UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 20-F
(Mark One)
| ¨ | REGISTRATION STATEMENT PURSUANT TO SECTION 12(b) OR (g) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
| OR | |||
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
| For the fiscal year ended | December 31, 2018 | ||
| OR | |||
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
| OR | |||
| ¨ | SHELL COMPANY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | ||
| Date of event requiring this shell company report________________________ | |
| For the transition period from__________ to ___________ |
Commission file number 001-36288
| Akari Therapeutics, PLC |
| (Exact name of Registrant as specified in its charter) |
| The Laws of England and Wales |
| (Jurisdiction of incorporation or organization) |
|
75/76 Wimpole Street London W1G 9RT United Kingdom |
| (Address of principal executive offices) |
|
Clive Richardson Interim Chief Executive Officer 75/76 Wimpole Street London W1G 9RT United Kingdom |
| (Name, Telephone, E-mail and/or Facsimile number and Address of Company Contact Person) |
| Securities registered or to be registered pursuant to Section 12(b) of the Act. | ||||
| Title of each class | Name of each exchange on which registered | |||
| American Depositary Shares, each representing 100 Ordinary Shares, par value £0.01 per share |
The Nasdaq Capital Market | |||
| Ordinary Shares, £0.01 par value per share* |
Securities registered or to be registered pursuant to Section 12(g) of the Act. None
Securities for which there is a reporting obligation pursuant to Section 15(d) of the Act. None
The number of outstanding shares of each of the issuer’s classes of capital or common stock as of the close of the period covered by the annual report: 1,580,693,413 Ordinary Shares, £0.01 par value per share
| * | Not for trading, but only in connection with the registration of American Depositary Shares representing such Ordinary Shares pursuant to the requirements of the Securities and Exchange Commission. |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
¨ Yes x No
If this report is an annual or transition report, indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934.
¨ Yes x No
Note – Checking the box above will not relieve any registrant required to file reports pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 from their obligations under those Sections.
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
x Yes ¨ No
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
x Yes ¨ No
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or an emerging growth company. See definition of “accelerated filer,” “large accelerated filer,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer x |
| Emerging growth company ¨ |
If an emerging growth company that prepares its financial statements in accordance with U.S. GAAP, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Ϯ The term “new or revised financial accounting standard” refers to any update issued by the Financial Accounting Standards Board to its Accounting Standards Codification after April 5, 2012.
Indicate by check mark which basis of accounting the registrant has used to prepare the financial statements included in this filing:
| U.S. GAAP x | International Financial Reporting Standards as issued by the International Accounting Standards Board ¨ | Other ¨ |
If “Other” has been checked in response to the previous question, indicate by check mark which financial statement item the registrant has elected to follow.
¨ Item 17 ¨ Item 18
If this is an annual report, indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
¨ Yes x No
(APPLICABLE ONLY TO ISSUERS INVOLVED IN BANKRUPTCY PROCEEDINGS DURING THE PAST FIVE YEARS)
Indicate by check mark whether the registrant has filed all documents and reports required to be filed by Sections 12, 13 or 15(d) of the Securities Exchange Act of 1934 subsequent to the distribution of securities under a plan confirmed by a court.
¨ Yes ¨ No
TABLE OF CONTENTS
INTRODUCTION
Unless the context otherwise requires, all references to “Akari,” “we,” “us,” “our,” the “Company” and similar designations refer to Akari Therapeutics, PLC and its subsidiaries.
Market data and certain industry data and forecasts used throughout this Annual Report on Form 20-F were obtained from sources we believe to be reliable, including market research databases, publicly available information, reports of governmental agencies, and industry publications and surveys. We have relied on certain data from third-party sources, including internal surveys, industry forecasts, and market research, which we believe to be reliable based on our management's knowledge of the industry. While we are not aware of any misstatements regarding the industry data presented in this Annual Report, our estimates involve risks and uncertainties and are subject to change based on various factors, including those discussed under the heading "Risk Factors" and elsewhere in this prospectus.
All trademarks, service marks, trade names and registered marks used in this report are trademarks, trade names or registered marks of their respective owners.
Statements made in this Annual Report on Form 20-F concerning the contents of any agreement, contract or other document are summaries of such agreements, contracts or documents and are not complete description of all of their terms. If we filed any of these agreements, contracts or documents as exhibits to this Report or to any previous filing with the Securities and Exchange Commission, or SEC, you may read the document itself for a complete understanding of its terms.
This Annual Report on Form 20-F contains forward-looking statements. All statements other than statements of historical facts contained in this Annual Report on Form 20-F, including statements regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements.
Words such as “may,” “anticipate,” “estimate,” “expects,” “projects,” “intends,” “plans,” “believes” and words and terms of similar substance used in connection with any discussion of future operating or financial performance, identify forward-looking statements. Forward-looking statements represent management’s present judgment regarding future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially from those described in the forward-looking statements.
Such risks and uncertainties include, but are not limited to:
| · | our needs for additional capital to fund our operations and our inability to obtain additional capital on acceptable terms, or at all; |
| · | our ability to continue as a going concern; |
| · | uncertainties of cash flows and inability to meet working capital needs; |
| · | an inability or delay in obtaining required regulatory approvals for Nomacopan (Coversin) and any other product candidates, which may result in unexpected cost expenditures; |
| · | our ability to obtain orphan drug designation in additional indications; |
| · | risks inherent in drug development in general; |
| · | uncertainties in obtaining successful clinical results for Nomacopan (Coversin) and any other product candidates and unexpected costs that may result therefrom; |
| 1 |
| · | difficulties enrolling patients in our clinical trials; |
| · | failure to realize any value of Nomacopan (Coversin) and any other product candidates developed and being developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; |
| · | inability to develop new product candidates and support existing product candidates; |
| · | the approval by the FDA and EMA and any other similar foreign regulatory authorities of other competing or superior products brought to market; |
| · | risks resulting from unforeseen side effects; |
| · | risk that the market for Nomacopan (Coversin) may not be as large as expected; |
| · | risks associated with the departure of our former Chief Executive Officers and other executive officers; |
| · | risks associated with the SEC investigation; |
| · | inability to obtain, maintain and enforce patents and other intellectual property rights or the unexpected costs associated with such enforcement or litigation; |
| · | inability to obtain and maintain commercial manufacturing arrangements with third party manufacturers or establish commercial scale manufacturing capabilities; |
| · | the inability to timely source adequate supply of our active pharmaceutical ingredients from third party manufacturers on whom the company depends; and |
| · | unexpected cost increases and pricing pressures. |
In light of these assumptions, risks and uncertainties, the results and events discussed in the forward-looking statements contained in this Annual Report on Form 20-F might not occur. We are not under any obligation, and we expressly disclaim any obligation, to update or alter any forward-looking statements, whether as a result of new information, future events or otherwise. All subsequent forward-looking statements attributable to us or to any person acting on our behalf are expressly qualified in their entirety by the cautionary statements contained or referred to in this section.
We have obtained the statistical data, market data and other industry data and forecasts used throughout this Annual Report on Form 20-F from publicly available information. We have not sought the consent of the sources to refer to the publicly available reports in this Annual Report on Form 20-F.
You should read this Annual Report on Form 20-F and the documents that we have filed as exhibits to the Annual Report on Form 20-F with the understanding that our actual future results may be materially different from what we expect. We do not assume any obligation to update any forward-looking statements whether as a result of new information, future events or otherwise, except as required by applicable law.
| ITEM 1. | IDENTITY OF DIRECTORS, SENIOR MANAGEMENT AND ADVISERS |
Not applicable.
| 2 |
| ITEM 2. | OFFER STATISTICS AND EXPECTED TIMETABLE |
Not applicable.
| ITEM 3. | KEY INFORMATION |
| A. | Selected Financial Data |
The following selected financial data should be read in conjunction with “Item 5 Operating and Financial Review and Prospects” and the Financial Statements and Notes thereto included elsewhere in this Annual Report. The selected consolidated financial data in this section is not intended to replace the consolidated financial statements and is qualified in its entirety thereby.
We have derived the balance sheet data as of December 31, 2018 and 2017 and the consolidated statement of operations data for the years ended December 31, 2018, 2017 and 2016 from our audited financial statements included elsewhere in this Annual Report. We have derived the balance sheet data as of December 31, 2016, 2015 and 2014 and the consolidated statement of operations data for the year ended December 31, 2015 and 2014 from our audited financial statements not included in this Annual Report. Our financial statements have been prepared in accordance with US GAAP.
Certain factors that affect the comparability of the information set forth in the following table are described in Item 5 “Operating and Financial Review and Prospects” and the Consolidated Financial Statements and related notes thereto included elsewhere in this Annual Report.
| BALANCE SHEET DATA | As of December 31, | |||||||||||||||||||
| (In United States Dollars) | 2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||
| Total current assets | $ | 7,454,322 | $ | 28,813,086 | $ | 45,633,781 | $ | 69,658,487 | $ | 3,335,249 | ||||||||||
| Total assets | 8,029,554 | 29,050,343 | 45,873,678 | 69,893,562 | 3,394,666 | |||||||||||||||
| Total current liabilities | 4,918,267 | 11,848,369 | 11,714,768 | 21,124,968 | 1,171,368 | |||||||||||||||
| Total liabilities | 4,918,267 | 11,896,372 | 11,771,128 | 21,174,037 | 1,171,368 | |||||||||||||||
| Capital stock | 23,651,277 | 22,927,534 | 18,340,894 | 18,340,894 | 11,210,804 | |||||||||||||||
| Shareholders’ equity | 3,111,287 | 17,153,971 | 34,102,550 | 48,719,525 | 2,223,298 | |||||||||||||||
| STATEMENT OF OPERATIONS DATA | ||||||||||||||||||||
| (In United States Dollars) | For the Year Ended December 31, | |||||||||||||||||||
| 2018 | 2017 | 2016 | 2015 | 2014 | ||||||||||||||||
| Research and development | $ | 11,795,376 | $ | 23,285,279 | $ | 17,306,001 | $ | 5,799,076 | $ | 1,616,204 | ||||||||||
| General and administrative | 10,896,158 | 11,798,910 | 9,940,557 | 5,502,214 | 303,095 | |||||||||||||||
| Litigation settlement (gain) loss | (2,700,000 | ) | 2,700,000 | - | - | - | ||||||||||||||
| Excess consideration | - | - | - | 19,293,280 | - | |||||||||||||||
| Total operating expenses | 19,991,534 | 37,784,189 | 27,246,558 | 30,584,570 | 1,919,299 | |||||||||||||||
| Other expense (income) | (3,524,754 | ) | (2,384,932 | ) | (9,105,561 | ) | 14,732,962 | 28,254 | ||||||||||||
| Net loss | (16,466,780 | ) | (35,399,257 | ) | (18,140,997 | ) | (45,317,532 | ) | (1,947,553 | ) | ||||||||||
| Net basic and diluted loss per ordinary share | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.02 | ) | $ | (0.05 | ) | $ | (0.02 | ) | |||||
| Weighted average number of ordinary shares (basic and diluted) | 1,540,309,840 | 1,247,293,388 | 1,177,693,383 | 852,088,530 | 85,515,588 | |||||||||||||||
| OTHER FINANCIAL DATA | For the Year Ended December 31, | |||||||||||||||||||
| (In United States Dollars) | 2018 | 2017 | 2016 | 2015 | 2014 | |||||||||||||||
| Net cash used in operating activities | $ | (22,580,240 | ) | $ | (31,598,708 | ) | $ | (24,624,535 | ) | $ | (4,965,583 | ) | $ | (1,476,824 | ) | |||||
| Net cash provided by (used in) investing activities | - | 9,985,078 | (10,066,632 | ) | 1,391,798 | - | ||||||||||||||
| Net cash provided by financial activities | 306,232 | 15,671,881 | - | 69,044,419 | 4,235,376 | |||||||||||||||
| 3 |
| B. | Capitalization and Indebtedness |
Not applicable.
| C. | Reasons for the Offer and Use of Proceeds |
Not applicable.
| D. | Risk Factors |
Except for the historical information contained herein or incorporated by reference, this Annual Report on Form 20-F and the information incorporated by reference contains forward-looking statements that involve risks and uncertainties. These statements include projections about our accounting and finances, plans and objectives for the future, future operating and economic performance and other statements regarding future performance. These statements are not guarantees of future performance or events. Our actual results could differ materially from those discussed in this Annual Report on Form 20-F. Factors that could cause or contribute to these differences include, but are not limited to, those discussed in the following section, as well as those discussed in Item 5 entitled “Operating and Financial Review and Prospects” and elsewhere throughout this Annual Report on Form 20-F and in any documents incorporated in this Annual Report on Form 20-F by reference.
You should consider carefully the following risk factors, together with all of the other information included or incorporated in this Annual Report on Form 20-F. If any of the following risks, either alone or taken together, or other risks not presently known to us or that we currently believe to not be significant, develop into actual events, then our business, financial condition, results of operations or prospects could be materially adversely affected. If that happens, the market price of our American Depositary Shares, or ADSs, could decline, and shareholders may lose all or part of their investment.
Risks Relating to Our Financial Position and Our Business
We have a history of operating losses and cannot give assurance of future revenues or operating profits; investors may lose their entire investment.
We do not expect to generate revenue or profitability that is necessary to finance our operations in the short term. We incurred net losses of $16,466,780 and $35,399,257 for the years ended December 31, 2018 and 2017, respectively. In addition, our accumulated deficit as of December 31, 2018 and 2017 was $126,803,647 and $110,336,867, respectively. Losses have principally resulted from costs incurred for manufacturing, clinical trial and preclinical activities and general and administrative expenses. We have funded our operations primarily through the private placement and public offering of equity securities. As of December 31, 2018, we had cash of $5,446,138.
To date, we have not commercialized any products or generated any revenues from the sale of products, and absent the realization of sufficient revenues from product sales, we may never attain profitability in the future. We expect to incur significant losses for the foreseeable future as we continue to conduct research and development, clinical testing, regulatory compliance activities and, if Nomacopan (Coversin) or other future product candidates receive regulatory approval, sales and marketing activities.
Our failure to become and remain profitable would depress the market price of the ADSs and could impair our ability to raise capital, expand our business, diversify our product offerings or continue our operations. If we continue to suffer losses as we have in the past, investors may not receive any return on their investment and may lose their entire investment.
| 4 |
Our auditor’s report on our financial statements states that our recurring operating losses, negative cash flows and dependence on additional financial support raises substantial doubt about our ability to continue as a going concern, which may have a detrimental effect on our ability to obtain additional funding.
The report of our U.S. independent registered public accounting firm on our financial statements for the period ended December 31, 2018, includes an explanatory paragraph raising substantial doubt about our ability to continue as a going concern as a result of our recurring losses from operations and net capital deficiency. Our future is dependent upon our ability to obtain financing in the future. This opinion could materially limit our ability to raise funds. If we fail to raise sufficient capital when needed, we will not be able to complete our business plan. As a result we may have to liquidate our business and you may lose your investment in our ADSs.
We will require additional capital to fund our operations, and if we are unable to obtain such capital, we will be unable to successfully develop and commercialize any product candidates.
As of December 31, 2018, we had cash of $5,446,138. On September 26, 2018, we entered into a securities purchase agreement, or Purchase Agreement, with Aspire Capital Fund, LLC, or Aspire Capital which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $19.3 million of our ADSs. See “Item 5B. Operating and Financial Review and Prospects — Liquidity and Capital Resources — Aspire Capital Financing Arrangement”. We will require additional capital in order to develop and commercialize our current product candidates or any product candidates that we acquire, if any. There can be no assurance that additional funds will be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may be required to terminate or delay development for one or more of our product candidates.
The extent to which we utilize the Purchase Agreement with Aspire Capital as a source of funding will depend on a number of factors, including the prevailing market price of our ADSs, the volume of trading in our ADSs and the extent to which we are able to secure funds from other sources. The number of ADSs that we may sell to Aspire Capital under the Purchase Agreement on any given day and during the term of the agreement is limited. Additionally, we and Aspire Capital may not effect any sales of ADSs under the Purchase Agreement during the continuance of an event of default or on any trading day that the closing sale price of our ADSs is less than $0.25 per share.
The amount and timing of any expenditure needed will depend on numerous factors, some of which are outside our control, including:
| · | the type, number, scope, progress, expansion costs, results of and timing of our ongoing or future clinical trials or the need for additional clinical trials of Nomacopan (Coversin) bullous pemphigoid, or BP, atopic keratoconjunctivitis, or AKC, thrombotic microangiopathy, or TMA, paroxysmal nocturnal hemoglobinuria, or PNH, or any other indications or product candidates which we are pursuing or may choose to pursue in the future; |
| · | the costs of obtaining, maintaining and enforcing our patents and other intellectual property rights; |
| · | the costs and timing of obtaining or maintaining manufacturing for Nomacopan (Coversin) for BP, AKC, TMA, PNH or any other indications or product candidates, including commercial manufacturing if any product candidate is approved; |
| · | the costs and timing of establishing sales marketing, and reimbursement capabilities and enhanced internal controls over financial reporting; |
| · | the terms and timing of establishing and maintaining collaborations, license agreements and other partnerships; |
| · | costs associated with any new product candidates that we may develop, in-license or acquire; |
| · | the effect of competing technological and market developments; and |
| · | the costs associated with being a public company. |
We have not sold any products, and we do not expect to sell or derive revenue from any product sales for the foreseeable future. We may seek additional funding through future debt and equity financing, as well as potential additional collaborations or strategic partnerships with other companies or through non-dilutive financings. Additional funding may not be available to us on acceptable terms or at all. General market conditions may make it very difficult for us to seek financing from the capital markets and our SEC investigation could impact the availability or cost of future financings. We may be required to relinquish rights to our technologies or product candidates, or grant licenses on terms that are not favorable to us, in order to raise additional funds through alliance, joint venture or licensing arrangements. In addition, the terms of any financing may adversely affect the holdings or the rights of our shareholders and the issuance of additional shares by us, or the possibility of such issuance, may cause the market price of our shares to decline.
| 5 |
If we are unable to obtain funding on a timely basis, we will be unable to complete ongoing and planned clinical trials for Nomacopan (Coversin) and we may be required to significantly curtail some or all of our activities. We also could be required to seek funds through arrangements with collaborative partners or otherwise that may require us to relinquish rights to our product candidates or some of our technologies or otherwise agree to terms unfavorable to us.
Future sales and issuances of the ADSs or rights to purchase ADSs and any equity financing that we pursue, could result in significant dilution of the percentage ownership of our shareholders and could cause our ADS price to fall.
We may seek additional capital through a combination of private and public equity offerings, debt financings or strategic partnerships licensing arrangements. In any financing transaction, we may sell ordinary shares or ADSs, convertible securities or other equity securities. To the extent that we raise additional funds by issuing equity securities (including up to $19.3 million of ADSs to Aspire Capital Fund, LLC, or Aspire Capital, pursuant to our financing arrangement with Aspire Capital), our shareholders may experience significant dilution. See “Item 5B. Operating and Financial Review and Prospects — Liquidity and Capital Resources — Aspire Capital Financing Arrangement”. To the extent that we raise additional capital through sales of ADSs to Aspire Capital, or the sale of equity or convertible debt securities by any other means, existing ownership interests will be diluted. The sale of a substantial number of ADSs by Aspire Capital, or anticipation of such sales, could cause the trading price of our ADSs to decline or make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise desire. However, we have the right to control the timing and amount of sales of ADSs to Aspire Capital, and we may terminate the financing arrangement at any time, at our discretion, without any penalty or cost to us.
Risks Related to the Clinical Development and Regulatory Approval of Our Product Candidates
Our business depends on the success of Nomacopan (Coversin), which is still under development. If we are unable to obtain regulatory approval for or successfully commercialize Nomacopan (Coversin), our business will be materially harmed.
Nomacopan (Coversin) has been the sole focus of our product development. Successful continued development and ultimate regulatory approval of Nomacopan (Coversin) for at least one autoimmune disease is critical to the future success of our business. We have invested, and will continue to invest, a significant portion of our time and financial resources in the development of Nomacopan (Coversin). We will need to raise sufficient funds for, and successfully enroll and complete, our ongoing clinical development program for Nomacopan (Coversin) and for our planned clinical development program for Nomacopan (Coversin) in other indications. The future regulatory and commercial success of this product candidate is subject to a number of risks, including the following:
| · | we may not have sufficient financial and other resources to complete the necessary clinical trials for Nomacopan (Coversin); |
| · | we may not be able to obtain adequate evidence of efficacy and safety for Nomacopan (Coversin); |
| · | we do not know the degree to which Nomacopan (Coversin) will be accepted as a therapy, even if approved; |
| · | in our clinical programs, we may experience difficulty in enrollment, variability in patients, adjustments to clinical trial procedures and the need for additional clinical trial sites, which could delay our clinical trial progress; |
| · | our reliance on a sole manufacturer to supply the drug product formulation of Nomacopan (Coversin) that is being used in our clinical trials; |
| · | the results of our clinical trials may not meet the level of statistical or clinical significance required by the FDA, EMA or comparable foreign regulatory bodies for marketing approval; |
| · | patients in our clinical trials may die or suffer other adverse effects for reasons that may or may not be related to Nomacopan (Coversin), which could delay or prevent further clinical development; |
| 6 |
| · | the standards implemented by clinical or regulatory agencies may change at any time; |
| · | the FDA, EMA or foreign clinical or regulatory agencies may require efficacy endpoints for a clinical trial for the treatment of BP, AKC, TMA-HSCT, or PNH that differ from the endpoints of our planned current or future trials, which may require us to conduct additional clinical trials; |
| · | the mechanism of action of Nomacopan (Coversin) is complex and we do not know the degree to which it will translate into a medical benefit in certain indications; |
| · | our intellectual property rights may not be patentable, valid or enforceable; and |
| · | we may not be able to obtain, maintain or enforce our patents and other intellectual property rights. |
Of the large number of drugs in development in the pharmaceutical industry, only a small percentage results in the submission of a new drug application, or NDA, to the FDA, or a marketing authorization application, or MAA, to the EMA and even fewer are approved for commercialization. Furthermore, even if we do receive regulatory approval to market Nomacopan (Coversin), any such approval may be subject to limitations on the indicated uses or patient populations for which we may market the product. Accordingly, even if we are able to obtain the requisite financing to continue to fund our development programs, we cannot assure you that Nomacopan (Coversin) will be successfully developed or commercialized. If we or any of our future development partners are unable to develop, or obtain regulatory approval for, or, if approved, successfully commercialize Nomacopan (Coversin), we may not be able to generate sufficient revenue to continue our business.
If we encounter difficulties enrolling patients in our clinical trials, our clinical development activities could be delayed or otherwise adversely affected.
We may not be able to initiate or continue clinical trials required by the FDA, EMA or other foreign regulatory agencies for Nomacopan (Coversin) if we are unable to locate and enroll a sufficient number of eligible patients to participate in these clinical trials. We will be required to identify and enroll a sufficient number of patients with BP, AKC, TMA, PNH and other rare and orphan autoimmune and inflammatory diseases for each of our ongoing and planned clinical trials of Nomacopan (Coversin) in these indications. To date, we have experienced delays in enrollment of patients in our clinical trials due in particular to the fact that we are targeting a small patient population with a rare disease or indication.
Patient enrollment is affected by other factors, including:
| · | severity of the disease under investigation; |
| · | design of the clinical trial protocol; |
| · | size and nature of the patient population; |
| · | eligibility criteria for the trial in question; |
| · | perceived risks and benefits of the product candidate under trial; |
| · | proximity and availability of clinical trial sites for prospective patients; |
| · | availability of competing therapies and clinical trials; |
| · | clinicians’ and patients’ perceptions as to the potential advantages of the drug being studied in relation to other available therapies, including any new drugs that may be approved for the indications we are investigating; |
| 7 |
| · | efforts to facilitate timely enrollment in clinical trials; |
| · | patient referral practices of physicians; and |
| · | our ability to monitor patients adequately during and after treatment. |
Further, there are only a limited number of specialist physicians that treat patients with these diseases. We also may encounter difficulties in identifying and enrolling such patients with a stage of disease appropriate for our ongoing or future clinical trials. In addition, the process of finding and diagnosing patients may prove costly. Our inability to enroll a sufficient number of patients for any of our clinical trials would result in significant delays or may require us to abandon one or more clinical trials.
If clinical trials or regulatory approval processes for Nomacopan (Coversin) are prolonged, delayed or suspended, we may be unable to commercialize Nomacopan (Coversin) on a timely basis.
We cannot predict whether we will encounter problems with any of our completed, ongoing or planned clinical trials that will cause us, or any regulatory authority, to delay or suspend those clinical trials or delay the completion of our ongoing and planned clinical trials and negatively impact our ability to obtain regulatory approval for, and to market and sell, a particular product candidate, including:
| · | conditions imposed on us by the FDA, EMA or another foreign regulatory authority regarding the scope or design of our clinical trials; |
| · | insufficient supply of our product candidates or other materials necessary to conduct and complete our clinical trials; |
| · | slow enrollment and retention rate of subjects in our clinical trials; and |
| · | serious and unexpected drug-related side effects related to the product candidate being tested. |
Commercialization may be delayed by the imposition of additional conditions on our clinical trials by the FDA, EMA or any other applicable foreign regulatory authority or the requirement of additional supportive studies by the FDA, EMA or such foreign regulatory authority.
We do not know whether our clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, if at all. However, to date, we have encountered delays in enrollment in our clinical trials. Delays in our clinical trials will result in increased development costs for our product candidates, and our financial resources may be insufficient to fund any incremental costs. In addition, if our clinical trials are delayed, our competitors may be able to bring products to market before we do and the commercial viability of our product candidates could be limited.
The efficacy of Nomacopan (Coversin) may not be known until advanced stages of testing, after we have incurred significant product development costs which may not be recoverable.
Nomacopan (Coversin) may fail to show the desired safety and efficacy at any phase in the clinical development program. Good efficacy in animal models of the target indication are no guarantee of success in human clinical trials. Often there is no adequate animal model of a human disease, such as PNH. As a result, the first definitive proof of efficacy may not occur until clinical trials in humans. In our Phase II PNH trial, while we determined that overall the trial met the primary endpoint, individually two of the seven patients who completed the trial did not meet the primary endpoint (which was assessed at day 28) and an additional patient who withdrew from the trial on day 43 also did not meet the primary endpoint. If Nomacopan (Coversin) does not demonstrate adequate efficacy, its development may be delayed or terminated, which could have a material adverse effect on our financial condition and results of operation.
| 8 |
Results of earlier preclinical studies or clinical trials may not be predictive of advancement to the next phase of development.
Completion of preclinical studies or clinical trials does not guarantee that we will initiate additional studies or trials for our product candidates. If further studies or trials are initiated, earlier preclinical studies or clinical trial may not predict the scope and phase of further trials, that these further studies or trials will be completed, or that if these further studies or trials are completed, that the design or results will provide a sufficient basis to apply for or receive regulatory approvals or to commercialize products. Results of clinical trials could be inconclusive, requiring additional or repeat trials. Data obtained from preclinical studies and clinical trials is subject to varying interpretations that could delay, limit or prevent regulatory approval. If the design or results achieved in our clinical trials are insufficient to proceed to further trials or to regulatory approval of our product candidates, we could be materially adversely affected. Failure of a clinical trial to achieve its pre-specified primary endpoint generally increases the likelihood that additional studies or trials will be required if we determine to continue development of the product candidate, reduces the likelihood of timely development of and regulatory approval to market the product candidate, and may decrease the chances for successfully achieving the primary endpoint in scientifically similar indications.
The route of administration or dose for Nomacopan (Coversin) may be inadequate.
Unsatisfactory drug availability due to problems relating to the route of administration or the target tissue availability of the drug is another potential cause of lack of efficacy of Nomacopan (Coversin), if and when it is commercialized. Complement component C5, the target of Nomacopan (Coversin), is predominantly found in blood. For AKC, BP, TMA-HSCT and PNH, Nomacopan (Coversin) is being administered subcutaneously. For AKC, Nomacopan (Coversin) is being administered topically which may also prove to be unfeasible which could delay commercialization of Nomacopan (Coversin) and result in significant additional costs to us.
Long-term animal toxicity and long-term human safety studies of Nomacopan (Coversin) could result in adverse results.
While we have conducted toxicity studies in certain animals with no observed adverse effect at the highest dose tested, we intend to conduct further long-term animal toxicity studies, including reproductive and carcinogenicity studies, and are currently conducting a long-term human safety study of Nomacopan (Coversin). Such tests may show that Nomacopan (Coversin) results in serious adverse events, is not as effective as we expected, or other adverse results. If animal toxicity and human safety tests do not yield favorable results, we may be required to abandon our development of Nomacopan (Coversin), which could have a material adverse effect on our financial condition and results of operation.
Chronic dosing of patients with Nomacopan (Coversin) could lead to an immune response that causes adverse reactions or impairs the activity of the drug.
There is a risk that chronic dosing of patients with Nomacopan (Coversin) may lead to an immune response that causes adverse reactions or impairs the activity of the drug. Patients may develop an allergic reaction to the drug and/or develop antibodies directed at the drug. Impaired drug activity could be caused by neutralization of the drug’s inhibitory activity or by an increased rate of clearance of the drug from circulation.
One potential toxic side effect of Nomacopan (Coversin) that has occurred in patients receiving Soliris® (eculizumab), a humanized antibody against complement component C5, may include the inhibition of the terminal complement system, which can result in an increased incidence of meningitis. As a result, we expect that patients receiving Nomacopan (Coversin) would also receive meningitis immunization and prophylactic antibiotics as indicated.
Nomacopan (Coversin) has a secondary binding site that sequesters LTB4. LTB4 synthesis from eicosanoid fatty acids can be induced by a variety of triggers including complement. LTB4 is a pro-inflammatory mediator which attracts and activates white blood cells at the area of inflammation. LTB4 inhibition may lead to positive anti-inflammatory benefits, but another potential cause of undesired side effects is that the reduction of these neutrophil attractant properties may include increased risk of infection, among others.
Any immune response that causes adverse reactions or impairs the activity of the drug could cause a delay in or termination of our development of Nomacopan (Coversin), which would have a material adverse effect on our financial condition and results of operation.
| 9 |
If Nomacopan (Coversin) is not convenient for patients to use, then potential sales may decrease materially.
Nomacopan (Coversin) may require refrigerated storage prior to use and will likely require self-injection. If the drug product is not stable at temperatures of between four and eight degrees Celsius, then the drug product may need to be defrosted before use, which patients could view as inconvenient, causing sales to decrease. In addition, if Nomacopan (Coversin) shows a lack of long-term stability at low storage temperatures, this may negatively impact our ability to manage the commercial supply chain, which could result in us having to refund customers or replace products that are unstable, which could materially increase our costs and have a material adverse effect on our financial condition and results of operation.
Because Nomacopan (Coversin) has not yet received regulatory approval, it is difficult to predict the time and cost of development and our ability to successfully complete clinical development and obtain the necessary regulatory approvals for commercialization.
Nomacopan (Coversin) has not yet received regulatory approval for the treatment of any indications, and unexpected problems may arise that could cause us to delay, suspend or terminate our development efforts. To date, only a limited number of patients have been enrolled in our clinical trials. Larger scale trials will be required to obtain regulatory approval and the efficacy or non-efficacy of Nomacopan (Coversin) will ultimately be determined by the applicable regulatory agencies. The long-term safety consequences of inhibition of C5 and/or LTB4 with Nomacopan (Coversin) is not known. Regulatory approval of product candidates such as Nomacopan (Coversin) can be more expensive and take longer than approval for candidates for the treatment of more well understood diseases with previously approved products.
We have obtained orphan drug status for Nomacopan (Coversin) in Guillain-Barré syndrome, or GBS, and PNH both in the United States and the EU, but we may be unable to maintain the benefits associated with orphan drug status, including market exclusivity.
In the United States, orphan drug designation entitles a party to financial incentives such as opportunities for grant funding towards clinical trial costs, tax advantages, and user-fee waivers. After the FDA grants orphan drug designation, the generic identity of the drug and its potential orphan use are disclosed publicly by the FDA. Although we have received orphan drug designation for Nomacopan (Coversin) in GBS and PNH and intend to seek orphan product designation for Nomacopan (Coversin) in further indications, we may never receive such additional designations and we are not currently pursuing a clinical development program targeting GBS.
If a product that has orphan drug designation subsequently receives the first FDA approval for a particular active ingredient for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications, including a biologics license application, or BLA, to market the same biologic for the same indication for seven years, except in limited circumstances such as a showing of clinical superiority to the product with orphan product exclusivity or if the FDA finds that the holder of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Even if we were to obtain orphan drug designation for Nomacopan (Coversin) for a particular indication, we may not be the first to obtain marketing approval for any particular orphan indication due to the uncertainties associated with developing pharmaceutical products. If we do obtain exclusive marketing rights in the United States, they may be limited if we seek approval for an indication broader than the orphan designated indication, and may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of the relevant patients. Further, exclusivity may not effectively protect the product from competition because different drugs with different active moieties can be approved for the same condition. Even after an orphan drug is approved, the FDA can subsequently approve a drug with the same active moiety for the same condition if the FDA concludes that the later drug is safer, more effective, or makes a major contribution to patient care. Furthermore, the FDA can waive orphan exclusivity if we are unable to manufacture sufficient supply of our product.
In the EU, where a marketing authorization in respect of an orphan medicinal product is granted, the Agency and the Member States shall not, for a period of 10 years, accept another application for a marketing authorization, or grant a marketing authorization or accept an application to extend an existing marketing authorization, for the same therapeutic indication, in respect of a similar medicinal product. A marketing authorization may be granted, for the same therapeutic indication, to a similar medicinal product if: (i) the holder of the marketing authorization for the original orphan medicinal product has given his consent to the second applicant, or; (ii) the holder of the marketing authorization for the original orphan medicinal product is unable to supply sufficient quantities of the medicinal product, or; (iii) the second applicant can establish in the application that the second medicinal product, although similar to the orphan medicinal product already authorized, is safer, more effective or otherwise clinically superior.
| 10 |
The receipt of orphan drug designation status does not change the regulatory requirements or process for obtaining marketing approval and designation does not mean that marketing approval will be received.
We may seek a breakthrough therapy designation from the FDA for Nomacopan (Coversin). Such designation or a similar designation from other national or international regulatory agencies, may not lead to a faster development or regulatory review or approval process, and it does not increase the likelihood that Nomacopan (Coversin) or any other product candidates will receive marketing approval.
We may seek a breakthrough therapy designation for Nomacopan (Coversin). A breakthrough therapy is defined as a product that is intended, alone or in combination with one or more other drugs, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. Designation as a breakthrough therapy is within the discretion of the FDA. Receipt of a breakthrough therapy designation for Nomacopan (Coversin) may not result in a faster development process, review or approval compared to products considered for approval under conventional FDA procedures and does not assure ultimate approval by the FDA. In addition, even if Nomacopan (Coversin) qualifies as a breakthrough therapy, the FDA may later decide that it no longer meets the conditions for qualification.
Even if we obtain FDA approval of Nomacopan (Coversin), we or our partners may never obtain approval or commercialize our product candidates outside of the United States and, conversely, even if we obtain regulatory approval of Nomacopan (Coversin) in the EU, we or our partners may never obtain approval or commercialize our product candidates outside the EU.
In order to market any products in a country, we must establish and comply with numerous and varying regulatory requirements of other countries regarding clinical trial design, safety and efficacy. Clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not mean that regulatory approval will be obtained in any other country. Approval procedures vary among countries and can involve additional product testing and validation and additional administrative review periods. Seeking regulatory approvals in other countries could result in significant delays, difficulties and costs for us, and may require additional preclinical studies or clinical trials which would be costly and time consuming and could delay or prevent introduction of Nomacopan (Coversin) in those countries. We rely on contract research organizations for experience in obtaining regulatory approval in international markets. If we or our partners fail to comply with regulatory requirements or to obtain and maintain required approvals, our target market will be reduced and our ability to realize the full market potential of Nomacopan (Coversin) will be harmed.
If we or our partners market products in a manner that violates fraud and abuse and other healthcare laws, or if we or they violate government price reporting laws, we or our partners may be subject to administrative civil and/or criminal penalties.
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare laws, including those commonly referred to as “fraud and abuse” laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws include, among others, false claims and anti-kickback statutes. At such time, if ever, as we or any of our partners market any of our future approved products, it is possible that some of the business activities of us and/or our partners could be subject to challenge under one or more of these laws.
Federal false claims, false statements and civil monetary penalties laws prohibit any person from knowingly presenting, or causing to be presented, a false claim for payment to the federal government or to get a false claim paid. The federal healthcare program anti-kickback statute prohibits, among other things, knowingly and willfully offering, paying, soliciting or receiving remuneration to induce, or in return for, purchasing, leasing, ordering or arranging for the purchase, lease or order of any healthcare item or service reimbursable under Medicare, Medicaid or other federally financed healthcare programs. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. Although there are several statutory exceptions and regulatory safe harbors protecting certain common activities from prosecution, they are drawn narrowly, and practices that involve remuneration intended to induce prescribing, purchasing or recommending may be subject to scrutiny if they do not qualify for an exception or safe harbor.
| 11 |
In addition, we and/or our partners may be subject to data privacy and security regulation, including the Health Insurance Portability and Accountability Act of 1996, or HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, or HITECH, and their respective implementing regulations, which impose specified requirements relating to the privacy, security and transmission of individually identifiable health information.
Most states also have statutes or regulations similar to these federal laws, which may apply to items such as pharmaceutical products and services reimbursed by private insurers. We and/or our partners may be subject to administrative, civil and criminal sanctions for violations of any of these federal and state laws.
Our employees, principal investigators, consultants, commercial partners or vendors may engage in misconduct or other improper activities, including non-compliance with regulatory standards.
We are also exposed to the risk of employees, independent contractors, principal investigators, consultants, commercial partners or vendors engaging in fraud or other misconduct. Misconduct by employees, independent contractors, principal investigators, consultants, commercial partners and vendors could include intentional failures to comply with European Union, or EU, regulations, to provide accurate information to the EMA or EU Member States authorities or to comply with manufacturing or quality standards we have or will have established. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices such as promotion of products by medical practitioners. Of general application are the European Anti-Fraud Office Regulation 883/2013, and the UK Bribery Act 2010. Under the latter, a commercial organization can be guilty of the offence if the bribery is carried out by an employee, agent, subsidiary, or another third-party, and the location of the third-party is irrelevant to the prosecution. The advertising of medicinal products in the EU is regulated by Title VIII of European Directive 2001/83/EC. The corresponding UK implementing legislation is Part 14 of the Human Medicines Regulations 2012 (S.I. 2012/1916 as amended). Such laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Misconduct could also involve the improper use of information obtained in the course of clinical studies, which could result in regulatory sanctions and serious and irreparable harm to our reputation.
This could also apply with respect to data privacy. In the EU, on May 25, 2018 the EU Directive 95/46/EEC and Data Protection Act 1998 were replaced by the General Data Protection Regulation (EU) 2016/679, or GDPR, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data and, in the UK, the Data Protection Act 2018. The GDPR applies directly in all Member States (including the UK) from May 25, 2018 and applies to companies with an establishment in the EEA and to certain other companies not in the EEA that offer or provide goods or services to individuals located in the EEA or monitor the behavior of individuals located in the EEA. The GDPR implements more stringent operational requirements for controllers of personal data, including, for example, expanded disclosures about how personal information is to be used, limitations on retention of information, increased requirements pertaining to health data and pseudonymized (i.e., key-coded) data, increased cyber security requirements, mandatory data breach notification requirements and higher standards for controllers to demonstrate that they have obtained a valid legal basis for certain data processing activities. The activities of data processors are being regulated for the first time, and require companies undertaking processing activities to offer certain guarantees in relation to the security of such processing and the handling of personal data. Contracts with data processors will also need to be updated to include certain terms prescribed by the GDPR, and negotiating such updates may not be fully successful in all cases. The GDPR provides that EU Member States may make their own further laws and regulations in relation to the processing of genetic, biometric or health data, which could result in differences between Member States, limit our ability to use and share personal data or could cause our costs to increase, and harm our business and financial condition. We are also subject to evolving and strict rules on the transfer of personal data out of the European Union to the United States. Further prospective revision of the Directive on privacy and electronic communications (Directive 2002/58/EC), or ePrivacy Directive, may affect our marketing communications. Failure to comply with EU laws, including failure under the GDPR, Data Protection Act 2018, ePrivacy Directive and other laws relating to the security of personal data may result in fines up to €20,000,000 or up to 4% of the total worldwide annual turnover of the preceding financial year, if greater, and other administrative penalties including criminal liability, which may be onerous and adversely affect our business, financial condition, results of operations and prospects. Failure to comply with the GDPR and related laws may also give risk to increase risk of private actions from data subjects and consumer not-for-profit organizations, including a new form of class action that is available under the GDPR. Compliance with the GDPR requires a rigorous and time-intensive process that may increase our cost of doing business or require us to change our business practices, and despite those efforts, there is a risk that we may be subject to the aforementioned fines and penalties, litigation, and reputational harm in connection with any European activities. If no transitional arrangements are agreed by the UK government, the GDPR will no longer form part of UK law after the withdrawal of the UK from the European Union. On such event, the Data Protection Act 2018, will continue to apply and the extra-territorial aspects of the GDPR will apply but there may be an adverse effect on the ease to which personal data can be transferred from and to the remaining EU Member States. In particular, the two-way free flow of personal data currently in place between the UK and EEA countries will cease to apply if the UK leaves the EU without a withdrawal agreement that specifically provides for the continued flow of personal data.
| 12 |
It is not always possible to identify and deter misconduct by employees or other parties. The precautions we take to detect and prevent this activity may not protect us from legal or regulatory action resulting from a failure to comply with applicable laws or regulations. Misconduct by our employees, principal investigators, consultants, commercial partners or vendors could result in significant financial penalties, criminal sanctions and thus have a material adverse effect on our business, including through the imposition of significant fines or other sanctions, and our reputation.
Risks Related to our Intellectual Property
Our success depends on our ability to protect our intellectual property and our proprietary technologies.
Our commercial success depends in part on our ability to obtain and maintain patent protection and trade secret protection for our product candidates, proprietary technologies, and their uses as well as our ability to operate without infringing upon the proprietary rights of others. We can provide no assurance that our patent applications or those of our licensors will result in additional patents being issued or that issued patents will afford sufficient protection against competitors with similar technologies, nor can there be any assurance that the patents issued will not be infringed, designed around or invalidated by third parties. Even issued patents may later be found unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for our proprietary rights is uncertain. Only limited protection may be available and may not adequately protect our rights or permit us to gain or keep any competitive advantage. Composition-of-matter patents on the biological or chemical active pharmaceutical ingredients are generally considered to offer the strongest protection of intellectual property and provide the broadest scope of patent protection for pharmaceutical products, as such patents provide protection without regard to any method of use or any method of manufacturing. While we have issued composition-of-matter patents in the United States and other countries for Nomacopan (Coversin), we cannot be certain that the claims in our issued composition-of-matter patents will not be found invalid or unenforceable if challenged. We cannot be certain that the claims in any patent applications covering composition-of-matter or formulations of our product candidates that are pending, or that we may file, will be considered patentable by the United States Patent and Trademark Office, or USPTO, and courts in the United States or by the patent offices and courts in foreign countries, nor can we be certain that the claims in our issued composition-of-matter patents will not be found invalid or unenforceable if challenged. Even if any patent applications that we may file relating to specific formulations of our product candidates issue as patents, formulation patents protect a specific formulation of a product and may not be enforced against competitors making and marketing a product that has the same active pharmaceutical ingredient in a different formulation. Method-of-use patents protect the use of a product for the specified method or for treatment of a particular indication. This type of patents may not be enforced against competitors making and marketing a product that has the same active pharmaceutical ingredient but is used for a method not included in the patent. Moreover, even if competitors do not actively promote their product for our targeted indications, physicians may prescribe these products “off-label.” Although off-label prescriptions may infringe or contribute to the infringement of method-of-use patents, the practice is common and such infringement is difficult to prevent or prosecute.
Our issued patents for Nomacopan (Coversin) and its uses are expected to expire between 2024 and 2031 (excluding any patent term adjustment or potential patent term extension). Our pending patent applications for Nomacopan (Coversin) and its uses, if issued, are expected to expire at various times that range from 2024 to 2039 (excluding any potential patent term adjustment or extension).
The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that we or any of our future development partners will be successful in protecting our product candidates by obtaining and defending patents. These risks and uncertainties include the following:
| · | the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process. There are situations in which noncompliance can result in abandonment or lapse of a patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. In such an event, competitors might be able to enter the market earlier than would otherwise have been the case; |
| 13 |
| · | patent applications may not result in any patents being issued; |
| · | patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable or otherwise may not provide any competitive advantage; |
| · | our competitors, many of whom have substantially greater resources and many of whom have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or eliminate our ability to make, use, and sell our potential product candidates; |
| · | there may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns; and |
| · | countries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing product candidates. |
In addition, we rely on the protection of our trade secrets and proprietary know-how. Although we have taken steps to protect our trade secrets and unpatented know-how, including entering into confidentiality agreements with third parties, and confidential information and inventions agreements with employees, consultants and advisors, we cannot provide any assurances that all such agreements have been duly executed, and third parties may still obtain this information or may come upon this or similar information independently. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating its trade secrets. If any of these events occurs or if we otherwise lose protection for our trade secrets or proprietary know-how, our business may be harmed.
Others may claim an ownership interest in our intellectual property which could expose it to litigation and have a significant adverse effect on its prospects.
A third party may claim an ownership interest in one or more of our patents or other intellectual property. A third party could bring legal actions against us and seek monetary damages and/or enjoin clinical testing, manufacturing and marketing of the affected product or products. We cannot guarantee that a third-party will not assert a claim or an interest in any of such patents or intellectual property. If we become involved in any litigation, it could consume a substantial portion of our resources, and cause a significant diversion of effort by our technical and management personnel. If any of these actions are successful, in addition to any potential liability for damages, we could be required to obtain a license to continue to manufacture or market the affected product, in which case we may be required to pay substantial royalties or grant cross-licenses to our patents. We cannot, however, assure you that any such license will be available on acceptable terms, if at all. Ultimately, we could be prevented from commercializing a product, or be forced to cease some aspect of our business operations as a result of claims of patent infringement or violation of other IP rights, Further, the outcome of IP litigation is subject to uncertainties that cannot be adequately quantified in advance, including the demeanor and credibility of witnesses and the identity of the adverse party. This is especially true in IP cases that may turn on the testimony of experts as to technical facts upon which experts may reasonably disagree.
Risks Related to our Business Operations
We are under an investigation by the SEC, which could divert management’s focus, result in substantial investigation expenses, monetary fines and other possible remedies and have an adverse impact on our reputation and financial condition and results of operations.
We voluntarily reported to the SEC the circumstances leading to the withdrawal of the Edison Report and the outcome of our special committee’s investigation. In response, the SEC requested certain documents from us with respect to the matters we reported. We have been cooperating with the SEC’s requests for information. On June 5, 2018, we received a subpoena from the SEC which requested further documents and information primarily related to our Phase II PNH clinical trial of Nomacopan (Coversin). We are in the process of responding to the subpoena and will continue to cooperate with the SEC. We cannot predict what, if any, actions the SEC may take or the timing or duration of the investigation. If the SEC were to conclude that enforcement action is appropriate, we could be required to pay civil penalties and fines, and the SEC could impose other sanctions against us or against our current and former officers and directors. In addition, our board of directors, management and employees may expend a substantial amount of time on the SEC investigation, diverting resources and attention that would otherwise be directed toward our operations and implementation of our business strategy, all of which could materially adversely affect our business, financial condition, results of operations or cash flows. Furthermore, while the SEC has informed us that the investigation should not be construed as an indication by the SEC or its staff that any violation of law has occurred, nor as a reflection upon any person, entity or security, publicity surrounding the foregoing, or any SEC enforcement action or settlement as a result of the SEC’s investigation, even if ultimately resolved favorably for us, could have an adverse impact on our reputation, business, financial condition, results of operations or cash flows.
| 14 |
We currently have no marketing, sales or distribution infrastructure with respect to Nomacopan (Coversin). If we are unable to develop our sales, marketing and distribution capability on our own or through collaborations with marketing partners, we will not be successful in commercializing our product candidates.
We currently have no marketing, sales or distribution capabilities and have limited sales or marketing experience within our organization. If our product candidate Nomacopan (Coversin) is approved, we intend either to establish a sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize Nomacopan (Coversin), or to outsource this function to a third party. Either of these options would be expensive and time consuming. Some or all of these costs may be incurred in advance of any approval of Nomacopan (Coversin). In addition, we may not be able to hire a sales force in the United States or other target market that is sufficient in size or has adequate expertise in the medical markets that we intend to target. Any failure or delay in the development of our or third parties’ internal sales, marketing and distribution capabilities would adversely impact the commercialization of Nomacopan (Coversin) and other future product candidates.
With respect to our existing and future product candidates, we may choose to collaborate with third parties that have direct sales forces and established distribution systems, either to augment or to serve as an alternative to our own sales force and distribution systems. Any future product revenue may be lower than if it directly marketed or sold any approved products. In addition, any revenue we receive will depend in whole or in part upon the efforts of these third parties, which may not be successful and are generally not within our control. If we are unable to enter into these arrangements on acceptable terms or at all, we may not be able to successfully commercialize any approved products. If we are not successful in commercializing any approved products, our future product revenue will suffer and we may incur significant additional losses.
We only have a limited number of employees to manage and operate our business.
As of December 31, 2018, we had thirteen full-time employees and one full-time equivalent consultant. Our focus on the development of Nomacopan (Coversin) requires us to optimize cash utilization and to manage and operate our business in a highly efficient manner. We cannot assure you that we will be able to hire and/or retain adequate staffing levels to develop Nomacopan (Coversin) or run our operations and/or to accomplish all of the objectives that we otherwise would seek to accomplish.
Our industry is highly competitive, and our product candidates may become obsolete.
We are engaged in a rapidly evolving field. Competition from other pharmaceutical companies, biotechnology companies and research and academic institutions is intense and likely to increase. Many of those companies and institutions have substantially greater financial, technical and human resources than us. Those companies and institutions also have substantially greater experience in developing products, conducting clinical trials, obtaining regulatory approval and in manufacturing and marketing pharmaceutical products. Our competitors may succeed in obtaining regulatory approval for their products more rapidly than we do. Competitors have developed or are in the process of developing technologies that are, or in the future may be, the basis for competitive products, such as Alexion Pharmaceuticals’ Soliris ® (eculizumab). Our competitors may succeed in developing products that are more effective and/or cost competitive than those we are developing, or that would render our product candidates less competitive or even obsolete. In addition, one or more of our competitors may achieve product commercialization or patent protection earlier than us, which could materially adversely affect our business.
| 15 |
If the FDA or other applicable regulatory authorities approve generic products that compete with any of our or any of our partners’ product candidates, the sales of our product candidates would be adversely affected.
Once an NDA or marketing authorization application outside the United States is approved, the product covered thereby becomes a “listed drug” that can, in turn, be cited by potential competitors in support of approval of an abbreviated new drug application in the United States. Agency regulations and other applicable regulations and policies provide incentives to manufacturers to create modified, non-infringing versions of a drug to facilitate the approval of an abbreviated new drug application or other application for generic substitutes in the United States and in nearly every pharmaceutical market around the world. These generic equivalents, which must meet the same quality standards as branded pharmaceuticals, would be significantly less costly to bring to market than our product candidates, and companies that produce generic equivalents are generally able to offer their products at lower prices. Thus, after the introduction of a generic competitor, a significant percentage of the sales of any branded product is typically lost to the generic product. Accordingly, competition from generic equivalents to our or any of our partners’ future products, if any, could materially adversely impact our future revenue, profitability and financial condition.
If physicians and patients do not accept our future products or if the market for indications for which any product candidate is approved is smaller than expected, we may be unable to generate significant revenue, if any.
Even if any of our product candidates obtain regulatory approval, they may not gain market acceptance among physicians, patients, and third-party payers. Physicians may decide not to recommend its treatments for a variety of reasons including:
| · | timing of market introduction of competitive products; |
| · | demonstration of clinical safety and efficacy compared to other products; |
| · | cost-effectiveness; |
| · | limited or no coverage by third-party payers; |
| · | convenience and ease of administration; |
| · | prevalence and severity of adverse side effects; |
| · | restrictions in the label of the drug; |
| · | other potential advantages of alternative treatment methods; and |
| · | ineffective marketing and distribution support of any future products. |
If any of our product candidates are approved, but fail to achieve market acceptance or such market is smaller than anticipated, we may not be able to generate significant revenue and our business would suffer.
The uncertainty associated with pharmaceutical reimbursement and related matters may adversely affect our business.
