[***] Certain information in this document has been excluded pursuant to Regulation S-K, Item 601(b)(10). Such excluded information is not material and would likely cause competitive harm to the registrant if publicly disclosed.
Exhibit 10.8
NON-EXCLUSIVE LICENSE AGREEMENT
between
Heart Test Laboratories, Inc.
and
Icahn School of Medicine at Mount Sinai
TABLE OF CONTENTS |
||
1. |
DEFINITIONS |
2 |
2. |
LICENSE GRANT |
10 |
3. |
DUE DILIGENCE |
11 |
4. |
FEES, ROYALTIES, AND PAYMENTS |
12 |
5. |
INTENTIONALLY RESERVED |
13 |
6. |
REPORTS |
13 |
7. |
CONFIDENTIALITY; PUBLICITY; USE OF NAME |
16 |
8. |
PATENT PROSECUTION |
18 |
9. |
REPRESENTATIONS; DISCLAIMER OF WARRANTIES; LIMITATION OF LIABILITIES |
18 |
10. |
INDEMNIFICATION |
20 |
11. |
INSURANCE |
21 |
12. |
TERM AND TERMINATION |
22 |
13. |
EFFECT OF TERMINATION |
23 |
14. |
ADDITIONAL PROVISIONS |
24 |
Exhibit A: Securities Purchase Agreement
Exhibit B: Licensed Patents
Exhibit C: Link to Licensee 10-K
Exhibit D: Initial Development Plan
Exhibit E: Form of Quarterly Royalty Report
1
Non-Exclusive License Agreement
This Non-Exclusive License Agreement (this “Agreement”) is by and between Icahn School of Medicine at Mount Sinai, a New York not-for-profit education corporation, with a principal place of business at One Gustave L. Levy Place, New York, NY 10029 (“Mount Sinai”) and Heart Test Laboratories, Inc. d/b/a HeartSciences a Texas corporation, with a principal place of business at 550 Reserve Street, Suite 360, Southlake, TX 76092 (referred to herein as (“Licensee”). This Agreement shall become effective upon the Closing (the “Effective Date”), which shall be deemed the Closing Date. Mount Sinai and Licensee are each referred to herein as a “Party” and collectively as the “Parties.” Terms not defined herein shall have the meaning ascribed to them in the Securities Purchase Agreement between the Parties, executed contemporaneously herewith (the “SPA”) and annexed hereto as Exhibit A.
WHEREAS, Mount Sinai has created and owns certain intellectual property relating to screening for and diagnosis of cardiovascular disease;
WHEREAS, Licensee wishes to obtain from Mount Sinai certain rights to such intellectual property and to develop and commercialize Licensed Products (as defined below);
WHEREAS, Mount Sinai has determined that the exploitation of the intellectual property by Licensee subject to the terms and conditions of this Agreement is in the best interest of Mount Sinai, consistent with Mount Sinai’s educational, research, and public health missions and goals; and
WHEREAS, the Parties are contemporaneously entering into additional non-exclusive licenses (the “Non-Exclusive Licenses”) and exclusive licenses (the “Exclusive Licenses”) with respect to screening for and diagnosis of cardiovascular disease;
WHEREAS, the Parties are contemporaneously entering into the SPA pursuant to which, in consideration for entering into this Agreement, Licensee wishes to issue to Mount Sinai certain equity securities of the Licensee upon the Closing Date;
NOW THEREFORE, in consideration of the mutual rights and obligations contained in this Agreement, and intending to be legally bound, the Parties agree as follows:
1. DEFINITIONS
1.1. “Calendar Year” means January 1 through December 31 of a given year.
1.2. “Change of Control” means, with respect to any entity, the occurrence of any one or more of the following: (1) the acquisition, whether directly, indirectly, beneficially or of record, by any individual, entity or group of fifty percent (50%) or more of the ownership of such entity, whether by merger, consolidation, sale or other transfer of Licensee Shares in a single transaction or series of related transactions (other than (i) a bona fide equity financing by such entity for capital raising purposes and (ii) a transaction where one or more of the equity owners of such entity who controlled a majority of the outstanding voting securities prior to the transaction are the holders of a majority of the voting securities of the entity that survives such transaction); or (2) the sale, lease,
2
transfer, exclusive license or other disposition, in a single transaction or series of related transactions, by such entity of all or substantially all the assets of such entity.
1.3. “Combination Product” means a Licensed Product that: (a) is sold, combined or bundled with one or more algorithms that are not licensed to Licensee by Mount Sinai (in this Agreement or any other agreement), in addition to the Non-Exclusively Licensed Technical Information and rights therein; and (b) the additional algorithm is capable of being sold as a separate product or service. Notwithstanding the foregoing, to qualify as a Combination Product, such product or service and all its components (e.g. algorithms) must be sold together as a single product and invoiced as one product.
1.4. “Commercial Sale” means any bona fide transaction with a Third Party for which consideration is received for the sale, use, lease, transfer or other disposition of a Licensed Product by or on behalf of Licensee, including without limitation, through any subscription model. Commercial Sale is deemed completed when a bona fide transaction qualifies as a Gross Sale.
1.5. “Commercialization” means any and all activities related to the manufacturing, promotion, distribution, marketing, offering for sale and selling of or otherwise granting rights to a product, including advertising, educating, planning, obtaining, supporting and maintaining pricing and reimbursement approvals and Regulatory Authorizations, managing and responding to adverse events involving the product, pricing, price reporting, marketing, promoting, detailing, storing, handling, shipping, distributing, importing, exporting, using, offering for sale, or selling a product anywhere in the world. Commercialization excludes Development activities. When used as a verb, “Commercialize” means to engage in Commercialization.
1.6. “Commercially Reasonable Efforts” means, with respect to Licensee’s obligations under this Agreement, the diligent and continuous dedication and expenditure of efforts, money, personnel, and other resources, consistent with those that companies of similar size, type, characteristics, and position, reasonably necessary, as soon as reasonably practicable, to fulfill Licensees obligations under this Agreement including the obligation to utilize the licensed rights to Exploit the Licensed Product. Such efforts must be reasonably documented and be reasonably consistent with those that companies of similar size, type, circumstance, and position have reasonably used in actively, diligently and successfully pursuing the research, Development, Manufacturing or Commercialization of a similarly situated , product, or service at a similar stage of Development or Commercialization as the applicable Licensed Product. At a minimum, Commercially Reasonable Efforts shall require material compliance with the Initial Development Plan and subsequent Development Plans as updated, that are submitted to Mount Sinai by Licensee as required under this Agreement. In determining Commercially Reasonable Efforts with respect to a particular Licensed Product, Licensee may not reduce such efforts due to the competitive, regulatory or other impact of any other product, or asset that is Developed, Commercialized, or Controlled by Licensee.
1.7. “Confidential Information” shall have the meaning assigned in Article 7.
1.8. “Control” or “Controlled” shall mean, with respect to any Patent, Non-Exclusively Licensed Technical Information, Know-How, or other intellectual property right or other intangible property, an Entity’s ownership or the possession (whether by ownership, license
3
or “control” over an affiliated entity having possession by ownership or license) of the ability to grant access to, or a license or sublicense to, such Patent, Non-Exclusively Licensed Technical Information, Know-How, rights or property. For purposes of this definition, “control” and its various forms means the possession, directly or indirectly, of the power to direct or cause the direction of the management and policies of such Entity, whether through ownership of voting securities, by contract or otherwise. Without limiting the generality of the foregoing, the Licensee will be deemed to control another Entity if the Licensee owns or directly or indirectly controls more than fifty percent (50%) of the voting stock or other securities of the Entity.
1.9. “Development” means any and all activities related to researching or developing a product or process or service, including preclinical and clinical research, testing and development activities relating to the discovery and/or development of product or process candidates and submission of information and applications to a Regulatory Authority, including toxicology, pharmacology, and other discovery, optimization, and preclinical efforts, test method development and stability testing, manufacturing process development, formulation development, upscaling, validation, delivery system development, quality assurance and quality control development, statistical analysis, managing and responding to adverse events involving a product. When used as a verb, “Develop” means to engage in Development.
1.10. “Development Plan” means the then-current version of Licensee’s plan for the Exploitation by Licensee of the Licensed Patents, Non-Exclusively Licensed Technical Information, and Know-How as such plan may be adjusted or updated from time to time e.g. as contemplated by Section 3.1. For clarity no updated Development Plan will be effective until agreed to in writing by both Parties.
1.11. “Derivative Work(s)” means any improvement, product, service, software, or algorithm created by or on behalf of Licensee, that is based upon or created in whole or in part through, the decompiling, reverse engineering, use or analysis of, or that includes or incorporates any portion of, the Mount Sinai Technology. For avoidance of doubt, all Derivative Works shall be Licensed Products.
1.12. “EMA” means the European Medicines Agency or any successor Entities thereto.
1.13. “Entity” means a corporation, an association, a joint venture, a partnership, a trust, a business, an institution, an individual, a government or political subdivision thereof, including an agency, or any other organization that can exercise independent legal standing.
1.14. “Exploit” means, collectively, to Develop and Commercialize, including to Manufacture, to have Developed, to have Manufactured, to have Commercialized, and otherwise to commercially exploit. “Exploitation” has a correlative meaning.
