UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 8-K
CURRENT REPORT PURSUANT
TO SECTION 13 OR 15(D) OF THE
SECURITIES EXCHANGE ACT OF 1934
Date of report (Date of earliest event reported): December 17, 2015
Microlin Bio, Inc.
(Exact name of small business issuer as specified in its charter)
| Delaware | 45-4507811 |
| (State or other jurisdiction of incorporation or organization) | (IRS Employer Identification No.) |
|
135 East 57th Street, 24th Floor, New York, NY 10022 |
| (Address of principal executive offices) |
|
(646) 406-6243 |
| (Issuer’s telephone number) |
|
American Boarding Company, 358 Frankfort Street, Daly City, CA 94104 (Former name or former address, if changed since last report) |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions (see General Instruction A.2. below):
[ ] Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
[ ] Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
[ ] Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
[ ] Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))

| 2 |
Section 1 – Registrant’s Business and Operations
Item 1.01 Entry Into a Material Definitive Agreement
On December 17, 2015, we entered into an Agreement and Plan of Merger (the “Merger Agreement”) with Microlin Bio, Inc., a private Delaware corporation (“Microlin”), and our subsidiary formed for the purposes of the transaction, Microlin Merger Sub, Inc. (the “Merger Sub”). Pursuant to the Merger Agreement, Microlin merged with and into the Merger Sub, which resulted in Microlin becoming our wholly-owned subsidiary (the “Acquisition”). Immediately following the Acquisition, the Merger Sub was merged with and into our Corporation. In connection with this subsequent subsidiary merger, we changed our corporate name to “Microlin Bio, Inc.”
In addition, pursuant to the terms and conditions of the Merger Agreement:
| • | The holders of all of the capital stock of Microlin issued and outstanding immediately prior to the closing of the Acquisition exchanged their shares on a pro-rata basis for 19,000,000 newly-issued shares of our common stock. |
| • | Following the closing of the Acquisition, four of our shareholders canceled and returned a total of 8,635,000 shares of common stock. |
| • | As a result, immediately following the Acquisition, there were 20,000,000 shares of our common stock issued and outstanding. |
| • | Our officers and directors immediately prior to the Acquisition, Reza Noorkayhani and Joseph Marshall, resigned from the board and from all offices. |
| • | Joseph Hernandez, who was the sole director of Microlin prior to the Acquisition, was appointed as our new sole director, effective ten (10) days after mailing to shareholders of our Schedule 14f-1 regarding the change in our board, which was filed with the Commission and mailed to shareholders of record on December 16, 2015. |
| • | Our board also appointed the following new officers and directors, each of who had served in the same capacity as an officer of Microlin prior to the acquisition: |
- Joseph Hernandez, Executive Chairman, Chief Executive Officer, and Chief Financial Officer
- Bruce Galton, Chief Operating Officer
| • | Concurrently with the Acquisition, the holders of certain of our issued and outstanding promissory notes in the total amount of $87,844 agreed to amend the notes to remove the conversion features contained therein. In addition, we have committed to pay all of our pre-Acquisition liabilities, up to a total maximum amount of $90,000, within 120 days of the Acquisition. Our former CEO, Reza Noorkayhani, has agreed to indemnify us for any pre-Acquisition liabilities in excess of $90,000. |
As of the date of the Merger Agreement and currently, there are no material relationships between us or any of our affiliates and Microlin, other than with regard to the Merger Agreement.
The foregoing description of the Merger Agreement does not purport to be complete and is qualified in its entirety by reference to the complete text of the Merger Agreement, which is filed as Exhibit 2.1 hereto and incorporated herein by reference.
| 3 |
Section 2 – Financial Information
Item 2.01. Completion of Acquisition or Disposition of Assets
Information about the Company and the principal terms of the Acquisition are set forth below.
The Acquisition. On December 17, 2015, in accordance with the Merger Agreement, Microlin merged with and into the Merger Sub, which resulted in Microlin becoming our wholly-owned subsidiary. Immediately following the Acquisition, the Merger Sub was merged with and into our corporation. In exchange for all of the issued and outstanding shares of Microlin, the shareholders of Microlin received a total of 19,000,000 shares of our common stock.
There were 9,635,000 shares of our common stock outstanding before giving effect to the stock issuances in the Acquisition and the cancellation of 8,635,000 shares by four of our shareholders. Following these events, there were 20,000,000 shares outstanding, including:
Shares Held by:
19,000,000 former Microlin shareholders
1,000,000 existing shareholders
20,000,000
The issuance of shares of our common stock to the former holders of Microlin’s capital stock and convertible debentures in connection with the Acquisition was not registered under the Securities Act of 1933, as amended (the “Securities Act”), but was performed in reliance upon the exemption from registration provided by Section 4(2) of the Securities Act and/or Regulation D promulgated under that section, which exempts transactions by an issuer not involving any public offering.
Prior to the Acquisition, there were no material relationships between us and Microlin, or any of their respective affiliates, directors or officers, or any associates of their respective officers or directors.
General Changes Resulting from the Acquisition. Following the Acquisition, we intend to carry on the business of Microlin as our sole line of business. We have relocated our principal executive offices to 135 East 57th Street, 24th Floor, New York, NY 10022. Our telephone number is now (646) 406-6243.
Changes to the Board of Directors. Reza Noorkayhani and Joseph Marshall resigned from the board and from all offices. Joseph Hernandez, who was the sole director of Microlin prior to the Acquisition, was appointed as our new sole director, effective ten (10) days after mailing to shareholders of our Schedule 14f-1 regarding the change in our board, which was filed with the Commission and mailed to shareholders of record on December 16, 2015.
All directors hold office for one-year terms until the election and qualification of their successors. Officers are elected by the board of directors and serve at the discretion of the board.
| 4 |
Accounting Treatment; Change of Control. The Acquisition is being accounted for as a “reverse acquisition,” as the stockholders of Microlin possess majority voting control of the company immediately following the Acquisition and now control our board of directors. Microlin is deemed to be the accounting acquirer in the reverse acquisition. Consequently, the assets and liabilities and the historical operations of Microlin prior to the Merger will be reflected in the financial statements and will be recorded at the historical cost basis of Microlin. Our consolidated financial statements after completion of the Acquisition will include the assets and liabilities of both companies, the historical operations of Microlin, and our operations from the closing date of the Acquisition. Following the Acquisition our fiscal year-end has been changed from December 31 to September 30. As a result of the issuance of the shares of our common stock pursuant to the Acquisition, a change in control of the Company occurred on December 17, 2015. Except as described herein, no arrangements or understandings exist among present or former controlling stockholders with respect to the election of members of our board of directors and, to our knowledge, no other arrangements exist that might result in a future change of control of the Company. We will continue to be a “small business issuer,” as defined under the Securities Exchange Act of 1934, as amended (the “Exchange Act”), following the Acquisition.
We were incorporated as American Boarding Company in the State of Delaware on January 27, 2013. American Boarding Company was originally a real estate based company with a principle business objective of acquisition, design, development, lease, and management services of student housing communities located within close proximity of colleges and universities in the United States.
On December 17, 2015, we entered into the Merger Agreement with Microlin, whereby we acquired all of the issued and outstanding common stock of Microlin through a subsidiary. Following the Acquisition, we merged the subsidiary with and into our corporation, and changed our name to “Microlin Bio, Inc.” as part of that process. As a consequence of the Acquisition, we will no longer pursue our former business plan.
As a result of entering into the Merger Agreement, we are now a biotechnology company focused on microRNA based therapeutics for Oncology.
| 5 |
Cautionary Note Regarding Forward-Looking Statements
The words ‘‘anticipate,’’ ‘‘believe,’’ ‘‘could,’’ ‘‘estimate,’’ ‘‘expect,’’ ‘‘intend,’’ ‘‘may,’’ ‘‘plan,’’ ‘‘potential,’’ ‘‘predict,’’ ‘‘project,’’ ‘‘should,’’ ‘‘target,’’ ‘‘will,’’ ‘‘would’’ or the negative of those terms, and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, but are not limited to, statements about:
- the initiation, cost, timing, progress and results of our preclinical research and development activities;
- our compliance with the terms of our in-license agreements, including our exclusive in-license agreements with OSIF;
- our ability to generate revenue;
- Our ability to obtain additional financing;
- our ability to continue as a going concern;
- our ability to obtain or maintain the rights to microRNA intellectual property and to successfully enforce our intellectual property rights;
- the safety, efficacy and market acceptance of microRNA technology and our other product candidates;
- regulatory approval of our product candidates and future product candidates we may develop, and the labeling under any approval we may obtain;
- our ability to obtain orphan drug designation for LumiralinTM and OmiralinTM, two of our product candidates;
- future regulation by the FDA;
- our ability to bring our product candidates to clinical trial;
- regulatory developments;
- the development, success or failure of competing drugs that are or become available;
- the potential markets for our product candidates and our ability to serve and compete in those markets;
- our ability to build and maintain strategic partnerships;
- our compliance with environmental, health and safety laws and regulations such as federal and state healthcare fraud and abuse laws and regulations;
- our ability to successfully defend any product liability claims;
- the performance of our third party partners, including third party manufacturers and suppliers;
- our ability to obtain reimbursement coverage by third party payors for our product candidates;
- our ability to obtain sufficient product liability insurance coverage;
- our ability to obtain formulary approval;
| 6 |
- the production of our product candidates within a viable cost structure;
- our ability to recruit and maintain key scientific, management personnel, board members or employees;
- successful development of our sales and marketing capabilities;
- our ability to successfully defend against any lawsuits or claims against us in the ordinary course of business;
- the occurrence of system failures, natural or man-made disasters or terrorist attacks disrupting our ability to operate;
- our ability to establish and maintain effective disclosure controls and procedures; and
- the accuracy of our estimates regarding expenses, future revenues, capital requirements and need for additional financing.
These forward-looking statements are only predictions and we may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, so you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from plans, intentions and expectations disclosed in the forward-looking statements that we make. We have based these forward-looking statements largely on our current expectations and projections about future events, and trends that we believe may affect our business, financial condition and operating results. We operate in a very competitive and rapidly changing environment and it is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. We have included important factors in the cautionary statements included in this Current Report, particularly in the ‘‘Risks Factors’’ section, that could cause actual future results or events to differ materially from the forward- looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments we may make.
The forward-looking statements in this Current Report represent our views as of the date of this Current Report. We anticipate that subsequent events and developments will cause our views to change. However, although we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should, therefore, not rely on these forward- looking statements as representing our views as of any date subsequent to the date of this Current Report. You should read this report and the documents that we reference in this report and have filed as exhibits completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of the forward-looking statements in this Current Report by these cautionary statements.
Overview
We are a development stage emerging therapeutic company focusing on microRNA and its role in oncology. MicroRNAs are naturally-occurring RNA molecules (composed of 19 to 25 nucleotides) that do not encode proteins but instead regulate gene expression and play a critical role in regulating networks of biological pathways. MicroRNAs were discovered fairly recently, and scientific research has shown that the improper balance of microRNAs is linked to many diseases, including cancer. MicroRNAs represent targets for a new class of therapeutics, which we believe may be more effective in the treatment of cancer, and diseases in general, with the potential for mitigated side effects in comparison with conventional small molecule drugs. We are working to develop and market, antimiR therapies, and miR replacement therapies. Our ultimate goal is to change the field of cancer treatment, minimize unnecessary healthcare costs and improve patients’ quality of life. We are focused on four types of cancer: lung, ovarian, colorectal and prostate.
| 7 |
We have in-licensed a broad intellectual property portfolio of granted and pending patents and patent applications. In September 2013, we signed five exclusive licensing agreements with OSIF for approximately 138 pending patent applications and 132 granted patents covering numerous microRNAs. In addition, we licensed a novel delivery technology, which we call QTsomeTM. We have assembled a scientific advisory board with deep expertise in microRNA biology and broad business experience in biotechnology and drug development. We have also initiated the preclinical development of our lead therapeutic product candidate, LumiralinTM, including in vitro and in vivo animal studies. We believe that there is a significant clinical need for the type of therapeutics we are seeking to develop for lung, ovarian, colorectal and prostate cancers. We believe that these elements will allow us to successfully compete against existing companies who focus on microRNA therapeutics, even though our competitors are more established and have greater resources at their disposal.
Our Strategy
Our strategy thus far has been to build a company that has a limited infrastructure with flexibility to react to a wide variety of potential outcomes of our validation, clinical and preclinical work. Since we are an early stage company, being able to change direction easily, quickly and at minimal cost allows us flexibility which will be critical to the success of our company. We will need to react to critical events that will determine the course of our company, including the success and timing of our fundraising efforts, the results of our preclinical work, and the various stages of our clinical trials. Based on the outcomes of these events, we may need to alter our development plan and advance certain clinical programs over others based on resource constraint or clinical viability.
We intend for our clinical therapeutics programs to focus on areas of oncology where the current standard of care offers limited hope for patients suffering from cancer. Our product candidates are intended to employ a novel mechanism that could, alone or in combination, potentially help to eradicate or slow the progression of cancer.
A key component to our strategy is engaging seasoned executives to manage our outsourcing strategy. Another key component is our relationship with OSU. We believe OSU has been in the forefront of the discovery of microRNAs and their role in cancer. We intend to partner with OSU on several fronts, including on the preclinical work, the development of the delivery technology and on the clinical trials development. Further, one of our scientific advisors is a current professors at OSU. We view this relationship as a key component to our strategy.
Our strategy is to outsource as many elements of the development and manufacturing process as possible, which is a trend in the biotechnology industry. We believe this outsourcing strategy will allow us to avoid capital expenditures, keep fixed costs to a minimum and maximize the flexibility of our programs as we gather data and determine to which programs we should devote our resources. Outsourcing our developmental functions eliminates the need to establish a sizeable research and development facility, which is time consuming and capital intensive. We intend to work with consultants, CROs and CMOs to perform the critical work for our development and manufacturing processes. Under this model, we can leverage the expertise of established consultants, the capabilities of partner academic institutions and the experience of CROs and CMOs, resulting in an overall efficient process for drug development. To execute this strategy, we intend to work mostly as a virtual company, with members of our management team working remotely to execute our strategic plan, carry out operational management tasks and coordinate activities at the sites of our consultants, CROs and CMOs.
As part of this strategy, we currently do not own any facilities and plan instead to enter into as many consulting agreements, as opposed to employment agreements, as possible. We also plan to engage regulatory consultants to help us work through the various stringent regulations required by the relevant regulatory authorities, in particular the FDA, to acquire regulatory approval and comply with any post-approval regulatory requirements.
| 8 |
An Overview of microRNA
The Biology of microRNA
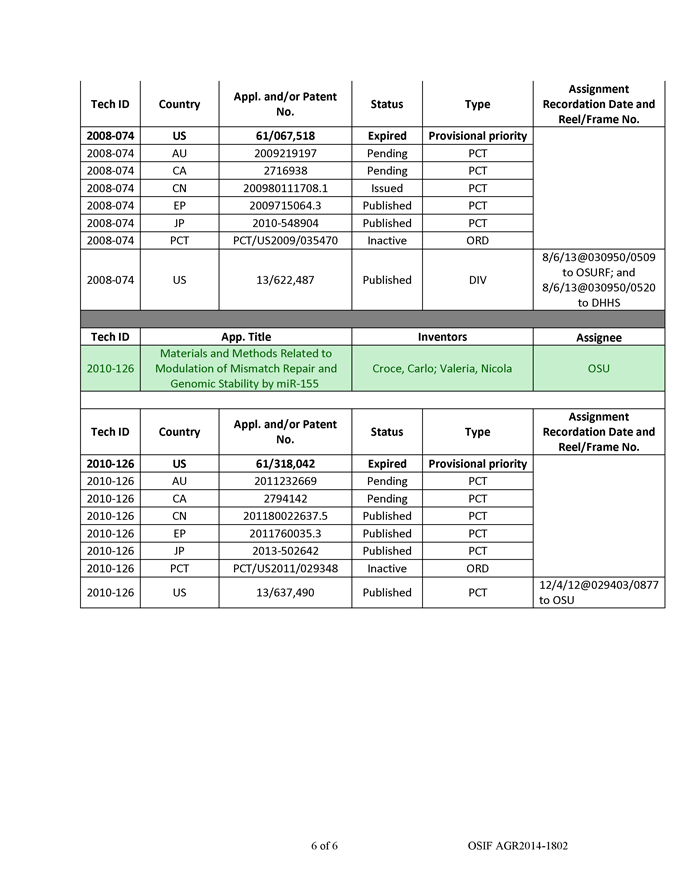
In a cell, DNA transcribed into messenger RNA (mRNA) as well as non-coding RNA. mRNA is ‘‘translated’’ into proteins, which performs various cellular functions. In contrast, microRNAs (miRNAs or miRs) are non-coding RNAs (ncRNAs) that regulate the expression of genes. First, miRNA genes are transcribed to long primary transcripts that are long precursors (‘‘pri-miRNA’’) and then processed in the nucleus by an enzyme called Drosha into another precursor (‘‘pre-miRNA’’). The pre-miRNA is then transported into the cytoplasm by exportin-5, where it is further cut into 19 to 25 nt double-stranded miRNA: miRNA* duplexes. Next, miRNA: miRNA duplexes are incorporated into RNA-Induced Silencing Complexes (RISC), followed by unwinding of the duplex and retention of the mature miRNA strand in the RISC, while the complementary miRNA* strand is degraded. The mature miRNA serves as a guide molecule for RISC by directing it to partially complementary sites located predominantly in the 3’ untranslated regions (UTRs) of target mRNAs, resulting in translational repression and/or mRNA degradation of the target mRNAs (Figure 1). Over 2,000 potential microRNAs have been identified thus far, more than one-third of all human genes are predicted to be regulated by microRNAs, and each miRNA can regulate hundreds of mRNA and ncRNA targets. Since a single miRNA can regulate an entire network of genes, miRNAs operate as master regulators of the genome.
Figure 1. Mechanism of gene regulation by miRNA
Small-interfering-RNA (siRNA) and miRNAs are similar in that both utilize the RISC to reduce target gene expression. However, there are some important differences between them. siRNAs are mostly exogenous molecules custom-designed to target a specific gene. In contrast, miRNAs are naturally expressed by cells and are regulatory molecules with multiple mRNA gene targets. The multiple mRNA targets of each microRNA have been selected by evolution and thus often play important roles in cellular pathways and networks of pathways that control specific functions. Studies have shown that changes in miRNA level in tissues and blood may be linked to various diseases, including cancer.
| 9 |
The discovery of miRNAs has opened up new opportunities in the discovery of new biomarkers and new drugs. Because levels of miRNAs are altered in many diseases, they can act as biomarkers for these diseases for their diagnosis and prognosis, for prediction of response to therapy, and for post-treatment monitoring. In addition, pathological changes caused by miRNA dysregulation can potentially be reversed by miR-based therapy. There are multiple approaches for miR-based therapy. One is miR-replacement therapy (MRT), in which an exogenous ‘‘miRNA mimic’’ for a miRNA that has a reduced level of expression is introduced. Another approach is antimiR therapy (AMT) in which an inhibitor for a miRNA that is overexpressed is introduced. These miRNA-modulating agents are synthetic oligonucleotides with sequences that are identical or complementary to the targeted miRNAs.
Designing of MRT or AMT therapeutic candidates is relatively straight forward because the sequences of the target miRNAs are already known. Because miRNA mimics and antimiRs are sequence specific, compared to the conventional small molecule chemotherapy, fewer adverse sides effects are expected for miRNA-based therapies along with much greater probability of hitting their targets. Many targets that were previously deemed undruggable can now be targeted using AMT or MRT. Finally, blood-based miRNA biomarker tests and miR-based therapeutics can be used in combination because patients most likely to benefit from therapy can be identified based on their miR expression profile and can then be treated with the corresponding miR-based therapy.
The Role of microRNA in Cancer
The role of miRNAs in cancer development was first reported in 2002 by Dr. Carlo Croce, who is a member of the National Academy of Sciences, Professor and Chairman of the Department of Molecular Virology, Immunology and Medical Genetics at the OSU College of Medicine. miRNAs expression patterns are altered in all types of cancer, with each cancer type displaying a distinctive signature miR expression pattern. miR expression profiling, therefore, potentially enables differentiation of the type of the tumor and its tissue of origin. It has been shown that changes in miRNAs can promote tumor development by increasing the expression of oncogenes that promote tumor growth and by decreasing the expression of tumor-suppressor genes. MiRNAs have been linked to tumor development, invasion, metastasis, new blood vessel generation, and tumor resistance to chemotherapy and radiotherapy. Each miR can affect multiple tumor types whereas multiple miRs may be changed in each tumor type. For example, miR-21 is increased in glioma, breast, lung, prostate, colon, stomach, esophageal, and cervical cancers, as well as diffuse large B-cell lymphoma. Meanwhile, inhibition of miR-21 has been shown to inhibit tumor growth by increasing the expression of certain tumor suppressors.
Our microRNA Product Platform
Scientists have shown in animal models that AMT inhibiting oncogenic miRs that are increased in a tumor, such as a miR-21 and members of miR-17-92 cluster, can inhibit tumor growth, tumor cell invasion, new blood vessel development (angiogenesis), and/or reverse resistance to chemotherapy and radiotherapy treatments. AntimiRs are ‘‘mirror images’’ of their miR targets and act by forming a stable complex with them, thereby inhibiting their biological function. Conversely, MRT replacing tumor suppressor miRNAs that are decreased in a tumor, such as miR-484 and miR-34a, with synthetic miR mimics can similarly achieve therapeutic benefits. Therefore, AMR and MRT and their combinations constitute miR-based therapies, a novel modality for the treatment of cancer.
We plan to develop four miR-based therapeutics: LumiralinTM (antimiR-21 AMT), OmiralinTM (miR-484 MRT), ColomiralinTM (antimiR-17-5p AMT), and PromiralinTM (miR-34 mimic MRT). Since both miR-21 and miR-17-5p are elevated in many types of cancer, LumiralinTM and ColomiralinTM may be useful in treating multiple tumor types. miR-34 is also reduced in multiple tumor types, so PromiralinTM will also have therapeutic potential in multiple tumor types. miR-based therapeutics can potentially be used in combination with each other in a common delivery vehicle. Furthermore, because of their ability to decrease therapeutic resistance, miR-based therapy could potentially be combined with standard chemotherapy or radiotherapy and could potentially produce a synergistic effect.
We have in-licensed a portfolio of approximately 138 pending patent applications and 132 granted patents covering various aspects of applications of miRNAs in cancer from OSIF. The subject matter covered by these patents was developed, in whole or in part, by Dr. Carlo Croce, a pioneer and recognized leader in the field of miRNA, and by Dr. Robert Lee, a recognized expert on nanoparticle-based miRNA delivery technology. Dr. Robert Lee is a member of our scientific advisory board and a consultant to the company. The other members of our scientific advisory board also have extensive experience in miRNA biology and in oligonucleotide therapeutics design and development.
In contrast to conventional chemotherapy, miR-based therapy is biomarker-driven and its miR targets are expected to affect entire gene-regulatory networks that are believed to serve as the master switches of tumor survival. The networks usually control several different points in the course of cancer development. Therefore, miR-based therapy is likely to be more effective with fewer side effects. Rather than screening a large library of hundreds of thousands of compounds for lead identification, which is routinely done in traditional drug discovery, miR-based drug discovery is relatively straightforward because the therapeutic oligonucleotide sequence is defined by that of the miR target. The drug directly targets the dysregulated microRNA based on sequence complementarity. If successfully developed, manufactured and commercialized with all the required regulatory approvals, we believe this has the potential to alter the landscape of the oncology market.
| 10 |
Delivery Technology for miR-Based Therapeutics
A significant challenge to clinical development of miR-based therapeutics is the need for a delivery system. This is because human serum is rich in nucleases and the cell membrane is a formidable barrier to the intracellular delivery of oligonucleotides, which makes it difficult to bring the therapeutics to the target site. Other factors, such as renal and reticuloendothelial system clearance, also hinder delivery. The problem of delivery has been a major impediment in previous efforts toward clinical development of siRNA therapeutics and antisense oligonucleotides, as shown by the limited success of these classes of agents in the clinic.