Market acceptance and sales of any one or more of our product candidates will depend on reimbursement policies and may be affected by future healthcare reform measures in the United States and in foreign jurisdictions. Government authorities and third-party payers, such as private health insurers and health maintenance organizations, decide which drugs they will cover and establish payment levels. We cannot be certain that reimbursement will be available for any of our product candidates. Also, we cannot be certain that reimbursement policies will not reduce the demand for, or the price paid for, any future products. The insurance coverage and reimbursement status of newly-approved products for orphan diseases is particularly uncertain, and failure to obtain or maintain adequate coverage and reimbursement for Nomacopan (Coversin) or any other product candidates could limit our ability to generate revenue.
| 16 |
The United States and several foreign jurisdictions are considering, or have already enacted, a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell any future products profitably. There is significant interest in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access to healthcare. In the United States, the pharmaceutical industry has been a particular focus of these efforts and has been significantly affected by major legislative initiatives. We expect to experience pricing pressures in connection with the sale of any products that we develop due to the trend toward managed healthcare, increasing influence of health maintenance organizations and additional legislative proposals.
In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively, PPACA, became law in the United States. Since its enactment, there have been judicial and Congressional challenges to certain aspects of the PPACA. Congress and President Trump have expressed their intentions to repeal or repeal and replace the PPACA. President Trump issued an Executive Order and both chambers of Congress passed bills, all with the goal of fulfilling their intensions. However, to date, the Executive Order has had limited effect and the Congressional activities have not resulted in the passage of a law. If a law is enacted, many if not all of the provisions of the PPACA may no longer apply to prescription drugs. While we are unable to predict what changes may ultimately be enacted, to the extent that future changes affect how any future products are paid for and reimbursed by government and private payers our business could be adversely impacted. On December 14, 2018, a federal district court in Texas ruled that the PPACA is unconstitutional as a result of the Tax Cuts and Jobs Act, the federal income tax reform legislation previously passed by Congress and signed by President Trump on December 22, 2017, that eliminated the individual mandate portion of the PPACA. The case, Texas, et al, v. United States of America, et al., (N.D. Texas), is an outlier, and the ruling has been stayed by the ruling judge. We are not able to state with any certainty what the impact of this court decision will be on our business pending further court action and possible appeals.
In the fourth quarter of 2018, the Trump Administration announced initiatives that it asserted are intended to result in purportedly lower drug prices. The first initiative, announced on October 15, 2018, involved the plan to a new federal regulation that would require pharmaceutical manufacturers to disclose the list prices of their respective prescription drugs in their television advertisements for their products if the list price is greater than $35 USD. With respect to the second initiative, on October 25, 2018, the Centers for Medicaid and Medicare Services gave Advance Notice of Proposed Rulemaking to propose the implementation of an “International Pricing Index” model for Medicare Part B drugs and biologicals (single source drugs, biologicals, and biosimilars). Public comments were due on December 31, 2018 with a proposed rule theoretically being offered as early as Spring 2019 with target implementation of a 5 year pilot program beginning in Spring 2020. While these initiatives have not been put into effect, we are not in a position to know at this time whether they will ever become law or what impact the enactment either of these proposals would have on our business.
In February 2019, the Department of Health and Human Services has proposed a regulation that would significantly restrict the availability of certain regulatory safe harbors under the federal Anti-Kickback Statute that are used to facilitate certain types of transactions between manufacturers and pharmacy benefits managers that play a significant role in the pharmaceutical distribution chain. These changes to the Discount Safe Harbors available under the Anti-Kickback Statute would reduce some of the protections currently available to manufacturers that pay negotiated rebates to pharmacy benefits managers in exchange for these “PBMs” agreeing to include drugs and biologics on the formularies of the PBM’s downstream customers, primarily the health plans that insure patients for both private commercial plans and government-sponsored plans. While we do not know whether the Trump Administration will be successful in implementing this proposed regulation, its successful implementation could have an impact on both our commercial supply arrangements with health plans and our supply arrangements to health plans that serve beneficiaries of federal health care programs such as Medicare Part D.
As part of its reform of the 340B discount drug program, on October 31, 2018, the Health Resources and Services Administration (HRSA) at the U.S. Department of Health and Human Services (HHS) issued a notice of proposed rulemaking to move up the effective date of a final rule that would give HHS authority to impose Civil Monetary Penalties on pharmaceutical manufacturers who knowingly and intentionally charged a covered entity more than the statutorily allowed ceiling price for a covered outpatient drug. The final rule is intended to encourage compliance by manufacturers in offering the mandatory 340B ceiling purchase price to eligible purchasers, such as certain qualified health systems or individual hospitals.
| 17 |
If any product liability lawsuits are successfully brought against us or any of our collaborative partners, we may incur substantial liabilities and may be required to limit commercialization of our product candidates.
We face an inherent risk of product liability lawsuits related to the testing of our product candidates in seriously ill patients and will face an even greater risk if product candidates are approved by regulatory authorities and introduced commercially. Product liability claims may be brought against us or our partners by participants enrolled in our clinical trials, patients, health care providers or others using, administering or selling any of our future approved products. If we cannot successfully defend ourselves against any such claims, we may incur substantial liabilities, which may result in:
| · | decreased demand for any of our future approved products; |
| · | injury to our reputation; |
| · | withdrawal of clinical trial participants; |
| · | termination of clinical trial sites or entire trial programs; |
| · | significant litigation costs; |
| · | substantial monetary awards to or costly settlements with patients or other claimants; |
| · | product recalls or a change in the indications for which they may be used; |
| · | loss of revenue; |
| · | diversion of management and scientific resources from our business operations; and |
| · | the inability to commercialize our product candidates. |
If any of our product candidates are approved for commercial sale, we will be highly dependent upon consumer perceptions of us and the safety and quality of our products. We could be adversely affected if we are subject to negative publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies.
Although we currently carry clinical trial insurance, the amount of such insurance coverage may not be adequate. In addition, we will need to obtain more comprehensive insurance and increase our insurance coverage when we begin the commercialization of our product candidates. Insurance coverage is becoming increasingly expensive. As a result, we may be unable to maintain or obtain sufficient insurance at a reasonable cost to protect us against losses that could have a material adverse effect on our business.
We enter into various contracts in the normal course of our business in which we indemnify the other party to the contract. In the event we have to perform under these indemnification provisions, it could have a material adverse effect on our business, financial condition and results of operations.
In the normal course of business, we periodically enter into academic, commercial, service, collaboration, licensing, consulting, investor relations and other agreements that contain indemnification provisions. With respect to our academic and other research agreements, we typically indemnify the institution and related parties from losses arising from claims relating to the products, processes or services made, used, sold or performed pursuant to the agreements for which we have secured licenses, and from claims arising from our or our sublicensees’ exercise of rights under the agreement. With respect to our commercial agreements, we indemnify our vendors from any third-party product liability claims that could result from the production, use or consumption of the product, as well as for alleged infringements of any patent or other intellectual property right by a third party. With respect to investor relations agreements, we may indemnify the counterparty for losses resulting from our negligence or our supply of inaccurate information.
| 18 |
Should our obligation under an indemnification provision exceed applicable insurance coverage or if we were denied insurance coverage, our business, financial condition and results of operations could be adversely affected. Similarly, if we are relying on a collaborator to indemnify us and the collaborator is denied insurance coverage or the indemnification obligation exceeds the applicable insurance coverage and does not have other assets available to indemnify us, our business, financial condition and results of operations could be adversely affected.
Our business and operations would suffer in the event of computer system failures or security breaches.
Despite the implementation of security measures, our internal computer systems, and those of our contract research organizations, or CROs and other third parties on which we rely, are vulnerable to damage from computer viruses, unauthorized access, cyber-attacks, natural disasters, fire, terrorism, war, and telecommunication and electrical failures. If such an event were to occur and interrupt our operations, it could result in a material disruption of our drug development programs. For example, the loss of clinical trial data from ongoing or planned clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to our data or applications, loss of trade secrets or inappropriate disclosure of confidential or proprietary information, including protected health information or personal data of employees or former employees, access to our clinical data, or disruption of the manufacturing process, we could incur liability and the further development of our drug candidates could be delayed. We may also be vulnerable to cyber-attacks by hackers or other malfeasance. This type of breach of our cybersecurity may compromise our confidential information and/or our financial information and adversely affect our business or result in legal proceedings. Further, these cybersecurity breaches may inflict reputational harm upon us that may result in decreased market value and erode public trust.
If we fail to develop and commercialize other product candidates, we may be unable to grow our business.
Although the development and commercialization of Nomacopan (Coversin) is our primary focus, as part of our longer-term growth strategy, we plan to evaluate the development and commercialization of other therapies related to immune-mediated, inflammatory, orphan and other diseases. We may from time to time evaluate internal opportunities from our current product candidates, and also may choose to in-license or acquire other product candidates as well as commercial products to treat patients suffering from immune-mediated, orphan or other disorders with high unmet medical needs and limited treatment options. These other product candidates will require additional, time-consuming development efforts prior to commercial sale, including preclinical studies, clinical trials and approval by the FDA, EMA and/or applicable foreign regulatory authorities. All product candidates are prone to the risks of failure that are inherent in pharmaceutical product development, including the possibility that the product candidate will not be shown to be sufficiently safe and effective for approval by regulatory authorities. In addition, we cannot assure you that any such products that are approved will be manufactured or produced economically, successfully commercialized or widely accepted in the marketplace or be more effective than other commercially available alternatives.
The departure of former Chief Executive Officers and other executive officers and our searches for, and appointments of, a permanent Chief Executive Officer creates uncertainties and could have a material adverse impact on our business.
Our success depends, and will likely continue to depend, upon our ability to hire and retain the services of our current executive officers, directors, principal consultants and others. In addition, we have established relationships with universities and research institutions which have historically provided, and continue to provide, us with access to research laboratories, clinical trials, facilities and patients. The loss of the services of any of these individuals or institutions has had and could have a material adverse effect on our business.
On May 8, 2018, David Horn Solomon resigned as Chief Executive Officer and member of our board. Dr. Solomon’s resignation followed the results of an investigation conducted, with the assistance of an independent law firm, which revealed that Dr. Solomon incurred personal charges on the Company’s corporate credit cards in violation of Company policy. Clive Richardson, who was then serving as our Chief Operating Officer, was appointed to serve as our Interim Chief Executive Officer while we seek a permanent Chief Executive Officer. Previously, in December 2017 our former Chief Legal & Compliance Officer was terminated without cause and in May 2017 our former Chief Executive Officer who preceded David Horn Solomon also resigned. We face significant competition for executives with the qualifications and experience we are seeking. There can be no assurances concerning the timing or outcome of our search for a new permanent Chief Executive Officer or any other executive officer.
| 19 |
Changes in our organization as a result of recent departures have been harmful to our reputation and had a disruptive impact on our ability to implement our strategy and have had an adverse effect on our business, internal controls, financial condition and results of operations. As a result of the recent changes in our management team, our existing management team has taken on substantially more responsibility, which has resulted in greater workload demands and could divert their attention away from certain key areas of our business. Management transition inherently causes some loss of institutional knowledge, which can negatively affect strategy and execution. Until we find and integrate a replacement permanent Chief Executive Officer, and unless such replacement is able to succeed in the position, we may be unable to successfully manage and grow our business, and our results of operations, internal controls and financial condition could suffer as a result. We do not carry "key person" life insurance policies on any of our employees. Our future success also depends on our continuing ability to identify, hire, train and retain other highly qualified technical and managerial personnel. Competition for these personnel is intense, especially in the life science industry.
Class action lawsuits against us could lead to adverse outcomes.
We have in the past been, and may in the future become subject to class action litigation. In May 2017, putative class actions asserting violations of Sections 10(b) and 20(a) of the Exchange Act, based primarily on our press releases or statements concerning the Phase II PNH trial of Nomacopan (Coversin) and a report issued on April 26, 2017 titled “Akari’s Coversin matches Soliris in Phase II”, or the Edison Report, by Edison Investment Research Ltd, or Edison, about us and actions taken by us after the report was issued were commenced in the U.S. District Court for the Southern District of New York against us, a former Chief Executive Officer and our Chief Financial Officer. These actions were consolidated, and plaintiffs amended their pleadings to include our Executive Chairman and Edison as defendants. On June 8, 2018, the parties entered into a memorandum of understanding to settle plaintiffs’ claims for a total payment of $2.7 million in cash. On July 26, 2018, plaintiffs filed a notice with the Court voluntarily dismissing Edison from the action. On August 3, 2018, the remaining parties executed and filed a stipulation and agreement of settlement (the terms of which were consistent with the memorandum of understanding). On August 7, 2018, the Court granted plaintiffs’ motion for preliminary approval of the settlement, and on November 28, 2018, following a hearing with the parties, the court ordered final approval of the settlement. Plaintiffs subsequently moved to distribute the settlement funds to the class, and the Court granted plaintiffs’ motion on February 4, 2019. Separately, Edison Investment Research Ltd. sought indemnification from us including reimbursement of all legal expenses that Edison incurs in connection with the securities class action (to which, as discussed above, Edison was added as a defendant) and lost profits from customer relationships that Edison claims it lost as a result of the retraction of the Edison Report. The parties have finalized and consummated a settlement and the settlement payment has been made. If we become subject to any future class action litigation, we could incur substantial costs not covered by our liability insurance, suffer a significant adverse impact on our reputation and this could divert management’s attention and resources from other priorities, any of which could have a material adverse effect on our business. In addition, any of these matters could require payments that are not covered by, or exceed the limits of, our available liability insurance, which could have a material adverse effect on our business, financial condition and results of operation.
Risks Related to Our Reliance on Third Parties
If the third parties on which we rely for our clinical trials and results do not perform our clinical trial activities in accordance with good clinical practices and related regulatory requirements, we may be unable to obtain regulatory approval for or commercialize our product candidates.
We use and heavily rely on third-party service providers to conduct and/or oversee the clinical trials of our product candidates and expect to continue to do so for the foreseeable future. Nonetheless, we are responsible for confirming that each of our clinical trials is conducted in accordance with the FDAs and/or EMA’s requirements and its general investigational plan and protocol.
The FDA and EMA require us and our third-party service providers to comply with regulations and standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Third parties may not complete activities on schedule or conduct our clinical trials in accordance with regulatory requirements or the respective trial plans and protocols. In addition, third parties may not be able to repeat their past successes in clinical trials. The third parties’ failure to carry out their obligations could delay or prevent the development, approval and commercialization of our product candidates or result in enforcement action against us.
| 20 |
Use of third parties to manufacture our product candidates may increase the risk that we will not have sufficient quantities of our product candidates, products, or necessary quantities at an acceptable cost.
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of our product candidates, and we lack the resources and the capabilities to do so. As a result, we currently rely on third parties for supply of the active pharmaceutical ingredients, or API, in our product candidates. Our strategy is to outsource all manufacturing of our product candidates and products to third parties.
We currently engage a third-party manufacturer to provide clinical material of the API, lyophilization, release testing and fill and finish services for the final drug product formulation of Nomacopan (Coversin) that is being used in our clinical trials. Although we believe that there are several potential alternative manufacturers who could manufacture Nomacopan (Coversin), we may incur added costs and delays in identifying and qualifying any such replacement. In addition, we have not yet concluded a commercial supply contract with any commercial manufacturer. There is no assurance that we will be able to timely secure needed supply arrangements on satisfactory terms, or at all. Our failure to secure these arrangements as needed could have a material adverse effect on our ability to complete the development of our product candidates or, to commercialize them. We may be unable to conclude agreements for commercial supply with third-party manufacturers, or may be unable to do so on acceptable terms. There may be difficulties in scaling up to commercial quantities and formulation of Nomacopan (Coversin) and the costs of manufacturing could be prohibitive.
Even if we are able to establish and maintain arrangements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
| · | reliance on third-parties for manufacturing process development, regulatory compliance and quality assurance; |
| · | limitations on supply availability resulting from capacity and scheduling constraints of third-parties; |
| · | the possible breach of manufacturing agreements by third-parties because of factors beyond our control; and |
| · | the possible termination or non-renewal of the manufacturing agreements by the third-party, at a time that is costly or inconvenient to us. |
If we do not maintain our key manufacturing relationships, we may fail to find replacement manufacturers or develop our own manufacturing capabilities, which could delay or impair our ability to obtain regulatory approval for our products. If we do find replacement manufacturers, we may not be able to enter into agreements with them on terms and conditions favorable to us and there could be a substantial delay before new facilities could be qualified and registered with the FDA and other foreign regulatory authorities.
The FDA, EMA and other foreign regulatory authorities require manufacturers to register manufacturing facilities. The FDA and corresponding foreign regulators also inspect these facilities to confirm compliance with current good manufacturing practices, or cGMPs. Contract manufacturers may face manufacturing or quality control problems causing drug substance production and shipment delays or a situation where the contractor may not be able to maintain compliance with the applicable cGMP requirements. Any failure to comply with cGMP requirements or other FDA, EMA and comparable foreign regulatory requirements could adversely affect our clinical research activities and our ability to develop our product candidates and market our products following approval.
If our third-party manufacturer of Nomacopan (Coversin) is unable to increase the scale of its production of Nomacopan (Coversin), and/or increase the product yield of its manufacturing, then our costs to manufacture the product may increase and commercialization may be delayed.
In order to produce sufficient quantities of Nomacopan (Coversin) to meet the demand for clinical trials and subsequent commercialization, our third party manufacturer of Nomacopan (Coversin) will be required to increase its production and optimize its manufacturing processes while maintaining the quality of the product. The transition to larger scale production could prove difficult. In addition, if our third party manufacturer is not able to optimize its manufacturing process to increase the product yield for Nomacopan (Coversin), or if it is unable to produce increased amounts of Nomacopan (Coversin) while maintaining the quality of the product, then we may not be able to meet the demands of clinical trials or market demands, which could decrease our ability to generate profits and have a material adverse impact on our business and results of operation.
| 21 |
Risks Related to our Ordinary Shares and ADSs
Ownership of our ADSs and/or ordinary shares involves a high degree of risk.
Investing in and owning our ADSs and ordinary shares involve a high degree of risk. Shareholders should read carefully the risk factors provided within this section, as well as our public documents filed with the SEC, including the financial statements therein.
If we are deemed or become a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in 2019 or in any prior or subsequent years, there may be negative tax consequences for U.S. taxpayers that are holders of our ADSs.
We will be treated as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes in any taxable year in which either (i) at least 75% of our gross income is “passive income” or (ii) on average at least 50% of our assets by value produce passive income or are held for the production of passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. In determining whether a non-U.S. corporation is a PFIC, a proportionate share of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
We believe we were not a PFIC for 2018. Because the PFIC determination is highly fact sensitive, there can be no assurance that we will not be a PFIC for 2019 or for any other taxable year. If we were to be characterized as a PFIC for U.S. federal income tax purposes in any taxable year during which a U.S. shareholder owns our ADSs, and such U.S. shareholder does not make an election to treat us as a “qualified electing fund,” or QEF, or make a “mark-to-market” election, then “excess distributions” to such U.S. shareholder, and any gain realized on the sale or other disposition of our ADSs will be subject to special rules. Under these rules: (i) the excess distribution or gain would be allocated ratably over the U.S. shareholder’s holding period for ADSs; (ii) the amount allocated to the current taxable year and any period prior to the first day of the first taxable year in which we were a PFIC would be taxed as ordinary income; and (iii) the amount allocated to each of the other taxable years would be subject to tax at the highest rate of tax in effect for the applicable class of taxpayer for that year, and an interest charge for the deemed deferral benefit would be imposed with respect to the resulting tax attributable to each such other taxable year. In addition, if the U.S. Internal Revenue Service determines that we are a PFIC for a year with respect to which we have determined that we were not a PFIC, it may be too late for a U.S. shareholder to make a timely QEF or mark-to-market election. U.S. shareholders who hold our ADSs during a period when we are a PFIC will be generally subject to the foregoing rules, even if we cease to be a PFIC in subsequent years, subject to certain exceptions, including for U.S. shareholders who made a timely QEF or mark-to-market election. A U.S. shareholder can make a QEF election by completing the relevant portions of and filing IRS Form 8621 in accordance with the instructions thereto. A QEF election generally may not be revoked without the consent of the IRS. If an investor provides reasonable notice to us that it has determined to make a QEF election, we intend to provide annual financial information to such investor as may be reasonably required for purposes of filing United States federal income tax returns in connection with such QEF election.
Our ADSs may be involuntarily delisted from trading on The Nasdaq Capital Market if we fail to comply with the continued listing requirements. A delisting of our ADSs is likely to reduce the liquidity of our ADSs and may inhibit or preclude our ability to raise additional financing.
Nasdaq requires us to meet certain financial, public float, bid price and liquidity standards on an ongoing basis in order to continue the listing of our ADSs. Generally, we must maintain a minimum amount of stockholders equity (generally $2.5 million) and a minimum closing bid price (generally $1.00). If we fail to meet any of the continuing listing requirements, our ADSs may be subject to delisting and we may become subject to delisting proceedings. If our ADSs are delisted and we are not able to list our ADSs on another national securities exchange, we expect our securities would be quoted on an over-the-counter market. If this were to occur, our stockholders could face significant material adverse consequences, including limited availability of market quotations for our ADSs and reduced liquidity for the trading of our securities. In addition, we could experience a decreased ability to issue additional securities and obtain additional financing in the future. There can be no assurance that an active trading market for our ADSs will develop or be sustained. We may choose to raise additional capital in order to increase our stockholders’ equity in order to meet the Nasdaq continued listing standards. Any additional equity financings may be financially dilutive to, and will be dilutive from an ownership perspective to our stockholders, and such dilution may be significant based upon the size of such financing. Additionally, we cannot assure that such funding will be available on a timely basis, in needed quantities, or on terms favorable to us, if at all.
| 22 |
A limited public market exists for our securities and we cannot assure you that our securities will continue to be listed on the Nasdaq Capital Market or any other securities exchange or that an active trading market will ever develop for any of our securities.
Our ADSs were approved for listing and began trading on the Nasdaq Capital Market under the symbol “CLTX” on January 31, 2014 and commenced trading under the symbol “AKTX” commencing on September 21, 2015. An active trading market for our shares has not fully developed and, even if it does, it may not be sustained. In addition, we cannot assure you that we will be successful in meeting the continuing listing standards of the Nasdaq Capital Market and cannot assure you that our ADSs will be listed on a national securities exchange. If an active market for our ADS does not develop or is not sustained, it may be difficult for investors to sell their shares without depressing the market price for the shares or at all. Further, an inactive market may also impair our ability to raise capital and may impair our ability to enter into strategic partnerships or acquire companies or products by using our ADSs or ordinary shares as consideration.
The market price of our ADSs may be volatile and may fluctuate in a way that is disproportionate to our operating performance.
Our stock price may experience substantial volatility as a result of a number of factors. The market prices for securities of biotechnology companies in general have been highly volatile and may continue to be so in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our ADSs:
| · | sales or potential sales of substantial amounts of our ordinary shares or ADSs; |
| · | delay or failure in initiating, enrolling, or completing pre-clinical or clinical trials or unsatisfactory results of these trials or events reported in any of our current or future clinical trials; |
| · | announcements about us or about our competitors, including clinical trial results, regulatory approvals or new product introductions; |
| · | developments concerning our licensors or product manufacturers; |
| · | litigation and other developments relating to our patents or other proprietary rights or those of our competitors; |
| · | developments relating to the SEC investigation; |
| · | conditions in the pharmaceutical or biotechnology industries; |
| · | variations in our anticipated or actual operating results; |
| · | governmental regulation and legislation. |
| · | variations in our anticipated or actual operating results; |
| · | change in securities analysts’ estimates of our performance, or our failure to meet analysts’ expectations; |
| 23 |
| · | whether, to what extent and under what conditions the FDA or EMA will permit us to continue developing our product candidates, if at all, and if development is continued, any reports of safety issues or other adverse events observed in any potential future studies of these product candidates; |
| · | adverse publicity; |
| · | our ability to enter into new collaborative arrangements with respect to our product candidates; |
| · | the terms and timing of any future collaborative, licensing or other arrangements that we may establish; |
| · | our ability to raise additional capital to carry through with our clinical development plans and current and future operations and the terms of any related financing arrangements; |
| · | the timing of achievement of, or failure to achieve, our and any potential future collaborators’ clinical, regulatory and other milestones, such as the commencement of clinical development, the completion of a clinical trial or the receipt of regulatory approval; |
| · | announcement of FDA or EMA approval or non-approval of our product candidates or delays in or adverse events during the FDA or EMA review process; |
| · | actions taken by regulatory agencies with respect to our product candidates or products, our clinical trials or our sales and marketing activities, including regulatory actions requiring or leading to restrictions, limitations and/or warnings in the label of an approved product candidate; |
| · | uncontemplated problems in the supply of the raw materials used to produce our product candidates; |
| · | the commercial success of any product approved by the FDA, EMA or any other foreign counterpart; |
| · | introductions or announcements of technological innovations or new products by us, our potential future collaborators, or our competitors, and the timing of these introductions or announcements; |
| · | market conditions for equity investments in general, or the biotechnology or pharmaceutical industries in particular; |
| · | we may have limited or very low trading volume that may increase the volatility of the market price of our ADSs; |
| · | regulatory developments in the United States and foreign countries; |
| · | changes in the structure or reimbursement policies of health care payment systems; |
| · | any intellectual property infringement lawsuit involving us; |
| · | actual or anticipated fluctuations in our results of operations; |
| · | changes in financial estimates or recommendations by securities analysts; |
| · | hedging activity that may develop regarding our ADSs; |
| · | regional or worldwide recession; |
| · | sales of large blocks of our ordinary shares or ADSs; |
| · | sales of our ordinary shares or ADSs by our executive officers, directors and significant shareholders; |
| 24 |
| · | managerial costs and expenses; |
| · | changes in accounting principles; and |
| · | the loss of any of our key scientific or management personnel. |
The stock markets in general, and the markets for biotechnology stocks in particular, have experienced significant volatility that has often been unrelated to the operating performance of particular companies. The financial markets continue to face significant uncertainty, resulting in a decline in investor confidence and concerns about the proper functioning of the securities markets, which decline in general investor confidence has resulted in depressed stock prices for many companies notwithstanding the lack of a fundamental change in their underlying business models or prospects. These broad market fluctuations may adversely affect the trading price of our ADSs.
In the past, class action litigation has often been instituted against companies whose securities have experienced periods of volatility in market price. Any such litigation brought against us, could result in substantial costs, which could hurt our financial condition and results of operations and divert management’s attention and resources, which could result in delays of our clinical trials or commercialization efforts.
Insiders have control over us which could delay or prevent a change in corporate control or result in the entrenchment of management and/or the board of directors.
As of December 31, 2018, our directors and executive officers, together with their affiliates and related persons, beneficially own, in the aggregate, approximately 51.6% of our outstanding ordinary shares. RPC Pharma Limited, or RPC, which is controlled by our chairman Dr. Ray Prudo, beneficially owns approximately 49.5% of our outstanding ordinary shares. Accordingly, these shareholders, if acting together, or Dr. Prudo, individually, may have the ability to impact the outcome of matters submitted to our shareholders for approval, including the election and removal of directors and any merger, consolidation, or sale of all or substantially all of our assets. In addition, these persons may have the ability to influence the management and affairs of our company. Accordingly, this concentration of ownership may harm the market price of our ADSs by:
| · | delaying, deferring, or preventing a change in control; |
| · | entrenching our management and/or the board of directors; |
| · | impeding a merger, consolidation, takeover, or other business combination involving us; or |
| · | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
Future sales and issuances of our ordinary shares or ADSs or rights to purchase ordinary shares or ADSs pursuant to our equity incentive plans could result in additional dilution of the percentage ownership of our shareholders and could cause our share price to fall.
We expect that significant additional capital will be needed in the future to continue our planned operations. To the extent we raise additional capital by issuing equity securities, our shareholders may experience substantial dilution. We may sell ordinary shares (which may be represented by ADSs), convertible securities or other equity securities in one or more transactions at prices and in a manner we determine from time to time. If we sell ordinary shares, convertible securities or other equity securities in more than one transaction, investors may be materially diluted by subsequent sales. Such sales may also result in material dilution to our existing shareholders, and new investors could gain rights superior to our existing shareholders.
| 25 |
Sales of a substantial number of our ADSs by our existing shareholders in the public market could cause our stock price to fall.
Sales of a substantial number of our ADSs in the public market or the perception that these sales might occur, could significantly reduce the market price of our ADSs and impair our ability to raise adequate capital through the sale of additional equity securities.
As a result of the delayed filing of our Annual Report on Form 20-F for the period ended December, 31, 2017 with the SEC, we are not currently eligible to use a registration statement on Form F-3 to register the offer and sale of securities, which may adversely affect our ability to raise future capital in a timely manner.
As previously disclosed in our press release and reported on our Form 6-K filed May 21, 2018, we received a deficiency letter from Nasdaq on May 17, 2018 indicating that, as a result of not filing our Annual Report on Form 20-F for the year ended December 31, 2017, or the Late Annual Report, in a timely manner, we were not in compliance with Nasdaq Listing Rule 5250(c)(1) for continued listing.
Although we have adequately remedied our non-compliance with Nasdaq’s listing rules, we are not currently eligible to register the offer and sale of our securities using a registration statement on Form F-3 and we will not become eligible until we have timely filed certain periodic reports required under the Exchange Act for 12 consecutive calendar months. There can be no assurance when we will meet this requirement, which depends in part upon our ability to file our periodic reports on a timely basis in the future.
The vote by the United Kingdom to leave the European Union could adversely affect our business, financial condition, results of operations and prospects.
On March 29, 2017, the Prime Minister of the United Kingdom submitted formal notice to the European Union in order to trigger Article 50 of the Treaty on European Union. This is the formal mechanism which begins the two-year process of negotiating the UK’s exit from the EU, and to determine the future terms of the UK’s relationship with the EU, including the terms of trade between the UK and the EU, and potentially other countries. The effects of the UK exiting the EU (commonly referred to as Brexit) will depend on any agreements the UK makes to retain access to EU markets. In addition, Brexit could lead to legal uncertainty and potentially divergent national laws and regulations as the UK determines which EU laws to replace or replicate. Brexit could adversely affect economic or market conditions in the UK, Europe or globally and could contribute to instability in global financial markets, in particular until there is more certainty as to the outcome of the aforementioned decisions and negotiations. Exports from the United Kingdom may incur increased duties and tariffs following Brexit, or Brexit could result in the regulatory compliance and patent costs associated with our business compliance costs increasing significantly so as to adversely affect our financial condition, results of operations and prospects. Our regulatory compliance costs may increase as a result of Brexit. Following an exit by the UK from the EU, we may be required to register any current EU-wide patents separately with the UK Intellectual Property Office, which could require significant additional expense. Further, our regulatory compliance costs may increase as a result of Brexit, as on an exit from the EU, the UK may cease compliance with the EU and the EMA’s legislative regime for medicines, their research, development and commercialization. Any changes to such regulatory regimes could require us to comply with separate regimes in the UK and the EU, or to develop new policies and procedures or reorganize our operations, any of which could increase our compliance costs. Brexit has also led to a decrease in the value of pounds sterling against the U.S. dollar, as well as general volatility in currency exchange markets. The challenges faced by the UK following Brexit could result in an overall decline in trade and economic growth and/or an increase in economic volatility and therefore may affect the attractiveness of the UK as a leading centre for business and commerce. Any of the aforementioned possible effects of Brexit, and others that the we cannot anticipate, may materially adversely affect our business, financial condition, results of operations and prospects.
Provisions in our Articles of Association and under English law could make an acquisition of our company more difficult and may prevent attempts by our shareholders to replace or remove our organization management.
Provisions in our Articles of Association may delay or prevent an acquisition or a change in management. These provisions include a staggered board and prohibition on actions by written consent of our shareholders. Although we believe these provisions collectively will provide for an opportunity to receive higher bids by requiring potential acquirors to negotiate with our board of directors, they would apply even if the offer may be considered beneficial by some shareholders. In addition, these provisions may frustrate or prevent any attempts by our shareholders to replace or remove then current management by making it more difficult for shareholders to replace members of the board of directors, which is responsible for appointing the members of management.
| 26 |
We do not anticipate paying cash dividends, and accordingly, shareholders must rely on the appreciation in our ADSs for any return on their investment.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Therefore, the success of an investment in our ADSs will depend upon any future appreciation in their value. There is no guarantee that our ADSs will appreciate in value or even maintain the price at which our shareholders have purchased their shares.
We identified material weaknesses in our internal controls over financial reporting in fiscal year 2017 which were subsequently remediated. If additional material weaknesses or significant deficiencies in our internal control over financial reporting are identified in the future, or if we fail to maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results or prevent fraud, which could adversely affect our stock price and negatively impact our results of operations.
The Sarbanes-Oxley Act of 2002 requires, among other things, that we maintain effective internal control over financial reporting and disclosure controls and procedures. In particular, we are required, under Section 404 of the Sarbanes-Oxley Act of 2002, to perform system and process evaluations and testing of our internal control over financial reporting to allow management to report on the effectiveness of our internal control over financial reporting. This assessment must include disclosure of any material weaknesses in our internal control over financial reporting identified by our management. A material weakness is a control deficiency, or combination of control deficiencies, in internal control over financial reporting that results in more than a reasonable possibility that a material misstatement of annual or interim consolidated financial statements will not be prevented or detected on a timely basis.
We previously identified material weaknesses in our internal control over financial reporting in fiscal year 2017, as discussed in “Item 15. Controls and Procedures” of this Annual Report on Form 20-F and our Annual Report on Form 20-F for fiscal year 2017. These material weaknesses were remediated, and management concluded that its internal control over financial reporting was effective as of December 31, 2018. However, any failure to maintain internal control over financial reporting could severely inhibit our ability to accurately report our financial condition, results of operations or cash flows. If we are unable to remedy any future material weaknesses and conclude that our internal control over financial reporting is effective, or if our independent registered public accounting firm determines we have a material weakness or significant deficiency in our internal control over financial reporting, we could lose investor confidence in the accuracy and completeness of our financial reports, the market price of our ordinary shares could decline, and we could be subject to sanctions or investigations by the Nasdaq Stock Market, the SEC or other regulatory authorities. Failure to remedy any material weakness in our internal control over financial reporting, or to implement or maintain other effective control systems required of public companies, could also restrict our future access to the capital markets.
Failure to maintain our disclosure controls and procedures could impair our ability to accurately report our financial condition, results of operations or cash flows, which may adversely affect investor confidence in us.
Our management previously concluded that our disclosure controls and procedures for the fiscal year ended December 31, 2017 were not effective for the fiscal year ended December 31, 2017 due to the material weaknesses noted above. See “Item 15. Controls and Procedures.” Although these material weaknesses were remediated, if we fail to maintain our effective disclosure controls and procedures, we may not be able to accurately report our financial condition, results of operations or cash flows on a timely basis, which may adversely affect investor confidence in us.
Our disclosure controls and internal controls and procedures may not prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system reflects that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all our control issues and instances of fraud, if any, have been or will be detected. These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur simply because of error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by circumvention of the internal control procedures. The design of any system of controls also is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions; over time, a control may become inadequate because of changes in conditions, or the degree of compliance with the policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and may not be detected.
| 27 |
We incur significant costs and demands upon management as a result of complying with the laws and regulations affecting public companies, which could harm our operating results.
As a public company, we incur significant legal, accounting and other expenses, including costs associated with public company reporting requirements. We also incur costs associated with current corporate governance requirements, including requirements under Section 404 and other provisions of the Sarbanes-Oxley Act of 2002, as well as rules implemented by the SEC and the Nasdaq Stock Market. The expenses incurred by public companies for reporting and corporate governance purposes have increased dramatically in recent years.
We are a “foreign private issuer” and as a result of this and other reduced disclosure requirements applicable to foreign private issuers, our ADSs may be less attractive to investors.
On July 1, 2016, we became a foreign private issuer having previously lost this status at the end of 2014. As a foreign private issuer, we are not subject to the same requirements that are imposed upon U.S. domestic issuers by the SEC. Under the Exchange Act, we are subject to reporting obligations that, in certain respects, are less detailed and less frequent than those of U.S. domestic reporting companies. For example, we will not be required to issue proxy statements that comply with the requirements applicable to U.S. domestic reporting companies. We will also have four months after the end of each fiscal year to file our annual reports with the SEC and will not be required to file current reports as frequently or promptly as U.S. domestic reporting companies. Furthermore, our officers, directors, and principal shareholders will be exempt from the requirements to report transactions in our equity securities and from the short-swing profit liability provisions contained in Section 16 of the Exchange Act. These exemptions and leniencies, along with other corporate governance exemptions resulting from our ability to rely on home country rules, will reduce the frequency and scope of information and protections to which you may otherwise have been eligible in relation to a U.S. domestic reporting companies. If we were to lose our foreign private issuer status, the regulatory and compliance costs to us under U.S. securities laws as a U.S. domestic issuer will be significantly more than costs we incur as a foreign private issuer.
U.S. investors may not be able to enforce their civil liabilities against our company or certain of our directors, controlling persons and officers.
It may be difficult for U.S. investors to bring and/or effectively enforce suits against our company outside of the United States. We are a public limited company incorporated in England and Wales under the Companies Act 2006, as amended, or the Company Act. A majority of our directors are not residents of the United States, and all or substantial portions of their assets are located outside of the United States. As a result, it may be difficult for U.S. holders of our ordinary shares or ADSs to effect service of process on these persons within the United States or to make effective recovery in the United States by enforcing any judgments rendered against them. In addition, if a judgment is obtained in the U.S. courts based on civil liability provisions of the U.S. federal securities laws against us or our directors or officers, it may, depending on the jurisdiction, be difficult to enforce the judgment in the non-U.S. courts against us and any of our non-U.S. resident executive officers or directors. Accordingly, U.S. shareholders may be forced to bring legal proceedings against us and our respective directors and officers under English law and in the English courts in order to enforce any claims that they may have against us or our directors and officers. The enforceability of a U.S. judgment in the United Kingdom will depend on the particular facts of the case as well as the laws and treaties in effect at the time. The United States and the United Kingdom do not currently have a treaty providing for reciprocal recognition and enforcement of judgments (other than arbitration awards) in civil and commercial matters. Nevertheless, it may be difficult for U.S. shareholders to bring an original action in the English courts to enforce liabilities based on the U.S. federal securities laws against us and any of our non-U.S. resident executive officers or directors.
The rights of our shareholders may differ from the rights typically offered to shareholders of a U.S. corporation.
We are incorporated under English law. The rights of holders of ordinary shares and, therefore, certain of the rights of holders of ADSs, are governed by English law, including the provisions of the Companies Act, and by our Articles of Association. These rights differ in certain respects from the rights of shareholders in typical U.S. corporations.
| 28 |
Provisions in the UK City Code on Takeovers and Mergers may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our shareholders.
The UK City Code on Takeovers and Mergers, or the Takeover Code, applies, among other things, to an offer for a public company whose registered office is in the United Kingdom and whose securities are not admitted to trading on a regulated market in the United Kingdom if the company is considered by the Panel on Takeovers and Mergers, or the Takeover Panel, to have its place of central management and control in the United Kingdom. This is known as the “residency test.” The test for central management and control under the Takeover Code is different from that used by the UK tax authorities. Under the Takeover Code, the Takeover Panel will determine whether we have our place of central management and control in the United Kingdom by looking at various factors, including the structure of our board of directors, the functions of the directors and where they are resident.
If at the time of a takeover offer the Takeover Panel determines that we have our place of central management and control in the United Kingdom, we would be subject to a number of rules and restrictions, including but not limited to the following: (1) our ability to enter into deal protection arrangements with a bidder would be extremely limited; (2) we may not, without the approval of our shareholders, be able to perform certain actions that could have the effect of frustrating an offer, such as issuing shares or carrying out acquisitions or disposals; and (3) we would be obliged to provide equality of information to all bona fide competing bidders.
Holders of ADSs must act through the depositary to exercise their rights as shareholders of our company.
Holders of our ADSs do not have the same rights of our shareholders and may only exercise the voting rights with respect to the underlying ordinary shares in accordance with the provisions of the deposit agreement for the ADSs. Under our Articles of Association, the minimum notice period required to convene an Annual General Meeting is no less than 21 clear days’ notice and 14 clear days’ notice for a general meeting (unless, in the case of an annual general meeting all members entitled to attend and vote at the meeting, or in the case of a general meeting, a majority of the members entitled to attend and vote who hold not less than 95% of the voting shares (excluding treasury shares), agree to shorter notice). When a general meeting is convened, holders of our ADSs may not receive sufficient notice of a shareholders’ meeting to permit them to withdraw their ordinary shares to allow them to cast their vote with respect to any specific matter. In addition, the depositary and its agents may not be able to send voting instructions to holders of our ADSs or carry out their voting instructions in a timely manner. We will make all reasonable efforts to cause the depositary to extend voting rights to holders of our ADSs in a timely manner, but we cannot assure them that they will receive the voting materials in time to ensure that they can instruct the depositary to vote their ADSs. Furthermore, the depositary and its agents will not be responsible for any failure to carry out any instructions to vote, for the manner in which any vote is cast or for the effect of any such vote. As a result, holders of our ADSs may not be able to exercise their right to vote and they may lack recourse if their ADSs are not voted as they requested. In addition, in the capacity as an ADS holder, they will not be able to call a shareholders’ meeting.
Holders of our ADSs may be subject to limitations on transfers of ADSs.
ADSs are transferable on the books of the depositary. However, the depositary may close its transfer books at any time or from time to time when it deems expedient in connection with the performance of its duties. In addition, the depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary deems it advisable to do so because of any requirement of law or of any government or governmental body, or under any provision of the deposit agreement, or for any other reason.
The rights of holders of our ADSs to participate in any future rights offerings may be limited, which may cause dilution to their holdings and they may not receive cash dividends if it is impractical to make them available to them.
We may from time to time distribute rights to our shareholders, including rights to acquire our securities. However, we cannot make rights available to holders of our ADSs in the United States unless we register the rights and the securities to which the rights relate under the Securities Act or an exemption from the registration requirements is available. Also, under the deposit agreement, the depositary will not make rights available to holders of our ADSs unless either both the rights and any related securities are registered under the Securities Act, or the distribution of them to ADS holders is exempted from registration under the Securities Act. We are under no obligation to file a registration statement with respect to any such rights or securities or to endeavor to cause such a registration statement to be declared effective. Moreover, we may not be able to establish an exemption from registration under the Securities Act. Accordingly, holders of our ADSs may be unable to participate in our rights offerings and may experience dilution in their holdings.
In addition, the depositary has agreed to pay to holders of our ADSs the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. Holders of our ADSs will receive these distributions in proportion to the number of ordinary shares their ADSs represent. However, the depositary may, at its discretion, decide that it is inequitable or impractical to make a distribution available to any holders of ADSs. For example, the depositary may determine that it is not practicable to distribute certain property through the mail, or that the value of certain distributions may be less than the cost of mailing them. In these cases, the depositary may decide not to distribute such property and holders of our ADSs will not receive any such distribution.
| ITEM 4. | INFORMATION ON THE COMPANY |
| A. | History and Development of the Company |
Our Corporate History
Our legal and commercial name is Akari Therapeutics, PLC. We were originally established as a private limited company under the laws of England and Wales on October 7, 2004 under the name Freshname No. 333 Limited. On January 19, 2005, we changed our name to Morria Biopharmaceuticals Limited and on February 3, 2005, we completed a reverse merger with Morria Biopharmaceuticals Inc., or Morria, a Delaware corporation, in which Morria became our wholly-owned subsidiary and we re-registered as a non-traded public limited company under the laws of England and Wales. Morria was dedicated to the discovery and development of novel, first-in-class, non-steroidal, synthetic anti-inflammatory drugs. On March 22, 2011, we incorporated an Israeli subsidiary, Morria Biopharma Ltd. On June 25, 2013, we changed our name to Celsus Therapeutics PLC and on October 13, 2013 Morria was renamed Celsus Therapeutics Inc. As of the date of this report, Celsus Therapeutics Inc. and Morria Biopharma Ltd. do not conduct any operations.
| 29 |
On September 18, 2015, we completed an acquisition of all of the capital stock of Volution Immuno Pharmaceuticals SA, or Volution, a private Swiss company, from RPC Pharma Limited, or RPC, Volution’s sole shareholder, in exchange for our ordinary shares, in accordance with the terms of a Share Exchange Agreement, dated as of July 10, 2015. In connection with the acquisition, our name was changed to Akari Therapeutics, PLC and the combined company focused on the development and commercialization of life-transforming treatments for a range of rare and orphan autoimmune and inflammatory diseases caused by dysregulation of complement C5.
Our ADSs have been listed on the Nasdaq Capital Market under the symbol “AKTX” since September 21, 2015 and under the symbol “CLTX” from January 31, 2014 until September 18, 2015. Prior to that, our ADSs were quoted on the OTCQB under the symbol “CLSXD” from January 3, 2014 to January 30, 2014 and were quoted on the OTCQB under the symbol “CLSXY” from September 16, 2013 until January 2, 2014 and under the symbol “MRRBY” from February 19, 2013 to September 15, 2013. Effective January 3, 2014, our ratio of ADSs to ordinary shares changed from one ADS per each two ordinary shares to one ADS per each ten ordinary shares and, effective as of September 17, 2015, our ratio of ADSs to ordinary shares changed from one ADS per each ten ordinary shares to one ADS per each one hundred ordinary shares. Currently, each ADS represents by one hundred ordinary shares.
Our principal office is located at 75/76 Wimpole Street, London W1G 9RT, United Kingdom, and our telephone number is +44 20 8004 0270. Our website address is www.akaritx.com. The information contained on, or that can be accessed through, our website is neither a part of nor incorporated into this Annual Report. We have included our website address in this annual report solely as an inactive textual reference. Puglisi & Associates, or Puglisi, serves as our agent for service of process in the United States. Puglisi’s address is 850 Library Avenue, Suite 204, Newark, Delaware 19711.
We use our website (www.akaritx.com) as a channel of distribution of Company information. The information we post through this channel may be deemed material. Accordingly, investors should monitor our website, in addition to following our press releases, SEC filings and public conference calls and webcasts. The contents of our website are not, however, a part of this Annual Report.
Capital Expenditures
Our capital expenditures for the years ended December 31, 2018, 2017 and 2016 were $0, $36,885 and $54,750, respectively. Our current capital expenditures primarily consist of computer equipment.
| B. | Business Overview |
We are a clinical-stage biopharmaceutical company focused on developing inhibitors of acute and chronic inflammation, specifically the complement system, the eicosanoid or leukotriene system and the bioamine system for the treatment of rare and orphan diseases. Each of these systems has scientifically well-supported causative roles in the diseases being targeted by us. We believe that blocking early mediators of inflammation will prevent initiation and continual amplification of the processes that cause certain diseases.
Ticks have undergone 300 million years of natural selection to produce inhibitors that bind tightly to key highly-conserved inflammatory mediators, are generally well tolerated in humans, and remain fully functional when a host is repeatedly exposed to the molecule. Our molecules are derived from these inhibitors.
Our lead product candidate, Coversin, which was recently renamed Nomacopan following its acceptance by as an International Nonproprietary Name by the World Health Organization, is a second-generation complement inhibitor, acts on complement component-C5, preventing release of C5a and formation of C5b–9 (also known as the membrane attack complex, or MAC), and independently also inhibits leukotriene B4, or LTB4, activity, both elements that are co-located as part of the immune/inflammatory response. Nomacopan (Coversin) is a recombinant small protein (16,740 Da) derived from a protein originally discovered in the saliva of the Ornithodoros moubata tick, where it modulates the host immune system to allow the parasite to feed without alerting the host to its presence or provoking an immune response.
| 30 |
Nomacopan (Coversin) has received orphan drug status from the U.S. Food and Drug Administration, or the FDA, and the European Medicines Agency, or the EMA, for paroxysmal nocturnal haemoglobinuria, or PNH, and Guillain Barré Syndrome, or GBS. Orphan drug designation provides us with certain benefits and incentives, including a period of marketing exclusivity if regulatory approval of the drug is ultimately received for the designated indication. The receipt of orphan drug designation status does not change the regulatory requirements or process for obtaining marketing approval and the designation does not mean that marketing approval will be received. We intend to apply in the future for orphan drug designation in additional indications we deem appropriate.
On March 29, 2017, we received notice from the U.S. Food and Drug Administration (FDA) of fast track designation for the investigation of Nomacopan (Coversin) for the treatment of PNH in patients who have polymorphisms conferring Soliris® (eculizumab) resistance. The fast track program was created by the FDA to facilitate the development and expedite the review of new drugs which show promise in treating a serious or life-threatening disease and address an unmet medical need. Drugs that receive this designation benefit from more frequent communications and meetings with the FDA to review the drug’s development plan including the design of the proposed clinical trials, use of biomarkers and the extent of data needed for approval. Drugs with fast track designation may also qualify for priority review to expedite the FDA review process, if relevant criteria are met.
Our clinical targets for Nomacopan (Coversin) are diseases where complement dysregulation is the key driver such as: paroxysmal nocturnal hemoglobinuria or PNH and thrombotic microangiopathy post bone marrow transplant or TMA-HSCT, as well as a range of inflammatory diseases where the inhibition of both C5 and LTB4 are implicated, including bullous pemphigoid, or BP, and atopic keratoconjunctivitis, or AKC.
Other compounds in our pipeline include engineered versions of Nomacopan (Coversin) that potentially decrease the frequency of administration, improve potency, or allow for specific tissue targeting, as well as new proteins targeting LBT4 alone, as well as bioamine inhibitors (for example, anti-histamines). In general, these inhibitors act as ligand binding compounds, which may provide additional benefit versus other modes of inhibition. For example, off-target effects are less likely with ligand capture. One example of this benefit is seen with LTB4 inhibition where compounds that have targeted the production of leukotrienes can inhibit both the production of pro-inflammatory as well as anti-inflammatory leukotrienes—often diminishing the potential benefit of the drug on the inflammatory system. Nomacopan (Coversin) has demonstrated that, by capturing LTB4, it is limited to disrupting the white blood cell activation and attraction aspects, without interfering with the anti-inflammatory benefits of other leukotrienes.
Nomacopan (Coversin) is much smaller than typical antibodies currently used in therapeutic treatment. Nomacopan (Coversin) can be self-administered by subcutaneous injection, much like an insulin injection, which we believe will provide considerable benefits in terms of patient convenience. We believe that the subcutaneous formulation of Nomacopan (Coversin) as an alternative to intravenous infusion may accelerate patient uptake if Nomacopan (Coversin) is approved by regulatory authorities for commercial sale. Patient surveys contracted by us suggest that many patients would prefer to self-inject daily than undergo intravenous infusions. Additionally, Nomacopan (Coversin)’s bio-physical properties allow it to be potentially used in a variety of formulations, some of which may enable therapeutic use via topical or inhaled routes of administration.
Clinical Development Program — Past and Future
Phase Ia Single Ascending Dose Trial
Nomacopan (Coversin) entered clinical development in 2013 when a Phase Ia clinical trial was initiated under a Clinical Trials Authorisation (CTA) issued by the Medicines and Healthcare products Regulatory Agency (MHRA), an executive agency of the Department of Health in the United Kingdom. The primary objective of this single ascending dose, first-in-man study was to explore the safety profile of Nomacopan (Coversin) in 24 subjects. The drug was well tolerated, and no serious or dose-related adverse events were reported. The secondary objective of this Phase Ia clinical trial was to examine the effect of Nomacopan (Coversin) on complement activity at the highest, therapeutic dose. This showed that the peak onset of action was about nine hours after injection, and that the effect of a single dose was detectable for more than 96 hours. The effects were consistent between all subjects and showed 100% inhibition of the complement system within 12 hours.
| 31 |
Phase 1b Dose Range Finding Trial
A Phase Ib repeat dose study was initiated in the first quarter of 2016. In this double-blind, randomized Phase Ib trial, each cohort of six normal healthy volunteers was given either a loading dose of subcutaneous placebo twice a day for two days followed by five days of a single daily placebo dose (n=2) or a loading dose of 30 mg of subcutaneous Nomacopan (Coversin) twice a day for two days followed by five days of a single daily subcutaneous maintenance dose (n=4) of either 15 mg, 22.5 mg or 30 mg.
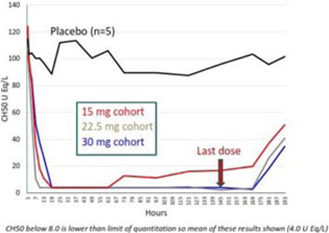
Data from the 22.5 mg once daily maintenance cohort and 30 mg once daily maintenance cohort demonstrated that subcutaneous Nomacopan (Coversin) achieved complete complement inhibition (Elisa CH50 < 8 Eq/ml, lower limit of quantification) within the first day, and demonstrated complete complement inhibition at the end of dosing on day seven whether measured using the ELISA or lytic CH50 assays.
