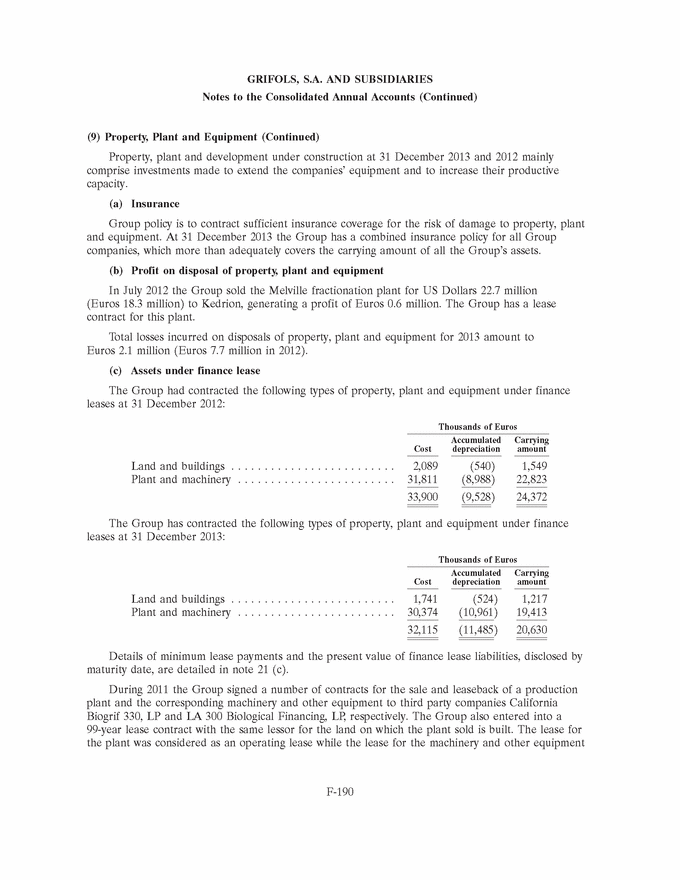
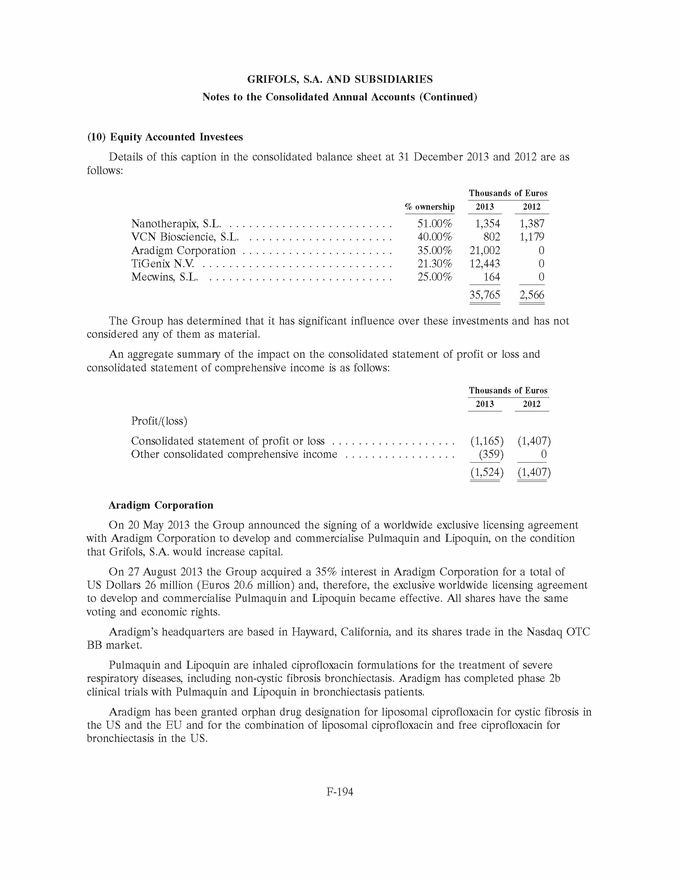
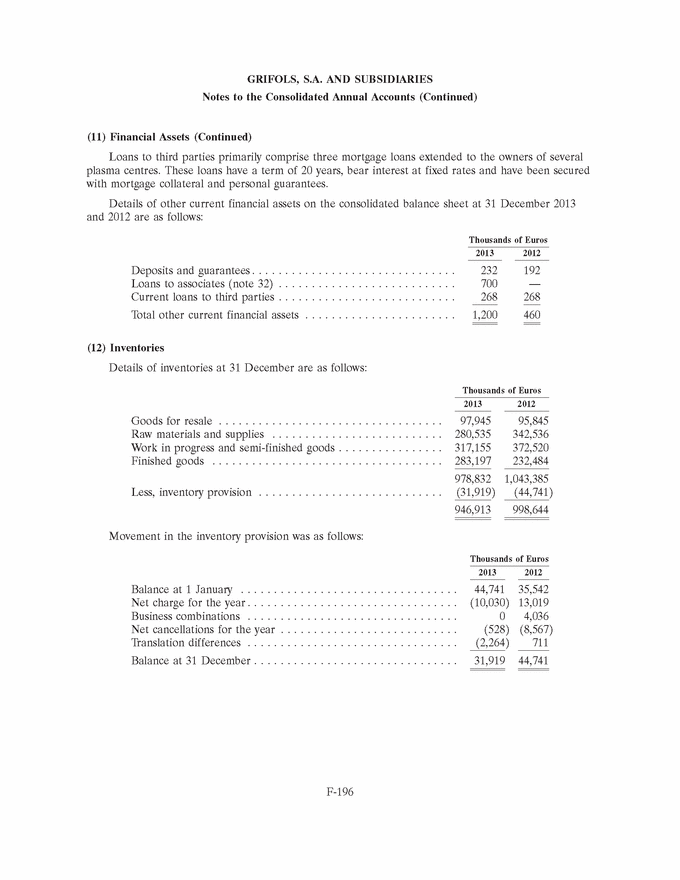
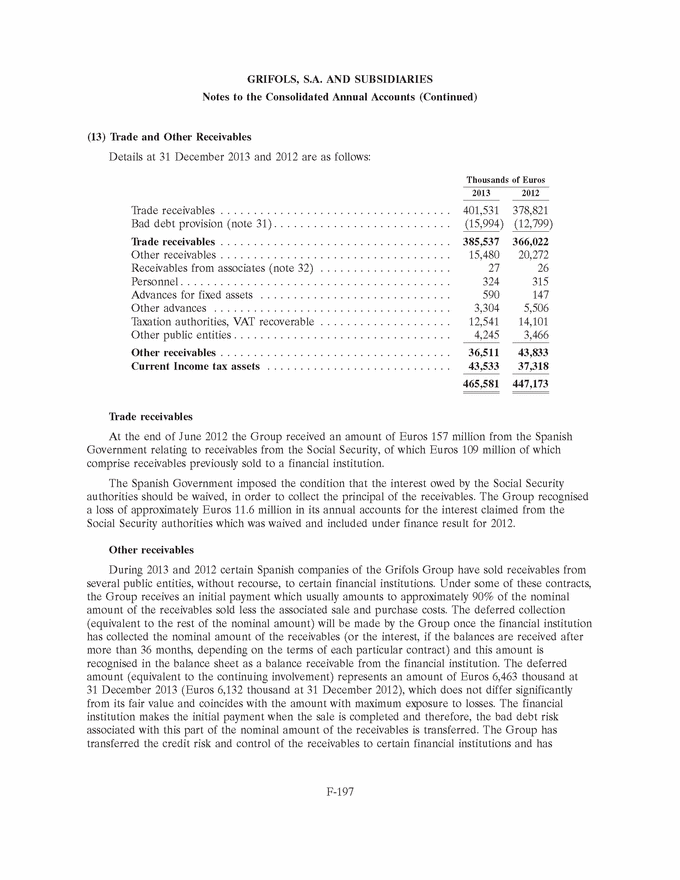
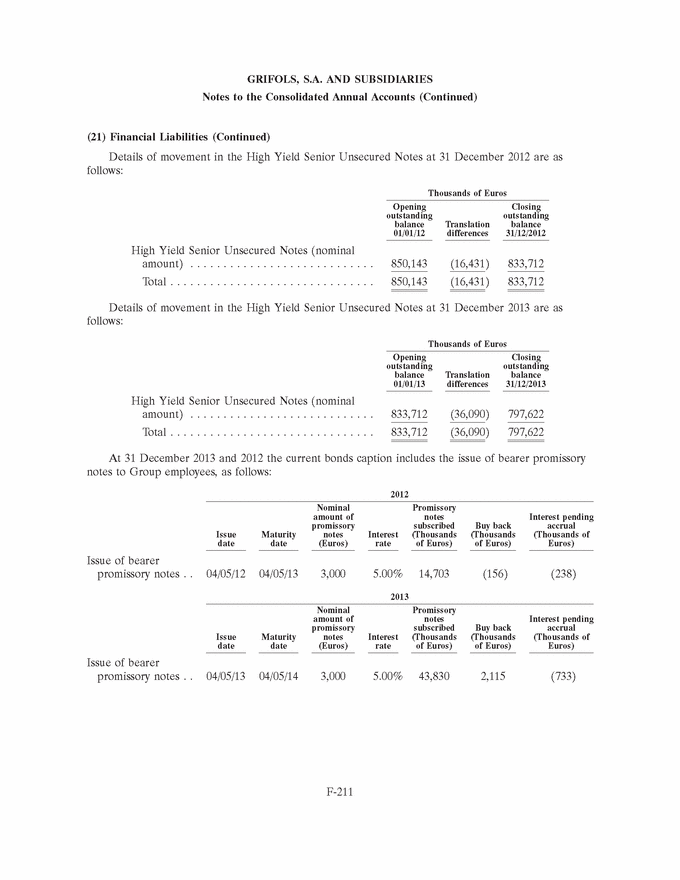
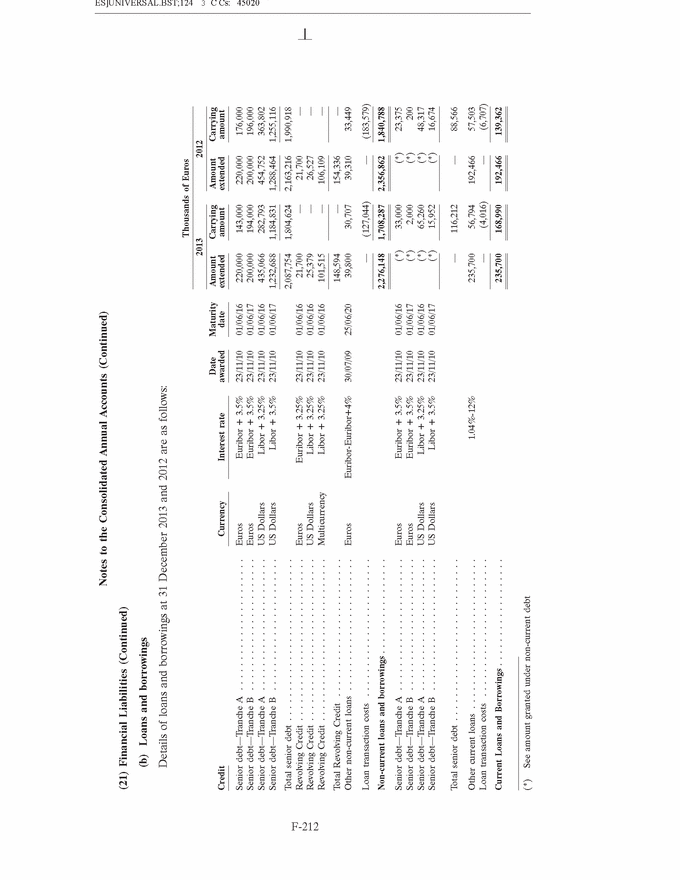
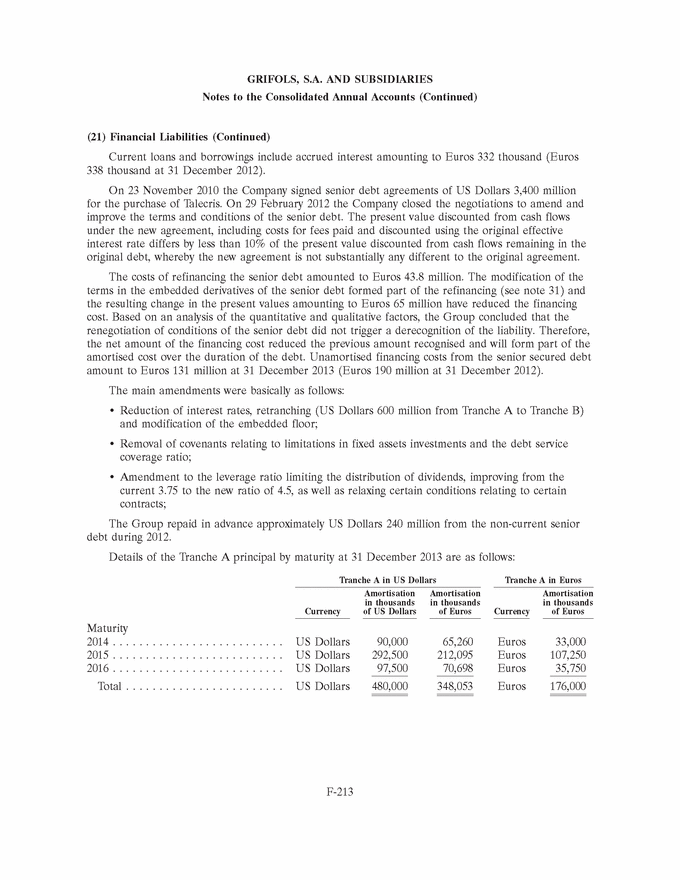
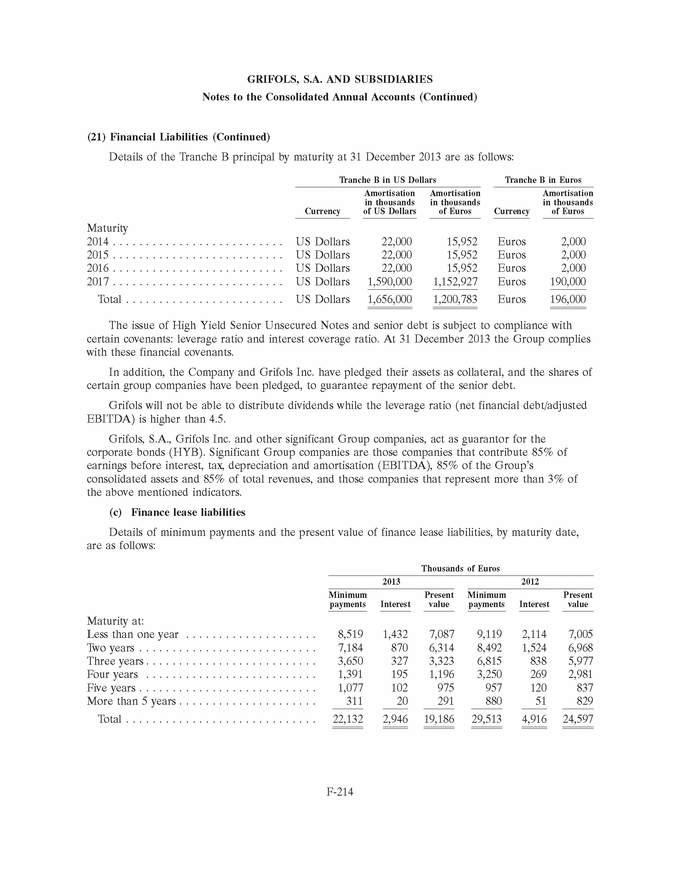
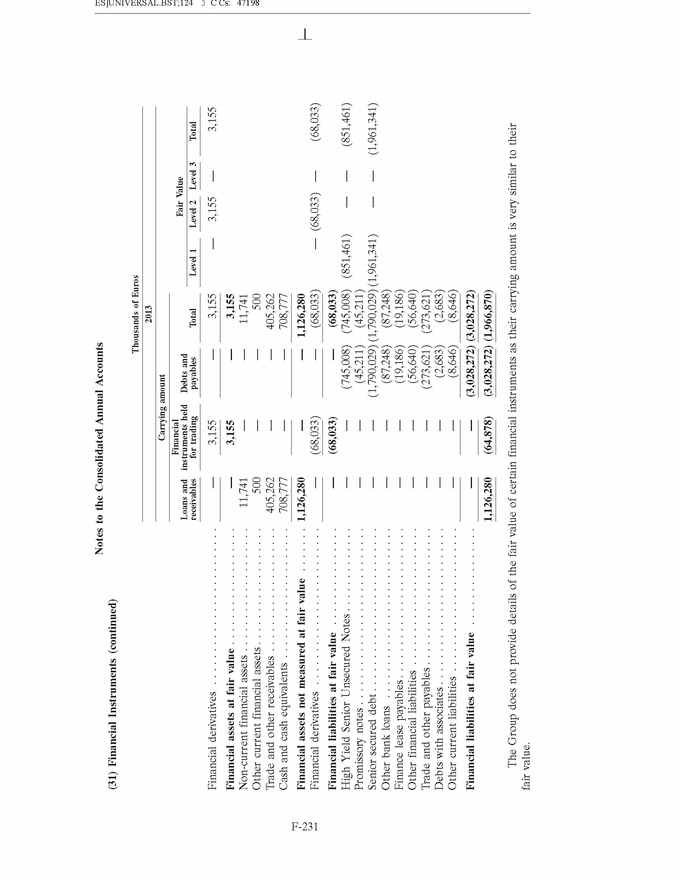
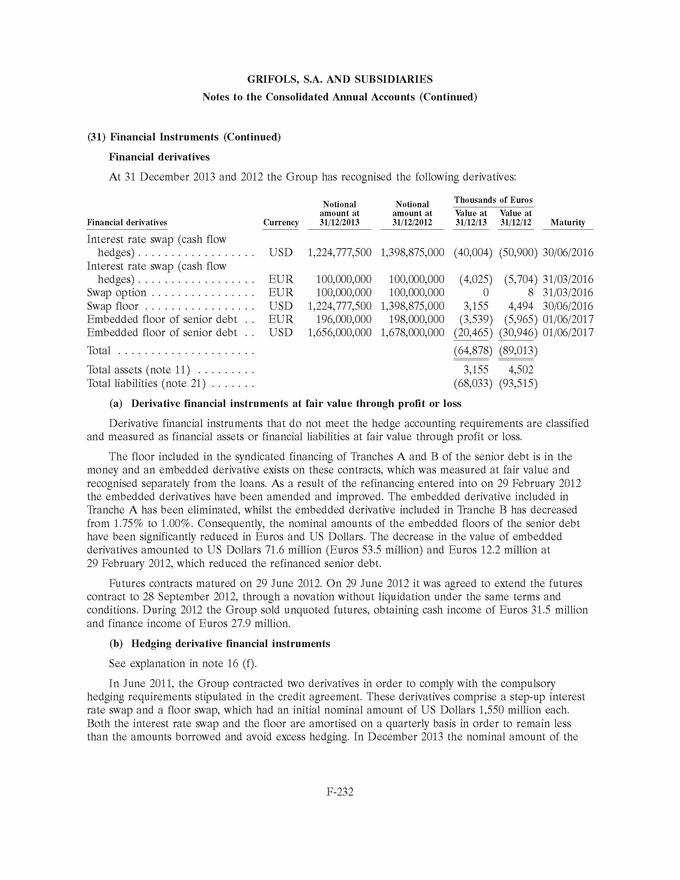
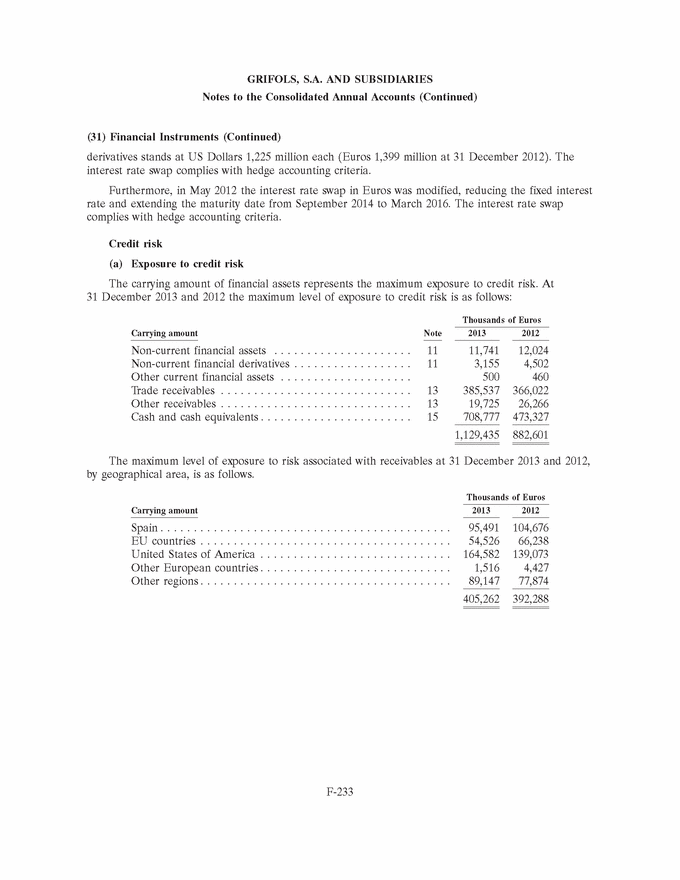
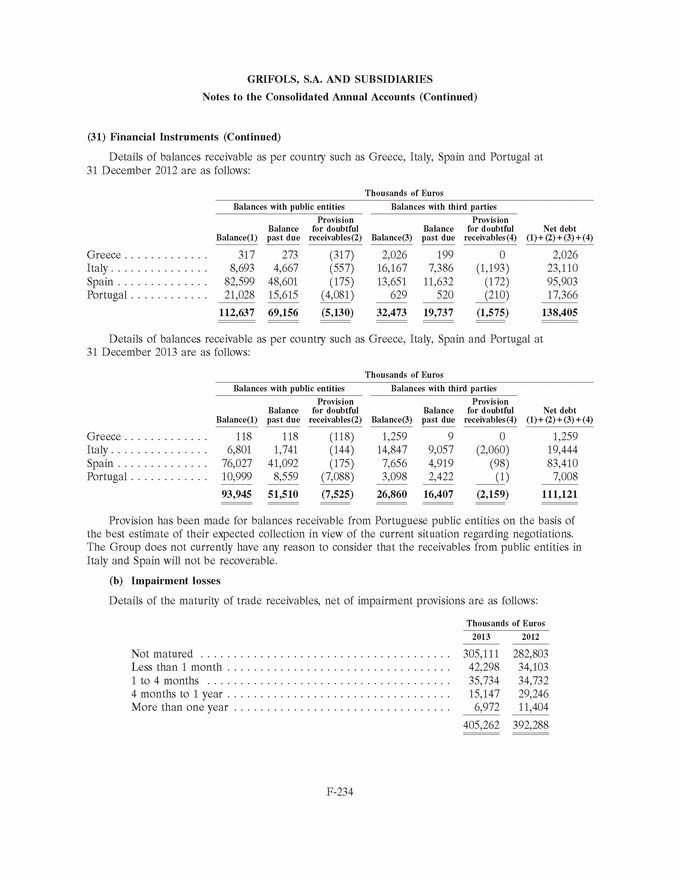
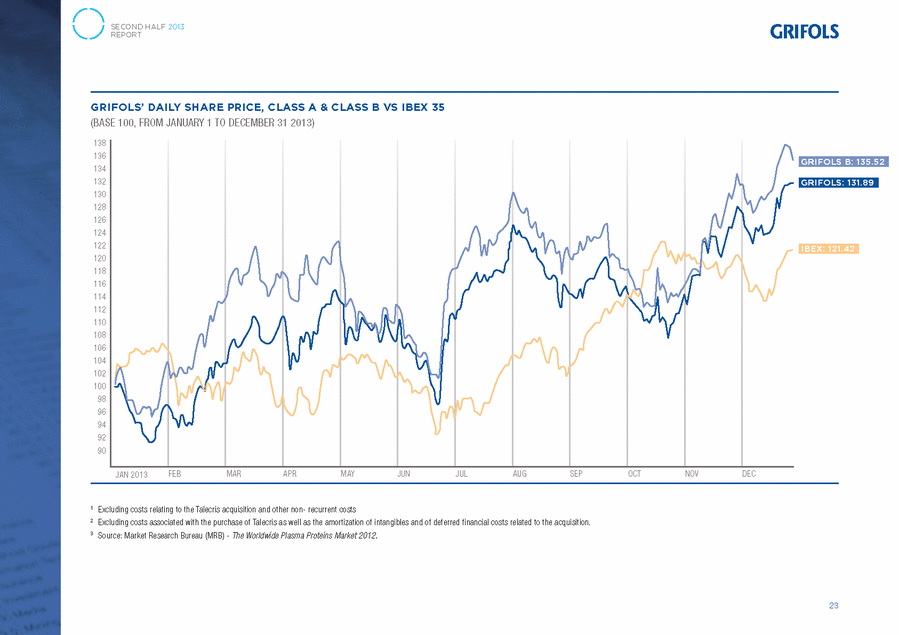
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 6-K
REPORT OF FOREIGN ISSUER
PURSUANT TO RULE 13a-16 OR 15d-16 OF THE
SECURITIES EXCHANGE ACT OF 1934
For the month February 2014
(Commission File No. 001-35193)
Grifols, S.A.
(Translation of registrant’s name into English)
Avinguda de la Generalitat, 152-158
Parc de Negocis Can Sant Joan
Sant Cugat del Valles 08174
Barcelona, Spain
(Address of registrant’s principal executive office)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F.
Form 20-F x Form 40-F o
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (1):
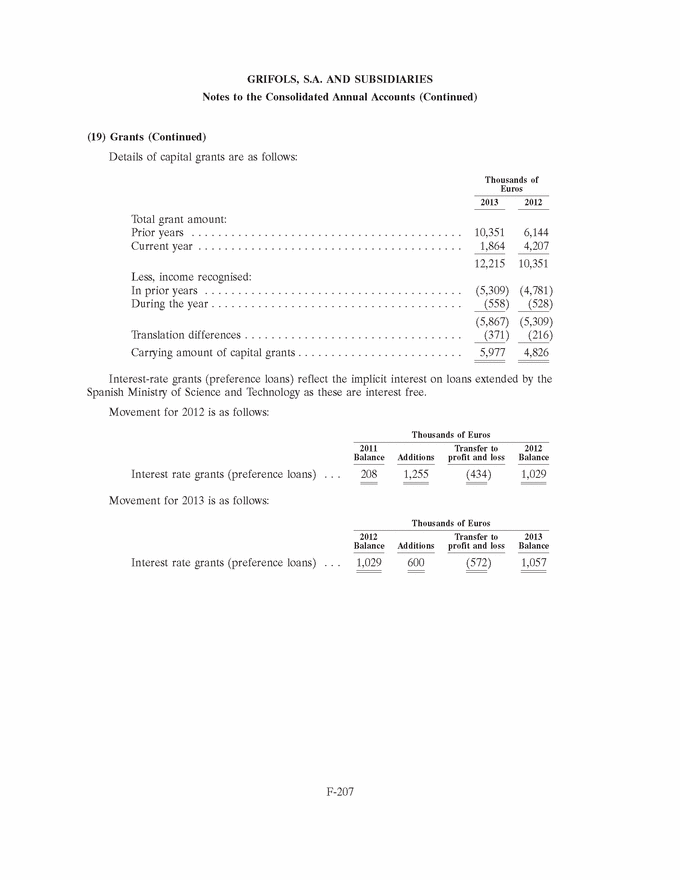
Yes o No x
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101 (b) (7):
Yes o No x
Indicate by check mark whether the registrant by furnishing the information contained in this Form is also thereby furnishing the information to the Commission pursuant to Rule 12g3-2(b) under the Securities Exchange Act of 1934.
Yes o No x
If “Yes” is marked, indicate below the file number assigned to the registrant in connection with Rule 12g3-2(b): 82- . .
Grifols, S.A.
TABLE OF CONTENTS
|
Item |
|
|
|
Sequential Page Number |
|
|
|
|
|
|
|
1 |
|
Consolidated Annual Accounts for the year ended December 31, 2013. |
|
2 |
|
|
|
|
|
|
|
2. |
|
Second Half 2013 Report. |
|
|
|
|
|
|
|
|
|
3. |
|
Press release, dated February 27, 2014. |
|
|
Grifols Conso CCAA
|
|
[LOGO] |
|
|
[LOGO] |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Annual Accounts 31 December 2013 and 2012 SUMMARY • Consolidated financial statements • Consolidated Balance Sheets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-142 • Consolidated Statements of Profit or Loss . . . . . . . . . . . . . . . . . . . . . . . . . . . F-144 • Consolidated Statements of Comprehensive Income . . . . . . . . . . . . . . . . . . . . F-145 • Consolidated Statements of Cash Flows . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-146 • Consolidated Statements of Changes in Equity . . . . . . . . . . . . . . . . . . . . . . . . F-147 • Notes to the Annual Accounts (1) Nature, Principal Activities and Subsidiaries . . . . . . . . . . . . . . . . . . . . . . . . . . F-148 (2) Basis of Presentation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-148 (3) Business Combinations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-154 (4) Significant Accounting Policies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-159 (5) Financial Risk Management Policy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-180 (6) Segment Reporting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-184 (7) Goodwill . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-186 (8) Other Intangible Assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-188 (9) Property, Plant and Equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-189 (10) Equity-Accounted Investees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-194 (11) Financial Assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-195 (12) Inventories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-196 (13) Trade and Other Receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-197 (14) Other Current Assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-198 (15) Cash and Cash Equivalents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-198 (16) Equity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-199 (17) Earnings per Share . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-204 (18) Non-Controlling Interests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-205 (19) Grants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-206 (20) Provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-208 (21) Financial Liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-209 (22) Trade and Other Payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-216 (23) Other Current Liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-216 (24) Net Revenues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-217 (25) Personnel Expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-219 (26) Expenses by Nature . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-220 (27) Finance Result . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-222 (28) Taxation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-222 (29) Operating Leases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-227 (30) Other Commitments with Third Parties and Other Contingent Liabilities . . . . . F-227 (31) Financial Instruments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-230 (32) Balances and Transactions with Related Parties . . . . . . . . . . . . . . . . . . . . . . . F-238 (33) Environmental Issues . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-240 (34) Other Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-241 (35) Events After the Reporting Period . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-242 F-140 |
|
|
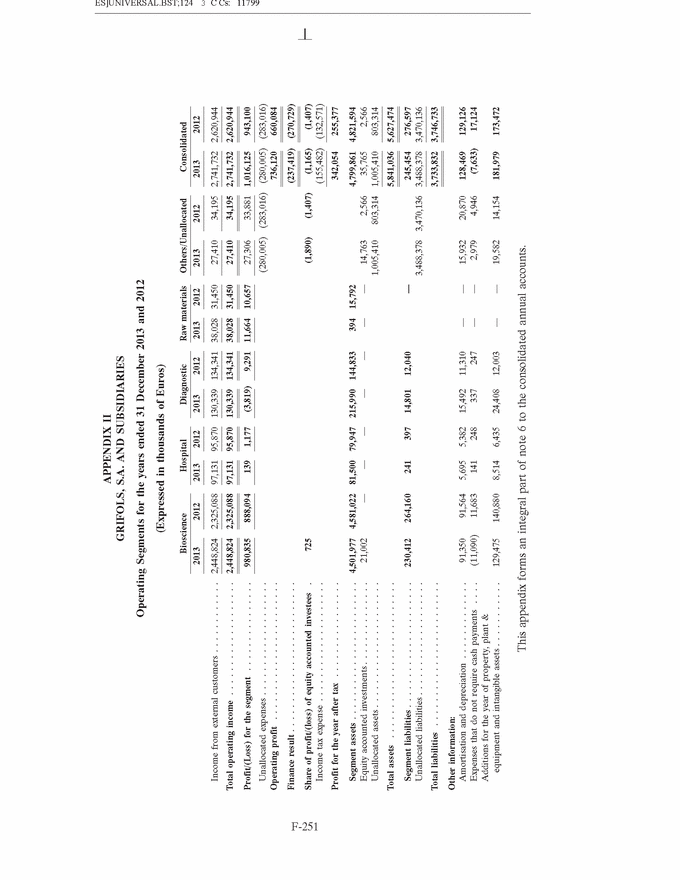
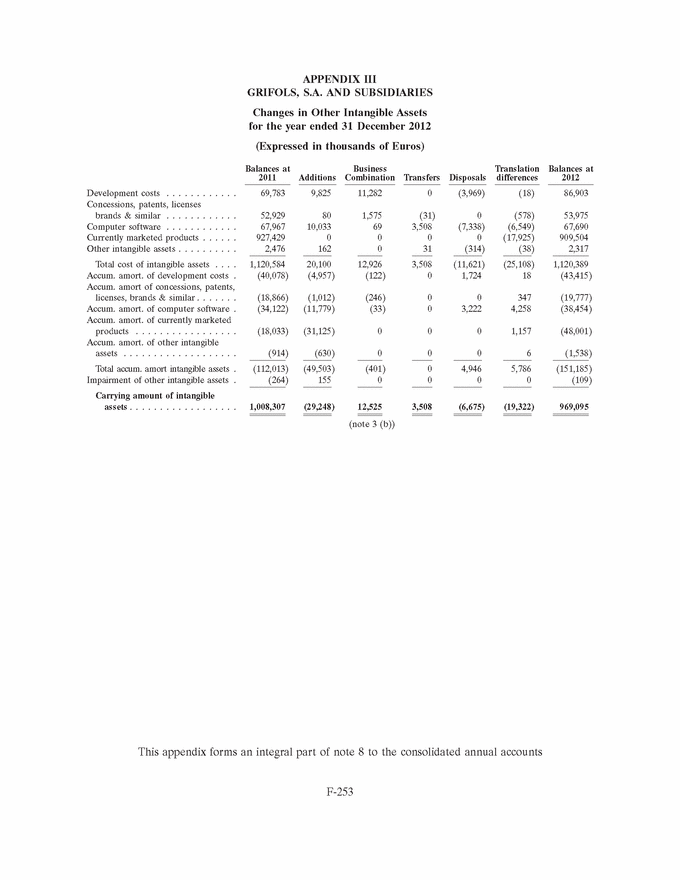
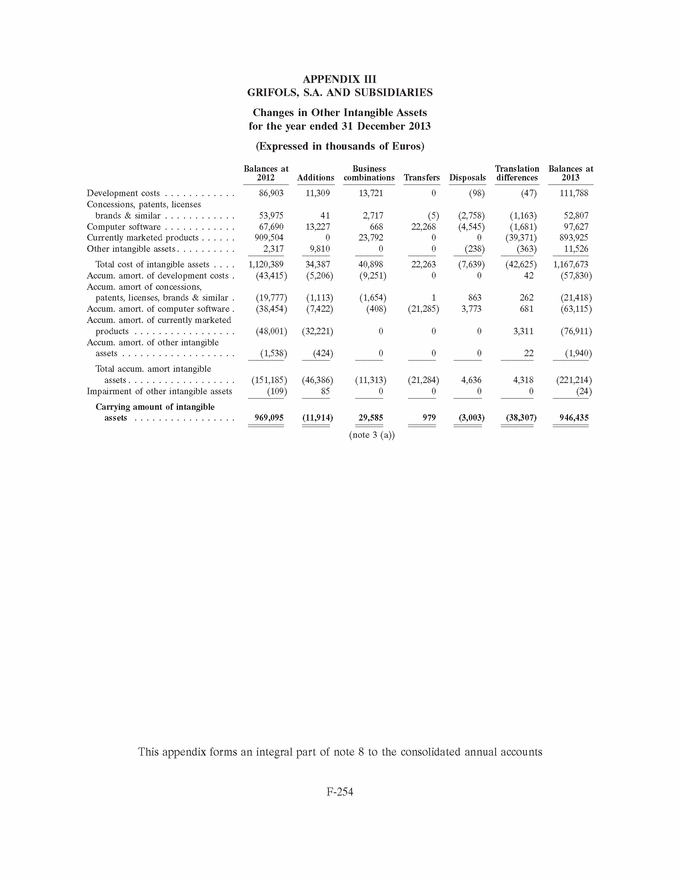
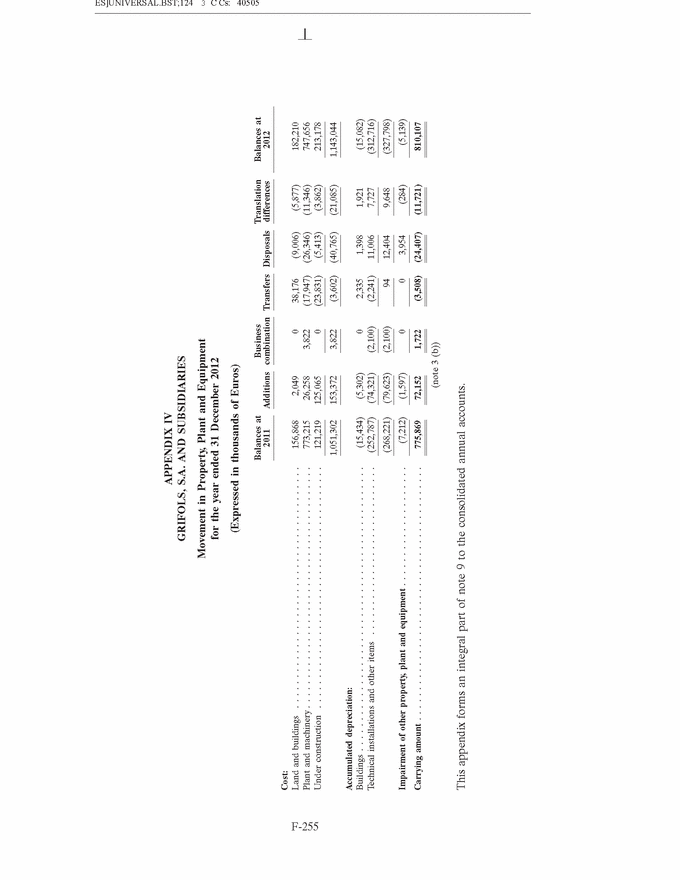
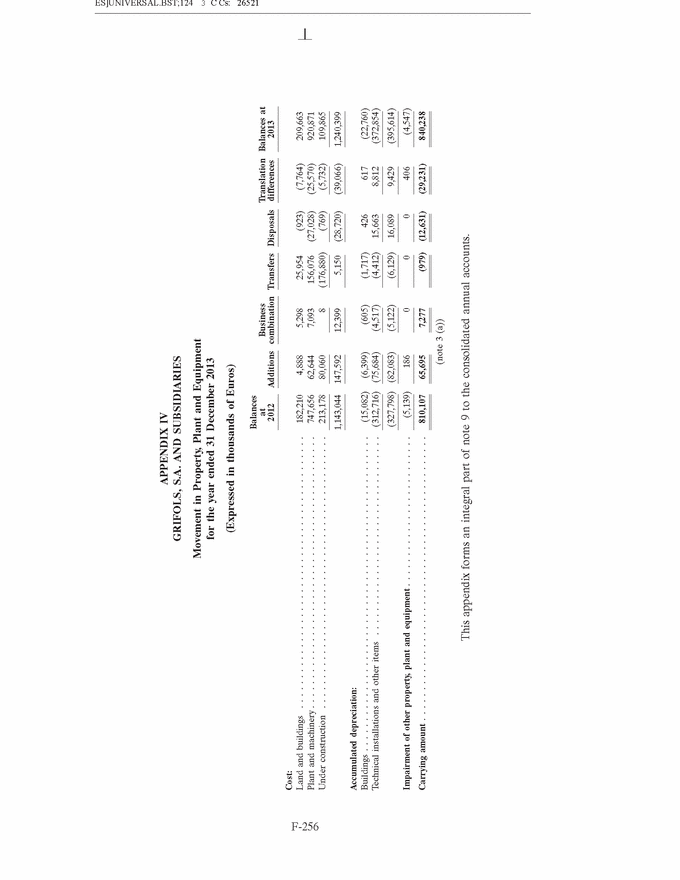
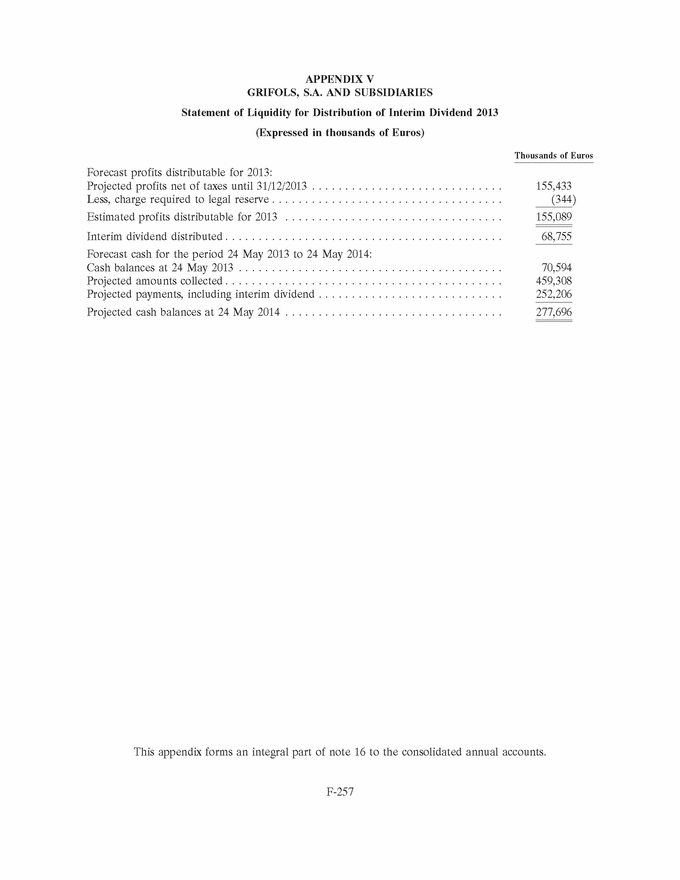
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Annual Accounts 31 December 2013 and 2012 SUMMARY • Appendices • Appendix I Information on Group Companies, Associates and Other . . . . . . . F-245 • Appendix II Operating Segments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . F-251 • Appendix III Movement in Other Intangible Assets . . . . . . . . . . . . . . . . . . . . F-253 • Appendix IV Movement in Property, Plant and Equipment . . . . . . . . . . . . . . . F-255 • Appendix V Statement of Liquidity for Distribution of Interim Dividend 2013 . F-277 F-141 |
|
|
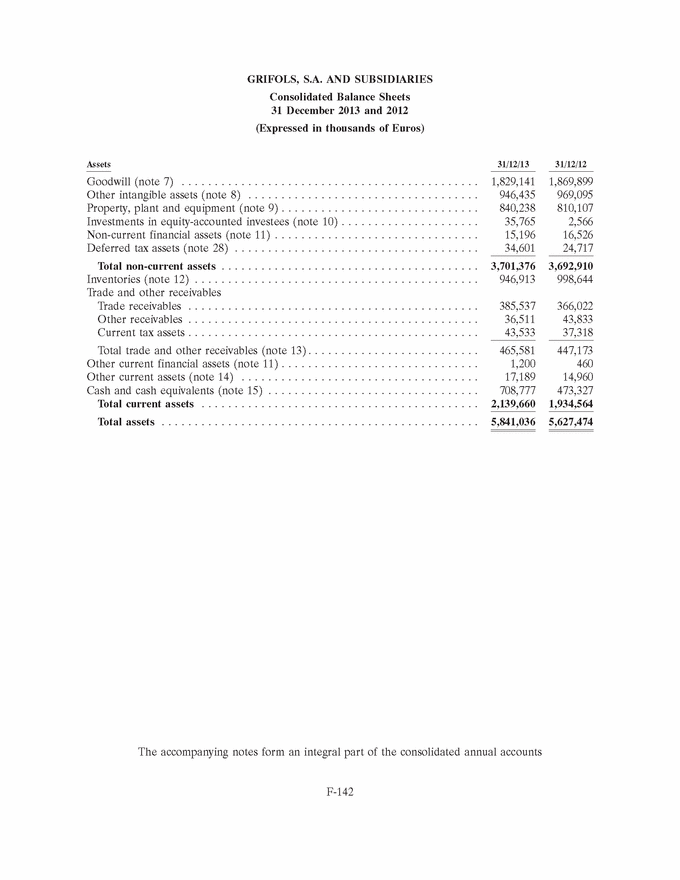
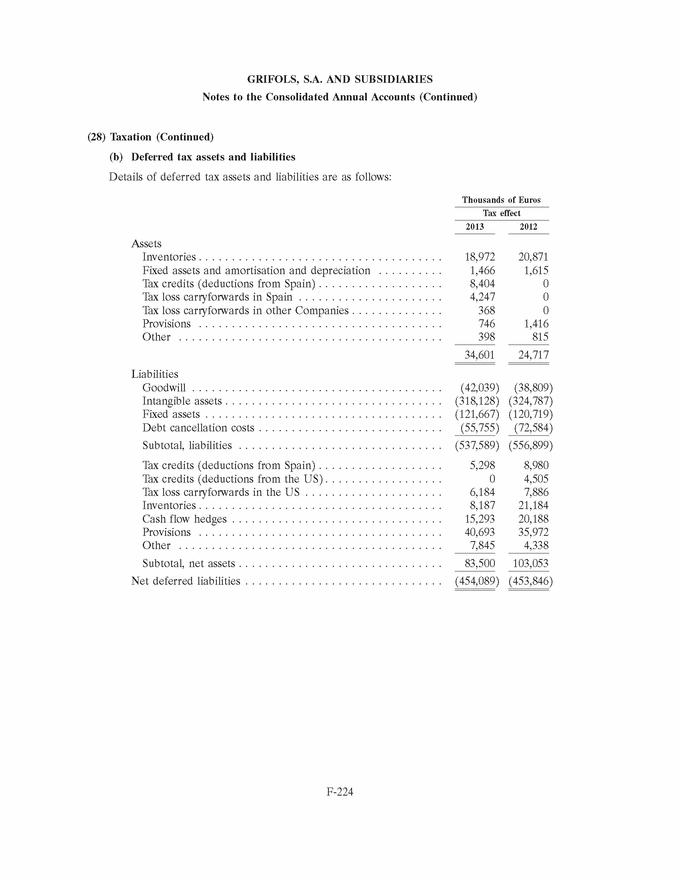
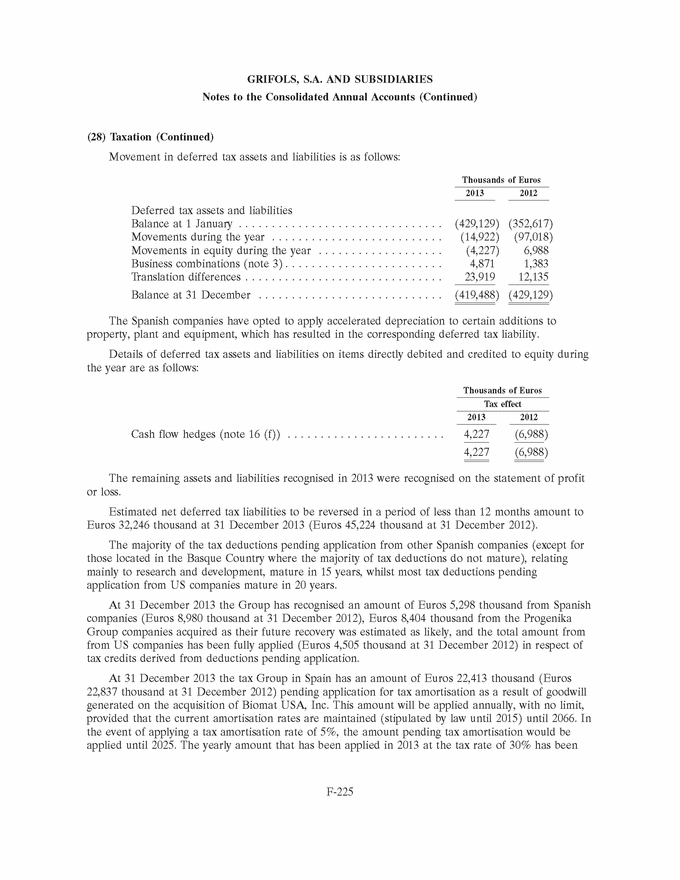
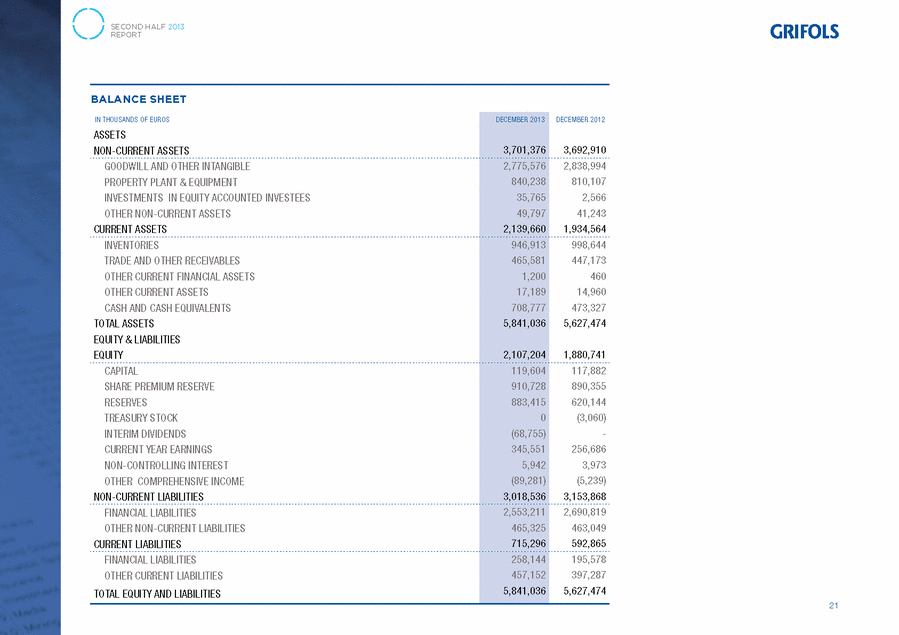
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Balance Sheets 31 December 2013 and 2012 (Expressed in thousands of Euros) Assets 31/12/13 31/12/12 Goodwill (note 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,829,141 1,869,899 Other intangible assets (note 8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 946,435 969,095 Property, plant and equipment (note 9) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 840,238 810,107 Investments in equity-accounted investees (note 10) . . . . . . . . . . . . . . . . . . . . . 35,765 2,566 Non-current financial assets (note 11) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15,196 16,526 Deferred tax assets (note 28) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 34,601 24,717 Total non-current assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,701,376 3,692,910 Inventories (note 12) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 946,913 998,644 Trade and other receivables Trade receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 385,537 366,022 Other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36,511 43,833 Current tax assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43,533 37,318 Total trade and other receivables (note 13) . . . . . . . . . . . . . . . . . . . . . . . . . . 465,581 447,173 Other current financial assets (note 11) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,200 460 Other current assets (note 14) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17,189 14,960 Cash and cash equivalents (note 15) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 708,777 473,327 Total current assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,139,660 1,934,564 Total assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,841,036 5,627,474 The accompanying notes form an integral part of the consolidated annual accounts F-142 |
|
|
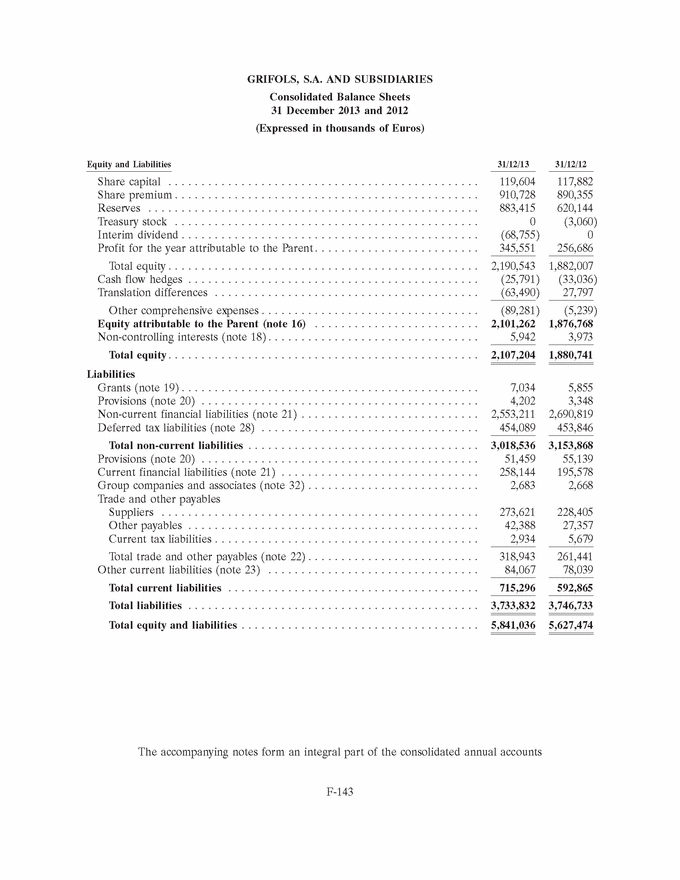
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Balance Sheets 31 December 2013 and 2012 (Expressed in thousands of Euros) Equity and Liabilities 31/12/13 31/12/12 Share capital . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 119,604 117,882 Share premium . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 910,728 890,355 Reserves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 883,415 620,144 Treasury stock . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0 (3,060) Interim dividend . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (68,755) 0 Profit for the year attributable to the Parent . . . . . . . . . . . . . . . . . . . . . . . . . 345,551 256,686 Total equity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,190,543 1,882,007 Cash flow hedges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (25,791) (33,036) Translation differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (63,490) 27,797 Other comprehensive expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (89,281) (5,239) Equity attributable to the Parent (note 16) . . . . . . . . . . . . . . . . . . . . . . . . . 2,101,262 1,876,768 Non-controlling interests (note 18) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,942 3,973 Total equity . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,107,204 1,880,741 Liabilities Grants (note 19) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7,034 5,855 Provisions (note 20) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,202 3,348 Non-current financial liabilities (note 21) . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,553,211 2,690,819 Deferred tax liabilities (note 28) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 454,089 453,846 Total non-current liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,018,536 3,153,868 Provisions (note 20) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51,459 55,139 Current financial liabilities (note 21) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 258,144 195,578 Group companies and associates (note 32) . . . . . . . . . . . . . . . . . . . . . . . . . . 2,683 2,668 Trade and other payables Suppliers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 273,621 228,405 Other payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42,388 27,357 Current tax liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,934 5,679 Total trade and other payables (note 22) . . . . . . . . . . . . . . . . . . . . . . . . . . 318,943 261,441 Other current liabilities (note 23) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84,067 78,039 Total current liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 715,296 592,865 Total liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,733,832 3,746,733 Total equity and liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,841,036 5,627,474 The accompanying notes form an integral part of the consolidated annual accounts F-143 |
|
|
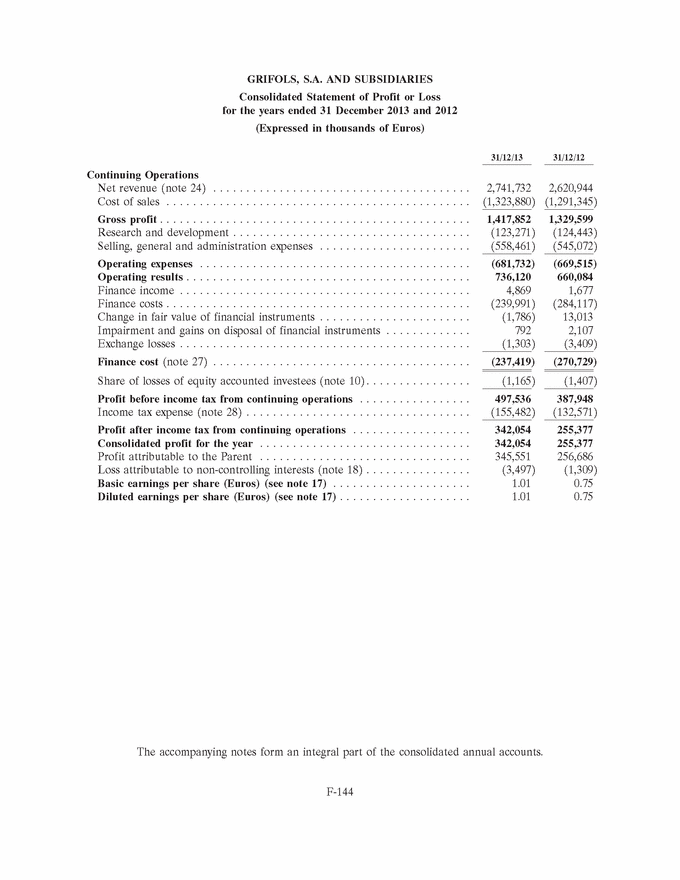
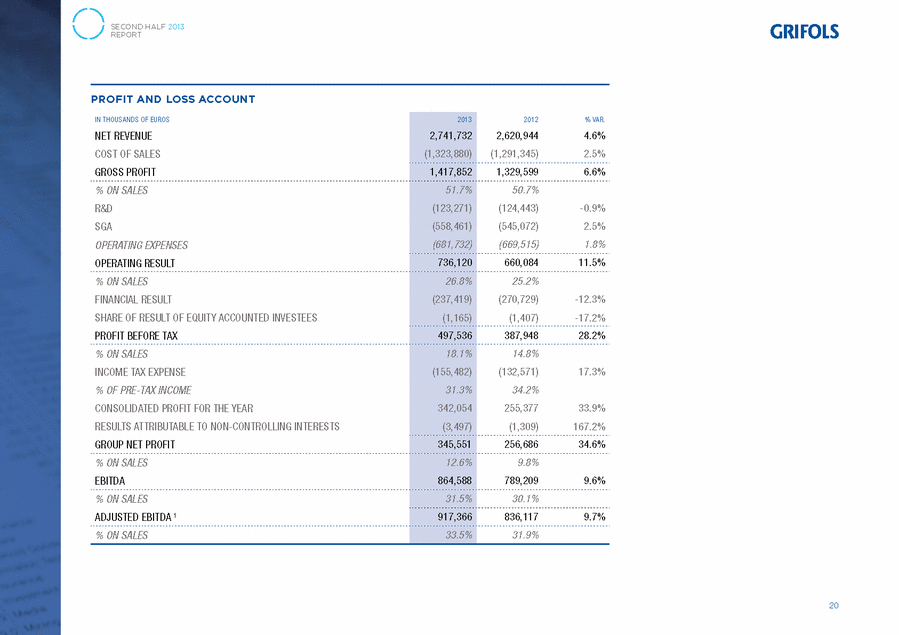
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Statement of Profit or Loss for the years ended 31 December 2013 and 2012 (Expressed in thousands of Euros) 31/12/13 31/12/12 Continuing Operations Net revenue (note 24) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,741,732 2,620,944 Cost of sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,323,880) (1,291,345) Gross profit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,417,852 1,329,599 Research and development . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (123,271) (124,443) Selling, general and administration expenses . . . . . . . . . . . . . . . . . . . . . . . (558,461) (545,072) Operating expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (681,732) (669,515) Operating results . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 736,120 660,084 Finance income . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,869 1,677 Finance costs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (239,991) (284,117) Change in fair value of financial instruments . . . . . . . . . . . . . . . . . . . . . . . (1,786) 13,013 Impairment and gains on disposal of financial instruments . . . . . . . . . . . . . 792 2,107 Exchange losses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,303) (3,409) Finance cost (note 27) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (237,419) (270,729) Share of losses of equity accounted investees (note 10) . . . . . . . . . . . . . . . . (1,165) (1,407) Profit before income tax from continuing operations . . . . . . . . . . . . . . . . . 497,536 387,948 Income tax expense (note 28) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (155,482) (132,571) Profit after income tax from continuing operations . . . . . . . . . . . . . . . . . . 342,054 255,377 Consolidated profit for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 342,054 255,377 Profit attributable to the Parent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 345,551 256,686 Loss attributable to non-controlling interests (note 18) . . . . . . . . . . . . . . . . (3,497) (1,309) Basic earnings per share (Euros) (see note 17) . . . . . . . . . . . . . . . . . . . . . 1.01 0.75 Diluted earnings per share (Euros) (see note 17) . . . . . . . . . . . . . . . . . . . . 1.01 0.75 The accompanying notes form an integral part of the consolidated annual accounts. F-144 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Statements of Comprehensive Income for the years ended 31 December 2013 and 2012 (Expressed in thousands of Euros) 31/12/13 31/12/12 Consolidated profit for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 342,054 255,377 Other comprehensive expenses Items for reclassification to profit or loss Translation differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (91,610) (31,016) Equity-accounted investees (note 10) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (359) 0 Cash flow hedges—effective part of changes in fair value . . . . . . . . . . . . . . . . . . . 22,943 (25,140) Cash flow hedges—amounts taken to profit and loss . . . . . . . . . . . . . . . . . . . . . . . (11,471) 6,300 Tax effect . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (4,227) 6,988 Other comprehensive expenses for the year, after tax . . . . . . . . . . . . . . . . . . . . . . (84,724) (42,868) Total comprehensive income for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 257,330 212,509 Total comprehensive income attributable to the Parent . . . . . . . . . . . . . . . . . . . . . 261,509 213,831 Total comprehensive expense attributable to the non-controlling interests . . . . . . . . (4,179) (1,322) The accompanying notes form an integral part of the consolidated annual accounts. F-145 |
|
|
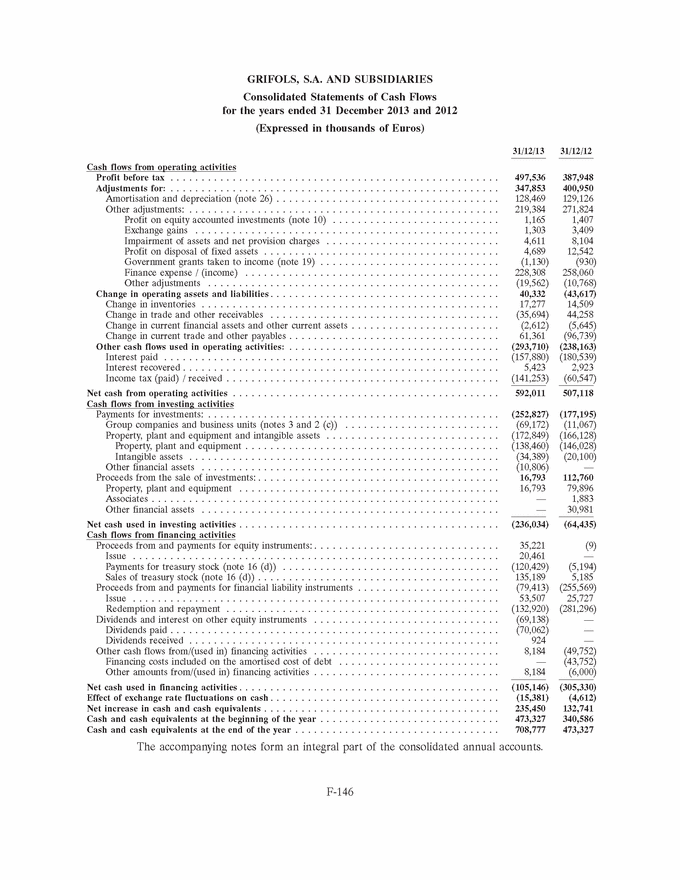
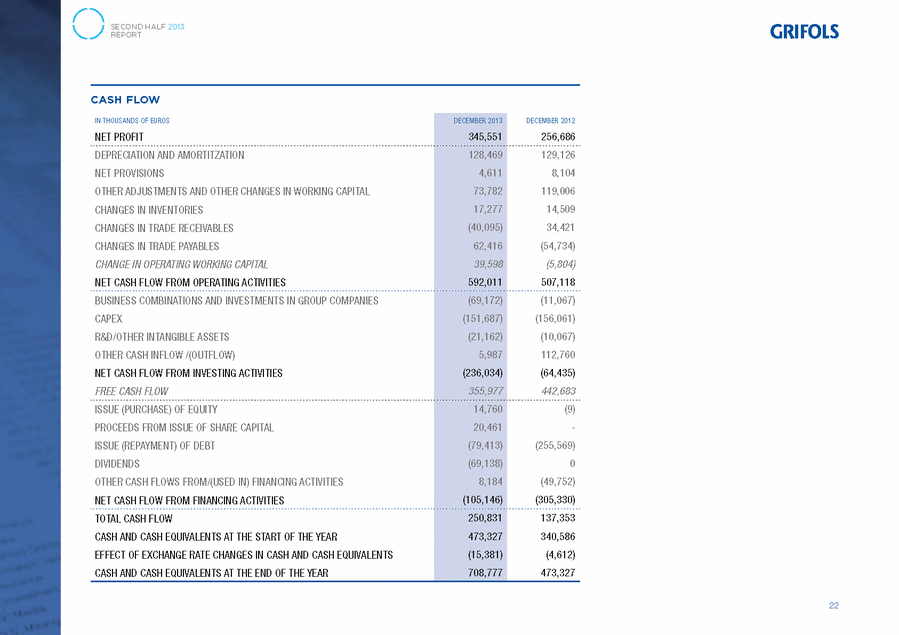
GRIFOLS, S.A. AND SUBSIDIARIES Consolidated Statements of Cash Flows for the years ended 31 December 2013 and 2012 (Expressed in thousands of Euros) 31/12/13 31/12/12 Cash flows from operating activities Profit before tax . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 497,536 387,948 Adjustments for: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 347,853 400,950 Amortisation and depreciation (note 26) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 128,469 129,126 Other adjustments: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 219,384 271,824 Profit on equity accounted investments (note 10) . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,165 1,407 Exchange gains . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,303 3,409 Impairment of assets and net provision charges . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,611 8,104 Profit on disposal of fixed assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,689 12,542 Government grants taken to income (note 19) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,130) (930) Finance expense / (income) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 228,308 258,060 Other adjustments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (19,562) (10,768) Change in operating assets and liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40,332 (43,617) Change in inventories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17,277 14,509 Change in trade and other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (35,694) 44,258 Change in current financial assets and other current assets . . . . . . . . . . . . . . . . . . . . . . . . (2,612) (5,645) Change in current trade and other payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61,361 (96,739) Other cash flows used in operating activities: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (293,710) (238,163) Interest paid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (157,880) (180,539) Interest recovered . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,423 2,923 Income tax (paid) / received . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (141,253) (60,547) Net cash from operating activities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 592,011 507,118 Cash flows from investing activities Payments for investments: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (252,827) (177,195) Group companies and business units (notes 3 and 2 (c)) . . . . . . . . . . . . . . . . . . . . . . . . . (69,172) (11,067) Property, plant and equipment and intangible assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . (172,849) (166,128) Property, plant and equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (138,460) (146,028) Intangible assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (34,389) (20,100) Other financial assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (10,806) — Proceeds from the sale of investments: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16,793 112,760 Property, plant and equipment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16,793 79,896 Associates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . — 1,883 Other financial assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . — 30,981 Net cash used in investing activities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (236,034) (64,435) Cash flows from financing activities Proceeds from and payments for equity instruments: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35,221 (9) Issue . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 20,461 — Payments for treasury stock (note 16 (d)) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (120,429) (5,194) Sales of treasury stock (note 16 (d)) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 135,189 5,185 Proceeds from and payments for financial liability instruments . . . . . . . . . . . . . . . . . . . . . . . (79,413) (255,569) Issue . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53,507 25,727 Redemption and repayment . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (132,920) (281,296) Dividends and interest on other equity instruments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (69,138) — Dividends paid . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (70,062) — Dividends received . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 924 — Other cash flows from/(used in) financing activities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,184 (49,752) Financing costs included on the amortised cost of debt . . . . . . . . . . . . . . . . . . . . . . . . . . — (43,752) Other amounts from/(used in) financing activities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,184 (6,000) Net cash used in financing activities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (105,146) (305,330) Effect of exchange rate fluctuations on cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (15,381) (4,612) Net increase in cash and cash equivalents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 235,450 132,741 Cash and cash equivalents at the beginning of the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 473,327 340,586 Cash and cash equivalents at the end of the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 708,777 473,327 The accompanying notes form an integral part of the consolidated annual accounts. F-146 |
|
|
LES]UNIVERSAL.BST;124 F-147 for the years ended 31 December 2013 and 2012 (Expressed in thousands of Euros) Attributable to equity holders of the Parent Other comprehensive income Profit Equity Non- Share Share attributable Interim Treasury Translation Cash flow attributable controlling Capital Premium Reserves to Parent dividend stock differences hedges to Parent interests Equity Balance at 31 December 2011 . . . . . . . . . . . . . . . . . . . 117,882 890,355 568,274 50,307 — (1,927) 58,800 (21,184) 1,662,507 2,487 1,664,994 Translation differences . . . . . . . . . . . . . . . . . . . . . — — — — — — (31,003) — (31,003) (13) (31,016) Cash flow hedges . . . . . . . . . . . . . . . . . . . . . . . . . — — — — — — — (11,852) (11,852) — (11,852) Other comprehensive expense for the year . . . . . . . . . — — — — — — (31,003) (11,852) (42,855) (13) (42,868) Profit/(loss) for the year . . . . . . . . . . . . . . . . . . . . . — — — 256,686 — — — — 256,686 (1,309) 255,377 Total comprehensive income/(expense) for the year . . . . — — — 256,686 — — (31,003) (11,852) 213,831 (1,322) 212,509 Other changes . . . . . . . . . . . . . . . . . . . . . . . . . . — — 1,563 — — (1,133) — — 430 (59) 371 Acquisition of non-controlling interest (note 3 (b)) . . . . — — — — — — — — — 2,867 2,867 Distribution of 2011 profit Reserves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . — — 50,307 (50,307) — — — — — — — Operations with equity holders or owners . . . . . . . . . . — — 51,870 (50,307) — (1,133) — — 430 2,808 3,238 Balance at 31 December 2012 . . . . . . . . . . . . . . . . . . . 117,882 890,355 620,144 256,686 — (3,060) 27,797 (33,036) 1,876,768 3,973 1,880,741 Translation differences . . . . . . . . . . . . . . . . . . . . . (91,287) (91,287) (682) (91,969) Cash flow hedges (note 16 (f)) . . . . . . . . . . . . . . . . . — — — — — — — 7,245 7,245 — 7,245 Other comprehensive income/(expense) for the year . . . . — — — — — — (91,287) 7,245 (84,042) (682) (84,724) Profit/(loss) for the year . . . . . . . . . . . . . . . . . . . . . — — — 345,551 — — — — 345,551 (3,497) 342,054 Total comprehensive income/(expense) for the year . . . . — — — 345,551 — — (91,287) 7,245 261,509 (4,179) 257,330 Net change in treasury stock (note 16 (d)) . . . . . . . . . — — 11,806 — — 3,060 — — 14,866 — 14,866 Capital increase January 2013 (note 16 (a)) . . . . . . . . . 1,633 — (1,665) — — — — — (32) — (32) Capital increase April 2013 (note 16 (a)) . . . . . . . . . . 89 20,373 (375) — — — — — 20,087 — 20,087 Acquisition of non-controlling interests (note 16 (c)) . . . — — (2,800) — — — — — (2,800) 2,895 95 Acquisition of non-controlling interests in investees (note 3 (a)) . . . . . . . . . . . . . . . . . . . . . . . . . . . — — — — — — — — — 1,712 1,712 Other changes . . . . . . . . . . . . . . . . . . . . . . . . . . — — 2 — — — — — 2 1,541 1,543 Interim dividend . . . . . . . . . . . . . . . . . . . . . . . . . — — 924 — (68,755) — — — (67,831) — (67,831) — Distribution of 2012 profit . . . . . . . . . . . . . . . . . . . . . — Reserves . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . — — 255,379 (255,379) — — — — — — — Dividends (Class B shares) . . . . . . . . . . . . . . . . . . . — — (1,307) — — — — (1,307) — (1,307) Operations with equity holders or owners . . . . . . . . . . 1,722 20,373 263,271 (256,686) (68,755) 3,060 — — (37,015) 6,148 (30,867) Balance at 31 December 2013 . . . . . . . . . . . . . . . . . . . 119,604 910,728 883,415 345,551 (68,755) — (63,490) (25,791) 2,101,262 5,942 2,107,204 The accompanying notes form an integral part of the consolidated annual accounts. 3 C Cs: 24045 |
|
|
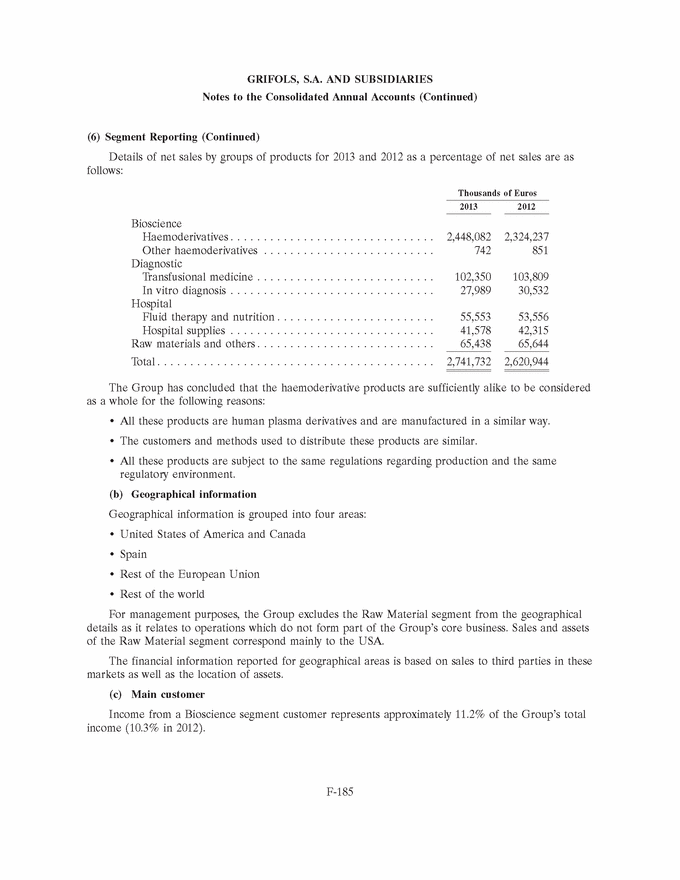
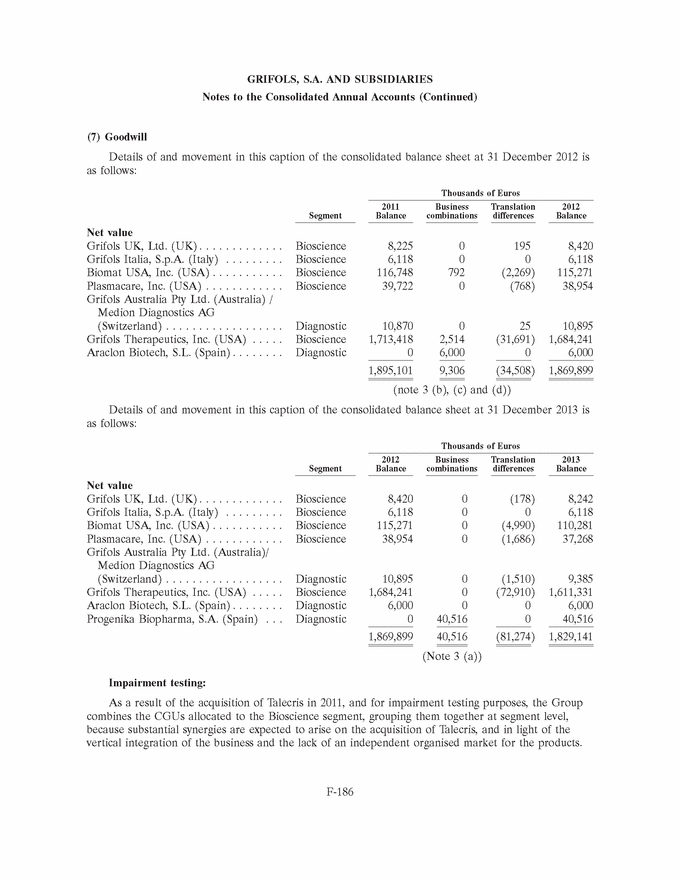
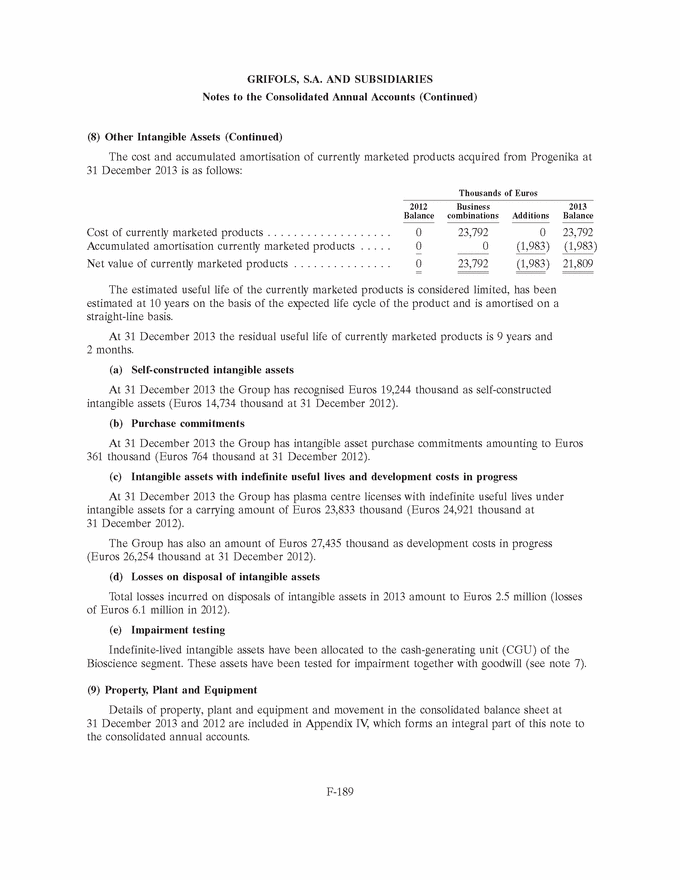
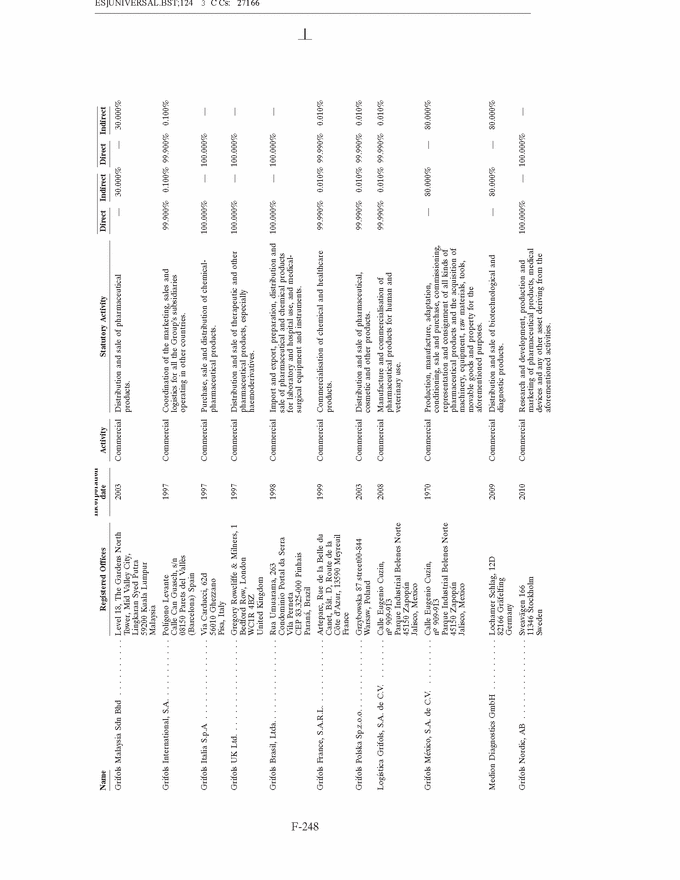
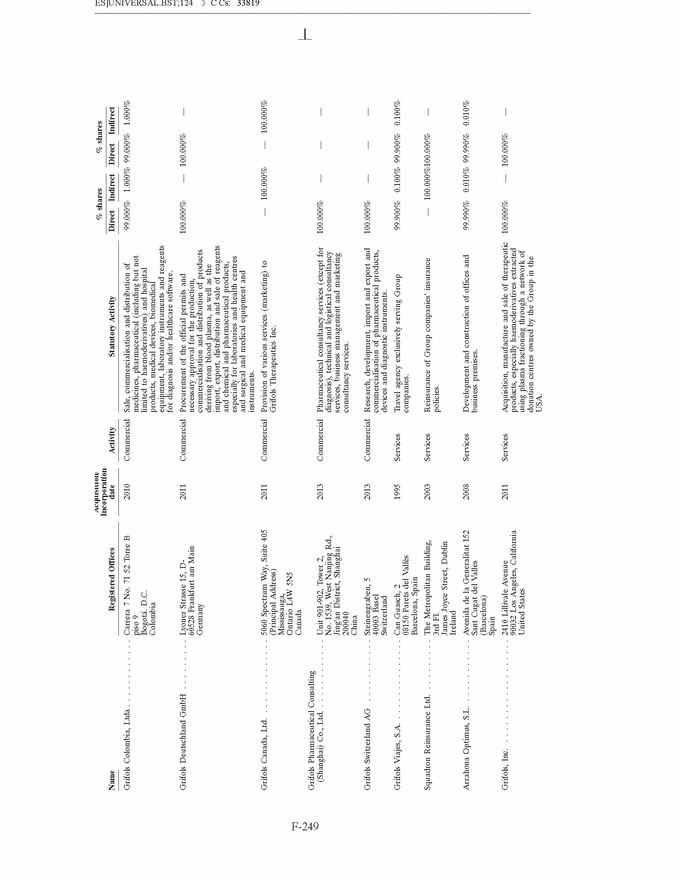
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (1) Nature, Principal Activities and Subsidiaries Grifols, S.A. (hereinafter the Company) was incorporated with limited liability under Spanish law on 22 June 1987. Its registered and tax offices are in Barcelona. The Company’s statutory activity consists of providing corporate and business administrative, management and control services, as well as investing in assets and property. Its principal activity involves rendering administrative, management and control services to its subsidiaries. On 17 May 2006 the Company completed its flotation on the Spanish securities market, which was conducted through the public offering of 71,000,000 ordinary shares of Euros 0.50 par value each and a share premium of Euros 3.90 per share. The total capital increase (including the share premium) amounted to Euros 312.4 million, equivalent to a price of Euros 4.40 per share. The Company’s shares were floated on the Spanish stock exchange IBEX-35 index on 2 January 2008. All of the Company’s shares are listed on the Barcelona, Madrid, Valencia and Bilbao securities markets and on the Spanish Automated Quotation System (SIBE/Continuous Market). On 2 June 2011, Class B non-voting shares were listed on the NASDAQ (USA) and on the Spanish Automated Quotation System (SIBE/Continuous Market) (see note 16). In November 2011 the Company registered its High-Yield Senior Unsecured Corporate Bonds at the Securities Exchange Commission (SEC) (see note 21). Grifols, S.A. is the Parent of the subsidiaries listed in Appendix I of this note to the consolidated annual accounts. Grifols, S.A. and subsidiaries (hereinafter the Group) act on an integrated basis and under common management and their principal activity is the procurement, manufacture, preparation and sale of therapeutic products, especially haemoderivatives. The main factory locations of the Group’s Spanish companies are in Barcelona, Parets del Vall´es (Barcelona) and Torres de Cotilla (Murcia), while the US companies are located in Los Angeles, (California, USA) and Clayton (North Carolina, USA). (2) Basis of Presentation The consolidated annual accounts have been prepared on the basis of the accounting records of Grifols, S.A. and of the Group companies. The consolidated annual accounts for 2013 have been prepared under International Financial Reporting Standards as adopted by the European Union (IFRS-EU) and other legislative provisions contained in the applicable legislation governing financial information to present fairly the consolidated equity and consolidated financial position of Grifols, S.A. and subsidiaries at 31 December 2013, as well as the consolidated results from their operations, consolidated cash flows and consolidated changes in equity for the year then ended. The Group adopted IFRS-EU for the first time on 1 January 2004 and on the same date applied IFRS 1 ‘‘First-time adoption of International Financial Reporting Standards’’. The directors of the Company consider that the consolidated annual accounts for 2013 authorised for issue on 21 February 2014 will be approved with no changes. F-148 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (2) Basis of Presentation (Continued) (a) Comparative information These consolidated annual accounts have been prepared following IFRS-EU consistently and present for comparative purposes for each individual caption in the consolidated balance sheet, consolidated statement of profit or loss, consolidated statement of comprehensive income, consolidated statement of cash flows, consolidated statement of changes in equity and consolidated notes, comparative figures for the previous year, which have been obtained through consistent application of IFRS-EU. As a result of the application of IAS 1 as amended, the Group has changed the presentation of the items included in the statement of comprehensive income to show separately those that will be transferred to the statement of profit or loss in the future from those which will not. Comparative information has been adapted in this regard. Furthermore, in accordance with the transitional provisions of IFRS 13, the comparative information for 2012 does not include the information required by this rule breakdown. (b) Relevant accounting estimates, assumptions and judgements used when applying accounting principles The preparation of the consolidated annual accounts in conformity with IFRS-EU requires management to make judgements, estimates and assumptions that affect the application of Group accounting policies. The following notes include a summary of the relevant accounting estimates and judgements used to apply accounting policies which have the most significant effect on the amounts recognised in the consolidated annual accounts. • The assumptions used for calculation of the fair value of financial instruments, in particular, financial derivatives. Financial derivatives are measured based on observable market data (level 2 of fair value hierarchy) (see notes 4(k) and 31). The High Yiel Senior Unsecured Notess and senior secured debt are valued at their quoted price in active markets (level 1 in the fair value hierarchy). Regarding the valuation of derivative instruments, the selection of the appropriate data within the alternatives requires the use of judgement in qualitative factors such as, which methodology and valuation models are used, and in quantitative factors, data required to be included within the chosen models. • The assumptions used to test non-current assets and goodwill for impairment. Relevant cash generating units are tested annually for impairment. These are based on risk-adjusted future cash flows discounted using appropriate interest rates. The key assumptions used are specified in note 7. Assumptions relating to risk-adjusted future cash flows and discount rates are based on business forecasts and are therefore inherently subjective. Future events could cause a change in business forecasts, with a consequent adverse effect on the future results of the Group. To the extent considered a reasonably possible change in key assumptions could result in an impairment of goodwill, a sensitivity analysis has been disclosed to show the effect of changes to these assumptions and the effect of the cash generating unit (CGU) on the recoverable amount. • Useful lives of property, plant and equipment and intangible assets. The estimated useful lives of each category of property, plant and equipment and intangible assets are set out in notes 4(g) and 4(h). Although estimates are calculated by the Company’s management based on the best information available at 31 December 2013, future events may require changes to these estimates in subsequent years. Given the large number of individual items of property, plant and F-149 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (2) Basis of Presentation (Continued) equipment it is not considered likely that a reasonably possible change in the assumptions would lead to a material adverse effect. Potential changes to the useful lives of intangible assets are mainly related to the currently marketed products and the useful lives will depend on the life cycle of the same. No significant changes to useful lives are expected. Adjustments made in subsequent years are recognised prospectively. • Evaluation of the effectiveness of hedging derivatives. The key assumption relates to the measurement of the effectiveness of the hedge. Hedge accounting is only applicable when the hedge is expected to be highly effective at the inception of the hedge and, in subsequent years, in achieving offsetting changes in fair value or cash flows attributable to the hedged risk, throughout the period for which the hedge was designated (prospective analysis) and the actual effectiveness, which can be reliably measured, is within a range of 80%-125% (retrospective analysis) (see notes 4(l), 16(f) and 31). • Evaluation of the nature of leases (operating or finance). The Group analyses the conditions of the lease contracts at their inception in order to conclude if the risks and rewards have been transferred (see note 4(j) and 9(c)). If the lease contract is renewed or amended the Group conducts a new evaluation. • Assumptions used to determine the fair value of assets, liabilities and contingent liabilities related to business combinations. Details of the fair value methods used by the Group are provided in note 3. • Evaluation of the capitalisation of development costs (see note 4(h)). The key assumption is related to the estimation of sufficient future economic benefits of the projects. • Evaluation of provisions and contingencies. Key assumptions relate to the evaluation of the likelihood of an outflow of resources due to a past event, as well as to the evaluation of the best estimate of the likely outcome. These estimates take into account the specific circumstances of each dispute and relevant external advice and therefore are inherently subjective and could change substantially over time as new facts arise and each dispute progresses. Details of the status of various uncertainties involved in significant unresolved disputes are set out in note 30. • Evaluation of the recoverability of receivables from public entities in countries facing liquidity problems, specifically in Italy, Portugal and Spain. The key assumption is the estimation of the amounts expected to be collected from these public entities (see notes 5 and 31). • Evaluation of the recoverability of tax credits, including tax loss carryforwards and rights for deductions. Deferred tax assets are recognised to the extent that future taxable profits will be available against which the temporary differences can be utilised, based on management’s assumptions relating to the amount and timing of future taxable profits. Capitalisation of deferred tax assets relating to investments in Group companies depends on whether they will reverse in the foreseeable future (see notes 4(t) and 28). No changes have been made to prior year judgements relating to existing uncertainties. The Group is also exposed to interest rate and currency risks. Refer to sensitivity analysis in note 31. F-150 |
|
|
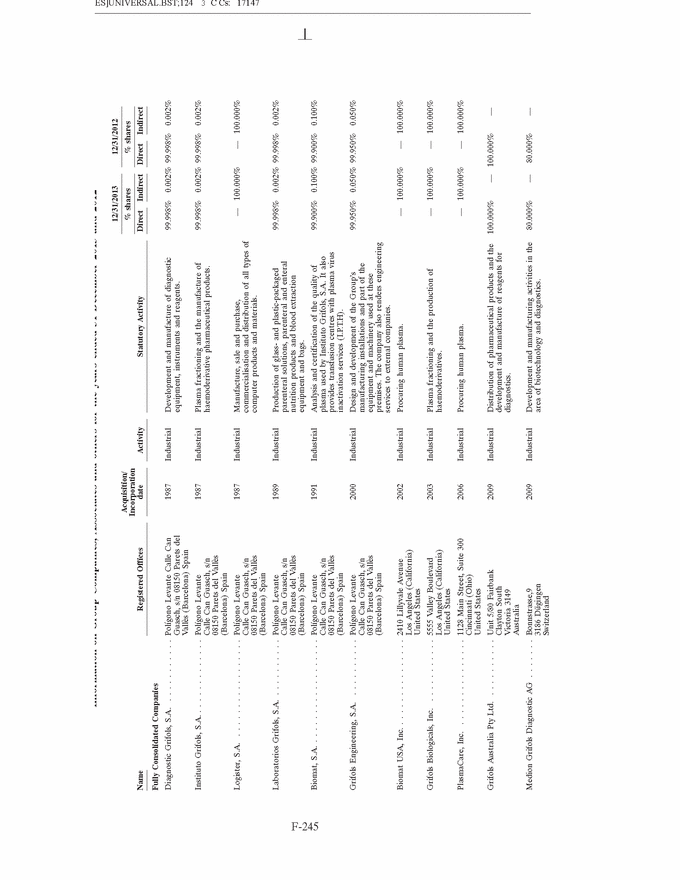
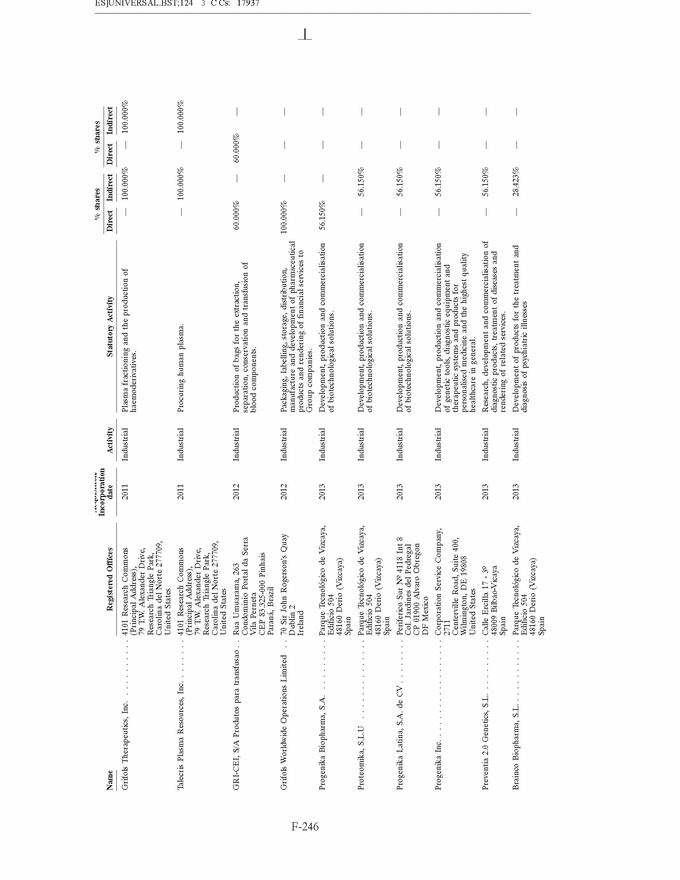
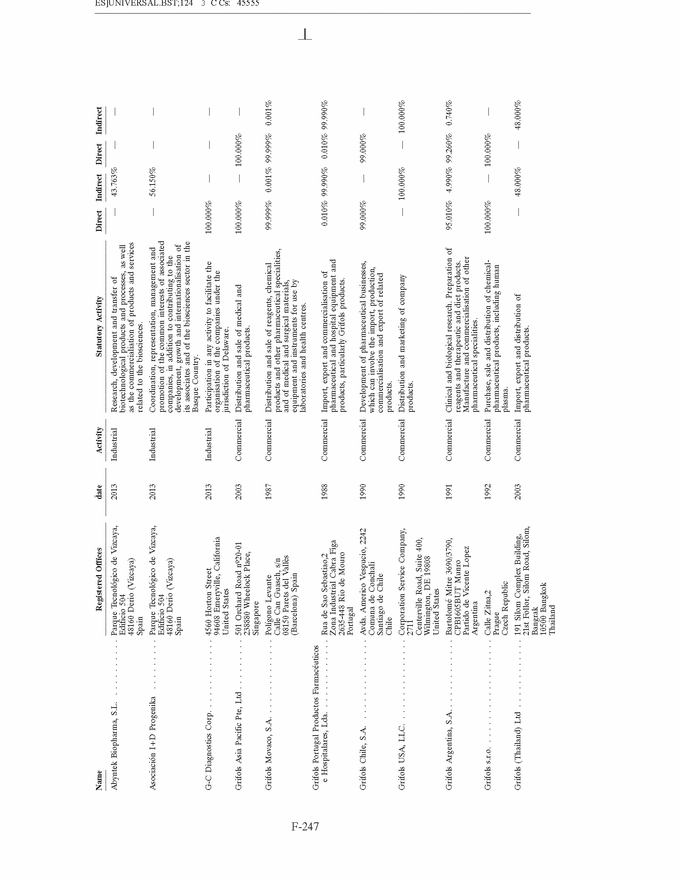
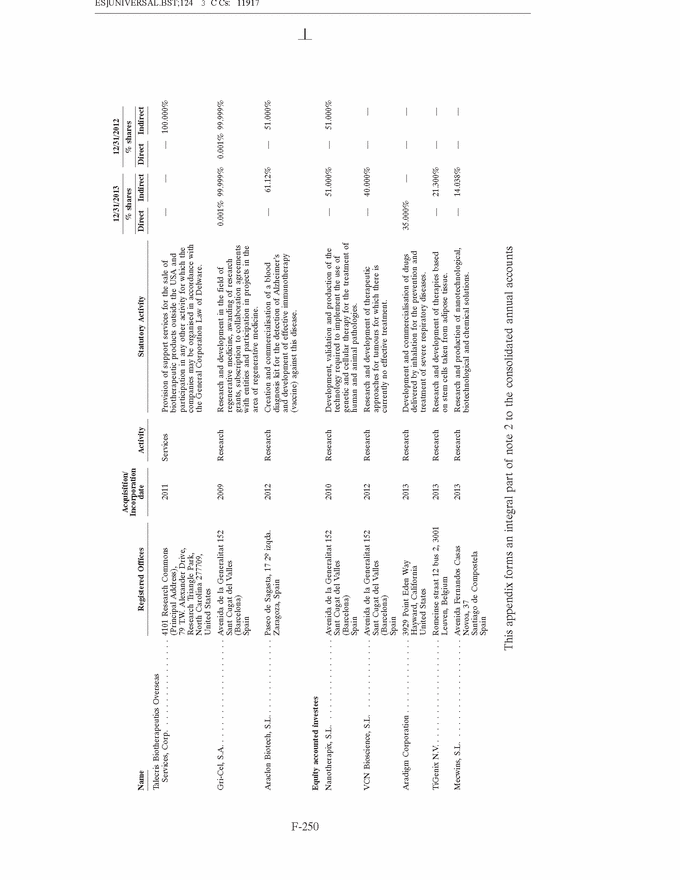
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (2) Basis of Presentation (Continued) Grifols management does not consider that there are any assumptions or causes for uncertainty in the estimates which could imply a significant risk of material adjustments arising in the next financial year. (c) Basis of consolidation Appendix I shows details of the percentages of direct or indirect ownership of subsidiaries by the Company at 31 December 2013 and 2012, as well as the consolidation method used in each case for preparation of the accompanying consolidated annual accounts. Subsidiaries in which the Company directly or indirectly owns the majority of equity or voting rights have been fully consolidated. Associates in which the Company owns between 20% and 50% of share capital and over which it has no control but does have significant influence, have been accounted for under the equity method. Although the Group holds 30% of the shares with voting rights of Grifols Malaysia Sdn Bhd, it controls the majority of the economic and voting rights of Grifols Malaysia Sdn Bhd through a contract with the other shareholder and a pledge on its shares. Grifols (Thailand) Ltd. has two classes of shares and it grants the majority of voting rights to the class of shares held by the Group. On 9 March 2010 one of the Group companies acquired 51% of Nanotherapix, S.L., a technologically based company which engages in advisory services, training of researchers, design and development of technologies, services, know-how, molecules and products applied to biotechnology, biomedicine and pharmaceutical fields. The acquisition of Nanotherapix, S.L. has been treated as an equity-accounted joint venture, as the company’s strategic and operational decisions require shareholder approval and Grifols does not avail of the majority of the members of the board of directors. Changes in subsidiaries In 2013 Grifols incorporated the following companies: • G-C Diagnostics Corp. (USA) • Grifols Switzerland AG (Switzerland) • Grifols Pharmaceutical Consulting (Shanghai) Co. Ltd (China) • Grifols Worldwide Operations, Ltd (Ireland) On 27 February 2013 the Group acquired shares representing 60% of the economic and voting rights (56.1% after Ekarpen capital increase) of the Spanish biotechnology group of companies headed by Progenika Biopharma, S.A. (hereinafter Progenika) for an amount of Euros 37,010 thousand (see note 3(a)). During the second half of 2013 Talecris Biotherapeutics Overseas Services, Corp. was wound up. The assets and liabilities of these companies have been integrated into Grifols Therapeutics, Inc. On 29 February 2012 and in relation to the strategic R&D priorities of the Group, Grifols acquired 51% of the capital of Araclon Biotech, S.L. for a total of Euros 8,259 thousand (see note 3 (b)). As explained in note 16 (c), in May 2013 Araclon Biotech, S.L. carried out a share capital increase of Euros 7 million, Euros 6.9 million of which were subscribed by the Group. F-151 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (2) Basis of Presentation (Continued) During the first half of 2012, Grifols incorporated a new company, under the name Gri-Cei, S/A Produtos para transfus˜ao with the Brazilian company CEI Comercio Exporta¸c˜ao e Importa¸c˜ao de Materiais M´edicos, Ltda in which Grifols owns 60% of shares and has the control of the company. Gri-Cei was established in order to manufacture bags for extraction, separation, conservation and transfusion of blood components in Brazil. During 2013 Grifols, S.A. carried out a share capital increase of Euros 2,320 thousand. During the third quarter of 2012 all of the Australian companies have been wound up, with the exception of Grifols Australia Pty Ltd. The assets and liabilities of these companies have been integrated into Grifols Australia Pty. Ltd. Changes in associates On 19 November 2013, the Group company Gri-Cel, S.A., which is the affiliate that centralises the Company’s investments in R&D companies and projects in fields of medicine other than its core business, acquired 21.3% of TiGenix N.V. for a total of Euros 12,443 thousand. This investment has been accounted for using the equity method. On 20 May 2013 the Group announced the signing of a worldwide exclusive licensing agreement with Aradigm Corporation to develop and commercialise Pulmaquin and Lipoquin, on the condition that Grifols, S.A. would increase capital. On 27 August 2013 the Group acquired a 35% interest in Aradigm Corporation for a total of US Dollars 26 million (Euros 20.6 million) and, therefore, the exclusive worldwide licensing agreement to develop and commercialise Pulmaquin and Lipoquin became effective (see note 10). All shares have the same voting and economic rights. On 6 July 2012, the Group company Gri-Cel, S.A. acquired 40% of the capital of VCN Bioscience, S.L. for a total of Euros 1,500 thousand. This investment has been accounted for using the equity method. VCN Bioscience, S.L. is specialised in the research and development of new therapeutic approaches for tumours based on the use of oncologic viruses. Grifols has committed under certain conditions to finance VCN Bioscience, S.L.’s on-going projects for a minimum amount of Euros 5 million and it can result in Grifols increasing its share in the capital of VCN Bioscience, S.L. (d) Amendments to IFRS-EU in 2013 The following standards came into effect in 2013 and have therefore been taken into account when drawing up the consolidated annual accounts: • Amendments to IFRS 1:—Government Loans. Effective date 1 January 2013. • Amendments to IAS 1 Presentation of Components of Other Comprehensive Income. Effective for annual periods beginning on or after 1 July 2012. • IAS 19 Employee Benefits Effective for annual periods beginning on or after 1 January 2013. • IAS 27 Separate Financial Statements. Effective for annual periods beginning on or after 1 January 2014 (earlier application is permitted). • IAS 28 Investments in Associates and Joint Ventures. Effective for annual periods beginning on or after 1 January 2014 (earlier application is permitted). F-152 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (2) Basis of Presentation (Continued) • Amendments to IFRS 7: Offsetting Financial Assets and Financial Liabilities: Disclosure: Effective date 1 January 2013. • IFRS 10 Consolidated Financial Statements. Effective for annual periods beginning on or after 1 January 2014 (earlier application is permitted). • IFRS 11 Joint Arrangements. Effective for annual periods beginning on or after 1 January 2014 (earlier application is permitted). • IFRS 12 Disclosures of Interests in Other Entities. Effective for annual periods beginning on or after 1 January 2014 (earlier application is permitted). • Consolidated financial statements, joint arrangements and disclosure of interests in other entities: Transition guidance (issued on 28 June 2012). Improvements to IFRSs 10, 11 and 12. Effective on 1 January 2014 (earlier application is permitted). • IFRS 13 Fair Value Measurement. Effective for annual periods beginning on or after 1 January 2013. • Improvements to IFRSs (2009-2011) issued on 17 May 2012 (effective on 1 January 2013). The application of these standards and interpretations has had no material impact on these consolidated annual accounts. In regards to IAS 1 as amended, which requires the separate presentation of other income and expense recognized directly in equity that can be transferred to the statement of profit or loss in the future from those which can not be transferred, the consolidated statement of comprehensive income has been adapted for the year 2013 as well as w for the comparative figures. The European Union has adopted the following standards, which are obligatory for annual periods beginning on or after 1 January 2014: • IAS 32 Financial Instruments: Presentation: Amendments to Offsetting Financial Assets and Financial Liabilities. Effective for annual periods beginning on or after 1 January 2014. • Amendments to IAS 36: Disclosure of impairment of assets (effective 1 January 2014) • Amendment to IAS 39: Novation of derivatives and continuation of hedge accounting (effective 1 January 2014). • Investment Entities. Amendments to IFRS 10, IFRS 12 and IAS 27, Investment companies. Effective on 1 January 2014. The standards issued by the IASB and pending adoption by the European Union and which are mandatory for annual periods beginning on or after 1 January 2014 are as follows: • IFRIC 21 Levies. Effective for annual periods beginning on or after 1 January 2014. • IAS 19 Employee Benefits. Defined benefit pension plans. Effective for annual periods beginning on or after 1 January 2014. • Improvements to IFRS (2010-2012). Effective for annual periods beginning on or after 1 July 2014. F-153 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (2) Basis of Presentation (Continued) • Improvements to IFRS (2011-2013). Effective for annual periods beginning on or after 1 July 2014. At the date of issue of these consolidated annual accounts it is not expected that the standards or interpretations published by the International Accounting Standards Board (IASB), pending adoption by the European Union, will have a significant effect on the Group’s consolidated annual accounts. (3) Business Combinations 2013 (a) Progenika Biopharma On 27 February 2013 the Group acquired shares representing 60% of the economic and voting rights (56.1% after Ekarpen capital increase mentioned below) of the Spanish biotechnology group of companies headed by Progenika Biopharma, S.A. (hereinafter Progenika) for an amount of Euros 37,010 thousand. The acquisition was paid through the following: • 50% of the purchase price has been paid in exchange for 884,997 Class B non-voting Grifols shares, with a fair value of Euros 20.91 per share. The Group granted to the selling shareholders the option to resell the Class B shares at the same price during the first five days following the acquisition date. Selling shareholders representing 879,913 shares executed this option, and the cash paid amounted to Euros 18,399 thousand, being considered as cash for investment activities in the statement of cash flows. • The remaining 50% of the price has been paid in cash (Euros 18,505 thousand). The non-voting Grifols Class B shares have been provided by a related party under a loan agreement signed on 12 February 2013 (see note 32). On 16 April 2013, the Company´s share capital has been increased in the nominal amount of Euros 88,499.70 by issuing and placing in circulation 884,997 new Class B shares without voting rights. The share capital increase has enabled Grifols to issue the number of shares needed to pay the price for the acquisition of Progenika in shares and thus return the Lender the non-voting shares that were lent pursuant to the provisions of the Loan Agreement (see note 16). Additionally, the Group and the selling shareholders have granted each other call and put option rights over the shares representing 35% (32.9% after Ekarpen capital increase mentioned below) of the remaining share capital held by the aforementioned sellers, with may be exercised in three years. The purchase price of the shares subject to the put and call option amounts to Euros 21,701 thousand, increased at the rate of 5% per annum and has been treated as a financial liability (see note 21 (e)). The conditions of the payment of these shares will be the same as the initial acquisition. Grifols, Progenika and the investment vehicle EKARPEN SPE, S.A. (hereinafter ‘‘Ekarpen’’), owned by the Basque Government, Kutxabank, Caja Laboral—Euskadiko Kutxa, Lagun Aro and the Provincial Governments of the Basque Country, have agreed that Ekarpen subscribes a share capital increase pursuant to which, for an amount of Euros 5,000 thousand, Ekarpen has received new shares representing approximately 6.5% of the share capital of Progenika. These shares are subject to a call and put option which may be exercised at the end of a 5-year period for a purchase price of Euros 5,000 thousand and has been treated as financial liability (see note 21 (e)). The call option has premium costs of Euros 300 thousand for each of the 5-year period. F-154 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (3) Business Combinations (Continued) As the non-controlling shareholders do not have present access to the economic benefits associated with the underlying ownership interests related to shares under the put and call options, the Group has applied the anticipated-acquisition method. Under this method, Grifols recognises the contract as an anticipated acquisition of the underlying non-controlling interest, as if the put option had already been exercised by the non-controlling shareholders. Progenika specialises in the development of technology for personalised medicine, focusing on the design and manufacture of in-vitro genome and proteome-based diagnostic tests, disease prognosis and prediction and monitoring of responses to pharmacological treatment. It has also developed its own technology for the production of DNA chips for diagnosis and prognosis and it is an international leader in this field. In particular, Progenika has pioneered the development of molecular biology tests for the performance of transfusional compatibility studies. Details of the aggregate business combination cost, the fair value of the net assets acquired and goodwill at the acquisition date (or the amount by which the business combination cost exceeds the fair value of the net assets acquired) are provided below: Thousands of Euros Payment in cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18,505 Payment in Class B shares . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18,505 Deferred acquisition costs (put and call option) . . . . . . . . . . . . . . 26,701 Total cost of the business combination . . . . . . . . . . . . . . . . . . . . . 63,711 Fair value of net assets acquired . . . . . . . . . . . . . . . . . . . . . . . . . 23,195 Goodwill (note 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40,516 Payment in cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36,904 Cash and cash equivalents of the acquired company . . . . . . . . . . . (2,283) Net cash outflow for the acquisition . . . . . . . . . . . . . . . . . . . . . . 34,621 Had the acquisition taken place at 1 January 2013, the Group’s revenue and consolidated profit for the year ended 31 December 2013 would not have varied significantly. F-155 |
|
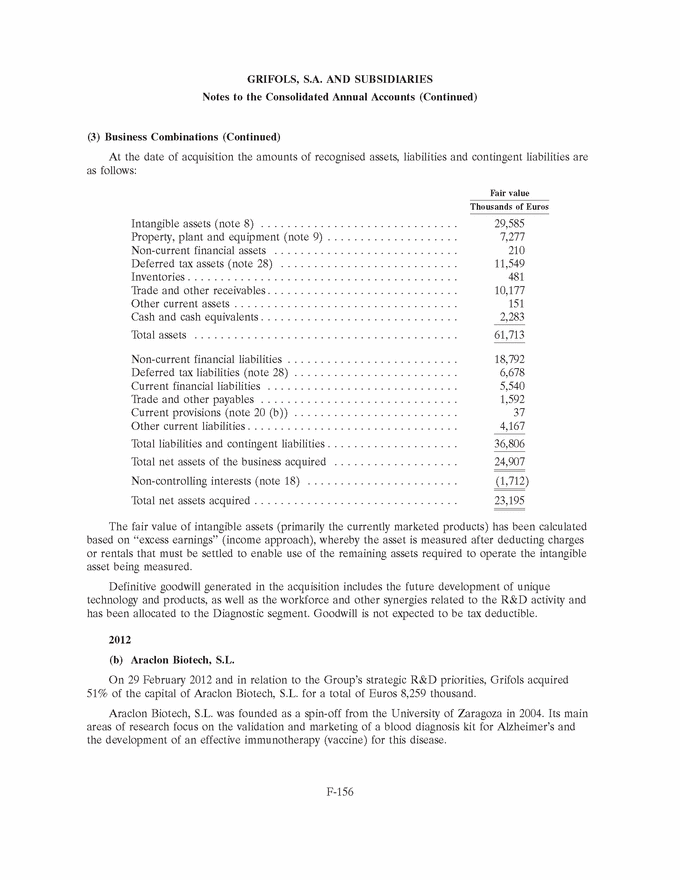
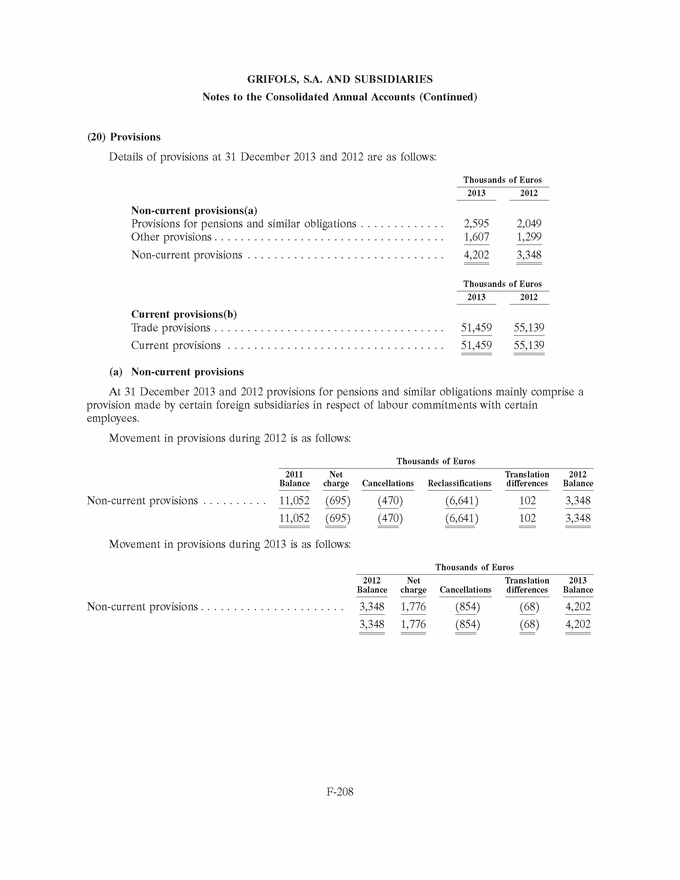
|
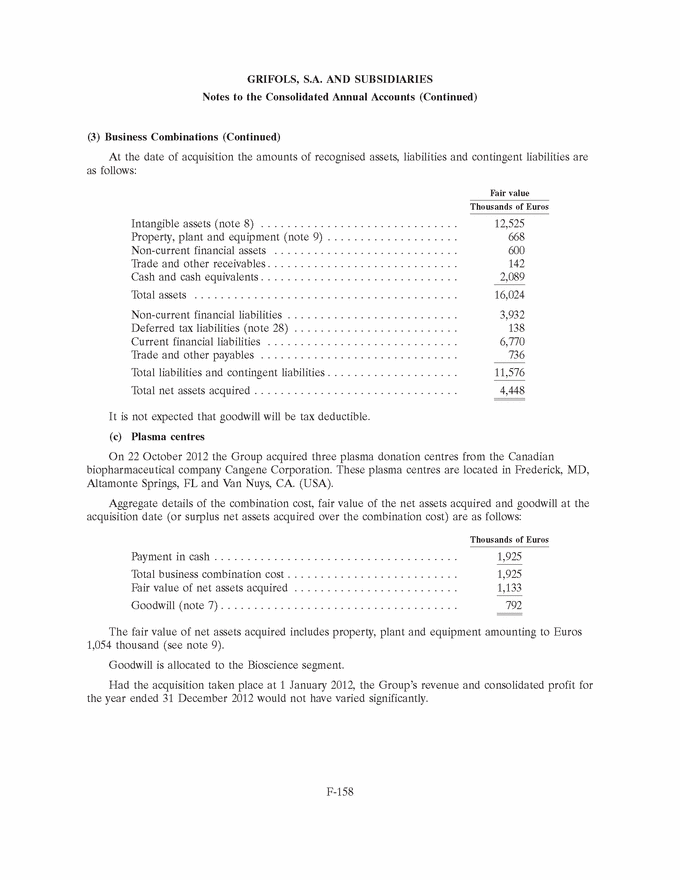
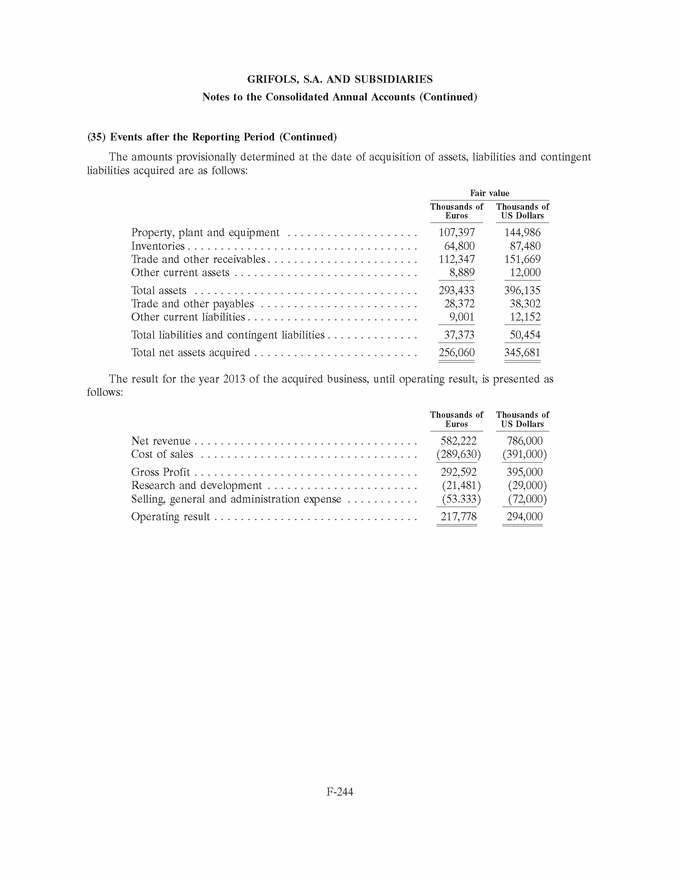
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (3) Business Combinations (Continued) At the date of acquisition the amounts of recognised assets, liabilities and contingent liabilities are as follows: Fair value Thousands of Euros Intangible assets (note 8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29,585 Property, plant and equipment (note 9) . . . . . . . . . . . . . . . . . . . . 7,277 Non-current financial assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . 210 Deferred tax assets (note 28) . . . . . . . . . . . . . . . . . . . . . . . . . . . 11,549 Inventories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 481 Trade and other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10,177 Other current assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 151 Cash and cash equivalents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,283 Total assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 61,713 Non-current financial liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . 18,792 Deferred tax liabilities (note 28) . . . . . . . . . . . . . . . . . . . . . . . . . 6,678 Current financial liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,540 Trade and other payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,592 Current provisions (note 20 (b)) . . . . . . . . . . . . . . . . . . . . . . . . . 37 Other current liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,167 Total liabilities and contingent liabilities . . . . . . . . . . . . . . . . . . . . 36,806 Total net assets of the business acquired . . . . . . . . . . . . . . . . . . . 24,907 Non-controlling interests (note 18) . . . . . . . . . . . . . . . . . . . . . . . (1,712) Total net assets acquired . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23,195 The fair value of intangible assets (primarily the currently marketed products) has been calculated based on ‘‘excess earnings’’ (income approach), whereby the asset is measured after deducting charges or rentals that must be settled to enable use of the remaining assets required to operate the intangible asset being measured. Definitive goodwill generated in the acquisition includes the future development of unique technology and products, as well as the workforce and other synergies related to the R&D activity and has been allocated to the Diagnostic segment. Goodwill is not expected to be tax deductible. 2012 (b) Araclon Biotech, S.L. On 29 February 2012 and in relation to the Group’s strategic R&D priorities, Grifols acquired 51% of the capital of Araclon Biotech, S.L. for a total of Euros 8,259 thousand. Araclon Biotech, S.L. was founded as a spin-off from the University of Zaragoza in 2004. Its main areas of research focus on the validation and marketing of a blood diagnosis kit for Alzheimer’s and the development of an effective immunotherapy (vaccine) for this disease. F-156 |
|
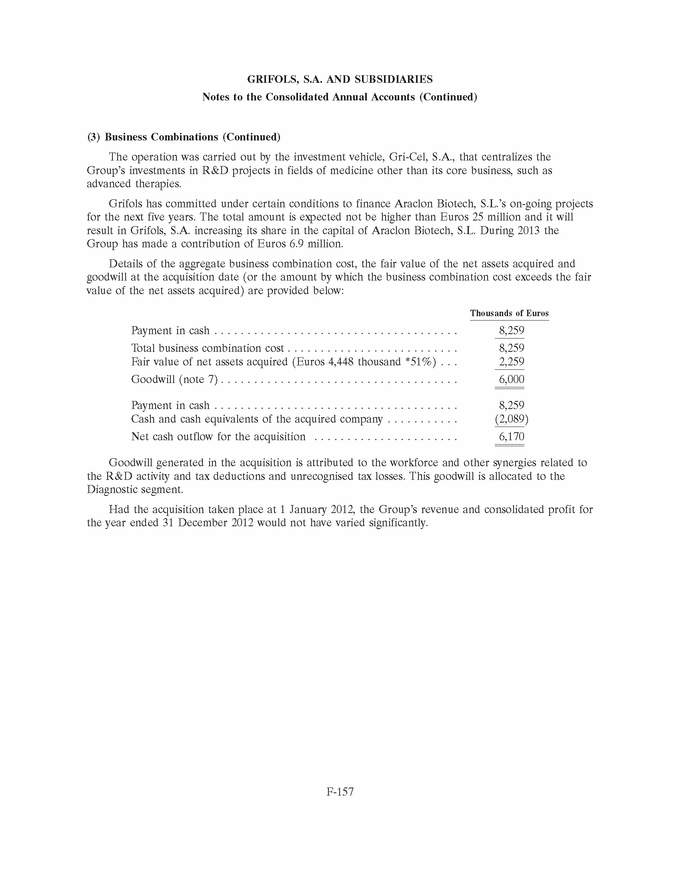
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (3) Business Combinations (Continued) The operation was carried out by the investment vehicle, Gri-Cel, S.A., that centralizes the Group’s investments in R&D projects in fields of medicine other than its core business, such as advanced therapies. Grifols has committed under certain conditions to finance Araclon Biotech, S.L.’s on-going projects for the next five years. The total amount is expected not be higher than Euros 25 million and it will result in Grifols, S.A. increasing its share in the capital of Araclon Biotech, S.L. During 2013 the Group has made a contribution of Euros 6.9 million. Details of the aggregate business combination cost, the fair value of the net assets acquired and goodwill at the acquisition date (or the amount by which the business combination cost exceeds the fair value of the net assets acquired) are provided below: Thousands of Euros Payment in cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,259 Total business combination cost . . . . . . . . . . . . . . . . . . . . . . . . . . 8,259 Fair value of net assets acquired (Euros 4,448 thousand *51%) . . . 2,259 Goodwill (note 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6,000 Payment in cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,259 Cash and cash equivalents of the acquired company . . . . . . . . . . . (2,089) Net cash outflow for the acquisition . . . . . . . . . . . . . . . . . . . . . . 6,170 Goodwill generated in the acquisition is attributed to the workforce and other synergies related to the R&D activity and tax deductions and unrecognised tax losses. This goodwill is allocated to the Diagnostic segment. Had the acquisition taken place at 1 January 2012, the Group’s revenue and consolidated profit for the year ended 31 December 2012 would not have varied significantly. F-157 |
|
|
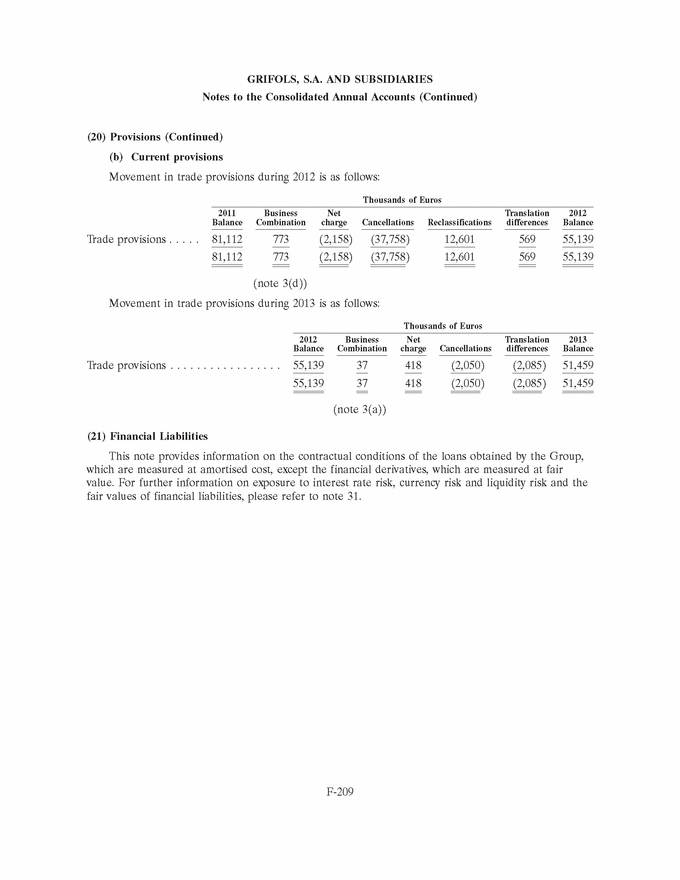
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (3) Business Combinations (Continued) At the date of acquisition the amounts of recognised assets, liabilities and contingent liabilities are as follows: Fair value Thousands of Euros Intangible assets (note 8) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12,525 Property, plant and equipment (note 9) . . . . . . . . . . . . . . . . . . . . 668 Non-current financial assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . 600 Trade and other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 142 Cash and cash equivalents . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,089 Total assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16,024 Non-current financial liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . 3,932 Deferred tax liabilities (note 28) . . . . . . . . . . . . . . . . . . . . . . . . . 138 Current financial liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6,770 Trade and other payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 736 Total liabilities and contingent liabilities . . . . . . . . . . . . . . . . . . . . 11,576 Total net assets acquired . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,448 It is not expected that goodwill will be tax deductible. (c) Plasma centres On 22 October 2012 the Group acquired three plasma donation centres from the Canadian biopharmaceutical company Cangene Corporation. These plasma centres are located in Frederick, MD, Altamonte Springs, FL and Van Nuys, CA. (USA). Aggregate details of the combination cost, fair value of the net assets acquired and goodwill at the acquisition date (or surplus net assets acquired over the combination cost) are as follows: Thousands of Euros Payment in cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,925 Total business combination cost . . . . . . . . . . . . . . . . . . . . . . . . . . 1,925 Fair value of net assets acquired . . . . . . . . . . . . . . . . . . . . . . . . . 1,133 Goodwill (note 7) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 792 The fair value of net assets acquired includes property, plant and equipment amounting to Euros 1,054 thousand (see note 9). Goodwill is allocated to the Bioscience segment. Had the acquisition taken place at 1 January 2012, the Group’s revenue and consolidated profit for the year ended 31 December 2012 would not have varied significantly. F-158 |
