fv4
As filed with the Securities and Exchange
Commission on October 24, 2011
Registration
No. 333-
UNITED STATES SECURITIES AND
EXCHANGE COMMISSION
Washington, D.C.
20549
Form F-4
REGISTRATION
STATEMENT
UNDER
THE SECURITIES ACT OF
1933
GRIFOLS, S.A.
(Exact name of registrant as
specified in its charter)
| |
|
|
|
|
|
Spain
|
|
2834
|
|
Not applicable
|
(Jurisdiction of
incorporation or organization)
|
|
(Primary Standard Industrial
Classification Code Number)
|
|
(I.R.S. Employer
Identification Number)
|
(FOR CO-REGISTRANTS, PLEASE SEE “TABLE OF
CO-REGISTRANTS”
ON THE FOLLOWING PAGE)
Avinguda
de la Generalitat,
152-158
Parc de Negocis Can Sant Joan
Sant Cugat del Vallès 08174
Barcelona, Spain
(Address, including zip code,
and telephone number, including area code, of registrants’
principal executive offices)
David Ian Bell
General Counsel
Grifols Inc.
2410 Lillyvale Ave
Los Angeles, CA
90032-3514
(323) 227-7540
(Name, address, including zip
code, and telephone number, including area code, of agent for
service)
Copies to:
| |
|
|
Julie M. Allen, Esq.
Proskauer Rose LLP
Eleven Times Square
New York, New York 10036
Telephone:
(212) 969-3000
Facsimile:
(212) 969-2900
|
|
Tomás Dagá
Raimon Grifols
Osborne Clarke S.L.P.
Avenida Diagonal, 477
Planta 20, 08036 Barcelona, Spain
Tel: +34 93 419 1818
|
Approximate date of commencement of the proposed sale to the
public: As soon as practicable after the
effective date of this Registration Statement.
If the securities being registered on this form are being
offered in connection with the formation of a holding company
and there is compliance with General Instruction G, check
the following
box. o
If this form is filed to register additional securities for an
offering pursuant to Rule 462(b) under the Securities Act
of 1933, check the following box and list the Securities Act
registration statement number of the earlier effective
registration statement for the same
offering. o
If this form is a post-effective amendment filed pursuant to
Rule 462(d) under the Securities Act of 1933, check the
following box and list the Securities Act registration statement
number of the earlier effective registration statement for the
same
offering. o
Indicate by check mark whether the registrant is a large
accelerated filer, an accelerated filer, a non-accelerated
filer, or a smaller reporting company. See the definitions of
“large accelerated filer,” “accelerated
filer” and “smaller reporting company” in Rule
12b-2 of the Exchange Act.
|
|
|
|
|
| Large
accelerated
filer o
|
Accelerated
filer o
|
Non-accelerated
filer þ
|
Smaller
reporting
company o
|
|
(Do not check if smaller reporting company)
If applicable, place an X in the box to designate the
appropriate rule provision relied upon in conducting this
transaction: Exchange Act Rule 13e-4(i) (Cross-Border Issuer
Tender
Offer) o Exchange
Act Rule 14d-1(d) (Cross-Border Third-Party
Offer) o
CALCULATION
OF REGISTRATION FEE
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proposed Maximum
|
|
|
|
Proposed Maximum
|
|
|
|
Amount of
|
|
Title of Each Class of
|
|
|
Amount to be
|
|
|
|
Offering
|
|
|
|
Aggregate
|
|
|
|
Registration
|
|
|
Securities to be Registered
|
|
|
Registered(1)
|
|
|
|
Price per Note(1)
|
|
|
|
Offering Price(1)
|
|
|
|
Fee
|
|
|
8.25% Senior Notes due 2018
|
|
|
$
|
1,100,000,000
|
|
|
|
|
100
|
%
|
|
|
$
|
1,100,000,000
|
|
|
|
$
|
126,060
|
|
|
Guarantee of 8.25% Senior Notes due 2018(2)
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) |
|
Estimated solely for the purpose of calculating the registration
fee pursuant to Rule 457 under the Securities Act of 1933,
as amended (the “Securities Act”). |
| |
|
(2) |
|
Pursuant to Rule 457(n) under the Securities Act, no
separate fee is payable with respect to the guarantee. |
The Registrants hereby amend this Registration Statement on
such date or dates as may be necessary to delay its effective
date until the Registrants shall file a further amendment that
specifically states that this Registration Statement shall
thereafter become effective in accordance with Section 8(a)
of the Securities Act of 1933, as amended, or until this
Registration Statement shall become effective on such date as
the Securities and Exchange Commission, acting pursuant to said
Section 8(a), determines.
TABLE OF
CO-REGISTRANTS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Address, Including Zip
|
|
|
|
State or Other
|
|
|
|
|
|
Code and Telephone
|
|
|
|
Jurisdiction of
|
|
Primary Standard
|
|
I.R.S. Employer
|
|
Number, Including Area
|
|
|
|
Incorporation or
|
|
Industrial
|
|
Identification
|
|
Code, of Principal
|
|
Exact Name as Specified in Its Charter
|
|
Organization
|
|
Classification Number
|
|
Number
|
|
Executive Offices
|
|
|
|
Grifols
Inc.(1)
|
|
Virginia,
United States
|
|
2834
|
|
20-2533768
|
|
2410 Lillyvale Ave.,
Los Angeles, CA 90032,
(323) 225-2221
|
|
Grifols Biologicals Inc.
|
|
Delaware,
United States
|
|
2834
|
|
13-4253630
|
|
5555 Valley Boulevard,
Los Angeles, CA 90032,
(323) 225-2221
|
|
Biomat USA, Inc.
|
|
Delaware,
United States
|
|
8099
|
|
95-4343492
|
|
2410 Lillyvale Ave.,
Los Angeles, CA 90032,
(323) 225-2221
|
|
Grifols Therapeutics Inc.
|
|
Delaware,
United States
|
|
2834
|
|
34-2032472
|
|
4101 Research Commons
79 T.W. Alexander Drive,
Research Triangle Park,
North Carolina 27709
(919) 316-6300
|
|
Talecris Plasma Resources, Inc.
|
|
Delaware,
United States
|
|
8099
|
|
20-5444433
|
|
4101 Research Commons
79 T.W. Alexander Drive,
Research Triangle Park,
North Carolina 27709
(919) 316-6300
|
|
Instituto Grifols, S.A.
|
|
Spain
|
|
2834
|
|
N/A
|
|
Polígono Levante,
calle Can Guasch s/n 08150
Parets del Vallés, Barcelona, Spain
(34) 93 5710200
|
|
Diagnostic Grifols, S.A.
|
|
Spain
|
|
3826
|
|
N/A
|
|
Polígono Levante,
calle Can Guasch s/n, 08150
Parets del Vallés, Barcelona, Spain
(34) 93 5710400
|
|
Movaco, S.A.
|
|
Spain
|
|
3826
|
|
N/A
|
|
Polígono Levante,
calle Can Guasch s/n, 08150
Parets del Vallés, Barcelona, Spain
(34) 935710200
|
|
Laboratorios Grifols, S.A.
|
|
Spain
|
|
5122
|
|
N/A
|
|
Polígono Levante,
calle Can Guasch s/n, 08150
Parets del Vallés, Barcelona, Spain
(34) 93 5710100
|
|
Grifols Italia, S.p.A.
|
|
Italy
|
|
2834
|
|
N/A
|
|
Via Carducci, 62D, Loc. La Fontina
56010 San Giuliano Terme (PI)
Frazione Ghezzano, Italy
(39) 050 8798341
|
|
Grifols Deutschland GmbH
|
|
Germany
|
|
2834
|
|
N/A
|
|
Lyoner Strasse 15
60528 Frankfurt am Main,
Germany (49) 6103 75020
|
|
|
|
|
(1) |
|
Grifols Inc. is the Issuer of the new notes offered hereby. The
other listed registrants, including Grifols, S.A., are
Guarantors of the new notes. |
The information
in this prospectus is not complete and may be changed. We may
not sell these securities until the registration statement filed
with the Securities and Exchange Commission is effective. This
prospectus is not an offer to sell these securities and it is
not soliciting an offer to buy these securities in any state or
jurisdiction where the offer or sale is not permitted.
|
SUBJECT TO COMPLETION, DATED
OCTOBER 24, 2011
PROSPECTUS
Grifols Inc.
Offer to Exchange up
to
U.S.$1,100,000,000 principal
amount of 8.25% Senior Notes due 2018
For Any and All Outstanding
Unregistered
U.S.$1,100,000,000 principal
amount of 8.25% Senior Notes due 2018
MATERIAL
TERMS OF THE EXCHANGE OFFER
|
|
|
| |
•
|
The existing notes were originally issued by Giant Funding
Corp., an escrow company formed solely for the purpose of
issuing the existing notes, on January 21, 2011. Giant
Funding Corp. merged into Grifols Inc., our wholly owned
subsidiary, and Grifols Inc. assumed its obligations under the
existing notes and the related indenture on June 1, 2011.
The exchange notes will represent the same debt as the existing
notes and Grifols Inc. will issue the exchange notes under the
same indenture.
|
| |
| |
•
|
The terms of the exchange notes are substantially identical to
the existing notes, except that the transfer restrictions and
registration rights relating to the existing notes will not
apply to the exchange notes and the exchange notes will not
provide for the payment of special interest under circumstances
related to the timing and completion of the exchange offer.
|
| |
| |
•
|
We are making the exchange offer to satisfy your registration
rights, as a holder of existing notes.
|
| |
| |
•
|
The exchange offer expires at 5:00 p.m., New York City
time,
on ,
2011, unless extended.
|
| |
| |
•
|
Subject to the satisfaction or waiver of specified conditions,
we will exchange your validly tendered unregistered existing
notes that have not been withdrawn prior to the expiration of
the exchange offer for an equal principal amount of exchange
notes that have been registered under the Securities Act of
1933, as amended, or the Securities Act.
|
| |
| |
•
|
The exchange offer is not subject to any condition other than
that the exchange offer not violate applicable law or any
applicable interpretation of the staff of the Securities and
Exchange Commission, or the SEC, and other customary conditions.
|
| |
| |
•
|
You may withdraw your tender of notes at any time before the
exchange offer expires.
|
| |
| |
•
|
The exchange of notes should not be a taxable exchange for
U.S. federal income tax purposes.
|
| |
| |
•
|
We will not receive any proceeds from the exchange offer.
|
| |
| |
•
|
Any outstanding existing notes not validly tendered will remain
subject to existing transfer restrictions.
|
| |
| |
•
|
The exchange notes will not be traded on any national securities
exchange and, therefore, we do not anticipate that an active
public market in the exchange notes will develop.
|
Each broker-dealer that receives exchange notes for its own
account pursuant to the exchange offer must acknowledge that it
will deliver a prospectus in connection with any resale of such
exchange notes. A broker-dealer that is issued exchange notes
for its own account in exchange for existing notes that were
acquired by such broker-dealer as a result of market-making or
other trading activities may use this prospectus, as
supplemented or amended, for an offer to resell, resale or other
retransfer of the exchange notes issued to it in the exchange
offer.
Please refer to “Risk Factors” beginning on
page 34 of this prospectus for certain important
information.
Neither the Securities and Exchange Commission nor any state
securities commission has approved or disapproved of the notes
to be issued in the exchange offer or passed upon the adequacy
or accuracy of this prospectus. Any representation to the
contrary is a criminal offense.
The date of this
prospectus ,
2011
ABOUT
THIS PROSPECTUS
This prospectus is part of a registration statement that we have
filed with the SEC. This prospectus does not contain all of the
information included in the registration statement. The
registration statement filed with the SEC includes exhibits that
provide more details about the matters discussed in this
prospectus. You should carefully read this prospectus, the
related exhibits filed with the SEC and any prospectus
supplement, together with the additional information described
below under the heading “Where You Can Find More
Information.” We will provide without charge to each person
to whom a copy of this prospectus is delivered, upon written or
oral request of that person, a copy of any and all of this
information. Requests for copies should be directed to Investor
Relations, Grifols, S.A., Avinguda de la Generalitat,
152-158,
Parc de Negocis Can Sant Joan, 08174 Sant Cugat del Vallés,
Barcelona, Spain; (34) 935 710 500. You should request this
information at least five business days in advance of the date
on which you expect to make your decision with respect to the
exchange offer. In any event, in order to obtain timely
delivery, you must request this information prior
to ,
2011, which is five business days before the expiration date of
the exchange offer. Our website address is
http://www.grifols.com.
Our website is not a part of this prospectus.
You should rely only on the information contained in this
prospectus and in any accompanying prospectus supplement. We
have not authorized any other person to provide you with
different information. If anyone provides you with different or
inconsistent information, you should not rely on it. You should
assume that the information appearing in this prospectus and any
prospectus supplement is accurate only as of the date on the
front cover of those documents. We do not imply that there has
been no change in the information contained in this prospectus
or in our affairs since that date by delivering this prospectus.
As used in this prospectus, unless the context otherwise
requires or as is otherwise indicated:
|
|
|
| |
•
|
all references in this document to “Grifols,” the
“Company,” “we,” “us,” and
“our” refer to Grifols, S.A., a company (sociedad
anónima) organized under the laws of Spain, and our
consolidated subsidiaries; for periods after the acquisition
these terms include the acquired Talecris operations;
|
| |
| |
•
|
all references to “the Issuer” refer to Grifols Inc.,
a Virginia corporation and wholly owned subsidiary of Grifols,
S.A.;
|
| |
| |
•
|
all references to “Talecris” refer to Talecris
Biotherapeutics Holdings Corp., a Delaware corporation, and its
consolidated subsidiaries, as existing prior to the closing of
the acquisition; and
|
| |
| |
•
|
all references to the “acquisition” refer to our
acquisition of Talecris, consummated on June 1, 2011.
|
Each broker-dealer that receives exchange notes for its own
account pursuant to the exchange offer must acknowledge that it
will deliver a prospectus in connection with any resale of such
exchange notes. The letter of transmittal relating to the
exchange offer states that by so acknowledging and by delivering
a prospectus, a broker-dealer will not be deemed to admit that
it is an “underwriter” within the meaning of the
Securities Act of 1933, or the Securities Act. This prospectus,
as it may be amended or supplemented from time to time, may be
used by a broker-dealer in connection with resales of exchange
notes received in exchange for existing notes where such
existing notes were acquired by such broker-dealer as a result
of market-making activities or other trading activities. We have
agreed that, for a period of up to 180 days after the
consummation of the exchange offer, we will make this prospectus
available to any broker-dealer, at such broker-dealer’s
request, for use in connection with any such resale. See
“Plan of Distribution.”
NOTICE
REGARDING PRESENTATION OF FINANCIAL INFORMATION
The basis of presentation of financial information of Grifols in
this document is in accordance with International Financial
Reporting Standards (“IFRS”), as issued by the
International Accounting Standards Board (the “IASB”),
unless indicated otherwise. In this prospectus, unless otherwise
specified or the context otherwise requires, references to
“$” and “dollars” are to United States
(“U.S.”) Dollars. Talecris has been included in the
consolidated financial statements of Grifols from June 1,
2011, the date of the closing of the acquisition. Prior to
June 1, 2011, Talecris reported on their own financials
statements, which are included elsewhere in this prospectus.
ii
NOTICE TO
NEW HAMPSHIRE RESIDENTS
NEITHER THE FACT THAT A REGISTRATION STATEMENT OR AN
APPLICATION FOR A LICENSE HAS BEEN FILED UNDER
RSA 421-B
WITH THE STATE OF NEW HAMPSHIRE NOR THE FACT THAT A SECURITY IS
EFFECTIVELY REGISTERED OR A PERSON IS LICENSED IN THE STATE OF
NEW HAMPSHIRE CONSTITUTES A FINDING BY THE SECRETARY OF STATE OF
THE STATE OF NEW HAMPSHIRE THAT ANY DOCUMENT FILED UNDER
RSA 421-B
IS TRUE, COMPLETE AND NOT MISLEADING. NEITHER ANY SUCH FACT NOR
THE FACT THAT AN EXEMPTION OR EXCEPTION IS AVAILABLE FOR A
SECURITY OR A TRANSACTION MEANS THAT THE SECRETARY OF STATE OF
THE STATE OF NEW HAMPSHIRE HAS PASSED IN ANY WAY UPON THE MERITS
OR QUALIFICATIONS OF, OR RECOMMENDED OR GIVEN APPROVAL TO, ANY
PERSON, SECURITY OR TRANSACTION. IT IS UNLAWFUL TO MAKE, OR
CAUSE TO BE MADE, TO ANY PROSPECTIVE PURCHASER, CUSTOMER OR
CLIENT ANY REPRESENTATION INCONSISTENT WITH THE PROVISIONS OF
THIS PARAGRAPH.
INDUSTRY
AND MARKET DATA
We obtained the market and competitive position data used
throughout this prospectus from our own research, surveys or
studies conducted by third parties and industry or general
publications. Industry publications and surveys generally state
that they have obtained information from sources believed to be
reliable, but do not guarantee the accuracy and completeness of
such information. While we believe that each of these studies
and publications is reliable, we have not independently verified
such data, and we do not make any representation as to the
accuracy of such information. Similarly, we believe our internal
research is reliable, but it has not been verified by any
independent sources. All references to the North American market
throughout this prospectus refer only to the United States and
Canada.
TRADEMARKS
AND SERVICE MARKS
We own or have the rights to various trademarks and trade names
that we use in conjunction with the operation of our business
including, but not limited to, Alphanate, Flebogamma, Gamunex,
Grifols, Prolastin and Talecris. Q-Coagulometer is a registered
design mark of Grifols. We pursue registration of our important
service marks and trademarks and vigorously oppose any
infringement upon them. In this prospectus, we also refer to
product names, trademarks, trade names and service marks that
are the property of other companies. Each of the trademarks,
trade names or service marks of other companies appearing in
this prospectus belongs to its owner. The use or display of
other parties’ trademarks, trade names or service marks is
not intended to and does not imply a relationship with, or
endorsement or sponsorship by us of, the product, trademark,
trade name or service mark owner, unless we otherwise expressly
indicate.
ENFORCEABILITY
OF CIVIL LIABILITIES UNDER U.S. SECURITIES LAW
Grifols, S.A., the Parent Guarantor, is a company (sociedad
anónima) organized under the laws of Spain and most of
the Subsidiary Guarantors (as defined herein) are also
incorporated outside of the United States. A substantial portion
of the assets of the Parent Guarantor and the assets of the
Subsidiary Guarantors are located outside of the United States.
In addition, nearly all of the directors and officers of the
Parent Guarantor and certain of the Subsidiary Guarantors’
directors and officers are nationals or residents of countries
other than the United States, and all or a substantial portion
of such persons’ assets are located outside the
United States. As a result, it may be difficult for
investors to affect service of process within the United States
upon the Parent Guarantor or certain Subsidiary Guarantors or
their directors or officers with respect to matters
iii
arising under the Securities Act or to enforce against them
judgments of courts of the United States predicated upon civil
liability under the Securities Act. It may also be difficult to
recover fully in the United States on any judgment rendered
against such persons or the Parent Guarantor or certain of the
Subsidiary Guarantors.
We are advised by Spanish legal counsel, Osborne Clarke, S.L.P.,
(i) that there is doubt as to the enforceability in Spain
in original actions, or in actions for enforcement of judgments
of U.S. courts of liabilities predicated solely upon the
securities laws of the United States and (ii) that any
final and binding judgment obtained against us in the United
States would be recognized and enforced by the courts of Spain
in accordance with the Law of Civil Procedure (Ley de
Enjuiciamiento Civil) if the appropriate order
(exequatur) were obtainable, for which prior to the time
such judgment is introduced into a Spanish court for
enforcement, there should be no material contradiction or
incompatibility between the referred judgment with a judgment
rendered or judicial proceedings outstanding in Spain, and
(a) according to the provisions of any applicable treaty
(there is none currently in existence with the United States),
or (b) in the absence of any such treaty, if it could be
proven that the jurisdiction where the foreign judgment was
rendered recognizes Spanish judgments on a reciprocal basis and
the judgment satisfies other requirements such as not being
incompatible or infringing the Spanish public order or policy.
If such reciprocity cannot be proven, the requisite order
(exequatur) would not be granted by the Spanish courts.
CAUTIONARY
STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains a number of forward-looking statements,
including statements about our financial condition, results of
operations, and prospects. Forward-looking statements are
typically identified by words such as “may,”
“anticipate,” “believe,”
“estimate,” “predict,” “expect,”
“intend,” “forecast,” “will,”
“would,” “should” or the negative of such
terms or other variations on such terms or comparable or similar
words or expressions.
These forward-looking statements reflect, as applicable, our
management’s current beliefs, assumptions and expectations
and are subject to a number of factors that may cause actual
results to differ materially. These factors include but are not
limited to:
|
|
|
| |
•
|
risks related to the notes and the guarantees;
|
| |
| |
•
|
our significant indebtedness;
|
| |
| |
•
|
our ability to service our indebtedness, which in turn depends
on our ability to generate cash;
|
| |
| |
•
|
the subordinated nature of the notes and guarantees;
|
| |
| |
•
|
the restrictive covenants governing our debt;
|
| |
| |
•
|
Federal and state statutes permitting courts to void guarantees
under certain circumstances;
|
| |
| |
•
|
Bankruptcy law limitations on the amounts payable to note
holders;
|
| |
| |
•
|
limitations on the enforcement of civil liabilities under
U.S. securities laws;
|
| |
| |
•
|
extensive environmental, health and safety laws and regulations;
|
| |
| |
•
|
implementation of health care reform law in the U.S.;
|
| |
| |
•
|
potential decreases or limitations on reimbursement for
purchasers of our products;
|
| |
| |
•
|
our ability to maintain compliance with government regulations
and licenses, including those related to plasma collection,
production, and marketing;
|
| |
| |
•
|
regulatory actions or lawsuits brought under federal or state
laws;
|
| |
| |
•
|
risks related to compliance with the Sarbanes-Oxley Act of 2002
and our internal controls over financial reporting;
|
| |
| |
•
|
product concentration risk;
|
iv
|
|
|
| |
•
|
the risks associated with the potential damage or contamination
of plasma, our main raw material;
|
| |
| |
•
|
side effects associated with our products;
|
| |
| |
•
|
our adherence to cGMP;
|
| |
| |
•
|
our ability to procure adequate quantities of plasma and other
materials which are acceptable for use in our manufacturing
processes;
|
| |
| |
•
|
increased competition in our industry;
|
| |
| |
•
|
the impact of competitive products and pricing and actions of
competitors;
|
| |
| |
•
|
fluctuations in the balance between supply and demand with
respect to the market for plasma-derived products;
|
| |
| |
•
|
potential product liability claims or product recalls involving
our products;
|
| |
| |
•
|
the impact of our substantial capital expenditures;
|
| |
| |
•
|
market risks, such as interest rate risk and foreign exchange
rate risk;
|
| |
| |
•
|
the unprecedented volatility in the global economy and
fluctuations in the financial markets;
|
| |
| |
•
|
unexpected shut-downs of our manufacturing and storage
facilities or delays in opening new facilities;
|
| |
| |
•
|
disruptions in our distribution channels;
|
| |
| |
•
|
our ability to identify growth opportunities for existing
products and to identify and develop new product candidates
through our research and development activities;
|
| |
| |
•
|
our ability to protect our intellectual property rights and
defend against allegations of infringement by others;
|
| |
| |
•
|
our ability to resume or replace sales to countries affected by
the ongoing Foreign Corrupt Practices Act (“FCPA”)
investigation;
|
| |
| |
•
|
potential sanctions, if any, that the Department of Justice
(“DOJ”) or other federal agencies, may impose on us as
a result of the ongoing FCPA investigation;
|
| |
| |
•
|
the impact of the pending investigation into compliance with the
Pharmaceutical Pricing Agreement (“PPA”) under the
Public Health Service Program; and
|
| |
| |
•
|
other factors that are set forth below under the section
entitled “Risk Factors.”
|
Because these forward-looking statements are subject to
assumptions and uncertainties, actual results may differ
materially from those expressed or implied by these
forward-looking statements. You are cautioned not to place undue
reliance on these statements, which speak only as of the date of
this prospectus. Forward-looking statements are not guarantees
of future performance. They have not been reviewed by our
auditors.
All written and oral forward-looking statements attributable to
us or any person acting on our behalf are expressly qualified in
their entirety by the cautionary statements contained or
referred to in this prospectus. Except as required by law, we do
not assume any obligation to update any forward-looking
statements after the date of this prospectus as a result of new
information or future events or developments.
v
SUMMARY
This summary highlights selected information appearing
elsewhere in this prospectus. As a result, it is not complete
and does not contain all of the information that you should
consider before exchanging your notes. You should read the
following summary in conjunction with the more detailed
information included herein.
Our
Company
We are a leading global specialty biopharmaceutical company that
develops, manufactures and distributes a broad range of plasma
derivative products. These protein-based therapies extend and
enhance the lives of individuals who suffer from chronic and
acute, often life-threatening, conditions, such as primary and
secondary immunological deficiencies, chronic inflammatory
demyelinating polyneuropathy (“CIDP”), alpha-1
antitrypsin deficiency and related emphysema, immune-mediated
idiopathic thrombocytopenic purpura (“ITP”), Guillain
Barré syndrome, Kawasaki disease, allogeneic bone marrow
transplants, CPI, hemophilia A and B, von Willebrand disease,
traumatic or hemorrhagic shock and severe burns. We also
specialize in providing infusion solutions, nutrition products,
medical devices, diagnostic instrumentation and reagents for use
in hospitals and clinics.
On June 1, 2011, we acquired 100% of the share capital of
Talecris Biotherapeutics Holdings Corp., a Delaware corporation,
making us one of the world’s largest producers of plasma
derivative products. For a discussion of our acquisition of
Talecris, see the section below entitled
“Business — The Acquisition and Related Financing
Events.”
