Inc. and Mint Organics Florida were 22.5% and 4.0%, respectively. As of October 31, 2018, the non-controlling interests representing the minority interest’s share of both Mint Organics and Mint Organics Florida equity was $42,977.
| 97 |
NOTE 16 – LIABILITIES ATTRIBUTABLE TO DISCONTINUED OPERATIONS
During September 2015, the Company formed Ethan NY for the purpose of selling clothing and accessories through a retail store. During June 2016, the Ethan NY operations were closed.
The following summarizes the carrying amounts of the assets and liabilities of Ethan NY at October 31, 2019 and 2018 (see Note 14):
| October 31, | ||||||||
| 2019 | 2018 | |||||||
| Assets | $ | – | $ | – | ||||
| Liabilities: | ||||||||
| Accounts Payable | $ | 94,835 | $ | 94,835 | ||||
| Accrued Expenses | 31,016 | 31,016 | ||||||
| $ | 125,851 | $ | 125,851 | |||||
NOTE 17 - SEGMENT INFORMATION
For the years ended October 31, 2019 and 2018, the Company operated only one operating segment.
NOTE 18 – SUBSEQUENT EVENTS
Several subsequent events are disclosed in Notes 4, 5, 8, 9, 12, 13, 14, and 15. There were no other subsequent events for disclosure purposes.
| 98 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
As previously reported in a Form 8-K filed on August 16, 2018, effective July 1, 2018, our principal independent accountants, GBH CPAs, PC (“GBH”) completed the combination of its practice into Marcum LLP (“Marcum”). As a result of the aforementioned, on August 13, 2018, we formally accepted the resignation of GBH and engaged Marcum as its independent registered public accountants. The engagement of Marcum was approved by our board of directors.
In connection with the foregoing change in accountants, there was no disagreement of the type described in paragraph (a)(1)(iv) if Item 304 of Regulation S-K or any reportable event as described in paragraph (a)(1)(v) of such Item.
ITEM 9A. CONTROLS AND PROCEDURES.
Evaluation of Controls and Procedures.
In accordance with Exchange Act Rules 13a-15 and 15d-15, our management is required to perform an evaluation under the supervision and with the participation of the Company’s management, including the Company’s principal executive and principal financial officers, or persons performing similar functions, of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as of the end of the period.
Based on their evaluation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act) as of October 31, 2019, our Principal Executive Officer and Principal Financial Officer have concluded that our disclosure controls and procedures were not effective to provide reasonable assurance that information we are required to disclose in reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934, as amended, as a process designed by, or under the supervision of, the Company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the Company’s board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America and includes those policies and procedures that: pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of the assets of the company; provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of the inherent limitations of internal control, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk.
| 99 |
As of October 31, 2019, management assessed the effectiveness of our internal control over financial reporting based on the criteria for effective internal control over financial reporting established in Internal Control-Integrated Framework of 2013 issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) and SEC guidance on conducting such assessments.
Based on that evaluation under this framework, our management concluded that as of October 31, 2019, our internal control over financial reporting was not effective because of the following material weaknesses:
| § | Due to our small number of employees and resources, we have limited segregation of duties, as a result of which there is insufficient independent review of duties performed. |
| § | Due to our small number of employees and resources, we have limited segregation of duties, as a result of which do not have the ability to implement internal controls over the granting of access to our IT environment. |
| § | As a result of the limited number of accounting personnel, we rely on inexperienced staff and outside consultants for the preparation of our financial reports, including tax preparation, which could lead to overlooking items requiring disclosure. |
| § | The Company’s Board of Directors at October 31, 2019 were solely comprised of two outside directors and the remaining directors served also as the executive management of the Company. The Board does not have an audit committee or an independent audit committee financial expert nor did it have either one at October 31, 2019. While not being legally obligated to have an audit committee or independent audit committee financial expert, it is the management’s view that to have an audit committee, comprised of independent board members, and an independent audit committee financial expert, is an important entity-level control over the Company’s financial statements. |
| § | The Company did not file this Annual Report on Form 10-K or the three quarterly reports on Form 10-Q for the fiscal quarters January 31, 2019, April 30, 2019 and July 31, 2019 by their required due dates. In addition, the Company has not filed the three quarterly reports on Form 10-Q for the fiscal quarters ended January 31, 2020, April 30, 2020 and July 31, 2020 within the appropriate filing deadlines. The Company has been delinquent in its filings with the SEC under the Securities Exchange Act of 1934, as amended. This delinquency is due to the Company’s limited financial and personnel resources. These delays limit the Company’s ability to timely analyze and identify potential operational and disclosure transactions within management and to comply with financial reporting regulations. |
Management’s Remediation Initiatives
In an effort to remediate the identified material weaknesses and other deficiencies and enhance our internal controls, in addition to the engagement of Ian T. Bothwell as the Chief Financial Officer (Principal Financial and Accounting Officer) of the Company in November 2016, the Company in May 2019 hired a new accountant to fill the vacancy of the prior accountant that resigned in October 2018. In addition, the Company has increased its other administrative support personnel since May 2019. The Company also engaged outside tax consultants in December 2018 to assist in advising the Company in ongoing tax matters. During July and August 2020, the Company completed a private placement in the amount of $405,000 (“Sale”). The proceeds are to be used exclusively to fund the costs associated with the Company’s ongoing public company filing requirements, including audit, tax, valuation and legal fees.
If and when the Company obtains sufficient capital resources, the Company intends to hire additional personnel with sufficient U.S. GAAP knowledge and business experience and to segregate appropriate duties among them. The Company has also begun efforts to further automate its accounting, sales ordering and inventory management functions.
We also intend to appoint one or more independent members to our Board of Directors who shall also be appointed to a standing audit committee which will undertake the oversight in the establishment and monitoring of required internal controls and procedures such as reviewing and approving estimates and assumptions made by management. While we are actively seeking outside members, including candidates with accounting experience, we cannot provide any assurance that we will be successful. Given the size of our Company, lack of revenues and current lack of financing to continue with our business, it is unlikely that we will be able to hire any additional personnel or that independent directors will agree to join our Board until general economic conditions and our own business prospects improve significantly.
| 100 |
This annual report does not include an attestation report of our registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to temporary rules of the SEC that permit us to provide only management’s report in this annual report.
Changes in Internal Controls
No change in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) occurred during the fourth quarter ended October 31, 2019 that has materially affected, or is reasonably likely to materially affect, the Company’s internal control over financial reporting.
None.
| 101 |
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Directors and Executive Officers
Below are the names of and certain information regarding the Company’s current executive officers and directors:
| Name: | Age: | Position: | Director Since: |
| Albert Mitrani | 65 |
Chief Executive Officer, Chief Operating Officer, President, Secretary and Director (Principal Executive Officer) |
June 24, 2015 |
| Ian T. Bothwell | 60 |
Chief Financial Officer and Director (Principal Financial and Accounting Officer) |
September 11, 2019 March 8, 2017 - April 13, 2018 |
| Dr. Maria Ines Mitrani | 40 | Chief Science Officer, VP and Director |
August 14, 2019 November 4, 2016 - April 13, 2018 |
| Dr. George Shapiro | 59 | Director and Chief Medical Officer | February 7, 2019 |
| Dr. Allen Meglin | 61 | Director |
April 2, 2020 |
| Michael Carbonara | 37 | Director |
April 2, 2020 |
Mr. Manuel Iglesias and Mr. Robert Zucker both served as Directors of the Company during the fiscal year ended October 31, 2019 and both resigned as Directors of the Company on April 25, 2020 and April 15, 2020, respectively.
Directors are elected to serve until the next annual meeting of stockholders and until their successors are elected and qualified. Directors are elected by a plurality of the votes cast at the annual meeting of stockholders and hold office until the expiration of the term for which he or she was elected and until a successor has been elected and qualified.
A majority of the authorized number of directors constitutes a quorum of the Board of Directors for the transaction of business. The directors must be present at the meeting to constitute a quorum. However, any action required or permitted to be taken by the Board of Directors may be taken without a meeting if all members of the Board of Directors individually or collectively consent in writing to the action.
Executive officers are appointed by, and serve at the pleasure of, the Board of Directors of the Company, subject to any contractual arrangements.
Professional Experience
Albert Mitrani has been serving as our President, Secretary, Treasurer and a member of the Board of Directors since June 24, 2015. Mr. Mitrani has also been serving as our Chief Executive Officer since September 2019. Mr. Mitrani was also our Chief Executive Officer and Chairman of the Board from June 24, 2015 until April 13, 2018. Mr. Mitrani served as the Chief Executive Officer of Analytical Stem Cell Corp. from April 2014 through May 2015. Analytical Stem Cell was involved in stem cell research and patient treatment referral centers. From February 2012 through March 2014 Mr. Mitrani was the Chief Executive Officer of Americell Trinidad and the President of ASCAAC LLC (American Stem Cell) from March 2011 through January 2013. Mr. Mitrani was the Chief Executive Officer of American Cellular Center Quito Ecuador from 2009 through 2012.
| 102 |
Ian T. Bothwell was elected as a member of the Board of Directors of the Company effective September 11, 2019. Mr. Bothwell previously served as a member of the Board of Directors of the Company from March 8, 2017 until his resignation in April 2018, when the Company executed a Plan and Agreement of Reorganization. Mr. Bothwell serves as the Chief Financial Officer of the Company, a position he has held since November 4, 2016. From 2003 through November 2015, Mr. Bothwell served in various executive positions for Central Energy GP LLC, the general partner of Central Energy Partners LP, a previously publicly traded master limited partnership. From July 2007 through November 2015, Mr. Bothwell served as President and a director of Regional Enterprises, Inc. Since April 2007, Mr. Bothwell has served as the President and controlling member of Rover Advanced Technologies, LLC, a company formed to provide management solutions to the public transportation industry. Since 2015, Mr. Bothwell has also served as the President and controlling member of CountOnMe Inc., a company that provides software solutions for the educational industry. Mr. Bothwell received his Bachelor of Science in Business Administration from Boston University in 1984.
