Exhibit 99.1

SI-BONE Reports Preliminary Unaudited Revenue for Fourth Quarter and Full Year 2019
and Provides Full Year 2020 Revenue Guidance
SANTA CLARA, Calif. - January 9, 2019 - SI-BONE, Inc. (Nasdaq: SIBN), a medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, today announced preliminary unaudited revenue for the fourth quarter and full year 2019.
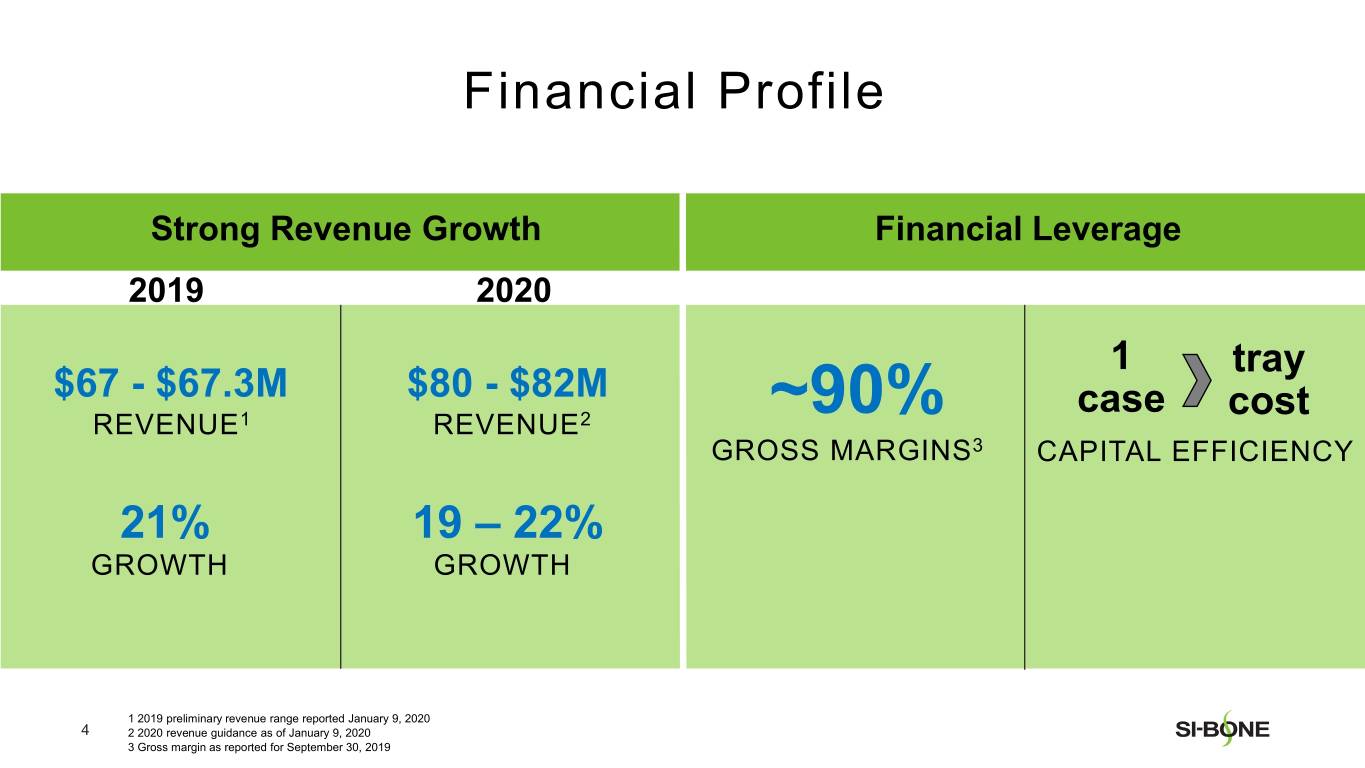
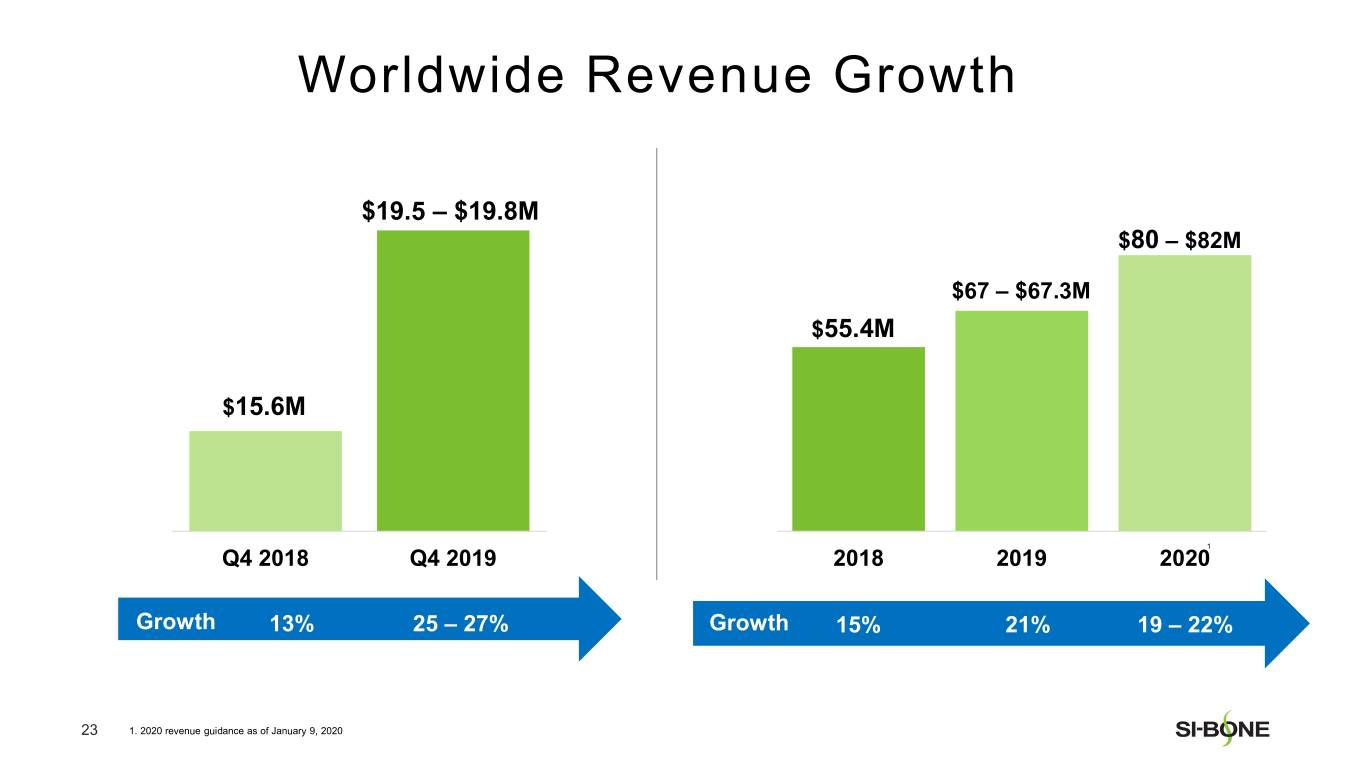