Our products are used by healthcare providers in more than 100
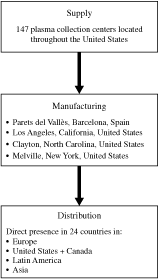
countries. As a result of the acquisition, we operate 147 plasma
collection centers, 145 of which are licensed by the United
States Food and Drug Administration (the “FDA”) and
two are awaiting FDA approval. Our plasma derivative products
are manufactured at our plasma fractionation plants near
Barcelona, Spain and in Los Angeles, California, Melville, New
York and Clayton, North Carolina. We have product licenses from
the FDA for the sale in the United States of intravenous
immunoglobulin (“IVIG”), alpha-1 proteinase inhibitor
(“A1PI”), albumin, anti-hemophilic blood clotting
factor (“Factor VIII”), blood clotting factor ix
(“Factor IX”), an antithrombin in plasma which
inactivates thrombin (“Antithrombin III”) and
prothrombin complex concentrates (“PTC”), as well as
licenses for the sale of these and other products in Canada,
Europe, Latin America and Asia.
For the fiscal year ended December 31, 2010 and the six
months ended June 30, 2011, we reported revenues of
€990.7 million and €635.3 million,
respectively. For the six months ended June 30, 2011, our
pro forma revenues, giving effect to the acquisition of Talecris
as of January 1, 2011, would have been
€1,139.4 million.
Our Class A and Class B (non-voting) shares are listed
on the Madrid, Barcelona, Bilbao and Valencia Stock Exchanges
(the “Spanish Stock Exchanges”) and quoted on the
Automated Quotation System of the Spanish Stock Exchanges under
the symbol “GRF” and “GRF.P,” respectively.
Our American Depository Shares (“ADSs”), evidenced by
American Depositary Receipts (“ADRs”), representing
our Class B shares are listed in the United States on
the NASDAQ Global Select Market (“NASDAQ”) under the
symbol “GRFS.”
Plasma
Industry Overview
Our key products are derived from human plasma. Plasma, a liquid
that accounts for approximately 50% of blood, is obtained after
blood is separated via centrifugation into plasma, red blood
cells, white blood cells and platelets. Collected plasma is
fractionated to isolate component proteins, which are then
purified. Proteins are the key component of plasma, accounting
for 7% of plasma’s composition. The proteins include
albumin, globulins, coagulation factors and other proteins. The
four largest selling plasma proteins, which together constituted
approximately 74% of plasma derived product sales in the world
in 2008 and 78% in the United States in 2010, are IVIG, Factor
VIII, albumin and A1PI. There are hundreds of proteins present
in plasma; however, only a handful of these proteins have been
utilized for therapeutic applications to date.
According to the Marketing Research Bureau (“MRB”),
the human plasma-derived products industry has demonstrated
revenue growth at a compound annual rate of approximately 6.8%
globally from 1994 through
1
2008, with worldwide sales of approximately $11.8 billion
in 2008. Sales in the United States have grown at a compound
annual rate of approximately 9% from 1993 through 2010, with
sales of $4.8 billion in 2010, representing a 3.2% increase
over 2009, according to the MRB. Although the industry has
experienced consistent worldwide growth in demand, a more
balanced supply and demand dynamic has moderated price
increases. Demand for plasma derivatives has grown substantially
through active management of disease, the discovery of new
therapeutic applications, the development of new products and
the increase in prophylactic use. The two main regions in the
world for the sales of plasma derivatives in 2008 were North
America (defined by the MRB as the United States and Canada) and
Europe, which together represent 73% of global sales of
plasma-derived therapies.
The policy of the World Health Organization and many European
jurisdictions is based on a recommendation that blood and its
derivatives be obtained from voluntary, altruistic donors.
Payment to donors is prohibited in most European countries;
however the United States permits payment to donors. Because of
this limitation, most European countries are unable to meet
their supply requirements and rely on the United States paid
donations to fill the supply gap. The United States supplies
approximately 60% of the world’s plasma. Effectively, the
United States only permits the sale of plasma derivative
products that have been manufactured with plasma collected in
the United States. In addition, plasma collected in the United
States can be used in plasma derivative products sold in most
world markets, whereas plasma collected in Europe is generally
used only in the country where it is obtained.
Operating
Divisions
We organize our business into four divisions: Bioscience,
Hospital, Diagnostic and Raw Materials. Subsequent to the
acquisition, Talecris’ operations have been incorporated
into our existing Bioscience division.
Bioscience. The Bioscience division includes
activities relating to the manufacture of plasma derivatives for
therapeutic use, including the reception, analysis, quarantine,
classification, fractionation and purification of plasma, and
the sale and distribution of end products. The main types of
plasma products manufactured by us are IVIG, Factor VIII, A1PI
and albumin. We also manufacture hyperimmune immunoglobulins,
Antithrombin III, Factor IX and PTC. The Bioscience division,
which accounts for a majority of our total net sales, accounted
for €773.4 million, or 78.1%, and
€521.5 million, or 82.1%, and of our total net sales
for the year ended December 31, 2010 and the six months
ended June 30, 2011, respectively.
Hospital. The Hospital division manufactures
and, in certain instances installs, products that are used by
and in hospitals, such as parenteral solutions and enteral and
parenteral nutritional fluids, which are sold almost exclusively
in Spain and Portugal, and which accounted for
€89.6 million, or 9.0%, and €49.3 million,
or 7.8%, of our total net sales for the year ended
December 31, 2010 and the six months ended June 30,
2011 respectively. We believe we are the leading provider of
intravenous therapy in Spain, with a 34% market share.
Diagnostic. The Diagnostic division focuses on
researching, developing, manufacturing and marketing in vitro
diagnostics products including analytical instruments and
reagents for diagnostics, as well as blood bank products. We
concentrate our Diagnostic business in three areas:
immunohematology, hemostasis and immunology. The Diagnostic
division’s main customers are blood donation centers,
clinical analysis laboratories and hospital immunohematology
services. The division also manufactures and distributes blood
collection bags and other disposables. The Diagnostic division
accounted for €109.1 million, or 11.0% and
€56.8 million, or 8.9%, of our total net sales for the
year ended December 31, 2010 and the six months ended
June 30, 2011, respectively.
Raw Materials. The Raw Materials division
includes the sale of intermediate pastes and plasma to third
parties, which accounted for €4.8 million, or 0.5%,
and €1.7 million, or 0.3%, of our total net sales for
the year ended December 31, 2010 and the six months ended
June 30, 2011, respectively. Sales of the Raw Materials
division are used to optimize inventory levels with the aim of
striking a better balance between plasma collections and
fractionation needs.
2
Competitive
Strengths
We believe we have a number of competitive strengths, including:
Global
Player with an Established Presence in the Two Largest Plasma
Derivatives Markets
We are the third largest producer of plasma products with
operations in more than 100 countries with a direct presence in
24 countries. We have an established presence in North America
and Europe, which are the two largest plasma derivatives sales
regions in the world, accounting for approximately
$8.6 billion or 73% of the $11.8 billion in total
worldwide sales in 2008. North America and the European Union
accounted for 81.8% of our total revenues in the Bioscience
division in the six months ended June 30, 2011. Our
acquisition of Talecris has allowed us to further expand our
presence in the United States. We also have a presence in fast
growing sales regions including Asia (Malaysia, China and
Thailand), Japan, Canada, Australia and Latin America (Mexico,
Colombia, Argentina, Chile and Brazil). In addition, we operate
nine manufacturing facilities located in the United States,
Spain, Mexico, Switzerland and Australia.
Vertically
Integrated Business Model with a Secure Supply of United States
Source Plasma
We are a vertically integrated global producer of plasma
derivatives. Our activities include sourcing raw material,
manufacturing various plasma derivative products and sales and
distribution of the final products to healthcare providers.
We have expanded our plasma collection network through a
combination of organic growth and acquisitions and the opening
of new plasma collection centers. Our acquisitions of SeraCare
(now renamed Biomat USA) in 2002, PlasmaCare, Inc. in 2006,
eight plasma collection centers from a subsidiary of Baxter
Healthcare Corporation in 2006, four plasma collection centers
from Bio-Medics, Inc. in 2007, and one plasma collection center
from Amerihealth Plasma LLC in 2008 have given us reliable
access to United States source plasma. In 2010, we collected
2.6 million liters of plasma from our plasma centers. Our
acquisition of Talecris in June 2011 expanded our network by an
additional 67 centers. Following the acquisition, we have 147
plasma collection centers in the United States, which in the six
months ended June 30, 2011, collected 2.8 million
liters of plasma combined.
By decreasing our reliance on third parties for plasma and
having a secure supply of United States source plasma, a
critical operational requirement in our business, we are able to
better ensure the availability of plasma for our manufacturing
needs, assure the quality of the plasma throughout the
manufacturing process, better control plasma costs and improve
our margins.
State-of-the-Art,
FDA-Approved Manufacturing Facilities in Spain and the United
States
We have
state-of-the-art
plasma derivatives manufacturing facilities that are highly
efficient and safe. All of our fractionation facilities, other
than the Melville facility, have European Medicine Agency
(“EMEA”) certification. Our plasma fractionation plant
located in Parets del Vallès, near Barcelona, Spain, is
licensed by the FDA for the production of albumin and IVIG. The
Parets del Vallès facility has a unique design that
separates the maintenance area from the clean areas required for
the fractionation and purification procedures. This design,
which we developed in-house, minimizes the risk of contamination
and reduces maintenance costs.
In July 2003, we acquired Alpha Therapeutic Corporation’s
plasma fractionation business, which included a plasma
fractionation plant in Los Angeles, California, certain
inventories, patents, FDA approved licenses for plasma
derivative products in the United States and product licenses
and marketing and distribution structures in Europe and Asia.
This acquisition helped us increase our fractionation capacity
and expand our presence in the United States market. We have
made significant capital investments in the plant, including the
construction of purification and aseptic filling areas for
coagulation factors and albumin, which were completed in 2006
and 2009, and an increase of the fractionation capacity by
0.7 million liters to 2.2 million liters, which was
approved by the FDA during 2009.
3
As a result of the acquisition of Talecris we obtained an
additional fractionation facility in Clayton, North Carolina.
The Clayton facility is one the world’s largest integrated
protein manufacturing sites, including fractionation,
purification and aseptic filling and finishing of plasma-derived
proteins with a fractionation capacity of approximately
2.6 million liters of plasma per year. Over the last
15 years, the facility in Clayton has benefited from
roughly $699 million of capital investment, including
compliance enhancements, general site infrastructure upgrades,
capacity expansions and new facilities, such as its
chromatographic purification facilities and its high-capacity
sterile filling facility.
In connection with the acquisition and pursuant to the Consent
Agreement (as defined below), Talecris’ Melville, New York
facility was sold to Kedrion S.p.A. We now lease and operate the
Melville facility to produce intermediate pastes which are
further purified into final products at the Clayton facility.
See “Business — The Acquisition and Related
Financing Events.” The Melville facility has a
fractionation capacity of approximately 1.6 million liters
of plasma per year, which, when combined with the added capacity
from the Clayton facility, has increased our fractionation
capacity by approximately 98%.
Our current production processes for IVIG and Factor VIII have
been approved by the FDA as has the use of the intermediate
pastes created as raw material at the Barcelona and Los Angeles
plants, giving us increased production efficiency and
flexibility. On July 21, 2011, we obtained FDA approval for
the utilization of the intermediate product Fraction II+III made
at our Los Angeles’ plant to be further purified at the
Clayton facility to obtain Gamunex, a product we acquired in the
acquisition of Talecris. We are pursuing additional
opportunities to use intermediates produced at our Grifols
plants in the previously Talecris-owned facilities as well as
intermediates from the previously Talecris-owned facilities in
our Grifols facilities.
Strong
Reputation for Safety and Reliable Service
We have never experienced a recall of any of our finished
biological products, although certain of our other products have
been subject to non-material recalls. Prior to the acquisition,
Talecris had four recalls of its finished biological products.
Our philosophy is that the health of the plasma donor and the
patient recipients of our drugs is the paramount consideration.
We believe that our safety philosophy is consistent with our
business objective of generating profit. We also believe that we
have a strong reputation for safety, making our products
particularly attractive to customers. Our vertically integrated
business model allows us to assure the safety and quality of our
plasma derivative products through the implementation of our own
safety standards throughout each stage of production.
We adopted and maintain rigorous safety standards that exceed
those required by health authorities in Europe and the United
States and actively invest in the continued improvement of our
manufacturing facilities and plasma fractionation process. In
2006, we developed a new sterile filling and purification area
for our Los Angeles, California plant, and developed a
nanofiltration area for our Parets del Vallès plant.
Additionally, we developed the nanofiltration method of viral
elimination of our IVIG and Antithrombin III products.
We require our management to adhere to a formal code of ethical
conduct. By signing the formal code of ethical conduct, our
managers commit to making our products the safest and most
effective on the market. The code imposes the obligation on each
manager to report any ethical concerns directly to our Board of
Directors. Our high safety standards and reliability have helped
us establish and maintain successful long-term relationships
with key customers and physicians worldwide. We believe the
strength of our reputation positions us favorably as we continue
to expand our business.
We maintain standards consistent with other industry
participants with regard to infectious disease screening and
quarantine of units. For example, source plasma inventory is
held for not less than 60 days. Some of our additional
safety policies include look-back procedures for seroconversion
and ongoing testing of donations for a
12-month
period after a negative donation. We have also introduced
innovative methods such as the PediGri system. This system
allows the physician to track the origin of the fractionated
product prescribed to patients back to the source donor,
providing full traceability of plasmatic raw material throughout
the plasma supply-chain.
4
High-Quality,
Industry-Leading Plasma Derivative and Diagnostic
Products
Our plasma derivative product portfolio includes a broad range
of reliable, high-quality products that improve patient care.
Our Factor VIII/von Willebrand Factor product is used both for
the treatment of hemophilia and von Willebrand disease. We
believe that the von Willebrand market segment will grow at a
higher rate than the overall Factor VIII market. In addition, we
offer our albumin product with a reduced aluminum content,
meeting European requirements and making our albumin product
more attractive to biotechnology companies and genetic labs, as
well as hospitals and physicians.
Our acquisition of Talecris expanded our portfolio of IVIG and
A1PI products. Gamunex IVIG, which was launched in North America
in 2003 as a premium
ready-to-use
liquid IVIG product, is one of the leading products in the IVIG
segment with a 24% share of United States sales in 2010
according to MRB. We believe Gamunex IVIG is considered to be
the industry benchmark due to a comprehensive set of
differentiated product characteristics that have positioned it
as the premium product in its category since its launch. We
manufacture the only IVIG products approved for CIDP in the
United States and Canada and, through mutual recognition
procedures, in 16 European countries. The CIDP indication
approval makes certain of the IVIG products that we manufacture
the only IVIG products approved for use in a neurological
indication in North America. According to an independent survey
by Harris Interactive, CIDP is the largest IVIG segment in the
United States, representing 29% of total unit volume. This same
survey estimated that the FDA’s approval of IVIG for CIDP
may double Gamunex’s licensed market access to 61% of total
United States IVIG unit volume. Further, the FDA granted Gamunex
IVIG orphan drug status, which provides marketing exclusivity
for the CIDP indication in the United States through September
2015. In addition, following the acquisition of Talecris we are
the world’s largest producer of A1PI, which is used for the
treatment of A1PI deficiency-related emphysema. In 2010,
Prolastin A1PI had a 64% share of sales in the United States. In
2008, it had an 87% share of sales in Europe, where it is
licensed in 15 countries, and it only competes with another
licensed A1PI product in France. Prolastin-C is also the only
licensed A1PI product in Canada.
We have focused our product offerings in the diagnostic market
in three distinct submarkets, immunohematology, hemostasis and
immunology. Our Diagnostic division has developed
state-of-the-art
automated analyzers for each of these submarkets. In the
immunohematology field, our Wadiana and Erytra analyzers are
fully automated instruments with high processing capacities for
pre-transfusion compatibility tests using the gel agglutination
technique. In the hemostasis field, our Q Coagulometer is an
automated instrument used for coagulation tests. In the
immunology field, our Triturus analyzers completely automate
enzyme immunoassays for hospital laboratories.
Over
70-Year
History of Successful Innovation
We have a strong track record as an innovator in the industry,
focusing on three areas: (i) discovering and developing new
products, (ii) researching new applications for existing
products and (iii) improving our manufacturing processes to
increase yields, safety and efficiency. For example, we
developed a unique fractionation design that reduces the risk of
contamination, reduces maintenance costs and increases the
amount of product extracted per liter of plasma. We have also
developed the first centrifugation unit for the automated
cleaning of blood cells, known as the Coombs test. In addition,
we were one of the first fractionators to conduct double viral
inactivation processes for Factor VIII and have designed and
implemented a new process for the sterile filling of vials that
reduces exposure to potential contaminants as compared to other
existing processes. We have recently developed a nanofiltration
method of viral inactivation for our IVIG and
Antithrombin III products. We believe that adoption of
novel policies and methodologies have raised industry standards
and made us a leader in safety and product quality.
Talecris was the developer of the first
ready-to-use
10% liquid IVIG product in North America and the first A1PI
product in the world. Talecris applied new developments in
protein purification, including caprylate and chromatography
technologies, to produce and sell a third-generation IVIG
product. Talecris’ next generation A1PI product,
Prolastin-C A1PI, was approved by the FDA and Health Canada. In
March 2010, Talecris
5
launched Prolastin-C A1PI in the United States and in the third
quarter of 2010 in Canada. Talecris completed the conversion of
its existing U.S. and Canadian Prolastin A1PI patients to
Prolastin-C A1PI during 2010. Presently, additional clinical
trials are being required by European authorities as a precursor
to Prolastin-C A1PI approval in Europe. In October 2010, the FDA
approved Gamunex-C for the subcutaneous route of administration
for the treatment of primary immunodeficiency. Additionally,
Talecris received approval for and we are proceeding with a
proof of concept trial for plasma-derived Plasmin to treat
ischemic stroke in six countries outside of the United States.
Experienced
and Committed Management Team
We have an experienced and committed management team. The
President and Chief Executive Officer, Victor Grifols Roura, is
the grandson of our founder and has held his current office for
over 26 years. The President of our Global Industrial
Division, Juan Ignacio Twose Roura, has been associated with
Grifols and our predecessor for more than 37 years. The
President of our Global Commercial Division, Ramón Riera,
has been associated with Grifols and our predecessor for more
than 33 years. Our Chief Financial Officer, Alfredo Arroyo,
has been associated with Grifols for five years. The President
of U.S. Operations and Chief Executive Officer of Grifols
Inc., Gregory Gene Rich, has been in the industry for nearly
31 years.
Our
Business Strategy
Our objective is to consolidate and expand our leadership
position in the plasma derivative product industry by employing
the following strategies:
Expand
Our Presence Internationally, Including in Emerging
Markets
Geographical diversification is a cornerstone of our strategy.
We currently operate in more than 100 countries with a direct
presence in 24 countries.
Certain sales regions are expected to experience significant
growth driven by enhanced socioeconomic conditions and more
informed patients who are demanding better quality medical care,
as well as increasing government healthcare spending on plasma
derivative products in some of these markets. In Latin America
and the Asia-Pacific region, we are expanding our presence by
establishing and strengthening relationships with distributors
as well as obtaining additional product licenses. Our presence
and experience in Latin America, in countries such as Mexico,
Colombia, Argentina, Chile and Brazil, where we have been
marketing and selling products for over 20 years, has
positioned us to benefit from this potential growth in both the
Bioscience and Diagnostic divisions. In addition, our
acquisition of product licenses and marketing and distribution
structures in Asia has helped accelerate the development of our
presence in that region. In the Asia-Pacific region, we have
established a presence through our subsidiaries and
representative offices in Malaysia, China, Thailand, Singapore
and Japan. Additionally, we have a license to market the latest
generation IVIG in Australia, which gives us the opportunity to
reach a country that has one of the highest levels of IVIG
consumption per capita.
Additionally, in March 2009, in a €25 million
transaction, we acquired 49% of the profit-sharing rights and
99% of the voting rights in Woolloomooloo Holdings Pty Ltd., the
holding company of an Australian-Swiss group that distributes
diagnostic products in Australia and Switzerland and has a
manufacturing facility in Switzerland, demonstrating our
continued focus on international expansion and acquisitions that
generate synergies. In August 2011, we acquired the remaining
outstanding capital stock and now hold 100% of the stock and
voting rights of the company.
Continue
Investment in Research and Development, Innovation and New
Facilities
Research and development is a significant aspect of our
business. We have recently increased investments in research and
development, in particular to develop the possible use of
albumin in treating Alzheimer’s disease, as well as other
projects relating to future biotechnological developments.
Recent product developments include Niuliva, an anti-hepatitis B
intravenous immunoglobin, launched in Italy, Spain and Latin
America at the start of 2010, and Flebogamma DIF, the latest
generation intravenous immunoglobulin, with licenses for
marketing in
6
the United States and Europe, with additional licenses expected
in Latin America and Asia. We spent more than
€36.6 million in 2010 on research and development, a
3.7% increase in comparison to 2009. As of June 30, 2011,
we had approximately 706 scientists and support staff dedicated
to research and development.
We are currently implementing a €690 million
investment plan over 2011 to 2015 of which we have approximately
€300 million still outstanding under the plan. As part
of the plan, we are constructing new fractionation facilities in
Clayton, North Carolina and Parets del Vallès, Barcelona.
Construction of and the receipt of the necessary approvals for
the new Clayton facility is expected to be completed in 2015. We
expect this new facility to increase our fractionation capacity
by six million liters. Construction of and the receipt of the
necessary approvals for the new Parets del Vallès facility
is expected to be completed in 2014 and will increase our
fractionation capacity by an additional one million liters. We
also began construction on a new plant in Murcia, Spain in 2009
that will enable us to increase the production capacity of
non-pvc parenteral solutions by 15 million units per year.
The facility is expected to be operational in 2012.
We are also focused on expanding our existing facilities. In
2010, we completed the construction of a new IVIG plant in Los
Angeles, United States, which we believe will be operational
within the next year. In 2009, we completed construction of a
new raw materials warehouse and new research and development and
control laboratories at our industrial complex in Parets del
Vallès, Barcelona and new corporate offices in Sant Cugat,
Barcelona. In 2010, we completed construction on a new
production plant in Barcelona for “fibrin glue”, a new
product combining human plasma proteins, fibrinogen and thrombin
which, when combined, act as a biological glue. Also in 2010, we
completed construction on a new plasma analysis laboratory in
the United States in San Marcos, Texas. This new laboratory
near our existing facilities will enable us to cope with the
increasing volumes of plasma that we must analyze.
Expand
Our Product Offerings
Our research and development team, whose work is primarily
concentrated on the Bioscience division, will continue to seek
to develop new plasma derivative products as well as new
applications for our existing plasma derivative products. We
also seek to leverage our plasma derivative product portfolio by
offering diagnostic and hospital products developed by our
research and development team or by premier healthcare companies
with which we maintain distribution agreements. We believe that
by increasing the number of products we offer, we can generate
higher revenues, diversify our product base and facilitate our
entry into new markets. In addition, we also believe that a
one-stop shopping approach offering a broader range of
complementary, high-quality products is particularly attractive
to our existing and potential customers.
We are pursuing new plasma products, including two versions of
Plasmin. We recently completed a Phase I clinical trial for
the use of one version of Plasmin for the treatment of
peripheral arterial occlusive disease (“aPAO”). In
addition we are applying a commercial process to another version
of Plasmin in order to produce a recombinant form of Plasmin to
treat ischemic stroke. Additionally, we are evaluating whether
to continue Talecris’ research into recombinant versions of
Factor VIII and A1PI through the use of human cell lines. If
successful, the development of these therapies could
significantly improve our revenue and profitability.
The integration of Talecris gave us (i) a broad range of
key products addressing a variety of therapeutic areas such as
neurology, immunology, pulmonology and hematology, among others
and (ii) enhanced our research and development pipeline
with complementary products and new recombinant projects that we
are evaluating, all of which we expect will further expand our
broad product offerings.
The
Acquisition and Related Financing Events
On June 1, 2011, pursuant to the Agreement and Plan of
Merger, dated as of June 6, 2010 (the “Merger
Agreement”), by and among Talecris, Merger Sub, Grifols,
S.A. and Grifols Inc., as amended by Amendment No. 1, dated
November 4, 2010, we completed our acquisition of Talecris,
for a total of $3.7 billion. The acquisition was effected
through (i) the merger of Talecris with and into Stream
Merger Sub, Inc., a Virginia corporation and wholly owned
subsidiary of Talecris (“Merger Sub”), and
(ii) the immediately subsequent merger of Grifols Inc., a
Delaware corporation and wholly owned subsidiary of Grifols,
S.A., with and into
7
Merger Sub, with Merger Sub continuing as the surviving
corporation and a wholly owned subsidiary of Grifols, S.A.
Merger Sub was subsequently renamed Grifols Inc. (the
“Issuer”).
In connection with the consummation of the acquisition, each
share of Talecris common stock, par value $0.01 per share, was
converted into the right to receive $19 in cash and 0.6485 (or
0.641 for Talecris directors and Talecris Holdings, LLC) of
a non-voting (Class B) share of Grifols, S.A. and cash
in lieu of fractional Class B shares and any cash
representing dividends or other distributions payable in
accordance with the Merger Agreement. ADSs representing our
Class B shares are listed on the NASDAQ Global Select
Market under the symbol “GRFS.”