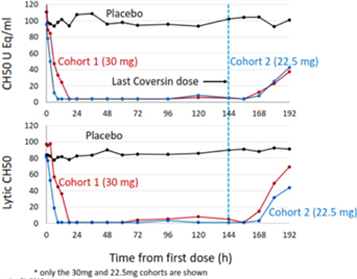
The data from the 15 mg once daily maintenance cohort demonstrated that subcutaneous Nomacopan (Coversin) achieved complete complement inhibition (Elisa CH50 < 8 Eq/ml, lower limit of quantification) within the first day following an ablating dose but by day three was unable to maintain complete complement inhibition at the 24-hour trough measurement. A final cohort of 4 healthy volunteers was given 22.5 mg of Nomacopan (Coversin) as a maintenance dose for 21 days. Complete complement inhibition was demonstrated at the end of the 21 day period of once daily dosing and there we no neutralizing antibodies detected. One volunteer receiving the Nomacopan (Coversin) in the 30 mg 7 day cohort stopped dosing on day three due to a non-serious adverse event possibly related to antibiotics administered for meningitis prophylaxis. The trial was conducted at Hammersmith Medicines Research Ltd, in London.
| 32 |
BP Clinical Program
We continue to develop Nomacopan (Coversin) in indications that take advantage of the dual-acting properties of the drug to inhibit both C5 and LTB4. In patients with BP there is evidence that both C5 and LTB4 have a central role in driving the disease. Ex vivo data, from a recent study at Lubeck University, in BP patients showed a pronounced accumulation of LTB4 and C5 and its activation products in the inflamed skin of bullous pemphigoid disease patients.
In 2018, we opened our first site for a six-week Phase IIa open label, single-arm trial to evaluate the safety and efficacy of Nomacopan (Coversin) in patients with mild to moderate BP. The primary endpoints of the trial were proportion of patients reporting grade 3, 4 and 5 adverse events which are related/possibly related to Nomacopan (Coversin) during the treatment period and secondary and other endpoints include, among others, Bullous Pemphigoid Disease Area Index (BPDAI) score. The Phase IIa clinical trial is currently recruiting at six sites across Europe.
Initial results from the first three patients showed that Nomacopan (Coversin), dosed daily subcutaneously, was well tolerated in three elderly patients (>65 years), and that there were no drug-related adverse events. Prior to treatment with Nomacopan (Coversin), two out of the three patients were already on topical corticosteroids (mometasone) while a third was naïve to steroid treatment. Steroids were reduced at weekly intervals so that by day 21 both patients were only treated with Nomacopan (Coversin). In the 7-11-day period prior to initiation on Nomacopan (Coversin), the two patients on steroids showed either no or minor improvement in their BPDAI global score (between 0% and 5%) and no improvement in blisters. By Day 7, 21 and 42 of treatment with Nomacopan (Coversin), the BPDAI global score fell by a mean of 31%, 45% and 52%, respectively. By Day 7, 21 and day 42 of treatment with Nomacopan (Coversin), blisters/erosions dropped by a mean of 45%, 75% and 87%, respectively.
As a result of this positive data, we intend to expand the trial to include the treatment of severe patients.
AKC Clinical Program
During the third quarter of 2018, we commenced a Phase I/II randomized, double blinded, placebo-controlled trial with an initial three patients (Part A) prior to the blinding (Part B) to evaluate the safety and efficacy of Nomacopan (Coversin) in patients with the inflammatory mediated eye disorder AKC. Results in a rodent model of Experimental Immune Conjunctivitis (EIC), undertaken at the world leading Moorfields Hospital Institute of Ophthalmology, showed that Nomacopan (Coversin) demonstrated significant anti-inflammatory activity. In this preclinical model of severe eye surface inflammation, Nomacopan (Coversin), applied topically, resulted in a statistically significant reduction (64%, p<0.001) in late phase inflammation versus placebo. We plan to provide interim data readouts for our AKC trial and present a poster showing Nomacopan’s (Coversin’s) effect in a preclinical model of autoimmune uveitis, a back of the eye orphan disease, at the Association for Research in Vision and Ophthalmology (ARVO) conference to be held in Vancouver, from April 28 to May 2, 2019.
| 33 |
TMA Clinical Program
Thrombotic microangiopathy following hematopietic stem cell transplant, or TMA-HSCT is an orphan condition with an estimated fatality rate of more than 80% in pediatric patients with the disease. A framework for a pivotal trial design for pediatric patients was agreed with the FDA in which the response to Nomacopan (Coversin) of selected, clinically meaningful treatment variables would be the primary endpoint. In September 2018, Akari announced that in the first two patients treated with Nomacopan (Coversin) as part of a UK named patient program it had observed a rapid reduction of the markers of complement activation as well as normalization of markers that are elevated in TMA (platelet count, red blood cell fragments, thrombocytopaenia, elevated LDH and hypertension).
We aim to commence a European and US trial in pediatric TMA-HSCT patients in the fourth quarter of 2019. TMA-HSCT is now planned as our gateway indication into the broad TMA space which includes a large number of related and poorly treated orphan diseases including atypical hemolytic uremic syndrome, or aHUS. In order to accommodate the new focus on the broader TMA space, the current aHUS Phase II program is being put on hold and will be reviewed in order to align with the wider TMA program.
PNH Clinical Program
Phase II PNH Eculizumab-Resistant Trials
In February 2016, we initiated an open-label Phase II single arm trial for PNH patients with eculizumab resistance due to C5 polymorphisms, pursuant to a clinical trial protocol approved by the European Union national regulatory authority The primary objective of the eculizumab-resistance program was to provide patients who have clinically demonstrated resistance to eculizumab with early access to Nomacopan (Coversin) as a potentially lifesaving alternative. The patient, who is being treated in the Netherlands, was entered into an open label protocol where safety and efficacy parameters are evaluated on an ongoing basis including measures of complement activity such as CH50 and biomarkers of hemolysis activity such as lactate dehydrogenase, or LDH. Results from the patient with PNH who was resistant to eculizimab due to a C5 polymorphism has demonstrated complete complement inhibition (Elisa CH50 < 8 Eq/ml, lower limit of quantification) and marked LDH reduction to around 1.5 times the upper limit of normal, or ULN. This patient has been transferred to the long-term safety and efficacy CONSERVE study and in aggregate has been treated for more than three years of therapy as of March 31, 2019 and has been self-administering Nomacopan (Coversin).
In May 2018, we enrolled a second PNH patient with eculizumab resistance due to C5 polymorphisms in an open-label six-month Phase II single arm trial. The trial was conducted at a study site in New York and the patient has entered into our long-term safety and efficacy CONSERVE study.
Phase II COBALT PNH Trial
In the fourth quarter of 2016, we commenced enrollment for a 90-day open-label Phase II, single-arm clinical trial in patients with PNH in five centers in the European Union, known as the COBALT trial. The trial was concluded in December 2017. We enrolled and treated eight patients with Nomacopan (Coversin)self-administered subcutaneous injections twice a day for approximately the first month and then switched to once daily injections. Of those eight patients, seven completed the 90-day trial while one patient with a suspected co-morbidity unrelated to treatment was withdrawn on day 43 of the trial. Following the ablating dose, the first five patients were dosed twice daily with either 15mg or 22.5mg of Nomacopan (Coversin)for approximately the first month and then once daily 30mg or 45 mg until end of the trial. Two of those patients were updosed from 30mg to 45mg once daily at Days 40 and 54, respectively and a third patient was updosed to 22.5mg twice daily at Day 24 and moved to 45mg once daily at Day 67. The final three patients recruited were dosed under a revised dosing regime in which following the ablating dose, were dosed twice daily with 22.5mg of Nomacopan (Coversin)for approximately the first month and then once daily 45mg until the end of the trial. The primary endpoint of the trial was defined as a reduction in LDH to ≤1.8 times the ULN, at day 28.
Results from the COBALT trial showed that patients were comfortable with self-administration of Nomacopan (Coversin). Those results showed that there were four serious adverse events, SAEs, but no SAEs related to Nomacopan (Coversin). The most commonly reported adverse events were mild self-limiting injection site reactions. All patients that completed the trial saw declines in lactate dehydrogenase, LDH, levels (although in some cases there were intermittent rises). The trial met its primary endpoint defined as an LDH of ≤1.8 X ULN at Day 28. LDH as a multiple of ULN (xULN) was 1.4, 2.2, 2.3, 1.3, 1.4, 2.7, 1.6, 1.3 at day 28 (n=8); 1.5, 2.1, 1.8, 2.2, 1.5, 1.4, 1.3 at day 60 (n=7); and 1.6, 2.4, 2.0, 2.5, 1.9, 1.5, 1.2 at day 90 (n=7). Of the seven patients who completed the study, six were transfusion-dependent prior to the trial. Of those six patients, three have not required transfusions while on Nomacopan (Coversin) during the trial and a fourth patient became transfusion-dependent while on CONSERVE.
Long-Term Safety and Efficacy CONSERVE Study
CONSERVE is a long-term safety and efficacy study for those patients who wish to continue on Nomacopan (Coversin) treatment following completion of a trial.
All seven patients that completed the COBALT trial opted to be enrolled into the CONSERVE study. As of March 31, 2019, all these patients have been receiving Nomacopan (Coversin) subcutaneously for over a year and a half in the aggregate (factoring in time spent on COBALT and CONSERVE). To date, there have been no drug-related serious adverse events reported and patients are self-administering. Two Polish patients have withdrawn from CONSERVE following the approval of eculizumab for reimbursement in Poland.
| 34 |
Phase III CAPSTONE PNH Trial and Planned ASSET PNH Trial
In March 2018, we opened our first site for CAPSTONE, a three-part, two-arm, randomized, open label, Phase III clinical trial of Nomacopan (Coversin) in transfusion dependent PNH patients in specific countries in Europe and other countries, where Soliris® is not the standard of care. The standard of care in these territories is blood transfusion with or without anticoagulation and/or any other concomitant medications for PNH that the patient may be taking. The trial is being run in three parts as follows: (i) in part one, eligible patients (who must be transfusion dependent) enter an up to 3-month observation period, (ii) in part two, a minimum of 30 patients that meet certain criteria in part one are randomized 1:1 to receive for 6 months either Coversin or the standard of care, and (iii) in part three, patients receiving Coversin will continue to receive Coversin plus standard of care for 3 months while patients receiving standard of care will receive Coversin for 3 months. Upon completion of the trial, patients will have the option of remaining on Coversin and being entered into our long-term safety and efficacy CONSERVE study. Following the ablating dose, patients receiving Coversin will be dosed twice daily with 22.5mg of Coversin for approximately the first month and then once daily 45mg until the end of the trial. The objective of the trial is to demonstrate efficacy of Nomacopan (Coversin) plus standard of care to standard of care in patients with uncontrolled haemolysis due to PNH and to assess safety and tolerability. The primary endpoint of the study is haemoglobin, or Hb, stabilization rate (defined as Hb greater than the set point for each patient defined during the pre-study randomization period) and the avoidance of PRBC transfusions during the treatment period, each as assessed at the end of part two. Secondary and other endpoints include LDH, CH50, quality of life and safety. The Phase III clinical trial is currently recruiting.
In addition to CAPSTONE we are considering to conduct ASSET, a Phase III clinical trial in PNH patients who have been treated with Soliris in the United States, Europe and other countries where Soliris is the current standard of care. Based on the FDA’s advice, we may decide to engage in additional discussions with the FDA.
| 35 |
Immunogenicity
We successfully completed a chronic (28 day) dosing experiment in mice to investigate whether daily subcutaneous administration of the expected therapeutic dose of Nomacopan (Coversin) induces an antibody response, and whether the antibodies neutralise complement inhibition by Nomacopan (Coversin). The data from this chronic dosing experiment showed Nomacopan (Coversin) was well-tolerated with no injection site allergic reactions or behavioral changes. Nomacopan (Coversin) can induce formation of low titre anti-drug IgG antibodies in mice after four weeks of daily inoculation, which is not uncommon, but these antibodies were not neutralising and had no effect on Nomacopan (Coversin)’s ability to inhibit complement.
No neutralizing antibodies have been detected, including in the healthy volunteers in the Phase Ia and Ib trials, the ongoing PNH patient treated for over three years in the eculizumab resistance trial and in the Phase II COBALT study and long-term safety and efficacy CONSERVE study.
Nomacopan (Coversin) LA: Once Weekly Formulation
Using PASylation®, a proprietary process of XL-protein GmbH, XL-protein has modified Nomacopan (Coversin) by adding a 600 amino acid proline/alanine/ serine (PAS) N-terminal fusion tag to generate PAS-Nomacopan (Coversin) (68kDa). The unstructured and uncharged PAS polypeptide increases the apparent molecular size to approximately 700kDa, slowing kidney clearance and extending the half-life.
Data from mouse, rat and dog studies of PAS-Nomacopan (Coversin) demonstrated that the expected terminal half-life in humans should be approximately 4 days. Based on these data, PK modeling supports that a once weekly dosing regimen is feasible. We intend to conduct a Phase I clinical study of PAS Nomacopan (Coversin) in the future based on our availability of resources.
In addition, new data in a pig model has shown a similar PK profile for the higher concentrated formulation to be used across our subcutaneous programs. This new highly concentrated formulation with small (0.3mL) volume and water-like viscosity is intended to allow ease of administration and increased patient comfort for use alongside a new auto-injector pen holding a week’s dosing stable at room temperature. We plan to initiate a Phase I clinical trial with a new auto-injector pen formulation in the second half of 2019.
Target Indications
| 36 |
Bullous Pemphigoid
Bullous pemphigoid, or BP, is an autoimmune blistering skin disease. BP is a serious condition with significant associated morbidity and mortality. Widespread tense and haemorrhagic blisters, skin erosions and severe itching cause patients a great deal of distress and pain.
Untreated, BP may be a self-limiting disease in a proportion of patients with periods of spontaneous remissions and exacerbations. In most patients who are treated, BP remits within 1.5-5 years but may recur once medication is stopped. Patients are often admitted to hospital for initial treatment. The estimates of admission rates for patients with BP vary, but they are generally high, thus representing a significant burden and cost to the healthcare systems, as well to the patients’ and their families /carers. The severity of symptoms and lesions in BP make treatment mandatory. Corticosteroids are often administered and frequent hospital visits are needed for dose adjustments.
Older age at onset and frail general condition are poor prognostic factors. Many available treatments are associated with toxicity and may be poorly tolerated in patients with BP. It is thought that, in the elderly population, corticosteroid treatment contributes to the high mortality rate. This is due, at least in part, to the significant adverse events associated with the use of steroids, such as hypertension, diabetes, infections and osteoporosis. Management of these conditions can be difficult and their treatment represents a significant burden. Therefore, the avoidance of systemic corticosteroids in this vulnerable group of patients is highly desirable and a safer, effective, evidence based alternative is needed.
Treatment should aim to control symptoms with minimum adverse effects where possible. Options are broadly divided into anti-inflammatory drugs, immunosuppressive or immunomodulating drugs, and procedures that aim to remove circulating pathogenic antibodies and inflammatory mediators. Corticosteroids are the most commonly used anti-inflammatory drugs given for treatment of BP. They are administered systemically, for example prednisolone, or topically for very potent steroids such as clobetasol. Anti-inflammatory antibiotics such as tetracyclines are used as well. Long-term use of potent steroids is associated with several adverse effects such as severe skin atrophy, osteoporosis, diabetes, glaucoma, cataract formation, weight gain, and psychologic disturbances. Intravenous immunoglobulins have been used as immunomodulatory agents in BP as well as other auto immune blistering skin diseases.
The choice of treatment depends on the individual patient’s circumstances especially the severity of the BP and presence of comorbidities. All of these treatment options have limited applicability due to reasons associated with efficacy, safety or both. And thus, there exists a need for a safe and effective therapy for BP patients.
Atopic Keratoconjunctivitis
Atopic keratoconjunctivitis, or AKC, is a severe allergic conjunctivitis which usually persists all year and for which the allergen(s) are usually unidentified. AKC, which is an orphan disease, mainly affects adults and is triggered by mast cell activation secondary to inflammatory stimuli including eosinophils, neutrophils and Th2-generated cytokines. Treatment is initially with topical lubricants, antihistamines, immunomodulators (e.g. ciclosporin A) and intermittent steroids but systemic immunotherapy may become necessary in patients unresponsive to topical therapy. AKC sufferers usually exhibit symptoms such as itching, burning, tearing, erythematosus and swollen eye lids. The disease may lead to corneal scarring and may also lead to vascularisation of the cornea. Both eyes are usually affected equally. There is substantial unmet need for topical agents that will prevent the progression of corneal involvement and the requirement for systemic immunomodulators.
Thrombotic Microangiopathy (TMAs)
TMAs are a group of diseases in which thrombosis occurs in small blood vessels as a result of damage to the endothelium (lining) of the vessels. This leads to haemolytic anaemia, low platelet count (thrombocytopaenia), end organ damage which may result in complications including renal failure, stroke and pulmonary hypertension. The major varieties of TMA are haemolytic uraemic syndrome (HUS), atypical haemolytic uraemic syndrome (aHUS), thrombotic thrombocytopaenic purpura (TTP), antiphospholipid syndrome (APS), disseminated intravascular coagulation (DIC), malignant hypertension, scleroderma renal failure and TMA associated with haemapoietc stem cell transplant (HSCT). The latter is often linked to toxicity of calcineurin inhibitors which are used to protect against graft versus host disease (GvHD). There are no currently approved drugs for the treatment of HSCT-TMA and, untreated, the condition in its more severe forms has a high risk of death.
Paroxysmal nocturnal haemoglobinuria (PNH)
PNH is an ultra-rare, life-threatening and debilitating disease of the blood with an estimated 8,000 – 10,000 patients globally. Due to an acquired genetic deficiency, uncontrolled complement activation in PNH patients allows their own complement system to attack and destroy blood cells, leading to life-threatening complications.
Patients with PNH suffer from chronic complement activation and destruction of some of their blood cells, known as hemolysis, caused by the C5 cleavage product C5b-9 (the membrane attack complex). This hemolysis is associated with further clinical symptoms and negative outcomes, including kidney disease, thrombosis (blood clots), liver dysfunction, fatigue, impaired quality of life, recurring pain, shortness of breath, pulmonary hypertension, intermittent episodes of dark-colored urine (hemoglobinuria), and anemia. When the destruction of red blood cells is sufficiently large, recurrent blood transfusions may be necessary.
Soliris®, (eculizumab) is the only FDA approved drug for the treatment of PNH. Before the introduction of eculizumab, PNH patients, many of whom were in young adulthood, faced a life of repeated blood transfusions, thromboembolic complications and typical life expectancies of only 8 – 10 years from diagnosis.
It has recently been discovered that a small but identifiable subgroup of eculizumab-treated patients have a C5 polymorphism affecting the eculizumab binding site which prevents high affinity binding and makes these patients resistant to treatment — but does not appear to affect binding by Nomacopan (Coversin) in those patients tested to date.
Other Indications
We are also conducting discovery and pre-clinical research into the use of an engineered, targeted version of Nomacopan (Coversin) for the treatment of other diseases such as Myasthenia Gravis.
Market Opportunity in Complement Mediated Diseases
The NIH estimates that approximately 23.5 million Americans may suffer from an autoimmune disorder, although this number may underestimate actual prevalence as it includes only 24 diseases for which good epidemiology studies were available. Researchers have identified 80 – 100 different autoimmune diseases and suspect at least 40 additional diseases of having an autoimmune basis. These diseases are chronic and can be life-threatening. Autoimmune disease is one of the top 10 leading causes of death in female children and women in all age groups up to 64 years of age. The NIH estimates annual direct health care costs for autoimmune diseases to be in the range of $100 billion.
| 37 |
Both the complement and leukotrine pathways work as part of the immune system to disable and clear out foreign invaders and unwanted cells, and as such, plays an important role in the pathology of many autoimmune diseases. The term “Complement Mediated Diseases” applies to diseases and conditions where a patient’s immune system attacks and destroys healthy body tissue by mistake, causing damage through its complement component and through mediators induced by complement activation. These diseases and conditions are often very rare, and include such diseases as PNH, aHUS, GBS, Myasthenia Gravis, transplant mediated organ rejection, glomerulopathies (kidney diseases), as well as numerous other disorders. While not all complement mediated diseases will respond to a direct C5 inhibitor, like Nomacopan (Coversin), there is a large market opportunity as demonstrated by eculizumab with approximately $3.6 billion in net sales in 2018.
In addition to those conditions listed, where complement activity is believed to be the primary driver of disease, there are many other poorly treated diseases where in addition to complement activation other inflammatory pathways are implicated. Examples of such diseases where both the complement and leukotriene pathways are both believed to play an active role include BP, AKC, vasculitis, trauma and several severe inflammatory lung conditions. An example of an approved leukotriene inhibitor is Zileuton, which is a treatment for severe asthma.
Competition in Complement and Leukotriene Mediated Diseases
The development and commercialization of new drugs is highly competitive. We will face competition with respect to all product candidates that we may develop or commercialize in the future from pharmaceutical and biotechnology companies worldwide. The key factors affecting the success of any approved product will be its efficacy, safety profile, drug interactions, method of administration, pricing, reimbursement and level of promotional activity relative to those of competing drugs.
Our potential competitors may have substantially greater financial, technical, and personnel resources than we do. In addition, many of these competitors have significantly greater commercial infrastructures. Our ability to compete successfully will depend largely on our ability to leverage our collective experience in drug discovery, development and commercialization to:
| · | discover and develop drugs that are differentiated from other products in the market, including eculizumab; |
| · | obtain patent and/or proprietary protection for our product candidates and technologies; |
| · | obtain required regulatory approvals; |
| · | obtain a commercial partner; |
| · | commercialize our product candidates, if approved; and |
| · | attract and retain high-quality research, development and commercial personnel. |
There has been a broad research effort in complement based therapy to date, with eculizumab being the first and only therapy approved that directly inhibits C5. Although there is currently less R&D effort in the leukotriene field there are approved leukotriene inhibitors such as Zileuton, which is a treatment for severe asthma. However, we are aware of certain other companies and academic institutions that are continuing their efforts to discover and develop alternate complement and/or C5 inhibitors, including Alexion, Appelis, Amyndus, Achillion, Omeros, Chemocentryx, True North InflaRx, and RA Pharmaceuticals.
Sales and Marketing
Because we are focused on discovery and development of drugs, we currently have no sales, marketing or distribution capabilities in order to commercialize Nomacopan (Coversin) or any approved product candidates. If our lead product candidate Nomacopan (Coversin) is approved, we intend either to establish a sales and marketing organization with technical expertise and supporting distribution capabilities to commercialize Nomacopan (Coversin), or to outsource this function to a third party. We will adopt a similar strategy for the other compounds in our pipeline.
| 38 |
Manufacturing
We currently rely on a third-party contract manufacturer, or CMO, which complies with FDA’s current good manufacturing practice requirements, for all of our clinical supplies, including active pharmaceutical ingredients, or APIs, drug substances and finished drug products for our preclinical research and clinical trials, including the Phase I/II trials for Nomacopan (Coversin). Analytical methods that define activity, identity, purity, sterility, endotoxin, and host cell related impurities have been established and qualified for release testing of drug substance. We successfully completed a commercial scale process batch in 2016 and manufactured multiple lots of Nomacopan (Coversin) in 2018. We expect that during 2019 we will need to manufacture additional lots of Nomacopan (Coversin).
We do not own or operate, and currently have no plans to establish, any manufacturing facilities. We currently rely, and expect to continue to rely, on CMOs for the manufacture of Nomacopan (Coversin) and any other product candidates that we may develop for larger scale preclinical and clinical testing, as well as for commercial quantities of any product candidates that are approved.
We currently expect initial commercial supplies of Nomacopan (Coversin) to be supplied as a dry powder reconstituted into a pen injector which will hold one week’s supply of Nomacopan (Coversin) stable at room temperature.
We plan to expand our relationship with the current CMO for commercial supplies of Nomacopan (Coversin), if and when it nears potential approval, and plan to examine alternate CMOs for secondary commercial supplies of Nomacopan (Coversin). and other pipeline molecules.
Intellectual Property
We will be able to protect our technology and products from unauthorized use by third parties only to the extent it is covered by valid and enforceable patents or is effectively maintained as trade secrets. Patents and other proprietary rights are thus an essential element of our business.
Our success will depend in part on our ability to obtain and maintain proprietary protection for our product candidates, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing its proprietary rights. Our policy is to seek to protect its proprietary position by, among other methods, filing U.S. and foreign patent applications related to its proprietary technology, inventions, and improvements that are important to the development of its business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
We own or have exclusive rights to six United States patents, 74 national patents in Europe derived from five patents granted by the European Patent Office and 22 foreign issued patents in other jurisdictions, and eight United States and 21 foreign or international pending patent applications, relating to the complement C5 inhibitor protein Nomacopan (Coversin) and to its use in the treatment of key disease indications, as well as to Nomacopan (Coversin) variants. Our current patent portfolio includes granted patents in the jurisdictions of United States, Canada, major European countries, Japan, China, Australia and New Zealand and pending applications in the jurisdictions of United States, Canada, Europe, Japan, China, Brazil, Israel, India, Republic of Korea, Mexico, Russia, Australia and New Zealand.
Issued patents in the US and other countries which cover our product candidate Nomacopan (Coversin) and its uses will expire between 2024 and 2031, excluding any patent term extensions that might be available following the grant of marketing authorizations. We have pending patent applications for our product candidate Nomacopan (Coversin) and its uses that, if issued, would expire in the United States and in countries outside of the United States between 2024 and 2039, excluding any patent term adjustment that might be available following the grant of the patent and any patent term extensions that might be available following the grant of marketing authorizations. These patent and patent applications relate to subject matters including: complement inhibitor molecule; methods for treating myasthenia gravis; methods for treating peripheral nerve disorders; methods for treating respiratory disorders; methods for treating viral infections of the respiratory tract; methods of treating complement-mediated diseases in patients with C5 polymorphisms, methods of treating acute graft versus host disease; Nomacopan (Coversin) for the treatment of cicatrizing eye inflammatory disorders; Nomacopan (Coversin) for the treatment of autoimmune blistering diseases; and Nomacopan (Coversin) variants lacking C5 binding.
| 39 |
Government Regulation
Government Regulation and Product Approval
Government authorities in the U.S., at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, marketing and export and import of products such as those that we are developing. A new drug must be approved by the FDA, generally through the new drug application, or NDA, process and a new biologic must be approved by the FDA through the biologics license application, or BLA, process before it may be legally marketed in the U.S. The animal and other non-clinical data and the results of human clinical trials performed under an Investigational New Drug application, or IND, and under similar foreign applications will become part of the NDA or BLA.
U.S. Drug Development Process
In the U.S., the FDA regulates drugs under the Federal Food, Drug, and Cosmetic Act, or FDCA, and in the case of biologics, also under the Public Health Service Act, or PHSA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local, and foreign statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, requesting product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement, or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us. The process required by the FDA before a drug or biologic may be marketed in the U.S. generally involves the following:
| · | completion of preclinical laboratory tests, animal studies and formulation studies according to Good Laboratory Practices or other applicable regulations; |
| · | submission to the FDA of an IND which must become effective before human clinical trials may begin; |
| · | performance of adequate and well-controlled human clinical trials according to Good Clinical Practices to establish the safety and efficacy of the proposed drug for its intended use; |
| · | submission to the FDA of an NDA or BLA; |
| · | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the drug is produced to assess compliance with current good manufacturing practice, or cGMP, to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| · | FDA review and approval of the NDA or BLA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. The sponsor will also include a protocol detailing, among other things, the objectives of the first phase of the clinical trials, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated, if the first phase lends itself to an efficacy evaluation. Some preclinical testing may continue even after the IND is submitted. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the clinical trial on a clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before the clinical trial can begin. Clinical holds also may be imposed by the FDA at any time before or during studies due to safety concerns or non-compliance.
| 40 |
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with good clinical practice regulations. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND, and progress reports detailing the results of the clinical trials must be submitted at least annually. In addition, timely safety reports must be submitted to the FDA and the investigators for serious and unexpected adverse events. An institutional review board, or IRB, responsible for the research conducted at each institution participating in the clinical trial must review and approve each protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each trial subject or his or her legal representative, monitor the study until completed and otherwise comply with IRB regulations.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| · | Phase I: The product candidate is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be too inherently toxic to ethically administer to healthy volunteers, the initial human testing may be conducted in patients. |
| · | Phase II: This phase involves studies in a limited patient population to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| · | Phase III: Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product candidate and provide, if appropriate, an adequate basis for product labeling. |
The FDA or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the research subjects or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients. Phase I, Phase II, and Phase III testing may not be completed successfully within any specified period, if at all.
During the development of a new drug, sponsors are given opportunities to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase II, and before an NDA or BLA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the end of Phase II meeting to discuss their Phase II clinical results and present their plans for the pivotal Phase III clinical trial that they believe will support approval of the new drug.
Concurrent with clinical trials, companies usually complete additional animal studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the manufacturer must develop methods for testing the identity, strength, quality and purity of the final drug. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
| 41 |
U.S. Review and Approval Processes
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling, and other relevant information are submitted to the FDA as part of an NDA or BLA requesting approval to market the product. The submission of an NDA or BLA is subject to the payment of user fees; a waiver of such fees may be obtained under certain limited circumstances. The FDA initially reviews all NDAs and BLAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. The FDA may request additional information rather than accept a NDA or BLA for filing. In this event, the NDA or BLA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing. Once the submission is accepted for filing, the FDA begins an in-depth substantive review. FDA may refer the NDA or BLA to an advisory committee for review, evaluation and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. The approval process is lengthy and often difficult, and the FDA may refuse to approve an NDA or BLA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data and information. Even if such data and information are submitted, the FDA may ultimately decide that the NDA or BLA does not satisfy the criteria for approval. Data obtained from clinical trials are not always conclusive and the FDA may interpret data differently than we interpret the same data. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. The FDA reviews a BLA to determine, among other things whether the product is safe, pure and potent and the facility in which it is manufactured, processed, packed or held meets standards designed to assure the product’s continued safety, purity and potency. Before approving an NDA or BLA, the FDA will inspect the facility or facilities where the product is manufactured. The FDA may issue a complete response letter, which may require additional clinical or other data or impose other conditions that must be met in order to secure final approval of the NDA or BLA, or an approval letter following satisfactory completion of all aspects of the review process.
NDAs or BLAs may receive either standard or priority review. Under current FDA review goals, standard review of an NDA for a new molecular entity (NME) or original BLA will be ten months from the date that the NDA or BLA is filed. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive a priority review of six months. Priority review does not change the standards for approval, but may expedite the approval process.
If a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial value of the product. In addition, the FDA may require a sponsor to conduct Phase IV testing which involves clinical trials designed to further assess a drug’s safety and effectiveness after NDA or BLA approval, and may require testing and surveillance programs to monitor the safety of approved products which have been commercialized.
The Food and Drug Administration Safety and Innovation Act, or FDASIA, which was enacted in 2012, made permanent the Pediatric Research Equity Act, or PREA, which requires a sponsor to conduct pediatric studies for most drugs and biologics with a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration. Under PREA, original NDAs, BLAs and supplements thereto, must contain a pediatric assessment unless the sponsor has received a deferral or waiver. The required assessment must assess the safety and effectiveness of the product for the claimed indications in all relevant pediatric subpopulations and support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The sponsor or FDA may request a deferral of pediatric studies for some or all of the pediatric subpopulations. A deferral may be granted for several reasons, including a finding that the drug or biologic is ready for approval for use in adults before pediatric studies are complete or that additional safety or effectiveness data needs to be collected before pediatric studies can begin. After April 2013, the FDA must send a non-compliance letter to any sponsor that fails to submit a required pediatric assessment within specified deadlines or fails to submit a timely request for approval of a pediatric formulation, if required.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of our drugs, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as partial compensation for effective patent term lost due to time spent during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA or BLA, plus the time between the submission date of an NDA or BLA and the approval of that application, except that the period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved drug may be extended, and the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
| 42 |
Pediatric exclusivity is another type of marketing exclusivity available in the U.S. The FDASIA made permanent the Best Pharmaceuticals for Children Act, or BPCA, which provides, under certain circumstances, for an additional six months of marketing exclusivity if a sponsor conducts clinical trials in children in response to a written request from the FDA, or a Written Request. If the Written Request does not include studies in neonates, the FDA is required to include its rationale for not requesting those studies. The FDA may request studies on approved or unapproved indications in separate Written Requests. The issuance of a Written Request does not require the sponsor to undertake the described studies.
Biologics Price Competition and Innovation Act of 2009
The Biologics Price Competition and Innovation Act of 2009, or BPCIA, amended the PHSA to create an abbreviated approval pathway for two types of “generic” biologics — biosimilars and interchangeable biologic products — and provides for a twelve-year exclusivity period for the first approved biological product, or reference product, against which a biosimilar or interchangeable application is evaluated; however if pediatric studies are performed and accepted by the FDA, the twelve-year exclusivity period will be extended for an additional six months. A biosimilar product is defined as one that is highly similar to a reference product notwithstanding minor differences in clinically inactive components and for which there are no clinically meaningful differences between the biological product and the reference product in terms of the safety, purity and potency of the product. An interchangeable product is a biosimilar product that may be substituted for the reference product without the intervention of the health care provider who prescribed the reference product.
The biosimilar applicant must demonstrate that the product is biosimilar based on data from: (1) analytical studies showing that the biosimilar product is highly similar to the reference product; (2) animal studies (including toxicity); and (3) one or more clinical studies to demonstrate safety, purity and potency in one or more appropriate conditions of use for which the reference product is approved. In addition, the applicant must show that the biosimilar and reference products have the same mechanism of action for the conditions of use on the label, route of administration, dosage and strength, and the production facility must meet standards designed to assure product safety, purity and potency.
An application for a biosimilar product may not be submitted until four years after the date on which the reference product was first approved. The first approved interchangeable biologic product will be granted an exclusivity period of up to one year after it is first commercially marketed, but the exclusivity period may be shortened under certain circumstances.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S., or more than 200,000 individuals in the U.S. and for which there is no reasonable expectation that the cost of developing and making available in the U.S. a drug for this type of disease or condition will be recovered from sales in the U.S. for that drug. Orphan drug designation must be requested before submitting an NDA or BLA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not itself convey any advantage in or shorten the duration of the regulatory review and approval process. If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very limited circumstances, for seven years. Orphan drug exclusivity, however, also could block the approval of one of our product candidates for seven years if a competitor obtains approval of the same drug, for the same designated orphan indication or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease.
The FDA also administers a clinical research grants program, whereby researchers may compete for funding to conduct clinical trials to support the approval of drugs, biologics, medical devices, and medical foods for rare diseases and conditions. A product does not have to be designated as an orphan drug to be eligible for the grant program. An application for an orphan grant should propose one discrete clinical study to facilitate FDA approval of the product for a rare disease or condition. The study may address an unapproved new product or an unapproved new use for a product already on the market.
| 43 |
Fast Track Designation and Accelerated Approval
The FDA is required to facilitate the development, and expedite the review of, drugs that it finds are intended for the treatment of a serious or life-threatening disease or condition for which there is no effective treatment and which demonstrate the potential to address unmet medical needs for the condition. Under the fast track program, the sponsor of a new product candidate may request that the FDA designate the product candidate for a specific indication as a fast track drug concurrent with, or after, the filing of the IND for the product candidate. The FDA must determine if the product candidate qualifies for fast track designation within 60 days of receipt of the sponsor’s request.
Under the fast track program, the FDA may designate a drug for fast-track status if it is intended to treat a serious or life-threatening illness and nonclinical or clinical data demonstrate the potential to address an unmet medical need. Similarly, the agency may designate a drug for accelerated approval if it treats a serious condition and generally provides meaningful therapeutic benefit to patients over existing treatments based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality, that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments.
In clinical trials, a surrogate endpoint is a measurement of laboratory or clinical signs of a disease or condition that substitutes for a direct measurement of how a patient feels, functions, or survives. Surrogate endpoints can often be measured more easily or more rapidly than other clinical endpoints. A product candidate approved on this basis is generally subject to rigorous post-marketing compliance requirements, including the completion of Phase 4 or post-approval clinical trials to confirm the effect on the clinical endpoint. Failure to conduct required post-approval studies, or confirm a clinical benefit during post-marketing studies, will allow the FDA to withdraw the drug from the market on an expedited basis. All promotional materials for product candidates approved under accelerated regulations are subject to prior review by the FDA.
In addition to other benefits such as the ability to use surrogate endpoints and engage in more frequent interactions with the FDA, the FDA may initiate review of sections of a fast track drug’s NDA or BLA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal for reviewing an application does not begin until the last section of the application is submitted. Additionally, the fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Post-Approval Requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems are identified after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMP and other laws and regulations. We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Future inspections by the FDA and other regulatory agencies may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things, record-keeping requirements, reporting of adverse experiences with the drug, providing the FDA with updated safety and efficacy information, drug sampling and distribution requirements, complying with certain electronic records and signature requirements, and complying with FDA promotion and advertising requirements. The FDA strictly regulates labeling, advertising, promotion and other types of information that may be disseminated about products that are placed on the market. Drugs may be promoted only for the approved indications and in accordance with the provisions of the approved label.
| 44 |
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the development, approval, manufacturing and marketing of products regulated by the FDA. It is impossible to predict whether further legislative changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Regulation and Marketing Authorization in the European Union
The process governing approval of medicinal products in the European Union follows essentially the same lines as in the United States and, likewise, generally involves satisfactorily completing each of the following:
| · | preclinical laboratory tests, animal studies and formulation studies all performed in accordance with the applicable EU Good Laboratory Practice regulations; |
| · | submission to the relevant national authorities of a clinical trial application, or CTA, which must be approved before human clinical trials may begin; |
| · | performance of adequate and well-controlled clinical trials to establish the safety and efficacy of the product for each proposed indication |
| · | submission to the relevant competent authorities of a MAA, which includes the data supporting safety and efficacy as well as detailed information on the manufacture and composition of the product in clinical development and proposed labelling; |
| · | satisfactory completion of an inspection by the relevant national authorities of the manufacturing facility or facilities, including those of third parties, at which the product is produced to assess compliance with strictly enforced current cGMP; |
| · | potential audits of the non-clinical and clinical trial sites that generated the data in support of the MAA; and |
| · | review and approval by the relevant competent authority of the MAA before any commercial marketing, sale or shipment of the product. |
Preclinical Studies
Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as studies to evaluate toxicity in animal studies, in order to assess the potential safety and efficacy of the product. The conduct of the preclinical tests and formulation of the compounds for testing must comply with the relevant EU regulations and requirements. The results of the preclinical tests, together with relevant manufacturing information and analytical data, are submitted as part of the CTA and MAA.
Clinical Trial Approval
Requirements for the conduct of clinical trials in the European Union including Good Clinical Practice, or GCP, are implemented in the Clinical Trials Directive 2001/20/EC and the GCP Directive 2005/28/EC. Pursuant to Directive 2001/20/EC and Directive 2005/28/EC, as amended, a system for the approval of clinical trials in the European Union has been implemented through national legislation of the member states. Under this system, approval must be obtained from the competent national authority of an EU member state in which a study is planned to be conducted, or in multiple member states if the clinical trial is to be conducted in a number of member states. To this end, a CTA is submitted, which must be supported by an investigational medicinal product dossier, or IMPD, and further supporting information prescribed by Directive 2001/20/EC and Directive 2005/28/EC and other applicable guidance documents. Furthermore, a clinical trial may only be started after a competent ethics committee has issued a favorable opinion on the clinical trial application in that country.
| 45 |
In April 2014, a new Clinical Trials Regulation, (EU) No 536/2014, or the New CT Regulation, was adopted which will replace the current Clinical Trials Directive 2001/20/EC. To ensure that the rules for clinical trials are identical throughout the European Union, the new EU clinical trials legislation was passed as a “regulation” that is directly applicable in all EU member states. All clinical trials performed in the European Union are required to be conducted in accordance with the Clinical Trials Directive 2001/20/EC until the new Clinical Trials Regulation (EU) No 536/2014 becomes applicable. Although the New CT Regulation entered into force on June 16, 2014 the timing of its application depends on the development of a fully functional EU clinical trials portal and database, which will be confirmed by an independent audit. The New CT Regulation becomes applicable six months after the European Commission publishes a notice of this confirmation. While currently estimated to occur in 2019, as at April 16, 2019 no such publication of the notice of confirmation has occurred. Despite not yet being in force, the New CT Regulation is being treated as the template with respect to the emphasis it places on the need for transparency, accuracy and fairness in reporting clinical data.
The New CT Regulation aims to harmonize, simplify and streamline the approval of clinical trials in the European Union. The main characteristics of the New CT Regulation include:
| · | A streamlined application procedure via a single entry point, the EU portal. |
| · | A single set of documents to be prepared and submitted for the application as well as simplified reporting procedures that will spare sponsors from submitting broadly identical information separately to various bodies and different member states. |
| · | A harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts. Part I is assessed jointly by all member states concerned. Part II is assessed separately by each member state concerned. |
| · | Strictly defined deadlines for the assessment of clinical trial application. |
| · | The involvement of the ethics committees in the assessment procedure in accordance with the national law of the member state concerned but within the overall timelines defined by the New CT Regulation. |
Marketing Authorization
Authorization to market a product in the member states of the European Union proceeds under one of four procedures: a centralized authorization procedure, a mutual recognition procedure, a decentralized procedure or a national procedure.
Centralized Authorization Procedure
The centralized procedure enables applicants to obtain a marketing authorization that is valid in all EU member states based on a single application. Certain medicinal products, including products developed by means of biotechnological processes, must undergo the centralized authorization procedure for marketing authorization, which, if granted by the European Commission, is automatically valid in all 28 EU member states. The EMA and the European Commission administer this centralized authorization procedure pursuant to Regulation (EC) No 726/2004.
Pursuant to Regulation (EC) No 726/2004, this procedure is mandatory for:
| · | medicinal products developed by means of one of the following biotechnological processes: |
| · | recombinant DNA technology; |
| · | controlled expression of genes coding for biologically active proteins in prokaryotes and eukaryotes including transformed mammalian cells; and |
| · | hybridoma and monoclonal antibody methods; |
| · | advanced therapy medicinal products as defined in Article 2 of Regulation (EC) No. 1394/2007 on advanced therapy medicinal products; |
| 46 |
| · | medicinal products for human use containing a new active substance that, on the date of effectiveness of this regulation, was not authorized in the European Union, and for which the therapeutic indication is the treatment of any of the following diseases: |
| · | acquired immune deficiency syndrome; |
| · | cancer; |
| · | neurodegenerative disorder; |
| · | diabetes; |
| · | auto-immune diseases and other immune dysfunctions; and |
| · | viral diseases; and |
| · | medicinal products that are designated as orphan medicinal products pursuant to Regulation (EC) No 141/2000. |
The centralized authorization procedure is optional for other medicinal products if they contain a new active substance or if the applicant shows that the medicinal product concerned constitutes a significant therapeutic, scientific or technical innovation or that the granting of authorization is in the interest of patients in the European Union.
Administrative Procedure
Under the centralized authorization procedure, the EMA’s Committee for Human Medicinal Products, or CHMP, serves as the scientific committee that renders opinions about the safety, efficacy and quality of medicinal products for human use on behalf of the EMA. The CHMP is composed of experts nominated by each member state’s national authority for medicinal products, with expert appointed to act as Rapporteur for the co-ordination of the evaluation with the possible assistance of a further member of the Committee acting as a Co-Rapporteur. After approval, the Rapporteur(s) continue to monitor the product throughout its life cycle. The CHMP has 210 days to adopt an opinion as to whether a marketing authorization should be granted. The process usually takes longer in case additional information is requested, which triggers clock-stops in the procedural timelines. The process is complex and involves extensive consultation with the regulatory authorities of member states and a number of experts. When an application is submitted for a marketing authorization in respect of a drug that is of major interest from the point of view of public health and in particular from the viewpoint of therapeutic innovation, the applicant may pursuant to Article 14(9) Regulation (EC) No 726/2004 request an accelerated assessment procedure. If the CHMP accepts such request, the time-limit of 210 days will be reduced to 150 days but it is possible that the CHMP can revert to the standard time limit for the centralized procedure if it considers that it is no longer appropriate to conduct an accelerated assessment. Once the procedure is completed, a European Public Assessment Report, or EPAR, is produced. If the opinion is negative, information is given as to the grounds on which this conclusion was reached. After the adoption of the CHMP opinion, a decision on the MAA must be adopted by the European Commission, after consulting the EU member states, which in total can take more than 60 days.
Conditional Approval
In specific circumstances, EU legislation (Article 14(7) Regulation (EC) No 726/2004 and Regulation (EC) No 507/2006 on Conditional Marketing Authorisations for Medicinal Products for Human Use) enables applicants to obtain a conditional marketing authorization prior to obtaining the comprehensive clinical data required for an application for a full marketing authorization. Such conditional approvals may be granted for product candidates (including medicines designated as orphan medicinal products) if (1) the risk-benefit balance of the product candidate is positive, (2) it is likely that the applicant will be in a position to provide the required comprehensive clinical trial data, (3) the product fulfills unmet medical needs and (4) the benefit to public health of the immediate availability on the market of the medicinal product concerned outweighs the risk inherent in the fact that additional data are still required. A conditional marketing authorization may contain specific obligations to be fulfilled by the marketing authorization holder, including obligations with respect to the completion of ongoing or new studies, and with respect to the collection of pharmacovigilance data. Conditional marketing authorizations are valid for one year, and may be renewed annually, if the risk-benefit balance remains positive, and after an assessment of the need for additional or modified conditions and/or specific obligations. The timelines for the centralized procedure described above also apply with respect to the review by the CHMP of applications for a conditional marketing authorization.
| 47 |
Marketing Authorization under Exceptional Circumstances
Under Article 14(8) Regulation (EC) No 726/2004, products for which the applicant can demonstrate that comprehensive data (in line with the requirements laid down in Annex I of Directive 2001/83/EC, as amended) cannot be provided (due to specific reasons foreseen in the legislation) might be eligible for marketing authorization under exceptional circumstances. This type of authorization is reviewed annually to reassess the risk-benefit balance. The fulfillment of any specific procedures/obligations imposed as part of the marketing authorization under exceptional circumstances is aimed at the provision of information on the safe and effective use of the product and will normally not lead to the completion of a full dossier/approval.
Market Authorizations Granted by Authorities of EU Member States
In general, if the centralized procedure is not followed, there are three alternative procedures as prescribed in Directive 2001/83/EC:
| · | The decentralized procedure allows applicants to file identical applications to several EU member states and receive simultaneous national approvals based on the recognition by E.U. member states of an assessment by a reference member state. |
| · | The national procedure is only available for products intended to be authorized in a single EU member state. |
| · | A mutual recognition procedure similar to the decentralized procedure is available when a marketing authorization has already been obtained in at least one EU member state. |
A marketing authorization may be granted only to an applicant established in the European Union.
Paediatric Studies
Prior to obtaining a marketing authorization in the European Union, applicants have to demonstrate compliance with all measures included in an EMA-approved Paediatric Investigation Plan, or PIP, covering all subsets of the paediatric population, unless the EMA has granted a product-specific waiver, a class waiver, or a deferral for one or more of the measures included in the PIP. The respective requirements for all marketing authorization procedures are set forth in Regulation (EC) No 1901/2006, which is referred to as the Pediatric Regulation. This requirement also applies when a company wants to add a new indication, pharmaceutical form or route of administration for a medicine that is already authorized. The Paediatric Committee of the EMA, or PDCO, may grant deferrals for some medicines, allowing a company to delay development of the medicine in children until there is enough information to demonstrate its effectiveness and safety in adults. The PDCO may also grant waivers when development of a medicine in children is not needed or is not appropriate, such as for diseases that only affect the elderly population.
Before a marketing authorization application can be filed, or an existing marketing authorization can be amended, the EMA determines that companies actually comply with the agreed studies and measures listed in each relevant PIP.
| 48 |
Periods of Authorization and Renewals
A marketing authorization is valid for five years in principle and the marketing authorization may be renewed after five years on the basis of a re-evaluation of the risk-benefit balance by the competent authority of the authorizing member state. To this end, the marketing authorization holder must provide the EMA or the competent authority with a consolidated version of the file in respect of quality, safety and efficacy, including all variations introduced since the marketing authorization was granted, at least six months before the marketing authorization ceases to be valid. Once renewed, the marketing authorization is valid for an unlimited period, unless the European Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal. Any authorization which is not followed by the actual placing of the drug on the EU market (in case of centralized procedure) or on the market of the authorizing member state within three years after authorization ceases to be valid (the so-called sunset clause).
Orphan Drug Designation and Exclusivity
Pursuant to Regulation (EC) No 141/2000 and Regulation (EC) No. 847/2000, the European Commission can grant such orphan medicinal product designation to products for which the sponsor can establish that it is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting not more than five in 10,000 people in the European Union, or a life threatening, seriously debilitating or serious and chronic condition in the European Union and that without incentives it is unlikely that sales of the drug in the European Union would generate a sufficient return to justify the necessary investment. In addition, the sponsor must establish that there is no other satisfactory method approved in the European Union of diagnosing, preventing or treating the condition, or if such a method exists, the proposed orphan drug will be of significant benefit to patients.
Orphan drug designation is not a marketing authorization. It is a designation that provides a number of benefits, including fee reductions, regulatory assistance, and the possibility to apply for a centralized EU marketing authorization, as well as ten years of market exclusivity following a marketing authorization. During this market exclusivity period, neither the EMA, the European Commission nor the member states can accept an application or grant a marketing authorization for a “similar medicinal product.” A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as those contained in an authorized orphan medicinal product and that is intended for the same therapeutic indication. The market exclusivity period for the authorized therapeutic indication may be reduced to six years if, at the end of the fifth year, it is established that the orphan designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. In addition, a competing similar medicinal product may, in limited circumstances, be authorized prior to the expiration of the market exclusivity period, including if it is shown to be safer, more effective or otherwise clinically superior to the already approved orphan drug. Furthermore, a product can lose orphan designation, and the related benefits, prior to obtaining a marketing authorization if it is demonstrated that the orphan designation criteria are no longer met.
Regulatory Data Protection
EU legislation also provides for a system of regulatory data and market exclusivity. According to Article 14(11) of Regulation (EC) No 726/2004, as amended, and Article 10(1) of Directive 2001/83/EC, as amended, upon receiving marketing authorization, new chemical entities approved on the basis of complete independent data package benefit from eight years of data exclusivity and an additional two years of market exclusivity. Data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a generic (abbreviated) application. During the additional two-year period of market exclusivity, a generic marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic medicinal product can be marketed until the expiration of the market exclusivity. The overall ten-year period will be extended to a maximum of eleven years if, during the first eight years of those ten years, the marketing authorization holder, or MAH, obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are held to bring a significant clinical benefit in comparison with existing therapies. Even if a compound is considered to be a new chemical entity and the innovator is able to gain the period of data exclusivity, another company nevertheless could also market another version of the drug if such company obtained marketing authorization based on an MAA with a complete independent data package of pharmaceutical test, preclinical tests and clinical trials. However, products designated as orphan medicinal products enjoy, upon receiving marketing authorization, a period of ten years of orphan market exclusivity—see also Orphan Drug Designation and Exclusivity. Depending upon the timing and duration of the EU marketing authorization process, products may be eligible for up to five years’ supplementary protection certificates, or SPCs, pursuant to Regulation (EC) No 469/2009. Such SPCs extend the rights under the basic patent for the drug.
| 49 |
Regulatory Requirements After a Marketing Authorization has been Obtained
If we obtain authorization for a medicinal product in the European Union, we will be required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products:
Pharmacovigilance and other requirements
We will, for example, have to comply with the EU’s stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. Other requirements relate, for example, to the manufacturing of products and APIs in accordance with good manufacturing practice standards. EU regulators may conduct inspections to verify our compliance with applicable requirements, and we will have to continue to expend time, money and effort to remain compliant. Non-compliance with EU requirements regarding safety monitoring or pharmacovigilance, and with requirements related to the development of products for the pediatric population, can also result in significant financial penalties in the European Union. Similarly, failure to comply with the EU’s requirements regarding the protection of individual personal data can also lead to significant penalties and sanctions. Individual EU member states may also impose various sanctions and penalties in case we do not comply with locally applicable requirements.
Manufacturing
The manufacturing of authorized drugs, for which a separate manufacturer’s license is mandatory, must be conducted in strict compliance with the EMA’s Good Manufacturing Practices, or GMP, requirements and comparable requirements of other regulatory bodies in the European Union, which mandate the methods, facilities and controls used in manufacturing, processing and packing of drugs to assure their safety and identity. The EMA enforces its current GMP requirements through mandatory registration of facilities and inspections of those facilities. The EMA may have a coordinating role for these inspections while the responsibility for carrying them out rests with the member states competent authority under whose responsibility the manufacturer falls. Failure to comply with these requirements could interrupt supply and result in delays, unanticipated costs and lost revenues, and could subject the applicant to potential legal or regulatory action, including but not limited to warning letters, suspension of manufacturing, seizure of product, injunctive action or possible civil and criminal penalties.