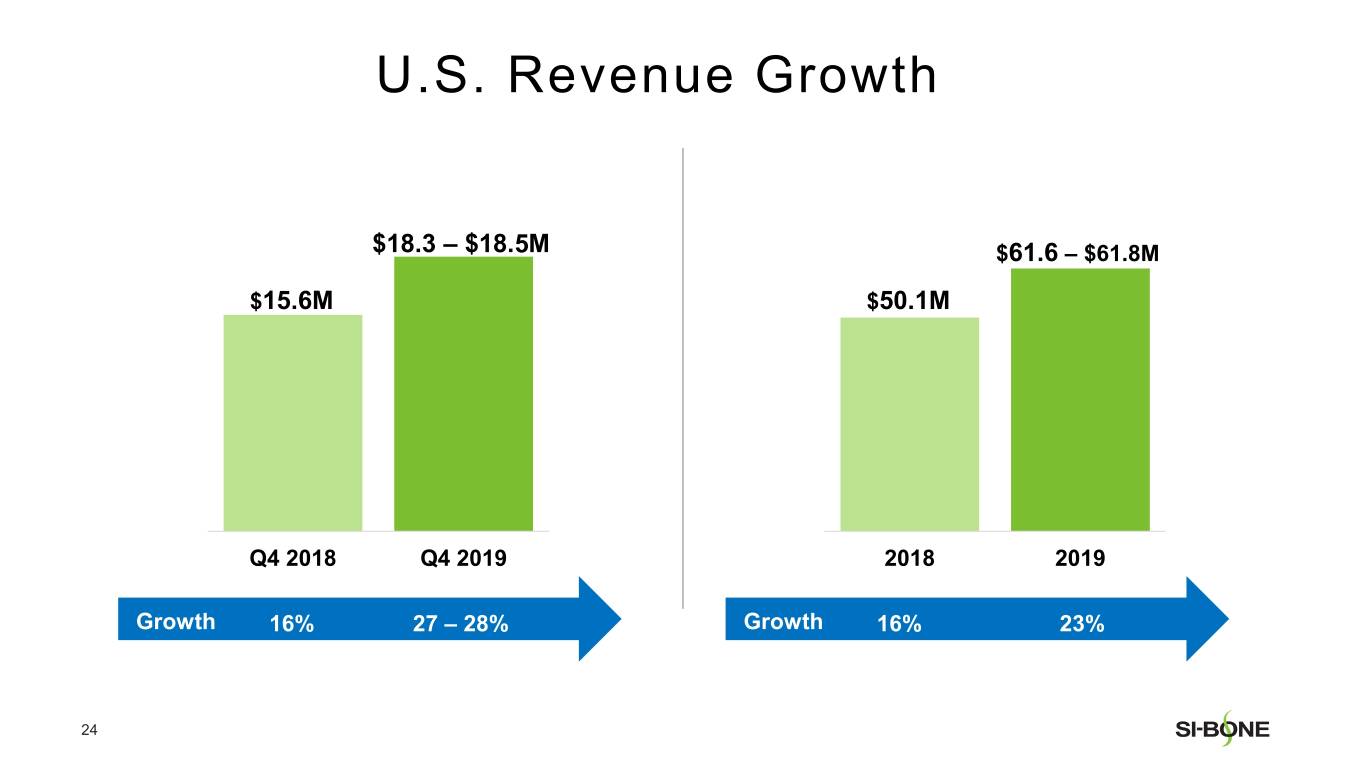
Preliminary and unaudited revenue for fourth quarter 2019 is expected to be in the range of $19.5-$19.8 million, reflecting growth of 25-27% compared to the prior year period. U.S. revenue is expected to be in the range of $18.3-$18.5 million, reflecting growth of 27-28% compared to the prior year period. International revenue is expected to be in the range of $1.2-$1.3 million.