On April 29, 2011, Grifols, S.A. and Talecris entered into
an Agreement Containing Consent Orders (the “Consent
Agreement”) with the staff of the Bureau of Competition of
the U.S. Federal Trade Commission (“FTC”) which
provided for certain conditions to the closing of the
acquisition. The FTC accepted the Consent Agreement for public
comment on May 31, 2011. The Consent Agreement required us
to divest certain assets to Kedrion S.p.A.
(“Kedrion”). As required by the Consent Agreement we
satisfied all necessary conditions within ten days of the
completion of the acquisition. Our next compliance report to the
FTC is due in 2012.
In order to finance the cash portion of the acquisition
consideration and repay existing indebtedness, on
November 23, 2010, we entered into a credit and guaranty
agreement, which provided for senior term loans aggregating
$2.5 billion and €440 million (the “Senior
Term Loans”) and revolving credit facilities in the amounts
of $50 million, €36.7 million and the
$200 million equivalent in multicurrencies (the
“Revolving Credit Facilities,” and together with the
Senior Term Loans, the “Senior Credit Facilities”). On
June 1, 2011, upon completion of the acquisition, we closed
the Senior Credit Facilities and the lenders funded their
commitments thereunder. As of June 30, 2011, no amounts
have been drawn on the Revolving Credit Facilities. For a
summary of the material terms of the Senior Credit Facilities,
see the section of this prospectus entitled
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations —
Indebtedness — Senior Credit Facilities.”
In addition, on January 21, 2011, Giant Funding Corp. (the
“Escrow Corp.”), an escrow company formed solely for
the purpose of issuing the existing notes to partially fund the
acquisition and repay existing indebtedness, completed a private
offering of the existing notes. The proceeds of the offering of
the existing notes were held in an escrow account pending
completion of the acquisition and the satisfaction of other
conditions. On June 1, 2011, upon completion of the
acquisition, Escrow Corp. was merged with and into the Issuer,
and the Issuer assumed all of Escrow Corp.’s obligations
under the existing notes and the indenture.
In addition, upon consummation of the acquisition, the Issuer,
the Parent Guarantor (as defined below) and the initial
Subsidiary Guarantors (as defined below) executed joinders to a
registration rights agreement that had been entered into by
Escrow Corp. and the initial purchasers of the existing notes on
January 21, 2011. Pursuant to the registration rights
agreement, the Issuer, the Parent Guarantor and the initial
Subsidiary Guarantors agreed, for the benefit of the holders of
the existing notes, at our cost, to file the registration
statement of which this prospectus forms a part and to complete
an exchange offer for the existing notes. For a summary of the
material terms of the notes, see the section of this prospectus
entitled “Management’s Discussion and Analysis of
Financial Condition and Results of Operations —
Indebtedness — 8.25% Senior Notes due 2018.”
8
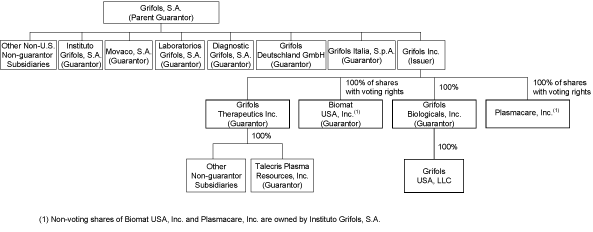
Corporate
Structure
The diagram below depicts, in simplified form, our corporate
structure. The diagram below does not reflect all subsidiaries
of Grifols, S.A. All company ownership is 100% unless otherwise
indicated.
Risk
Factors
Prospective investors should carefully consider the information
set out under “Risk Factors,” including, without
limitation, risks related to our business and risks related to
the healthcare industry, and other information set out in the
prospectus.
Our
Corporate Information
We were incorporated in Spain in 1987 under the name Grupo
Grifols, S.A. and changed our name to Grifols, S.A. in 2005. Our
principal executive offices are located at Avinguda de la
Generalitat,
152-158,
Parc de Negocis Can Sant Joan, Sant Cugat del Vallès,
08174, Barcelona, Spain. Our telephone number is
(34) 935-710-500.
Our internet address is www.grifols.com. We are not
including the information contained on or available through our
website as a part of, or incorporating such information by
reference into, this prospectus.
9
SELECTED
HISTORICAL CONSOLIDATED FINANCIAL DATA
The following is a summary of our historical consolidated
financial data for the periods ended and at the dates indicated
below. You are encouraged to read this information together with
the consolidated financial statements and the related footnotes
and the section entitled “Management’s Discussion and
Analysis of Financial Condition and Results of Operations”
included elsewhere in this prospectus.
The following table presents our consolidated financial data for
the periods and as of the dates indicated. Our consolidated
balance sheet data as of December 31, 2010 and 2009 and our
consolidated statement of operations data for the years ended
December 31, 2010, 2009 and 2008 is derived from our
audited consolidated financial statements for those years, which
are included elsewhere in this prospectus. Our consolidated
balance sheet data as of December 31, 2008, 2007 and 2006
and our consolidated statement of operations data for the years
ended December 31, 2007 and 2006 is derived from our
unaudited consolidated financial statements for those years,
which are not included in this prospectus.
The unaudited condensed consolidated interim financial data for
the six months ended June 30, 2011 and 2010 and as of
June 30, 2011 have been derived from our unaudited
condensed consolidated interim financial statements as of and
for the six months ended June 30, 2011, which are included
elsewhere in this prospectus. In our opinion, the unaudited
condensed consolidated interim financial statements have been
prepared on the same basis as our annual audited consolidated
financial statements and contain all material adjustments
(consisting of normal recurring accruals and adjustments)
necessary for a fair presentation of our financial position and
results of operations. Operating results for the six months
ended June 30, 2011 are not necessarily indicative of
results that may be expected for the year ending
December 31, 2011.
Our consolidated financial statements are presented in
accordance with International Financial Reporting Standards
(“IFRS”) as issued by the International Accounting
Standards Board (the “IASB”). For additional
information, see our consolidated financial statements and the
accompanying notes included elsewhere in this prospectus.
Consolidated
Balance Sheet Data:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
As of June 30,
|
|
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2011
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
ASSETS
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
150,820
|
|
|
|
150,243
|
|
|
|
158,567
|
|
|
|
174,000
|
|
|
|
189,448
|
|
|
|
2,281,696
|
|
|
Other intangible assets
|
|
|
60,850
|
|
|
|
57,223
|
|
|
|
57,756
|
|
|
|
69,385
|
|
|
|
78,299
|
|
|
|
128,474
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
211,670
|
|
|
|
207,466
|
|
|
|
216,323
|
|
|
|
243,385
|
|
|
|
267,747
|
|
|
|
2,410,170
|
|
|
Property, plant and equipment
|
|
|
184,993
|
|
|
|
201,332
|
|
|
|
301,009
|
|
|
|
371,705
|
|
|
|
434,131
|
|
|
|
639,735
|
|
|
Investments in equity accounted investees
|
|
|
253
|
|
|
|
243
|
|
|
|
374
|
|
|
|
383
|
|
|
|
598
|
|
|
|
3,546
|
|
|
Non-current financial assets
|
|
|
2,012
|
|
|
|
891
|
|
|
|
1,636
|
|
|
|
3,731
|
|
|
|
7,535
|
|
|
|
41,667
|
|
|
Deferred tax assets
|
|
|
41,452
|
|
|
|
34,110
|
|
|
|
34,297
|
|
|
|
33,395
|
|
|
|
34,889
|
|
|
|
139,435
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
440,380
|
|
|
|
444,042
|
|
|
|
553,639
|
|
|
|
652,599
|
|
|
|
744,900
|
|
|
|
3,234,553
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
|
235,475
|
|
|
|
270,659
|
|
|
|
373,098
|
|
|
|
484,462
|
|
|
|
527,865
|
|
|
|
997,826
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade receivables
|
|
|
173,053
|
|
|
|
174,351
|
|
|
|
186,324
|
|
|
|
207,840
|
|
|
|
224,355
|
|
|
|
405,450
|
|
|
Other receivables
|
|
|
22,588
|
|
|
|
28,624
|
|
|
|
43,443
|
|
|
|
39,540
|
|
|
|
44,032
|
|
|
|
48,971
|
|
|
Current income tax assets
|
|
|
3,710
|
|
|
|
2,402
|
|
|
|
5,428
|
|
|
|
7,802
|
|
|
|
14,607
|
|
|
|
41,029
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
|
199,351
|
|
|
|
205,377
|
|
|
|
235,195
|
|
|
|
255,182
|
|
|
|
282,994
|
|
|
|
495,450
|
|
|
Other current financial assets
|
|
|
6,232
|
|
|
|
7,600
|
|
|
|
6,680
|
|
|
|
8,217
|
|
|
|
12,946
|
|
|
|
19,254
|
|
|
Other current assets
|
|
|
5,353
|
|
|
|
6,201
|
|
|
|
5,259
|
|
|
|
7,345
|
|
|
|
80,628
|
|
|
|
13,344
|
|
|
Cash and cash equivalents
|
|
|
26,883
|
|
|
|
5,690
|
|
|
|
6,368
|
|
|
|
249,372
|
|
|
|
239,649
|
|
|
|
583,792
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
473,294
|
|
|
|
495,527
|
|
|
|
626,600
|
|
|
|
1,004,578
|
|
|
|
1,144,082
|
|
|
|
2,109,666
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Assets
|
|
|
913,674
|
|
|
|
939,569
|
|
|
|
1,180,239
|
|
|
|
1,657,177
|
|
|
|
1,888,982
|
|
|
|
5,344,219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As of December 31,
|
|
|
As of June 30,
|
|
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2011
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
EQUITY AND LIABILITIES
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
106,532
|
|
|
|
106,532
|
|
|
|
106,532
|
|
|
|
106,532
|
|
|
|
106,532
|
|
|
|
114,914
|
|
|
Reserves
|
|
|
284,092
|
|
|
|
316,440
|
|
|
|
369,471
|
|
|
|
436,705
|
|
|
|
525,406
|
|
|
|
1,460,037
|
|
|
Own shares
|
|
|
—
|
|
|
|
(28,893
|
)
|
|
|
(33,087
|
)
|
|
|
(677
|
)
|
|
|
(1,927
|
)
|
|
|
(1,927
|
)
|
|
Interim dividend
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(31,960
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Profit for the year attributable to the Parent
|
|
|
45,394
|
|
|
|
87,774
|
|
|
|
121,728
|
|
|
|
147,972
|
|
|
|
115,513
|
|
|
|
19,269
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
436,018
|
|
|
|
481,853
|
|
|
|
564,644
|
|
|
|
658,572
|
|
|
|
745,524
|
|
|
|
1,592,293
|
|
|
Available-for-sale
financial assets
|
|
|
(52
|
)
|
|
|
(152
|
)
|
|
|
(158
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(575
|
)
|
|
Cash flow hedges
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,948
|
)
|
|
|
(1,751
|
)
|
|
|
(2,331
|
)
|
|
Translation differences
|
|
|
(68,022
|
)
|
|
|
(98,516
|
)
|
|
|
(84,457
|
)
|
|
|
(90,253
|
)
|
|
|
(50,733
|
)
|
|
|
(88,734
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income
|
|
|
(68,074
|
)
|
|
|
(98,668
|
)
|
|
|
(84,615
|
)
|
|
|
(92,201
|
)
|
|
|
(52,484
|
)
|
|
|
(91,640
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity attributable to the Parent
|
|
|
367,944
|
|
|
|
383,185
|
|
|
|
480,029
|
|
|
|
566,371
|
|
|
|
693,040
|
|
|
|
1,500,653
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Minority interest
|
|
|
408
|
|
|
|
981
|
|
|
|
1,250
|
|
|
|
12,157
|
|
|
|
14,350
|
|
|
|
12,941
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Equity
|
|
|
368,352
|
|
|
|
384,166
|
|
|
|
481,279
|
|
|
|
578,528
|
|
|
|
707,390
|
|
|
|
1,513,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
4,819
|
|
|
|
4,545
|
|
|
|
2,353
|
|
|
|
2,311
|
|
|
|
2,088
|
|
|
|
1,815
|
|
|
Provisions
|
|
|
902
|
|
|
|
999
|
|
|
|
3,045
|
|
|
|
1,232
|
|
|
|
1,378
|
|
|
|
10,461
|
|
|
Non-current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
198,329
|
|
|
|
178,425
|
|
|
|
311,513
|
|
|
|
703,186
|
|
|
|
665,385
|
|
|
|
2,642,944
|
|
|
Other financial liabilities
|
|
|
12,646
|
|
|
|
11,064
|
|
|
|
12,542
|
|
|
|
12,552
|
|
|
|
10,474
|
|
|
|
72,400
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities
|
|
|
210,975
|
|
|
|
189,489
|
|
|
|
324,055
|
|
|
|
715,738
|
|
|
|
675,859
|
|
|
|
2,715,344
|
|
|
Deferred tax liabilities
|
|
|
45,862
|
|
|
|
43,794
|
|
|
|
51,969
|
|
|
|
60,325
|
|
|
|
79,141
|
|
|
|
140,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
262,558
|
|
|
|
238,827
|
|
|
|
381,422
|
|
|
|
779,606
|
|
|
|
758,466
|
|
|
|
2,867,695
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provisions
|
|
|
3,890
|
|
|
|
3,957
|
|
|
|
3,830
|
|
|
|
4,702
|
|
|
|
4,365
|
|
|
|
35,828
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
138,123
|
|
|
|
177,540
|
|
|
|
147,547
|
|
|
|
113,991
|
|
|
|
191,635
|
|
|
|
507,374
|
|
|
Other financial liabilities
|
|
|
31,583
|
|
|
|
9,555
|
|
|
|
9,685
|
|
|
|
12,230
|
|
|
|
18,236
|
|
|
|
17,336
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities
|
|
|
169,706
|
|
|
|
187,095
|
|
|
|
157,232
|
|
|
|
126,221
|
|
|
|
209,871
|
|
|
|
524,710
|
|
|
Debts with associates
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,162
|
|
|
|
2,352
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
67,401
|
|
|
|
90,790
|
|
|
|
107,613
|
|
|
|
120,909
|
|
|
|
160,678
|
|
|
|
266,393
|
|
|
Other payables
|
|
|
23,282
|
|
|
|
11,396
|
|
|
|
9,068
|
|
|
|
17,832
|
|
|
|
11,928
|
|
|
|
25,618
|
|
|
Current income tax liabilities
|
|
|
4,345
|
|
|
|
3,770
|
|
|
|
16,362
|
|
|
|
3,258
|
|
|
|
4,172
|
|
|
|
27,227
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables
|
|
|
95,028
|
|
|
|
105,956
|
|
|
|
133,043
|
|
|
|
141,999
|
|
|
|
176,778
|
|
|
|
319,238
|
|
|
Other current liabilities
|
|
|
14,140
|
|
|
|
19,568
|
|
|
|
23,433
|
|
|
|
26,121
|
|
|
|
30,950
|
|
|
|
80,802
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
282,764
|
|
|
|
316,576
|
|
|
|
317,538
|
|
|
|
299,043
|
|
|
|
423,126
|
|
|
|
962,930
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Liabilities
|
|
|
545,322
|
|
|
|
555,403
|
|
|
|
698,960
|
|
|
|
1,078,649
|
|
|
|
1,181,592
|
|
|
|
3,830,625
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Equity and Liabilities
|
|
|
913,674
|
|
|
|
939,569
|
|
|
|
1,180,239
|
|
|
|
1,657,177
|
|
|
|
1,888,982
|
|
|
|
5,344,219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
11
Consolidated
Statement of Operations Data:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the Six Months
|
|
|
|
|
For the Year Ended December 31,
|
|
|
Ended June 30,
|
|
|
|
|
2006
|
|
|
2007
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2010
|
|
|
2011
|
|
|
|
|
(In thousands of Euros, except share amounts)
|
|
|
|
|
Revenues
|
|
|
648,417
|
|
|
|
703,291
|
|
|
|
814,311
|
|
|
|
913,186
|
|
|
|
990,730
|
|
|
|
487,809
|
|
|
|
635,341
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
(21,631
|
)
|
|
|
16,882
|
|
|
|
31,058
|
|
|
|
73,093
|
|
|
|
45,749
|
|
|
|
41,209
|
|
|
|
2,757
|
|
|
Self-constructed non-current assets
|
|
|
12,472
|
|
|
|
19,860
|
|
|
|
25,794
|
|
|
|
41,142
|
|
|
|
33,513
|
|
|
|
16,051
|
|
|
|
32,346
|
|
|
Supplies
|
|
|
(181,541
|
)
|
|
|
(196,308
|
)
|
|
|
(206,738
|
)
|
|
|
(286,274
|
)
|
|
|
(304,818
|
)
|
|
|
(157,107
|
)
|
|
|
(175,142
|
)
|
|
Other operating income
|
|
|
380
|
|
|
|
2,322
|
|
|
|
1,289
|
|
|
|
1,443
|
|
|
|
1,196
|
|
|
|
631
|
|
|
|
1,009
|
|
|
Personnel expenses
|
|
|
(184,730
|
)
|
|
|
(209,049
|
)
|
|
|
(238,159
|
)
|
|
|
(273,168
|
)
|
|
|
(289,008
|
)
|
|
|
(141,972
|
)
|
|
|
(183,727
|
)
|
|
Other operating expenses
|
|
|
(143,477
|
)
|
|
|
(158,273
|
)
|
|
|
(192,288
|
)
|
|
|
(203,381
|
)
|
|
|
(205,260
|
)
|
|
|
(98,279
|
)
|
|
|
(155,532
|
)
|
|
Amortization and depreciation
|
|
|
(29,357
|
)
|
|
|
(31,528
|
)
|
|
|
(33,256
|
)
|
|
|
(39,554
|
)
|
|
|
(45,776
|
)
|
|
|
(21,434
|
)
|
|
|
(28,156
|
)
|
|
Transaction costs of Talecris business combination
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(16,999
|
)
|
|
|
(2,019
|
)
|
|
|
(38,607
|
)
|
|
Non-financial and other capital grants
|
|
|
531
|
|
|
|
282
|
|
|
|
2,941
|
|
|
|
1,188
|
|
|
|
728
|
|
|
|
550
|
|
|
|
742
|
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(574
|
)
|
|
|
(1,125
|
)
|
|
|
(1,991
|
)
|
|
|
(1,147
|
)
|
|
|
(372
|
)
|
|
|
681
|
|
|
|
(22,302
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
100,490
|
|
|
|
146,354
|
|
|
|
202,961
|
|
|
|
226,528
|
|
|
|
209,683
|
|
|
|
126,120
|
|
|
|
68,729
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
4,667
|
|
|
|
4,526
|
|
|
|
2,682
|
|
|
|
7,067
|
|
|
|
4,526
|
|
|
|
2,179
|
|
|
|
1,761
|
|
|
Finance expenses
|
|
|
(42,140
|
)
|
|
|
(23,523
|
)
|
|
|
(29,305
|
)
|
|
|
(27,087
|
)
|
|
|
(49,660
|
)
|
|
|
(25,285
|
)
|
|
|
(55,546
|
)
|
|
Change in fair value of financial instruments
|
|
|
1,480
|
|
|
|
829
|
|
|
|
(1,268
|
)
|
|
|
(587
|
)
|
|
|
(7,593
|
)
|
|
|
(15,404
|
)
|
|
|
13,945
|
|
|
Impairment of gains/(losses) on disposal of financial instruments
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(245
|
)
|
|
|
91
|
|
|
|
—
|
|
|
|
—
|
|
|
Exchange losses
|
|
|
(1,064
|
)
|
|
|
(4,618
|
)
|
|
|
(2,825
|
)
|
|
|
(1,733
|
)
|
|
|
1,616
|
|
|
|
1,970
|
|
|
|
(2,122
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income and expense
|
|
|
(37,057
|
)
|
|
|
(22,786
|
)
|
|
|
(30,716
|
)
|
|
|
(22,585
|
)
|
|
|
(51,020
|
)
|
|
|
(36,540
|
)
|
|
|
(41,962
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit of equity accounted investees
|
|
|
76
|
|
|
|
19
|
|
|
|
24
|
|
|
|
51
|
|
|
|
(879
|
)
|
|
|
(728
|
)
|
|
|
(807
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
63,509
|
|
|
|
123,587
|
|
|
|
172,269
|
|
|
|
203,994
|
|
|
|
157,784
|
|
|
|
88,852
|
|
|
|
25,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
(17,824
|
)
|
|
|
(35,239
|
)
|
|
|
(50,153
|
)
|
|
|
(56,424
|
)
|
|
|
(42,517
|
)
|
|
|
(23,022
|
)
|
|
|
(7,347
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
45,685
|
|
|
|
88,348
|
|
|
|
122,116
|
|
|
|
147,570
|
|
|
|
115,267
|
|
|
|
65,830
|
|
|
|
18,613
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
45,394
|
|
|
|
87,774
|
|
|
|
121,728
|
|
|
|
147,972
|
|
|
|
115,513
|
|
|
|
66,408
|
|
|
|
19,269
|
|
|
Profit attributable to minority interest
|
|
|
291
|
|
|
|
574
|
|
|
|
388
|
|
|
|
(402
|
)
|
|
|
(246
|
)
|
|
|
(578
|
)
|
|
|
(656
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
45,685
|
|
|
|
88,348
|
|
|
|
122,116
|
|
|
|
147,570
|
|
|
|
115,267
|
|
|
|
65,830
|
|
|
|
18,613
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per ordinary share (Euros)
|
|
|
0.24
|
|
|
|
0.41
|
|
|
|
0.58
|
|
|
|
0.71
|
|
|
|
0.54
|
|
|
|
0.31
|
|
|
|
0.09
|
|
|
Diluted earnings per ordinary share (Euros)
|
|
|
0.25
|
|
|
|
0.41
|
|
|
|
0.58
|
|
|
|
0.71
|
|
|
|
0.54
|
|
|
|
0.31
|
|
|
|
0.09
|
|
|
Cash dividend per ordinary share (Euros)
|
|
|
0.06
|
|
|
|
0.06
|
|
|
|
0.17
|
|
|
|
0.38
|
|
|
|
0.13
|
|
|
|
—
|
|
|
|
—
|
|
12
UNAUDITED
PRO FORMA CONDENSED COMBINED FINANCIAL INFORMATION
The following summary unaudited pro forma condensed combined
financial information is intended to illustrate the effect of
the transaction between Grifols and Talecris. We have accounted
for the transaction as an acquisition under IFRS No. 3
(revised), Business Combinations.
Presented below are the unaudited pro forma condensed combined
statements of income of Grifols for the year ended
December 31, 2010 and the six months ended June 30,
2011. The unaudited pro forma condensed combined income
statements for the year ended December 31, 2010 and the six
months ended June 30, 2011 have been prepared as though the
acquisition of Talecris occurred as of January 1, 2010.
The assumptions underlying the pro forma adjustments are
described in the accompanying notes.
The unaudited pro forma condensed combined financial information
has been prepared based upon information derived from the
following:
|
|
|
| |
•
|
The audited consolidated financial statements of Grifols as of
and for the year ended December 31, 2010, which have been
prepared in accordance with IFRS as issued by the IASB and
included elsewhere in this prospectus;
|
| |
| |
•
|
The unaudited condensed consolidated interim financial
statements of Grifols as of and for the six month period ended
June 30, 2011, which have been prepared in accordance with
IAS 34, Interim Financial Reporting and included
elsewhere in this prospectus;
|
| |
| |
•
|
The audited consolidated financial statements of Talecris as of
and for the year ended December 31, 2010, which have been
prepared in accordance with U.S. GAAP and are included
elsewhere in this prospectus. These consolidated financial
statements have been adjusted to IFRS and translated to euros
for purposes of presentation in the unaudited pro forma
condensed combined financial information.
|
| |
| |
•
|
The unaudited consolidated financial information of Talecris as
of and for the five month period ended May 31, 2011. This
consolidated financial information has been adjusted to IFRS and
translated to euros for purposes of presentation in the
unaudited pro forma condensed combined financial information.
|
The purchase price for the acquisition of Talecris was
€2.6 billion based on the per share merger
consideration of $19.00 in cash (translated to euros using a
U.S. dollar/euro exchange rate as of May 31, 2011 of
$1.4408 per €1.00) and 0.6485 (or 0.641 for Talecris
specified affiliated stockholders) of a non-voting
(Class B) ordinary share of Grifols and cash in lieu
of fractional Class B shares and any cash representing dividends
or other distributions payable in accordance with the Merger
Agreement, for each share of Talecris common stock held at the
time of the transaction. The value of the Class B shares has
been determined based on the average of the daily median trading
price of the ADSs on the NASDAQ Global Select Market from
June 6 through June 28, 2011 (€9.9). The Class B
shares started quotation on June 2, 2011. The purchase
price is calculated using Talecris diluted shares as of
May 27, 2011.
The transaction has been accounted for by Grifols using the
acquisition method pursuant to IFRS 3 (revised), Business
Combinations. Under the acquisition method, assets and
liabilities are recorded at their fair value on the date of
purchase and the total purchase price is allocated to the
tangible and intangible assets acquired and liabilities assumed.
As of the date of this prospectus, the valuation studies
necessary to finalize the fair values of the assets acquired and
liabilities assumed and the related allocation of the purchase
price have not been completed. Accordingly, Grifols has
allocated the difference between total estimated purchase price,
calculated as described under “Notes to Unaudited Pro Forma
Condensed Combined Financial Information”, and net assets
acquired at book value as of May 31, 2011 to
“Preliminary Goodwill”. A final determination of these
fair values will reflect, among other things, Grifols’
consideration of a final valuation based on the actual net
tangible and intangible assets, such as acquired in-process
research and development, customer relationships, developed and
core technology, intellectual property, patents and trade names
and contingent liabilities, that exist as of the closing date of
the acquisition. Any final adjustment will change the
13
allocation of the purchase price, which will affect the fair
value assigned to the assets and liabilities and could result in
a material change to the unaudited pro forma condensed combined
financial information.
