Exhibit 99.1

First Clinical Results from ReZolve2 Trial
San Diego, California and Sydney, Australia (Monday 28 October 2013, AEDT) — REVA Medical, Inc. (ASX: RVA) (“REVA” or the “Company”) announced initial clinical results from patients treated with its ReZolve2 sirolimus-eluting bioresorbable coronary scaffold at Transcatheter Therapeutics 2013 (“TCT”), the world’s leading interventional cardiology conference, which is being held this week in San Francisco, California. The ReZolve2 trial is currently enrolling up to 125 patients at multiple centers in Australia, Brazil, Europe, and New Zealand to provide the data needed to apply for European CE Marking. Patient enrollment was initiated in March 2013 and is currently ongoing.
Reporting on the results was Dr. Ricardo Costa from the Institute Dante Pazzanese of Cardiology in Sao Paulo, Brazil, a leading enrollment center for the trial. “I am pleased to report that in the first 65 patients treated with ReZolve2 who have completed 30-day follow-up, there has been no reported Major Adverse Coronary Events (MACE), with no incidences of ischemic target revascularization (TLR), myocardial infarction (heart attack), or stent thrombosis. In this preliminary analysis, the scaffold is performing well and investigators in the trial continue to appreciate the complete visibility of ReZolve2 as well as the ability to expand the scaffold to the desired implant size with a single inflation.” Dr. Costa also spoke about REVA’s pipeline products. These include new and thinner bioresorbable scaffolds for coronary and peripheral applications that feature the x-ray visibility and distinctive product features of the ReZolve platform.
“These early results in patients treated with ReZolve2 represent an important step toward CE Marking and eventual commercialization of REVA’s first commercial product,” commented REVA’s CEO, Bob Stockman. “We look forward to presenting 30-day data on the remaining patients enrolled in the trial as well as longer-term data at the Paris Course on Revascularization (“EuroPCR”), which will be held next May in Paris, France.”
The presentation materials delivered at the conference are attached hereto. These materials are also being filed with the U.S. Securities and Exchange Commission and are posted under the Investor Relations section of REVA’s website at www.revamedical.com
Dr. Costa will present 12-month data on patients enrolled in the RESTORE clinical trial, which is a pilot study evaluating the safety and performance of the first generation ReZolve® sirolimus-eluting bioresorbable coronary scaffold, during TCT’s bioresorbable oral abstract session on Tuesday, October 29, 2013 PDT.
HEAD OFFICE: 5751 Copley Drive, San Diego, CA 92111 · +1 (858) 966-3000 · +1 (858) 966-3099 (FAX) · www.revamedical.com
AUSTRALIAN OFFICE: Level 6, 175 Macquarie Street, Sydney, NSW 2000 · +61 2 9231 3322 · +61 9229 2727 (FAX) · ARBN 146 505 777
REVA Medical, Inc., is a foreign company incorporated in Delaware, USA, whose stockholders have limited liability.
About REVA
REVA is a development stage medical device company incorporated in Delaware, USA, that is focused on the development and eventual commercialization of its proprietary bioresorbable stent products. The ReZolve® product family, which is in a clinical study phase, combines REVA’s proprietary stent design with a proprietary polymer that is metabolized and cleared from the body. REVA’s anticipated initial commercial product, the ReZolve2 scaffold, is designed to offer full x-ray visibility, clinically relevant sizing, and a controlled and safe resorption rate. In addition, by early encapsulation of the stent in the artery tissue coupled with the loss of scaffold structure over time, the ReZolve2 scaffold may reduce the incidence of late forming blood clots or otherwise reduce long-term disease progression, potential benefits of bioresorbable scaffolds that have yet to be proven. REVA will require clinical results and regulatory approval before it can begin selling the ReZolve2 scaffold.