The critical importance of in vivo delivery is well-recognized in the field of siRNA therapeutics as a bottleneck in their clinical development. The approval of ISIS and Genzyme’s KynamroTM as the first systemically administered antisense oligonucleotide therapy in humans for familial hypercholesterolemia in early 2013 was a watershed event in the field of oligonucleotide therapeutics. However, many antisense drugs have performed poorly in the clinic, which may be attributed to inadequacy in the delivery system. On the other hand, if an effective delivery technology is found, it would have broad applications to many types of potential oligonucleotide therapies.
There are two general strategies to addressing the delivery problem of oligonucleotides: chemical modification and nanoparticle formulation. Our delivery approach is expected to incorporate both of these strategies, based on what we believe are strong scientific rationale and strong patent positions.
This dual approach can be seen in the example of one of our therapeutic product candidates, LumiralinTM (antimiR-21). The antimiR-21 oligonucleotide is defined as the drug substance or active pharmaceutical ingredient (API) for the product. To achieve optimum activity, modifications are incorporated to increase its affinity for the miR-21 target and endonuclease stability while reducing non-specific immune system activation which may lead to side effects. While with these modifications the antimiR design is considered ‘‘optimized’’ for an unformulated free or ‘‘naked’’ agent, previous studies have shown that free antimiRs are mostly taken up by the liver and that a high dose is required for therapeutic activity, which in turn leads to potential kidney and liver toxicity.
QTsomeTM is a proprietary lipid nanoparticle-based delivery platform developed by Dr. Robert Lee, a member of our scientific advisory board, and a Professor at the OSU College of Pharmacy. QTsomesTM comprise an optimum combination of two cationic lipids, one with a quaternary amine headgroup and the other with a tertiary amine headgroup. These components can provide a stable nanoparticle and facilitate efficient delivery of the antimiR into the cytoplasm of the target cell. In addition, QTsomesTM incorporate PEG-DPPE, which cause them to stay in the blood circulation for a long time and to passively target delivery to solid tumors based on the enhanced permeability and retention (EPR) effect. This EPR effect is due to the increased blood vessel permeability of solid tumors and reduced lymphatic drainage, which lead to higher QTsomeTM uptake by the tumor.
The benefits of QTsomesTM include high efficiency, low toxicity, rational design, and, in our belief, our strong patent position. QTsomesTM utilize off-the-shelf cationic lipids and helper lipid components that are available in cGMP grade from commercial suppliers. This removes a potential barrier to clinical translation. According to Dr. Lee, production of QTsomesTM can readily be up-scaled to gram- and kilogram levels using standard and scaleable pharmaceutical manufacturing technologies such as diafiltration, sterile filtration, and lyophilization. The feasibility of large-scale production is an important advantage of the QTsomesTM formulation. Due to its higher efficacy and tumor selectivity, a lower clinical dose would be required than free antimiRs or miR mimics, and we therefore expect reduced adverse side effects.
| 11 |
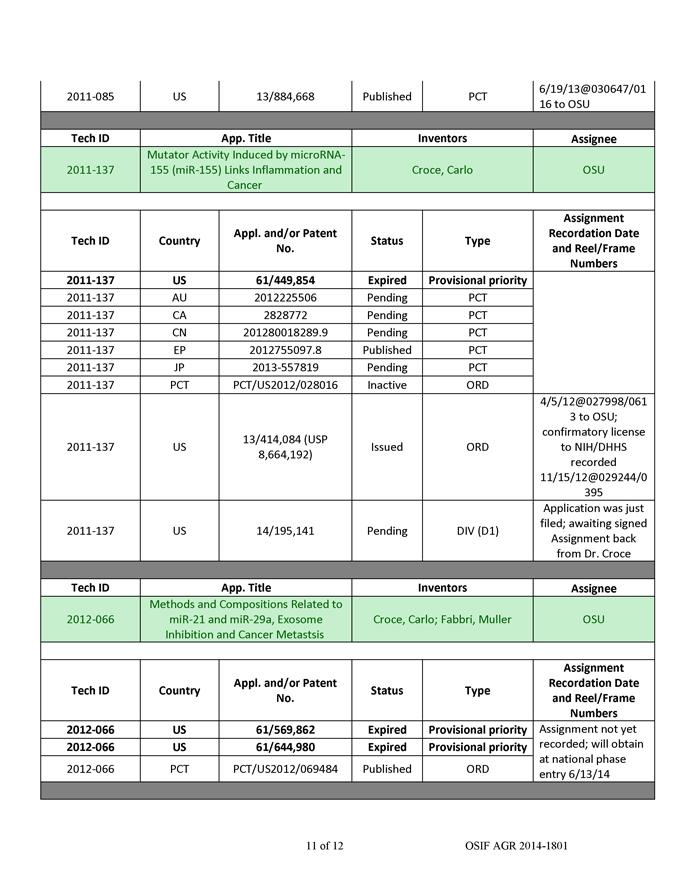
The QTsomeTM technology has been validated in Dr. Lee’s laboratory at OSU. Antimir-21 (AM-21) was shown to upregulate tumor suppressor genes RECK and PTEN in A549 lung cancer cells (Figure 2).
![[GRAPHIC MISSING]](image_002.jpg)
Figure 2. Upregulation of tumor suppressor genes in A549 lung cancer cells by AM-21
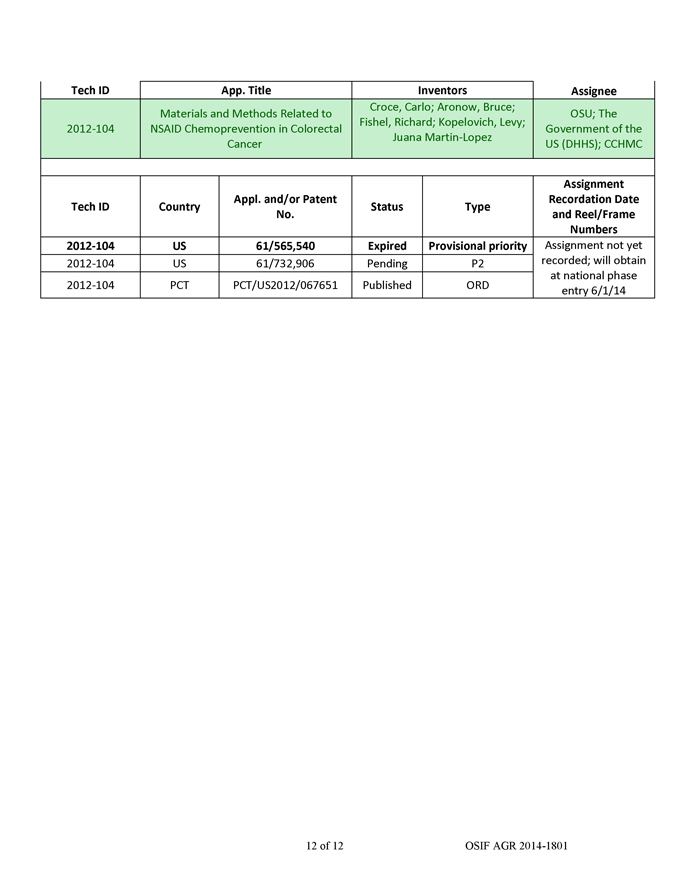
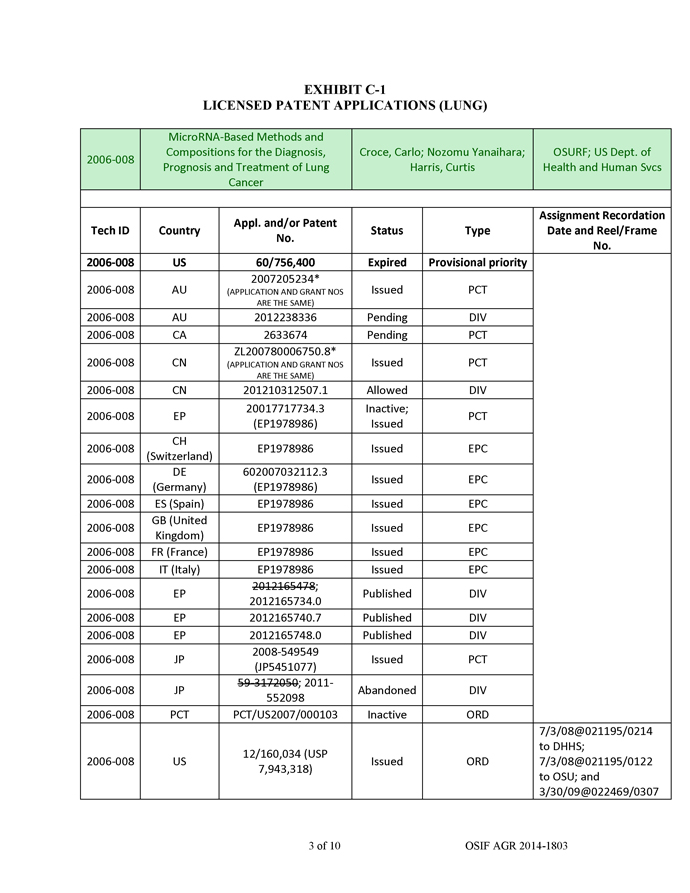
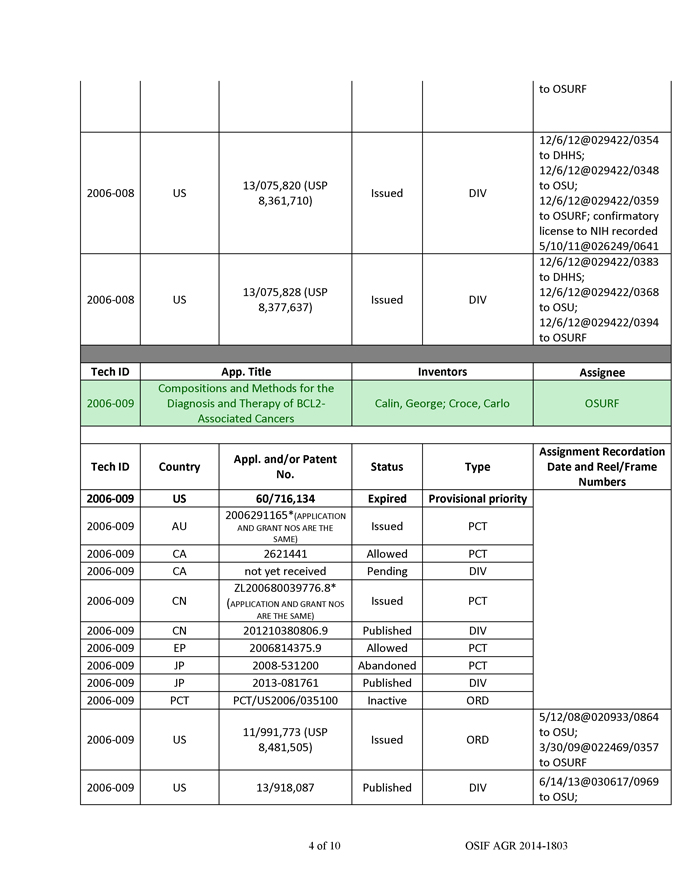
AM-21 were then loaded into QTsomesTM and evaluated in tumor cell lines and in a mouse tumor model. The QTsomesTM were small in size (~100 nm) based on dynamic light-scattering measurement and had a low surface charge. These properties are ideal for in vivo delivery. The QTsomesTM effectively inhibited miR-21’s biological functions and unregulated tumor suppressor genes RECK and PTEN in A549 cells and sensitized these cells to a chemotherapy agent paclitaxel (PTX) (Figure 3). Additionally, treatment of A549 cells with OTsomeTM/AM-21 resulted in a reduced capacity to migrate on a plastic dish (Figure 3a). This ability to reduce migration is often interpreted as a potential for the drug to be anti-metastatic. Furthermore, QTsomesTM carrying AM-21 completely inhibited the growth of A549 xenograft tumors in nude mice (Figure 3b) without causing overt toxicity to the mice as indicated by increased weight gain and no significant changes in organ weights in the treated group (data not shown).
Cell Viability (% of untreated cells)
![[GRAPHIC MISSING]](image_003.jpg)
Figure 3. QTsomeTM/AM-21 sensitized A549 cells to chemotherapy drug paclitaxel (PTX)
| 12 |
![[GRAPHIC MISSING]](image_004.jpg)
Figure 3a. QTsomeTM/AM-21 treatment of A549 lung cancer cells reduced their ability to migrate into an open space on a tissue culture dish caused by a scratch through a monolayer of cells.
![[GRAPHIC MISSING]](image_005.jpg)
Figure 3b. Inhibition of A549 xenograft tumor growth by QTsomeTM/AM-21. Mice were inoculated s.c. with A549 cells and randomized into two groups of seven after tumors reach >50 mm3 (~1 week after inoculation). The treatment group was injected intravenously with 2 mg/kg by i.v. injection 3 times in the first week and once a week thereafter.
| 13 |
QTsomeTM/AM-21 was also evaluated in KB tumor cells. As shown in Figure 4, QTsomesTM/AM-21 upregulated tumor suppressor gene PTEN over untreated control and more effectively than free AM-21. In a preliminary pilot study in vivo, intravenous delivery of an antimiR-21 formulated in QTsomesTM resulted in inhibition of miR-21 as demonstrated by upregulation of miR-21 target genes (Figure 5) and a trend toward tumor growth inhibition (data not shown). The study did not have large enough group sizes to detect the tumor growth inhibition with confidence, so larger follow up studies are underway.
![[GRAPHIC MISSING]](image_006.jpg)
Figure 4. Upregulation of tumor suppressor gene PTEN by QTsomeTM/AM-21 in KB cells.
Normalized gene expression (% of untreated tumors)
![[GRAPHIC MISSING]](image_007.jpg)
Figure 5. Upregulation of miR-21 target genes in KB cell xenograft tumors after delivery of QTsomeTM formulated antimiR-21.
| 14 |
Collectively, these results support the further development of QTsomeTM/AM-21 nanoparticles for lung cancer therapy, either alone or in combination with standard chemotherapy. We have recently identified early lead antimiR-21 compounds with a proprietary design that are more potent than the publicly available antimiR-21 compounds we used in the preclinical data here. We believe this will allow us to move forward into additional in vivo studies with proprietary compounds formulated in QTsomesTM while also performing more screens to identify additional lead compounds to enter the in vivo studies to ensure we identify the best potential clinical candidate.
Dr. Lee’s laboratory also made progress on designing enhanced versions of QTsomesTM (licensed by the company). It was found that adding the small peptide, gramicidin, into QTsomesTM was able to improve delivery in the presence of serum. These peptide-enhanced QTsomesTM were found to be very effective in delivery of both siRNA, which is similar to miR mimics, and antimiRs. In a study recently published in the Journal of Controlled Release, a targeted version of QTsomesTM loaded with antimiR-155, with enhancement by gramicidin addition, was shown to be highly active in blocking miR-155 function in liver cancer cells. In this study, a glycolipid, Lactosyl-DOPE, was also added to make these QTsomeTM nanoparticles selective for liver cancer cells, which express the asialoglycoprotein receptor. This improved both the efficiency of antimiR delivery and the selectivity for the targeted cells. The design of the antimiR incorporated chemical modifications (OMe and phosphorothioate), which improved the stability and bioactivity of the oligonucleotide. The particles synthesized were compact in size (73 nm), were slightly positively charged and had high encapsulation efficiency for the antimiR (88%). These are properties that are conducive to in vivo delivery. Treatment of the cancer cells greatly increased the expression of two tumor suppressor genes, CEBP/beta and FOXP3, by 4 and 16-fold, respectively. Importantly, these QTsomesTM were highly active when injected intravenously at a relatively low dose (1.5 mg/kg) into mice and induced similar biological effects in vivo, increasing expression of a tumor suppressor CEBP/beta and FOXP3 by 6.9 and 2.2-fold, respectively. We believe this is significant since in vivo delivery efficiency is the key towards development of a suitable delivery vehicle for clinical applications.
In summary, we have licensed a proprietary lipid nanoparticle-based miR delivery technology (QTsomeTM) for AMT and MRT from OSIF, which we believe will provide us with an advantage in addressing the problem of AMT and MRT delivery with respect to our proposed therapeutics.
Our miR-BASED THERAPEUTIC PRODUCT CANDIDATES
We intend to develop a series of miR-based AMT and MRT agents for lung, ovarian, colorectal and prostate cancers. The initial clinical development candidates are listed in the table below:
| Therapeutic Candidate | miR Target | Modality | Cancer Targets | ||||
| LumiralinTM | miR-21 | AMT | Lung | ||||
| OmiralinTM | miR-484 | MRT | Ovarian | ||||
| ColomiralinTM | miR-17-5p | AMT | Colorectal | ||||
| ProstamiralinTM | miR-34a | MRT | Prostate |
Market Opportunity
NSCLC, which includes adeno-, squamous-cell and large-cell carcinoma, is the leading cause of cancer deaths both in men and in women. Lung cancer causes more deaths than the next three most common cancers combined (colon, breast and prostate). According to the American Cancer Society, an estimated 160,340 Americans were expected to die from lung cancer in 2012, accounting for approximately 28 percent of all cancer deaths. According to a 2009 report by Global Industry Analysis, the global market for NSCLC therapeutics is set to reach $13 billion in year 2015, representing a large potential market.
Diagnosis and Current Treatment
Lung cancer is diagnosed using computed tomography (CT) X-ray-scan and tissue biopsy. Serum markers CEA and CYFRA 21-1 are often used as prognostic and predictive markers. Lung cancer treatment depends on the stage of
the disease. Early stage NSCLC is managed by surgery followed by combination chemotherapy. However, this is associated with serious side effects and limited response rates. A new molecularly targeted agent gifitinib (Iressa) has shown some early promise. However, approval of this drug was withdrawn by the FDA in 2005 due to lack of evidence that it extended life. Molecular classification of lung cancer patients is beginning to improve therapy for small subsets of patients. However, the majority of NSCLC patients (e.g., patents with mutated K-ras gene) still have no effective molecularly targeted therapy. Development of additional novel therapeutic and diagnostic strategies for NSCLC, therefore, is urgently needed to better match additional NSCLC patients to effective novel therapies. We believe that our product candidates are different from other molecularly targeted agents due to our intended targets and proprietary delivery methods.
| 15 |
LumiralinTM AMT
miR-21 may arguably be the most validated oncomiR in cancer. It is commonly elevated in many cancer types including lung, ovarian, colorectal, prostate, liver, kidney, breast and brain cancers. miR-21 targets tumor suppressor gene such as PTEN. AntimiR-21 AMT has been shown to inhibit tumor growth, migration and invasion, and to reverse chemotherapy and radiotherapy resistance of cancer cells.
miR-21 is the most frequently over-expressed miR over all cancer types and its genomic locus is amplified in some cancer types. Expression of miR-21 correlates with poor patient outcome in many cancer types, including lung cancer. miR-21 expression is known to be driven by several well characterized oncogenes including STAT3, K-ras, C-raf, and PI3K pathways. Inhibition of miR-21 with an anti-miR leads to reduced tumor formation and prolonged survival in a Ras driven model of HCC.
In one study, a mouse model was constructed in which miR-21 could be inducibly over-expressed in the hematopoietic system. When miR-21 was induced, hematopoietic organ grew to 20 times the normal size, which resulted in an acute lymphoma. After lymphomas were formed, miR-21 was shut off and the tumors completely regressed within 2 weeks. This demonstrates that these tumors were not only formed due solely to over-expression of miR-21, but the tumors were also ‘‘addicted’’ to the expression of miR-21.
In a K-ras mouse model, miR-21 expression was found to cooperate with K-ras to form lung cancer. In this model mutant K-ras was expressed only in the lungs. Knockout of miR-21 reduced tumor formation in the model, as shown in Figure 6.
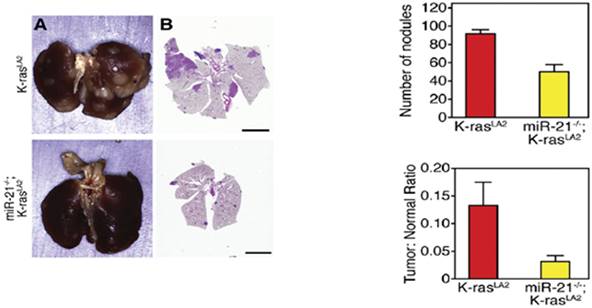
Figure 6. Knockout of miR-21 reduced tumor formation in a K-ras model (adapted from Hatley et al., 2010, Cancer Cell 18: 282-293)
We believe we have established a strong patent position on miR-21-based AMT therapy in lung cancer. Free antimiR-21 (without nanoparticles as carriers) is currently under clinical development by Regulus and Sanofi for hepatocellular carcinoma (HCC) and for kidney fibrosis. In comparison, our antimiR-21 AMT drug candidate, LumiralinTM, incorporates the proprietary delivery technology based on QTsomeTM lipid nanoparticles and we believe is likely to be more potent. LumiralinTM AMT is expected to have therapeutic activity in multiple tumor types, which we believe makes it a logical choice as a lead product for rapid clinical development. LumiralinTM AMT will be designed to be used by intravenous infusion either as a monotherapy or as a combination with standard chemotherapy in patients with recurrent disease, with extension of survival as the expected clinical endpoint. Because miR-21 is unregulated in many cancers including lung and ovarian, we will evaluate whether we can feasibly conduct human clinical trials in both lung and ovarian cancer patients and seek orphan drug status for LumiralinTM AMT.
| 16 |
Ovarian Cancer
Market Opportunity
Ovarian cancer is a leading cause of cancer death among women. The American Cancer Society estimates that approximately 22,000 new cases of ovarian cancer will be diagnosed in 2013 in the United States and approximately 14,000 of the diagnosed individuals will die. While the mortality rate of other major cancers have declined, the mortality rate for ovarian cancer changed little in the past 40 years, making it the most lethal of all gynecologic cancers. Ovarian cancer therapies are eligible for orphan drug status under the Orphan Drug Act of 1983, which provides seven years of market exclusivity. According to a 2013 report by BCC Research, the global ovarian cancer drug and diagnostic market is projected to reach $34.6 billion by 2018.
Diagnosis and Current Treatment
Ovarian cancer is diagnosed by physical exam, CT scan, ultrasound, PET scan, laparoscopy, colonoscopy, and biopsy. Serum CA-125 test is also routinely used in diagnosis and post-therapy monitoring. Early stage ovarian cancer can be effectively treated with surgery. However, most ovarian cancer patients are at an advanced stage at the time of diagnosis. The standard therapy for an ovarian cancer patient is platinum/taxane combination or Doxil therapy. Due to the frequent abdominal involvement of the disease, intraperitoneal chemotherapy can be added to intravenous chemotherapy. An antibody drug, Avastin, is sometimes added as well. There has been less success with molecularly characterizing ovarian cancer patients compared to many other cancer types and this is a factor in the poor response to current therapies. Given the poor survival rates of ovarian cancer patients and the prominent role of early diagnosis, development of new therapeutic and diagnostic strategies is urgently needed.
OmiralinTM MRT
It has been found that miR-484 expression is frequently lowered in ovarian carcinomas. This miR has been shown to regulate the mitochodrial network and programmed cell death. miR-484 MRT has been shown to inhibit tumor growth when combined with chemotherapy. We believe we have established a strong patent position on miR-484 based therapy in cancer. Our miR-484 mimic MRT drug candidate, OmiralinTM, incorporates the proprietary delivery technology based on QTsomeTM lipid nanoparticles. It is designed to be administered by intravenous infusion or intravenous/intraperitoneal combination, and can be used alone or in combination with standard chemotherapy in patients with recurrent disease, with extension of survival as the clinical endpoint.
The important role of miR-484 in cancer was discovered in Dr. Croce’s lab at OSU. miR-484 targets VEGF-B and VEGFR2 (along with many other genes) to block the angiogenic process needed by tumors.
