Filed Pursuant to Rule 424(b)(5)
Registration No.
333-223777
PROSPECTUS SUPPLEMENT
(To Prospectus dated March
28, 2018)
![]()
| Up to $25,000,000 |
| Common Stock |
We have entered into a Controlled Equity OfferingSM Sales Agreement, or the Sales Agreement, with Cantor Fitzgerald & Co., or Cantor Fitzgerald, relating to shares of our common stock offered by this prospectus supplement. In accordance with the terms of the Sales Agreement, we may offer and sell shares of our common stock from time to time through Cantor Fitzgerald, acting as sales agent, having an aggregate offering price of up to $25,000,000, pursuant to this prospectus supplement.
Our common stock is listed on The Nasdaq Capital Market under the symbol “ORGS.” On December 19, 2018, the last reported sale price of our common stock was $4.76 per share.
Sales of our common stock, if any, under this prospectus supplement and the accompanying prospectus may be made by any method deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act of 1933, as amended, or the Securities Act. Subject to the terms of the Sales Agreement, Cantor Fitzgerald will act as sales agent and use commercially reasonable efforts to sell on our behalf all of the shares of common stock requested to be sold by us, consistent with its normal trading and sales practices, on mutually agreed terms between Cantor Fitzgerald and us. There is no arrangement for funds to be received in any escrow, trust or similar arrangement.
Cantor Fitzgerald is entitled to compensation at a commission rate of 3.0% of the gross sales price per share sold under the Sales Agreement. See “Plan of Distribution” beginning on page S-13 for additional information regarding the compensation to be paid to Cantor Fitzgerald. In connection with the sale of shares of our common stock on our behalf, Cantor Fitzgerald will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor Fitzgerald will be deemed to be underwriting commissions or discounts.
|
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks that we have described on page S-6 of this prospectus supplement under the caption “Risk Factors”, in the documents incorporated by reference into this prospectus supplement and on page 7 of the accompanying base prospectus. |
| Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement is truthful or complete. Any representation to the contrary is a criminal offense. |

The date of this prospectus supplement is December 20, 2018.
TABLE OF CONTENTS
PROSPECTUS SUPPLEMENT
TABLE OF CONTENTS
PROSPECTUS
S-i
ABOUT THIS PROSPECTUS SUPPLEMENT
This prospectus supplement and the accompanying prospectus relate to the offering of our common stock. This prospectus supplement, the accompanying prospectus and any free writing prospectus we provide you, include or incorporate by reference important information about us, our common stock, and other matters you should know before investing. You should read this prospectus supplement, the accompanying prospectus and any free writing prospectus we provide you, as well as additional information described under “Where You Can Find More Information” and “Incorporation of Documents by Reference” in this prospectus supplement before making an investment decision.
This document is in two parts and is part of the registration statement (No. 333-223777) that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process. The first part is this prospectus supplement, which describes the terms of this offering and also adds to and updates information contained in the accompanying prospectus and the documents incorporated by reference in this prospectus supplement and the accompanying prospectus. The second part, the accompanying prospectus dated March 28, 2018, including the documents incorporated by reference therein, provides more general information, some of which may not apply to this offering. Generally, when we refer to this prospectus, we are referring to both parts of this document combined. To the extent there is a conflict between the information contained in this prospectus supplement, on the one hand, and the information contained in the accompanying prospectus or in any document incorporated by reference herein or therein that was filed with the SEC before the date of this prospectus supplement, on the other hand, you should rely on the information in this prospectus supplement. If any statement in one of these documents is inconsistent with a statement in another document having a later date“for example, a document incorporated by reference in the accompanying prospectus“the statement in the document having the later date modifies or supersedes the earlier statement.
You should rely only on this prospectus supplement, the accompanying prospectus and the information incorporated or deemed to be incorporated by reference in herein or therein or in any free writing prospectuses we provide you. We have not, and Cantor Fitzgerald has not, authorized anyone to provide you with information that is in addition to, or different from, that contained or incorporated by reference in this prospectus supplement or the accompanying prospectus or contained in any free writing prospectus we provide you. If anyone provides you with different or inconsistent information, you should not rely on it. We are not, and Cantor Fitzgerald is not, offering to sell securities in any jurisdiction where the offer or sale of such securities is not permitted as of the time of such sale. You should not assume that the information contained or incorporated by reference in this prospectus supplement or the accompanying prospectus or contained in any free writing prospectus we provide you is accurate as of any date other than as of the date of these respective documents or in the case of the documents incorporated by reference, the date of such documents regardless of the time of delivery of this prospectus supplement, the accompanying prospectus or any free writing prospectus we provide you or any sale of our common shares. Our business, financial condition, liquidity, results of operations, and prospects may have changed since those dates.
We further note that the representations, warranties and covenants made by us in any agreement that is filed as an exhibit to the registration statement to which the accompanying prospectus forms a part or to any document that is incorporated by reference in this prospectus supplement or the accompanying prospectus were made solely for the benefit of the parties to such agreement, including, in some cases, for the purpose of allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty or covenant to you. Moreover, such representations, warranties or covenants were accurate only as of the date when made. Accordingly, such representations, warranties and covenants should not be relied on as accurately representing the current state of our affairs.
Unless the context otherwise requires, “Orgenesis,” “the Company,” “we,” “us,” “our” and similar terms refer to Orgenesis Inc. and our subsidiaries. When we refer to “you” or “yours” we mean the investors and potential investors in the shares of common stock offered hereby.
Our trademarks include, without limitation, our name and corporate logo. Other service marks, trademarks and trade names contained in this prospectus supplement, the accompanying prospectus or the documents incorporated by reference herein and therein are the property of their respective owners.
S-1
PROSPECTUS SUPPLEMENT SUMMARY
The following is a summary of what we believe to be the most important aspects of our business and the offering of our securities under this prospectus supplement and the accompanying prospectus. We urge you to read this entire prospectus supplement and the accompanying prospectus, including the more detailed consolidated financial statements, notes to the consolidated financial statements and other information incorporated by reference from our other filings with the SEC, and the information included in any free writing prospectus we provide you. Investing in our securities involves risks. Therefore, carefully consider the risk factors set forth in this prospectus supplement, the accompanying prospectus and in any free writing prospectus we provide you, or incorporated by reference herein and therein, as well as other information in this prospectus supplement, the accompanying prospectus and in any free writing prospectus we provide you, as well as in the documents incorporated by reference herein and therein, before purchasing our securities. Each of the risk factors could adversely affect our business, operating results and financial condition, as well as adversely affect the value of an investment in our securities.
About Orgenesis
In recent years, there has been growth in the field of cellular biology enabling scientists to manipulate tissues and cells utilizing advanced genetic, epigenetic and other technologies such as selection, propagation, scaffolding and even 3D printing techniques. This scientific advancement is the basis of a new generation of therapeutics known as Advanced Therapeutic Medicinal Products, or ATMPs.
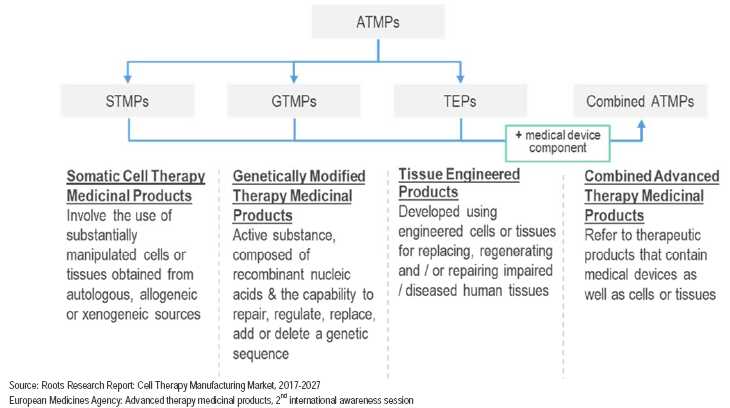
Autologous therapies are a class of ATMPs that are based on a personalized treatment to the patient, in which the manufacturing of the drug or the preparation of the treatment protocol is patient specific. Production of an autologous ATMP is based on a starting material derived from the patient•s own cells.
There are several major challenges in advancing such therapeutic technologies to a clinical stage:
| • |
Because these therapies are based on living cells, they cannot be sterilized throughout the manufacturing process, and, consequently, require production in highly regulated cleanrooms; | |
| • |
There is a need for small batch sizes, and in the case of autologous therapies, a batch per patient; | |
| • |
The manufacturing process must be adapted to a high volatility in the source material; |
S-2
| • |
Cells are highly sensitive to any change in their environment, which requires a highly controlled manufacturing environment; | |
| • |
In many cases, the resulting product cannot be frozen or stored for long periods, which provides for logistic challenges in supplying the product; | |
| • |
Regulatory as well as biological requirements may require localized manufacturing; | |
| • |
Initial development has historically been based on a small scale laboratory process; consequently, there is a need to develop an industrial process with standardized procedures and quality controls; and | |
| • |
As a new industry, there are regulatory challenges with regulatory agencies around the world in trying to implement new frameworks for these therapies. |
All of the above challenges require development of innovative manufacturing techniques and technologies, as well as logistic solutions that can provide such products in a scalable, medical grade industrial manner at a realistic affordable cost. The promise of such therapies is their potential to cure diseases and conditions which cannot be currently addressed. One such example is the latest drugs that have been approved for cancer treatment based on the CAR-T technology, which have had some success in treating patients with certain cancers, but are very expensive with limited supply capabilities.
Our goal is to face these challenges and to bridge the gap between science and clinical availability by developing technologies, platforms and providing services to the biotechnology and health industry.
We are a vertically integrated service and research company in the regenerative medicine industry with a focus on ATMP development, manufacturing and supply. Our company operates through two platforms including (i) an autologous ATMP focused Cellular Therapy (“CT”) development and supply platform and (ii) a Contract Development and Manufacturing Organization (“CDMO”) platform. Through our CT platform, we are focused on the development, advancement and supply of proprietary personalized cell therapy related technologies.
Through our CDMO platform, we are focused on manufacturing and development services for other biopharma companies. Our CDMO platform operates through our subsidiary Masthercell Global, Inc., a Delaware corporation (“Masthercell Global”), which currently holds the following subsidiaries: MaSTherCell S.A. in Belgium (our “Belgian Subsidiary”), Atvio Ltd. in Israel (our “Israeli Subsidiary”) and additional subsidiaries in South Korea and in the United States, each having unique knowhow and expertise for manufacturing in a multitude of cell types. As part of our U.S. activity, we intend to also have Masthercell Global set up a CDMO facility in the United States. We believe that, in order to provide the optimal service to our customers, we need to have a global presence. We target the international market as a key priority through our network of facilities that provide development, manufacturing and logistics services, utilizing our advanced quality management system and experienced staff. All these capabilities offered to third-parties are utilized for our internal development projects, with the goal of allowing us to potentially be able to bring new products to patients faster and in a cost-effective way. Masthercell Global strives to provide services that are all compliant with GMP requirements, ensuring identity, purity, stability, potency and robustness of cell therapy products for clinical phase I, II, III and through commercialization.
Our therapeutic development efforts are focused on advancing breakthrough scientific achievements in the field of autologous therapies which have a curative potential. We base our development on therapeutic collaborations and licensing with other pre-clinical and clinical-stage biopharma companies as well as direct collaboration with research and healthcare institutes. We are engaging in therapeutic collaborations and licensing (both directly and through our affiliated entity, Orgenesis Maryland Inc.) with other academic centers and research centers in order to pursue emerging technologies of other cell therapy products in such areas as cell-based immunotherapies, metabolic diseases, neurodegenerative diseases and tissue regeneration.
An example of such a therapy is our autologous trans-differentiation technology that demonstrates the capacity to induce a shift in the developmental fate of cells from the liver or other tissues and transdifferentiating them into “pancreatic beta cell-like” Autologous Insulin Producing (“AIP”) cells as a potential therapy for patients with Type 1 Diabetes, acute pancreatitis and other insulin deficient diseases. This technology, which has yet to be proven in human clinical trials, has shown in relevant animal models that the human derived AIP cells produce insulin in a glucose-sensitive manner. This trans-differentiation technology is licensed by our Israeli subsidiary and is based on the work of Professor Sarah Ferber, our Chief Scientific Officer and a researcher at Tel Hashomer Medical Research Infrastructure and Services Ltd. (“THM”) in Israel. Our development plan calls for conducting additional pre-clinical safety and efficacy studies with respect to diabetes and other potential indications prior to initiating human clinical trials. With respect to our trans-differentiation technology, we own or have exclusive rights to ten (10) United States and ten (10) foreign patents issued in Europe, Canada, Australia and Israel, six (6) pending applications in the United States, thirty-two (32) pending applications in foreign jurisdictions, including Europe, Australia, Brazil, Canada, China, Eurasia, Israel, Japan, South Korea, Mexico, and Singapore, and one (1) international PCT patent application, relating to (A) the trans-differentiation of cells (including hepatic cells) to cells having pancreatic “-cell phenotype and function, and (B) scaffolds and components thereof for use supporting growth of the trans-differentiated cells upon implantation, and use of the trans-differentiated cells with or without scaffolds in the treatment of degenerative pancreatic disorders including diabetes, pancreatic cancer, and pancreatitis.