Marketing and Promotion
The marketing and promotion of authorized drugs, including industry-sponsored continuing medical education and advertising directed toward the prescribers of drugs and/or the general public, are strictly regulated in the European Union under Directive 2001/83/EC. The applicable regulations aim to ensure that information provided by holders of marketing authorizations regarding their products is truthful, balanced and accurately reflects the safety and efficacy claims authorized by the EMA or by the competent authority of the authorizing member state. Failure to comply with these requirements can result in adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties.
Patent Term Extension
In order to compensate the patentee for delays in obtaining a marketing authorization for a patented product, a supplementary protection certificate, or SPC, may be granted extending the exclusivity period for that specific product by up to five years. Applications for SPCs must be made to the relevant patent office in each EU member state and the granted certificates are valid only in the member state of grant. An application has to be made by the patent owner within six months of the first marketing authorization being granted in the European Union (assuming the patent in question has not expired, lapsed or been revoked) or within six months of the grant of the patent (if the marketing authorization is granted first). In the context of SPCs, the term “product” means the active ingredient or combination of active ingredients for a medicinal product and the term “patent” means a patent protecting such a product or a new manufacturing process or application for it. The duration of an SPC is calculated as the difference between the patent’s filing date and the date of the first marketing authorization, minus five years, subject to a maximum term of five years.
A six-month pediatric extension of an SPC may be obtained where the patentee has carried out an agreed paediatric investigation plan, the authorized product information includes information on the results of the studies and the product is authorized in all member states of the EU. The six-month paediatric extension of SPCs is not available for orphan medicinal products, as such products benefit from a separate two year paediatric extension of orphan status and exclusivity. The six month paediatric extension of SPCs is, however, available for medicinal products which were originally designated as orphan medicinal products but were subsequently (voluntarily) removed from the EU’s Community Register of Orphan Medicinal Products.
| 50 |
Foreign Regulation
In addition to regulations in the United States and European Union, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA or EMA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we may commence clinical trials or market products in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA or EMA approval.
Pharmaceutical Pricing and Reimbursement
Sales of pharmaceutical products depend in significant part on the extent of coverage and reimbursement from government programs, including Medicare and Medicaid in the U.S., and other third party payers. Third party payers are sensitive to the cost of drugs and are increasingly seeking to implement cost containment measures to control, restrict access to, or influence the purchase of drugs, biologicals, and other health care products and services. Governments may regulate reimbursement, pricing, and coverage of products in order to control costs or to affect levels of use of certain products. Payers may restrict coverage of some products due to cost concerns, by various means such as using payer formularies under which only selected drugs are covered, variable co-payments that make drugs that are not preferred by the payer more expensive in terms of higher out-of-pocket expenses for patients, and by employing utilization management controls, such as discouraging patients’ use of copay coupons and discount cards and imposing requirements for prior authorization before a prescription can be billed or prior clinical failure on another type of treatment before a new product can be prescribed. Payers may especially impose these obstacles to coverage for higher-priced drugs in order to limit the payer’s cost for treatment of the disease. Consequently, any future products may be subject to payer-driven restrictions, rendering patients responsible for a higher percentage of the total cost of drugs in the outpatient setting. This could lower the demand for any future products if the increased patient out-of-pocket cost-sharing obligations are more than they can afford.
Medicare is a U.S. federal government insurance program that covers individuals aged 65 years or older, as well as individuals of any age with certain disabilities, and individuals with End-Stage Renal Disease. The primary Medicare programs that may affect reimbursement for Akari are Medicare Part B, which covers physician services and outpatient care, and Medicare Part D, which provides a voluntary outpatient prescription drug benefit. Medicare Part B provides limited coverage of certain outpatient drugs and biologicals that are reasonable and necessary for diagnosis or treatment of an illness or injury. Under Medicare Part B, reimbursement for most drugs is based on a fixed percentage above the applicable product’s average sales price, or ASP. Manufacturers calculate ASP based on a statutory formula and must report ASP information on a quarterly basis to the Centers for Medicare and Medicaid Services (CMS), the federal agency that administers Medicare and the Medicaid Drug Rebate Program. The current reimbursement rate for drugs and biologicals in both the hospital outpatient department setting and the physician office setting is ASP + 6%. The rate for the physician clinic setting is set by statute, but CMS has the authority to adjust the rate for the hospital outpatient setting on an annual basis. This reimbursement rate may decrease in the future. In both settings, the amount of reimbursement for a product’s usage is updated quarterly based on the manufacturer’s submission of new ASP information about its product or based on the submission of ASP information of each manufacturer that sells a product for which there are multiple competitors in that product market. On October 30, 2018, CMS issued an advance notice of proposed rulemaking (ANPRM) proposing a rule to set prices for certain drugs based on prices paid in other nations. Under the proposal, beginning in 2020 and extending until 2025, CMS would use a model called the IPI to allow Medicare to more closely align its Medicare payment amount for selected Part B drugs with prices paid in other nations and would allow for private-sector vendors to negotiate drug prices, take title to drugs, and compete for physician and hospital business. The current pricing model is based on average sales prices plus a 4.3 percent add-on payment. The IPI model would include mandatory participation from physician practices and hospital outpatient clinics that supply the included drugs. It is uncertain whether CMS the proposed rule will be issued.
Medicare Part D is a prescription drug benefit available to all Medicare beneficiaries. It is a voluntary benefit that is implemented through private plans under contractual arrangements with the federal government. Similar to pharmaceutical coverage through private health insurance, Part D plans negotiate discounts from drug manufacturers. Medicare Part D coverage is available through private plans, and the list of prescription drugs covered by Part D plans varies by plan. However, individual plans are required by statute to cover certain therapeutic categories and classes of drugs or biologicals and to have at least two drugs in each unique therapeutic category or class, with certain exceptions.
| 51 |
Medicare Part A covers inpatient hospital benefits. Hospitals typically receive a single payment for an inpatient stay depending on the Medicare Severity Diagnosis Related Group (MS-DRG) to which the inpatient stay is assigned. The MS-DRG for a hospital inpatient stay varies based on the patient’s condition. Hospitals generally do not receive separate payment for drugs and biologicals administered to patients during an inpatient hospital stay. As a result, hospitals may not have a financial incentive to utilize any future products for inpatients.
Beginning April 1, 2013, the Budget Control Act of 2011, Pub. L. No. 112-25, as amended by the American Taxpayer Relief Act of 2012, Pub. L. 112-240, required Medicare payments for all items and services, including drugs and biologicals, to be reduced by 2% under sequestration (i.e., automatic spending reductions). Subsequent legislation extended the 2% reduction, on average, to 2025. This 2% reduction in Medicare payments affects all Parts of the Medicare program and could impact any future sales of any future products.
On January 20, 2017, President Trump took office as the President of the United States. President Trump has stated that he intends to “repeal and replace” the Affordable Care Act, and Congress has taken initial steps to repeal the law. In December 2017, Congress passed and the President signed into law tax reform legislation that made significant changes to the Affordable Care Act including the repeal of the “individual mandate” that was in place to strongly encourage broad participation in the health insurance markets. Given these changes and other statements of political leaders, we cannot predict the ultimate impact on the Affordable Care Act and the subsequent effect on the pharmaceutical industry at this time. In the fourth quarter of 2018, the Trump Administration announced initiatives that it asserted are intended to result in purportedly lower drug prices. The first initiative, announced on October 15, 2018, involved the plan to a new federal regulation that would require pharmaceutical manufacturers to disclose the list prices of their respective prescription drugs in their television advertisements for their products if the list price is greater than $35 USD. With respect to the second initiative, on October 25, 2018, the Centers for Medicaid and Medicare Services gave Advance Notice of Proposed Rulemaking to propose the implementation of an “International Pricing Index” model for Medicare Part B drugs and biologicals (single source drugs, biologicals, and biosimilars). Public comments were due on December 31, 2018 with a proposed rule theoretically being offered as early as Spring 2019 with target implementation of a five year pilot program beginning in Spring 2020. While these initiatives have not been put into effect, we are not in a position to know at this time whether they will ever become law or what impact the enactment either of these proposals would have on our business.
In the fourth quarter of 2018, the Trump Administration announced initiatives that it asserted are intended to result in purportedly lower drug prices. The first initiative, announced on October 15, 2018, involved the plan to a new federal regulation that would require pharmaceutical manufacturers to disclose the list prices of their respective prescription drugs in their television advertisements for their products if the list price is greater than $35 USD. With respect to the second initiative, on October 25, 2018, the Centers for Medicaid and Medicare Services gave Advance Notice of Proposed Rulemaking to propose the implementation of an “International Pricing Index” model for Medicare Part B drugs and biologicals (single source drugs, biologicals, and biosimilars). Public comments were due on December 31, 2018 with a proposed rule theoretically being offered as early as Spring 2019 with target implementation of a five year pilot program beginning in Spring 2020. While these initiatives have not been put into effect, we are not in a position to know at this time whether they will ever become law or what impact the enactment either of these proposals would have on our business.
In February 2019, the Department of Health and Human Services has proposed a regulation that would significantly restrict the availability of certain regulatory safe harbors under the federal Anti-Kickback Statute that are used to facilitate certain types of transactions between manufacturers and pharmacy benefits managers that play a significant role in the pharmaceutical distribution chain. These changes to the Discount Safe Harbors available under the Anti-Kickback Statute would reduce some of the protections currently available to manufacturers that pay negotiated rebates to pharmacy benefits managers in exchange for these “PBMs” agreeing to include drugs and biologics on the formularies of the PBM’s downstream customers, primarily the health plans that insure patients for both private commercial plans and government-sponsored plans. While we do not know whether the Trump Administration will be successful in implementing this proposed regulation, its successful implementation could have an impact on both our commercial supply arrangements with health plans and our supply arrangements to health plans that serve beneficiaries of federal health care programs such as Medicare Part D.
| 52 |
As part of its reform of the 340B discount drug program, on October 31, 2018, the HRSA at HHS issued a notice of proposed rulemaking to move up the effective date of a final rule that would give HHS authority to impose Civil Monetary Penalties on pharmaceutical manufacturers who knowingly and intentionally charged a covered entity more than the statutorily allowed ceiling price for a covered outpatient drug. The final rule is intended to encourage compliance by manufacturers in offering the mandatory 340B ceiling purchase price to eligible purchasers, such as certain qualified health systems or individual hospitals.
Various states, such as California, have also taken steps to consider and enact laws or regulations that are intended to increase the visibility of the pricing of pharmaceutical products with the goal of reducing the prices at which we are able to sell our products. Because these various actual and proposed legislative changes are intended to operate on a state-by-state level rather than a national one, we cannot predict what the full effect of these legislative activities may be on our business in the future. Medicaid is a government health insurance program for low-income children, families, pregnant women, and people with disabilities. It is jointly funded by the federal and state governments, and it is administered by individual states within parameters established by the federal government. Coverage and reimbursement for drugs and biologics thus varies by state. Drugs and biologics may be covered under the medical or pharmacy benefit. State Medicaid programs may impose utilization management controls, such as prior authorization, step therapy, or quantity limits on drugs and biologics. Medicaid also includes the Medicaid Drug Rebate Program, under which, as a condition of coverage for our future products by the individual state Medicaid programs, we will be required to pay a retrospective rebate to each state Medicaid program for the quarterly utilization of our products by those respective state Medicaid programs we would be required to pay a rebate to each state Medicaid program for quantities of any future products that are dispensed to Medicaid beneficiaries and paid for by a state Medicaid program as a condition of having federal funds being made available to the states for any future products under Medicaid and Medicare Part B. Those rebates are based on pricing data that would be reported by us on a monthly and quarterly basis to CMS. These data include the average manufacturer price and the best price for each product we sell. As further described below under “U.S. Healthcare Reform and Other U.S. Healthcare Laws,” the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively, the PPACA, made significant changes to the Medicaid Drug Rebate Program that could negatively impact our results of operations.
Federal law requires that any company that participates in the Medicaid Drug Rebate Program also participate in the Public Health Service’s 340B drug discounted pricing program in order for federal funds to be available for the manufacturer’s drugs under Medicaid and Medicare Part B. The 340B pricing program requires participating manufacturers to agree to charge statutorily-defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. These 340B covered entities include a variety of community health clinics and other entities that receive health services grants from the Public Health Service as well as hospitals that serve a disproportionate share of low-income patients. The 340B ceiling price is calculated using a statutory formula, which is based on the average manufacturer price and rebate amount for the covered outpatient drug as calculated under the Medicaid Drug Rebate Program. Changes to the definition of average manufacturer price and the Medicaid rebate amount under PPACA and CMS’s issuance of final regulations implementing those changes also could affect the 340B ceiling price calculation for any future products and could negatively impact our results of operations. As described below under “U.S. Healthcare Reform and Other U.S. Healthcare Laws,” PPACA expanded the 340B program to include additional types of covered entities but exempts “orphan drugs” designated under section 526 of the FDCA from the ceiling price requirements for these newly-eligible entities. CMS has also implemented new regulations that further define and further expand which health care provider entities are eligible to purchase approved drugs at the discounted 340B prices. As part of its reform of the 340B program, on October 31, 2018, the HRSA at HHS issued a notice of proposed rulemaking to move up the effective date of a final rule that would give HHS authority to impose Civil Monetary Penalties on pharmaceutical manufacturers who knowingly and intentionally charged a covered entity more than the statutorily allowed ceiling price for a covered outpatient drug.
In order to be eligible to have products paid for with federal funds under the Medicaid and Medicare Part B programs and purchased by certain federal agencies and grantees, manufacturers must participate in the Department of Veterans Affairs Federal Supply Schedule, or FSS, pricing program, established by Section 603 of the Veterans Health Care Act of 1992, or VHCA. Under this program, we would be obligated to make our innovator “covered drugs” available for procurement on an FSS contract and charge a price to four federal agencies, Department of Veterans Affairs, Department of Defense, Public Health Service and Coast Guard, the so-called “Big Four” government purchasers, that is no higher than the statutory Federal Ceiling Price, or FCP. The FCP is based on the non-federal average manufacturer price, or Non-FAMP, which we would calculate and report to the Department of Veterans Affairs on a quarterly and annual basis. Under the Tricare Retail Pharmacy program, established by Section 703 of the National Defense Authorization Act for FY 2008 and related regulations, participating manufacturers pay quarterly rebates on utilization of innovator products that are dispensed through the Tricare Retail Pharmacy network to Tricare beneficiaries. The rebates are calculated as the difference between Annual Non-FAMP and FCP. The FCP is based on a weighted average non-federal average manufacturer price (Non-FAMP) which manufacturers are required to report on a quarterly and annual basis to the VA. If a company misstates Non-FAMPs or FCPs it must restate these figures and potentially refund to the government purchasers any overcharges that occurred.
| 53 |
Pursuant to the VHCA, knowing provision of false information in connection with a Non-FAMP filing can subject a manufacturer to penalties of one hundred seventy-eight thousand dollars for each item of false information.
Payers also are increasingly considering new metrics as the basis for reimbursement rates, such as ASP, average manufacturer price, and actual acquisition cost. The existing data for reimbursement based on these metrics is relatively limited, although certain states have begun to survey acquisition cost data for the purpose of setting Medicaid reimbursement rates. CMS surveys and publishes retail community pharmacy acquisition cost information in the form of National Average Drug Acquisition Cost files to provide state Medicaid agencies with a basis of comparison for their own reimbursement and pricing methodologies and rates. It may be difficult to project the impact of these evolving reimbursement mechanics on the willingness of payers to cover any future products.
FSS contracts are federal procurement contracts that include standard government terms and conditions, separate pricing for each product, and extensive disclosure and certification requirements. All items on FSS contracts are subject to a standard FSS contract clause that requires FSS contract price reductions under certain circumstances where pricing is reduced to an agreed “tracking customer.” Further, in addition to the “Big Four” agencies, all other federal agencies and some non-federal entities are authorized to access FSS contracts. FSS contractors are permitted to charge FSS purchasers other than the Big Four agencies “negotiated pricing” for covered drugs that is not capped by the FCP; instead, such pricing is negotiated based on a mandatory disclosure of the contractor’s commercial “most favored customer” pricing.
In addition, pursuant to regulations issued by the DoD TRICARE Management Activity, now the Defense Health Agency, to implement Section 703 of the National Defense Authorization Act for Fiscal Year 2008, participating manufacturers have each of their covered drugs is listed on a Section 703 Agreement under which they have agreed to pay rebates on covered drug prescriptions dispensed to TRICARE beneficiaries by TRICARE network retail pharmacies. Companies are required to list their innovator products on Section 703 Agreements in order for those products to be eligible for DoD formulary inclusion. The formula for determining the rebate is established in the regulations and our Section 703 Agreement and is based on the difference between the annual Non-FAMP and the FCP (as described above, these price points are required to be calculated by us under the VHCA).
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. Moreover, the requirements governing drug pricing and reimbursement vary widely from country to country. For example, in the EU the sole legal instrument at the EU level governing the pricing and reimbursement of medicinal products is Council Directive 89/105/EEC, or the Price Transparency Directive. The aim of the Price Transparency Directive is to ensure that pricing and reimbursement mechanisms established in EU member states are transparent and objective, do not hinder the free movement and trade of medicinal products in the EU and do not hinder, prevent or distort competition on the market. The Price Transparency Directive does not, however, provide any guidance concerning the specific criteria on the basis of which pricing and reimbursement decisions are to be made in individual EU member states. Neither does it have any direct consequence for pricing or levels of reimbursement in individual EU member states. The national authorities of the individual EU member states are free to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices and/or reimbursement of medicinal products for human use. Some individual EU member states adopt policies according to which a specific price or level of reimbursement is approved for the medicinal product. Other EU member states adopt a system of reference pricing, basing the price or reimbursement level in their territory either, on the pricing and reimbursement levels in other countries, or on the pricing and reimbursement levels of medicinal products intended for the same therapeutic indication. Furthermore, some EU member states impose direct or indirect controls on the profitability of the company placing the medicinal product on the market.
Health Technology Assessment, or HTA, of medicinal products is becoming an increasingly common part of the pricing and reimbursement procedures in some EU member states. These countries include the United Kingdom, France, Germany and Sweden. The HTA process in the EU member states is governed by the national laws of these countries. HTA is the procedure according to which the assessment of the public health impact, therapeutic impact and the economic and societal impact of the use of a given medicinal product in the national healthcare systems of the individual country is conducted. HTA generally focuses on the clinical efficacy and effectiveness, safety, cost, and cost-effectiveness of individual medicinal products as well as their potential implications for the national healthcare system. Those elements of medicinal products are compared with other treatment options available on the market.
| 54 |
The outcome of HTA may influence the pricing and reimbursement status for specific medicinal products within individual EU member states. The extent to which pricing and reimbursement decisions are influenced by the HTA of a specific medicinal product varies between the EU member states.
In 2011, Directive 2011/24/EU was adopted at the EU level. This Directive concerns the application of patients’ rights in cross-border healthcare. The Directive is intended to establish rules for facilitating access to safe and high-quality cross-border healthcare in the EU. Pursuant to Directive 2011/24/EU, a voluntary network of national authorities or bodies responsible for HTA in the individual EU Member States was established. The purpose of the network is to facilitate and support the exchange of scientific information concerning HTAs. In October 2016, the European Commission initiated a public consultation on strengthening EU cooperation on HTA. The consultation was closed in January 2017 and the report was published on May 15, 2017.
Following on from the consultation, on January 31, 2018, the European Commission put forward a proposed HTA Regulation. The proposed regulation covers new medicines and certain new medical devices, “providing the basis for permanent and sustainable cooperation at the EU level for joint clinical assessments in these areas.” Member states will be able to use common HTA tools, methodologies and procedures across the EU, working together in four main areas: 1) joint clinical assessments focusing on the most innovative health technologies with the most potential impact for patients; 2) joint scientific consultations whereby developers can seek advice from HTA authorities; 3) identification of emerging health technologies to identify promising technologies early; and 4) continuing voluntary cooperation in other areas.
Individual member states will continue to be responsible for assessing non-clinical (e.g., economic, social, ethical) aspects of health technology, and making decisions on pricing and reimbursement.
The proposed regulation will now be discussed by the European Parliament and the Council of Ministers. It is expected that if it is adopted and enters into force, it will become applicable three years later. Following the date of application, a further three-year period is envisaged to allow for a phase-in approach for member states to adapt to the new system.
U.S. Healthcare Reform and Other U.S. Healthcare Laws
In addition to FDA restrictions on marketing of pharmaceutical products, several other types of state and federal healthcare laws, including those commonly referred to as “fraud and abuse” laws have been applied in recent years to restrict certain marketing practices in the pharmaceutical industry. These laws may impact, among other things, our proposed sales, marketing and education programs. In addition, we may be subject to patient privacy regulation by both the U.S. federal government and the states in which we conduct our business. The laws that may affect our ability to operate include the following:
| · | The federal Anti-Kickback Statute prohibits, among other things, knowingly and willfully soliciting, offering, receiving, or paying any remuneration, directly or indirectly, in cash or in kind, to induce or reward purchasing, ordering or arranging for or recommending the purchase or order of any item or service for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. Liability may be established without a person or entity having actual knowledge of the federal Anti-Kickback Statute or specific intent to violate it. This statute has been interpreted to apply broadly to arrangements between pharmaceutical manufacturers on the one hand and prescribers, patients, purchasers and formulary managers on the other. In addition, PPACA amended the Social Security Act to provide that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. A conviction for violation of the Anti-Kickback Statute requires mandatory exclusion from participation in federal health care programs. Although there are a number of statutory exemptions and regulatory safe harbors protecting certain common activities from prosecution, the exemptions and safe harbors are drawn narrowly, and those activities may be subject to scrutiny or penalty if they do not qualify for an exemption or safe harbor. |
| 55 |
| · | The federal civil False Claims Act, or FCA, prohibits, among other things, knowingly presenting, or causing to be presented claims for payment of government funds that are false or fraudulent, or knowingly making, using or causing to be made or used a false record or statement material to such a false or fraudulent claim, or knowingly concealing or knowingly and improperly avoiding, decreasing, or concealing an obligation to pay money to the federal government. This statute also permits a private individual acting as a “whistleblower” to bring actions on behalf of the federal government alleging violations of the FCA and to share in any monetary recovery. The FCA prohibits anyone from knowingly presenting, conspiring to present, making a false statement in order to present, or causing to be presented, for payment to federal programs (including Medicare and Medicaid) claims for items or services, including drugs, that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. This law also prohibits anyone from knowingly underpaying an obligation owed to a federal program. Increasingly, U.S. federal agencies are requiring nonmonetary remedial measures, such as corporate integrity agreements in FCA settlements. The U.S. Department of Justice announced in 2016 its intent to follow the “Yates Memo,” taking a far more aggressive approach in pursuing individuals as FCA defendants in addition to the corporations. FCA liability is potentially significant in the healthcare industry because the statute provides for treble damages and mandatory penalties of $5,500 to $11,000 per false claim or statement ($10,781 to $21,563 per false claim or statement for penalties assessed after August 1, 2016 for violations occurring after November 2, 2015, and $10,957 to $21,916 per false claim or statement for penalties assessed after February 3, 2017 for violations occurring after November 2, 2015). Government enforcement agencies and private whistleblowers have investigated pharmaceutical companies for or asserted liability under the FCA for a variety of alleged promotional and marketing activities, such as providing free product to customers with the expectation that the customers would bill federal programs for the product; providing consulting fees and other benefits to physicians to induce them to prescribe products; engaging in promotion for “off-label” uses; and submitting inflated best price information to the Medicaid Rebate Program. |
| · | The federal False Statements Statute prohibits knowingly and willfully falsifying, concealing, or covering up a material fact or making any materially false, fictitious or fraudulent statement or representation, or making or using any false writing or document knowing the same to contain any materially false, fictitious or fraudulent statement or entry, in connection with the delivery of or payment for healthcare benefits, items, or services. |
| · | The federal Civil Monetary Penalties Law authorizes the imposition of substantial civil monetary penalties against an entity, such as a pharmaceutical manufacturer, that engages in activities including, among others (1) knowingly presenting, or causing to be presented, a claim for services not provided as claimed or that is otherwise false or fraudulent in any way; (2) arranging for or contracting with an individual or entity that is excluded from participation in federal healthcare programs to provide items or services reimbursable by a federal healthcare program; (3) violations of the federal Anti-Kickback Statute; or (4) failing to report and return a known overpayment. |
| · | The federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, imposes criminal and civil liability for knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, or knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false statement in connection with the delivery of, or payment for, healthcare benefits, items or services; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it in order to have committed a violation. |
| · | The majority of states also have statutes similar to the federal anti-kickback law and false claims laws that apply to items and services reimbursed under Medicaid and other state programs, or, in several states, that apply regardless of whether the payer is a government entity or a private commercial entity. |
| 56 |
| · | The federal Open Payments (Physician Payments Sunshine Act) program requires manufacturers of products for which payment is available under Medicare, Medicaid or the State Children’s Health Insurance Program, to track and report annually to the federal government (for disclosure to the public) certain payments and other transfers of value made to physicians and teaching hospitals as well as disclosure of payments and other transfers of value provided to physicians and teaching hospitals, and ownership and investment interests held by physicians and other healthcare providers and their immediate family members and applicable group purchasing organizations. In addition, several U.S. states and localities have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, and/or make periodic public disclosures on sales, marketing, pricing, clinical trials, and other activities. Other state laws prohibit certain marketing-related activities including the provision of gifts, meals or other items to certain healthcare providers. Many of these laws and regulations contain ambiguous requirements that government officials have not yet clarified. Given the lack of clarity in the laws and their implementation, our reporting actions could be subject to the penalty provisions of the pertinent federal and state laws and regulations. |
Sanctions under these federal and state healthcare laws may include civil monetary penalties, exclusion of a manufacturer’s products from reimbursement under government programs, monetary damages, criminal fines, disgorgement, additional reporting obligations and oversight if the manufacture becomes subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws, and individual imprisonment.
Federal and state authorities are continuing to devote significant attention and resources to enforcement of fraud and abuse laws within the pharmaceutical industry, and private individuals have been active in alleging violations of the law and bringing suits on behalf of the government under the FCA. For example, federal enforcement agencies recently have investigated certain pharmaceutical companies’ product and patient assistance programs, including manufacturer reimbursement support services, relationships with specialty pharmacies, and grants to independent charitable foundations.
The PPACA, was adopted in the U.S. in March 2010. This law substantially changes the way healthcare is financed by both governmental and private insurers in the U.S., and significantly impacts the pharmaceutical industry. PPACA contains a number of provisions that are expected to impact our business and operations. Changes that may affect our business include those governing enrollment in federal healthcare programs, reimbursement changes, rules regarding prescription drug benefits under the health insurance exchanges, expansion of the 340B program, expansion of state Medicaid programs, and fraud and abuse and enforcement. These changes will impact existing government healthcare programs and will result in the development of new programs, including Medicare payment for performance initiatives and improvements to the physician quality reporting system and feedback program.
PPACA contains several provisions that have or could potentially impact our business. PPACA made significant changes to the Medicaid Drug Rebate Program. Effective March 23, 2010, rebate liability expanded from fee-for-service Medicaid utilization to include the utilization of Medicaid managed care organizations as well. With regard to the amount of the rebates owed, PPACA increased the minimum Medicaid rebate from 15.1% to 23.1% of the average manufacturer price for most innovator products; changed the calculation of the rebate for certain innovator products that qualify as line extensions of existing drugs; and capped the total rebate amount for innovator drugs at 100% of the average manufacturer price. In addition, PPACA and subsequent legislation changed the definition of average manufacturer price. In early 2016, CMS issued final regulations to implement the changes to the Medicaid Drug Rebate Program under PPACA, which became effective on April 1, 2016. Finally, PPACA requires pharmaceutical manufacturers of branded prescription drugs to pay a branded prescription drug fee to the federal government. Each individual pharmaceutical manufacturer pays a prorated share of the branded prescription drug fee of $4,000 in 2017 (and set to increase in ensuing years), based on the dollar value of its branded prescription drug sales to certain federal programs identified in the law. Sales of “orphan drugs” are excluded from this fee. “Orphan drugs” are specifically defined for purposes of the fee. For each indication approved by the FDA for the drug, such indication must have been designated as orphan by the FDA under section 526 of the FDCA, an orphan drug tax credit under section 45C of the Internal Revenue Code must have been claimed with respect to such indication, and such tax credit must not have been disallowed by the Internal Revenue Service. Finally, the FDA must not have approved the drug for any indication other than an orphan indication for which a section 45C orphan drug tax credit was claimed (and not disallowed).
Additional provisions of PPACA may negatively affect manufacturer’s revenues in the future. For example, as part of PPACA’s provisions closing a coverage gap that currently exists in the Medicare Part D prescription drug program (commonly known as the “donut hole”), manufacturers of branded prescription drugs are required to provide a 50% discount on branded prescription drugs dispensed to beneficiaries within this donut hole.
| 57 |
PPACA also expanded the Public Health Service’s 340B drug pricing discount program. The 340B pricing program requires participating manufacturers to agree to charge statutorily-defined covered entities no more than the 340B “ceiling price” for the manufacturer’s covered outpatient drugs. PPACA expanded the 340B program to include additional types of covered entities: certain free-standing cancer hospitals, critical access hospitals, rural referral centers and sole community hospitals, each as defined by PPACA. PPACA exempts “orphan drugs” designated under section 526 of the FDCA, from the ceiling price requirements for these newly-eligible entities.
Finally, numerous federal and state laws, including state security breach notification laws, state health information privacy laws, and federal and state consumer protection laws govern the collection, use, and disclosure of personal information. In addition, most healthcare providers and research institutions with whom we collaborate are subject to privacy and security requirements under HIPAA, as amended by HITECH, and its implementing regulations. Although we are currently neither a “covered entity” nor a “business associate” under HIPAA, and these privacy and security requirements do not apply to us, the regulations may affect our interactions with healthcare providers, health plans, and research institutions from whom we obtain patient health information. Further, we could be subject to criminal penalties if we knowingly obtain individually identifiable health information from a HIPAA covered entity in a manner that is not authorized or permitted by HIPAA or for aiding and abetting the violation of HIPAA.
There is significant interest in the United States in promoting changes in healthcare systems with the stated goals of containing healthcare costs, improving quality and/or expanding access, including increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices, particularly with respect to drugs that have been subject to relatively large price increases over relatively short time periods. There have been several recent U.S. Congressional inquiries and proposed bills designed to, among other things, bring more transparency to drug pricing, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs. Legislative changes to PPACA also remain possible and appear likely in the 115th U.S. Congress and under the Trump Administration. Although multiple bills to repeal or repeal and replace portions of the PPACA have been introduced in 2017, none of these measure has successfully passed both houses of Congress. Congress may consider other legislation to repeal and replace elements of the PPACA or other health reform measures in the future.
Other Regulations
We are also subject to the U.S. Foreign Corrupt Practices Act, or FCPA, the U.K. Bribery Act, or Bribery Act, and other anticorruption laws and regulations pertaining to our financial relationships with foreign government officials. The FCPA prohibits U.S. companies and their representatives from paying, offering to pay, promising, or authorizing the payment of anything of value to any foreign government official, government staff member, political party, or political candidate to obtain or retain business or to otherwise seek favorable treatment. In many countries in which we operate, the healthcare professionals with whom we interact may be deemed to be foreign government officials for purposes of the FCPA. The Bribery Act, which applies to any company incorporated or doing business in the UK, prohibits giving, offering, or promising bribes in the public and private sectors, bribing a foreign public official or private person, and failing to have adequate procedures to prevent bribery amongst employees and other agents. Penalties under the Bribery Act include potentially unlimited fines for companies and criminal sanctions for corporate officers under certain circumstances. Liability in relation to breaches of the Bribery Act is strict. This means that it is not necessary to demonstrate elements of a corrupt state of mind. However, a defense of having in place adequate procedures designed to prevent bribery is available.
Recent years have seen a substantial increase in anti-bribery law enforcement activity by U.S. regulators, with more frequent and aggressive investigations and enforcement proceedings by both the DOJ and the SEC, increased enforcement activity by non-U.S. regulators, and increases in criminal and civil proceedings brought against companies and individuals. Increasing regulatory scrutiny of the promotional activities of pharmaceutical companies also has been observed in a number of EU member states. In Germany, a specific anti-corruption provision with regard to healthcare professionals was introduced in the Criminal Code in 2017.
Similar strict restrictions are imposed on the promotion and marketing of drug products in the EU, where a large portion of our non-U.S. business is conducted, and other territories. Laws in the EU, including in the individual EU member states, require promotional materials and advertising for drug products to comply with the product’s Summary of Product Characteristics, or SmPC, which is approved by the competent authorities. Promotion of a medicinal product which does not comply with the SmPC is considered to constitute off-label promotion. The off-label promotion of medicinal products is prohibited in the EU and in other territories. The promotion of medicinal products that are not subject to a marketing authorization is also prohibited in the EU. Laws in the EU, including in the individual EU member states, also prohibit the direct-to-consumer advertising of prescription-only medicinal products. Violations of the rules governing the promotion of medicinal products in the EU and in other territories could be penalized by administrative measures, fines and imprisonment. Furthermore, illegal advertising can be challenged by competitors, and as a result, can be prohibited by court and the responsible company can be obligated to pay damages to the competitor.
| 58 |
Interactions between pharmaceutical companies and physicians are also governed by strict laws, regulations, industry self-regulation codes of conduct and physicians’ codes of professional conduct in the individual EU member states. The provision of any inducements to physicians to prescribe, recommend, endorse, order, purchase, supply, use or administer a medicinal product is prohibited. A number of EU member states have introduced additional rules requiring pharmaceutical companies to publicly disclose their interactions with physicians and to obtain approval from employers, professional organizations and/or competent authorities before entering into agreements with physicians. These rules have been supplemented by provisions of related industry codes, including the EFPIA Disclosure Code on Disclosure of Transfers of Value from Pharmaceutical Companies to Healthcare Professionals and Healthcare Organizations and related codes developed at national level in individual EU member states. Additional countries may consider or implement similar laws and regulations. Violations of these rules could lead to reputational risk, public reprimands, and/or the imposition of fines or imprisonment. Our present and future business has been and will continue to be subject to various other laws and regulations. Laws, regulations and recommendations relating to safe working conditions, laboratory practices, the experimental use of animals, and the purchase, storage, movement, import and export and use and disposal of hazardous or potentially hazardous substances, including radioactive compounds, used in connection with our research work are or may be applicable to our activities. We cannot predict the impact of government regulation, which may result from future legislation or administrative action, on our business.
Legal Proceedings
On April 27, 2017, we issued a press release stating that Edison Investment Research Ltd., or Edison, has withdrawn its report issued April 26, 2017 titled “Akari’s Nomacopan (Coversin) matches Soliris in Phase II”, or the “Edison Report”, because it contains material inaccuracies, including without limitation, with respect to our interim analysis of our ongoing Phase II PNH trial of Nomacopan (Coversin). Investors were cautioned not to rely upon any information contained in the Edison Report and instead were directed to our press release issued on April 24, 2017 that discusses the interim analysis of our then ongoing Phase II PNH trial and other matters. Our Board of Directors established an ad hoc special committee of the Board to review the involvement, if any, of our personnel with the Edison Report, which was later retracted. Edison was retained by the Company to produce research reports about us. While that review was pending, Dr. Gur Roshwalb, a former Chief Executive Officer, was placed on administrative leave and Dr. Ray Prudo in his role as Executive Chairman temporarily assumed Dr. Roshwalb’s duties in his absence. Following that review, we determined that the Edison Report was reviewed and approved by Dr. Roshwalb, in contravention of Company policy. On May 29, 2017, Dr. Roshwalb submitted his resignation as Chief Executive Officer and member of our Board of Directors, effective immediately.
On May 12, 2017, a putative securities class action captioned Derek Da Ponte v. Akari Therapeutics, PLC, Gur Roshwalb, and Dov Elefant (Case 1:17-cv-03577) was filed in the U.S. District Court for the Southern District of New York against us, a former Chief Executive Officer and our Chief Financial Officer. The plaintiff asserted claims alleging violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, or the Exchange Act, based primarily on our press releases or statements issued between April 24, 2017 and May 11, 2017 concerning the Phase II PNH trial of Nomacopan (Coversin) and the Edison Report about us and actions taken by us after the report was issued. The purported class covers the period from March 30, 2017 to May 11, 2017. The complaint seeks unspecified damages and costs and fees. On May 19, 2017, an almost identical class action complaint captioned Shamoon v. Akari Therapeutics, PLC, Gur Roshwalb, and Dov Elefant (Case 1:17-cv-03783) was filed in the same court. On July 11-12, 2017, candidates to be lead plaintiff filed motions to consolidate the cases and appoint a lead plaintiff. On August 10, 2017, the court issued a stipulated order: (i) consolidating the class actions under the caption In re: Akari Therapeutics, PLC Securities Litigation (Case 1:17-cv-03577); and (ii) setting out schedule for plaintiffs to file a consolidated amended complaint and defendants to respond thereto. By order dated September 7, 2017, the court appointed lead plaintiffs for the class and lead plaintiffs’ counsel. On November 6, 2017, lead plaintiffs filed a consolidated amended complaint, or the CAC. While the CAC contains similar substantive allegations to the initial complaints, it adds two additional defendants, Ray Prudo and Edison Investment Research Ltd., and the purported class period was changed to April 24, 2017 through May 30, 2017. On January 10, 2018, at a hearing regarding the defendants’ impending motions to dismiss the CAC, the Court gave plaintiffs permission to file a second consolidated amended complaint, or the SCAC and established a briefing schedule for defendants’ motions to dismiss the SCAC. Pursuant to that schedule, plaintiffs’ SCAC was filed on January 31, 2018. All briefing on the motions to dismiss was completed on April 20, 2018. On June 8, 2018, the parties entered into a memorandum of understanding to settle plaintiffs’ claims for a total payment of $2.7 million in cash and on July 26, 2018, plaintiffs filed a notice with the Court voluntarily dismissing Edison from the action. On August 3, 2018, the remaining parties executed and filed a stipulation and agreement of settlement (the terms of which were consistent with the memorandum of understanding). On August 7, 2018, the Court granted plaintiffs’ motion for preliminary approval of the settlement, and on November 28, 2018, following a hearing with the parties, the court ordered final approval of the settlement. Plaintiffs subsequently moved to distribute the settlement funds to the class, and the Court granted plaintiffs’ motion on February 4, 2019. We recorded the $2.7 million SCAC litigation settlement loss in the consolidated statement of comprehensive loss in the year ended December 31, 2018, which is the period in which the lawsuits were originally filed. The $2.7 million SCAC settlement liability was recorded as a loss contingency in accrued expenses in our consolidated balance sheets as of December 31, 2018. On August 24, 2018, we received a $2.7 million payment from our directors’ and officers’ liability insurance provider, the sum of which was paid to an escrow account for the benefit of the settlement class on August 27, 2018. This was recorded as a gain in the consolidated statements of comprehensive loss during the third quarter of 2018.
| 59 |
Separately, Edison sought indemnification from us pursuant to its contract with us, including reimbursement of all legal expenses that Edison incurs in connection with the securities class action (to which, as discussed above, Edison was added as a defendant on November 6, 2017) and lost profits from customer relationships that Edison claims it lost as a result of the retraction of the Edison Report. The parties have finalized and consummated a settlement and the settlement payment has been made.
We voluntarily reported to the SEC the circumstances leading to the withdrawal of the Edison Report and the outcome of our special committee’s investigation. In response, the SEC requested certain documents from us with respect to the matters we reported. We have been cooperating with the SEC’s requests for information. On June 5, 2018, we received a subpoena from the SEC, which requested further documents and information primarily related to our Phase II clinical trial of Nomacopan (Coversin) in connection with an investigation of us that the SEC is conducting. We are in the process of responding to the subpoena and will continue to cooperate with the SEC.
Employees
As of December 31, 2018, we had thirteen full-time employees and one full-time equivalent consultant.
| C. | Organizational Structure |
Our corporate structure consists of Akari Therapeutics PLC and three subsidiaries, one of which is an indirect subsidiary. The following is a list of our subsidiaries:
| Name of Subsidiary | Jurisdiction | Activity | % Holding |
|||||
| Volution Immuno Pharmaceuticals SA | Switzerland | Research & Development | 100 | % | ||||
| Celsus Therapeutics Inc. | Delaware, USA | Inactive | 100 | % | ||||
| Morria Biopharma Ltd | Israel | Inactive | 100 | % | ||||
| D. | Property, Plant and Equipment |
We do not currently own any property. We previously leased 4,900 square feet of office space in New York, New York, for approximately $27,000 per month. The lease for this property was to expire in August 2019 but ended early in December 2018.
| 60 |
In addition, in January 2018, we entered into a sublease of approximately 7,070 square feet of office space in New York, New York with a lease term ending on July 29, 2022. Under the sublease, the monthly fixed rent amounts range from approximately $63,000 to $70,000 per month during the lease term for cumulative fixed rent of approximately $3.3 million. In addition to our monthly fixed rent obligations, we are responsible for providing a security deposit, and payment of certain operating costs and taxes associated with the premises. This lease ended early in December 2018.
We also lease office space in New York, New York on a month to month basis for approximately $2,700 per month.
We lease 1,260 square feet of office space in London, England, for approximately $12,000 per month. The lease for this property expired in March 2019 and is continuing on a month to month basis.
| Item 4A. | UNRESOLVED STAFF COMMENTS |
None.
| Item 5. | OPERATING AND FINANCIAL REVIEW AND PROSPECTS |
| A. | Operating Results |
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our consolidated financial statements and related notes appearing elsewhere in this Annual Report on Form 20-F. In addition to historical information, this discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Our actual results may differ materially from those anticipated in the forward-looking statements as a result of certain factors. We discuss factors that we believe could cause or contribute to these differences below and elsewhere in this report including those set forth under Item 3D ”Risk Factors” in this Annual Report on Form 20-F.
Overview
We are a clinical-stage biopharmaceutical company focused on developing inhibitors of acute and chronic inflammation, specifically the complement system, the eicosanoid system and the bioamine system for the treatment of rare and orphan diseases. Each of these systems has scientifically well-supported causative roles in the diseases we are targeting. We believe that blocking early mediators of inflammation will prevent initiation and continual amplification of the processes that cause certain diseases.
On October 20, 2017, we completed a public offering of an aggregate of 3,480,000 ADSs representing 348,000,000 ordinary shares for gross proceeds of $17.4 million at a price of $5.00 per ADS. In connection with the offering we incurred $1.7 million of share issuance costs for net proceeds of $15.7 million.
On September 26, 2018, we entered into a Purchase Agreement with Aspire Capital which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $20.0 million of our ADSs beginning on the effective date of a registration statement related to the transaction. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued 30,000,000 ordinary shares to Aspire Capital and sold to Aspire Capital 25,000,000 ordinary shares for $0.02 per share (equivalent to $2.00 per ADS). In March 2019, we issued an additional 5,000,000 ordinary shares for $0.0346 per share (equivalent to $3.46 per ADS) for gross proceeds of $173,000. See “Item 5B. Operating and Financial Review and Prospects — Liquidity and Capital Resources — Aspire Capital Financing Arrangement”.
Critical Accounting Policies and Use of Estimates
The preparation of the consolidated financial statements in conformity with United States generally accepted accounting principles, or U.S. GAAP, requires management to make estimates, judgments and assumptions. Our management believes that the estimates, judgments and assumptions used are reasonable based upon information available at the time they are made. These estimates, judgments and assumptions can affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the dates of the financial statements, and the reported amounts of expenses during the reporting period. Actual results could differ from those estimates.
| 61 |
Share-Based Compensation and Fair Value of Ordinary Shares
We account for awards of equity instruments issued to employees and directors under the fair value method of accounting and recognize such amounts in our Consolidated Statements of Comprehensive Loss. We measure compensation cost for all stock-based awards at fair value on the date of grant and recognize compensation expense in general administrative and research and development expenses in our Consolidated Statements of Comprehensive Loss using the straight-line method over the service period over which we expect the awards to vest.
We estimate the fair value of all time-vested options as of the date of grant using the Black-Scholes option valuation model, which was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. Option valuation models require the input of highly subjective assumptions, including the expected share price volatility, which we calculate based on the historical volatility of peer companies. We use a risk-free interest rate, based on U.S. Treasury instruments in effect at the time of the grant, for the period comparable to the expected term of the option. Given our limited history with share option grants and exercises, we use the “simplified” method in estimating the expected term, the period of time that options granted are expected to be outstanding, for our grants.
We classify our stock-based payments as either liability-classified awards or as equity-classified awards. We remeasure liability-classified awards to fair value at each balance sheet date until the award is settled. We measure equity-classified awards at their grant date fair value and do not subsequently remeasure them. We have classified our share-based payments which are settled in our ordinary shares as equity-classified awards and our share-based payments that are settled in cash as liability-classified awards. Compensation costs related to equity-classified awards generally are equal to the grant-date fair value of the award amortized over the vesting period of the award. The liability for liability-classified awards generally is equal to the fair value of the award as of the balance sheet date multiplied by the percentage vested at the time. We charge (or credit) the change in the liability amount from one balance sheet date to another to changes in fair value of options and warrants liabilities.
RPC Options
In connection with a short-term working capital loan from shareholders of approximately $3 million, the shareholders were granted options in RPC, equivalent to 15% of the current outstanding equity issued by RPC. The RPC options were accounted for in accordance with ASC 718, “Compensation-Stock Compensation”. The fair value of the RPC options is estimated using the fair value of Akari ordinary shares times RPC’s ownership in Akari ordinary shares times 15% and was initially valued at approximately $26 million. These options do not relate to the share capital of Akari. At December 31, 2017, the fair value of the options was $5,081,335. At December 2018, the fair value of the options was $1,842,424. The change in fair value of the options in the year ended December 31, 2018, was a decrease of $3,238,911 and was recognized as a change in fair value of option liabilities in the Consolidated Statement of Comprehensive Loss.
Functional Currency
The functional currency of Akari is U.S. dollars as that is the primary economic environment in which the Company operates as well as the currency in which it has been financed.
The reporting currency of the Company is U.S. Dollars. The Company translated its non-U.S. operations’ assets and liabilities denominated in foreign currencies into U.S. dollars at current rates of exchange as of the balance sheet date and income and expense items at the average exchange rate for the reporting period. Translation adjustments resulting from exchange rate fluctuations are recorded as foreign currency translation adjustments, a component of accumulated other comprehensive (loss) income. Gains or losses from foreign currency transactions and the remeasurement of intercompany balances are included in foreign currency exchange gains/(losses).
| 62 |
Results of Operations
For the Years Ended December 31, 2018 and December 31, 2017
Research and development expenses
Research and development expenses for the year ended December 31, 2018 were approximately $11,795,000 compared to approximately $23,285,000 for the year ended December 31, 2017. This 49% or $11,490,000 decrease was primarily due to lower expenses of approximately $7,400,000 for manufacturing as we had previously manufactured clinical trial material for supply through 2019, and an R&D tax credit of $3,800,000 which offset overall R&D expenses.
We expect our clinical expenses to increase in the future as we conduct additional trials to support the development of Nomacopan (Coversin), and advance other product candidates into pre-clinical and clinical development.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2018 were approximately $10,896,000 compared to approximately $11,799,000 for the year ended December 31, 2017. This 8% or $903,000 decrease was primarily due to lower expenses of approximately $1,084,000 for personnel expenses, $1,015,000 for stock-based non-cash compensation expense and $256,000 for recruiting offset by higher expenses of approximately $501,000 for professional fees, $409,000 for rent expense, $397,000 for insurance, and $143,00 for other miscellaneous expenses.
We expect our general and administrative expenses to increase due to increased legal, accounting and professional fees associated with being a publicly reporting company in the United States and rental expense associated with offices in the United States and London to support the Company’s operations and anticipated growth.
Litigation settlement gain
Litigation settlement gain for the year ended December 31, 2018 was $2,700,000. This relates to the receipt of funds from our insurance carrier in 2018 used to settle our securities class action lawsuit which was accrued for in 2017.
Other Income (expenses)
Other income for the year ended December 31, 2018 was approximately $3,525,000 compared to $2,385,000 for the year ended December 31, 2017. This change was primarily attributed to approximately $658,000 of higher income related to the change in the fair value of the stock option liabilities in 2018 than in 2017, and foreign exchange gains in 2018 of approximately $82,000 as compared to foreign exchange losses of $359,000 in 2017.
For the years ended December 31, 2017 and December 31, 2016
Research and development expenses
Research and development expenses for the year ended December 31, 2017 were approximately $23,285,000 compared to approximately $17,306,000 for the year ended December 31, 2016. This 35% or $5,979,000 increase was primarily due to higher expenses of approximately $2,035,000 for manufacturing, $1,633,000 for clinical trial expenses, $988,000 for consulting expense, $929,000 for personnel expenses and $134,000 for stock-based non-cash compensation expense.
We expect our clinical expenses to increase in the future as we conduct additional trials to support the development of Nomacopan (Coversin), and advance other product candidates into pre-clinical and clinical development.
General and administrative expenses
General and administrative expenses for the year ended December 31, 2017 were approximately $11,799,000 compared to approximately $9,941,000 for the year ended December 31, 2016. This 19% or $1,858,000 increase was primarily due to higher expenses of approximately $2,276,000 for legal, accounting and professional fees, $574,000 of personnel expenses and $276,000 of recruiting expenses offset by $1,332,000 for stock-based non-cash compensation expense.
| 63 |
We expect our general and administrative expenses to increase due to increased legal, accounting and professional fees associated with being a publicly reporting company in the United States and rental expense associated with offices in the United States and London to support the Company’s operations and anticipated growth.
Litigation settlement loss
Litigation settlement loss for the year ended December 31, 2017 was $2,700,000. This relates to the settlement agreement of our securities class action lawsuit which was approved by the court in 2019.
Other Income (expenses)
Other income for the year ended December 31, 2017 was approximately $2,385,000 compared to $9,106,000 for the year ended December 31, 2016. This change was primarily attributed to approximately $6,152,000 of lower income related to the change in the fair value of the stock option and warrant liabilities in 2016 than in 2017.
| B. | Liquidity and Capital Resources |
At December 31, 2018, we had $5,446,138 in cash. In addition, as of December 31, 2018, we had an accumulated deficit in the amount of $126,803,647. Since inception, we have funded our operations primarily through the sale of equity securities and debt financing. In October 2017, we completed a public offering of an aggregate of 3,480,000 ADSs representing 348,000,000 ordinary shares for gross proceeds of $17.4 million at a price of $5.00 per ADS. In connection with the offering, we incurred $1.7 million of share issuance costs for net proceeds of $15.7 million. On September 26, 2018, we entered into a Purchase Agreement with Aspire Capital which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $20.0 million of our ADSs beginning on the effective date of a registration statement related to the transaction. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued 30,000,000 ordinary shares to Aspire Capital and sold to Aspire Capital 25,000,000 ordinary shares for $0.02 per share (equivalent to $2.00 per ADS) for gross proceeds of $500,000. In March 2019, we issued an additional 5,000,000 ordinary shares for $0.0346 per share (equivalent to $3.46 per ADS) for gross proceeds of $173,000. To date, we have sold to Aspire Capital a total of $673,000 of ordinary shares and $19.3 million remains available for draw down under the Purchase Agreement. See “— Aspire Capital Financing Arrangement” below. In addition, in March 2019, we received research and development tax credit of approximately $4,900,000 for the year ended December 31, 2017 from the HM Revenues and Customs, or HMRC.
We have not yet generated any revenues and we expect to continue to incur net losses and negative cash flows for the foreseeable future. These net losses and negative cash flows have had, and will continue to have, an adverse effect on our shareholders’ equity and working capital. We believe our current cash is sufficient to fund future operations through the end of the second quarter of 2019 and we plan to raise additional funds from external sources or from Aspire Capital. This forecast of cash resources is forward-looking information that involves risks and uncertainties, and the actual amount of our expenses over the next twelve months could vary materially and adversely as a result of a number of factors, including the risks and uncertainties set forth in Item 3D under the heading “Risk Factors” of our Annual Report on Form 20-F for the year ended December 31, 2018.