1.15. “Fair Market Value” means (a) in the case of arm’s length sale of a Licensed Product, (i) the cash consideration that Licensee has realized from such sale, or (ii) if there have been no such sales or such sales have been insufficient, the cash consideration that Licensee would have realized from an unaffiliated, unrelated buyer in an arm’s length sale of Licensed Product in the same quantity, under the same terms, and at the same time and place as the sale for which Fair Market Value is being determined; (b) in the case of non‑cash consideration received in a sale of
4
a Licensed Product or in a transaction giving rise to Sublicense Income, the cash value of such consideration; or (c) in the case of determining the portion of proceeds from an issuance of equity to be included in Sublicense Income, the value of the issued equity as then most recently determined under U.S. Internal Revenue Code § 409A for purposes of the Licensee’s equity grants (or, if the class of equity issued has not then been so valued, then a value based on the value of a class of equity that has been so valued, taking into account differences between the rights and preferences of the class of equity issued and those of the class of equity then most recently valued).
1.16. “FDA” means the United States Food and Drug Administration or any successor Entities thereto.
1.17. “Field of Use” means screening for, or diagnosis of, cardiovascular disease in humans using electrocardiogram ECG data.
1.18. "Finished Product” means a Licensed Product sold, licensed, or otherwise transferred by the Licensee to a Third Party without modification by the Third Party.
1.19. “First Commercial Sale” means, on a Jurisdiction-by-Jurisdiction basis and Licensed Product-by-Licensed Product basis, the first time a Commercial Sale is made.
1.20. “Good Clinical Practices” means the then-current standards, practices and procedures for good clinical practices in the conduct of clinical trials, including adequate human subject protections, as promulgated or endorsed by the FDA and other applicable Governmental Authorities, such as set forth in, “International Conference on Harmonization - Guidance for Industry E6 Good Clinical Practice: Consolidated Guidance,” or as otherwise required by applicable Law.
1.21. “Good Laboratory Practices” means the then-current standards, practices and procedures for good laboratory practices by facilities that perform non-clinical (including pre-clinical) laboratory studies, as promulgated or endorsed by the FDA and other applicable Governmental Authorities, including as set forth in 21 C.F.R. Part 58, or as otherwise required by applicable Law.
1.22. “Good Manufacturing Practices” means the then-current standards, practices and procedures for the manufacture of drugs or medical devices, as applicable to the Licensed Products (including the practices of and methods to be used in, and the facilities or controls to be used for, the manufacture, processing, packaging, sterilizing, labeling, testing or holding of the Licensed Products), as promulgated or endorsed by the FDA and other applicable Governmental Authorities, including, as applicable, as set forth in 21 C.F.R. Parts 210, 211, and 820, or as otherwise required by applicable Law.
1.23. “Governmental Authority” means any supranational, national, federal, state, provincial, local or foreign Entity of any nature exercising executive, legislative, judicial, regulatory or administrative functions of or pertaining to government, including any governmental authority, agency, department, board, commission, court, tribunal, judicial body or instrumentality of any union of nations, federation, nation, state, municipality, county, locality or other political subdivision thereof.
5
1.24. “Gross Sales” means the gross amounts actually received from a Third Party by Licensee for Commercial Sales. A Licensed Product shall be considered sold for purposes of calculating Gross Sales when it is paid. In the event Licensee transfers a Licensed Product to a Third Party in a bona fide arm’s length transaction, for any consideration other than cash, then the Gross Sales price for such Licensed Product shall be deemed to be the standard invoice price then being invoiced by Licensee, as applicable, in an arm’s length transaction with similar companies. In the absence of such standard invoice price, then the Gross Sales price shall be the Fair Market Value of the Licensed Product.
1.25. “Health Care Law” means all applicable Laws relating in any way to patient care and human health and safety, including such Laws pertaining to: (a) the Development, Manufacture and Commercialization of drugs and medical devices, including, without limitation, the United States Food, Drug and Cosmetic Act, the Public Health Service Act, the regulations promulgated thereunder (including with respect to Good Clinical Practices, Good Laboratory Practices and Good Manufacturing Practices), and equivalent applicable Laws of other Governmental Authorities; and (b) the reimbursement and payment for health care products and services, including any United States federal health care program (as such term is defined in 42 U.S.C. § 1320a-7b(f)), and programs and arrangements pertaining to providers of health care products or services that are paid for by any Governmental Authority or other Entity, including the federal Anti‑Kickback Statute (42 U.S.C. § 1320a-7b(b)), the civil False Claims Act (31 U.S.C. § 3729 et seq.), the administrative False Claims Law (42 U.S.C. § 1320a-7b(a)), 42 U.S.C. § 1320a-7 and 42 U.S.C. § 1320a-7a, and the regulations promulgated pursuant to such statutes, Medicare (Title XVIII of the Social Security Act) and the regulations promulgated thereunder, Medicaid (Title XIX of the Social Security Act) and the regulations promulgated thereunder, and equivalent applicable Laws of other Governmental Authorities; and (c) the privacy and security of patient-identifying information, including, without limitation, the Health Insurance Portability and Accountability Act of 1996 (42 U.S.C. § 1320d et seq.) and the regulations promulgated thereunder and equivalent applicable Laws of other Governmental Authorities; in each of the foregoing (a) through (c), as may be amended from time to time.
1.26. “Initial Development Plan” means the initial Development Plan for the Exploitation by Licensee of the Licensed Patents, Non-Exclusively Licensed Technical Information, and Know-How, to be provided by Licensee to Mount Sinai within thirty (30) days of the Effective Date, which Development Plan shall be attached at Exhibit D, incorporated into and made part of this Agreement.
1.27. “Jurisdiction” means a geographic area (e.g. country or region) in which any Licensed Product is Exploited.
1.28. “Know-How” means any and all technical, scientific and other knowledge and information regarding technology, methods, processes, practices, formulae, assays, instructions, skills, techniques, procedures, experiences, ideas, technical assistance, designs, drawings, assembly procedures, specifications, data and/or results relating solely to Licensed Products, in all cases whether or not confidential, proprietary, patented, patentable, existing in written or electronic form developed in the laboratory of one or more inventors or authors of the Licensed Patents or Non-Exclusively Licensed Technical Information, prior to the Effective Date of this Agreement. For clarity, Know-How does not include any Exclusively Licensed Technical Information,
6
Non-Exclusively Licensed Technical Information, or any Patent rights of Mount Sinai, including without limitation, software, algorithms, or formulae, licensed to Licensee under any of the Exclusive Licenses or Non-Exclusive Licenses.
1.29. “Laws” means all active governmental constitutions, laws, statutes, ordinances, treaties, rules, common laws, rulings, regulations, orders, charges, directives, determinations, executive orders, writs, judgments, injunctions, decrees, restrictions or similar legally effective pronouncements of any Governmental Authority.
1.30. “Licensed Patents” means the Patents owned or Controlled by Mount Sinai as of the Effective Date and listed on Exhibit B hereto. Notwithstanding the preceding definition, Licensed Patents shall not include any Patent based in whole or part on research conducted after the Effective Date, except as otherwise agreed in a separate writing executed by the Parties.
1.31. “Licensed Product” means any product or service, the exploitation, development, manufacturing, commercialization, use, rental or lease of which (a) is covered by at least one Valid Claim or (b) arises from the use of, involves the use of, or incorporates, in whole or in part, any Non-Exclusively Licensed Technical Information.
1.32. “Licensed Product Data” means data (including clinical data) that is possessed, owned or Controlled by Licensee directly relating to any Licensed Product and generated after the Effective Date.
1.33. “Manufacturing” means all activities directed to sourcing of necessary raw materials, producing, processing, packaging, labeling, quality assurance testing, release of a Licensed Product or Licensed Product candidate, whether for Development or Commercialization. When used as a verb, “Manufacture” means to engage in Manufacturing.
1.34. “Mount Sinai Technology” means the Licensed Patents, Non-Exclusively Licensed Technical Information, and Know-How.
1.35. “Net Sales” means all Gross Sales of Licensed Product less the total of the following: deductions to the extent they are included in the gross invoiced sale price of the Licensed Product or otherwise directly paid or incurred by Licensee with respect to such sale of the Licensed Product:
(a) trade, cash and/or quantity discounts, retroactive price reductions, chargeback payments and rebates actually allowed and taken by purchasers of a Licensed Product or Third Party payors, including discounts and rebates to governmental payors or managed care organizations, their agencies, purchasers and reimbursers, and allowances or credits to Third Parties for rejections or returns that do not exceed the original invoice amount;
(b) taxes, tariffs, duties and governmental charges required by law that are applicable to the sale, transportation or delivery of Licensed Product that Licensee has to pay on such sales, transportation or delivery of Licensed Product (including annual fees due under Section 9008 of the United States Patient Protection and Affordable Care Act of 2010 (Pub. L. No. 111-48)); and
7
(c) required outbound transportation and insurance charges prepaid or allowed, but not separately reimbursed by the purchaser.
In the event Licensee transfers a Licensed Product to a Third Party in a bona fide arm’s length transaction, for any consideration other than cash, then the Net Sales price for such Licensed Product shall be deemed to be the standard invoice price then being invoiced by Licensee, as applicable, in an arm’s length transaction with similar companies. In the absence of such standard invoice price, then the Net Sales price shall be the Fair Market Value of the Licensed Product. Components of Net Sales shall be determined in the ordinary course of business using the accrual method of accounting in accordance with generally accepted accounting principles, consistently applied.
Any Write Offs that are at any time thereafter collected, in whatever amount, shall be deemed a Net Sale and will be subject to the running royalties pursuant to Section 4.1 hereunder.
No deductions shall be made from Net Sales for commissions paid to individuals whether they are (i) with independent sales agents or agencies or (ii) regularly employed by Licensee on its or their payroll, or (iii) for the cost of collections.