Low circulating levels of miR-484 in ovarian cancer patients can be used to predict patient response to chemotherapy. Meanwhile, increased expression of miR-484 sensitizes ovarian tumors to chemotherapy. Ovarian cancer can be potentially treated by ‘‘local’’ intraperitoneal delivery, in combination with chemotherapeutics.
| 17 |
Colorectal Cancer
Market Opportunity
Colorectal cancer is the third leading cause of cancer deaths in the United States among men and women. According to the American Cancer Society, it is estimated to cause 50,830 deaths in 2013. If diagnosed early, when the disease is local, 5-year survival rate is approximately 90%. However, only 40% are diagnosed at this early stage. According to a 2010 report by Global Data, the global colorectal cancer therapy market is projected to grow to $11.6 billion in 2016 with a CAGR of 9.8%.
Diagnosis and Current Treatment
Colorectal cancer is detected by colonoscopy, CT and PET scans, ultrasound, blood tests, and biopsy. Serum CEA and CA19-9 tests are also routinely used in monitoring of the disease. Early stage colon cancer can be effectively treated with surgery. However, a large portion of colon cancer patients are at an advanced stage at the time of diagnosis. The standard therapies for colorectal cancer include a combination of surgery, radiation therapy, and chemotherapy. Given the prevalence of colorectal cancer patient and the critical role of early diagnosis in the management of this disease, new therapeutic and diagnostic strategies are urgently needed.
ColomiralinTM AMT
miR-17-92 cluster consists of 6 microRNAs that are frequently elevated in lung, gastric, colon, liver, pancreatic, renal, prostate, and breast cancers. These are a family of microRNAs encoded in three genomic clusters. Because members of a microRNA ‘‘family’’ share the same seed sequence, it is possible to design a single anti-miR to inhibit multiple family members. The miR-17 family is the only family with at least two members in each cluster. The targets of the miR-17 family include important genes in cell cycle and cell surface receptor driven proliferation. The miR-17 family is well validated alone, but the compound nature of the clusters will require more work to determine the best AMT strategy. AntimiR-17-5p AMT has been shown to inhibit tumor growth. We believe we have a strong patent position on miR-17-5p based therapy in colorectal cancer. Our antimiR-17-5p AMT drug candidate, ColomiralinTM, incorporates the proprietary delivery technology based on QTsomeTM lipid nanoparticles. ColomiralinTM AMT is designed to be used by intravenous infusion as monotherapy or in combination with standard chemotherapy as a second-line therapy, with extension of survival as the clinical endpoint.
| 18 |
Prostate Cancer
Market Opportunity
Prostate cancer is very common and is the second leading cause of cancer death among men. The American Cancer Society estimates that approximately 238,590 new cases of prostate cancer will be diagnosed in the United States in 2013 and 29,720 of the diagnosed individuals will die from it. According to Decision Resources, a man has a 1 in 6 chance of diagnosis of prostate cancer in his lifetime. While the 5- year survival rate of local (Stage I, II) and regional (Stage III) disease is 100%, Stage IV prostate cancer with distant metastasis has a survival rate of only 28%. According to a report by Decision Resources, the global market for prostate cancer drug will grow from $3.6 billion in 2010 to $10.1 billion in 2020, a CAGR of
10.8%.
Diagnosis and Current Treatment
Prostate cancer is diagnosed through screening tests such as prostate-specific antigen (PSA) test and digital rectal exam, and confirmed by a biopsy. Treatment of prostate cancer includes radiation therapy, hormone therapy, orchiectomy, prostatectomy, cryoablation, high intensity focused ultrasound thermoablation, and chemotherapy. Since advanced prostate cancer is often resistant to standard chemotherapy drugs, novel therapeutic strategies are needed.
PromiralinTM MRT
miR-34a is a tumor suppressor microRNA that is significantly lowered in prostate cancer. The genomic locus of miR-34a is frequently methylated in tumors to silence expression and is also repressed by cell surface oncogenic receptor tyrosine kinases (RTKs). miR-34 potentiates the p53 tumor suppressor pathway and inhibits proliferation pathways downstream of oncogenic growth factors. The targets of miR-34a include CDK4, CDK8, Met, Myc and E2F3. Delivery of miR-34 mimics into tumor cells leads to a G1 cell cycle block. In prostate cancer, its expression is associated with less aggressive diseases. Accordingly, miR-34 MRT has been shown to inhibit tumor cell growth including in tumors with a mutated p53. miR-34 MRT, therefore, is a promising novel therapeutic modality against prostate cancer. Mirna Therapeutics, Inc. is conducting a Phase I clinical trial for MRX34 in patients with unresectable liver cancer or liver metastasis. MRX34 utilizes the SMARTICLES as the delivery vehicle, which is based on a proprietary ‘‘charge-reversing’’ lipid nanoparticle formulation. We believe we have a strong patent position on miR-34 MRT in prostate cancer. Our miR-34 mimic MRT candidate therapeutic, PromiralinTM, incorporates the proprietary delivery technology based on QTsomesTM lipid nanoparticles. PromiralinTM MRT is designed to be used by intravenous infusion as a monotherapy or in combination with standard chemotherapy in patients with metastatic disease, with extension of survival as the clinical endpoint.
| 19 |
OUR miR-BASED THERAPEUTIC PRODUCT PIPELINE
We have identified four miR-based therapeutic products (LumiralinTM AMT, OmiralinTM MRT, ColomiralinTM AMT, and PromiralinTM MRT) as our initial pipeline candidates. Lung, ovarian, colorectal, and prostate cancers have been selected as lead indications because of their established clinical need and large market potential. In addition, we believe we have strong intellectual property positions on these product candidates, which incorporate advanced delivery technology based on QTsomesTM. Each of the product candidates we have identified can target multiple tumor types given the prevalence of the corresponding miR targets in these tumors. In addition, it is possible to combine miR therapeutics to match a specific miR profile. For example, for a tumor that is high in miR-21 and low in miR-34, a therapy combination of LumiralinTM AMT and PromiralinTM MRT can be used to produce a synergistic effect. This miR combination can also be developed as a new drug product by combining the antimiR and miR-mimic into the same QTsomeTM lipid nanoparticle. This strategy can generate additional product candidates for our pipeline. Our therapeutic development strategy is to initially focus our resources on preclinical development and clinical translation of our current lead product candidate, LumiralinTM AMT, while continuing with validation of pipeline product candidates through preclinical research. We intend to seek strategic partners to develop pipeline product candidates.
OUR OUTSOURCING-BASED PRODUCT DEVELOPMENT STRATEGY
Outsourcing
An emerging trend in the biotechnology industry is to outsource developmental functions. This eliminates the need to establish a sizeable research and development facility, which is time consuming and capital intensive. By outsourcing our developmental functions, we are able to keep fixed costs to a minimum and flexibility to a maximum. Under this outsourcing model, we can leverage the established expertise of consultants and capabilities at partner academic institutions and CROs resulting in an overall efficient process for drug development. Our management will execute the strategic plan and carry out operational management and coordinate activities at sites of contractors and CROs. We are engaged in multiple discussions with CROs covering all stages of preclinical development and clinical trials. However, there is no assurance that we will enter into agreements with CROS or any other parties on acceptable terms, or at all.
Preclinical Research: Relationship with OSU
We have entered into license agreements with OSIF, an affiliate of OSU, covering approximately 138 pending patent applications and 132 granted patents covering various aspects of applications of miRNAs in cancer, as well as on miR therapeutic delivery, and as a result, we have developed a strategic relationship with OSU. The key inventor of the licensed delivery technology, Dr. Robert Lee, have extensive experience and resources to conduct preclinical research geared towards product development. Dr. Lee serves on our scientific advisory board and is a consultant to the company. Substantial complementary resources are available from the OSU Comprehensive Cancer Center, the OSU College of Medicine, the OSU College of Pharmacy, the NIH-funded Center for Clinical and Translational Sciences, the Drug Development Institute, and the NSF-funded Nanoscale Science and Engineering Center. We expect to enter into a contract with OSU and Dr. Lee pursuant to which Dr. Lee will serve as the Principal Investigator for our preclinical studies. However, we cannot provide any assurance that we will be successful in entering into such an agreement on acceptable terms, or at all.
| 20 |
Manufacturing of the Active Pharmaceutical Ingredient and the Therapeutic Product
A drug product is composed of active pharmaceutical ingredient (API) and excipients. The manufacturing of the drug product for clinical trial must be carried out under cGMP.
Sourcing of API
AntimicroRNAs and miR mimics are manufactured as custom synthetic oligonucleotides. These oligonucleotides will carry chemical modifications. For clinical development, the drug product needs to be synthesized at 100 − 1,000 gram-scale at a cGMP-compliant CMO. We are engaged in discussions and have obtained price quotes on the quantity of materials needed for preclinical development and clinical trials.
Chemical modifications will increase the potency of anti-miR compounds. Oligonucleotide therapeutic compounds have chemical modifications of the backbone or sugar of the oligonucleotide to increase potency and stability and reduce off target effects. Our QTsomeTM delivery technology, which protects the compounds in circulation, allows us more freedom around modifications. Our first library of potential anti miR-21 leads contains compounds with phosphorothioate and 2’-O-methyl modifications, both of which are in the public domain and will result in active compounds. In addition, we intend to enter into agreements with companies to license proprietary modifications that could further increase the potency of our lead compounds. We can provide no assurance, however, that we will be able to enter into such agreements on favorable terms, or at all. The resulting lead compounds that are identified in our ongoing product candidate screens will be unique chemical entities and will be proprietary to us.
Sourcing of Excipients
QTsomesTM include several key lipid ingredients. These will be acquired in cGMP-grade from excipient suppliers. Other excipients include USP grade glycine, trehalose are available from many suppliers. By design, for the QTsomesTM, all excipients are available through commercial suppliers.
| 21 |
Drug Product Manufacturing
We plan that QTsomeTM lipid nanoparticles will be manufactured at a cGMP compliant CMO. There are many CMOs to choose from, and we have engaged several CMOs in discussion. However we can provide no assurance that we will be able to enter into an agreement with a CMO on favorable terms, or at all. Each of these CMOs has established expertise on lipid-based formulations. Lipid nanoparticles are a relatively complicated formulation. Dr. Robert Lee has extensive expertise in the manufacturing of lipid nanoparticles and will advise us throughout this process. Processes and analytical methods developed in his laboratory at OSU and from the API CMO will be transferred to the CMO for making the drug product. When and if we are ready for manufacturing, the manufacturing process will be scaled up to the necessary scale for the clinical trials and performed under cGMP. Smaller non-GMP batches will be manufactured with the same product specifications prior to the clinical batch and used for IND-directed good laboratory practice (GLP) toxicology studies and stability studies. The manufacturing process includes continuous mixing, diafiltration, sterile filtration, aseptic filling, and lyophilization. The product specifications for quality control will include API content, particle size and polydispersity, zeta potential, pH, appearance and colloidal stability. In addition, stability, sterility, and pyrogen tests will be carried out. Data obtained will be incorporated into the IND in the section on Chemistry, Manufacturing, and Control (CMC).
Toxicology Testing
We expect that IND-directed toxicology testing will be carried out at a GLP-compliant toxicology CRO. There are many CROs to choose from and we have engaged a few CROs in discussion. However, we can provide no assurance that we will be able to enter into an agreement with any of these parties on acceptable terms, or at all. Materials used for the GLP toxicology study are to be provided by the CMO that produces the clinical trial material. However, a non-GMP batch manufactured to the same specifications can be used in the toxicology testing to accelerate the developmental process. The toxicology studies will include dose-escalation studies in rat and in a non-human primate and will include genotoxicity studies.
IND Filing with the FDA
We plan to retain a regulatory consultant to handle regulatory filings. During the development process, we expect to arrange a pre-IND meeting with the FDA to obtain input on various aspects of the product development plan. The regulatory consultant will help us organize preclinical efforts to ensure all preclinical work is completed in a timely fashion. As described above, the information relating to the Animal Pharmacology and Toxicology Studies is expected to be based on OSU studies and the GLP Toxicology Study data, which is expected to be collected by a CRO. Meanwhile, materials for the CMC section are expected to come from the CMO that manufactures the drug product. Finally, a section on Clinical Protocols and Investigator Information is expected to be generated in close collaboration with the OSU Division of Medical Oncology and the Clinical Trials Office. Clinical protocols are expected to be developed by physician scientists Dr. Miguel Villalona and Dr. John Hayes, who have extensive experience designing and leading Phase I clinical trials. If so, the clinical trial protocol will be reviewed and approved by OSU’s Institutional Review Board (IRB). In addition, we expect to draft an Investigator’s Brochure to be included in the IND application. The regulatory consultant will be expected to compile all relevant information and assemble the complete IND application and submit to the FDA and, if necessary, follow up with responses if questions are raised by the FDA.
Phase I Clinical Trials on Lumiralin in Lung Cancer and Ovarian Cancer Patients
We plan to retain the service of a CRO to conduct Phase I clinical trials at the OSU Comprehensive Cancer Center on our lead product candidate, LumiralinTM. The Phase I trial will be based on a 3x3 design of dose escalation plus an expansion cohort. This study will involve 12 − 36 patients. Patient blood samples will be analyzed for pharmacokinetics of the drug product and for biomarkers. The CRO and lead physician scientists at OSU will work closely to conduct trial planning and design, patient recruitment, data management, laboratory analysis, and reporting and oversight to ensure data integrity and adherence to good clinical practices (GCP).
| 22 |
OUR RELATIONSHIP WITH OSU
We have established relationships with multiple departments across OSU. In September 2013 we entered into five exclusive licensing agreements with OSIF, an affiliate of OSU, to exclusively in-license approximately 138 pending patent applications and 132 granted patents covering various aspects of applications of miRNAs in cancer as well as a novel delivery technology known as QTsomes. In addition, Dr. Robert Lee, the inventor of the QTsomeTM technology, serves as members of our scientific advisory board and is a consultant to the company.
We expect to engage Dr. Lee as the Principal Investigator for our preclinical studies, which will then be conducted in his lab at OSU. Throughout the manufacturing process, Dr. Lee will continue to advise and oversee the work with our third party contractor. Further, we expect to work with Dr. Lee and others at OSU in designing our clinical trials, which we expect will be carried out at the OSU Comprehensive Cancer Center. We cannot guarantee, however, that we will be able to enter into definitive agreements with these parties on acceptable terms, if at all.
We believe that our relationship with OSU will be beneficial to our company because of OSU’s deep research expertise and experience in the microRNA and oncology fields. Further, we expect that the OSU Comprehensive Cancer Center will provide us with guidance in running clinical trials as well as a large patient population pool.
OUR INTELLECTUAL PROPERTY
We have a portfolio of approximately 138 pending patent applications and 132 granted patents worldwide, which we have exclusively licensed from OSIF for use in the diagnosis, prognosis and therapy of certain types of cancer. The portfolio comprises 21 patent families having expiries between 2026 and 2035, not including any patent term adjustments or extensions, which may have been granted or which may be available, respectively. Protection of the intellectual property is being sought in major markets, including the United States, Europe, China, Japan, Australia and Canada. The portfolio embraces claims directed to composition of matter, methods of use, treatment, diagnosis, prognosis and/or stratification of patient populations and evaluation of cancer risk for ovarian, lung, colorectal and prostate cancers, including solid tumors and leukemia. Worldwide, the portfolio includes 6 granted patents directed to ovarian cancer, 37 granted patents directed to lung cancer, 92 granted patents directed to colorectal cancer and 16 granted patents directed to prostate cancer. Our product candidates are based upon the exploitation of these compositions and methods relating to the detection, measurement and/or stratification of microRNA levels or signatures in patients. Our intellectual property portfolio provides us with exclusive access to certain microRNAs and their applications in certain types of cancer as well as a proprietary delivery technology.
We believe that this intellectual property is critical to our business and we strive to strengthen and protect it. We also will rely on trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. Our success will depend significantly on our ability to maintain and defend our patents and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, and preserve the confidentiality of our trade secrets. We also rely on know-how, continuing technological innovation and in-licensing opportunities to develop, strengthen and maintain our proprietary position in the field of microRNA therapeutics. We intend to continue to seek opportunities to in-license relevant intellectual property as it is necessary to develop our product candidates and defend our competitive position in the marketplace.
COMPETITION
The biotechnology and pharmaceutical space is very competitive and rapidly changing, with new companies entering and working to develop new technologies and proprietary products. In addition, our field is tied to the advancement of the related fields of science, which continue to evolve and generate new insights and intellectual property.
We are aware of many competitors working in the therapeutic space for microRNA. Therapeutic competitors include miRagen Therapeutics, Inc., Mirna Therapeutics, Inc., and Regulus Therapeutics, Inc. These competitors compete with us in terms of recruiting talent, acquiring intellectual property and receiving potential funding during our development phase. Our competitors are better established, are further along in their development efforts and have greater resources at their disposal than us.
In addition, we expect that for each type of cancer for which we develop a therapeutic product candidate, we will compete against a different set of companies that market therapeutics for that given disease utilizing other approaches and technologies. For example, Mirna Therapeutics, Inc. is conducting a Phase I clinical trial for MRX34 in patients with unresectable liver cancer or liver metastasis. MRX34 utilizes the SMARTICLES as the delivery vehicle, which is based on a proprietary ‘‘charge-reversing’’ lipid nanoparticle formulation. The key competitive factors that will affect the success of any of our product candidates, if developed, manufactured and commercialized after obtaining all required regulatory approvals, are likely to be their safety, convenience, and efficacy.
| 23 |
GOVERNMENT REGULATION AND PRODUCT APPROVAL
Government authorities in both the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of products such as those we are developing. The process of developing and obtaining regulatory approvals for our product candidates, as well as the subsequent compliance with applicable federal, state, local and foreign statutes and regulations, require the expenditure of substantial time and financial resources.
U.S. Therapeutic Regulations
U.S. Drug Development Process
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or FDCA, and implementing regulations. Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with the appropriate federal, state, local and foreign laws and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial civil or criminal sanctions. FDA sanctions may include clinical holds, refusals to approve pending applications, withdrawal of an existing approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, debarment, restitution, disgorgement or civil or criminal penalties.
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
- completion of nonclinical laboratory tests, animal studies and formulation studies according to good laboratory practices, or GLP;
- submission to the FDA of an IND, which must become effective before human clinical trials may begin;
- performance of adequate and well-controlled human clinical trials according to the FDA’s regulations commonly referred to as GCPs, to establish the safety and efficacy of the proposed drug for its intended use;
- submission to the FDA of a NDA for a new drug;
- satisfactory completion of an FDA inspection of the manufacturing facility or facilities where the drug is produced to assess compliance with the FDA’s cGMP to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity;
- potential FDA audit of the nonclinical and clinical trial sites that generated the data in support of the NDA; and
- FDA review and approval of the NDA.
Before testing any compounds with potential therapeutic value in humans, the drug candidate enters the preclinical testing stage. Preclinical tests, also referred to as nonclinical studies, include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies to assess the potential safety and activity of the drug candidate. The conduct of the preclinical tests must comply with federal regulations and requirements including GLP. The sponsor must submit the results of the preclinical tests, together with manufacturing information, analytical data, any available clinical data or literature and a proposed clinical protocol, to the FDA as part of the IND. The FDA may also impose clinical holds on a drug candidate at any time before or during clinical trials due to safety concerns or non-compliance. Further, each clinical trial must be reviewed and approved by an independent institutional review board, or IRB, at or servicing each institution at which the clinical trial will be conducted. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits.
| 24 |
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
- Phase 1. The drug is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients.
- Phase 2. The drug is evaluated in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance, optimal dosage and dosing schedule.
- Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical trial sites. These clinical trials are intended to establish the overall risk/benefit ratio of the product and provide an adequate basis for product labeling. Generally, two adequate and well-controlled Phase 3 clinical trials are required by the FDA for approval of an NDA.
Post-approval clinical trials, sometimes referred to as Phase 4 clinical trials, may be conducted after initial marketing approval. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication. Annual progress reports detailing the results of the clinical trials must be submitted to the FDA and written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events or any finding from tests in laboratory animals that suggests a significant risk for human subjects. The FDA or the sponsor or its data safety monitoring board may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
U.S. Review and Approval Processes
The results of product development, nonclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of substantial user fees; a waiver of such fees may be obtained under certain limited circumstances. In addition, under the Pediatric Research Equity Act, an NDA or supplement to an NDA must contain data to assess the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective.
The FDA reviews all NDAs submitted to determine if they are substantially complete before it accepts them for filing. Once the submission is accepted for filing, the FDA begins an in-depth review of the NDA. Under the goals and policies agreed to by the FDA under the Prescription Drug User Fee Act, or PDUFA, the FDA targets 10 months in which to complete its initial review of a standard NDA and respond to the applicant, and six months for a priority NDA. The FDA often does not meet its PDUFA goal dates for standard and priority NDAs.
After the NDA submission is accepted for filing, the FDA reviews the NDA to determine, among other things, whether the proposed product is safe and effective for its intended use, and whether the product is being manufactured in accordance with cGMP to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer applications for novel drug or biological products or drug or biological products which present difficult questions of safety or efficacy to an advisory committee, typically a panel that includes clinicians and other experts, for review, evaluation and a recommendation as to whether the application should be approved and under what conditions. Before approving an NDA, the FDA will inspect the facilities at which the product is manufactured.
The NDA review and approval process is frequently lengthy and complex and the FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical data or other data and information. Even if such data and information is submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling. In addition, the FDA may require post marketing clinical trials, sometimes referred to as Phase 4 clinical trials testing, which involves clinical trials designed to further assess a drug safety and effectiveness and may require testing and surveillance programs to monitor the safety of approved products that have been commercialized.
| 25 |
U.S. Foreign Corrupt Practices Act
The U.S. Foreign Corrupt Practices Act, to which we are subject, prohibits corporations and individuals from engaging in certain activities to obtain or retain business or to influence a person working in an official capacity. It is illegal to pay, offer to pay or authorize the payment of anything of value to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business or to otherwise influence a person working in an official capacity.
Federal and State Fraud and Abuse Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal laws have been applied to restrict certain marketing practices in the biopharmaceutical industry in recent years. These laws include anti-kickback statutes and false claims statutes. We could face substantial penalties if we are unable to fully comply with these laws.
The federal healthcare program anti-kickback statute prohibits, among other things, persons or entities from knowingly and willfully offering, paying, providing, soliciting, or receiving remuneration, directly or indirectly, to induce or in return for purchasing, leasing, ordering, or arranging for the purchase, lease, or order of any healthcare item or service reimbursable under Medicare, Medicaid, or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on one hand and prescribers, purchasers, and formulary managers on the other. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchases, or recommendations may be subject to scrutiny if they do not qualify for an exemption or safe harbor. Our practices may not in all cases meet all of the criteria for safe harbor protection from anti-kickback liability.
The federal physician self-referral prohibition, commonly known as the Stark Law, prohibits physicians from referring Medicare or Medicaid patients to providers of ‘‘designated health services’’ with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exception applies.
Federal false claims laws prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, a false claim for payment from Medicare, Medicaid or other third-party payors to the federal government, or knowingly making, or causing to be made, a false statement to get a false claim paid. Recently, several pharmaceutical and other healthcare companies have been prosecuted under these laws for allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the company’s marketing of the product for unapproved, and thus non-reimbursable, uses. The majority of states also have statutes or regulations similar to the federal anti-kickback law and false claims laws, which apply to items and services reimbursed under Medicaid and other state programs, or, in several states, apply regardless of the payer. Sanctions under these federal and state laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, criminal fines, and imprisonment.
In addition, we may be subject to patient privacy regulation by both the federal government and the states in which we conduct our business. The laws that may affect our ability to operate include:
- HIPAA, which created new federal criminal statutes for knowingly and willfully executing a scheme to defraud any healthcare benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services;
- HIPAA, as amended by the Health Information Technology and Clinical Health Act, or HITECH, and its implementing regulations, which imposes certain requirements relating to the privacy, security and transmission of individually identifiable health information;
- the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients to providers of ‘‘designated health services’’ with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exemption applies; and
- state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts.