|
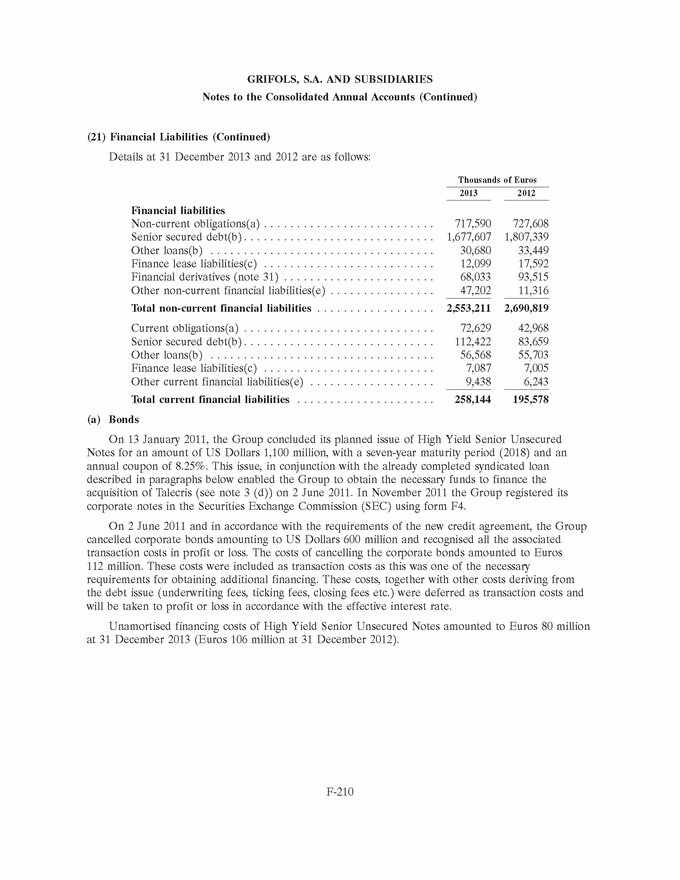
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (3) Business Combinations (Continued) 2011 (d) Talecris Biotherapeutics Holdings Corp and subsidiaries At 2 June 2011 the Group acquired 100% of the share capital of the US company Talecris Biotherapeutics Holdings Corporation. At this date, the Group did not have all the necessary information to determine the definitive fair value of intangible assets, liabilities and contingent liabilities acquired in the business combination of Talecris. During the second quarter of 2012 the Group obtained additional information on events and circumstances existing at the acquisition date which enabled it to accurately finalise the allocation of assets and liabilities. The allocation of the purchase price is therefore definitive. Goodwill increased by Euros 2,514 thousand (see note 7) due to a change in the valuation of inventories and the recognition of a current provision arising from an onerous contract, both of which are net of tax effect (Euros 3,759 thousand gross tax effect). Goodwill was allocated to Talecris Biotherapeutics, Inc, now named Grifols Therapeutics Inc, belonging to the Bioscience segment. (4) Significant Accounting Policies (a) Subsidiaries and associates Subsidiaries are entities, including special purpose entities (SPE), over which the Group exercises control, either directly or indirectly, through subsidiaries. Control exists when investors are exposed to variable returns from the subsidiaries and have the ability to affect those returns through their decisionmaking power over the subsidiary. The income, expenses and cash flows of subsidiaries are included in the consolidated annual accounts from the date of acquisition, which is when the Group takes control. Subsidiaries are excluded from the consolidated Group from the date on which control is lost. Transactions and balances with Group companies and unrealised gains or losses have been eliminated upon consolidation. The accounting policies of subsidiaries have been adapted to those of the Group for transactions and other events in similar circumstances. The financial statements of consolidated subsidiaries have been prepared as of the same date and for the same reporting period as the annual accounts of the Company. Associates are entities over which the Company, either directly or indirectly through subsidiaries, exercises significant influence. Significant influence is the power to participate in the financial and operating policy decisions of the investee but is not control or joint control over those entities. The existence of potential voting rights that are exercisable or convertible at the end of each reporting period, including potential voting rights held by the Group or other entities, are considered when assessing whether an entity has significant influence. Investments in associates are accounted for using the equity method from the date that significant influence commences until the date that significant influence ceases. Investments in associates are initially recognised at acquisition cost, including any cost directly attributable to the acquisition and any consideration receivable or payable contingent on future events or on compliance with certain conditions. F-159 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) The excess of the cost of the investment over the Group’s share of the fair values of the identifiable net assets is recognised as goodwill, which is included in the carrying amount of the investment. Any shortfall, once the cost of the investment and the identification and measurement of the associate’s net assets have been evaluated, is recognised as income when determining the investor’s share of the profit or loss of the associate for the year in which it was acquired. The accounting policies of associates have been harmonised in terms of timing and measurement, applying the policies described for subsidiaries. The Group’s share of the profit or loss of an associate from the date of acquisition is recognised as an increase or decrease in the value of the investments, with a credit or debit to share of the profit or loss for the year of equity-accounted associates in the consolidated statement of profit or loss (consolidated statement of comprehensive income). The Group’s share of other comprehensive income of associates from the date of acquisition is recognised as an increase or decrease in the investments in associates with a balancing entry recognised by nature in other comprehensive income. The distribution of dividends is recognised as a decrease in the value of the investment. The Group’s share of profit or loss, including impairment losses recognised by the associates, is calculated based on income and expenses arising from application of the acquisition method. The Group’s share of the profit or loss of an associate and changes in equity is calculated to the extent of the Group’s interest in the associate at year end and does not reflect the possible exercise or conversion of potential voting rights. However, the Group’s share is calculated taking into account the possible exercise of potential voting rights and other derivative financial instruments which, in substance, currently allow access to the economic benefits associated with the interests held, such as entitlement to a share in future dividends and changes in the value of associates. Information on the subsidiaries and associates included in the consolidated Group is presented in Appendix I. (b) Business combinations On the date of transition to IFRS-EU, 1 January 2004, the Group applied the exception permitted under IFRS 1 ‘‘First-time adoption of International Financial Reporting Standards’’, whereby only those business combinations performed as from 1 January 2004 have been recognised using the acquisition method. Entities acquired prior to that date were recognised in accordance with accounting prevailing at that time, taking into account the necessary corrections and adjustments at the transition date. The Group applies the revised IFRS 3 ‘‘Business combinations’’ in transactions made subsequent to 1 January 2010. The Group applies the acquisition method for business combinations. The acquisition date is the date on which the Group obtains control of the acquiree. Business combinations made subsequent to 1 January 2010 The cost of the business combination is calculated as the sum of the acquisition-date fair values of the assets transferred, the liabilities incurred or assumed, equity instruments issued and any additional consideration contingent on future events or the fulfilment of certain conditions, in exchange for control of the acquiree. F-160 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) The consideration paid excludes all amounts that do not form part of the exchange for the acquired business. Acquisition-related costs are accounted for as expenses when incurred. Share increase costs are recognised as equity when the increase takes place and borrowing costs are deducted from the financial liability when it is recognised. At the acquisition date the Group recognises at fair value the assets acquired and liabilities assumed. Liabilities assumed include any contingent liabilities that represent present obligations arising from past events for which the fair value can be reliably measured. The Group also recognises indemnification assets transferred by the seller at the same time and following the same measurement criteria as the item that is subject to indemnification from the acquired business, taking into consideration, where applicable, the insolvency risk and any contractual limit on the indemnity amount. This criterion does not include non-current assets or disposable groups of assets which are classified as held for sale, long-term defined benefit employee benefit liabilities, share-based payment transactions, deferred tax assets and liabilities and intangible assets arising from the acquisition of previously transferred rights. Assets and liabilities assumed are classified and designated for subsequent measurement in accordance with the contractual terms, economic conditions, operating or accounting policies and other factors that exist at the acquisition date, except for leases and insurance contracts. The excess between the consideration transferred and the value of net assets acquired and liabilities assumed, less the value assigned to non-controlling interests, is recognised as goodwill. Where applicable, any shortfall, after evaluating the consideration transferred, the value assigned to non-controlling interests and the identification and measurement of net assets acquired, is recognised in profit or loss. When a business combination has been provisionally determined, net identifiable assets have initially been recognised at their provisional value, and any adjustments made during the measurement period have been recorded as if they had been known at that date. Where applicable, comparative figures for the prior year have been restated. Adjustments to the provisional values only reflect information relating to events and circumstances existing at the acquisition date and which, had they been known, would have affected the amounts recognised at that date. Once this period has elapsed, adjustments are only made to initial values when errors must be corrected. Any potential benefits arising from tax losses and other deferred tax assets of the acquiree that have not been recorded as they did not qualify for recognition at the acquisition date, are accounted for as income tax revenue, provided the adjustments were not made during the measurement period. The contingent consideration is classified in accordance with underlying contractual terms as a financial asset or financial liability, equity instrument or provision. Provided that subsequent changes to the fair value of a financial asset or financial liability do not relate to an adjustment of the measurement period, they are recognised in consolidated profit or loss. The contingent consideration classified, where applicable, as equity is not subject to subsequent change, with settlement being recognised in equity. The contingent consideration classified, where applicable, as a provision is recognised subsequently in accordance with the relevant measurement standard. F-161 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) Business combinations made prior to 1 January 2010 The cost of the business combination is calculated as the sum of the acquisition-date fair values of the assets transferred, the liabilities incurred or assumed, and equity instruments issued by the Group, in exchange for control of the acquiree, plus any costs directly attributable to the business combination. Any additional consideration contingent on future events or the fulfilment of certain conditions is included in the cost of the combination provided that it is probable that an outflow of resources embodying economic benefits will be required and the amount of the obligation can be reliably estimated. Subsequent recognition of contingent considerations or subsequent variations to contingent considerations is recognised as a prospective adjustment to the cost of the business combination. Where the cost of the business combination exceeds the Group’s interest in the fair value of the identifiable net assets of the entity acquired, the difference is recognised as goodwill, whilst the shortfall, once the costs of the business combination and the fair values of net assets acquired have been reconsidered, is recognised in profit or loss. (c) Non-controlling interests Non-controlling interests in subsidiaries acquired after 1 January 2004 are recognised at the acquisition date at the proportional part of the fair value of the identifiable net assets. Non-controlling interests in subsidiaries acquired prior to the transition date were recognised at the proportional part of the equity of the subsidiaries at the date of first consolidation. Non-controlling interests are disclosed in the consolidated balance sheet under equity separately from equity attributable to the Parent. Non-controlling interests’ share in consolidated profit or loss for the year (and in consolidated comprehensive income for the year) is disclosed separately in the consolidated statement of profit or loss (consolidated statement of comprehensive income). The consolidated profit or loss for the year (consolidated comprehensive income) and changes in equity of the subsidiaries attributable to the Group and non-controlling interests after consolidation adjustments and eliminations, is determined in accordance with the percentage ownership at year end, without considering the possible exercise or conversion of potential voting rights. However, whether or not control exists is determined taking into account the possible exercise of potential voting rights and other derivative financial instruments which, in substance, currently allow access to the economic benefits associated with the interests held, such as entitlement to a share in future dividends and changes in the value of subsidiaries. Profit and loss and each component of other comprehensive income are assigned to equity attributable to shareholders of the Parent and to non-controlling interests in proportion to their interest, although this implies a balance receivable from non-controlling interests. Agreements signed between the Group and the non-controlling interests are recognised as a separate transaction. The increase and reduction of non-controlling interests in a subsidiary in which control is retained is recognised as an equity instrument transaction. Consequently, no new acquisition cost arises on increases nor is a gain recorded on reductions; rather, the difference between the consideration transferred or received and the carrying amount of the non-controlling interests is recognised in the reserves of the investor, without prejudice to reclassifying consolidation reserves and reallocating other comprehensive income between the Group and the non-controlling interests. When a Group’s interest F-162 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) in a subsidiary diminishes, non-controlling interests are recognised at their share of the net consolidated assets, including goodwill. (d) Joint arrangements Joint arrangements are those in which there is a contractual agreement to share the control over an economic activity, in such a way that the decisions over relevant activities require the unanimous consent of the Group and the remaining venturers. Investments in joint arrangements are accounted for using the equity method. The acquisition cost of investments in joint arrangements is determined consistently with that established for investments in associates. (e) Foreign currency transactions and balances (i) Functional and presentation currency The consolidated annual accounts are presented in thousands of Euros, which is the functional and presentation currency of the Parent. (ii) Foreign currency transactions, balances and cash flows Foreign currency transactions are translated into the functional currency using the previous month’s exchange rate for all transactions performed during the current month. This method does not differ significantly from applying the exchange rate at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies have been translated into thousands of Euros at the closing rate, while non-monetary assets and liabilities measured at historical cost have been translated at the exchange rate prevailing at the transaction date. Non-monetary assets measured at fair value have been translated into thousands of Euros at the exchange rate at the date that the fair value was determined. In the consolidated statement of cash flows, cash flows from foreign currency transactions have been translated into thousands of Euros at the exchange rates prevailing at the dates the cash flows occur. The effect of exchange rate fluctuations on cash and cash equivalents denominated in foreign currencies is recognised separately in the statement of cash flows as ‘‘Effect of exchange rate fluctuations on cash and cash equivalents’’. Exchange gains and losses arising on the settlement of foreign currency transactions and the translation into thousands of Euros of monetary assets and liabilities denominated in foreign currencies are recognised in profit or loss. (iii) Translation of foreign operations The translation into thousands of Euros of foreign operations for which the functional currency is not the currency of a hyperinflationary economy is based on the following criteria: • Assets and liabilities, including goodwill and net asset adjustments derived from the acquisition of the operations, including comparative amounts, are translated at the closing rate at the reporting date. F-163 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) • Income and expenses, including comparative amounts, are translated using the previous month’s exchange rate for all transactions performed during the current month. This method does not differ significantly from using the exchange rate at the date of the transaction; • Translation differences resulting from application of the above criteria are recognised in other comprehensive income. (f) Borrowing costs In accordance with IAS 23 ‘‘Borrowing Costs’’, since 1 January 2009 the Group recognises interest cost directly attributable to the purchase, construction or production of qualifying assets as an increase in the value of these assets. Qualifying assets are those which require a substantial period of time before they can be used or sold. To the extent that funds are borrowed specifically for the purpose of obtaining a qualifying asset, the amount of borrowing costs eligible for capitalisation is determined as the actual borrowing costs incurred, less any investment income on the temporary investment of those funds. Capitalised interest borrowing costs corresponding to general borrowing are calculated as the weighted average of the qualifying assets without considering specific funds.The amount of borrowing costs capitalised cannot exceed the amount of borrowing costs incurred during that period. The capitalised interest cost includes adjustments to the carrying amount of financial liabilities arising from the effective portion of hedges entered into by the Group. The Group begins capitalising borrowing costs as part of the cost of a qualifying asset when it incurs expenditures for the asset, interest is accrued, and it undertakes activities that are necessary to prepare the asset for its intended use or sale, and ceases capitalising borrowing costs when all or substantially all the activities necessary to prepare the qualifying asset for its intended use or sale are complete. Nevertheless, capitalisation of borrowing costs is suspended when active development is interrupted for extended periods. (g) Property, plant and equipment (i) Initial recognition Property, plant and equipment are recognised at cost or deemed cost, less accumulated depreciation and any accumulated impairment losses. The cost of self-constructed assets is determined using the same principles as for an acquired asset, while also considering the criteria applicable to production costs of inventories. Capitalised production costs are recognised by allocating the costs attributable to the asset to ‘‘Self-constructed non-current assets’’ in the consolidated statement of profit or loss. At 1 January 2004 the Group opted to apply the exemption regarding fair value and revaluation as deemed cost as permitted by IFRS 1 First time Adoption of International Financial Reporting Standards. (ii) Depreciation Property, plant and equipment are depreciated by allocating the depreciable amount of an asset on a systematic basis over its useful life. The depreciable amount is the cost or deemed cost of an asset, less its residual value. The Group determines the depreciation charge separately for each item for a component of property, plant and equipment with a cost that is significant in relation to the total cost of the asset. F-164 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) Property, plant and equipment are depreciated using the following criteria: Depreciation method Rates Buildings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Straight line 1% - 3% Other property, technical equipment and machinery . Straight line 10% Other property, plant and equipment . . . . . . . . . . . . Straight line 7% - 33% The Group reviews residual values, useful lives and depreciation methods at each financial year end. Changes to initially established criteria are accounted for as a change in accounting estimates. (iii) Subsequent recognition Subsequent to initial recognition of the asset, only those costs incurred which will probably generate future profits and for which the amount may reliably be measured are capitalised. Costs of day-to-day servicing are recognised in profit or loss as incurred. Replacements of property, plant and equipment which qualify for capitalisation are recognised as a reduction in the carrying amount of the items replaced. Where the cost of the replaced items has not been depreciated independently and it is not possible to determine the respective carrying amount, the replacement cost is used as indicative of the cost of items at the time of acquisition or construction. (iv) Impairment The Group tests for impairment and reversals of impairment losses on property, plant and equipment based on the criteria set out in note 4(i) below. (h) Intangible assets (i) Goodwill Goodwill is generated on the business combinations and is calculated using the criteria described in the section on business combinations. Goodwill is not amortised, but is tested for impairment annually or more frequently whenever there is an indication that goodwill may be impaired. Goodwill acquired in business combinations is allocated to the cash-generating units (CGUs) or groups of CGUs which are expected to benefit from the synergies of the business combination and the criteria described in note 7 are applied. After initial recognition, goodwill is measured at cost less any accumulated impairment losses. (ii) Internally generated intangible assets Any research and development expenditure incurred during the research phase of projects is recognised as an expense when incurred. Costs related with development activities are capitalised when: • The Group has technical studies that demonstrate the feasibility of the production process. • The Group has undertaken a commitment to complete production of the asset, to make it available for sale or internal use. • The asset will generate sufficient future economic benefits. F-165 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) • The Group has sufficient technical and financial resources to complete development of the asset and has devised budget control and cost accounting systems that enable monitoring of budgetary costs, modifications and the expenditure actually attributable to the different projects. The cost of internally generated assets is calculated using the same criteria established for determining production costs of inventories. The production cost is capitalised by allocating the costs attributable to the asset to self-constructed non-current assets in the consolidated statement of profit or loss. Expenditure on activities that contribute to increasing the value of the different businesses in which the Group as a whole operates is expensed when incurred. Replacements or subsequent costs incurred on intangible assets are generally recognised as an expense, except where they increase the future economic benefits expected to be generated by the assets. (iii) Other intangible assets Other intangible assets are carried at cost, or at fair value if they arise on business combinations, less accumulated amortisation and impairment losses. Intangible assets with indefinite useful lives are not amortised but tested for impairment at least annually. (iv) Intangible assets acquired in business combinations The cost of identifiable intangible assets acquired in the business combination of Talecris includes the fair value of the currently marketed products sold and which are classified in ‘‘Other intangible assets’’. The cost of identifiable intangible assets acquired in the business combination of Araclon Biotech, S.L. includes the fair value of research and development projects in progress. The cost of identifiable intangible assets acquired in the business combination of the Progenika Group includes the fair value of the currently marketed products sold and which are classified in ‘‘Other intangible assets’’ and ‘‘Development costs’’. (v) Useful life and amortisation rates The Group assesses whether the useful life of each intangible asset acquired is finite or indefinite. An intangible asset is regarded as having an indefinite useful life when there is no foreseeable limit to the period over which the asset will generate net cash inflows. Intangible assets with finite useful lives are amortised by allocating the depreciable amount of an asset on a systematic basis over its useful life, by applying the following criteria: Amortisation method Rates Development expenses . . . . . . . . . . . . . . . . . . . . . Straight line 20% - 33% Concessions, patents, licences, trademarks and similar . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Straight line 7% - 20% Computer software . . . . . . . . . . . . . . . . . . . . . . . . Straight line 16% - 33% Currently marketed products . . . . . . . . . . . . . . . . . Straight line 3% - 10% The depreciable amount is the cost or deemed cost of an asset, less its residual value. F-166 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) The Group does not consider the residual value of its intangible assets to be material. The Group reviews the residual value, useful life and amortisation method for intangible assets at each financial year end. Changes to initially established criteria are accounted for as a change in accounting estimates. (i) Impairment of goodwill, other intangible assets and other non-financial assets subject to depreciation or amortisation The Group evaluates whether there are indications of possible impairment losses on non-financial assets subject to amortisation or depreciation, to verify whether the carrying amount of these assets exceeds the recoverable amount. The Group tests goodwill, intangible assets with indefinite useful lives and intangible assets with finite useful lives that are not available for use for potential impairment at least annually, irrespective of whether there is any indication that the assets may be impaired. The recoverable amount of the assets is the higher of their fair value less costs of disposal and their value in use. An asset’s value in use is calculated based on an estimate of the future cash flows expected to derive from the use of the asset, expectations about possible variations in the amount or timing of those future cash flows, the time value of money, the price for bearing the uncertainty inherent in the asset and other factors that market participants would reflect in pricing the future cash flows deriving from the asset. Negative differences arising from comparison of the carrying amounts of the assets with their recoverable amounts are recognised in the consolidated statement of profit or loss.Recoverable amount is determined for each individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets. If this is the case, recoverable amount is determined for the cash-generating unit (CGU) to which the asset belongs. Impairment losses recognised for cash-generating units are first allocated to reduce, where applicable, the carrying amount of goodwill allocated to the CGU and then to the other assets of the CGU pro rata on the basis of the carrying amount of each asset. The carrying amount of each asset may not be reduced below the highest of its fair value less costs of disposal, its value in use and zero. At the end of each reporting period the Group assesses whether there is any indication that an impairment loss recognised in prior periods may no longer exist or may have decreased. Impairment losses on goodwill are not reversible. Impairment losses on other assets are only reversed if there has been a change in the estimates used to calculate the recoverable amount of the asset. A reversal of an impairment loss is recognised in consolidated profit or loss. The increased carrying amount of an asset attributable to a reversal of an impairment loss may not exceed the carrying amount that would have been determined, net of depreciation or amortisation, had no impairment loss been recognised. A reversal of an impairment loss for a CGU is allocated to the assets of each unit, except goodwill, pro rata with the carrying amounts of those assets. The carrying amount of an asset may not be increased above the lower of its recoverable amount and the carrying amount that would have been disclosed, net of amortisation or depreciation, had no impairment loss been recognised. F-167 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) (j) Leases (i) Lessee accounting records The Group has rights to use certain assets through lease contracts. Leases in which the Group assumes substantially all the risks and rewards incidental to ownership are classified as finance leases, otherwise they are classified as operating leases. • Finance leases At the commencement of the lease term, the Group recognises finance leases as assets and liabilities at the lower of the fair value of the leased asset and the present value of the minimum lease payments. Initial direct costs are added to the asset’s carrying amount. Minimum lease payments are apportioned between the finance charge and the reduction of the outstanding liability. The finance charge is allocated to each period during the lease term so as to produce a constant periodic rate of interest on the remaining balance of the liability. Contingent rents are recognised as an expense in the years in which they are incurred. • Operating leases Lease payments under an operating lease (excluding incentives) are recognised as an expense on a straight-line basis unless another systematic basis is representative of the time pattern of the user’s benefit. (ii) Leasehold investments Non-current investments in properties leased from third parties are recognised on the basis of the same criteria for property, plant and equipment. Investments are amortised over the lower of their useful lives and the term of the lease contract. The lease term is consistent with that established for recognition of the lease. (iii) Sale and leaseback transactions Any profit on sale and leaseback transactions that meet the conditions of a finance lease is deferred over the term of the lease. When the leaseback is classed as an operating lease: • If the transaction is established at fair value, any profit or loss on the sale is recognised immediately in the consolidated statement of profit or loss for the year. • If the sale price is below fair value, any profit or loss is recognised immediately in the consolidated statement of profit or loss. However, if the loss is compensated for by future lease payments at below market price, it is deferred in proportion to the lease payments over the period for which the asset is to be used. (k) Financial instruments (i) Classification of financial instruments Financial instruments are classified on initial recognition as a financial asset, a financial liability or an equity instrument in accordance with the substance of the contractual arrangement and the definitions of a financial liability, a financial asset and an equity instrument set out in IAS 32, Financial Instruments: Presentation. F-168 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) Financial instruments are classified into the following categories for valuation purposes: financial assets and financial liabilities at fair value through profit or loss, loans and receivables, held-to-maturity investments, available-for-sale financial assets and financial liabilities. Financial instruments are classified into different categories based on the nature of the instruments and the Group’s intentions on initial recognition. Regular way purchases and sales of financial assets are recognised using trade date accounting, i.e. when the Group commits itself to purchase or sell an asset. a) Financial assets at fair value through profit or loss Financial assets and financial liabilities at fair value through profit or loss are those which are classified as held for trading or which the Group designated as such on initial recognition. A financial asset or financial liability is classified as held for trading if it: • It is acquired or incurred principally for the purpose of selling or repurchasing it in the near term; • it forms part of a portfolio of identified financial instruments that are managed together and for which there is evidence of a recent pattern of short-term profit-taking, or • It is a derivative, except for a derivative that is a financial guarantee contract or a designated and effective hedging instrument. Financial assets and financial liabilities at fair value through profit or loss are initially recognised at fair value. Transaction costs directly attributable to the acquisition or issue are recognised as an expense when incurred. After initial recognition, they are recognised at fair value through profit or loss. The Group does not reclassify any financial assets or liabilities from or to this category while they are recognised in the consolidated balance sheet. b) Loans and receivables Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market, other than those classified in other financial asset categories. These assets are recognised initially at fair value, including transaction costs, and subsequently measured at amortised cost using the effective interest method. c) Available-for-sale financial assets Available-for-sale financial assets are non-derivative financial assets that are either designated specifically to this category or do not comply with requirements for classification in the above categories. Available-for-sale financial assets are initially recognised at fair value plus transaction costs directly attributable to the acquisition. After initial recognition, financial assets classified in this category are measured at fair value and any gain or loss, except for impairment losses, is accounted for in other comprehensive income recognised in equity. On disposal of the financial assets, amounts recognised in other comprehensive income or the impairment loss are reclassified to profit or loss. F-169 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) d) Financial assets and financial liabilities carried at cost Investments in equity instruments whose fair value cannot be reliably measured and derivative instruments that are linked to these instruments and that must be settled by delivery of such unquoted equity instruments, are measured at cost. Nonetheless, if the financial assets or liabilities can be reliably measured subsequently on an ongoing basis, they are accounted for at fair value and any gain or loss is recognised in accordance with their classification. (ii) Offsetting principles A financial asset and a financial liability are offset only when the Group currently has the legally enforceable right to offset the recognised amounts and intends either to settle on a net basis or to realise the asset and settle the liability simultaneously. (iii) Fair value The fair value is the amount for which an asset can be exchanged, or a liability settled, between knowledgeable, willing parties in an arm’s length transaction. The Group generally applies the following systematic hierarchy to determine the fair value of financial assets and financial liabilities: • Firstly, the Group applies the quoted prices of the most advantageous active market to which the entity has immediate access, adjusted where appropriate to reflect any differences in counterparty credit risk between instruments traded in that market and the one being valued. The quoted market price for an asset held or liability to be issued is the current bid price and, for an asset to be acquired or liability held, the asking price. If the Group has assets and liabilities with offsetting market risks, it uses mid-market prices as a basis for establishing fair values for the offsetting risk positions and applies the bid or asking price to the net open position as appropriate. • When current bid and asking prices are unavailable, the price of the most recent transaction is used, adjusted to reflect changes in economic circumstances. • Otherwise, the Group applies generally accepted measurement techniques using, insofar as is possible, market data and, to a lesser extent, specific Group data. (iv) Amortised cost The amortised cost of a financial asset or financial liability is the amount at which the financial asset or financial liability is measured at initial recognition minus principal repayments, plus or minus the cumulative amortisation using the effective interest method of any difference between that initial amount and the maturity amount, and minus any reduction for impairment or uncollectibility. (v) Impairment of financial assets carried at cost The amount of the impairment loss on assets carried at cost is measured as the difference between the carrying amount of the financial asset and the present value of estimated future cash flows discounted at the current market rate of return for a similar financial asset. Such impairment losses cannot be reversed and are therefore recognised directly against the value of the asset and not as an allowance account. F-170 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) (vi) Impairment of financial assets carried at amortised cost In the case of financial assets carried at amortised cost, the amount of the impairment loss is measured as the difference between the asset’s carrying amount and the present value of estimated future cash flows (excluding future credit losses that have not been incurred) discounted at the financial asset’s original effective interest rate. For variable income financial assets, the effective interest rate corresponding to the measurement date under the contractual conditions is used. The Group recognises impairment losses and unrecoverable loans and receivables and debt instruments by recognising an allowance account for financial assets. When impairment and uncollectibility are considered irreversible, their carrying amount is eliminated against the allowance account. The impairment loss is recognised in profit or loss and may be reversed in subsequent periods if the decrease can be objectively related to an event occurring after the impairment has been recognised. The loss can only be reversed to the limit of the amortised cost of the assets had the impairment loss not been recognised. The impairment loss is reversed against the allowance account. (vii) Impairment of available-for-sale financial assets When a decline in the fair value of an available-for-sale financial asset at fair value through profit or loss has been accounted for in other comprehensive income, the accumulative loss is reclassified from equity to profit or loss when there is objective evidence that the asset is impaired, even though the financial asset has not been derecognised. The impairment loss recognised in profit or loss is calculated as the difference between the acquisition cost, net of any reimbursements or repayment of the principal, and the present fair value, less any impairment loss previously recognised in profit or loss for the year. Impairment losses relating to investments in equity instruments are not reversible and are therefore recognised directly against the value of the asset and not as an allowance account. If the fair value of debt instruments increases and the increase can be objectively related to an event occurring after the impairment loss was recognised, the increase is recognised in profit or loss up to the amount of the previously recognised impairment loss and any excess is accounted for in other comprehensive income recognised in equity. (viii) Financial liabilities Financial liabilities, including trade and other payables, which are not classified at fair value through profit or loss, are initially recognised at fair value less any transaction costs that are directly attributable to the issue of the financial liability. After initial recognition, liabilities classified under this category are measured at amortised cost using the effective interest method. (ix) Derecognition of financial assets The Group applies the criteria for derecognition of financial assets to part of a financial asset or part of a group of similar financial assets or to a financial asset or group of similar financial assets. Financial assets are derecognised when the contractual rights to the cash flows from the financial asset expire or have been transferred and the Group has transferred substantially all the risks and rewards of ownership. Where the Group retains the contractual rights to receive cash flows, it only F-171 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) derecognises financial assets when it has assumed a contractual obligation to pay the cash flows to one or more recipients and if the following requirements are met: • Payment of the cash flows is conditional on their prior collection. • The Group is unable to sell or pledge the financial asset. • The cash flows collected on behalf of the eventual recipients are remitted without material delay and the Group is not entitled to reinvest the cash flows. This criterion is not applicable to investments in cash or cash equivalents made by the Group during the settlement period from the collection date to the date of required remittance to the eventual recipients, provided that interest earned on such investments is passed on to the eventual recipients. If the Group neither transfers nor retains substantially all the risks and rewards of ownership of the financial asset, it determines whether it has retained control of the financial asset. In this case: • If the Group has not retained control, it derecognises the financial asset and recognises separately as assets or liabilities any rights and obligations created or retained in the transfer. • If the Group has retained control, it continues to recognise the financial asset to the extent of its continuing involvement in the financial asset and recognises an associated liability. The extent of the Group’s continuing involvement in the transferred asset is the extent to which it is exposed to changes in the value of the transferred asset. The transferred asset and the associated liability are measured on a basis that reflects the rights and obligations that the Group has retained. The associated liability is measured in such a way that the carrying amount of the transferred asset and the associated liability is equal to the amortised cost of the rights and obligations retained by the Group, if the transferred asset is measured at amortised cost, or to the fair value of the rights and obligations retained by the Group, if the transferred asset is measured at fair value. The Group continues to recognise any income arising on the transferred asset to the extent of its continuing involvement and recognises any expense incurred on the associated liability. Recognised changes in the fair value of the transferred asset and the associated liability are accounted for consistently with each other in profit or loss or equity, following the general recognition criteria described previously, and are not offset. If the Group retains substantially all the risks and rewards of ownership of a transferred financial asset, the consideration received is recognised in liabilities. Transaction costs are recognised in profit or loss using the effective interest method. (x) Derecognition and modifications of financial liabilities A financial liability, or part of it, is derecognised when the Group either discharges the liability by paying the creditor, or is legally released from primary responsibility for the liability either by process of law or by the creditor. The exchange of debt instruments between the Group and the counterparty or substantial modifications of initially recognised liabilities are accounted for as an extinguishment of the original financial liability and the recognition of a new financial liability, providing the instruments have substantially different terms. The Group considers the terms are substantially different if the discounted present value of the cash flows under the new terms, including any fees paid net of any fees received and discounted using F-172 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) the original effective interest rate, is at least 10 per cent different from the discounted present value of the remaining cash flows of the original financial liability. If the exchange is accounted for as an extinguishment of the financial liability, any costs or fees incurred are recognised as part of the gain or loss on the extinguishment. If the exchange is not accounted for as an extinguishment, any costs or fees incurred adjust the carrying amount of the liability and are amortised over the remaining term of the modified liability. The difference between the carrying amount of a financial liability, or part of a financial liability, extinguished or transferred to another party and the consideration paid, including any non-cash assets transferred or liabilities assumed, is recognised in profit or loss. (l) Hedge accounting Derivative financial instruments are initially recognised using the same criteria as those described for financial assets and financial liabilities. Derivative financial instruments that do not meet the hedge accounting requirements are classified and measured as financial assets and financial liabilities at fair value through profit or loss. Derivative financial instruments which qualify for hedge accounting are initially measured at fair value. At the inception of the hedge the Group formally designates and documents the hedging relationships and the objective and strategy for undertaking the hedges. Hedge accounting is only applicable when the hedge is expected to be highly effective at the inception of the hedge and in subsequent years in achieving offsetting changes in fair value or cash flows attributable to the hedged risk, throughout the period for which the hedge was designated (prospective analysis) and the actual effectiveness, which can be reliably measured, is within a range of 80%-125% (retrospective analysis). (i) Cash flow hedges The Group recognises the portion of the gain or loss on the measurement at fair value of a hedging instrument that is determined to be an effective hedge in other comprehensive income. The ineffective portion and the specific component of the gain or loss or cash flows on the hedging instrument, excluding the measurement of the hedge effectiveness, are recognised with a debit or credit to finance costs or finance income. If a hedge of a forecast transaction subsequently results in the recognition of a financial asset or a financial liability, the associated gains or losses that were recognised in other comprehensive income are reclassified from equity to profit or loss in the same period or periods during which the asset acquired or liability assumed affects profit or loss and under the same caption of the consolidated statement of profit or loss (consolidated statement of comprehensive income). F-173 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) (m) Equity instruments The Group’s acquisition of equity instruments of the Parent is recognised separately at cost of acquisition in the consolidated balance sheet as a reduction in equity, regardless of the motive of the purchase. Any gains or losses on transactions with treasury equity instruments are not recognised in consolidated profit or loss. The subsequent redemption of Parent shares, where applicable, leads to a reduction in share capital in an amount equivalent to the par value of such shares. Any positive or negative difference between the cost of acquisition and the par value of the shares is debited or credited to accumulated gains. Transaction costs related with treasury equity instruments, including issue costs related to a business combination, are accounted for as a reduction in equity, net of any tax effect. (n) Inventories Inventories are measured at the lower of cost and net realisable value. The cost of inventories comprises all costs of purchase, costs of conversion and other costs incurred in bringing the inventories to their present location and condition. The costs of conversion of inventories include costs directly related to the units of production and a systematic allocation of fixed and variable production overheads that are incurred in converting materials into finished goods. The allocation of fixed indirect overheads is based on the higher of normal production capacity or actual production. The raw material used to produce haemoderivatives is human plasma, which is obtained from our donation centres using the plasmapheresis method. The cost of inventories includes the amount paid to plasma donors, or the amount billed by the seller when purchased from third parties, as well as the cost of products and devices used in the collection process, rental expenses and storage. This plasma has to be stored before use, which is an essential part of the production process. During the storage period, the plasma undergoes various virological tests and should be kept in quarantine in accordance with FDA and European Medicines Agency regulations, in order to guarantee that all the plasma is suitable for use in the production process. To the extent that plasma storage costs are necessary to the production process, they are included as cost of inventories. Indirect costs such as general management and administration costs are recognized as expenses in the period in which they are incurred The cost of raw materials and other supplies and the cost of merchandise are allocated to each inventory unit on a weighted average cost basis. The transformation cost is allocated to each inventory unit on a FIFO (first-in, first-out) basis. The Group uses the same cost model for all inventories of the same nature and with a similar use. Volume discounts extended by suppliers are recognised as a reduction in the cost of inventories when it is probable that the conditions for discounts to be received will be met. Discounts for prompt payment are recognised as a reduction in the cost of the inventories acquired. F-174 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) When the cost of inventories exceeds net realisable value, materials are written down to net realisable value, which is understood to be: • For raw materials and other supplies, replacement cost. Nevertheless, raw materials and other supplies are not written down below cost if the finished goods into which they will be incorporated are expected to be sold at or above cost of production. • Merchandise and finished goods, estimated selling price less costs to sell; • Work in progress, the estimated selling price of related finished goods, less the estimated costs of completion and the estimated costs necessary to make the sale; The previously recognised write-down is reversed against profit or loss when the circumstances that previously caused inventories to be written down no longer exist or when there is clear evidence of an increase in net realisable value because of changed economic circumstances. The reversal of the write-down is limited to the lower of the cost and revised net realisable value of the inventories. Writedowns may be reversed with a credit to ‘‘Changes in inventories of finished goods and work in progress’’ and ‘‘Supplies’’. (o) Cash and cash equivalents Cash and cash equivalents include cash on hand and demand deposits in financial institutions. They also include other short-term, highly liquid investments that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value. An investment normally qualifies as a cash equivalent when it has a maturity of less than three months from the date of acquisition. The Group classifies cash flows relating to interest received and paid as operating activities, and dividends received and distributed are classified under investing and financing activities, respectively. (p) Government grants Government grants are recognised when there is reasonable assurance that they will be received and that the Group will comply with the conditions attached. (i) Capital grants Outright capital grants are initially recognised as deferred income in the consolidated balance sheet. Income from capital grants is recognised as other income in the consolidated statement of profit or loss in line with the depreciation of the corresponding financed assets. (ii) Operating grants Operating grants received to offset expenses or losses already incurred, or to provide immediate financial support not related to future disbursements, are recognised as other income in the consolidated statement of profit or loss. (iii) Interest rate grants Financial liabilities comprising implicit assistance in the form of below-market interest rates are initially recognised at fair value. The difference between this value, adjusted where necessary for the issue costs of the financial liability and the amount received, is recognised as a government grant based on the nature of the grant awarded. F-175 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) (q) Employee benefits (i) Defined contribution plans The Group recognises the contributions payable to a defined contribution plan in exchange for a service in the period in which contributions are accrued. Accrued contributions are recognised as an employee benefit expense in the corresponding consolidated statement of profit or loss in the year that the contribution was made. (ii) Termination benefits Termination benefits payable that do not relate to restructuring processes in progress are recognised when the Group is demonstrably committed to terminating the employment of current employees prior to retirement date. The Group is demonstrably committed to terminating the employment of current employees when a detailed formal plan has been prepared and there is no possibility of withdrawing or changing the decisions made. (iii) Short-term employee benefits The Group recognises the expected cost of short-term employee benefits in the form of accumulating compensated absences when the employees render service that increases their entitlement to future compensated absences. In the case of non-accumulating compensated absences, the expense is recognised when the absences occur. The Group recognises the expected cost of profit-sharing and bonus plans when it has a present legal or constructive obligation to make such payments as a result of past events and a reliable estimate of the obligation can be made. (r) Provisions Provisions are recognised when the Group has a present obligation (legal or implicit) as a result of a past event; it is probable that an outflow of resources embodying economic benefits will be required to settle the obligation; and a reliable estimate can be made of the amount of the obligation. The amount recognised as a provision is the best estimate of the expenditure required to settle the present obligation at the