Dr. Maria Ines Mitrani was elected as a member of the Board of Directors of the Company effective August 14, 2019. Dr. Mitrani previously served as a member of the Board of Directors of the Company from November 4, 2016 until her resignation in April 2018, when the Company executed a Plan and Agreement of Reorganization. Dr. Mitrani is a cofounder of the Company and is its Chief Science Officer. Dr. Mitrani previously served as the Executive Vice President of Analytical Stem Cell from 2014 to 2015. From 2012 to 2014, Dr. Mitrani served as the Executive Vice President, Medical Tourism Coordinator and Patient Referral Coordinator of Americell Trinidad, LLC. From 2008 to 2014, Dr. Mitrani was with the American Stem Cell & Anti-Aging center where she co-founded the first autologous stem cell center in Quito, Ecuador. Dr. Mitrani received a degree in medicine from Universidad San Francisco de Quito, in Quito, Ecuador.
Dr. Mitrani is the spouse of Albert Mitrani, Chief Executive Officer, President, Chief Operating Officer, Co-Founder and a director of the Company.
Dr. George Shapiro was elected as a member of the Board of Directors of the Company effective February 2019. Since September 2018, Dr. Shapiro has served as the Company’s Chief Medical Officer. George C. Shapiro has been in practice for over 27 years. His career in medicine began in 1988 when he graduated from New York Medical College. An internship and residency then followed at Albert Einstein college of Medicine, after which, Dr. Shapiro completed a Cardiovascular Disease fellowship at Columbia University College of Physicians and Surgeons in 1994. Dr. Shapiro is currently a cardiologist in private practice.
Michael Carbonara was elected as a member of the Board of Directors of the Company effective April 2020. Since 2015. Mr. Carbonara has served as the Chief Executive Officer of the Phoenix Group, a company that provides international financial and banking services. In addition, Mr. Carbonara has successfully worked directly with financial regulators in Canada, Europe and Asia to establish regulated banking and payment institutions as well as a SICAV (Société d'investissement à Capital Variable) alternative investment fund. Mr. Carbonara currently serves on the board of directors of several private United States and international companies. Mr. Carbonara is a member of the Association of Certified Anti-Money Laundering Specialists® (“ACAMS”), the largest international membership organization dedicated to enhancing the knowledge skills and expertise of anti- money laundering/counter terrorist financing and financial crime detection and prevention professionals.
Mr. Carbonara received his Associates Degree in Business Administration in 2006. The Company believes that Mr. Carbonara’s financial and business experience, including his significant international business experience and expertise in financial technology, regulatory compliance, payments, cross border remittance and e-commerce consulting services make him qualified to be a member of the Board.
| 103 |
Dr. Allen Meglin was elected as a member of the Board of Directors of the Company effective April 2020. Since 2015. Since June 2019, Dr. Meglin has served on the Company’s Products and Technical Advisory Board. Since 2005, Dr. Meglin has served as a staff radiologist for Chatham Radiologists, P.A. a medical facility specializing in interventional radiology and musculoskeletal radiology. Dr. Meglin also serves as the Medical Director for Northeast Georgia Aesthetics and is the owner operator of several proprietorships involved in providing aesthetics, chiropractic and wellness services. Throughout his career, Dr Meglin has been a frequent lecturer and presenter, has issued many medical related publications, has served on the faculty and taught various courses at educational institutions, has participated in as a principal investigator in several clinical research studies, and holds several medical based patents. Dr. Meglin also currently serves on the board of directors of several private United States companies. Dr. Meglin is also a member of the American Heart Association - Scientific Council Committee, the American Academy of Regenerative Medicine and serves on the FDA’s education materials committee.
Dr. Meglin currently holds the following licenses and certifications:
| · | Registered Vascular Technologist, ARDMS |
| · | Certificate in Added Qualifications in Vascular and Interventional Radiology from the American Board of Radiology |
| · | National Board of Medical Examiners Diplomate |
| · | Medical License from the state of North Carolina |
Dr. Meglin earned a M.D from the University of Pittsburgh - School of Medicine, Pittsburgh, PA and completed his Diagnostic Radiology Residency from the Walter Reed Army Medical Center, Washington, DC. The Company believes that Dr. Meglin’s medical industry expertise make him qualified to be a member of the Board.
Family Relationships
Albert Mitrani, our President and Chief Executive Officer, and Dr. Maria Ines Mitrani, our Chief Science Officer, are spouses.
Involvement in Certain Legal Proceedings
None of our directors or executive officers has been involved in any of the following events during the past ten years:
| ● | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; | |
| ● | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offences); | |
| ● | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his or her involvement in any type of business, securities or banking activities; or | |
| ● | being found by a court of competent jurisdiction (in a civil action), the Commission or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Audit Committee
We currently do not have a separately standing Audit Committee due to our limited size and our Board performs the functions that would otherwise be performed by an Audit Committee.
| 104 |
Compensation Committee
The Company does not have a Compensation Committee due to our limited size and our Board performs the functions that would otherwise be performed by a Compensation Committee. Our Board intends to form a Compensation Committee when needed.
Other Committees
We do not currently have a separately designated standing nominating committee. Further, we do not have a policy with regard to the consideration of any director candidates recommended by security holders. To date, no security holders have made any such recommendations. The entire Board of Directors performs all functions that would otherwise be performed by committees. Given the present size of our Board, it is not practical for us to have committees other than those described above, or to have more than two directors on such committees. If we are able to grow our business and increase our operations, we intend to expand the size of our board and our committees and allocate responsibilities accordingly.
Code of Ethics
Due to our small size, we have not adopted a Code of Ethics and Business Conduct that applies to our officers, directors and employees. We intend to adopt a Code of Ethics and Business Conduct in the near future as we grow our operations and hire additional employees.
Compliance with Section 16(a) of the Securities Exchange Act of 1934
Section 16(a) of the Exchange Act requires our executive officers and directors and persons who own more than 10% of a registered class of our equity securities to file with the SEC initial statements of beneficial ownership, reports of changes in ownership and annual reports concerning their ownership of our common stock and other equity securities, on Forms 3, 4 and 5 respectively. Executive officers, directors and greater than 10% shareholders are required by the SEC regulations to furnish us with copies of all Section 16(a) reports that they file.
Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that all filing requirements applicable to our officers, directors and greater than 10% beneficial owners were complied with under Section 16 of the Exchange Act during the fiscal year ended October 31, 2019 and up through the date of this filing except as follows: Ian T. Bothwell filed a late Form 4 in August 2020 regarding the grant of warrants in February 2020 and the grant of common shares in May 2020 and a late Form 4 in September 2020 regarding the grant of common shares in August 2020 and September 2020; Dr. Allen Meglin filed a late Form 3 in August 2020 regarding his appointment as a director in March 2020 and a late Form 4 in August 2020 regarding the purchase of common shares in April 2020, May 2020 and July 2020 and a late Form 4 in September 2020 regarding the purchase of common shares in August 2020; Dr. Maria Ines Mitrani filed a late Form 4 in August 2020 regarding the grant of common shares in May 2020 and a late Form 4 in September 2020 regarding the grant of common shares in August 2020 and September 2020; Dr. George Shapiro filed a late Form 4 in August 2020 regarding the grant of common shares in May 2020 and a late Form 4 in September 2020 regarding the grant of common shares in August 2020 and September 2020; Robert W. Zucker filed a late Form 4 in August 2020 in connection with his grant of common shares in April 2020; and Albert Mitrani filed a late Form 4 in August 2020 regarding the grant of common shares in May 2020 and a late Form 4 in September 2020 regarding the grant of common shares in August 2020, September 2020c.n and as a result of being the spouse Dr. Maria Ines Mitrani, who was a reporting person from the issuances and exercises described above.
Michael Carbonara filed a late Form 3 in September 2020 regarding his appointment as a director in March 2020 and a late Form 4 in September 2020 regarding the purchase of common shares in April 2020.
| 105 |
ITEM 11. EXECUTIVE COMPENSATION
The following table sets forth information concerning the total compensation paid or accrued by the Company during the last two fiscal years indicated to (i) all individuals that served as the Company’s principal executive officer or acted in a similar capacity for the Company at any time during the fiscal year ended October 31, 2019; (ii) the two most highly compensated executive officers who were serving as executive officers of the Company at the end of the fiscal year ended October 31, 2019 whose total compensation exceeded $100,000; and (iii) up to two additional individuals for whom disclosure would have been provided pursuant to clause (ii) above but for the fact that the individual was not serving as an executive officer of the Company at the end of the fiscal year ended October 31, 2019.