S-3
Business Strategy
Our objective is to utilize our know-how and intellectual property in order to advance new autologous cell therapies to a clinical stage by leveraging and evolving our expertise to enable supply of such therapies. One of our strategies with our CT platform is to enable point-of-care (“POCare”) cell therapy supply for development as well as therapeutic treatment. We define POCare cell therapy as a process of collecting, processing and administering cells within the patient care setting, without the need to transfer the cells to be manufactured at a centralized facility. We believe the approach of decentralized manufacturing is an attractive proposition for personalized medicine because a POCare approach based on utilizing closed systems has the potential of reducing the required grade of clean room facilities, thus substantially reducing manufacturing costs. Furthermore, cell transportation, which is a high-risk and costly aspect of the supply chain, could potentially be minimized or eliminated.
While our POCare strategy is currently limited to early stage development, we intend to continue developing a global POCare network through our CT platform, with the goal of developing autologous cell therapies with regional partners. We believe that we will reduce costs by leveraging our joint ventures with local partners who bring strong regional networks. Such networks include partnerships with local hospitals which allows us to engage in continuous in-licensing of autologous therapies from academia and research institutes, co-development of hospital and academic-based therapies, and utilization of hospital networks for clinical development of therapies.
We intend to leverage our following capabilities in order to advance our POCare strategy:
| • | industrial manufacturing know-how and biological assay development; | |
| • | integration of higher automation levels; | |
| • | providing ingredients (assays, viruses, etc.) and technology components (e.g. sensors); | |
| • | implementation of manufacturing automation enabling closed systems; | |
| • | implementation of GMP quality systems; | |
| • | regulatory and clinical development support; and | |
| • | preclinical development and animal testing. |
We operate our CDMO and the CT platforms as two separate business segments.
Additional Information
We were incorporated in the state of Nevada on June 5, 2008 under the name Business Outsourcing Services, Inc. Effective August 31, 2011, we completed a merger with our subsidiary, Orgenesis Inc., a Nevada corporation, which was incorporated solely to effect a change in its name. As a result, the Company changed its name from “Business Outsourcing Services, Inc.” to “Orgenesis Inc.” Our principal executive offices are located at 20271 Goldenrod Lane, Germantown, MD 20876, and our telephone number is (480) 659-6404. Our website address is http://www.orgenesis.com. The information contained in, or accessible through, our website does not constitute a part of this prospectus supplement or the accompanying prospectus.
S-4
THE OFFERING
The following summary contains basic information about our common stock and the offering and is not intended to be complete. It does not contain all the information that may be important to you. For a more complete understanding of our common stock, you should read this prospectus supplement and the accompanying prospectus and the documents referred to herein and therein.
| Common stock offered by us |
Shares of our common stock having an aggregate offering price of up to $25,000,000. |
| Common stock to be outstanding after this offering |
Up to 18,872,363 shares (as more fully described in the notes following this table), assuming sales of 5,252,101 shares of our common stock in this offering at an assumed offering price of $4.76 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on December 19, 2018. The actual number of shares issued in connection with this offering will vary depending on how many shares of our common stock we choose to sell and the prices at which such sales occur. |
| Plan of Distribution |
An “at the market offering” of common stock that may be made from time to time through our sales agent, Cantor Fitzgerald. See “Plan of Distribution” beginning on page S-13 of this prospectus supplement. |
| Use of Proceeds |
We currently intend to use the net proceeds from this offering, if any, for working capital and general corporate purposes. See “Use of Proceeds” beginning on page S-10 of this prospectus supplement. |
| Risk Factors |
Your investment in shares of our common stock involves substantial risks. You should consider the matters referred to under the heading “Risk Factors” beginning on page S-6 in this prospectus supplement, and any documents incorporated by reference, for certain considerations relevant to an investment in our common stock. |
| Nasdaq Capital Market symbol |
“ORGS” |
The number of shares of our common stock to be outstanding after this offering is based on 14,915,507 shares of our common stock issued and outstanding as of August 31, 2018 and excludes:
| • |
2,451,876 shares of our common stock issuable upon the exercise of stock options outstanding as of August 31, 2018, at a weighted-average exercise price of $4.56 per share; | |
|
| ||
| • |
6,330,642 shares of our common stock issuable upon the exercise of warrants outstanding as of August 31, 2018, at a weighted-average exercise price of $6.29 per share; | |
|
| ||
| • |
1,120,257 shares of common stock available for future issuance under our 2017 Stock Incentive Plan as of August 31, 2018; and | |
|
| ||
| • |
494,880 shares of common stock available for future issuance under our 2012 Stock Incentive Plan as of August 31, 2018. |
S-5
RISK FACTORS
An investment in our common stock involves risks. You should carefully consider the risks described below, as well as the other information included or incorporated by reference in this prospectus supplement and the accompanying prospectus, including “Risk Factors” included in our Annual Report on Form 10-K for the year ended November 30, 2017, filed with the SEC on February 28, 2017 and our Quarterly Report on Form 10-Q for the quarter ended August 31, 2018, filed with the SEC on October 12, 2018, and in any subsequent Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, before making an investment decision. In addition, please read “About this Prospectus Supplement” in this prospectus supplement and “Special Note Regarding Forward-Looking Statements” in this prospectus supplement, where we describe additional uncertainties associated with our business and the forward-looking statements included or incorporated by reference in this prospectus supplement and the accompanying prospectus. If any of these risks occur, our business, financial condition, results of operations and future growth prospects could be materially and adversely affected. In these circumstances, the market price of our common stock could decline, and you may lose all or part of your investment. Please note that additional risks not currently known to us or that we currently deem immaterial may also impair our business and operations.
Risks Related to this Offering
You will incur immediate and substantial dilution as a result of this offering.
The assumed offering price per share of our common stock in this offering is substantially higher than the current net tangible book value per share of our common stock. Therefore, if you purchase shares of our common stock in this offering, you will pay a price per share that substantially exceeds our net tangible book value per share after this offering. Assuming the sale of $25,000,000 of our common stock in this offering, based on the assumed offering price of $4.76 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on December 19, 2018, and net tangible book value per share of our common stock of $15,402,000 as of August 31, 2018, if you purchase shares in this offering, you will suffer immediate and substantial dilution of $2.67 per share in the as adjusted net tangible book value of common stock purchased. See “Dilution” beginning on page S-11 of this prospectus supplement for a more detailed description of the dilution to new investors in this offering.
We have broad discretion in the use of net proceeds from this offering and may not use them effectively.
We currently intend to use the net proceeds, if any, from this offering for working capital and general corporate purposes. See “Use of Proceeds” beginning on page S-10 of this prospectus supplement for further detail. Although we currently intend to use the net proceeds, if any, from this offering in such a manner, we will have broad discretion in the application of such net proceeds. Our failure to apply these funds effectively could result in unfavorable returns and uncertainty about our prospects, each of which could cause the price of our common stock to decline. Pending their use, we may invest the net proceeds from this offering in a manner that does not produce income or loses value.
You may experience future dilution as a result of future equity offerings.
To raise additional capital, we may in the future offer additional shares of our common stock or other securities convertible into or exchangeable for our common stock at prices that may not be the same as the price per share in this offering. We may sell shares or other securities in any other offering at a price per share that is less than the price per share paid by investors in this offering, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering.
Sales of a significant number of shares of our common stock in the public markets, or the perception that such sales could occur, could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock in the public markets could occur at any time and could depress the market price of our common stock and impair our ability to raise capital through the sale of additional equity or equity-based securities. We cannot predict the effect that future sales of our common stock would have on the market price of our common stock.
Because we have no current plans to pay cash dividends on our common stock for the foreseeable future, you may not receive any return on investment unless you sell your common stock for a price greater than that which you paid for it.
S-6
We intend to retain future earnings, if any, for future operations and expansion of our business and have no current plans to pay any cash dividends for the foreseeable future. The declaration, amount and payment of any future dividends on shares of common stock will be at the sole discretion of our Board of Directors. Our Board of Directors may take into account general and economic conditions, our financial condition, and results of operations, our available cash and current and anticipated cash needs, capital requirements, contractual, legal, tax and regulatory restrictions, implications on the payment of dividends by us to our stockholders or by our subsidiaries to us and such other factors as our Board of Directors may deem relevant. In addition, our ability to pay dividends is limited by covenants of our existing and outstanding indebtedness and may be limited by covenants of any future indebtedness we or our subsidiaries incur. As a result, you may not receive any return on an investment in our common stock unless you sell our common stock for a price greater than that which you paid for it.
S-7
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein include “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Exchange Act. All statements, other than statements of historical facts, included in this prospectus supplement, the accompanying prospectus and the information incorporated by reference herein and therein regarding our strategy, future operations, future financial position, future revenue, projected costs, prospects, plans, objectives of management and expected market growth are forward-looking statements. The words “anticipate,” “believe,” “contemplate,” “estimate,” “expect,” “forecast,” “future,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. These forward-looking statements include, among other things, statements about:
Corporate
| • |
our ability to achieve profitability; | |
| • |
our ability to increase revenues and raise sufficient capital or strategic business arrangements to expand our CDMO global network; | |
| • |
our ability to grow the size and capabilities of our organization through further collaboration and strategic alliances; | |
| • |
our ability to manage the growth of our CDMO business; | |
| • |
our ability to attract and retain key scientific or management personnel and to expand our management team; the accuracy of estimates regarding expenses, future revenue, capital requirements, profitability, and needs for additional financing; | |
| • |
our belief that our therapeutic related developments have competitive advantages such as our cell trans-differentiation technology being developed by our Israeli Subsidiary and being able to compete favorably and profitably in the regenerative medicine sector; |
CDMO business
| • |
our ability to grow the business of Masthercell Global, including through the Belgian Subsidiary, which we acquired in our fiscal year 2015, as our principal contract development and manufacturing (CDMO) business and continues to act as such through Masthercell Global; | |
| • |
our ability to attract and retain customers; | |
| • |
our ability to expand and maintain our CDMO business through strategic alliances, joint ventures and internal growth; | |
| • |
our ability to fund the operational and capital requirements of the global expansion of Masthercell Global and our CDMO business; | |
| • |
our expectations regarding Masthercell Global•s expenses and revenue, including our expectations that our research and development expenses and selling, general and administrative expenses may increase in absolute dollars; | |
| • |
the successful integration of our clinical and CDMO strategy through Masthercell Global and its subsidiaries; | |
| • |
our ability to contract (through Masthercell Global and its subsidiaries) with third-party suppliers and manufacturers and their ability to perform adequately; | |
| • |
extensive industry regulation, and how that will continue to have a significant impact on our business, especially our product development, manufacturing and distribution capabilities; | |
| • |
the ability of Masthercell Global to receive future payments pursuant to the Stock Purchase Agreement with GPP-II; | |
| • |
our and our shareholders• ability to receive value on par with GPP-II upon its forced sale of Masthercell Global; | |
| • |
our ability to control, direct the activities of or hold any shares in Masthercell Global if GPP-II were to assume control of the Board of Directors of Masthercell Global or if it were to purchase our shares in Masthercell Global; | |
| • |
our ability to receive benefits or value from Masthercell Global in the event of a spin-off effectuated by GPP-II; | |
| • |
the significant potential dilution to our existing shareholders due to GPP-II•s option to exchange its Masthercell Global Preferred Stock for shares of our common stock; |
Cellular Therapy business (“CT”)
| • |
our ability to develop, through our Israeli Subsidiary and Belgian Subsidiary, to the clinical stage a new technology to transdifferentiate liver cells into functional insulin-producing cells, thus enabling normal glucose regulated insulin secretion, via cell therapy; | |
| • |
our ability to advance our therapeutic collaborations in terms of industrial development, clinical development, regulatory challenges, commercial partners and.manufacturing availability; |
S-8
| • |
our belief that our diabetes-related treatment seems to be safer than other options; | |
| • |
expectations regarding the ability of our Israeli Subsidiary and our Belgian Subsidiary to obtain additional and maintain intellectual property protection for our technology and therapies; | |
| • |
our ability to commercialize products in light of the intellectual property rights of others; | |
| • |
our ability to obtain funding necessary to start and complete such clinical trials; | |
| • |
our belief that Diabetes Mellitus will be one of the most challenging health problems in the 21st century and will have staggering health, societal and economic impact; | |
| • |
our relationship with Tel Hashomer Medical Research Infrastructure and Services Ltd. (“THM”) and the risk that THM may cancel the License Agreement; and | |
| • |
expenditures not resulting in commercially successful products. |
We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in the forward-looking statements we make. We have included important factors in the cautionary statements included in this prospectus supplement, the accompanying prospectus, and the information incorporated by reference herein and therein, particularly in the “Risk Factors” sections of this prospectus supplement, the accompanying prospectus and of our Annual Report on Form 10-K for the year ended November 30, 2017 and Quarterly Report on Form 10-Q for the quarter ended August 31, 2018, which are incorporated by reference herein, that could cause actual results or events to differ materially from the forward-looking statements that we make. Our forward-looking statements do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments that we may make.
S-9
USE OF PROCEEDS
We may issue and sell shares of our common stock having aggregate gross sales proceeds of up to $25,000,000 from time to time under this prospectus supplement and the accompanying prospectus. Because there is no minimum offering amount required pursuant to the Sales Agreement with Cantor Fitzgerald, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. Actual net proceeds will depend on the number of shares we sell and the prices at which such sales occur. There can be no assurance that we will sell any shares under or fully utilize the Sales Agreement with Cantor Fitzgerald as a source of financing.