end of the reporting period, taking into account all risks and uncertainties surrounding the amount to be recognised as a provision and, where the time value of money is material, the financial effect of discounting provided that the expenditure to be made each period can be reliably estimated. The discount rate is a pre-tax rate that reflects the time value of money and the specific risks for which future cash flows associated with the provision have not been adjusted at each reporting date. If it is not probable that an outflow of resources embodying economic benefits will be required to settle the obligation, the provision is reversed against the consolidated statement of profit or loss item where the corresponding expense was recognised. (s) Revenue recognition Revenue from the sale of goods or services is measured at the fair value of the consideration received or receivable. Revenue is presented net of VAT and any other amounts or taxes which are effectively collected on the behalf of third parties. Volume or other types of discounts for prompt F-176 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) payment are recognised as a reduction in revenues if considered probable at the time of revenue recognition. (i) Sale of goods The Group recognises revenue from the sale of goods when: • The Group has transferred to the buyer the significant risks and rewards of ownership of the goods. • It retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; • The amount of revenue and the costs incurred or to be incurred can be measured reliably; • It is probable that the economic benefits associated with the transaction will flow to the Group; and • The costs incurred or to be incurred in respect of the transaction can be measured reliably. The Group participates in the government-managed Medicaid programmes in the United States, accounting for Medicaid rebates by recognising an accrual at the time a sale is recorded for an amount equal to the estimated claims for Medicaid rebates attributable to the sale. Medicaid rebates are estimated based on historical experience, legal interpretations of the applicable laws relating to the Medicaid programme and any new information regarding changes in the programme regulations and guidelines that would affect rebate amounts. Outstanding Medicaid claims, Medicaid payments and inventory levels are analysed for each distribution channel and the accrual is adjusted periodically to reflect actual experience. While rebate payments are generally made in the following or subsequent quarter, any adjustments for actual experience have not been material. As is common practice in the sector, the purchase contracts signed by some customers with the Group entitle these customers to price discounts for a minimum purchase volume, volume discounts or prompt payment discounts. The Group recognises these discounts as a reduction in sales and receivables in the same month that the corresponding sales are invoiced based on the customer’s actual purchase figures or on past experience when the customer’s actual purchases will not be known until a later date. In the USA, the Group enters into agreements with certain customers to establish contract pricing for the products, which these entities purchase from the authorised wholesaler or distributor (collectively, wholesalers) of their choice. Consequently, when the products are purchased from wholesalers by these entities at the contract price which is less than the price charged by the Group to the wholesaler, the Group provides the wholesaler with a credit referred to as a chargeback. The Group records the chargeback accrual at the time of the sale. The allowance for chargebacks is based on Group’s estimate of the wholesaler inventory levels, and the expected sell-through of the products by the wholesalers at the contract price based on historical chargeback experience and other factors. The Group periodically monitors the factors that influence the provision for chargebacks, and makes adjustments when believes that actual chargebacks may differ from established allowances. These adjustments occur in a relatively short period of time. As these chargebacks are typically settled within 30 to 45 days of the sale, adjustments for actual experience have not been material. F-177 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) (ii) Services rendered Revenues associated with the rendering of service transactions are recognised by reference to the stage of completion at the consolidated balance sheet date when the outcome of the transaction can be estimated reliably. The outcome of a transaction can be estimated reliably when revenues, the stage of completion, the costs incurred and the costs to complete the transaction can be estimated reliably and it is probable that the economic benefits derived from the transaction will flow to the Group. When the outcome of the transaction involving the rendering of services cannot be estimated reliably, revenue is recognised only to the extent of costs incurred that are recoverable. (iii) Interest income Until June 2012 the Group has been recognising interest receivable from the different Social Security affiliated bodies, to which it provides goods or services, on an accrual basis, and only for those bodies to which historically claims have been made and from which interest has been collected. As a result of the terms imposed by the Spanish Government in 2012 regarding the waiver of late payment interest on overdue receivables, the Group modified its estimate regarding late payment interest. In this respect, since June 2012 the Group has been recognizing only late payment interest on receivables from Social Security affiliated bodies on the date on which delayed invoices are collected, as it is highly likely that they will be collected as of that date and if the Spanish Government has not imposed to waiver the late payment interests. (t) Income taxes The income tax expense or tax income for the year comprises current tax and deferred tax. Current tax is the amount of income taxes payable or recoverable in respect of the consolidated taxable profit or consolidated tax loss for the year. Current tax assets or liabilities are measured at the amount expected to be paid to or recovered from the taxation authorities, using the tax rates and tax laws that have been enacted or substantially enacted at the reporting date. Deferred tax liabilities are the amounts of income taxes payable in future periods in respect of taxable temporary differences, whereas deferred tax assets are the amounts of income taxes recoverable in future periods in respect of deductible temporary differences, the carryforward of unused tax losses, and the carryforward of unused tax credits. Temporary differences are differences between the carrying amount of an asset or liability in the balance sheet and its tax base. Current and deferred tax are recognised as income or an expense and included in profit or loss for the year, except to the extent that the tax arises from a transaction or event which is recognised, in the same or a different year, directly in equity, or from a business combination. (i) Taxable temporary differences Taxable temporary differences are recognised in all cases except where: • They arise from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither accounting profit nor taxable income. F-178 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) • They are associated with investments in subsidiaries over which the Group is able to control the timing of the reversal of the temporary difference and it is not probable that the temporary difference will reverse in the foreseeable future. (ii) Deductible temporary differences Deductible temporary differences are recognised provided that: • It is probable that sufficient taxable income will be available against which the deductible temporary difference can be utilised, unless the differences arise from the initial recognition of an asset or liability in a transaction that is not a business combination and, at the time of the transaction, affects neither accounting profit nor taxable income. • The temporary differences are associated with investments in subsidiaries to the extent that the difference will reverse in the foreseeable future and sufficient taxable income is expected to be generated against which the temporary difference can be offset. Tax planning opportunities are only considered when assessing the recoverability of deferred tax assets and if the Group intends to use these opportunities or it is probable that they will be utilised. (iii) Measurement Deferred tax assets and liabilities are measured at the tax rates that are expected to apply to the years when the asset is realised or the liability is settled, based on tax rates and tax laws that have been enacted or substantively enacted.The tax consequences that would follow from the manner in which the Group expects to recover or settle the carrying amount of its assets or liabilities are also reflected in the measurement of deferred tax assets and liabilities. At year end the Group reviews the fair value of deferred tax assets to write down the balance if it is not probable that sufficient taxable income will be available to apply the tax asset. Deferred tax assets which do not meet the above conditions are not recognised in the consolidated balance sheet. At year end the Group assesses whether deferred tax assets which were previously not recognised now meet the conditions for recognition. (iv) Offset and classification The Group only offsets current tax assets and current tax liabilities if it has a legally enforceable right to set off the recognised amounts and intends either to settle on a net basis, or to realise the asset and settle the liability simultaneously. The Group only offsets deferred tax assets and liabilities where it has a legally enforceable right, where these relate to income taxes levied by the same taxation authority and where the taxation authority permits the entity to settle on a net basis, or to realise the asset and settle the liability simultaneously for each of the future years in which significant amounts of deferred tax assets or liabilities are expected to be settled or recovered. Deferred tax assets and liabilities are recognised in the consolidated balance sheet under non-current assets or liabilities, irrespective of the expected date of recovery or settlement. F-179 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (4) Significant Accounting Policies (Continued) (u) Segment reporting An operating segment is a component of the Group that engages in business activities from which it may earn revenues and incur expenses, whose operating results are regularly reviewed by the Group’s chief operating decision maker to make decisions about resources to be allocated to the segment, assess its performance and, based on which, differentiated financial information is available. (v) Classification of assets and liabilities as current and non-current The Group classifies assets and liabilities in the consolidated balance sheet as current and non-current. Current assets and liabilities are determined as follows: • Assets are classified as current when they are expected to be realised or are intended for sale or consumption in the Group’s normal operating cycle, they are held primarily for the purpose of trading, they are expected to be realised within twelve months after the reporting date or are cash or a cash equivalent, unless the assets may not be exchanged or used to settle a liability for at least twelve months after the reporting date. • Liabilities are classified as current when they are expected to be settled in the Group’s normal operating cycle, they are held primarily for the purpose of trading, they are due to be settled within twelve months after the reporting date or the Group does not have an unconditional right to defer settlement of the liability for at least twelve months after the reporting date. • Financial liabilities are classified as current when they are due to be settled within twelve months after the reporting date, even if the original term was for a period longer than twelve months, and an agreement to refinance, or to reschedule payments, on a long-term basis is completed after the reporting date and before the consolidated annual accounts are authorised for issue. (w) Environmental issues The Group takes measures to prevent, reduce or repair the damage caused to the environment by its activities. Property, plant and equipment acquired by the Group for long-term use to minimise the environmental impact of its activity and protect and improve the environment, including the reduction and elimination of future pollution from the Group’s operations, are recognised as assets applying the measurement, presentation and disclosure criteria described in note 4(g). (5) Financial Risk Management Policy (a) General The Group is exposed to the following risks associated with the use of financial instruments: • Credit risk • Liquidity risk • Market risk: includes interest rate risk, currency risk and other price risks. This note provides information on the Group’s exposure to each of these risks, the Group’s objectives and procedures to measure and mitigate this risk, and the Group’s capital management F-180 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (5) Financial Risk Management Policy (Continued) strategy. More exhaustive quantitative information is disclosed in note 31 to the consolidated annual accounts. The Group’s risk management policies are established to identify and analyse the risks faced by the Group, define appropriate risk limits and controls and to control risks and comply with limits. Risk management policies and procedures are reviewed regularly so that they reflect changes in market conditions and the Group’s activities. The Group’s management procedures and rules are designed to create a strict and constructive control environment in which all employees understand their duties and obligations. The Group’s Audit Committee supervises how management controls compliance with the Group’s risk management procedures and policies and reviews whether the risk management policy is suitable considering the risks to which the Group is exposed. This committee is assisted by Internal Audit which acts as supervisor. Internal Audit performs regular and ad hoc reviews of the risk management controls and procedures and reports its findings to the Audit Committee. Credit risk Credit risk is the risk to which the Group is exposed in the event that a customer or a counterparty to a financial instrument fails to discharge a contractual obligation, and mainly results from trade receivables and the Group’s investments in financial assets. Trade receivables The Group does not predict any significant insolvency risks as a result of delays in receiving payment from some European countries due to their current economic situation. The main risk in these countries is that of delayed payments, which is mitigated through the possibility of claiming interest as foreseen by prevailing legislation. During 2012, as a result of the condition imposed by the Spanish Government to waive late payment interest on past due receivables, the Group recognised a loss due to the waiving of interest owed by the Social Security (see note 13). No significant bad debt or late payment issues have been detected for sales to private entities. The Group recognises impairment based on its best estimate of the losses incurred on trade and other receivables. The main impairment losses recognised are due to specific losses relating to individually identified risks. At year end, these impairment losses are immaterial. Details of exposure to credit risk are disclosed in note 31. Liquidity risk Liquidity risk is the risk that the Group cannot meet its financial obligations as they fall due. The Group’s approach to managing liquidity is to ensure where possible, that it always has sufficient liquidity to settle its obligations at the maturity date, both in normal conditions and in times of tension, to avoid incurring unacceptable losses or tarnishing the Group’s reputation. The Group manages liquidity risk on a prudent basis, based on availability of cash and sufficient committed unused long-term credit facilities, enabling the Group to implement its business plans and carry out operations using stable and secure sources of financing. On 29 February 2012 the Group finished amending the terms and conditions of the senior debt agreement entered into in November 2010. In addition to the improved conditions and the elimination F-181 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (5) Financial Risk Management Policy (Continued) of certain bank covenants, the Group repaid in advance approximately US Dollars 240 million from the non-current senior debt. Subsequent to this re-financing, the Group has a Euros, US Dollars and Multicurrency revolving credit facility of Euros 149 million at 31 December 2013 (unused at year end) and a non-current loan consisting of two tranches amounting to US Dollars 2,649 million. The Group also has US Dollars 1,100 million (Euros 797 million) of corporate bonds issued in January 2011. At 31 December 2013 the Group has total cash and cash equivalents of Euros 709 million. The Group also has approximately Euros 340 million in unused credit facilities, including Euros 149 million on the revolving credit facility. Market risk Market risk comprises the risk of changes in market prices, for example, exchange rates, interest rates, or the prices of equity instruments affecting the Group’s revenues or the value of financial instruments it holds. The objective of managing market risk is to manage and control the Group’s exposure to this risk within reasonable parameters at the same time as optimising returns. (i) Currency risk The Group operates internationally and is therefore exposed to currency risk when operating with foreign currencies, especially with regard to the US Dollars. Currency risk is associated with future commercial transactions, recognised assets and liabilities, and net investments in foreign operations. The Group holds significant investments in foreign operations, the net assets of which are exposed to currency risk. The conversion risk affecting net assets of the Group’s foreign operations in US Dollars is mitigated primarily through borrowings in this foreign currency. The Group’s main exposure to currency risk is due to the US Dollars, which is used in a significant percentage of transactions in foreign functional currencies. Details of the Group’s exposure to currency risk at 31 December 2013 and 2012 of the most significant financial instruments are shown in note 31. (ii) Interest rate risk The Group’s interest rate risks arise from current and non-current borrowings. Borrowings at variable interest rates expose the Group to cash flow interest rate risks. Fixed-rate borrowings expose the Group to fair value interest rate risk. The purpose of managing interest-rate risk is to balance the debt structure, maintaining part of borrowings at fixed rates and hedging part of variable rate debt. The Group manages cash flow interest rate risks through variable to fixed interest rate swaps. A significant part of the financing obtained accrues interest at fixed rates. This fixed interest debt (corporate bond) amounts to US Dollars 1,100 million, which represents approximately 29% of the Group’s total debt in US Dollars. For the remaining senior debt in US Dollars, which totals US Dollars 2,136 million, the Group has partially contracted a variable to fixed interest rate swap. At 31 December 2013 the nominal part of this hedging instrument amounts to US Dollars 1,225 million. This nominal part will decrease over the F-182 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (5) Financial Risk Management Policy (Continued) term of the debt, based on the scheduled repayments of the principal. The purpose of these swaps is to convert borrowings at variable interest rates into fixed interest rate debt. Through these swaps the Group undertakes to exchange the difference between fixed interest and variable interest with other parties periodically. The difference is calculated based on the contracted notional amount (see notes 16 (f) and 31). The notional amount of the swap contracted by the Group hedges 57% (63% at 31 December 2012) of the senior variable interest rate debt denominated in US Dollars at 31 December 2013. Senior debt in Euros represents approximately 14% of the Group’s total debt at 31 December 2013 (14% at 31 December 2012). The total senior debt is at variable rates. In order to manage the cash flow interest rate risks a hedging operation has taken place by contracting derivative financial instruments consisting of variable to fixed interest rate swaps. The nominal part of this hedging instrument amounts to Euros 100 million, representing hedging of 27% (25% at 31 December 2012) of the senior variable interest rate debt denominated in Euros at 31 December 2013 (see notes 16 (f) and 31). The fair value of interest rate swaps contracted to reduce the impact of rises in variable interest rates (Libor and Euribor) is accounted for on a monthly basis. These derivative financial instruments comply with hedge accounting requirements. Total fixed-interest debt plus interest rate hedging represent a total of 66% of debt at 31 December 2013. (iii) Market Price risk Price risk affecting raw materials is mitigated by the vertical integration of the haemoderivatives business in a sector which is highly concentrated. The Group has signed two unquoted futures contracts, the underlying asset of which is shares in Grifols, S.A. and it was therefore exposed to risk of value fluctuations. These contracts were settled during 2012 (see note 31). (b) Capital management The directors’ policy is to maintain a solid capital base in order to ensure investor, creditor and market confidence and sustain future business development. The board of directors defines and proposes the level of dividends paid to shareholders. The directors consider various arguments to calculate capital structure: • The directors control capital performance using rates of returns on equity (ROE). In 2013, the ROE stood at 16% (14% in December 2012). The ROE is calculated by dividing profit attributable to the Parent by the equity attributable to the Parent. • In accordance with the senior secured debt contract, at 31 December 2013 the net financial debt should be 3.6 times lower than adjusted EBITDA. In 2013 the leverage ratio is 2.28 times adjusted EBITDA (2.87 times adjusted EBITDA at 31 December 2012). • Consideration of the Company’s credit rating (see note 21). The Group has no share-based payment schemes for employees. F-183 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (5) Financial Risk Management Policy (Continued) At 31 December 2013 the Group holds no treasury stock (at 31 December 2012 it had treasury stock equivalent to 0.05% of share capital). The Group does not have a formal plan for repurchasing shares. In accordance with the senior unsecured debt contract, Grifols will not be able to distribute dividends while the leverage ratio (net financial debt/adjusted EBITDA) is higher than 4.5. (6) Segment Reporting In accordance with IFRS 8 ‘‘Operating Segments’’, financial information for operating segments is reported in the accompanying Appendix II, which forms an integral part of this note to the consolidated annual accounts. Group companies are divided into three areas: companies from the industrial area, companies from the commercial area and companies from the services area. Within each of these areas, activities are organised based on the nature of the products and services manufactured and marketed. Assets, liabilities, income and expenses for segments include directly and reliably attributable items. Items which are not attributed to segments by the Group are: • Balance sheet: cash and cash equivalents, other receivables, public entities, deferred tax assets and liabilities, loans and borrowings and certain payables. • Statement of profit or loss: general administration expenses, finance result and income tax. There have been no significant inter-segment sales. (a) Operating segments The operating segments defined by the steering committee are as follows: • Bioscience: including all activities related with products deriving from human plasma for therapeutic use. • Hospital: comprising all non-biological pharmaceutical products and medical supplies manufactured by Group companies earmarked for hospital pharmacy. Products related with this business which the Group does not manufacture but markets as supplementary to its own products are also included. • Diagnostic: including the marketing of diagnostic testing equipment, reagents and other equipment, manufactured by Group or other companies. • Raw materials: including sales of intermediate biological products and the rendering of manufacturing services to third party companies. F-184 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (6) Segment Reporting (Continued) Details of net sales by groups of products for 2013 and 2012 as a percentage of net sales are as follows: Thousands of Euros 2013 2012 Bioscience Haemoderivatives . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,448,082 2,324,237 Other haemoderivatives . . . . . . . . . . . . . . . . . . . . . . . . . . 742 851 Diagnostic Transfusional medicine . . . . . . . . . . . . . . . . . . . . . . . . . . . 102,350 103,809 In vitro diagnosis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27,989 30,532 Hospital Fluid therapy and nutrition . . . . . . . . . . . . . . . . . . . . . . . . 55,553 53,556 Hospital supplies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41,578 42,315 Raw materials and others . . . . . . . . . . . . . . . . . . . . . . . . . . . 65,438 65,644 Total . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,741,732 2,620,944 The Group has concluded that the haemoderivative products are sufficiently alike to be considered as a whole for the following reasons: • All these products are human plasma derivatives and are manufactured in a similar way. • The customers and methods used to distribute these products are similar. • All these products are subject to the same regulations regarding production and the same regulatory environment. (b) Geographical information Geographical information is grouped into four areas: • United States of America and Canada • Spain • Rest of the European Union • Rest of the world For management purposes, the Group excludes the Raw Material segment from the geographical details as it relates to operations which do not form part of the Group’s core business. Sales and assets of the Raw Material segment correspond mainly to the USA. The financial information reported for geographical areas is based on sales to third parties in these markets as well as the location of assets. (c) Main customer Income from a Bioscience segment customer represents approximately 11.2% of the Group’s total income (10.3% in 2012). F-185 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (7) Goodwill Details of and movement in this caption of the consolidated balance sheet at 31 December 2012 is as follows: Thousands of Euros 2011 Business Translation 2012 Segment Balance combinations differences Balance Net value Grifols UK, Ltd. (UK) . . . . . . . . . . . . . Bioscience 8,225 0 195 8,420 Grifols Italia, S.p.A. (Italy) . . . . . . . . . Bioscience 6,118 0 0 6,118 Biomat USA, Inc. (USA) . . . . . . . . . . . Bioscience 116,748 792 (2,269) 115,271 Plasmacare, Inc. (USA) . . . . . . . . . . . . Bioscience 39,722 0 (768) 38,954 Grifols Australia Pty Ltd. (Australia) / Medion Diagnostics AG (Switzerland) . . . . . . . . . . . . . . . . . . Diagnostic 10,870 0 25 10,895 Grifols Therapeutics, Inc. (USA) . . . . . Bioscience 1,713,418 2,514 (31,691) 1,684,241 Araclon Biotech, S.L. (Spain) . . . . . . . . Diagnostic 0 6,000 0 6,000 1,895,101 9,306 (34,508) 1,869,899 (note 3 (b), (c) and (d)) Details of and movement in this caption of the consolidated balance sheet at 31 December 2013 is as follows: Thousands of Euros 2012 Business Translation 2013 Segment Balance combinations differences Balance Net value Grifols UK, Ltd. (UK) . . . . . . . . . . . . . Bioscience 8,420 0 (178) 8,242 Grifols Italia, S.p.A. (Italy) . . . . . . . . . Bioscience 6,118 0 0 6,118 Biomat USA, Inc. (USA) . . . . . . . . . . . Bioscience 115,271 0 (4,990) 110,281 Plasmacare, Inc. (USA) . . . . . . . . . . . . Bioscience 38,954 0 (1,686) 37,268 Grifols Australia Pty Ltd. (Australia)/ Medion Diagnostics AG (Switzerland) . . . . . . . . . . . . . . . . . . Diagnostic 10,895 0 (1,510) 9,385 Grifols Therapeutics, Inc. (USA) . . . . . Bioscience 1,684,241 0 (72,910) 1,611,331 Araclon Biotech, S.L. (Spain) . . . . . . . . Diagnostic 6,000 0 0 6,000 Progenika Biopharma, S.A. (Spain) . . . Diagnostic 0 40,516 0 40,516 1,869,899 40,516 (81,274) 1,829,141 (Note 3 (a)) Impairment testing: As a result of the acquisition of Talecris in 2011, and for impairment testing purposes, the Group combines the CGUs allocated to the Bioscience segment, grouping them together at segment level, because substantial synergies are expected to arise on the acquisition of Talecris, and in light of the vertical integration of the business and the lack of an independent organised market for the products. F-186 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (7) Goodwill (Continued) Because the synergies benefit the Bioscience segment globally they cannot be allocated to individual CGUs. The Bioscience segment represents the lowest level to which goodwill is allocated and is subject to control by Group management for internal control purposes. For the remaining segments CGUs identified by management are tested for impairment. The following CGUs have been identified in the Diagnostic segment as a result of the business combinations carried out by the Group: • Australia-Medion • Progenika • Araclon The recoverable amount of the CGUs was calculated based on their value in use, with the exception of Araclon Biotech, S.L and Progenika, the recoverable amount of which has been determined on the basis of fair value less costs of disposal due to the fact that the transaction is recent. The level of hierarchy used to determine the fair value has been level 2. This value in use and fair value calculations use cash flow projections for five years based on the financial budgets approved by management. Cash flows estimated as of the year in which stable growth in the CGU has been reached are extrapolated using the estimated growth rates indicated below. The key assumptions used in calculating impairment of the CGUs for 2012 were as follows: Perpetual growth rate Pre-tax discount rate Bioscience . . . . . . . . . . . . . . . . . . . . . . . . . . 2% 11.33% Diagnostic—Australia . . . . . . . . . . . . . . . . . . 2% 10.55% The key assumptions used in calculating impairment of the CGUs for 2013 have been as follows: Perpetual growth rate Pre-tax discount rate Bioscience . . . . . . . . . . . . . . . . . . . . . . . . . . 2% 10.60% Diagnostic—Australia . . . . . . . . . . . . . . . . . . 2% 9.05% Management determined budgeted gross margins based on past experience, investments in progress which would imply significant growth in production capacity and its forecast international market development. Perpetual growth rates are coherent with the forecasts included in industry reports. The discount rate used reflects specific risks related to the CGU. As the recoverable amount of the Bioscience CGU is much higher than the carrying amount of the Bioscience segment’s net assets, specific information from the impairment test sensitivity analysis is not included. Based on the results of the impairment test performed on the Australia-Medion CGU, the Group recognised impairment of Euros 13 million for goodwill in 2011. On the basis of the impairment test for 2013, a 4.5% reduction in the gross margin of projections would mean that the value in use of the business would be equal to the carrying amount of the CGU’s assets (reduction of 15% in the gross margin of projections for 2012). At 31 December 2013 Grifols’ stock market capitalisation totals Euros 10,790 million (Euros 7,784 million at 31 December 2012). F-187 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (8) Other Intangible Assets Details of other intangible assets and movement during the years ended 31 December 2013 and 2012 are included in Appendix III, which forms an integral part of these notes to the consolidated annual accounts. Intangible assets mainly include currently marketed products. Identifiable intangible assets correspond to Gamunex and have been recognised at fair value at the acquisition date of Talecris and classified as currently marketed products (see note 3(d)). Intangible assets acquired from Progenika mainly include currently marketed products. Identifiable intangible assets correspond to blood, immunology and cardiovascular genotyping. These assets have been recognised at fair value at the acquisition date of Progenika and classified as currently marketed products (see note 3(a)). The cost and accumulated amortisation of currently marketed products acquired from Talecris at 31 December 2012 is as follows: Thousands of Euros 2011 Translation 2012 Balance Additions differences Balance Cost of currently marketed products—Gamunex . . . . . . . . . . 927,429 0 (17,925) 909,504 Accumulated amortisation of currently marketed products— Gamunex . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (18,033) (31,125) 1,157 (48,001) Net value of currently marketed products—Gamunex . . . . . . 909,396 (31,125) (16,768) 861,503 The cost and accumulated amortisation of currently marketed products acquired from Talecris at 31 December 2013 is as follows: Thousands of Euros 2012 Translation 2013 Balance Additions differences Balance Cost of currently marketed products—Gamunex . . . . . . . . . . 909,504 0 (39,371) 870,133 Accumulated amortisation of currently marketed products— Gamunex . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (48,001) (30,238) 3,311 (74,928) Net value of currently marketed products—Gamunex . . . . . . 861,503 (30,238) (36,060) 795,205 Intangible assets recognised relate to currently marketed products acquired from Talecris and comprise the rights on the Gamunex product, its commercialisation and distribution licence, trademark, as well as relations with hospitals. Each of these components are closely linked and fully complementary, are subject to similar risks and have a similar regulatory approval process. The estimated useful life of the currently marketed products is considered limited, has been estimated at 30 years on the basis of the expected life cycle of the product (Gamunex) and is amortised on a straight-line basis. At 31 December 2013 the residual useful life of currently marketed products is 27 years and 5 months (28 years and 5 months at 31 December 2012). F-188 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (8) Other Intangible Assets (Continued) The cost and accumulated amortisation of currently marketed products acquired from Progenika at 31 December 2013 is as follows: Thousands of Euros 2012 Business 2013 Balance combinations Additions Balance Cost of currently marketed products . . . . . . . . . . . . . . . . . . . 0 23,792 0 23,792 Accumulated amortisation currently marketed products . . . . . 0 0 (1,983) (1,983) Net value of currently marketed products . . . . . . . . . . . . . . . 0 23,792 (1,983) 21,809 The estimated useful life of the currently marketed products is considered limited, has been estimated at 10 years on the basis of the expected life cycle of the product and is amortised on a straight-line basis. At 31 December 2013 the residual useful life of currently marketed products is 9 years and 2 months. (a) Self-constructed intangible assets At 31 December 2013 the Group has recognised Euros 19,244 thousand as self-constructed intangible assets (Euros 14,734 thousand at 31 December 2012). (b) Purchase commitments At 31 December 2013 the Group has intangible asset purchase commitments amounting to Euros 361 thousand (Euros 764 thousand at 31 December 2012). (c) Intangible assets with indefinite useful lives and development costs in progress At 31 December 2013 the Group has plasma centre licenses with indefinite useful lives under intangible assets for a carrying amount of Euros 23,833 thousand (Euros 24,921 thousand at 31 December 2012). The Group has also an amount of Euros 27,435 thousand as development costs in progress (Euros 26,254 thousand at 31 December 2012). (d) Losses on disposal of intangible assets Total losses incurred on disposals of intangible assets in 2013 amount to Euros 2.5 million (losses of Euros 6.1 million in 2012). (e) Impairment testing Indefinite-lived intangible assets have been allocated to the cash-generating unit (CGU) of the Bioscience segment. These assets have been tested for impairment together with goodwill (see note 7). (9) Property, Plant and Equipment Details of property, plant and equipment and movement in the consolidated balance sheet at 31 December 2013 and 2012 are included in Appendix IV, which forms an integral part of this note to the consolidated annual accounts. F-189 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (9) Property, Plant and Equipment (Continued) Property, plant and development under construction at 31 December 2013 and 2012 mainly comprise investments made to extend the companies’ equipment and to increase their productive capacity. (a) Insurance Group policy is to contract sufficient insurance coverage for the risk of damage to property, plant and equipment. At 31 December 2013 the Group has a combined insurance policy for all Group companies, which more than adequately covers the carrying amount of all the Group’s assets. (b) Profit on disposal of property, plant and equipment In July 2012 the Group sold the Melville fractionation plant for US Dollars 22.7 million (Euros 18.3 million) to Kedrion, generating a profit of Euros 0.6 million. The Group has a lease contract for this plant. Total losses incurred on disposals of property, plant and equipment for 2013 amount to Euros 2.1 million (Euros 7.7 million in 2012). (c) Assets under finance lease The Group had contracted the following types of property, plant and equipment under finance leases at 31 December 2012: Thousands of Euros Accumulated Carrying Cost depreciation amount Land and buildings . . . . . . . . . . . . . . . . . . . . . . . . . 2,089 (540) 1,549 Plant and machinery . . . . . . . . . . . . . . . . . . . . . . . . 31,811 (8,988) 22,823 33,900 (9,528) 24,372 The Group has contracted the following types of property, plant and equipment under finance leases at 31 December 2013: Thousands of Euros Accumulated Carrying Cost depreciation amount Land and buildings . . . . . . . . . . . . . . . . . . . . . . . . . 1,741 (524) 1,217 Plant and machinery . . . . . . . . . . . . . . . . . . . . . . . . 30,374 (10,961) 19,413 32,115 (11,485) 20,630 Details of minimum lease payments and the present value of finance lease liabilities, disclosed by maturity date, are detailed in note 21 (c). During 2011 the Group signed a number of contracts for the sale and leaseback of a production plant and the corresponding machinery and other equipment to third party companies California Biogrif 330, LP and LA 300 Biological Financing, LP, respectively. The Group also entered into a 99-year lease contract with the same lessor for the land on which the plant sold is built. The lease for the plant was considered as an operating lease while the lease for the machinery and other equipment F-190 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (9) Property, Plant and Equipment (Continued) was considered a finance lease, taking into account the terms of the related purchase option (see note 9f (ii)). (d) Self—constructed property, plant and equipment At 31 December 2013 the Group has recognised Euros 41,134 thousand as self -constructed property, plant and equipment (Euros 36,877 thousand at 31 December 2012). (e) Purchase commitments At 31 December 2013 the Group has property, plant and equipment purchase commitments amounting to Euros 35,956 thousand (Euros 24,774 thousand at 31 December 2012). (f) Sale and leaseback of buildings (i) Sale and leaseback of Spanish properties On 10 May 2011 the Group sold five properties located in Spain to Gridpan Invest, S.L., a wholly owned subsidiary of Scranton Enterprises, B.V., a shareholder of Grifols, S.A., for Euros 80.4 million (see note 32). These properties related to non-core assets such as offices, warehouses and factory premises. Two of the properties were sold together with their related mortgage loans for a total of Euros 53.5 million. As a result of this operation, the Group incurred a net loss of Euros 7.4 million in 2011, which included Euros 2 million in brokerage fees paid to a related company. The prices paid for the properties were established based on appraisals made by independent appraisers. At the same time, operating lease agreements for the aforementioned properties were entered into with Gridpan Invest, S.L., the key terms of which were as follows: • Compulsory initial term of five years • Initial rent established at market prices and subject to annual review, based on the percentage variation in the Spanish Consumer Price Index (CPI) • Automatic extensions for five-year periods that can be terminated by either party by advance six months notice. • Upon vacating the premises, Grifols will be compensated by the lessor for any on-site assets in which it has invested, insofar as these have a residual value and are not recoverable by Grifols. Grifols also signed a call option on the shares of Gridpan Invest, S.L., which is exercisable between 10 May 2016 and 10 May 2017 and for which no consideration was required. The strike price will be calculated as the exercise date market value, as determined by independent appraisers. The rental expense incurred by the Group in 2013 for these contracts amounted to Euros 8,210 thousand (Euros 8,020 thousand during 2012), coinciding fully with the minimum contractual payments. (ii) Sale and leaseback of properties, machinery and other equipment in the USA F-191 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (9) Property, Plant and Equipment (Continued) Los Angeles, CA, USA On 9 June 2011 the Group signed various contracts for the sale and leaseback of a production plant located in Los Angeles, CA, USA with its machinery and other equipment to institutional investors California Biogrif 330, LP and LA 300 Biological Financing, LP, respectively. The Group also entered into a 99-year lease contract with the same lessor for the land on which the plant sold is built. An amount of US Dollars 35.4 million (Euros 24.6 million) was received for the sale of the plant, whilst an amount of US Dollars 23.8 million (Euros 16.5 million) was received for the sale of the machinery and other equipment. The plant lease was considered an operating lease whilst the lease on the machinery and other equipment was considered a finance lease in accordance with the terms of the purchase option. As a result of the sale of the plant, the Group incurred a net loss of US Dollars 2.4 million in 2011 (Euros 1.3 million), mainly due to the expenses incurred by the Group during the operation. The main terms of the plant operating lease contract are as follows: • Compulsory initial term of 20 years • Initial rent established at market prices and subject to an annual 3% increase. On the first day of the sixth year, the rent remaining up until the 20th year will be paid in advance. • Option to extend the lease by a ten-year period at the discretion of the Grifols Group. • Awarding of purchase options in the sixth and 20th years at a market price to be determined by independent appraisers. The main terms of the finance lease contract for the machinery and other equipment are: a compulsory term of five years and sixty (60) monthly payments of US Dollars 529 thousand (Euros 369 thousand). The lease contract is non-extendable and anticipates the repurchase of the machinery and other equipment for the amount of US Dollars 1 on expiry of the lease term. The rental expense incurred by the Group in 2013 for the operating lease contracts amounted to Euros 1,812 thousand (Euros 1,878 thousand in 2012), coinciding fully with the minimum contractual payments. North Carolina, NC, USA On 29 December 2011, the Group signed a number of contracts for the sale and leaseback of certain buildings and equipment under construction (jointly denominated ‘‘New Fractionation Facility’’ or ‘‘NFF’’), located in Clayton, North Carolina (USA), with the related company Scranton Enterprises USA, Inc, (hereinafter ‘‘Scranton’’) (see note 32). The sale price was US Dollars 199 million (Euros 152 million), which has been collected as follows: • In December 2011 the Group received US Dollars 115 million (Euros 88 million). • In June 2012 the Group received the whole outstanding amount for a total of US Dollars 84 million (Euros 67 million). F-192 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (9) Property, Plant and Equipment (Continued) As a result of the transaction, the Group recognised a net loss of US Dollars 12.1 million (Euros 8,9 million) in 2011, primarily due to the brokerage fees paid to a related company, which amounted to US Dollars 10 million. The main terms of the operating lease contract for the building are as follows: • Compulsory initial lease term: eight years • The annual rent was established at a minimum of US Dollars 20.5 million, subject to annual increases in line with inflation. • Option enabling Grifols to renew and extend the contract for a further five years. • Automatic renewal for additional five-year periods unless one of the parties gives six months’ notice to the contrary. • Upon vacating the premises, Grifols will be compensated by the lessor for any on-site assets in which it has invested, insofar as these have a residual value and are not recoverable by Grifols. • Scranton Enterprises USA Inc. has required Grifols to lodge a cash or bank guarantee of US Dollars 25 million. The main terms of the lease contract for the land on which the NFF building is located are as follows: • Initial lease period: 99 years • The annual rent has been established at a minimum of US Dollars 1 per year. The Group contracted a call option on the shares of Scranton Investments, B.V., a shareholder of Scranton Enterprises USA, Inc. This option, which had a cost of US Dollars 4 million (see note 11), can be exercised on the date on which the license is granted by the Food and Drug Administration (FDA), at five and ten years from that date, and on the expiry date of the lease contract. The purchase price will vary depending on the market value determined on the date the option is exercised. The rental expense incurred by the Group in 2013 for the operating lease contracts amounted to Euros 15,811 thousand (Euros 16,037 thousand in 2012), coinciding fully with the minimum contractual payments. (g) Impairment One of the CGUs forming part of the Hospital segment has been tested for impairment due to the decrease in the results of the segment and no impairment has been observed. The recoverable amount of the mentioned CGUs is calculated based on the fair value less cost of disposal, using cash flow projections based on 5-year financial budgets approved by management. Cash flows estimated as of the year in which stable growth has been reached in the CGU are extrapolated using a pre-tax discount rate of 10.4% and a perpetual growth rate of 2%. F-193 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (10) Equity Accounted Investees Details of this caption in the consolidated balance sheet at 31 December 2013 and 2012 are as follows: Thousands of Euros % ownership 2013 2012 Nanotherapix, S.L. . . . . . . . . . . . . . . . . . . . . . . . . . 51.00% 1,354 1,387 VCN Biosciencie, S.L. . . . . . . . . . . . . . . . . . . . . . . 40.00% 802 1,179 Aradigm Corporation . . . . . . . . . . . . . . . . . . . . . . . 35.00% 21,002 0 TiGenix N.V. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 21.30% 12,443 0 Mecwins, S.L. . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25.00% 164 0 35,765 2,566 The Group has determined that it has significant influence over these investments and has not considered any of them as material. An aggregate summary of the impact on the consolidated statement of profit or loss and consolidated statement of comprehensive income is as follows: Thousands of Euros 2013 2012 Profit/(loss) Consolidated statement of profit or loss . . . . . . . . . . . . . . . . . . . (1,165) (1,407) Other consolidated comprehensive income . . . . . . . . . . . . . . . . . (359) 0 (1,524) (1,407) Aradigm Corporation On 20 May 2013 the Group announced the signing of a worldwide exclusive licensing agreement with Aradigm Corporation to develop and commercialise Pulmaquin and Lipoquin, on the condition that Grifols, S.A. would increase capital. On 27 August 2013 the Group acquired a 35% interest in Aradigm Corporation for a total of US Dollars 26 million (Euros 20.6 million) and, therefore, the exclusive worldwide licensing agreement to develop and commercialise Pulmaquin and Lipoquin became effective. All shares have the same voting and economic rights. Aradigm’s headquarters are based in Hayward, California, and its shares trade in the Nasdaq OTC BB market. Pulmaquin and Lipoquin are inhaled ciprofloxacin formulations for the treatment of severe respiratory diseases, including non-cystic fibrosis bronchiectasis. Aradigm has completed phase 2b clinical trials with Pulmaquin and Lipoquin in bronchiectasis patients. Aradigm has been granted orphan drug designation for liposomal ciprofloxacin for cystic fibrosis in the US and the EU and for the combination of liposomal ciprofloxacin and free ciprofloxacin for bronchiectasis in the US. F-194 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (10) Equity Accounted Investees (Continued) Grifols and Aradigm have agreed to advance the formulations of Pulmaquin and Lipoquin into phase III clinical trials in bronchiestasis. Pulmaquin will complement Grifols’ existing pulmonary business activity. Grifols will be responsible for all development and clinical expenses up to a maximum of US Dollars 65 million for the bronchiectasis indication. Aradigm will be entitled to receive cash payments of up to a maximum of US Dollars 25 million from Grifols, upon achievement of development milestones. Grifols will be responsible for all commercialisation activities and will pay Aradigm royalties on worldwide sales of products. In relation to this agreement, Grifols has paid an amount of US Dollars 13 million (Euros 9 million) as upfront licensing fees, which have been capitalised under ‘‘Other intangible assets’’ at 31 December 2013. The acquisition of Aradigm is accounted for as an ‘‘Investment in equity-accounted investee’’, as Grifols does not control the decisions regarding relevant activities nor the governing bodies of the company. TiGenix N.V. On 19 November 2013, the Group company Gri-Cel, S.A., acquired 21.3%, through the subscription of a capital increase with exclusion of preferential subscription right, of the biotechnology company TiGenix N.V. (hereinafter TiGenix), which is listed on NYSE Euronext Brussels (TIG), with head office in Lovaina and offices in Madrid and Sittard-Geleen (the Netherlands). TiGenix holds a 100% interest in TiGenix, S.A. (formerly Cellerix, S.A.), which engages in research and development of stem cells taken from fatty tissue. Phase III clinical trials are currently at an advanced stage for the treatment of complex perianal fistulas in patients with Crohn’s disease (‘‘Cx601’’), and the product achieved orphan drug status from the European Medicines Agency. The agreement with TiGenix envisages the appointment of two directors by Grifols and a preferential right to negotiate the development and commercialisation of any product owned by TiGenix (with the exception of ChondroCelect). The price paid for 21.30% of TiGenix was Euros 12 million. (11) Financial Assets Details of non-current financial assets on the consolidated balance sheet at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Non-current deposits and guarantees . . . . . . . . . . . . . . . . . . . . . . 3,414 3,203 Non-current derivatives (note 31) . . . . . . . . . . . . . . . . . . . . . . . . 3,155 4,502 Loans to third parties . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,962 5,420 Loan to associates (note 32) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 300 — Other non-current financial assets (note 9 (f) ii) . . . . . . . . . . . . . 3,365 3,401 Total non-current financial assets . . . . . . . . . . . . . . . . . . . . . . . . 15,196 16,526 F-195 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (11) Financial Assets (Continued) Loans to third parties primarily comprise three mortgage loans extended to the owners of several plasma centres. These loans have a term of 20 years, bear interest at fixed rates and have been secured with mortgage collateral and personal guarantees. Details of other current financial assets on the consolidated balance sheet at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Deposits and guarantees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 232 192 Loans to associates (note 32) . . . . . . . . . . . . . . . . . . . . . . . . . . . 700 — Current loans to third parties . . . . . . . . . . . . . . . . . . . . . . . . . . . 268 268 Total other current financial assets . . . . . . . . . . . . . . . . . . . . . . . 1,200 460 (12) Inventories Details of inventories at 31 December are as follows: Thousands of Euros 2013 2012 Goods for resale . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97,945 95,845 Raw materials and supplies . . . . . . . . . . . . . . . . . . . . . . . . . . 280,535 342,536 Work in progress and semi-finished goods . . . . . . . . . . . . . . . . 317,155 372,520 Finished goods . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 283,197 232,484 978,832 1,043,385 Less, inventory provision . . . . . . . . . . . . . . . . . . . . . . . . . . . . (31,919) (44,741) 946,913 998,644 Movement in the inventory provision was as follows: Thousands of Euros 2013 2012 Balance at 1 January . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 44,741 35,542 Net charge for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (10,030) 13,019 Business combinations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0 4,036 Net cancellations for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . (528) (8,567) Translation differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (2,264) 711 Balance at 31 December . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31,919 44,741 F-196 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (13) Trade and Other Receivables Details at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Trade receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 401,531 378,821 Bad debt provision (note 31) . . . . . . . . . . . . . . . . . . . . . . . . . . . (15,994) (12,799) Trade receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 385,537 366,022 Other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15,480 20,272 Receivables from associates (note 32) . . . . . . . . . . . . . . . . . . . . 27 26 Personnel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 324 315 Advances for fixed assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 590 147 Other advances . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,304 5,506 Taxation authorities, VAT recoverable . . . . . . . . . . . . . . . . . . . . 