SUMMARY COMPENSATION TABLE
Name and Principal Position |
Fiscal Year |
Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($) | Non-equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) |
All Other Consideration ($) | Total Actually Received ($) | |||||||||||||||||||||||||||
| Manuel Iglesias, | 2019 | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | |||||||||||||||||||||||||||
| CEO (1), (7) | 2018 | -0- | -0- | 2,758,071 | (1) | -0- | -0- | -0- | -0- | 2,758,071 | ||||||||||||||||||||||||||
| Albert Mitrani - CEO, President | 2019 | 339,852 | (8) | -0- | -0- | -0- | -0- | -0- | 50,205 | (13) | 390,057 | |||||||||||||||||||||||||
| Secretary and Treasurer (2) (7) | 2018 | 264,343 | (8) | -0- | (14) | -0- | -0- | -0- | -0- | 27,503 | (13) | 291,846 | ||||||||||||||||||||||||
| Dr. Maria I. Mitrani, VP and | 2019 | 277,083 | (9) | -0- | -0- | -0- | -0- | -0- | -0- | 277,083 | ||||||||||||||||||||||||||
| Chief Science Officer (3) (7) | 2018 | 224,726 | (9) | -0- | (15) | 25,942 | (3) | 238,500 | (3) | -0- | -0- | -0- | 489,168 | |||||||||||||||||||||||
| Ian T. Bothwell, | 2019 | 277,083 | (10) | -0- | -0- | -0- | -0- | -0- | -0- | 277,083 | ||||||||||||||||||||||||||
| Chief Financial Officer (4) (7) | 2018 | 251,685 | (10) | -0- | (16) | 57,975 | (4) | 533,000 | (4) | -0- | -0- | -0- | 842,660 | |||||||||||||||||||||||
| Dr. Bruce Werber, | 2019 | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | |||||||||||||||||||||||||||
| Chief Operating Officer (5) | 2018 | 95,671 | (11) | -0- | (17) | 83,250 | (5) | -0- | -0- | -0- | -0- | 178,921 | ||||||||||||||||||||||||
| Terrell Suddarth, | 2019 | -0- | -0- | -0- | -0- | -0- | -0- | -0- | -0- | |||||||||||||||||||||||||||
| Chief Technology Officer (6) | 2018 | 79,726 | (12) | -0- | (18) | 83,250 | (6) | -0- | -0- | -0- | -0- | 162,976 | ||||||||||||||||||||||||
| (1) | Mr. Iglesias was appointed as the Chief Executive Office and principal executive officer in April 2018. Mr. Iglesias was removed as Chief Executive Officer and principal executive officer in September 2019. Mr. Iglesias did not receive any salary for his services during the years ended October 31, 2019 and 2018. In connection with the Reorganization, Management and Business Associates, LLC, an entity of which Manuel E. Iglesias has voting and dispositive control received 222.425,073 shares of common stock. The Company recorded $2,758,071 of stock compensation expense associated with the issuance of the shares referred to above and Mr. Iglesias’s agreement to serve as the Company’s Chief Executive Officer. |
| 106 |
| (2) | Albert Mitrani was appointed as the Chief Executive Officer, President, Secretary and Treasurer of the Company on June 24, 2015. He was replaced as Chief Executive Officer in April 2018. He was appointed as Chief Executive Officer and principal executive officer in September 2019. |
| (3) | Dr. Maria I. Mitrani is Albert Mitrani’s wife. Dr. Maria I. Mitrani was appointed as the Vice President and Chief Science Officer of the Company on November 4, 2016. During April 2018, Dr. Mitrani’s employment agreement was amended to provide for a reduction in the exercise price of her outstanding warrants to $0.001 per share in the event of a change in control. The value of the warrant modification was $238,500 which vested at the time of the Reorganization when the change in control event was triggered. Effective April 13, 2018, Dr. Mitrani was granted 2,092,105 shares of common stock of the Company with a grant value of $25,942. See Notes 12 and 13 to the October 31, 2018 audited consolidated financial statements for a description of the assumptions used in determining the value of the warrants modified and the stock granted. |
| (4) | Ian Bothwell was appointed as the Chief Financial Officer of the Company on November 4, 2016. During the year ended October 31, 2018, $156,615 of the costs associated with the warrants previously granted to Mr. Bothwell were vested. During April 2018, Mr. Bothwell’s employment agreement was amended to provide for a reduction in the exercise price of his warrants to $0.001 per share in the event of a change in control. The value of the warrant modification was $533,000 which vested at the time of the Reorganization when the change in control event was triggered. Effective April 13, 2018, Mr. Bothwell was granted 4,675,439 shares of common stock of the Company with a grant value of $57,975. See Notes 12 and 13 to the October 31, 2018 audited consolidated financial statements for a description of the assumptions used in determining the value of warrants modified and the stock granted. |
| (5) | Dr. Bruce Werber was appointed as the Chief Operating Officer of the Company on November 4, 2016. Dr. Werber resigned in February 2018. In connection with Dr. Werber’s resignation in February 2018, Dr. Werber forfeited all accrued and unpaid compensation owed as of the date of the Sale and all the warrants he previously received in connection with his employment agreement in exchange for receiving 7,500,000 shares of common stock with a grant value of $83,250. |
| (6) | Terrell Suddarth was appointed as the Chief Technology Officer of the Company on March 8, 2017. Mr. Suddarth resigned in February 2018. In connection with Mr. Suddarth’s resignation in February 2018, Mr. Suddarth forfeited all accrued and unpaid compensation owed as of the date of the Sale and all the warrants he previously received in connection with his employment agreement in exchange for receiving 7,500,000 shares of common stock with a grant value of $83,250. |
| (7) | Effective April 13, 2018, in connection with Reorganization, Manuel Iglesias replaced Albert Mitrani as the Chief Executive Officer of the Company, Ian Bothwell resigned from the Board of Directors of the Company and Dr. Maria Mitrani resigned from the Board of Directors of the Company. In addition, effective April 13, 2018, Albert Mitrani, Ian Bothwell, and Dr. Maria Mitrani, each agreed to terminate their respective executive employment agreements, dated November 4, 2016, as amended, in favor of new employment agreements (“April 2018 Executive Employment Agreements”) and Albert Mitrani, Ian Bothwell and Dr. Maria Mitrani each agreed to release the Company for all amounts owing to them for unpaid salaries through the Effective Date and advances and/or expenses incurred prior to December 31, 2017. |
| (8) | $132,105 and $12,659 of salary and commissions were accrued and unpaid at October 31, 2019 and 2018, respectively. During April 2018 in connection with the Reorganization, a total of $480,304 of accrued and unpaid salary was forfeited and written off. |
| (9) | $129,613 and $22,210 of salary was accrued and unpaid at October 31, 2019 and 2018, respectively. During April 2018 in connection with the Reorganization, a total of $401,089 of accrued and unpaid salary was forfeited and written off. |
| (10) | $321,907 and $44,824 of salary was accrued and unpaid at October 31, 2019 and 2018, respectively. During April 2018 in connection with the Reorganization, a total of $486,938 of accrued and unpaid salary was forfeited and written off. |
| (11) | $0 of salary was accrued and unpaid at October 31, 2019 and 2018. During February 2018 in connection with the Sale, a total of $467,670 of accrued and unpaid salary was forfeited and written off. |
| (12) | $0 of salary was accrued and unpaid at October 31, 2019 and 2018. During February 2018 in connection with the Sale, a total of $271,818 of accrued and unpaid salary was forfeited and written off. |
| (13) | Albert Mitrani’s and his wife, Dr. Maria I. Mitrani, received benefits totaling approximately $50,205 and $27,503 during the fiscal year ended October 31, 2019 and 2018, respectively. |
| (14) | The unpaid signing bonus in the amount of $100,000 was forfeited and written off in connection with the Reorganization in April 2018. |
| (15) | The unpaid signing bonus in the amount of $50,000 was forfeited and written off in connection with the Reorganization in April 2018. |
| (16) | The unpaid signing bonus in the amount of $35,000 was forfeited and written off in connection with the Reorganization in April 2018. |
| (17) | The unpaid signing bonus in the amount of $35,000 was forfeited and written off in connection with the Sale in February 2018. |
| (18) | The unpaid signing bonus in the amount of $35,000 and $105,000 in additional bonus was forfeited and written off in connection with the Sale in February 2018. |
| 107 |
We have no plans in place and have never maintained any plans that provide for the payment of retirement benefits or benefits that will be paid primarily following retirement including, but not limited to, tax qualified deferred benefit plans, supplemental executive retirement plans, tax-qualified deferred contribution plans and nonqualified deferred contribution plans.
Outstanding Equity Awards at Fiscal Year-End
There were no outstanding equity awards as of October 31, 2019. The Company has securities authorized for issuance under the 2020 Plan, the Board Plan and the MCPP that were granted subsequent to October 31, 2020.