For the avoidance of doubt, disposal of any Licensed Product without charge for use in any clinical trials, as free samples, or under compassionate use, patient assistance, named patient or test marketing programs or non-registrational studies or other similar programs or studies where Licensed Product is supplied or delivered without charge, shall not result in any Net Sales. No Licensed Product donated by Licensee to non-profit institutions or government agencies for a non-commercial purpose shall result in any Net Sales.
If Licensee sells, leases or otherwise Commercializes any Licensed Product at a reduced fee or price for the purpose of promoting other products, goods or services (except when those other products, goods or services are solely other Licensed Products from Mount Sinai) or for the purpose of facilitating the sale, license or lease of other products, goods or services (except when those other products, goods or services are solely other Licensed Products from Mount Sinai), then notwithstanding anything herein to the contrary, Mount Sinai shall be entitled to payments under Article 4 based upon the Fair Market Value of the Licensed Product.
In the event that Licensee contracts with a Third Party (whether such Third Party is a Third Party authorized representative, importer or distributor) for such Third Party to sell Finished Products to Third Party end users (including hospitals, healthcare institutions or direct sale to patients (as opposed to resale)), such Third Party shall be considered a “Distributor” and Net Sales shall be calculated off the price charged for such Finished Product by the Distributor to the Third Party end user.
1.1. “Non-Exclusively Licensed Technical Information” means the software, algorithms, formulae, and other technology and information identified as Non-Exclusively Licensed Technical Information in Exhibit B hereto, together with all copyright protection therein. For clarity, Non-Exclusively Licensed Technical Information includes any information contained within Licensed Patents.
8
1.2. “Open Source License Terms” means terms in any license agreement or license grant that require, as a condition of use, modification and/or distribution of a work:
i. the making available of Source Code, design descriptions or other materials for modification, or
ii. the granting of permission for creating derivative works, or
iii. the reproduction of certain notices or license terms in derivative works or accompanying documentation, or
iv. the granting of a royalty-free license to any party under intellectual property rights regarding the work and/or any work that contains, is combined with, requires, or otherwise is based on the work.
1.3. “Open Source Software” means any software that is licensed under Open Source License Terms.
1.4. “Patents” means (a) United States and foreign patents and/or patent applications; (b) any and all patents issuing from the foregoing; (c) any and all claims of continuation-in-part applications that claim priority to the United States patent applications, but only where such claims are directed to inventions disclosed in the manner provided in the first paragraph of 35 U.S.C. § 112 in such United States patent applications, and such claims in any patents issuing from such continuation-in-part applications; (d) any and all foreign patent applications, foreign patents, or related foreign patent documents that claim priority to the patents and/or patent applications; and (e) any and all divisionals, continuations, reissues, re-examinations, renewals, substitutions, and extensions of the foregoing.
1.5. “Prosecution” means the filing, preparation, prosecution (including any interferences, reissue proceedings, reexaminations, and oppositions), extension, term adjustment, and maintenance of Licensed Patents. When used as a verb, “Prosecute” means to engage in Prosecution.
1.6. “Quarter” means each three-month period beginning on January 1, April 1, July 1 and October 1 of each Calendar Year; provided, however, that as it relates to the Commercial Sale of Licensed Products, the first Quarter shall be comprised of the time period beginning on the date of First Commercial Sale and ending at the end of the Quarter during which such First Commercial Sale occurs. “Quarterly” means once during each Quarter.
1.7. “Quarterly Reports” shall have the meaning assigned in Article 6.
1.8. “Regulatory Approval” means, with respect to a country or other jurisdiction, all approvals, licenses, clearances, marks, registrations, authorizations certificates, exemptions, consents, franchises, concessions, notices or other like item of or issued by any Governmental Authority, from the relevant Governmental Authority necessary or useful to commercially distribute, sell or market a Licensed Product in such country or other applicable jurisdiction (not
9
including any applicable pricing and governmental reimbursement approvals unless legally required to market the Licensed Product in a country or other applicable jurisdiction).
1.9. “Regulatory Authority” means any applicable Governmental Authority involved in granting Regulatory Approval for, and responsible for the regulation of, the Licensed Product in any Jurisdiction, including the FDA, EMA, and any corresponding Governmental Authority.
1.10. “Royalty Term” means, on a Licensed Product-by-Licensed Product and jurisdiction-by-jurisdiction basis, starting with the date of the First Commercial Sale of such Licensed Product in such jurisdiction until Licensee notifies Mount Sinai in writing that it has ceased marketing and made its last Commercial Sale of a Licensed Product.
1.11. “Source Code” means the compilable and human-readable version of software, including without limitation, all comments and procedural code, associated flow charts, concepts, algorithms, instructions and all related preparatory materials.
1.12. “Term” means from the Effective Date until Licensee notifies Mount Sinai in writing that it has ceased marketing and made its last Commercial Sale of a Licensed Product.
1.13. “Territory” means worldwide.
1.14. “Third Party” means any Entity other than a Party.
1.15. “Valid Claim” means (a) an unexpired claim of an issued Patent within the Licensed Patents that has not been ruled unpatentable, invalid or unenforceable by a final and unappealable decision of a court or other competent authority in the subject Jurisdiction; or (b) a pending claim of a Patent application within the Licensed Patents.
2. LICENSE GRANT
2.1. Non-Exclusive Patent License. Subject to the terms and conditions set forth herein, immediately upon, and contemporaneously with, the Closing Date, and without further action by the Parties, Mount Sinai hereby grants to Licensee a non-sublicensable, non-transferable, royalty-bearing, non-exclusive license to the Licensed Patents identified in Exhibit B, solely to Exploit Licensed Products in the Field of Use, during the Term, throughout the Territory. For clarity and avoidance of doubt, this license grant does not permit Licensee to disclose or transfer any rights in the Licensed Patents to any Third Party.
2.2. Non-Exclusive License to Non-Exclusively Licensed Technical Information. Subject to the terms and conditions set forth herein, immediately upon, and contemporaneously with, the Closing Date, and without further action by the Parties, Mount Sinai hereby grants to Licensee a non-sublicensable, non-transferable, royalty-bearing, non-exclusive license to the Non-Exclusively Licensed Technical Information identified in Exhibit B, solely to Exploit Licensed Products in the Field of Use, during the Term, throughout the Territory. For clarity and avoidance of doubt, this license grant does not permit Licensee to disclose or transfer any rights in the Non-Exclusively Licensed Technical Information to any Third Party.
10
2.3. Non-exclusive Know-How License. Subject to the terms and conditions set forth herein, Mount Sinai hereby grants to Licensee a non-sublicensable, non-transferable, royalty-bearing non-exclusive license to the Mount Sinai Know-How, solely to the extent Mount Sinai agrees it is reasonably required to exploit the non-exclusive rights granted in Sections 2.1 and 2.2 in the Field of Use, during the Term, and throughout the Territory.
2.4. Retained Rights. The grants provided hereunder are: (a) non-exclusive and therefore Mount Sinai retains all rights to use and otherwise exploit such rights and permit others to use and otherwise exploit such rights non-exclusively in the Field of Use and non-exclusively or exclusively outside the Field of Use, including via licensing, for any purpose; and (b) subject to and contingent upon Licensee’s compliance with all of its obligations hereunder including, but not limited to, the payment by Licensee to Mount Sinai of all consideration required under this Agreement.
2.5. Government Rights. All rights and licenses granted by Mount Sinai to Licensee under this Agreement are subject to (a) any limitations imposed by the terms of any grant, contract or cooperative agreement by any Governmental Authority applicable to the technology that is the subject of this Agreement, and (b) applicable requirements of 35 U.S.C. § 200 et seq., as amended, and implementing regulations and policies. Without limitation of the foregoing, Licensee agrees that, to the extent required under 35 U.S.C. § 204, any Licensed Product used, sold, distributed, rented or leased by Licensee in the United States will be Manufactured substantially in the United States.
2.6. No Implied Licenses. Except as expressly provided under this Article 2, no right or license is granted under this Agreement (expressly or by implication or estoppel) by Mount Sinai to Licensee under any tangible or intellectual property, materials, patent, patent application, trademark, copyright, technical information, data, or other proprietary right.
3. DUE DILIGENCE
3.1. Development Plan. The Initial Development Plan shall be provided by Licensee to Mount Sinai within thirty (30) days of the Effective Date and become a part of this Agreement upon the written consent of the Parties. With respect to each Calendar Year following the Effective Date, Licensee shall deliver to Mount Sinai an annual updated Development Plan in accordance with Section 6.5, which shall set forth in reasonable detail the planned Development activities for such Calendar Year and the subsequent Calendar Year, as well as the anticipated timeline and budget for such activities. Such updated Development Plan shall replace the prior Development Plan and become incorporated into and a part of this Agreement only upon written approval of Mount Sinai of such updated Development Plan. Licensee has not fulfilled its obligations under this Section 3.1 until such approval of Mount Sinai of such updated Development Plan is provided. Licensee will promptly provide additional information as reasonably requested by Mount Sinai.
3.2. Commercially Reasonable Efforts. Throughout the Term and at Licensee’s sole cost and expense, Licensee shall use no less than Commercially Reasonable Efforts to Develop and Commercialize the Licensed Products in the Field of Use and Territory as soon as reasonably practicable. Licensee shall maintain such active diligent Commercially Reasonable Efforts to
11
Develop and Commercialize the Licensed Products at all times throughout the Term. Solely seeking a Sublicense is not Commercially Reasonable Efforts.