We currently intend to use the net proceeds, if any, from sales of shares of our common stock covered by this prospectus supplement and the accompanying prospectus for working capital and general corporate purposes, which may include, among other things, the funding of capital and other expenditures, including to expand our manufacturing capabilities, and the acquisition or in-license of additional therapeutics, product candidates or technology.
Our expected use of net proceeds, if any, from the sale of shares of common stock pursuant to the Sales Agreement with Cantor Fitzgerald represents our intentions based upon our present plans and business conditions as of the date of this prospectus supplement, which could change in the future as our plans and business conditions evolve. The amounts and timing of our actual expenditures will depend upon numerous factors, including, but not limited to:
| • |
initiation, progress, timing, costs and results of preclinical studies and clinical trials of our cell therapy product candidates; | |
| • |
our obligation to make royalty and non-royalty sublicense payments to third-party licensors, if any, under our licensing agreements; | |
| • |
the timing, receipt, and amount of milestone payments or royalties, if any, from any of our product candidates; | |
| • |
the number and characteristics of product candidates that we discover or in-license and develop; | |
| • |
the outcome, timing and cost of seeking regulatory review by the FDA and comparable foreign regulatory authorities, including the potential for the FDA or comparable foreign regulatory authorities to require that we perform more studies than those that we currently expect; | |
| • |
the costs of filing, prosecuting, defending and enforcing any patent claims and maintaining and enforcing other intellectual property rights; | |
| • |
subject to receipt of marketing approval, revenue, if any, received from commercial sales of any products; | |
| • |
the costs and timing of the implementation of commercial-scale manufacturing activities; | |
| • |
the costs and timing of establishing sales, marketing and distribution capabilities for any product candidates for which we may receive regulatory approval; and | |
| • |
the costs of operating as a public company. |
As a result, our management will have broad discretion over the use of the net proceeds from this offering, if any. We may temporarily invest the net proceeds in investment-grade, interest-bearing securities pending their use for the purposes described above. We have not determined the amount of net proceeds to be used specifically for such purposes and, as a result, management will retain broad discretion over the allocation of net proceeds from this offering, if any.
S-10
DILUTION
If you invest in this offering, your ownership interest will be diluted to the extent of the difference between the public offering price per share and the as adjusted net tangible book value per share after giving effect to this offering. We calculate net tangible book value per share by dividing the net tangible book value, which is tangible assets less total liabilities, by the number of outstanding shares of our common stock. Dilution represents the difference between the amount per share paid by purchasers of shares in this offering and the as adjusted net tangible book value per share of our common stock immediately after giving effect to this offering. Our net tangible book value as of August 31, 2018 was approximately $15,402,000, or $1.13 per share.
After giving effect to the sale of our common stock pursuant to this prospectus supplement and accompanying prospectus in the aggregate amount of $25,000,000 at an assumed offering price of $4.76 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on December 19, 2018, and after deducting offering commissions and estimated expenses payable by us in the aggregate, our net tangible book value as of August 31, 2018 would have been $39,602,000, or $2.10 per share of common stock. This represents an immediate increase in the net tangible book value of $0.97 per share to our existing stockholders and an immediate dilution in net tangible book value of $2.67 per share to new investors. The following table illustrates this per share dilution:
| Assumed offering price per share | $ | 4.76 | |
| Net tangible book value per share as of August 31, 2018 | $ | 15,402,000 | |
| Increase per share attributable to this offering | 0.97 | ||
| As adjusted net tangible book value per share as of August 31, 2018 after giving effect to this offering | 2.10 | ||
| Dilution per share to new investors in this offering | $ | 2.67 |
The table above assumes for illustrative purposes that an aggregate of 5,252,101 shares of our common stock are sold at a price of $4.76 per share, which was the last reported sale price of our common stock on the Nasdaq Capital Market on December 19, 2018, for aggregate gross proceeds of $25,000,000. The shares sold in this offering, if any, will be sold from time to time at various prices. An increase of $1.00 per share in the price at which the shares are sold from the assumed offering price of $4.76 per share shown in the table above, assuming all of our common stock in the aggregate amount of $25,000,000 during the term of the Sales Agreement with Cantor Fitzgerald is sold at that price, would increase our as adjusted net tangible book value per share after the offering to $2.21 per share and would increase the dilution in net tangible book value per share to new investors in this offering to $3.56 per share, after deducting offering commissions and estimated expenses payable by us. A decrease of $1.00 per share in the price at which the shares are sold from the assumed offering price of $4.76 per share shown in the table above, assuming all of our common stock in the amount of $25,000,000 during the term of the Sales Agreement with Cantor Fitzgerald is sold at that price, would decrease our as adjusted net tangible book value per share after the offering to $1.95 per share and would decrease the dilution in net tangible book value per share to new investors in this offering to $1.81 per share, after deducting offering commissions and estimated offering expenses payable by us. This information is supplied for illustrative purposes only and may differ based on the actual offering price and the actual number of shares offered.
The above discussion and table are based on 14,915,507 shares of our common stock issued and outstanding as of August 31, 2018 and exclude:
| • |
2,451,876 shares of our common stock issuable upon the exercise of stock options outstanding as of August 31, 2018, at a weighted-average exercise price of $4.56 per share; | |
|
| ||
| • |
6,330,642 shares of our common stock issuable upon the exercise of warrants outstanding as of August 31, 2018, at a weighted-average exercise price of $6.29 per share; | |
|
| ||
| • |
1,120,257 shares of common stock available for future issuance under our 2017 Stock Incentive Plan as of August 31, 2018; and | |
|
| ||
|
• |
494,880 shares of common stock available for future issuance under our 2012 Stock Incentive Plan as of August 31, 2018. |
S-11
any shares of common stock that could potentially be issuable to GPP-II in connection with its right to exchange its preferred stock in Masthercell Global for shares of our common stock under certain conditions. To the extent that any options are exercised, new equity awards are granted under our equity incentive plans or we otherwise issue additional shares of common stock in the future, investors purchasing shares in this offering could experience further dilution. In addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or equity-based securities, the issuance of these securities could result in further dilution to our stockholders.
S-12
PLAN OF DISTRIBUTION
We have entered into a Controlled Equity OfferingSM Sales Agreement, or the Sales Agreement, with Cantor Fitzgerald & Co., or Cantor Fitzgerald, under which we may offer and sell shares of our common stock having an aggregate gross sales price of up to $25,000,000 from time to time through Cantor Fitzgerald acting as agent. The Sales Agreement has been filed as an exhibit to a Current Report on Form 8-K and incorporated by reference into this prospectus supplement and the accompanying prospectus.
Upon delivery of a placement notice and subject to the terms and conditions of the Sales Agreement, Cantor Fitzgerald may offer and sell shares of our common stock by any method permitted by law deemed to be an “at the market offering” as defined in Rule 415(a)(4) promulgated under the Securities Act. We may instruct Cantor Fitzgerald not to sell common stock if the sales cannot be effected at or above the price designated by us from time to time. We or Cantor Fitzgerald may suspend the offering of common stock upon notice and subject to other conditions.
We will pay Cantor Fitzgerald commissions, in cash, for its services in acting as agent in the sale of our common stock. Cantor Fitzgerald is entitled to compensation at a commission rate of 3.0% of the gross sales price per share sold under the Sales Agreement. Because there is no minimum offering amount required as a condition to close this offering, the actual total public offering amount, commissions and proceeds to us, if any, are not determinable at this time. We have also agreed to reimburse Cantor Fitzgerald for certain specified expenses, including the reasonable and documented fees and disbursements of its legal counsel in an amount not to exceed $50,000. We estimate that the total expenses for the offering under this prospectus supplement, excluding compensation and reimbursements payable to Cantor Fitzgerald under the terms of the Sales Agreement, will be approximately $100,000.
Settlement for sales of shares of common stock will occur on the second business day following the date on which any sales are made, or on some other date that is agreed upon by us and Cantor Fitzgerald in connection with a particular transaction, in return for payment of the net proceeds to us. Sales of our common stock as contemplated in this prospectus supplement will be settled through the facilities of The Depository Trust Company or by such other means as we and Cantor Fitzgerald may agree upon. There is no arrangement for funds to be received in an escrow, trust or similar arrangement.
Cantor Fitzgerald will use its commercially reasonable efforts, consistent with its sales and trading practices, to solicit offers to purchase the shares of common stock under the terms and subject to the conditions set forth in the Sales Agreement. In connection with the sale of the shares of common stock on our behalf, Cantor Fitzgerald will be deemed to be an “underwriter” within the meaning of the Securities Act and the compensation of Cantor Fitzgerald will be deemed to be underwriting commissions or discounts. We have agreed to provide indemnification and contribution to Cantor Fitzgerald (and its partners, members, directors, officers, employees and agents) against certain civil liabilities, including liabilities under the Securities Act.
The offering of shares of our common stock pursuant to the Sales Agreement will terminate upon the termination of the Sales Agreement as permitted therein. We and Cantor Fitzgerald may each terminate the Sales Agreement at any time upon ten days• prior notice.
Cantor Fitzgerald and its affiliates may in the future provide various investment banking, commercial banking and other financial services for us, our subsidiaries and our affiliates, for which services they may in the future receive customary fees. To the extent required by Regulation M, Cantor Fitzgerald will not engage in any market making activities involving our common stock while the offering is ongoing under this prospectus supplement.
This prospectus supplement and the accompanying prospectus in electronic format may be made available on a website maintained by Cantor Fitzgerald and Cantor Fitzgerald may distribute this prospectus supplement and the accompanying prospectus electronically.
S-13
LEGAL MATTERS
The validity of the shares of common stock offered hereby will be passed upon for us by Pearl Cohen Zedek Latzer Baratz LLP. Mintz, Levin, Cohn, Ferris, Glovsky and Popeo, P.C., New York, New York and Pearl Cohen Zedek Latzer Baratz LLP are advising us with respect to certain securities law and corporate law matters in connection with the offering. Cantor Fitzgerald & Co. is being represented in connection with this offering by Cooley LLP, New York, New York.
EXPERTS
The consolidated financial statements of Orgenesis Inc. and its subsidiaries as of November 30, 2017 and 2016 and for each of the years in the two-year period ended November 30, 2017 incorporated in this prospectus supplement and the accompanying prospectus by reference from the Orgenesis Inc. Annual Report on Form 10-K for the year ended November 30, 2017 have been audited by Kesselman & Kesselman, a member firm of PricewaterhouseCoopers International Limited, an independent registered public accounting firm, as stated in their report thereon incorporated herein by reference, and have been incorporated in this prospectus supplement, the accompanying prospectus and registration statement in reliance upon such report and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities offered by this prospectus supplement. This prospectus supplement and the accompanying prospectus, which are part of the registration statement, omits certain information, exhibits, schedules and undertakings set forth in the registration statement, as permitted by the SEC. For further information pertaining to us and the securities offered in this prospectus supplement, reference is made to that registration statement and the exhibits and schedules to the registration statement. Statements contained in this prospectus supplement and the accompanying prospectus as to the contents or provisions of any documents referred to in this prospectus are not necessarily complete, and in each instance where a copy of the document has been filed as an exhibit to the registration statement, reference is made to the exhibit for a more complete description of the matters involved.
We are subject to the reporting requirements of the Exchange Act and file annual, quarterly and current reports, proxy statements and other information with the SEC. SEC filings are available at the SEC•s web site at http://www.sec.gov. Our common stock is listed on the Nasdaq Capital Market under the symbol “ORGS.” General information about our company, including our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as any amendments and exhibits to those reports, are available free of charge through our website at http://www.orgenesis.com as soon as reasonably practicable after we file them with, or furnish them to, the SEC. Information on, or than can be accessed through, our website is not incorporated into this prospectus supplement or other securities filings and is not a part of these filings.