If our operations are found to be in violation of any of the laws and regulations described above or any other governmental laws or regulations that apply to us, we may be subject to penalties, including civil and criminal penalties, damages, fines, exclusion from participation in Medicare, Medi-Cal or other state or federal health care programs and/or the curtailment or restructuring of our operations, and we could be required to refund payments received by us. Any of the foregoing consequences could have a material adverse effect to our business, results of operations and financial condition.
| 26 |
EMPLOYEES
Currently, our only employee is our founder, Chief Executive Officer and Executive Chairman, Joseph Hernandez. Mr. Hernandez, together with several select consultants and scientific advisory board members, has overseen the negotiation of our intellectual property licenses, building of our management and scientific team and relationships with third parties, and development of our strategic plan. We expect to convert many of our current consultants to full time employees post financing.
FACILITIES
Our corporate headquarters are located in New York, New York. We lease part of an office space and shared facilities, which in total encompasses approximately 2,000 square feet. Our lease began on May 1, 2014 and expires on June 30, 2016. Our monthly rent payments are $8,000 and our future minimum annual rental payments total $32,000 in fiscal year ending 2014, $96,000 in fiscal year ending 2015 and $72,000 in fiscal year ending 2016. These payments continue to accrue. We currently do not own or lease any laboratory space. We believe that our current facility (and future facility) is suitable and adequate to meet our needs and that additional space for our office and laboratory needs will be available as and when needed.
LEGAL PROCEEDINGS
From time to time, we may become subject to various legal proceedings and claims that arise in the ordinary course of our business activities, especially since the biotechnology and pharmaceutical space is particularly litigious. Although the results of litigation and claims cannot be predicted with certainty, we are not currently party to any claim or litigation the outcome of which, if determined adversely to us, would individually or in the aggregate be reasonably expected to have a material adverse effect on our business.
Management’s Discussion and Analysis
THE FOLLOWING DISCUSSION SHOULD BE READ TOGETHER WITH THE INFORMATION CONTAINED IN THE FINANCIAL STATEMENTS AND RELATED NOTES INCLUDED ELSEWHERE IN THIS CURRENT REPORT ON FORM 8-K.
Plan of Operations
Since our inception, we have focused on identifying product candidates, obtaining in-licenses for critical intellectual property rights, recruiting a scientific advisory board, formulating a development plan for our product candidates and initiating preclinical studies for our lead product candidate for lung cancer, LumiralinTM, including in vitro and in vivo animal studies.
We plan to perform preclinical and toxicology studies as well as work to initiate our manufacturing with CROs or at OSU. Overall, we do not plan to engage any employees for the development process, but may hire consultants or utilize the services of other third parties as necessary. We have developed business relationships that will likely be critical to us throughout our development process as we advance in our programs. Based on our relationship with Dr. Robert Lee, an advisor and consultant to the company, we expect to complete our preclinical work at Dr. Lee’s laboratory at OSU. Finally, we have a relationship with the OSU Comprehensive Cancer Center, which is expected to conduct our initial clinical trials for our therapeutic product candidates. We cannot provide any assurance that we will enter into agreements with these parties on acceptable terms, or at all.
Initially, we plan to continue preclinical studies on our lead product candidate for lung cancer, LumiralinTM, including in vitro and in vivo animal studies. We also intend to validate our delivery technology which, if successfully developed, could be used for any of our four product candidate applications.
Our therapeutic product candidates face a long road to commercialization. Before filing an IND and initiating our clinical trial programs, we must successfully complete the requisite preclinical and toxicology studies and engage CMC manufacturing for all therapeutic product candidates, and the pre-IND work for one therapeutic product candidate. We expect to complete our preclinical development of LumiralinTM by the end of 2016, at which time we will be able to file an IND and initiate clinical trials for LumiralinTM at the OSU Comprehensive Care Center. We can provide no assurance, however, that we will be able to complete the preclinical development of LumiralinTM or any of our other therapeutic product candidates.
We also intend to validate our delivery technology which, if successfully developed, could be used for any of our four product candidate applications.
For our therapeutic product candidates, there are a number of risks associated with the development and subsequent marketing of our candidate products, and we may never be able to successfully obtain approval or become profitable in the anticipated timeframe, if at all. For a more comprehensive discussion of these risks, please refer to the ‘‘Risk Factors’’ section of this Current Report.
| 27 |
Results of Operations of Microlin, Bio, Inc. for the Fiscal Years Ended September 30, 2015 and September 30, 2015
We have not earned any revenues since the inception or our operations. During the fiscal year ended September 30, 2015, we incurred total expenses and a net loss of $1,965,631. Our expenses consisted of research and development expenses (principally the costs to obtain our patent portfolio) in the amount of $894,528, general and administrative expenses of $985,485, and interest expense of $85,617. By comparison, during the fiscal year ended September 30, 2014, we incurred expenses and a net loss of $4,469,236. Our expenses during the fiscal year ended September 30, 2014 consisted of research and development (also principally costs to obtain our patent portfolio) of $1,631,234, general and administrative expenses of $2,753,233, and interest expense of $84,769.
We were incorporated on July 30, 2013, and there is limited financial information about us upon which to base an evaluation of our performance. We are in development stage operations, have minimal assets and have not generated any revenues. We have incurred net losses in each month since our inception in July 2013 and have an accumulated deficit since inception of approximately $12,289,000. We expect to continue to incur significant losses in at least our first five years of operation and anticipate that our expenses will increase substantially as we:
| · | initiate, accelerate and expand our research and development efforts, including our clinical development activities; |
| · | add personnel to support our product development and, at a later time, commercialization efforts; |
| · | maintain, expand and protect our intellectual property portfolio; and |
| · | operate as a public company. |
We have incurred substantial research and development costs, consisting principally of our technology license costs, and these costs did not meet the capitalization criteria under US GAAP and therefore been expensed. We expect our research and development and specifically our clinical development expenses to increase for the foreseeable future as we continue the development of LumiralinTM and initiate the development our other product candidates and advance into clinical trials. Specifically, we expect to devote our capital primarily to the expense of the preclinical studies as well as seeking to commence Phase I of clinical trials, which may include the expense of manufacturing our therapeutic product candidates, patient expense and administrative expense. As we increase our development activity, we will need to increase the size of our management team to oversee the management of this work. If we are able to commercialize our therapeutic product candidates, we will also need to engage additional personnel to oversee the commercialization efforts.
Further, our intellectual property is central to our business, and we may need to acquire additional intellectual property in order to create a viable product. We may need to expend resources protecting our exclusive intellectual property rights from misuse by competitors and other parties through litigation or other legal action.
In addition, operating as a public company requires a number of compliance efforts, including financial reporting and other disclosures, and we will incur significant expense as a public company.
| 28 |
Liquidity and Capital Resources
As of September 30, 2015, we had limited cash on hand and working capital deficit of $9,651,201. Our current liabilities consisted of $1,652,183 in accounts payable, accrued expenses of $4,377,727, notes payable to OSIF in the amount of $2,446,213, loans from our founder, Joseph Hernandez, in the amount of $430,379, and derivative liability resulting from certain equity anti-dilution rights held by OSIF in the amount of $752,700.
Sources of Liquidity
We have incurred losses and cumulative negative cash flows from operations since our inception. We anticipate that we will continue to incur losses for at least the next five years. We expect that our research and development and general and administrative expenses will continue to increase and, as a result, we will need additional capital to fund our operations. We will seek additional financing through equity offerings, debt financings, government or other third-party funding and other collaborations, strategic partnerships and licensing arrangements. However, there is no guarantee that we will be able to obtain additional financing on favorable terms, or at all.
From our inception, we have funded our operations principally with an advances from Joseph Hernandez, our founder, Chief Executive Officer and Executive Chairman. As of September 30, 2015, these advances totaled $430,379. The advances are short-term, non-collateralized, and non-interest bearing.
Cash Flows
For the periods ended September 30, 2015 and September 30, 2014, our cash flows were $0 and $1, respectively.
Future Funding Requirements
To date, we have not generated any revenue. We do not know when, or if, we will generate any revenue. At the same time, we expect our expenses to increase in connection with our ongoing development activities, particularly as we continue the research and development of our lead therapeutic product candidate, LumiralinTM, and initiate the research and development of our other product candidates. In addition, we expect to incur additional costs associated with operating as a public company and hiring key personnel.
We expect to devote substantially all of the capital we may raise to the preclinical and initial clinical development of our therapeutic product candidates, the validation of our delivery technology, as well as the acquisition of other intellectual property rights as necessary for our business. The exact amount of cash required to move our business forward is difficult to forecast and will depend on many factors, including:
| · | the need to expand our research and develop activities; |
| · | the timing of the initiation and completion of therapeutic preclinical studies, clinical studies and the cost of these studies; |
| · | clinical trial design and the associated number of patients; |
| · | the outcome, costs and timing of seeking and obtaining FDA and other regulatory approvals; |
| 29 |
| · | results of our CLIA validation, preclinical studies and clinical work and the number of product candidates that we choose to advance as a result; |
| · | the dynamics of the regulatory space; |
| · | our need and ability to hire additional management and scientific personnel; |
| · | the costs of acquiring, licensing and enforcing intellectual property rights; |
| · | timing and costs associated with our marketing efforts; and |
| · | the economic and other terms, timing and success of our existing licensing arrangements and any collaboration, licensing or other arrangements into which we may enter in the future. |
Until such time, if ever, as we can generate substantial revenue from product sales, we expect to finance our cash needs through a combination of equity offerings, debt financings, government or other third-party funding and other collaborations, strategic partnerships and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, the ownership interests of our common stockholders will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect the rights of our common stockholders. Debt financing, if available, may involve agreements that include restrictive covenants that limit or restrict our ability to take specific actions, such as incurring additional debt, making capital expenditures, declaring dividends, or acquiring or disposing of certain assets. If we raise additional funds through other third-party agreements, such as licensing arrangements, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs or product candidates or to grant licenses on terms that may not be favorable to us. We can provide no assurance, however, that we will be able to obtain additional financing on favorable terms, or at all.
License Agreements with OSIF
In September 2013, we entered into five patent and technology license agreements with OSIF, whereby we obtained certain intellectual property rights (OSIF Patent Rights), from OSIF relating to miR diagnostics, prognostics and therapeutics for lung cancer, colorectal cancer, prostate cancer and ovarian cancer, as well as a novel nucleic acid delivery technology that may be used to deliver miR therapies to cancer cells. The term of each agreement continues until the last to expire of the applicable patent rights licensed thereunder. In consideration of the licenses:
1. We paid OSIF a total non-refundable license initiation fee of $25,000 ($5,000 for each of the five licensing agreements) and is required to pay an additional fee of $500,000 ($95,000 for each of the four licensing agreements and $120,000 for one of the five licensing agreements) in year 2;
2. We issued 280,000 shares of common stock which have been assigned amounts equivalent to the estimated fair value of the securities issued during the year ended September 30, 2013.
3. OSIF received anti-dilution rights equal to 7% of our issued and outstanding share capital of the Company on a fully diluted basis. In January 2014, we issued an additional 30,258 common shares to OSIF pursuant to anti-dilution provisions in our licensing agreements with OSIF. The right shall lapse following a raise of at least $10,000,000 in a single transaction or a series of transactions of equity financing. The anti-dilution provision was determined to be a derivative liability, as the timing and number of shares which may be issuable are not determinable at September 6, 2013 (date of the OSIF agreements). The maximum liability of the instrument of $752,700 was charged to research and development expense during the year ended September 30, 2013. This liability is based upon the estimated value of shares potentially required to be issued under such agreements and is considered a level 3 measurement.
| 30 |
4. We executed a term note with OSIF in the amount of approximately $2,363,000. The terms of the note require four payments of principal and interest in fixed installments of $100,000 beginning October 31, 2013 through December 31, 2014, and eight payments of principal and interest in fixed installments of approximately $273,000 beginning June 30, 2015 through March 31, 2017. The entire amount becomes immediately due in the event that we receive external funding of at least $10,000,000. Interest is charged at a 3.5% interest rate. In the event we do not make note payments as required, the agreements are technically in default with the patent portfolio reverting back to OSIF. In January 2014, we executed an amendment to one of the license agreements which increases the number of patents for which we have exclusive licensing rights. As consideration for the additional patents, we have agreed to pay additional upfront licensing costs of $25,000 and have agreed to increase the term note from approximately $2,363,000 to $2,446,000. Such amounts have not yet been paid. The execution of the note payable resulted in charges to research and development expense of approximately $83,000 for the year ended September 30, 2013. At September 30, 2015 and 2014, we have not made the payments as required under the license agreement. Although OSIF has informally accepted the deferral of the payments currently due until consummation of external funding by the Company, we have recorded the entire amount as current as of September 30, 2015 and 2014 due to the technical default.
5. We will pay OSIF annual license maintenance fees of $95,000 (in contract year 3), $35,000 (in contract year 4), $60,000 (in contract year 5) and $10,000 (each contract year thereafter);
6. We may be required to make future milestone payments upon the achievement of various milestones relating to regulatory approval or commercial events under the five licensing agreements plus additional occurrence specific costs which will depend on international and commercial growth; and
7. We will be required to pay OSIF a varying low single digit royalty rate of net sales relating to the licensed products. The license agreements also require annual minimum royalties, as defined in the agreements, beginning in the contract year ending December 31, 2015 and continuing in all contract years thereafter (minimum royalty payments will escalate annually through contract year December 31, 2019).
Under the terms of the license agreements, we are allowed to grant sublicenses or assign the license agreements to third parties. If the grant of a sublicense occurs, then we will be obligated to pay OSIF a varying percentage of all payments received from the sublicensees. If an assignment of any of the license agreements occur, we will be obligated to pay the greater of a specified amount or percentage, as defined in the agreements, of the gross consideration of the total transaction for contract years beginning December 31, 2014 and continuing in all contract years thereafter. The term of each agreement continues until the last to expire of the applicable patent rights licensed thereunder. We may terminate the agreements at any time upon 90 days' prior written notice. Four of the agreements require us to pay a termination penalty of $2,500,000 if the agreement is terminated by us in the first two years of the contract.
Going Concern
We have a working capital deficit and have suffered recurring losses from operations. For these reasons, our auditor has raised substantial doubt about our ability to continue as a going concern.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, results or operations, liquidity, capital expenditures or capital resources that is deemed material.
Critical Accounting Policies
In December 2001, the SEC requested that all registrants list their most “critical accounting polices” in the Management Discussion and Analysis. The SEC indicated that a “critical accounting policy” is one which is both important to the portrayal of a company’s financial condition and results, and requires management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. As of September 30, 2015, we believe the following policies currently fit this definition:
| 31 |
Equity-based compensation:
The Company recognizes compensation expense for all equity-based payments. Stock based compensation issued to employees is accounted for under Accounting Standard Codification (“ASC”) ASC 718-10, Compensation— Share Compensation” (“ASC 718-10”). The Company utilizes the Black- Scholes valuation method to recognize compensation expense over the vesting period. Certain assumptions need to be made with respect to utilizing the Black-Scholes valuation model, including the expected life, volatility, risk-free interest rate and anticipated forfeiture of the stock options.
The Company accounts for stock-based transactions with non-employees in which services are received in exchange for the equity instruments based upon the fair value of the equity instruments issued, in accordance with Accounting Standards Codification (“ASC”) 718-10 and ASC 505-50, Equity-Based Payments to Non-Employees. The value of such options, computed utilizing the Black-Scholes valuation model, is remeasured at the end of each period for any change in fair value from the previous valuation until the award vests. The Company begins expensing the awards to non-employees at the service commencement date.
Accounting for share-based compensation granted by the Company requires fair value estimates of the equity instrument granted. If the Company’s estimate of the fair value of stock-based compensation is too high or too low, it will have the effect of overstating or understating expenses. When stock-based grants are granted in exchange for the receipt of goods or services, the Company estimates the value of the stock-based compensation based upon the value of its common stock.
Derivative Instrument:
The Company accounts for its derivative instruments in accordance with ASC 815-10, “Derivatives and Hedging”. ASC 815-10 establishes accounting and reporting standards requiring that derivative instruments, including derivative instruments embedded in other contracts, be recorded on the balance sheet as either an asset or liability measured at its fair value. ASC 815-10 also requires that changes in the fair value of derivative instruments be recognized currently in results of operations unless specific hedge accounting criteria are met. The Company has not entered into hedging activities to date but has entered into a derivative (Note D).
Research and development:
One of the most significant estimates or judgments that the Company makes is whether to capitalize or expense patent and license costs. The Company makes this judgment based on whether the technology has alternative future uses, as defined in ASC 730, “Research and Development.” The Company’s patent portfolio consists of a large number of compounds and methodologies, substantially all of which are in the very early stage of development, and there is significant uncertainty as to future use and ability to make required payments to the licensor. Based on this consideration, the Company expensed payments made to in-license such patent portfolio. These costs are reflected in research and development expense in the statement of operations.
| 32 |
The following are certain identifiable risk factors for Microlin’s business operations. Risk factors related to our former business operations have been excluded but can be found in prior filings with the Securities and Exchange Commission.
Risks Related to Our Financial Condition and Capital Requirements
We have a very limited operating history, have minimal assets, have incurred significant losses since our inception and anticipate that we will continue to incur significant losses for the foreseeable future.
We are a developmental stage emerging therapeutics company with minimal assets and a very limited operating history. Since our inception in July 2013, our operations have focused primarily on acquiring and in-licensing critical intellectual property rights from the Ohio State Innovation Foundation (OSIF), recruiting a management team and a scientific advisory board, preclinical development activities, as well as developing strategic company plans to identify and develop our therapeutics and bring them to market. We have only initiated preclinical studies on our lead therapeutic product candidate for lung cancer, LumiralinTM, including in vitro and in vivo animal studies, and have not yet initiated any clinical trials or obtained regulatory approvals for any of our commercial product candidates, or generated any revenue. We expect to continue incurring significant expenses and increasing operating losses for the foreseeable future. Our prior losses, combined with expected future losses, may have a material adverse effect on business, results of operations and financial condition.
We are unable to predict the timing and amount of additional expenses that we will incur in our efforts to generate revenue and become profitable, if we are successful at all. We anticipate that our expenses will continue to increase significantly in the foreseeable future due to the high costs associated with developing therapeutic product candidates. We intend to devote a significant amount of our capital to preclinical research and development of product candidates, acquisitions or in-licensing of intellectual property, if necessary, initiating clinical trials and seeking regulatory approval, complying with federal and state regulatory requirements, developing our intellectual property portfolio and hiring additional personnel to support our growth as a public company. Even if we obtain regulatory approval to market a product candidate, or obtain or develop critical intellectual property rights, our future revenues will depend on our ability to achieve sufficient market acceptance and adequate market share for our product candidates in those markets. If we continue to incur significant operating losses without the ability to offset such losses with revenue streams or the raising of additional capital, our business, results of operations and financial condition will be materially adversely affected.
We may be unable to fulfill our contractual commitments to OSIF. As a result, we may incur significant financial fees and may be required to terminate our license agreements with OSIF, which would have a material adverse effect on our business, results of operations and financial condition.
In September 2013, we entered into five patent and technology license agreements with OSIF, whereby we obtained the exclusive, worldwide rights and license to use, develop, manufacture, market and commercialize certain intellectual property rights. Each agreement requires us to make payments to OSIF upon the achievement of certain developmental and commercialization milestones relating to the licensed intellectual property rights. In addition, we will be required to pay to OSIF a royalty on the net sales of products and services whether or not they utilize the intellectual property rights. As we develop our product candidates, we expect to incur significant expenses and increasing operating losses for the foreseeable future without the ability to generate enough revenue to offset such expenses and losses. If we are unable to satisfy our milestone and royalty payment obligations to OSIF, we would be in default under the license agreements and may be required to return the licensed intellectual property rights to OSIF, which would have a material adverse effect on our business, results of operations and financial condition.
We have the right to terminate any of the license agreements upon prior written notice to OSIF. However, upon our breach of certain provisions in the license agreements, OSIF has the option to terminate the applicable agreement, change the applicable field of use or territory or change the license granted from an exclusive license to a non-exclusive license. We may be unable to pay the termination fee required under the agreements if we terminate one of the four miR-related agreements, which could cause OSIF to pursue a claim against us in court and cause us to incur additional costs. In addition, if we commit a breach and OSIF decides to terminate an agreement or modify the applicable field of use or territory in a license agreement, we may be unable to continue developing our product candidates and may never generate any revenue. If OSIF changes a license or all of the licenses to a non-exclusive license, our competitors may be able to license the same intellectual property rights on more favorable terms. Any of these events would have a material adverse effect on our business, results of operations and financial condition.
The terms of the license agreements, as amended, also require us to reimburse OSIF pursuant to a scheduled payment plan for patent costs, which amount to approximately $2,446,000 in the aggregate (including $200,000 in scheduled payments we have yet to make) as well as future patent costs to maintain the patents, which can be substantial. The unpaid patent costs accrue interest at a rate of 3.5% per annum.
| 33 |
We have minimal assets and have never generated any revenue. As a result, our ability to reduce our losses and reach profitability is unproven, and we may never achieve or sustain profitability.
Our company has two potential sources for revenue: out-licensing of our intellectual property, therapeutic revenue, and long-term partnership deals. As an emerging growth company with minimal assets and a limited operating history, we have yet to generate any revenue from these potential sources and do not expect to be profitable in the foreseeable future, if ever. Our ability to generate future revenues depends on our success in:
| · | building our intellectual property portfolio by acquiring or in-licensing critical intellectual property rights; |
| · | completing research and preclinical development of our product candidates; |
| · | seeking and obtaining regulatory approvals of our therapeutic product candidates on a timely basis; |
| · | entering into and maintaining long-term relationships with strategic partners on favorable terms; |
| · | solidifying a commercialization process or producing a product at a reasonable cost by scaling up our manufacturing process; |
| · | launching a product candidate for which we obtain regulatory and marketing approval and obtaining market acceptance of such product; |
| · | building a sales force that can adequately create a distribution for our product; |
| · | addressing regulatory changes and market developments in a cost-efficient manner; |
| · | identifying and testing new product candidates; |
| · | attracting, hiring and retaining qualified personnel; and |
| · | addressing competing businesses and products in the market. |
Due to the uncertainties of clinical trials and pharmaceutical development, we may never generate revenue from product sales, or be able to predict the amount of additional expense we will incur in our efforts to achieve product sales. We may never become profitable if our product candidates or fail in clinical trials or do not gain regulatory approval or market acceptance. If we are able to obtain regulatory approval of a product candidate and bring it to market, we will incur significant costs that may substantially offset any revenue generated from the sale of such product. In addition, the market may not accept our product candidates or another competing business may infringe on our intellectual property and develop a similar and more cost efficient product. Even if we are able to successfully commercialize any potential product, we may never become profitable and may need to turn to alternative sources of funding to continue our operation, which may have a material adverse effect on our business, results of operations and financial condition.
| 34 |
We will require substantial financing in order to pursue our business plan, which may not be available on acceptable terms, or at all.
We are currently undergoing preclinical research and development of product candidates and will need to raise additional capital in our efforts to advance such product candidates through clinical trials and ultimately bring them to market. Developing therapeutics is expensive and, as products move through the regulatory process to commercialization, unpredictable costs often arise. We expect that the cost for preclinical development of our four therapeutic product candidates up to Phase 1 will be approximately $10 to $14 million.
The process of raising capital could divert the attention of our management from the day-to-day operations of the company, which could delay or detract our ability to commercialize our product candidates, out-license our intellectual property or form strategic partnerships that are critical to our business. We may seek funding through a combination of equity offerings, debt financings, government or other third-party arrangements and other collaborations, strategic alliances and licensing arrangements. We may be forced to accept capital on non-ideal or onerous terms, which could adversely affect the holdings or the rights of our stockholders. If we are unable to obtain capital on a timely basis, on favorable terms, or at all, we may be required to significantly delay or discontinue one or more of our research or development programs, which could have a material adverse effect on the company. We may also be required to relinquish rights to some of our technologies or product candidates. Any of these events may have a material adverse effect on our business, results of operations and financial condition.
Our common stock could become diluted and the market price of our common stock could decline if we raise capital through equity or debt that converts into equity.