3.3. Due Diligence Events. In addition, Licensee shall perform at least the following obligations as part of its Commercially Reasonable Efforts to Develop and Commercialize the Licensed Products required under this Article 3:
(a) Materially perform the activities set forth in the applicable Development Plan, unless bona fide circumstances arise that make the achievement of the Development Plan impractical or the Licensee is unable to perform its obligations pursuant to the Development Plan due to the actions or omissions of Mount Sinai.
(b) Licensee raises Additional Financing pursuant to the terms and conditions of the SPA.
3.4 Failure to Achieve Due Diligence Events. If Licensee fails to exercise Commercially Reasonable Efforts to achieve the above due diligence obligation or, if despite consistent use of Commercially Reasonable Efforts, Licensee is unable to achieve the due diligence events set forth in Section 3.3 above, then Mount Sinai at its option, in its sole discretion, may: (a) terminate this License in whole or in part immediately upon provision of written notice to Licensee; (b) meet with License to arrange for revision of the due diligence events; or (c) require that Licensee sublicense the License in whole or in part to a party selected by Mount Sinai. It is agreed and understood that in the event Licensee fails to achieve the due diligence events set forth in Section 3.3 above and has not consistently used Commercially Reasonable Efforts to do so, then Mount Sinai may exercise any and all remedies available at law or otherwise.
4. FEES, ROYALTIES, AND PAYMENTS
4.1. Running Royalties. As additional consideration for the license and other rights granted under this Agreement, during the Royalty Term, Licensee shall pay to Mount Sinai the annual percentage of Net Sales on a Licensed Product-by-Licensed Product basis as follows:
Worldwide Net Sales for the Applicable Calendar Year in the Territory |
Running Royalty Percentage |
For Net Sales of a Licensed Product exploiting a Valid Claim |
[***]% |
For Net Sales of a Licensed Product not exploiting a Valid Claim |
[***]% |
For the avoidance of doubt, the running royalties outlined in Section 4.1 above are payable on an annual worldwide Net Sales basis, cumulative for each Calendar Year, within thirty (30) days of December 31st of each Calendar Year.
If a Licensed Product is sold, combined or bundled as a single product with one or more other products licensed to Licensee by Mount Sinai (in this Agreement or any other agreement) and
12
invoiced as one product, then the running royalty payable across all such licenses shall be the highest applicable royalty percentage and not the aggregate of each license running royalty added together.
4.2. Combination Products. In the event that a Licensed Product is sold as a Combination Product, the Net Sales of such Licensed Product for the purpose of calculating royalties owed under this Agreement shall be determined on a country-by-country basis by multiplying the Net Sales of such Combination Product as defined in Section 1.3, by the fraction A/(A+B), where “A” is the average sale price of the Licensed Product in the relevant country if sold separately, and “B” is the average sale price of additional algorithm(s) in such country, if sold separately. Regarding prices comprised in the average price when sold separately referred to above, if these are available for different use volumes of the Licensed Product or additional algorithm(s) than those that are included in the Combination Product, then Licensee shall be entitled to make a proportional adjustment to such prices in calculating the royalty-bearing Net Sales of the Combination Product. In the event that the additional algorithm(s) are other Licensed Products from Mount Sinai the total Net Sales of the Combination Product shall be pro-rated between the number of such Licensed Products sold. In the event that separate sales of products or services incorporating the additional algorithm were not made during the preceding calendar Quarter, then the Net Sales on the Combination Products shall be reasonably allocated between such Licensed Product and such additional algorithm based upon their relative value and proprietary protection as mutually agreed upon in good faith by Mount Sinai and Licensee. For clarity, notwithstanding anything else in this Section or elsewhere in this Agreement, regardless of any royalty deductions made for Combination Products under this Section 4.2, the royalty paid to Mount Sinai on the Net Sale of any Licensed Product shall not be reduced to an amount lower than two percent (2%) of the relevant Net Sale.
5. INTENTIONALLY RESERVED
6. REPORTS
6.1. Reporting of First Commercial Sale. In addition to the Quarterly Reports required under Section 6.3, Licensee shall provide a written report to Mount Sinai setting forth the date of First Commercial Sale in each Jurisdiction within thirty (30) days of the occurrence thereof.
6.2. Reporting of Regulatory Approvals. In addition to the Quarterly reports required under Section 6.3, Licensee shall provide a written report to Mount Sinai setting forth the date of each Regulatory Approval in each Jurisdiction within three (3) days of the occurrence thereof.
6.3. Quarterly Royalty Report. Within thirty (30) days after the Quarter in which any First Commercial Sale occurs, and within thirty (30) days after each Quarter thereafter, Licensee shall provide Mount Sinai with a written report detailing the amount of Gross Sales from Commercial Sales of Licensed Products during the preceding Quarter, the amount of Net Sales made during such Quarter and the royalty payments due to Mount Sinai for such Quarter pursuant to Article 4 (each such report, a “Quarterly Report”). Each Quarterly Report shall include at least the following:
13
(a) accounting for Net Sales, detailing the Gross Sales and specifying the deductions taken to arrive at Net Sales, listed by Licensed Product and by Jurisdiction;
(b) total royalty payments due to Mount Sinai by Licensed Product and by Jurisdiction.
6.4. Each Quarterly Report shall be in substantially similar form as Exhibit E, attached hereto or to such other form as Mount Sinai may provide from time to time. Each Quarterly Report shall be certified as true and correct by an officer of Licensee and be reasonably acceptable to Mount Sinai. With each Quarterly Report submitted, Licensee shall pay to Mount Sinai the royalties and fees due and payable under this Agreement, to the extent not already paid pursuant to Article 4. If no royalties or fees are due and payable, Licensee shall so report. Licensee’s failure to timely submit to Mount Sinai payment or a Quarterly Report substantially in the required form will constitute a material breach of this Agreement permitting Mount Sinai to terminate this Agreement in full pursuant to Section 12.1(a) hereof in addition to Mount Sinai’s right to exercise any and all remedies at law or otherwise.
6.5. Annual Progress Report and Development Plan. Within fifteen (15) business days of the beginning of each Calendar Year following the Effective Date while a Licensed Product is under development, Licensee shall submit to Mount Sinai (a) an updated Development Plan, in accordance with Section 3.1 and (b) a written report covering Licensee’s progress evidencing no less than Commercially Reasonable Efforts regarding: (i) development and testing of all Licensed Products; (ii) achieving the due diligence events specified in Section 3.3; and (iii) preparing, filing, and obtaining and maintaining of any Regulatory Approvals; and (iv) plans for the upcoming year related to commercializing the Licensed Product(s) (an “Annual Progress Report”). For clarity, SEC and other regulatory filings, marketing authorizations, and/or press releases are not sufficient to satisfy the requirements of the Annual Progress Report.
6.6. Payment and Currency. All dollar amounts referred to in this Agreement are expressed in United States dollars and Licensee shall make all payments due to Mount Sinai in U.S. Dollars, without deduction of exchange, collection, wiring fees, bank fees, or any other charges, in accordance with the appropriate sections requiring payments. Each payment will reference Agreement AGR-31512. All payments to Mount Sinai will be made in U.S. Dollars by wire transfer or check payable to the Icahn School of Medicine at Mount Sinai and sent to:
By Electronic Transfer:
Bank Name: JPMorgan Chase Manhattan Bank |
By Check: Payable to: |
14
028000024 |
|
6.7. Currency Exchange; Taxes. For converting any Net Sales made in a currency other than United States Dollars, the Parties will use the conversion rate published in the Wall Street Journal Eastern US Edition conversion rate, or other industry standard conversion rate approved in writing by Mount Sinai for the last day of the Quarter for which such royalty payment is due. All applicable currency exchange taxes and other currency exchange charges such as currency exchange duties, customs, tariffs, imposts and government-imposed currency exchange surcharges shall be borne by Licensee and will not be deducted from payments due to Mount Sinai.
6.8. Late Payments. In the event royalty payments or other fees are not received by Mount Sinai when due hereunder, Licensee shall pay to Mount Sinai interest charges that will accrue interest until paid at a rate equal to one (1) percentage point above the U.S. Prime Rate, as reported in the Wall Street Journal, Eastern Edition from time-to-time (or the maximum allowed by Law, if less), calculated on the number of days such payment is overdue.
6.9. Records and Audit Rights. Licensee shall keep complete, true and accurate records and books containing all particulars that may be necessary for the purpose of showing the amounts payable to Mount Sinai hereunder. Copies of all such records and books shall be kept at Licensee’s principal place of business or the principal place of business of the appropriate division of Licensee to which this Agreement relates. The records for each Quarter will be maintained for at least five (5) years after the Calendar Year in which the applicable report was submitted to Mount Sinai. Such books and the supporting data shall be open to inspection by Mount Sinai, its contractors or agents at all reasonable times for a term of five (5) years following the end of the Calendar Year to which they pertain, for the purpose of verifying Licensee’s royalty statement or compliance in other respects with this Agreement. Such access will be available to Mount Sinai, its contractors or agents upon not less than seven (7) days written notice to Licensee not more than twice each Calendar Year during the Term and once per Calendar Year after the expiration or termination of this Agreement. Should such inspection lead to the discovery of at least a five percent (5%) or five thousand dollar ($5,000) discrepancy in reporting to Mount Sinai’s detriment (whichever is greater), Licensee agrees to pay the full cost of such inspection. Whenever Licensee has its books and records audited by an independent certified public accountant with respect to any Quarter in which amounts are payable to Mount Sinai hereunder, Licensee will, within thirty (30) days of the conclusion of such audit, provide Mount Sinai with a written statement, certified by said auditor, setting forth the calculation of royalties, fees, and other payments due to Mount Sinai over the time period audited as determined from the books and records of such Entity, together with the payment of any outstanding amounts due to Mount Sinai. For clarity, any amounts shown
15
to be owed pursuant to any audits conducted under this Section but unpaid will be due immediately and payable by Licensee within sixty (60) days after receipt of the auditor’s report.