INCORPORATION OF DOCUMENTS BY REFERENCE
The SEC allows us to incorporate by reference in this prospectus supplement and the accompanying prospectus much of the information we file with the SEC, which means that we can disclose important information to you by referring you to those publicly available documents. The information that we incorporate by reference in this prospectus supplement and the accompanying prospectus is considered to be part of this prospectus supplement and the accompanying prospectus. Because we are incorporating by reference future filings with the SEC, this prospectus supplement and the accompanying prospectus is continually updated and those future filings may modify or supersede some of the information included or incorporated in this prospectus supplement and the accompanying prospectus. This means that you must look at all of the SEC filings that we incorporate by reference to determine if any of the statements in this prospectus supplement, the accompanying prospectus or in any document previously incorporated by reference have been modified or superseded. This prospectus supplement and the accompanying prospectus incorporate by reference the documents listed below (File No. 000-54329 and File No. 001-38416) and any future filings we make with the SEC under Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act (in each case, other than those documents or the portions of those documents not deemed to be filed) until the offering of the securities under the registration statement is terminated or completed:
| • |
our Annual Report on Form 10-K for the year ended November 30, 2017 filed with the SEC on February 28, 2018; | |
|
| ||
| • |
our Quarterly Reports on Form 10-Q for the quarters ended February 28, 2018, May 31, 2018 and August 31, 2018, filed with the SEC on April 16, 2018, July 17, 2018 and October 12, 2018, respectively; | |
|
| ||
| • |
the portions of our definitive proxy statement on Schedule 14A filed with the SEC on September 21, 2018 that are deemed “filed” with the SEC under the Exchange Act; |
S-14
| • |
our Current Reports on Form 8-K filed with the SEC on March 21, 2018, April 30, 2018, May 18, 2018, June 25, 2018, June 29, 2018, July 5, 2018, October 25, 2018, November 20, 2018, November 28, 2018, December 6, 2018 and December 20, 2018; | |
|
| ||
| • |
the description of our common stock contained in our Registration Statement on Form 8-A filed on March 8, 2018, including any amendments or reports filed for the purpose of updating such description; and | |
|
| ||
| • |
all reports and other documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 and 15(d) of the Exchange Act after the date of this prospectus supplement and prior to the termination or completion of the offering of securities under this prospectus supplement shall be deemed to be incorporated by reference in this prospectus and to be a part hereof from the date of filing such reports and other documents. |
Any statement contained in this prospectus supplement or in a document incorporated or deemed to be incorporated by reference into this prospectus supplement will be deemed to be modified or superseded for purposes of this prospectus supplement to the extent that a statement contained in this prospectus supplement or any other subsequently filed document that is deemed to be incorporated by reference into this prospectus supplement modifies or supersedes the statement. Any statement so modified or superseded will not be deemed, except as so modified or superseded, to constitute a part of this prospectus supplement.
You may request, orally or in writing, a copy of any or all of the documents incorporated herein by reference. These documents will be provided to you at no cost, by contacting:
Orgenesis Inc.
20271 Goldenrod Lane
Germantown, Maryland
20876
Attention: Secretary
Telephone: (480) 659-6404
You should rely only on information contained in, or incorporated by reference into, this prospectus supplement and the accompanying prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus supplement or incorporated by reference in this prospectus supplement. We are not making offers to sell the securities in any jurisdiction in which such an offer or solicitation is not authorized or in which the person making such offer or solicitation is not qualified to do so or to anyone to whom it is unlawful to make such offer or solicitation.
S-15
PROSPECTUS
ORGENESIS INC.
$100,000,000
Common Stock, Debt Securities, Warrants, Subscription Rights and Units
We may offer and sell up to $100,000,000 in the aggregate of any combination of the securities described in this prospectus, either individually or in units from time to time in one or more offerings. We will provide the specific terms of these offerings and securities in one or more supplements to this prospectus. We may also authorize one or more free writing prospectuses to be provided to you in connection with these offerings. The prospectus supplement and any related free writing prospectus may also add, update or change information contained in this prospectus. You should carefully read this prospectus, the applicable prospectus supplement and any related free writing prospectus, as well as any documents incorporated by reference, before you buy any of the securities being offered.
Our common stock is listed on the Nasdaq Capital Market under the symbol “ORGS.” On March 16, 2018, the last reported sale price of our common stock was $13.40 per share. The applicable prospectus supplement will contain information, where applicable, as to other listings, if any, on The Nasdaq Capital Market or other securities exchange of the securities covered by the prospectus supplement.
Investing in our securities involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” contained on page 9 of this prospectus and in the applicable prospectus supplement and in any free writing prospectuses we have authorized for use in connection with a specific offering, and under similar headings in the documents that are incorporated by reference into this prospectus.
This prospectus may not be used to consummate a sale of securities unless accompanied by a prospectus supplement.
The securities may be sold directly by us to investors, through agents designated from time to time or to or through agents, underwriters or dealers, on a continuous or delayed basis. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. If any agents, underwriters or dealers are involved in the sale of any securities with respect to which this prospectus is being delivered, the names of such agents, underwriters or dealers and any applicable fees, commissions, discounts and over-allotment options will be set forth in a prospectus supplement. The price to the public of such securities and the net proceeds we expect to receive from such sale will also be set forth in a prospectus supplement.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is March 28, 2018.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement on Form S-3 that we filed with the Securities and Exchange Commission, or SEC, using a “shelf” registration process. Under this shelf registration statement, we may offer and sell any combination of the securities described in this prospectus for total gross proceeds of up to $100,000,000 from time to time in one or more offerings.
Each time we offer securities under this prospectus, we will provide a prospectus supplement that will contain more specific information about the terms of that offering. We may also authorize one or more free writing prospectuses to be provided to you that may contain material information relating to these offerings. The prospectus supplement and any related free writing prospectus that we may authorize to be provided to you may also add, update or change any of the information contained in this prospectus or in the documents that we have incorporated by reference into this prospectus. We urge you to read carefully this prospectus, any applicable prospectus supplement and any free writing prospectuses we have authorized for use in connection with a specific offering, together with the information incorporated herein by reference as described under the section entitled “Incorporation by Reference,” before buying any of the securities being offered.
This prospectus may not be used to consummate a sale of securities unless it is accompanied by a prospectus supplement
You should rely only on the information contained in, or incorporated by reference into, this prospectus and any applicable prospectus supplement, along with the information contained in any free writing prospectuses we have authorized for use in connection with a specific offering. We have not authorized anyone to provide you with different or additional information. This prospectus is an offer to sell only the securities offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so.
The information appearing in this prospectus, any applicable prospectus supplement or any related free writing prospectus is accurate only as of the date on the front of the document and that any information we have incorporated by reference is accurate only as of the date of the document incorporated by reference, regardless of the time of delivery of this prospectus, any applicable prospectus supplement or any related free writing prospectus, or any sale of a security. Our business, financial condition, results of operations and prospects may have changed since those dates.
This prospectus incorporates by reference market data, industry statistics and other data that have been obtained from, or compiled from, information made available by third parties. We have not independently verified their data. This prospectus and the information incorporated herein by reference includes trademarks, service marks and trade names owned by us or other companies. All trademarks, service marks and trade names included or incorporated by reference into this prospectus, any applicable prospectus supplement or any related free writing prospectus are the property of their respective owners.
This prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled “Where You Can Find More Information.”
1
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus or incorporated by reference in this prospectus and does not contain all of the information that you need to consider in making your investment decision. You should carefully read the entire prospectus (as supplemented and amended), including the financial data and related notes, risk factors and other information incorporated by reference in this prospectus, before making an investment decision. Unless otherwise mentioned or unless the context requires otherwise, all references in this prospectus to “Orgenesis,” “the company,” “we,” “us,” “our” or similar references mean Orgenesis Inc.
Orgenesis Inc.
We are a service and research company in the field of the regenerative medicine industry with a focus on cell therapy development and manufacturing for advanced medicinal products serving the regenerative medicine industry. In addition, we are focused on developing novel and proprietary cell therapy trans-differentiation technologies for the treatment of diabetes.
A large number of diseases still face unmet needs and biologics• efficacy is not always sufficient. Cell therapy has a larger therapeutic effect as it is based on augmenting, repairing, replacing or regenerating organs and tissues “ leveraging the human body•s capacity to heal. It therefore holds the promise to be able to cure diseases that present both a major health care and economic burden, such as cancer, diabetes and cardiovascular diseases. But if the regenerative market is to realize its full potential, manufacturing and logistics need to be in place to ensure these products• safety, potency and consistency at an economically sustainable cost. We have built up our long-term strategy on this industry assessment.
Our vertically integrated manufacturing capabilities are being used to serve emerging technologies of cell therapy clients in such areas as cell-based cancer immunotherapies and neurodegenerative diseases and also to optimize our abilities to scale-up our proprietary technologies for clinical trials and eventual commercialization of our proposed diabetes treatment. Our hybrid business model combining our own proprietary cell therapy trans-differentiation technologies for the treatment of diabetes and a revenue-generating contract development and manufacturing service business provides us with unique capabilities and supports our mission of accelerating the development and marketing breakthrough life-improving medical treatments.
MaSTherCell, S.A., our Belgian subsidiary, is a contract development manufacturing organization (“CDMO”), specialized in cell therapy development for advanced medicinal products. In the last decade, cell therapy and regenerative medicine products have gained significant importance, particularly in the fields of ex-vivo gene therapy and immunotherapy. While academic and industrial research has triggered scientific development in the sector, manufacturing capabilities and industrialization expertise remains insufficient. Formed in 2011 as a spinoff of the Université Libre de Bruxelles (ULB), MaSTherCell has assembled a recognized team of experts and talents, attract and retains first class customers. We acquired MaSTherCell in March 2015.
We have leveraged the recognized expertise and experience in cell process development and manufacturing of MaSTherCell, and our international joint ventures, in Israel and Korea, to build a global and fully integrated network in the cell therapy development and manufacturing area. We believe that cell therapy companies need to be global in order to truly succeed. We target the international manufacturing market as a key priority through joint-venture agreements that provide development capabilities, along with manufacturing facilities and experienced staff. All of these capabilities offered to third-parties will be mobilized for our internal development projects, allowing us to be in a position to bring new products to the patients faster and in a cost-efficient way.
Our trans-differentiation technologies for treating diabetes, which we will refer to as our cellular therapy (“CT”) business, is based on the technology licensed by our Israeli subsidiary, Orgenesis Ltd., that demonstrates the capacity to induce a shift in the developmental fate of cells from the liver and differentiating (converting) them into “pancreatic beta cell-like” insulin producing cells for patients with Type 1 Diabetes. Our trans-differentiation technologies technology for diabetes is from the work of Prof. Sarah Ferber, our Chief Science Officer and a researcher at Tel Hashomer Medical Center, a leading medical hospital and research center in Israel (“THM”). Improved over the years, this research demonstrates the capacity to induce a shift in the developmental fate of cells from the liver and transdifferentiating them into “pancreatic beta cell-like” Autologous Insulin Producing (“AIP”) cells. Moreover, those cells were found to be resistant to autoimmune attack and to produce insulin in a glucose-sensitive manner in relevant animal models which significantly broadens the potential of the technology for other therapeutics areas. Our development plan calls for conducting additional preclinical safety and efficacy studies with respect to diabetes and other potential indications prior to initiating clinical trials. In parallel, we work on establishing the GMP manufacturing process which development is already accomplished.
2
We operate our CT and CDMO businesses as two separate segments.
Recent Developments
On November 15, 2017, we, MaSTherCell and the Belgian Sovereign Funds Société Fédérale de Participations et d'Investissement (“SFPI”) entered into a Subscription and Shareholders Agreement (the “Agreement”) pursuant to which SFPI made an equity investment in MaSTherCell in the aggregate amount of €5million (approximately $5.9 million), for approximately 19.7% of MaSTherCell. The equity investment included the conversion of the then outstanding loan of €1 million (approximately $1.1 million) plus accrued interest in the approximate amount of €70 thousand (approximately $77,000), previously made by SFPI to MaSTherCell (the “Loan Amount”).
Under the Agreement, an initial subscription amount of €2 million (approximately $2.3 million) was paid and the outstanding Loan Amount been converted. The balance of approximately €2 million is payable as needed by MaSTherCell and called in by the board of directors of MaSTherCell. The proceeds of the investment will be used to expand MaSTherCell•s facilities in Belgium by the addition of five new cGMP manufacturing cleanrooms.
This expansion will position MaSTherCell as the European hub for the Company•s continental activities and strengthen its leading position in cell and gene manufacturing. The state-of-the-art design enables MaSTherCell to offer full flexibility for production and process development.
On November 16, 2017, we implemented a one for twelve (1:12) reverse stock split of our issued, outstanding and authorized common stock. The reverse stock split was implemented by us in connection with our application to list our common stock on the Nasdaq Capital Market and was necessary in order to satisfy the initial listing bid price requirements.
On January 22, 2018, we announced a strategic organizational regrouping of our CDMO global manufacturing services offerings. The planned CDMO regrouping will utilize the global MaSTherCell brand, except for Atvio Biotech Ltd. for its unique technology and innovation activity located in Israel and serving the global cell & gene therapy markets. The primary purpose of the strategic regrouping is to create a more efficient CDMO corporate organization that can optimally utilize resources and more efficiently broaden, streamline and harmonize the CDMO service offerings on a global basis utilizing the quality and standards of Belgian-based subsidiary, MaSTherCell S.A. In connection with this and in order to align our CDMO activities, we plan to transfer to a newly formed and wholly-owned Delaware-based holding company, named MaSTherCell Global Inc., our interests in MaSTherCell S.A., as well as in our joint venture partners in Israel and Korea. When successfully concluded, of which no assurance can be provided, each of MaSTherCell S.A., our flagship Belgian based CDMO subsidiary, Atvio Biotech Ltd., our Israel based CDMO partner and CureCell Co. Ltd., our Korea based CDMO partners, will be direct subsidiaries of MaSTherCell Global Inc.
On March 8, 2018, our application to list our common stock on the Nasdaq Capital Market was approved and on March 13, 2018, our stock began trading on the Nasdaq Capital Market.