The unaudited pro forma condensed combined financial information
is presented for information purposes only and reflects
estimates and assumptions made by Grifols’ management that
it considers reasonable. It does not purport to represent what
Grifols’ actual results of operations or financial
condition would have been had the acquisition occurred on the
dates indicated, nor is it necessarily indicative of future
results of operations or financial condition. The unaudited pro
forma adjustments give effect to events that are directly
attributable to the acquisition and are factually supportable.
In addition to the matters noted above, the unaudited condensed
combined pro forma financial information does not reflect the
effect of anticipated synergies and efficiencies associated with
combining Grifols and Talecris.
Material nonrecurring profits and losses that result directly
from the transaction have not been included in the unaudited pro
forma condensed combined statement of income. Furthermore, the
costs and the transaction costs related to the incremental
funding needed in relation to the transaction in the form of
additional debt have been included in the unaudited pro forma
condensed combined statement of income. For a discussion of the
such items, see “Notes to Unaudited Pro Forma Condensed
Combined Financial Information”.
The unaudited condensed combined pro forma financial information
should be read in conjunction with the information contained in
“Selected Historical Consolidated Financial Data”,
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations” and the consolidated
financial statements of Grifols and Talecris appearing elsewhere
in this prospectus.
14
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF INCOME
Six Month Period Ended June 30, 2011
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Historical
|
|
|
Historical
|
|
|
|
|
|
|
|
|
|
|
|
GRIFOLS IFRS
|
|
|
TALECRIS IFRS
|
|
|
|
|
|
|
|
|
|
|
|
Six Month Period
|
|
|
Five Month Period
|
|
|
|
|
|
|
|
|
|
|
|
Ended June 30
|
|
|
Ended May 31
|
|
|
Pro forma
|
|
|
|
Pro Forma
|
|
|
|
|
2011
|
|
|
2011
|
|
|
Adjustments
|
|
|
|
Combined
|
|
|
|
|
(Note 1)
|
|
|
(Note 2 )
|
|
|
(Note 3)
|
|
|
|
GRIFOLS
|
|
|
|
|
(In thousands of Euros, except share amounts)
|
|
|
Revenues
|
|
|
635,341
|
|
|
|
504,051
|
|
|
|
|
|
|
|
|
1,139,392
|
|
|
Changes in inventories of finished goods and work in
|
|
|
2,757
|
|
|
|
(21,862
|
)
|
|
|
|
|
|
|
|
(19,105
|
)
|
|
Self-constructed non-current assets
|
|
|
32,346
|
|
|
|
6,192
|
|
|
|
|
|
|
|
|
38,538
|
|
|
Supplies
|
|
|
(175,142
|
)
|
|
|
(91,731
|
)
|
|
|
|
|
|
|
|
(266,873
|
)
|
|
Other operating income
|
|
|
1,009
|
|
|
|
—
|
|
|
|
|
|
|
|
|
1,009
|
|
|
Personnel expenses
|
|
|
(183,727
|
)
|
|
|
(149,571
|
)
|
|
|
14,810
|
(c)
|
|
|
|
(318,488
|
)
|
|
Other operating expenses
|
|
|
(155,532
|
)
|
|
|
(125,456
|
)
|
|
|
|
|
|
|
|
(280,988
|
)
|
|
Amortisation and depreciation
|
|
|
(28,156
|
)
|
|
|
(16,387
|
)
|
|
|
|
|
|
|
|
(44,543
|
)
|
|
Transition costs of Talecris business combination
|
|
|
(38,607
|
)
|
|
|
(16,756
|
)
|
|
|
55,363
|
(b)
|
|
|
|
—
|
|
|
Non-financial and other capital grants
|
|
|
742
|
|
|
|
—
|
|
|
|
|
|
|
|
|
742
|
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(22,302
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
(22,302
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
68,729
|
|
|
|
88,480
|
|
|
|
70,173
|
|
|
|
|
227,382
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
1,761
|
|
|
|
146
|
|
|
|
|
|
|
|
|
1,907
|
|
|
Finance expenses
|
|
|
(55,546
|
)
|
|
|
(13,163
|
)
|
|
|
(45,898
|
)(a)
|
|
|
|
(114,607
|
)
|
|
Change in fair value of financial instruments
|
|
|
13,945
|
|
|
|
1,880
|
|
|
|
|
|
|
|
|
15,825
|
|
|
Exchange gains/(losses)
|
|
|
(2,122
|
)
|
|
|
(2,989
|
)
|
|
|
|
|
|
|
|
(5,111
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial income and expense
|
|
|
(41,962
|
)
|
|
|
(14,126
|
)
|
|
|
(45,898
|
)
|
|
|
|
(101,986
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of (loss) profit of equity accounted investees
|
|
|
(807
|
)
|
|
|
234
|
|
|
|
|
|
|
|
|
(573
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing Operations
|
|
|
25,960
|
|
|
|
74,588
|
|
|
|
24,275
|
|
|
|
|
124,823
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax (expense) benefit
|
|
|
(7,347
|
)
|
|
|
(24,991
|
)
|
|
|
(9,152
|
)(d)
|
|
|
|
(41,490
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
18,613
|
|
|
|
49,597
|
|
|
|
15,123
|
|
|
|
|
83,333
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
19,269
|
|
|
|
49,597
|
|
|
|
15,123
|
|
|
|
|
83,989
|
|
|
Profit attributable to minority interest
|
|
|
(656
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
(656
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the three month period
|
|
|
18,613
|
|
|
|
49,597
|
|
|
|
15,123
|
|
|
|
|
83,333
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per share
|
|
|
0.09
|
|
|
|
|
|
|
|
|
|
|
|
|
0.39
|
|
|
Weighted average number of share in issue(B)
|
|
|
213,064,899
|
|
|
|
|
|
|
|
|
|
|
|
|
213,064,899
|
|
|
Diluted earnings per share
|
|
|
0.09
|
|
|
|
|
|
|
|
|
|
|
|
|
0.39
|
|
|
Weighted average number of share on fully diluted basis
|
|
|
213,064,899
|
|
|
|
|
|
|
|
|
|
|
|
|
213,064,899
|
|
|
|
| (B) |
The weighted average number of shares outstanding during the
period has been adjusted to give effect to shares issued as
consideration for the transaction as if the acquisition had
taken place as of January 1, 2010.
|
15
UNAUDITED
PRO FORMA CONDENSED COMBINED STATEMENT OF INCOME
Year Ended December 31, 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Pro forma
|
|
|
|
|
|
|
|
|
GRIFOLS IFRS
|
|
|
TALECRIS IFRS
|
|
|
Adjustments
|
|
|
|
Pro Forma
|
|
|
|
|
(Note 1)
|
|
|
(Note 2)
|
|
|
(Note 3)
|
|
|
|
GRIFOLS
|
|
|
|
|
(In thousands of Euros, except share amounts)
|
|
|
Revenues
|
|
|
990,730
|
|
|
|
1,207,766
|
|
|
|
|
|
|
|
|
2,198,496
|
|
|
Changes in inventories of finished goods and work in
|
|
|
45,749
|
|
|
|
27,932
|
|
|
|
|
|
|
|
|
73,681
|
|
|
Self-constructed non-current assets
|
|
|
33,513
|
|
|
|
15,292
|
|
|
|
|
|
|
|
|
48,805
|
|
|
Supplies
|
|
|
(306,859
|
)
|
|
|
(319,563
|
)
|
|
|
|
|
|
|
|
(626,422
|
)
|
|
Other operating income
|
|
|
1,196
|
|
|
|
—
|
|
|
|
|
|
|
|
|
1,196
|
|
|
Personnel expenses
|
|
|
(289,008
|
)
|
|
|
(352,803
|
)
|
|
|
12,422
|
(c)
|
|
|
|
(629,389
|
)
|
|
Other operating expenses
|
|
|
(220,218
|
)
|
|
|
(292,504
|
)
|
|
|
37,911
|
(b)
|
|
|
|
(474,811
|
)
|
|
Amortisation and depreciation
|
|
|
(45,776
|
)
|
|
|
(39,235
|
)
|
|
|
|
|
|
|
|
(85,011
|
)
|
|
Non-financial and other capital grants
|
|
|
728
|
|
|
|
—
|
|
|
|
|
|
|
|
|
728
|
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(372
|
)
|
|
|
(1,124
|
)
|
|
|
|
|
|
|
|
(1,496
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
209,683
|
|
|
|
245,761
|
|
|
|
50,333
|
|
|
|
|
505,777
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PCA Adjustment
|
|
|
|
|
|
|
(32,946
|
)
|
|
|
|
|
|
|
|
(32,946
|
)
|
|
Finance income
|
|
|
4,526
|
|
|
|
255
|
|
|
|
|
|
|
|
|
4,781
|
|
|
Finance expenses
|
|
|
(49,660
|
)
|
|
|
(34,301
|
)
|
|
|
(113,978
|
)(a)
|
|
|
|
(197,939
|
)
|
|
Change in fair value of financial instruments
|
|
|
(7,593
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
(7,593
|
)
|
|
Impairment of gains/(losses) on disposal of financial instruments
|
|
|
91
|
|
|
|
—
|
|
|
|
|
|
|
|
|
91
|
|
|
Exchange gains
|
|
|
1,616
|
|
|
|
3,565
|
|
|
|
|
|
|
|
|
5,181
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial income and expense
|
|
|
(51,020
|
)
|
|
|
(63,427
|
)
|
|
|
(113,978
|
)
|
|
|
|
(228,425
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of (loss) profit of equity accounted investees
|
|
|
(879
|
)
|
|
|
747
|
|
|
|
|
|
|
|
|
(132
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
157,784
|
|
|
|
183,081
|
|
|
|
(63,645
|
)
|
|
|
|
277,220
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax (expense) benefit
|
|
|
(42,517
|
)
|
|
|
(59,543
|
)
|
|
|
23,994
|
(d)
|
|
|
|
(78,066
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
115,267
|
|
|
|
123,538
|
|
|
|
(39,651
|
)
|
|
|
|
199,154
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
115,513
|
|
|
|
123,538
|
|
|
|
(39,651
|
)
|
|
|
|
199,400
|
|
|
Profit attributable to minority interest
|
|
|
(246
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
(246
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
115,267
|
|
|
|
123,538
|
|
|
|
(39,651
|
)
|
|
|
|
199,154
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per share
|
|
|
0.54
|
|
|
|
|
|
|
|
|
|
|
|
|
0.94
|
|
|
Weighted average number of share in issue(B)
|
|
|
212,909,162
|
|
|
|
|
|
|
|
|
|
|
|
|
212,909,162
|
|
|
Diluted earnings per share
|
|
|
0.54
|
|
|
|
|
|
|
|
|
|
|
|
|
0.94
|
|
|
Weighted average number of share on fully diluted basis
|
|
|
212,909,162
|
|
|
|
|
|
|
|
|
|
|
|
|
212,909,162
|
|
|
|
| (B) |
The weighed averaged number of shares outstanding during the
period has been adjusted to give effect to shares issued as
consideration for the transaction as if the acquisition had
taken place as of January 1, 2010.
|
16
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION
|
|
|
1.
|
Historical
Grifols Information
|
Represents the historical condensed consolidated statement of
income for the six month period ended June 30, 2011 and for
the year ended December 31, 2010, which have been extracted
from the financial statements of Grifols, as follows:
|
|
|
| |
•
|
unaudited condensed consolidated interim financial statements as
of and for the six month period ended June 30,
2011; and
|
| |
| |
•
|
audited consolidated financial statements as of and for the year
ended December 31, 2010.
|
The condensed consolidated statement of income of Grifols for
the six month period ended June 30, 2011, include
Talecris’ figures from the date of the acquisition
June 2, 2011.
|
|
|
2.
|
Talecris
Reconciliation to IFRS in Euros
|
Talecris’ unaudited consolidated income statement for the
five-month period ended May 31, 2011 and the audited
consolidated financial statements for the year ended
December 31, 2010 have been prepared in accordance with
U.S. GAAP, which differs in certain material respects from
IFRS. In addition, certain reclassifications are required to
conform the presentation of Talecris’ historical financial
information to that of Grifols under IFRS. Furthermore, the
referenced Talecris’ financial statements were prepared in
U.S. dollars and have been translated to euros, the
currency in which the Grifols financial statements have been
prepared. The effects of the applications of IFRS,
reclassifications and translation to euros are as follows:
17
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
Statement
of Income Date:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Statement of Income Five Month Period Ended May 31,
2011
|
|
|
|
|
Historical
|
|
|
|
|
|
|
|
Historical
|
|
|
|
|
|
|
|
|
Ex.
|
|
|
|
Historical
|
|
|
|
|
TALECRIS
|
|
|
Reclassifications
|
|
|
|
|
TALECRIS
|
|
|
Inventory
|
|
|
Capitalize
|
|
|
Income
|
|
|
TALECRIS
|
|
|
Rate
|
|
|
|
TALECRIS
|
|
|
|
|
US GAAP Cost
|
|
|
to Cost
|
|
|
|
|
US GAAP
|
|
|
Recovery
|
|
|
R&D Cost
|
|
|
Taxes
|
|
|
IFRS Cost
|
|
|
(h)
|
|
|
|
IFRS as Shown
|
|
|
|
|
by Function
|
|
|
by Nature(f)
|
|
|
|
|
Cost by Nature
|
|
|
(b)
|
|
|
(c)
|
|
|
(e)
|
|
|
by Nature
|
|
|
1.3721
|
|
|
|
in the Pro forma
|
|
|
|
|
A
|
|
|
B
|
|
|
|
|
A+B
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands of $)
|
|
|
|
|
(in thousands of $)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(in thousands of €)
|
|
|
Net revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product
|
|
|
691,609
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
691,609
|
|
|
|
|
|
|
Revenues
|
|
|
691,609
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
691,609
|
|
|
|
|
|
|
|
|
504,051
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
(399,219
|
)
|
|
|
399,219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
292,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
(135,617
|
)
|
|
|
135,617
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
(32,208
|
)
|
|
|
32,208
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(167,375
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
125,015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(29,635
|
)
|
|
Changes in inventories of finished goods and work in progress
|
|
|
(29,635
|
)
|
|
|
(362
|
)
|
|
|
|
|
|
|
|
|
|
|
(29,997
|
)
|
|
|
|
|
|
|
|
(21,862
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,199
|
|
|
Self-constructed non-current assets
|
|
|
4,199
|
|
|
|
|
|
|
|
4,297
|
|
|
|
|
|
|
|
8,496
|
|
|
|
|
|
|
|
|
6,192
|
|
|
|
|
|
|
|
|
|
(125,864
|
)
|
|
Supplies
|
|
|
(125,864
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(125,864
|
)
|
|
|
|
|
|
|
|
(91,731
|
)
|
|
|
|
|
|
|
|
|
(205,227
|
)
|
|
Personnel expenses
|
|
|
(205,227
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(205,227
|
)
|
|
|
|
|
|
|
|
(149,571
|
)
|
|
|
|
|
|
|
|
|
(172,138
|
)
|
|
Other operating expenses
|
|
|
(172,138
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(172,138
|
)
|
|
|
|
|
|
|
|
(124,456
|
)
|
|
|
|
|
|
|
|
|
(13,417
|
)
|
|
Amortization and depreciation
|
|
|
(13,417
|
)
|
|
|
|
|
|
|
(9,067
|
)
|
|
|
|
|
|
|
(22,484
|
)
|
|
|
|
|
|
|
|
(16,387
|
)
|
|
|
|
|
|
|
|
|
(22,991
|
)
|
|
Transition costs of Talecris business combin
|
|
|
(22,991
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(22,991
|
)
|
|
|
|
|
|
|
|
(16,756
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
126,536
|
|
|
|
(362
|
)
|
|
|
(4,770
|
)
|
|
|
—
|
|
|
|
144,395
|
|
|
|
|
|
|
|
|
88,480
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other non-operating (expense) income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
(17,859
|
)
|
|
|
17,859
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Equity in earnings of affiliate
|
|
|
320
|
|
|
|
(320
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(17,539
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(825
|
)
|
|
PCA judgment
|
|
|
(825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(825
|
)
|
|
|
|
|
|
|
|
(601
|
)
|
|
|
|
|
|
|
|
|
202
|
|
|
Finance income
|
|
|
201
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
201
|
|
|
|
|
|
|
|
|
146
|
|
|
|
|
|
|
|
|
|
(17,236
|
)
|
|
Finance expenses
|
|
|
(17,236
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(17,236
|
)
|
|
|
|
|
|
|
|
(12,562
|
)
|
|
|
|
|
|
|
|
|
2,580
|
|
|
Changes in FV of financial instruments
|
|
|
2,580
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,580
|
|
|
|
|
|
|
|
|
1,880
|
|
|
|
|
|
|
|
|
|
(4,101
|
)
|
|
Exchange gains
|
|
|
(4,101
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(4,101
|
)
|
|
|
|
|
|
|
|
(2,989
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial income and expense
|
|
|
(19,381
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(19,381
|
)
|
|
|
|
|
|
|
|
(14,126
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
321
|
|
|
Share of profit of equity accounted investees
|
|
|
321
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
321
|
|
|
|
|
|
|
|
|
234
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
107,476
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
107,476
|
|
|
|
(362
|
)
|
|
|
(4,770
|
)
|
|
|
—
|
|
|
|
125,335
|
|
|
|
|
|
|
|
|
74,588
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Provision) benefit for income taxes
|
|
|
(35,882
|
)
|
|
|
0
|
|
|
Income tax (expense) benefit
|
|
|
(35,882
|
)
|
|
|
|
|
|
|
|
|
|
|
1,593
|
|
|
|
(34,289
|
)
|
|
|
|
|
|
|
|
(24,991
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
71,594
|
|
|
|
|
|
|
continuing operations
|
|
|
71,594
|
|
|
|
(362
|
)
|
|
|
(4,770
|
)
|
|
|
1,593
|
|
|
|
91,046
|
|
|
|
|
|
|
|
|
49,597
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
18
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Statement of Income of the year ended December 31,
2010
|
|
|
|
|
Historical
|
|
|
|
|
|
|
|
Historical
|
|
|
Adjustments to IFRS
|
|
|
|
|
|
Ex.
|
|
|
|
Historical
|
|
|
|
|
TALECRIS
|
|
|
Reclassifications
|
|
|
|
|
TALECRIS
|
|
|
Debt
|
|
|
Inventory
|
|
|
Capitalize
|
|
|
Shared Based
|
|
|
Income
|
|
|
TALECRIS
|
|
|
Rate
|
|
|
|
TALECRIS
|
|
|
|
|
US GAAP Cost
|
|
|
to Cost
|
|
|
|
|
US GAAP
|
|
|
Issuance
|
|
|
Recovery
|
|
|
R&D Cost
|
|
|
Payments
|
|
|
Taxes
|
|
|
IFRS Cost
|
|
|
(g)
|
|
|
|
IFRS as Shown
|
|
|
|
|
by Function
|
|
|
by Nature(f)
|
|
|
|
|
Cost by Nature
|
|
|
Costs(a)
|
|
|
(b)
|
|
|
(c)
|
|
|
(d)
|
|
|
(e)
|
|
|
by Nature
|
|
|
1.3261
|
|
|
|
in the Pro forma
|
|
|
|
|
A
|
|
|
B
|
|
|
|
|
A+B
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(In thousands of $)
|
|
|
|
|
(In thousands of $)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(In thousands of €)
|
|
|
Net revenue
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product
|
|
|
1,601,619
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
1,601,619
|
|
|
|
|
|
|
Revenues
|
|
|
1,601,619
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,601,619
|
|
|
|
|
|
|
|
|
1,207,766
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cost of goods sold
|
|
|
(911,976
|
)
|
|
|
911,976
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
689,643
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general and administrative
|
|
|
(287,011
|
)
|
|
|
287,011
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development
|
|
|
(69,649
|
)
|
|
|
69,649
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(356,660
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
332,983
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
37,647
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
37,647
|
|
|
|
|
|
|
|
(606
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
37,041
|
|
|
|
|
|
|
|
|
27,932
|
|
|
|
|
|
|
|
|
|
8,610
|
|
|
Self-constructed non-current assets
|
|
|
8,610
|
|
|
|
|
|
|
|
|
|
|
|
11,669
|
|
|
|
|
|
|
|
|
|
|
|
20,279
|
|
|
|
|
|
|
|
|
15,292
|
|
|
|
|
|
|
|
|
|
(423,772
|
)
|
|
Supplies
|
|
|
(423,772
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(423,772
|
)
|
|
|
|
|
|
|
|
(319,563
|
)
|
|
|
|
|
|
|
|
|
(470,437
|
)
|
|
Personnel expenses
|
|
|
(470,437
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,585
|
|
|
|
|
|
|
|
(467,852
|
)
|
|
|
|
|
|
|
|
(352,803
|
)
|
|
|
|
|
|
|
|
|
(387,890
|
)
|
|
Other operating expenses
|
|
|
(387,890
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(387,890
|
)
|
|
|
|
|
|
|
|
(292,504
|
)
|
|
|
|
|
|
|
|
|
(36,030
|
)
|
|
Amortization and depreciation
|
|
|
(36,030
|
)
|
|
|
|
|
|
|
|
|
|
|
(16,000
|
)
|
|
|
|
|
|
|
|
|
|
|
(52,030
|
)
|
|
|
|
|
|
|
|
(39,235
|
)
|
|
|
|
|
|
|
|
|
(1,491
|
)
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(1,491
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,491
|
)
|
|
|
|
|
|
|
|
(1,124
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
328,256
|
|
|
|
—
|
|
|
|
(606
|
)
|
|
|
(4,331
|
)
|
|
|
2,585
|
|
|
|
—
|
|
|
|
325,904
|
|
|
|
|
|
|
|
|
245,761
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other non-operating (expense) income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
(45,837
|
)
|
|
|
45,837
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
PCA judgment
|
|
|
(43,690
|
)
|
|
|
43,690
|
|
|
PCA judgment
|
|
|
(43,690
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(43,690
|
)
|
|
|
|
|
|
|
|
(32,946
|
)
|
|
Equity in earnings of affiliate
|
|
|
991
|
|
|
|
(991
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(88,536
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
338
|
|
|
Finance income
|
|
|
338
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
338
|
|
|
|
|
|
|
|
|
255
|
|
|
|
|
|
|
|
|
|
(46,175
|
)
|
|
Finance expenses
|
|
|
(46,175
|
)
|
|
|
688
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(45,487
|
)
|
|
|
|
|
|
|
|
(34,301
|
)
|
|
|
|
|
|
|
|
|
4,727
|
|
|
Exchange gains
|
|
|
4,727
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,727
|
|
|
|
|
|
|
|
|
3,565
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
expense
|
|
|
(84,800
|
)
|
|
|
688
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(84,112
|
)
|
|
|
|
|
|
|
|
(63,427
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
991
|
|
|
Share of profit of equity accounted investees
|
|
|
991
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
991
|
|
|
|
|
|
|
|
|
747
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
244,447
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
244,447
|
|
|
|
688
|
|
|
|
(606
|
)
|
|
|
(4,331
|
)
|
|
|
2,585
|
|
|
|
—
|
|
|
|
242,783
|
|
|
|
|
|
|
|
|
183,081
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Provision) benefit for income taxes
|
|
|
(78,379
|
)
|
|
|
0
|
|
|
Income tax (expense) benefit
|
|
|
(78,379
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(581
|
)
|
|
|
(78,960
|
)
|
|
|
|
|
|
|
|
(59,543
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
166,068
|
|
|
|
|
|
|
continuing operations
|
|
|
166,068
|
|
|
|
688
|
|
|
|
(606
|
)
|
|
|
(4,331
|
)
|
|
|
2,585
|
|
|
|
(581
|
)
|
|
|
163,823
|
|
|
|
|
|
|
|
|
123,538
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
19
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
Adjustments
for IFRS:
For purpose of the unaudited pro forma condensed combined
financial information, the historical financial information of
Talecris has been converted to IFRS. The adjustments deemed
necessary to carry out the conversion are:
The debt issuance cost adjustment reflects both (i) amounts
capitalized under U.S. GAAP related to a 2009 Revolving
Loan amendment that would be charged to operating expenses under
IFRS; and (ii) amounts reported as long-term assets under
U.S. GAAP reclassified as a reduction of long-term debt
under IFRS. This adjustment resulted in an increase in profit
for 2010 of $0.688 million.
|
|
|
(b)
|
Inventory
Impairment Recoveries
|
According to U.S. GAAP, inventory impairment provisions
create a new cost basis for inventory. These provisions are only
recoverable upon the sale of the related inventory. IFRS permits
a recovery of previous inventory impairment charges at the time
the related inventory asset value is determined to be
recoverable. The IFRS adjustment resulted in a decrease in
profit for 2010 of $0.606 million and of
$0.362 million in profit for the five-month period ended
May 31, 2011.
|
|
|
(c)
|
Capitalized
Research and Development Costs
|
Talecris expensed all research and development costs under
U.S. GAAP. Certain development project costs, which
represent a probable future economic benefit that may be
capitalized under IFRS and amortized over the expected useful
economic life of the asset, are captured in this adjustment.
This adjustment resulted in a decrease in profit for 2010 of
$4.431 million and of $4.770 million for the five-month
period ended May 31, 2011.
Talecris’ Long Term Incentive Plan provided for the grant
of awards in the form of incentive stock options, nonqualified
stock options, share appreciation rights, restricted stock,
restricted stock units, unrestricted shares of common stock,
deferred shared units and performance awards. Talecris’
employees, directors and consultants were eligible to receive
awards under the 2009 Plan.