7. CONFIDENTIALITY; PUBLICITY; USE OF NAME
7.1. “Confidential Information” means any and all information of a Party (the “Disclosing Party”), or such information of such Party’s or of Third Parties provided on behalf of such Party to the other Party (“Receiving Party”), that is disclosed in tangible form marked as “confidential” upon disclosure or, if disclosed in oral or other intangible form, is identified as confidential at the time of disclosure and summarized in a writing that is marked as “confidential” and provided to the Receiving Party within thirty (30) days of the intangible disclosure, provided however that failure to so mark, identify, or summarize shall not alter the status of such information as Confidential Information if a reasonable person would, based on the content and/or context of the disclosure, recognize such disclosure was intended as confidential. Notwithstanding the foregoing, Confidential Information shall not include information that the Receiving Party can demonstrate by written and/or electronic records: (i) is available to the public at the time of disclosure hereunder or, after disclosure, becomes a part of the public domain by publication or otherwise, through no breach by the Receiving Party; (ii) is already properly possessed by the Receiving Party prior to receipt from the Disclosing Party; (iii) was received by the Receiving Party without obligation of confidentiality or limitation on use from a Third Party who had the lawful right to disclose such information; or (iv) was independently developed by or for the Receiving Party by any person or persons who had no knowledge or benefit of the Disclosing Party’s Confidential Information, where the written or electronic records demonstrating such exception were created contemporaneously with such independent development.
7.2. Confidentiality. The Receiving Party shall maintain in confidence and not disclose to any Third Party any of Disclosing Party’s Confidential Information, using the same degree of care it uses to protect its own confidential information of a similar nature but in no event using less than a reasonable degree of care. The Receiving Party will use Disclosing Party’s Confidential Information solely as required to undertake its rights and obligations under this Agreement (the “Purpose”) and only during the Term. For clarity, except as provided for herein, the Purpose expressly excludes any use of Disclosing Party’s Confidential Information for (i) regulatory or patent filing purposes other than in express support of Licensed Products as permitted hereunder, or (ii) for initiation or pursuit of any proceeding to challenge the patentability, validity, or enforceability of any patent application or issued patent (or any portion thereof) that is owned or Controlled by Disclosing Party (including, e.g., via pre-issuance submissions, post grant review, or inter partes review). Any such excluded use is hereby deemed a material breach of this Agreement and in such event, notwithstanding anything to the contrary herein, the non-breaching Party shall have the right to terminate this Agreement immediately upon notice to the breaching Party and seek resolution of such dispute in any court of competent jurisdiction notwithstanding any provisions herein regarding resolution of disputes between the Parties; in addition to any other relief granted to the non-breaching Party, the breaching Party shall pay to the non-breaching Party all costs such non-breaching Party incurs in such proceeding including in defense of such patent application or patent. Any such payment shall be made within thirty (30) days of written demand. The Receiving Party will ensure that its employees and independent contractors (“Recipient Individuals”) have access to Disclosing Party’s Confidential Information only on a need to know
16
basis, are informed of all the obligations attaching to such Confidential Information in advance of being given access to it, and are required to comply with such Receiving Party’s obligations under this Agreement Receiving Party shall be fully responsible to Disclosing Party for such compliance by its Recipient Individuals. If such Recipient Individual is not an employee of a Party hereto, then Recipient will enter into a legally binding confidentiality agreement with provisions at least as strict as the confidentiality obligations and use restrictions herein, with such Recipient Individual prior to Disclosing Party’s Confidential Information to such Recipient Individual, and Receiving Party will be fully responsible to Disclosing Party for compliance with such obligations and restrictions by such Recipient Individual.
7.3. Notwithstanding the above Section 7.2, the Receiving Party may disclose Disclosing Party’s Confidential Information to the limited extent required by Law, court order, other governmental authority with jurisdiction, provided that the Receiving Party (a) promptly provides the Disclosing Party, to the extent legally permissible, with written notice of such requirement, (b) uses no less than reasonable efforts to obtain confidential treatment of such Disclosing Party’s Confidential Information by such court or governmental authority, and (c) cooperates, at the Disclosing Party’s written request and expense, with the Disclosing Party’s legal efforts to prevent or limit the scope of such required disclosure; the Receiving Party shall in all other respects continue to hold such Confidential Information as confidential and subject to all obligations of this Article 7. The Receiving Party’s obligations of confidentiality and non-use restrictions as set forth in this Article 7 shall remain in effect for a period of five (5) years from receipt of the Confidential Information from the Disclosing Party.
7.4. Each Party agrees to treat the terms and conditions of this Agreement as the Confidential Information of the other Party, provided however that in addition to the above exceptions, each Party shall be free to disclose any of the terms of this Agreement (i) to the extent that a Party is advised by its counsel that it is required to do so by the regulations or rules of any relevant stock exchange, (ii) to its accountants, attorneys and other professional advisors, provided that (l) in the case of any disclosure under clause (ii) above, the recipient(s) are obligated and do so undertake to keep such terms of this Agreement confidential to the same extent as said Party (said Party being fully responsible to the other Party for such recipients’ compliance), and (2) in the case of disclosure under clause (i), such disclosure shall be in accordance with Section 7.3.
7.5. Publicity. The Parties may issue a press release only upon mutual written agreement and, if so, will cooperate to determine the timing and content of such press release.
7.6. Use of Either Party’s Name. Except as required by law or regulation, neither Party nor its employees or agents may use the name, logo, seal, trademark, or service mark of the other Party, including any school or organization of Mount Sinai, or, any faculty member, student, employee, officer, director, trustee, or other representative of such other Party in the context of their employment or association with such other Party (or any adaptation of any of the foregoing) (collectively, “Name”) without the prior written consent of such other Party, which consent will be granted or denied in the sole discretion of the Party whose name is sought to be used. In the event consent is granted, the requesting Party shall comply with any restrictions placed upon such use by the Party granting consent. Any request for use of Mount Sinai’s Name must be first submitted to and approved in writing by Mount Sinai Innovation Partners.
17
8. PATENT PROSECUTION
8.1. Patent Prosecution. Mount Sinai shall control Prosecution of the Licensed Patents, having the sole and exclusive right to Prosecute the Licensed Patents and the select patent counsel and shall be responsible for associated costs. For avoidance of doubt, Mount Sinai’s Prosecution rights include, without limitation, the right to decide in which jurisdictions to file applications for the Licensed Patents and whether, at any point during prosecution, to abandon the Licensed Patents, as well as all other rights respecting Prosecution of the Licensed Patents. Licensee shall have no right to receive copies of any correspondence with patent counsel or any patent office respecting Prosecution of the Licensed Patents.
8.2. Patent Extension. Licensee shall, within three (3) days of the triggering event, notify Mount Sinai of any Regulatory Approval for any Licensed Product for which an application for Patent term extension may be based, including with respect to any Third Party product, or any other event in any Jurisdiction that would enable Mount Sinai or Licensee as appropriate to apply for Patent term extension or other regulatory or marketing exclusivity or extension thereof in any Jurisdiction. Licensee agrees to cooperate fully with Mount Sinai to provide any information or documentation necessary to support an application for Patent term extension or other regulatory or marketing exclusivity.
9. REPRESENTATIONS; DISCLAIMER OF WARRANTIES; LIMITATION OF LIABILITIES
9.1. Certain Representations. Each Party represents to the other Party that, as of the Effective Date:
(a) it has the full right, power and authority to enter into this Agreement and to perform its obligations hereunder; and
(b) this Agreement has been duly authorized and executed by it and is legally binding upon it, enforceable in accordance with its terms, and does not conflict with any agreement, instrument or understanding, oral or written, to which it is a party or by which it may be bound, nor violate any applicable Law or applicable regulation of any court, governmental body or administrative or other agency having jurisdiction over it.
9.2. Health Care Law. Licensee represents, warrants, and covenants to Mount Sinai that:
(a) Licensee and its agents and employees who are or shall be involved in the performance of this Agreement, have not been, and during the Term of this Agreement shall not be, debarred, excluded or disqualified (or convicted of any crime or engaged in any conduct for which debarment, exclusion or disqualification is mandated) under any Health Care Law, including pursuant to 21 U.S.C. § 335a;
(b) to its reasonable knowledge, no Third Party that, on behalf of Licensee, has been or during the Term of this Agreement will be, involved in the Development, Manufacture or Commercialization of the Licensed Products (each a “Licensee Partner”), has been or will be
18
debarred, excluded or disqualified (or convicted of any crime or engaged in any conduct for which debarment, exclusion or disqualification is mandated) under any Health Care Law, including pursuant to 21 U.S.C. § 335a;
(c) Licensee and its agents and employees involved in the performance of this Agreement, and Licensee Partners, shall perform this Agreement in full compliance with all applicable Health Care Laws; and
(d) Licensee shall notify Mount Sinai in writing immediately in the event of a violation of any of the foregoing, and shall, with respect to any Entity involved in such violation, promptly remove such Entity from performing any role under this Agreement.
9.3. DISCLAIMER OF WARRANTIES. THE LICENSED PATENTS, NON-EXCLUISVELY LICENSED TECHNICAL INFORMATION, AND KNOW-HOW, LICENSED PRODUCTS, AND ANY OTHER TECHNOLOGY OR INFORMATION PROVIDED OR LICENSED UNDER THIS AGREEMENT ARE PROVIDED ON AN “AS IS” BASIS. MOUNT SINAI MAKES NO REPRESENTATIONS OR WARRANTIES, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO ANY WARRANTY OF ACCURACY, COMPLETENESS, NONINFRINGEMENT, PERFORMANCE, MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, COMMERCIAL UTILITY, SCOPE, OR TITLE WITH RESPECT THERETO.