We may raise funds through the issuance of equity or debt securities which convert into equity. The issuance of these securities could substantially dilute the holdings of our stockholders. Furthermore, the terms of these securities may contain liquidation preferences or other preferences that adversely affect the rights of stockholders. The market price of our common stock may also decline if we issue additional securities, whether equity or debt. If we incur additional debt by issuing debt securities, a significant portion of our cash flow may be committed to the payment of principal and interest to cover such indebtedness, and we may be subject to restrictive financial covenants which inhibit our ability to incur additional debt, issue additional equity or license, acquire or dispose of strategic assets or intellectual property rights. Any of these developments could have a material adverse effect on our business, results of operations and financial condition.
Our recurring losses from operations may raise substantial doubt regarding our ability to continue as a going concern.
Our recurring losses from operations and lack of funding raise substantial doubt about our ability to continue as a going concern. As of September 30, 2015, we had not funded the company and as of September 30, 2015, we limited cash on hand. Joseph Hernandez has paid, and continues to pay, certain bills on our behalf. If not funded, we will not be able to meet certain obligations, including our obligations to OSIF under license agreements (including certain patent costs which remain unpaid), as they become due. Sufficient financing may not be available to us when needed to allow us to continue as a going concern. The perception that we may not be able to continue as a going concern may cause others to choose not to deal with us due to concerns about our ability to meet our contractual obligations, which could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Development of Our Product Candidates
We are highly dependent on the success of microRNA technology and we may not be able to develop the technology, successfully obtain regulatory or marketing approval for, or successfully commercialize, our therapeutic product candidates.
Our therapeutic focus is entirely based on microRNA technology, including the application of microRNA technology in cancer, and the success of our company is based on the viability of this technology and the development of our product candidates. Since our inception, we have focused our efforts on acquiring strategic intellectual property related to microRNA technology, recruiting a management team and board of directors, who have agreed to serve upon consummation of this offering, and establishing a scientific advisory board with substantial experience working with microRNA. Neither we nor any other company has received FDA regulatory approval to market therapeutics targeting microRNAs. The scientific evidence to support the feasibility of developing product candidates based on these discoveries is both preliminary and limited. The evidence that these microRNAs will work as a therapeutic is limited to mostly animal data and in vitro data, which does not reflect the full human in vivo condition. The effect of microRNAs as a therapeutic in humans has not been extensively tested and, as a result, the long-term safety, toxicology and efficacy of the therapy with respect to humans remains unknown. If our microRNA technology is found to be unsafe in humans, or if it never receives regulatory approval for commercialization, we will never be able bring our product candidates to market and may never become profitable.
| 35 |
Further, our efforts are concentrated not only in microRNA technology, but also in its application in cancer. Our current strategy and licensed intellectual property is focused on targeting microRNA in only four types of cancer. This lack of diversification increases the risk associated with the ownership of our common stock. If we are unsuccessful in developing and commercializing microRNA technology and its application in these four types of cancer, we may have to alter the scope and direction of our company and steer away from the intellectual property we have acquired as well as the core capabilities of our management team and scientific advisory board. Without successful commercialization of our potential therapeutic products, we may never become profitable, which would have a material adverse effect on our business, results of operations and financial condition.
It is difficult to predict the time and cost of developing a viable microRNA-based product since it is a novel technology.
MicroRNA-based technologies are novel and relatively unproven. MicroRNAs are novel elements in the human genome, just recently understood, and their role in cancer was discovered less than ten years ago. The scientific knowledge related to microRNAs, their intracellular mechanism and their role in various forms of cancer continues to evolve and grow. For these reasons, it is difficult to predict the time and expense of developing a microRNA-based therapeutic product for cancer. This uncertainty may make it difficult for us to budget accurately, raise capital and hire appropriately, which are all elements critical to the success of our business. Delays and costs that are greater than expected may have a material adverse effect on our business, results of operations and financial condition.
All of our programs are still in preclinical development. Validation, preclinical testing and future clinical trials may not be successful or may experience delays, which would have a material adverse effect on our business, results of operation and financial condition.
To date, we have devoted our resources to identifying and developing product candidates and acquiring and in-licensing the intellectual property upon which we are building our clinical therapeutic product candidates. Our clinical therapeutic product candidates are currently in a preclinical development phase. We have only initiated preclinical studies on our lead therapeutic product candidate for lung cancer, LumiralinTM, including in vitro and in vivo animal studies. None of our product candidates have generated any revenue.
We may not be able to successfully complete the preclinical testing necessary to advance our therapeutic product candidates into clinical development, including animal pharmacology and toxicity studies. The results of any preclinical work may indicate that our therapeutic product candidates do not have the safety or efficacy necessary to file an Investigational New Drug (IND) with the FDA in order to move our product on to the clinical development process.
We expect to work with third party sites to conduct our clinical trials. If we are unable to sign up enough sites to conduct the trials, or manage the sites properly, there could be delays, increased costs or termination of the clinical trials all together.
Once we initiate the clinical development of our product candidates, it may be difficult to identify and qualify patients to participate in future clinical trials for our product candidates, and the timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing as well as completion of required follow-up periods. If patients are unwilling to participate in our clinical trials due to concerns over the safety of the product or for other reasons, the timeline for conducting the trials and obtaining regulatory approval may be delayed. Furthermore, we may also compete for patients with other companies conducting similar clinical trials. Any delays in our future clinical trials could result in increased costs, delays in product development or termination of the clinical trials altogether.
Any of these events could have a material adverse effect on our business, results of operations and financial condition.
We may fail to obtain orphan drug status for our product candidates.
We intend to seek orphan drug status from the FDA for LumiralinTM and OmiralinTM, two of our product candidates, for the treatment of ovarian cancer. In addition, we may seek orphan drug status for our other product candidates to the extent such product candidates are developed for ovarian cancer. Ovarian cancer therapies are eligible for orphan drug status under the Orphan Drug Act of 1983. The orphan drug status gives the manufacturer specific financial incentives to develop a pharmacological agent. If a product that has an orphan drug designation receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan drug exclusivity, which means that the FDA may not approve any other applications to market the same medication for the same indication, except in very limited circumstances, for seven years. Failure to obtain an orphan drug designation for LumiralinTM and/or OmiralinTM may have a material adverse effect on our business, results of operations and financial condition.
| 36 |
We may fail to demonstrate the safety and efficacy of our therapeutic product candidates in accordance with regulatory standards and may incur delays and substantial costs in our clinical trials.
In order to commercialize our therapeutic product candidates, we must conduct extensive clinical trials demonstrating the safety and efficacy of our product candidates in humans. The clinical testing process is expensive, difficult to design and implement, will take many years to complete and is unpredictable in both its duration and outcome. A failure of one or more clinical trials can occur at any stage of testing. There is a high failure rate for drugs and biological products proceeding through clinical trials. The research, testing, manufacturing, labeling, packaging, storage, approval, sale, marketing, advertising and promotion, pricing, export, import and distribution of drug products are subject to extensive regulation by the FDA and other regulatory authorities in the United States and other countries, which regulations differ from country to country. We are not permitted to market our product candidates as a prescription pharmaceutical product in the United States until we receive approval of a New Drug Application (NDA), from the FDA, or in any foreign countries until we receive the requisite approval from such countries. In the United States, the FDA generally requires the completion of preclinical testing and clinical trials of each drug to establish its safety and efficacy and extensive pharmaceutical development to ensure its quality before an NDA is approved. Regulatory authorities in other jurisdictions impose similar requirements. Of the large number of drugs in development, only a small percentage result in the submission of an NDA to the FDA and even fewer are eventually approved for commercialization. We have not submitted an NDA to the FDA or comparable applications to other regulatory authorities. Preclinical and clinical data is often susceptible to varying interpretations and types of analyses and regulatory authorities may fail to approve our product. In addition, even if we successfully complete early clinical trials, such results may not be indicative of the success or results of our later clinical trials.
Our successful completion of clinical trials may be materially adversely affected by many factors, including:
| · | ineffective trial design and disagreement with the FDA on final trial design; |
| · | imposition of a clinical hold following an inspection of our clinical trial operations by the FDA or other regulatory authorities; |
| · | difficulties or delays in reaching an agreement with a contract research organization (CRO), and clinical trial sites; |
| · | delays in obtaining required institutional review board (IRB) approval for each trial site; |
| · | data collected from clinical trials may not be sufficient to support the submission of a NDA or other submission or to obtain regulatory approval in the United States or elsewhere; |
| · | delays or difficulties in recruiting suitable patients to participate in clinical trials; |
| · | delays in manufacturing or delivering products and materials to clinical trial sites; |
| · | delays or difficulties caused by lack of patient adherence to treatment or post-treatment follow-up; |
| · | delays caused by patients dropping out of a trial and the need for recruiting additional patients; and |
| · | delays caused by clinical sites dropping out of the trial and the time required to recruit a new site. |
Any of the these delays or difficulties could cause us to be delayed in obtaining marketing approval from regulatory authorities, if at all, or allow us to obtain approval for specific indications or patient populations that are not as broad as currently targeted. In addition, such delays or difficulties may cause our development costs or our time to bring our product candidates to market to increase, may weaken our competitive positioning in the market and may have a material adverse effect on our business, results of operations and financial condition.
| 37 |
Any of our therapeutic product candidates may cause adverse effects or have properties that could delay or prevent their regulatory approval or limit the scope of any specific indications or market acceptance.
Adverse events caused by our potential therapeutic product candidates could cause interruptions, delays or the halting of our clinical trials. If adverse effects are observed in any clinical trials for our therapeutic product candidates, we may be unable to obtain timely, or any, regulatory approval of our therapeutic product candidates. Adverse effects caused by our product candidates could also subject us to litigation and liability, which could have a material adverse effect on our business, results of operations and financial condition.
In addition, if any of our product candidates are approved for commercialization and are found to cause serious or unpredicted side effects, serious consequences may result, including but not limited to, the withdrawal of marketing approval by regulatory authorities, restrictions on distribution by regulatory authorities, the need to conduct additional clinical trials, litigation and potential liability for personal injury to patients and damage to our reputation. Furthermore, our ability to achieve and/or maintain profitability may be permanently impaired. Any of these events could have a material adverse effect on our business, results of operations and financial condition.
We cannot predict if or when we will receive regulatory approval to commercialize a therapeutic product candidate.
We cannot commercialize a product candidate until the appropriate regulatory authorities, such as the FDA or a state regulating authority, have reviewed and approved the product candidate. Even if our product candidates demonstrate safety and efficacy in clinical trials, regulatory agencies may not complete their review processes in a timely manner, and we may not be able to obtain timely regulatory approval. We may never be able to receive regulatory approval for our product candidates at all. Additional delays may result if an FDA advisory committee or other regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials and the review process. Regulatory agencies also may approve a product candidate for fewer or more limited indications than requested or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our treatment candidates. Delays or failure to obtain necessary regulatory approvals could have a material adverse effect on our business, results of operations and financial condition.
Even if we obtain regulatory approval for a product candidate, we will remain subject to extensive regulatory scrutiny.
Even if we obtain regulatory approval in the United States for our product candidates, the FDA and/or other appropriate regulatory agencies may still impose significant restrictions or delays, including restriction of patient population or indications or additional costly studies. Any changes to the approved product or its labeling or manufacturing process would require FDA approval. Any advertisements or promotions must comply with FDA regulations and are subject to FDA review as well as state and federal laws. Drug product manufacturers are subject to continual review and inspection by the FDA and other regulatory authorities to comply with cGMP. If the FDA or other regulatory authority finds previously undiscovered compliance issues with products, such as unanticipated adverse effects or issues with the manufacturing facility, the FDA or other regulatory authority may:
| · | issue a warning letter asserting that we are in violation of law; |
| · | seek an injunction; |
| · | impose civil or criminal penalties or monetary fines; |
| · | suspend or withdraw regulatory approval; |
| · | suspend currently ongoing clinical trials; |
| · | refuse any pending applications; |
| · | seize product; or |
| · | prohibit us from entering into beneficial or necessary contracts such as supply or government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response, could result in litigation and litigation-related expense and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenue, which would have a material adverse effect on our business, results of operations and financial condition.
| 38 |
In addition, even if we obtain regulatory approvals, the timing or scope of any approvals may prohibit or reduce our ability to commercialize our product candidates successfully. For example, if the approval process takes too long, we may miss market opportunities and give other companies the ability to develop competing products or establish market dominance. Any regulatory approval that we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render our products not commercially viable. For example, regulatory authorities may approve our product candidates for fewer or more limited indications than we request, may not approve the price we intend to charge for our products, may grant approval contingent on the performance of costly post-marketing clinical trials, or may approve our products with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that indication. Further, the FDA may place conditions on approvals including potential requirements or risk management plans and the requirement for a Risk Evaluation and Mitigation Strategy (REMS) to assure the safe use of the drug. If the FDA concludes a REMS is needed, the sponsor of the NDA must submit a proposed REMS; the FDA will not approve the NDA without an approved REMS, if required. A REMS could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of our product candidates.
Moreover, product approvals may be withdrawn for non-compliance with regulatory standards or if problems occur following the initial marketing of the product. Any of the foregoing scenarios could materially harm the commercial success of our product candidates and have a material adverse effect on our business, results of operations and financial condition.
Reimbursement decisions by third-party payors may have an adverse effect on pricing and market acceptance. If there is not sufficient reimbursement for our product candidates, when and if developed and approved, we may not be able to successfully commercialize our product candidates.
Market acceptance and sales of our product candidates, when and if developed and approved, will depend on reimbursement policies and may be affected, among other things, by future healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which drugs they will cover and establish payment levels. Reimbursement by a third party payor may depend on a number of factors, including whether lower cost alternatives are available. Since each third party payor makes its own decision as to whether to establish a policy or enter into a contract to reimburse our product candidates, seeking these approvals is a time-consuming and costly process, and we may never secure reimbursement from any third party payors. Reimbursement may not be available for our product candidates. In addition, reimbursement
policies may reduce the demand for, or the price paid for, our product candidates. If reimbursement is not available or is available on a limited basis, we may not be able to successfully commercialize our product candidates. Further, physicians may not order or prescribe our clinical therapeutic product candidates unless third party payors reimburse a substantial portion of the price.
In addition, hospital formulary approval and reimbursement may not be available for our therapeutic product candidates, which could make it difficult for us to sell products profitably. In order for government or third party payors to reimburse hospitals for our therapeutic product candidates, such product candidates must be added to the hospital formularies. Obtaining formulary approval can be an expensive and time consuming process and we may not be able to obtain such approval. Failure to obtain timely formulary approval may limit our commercial success.
In the United States, the Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (MMA) changed the way Medicare covers and pays for pharmaceutical products. The legislation established Medicare Part D, which expanded Medicare coverage for outpatient prescription drug purchases by the elderly but provided authority for limiting the number of drugs that will be covered in any therapeutic class. The MMA also introduced a new reimbursement methodology based on average sales prices for physician-administered drugs. Any negotiated prices for our product candidates covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain in the United States. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payors.
The United States and several other jurisdictions are considering, or have already enacted, a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our product candidates profitably. Among policy makers and payors in the United States and elsewhere, there is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access to healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. We expect to experience pricing pressures in connection with the sale of any products that we develop, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative proposals.
| 39 |
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, ‘‘ACA’’) became law in the United States. The goal of ACA is to reduce the cost of health care and substantially change the way health care is financed by both governmental and private insurers. Although we cannot predict what impact on federal reimbursement policies this legislation will have in general or on our business specifically, the ACA may result in downward pressure on pharmaceutical reimbursement, which could negatively affect market acceptance of our product candidates. In addition, some members of the U.S. Congress have been seeking to overturn at least portions of the ACA and we expect they will continue to review and assess this legislation and alternative health care reform proposals. We cannot predict whether new proposals will be made or adopted, when they may be adopted or what impact they may have on us if they are adopted.
Any of these developments and events could have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Our Reliance on Third Parties
We plan to rely on third parties to conduct our preclinical testing and clinical trial design and management for our potential therapeutic products, and these third parties may not perform satisfactorily.
We have a relationship with a laboratory at OSU for preclinical testing of our therapeutic product candidates and with the OSU Comprehensive Cancer Center for clinical trial design and management. Our success in developing our potential therapeutic product candidates is dependent on the success of our relationships with these third parties. These third parties could be unable or unwilling to meet our needs and timelines for clinical trial design and management for a number of reasons, including, but not limited to:
| · | turnover of staff; |
| · | unexpected and unprojected costs; |
| · | new leadership with a changing focus for the organization; |
| · | occurrence of unexpected events which may delay completion of the work; |
| · | difficulties in obtaining internal review board approval; |
| · | difficulties in gaining access to necessary materials and samples; or |
| · | difficulties in recruiting suitable patients for clinical trials. |
If we enter into formal agreements with either of these third parties for preclinical testing or clinical trial design and management, either party may fail to successfully carry out their contractual obligations, meet expected deadlines or conduct studies in accordance with regulatory requirements or our stated protocols, causing us to delay or stop the completion of the preclinical studies and clinical trials required for approval of our product candidates. We may also face liability for their failure to comply with regulatory requirements. Either of these third parties may also decide to end their relationship with us at any time upon the occurrence of certain conditions. If we need to enter into alternative arrangements, our product candidates development activities may be critically delayed, which could have a material adverse effect on our business, results of operations and financial condition. In addition, we may be unable to enter into agreements with the OSU Comprehensive Cancer Center or with other third parties on favorable terms, or at all.
The concentration of our clinical efforts with OSU also means that our success is closely tied to a single relationship. If OSU were to terminate this relationship, or if adverse effects beyond our control caused the University to neglect its contractual obligations to us, our business would be materially impaired. Specifically, if OSIF terminates our license agreements whereby we have exclusively in-licensed a portfolio of issued and pending patents, our business, results of operations and financial condition may suffer materially, as this intellectual property in central to the therapeutic parts of our business. Without access to this intellectual property, we may be unable to continue in the clinical development or commercialization processes, which would have a material adverse effect on our business, results of operations and financial condition.
| 40 |
We intend to rely on third party manufacturers to produce the preclinical, clinical trial, and commercial supply of our product candidates.
We do not currently have, nor do we plan to acquire, the capability or infrastructure to manufacture the active pharmaceutical ingredient (API) in our product candidates for use in our clinical trials or for commercial products, if any. In addition, we do not have the capability to encapsulate our product candidates as a finished drug product for commercial distribution. We intend to rely on third parties to manufacture the supplies for our preclinical and clinical trials for our potential therapeutic products. Reliance on third-party manufacturers creates exposure to risks to which we would not be subject if we manufactured the product candidates ourselves, including:
| · | the inability to meet any product specifications and quality requirements consistently; |
| · | a delay or inability to procure or expand manufacturing capacity as necessary; |
| · | issues with product quality as manufacturing production scales up; |
| · | the inability to negotiate with suppliers on reasonable terms; |
| · | reliance on certain sources for raw materials and in some cases single sources; |
| · | operations of our third party manufactures could be disrupted by conditions unrelated to our business or condition; |
| · | failure to deliver products in a timely manner; |
| · | increased costs that cannot be controlled; and |
| · | non-compliance with regulatory requirements, including cGMP standards. |
Any of these events could lead to delays in the development of our product candidates, including delays in our preclinical and clinical trials, or it could impact our ability to successfully commercialize our current product candidates or any future products. Some of these events could be the basis for action from regulatory authorities, including injunction, or partial or total suspension of our production. In addition, we may be unable to enter into agreements with suppliers or manufacturers on favorable terms, or at all.
Additionally, to the extent we rely on third parties as sole suppliers of critical materials used in our development and production process, we may not be able to find alternative sources if our suppliers are no longer able to provide these materials. If we are unable to procure such materials from alternative sources, we could experience an interruption to our operations. Such an interruption could slow or stop our development processes and cause us to fail to meet certain critical milestones, impair customer and third party relationships, lead to the termination of our license agreements and negatively affect our ability to generate revenue, which may have a material adverse effect on our business, results of operations and financial condition.
The facilities used by our contract manufacturers to manufacture our product candidates must be approved by the FDA pursuant to inspections that will be conducted after we submit our NDA to the FDA. We will not control the manufacturing process of, and will be completely dependent on, our contract manufacturing partners for compliance with cGMPs for manufacture of both active drug substances and finished drug products. These cGMP regulations cover all aspects of the manufacturing, testing, quality control and record keeping relating to our product candidates. If our contract manufacturers cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the FDA or others, they will not be able to secure and/or maintain regulatory approval for their manufacturing facilities. If the FDA or a comparable foreign regulatory authority does not approve these facilities for the manufacture of our product candidates or if it withdraws any such approval in the future, we may need to find alternative manufacturing facilities, which would significantly impact our ability to develop, obtain regulatory approval for or market our product candidates, if approved.
| 41 |
Our contract manufacturers will be subject to ongoing periodic unannounced inspections by the FDA and corresponding state and foreign agencies for compliance with cGMPs and similar regulatory requirements. We will not have control over our contract manufacturers’ compliance with these regulations and standards.
Failure by any of our contract manufacturers to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, failure to grant approval to market our product candidates, delays, suspensions or withdrawals of approvals, operating restrictions and criminal prosecutions. In addition, we have no control over the ability of our contract manufacturers to maintain adequate quality control, quality assurance and qualified personnel. Failure by our contract manufacturers to comply with or maintain any of these standards could adversely affect our ability to develop, obtain regulatory approval for or market our product candidates. Any of these events could have a material adverse effect on
our business, results of operations and financial condition.
Our reliance on third-party relationships will subject us to the downstream effects that any of our future partners’ may experience.
Our business model is to outsource processes and operations wherever possible and we, therefore, expect be dependent on third parties for multiple aspects critical to our business. As such, any events or changes that have an effect on the third parties that we work with will likely have an effect on our business as well. Events for our third party partners that could have a significant effect on our business include:
| · | employee turnover causing interruptions or delays in production; |
| · | leadership change causing shift in organizational goals; |
| · | bankruptcy or other financial issues halting or shutting down operations; |
| · | surges in costs triggering renegotiation of contracts or inability to renew contracts on mutually satisfactory terms; or |
| · | a natural disaster which halts operations. |
We may not be able to anticipate the effect such events or changes will have on our business or our relationship with our third party partners. If such events or changes materially affect the ability of our third party partners to adequately perform their contractual obligations, we may be unable to complete our clinical trials or product development in a timely and cost-effective manner, which may affect our ability to become profitable and have a material adverse effect our business, results of operations and financial condition.
| 42 |
Interruptions in the supply of product or inventory loss may have a material adverse effect on our business, results of operations and financial condition.
Our product candidates will likely be manufactured and distributed using technically complex processes requiring specialized facilities, specific raw materials and other production constraints. The complexity of these processes, as well as strict company and government standards for the manufacture and storage of our future products, will subject us to production risks. While product batches released for use in clinical trials will undergo sample testing, some defects may only be identified following product release. In addition, process deviations or unanticipated effects of approved process changes may result in these intermediate products not complying with stability requirements or specifications. Our product candidates will also need to be stored and transported at temperatures within a certain range. If environmental conditions deviate, our product candidates’ remaining shelf-lives could be impaired or their efficacy and safety could be adversely affected. The occurrence or suspected occurrence of production and distribution difficulties can lead to lost inventories, and in some cases, product recalls, with consequential reputational damage and the risk of product liability. The investigation and remediation of any identified problems can cause production delays, substantial expense, lost sales and delays of new product launches. Any interruption in the supply of finished products or the loss thereof and any unforeseen failure in the storage of the product or loss in supply could delay our clinical trials and, if our product candidates are approved for commercialization, result in a loss of our market share. Any of these events could have a material adverse effect on our business, results of operations and financial condition.
We may never form any long-term partnership deals due to our clinical program results, the perceived value of our intellectual property or the competitive environment of therapeutics.
We may never be able to engage a pharmaceutical or biotech partner to move forward and form a long-term partnership. Our clinical data may not be clinically relevant to potential partners or the results of our preclinical and clinical work may not be sufficient to develop clinically viable products. Potential partners may not perceive any value in the intellectual property that we have licensed, or the competitive environment may change such that our intellectual property or clinical programs are believed to be lacking in value or not having a market opportunity. Our inability to form such partnerships, as needed, may have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Our Intellectual Property
Our failure to comply with our obligations in the agreements under which we license intellectual property rights from third parties could cause us to lose license rights that are critical to our business.