For the year ended December 31, 2018, we reported a net loss of $16,466,780 and we expect to continue to incur substantial losses over the next several years during our development phase. Our independent registered public accounting firm, in its report on our audited financial statements for the year ended December 31, 2018 expressed substantial doubt about our ability to continue as a going concern. To fully execute our business plan, we will need, among other things, to complete our research and development efforts and clinical and regulatory activities. These activities may take several years and will require significant operating and capital expenditures in the foreseeable future. There can be no assurance that these activities will be successful. If we are not successful in these activities or there is not a favorable resolution of the SEC investigation, it could delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development activities. To fund our capital needs, we plans to raise funds through equity or debt financings or other sources, such as strategic partnerships and alliance and licensing arrangements, and in the long term, from the proceeds from sales. Additional funds may not be available when we need them, on terms that are acceptable to it, or at all. To the extent that we raise additional funds by issuing equity securities, our shareholders may experience significant dilution. There can be no assurance that we will be successful in obtaining an adequate level of financing needed for our long-term research and development activities. If we are unable to raise sufficient capital resources, we will not be able to continue the development of all of our products or may be required to delay part of our development programs and significantly reduce our activities in order to maintain our operations. These matters raise substantial doubt about the Company’s ability to continue as a going concern.
| 64 |
Aspire Capital Financial Arrangement
On September 26, 2018, we entered into the Purchase Agreement with Aspire Capital which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $20.0 million of our ADSs, beginning during a 30-month period beginning on the effective date of a registration statement related to the transaction. Concurrently with entering into the Purchase Agreement, we also entered into a registration rights agreement with Aspire Capital, or the Registration Rights Agreement, in which we agreed to file one or more registration statements, as permissible and necessary to register under the Securities Act, the sale of our securities that have been and may be issued to Aspire Capital under the Purchase Agreement. Subsequently on October 9, 2018, we filed a registration statement on Form F-1 to register the resale of such securities and such registration statement was declared effective on March 4, 2019.
Under the Purchase agreement, after the SEC has declared effective the registration statement referred to above, on any trading day selected by us, we have the right, in our sole discretion, to present Aspire Capital with a purchase notice, each, a Purchase Notice, directing Aspire Capital (as principal) to purchase up to 150,000 ADSs per business day and up to $20.0 million of our ADSs in the aggregate at a per share price, or the Purchase Price, equal to the lesser of:
| • | the lowest sale price of our ADSs on the purchase date; or |
| • | the arithmetic average of the three (3) lowest closing sale prices for the ADSs during the ten (10) consecutive business days ending on the business day immediately preceding such Purchase Date (to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction). |
In addition, on any date on which we submit a Purchase Notice to Aspire Capital in an amount of 150,000 ADSs, the Company also has the right, in its sole discretion, to present Aspire Capital with a volume-weighted average price purchase notice, each, a VWAP Purchase Notice, directing Aspire Capital to purchase an amount of ADSs equal to up to 30% of the aggregate shares of our ADSs traded on our principal market on the next trading day, or the VWAP Purchase Date, subject to a maximum number of 250,000 ADSs. The purchase price per share pursuant to such VWAP Purchase Notice is generally 97% of the volume-weighted average price for our ADSs traded on our principal market on the VWAP Purchase Date.
The Purchase Price will be adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the period(s) used to compute the Purchase Price. We may deliver multiple Purchase Notices and VWAP Purchase Notices to Aspire Capital from time to time during the term of the Purchase Agreement, so long as the most recent purchase has been completed.
The Purchase Agreement provides that we and Aspire Capital shall not effect any sales under the Purchase Agreement on any purchase date where the closing sale price of our ADSs is less than $0.25. There are no trading volume requirements or restrictions under the Purchase Agreement, and we will control the timing and amount of sales of our ADSs to Aspire Capital. Aspire Capital has no right to require any sales by us, but is obligated to make purchases from us as directed by us in accordance with the Purchase Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the Purchase Agreement. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, we issued to Aspire Capital 30,000,000 ordinary shares of us, the Commitment Shares, and sold to Aspire Capital 25,000,000 ordinary shares, or the Initial Shares, for $0.02 per share (equivalent to $2.00 per ADS). The Purchase Agreement may be terminated by us at any time, at its discretion, without any cost to us. Aspire Capital has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging of our securities during any time prior to the termination of the Purchase Agreement. Any proceeds we receive under the Purchase Agreement are expected to be used for working capital and general corporate purposes.
In addition to the 30,000,000 Commitment Shares and the 25,000,000 Initial Shares sold to Aspire Capital in September 2018 for gross proceeds of $500,000, we sold 5,000,000 ordinary shares to Aspire Capital in March 2019 at $0.0346 per share in accordance with the terms of the Purchase Agreement for gross proceeds of $173,000.
| 65 |
Cash Flows
Net cash used in operating activities was $22,580,000 during the year ended December 31, 2018 compared to $31,599,000 during the year ended December 31, 2017. Net cash flow used in operating activities was primarily attributed to our ongoing research activities to support Nomacopan (Coversin), including manufacturing, clinical trial and preclinical activities.
Net cash used in operating activities was $31,599,000 during the year ended December 31, 2017 compared to $24,625,000 during the year ended December 31, 2016. Net cash flow used in operating activities was primarily attributed to our ongoing research activities to support Nomacopan (Coversin), including manufacturing, clinical trial and preclinical activities.
Net cash provided by investing activities was $0 during the year ended December 31, 2018 compared to $9,985,000 cash provided by investing activities derived from the maturities of short-term investments during the year ended December 31, 2017.
Net cash provided by investing activities was $9,985,000 during the year ended December 31, 2017 compared to cash used by investing activities of $10,067,000 during the year ended December 31, 2016, which was cash used to purchase office equipment and short-term investments offset by maturities of short-term securities.
Net cash provided by financing activities was $306,000 during the year ended December 31, 2018 compared to $15,672,000 during the year ended December 31, 2017.
Net cash provided by financing activities was $15,672,000 during the year ended December 31, 2017. In the year ended December 31, 2016, we had no financing activity.
| C. | Research and Development, Patents and Licenses |
Our research and development expenditures were approximately $11,795,000, $23,285,000 and $17,306,000 for the years ended December 31, 2018, 2017 and 2016 respectively. Most of such research and development expenditures were in the form of payments to third parties to carry out our manufacturing, pre-clinical and clinical research activities.
We incurred the following research and development expenses for the years ended December 31, 2018, 2017 and 2016:
| Years ended December 31, | ||||||||||||
| (in $000’s) | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| Direct Expenses: | ||||||||||||
| Nomacopan (Coversin) | $ | 6,961 | $ | 14,063 | $ | 12,087 | ||||||
| Clinical trials | 4,030 | 3,915 | 1, 982 | |||||||||
| Other | 1,000 | 1,572 | 2,161 | |||||||||
| Total direct expenses | 11,991 | 19,550 | 16,230 | |||||||||
| Indirect Expenses: | ||||||||||||
| Staffing | 2,407 | 2,001 | 1,062 | |||||||||
| Other indirect | 1,191 | 1,734 | 14 | |||||||||
| Total indirect expenses | 3,598 | 3,735 | 1,076 | |||||||||
| Tax credits | (3,794 | ) | - | - | ||||||||
| Total Research and Development | $ | 11,795 | $ | 23,285 | $ | 17,306 | ||||||
| 66 |
| D. | Trend Information |
We are a development stage company and it is not possible for us to predict with any degree of accuracy the outcome of our research, development or commercialization efforts. As such, it is not possible for us to predict with any degree of accuracy any significant trends, uncertainties, demands, commitments or events that are reasonably likely to have a material effect on our net sales or revenues, income from continuing operations, profitability, liquidity or capital resources, or that would cause financial information to not necessarily be indicative of future operating results or financial condition. However, to the extent possible, certain trends, uncertainties, demands, commitments and events are identified in the preceding subsections of this Item 5.
| E. | Off-balance Sheet Arrangements |
We currently do not have any off-balance sheet arrangements.
| F. | Contractual Obligations |
The following table summarizes our significant contractual obligations as of December 31, 2018:
| Contractual Obligations | Total |
Less than 1 year |
1 – 3 years |
3-5 years |
More than 5 years |
|||||||||||||||
| Lease of office space (1) | $ | 33,000 | $ | 33,000 |
$ | - | $ | - | $ | - | ||||||||||
| Total | $ | 33,000 |
$ | 33,000 | $ | - | $ | - | $ | - | ||||||||||
| (1) | In March 2014, we entered into a lease agreement for offices in London which was amended January 1, 2016. The lease term commenced on December 1, 2014 and expired in March 2019 which we intend to renew and are currently leasing on the same terms of the lease. The lease can be cancelled early by either party upon 3 months’ notice. |
We also had a five-year lease for offices in New York, New York effective July 2014. The lease ended early in December 2018. In January 2018, we entered into a sublease of office space in New York, New York for an approximately four-year term which also ended early in December 2018. We currently lease office space in New York, New York on a month to month basis.
| Item 6. | DIRECTORS, SENIOR MANAGEMENT AND EMPLOYEES |
| A. | Directors and Senior Management |
The following table presents the names of the current members of our board of directors and executive officers.
| Name | Age | Position | ||
| James Hill, M.D. | 73 | Class A Director | ||
| Stuart Ungar, M.D. | 75 | Class A Director | ||
| David Byrne | 59 | Class A Director | ||
| Donald Williams | 60 | Class A Director | ||
| Michael Grissinger | 65 | Class A Director | ||
| Dr. Peter Feldschreiber | 75 | Class A Director | ||
| Clive Richardson | 54 | Class B Director —Interim Chief Executive Officer and Chief Operating Officer | ||
| Ray Prudo | 74 | Class C Director — Executive Chairman of the Board | ||
| Dov Elefant | 51 | Chief Financial Officer |
| 67 |
Biographical information of the members of our board of directors and executive officers is set forth below.
James Hill, M.D., age 73, has served as a member of our board of directors since September 2015. Prior to joining our board of directors, Dr. Hill was a non-executive director and Chairman of Genetix Group Plc from 2001 to 2009, an AIM listed company providing scientists with intelligent solutions for cell imaging and analysis. Previously Dr. Hill was a director and Senior Vice President of Corporate Affairs with SmithKline Beecham, from 1994 to 2001, with global responsibility for Investor Relations, Government Affairs, Communication and was a member of the corporate management team which oversaw corporate strategy. Dr. Hill’s prior experience was in the field of strategic product development working closely with research and development and the global markets. Dr. Hill qualified in medicine at Guys Hospital and became a fellow to The Royal Colleges of Physicians in both London and Edinburgh and was earlier awarded a Hunterian Professorship by The Royal College of Surgeons in England.
Stuart Ungar, M.D., age 75, has served as a member of our board of directors since September 2015. Dr. Ungar is currently a member of our board of directors. After pursuing post-graduate studies in Internal Medicine and research in neuro-pharmacology at The Royal Post-Graduate Medical School, UK, Dr. Ungar was in practice as an Internist at The Princess Grace Hospital, London. Following fifteen years of practice he, jointly with Dr. Raymond Prudo, founded The Doctors Laboratory PLC (TDL), a general pathology laboratory, which provided analytical services to clinicians and pharmaceutical organizations throughout the United Kingdom and abroad. During his tenure as Chairman and Board Director, The Doctors Laboratory PLC grew from a start-up to become one of the largest pathology laboratories in the United Kingdom. It was sold to Sonic Healthcare, a quoted Australian PLC in 2002. Dr. Ungar studied medicine and biochemistry in the University of London at the Royal Free Hospital School of Medicine. As a post-graduate he was admitted to The Royal College of Physicians of the United Kingdom. Dr. Ungar is a Life Fellow of The Royal Society of Medicine and a founder and former Vice-President of The Independent Doctors Federation.
David Byrne, age 59, has served as a member of our board of directors since June 2016. Mr. Byrne is currently Group Chief Executive Officer of Sonic Healthcare UK Group, the United Kingdom’s largest NGO clinical diagnostics organization, a position that he has held since 1997. Mr. Byrne is also the CEO of The Doctors Laboratory which is a subsidiary of Sonic. Mr. Byrne also currently serves as a Main Board Director for CIS Healthcare Limited and served as a Main Board Finance Director for Clinisys Solutions Ltd from 2000 to 2007. He is a UK Chartered Certified Accountant with over 25 years’ experience in corporate finance and developing early stage biotechnology and medical services companies.
Donald Williams, age 60, has served as a member of our board of directors since June 2016. Mr. Williams is a 35-year veteran of the public accounting industry who retired in 2014. Mr. Williams spent 18 years as a partner at Ernst & Young and the last seven years as a partner at Grant Thornton. Mr. Williams’ career focused on private and public companies in the technology and life sciences sectors. During the last seven years at Grant Thornton, he served as the National Leader of Grant Thornton’s Life Sciences Practice and the Managing Partner of the San Diego Office. He was the lead partner for both Ernst & Young and Grant Thornton on multiple initial public offerings; secondary offerings; private and public debt financings; as well as numerous mergers and acquisitions. Mr. Williams serves as a director of Alphatec Holdings, Inc., Impedimed Limited and Adhera Therapeutics, Inc. (formerly Marina Biotech, Inc.). Mr. Williams served on the board of directors and is past President and Chairman of the San Diego Venture Group and has served on the board of directors of Proove Biosciences and various charitable organizations in the communities in which he has lived. Mr. Williams is a graduate of Southern Illinois University with a B.S. degree.
Michael Grissinger, age 65, has served as a member of our board of directors since January 2018. Mr. Grissinger spent 22 years at Johnson & Johnson, retiring in 2018. During his Johnson and Johnson tenure, Mr. Grissinger served in a variety of senior-level management roles including Vice President- Corporate Development, Vice President- Worldwide Business Development & Licensing, as well as Vice President and Head- Mergers & Acquisitions for the pharmaceuticals group. Prior to Johnson & Johnson, Mr. Grissinger spent 12 years at Ciba-Geigy in finance, marketing, and business development roles. In addition to Akari, Mr. Grissinger also serves as a member of the board of directors of resTORbio and Atrin Pharmaceuticals. Mr. Grissinger holds a B. Sc. in Chemistry from Juniata College and an MBA from Temple University- Fox School of Business.
| 68 |
Dr. Peter Feldschreiber, age 75, has served as a member of our board of directors since January 2018. Dr. Feldschreiber is dual qualified as a physician and barrister with extensive experience both in the pharmaceutical industry and healthcare law. Since 2004, Dr. Feldschreiber has been a member of 4 New Square chambers in Lincoln’s Inn. He has over 20 years’ experience in the pharmaceutical industry including 10 years’ as European Medical Director at Proctor and Gamble Limited and he has held appointments as Senior Medical Assessor and Special Litigation Coordinator to the Commission on Human Medicines, a U.K. government advisory body, as well as the Committee on Safety of Devices, Medicines, and Healthcare Products Regulatory Agency, part of the U.K. government’s Department of Health. Dr. Feldschreiber is General Editor of the Law and Regulation of Medicines (Oxford University Press) and Consultant Editor for the section on Medicinal Products and Drugs in the Fifth Edition of Halsbury’s Laws of England. Dr. Feldschreiber holds a B.Sc. MB.BS from Kings College Hospital Medical School, University of London, is a Fellow of the Faculty of Pharmaceutical Medicine Royal College of Physicians and holds an LLB Hons. from Thames Valley University.
Clive Richardson, age 54, has served as our Chief Operating Officer and member of our board of directors since September 2015 and as our Interim Chief Executive Officer since May 2018. Prior to that, Mr. Richardson was Head of Operations for Volution, a position he has held from January 2014 until September 2015 and before that he was a consultant to Volution’s predecessor company, Varleigh Immuno Pharmaceuticals, since inception in 2007. Prior to working for Volution and Varleigh, Mr. Richardson served as a member of the board of directors for a range of international healthcare companies, including CIS Healthcare Ltd. and Clinisys Ltd. Mr. Richardson was formerly Head of Equities Research for Investec Bank, and worked as a strategy consultant for L.E.K. Consulting. Mr. Richardson holds an M.A. in Zoology from Trinity College, Oxford University.
Ray Prudo, M.D. age 74, has served as our Executive Chairman since September 2015. Dr. Prudo has been an active investor and developer of healthcare companies for 25 years. Dr. Prudo is the Founder, Chairman, and Chief Executive Officer of Volution and its predecessor company, Varleigh Immuno Pharmaceuticals, since inception in 2007. He is currently a board member of several UK healthcare companies. Dr. Prudo holds an MBBS from the University of London, and an FRCP(C) from the Royal College of Physicians and Surgeons of Canada.
Dov Elefant, age 51, has served as our Chief Financial Officer since January 2012. From March 2011 until January 2012, he was Chief Financial Officer of Althera Medical Ltd. and from March 2009 to February 2011 he performed consulting services to a number of companies. He was also the Corporate Controller, from March 2007 to February 2009 for Lev Pharmaceuticals, which was acquired by ViroPharma in 2008, Controller and Vice President of Finance and Administration at EpiCept Corporation from December 1999 to March 2007, Assistant Controller at Tetragenex Pharmaceuticals from November 1998 to October 1999 and held other accounting and finance roles from March 1991 to October 1998. Mr. Elefant holds a B.S. in accounting from Yeshiva University.
| B. | Compensation |
Summary Compensation Table
The following table shows the compensation paid or accrued during the last two fiscal years ended December 31, 2018 and 2017 to (1) our Executive Chairman, (2) our Interim Chief Executive Officer and Chief Operating Officer, (3) our Chief Financial Officer, (4) our former Chief Executive Officers, and (5) our former Chief Legal & Compliance Officer and Secretary.
| 69 |
| Name and Principal Position |
Year | Salary ($) |
Bonus ($) |
Stock Awards ($) |
Option Awards ($) (5) |
Non-Equity Incentive Plan Compensation ($) |
Nonqualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
|||||||||||||||||||||||||||
| Ray Prudo, | 2018 | 212,180 | 106,090 | — | — | — | — | — | 318,270 | |||||||||||||||||||||||||||
| Executive | ||||||||||||||||||||||||||||||||||||
| Chairman | 2017 | 206,000 | 103,000 | — | — | — | — | — | 309,000 | |||||||||||||||||||||||||||
| Clive | 2018 | 354,405 | 131,755 | — | 253,123 | — | — | 34,683 | (2) | 773,966 | ||||||||||||||||||||||||||
| Richardson, | ||||||||||||||||||||||||||||||||||||
| Interim Chief Executive Officer and Chief Operating Officer | 2017 | 332,507 | 135,991 | — | 113,272 | — | — | 32,650 | (2) | 614,420 | ||||||||||||||||||||||||||
| Dov Elefant, | 2018 | 264,000 | 66,000 | — | — | — | — | 13,691 | (2) | 343,691 | ||||||||||||||||||||||||||
| Chief Financial | ||||||||||||||||||||||||||||||||||||
| Officer | 2017 | 240,000 | 60,000 | — | 12,586 | — | — | 7,822 | (2) | 320,408 | ||||||||||||||||||||||||||
| David Horn | 2018 | 183,371 | — | — | — | — | — | 4,261 | (4) | 187,632 | ||||||||||||||||||||||||||
| Solomon Ph.D., | ||||||||||||||||||||||||||||||||||||
| Former Chief Executive Officer(3) | 2017 | 173,611 | 119,041 | — | 654,459 | — | — | 197,879 | (4) | 1,144,990 | ||||||||||||||||||||||||||
| Gur Roshwalb, | 2018 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| M.D., Former | ||||||||||||||||||||||||||||||||||||
| Chief Executive Officer (5) | 2017 | 187,500 | — | — | — | — | — | 5,763 | (6) | 193,263 | ||||||||||||||||||||||||||
| Robert Shaw, | 2018 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||
| Former Chief | ||||||||||||||||||||||||||||||||||||
| Legal & Compliance Officer and Secretary (7) | 2017 | 281,745 | — | — | 45,309 | — | — | 478,401 | (8) | 805,455 | ||||||||||||||||||||||||||
| (1) | These amounts represent the aggregate grant date fair value for option awards for fiscal years 2018 and 2017, respectively, computed in accordance with FASB ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in our Financial Statements, included in our Annual Report on Form 20-F for the years ended December 31, 2018 and 2017. |
| (2) | Consists of company contributions to a 401K plan or pension scheme. |
| (3) | Dr. Solomon was appointed as our Chief Executive Officer on August 28, 2017 and resigned as Chief Executive Officer on May 8, 2018. |
| (4) | Consists of (i) $193,750 in 2017 in interim housing and related expenses paid on behalf of Dr. Solomon, and (ii) $1,000 per month allowance. See “Item 6 B. Directors, Senior Management and Employees—Compensation”. |
| (5) | Dr. Roshwalb resigned as Chief Executive Officer on May 29, 2017. |
| (6) | Consists of accrued vacation paid. |
| (7) | Mr. Shaw was terminated without cause as Chief Legal & Compliance Officer and Secretary as of January 3, 2018. |
| 70 |
| (8) | Consists of (i) $8,105 of company contribution to a 401K plan, (ii) $14,269 of accrued vacation, and (iii) $456,027 of severance related payments that accrued in 2017. See “Item 6 B. Directors, Senior Management and Employees—Compensation”. |
Narrative Disclosure to Summary Compensation Table
Ray Prudo. On September 21, 2015, in connection with the Acquisition, we entered into an agreement with our Executive Chairman and director, Ray Prudo. The agreement was effective as of September 18, 2015. Under the agreement, Dr. Prudo will hold office as Chairman and Class C Director for a three-year term and thereafter will seek reappointment in accordance with our Articles of Association. Dr. Prudo’s appointment is subject to early termination in accordance with the Articles of Association or if he ceases to be a director.
Dr. Prudo’s current annual salary is $400,000 beginning January 1, 2019. Upon termination of Dr. Prudo’s appointment, he shall be entitled to any accrued but unpaid base salary and expense reimbursement. Under the agreement, Dr. Prudo is required to maintain the confidentiality of our confidential information.
In a separately entered into agreement with Dr. Prudo on September 2015, Dr. Prudo is entitled to an additional cash payment each calendar year in the discretion of our board of directors based on Dr. Prudo’s performance. Dr. Prudo’s annual cash bonus is currently set at 50% of base salary.
Clive Richardson. On September 21, 2015, in connection with the Acquisition, we entered into an executive employment agreement with our Chief Operating Officer and director, Clive Richardson. The employment agreement was effective as of September 16, 2015 and shall continue in effect until terminated by either party upon at least six months’ prior written notice. On May 8, 2018, Mr. Richardson was appointed Interim Chief Executive Officer.
Mr. Richardson’s current annual base salary is £337,428 (approximately $430,000), beginning January 1, 2019. Mr. Richardson is also entitled to an annual cash bonus with a target of 40% of base salary, provided that the actual amount of such bonus may be greater or less that the target amount. The employment agreement also provides that Mr. Richardson is entitled to a stock option grant to purchase 16,271,850 ordinary shares (equivalent to 162,718 ADSs). This option was granted under our 2014 Plan on September 21, 2015, has a ten-year term, is exercisable at a price equal to $0.3221 per share (or $32.21 per ADS) and vests ratably on a semi-annual basis over four years, with a minimum 25% vesting, subject to acceleration in the case of change of control or non-renewal of the employment agreement.
Upon termination of Mr. Richardson’s employment by us for cause, then he shall be entitled to any accrued amounts due at the date of termination. In other instances of termination of Mr. Richardson’s employment, he shall be entitled to receive an amount equal to 18 months of base salary in effect before the employment terminates, plus one and a half times the target annual performance bonus to which Mr. Richardson may have been entitled to for the year in which the employment terminates; provided our valuation in a change of control transaction is greater than our valuation at the time of the Acquisition.
The employment agreement also contains restrictive covenants for our benefit and Mr. Richardson is required to maintain the confidentiality of our confidential information.
In October 2018, we entered into an Option and Bonus Letter with Mr. Richardson which granted Mr. Richardson options to purchase 20,000,000 ordinary shares (equivalent to 200,000 ADSs) subject to the terms and conditions of our 2014 Plan in consideration for his new appointment as interim Chief Executive Officer. The Option and Bonus Letter also entitles Mr. Richardson to a cash bonus of three times (3x) his annual salary and benefits in the event of a sale transaction.
Dov Elefant. On September 21, 2015, in connection with the Acquisition, we entered into an executive employment agreement with our Chief Financial Officer, Dov Elefant. The employment agreement was effective as of September 18, 2015, and has a term of one year with automatic renewals for successive one-year periods unless terminated by either party upon three-months’ notice prior to the expiration of the current term. We are entitled to terminate the employment agreement immediately upon notice in the case of termination for cause or Mr. Elefant’s death or disability and upon 30 days’ prior written notice for any other reason. Mr. Elefant is entitled to terminate the employment agreement with or without good reason upon 30 days’ prior written notice.
Mr. Elefant’s current annual base salary is $282,480, beginning January 1, 2019. Mr. Elefant is also entitled to an annual cash bonus with a target of 25% of base salary, provided that the actual amount of such bonus may be greater or less that the target amount. The employment agreement also provides that Mr. Elefant is entitled to a stock option grant to purchase 4,067,963 ordinary shares (equivalent to 40,679 ADSs). This option was granted under our 2014 Plan on September 21, 2015, has a ten-year term, is exercisable at a price equal to $0.3221 per share (or $32.21 per ADS) and vests ratably on a semi-annual basis over four years, subject to acceleration in the case of change of control or non-renewal of the employment agreement.
| 71 |
Upon termination of Mr. Elefant’s employment by us for cause or by Mr. Elefant without good reason or in the case of Mr. Elefant’s disability or death then he shall be entitled to any accrued but unpaid base salary and expense reimbursement.
Upon termination of Mr. Elefant’s employment without cause or by Mr. Elefant for good reason, in addition to any accrued but unpaid base salary and expense reimbursement, he shall be entitled to receive an amount equal to 12 months of base salary in effect before the employment terminates, plus the greater of the actual or target annual performance bonus to which Mr. Elefant may have been entitled to for the year in which the employment terminates. Upon termination of Mr. Elefant’s employment upon non-renewal of the term, in addition to any accrued but unpaid base salary and expense reimbursement, he shall be entitled to receive an amount equal to 12 months of base salary in effect before the employment terminates. In each such instance of termination, Mr. Elefant shall also be entitled to an amount equal to our share of the medical insurance premium that we pay for Mr. Elefant under our health care plan for 12 months following the date of termination.
Upon termination of Mr. Elefant’s employment by us without cause or by Mr. Elefant for good reason within one year of a change of control in addition to any accrued but unpaid base salary and expense reimbursement, he shall be entitled to receive an amount equal to 18 months of base salary in effect before the employment terminates, plus one and a half times the target annual performance bonus to which Mr. Elefant may have been entitled to for the year in which the employment terminates; provided our valuation in a change of control transaction is greater than our valuation at the time of the Acquisition. In such instance, Mr. Elefant shall also be entitled to an amount equal to our share of the medical insurance premium that we pay for Mr. Elefant under our health care plan for 18 months following the date of termination.
The employment agreement also contains restrictive covenants for our benefit and Mr. Elefant is required to maintain the confidentiality of our confidential information.
Dr. David Horn Solomon. On August 18, 2017, Dr. Solomon was appointed to the Board, effective as of August 28, 2017, as a Class A director until the 2018 annual meeting of the Company’s shareholders. Dr. Solomon was also hired, pursuant to the Executive Employment Agreement, dated August 18, 2017 to be the Chief Executive Officer of the Company, effective August 28, 2017. On May 8, 2018, Dr. Solomon resigned as Chief Executive Officer and director.
Dr. Solomon’s annual base salary was $515,000, reflecting a 3% increase beginning January 1, 2018, and subject to review on an annual basis. Dr. Solomon was entitled to a one-time cash bonus of $50,000 in 2017 plus relocation expenses of up to $80,000 with a gross up for taxes, and a monthly perquisite payment of $1,000. Under the employment agreement, Dr. Solomon was required to relocate his primary residence to and be resident in the New York metropolitan area by August 1, 2018. Dr. Solomon was also eligible to receive an annual cash bonus with a target of 40% of base salary, provided that the actual amount of such bonus shall be determined by the Board or an appropriate committee thereof in its sole discretion. Any bonus for a year in which Dr. Solomon was employed for less than the full year will be prorated. The employment agreement also provided that Dr. Solomon is entitled to a stock option to purchase 26,000,000 Ordinary Shares (equivalent to 260,000 ADSs) under the Company’s Amended and Restated 2014 Equity Incentive Plan. The option had a term of ten years with an exercise price equal to the closing price of the grant date vesting ratably on an annual basis over four years, beginning on the grant date, provided that Dr. Solomon remains employed with the Company, and subject to acceleration in the case of change of control. These options were granted on September 1, 2017. The employment agreement also contained restrictive covenants for our benefit and Dr. Solomon is required to maintain the confidentiality of our confidential information.
On September 14, 2017, we and Dr. Solomon entered into an amendment to the employment agreement amending provisions relating to Dr. Solomon’s relocation to New York. Under the amendment, it was agreed, among other things, that Dr. Solomon would relocate his primary residence and be resident in New York on September 15, 2017 and in lieu of certain relocation expenses originally agreed to in the employment agreement, we would pay up to $193,750 in interim housing and related expenses (including deposits) subject to repayment in certain circumstances. Under the amendment, Dr. Solomon is required to repay $39,500 in deposits at the end of the rental term which expires September 14, 2018, and as a result of Dr. Solomon’s resignation is required to repay to us $18,750 in interim housing and related expenses.
| 72 |
In connection with Dr. Solomon’s resignation, on May 8, 2018, we and Dr. Solomon entered into an Employment Separation and General Release Agreement dated July 18, 2018, or the Solomon Separation Agreement. Pursuant to the terms of the Solomon Separation Agreement, the parties agreed that Dr. Solomon’s employment was terminated by reason of Dr. Solomon’s resignation on May 8, 2018, or the Solomon Separation Date. Dr. Solomon is entitled to receive, to the extent entitled, any unpaid base salary, vested benefits, accrued but unused vacation time and expense reimbursement through the Solomon Separation Date and, under certain circumstances, reimbursement of up to $5,000 for legal fees incurred in connection with the Solomon Separation Agreement. The Solomon Separation Agreement provided that the 26,000,000 stock options that were granted to Dr. Solomon, all of which were unvested, were forfeited as of the Solomon Separation Date, with no compensation or other payment due to Dr. Solomon. In addition, the Solomon Separation Agreement provided that if Dr. Solomon executed the Solomon Separation Agreement within 21 days of the date of the agreement and did not exercise his right to revoke, we agreed to enter into a promissory note, or the Separation Consideration, with a principal sum of $93,202.24, due with all accrued but unpaid interest on November 8, 2019, which would fully satisfy any amounts owed by Dr. Solomon to us as of the Solomon Separation Date arising out of (a) charges made by Dr. Solomon to the Company credit card, and (b) repayment of interim housing and related expenses under the amendment described above. The Solomon Separation Agreement also contains continuing confidentiality restrictions, a release and a covenant not to sue. Dr. Solomon signed the Solomon Separation Agreement effective May 8, 2018.
Gur Roshwalb, M.D. On September 21, 2015, in connection with the Acquisition, we entered into an executive employment agreement with our former Chief Executive Officer and director, Gur Roshwalb. Dr. Roshwalb resigned as Chief Executive Officer on May 29, 2017. Dr. Roshwalb’s most recent annual base salary was $450,000 which was subject to review on an annual basis. Dr. Roshwalb was also entitled to an annual cash bonus with a target of 40% of base salary, provided that the actual amount of such bonus may be greater or less that the target amount. The employment agreement also provided that Dr. Roshwalb was entitled to a stock option grant to purchase 32,543,700 Ordinary Shares (equivalent to 325,437 ADSs). This option was granted under our 2014 Equity Incentive Compensation Plan, or the 2014 Plan, on September 21, 2015, had a ten-year term, was exercisable at a price equal to $0.3221 per share (or $32.21 per ADS) and vested ratably on a semi-annual basis over four years, with a minimum 25% vesting, subject to acceleration in the case of change of control or non-renewal of the employment agreement. The employment agreement further provided that Dr. Roshwalb shall receive no additional compensation for his service on the board of directors. The employment agreement also contained restrictive covenants for our benefit and Dr. Roshwalb is required to maintain the confidentiality of our confidential information.
Robert Shaw. On March 23, 2016, we entered into an executive employment agreement with our former Chief Legal & Compliance Officer and Secretary, Robert Shaw which was subsequently amended on February 15, 2017 and August 2, 2017. Mr. Shaw was terminated without cause as of January 3, 2018. Mr. Shaw’s most recent annual base salary was $318,000 which was subject to review on an annual basis. From February 15, 2017, Mr. Shaw was also entitled to an annual cash bonus with a target of 40% of base salary, provided that the actual amount of such bonus may be greater or less that the target amount. The employment agreement also provided that Mr. Shaw was entitled to a stock option grant to purchase 1,800,000 Ordinary Shares (equivalent to 18,000 ADSs). This option was granted under our 2014 Plan on March 23, 2016, had a ten-year term, is exercisable at a price equal to $.0141 per share (or $14.10 per ADS) and vested ratably on a semi-annual basis over four years, subject to acceleration in the case of change of control or non-renewal of the employment agreement.
In connection with Mr. Shaw’s termination, on January 26, 2018, we and Mr. Shaw entered into an Employment Separation and General Release Agreement dated January 4, 2018, or the Shaw Separation Agreement. Pursuant to the terms of the Shaw Separation Agreement, the parties agreed that Mr. Shaw’s employment was terminated without cause effective December 4, 2017, or the Separation Date, and that he will receive unpaid base salary through January 3, 2018, our portion of health insurance premiums paid for Mr. Shaw through January 3, 2018 and accrued but unused vacation time and expense reimbursement through the Separation Date. In addition, if Mr. Shaw executed the Shaw Separation Agreement within 60 days of the Separation Date and did not exercise his right to revoke, we agreed to (i) pay Mr. Shaw $445,200 as severance pay, (ii) pay Mr. Shaw $10,827, which is an amount equal to our share of the health insurance premiums paid for Mr. Shaw while he was an employee for a period of twelve (12) months following the Separation Date (in both cases less applicable tax withholdings and deductions) and (iii) subject to the approval of the administrator in accordance with the 2014 Incentive Plan, modify the terms of the outstanding option agreements granted to Mr. Shaw on March 23, 2016 and September 1, 2017 such that each tranche of unvested options in the option agreements will continue to vest on the same schedules through September 23, 2018. The Separation Agreement also contains mutual non-disparagement provisions and a release and covenant not to sue. Mr. Shaw signed the Shaw Separation Agreement effective January 3, 2018.
| 73 |
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding all outstanding equity awards for (1) our Executive Chairman, (2) our Interim Chief Executive Officer and Chief Operating Officer and (3) our Chief Financial Officer as of December 31, 2018:
| Option Awards | ||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options (#) Exercisable(1) | Number of Securities Underlying Unexercised Options (#) Unexercisable(1) | Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) | Option Exercise Price ($) | Option Expiration Date | |||||||||||||||
| Ray Prudo | — | — | — | — | — | |||||||||||||||
| Clive Richardson | 13,220,878 | (2) | 3,050,972 | (2) | — | 0.3221 | 9/21/2025 | |||||||||||||
| 1,125,000 | (3) | 3,375,000 | (3) | — | 0.035992 | 9/1/2027 | ||||||||||||||
| — | 20,000,000 | (4) | — | 0.0187 | 8/15/2028 | |||||||||||||||
| Dov Elefant | 40,000 | (5) | — | — | 1.56 | 1/11/2022 | ||||||||||||||
| 100,000 | (6) | — | — | 2.00 | 7/1/2023 | |||||||||||||||
| 40,000 | (7) | — | — | 0.75 | 2/5/2024 | |||||||||||||||
| 3,305,220 | (2) | 762,743 | (2) | — | 0.3221 | 9/21/2025 | ||||||||||||||
| 125,000 | (3) | 375,000 | (3) | — | 0.035992 | 9/1/2027 | ||||||||||||||
| (1) | Amounts represent options to purchase ordinary shares. |
| (2) | These options were granted on September 21, 2015, in connection with the Acquisition, have a ten-year term and vest over a four-year period with one-sixteenth of the options granted vesting every three months beginning September 21, 2015, through 2019. |
| (3) | These options were granted on September 1, 2017, have a ten-year term and vest annually over a four-year period. |
| (4) | These options were granted on August 15, 2018, have a ten-year term and vest annually over a four-year period. |
| (5) | These options were granted on January 11, 2012, have a ten-year term and were fully exercisable on January 11, 2013. |
| (6) | These options were granted on September 24, 2013, have a ten-year term and were fully exercisable on July 1, 2014. |
| (7) |
These options were granted on February 5, 2014, have a ten-year term and were fully exercisable on May 31, 2014. |
Director Compensation
Our director compensation program is administered by our board of directors with the assistance of the compensation committee. The compensation committee conducts an annual review of director compensation and makes recommendations to the board with respect thereto.
| 74 |
The shareholders approved our Directors Renumeration Policy on July 14, 2017 to provide a framework for the Director’s compensation package. In addition, the Company has a non-employee director compensation policy, which was amended and restated on November 19, 2015 and was subsequently amended on June 29, 2016, January 26, 2017 and on January 23, 2018. On January 8, 2019, our board approved a 3% increase in cash compensation and increased committee membership fees. As a result, our non-employee directors will be compensated for service on our board of directors as follows in 2019:
| · | an annual retainer for service on the board of directors of $39,338; |
| · | an annual retainer for service as a member of the compensation committee and nominating and governance committee of $5,305; |
| · | an annual retainer for service as a member of the audit committee of $7,500; |
| · | for the chairman of the compensation committee, and nominating and governance committee, an annual retainer of $10,609; |
| · | for the chairperson of the audit committee, an annual retainer of $17,500; |
| · | for service on the board, an annual grant of a stock option to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) or Annual Stock Option; the grant is made each year on the date of the Company’s Annual General Meeting of shareholders, or AGM, on the date of the first meeting of the board held following the AGM, or on any date following such AGM as may be recommended by the compensation committee and approved by the Board; |
| · | for each new non-employee director that is appointed to the board of directors, an initial grant of a stock option to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs), or Initial Stock Option Grant; if the non-employee director was not previously appointed to the board and is first elected at the Company’s AGM, the grant shall be made on the date of the Company’s AGM, on the date of the first meeting of the board held following the AGM, or on any date following such AGM as may be recommended by the compensation committee and approved by the board; if the non-employee director is appointed to the board in advance of the next AGM and will stand for election at that AGM, the grant shall be made at the meeting of the board at which he or she is appointed and, if not made at such meeting, shall be made promptly following such meeting by written unanimous consent of the board; and if the non-employee director is first appointed to the board in advance of the next AGM and will not stand for election at that AGM, the number of shares in the grant and the vesting schedule shall be determined by the compensation committee, taking into account the term of the appointment and the grant shall be made at the meeting of the board at which he or she is appointed and, if not made at such meeting, shall be made promptly following such meeting by written unanimous consent of the board; |
| · | unless otherwise specified by the board or the compensation committee at the time of grant, each Annual Stock Option granted under this policy shall (i) vest in full on the date of the next AGM following the date of grant, subject to the non-employee director’s continued service on the Board; (ii) have an exercise price equal to the fair market value of the Company’s ordinary shares as determined in the 2014 Plan on the grant date; (iii) terminate ten years after the grant date, (iv) become fully vested immediately prior to a change of control and (v) contain such other terms and conditions as set forth in the form of option agreement approved by the board or the compensation committee prior to the grant date; and |
| · | unless otherwise specified by the board or the compensation committee at the time of grant, each Initial Stock Option Grant for newly appointed or elected directors granted under the non-employee director compensation policy shall (i) vest ratably in three equal installments with the first installment vesting on the date of the first AGM following the date of the grant, and with the second and third installments vesting, respectively, on the dates of the second and third AGMs following the date of the grant, subject to the non-employee director’s continued service on the board; (ii) have an exercise price equal to the fair market value of the Company’s ordinary shares as determined in the 2014 Plan on the grant date; (iii) terminate ten years after the grant date, (iv) become fully vested immediately prior to a change of control and (v) contain such other terms and conditions as set forth in the form of option agreement approved by the board or the compensation committee prior to the grant date; and |
| 75 |
| · | each of these options shall be granted under our 2014 Plan, terminate ten years after the grant date and become fully vested immediately prior to a change of control. |
All directors are eligible to receive reimbursement for reasonable out-of-pocket expenses incurred in connection with attendance at meetings of our board of directors, and our non-employee directors are also eligible to receive reimbursement, upon approval of the board of directors or a committee thereof, for reasonable out-of-pocket expenses incurred in connection with attendance at various conferences or meetings with our management.
The following table sets forth information regarding the total compensation awarded to, earned by or paid to each of our non-employee directors during the year ended December 31, 2018 for their service on our board of directors. Dr. Prudo, our Executive Chairman, Clive Richardson, our Interim Chief Executive Officer and Chief Operating Officer and Dr. David Horn Solomon, did not receive any additional compensation for their service as a director during 2018. The compensation that we pay to the foregoing persons is discussed in the “Summary Compensation Table” above.
| Name | Fees Earned or Paid in Cash ($) |
Option Awards ($) (1) |
Total ($) |
|||||||||
| James Hill, M.D. (2) | $ | 58,792 | $ | 16,869 | $ | 75.661 | ||||||
| Stuart Ungar (3) | 48,492 | 16,869 | 65,361 | |||||||||
| David Byrne (4) | 48,492 | 16,869 | 65,361 | |||||||||
| Donald Williams (5) | 53,642 | 25,003 | 61,372 | |||||||||
| Robert Ward (6) | 36,369 | - | 36,369 | |||||||||
| Pete Feldschreiber (7) | 35,890 | 49,304 | 85,194 | |||||||||
| Michael Grissinger (8) | 35,890 | 49,304 | 85,194 | |||||||||
| (1) | These amounts represent the aggregate grant date fair value of options granted to each director during the year ended December 31, 2018 computed in accordance with ASC Topic 718. A discussion of the assumptions used in determining grant date fair value may be found in our Audited Financial Statements, included in our Annual Report on Form 20-F for the fiscal year ended December 31, 2018. |
| (2) | As of December 31, 2018, options to purchase an aggregate of 5,200,000 ordinary shares (equivalent to 52,000 ADSs) were outstanding, of which 3,900,000 ordinary shares (equivalent to 39,000 ADSs) were exercisable. |
| (3) | As of December 31, 2018, options to purchase an aggregate of 5,200,000 ordinary shares (equivalent to 52,000 ADSs) were outstanding, of which 3,900,000 ordinary shares (equivalent to 39,000 ADSs) were exercisable. |
| (4) | As of December 31, 2018, options to purchase an aggregate of 5,200,000 ordinary shares (equivalent to 52,000 ADSs) were outstanding, of which 3,466,666 ordinary shares (equivalent to 34,666 ADSs) were exercisable. |
| (5) | As of December 31, 2018, options to purchase an aggregate of 5,950,000 ordinary shares (equivalent to 59,500 ADSs) were outstanding, of which 3,466,666 ordinary shares (equivalent to 34,666 ADSs) were exercisable. |
| (6) | Mr. Ward did not stand for re-election at the 2018 annual general meeting and accordingly ceased to serve as a director as of September 19, 2018. As of December 31, 2018, no options were outstanding. |
| 76 |
| (7) | As of December 31, 2018, options to purchase an aggregate of 2,600,000 ordinary shares (equivalent to 26,000 ADSs) were outstanding, none of which were exercisable. |
| (8) | As of December 31, 2018, options to purchase an aggregate of 2,600,000 ordinary shares (equivalent to 26,000 ADSs) were outstanding, none of which were exercisable. |
| C. | Board Practices |
Our Articles of Association, as amended, provide that our business is to be managed by the board of directors (subject to any directions made by the members of the Company by special shareholder resolution). Our board of directors is divided into three classes for purposes of election (Class A Directors, who serve a one year term before being subject to re-election at the Company’s annual general meeting; Class B Directors, who serve a two year term before being subject to re-election at the annual general meeting; and Class C Directors who serve a three year term before being subject to re-election at the annual general meeting, provided also that in any two year period, a majority of the board must stand for re-election). Our board of directors currently consists of eight members: James Hill, M.D., Stuart Ungar, M.D., David Byrne, Donald Williams, Michael Grissinger and Peter Feldschreiber currently serve as Class A directors; Clive Richardson currently serves as a Class B director and Ray Prudo currently serves as a Class C director. Ray Prudo serves as Executive Chairman of our board of directors.
Subject to certain exceptions, the rules of Nasdaq permit a foreign private issuer to follow its home country practice in lieu of listing requirements of Nasdaq. The committees of our board of directors consist of an audit committee, a compensation committee and a nominating and corporate governance committee.
Audit Committee
Our audit committee currently consists of three members, appointed by the board of directors: Donald Williams, James Hill, M.D. and David Byrne, all of whom are independent within the meaning of SEC corporate governance rules of independence for purposes of the audit committee. Mr. Williams is the chairman of our audit committee. Our board of directors has determined that Mr. Williams is the audit committee financial expert.
Compensation Committee
Our compensation committee currently consists of three members, appointed by the board of directors: James Hill, M.D., Stuart Ungar, M.D. and David Byrne, all of whom are independent within the meaning of SEC corporate governance rules of independence for purposes of the compensation committee. Dr. Hill is the chairman of our compensation committee.
Nominating and Corporate Governance Committee
Our nominating and corporate governance committee currently consists of three members, appointed by our board of directors: Dr. Peter Feldschreiber, James Hill, M.D., and Stuart Ungar, M.D., all of whom are independent within the meaning of SEC corporate governance rules of independence for purposes of the nominating and corporate governance committee. Dr. Feldschreiber is chairman of our nominating and corporate governance committee.
None of our non-employee directors have any service contracts with Akari or any of our subsidiaries that provide for benefits upon termination of employment.
| D. | Employees |
As of December 31, 2018, we had thirteen full-time employees and one full-time equivalent consultant. Seven of our employees were engaged in research and development and six employees and one consultant were engaged in management, administration and finance. Eleven are located in England and three are located in the United States.
None of our employees are members of labor unions.
| 77 |
| E. | Share Ownership |
Information with respect to share ownership of members of our board of directors and executive officers is included in “Item 7. Major Shareholders and Related Party Transactions”
Option Plans
2014 Plan
In order to retain qualified individuals, the Board previously established the 2007 Stock Option Plan. We currently have issued options representing 180,000 ordinary shares under the 2007 Stock Option Plan.
Our board of directors approved the 2014 Equity Incentive Plan, or the 2014 Plan. The 2014 Plan was most recently amended by our shareholders on September 19, 2018. The shareholders approved the 2014 Plan on June 19, 2014. The purpose of the 2014 Plan is to enable us to continue to attract and retain professional personnel for the purposes of executing our clinical development plan. The material terms of the 2014 are set forth below.
The option plan is administered by our board of directors and grants are made pursuant thereto by the compensation committee. The aggregate number of ordinary shares that may be issued upon exercise of options under the 2014 Plan shall be the sum of: (i) 183,083,207 ordinary shares and (ii) any ordinary shares that are represented by awards granted under the Company’s 2007 Stock Option Plan that are forfeited, expire or are cancelled without delivery of ordinary shares or which result in the forfeiture of ordinary shares back to the Company on or after June 19, 2014, or the equivalent of such number of ordinary shares after the administrator, in its sole discretion, has interpreted the effect of any stock split, stock dividend, combination, recapitalization or similar transaction in accordance with the 2014 Plan; provided, however, that no more than 480,000 ordinary shares shall be added to the 2014 Plan pursuant to subsection (ii). Options may be granted at any time. As of December 31, 2018, options to purchase 94,096,998 of our ordinary shares were outstanding under the 2014 Plan. Unless sooner terminated, the Plan shall expire on April 30, 2024.
The per share exercise price for the shares to be issued pursuant to the exercise of an option shall be such price as determined by the board of directors and set forth in the individual option agreement, subject to any guidelines as may be determined by the board of directors from time to time, provided, however, that the exercise price shall be not less than the par value of the shares underlying the option, and subject to other conditions set forth in the 2014 Plan.
Options are exercisable pursuant to the terms under which they were awarded and subject to the terms and conditions of the 2014 Plan. In general, an option, or any part thereof, may not be exercised unless the optionee is then a service provider of our company or any parent or subsidiary thereof (as each such term is defined in the 2014 Plan). Any tax consequences arising from the grant or exercise of any option from the payment for shares covered thereby, the sale or disposition of such shares and any other expenses are the responsibility of the optionee unless otherwise required by applicable law.
On January 23, 2017, the compensation committee approved a UK Sub-Plan of the 2014 Plan which offers UK residents the opportunity to acquire interests in us in a similar manner to that offered to US employees. The UK Sub-Plan requires that only employees may receive grants, and that all options granted under the UK Sub-Plan must be designated as “non-tax-advantaged Options” under UK law. Options granted under the UK Sub-Plan are exercisable pursuant to the terms of their award and are subject to the terms of the 2014 Plan generally, except where otherwise stated. The UK Sub-Plan differs from the 2014 Plan only in UK-specific provisions regarding a valid disposition to survivors and compliance with local UK laws regarding securities, treatment as compensation, tax matters, data privacy, and third party rights.
Grants to non-employee directors who are based in the UK or who are UK resident taxpayers are made pursuant to the 2014 Plan in accordance with the UK Appendix to the Amended and Restated Non-Employee Director Compensation Policy which was approved by the compensation committee on January 23, 2017. The UK Appendix, as with the Sub-Plan for employees, differs from the Non-Employee Director Compensation Policy by providing specific UK-specific provisions. All options granted to UK non-employee directors or resident taxpayers are, as with the Sub-Plan for employees, designated as “non-tax-advantaged options” under UK law, exercisable pursuant to the terms of the award and the 2014 Plan, and provide for UK specific provisions regarding survivorship, securities, compensation, tax, data privacy, and third party rights laws.
| 78 |
In addition, on January 26, 2017, our board of directors approved a CSOP Sub Plan as recommended to the board by the compensation committee. The CSOP Sub-Plan modifies the 2014 Plan to qualify as a Schedule 4 CSOP Scheme as defined under the UK’s Income Tax (Earnings & Pensions) Act 2003, or ITEPA. The purpose of the CSOP Sub-Plan is to allow options granted to UK resident employees and directors to qualify as a Schedule 4 CSOP Scheme under governing law and to therefore qualify for certain tax advantages under ITEPA. CSOP options are effectuated by the execution of a deed of grant by the Company evidencing shares, option exercise price, restrictions, and other terms or conditions. Grants are limited under the CSOP Sub-Plan such that an employee cannot hold more than £30,000 in value, measured as of grant date. In the event of redundancy, retirement, a relevant transfer within the meaning of the Protection of Employment regulations, or a cease of control (as defined in Section 719 ITEPA) under governing UK law, the recipient may exercise any CSOP option that has become exercisable but has not yet been exercised for a period of six months. A Change of Control under UK law will similarly permit option exercise.
2007 Stock Option Plan
On August 28, 2007, our board of directors approved the 2007 Stock Option Plan, or the 2007 Stock Option Plan, as amended on April 26, 2012, June 20, 2012 and April 29, 2013. The shareholders approved the 2007 Stock Option Plan on June 20, 2013. The purpose of the 2007 Stock Option Plan was to provide an additional incentive to employees, officers, directors, consultants and other service providers of the Company and any parent or subsidiary of the Company (each as defined in the 2007 Stock Option Plan) to further the growth, development and financial success of the company by providing them with opportunities to purchase shares pursuant to the 2007 Stock Option Plan and to promote the success of the business. The material terms of the 2007 Stock Option Plan are set forth below.
The option plan was administered by the board of directors and grants were made pursuant thereto by the compensation committee. The aggregate number of ordinary shares that could be issued upon exercise of options under the 2007 Stock Option Plan could not exceed 5,865,000 ordinary shares. The board of directors could have, at any time during the term of the 2007 Stock Option Plan, increased the number of shares available for grant under the 2007 Stock Option Plan. As of August 28, 2017, options to purchase 480,000 of our ordinary shares were outstanding under the 2007 Stock Option Plan when the Plan expired on the tenth anniversary of its effective date. As of December 31, 2018, options to purchase 180,000 of our ordinary shares were outstanding under the 2007 Stock Option Plan.
The per share exercise price for the shares issued pursuant to the exercise of an option was such price as determined by the board of directors and set forth in the individual option agreement, subject to any guidelines as may have been determined by the board of directors from time to time, provided, however, that the exercise price could not be less than the par value of the shares underlying the option, and subject to other conditions set forth in the 2007 Stock Option Plan.
Options were exercisable pursuant to the terms under which they were awarded and were subject to the terms and conditions of the 2007 Stock Option Plan. In general, an option, or any part thereof, could not be exercised unless the optionee was then a service provider of our company or any parent or subsidiary thereof (as each such term is defined in the 2007 Stock Option Plan). Any tax consequences arising from the grant or exercise of any option from the payment for shares covered thereby, the sale or disposition of such shares and any other expenses was the responsibility of the optionee unless otherwise required by applicable law.
Equity Award Grant Policy
Based on the recommendation of the compensation committee, our board of directors adopted an Equity Award Grant Policy on January 26, 2017, which sets forth policies for us to follow when we grant stock options, shares of restricted stock, restricted stock units or other equity-based awards to employees of the Company or its subsidiaries.