9.4. DISCLAIMER OF LIABILITIES. MOUNT SINAI WILL NOT BE LIABLE TO LICENSEE, ITS SUCCESSORS OR ASSIGNS, OR TO ANY THIRD PARTY WITH RESPECT TO ANY CLAIM ARISING FROM OR ATTRIBUTABLE TO USE BY LICENSEE OF THE MOUNT SINAI TECHNOLOGY, KNOW-HOW, LICENSED PRODUCTS, OR ANY OTHER TECHNOLOGY OR INFORMATION PROVIDED OR LICENSED UNDER THIS AGREEMENT, OR ARISING FROM THE DEVELOPMENT, TESTING, MANUFACTURE, USE OR SALE OF LICENSED PRODUCTS, OR FOR LOST PROFITS, BUSINESS INTERRUPTION, INCIDENTIAL, INDIRECT, SPECIAL, CONSEQUENTIAL OR OTHER DAMAGES OF ANY KIND.
9.5. WITHOUT LIMITING THE GENERALITY OF ANYTHING IN THIS ARTICLE 9, NOTHING IN THIS AGREEMENT SHALL BE CONSTRUED AS:
(a) A WARRANTY OR REPRESENTATION BY MOUNT SINAI THAT ANYTHING MADE, USED, SOLD, OFFERED FOR SALE, DISTRIBUTED, OR AS APPLICABLE PUBLICLY PERFORMED, PUBLICLY DISPLAYED, DERIVED FROM, OR OTHERWISE DISPOSED OF PURSUANT TO ANY LICENSE GRANTED UNDER THIS AGREEMENT IS OR WILL BE FREE FROM INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS;
(b) AN OBLIGATION BY MOUNT SINAI TO BRING OR PROSECUTE ACTIONS OR SUITS AGAINST THIRD PARTIES FOR INFRINGEMENT, MISAPPROPRIATION, OR OTHER SIMILAR CAUSES OF ACTION RELATED TO THE MOUNT SINAI TECHNOLOGY, OR
19
(c) CONFERRING BY IMPLICATION, ESTOPPEL OR OTHERWISE ANY LICENSE OR RIGHTS UNDER ANY INTELLECTUAL PROPERTY RIGHTS OF MOUNT SINAI OTHER THAN AS AND TO THE EXTENT EXPRESSLY SET FORTH HEREIN
10. INDEMNIFICATION
10.1. Indemnification. Licensee will indemnify, hold harmless, and at Mount Sinai’s option, shall defend Mount Sinai, and its trustees, officers, faculty, agents, employees and students (each, an “Indemnified Party”) from and against any and all claims, actions, liabilities, losses, damages, judgments, costs or expenses suffered or incurred by the Indemnified Parties, including attorneys’ fees and related costs (collectively, “Liabilities”), arising out of or resulting from:
(a) the exercise of any license granted under this Agreement;
(b) any breach of this Agreement by Licensee, any of Licensee’s officers, directors, employees or agents; ;
(c) the enforcement of this Article 10 by any Indemnified Party; and/or
(d) any willful misconduct, negligent act or omission of Licensee or any of Licensee’s officers, directors, employees or agents, with respect to its obligations hereunder or with respect to applicable law or regulation;
except in each case to the extent such Liabilities result solely from the gross negligence or willful misconduct of an Indemnified Party. Liabilities under this Section include, but are not limited to, Liabilities arising in connection with: (i) the use by a Third Party of a Licensed Product that was Developed, Manufactured or Commercialized by Licensee, vendors or Third Parties; (ii) a claim by a Third Party that the Mount Sinai Technology, or the design, composition, or Exploitation of any Licensed Product infringes or violates or appropriates any patent, copyright, trade secret, trademark or other intellectual property right of such Third Party; (iii) clinical trials or studies conducted by or on behalf of Licensee, vendors or associated Third Parties relating to the Mount Sinai Technology or Licensed Products, such as claims by or on behalf of a human subject of any such trial or study; or (iv) a failure to perform under this Agreement or any Sublicense in material compliance with all applicable Laws, including, without limitation, all Health Care Laws.
10.2. Indemnification Procedure. Indemnified Party will (a) promptly provide Licensee with written notice of any Liability that is indemnifiable under this Article 10, (b) giving Licensee sole control over the defense and settlement of such Liabilities, and (c) giving Licensee, at Licensee’s request and expense, all reasonably requested information and assistance to assist in the defense and settlement of any such Liabilities, provided, however, that Licensee shall not settle or compromise any Liabilities in any manner that may impose restrictions or obligations on any Indemnified Party, or that grants any rights to the Licensed Patents, Non-Exclusively Licensed Technical Information, Know-How, or Licensed Products or that concedes any fault or wrongdoing on the part of Indemnified Party, without the Indemnified Party’s prior written consent (not to be unreasonably withheld, conditioned, or delayed). If Licensee fails or declines to assume the defense against any claim or action within thirty (30) days after notice thereof, then Mount Sinai may assume and control the defense of such claim or action for the account and at the risk
20
of Licensee, and any Liabilities related to such claim or action will be conclusively deemed a liability of Licensee. The indemnification rights of the Indemnified Parties under this Article 10 are in addition to all other rights that an Indemnified Party may have at law, in equity or otherwise.
11. INSURANCE
11.1. Coverages. Licensee will procure and maintain insurance policies for the following coverages with respect to personal injury, bodily injury, property damage and contractual liability arising out of Licensee’s performance under this Agreement as follows: (a) during the Term, comprehensive general liability, including broad form and contractual liability, in a minimum amount of One Million U.S. Dollars ($1,000,000 USD) combined single limit per occurrence and in the aggregate, written on an occurrence-basis, with no deductible, containing a separation of insureds provision, with additional coverage for broad form and contractual liability, completed operations; (b) prior to the commencement of clinical trials involving Licensed Products, clinical trials coverage in a minimum amount of One Million U.S. Dollars ($1,000,000 USD) combined single limit per occurrence and in the aggregate; and (c) prior to the sale of the first Licensed Product, product liability coverage, in a minimum amount of Three Million U.S. Dollars ($3,000,000 USD) combined single limit per occurrence and in the aggregate. Mount Sinai may review periodically the adequacy of the minimum amounts of insurance for each type of coverage required by this Article 11, and Mount Sinai reserves the right to request Licensee to adjust the limits accordingly, such request not to be unreasonably delayed, conditioned, or denied.
11.2. Other Requirements. Any policies of insurance required by Section 11.1 will be issued by an insurance carrier with an A.M. Best rating of “A minus” or better and will name Mount Sinai as an additional insured, on a primary and non-contributory basis, with respect to Licensee’s performance under this Agreement. Licensee will provide Mount Sinai with insurance certificates evidencing the required coverage within thirty (30) days after the commencement of each policy period and any renewal periods. Each certificate will provide that the insurance carrier will notify Mount Sinai in writing at least thirty (30) days prior to the cancellation or material change in coverage.
11.3. For clarity, the insurance coverage required by this Article 11 is the total coverage required of Licensee with respect to this Agreement and all of the Non-Exclusive Licenses and Exclusive Licenses in the aggregate.
12. TERM AND TERMINATION
12.1. Termination by Mount Sinai.
(a) For Cause. Mount Sinai may give written notice of default to Licensee, if Licensee: (i) materially breaches any obligation, covenant, condition, or undertaking of this Agreement to be performed by it hereunder (including e.g. if Licensee should cease or fail to undertake Commercially Reasonable Efforts with respect to Licensed Products, fail to make any payment at the time such payment is due, fail to maintain the insurance coverage required hereunder, or fail to timely and sufficiently submit any Quarterly Report, Annual Progress Report, or Development Plan); (ii) fails to timely provide the initial Development Plan or any annual updated Development Plan; or (iii) fails to achieve any Diligence event described in Section 3.3.
21
If Licensee should fail to cure such default within ninety (90 days of such written notice, then Mount Sinai shall have the right to terminate this Agreement, and all of the rights, privileges, and license granted hereunder, with immediate effect at the end of such ninety (90) days.
(b) Failure to Raise Required Additional Financing. If Licensee fails to raise Additional Financing as set forth in the SPA, then Mount Sinai shall have the right to terminate this Agreement immediately upon written notice to Licensee, without opportunity to cure.
(c) Event of Bankruptcy. If Licensee experiences an Event of Bankruptcy, then Licensee shall notify Mount Sinai immediately. For purposes of this provision, the term “Event of Bankruptcy” means, with respect to a Party: (a) filing by such Party in any court or agency pursuant to any statute or regulation of any state or country, a petition in bankruptcy or insolvency or for reorganization or for an arrangement or for the appointment of a receiver or trustee of the Party or of its assets; (b) such Party being served with an involuntary petition against such Party, filed in any insolvency proceeding, where such petition has not been dismissed within sixty (60) days after the filing thereof; (c) such Party proposing or being a party to any dissolution or liquidation of such Party; or (d) such Party making a general assignment for the benefit of creditors. Mount Sinai has the right to immediately terminate this Agreement after sixty (60) days of such notice of an Event of Bankruptcy provided that Licensee has not provided to Mount Sinai sufficient documentation that demonstrates Licensee is no longer under an Event of Bankruptcy.