Forward-Looking Statements
This announcement contains or may contain forward-looking statements that are based on management’s beliefs, assumptions and expectations and on information currently available to management. All statements that are not statements of historical fact, including those statements that address future operating performance and events or developments that we expect or anticipate will occur in the future, are forward-looking statements, such as those statements regarding our ability to obtain the regulatory approvals required to market our ReZolve® scaffold, our ability to timely and successfully complete our clinical trials, our ability to protect our intellectual property position, our ability to commercialize our products if and when approved, our ability to develop and commercialize new products, and our estimates regarding our capital requirements and financial performance, including profitability. You should not place undue reliance on these forward-looking statements. Although management believes these forward-looking statements are reasonable as and when made, forward-looking statements are subject to a number of risks and uncertainties that may cause our actual results to vary materially from those expressed in the forward-looking statements, which risks and uncertainties are described in the “Risk Factors” section of our Annual Report on Form 10-K filed with the United States Securities and Exchange Commission (the “SEC”) on 28 February 2013. Any forward-looking statements in this announcement speak only as of the date when made. REVA does not assume any obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events, or otherwise.
|
United States |
Australia |
|
|
|
|
Investor and Media Enquiries: |
Investor Enquiries: |
|
Cheryl Liberatore |
Kim Jacobs |
|
Director, Investor Relations and Marketing |
Inteq Limited |
|
REVA Medical, Inc. |
+61 2 9229 2700 |
|
+1 858 966-3045 |
|
|
|
Media Enquiries: |
|
|
Haley Price or Rebecca Wilson |
|
|
Buchan Consulting |
|
|
+61 3 9866 4722 |
|
|
ReZolve® Bioresorbable Coronary Scaffold REVA Medical Clinical Program Update Dr. Ricardo Costa Institute Dante Pazzanese of Cardiology Sao Paulo, Brazil |
|
|
Disclosure Statement of Financial Interest I, Ricardo Costa, DO NOT have a financial interest/arrangement or affiliation with one or more organizations that could be perceived as a real or apparent conflict of interest in the context of the subject of this presentation. |
|
|
ReZolve® Sirolimus-Eluting Bioresorbable Coronary Scaffold Drug-Eluting (Sirolimus) Radiopaque Strong and Resilient Polymer Unique Slide & Lock Design Desaminotyrosine -derived polycarbonate material that is radiopaque Limited to clinical investigation only; not approved for sale or distribution |
|
|