Executive Employment Agreements
Effective November 4, 2016, the Company entered into executive employment agreements with Albert Mitrani, Dr. Maria Mitrani, Bruce Werber, and Ian Bothwell. On March 8, 2017, the Company entered into an executive employment agreement with Terrell Suddarth, and amended the employment agreements of Dr. Mitrani, Dr. Werber and Mr. Bothwell. On February 5, 2018, Dr. Werber’s and Mr. Suddarth’s’ employment agreements were terminated in connection with the Sale. On April 6, 2018, the Company amended Mr. Bothwell’s and Dr. Mitrani’s employment agreement, which provided among other things, that in the event of an occurrence of a change in control or termination of the employment (as defined in agreement) pursuant to the terms thereof, the exercise price for all outstanding warrants granted to Mr. Bothwell and Dr. Mitrani to purchase common stock of the Company during the term of the agreement shall be reduced to $0.001 per share. In addition, Mr. Bothwell’s employment agreement was amended to increase the initial term and the automatic renewal term provided for in the employment agreement from three years to five years, increased the amount of automobile expense allowance and removed the cap for the reimbursement of office related expenses. Collectively, the aforementioned executive employment agreements are referred to as the FY 2017 Executive Employment Agreements.
In connection with Sale, Werber and Suddarth each entered into a Separation and General Release Agreement with the Company effective upon the closing of the Sale which provided for the immediate resignation of Werber and Suddarth of all their respective executive and Board of Director positions held with the Company and/or any of the Company’s subsidiaries, and the termination and settlement of all obligations of each party to the other pursuant to the respective employment agreements, including the release of all rights the Company may have held in any intellectual property of Werber and Suddarth and any non-compete restrictions on Werber and Suddarth. In connection with such releases, Werber and Suddarth each agreed to forfeit all warrants previously granted and outstanding (a total of 77,150,000 warrants to purchase shares of common stock of the Company), forfeit any and all accrued and unpaid amounts owing under the employment agreements for past due wages, benefits, severance obligations, unreimbursed expenses and any other obligations owing to one another as of the date of the Sale totaling $906,515 in exchange for a grant of 7,500,000 shares of restricted common stock of the Company to each of Werber and Suddarth (the grant date fair value of the newly issued shares issued to each of Werber and Suddarth was $83,250).
In connection with the Reorganization, Mr. Mitrani’s, Dr. Mitrani’s and Mr. Bothwell’s FY 2017 Executive Employment Agreements were terminated in favor of newly executed employment agreements (collectively referred to as the April 2018 Executive Employment Agreements). In addition, as a condition of the Reorganization, Mr. Mitrani, Mr. Bothwell and Dr. Mitrani each agreed to release the Company for all amounts owed to them for unpaid salaries through the Effective Date and unpaid advances and/or expenses incurred prior to December 31, 2017 totaling $1,636,808. The significant terms provided for in the FY 2017 Executive Employment Agreements and the April 2018 Executive Employment Agreements are summarized below:
April 2018 Executive Employment Agreements
General
Pursuant to Albert Mitrani’s April 2018 Executive Employment Agreement, Mr. Mitrani serves as the Company’s President and Chief Operating Officer. Mr. Mitrani’s base annual salary is $162,500, which shall accrue commencing on the Effective Date and shall be payable in equal semi-monthly installments, commencing May 1, 2018, in arrears. The base salary shall be reviewed at least annually by the Board and the Board may, but shall not be required to, increase the base salary during the Employment Term. Mr. Mitrani is also entitled to a commission on all sales attributable to him (i.e., excluding existing customers of the Company at the time of the Reorganization) at the rate of five percent (5%) of the "Net Sales" as defined in the agreement and an expense allowance of $5,000 per month.
| 108 |
Pursuant to Ian Bothwell’s April 2018 Executive Employment Agreement, Mr. Bothwell continues to serve as the Company’s Chief Financial Officer. Mr. Bothwell’s base annual salary is $162,500, which shall accrue commencing on the Effective Date and shall be payable in equal semi-monthly installments, commencing May 1, 2018, in arrears. The base salary shall be reviewed at least annually by the Board and the Board may, but shall not be required to, increase the base salary during the Employment Term. Mr. Bothwell has not been paid salary since July 2018.
Pursuant to Dr. Maria Mitrani’s April 2018 Executive Employment Agreement, Dr. Mitrani continues to serve as the Company’s Chief Science Officer. Dr. Mitrani’s base annual salary is $162,500, which shall accrue commencing on the Effective Date and shall be payable in equal semi-monthly installments, commencing May 1, 2018, in arrears. The base salary shall be reviewed at least annually by the Board and the Board may, but shall not be required to, increase the base salary during the Employment Term.
Term
The term of each of the April 2018 Executive Employment Agreements commences as of the Effective Date and continues until December 31, 2020 (Mr. Bothwell) or December 31, 2023 (Mr. Mitrani and Dr. Mitrani) (“Initial Term”), unless terminated earlier pursuant to the terms of the April 2018 Executive Employment Agreement; provided that on such expiration of the Initial Term, and each annual anniversary thereafter (such date and each annual anniversary thereof, a “Renewal Date”), the agreement shall be deemed to be automatically extended, upon the same terms and conditions, for successive periods of one year, unless either party provides written notice of its intention not to extend the term of the April 2018 Executive Employment Agreement at least 90 days’ prior to the applicable renewal Date. The period during which the Executive is employed by the Company hereunder is hereinafter referred to as the “Employment Term.”
Unpaid Advances
The Company was required to repay the unpaid advances subsequent to December 31, 2017, and the unreimbursed expenses incurred subsequent to December 31, 2017, on May 15, 2018. Such payments were not made as required.
Fringe Benefits and Perquisites
During the Employment Term, each Executive shall be entitled to fringe benefits and perquisites consistent with the practices of the Company, and to the extent the Company provides similar benefits or perquisites (or both) to similarly situated executives of the Company.
Termination
The Company may terminate the April 2018 Executive Employment Agreement at any time for good cause, as defined in the April 2018 Executive Employment Agreement, including, the Executive’s death, disability, Executive’s willful and intentional failure or refusal to follow reasonable instructions of the Company’s Board of Directors, reasonable and material policies, standards and regulations of the Company’s Board of Directors or management.
| 109 |
Amendments To The April 2018 Executive Employment Agreements
February 26, 2020 Amendment
On February 26, 2020, the Company agreed to modify the employment agreement of Mr. Ian T. Bothwell, the Company’s Chief Financial Officer to provide Mr. Bothwell with:
| a) | an extension to his employment agreement dated April 13, 2018 from December 2020 to December 2023 consistent with other executives of the Company; and |
| b) | and a one-time bonus in the form of a fully vested cashless warrant to purchase 7,500,000 shares of common stock of the Company, exercisable for ten years at an exercise price of $0.28 per share, the closing price of the common stock on the date of the grant. |
On February 26, 2020, pursuant to the respective employment agreements with each of the Company’s executive officers, the Board granted each of Mr. Albert Mitrani, Dr. Maria Mitrani and Mr. Ian Bothwell a cash bonus of $37,500 for the calendar year ended December 31, 2019.
April 25, 2020 Amendment
On April 25, 2020, the Company agreed to amend and revise the each of Albert Mitrani, Ian Bothwell and Dr. Maria I. Mitrani, (individually each of A. Mitrani, Bothwell and Dr. Mitrani are referred to as an “Executive” and collectively the “Executives”) April 2018 Executive Employment Agreements. The primary amended terms associated with the agreements for each Executive were substantially similar and consisted of the following:
| Term: | An extension to the term of the employment agreements dated April 13, 2018 from December 31, 2023 to December 31, 2025. | |
| Base Salary: | An increase in base annual salary from $162,500 to $300,000. The amended salary amount of $300,000 shall be retroactively adjusted to commence as of January 1, 2019. The increased annual salary of $137,500 (“Incremental Salary”) over the prior annual salary amount of $162,500 (“Original Base Salary”) shall only be paid only upon there being sufficient available cash. Beginning July 1, 2020, at the sole option of the Executive, any portion of unpaid Original Base Salary for periods after January 1, 2020, including unpaid bonus salary, may be converted by Executive into common stock at a conversion rate equal to the average trading price during the month in which the accrued salary pertains. For any unpaid Original Base Salary that existed prior to January 1, 2020, including unpaid bonus salary, the amounts may be converted at a conversion price using the closing trading price of the stock on the last trading day in December 2019.
Beginning December 1, 2020, at the sole option of the Executive, all unpaid Incremental Salary for periods after January 1, 2020 may be converted by the Executive into common stock at a conversion rate equal to the average trading price during the month in which the accrued salary pertains. For any unpaid Incremental Salary that existed prior to January 1, 2020, the amounts may be converted at a conversion price using the closing trading price of the stock on the last trading day in December 2019.
Until such time as the Executive elects to convert, the accrued and unpaid salary, including Original Base Salary and Incremental Salary shall remain an obligation of the Company. |
| 110 |
Severance Provisions:
Company termination without cause, Executive for good reason:
| a) | All existing accrued obligations existing at time of termination shall be paid to Executive. |
| b) | Any unvested equity grants in favor of Executive shall immediately become fully vested and any pending grants pursuant to the MCPP eligible to be issued to Executive shall be granted to Executive, regardless of whether the associated milestone were achieved prior to termination, |
| c) | Executive shall be entitled to a cash payment equal to his unpaid base salary for the remaining term in effect at time of the time of the termination or an amount equal to four times (4x's) the base salary in effect at the time of termination, whichever is greater, |
| d) | Executive shall be entitled to a cash payment equal to his 200% of the prior year’s cash or stock bonus (excluding any stock grants received pursuant to the MCPP). |
Change In Control: In the event of a Change in Control and the Executive’s employment agreement is not extended for period of five years from the date of the Change in Control with all other terms and conditions of the agreement remaining the same, then the Executive may terminate the agreement for good reason and all respective severance terms as provided for a termination by Executive for good reason described in clause 1 above shall be provided to Executive.