The grants are divided into annual grants, made on the last business day in March, new hire grants, to our new hires, and performance grants to existing employees in recognition of performance. The policy standardizes employee grants and delegates authority to the Executive Chairman and CEO to make grants other than (a) grants of more than 1,000,000 ordinary shares or share-equivalents to one individual on one date; (b) new hire or performance grants to a person who is reasonably expected to be an executive officer or director upon hire or promotion; (c) any grant which would exceed 1,000,000 ordinary shares or share equivalents to any one individual in a calendar year; or (d) any grant with an exercise price less than fair market value on the date. These non-delegated grants remain in the sole authority of the compensation committee.
| 79 |
Pricing will be based on the closing market price on the Nasdaq Capital Market for all such grants. All new-hire grants must be made by a writing sent to the recipient stating the terms and conditions and clarifying that the equity award is subject to approval, whether by the delegated authority pursuant to the policy or by the compensation committee or the board itself.
| Item 7. | MAJOR SHAREHOLDERS AND RELATED PARTY TRANSACTIONS |
| A. | Major Shareholders |
The following table sets forth certain information with respect to the beneficial ownership of our ordinary shares as of March 31, 2019, the latest practicable date for inclusion in this annual report (except where otherwise indicated), for:
| · | each person, or group of affiliated persons, who are known by us to beneficially own more than 5% of our outstanding ordinary shares; |
| · | each member of our board of directors; |
| · | each of our other executive officers; and |
| · | all of our directors and executive officers as a group. |
The number of shares beneficially owned by each entity, person, director or executive officer is determined under the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under such rules, beneficial ownership includes any shares as to which the individual has the sole or shared voting power or investment power and also any shares that the individual has the right to acquire within 60 days of March 31, 2019, through the exercise of any option or other right. Unless otherwise indicated, each person has sole investment and voting power, or shares such powers with his or her spouse, with respect to the shares set forth in the following table.
Ordinary shares that may be acquired by an individual or group within 60 days of March 31, 2019, pursuant to the exercise of options or warrants, are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be outstanding for the purpose of computing the percentage ownership of any other person shown in the table. The percentage of ownership is based on 1,585,693,413 shares of ordinary shares outstanding on March 31, 2019, adjusted as required by the rules promulgated by the SEC to determine beneficial ownership. Akari does not know of any arrangements, including any pledge by any person of securities of Akari, the operation of which may at a subsequent date result in a change of control of Akari. Unless otherwise noted, the address of each director and executive officer of Akari is: c/o Akari Therapeutics, Plc, 75/76 Wimpole Street, London W1G 9RT.
Number of Ordinary Beneficially Owned (1) | Percentage of Ordinary Beneficially | |||||||
| Directors and Executive Officers | ||||||||
| Ray Prudo | 782,345,600 | (1) | 49.3 | % | ||||
| Clive Richardson | 15,362,869 | (2) | * | |||||
| James Hill, M.D. | 3,900,000 | (3) | * | |||||
| Stuart Ungar, M.D. | 3,900,000 | (4) | * | |||||
| Dov Elefant | 3,864,468 | (5) | * | |||||
| David Byrne | 3,900,000 | (6) | * | |||||
| Donald Williams | 3,466,666 | (7) | * | |||||
| Michael Grissinger | 325,000 | (8) | * | |||||
| Peter Feldschreiber | 325,000 | (8) | * | |||||
| All directors and officers as a group (10 persons) | 51.5 | % | ||||||
| 5% or More Shareholders | ||||||||
| RPC Pharma Limited (10) | 782,345,600 | 49.3 | % | |||||
* Represents beneficial ownership of less than 1% of our outstanding ordinary shares.
| 80 |
| (1) | Represents the entire holdings of RPC Pharma Limited. Dr. Prudo has voting and dispositive control over the ordinary shares held by RPC Pharma Limited and owns approximately 71% of RPC’s outstanding shares (including option grants), including 10.64% of RPC’s outstanding shares held in trust for Dr. Ungar. Dr. Prudo disclaims beneficial ownership except to the extent of his actual pecuniary interest in such shares. |
| (2) | Consists of options to purchase 14,237,869 ordinary shares (equivalent to 14,237 ADSs) at an exercise price of $0.3221 per share (or $32.21 per ADS), which expire on September 21, 2025 and options to purchase 1,125,000 ordinary shares (equivalent to 11,250 ADSs) at an exercise price of $0.035992 per share (or $3.5992 per ADS) and which expire on September 1, 2027. Does not include (i) options to purchase 2,033,981 ordinary shares (equivalent to 20,339 ADSs) at an exercise price of $0.3221 per share (or $32.21 per ADS) and which expire on September 21, 2025 that vest in more than 60 days from March 31, 2019, (ii) options to purchase 3,375,000 ordinary shares (equivalent to 33,750 ADSs) at an exercise price of $0.035992 per share (or $3.5992 per ADS) and which expire on September 1, 2027 that vest in more than 60 days from March 31, 2019, and (iii) options to purchase 20,000,000 ordinary shares (equivalent to 200,000 ADSs) at an exercise price of $0.0187 per share (or $1.87 per ADS) and which expire on August 15, 2028 that vest in more than 60 days from March 31, 2019. |
| (3) | Consists of (i) options to purchase 231,278 ordinary shares (equivalent to 2,312 ADSs) at an exercise price of $0.3221 per share (or $32.21 per ADS), which expire on September 21, 2025, (ii) options to purchase 1,068,722 ordinary shares (equivalent to 10,687 ADSs) at an exercise price of $0.1917 per share (or $19.17 per ADS), which expire on November 25, 2025, (iii) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.145 per share (or $14.50 per ADS), which expire on June 29, 2026, and (iv) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0504 per share (or $5.04 per ADS) and which expire on June 28, 2027. Does not include options to purchase 1,300,00 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0208 per share (or $2.08 per ADS) and which expire on September 19, 2028 that vest in more than 60 days from March 31, 2019. Also, does not include shares held by RPC Pharma Limited of which he has a pecuniary interest. Dr. Hill disclaims beneficial ownership of the shares held by RPC Pharma Limited. |
| (4) | Consists of (i) options to purchase 211,278 ordinary shares (equivalent to 2,112 ADSs) at an exercise price of $0.3221 per share (or $32.21 per ADS), which expire on September 21, 2025, (ii) options to purchase 1,088,722 ordinary shares (equivalent to 10,887 ADSs) at an exercise price of $0.1917 per share (or $19.17 per ADS), which expire on November 25, 2025, (iii) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.145 per share (or $14.50 per ADS), which expire on June 29, 2026, and (iv) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0504 per share (or $5.04 per ADS) and which expire on June 28, 2027. Does not include options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0208 per share (or $2.08 per ADS) and which expire on September 19, 2028 that vest in more than 60 days from March 31, 2019. Also, does not include shares held by RPC Pharma Limited of which he has a pecuniary interest. Dr. Ungar disclaims beneficial ownership of the shares held by RPC Pharma Limited. |
| 81 |
| (5) | Consists of (i) options to purchase 40,000 ordinary shares (equivalent to 400 ADSs) at an exercise price of $1.56 per share (or $156.00 per ADS), which expire on January 11, 2022, (ii) options to purchase 100,000 ordinary shares (equivalent to 1,000 ADSs) at an exercise price of $2.00 per share (or $200.00 per ADS), which expire on July 1, 2023, (iii) options to purchase 40,000 ordinary shares (equivalent to 400 ADSs) at an exercise price of $0.75 per share (or $75.00 per ADS), which expire on February 5, 2024, (iv) options to purchase 3,559,468 ordinary shares (equivalent to 35,594 ADSs) at an exercise price of $0.3221 per share (or $32.21 per ADS), which expire on September 21, 2025 and (v) options to purchase 125,000 ordinary shares (equivalent to 1,250 ADSs) at an exercise price of $0.035982 per share (or $3.5992 per ADS), which expire on September 1, 2027. Does not include (i) options to purchase 508,495 ordinary shares (equivalent to 5,084 ADSs) at an exercise price of $0.3221 per share (or $32.21 per ADS) and which expire on September 21, 2025 that vest in more than 60 days from March 31, 2019, and (ii) options to purchase 375,000 ordinary shares (equivalent to 3,750 ADSs) at an exercise price of $0.035992 per share (or $3.5992 per ADS) and which expire on September 1, 2027 that vest in more than 60 days from March 31, 2019. |
| (6) | Consists of (i) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.1796 per share (or $17.96 per ADS), which expire on April 22, 2026, (ii) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.145 per share (or $14.50 per ADS), which expire on June 29, 2026, and (iii) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0504 per share (or $5.04 per ADS) and which expire on June 28, 2027. Does not include options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0208 per share (or $2.08 per ADS) and which expire on September 19, 2028 that vest in more than 60 days from March 31, 2019. Also, does not include shares held by RPC Pharma Limited of which he has a pecuniary interest. Mr. Byrne disclaims beneficial ownership of the shares held by RPC Pharma Limited. |
| (7) | Consists of (i) options to purchase 2,166,666 ordinary shares (equivalent to 2,166 ADSs) at an exercise price of $0.145 per share (or $14.50 per ADS), which expire on June 29, 2026, and (ii) 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0504 per share (or $5.04 per ADS) and which expire on June 28, 2027. Does not include (i) options to purchase 433,334 ordinary shares (equivalent to 4,333 ADSs) at an exercise price of $0.145 per share (or $14.50 per ADS) and which expire on June 29, 2026 that vest in more than 60 days from March 31, 2019, (ii) options to purchase 1,300,00 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0208 per share (or $2.08 per ADS) and which expire on September 19, 2028 that vest in more than 60 days from March 31, 2019 and (iii) options to purchase 750,000 ordinary shares (equivalent to 7,500 ADSs) at an exercise price of $0.0175 per share (or $1.75 per ADS) and which expire on November 16, 2028 that vest in more than 60 days from March 31, 2019. |
| (8) | Consists of options to purchase 325,000 ordinary shares (equivalent to 3,250 ADSs) at an exercise price of $0.0347 per share (or $3.47 per ADS) and which expire on January 23, 2028 that vest in more than 60 days from March 31, 2019. Does not include (i) options to purchase 975,000 ordinary shares (equivalent to 9,750 ADSs) at an exercise price of $0.0347 per share (or $3.47 per ADS) and which expire on January 23, 2028 that vest in more than 60 days from March 31, 2019 and (ii) options to purchase 1,300,000 ordinary shares (equivalent to 13,000 ADSs) at an exercise price of $0.0208 per share (or $2.08 per ADS) and which expire on September 19, 2028 that vest in more than 60 days from March 31, 2019. |
| 82 |
| (10) | The principal business office of RPC Pharma Limited is c/o Landmark Fiduciare (Suisse) SA, 6 Place des Eaux-Vives, P.O. Box 3461, Geneva, V8 1211, Switzerland. |
Deutsche Bank Trust Company Americas, or Deutsche Bank, is the holder of record for the company’s ADR program, pursuant to which each ADS represents 100 ordinary shares. As of March 31, 2019, Deutsche Bank held 1,580,911,829 ordinary shares representing 97.3% of the issued share capital held at that date. Certain of these ordinary shares were held by brokers or other nominees. As a result, the number of holders of record or registered holders in the United States is not representative of the number of beneficial holders or of the residence of beneficial holders.
To our knowledge, the only significant changes in the percentage ownership held by our more than 5% shareholders as reported in our annual reports during the past three years are as follows: (i) the ownership percentage of RPC Pharma Limited decreased by 12% to 49.3% from 61.3% as of April 21, 2016, and (ii) the ownership percentage of Deerfield Management Company, L.P. decreased to under 5% in 2017 from 9.8% as of April 21, 2016.
| B. | Related Party Transactions |
The following discloses, since January 1, 2016, certain related party transactions involving us. The descriptions provided below are summaries of the terms of such agreements, do not purport to be complete and are qualified in their entirety by the complete agreements.
Employment and Consulting Agreements
We have or have had employment, consulting or related agreements with each member of our senior management and employee-directors. See “Item 6. Directors, Senior Management and Employees—Compensation”.
Options
We have granted options to purchase our ordinary shares to certain of our officers and directors. See “Item 6.B. Compensation” and “Item 7.A.Major Shareholders”. We describe our option plans under “Item 6.E. Share Ownership” and “Item 7.A. Major Shareholders”.
Office Lease
We lease our UK office space from The Doctors Laboratory, or TDL, and have incurred expenses of approximately $139,000, $141,000 and $142,000 plus VAT during the years ended December 31, 2018, December 31, 2017 and December 31, 2016, respectively. David Byrne, a non-employee director of ours is also the CEO of TDL.
Indemnification Agreements
To the extent permitted by the U.K. Companies Act 2006, we are empowered to indemnify our directors against any liability they incur by reason of their directorship. In addition to such indemnification, we provide our directors and executive officers with directors’ and officers’ liability insurance.
| C. | Interests of Experts and Counsel. |
Not applicable.
| Item 8. | FINANCIAL INFORMATION |
| A. | Consolidated Statements and Other Financial Information |
See “Item 18. Financial Statements,” which contains our financial statements prepared in accordance with United States GAAP.
| B. | Legal Proceedings |
See “Item 4 B. Business Overview—Legal Proceedings”.
| 83 |
| C. | Dividend Policy |
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Therefore, the success of an investment in our ordinary shares or ADSs will depend upon any future appreciation in their value. There is no guarantee that our ordinary shares or ADSs will appreciate in value or even maintain the price at which our shareholders have purchased their shares.
| D. | Significant Changes |
A discussion of the significant changes in our business can be found under “Item 4. Information on the Company—A. History and Development of the Company.”
| Item 9. | THE OFFER AND LISTING |
| A. | Offering and Listing Details |
Our ADSs have been listed on the Nasdaq Capital Market under the symbol “AKTX” since September 21, 2015 and under the symbol “CLTX” from January 31, 2014 until September 18, 2015. Prior to that, our ADSs were quoted on the OTCQB under the symbol “CLSXD” from January 3, 2014 to January 30, 2014 and were quoted on the OTCQB under the symbol “CLSXY” from September 16, 2013 until January 2, 2014 and under the symbol “MRRBY” from February 19, 2013 to September 15, 2013. Effective January 3, 2014, our ratio of ADSs to ordinary shares changed from one ADS per each two ordinary shares to one ADS per each ten ordinary shares and, effective as of September 17, 2015, our ratio of ADSs is to ordinary shares changed from one ADS per each ten ordinary shares to one ADS per each one hundred ordinary shares. Currently, each ADS represents by one hundred ordinary shares.
| B. | Plan of Distribution |
Not applicable.
| C. | Markets |
See “Offering and Listing Details” above.
| D. | Selling Shareholders |
Not applicable.
| E. | Dilution |
Not applicable.
| F. | Expenses of the Issue |
Not applicable.
| Item 10. | ADDITIONAL INFORMATION |
| A. | Share Capital |
Our board of directors is generally authorized to issue up to 10,000,000,000 ordinary shares of £0.01 each until June 28, 2022, without seeking shareholder approval, subject to certain limitations. As of March 31, 2019, there were 1,585,693,413 ordinary shares outstanding, outstanding options to purchase 94,096,988 ordinary shares and 88,986,209 ordinary shares available for future issuance under our 2014 Equity Incentive Plan. All of our existing issued ordinary shares are fully paid. Accordingly, no further capital may be required by us from the holders of such shares.
| 84 |
The rights and restrictions to which the ordinary shares will be subject are prescribed in our Articles of Association. Our Articles of Association permit our board of directors, with shareholder approval, to determine the terms of any preferred shares that we may issue. Our board of directors is authorized, having obtained the consent of the shareholders, to provide from time to time for the issuance of other classes or series of shares and to establish the characteristics of each class or series, including the number of shares, designations, relative voting rights, dividend rights, liquidation and other rights, redemption, repurchase or exchange rights and any other preferences and relative, participating, optional or other rights and limitations not inconsistent with applicable law.
English law does not recognize fractional shares held of record. Accordingly, our Articles of Association do not provide for the issuance of fractional ordinary shares, and our official English share register will not reflect any fractional shares.
We are not permitted under English law to hold our own ordinary shares unless they are repurchased by us and held in treasury.
During the last three years, we have issued an aggregate of 403,000,030 ordinary shares and options to purchase an aggregate of 114,100,000 ordinary shares.
| B. | Memorandum and Articles of Association |
The following summarizes the material rights of holders of ordinary shares, as set out in our Articles of Association. The following summary is qualified in its entirety by reference to the Companies Act and to our Articles of Association, which is filed as an exhibit to our Form 6-K filed with the SEC on July 18, 2017, which is incorporated by reference.
We were originally established as a private limited company under the laws of England and Wales on October 7, 2004 under the name Freshname No. 333 Limited. On January 19, 2005, we changed our name to Morria Biopharmaceuticals Limited and on February 3, 2005, we completed a reverse merger with Morria Biopharmaceuticals Inc., or Morria, a Delaware corporation, in which Morria became our wholly-owned subsidiary and we re-registered as a non-traded public limited company under the laws of England and Wales. Morria was dedicated to the discovery and development of novel, first-in-class, non-steroidal, synthetic anti-inflammatory drugs. On March 22, 2011, we incorporated an Israeli subsidiary, Morria Biopharma Ltd. On June 25, 2013, we changed our name to Celsus Therapeutics PLC and on October 13, 2013 Morria was renamed Celsus Therapeutics Inc. On September 25, 2015, we further changed our name to “Akari Therapeutics, PLC”. As such our affairs are governed by our Articles of Association and the English law.
In the following summary, a “shareholder” is the person registered in our register of members as the holder of the relevant securities. For those ordinary shares that have been deposited in our ADS facility pursuant to our deposit agreement with Deutsche Bank Trust Company Americas, Deutsche Bank Trust Company Americas or its nominee is deemed the shareholder.
Issuance of Options and Warrants
Our Articles of Association provide that, subject to any shareholder approval requirement under any laws, regulations or the rules of any stock exchange to which we are subject, our board of directors is authorized, from time to time, in its discretion, to grant such persons, for such periods and upon such terms as it deems advisable, options to purchase such number of shares of any class or classes or of any series of any class as our board of directors may deem advisable, and to cause warrants or other appropriate instruments evidencing such options to be issued. The Companies Act provides that directors may issue options or warrants without shareholder approval once authorized to do so by the Articles of Association or an ordinary resolution of shareholders. Our board of directors may issue shares upon exercise of options or warrants without shareholder approval or authorization, up to the relevant authorized share capital limit.
| 85 |
Dividends
Our Articles of Association provide that our board of directors may, subject to the applicable provisions of the Companies Act, from time to time, declare such dividend as may appear to the board of directors to be justified by the distributable profits of the company. Subject to the rights of the holders of shares with preferential or other special rights that may be authorized in the future, holders of ordinary shares are entitled to receive dividends according to their rights and interest in our distributable profits. Dividends, to the extent declared, are distributed according to the proportion of the nominal value paid up on account of the shares held at the date so appointed by the Company, without regard to the premium paid in excess of the nominal value, if any. A company may only distribute a dividend out of the company’s distributable profits, as defined under the Companies Act.
Any dividend unclaimed after a period of twelve years from the date of declaration of such dividend shall be forfeited and shall revert to us. In addition, the payment by the board of directors of any unclaimed dividend, interest or other sum payable on or in respect of an ordinary share into a separate account shall not constitute us as a trustee in respect thereof.
Rights in a Liquidation
In the event of our liquidation, subject to applicable law, after satisfaction of liabilities to creditors, our assets will be distributed to the holders of ordinary shares in proportion to their respective holdings. This liquidation right may be affected by the grant of preferential dividends or distribution rights to the holders of a class of shares with preferential rights that may be authorized in the future.
Voting Rights
Holders of ordinary shares have one vote for each ordinary share held on all matters submitted to a vote of shareholders. These voting rights may be affected by the grant of any special voting rights to the holders of a class of shares with preferential rights that may be authorized in the future.
The ordinary shares do not have cumulative voting rights in the election of directors. As a result, holders of ordinary shares that represent more than 50% of the voting power at the general meeting of shareholders, in person or by proxy, have the power to elect all the directors whose positions are being filled at that meeting to the exclusion of the remaining shareholders. At every annual general meeting, one third of the directors who are subject to retirement by rotation, or as near to it as may be, will retire from office. In any two-year period, a majority of the directors must stand for re-election or replacement. In the event that this majority has not been met and the number of directors eligible for retirement by rotation under the provision of our Articles of Association are not met, any further directors to retire are those who have been in office the longest since their last appointment or re-appointment, but as between persons who became or were last re-appointed directors on the same day, those to retire are determined by the Board of Directors at the recommendation of the Chairman. A retiring director is eligible for re-appointment, subject to the terms of our Articles of Association.
The actions necessary to change the rights of holders of the ordinary shares are as follows: the rights of the shareholders would need to be altered by way of a special resolution requiring 75% vote of the shareholders who are present and voting in person or by proxy. In order to change the rights of a separate class of shares, it will require such a vote by shareholders of that class of shares.
Preemptive Rights
There are no rights of pre-emption under our Articles of Association in respect of transfers of issued ordinary shares. In certain circumstances, our shareholders have preemptive rights with respect to new issuances of equity securities. However our board of directors is generally authorized to allot equity securities for cash without triggering shareholder preemptive rights, provided that this power shall (i) be limited to the allotment of equity securities up to an aggregate nominal amount of £100,000,000; and (ii) expire (unless previously revoked or varied by us), on June 28, 2022.
Transfer of Shares
Fully paid ordinary shares are issued in registered form and may be transferred pursuant to our Articles of Association, unless such transfer is restricted or prohibited by another instrument and subject to applicable securities laws. The Articles of Association state that the directors of the Company may refuse to authorize a transfer of shares if the shares in question have not been paid in full and are therefore only partly paid.
| 86 |
Limitation on Owning Securities
Our Articles of Association do not restrict in any way the ownership or voting of ordinary shares by non-residents. If the company serves a demand on a person under section 793 to the Companies Act 2006, that person will be required to disclose any interest he has in the shares of the company.
Fiduciary Duties of Office Holders
The Companies Act imposes a duty of care and a duty of loyalty on all office holders of a company. The duty of care requires an office holder to act with the standard of skills with which a reasonable office holder in the same position would have acted under the same circumstances. The duty of care includes a duty to use reasonable means to obtain:
| · | information regarding the business advisability of a given action brought for his or her approval or performed by him or her by virtue of his or her position; and |
| · | all other information of importance pertaining to the aforesaid actions. |
The duty of loyalty requires an office holder to act in good faith and for the benefit of the company and includes a duty to:
| · | refrain from any act involving a conflict of interest between the fulfillment of his or her role in the company and the fulfillment of any other role or his or her personal affairs; |
| · | refrain from any activity that is competitive with the business of the company; |
| · | refrain from exploiting any business opportunity of the company with the aim of obtaining a personal gain for himself or herself or others; and |
| · | disclose to the company all information and provide it with all documents relating to the company’s affairs which the office holder has obtained due to his position in the company. |
Under equity, directors have owed fiduciary duties to their companies. Chapter 2 of Part 10 of the Companies Act 2006 (2006 Act) codifies certain of those duties. The relevant statutory duties under the 2006 Act are:
| · | to act within powers; |
| · | to promote the success of the company; |
| · | to exercise independent judgment; |
| · | to avoid conflicts of interest; |
| · | not to accept benefits from third parties; and |
| · | to declare an interest in a proposed transaction or arrangement. |
In addition, the general principles of fiduciary duties as set out in common law continue in place in respect of Directors. The general four principles of fiduciary duties are:
| · | No conflict: A must not place himself in a position where his own interests conflict with those of B or where there is a real possibility that this will happen. This is also known as conflict of duty or conflict of interest. |
| · | No-profit: A must not profit from his position at the expense of B. This is also known as misuse of property held in a fiduciary capacity. |
| 87 |
| · | Undivided loyalty: A fiduciary owes undivided loyalty to his beneficiary. Rather confusingly, this is sometimes called conflict of duty. A must not place himself in a position where his duty to another customer conflicts with his duty to B. |
| · | Confidentiality: A must use or disclose information obtained in confidence from B for the benefit only of B. |
In the corporate realm, these have been refined as follows:
| · | Duty to act in good faith in the best interests of the company: A director had to act at all times in good faith in what he considered was the best interests of the company. |
| · | Duty to act within the powers conferred by the company’s memorandum and articles of association and to exercise powers for proper purposes: A director could not cause the company to undertake activities outside that permitted by the company’s constitutional documents, or exercise his powers for any “improper purpose”. |
| · | Duty to avoid conflicting interests and duties: A director was obliged to avoid placing himself in a position where there was a conflict, or possible conflict, between the duties which he owed to the company and either his personal interests or other duties which he owed to a third party. |
| · | Duty not to make unauthorized profits: A director was under a duty to account for any personal profit made by virtue of his directorship unless the prof it was authorized by shareholder resolution or was in accordance with the company’s articles. The duty to account was strict, and did not depend on fraud or lack of good faith, or on the company suffering any loss. |
Standard of Care
A director had to take such actions as would be taken by “a reasonably diligent person,” having both:
| · | the general knowledge, skill and experience that may reasonably be expected of a person carrying out the same functions as are carried out by that director in relation to the company; and |
| · | the general knowledge, skill and experience that that director has. |
Disclosure of Personal Interests of an Officer Holder
The Companies Act requires that an office holder disclose to the Company any direct or indirect personal interest that he or she may have, and all related material information and documents known to him or her, in connection with any existing or proposed transaction by the company. The disclosure is required to be made promptly and in any event, no later than the board of directors meeting in which the transaction is first discussed.
Section 177 of the Companies Act requires any transaction in which a director has an interest to be declared, and not only those that are extraordinary transactions.
| 88 |
Disclosure of Conflicts of Interests
Except as provided in our Articles of Association, a director may not vote at a meeting of the board or of a committee of the board on any resolution concerning a matter:
| · | in which he has (either alone or together with any person connected with him, as provided in the Companies Act) a material interest, other than an interest in shares or debentures or other securities of or in the company; and |
| · | subject to the Companies Act, which conflicts or may conflict with the interests of the Company. |
A director is not counted in the quorum at a meeting in relation to any resolution on which he is debarred from voting.
Notwithstanding the foregoing, a director is entitled to vote and be counted in the quorum in respect of any resolution concerning any of the following matters:
| · | the giving of any security, guarantee or indemnity to a third party in respect of a debt or obligation of Celsus or any of our subsidiaries for which he himself has assumed responsibility in whole or in part under a guarantee or indemnity or by the giving of security; |
| · | any proposal concerning an offer of shares or debentures or other securities of or by Celsus or any of our subsidiaries for subscription or purchase in which offer he is or is to be interested as a participant as the holder of such shares, debentures or other securities or in its underwriting or sub-underwriting; |
| · | any contract, arrangement, transaction or other proposal concerning any other company in which he holds an interest not representing one per cent. or more of any class of the equity share capital (calculated exclusive of any shares of that class held as treasury shares) of such company, or of any third company through which his interest is derived, or of the voting rights available to members of the relevant company, any such interest being deemed for the purpose of this regulation to be a material interest in all circumstances; |
| · | any contract, arrangement, transaction or other proposal concerning the adoption, modification or operation of a superannuation fund or retirement, death or disability benefits scheme under which he may benefit and which has been approved by or is subject to and conditional upon approval by Her Majesty’s Revenue & Customs; |
| · | any contract, arrangement, transaction or proposal concerning the adoption, modification or operation of any scheme for enabling employees, including full time executive directors of Celsus or any of our subsidiaries to acquire shares of Celsus or any arrangement for the benefit of employees of Celsus or any of our subsidiaries, which does not award him any privilege or benefit not awarded to the employees to whom such scheme relates; or |
| · | any contract, arrangement, transaction or proposal concerning insurance which Celsus proposes to maintain or purchase for the benefit of directors or for the benefit of persons including directors. |
Article 26 of the Articles of Association states, that the board may authorize any matter which may otherwise involve a director breaching his duties under certain sections of the Companies Act 2006 to avoid conflicts of interest.
Any director (including the director which has the conflict) may propose that such conflicted director be authorized in relation to any matter which is the subject of such a conflict. The director with the conflict will not count towards the quorum at the meeting at which the conflict is considered and may not vote on any resolution authorizing the conflict. Where the board gives authority in relation to such a conflicts, the board may impose such terms on the relevant director as it deems appropriate.
| 89 |
Directors’ and Officers’ Compensation
The Companies Act requires that a resolution approving provisions to appoint a director for a fixed period of more than two years must not be passed unless a memorandum setting out the proposed contract incorporating the provision is made available to members: in the case of a resolution at a meeting, by being made available for inspection by members of the company both (i) at the company’s registered office for not less than 15 days ending with the date of the meeting, and (ii) at the meeting itself.
Directors’ Borrowing Powers
Our board of directors may, from time to time, in its discretion, cause us to borrow or secure the payment of any sum or sums of money for the purposes of our company.
Retirement of Directors
We do not have any age limitations for our directors, nor do we have mandatory retirement as a result of reaching a certain age.
Share Qualification of Directors
No shareholding qualification is required by a director.
Redemption Provisions.
We may, subject to applicable law and to our Articles of Association, issue redeemable preference shares and redeem the same.
Capital Calls.
Under our Articles of Association and the Companies Act, the liability of our shareholders is limited to the nominal value (i.e. par). The board of directors has the authority to make calls upon the shareholders in respect of any money unpaid on their shares and each shareholder shall pay to us as required by such notice the amount called on his shares. If a call remains unpaid after it has become due and payable, and the fourteen days’ notice provided by the board of directors has not been complied with, any share in respect of which such notice was given may be forfeited by a resolution of the board.
No Sinking Fund
Our ordinary shares do not have sinking fund provisions.
Modification of Rights
Subject to the provisions of the Companies Act, if at any time our capital is divided into different classes of shares, the rights attached to any class may be varied or abrogated with the consent in writing of the holders of at least three-fourths in nominal value of that class or with the sanction of a special resolution passed at a separate meeting of the holders of that class, but not otherwise. The quorum at any such meeting is two or more persons holding, or representing by proxy, at least one-third in nominal value of the issued shares in question.
Transfer Restrictions
Upon the listing of our shares on a Regulated Market (as defined by the Financial Services and Markets Act 2000, the AIM market of the London Stock Exchange, the New York Stock Exchange, the NYSE American, Nasdaq and similar securities exchanges), the Board may decide that up to 100% of each shareholders’ free shares (i.e. unrestricted shares under the applicable rules and regulations) shall be restricted to sale or transfer according to the following provisions, such shares as restricted by the Board being Restricted Shares: (i) during the first six months commencing on the date of the listing, no transfer of Restricted Shares is permitted; (ii) as of the seventh and eighth month following the date of the listing, such a shareholder may transfer shares that constitute up to 12.5% of his Restricted Shares per month; and (iii) as of the ninth month following the date of the listing, the remaining Restricted Shares are no longer considered restricted.
| 90 |
Shareholders’ Meetings and Resolutions
Pursuant to our Articles of Association, the quorum required for an ordinary meeting of shareholders consists of at least two shareholders present in person or by proxy, who hold shares conferring in the aggregate more than 15% of our voting power. If at any time the Company has only one shareholder, such shareholder, in person, by proxy or, if a corporation, by its representative, shall constitute a quorum. A meeting adjourned for lack of a quorum generally is adjourned to the same day in the following week at the same time and place or any time and place as the chairman of the board may designate. Furthermore, the board of the company may call a general meeting whenever they think fit. If the Board, in its absolute discretion, considers that it is impractical or unreasonable for any reason to hold a general meeting on the date or at the time or place specified in the notice calling the general meeting, it may postpone the general meeting to another date, time and/or place.
Under the Companies Act, each shareholder of record must be provided at least 14 calendar days prior to the notice of any general shareholders’ meeting and 21 days prior to the notice of an annual general meeting. Subject to the provisions of the Companies Act, our annual general meeting will be held at such time and place or places as our board may determine. Our board may call a general meeting whenever it thinks fit, and must do so when required under the Companies Act. General meetings must also be convened on such requisition, or in default may be convened by such requisitionists or by court order, as provided by the Companies Act.
Voting at any general meeting of shareholders is by a show of hands, unless a poll is demanded. A poll may be demanded by:
| · | the chairman of the meeting; |
| · | at least five shareholders entitled to vote at the meeting; |
| · | any shareholder or shareholders representing in the aggregate not less than one-tenth of the total voting rights of all shareholders entitled to vote at the meeting; or |
| · | any shareholder or shareholders holding shares conferring a right to vote at the meeting on which there have been paid up sums in the aggregate equal to not less than one-tenth of the total sum paid up on all the shares conferring that right. |
In a vote by a show of hands, every shareholder who is present in person or by proxy at a general meeting has one vote. In a vote on a poll, every shareholder who is present in person or by proxy shall have one vote for every share of which they are registered as the holder (provided that no shareholder shall have more than one vote on a show of hands notwithstanding that he may have appointed more than one proxy to vote on his behalf). The quorum for a shareholders’ meeting is a minimum of two persons holding at least 15% of the share capital, present in person or by proxy. To the extent the Articles of Association provide for a vote by a show of hands in which each shareholder has one vote, this differs from U.S. law, under which each shareholder typically is entitled to one vote per share at all meetings.
Holders of ADSs are also entitled to vote by supplying their voting instructions to Deutsche Bank Trust Company Americas who will vote the ordinary shares represented by their ADSs in accordance with their instructions. The ability of Deutsche Bank Trust Company Americas to carry out voting instructions may be limited by practical and legal limitations, the terms of our Articles of Association, and the terms of the ordinary shares on deposit. We cannot assure the holders of our ADSs that they will receive voting materials in time to enable them to return voting instructions to Deutsche Bank Trust Company Americas a timely manner.
Unless otherwise required by law or the Articles of Association, voting in a general meeting is by ordinary resolution. An ordinary resolution is approved by a majority vote of the shareholders present at a meeting at which there is a quorum. Examples of matters that can be approved by an ordinary resolution include:
| 91 |
| · | the election of directors; |
| · | the approval of financial statements; |
| · | the declaration of final dividends; |
| · | the appointment of auditors; |
| · | the increase of authorized share capital; or |
| · | the grant of authority to issue shares. |
A special resolution or an extraordinary resolution requires the affirmative vote of not less than three-fourths of the eligible votes. Examples of matters that must be approved by a special resolution include modifications to the rights of any class of shares, certain changes to the Articles of Association, or our winding-up.
Limitation on Owning Securities
Our Articles of Association do not restrict in any way the ownership or voting of ordinary shares by non-residents. Furthermore, there is no longer an obligation of a shareholder of a UK company which is a non-listed (in the UK or EU) company to voluntarily disclose his shareholding unless, required to do so by the company. If the company serves a demand on a person under section 793 to the Companies Act 2006, that person will be required to disclose any interest he has in the shares of the company.
Change in Control
We can issue additional shares with any rights or restrictions attached to them as long as not restricted by any rights attached to existing shares. These rights or restrictions can be decided by the directors so long as there is no conflict with any resolution passed by the shareholders. The ability of the directors to issue shares with rights or restrictions that are different than those attached to the currently outstanding ordinary shares could have the effect of delaying, deferring or preventing change of control of our company.
In addition, as discussed above under “Directors and Senior Management”, our board of directors is divided into three classes for purposes of election. One class is elected at each annual meeting of stockholders to serve for a three-year term. Because this would prevent shareholders from replacing the entire board at a single meeting, this provision could also have the effect of delaying, deferring or preventing a change in control of our company.
We may in the future be subject to the UK Takeover Code which is not binding on our company at the present time. Nevertheless, the UK Takeover Code could apply to our company under certain circumstances in the future and if that were to occur, each shareholder who is to acquire more than 29.9% of our issued and outstanding shares could, in most circumstances, be required to make an offer for all the shares in our company under the terms of the UK Takeover Code.
Differences in Corporate Law Between England and the State Of Delaware
As a public limited company incorporated under the laws of England and Wales, the rights of our shareholders are governed by applicable English law, including the Companies Act, and not by the law of any U.S. state. As a result, our directors and shareholders are subject to different responsibilities, rights and privileges than are applicable to directors and shareholders of U.S. corporations. We have set below a summary of the differences between the provisions of the Companies Act applicable to us and the Delaware General Corporation Law relating to shareholders’ rights and protections. This summary is not intended to be a complete discussion of the respective rights and it is qualified in its entirety by reference to English law, Delaware law and our Articles of Association. Before investing, you should consult your legal advisor regarding the impact of English corporate law on your specific circumstances and reasons for investing. The summary below does not include a description of rights or obligations under the U.S. federal securities laws or Nasdaq listing requirements. You are also urged to carefully read the relevant provisions of the Delaware General Corporation Law and the Companies Act for a more complete understanding of the differences between Delaware and English law.
| 92 |
| Delaware | England | |||
| Number of Directors | Under Delaware law, a corporation must have at least one director and the number of directors shall be fixed by or in the manner provided in the bylaws, unless specified in the certificate of incorporation. | Under the Companies Act, a public limited company must have at least two directors and the number of directors may be fixed by or in the manner provided in a company’s articles of association. | ||
| Removal of Directors | Under Delaware law, directors may be removed from office, with or without cause, by a majority shareholder vote, except (a) in the case of a corporation whose board is classified, shareholders may effect such removal only for cause, unless otherwise provided in the certificate of incorporation, and (b) in the case of a corporation having cumulative voting, if less than the entire board is to be removed, no director may be removed without cause if the votes cast against his or her removal would be sufficient to elect him or her if then cumulatively voted at an election of the entire board of directors, or, if there are classes of directors, at an election of the class of directors of which he or she is a part. | Under the Companies Act, shareholders may remove a director without cause by an ordinary resolution (which is passed by a simple majority of those voting in person or by proxy at a general meeting) irrespective of any provisions of any service contract the director has with the company, provided that 28 clear days’ notice of the resolution is given to the company and certain other procedural requirements under the Companies Act are followed (such as allowing the director to make representations against his or her removal at the meeting and/or in writing). | ||
| Vacancies on the Board of Directors | Under Delaware law, vacancies and newly created directorships may be filled by a majority of the directors then in office (even though less than a quorum) or by a sole remaining director unless otherwise provided in the certificate of incorporation or bylaws of the corporation. | Under English law, the procedure by which directors (other than a company’s initial directors) are appointed is generally set out in a company’s articles of association, provided that where two or more persons are appointed as directors of a public limited company by resolution of the shareholders, resolutions appointing each director must be voted on individually unless a resolution of the shareholders that such resolutions do not have to be voted on individually is first agreed to by the meeting without any vote being given against it. | ||
| Annual General Meeting | Under Delaware law, the annual meeting of shareholders shall be held at such place, on such date and at such time as may be designated from time to time by the board of directors or as provided in the certificate of incorporation or by the bylaws. | Under the Companies Act, a public limited company must hold an annual general meeting each year. This meeting must be held within six months beginning with the day following the company’s accounting reference date. | ||
| General Meeting | Under Delaware law, special meetings of the shareholders may be called by the board of directors or by such person or persons as may be authorized by the certificate of incorporation or by the bylaws. | Under the Companies Act, a general meeting of the shareholders of a public limited company may be called by the directors. Shareholders holding at least 5% of the paid-up capital (excluding any paid-up capital held as treasury shares) of the company carrying voting rights at general meetings can also require the directors to call a general meeting. |
| 93 |
| Delaware | England | |||
| Notice of General Meetings | Under Delaware law, written notice of any meeting of the shareholders must be given to each shareholder entitled to vote at the meeting not less than ten nor more than 60 days before the date of the meeting and shall specify the place, date, hour and purpose or purposes of the meeting. |
The Companies Act provides that a general meeting (other than an adjourned meeting) must be called by notice of: • in the case of an annual general meeting, at least 21 days; and • in any other case, at least 14 days. | ||
| The company’s articles of association may provide for a longer period of notice and, in addition, certain matters (such as the removal of directors or auditors) require special notice, which is 28 clear days’ notice. The shareholders of a company may in all cases consent to a shorter notice period, the proportion of shareholders’ consent required being 100% of those entitled to attend and vote in the case of an annual general meeting and, in the case of any other general meeting, a majority in number of the members having a right to attend and vote at the meeting, being a majority who together hold not less than 95% in nominal value of the shares giving a right to attend and vote at the meeting. | ||||
| Quorum | The certificate of incorporation or bylaws may specify the number of shares, the holders of which shall be present or represented by proxy at any meeting in order to constitute a quorum, but in no event shall a quorum consist of less than 1/3 of the shares entitled to vote at the meeting. In the absence of such specification in the certificate of incorporation or bylaws, a majority of the shares entitled to vote, present in person or represented by proxy, shall constitute a quorum at a meeting of shareholders. | Subject to the provisions of a company’s articles of association, the Companies Act provides that two shareholders present at a meeting (in person or by proxy) shall constitute a quorum. | ||
| Proxy | Under Delaware law, at any meeting of shareholders, a shareholder may designate another person to act for such shareholder by proxy, but no such proxy shall be voted or acted upon after three years from its date, unless the proxy provides for a longer period. | Under the Companies Act, at any meeting of shareholders, a shareholder may designate another person to attend, speak and vote at the meeting on their behalf by proxy (or, in the case of a shareholder which is a corporate body, by way of a corporate representative). |
| 94 |
| Delaware | England | |||
| Issue of New Shares | Under Delaware law, if the company’s certificate of incorporation so provides, the directors have the power to authorize additional stock. The directors may authorize capital stock to be issued for consideration consisting of cash, any tangible or intangible property or any benefit to the company or any combination thereof. |
Under the Companies Act, the directors of a company must not exercise any power to allot shares or grant rights to subscribe for, or to convert any security into, shares unless they are authorized to do so by the company’s articles of association or by an ordinary resolution of the shareholders.
Any authorization given must state the maximum amount of shares that may be allotted under it and specify the date on which it will expire, which must be not more than five years from the date the authorization was given. The authority can be renewed by a further resolution of the shareholders. | ||
| Pre-emptive Rights | Under Delaware law, unless otherwise provided in a corporation’s certificate of incorporation, a stockholder does not, by operation of law, possess pre-emptive rights to subscribe to additional issuances of the corporation’s stock. | Under the Companies Act, “equity securities” (being (i) shares in the company other than shares that, with respect to dividends and capital, carry a right to participate only up to a specified amount in a distribution (“ordinary shares”) or (ii) rights to subscribe for, or to convert securities into, ordinary shares) proposed to be allotted for cash must be offered first to the existing equity shareholders in the company in proportion to the respective nominal value of their holdings, unless an exception applies or a special resolution to the contrary has been passed by shareholders in a general meeting or the articles of association provide otherwise in each case in accordance with the provisions of the Companies Act. | ||
| Liability of Directors and Officers | Under Delaware law, a corporation’s certificate of incorporation may include a provision eliminating or limiting the personal liability of a director to the corporation and its shareholders for monetary damages arising from a breach of fiduciary duty as a director. However, no provision can limit the liability of a director for: | Under the Companies Act, any provision (whether contained in a company’s articles of association or any contract or otherwise) that purports to exempt a director of a company (to any extent) from any liability that would otherwise attach to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company is void. |
| 95 |
| Delaware | England | |||
|
• any breach of the director’s duty of loyalty to the corporation or its shareholders; • acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; • willful or negligent payment of unlawful dividends or stock purchases or redemptions; or • any transaction from which the director derives an improper personal benefit. |
Any provision by which a company directly or indirectly provides an indemnity (to any extent) for a director of the company or of an associated company against any liability attaching to him in connection with any negligence, default, breach of duty or breach of trust in relation to the company of which he or she is a director is also void except as permitted by the Companies Act, which provides exceptions for the company to: (i) purchase and maintain insurance against such liability; (ii) provide a “qualifying third party indemnity” (being an indemnity against liability incurred by the director to a person other than the company or an associated company. Such indemnity must not cover fines imposed in criminal proceedings, penalties imposed by regulatory bodies arising out of non-compliance with regulatory requirements, the defense costs of criminal proceedings where the director is found guilty, the defense costs of civil proceedings successfully brought against the director by the company or an associated company, and the costs of unsuccessful applications by the director for relief); and (iii) provide a “qualifying pension scheme indemnity” (being an indemnity against liability incurred in connection with the company’s activities as trustee of an occupational pension plan). | |||
| Voting Rights | Delaware law provides that, unless otherwise provided in the certificate of incorporation, each shareholder of record is entitled to one vote for each share of capital stock held by such shareholder. | Under English law, unless a poll is demanded by the shareholders of a company or is required by the Chairman of the meeting or the company’s articles of association, shareholders shall vote on all resolutions on a show of hands. |
| 96 |
| Delaware | England | |||
| Under the Companies Act, a poll may be demanded by: (i) not fewer than five shareholders having the right to vote on the resolution; (ii) any shareholder(s) representing at least 10% of the total voting rights of all the shareholders having the right to vote on the resolution (excluding any voting rights attached to treasury shares); or (iii) any shareholder (s) holding shares in the company conferring a right to vote on the resolution being shares on which an aggregate sum has been paid up equal to not less than 10% of the total sum paid up on all the shares conferring that right. A company’s articles of association may provide more extensive rights for shareholders to call a poll. | ||||
| Under English law, an ordinary resolution is passed on a show of hands if it is approved by a simple majority (more than 50%) of the votes cast by shareholders present (in person or by proxy) and entitled to vote. If a poll is demanded, an ordinary resolution is passed if it is approved by holders representing a simple majority of the total voting rights of shareholders present (in person or by proxy) who (being entitled to vote) vote on the resolution. Special resolutions require the affirmative vote of not less than 75% of the votes cast by shareholders present (in person or by proxy) at the meeting. | ||||
| Variation of Class Rights | Under Delaware law, the holders of the outstanding shares of a class shall be entitled to vote as a class upon a proposed amendment, whether or not entitled to vote thereon by the certificate of incorporation, if the amendment would increase or decrease the aggregate number of authorized shares of such class, increase or decrease the par value of the shares of such class, or alter or change the powers, preferences or special rights of the shares of such class so as to affect them adversely. |
The Companies Act provides that rights attached to a class of shares may only be varied or abrogated in accordance with provision in the company’s articles for the variation or abrogation of those rights or, where the company’s articles contain no such provision, if the holders of shares of that class consent to the variation or abrogation. Consent for these purposes means: • consent in writing from the holders of at least 75% in nominal value of the issued shares of that class (excluding any shares held as treasury shares); or • a special resolution passed at a separate meeting of the holders of that class sanctioning the variation. |
| 97 |
| Delaware | England | |||
| The Companies Act provides that the quorum for a class meeting is not less than two persons holding or representing by proxy at least one-third of the nominal value of the issued shares of that class. Following a variation of class rights, shareholders who amount to not less than 15% of the shareholders of the class in question who did not approve the variation may apply to court to have the variation cancelled. Any application must be made within 21 days of the variation. The court may cancel the variation if it is satisfied having regard to all the circumstances of the case that the variation would unfairly prejudice the shareholders of the class represented by the applicant. | ||||
| Shareholder Vote on Certain Transactions |
Generally, under Delaware law, unless the certificate of incorporation provides for the vote of a larger portion of the stock, completion of a merger, consolidation, sale, lease or exchange of all or substantially all of a corporation’s assets or dissolution requires: • the approval of the board of directors; and • approval by the vote of the holders of a majority of the outstanding stock or, if the certificate of incorporation provides for more or less than one vote per share, a majority of the votes of the outstanding stock of a corporation entitled to vote on the matter. |
The Companies Act provides for schemes of arrangement, which are arrangements or compromises between a company and any class of shareholders or creditors and used in certain types of reconstructions, amalgamations, capital reorganizations or takeovers. These arrangements require: • the approval at a shareholders’ or creditors’ meeting convened by order of the court, of a majority in number of shareholders or creditors representing 75% in value of the capital held by, or debt owed to, the class of shareholders or creditors, or class thereof present and voting, either in person or by proxy; and | ||
|
Under Delaware law, a contract or transaction between the company and one or more of its directors or officers, or between the company and any other organization in which one or more of its directors or officers, are directors or officers, or have a financial interest, shall not be void solely for this reason, or solely because the director or officer participates in the meeting of the board which authorizes the contract or transaction, or solely because any such director’s or officer’s votes are counted for such purpose, if: • the material facts as to the director’s or officer’s relationship or interest and as to the contract or transaction are disclosed or are known to the board, and the board in good faith authorizes the contract or transaction by the affirmative votes of a majority of the disinterested directors, even though the disinterested directors be less than a quorum; |
• the approval of the court. Once approved, sanctioned and effective, all shareholders and creditors of the relevant class and the company are bound by the terms of the scheme. The Companies Act also contains certain provisions relating to transactions between a director and the company, including transactions involving the acquisition of substantial non-cash assets from a director or the sale of substantial noncash assets to a director, and loans between a company and a director or certain connected persons of directors. If such transactions meet certain thresholds set out within the Companies Act the approval of shareholders by ordinary resolution will be required. |
| 98 |
| Delaware | England | |||
|
• the material facts as to the director’s or officer’s relationship or interest and as to the contract or transaction are disclosed or are known to the shareholders entitled to vote thereon, and the contract or transaction is specifically approved in good faith by vote of the shareholders; or • the contract or transaction is fair as to the corporation as of the time it is authorized, approved or ratified, by the board of directors, a committee or the shareholders. |
||||
| Standard of Conduct for Directors | Delaware law does not contain specific provisions setting forth the standard of conduct of a director. The scope of the fiduciary duties of directors is generally determined by the courts of the State of Delaware. In general, directors have a duty to act without self-interest, on a well-informed basis and in a manner they reasonably believe to be in the best interest of the shareholders. Directors of a Delaware corporation owe fiduciary duties of care and loyalty to the corporation and to its shareholders. The duty of care generally requires that a director act in good faith, with the care that an ordinarily prudent person would exercise under similar circumstances. Under this duty, a director must inform himself or herself of all material information reasonably available regarding a significant transaction. The duty of loyalty requires that a director act in a manner he or she reasonably believes to be in the best interests of the corporation. The director must not use his or her corporate position for personal gain or advantage. In addition, under Delaware law, when the board of directors of a Delaware corporation approves the sale or break-up of a corporation, the board of directors may, in certain circumstances, have a duty to obtain the highest value reasonably available to the shareholders. |
Under English law, a director owes various statutory and fiduciary duties to the company, including: • to act in the way he or she considers, in good faith, would be most likely to promote the success of the company for the benefit of its shareholders as a whole; • to avoid a situation in which he or she has, or can have, a direct or indirect interest that conflicts, or possibly conflicts, with the interests of the company; • to act in accordance with the company’s constitution and only exercise his or her powers for the purposes for which they are conferred; • to exercise independent judgment; • to exercise reasonable care, skill and diligence; • not to accept benefits from a third party conferred by reason of his or her being a director or doing (or not doing) anything as a director; and • a duty to declare any interest that he or she has, whether directly or indirectly, in a proposed or existing transaction or arrangement with the company. |
| 99 |
| Delaware | England | |||
| Shareholder Suits |
Under Delaware law, a shareholder may initiate a derivative action to enforce a right of a corporation if the corporation fails to enforce the right itself. The complaint must: • state that the plaintiff was a shareholder at the time of the transaction of which the plaintiff complains or that the plaintiff’s shares thereafter devolved on the plaintiff by operation of law; and • allege with particularity the efforts made by the plaintiff to obtain the action the plaintiff desires from the directors and the reasons for the plaintiff’s failure to obtain the action; or • state the reasons for not making the effort. Additionally, the plaintiff must remain a shareholder through the duration of the derivative suit. |
Under English law, generally, the company, rather than its shareholders, is the proper claimant in an action in respect of a wrong done to the company or where there is an irregularity in the company’s internal management. Notwithstanding this general position, the Companies Act provides that (i) a court may allow a shareholder to bring a derivative claim (that is, an action in respect of and on behalf of the company) in respect of a cause of action arising from a director’s negligence, default, breach of duty or breach of trust, subject to complying with the procedural requirements under the Companies Act and (ii) a shareholder may bring a claim for a court order where the company’s affairs have been or are being conducted in a manner that is unfairly prejudicial to some or all of its shareholders. |
Other U.K. Law Considerations
Squeeze-Out
Under the Companies Act, if a takeover offer (as defined in Section 974 of the Companies Act) is made for the shares of a company and the offeror were to acquire, or unconditionally contract to acquire: (i) not less than 90% in value of the shares to which the takeover offer relates (the “Takeover Offer Shares”); and (ii) where those shares are voting shares, not less than 90% of the voting rights attached to the Takeover Offer Shares, the offeror could acquire compulsorily the remaining 10% within three months of the day after the last day on which its offer can be accepted. It would do so by sending a notice to outstanding shareholders telling them that it will acquire compulsorily their Takeover Offer Shares and then, six weeks later, it would execute a transfer of the outstanding Takeover Offer Shares in its favor and pay the consideration to the company, which would hold the consideration on trust for outstanding shareholders. The consideration offered to the shareholders whose Takeover Offer Shares are acquired compulsorily under the Companies Act must, in general, be the same as the consideration that was available under the takeover offer.