12,541 14,101 Other public entities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,245 3,466 Other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36,511 43,833 Current Income tax assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43,533 37,318 465,581 447,173 Trade receivables At the end of June 2012 the Group received an amount of Euros 157 million from the Spanish Government relating to receivables from the Social Security, of which Euros 109 million of which comprise receivables previously sold to a financial institution. The Spanish Government imposed the condition that the interest owed by the Social Security authorities should be waived, in order to collect the principal of the receivables. The Group recognised a loss of approximately Euros 11.6 million in its annual accounts for the interest claimed from the Social Security authorities which was waived and included under finance result for 2012. Other receivables During 2013 and 2012 certain Spanish companies of the Grifols Group have sold receivables from several public entities, without recourse, to certain financial institutions. Under some of these contracts, the Group receives an initial payment which usually amounts to approximately 90% of the nominal amount of the receivables sold less the associated sale and purchase costs. The deferred collection (equivalent to the rest of the nominal amount) will be made by the Group once the financial institution has collected the nominal amount of the receivables (or the interest, if the balances are received after more than 36 months, depending on the terms of each particular contract) and this amount is recognised in the balance sheet as a balance receivable from the financial institution. The deferred amount (equivalent to the continuing involvement) represents an amount of Euros 6,463 thousand at 31 December 2013 (Euros 6,132 thousand at 31 December 2012), which does not differ significantly from its fair value and coincides with the amount with maximum exposure to losses. The financial institution makes the initial payment when the sale is completed and therefore, the bad debt risk associated with this part of the nominal amount of the receivables is transferred. The Group has transferred the credit risk and control of the receivables to certain financial institutions and has F-197 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (13) Trade and Other Receivables (Continued) therefore derecognised the asset transferred, as the risks and rewards inherent to ownership have not been substantially retained. Certain foreign Group companies have also entered into a contract to sell receivables without recourse to various financial institutions. Total balances receivable without recourse sold to financial institutions through the aforementioned contracts in 2013 amount to Euros 244 million (Euros 197 million in 2012). The finance cost of these operations for the Group totals approximately Euros 6,972 thousand which has been recognised under finance result in the consolidated statement of profit or loss for 2013 (Euros 7,406 thousand in 2012) (see note 27). Details of balances with related parties are shown in note 32. (14) Other Current Assets Details of this caption of the consolidated balance sheet at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Prepaid expenses—professional services . . . . . . . . . . . . . . . . . . 5,262 5,436 Prepaid expenses—insurance . . . . . . . . . . . . . . . . . . . . . . . . . . 3,110 4,063 Prepaid expenses—leases . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,704 2,357 Other prepaid expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,113 3,104 Total other current assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17,189 14,960 (15) Cash and Cash Equivalents Details of this caption of the consolidated balance sheet at 31 December 2013 and 2012 are as follows: 2013 2012 Current deposits . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 283,546 296,437 Cash in hand and at banks . . . . . . . . . . . . . . . . . . . . . . . . . . . . 425,231 176,890 Total cash and cash equivalents . . . . . . . . . . . . . . . . . . . . . . . . . 708,777 473,327 During 2013 the Group recognised the following transactions which did not require the use of cash and/or cash equivalents: • Put and call options relating to the acquisition of Progenika Biopharma, S.A. (see note 3 (a)). • Loan of class B shares to a related party (see note 16). • Issue of new shares on 4 January 2013 (see note 16). F-198 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (16) Equity Details of consolidated equity and movement are shown in the consolidated statement of changes in equity. (a) Share capital On 4 December 2012, the shareholders of Grifols approved a share capital increase through the issue of 16,328,212 new Class B non-voting shares, with a charge to voluntary reserves. This issue was executed in a public deed on 4 January 2013 and the shares were admitted for trading on the four Spanish stock exchanges and the Spanish Automated Quotation System on 14 January 2013. On 16 April 2013 Grifols increased its share capital by issuing 884,997 Class B non-voting shares of Euros 0.10 par value each, with a share premium of Euros 23.02 per share. Therefore, the total amount of the share capital increase has been Euros 20,461 thousand, of which Euros 88 thousand corresponds to the par value and Euros 20,373 thousand to share premium. The board of directors has agreed to suppress the pre-emptive subscription rights in connection with the share capital increase. The aforementioned share capital increase has enabled Grifols to return to the lender the non-voting shares to comply with the commitment with the vendors of Progenika shares pursuant to the provisions of the share loan agreement signed in February 2013 (see note 3 (a) and section (d) of this note and note 32). At 31 December 2013, the Company’s share capital amounts to Euros 119,603,705 and comprises: • Class A shares: 213,064,899 ordinary shares of Euros 0.50 par value each, subscribed and fully paid and of the same class and series. • Class B shares: 130,712,555 non-voting preference shares of 0.10 Euros par value each, of the same class and series, and with the preferential rights set forth in the Company’s by-laws. The main characteristics of the Class B shares are as follows: • Each Class B share entitles its holder to receive a minimum annual preferred dividend out of the distributable profits at the end of each year equal to Euros 0.01 per Class B share provided that the aggregate preferred dividend does not exceed the distributable profits of that year and a distribution of dividends has been approved by the Company’s shareholders. This preferred dividend is not cumulative if sufficient distributable profits are not obtained in the period. • Each Class B share is entitled to receive, in addition to the above-mentioned preferred dividend, the same dividends and other distributions as for one Grifols ordinary share. • Each Class B share entitles the holder to its redemption under certain circumstances, if a takeover bid for all or part of the shares in the Company has been made, except if holders of Class B shares have been entitled to participate in the bid on the same terms as holders of Class A shares. The redemption terms and conditions reflected in the Company’s by-laws limit the amount that may be redeemed, requiring that sufficient distributable reserves be available, and limit the percentage of shares to be redeemed in line with the ordinary shares to which the bid is addressed. • In the event the Company were to be wound up and liquidated, each Class B share entitles the holder to receive, before any amounts are paid to holders of ordinary shares, an amount equal to the sum of (i) the par value of the Class B share, and (ii) the share premium paid for the F-199 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (16) Equity (Continued) Class B share when it was subscribed. In addition to the Class B liquidation preference amount, each holder is entitled to receive the same liquidation amount that is paid for each ordinary share. These shares are freely transferable. Since 23 July 2012 the ADSs (American Depositary Shares) representing Grifols’ Class B shares (non-voting shares) have had an exchange ratio of 1:1 in relation to Class B shares, ie.1 ADS represents 1 Class B share. The previous rate was 2 ADS per 1 Class B share. The Company’s knowledge of its shareholders is based on information provided voluntarily or in compliance with applicable legislation. According to the information available to the Company, there are no interests representing more than 10% of the Company’s total capital at 31 December 2013 and 2012: Percentage ownership 2013 2012 Capital Research and Management Company . . . . . . . . . . . . . 9.98% 9.98% Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 90.02% 90.02% 100.00% 100.00% At 31 December 2013 and 2012, the number of outstanding shares is equal to the total number of Company shares, less treasury stock. Movement in outstanding shares during 2012 is as follows: Class A shares Class B shares Balance at 1 January 2012 . . . . . . . . . . . . . . . . . . . . . . 212,906,573 113,483,514 (Acquisition) / disposal of treasury stock . . . . . . . . . . . . 0 (250) Balance at 31 December 2012 . . . . . . . . . . . . . . . . . . . 212,906,573 113,483,264 (note 16 (d)) Movement in outstanding shares during 2013 is as follows: Class A shares Class B shares Balance at 1 January 2013 . . . . . . . . . . . . . . . . . . . . . . 212,906,573 113,483,264 Capital increase with charge to reserves . . . . . . . . . . . . 0 17,213,209 (Acquisition) / disposal of treasury stock . . . . . . . . . . . . 158,326 15,429 Balance at 31 December 2013 . . . . . . . . . . . . . . . . . . . 213,064,899 130,711,902 (note 16 (d)) (b) Share premium Movement in the share premium is described in the consolidated statement of changes in equity, which forms an integral part of this note to the consolidated annual accounts. F-200 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (16) Equity (Continued) (c) Reserves The drawdown of accumulated gains is subject to legislation applicable to each of the Group companies. At 31 December 2013, Euros 49,601 thousand equivalent to the carrying amount of development costs pending amortisation of certain Spanish companies (Euros 33,097 thousand at 31 December 2012) (see note 8) are, in accordance with applicable legislation, restricted reserves which cannot be distributed until these development costs have been amortised. In February 2013 a related party lent the Group 884,997 Class B shares with a fair value of Euros 18 million, which were used to acquire Progenika (see note 3(a)). Under the Class B share loan agreement, the Group had the commitment to return the same number of Class B shares on or before 31 December 2013. On 16 April 2013 share capital was increased by a nominal amount of Euros 88,499.70, and has enabled Grifols to return the non-voting shares to the lender. In May 2013 Araclon Biotech, S.L. increased capital by an amount of Euros 7 million, Euros 6.9 million of which were subscribed by the Group. As a result, the Group has increased its investment from 51% to 61.12%. The difference between the share capital increase carried out by the Group and the non-controlling interest has been recognised as a Euros 2.8 million decrease in reserves. In November 2013 the Company sold 4,402,986 treasury stocks (ADSs), generating a profit of Euros 11.2 million, recognised in reserves. At 31 December 2013 and 2012 reserves include the IFRS-EU first-time adoption revaluation reserves and legal reserve of certain Group companies. Legal reserve Companies in Spain are obliged to transfer 10% of each year’s profits to a legal reserve until this reserve reaches an amount equal to 20% of share capital. This reserve is not distributable to shareholders and may only be used to offset losses if no other reserves are available. Under certain conditions it may be used to increase share capital provided that the balance left on the reserve is at least equal to 10% of the nominal value of the total share capital after the increase. At 31 December 2013 the legal reserve of the Company amounts to Euros 23,576 thousand (Euros 21,323 thousand at 31 December 2012). Distribution of the legal reserves of Spanish companies is subject to the same restrictions as those of the Company and at 31 December 2013 the balance of the legal reserve of other Spanish companies amounts to Euros 2,113 thousand (Euros 2,106 thousand at 31 December 2012). Other foreign Group companies have a legal reserve amounting to Euros 587 thousand at 31 December 2013 and 2012. F-201 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (16) Equity (Continued) (d) Treasury stock Movement in Class A treasury stock during 2012 is as follows: No. class A shares Thousands of Euros Balance at 1 January 2012 . . . . . . . . . . . . . . . . . 158,326 1,927 Acquisition of class A shares . . . . . . . . . . . . . . . 210,257 5,192 Disposal of class A shares . . . . . . . . . . . . . . . . . (210,257) (4,061) Balance at 31 December 2012 . . . . . . . . . . . . . . 158,326 3,058 Movement in Class B treasury stock during 2012 is as follows: No. class B shares Thousands of Euros Balance at 1 January 2012 . . . . . . . . . . . . . . . . . 15,832 0 Acquisition of class B shares . . . . . . . . . . . . . . . 250 2 Balance at 31 December 2012 . . . . . . . . . . . . . . 16,082 2 Movement in Class A treasury stock during 2013 is as follows: No. class A shares Thousands of Euros Balance at 1 January 2013 . . . . . . . . . . . . . . . . . 158,326 3,058 Acquisition of class A shares . . . . . . . . . . . . . . . 448,802 11,040 Disposal of class A shares . . . . . . . . . . . . . . . . . (607,128) (14,098) Balance at 31 December 2013 . . . . . . . . . . . . . . 0 0 Movement in Class B treasury stock during 2013 is as follows: No. class B shares Thousands of Euros Balance at 1 January 2013 . . . . . . . . . . . . . . . . . 16,082 2 Cash acquisition of class B shares . . . . . . . . . . . 6,177,372 127,788 Non-cash acquisition of class B shares . . . . . . . . 884,997 17,744 Cash disposal of class B shares . . . . . . . . . . . . . (5,307,804) (107,329) Non-cash disposal of class B shares . . . . . . . . . . (1,769,994) (38,205) Balance at 31 December 2013 . . . . . . . . . . . . . . 653 0 On 11 March 2013 Grifols S.A. purchased 4,402,986 of its American Depositary Shares (‘‘ADSs’’) from various funds managed by Cerberus Capital Management, L.P. and/or its affiliated advisory entities for a total of Euros 88.9 million (US Dollars 118.9 million, or US Dollars 27 per ADS). Grifols originally issued the ADSs to Cerberus in June 2011 in connection with the acquisition of Talecris Biotherapeutics Corp. Cerberus was the majority shareholder of Talecris. In November 2013, the Company sold all the ADSs forming part of its treasury stock. The sale generated a profit of Euros 11.2 million, which has been recognised in reserves. F-202 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (16) Equity (Continued) Cash acquisitions Class B include the purchase of the Class B shares from the vendor shareholders of Progenika for which Grifols exercised the cash option for an amount of Euros 18,399 thousand. This amount has been considered as cash used in investing activities in the statement of cash flows. Cash acquisitions also include purchases of Class B shares issued on 16 April 2013 and subscribed by a financial institution (see section (a) of this note). Non-cash acquisitions and disposals of Class B shares include a share loan transaction entered into with a related party (see note 32). Subsequent disposals include Class B shares exchanged in the acquisition of Progenika Biopharma, S.A. (see notes 3(a) and 15). Cash obtained through disposals of Class A and B shares amounts to Euros 15,286 thousand and Euros 119,903 thousand, respectively. The Parent held Class A and B treasury stock equivalent to 0.05% of its capital at 31 December 2012. The Parent does not hold any Class A treasury stock at 31 December 2013. (e) Distribution of profit The profits of Grifols, S.A. and subsidiaries will be distributed as agreed by respective shareholders at their general meetings. Grifols will not be able to distribute dividends while the leverage ratio (net financial debt/adjusted EBITDA) is higher than 4.5. The proposed distribution of profit of the Parent Grifols, S.A. for the years ended 31 December 2013 and the distribution approved for the year 2012 is as follows: Thousands of Euros 2013 2012 Legal reserve . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 344 2,254 Voluntary reserve . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29,189 48,808 Preferred dividend . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,307 1,307 Interim dividend . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68,755 0 Dividend . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68,756 0 Profit of the Parent . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 168,351 52,369 The following dividends were paid in 2013: 2013 Euros per Thousands of % of par value share Euros Ordinary shares (interim dividend) . . . . . . . . . 40% 0.2 42,612 Non-voting shares (interim dividend) . . . . . . . 200% 0.2 26,143 Non-voting shares (preferred dividend) . . . . . . 10% 0.01 1,307 Total dividends paid . . . . . . . . . . . . . . . . . . . . 70,062 F-203 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (16) Equity (Continued) At the general meeting held on 24 May 2013, the shareholders of Grifols approved the distribution of interim dividend for 2013 of Euros 0.20 for each Class A and B share, recognising a total of Euros 68,755 thousand as interim dividend. These amounts to be distributed did not exceed the profits generated by the Company since the end of the last reporting period, less the estimated income tax payable on these profits, in accordance with article 277 of the Revised Spanish Companies Act. The Statement of Liquidity for Distribution of Interim Dividend 2013 prepared in accordance with legal requirements and which shows the existence of sufficient liquidity to be able to distribute the aforementioned dividend is provided in Appendix V. At a general meeting held on 24 May 2013 the shareholders approved the distribution of a preferred dividend of Euros 0.01 for every Class B non-voting share. The distribution of the profit for the year ended 31 December 2012 is presented in the consolidated statement of changes in equity. (f) Cash flow hedges In June and October 2011 Grifols contracted variable to fixed interest-rate swaps for initial nominal amounts of US Dollars 1,550 million and Euros 100 million, respectively, to hedge interest-rate risk on its senior debt. The Group has recognised these financial derivatives as cash flow hedges (see notes 5 (a) and 31). Ineffective cash flow hedges recognised as finance income and cost in the consolidated statement of profit or loss (consolidated statement of comprehensive income) for 2013 amount to Euros 1,015 thousand (Euros 226 thousand in 2012). (17) Earnings Per Share The calculation of basic earnings per share is based on the profit for the year attributable to the shareholders of the Parent divided by the weighted average number of ordinary shares in circulation throughout the year, excluding treasury stock. Details of the calculation of basic earnings per share are as follows: Thousands of Euros 2013 2012 Profit for the period attributable to equity holders of the Parent (thousands of Euros) . . . . . . . . . . . . . . . . . . . . 345,551 256,686 Weighted average number of ordinary shares outstanding . . 340,505,298 342,701,194 Basic earnings per share (Euros per share) . . . . . . . . . . . 1.01 0.75 F-204 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (17) Earnings Per Share (Continued) The weighted average number of ordinary shares issued (basic and diluted) is determined as follows: No. of shares 2013 2012 Ordinary shares outstanding at 1 January . . . . . . . . . . . . 342,708,823 342,709,051 Effect of shares issued . . . . . . . . . . . . . . . . . . . . . . . . . 627,984 0 Effect of treasury stock . . . . . . . . . . . . . . . . . . . . . . . . . (2,831,509) (7,857) Weighted average number of ordinary shares outstanding at 31 December . . . . . . . . . . . . . . . . . . . . . . . . . . . . 340,505,298 342,701,194 Diluted earnings per share are calculated by dividing profit for the year attributable to shareholders of the Parent by the weighted average number of ordinary shares in circulation considering the diluting effects of potential ordinary shares. At 31 December 2013 and 2012 basic and diluted earnings per share are the same as no potential diluting effects exist. (18) Non-Controlling Interests Details of non-controlling interests and movement at 31 December 2012 are as follows: Thousands of Euros Business combinations/ Additions to 2011 consolidated Translation 2012 Balance Additions Group Disposals differences Balance Grifols (Thailand) Pte Ltd . . . . . . . . . 1,770 22 0 (59) 28 1,761 Grifols Malaysia Sdn Bhd . . . . . . . . . . 717 (16) 0 0 12 713 Araclon Biotech, S.A. . . . . . . . . . . . . 0 (1,316) 2,188 0 0 872 Medion Grifols Diagnostic AG . . . . . . 0 23 0 0 5 28 GRI-CEI S/A Productos para transfusao . . . . . . . . . . . . . . . . . . . . 0 (22) 679 0 (58) 599 2,487 (1,309) 2,867 (59) (13) 3,973 F-205 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (18) Non-Controlling Interests (Continued) Details of non-controlling interests and movement at 31 December 2013 are as follows: Thousands of Euros Business combinations/ Additions to 2012 consolidated Capital Translation 2013 Balance Additions Group increases Disposals differences Balance Grifols (Thailand) Pte Ltd . . . . . . . . . 1,761 (18) 0 0 (6) (183) 1,554 Grifols Malaysia Sdn Bhd . . . . . . . . . . 713 74 0 0 0 (86) 701 Araclon Biotech, S.L. . . . . . . . . . . . . 872 (2,955) 0 2,895 0 0 812 Medion Grifols Diagnostic AG . . . . . . 28 (309) 0 0 0 (1) (282) GRI-CEI S/A Productos para transfusao . . . . . . . . . . . . . . . . . . . 599 (5) 0 1,547 0 (420) 1,721 Progenika Biopharma, S.A. . . . . . . . . 0 14 1,093 0 0 8 1,115 Brainco Biopharma, S.L. . . . . . . . . . . 0 (283) 664 0 0 0 381 Abyntek Biopharma, S.L. . . . . . . . . . . 0 (15) (45) 0 0 0 (60) 3,973 (3,497) 1,712 4,442 (6) (682) 5,942 (note 3 (a)) (19) Grants Details are as follows: Thousands of Euros 2013 2012 Capital grants . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,977 4,826 Interest rate grants (preference loans) . . . . . . . . . . . . . . . . . . . . . . . 1,057 1,029 7,034 5,855 F-206 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (19) Grants (Continued) Details of capital grants are as follows: Thousands of Euros 2013 2012 Total grant amount: Prior years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10,351 6,144 Current year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,864 4,207 12,215 10,351 Less, income recognised: In prior years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (5,309) (4,781) During the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (558) (528) (5,867) (5,309) Translation differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (371) (216) Carrying amount of capital grants . . . . . . . . . . . . . . . . . . . . . . . . . 5,977 4,826 Interest-rate grants (preference loans) reflect the implicit interest on loans extended by the Spanish Ministry of Science and Technology as these are interest free. Movement for 2012 is as follows: Thousands of Euros 2011 Transfer to 2012 Balance Additions profit and loss Balance Interest rate grants (preference loans) . . . 208 1,255 (434) 1,029 Movement for 2013 is as follows: Thousands of Euros 2012 Transfer to 2013 Balance Additions profit and loss Balance Interest rate grants (preference loans) . . . 1,029 600 (572) 1,057 F-207 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (20) Provisions Details of provisions at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Non-current provisions(a) Provisions for pensions and similar obligations . . . . . . . . . . . . . 2,595 2,049 Other provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,607 1,299 Non-current provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,202 3,348 Thousands of Euros 2013 2012 Current provisions(b) Trade provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51,459 55,139 Current provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51,459 55,139 (a) Non-current provisions At 31 December 2013 and 2012 provisions for pensions and similar obligations mainly comprise a provision made by certain foreign subsidiaries in respect of labour commitments with certain employees. Movement in provisions during 2012 is as follows: Thousands of Euros 2011 Net Translation 2012 Balance charge Cancellations Reclassifications differences Balance Non-current provisions . . . . . . . . . . 11,052 (695) (470) (6,641) 102 3,348 11,052 (695) (470) (6,641) 102 3,348 Movement in provisions during 2013 is as follows: Thousands of Euros 2012 Net Translation 2013 Balance charge Cancellations differences Balance Non-current provisions . . . . . . . . . . . . . . . . . . . . . . 3,348 1,776 (854) (68) 4,202 3,348 1,776 (854) (68) 4,202 F-208 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (20) Provisions (Continued) (b) Current provisions Movement in trade provisions during 2012 is as follows: Thousands of Euros 2011 Business Net Translation 2012 Balance Combination charge Cancellations Reclassifications differences Balance Trade provisions . . . . . 81,112 773 (2,158) (37,758) 12,601 569 55,139 81,112 773 (2,158) (37,758) 12,601 569 55,139 (note 3(d)) Movement in trade provisions during 2013 is as follows: Thousands of Euros 2012 Business Net Translation 2013 Balance Combination charge Cancellations differences Balance Trade provisions . . . . . . . . . . . . . . . . . 55,139 37 418 (2,050) (2,085) 51,459 55,139 37 418 (2,050) (2,085) 51,459 (note 3(a)) (21) Financial Liabilities This note provides information on the contractual conditions of the loans obtained by the Group, which are measured at amortised cost, except the financial derivatives, which are measured at fair value. For further information on exposure to interest rate risk, currency risk and liquidity risk and the fair values of financial liabilities, please refer to note 31. F-209 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (21) Financial Liabilities (Continued) Details at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Financial liabilities Non-current obligations(a) . . . . . . . . . . . . . . . . . . . . . . . . . . 717,590 727,608 Senior secured debt(b) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,677,607 1,807,339 Other loans(b) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30,680 33,449 Finance lease liabilities(c) . . . . . . . . . . . . . . . . . . . . . . . . . . 12,099 17,592 Financial derivatives (note 31) . . . . . . . . . . . . . . . . . . . . . . . 68,033 93,515 Other non-current financial liabilities(e) . . . . . . . . . . . . . . . . 47,202 11,316 Total non-current financial liabilities . . . . . . . . . . . . . . . . . . 2,553,211 2,690,819 Current obligations(a) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 72,629 42,968 Senior secured debt(b) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 112,422 83,659 Other loans(b) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 56,568 55,703 Finance lease liabilities(c) . . . . . . . . . . . . . . . . . . . . . . . . . . 7,087 7,005 Other current financial liabilities(e) . . . . . . . . . . . . . . . . . . . 9,438 6,243 Total current financial liabilities . . . . . . . . . . . . . . . . . . . . . 258,144 195,578 (a) Bonds On 13 January 2011, the Group concluded its planned issue of High Yield Senior Unsecured Notes for an amount of US Dollars 1,100 million, with a seven-year maturity period (2018) and an annual coupon of 8.25%. This issue, in conjunction with the already completed syndicated loan described in paragraphs below enabled the Group to obtain the necessary funds to finance the acquisition of Talecris (see note 3 (d)) on 2 June 2011. In November 2011 the Group registered its corporate notes in the Securities Exchange Commission (SEC) using form F4. On 2 June 2011 and in accordance with the requirements of the new credit agreement, the Group cancelled corporate bonds amounting to US Dollars 600 million and recognised all the associated transaction costs in profit or loss. The costs of cancelling the corporate bonds amounted to Euros 112 million. These costs were included as transaction costs as this was one of the necessary requirements for obtaining additional financing. These costs, together with other costs deriving from the debt issue (underwriting fees, ticking fees, closing fees etc.) were deferred as transaction costs and will be taken to profit or loss in accordance with the effective interest rate. Unamortised financing costs of High Yield Senior Unsecured Notes amounted to Euros 80 million at 31 December 2013 (Euros 106 million at 31 December 2012). F-210 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (21) Financial Liabilities (Continued) Details of movement in the High Yield Senior Unsecured Notes at 31 December 2012 are as follows: Thousands of Euros Opening Closing outstanding outstanding balance Translation balance 01/01/12 differences 31/12/2012 High Yield Senior Unsecured Notes (nominal amount) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 850,143 (16,431) 833,712 Total . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 850,143 (16,431) 833,712 Details of movement in the High Yield Senior Unsecured Notes at 31 December 2013 are as follows: Thousands of Euros Opening Closing outstanding outstanding balance Translation balance 01/01/13 differences 31/12/2013 High Yield Senior Unsecured Notes (nominal amount) . . . . . . . . . . . . . . . . . . . . . . . . . . . . 833,712 (36,090) 797,622 Total . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 833,712 (36,090) 797,622 At 31 December 2013 and 2012 the current bonds caption includes the issue of bearer promissory notes to Group employees, as follows: 2012 Nominal Promissory amount of notes Interest pending promissory subscribed Buy back accrual Issue Maturity notes Interest (Thousands (Thousands (Thousands of date date (Euros) rate of Euros) of Euros) Euros) Issue of bearer promissory notes . . 04/05/12 04/05/13 3,000 5.00% 14,703 (156) (238) 2013 Nominal Promissory amount of notes Interest pending promissory subscribed Buy back accrual Issue Maturity notes Interest (Thousands (Thousands (Thousands of date date (Euros) rate of Euros) of Euros) Euros) Issue of bearer promissory notes . . 04/05/13 04/05/14 3,000 5.00% 43,830 2,115 (733) F-211 |
|
|
LES]UNIVERSAL.BST;124 F-212 Notes to the Consolidated Annual Accounts (Continued) (21) Financial Liabilities (Continued) (b) Loans and borrowings Details of loans and borrowings at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Date Maturity Amount Carrying Amount Carrying Credit Currency Interest rate awarded date extended amount extended amount Senior debt—Tranche A . . . . . . . . . . . . . . . . . . . . . . Euros Euribor + 3.5% 23/11/10 01/06/16 220,000 143,000 220,000 176,000 Senior debt—Tranche B . . . . . . . . . . . . . . . . . . . . . . Euros Euribor + 3.5% 23/11/10 01/06/17 200,000 194,000 200,000 196,000 Senior debt—Tranche A . . . . . . . . . . . . . . . . . . . . . . US Dollars Libor + 3.25% 23/11/10 01/06/16 435,066 282,793 454,752 363,802 Senior debt—Tranche B . . . . . . . . . . . . . . . . . . . . . . US Dollars Libor + 3.5% 23/11/10 01/06/17 1,232,688 1,184,831 1,288,464 1,255,116 Total senior debt . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,087,754 1,804,624 2,163,216 1,990,918 Revolving Credit . . . . . . . . . . . . . . . . . . . . . . . . . . . Euros Euribor + 3.25% 23/11/10 01/06/16 21,700 — 21,700 — Revolving Credit . . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars Libor + 3.25% 23/11/10 01/06/16 25,379 — 26,527 — Revolving Credit . . . . . . . . . . . . . . . . . . . . . . . . . . . Multicurrency Libor + 3.25% 23/11/10 01/06/16 101,515 — 106,109 — Total Revolving Credit . . . . . . . . . . . . . . . . . . . . . . . 148,594 — 154,336 — Other non-current loans . . . . . . . . . . . . . . . . . . . . . . Euros Euribor-Euribor+4% 30/07/09 25/06/20 39,800 30,707 39,310 33,449 Loan transaction costs . . . . . . . . . . . . . . . . . . . . . . . — (127,044) — (183,579) Non-current loans and borrowings . . . . . . . . . . . . . . . . 2,276,148 1,708,287 2,356,862 1,840,788 Senior debt—Tranche A . . . . . . . . . . . . . . . . . . . . . . Euros Euribor + 3.5% 23/11/10 01/06/16 (*) 33,000 (*) 23,375 Senior debt—Tranche B . . . . . . . . . . . . . . . . . . . . . . Euros Euribor + 3.5% 23/11/10 01/06/17 (*) 2,000 (*) 200 Senior debt—Tranche A . . . . . . . . . . . . . . . . . . . . . . US Dollars Libor + 3.25% 23/11/10 01/06/16 (*) 65,260 (*) 48,317 Senior debt—Tranche B . . . . . . . . . . . . . . . . . . . . . . US Dollars Libor + 3.5% 23/11/10 01/06/17 (*) 15,952 (*) 16,674 Total senior debt . . . . . . . . . . . . . . . . . . . . . . . . . . . — 116,212 — 88,566 Other current loans . . . . . . . . . . . . . . . . . . . . . . . . . 1.04%-12% 235,700 56,794 192,466 57,503 Loan transaction costs . . . . . . . . . . . . . . . . . . . . . . . — (4,016) — (6,707) Current Loans and Borrowings . . . . . . . . . . . . . . . . . . 235,700 168,990 192,466 139,362 (*) See amount granted under non-current debt 3 C Cs: 45020 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (21) Financial Liabilities (Continued) Current loans and borrowings include accrued interest amounting to Euros 332 thousand (Euros 338 thousand at 31 December 2012). On 23 November 2010 the Company signed senior debt agreements of US Dollars 3,400 million for the purchase of Talecris. On 29 February 2012 the Company closed the negotiations to amend and improve the terms and conditions of the senior debt. The present value discounted from cash flows under the new agreement, including costs for fees paid and discounted using the original effective interest rate differs by less than 10% of the present value discounted from cash flows remaining in the original debt, whereby the new agreement is not substantially any different to the original agreement. The costs of refinancing the senior debt amounted to Euros 43.8 million. The modification of the terms in the embedded derivatives of the senior debt formed part of the refinancing (see note 31) and the resulting change in the present values amounting to Euros 65 million have reduced the financing cost. Based on an analysis of the quantitative and qualitative factors, the Group concluded that the renegotiation of conditions of the senior debt did not trigger a derecognition of the liability. Therefore, the net amount of the financing cost reduced the previous amount recognised and will form part of the amortised cost over the duration of the debt. Unamortised financing costs from the senior secured debt amount to Euros 131 million at 31 December 2013 (Euros 190 million at 31 December 2012). The main amendments were basically as follows: • Reduction of interest rates, retranching (US Dollars 600 million from Tranche A to Tranche B) and modification of the embedded floor; • Removal of covenants relating to limitations in fixed assets investments and the debt service coverage ratio; • Amendment to the leverage ratio limiting the distribution of dividends, improving from the current 3.75 to the new ratio of 4.5, as well as relaxing certain conditions relating to certain contracts; The Group repaid in advance approximately US Dollars 240 million from the non-current senior debt during 2012. Details of the Tranche A principal by maturity at 31 December 2013 are as follows: Tranche A in US Dollars Tranche A in Euros Amortisation Amortisation Amortisation in thousands in thousands in thousands Currency of US Dollars of Euros Currency of Euros Maturity 2014 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 90,000 65,260 Euros 33,000 2015 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 292,500 212,095 Euros 107,250 2016 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 97,500 70,698 Euros 35,750 Total . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 480,000 348,053 Euros 176,000 F-213 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (21) Financial Liabilities (Continued) Details of the Tranche B principal by maturity at 31 December 2013 are as follows: Tranche B in US Dollars Tranche B in Euros Amortisation Amortisation Amortisation in thousands in thousands in thousands Currency of US Dollars of Euros Currency of Euros Maturity 2014 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 22,000 15,952 Euros 2,000 2015 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 22,000 15,952 Euros 2,000 2016 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 22,000 15,952 Euros 2,000 2017 . . . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 1,590,000 1,152,927 Euros 190,000 Total . . . . . . . . . . . . . . . . . . . . . . . . US Dollars 1,656,000 1,200,783 Euros 196,000 The issue of High Yield Senior Unsecured Notes and senior debt is subject to compliance with certain covenants: leverage ratio and interest coverage ratio. At 31 December 2013 the Group complies with these financial covenants. In addition, the Company and Grifols Inc. have pledged their assets as collateral, and the shares of certain group companies have been pledged, to guarantee repayment of the senior debt. Grifols will not be able to distribute dividends while the leverage ratio (net financial debt/adjusted EBITDA) is higher than 4.5. Grifols, S.A., Grifols Inc. and other significant Group companies, act as guarantor for the corporate bonds (HYB). Significant Group companies are those companies that contribute 85% of earnings before interest, tax, depreciation and amortisation (EBITDA), 85% of the Group’s consolidated assets and 85% of total revenues, and those companies that represent more than 3% of the above mentioned indicators. (c) Finance lease liabilities Details of minimum payments and the present value of finance lease liabilities, by maturity date, are as follows: Thousands of Euros 2013 2012 Minimum Present Minimum Present payments Interest value payments Interest value Maturity at: Less than one year . . . . . . . . . . . . . . . . . . . . 8,519 1,432 7,087 9,119 2,114 7,005 Two years . . . . . . . . . . . . . . . . . . . . . . . . . . . 7,184 870 6,314 8,492 1,524 6,968 Three years . . . . . . . . . . . . . . . . . . . . . . . . . . 3,650 327 3,323 6,815 838 5,977 Four years . . . . . . . . . . . . . . . . . . . . . . . . . . 1,391 195 1,196 3,250 269 2,981 Five years . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,077 102 975 957 120 837 More than 5 years . . . . . . . . . . . . . . . . . . . . . 311 20 291 880 51 829 Total . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 22,132 2,946 19,186 29,513 4,916 24,597 F-214 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (21) Financial Liabilities (Continued) (d) Credit rating On 15 July 2013 Moody’s Investors Service upgraded Grifols’ corporate credit rating to Ba2, its senior debt rating to Ba1 and its corporate bond rating to B1, with a positive outlook. As a result of the acquisition of the transfusion diagnostics and immunology business from the Swiss company Novartis International ADG on 21 November 2013, Moody’s Investors Service re-confirmed Grifols’ corporate rating and changed the outlook to negative. On 13 November 2013 Standard & Poor’s confirmed the global corporate credit rating of Grifols, issued on 1 August 2012, at BB, with senior secured debt being BB+ and the corporate bond being B+. All the ratings have a stable outlook. (e) Other financial liabilities At 31 December 2013 other financial liabilities include interest-free loans extended by governmental institutions amounting to Euros 22,282 thousand (Euros 12,660 thousand at 31 December 2012). The portion of the loans considered a grant and still to be taken to profit or loss amounts to Euros 1,057 thousand (Euros 1,029 thousand at 31 December 2012) (see note 19). At 31 December 2013 other non-current financial liabilities include Euros 27,624 thousand relating to the put and call option extended by the Group and the shareholders of Progenika (see note 3(a)). At 31 December 2013 and 2012 other current financial liabilities also include approximately Euros 3,955 thousand and Euros 2,631 thousand, respectively, which have been collected directly from Social Security affiliated bodies and transferred to financial institutions (see note 13). Details of the maturity of other financial liabilities are as follows: Thousands of Euros 2013 2012 Maturity at: Up to one year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9,438 6,243 Two years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,195 3,092 Three years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26,242 2,396 Four years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,318 1,950 Five years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7,352 1,572 Over five years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6,095 2,306 56,640 17,559 F-215 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (22) Trade and Other Payables Details are as follows: Thousands of Euros 2013 2012 Suppliers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 273,621 228,405 VAT payable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,608 5,518 Tax authorities, withholdings payable . . . . . . . . . . . . . . . . . . . . . 4,062 3,798 Social security payable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,938 3,745 Other public entities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23,780 14,296 Other payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42,388 27,357 Current tax liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,934 5,679 318,943 261,441 Suppliers Details of balances with related parties are shown in note 32. The Group’s exposure to currency risk and liquidity risk associated with trade and other payables is described in note 31. Late payments to suppliers in Spain. ‘‘Reporting Requirement’’ Third Additional Provision of Law 15/2010 of 5 July: Payments made and outstanding at the reporting date 2013 2012 Thousands of Euros % Thousands of Euros % Within maximum legal period . . . . . . . . . . . . . . . . . . 118,728 38% 128,423 40% Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 193,173 62% 195,509 60% Total payments for the year . . . . . . . . . . . . . . . . . . . 311,901 100% 323,932 100% Average weighted payment period exceeded (days) . . 46 — 25 — Late payments exceeding the maximum legal period at balance sheet date (Thousands of Euros) . . . . . . 16,853 8,728 (23) Other Current Liabilities Details at 31 December are as follows: Thousands of Euros 2013 2012 Salaries payable . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 75,421 75,122 Other payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,183 2,917 Deferred income . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,395 0 Advances received . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,068 0 Other current liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84,067 78,039 F-216 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (24) Net revenues Net revenues are mainly generated by the sale of goods. The distribution of net consolidated revenues for 2013 and 2012 by segment is as follows: Thousands of Euros 2013 2012 Bioscience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,448,824 2,325,088 Diagnostic . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 130,339 134,342 Hospital . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 97,131 95,870 Raw materials and others . . . . . . . . . . . . . . . . . . . . . . . . . . . 65,438 65,644 2,741,732 2,620,944 The geographical distribution of net consolidated revenues is as follows: Thousands of Euros 2013 2012 USA and Canada . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,707,620 1,658,548 Spain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 207,922 212,983 European Union . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 361,905 346,345 Rest of the world . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 426,257 371,618 Sub total . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,703,704 2,589,494 Raw materials . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38,028 31,450 Consolidated . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,741,732 2,620,944 Details of discounts and other reductions to gross income are as follows: Thousands of Euros 2013 2012 Gross sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,915,496 2,741,405 Chargebacks . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (58,065) (34,102) Cash discount . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (28,831) (27,447) Volume rebates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (50,505) (29,391) Medicare and Medicaid . . . . . . . . . . . . . . . . . . . . . . . . . . (18,961) (16,332) Other discounts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (17,402) (13,189) Net sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,741,732 2,620,944 F-217 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (24) Net revenues (Continued) Movement in discounts and other reductions to gross income during 2012 were as follows: Thousands of Euros Cash Volume Medicare/ Other Chargebacks discount rebates Medicaid discounts Total Balance sheet at 31 December 2011 . . . 3,537 1,786 8,431 8,708 679 23,141 Current estimate related to sales made in current and prior year . . . . . . . . . 34,102 27,447 29,391 16,332 13,189 120,461 (1) (Actual returns or credits in current period related to sales made in current period) . . . . . . . . . . . . . . . . (27,655) (25,277) (20,345) (10,212) (13,189) (96,678)(2) (Actual returns or credits in current period related to sales made in prior periods) . . . . . . . . . . . . . . . . . . . . . . (3,663) (1,645) (9,841) (8,495) (679) (24,323)(3) Translation differences . . . . . . . . . . . . . (15) (191) 2,683 451 (30) 2,898 Balance sheet at 31 December 2012 . . . 6,306 2,120 10,319 6,784 (30) 25,499 Movement in discounts and other reductions to gross income during 2013 were as follows: Thousands of Euros Cash Volume Medicare/ Other Chargebacks discounts rebates Medicaid discounts Total Balance sheet at 31 December 2012 . . 6,306 2,120 10,319 6,784 (30) 25,499 Current estimate related to sales made in current and prior year . . . . . . . . . 58,065 28,831 50,505 18,961 17,402 173,764 (1) (Actual returns or credits in current period related to sales made in current period) . . . . . . . . . . . . . . . . (41,209) (25,428) (33,510) (15,948) (17,167) (133,262)(2) (Actual returns or credits in current period related to sales made in prior periods) . . . . . . . . . . . . . . . . . . . . . (5,201) (2,112) (8,252) (1,901) 27 (17,439)(3) Translation differences . . . . . . . . . . . . (983) (144) (765) (339) (22) (2,253) Balance sheet at 31 December 2013 . . 16,978 3,267 18,297 7,557 210 46,309 (1) Net impact on statement of profit or loss: estimate for current period plus prior year’s adjustments. Adjustments made during the year corresponding to prior year’s estimates have not been significant. (2) Amounts credited and posted against provisions for current period. (3) Amounts credited and posted against provisions for prior period. F-218 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (25) Personnel Expenses Details of personnel expenses by function are as follows: Thousands of Euros 2013 2012 Cost of sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 412,660 410,382 Research and development . . . . . . . . . . . . . . . . . . . . . . . . . . . 57,012 59,925 Selling, general & administrative expenses . . . . . . . . . . . . . . . . 203,944 193,631 673,616 663,938 Details by nature are as follows: Thousands of Euros 2013 2012 Salaries and wages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 549,703 534,554 Contributions to pension plans (note 30) . . . . . . . . . . . . . . . . . 10,233 10,637 Other social charges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14,059 13,803 Social Security . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 99,621 104,944 673,616 663,938 The average headcount during 2013 and 2012, by department, was approximately as follows: Average headcount 2013 2012 Manufacturing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9,095 8,571 Research & development—technical area . . . . . . . . . . . . . . . . . 666 693 Administration and others . . . . . . . . . . . . . . . . . . . . . . . . . . . . 874 777 General management . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 149 147 Marketing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 147 133 Sales and distribution . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 848 787 11,779 11,108 F-219 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (25) Personnel Expenses (Continued) The headcount of the Group and the Company’s board of directors at 31 December 2012, by gender, is as follows: 2012 Total number Male Female of employees Directors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 1 11 Manufacturing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,991 4,801 8,792 Research & development—technical area . . . . . . . . . . . 280 388 668 Administration and others . . . . . . . . . . . . . . . . . . . . . . 451 410 861 General management . . . . . . . . . . . . . . . . . . . . . . . . . 76 80 156 Marketing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70 66 136 Sales and distribution . . . . . . . . . . . . . . . . . . . . . . . . . 469 325 794 5,347 6,071 11,418 The headcount of the Group and the Company’s board of directors at 31 December 2013, by gender, is as follows: 2013 Total number Male Female of employees Directors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 10 2 12 Manufacturing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,295 5,516 9,811 Research & development—technical area . . . . . . . . . . . 266 420 686 Administration and others . . . . . . . . . . . . . . . . . . . . . . 491 417 908 General management . . . . . . . . . . . . . . . . . . . . . . . . . 82 91 173 Marketing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 76 83 159 Sales and distribution . . . . . . . . . . . . . . . . . . . . . . . . . 503 363 866 5,723 6,892 12,615 (26) Expenses by Nature (a) Amortisation and depreciation Expenses for the amortisation and depreciation of intangible assets and property, plant and equipment, incurred during 2013 and 2012 classified by functions are as follows: Thousands of Euros 2013 2012 Cost of sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 69,091 66,200 Research and development . . . . . . . . . . . . . . . . . . . . . . . . . . . 12,018 9,693 Selling, general & administrative expenses . . . . . . . . . . . . . . . . 47,360 53,233 128,469 129,126 F-220 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (26) Expenses by Nature (Continued) (b) Other operating income and expenses Other operating expenses and income incurred during 2013 and 2012 by function are as follows: Thousands of Euros 2013 2012 Cost of sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 202,860 210,817 Research and development . . . . . . . . . . . . . . . . . . . . . . . . . . . 54,854 54,673 Selling, general & administrative expenses . . . . . . . . . . . . . . . . 344,215 308,738 601,929 574,228 Details by nature are as follows: Thousands of Euros 2013 2012 Change in trade provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,168 9,135 Professional services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 121,467 99,641 Commissions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18,327 19,780 Supplies and auxiliary materials . . . . . . . . . . . . . . . . . . . . . . . . 78,993 80,461 Operating leases (note 29) . . . . . . . . . . . . . . . . . . . . . . . . . . . 69,522 67,991 Freight . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54,177 52,280 Repair and maintenance expenses . . . . . . . . . . . . . . . . . . . . . . 55,242 50,256 Advertising . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 48,115 43,429 Insurance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16,178 16,745 Royalties . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3,831 5,824 Travel expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33,258 27,353 External services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43,681 49,222 Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 53,970 52,111 Other operating expenses . . . . . . . . . . . . . . . . . . . . . . . . . . . . 601,929 574,228 F-221 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (27) Finance Result Details are as follows: Thousands of Euros 2013 2012 Finance income . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,869 1,677 Finance costs from High Yield Senior Unsecured Notes (note 21) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (91,002) (96,711) Finance costs from senior debt -tranche A (note 21) . . . . . . . . (38,308) (58,731) Finance costs from senior debt -tranche B (note 21) . . . . . . . . (95,172) (103,687) Finance costs from sale of receivables (note 13) . . . . . . . . . . . (6,972) (7,406) Capitalised interest . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9,131 7,344 Other finance costs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (17,668) (24,926) Finance costs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (239,991) (284,117) Change in fair value of financial derivatives (note 31) . . . . . . . (1,786) 13,013 Impairment and gains/(losses) on disposal of financial instruments . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 792 2,107 Exchange differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,303) (3,409) Finance result . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (237,419) (270,729) During 2013 the Group has capitalised interest at a rate of between 4.4% and 6.2% based on the financing received (between 4.7% and 6.5% during 2012) (see note 4 (f)). (28) Taxation Grifols, S.A. is authorised to present a consolidated tax return with Diagnostic Grifols, S.A., Movaco, S.A., Laboratorios Grifols, S.A., Instituto Grifols, S.A., Logister, S.A., Biomat, S.A., Grifols Viajes, S.A., Grifols International, S.A., Grifols Engineering, S.A., Arrahona Optimus, S.L. and Gri-Cel, S.A. Grifols, S.A., in its capacity as Parent, is responsible for the presentation and payment of the consolidated tax return. Under prevailing tax law, the Spanish companies pay 30% tax, which may be reduced by certain deductions. The North American company Grifols Inc. is also authorised to present consolidated tax returns in the USA with Grifols Biologicals Inc., Grifols USA, LLC., Biomat USA, Inc., Plasmacare, Inc, Grifols Therapeutics Inc. and Talecris Plasma Resources, Inc. The profits of the companies domiciled in the USA, determined in accordance with prevailing tax legislation, are subject to tax of approximately 37.5% of taxable income, which may be reduced by certain deductions. F-222 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (28) Taxation (Continued) (a) Reconciliation of accounting and taxable income Details of the income tax expense and income tax related to profit for the year are as follows: Thousands of Euros 2013 2012 Profit for the year before income tax . . . . . . . . . . . . . . . . . . . . 497,536 387,948 Tax at 30%. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 149,261 116,384 Permanent differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (3,771) 3,965 Effect of different tax rates in US companies . . . . . . . . . . . . . . 23,216 24,291 Effect of different tax rates in other countries . . . . . . . . . . . . . 5,734 3,172 Tax credits (deductions) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (24,465) (16,632) Prior year income tax expense . . . . . . . . . . . . . . . . . . . . . . . . . (2,175) (1,677) Other income tax expenses/(income) . . . . . . . . . . . . . . . . . . . . 