(d) Cessation of Business. If Licensee at any time (i) ceases to carry on its business with respect to the rights granted in this Agreement, (ii) liquidates all or a material portion of its assets or business locations, (iii) employs an agent or other third party to conduct a program of closings, liquidations or sales of any material portion of its business, (iv) is no longer a Going Concern, or (v) ceases to be listed on a public stock exchange (other than in connection with a Change of Control), then this Agreement shall terminate immediately upon written notice by Mount Sinai, without opportunity to cure. “Going Concern” is defined by the Auditing Standards Board SAS No. 132.
(e) Challenge of Patents. Licensee acknowledges and agrees that nothing herein shall be construed as preventing it from challenging the validity or enforceability of the Licensed Patents at any time. In the event that Licensee shall, however, challenge the validity or enforceability of any of the Licensed Patents in any forum through any means, or otherwise indicate the remittance of any payment due under this Agreement is made under protest or with any objection, Licensee agrees that Mount Sinai shall have the right, but not the obligation, in addition to any other remedy it may have available to it at law and/or in equity, to terminate this Agreement immediately upon providing written notice of the same to Licensee. Mount Sinai in response to such challenge by Licensee or following termination by Mount Sinai under this subsection may seek redress in any court of competent jurisdiction in its sole discretion notwithstanding any other provision of this Agreement.
12.2. Termination by Licensee. Licensee may terminate this Agreement, in whole or in part, at any time, without cause, by giving written notice thereof to Mount Sinai. Such termination shall become effective sixty (60) days after such notice and all of Licensee’s rights associated therewith shall cease as of such effective date.
22
12.3 Change of Control. For avoidance of doubt, a Change of Control shall not trigger termination of this Agreement.
13. EFFECT OF TERMINATION
13.1. Continuing Obligations of Licensee. Upon expiration or termination of this Agreement, Licensee shall promptly (within seven (7) business days) return to Mount Sinai or destroy all Know-How, Non-Exclusively Licensed Technical Information, and Licensed Products existing in tangible form or copied in written or other tangible form by Licensee. In the event of destruction, Licensee shall certify in a writing signed by Licensee’s authorized signatory within seven (7) business days of such destruction, that all such Know-How, Non-Exclusively Licensed Technical Information, and Licensed Products have been destroyed. Termination or expiration of this Agreement shall not relieve Licensee of any monetary or any other obligation or liability accrued hereunder prior to the effective date of such termination or expiration, or rescind or give rise to any right to rescind any payments made or other consideration given to Mount Sinai hereunder prior to the effective date of such termination; nor shall such termination or expiration affect in any manner any rights of Mount Sinai arising under this Agreement prior to the date of such termination. Licensee shall pay all costs incurred by Mount Sinai in enforcing any obligation of Licensee or accrued right of Mount Sinai including, but not limited to, attorney’s fees.
13.2. Survival of Terms. In addition to any provision which by its terms contemplates performance after the Term, the following provisions shall survive the expiration or termination of this Agreement: Articles 1 (Definitions), 4 (Fees, Royalties, and Payments), 6 (Reports), 7 (Confidentiality; Publicity; Use of Name), 9 (Representations; Disclaimer of Warranties; Limitation of Liabilities), 10 (Indemnification), 11 (Insurance), 13 (Effect of Termination), and 14 (Additional Provisions).
13.3. Licensed Product Data. A copy of all Licensed Product Data must be transferred to Mount Sinai within forty-five (45) days of termination of this Agreement for any reason and shall become the sole property of Mount Sinai. Mount Sinai shall have a non-exclusive, world-wide, perpetual, non-cancelable, royalty-free, fully paid-up license, with right to sublicense, to use such Licensed Product Data to further advance the development of Mount Sinai technologies (e.g. the Licensed Patents, Non-Exclusively Licensed Technical Information, and Know-How).
14. ADDITIONAL PROVISIONS
14.1. Regulatory. Licensee acknowledges that the Mount Sinai Technology has not received any governmental approval, clearance, or similar designation (“Approvals”), does not necessarily satisfy the requirements of any governmental body or other organization, and has not been validated for clinical or diagnostic use, for safety and effectiveness, or for any other specific use or application. Licensee is solely responsible for compliance with any and all applicable laws, rules and regulations, and governmental policies that pertain to Licensee’s use of the Mount Sinai Technology, including, but not limited to, obtaining any necessary Approvals and conforming with any regional, territorial or other regulatory requirements, or the requirements.
14.2. Independent Contractors. The Parties are independent contractors. Nothing contained in this Agreement is intended to create an agency, partnership or joint venture between
23
the Parties. At no time will either Party make commitments or incur any charges or expenses for or on behalf of the other Party.
14.3. Compliance with Laws. Licensee must comply with all prevailing Laws that apply to its activities or obligations under this Agreement. For example, Licensee will comply with applicable United States export Laws. The transfer of certain technical data and commodities may require a license from the applicable agency of the United States government and/or written assurances by Licensee that Licensee will not export data or commodities to certain foreign countries without prior approval of the agency. Mount Sinai does not represent that no license is required, or that, if required, the license will issue.
14.4. Export Control. Licensee acknowledges that the export, re-export, or transfer of certain technology, software, or hardware (collectively, “Items”) may be subject to applicable laws and regulations of the United States and other jurisdictions, including but not limited to the U.S. Department of Commerce’s Export Administration Regulations (“EAR”), as set forth in 15 C.F.R. 730-774, the U.S. Department of State’s International Traffic in Arms Regulations (“ITAR”), as set forth in 22 C.F.R. 120-130, and the economic sanctions programs administered by the U.S. Department of Treasury’s Office of Foreign Assets Control (“OFAC”), as set forth in 31 C.F.R. 500-598 and certain executive orders (collectively, “Trade Control Laws”); as well as the laws and regulations of the U.S. Food and Drug Administration (“FDA”), U.S. Department of Agriculture (“USDA”), and U.S. Centers for Disease Control and Prevention (“CDC”). Notwithstanding any other provision of this Agreement, Mount Sinai and Licensee agree to comply fully with all applicable Trade Control Laws in the performance of this Agreement and the parties will not cause each other to be in violation of applicable Trade Control Laws. Neither Mount Sinai nor Licensee shall be required to take, or to refrain from taking, any action or obligation under this Agreement, where to do so would be inconsistent with or potentially violate or incur a penalty under applicable Trade Control Laws or other applicable laws, including but not limited to the anti-boycott laws administered by the U.S. Commerce and Treasury Departments. Mount Sinai shall have the sole discretion to refrain from being directly or indirectly involved in the provision of Items that may be prohibited by applicable Trade Control Laws. Licensee represents and warrants that Licensee, any parent, subsidiary, or affiliate of Licensee, any of its sub-distributors, and agents deployed in connection with the sale, supply, and delivery of any Items or services covered by the Agreement are not (i) on any list of designated parties created and maintained in line with Trade Control Laws by any country or intergovernmental or supranational organization or otherwise targeted by sanctions regimes including but not limited to those administered by the United States (“Restricted Parties”); (ii) located in, organized under the laws of, or ordinarily resident in any country or territory subject to comprehensive territorial sanctions regimes including but not limited to those administered by the United States (at present, applicable for Cuba, Iran, North Korea, and Syria, as well as the Crimea region and the so-called Donetsk People’s Republic and the Luhansk People’s Republic) (“Restricted Territories”); (iii) part of any government, including its agencies and instrumentalities, that are targeted by sanctions regimes including but not limited to those administered by the United States (“Sanctioned Government”); or (iv) owned (at 50% or more) or controlled, directly or indirectly, individually or in the aggregate, by a Restricted Party or Sanctioned Government. Licensee hereby acknowledges and confirms that, unless specifically authorized by Mount Sinai and in compliance with all applicable Trade Control Laws, the Licensee
24
will not export, re-export, or transfer, directly or indirectly through third parties or otherwise, any Items or services covered by the Agreement to any Restricted Party or Sanctioned Country.
14.5. Marking. Licensee shall comply with any marking requirements of the intellectual property Laws of the applicable countries in the Territory, and particularly agrees to permanently and legibly mark all Licensed Products made, used, reproduced, or sold under the terms of this Agreement, or their respective containers.
14.6. Modification, Waiver and Remedies. This Agreement may only be modified by a written amendment that is executed by an authorized representative of each Party. Any waiver must be express and in writing. No waiver by either Party of a breach by the other Party will constitute a waiver of any different or succeeding breach. Unless otherwise specified, all remedies are cumulative.
14.7. Assignment. This Agreement, and every part of it, is a personal contract between the Parties. None of the rights or obligations or other interests of Licensee hereunder may be assigned or otherwise transferred by Licensee, either directly or by merger or operation of Law and any such assignment granted, or purported to be granted, shall be null and void.
14.8. Notices. Except as otherwise expressly set forth herein, any notice or other required communication under this Agreement (each, a “Notice”) must be in writing, addressed to the Party’s respective Notice Address, and delivered personally or by globally recognized express delivery service, charges prepaid. A Notice will be deemed delivered and received: (a) in the case of personal delivery, on the date of such delivery; and (b) in the case of a globally recognized express delivery service, five (5) days from transmittal by Mount Sinai to the address below. The “Notice Address” of each Party is as follows:
if to Mount Sinai, to: |
Icahn School of Medicine at Mount Sinai |
and a copy of legal notices only to: |
Icahn School of Medicine at Mount Sinai Place, One Gustave L. Levy Box 1099, New York, NY 10029 |
if to Licensee, to: |
Heart Test Laboratories, Inc. d/b/a HeartSciences 550 Reserve Street, Suite 360 Southlake, TX 76092 Attention: Chief Financial Officer
|
14.9. Severability and Reformation. If any provision of this Agreement is held to be invalid or unenforceable by a court of competent jurisdiction, then the remaining provisions of this Agreement will remain in full force and effect. Such invalid or unenforceable provision will be revised by such court to be a valid or enforceable provision that comes as close as permitted by Law to the Parties’ original intent.