Sell-Out
The Companies Act also gives minority shareholders a right to be bought out in certain circumstances by an offeror who has made a takeover offer (as defined in Section 974 of the Companies Act). If a takeover offer related to all the shares of a company and, at any time before the end of the period within which the offer could be accepted, the offeror held or had agreed to acquire not less than 90% of the shares to which the offer relates, any holder of the shares to which the offer related who had not accepted the offer could by a written communication to the offeror require it to acquire those shares. The offeror is required to give any shareholder notice of his or her right to be bought out within one month of that right arising. The offeror may impose a time limit on the rights of the minority shareholders to be bought out, but that period cannot end less than three months after the end of the acceptance period. If a shareholder exercises his or her rights, the offeror is bound to acquire those shares on the terms of the offer or on such other terms as may be agreed.
| 100 |
Disclosure of Interest in Shares
Pursuant to Part 22 of the Companies Act, a company is empowered by notice in writing to require any person whom the company knows to be, or has reasonable cause to believe to be, interested in the company’s shares or at any time during the three years immediately preceding the date on which the notice is issued to have been so interested, within a reasonable time to disclose to the company details of that person’s interest and (so far as is within such person’s knowledge) details of any other interest that subsists or subsisted in those shares. If a shareholder defaults in supplying the company with the required details in relation to the shares in question (the “Default Shares”), the shareholder shall not be entitled to vote or exercise any other right conferred by membership in relation to general meetings. Where the Default Shares represent 0.25% or more of the issued shares of the class in question, in certain circumstances the directors may direct that:
| (i) | any dividend or other money payable in respect of the Default Shares shall be retained by the company without any liability to pay interest on it when such dividend or other money is finally paid to the shareholder; and/or |
| (ii) | no transfer by the relevant shareholder of shares (other than a transfer approved in accordance with the provisions of the company’s articles of association) may be registered (unless such shareholder is not in default and the transfer does not relate to Default Shares). |
Dividends
Under English law, before a company can lawfully make a distribution, it must ensure that it has sufficient distributable reserves. A company’s distributable reserves are its accumulated, realized profits, so far as not previously utilized by distribution or capitalization, less its accumulated, realized losses, so far as not previously written off in a reduction or reorganization of capital duly made. In addition to having sufficient distributable reserves, a public company will not be permitted to make a distribution if, at the time, the amount of its net assets (that is, the aggregate of the company’s assets less the aggregate of its liabilities) is less than the aggregate of its issued and paid-up share capital and undistributable reserves, or if the distribution would result in the amount of its net assets being less than that aggregate.
Purchase of Own Shares
Under English law, a public limited company may purchase its own shares only out of the distributable profits of the company or the proceeds of a new issue of shares made for the purpose of financing the purchase, provided that it is not restricted from doing so by its articles. A public limited company may not purchase its own shares if as a result of the purchase there would no longer be any issued shares of the company other than redeemable shares or shares held as treasury shares. Shares must be fully paid in order to be repurchased.
Subject to the foregoing, because Nasdaq is not a “recognized investment exchange” under the Companies Act, a company may purchase its own fully paid shares only pursuant to a purchase contract authorized by ordinary resolution of the holders of its ordinary shares before the purchase takes place. Any authority will not be effective if any shareholder from whom the company proposes to purchase shares votes on the resolution and the resolution would not have been passed if such shareholder had not done so. The resolution authorizing the purchase must specify a date, not being later than five years after the passing of the resolution, on which the authority to purchase is to expire.
A share buy back by a company of its ordinary shares will give rise to U.K. stamp duty at the rate of 0.5% of the amount or value of the consideration payable by the company, and such stamp duty will be paid by the company. Our Articles of Association do not have conditions governing changes in our capital which are more stringent than those required by law.
| 101 |
Statutory Pre-Emption Rights
Under English law, a company must not allot equity securities to a person on any terms unless the following conditions are satisfied:
| (i) | it has made an offer to each person who holds ordinary shares in the company to allot to them on the same or more favorable terms a proportion of those securities that is as nearly as practicable equal to the proportion in nominal value held by them of the ordinary share capital of the company; and |
| (ii) | the period during which any such offer may be accepted has expired or the company has received notice of the acceptance or refusal of every offer so made. |
For these purposes “equity securities” means ordinary shares in the company or rights to subscribe for, or to convert securities into, ordinary shares in the company. “Ordinary shares” means shares other than shares that, with respect to dividends and capital, carry a right to participate only up to a specified amount in a distribution. The statutory pre-emption rights are subject to certain exceptions, including the issue of ordinary shares for non-cash consideration, an allotment of bonus shares and the allotment of equity securities pursuant to an employees’ share scheme. The statutory pre-emption rights may also be disapplied with the approval of 75% of shareholders.
U.K. City Code On Takeovers And Mergers
The UK City Code on Takeovers and Mergers, or the Takeover Code, applies, among other things, to an offer for a public company whose registered office is in the United Kingdom and whose securities are not admitted to trading on a regulated market in the United Kingdom if the company is considered by the Panel on Takeovers and Mergers, or the Takeover Panel, to have its place of central management and control in the United Kingdom. This is known as the “residency test.” The test for central management and control under the Takeover Code is different from that used by the UK tax authorities. Under the Takeover Code, the Takeover Panel will determine whether we have our place of central management and control in the United Kingdom by looking at various factors, including the structure of our board of directors, the functions of the directors and where they are resident.
If at the time of a takeover offer the Takeover Panel determines that we have our place of central management and control in the United Kingdom, we would be subject to a number of rules and restrictions, including but not limited to the following: (1) our ability to enter into deal protection arrangements with a bidder would be extremely limited; (2) we may not, without the approval of our shareholders, be able to perform certain actions that could have the effect of frustrating an offer, such as issuing shares or carrying out acquisitions or disposals; and (3) we would be obliged to provide equality of information to all bona fide competing bidders.
Further, the Takeover Code contains certain rules in respect of mandatory offers. Under Rule 9 of the Takeover Code, if a person: (a) acquires an interest in our shares which, when taken together with shares in which he or persons acting in concert with him are interested, carries 30% or more of the voting rights of our shares; or (b) who, together with persons acting in concert with him, is interested in shares that in the aggregate carry not less than 30% and not more than 50% of the voting rights in us, acquires additional interests in shares that increase the percentage of shares carrying voting rights in which that person is interested, the acquirer and depending on the circumstances, its concert parties, would be required (except with the consent of the Takeover Panel) to make a cash offer for our outstanding shares at a price not less than the highest price paid for any interests in the shares by the acquirer or its concert parties during the previous 12 months.
| C. | Material Contracts |
Set forth below are summaries of material agreements to which we are a party, other than contracts entered into in the ordinary course of business, that have been in effect since March 15, 2016. In addition to the agreements described below, we also enter into agreements with clinical research organizations, or CROs, for the conduct of our clinical trials. The descriptions provided below do not purport to be complete and are qualified in their entirety by the complete agreements, which are attached as exhibits to this Annual Report.
| 102 |
Relationship Agreement
As a condition to the closing of the Acquisition, we and RPC entered into a Relationship Agreement, which provided, subject to closing of the Acquisition, the right for RPC to appoint the following number of directors to our board of directors in relation to the percentage of our Ordinary Shares held in aggregate by RPC from time to time: (i) two class A directors if RPC holds 25% or more of our Ordinary Shares; (ii) one class A director if RPC holds 10% or more but less than 25% of our Ordinary Shares; and (iii) no directors if RPC holds less than 10% of our Ordinary Shares.
Additionally, where such right to appoint a director falls away, RPC is obliged to procure the resignation of the relevant director as soon as practicably possible thereafter at no cost to us. Unless otherwise agreed by our board of directors, the directors appointed by RPC shall be class A directors.
Employment and Consulting Agreements
See “Item 6. Directors, Senior Management and Employees—Compensation—Employment and Consulting Agreements”.
| D. | Exchange Controls |
There are currently no U.K. laws, decrees or regulations that restrict the export or import of capital, including, but not limited to, foreign exchange controls, or that affect the remittance of dividends or other payments to non-U.K. residents or to U.S. holders of our securities except as otherwise set forth in “Taxation” below. There are no limitations under our Memorandum and Articles of Association restricting voting or shareholding.
| E. | Taxation |
The following summary contains a description of certain United Kingdom and United States federal income tax consequences of the acquisition, ownership and disposition of our ordinary shares or ADSs to a U.S. Holder (as defined below) of our ordinary shares or ADSs and, to the limited extent discussed below, a Non-U.S. Holder (as defined below) of our ordinary shares or ADSs. The summary is based upon the tax laws of the United Kingdom and the United States and the respective regulations thereunder as of the date hereof, which are subject to change.
For purposes of this description, a “U.S. Holder” includes any beneficial owner of our ordinary shares or ADSs that is, for U.S. federal income tax purposes:
| · | a citizen or individual resident of the United States; |
| · | a corporation (or other entity treated as a corporation for U.S. federal income tax purposes) created or organized in or under the laws of the United States or organized under the laws of any state thereof, or the District of Columbia; |
| · | an estate, the income of which is subject to U.S. federal income taxation regardless of its source; or |
| · | a trust if (1) a court within the United States is able to exercise primary supervision over its administration and one or more United States persons have the authority to control all of the substantial decisions of such trust; or (2) such trust has a valid election in effect to be treated as a United States person for U.S. federal income tax purposes. |
A “Non-U.S. Holder” is any beneficial owner of our ordinary shares or ADSs that is not a U.S. Holder.
| 103 |
This section does not purport to be a comprehensive description of all of the tax considerations that may be relevant to any particular investor. This discussion assumes that you are familiar with the tax rules applicable to investments in securities generally, and with any special rules to which you may be subject. In particular, the discussion deals only with investors that will hold our ordinary shares or ADSs as capital assets (generally, property held for investment), and does not address the tax treatment of investors that are subject to special rules, such as banks, financial institutions, insurance companies, dealers or traders in securities or currencies, persons that elect mark-to- market treatment, tax-exempt entities or government organizations , real estate investment trusts, regulated investment companies, grantor trusts, individual retirement and other tax-deferred accounts, persons that received our ordinary or ADS shares as compensation for the performance of services, persons who own, directly, indirectly through non-U.S. entities or by attribution by application of the constructive ownership rules of section 958(b) of the United States Internal Revenue Code of 1986, or the Code, 10% or more of our total voting power or value, persons that are residents of the U.K. for U.K. tax purposes or that conduct a business or have a permanent establishment in the U.K., persons that hold our ordinary shares or ADSs as a position in a straddle, hedging, conversion, integration, constructive sale or other risk reduction transaction, certain former citizens or long-term residents of the U.S., partnerships and their partners and persons whose functional currency is not the U.S. dollar. This discussion is based on laws, treaties, judicial decisions, and regulatory interpretations in effect on the date hereof, all of which are subject to change, as well as, in the United States, the Code, administrative pronouncements, judicial decisions, and final, temporary and proposed Treasury regulations, all as of the date hereof, any of which is subject to change, possibly with retroactive effect. Additionally, the summary below regarding U.S. federal income tax does not address any U.S. state or local tax considerations or any U.S. federal estate, gift or alternative minimum tax considerations or any U.S. federal tax considerations other than U.S. federal income tax considerations.
If a partnership (or any other entity treated as a partnership for U.S. federal income tax purposes) holds our ordinary shares or ADSs, the tax treatment of a partner will generally depend upon the status of the partner and upon the activities of the partnership.
We will not seek a ruling from the U.S. Internal Revenue Service, or IRS, with regard to the U.S. federal income tax treatment of an investment in our ordinary shares or ADSs, and we cannot assure you that the IRS will agree with the conclusions set forth below.
You are urged to consult with your own advisers regarding the tax consequences of the acquisition, ownership, and disposition of our ordinary shares or ADSs in the light of your particular circumstances, including the effect of any state, local, or other national laws.
United Kingdom tax considerations
Taxation of dividends
Under current U.K. tax law, no tax is required to be withheld in the United Kingdom at source from cash dividends paid to U.S. resident holders.
Taxation of Capital Gains
Subject to the comments in the following paragraph, a holder of our ordinary shares or ADSs who, for U.K. tax purposes, is not resident in the U.K. will not be liable for U.K. taxation on capital gains realized on the disposal of our ordinary shares or ADS unless at the time of the disposal:
| · | the holder carries on a trade, or in the case of an individual, a profession or vocation in the United Kingdom through, in the case of an individual, a branch or agency, or, in the case of a company, a permanent establishment, and |
| · | our ordinary shares or ADSs are or have been used, held, or acquired for the purpose of such trade, profession, vocation, branch, agency or permanent establishment. |
A holder of our ordinary shares or ADSs who (1) is an individual who has ceased to be resident for U.K. tax purposes in the United Kingdom, (2) was solely resident for U.K. tax purposes in the United Kingdom for at least four out of the seven U.K. tax years immediately preceding the year in which he or she ceased to be resident in the United Kingdom, (3) only remains non-resident in the United Kingdom for a period of five years or less and (4) disposes of his or her ordinary shares or ADSs during that period may also be liable, upon returning to the United Kingdom, for U.K. tax on capital gains, subject to any available exemption or relief, even though he or she was not resident in the United Kingdom at the time of the disposal.
| 104 |
Inheritance Tax
Our ordinary shares or ADSs are assets situated in the United Kingdom for the purposes of U.K. inheritance tax (the equivalent of U.S. estate and gift tax). Subject to the discussion of the U.K.-U.S. estate tax treaty in the next paragraph, U.K. inheritance tax may apply (subject to any available reliefs) if an individual who holds our ordinary shares or ADSs gifts them or dies even if he or she is neither domiciled in the United Kingdom nor deemed to be domiciled there under U.K. law. For inheritance tax purposes, a transfer of our ordinary shares or ADSs at less than full market value may be treated as a gift for these purposes. Special inheritance tax rules apply (1) to gifts if the donor retains some benefit, (2) to close companies and (3) to trustees of settlements.
However, as a result of the U.K.-U.S. estate tax treaty, our ordinary shares or ADSs held by an individual who is domiciled in the United States for the purposes of the U.K.-U.S. estate tax treaty and who is not a U.K. national will not be subject to U.K. inheritance tax on that individual’s death or on a gift of our ordinary shares or ADSs unless the ordinary shares or ADSs:
| · | are part of the business property of a permanent establishment in the United Kingdom, or |
| · | pertain to a fixed base in the United Kingdom used for the performance of independent personal services. |
The U.K.-U.S. estate tax treaty provides a credit mechanism if our ordinary shares or ADSs are subject to both U.K. inheritance tax and to U.S. estate and gift tax.
U.K. Stamp Duty and Stamp Duty Reserve Tax (SDRT)
U.K. legislation provides that SDRT is chargeable at 1.5% on the issuance of a depositary receipt for U.K. shares or securities, or the issuance of such shares or securities into a clearance system. HMRC currently accepts that these provisions contravene European Union law, and accordingly does not seek to enforce SDRT on issues of UK shares and securities to depositary receipt issuers and clearance services anywhere in the world. The UK government has indicated that it will not seek to reimpose such a charge when the United Kingdom leaves the European Union, which is expected to happen in March 2019, although no legislation has been introduced to repeal the charging provision. HMRC still contends that stamp duty/SDRT at 1.5% is payable on transfers (by sale or otherwise) of shares and securities to depository receipt systems or clearance services that are not an integral part of an issue of share capital.
Transfers of ADSs do not attract stamp duty or SDRT.
Transfer of shares in registered form
A transfer of shares in registered form would attract ad valorem stamp duty generally at the rate of 0.5% of the purchase price of the shares. There is no charge to ad valorem stamp duty on gifts.
SDRT would generally be payable on an unconditional agreement to transfer shares in registered form at 0.5% of the amount or value of the consideration for the transfer, but is repayable if, within six years of the date of the agreement, an instrument transferring the shares is executed or, if the SDRT has not been paid, the liability to pay the tax (but not necessarily interest and penalties) would be cancelled.
Transfer of ADSs
No U.K stamp duty will be payable on a written instrument transferring an ADS or on a written agreement to transfer an ADS provided that the instrument of transfer or the agreement to transfer is executed and remains at all times outside the United Kingdom. Where these conditions are not met, the transfer of, or agreement to transfer, an ADS could, depending on the circumstances, attract a charge to U.K. stamp duty at the rate of 0.5% of the value of the consideration given in connection with the transfer.
No SDRT will be payable in respect of an agreement to transfer an ADS.
| 105 |
There is generally no charge to ad valorem stamp duty on gifts. However, transfers of, and unconditional agreements to transfer shares in registered form for nil or non-monetary consideration to a connected person (within the meaning of section 1122 U.K. Corporation Tax Act 2010) on or after 29 October 2018 which are listed on a regulated market, a multilateral trading facility or a recognized foreign exchange (each within the meaning of sections 80B and 88B of the Finance Act 1986) will attract stamp duty and SDRT respectively on the market value of the shares. It is possible that Nasdaq could be either a regulated market or a multilateral trading facility for these purposes, although the position has not been confirmed. As such, any transfers of, or unconditional agreements to transfer, shares in registered form in these circumstances may attract stamp duty and SDRT respectively.
United States federal income tax considerations
Ownership of ADSs
For U.S. federal income tax purposes, a holder of ADSs generally will be treated as the owner of the ordinary shares represented by such ADSs. Gain or loss will generally not be recognized on account of exchanges of ordinary shares for ADSs, or of ADSs for ordinary shares. References to ordinary shares in the discussion below are deemed to include ADSs, unless context otherwise requires.
U.S. Taxation of Distributions
Subject to the discussion below under “Passive Foreign Investment Company Rules,” the gross amount of any distributions made by us to a U.S. Holder will generally be subject to U.S. federal income tax as dividend income to the extent paid or deemed paid out of our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. Such dividends will not be eligible for the dividends received deduction generally allowed to U.S. corporations with respect to dividends received from other U.S. corporations. To the extent that an amount received by a U.S. Holder exceeds its allocable share of our current and accumulated earnings and profits, such excess would, subject to the discussion below, be treated first as a tax-free return of capital which will reduce such U.S. Holder’s tax basis in his ordinary shares or ADSs and then, to the extent such distribution exceeds such U.S. Holder’s tax basis, it will be treated as capital gain. We have not maintained and do not plan to maintain calculations of earnings and profits under U.S. federal income tax principles. Accordingly, it is unlikely that U.S. Holders will be able to establish whether a distribution by us is in excess of our and accumulated earnings and profits (as computed under U.S. federal income tax principles). Thus, it is expected that any distribution will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain under the rules described above. The amount of any distribution of property other than cash will be the fair market value of that property on the date of distribution.
Subject to applicable holding period (which generally requires our ordinary shares to be held for at least 61 days without protection from the risk of loss during the 121-day period beginning 60 days before the ex-dividend date) and other limitations, the U.S. Dollar amount of dividends received on our ordinary shares or ADSs by certain non-corporate U.S. Holders are currently subject to taxation at a maximum rate of 20% if the dividends are “qualified dividends” and certain other requirements are met. Dividends paid on our ordinary shares or ADSs will be treated as qualified dividends if: (i) we are eligible for the benefits of the U.S.-U.K. Tax Treaty (as defined below) or the ordinary shares or ADSs are readily tradable on an established U.S. securities market and (ii) we were not, in the year prior to the year in which the dividend was paid, and are not, in the year in which the dividend is paid, a PFIC. Our ADSs are listed on the Nasdaq Capital Market, which is an established securities market in the United States, and we expect the ADSs to be readily tradable on the Nasdaq Capital Market. However, there can be no assurance that the ADSs will be considered readily tradable on an established securities market in the United States in later years. The Company, which is incorporated under the laws of England and Wales, believes that it qualifies as a resident of the United Kingdom for the purposes of, and is eligible for the benefits of, the Convention between the Government of the United States of America and the Government of the United Kingdom of Great Britain and Northern Ireland for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income and Capital Gains, signed on July 24, 2001, or the U.S.-U.K. Tax Treaty, although there can be no assurance in this regard. Further, the IRS has determined that the U.S.-U.K. Tax Treaty is satisfactory for purposes of the qualified dividend rules and that it includes an exchange-of-information program. Based on the foregoing, we expect to be considered a qualified foreign corporation under the Code. Accordingly, dividends paid by us to non-corporate U.S. Holders with respect to shares that meet the minimum holding period and other requirements are expected to be treated as “qualified dividend income.” However, dividends paid by us will not qualify for the 20% maximum U.S. federal income tax rate if we are treated, for the tax year in which the dividends are paid or the preceding tax year, as a “passive foreign investment company” for U.S. federal income tax purposes, as discussed below. Although we currently believe that distributions on our ordinary shares or ADSs that are treated as dividends for U.S. federal income tax purposes should constitute qualified dividends, no assurance can be given that this will be the case. U.S. Holders should consult their tax advisors regarding the tax rate applicable to dividends received by them with respect to our ordinary shares or ADSs, as well as the potential treatment of any loss on a disposition of our ordinary shares or ADSs as long-term capital loss regardless of the U.S. Holders’ actual holding period for our ordinary shares or ADSs.
| 106 |
The U.S. Treasury Department has announced its intention to issue rules regarding when and to what extent holders of ADSs will be permitted to rely on certifications from issuers to establish that dividends paid on shares to which such ADSs relate are treated as qualified dividends. Because such procedures have not yet been issued, it is not clear whether we will be able to comply with them.
For foreign tax credit computation purposes, dividends will generally constitute foreign source income, and with certain exceptions, will constitute “passive category income.”
The additional 3.8% “net investment income tax” (described below) may apply to dividends received by certain U.S. Holders who meet certain modified adjusted gross income thresholds.
U.S. Taxation upon Sale or Other Disposition
Subject to the discussion under “Passive Foreign Investment Company Rules” below, gain or loss realized by a U.S. Holder on the sale or other taxable disposition of our ordinary shares or ADSs will be subject to U.S. federal income taxation as capital gain or loss in an amount equal to the difference between the U.S. Holder’s adjusted tax basis in our ordinary shares or ADSs and the amount realized on the disposition. Such gain or loss generally will be treated as long-term capital gain or loss if our ordinary shares or ADSs have been held for more than one year at the time of the sale or disposition. Any such gain or loss realized will generally be treated as U.S. source gain or loss. In the case of a non-corporate U.S. Holder, long-term capital gains are currently eligible for federal income tax at preferential rates. The deductibility of capital losses is subject to significant limitations.
For a cash basis taxpayer, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the settlement date of the purchase or sale. In that case, no foreign currency exchange gain or loss will result from currency fluctuations between the trade date and the settlement date of such a purchase or sale. An accrual basis taxpayer, however, may elect the same treatment required of cash basis taxpayers with respect to purchases and sales of the ADSs that are traded on an established securities market, provided the election is applied consistently from year to year. Such election may not be changed without the consent of the IRS. For an accrual basis taxpayer who does not make such election, units of foreign currency paid or received are translated into U.S. dollars at the spot rate on the trade date of the purchase or sale. Such an accrual basis taxpayer may recognize exchange gain or loss based on currency fluctuations between the trade date and settlement date. Any foreign currency gain or loss a U.S. Holder realizes will be U.S. source ordinary income or loss. U.S. Holders should consult their own tax advisors regarding the U.S. federal income tax consequences of receiving currency other than U.S. dollars upon the disposition of their ordinary shares or ADSs.
The maximum individual rate for long-term capital gain is currently 20%.
The additional 3.8% “net investment income tax” (described below) may apply to gains recognized upon the sale or other taxable disposition of our ordinary shares or ADSs by certain U.S. Holders who meet certain modified adjusted gross income thresholds.
Medicare Tax
Certain U.S. Holders including individuals, estates and trusts are subject to a Medicare tax of 3.8% on “net investment income,” which includes dividends, interest, and capital gain from the sale of investment securities, adjusted for certain deductions properly allocated to such investment income. The Medicare tax will apply to the lesser of such net investment income or the excess of the taxpayer’s adjusted gross income (with certain modifications) over a specified amount. The specified amount is $250,000 for married individuals filing jointly, $125,000 for married individuals filing separately, and $200,000 for single individuals. U.S. Holders should consult with their own tax advisers regarding the application of the net investment income tax to them as a result of their investment in our ADSs or ordinary shares.
| 107 |
Passive Foreign Investment Company Rules
Based on the nature of our present business operations, assets and income, we believe that for the year 2018, we were not a PFIC. However, because the PFIC determination is highly fact intensive, there can be no assurance that we will not be a PFIC for 2019 or for any other taxable year. A separate determination must be made after the close of each taxable year as to whether we are a PFIC for that year. As a result, our PFIC status may change from year to year.
We would be a PFIC for U.S. federal income tax purposes in any taxable year if 75% or more of our gross income would be passive income, or on average at least 50% of the gross value of our assets is held for the production of, or produces, passive income. Passive income for this purpose generally includes, among other things, certain dividends, interest, royalties, rents and gains from commodities and securities transactions and from the sale or exchange of property that gives rise to passive income. Passive income also includes amounts derived by reason of the temporary investment of funds, including those raised in a public offering. Assets that produce or are held for the production of passive income include cash, even if held as working capital or raised in a public offering, marketable securities and other assets that may produce passive income. In making the above determination, we are treated as earning our proportionate share of any income and owning our proportionate share of any asset of any company in which we are considered to own, directly or indirectly, 25% or more of the shares by value. If we were considered a PFIC at any time when a U.S. Holder held our ordinary shares or ADSs, we generally should continue to be treated as a PFIC with respect to that U.S. Holder, and the U.S. Holder generally will be subject to special rules with respect to (a) any gain realized on the disposition of our ordinary shares or ADSs and (b) any “excess distribution” by us to the U.S. Holder in respect of our ordinary shares or ADSs. Generally, a distribution during a taxable year to a U.S. Holder with respect to ordinary shares would be treated as an “excess distribution” to the extent that the distribution plus all other distributions received (or deemed to be received) by the U.S. Holder during the taxable year with respect to such ordinary shares, is greater than 125% of the average annual distributions received by the U.S. Holder with respect to such ordinary shares during the three preceding years (or during such shorter period as the U.S. Holder may have held the ordinary shares or ADSs). Under the PFIC rules: (i) the gain or excess distribution would be allocated ratably over the U.S. Holder’s holding period for our ordinary shares or ADSs, (ii) the amount allocated to the taxable year in which the gain or excess distribution was realized or to any year before we became a PFIC would be taxable as ordinary income and (iii) the amount allocated to each other taxable year would be subject to tax at the highest tax rate in effect in that year and an interest charge generally applicable to underpayments of tax would be imposed in respect of the tax attributable to each such year. Because a U.S. Holder that is a direct (and in certain cases indirect) shareholder of a PFIC is deemed to own its proportionate share of interests in any lower-tier PFICs, U.S. Holders should be subject to the foregoing rules with respect to any of our subsidiaries characterized as PFICs, if we are deemed a PFIC.
In the event we are treated as a PFIC for any taxable year, the tax consequences under the default PFIC regime described above could be avoided by either a “mark-to-market” or “qualified electing fund” election. If our ordinary shares or ADSs are considered “marketable stock,” a U.S. Holder may elect to “mark-to-market” its ADSs provided the U.S. Holder completes and files IRS Form 8621 in accordance with the relevant instructions and related Treasury Regulations. A U.S. Holder making a mark-to-market election (if the eligibility requirements for such an election were satisfied) generally would not be subject to the PFIC rules discussed above, except with respect to any portion of the holder’s holding period that preceded the effective date of the election. Instead, such U.S. Holder would generally include in income any excess of the fair market value of the ordinary shares or ADSs at the close of each tax year over its adjusted basis in the ordinary shares or ADSs. If the fair market value of the ordinary shares of ADSs had depreciated below the U.S. Holders adjusted basis at the close of the tax year, the U.S. Holder may generally deduct the excess of the adjusted basis of the ordinary shares or ADSs over its fair market value at that time. However, such deductions generally would be limited to the net mark-to-market gains, if any, that the U.S. Holder included in income with respect to such ordinary shares or ADSs in prior years. Income recognized and deductions allowed under the mark-to-market provisions, as well as any gain or loss on the disposition of ordinary shares or ADSs with respect to which the mark-to-market election is made, is treated as ordinary income or loss (except that loss is treated as capital loss to the extent the loss exceeds the net mark-to-market gains, if any, that a U.S. Holder included in income with respect to such ordinary share or ADSs in prior years). Gain or loss from the disposition of ordinary shares or ADSs (as to which a “mark-to-market” election was made) in a year in which we are no longer a PFIC, will be capital gain or loss. Our ordinary shares or ADSs should be considered “marketable stock” if they traded at least 15 days during each calendar quarter of the relevant calendar year in more than de minimis quantities. Any such mark to market election would not be available for a lower-tier PFIC.
| 108 |
Alternatively, a U.S. Holder making a valid and timely “qualified electing fund” or “QEF” election generally would not be subject to the default PFIC regime discussed above. Instead, for each PFIC year to which such an election applied, the electing U.S. Holder would be subject to U.S. federal income tax on the electing U.S. Holder’s pro rata share of our net capital gain and ordinary earnings, regardless of whether such amounts were actually distributed to the electing U.S. Holder. Any gain on sale or other disposition of a U.S. Holder’s ordinary shares or would be treated as capital, and the interest penalty will not be imposed. If an investor provides reasonable notice to us that it has determined to make a QEF election, we intend to provide annual financial information to such investor as may be reasonably required for purposes of filing United States federal income tax returns in connection with such QEF election.
U.S. Holders are urged to consult their tax advisors about the PFIC rules, including the advisability, procedure and timing of making a mark-to-market election and the U.S. Holder’s eligibility to file such an election (including whether our ordinary shares or ADSs are treated as “marketable stock” for such purpose). A U.S. Holder will be required to file Internal Revenue Service Form 8621 if such U.S. Holder owns our ordinary shares or ADSs in any year in which we are classified as a PFIC.
Information reporting and backup withholding
A U.S. Holder may be subject to information reporting to the IRS and possible backup withholding with respect to dividends paid on, or proceeds of the sale or other disposition of our ordinary shares or ADSs unless such U.S. Holder is a corporation or qualifies within certain other categories of exempt recipients or in the case of backup withholding provides a taxpayer identification number and certifies as to no loss of exemption from backup withholding and otherwise complies with applicable requirements of the backup withholding rules. Amounts withheld under these rules may be credited against the U.S. Holder’s U.S. federal income tax liability and a U.S. Holder may obtain a refund of any excess amounts withheld under the backup withholding rules by filing the appropriate IRS forms and furnishing any required information. A U.S. Holder who does not provide a correct taxpayer identification number may be subject to penalties imposed by the IRS.
A Non-U.S. Holder generally will not be subject to information reporting or backup withholding with respect to dividends on our ordinary shares or ADSs, unless payment is made through a paying agent (or office) in the United States or through certain U.S.-related financial intermediaries. However, a Non-U.S. Holder generally may be subject to information reporting and backup withholding with respect to the payment within the United States of dividends on our ordinary shares or ADSs, unless such Non-U.S. Holder provides a taxpayer identification number, certifies under penalties of perjury as to its foreign status, or otherwise establishes an exemption.
Certain reporting requirements
Certain U.S. Holders are required to file IRS Form 926, Return by U.S. Transferor of Property to a Foreign Corporation, and certain U.S. Holders may be required to file IRS Form 5471, Information Return of U.S. Persons With Respect to Certain Foreign Corporations, reporting transfers of cash or other property to us and information relating to the U.S. Holder and us. Substantial penalties may be imposed upon a U.S. Holder that fails to comply. See also the discussion regarding Form 8621, Information Return by a Shareholder of a Passive Foreign Investment Company or Qualified Electing Fund, above.
In addition, certain U.S. Holders must report information on IRS Form 8938, Statement of Specified Foreign Financial Assets, with respect to their investments in certain “foreign financial assets,” which would include an investment in our ordinary shares, if the aggregate value of all of those assets exceeds $50,000 on the last day of the taxable year (and in some circumstances, a higher threshold). This reporting requirement applies to individuals and certain U.S. entities.
U.S. Holders who fail to report required information could become subject to substantial penalties. U.S. Holders should consult their tax advisors regarding the possible implications of these reporting requirements arising from their investment in our ordinary shares or ADSs.
| F. | Dividends and Paying Agents |
Not applicable.
| 109 |
| G. | Statement by Experts |
Not applicable.
| H. | Documents on Display |
We are currently subject to the information and periodic reporting requirements of the Exchange Act, and file periodic reports and other information with the SEC through its electronic data gathering, analysis and retrieval (EDGAR) system. The SEC maintains an Internet website that contains reports and other information regarding issuers that file electronically with the SEC. You may read and copy this annual report, including the related exhibits and schedules, and any document we file with the SEC at http://www.sec.gov.
As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file annual, quarterly and current reports and financial statements with the SEC as frequently or as promptly as United States companies whose securities are registered under the Exchange Act.
| . | Subsidiary Information |
Not applicable.
| Item 11. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT RISK |
You should read the following information in conjunction with Item 5, “Operating and Financial Review and Prospects;” Item 3, “Risk Factors;” and our consolidated financial statements, including the related notes thereto, including Note 2, both of which are included elsewhere in this document. The following discussion about our financial risk management activities includes “forward-looking statements” that involve risks and uncertainties. Actual results could differ materially from those projected in these forward-looking statements.
Risk Management Framework
We are exposed to a variety of risks, including changes in foreign currency exchange risk and interest rates.
Currency Exchange Rate Sensitivity
The results of our operations are subject to currency transactional risk. Operating results and financial position are reported in local currencies and then translated into United States dollars at the applicable exchange rate for preparation of our consolidated financial statements. The fluctuation of the U.S. dollar in relation to the British Pound, Euro and Swiss Franc will therefore have an impact upon profitability of our operations and may also affect the value of our assets and the amount of shareholders’ equity.
Our functional currency is the United States dollar and our activities are predominantly executed using both the U.S. dollar, Euro and British Pound. We have done a limited number of financings, and we are not subject to significant operational exposures due to fluctuations in these currencies. We have not entered into any agreements, or purchased any instruments, to hedge any possible currency risks at this time.
Interest Rate Sensitivity
We currently have no short-term or long-term debt requiring interest payments. This does not require us to consider entering into any agreements or purchasing any instruments to hedge against possible interest rate risks at this time. Our interest-earning investments are short-term. Thus, any reductions in future income or carrying values due to future interest rate declines are believed to be immaterial.
| 110 |
| Item 12. | DESCRIPTION OF SECURITIES OTHER THAN EQUITY SECURITIES |
| A. | Debt Securities |
Not applicable.
| B. | Warrants and Rights |
Not applicable.
| C. | Other Securities |
Not applicable.
| D. | American Depositary Shares |
Deutsche Bank Trust Company Americas, as depositary, will register and deliver the ADSs. Each ADS will represent ownership of one hundred (100) ordinary shares deposited with State Street Bank & Trust Company, having its principal office at 525 Ferry Road, Crewe Toll, Edinburgh, EH5 2AW Scotland, as custodian for the depositary. Each ADS will also represent ownership of any other securities, cash or other property which may be held by the depositary. The depositary’s corporate trust office at which the ADSs will be administered is located at 60 Wall Street, New York, NY 10005, USA. The principal executive office of the depositary is located at 60 Wall Street, New York, NY 10005, USA.
Fees and Charges
As a holder ADSs, you will be required to pay the following service fees to the depositary bank:
| Service: | Fee: | |
| Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property | Up to $0.05 per ADS issued | |
| Cancellation of ADSs, including in the case of termination of the deposit agreement | Up to $0.05 per ADS cancelled | |
| Distribution of cash dividends or other cash distributions | Up to $0.05 per ADS held | |
| Distribution of ADSs pursuant to share dividends, free share distributions or exercise of rights | Up to $0.05 per ADS held | |
| Distribution of securities other than ADSs or rights to purchase ADSs additional ADSs | A fee equivalent to the fee that would be payable if securities distributed to you had been ordinary shares and the ordinary shares had been deposited for issuance of ADSs | |
| Depositary services | Up to $0.05 per ADS held on the applicable record date(s) established by the depositary bank | |
| Transfer of ADRs | $1.50 per certificate presented for transfer |
As an ADS holder, you will also be responsible to pay certain fees and expenses incurred by the depositary bank and certain taxes and governmental charges such as:
| 111 |
| · | Fees for the transfer and registration of ordinary shares charged by the registrar and transfer agent for the ordinary shares in the United Kingdom (i.e., upon deposit and withdrawal of ordinary shares). |
| · | Expenses incurred for converting foreign currency into U.S. dollars. |
| · | Expenses for cable, telex and fax transmissions and for delivery of securities. |
| · | Taxes and duties upon the transfer of securities, including any applicable stamp duties, any stock transfer charges or withholding taxes (i.e., when ordinary shares are deposited or withdrawn from deposit). |
| · | Fees and expenses incurred in connection with the delivery or servicing of ordinary shares on deposit. |
| · | Fees and expenses incurred in connection with complying with exchange control regulations and any other regulatory requirements that are not currently applicable but may arise or become applicable to Ordinary Shares, deposited securities, ADSs and ADRs. |
| · | Any applicable fees and penalties thereon. |
| · | The depositary fees payable upon the issuance and cancellation of ADSs are typically paid to the depositary bank by the brokers (on behalf of their clients) receiving the newly issued ADSs from the depositary bank and by the brokers (on behalf of their clients) delivering the ADSs to the depositary bank for cancellation. The brokers in turn charge these fees to their clients. Depositary fees payable in connection with distributions of cash or securities to ADS holders and the depositary services fee are charged by the depositary bank to the holders of record of ADSs as of the applicable ADS record date. |
The depositary fees payable for cash distributions are generally deducted from the cash being distributed or by selling a portion of distributable property to pay the fees. In the case of distributions other than cash (i.e., share dividends, rights, etc.), the depositary bank charges the applicable fee to the ADS record date holders concurrent with the distribution. In the case of ADSs registered in the name of the investor (whether certificated or uncertificated in direct registration), the depositary bank sends invoices to the applicable record date ADS holders. In the case of ADSs held in brokerage and custodian accounts (via DTC), the depositary bank generally collects its fees through the systems provided by DTC (whose nominee is the registered holder of the ADSs held in DTC) from the brokers and custodians holding ADSs in their DTC accounts. The brokers and custodians who hold their clients’ ADSs in DTC accounts in turn charge their clients’ accounts the amount of the fees paid to the depositary banks.
In the event of refusal to pay the depositary fees, the depositary bank may, under the terms of the deposit agreement, refuse the requested service until payment is received or may set off the amount of the depositary fees from any distribution to be made to the ADS holder.
The depositary has agreed to reimburse us for a portion of certain expenses we incur that are related to establishment and maintenance of the American Depository Receipt, or ADR, program, including investor relations expenses. There are limits on the amount of expenses for which the depositary will reimburse us, but the amount of reimbursement available to us is not related to the amounts of fees the depositary collects from investors. Further, the depositary has agreed to reimburse us certain fees payable to the depositary by holders of ADSs. Neither the depositary nor we can determine the exact amount to be made available to us because (i) the number of ADSs that will be issued and outstanding, (ii) the level of service fees to be charged to holders of ADSs and (iii) our reimbursable expenses related to the program are not known at this time.
| 112 |
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until such taxes or other charges are paid. It may apply payments owed to you or sell deposited securities represented by your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to you any net proceeds, or send to you any property, remaining after it has paid the taxes. You agree to indemnify us, the depositary, the custodian and each of our and their respective agents, directors, employees and affiliates for, and hold each of them harmless from, any claims with respect to taxes (including applicable interest and penalties thereon) arising from any tax benefit obtained for you.
| Item 13. | DEFAULTS, DIVIDEND ARREARAGES AND DELINQUENCIES |
| A. | Defaults |
None.
| B. | Arrears and Delinquencies |
None.
| Item 14. | MATERIAL MODIFICATIONS TO THE RIGHTS OF SECURITY HOLDERS AND USE OF PROCEEDS |
None.
| Item 15. | CONTROLS AND PROCEDURES |
| (a) | Disclosure Controls and Procedures |
We performed an evaluation of the effectiveness of our disclosure controls and procedures that are designed to ensure that information required to be disclosed in this annual report and filed with the SEC is recorded, processed, summarized and reported timely within the time period specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by an issuer in the reports that it files or submits under the Exchange Act, is accumulated and communicated to the issuer’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure. There can be no assurance that our disclosure controls and procedures will detect or uncover all failures of persons within our Company to disclose information otherwise required to be set forth in our reports. Nevertheless, our disclosure controls and procedures are designed to provide reasonable assurance of achieving the desired control objectives. Based on our evaluation, our principal executive officer and principal financial officer, have concluded that our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15(d)-15(e) of the Exchange Act) as of the end of the period covered by this annual report are effective at such reasonable assurance level.
| (b) | Management’s Annual Report on Internal Control over Financial Reporting |
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Our management conducted an evaluation of the effectiveness of our internal control over financial reporting as of December 31, 2018, based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (2013 framework). Based on this evaluation, management concluded that our internal control over financial reporting was effective as of December 31, 2018.
| 113 |
| (c) | Attestation Report of Registered Public Accounting Firm |
This report does not include an attestation report of our registered public accounting firm as we are not an accelerated filer or a large accelerated filer.
| (d) | Changes in Internal Controls over Financial Reporting |
In our annual report for the year ended December 31, 2017, we identified and disclosed material weaknesses in our internal control over financial reporting as a result of (i) our former Chief Executive Officer violating our Code of Conduct and Ethics by utilizing the Company credit card for personal expenses and exceeding his delegation of authority by entering into unauthorized agreements with third parties; and (ii) having ineffective internal controls related to the evaluation of the accounting principles and disclosure requirements for loss contingencies. We implemented the following remedial actions in response to the material weaknesses: (i) on May 8, 2018, our former Chief Executive Officer resigned as Chief Executive Officer and from the board of directors and in his place we appointed Clive Richardson to serve as our interim Chief Executive Officer, (ii) we cancelled all corporate credit cards, (iii) we reinforced, through communication and training of our employees, executive officers and directors the requirement to comply with the letter and the spirit of our Code of Conduct and Ethics and authority limits of senior management, and (iv) our Chief Financial Officer undertook a comprehensive review of the procedures to be followed by the Company for establishing significant and judgmental reserves, including reserves for litigation including the formation of a financial disclosure committee which meets quarterly.
Based on testing performed by management, we believe the implemented controls are operating effectively and the prior year material weaknesses have been remediated as of December 31, 2018. There were no other changes in our internal control over financial reporting that occurred during the year ended December 31, 2018 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 16. | [RESERVED] |
| Item 16A. | Audit Committee Financial Expert |
Our board of directors has determined that Mr. Donald Williams is the audit committee financial expert.
| Item 16B. | Code of Ethics |
We have adopted a code of ethics that applies to all of our employees, including our chief executive officer and chief financial and accounting officer. The text of the code of conduct is posted in the “Investor Relations” section of our website at www.akaritx.com. Disclosure regarding any amendments to, or waivers from, provisions of the code of conduct that apply to our directors, principal executive and financial officers will be included in a Current Report on Form 6-K within four business days following the date of the amendment or waiver, unless website posting or the issuance of a press release of such amendments or waivers is then permitted by the rules of the Nasdaq stock market.
| Item 16C. | Principal Accountant Fees and Services |
BDO USA, LLP served as our independent registered public accounting firm and audited our financial statements for the years ended December 31, 2017 and 2018.
| 2018 | 2017 | |||||||
| Audit Fees (1) | $ | 200,000 | $ | 190,000 | ||||
| Audit-Related Fees (2) | 26,850 | 207,450 | ||||||
| Tax Fees (3) | - | 25,600 | ||||||
| Total | $ | 226,850 | $ | 423,050 | ||||
| (1) | Audit fees consisted of audit work performed in the preparation of the consolidated financial statements, interim review procedures as well as work generally only the independent registered public accounting firm can reasonably be expected to provide, such as statutory audits. |
| (2) | Audit related services relate to work regarding a public listing, offering and additional audit procedures. |
| (3) | Tax fees relate to tax compliance planning and advice. |
Pre-Approval policies and procedures
The audit committee reviews and pre-approves all audit and non-audit services performed by its independent registered public accounting firm, as well as the fees charged for such services. All fees incurred for the year ended 2018 for services rendered by BDO were approved in accordance with these policies. In its review of non-audit service fees, the audit committee considers, among other things, the possible impact of the performance of such services on the auditor’s independence. The audit committee has determined that the non-audit services performed by BDO for the year ended December 31, 2018 were compatible with maintaining the auditor’s independence.
| 114 |
| Item 16D. | Exemptions from the Listing Standards for Audit Committees |
Not applicable.
| Item 16E. | Purchases of Equity Securities by the Issuer and Affiliated Purchasers |
Not applicable.
| Item 16F. | Change in Registrant’s Certifying Accountant |
Not applicable.
| Item 16G. | Corporate Governance |
We rely on a provision in Nasdaq's Listed Company Manual that allows us to follow English corporate law and the Companies Act 2006 with regard to certain aspects of corporate governance. This allows us to follow certain corporate governance practices that differ in significant respects from the corporate governance requirements applicable to U.S. companies listed on Nasdaq.
In accordance with English corporate law and the Companies Act 2006 and practice and subject to the exemption set forth in Rule 5615 of the Marketplace Rules of the Nasdaq Stock Market, we follow the provisions of the Companies Act, rather than the Marketplace Rules of the Nasdaq Stock Market, with respect to the following requirements:
| · | We do not follow Nasdaq’s requirements to seek shareholder approval for the implementation of certain equity compensation plans, the issuances of ordinary shares under such plans, or in connection with certain private placements of equity securities. In accordance with U.K. law, we are not required to seek shareholder approval to allot ordinary shares in connection with applicable employee equity compensation plans. We will follow U.K. law with respect to any requirement to obtain shareholder approval prior to any private placements of equity securities. |
As a foreign private issuer, we are permitted to, and we will, follow home country practice in lieu of the above requirements.
Because we are a foreign private issuer, our directors and senior management are not subject to short-swing profit and insider trading reporting obligations under Section 16 of the U.S. Securities Exchange Act of 1934, as amended, or Exchange Act. They are, however, subject to the obligations to report changes in share ownership under Section 13 of the Exchange Act and related SEC rules.
| Item 16H. | Mine Safety Disclosures |
Not applicable.
| 115 |
| Item 17. | FINANCIAL STATEMENTS |
We have responded to Item 18 in lieu of this item.
| Item 18. | FINANCIAL STATEMENTS |
Financial Statements are filed as part of this annual report. See page F-1.
| 116 |
| Item 19. | EXHIBITS |
| 117 |
| 10.21+ | Option and Bonus Letter between the Company and Clive Richardson dated October 19, 2018* |
| 21.1 | List of subsidiaries (incorporated by reference to the exhibit previously filed with the Registrant’s Annual Report on Form 20-F filed on July 18, 2018) | |
| 23.1 | Consent of registered public accounting firm* | |
| 31.1 | Certification of Chief Executive Officer* | |
| 31.2 | Certification of the Chief Financial Officer* | |
| 32.1 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |
| 32.2 | Certification pursuant to Section 906 of the Sarbanes-Oxley Act of 2002* | |
| 101.INS | XBRL Instance Document* | |
| 101.SCH | XBRL Taxonomy Schema Linkbase Document* | |
| 101.CAL | XBRL Taxonomy Calculation Linkbase Document* | |
| 101.DEF | XBRL Taxonomy Definition Linkbase Document* | |
| 101.LAB | XBRL Taxonomy Labels Linkbase Document* | |
| 101.PRE | XBRL Taxonomy Presentation Linkbase Document* |
+ Indicates management contract or compensatory plan.