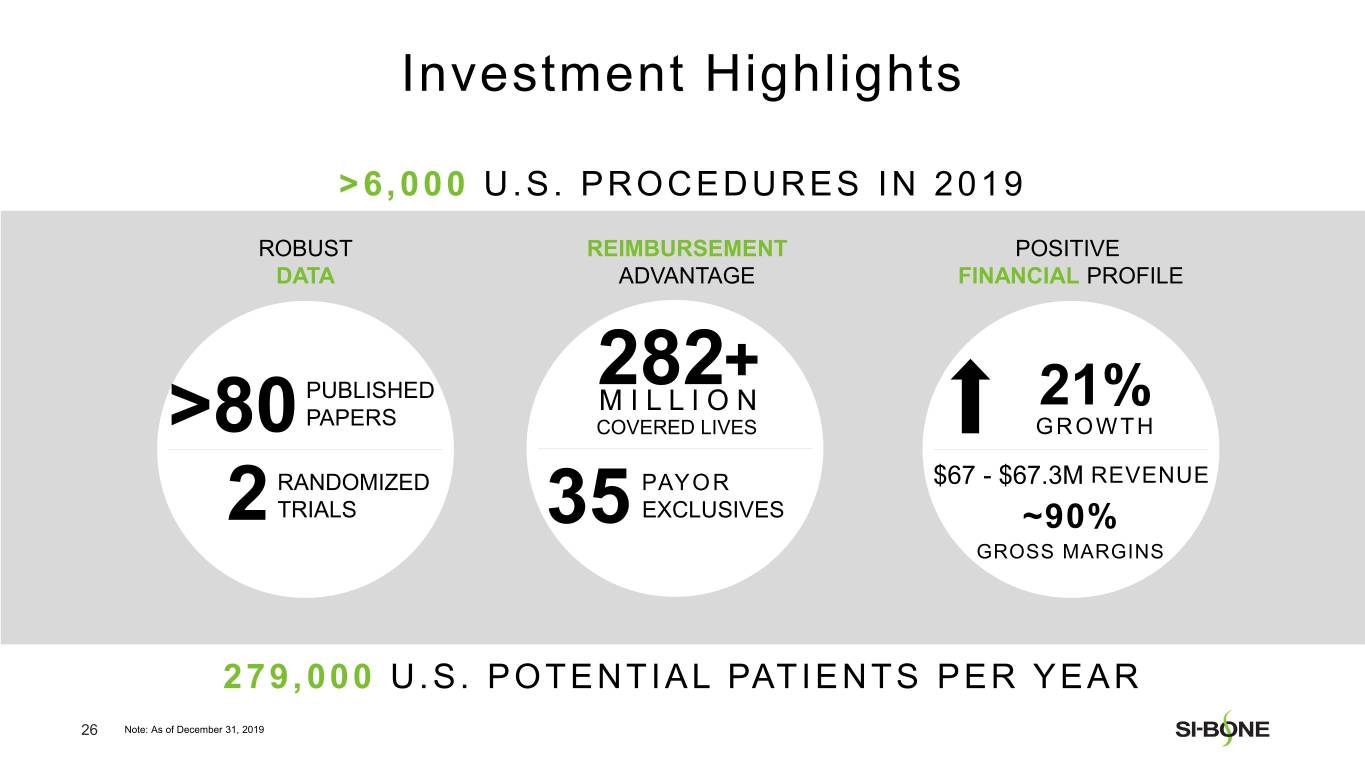
Preliminary and unaudited revenue for full year 2019 is expected to be in the range of $67.0-$67.3 million, reflecting growth of approximately 21% over full year 2018. U.S. revenue is expected to be in the range of $61.6-$61.8 million, reflecting growth of approximately 23% compared to the prior year period. International revenue is expected to be in the range of $5.4-$5.5 million.
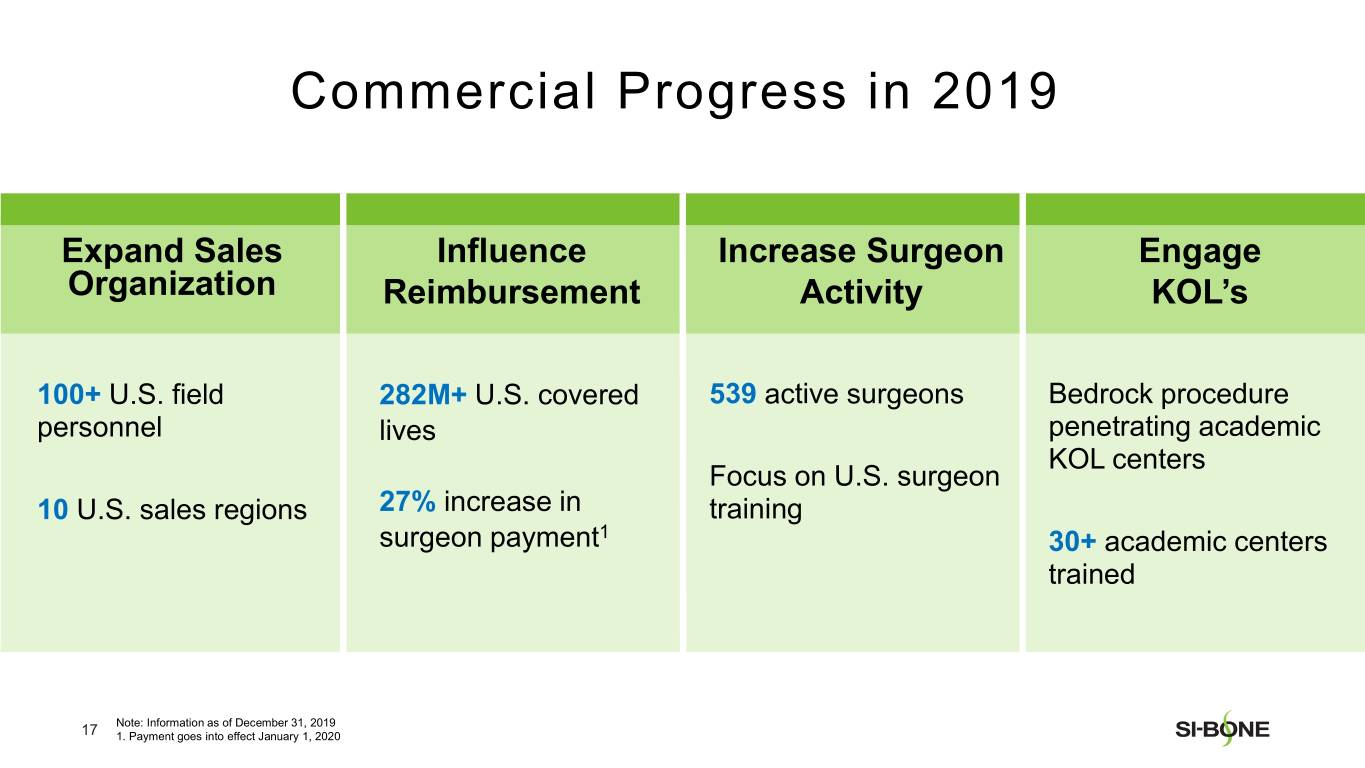
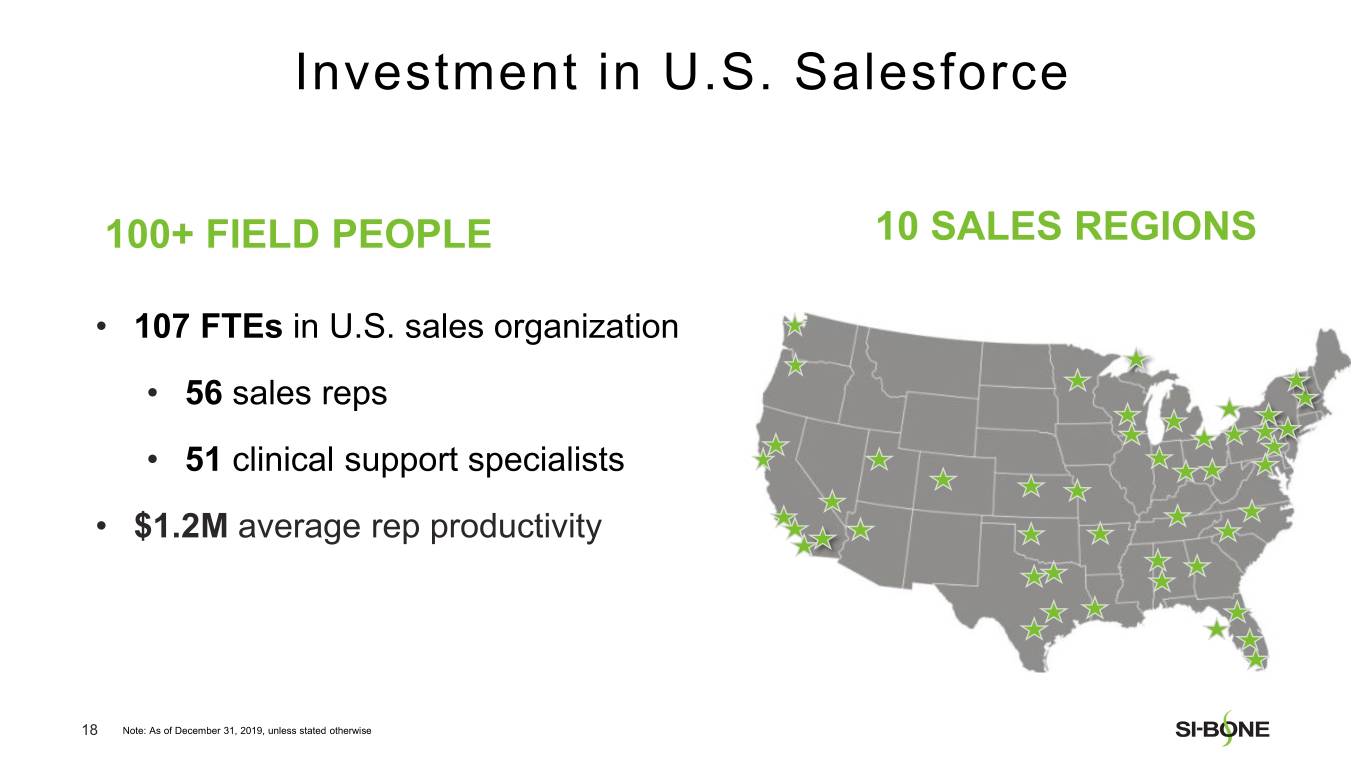
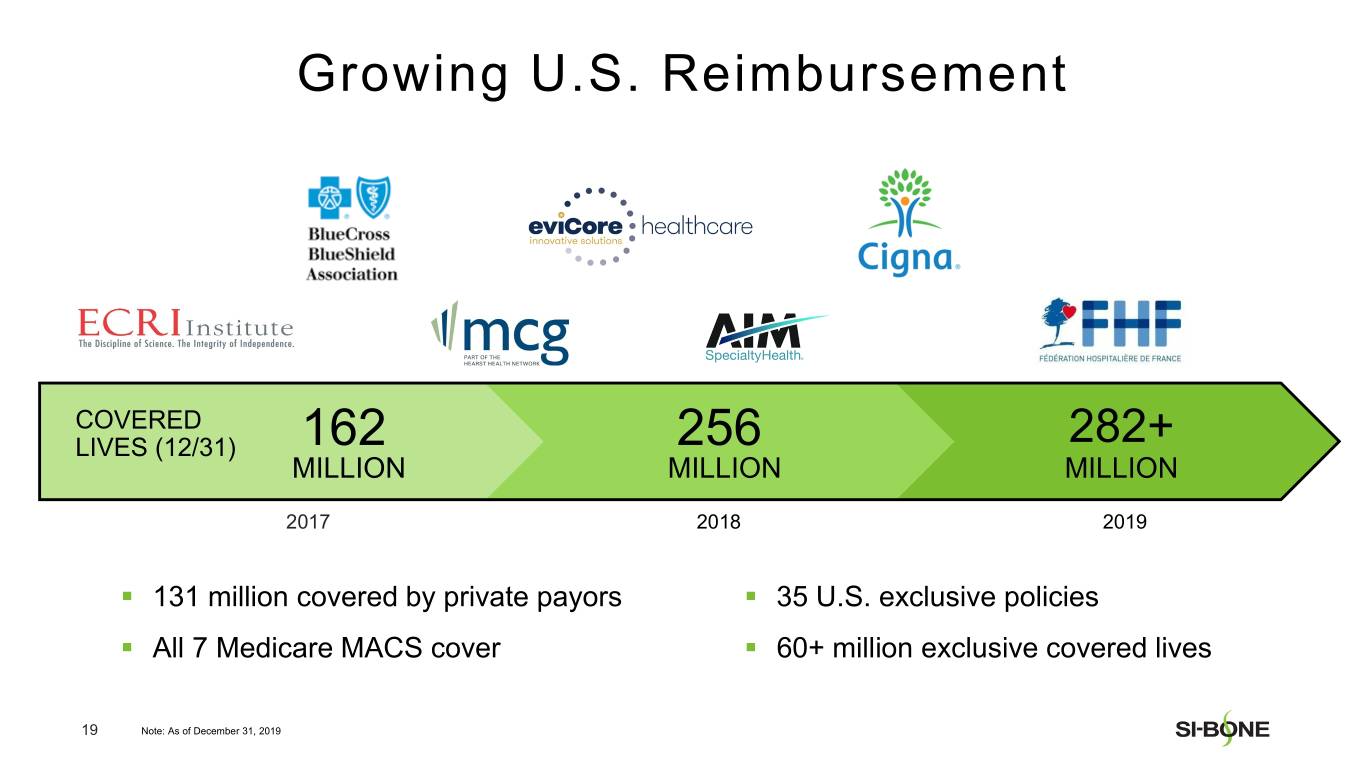
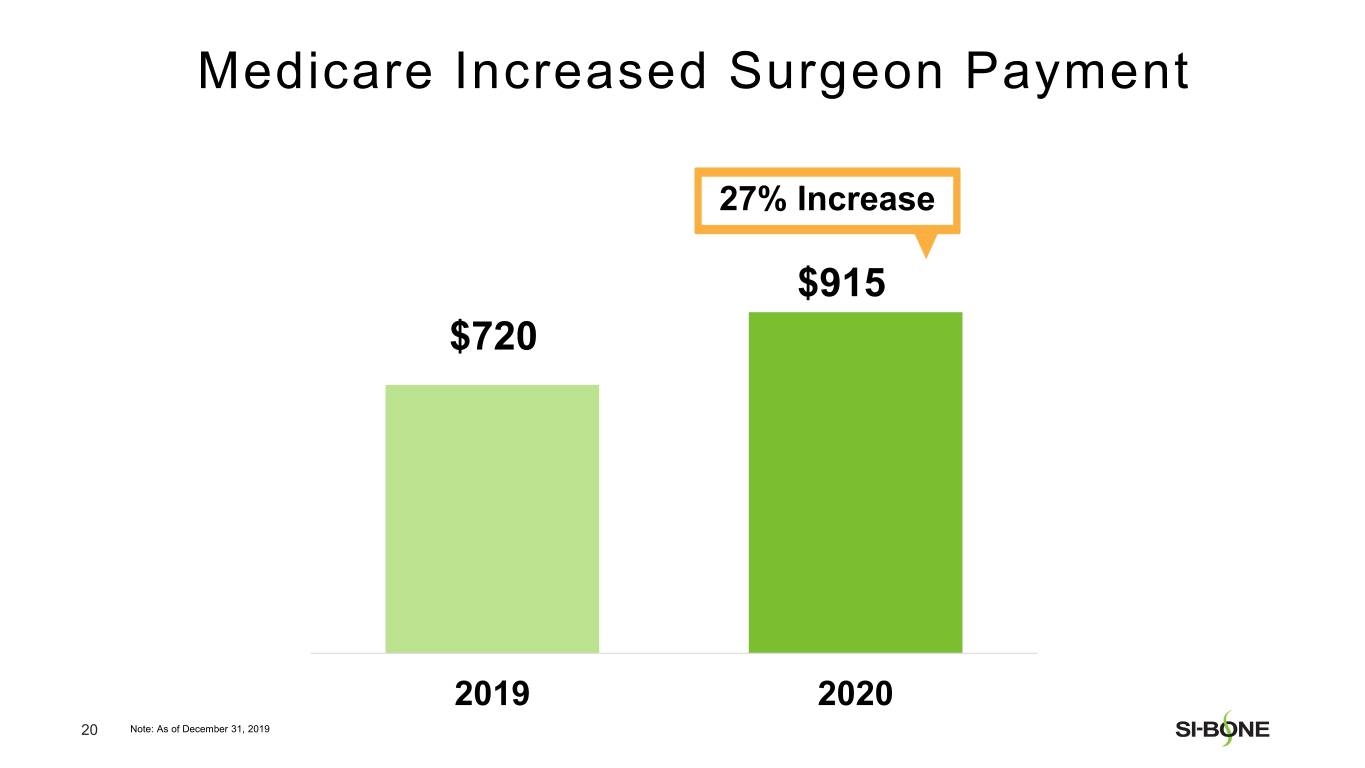
“We are pleased with our strong revenue performance in the fourth quarter, resulting from U.S. investments in sales force hiring and surgeon training throughout the year,” said Jeff Dunn, President, Chief Executive Officer, and Chairman of SI-BONE. “In addition, major U.S. commercial payors like Cigna continue to establish positive coverage policies for minimally invasive SI joint fusion based upon the strength of our clinical data, totaling over 282 million covered lives in the U.S. as of year-end. We are well positioned to deliver continued U.S. growth based upon execution of our commercial strategy while making improvements to our international business.”
The fourth quarter and full year 2019 revenue included in this release are preliminary and prior to the completion of SI-BONE's financial closing procedures and audit procedures by its external auditors and therefore may be subject to adjustment. SI-BONE expects to provide fourth quarter and full year 2019 financial results during its fourth quarter 2019 earnings call in March 2020.
2020 Financial Guidance
SI-BONE expects full year 2020 revenue to be in the range of $80-$82 million, representing growth of approximately 19-22% over preliminary and unaudited full year 2019 revenue.