Executive termination due to disability, death, or non-renewal by Company:
| a) | All existing accrued obligations existing at time of termination shall be paid to Executive. |
| b) | Any unvested equity grants in favor of Executive shall immediately become fully vested and any pending grants pursuant to the MCPP eligible to be issued to Executive shall be granted to Executive, regardless of whether the associated milestone were achieved prior to termination. |
| c) | Executive shall be entitled to a cash payment equal to 299% of Executive’s base salary in effect at the time of termination, plus a gross up amount to cover Executive’s tax liability associated with such payment. |
| d) | 200% of the prior years cash or stock bonus (excluding MCPP performance stock grants). |
June 29, 2020 Amendment
On June 29, 2020, the board of directors of the Company agreed to further amend and revise the April 2018 Executive Employment Agreements for each of Executives. The primary amended terms associated with the agreements for each Executive were substantially similar and consisted of the following:
| Base Salary: | An increase in the Executives annual base annual salary upon such time that the Company achieves monthly revenues in the amounts provided below, provided such monthly revenue increase occurs for four consecutive months. Upon the achievement of the defined salary milestone, the salary adjustment will be retroactive to the first month in which the salary threshold was met. Any adjustment pursuant to this provision shall not be reduced for any future reduction in revenues that may occur. |
| Monthly Revenues (in millions) | Base Salary Increase | |||||
| $ | 1.00 | $ | 130,000 | |||
| $ | 1.50 | $ | 200,000 | |||
| $ | 2.00 | $ | 275,000 | |||
| $ | 3.50 | $ | 630,000 | |||
| $ | 5.00 | $ | 900,000 | |||
| 111 |
Peter Taddeo
Pursuant to an employment agreement entered into effective May 1, 2017, with Mr. Taddeo (“Taddeo”) and Mint Organics (“Taddeo Employment Agreement”), Mr. Taddeo shall serve as the Chief Executive Officer of Mint Organics (“Mint CEO”) and a member of the Board of Directors of Mint Organics (“Mint Board”). The employment term shall be for three years, unless terminated earlier pursuant to the terms of the agreement, and thereafter deemed to be automatically extended, upon the same terms and conditions, for successive periods of one year, unless either party provides written notice of its intention not to extend the term at least 90 days prior to the applicable renewal date. The Mint CEO’s base annual salary is $180,000 during the period prior to Mint Organics, through one of its subsidiaries, or by other means, obtains or acquires access for a license from a state to dispense cannabis which shall accrue commencing as of the effective date and shall be payable upon Mint Organics generating sufficient net revenue or obtaining sufficient third party financing; and thereafter payable in periodic installments in accordance with Mint Organics customary payroll practices, but no less frequently than monthly. The Mint CEO’s base salary shall automatically be adjusted to an annual rate of base salary of $250,000 once the license is obtained. The base salary shall be reviewed at least annually by the Mint Board and the Mint Board may, but shall not be required to, increase the base salary during the employment term. In connection with the execution of the agreement, Mint Organics agreed to pay the Mint CEO a $25,000 signing bonus which shall be accrued and paid by Mint Organics upon Mint Organics having sufficient cash flow. The agreement also contains terms regarding eligibility for future annual bonuses, annual equity awards under Mint Organics’ equity plan, if any, fringe benefits and perquisites consistent with the practices Mint Organics (including health and dental insurance, an automobile expense allowance of $1,000 per month, and reimbursement for all reasonable and necessary out-of-pocket business, entertainment and travel expenses incurred by the Mint CEO in accordance with Mint Organics’ expense reimbursement policies. Mint Organics may terminate the agreement at any time with or without “Cause” and the Mint CEO may resign at any time with or without “Good Reason” (as defined in the agreement). The nature of the obligations owing to the Mint CEO upon termination is more fully described in the agreement. In connection with the execution of the agreement, the Company granted the Mint CEO 1,000,000 shares of unregistered common stock of Organicell, which vested on December 31, 2017.
On April 6, 2018, Peter Taddeo resigned as a member of the Board of Directors of the Company and as the Chief Executive Officer and member of the board of directors of the Mint Organics Entities. In connection with Mr. Taddeo’s resignation, Mr. Taddeo entered into a Separation and General Release Agreement (“Taddeo Separation Agreement”) whereby Mr. Taddeo agreed to release the Mint Organics Entities from all obligations in connection with the Taddeo Agreement and all other agreements and/or financial obligations between the parties related to the Taddeo’s employment or services performed with any of Mint Organics Entities totaling $156,568. In consideration for Taddeo entering into the Taddeo Separation Agreement, the Mint Organics Entities paid Taddeo $5,000 and Mr. Bothwell paid $3,000 to Taddeo for the purchase of the 1,000,000 shares of common stock of the Company that were granted to Taddeo in connection with the Taddeo Agreement. Contemporaneously with the execution of the Taddeo Separation Agreement, the Company and Mr. Taddeo entered into a Share Purchase and General Release Agreement whereby the Company agreed to purchase from Mr. Taddeo his 150 shares of Mint Series A Preferred Stock for an aggregate purchase price of $40,000.
Resignation, Retirement, Other Termination, or Change in Control Arrangements
Our current executive officers Albert Mitrani, Dr. Maria Mitrani and Ian Bothwell have employment agreements that provide for payments to executives at, following, or in connection with the resignation, retirement or other termination of our directors or executive officers, or a change in control of our company or a change in our executive officers’ responsibilities during the term of their employment and/or following a change in control.
We have no contract, agreement, plan or arrangement, whether written or unwritten, that provides for payments to our directors at, following, or in connection with the resignation, retirement or other termination of our directors, or a change in control of our company or a change in our directors’ responsibilities following a change in control.
Director Compensation
No director received or accrued any compensation for his or her services as a director during the fiscal year ended October 31, 2019.
| 112 |
On February 26, 2020, the Company established the Board Stock Compensation Plan (“Board Plan”) which provides compensation for non-executive Board members for participation in Board meetings retroactive to November 1, 2019. The Board Plan provides for a grant of $7,500 in equivalent shares of common stock (based on trading price at the end of the applicable current quarter) on the last day of each respective fiscal quarter that a member attends at least 75% of all meetings held during such quarter and in which a minimum of 1 meeting is held, for a maximum annual compensation amount of $30,000 per year per member. In addition, Board members that participate on future board committees will also be eligible to receive additional compensation for serving on such committees, in amounts to be determined by the Board. The maximum aggregate number of shares that are currently authorized to be issued pursuant to the Board Plan is 5,000,000 shares.
On April 15, 2020, the Company issued 236,808 shares of common stock to a non-executive Board member in accordance with the Board Plan.
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
Beneficial ownership is determined in accordance with the rules of the Securities and Exchange Commission and generally includes voting or investment power with respect to securities. In accordance with Securities and Exchange Commission rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the applicable table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
The following table sets forth information with respect to the beneficial ownership of our common stock as of September 30, 2020, by (i) each stockholder known by us to be the beneficial owner of more than 5% of our outstanding voting capital stock, (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our capital stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company or any of its parents, the operation of which may at a subsequent date result in a change in control of the Company.
The percentages below are calculated based on 875,194,450 shares of common stock outstanding as of September 30, 2020. Except as noted, the business address of the persons listed below is c/o Organicell Regenerative Medicine, Inc. at 4045 Sheridan Ave., #239, Miami Beach, FL 33140.