The Securities We May Offer
We may offer shares of our common stock, various series of debt securities and warrants to purchase any of such securities, either individually or in units, with a total value of up to $100,000,000 from time to time under this prospectus at prices and on terms to be determined by market conditions at the time of offering. This prospectus provides you with a general description of the securities we may offer. Each time we offer a type or series of securities, we will provide a prospectus supplement that will describe the specific amounts, prices and other important terms of the securities, including, to the extent applicable:
3
| • |
aggregate principal amount or aggregate offering price; | |
|
| ||
| • |
maturity, if applicable; | |
|
| ||
| • |
original issue discount, if any; | |
|
| ||
| • |
rates and times of payment of interest, if any; | |
|
| ||
| • |
redemption, conversion, exchange or sinking fund terms, if any; | |
|
| ||
| • |
conversion or exchange prices or rates, if any, and, if applicable, any provisions for changes to or adjustments in the conversion or exchange prices or rates and in the securities or other property receivable upon conversion or exchange; | |
|
| ||
| • |
ranking; | |
|
| ||
| • |
restrictive covenants, if any; | |
|
| ||
| • |
voting or other rights, if any; and | |
|
| ||
| • |
important federal income tax considerations. |
The prospectus supplement and any related free writing prospectus that we may authorize to be provided to you also may add, update or change information contained in this prospectus or in documents we have incorporated by reference into this prospectus. However, no prospectus supplement will offer a security that is not registered and described in this prospectus at the time of the effectiveness of the registration statement of which this prospectus is a part.
We may sell the securities directly to or through underwriters, dealers or agents. We, and our underwriters or agents, reserve the right to accept or reject all or part of any proposed purchase of securities. If we do offer securities through underwriters or agents, we will include in the applicable prospectus supplement:
| • | the names of those underwriters or agents; | |
| • | applicable fees, discounts and commissions to be paid to them; | |
| • | details regarding over-allotment options, if any; and | |
| • | the net proceeds to us. |
The following is a summary of the securities we may offer with this prospectus.
Common Stock. We have authorized 145,833,334 shares of common stock, par value $0.0001 per share, of which as of February 28, 2018, 10,273,644 shares are outstanding. We may offer shares of our common stock either alone or underlying other registered securities convertible into or exercisable for our common stock from time to time. Holders of our common stock are entitled to one vote per share for the election of directors and on all other matters that require stockholder approval. In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to share ratably in the assets remaining after payment of liabilities. Currently, we do not pay any dividends. Our common stock does not carry any preemptive rights enabling a holder to subscribe for, or receive shares of, any class of our common stock or any other securities convertible into shares of any class of our common stock, or any redemption rights.
4
Debt Securities. We may offer debt securities from time to time, in one or more series, as either senior or subordinated debt or as senior or subordinated convertible debt. The senior debt securities will rank equally with any other unsubordinated debt that we may have and may be secured or unsecured. The subordinated debt securities will be subordinate and junior in right of payment, to the extent and in the manner described in the instrument governing the debt, to all or some portion of our indebtedness. Any convertible debt securities that we issue will be convertible into or exchangeable for our common stock or other securities of ours. Conversion may be mandatory or at your option and would be at prescribed conversion rates.
Any debt securities will be issued under one or more documents called indentures, which are contracts between us and a trustee for the holders of the debt securities. In this prospectus, we have summarized certain general and standard features of the debt securities we may issue. We urge you, however, to read the prospectus supplements related to the series of debt securities being offered, as well as the complete indentures that contain the terms of the debt securities. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference into such registration statement from a Current Report on Form 8-K that we file with the SEC, the forms of indentures and any supplemental indentures and the forms of debt securities containing the terms of debt securities we are offering before the issuance of any series of debt pursuant to the Registration Statement of which this prospectus forms a part.
Warrants. We may offer warrants for the purchase of our common stock, and/or debt securities in one or more series, from time to time. We may issue warrants independently or together with common stock, and/or debt securities and the warrants may be attached to or separate from those securities.
The warrants will be evidenced by warrant certificates issued under one or more warrant agreements, which are contracts between us and an agent for the holders of the warrants. In this prospectus, we have summarized certain general and standard features of the warrants. We urge you, however, to read the prospectus supplements related to the series of warrants being offered, as well as the warrant agreements and warrant certificates that contain the terms of the warrants. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference into such registration statement from a Current Report on Form 8-K that we file with the SEC, the form of warrant agreements and form of warrant certificates relating to warrants for the purchase of common stock and debt securities we are offering before the issuance of any such warrants pursuant to the Registration Statement of which this prospectus forms a part.
Subscription Rights. We may issue subscription rights to purchase our securities. These subscription rights may be issued independently or together with any other security and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The applicable prospectus supplement will describe the specific terms of any offering of subscription rights for which this prospectus is being delivered, including the type of security underlying the subscription rights, the price payable for the subscription rights, the number of rights to be issued to each shareholder, the expiration date of the subscription rights as well as any other operative terms.
Units. We may offer units consisting of common stock, debt securities and/or warrants to purchase any of such securities in one or more series. In this prospectus, we have summarized certain general and standard features of the units. We urge you, however, to read the prospectus supplements related to the series of units being offered, as well as the unit agreements that contain the terms of the units. We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a Current Report on Form 8-K that we file with the SEC, the form of unit agreement and any supplemental agreements that describe the terms of the series of units we are offering before the issuance of the related series of units pursuant to the Registration Statement of which this prospectus forms a part.
We will evidence each series of units by unit certificates that we will issue under a separate agreement. We will enter into the unit agreements with a unit agent. Each unit agent will be a bank or trust company that we select.
5
We will indicate the name and address of the unit agent in the applicable prospectus supplement relating to a particular series of units.
THIS PROSPECTUS MAY NOT BE USED TO CONSUMMATE A SALE OF ANY SECURITIES UNLESS ACCOMPANIED BY A PROSPECTUS SUPPLEMENT.
6
RISK FACTORS
Investing in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks and uncertainties described under the heading “Risk Factors” contained in the applicable prospectus supplement and any related free writing prospectus, and discussed under the section entitled “Risk Factors” contained in our most recent annual report on Form 10-K and in our most recent quarterly report on Form 10-Q, as well as any amendments thereto reflected in subsequent filings with the SEC, which are incorporated by reference into this prospectus in their entirety, together with other information in this prospectus, the documents incorporated by reference and any free writing prospectus that we may authorize for use in connection with a specific offering. The risks described in these documents are not the only ones we face, but those that we consider to be material. There may be other unknown or unpredictable economic, business, competitive, regulatory or other factors that could have material adverse effects on our future results. Past financial performance may not be a reliable indicator of future performance, and historical trends should not be used to anticipate results or trends in future periods. If any of these risks actually occurs, our business, financial condition or results of operations could be seriously harmed. This could cause the trading price of our securities to decline, resulting in a loss of all or part of your investment. Please also read carefully the section below entitled “Special Note Regarding Forward-Looking Statements.”
7
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus and the documents incorporated by reference contain forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act. These statements are based on our management•s beliefs and assumptions and on information currently available to us. Discussions containing these forward-looking statements may be found, among other places, in the sections entitled “Business,” “Risk Factors” and “Management•s Discussion and Analysis of Financial Condition and Results of Operations” incorporated by reference from our most recent annual report on Form 10-K and in our most recent quarterly report on Form 10-Q, as well as any amendments thereto reflected in subsequent filings with the SEC.
Examples of forward-looking statements in this prospectus include, but are not limited to, our expectations regarding our business strategy, business prospects, operating results, operating expenses, working capital, liquidity and capital expenditure requirements. Important assumptions relating to the forward-looking statements include, among others, assumptions regarding demand for our products, the cost, terms and availability of components, pricing levels, the timing and cost of capital expenditures, competitive conditions and general economic conditions. These statements are based on our management•s expectations, beliefs and assumptions concerning future events affecting us, which in turn are based on currently available information. These assumptions could prove inaccurate. Although we believe that the estimates and projections reflected in the forward-looking statements are reasonable, our expectations may prove to be incorrect.
Corporate
| • |
Our ability to achieve profitability; | |
|
| ||
| • |
our ability to raise sufficient capital or establish strategic business arrangements to expand our CDMO global network; | |
|
| ||
| • |
our ability to grow the size and capabilities of our organization through further collaboration and strategic alliances; | |
|
| ||
| • |
our ability to attract and retain key scientific or management personnel and to expand our management team; | |
|
| ||
| • |
the accuracy of estimates regarding expenses, future revenue, capital requirements, profitability, and needs for additional financing; | |
|
| ||
| • |
our belief that one of our principal competitive advantages is our cell trans-differentiation technology being developed by our Israeli Subsidiary and being able to compete favorably and profitably as a Contract Development and Manufacturing Organization (“CDMO”) in the regenerative medicine sector; |
CDMO business
| • |
our ability to grow the business of MaSTherCell, which we acquired in our fiscal year 2015, as our principal CDMO business; | |
|
| ||
| • |
our ability to attract and retain customers; | |
|
| ||
| • |
our ability to expand and maintain our CDMO business through strategic alliances and joint-ventures; | |
|
| ||
| • |
our ability to fund the operational and capital requirements of our CDMO business and its global expansion; |
8
| • |
our expectations regarding our expenses and revenue, including our expectations that our research and development expenses and selling, general and administrative expenses may increase in absolute dollars; | |
|
| ||
| • |
the successful integration of our clinical and CDMO strategy; | |
|
| ||
| • |
our ability to contract with third-party suppliers and manufacturers and their ability to perform adequately; | |
|
| ||
| • |
extensive industry regulation, and how that will continue to have a significant impact on our business, especially our product development, manufacturing and distribution capabilities; and |
Cellular Therapy business (“CT”)
| • |
our ability to develop, through our Israeli Subsidiary and Belgian Subsidiary, to the clinical stage a new technology to transdifferentiate liver cells into functional insulin-producing cells, thus enabling normal glucose regulated insulin secretion, via cell therapy; | |
| • |
our belief that our diabetes-related treatment seems to be safer than other options; | |
| • |
our expectations regarding the ability of our Israeli Subsidiary and our Belgian Subsidiary to obtain additional and maintain intellectual property protection for our technology and therapies; | |
| • |
our ability to commercialize products in light of the intellectual property rights of others; | |
| • |
our ability to fund and start and complete such clinical trials; | |
| • |
our belief that Diabetes Mellitus will be one of the most challenging health problems in the 21st century and will have staggering health, societal and economic impact; | |
| • |
our relationship with Tel Hashomer Medical Research Infrastructure and Services Ltd. (“THM”) and the risk that THM may cancel the License Agreement; and | |
| • |
expenditures not resulting in commercially successful products. |
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity or performance. Moreover, neither we nor any other person assumes responsibility for the accuracy and completeness of these forward-looking statements. The company is under no duty to update any forward-looking statements after the date of this report to conform these statements to actual results.
9
USE OF PROCEEDS
Except as described in any applicable prospectus supplement or in any free writing prospectuses we have authorized for use in connection with a specific offering, we currently anticipate using the net proceeds from the sale of securities under this prospectus, if any, for the expansion and consolidation of our CDMO global network, clinical development and other research and development activities and for working capital and general corporate purposes. As of the date of this prospectus, we cannot specify with certainty all of the particular uses of the proceeds from the sale of the securities under this prospectus. Accordingly, we will retain broad discretion over the use of such proceeds. Pending these uses, we intend to invest the net proceeds in investment-grade, interest-bearing instruments.
10
DESCRIPTION OF CAPITAL STOCK
General
As of the date of this prospectus, our authorized capital stock consists of 145,833,334 shares of common stock, $0.0001 par value per share. As of February 28, 2018, there were 10,273,644 shares of our common stock outstanding.
On November 16, 2017, we effected a one-for-twelve (1:12) reverse stock split of our outstanding common stock. At the effective time of the reverse stock split, every twelve shares of our issued and outstanding common stock was automatically combined and reclassified into one issued and outstanding share of common stock. No fractional shares of our common stock were issued and each holder of our common stock who would otherwise have been entitled to a fraction of a share of our common stock received one share. In addition, as a result of the reverse stock split, proportionate adjustments were made to the per share exercise price and/or the number of shares issuable upon the exercise or vesting of all stock options and warrants issued by us and outstanding immediately prior to the effective time of the reverse stock split, which resulted in a proportionate increase in the exercise price of all such stock options and warrants. The number of shares reserved for issuance under our equity compensation plans immediately prior to the effective time of the reverse stock split was reduced proportionately. All share and per share information included in this prospectus has been retroactively adjusted to give effect to the reverse stock split.
The following summary description of our capital stock is based on the provisions of our certificate of incorporation and bylaws, the applicable provisions of the Nevada Revised Business Corporations Act, and the agreements described below. This information may not be complete in all respects and is qualified entirely by reference to the provisions of our certificate of incorporation and bylaws, Nevada law and such agreements. For information on how to obtain copies of our certificate of incorporation, bylaws and such agreements, which are exhibits to the registration statement of which this prospectus is a part, see the section entitled “Where You Can Find More Information.”
Common Stock
Each share of common stock entitles the holder to one vote on all matters submitted to a vote of the stockholders including the election of directors. Except as otherwise required by law the holders of our common stock possess all voting power. According to our bylaws, in general, each director is to be elected by a majority of the votes cast with respect to the directors at any meeting of our stockholders for the election of directors at which a quorum is present. According to our bylaws, in general, the affirmative vote of a majority of the shares represented at the meeting and entitled to vote on any matter (which shares voting affirmatively also constitute at least a majority of the required quorum), except for the election of directors, is to be the act of our stockholders. Our bylaws provide that stockholders holding at least 33.3% of the shares entitled to vote, represented in person or by proxy, constitute a quorum at the meeting of our stockholders. Our bylaws also provide that any action which may be taken at any annual or special meeting of our stockholders may be taken without a meeting and without prior notice if a consent in writing, setting forth the action so taken, is signed by the holders of outstanding shares having not less than the minimum number of votes that would be necessary to authorize or take such action at a meeting at which all shares entitled to vote thereon were present and voted.