About SI-BONE
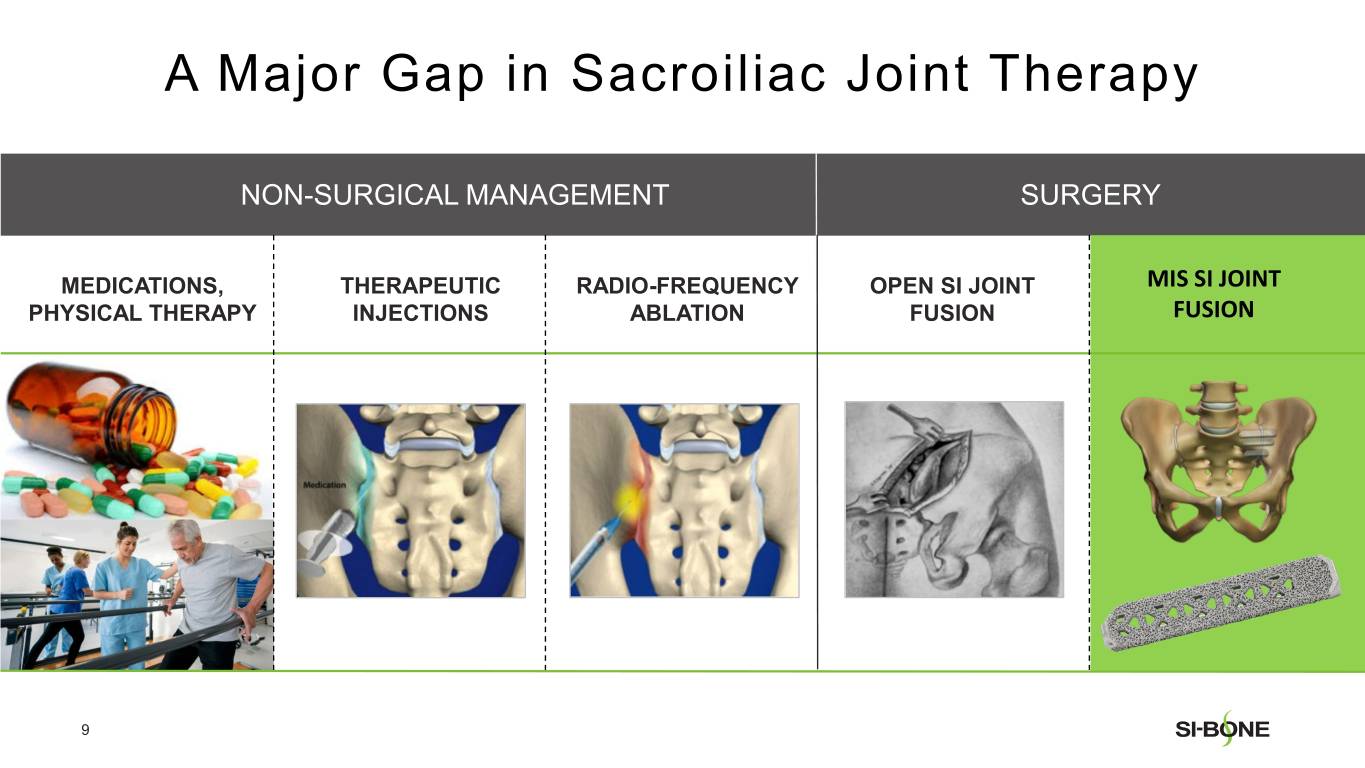
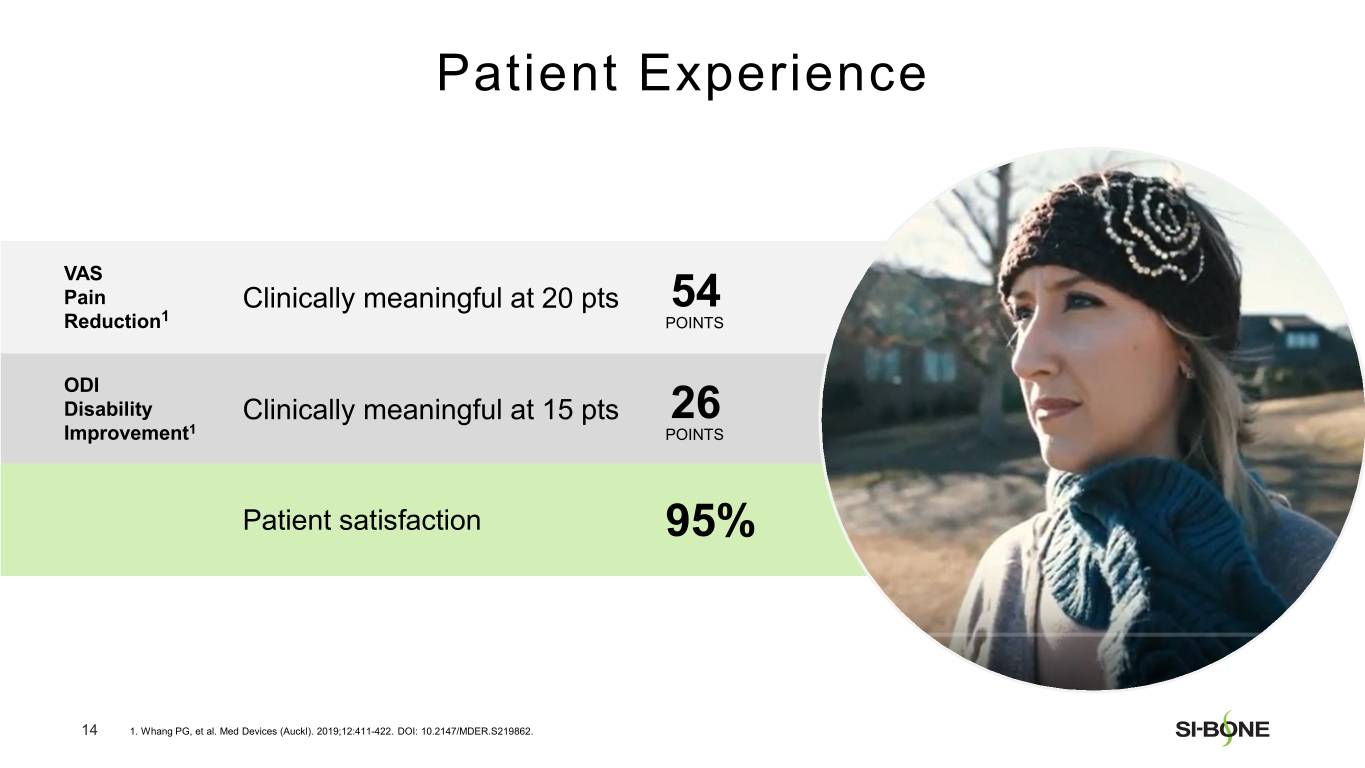
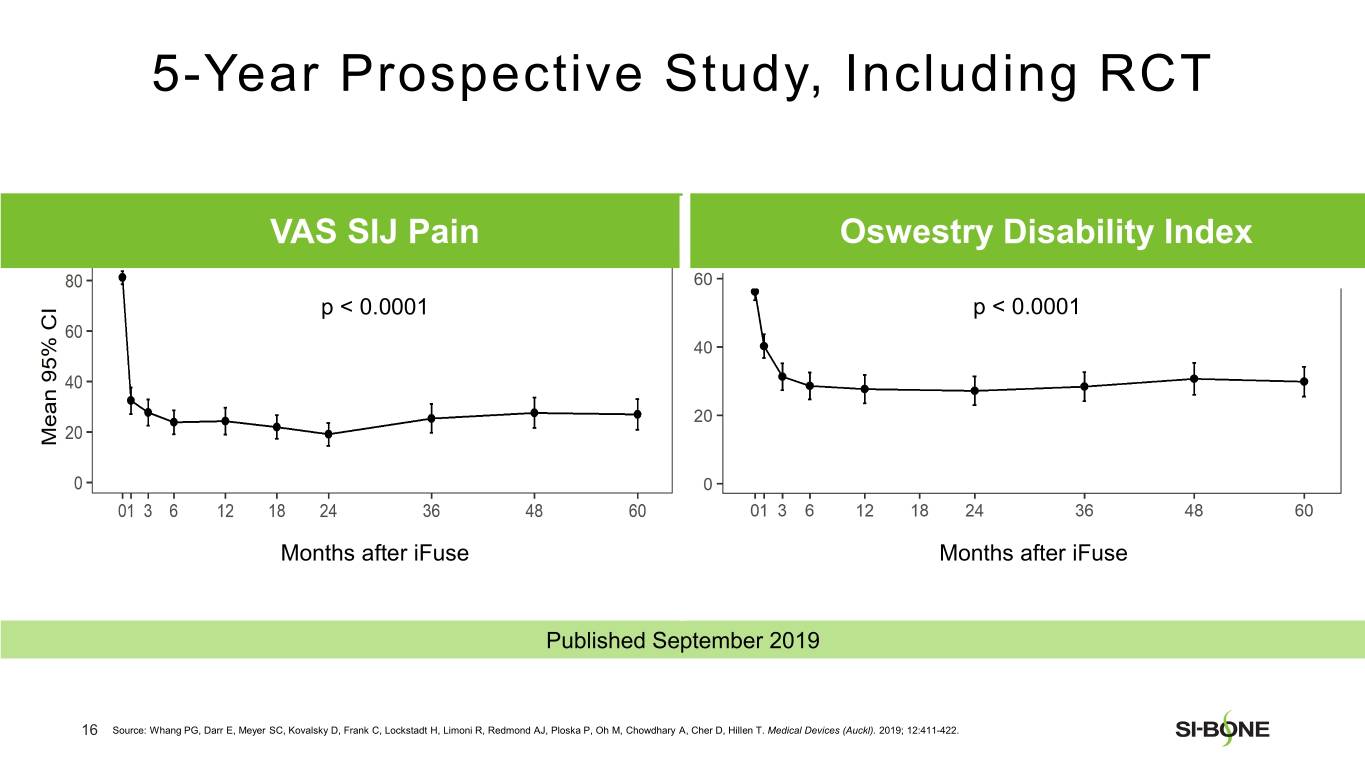
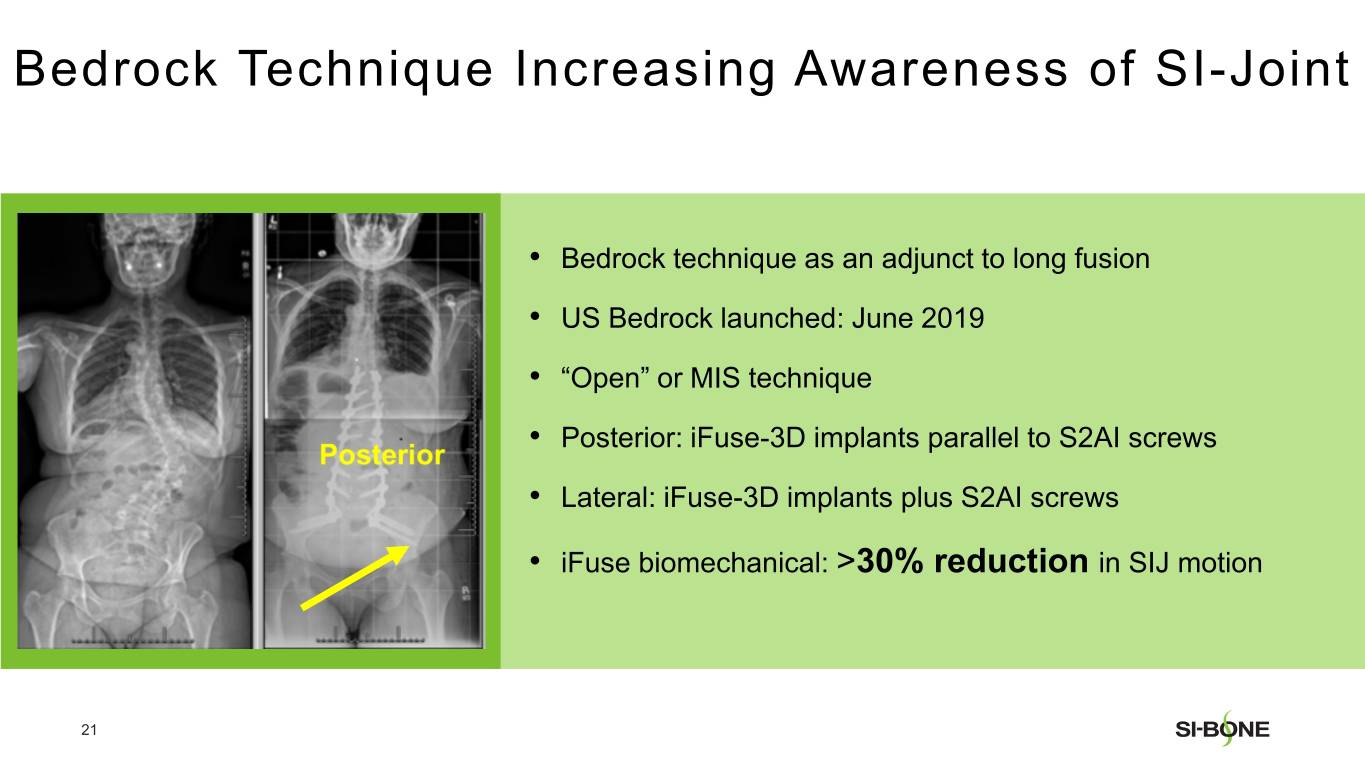
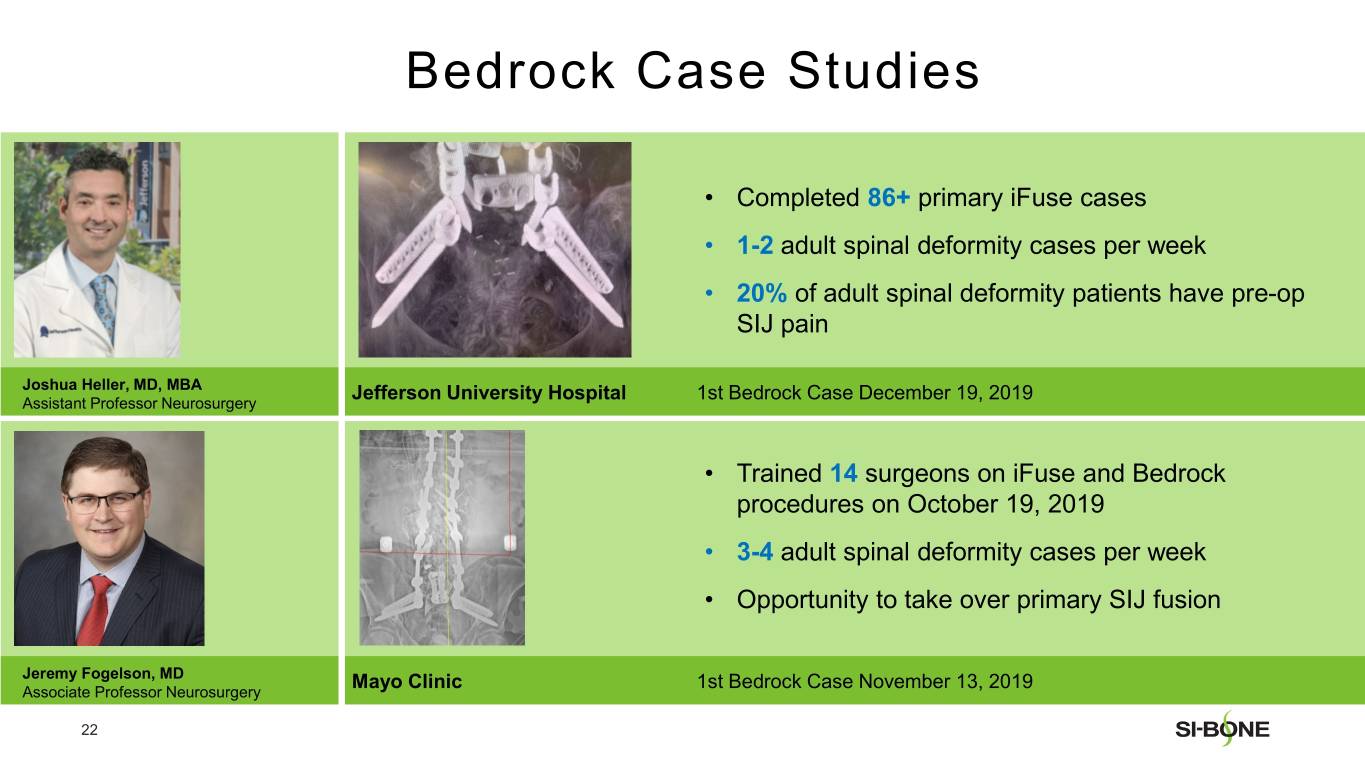
SI-BONE is a medical device company that pioneered minimally invasive surgery of the SI joint with the iFuse Implant System. Studies have shown that the SI joint can be a source of pain in 15% to 30% of chronic low back pain. The iFuse Implant™, commercially available since 2009, is the only SI joint fusion device supported by multiple prospective clinical studies, including two RCTs, showing improved pain, patient function and quality of life resulting from treatment. There are over 80 peer-reviewed publications demonstrating the safety, durable effectiveness, and biomechanical and economic benefits unique to the iFuse Implant (www.si-bone.com/results). This body of evidence has enabled multiple government and private insurance payors to establish coverage of the SI joint fusion procedure exclusively when performed with the iFuse Implant System.