| NAME | TITLE | COMMON SHARES | PERCENTAGE (1) |
| Officer and Directors | |||
| Albert Mitrani (3) | Chief Executive Officer, President and Director | 197,955,190 | 22.43% |
| Maria Ines Mitrani (4) | Chief Science Officer and Director | 197,955,190 | 22.43% |
| Ian Bothwell (5) | Chief Financial Officer and Director | 112,800,000 | 12.78% |
| George Shapiro | Chief Medical Officer and Director | 62,500,000 | 7.08% |
| Michael Carbonara (2) | Director | 45,000,000 | 5.10% |
| Allen Meglin | Director | 12,964,180 | 1.47% |
| All officers and directors as a group (6 persons) (6) | -- |
431,219,370
|
48.85% |
| 5% Stockholders (7) | |||
| Management and Business Associates Inc. (8) | -- | 215,425,073 | 24.41% |
| 113 |
| (1) | Based on 875,194,450 shares of common stock outstanding as of September 30, 2020 and 7,500,000 warrants to purchase 7,500,000 shares of common stock of the Company. |
| (2) | Held indirectly by Republic Asset Holdings LLC, an entity of which Michael Carbonara has voting and dispositive control. 102 NE 2nd Street, Boca Raton, FL 33432. |
| (3) | Includes 73,850,000 shares of common stock held by Maria Mitrani, Albert Mitrani’s wife. |
| (4) | Includes 124,105,190 shares of common stock held by Albert Mitrani, Maria Mitrani’s husband. |
| (5) | Includes 7,500,000 warrants to purchase 7,500,000 shares of common stock of the Company. |
| (6) | Includes 7,500,000 warrants to purchase 7,500,000 shares of common stock of the Company. |
| (7) | The Company has not received any filings by a third party indicating beneficial ownership of more than 5% of our outstanding voting capital stock that are not listed herein. |
| (8) | 2060 Dartmouth Ave. N, St. Petersburg, Fl 33713. |
Securities Authorized for Issuance under Equity Compensation Plans
2020 Plan
On February 26, 2020, the Company established the 2020 Stock Incentive Plan (“2020 Plan”). The 2020 Plan permits the grant of options, appreciation rights, dividend equivalent right and restricted common stock of the Company (“Award”) to any person who is an employee or director of, or consultant to the Company. The maximum aggregate number of shares that may be issued pursuant to all Awards is 50,000,000 shares, plus an annual increase to be added on the first day of the calendar year beginning January 1, 2021 equal to (i) the greater of such number of shares as (A) will set the maximum number of shares that may be issued pursuant to all Awards equal to 15% of the number of Shares outstanding as of such date; or (B) 2% of the number of shares outstanding as of such date; or (ii) a lesser number of shares determined by the administrator of the 2020 Plan (“Administrator”) in good faith. The maximum aggregate number of shares available for grant of shares and/or incentive stock options shall be 25,000,000 shares, increased on the first day of the calendar year beginning January 1, 2021, in a number of Shares proportionate to the increase in the total number of shares that may be issued pursuant to all Awards under the Plan.
The Plan shall be administered by (A) the board of the directors of the Company (“Board”) or (B) a committee (“Committee”) designated by the Board, which Committee shall be constituted in such a manner as to satisfy the applicable laws and to permit such grants and related transactions under the Plan to be exempt from Section 16(b) of the Exchange Act in accordance with Rule 16b-3. Once appointed, such Committee shall continue to serve in its designated capacity until otherwise directed by the Board. The Board may at any time amend, suspend or terminate the Plan; provided, however, that no such amendment shall be made without the approval of the Company’s shareholders to the extent such approval is required by applicable laws.
The Company has yet to appoint the Administrator for the Plan and no Awards have yet to be granted under the Plan.
Board Stock Compensation Plan
On February 26, 2020, the Company established the Board Stock Compensation Plan (“Board Plan”) which provides compensation for non-executive Board members for participation in Board meetings retroactive to November 1, 2019. The Board Plan provides for a grant of $7,500 in equivalent shares of common stock (based on trading price at the end of the applicable current quarter) on the last day of each respective fiscal quarter that a member attends at least 75% of all meetings held during such quarter and in which a minimum of 1 meeting is held, for a maximum annual compensation amount of $30,000 per year per member. In addition, Board members that participate on future board committees will also be eligible to receive additional compensation for serving on such committees, in amounts to be determined by the Board. The maximum aggregate number of shares that are currently authorized to be issued pursuant to the Board Plan is 5,000,000 shares.
On April 15, 2020, the Company issued 236,808 shares of common stock to a non-executive Board member in accordance with the Board Plan.
| 114 |
Management and Consultants Performance Stock Plan
On April 25, 2020, the Company approved the adoption of the Management and Consultants Performance Stock Plan (“MCPP”) providing for the grant to current senior executive members of management and third-party consultants of an aggregate of approximately 205,000,000 shares of common stock of the Company (“Shares”) based on the achievement of certain defined operational performance milestones (“Milestones”).
On June 29, 2020, the Board amended the MCPP, providing for the additional grant of common stock of the Company to the current senior executive members of management and the current non-executive members of the Board based on the Company completing any transaction occurring while employed and/or serving as a member of the Board, respectively, that results in a change in control of the Company or any sale of substantially all the assets of the Company (“Transaction”) which upon after giving effect to such issuance of shares below, corresponds to a minimum pre-Transaction fully diluted price per share of the Company’s common stock in the amounts indicated below.
| Pre-Transaction Price Per Share Valuation (a) | Executive Bonus Shares Issued (b) | Non-executive Board Bonus Shares Issued (c) | ||||||||
| $ | 0.22 | 40,000,000 | 2,000,000 | |||||||
| $ | 0.34 | 60,000,000 | 3,000,000 | |||||||
| $ | 0.45 | 80,000,000 | 4,000,000 | |||||||
| $ | 0.54 | 100,000,000 | 5,000,000 | |||||||
| (a) | proforma for issuance of all shares to be issued pursuant to the MCPP and other in the money contingent share issuances | |
| (b) | per each executive consisting of Albert Mitrani, Dr. Mari Mitrani, Ian Bothwell, and Dr. George Shapiro | |
| (c) | per each non-executive Board member consisting of Dr. Allen Meglin and Michael Carbonara |
On August 14, 2020, the Board amended the MCPP, providing for the additional grant of common stock of the Company to each Dr. Maria I. Mitrani and Ian Bothwell based on the Company obtaining aggregate gross fundings (grants for research and development and clinical trials, purchase contracts for Company products, debt and/or equity financings) or other financial awards during the term of employment with the Company based on the amounts indicated below:
Aggregate Funding Amount | Shares | |||||||||
| From | To | |||||||||
| $ | 2,500,000 | $ | 5,000,000 | 5,000,000 | ||||||
| $ | 5,000,001 | $ | 10,000,000 | 10,000,000 | ||||||
| $ | 10,000,001 | $ | 30,000,000 | 30,000,000 | ||||||
On September 23, 2020, the Board amended the MCPP, providing for the grant of common stock of the Company of 15.0 million, 7.5 million and 15.0 million shares of common stock of the Company, respectively, to each Albert Mitrani, Dr. Maria I. Mitrani and Ian Bothwell upon such time that the Company’s common stock trades above $0.25 per share, $0.50 per share and $0.75 per share, respectively, for 30 consecutive trading days subsequent to March 31, 2021 and provided such milestone occurs during the term of employment with the Company.
| 115 |
In addition, each of the current executives were entitled to receive an additional 7 million shares, which when combined with all previous IND and/or eIND’s Milestones previously issued under the MCPP of 43 million shares, represents the total of all incentive shares to be issued to each executive in connection with the combined thirteen IND’s and/or eIND’s Milestones achieved through September 23, 2020. In the future, each of the current executives shall be entitled to receive 5 million shares as a performance incentive for each IND and/or “Expanded Access” approval (and excluding all eIND’s) received by the Company that involve more than 15 patients and provided such milestone occurs during the term of employment with the Company.
Pursuant to the MCPP, as of September 23, 2020, a total of 233,000,000 shares have been issued and approximately 582,500,000 shares are authorized to be issued under the MCPP subject to the achievement of the defined contingent performance based milestones described above and provided the milestones are achieved while the individual is employed and/or serving as a member of the Board:
| MCPP | MCPP | |||||||||||
| MCPP | Remaining | Total | ||||||||||
| Shares | Shares | Shares | ||||||||||
| Name | Awarded | Available | Approved | |||||||||
| Albert Mitrani | 50,000,000 | 137,500,000 | 187,500,000 | |||||||||
| Ian Bothwell | 50,000,000 | 167,500,000 | 217,500,000 | |||||||||
| Dr. Maria I. Mitrani | 50,000,000 | 167,500,000 | 217,500,000 | |||||||||
| Dr. George Shapiro | 50,000,000 | 100,000,000 | 150,000,000 | |||||||||
| Dr. Allen Meglin | – | 5,000,000 | 5,000,000 | |||||||||
| Michael Carbonara | – | 5,000,000 | 5,000,000 | |||||||||
| Consultants | 33,000,000 | – | 33,000,000 | |||||||||
| Total | 233,000,000 | 582,500,000 | 815,500,000 | |||||||||
| Plan category | Number
of securities to | Weighted-average | Number
of securities remaining | |||||||||
| 2020 Plan | -0- | -0- | 50,000,000 | |||||||||
| Board Stock Compensation Plan | -0- | -0- | 4,763,192 | |||||||||
| Management And Consultants Performance Stock Plan | -0- | -0- | 582,500,000 | |||||||||
| 116 |
Changes in Control
On April 23, 2018, the Company and Management and Business Associates, LLC, a Florida limited liability company (“MBA”), executed a Plan and Agreement of Reorganization (“Reorganization”) whereby the Company agreed to issue to MBA an aggregate of 222,425,073 shares of common stock, par value $0.001 per share of the Company, representing at the time, 51% of the outstanding shares of common stock of the Company on fully-diluted basis, for $0.001 per share (or an aggregate of $222,425), in consideration for Mr. Manuel Iglesias’ agreement to serve as the Company’s Chief Executive Officer and a member of the Board of the Company. The Reorganization was retroactive as of April 13, 2018 (“Effective Date”). The Reorganization also provided for the cancelation and termination of the Company’s previously issued and outstanding Series A Preferred Stock and Series B Preferred Stock. As a result of the Reorganization, MBA acquired at the time a controlling interest of the Company. Manuel E. Iglesias is the sole Manager of MBA and thus may be deemed to control MBA. Currently MBA’s beneficial ownership of the Company is 24.6%
As of September 30, 2020, there are 875,194,450 shares of common stock outstanding, of which 395,719,370 shares of common stock (approximately 47.07% of the outstanding shares of common stock) are owned and/or controlled by our Board and executive officers, Albert Mitrani, Ian T. Bothwell, Dr. Maria Mitrani, Dr. George Shapiro, Michael Carbonara and Dr. Allen Meglin, and two of the members of management are spouses, Albert Mitrani and Dr. Maria Mitrani. In addition, all four of our executive officers are also members of the Board of Directors, which currently consists of six members. In addition, our executive officers may receive additional stock grants in the future based on the achievement of certain performance milestones and from the conversion of unpaid compensation into common stock, which if fully issued would provide our Board and executive officers with over 51% of the outstanding shares of common stock outstanding. As a result, the foregoing persons have the ability to significantly influence the outcome of issues submitted to our stockholders. Although our officers and directors have a fiduciary obligation to the Company stockholders, their interests may not always coincide with our interests or the interests of other stockholders. As a consequence, it may be difficult for the other stockholders to remove our management. The ownership of these officers/directors could also deter unsolicited takeovers, including transactions in which stockholders might otherwise receive a premium for their shares over then current market prices.