* Filed herewith
| 118 |
The registrant hereby certifies that it meets all of the requirements for filing on Form 20-F and that it has duly caused and authorized the undersigned to sign this annual report on its behalf.
| Akari Therapeutics, PLC | |||
| By: | /s/ Clive Richardson | ||
| Name: | Clive Richardson | ||
| Title: | Interim Chief Executive Officer and Chief Operating Officer | ||
Date: April 23, 2019
| 119 |
AKARI THERAPEUTICS, PLC
CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2018
U.S. DOLLARS
INDEX
Report of Independent Registered Public Accounting Firm
Shareholders and Board of Directors
Akari Therapeutics, Plc
London, United Kingdom
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Akari Therapeutics, Plc (the “Company”) and subsidiaries as of December 31, 2018 and 2017, the related consolidated statements of comprehensive loss, shareholders’ equity, and cash flows for each of the three years in the period ended December 31, 2018, and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company and subsidiaries at December 31, 2018 and 2017, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
Going Concern Uncertainty
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 1 to the consolidated financial statements, the Company has suffered recurring losses from operations that raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 1. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
| /s/ BDO USA, LLP | |
| We have served as the Company's auditor since 2016. | |
| New York, New York | |
| April 23, 2019 |
| F-1 |
CONSOLIDATED BALANCE SHEETS
(in U.S. Dollars, except share data)
| December 31, 2018 | December 31, 2017 | |||||||
| Assets | ||||||||
| Current Assets: | ||||||||
| Cash | $ | 5,446,138 | $ | 28,106,671 | ||||
| Prepaid expenses and other current assets | 1,423,184 | 706,415 | ||||||
| Deferred financing costs | 585,000 | - | ||||||
| Total Current Assets | 7,454,322 | 28,813,086 | ||||||
| Restricted cash | 521,829 | 142,235 | ||||||
| Property and equipment, net | 20,425 | 55,898 | ||||||
| Patent acquisition costs, net | 32,978 | 39,124 | ||||||
| Total Assets | $ | 8,029,554 | $ | 29,050,343 | ||||
| Liabilities and Shareholders' Equity | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable | $ | 1,586,285 | $ | 1,971,161 | ||||
| Accrued expenses | 1,489,558 | 4,795,873 | ||||||
| Liabilities related to options | 1,842,424 | 5,081,335 | ||||||
| Total Current Liabilities | 4,918,267 | 11,848,369 | ||||||
| Other long-term liability | - | 48,003 | ||||||
| Total liabilities | 4,918,267 | 11,896,372 | ||||||
| Commitments and Contingencies | ||||||||
| Shareholders' Equity: | ||||||||
| Share capital of £0.01 par value | ||||||||
| Authorized: 10,000,000,000 and 5,000,000,000 ordinary shares; issued and outstanding: 1,580,693,413 and 1,525,693,393 at December 31, 2018 and 2017, respectively | 23,651,277 | 22,927,534 | ||||||
| Additional paid-in capital | 106,616,083 | 104,799,550 | ||||||
| Accumulated other comprehensive loss | (352,426 | ) | (236,246 | ) | ||||
| Accumulated deficit | (126,803,647 | ) | (110,336,867 | ) | ||||
| Total Shareholders' Equity | 3,111,287 | 17,153,971 | ||||||
| Total Liabilities and Shareholders' Equity | $ | 8,029,554 | $ | 29,050,343 | ||||
See notes to consolidated financial statements.
| F-2 |
CONSOLIDATED STATEMENTS OF COMPREHENSIVE LOSS
For the Years Ended December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| Operating Expenses: | ||||||||||||
| Research and development costs | $ | 11,795,376 | $ | 23,285,279 | $ | 17,306,001 | ||||||
| General and administrative expenses | 10,896,158 | 11,798,910 | 9,940,557 | |||||||||
| Litigation settlement (gain) loss | (2,700,000 | ) | 2,700,000 | - | ||||||||
| Total Operating Expenses | 19,991,534 | 37,784,189 | 27,246,558 | |||||||||
| Loss from Operations | (19,991,534 | ) | (37,784,189 | ) | (27,246,558 | ) | ||||||
| Other Income (Expenses): | ||||||||||||
| Interest income | 222,256 | 175,393 | 143,195 | |||||||||
| Changes in fair value of option liabilities - gains | 3,238,911 | 2,581,473 | 8,733,350 | |||||||||
| Foreign currency exchange gains (losses) | 81,501 | (358,540 | ) | 272,985 | ) | |||||||
| Other expenses | (17,914 | ) | (13,394 | ) | (43,969 | ) | ||||||
| Total Other Income (Expenses) | 3,524,754 | 2,384,932 | 9,105,561 | |||||||||
| Net Loss | (16,466,780 | ) | (35,399,257 | ) | (18,140,997 | ) | ||||||
| Other Comprehensive (Loss) Income: | ||||||||||||
| Foreign Currency Translation Adjustment | (116,180 | ) | 43,851 | (436,577 | ) | |||||||
| Comprehensive Loss | $ | (16,582,960 | ) | $ | (35,355,406 | ) | $ | (18,577,574 | ) | |||
| Loss per ordinary share (basic and diluted) | $ | (0.01 | ) | $ | (0.03 | ) | $ | (0.02 | ) | |||
| Weighted average ordinary shares (basic and diluted) | 1,540,309,840 | 1,247,293,388 | 1,177,693,383 | |||||||||
See notes to and consolidated financial statements.
| F-3 |
CONSOLIDATED STATEMENT OF CHANGES IN SHAREHOLDERS' EQUITY
As of and for the Years Ended December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| Accumulated | ||||||||||||||||||||||||
| Additional | Other | |||||||||||||||||||||||
| Share Capital | Paid-in | Comprehensive | Accumulated | |||||||||||||||||||||
| Shares | Amount | Capital | Income (Loss) | Deficit | Total | |||||||||||||||||||
| Shareholders' Equity, January 1, 2016 | 1,177,693,383 | $ | 18,340,894 | $ | 87,018,764 | $ | 156,480 | $ | (56,796,613 | ) | $ | 48,719,525 | ||||||||||||
| Stock-based compensation | - | - | 3,933,222 | - | - | 3,933,222 | ||||||||||||||||||
| Dissolution of subsidiary | - | - | 27,377 | - | - | 27,377 | ||||||||||||||||||
| Comprehensive Loss | - | - | - | (436,577 | ) | (18,140,997 | ) | (18,577,574 | ) | |||||||||||||||
| Shareholders' Equity, December 31, 2016 | 1,177,693,383 | 18,340,894 | 90,979,363 | (280,097 | ) | (74,937,610 | ) | 34,102,550 | ||||||||||||||||
| Stock-based compensation | - | - | 2,734,946 | - | - | 2,734,946 | ||||||||||||||||||
| Issuance of share capital upon conversion of deferred shares | 10 | - | - | - | - | - | ||||||||||||||||||
| Issuance of share capital related to financing, net of issuance costs | 348,000,000 | 4,586,640 | 11,085,241 | - | - | 15,671,881 | ||||||||||||||||||
| Comprehensive Income (Loss) | - | - | 43,851 | (35,399,257 | ) | (35,355,406 | ) | |||||||||||||||||
| Shareholders' Equity, December 31, 2017 | 1,525,693,393 | 22,927,534 | 104,799,550 | (236,246 | ) | (110,336,867 | ) | 17,153,971 | ||||||||||||||||
| Stock-based compensation | - | - | 1,649,044 | - | - | 1,649,044 | ||||||||||||||||||
| Issuance of share capital to directors | 20 | - | - | - | - | - | ||||||||||||||||||
| Issuance of share capital related to financing, net of issuance costs | 55,000,000 | 723,743 | 167,489 | - | - | 891,232 | ||||||||||||||||||
| Comprehensive income loss | - | - | - | (116,180 | ) | (16,466,780 | ) | (16,582,960 | ) | |||||||||||||||
| Shareholders’ Equity, December 31, 2018 | 1,580,693,413 | $ | 23,651,277 | $ | 106,616,083 | $ | (352,426 | ) | $ | (126,803,647 | ) | $ | 3,111,287 | |||||||||||
See notes to consolidated financial statements.
| F-4 |
CONSOLIDATED STATEMENTS OF CASH FLOWS
For the Years Ended December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| Cash Flows from Operating Activities: | ||||||||||||
| Net loss | $ | (16,466,780 | ) | $ | (35,399,257 | ) | $ | (18,140,997 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation and amortization | 35,848 | 42,400 | 39,944 | |||||||||
| Stock-based compensation | 1,649,044 | 2,734,946 | 3,933,222 | |||||||||
| Litigation settlement loss | - | 2,700,000 | - | |||||||||
| Changes in fair value of the liability for options and warrants - gains | (3,238,911 | ) | (2,581,473 | ) | (8,733,350 | ) | ||||||
| Foreign currency exchange (losses) gains | (104,719 | ) | 93,402 | (272,985 | ) | |||||||
| Changes in operating assets and liabilities: | ||||||||||||
| (Increase) decrease in assets: | ||||||||||||
| Prepaid expenses and other current assets | (716,859 | ) | 807,408 | (785,876 | ) | |||||||
| (Decrease) increase in liabilities: | ||||||||||||
| Accounts payable and accrued expenses | (3,689,860 | ) | 12,222 | (671,784 | ) | |||||||
| Other liabilities | (48,003 | ) | (8,357 | ) | 7,291 | |||||||
| Total adjustments | (6,113,460 | ) | 3,800,548 | (6,483,538 | ) | |||||||
| Net Cash Used in Operating Activities | (22,580,240 | ) | (31,598,709 | ) | (24,624,535 | ) | ||||||
| Cash Flows from Investing Activities: | ||||||||||||
| Purchase of property and equipment | - | (36,885 | ) | (54,750 | ) | |||||||
| Purchase of short-term investments | - | - | (46,120,172 | ) | ||||||||
| Maturities of short-term investments | - | 10,021,963 | 36,098,209 | |||||||||
| Receivable from related party | - | - | 10,081 | |||||||||
| Net Cash Provided by (Used in) Investing Activities | - | 9,985,078 | (10,066,632 | ) | ||||||||
| Cash Flows from Financing Activities: | ||||||||||||
| Net proceeds from issuance of shares | 306,232 | 15,671,881 | - | |||||||||
| Net Cash Provided by Financing Activities | 306,232 | 15,671,881 | - | |||||||||
| Effect of Exchange Rates on Cash and Restricted Cash | (6,931 | ) | (50,324 | ) | (129,927 | ) | ||||||
| Net Decrease in Cash and Restricted Cash | (22,280,939 | ) | (5,992,074 | ) | (34,821,094 | ) | ||||||
| Cash and Restricted Cash, beginning | 28,248,906 | 34,240,980 | 69,062,074 | |||||||||
| Cash and Restricted Cash, end | $ | 5,967,967 | $ | 28,248,906 | $ | 34,240,980 | ||||||
| Supplemental Disclosures of Cash Flow Information: | ||||||||||||
| Deferred financing costs | $ | 585,000 | $ | - | $ | - | ||||||
See notes to consolidated financial statements.
| F-5 |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 1 – Nature of Business |
Akari Therapeutics, Plc, (the “Company” or “Akari”), formerly Celsus Therapeutics Plc (“Celsus”), is incorporated in the United Kingdom. The Company is a clinical-stage biopharmaceutical company focused on developing inhibitors of acute and chronic inflammation, specifically the complement system, the eicosanoid system and the bioamine system for the treatment of rare and orphan diseases.
The accompanying financial statements have been prepared in conformity with U.S. GAAP, assuming that the Company will continue to operate as a going concern. As of December 31, 2018, the Company has an accumulated deficit of $126,803,647, cash of $5,446,138 and negative cash flows from operating activities in the amount of $22,580,240. On October 20, 2017, the Company sold an aggregate of 3,480,000 American Depositary Shares (“ADSs”) representing 348,000,000 ordinary shares, par value £0.01 (“Ordinary Shares”) for gross proceeds of $17.4 million at $5.00 per ADS with issuance costs of approximately $1.7 million. On September 26, 2018, the Company entered into a securities purchase agreement (the “Purchase Agreement”) with Aspire Capital Fund, LLC, an Illinois limited liability company (“Aspire Capital”), which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $20.0 million of the Company’s ADSs over the 30-month term of the Purchase Agreement. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, the Company issued 30,000,000 Ordinary Shares to Aspire Capital and sold to Aspire Capital 25,000,000 Ordinary Shares for $0.02 per share (equivalent to $2.00 per ADS) (See Note 4). The Company believes its current capital resources are sufficient to support its operations through the end of the second quarter of 2019 without giving effect to the sale of additional shares to Aspire Capital under the Purchase Agreement.
The Company’s activities since inception have consisted of raising capital and performing research and development activities. As of December 31, 2018, principal commercial operations have not commenced. The Company is subject to a number of risks similar to those of clinical stage companies, including dependence on key individuals, uncertainty of product development and generation of revenues, dependence on outside sources of capital, risks associated with clinical trials of products, dependence on third-party collaborators for research operations, need for regulatory approval of products, risks associated with protection of intellectual property, and competition with larger, better-capitalized companies. In addition, the Company is subject to risks related to an SEC investigation.
For the year ended December 31, 2018, the Company reported a net loss of $16,466,780 and expects to continue to incur substantial losses over the next several years during its development phase. To fully execute its business plan, the Company will need, among other things, to complete its research and development efforts and clinical and regulatory activities. These activities may take several years and will require significant operating and capital expenditures in the foreseeable future. There can be no assurance that these activities will be successful. If the Company is not successful in these activities or there is not a favorable resolution of the SEC investigation it could delay, limit, reduce or terminate preclinical studies, clinical trials or other research and development activities. To fund its capital needs, the Company plans to raise funds through equity or debt financings or other sources, such as strategic partnerships and alliance and licensing arrangements, and in the long term, from the proceeds from sales. Additional funds may not be available when the Company needs them, on terms that are acceptable to it, or at all. These matters raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements do not include any adjustments to the carrying amounts and classifications of assets and liabilities that would result if the Company was unable to continue as a going concern.
| F-6 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 2 – Summary of Significant Accounting Policies |
Principles of Consolidation – The Consolidated Financial Statements include the accounts of the Company and Volution Immuno Pharmaceuticals SA, a private Swiss company, its wholly-owned subsidiary. All intercompany transactions have been eliminated.
Foreign Currency – The functional currency of the Company is U.S. dollars as that is the primary economic environment in which the Company operates as well as the currency in which it has been financed.
The reporting currency of the Company is U.S. Dollars. The Company translated its non-U.S. operations’ assets and liabilities denominated in foreign currencies into U.S. dollars at current rates of exchange as of the balance sheet date and income and expense items at the average exchange rate for the reporting period. Translation adjustments resulting from exchange rate fluctuations are recorded as foreign currency translation adjustments, a component of accumulated other comprehensive loss. Gains or losses from foreign currency transactions are included in foreign currency exchange gains/(losses).
Use of Estimates – The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and judgments that may affect the reported amounts of assets, liabilities, equity, revenue, expenses and related disclosure of contingent assets and liabilities. Management’s estimates and judgments include assumptions used in the evaluation of impairment and useful lives of intangible assets (patents), accrued liabilities, deferred income taxes, liabilities related to stock options and warrants, stock-based compensation and various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ from those estimates under different assumptions or conditions.
Fair Value Measurements – The carrying amounts of financial instruments, including cash, restricted cash, accounts payable and accrued expenses approximate fair value due to their short-term maturities.
The Company’s liabilities related to options relate to equity and debt financing rounds and options related to RPC Pharma Limited (“RPC”), Akari’s majority shareholder, and are recognized on the balance sheet at their fair value, with changes in the fair value accounted for in the Statements of Comprehensive Loss and included in changes in fair value of option liabilities gains.
Cash – The Company considers all highly-liquid investments with original maturities of 90 days or less at the time of acquisition to be cash equivalents. The Company had no cash equivalents as of December 31, 2018 and December 31, 2017.
Restricted cash - Restricted cash is collateral for a letter of credit related to the Company’s former office leases.
Prepaid Expenses and Other Current Assets – Prepaid expenses and other current assets consist principally of VAT receivables and prepaid expenses.
Deferred Financing Costs – Deferred financing costs relate to the upfront commitment fee paid to Aspire Capital in the form of Ordinary Shares and are included in current assets. They are amortized proportionally as the Company sells shares to Aspire Capital.
Property and equipment, net – Property and equipment are stated at cost, net of accumulated depreciation. Depreciation is calculated using the straight-line method over the estimated useful lives of the assets at the following annual rates:
| Years | |||
| Computers, peripheral, and scientific equipment | 3 | ||
| Office furniture and equipment | 3 |
Depreciation expense for the years ended December 31, 2018, 2017 and 2016 was $35,473, $39,351 and $36,898, respectively.
| F-7 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 2 – Summary of Significant Accounting Policies (cont.) |
Long-Lived Assets – The Company reviews all long-lived assets for impairment whenever events or circumstances indicate the carrying amount of such assets may not be recoverable. Recoverability of assets to be held or used is measured by comparison of the carrying value of the asset to the future undiscounted net cash flows expected to be generated by the asset. If such asset is considered to be impaired, the impairment recognized is measured by the amount by which the carrying value of the asset exceeds the discounted future cash flows expected to be generated by the asset.
Patent Acquisition Costs – Patent acquisition costs and related capitalized legal fees are amortized on a straight-line basis over the shorter of the legal or economic life. The estimated useful life is 22 years. The Company expenses costs associated with maintaining and defending patents subsequent to their issuance in the period incurred. Amortization of patent acquisition costs for the years ended December 31, 2018, 2017 and 2016 was $375, $3,049 and $3,046, respectively.
Accrued Expenses – As part of the process of preparing the consolidated financial statements, it requires the estimate of accrued expenses. This process involves identifying services that third parties have performed on the Company’s behalf and estimating the level of service performed and the associated cost incurred on these services as of each balance sheet date in the Company’s consolidated financial statements. Examples of estimated accrued expenses include contract service fees in conjunction with pre-clinical and clinical trials, professional service fees and contingent liabilities. In connection with these service fees, the Company’s estimates are most affected by its understanding of the status and timing of services provided relative to the actual services incurred by the service providers. In the event that the Company does not identify certain costs that have been incurred or it under or over-estimates the level of services or costs of such services, the Company’s reported expenses for a reporting period could be understated or overstated. The date on which certain services commence, the level of services performed on or before a given date, and the cost of services are often subject to the Company’s estimation and judgment. The Company makes these judgments based upon the facts and circumstances known to it in accordance with U.S. GAAP.
Research and Development Expenses – Costs associated with research and development are expensed as incurred. Research and development expenses include, among other costs, personnel expenses, costs incurred by outside laboratories, manufacturers’ and other accredited facilities in connection with clinical trials and preclinical studies. Research and development expenses for the years ended December 31, 2018, 2017 and 2016 were $11,795,376, $23,285,279 and $17,306,001, respectively. The Company accounts for research and development tax credits at the time its realization becomes probable. In September 2016 and March 2018, the Company realized research and development tax credits of $577,671 and $3,794,094, respectively, that was recorded as a credit to research and development costs in the Consolidated Statements of Comprehensive Loss (See Note 8). The Company did not realize research and development credits in 2017.
Stock-Based Compensation Expense – Stock-based compensation expense is recorded using the fair-value based method for all awards granted. Compensation costs for stock options and awards is recorded in earnings (loss) over the requisite service period based on the fair value of those options and awards. For employees, fair value is estimated at the grant date, and for non-employees, fair value is re-measured at each reporting date as required by ASC 718, “Compensation-Stock Compensation,” and ASC 505-50, “Equity-Based Payments to Non-Employees.” Fair values of awards granted under the share option plans are estimated using a Black-Scholes option pricing model. The determination of fair value for stock-based awards on the date of grant using an option pricing model requires management to make certain assumptions regarding a number of complex and subjective variables. The Company classifies its stock-based payments as either liability-classified awards or as equity-classified awards. The Company remeasures liability-classified awards to fair value at each balance sheet date until the award is settled. The liability for liability-classified awards generally is equal to the fair value of the award as of the balance sheet date multiplied by the percentage vested at the time. The Company charges (or credits) the change in the liability amount from one balance sheet date to another to changes in fair value of option/warrant liabilities gain (loss). The Company accounts for awards of equity instruments issued to employees and directors under the fair value method of accounting and recognizes such amounts, upon vesting, in general administrative or research and development expenses within its Consolidated Statements of Comprehensive Loss.
Concentration of Credit Risk – Financial instruments that subject the Company to credit risk consist of cash. The Company maintains cash with well-capitalized financial institutions. At times, those amounts may exceed insured limits. The Company has no significant concentrations of credit risk.
| F-8 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 2 – Summary of Significant Accounting Policies (cont.) |
Income Taxes – The Company accounts for income taxes in accordance with the accounting rules that require an asset and liability approach to accounting for income taxes based upon the future expected values of the related assets and liabilities. Deferred income tax assets and liabilities are determined based on the differences between the financial reporting and tax bases of assets and liabilities and for tax loss and credit carry forwards and are measured using the expected tax rates estimated to be in effect when such basis differences reverse. Valuation allowances are established, if necessary, to reduce the deferred tax asset to the amount that will, more likely than not, be realized. The Company has recorded a full valuation allowance on its deferred tax assets as of December 31, 2018 and December 31, 2017.
2017 U.S. Tax Reform – On December 22, 2017, the Tax Cuts and Jobs Act (the “TCJA”) was signed into United States law. The TCJA includes a number of changes to existing tax law, including, among other things, a permanent reduction in the federal corporate income tax rate from 34% to 21%, effective as of January 1, 2018, as well as limitation of the deduction for net operating losses to 80% of annual taxable income and elimination of net operating loss carrybacks, in each case, for losses arising in taxable years beginning after December 31, 2017 (though any such net operating losses may be carried forward indefinitely). The staff of the Securities and Exchange Commission issued Staff Accounting Bulletin No. 118 to address the application of U.S. GAAP in situations when a registrant does not have the necessary information available, prepared or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the TCJA. In connection with the Company’s completed analysis of the impact of the TCJA, the tax rate change resulted in (i) a reduction in the gross amount of the Company’s deferred tax assets recorded as of December 31, 2017, without an impact on the net amount of its deferred tax assets, which are reported with a full valuation allowance, and (ii) no income tax expense or benefit being recognized as of the enactment date of the TCJA.
Uncertain Tax Positions – The Company follows the provisions of ASC 740 “Accounting for Uncertainty in Income Taxes”, which prescribes recognition thresholds that must be met before a tax position is recognized in the financial statements and provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. Under ASC 740 “Accounting for Uncertainty in Income Taxes,” an entity may only recognize or continue to recognize tax positions that meet a “more-likely-than-not” threshold. Interest and penalties related to uncertain tax positions are recognized as income tax expense. At December 31, 2018 and December 31, 2017, the Company had no uncertain tax positions.
Earnings (Loss) Per Share – Basic earnings (loss) per ordinary share is computed by dividing net income (loss) available to ordinary shareholders by the weighted-average number of Ordinary Shares outstanding during the period. Diluted earnings (loss) per ordinary share is computed by dividing net income (loss) available to ordinary shareholders by the sum of (1) the weighted-average number of Ordinary Shares outstanding during the period, (2) the dilutive effect of the assumed exercise of options and warrants using the treasury stock method and (3) the dilutive effect of other potentially dilutive securities.
Comprehensive Income (Loss) – Comprehensive loss is defined as the change in equity of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. The Company’s other comprehensive loss is comprised of foreign currency translation adjustments.
The following table provides details with respect to changes in accumulated other comprehensive loss, which is comprised of foreign currency translation adjustments, as presented in the balance sheets at December 31, 2018 and 2017:
| Balance January 1, 2016 | $ | 156,480 | ||
| Net current period other comprehensive income | (436,577 | ) | ||
| Balance December 31, 2016 | (280,097 | ) | ||
| Net current period other comprehensive loss | 43,851 | |||
| Balance December 31, 2017 | (236,246 | ) | ||
| Net current period other comprehensive income | (116,180 | ) | ||
| Balance December 31, 2018 | $ | (352,426 | ) |
| F-9 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 2 – Summary of Significant Accounting Policies (cont.) |
Recent Accounting Pronouncements
Adopted during year –
In March 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2016-09, Improvements to Employee Share-Based Payment Accounting (“ASU No. 2016-09”). ASU 2016-09 simplifies various aspects related to how share-based payments are accounted for and presented in the consolidated financial statements. The amendments include income tax consequences, the accounting for forfeitures, classification of awards as either equity or liabilities, and classification on the statement of cash flows. The guidance is effective for annual periods beginning after December 15, 2016, and interim periods within those annual periods. The adoption of this standard in 2017 did not have a material impact on the Company’s financial position, results of operations or related financial statement disclosures.
In May 2014, the FASB issued ASU No. 2014-09, Revenue from Contracts with Customers (“ASU 2014-09”), which supersedes nearly all existing revenue recognition guidance under GAAP. The core principle of ASU 2014-09 is to recognize revenues when promised goods or services are transferred to customers in an amount that reflects the consideration to which an entity expects to be entitled for those goods or services. ASU 2014-09 defines a five-step process to achieve this core principle and, in doing so, more judgment and estimates may be required within the revenue recognition process than are required under existing GAAP. The adoption of this standard in 2018 did not have a material impact on the Company’s financial position and results of operations. The future impact of ASU 2014-09 will be dependent on the nature of the Company’s future revenue contracts and arrangements, if any.
In November 2016, the FASB issued ASU 2016-18, Restricted Cash, which requires that restricted cash be included with cash and cash equivalents when reconciling the beginning and ending total amounts shown on the statement of cash flows. This guidance is effective for fiscal years beginning after December 15, 2017, and interim periods within fiscal years beginning after December 15, 2019 and should be applied using a retrospective transition method to each period presented. Early adoption is permitted, including adoption in an interim period. If an entity early adopts the amendments in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period. The Company currently presents changes in restricted cash within investing activities, and so the adoption of this guidance will result in changes in net cash flows from investing activities and to certain beginning and ending cash and cash equivalent totals shown on Consolidated Statements of Cash Flows. The adoption of this standard will not have a material impact on its statements of cash flows.
In August 2016, the FASB issued ASU 2016-15, Classification of Certain Cash Receipts and Cash Payments, which addresses eight specific cash flow issues with the objective of reducing the existing diversity in practice, including presentation of cash flows relating to contingent consideration payments, proceeds from the settlement of insurance claims, and debt prepayment or debt extinguishment costs, among other matters. This guidance is effective for fiscal years beginning after December 15, 2017, and interim periods within fiscal years beginning after December 15, 2019. Early adoption is permitted, including adoption in an interim period. If adopted in an interim period, any adjustments should be reflected as of the beginning of the fiscal year that includes that interim period. Adoption of this guidance is required to be applied using a retrospective transition method to each period presented, unless impracticable to do so. The adoption of this standard did not have a material impact on its statements of cash flows or related financial statement disclosures.
Not yet adopted –
In February 2016, the FASB issued ASU No. 2016-02, Leases (“ASU 2016-02”). ASU 2016-02 establishes a right-of-use (ROU) model that requires a lessee to record a ROU asset and a lease liability on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the income statement. The new standard is effective for fiscal years beginning after December 15, 2018, including interim periods within those fiscal years. A modified retrospective transition approach is required for lessees for capital and operating leases existing at, or entered into after, the beginning of the earliest comparative period presented in the financial statements, with certain practical expedients available. The adoption of this standard in 2019 will not have a material impact on the Company’s financial position, results of operations or related financial statement disclosures since it does not have a lease with a term longer than 12 months.
| F-10 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 2 – Summary of Significant Accounting Policies (cont.) |
In October 2016, the FASB issued ASU 2016-16, Intra-Entity Transfers of Assets Other Than Inventory. This guidance removes the prohibition in ASC 740 against the immediate recognition of the current and deferred income tax effects of intra-entity transfers of assets other than inventory. This guidance is intended to reduce the complexity of U.S. GAAP and diversity in practice related to the tax consequences of certain types of intra-entity asset transfers, particularly those involving intellectual property. This guidance is effective for annual reporting periods beginning after December 15, 2018, and interim periods within fiscal years beginning after December 15, 2019. Early adoption is permitted. The Company is currently evaluating the impact of this guidance on its consolidated financial statements.
|
NOTE 3 – Fair Value Measurements |
Fair value of financial instruments:
The estimated fair value of financial instruments has been determined by the Company using available market information and valuation methodologies. Considerable judgment is required in estimating fair values. Accordingly, the estimates may not be indicative of the amounts the Company could realize in a current market exchange.
The carrying amounts of cash, restricted cash, accounts payable and accrued expenses approximate their fair value due to the short-term maturity of such instruments.
Fair value is an exit price, representing the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or a liability. As a basis for considering such assumptions, ASC 820, Fair Value Measurements and Disclosures establishes a three-tier value hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value:
Level 1 - quoted prices in active markets for identical assets or liabilities;
Level 2 - inputs other than Level 1 that are observable, either directly or indirectly, such as quoted prices in active markets for similar assets or liabilities, quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities; or
Level 3 - unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
| F-11 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 3 – Fair Value Measurements (cont.) |
The fair value hierarchy also requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value.
In accordance with ASC No. 820, the Company measures its liabilities related to options on a recurring basis at fair value. The liabilities related to options are classified within Level 3 value hierarchy because the liabilities are based on present value calculations and external valuation models whose inputs include market interest rates, estimated operational capitalization rates, volatilities and illiquidity. Unobservable inputs used in these models are significant.
In June 2015, the Company raised short-term working capital in the form of loans from shareholders of approximately $3 million with the loans carrying with it, options in RPC, equivalent to 15% of the current outstanding equity issued by RPC. RPC is a private company that is a majority shareholder of the Company. The RPC options were accounted for in accordance with ASC 718, Compensation – Stock Compensation. The fair value of the RPC options was estimated using the fair value of Akari Ordinary Shares times RPC’s ownership in Akari Ordinary Shares times 15% and was initially valued at approximately $26 million. These options do not relate to the share capital of Akari. The exact terms of these options have not been finalized.
The fair value of the RPC options was $1,842,424 and $5,081,335 as of December 31, 2018 and December 31, 2017, respectively. The fair value of the RPC options for the year ended December 31, 2018 decreased by $3,238,911 and the change, which represents a gain, was recognized as change in fair value of option/warrant liabilities gains in the Consolidated Statements of Comprehensive Loss. The Company accounts for the RPC options as a liability in accordance with ASC 815-40-25, Accounting for Derivative Financial Instruments Indexed to, and Potentially Settled in, a Company’s Own Stock and ASC 815-40-15, Determining Whether an Instrument (or Embedded Feature) Is Indexed to an Entity’s Own Stock.
The Company’s financial assets and liabilities measured at fair value on a recurring basis, consisted of the following instruments as of the following dates:
| December 31, | December 31, | |||||||
| 2018 | 2017 | |||||||
| RPC options | $ | 1,842,424 | $ | 5,081,335 | ||||
Fair value measurements using significant unobservable inputs (Level 3):
| Fair value of liabilities related to stock options | ||||
| Balance at December 31, 2016 | 7,662,808 | |||
| Changes in values of liabilities related to options | (2,581,473 | ) | ||
| Balance at December 31, 2017 | 5,081,335 | |||
| Changes in values of liabilities related to options | (3,238,911 | ) | ||
| Balance at December 31, 2018 | $ | 1,842,424 | ||
| F-12 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| NOTE 4 – Shareholders’ Equity |
Share Capital – The Company has 10,000,000,000 and 5,000,000,000 Ordinary Shares of authorized capital and 1,580,693,413 and 1,525,693,393 Ordinary Shares outstanding at December 31, 2018 and December 31, 2017, respectively.
On June 26, 2017, previously issued Deferred B Shares and Deferred C Shares were converted into 10 Ordinary Shares.
On October 20, 2017, the Company issued an aggregate of 3,480,000 ADSs representing 348,000,000 Ordinary Shares for gross proceeds of $17.4 million with issuance costs of approximately $1.7 million generating net proceeds of approximately $15.7 million.
On July 19, 2018, the Company issued 10 Ordinary Shares each to two directors.
Purchase Agreement and Registration Rights Agreement with Aspire Capital
On September 26, 2018, the Company entered into a Purchase Agreement with Aspire Capital, which provides that, upon the terms and subject to the conditions and limitations set forth therein, Aspire Capital is committed to purchase up to an aggregate of $20.0 million of the Company’s ADS, with each ADS representing one hundred (100) Ordinary Shares, during a 30-month period beginning on the effective date of a registration statement related to the transaction. Concurrently with entering into the Purchase Agreement, the Company also entered into a registration rights agreement with Aspire Capital (the “Registration Rights Agreement”), in which the Company agreed to file one or more registration statements, as permissible and necessary to register under the Securities Act of 1933, as amended (the “Securities Act”), the sale of the Company’s securities that have been and may be issued to Aspire Capital under the Purchase Agreement.
Under the Purchase Agreement, after the Securities and Exchange Commission (the “SEC”) has declared effective the registration statement referred to above (which occurred in February 2019), on any trading day selected by the Company, the Company has the right, in its sole discretion, to present Aspire Capital with a purchase notice (each, a “Purchase Notice”), directing Aspire Capital (as principal) to purchase up to 150,000 ADSs per business day and up to $20.0 million of the Company’s ADSs in the aggregate at a per share price (the “Purchase Price”) equal to the lesser of:
| · | the lowest sale price of the Company’s ADSs on the purchase date; or |
| · | the arithmetic average of the three (3) lowest closing sale prices for the ADSs during the ten (10) consecutive business days ending on the business day immediately preceding such Purchase Date (to be appropriately adjusted for any reorganization, recapitalization, non-cash dividend, stock split, reverse stock split or other similar transaction). |
In addition, on any date on which the Company submits a Purchase Notice to Aspire Capital in an amount of 150,000 ADSs, the Company also has the right, in its sole discretion, to present Aspire Capital with a volume-weighted average price purchase notice (each, a “VWAP Purchase Notice”) directing Aspire Capital to purchase an amount of ADSs equal to up to 30% of the aggregate shares of the Company’s ADSs traded on its principal market on the next trading day (the “VWAP Purchase Date”), subject to a maximum number of 250,000 ADSs. The purchase price per share pursuant to such VWAP Purchase Notice is generally 97% of the volume-weighted average price for the Company’s ADSs traded on its principal market on the VWAP Purchase Date.
The Purchase Price will be adjusted for any reorganization, recapitalization, non-cash dividend, stock split, or other similar transaction occurring during the period(s) used to compute the Purchase Price. The Company may deliver multiple Purchase Notices and VWAP Purchase Notices to Aspire Capital from time to time during the term of the Purchase Agreement, so long as the most recent purchase has been completed.
The Purchase Agreement provides that the Company and Aspire Capital shall not effect any sales under the Purchase Agreement on any purchase date where the closing sale price of the Company’s ADSs is less than $0.25. There are no trading volume requirements or restrictions under the Purchase Agreement, and the Company will control the timing and amount of sales of the Company’s ADSs to Aspire Capital. Aspire Capital has no right to require any sales by the Company but is obligated to make purchases from the Company as directed by the Company in accordance with the Purchase Agreement. There are no limitations on use of proceeds, financial or business covenants, restrictions on future fundings, rights of first refusal, participation rights, penalties or liquidated damages in the
| F-13 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| NOTE 4 – Shareholders’ Equity (cont.) |
Purchase Agreement. In consideration for entering into the Purchase Agreement, concurrently with the execution of the Purchase Agreement, the Company issued to Aspire Capital 30,000,000 Ordinary Shares of the Company (the “Commitment Shares”) and sold to Aspire Capital 25,000,000 Ordinary Shares (the “Initial Shares”) for $0.02 per share (equivalent to $2.00 per ADS). The Company recorded the value of the Commitment shares as deferred financing costs and included the costs in current assets. They are amortized proportionally as the Company sells shares to Aspire. As of December 31, 2018, the Company recognized $15,000 of such costs and included the costs in additional paid-in capital. The Purchase Agreement may be terminated by the Company at any time, at its discretion, without any cost to the Company. Aspire Capital has agreed that neither it nor any of its agents, representatives and affiliates shall engage in any direct or indirect short-selling or hedging of the Company’s securities during any time prior to the termination of the Purchase Agreement. Any proceeds the Company receives under the Purchase Agreement are expected to be used for working capital and general corporate purposes.
Share option plan –
In accordance with the Company’s 2014 Equity Incentive Plan (the “Plan”), the number of shares that may be issued upon exercise of options under the Plan shall not exceed 183,083,207 Ordinary Shares. At December 31, 2018, 88,986,209 Ordinary Shares are available for future issuance under the Plan. The option plan is administered by the Company’s board of directors and grants are made pursuant thereto by the compensation committee. The per share exercise price for the shares to be issued pursuant to the exercise of an option shall be such price equal to the fair market value of the Company’s Ordinary Shares on the grant date and set forth in the individual option agreement. Options expire ten years after the grant date and typically vest over one to four years.
The following is a summary of the Company’s share option activity and related information for employees and directors for the years ended December 31, 2018, 2017 and 2016:
| Number of shares | Weighted average exercise price | Weighted average grant date fair value | Weighted average remaining contractual term (in years) | Aggregate intrinsic value | ||||||||||||||||
| Options outstanding as of January 1, 2016 | 61,362,198 | $ | 0.34 | - | ||||||||||||||||
| Changes during the period: | ||||||||||||||||||||
| Granted | 22,700,000 | $ | 1.14 | $ | 0.09 | |||||||||||||||
| Forfeited | (4,690,000 | ) | $ | 0.21 | $ | 0.12 | ||||||||||||||
| Options outstanding at December 31, 2016 | 79,372,198 | $ | 0.29 | - | ||||||||||||||||
| Changes during the period: | ||||||||||||||||||||
| Granted | 50,700,000 | $ | 0.05 | $ | 0.03 | |||||||||||||||
| Forfeited | (40,010,200 | ) | $ | 0.32 | $ | 0.19 | ||||||||||||||
| Options outstanding at December 31, 2017 | 90,061,998 | $ | 0.15 | $ | 266,011 | |||||||||||||||
| Changes during the period: | ||||||||||||||||||||
| Granted | 41,150,000 | $ | 0.02 | $ | 0.01 | |||||||||||||||
| Forfeited | (37,115,000 | ) | $ | 0.07 | $ | 0.05 | ||||||||||||||
| Options outstanding at December 31, 2018 | 94,096,998 | $ | 0.12 | 8.4 | $ | - | ||||||||||||||
| Exercisable options at December 31, 2018 | 39,272,865 | $ | 0.21 | 7.3 | $ | - | ||||||||||||||
The Company measures compensation cost for all share-based awards at fair value on the date of grant and recognizes compensation expense in general administrative and research and development expenses within its Consolidated Statements of Comprehensive Loss using the straight-line method over the service period over which it expects the awards to vest.
| F-14 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 4 – Shareholders’ Equity (cont.) |
The Company estimates the fair value of all time-vested options as of the date of grant using the Black-Scholes option valuation model, which was developed for use in estimating the fair value of traded options that have no vesting restrictions and are fully transferable. Option valuation models require the input of highly subjective assumptions, including the expected share price volatility, which is calculated based on the historical volatility of peer companies. The Company uses a risk-free interest rate, based on the U.S. Treasury instruments in effect at the time of the grant, for the period comparable to the expected term of the option. Given its limited history with share option grants and exercises, the Company uses the “simplified” method in estimating the expected term, the period of time that options granted are expected to be outstanding, for its grants.
The Company classifies its stock-based payments as either liability-classified awards or as equity-classified awards. The Company re-measures liability-classified awards to fair value at each balance sheet date until the award is settled. The Company measures equity-classified awards at their grant date fair value and does not subsequently re-measure them. The Company has classified its stock-based payments, which are settled in ordinary shares as equity-classified awards, and share-based payments that are settled in cash as liability-classified awards. Compensation costs related to equity-classified awards generally are equal to the grant-date fair value of the award amortized over the vesting period of the award. The liability for liability-classified awards generally is equal to the fair value of the award as of the balance sheet date multiplied by the percentage vested at the time. The Company charges (or credits) the change in the liability amounts from one balance sheet date to another to stock-based compensation expense.
Below are the assumptions used for the options granted during the years ended December 31, 2018, 2017 and 2016:
| December 31, | ||||||||||||||
| 2018 | 2017 | 2016 | ||||||||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||||
| Expected volatility | 70.52%-82.23 | % | 78.77%-82.03 | % | 74.18%-80.71 | % | ||||||||
| Risk-free interest | 2.49%-3.13 | % | 1.21%-2.21 | % | 1.03%-1.52 | % | ||||||||
| Expected life | 5.5-6.25 years | 5.5-6.25 years | 5.5-6.25 years | |||||||||||
The following is a summary of the Company’s share options granted separated into ranges of exercise price:
| Exercise price (range) ($) | Options outstanding at December 31, 2018 | Weighted average remaining contractual life (years) | Weighted average exercise price ($) | Options exercisable at December 31, 2018 | Remaining contractual life (years for exercisable options) | Weighted average exercise price ($) | ||||||||||||||||||
| 0.02-0.05 | 54,300,000 | 9.39 | 0.03 | 7,187,500 | 8.54 | 0.05 | ||||||||||||||||||
| 0.12-0.19 | 18,834,629 | 7.32 | 0.15 | 14,936,711 | 7.29 | 0.16 | ||||||||||||||||||
| 0.32 | 20,782,369 | 6.72 | 0.32 | 16,968,654 | 6.72 | 0.32 | ||||||||||||||||||
| 1.56-2.00 | 180,000 | 4.43 | 1.62 | 180,000 | 4.43 | 1.62 | ||||||||||||||||||
| 94,096,998 | 39,272,865 | |||||||||||||||||||||||
During the years ended December 31, 2018, 2017 and 2016, the Company recorded approximately $1,649,000, $2,735,000 and $3,933,000, respectively, in stock-based compensation expenses for employees and directors. At December 31, 2018, there was approximately $1,665,000 of unrecognized compensation cost related to unvested share-based compensation arrangements granted under the Company’s share option plans which the Company expects to recognize over 1.7 years.
Warrants to service providers and investors – At December 31, 2018 there were no warrants outstanding. During the twelve months ended December 31, 2018, 399,160 warrants to purchase Ordinary Shares expired.
| F-15 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 5 – Related Party Transactions |
Office Lease - A non-employee director of the Company is also the CEO of The Doctors Laboratory (“TDL”). The Company leases its UK office space from TDL and has incurred expenses of approximately $139,000, $141,000 and $141,000 plus VAT during the years ended December 31, 2018, 2017 and 2016, respectively (see Note 6).
| NOTE 6 – Commitments and Contingencies |
Loss contingencies - On April 27, 2017, the Company issued a press release stating that Edison Investment Research Ltd. (“Edison”) had withdrawn its report issued April 26, 2017 titled “Akari’s Coversin matches Soliris in Phase II” (the “Edison Report”) because it contained material inaccuracies, including, without limitation, with respect to the Company’s interim analysis of its ongoing Phase II PNH trial of Coversin. Investors were cautioned not to rely upon any information contained in the Edison Report and instead were directed to the Company’s press release issued on April 24, 2017 that discusses the interim analysis of the Company’s then ongoing Phase II PNH trial and other matters. The Company’s Board of Directors established an ad hoc special committee of the Board to review the involvement, if any, of its personnel with the Edison Report, which was later retracted. Edison was retained by the Company to produce research reports about the Company. While that review was pending, Dr. Gur Roshwalb, the Company’s former Chief Executive Officer, was placed on administrative leave and Dr. Ray Prudo in his role as Executive Chairman temporarily assumed Dr. Roshwalb’s duties in his absence. Following that review, the Company determined that the Edison Report was reviewed and approved by Dr. Roshwalb, in contravention of Company policy. On May 29, 2017, Dr. Roshwalb submitted his resignation as Chief Executive Officer and member of the Company’s Board of Directors, effective immediately.
On May 12, 2017, a putative securities class action captioned Derek Da Ponte v. Akari Therapeutics, PLC, Gur Roshwalb, and Dov Elefant (Case 1:17-cv-03577) was filed in the U.S. District Court for the Southern District of New York against the Company, its former Chief Executive Officer, and its Chief Financial Officer. The plaintiff asserted claims alleging violations of Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 (the “Exchange Act”), based primarily on the Company’s press releases or statements issued between April 24, 2017 and May 11, 2017 concerning the Phase II PNH trial of Nomacopan (Coversin) and the Edison Report about the Company and actions taken by it after the report was issued. The purported class covers the period from March 30, 2017 to May 11, 2017. The complaint seeks unspecified damages and costs and fees. On May 19, 2017, an almost identical class action complaint captioned Shamoon v. Akari Therapeutics, PLC, Gur Roshwalb, and Dov Elefant (Case 1:17-cv-03783) was filed in the same court. On July 11-12, 2017, candidates to be lead plaintiff filed motions to consolidate the cases and appoint a lead plaintiff. On August 10, 2017, the court issued a stipulated order: (i) consolidating the class actions under the caption In re: Akari Therapeutics, PLC Securities Litigation (Case 1:17-cv-03577); and (ii) setting out a schedule for plaintiffs to file a consolidated amended complaint and defendants to respond thereto.
By order dated September 7, 2017, the court appointed lead plaintiffs for the class and lead plaintiffs’ counsel. On November 6, 2017, lead plaintiffs filed a consolidated amended complaint (the “CAC”). While the CAC contains similar substantive allegations to the initial complaints, it adds two additional defendants, Ray Prudo and Edison Investment Research Ltd., and the purported class period was changed to April 24, 2017 through May 30, 2017. On January 10, 2018, at a hearing regarding the defendants’ impending motions to dismiss the CAC, the Court gave plaintiffs permission to file a second consolidated amended complaint (the “SCAC”) and established a briefing schedule for defendants’ motions to dismiss the SCAC. Pursuant to that schedule, plaintiffs’ SCAC was filed on January 31, 2018. All briefing on the motions to dismiss was completed on April 20, 2018.
On May 9, 2018, the parties engaged in a mediation session and came to an agreement in principle to settle the dispute. On June 8, 2018, the parties entered into a memorandum of understanding. A memorandum of understanding is not a definitive settlement agreement, which must be approved by the Court. By the terms of the memorandum, the parties agreed in principle to a total payment of $2.7 million in cash. The Company recorded the $2.7 million SCAC litigation settlement loss in the Consolidated Statement of Comprehensive Loss in the year ended December 31, 2017, which is the period in which the lawsuits were originally filed. The $2.7 million SCAC settlement liability was recorded as a loss contingency in accrued expenses in the Company’s Consolidated Balance Sheets as of December 31, 2017. On July 26, 2018, plaintiffs filed a notice with the Court voluntarily dismissing Edison from the action. On August 3, 2018, the remaining parties executed and filed a stipulation and agreement of settlement (the terms of which were consistent with the memorandum of understanding). On August 7, 2018, the Court granted plaintiffs’ motion for preliminary approval of the settlement, and on November 28, 2018, following a hearing with the parties, the court ordered final approval of the settlement. Plaintiffs subsequently moved to distribute the settlement funds to the class, and the Court granted plaintiffs’ motion on February 4, 2019.
| F-16 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| NOTE 6 – Commitments and Contingencies (cont.) |
On August 24, 2018, the Company received a $2.7 million payment from its directors’ and officers’ liability insurance provider, the sum of which was paid to an escrow account for the benefit of the settlement class on August 27, 2018. This was recorded as a gain in the Consolidated Statements of Comprehensive Loss during the third quarter of 2018.
Separately, Edison has sought indemnification from the Company pursuant to its contract with the Company, including reimbursement of all legal expenses that Edison incurs in connection with the securities class action (to which, as discussed above, Edison was added as a defendant on November 6, 2017) and lost profits from customer relationships that Edison claims it lost as a result of the retraction of the Edison Report. The parties have come to an agreement and settled the dispute for an immaterial amount to the Company’s operations and cash flows.
The Company voluntarily reported to the SEC the circumstances leading to the withdrawal of the Edison Report and the outcome of its special committee’s investigation. In response, the SEC requested certain documents from the Company with respect to the matters it reported. The Company is cooperating with the SEC’s requests for information. On June 5, 2018, the Company received a subpoena from the SEC, which requested further documents and information primarily related to the Company’s Phase II clinical trial of Nomacopan (Coversin) in connection with an investigation of the Company that the SEC is conducting. The Company is in the process of responding to the subpoena and will continue to cooperate with the SEC.
Lease commitment – In March 2014, the Company entered into a lease agreement for offices in London which was amended January 1, 2016. The lease term commenced on December 1, 2014 and expired in March 2019, which we intend to renew and are currently leasing on the same terms of the lease. The lease can be cancelled early by either party upon 3 months’ notice (See Note 5).
The Company also had a five-year lease for offices in New York, New York effective July 2014. The lease ended early in December 2018. In January 2018, the Company entered into a sublease of office space in New York, New York for an approximately four-year term, which ended early in December 2018. The Company currently leases office space in New York, New York on a month-to-month basis.
For the years ended December 31, 2018, 2017 and 2016, the Company incurred rental expense in the amount of approximately $814,000, $446,000 and $445,000, respectively.
|
NOTE 7 – Loss Per Share |
Basic loss per Ordinary Share is computed by dividing net loss available to ordinary shareholders by the weighted-average number of Ordinary Shares outstanding during the period. Diluted loss per ordinary share is computed by dividing net loss available to ordinary shareholders by the sum of (1) the weighted-average number of Ordinary Shares outstanding during the period, (2) the dilutive effect of the assumed exercise of share options using the treasury stock method, and (3) the dilutive effect of other potentially dilutive securities.
The following is the calculation of the basic and diluted weighted average shares outstanding for the years ended December 31, 2018, 2017 and 2016, respectively:
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| Company posted | Net loss | Net loss | Net loss | |||||||||
| Basic weighted average shares outstanding | 1,540,309,840 | 1,247,293,388 | 1,177,693,383 | |||||||||
| Dilutive effect of Ordinary Share equivalents | None | None | None | |||||||||
| Dilutive weighted average shares outstanding | 1,540,309,840 | 1,247,293,388 | 1,177,693,383 | |||||||||
For purposes of the diluted net loss per share calculation, share options and warrants are considered to be potentially dilutive securities and are excluded from the calculation of diluted net loss per share because their effect would be anti-dilutive. Therefore, basic and diluted net loss per share was the same for the periods presented due to the Company’s net loss position.
| F-17 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 7 – Loss Per Share (cont.) |
The following table shows the number of share equivalents that were excluded from the computation of diluted loss per share for the respective periods because the effect would have been anti-dilutive:
| Years Ended December 31, | ||||||||||||
| 2018 | 2017 | 2016 | ||||||||||
| Total share options | 94,096,998 | 89,611,998 | 79,372,198 | |||||||||
| Total warrants-equity classified | - | 399,160 | 1,782,246 | |||||||||
| Total warrants-liability classified | - | - | 5,806,280 | |||||||||
| Total share options and warrants | 94,096,998 | 90,011,158 | 86,960,724 | |||||||||
|
NOTE 8 – Taxes |
Tax rates:
The Company is incorporated in England. The effective corporate tax rate applying to a company that is incorporated in England on December 31, 2018 is 19%, reduced from 19.25% from December 31, 2017.
Tax assessment:
The periods open to inquiry by the United Kingdom (“UK”) tax authorities are 2017 and 2018. The Company has not been issued final tax assessments since its establishment in Switzerland.
Deferred taxes:
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Management currently believes that since the Company has a history of losses, it is more likely than not that the deferred tax assets relating to the loss carryforwards and other temporary differences will not be realized in the foreseeable future. Therefore, the Company provided a full valuation allowance to reduce the deferred tax assets.
The main reconciling item between the statutory tax rate of the Company and the effective tax rate is the recognition of valuation allowances in respect of deferred taxes relating to accumulated net operating losses carried forward due to the uncertainty of the realization of such deferred taxes.
At December 31, 2018 and 2017, there were no known uncertain tax positions. The Company has not identified any tax positions for which it is reasonably possible that a significant change will occur during the next 12 months.
The components of income/(loss) before income taxes are as follows:
| December 31, 2018 | December 31, 2017 | December 31, 2016 | ||||||||||
| Domestic (UK) | $ | (16,600,376 | ) | $ | (35,295,613 | ) | $ | (17,956,167 | ) | |||
| Foreign | 133,596 | (103,644 | ) | (184,830 | ) | |||||||
| Loss before income tax | $ | (16,466,780 | ) | $ | (35,399,257 | ) | $ | (18,140,997 | ) | |||
| F-18 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
|
NOTE 8 – Taxes (cont.) |
Income taxes relating to the Company’s operations are as follows:
| December 31, 2018 | December 31, 2017 | December 31, 2016 | ||||||||||
| Current income taxes | ||||||||||||
| Domestic (UK) | - | - | - | |||||||||
| Foreign | - | - | - | |||||||||
| Deferred income taxes | - | - | - | |||||||||
| Domestic (UK) | - | - | - | |||||||||
| Foreign | - | - | - | |||||||||
| Income tax expense/recovery |
Income taxes at the UK statutory rate compared to the Company’s income tax expenses as reported are as follows:
| December 31, 2018 |
December 31, 2017 |
December 31, 2016 |
||||||||||
| Net loss before income tax | $ | (16,466,780 | ) | $ | (35,399,257 | ) | $ | (18,140,997 | ) | |||
| Statutory rate | 19.00 | % | 19.25 | % | 20.00 | % | ||||||
| Expected income tax recovery | (3,128,688 | ) | (6,814,357 | ) | (3,628,199 | ) | ||||||
| Impact on income tax expense/recovery from | ||||||||||||
| Change in valuation allowance | (1,159,720 | ) | 4,210,615 | 1,336,161 | ||||||||
| Permanent differences - liability related to options and warrants | - | (6,706 | ) | (130,061 | ) | |||||||
| Tax rate difference in foreign jurisdictions | 2,686,296 | 801,056 | 80,986 | |||||||||
| Change of tax rate due to U.S. tax reform | - | 2,506,519 | - | |||||||||
| Change of tax rate from prior year | 1,602,112 | (697,127 | ) | 2,341,113 | ||||||||
| Income tax expense | - | - | - | |||||||||
The Company’s deferred tax assets and liabilities consist of the following:
| December 31, 2018 |
December 31, 2017 |
|||||||
| Deferred tax assets | ||||||||
| Stock-based compensation | 1,590,703 | 1,464,527 | ||||||
| Litigation settlement loss | - | 513,000 | ||||||
| Stock option and warrant revaluation | 313,213 | 965,454 | ||||||
| Tax loss carry forward | 17,037,739 | 17,158,394 | ||||||
| Valuation allowance | (18,941,655 | ) | (20,101,375 | ) | ||||
| Deferred tax assets/liabilities | - | - | ||||||
The Company has reviewed its tax positions for its past domestic and foreign tax filings and has not identified any uncertain income tax positions. The Company’s position is to record penalties and interest on any uncertain tax position, if any, and record such items to general and administrative expense.
| F-19 |
AKARI THERAPEUTICS, Plc
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2018, 2017 and 2016
(in U.S. Dollars)
| NOTE 8 – Taxes (cont.) |
At December 31, 2018, the Company had approximately $79.2 million of UK operating loss carryforwards to reduce future UK taxable income. These carryforwards to do not expire. At December 31, 2018, the Company had approximately $20.6 million of foreign operating loss carryforwards in the US and Switzerland. Carryforwards in the US totaling approximately $5.2 million expire beginning in 2032 and current year carryforwards totaling $7.2 million do not expire. The carryforwards in Switzerland expire in seven years. The Company has not conducted a formal analysis and the net operating loss carryovers may be subject to limitation under Internal Revenue Code Section 382 should there have been a greater than 50% ownership change as determined under the regulations.
Research and development credits:
The Company carries out extensive research and development activities and may benefit from the UK research and development tax relief regime, whereby the Company can receive an enhanced UK tax deduction on its research and development activities. Where the Company is loss making for the period it can elect to surrender taxable losses for a refundable tax credit. The losses available to surrender are equal to the lower of the sum of the research and development qualifying expenditure and enhanced tax deduction and the Company’s taxable losses for the period with the tax credit for December 31, 2018 available at a rate of 14.5%. The credit therefore gives a cash flow advantage to Company at a lower rate than would be available if the enhanced losses were carried forward and relieved against future taxable profits.
Qualifying expenditures comprise of chemistry and manufacturing consumables, employment costs for research staff, clinical trials management, and other subcontracted research expenditures.
In December 2017, the Company filed a research and development tax credit claim for 2016 tax year. The credit claim was in the amount of approximately $3,800,000 for the year ended December 31, 2016, was received in March 2018, and was recorded as a credit to research and development costs in the Consolidated Statements of Comprehensive Loss in March 2018. In October 2018, the Company filed a research and development tax credit claim for 2017 tax year. The credit claim was in the amount of approximately $4,900,000 for the year ended December 31, 2017, was received in March 2019, and was recorded as a credit to research and development costs in the Consolidated Statements of Comprehensive Loss in March 2019. Due to the uncertainty of the approval of these tax credit claims and the potential that an election for a tax credit in the form of cash is not made, the Company did not record a related receivable at December 31, 2018.
| NOTE 9 – Segment Information |
The chief operating decision maker (“CODM”) is the Company’s CEO. Neither the CODM nor the Company’s directors receive disaggregated financial information about the locations in which research and development is occurring. Therefore, the Company considers that it has only one reporting segment.
The following table presents the Company’s tangible fixed assets by geographic region:
| December 31, 2018 | December 31, 2017 | |||||||
| United States | $ | 5,735 | $ | 22,103 | ||||
| United Kingdom | 14,690 | 33,795 | ||||||
| Total | $ | 20,425 | $ | 55,898 | ||||
| NOTE 10 – Subsequent Event |
On March 29, 2019, the Company sold to Aspire Capital 5,000,000 Ordinary Shares of the Company for $0.0346 per share (equivalent to $3.46 per ADS) for gross proceeds of $173,000.
| F-20 |