Our articles of incorporation and bylaws do not provide for cumulative voting in the election of directors. Because the holders of our common stock do not have cumulative voting rights and directors are generally to be elected by a majority of the votes casts with respect to the directors at any meeting of our stockholders for the election of directors, holders of more than fifty percent, and in some cases less than 50%, of the issued and outstanding shares of our common stock can elect all of our directors.
Dividend Rights
The holders of our common stock are entitled to receive such dividends as may be declared by our board of directors out of funds legally available for dividends. Our board of directors is not obligated to declare a dividend. Any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, future earnings, the operating and financial condition of our company, its capital requirements, general business conditions and other pertinent factors. We do not anticipate that dividends will be paid in the foreseeable future.
11
Miscellaneous Rights and Provisions
In the event of our liquidation or dissolution, whether voluntary or involuntary, each share of our common stock is entitled to share ratably in any assets available for distribution to holders of our common stock after satisfaction of all liabilities.
Our common stock is not convertible or redeemable and has no preemptive, subscription or conversion rights. There are no conversions, redemption, sinking fund or similar provisions regarding our common stock.
Our common stock, after the fixed consideration thereof has been paid or performed, are not subject to assessment, and the holders of our common stock are not individually liable for the debts and liabilities of our company.
Our bylaws provide that our board of directors may amend our bylaws by a majority vote of our board of directors including any bylaws adopted by our stockholders, but our stockholders may from time to time specify particular provisions of these bylaws, which must not be amended by our board of directors. Our current bylaws were adopted by our board of directors. Therefore, our board of directors can amend our bylaws to make changes to the provisions relating to the quorum requirement and votes requirements to the extent permitted by the Nevada Revised Statutes.
Anti-Takeover Provisions
Some features of the Nevada Revised Statutes, which are further described below, may have the effect of deterring third parties from making takeover bids for control of our company or may be used to hinder or delay a takeover bid. This would decrease the chance that our stockholders would realize a premium over market price for their shares of common stock as a result of a takeover bid.
Acquisition of Controlling Interest
The Nevada Revised Statutes contain provisions governing the acquisition of a controlling interest of certain Nevada corporations. These provisions provide generally that any person or entity that acquires in excess of a specified percentage of the outstanding voting shares of a Nevada corporation may be denied voting rights with respect to the acquired shares, unless the holders of a majority of the voting power of the corporation, excluding shares of which such acquiring person or entity, an officer or a director of the corporation, and an employee of the corporation exercises voting rights, elect to restore such voting rights in whole or in part. These provisions apply whenever a person or entity acquires shares that, but for the operation of these provisions, would bring voting power of such person or entity in the election of directors within any of the following three ranges:
| 1. |
20% or more but less than 33 1/3%; | |
| 2. |
33 1/3% or more but less than or equal to 50%; or | |
| 3. |
more than 50%. |
The stockholders or board of directors of a corporation may elect to exempt the stock of the corporation from these provisions through adoption of a provision to that effect in the articles of incorporation or bylaws of the corporation. Our articles of incorporation and bylaws do not exempt our common stock from these provisions.
These provisions are applicable only to a Nevada corporation, which:
12
| 1. |
has 200 or more stockholders of record, at least 100 of whom have addresses in Nevada appearing on the stock ledger of the corporation; and | |
| 2. |
does business in Nevada directly or through an affiliated corporation. |
At this time, we do not have 100 stockholders of record who have addresses in Nevada appearing on the stock ledger of our company nor do we conduct any business in Nevada, either directly or through an affiliated corporation. Therefore, we believe that these provisions do not apply to acquisitions of our shares and will not until such time as these requirements have been met. At such time as they may apply to us, these provisions may discourage companies or persons interested in acquiring a significant interest in or control of our company, regardless of whether such acquisition may be in the interest of our stockholders.
Combination with Interested Stockholder
The Nevada Revised Statutes contain provisions governing the combination of any Nevada corporation that has 200 or more stockholders of record with an interested stockholder. As of February 28, 2018, we had approximately 75 stockholders of record. Therefore, we believe that these provisions do not apply to us and will not until such time as these requirements have been met. At such time as they may apply to us, these provisions may also have the effect of delaying or making it more difficult to effect a change in control of our company.
A corporation affected by these provisions may not engage in a combination within three years after the interested stockholder acquires his, her or its shares unless the combination or purchase is approved by the board of directors before the interested stockholder acquired such shares. Generally, if approval is not obtained, then after the expiration of the three-year period, the business combination may be consummated with the approval of the board of directors before the person became an interested stockholder or a majority of the voting power held by disinterested stockholders, or if the consideration to be received per share by disinterested stockholders is at least equal to the highest of:
| 1. |
the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or within three years immediately before, or in, the transaction in which he, she or it became an interested stockholder, whichever is higher; | |
| 2. |
the market value per share on the date of announcement of the combination or the date the person became an interested stockholder, whichever is higher; or | |
| 3. |
if higher for the holders of preferred stock, the highest liquidation value of the preferred stock, if any. |
Generally, these provisions define an interested stockholder as a person who is the beneficial owner, directly or indirectly of 10% or more of the voting power of the outstanding voting shares of a corporation. Generally, these provisions define combination to include any merger or consolidation with an interested stockholder, or any sale, lease, exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions with an interested stockholder of assets of the corporation having:
| 1. |
an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation; | |
| 2. |
an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation; or | |
| 3. |
representing 10% or more of the earning power or net income of the corporation. |
13
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Securities Transfer Corporation located at 2591 Dallas Parkway, Suite 102, Frisco, TX 75034.
14
DESCRIPTION OF DEBT SECURITIES
The following description, together with the additional information we include in any applicable prospectus supplements, summarizes the general terms and provisions of the debt securities that we may offer under this prospectus. While the terms we have summarized below will generally apply to any future debt securities we may offer under this prospectus, we will describe the particular terms of any debt securities that we may offer in more detail in the applicable prospectus supplement. The terms of any debt securities we offer under a prospectus supplement may differ from the terms we describe below. However, no prospectus supplement shall fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness. As of the date of this prospectus, we have no outstanding registered debt securities.
We may issue one or more series of notes under indentures, which we will enter into with the trustee to be named therein. If we issue debt securities, we will file these documents as exhibits to the registration statement of which this prospectus is a part, or incorporate them by reference from a Current Report on Form 8-K that we file with the SEC. We use the term “indentures” to refer to any and all indentures that we may enter into with respect to debt securities issued and sold pursuant to this Registration Statement.
The indentures will be qualified under the Trust Indenture Act of 1939. We use the term “debenture trustee” to refer to either the senior trustee or the subordinated trustee, as applicable.
The following summaries of material provisions of the debt securities are subject to, and qualified in their entirety by reference to, all the provisions of the indenture applicable to a particular series of debt securities. We urge you to read the applicable prospectus supplements related to the debt securities that we sell under this prospectus, as well as the complete indentures that contain the terms of the debt securities. Except as we may otherwise indicate, the terms of the senior and the subordinated indentures are identical.
General
The indentures may limit the aggregate principal amount of the debt securities which we may issue and will provide that we may issue the debt securities from time to time in one or more series. The indentures may or may not limit the amount of our other indebtedness or the debt securities which we may issue. The particular terms of each series of debt securities will be described in a prospectus supplement relating to such series, including any pricing supplement. The prospectus supplement will set forth:
| • |
the title; | |
|
| ||
| • |
the principal amount being offered, and, if a series, the total amount authorized and the total amount outstanding; | |
|
| ||
| • |
any limit on the amount that may be issued; | |
|
| ||
| • |
whether or not we will issue the series of debt securities in global form and, if so, the terms and who the depositary will be; | |
|
| ||
| • |
the maturity date; | |
|
| ||
| • |
whether and under what circumstances, if any, we will pay additional amounts on any debt securities held by a person who is not a United States person for tax purposes, and whether we can redeem the debt securities if we have to pay such additional amounts; | |
|
| ||
| • |
the annual interest rate, which may be fixed or variable, or the method for determining the rate, the date interest will begin to accrue, the dates interest will be payable and the regular record dates for interest payment dates or the method for determining such dates; |
15
| • |
whether or not the debt securities will be secured or unsecured, and the terms of any secured debt; | |
|
| ||
| • |
the terms of the subordination of any series of subordinated debt; | |
|
| ||
| • |
the place where payments will be payable; | |
|
| ||
| • |
restrictions on transfer, sale or other assignment, if any; | |
|
| ||
| • |
our right, if any, to defer payment of interest and the maximum length of any such deferral period; | |
|
| ||
| • |
the date, if any, after which, the conditions upon which, and the price at which we may, at our option, redeem the series of debt securities pursuant to any optional or provisional redemption provisions, and any other applicable terms of those redemption provision. | |
|
| ||
| • |
the date, if any, on which, and the price at which we are obligated, pursuant to any mandatory sinking fund or analogous fund provisions or otherwise, to redeem, or at the holder’s option to purchase, the series of debt securities and the currency or currency unit in which the debt securities are payable; | |
|
| ||
| • |
whether the indenture will restrict our ability to: |
| • |
incur additional indebtedness; | |
|
| ||
| • |
issue additional securities; | |
|
| ||
| • |
create liens; | |
|
| ||
| • |
pay dividends and make distributions in respect of our capital stock; | |
|
| ||
| • |
redeem capital stock; | |
|
| ||
| • |
place restrictions on our subsidiaries’ ability to pay dividends, make distributions or transfer assets; | |
|
| ||
| • |
make investments or other restricted payments; | |
|
| ||
| • |
sell or otherwise dispose of assets; | |
|
| ||
| • |
enter into sale-leaseback transactions; | |
|
| ||
| • |
engage in transactions with stockholders and affiliates; | |
|
| ||
| • |
issue or sell stock of our subsidiaries; or | |
|
| ||
| • |
effect a consolidation or merger; |
| • |
whether the indenture will require us to maintain any interest coverage, fixed charge, cash flow-based, asset-based or other financial covenants; | |
|
| ||
| • |
a discussion of any material or special United States federal income tax considerations applicable to the debt securities; | |
|
| ||
| • |
information describing any book-entry features; |
16
| • |
provisions for a sinking fund purchase or other analogous fund, if any; | |
|
| ||
| • |
whether the debt securities are to be offered at a price such that they will be deemed to be offered at an “original issue discount” as defined in paragraph (a) of Section 1273 of the Internal Revenue Code; | |
|
| ||
| • |
the procedures for any auction and remarketing, if any; | |
|
| ||
| • |
the denominations in which we will issue the series of debt securities, if other than denominations of $1,000 and any integral multiples; | |
|
| ||
| • |
if other than dollars, the currency in which the series of debt securities will be denominated; and | |
|
| ||
| • |
any other specific terms, preferences, rights or limitations of, or restrictions on, the debt securities, including any events of default that are in addition to those described in this prospectus or any covenants provided with respect to the debt securities that are in addition to those described above, and any terms which may be required by us or advisable under applicable laws or regulations or advisable in connection with the marketing of the debt securities. |
Conversion or Exchange Rights
We will set forth in the prospectus supplement the terms on which a series of debt securities may be convertible into or exchangeable for common stock or other securities of ours or a third party, including the conversion or exchange rate, as applicable, or how it will be calculated, and the applicable conversion or exchange period. We will include provisions as to whether conversion or exchange is mandatory, at the option of the holder or at our option. We may include provisions pursuant to which the number of our securities or the securities of a third party that the holders of the series of debt securities receive upon conversion or exchange would, under the circumstances described in those provisions, be subject to adjustment, or pursuant to which those holders would, under those circumstances, receive other property upon conversion or exchange, for example in the event of our merger or consolidation with another entity.
Consolidation, Merger or Sale
The description of the debt securities in the prospectus supplement or the indentures may provide that we may not consolidate or amalgamate with or merge into any person or convey, transfer or lease our properties or assets as an entirety or substantially as an entirety to any person, and we may not permit any person to consolidate or amalgamate with or merge into us, or convey, transfer or lease its properties and assets as an entirety or substantially as an entirety to us, unless:
| • |
immediately after giving effect to the transaction, no event of default, and no event which after notice or lapse of time or both would | |
| • |
certain other conditions are met. |
If the debt securities are convertible for our other securities, the person with whom we consolidate or merge or to whom we sell all of our property must make provisions for the conversion of the debt securities into securities that the holders of the debt securities would have received if they had converted the debt securities before the consolidation, merger or sale.