7,682 3,068 Total income tax expense . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155,482 132,571 Deferred tax . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14,922 97,018 Current tax . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 140,560 35,553 Total income tax expense . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155,482 132,571 F-223 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (28) Taxation (Continued) (b) Deferred tax assets and liabilities Details of deferred tax assets and liabilities are as follows: Thousands of Euros Tax effect 2013 2012 Assets Inventories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 18,972 20,871 Fixed assets and amortisation and depreciation . . . . . . . . . . 1,466 1,615 Tax credits (deductions from Spain) . . . . . . . . . . . . . . . . . . . 8,404 0 Tax loss carryforwards in Spain . . . . . . . . . . . . . . . . . . . . . . 4,247 0 Tax loss carryforwards in other Companies . . . . . . . . . . . . . . 368 0 Provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 746 1,416 Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 398 815 34,601 24,717 Liabilities Goodwill . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (42,039) (38,809) Intangible assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (318,128) (324,787) Fixed assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (121,667) (120,719) Debt cancellation costs . . . . . . . . . . . . . . . . . . . . . . . . . . . . (55,755) (72,584) Subtotal, liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (537,589) (556,899) Tax credits (deductions from Spain) . . . . . . . . . . . . . . . . . . . 5,298 8,980 Tax credits (deductions from the US) . . . . . . . . . . . . . . . . . . 0 4,505 Tax loss carryforwards in the US . . . . . . . . . . . . . . . . . . . . . 6,184 7,886 Inventories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,187 21,184 Cash flow hedges . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15,293 20,188 Provisions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 40,693 35,972 Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7,845 4,338 Subtotal, net assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 83,500 103,053 Net deferred liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (454,089) (453,846) F-224 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (28) Taxation (Continued) Movement in deferred tax assets and liabilities is as follows: Thousands of Euros 2013 2012 Deferred tax assets and liabilities Balance at 1 January . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (429,129) (352,617) Movements during the year . . . . . . . . . . . . . . . . . . . . . . . . . . (14,922) (97,018) Movements in equity during the year . . . . . . . . . . . . . . . . . . . (4,227) 6,988 Business combinations (note 3) . . . . . . . . . . . . . . . . . . . . . . . . 4,871 1,383 Translation differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 23,919 12,135 Balance at 31 December . . . . . . . . . . . . . . . . . . . . . . . . . . . . (419,488) (429,129) The Spanish companies have opted to apply accelerated depreciation to certain additions to property, plant and equipment, which has resulted in the corresponding deferred tax liability. Details of deferred tax assets and liabilities on items directly debited and credited to equity during the year are as follows: Thousands of Euros Tax effect 2013 2012 Cash flow hedges (note 16 (f)) . . . . . . . . . . . . . . . . . . . . . . . . 4,227 (6,988) 4,227 (6,988) The remaining assets and liabilities recognised in 2013 were recognised on the statement of profit or loss. Estimated net deferred tax liabilities to be reversed in a period of less than 12 months amount to Euros 32,246 thousand at 31 December 2013 (Euros 45,224 thousand at 31 December 2012). The majority of the tax deductions pending application from other Spanish companies (except for those located in the Basque Country where the majority of tax deductions do not mature), relating mainly to research and development, mature in 15 years, whilst most tax deductions pending application from US companies mature in 20 years. At 31 December 2013 the Group has recognised an amount of Euros 5,298 thousand from Spanish companies (Euros 8,980 thousand at 31 December 2012), Euros 8,404 thousand from the Progenika Group companies acquired as their future recovery was estimated as likely, and the total amount from from US companies has been fully applied (Euros 4,505 thousand at 31 December 2012) in respect of tax credits derived from deductions pending application. At 31 December 2013 the tax Group in Spain has an amount of Euros 22,413 thousand (Euros 22,837 thousand at 31 December 2012) pending application for tax amortisation as a result of goodwill generated on the acquisition of Biomat USA, Inc. This amount will be applied annually, with no limit, provided that the current amortisation rates are maintained (stipulated by law until 2015) until 2066. In the event of applying a tax amortisation rate of 5%, the amount pending tax amortisation would be applied until 2025. The yearly amount that has been applied in 2013 at the tax rate of 30% has been F-225 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (28) Taxation (Continued) Euros 424 thousand (Euros 424 thousand in 2012). The Group has recognised a deferred tax liability of Euros 16,120 thousand for the tax amortisation of goodwill at 31 December 2013 (Euros 15,696 thousand at 31 December 2012). At 31 December 2013 the tax Group in Spain has an amount of Euros 9,342 thousand (Euros 9,471 thousand at 31 December 2012) pending application for tax amortisation as a result of goodwill generated on the acquisition of Plasmacare, Inc. This amount will be deducted annually, with no limit, provided that the current amortization rates are maintained (stipulated by law until 2015) until 2086. In the event of applying a tax amortization rate of 5% the amount pending tax amortization would be applied until 2029. The yearly amount that has been applied in 2013 at the tax rate of 30% has been Euros 128 thousand (Euros 128 thousand in 2012). The Group has recognised a deferred tax liability of Euros 3,485 thousand for the tax amortization of goodwill at 31 December 2013 (Euros 3,356 thousand at 31 December 2012). At 31 December 2013 the Group has recognised tax loss carryforwards of Euros 6,184 thousand (Euros 7,886 thousand at 31 December 2012). These tax credits derive from the US companies and are available for 20 years from their date of origin. At 31 December 2013 the Group has recognised loss carryforwards of Euros 4,247 thousand from Progenika Group companies acquired which are pending offset and have no maturity date. The Group has not recognised as deferred tax assets the tax effect of the tax loss carryforwards of Group companies, which amount to Euros 35,657 thousand (Euros 12,456 thousand at 31 December 2012). The rise for 2013 is mainly due to the Euros 15,205 thousand losses from the Progenika Group companies. (c) Years open to inspection Under prevailing legislation, taxes cannot be considered to be definitively settled until the returns filed have been inspected by the taxation authorities, or the prescription period has elapsed. The Group has the following tax inspections underway: • Log´istica Grifols, S.A. de CV: Tax report on the financial statements for 2005 and 2006. • Grifols Inc. and subsidiaries: notification of an inspection of federal income tax for the year ended 1 June 2011. • Grifols Inc. and subsidiaries: notification of an inspection of federal income tax for the years ended 31 December 2010 and 31 December 2011. Group management does not expect any significant liability to derive from these inspections. No significant liabilities have arisen from completion of the tax inspection in 2013 of California franchise tax for 2009 to 2010 in Grifols Inc. (formerly Talecris Biotherapeutics Holdings Corp and subsidiaries). No significant liabilities have arisen from completion of the tax inspection in 2013 of Indiana income tax for 2009 to 2011 in Talecris Plasma Resources, Inc. F-226 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (29) Operating Leases (a) Operating leases (as lessee) At 31 December 2013 and 2012 the Group leases buildings from third parties under operating leases. In addition to the lease contracts described in note 9 (f (i)), the Group has warehouses and buildings contracted under operating lease. The duration of these lease contracts ranges from between 1 to 30 years. Contracts may be renewed on termination. Lease instalments are adjusted periodically in accordance with the price index established in each contract. One Group company has entered into lease contracts which include contingent rents. These contingent rents have been based on production capacity, surface area used and the real estate market and are expensed on a straight line basis. Operating lease instalments of Euros 69,522 thousand have been recognised as an expense for the year at 31 December 2013 (Euros 67,991 thousand at 31 December 2012) and comprise minimum lease payments. Future minimum payments on non-cancellable operating leases at 31 December 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Maturity at: Up to one year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52,520 54,080 Between 1 and 5 years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 156,413 171,315 More than 5 years . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 52,708 67,864 Total future minimum payments . . . . . . . . . . . . . . . . . . . . . . . 261,641 293,259 (b) Operating leases (as lessor) At 31 December 2013 and 2012 the Group has no lease contracts as lessor. (30) Other Commitments with Third Parties and Other Contingent Liabilities (a) Guarantees The Group has no significant guarantees extended to third parties. (b) Guarantees committed with third parties The Group has no significant guarantees extended to third parties. (c) Obligations with personnel The Group’s annual contribution to defined contribution pension plans of Spanish Group companies for 2013 has amounted to Euros 595 thousand (Euros 558 thousand for 2012). In successive years this contribution will be defined through labour negotiations. In the event that control is taken of the Company, the Group has agreements with 89 employees/ directors/senior management whereby they can unilaterally rescind their employment contracts with the Company and are entitled to termination benefits ranging from 2 to 5 years salary. F-227 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (30) Other Commitments with Third Parties and Other Contingent Liabilities (Continued) The Group has contracts with five directors entitling them to termination benefits ranging from one to two years of their salary due to various circumstances. Savings plan and profit-sharing plan The Group has a defined contribution plan (savings plan), which qualifies as a deferred salary arrangement under Section 401 (k) of the Internal Revenue Code (IRC). Once eligible, employees may elect to contribute a portion of their salaries to the savings plan, subject to certain limitations. The Group matches 100% of the first 3% of employee contributions and 50% of the next 2%. Group and employee contributions are fully vested when contributed. The plan assets are held in trust and invested as directed by the plan participants. The total cost of matching contributions to the savings plan was US Dollars 11.2 million for 2013 (US Dollars 11.3 million for 2012). The recognition of the cost of these contributions is consistent with each participant’s salary. Other plans The Group has a defined benefit pension plan for certain Talecris Biotherapeutics, GmbH employees in Germany as required by statutory law. The pension cost relating to this plan was not material for the periods presented. (d) Purchase commitments Details of the Group’s commitments mainly to purchase plasma at 31 December 2013 are as follows: Thousands of Euros 2014 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 84,814 2015 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 73,977 2016 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68,668 2017 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 39,407 2018 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38,658 2019 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2,478 (e) Judicial procedures and arbitration Details of legal proceedings in which the Company or Group companies are involved are as follows: Catalan haemophiliacs Instituto Grifols, S.A. was notified in 2007 of a claim for maximum damages of Euros 12,960 thousand filed by a group of 100 Catalan haemophiliacs against all plasma fractionation companies. During 2008 this claim was rejected, and the ruling appealed. Notification was published on 21 January 2011 that on 18 January 2011 the Barcelona Provincial Court had rejected the haemophiliacs’ claim. An appeal was subsequently filed by the counterparty in the Catalan High Court, which was rejected. The Group is currently awaiting the ruling on the appeal filed again by the group of haemophiliacs at the Spanish Supreme Court. F-228 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (30) Other Commitments with Third Parties and Other Contingent Liabilities (Continued) Foreign Corrupt Practices Act (FCPA) The Group is carrying out an internal investigation, already started prior to the acquisition of Talecris, in relation to possible breaches of the Foreign Corrupt Practices Act (FCPA) of which Talecris was aware in the context of a review unrelated to this matter. This FCPA investigation is being carried out by an external legal advisor. In principle, the investigation has been focused on sales to certain Central and Eastern European countries, specifically Belarus and Russia, although trading practices in Brazil, China, Georgia, Iran and Turkey are also being investigated, in addition to other countries considered necessary. In July 2009, the Talecris Group voluntarily contacted the U.S. Department of Justice (DOJ) to inform them of an internal investigation that the Group was carrying out regarding possible breaches of the FCPA in certain sales to certain central and East European countries and to offer the Group’s collaboration in any investigation that the DOJ wanted to carry out. As a result of this investigation the Group suspended shipments to some of these countries. In certain cases, the Group had safeguards in place which led to terminating collaboration with consultants and suspending or terminating relations with distributors in those countries under investigation as circumstances warranted As a consequence of the investigation, the agreement with Talecris’ Turkish distributor was terminated and a settlement agreement has been reached between the parties. In November 2012, the Group was notified by the DOJ that the proceedings would be closed, without prejudice to the fact that they could be re-opened in the future should new information arise. The Group continues with the in-depth review of potential irregular practices. Furthermore an investigation has been opened in Italy, in relation with the criminal prosecution in Naples against 5 employees of the Company, including the former General Manager. The Company and its legal advisors consider this investigation will be limited to the individual employees and the likelihood is remote this issue will affect the Company. The legal advisors recommend limiting disclosure of the aforementioned information in these consolidated annual accounts, because the matter is currently under legal dispute. F-229 |
|
|
LES]UNIVERSAL.BST;124 F-230 (31) Financial Instruments Classification Disclosure of financial instruments by nature, category and fair value is as follows: Thousands of Euros 2012 Carrying amount Financial Fair Value Loans and instruments held Debts and receivables for trading payables Total Level 1 Level 2 Level 3 Total Financial derivatives . . . . . . . . . . . . . . . . . . . . . . . . . — 4,502 — 4,502 — 4,502 — 4,502 Financial assets at fair value . . . . . . . . . . . . . . . . . . — 4,502 — 4,502 Non-current financial assets . . . . . . . . . . . . . . . . . . . 12,024 — — 12,024 Other current financial assets . . . . . . . . . . . . . . . . . . 460 — — 460 Trade and other receivables . . . . . . . . . . . . . . . . . . . 392,288 — — 392,288 Cash and cash Equivalents . . . . . . . . . . . . . . . . . . . . 473,327 — — 473,327 Financial assets not measured at fair value . . . . . . . . 878,099 — — 878,099 Financial derivatives . . . . . . . . . . . . . . . . . . . . . . . . . — (93,515) — (93,515) — (93,515) — (93,515) Financial liabilities at fair value . . . . . . . . . . . . . . . . — (93,515) — (93,515) High Yield Senior Unsecured Notes . . . . . . . . . . . . . — — (756,267) (756,267) (918,195) — — (918,195) Promissory notes . . . . . . . . . . . . . . . . . . . . . . . . . . . — — (14,309) (14,309) Secured senior debt . . . . . . . . . . . . . . . . . . . . . . . . . — — (1,890,998) (1,890,998) (2,129,522) — — (2,129,522) Other bank loans . . . . . . . . . . . . . . . . . . . . . . . . . . . — — (89,152) (89,152) Finance lease payables . . . . . . . . . . . . . . . . . . . . . . . — — (24,597) (24,597) Other financial liabilities . . . . . . . . . . . . . . . . . . . . . — — (17,559) (17,559) Trade and other payables . . . . . . . . . . . . . . . . . . . . . — — (228,405) (228,405) Debts with associates . . . . . . . . . . . . . . . . . . . . . . . . — — (2,668) (2,668) Other current liabilities . . . . . . . . . . . . . . . . . . . . . . — — (2,917) (2,917) Financial liabilities at fair value . . . . . . . . . . . . . . . . — — (3,026,872) (3,026,872) 878,099 (89,013) (3,026,872) (2,237,786) The Group does not provide details of the fair value of certain financial instruments as their carrying amount is very similar to their fair value. 3 C Cs: 20027 |
|
|
LES]UNIVERSAL.BST;124 F-231 Notes to the Consolidated Annual Accounts (31) Financial Instruments (continued) Thousands of Euros 2013 Carrying amount Financial Fair Value Loans and instruments held Debts and receivables for trading payables Total Level 1 Level 2 Level 3 Total Financial derivatives . . . . . . . . . . . . . . . . . . . . . . . . — 3,155 — 3,155 — 3,155 — 3,155 Financial assets at fair value . . . . . . . . . . . . . . . . . . — 3,155 — 3,155 Non-current financial assets . . . . . . . . . . . . . . . . . . . 11,741 — — 11,741 Other current financial assets . . . . . . . . . . . . . . . . . . 500 — — 500 Trade and other receivables . . . . . . . . . . . . . . . . . . . 405,262 — — 405,262 Cash and cash equivalents . . . . . . . . . . . . . . . . . . . . 708,777 — — 708,777 Financial assets not measured at fair value . . . . . . . 1,126,280 — — 1,126,280 Financial derivatives . . . . . . . . . . . . . . . . . . . . . . . . — (68,033) — (68,033) — (68,033) — (68,033) Financial liabilities at fair value . . . . . . . . . . . . . . . — (68,033) — (68,033) High Yield Senior Unsecured Notes . . . . . . . . . . . . . — — (745,008) (745,008) (851,461) — — (851,461) Promissory notes . . . . . . . . . . . . . . . . . . . . . . . . . . . — — (45,211) (45,211) Senior secured debt . . . . . . . . . . . . . . . . . . . . . . . . . — — (1,790,029) (1,790,029) (1,961,341) — — (1,961,341) Other bank loans . . . . . . . . . . . . . . . . . . . . . . . . . . — — (87,248) (87,248) Finance lease payables . . . . . . . . . . . . . . . . . . . . . . . — — (19,186) (19,186) Other financial liabilities . . . . . . . . . . . . . . . . . . . . . — — (56,640) (56,640) Trade and other payables . . . . . . . . . . . . . . . . . . . . . — — (273,621) (273,621) Debts with associates . . . . . . . . . . . . . . . . . . . . . . . . — — (2,683) (2,683) Other current liabilities . . . . . . . . . . . . . . . . . . . . . . — — (8,646) (8,646) Financial liabilities at fair value . . . . . . . . . . . . . . . — — (3,028,272) (3,028,272) 1,126,280 (64,878) (3,028,272) (1,966,870) The Group does not provide details of the fair value of certain financial instruments as their carrying amount is very similar to their fair value. 3 C Cs: 47198 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) Financial derivatives At 31 December 2013 and 2012 the Group has recognised the following derivatives: Thousands of Euros Notional Notional amount at amount at Value at Value at Financial derivatives Currency 31/12/2013 31/12/2012 31/12/13 31/12/12 Maturity Interest rate swap (cash flow hedges) . . . . . . . . . . . . . . . . . . USD 1,224,777,500 1,398,875,000 (40,004) (50,900) 30/06/2016 Interest rate swap (cash flow hedges) . . . . . . . . . . . . . . . . . . EUR 100,000,000 100,000,000 (4,025) (5,704) 31/03/2016 Swap option . . . . . . . . . . . . . . . . EUR 100,000,000 100,000,000 0 8 31/03/2016 Swap floor . . . . . . . . . . . . . . . . . USD 1,224,777,500 1,398,875,000 3,155 4,494 30/06/2016 Embedded floor of senior debt . . EUR 196,000,000 198,000,000 (3,539) (5,965) 01/06/2017 Embedded floor of senior debt . . USD 1,656,000,000 1,678,000,000 (20,465) (30,946) 01/06/2017 Total . . . . . . . . . . . . . . . . . . . . . (64,878) (89,013) Total assets (note 11) . . . . . . . . . 3,155 4,502 Total liabilities (note 21) . . . . . . . (68,033) (93,515) (a) Derivative financial instruments at fair value through profit or loss Derivative financial instruments that do not meet the hedge accounting requirements are classified and measured as financial assets or financial liabilities at fair value through profit or loss. The floor included in the syndicated financing of Tranches A and B of the senior debt is in the money and an embedded derivative exists on these contracts, which was measured at fair value and recognised separately from the loans. As a result of the refinancing entered into on 29 February 2012 the embedded derivatives have been amended and improved. The embedded derivative included in Tranche A has been eliminated, whilst the embedded derivative included in Tranche B has decreased from 1.75% to 1.00%. Consequently, the nominal amounts of the embedded floors of the senior debt have been significantly reduced in Euros and US Dollars. The decrease in the value of embedded derivatives amounted to US Dollars 71.6 million (Euros 53.5 million) and Euros 12.2 million at 29 February 2012, which reduced the refinanced senior debt. Futures contracts matured on 29 June 2012. On 29 June 2012 it was agreed to extend the futures contract to 28 September 2012, through a novation without liquidation under the same terms and conditions. During 2012 the Group sold unquoted futures, obtaining cash income of Euros 31.5 million and finance income of Euros 27.9 million. (b) Hedging derivative financial instruments See explanation in note 16 (f). In June 2011, the Group contracted two derivatives in order to comply with the compulsory hedging requirements stipulated in the credit agreement. These derivatives comprise a step-up interest rate swap and a floor swap, which had an initial nominal amount of US Dollars 1,550 million each. Both the interest rate swap and the floor are amortised on a quarterly basis in order to remain less than the amounts borrowed and avoid excess hedging. In December 2013 the nominal amount of the F-232 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) derivatives stands at US Dollars 1,225 million each (Euros 1,399 million at 31 December 2012). The interest rate swap complies with hedge accounting criteria. Furthermore, in May 2012 the interest rate swap in Euros was modified, reducing the fixed interest rate and extending the maturity date from September 2014 to March 2016. The interest rate swap complies with hedge accounting criteria. Credit risk (a) Exposure to credit risk The carrying amount of financial assets represents the maximum exposure to credit risk. At 31 December 2013 and 2012 the maximum level of exposure to credit risk is as follows: Thousands of Euros Carrying amount Note 2013 2012 Non-current financial assets . . . . . . . . . . . . . . . . . . . . . 11 11,741 12,024 Non-current financial derivatives . . . . . . . . . . . . . . . . . . 11 3,155 4,502 Other current financial assets . . . . . . . . . . . . . . . . . . . . 500 460 Trade receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13 385,537 366,022 Other receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13 19,725 26,266 Cash and cash equivalents . . . . . . . . . . . . . . . . . . . . . . . 15 708,777 473,327 1,129,435 882,601 The maximum level of exposure to risk associated with receivables at 31 December 2013 and 2012, by geographical area, is as follows. Thousands of Euros Carrying amount 2013 2012 Spain . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 95,491 104,676 EU countries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 54,526 66,238 United States of America . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 164,582 139,073 Other European countries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,516 4,427 Other regions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 89,147 77,874 405,262 392,288 F-233 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) Details of balances receivable as per country such as Greece, Italy, Spain and Portugal at 31 December 2012 are as follows: Thousands of Euros Balances with public entities Balances with third parties Provision Provision Balance for doubtful Balance for doubtful Net debt Balance(1) past due receivables(2) Balance(3) past due receivables(4) (1)+(2)+(3)+(4) Greece . . . . . . . . . . . . . 317 273 (317) 2,026 199 0 2,026 Italy . . . . . . . . . . . . . . . 8,693 4,667 (557) 16,167 7,386 (1,193) 23,110 Spain . . . . . . . . . . . . . . 82,599 48,601 (175) 13,651 11,632 (172) 95,903 Portugal . . . . . . . . . . . . 21,028 15,615 (4,081) 629 520 (210) 17,366 112,637 69,156 (5,130) 32,473 19,737 (1,575) 138,405 Details of balances receivable as per country such as Greece, Italy, Spain and Portugal at 31 December 2013 are as follows: Thousands of Euros Balances with public entities Balances with third parties Provision Provision Balance for doubtful Balance for doubtful Net debt Balance(1) past due receivables(2) Balance(3) past due receivables(4) (1)+(2)+(3)+(4) Greece . . . . . . . . . . . . . 118 118 (118) 1,259 9 0 1,259 Italy . . . . . . . . . . . . . . . 6,801 1,741 (144) 14,847 9,057 (2,060) 19,444 Spain . . . . . . . . . . . . . . 76,027 41,092 (175) 7,656 4,919 (98) 83,410 Portugal . . . . . . . . . . . . 10,999 8,559 (7,088) 3,098 2,422 (1) 7,008 93,945 51,510 (7,525) 26,860 16,407 (2,159) 111,121 Provision has been made for balances receivable from Portuguese public entities on the basis of the best estimate of their expected collection in view of the current situation regarding negotiations. The Group does not currently have any reason to consider that the receivables from public entities in Italy and Spain will not be recoverable. (b) Impairment losses Details of the maturity of trade receivables, net of impairment provisions are as follows: Thousands of Euros 2013 2012 Not matured . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 305,111 282,803 Less than 1 month . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 42,298 34,103 1 to 4 months . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 35,734 34,732 4 months to 1 year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15,147 29,246 More than one year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6,972 11,404 405,262 392,288 F-234 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) Unimpaired receivables that are past due mainly relate to public entities. Movement in the inventory provision was as follows: Thousands of Euros 2013 2012 Opening balance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12,799 8,871 Business combinations . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 722 0 Net charge for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4,750 5,248 Net cancellations for the year . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,617) (1,248) Translation differences . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (581) (72) Closing balance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16,073 12,799 An analysis of the concentration of credit risk is provided in note 5 (a). Liquidity risk The management of the liquidity risk is explained in note 5. Details of the contractual maturity dates of financial liabilities including committed interest calculated using interest rate forward curves are as follows: Thousands of Euros Carrying More amount at Contractual 6 months than five Carrying amount Note 31/12/2012 flows or less 6-12 months 1-2 years 2-5 years years Financial liabilities Bank loans . . . . . . . . . . . 21 1,980,150 2,509,660 135,776 99,268 209,243 2,054,190 11,183 Other financial liabilities . 21 17,559 19,636 4,496 1,824 3,508 6,699 3,109 Bonds and other marketable securities . . 21 770,576 1,226,319 48,700 34,391 68,781 206,344 868,103 Finance lease payables . . 21 24,597 24,597 3,689 3,316 6,968 9,795 829 Payable to associates . . . . 32 2,668 2,668 2,668 0 0 0 0 Payable to suppliers . . . . 22 228,405 228,405 228,286 119 0 0 0 Other current liabilities . . 23 2,917 2,917 2,843 74 0 0 0 Derivative financial liabilities . . . . . . . . . . . 21 36,911 31,412 3,890 3,994 7,676 15,852 0 Financial liabilities for hedging derivatives . . . 21 56,604 56,953 4,770 8,899 19,242 24,042 0 Total . . . . . . . . . . . . . . . 3,120,387 4,102,567 435,118 151,885 315,418 2,316,922 883,224 F-235 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) Thousands of Euros Carrying More amount at Contractual 6 months than five Carrying amount Note 31/12/2013 flows or less 6-12 months 1-2 years 2-5 years years Financial liabilities Bank loans . . . . . . . . . . . 21 1,877,277 2,256,838 146,822 105,206 416,706 1,581,963 6,141 Other financial liabilities . 21 56,640 56,640 5,739 3,699 4,195 36,911 6,096 Bonds and other marketable securities . . 21 790,219 1,138,951 78,114 32,902 65,804 962,131 0 Finance lease payables . . 21 19,186 20,787 4,164 3,912 6,861 5,559 291 Payable to associates . . . . 32 2,683 2,683 2,683 0 0 0 0 Payable to suppliers . . . . 22 273,621 273,621 272,829 792 0 0 0 Other current liabilities . . 23 8,646 8,647 7,664 983 0 0 0 Derivative financial liabilities . . . . . . . . . . . 21 24,004 45,876 4,524 14,070 27,282 0 0 Financial liabilities for hedging derivatives . . . 21 44,029 25,637 3,573 8,475 11,727 1,862 0 Total . . . . . . . . . . . . . . . 3,096,305 3,829,680 526,112 170,039 532,575 2,588,426 12,528 Currency risk The Group’s exposure to currency risk is as follows: Thousands of Euros 2012 Euros(*) US Dollars(**) Trade receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68 3,107 Receivables from Group companies . . . . . . . . . . . . . . . . . . 0 45 Loans to Group companies . . . . . . . . . . . . . . . . . . . . . . . . . 0 6 Cash and cash equivalents . . . . . . . . . . . . . . . . . . . . . . . . . 858 24,977 Trade payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,508) (2,684) Payables to Group companies . . . . . . . . . . . . . . . . . . . . . . . (7,357) (56,405) Loans to Group companies . . . . . . . . . . . . . . . . . . . . . . . . . (8,929) 0 Balance sheet exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . (16,868) (30,954) (*) Balances in Euros in subsidiaries with US Dollars functional currency (**) Balances in US Dollars in subsidiaries with Euros functional currency F-236 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) Thousands of Euros 2013 Euros(*) US Dollars(**) Trade receivables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 267 2,637 Receivables from Group companies . . . . . . . . . . . . . . . . . . . 28,472 5,898 Loans to Group companies . . . . . . . . . . . . . . . . . . . . . . . . . 0 204,480 Cash and cash Equivalents . . . . . . . . . . . . . . . . . . . . . . . . . 16,524 95,177 Trade payables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (602) (15,730) Payables to Group companies . . . . . . . . . . . . . . . . . . . . . . . (7,502) (19,359) Loans to Group companies . . . . . . . . . . . . . . . . . . . . . . . . . 28,411 (135,418) Balance sheet exposure . . . . . . . . . . . . . . . . . . . . . . . . . . . 65,570 137,685 (*) Balances in Euros in subsidiaries with US Dollars functional currency (**) Balances in US Dollars in subsidiaries with Euros functional currency The most significant exchange rates applied at 2013 and 2012 year ends are as follows: Closing exchange rate 2013 2012 Euros US Dollars . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1.3791 1.3194 A sensitivity analysis for foreign exchange fluctuations is as follows: Had the US Dollar strengthened by 10% against the Euro at 31 December 2013, equity would have increased by Euros 204,191 thousand (Euros 145,895 thousand at 31 December 2012) and profit would have increased by Euros 20,326 thousand (at 31 December 2012 it would have decreased by Euros 4,782 thousand). This analysis assumes that all other variables are held constant, especially that interest rates remain constant. A 10% weakening of the US Dollar against the Euro at 31 December 2013 and 2012 would have had the opposite effect for the amounts shown above, all other variables being held constant. F-237 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (31) Financial Instruments (Continued) Interest rate risk (a) Interest-rate profile To date, the profile of interest on interest-bearing financial instruments is as follows: Thousands of Euros 2013 2012 Fixed-interest financial instruments Financial assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5,230 5,688 Financial liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (817,843) (770,576) (812,613) (764,888) Variable-interest financial instruments Financial liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (1,896,463) (2,004,747) (1,896,463) (2,004,747) (2,709,076) (2,769,635) (b) Sensitivity analysis Had the interest rate curve at 31 December 2013 been 100 basis points higher, the interest expense would have increased by Euros 9.7 million, the finance cost due to changes in the value of derivatives would have been Euros 10.4 million lower and equity would have increased by Euros 18.8 million as a result of changes in derivatives to which hedge accounting is applied. Had the interest rate at 31 December 2012 been 100 basis points higher, the interest expense would have increased by Euros 6.2 million, the finance cost due to changes in the value of derivatives would have been Euros 23.6 million lower and equity would have increased by Euros 27.8 million as a result of changes in derivatives to which hedge accounting is applied. (32) Balances and Transactions with Related Parties Details of balances with related parties are as follows: Thousands of Euros 2013 2012 Receivable from associates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27 26 Loans to associates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,000 0 Debts with associates . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (2,683) (2,668) Debts with key management personnel . . . . . . . . . . . . . . . . . . . . (4,017) (1,250) Payables to members of the board of directors . . . . . . . . . . . . . . (400) (458) Payables to other related parties . . . . . . . . . . . . . . . . . . . . . . . . (7,906) (5,969) (13,979) (10,319) Payables are included in suppliers and trade payables (see note 22). F-238 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (32) Balances and Transactions with Related Parties (Continued) (a) Group transactions with related parties Group transactions with related parties during 2012 were as follows: Thousands of Euros Key management Other related Board of directors Associates personnel parties of the Company Net sales . . . . . . . . . . . . . 186 — — — Other service expenses . . . — — (6,072) (1,270) Operating lease expenses (note 9) . . . . . . . . . . . . — — (24,057) — Remuneration . . . . . . . . . . — (7,871) — (3,688) 186 (7,871) (30,129) (4,958) Group transactions with related parties during 2013 are as follows: Thousands of Euros Key management Other related Board of directors Associates personnel parties of the Company Net sales . . . . . . . . . . . . . 263 — — — Other service expenses . . . — — (5,849) (1,269) Operating lease expenses (note 9) . . . . . . . . . . . . — — (23,985) — Remuneration . . . . . . . . . . — (9,130) — (4,405) R&D agreements . . . . . . . (9,802) Finance costs . . . . . . . . . . (36) — (210) — (9,575) (9,130) (30,044) (5,674) Every year the Group contributes 0.7% of its profits before tax to a non-profit organisation. ‘‘Other service expenses’’ include contributions to non-profit organisations totalling Euros 2,779 thousand in 2013 (Euros 3,012 thousand in 2012). Interest expense to related parties for the year 2013 include interest accrued on the loan of Class B shares (see note 3 (a) and 16). During 2011 one of the Company’s directors signed a three-year consulting services contract. The director will receive annual fees of US Dollars 1 million for these services and an additional bonus of US Dollars 2 million for complying with certain conditions. Directors representing shareholders interests have received remuneration of Euros 100 thousand during 2013 and 2012. The Group has not extended any advances or loans to the members of the board of directors or key management personnel nor has it assumed any guarantee commitments on their behalf.It has also not assumed any pension or life insurance obligations on behalf of former or current members of the board of directors or key management personnel. In addition, certain Company directors and key management personnel have termination benefit commitments (see note 30 (c)). F-239 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (32) Balances and Transactions with Related Parties (Continued) (b) Investments and positions held by directors of the Parent in other companies and related parties The directors and related parties do not hold any investments, nor do they hold positions or carry out functions or activities in companies with an identical, similar or complementary statutory activity to that of the Company. (33) Environmental Issues The most significant systems, equipment and fixtures for the protection and improvement of the environment at 31 December 2012 are as follows: Thousands of Euros Accumulated Project Cost depreciation Net value Waste water treatment . . . . . . . . . . . . . . . . . . . . . . 4,215 (759) 3,456 Waste management . . . . . . . . . . . . . . . . . . . . . . . . . 3,482 (850) 2,632 Reduction of electricity consumption . . . . . . . . . . . . 7,969 (456) 7,513 Reduction of water consumption . . . . . . . . . . . . . . . 6,104 (1,161) 4,943 Energy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 869 (1) 868 Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 118 0 118 22,757 (3,227) 19,530 The most significant systems, equipment and fixtures for the protection and improvement of the environment at 31 December 2013 are as follows: Thousands of Euros Accumulated Project Cost depreciation Net value Waste water treatment . . . . . . . . . . . . . . . . . . . . . . 5,977 (1,353) 4,624 Waste management . . . . . . . . . . . . . . . . . . . . . . . . . 4,693 (770) 3,923 Reduction of electricity consumption . . . . . . . . . . . . 8,610 (2,081) 6,529 Reduction of water consumption . . . . . . . . . . . . . . . 6,412 (1,541) 4,871 Energy . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 887 (37) 850 Other . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,999 (38) 1,961 28,578 (5,820) 22,758 Expenses incurred by the Group for protection and improvement of the environment during 2013 totalled approximately Euros 9,659 thousand (Euros 1,240 thousand during 2012). The Group considers that the environmental risks are adequately controlled by the procedures currently in place. The Group has received environmental grants of Euros 1,383 thousand during 2013 (Euros 1,062 thousand during 2012). F-240 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (34) Other Information Audit fees: KPMG Auditores, S.L. has invoiced the following fees and expenses for professional services during 2013 and 2012: Thousands of Euros 2013 2012 Audit services for accounts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 499 449 Other assurance services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 997 965 Other services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 267 38 1,763 1,452 Audit services detailed in the above table include the total fees for services rendered in 2013 and 2012, irrespective of the date of invoice. Fees and expenses for professional services rendered by other firms of the KPMG Europe LLP Group for 2013 and 2012 are as follows: Thousands of Euros 2013 2012 Audit services for accounts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 143 126 Other assurance services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 46 45 Tax fees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8 11 Other services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . — — 197 182 Other entities affiliated to KPMG International have invoiced the Group for the following fees and expenses for professional services during 2013 and 2012: Thousands of Euros 2013 2012 Audit services for accounts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,346 1,302 Other assurance services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 355 356 Tax fees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15 21 Other services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 30 33 1,746 1,712 F-241 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (34) Other Information (Continued) Other audit firms have invoiced the Group for the following fees and expenses for professional services during 2013 and 2012: Thousands of Euros 2013 2012 Audit services for accounts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32 23 Other assurance services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13 8 Tax fees . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 45 — Other services . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 51 52 141 83 (35) Events after the Reporting Period Signing of a bridge loan for the acquisition of Novartis’ Diagnostic unit On 3 January 2014 the Group signed a US Dollars 1,500 million bridge loan, fully entered into in equal parts by Nomura, BBVA and Morgan Stanley. This loan was taken out to pay for the acquisition of Novartis’ Diagnostic unit relating to transfusional medicine and immunology. There are no financial restrictions on this loan relating to Grifols dividends or investments. Closing of the purchase of Novartis’ Diagnostic unit On 9 January 2014 the Group acquired the transfusion medicine and immunology Diagnostic unit of the Swiss company Novartis International AG for approximately US Dollars 1,650 million (Euros 1,222 million). This transaction has been structured through a newly-created 100% Grifols-owned subsidiary, G-C Diagnostics Corp. (USA) and this transaction has been financed through a US Dollars 1,500 million bridge loan. Grifols will expand its portfolio by including Novartis’ diagnostic products for transfusion medicine and immunology, including its highly innovative, market-leading NAT technology (Nucleic Acid Amplification Techniques), instrumentation and equipment for blood screening, specific software and reagents. The assets acquired include patents, brands and licenses, together with the production plant at Emeryville (California, United States) and commercial offices in United States, Switzerland and Hong Kong (for the Asia-Pacific region) among others. This strategic operation will strengthen Grifols’ Diagnostic division, particularly in the US, with a very strong and specialized commercial organisation. It will also diversify Grifols’ business by promoting an activity area that complements the Bioscience division. The diagnostic business being purchased from Novartis, focused on guaranteeing the safety of blood donations for transfusions or to be used in the production of plasma derivatives, complements and expands Grifols’ existing product range. Grifols will become a vertically integrated company able to provide solutions for blood and plasma donor centres, with the most complete product portfolio in the immunohaematology field, including reagents using gel technology, multicard and the new genotyping technologies from Progenika. F-242 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (35) Events after the Reporting Period (Continued) Grifols’ workforce will increase by approximately 550 employees, after taking on the employees of Novartis. At the date of issue of these consolidated annual accounts the Group did not have all the necessary information to determine the definitive fair value of intangible assets, liabilities and contingent liabilities acquired in the business combination. Details of the aggregate business combination cost, the provisional fair value of the net assets acquired and provisional goodwill at the acquisition date (or the amount by which the business combination cost exceeds the fair value of the net assets acquired) are provided below. The values shown in the table below should be considered provisional. For practical purposes, for the present transaction, the exchange rate Euro / Dollar 1.35 was used for all purposes. The Novartis’ Diagnostic business did not operate as a separate legal entity or segment, so the acquired business was structured as an asset deal, with the exception of the Hong Kong subsidiary, which was acquired via a share deal. For this reason the combined financial information presented in respect to the acquired business has been prepared based on ‘‘carve-out’’ financial information, using the directly attributable assets and liabilities and the historical results of operations, which include allocations of expenses attributable to the acquired business from the Diagnostic division within the Novartis Group. The combined financial information is therefore not indicative of the results of operations or financial position that would have occurred if the carve-out Diagnostic division had been a separate stand-alone entity during the year presented, or of the future results of the carve-out Diagnostic division. Thousands of Thousands of Euros US Dollars Cost of the business combination Payment in cash . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1,222,222 1,650,000 Total business combination cost . . . . . . . . . . . . . . . . . . . . 1,222,222 1,650,000 Fair value of net assets acquired . . . . . . . . . . . . . . . . . . . 256,060 345,681 Goodwill (excess of the cost of the business combination over the fair value of net assets acquired) . . . . . . . . . . . 966,162 1,304,319 Provisional goodwill generated in the acquisition is attributed to the synergies, workforce and other expected benefits from the business combination of the assets and activities of the Group. The expenses incurred in this transaction in 2013 amount to approximately Euros 19 million. F-243 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES Notes to the Consolidated Annual Accounts (Continued) (35) Events after the Reporting Period (Continued) The amounts provisionally determined at the date of acquisition of assets, liabilities and contingent liabilities acquired are as follows: Fair value Thousands of Thousands of Euros US Dollars Property, plant and equipment . . . . . . . . . . . . . . . . . . . . 107,397 144,986 Inventories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 64,800 87,480 Trade and other receivables . . . . . . . . . . . . . . . . . . . . . . . 112,347 151,669 Other current assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . 8,889 12,000 Total assets . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 293,433 396,135 Trade and other payables . . . . . . . . . . . . . . . . . . . . . . . . 28,372 38,302 Other current liabilities . . . . . . . . . . . . . . . . . . . . . . . . . . 9,001 12,152 Total liabilities and contingent liabilities . . . . . . . . . . . . . . 37,373 50,454 Total net assets acquired . . . . . . . . . . . . . . . . . . . . . . . . . 256,060 345,681 The result for the year 2013 of the acquired business, until operating result, is presented as follows: Thousands of Thousands of Euros US Dollars Net revenue . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 582,222 786,000 Cost of sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (289,630) (391,000) Gross Profit . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 292,592 395,000 Research and development . . . . . . . . . . . . . . . . . . . . . . . (21,481) (29,000) Selling, general and administration expense . . . . . . . . . . . (53.333) (72,000) Operating result . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 217,778 294,000 F-244 |
|
|
LES]UNIVERSAL.BST;124 F-245 Information on Group Companies, Associates and others for the years ended 31 December 2013 and 2012 12/31/2013 12/31/2012 Acquisition/ % shares % shares Incorporation Name Registered Offices date Activity Statutory Activity Direct Indirect Direct Indirect Fully Consolidated Companies Diagnostic Grifols, S.A. . . . . . . . . . . . Pol´igono Levante Calle Can 1987 Industrial Development and manufacture of diagnostic 99.998% 0.002% 99.998% 0.002% Guasch, s/n 08150 Parets del equipment, instruments and reagents. Vall`es (Barcelona) Spain Instituto Grifols, S.A. . . . . . . . . . . . . . Pol´igono Levante 1987 Industrial Plasma fractioning and the manufacture of 99.998% 0.002% 99.998% 0.002% Calle Can Guasch, s/n haemoderivative pharmaceutical products. 08150 Parets del Vall`es (Barcelona) Spain Logister, S.A. . . . . . . . . . . . . . . . . . Pol´igono Levante 1987 Industrial Manufacture, sale and purchase, — 100.000% — 100.000% Calle Can Guasch, s/n commercialisation and distribution of all types of 08150 Parets del Vall`es computer products and materials. (Barcelona) Spain Laboratorios Grifols, S.A. . . . . . . . . . . Pol´igono Levante 1989 Industrial Production of glass- and plastic-packaged 99.998% 0.002% 99.998% 0.002% Calle Can Guasch, s/n parenteral solutions, parenteral and enteral 08150 Parets del Vall`es nutrition products and blood extraction (Barcelona) Spain equipment and bags. Biomat, S.A. . . . . . . . . . . . . . . . . . . Pol´igono Levante 1991 Industrial Analysis and certification of the quality of 99.900% 0.100% 99.900% 0.100% Calle Can Guasch, s/n plasma used by Instituto Grifols, S.A. It also 08150 Parets del Vall`es provides transfusion centres with plasma virus (Barcelona) Spain inactivation services (I.P.T.H). Grifols Engineering, S.A. . . . . . . . . . . Pol´igono Levante 2000 Industrial Design and development of the Group’s 99.950% 0.050% 99.950% 0.050% Calle Can Guasch, s/n manufacturing installations and part of the 08150 Parets del Vall`es equipment and machinery used at these (Barcelona) Spain premises. The company also renders engineering services to external companies. Biomat USA, Inc. . . . . . . . . . . . . . . . 2410 Lillyvale Avenue 2002 Industrial Procuring human plasma. — 100.000% — 100.000% Los Angeles (California) United States Grifols Biologicals, Inc. . . . . . . . . . . . 5555 Valley Boulevard 2003 Industrial Plasma fractioning and the production of — 100.000% — 100.000% Los Angeles (California) haemoderivatives. United States PlasmaCare, Inc. . . . . . . . . . . . . . . . 1128 Main Street, Suite 300 2006 Industrial Procuring human plasma. — 100.000% — 100.000% Cincinnati (Ohio) United States Grifols Australia Pty Ltd. . . . . . . . . . . Unit 5/80 Fairbank 2009 Industrial Distribution of pharmaceutical products and the 100.000% — 100.000% — Clayton South development and manufacture of reagents for Victoria 3149 diagnostics. Australia Medion Grifols Diagnostic AG . . . . . . . Bonnstrasse,9 2009 Industrial Development and manufacturing activities in the 80.000% — 80.000% — 3186 D¨ugingen area of biotechnology and diagnostics. Switzerland 3 C Cs: 17147 |
|
|
LES]UNIVERSAL.BST;124 F-246 Acquisition/ % shares % shares Incorporation Name Registered Offices date Activity Statutory Activity Direct Indirect Direct Indirect Grifols Therapeutics, Inc. . . . . . . . . . . 4101 Research Commons 2011 Industrial Plasma fractioning and the production of — 100.000% — 100.000% (Principal Address), haemoderivatives. 79 T.W. Alexander Drive, Research Triangle Park, Carolina del Norte 277709, United States Talecris Plasma Resources, Inc. . . . . . . . 4101 Research Commons 2011 Industrial Procuring human plasma. — 100.000% — 100.000% (Principal Address), 79 T.W. Alexander Drive, Research Triangle Park, Carolina del Norte 277709, United States GRI-CEI, S/A Produtos para transfusao . . Rua Umuarama, 263 2012 Industrial Production of bags for the extraction, 60.000% — 60.000% — Condominio Portal da Serra separation, conservation and transfusion of Vila Perneta blood components. CEP 83.325-000 Pinhais Paran´a, Brazil Grifols Worldwide Operations Limited . . 70 Sir John Rogerson’s Quay 2012 Industrial Packaging, labelling, storage, distribution, 100.000% — — — Dublin 2 manufacture and development of pharmaceutical Ireland products and rendering of financial services to Group companies. Progenika Biopharma, S.A. . . . . . . . . . Parque Tecnol´ogico de Vizcaya, 2013 Industrial Development, production and commercialisation 56.150% — — — Edificio 504 of biotechnological solutions. 48160 Derio (Vizcaya) Spain Proteomika, S.L.U . . . . . . . . . . . . . . Parque Tecnol´ogico de Vizcaya, 2013 Industrial Development, production and commercialisation — 56.150% — — Edificio 504 of biotechnological solutions. 48160 Derio (Vizcaya) Spain Progenika Latina, S.A. de CV . . . . . . . . Periferico Sur Nº 4118 Int 8 2013 Industrial Development, production and commercialisation — 56.150% — — Col. Jardines del Pedregal of biotechnological solutions. CP 01900 Alvaro Obregon DF Mexico Progenika Inc. . . . . . . . . . . . . . . . . . Corporation Service Company, 2013 Industrial Development, production and commercialisation — 56.150% — — 2711 of genetic tools, diagnostic equipment and Centerville Road, Suite 400, therapeutic systems and products for Wilmington, DE 19808 personalised medicine and the highest quality United States healthcare in general. Preventia 2.0 Genetics, S.L. . . . . . . . . . Calle Ercilla 17 - 3º 2013 Industrial Research, development and commercialisation of — 56.150% — — 48009 Bilbao-Vicaya diagnostic products, treatment of diseases and Spain rendering of related services. Brainco Biopharma, S.L. . . . . . . . . . . . Parque Tecnol´ogico de Vizcaya, 2013 Industrial Development of products for the treatment and — 28.423% — — Edificio 504 diagnosis of psychiatric illnesses 48160 Derio (Vizcaya) Spain 3 C Cs: 17937 |
|
|