The fair value of Talecris’ time-based equity awards was
amortized to compensation expense on a straight-line basis over
the vesting period under U.S. GAAP, while such awards are
amortized on an accelerated methodology under IFRS including the
remeasurement of annual compensation expense at year end for the
succeeding year. This adjustment resulted in a decrease in
personnel expenses of $2.585 million in 2010.
The income tax adjustment reflects the application of the
Talecris 2009 and 2010 consolidated effective income tax rate
determined under U.S. GAAP to (i) all the adjustments
for IFRS that are not permanent differences for U.S. income
tax provision purposes; (ii) the determination of any
deferred tax assets for share-based compensation using the
intrinsic value of share-based compensation considering the
graded vesting methodology under IFRS and reflecting the best
estimate of the actual tax benefits reasonably expected to be
realized when the options are exercised; and (iii) the
difference in the tax basis of intercompany profits on sales to
Talecris GmbH.
The IFRS adjustment resulted in a decrease in profit in 2010 of
$0.581 million and an increase of $1.593 million for
the five month period ended May 31, 2011.
20
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
Talecris’ U.S. GAAP consolidated statements of
income for the year 2010 and for the five-month period ended
May 31, 2011 were prepared on a cost by activity format and
the reclassification adjustments reflected are necessary to
report the statement of income in accordance with a cost by
nature format under IFRS.
Currency
Translation
(g) Translation
to Euros
Talecris’ U.S. dollar consolidated statements of
income for the year 2010 and for the five-month period ended
May 31, 2011 were translated to euros using the average
euro/U.S. dollar exchange rate for the year 2010 and for
the five-month period ended May 31, 2011, respectively.
Adjustments
to reflect the accounting for the acquisition of Talecris by
Grifols
|
|
|
(a)
|
Debt
interest expenses
|
The acquisition of Talecris by Grifols occurred on June 1,
2011. Upon the consummation of the transaction, holders of
Talecris common stock received, for each share of Talecris
common stock held at the time of the transaction, a combination
of (1) $19.00 in cash and (2) 0.6485 (or 0.641 for
specified Talecris affiliated stockholders) of a non-voting
(Class B) ordinary share of Grifols and cash in lieu
of fractional Class B shares and any cash representing
dividends or other distributions payable in accordance with the
Merger Agreement.
The components of the purchase price are as follows:
| |
|
|
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
Purchase price:
|
|
|
|
|
|
Cash(i)
|
|
|
1,763,601
|
|
|
Fair value of shares issued(ii)
|
|
|
829,799
|
|
|
|
|
|
|
|
|
|
|
|
2,593,400
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Cash Consideration per Talecris
Share($)(1)
|
|
|
19
|
|
|
|
|
|
|
Total Talecris Shares Outstanding
|
|
|
126,107,617
|
|
|
|
|
|
|
Cash for Talecris Shares Outstanding($)
|
|
|
2,396,044,723
|
|
|
|
|
|
|
Cash for Talecris Options / Awards (Net Method)($)
|
|
|
76,327,737
|
|
|
|
|
|
|
Cash in Lieu for Fractional Talecris Options /Awards (Net
Method)($)
|
|
|
4,525
|
|
|
|
|
|
|
Cash for Talecris Options / Awards (Net Method)($)
|
|
|
76,332,262
|
|
|
|
|
|
|
Additional Cash Consideration per Fractional Share($)
|
|
|
19.693
|
|
|
|
|
|
|
Fractional Shares (from Talecris Shares Outstanding)
|
|
|
21.9095
|
|
|
|
|
|
|
Cash for Fractional Shares (Talecris
Shares Outstanding)($)
|
|
|
431
|
|
|
|
|
|
|
Cash($)
|
|
|
2,472,377,417
|
|
|
|
|
|
|
Taxes Withheld on Cash Consideration (Options / Awards)($)
|
|
|
41,142,748
|
|
|
|
|
|
|
Taxes Withheld on Stock Consideration (Options / Awards)($)
|
|
|
27,476,748
|
|
|
|
|
|
|
Cash for Tax Payments (Options / Awards)($)
|
|
|
68,619,495
|
|
|
|
|
|
|
Total Cash($)
|
|
|
2,540,996,912
|
|
|
|
|
|
|
Exchange rate
$/Euros(2)
|
|
|
1.4408
|
|
|
|
|
|
|
Cash in thousands of euros
|
|
|
|
|
|
|
1,763,601
|
|
21
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
|
|
|
|
(1)
|
|
Based on the Merger Agreement
|
| |
|
(2)
|
|
Exchange rate $/Euro at
May 31, 2011 based on the exchange rate included elsewhere
in this prospectus.
|
|
|
|
(ii)
|
Estimated
fair value of shares issued
|
| |
|
|
|
|
|
|
|
|
|
Talecris Shares Outstanding (Affiliated Shareholders)
|
|
|
62,517,962
|
|
|
|
|
|
|
Exchange Ratio (Talecris Affiliated
Shareholders)(1)
|
|
|
0.6410
|
|
|
|
|
|
|
Grifols Class B Shares (Talecris Affiliated Shareholders)
|
|
|
40,074,014
|
|
|
|
|
|
|
Fractional Shares (Talecris Affiliated Shares Outstanding)
|
|
|
(3.6420
|
)
|
|
|
|
|
|
Grifols Class B Shares to be Issued (Talecris Affiliated
Shareholders)
|
|
|
40,074,010
|
|
|
|
|
|
|
Talecris Shares Outstanding (Unaffiliated Shareholders)
|
|
|
63,589,655
|
|
|
|
|
|
|
Exchange Ratio (Talecris Unaffiliated
Shareholders)(2)
|
|
|
0.6485
|
|
|
|
|
|
|
Grifols Class B Shares (Talecris Unaffiliated Shareholders)
|
|
|
41,237,891
|
|
|
|
|
|
|
Fractional Shares (Talecris Unaffiliated Shares Outst.)
|
|
|
(18.2675
|
)
|
|
|
|
|
|
Grifols Class B Shares to be Issued (Talecris Unaffiliated
Shareholders)
|
|
|
41,237,873
|
|
|
|
|
|
|
Grifols Class B Shares for Talecris Shares Outstanding
(included Affiliated/Unaffil.)
|
|
|
81,311,883
|
|
|
|
|
|
|
Grifols Class B Shares for Talecris Options/Awards (Net Method)
|
|
|
2,499,805
|
|
|
|
|
|
|
Total Grifols Class B Shares (to be Issued by Grifols)
|
|
|
83,811,688
|
|
|
|
|
|
|
Price of Grifols Class B
Shares($)(3)
|
|
|
14.2650
|
|
|
|
|
|
|
Estimated fair value of Class B Shares issued in thousands of
$
|
|
|
1,195,574
|
|
|
|
|
|
|
Exchange rate
$/Euros(4)
|
|
|
1.4408
|
|
|
|
|
|
|
Estimated fair value of Class B Shares issued in thousands of
Euros
|
|
|
|
|
|
|
829,799
|
|
|
|
|
|
(1)
|
|
Based on the Merger Agreement
|
| |
|
(2)
|
|
Based on Amendment No. 1 to
the Merger Agreement
|
| |
|
(3)
|
|
The fair value of the Class B
shares has been determined based on the average of the daily
median trading price of the ADSs on the NASDAQ Global Select
Market from June 6 through June 28, 2011. The Class B shares
started quotation on June 2, 2011.
|
| |
|
(4)
|
|
Exchange rate $/Euro at
May 31, 2011 based on the exchange rate included elsewhere
in this prospectus.
|
|
|
|
(iii)
|
Preliminary
Goodwill
|
The preliminary purchase price allocation to assets and
liabilities is assumed to be as follows:
| |
|
|
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
Net assets acquired provisional (book value) as at May 31,
2011
|
|
|
469,318
|
|
|
Purchase price
|
|
|
2,593,400
|
|
|
|
|
|
|
|
|
Preliminary
goodwill(1)(2)
|
|
|
2,124,082
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
As of the date of the preparation
of this unaudited pro forma condensed combined financial
information, a purchase price allocation, in accordance with
IFRS 3 (revised), could not be completed. When the purchase
price allocation is completed, other intangible assets may be
identified (i.e. licenses, in process research and development,
customer relationships, etc.), which could result in a reduction
of goodwill. The identification of such intangible assets could
generate an amortization charge, which could have an impact on
the unaudited pro forma condensed combined statement of income.
|
| |
|
|
|
Based on preliminary assumptions
for the fair value adjustments of tangible and intangible assets
in connection with the purchase price allocation, such
allocation represents approximately 40% of the estimated
goodwill of €2,124 million as of June 30, 2011.
On this basis the amount of the annual depreciation and
amortization expense net of the tax effect (calculated based on
statutory rates of 37.7%) would be approximately between
€50 million and €60 million.
|
22
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
|
|
|
|
|
|
For purposes of a potential income
statement adjustment, the depreciation expense of assets was
estimated based on the following residual lives, approximately
20 to 25 years for buildings, 5 to 8 years for
machinery and equipment and 20 to 30 years for intangible
assets. Those estimated useful lives are management’s best
estimate based on the available information.
|
| |
|
(2)
|
|
Goodwill will be denominated in
U.S. Dollars and will be approximately $3.060 billion. The
effect of future currency fluctuations between the Euro, which
is the functional currency of Grifols, and the U.S. Dollar will
be accounted for as a currency translation adjustment.
|
The cash required is the following:
| |
|
|
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
Cash and cash equivalents at Grifols
|
|
|
149,693
|
|
|
Incremental debts
|
|
|
1,613,908
|
|
|
|
|
|
|
|
|
Purchase price in cash in connection with the transaction
|
|
|
1,763,601
|
|
|
|
|
|
|
|
|
|
|
(v)
|
Interest
expenses on the incremental debt in relation to the
acquisition
|
The financial liabilities of Grifols, S.A. and subsidiaries as
at June 30, 2011 amount to €3,240 million
($4,683 million) included the incremental debt issued in
June 2011 in relation to the acquisition of Talecris for an
amount of €1,614 million ($2,325 million). The
interest expense on this incremental debt of
€113.978 million in 2010 and €45.898 million
for the five month period ended May 31, 2011 were adjusted
in the pro forma condensed combined statement of income for the
year 2010 and six month period ended June 30, 2011 to give
the effect that the transaction occurred on January 1, 2010.
| |
|
|
|
|
|
|
|
|
|
|
|
|
Calculation of interest
|
|
|
|
|
|
|
|
|
|
expenses on the incremental debt in
|
|
|
|
|
Exchange
|
|
|
Euros
|
|
|
relation to the acquisition
|
|
$ Thousands
|
|
|
rate $/Euros
|
|
|
Thousands
|
|
|
|
|
Purchase price in cash in connection with the transaction
|
|
|
2,540,997
|
|
|
|
1.4408
|
|
|
|
1,763,601
|
|
|
Cash and cash equivalents at Talecris
|
|
|
(215,678
|
)
|
|
|
1.4408
|
|
|
|
(149,693
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Incremental debt to be issued in relation to the acquisition
|
|
|
2,325,319
|
|
|
|
|
|
|
|
1,613,908
|
|
|
Average interest
rate(1)
|
|
|
6.50
|
%
|
|
|
|
|
|
|
|
|
|
Period of five months ended May 31, 2011 finance expenses
|
|
|
62,977
|
|
|
|
1.3721
|
|
|
|
45,898
|
|
|
2010 Annual finance expenses
|
|
|
151,146
|
|
|
|
1.3261
|
|
|
|
113,978
|
|
|
|
|
|
(1)
|
|
Assumed weighted average interest
rate on incremental debt. A 25 basis point increase in the
interest rate on the incremental debt would increase interest
expense on an annual basis by €4.4 million.
|
(b) Acquisition
related costs
Acquisition related costs are estimated as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols
|
|
|
Talecris
|
|
|
Total
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
Acquisition related costs
2010(2)
|
|
|
17,000
|
|
|
|
20,911
|
|
|
|
37,911
|
|
|
Acquisition related costs
2011(2)
|
|
|
38,607
|
|
|
|
16,756
|
|
|
|
55,363
|
|
|
Related with debt
issuance(1)
|
|
|
152,880
|
|
|
|
|
|
|
|
152,880
|
|
|
Related with capital increase and transaction related expenses
|
|
|
2,264
|
|
|
|
|
|
|
|
2,264
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total acquisition related costs considered in the pro formas
|
|
|
210,751
|
|
|
|
37,667
|
|
|
|
248,418
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The acquisition costs already
incurred in the year 2010 and in the six month period ended
June 30, 2011 amounts to €37.911 million (Grifols
€17.000 and Talecris $27.730 million
(€20.911 million)) and €55.363 million
(Grifols €38.607 and Talecris $22.991 million
(€16.756 million)) respectively were adjusted in the
unaudited pro forma
|
23
NOTES TO
UNAUDITED PRO FORMA CONDENSED COMBINED FINANCIAL
INFORMATION — (Continued)
|
|
|
|
|
|
condensed combined statement of
income for the year 2010 and six month period ended
June 30, 2011 to give the effect that the transaction
occurred on January 1, 2010.
|
| |
|
(1)
|
|
The acquisition costs related to
debt issuance are estimated based on the incremental debt
|
| |
|
(2)
|
|
These acquisition costs may relate
to the income statement; however, as they could represent
material non-recurring charges that result directly from the
transaction, these expenses have not been included in the
unaudited condensed combined pro forma statement of income.
|
Pro
forma adjustments to Talecris’ Unaudited Condensed Combined
Statement of Income
This adjustment includes the following items:
|
|
|
| |
•
|
Non-cash equity compensation expense of
€10.845 million in 2010 and €11.252 million
for the five month period ended May 31, 2011. The Merger
Agreement contemplated that, upon completion of the acquisition,
all the Talecris stock based awards would become fully vested
and be cancelled. As this is a material nonrecurring charge that
results directly from the transaction, these expenses have not
been included in the corresponding unaudited condensed combined
pro forma statements of income for the periods presented.
|
| |
| |
•
|
Compensation expense associated with special recognition bonus
awards and retention related costs including fringe benefits
granted to certain Talecris employees and senior executives of
€1.577 million in 2010 and €3.558 million
for the five-month period ended May 31, 2011 paid upon the
transaction closing. As this is a material nonrecurring charge
that results directly from the transaction, this expense has not
been included in the unaudited pro forma condensed combined
statement of income for the periods presented.
|
The tax effect on the pro forma adjustments has been calculated
using the U.S. statutory Federal income tax rate of 37.7%.
24
COMPARATIVE
MARKET PRICE AND DIVIDEND INFORMATION
Our share capital consists of two separate classes of shares:
Class A shares and Class B shares. The Class A
shares, comprised of 213,064,899 ordinary voting shares, have a
nominal value of €0.50 each. The Class B shares,
comprised of 83,811,688 non-voting shares, have a nominal value
of €0.10 each.
Our Class A shares have been listed on the Spanish Stock
Exchanges since May 2006 and are quoted on the Automated
Quotation System under the ticker symbol “GRF”.
Fluctuations in the exchange rate between the Euro and the
U.S. Dollar will affect the U.S. Dollar equivalent of
the Euro price of our Class A shares on the Spanish Stock
Exchanges.
Our Class B (non-voting) shares were issued and delivered
by us to holders of Talecris common stock as partial
consideration in connection with the acquisition on June 1,
2011. Our Class B shares have been traded on the NASDAQ
Global Select Market in the form of ADSs, evidenced by ADRs,
since June 2, 2011 under the ticker symbol
“GRFS.” Each ADS represents one-half (0.5) of one
Class B share.
Price
Range of Our Class A Shares
The following table sets forth the high and low closing prices,
in Euros, for our Class A shares for the periods indicated,
as reported on the Automated Quotation System:
| |
|
|
|
|
|
|
|
|
|
|
|
Class A Shares
|
|
|
|
|
High
|
|
|
Low
|
|
|
|
|
(Euros)
|
|
|
|
|
Fiscal Year 2006
|
|
|
|
|
|
|
|
|
|
Annual (from May 17, 2006)
|
|
|
10.08
|
|
|
|
4.99
|
|
|
Fiscal Year 2007
|
|
|
|
|
|
|
|
|
|
Annual
|
|
|
18.25
|
|
|
|
10.11
|
|
|
Fiscal Year 2008
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
|
16.63
|
|
|
|
13.28
|
|
|
Second Quarter
|
|
|
20.53
|
|
|
|
15.77
|
|
|
Third Quarter
|
|
|
20.53
|
|
|
|
17.67
|
|
|
Fourth Quarter
|
|
|
17.69
|
|
|
|
11.76
|
|
|
Fiscal Year 2009
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
|
14.29
|
|
|
|
10.30
|
|
|
Second Quarter
|
|
|
13.45
|
|
|
|
11.07
|
|
|
Third Quarter
|
|
|
13.19
|
|
|
|
11.92
|
|
|
Fourth Quarter
|
|
|
12.88
|
|
|
|
11.01
|
|
|
Fiscal Year 2010
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
|
12.44
|
|
|
|
10.12
|
|
|
Second Quarter
|
|
|
11.60
|
|
|
|
8.44
|
|
|
Third Quarter
|
|
|
10.93
|
|
|
|
8.32
|
|
|
Fourth Quarter
|
|
|
12.45
|
|
|
|
8.11
|
|
|
Fiscal Year 2011
|
|
|
|
|
|
|
|
|
|
First Quarter
|
|
|
12.49
|
|
|
|
9.85
|
|
|
April
|
|
|
13.48
|
|
|
|
12.12
|
|
|
May
|
|
|
14.44
|
|
|
|
13.18
|
|
|
June
|
|
|
14.49
|
|
|
|
12.89
|
|
|
July
|
|
|
15.30
|
|
|
|
13.81
|
|
|
August
|
|
|
15.80
|
|
|
|
12.70
|
|
|
September
|
|
|
14.61
|
|
|
|
13.24
|
|
|
October (through October 21)
|
|
|
14.50
|
|
|
|
13.44
|
|
25
Price
Range of the ADSs Representing Our Class B Shares
The following table sets forth the high and low closing prices,
in U.S. dollars, for the ADSs representing our Class B
shares for the periods indicated, as reported by NASDAQ:
| |
|
|
|
|
|
|
|
|
|
|
|
Class B
|
|
|
|
ADSs
|
|
|
|
High
|
|
Low
|
|
|
|
(U.S. Dollars)
|
|
|
|
Fiscal Year 2011
|
|
|
|
|
|
|
|
|
|
June (from June
2nd)
|
|
|
8.50
|
|
|
|
6.85
|
|
|
July
|
|
|
8.16
|
|
|
|
7.24
|
|
|
August
|
|
|
8.07
|
|
|
|
6.33
|
|
|
September
|
|
|
6.99
|
|
|
|
5.30
|
|
|
October (through October 21)
|
|
|
6.75
|
|
|
|
6.00
|
|
Dividends
Class A
Shares
The following table sets forth the dividends per Class A
share paid by us with respect to the fiscal years ended
December 31, 2009 and December 31, 2008. We have
recently paid ordinary annual dividends to our stockholders.
Interim dividends are normally declared and paid by our Board of
Directors on account of the results of the then-current fiscal
year.
Cash dividends are paid by us in euros, and exchange rate
fluctuations between the euro and the U.S. dollar will
affect the U.S. dollar amounts received by holders of our
new ADSs.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross per Share
|
|
Net per Share
|
|
Year
|
|
Type
|
|
Payment Date
|
|
Amount
|
|
Amount(1)
|
|
|
|
|
|
|
|
(Euros)
|
|
(Euros)
|
|
|
|
2009
|
|
Ordinary
|
|
July 1, 2010
|
|
|
0.12789208
|
|
|
|
0.10359258
|
|
|
|
|
Interim
|
|
December 18, 2009
|
|
|
0.15305538
|
|
|
|
0.12550541
|
|
|
2008
|
|
Ordinary
|
|
July 2, 2009
|
|
|
0.23209553
|
|
|
|
0.19031833
|
|
|
|
|
|
(1)
|
|
Net of Spanish withholding tax. The
Spanish withholding tax rate was 18% in 2008 and 2009 and 19% in
2010.
|
As of May 24, 2011, the most recent date for which it was
practicable to obtain this information, there were 21,731
registered holders of our Class A shares.
The terms of our Senior Credit Facilities contain limitations on
our ability to pay ordinary dividends going forward, and we do
not expect to be able to declare dividends in cash at historical
levels for some period of time following the completion of the
acquisition. For a further discussion of the terms of our Senior
Credit Facilities, see “Management’s Discussion and
Analysis of Financial Condition and Results of
Operations — Liquidity and Capital Resources.”
Class B
Shares
We have not issued a dividend to our Class B stockholders
since we issued our Class B shares on June 1, 2011.
Each Class B share entitles its holder to receive a minimum
annual preferred dividend out of the distributable profits at
the end of each fiscal year the share is outstanding equal to
€0.01 per Class B share. In any given fiscal year, we
will pay a preferred dividend to the holders of the Class B
shares before any dividend out of the distributable profits for
such fiscal year is paid to the holders of Class A shares.
The preferred dividend on all issued Class B shares will be
paid by us within the nine months following the end of that
fiscal year, in an amount not to exceed the distributable
profits obtained during that fiscal year.
26
EXCHANGE
RATES
The following tables show, for the periods indicated, the
exchange rate between the U.S. Dollar and the Euro. This
information is provided solely for your information and we do
not represent that Euros could be converted into
U.S. Dollars at these rates or at any other rate, during
the periods indicated or at any other time. These rates are not
the rates used by us in the preparation of our consolidated
financial statements included in this prospectus.
As used in this prospectus, the term “Noon Buying
Rate” refers to the rate of exchange for euros, expressed
in U.S. dollars per euro, in the City of New York for cable
transfers payable in foreign currencies as certified by the
Federal Reserve Bank of New York for customs purposes. The Noon
Buying Rate certified by the New York Federal Reserve Bank for
the euro on October 14, 2011 was $1.3861 = €1.00. The
following tables describe, for the periods and dates indicated,
information concerning the Noon Buying Rate for the Euro.
Amounts are expressed in U.S. Dollars per €1.00.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Period
|
|
Average
|
|
|
|
|
|
Annual Data (Year Ended December 31,)
|
|
End ($)
|
|
Rate ($)(1)
|
|
High ($)
|
|
Low ($)
|
|
|
|
2006
|
|
|
1.3197
|
|
|
|
1.2661
|
|
|
|
1.3327
|
|
|
|
1.1860
|
|
|
2007
|
|
|
1.4603
|
|
|
|
1.3797
|
|
|
|
1.4862
|
|
|
|
1.2904
|
|
|
2008
|
|
|
1.3919
|
|
|
|
1.4698
|
|
|
|
1.6010
|
|
|
|
1.2446
|
|
|
2009
|
|
|
1.4332
|
|
|
|
1.3955
|
|
|
|
1.5100
|
|
|
|
1.2547
|
|
|
2010
|
|
|
1.3269
|
|
|
|
1.3261
|
|
|
|
1.4536
|
|
|
|
1.1959
|
|
|
Interim Data (Six Months Ended June 30,)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
1.2291
|
|
|
|
1.3183
|
|
|
|
1.4536
|
|
|
|
1.1959
|
|
|
2011
|
|
|
1.4523
|
|
|
|
1.4211
|
|
|
|
1.4875
|
|
|
|
1.2944
|
|
Source: Federal Reserve Bank of New York
|
|
|
|
(1) |
|
The average of the Noon Buying Rates for the euro on the last
day reported of each month during the relevant period. |
| |
|
|
|
|
|
|
|
|
|
Recent Monthly Data
|
|
High ($)
|
|
Low ($)
|
|
|
|
March 2011
|
|
|
1.4212
|
|
|
|
1.3813
|
|
|
April 2011
|
|
|
1.4821
|
|
|
|
1.4211
|
|
|
May 2011
|
|
|
1.4875
|
|
|
|
1.4015
|
|
|
June 2011
|
|
|
1.4675
|
|
|
|
1.4155
|
|
|
July 2011
|
|
|
1.4508
|
|
|
|
1.4014
|
|
|
August 2011
|
|
|
1.4510
|
|
|
|
1.4158
|
|
|
September 2011
|
|
|
1.4283
|
|
|
|
1.3518
|
|
|
October 2011 (through October 14)
|
|
|
1.3861
|
|
|
|
1.3281
|
|
27
SUMMARY
OF THE EXCHANGE OFFER
In this prospectus, except as otherwise indicated,
“existing notes” refers to our 8.25% Senior Notes
due 2018 issued on January 21, 2011, “exchange
notes” refers to the 8.25% Senior Notes due 2018
offered hereby and “notes” refers to the existing
notes and the exchange notes, collectively.
The following summary contains basic information about the
exchange offer and the exchange notes. It does not contain all
the information that may be important to you. For a complete
understanding of the exchange notes, please refer to the
sections of this prospectus entitled “The Exchange
Offer” and “Description of the Notes.”
|
|
|
|
The Offering of the Exchange Notes |
|
The existing notes were originally issued by Giant Funding Corp.
(the “Escrow Corp.”), an escrow company formed solely
for the purpose of issuing the existing notes, on
January 21, 2011 in an offering not registered under the
Securities Act. On June 1, 2011, upon consummation of the
acquisition, Escrow Corp. was merged with and into the Issuer,
and the Issuer assumed all of Escrow Corp.’s obligations
under the existing notes and the indenture governing the
existing notes. In addition, upon consummation of the
acquisition, the Issuer, the Parent Guarantor (as defined below)
and the initial Subsidiary Guarantors (as defined below)
executed joinders to a registration rights agreement that had
been entered into by Escrow Corp. and the initial purchasers of
the existing notes on January 21, 2011. Pursuant to the
registration rights agreement, the Issuer, the Parent Guarantor
and the Subsidiary Guarantors party thereto agreed, for the
benefit of the holders of the existing notes, at our cost, to
file the registration statement of which this prospectus forms a
part and to complete an exchange offer for the existing notes.