The iFuse Implant System is intended for sacroiliac fusion for conditions including sacroiliac joint dysfunction that is a direct result of sacroiliac joint disruption and degenerative sacroiliitis. This includes conditions whose symptoms began during pregnancy or in the peripartum period and have persisted postpartum for more than 6 months. The iFuse Implant System is also intended for sacroiliac fusion to augment stabilization and immobilization of the sacroiliac joint in skeletally mature patients undergoing sacropelvic fixation as part of a lumbar or thoracolumbar fusion. There are potential risks associated with the iFuse Implant System. It may not be appropriate for all patients and all patients may not benefit.
For additional information on the company or the products including risks and benefits, please visit www.si-bone.com.
Forward-Looking Statements
The preliminary unaudited financial results, and statements regarding SI-BONE's continued growth and financial outlook, contained in this press release are "forward-looking" statements. These forward-looking statements are based on SI-BONE's current expectations and inherently involve significant risks and uncertainties and are subject to quarter-end closing adjustments. These risks include SI-BONE's preliminary fourth quarter and full year 2019 revenue, which are subject to continued review by SI-BONE and its auditors and significant adjustments may be made before final results are determined, and future performance is subject to SI-BONE's ability to expand its sales and marketing capabilities and increase demand for iFuse, expand geographically, and obtain favorable coverage and reimbursement determinations from third-party payors. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of the risks and uncertainties, many of which are described in the company's filings on Form 10-K and Form 10-Q, and the company’s other filings with the Securities and Exchange Commission (SEC) available at the SEC's Internet site (www.sec.gov), especially under the caption "Risk Factors". SI-BONE does not undertake any obligation to update forward-looking statements and expressly disclaims any obligations or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein, except as required by law.
Investor Contact:
Lynn Lewis or Carrie Mendivil
investors@SI-BONE.com
Media Contact:
Joe Powers
jpowers@si-bone.com