Events of Default under the Indenture
Each of the following constitute reasonably standard events of default that may be included in any finalized indenture or prospectus supplement as constituting an event of default with respect to any series of debt securities that we may issue:
17
| • |
if we fail to pay interest when due and payable and our failure continues for 30 days and the time for payment has not been extended or deferred; | |
|
| ||
| • |
if we fail to pay the principal, sinking fund payment or premium, if any, when due and payable and the time for payment has not been extended or delayed; | |
|
| ||
| • |
if we fail to observe or perform any other covenant contained in the debt securities or the indentures, other than a covenant specifically relating to another series of debt securities, and our failure continues for 90 days after we receive notice from the debt trustee or holders of at least 25% in aggregate principal amount of the outstanding debt securities of the applicable series; | |
|
| ||
| • |
if specified events of bankruptcy, insolvency or reorganization occur; and | |
|
| ||
| • |
any other event of default provided in or pursuant to the applicable indenture or prospectus supplement with respect to the debt securities of that series. |
if an event of default with respect to debt securities of any series occurs and is continuing, other than an event of default in the event of bankruptcy, insolvency or reorganization, the debenture trustee or the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series, by notice to us in writing, and to the debenture trustee if notice is given by such holders, may declare the unpaid principal of, premium, if any, and accrued interest, if any, due and payable immediately. If an event of default due to bankruptcy, insolvency or reorganization occurs with respect to us, the principal amount of and accrued interest, if any, of each issue of debt securities then outstanding shall be due and payable without any notice or other action on the part of the debenture trustee or any holder.
The holders of a majority in principal amount of the outstanding debt securities of an affected series may waive any default or event of default with respect to the series and its consequences, except defaults or events of default regarding payment of principal, premium, if any, or interest, unless we have cured the default or event of default in accordance with the indenture.
Subject to the terms of the indentures, if an event of default under an indenture shall occur and be continuing, the debenture trustee will be under no obligation to exercise any of its rights or powers under such indenture at the request or direction of any of the holders of the applicable series of debt securities, unless such holders have offered the debenture trustee reasonable indemnity. The holders of a majority in principal amount of the outstanding debt securities of any series will have the right to direct the time, method and place of conducting any proceeding for any remedy available to the debenture trustee, or exercising any trust or power conferred on the debenture trustee, with respect to the debt securities of that series, provided that:
| • |
the direction so given by the holder is not in conflict with any law or the applicable indenture; and | |
|
| ||
| • |
subject to its duties under the Trust Indenture Act of 1939, the debenture trustee need not take any action that might involve it in personal liability or might be unduly prejudicial to the holders not involved in the proceeding. |
A holder of the debt securities of any series will only have the right to institute a proceeding under the indentures or to appoint a receiver or trustee, or to seek other remedies if:
| • |
the holder has given written notice to the debenture trustee of a continuing event of default with respect to that series; | |
|
| ||
| • |
the holders of at least 25% in aggregate principal amount of the outstanding debt securities of that series have made written request, and such holders have offered reasonable indemnity to the debenture trustee to institute the proceeding as trustee; and |
18
|
• |
the debenture trustee does not institute the proceeding, and does not receive from the holders of a majority in aggregate principal amount of the outstanding debt securities of that series other conflicting directions within 90 days after the notice, request and offer. |
These limitations do not apply to a suit instituted by a holder of debt securities if we default in the payment of the principal, premium, if any, or interest on, the debt securities.
We will periodically file statements with the debenture trustee regarding our compliance with specified covenants in the indentures.
Modification of Indenture; Waiver
We and the debenture trustee may change an indenture without the consent of any holders with respect to specific matters, including:
| • |
to fix any ambiguity, defect or inconsistency in the indenture; | |
|
| ||
| • |
to comply with the provisions described above under “Consolidation, Merger or Sale”; | |
|
| ||
| • |
to comply with any requirements of the SEC in connection with the qualification of any indenture under the Trust Indenture Act of 1 | |
|
| ||
| • |
to evidence and provide for the acceptance of appointment by a successor trustee; | |
|
| ||
| • |
to provide for uncertificated debt securities and to make all appropriate changes for such purpose; | |
|
| ||
| • |
to add to, delete from, or revise the conditions, limitations and restrictions on the authorized amount, terms or purposes of issuance, authorization and delivery of debt securities or any series, as set forth in the indenture; | |
|
| ||
| • |
to provide for the issuance of and establish the form and terms and conditions of the debt securities of any series as provided under “General,” to establish the form of any certifications required to be furnished pursuant to the terms of the indenture or any series of debt securities, or to add to the rights of the holders of any series of debt securities; | |
|
| ||
| • |
to add to our covenants such new covenants, restrictions, conditions or provisions for the protection of the holders, to make the occurrence, or the occurrence and the continuance, of a default in any such additional covenants, restrictions, conditions or provision an event of default, or to surrender any of our rights or powers under the indenture; or | |
|
| ||
| • |
to change anything that does not materially adversely affect the interests of any holder of debt securities of any series. |
In addition, under the indentures, the rights of holders of a series of debt securities may be changed by us and the debenture trustee with the written consent of the holders of at least a majority in aggregate principal amount of the outstanding debt securities of each series that is affected. However, we and the debenture trustee may only make the following changes with the consent of each holder of any outstanding debt securities affected:
| • | extending the fixed maturity of the series of debt securities; | |
| • | reducing the principal amount, reducing the rate of or extending the time of payment of interest, or reducing any premium payable upon the redemption of any debt securities; or |
19
| • | reducing the percentage of debt securities, the holders of which are required to consent to any amendment, supplement, modification or waiver. |
Discharge
Each indenture will provide that we can elect to be discharged from our obligations with respect to one or more series of debt securities, except for obligations to:
| • |
register the transfer or exchange of debt securities of the series; | |
|
| ||
| • |
replace stolen, lost or mutilated debt securities of the series; | |
|
| ||
| • |
maintain paying agencies; | |
|
| ||
| • |
hold monies for payment in trust; | |
|
| ||
| • |
recover excess money held by the debenture trustee; | |
|
| ||
| • |
compensate and indemnify the debenture trustee; and | |
|
| ||
| • |
appoint any successor trustee. |
In order to exercise our rights to be discharged, we must deposit with the debenture trustee money or government obligations sufficient to pay all the principal of, any premium, if any, and interest on, the debt securities of the series on the dates payments are due.
Form, Exchange and Transfer
We will issue the debt securities of each series only in fully registered form without coupons and, unless we otherwise specify in the applicable prospectus supplement, in denominations of $1,000 and any integral multiple thereof. The indenture will provide that we may issue debt securities of a series in temporary or permanent global form and as book-entry securities that will be deposited with, or on behalf of, The Depository Trust Company, New York, New York, known as DTC, or another depositary named by us and identified in a prospectus supplement with respect to that series. See “Legal Ownership of Securities” for a further description of the terms relating to any book-entry securities.
At the option of the holder, subject to the terms of the indentures and the limitations applicable to global securities described in the applicable prospectus supplement, the holder of the debt securities of any series can exchange the debt securities for other debt securities of the same series, in any authorized denomination and of like tenor and aggregate principal amount.
Subject to the terms of the indentures and the limitations applicable to global securities set forth in the applicable prospectus supplement, holders of the debt securities may present the debt securities for exchange or for registration of transfer, duly endorsed or with the form of transfer endorsed thereon duly executed if so required by us or the security registrar, at the office of the security registrar or at the office of any transfer agent designated by us for this purpose. Unless otherwise provided in the debt securities that the holder presents for transfer or exchange, we will make no service charge for any registration of transfer or exchange, but we may require payment of any taxes or other governmental charges.
We will name in the applicable prospectus supplement the security registrar, and any transfer agent in addition to the security registrar, that we initially designate for any debt securities. We may at any time designate additional transfer agents or rescind the designation of any transfer agent or approve a change in the office through which any transfer agent acts, except that we will be required to maintain a transfer agent in each place of payment for the debt securities of each series.
20
If we elect to redeem the debt securities of any series, we will not be required to:
| • |
issue, register the transfer of, or exchange any debt securities of any series being redeemed in part during a period beginning at the opening of business 15 days before the day of mailing of a notice of redemption of any debt securities that may be selected for redemption. | |
| • |
register the transfer of or exchange any debt securities so selected for redemption, in whole or in part, except the unredeemed portion of any debt securities we are redeeming in part. |
Information Concerning the Debenture Trustee
The debenture trustee, other than during the occurrence and continuance of an event of default under an indenture, will undertake to perform only those duties as are specifically set forth in the applicable indenture. Upon an event of default under an indenture, the debenture trustee must use the same degree of care as a prudent person would exercise or use in the conduct of his or her own affairs. Subject to this provision, the debenture trustee is under no obligation to exercise any of the powers given it by the indentures at the request of any holder of debt securities unless it is offered reasonable security and indemnity against the costs, expenses and liabilities that it might incur.
Payment and Paying Agents
Unless we otherwise indicate in the applicable prospectus supplement, we will make payment of the interest on any debt securities on any interest payment date to the person in whose name the debt securities, or one or more predecessor securities, are registered at the close of business on the regular record date for the interest.
We will pay principal of and any premium and interest on the debt securities of a particular series at the office of the paying agents designated by us, except that, unless we otherwise indicate in the applicable prospectus supplement, we may make interest payments by check which we will mail to the holder or by wire transfer to certain holders. Unless we otherwise indicate in a prospectus supplement, we will designate the corporate office of the debenture trustee in the City of Dallas, Texas as our sole paying agent for payments with respect to debt securities of each series. We will name in the applicable prospectus supplement any other paying agents that we initially designate for the debt securities of a particular series. We will maintain a paying agent in each place of payment for the debt securities of a particular series.
All money we pay to a paying agent or the debenture trustee for the payment of the principal of or any premium or interest on any debt securities which remains unclaimed at the end of two years after such principal, premium or interest has become due and payable will be repaid to us, and the holder of the debt security thereafter may look only to us for payment thereof.
Subordination of Subordinated Debt Securities
The subordinated debt securities will be subordinate and junior in priority of payment to certain of our other indebtedness to the extent described in a prospectus supplement. The indentures will not limit the amount of indebtedness which we may incur, including senior indebtedness or subordinated indebtedness, and will not limit us from issuing any other debt, including secured debt or unsecured debt.
21
DESCRIPTION OF WARRANTS
The following description, together with the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions of the warrants that we may offer under this prospectus. While the terms we have summarized below will apply generally to any warrants that we may offer under this prospectus, we will describe the particular terms of any series of warrants in more detail in the applicable prospectus supplement. The terms of any warrants offered under a prospectus supplement may differ from the terms described below. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a Current Report on Form 8-K that we file with the SEC, the form of warrant agreement, including a form of warrant certificate, that describes the terms of the particular series of warrants we are offering before the issuance of the related series of warrants. The following summaries of material provisions of the warrants and the warrant agreements are subject to, and qualified in their entirety by reference to, all the provisions of the warrant agreement and warrant certificate applicable to a particular series of warrants. We urge you to read the applicable prospectus supplements related to the particular series of warrants that we sell under this prospectus, as well as the complete warrant agreements and warrant certificates that contain the terms of the warrants.
General
We will describe in the applicable prospectus supplement the terms relating to a series of warrants, including:
| • |
the offering price and aggregate number of warrants offered; | |
|
| ||
| • |
the currency for which the warrants may be purchased; | |
|
| ||
| • |
if applicable, the designation and terms of the securities with which the warrants are issued and the number of warrants issued with each such security or each principal amount of such security; | |
|
| ||
| • |
if applicable, the date on and after which the warrants and the related securities will be separately transferable; | |
|
| ||
| • |
in the case of warrants to purchase debt securities, the principal amount of debt securities purchasable upon exercise of one warrant and the price at which, and currency in which, this principal amount of debt securities may be purchased upon such exercise; | |
|
| ||
| • |
in the case of warrants to purchase common stock, the number of shares of common stock may be, purchasable upon the exercise of one warrant and the price at which these shares may be purchased upon such exercise; | |
|
| ||
| • |
the effect of any merger, consolidation, sale or other disposition of our business on the warrant agreements and the warrants; | |
|
| ||
| • |
the terms of any rights to redeem or call the warrants; | |
|
| ||
| • |
any provisions for changes to or adjustments in the exercise price or number of securities issuable upon exercise of the warrants; | |
|
| ||
| • |
the dates on which the right to exercise the warrants will commence and expire; | |
|
| ||
| • |
the manner in which the warrant agreements and warrants may be modified; |
22
| • | federal income tax consequences of holding or exercising the warrants; | |
| • | the terms of the securities issuable upon exercise of the warrants; and | |
| • | any other specific terms, preferences, rights or limitations of or restrictions on the warrants. |
Before exercising their warrants, holders of warrants will not have any of the rights of holders of the securities purchasable upon such exercise, including:
| • |
in the case of warrants to purchase debt securities, the right to receive payments of principal of, or premium, if any, or interest on, the debt securities purchasable upon exercise or to enforce covenants in the applicable indenture; and | |
|
| ||
| • |
in the case of warrants to purchase common stock, the rights of common stock holders such as, but not limited to, the right to participate in voting on shareholder and/or company matters. |
Exercise of Warrants
Each warrant will entitle the holder to purchase the securities that we specify in the applicable prospectus supplement at the exercise price that we describe in the applicable prospectus supplement. Unless we otherwise specify in the applicable prospectus supplement, holders of the warrants may exercise the warrants at any time up to the specified time on the expiration date that we set forth in the applicable prospectus supplement. After the close of business on the expiration date, unexercised warrants will become void.