This exchange offer is intended to satisfy that obligation. |
| |
|
The Exchange Offer |
|
We are offering to exchange the exchange notes that have been
registered under the Securities Act for the existing notes. As
of this date, there is an aggregate principal amount of
$1,100,000,000 of our existing notes outstanding. |
| |
|
Required Representations |
|
In order to participate in this exchange offer, you will be
required to make certain representations to us in a letter of
transmittal, including that: |
| |
|
|
|
• any exchange notes will be acquired by you in the
ordinary course of your business;
|
| |
|
|
|
• you have not engaged in and do not intend to engage
in, and do not have an arrangement or understanding with any
person to participate in, a distribution of the exchange notes;
and
|
| |
|
|
|
• you are not an “affiliate” of our company
or any of our subsidiaries, as that term is defined in
Rule 405 of the Securities Act.
|
| |
|
|
|
If our belief is inaccurate and you transfer any exchange note
issued to you in the exchange offer without delivering a
prospectus meeting the requirements of the Securities Act or
without an exemption from registration of your exchange notes
from such requirements, you may incur liability under the
Securities Act. We do not assume, or indemnify you against, any
such liability. The SEC has not considered this exchange offer
in the context of a no-action letter, and we cannot be sure that
the SEC would make the |
28
|
|
|
|
|
|
same determination with respect to this exchange offer as it has
in other circumstances. |
| |
|
|
|
Each broker-dealer that is issued exchange notes for its own
account in exchange for existing notes that were acquired by
such broker-dealer as a result of market-making or other trading
activities also must acknowledge that it has not entered into
any arrangement or understanding with us or any of our
affiliates to distribute the exchange notes and will deliver a
prospectus meeting the requirements of the Securities Act in
connection with any resale of the exchange notes issued in the
exchange offer. |
| |
|
|
|
We have agreed in the registration rights agreement that a
broker-dealer may use this prospectus for an offer to resell,
resale or other retransfer of the exchange notes issued to it in
the exchange offer. |
| |
|
Expiration Date |
|
The exchange offer will expire at 5:00 p.m., New York City
time,
on ,
2011, unless extended, in which case the term “expiration
date” shall mean the latest date and time to which we
extend the exchange offer. |
| |
|
Conditions to the Exchange Offer |
|
The exchange offer is subject to certain customary conditions,
which may be waived by us. The exchange offer is not conditioned
upon any minimum principal amount of existing notes being
tendered. |
| |
|
Procedures for Tendering Existing Notes |
|
If you wish to tender existing notes, you must (a)(1) complete,
sign and date the letter of transmittal, or a facsimile of it,
according to its instructions and (2) send the letter of
transmittal, together with your existing notes to be exchanged
and other required documentation, to the Exchange Agent (as
defined below) at the address provided in the letter of
transmittal; or (b) tender through the Depository Trust
Company (“DTC”) pursuant to DTC’s Automated
Tender Offer Program, or ATOP system. The letter of transmittal
or a valid agent’s message through ATOP must be received by
the Exchange Agent by 5:00 p.m., New York City time,
on the expiration date. See “The Exchange Offer —
Procedures for Tendering,” and “— Book-Entry
Tender.” By executing the letter of transmittal, you are
representing to us that you are acquiring the exchange notes in
the ordinary course of your business, that you are not
participating, do not intend to participate and have no
arrangement or understanding with any person to participate in
the distribution of exchange notes, and that you are not an
“affiliate” of ours. See “The Exchange
Offer — Procedures for Tendering,” and
“— Book-Entry Tender.” |
| |
|
|
|
Do not send letters of transmittal and certificates representing
existing notes to us. Send these documents only to the Exchange
Agent. See “The Exchange Offer — Procedures for
Tendering” for more information. |
| |
|
Special Procedures for Beneficial Owners |
|
If you are the beneficial owner of book-entry interests and your
name does not appear on a security position listing of DTC as
the holder of the book-entry interests or if you are a
beneficial owner whose existing notes are registered in the name
of a broker, dealer, |
29
|
|
|
|
|
|
commercial bank, trust company or other nominee, and you wish to
tender your existing notes in the exchange offer, you should
contact the registered holder promptly and instruct the
registered holder to tender on your behalf. If you are a
beneficial owner and wish to tender on your own behalf, you
must, before completing and executing the letter of transmittal
and delivering your existing notes, either make appropriate
arrangements to register ownership of the existing notes in your
name or obtain a properly completed bond power from the
registered holder. See “The Exchange Offer —
Procedure if the Existing Notes Are Not Registered in Your
Name,” and “— Beneficial Owner Instructions
to Holders of Existing Notes.” The transfer of registered
ownership may take considerable time and may not be possible to
complete before the expiration date. |
| |
|
Guaranteed Delivery Procedures |
|
If you wish to tender existing notes and time will not permit
the documents required by the letter of transmittal to reach the
exchange agent prior to the expiration date, or the procedure
for book-entry transfer cannot be completed on a timely basis,
you must tender your existing notes according to the guaranteed
delivery procedures described under “The Exchange
Offer — Guaranteed Delivery Procedures.” |
| |
|
Acceptance of Existing Notes and Delivery of Exchange
Notes |
|
Subject to the conditions described under “The Exchange
Offer — Conditions,” we will accept for exchange
any and all existing notes which are validly tendered in the
exchange offer and not withdrawn, prior to 5:00 p.m., New
York City time, on the expiration date. |
| |
|
Interest on Existing Notes |
|
Interest will not be paid on existing notes that are tendered
and accepted for exchange in the exchange offer. |
| |
|
Withdrawal Rights |
|
You may withdraw your tender of existing notes at any time prior
to 5:00 p.m., New York City time, on the expiration date,
subject to compliance with the procedures for withdrawal
described in this prospectus under the heading “The
Exchange Offer — Withdrawal of Tenders.” |
| |
|
Federal Income Tax Consequences |
|
For a discussion of the material federal income tax
considerations relating to the exchange of existing notes for
the exchange notes as well as the ownership of the exchange
notes, see “Certain Material United States Federal Income
Tax Considerations.” |
| |
|
Exchange Agent |
|
The Bank of New York Mellon Trust Company, N.A. is serving
as the exchange agent (the “Exchange Agent”). The
address, telephone number and facsimile number of the exchange
agent are set forth in this prospectus under the heading
“The Exchange Offer — Exchange Agent.” The
Bank of New York Mellon Trust Company, N.A. is also the
trustee under the indenture, as supplemented, among the Issuer,
the Parent Guarantor, the Subsidiary Guarantors party thereto
and The Bank of New York Mellon Trust Company, N.A.
governing the notes, as described under “Description of the
Notes.” |
30
|
|
|
|
Consequences of Failure to Exchange the Existing Notes |
|
If you do not exchange existing notes for exchange notes, you
will continue to be subject to the restrictions on transfer
provided in the existing notes and in the indenture governing
the existing notes. In general, the unregistered existing notes
may not be offered or sold, unless they are registered under the
Securities Act, except pursuant to an exemption from, or in a
transaction not subject to, the Securities Act and applicable
state securities laws. |
| |
|
|
|
In addition, after the consummation of the exchange offer, it is
anticipated that the outstanding principal amount of the
existing notes available for trading will be significantly
reduced. The reduced float will adversely affect the liquidity
and market price of the existing notes. A smaller outstanding
principal amount at maturity of existing notes available for
trading may also tend to make the price more volatile. |
| |
|
Use of Proceeds |
|
We will not receive any proceeds from the issuance of the
exchange notes in exchange for the existing notes. |
| |
|
Fees and Expenses |
|
We will pay all fees and expenses related to this exchange offer. |
31
SUMMARY
OF THE TERMS OF THE EXCHANGE NOTES
The summary below describes the principal terms of the
exchange notes. Certain of the terms described below are subject
to important limitations and exceptions. See the section
entitled “Description of the Notes” of this prospectus
for a more detailed description of the terms of the notes,
including the exchange notes, and the indenture governing the
notes.
|
|
|
|
Issuer |
|
Grifols Inc., a Virginia corporation (the “Issuer”). |
| |
|
Securities Offered |
|
$1,100,000,000 aggregate principal amount of 8.25% senior
notes due 2018. |
| |
|
Maturity Date |
|
February 1, 2018. |
| |
|
Interest Rate |
|
8.25% per year. |
| |
|
Interest Payment Dates |
|
February 1 and August 1, beginning on February 1, 2012. |
| |
|
Guarantees |
|
The exchange notes will be fully and unconditionally guaranteed
on a joint and several senior unsecured basis by Grifols, S.A.,
a company incorporated under the laws of the Kingdom of Spain
(the “Parent Guarantor”) and each of the Parent
Guarantor’s existing and future direct or indirect
subsidiaries that guarantee our Senior Credit Facilities (other
than any foreign subsidiary of Grifols Inc.) (each a
“Subsidiary Guarantor” and together with the Parent
Guarantor, the “Guarantors”). On the issue date of the
exchange notes, the Subsidiary Guarantors will consist of:
Grifols Biologicals Inc., Biomat USA, Inc., Grifols Therapeutics
Inc., Talecris Plasma Resources, Inc., Instituto Grifols, S.A.,
Diagnostic Grifols, S.A., Movaco, S.A., Laboratorios Grifols,
S.A., Grifols Italia, S.p.A., and Grifols Deutschland GmbH. |
| |
|
Ranking |
|
The exchange notes will be senior unsecured obligations of the
Issuer and will: |
| |
|
|
|
• rank equally in right of payment to all of the
Issuer’s existing and future senior indebtedness;
|
| |
|
|
|
• be effectively subordinated in right of payment to
the Issuer’s secured indebtedness (including its
obligations under our Senior Credit Facilities), to the extent
of the value of the collateral securing such indebtedness; and
|
| |
|
|
|
• be structurally subordinated to all existing and
future liabilities of each non-guarantor subsidiary of the
Parent Guarantor.
|
| |
|
|
|
The guarantees will be the senior unsecured obligations of the
Guarantors and will: |
| |
|
|
|
• rank equally in right of payment to all existing and
future senior indebtedness of the Guarantors;
|
| |
|
|
|
• be effectively subordinated in right of payment to
the Guarantors’ secured indebtedness (including their
obligations under our Senior Credit Facilities), to the extent
of the value of the collateral securing such indebtedness; and
|
| |
|
|
|
• be structurally subordinated to all existing and
future liabilities of each non-guarantor subsidiary of Parent
Guarantor.
|
32
|
|
|
|
Optional Redemption |
|
The Issuer may redeem some or all of the exchange notes at any
time prior to February 1, 2014 at a price equal to 100% of
the principal amount of the exchange notes plus a
“make-whole” premium as set forth under
“Description of the Notes — Optional
Redemption.” Additionally, the Issuer may redeem the
exchange notes, in whole or in part, at any time on and after
February 1, 2014 at the redemption prices set forth under
“Description of the Notes — Optional
Redemption.” |
| |
|
Optional Redemption After Equity Offerings |
|
The Issuer may redeem up to 35% of the notes with money raised
in one or more equity offerings by the Parent Guarantor at any
time (which may be more than once) prior to February 1,
2014, as long as at least 65% of the aggregate principal amount
of notes issued under the indenture remains outstanding
immediately following any such offerings. See “Description
of the Notes — Optional Redemption.” |
| |
|
Change of Control Offer |
|
If we experience a change of control, the Issuer must give
holders of the exchange notes the opportunity to sell the Issuer
their exchange notes at 101% of their face amount, plus accrued
and unpaid interest. See “Description of the
Notes — Repurchase at the Option of
Holders — Change of Control.” |
| |
|
Certain Indenture Provisions |
|
The exchange notes will be, and the existing notes are, governed
by an indenture containing covenants that, among other things,
restrict the Parent Guarantor’s ability and the ability of
its restricted subsidiaries, including the Issuer, to: |
| |
|
|
|
• pay dividends or make certain other restricted
payments or investments;
|
| |
|
|
|
• incur additional indebtedness or provide guarantees
of indebtedness and issue disqualified stock;
|
| |
|
|
|
• create liens on assets;
|
| |
|
|
|
• merge, consolidate or sell all or substantially all
of our and our restricted subsidiaries’ assets;
|
| |
|
|
|
• enter into certain transactions with affiliates;
|
| |
|
|
|
• create restrictions on dividends or other payments
by our restricted subsidiaries; and
|
| |
|
|
|
• create guarantees of indebtedness by restricted
subsidiaries.
|
| |
|
|
|
These covenants are subject to a number of important limitations
and exceptions. See “Description of the Notes —
Certain Covenants.” |
| |
|
No Prior Market for Exchange Notes |
|
The exchange notes will be new securities for which there is no
market. We cannot assure you a liquid market for the exchange
notes will develop or be maintained. |
33
RISK
FACTORS
You should carefully consider each of the following risk
factors and all of the other information set forth in this
prospectus prior to participating in the exchange offer. Any of
the following risks could materially and adversely affect our
business, financial condition or results of operations. They are
not, however, the only risks we face. Additional risks and
uncertainties not presently known to us or that we currently
believe not to be material may also adversely affect our
business, financial condition or results of operations. If that
were to occur, the trading price of the notes would likely
decline and we may not be able to make payments of interest and
principal on the notes, and you may lose all or part of your
original investment.
Risks
Related to the Exchange Offer
If you do
not properly tender your existing notes, you will continue to
hold unregistered existing notes and your ability to transfer
existing notes will continue to be subject to any applicable
transfer restrictions, which may adversely affect their market
price.
If you do not properly tender your existing notes for exchange
notes in the exchange offer, you will continue to be subject to
any applicable restrictions on the transfer of your existing
notes. In general, the existing notes may not be offered or sold
unless they are registered under the Securities Act, as well as
applicable state securities laws, or the offer or sale is exempt
from registration thereunder. We do not intend to register
resales of the existing notes under the Securities Act. You
should refer to “The Exchange Offer — Procedures
For Tendering” for information about how to tender your
existing notes. The tender of existing notes under the exchange
offer will reduce the outstanding amount of the existing notes,
which may have an adverse effect upon, and increase the
volatility of, the market prices of the existing notes due to a
reduction in liquidity.
Lack of
an active market for the exchange notes may adversely affect the
liquidity and market price of the exchange notes.
There is no existing market for the exchange notes. We do not
intend to apply for a listing of the exchange notes on any
securities exchange. We do not know if an active public market
for the exchange notes will develop or, if developed, will
continue. If an active public market does not develop or is not
maintained, the market price and liquidity of the exchange notes
may be adversely affected. We cannot make any assurances
regarding the liquidity of the market for the exchange notes,
the ability of holders to sell their exchange notes or the price
at which holders may sell their exchange notes. Further, the
liquidity and the market price of the exchange notes may be
adversely affected by changes in the overall market for
securities similar to the exchange notes, by changes in our
business, financial condition or results of operations and by
changes in conditions in our industry. In addition, if a large
amount of existing notes are not tendered or are tendered
improperly, the limited amount of exchange notes that would be
issued and outstanding after we consummate the exchange offer
could adversely affect the market price of such exchange notes.
The
market price for the exchange notes may be volatile.
Historically, the market for non-investment grade debt has been
subject to disruptions that have caused substantial volatility
in the prices of securities similar to the exchange notes
offered hereby. The market for the exchange notes, if any, may
be subject to similar disruptions and any such disruptions may
adversely affect the prices at which you may sell your exchange
notes. In addition, once issued, the exchange notes may trade at
a discount from the initial offering price of the existing
notes, depending upon prevailing interest rates, the market for
similar notes, our performance and other factors.
The
issuance of the exchange notes may adversely affect the market
for the existing notes.
To the extent the existing notes are tendered and accepted in
the exchange offer, the trading market for the untendered and
tendered but unaccepted existing notes could be adversely
affected. Because we anticipate that most holders of the
existing notes will elect to exchange their existing notes for
exchange notes due to the
34
absence of restrictions on the resale of exchange notes under
the Securities Act, we anticipate that the liquidity of the
market for any existing notes remaining after the completion of
this exchange offer may be substantially limited. Please refer
to the section in this prospectus entitled “The Exchange
Offer — Consequences of Failure to Exchange.”
Late
deliveries of existing notes and other required documents could
prevent you from exchanging your existing notes.
Holders are responsible for complying with all procedures of the
exchange offer. The issuance of exchange notes in exchange for
existing notes will occur only upon completion of the procedures
described in “The Exchange Offer — Procedures For
Tendering”. Therefore, holders of existing notes who wish
to exchange them for exchange notes should allow sufficient time
for timely completion of the exchange procedures. Neither we nor
the exchange agent are obligated to extend the exchange offer or
notify you of any failure to follow the proper procedures or
waive any defect if you fail to follow the proper procedures.
If you
are a broker-dealer, your ability to transfer the exchange notes
may be restricted.
A broker-dealer that purchased existing notes for its own
account as part of market making or trading activities must
comply with the prospectus delivery requirements of the
Securities Act when it sells the exchange notes. Our obligation
to make this prospectus available to broker-dealers is limited.
Consequently, we cannot guarantee that a current prospectus will
be available to broker-dealers wishing to resell their exchange
notes.
Risks
Related to the Notes
Our
substantial level of indebtedness could adversely affect our
financial condition, restrict our ability to react to changes to
our business, and prevent us from fulfilling our obligations
under our debt.
As a result of the acquisition and related financing
transactions we are a highly leveraged company. Our substantial
level of indebtedness increases the risk that we may be unable
to generate sufficient cash to pay amounts due in respect to our
indebtedness. As of June 30, 2011, we had $1.1 billion
in aggregate principal amount of the notes, $2.5 billion
and €440 million of indebtedness outstanding under our
Senior Term Loans and the ability to borrow an additional
$50 million, €36.7 million and the
$200 million equivalent in multicurrencies under our
Revolving Credit Facilities. In addition, as of June 30,
2011, we had €93.1 million of indebtedness under other
bank loans and €37.6 in capital lease obligations. See the
section in this prospectus entitled “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations — Liquidity and Capital Resources” for
more detailed information.
Our high level of indebtedness could have significant adverse
effects on our business, including the following:
|
|
|
| |
•
|
our high level of indebtedness makes it more difficult for us to
satisfy our obligations with respect to the notes;
|
| |
| |
•
|
our high level of indebtedness makes us more vulnerable to
economic downturns and adverse developments in our business;
|
| |
| |
•
|
our ability to obtain additional financing for working capital,
capital expenditures, acquisitions or general corporate purposes
may be impaired;
|
| |
| |
•
|
we must use a substantial portion of our cash flow from
operations to pay interest on the notes and our other
indebtedness, which will reduce the funds available to us for
operations and other purposes;
|
| |
| |
•
|
all of the indebtedness outstanding under our purchase money
indebtedness, equipment financing and real estate mortgages will
have a prior ranking claim on the underlying assets;
|
| |
| |
•
|
our ability to fund a change of control offer may be limited;
|
35
|
|
|
| |
•
|
our high level of indebtedness could place us at a competitive
disadvantage compared to our competitors that may have
proportionately less debt;
|
| |
| |
•
|
our flexibility in planning for, or reacting to, changes in our
business and the industry in which we operate may be
limited; and
|
| |
| |
•
|
we may be restricted from making strategic acquisitions or
exploiting other business opportunities.
|
We expect to use cash flow from operations to pay our expenses,
amounts due under the notes and our other outstanding
indebtedness. Our ability to make these payments depends on our
future performance, which will be affected by financial,
business, economic and other factors, many of which we cannot
control. Our business may not generate sufficient cash flow from
operations in the future and our anticipated growth in revenue
and cash flow may not be realized, either or both of which could
result in our being unable to repay indebtedness, including the
notes, or to fund other liquidity needs. If we do not have
enough money, we may be required to refinance all or part of our
then-existing debt (including the notes), sell assets or borrow
more money. We may not be able to accomplish any of these
alternatives on terms acceptable to us, or at all. In addition,
the terms of existing or future debt agreements, including the
indenture governing the notes, may restrict us from adopting any
of these alternatives. The failure to generate sufficient cash
flow or to achieve any of these alternatives could materially
and adversely affect the value of the notes and our ability to
pay the amounts due under the notes.
Despite
our substantial indebtedness, we may still incur significantly
more debt. This could exacerbate the risks associated with our
substantial leverage.
We may be able to incur significant additional indebtedness in
the future. Although the indenture that governs the notes and
the Senior Credit Facilities contain restrictions on the
incurrence of additional indebtedness, these restrictions are
subject to a number of qualifications and exceptions and the
additional indebtedness incurred in compliance with these
exceptions could be substantial. In addition, as of
June 30, 2011, we had $50 million,
€36.7 million and the $200 million equivalent in
multicurrencies available for additional borrowing under our
Revolving Credit Facilities. If we incur additional
indebtedness, the risks related to our business associated with
our high level of debt could intensify. For more information
regarding our indebtedness, see the section in this prospectus
entitled “Management’s Discussion and Analysis of
Financial Condition and Results of Operations
— Liquidity and Capital Resources.”
To service our indebtedness and other obligations, we will
require a significant amount of cash. Our ability to generate
cash depends on many factors beyond our control.
Our ability to make payments on or to refinance our
indebtedness, including the notes, and to fund working capital
needs and planned capital expenditures will depend on our
ability to generate cash in the future. A significant reduction
in our operating cash flows resulting from changes in economic
conditions, increased competition or other events beyond our
control could increase the need for additional or alternative
sources of liquidity and could have a material adverse effect on
our business, financial condition, results of operations and
prospects and our ability to service our debt and other
obligations. If we are unable to service our indebtedness, we
will be forced to adopt an alternative strategy that may include
actions such as reducing capital expenditures, selling assets,
restructuring or refinancing our indebtedness or seeking
additional equity capital. We cannot assure you that any of
these alternative strategies could be affected on satisfactory
terms, if at all, or that they would yield sufficient funds to
make required payments on the notes and our other indebtedness.
We cannot assure you that our business will generate sufficient
cash flows from operations or that future borrowings will be
available to us under the Senior Credit Facilities or otherwise
in an amount sufficient to enable us to pay our indebtedness,
including the notes, or to fund our other liquidity needs. We
may need to refinance all or a portion of our indebtedness,
including the notes, on or before the maturity of the debt. We
cannot assure you that we will be able to refinance any of our
indebtedness, including our Senior Credit Facilities and the
notes, on commercially reasonable terms or at all.
36
If we
default on our obligations to pay our indebtedness, we may not
be able to make payments on the notes.
Any default under the agreements governing our indebtedness,
including a default under our Senior Credit Facilities, that is
not waived by the required lenders, and the remedies sought by
the holders of such indebtedness, could prevent us from paying
principal, premium, if any, and interest on the notes and
substantially decrease the market value of the notes. If we are
unable to generate sufficient cash flow and are otherwise unable
to obtain funds necessary to meet required payments of
principal, premium, if any, and interest on our indebtedness, or
if we otherwise fail to comply with the various covenants,
including financial and operating covenants, in the instruments
governing our indebtedness (including covenants in our Senior
Credit Facilities and the indenture governing the notes), we
could be in default under the terms of the agreements governing
such indebtedness. In the event of such default, the holders of
such indebtedness could elect to declare all the funds borrowed
thereunder to be due and payable, together with accrued and
unpaid interest, the lenders under our Senior Credit Facilities
could elect to terminate their commitments thereunder, cease
making further loans and institute foreclosure proceedings
against our assets, and we could be forced into bankruptcy or
liquidation. If our operating performance declines, we may in
the future need to obtain waivers from the required lenders
under the Senior Credit Facilities to avoid being in default. If
we breach our covenants under the Senior Credit Facilities and
seek a waiver, we may not be able to obtain a waiver from the
required lenders. If this occurs, we would be in default under
our Senior Credit Facilities, the lenders could exercise their
rights as described above, and we could be forced into
bankruptcy or liquidation.
The notes
and the guarantees will be unsecured and effectively
subordinated to the Issuer and the Guarantors’ existing and
future secured indebtedness.
The notes and the guarantees will be general unsecured
obligations ranking effectively junior in right of payment to
all of the existing and future secured indebtedness of the
Issuer and each Guarantor, including indebtedness under the
Senior Credit Facilities. Additionally, the indenture governing
the notes permits the Issuer and the Guarantors to incur
additional secured indebtedness in the future. In the event that
the Issuer or any Guarantor is declared bankrupt, becomes
insolvent or is liquidated or reorganized, any indebtedness that
is effectively senior to the notes and the guarantees will be
entitled to be paid in full from the assets of the Issuer or the
Guarantors, as applicable, securing such indebtedness before any
payment may be made with respect to the notes or the affected
guarantees. Holders of the notes will participate ratably with
all holders of our unsecured indebtedness that is deemed to be
of the same class as the notes, and potentially with all of our
other general creditors, based upon the respective amounts owed
to each holder or creditor, in our remaining assets.
As of June 30, 2011, the notes and the guarantees are
effectively subordinated to $2.5 billion and
€440 million of senior secured indebtedness under the
Senior Term Loans (excluding $50 million,
€36.7 million and the $200 million equivalent in
multicurrencies of undrawn revolving commitments under our
Revolving Credit Facilities).
Claims of
note holders will be structurally subordinated to claims of
creditors of the Parent Guarantor’s subsidiaries that do
not guarantee the notes.