25
14.10. Headings and Counterparts. The headings of the articles and sections included in this Agreement are inserted for convenience only and are not intended to affect the meaning or interpretation of this Agreement. This Agreement may be executed in several counterparts, and execution signatures may be exchanged electronically including by facsimile or as scanned e‑mail attachments, and signatures so exchanged shall be considered as original for all purposes and taken together will constitute one and the same instrument.
14.11. Governing Law. This Agreement will be governed and construed in accordance with the Laws of the State of New York, without giving effect to the conflict of law provisions of any jurisdiction.
14.12. Dispute Resolution; Venue. Except as expressly stated otherwise herein, if a dispute arises between the Parties concerning any right or duty under this Agreement, then the Parties will confer, as soon as practicable, in an attempt to resolve the dispute amicably. If the Parties are unable to resolve the dispute amicably, the Parties each hereby irrevocably submit to the exclusive jurisdiction of the state and federal courts located in the borough of Manhattan, New York, New York.
14.13. Integration. This Agreement, together with all attached Exhibits, contains the entire agreement between the Parties with respect to the Mount Sinai Technology, and supersedes all other oral or written representations, statements, or agreements with respect to such subject matter, including but not limited to, the term sheet exchanged prior to this Agreement. For clarity, this Section 14.13 does not supersede the other Exclusive Licenses or Non-Exclusive Licenses.
14.14. Force Majeure. If either Party fails to fulfill its obligations hereunder (other than an obligation for the payment of money), when such failure is due to an act of God, or other circumstances beyond its reasonable control, including but not limited to fire, flood, civil commotion, riot, war (declared and undeclared), revolution, or embargoes, then said failure shall be excused for the duration of such event and for such a time thereafter as is reasonable to enable the parties to resume performance under this Agreement, provided however, that in no event shall such time extend for a period of more than one hundred eighty (180) days.
14.15. Certain Conventions. Any reference in this Agreement to an Article, Section, subsection, paragraph, clause or Exhibit shall be deemed to be a reference to an Article, Section, subsection, paragraph, clause or Exhibit, of or to, as the case may be, this Agreement, unless otherwise indicated. Unless the context of this Agreement otherwise requires, (a) all definitions set forth herein shall be deemed applicable whether the words defined are used herein with initial capital letters in the singular or the plural, (b) the word “will” shall be construed to have the same meaning and effect as the word “shall,” (c) any definition of or reference to any agreement, instrument or other document herein shall be construed as referring to such agreement, instrument or other document as from time to time amended, supplemented or otherwise modified (subject to any restrictions on such amendments, supplements or modifications set forth herein), (d) any reference herein to any Party shall be construed to include the Party’s successors and assigns, (e) the word “notice” shall mean notice in writing (whether or not specifically stated) and shall include notices, consents, approvals and other written communications contemplated under this Agreement, (f) provisions that require that a Party or the Parties “agree,” “consent” or “approve” or the like shall require that such agreement, consent or approval be specific and in writing,
26
whether by written agreement, letter, approved minutes or otherwise (but excluding e-mail and instant messaging), (g) references to any specific Law, rule or regulation, or article, section or other division thereof, shall be deemed to include the then-current amendments thereto or any replacement or successor Law, rule or regulation thereof, (h) words of any gender include each other gender, (j) words such as “herein,” “hereof” and “hereunder” refer to this Agreement as a whole and not merely to the particular provision in which such words appear, (i) the words “include,” “includes” and “including” shall be deemed to be followed by the phrase “but not limited to,” “without limitation,” “inter alia” or words of similar import, and (j) “days” shall mean “calendar days.” In the event an ambiguity or question of intent or interpretation arises, this Agreement shall be construed as if drafted jointly by the Parties and no presumption or burden of proof shall arise favoring or disfavoring any Party by virtue of the authorship of any of the provisions of this Agreement.
14.16. Business Day Requirements. In the event that any notice or other action is required to be taken by a Party under this Agreement on a day that is not a business day, then such notice or other action shall be deemed to be required to be taken on the next occurring business day.
14.17. Exhibits. All Exhibits hereto are hereby incorporated into and made a part of this Agreement.
[Signature Page Follows]
27
IN WITNESS WHEREOF, the Parties have executed this Agreement as of the Effective Date.
HEART TEST LABORATORIES, INC.: BY: NAME: TITLE: |
ICAHN SCHOOL OF MEDICINE AT Mount Sinai: BY:
NAME:
TITLE: |
Exhibit A
Securities Purchase Agreement
Exhibit B
Licensed Patents
Tech ID |
Title |
Serial Number |
Status |
Country |
File Date |
230103G |
HeartBEiT: Vision Transformers improve diagnostic performance for electrocardiograms |
63/468,435 |
Pending |
US |
5/23/2023 |
Non-Exclusively Licensed Technical Information
[***] HeartBEiT: Vision Transformers improve diagnostic performance for electrocardiograms
Inventors
• [***]
Description
• The first vision-based waveform transformer that can be used to develop specialized models for ECG analysis especially at low sample sizes.
Publication
• [***]
Exhibit C
Link to Licensee 10-K
https://dd7pmep5szm19.cloudfront.net/2729/0000950170-23-033424.htm
Exhibit D
Initial Development Plan
[To be added within thirty (30) days of the Effective Date in accordance with the Agreement.]
Exhibit E
Form of Quarterly Royalty Report
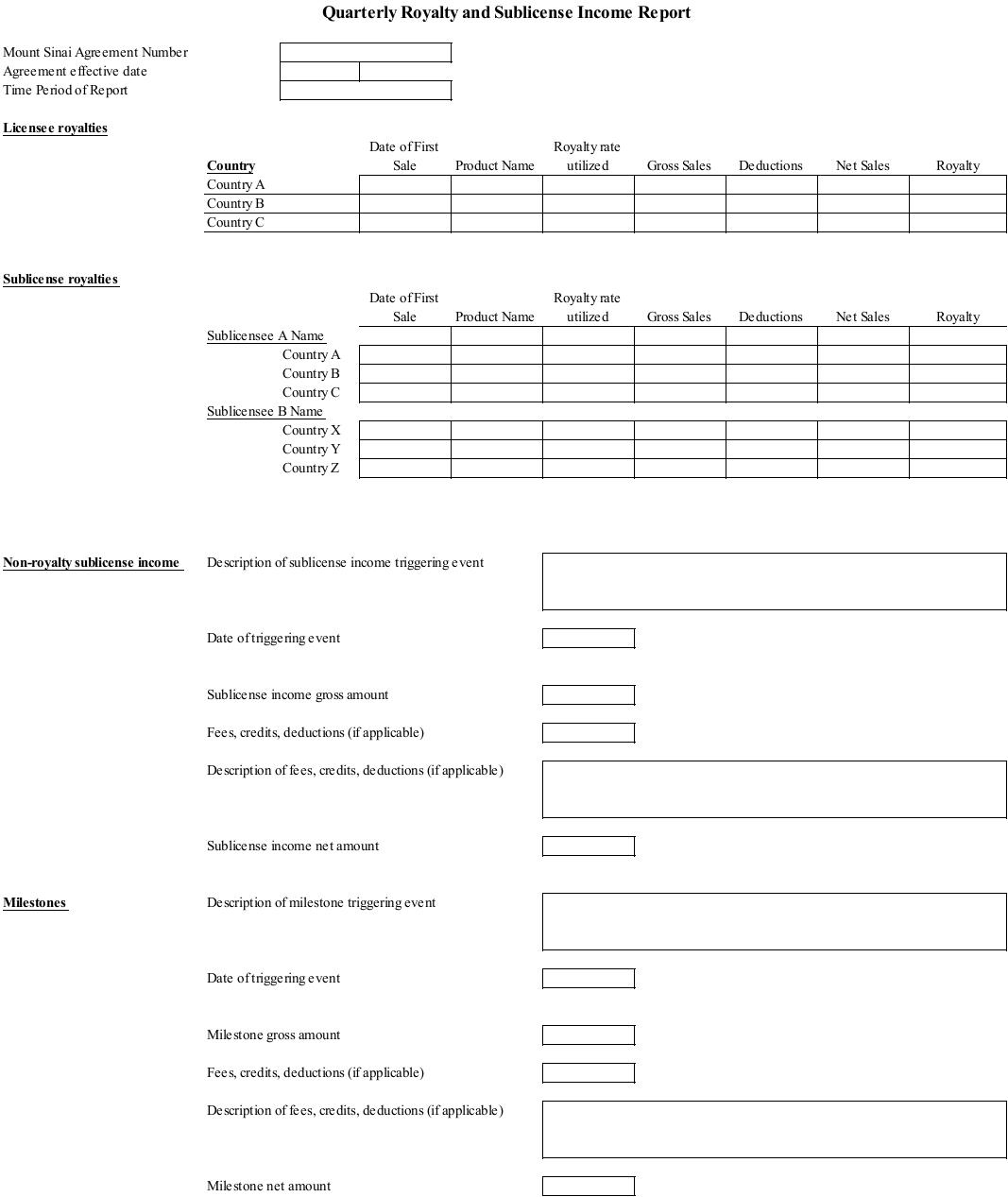
* Please add additional pages or line items as necessary.