Novel Approach for Scaffold Performance Proprietary Polymer Desaminotyrosine-derived polycarbonate Biocompatible for safe resorption X-ray visible to aid in scaffold placement Strong with broad degradation tunability Standard handling (not temp sensitive, no refrig.) High Performance Design Traditional inflation (not stepped) Strength to treat real world lesions Expansion to higher diameters without breaking (3.0 diameter can go up to 3.9 mm during postdilatation) Conforms to vessel shape ReZolve2 ReZolve2 Metal |
|
|
Resorption Pathway * Pietrzak WS. Craniofacial Surg 1997;2:92-96 1.0 . 0.9 . 0.4 . 0.8 . 0.3 . 0.7 . 0.2 . 0.6 . 0.1 . 0.5 . 0 . Time ~ 4 Years Generalized Polymer Degradation Curves* Hydrolysis REVA Polymer Molecular Weight Mass Loss I2DAT, Tyrosol, CO2 Excretion H20 ~85% MW degradation by 12 months Absorption and excretion by 3-4 years |
|
|
Demonstrated Safety ReZolve Scaffold Degradation 1 Month 12 Months 3 Months 48 Months •Pre-clinical studies evaluated in 100s of animals over 7 years •Benign degradation process •Full vasomotion restoration by ~ 12 months •Absorption and excretion within 48 months |
|
|
Degrading and Resorbing Struts SEM Time Lapse Non-Degraded 90+% Resorbed Highly Degraded & Resorbing 0 - 3 Months 6 - 24 Months 36 - 48 Months |
|
|
Release Kinetics of Sirolimus Elution profile comparable to commercially successful products 80ug of Sirolimus abluminally coated on 3.0 x 18mm scaffold |
|
|
ReZolve (gen. 1) Pilot Clinical Trial ReZolve Sirolimus-Eluting Bioresorbable Coronary Scaffold Initiated December 2011 • Enrolled 26 Patients • Primary Endpoint(s): .Freedom from ischemic-driven target lesion revascularization at 6 months .Quantitative measurements at 12 months (QCA/IVUS) |
|
|
Scaffold Specifications •ReZolve (gen. 1) .3.0 mm x 18 mm .7 Fr. Profile .80.mg Sirolimus . Fully radiopaque .Sheathed rapid exchange delivery system .Balloon expandable .No special storage or handling ReZolve Scaffold Clinical Program |
|
|
ReZolve Scaffold Case Sample Serial Imaging Assessment SUMMARY Currently asymptomatic (protocol FU) Clinical Presentation Patient Demographics Age: 63 Gender: Male Type II Diabetes Dyslipidemia Hypertension Risk Factors Stable angina CCS 2 . ReZolve (REVA) bioabsorbable scaffold to OM (June/2012) Past Medical History |
|
|
BASELINE ANGIOGRAPHY SCAFFOLD DEPLOYMENT (3.0 x 18mm) POST DILATATION (3.5 x15mm) Instituto Dante Pazzanese de Cardiologia São Paulo, Brazil Rezolve Scaffold Case Sample |
|
|
ReZolve Scaffold Case Sample Baseline FINAL RESULT Acute ISA A B C A B C A B C Acute ISA |
|
|
4 - Month Angiographic Follow-up |
|
|
4-Month OCT Evaluation Resolved Malapposition |
|
|
Main Arena São Paulo, Brazil Instituto Dante Pazzanese de Cardiologia 3D FD-OCT - 4 Month Follow-up Proximal Distal |
|
|
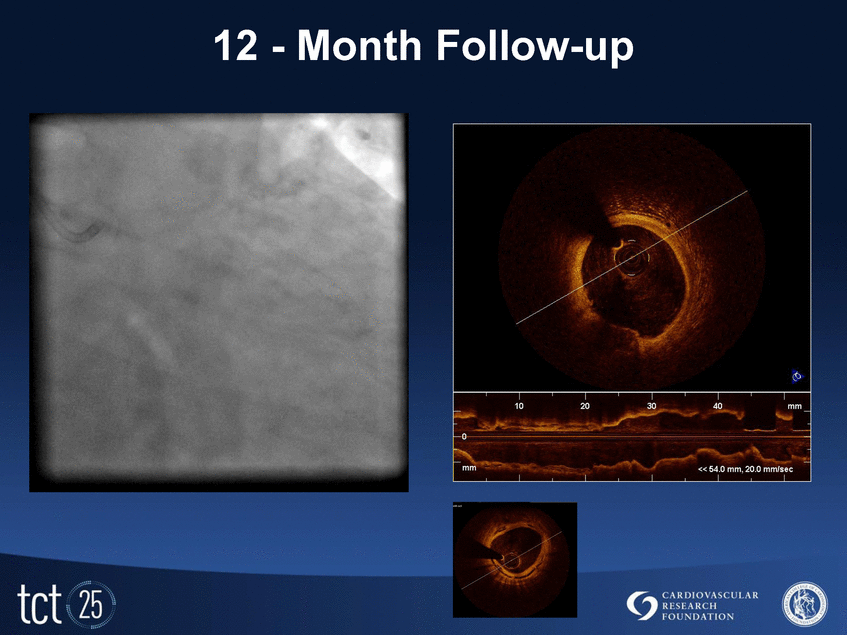
12 - Month Follow-up |
|
|
ReZolve Pilot Clinical Trial Acute Procedural Outcomes Technical Success (1) 85% n=26 Acute Procedural Success (2) 100% n=22 Clinical Procedural Success (3) 100% n=22 (1) Defined as successful delivery and deployment of the device. Four devices not delivered primarily due to profile (2) Defined as technical success with residual stenosis <20%. Estimated average pre-treatment stenosis = 70% (3) Defined as acute procedure success without the occurrence of MACE through 30 days |