We have built our portfolio of intellectual property rights through exclusive in-licensing deals with OSIF that are important to the development of our business and we expect that we may need to enter into additional license agreements in the future. Our existing license agreements impose certain developmental and commercial milestones which we must meet in order to maintain the exclusivity of our licenses. We are also obligated under the license agreements to make certain payments to OSIF, including payment for the remaining balance of the upfront fee for each license agreement, which we have agreed with OSIF to pay from the net proceeds of this offering. Our failure to meet the terms of these license agreements could result in non-exclusivity or revocation of our intellectual property rights, which would have a material adverse effect on our business, results of operations and financial condition.
We expect that we will need to enter into additional licensing agreements in the future as we develop and build our therapeutic product candidates and move them towards commercialization. We may be unable to obtain key licenses on commercially reasonable terms, if at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected therapeutic product candidates, which would have a material adverse effect on our business, results of operations and financial condition.
Licensing intellectual property is critical to our business and involves complex legal, business and scientific issues. Disputes may arise between us and our licensors regarding intellectual property subject to a license agreement, including disputes related to the scope of rights granted under the agreement. Disputes with our licensors may hinder our ability to maintain our current licensing arrangements on acceptable terms, which may jeopardize the successful development and commercialization of the affected therapeutic product candidates and have a material adverse effect on our business, results of operations and financial condition.
| 43 |
We may not be successful in obtaining or maintaining the rights to microRNA intellectual property, which would have a material adverse effect on our business, results of operations and financial condition.
We currently have the rights to intellectual property related to certain microRNAs through licenses from third parties to develop therapeutic and diagnostic product candidates. Because our programs may involve additional therapeutic or diagnostic product candidates that may require the use of proprietary rights held by third parties, the growth of our business will likely depend in part on our ability to acquire, in-license or maintain these proprietary rights.
In order to protect the intellectual property to which we have rights, we rely on a combination of issued and pending patents which we have licensed, patent applications, trade secret protection and confidentiality agreements with various parties. The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we in-license or may own in the future may fail to result in issued patents with claims that cover the products in the United States or in other foreign countries. There is no assurance that all of the potentially relevant prior art relating to our licensed and future patents and patent applications has been or will be found, which can invalidate a patent or prevent a patent from issuing based on a pending patent application. Even if patents do successfully issue, third parties may challenge their validity, enforceability or scope, which may result in such patents being narrowed or invalidated. Furthermore, even if they are unchallenged, our licensed and future patents and patent applications may not adequately protect our intellectual property or prevent others from designing around our claims.
If the patent applications we hold or have in-licensed with respect to our programs or product candidates fail to issue or if their breadth or strength of protection is threatened, it could dissuade companies from collaborating with us to develop product candidates, and threaten our ability to commercialize, future products. We may never have a patent issued and our patents may be found to be invalid and unenforceable or threatened by third parties.
We may also be unable to acquire or in-license any additional third-party intellectual property rights due to:
| · | companies that perceive us to be a competitor being unwilling to license; |
| · | an inability to come to mutually agreeable terms with universities, companies or other third parties who hold critical intellectual property rights; |
| · | delays in coming to agreeable terms; and |
| · | competitors with greater resources and a more established business seeking to license the same rights. |
In addition, the United States Patent and Trade Office (USPTO) could decide not to grant us intellectual property rights to currently pending patent applications which will be integral to our business, which may have a material adverse effect on the development of our product candidates. Our failure to acquire the necessary intellectual property rights to commercialize viable therapeutic product candidates in a reasonable timeframe could have a material adverse effect on our business, results of operations and financial condition.
Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we may not be the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications, an interference or derivation proceeding in the United States can be initiated by a third party to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available; however, the life of a patent, and the protection it affords, is limited. Once the patent life has expired for a product, we may be open to competition from generic medications.
| 44 |
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable, processes for which patents are difficult to enforce and any other elements of our drug discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. Although we expect our employees to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we face risks of not all such agreements being duly executed or enforceable, our trade secrets and other confidential proprietary information being disclosed, and competitors otherwise gaining access to our trade secrets or independently developing substantially equivalent information and techniques. In addition, others may independently discover our trade secrets and proprietary information.
Further, the laws of some foreign countries do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our market.
Any of these events may have a material adverse effect on our business, results of operations and financial condition.
We may be involved in lawsuits relating to intellectual property, either to protect or enforce any future patents we may develop or the patents of our licensors, or to defend against third party claims of intellectual property infringement, which may prevent or delay our development and commercialization efforts and could be expensive, time consuming and unsuccessful.
Disputes relating to ownership of intellectual property in the biotechnology and pharmaceutical fields often lead to litigation. We may choose or be forced by our licensors to file lawsuits against third parties to protect against infringement of our large portfolio of licensed intellectual property rights which we would have to fully fund or risk losing exclusivity to those patents involved to any alleged infringer, or any intellectual property we may develop in the future. In addition, there may be third party claims filed against us asserting that we are employing third party proprietary technology without the appropriate authorizations, such as claims related to the materials, formulations, manufacturing processes or treatment protocol for our therapeutic product candidates which we would also be forced to fund pursuant to our license agreements. Litigation can be expensive and time-consuming, cause a substantial diversion of our resources and time and could distract management focus from critical decisions, lead to disclosure of our confidential information, and damage the perception of our business by investors and potential partners, causing a decline in the price of our common stock.
Legal proceedings may never result in a favorable outcome for our business. In an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable and/or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents or the patents of our licensors do not cover the technology in question. A third-party defendant may also request post reexamination, grant review or inter parties review by the USPTO of any patent we assert.
An adverse result in any litigation or defense proceedings could put one or more of our licensed patents or any future patents we develop at risk of being invalidated or interpreted narrowly and could put any of our future patent applications at risk of not issuing, which would have material adverse effect on our business, results of operations and financial condition as our intellectual property is a fundamental part of what distinguishes us from competitors.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our therapeutic product candidates. Because patent applications can take many years to issue, third parties may have currently pending patent applications which may later result in issued patents that our product candidates may infringe, or which such third parties claim are infringed by the use of our technologies. If any third-party patents are held by a court of competent jurisdiction to cover any aspect of the manufacturing process for any of our therapeutic product candidates, any molecules formed during the manufacturing process, or any final therapeutic product candidate, including the formulation or method of use of such product candidate, the holders of any such patents may be able to block our ability to commercialize such product candidates unless we obtained a license under the applicable patents, or until such patents expire. In any such case, such a license may not be available on commercially reasonable terms, or at all.
| 45 |
Parties making claims against us for infringement of their intellectual property rights may obtain injunctive or other equitable relief, which could effectively block our ability to develop one or more of our product candidates. In the event of a successful claim of infringement against us, we could be required to redesign our infringing product candidates or obtain a license from such third party to continue developing and/or commercializing these products candidates. We may be unable to obtain any required license on commercially reasonable terms, or at all. Even if we are able to obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us. It may be impossible to redesign our product candidates and technology, or it may require substantial time and monetary expenditure, which could force us to cease commercialization of one or more of our product candidates, or some of our business operations, which could have a material adverse effect on our business, results of operations and financial condition. In addition, in any such proceeding, we may be required to pay substantial damages, including treble damages and attorneys’ fees in the event we are found liable for willful infringement.
Interference or derivation proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to any of our future patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms, or at all. Our defense of litigation or interference proceedings may fail and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States. Any of these developments may have a material adverse effect on our business, results of operations and financial condition.
Risks Related to Commercialization of Our Product Candidates
We face significant competition from other biotechnology and pharmaceutical companies and our business, results of operations and financial condition will suffer if we fail to compete effectively.
The biotechnology and pharmaceutical space is highly competitive. We have competitors in the therapeutic space in the United States and internationally. We are aware of several other companies with microRNA-based therapeutics, including miRagen Therapeutics, Inc., Regulus Therapeutics, Inc., and Mirna Therapeutics, Inc.. Many of these and other competitors draw from substantially greater financial, scientific and other resources, including a large development staff, a built-out operation, experienced manufacturers as well as stronger and more strategic relationships and partnerships within the industry.
Any of our competitors could succeed in developing a therapeutic product that is more effective, less costly or more marketable or integrates more easily into the standard of care. Our competitors could commercialize and market their products sooner than us, and we could have difficulty in converting physicians to our product. Once other competing products are established, our product candidates would have to show incremental efficacy or price point advantage in order to convert physicians to our product candidates. The inability to do so, or the extra effort required to do so, could have a material adverse effect on our business, results of operations and financial condition.
The commercial success of our product candidates will depend upon the degree of market acceptance by the medical community, including physicians, patients and third party payors.
The commercial success of our product candidates will depend in part on the medical community, patients and third-party payors accepting microRNA therapeutic products as medically useful, cost-effective and safe. If our product candidates do not achieve an adequate level of market acceptance, we may not generate meaningful or any product revenue and may never become profitable. The degree of market acceptance of these product candidates, if approved for commercial sale, will depend on a number of factors, including:
| · | the potential and perceived efficacy and advantages of such product candidate as compared to alternative treatments; |
| · | the clinical indications for which the product candidate is approved; |
| · | the prevalence and severity of any side effects, including any limitations or warnings contained in a product’s approved labeling; |
| · | relative convenience and ease of administration; |
| · | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; |
| · | acceptance by physicians and patients of the product as a safe and effective treatment; |
| · | the existence of other microRNA therapeutic products; |
| 46 |
| · | the safety of such product candidate seen in a broader patient group; |
| · | the cost of treatment in relation to alternative treatments; |
| · | the strength of marketing and distribution support and timing of market introduction of competitive products; |
| · | the effectiveness of our sales and marketing efforts; |
| · | publicity concerning our product candidates or competing products and treatments; and |
| · | sufficient third-party insurance coverage or reimbursement. |
Even if a potential product candidate displays a favorable efficacy and safety profile in preclinical studies and clinical trials, market acceptance of the product will not be known until after it is launched. Our efforts to educate the medical community and third-party payors on the benefits of the product candidates may require significant resources and may never be successful. Such efforts to educate the marketplace may require more resources than are required by the conventional technologies marketed by our competitors. If we do not obtain significant additional resources or our product candidates are unable to achieve the level of acceptance by all of these parties, our sales volume will likely suffer and our business, results of operations and financial condition could suffer as well.
Furthermore, market acceptance and sales of any future product candidates that we develop will depend on reimbursement policies and may be affected by future healthcare reform measures. Government authorities and third party payors, such as private health insurers, hospitals and health maintenance organizations, decide which drugs they will pay for and establish reimbursement levels. Reimbursement may not be available for any future product candidates. Also, reimbursement amounts may reduce the demand for, or the price of, our future product candidates. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize future product candidates that we develop.
Any of these events would have a material adverse effect on our business, results of operations and financial condition.
If we are unable to establish sales and marketing capabilities to effectively sell our product candidates, we may be unable to generate any revenues.
We currently do not have a sales force available to market either our potential therapeutic product candidates. In order to generate revenues from an approved product candidate, we will either have to build our own sales and marketing team or contract with third parties to create a sales and marketing function. We may be unable to create a team or a capability that will effectively be able to market our product candidates at the volume and price point that we require for the success of our business. Our sales force will likely be competing with those of companies with more sales and marketing experience and which have access to better resources, financial or otherwise. We may be unable to build an effective sales and marketing team, which may materially affect our profitability and materially adversely impact our business, results of operations and financial condition.
If we are not successful in recruiting sales and marketing personnel or in building a sales and marketing infrastructure, or if we do not successfully enter into appropriate collaboration arrangements, we will have difficulty successfully commercializing our product candidates, which would have a material adverse effect on our business, operating results and financial condition. Outside the United States, we intend to commercialize our product candidates by entering into collaboration agreements with pharmaceutical partners. We may not be able to enter into such agreements on terms acceptable to us, or at all. In addition, even if we enter into such relationships, we may have limited or no control over the sales, marketing and distribution activities of these third parties. Our future revenues may depend heavily on the success of the efforts of these third parties.
| 47 |
We may not be able to produce our therapeutic product candidates within a viable cost structure.
Even if we are able to prove safety and efficacy for our product candidates and obtain regulatory approval, we may fail to produce our therapeutic product candidates within a reasonable cost structure. As a result, we may not be able to market our product candidates at a price point that will allow us to be profitable. Further, as we move through the validation and development processes, we may need to incorporate additional materials into our product candidates to meet regulatory guidelines. These additional materials may raise our cost of production in a way that we cannot anticipate. If our production costs are elevated, it is possible that we will be unable to offset such costs, which may negatively affect our profitability and materially adversely impact our business, results of operations and financial condition.
Risk Related to Our Business Operations and Industry
Our future success depends on our ability to attract and retain talent for our management team, board of directors and staff.
We are highly dependent on the members of our executive management team to drive the success of our business. As the corporation has only been in existence for less than three years, we currently only have one employee (our Chief Executive Officer) and several consultants. We face a risk that one or more individuals who have agreed to serve as members of our management team upon the consummation of this offering may be unwilling or unable to do so. The loss of any of the members of our management team could adversely affect our operations and could cause significant delays in the development of our product candidates. The search for a replacement could be costly, time consuming and distracting from our core business. Recruiting and retaining our non-managerial employees is also critical for our business, including scientific and technical personnel. There is currently a dearth of qualified executives and skilled personnel in our industry, and as a result, competition for such talent will be very strong between us and our competitors and turnover rates may be high. Any difficulties in these areas could compromise our validation, development or commercial processes and have a material adverse effect on our business, results of operations and financial condition.
We have scientific and clinical advisors and consultants who assist us in formulating our research, development and clinical strategies. These advisors are not full-time employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us and typically they will not enter into non-compete agreements with us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. Any difficulties in these areas could have a material adverse effect on our business, results of operations and financial condition.
As our business evolves and grows in scale, we will have to expand our organization and may face difficulties in managing this growth, which could slow or halt our operations.
Our business is currently in its very early stages, and we expect that our headcount and number of functions will grow significantly as we progress through the validation and development process and the potential commercialization of our product candidates. This will require us to recruit, maintain, manage and motivate additional employees. Given the shortage of skilled employees in our field, it may be difficult to recruit the necessary number of additional employees.
In addition, we may be unable to effectively manage the growth of our organization, which may result in weakness or inefficiencies in our infrastructure. Expansion may also require significant capital expenditure that could require us to seek additional capital. This expansion could divert the focus of our management and employees from our core business and divert our financial resources. If we do not effectively manage our growth, this could have negative consequences on our ability to commercialize and effectively market our product candidates, which could have a material adverse effect on our business, results of operations and financial results.
Our employees, consultants and commercial partners may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements and insider trading, which could have a material adverse effect on our business, results of operation and financial results.
We are exposed to the risk of fraudulent conduct or other illegal activity by our employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional recklessness and/or negligent conduct that fails to: comply with FDA regulations or regulations from other authorities, provide accurate information to the FDA, comply with healthcare fraud and abuse laws in the United States, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Employee misconduct could also involve the improper use of information obtained in the course of clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. Such misconduct could lead to governmental investigations or other claims or lawsuits. If we face any of these actions and are not able to successfully defend ourselves, we could face significant penalties or sanctions and damage to our reputation, which could have a material adverse effect on our business, results of operations and financial condition.
| 48 |
We face potential product liability, and, if successful claims are brought against us, we may incur substantial litigation, costs and damage to our reputation.
The use or misuse of our product candidates in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims may be brought against us by a number of parties, including physicians, patients, biotechnology or pharmaceutical companies, hospitals, manufacturing personnel, or anyone who comes into contact with our product or our personnel. There is a risk that our product candidates may induce adverse effects or fail to perform as intended. If we are unable to successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| · | harm to our business and product reputations; |
| · | withdrawal of clinical trial participants; |
| · | substantial costs due to litigation, monetary awards to patients or other claimants; |
| · | inability to commercialize our future products; |
| · | decrease in demand for our candidate products; and |
| · | product recalls, withdrawals or labeling, marketing or promotional restrictions. |
Upon the consummation of this offering, we plan to obtain, but do not currently have in place, limited product liability insurance coverage for our preclinical and any subsequent clinical activities in the United States. Our insurance coverage, once in place, may not reimburse us or may not be sufficient to reimburse us for any expenses or losses we may suffer. In addition, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to product liability. A successful product liability claim or series of claims brought against us, particularly if judgments exceed our insurance coverage, or if brought at the time when we do not have such insurance coverage, could have a material adverse effect on our business, results of operations and financial condition.
Interruptions to our business could delay the development, validation, production and sale of our product candidates.
Our headquarters are in New York City, NY and our clinical trials are expected to be conducted in Columbus, OH. If we enter into this relationship, we will be vulnerable to natural disasters in any of these locations or other disasters such as terrorist attacks, which is a particular threat to New York City where our headquarters are located.
Columbus, OH, is prone to tornadoes and other natural disasters. We do not have insurance against natural disasters and such an event could cause a significant delay in our operations, which could have a material adverse effect on our business, results of operations and financial condition.
In addition, as described above, our strategy is to outsource as many of our development and manufacturing functions as possible. Currently, we are still in discussions to determine which third party suppliers we will engage. However, once we engage with various third parties, we are also exposed to the risks of interruptions to their businesses, which could cause interruptions in our own business, delay our operations and have a material adverse effect on our business, results of operations and financial condition.
| 49 |
If we fail to comply with environmental, health and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on our business, results of operations and financial condition.
We are subject to numerous environmental, health and safety laws and regulations, including those governing laboratory procedures and the handling, use, storage, treatment and disposal of hazardous materials and wastes. For our research and manufacturing operations, we intend to contract with third parties and cannot completely eliminate the risk that such third parties will be non-compliant with these regulations. We could be held liable for damages as well as incur fines or penalties, which could create additional costs and materially harm our business. In addition, we could suffer damage to our reputation, which could affect our ability to successfully build and grow our business. Any of these events could have a material adverse effect on our business, results of operations and financial condition.
We are subject to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
We are subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These health care laws and regulations include, for example:
| · | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or services for which payment may be made under a federal health care program such as the Medicare and Medicaid programs; |
| · | the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients to providers of ‘‘designated health services’’ with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exception applies; |
| · | the federal Health Insurance Portability and Accountability Act of 1996 (HIPAA), which established federal crimes for knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services; |
| · | federal false claims laws, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid, or other third-party payors that are false or fraudulent, and which may apply to entities like us to the extent that our interactions with customers may affect their billing or coding practices; and |
| · | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers. |
If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including civil and criminal penalties, damages and fines, and/or exclusion from participation in Medicare, Medi-Cal or other state or federal health care programs, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could have a material adverse effect on our business, results of operations and financial condition.
Our business, results of operations and financial condition would suffer in the event of system failures.
Our internal computer systems and those of our current and potential collaborators, contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. If any material system failure, accident or security breach were to occur and cause interruptions to our operations, it could result in a material disruption of our development programs and our business operations. For example, the loss of clinical trial data from future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Confidential information sensitive to the development of our product candidates could also be disclosed. Likewise, we intend to rely on third parties to manufacture our product candidates and conduct our clinical trials, and similar events relating to their computer systems could also have a material adverse effect on our business, results of operations and financial condition. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liabilityand the further development of our product candidates could be delayed, which could have a material adverse effect on our business, results of operations and financial condition.
| 50 |
We may become subject to litigation, which could materially adversely affect us.
We may become subject to litigation, including claims relating to our operations, security offerings and otherwise in the ordinary course of business. Although we intend to defend any such lawsuits vigorously, any such claims and lawsuits could divert our management’s attention, cause us to incur defense costs, and could result in judgments against us. We cannot be certain of the ultimate outcomes of any claims that may arise, and any significant judgments could materially adversely impact us.
Risks Related to Ownership of Our Common Stock
If securities or industry analysts do not publish research or publish inaccurate or unfavorable research about our business, our share price and trading volume could decline.
The trading market for our common stock will depend on the research and reports that securities or industry analysts publish about us or our business. We do not have any control over these analysts. There can be no assurance that analysts will cover us or provide favorable coverage. If one or more of the analysts who cover us downgrade our stock or change their opinion of our stock, our share price would likely decline. If one or more of these analysts cease coverage of our company or fail to regularly publish reports on us, we could lose visibility in the financial markets, which could cause our share price or trading volume to decline.
Our shares are held by two principal stockholders who may be able to exert significant control over the company. Joseph Hernandez, our Chief Executive Officer and Executive Chairman, and OSIF currently hold all issued and outstanding shares of our common stock.
Joseph Hernandez, our Chief Executive Officer and Executive Chairman, and OSIF beneficially own a total of 95% of all of our issued and outstanding shares of our common stock. These stockholders may be able to determine all matters requiring stockholder approval, and, if acting together, may be able to control the amending of our organizational documents, the election of directors or the approval of corporate transaction. This control may deter investors or unsolicited acquisition proposals or offers for our common stock or our business that you may believe are in your best interest as a stockholder. Any of these events could have a material adverse effect on our business, results of operations and financial condition.
We are an ‘‘emerging growth company,’’ and the reduced reporting requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an ‘‘emerging growth company,’’ as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not ‘‘emerging growth companies,’’ including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any fiscal year end before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of fiscal year end or, if we issue more than $1.0 billion in non-convertible debt during any three year period before that time, we would cease to be an emerging growth company immediately.
Even after we no longer qualify as an emerging growth company, we may still qualify as a ‘‘smaller reporting company,’’ which would allow us to take advantage of many of the same exemptions from disclosure requirements, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements. Some investors may find our common stock less attractive because we rely on these exemptions, there may be a less active trading market for our common stock and our stock price may be more volatile.
We have elected not to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2) of the JOBS Act that allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. Section 107 provides that our decision to opt out of the extended transition period for complying with new or revised accounting standards is irrevocable.
| 51 |
If we are unable to establish and maintain effective disclosure controls and procedures, including an effective system of internal control over financial reporting, our business, results of operations and financial condition could be materially adversely affected.
We must maintain effective disclosure controls and procedures, including internal control over financial reporting, to provide reliable financial reports and to prevent and detect fraud and other improprieties. Since inception we have had a limited number of transactions and have not yet established an internal control system. Accordingly, we do not have effective internal controls over financial reporting. If we are unable to maintain effective disclosure controls and procedures, including an effective system of internal control over financial reporting, our investors could lose confidence in our reported financial information. Any such loss of confidence would have a negative effect on the market price of our stock, which could have a material adverse effect on our business, results of operations and financial condition.
We will face increased administrative costs as a public company.
As a result of the Acquisition, Microlin has become a public reporting company. We will incur significant legal, accounting and other expenses in order to comply with the Sarbanes-Oxley Act and other rules implemented by the SEC that we did not incur as a private company. Although we are exempt from certain regulations as an ‘‘emerging growth company’’ as well as a ‘‘smaller reporting company,’’ we are still subject to multiple regulations for which compliance will require significant financial resource and management and employees. For example, we will adopt additional internal controls and disclosures controls and procedures, we may pay higher rates for director and officer liability insurance, and we may incur internal and external costs of preparing and distributing periodic public reports in compliance with our obligations under the federal securities laws. An increase in any of these costs could have a material adverse effect on our business, results of operations and financial condition.
Future sales and issuances of our common stock or rights to purchase common stock could result in additional dilution for our stockholders and could cause our stock price to fall.
We expect that we will need significant additional capital in the future to continue along our development process towards approval and subsequent commercialization of our therapeutic product candidates. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. We may sell common stock, convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell common stock, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. These sales may also result in material dilution to our existing stockholders, and new investors could gain rights superior to our existing stockholders.
In addition, the terms of our licensing agreements with OSIF provide that OSIF is to hold a 7% equity interest in the company on a fully-diluted basis until we raise at least $10,000,000 in a single transaction or a series of transactions of equity financing. In the event we raise more than $10,000,000 of equity financing, the licensing agreements grant OSIF the right to participate in any sale of equity securities on the same terms as other purchasers so as to maintain its equity interest in the company. The issuance of additional shares to OSIF pursuant to these anti-dilution provisions may result in dilution to our stockholders, especially in instances where our stockholders may not be provided the opportunity to participate in a sale of equity securities or choose not to participate.