LES]UNIVERSAL.BST;124 F-247 p Name Registered Offices date Activity Statutory Activity Direct Indirect Direct Indirect Abyntek Biopharma, S.L. . . . . . . . . . . Parque Tecnol´ogico de Vizcaya, 2013 Industrial Research, development and transfer of — 43.763% — — Edificio 504 biotechnological products and processes, as well 48160 Derio (Vizcaya) as the commercialiation of products and services Spain related to the biosciences. Asociaci´on I+D Progenika . . . . . . . . . Parque Tecnol´ogico de Vizcaya, 2013 Industrial Coordination, representation, management and — 56.150% — — Edificio 504 promotion of the common interests of associated 48160 Derio (Vizcaya) companies, in addition to contributing to the Spain development, growth and internationalisation of its associates and of the biosciences sector in the Basque Country. G-C Diagnostics Corp. . . . . . . . . . . . . 4560 Horton Street 2013 Industrial Participation in any activity to facilitate the 100.000% — — — 94608 Emeryville, California organisation of the companies under the United States jurisdiction of Delaware. Grifols Asia Pacific Pte, Ltd . . . . . . . . . 501 Orchard Road nº20-01 2003 Commercial Distribution and sale of medical and 100.000% — 100.000% — 238880 Wheelock Place, pharmaceutical products. Singapore Grifols Movaco, S.A. . . . . . . . . . . . . . Pol´igono Levante 1987 Commercial Distribution and sale of reagents, chemical 99.999% 0.001% 99.999% 0.001% Calle Can Guasch, s/n products and other pharmaceutical specialities, 08150 Parets del Vall`es and of medical and surgical materials, (Barcelona) Spain equipment and instruments for use by laboratories and health centres. Grifols Portugal Productos Farmac´euticos e Hospitalares, Lda. . . . . . . . . . . . . Rua de Sao Sebastiao,2 1988 Commercial Import, export and commercialisation of 0.010% 99.990% 0.010% 99.990% Zona Industrial Cabra Figa pharmaceutical and hospital equipment and 2635-448 Rio de Mouro products, particularly Grifols products. Portugal Grifols Chile, S.A. . . . . . . . . . . . . . . Avda. Americo Vespucio, 2242 1990 Commercial Development of pharmaceutical businesses, 99.000% — 99.000% — Comuna de Conchali which can involve the import, production, Santiago de Chile commercialisation and export of related Chile products. Grifols USA, LLC. . . . . . . . . . . . . . . Corporation Service Company, 1990 Commercial Distribution and marketing of company — 100.000% — 100.000% 2711 products. Centerville Road, Suite 400, Wilmington, DE 19808 United States Grifols Argentina, S.A. . . . . . . . . . . . . Bartolom´e Mitre 3690/3790, 1991 Commercial Clinical and biological research. Preparation of 95.010% 4.990% 99.260% 0.740% CPB1605BUT Munro reagents and therapeutic and diet products. Partido de Vicente Lopez Manufacture and commercialisation of other Argentina pharmaceutical specialities. Grifols s.r.o. . . . . . . . . . . . . . . . . . . Calle Zitna,2 1992 Commercial Purchase, sale and distribution of chemical- 100.000% — 100.000% — Prague pharmaceutical products, including human Czech Republic plasma. Grifols (Thailand) Ltd . . . . . . . . . . . . 191 Silom Complex Building, 2003 Commercial Import, export and distribution of — 48.000% — 48.000% 21st Follor, Silom Road, Silom, pharmaceutical products. Bangrak 10500 Bangkok Thailand 3 C Cs: 45555 |
|
|
LES]UNIVERSAL.BST;124 F-248 Incorporation Name Registered Offices date Activity Statutory Activity Direct Indirect Direct Indirect Grifols Malaysia Sdn Bhd . . . . . . . . . . Level 18, The Gardens North 2003 Commercial Distribution and sale of pharmaceutical — 30.000% — 30.000% Tower, Mid Valley City, products. Lingkaran Syed Putra 59200 Kuala Lumpur Malaysia Grifols International, S.A. . . . . . . . . . . Pol´igono Levante 1997 Commercial Coordination of the marketing, sales and 99.900% 0.100% 99.900% 0.100% Calle Can Guasch, s/n logistics for all the Group’s subsidiaries 08150 Parets del Vall`es operating in other countries. (Barcelona) Spain Grifols Italia S.p.A . . . . . . . . . . . . . . Via Carducci, 62d 1997 Commercial Purchase, sale and distribution of chemical- 100.000% — 100.000% — 56010 Ghezzano pharmaceutical products. Pisa, Italy Grifols UK Ltd. . . . . . . . . . . . . . . . . Gregory Rowcliffe & Milners, 1 1997 Commercial Distribution and sale of therapeutic and other 100.000% — 100.000% — Bedford Row, London pharmaceutical products, especially WC1R 4BZ haemoderivatives. United Kingdom Grifols Brasil, Ltda. . . . . . . . . . . . . . . Rua Umuarama, 263 1998 Commercial Import and export, preparation, distribution and 100.000% — 100.000% — Condominio Portal da Serra sale of pharmaceutical and chemical products Vila Perneta for laboratory and hospital use, and medical- CEP 83.325-000 Pinhais surgical equipment and instruments. Paran´a, Brazil Grifols France, S.A.R.L. . . . . . . . . . . . Arteparc, Rue de la Belle du 1999 Commercial Commercialisation of chemical and healthcare 99.990% 0.010% 99.990% 0.010% Canet, Bˆat. D, Route de la products. Cˆote d’Azur, 13590 Meyreuil France Grifols Polska Sp.z.o.o. . . . . . . . . . . . . Grzybowska 87 street00-844 2003 Commercial Distribution and sale of pharmaceutical, 99.990% 0.010% 99.990% 0.010% Warsaw, Poland cosmetic and other products. Log´istica Grifols, S.A. de C.V. . . . . . . . Calle Eugenio Cuzin, 2008 Commercial Manufacture and commercialisation of 99.990% 0.010% 99.990% 0.010% nº 909-913 pharmaceutical products for human and Parque Industrial Belenes Norte veterinary use. 45150 Zapop´an Jalisco, Mexico Grifols M´exico, S.A. de C.V. . . . . . . . . Calle Eugenio Cuzin, 1970 Commercial Production, manufacture, adaptation, — 80.000% — 80.000% nº 909-913 conditioning, sale and purchase, commissioning, Parque Industrial Belenes Norte representation and consignment of all kinds of 45150 Zapop´an pharmaceutical products and the acquisition of Jalisco, Mexico machinery, equipment, raw materials, tools, movable goods and property for the aforementioned purposes. Medion Diagnostics GmbH . . . . . . . . . Lochamer Schlag, 12D 2009 Commercial Distribution and sale of biotechnological and — 80.000% — 80.000% 82166 Gr¨afelfing diagnostic products. Germany Grifols Nordic, AB . . . . . . . . . . . . . . Sveav¨agen 166 2010 Commercial Research and development, production and 100.000% — 100.000% — 11346 Stockholm marketing of pharmaceutical products, medical Sweden devices and any other asset deriving from the aforementioned activities. 3 C Cs: 27166 |
|
|
LES]UNIVERSAL.BST;124 F-249 Acquisition/ % shares % shares Incorporation Name Registered Offices date Activity Statutory Activity Direct Indirect Direct Indirect Grifols Colombia, Ltda . . . . . . . . . . . . Carrera 7 No. 71 52 Torre B 2010 Commercial Sale, commercialisation and distribution of 99.000% 1.000% 99.000% 1.000% piso 9 medicines, pharmaceutical (including but not Bogot´a. D.C. limited to haemoderivatives) and hospital Colombia products, medical devices, biomedical equipment, laboratory instruments and reagents for diagnosis and/or healthcare software. Grifols Deutschland GmbH . . . . . . . . . Lyoner Strasse 15, D- 2011 Commercial Procurement of the official permits and 100.000% — 100.000% — 60528 Frankfurt am Main necessary approval for the production, Germany commercialisation and distribution of products deriving from blood plasma, as well as the import, export, distribution and sale of reagents and chemical and pharmaceutical products, especially for laboratories and health centres and surgical and medical equipment and instruments. Grifols Canada, Ltd. . . . . . . . . . . . . . 5060 Spectrum Way, Suite 405 2011 Commercial Provision of various services (marketing) to — 100.000% — 100.000% (Principal Address) Grifols Therapeutics Inc. Mississauga, Ontario L4W 5N5 Canada Grifols Pharmaceutical Consulting (Shanghai) Co., Ltd. . . . . . . . . . . . . Unit 901-902, Tower 2, 2013 Commercial Pharmaceutical consultancy services (except for 100.000% — — — No. 1539, West Nanjing Rd., diagnosis), technical and logistical consultancy Jing’an District, Shanghai services, business management and marketing 200040 consultancy services. China Grifols Switzerland AG . . . . . . . . . . . Steinengraben, 5 2013 Commercial Research, development, import and export and 100.000% — — — 40003 Basel commercialisation of pharmaceutical products, Switzerland devices and diagnostic instruments. Grifols Viajes, S.A. . . . . . . . . . . . . . . Can Guasch, 2 1995 Services Travel agency exclusively serving Group 99.900% 0.100% 99.900% 0.100% 08150 Parets del Vall`es companies. Barcelona, Spain Squadron Reinsurance Ltd. . . . . . . . . . The Metropolitan Building, 2003 Services Reinsurance of Group companies’ insurance — 100.000%100.000% — 3rd Fl. policies. James Joyce Street, Dublin Ireland Arrahona Optimus, S.L. . . . . . . . . . . . Avenida de la Generalitat 152 2008 Services Development and construction of offices and 99.990% 0.010% 99.990% 0.010% Sant Cugat del Valles business premises. (Barcelona) Spain Grifols, Inc. . . . . . . . . . . . . . . . . . . 2410 Lillivale Avenue 2011 Services Acquisition, manufacture and sale of therapeutic 100.000% — 100.000% — 90032 Los Angeles, California products, especially haemoderivatives extracted United States using plasma fractioning through a network of donation centres owned by the Group in the USA. 3 C Cs: 33819 |
|
|
LES]UNIVERSAL.BST;124 F-250 12/31/2013 12/31/2012 Acquisition/ % shares % shares Incorporation Name Registered Offices date Activity Statutory Activity Direct Indirect Direct Indirect Talecris Biotherapeutics Overseas Services, Corp. . . . . . . . . . . . . . . . 4101 Research Commons 2011 Services Provision of support services for the sale of — — — 100.000% (Principal Address), biotherapeutic products outside the USA and 79 T.W. Alexander Drive, participation in any other activity for which the Research Triangle Park, companies may be organised in accordance with North Carolina 277709, the General Corporation Law of Delware. United States Gri-Cel, S.A. . . . . . . . . . . . . . . . . . . Avenida de la Generalitat 152 2009 Research Research and development in the field of 0.001% 99.999% 0.001% 99.999% Sant Cugat del Valles regenerative medicine, awarding of research (Barcelona) grants, subscription to collaboration agreements Spain with entities and participation in projects in the area of regenerative medicine. Araclon Biotech, S.L. . . . . . . . . . . . . . Paseo de Sagasta, 17 2º izqda. 2012 Research Creation and commercialisation of a blood — 61.12% — 51.000% Zaragoza, Spain diagnosis kit for the detection of Alzheimer’s and development of effective immunotherapy (vaccine) against this disease. Equity accounted investees Nanotherapix, S.L. . . . . . . . . . . . . . . Avenida de la Generalitat 152 2010 Research Development, validation and production of the — 51.000% — 51.000% Sant Cugat del Valles technology required to implement the use of (Barcelona) genetic and cellular therapy for the treatment of Spain human and animal pathologies. VCN Bioscience, S.L. . . . . . . . . . . . . Avenida de la Generalitat 152 2012 Research Research and development of therapeutic — 40.000% — — Sant Cugat del Valles approaches for tumours for which there is (Barcelona) currently no effective treatment. Spain Aradigm Corporation . . . . . . . . . . . . . 3929 Point Eden Way 2013 Research Development and commercialisation of drugs 35.000% — — — Hayward, California delivered by inhalation for the prevention and United States treatment of severe respiratory diseases. TiGenix N.V. . . . . . . . . . . . . . . . . . . Romeinse straat 12 bus 2, 3001 2013 Research Research and development of therapies based — 21.300% — — Leuven, Belgium on stem cells taken from adipose tissue. Mecwins, S.L. . . . . . . . . . . . . . . . . . Avenida Fernandos Casas 2013 Research Research and production of nanotechnological, — 14.038% — — Novoa, 37 biotechnological and chemical solutions. Santiago de Compostela Spain This appendix forms an integral part of note 2 to the consolidated annual accounts 3 C Cs: 11917 |
|
|
LES]UNIVERSAL.BST;124 F-251 APPENDIX II GRIFOLS, S.A. AND SUBSIDIARIES Operating Segments for the years ended 31 December 2013 and 2012 (Expressed in thousands of Euros) Bioscience Hospital Diagnostic Raw materials Others/Unallocated Consolidated 2013 2012 2013 2012 2013 2012 2013 2012 2013 2012 2013 2012 Income from external customers . . . . . . . . . . . . 2,448,824 2,325,088 97,131 95,870 130,339 134,341 38,028 31,450 27,410 34,195 2,741,732 2,620,944 Total operating income . . . . . . . . . . . . . . . . . . . 2,448,824 2,325,088 97,131 95,870 130,339 134,341 38,028 31,450 27,410 34,195 2,741,732 2,620,944 Profit/(Loss) for the segment . . . . . . . . . . . . . . . 980,835 888,094 139 1,177 (3,819) 9,291 11,664 10,657 27,306 33,881 1,016,125 943,100 Unallocated expenses . . . . . . . . . . . . . . . . . . . (280,005) (283,016) (280,005) (283,016) Operating profit . . . . . . . . . . . . . . . . . . . . . . . 736,120 660,084 Finance result . . . . . . . . . . . . . . . . . . . . . . . . . (237,419) (270,729) Share of profit/(loss) of equity accounted investees . 725 (1,890) (1,407) (1,165) (1,407) Income tax expense . . . . . . . . . . . . . . . . . . . . (155,482) (132,571) Profit for the year after tax . . . . . . . . . . . . . . . . 342,054 255,377 Segment assets . . . . . . . . . . . . . . . . . . . . . . . 4,501,977 4,581,022 81,500 79,947 215,990 144,833 394 15,792 4,799,861 4,821,594 Equity accounted investments . . . . . . . . . . . . . . 21,002 — — — — — — — 14,763 2,566 35,765 2,566 Unallocated assets . . . . . . . . . . . . . . . . . . . . . 1,005,410 803,314 1,005,410 803,314 Total assets . . . . . . . . . . . . . . . . . . . . . . . . . . 5,841,036 5,627,474 Segment liabilities . . . . . . . . . . . . . . . . . . . . . 230,412 264,160 241 397 14,801 12,040 — 245,454 276,597 Unallocated liabilities . . . . . . . . . . . . . . . . . . . 3,488,378 3,470,136 3,488,378 3,470,136 Total liabilities . . . . . . . . . . . . . . . . . . . . . . . . 3,733,832 3,746,733 Other information: Amortisation and depreciation . . . . . . . . . . . . . 91,350 91,564 5,695 5,382 15,492 11,310 — — 15,932 20,870 128,469 129,126 Expenses that do not require cash payments . . . . (11,090) 11,683 141 248 337 247 — — 2,979 4,946 (7,633) 17,124 Additions for the year of property, plant & equipment and intangible assets . . . . . . . . . . . 129,475 140,880 8,514 6,435 24,408 12,003 — — 19,582 14,154 181,979 173,472 This appendix forms an integral part of note 6 to the consolidated annual accounts. 3 C Cs: 11799 |
|
|
LES]UNIVERSAL.BST;124 F-252 APPENDIX II GRIFOLS, S.A. AND SUBSIDIARIES Reporting by geographical area for the years ended 31 December 2013 and 2012 (Expressed in thousands of Euros) Rest of Spain European Union USA + Canada Rest of World Subtotal Raw material Consolidated 2013 2012 2013 2012 2013 2012 2013 2012 2013 2012 2013 2012 2013 2012 Net Revenue . . . . . . . . . . . . . . . . . 207,922 212,983 361,905 346,345 1,707,620 1,658,548 426,257 371,618 2,703,704 2,589,494 38,028 31,450 2,741,732 2,620,944 Assets by geographical area . . . . . . . 933,722 759,670 280,510 126,041 4,487,429 4,573,400 138,981 152,571 5,840,642 5,611,682 394 15,792 5,841,036 5,627,474 Other information: Additions for the year of property, plant & equipment and intangible assets . . . . . . . . . . . . . . . . . . . 55,978 51,014 14,847 3,081 106,274 114,109 4,880 5,268 181,979 173,472 — — 181,979 173,472 This appendix forms an integral part of note 6 to the consolidated annual accounts. 3 C Cs: 60262 |
|
|
APPENDIX III GRIFOLS, S.A. AND SUBSIDIARIES Changes in Other Intangible Assets for the year ended 31 December 2012 (Expressed in thousands of Euros) Balances at Business Translation Balances at 2011 Additions Combination Transfers Disposals differences 2012 Development costs . . . . . . . . . . . . 69,783 9,825 11,282 0 (3,969) (18) 86,903 Concessions, patents, licenses brands & similar . . . . . . . . . . . . 52,929 80 1,575 (31) 0 (578) 53,975 Computer software . . . . . . . . . . . . 67,967 10,033 69 3,508 (7,338) (6,549) 67,690 Currently marketed products . . . . . . 927,429 0 0 0 0 (17,925) 909,504 Other intangible assets . . . . . . . . . . 2,476 162 0 31 (314) (38) 2,317 Total cost of intangible assets . . . . 1,120,584 20,100 12,926 3,508 (11,621) (25,108) 1,120,389 Accum. amort. of development costs . (40,078) (4,957) (122) 0 1,724 18 (43,415) Accum. amort of concessions, patents, licenses, brands & similar . . . . . . . (18,866) (1,012) (246) 0 0 347 (19,777) Accum. amort. of computer software . (34,122) (11,779) (33) 0 3,222 4,258 (38,454) Accum. amort. of currently marketed products . . . . . . . . . . . . . . . . . (18,033) (31,125) 0 0 0 1,157 (48,001) Accum. amort. of other intangible assets . . . . . . . . . . . . . . . . . . . (914) (630) 0 0 0 6 (1,538) Total accum. amort intangible assets . (112,013) (49,503) (401) 0 4,946 5,786 (151,185) Impairment of other intangible assets . (264) 155 0 0 0 0 (109) Carrying amount of intangible assets . . . . . . . . . . . . . . . . . . 1,008,307 (29,248) 12,525 3,508 (6,675) (19,322) 969,095 (note 3 (b)) This appendix forms an integral part of note 8 to the consolidated annual accounts F-253 |
|
|
APPENDIX III GRIFOLS, S.A. AND SUBSIDIARIES Changes in Other Intangible Assets for the year ended 31 December 2013 (Expressed in thousands of Euros) Balances at Business Translation Balances at 2012 Additions combinations Transfers Disposals differences 2013 Development costs . . . . . . . . . . . . 86,903 11,309 13,721 0 (98) (47) 111,788 Concessions, patents, licenses brands & similar . . . . . . . . . . . . 53,975 41 2,717 (5) (2,758) (1,163) 52,807 Computer software . . . . . . . . . . . . 67,690 13,227 668 22,268 (4,545) (1,681) 97,627 Currently marketed products . . . . . . 909,504 0 23,792 0 0 (39,371) 893,925 Other intangible assets . . . . . . . . . . 2,317 9,810 0 0 (238) (363) 11,526 Total cost of intangible assets . . . . 1,120,389 34,387 40,898 22,263 (7,639) (42,625) 1,167,673 Accum. amort. of development costs . (43,415) (5,206) (9,251) 0 0 42 (57,830) Accum. amort of concessions, patents, licenses, brands & similar . (19,777) (1,113) (1,654) 1 863 262 (21,418) Accum. amort. of computer software . (38,454) (7,422) (408) (21,285) 3,773 681 (63,115) Accum. amort. of currently marketed products . . . . . . . . . . . . . . . . . (48,001) (32,221) 0 0 0 3,311 (76,911) Accum. amort. of other intangible assets . . . . . . . . . . . . . . . . . . . (1,538) (424) 0 0 0 22 (1,940) Total accum. amort intangible assets . . . . . . . . . . . . . . . . . . (151,185) (46,386) (11,313) (21,284) 4,636 4,318 (221,214) Impairment of other intangible assets (109) 85 0 0 0 0 (24) Carrying amount of intangible assets . . . . . . . . . . . . . . . . . 969,095 (11,914) 29,585 979 (3,003) (38,307) 946,435 (note 3 (a)) This appendix forms an integral part of note 8 to the consolidated annual accounts F-254 |
|
|
LES]UNIVERSAL.BST;124 F-255 APPENDIX IV GRIFOLS, S.A. AND SUBSIDIARIES Movement in Property, Plant and Equipment for the year ended 31 December 2012 (Expressed in thousands of Euros) Balances at Business Translation Balances at 2011 Additions combination Transfers Disposals differences 2012 Cost: Land and buildings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 156,868 2,049 0 38,176 (9,006) (5,877) 182,210 Plant and machinery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 773,215 26,258 3,822 (17,947) (26,346) (11,346) 747,656 Under construction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 121,219 125,065 0 (23,831) (5,413) (3,862) 213,178 1,051,302 153,372 3,822 (3,602) (40,765) (21,085) 1,143,044 Accumulated depreciation: Buildings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (15,434) (5,302) 0 2,335 1,398 1,921 (15,082) Technical installations and other items . . . . . . . . . . . . . . . . . . . . . . . . . . . . (252,787) (74,321) (2,100) (2,241) 11,006 7,727 (312,716) (268,221) (79,623) (2,100) 94 12,404 9,648 (327,798) Impairment of other property, plant and equipment . . . . . . . . . . . . . . . . . . . . (7,212) (1,597) 0 0 3,954 (284) (5,139) Carrying amount . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 775,869 72,152 1,722 (3,508) (24,407) (11,721) 810,107 (note 3 (b)) This appendix forms an integral part of note 9 to the consolidated annual accounts. 3 C Cs: 40505 |
|
|
LES]UNIVERSAL.BST;124 F-256 APPENDIX IV GRIFOLS, S.A. AND SUBSIDIARIES Movement in Property, Plant and Equipment for the year ended 31 December 2013 (Expressed in thousands of Euros) Balances at Business Translation Balances at 2012 Additions combination Transfers Disposals differences 2013 Cost: Land and buildings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 182,210 4,888 5,298 25,954 (923) (7,764) 209,663 Plant and machinery . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 747,656 62,644 7,093 156,076 (27,028) (25,570) 920,871 Under construction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 213,178 80,060 8 (176,880) (769) (5,732) 109,865 1,143,044 147,592 12,399 5,150 (28,720) (39,066) 1,240,399 Accumulated depreciation: Buildings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (15,082) (6,399) (605) (1,717) 426 617 (22,760) Technical installations and other items . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (312,716) (75,684) (4,517) (4,412) 15,663 8,812 (372,854) (327,798) (82,083) (5,122) (6,129) 16,089 9,429 (395,614) Impairment of other property, plant and equipment . . . . . . . . . . . . . . . . . . . . . . . . . (5,139) 186 0 0 0 406 (4,547) Carrying amount . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 810,107 65,695 7,277 (979) (12,631) (29,231) 840,238 (note 3 (a)) This appendix forms an integral part of note 9 to the consolidated annual accounts. 3 C Cs: 26521 |
|
|
APPENDIX V GRIFOLS, S.A. AND SUBSIDIARIES Statement of Liquidity for Distribution of Interim Dividend 2013 (Expressed in thousands of Euros) Thousands of Euros Forecast profits distributable for 2013: Projected profits net of taxes until 31/12/2013 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155,433 Less, charge required to legal reserve . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . (344) Estimated profits distributable for 2013 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 155,089 Interim dividend distributed . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 68,755 Forecast cash for the period 24 May 2013 to 24 May 2014: Cash balances at 24 May 2013 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 70,594 Projected amounts collected . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 459,308 Projected payments, including interim dividend . . . . . . . . . . . . . . . . . . . . . . . . . . . . 252,206 Projected cash balances at 24 May 2014 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 277,696 This appendix forms an integral part of note 16 to the consolidated annual accounts. F-257 |
|
|
CONSOLIDATED DIRECTORS’ REPORT 2013 To the Shareholders: Grifols is a sound, growing company with a clearly defined mission: improving people’s health and welfare. The company’s capitalisation at the end of 2013(1) was Euros 10,790 million and our financial results and the milestones achieved in terms of production and commercialisation of our products, R&D, Human Resources or the environment, are evidence of how Grifols was managed in 2013 and the added value generated by our Company throughout the year. 1.—THE COMPANY’S POSITION In 2013 Grifols maintained its position as the third largest producer of plasma derivatives in the world, with a global market share of approximately 20%(2). The Group’s main products are leaders in global sales(2): Product—plasma derivative Market share World ranking Polyvalent IVIG (intravenous immunoglobulin) . . . . . . . . 27% 1 Alpha-1 Antitrypsin . . . . . . . . . . . . . . . . . . . . . . . . . . . . 66% 1 Coagulation factor VIII . . . . . . . . . . . . . . . . . . . . . . . . . 18% 2 Albumin . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 14% 3 The company has achieved an excellently and competitive position in its three diagnostics specialities: transfusions, immunology and haemostasis. In addition, the recent(3) acquisition of Novartis’ diagnostics unit for transfusions and immunology will allow the Group to grow further in the diagnostics segment from 2014, improving its range of equipment and reagents in the United States and other key markets and increasing the contribution from the Diagnostic division, which could generate close to 20% of total revenue. The Hospital division retains its leading position in Spain as a provider of intravenous solutions and the company intends to continue internationalising this division. Grifols’ main lines of business (Bioscience, Diagnostic and Hospital) are sound and consolidated. The confidence of shareholders and investors has enabled Grifols to continue its investment activity in 2013 to maintain its leading position in innovation, developing technology and improving production processes by investing Capex, but also looking for differentiating factors that generate added value. The company has an ambitious R&D plan and has made various strategic acquisitions over the course of the year. Some of the transactions will produce immediate results (Novartis’ diagnostics unit) and others form part of the strategy towards a sustainable and future projection (Progenika, Aradigm and TiGenix). Therefore, Grifols’ management has focused on two main areas: consolidating areas of recurring business and unlocking new opportunities for future growth by acquiring interests in research companies to guarantee and finance the viability of their R&D projects. (1) Market capitalisation calculated using closing prices of Class A and B shares in Grifols at 31 December 2013, the last trading day in 2013. (2) Source: Market Research Bureau (MRB)—The Worldwide Plasma Proteins Market 2012. (3) The acquisition was finalised in January 2014. F-258 |
|
|
2.—BUSINESS PERFORMANCE AND RESULTS INCOME STATEMENT: KEY INDICATORS • Sales performance: revenues of Euros 2,742 million Grifols closed 2013 with revenues of Euros 2,741.7 million, an increase of 4.6% on the prior year. The geographical diversification of sales limited the effect of volatility in exchange rates, in particularly the Euro-Dollar, and revenues grew by 7.4% at a constant exchange rate (cc). Boosting sales in regions which have been less affected by austerity measures, with shorter collection periods and better margins continued to be one of the company’s core strategies in 2013r. As a result, Grifols remained very active on international markets during the period, where it generated 92.4% of its revenues. Sales outside Spain rose by 5.2% (8.2% at a constant exchange rate) on 2012, to Euros 2,533.8 million. Breakdown of sales in 2013 by geographical areas: % var % of % of (constant (Thousands of Euros) 2013 sales 2012 sales % Var currency) EU . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 569,827 20.8% 559,327 21.3% 1.9% 2.2% US & Canada . . . . . . . . . . . . . . . . . . . . . 1,707,620 62.3% 1,658,548 63.3% 3.0% 6.1% RoW (Rest of the World) . . . . . . . . . . . . . 426,257 15.5% 371,619 14.2% 14.7% 19.5% SUBTOTAL . . . . . . . . . . . . . . . . . . . . . . 2,703,704 98.6% 2,589,494 98.8% 4.4% 7.2% RAW MATERIALS . . . . . . . . . . . . . . . . . 38,028 1.4% 31,450 1.2% 20.9% 23.5% TOTAL . . . . . . . . . . . . . . . . . . . . . . . . . 2,741,732 100.0% 2,620,944 100.0% 4.6% 7.4% In the European Union sales performance has confirmed the expected recovery. Recurring revenues, excluding Spain, grew by 4.5% to Euros 361.9 million. In Spain declines in sales of Diagnostic and Hospital products and services as a result of the cuts to public health service spending—which have reduced the number of operations and diagnostic tests performed at hospitals—have limited the growth in the region. The decline in Spain has slowed when compared to 2012, to 2.4%, with a turnover of Euros 207.9 million, representing 7.6% of total revenues. In the United States and Canada the sales performance of the main plasma proteins was good in terms of volumes, with double-digit growth in albumin and factor VIII. In Canada, activity was stable following the renegotiation of the contracts with the Canadian Blood Services (CBS) and H´ema-Qu´ebec. Grifols remains the country’s main supplier of haemoderivatives and plasma fractionator. The mid-year restructuring has also provided the company’s business in the country with more operating flexibility. Sales of plasma proteins in the United States have gradually risen, and the company has achieved record turnover growth in absolute terms of 7.9% (11.2% at a constant exchange rate) on a quarterly basis in the world’s largest haemoderivatives market. In addition, Grifols has bolstered its presence in the US with new products and services in areas such as Hospital Logistics (Hospital division) and the Diagnostic Division, as the FDA has approved a number of reagents and instruments for blood typing which are fundamental for its commercialisation. The Group’s total sales in the United States grew by 6.3% (9.6% cc) in 2013. The most dynamic areas remain those outside the European Union and North America. Overall, RoW sales (Rest of the World excluding Raw Materials) grew by 14.7% (19.5% cc). For Grifols, these emerging regions are the ones with the greatest growth potential. The company has strengthened its presence in markets such as the Middle East, opening a new branch office in Dubai; or China, where Grifols’ sales have grown rapidly over the past three years, and the company expects to consolidate its position as a key player in immunohaematology and a supplier of albumin, the only plasma protein which can be exported at present; or Brazil, where construction has started on a plant to manufacture F-259 |
|
|
bags for extraction and storage of blood components. Market research in countries such as Turkey, India and Russia has also progressed. Currently, Grifols has commercial presence in 25 countries through its subsidiaries and sells its products in close to 100 countries. Internationalisation is a core strategy for the company, and in 2013 it continued to implement measures to bolster this expansion. These measures included: • Opening a branch office in Dubai to manage the business in the Middle East. • Centralising and integrating logistical operations in a new centre which will be built in Ireland, allowing the company to optimise its distribution infrastructure, increase the efficiency of its operations and promote cost savings. • Converting the branch office in China (Shanghai) into a commercial subsidiary of the Group, providing the legal structure needed to develop and expand in this market in the coming years. Grifols’ organic growth will also come from strengthening the products and services offered by its three divisions in its main markets. The company’s has followed a commercial strategy of integration to complement its range of plasma protein therapies with Diagnostic (Diagnostic division) and Hospital logistics (Hospital division) products and services. In November 2013 this strategy led to an agreement to acquire Novartis’ transfusion and immunology diagnostics business, which focuses on guaranteeing the safety of the blood donated for transfusions. This transaction(4) has allowed Grifols to complement and expand the range of products and services offered by its Diagnostic division, becoming a vertically integrated company, capable of offering solutions to blood donation centres with broadest range of products for immunohaematology, including gel-based reagents, multicards and the innovative genotyping technology Progenika, company acquired in 2013. The acquisition will also strengthen Grifols’ presence in the United States, with a sound and specialised sales network. The acquisition of Novartis’ transfusion and immunology diagnostics unit has not impacted 2013 figures, and the weights of the divisions did not change. Breakdown of sales in 2013 by divisions: % var (constant (Thousands of Euros) 2013 %sales 2012 %sales % Var currency) BIOSCIENCE DIVISION . . . . . . . . . . . . . 2,448,824 89.3% 2,325,088 88.7% 5.3% 8.2% HOSPITAL DIVISION . . . . . . . . . . . . . . . 97,131 3.5% 95,870 3.7% 1.3% 2.6% DIAGNOSTIC DIVISION . . . . . . . . . . . . 130,339 4.8% 134,342 5.1% 3.0% 1.0% RAW MATERIALS AND OTHERS . . . . . . 65,438 2.4% 65,644 2.5% 0.3% 1.6% TOTAL . . . . . . . . . . . . . . . . . . . . . . . . . 2,741,732 100.0% 2,620,944 100.0% 4.6% 7.4% Grifols’ main growth driver in 2013 was the Bioscience division. The volume of sales of plasmaderived biologic medical products in a scenario of stable prices drove revenues, with an upward trend in United States, the European Union and RoW. The Hospital division’s revenues rose slightly as a result of the gradual internationalisation of this line of business. Excluding the Spanish market, the division’s sales grew by more than 45%. The Diagnostic division’s turnover was stable in absolute terms at Euros 130 million as a result of increased reagent sales in emerging markets among other factors, and the sales decline slowed during the fourth quarter of 2013. (4) The acquisition was completed in January 2014. F-260 |
|
|
Revenues from the Raw Materials & Others division, which represents approximately 2.4% of total revenues, were almost flat. These revenues include royalties, work carried out for third parties by Grifols Engineering and revenues from the manufacturing agreements with Kedrion that have continued to decline, as expected. • Sound results: margins and profits continue to improve In 2013 the company worked hard to increase its efficiency and competitiveness, leading to a notable improvement in operating margins. The EBITDA margin was 140 basis points (bps) higher, and at the end of the year represented 31.5% of revenues. The adjusted EBITDA margin(5) rose by 160bp to 33.5% of revenues. The sales performance of the main plasma proteins sold by the Group, the optimisation of raw material and manufacturing costs through more flexible production processes have together increased the profitability per litre of plasma. This was reflected in gross margins growing by 6.6% to 51.7% of revenues. Operating expenses related to administration and general services were stable. These two factors have driven growth of 9.6% in EBITDA compared to 2012 to Euros 864.6 million. On an adjusted basis EBITDA(5) grew by 9.7% to Euros 917.4 million. In terms of more efficient production of plasma derivatives, the company has continued to work towards greater flexibility and scalability in its production processes, to constantly adapt to market conditions. In order to achieve this Grifols has focused on both increasing its protein fractionation and purification capacities and on making the processes more flexible. The aim is to be able to purify and fill the intermediate fractions generated during the first stage of the manufacturing process at any of the three plants of the Group, for which Grifols requires FDA and EMA licenses. In this regard, in 2013 Grifols obtained FDA approval to use Fraction IV-1 (intermediate product) obtained at the Los Angeles plant (California, US) in the production (purification and dosage) of Alpha-1 Antitrypsin (Prolastin -C) using the Clayton plant’s method (North Carolina, US). Authorisation was also obtained to use Fraction II+III obtained in the plant in Parets del Vall`es (Barcelona, Spain) to produce IVIG (Gamunex and Gamunex -C) at the Clayton plant in the future. Grifols continues to work towards obtaining an FDA licence to use the cryoprecipitate obtained in Clayton in purification of the factor VIII at the plants in Los Angeles. The financing conditions negotiated at the start of 2012 have also contributed to a 12.3% reduction in finance results, down to Euros 237.4 million from Euros 270.7 million in 2012. Moreover, the R&D deductions for 2012, received in the first quarter of 2013 and the combined State Corporate Tax return filed by the North Carolina (US) companies have reduced the effective tax rate. Overall, the Group’s 2013 net profit rose by 34.6% to Euros 345.6 million, 12.6% of the Group’s revenues. (5) Does not include costs related to the Talecris acquisition or other non-recurrent costs. F-261 |
|
|
Key indicators in 2013 (Thousands of Euros) 2013 2012 % var. TOTAL NET REVENUES . . . . . . . . . . . . . . . . . . . 2,741,732 2,620,944 4.6% EBITDA . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 864,588 789,209 9.6% % sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31.5% 30.1% ADJUSTED EBITDA* . . . . . . . . . . . . . . . . . . . . . 917,366 836,117 9.7% % sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 33.5% 31.9% GROUP NET PROFIT . . . . . . . . . . . . . . . . . . . . . 345,551 256,686 34.6% % sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 12.6% 9.8% ADJUSTED GROUP NET PROFIT** . . . . . . . . . . 450,021 364,671 23.4% % sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 16.4% 13.9% * Does not include costs related to the Talecris acquisition or other non-recurrent costs ** Does not include costs related to the Talecris acquisition, amortisation of intangibles or deferred finance costs related to the acquisition KEY BALANCE SHEET INDICATORS AND CASH FLOWS At December 2013 total consolidated assets amount to Euros 5,841.0 million, with no significant changes in relation to the Euros 5,627.5 million reported at December 2012. The main investments during 2013 were the stakes acquired in Progenika, Aradigm and TiGenix. The gradual deleveraging, the sound results obtained and the positive performance of cash flows have strengthened the balance sheet in 2013. • Improvement in inventory turnover and average collection period The improvements in efficiency have also been reflected in stock management and safety stock controls, resulting in a gradual reduction in inventories of 5.2%, as forecast. Stock turnover has also decreased from 281 days in December 2012 to 262 days at the end of 2013. Optimization of working capital management has continued as a consequence of the Group’s greater exposure to countries with shorter collection periods. Grifols’ average collection period was stable at 52 days, the same level as in December 2012. • Strong generation of cash flows, providing the solvency to make strategic investments The Group’s cash positions after dividend, debt and interest payments, have risen to Euros 708.8 million, well above the Euros 473.3 million reported in 2012,. Generation of operating cash flows was particularly strong, at Euros 592.0 million at December 2013. The higher profits obtained and the improvement and control of financing activities allowed the Group to reduce its financial cash flow requirements by more than Euros 200 million, increasing the cash used in investment activities to guarantee long-term growth. In this regard, the Company invested Euros 236.0 million in 2013, including the various acquisitions made over the course of the year and in line with its Capex plan. • Debt reduction and improved credit ratings Grifols’ net financial debt at December 2013 stood at Euros 2,087.2 million, a ratio of 2.28 times adjusted EBITDA(6) and lower than the ratio of 2.87 recorded at December 2012. Over the course of the year, the Group’s net financial debt has been reduced by Euros 308.9 million. F-262 |
|
|
This priority objective of continuing to decrease debt, together with the high sustainable levels of operating activity and ongoing progress in achieving improvements related to the acquisition of Talecris contributed to Standard & Poor’s ratifying its credit rating for Grifols and Moody’s upgrading its rating. Credit ratings at December 2013: Moody’s Standard & Poors Senior secured debt . . . . . . . . . . . . . . . . . . . . . . . . . . . . Ba1 BB+ Corporate rating . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Ba2 BB Senior unsecured debt . . . . . . . . . . . . . . . . . . . . . . . . . . B1 B+ Outlook . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Negative Stable • Equity Grifols equity increased to Euros 2,107.2 million in 2013, mainly as a result of the profits obtained during the period. To December 2013, Grifols’ share capital amounted to Euros 119.6 million, represented by 213,064,899 ordinary shares (Class A) with a par value of Euros 0.50 per share and 130,712,555 shares without voting rights (Class B) with a par value of Euros 0.10 per share. During the course of the year share capital was increased twice by issuing shares without voting rights (Class B): 1. In January 2013, the agreement adopted by Grifols’ shareholders at the extraordinary general meeting of 4 December 2012 was implemented, and share capital was increased by Euros 1.6 million by issuing 16,328,212 new shares. These bonus shares were distributed to shareholders in the proportion of 1 new Class B share for every 20 original shares, irrespective of whether they were Class A or Class B, as an alternative form of remuneration to cash dividends. 2. In April 2013, share capital was increased to acquire Progenika Biopharma by Euros 20.5 million, by issuing 884,997 new Class B shares without voting rights. Grifols’ ordinary shares (Class A) are listed on the Spanish stock exchange electronic trading system and form part of the IBEX-35 (GRF) index, while shares without voting rights (Class B) are also listed on the Spanish stock exchange (GRF.P) and on the US NASDAQ stock exchange (GRFS) through ADRs (American Depositary Receipts). In 2013 Grifols resumed payment of a cash dividend as remuneration for all its shareholders (Class A and Class B shares). The payment corresponding to 2013 will be made in two parts: an interim dividend and a complementary dividend. In the second quarter of 2013 the company paid an ordinary dividend for a gross amount of Euros 0.20 for each Class A and Class B share, on account of 2013 results, totalling Euros 68.75 million, as reflected in the Group accounts. The Group plans to pay a final complementary on 2013 earnings. Grifols’ dividend policy has not changed and its pay-out ratio remains 40% of net profit, the level prior to the acquisition of Talecris. PERFORMANCE BY BUSINESS AREA: DIVISION ANALYSIS • Bioscience Division: 89.3% of Grifols’ income The Bioscience Division has generated 89.3% of Grifols’ turnover and sales totalled Euros 2,448.8 million. Over 95% of sales were concentrated in the international markets. F-263 |
|
|
By volume,(6) mention should be made of the almost 30% growth in albumin sales bolstered by the United States and China, which has the highest demand for this protein in Asia. Also worth noting is the rise in alpha-1 antitrypsin, with double digit volume growth. Grifols is the world’s benchmark for this plasma protein and sales in 2013 were particularly significant in countries such as Canada, the United States, Germany and Spain, where sales of Prolastin have just commenced. In the US and Canadian market the commercial pulmonology and haematology teams have focused their efforts on promoting the diagnosis and identification of patients with a AAT deficiency (emphysema and chronic bronchitis), asthma, liver cirrhosis or chronic hepatitis, among others. In 2013 Grifols strengthened its presence in the area of respiratory illness and is seeking to establish a respiratory care franchise to specifically tackle this significant therapeutic area. To this end Grifols has its own line of haemoderivative products; Prolastin and Prolastin-C and the world licence for Pulmaquin and Lipoquin , medication for the treatment of severe respiratory conditions, including bronchiectasis not associated with cystic fibrosis (BE), for which the phase 2b clinical trials have already been completed. Grifols has the exclusive right to distribute these compounds following the acquisition of 35% of Aradigm Corporation. Hospital Plasma Service Grifols also provides a specific service for the Inactivation of Plasma for Hospital Transfusions (IPTH). In 2013 36,209 units were inactivated. In 2013 the Regional Government of Murcia (Spain) awarded the company the contract to manufacture medication derived from plasma using the surpluses from the Centro Regional de Hemodonaci´on. This contract will enable 55,000 units of plasma a year to be processed and the finished plasma products will be delivered to various hospitals in the region. Raw materials With regard to raw materials, in 2013 the Grifols centres in the US collected 6.4 million litres of plasma which, in line with the company’s optimisation strategy, represents a 10.3% increase. At 2013 year end Grifols’ plasmapheresis centres in the US received more than 26,000 donations of plasma a day. In this area, the company’s efforts during the year have focused on differentiation, in order to add value to the donors. One of the main initiatives taken is the pilot scheme at 20 plasma donor centres to remunerate donors with a cash card which is expected to be implemented in all the centres in 2014. These types of initiatives have been possible following the completion of the integration process of the logistic, economic and financial activities of all the plasma centres in a single management system, which represents an important step in the unification of the organisation’s systems. Once the plasma samples have been received they are analysed again. They are subject to a minimum of 10 serological and PCR tests at the Grifols laboratories in San Marcos and Austin (Texas- United States). These laboratories are equipped to analyse over 15 million plasma donations a year. Process security, quality and control systems The security of processes and products is of paramount importance to Grifols as is the implementation of solid quality and control systems to gain a competitive advantage. The most noteworthy improvements in the year are: the validation of the results of the ELISA analyses through the Architect system both for mini-pools and complete fractioning pools. This system enables 3 results to be obtained from the same piece of equipment, thereby optimising analysis times. It also permits the automatic and combined detection of parvovirus B19V and hepatitis A (HAV) using the TIGRIS technique in mini-pools (16 units). (6) Volume refers to grams/units sold and not the monetary amount thereof. F-264 |
|
|
Grifols Engineering is developing a system to automate the process of obtaining plasma donation samples, the Company is also studying the possibility of implementing an RFID identification system in all its plasma bottles with a view to monitoring them throughout the entire supply chain simplifying the process of handling the units. Main indicators of activity in 2013 2013 No. of plasmapheresis centres . . . . . . . . 150 No. of plasma donations/day . . . . . . . . . +26,000 No. of donation analyses . . . . . . . . . . . . +15 million donations Litres of plasma obtained . . . . . . . . . . . 6.4 million litres Number of fractioning plants . . . . . . . . . 3 plants Fractionation capacity . . . . . . . . . . . . . . Euros 8.5 million litres/year • Diagnostic division: 4.8% of income In 2013 the Diagnostic Division recorded sales of Euros 130.3 million, of which almost 80% were made outside of Spain. This business area represents approximately 4.8% of Grifols invoicing, although three important milestones in 2013 will have an immediate impact on the future of the division: 1. The purchase of the transfusion and immunological diagnostic unit of Novartis, which will enable the expansion of the division’s portfolio of equipment and reagents making Grifols the only company capable of offering comprehensive solutions to the blood donation centres in the most efficient and secure manner with full control over the transfusion process: from donation to transfusion. 2. The acquisition of 60% of the biotech company Progenika Biopharma in March 2013, serving to reinforce the portfolio of products and acquire the most innovative technology as this company is a global pioneer in the development of molecular biology testing for the performance of studies to check for transfusion compatibility. 3. The approval by the FDA of the reagents for immunohaematological typing (gel card) and the instruments required for their use (incubator, centrifuge and the automatic instruments WADiana and Erytra ). 2013 has been a year of transition. The division has been highly affected by the planned termination of a contract for the distribution of third party products with Ortho Diagnostic in the United States and efforts have focused on achieving the licenses and authorisations required to sell directly the products and technology derived from both the new companies acquired and the solid organic R&D portfolio. Prospects are favourable and the positive trend is already visible in the results obtained in the fourth quarter of 2013, with a growth in sales of 1.3% (5.2% cc). The diagnostic division is estimated to generate approximately 20% of the total business of Grifols from 2014 onwards following the inclusion of assets contributed by Novartis. By products and area of specialisation, the DG Gel cards for determining blood type have continued to be the engine of growth of this division. Significant sales have been achieved in emerging countries, thereby offsetting the slump in Turkey following a change in distributor. Furthermore, the FDA has approved the DG Gel 8 system developed by Grifols for antigen blood typing and pre-transfusion compatibility tests. With regard to instrumentation, the first automatic analysers of blood type (Erytra ) were installed in Japan and Qatar and sales of the coagulometer Q commenced in the demanding Italian market. Also worthy of note is the completion of the first series of coagulometer Q Smart which offers new solutions for haemostasis in emerging markets such as Brazil, Chile, Bulgaria or Turkey. Among the new products launched worth noting is the presentation of the AlphaKit QuickScreen, a device that F-265 |
|
|
screens for deficiencies in alpha-1 antitrypsin, a rare, hereditary disease that is under-diagnosed and tends to be confused with COPD in adults. This system enables doctors to detect, in a matter of minutes using just a few drops of blood, whether the person carries the Z protein, which is responsible for 95% of the severe cases of this disease. The teams have continued to adapt to the specificities of each market: a version 1.0 of the Erytra analyser was designed and developed specifically for the US market, and received FDA approval in the first quarter of 2014; a new version 3.01 was created together with a new pack of techniques (v2.0) to automate new reagents and tailor the analyser to demand. In the area of immunology the first phase of testing of the new generation of Triturus analyser has been completed while the maintenance of the Triturus analysers currently installed continues together with component obsolescence management. The haemostasis area continued to expand its range of reagents. The main reagents introduced were as follows: DG -Chrom PC, Grifols’ own chromogenic assay kit for protein C and DG -TT L human, Liquid Human Thrombin for Thrombin Time. With regard to Progenika Biopharma, a company majority owned by Grifols, the group initiated the approval process for the new version of IDCore with the FDA and with the European authorities (EMA) to obtain EC accreditation. Both authorisations are expected to be obtained in 2014. Grifols also launched SeqProLipo , a product in the cardiovascular line for the diagnosis of familial hypercholesterolemia that incorporates Next Generation Sequencing technology and replaces LipoChip . Also noteworthy is a cooperation agreement signed with the reputed network of laboratories in the US, LabCorp, which will use the Progenika reagents. Finally, mention should be made of the approval in Mexico for the commercialisation of the Intercept Blood System in this country. This device enables the inactivation of pathogens in plasma and platelet components and Grifols is the exclusive distributor thereof. • Hospital division: 3.5% of Grifols’ revenues The Hospital division increased its turnover by 1.3% (2.6% at a constant exchange rate) in 2013 as a result of Grifols’ internationalisation strategy, which has led to a rise in sales in foreign markets of more than 45% and has mitigated the cuts to public health care spending in Spain. The Hospital division’s revenues in 2013 represented 3.5% of Grifols’ total revenues. The growth in the Hospital Logistics area in Latin America, new agreements to produce injectable pharmaceuticals for third parties and the launch of new products have all boosted the division’s strategy of internationalisation, offsetting the slump in sales in Spain. The production agreements with third parties include: • An agreement with Cadence Pharmaceuticals to produce its OFIRMEV paracetamol in a flexible container for intravenous infusion. • An agreement with Cumberland Pharmaceuticals to sell the first ibuprofen in a flexible container for intravenous infusion. Grifols has exclusive distributor rights for Spain, Portugal, Argentina, Chile, Brazil, Ecuador, Peru and Uruguay. In the fluid therapy area, the production for third parties includes: • The launch of two formulas of a pharmaceutical for treating bone diseases in the European Union and the United States. • The start of production of the Hospital division’s first FDA-licensed product to be sold in the US market. The product is the Zoledronic acid intravenous compound, which will be produced by Grifols for a US multinational and sold globally. • Completion of development of a painkiller in a polypropylene bag for the US market. F-266 |
|
|
• The start of development of three new products: a specific painkiller, also for the US market, an NSAID for the European and US markets and a Grifill for phase III clinical trials in collaboration with Cerus. In fluid therapy, in terms of in-house products, new ready-to-use potassium solutions have been launched in different formats: Flebolex Luer bags with needle-free connection and version 3.0 of Grifill . In Nutrition, a parenteral lipid solution has been launched and in Blood Bank the European body IMPD (Investigational Medicinal Product Dossiers) has authorised the start of clinical trials in Italy to obtain approval of the erythrocyte inactivation set. This new trial comes on top of those already being carried out in France, Germany and the United States. In Hospital Logistics, one of the areas with the greatest potential for international expansion, the first automated carousel system has been installed for a hospital pharmacy in the US, specifically in Emory University Hospital (Atlanta). This system allows for control of inventories of medication and hospital products, facilitating procurement processes, optimising space and time. 3.—LIQUIDITY AND CAPITAL RESOURCES The Group’s main liquidity and capital requirements are to cover operating expenses, capital expenditure (Capex), including maintenance and construction of manufacturing facilities, and payment of debt. Historically, the Company has covered its liquidity and capital requirements with own funds originating from its manufacturing activities and external financing. At December 2013, Grifols’ cash position was Euros 708.8 million, although the Company has an additional Euros 340.6 million available in undrawn credit facilities. Cash flows from operating activities In 2013 cash flows from operating activities amounted to Euros 592 million. The main factors affecting working capital were: • Trade and other receivables increased by Euros 35.7 million, although the average collection period was stable compared to December 2012 at 52 days. • Inventory levels decreased by Euros 17.3 million, as a result of improved inventory management and a decrease in safety stock. Inventory turnover was 262 days compared to 281 in 2012. • Trade and other payables rose by Euros 61.4 million. Cash flows from investing activities The net cash flows used in investing activities in 2013 amounted to Euros 236 million. The investments made in 2013 include the acquisition of 21.30% of TiGenix for Euros 12.4 million, 35% of the biotech company Aradigm for Euros 20.6 million (USD 25.7 million) , including acquisition costs, and 60% of Progenika for a net amount of Euros 34.6 million. Cash flows from financing activities Cash flows used in financing activities amounted to Euros 105.1 million and include a Euros 79.4 million net payment of debt and a Euros 69 million interim dividend payment on account of 2013 earnings made in June 2013. F-267 |
|
|