We are not aware of any other arrangements, including any pledge by any person of our securities, the operation of which may result in a change in control of the Company. However, pursuant to our Articles of Incorporation, our board has the authority, without further stockholder approval, to provide for the issuance of up to 10 million shares of our preferred stock in one or more series and to determine the dividend rights, conversion rights, voting rights, rights in terms of redemption, liquidation preferences, the number of shares constituting any such series and the designation of such series. Our Board has the power to afford preferences, powers and rights (including voting rights) to the holders of any preferred stock preferences, such rights and preferences being senior to the rights of holders of common stock.
Pursuant to our Second Amended and Restated Bylaws, the consent of a “supermajority” (as defined the Bylaws and dependent on how many directors there are at the time) of the Board is required for various actions which might be taken in connection with delaying or preventing a change in control of the Company desired by a majority of our Board of Directors, including, but not limited to, (i) the sale, exchange or other disposition of the Company’s assets with an aggregate value of at least $100,000 or all, or substantially all, of the Company’s assets, whichever is less, occurring as part of a single transaction or plan, or in multiple transactions over a six (6) month period, except in the orderly liquidation and winding up of the business of the Company upon its duly authorized dissolution, (ii) the acquisition of the stock or assets of another entity or the merger therewith, regardless of the nature or amount of consideration given therefor. Other than the foregoing, there are no provisions in our Articles of Incorporation or Bylaws that would delay, defer or prevent a change in control of our Company.
| 117 |
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE.
Under Rule 404 of Regulation S-K, we are required to describe any transaction, since the beginning of the fiscal year ended October 31, 2018, or any currently proposed transaction, in which the Company was or is to be a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000 or one percent (1%) of the average of the Company’s total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s common stock, or an immediate family member of any of those persons.
Effective February 5, 2018, Dr. Werber’s and Mr. Suddarth’s executive employment agreements were terminated and the parties entered into a settlement agreement providing for the release of all obligations owed to Dr. Werber and Mr. Suddarth as of the date of the Sale in exchange for each receiving a grant for 7,500,000 shares of common stock of the Company. On April 6, 2018, Dr. Mitrani’s and Mr. Bothwell’s executive employment agreements were amended to modify the exercise price of their outstanding warrants under certain conditions. Effective April 13, 2018, Mr. Mitrani’s, Dr. Mitrani’s and Mr. Bothwell’s executive employment agreements were terminated and replaced with new executive employment agreements. On February 26, 2020, April 25, 2020 and June 29, 2020, Mr. Mitrani’s, Dr. Mitrani’s and Mr. Bothwell’s employments agreement were further amended.
In connection with the Reorganization, Mr. Bothwell and Dr. Mitrani each agreed to exercise on a cashless basis all of their warrants to purchase 53,300,000 and 23,850,000 shares of common stock of the Company, respectively. Based on the closing price of the Company’s common stock on the Effective Date of $0.012 per share and the warrant exercise price of $0.001 per share, Mr. Bothwell and Dr. Mitrani were required to use 4,675,439 and 2,092,105 shares of common stock received from the exercise of the warrants, respectively, to pay for the exercise price for exercising all of the warrants.
Effective April 13, 2018, Mr. Bothwell and Dr. Mitrani were each granted 4,675,439 and 2,092,105 shares of common stock of the Company, respectively. The newly granted shares vest immediately and were valued at $57,975 and $25,942, respectively, based on the closing trading price of the common stock on the effective date of the grant.
In connection with the previous appointment of an independent member to the Board of Directors of the Company, during August 2019, the Board approved the issuance to the director of 5,000,000 shares of unregistered common stock valued at $0.028 per share, the closing price of the common stock of the Company on the grant date.
Effective February 26, 2020, Mr. Bothwell was granted cashless warrants to purchase 7,500,000 shares of common stock of the Company. The newly granted warrants vest immediately, have an exercise price of $0.028 per share and are exercisable for ten years from the effective date of the grant.
During April 2020, June 2020, August 2020 and September 2020, each of the current executives of the Company, Albert Mitrani, Dr. Mari Mitrani, Ian Bothwell and George Shapiro (“Current Executives”) were granted rights under the Management and Consultant Performance Plan (“MCPP”) to receive common stock of the Company based on the achievement of certain defined milestones. In addition, during June 2020, each of the current non-executive members of the Board were granted rights under the MCPP to receive common stock of the Company based on the achievement of certain defined milestones.
The Company’s corporate administrative offices are leased from MariLuna, LLC, a Florida limited liability company which is owned by Dr. Mitrani. The term of the lease has been extended through June 2023. The current monthly rent is $2,900 and beginning July 2020, the monthly rent increases to $3,500. The Company paid a security deposit of $5,000.
In connection with Mr. Bothwell’s executive employment agreements, the Company agreed to reimburse Rover Advanced Technologies, LLC, a company owned and controlled by Mr. Bothwell for office rent and other direct expenses (phone, internet, copier and direct administrative fees, etc.).
On February 5, 2018, in connection with the Sale, all amounts owed to the Mr. Bothwell and Dr. Werber in connection with the SPA were repaid.
| 118 |
On February 5, 2018, in connection with Dr. Werber’s resignation and termination, Dr. Werber agreed to the forfeit and the cancellation of the 100 shares of the Series A Preferred Stock previously issued. Effective April 13, 2018, in connection with the Reorganization, Mr. Mitrani, Mr. Bothwell and Dr. Mitrani each agreed to the forfeit and cancellation of their 100 shares of the Series A Preferred Stock.
On April 6, 2018, Peter Taddeo resigned as a member of the Board of Directors of the Company and as the Chief Executive Officer and member of the board of directors of the Mint Organics Entities. In connection with Mr. Taddeo’s resignation, Mr. Taddeo entered into a Separation and General Release Agreement (“Taddeo Separation Agreement”) whereby Mr. Taddeo agreed to release the Mint Organics Entities from all obligations in connection with the Taddeo Agreement and all other agreements and/or financial obligations between the parties related to the Taddeo’s employment or services performed with any of Mint Organics Entities. In consideration for Taddeo entering into the Taddeo Separation Agreement, the Mint Organics Entities paid Taddeo $5,000 and Mr. Bothwell paid $3,000 to Taddeo for the purchase of the 1,000,000 shares of common stock of the Company that were granted to Taddeo in connection with the Taddeo Agreement. Contemporaneously with the execution of the Taddeo Separation Agreement, the Company and Mr. Taddeo entered into a Share Purchase and General Release Agreement whereby the Company agreed to purchase from Mr. Taddeo his 150 shares of Mint Series A Preferred Stock for an aggregate purchase price of $40,000.
On May 1, 2019, the Company and Mint Organics entered into an exchange agreement whereby the Company agreed to acquire the 150 shares of Mint Series A Preferred Stock and the 150,000 warrants to purchase shares of common stock of the Company originally issued to Mr. Wayne Rohrbaugh in connection with the initial capitalization of Mint Organics in exchange for 4,400,000 shares of common stock of the Company.
On April 23, 2018, the Company and MBA executed a Plan and Agreement of Reorganization. As a result of the Reorganization, MBA acquired at the time a controlling interest of the Company. Manuel E. Iglesias is the sole Manager of MBA and thus may be deemed to control MBA.
From time to time, Mr. Iglesias and Mr. Bothwell and/or their respective affiliates have advanced funds to the Company to pay for certain expenses of the Company. As of October 31, 2019, $220,897 and $48,184 are owed to Mr. Iglesias and Mr. Bothwell and/or their respective affiliates, respectively. In addition, the Company has not provided Mr. Bothwell required salary payments since July 2018. At October 31, 2019, salary amounts owed to Albert Mitrani, Dr. Mari Mitrani and Ian Bothwell were $132,105, $129,613 and $321,907, respectively.
Mr. Iglesias has provided a personal guaranty in connection with amounts required to paid under the Credit Facility.