|
|
ReZolve Pilot Clinical Trial •Interim data on 8 patients presented at PCR in May .0.20 mm late lumen loss (1) One additional TLR directly related to protocol deviation at implant (2) Patient completed 6-month follow-up with negative stress test •All 22 patients have now completed 12-month follow-up .Updated data set to be presented on Tuesday at 3:00 p.m. .Bioresorbable Abstract Session •All patients will be followed for an additional four years Preliminary MACE Data 30- and 90-Day Results 0 MACE Events n=22 6-Month Results 1 study-related TLR (1) n=22 12-Month Preliminary Results 2 study-related TLRs and 1 death (2) n=13 |
|
|
ReZolve2 Clinical Program CE trial initiated March 2013 •Evaluating Safety & Efficacy in up to 125 patients •Vessel/Lesion Criteria .2.75mm to 3.3mm AVD .Scaffold expandable up to 4.0 mm .Lesions up to 14 mm in length •Primary Endpoint(s) .MACE at 6 & 12 months .Late Lumen Loss at 9 months |
|
|
ReZolve Clinical Program Scaffold Specifications •ReZolve (gen. 1) .3.0 mm x 18 mm .7 Fr. Profile .80mg Sirolimus .Fully radiopaque .Sheathed rapid exchange delivery system .Balloon expandable .No special storage or handling •ReZolve2 .3.0 mm x 18 mm .6 Fr. Profile .80mg Sirolimus .Fully radiopaque .Non-Sheathed rapid exchange delivery system .Balloon expandable .~30% higher radial strength .No special storage or handling |
|
|
ReZolve2 Case Example Radial Delivery Pre-Implant Post-Implant |
|
|
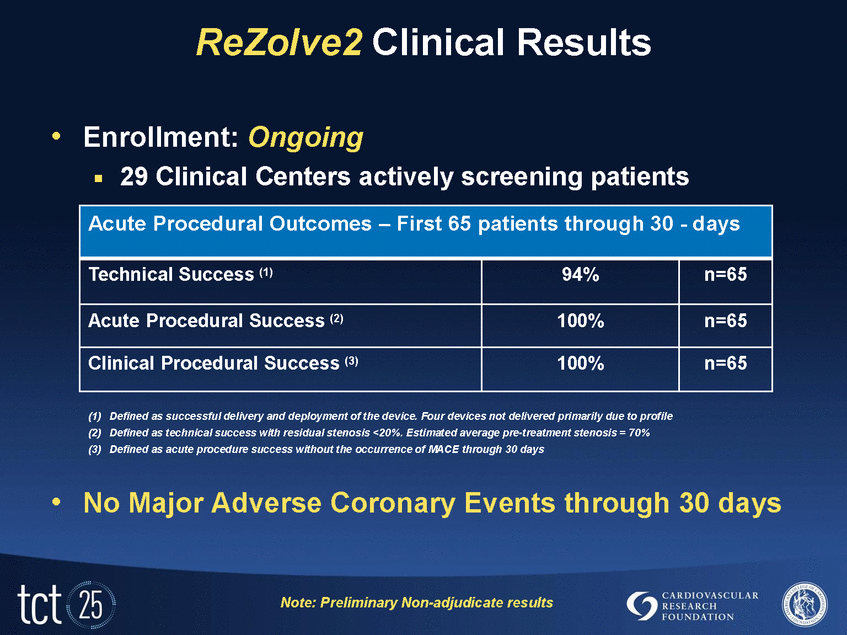
ReZolve2 Clinical Results •Enrollment: Ongoing .29 Clinical Centers actively screening patients •No Major Adverse Coronary Events through 30 days Note: Preliminary Non-adjudicate results Acute Procedural Outcomes – First 65 patients through 30 - days Technical Success (1) 94% n=65 Acute Procedural Success (2) 100% n=65 Clinical Procedural Success (3) 100% n=65 (1)Defined as successful delivery and deployment of the device. Four devices not delivered primarily due to profile (2)Defined as technical success with residual stenosis <20%. Estimated average pre-treatment stenosis = 70% (3)Defined as acute procedure success without the occurrence of MACE through 30 days |
|
|
REVA Pipeline Unique Slide & Lock design Complete scaffold visibility Exceptional strength for challenging lesions Accurate placement in complex anatomy ReZolve2 Scaffold Next Coronary Scaffold REVA Metal DES Uniform strut thickness (~150 µm) Complete scaffold visibility Minimal recoil Single-step inflation Enhanced deliverability Future Scaffolds High performance biomaterials Ultra-thin coronary scaffold (~100 µm strut thickness) Self-expanding scaffold for SFA Current bench-level evaluation ongoing REVA’s pipeline addresses future clinical requirements for BRS |
|
|
Thank you |