4.—RISKS AND UNCERTAINTIES The financial crisis, whose effects were already mentioned in the 2008 annual report, is still affecting the countries in which Grifols operates. It remains difficult to predict whether there will be any further changes in the public health systems that could affect the Company’s activity. The Group’s future results could be influenced by events relating to its own activity, such as shortages of raw materials for the manufacture of its products, the introduction of competing products or changes in legislation regulating the markets in which it operates. However, at the date of preparation of these annual accounts, Grifols has adopted the measures it considers necessary to mitigate the possible effects of these events. 5—EVENTS AFTER THE REPORTING PERIOD BRIDGE LOAN TO ACQUIRE THE DIAGNOSTIC DIVISION FROM NOVARTIS On 3 January 2014 Grifols signed a bridge loan of USD 1,500 million extended in equal amounts by Nomura, BBVA and Morgan Stanley. The loan is to finance the acquisition of Novartis’ transfusion medicine and immunology diagnostic division. The loan imposes no financial restrictions on Grifols in respect of dividends or investments. This bridge loan is a temporary, short-term finance. Grifols plans to optimise its finance costs by restructuring its debt in 2014, including this bridge loan. COMPLETION OF THE PURCHASE OF THE NOVARTIS DIAGNOSTIC DIVISION On 9 January 2014 the acquisition of the blood transfusion and immunology diagnostic division from the Swiss company Novartis (Novartis International AG) for USD 1,650 million (Euros 1,222 million) was completed. The transaction went ahead with the same terms and conditions announced on 11 November 2013 after receiving the necessary legal and regulatory authorisations. The transaction was carried out through the newly created, wholly owned Grifols’ subsidiary, G-C Diagnostics Corp. The Company expects the Diagnostic division to generate close to 20% of total Group revenues, compared to the 4% it currently represents. With this transaction Grifols is speeding up its new growth strategy that is based on promoting complementary business areas. Approximately 550 employees will join Grifols’ workforce from Novartis. 6—INVESTMENT ACTIVITIES: R&D, CAPEX, ACQUISITIONS AN EXTENSIVE R&D PROJECT PORTFOLIO According to Forbes magazine, Grifols is among the 100 most innovative companies in the world and its commitment to research is evident from its 2013 results. Its commitment is expressed both through a sound R&D investment policy, with 4.5% or more than Euros 123.3 million of its sales revenues earmarked for this area in 2013, and through the acquisition of shares in R&D companies and projects in fields of medicine other than Grifols’ core activity to thus ensure ongoing initiatives. This year Grifols invested more in R&D than in 2012 and the portfolio of projects in progress, including those of its investees such as Progenika, Araclon or Nanotherapix is the most extensive and diverse in all the Group’s history. • Main events in 2013 • Enrolment of the first patients in the AMBAR trials (Alzheimer Management by Albumin Replacement). This multicentre clinical trial involves combining plasma replacement and hemapherisis treatment with the administration of plasma proteins, mainly albumin at different intervals and in varying doses, to treat Alzheimer’s. F-268 |
|
|
In 2013 the trial protocol was completed and patients are now being included in the trials in Spain and USA. The first results are expected to be available in 2015. • Presentation of the SPARK study results in the annual conference of the American Thoracic Society (ATS) in May. The study shows that higher doses of Prolastin -C regulate alpha-1 antitrypsin levels in patients with a hereditary deficiency of this protein, a rare disorder affecting approximately 200,000 people in Europe and USA. In 2013, the Company also started a second trial: the SPARTA study, which will make it possible to measure the level of lung tissue preservation obtained with the use of Prolastin -C. The first patient has already signed up for this trial. • Commencement of the SPIRIT study (Study of Plasma-derived factor VIII/VWF in Immune tolerance Induction Therapy) Register of haemophilic patients with inhibitors in the United States to gather data on the efficiency and safety of treatments with Grifols’ plasma-derived factor VIII/VWF. The results will help to improve the immune tolerance induction therapy (ITI) in patients that develop inhibitors to factor VIII. • Authorisation of the Spanish Agency for Medication and Healthcare Products (AEMPS) for phase I of the clinical trials of the Alzheimer vaccination being developed by Grifols at its company, Araclon Biotech. In this phase its safety and tolerability for humans will be assessed, although not its efficiency, representing a first milestone in the project’s advancement. • Other lines of research underway: • Albumin in hepatology: A clinical trial is currently underway to evaluate the effect of prolonged administering of Grifols’ human albumin 20% on cardiovascular and renal functions in patients with advanced cirrhosis and ascites. • Biological fibrin glue Grifols has embarked upon a new area of research with its interdisciplinary R&D project on biosurgery. This research is focused on developing a biological glue with healing or sealing properties for vascular, parenchymal, and soft tissue surgery. It amounts to the development of new uses for plasma proteins, beyond the traditional replacement therapies. Four clinical trials are currently underway, two in vascular surgery and two in non-vascular surgery (parenchymal and soft tissue surgery), in Europe, Canada and the United States. The last patient in the European clinical trial, focusing on vascular surgery, was treated in 2013; therefore, the trial is expected to be completed in the second half of 2014. In 2013 three additional trials required by the FDA were started to obtain approval in USA. • Other research underway: The trials on the use of plasmin in cases of acute peripheral arterial occlusion are ongoing and the clinical trials to evaluate the safety and tolerance of treating cystic fibrosis with preparations such as an inhaled formulation of alpha 1-antitrypsin are at phase II, among others. • Research planned: In 2013 the necessary documentation to commence clinical trials of new products such as topical Thrombin and intravenous fibrinogen, the trials to test the efficiency of IVIG Flebogamma DIF in new areas such as the treatment of post-polio syndrome. Finally, the plans to use alpha-1 antitrypsin to treat type-1 diabetes (juvenile diabetes) are also of major importance. F-269 |
|
|
For another consecutive year, Grifols’ R&D activity has been rated ‘‘Excellent’’ by the Spanish Profarma Plan. The Spanish Profarma Plan is a joint programme set up by the Ministry of Industry, Tourism and Commerce, the Ministry of Health and Social Policy and the Ministry of Economy and Competitiveness aimed at promoting scientific research, development and technological innovation in the pharmaceutical industry. Grifols’ scientists’ commitment to excellence and innovation is essential to develop safe and efficient haemoderivative products in our therapeutic field. This daily effort is complemented by an international collaboration network with private and public research institutions, leaders in their field. • Collaboration with third parties In 2013 Grifols increased its collaboration with the European Consortium for the Study of Chronic Liver Failure, with a contribution of three million Euros over the next four years, further to the two million Euros already contributed since 2009. It has also signed an agreement with the Vall d’Hebron Research Institute (VHIR) to create a centre of excellence for research and training in the diagnosis, treatment and monitoring of patients with alpha 1-antitrypsin deficiency (DAAT). As part of Grifols’ global strategy to investigate Alzheimer’s, the Company has entered a long-term collaboration agreement with the ACE Foundation to finance the development of the ‘‘Barcelona Alzheimer Treatment & Research Centre’’. The centre designed by this foundation is conceived as an independent space to promote and facilitate the diagnosis, treatment and biomedical research of Alzheimer’s and the first project it will host is Grifols’ AMBAR study. • Promoting research through awards and grants • Sponsorship of the European Alpha-1 Antitrypsin Laurell (eALTA) research programme, which supports work that contributes to a better understanding and treatment of alpha 1-antitrypsin (AAT) deficiency. The research projects that received the award were announced at the annual congress of the European Respiratory Society held in Barcelona in September. • VI edition of the Mart´in Villar Research Awards, whereby Grifols supports research in the haemostasis field. • Sponsorship of two Fulbright Program grants, considered to be one of the most prestigious in the world. The program provides funds to extend postgraduate studies in USA. Grifols’ collaboration will finance two Grifols/Fulbright grants for two years. Priority will be given to the candidates who have passed the admission tests for the grants and present projects in study areas which are related to Grifols’ activities. CAPITAL INVESTMENTS (CAPEX) In 2013 Grifols had met most of its CAPEX targets up to 2015. Therefore, the Company continued with its planned investments and earmarked a total of Euros 151.7 million to expand and improve its production facilities in both Spain and the United States. From 2014 until 2016 the Group will invest around Euros 450 million. • Bioscience Division: increased fractionation and plasma protein purification capacity The Bioscience Division has absorbed a substantial proportion of the investment plan, which involves improving the structure of plasma procurement centres in the United States and gradually expanding production facilities. In 2013 the construction and validation of the new plasma fractionation plant in Parets del Vall`es (Barcelona, Spain) with a fractionation capacity of 2.2 million litres/year has been completed. The F-270 |
|
|
Company expects to obtain final approval for its start up during the first half of 2014. Throughout 2013, Grifols also worked intensely on the validation processes of intermediate product batches obtained in the new plasma fractionation plant (NFF-North Fractionation Facility) in Clayton (North Carolina, USA). This is a major milestone as Grifols will be able to expand its plasma fractionation capacity and supply of intermediate products from the equivalent of 2.5 million litres of plasma in 2013 up to a maximum of 6 million litres. When both plants are on line Grifols will have a total installed plasma fractionation capacity of 12.5 million litres/year. Investment in plasma protein purification has also been continued. In the first quarter of 2013 the EMA authorised the expansion of the albumin purification plant in Parets del Vall`es, thus its albumin purification capacity can be increased by 2.2 million litres. Construction is also going ahead with the new alpha-1 antitrypsin (Prolastin ) purification area in the same industrial complex. In USA the construction of the new IVIG purification plant in Los Angeles has been completed and the validation process has commenced. This plant has an initial purification capacity of 2 million litres, which could be expanded to 4 million in a second phase, and it will be operational in 2015. The investments to increase the production capacity of the albumin purification facilities in Los Angeles to 4.5 million litres are ongoing and in Clayton the new albumin purification plant will have an initial capacity of 2.8 million litres, which could be expanded in the future. Among all the validation processes that of the new facilities and equipment in the freeze-drying and dosage areas in Parets del Vall`es plant is particularly important and FDA approval is expected to be obtained during the second half of 2014. Part of the investments has also been earmarked to areas such as expanding and relocating plasma donation centres; improving infrastructures related to the classification, preparation and storage of raw materials. Finally, in 2013 management of the plasma fractionation plant in Melville (New York—USA) was transferred to Kedrion. Grifols has, therefore, complied with the conditions imposed by the US anti-trust agency, the Federal Trade Commission (FTC), for the acquisition of Talecris. • Hospital Division Of particular note in the Hospital Division is the commencement of the building work in the industrial complex of Las Torres de Cotillas (Murcia-Spain) on the new facilities (phase IV) to manufacture bags for the extraction and conservation of blood components. An investment of Euros 6.9 million is planned that will take production capacity to 9 million units. At this plant, where Grifols also produces intravenous saline solution in flexible packaging, three new fully automated production lines of this product, included in phase III, were brought into operation during 2013. As a result production processes have been optimised. The works to extend the fully automated warehouse have also been completed. It is expected to come on line in the first quarter of 2014, making the Murcia plant one of Grifols’ three major logistics platforms in Spain. Work has begun at the Parets del Vall`es site (Barcelona, Spain) to boost the third-party manufacturing area, with the aim of achieving greater flexibility and production capacity in the manufacturing of vials. • Diagnostic In the Diagnostic division a salient point is the incorporation of a production line for DG Gel cards in the Grifols’ plant in Dudingen (Switzerland). F-271 |
|
|
Finally, various investments have been made in Grifols’ investees; one of the most important being the concentration of Araclon Biotech’s activity in a single site in Zaragoza (Spain) to set the foundations for the future. The construction work is also ongoing at the new plant in Brazil which will manufacture bags for the extraction and conservation of blood components. This project has a budget of Euros 5 million and is being carried out by a new company called Gri-Cei, which is 60%/40% owned by Grifols and the Brazilian company Com´ercio Exporta¸c˜ao e Importa¸c˜ao de Materiais M´edicos Ltda (CEI). Construction is expected to take two years. Once operative, it will enable Grifols to increase production capacity and strengthen its direct commercial presence in Latin America. ACQUISITIONS IN 2013 • Acquisition of 21.43% of the biotechnology company TiGenix, which specialises in cell therapy In the last quarter of 2013 Grifols acquired a 21.43% stake in the biotechnology company TiGenex through its subsidiary Gri-Cel for Euros 12.4 million. The acquisition was entirely funded with Group capital and follows the strategy of holding an interest in research projects and companies to ensure the continuity of the initiatives being developed. This falls within the framework of Grifols’ commitment to the field of advanced therapy and personalised medicine, where it already holds an interest in VCN Bioscience (cancer/oncolytic adenovirus therapies) and Nanotherapix (genetic therapies based on autologous cells). TiGenex specialises in cell therapy. To be precise it uses a validated eASCs (expanded Adipose derived stem cells) platform to treat autoimmune and inflammatory diseases. At the present time it has one product used to regenerate knee cartilage and it is developing three others. • A 35% stake in Aradigm Corporation as part of a global strategic agreement In the second quarter Grifols acquired 35% of the capital of the US pharmaceutical company, Aradigm Corporation (OTC BB:ARDM.OB) which specialises in the development and marketing of inhaled pharmaceutical products to treat and prevent chronic respiratory diseases, such as non-cystic fibrosis bronchiectasis and cystic fibrosis, among others. The transaction was carried out through the subscription of USD 25.7 million (EURO 20.6 million) of share capital of a total capital increase of USD 40.7 million. The acquisition is part of a wider strategic agreement that also grants Grifols the exclusive worldwide licence to sell the inhaled ciprofloxacin compound (Pulmaqu´in and Lipoquin ) to treat chronic respiratory diseases, including non-cystic fibrosis bronchiectasis, for which the phase 2b clinical trials have been completed. • A 60% stake in Progenika Biopharma, specialised in the development of technology for personalised medicine In the first quarter of 2013 Grifols bought 60% of the capital of the Basque biotechnology company, Progenika Biopharma, for Euros 37 million. The transaction was carried out with a cash payment of 50% of the purchase price and the other 50% was settled in Grifols non-voting shares (Class B). Progenika specialises in developing technology for personalised medicine and focuses on the design and production of genomic and proteomic tests mainly for in vitro diagnosis. It has also developed its own technology to produce molecular diagnosis and prognosis tests and is one of the most advanced companies in this field internationally. Progenika is in fact a worldwide pioneer in the development of molecular biology tests to verify transfusion compatibility. F-272 |
|
|
With this acquisition Grifols has strengthened its commitment to research and development in its Diagnostic Division and has added the most advanced technology to its product portfolio in the immunohaematology area. Since 2010 Grifols has held the worldwide distribution rights (except Mexico) for the Progenika BLOODchip blood genotyping test, which makes the provision of compatible blood units between donor and recipient easier while making transfusions safer. 8—ACQUISITION AND DISPOSAL OF TREASURY STOCK Transactions with treasury stocks during 2013 are described in the notes to the consolidated accounts accompanying this report. 9—OTHER IMPORTANT INFORMATION HUMAN RESOURCES Two of the main human resources initiatives have been to maintain jobs and back the development of professionals working at Grifols to support the Company’s growth. Grifols’ average accumulated headcount in 2013 stood at 11,779 employees, up 6% on 2012. The headcount at the various Group companies has increased in all regions; however the 6.6% rise in the workforce in Spain, taking the total number of employees to 2,637 employees, is particularly significant. Also in 2013 Grifols consolidated its benchmark position as a model employer, with average length of service at six years. It provides equal opportunities for male and female staff, with an almost equal distribution by gender (45% men and 55% women), and an average age of 38 years. The number of courses, participants and total hours spent on training have all soared compared to 2012, while more emphasis has been placed on technical and scientific training, the development of personal and business skills, quality and GMPs or safety and the environment, among others. At a strategic level, the main focus of the HR Department’s training efforts in 2013 has been on employee health and safety. This commitment to employee health and safety is demonstrated in two key issues: the identification of design risks in facilities to avoid these as much as possible and risk management to mitigate those which cannot be eliminated. As part of this commitment a guide has been drawn up to perform audits of the health and safety (OHS) management systems, based on the OHSAS 18001 standard. The heads of health and safety in the USA, where 78% of Grifols’ average workforce is based, have received the first training courses in this subject. This is the first step to be internal auditors. Training will gradually be extended to the other subsidiaries. In fact, Germany has been the first subsidiary where a corporate audit of OHS has been performed. In 2013 the SAP Talent module has been implemented as a tool to standardise performance evaluations worldwide. More than 2,000 employees in Spain, USA, UK and China have received specific training in people management. This training will gradually be rolled out to the Group’s other subsidiaries and production centres, as has occurred during 2013 with the SAP Training module implemented in 2012. During the year the online training platform, Campus Grifols, has been expanded to be able to reach all Group employees in a personalised manner. ENVIRONMENTAL MANAGEMENT In 2013 the environmental programme implemented by the Company for 2011-2013 was completed, achieving more than 80% of the objectives set. Results demonstrate the importance and F-273 |
|
|
effectiveness of the energy efficiency, emissions reduction, and waste management measures adopted in all areas of activity, above all in the production units. In fact in 2013 T ¨ UV Rheinland has renewed all the environmental management certificates based on the ISO 14001 standard in all the Group companies located in Spain. Also the US Environmental Stewardship Council has awarded the environmental management system of the Clayton plant (North Carolina, USA) the category of ‘‘partner’’. Among the most salient measures adopted in 2013 are: • At the Parets del Vall`es plant (Barcelona, Spain), the use of CO2 to purify waste water and innovative eco-efficient solutions in the new installations, which have led to an annual saving of 4,600 MWh in electricity and 7,800 MWh in natural gas. The implementation of an improved segregation process for bio-sanitary waste in the laboratories has led to a 70% reduction in the waste generated. The installation of a new ethanol rectification tower which will double the present distillation capacity has also been completed and is currently at the validation stage. • At the Los Angeles plant (California, USA) the ethanol distillation tower is now operational, meaning that 1.4 million litres of this compound can be recycled each year instead of being treated as waste. The new IVIG purification plant, currently at the validation stage, incorporates the latest technology to reduce the impact of its activity on the environment, in terms of both water and energy resources. Of particular note among these are; the automated reactor cleaning systems (CIP), the installation of frequency converters, high efficiency pumps or pipe lagging. • At the Clayton plant (North Carolina, USA) the cold storage units that use R22 refrigerant gas have been replaced by others that use gases that are not harmful to the ozone layer. Also in an external plant an alternative waste recovery treatment of aqueous solution of polyethylene glycol has been started, using anaerobic digestion to produce biogas. Grifols has once again taken part in the Carbon Disclosure Project (CDP), the aim of which is to recognise the steps taken by the various participating companies to cut gas emissions and mitigate the risks of climate change. This programme represents 722 institutional investors with assets valued at USD 87 billion. In 2013 Grifols obtained 90 points out of a possible 100, two more than in 2012, placing it 15th in the ranking of the best valued companies among 125 major companies from Spain and Portugal, and the top company in the health sector. Grifols 2013 Annual Corporate Governance Report is part of this directors’ report. The report is available in the website of the Spanish Financial Markets Regulator (Comisi´on Nacional del Mercado de Valores—CNMV) and in the Grifols’ website from the date of publication of the annual financial statements. Section E of the aforementioned report includes an analysis of Risk Controls and Management Systems of the company and section F includes details of the Internal Control and Risk Management Systems in Relation to the financial information issuing process (‘‘SCIIF’’). F-274 |
|
|
GRIFOLS, S.A. AND SUBSIDIARIES At their meeting held on 21 February 2014, pursuant to legal requirements, the Directors of Grifols, S.A. authorised for issue the consolidated annual accounts and consolidated directors’ report for the period from 1 January 2013 to 31 December 2013. The consolidated annual accounts comprise the documents that precede this certification. Victor Grifols Roura Ram´on Riera Roca Juan Ignacio Twose Roura Chairman Board member Board member Tom´as Dag`a Gelabert Thortol Holding B.V. Thomas Glanzmann (J.A. Grifols G.) Board member Board member Board member Edgar Dalzell Jannotta Anna Veiga Lluch Luis Isasi Fern´andez de Bobadilla Board member Board member Board member Steven F. Mayer W. Brett Ingersoll Belen Villalonga Moren´es Board member Board member Board member Raimon Grifols Roura Secretary to the Board F-275 |
|
|
SECOND HALF 2013 REPORT |
|
|
2 SECOND HALF 2013 REPORT The facts and figures contained in this report which do not refer to historical data are “projections and forward looking statements”. The words and expressions like “believe”, “hope”, “anticipate”, “predict”, “expect”, “intend”, “should”, “try to achieve”, “estimate”, “future” and similar expressions, insofar as they are related to Grifols Group, are used to identify projections and forward-looking statements. These expressions reflect the assumptions, hypothesis, expectations and anticipations of the management team at the date of preparation of this report, which are subject to a number of factors that could make the real results differ considerably. The future results of Grifols Group could be affected by events related to its own activity, such as shortages of raw materials for the manufacture of its products, the launch of competitive products or changes in the regulations of markets in which it operates, among others. At the date of preparation of this report Grifols Group has adopted the measures it considers necessary to offset the possible effects of these events. Grifols, S.A. does not assume any obligation to publicly inform, review or update any projections and forward-looking statements to adapt them to facts or circumstances following the preparation of this report, except as specifically required by law. This document does not constitute an offer or invitation to purchase or subscribe shares, in accordance with the provisions of the Spanish Securities Market Law 24/1988, of July 28, the Royal Decree-Law 5/2005, of March 11, and/or Royal Decree 1310/2005, of November 4, and its implementing regulations. DISCLAIMER THIS IS A TRANSLATION OF A SPANISH LANGUAGE ANNOUNCEMENT FILED WITH THE CNMV. IN CASE OF DISCREPANCIES, THE SPANISH VERSION WILL PREVAIL. |
|
|
3 SECOND HALF 2013 REPORT 2013 KEY FIGURES: 1 Excluding costs relating to the Talecris acquisition and other non-recurrent costs 2 Excluding costs associated with the purchase of Talecris as well as the amortization of intangibles and of deferred financial costs related to the acquisition. 3 Source: Market Research Bureau (MRB) - The Worldwide Plasma Proteins Market 2012. 2013 INVESTING FOR FUTURE GROWTH. GREATER DIVERSIFICATION, NEW OPPORTUNITIES 2013 results support the soundness of a growing company that continues to work towards its mission: improving people's health and wellbeing. The company's capitalisation at the end of 2013 was Euros 10,790 million and our financial results and the milestones achieved in terms of production and commercialisation of our products, R&D, Human Resources or the environment, evidence how Grifols was managed in 2013 and the added value generated by our Company throughout the year. NET REVENUES: Euros 2, 741.7 million +4,6% growth +7,4% constant currency (cc) • Bioscience leads the growth +5.3% (+8.2% cc). • Volume growth in all proteins and stable prices. Adjusted 1 EBITDA grows +9.7% to Euros 917.4 million • Stabilization of SG&A expenses. Adjusted1 EBITDA margin increases 160 basis points (bps) reaching 33.5% of revenues • Management focus on efficiency and competitiveness. • Process’ flexibilization and plasma optimization. NET PROFIT: Euros 345.6 million, +34.6% growth and 12.6% over sales • Operating margin improvements. • 12.3% decrease in financial result. NET FINANCIAL DEBT: Euros 2,087.2 million de euros • Leverage ratio 2.28 times over adjusted1 EBITDA below the 2.87 times as of December 2012. • Euros 308.9 million Net Debt reduction and credit rating upgrade. CASH of Euros 708.8 million with Euros 592.0 million from operational activities • Strong operating cash flow generation to undertake strategic investments. • Euros 236.0 million of cash dedicated to acquisitions and CAPEX. DIVIDEND: The company returns to cash dividend payments • Committed to a 40% dividend pay-out over net profits. EMPLOYEES: 11,779 • 6% increase in the average accumulated headcount. • More training provided in the Grifols Academies. |
|
|
4 SECOND HALF 2013 REPORT 1. COMPANY'S POSITION IN 2013 In 2013 Grifols maintained its position as the third largest producer of plasma derivatives in the world, with a global market share of approximately 20%3. The Group's main products are worldwide sales3 leaders: The company has achieved an excellent and competitive position in its three diagnostics specialities: transfusions, immunology and haemostasis. In addition, the recent acquisition of Novartis' diagnostics unit for transfusions and immunology, completed in January 2014, will allow the Group to grow further in the diagnostics segment, improving its range of equipment and reagents in the United States and other key markets and increasing the contribution from the Diagnostic division, which could generate close to 20% of total revenue. The Hospital division retains its leading position in Spain as a provider of intravenous solutions and continues with its internationalization as a key priority Grifols’ main lines of business (Bioscience, Diagnostic and Hospital) are sound and consolidated. Grifols' management has focused on two main areas: consolidating areas of recurring business and unlocking new opportunities for future growth by acquiring interests in research companies to guarantee and finance the viability of their R&D projects. The confidence of shareholders and investors has enabled Grifols to continue its investment activity in 2013 to maintain its leading position in innovation: developing technology and improving production processes by investing Capex, but also looking for differentiating factors that generate added value. The company has an ambitious R&D plan and has made several strategic acquisitions over the course of the year. Some of the transactions will produce immediate results (Novartis' diagnostics unit) and others are part of the strategy towards a sustainable and future projection (Progenika, Aradigm and TiGenix). PLASMA DERIVATIVE MARKET SHARE GLOBAL RANKING Polyvalent IVIG (intravenous immunoglobulin) 27% 1 Alpha-1 Antitrypsin 66% 1 Coagulation Factor VIII 18% 2 Albumin 14% 3 |
|
|
5 SECOND HALF 2013 REPORT 2. 2013 results - BUSINESS PERFORMANCE PROFIT AND LOSS ACCOUNT: KEY INDICATORS Sales performance: Euros 2,742 million revenues Grifols closed 2013 with revenues of Euros 2,741.7 million, an increase of 4.6% on the prior year. The geographical diversification of sales limited the effect of volatility in exchange rates, in particularly the Euro-Dollar, and revenues grew by 7.4% cc. Boosting sales in regions which have been less affected by austerity measures, with shorter collection periods and better margins continued to be one of the company's core strategies in 2013. As a result, Grifols remained very active on international markets during the period, where it generated 92.4% of its revenues. Sales outside Spain rose by 5.2% (8.2% cc) compared to 2012, to Euros 2,533.8 million. In the European Union sales performance has confirmed the expected recovery. Recurring revenues, excluding Spain, grew by 4.5% to Euros 361.9 million. In Spain declines in sales of Diagnostic and Hospital products and services as a result of the cuts to public health service spending - which have reduced the number of operations and diagnostic tests performed at hospitals - have limited the growth in the region. The decline in Spain has decelerated when compared to 2012, to 2.4%, with a turnover of Euros 207.9 million, representing 7.6% of total revenues. In the United States and Canada the sales performance of the main plasma proteins was good in terms of volumes, with double-digit growth in albumin and factor VIII. In * Constant currency (CC) excludes the impact of exchange rate movements. Canada, activity was stable following the renegotiation of the contracts with the Canadian Blood Services (CBS) and Héma-Québec. Grifols remains the country's main supplier of haemoderivatives and plasma fractionator. The mid-year restructuring has also provided the company's business in the country with more operating flexibility. Sales of plasma proteins in the United States have gradually risen on a quarterly basis and the company has achieved record turnover growth in absolute terms of 7.9% (11.2% cc) in the world's largest haemoderivatives market. In addition, Grifols has bolstered its presence in the USA with new products and services in areas such as Hospital Logistics (Hospital division) and the Diagnostic Division, as the FDA has approved a number of reagents and instruments for blood typing which are fundamental for its commercialisation. The Group's total sales in the United States grew by 6.3% (9.6% cc) in 2013. The most dynamic areas remain those outside the European Union and North America. Overall, RoW sales (Rest of the World excluding Raw Materials) grew by 14.7% (19.5% cc). For Grifols, these emerging regions have the greatest growth potential. The company has strengthened its presence in markets such as the Middle East, opening a new branch office in Dubai; or China, where Grifols' sales have grown rapidly over the past three years, and where the company expects to consolidate its position as a key player in immunohaematology and as a supplier of albumin, the only plasma protein which can be exported at present; or Brazil, where construction has started on a plant to manufacture bags for extraction and storage of blood components. Market research in countries such as Turkey, India and Russia has also progressed. Currently, Grifols has commercial presence in 25 countries through its subsidiaries and sells its products in close to 100 countries. Internationalisation is a core strategy for the company, and in 2013 it continued to implement measures to bolster this expansion. These measures included: • Opening a branch office in Dubai, to manage the business in the Middle East. 2013 - SALES BY region IN THOUSANDS OF EUROS 2013 %SALES 2012 %SALES % VAR % VAR CC* EU 569,827 20.8% 559,327 21.3% 1.9% 2.2% US+CANADA 1,707,620 62.3% 1,658,548 63.3% 3.0% 6.1% R.O.W. 426,257 15.5% 371,619 14.2% 14.7% 19.5% SUBTOTAL 2,703,704 98.6% 2,589,494 98.8% 4.4% 7.2% RAW MATERIALS 38,028 1.4% 31,450 1.2% 20.9% 23.5% TOTAL 2,741,732 100.0% 2,620,944 100.0% 4.6% 7.4% |
|
|
6 SECOND HALF 2013 REPORT • Centralising and integrating logistical operations in a new centre which will be built in Ireland, allowing the company to optimise its distribution infrastructure, increase the efficiency of its operations and promote cost savings. • Converting the branch office in China (Shanghai) into a commercial subsidiary of the Group, providing the legal structure needed to develop and expand in this market in the coming years. Grifols' organic growth will also come f rom strengthening the products and services offered by its three divisions in its main markets. The company’s has followed a commercial strategy of integration to complement its range of plasma protein therapies with Diagnostic (Diagnostic division) and Hospital logistics (Hospital division) products and ser vices. In November 2013 this strategy led to an agreement to acquire Novartis' transfusion and immunology diagnostics business, which focuses on guaranteeing the safety of the blood donated for transfusions. The transaction, completed in January 2014, has allo wed Grifols to complement and expand the range of products and services offered by its Diagnostic division, becoming a vertically integrated company, capable of offering solutions to blood donation centres with broadest range of products for immunohaematology, including gel-based reagents, multicards and the innovative genotyping technology Progenika, company acquired in 2013. The acquisition will also strengthen Grifols' presence in the United States, with a sound and specialised sales network. The acquisition of Novartis' transfusion and immunology diagnostics unit has not impacted 2013 figures, and the weights of the divisions did not change. Grifols' main growth driver in 2013 was the Bioscience division. The volume of sales of plasma products in a scenario of stable prices drove revenues, with an upward trend in United States, the European Union and ROW. The Hospital division's revenues rose slightly as a result of the gradual internationalisation of this line of business. Excluding the Spanish market, the division's sales grew over 45%. The Diagnostic division's turnover was stable in absolute terms at Euros 130 million as a result of increased reagent sales in emerging markets among other factors, and the sales decline slowed during the fourth quarter of 2013. Revenues from the Raw Materials & Others division, which represents approximately 2.4% of total revenues, were almost flat. These revenues include royalties, work carried out for third parties by Grifols Engineering and revenues from the manufacturing agreements with Kedrion that have continued to decline as expected. Sound results: margins and profits continue to improve In 2013 the company worked hard to increase its efficiency and competitiveness, leading to a notable improvement in operating margins. The EBITDA margin was 140 bps higher, and at the end of the year represented 31.5% of revenues. The adjusted EBITDA margin 1, rose by 160 bps to 33.5% of revenues. The sales performance of the main plasma proteins sold by the Group and the optimisation of raw material and manufacturing costs through more flexible production processes have together increased the profitability per litre of plasma. This was reflected in gross margins gro wing by 6.6% to 51.7% of revenues. Operating expenses related to administration and general services were stable. These two factors have driven growth of 9.6% in EBITDA compared to 2012 to Euros 864.6 million. On an adjusted basis EBITDA1 grew by 9.7% to Euros 917.4 million. * Constant currency (CC) excludes the impact of exchange rate movements. 2013 - SALES BY DIVISION IN THOUSANDS OF EUROS 2013 %SALES 2012 %SALES % VAR % VAR CC* BIOSCIENCE DIVISION 2,448,824 89.3% 2,325,088 88.7% 5.3% 8.2% HOSPITAL DIVISION 97,131 3.5% 95,870 3.7% 1.3% 2.6% DIAGNOSTIC DIVISION 130,339 4.8% 134,342 5.1% -3.0% -1.0% RAW MATERIALS AND OTHERS 65,438 2.4% 65,644 2.5% -0.3% 1.6% TOTAL 2,741,732 100.0% 2,620,944 100.0% 4.6% 7.4% |
|
|
7 SECOND HALF 2013 REPORT In terms of more efficient production of plasma derivatives, the company has continued to work towards greater flexibility and scalability in its production processes, to constantly adapt to market conditions. In order to achieve this Grifols has focused on both increasing its protein fractionation and purification capacities and on making the processes more flexible. The aim is to be able to purify and fill the intermediate fractions generated during the first stage of the manufacturing process at any of the three plants of the Group, for which Grifols requires FDA and EMA licenses. In this regard, in 2013 Grifols obtained FDA a pproval to use Fraction IV-1 (intermediate product) obtained a t the Los Angeles plant (California, USA) in the production (purification and dosage) of Alpha-1 Antitrypsin (Prolastin®-C) using the Clayton plant’s method (North Carolina, USA). Authorisation was also obtained to use Fraction II+III obtained in the plant in Parets del Vallès (Barcelona, Spain) to produce IVIG (Gamunex® and Gamunex®-C) at the Clayton plant in the future. Grifols continues to work towards obtaining an FDA licence to use the cryoprecipitate obtained in Clayton in purification of the factor VIII at the plants in Los Angeles. The financing conditions negotiated at the start of 2012 have also contributed to a 12.3% reduction in finance results, down to Euros 237.4 million from Euros 270.7 million in 2012. Moreover, the R&D deductions for 2012, received in the first quarter of 2013 and the combined Sta te Corporate Tax return filed by the North Carolina (USA) companies ha ve reduced the effective tax rate. Overall, the Group's 2013 net profit rose by 34.6% to Euros 345.6 million, 12.6% of the Group's revenues. 1 Excluding costs relating to the Talecris acquisition and other non- recurrent costs 2 Excluding costs relating to the Talecris acquisition, as well as the amortisation of intangibles and the amortization of deferred finance costs related to the acquisition 2013 KEY FINANCIAL INDICATORS IN MILLIONS OF EUROS 2013 2012 % VAR. NET REVENUES (NR) 2,741.7 2,620.9 4.6% EBITDA 864.6 789.2 9.6% % NR 31.5% 30.1% ADJUSTED1 EBITDA 917.4 836.1 9.7% % NR 33.5% 31.9% NET PROFIT 345.6 256.7 34.6% % NR 12.6% 9.8% ADJUSTED2 NET PROFIT 450.0 364.7 23.4% % NR 16.4% 13.9% |
|
|
8 SECOND HALF 2013 REPORT BALANCE SHEET - KEY INDICATORS At December 2013 total consolidated assets amount to Euros 5,841.0 million, with no significant changes in relation to the Euros 5,627.5 million reported at December 2012. The main investments during 2013 were the stakes acquired in Progenika, Aradigm and TiGenix. The gradual deleveraging, the sound results obtained and the positive performance of cash flows have strengthened the balance sheet in 2013. Improvement of inventory turnover and average collection periods The improvements in efficiency have also been reflected in stock management and safety stock controls, resulting in a gradual reduction in inventories of 5.2%, as forecast. Stock turnover has also decreased from 281 days in December 2012 to 262 days at the end of 2013. Optimization of working capital management has continued as a consequence of the Group’s greater exposure to countries with shorter collection periods. Grifols’ average collection period was stable at 52 days, the same level as in December 2012. Debt leverage reduction and credit rating upgrades Grifols’ net financial debt at December 2013 stood at Euros 2,087.2 million, a ratio of 2.28 times adjusted EBITDA1 and lower than the ratio of 2.87 recorded at December 2012. Over the course of the year, the Group's net financial debt has been reduced by Euros 308.9 million. This priority objective of continuing to decrease debt, together with the high sustainable levels of operating activity and ongoing progress in achieving improvements related to the acquisition of Talecris contributed to Standard & Poor’s ratifying its credit rating for Grifols and Moody’s upgrading its rating. Credit ratings at December 2013: Equity Grifols equity increased to Euros 2,107.2 million in 2013, mainly as a result of the profits obtained during the period. To December 2013, Grifols’ share capital amounted to Euros 119.6 million, represented by 213,064,899 ordinary shares (Class A) with a par value of Euros 0.50 per share and 130,712,555 shares without voting rights (Class B) with a par value of Euros 0.10 per share. During the course of the year share capital was increased twice by issuing non- voting shares (Class B): 1. In January 2013, the agreement adopted by Grifols' shareholders at the extraordinary general meeting of 4 December 2012 was implemented, and share capital was increased by Euros 1.6 million by issuing 16,328,212 new shares. These bonus shares were distributed to shareholders in the proportion of 1 new Class B share for every 20 original shares, irrespective of whether they were Class A or Class B, as an alternative form of remuneration to cash dividends. 2. In April 2013, share capital was increased to acquire Progenika Biopharma by Euros 20.5 million, by issuing 884,997 new Class B shares without voting rights. Grifols’ ordinary shares (Class A) are listed on the Spanish stock exchange and are a component of the IBEX-35 (GRF) index, while shares without voting rights (Class B) are also listed on the Spanish stock exchange (GRF.P) and on the USA NASDAQ stock exchange (GRFS) through ADR’s (American Depositary Receipts). In 2013 Grifols resumed payment of cash dividends as remuneration for all its shareholders (Class A and Class B shares). The payment corresponding to 2013 will be made in two parts: an interim dividend and a complementary dividend. In the second quarter of 2013 the company paid an ordinary dividend for a gross amount of Euros 0.20 for each Class A and Class B share, on account of 2013 results, totalling Euros 68.75 million, as reflected in the Group accounts. The Group plans to pay a final complementary on 2013 earnings. Grifols' dividend policy has not changed and its pay-out ratio remains 40% of net profit, the level prior to the acquisition of Talecris. MOODY’S STANDARD & POORS Senior secured debt Ba1 BB+ Corporate rating Ba2 BB Senior unsecured debt B1 B+ Outlook Negative Stable |
|
|
9 SECOND HALF 2013 REPORT LIQUIDITY AND CAPITAL RESOURCES The Group's main liquidity and capital requirements relate to operating expenditures, capital investments (CAPEX), both for maintenance and construction of manufacturing facilities, and repayment of debt. Historically, Grifols has financed its liquidity and capital requirements through internally generated cash flows from its operating activities and external financing. As of December 2013, the Group’s cash positions after dividend, debt and interest payments, have risen to Euros 708.8 million, well above the Euros 473.3 million reported in 2012. In addition, the Company has Euros 340,6 million of additional cash available under undrawn credit Facilities. Strong generation of operating cash flows In 2013 cash flows from operating activities amounted to Euros 592 million. The main factors affecting working capital were: • Trade and other receivables increased by Euros 35.7 million, although the average collection period was stable compared to December 2012 at 52 days. • Inventory levels decreased by Euros 17.3 million, as a result of improved inventory management and a decrease in the need of safety stock. Inventory turnover was 262 days compared to 281 in 2012. • Trade and other payables rose by Euros 61.4 million. Additional Cash flows for strategic investments Growing profits and improvement and control of financing activities have contributed to the reduction in more than Euros 200 million of financing cashflow needs, freeing cash for investment activities in order to guarantee the long term growth of the company. Net cash flows of Euros 236.0 million were used in investing activities in 2013. The main acquisitions made in 2013 include the acquisition of 21.30% of TiGenix for Euros 12.4 million, 35% of the biotech company Aradigm for Euros 20.6 million (USD 25.7 million), including acquisition costs, and 60% of Progenika for Euros 37 million (Euros 34.6 million net of cash and cash equivalents). Cash flows have also been invested in CAPEX activities used in line with Grifols’ CAPEX plan. Cash flows for financing activities Cash flows used in financing activities amounted to Euros 105.1 million and include a Euros 79.4 million net payment of debt and a Euros 69 million interim dividend payment on account of 2013 earnings made in June 2013. |
|
|
10 SECOND HALF 2013 REPORT 3. FOURTH QUARTER OF 2013 - MAIN INDICATORS Grifols’ reported sales from October to December 2013 were Euros 695.2 million rising 5.1% (9,7% cc) compared to Euros 661.4 million obtained during the same period of the preceding year. The Bioscience division totaled Euros 627.4 million, contributing 90.2% to sales revenue and growth of 6.3%. The Diagnostic division generated Euros 32.5 millions, while Hospital accounted for Euros 22.8 million. These figures represent 4.7% and 3.3% of the group’s total income, respectively. By geographical region, the United States and Canada led growth in sales, with recurring sales (excluding Raw Materials) of Euros 440.2 million, equivalent to 63.3% of revenues. Europe with Euros 137.9 million and other regions (ROW) with Euros 112.5 million accounted for 19.9% and 16.2% of total income, respectively. Revenues were positive in all divisions and in all regions where the company is present, supporting the positive trend expected in the Diagnostic Division in the fourth quarter. * Constant currency (CC) excludes the impact of exchange rate movements. 4Q 2013 - SALES BY DIVISION IN THOUSANDS OF EUROS 4Q 2013 %SALES 4Q 2012 %SALES % VAR % VAR CC* BIOSCIENCE DIVISION 627,435 90.2% 590,288 89.2% 6.3% 11.0% HOSPITAL DIVISION 22,793 3.3% 21,728 3.4% 4.9% 7.8% DIAGNOSTIC DIVISION 32,471 4.7% 32,058 4.8% 1.3% 5.2% RAW MATERIALS AND OTHERS 12,471 1.8% 17,354 2.6% -28.1% -25.8% TOTAL 695,170 100.0% 661,428 100.0% 5.1% 9.7% 4Q 2013 - SALES BY region IN THOUSANDS OF EUROS 4Q 2013 %SALES 4Q 2012 %SALES % VAR % VAR CC* EU 137,973 19.9% 132,158 20.0% 4.4% 4.9% US+CANADA 440,170 63.3% 419,308 63.4% 5.0% 10.0% R.O.W. 112,538 16.2% 102,752 15.5% 9.5% 17.5% SUBTOTAL 690,681 99.4% 654,218 98.9% 5.6% 10.1% RAW MATERIALS 4,489 0.6% 7,210 1.1% -37.7% -34.8% TOTAL 695,170 100.0% 661,428 100.0% 5.1% 9.7% |
|
|
11 SECOND HALF 2013 REPORT 4. DIVISION ANALYSIS: PERFORMANCE BY BUSINESS AREA BIOSCIENCE DIVISION: 89.3% OF GRIFOLS' REVENUES The Bioscience Division has generated 89.3% of Grifols’ turnover and sales totalled Euros 2,448.8 million. Over 95% of sales were concentrated in the international markets. By volume, mention should be made to the approximately 30% growth in albumin sales bolstered by the United States and China, which has the highest demand for this protein in Asia. Also worth noting is the rise in alpha-1 antitrypsin, with double digit volume growth. Grifols is the world’s benchmark for this plasma protein and sales in 2013 were particularly significant in countries such as Canada, the United States, Germany and Spain, where sales of Prolastin® have just commenced. In the USA and Canadian market the commercial pulmonology and haematology teams have focused their efforts on promoting the diagnosis and identification of patients with a AAT deficiency (emphysema and chronic bronchitis), asthma, liver cirrhosis or chronic hepatitis, among others. In 2013 Grifols strengthened its presence in the area of respiratory illness and is seeking to establish a respiratory care franchise to specifically tackle this significant therapeutic area. To this end Grifols has its own line of haemoderivative products; Prolastin® and Prolastin-C® and the world licence for Pulmaquin™ and Lipoquin™, medication for the treatment of severe respiratory conditions, including bronchiectasis not associated with cystic fibrosis (BE), for which the phase 2b clinical trials have already been completed. Grifols has the exclusive right to distribute these compounds following the acquisition of 35% of Aradigm Corporation. Hospital Plasma Service Grifols also provides a specific service for the Inactivation of Plasma for Hospital Transfusions (IPTH). In 2013, 36,209 units were inactivated. In 2013 the Regional Government of Murcia (Spain) awarded the company the contract to manufacture medication derived from plasma using the excess from the Centro Regional de Hemodonación. This contract will enable 55,000 units of plasma a year to be processed and the finished plasma products will be delivered to various hospitals in the region. Raw Material In 2013 Grifols’ plasma centers in the USA received over 26,000 daily donations and collected 6.4 million liters of plasma which, in line with the company's optimization strategy, represents a 10.3% increase. An important step in the unification of managements systems is the completion of the integration process for all the logistic, economic and financial activities of all the plasma centres under a single management system. Grifols has two laboratories, in San Marcos and Austin (Texas –United States) for testing the plasma samples. The plasma samples are subjected to a minimum of 10 serological and PCR/NAT tests. These laboratories are equipped to analyse over 15 million plasma donations a year. Process security, quality and control systems The security of processes and products is of paramount importance to Grifols as is the implementation of solid quality and control systems to gain a competitive advantage. The most noteworthy improvements in the year are: the validation of the results of the ELISA analyses through the Architect system both for mini-pools and complete fractioning pools. This system enables 3 results to be obtained from the same piece of equipment, thereby optimising analysis times. It also permits the automatic and combined detection of parvovirus B19V and hepatitis A (HAV) using the TIGRIS technique in mini-pools (16 units). Grifols Engineering is developing a system to automate the process of obtaining plasma donation samples; the Company is also studying the possibility of implementing an RFID identification system in all its plasma bottles with a view to monitoring them throughout the entire supply chain simplifying the process of handling the units. 2013 - key operating indicators 2013 No. of plasmapheresis centres 150 No. of plasma donations/day + 26,000 No. of donation analyses (annual capacity) + 15 million donations Litres of plasma obtained 6.4 million litres Number of fractioning plants 3 plants Fractionation capacity Euros 8.5 million litres/year |
|
|
12 SECOND HALF 2013 REPORT acquired and the solid organic R&D portfolio. Prospects are favourable and the positive trend is already visible in the results obtained in the fourth quarter of 2013, with a growth in sales of 1.3% (5.2% cc). The diagnostic division will generate approximately 20% of the total business of Grifols from 2014 onwards following the integration of the Novartis Diagnostic unit acquired. Product and area analysis The DG Gel® cards for blood typing and pre-transfusion compatibility test have continued to be the growth driver of this division. Significant sales have been achieved in emerging countries. Furthermore, the FDA has approved the DG Gel® 8 system specifically developed for the United States by Grifols for antigen blood typing and pre-transfusion compatibility tests. With regard to instrumentation, the first automatic analysers of blood type (Erytra®) were installed in Japan and Qatar and sales of the coagulometer Q® commenced in the Italian market. Also worth noting is the completion of the first series of coagulometer Q Smart® which offers new solutions for haemostasis in emerging markets such as Brazil, Chile, Bulgaria or Turkey. The teams have continued to adapt to the specificities of each market: a version of the Erytra® analyser was designed and developed specifically for the USA market, and received FDA approval in the first quarter of 2014; and a new version and technical packs to automate new reagents were created. Among the new products launched worth noting is the presentation of the Alphakit® QuickScreen, a device that screens for deficiencies in alpha-1 antitrypsin, a rare, hereditary disease that is under-diagnosed and tends to be confused with COPD in adults. This system enables doctors to detect, in a matter of minutes using just a few drops of blood, whether the person carries the Z protein, which is responsible for 95% of the severe cases of this disease. DIAGNOSTIC DIVISION: 4.8% OF REVENUES In 2013 the Diagnostic Division recorded sales of Euros 130.3 million, of which almost 80% were made outside of Spain. This business area represents approximately 4.8% of Grifols sales, although three important milestones in 2013 will have an immediate impact on the future of the division: 1. The purchase of the transfusion and immunological diagnostic unit of Novartis, which will enable the expansion of the division's portfolio of equipment and reagents making Grifols the only company capable of offering comprehensive solutions to the blood donation centres in the most efficient and secure manner with full control over the transfusion process: from donation to transfusion. 2. The acquisition of 60% of the biotech company Progenika Biopharma in March 2013, to reinforce the portfolio of products and acquire the most innovative technology. This company is a global pioneer in the development of molecular biology testing for the performance of studies to check for transfusion compatibility and in the development of immunological tests to monitor biological drugs. 3. The approval by the FDA of the reagents for blood typing (gel card) and the instruments required for their use (incubator, centrifuge and the automatic instrument WADiana® and Erytra®). 2013 has been a year of transition. The division has been highly affected by the planned termination of the distribution contract with Ortho Diagnostic in the United States and efforts have focused on achieving the licenses and authorisations required to sell directly the products and technology derived from both the new companies |