During April 2020 through May 2020, the Company sold 11,000,000 shares of common stock to Dr. Allen Meglin, a director of the Company at $0.02 per share for an aggregate purchase price of $220,000. During July and August 2020, the Company sold an additional 1,166,666 shares and 422,514 shares of common stock to Dr. Allen Meglin at $0.03 per share and $0.10 per share, respectively, for an aggregate purchase price of $77,251.
On April 27, 2020, the Company sold 5,000,000 shares of common stock to Republic Asset Holdings LLC., a Company controlled by Michael Carbonara, a director of the Company, at $0.02 per share for an aggregate purchase price of $100,000.
During September 2018, in consideration for Dr. George Shapiro agreeing to serve as the Company’s Chief Medical Officer (“CMO”) and render other medical consulting and advisory services to the Company, the Board approved the issuance to the CMO of 2,500,000 shares of common stock. In connection with the CMO’s appointment to the Board of Directors of the Company during February 2019, during February 2019 and August 2019, the Board approved the issuance to the CMO of 2,000,000 and 3,000,000 shares, respectively, of common stock. On February 26, 2020, the Company agreed to immediately grant the CMO 5,000,000 shares of common stock in recognition of past services provided to the Company through February 2020. In addition the Company agreed to enter into a consulting agreement with the CMO to provide ongoing services to the Company. The CMO will receive compensation of $82,250 annually, commencing March 1, 2020. The term of the consulting agreement is one year, with automatic renewals for annual periods thereafter unless prior written notice is provided by either party of the desire to terminate.
| 119 |
For the year ended October 31, 2019 and 2018, the total amount of sales to customers related to our board of director members and/or employees of the Company totaled $71,650 and $19,550, respectively.
In connection with Mr. Robert Zucker’s resignation from the Board of Directors of the Company in April 2020, the Board approved the issuance to Mr. Zucker of 736,808 shares of unregistered common stock of the Company.
Director Independence
We are not currently subject to listing requirements of any national securities exchange or inter-dealer quotation system which has requirements that a majority of the Board of Directors be “independent” and, as a result, we are not at this time required to have our Board of Directors comprised of a majority of “independent directors.” Nevertheless, we believe that both Michael Carbonara and Dr. Allen Meglin qualify as “independent” under the applicable standards of the SEC and the NASDAQ stock market.
ITEM 14. PRINCIPAL ACCOUNTING FEES AND SERVICES.
As previously reported in a Form 8-K filed on August 16, 2018, effective July 1, 2018, our principal independent accountants, GBH CPAs, PC (“GBH”) completed the combination of its practice into Marcum LLP (“Marcum”).
Audit Fees
The aggregate fees billed the Company for the fiscal years ended October 31, 2019 and October 31, 2018 for professional services rendered by our principal accountants for their audit of our annual financial statements and review of financial statements included in our quarterly reports or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagements for those fiscal years were:
Marcum LLP
| Fiscal Year Ended October 31, 2019: | $ | 150,000 | ||
| Fiscal Year Ended October 31, 2018: | $ | 175,000 |
Audit-Related Fees
The aggregate fees billed the Company for the fiscal years ended October 31, 2019 and 2018 for assurance and related services by the principal accountant that are reasonably related to the performance of the audit or review of the registrant’s financial statements and are not reported under Item 9(e)(1) of Schedule 14A.
| Fiscal Year Ended October 31, 2019: | $ | – | ||
| Fiscal Year Ended October 31, 2018: | $ | – |
Tax Fees
The aggregate fees billed the Company for the fiscal years ended October 31, 2019 and 2018 for professional services rendered by the principal accountants for tax compliance, tax advice, and tax planning.
| Fiscal Year Ended October 31, 2019: | $ | – | ||
| Fiscal Year Ended October 31, 2018: | $ | – |
| 120 |
All Other Fees
The aggregate fees billed the Company for the fiscal years ended October 31, 2019 and 2018 for products and services provided by the principal accountants, other than the services reported in Items 9(e)(1) through 9(e)(3) of Schedule 14A.
| Fiscal Year Ended October 31, 2019: | $ | – | ||
| Fiscal Year Ended October 31, 2018: | $ | – |
Pre-Approval Policies and Procedures
We have not used Marcum for financial information system design and implementation. These services, which include designing or implementing a system that aggregates source data underlying the financial statements or generates information that is significant to our financial statements, are provided internally or by other service providers. We did not engage Marcum to provide compliance outsourcing services.
Our board of directors pre-approves all services provided by our independent auditors. All of the above services and fees were reviewed and approved by the board of directors either before or after the respective services were rendered. The board of directors has considered the nature and amount of fees billed by Marcum and believes that the provision of services for activities unrelated to the audit is compatible with maintaining our independence.
| 121 |
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES.
| Exhibit No: | Description: | |
| 2.1 | Plan and Agreement of Reorganization, dated April 23, 2018, between Management and Business Associates, LLC and Biotech Products Services and Research, Inc. (Filed as an exhibit to the Registrant’s Form 8-K filed on April 26, 2018 and incorporated by reference herein) | |
| 3.1 | Articles of Incorporation, as amended (Filed as an exhibit to Registration Statement on Form S-1 filed on September 4, 2012 (File No: 333-183710) and incorporated by reference herein) | |
| 3.2 | Certificate of Amendment to the Articles of Incorporation (Filed as an exhibit to Form 8-K filed on November 3, 2015 and incorporated by reference herein) | |
| 3.3 | Amendment to the Certificate of Incorporation of Biotech Products Services and Research, Inc., filed with the Secretary of State of Nevada on July 22, 2017, effective July 10, 2017 (Filed as an exhibit to Form 10-K for the fiscal year ended October 31, 2017 filed on July 7, 2018 and incorporated by reference herein) | |
| 3.4 | Series A Non-Convertible Preferred Stock Certificate of Designation, effective November 1, 2016 (Filed as an exhibit to the Registrant’s Form 8-K filed on November 3, 2016 and incorporated by reference herein) | |
| 3.5 | Amendment to Certificate of Designation of Series A Non-Convertible Preferred Stock of Biotech Products Services and Research, Inc. (Filed as an exhibit to the Registrant’s Form 8-K filed on March 15, 2017 and incorporated by reference herein) | |
| 3.6 | Series B Convertible Preferred Stock Certificate of Designation, effective November 1, 2016 (Filed as an exhibit to the Registrant’s Form 8-K filed on November 3, 2016 and incorporated by reference herein) | |
| 3.7 | Amendment to the Certificate of Incorporation of Biotech Products Services and Research, Inc., filed with the Secretary of State of Nevada on May 21, 2018, effective June 20, 2018 (Filed as an exhibit to the Registrant’s Form 10-K filed on November 1, 2018 and incorporated by reference herein) | |
| 3.8 | Certificate of Correction filed with the Secretary of State of Nevada on June 18, 2018(Filed as an exhibit to the Registrant’s Form 10-K filed on November 1, 2018 and incorporated by reference herein) | |
| 3.9 | Certificate of Withdrawal filed with the Secretary of State of Nevada on June 14, 2018 (Filed as an exhibit to the Registrant’s Form 10-K filed on November 1, 2018 and incorporated by reference herein) | |
| 3.10 | Amended and Restated By-laws of Biotech Products Services and Research, Inc. (Filed as an exhibit to the Registrant’s Form 8-K filed on March 15, 2017 and incorporated by reference herein) | |
| 3.11 | Second Amended and Restated By-laws of Biotech Products Services and Research, Inc.(Filed as an exhibit to the Registrant’s Form 8-K filed on December 18, 2017 and incorporated by reference herein) | |
| 3.12 | Certificate of Amendment to the Articles of Incorporation filed with the Secretary of State of Nevada on June 24, 2020, effective June 24, 2020. (Filed as an exhibit to Form 8-K filed on July 14, 2020 and incorporated by reference herein) |
| 122 |
| 123 |
| 124 |
| * | Filed herewith. |
| ** | Pursuant to Rule 406T of Regulation S-T, these interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934 and otherwise are not subject to liability under those sections. |
| 125 |
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| ORGANICELL REGENERATIVE MEDICINE, INC. | ||
| By: | /s/ Albert Mitrani | |
| Albert Mitrani | ||
| Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| October 15, 2020 | ||
| By: | /s/ Ian T. Bothwell | |
| Ian T. Bothwell | ||
| Chief Financial Officer | ||
|
(Principal Financial and Accounting Officer)
October 15, 2020
| ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated:
| Signature | Title | Date | ||
|
/s/ Albert Mitrani |
Chief Executive Officer, President, Chief Operating Officer and Secretary, Director (Principal Executive Officer) | October 15, 2020 | ||
| Albert Mitrani | ||||
| /s/ Ian T. Bothwell | Chief Financial Officer, Director | October 15, 2020 | ||
| Ian T. Bothwell | (Principal Financial and Accounting Officer) | |||
| /s/ Maria Ines Mitrani | Chief Science Officer, Director | October 15, 2020 | ||
| Maria Ines Mitrani |
| /s/ George Shapiro | Chief Medical Officer, Director | October 15, 2020 | ||
| George Shapiro |
| /s/ Allen Meglin | Director | October 15, 2020 | ||
| Allen Meglin | ||||
| /s/ Michael Carbonara | Director | October 15, 2020 | ||
| Michael Carbonara |
| 126 |