We do not intend to pay cash dividends on our common stock so any returns on our common stock will be limited to an appreciation in the value of our common stock, if any.
We have never declared or paid any cash dividends on our common stock and do not currently intend to declare or pay any cash dividends for the foreseeable future. As a result, stockholders may only receive a return on our common stock if the market price appreciates in value. Investors seeking cash dividends should not invest in our common stock.
If we fail to remain current on our reporting requirements, we could be removed from quotation on the OTCQB, which would limit the ability of broker-dealers to sell our securities and the ability of stockholders to sell their securities in the secondary market.
Companies trading on the OTCQB, such as us, must be reporting issuers with the Securities and Exchange Commission and must be current in their reports in order to maintain price quotation privileges on the OTCQB tier of the electronic quotation system operated by OTC Markets, Inc. As a result, the market liquidity for our securities could be severely adversely affected by limiting the ability of broker-dealers to sell our securities and the ability of stockholders to sell their securities in the secondary market.
| 52 |
Because our common stock could be deemed a low-priced “Penny” stock, it would be cumbersome for brokers and dealers to trade in our common stock, making the market for our common stock less liquid and negatively affect the price of our stock.
We may be subject to certain provisions of the Securities Exchange Act of 1934, commonly referred to as the “penny stock” as defined in Rule 3a51-1. A penny stock is generally defined to be any equity security that has a market price less than $5.00 per share, subject to certain exceptions. If our stock is deemed to be a penny stock, trading will be subject to additional sales practice requirements of broker-dealers. These require a broker-dealer to:
| • | Deliver to the customer, and obtain a written receipt for, a disclosure document; |
| • | Disclose certain price information about the stock; |
| • | Disclose the amount of compensation received by the broker-dealer or any associated person of the broker-dealer; |
| • | Send monthly statements to customers with market and price information about the penny stock; and |
| • | In some circumstances, approve the purchaser’s account under certain standards and deliver written statements to the customer with information specified in the rules. |
Consequently, penny stock rules may restrict the ability or willingness of broker-dealers to trade and/or maintain a market in our common stock. Also, prospective investors may not want to get involved with the additional administrative requirements, which may have a material adverse effect on the trading of our shares.
Because we became a public company through a reverse acquisition, we may not be able to attract the attention of major brokerage firms.
There may be risks associated with our becoming public through a reverse acquisition. Securities analysts of major brokerage firms may not provide coverage of our Company since there is no incentive to brokerage firms to recommend the purchase of our common stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of our post-merger company in the future.
| 53 |
Directors and Executive Officers
The following table sets forth information regarding the members of our board of directors and our executive officers and other significant employees. All of our officers and directors were appointed on the effective date of the Merger. All of our directors hold office until the next annual meeting of stockholders and their successors are duly elected and qualify. Executive officers serve at the request of the board of directors.
|
Name |
Age | Office(s) held |
| Joseph Hernandez | 43 | President, Chief Executive Officer (CEO), Chief Financial Officer (CFO), Executive Chairman, and Director |
| Bruce Galton | 63 | Chief Operating Officer (COO) |
| Richard S. Dondero | 65 | Vice President of Clinical Development |
| Sonya Zabludoff, Ph.D. | 52 | Head of Preclinical Drug Discovery |
| John Bonfiglio, Ph.D. | 60 | Director |
Set forth below is a brief description of the background and business experience of each of our current executive officers and directors.
Joseph Hernandez is our newly-appointed President, CEO, CFO, Executive Chairman, and sole member of our Board of Directors. Mr. Hernandez is the founder of several biotechnology companies, including Prolias Technologies, Inc., a molecular diagnostics company focusing on thyroid cancer, where he has served as a director since 2010, and Innovative Biosensors, Inc., a MIT-based technology company that develops, manufactures and markets rapid pathogen detection and diagnostic systems comprised of biosensors, where he served as President and Chief Executive Officer from 2003 until 2009. From July 2010 to March 2012, Mr. Hernandez also served as President and Chief Executive Officer of Signal Genetics, LLC, a privately-held company focused on oncology and the development of innovative diagnostic services. Additionally, Mr. Hernandez currently serves as the Executive Chairman of Ember Therapeutics, a late stage biotech company focusing on osteoarthritis since 2014. Mr. Hernandez has held numerous business development and marketing roles, having served in such roles at Digene Corp. (which has since been acquired by Qiagen NV (QGEN)), a molecular diagnostics company that develops, manufactures and markets gene-based testing systems for the screening, monitoring and diagnosis of certain human diseases, such as women’s cancers and infectious diseases, from 2001 to 2003, and at Affymetrix, Inc. (AFFX), a publicly-held Silicon Valley biotech company that develops DNA microarrays, from 2000 to 2001. From 1998 to 2000, Mr. Hernandez worked in sales and marketing for the publicly-held pharmaceutical company Merck & Co. (MRK). Mr. Hernandez was recently a member of the board of directors of MdBIO, a life science industry association based in the Mid-Atlantic region, and a member of the board of directors of Shady Grove Hospital. Mr. Hernandez holds an M.S. in Microbiology and Molecular Genetics from the University of Florida, an MBA from the University of Florida and a B.S. in Neuroscience from the University of Florida.
Bruce C. Galton, Bruce Galton has served as President and CEO and a Director of Senesco Technologies, Inc. a gene technology company pursuing oncology and anti-inflammatory applications (now Sevion Therapeutics, Inc. SVON), President and Chief Operating Officer and a Director of Annovis, Inc. which made specialty chemicals for DNA synthesis and was acquired by Transgenomic, Inc. and President and Chief Executive Officer of Cistron Biotechnology, Inc., which pursued cytokines and cytokine antibodies until it was acquired by CellTech. Prior to that, Mr. Galton was employed by Becton Dickinson and Company in a variety of financial positions including international treasury, financial manager of a domestic assay kit manufacturing division and as financial manager of an international lab equipment sales division. Currently, Mr. Galton is the principal of Galton Consulting, LLC, a life science advisory firm.
Richard S. Dondero. Richard S. Dondero has agreed to serve as our Vice President of Clinical Development. Mr. Dondero most recently was Senesco Technologies’ Vice President of Research and Development (2004-2014). Senesco’s platform technology is based upon the identification and characterization of specific genes that regulate cell death in plants (senescence) and humans (apoptosis). From July 2002 to July 2004, Mr. Dondero was a Group Leader in Proteomics Operations for Molecular Staging Inc., a company engaged in Bio-Marker Discovery and Whole Genome Amplification. From 1985 to 2001, Mr. Dondero held positions of increasing responsibility including Vice President of Operations at Cistron Biotechnology. Cistron activities were focused in the research and development of certain pro-inflammatory cytokines which are important mediators in a number of disease states. Prior to Cistron, Mr. Dondero was employed at Johnson & Johnson and Becton Dickerson. Mr. Dondero received his Masters of Science degree in Biology from Seton Hall University and Bachelor of Arts degree in Biology from New Jersey City University.
| 54 |
Dr. Sonya Zabludoff. Sonya Zabludoff, Ph.D., has agreed to serve as our Head of Preclinical Drug Discovery. Dr. Zabludoff has extensive experience as a scientific director in preclinical drug discovery and has worked in both large pharmaceutical companies and biotech. In 2012, she joined Regulus Therapeutics Inc. (RGLS), a publicly-held biopharmaceutical company focused on the development of microRNA-based drugs targeting fibrosis, metabolism and cardiovascular diseases, cancer, HCV and immune-related diseases, and served as the Senior Director of Oncology. Dr. Zabludoff previously served as Senior Director of Oncology at Pfizer, Associate Director of Oncology at AstraZeneca and Senior Scientist in Oncology at Novartis. Dr. Zabludoff was a post-doctoral fellow at California Institute of Technology, received her Ph.D. in Reproductive Biology from the Johns Hopkins University and a B.S. in Biology from University of Rochester.
John N. Bonfiglio, Ph.D. has agreed to serve as a member of our board of directors. Dr. Bonfiglio has served as the President, Chief Executive Officer and a director of Oragenics, Inc. (OGEN), a publicly-traded biotechnology company focused on the development of novel and innovative therapeutics for oral health, since May 2011. Dr. Bonfiglio previously served as the Chief Executive Officer, President and as a director of Transdel Pharmaceuticals, Inc., a specialty pharmaceutical company that developed non-invasive, topically delivered products, between May 2010 and April 2011. From January 2007 to March 2010, Dr. Bonfiglio served as the President and Chief Executive Officer of Argos Therapeutics, Inc., a privately-held biopharmaceutical company focused on the development and commercialization of personalized immunotherapies for the treatment of cancer and infectious diseases. From November 2005 to December 2006, he served as an independent consultant to two medical device companies, a therapeutic company and a medical communications company. From January 2003 to October 2005, Dr. Bonfiglio served as the Chief Executive Officer of The Immune Response Corporation, an immuno-pharmaceutical company focused on developing products to treat autoimmune and infectious diseases. From 2001 to 2002, he was the Chief Operating Officer and Executive Vice President of Cypress Biosciences, Inc., a company providing therapeutics and personalized medicine services, and from 1997 to 2001 he served as the Chief Executive Officer and President of Peregrine Pharmaceuticals, Inc. (PPHM), a publicly-held biopharmaceutical company developing first-in-class monoclonal antibodies for the treatment of cancer and viral infections. Dr. Bonfiglio has also held senior management positions with Baxter Healthcare Corporation (BAX) and Allergan, Inc. (AGN). Dr. Bonfiglio received his B.S. in Chemistry from the State University of New York at Stony Brook in 1976 and earned his Ph.D. in Synthetic Organic Chemistry from the University of California at San Diego in 1980. He later went on to serve as a postdoctoral fellow in organometallic chemistry at the University of California at Berkeley in 1981 and earned an M.S. in Business Administration from Pepperdine University in 1992.
Scientific Advisory Board
Our executive team is supported by our scientific advisory board, the members of which include scientists experienced in the fields of microRNA and cancer biology.
Robert Lee, Ph.D., Professor of Pharmaceutics at the OSU College of Pharmacy, has experience in developing novel targeted drug delivery systems for cancer based on lipid and polymer nanoparticles, including novel nanocarrier formulations of oligonucleotides Dr. Lee also has previous experience in the private sector, in which he served as the Vice President of Research and Development at Endocyte, Inc. (ECYT), a publicly-held biopharmaceutical company focused on the development of targeted therapies for the treatment of cancer and other serious diseases.
Philip Tsichlis, M.D., Professor of Hematology and Oncology at the Tufts University School of Medicine, serves as the Executive Director of the Molecular Oncology Research Institute at the Tufts Medical Center. Dr. Tsichlis is an expert in various molecular pathways involved in cancer.
Sakari Kauppinen, Ph.D., Professor at the Department of Haematology, Aalborg University Hospital in Denmark, is an expert in miRNA research and discovery and development of miRNA-based therapeutics. Dr. Kauppinen also owns a firm which consults to pharmaceutical companies and previously served as the Senior Director of microRNA Research at Santaris Pharma. Dr. Kauppinen has published 90 scientific papers and is co-inventor on 60 patent applications.
George Calin, M.D., Ph.D., is both a Professor for the Department of Experimental Therapeutics and Co- Director of The RNA Interference and non-coding RNA Center at the The University of Texas MD Anderson Cancer Center. Dr. Calin has hundreds of scientific publications and a strong focus on microRNA biology.
| 55 |
Directors
Our bylaws authorize no less than one (1) and no more than twelve (12) directors. We currently have one director. Pursuant to the terms of the Acquisition, Joseph Hernandez, who prior to the Merger was the sole director of
Microlin, was appointed as our director. It is our intention to immediately expand the Board of Directors with Industry Veterans.
All directors hold office for one-year terms until the election and qualification of their successors. Officers are elected by the board of directors and serve at the discretion of the board.
There are no family relationships between or among the directors, executive officers or persons nominated or chosen by the Company to become directors or executive officers.
Committees of the Board
We do not currently have a compensation committee, executive committee, or stock plan committee.
Audit Committee
We do not have a separately-designated standing audit committee. The entire Board of Directors performs the functions of an audit committee, but no written charter governs the actions of the Board when performing the functions of what would generally be performed by an audit committee. The Board approves the selection of our independent accountants and meets and interacts with the independent accountants to discuss issues related to financial reporting. In addition, the Board reviews the scope and results of the audit with the independent accountants, reviews with management and the independent accountants our annual operating results, considers the adequacy of our internal accounting procedures and considers other auditing and accounting matters including fees to be paid to the independent auditor and the performance of the independent auditor. Our Board of Directors, which performs the functions of an audit committee, does not have a member who would qualify as an “audit committee financial expert” within the definition of Item 407(d)(5)(ii) of Regulation S-K.
Nomination Committee
Our Board of Directors does not maintain a nominating committee. As a result, no written charter governs the director nomination process. Our size and the size of our Board, at this time, do not require a separate nominating committee.
When evaluating director nominees, our directors consider the following factors:
· The appropriate size of our Board of Directors;
· Our needs with respect to the particular talents and experience of our directors;
· The knowledge, skills and experience of nominees, including experience in finance, administration or public service, in light of prevailing business conditions and the knowledge, skills and experience already possessed by other members of the Board;
· Experience in political affairs;
· Experience with accounting rules and practices; and
· The desire to balance the benefit of continuity with the periodic injection of the fresh perspective provided by new Board members.
Our goal is to assemble a Board that brings together a variety of perspectives and skills derived from high quality business and professional experience. In doing so, the Board will also consider candidates with appropriate non-business backgrounds.
Other than the foregoing, there are no stated minimum criteria for director nominees, although the Board may also consider such other factors as it may deem are in our best interests as well as our stockholders. In addition, the Board identifies nominees by first evaluating the current members of the Board willing to continue in service. Current members of the Board with skills and experience that are relevant to our business and who are willing to continue in service are considered for re-nomination. If any member of the Board does not wish to continue in service or if the Board decides not to re-nominate a member for re-election, the Board then identifies the desired skills and experience of a new nominee in light of the criteria above. Current members of the Board are polled for suggestions as to individuals meeting the criteria described above. The Board may also engage in research to identify qualified individuals. To date, we have not engaged third parties to identify or evaluate or assist in identifying potential nominees, although we reserve the right in the future to retain a third party search firm, if necessary. The Board does not typically consider shareholder nominees because it believes that its current nomination process is sufficient to identify directors who serve our best interests.
Code of Ethics
As of September 30, 2015, we had not adopted a Code of Ethics for Financial Executives, which would include our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions.
| 56 |
Compensation Discussion and Analysis
Joseph Hernandez
We entered into an Employment Agreement with Mr. Hernandez in July 2013. Pursuant to the agreement, Mr. Hernandez is paid an annual base salary of $460,000. This salary is subject to an annual review by our board of directors. Mr. Hernandez is also eligible for an annual performance-based cash bonus equivalent to 50% of his base salary, subject to the discretion of our board of directors and attainment by our company of reasonable performance goals approved by the board of directors in its sole discretion. Mr. Hernandez is also entitled to reimbursement from our company for his out-of-pocket cost for life and disability insurance premiums in an amount not to exceed $10,000 annually. Mr. Hernandez’s agreement also provides for a grant of stock options exercisable for an aggregate of 60,000 shares of our common stock at an exercise price of $6.33 per share.
Upon termination of Mr. Hernandez’s employment for Cause or at Mr. Hernandez’s election other than for Good Reason, he will receive payment for any accrued but unpaid salary through the date of termination and any amount arising from his participation in or benefits under any employee benefit plans, programs, or arrangements. If Mr. Hernandez’s employment is terminated by us other than for Cause or is terminated by Mr. Hernandez for Good Reason, we will make a lump sum payment to Mr. Hernandez in the amount equal to twenty-four (24) months of his salary as in effect immediately prior to the date of termination, in addition to payment for any accrued but unpaid salary through the date of termination and any amount arising from his participation in or benefits under any employee benefit plans, programs, or arrangements.
Under the employment agreement, ‘‘Cause’’ is defined as (a) a material breach of fiduciary duty or material breach of the terms of the employment agreement or any other agreement between Mr. Hernandez and us (including without limitation any agreements regarding confidentiality, inventions assignment and non-competition), which, in the case of a material breach of the terms of the employment agreement, remains uncured for a period of sixty (60) days following receipt of written notice from our Board of Directors specifying the nature of such breach; (b) the commission by Mr. Hernandez of any act of embezzlement, fraud, larceny, or theft on or from us; (c) substantial and continuing neglect or inattention by Mr. Hernandez of the duties of his employment, refusal to perform the lawful and reasonable directives of the Board of Directors or the willful misconduct or gross negligence of Mr. Hernandez in connection with the performance of such duties which remains uncured for a period of sixty (60) days following receipt of written notice from the Board of Directors specifying the nature of such breach; (d) the commission by Mr. Hernandez of any crime involving moral turpitude or a felony; and (e) Mr. Hernandez’s performance or omission of any act which, in the judgment of the Board of Directors, if known to our customers, clients, stockholders or any of our regulators, would have a material and adverse impact on our business.
‘‘Good Reason’’ is defined as (a) a material breach of the agreement by us; (b) a material and substantial reduction of Mr. Hernandez’s responsibilities that is inconsistent with Mr. Hernandez’s status as a senior executive with us; or (c) the requirement by us that Mr. Hernandez perform any act or refrain from performing any act that would be in violation of applicable law.
Bruce C. Galton
We entered into a Consulting Agreement with Mr. Galton on May 21, 2015 (the “Agreement”). Under the Agreement, Mr. Galton has agreed to serve as our COO for a period of one (1) year. Mr. Galton will receive consulting fees of $187.50 per hour, and has agreed to devote at least twenty (20) hours per week to the company. In addition, the Agreement calls for Mr. Galton to be issued options to purchase 20,000 of our common stock, which shall vest in equal installments over four (4) years.
Richard S. Dondero
We entered into a Consulting Agreement with Mr. Dondero on May 29, 2015 (the “Agreement”). Under the Agreement, Mr. Dondero has agreed to serve as our Vice President of Clinical Development for a period of one (1) year. Mr. Dondero will receive consulting fees of $150 per hour, and has agreed to devote at least twenty (20) hours per week to the company. In addition, the Agreement calls for Mr. Dondero to be issued options to purchase 20,000 of our common stock, which shall vest in equal installments over four (4) years.
Sonya Zabludoff
We entered into a Consulting Agreement with Ms. Zabludoff on June 8, 2015 (the “Agreement”). Under the Agreement, Ms. Zabludoff has agreed to serve as our head of pre-clinical development for a period of one (1) year. Ms. Zabludoff will receive consulting fees of $225 per hour, and has agreed to devote at least twenty (20) hours per week to the company. In addition, the Agreement calls for Ms. Zabludoff to be issued options to purchase 10,000 of our common stock, which shall vest in equal installments over four (4) years.
| 57 |
2013 Equity Incentive Plan
Pursuant to the terms of the Merger Agreement, we have adopted Microlin’s 2013 Equity Incentive Plan (the “Plan”). The Plan provides for the grant of incentive stock options, or ISOs, within the meaning of Section 422 of the IRC, nonstatutory stock options, or NSOs, stock appreciation rights, restricted stock awards, restricted stock unit awards, share-based awards, and other forms of equity compensation, or collectively, stock awards, all of which may be granted to employees, including officers, and to non-employee directors and consultants. ISOs may be granted only to employees. All other awards may be granted to employees, including officers, and to non-employee directors and consultants.
Share Reserve
The aggregate number of shares of our common stock that may be issued pursuant to stock awards under the Plan is 500,000 shares. As of the date of this Current Report, stock options to purchase 182,000 shares of our common stock are outstanding under the Plan.
If a stock award is granted under the Plan but shares of our common stock are not acquired pursuant to the stock award (due to expiration, forfeiture, cancelation, surrender or termination of the stock award, cash settlement of the stock award, or otherwise), the shares not acquired again will become available for subsequent issuance under the Plan.
Administration
Our board of directors has the authority to administer the Plan. Our board of directors may delegate its authority to administer the Plan to our compensation committee. Our board of directors may also delegate to one or more of our officers (1) the authority to designate employees to be recipients of certain stock awards, and determine the number of shares of common stock to be subject to such stock awards, and (2) such other authority under the Plan as the board may determine. Subject to the terms of the Plan, our board of directors or the authorized delegate, referred to herein as the plan administrator, determines recipients, dates of grant, the numbers and types of stock awards to be granted and the terms and conditions of the stock awards, including the period of their exercisability and vesting schedule applicable to a stock award. Subject to the limitations set forth below, the plan administrator will also determine the exercise price, strike price or purchase price of awards granted and the types of consideration to be paid for the award.
The plan administrator has the power to modify outstanding awards under the Plan. Subject to the terms of the Plan, the plan administrator has the authority, at any time, to (1) provide that all or a portion of a stock award may be exercised, (2) provide that all or part of any time-based vesting restrictions shall lapse or that all or part of any performance-based criteria shall be deemed to be satisfied, or (3) waive any other limitation or requirement under any stock award.
Stock Options
Incentive and nonstatutory stock options are granted pursuant to stock option agreements adopted by the plan administrator. The plan administrator determines the exercise price for a stock option, within the terms and conditions of the Plan, provided that the exercise price of a stock option generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Options granted under the Plan vest at the rate specified by the plan administrator.
The plan administrator determines the term of stock options granted under the Plan, up to a maximum of 10 years. Unless the terms of an optionee’s stock option agreement provides otherwise, if an optionee’s service relationship with us, or any of our affiliates, ceases for any reason other than disability, death or cause, the optionee may generally exercise any vested options for a period of three months following the cessation of service. The option term may be extended in the event that exercise of the option following such a termination of service is prohibited by applicable securities laws or our insider trading policy. If an optionee’s service relationship with us, or any of our affiliates, ceases due to disability or death, the optionee or a beneficiary may generally exercise any vested options for a period of 12 months after the termination of the service relationship. In the event of a termination for cause, options generally terminate immediately upon the termination of the individual for cause. In no event may an option be exercised beyond the expiration of its term.
Acceptable consideration for the purchase of common stock issued upon the exercise of a stock option will be determined by the plan administrator and may include (1) cash or its equivalent, (2) a cashless exercise, (3) the tender of shares of our common stock previously owned by the optionee, (4) a net exercise of the option if it is a nonstatutory option, and (5) other legal consideration approved by the plan administrator.
Unless the plan administrator provides otherwise, options generally are not transferable except by will, the laws of descent and distribution, or pursuant to a domestic relations order. An optionee may designate a beneficiary, however, who may exercise the option following the optionee’s death.
| 58 |
Tax Limitations on Incentive Stock Options
The aggregate fair market value, determined at the time of grant, of our common stock with respect to ISOs that are exercisable for the first time by an optionee during any calendar year under all of our stock plans may not exceed $100,000. Options or portions thereof that exceed such limit will generally be treated as NSOs. No ISO may be granted to any person who, at the time of the grant, owns or is deemed to own stock possessing more than 10% of our total combined voting power or that of any of our affiliates unless (1) the option exercise price is at least 110% of the fair market value of the stock subject to the option on the date of grant, and (2) the term of the ISO does not exceed five years from the date of grant.
Restricted Stock Awards
Restricted stock awards may be granted, on terms and conditions determined by the plan administrator, pursuant to restricted stock award agreements adopted by the plan administrator. During any restriction period, the restricted shares are not transferable, but may entitle the holder to voting rights and a right to dividends and distributions paid with respect to the restricted shares.
Restricted Stock Unit Awards
Restricted stock unit awards may be granted, on terms and conditions determined by the plan administrator, pursuant to restricted stock unit award agreements adopted by the plan administrator. Restricted stock unit awards may be granted in consideration for any form of legal consideration. A restricted stock unit award may be settled by cash, delivery of stock, a combination of cash and stock as deemed appropriate by the plan administrator, or in any other form of consideration set forth in the restricted stock unit award agreement. Dividend equivalents may be credited in respect of shares covered by a restricted stock unit award.