Holders of the warrants may exercise the warrants by delivering the warrant certificate representing the warrants to be exercised together with specified information, and paying the required amount to the warrant agent in immediately available funds, as provided in the applicable prospectus supplement. We will set forth on the reverse side of the warrant certificate and in the applicable prospectus supplement the information that the holder of the warrant will be required to deliver to the warrant agent.
Upon receipt of the required payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the applicable prospectus supplement, we will issue and deliver the securities purchasable upon such exercise. If fewer than all of the warrants represented by the warrant certificate are exercised, then we will issue a new warrant certificate for the remaining amount of warrants. If we so indicate in the applicable prospectus supplement, holders of the warrants may surrender securities as all or part of the exercise price for warrants.
Enforceability of Rights by Holders of Warrants
Each warrant agent will act solely as our agent under the applicable warrant agreement and will not assume any obligation or relationship of agency or trust with any holder of any warrant. A single bank or trust company may act as warrant agent for more than one issue of warrants. A warrant agent will have no duty or responsibility in case of any default by us under the applicable warrant agreement or warrant, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a warrant may, without the consent of the related warrant agent or the holder of any other warrant, enforce by appropriate legal action its right to exercise, and receive the securities purchasable upon exercise of, its warrants.
23
Outstanding Warrants and Options
As of February 28, 2018, we had outstanding warrants to purchase an aggregate of 2,625,402 shares of our common stock having a per share exercise price ranging between $6.00 and $10.20. These warrants have a three-year term, the latest expiring on December 27, 2020. In addition, as of such date we also had outstanding options issued to employees and other service providers for an aggregate of 2,004,435 shares of our common stock having a share exercise price ranging between $ 0.0012 and $ 16.80.
24
DESCRIPTION OF SUBSCRIPTION RIGHTS
The following is a general description of the terms of the subscription rights we may issue from time to time. Particular terms of any subscription rights we offer will be described in the prospectus supplement or free writing prospectus relating to such subscription rights, and may differ from the terms described herein.
We may issue subscription rights to purchase our securities. These subscription rights may be issued independently or together with any other security offered hereby and may or may not be transferable by the stockholder receiving the subscription rights in such offering. In connection with any offering of subscription rights, we may enter into a standby arrangement with one or more underwriters or other purchasers pursuant to which the underwriters or other purchasers may be required to purchase any securities remaining unsubscribed for after such offering.
The applicable prospectus supplement will describe the specific terms of any offering of subscription rights for which this prospectus is being delivered, including the following:
| • |
whether common stock, or warrants for those securities will be offered under the stockholder subscription rights; | |
| • |
the price, if any, for the subscription rights; | |
| • |
the exercise price payable for each security upon the exercise of the subscription rights; | |
| • |
the number of subscription rights issued to each stockholder; | |
| • |
the number and terms of the securities which may be purchased per each subscription right; | |
| • |
the extent to which the subscription rights are transferable; | |
| • |
any other terms of the subscription rights, including the terms, procedures and limitations relating to the exchange and exercise of the subscription rights; | |
| • |
the date on which the right to exercise the subscription rights shall commence, and the date on which the subscription rights shall expire; | |
| • |
the extent to which the subscription rights may include an over-subscription privilege with respect to unsubscribed securities; | |
| • |
if appropriate, a discussion of material U.S. federal income tax considerations; and | |
| • |
if applicable, the material terms of any standby underwriting or purchase arrangement entered into by us in connection with the offering of subscription rights. |
The description in the applicable prospectus supplement of any subscription rights we offer will not necessarily be complete and will be qualified in its entirety by reference to the applicable subscription rights certificate or subscription rights agreement, which will be filed with the SEC if we offer subscription rights.
25
DESCRIPTION OF UNITS
The following description, together with the additional information we may include in any applicable prospectus supplements, summarizes the material terms and provisions of the units that we may offer under this prospectus. While the terms we have summarized below will apply generally to any units that we may offer under this prospectus, we will describe the particular terms of any series of units in more detail in the applicable prospectus supplement. The terms of any units offered under a prospectus supplement may differ from the terms described below. However, no prospectus supplement will fundamentally change the terms that are set forth in this prospectus or offer a security that is not registered and described in this prospectus at the time of its effectiveness.
We will file as exhibits to the registration statement of which this prospectus is a part, or will incorporate by reference from a Current Report on Form 8-K that we file with the SEC, the form of unit agreement that describes the terms of the series of units we are offering, and any supplemental agreements, before the issuance of the related series of units. The following summaries of material terms and provisions of the units are subject to, and qualified in their entirety by reference to, all the provisions of the unit agreement and any supplemental agreements applicable to a particular series of units. We urge you to read the applicable prospectus supplements related to the particular series of units that we sell under this prospectus, as well as the complete unit agreement and any supplemental agreements that contain the terms of the units.
General
We may issue units comprised of one or more debt securities, shares of common stock and warrants in any combination. Each unit will be issued so that the holder of the unit is also the holder of each security included in the unit. Thus, the holder of a unit will have the rights and obligations of a holder of each included security. The unit agreement under which a unit is issued may provide that the securities included in the unit may not be held or transferred separately, at any time or at any time before a specified date.
We will describe in the applicable prospectus supplement the terms of the series of units, including:
| • |
the designation and terms of the units and of the securities comprising the units, including whether and under what circumstances those securities may be held or transferred separately; | |
| • |
any provisions of the governing unit agreement that differ from those described below; and | |
| • |
any provisions for the issuance, payment, settlement, transfer or exchange of the units or of the securities comprising the units. |
The provisions described in this section, as well as those described under “Description of Capital Stock,” “Description of Debt Securities” and “Description of Warrants” will apply to each unit and to any common stock, debt security or warrant included in each unit, respectively.
Issuance in Series
We may issue units in such amounts and in numerous distinct series as we determine.
26
Enforceability of Rights by Holders of Units
Each unit agent will act solely as our agent under the applicable unit agreement and will not assume any obligation or relationship of agency or trust with any holder of any unit. A single bank or trust company may act as unit agent for more than one series of units. A unit agent will have no duty or responsibility in case of any default by us under the applicable unit agreement or unit, including any duty or responsibility to initiate any proceedings at law or otherwise, or to make any demand upon us. Any holder of a unit may, without the consent of the related unit agent or the holder of any other unit, enforce by appropriate legal action its rights as holder under any security included in the unit.
27
PLAN OF DISTRIBUTION
We may sell the securities to or through underwriters or dealers, through agents, or directly to one or more purchasers. A prospectus supplement or supplements will describe the terms of the offering of the securities, including, to the extent applicable:
| • | the name or names of any underwriters or agents; | |
| • | the purchase price of the securities and the proceeds we will receive from the sale; | |
| • | any over-allotment options under which underwriters may purchase additional securities from us; | |
| • | any agency fees or underwriting discounts and other items constituting agents’ or underwriters’ compensation; | |
| • | any public offering price; | |
| • | any discounts or concessions allowed or re-allowed or paid to dealers; and | |
| • | any securities exchange or market on which the securities may be listed. We may distribute the securities from time to time in one or more transactions at: | |
| • | fixed price or prices, which may be changed from time to time; | |
| • | market prices prevailing at the time of sale; | |
| • | prices related to such prevailing market prices; or | |
| • | negotiated prices. |
Underwriters
If we use underwriters for a sale of securities, the underwriters will acquire the securities for their own account. The underwriters may resell the securities in one or more transactions, including negotiated transactions, at a fixed public offering price or at varying prices determined at the time of sale. The obligations of the underwriters to purchase the securities will be subject to the conditions set forth in the applicable underwriting agreement. We may offer the securities to the public through underwriting syndicates represented by managing underwriters or by underwriters without a syndicate. Subject to certain conditions, the underwriters will be obligated to purchase all the securities of the series offered if they purchase any of the securities of that series. We may change from time to time any public offering price and any discounts or concessions the underwriters allow or pay to dealers. We may use underwriters with whom we have a material relationship. We will describe the nature of any such relationship in any applicable prospectus supplement naming any such underwriter. Only underwriters we name in the prospectus supplement are underwriters of the securities offered by the prospectus supplement. We may provide agents and underwriters with indemnification against civil liabilities related to offerings under this prospectus, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities.
Agents
We may designate agents who agree to use their reasonable efforts to solicit purchases of our securities for the period of their appointment or to sell our securities on a continuing basis. We will name any agent involved in the offering and sale of securities and we will describe any commissions we will pay the agent in the applicable prospectus supplement. Unless the prospectus supplement states otherwise, our agent will act on a best-efforts basis for the period of its appointment.
28
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
We may provide agents and underwriters with indemnification against civil liabilities related to this offering, including liabilities under the Securities Act, or contribution with respect to payments that the agents or underwriters may make with respect to these liabilities. Agents and underwriters may engage in transactions with, or perform services for, us in the ordinary course of business. In addition, the agents’ and underwriters’ commissions, discounts or concessions may qualify as underwriters’ compensation under the Securities Act and the rules of the Financial Industry Regulatory Authority, Inc., or FINRA
Direct Sales
We may also sell securities directly to one or more purchasers without using underwriters or agents.
Trading Markets and Listing of Securities
Unless otherwise specified in the applicable prospectus supplement, each class or series of securities will be a new issue with no established trading market, other than our common stock, which is currently listed on the Nasdaq Capital Market. We may elect to list any other class or series of securities on any exchange or market, but we are not obligated to do so. It is possible that one or more underwriters may make a market in a class or series of securities, but the underwriters will not be obligated to do so and may discontinue any market making at any time without notice. We cannot give any assurance as to the liquidity of the trading market for any of the securities.
Stabilization Activities
Any underwriter may engage in overallotment, stabilizing transactions, short covering transactions and penalty bids in accordance with Regulation M under the Exchange Act. Overallotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum. Short covering transactions involve purchases of the securities in the open market after the distribution is completed to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of these activities at any time.
29
LEGAL MATTERS
Unless otherwise indicated in the applicable prospectus supplement, the validity of the securities offered by this prospectus, and any supplement thereto, will be passed upon for us by Pearl Cohen Zedek Latzer Baratz LLP.
30
EXPERTS
The financial statements incorporated in this Prospectus by reference to the Annual Report on Form 10-K for the year ended November 30, 2017 have been so incorporated in reliance on the report of Kesselman & Kesselman, an independent registered public accounting firm and a member firm of PricewaterhouseCoopers International Limited, given on the authority of said firm as experts in auditing and accounting.
31
WHERE YOU CAN FIND MORE INFORMATION
We are a reporting company and file annual, quarterly and current reports, proxy statements and other information with the SEC. We have filed with the SEC a registration statement on Form S-3 under the Securities Act with respect to the securities we are offering under this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits to the registration statement. For further information with respect to us and the securities we are offering under this prospectus, we refer you to the registration statement and the exhibits filed as a part of the registration statement. You may read and copy the registration statement, as well as our reports, proxy statements and other information, at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for more information about the operation of the public reference room. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding issuers that file electronically with the SEC, including Orgenesis. The SEC’s website can be found at www.sec.gov. We maintain a website at www.orgenesis.com. Information found on, or accessible through, our website is not a part of, and is not incorporated into, this prospectus, and you should not consider it part of this prospectus.
32
INCORPORATION BY REFERENCE
The SEC allows us to incorporate by reference the information we file with it, which means that we can disclose important information to you by referring you to another document that we have filed separately with the SEC. You should read the information incorporated by reference because it is an important part of this prospectus. Information in this prospectus supersedes information incorporated by reference that we filed with the SEC prior to the date of this prospectus, while information that we file later with the SEC will automatically update and supersede the information in this prospectus. We incorporate by reference into this prospectus and the registration statement of which this prospectus is a part the information or documents listed below that we have filed with the SEC (Commission File No. 000-54329):
We incorporate by reference into this prospectus the documents listed below:
| • | our annual report on Form 10-K for the year ended November 30, 2017 filed on February 28, 2018; | |
| • | our current reports on Form 8-K filed on each of January 9, 2018 and January 24, 2018; and | |
| • | the description of our common stock in our registration statement on Form 8-A12b filed with the SEC on March 8, 2018; and |
We also incorporate by reference any future filings (other than current reports furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits filed on such form that are related to such items unless such Form 8-K expressly provides to the contrary) made with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including those made after the date of the initial filing of the registration statement of which this prospectus is a part and prior to effectiveness of such registration statement, until we file a post-effective amendment that indicates the termination of the offering of the securities made by this prospectus and will become a part of this prospectus from the date that such documents are filed with the SEC. Information in such future filings updates and supplements the information provided in this prospectus. Any statements in any such future filings will automatically be deemed to modify and supersede any information in any document we previously filed with the SEC that is incorporated or deemed to be incorporated herein by reference to the extent that statements in the later filed document modify or replace such earlier statements.
We will furnish without charge to each person, including any beneficial owner, to whom a prospectus is delivered, upon written or oral request, a copy of any or all of the documents incorporated by reference into this prospectus but not delivered with the prospectus, including exhibits that are specifically incorporated by reference into such documents. You should direct any requests for documents to Orgenesis, Inc., Attention: Corporate Secretary, 20271 Goldenrod Lane, Germantown MD 20876. Our phone number is (480) 659-6404.
33
|
|
| Up to $25,000,000 |
| Common Stock |
| PROSPECTUS SUPPLEMENT |
 |
| December 20, 2018 |