|
|
13 SECOND HALF 2013 REPORT In the area of immunology the first phase of validation of the new generation Triturus® analyser has been completed. The haemostasis area continued to expand its range of reagents. The main reagents introduced were: DG®-Chrom PC, Grifols’ own chromogenic assay kit for protein C and DG®-TT L human, Liquid Human Thrombin for Thrombin Time. The group initiated the approval process for the new version of IDCore®XT, a Progenika Biopharma product, with the FDA and with the European authorities (EMA) to obtain the EC accreditation. And there is a cooperation agreement in place with Lab Corp, a network of laboratories in the Unites States, for them to use the Progenika reagents. HOSPITAL DIVISION: 3.5% OF GRIFOLS' REVENUES The Hospital division increased its turnover by 1.3% (2.6% cc) in 2013 as a result of Grifols' internationalisation strategy, which has led to sales rising in foreign markets by more than 45% and has mitigated the cuts to public health care spending in Spain. The Hospital division's revenues in 2013 represented 3.5% of Grifols’ total revenues. The growth in the Hospital Logistics area in Latin America, new agreements to produce injectable pharmaceuticals for third parties and the launch of new products have all boosted the division's strategy of internationalisation, offsetting the slump in sales in Spain. The production agreements with third parties include: • Agreement with Cadence Pharmaceuticals to produce its OFIRMEV® paracetamol in a flexible container for intravenous infusion. • Agreement with Cumberland Pharmaceuticals to sell the first ibuprofen in a flexible container for intravenous infusion. Grifols has exclusive distribution rights for Spain, Portugal, Argentina, Chile, Brazil, Ecuador, Peru and Uruguay. In the fluid therapy area, the production for third parties includes: • Two formulas of a pharmaceutical for treating bone diseases in the European Union and the United States. • Production of the Hospital division's first FDA-licensed product to be sold in the USA market. The product is the Zoledronic acid intravenous compound, which will be produced by Grifols for a US multinational and sold globally. • Completion of development of a painkiller in a polypropylene bag for the USA market. • Commencement of three new development projects: a specific painkiller, also for the USA market, an NSAID for the European and USA markets and a Grifill® for phase III clinical trials in collaboration with Cerus. In fluid therapy, in terms of in-house products, new readyto- use potassium solutions have been launched in different formats: Flebolex Luer® bags with needle-free connection and version 3.0 of Grifill®. In Nutrition, a parenteral lipid solution has been launched and in Blood Bank the European body IMPD (Investigational Medicinal Product Dossiers) has authorised the start of clinical trials in Italy to obtain approval of the erythrocyte inactivation set. This new trial comes on top of those already being carried out in France, Germany and the United States. In Hospital Logistics, one of the areas with the greatest potential for international expansion, the first automated carousel system has been installed for a hospital pharmacy in the US, specifically in Emory University Hospital (Atlanta). This system allows for control of inventories of medication and hospital products, facilitating procurement processes, optimising space and time. |
|
|
14 SECOND HALF 2013 REPORT 5. INVESTMENT ACTIVITIES: R&D, CAPEX, ACQUISITIONS AN EXTENSIVE R&D PROJECT PORTFOLIO According to Forbes magazine, Grifols is among the 100 most innovative companies in the world and its commitment to research is evident from its 2013 results. This is expressed both through a sound R&D investment policy, with 4.5% or more than Euros 123.3 million of its sales revenues earmarked for this area in 2013, and through the acquisition of shares in R&D companies and projects in fields of medicine other than Grifols’ core activity to thus ensure ongoing initiatives. Grifols total R&D investment during the year including both its portfolio of projects in progress, and those of its investees such as Progenika, Araclon or Nanotherapix is the most extensive and diverse in the entire Group's history. Main events in 2013 • Enrolment of the first patients in the AMBAR trials (Alzheimer Management by Albumin Replacement). This multicentre clinical trial involves combining plasma replacement and hemapherisis treatment with the administration of plasma proteins, mainly albumin at different intervals and in varying doses, to treat Alzheimer’s. In 2013 the trial protocol was completed and patients are now being included in the trials in Spain and United States. The first results are expected to be available in 2015. • Presentation of the SPARK study results in the annual conference of the American Thoracic Society (ATS) in May. The study shows that higher doses of Prolastin®-C regulate alpha-1 antitrypsin levels in patients with a hereditary deficiency of this protein, a rare disorder affecting approximately 200,000 people in Europe and USA. In 2013, the Company also started a second trial: the SPARTA study, which will make it possible to measure the level of lung tissue preservation obtained with the use of Prolastin®-C. The first patient has already signed up for this trial. • Commencement of the SPIRIT study (Study of Plasmaderived factor VIII/VWF in Immune tolerance Induction Therapy) Register of haemophilic patients with inhibitors in the United States to gather data on the efficiency and safety of treatments with Grifols' plasma-derived factor VIII/VWF. The results will help to improve the immune tolerance induction therapy (ITI) in patients that develop inhibitors to factor VIII. • Authorisation of the Spanish Agency for Medication and Healthcare Products (AEMPS) for phase I of the clinical trials of the Alzheimer vaccination being developed by Grifols through its company, Araclon Biotech. Safety and tolerability for humans will be assessed in this phase but not its efficiency. This is a first milestone in the project's advancement. Main lines of research • Albumin in hepatology: A clinical trial is currently underway to evaluate the effect of prolonged administering of Grifols' human albumin 20% on cardiovascular and renal functions in patients with advanced cirrhosis and ascites. • Biological fibrin sealant: Grifols has embarked upon a new area of research with its interdisciplinary R&D project on biosurgery. This research is focused on developing a biological sealant with healing or sealing properties for vascular, parenchymal, and soft tissue surgery. It amounts to the development of new uses for plasma proteins, beyond the traditional replacement therapies. Four clinical trials are currently underway, two in vascular surgery and two in nonvascular surgery (parenchymal and soft tissue surgery), in Europe, Canada and the United States. The last patient in the European clinical trial, focusing on vascular surgery, was treated in 2013; therefore, the trial is expected to be completed in the second half of 2014. In 2013 three additional trials required by the FDA were started to obtain approval in USA. • Other research: The trials on the use of plasmin in cases of acute peripheral arterial occlusion are ongoing and the clinical trials to evaluate the safety and tolerance of treating cystic fibrosis with preparations such as an inhaled formulation of alpha 1-antitrypsin are at phase II, among others. In 2013 the necessary documentation to commence clinical trials of new products such as topical Thrombin and intravenous fibrinogen was presented as well as the trials to test the efficiency of IVIG Flebogamma® DIF in new areas such as the treatment of post-polio syndrome. Finally, the plans to use Alpha-1 antitrypsin to treat type-1 diabetes (juvenile diabetes) are also of major importance. |
|
|
15 SECOND HALF 2013 REPORT For another consecutive year, Grifols' R&D activity has been rated “Excellent” by the Spanish Profarma Plan. The Spanish Profarma Plan is a joint programme set up by the Ministry of Industry, the Ministry of Health and the Ministry of Economy aimed at promoting scientific research, development and technological innovation in the pharmaceutical industry. Grifols' scientists' commitment to excellence and innovation is essential to develop safe and efficient haemoderivative products in our therapeutic field. This daily effort is complemented by an international collaboration network with private and public research institutions, leaders in their field. Collaboration with third parties In 2013 Grifols increased its collaboration with the European Consortium for the Study of Chronic Liver Failure, with a contribution of three million Euros over the next four years, further to the two million Euros already contributed since 2009. It has also signed an agreement with the Vall d'Hebron Research Institute (VHIR) to create a centre of excellence for research and training in the diagnosis, treatment and monitoring of patients with alpha 1-antitrypsin deficiency (DAAT). As part of Grifols’ global strategy to investigate Alzheimer’s, the Company has entered a long-term collaboration agreement with the ACE Foundation to finance the development of the “Barcelona Alzheimer Treatment & Research Centre”. The centre designed by this foundation is conceived as an independent space to promote and facilitate the diagnosis, treatment and biomedical research of Alzheimer’s and the first project it will host is Grifols’ AMBAR study. Promoting research through awards and grants • Sponsorship of the European Alpha-1 Antitrypsin Laurell (eALTA) research programme, which supports work that contributes to a better understanding and treatment of Alpha 1-antitrypsin (DAAT) deficiency. The research projects that received the award were announced at the annual congress of the European Respiratory Society held in Barcelona in September. • VI edition of the Martín Villar Research Awards, whereby Grifols supports research in the haemostasis field. • Sponsorship of two Fulbright Program grants considered to be one of the most prestigious in the world. The program provides funds to extend postgraduate studies in USA. Grifols’ collaboration will finance two Grifols/Fulbright grants for two years. Priority will be given to the candidates who have passed the admission tests for the grants and present projects in study areas which are related to Grifols’ activities. CAPITAL INVESTMENTS (CAPEX) In 2013 Grifols had met most of its CAPEX plan up to 2015. Therefore, the Company continued with its planned investments and earmarked a total of Euros 151.7 million to expand and improve its production facilities in both Spain and the United States. From 2014 until 2016 the Group will invest around Euros 450 million. Bioscience Division: increased plasma proteins’ fractionation and purification capacity The Bioscience Division has absorbed a substantial proportion of the investment plan, which involves improving the structure of plasma procurement centres in the United States and gradually expanding production facilities. In 2013 the construction and validation of the new plasma fractionation plant in Parets del Vallès (Barcelona, Spain) with a fractionation capacity of 2.2 million litres/year has been completed. The Company expects to obtain final approval for its start up during the first half of 2014. Throughout 2013, Grifols also worked intensely on the validation processes of intermediate product batches obtained in the new plasma fractionation plant (NFF-North Fractionation Facility) in Clayton (North Carolina, USA). This is a major milestone as Grifols will be able to expand its plasma fractionation capacity and supply of intermediate products from the equivalent of 2.5 million litres of plasma in 2013 up to a maximum of 6 million litres. When both plants are on line Grifols will have a total installed plasma fractionation capacity of 12.5 million litres/year. Investment in plasma protein purification has also continued. In the first quarter of 2013 the EMA authorised the expansion |
|
|
16 SECOND HALF 2013 REPORT of the albumin purification plant in Parets del Vallès, thus its albumin purification capacity can be increased by 2.2 million litres. Construction of the new alpha-1 antitrypsin (Prolastin®) purification area in the same industrial complex continues. In USA the construction of the new IVIG purification plant in Los Angeles has been completed and the validation process is underway. This plant has an initial purification capacity of 2 million litres, which could be expanded to 4 million in a second phase, and it will be operational in 2015. The investments to increase the production capacity of the albumin purification facilities in Los Angeles to 4.5 million litres are ongoing and in Clayton the new albumin purification plant will have an initial capacity of 2.8 million litres, which could be expanded in the future. The validation processes of the new facilities and equipment in the freeze-drying and dosage areas in Parets del Vallès plant continues and the FDA approval is expected to be obtained during the second half of 2014. Part of the investments have been earmarked to expanding and relocating plasma donation centres in order to provide more comfort to donors and improve infrastructures related to the classification, preparation and storage of raw materials. Finally, in 2013 management of the plasma fractionation plant in Melville (New York - USA) was transferred to kedrion. Grifols has, therefore, complied with the conditions imposed by the USA anti-trust agency, the Federal Trade Commission (FTC), for the acquisition of Talecris. Hospital Division: Investments focus on Barcelona and Murcia (Spain) Civil work has commenced in the new industrial complex (phase IV) of Las Torres de Cotillas (Murcia-Spain) to manufacture bags for the extraction and conservation of blood components. Taking production capacity to 9 million units will require an investment of Euros 6.9 million approximately. Three new fully automated production lines of intravenous saline solution in flexible polypropylene were brought into operation during 2013 in this plant. The extension works to the fully automated warehouse have also been completed. It is expected to come on line in the first quarter of 2014. The Murcia plant will become one of Grifols’ three major logistics platforms in Spain. Work has begun at the Parets del Vallès site (Barcelona, Spain) to boost the third-party manufacturing area, with the aim of achieving greater flexibility and production capacity in the manufacturing of injectable vials. Diagnostic: financing and providing support to its acquired companies A new production line for DG Gel® cards was incorporated in the Grifols' plant in Düdingen (Switzerland). The construction work is also ongoing at the new plant in Brazil which will manufacture bags for the extraction and conservation of blood components. Approximately Euros 5 million will be invested in the plant, that is being managed by a new company called Gri-Cei, 60% owned by Grifols and 40% owned by the Brazilian company Comércio Exportação e Importação de Materiais Médicos Ltda (CEI). Construction is expected to take two years. Once operative, it will enable Grifols to increase production capacity and strengthen its direct commercial presence in Latin America. Several investments have been made in Grifols' investees; among them the reallocation of all Araclon Biotech's activity and laboratories in a single site in Zaragoza (Spain). |
|
|
17 SECOND HALF 2013 REPORT ACQUISITIONS IN 2013 Acquisition of 21.43% of the biotechnology company TiGenix, specialised in cell therapy In the last quarter of 2013 Grifols acquired a 21.43% stake in the biotechnology company TiGenex through its subsidiary Gri-Cel for Euros 12.4 million. The acquisition was entirely funded with Group capital and follows the strategy of holding an interest in research projects and companies to ensure the continuity of the initiatives being developed. This falls within the framework of Grifols' commitment to the field of advanced therapy and personalised medicine, where it already holds an interest in VCN Bioscience (cancer/oncolytic adenovirus therapies) and Nanotherapix (genetic therapies based on autologous cells). TiGenix specialises in cell therapy. It uses a validated eASCs (expanded Adipose derived stem cells) platform to treat autoimmune and inflammatory diseases. At the present time it has one product used to regenerate knee cartilage and it is developing three others. A 35% stake in Aradigm Corporation as part of a global strategic agreement In the second quarter Grifols acquired 35% of the capital of the US pharmaceutical company, Aradigm Corporation (OTC BB:ARDM.OB) which specialises in the development and marketing of inhaled pharmaceutical products to treat and prevent chronic respiratory diseases, such as non-cystic fibrosis bronchiectasis and cystic fibrosis, among others. The transaction was carried out through the subscription of USD 25.7 million (Euros 20.6 milllion) of share capital of a total capital increase of USD 40.7 million. The acquisition is part of a wider strategic agreement that also grants Grifols the exclusive worldwide future licence to sell the inhaled ciprofloxacin compound (Pulmaquín™ and Lipoquin™) to treat chronic respiratory diseases, including non-cystic fibrosis bronchiectasis, for which the phase 2b clinical trials have been completed. A 60% stake in Progenika Biopharma, specialised in the development of technology for personalised medicine In the first quarter of 2013 Grifols bought 60% of the capital of the Spanish biotechnology company, Progenika Biopharma, for Euros 37 million (Euros 34.6 million net of cash and cash equivalents). The transaction was carried out with a cash payment of 50% of the purchase price and the other 50% was settled in Grifols non-voting shares (Class B). Progenika specialises in developing technology for personalised medicine and focuses on the design and production of genomic and proteomic tests mainly for in vitro diagnosis. It has also developed its own technology to produce molecular diagnosis and prognosis tests and is one of the most advanced companies in this field internationally. Progenika is in fact a worldwide pioneer in the development of molecular biology tests to verify transfusion compatibility. With this acquisition Grifols has strengthened its commitment to research and development in its Diagnostic Division and has added the most advanced technology to its product portfolio in the immunohaematology area. Since 2010 Grifols has held the worldwide distribution rights (except Mexico) for the Progenika BLOODchip® blood genotyping test, which makes the provision of compatible blood units between donor and recipient easier while making transfusions safer. |
|
|
18 SECOND HALF 2013 REPORT 6. OTHER RELEVANT INFORMATION HUMAN RESOURCES Grifols’ average accumulated headcount in 2013 stood at 11,779 employees, up 6% on 2012. The headcount at the various Group companies has increased in all regions; however the 6.6% rise in the workforce in Spain, taking the total number of employees to 2,637 employees, is particularly significant. Job security and employee professional development have been the two strategic priorities of human resources. The number of courses, participants and total hours spent on training have all increased compared to 2012, while more emphasis has been placed on technical and scientific training, and the development of personal and business skills, quality and GMPs, safety and environmental matters, among others. The SAP Talent module has been implemented during 2013 as a tool to standardise performance evaluations worldwide and the number of e-learning courses available through Campus Grifols, the company’s online training platform has increased. ENVIRONMENTAL MANAGEMENT In 2013 the environmental programme implemented by the Company for 2011-2013 was completed, achieving more than 80% of the objectives set. Results demonstrate the importance and effectiveness of the energy efficiency, emissions reduction, and waste management measures adopted in all areas of activity, above all in the production units. Among the most relevant measures adopted in 2013 are • At the Parets del Vallès plant (Barcelona, Spain), the use of CO2 to purify waste water and innovative eco-efficient solutions in the new installations, which have led to an annual saving of 4,600 MWh in electricity and 7,800 MWh in natural gas. The implementation of an improved segregation process for biosanitary waste in the laboratories has led to a 70% reduction in the waste generated. The installation of a new ethanol rectification tower which will double the present distillation capacity has also been completed and is currently at the validation stage. • At the Los Angeles plant (California, USA) the ethanol distillation tower is now finalised, meaning that 1.4 million litres of this compound can be recycled each year instead of being treated as waste. • At the Clayton plant (North Carolina, USA) the cold storage units that use R22 refrigerant gas have been replaced by others that use gases that are not harmful to the ozone layer. Also in an external plant an alternative waste recovery treatment of aqueous solution of polyethylene glycol has been started, using anaerobic digestion to produce biogas. Grifols has once again taken part in the Carbon Disclosure Project (CDP), the aim of which is to recognise the steps taken by the various participating companies to cut gas emissions and mitigate the risks of climate change. This programme represents 722 institutional investors with assets valued at USD 87 billion. In 2013 Grifols obtained 90 points out of a possible 100, two more than in 2012, placing it 15th in the ranking of the best valued companies among 125 major companies from Spain and Portugal, and the top company in the health sector. |
|
|
19 SECOND HALF 2013 REPORT 7. STOCK PERFORMANCE 2013 was an exceptional year for the equity markets and the IBEX 35 was not an exception increasing its value by 21.42%. Grifols Class A shares revaluation beat the Spanish reference index by increasing its value 32% in 2013. Shares started the year at Euros 26.36 per share and closed on the 31 of December at Euros 34.77 per share. Grifols has two types of shares publicly listed. Class A shares are listed in the Spanish Stock Exchange and a component of the main index, IBEX-35. Class B or non-voting shares are also listed in the Spanish Stock exchange and in NASDAQ in the United States via ADR’s (American Depository Receipts). Class B shares also beat the IBEX with a revaluation close to 36%, from Euros 19.10 per share to Euros 25.89 per share. The ADR listed in NASDAQ experienced a 39.90% revaluation reaching US dollars 36.12 per share on the last trading day of December 2013. 8. EVENTS AFTER THE REPORTING PERIOD SIGNING OF BRIDGE LOAN TO ACQUIRE THE DIAGNOSTIC DIVISION FROM NOVARTIS On 3 January 2014 Grifols signed a bridge loan of USD 1,500 million funded in equal amounts by Nomura, BBVA and Morgan Stanley. The loan is to finance the acquisition of Novartis' transfusion medicine and immunology diagnostic division. The loan imposes no financial restrictions on Grifols in respect of dividends or investments. This bridge loan is a temporary, short-term finance. Grifols plans to optimise its finance costs by restructuring its debt in 2014, including this bridge loan. COMPLETION OF THE PURCHASE OF THE NOVARTIS DIAGNOSTIC DIVISION On 9 January 2014 the group completed the acquisition of the blood transfusion medicine and immunology Diagnostic unit of the Swiss company Novartis International AG for USD 1,675 million (Euros 1,222 million). The transaction was completed with the same terms and conditions announced on 11 November 2013, after receiving the necessary legal and regulatory authorisations. The transaction has been carried out through the newly created, wholly owned Grifols’ subsidiary, G-C Diagnostics Corp (USA). The Company expects the Diagnostic division to generate closed to 20% of total Group revenues subsequent to the transaction, compared to the 4% it currently represents. With this transaction Grifols is speeding up its new growth strategy based on promoting complementary business areas. Approximately 550 employees will join Grifols’ workforce from Novartis. |
|
|
20 SECOND HALF 2013 REPORT PROFIT AND LOSS ACCOUNT IN THOUSANDS OF EUROS 2013 2012 % VAR. NET REVENUE 2,741,732 2,620,944 4.6% COST OF SALES (1,323,880) (1,291,345) 2.5% GROSS PROFIT 1,417,852 1,329,599 6.6% % ON SALES 51.7% 50.7% R&D (123,271) (124,443) -0.9% SGA (558,461) (545,072) 2.5% OPERATING EXPENSES (681,732) (669,515) 1.8% OPERATING RESULT 736,120 660,084 11.5% % ON SALES 26.8% 25.2% FINANCIAL RESULT (237,419) (270,729) -12.3% SHARE OF RESULT OF EQUITY ACCOUNTED INVESTEES (1,165) (1,407) -17.2% PROFIT BEFORE TAX 497,536 387,948 28.2% % ON SALES 18.1% 14.8% INCOME TAX EXPENSE (155,482) (132,571) 17.3% % OF PRE-TAX INCOME 31.3% 34.2% CONSOLIDATED PROFIT FOR THE YEAR 342,054 255,377 33.9% RESULTS ATTRIBUTABLE TO NON-CONTROLLING INTERESTS (3,497) (1,309) 167.2% GROUP NET PROFIT 345,551 256,686 34.6% % ON SALES 12.6% 9.8% EBITDA 864,588 789,209 9.6% % ON SALES 31.5% 30.1% ADJUSTED EBITDA 1 917,366 836,117 9.7% % ON SALES 33.5% 31.9% |
|
|
21 SECOND HALF 2013 REPORT BALANCE SHEET IN THOUSANDS OF EUROS DECEMBER 2013 DECEMBER 2012 ASSETS NON-CURRENT ASSETS 3,701,376 3,692,910 GOODWILL AND OTHER INTANGIBLE 2,775,576 2,838,994 PROPERTY PLANT & EQUIPMENT 840,238 810,107 INVESTMENTS IN EQUITY ACCOUNTED INVESTEES 35,765 2,566 OTHER NON-CURRENT ASSETS 49,797 41,243 CURRENT ASSETS 2,139,660 1,934,564 INVENTORIES 946,913 998,644 TRADE AND OTHER RECEIVABLES 465,581 447,173 OTHER CURRENT FINANCIAL ASSETS 1,200 460 OTHER CURRENT ASSETS 17,189 14,960 CASH AND CASH EQUIVALENTS 708,777 473,327 TOTAL ASSETS 5,841,036 5,627,474 EQUITY & LIABILITIES EQUITY 2,107,204 1,880,741 CAPITAL 119,604 117,882 SHARE PREMIUM RESERVE 910,728 890,355 RESERVES 883,415 620,144 TREASURY STOCK 0 (3,060) INTERIM DIVIDENDS (68,755) - CURRENT YEAR EARNINGS 345,551 256,686 NON-CONTROLLING INTEREST 5,942 3,973 OTHER COMPREHENSIVE INCOME (89,281) (5,239) NON-CURRENT LIABILITIES 3,018,536 3,153,868 FINANCIAL LIABILITIES 2,553,211 2,690,819 OTHER NON-CURRENT LIABILITIES 465,325 463,049 CURRENT LIABILITIES 715,296 592,865 FINANCIAL LIABILITIES 258,144 195,578 OTHER CURRENT LIABILITIES 457,152 397,287 TOTAL EQUITY AND LIABILITIES 5,841,036 5,627,474 |
|
|
22 SECOND HALF 2013 REPORT CASH FLOW IN THOUSANDS OF EUROS DECEMBER 2013 DECEMBER 2012 NET PROFIT 345,551 256,686 DEPRECIATION AND AMORTITZATION 128,469 129,126 NET PROVISIONS 4,611 8,104 OTHER ADJUSTMENTS AND OTHER CHANGES IN WORKING CAPITAL 73,782 119,006 CHANGES IN INVENTORIES 17,277 14,509 CHANGES IN TRADE RECEIVABLES (40,095) 34,421 CHANGES IN TRADE PAyABLES 62,416 (54,734) chANGE IN OPERATING WORkING cAPITAL 39,598 (5,804) NET CASH FLOW FROM OPERATING ACTIVITIES 592,011 507,118 BUSINESS COMBINATIONS AND INVESTMENTS IN GROUP COMPANIES (69,172) (11,067) CAPEX (151,687) (156,061) R&D/OTHER INTANGIBLE ASSETS (21,162) (10,067) OTHER CASH INFLOW /(OUTFLOW) 5,987 112,760 NET CASH FLOW FROM INVESTING ACTIVITIES (236,034) (64,435) FREE CASH FLOW 355,977 442,683 ISSUE (PURCHASE) OF EQUITY 14,760 (9) PROCEEDS FROM ISSUE OF SHARE CAPITAL 20,461 - ISSUE (REPAYMENT) OF DEBT (79,413) (255,569) DIVIDENDS (69,138) 0 OTHER CASH FLOWS FROM/(USED IN) FINANCING ACTIVITIES 8,184 (49,752) NET CASH FLOW FROM FINANCING ACTIVITIES (105,146) (305,330) TOTAL CASH FLOW 250,831 137,353 CASH AND CASH EQUIVALENTS AT THE START OF THE YEAR 473,327 340,586 EFFECT OF EXCHANGE RATE CHANGES IN CASH AND CASH EQUIVALENTS (15,381) (4,612) CASH AND CASH EQUIVALENTS AT THE END OF THE YEAR 708,777 473,327 |
|
|
23 SECOND HALF 2013 REPORT GRIFOLS’ DAILY SHARE PRICE, CLASS A & CLASS B VS IBEX 35 142 140 138 136 134 132 130 128 126 124 122 120 118 116 114 112 110 108 106 104 102 100 98 96 94 92 90 JAN 2013 FEB MAR SEP OCT NOV DEC JUN JUL AUG APR MAY grifols B: 135.52 grifols: 131.89 iBex: 121.42 (BASE 100, FROM JANUARy 1 TO DECEMBER 31 2013) 1 Excluding costs relating to the Talecris acquisition and other non- recurrent costs 2 Excluding costs associated with the purchase of Talecris as well as the amortization of intangibles and of deferred financial costs related to the acquisition. 3 Source: Market Research Bureau (MRB) - The Worldwide Plasma Proteins Market 2012. |
|
|
1 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. Grifols’ sales rose by 4.6% to Euros 2,741.7 million in 2013 · Bioscience leads the growth with +5.3% (+8.2% cc1) sales increase driven by volume growth in all plasma derivative products and stable prices. · Net profit rose by 34.6% to Euros 345.6 million. A 12.3% reduction in finance results improves earnings. · Adjusted EBITDA2 grew by 9.7% to Euros 917.4 million. · Efficiencies and competitiveness derived from process flexibilization and the optimisation of plasma contributed to the 160 bps3 increase in adjusted2 EBITDA margin, which rose to 33.5% of revenues. · Net financial debt at December 2013 stood at Euros 2,087.2 million, 2.28 times adjusted2 EBITDA and lower than the ratio of 2.87 at December 2012. · Grifols resumes payment of cash dividends and keeps its commitment to a pay-out ratio of 40% of net profit. Barcelona, February 27th 2014.- Grifols (MCE:GRF, MCE:GRF.P and NASDAQ:GRFS) closed 2013 with revenues of Euros 2,741.7 million, an increase of 4.6% on the prior year. The geographical diversification of sales limited the effect of volatility in exchange rates, in particularly the Euro-Dollar, and revenues grew by 7.4% cc. I n 2013 Grifols maintained its position as the third largest producer of plasma derivatives (Bioscience Division) in the world, with a global market share of approximately 20%4. The company has achieved an excellent and competitive position in the three diagnostics specialities it operates (Diagnostic Division). The recent acquisition of Novartis' transfusions and immunology diagnostics unit, completed in January 2014, will allow the Group to grow further in the diagnostics segment from 2014. The Hospital division retains its leading position in Spain as a provider of intravenous solutions. Boosting sales in regions which have been less affected by austerity measures, with shorter collection periods and better margins continued to be one of the company's core strategies in 2013. As a result, Grifols remained very active on international markets during the period, where it generated 92.4% of its revenues. Sales outside Spain rose by 5.2% (8.2% cc) compared to 2012, reaching Euros 2,533.8 million. 1 CC – Constant currency 2 Excluding costs relating to the Talecris acquisition and other non- recurrent costs 3 Bps – basis points 4 Source: Market Research Bureau (MRB) – The Worldwide Plasma Proteins Market 2012. |
|
|
2 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. Sound results: margins and profits continue to improve The EBITDA margin was 140 bps higher, and at the end of the year represented 31.5% of revenues. The adjusted EBITDA margin5 rose by 160 bps to 33.5% of revenues. The sales performance of the main plasma proteins sold by the Group and the optimisation of raw material and manufacturing costs through more flexible production processes have together increased the profitability per litre of plasma. This was reflected in gross margins growing by 6.6% to 51.7% of revenues. Operating expenses related to administration and general services were stable. Both factors have contributed to a 9.6% increase in EBITDA compared to 2012 to Euros 864.6 million. On an adjusted basis EBITDA5 increased by 9.7% to Euros 917.4 million. The company has continued to work towards greater flexibility and scalability in its production processes to achieve efficiencies in the manufacturing of plasma derivatives, and to constantly adapt to market conditions. To achieve this, Grifols has focused on both, increasing its protein fractionation and purification capacities and on making these processes more flexible. The aim is to be able to purify and fill the intermediate fractions generated during the first stage of the manufacturing process (fractionation) at any of the three plants of the Group, for which Grifols requires FDA and EMA licenses. The financing conditions negotiated at the start of 2012 have contributed to a 12.3% reduction in financial results, down to Euros 237.4 million from Euros 270.7 million in 2012. 2012 R&D deductions received in the first quarter of 2013 and the filing of a combined State Corporate Tax return for the subsidiaries of North Carolina (USA) have reduced the effective tax rate. As a result the Group's net profit for 2013 rose by 34.6% to Euros 345.6 million, 12.6% of the Group's revenues. 2013 - Key financial indicators (Thousands of Euros) 2013 2012 % var. % var. cc TOTAL NET REVENUES (NR) 2,741,732 2,620,944 4.6% 7.4% BIOSCIENCE DIVISION 2,448,824 2,325,088 5.3% 8.2% HOSPITAL DIVISION 97,131 95,870 1.,3% 2.,6% DIAGNOSTIC DIVISION 130,339 134,342 -3.0% -1.,0% RAW MATERIALS AND OTHERS 65,438 65,644 -0.3% 1.6% EBITDA 864,588 789,209 9.6% % NR 31.5% 30.1% 140 bps ADJUSTED EBITDA* 917,366 836,117 9.7% % NR 33.5% 31.9% 160 bps GROUP NET PROFIT 345,551 256,686 34.6% % NR 12.6% 9.8% ADJUSTED GROUP NET PROFIT** 450,021 364,671 23.4% % NR 16.4% 13.9% * Excluding costs relating to the Talecris acquisition and other non- recurrent costs * * Excluding costs relating to the Talecris acquisition, as well as the amortisation of intangibles and the amortization of deferred finance costs related to the acquisition |
|
|
3 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. Sales increase in all regions and Grifols generates 92.4% of its revenues on international markets 2013 sales by region (Thousands of Euros) 2013 % of sales 2012 % of sales % Var % var cc EU 569,827 20.8% 559,327 21.3% 1.9% 2.2% US & Canada 1,707,620 62.3% 1,658,548 63.3% 3.0% 6.1% RoW (Rest of the World) 426,257 15.5% 371,619 14.2% 14.7% 19.5% SUBTOTAL 2,703,704 98.6% 2,589,494 98.8% 4.4% 7.2% RAW MATERIALS 38,028 1.4% 31,450 1.2% 20.9% 23.5% TOTAL 2,741,732 100.0% 2,620,944 100.0% 4.6% 7.4% I n the European Union sales performance has confirmed the expected recovery. Recurring revenues, excluding Spain, grew by 4.5% to Euros 361.9 million. In Spain declines in sales of Diagnostic and Hospital products and services as a result of the cuts to public health service spending – which have reduced the number of operations and diagnostic tests performed at hospitals – have limited the growth in the region. The decline in Spain has decelerated when compared to 2012, to 2.4%, with a turnover of Euros 207.9 million, representing 7.6% of total revenues. In the United States and Canada the sales performance of the main plasma proteins was good in terms of volumes, with double-digit growth in albumin and factor VIII. In Canada, activity was stable following the renegotiation of the contracts with the Canadian Blood Services (CBS) and Héma-Québec. Grifols remains the country's main supplier of plasma products and plasma fractionator. Sales of plasma proteins in the United States have gradually risen on a quarterly basis achieving record sales in absolute terms and growth of 7.9% (11.2% cc) in the world's largest plasma derivatives market. In addition, Grifols has bolstered its presence in the USA with new products and services in areas such as Hospital Logistics (Hospital division) and the Diagnostic Division, as the FDA has approved a number of reagents and instruments for blood typing which are fundamental for its commercialisation. The Group's total sales in the United States grew by 6.3% (9.6% cc) in 2013. The most dynamic areas remain those outside the European Union and North America. Overall, RoW sales (Rest of the World excluding Raw Materials) grew by 14.7% (19.5% cc). For Grifols, these emerging regions have the greatest growth potential. The company has strengthened its presence in markets such as the Middle East, opening a new branch office in Dubai; or China, where Grifols' sales have grown rapidly over the past three years, and where the company expects to consolidate its position as a key player in immunohaematology and as a supplier of albumin, the only plasma protein which can be exported at present; or Brazil, where construction has started on a plant to manufacture bags for extraction and storage of blood components. Market research in countries such as Turkey, India and Russia has also progressed. Sales performance by division Grifols' organic growth will come from strengthening the products and services offered by its three divisions in its main markets. The company has followed an integration |
|
|
4 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. commercial strategy to complement its plasma protein therapies with diagnostic (Diagnostic division) and hospital logistics (Hospital division) products and services. I n November 2013 this strategy led to an agreement to acquire Novartis' transfusion and immunology diagnostics business, which focuses on guaranteeing the safety of the blood donated for transfusions. The acquisition of Novartis' transfusion and immunology diagnostics unit has not impacted 2013 figures, and the weights of the divisions did not change. · Bioscience Division: 89.3% of Grifols’ revenues The Bioscience Division continues to drive the Group’s growth. Volume growth in a stable price scenario has boosted revenues with a positive trend in the United States, EU and ROW. The Bioscience Division sales totalled Euros 2,448.8 million. Over 95% of sales were concentrated in the international markets. By volume,6 mention should be made to the approximately 30% growth in albumin sales bolstered by the United States and China, which has the highest demand for this protein in Asia. Also worth noting is the rise in alpha-1 antitrypsin, with double digit volume growth. Grifols is the world’s benchmark for this plasma protein and sales in 2013 were particularly significant in countries such as Canada, the United States, Germany and Spain, where sales of Prolastin® have just commenced. I n 2013 Grifols strengthened its presence in the area of respiratory illness and is seeking to establish a respiratory care franchise to specifically tackle this significant therapeutic area. To this end Grifols has its own line of haemoderivative products; Prolastin® and Prolastin-C® and the world licence for Pulmaquin™ and Lipoquin™. Grifols has the exclusive right to distribute these compounds following the acquisition of 35% of Aradigm Corporation. · Diagnostic Division: 4.8% of revenues In 2013 the Diagnostic Division recorded sales of Euros 130.3 million, of which almost 80% were made outside of Spain. This business area represents approximately 4.8% of Grifols sales, although three important milestones in 2013 will have an immediate impact on the future of the division: 1. The acquisition of the transfusion and immunological diagnostic unit of Novartis, which will enable the expansion of the division's portfolio of equipment and reagents making Grifols the only company capable of offering comprehensive solutions to the blood donation centres in the most efficient and secure manner with full control over the transfusion process: from donation to transfusion. 2. The acquisition of 60% of the biotech company Progenika Biopharma in March 2013, to reinforce the portfolio of products and acquire the most innovative technology. This company is a global pioneer in the development of molecular biology testing for the performance of studies to check for transfusion compatibility and in the development of immunological tests to monitor biological drugs. 3. The approval by the FDA of the reagents for blood typing (gel card) and the instruments required for their use (incubator, centrifuge and the automatic instrument WADiana® and Erytra®). 6 Volume refers to grams/units sold and not the monetary amount |
|
|
5 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. · Hospital Division: 3.5% of Grifols’ revenues The Hospital division increased its turnover by 1.3% (2.6% cc) in 2013 as a result of Grifols' internationalisation strategy, which has led to sales rising in foreign markets by and has mitigated the cuts to public health care spending in Spain. Sales of the Hospital division excluding Spain increased over 45% during the year. The growth in the Hospital Logistics area in Latin America, new agreements to produce injectable pharmaceuticals for third parties and the launch of new products have all boosted the division's strategy of internationalisation, offsetting the slump in sales in Spain. The production agreements with third parties include the agreement with Cadence Pharmaceuticals to produce its OFIRMEV® paracetamol in a flexible container for intravenous infusion and the Agreement with Cumberland Pharmaceuticals to sell the first ibuprofen in a flexible container for intravenous infusion. The division has also completed the development of a painkiller in a polypropylene bag for the US market, and has started the development of three new products: a specific painkiller, also for the US market, an NSAID for the European and US markets and a Grifill® to be used in phase III clinical trials in collaboration with Cerus. I n Hospital Logistics, one of the areas with the greatest potential for international expansion, the first automated carousel system was installed in the Unites States, at the Emory University Hospital’s pharmacy (Atlanta). This system allows the control of drugs’ inventories and hospital products, facilitating procurement processes, optimizing space and time. Balance Sheet - Key Indicators · Euros 309 million debt reduction and credit rating upgrades At December 2013 total consolidated assets amount to Euros 5,841.0 million, with no significant changes in relation to the Euros 5,627.5 million reported at December 2012. Grifols’ net financial debt at December 2013 stood at Euros 2,087.2 million, a ratio of 2.28 times adjusted EBITDA6 and lower than the ratio of 2.87 recorded at December 2012. Over the course of the year, the Group's net financial debt has been reduced by Euros 308.9 million. This priority objective of continuing to decrease debt, together with the high sustainable levels of operating activity and ongoing progress in achieving improvements related to the acquisition of Talecris contributed to Standard & Poor’s ratifying its credit rating for Grifols and Moody’s upgrading its rating. Credit ratings at December 2013: Moody’s Standard & Poors Senior secured debt Ba1 BB+ Corporate rating Ba2 BB Senior unsecured debt B1 B+ Outlook Negative Stable |
|
|
6 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. The cash position after dividend, debt and interest payments at December 2013 was Euros 708.8 million, well above Euros 473.3 million reported in 2012. Generation of operating cash flows was particularly strong, at Euros 592.0 million at year end. Growing profits and financing discipline have contributed to the reduction in more than Euros 200 million of financing cashflow needs, freeing cash for investment activities in order to guarantee the long term growth of the company. In this regard, the Company invested Euros 236.0 million in 2013, including the various acquisitions made over the course of the year and in compliance with the Capex plan. · Equity Grifols equity increased to Euros 2,107.2 million in 2013, mainly as a result of profits obtained during the period. As at December 2013, Grifols’ share capital amounted to Euros 119.6 million, represented by 213,064,899 ordinary shares (Class A) with a par value of Euros 0.50 per share and 130,712,555 shares without voting rights (Class B) with a par value of Euros 0.10 per share. I n 2013 Grifols resumed payment of cash dividends as remuneration for all its shareholders (Class A and Class B shareholders). Grifols' dividend policy has not changed and its pay-out ratio remains at 40% of net profit, the level prior to the acquisition of Talecris. F ourth quarter of 2013 – Key indicators Grifols’ reported sales from October to December 2013 were Euros 695.2 million rising 5.1% (9.7% cc) compared to Euros 661.4.1 million obtained during the same period of the preceding year. The Bioscience division totaled Euros 627.4 million, contributing 90.2% to sales revenue and growth of 6.3%. The Diagnostic division generated Euros 32.5 millions, while Hospital accounted for Euros 22.8 million. These figures represent 4.7% and 3.3% of the group’s total income, respectively. By geographical region, the United States and Canada led growth in sales, with recurring sales (excluding Raw Materials) of Euros 440.2 million, equivalent to 63.3% of revenues. Europe with Euros 137.9 million and other regions (ROW) with Euros 112.5 million accounted for 19.9% and 16.2% of total income, respectively. Revenues were positive in all divisions and in all regions where the company is present, supporting the positive trend expected in the Diagnostic Division in the fourth quarter. Investment activities: R&D, CAPEX, Acquisitions · An extensive R&D Project’s portfolio According to Forbes magazine, Grifols is among the 100 most innovative companies in the world and its commitment to research is evident from its 2013 results. This is expressed both through a sound R&D investment policy, with 4.5% or more than Euros 123.3 million of its sales revenues earmarked for this area in 2013, and through the acquisition of shares in R&D companies and projects in fields of medicine other than Grifols’ core activity to thus ensure ongoing initiatives. Grifols total R&D investment during the year including both its |
|
|
7 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. portfolio of projects in progress, and those of its investees such as Progenika, Araclon or Nanotherapix is the most extensive and diverse in the entire Group's history. Some of the main events have been the enrolment of the first patients to the AMBAR trials (Alzheimer Management by Albumin Replacement) that involves combining plasma exchange and its replacement with albumin to treat Alzheimer’s and the authorisation of the Spanish Agency for Medication and Healthcare Products (AEMPS) to start phase I of the clinical trial of the Alzheimer vaccination being developed by Grifols through its company, Araclon Biotech. Safety and tolerability for humans, but not efficiency, will be assessed in this phase. This represents a first milestone in the project's development. Grifols has several ongoing R&D projects in different areas, among others the use of Albumin in hepatology, the development of biological fibrin glue with healing or sealing properties for surgery, etc. · Capital Investments (CAPEX) Euros 450 million from 2014 to 2016 I n 2013 Grifols has completed most of its CAPEX plan up to 2015. The Company continued with its planned investments and earmarked a total of Euros 151.7 million to expand and improve its production facilities in both Spain and the United States. From 2014 until 2016 the Group will invest around Euros 450 million. The Bioscience Division has absorbed a substantial proportion of the investment plan, which involves improving the structure of plasma procurement centres in the United States and gradually expanding production facilities. I n 2013 the construction and validation of the new plasma fractionation plant in Parets del Vallès (Barcelona, Spain) with a fractionation capacity of 2.2 million litres/year has been completed. The Company expects to obtain final approval during the first half of 2014. Throughout 2013, Grifols also worked intensely on the validation processes of intermediate product batches obtained in the new plasma fractionation plant in Clayton (North Carolina, USA). When both plants are on line Grifols will have a total installed plasma fractionation capacity of 12.5 million litres/year. I nvestment in plasma protein purification has also continued. The new albumin purification plant in Parets del Vallès will increase albumin purification capacity by 2.2 million litres and construction of the new alpha-1 antitrypsin (Prolastin®) purification area in the same industrial complex continues. I n the Unites States investments to increase the production capacity of the albumin purification facilities in Los Angeles to 4.5 million litres and in Clayton where the new albumin purification plant will have an initial capacity of 2.8 million litres, which could be expanded in the future, are ongoing. Part of the investments has been earmarked to expanding and relocating plasma donation centres. The Hospital Division has commenced civil works in the new industrial complex (phase IV) of Las Torres de Cotillas (Murcia-Spain) to manufacture bags for the extraction and conservation of blood components. The new plant will increase production capacity to 9 million units and it will require an investment of Euros 6.9 million approximately. The Diagnostic Division incorporated a new production line for DG Gel® cards in the Grifols' plant in Düdingen (Switzerland). The construction work is also ongoing at the new plant in Brazil which will manufacture bags for the extraction and conservation of blood |
|
|
8 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. components. Approximately Euros 5 million will be invested in the new company called Gri-Cei, 60% owned by Grifols and 40% owned by the Brazilian company Comércio Exportação e Importação de Materiais Médicos Ltda (CEI). Construction is expected to take two years. Once operative, it will enable Grifols to increase production capacity and strengthen its direct commercial presence in Latin America. · Acquisitions in 2013 A 35% stake in Aradigm Corporation. In the second quarter Grifols acquired 35% of the capital of the company, which specialises in the development and marketing of inhaled pharmaceutical products to treat and prevent chronic respiratory diseases, such as noncystic fibrosis bronchiectasis and cystic fibrosis, among others. The transaction was carried out through the subscription of USD 25.7 million (Euros 20.6 million) of share capital of a total capital increase of USD 40.7 million and it is part of a wider strategic agreement. A 60% stake in Progenika Biopharma, specialised in the development of technology for personalised medicine, for Euros 37 million (Euro 34.6 million net of cash and cash equivalents). The acquisition price was met by a 50% cash payment and 50% Grifols nonvoting shares (Class B). Progenika has pioneered the development of molecular biology tests for pre transfusion compatibility. Acquisition of 21.43% of the biotechnology company TiGenix, for Euros 12.4 million. The acquisition was entirely funded with internal funds. TiGenix is specialised in cell therapy and the investment follows Grifols’ strategy of holding an interest in research projects in the fields of advance therapies and personalized medicine. Completion of the acquisition of the Novartis’ Diagnostic unit. On January 2014 the group completed the acquisition of the blood transfusion medicine and immunology Diagnostic unit of the Swiss company Novartis for USD 1,675 million (Euros 1,222 million). The transaction was completed with the same terms and conditions announced on 11 November 2013, once all the necessary legal and regulatory authorisations were received. The transaction has been carried out through the newly created, wholly owned Grifols’ subsidiary, G-C Diagnostics Corp (USA) Human Resources Grifols’ average accumulated headcount in 2013 stood at 11,779 employees, up 6% on 2012. The headcount has increased in all regions where the company has a presence. In Spain the number of employees increased by 6.6% over the groups average. Job security and employee professional development have been the two strategic priorities of human resources during the year. Environmental Management I n 2013 the environmental programme implemented by the Company for 2011-2013 was completed, achieving over 80% of its objectives. Results demonstrate the importance and effectiveness of the energy efficiency, emissions reduction, and waste management measures adopted in all areas of activity, above all in the production units. |
|
|
9 Free translation from the original in Spanish. In the event of discrepancy, the Spanish-language version prevails. About Grifols Grifols is a global healthcare company with a 70-year legacy of improving people’s health and well being through the development of life-saving plasma medicines, hospital pharmacy products and diagnostic technology for clinical use. The company is present in more than 100 countries worldwide and its headquarters are located in Barcelona, Spain. Grifols is a leader in plasma collection with a network of 150 plasma donation centers in the U.S., and a leading producer of plasma-derived biological medicines. The company provides a comprehensive range of solutions for in vitro diagnostics for clinical laboratories, blood banks and blood transfusion services, and it is a point of reference in the field of transfusion medicine. In 2013, sales exceeded 2,740 million Euros with a headcount of 13,200 employees Annually, Grifols allocates approximately 5% of income to R&D; this investment is evidence of its commitment to scientific progress. The company’s class A shares are listed on the Spanish Stock Exchange, where they are part of the Ibex-35 (MCE:GRF). Its non-voting class B shares are listed on the Mercado Continuo (MCE:GRF.P) and on the U.S. NASDAQ via ADRs (NASDAQ: GRFS). For more information visit www.grifols.com DISCLAIMER The facts and figures contained in this report which do not refer to historical data are “projections and forward-looking statements”. The words and expressions like “believe”, “hope”, “anticipate”, “predict”, “expect”, “intend”, “should”, “try to achieve”, “estimate”, “future” and similar expressions, insofar as they are related to Grifols Group, are used to identify projections and forward-looking statements. These expressions reflect the assumptions, hypothesis, expectations and anticipations of the management team at the date of preparation of this report, which are subject to a number of factors that could make the real results differ considerably. The future results of Grifols Group could be affected by events related to its own activity, such as shortages of raw materials for the manufacture of its products, the launch of competitive products or changes in the regulations of markets in which it operates, among others. At the date of preparation of this report Grifols Group has adopted the measures it considers necessary to offset the possible effects of these events. Grifols, S.A. does not assume any obligation to publicly inform, review or update any projections and forward-looking statements to adapt them to facts or circumstances following the preparation of this report, except as specifically required by law. This document does not constitute an offer or invitation to purchase or subscribe shares, in accordance with the provisions of the Spanish Securities Market Law 24/1988, of July 28, the Royal Decree-Law 5/2005, of March 11, and/or Royal Decree 1310/2005, of November 4, and its implementing regulations. |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereto duly authorized.
|
|
Grifols, S.A. | ||
|
|
| ||
|
|
| ||
|
|
By: |
/s/ David I. Bell | |
|
|
|
Name: |
David I. Bell |
|
|
|
Title: |
Authorized Signatory |
|
|
| ||
|
Date: February 27, 2014 |
| ||