Stock Appreciation Rights
Stock appreciation rights may be granted, on terms and conditions determined by the plan administrator, pursuant to stock appreciation grant agreements adopted by the plan administrator. The plan administrator determines the strike price for a stock appreciation right, which generally cannot be less than 100% of the fair market value of our common stock on the date of grant. Upon the exercise of a stock appreciation right, we will pay the participant an amount equal to the product of (1) the excess of the per share fair market value of our common stock on the date of exercise over the strike price, multiplied by (2) the number of shares of common stock with respect to which the stock appreciation right is exercised. A stock appreciation right granted under the Plan vests at the rate specified in the stock appreciation grant agreement as determined by the plan administrator.
Other Stock Award
The plan administrator may grant other awards based in whole or in part by reference to our common stock. The plan administrator will set the number of shares under the stock award and all other terms and conditions of such awards.
Changes to Capital Structure
In the event that there is a specified type of change in our capital structure, such as a stock split or recapitalization, appropriate adjustments will be made to (1) the class and maximum number of shares reserved for issuance under the Plan, (2) the class and maximum number of shares that may be issued upon the exercise of ISOs, (3) the class and maximum number of shares subject to stock awards that can be granted in a calendar year (as established under the Plan pursuant to Section 162(m) of the IRC) and (4) the class and number of shares and exercise price, strike price, or purchase price, if applicable, of all outstanding stock awards.
Change in Control
The Plan provides that the plan administrator may take actions that it deems necessary with respect to any stock awards, including the acceleration of vesting, settlement and exercisability in the event of a change in control. For example, a stock award may provide for accelerated vesting upon the participant’s termination without cause or resignation for good reason in connection with a change in control. In the absence of such action or provision, no such acceleration of the stock award will occur. Under the Plan, a change in control is generally (1) the acquisition by a person or entity of more than 50% of our combined voting power other than by merger, consolidation or similar transaction; (2) a consummated merger, consolidation or similar transaction immediately after which our stockholders cease to own more than 50% of the combined voting power of the surviving entity; or (3) a sale of all or substantially all of our assets to a person or entity that is not our affiliate or controlled by the our stockholders.
Amendment and Termination
Our board of directors has the authority to amend, suspend, or terminate our Plan, provided that such action does not materially impair the existing rights of any participant without such participant’s written consent. No stock awards may be granted after the tenth anniversary of the effective date of the Plan.
| 59 |
Summary Compensation Table
The table below summarizes all compensation awarded to, earned by, or paid to each named executive officer for our last two completed fiscal years for all services rendered to us.
| SUMMARY COMPENSATION TABLE | ||||||||||
|
Name and principal position |
Year |
Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($) |
Non-Equity Incentive Plan Compensation ($) |
Nonqualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) | |
| Joseph Hernandez, CEO, CFO, Exec. Chairman |
2014 2013 |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a | |
| Bruce C. Galton, COO |
2014 2013 |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a | |
| Richard S. Dondero, V.P. of Clinical Dev. |
2014 2013 |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a | |
| Sonya Zabludoff, Head of Pre-Clinical Dev. |
2014 2013 |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a |
n/a n/a | |
| Reza Noorkayhani, former officer |
2014 2013 |
0 0 |
0 0 |
180,000 0 |
0 0 |
0 0 |
0 0 |
0 0 |
180,000 0 | |
| Joseph Marshall, former officer |
2014 2013 |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 |
0 0 | |
Narrative Disclosure to the Summary Compensation Table
Our current executive officers were appointed in connection with the recent Acquisition and did not serve during our last two fiscal years.
Our employment and compensation arrangement with our current named executive officers are as set forth above under “Compensation Discussion and Analysis.”
| 60 |
Stock Option Grants
We have not granted any stock options to the executive officers or directors since our inception. Microlin Bio, Inc. has issued options to its officers and directors as follows:
| Name | # of Shares |
| Joseph Hernandez | 60,000 |
| Bruce Galton | 20,000 |
| Richard S. Dondero | 20,000 |
| Sonya Zabludoff | 10,000 |
| John Bonfiglio | 20,000 |
Outstanding Equity Awards At Fiscal Year-end Table
The table below summarizes all unexercised options, stock that has not vested, and equity incentive plan awards for each named executive officer outstanding as of the end of our last completed fiscal year.
| OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | |||||||||
| OPTION AWARDS | STOCK AWARDS | ||||||||
|
Name |
Number of Securities Underlying Unexercised Options (#) Exercisable |
Number of Securities Underlying Unexercised Options (#) Unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
Number of Shares or Shares of Stock That Have Not Vested (#) |
Market Value of Shares or Shares of Stock That Have Not Vested ($) |
Equity Incentive Plan Awards: Number of Unearned Shares, Shares or Other Rights That Have Not Vested (#) |
Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Shares or Other Rights That Have Not Vested (#) |
| Joseph Hernandez, CEO, CFO, Exec. Chairman | - | - | - | - | - | - | - | - | - |
| Bruce C. Galton, COO | - | - | - | - | - | - | - | - | - |
| Richard S. Dondero, V.P. of Clinical Dev. | - | - | - | - | - | - | - | - | - |
| Sonya Zabludoff, Head of Pre-Clinical Dev. | - | - | - | - | - | - | - | - | - |
| Reza Noorkayhani, former officer | - | - | - | - | - | - | - | - | - |
| Joseph Marshall, former officer | - | - | - | - | - | - | - | - | - |
| 61 |
Compensation of Directors Table
The table below summarizes all compensation paid to our directors for our last completed fiscal year.
| DIRECTOR COMPENSATION | |||||||
Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards ($) |
Non-Equity Incentive Plan Compensation ($) |
Non-Qualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
| Joseph Hernandez | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| John Bonfiglio | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
| Reza Noorkayhani, former director | -0 | -0 | -0 | -0 | -0 | -0 | -0 |
| Joseph Marshall, former director | -0 | -0 | -0 | -0 | -0 | -0 | -0 |
Narrative Disclosure to the Director Compensation Table
We have not previously provided any compensation to directors for their service as directors. Our new directors, Joseph Hernandez and John Bonfiglio, did not serve during our last fiscal year.
Security Ownership of Certain Beneficial Owners and Management
The following table sets forth the beneficial ownership of our capital stock by each executive officer and director, by each person known by us to beneficially own more than 5% of any class of stock and by the executive officers and directors as a group. Except as otherwise indicated, all Shares are owned directly and the percentage shown is based on 20,000,000 shares common stock issued and outstanding following the Acquisition and the related events described herein. All addresses are c/o Microlin Bio, Inc. 135 East 57th Street, 24th Floor, New York, NY 10022 unless otherwise stated.
| Title of class |
Name and address of beneficial owner (1) |
Amount of beneficial ownership |
Percent
|
| Current Executive Officers & Directors: | |||
| Common Stock | Joseph Hernandez | 17,597,000(2) | 87.99% |
| Common Stock | Bruce Galton | 20,000(3) | 0.1% |
| Common Stock | Richard S. Dondero | 20,000(4) | 0.1% |
| Common Stock | Sonya Zabludoff | 10,000(5) | 0.05% |
| Common Stock | John Bonfiglio | 20,000(6) | 0.1% |
| Total of All Current Directors and Officers: | |||
| Common Stock | 17,667,000 | 88.34% | |
| More than 5% Beneficial Owners | |||
| Common Stock |
Ohio State University Foundation, Inc. 1524 North High Street Columbus, OH 43201 |
1,463,000 | 7.32% |
| (1) |
As used in this table, "beneficial ownership" means the sole or shared power to vote, or to direct the voting of, a security, or the sole or shared investment power with respect to a security (i.e., the power to dispose of, or to direct the disposition of, a security). In addition, for purposes of this table, a person is deemed, as of any date, to have "beneficial ownership" of any security that such person has the right to acquire within 60 days after such date. |
| (2) | The total shares for Joseph Hernandez include 17,537,000 shares of common stock and options to purchase 60,000 shares of common stock at a price of $6.33, exercisable for 10 years |
| (3) | Includes options to purchase 20,000 shares of common stock |
| (4) | Includes options to purchase 20,000 shares of common stock |
| (5) | Includes options to purchase 10,000 shares of common stock |
| (6) | Includes options to purchase 20,000 shares of common stock |
| 62 |
Certain Relationships and Related Transactions and Director Independence
The following is a description of transactions that were entered into with our executive officers, directors or greater than 5% stockholders since our inception on July 30, 2013.
Since our inception on July 30, 2013 through March 31, 2014, we have funded our operations through non-interest bearing, unsecured advances from Joseph Hernandez, our founder, Chief Executive Officer and Executive Chairman. As of September 30, 2015 , Mr. Hernandez had advanced us $430,379.
On July 30, 2013, in connection with our incorporation, we issued 3,720,000 shares of common stock to Joseph Hernandez, our founder, Chief Executive Officer and Executive Chairman. We also entered into an Employment Agreement with Mr. Hernandez in July 2013. For a description of the terms of Mr. Hernandez’s Employment Agreement, please see Compensation Discussion and Analysis, above.
If approved by our board of directors, we may enter into certain business transactions with a CRO or other entities controlled, directly or indirectly, by Joseph Hernandez, our founder, Chief Executive Officer and Executive Chairman.
We have entered into Consulting Agreements with Mr. Galton, Mr. Dondero, and Ms. Zabludoff as set forth above.
Director Independence
We are not a “listed issuer” within the meaning of Item 407 of Regulation S-K and there are no applicable listing standards for determining the independence of our directors. Applying the definition of independence set forth in Rule 4200(a)(15) of The Nasdaq Stock Market, Inc., we do not believe that we currently have any independent directors.
Description of Securities
Our authorized capital stock consists of 90,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of preferred stock, par value $0.001 per share. Immediately following the Acquisition and the events reported herein, there were 20,000,000 shares of our common stock issued and outstanding and no shares of preferred stock issued and outstanding
Common Stock
The holders of common stock are entitled to one vote per share. Our certificate of incorporation does not provide for cumulative voting. The holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the board of directors out of legally available funds. However, the current policy of the board of directors is to retain earnings, if any, for operations and growth. Upon liquidation, dissolution or winding-up, the holders of common stock are entitled to share ratably in all assets that are legally available for distribution. The holders of common stock have no preemptive, subscription, redemption or conversion rights.
In the event of any merger or consolidation with or into another company in connection with which shares of our common stock are converted into or exchangeable for shares of stock, other securities or property (including cash), all holders of our common stock will be entitled to receive the same kind and amount of shares of stock and other securities and property (including cash). Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock.
| 63 |
Preferred Stock
Our board of directors is authorized by our articles of incorporation to divide the authorized shares of our preferred stock into one or more series, each of which must be so designated as to distinguish the shares of each series of preferred stock from the shares of all other series and classes. Our board of directors is authorized, within any limitations prescribed by law and our articles of incorporation, to fix and determine the designations, rights, qualifications, preferences, limitations and terms of the shares of any series of preferred stock including, but not limited to, the following:
- The number of shares constituting that series and the distinctive designation of that series, which may be by distinguishing number, letter or title;
- The dividend rate on the shares of that series, whether dividends will be cumulative, and if so, from which date(s), and the relative rights of priority, if any, of payment of dividends on shares of that series;
- Whether that series will have voting rights, in addition to the voting rights provided by law, and, if so, the terms of such voting rights;
- Whether that series will have conversion privileges, and, if so, the terms and conditions of such conversion, including provision for adjustment of the conversion rate in such events as the Board of Directors determines;
- Whether or not the shares of that series will be redeemable, and, if so, the terms and conditions of such redemption, including the date or date upon or after which they are redeemable, and the amount per share payable in case of redemption, which amount may vary under different conditions and at different redemption dates;
- Whether that series will have a sinking fund for the redemption or purchase of shares of that series, and, if so, the terms and amount of such sinking fund;
- The rights of the shares of that series in the event of voluntary or involuntary liquidation, dissolution or winding up of the corporation, and the relative rights of priority, if any, of payment of shares of that series;
- Any other relative rights, preferences and limitations of that series.
Provisions in Our Articles of Incorporation and By-Laws That Would Delay, Defer or Prevent a Change in Control
Our articles of incorporation authorize our board of directors to issue a class of preferred stock commonly known as a "blank check" preferred stock. Specifically, the preferred stock may be issued from time to time by the board of directors as shares of one (1) or more classes or series. Our board of directors, subject to the provisions of our Articles of Incorporation and limitations imposed by law, is authorized to adopt resolutions; to issue the shares; to fix the number of shares; to change the number of shares constituting any series; and to provide for or change the following: the voting powers; designations; preferences; and relative, participating, optional or other special rights, qualifications, limitations or restrictions, including the following: dividend rights, including whether dividends are cumulative; dividend rates; terms of redemption, including sinking fund provisions; redemption prices; conversion rights and liquidation preferences of the shares constituting any class or series of the preferred stock.
In each such case, we will not need any further action or vote by our shareholders. One of the effects of undesignated preferred stock may be to enable the board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a tender offer, proxy contest, merger or otherwise, and thereby to protect the continuity of our management. The issuance of shares of preferred stock pursuant to the board of director's authority described above may adversely affect the rights of holders of common stock. For example, preferred stock issued by us may rank prior to the common stock as to dividend rights, liquidation preference or both, may have full or limited voting rights and may be convertible into shares of common stock. Accordingly, the issuance of shares of preferred stock may discourage bids for the common stock at a premium or may otherwise adversely affect the market price of the common stock.
| 64 |
Delaware Anti-Takeover Laws
We are subject to the provisions of Section 203 of the Delaware General Corporation Law, which applies to "business combinations" such as a merger, asset or stock sale or other transaction that result in financial benefit to an "interested stockholder". An "interested stockholder" is a person who, together with affiliates and associates, owns, or within three years prior, did own, 15% or more of a corporation's outstanding voting stock. Section 203 generally prohibits a publicly held Delaware corporation from engaging in a "business combination” with an "interested stockholder" for a period of three years following the time that the stockholder became an interested stockholder, unless:
- prior to entering into the business combination, the board of directors of the corporation approved either the business combination or the transaction that resulted in the stockholder becoming an interested stockholder;
- upon consummation of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the number of shares outstanding, those shares owned by persons who are directors and also officers, and employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
- on or subsequent to that time, the business combination is approved by the board of directors and authorized at an annual or special meeting of stockholders, and not by written consent, by the affirmative vote of at least two-thirds of the outstanding voting stock that is not owned by the interested stockholder.
This provision may have the effect of delaying, deterring or preventing a change in control over us without further actions by our stockholders.
Options and Warrants
In connection with the Acquisition, we adopted Microlin’s 2013 Equity Incentive Plan (the “Plan”) and have agreed to assume the terms of all stock options issued thereunder.
The following is a summary of the status of stock options outstanding at September 30, 2015:
| Outstanding Options | Exercisable Options | ||
| Exercise Price | Number |
Weighted Average Contractual Life |
Number |
| $6.325 | 182,000 | 8.25 years | 81,583 |
OSIF Anti-Dilution and Conversion Rights
In connection with the Acquisition, we adopted and assumed certain non-dilution rights and conversion rights granted by Microlin to the Ohio State University Foundation, Inc. (“OSIF”). Under its License Agreements with Microlin, OSIF received anti-dilution rights equal to 7% of the company’s issued and outstanding share capital on a fully diluted basis. The right shall lapse following a raise of at least $10,000,000 in a single transaction or a series of transactions of equity financing.
In addition, our agreements with OSIF, as amended, provide for certain rights to convert amounts due under the agreements to common stock, with the conversion prices expressed in terms of an initial public offering price. The details of the agreements are subject to ongoing discussion with OSIF. In connection with the Acquisition, we also adopted and assumed all equity conversion rights currently enjoyed by OSIF under its agreements with Microlin.
| 65 |
Market Price and Dividends
Microlin is, and has always been, a privately-held company. There has never been a public market for the securities of Microlin has never declared or paid any cash dividends on its capital stock. In addition, there has never been a trading market for Microlin’s common stock.
Indemnification of Directors and Officers
Our officers and directors are indemnified as provided by Delaware General Corporation Law and our bylaws.
Under the governing Delaware General Corporation Law, director immunity from liability to a company or its shareholders for monetary liabilities applies automatically unless it is specifically limited by a company's Certificate of Incorporation. Our Certificate of Incorporation do not contain any limiting language regarding director immunity from liability. Excepted from this immunity are:
| 1. | a willful failure to deal fairly with the company or its shareholders in connection with a matter in which the director has a material conflict of interest; |
| 2. | a violation of criminal law (unless the director had reasonable cause to believe that his or her conduct was lawful or no reasonable cause to believe that his or her conduct was unlawful); |
| 3. | a transaction from which the director derived an improper personal profit; and |
| 4. | willful misconduct. |
Our bylaws provide that we will indemnify our directors and officers to the fullest extent not prohibited by Delaware General Corporation Law; provided, however, that we may modify the extent of such indemnification by individual contracts with our directors and officers; and, provided, further, that we shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless:
| 1. | such indemnification is expressly required to be made by law; |
| 2. | the proceeding was authorized by our Board of Directors; |
| 3. | such indemnification is provided by us, in our sole discretion, pursuant to the powers vested us under Delaware law; or; |
| 4. | such indemnification is required to be made pursuant to the bylaws. |
Our bylaws provide that we will advance to any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, by reason of the fact that he is or was a director or officer, of the company, or is or was serving at the request of the company as a director or executive officer of another company, partnership, joint venture, trust or other enterprise, prior to the final disposition of the proceeding, promptly following request therefore, all expenses incurred by any director or officer in connection with such proceeding upon receipt of an undertaking by or on behalf of such person to repay said amounts if it should be determined ultimately that such person is not entitled to be indemnified under our bylaws or otherwise.
Our bylaws provide that no advance shall be made by us to an officer of the company, except by reason of the fact that such officer is or was a director of the company in which event this paragraph shall not apply, in any action, suit or proceeding, whether civil, criminal, administrative or investigative, if a determination is reasonably and promptly made: (a) by the board of directors by a majority vote of a quorum consisting of directors who were not parties to the proceeding, or (b) if such quorum is not obtainable, or, even if obtainable, a quorum of disinterested directors so directs, by independent legal counsel in a written opinion, that the facts known to the decision-making party at the time such determination is made demonstrate clearly and convincingly that such person acted in bad faith or in a manner that such person did not believe to be in or not opposed to the best interests of the company.
| 66 |
Trading Information
Our common stock is quoted under the symbol “AMIB” on the OTC Pink Current (SEC Reporting) tier of the market operated by OTC Markets Group, Inc. Due to our recent name change, we expect our trading symbol to change in the near future.
The following tables set forth the range of high and low prices for our common stock for the each of the periods indicated as reported by OTC Markets Group. These quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| Fiscal Year Ending December 31, 2015 | ||||||||||
| Quarter Ended | High $ | Low $ | ||||||||
| September 30, 2015 | $ | 0.40 | $ | 0.40 | ||||||
| June 30, 2015 | $ | 0.51 | $ | 0.40 | ||||||
| March 31, 2015 | $ | 1.45 | $ | 0.50 | ||||||
| Fiscal Year Ending December 31, 2014 | ||||||||||
| Quarter Ended | High $ | Low $ | ||||||||
| December 31, 2014 | $ | 1.50 | $ | 0.51 | ||||||
| September 30, 2014 | 1.50 | 0.25 | ||||||||
| June 30, 2014 | n/a | n/a | ||||||||
| March 31, 2015 | n/a | n/a | ||||||||
The last quoted price of our common stock was $0.40 per share on December 18, 2015.
Penny Stock
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a market price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock, to deliver a standardized risk disclosure document prepared by the SEC, that: (a) contains a description of the nature and level of risk in the market for penny stocks in both public offerings and secondary trading; (b) contains a description of the broker's or dealer's duties to the customer and of the rights and remedies available to the customer with respect to a violation of such duties or other requirements of the securities laws; (c) contains a brief, clear, narrative description of a dealer market, including bid and ask prices for penny stocks and the significance of the spread between the bid and ask price; (d) contains a toll-free telephone number for inquiries on disciplinary actions; (e) defines significant terms in the disclosure document or in the conduct of trading in penny stocks; and (f) contains such other information and is in such form, including language, type size and format, as the SEC shall require by rule or regulation.
The broker-dealer also must provide, prior to effecting any transaction in a penny stock, the customer with (a) bid and offer quotations for the penny stock; (b) the compensation of the broker-dealer and its salesperson in the transaction; (c) the number of shares to which such bid and ask prices apply, or other comparable information relating to the depth and liquidity of the market for such stock; and (d) a monthly account statement showing the market value of each penny stock held in the customer's account.
In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from those rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser's written acknowledgment of the receipt of a risk disclosure statement, a written agreement as to transactions involving penny stocks, and a signed and dated copy of a written suitability statement.
These disclosure requirements may have the effect of reducing the trading activity for our common stock. Therefore, stockholders may have difficulty selling our securities.
| 67 |
Holders of Our Common Stock
As of December 22, 2015 we had 20,000,000 shares of our common stock issued and outstanding, held by thirty (30) shareholders of record.
Transfer Agent
The transfer agent for our common stock is VStock Tranfer, LLC.
Section 3 – Securities and Trading Markets
Item 3.02. Unregistered Sales of Equity Securities
In connection with the Acquisition, the previous shareholders of Microlin received 19,000,000 shares of our common stock. The 19,000,000 shares of our common stock which were issued to the former holders of common stock of Microlin on the effective date of the Acquisition were issued in reliance on the exemption from registration afforded by Section 4(2) of the Securities Act.
Section 5 – Corporate Governance and Management
Item 5.01. Changes in Control of Registrant
Reference is made to the disclosure set forth under Item 2.01 of this Current Report on Form 8-K, which disclosure is incorporated herein by reference.
Item 5.02. Departure of Directors or Principal Officers; Election of Directors; Appointment of Principal Officers
At the effective time of the Acquisition, Reza Noorkayhani resigned as our Chief Executive Officer, Chief Financial Officer, and President, and Joseph Marshall resigned as our Chief Operations Officer, and Secretary. In addition, Mr. Noorkayhani and Mr. Marshall resigned from our Board of Directors effective ten (10) days after mailing to shareholders of our Schedule 14f-1 regarding the change in our board, which was filed with the Commission and mailed to shareholders of record on December 16, 2015. There was no known disagreement with Mr. Noorkayhani and Mr. Marshall on any matter relating to our operations, policies, or practices. Pursuant to the terms of the Merger Agreement, our new directors and officers are as set forth herein. Reference is made to the disclosure set forth under Item 2.01 of this Current Report on Form 8-K, which disclosure is incorporated herein by reference.
Item 5.03 Amendments to Articles of Incorporation or Bylaws; Change in Fiscal Year
In connection with the Acquisition, the Company’s fiscal year end has changed from December 31 to September 30, effective for fiscal year 2015.
In addition, in connection with this subsequent subsidiary merger discussed herein, we changed our corporate name to “Microlin, Bio, Inc.”
Item 5.06 Change in Shell Company Status
As a result of the Acquisition and related transactions as described herein, to the extent that we were previously considered a “shell company” as defined in Rule 12b-2, we have ceased to be a shell company. The material terms of the transaction are described herein.
In addition, to the extent required under applicable regulations, the information contained in this Current Report is intended to provide "Form 10 information" within the meaning of Rule 144(i)(3) under the Securities Act of 1933.
| 68 |
Section 9 – Financial Statements and Exhibits
Item 9.01. Financial Statements and Exhibits
Financial Statements of Businesses Acquired. In accordance with Item 9.01(a), the audited financial statements of our accounting predecessor, Microlin Bio, Inc., a Delaware corporation, for the years ended September 30, 2015 and 2014, are filed with this Current Report on Form 8-K as Exhibit 99.1.
Pro Forma Financial Information. In accordance with Item 9.01(b), our pro forma financial combined statements are filed in this Current Report on Form 8-K as Exhibit 99.2.
(c) Exhibits.
The exhibits listed in the following Exhibit Index are filed as part of this Current Report on Form 8-K.
*Documents redacted; the company will request confidential treatment
| 69 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
Microlin Bio, Inc.
/s/ Joseph Hernandez
Joseph Hernandez
Executive Chairman and CEO
Date: December 17, 2015
| 70 |