The notes are not and in the future may not be guaranteed by all
of the Parent Guarantor’s subsidiaries. For example, the
Parent Guarantor’s subsidiaries that do not guarantee the
Senior Credit Facilities and any foreign subsidiary of Grifols
Inc. are not required to guarantee the notes. Accordingly,
claims of holders of the notes will be structurally subordinated
to the claims of creditors of these non-guarantor subsidiaries,
including trade creditors. All obligations of non-guarantor
subsidiaries will have to be satisfied before any of the assets
of these subsidiaries would be available for distribution, upon
liquidation or otherwise, to the Issuer or a Guarantor of the
notes. Furthermore, the guarantees are subject to release under
certain circumstances. In the event of the liquidation,
dissolution, reorganization, bankruptcy or similar proceeding of
the business of a subsidiary that is not a Guarantor, creditors
of that subsidiary would generally have the right to be paid in
full before any distribution is made to us or the holders of the
notes. In any of these events, we may not have sufficient assets
to pay amounts due on the notes with respect to the assets of
that subsidiary.
37
Covenants
in our debt agreements restrict our business in many
ways.
The indenture governing the notes and the Senior Credit
Facilities contain various covenants that limit our ability to,
among other things:
|
|
|
| |
•
|
incur or assume liens or additional debt or provide guarantees
in respect of obligations of other persons;
|
| |
| |
•
|
issue redeemable stock and preferred equity;
|
| |
| |
•
|
pay dividends or distributions or redeem or repurchase capital
stock;
|
| |
| |
•
|
prepay, redeem or repurchase debt;
|
| |
| |
•
|
make loans, investments and capital expenditures;
|
| |
| |
•
|
enter into agreements that restrict distributions from our
subsidiaries;
|
| |
| |
•
|
sell assets and capital stock of our subsidiaries;
|
| |
| |
•
|
enter into certain transactions with affiliates; and
|
| |
| |
•
|
consolidate or merge with or into, or sell substantially all of
our assets to, another person.
|
A breach of any of these covenants could result in a default
under our Senior Credit Facilities
and/or the
indenture governing the notes. Upon the occurrence of an event
of default under the Senior Credit Facilities, the lenders could
elect to declare all amounts outstanding under the Senior Credit
Facilities to be immediately due and payable and terminate all
commitments to extend further credit. If we were unable to repay
those amounts, the lenders could proceed against the collateral
granted to them to secure that indebtedness. We have pledged a
significant portion of our assets as collateral under the Senior
Credit Facilities. If the lenders under the Senior Credit
Facilities accelerate the repayment of borrowings, we may not
have sufficient assets to repay the Senior Credit Facilities and
our other indebtedness, including the notes. Our borrowings
under the Senior Credit Facilities are at variable rates of
interest and expose us to interest rate risk. If interest rates
increase, our debt service obligations on the variable rate
indebtedness would increase even though the amount borrowed
remained the same, and our net income would decrease. See the
section in this prospectus entitled “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations — Liquidity and Capital Resources” for
more detailed information.
We may
not be able to satisfy our obligations to holders of the notes
upon a change of control or sale of assets.
Upon the occurrence of a change of control, as defined in the
indenture governing the notes, we will be required to offer to
purchase the notes at a price equal to 101% of the principal
amount of such notes, together with any accrued and unpaid
interest, to the date of purchase. See “Description of the
Notes — Repurchase at the Option of
Holders — Change of Control.”
Upon the occurrence of an asset sale, as defined in the
indenture, we will be required to offer to purchase the notes at
a price equal to 100% of the principal amount of such notes,
together with any accrued and unpaid interest, to the date of
purchase. See “Description of the Notes —
Repurchase at the Option of Holders — Asset
Sales.”
We cannot assure you that, if a change of control offer or asset
sale offer is made, we will have available funds sufficient to
pay the change of control purchase price or asset sale purchase
price for any or all of the notes that might be delivered by
holders of the notes seeking to accept the change of control
offer or asset sale offer. If we are required to purchase notes
pursuant to a change of control offer or asset sale offer, we
would be required to seek third-party financing to the extent we
do not have available funds to meet our purchase obligations.
There can be no assurance that we will be able to obtain such
financing on acceptable terms to us or at all. Accordingly, none
of the holders of the notes may receive the change of control
purchase price or asset sale purchase price for their notes. Our
failure to make or consummate the change of control offer or
38
asset sale offer, or to pay the change of control purchase price
or asset sale purchase price when due, will give the holders of
the notes the rights described in “Description of the
Notes — Events of Default and Remedies.”
In addition, the events that constitute a change of control or
asset sale under the indenture may also be events of default
under our Senior Credit Facilities. These events may permit the
lenders under our Senior Credit Facilities to accelerate the
debt outstanding thereunder and, if such debt is not paid, to
enforce security interests in our specified assets, thereby
limiting our ability to raise cash to purchase the notes and
reducing the practical benefit of the
offer-to-purchase
provisions to the holders of the notes.
The
trading prices of the notes will be directly affected by our
ratings with major credit rating agencies, the prevailing
interest rates being paid by companies similar to us, and the
overall condition of the financial and credit markets.
The trading prices of the notes in the secondary market will be
directly affected by our ratings with major credit rating
agencies, the prevailing interest rates being paid by companies
similar to us, and the overall condition of the financial and
credit markets. It is impossible to predict the prevailing
interest rates or the condition of the financial and credit
markets. Credit rating agencies continually revise their ratings
for companies that they follow, including us. Any ratings
downgrade could adversely affect the trading price of the notes
or the trading market for the notes, to the extent a trading
market for the notes develops. The condition of the financial
and credit markets and prevailing interest rates have fluctuated
in the past and are likely to fluctuate in the future.
Our
ability to make payments on the notes depends on our ability to
receive dividends and other distributions from our
subsidiaries.
Our principal assets are the equity interests that we hold in
our operating subsidiaries. As a result, we are dependent on
dividends and other distributions from our subsidiaries to
generate the funds necessary to meet our financial obligations,
including the payment of principal and interest on our
outstanding debt. Our subsidiaries may not generate sufficient
cash from operations to enable us to make principal and interest
payments on our indebtedness, including the notes. In addition,
any payment of dividends, distributions, loans or advances to us
by our subsidiaries could be subject to restrictions on
dividends or, in the case of foreign subsidiaries, restrictions
on repatriation of earnings under applicable local law and
monetary transfer restrictions in the jurisdictions in which our
subsidiaries operate. In addition, payments to us by our
subsidiaries will be contingent upon our subsidiaries’
earnings. Our subsidiaries are permitted under the terms of our
indebtedness, including the indenture governing the notes, to
incur additional indebtedness that may restrict payments from
those subsidiaries to us. We cannot assure you that agreements
governing current and future indebtedness of our subsidiaries
will permit those subsidiaries to provide us with sufficient
cash to fund payments on the notes when due.
Our subsidiaries are legally distinct from us and, except for
existing and future subsidiaries that will be Guarantors of the
notes, have no obligation, contingent or otherwise, to pay
amounts due on our debt or to make funds available to us for
such payment.
A
guarantee could be voided if it constitutes a fraudulent
transfer under U.S. bankruptcy or similar state law, which would
prevent the holders of the notes from relying on that Guarantor
to satisfy claims.
The notes will be fully and unconditionally guaranteed on a
joint and several senior unsecured basis by the Parent Guarantor
and each of the Parent Guarantor’s existing and future
direct or indirect subsidiaries that guarantee the Senior Credit
Facilities. The guarantees may be subject to review under
U.S. federal bankruptcy law and comparable provisions of
state fraudulent conveyance laws if a bankruptcy or another
similar case or lawsuit is commenced by or on behalf of the
Issuer’s or a Guarantor’s unpaid creditors or another
authorized party. Under these laws, if a court were to find
that, at the time any Guarantor issued a guarantee of the notes,
either it issued the guarantee to delay, hinder or defraud
present or future creditors, or it received less than reasonably
equivalent value or fair consideration for issuing the guarantee
and at the time:
|
|
|
| |
•
|
it was insolvent or rendered insolvent by reason of issuing the
guarantee;
|
39
|
|
|
| |
•
|
it was engaged, or about to engage, in a business or transaction
for which its remaining unencumbered assets constituted
unreasonably small capital to carry on its business;
|
| |
| |
•
|
it intended to incur, or believed that it would incur, debts
beyond its ability to pay as they mature; or
|
| |
| |
•
|
it was a defendant in an action for money damages, or had a
judgment for money damages docketed against it if, in either
case, after final judgment, the judgment is unsatisfied,
|
then the court could void the obligations under the guarantee,
subordinate the guarantee of the notes to other debt or take
other action detrimental to holders of the notes.
We cannot be sure as to the standard that a court would use to
determine whether a Guarantor was solvent at the relevant time,
or, regardless of the standard that the court uses, that the
issuance of the guarantees would not be voided or that the
guarantees would not be subordinated to other debt. If such a
case were to occur, the guarantee could also be subject to the
claim that, since the guarantee was incurred for our benefit,
and only indirectly for the benefit of the Guarantor, the
obligations of the applicable Guarantor were incurred for less
than fair consideration. A court could thus void the obligations
under the guarantee, subordinate the guarantee to the applicable
guarantor subsidiary’s other debt or take other action
detrimental to holders of the notes. If a court were to void a
guarantee, you would no longer have a claim against the
Guarantor. Sufficient funds to repay the notes may not be
available from other sources, including the remaining
Guarantors, if any. In addition, the court might direct you to
repay any amounts that you already received from or are
attributable to the Guarantor.
Each Guarantee contains a provision intended to limit the
Guarantor’s liability to the maximum amount that it could
incur without causing the incurrence of obligations under its
Guarantee to be a fraudulent transfer. This provision may not be
effective to protect the Guarantees from being voided under
fraudulent transfer law.
The
Guarantors may be unable to fulfill their obligations under
their guarantees.
We expect that the Guarantors will use cash flow from operations
to pay amounts due, if any, pursuant to their guarantees of the
notes. The ability of the Guarantors to make these payments
depends on our future performance, which will be affected by
financial, business, economic, and other factors, many of which
we cannot control. Such Guarantors’ businesses may not
generate sufficient cash flow from operations in the future and
their anticipated growth in revenue and cash flow may not be
realized, either or both of which could result in their being
unable to honor their guarantees or to fund other liquidity
needs. If the Guarantors do not have enough money, they may be
required to refinance all or part of their then-existing debt,
sell assets or borrow more money. They may not be able to
accomplish any of these alternatives on terms acceptable to
them, or at all. In addition, the terms of existing or future
debt agreements, including our Senior Credit Facilities and the
indenture that governs the notes, may restrict the Guarantors
from adopting any of these alternatives. The failure of the
Guarantors to generate sufficient cash flow or to achieve any of
these alternatives could materially and adversely affect the
value of the notes and the ability of such Guarantors to pay the
amounts due under their guarantees, if any.
Interest
on the notes may not be deductible by us for United States
federal income tax purposes.
The deductibility of interest is subject to many limitations
under the Internal Revenue Code. We may not be able to deduct,
in whole or in part, the interest on the notes. The availability
of an interest deduction on the notes was not determinative in
the issuance of the notes.
Limitations
on guarantees under the laws of foreign jurisdictions may
adversely affect the validity and enforceability of the
guarantees of the notes.
The laws of certain of the jurisdictions in which the Guarantors
are organized limit their ability to guarantee debt of a related
company. These limitations arise under various provisions or
principles of corporate law. If these limitations were not
observed, the guarantees could be subject to legal challenge. In
these jurisdictions, the guarantees contain language limiting
the amount of debt guaranteed so that applicable local law
restrictions will not be violated. Accordingly if you were to
enforce the guarantees by a Guarantor in one
40
of these jurisdictions, your claims are likely to be limited. In
some cases, where the amount that can be guaranteed is limited
by reference to the net assets and legal capital of the
Guarantor or by reference to the outstanding debt owed by the
relevant Guarantor to an issuer under intercompany loans that
amount might have reached zero or close to zero at the time of
any insolvency or enforcement. Furthermore, although we believe
that the guarantees by these Guarantors will be validly given in
accordance with local law restrictions, there can be no
assurance that a third-party creditor would not challenge these
guarantees and prevail in court.
Local
insolvency laws may not be as favorable to you as U.S.
bankruptcy laws or those of another jurisdiction with which you
are familiar.
Most of the Guarantors are organized outside of the United
States. The insolvency laws of these jurisdictions may not be as
favorable to your interests as the laws of the United States or
other jurisdictions with which you are familiar. In the event
that any one or more of the Guarantors or any other of our
subsidiaries experienced financial difficulty, it is not
possible to predict with certainty in which jurisdiction or
jurisdictions insolvency or similar proceedings would be
commenced, or the outcome of such proceedings.
Your
ability to enforce civil liabilities under U.S. securities laws
may be limited.
The Parent Guarantor is a company organized under the laws of
Spain and most of the Subsidiary Guarantors are also
incorporated outside of the United States. A substantial portion
of the assets of the Parent Guarantor and the assets of the
Subsidiary Guarantors are located outside of the United States.
In addition, nearly all of the directors and officers of the
Parent Guarantor and certain of the Subsidiary Guarantors’
directors and officers are nationals or residents of countries
other than the United States, and all or a substantial portion
of such persons’ assets are located outside the United
States. As a result, it may be difficult for investors to affect
service of process within the United States upon the Parent
Guarantor or certain Subsidiary Guarantors or their directors or
officers with respect to matters arising under the Securities
Act or to enforce against them judgments of courts of the United
States predicated upon civil liability under the Securities Act.
It may also be difficult to recover fully in the United States
on any judgment rendered against such persons or the Parent
Guarantor or certain of the Subsidiary Guarantors.
In addition, there is doubt as to the enforceability in Spain in
original actions, or in actions for enforcement of judgments of
United States courts of liabilities, predicated solely upon
the securities laws of the United States. If a judgment was
obtained outside Spain and efforts were made to enforce the
judgment in Spain, there is some doubt that Spanish courts would
agree to recognize and enforce a foreign judgment. Accordingly,
even if you obtain a favorable judgment in a United
States court, you may be required to re-litigate your claim
in Spain. See “Enforceability of Civil Liabilities Under
U.S. Securities Laws.”
Risks
Relating to the Healthcare Industry
The
implementation of the 2010 health care reform law in the United
States may adversely affect our business.
Through the March 2010 adoption of the Patient Protection and
Affordable Care Act and the companion Healthcare and Education
Reconciliation Act in the United States, which is referred to as
the “healthcare reform law,” substantial changes are
being made to the current system for paying for healthcare in
the United States, including programs to extend medical benefits
to millions of individuals who currently lack insurance
coverage. The changes contemplated by health care reform law are
subject to rule-making and implementation timelines that extend
for several years, and this uncertainty limits our ability to
forecast changes that may occur in the future. However,
implementation has already begun with respect to certain
significant cost-savings measures under the healthcare reform
law, for example with respect to several government healthcare
programs that cover the cost of our products, including
Medicaid, Medicare Parts B and D and the 340B/PHS program, and
these efforts could have a materially adverse impact on our
financial performance.
For example, with respect to Medicaid, in order for a drug
manufacturer’s products to be reimbursed by federal funding
under Medicaid, the manufacturer must enter into a Medicaid drug
rebate agreement with the
41
Secretary of the United States Department of Health and Human
Services, and pay certain rebates to the states based on
utilization data provided by each state to the manufacturer and
to the Centers for Medicare & Medicaid Services, which
is referred to as CMS, and pricing data provided by the
manufacturer to the federal government. The states share this
savings with the federal government, and sometimes implement
their own additional supplemental rebate programs. Under the
Medicaid drug rebate program, the rebate amount for most branded
drugs was previously equal to a minimum of 15.1% of the Average
Manufacturer Price, which is referred to as AMP, or AMP less
Best Price, which is referred to as AMP less BP, whichever is
greater. Effective January 1, 2010, the healthcare reform
law generally increases the size of the Medicaid rebates paid by
drug manufacturers for single source and innovator multiple
source (brand name) drugs from a minimum of 15.1% to a minimum
of 23.1% of the AMP, subject to certain exceptions, for example,
for certain clotting factors, the increase is limited to a
minimum of 17.1% of the AMP. For non-innovator multiple source
(generic) drugs, the rebate percentage is increased from a
minimum of 11.0% to a minimum of 13.0% of AMP. In 2010, the
healthcare reform law also newly extended this rebate obligation
to prescription drugs covered by Medicaid managed care
organizations. These increases in required rebates may adversely
affect our financial performance.
In addition, the statutory definition of AMP changed in 2010 as
a result of the new healthcare reform law. While in November
2010 CMS withdrew previously issued regulations defining AMP and
stated that new regulations would be forthcoming, no such
regulations have yet been issued. We believe we are making
reasonable assumptions regarding our reporting obligations with
respect to this new definition, but in the absence of
regulations from CMS the adequacy of our assumptions is not
certain. Once CMS issues regulations to clarify the calculation
of AMP, we may determine that our assumptions require amendment
to comply with the regulatory definition of AMP, and it is
possible we may need to restate or correct our prior reported
AMPs, which could, for example, result in rebate liability for
past quarters or other adverse consequences.
The Healthcare Reform Law also creates new rebate obligations
for our products under Medicare Part D, a partial,
voluntary prescription drug benefit created by the United States
federal government primarily for persons 65 years old and
over. The Part D drug program is administered through
private insurers that contract with CMS. Beginning in 2011, the
healthcare reform law generally requires that in order for a
drug manufacturer’s products to be reimbursed under
Medicare Part D, the manufacturer must enter into a
Medicare Coverage Gap Discount Program agreement with the
Secretary of the United States Department of Health and Human
Services, and reimburse each Medicare Part D plan sponsor
an amount equal to 50% savings for the manufacturer’s brand
name drugs and biologics which the Part D plan sponsor has
provided to its Medicare Part D beneficiaries who are in
the “donut hole” (or a gap in Medicare Part D
coverage for beneficiaries who have expended certain amounts for
drugs). The Part D plan sponsor is responsible for
calculating and providing the discount directly to its
beneficiaries and for reporting these amounts paid to CMS’s
contractor, which notifies drug manufacturers of the rebate
amounts it must pay to each Part D plan sponsor. The rebate
requirement could adversely affect our financial performance,
particularly if contracts with Part D plans cannot be
favorably renegotiated or the Part D plan sponsors fail to
accurately calculate payments due in a manner that overstates
our rebate obligation.
The availability of federal funds to pay for our products under
the United States Medicaid and Medicare Part B programs
requires that we extend discounts under the 340B/PHS program,
and changes to this program under the healthcare reform law
could adversely affect our financial performance. The 340B/PHS
program extends discounts to a variety of community health
clinics and other entities that receive health services grants
from the PHS, as well as hospitals that serve a disproportionate
share of certain low income individuals, and the healthcare
reform law expanded the number of qualified 340B entities
eligible to purchase products for outpatient use, adding certain
cancer centers, children’s hospitals, critical access
hospitals and rural referral centers. The PHS price (or
“ceiling price”) cannot exceed the AMP (as reported to
CMS under the Medicaid drug rebate program) less the Medicaid
unit rebate amount. We have entered into a Pharmaceutical
Pricing Agreement with the government in which we have agreed to
participate in the 340B/PHS program by charging eligible
entities no more than the PHS ceiling price for drugs intended
for outpatient use. The healthcare reform law imposes a
“must sell” obligation on manufacturers that will
require manufacturers to offer their products to eligible
entities at legally mandated discount prices if such products
are “made available to any
42
other purchaser at any other price.” The healthcare reform
law imposes this obligation by requiring HHS to add the
requirement to the 340B/PHS program agreements that
manufacturers must execute with HHS. Rulemaking to implement
this obligation is not yet complete, and as a result the full
impact of the changes is not yet certain. For example, certain
biological products we sell are subject to shortages on
occasion, and we do not believe that this new provision would
require us to provide a supply advantage to covered entities. If
implementing regulations do not reflect this interpretation of
the law, it could adversely affect our financial performance.
The healthcare reform law also introduced a biosimilar pathway
that will permit companies to obtain FDA approval of generic
versions of existing biologics based upon reduced documentation
and data requirements deemed sufficient to demonstrate safety
and efficacy than are required for the pioneer biologics. The
new law provides that a biosimilar application may be submitted
as soon as 4 years after the reference product is first
licensed, and that the FDA may not make approval of an
application effective until 12 years after the reference
product was first licensed. With the likely introduction of
biosimilars in the United States, we expect in the future to
face greater competition from biosimilar products, including a
possible increase in patent challenges. The FDA has reported
meeting with sponsors who are interested in developing
biosimilar products, and is developing regulations to implement
the abbreviated regulatory review pathway.
Regarding access to our products, the healthcare reform law
established and provided significant funding for a
Patient-Centered Outcomes Research Institute to coordinate and
fund Comparative Effectiveness Research (CER). While the
stated intent of CER is to develop information to guide
providers to the most efficacious therapies, outcomes of CER
could influence the reimbursement or coverage for therapies that
are determined to be less cost-effective than others. Should any
of our products be determined to be less cost-effective than
alternative therapies, the levels of reimbursement for these
products, or the willingness to reimburse at all, could be
impacted, which could materially impact our financial results.
We could
be adversely affected if other government or private third-party
payors decrease or otherwise limit the amount, price, scope or
other eligibility requirements for reimbursement for the
purchasers of our products.
Prices in many countries, including many in Europe, are subject
to local regulation and certain pharmaceutical products, such as
plasma derivative products, are subject to price controls in
several of our principal markets, including Spain and countries
within the European Union. In the United States, where pricing
levels for our products are substantially established by
third-party payors, if payors reduce the amount of reimbursement
for a product, it may cause groups or individuals dispensing the
product to discontinue administration of the product, to
administer lower doses, to substitute lower cost products or to
seek additional price-related concessions. These actions could
have a negative effect on financial results, particularly in
cases where our products command a premium price in the
marketplace, or where changes in reimbursement induce a shift in
the site of treatment. The existence of direct and indirect
price controls and pressures over our products have affected,
and may continue to materially adversely affect, our ability to
maintain or increase gross margins.
In the United States, for example, beginning in 2005, the
Medicare drug reimbursement methodology for physician and
hospital outpatient payment schedules changed to Average Sales
Price, which is referred to as ASP, +6%. This payment was
based on a volume-weighted average of all brands under a common
billing code. Medicare payments to physicians between the fourth
quarter of 2004 and the first quarter of 2005 dropped 14% for
both the powder and liquid forms of intravenous immune globulin,
which is referred to as IVIG. Medicare payments to hospitals
fell 45% for powder IVIG and 30% for liquid IVIG between the
fourth quarter of 2005 and the first quarter of 2006. The
Medicare reimbursement changes resulted in the shift of a
significant number of Medicare IVIG patients to hospitals from
physicians’ offices beginning in 2005 as many physicians
could no longer recover their costs of obtaining and
administering IVIG in their offices and clinics. After 2006,
some hospitals reportedly began to refuse providing IVIG to
Medicare patients due to reimbursement rates that were below
their acquisition costs. Subsequent changes have improved some
of these Medicare reimbursement issues, although there has been
variability in the reimbursement level for separately payable,
non-pass-through drugs and biologicals in the hospital
outpatient setting over the past few years, making
43
financial performance predictions difficult. On January 1,
2008, the CMS reduced the reimbursement for these separately
covered drugs and biologicals, including IVIG, in the hospital
outpatient setting from ASP +6% to ASP +5%, using 2006 Medicare
claims data as a reference for this reduction. In addition, CMS
reduced a hospital add-on payment from $75 to $38 per infusion.
Beginning January 1, 2009 CMS further reduced the hospital
outpatient reimbursement for separately covered outpatient
drugs, including IVIG, to ASP +4%, and eliminated the add-on
payment. For 2010, the rate remained as ASP+4%, based on a
cost-based methodology that also involved reallocating certain
overhead costs from packaged drugs to unpackaged drugs. In 2011,
relying on the 2010 methodology, CMS increased the rate to ASP
+5%. For 2012, as part of a July 2011 proposed rule issued in
connection with the Medicare hospital outpatient prospective
payment system, CMS has proposed continuing to rely on the
2010-2011
methodology, which according to CMS would result in a rate of
ASP +4% or lower as of January 1, 2012. Depending upon
their magnitude, which is currently not known, the decreases in
reimbursement rates proposed for 2012 could adversely affect
financial performance.
Also, the intended use of a drug product by a physician can
affect pricing. Physicians frequently prescribe legally
available therapies for uses that are not described in the
product’s labeling and that differ from those tested in
clinical studies and approved by the FDA or similar regulatory
authorities in other countries. These unapproved (also known as
“off-label”) uses are common across medical
specialties, and physicians may believe such off-label uses
constitute the preferred treatment or treatment of last resort
for many patients in varied circumstances. We believe that a
significant portion of our IVIG volume may be used to fill
physician prescriptions for indications not approved by the FDA
or similar regulatory authorities. If reimbursement for
off-label uses of products, including IVIG, is reduced or
eliminated by Medicare or other third-party payors, including
those in the United States or the European Union, we could be
adversely affected. For example, CMS could initiate an
administrative procedure known as a National Coverage
Determination (NCD) by which the agency determines which uses of
a therapeutic product would be reimbursable under Medicare and
which uses would not. This determination process can be lengthy,
thereby creating a long period during which the future
reimbursement for a particular product may be uncertain. High
levels of spending on IVIG products, along with increases in
IVIG prices, increased IVIG utilization and the high proportion
of off-label uses, may increase the risk of regulation of IVIG
reimbursement by CMS. On the state level, similar limits could
be proposed for therapeutic products covered under Medicaid.
With respect to pricing, for many payors, including private
health insurers and self-insured health plans, as well as
Medicare Part D plans and some state Medicaid programs,
outpatient pharmaceuticals are often reimbursed based upon a
discount calculated off of a pricing benchmark called
“Average Wholesale Price,” which is referred to as
AWP. AWP is a list price that has been calculated and published
by private third-party publishers (such as First DataBank,
Thomson Reuters (Red Book) and Wolters Kluwer (Medi-Span)). AWP
does not reflect actual transactions in the distribution chain
(e.g., the publishers do not base the figure on actual
transaction prices, including any prompt pay or other discounts,
rebates or price reductions). Often, publishers calculate AWP
based upon a standard markup of, for example, 20% over another
list price that is reported by drug manufacturers to the
publishers. This list price is called “Wholesale
Acquisition Cost,” which is referred to as WAC. WAC is
generally understood in the industry to be the list price drug
manufacturers have for their drug wholesaler customers and, like
AWP, is not calculated based on actual transaction prices,
including any prompt pay or other discounts, rebates or price
reductions. We do not publish AWPs for any of our products, and
we report WACs for our products. We may be at a competitive
disadvantage where providers are reimbursed on an AWP basis and
competitors’ products are reimbursed based on a higher AWP
than the corresponding AWP for our product. The use of AWP and
WAC as pricing benchmarks has been subject to legal challenge by
both government officials and private citizens, often based on
claims that the benchmarks were used in a misleading manner,
thus defrauding consumers and third-party payors. It is possible
that we, as a reporter of WAC, could be challenged on this
basis. Additionally, the settlement of class action litigation
against First DataBank and others has resulted in the downward
revision of certain reported AWP listings (to a level of 20%
over WAC). Issues regarding AWP have contributed to suggestions
to eliminate its use as a drug pricing benchmark. With respect
to the major private publishers of AWP, First DataBank has
reported that it will no longer publish AWP as of
September 26, 2011, while Thomson Reuters (Red Book) and
Wolters Kluwer (Medi-Span) have reported that they will continue
to publish AWP.
44
Certain
of our business practices are subject to scrutiny by regulatory
authorities, as well as to lawsuits brought by private citizens
under federal and state laws. Failure to comply with applicable
law or an adverse decision in lawsuits may result in adverse
consequences to us.
The laws governing our conduct in the United States are
enforceable by criminal, civil and administrative penalties.
Violations of laws such as the Federal Food, Drug and Cosmetic
Act, the False Claims Act and the Anti-Kickback Law and the
Public Health Service Act, and any regulations promulgated under
their authority, may result in jail sentences, fines or
exclusion from federal and state programs, as may be determined
by Medicare, Medicaid and the Department of Defense and other
regulatory authorities as well as by the courts. There can be no
assurance that our activities will not come under the scrutiny
of regulators and other government authorities or that our
practices will not be found to violate applicable laws, rules
and regulations or prompt lawsuits by private citizen
“relators” under federal or state false claims laws.
For example, under the Anti-Kickback Law, and similar state laws
and regulations, even common business arrangements, such as
discounted terms and volume incentives for customers in a
position to recommend or choose drugs and devices for patients,
such as physicians and hospitals, can result in substantial
legal penalties, including, among others, exclusion from the
Medicare and Medicaid programs, and arrangements with referral
sources must be structured with care to comply with applicable
requirements. Also, certain business practices, such as
consulting fees to healthcare providers, sponsorship of
educational or research grants, charitable donations,
interactions with healthcare providers that prescribe products
for uses not approved by the FDA and financial support for
continuing medical education programs, must be conducted within
narrowly prescribed and controlled limits to avoid any
possibility of wrongfully influencing healthcare providers to
prescribe or purchase particular products or as a reward for
past prescribing. Under the U.S. healthcare reform law,
such payments by pharmaceutical manufacturers to United States
healthcare practitioners and academic medical centers must be
publicly disclosed starting with payments made in calendar year
2012. A number of states have similar laws in place. Additional
and stricter prohibitions could be implemented by federal and
state authorities. Where such practices have been found to be
improper incentives to use such products, government
investigations and assessments of penalties against
manufacturers have resulted in substantial damages and fines.
Many manufacturers have been required to enter into consent
decrees or orders that prescribe allowable corporate conduct.
Failure to satisfy requirements under the Federal Food, Drug and
Cosmetic Act can also result in penalties, as well as
requirements to enter into consent decrees or orders that
prescribe allowable corporate conduct. In this regard, our blood
plasma fractionation center in Los Angeles, California is
managed through a consent decree that was entered into in
February 1998 based on action by the FDA and
U.S. Department of Justice (“DOJ”) addressing
Federal Food, Drug and Cosmetic Act violations committed by the
former owner of the center, Alpha Therapeutic Corporation. As a
result of this consent decree, the Los Angeles establishment is
subject to strict FDA audits and may only sell products
manufactured in the center subsequent to prior authorization.
Adverse consequences can also result from failure to comply with
the requirements of the 340B/PHS program under the Public Health
Service Act, which extends discounts to a variety of community
health clinics and other entities that receive health services
grants from the PHS. For example, the healthcare reform law
requires the Secretary of HHS to develop and issue regulations
for the 340B/PHS program establishing standards for the
imposition of sanctions in the form of civil monetary penalties
(“CMP”) for manufacturers that knowingly and
intentionally overcharge a covered entity for a 340B drug. The
CMP can be up to $5,000 for each instance of overcharging a
covered entity. HHS has never had CMP authority that addresses
this area, and has not yet issued final regulations to implement
this new penalty provision, and accordingly the impact of this
CMP provision is uncertain. However, in an advance notice of
proposed rulemaking and request for comments, HHS’ Health
Resources and Services Administration (“HRSA”) has
suggested that HRSA is considering imposing CMPs on
manufacturers in certain cases where a covered entity has been
unable to find a given product at a 340B price and has instead
purchased the drug outside of the 340B Program at a price
greater than the ceiling price. Certain of our products are
subject to shortages and allocation issues can arise.
Accordingly, if HRSA adopts a CMP interpretation of this kind it
could potentially have adverse consequences on our financial
performance.
45
In addition, and prior to the enactment of the new healthcare
reform law, some government regulators have suggested that
allocation issues linked to product shortages could give rise to
potential 340B/PHS program violations. In November 2009, the
United States Attorney’s Office for the Eastern District of
Pennsylvania commenced an investigation of Talecris, with
respect to its method of allocating its IVIG product, Gamunex,
as available for sale at the PHS price to covered entities. We
are cooperating with the ongoing investigation and intend to
respond to information requests from the United States
Attorney’s Office. We believe that we have complied with
the terms of the Pharmaceutical Pricing Agreement
(“PPA”) and federal law, but an adverse outcome in
this investigation could have a material adverse effect on our
results of operation.
In addition, while regulatory authorities generally do not
regulate physicians’ discretion in their choice of
treatments for their patients, they do restrict communications
by manufacturers on unapproved uses of approved drugs or on the
potential safety and efficacy of unapproved products in
development. Companies in the United States, Canada and European
Union cannot promote approved products for other indications
that are not specifically approved by the competent regulatory
authorities (e.g., FDA in the United States), nor can companies
promote unapproved products. In limited circumstances, companies
may disseminate to physicians information regarding unapproved
uses of approved products or results of studies involving
investigational products. If such activities fail to comply with
applicable regulations and guidelines of the various regulatory
authorities, we may be subject to warnings from, or enforcement
action by, these authorities. Furthermore, if such activities
are prohibited, it may harm demand for our products.
Promotion of unapproved drugs or devices or unapproved
indications for a drug or device is a violation of the Federal
Food, Drug and Cosmetic Act and subjects us to civil and
criminal sanctions. Furthermore, sanctions under the Federal
False Claims Act have recently been brought against companies
accused of promoting off-label uses of drugs, because such
promotion induces the use and subsequent claims for
reimbursement under Medicare and other federal programs. Similar
actions for off-label promotion have been initiated by several
states for Medicaid fraud. The U.S. healthcare reform law
significantly strengthened provisions of the Federal False
Claims Act, Medicare and Medicaid Anti-Kickback provisions, and
other health care fraud provisions, leading to the possibility
of greatly increased qui tam suits by relators for
perceived violations. Violations or allegations of violations of
the foregoing restrictions could materially and adversely affect
our business.
We are required to report detailed pricing information, net of
included discounts, rebates and other concessions, to CMS for
the purpose of calculating national reimbursement levels,
certain federal prices and certain federal and state rebate
obligations. We have established systems for collecting and
reporting this data accurately to CMS and have instituted a
compliance program to assure that the information collected is
complete in all respects. If we report pricing information that
is not accurate to the federal government, we could be subject
to fines and other sanctions that could adversely affect their
business
To market and sell our products outside of the United States, we
must obtain and maintain regulatory approvals and comply with
regulatory requirements in such jurisdictions. The approval
procedures vary among countries in complexity and timing. We may
not obtain approvals from regulatory authorities outside the
United States on a timely basis, if at all, which would preclude
us from commercializing products in those markets. In addition,
some countries, particularly the countries of the European
Union, regulate the pricing of prescription pharmaceuticals. In
these countries, pricing discussions with governmental
authorities can take considerable time after the receipt of
marketing approval for a product. To obtain reimbursement or
pricing approval in some countries, we may be required to
conduct a clinical trial that compares the cost-effectiveness of
their product candidate to other available therapies. Such
trials may be time-consuming and expensive, and may not show an
advantage in efficacy for our products. If reimbursement of our
products is unavailable or limited in scope or amount, or if
pricing is set at unsatisfactory levels, in either the United
States or the European Union, we could be adversely affected.
Also, under the United States Foreign Corrupt Practices Act,
referred to as FCPA, the United States has increasingly focused
on regulating the conduct by United States businesses occurring
outside of the United States, generally prohibiting remuneration
to foreign officials for the purpose of obtaining or retaining
business.
46
To enhance compliance with applicable health care laws, and
mitigate potential liability in the event of noncompliance,
regulatory authorities, such as the United States Health and
Human Services Department Office of Inspector General
(“OIG”), have recommended the adoption and
implementation of a comprehensive health care compliance program
that generally contains the elements of an effective compliance
and ethics program described in Section 8B2.1 of the United
States Sentencing Commission Guidelines Manual. Increasing
numbers of United States-based pharmaceutical companies have
such programs. While we have adopted U.S. healthcare
compliance and ethics programs that generally incorporate the
OIG’s recommendations, and train our applicable
U.S. employees in such compliance, having such a program
can be no assurance that we will avoid any compliance issues.
We are
subject to extensive environmental, health and safety laws and
regulations.
Our business involves the controlled use of hazardous materials,
various biological compounds and chemicals. The risk of
accidental contamination or injury from these materials cannot
be eliminated. If an accident, spill or release of any regulated
chemicals or substances occurs, we could be held liable for
resulting damages, including, for investigation, remediation and
monitoring of the contamination, including natural resource
damages, the costs of which could be substantial. We are also
subject to numerous environmental, health and workplace safety
laws and regulations, including those governing laboratory
procedures, exposure to blood-borne pathogens and the handling
of biohazardous materials and chemicals. Although we maintain
workers’ compensation insurance to cover the costs and
expenses that may be incurred due to injuries to our employees
resulting from the use of these materials, this insurance may
not provide adequate coverage against potential liabilities. We
do not maintain insurance for environmental liability or toxic
tort claims that may be asserted against us for claims arising
in the United States. Additional or more stringent federal,
state or local laws and regulations affecting our operations may
be adopted in the future. We may incur substantial capital costs
and operating expenses to comply with any of these laws or
regulations and the terms and conditions of any permits required
pursuant to such laws and regulations, including costs to
install new or updated pollution control equipment, modify our
operations or perform other corrective actions at our respective
facilities. In addition, fines and penalties may be imposed for
noncompliance with environmental and health and safety laws and
regulations or for the failure to have or comply with the terms
and conditions of required environmental permits.
Internationally, the Kyoto Protocol to the United Nations
Framework Convention on Climate Change, which is commonly called
the Kyoto Protocol, became effective in February 2005. Adopted
by some of the countries in which we operate, the Kyoto Protocol
requires the implementation of national programs to reduce
emissions of certain gases, generally referred to as greenhouse
gases, which contribute to global warming. Climate
change-related legislation has also passed the U.S. House
of Representatives, which, if enacted by the full Congress,
would limit and reduce greenhouse gas emissions from large
emitters of greenhouse gasses through a
“cap-and-trade”
system of allowances and credits and other provisions. Moreover,
the Environmental Protection Agency, which is referred to as the
EPA, issued a finding that the current and projected
concentrations of certain greenhouse gases in the atmosphere,
including carbon dioxide, which is referred to as
CO2,
threaten the public health and welfare of current and future
generations. While this finding in itself does not impose any
requirements on our industry, it authorizes the EPA to regulate
directly greenhouse gas emissions through a rule-making process.
Existing legislation and the future passage of climate control
legislation or regulations that restrict emissions of greenhouse
gases in the areas in which we operate could result in adverse
financial and operational impacts on our respective business.
Plasma
collection and manufacturing, and the manufacture of drugs,
biologicals and devices, are heavily regulated.
Our business is heavily regulated in all jurisdictions where we
collect plasma or manufacture or sell our products. In
particular, plasma collection activities in the United States
are regulated by the FDA, which requires a licensing and
certification process for each plasma collection center prior to
opening and conducts periodic inspections of facilities and
processes. Many states also regulate plasma collection, imposing
similar obligations and additional inspections and audits. In
addition, the marketing and sale of a pharmaceutical
47
product such as plasma derivatives and parenteral solutions are
subject to the prior registrations, listings, licenses and
approvals of such products with the competent authorities of the
jurisdiction where the product is to be marketed and sold,
including compliance with promotion, labeling and advertising
requirements. Our manufacturing facilities located near
Barcelona, Spain, in Los Angeles, California, Melville, New York
and in Clayton, North Carolina, must meet strict European Union
and FDA rules as well. Our
U.S.-based
manufacturing facilities must also comply with applicable state
laws. U.S. plasma centers collecting plasma for manufacture
into products to be distributed in the European Union must also
be approved by the competent European Health Authority.
Collection centers and manufacturing facilities are subject to
periodic inspections by regulatory authorities. Our subsidiary,
PlasmaCare, Inc., was issued an FDA Warning Letter in 2007, with
respect to a plasma collection facility located in Cincinnati,
Ohio. The Warning Letter reported certain compliance deviations
from FDA standards at the facility. The conditions were
corrected to the satisfaction of the FDA. The consequences of
adverse findings following inspections can be more serious, such
as the temporary shutdown of such center or facility, the loss
of that center’s or facility’s license because of
alleged noncompliance with applicable requirements, a voluntary
or mandatory recall of finished product released to the market,
or the destruction of inventory. These more serious consequences
are often highly public and may also prompt private products
liability lawsuits, additional regulatory enforcement actions,
the imposition of substantial fines or penalties by regulatory
authorities, and damage to the reputation and public image of
the collection or manufacturing facility.
Prior to the acquisition Talecris had voluntarily recalled
plasma products that had been released to the market in an
effort to address drug safety issues. Since its formation in
2005, Talecris had four recalls of finished biological products.
The products involved were: Plasma Protein Fraction (Human) 5%
USP,
Plasmanate®,
Lot Number: 26N39N1; Antihemophilic Factor (Human), Koate
DVI®;
Lot Numbers: 26N7802, 26N6XW1, 26N6N01, 26N7H01; Rho(D) Immune
Globulin (Human); HyperRHO
S/D®,
Mini-Dose, Lot Number: 26N7XX1; and
Plasbumin-5®,
Albumin (Human) 5%, USP, Lot Number: 26N9P21. In addition,
plasma unit look backs and retrievals are routinely handled when
new information relevant to donor or plasma suitability is
received after a donation is collected. Plasma unit retrievals
are also triggered if units were distributed that should have
been rejected by the plasma center. A minority of unit
retrievals are required to be reported to the FDA as Biological
Product Deviation Reports (“BPDRs”), and a relatively
small number are classified by the FDA as recalls. We have been
and also may be in the future involved in voluntary recalls
involving certain devices.
In addition, the FDA conducts ongoing monitoring and
surveillance of advertising and promotional matter used by
manufacturers to sell and promote their products. The FDA
assesses these materials for compliance with the FDCA,
regulations on misbranding and other requirements, for example,
assessing if information about the risks and benefits of
regulated products are communicated in a truthful, accurate,
science-based, non-misleading and balanced manner. In 2005, the
FDA issued us a Warning Letter with respect to our product,
Flebogamma, indicating that a brochure was misleading for
failing to reveal material facts regarding risks associated with
the product, and therefore misbranded Flebogamma in violation of
the FDCA. In addition, the FDA issued Untitled Letters to
Talecris on three occasions prior to the acquisition, requesting
that Talecris change advertising materials on the basis that
they were inconsistent with the package insert for the product.
All of these matters were addressed to the satisfaction of the
FDA.
In particular, the manufacturing processes for our products are
governed by detailed and constantly evolving federal and
sometimes state regulations that set forth cGMP for drugs and
devices manufactured or distributed in the United States. We
monitor compliance with these evolving procedures and
regulations to help assure compliance, but failure to adhere to
established procedures or regulations, or to meet a
specification, could require that a product or material be
rejected and destroyed, and could result in adverse regulatory
actions against us. As a result of routine inspections by
regulatory health authorities, we have been issued observations,
for example, Form 483 FDA Inspection Observations, with
regard to cGMP compliance. While these issues have been
corrected, no assurances can be provided that we will avoid
citation for deficiencies in the future. If serious deficiencies
are noted or recur, compliance may be costly and difficult to
achieve, and consequences may include the need to recall product
or suspend operations until appropriate
48
measures can be implemented. Also, certain deviations from
procedures must be reported to the FDA, and even if we determine
that the deviations were not material, the FDA could require us
to take similar measures.
Existing
government regulation and its interpretation may change or the
requirements of different jurisdictions may become less uniform,
thereby making compliance more expensive or reducing profit
margins.
Changes in the regulation of our activities, such as increased
regulation affecting plasma collection activity, or new
regulation, such as regulation of compensation paid to plasma
donors or the prices charged to customers in the European Union
or the United States or other jurisdictions in which we operate,
could materially adversely affect the business. Currently, we
are not subject to limits on compensation paid to plasma donors
or product price controls in the United States market, but we
cannot assure you this will remain the case. In addition, the
requirements of different jurisdictions in which we operate may
become less uniform, creating a greater administrative burden
and generating additional costs. Any such regulatory changes
could have a material adverse effect on our business, results of
operations and financial condition.
Risks
Relating to Our Business
We are a
foreign private issuer under the rules and regulations of the
SEC and, thus, are exempt from a number of rules under the
Exchange Act and are permitted to file less information with the
SEC than a company incorporated in the United States.
As a foreign private issuer under the Exchange Act, we are
exempt from certain rules under the Exchange Act, including the
proxy rules, which impose certain disclosure and procedural
requirements for proxy solicitations. Moreover, we are not
required to file periodic reports and financial statements with
the SEC as frequently or as promptly as U.S. companies with
securities registered under the Exchange Act; we are not
required to file financial statements prepared in accordance
with U.S. GAAP; and we are not required to comply with
Regulation FD, which imposes certain restrictions on the
selective disclosure of material information. In addition, our
officers, directors and principal shareholders are not subject
to the reporting and short-swing profit recovery provisions of
Section 16 of the Exchange Act and the rules under the
Exchange Act with respect to their purchases and sales of our
Class A or Class B shares. Accordingly, you may receive
less information about us than you would receive about a company
incorporated in the United States and be afforded less
protection under the U.S. federal securities laws than you
would be afforded with respect to a company incorporated in the
United States. If we lose our status as a foreign private issuer
at some future time, we will no longer be exempt from such rules
and, among other things, will be required to file periodic
reports and financial statements as if we were a company
incorporated in the United States. The costs incurred in
fulfilling these additional regulatory requirements could be
substantial.
Additionally, pursuant to NASDAQ Listing Rules, as a foreign
private issuer we may elect to follow our home country practice
in lieu of the corporate governance requirements of the
Rule 5600 Series, with the exception of those rules which
are required to be followed pursuant to the provisions of
Listing Rule 5615(a)(3). We have elected to follow Spanish
practices in lieu of the requirements of the Rule 5600
Series to the extent permitted under NASDAQ Listing
Rule 5615(a)(3). We disclose on our website each
requirement that we do not follow and describe the Spanish
practices followed by us in lieu of such requirements. Our
website is
http://www.grifols.com.
The information provided on our website is not part of this
prospectus and is not incorporated herein by reference.
We may be
exposed to potential risks relating to our internal controls
over financial reporting and our ability to have those controls
attested to by our independent auditors.
Our management is responsible for establishing and maintaining
adequate internal control over financial reporting. Our internal
control over financial reporting is a process designed to
provide reasonable assurance regarding the reliability of
financial reporting and the preparation of financial statements
in accordance with IFRS. Under the provisions of
Section 404 of the Sarbanes-Oxley Act of 2002, we will be
required to include
49
a report by our management on the effectiveness of our internal
control over financial reporting beginning with our Annual
Report on
Form 20-F
for the fiscal year ending December 31, 2011. This report
must contain an assessment by management of the effectiveness of
our internal control over financial reporting as of the end of
our fiscal year and a statement as to whether or not our
internal control over financial reporting is effective. Our
annual report for the fiscal year ending December 31, 2011
must also contain a statement that our independent registered
public accountants have issued an attestation report on the
effectiveness of our internal control over financial reporting.
Our management may conclude that our internal controls over
financial reporting are not effective. Even if our management
concludes that our internal controls over financial reporting
are effective, our independent registered public accounting firm
may still issue a report that is qualified if it is not
satisfied with our controls or the level at which our controls
are documented, designed, operated or reviewed, or if it
interprets the relevant requirements differently from us. We can
provide no assurance that we will be in compliance with all of
the requirements imposed by SOX 404 or that we will receive a
positive attestation from our independent auditors. In the event
we identify significant deficiencies or material weaknesses in
our internal controls that we cannot remediate in a timely
manner or we are unable to receive a positive attestation from
our independent auditors with respect to our internal controls,
investors and others may lose confidence in the reliability of
our financial statements. If we are not in compliance with all
of the requirements imposed by SOX 404 our Annual Report on
Form 20-F
for the fiscal year ending December 31, 2011, we may face
delisting proceedings by NASDAQ. Any of these possible outcomes
could result in an adverse reaction in the financial marketplace
due to a loss of investor confidence in the reliability of our
reporting processes, which could adversely affect the market for
our ADRs or the notes. Our inability to conclude that our
internal control over financial reporting is effective would
also adversely affect the results of the periodic management
evaluations of our disclosure controls and procedures and
internal control over financial reporting that will be required
under the Sarbanes-Oxley Act of 2002.
In addition, in order to maintain and improve our disclosure
controls and procedures and internal control over financial
reporting, significant resources and management oversight will
be required. This may divert management’s attention from
other business concerns, which could have a material adverse
effect on our business, financial condition, results of
operations and cash flows. Until we are required to comply with
these requirements, we will not have comparable procedures in
place as compared to companies already subject to Sarbanes-Oxley.
Our
manufacturing processes are complex and involve biological
intermediates that are susceptible to contamination and
variations in yield.
Plasma is a raw material that is susceptible to damage and
contamination and may contain human pathogens, any of which
would render the plasma unsuitable as raw material for further
manufacturing. For instance, improper storage of plasma by us or
third-party suppliers, if any, may require us to destroy some of
our raw material. If unsuitable plasma is not identified and
discarded prior to the release of the plasma to our
manufacturing process, it may be necessary to discard
intermediate or finished product made from that plasma or to
recall any finished product released to the market, resulting in
a charge to cost of goods sold.
The manufacture of our plasma products is an extremely complex
process of fractionation, purification, filling and finishing.
Our products can become non-releasable or otherwise fail to meet
our specifications through a failure of one or more of our
product testing, manufacturing, process controls and quality
assurance processes. We may detect instances in which an
unreleased product was produced without adherence to our
manufacturing procedures, or plasma used in our production
process was not collected or stored in a compliant manner
consistent with our cGMP or other regulations. Such an event of
noncompliance would likely result in our determination that the
impacted products should not be released and therefore should be
destroyed. For example, a malfunction of the Gamunex IVIG
chromatography system just prior to Talecris’ formation
transaction in 2005 resulted in the processing of IVIG products
containing elevated levels of antibodies for over one month.
Talecris’ total cost related to this incident, including
the costs of product loss, investigation, testing, disposal, and
other remedial actions, was approximately $41.6 million.
Talecris subsequently recovered from Bayer $10.7 million
through its 2005 working capital adjustment and
$9.0 million in the first quarter of 2007 through a
settlement.
50
Once we have manufactured our plasma-derived products, they must
be handled carefully and kept at appropriate temperatures. Our
failure, or the failure of third parties that supply, ship or
distribute our products, to properly care for those products may
require that those products be destroyed.
While we expect to write off small amounts of
work-in-process
inventories in the ordinary course of business due to the
complex nature of plasma, our processes and our products,
unanticipated events may lead to write-offs and other costs
materially in excess of our expectations. We have in the past
had issues with product quality and purity that have caused us
to write off the value of our product. Such write-offs and other
costs could cause material fluctuations in our profitability.
Furthermore, contamination of our products could cause
investors, consumers or other third parties with whom we conduct
business to lose confidence in the reliability of our
manufacturing procedures, which could adversely affect sales and
profits. In addition, faulty or contaminated products that are
unknowingly distributed could result in patient harm, threaten
the reputation of our products and expose us to product
liability damages and claims.
Additionally, due to the nature of plasma there will be
variations in the biologic properties of the plasma we collect
or purchase for fractionation that may result in fluctuations in
the obtainable yield of desired fractions, even if cGMP is
followed. Lower yields may limit production of our
plasma-derived products due to capacity constraints. If these
batches of plasma with lower yields impact production for
extended periods, it may reduce the total capacity of product
that we could market and increase our cost of goods sold, thus
reducing our profitability.
We must
continually monitor the performance of our products once
approved and marketed for signs that their use may elicit
serious and unexpected side effects, which could jeopardize our
ability to continue marketing our products. We may also be
required to conduct post-approval clinical trials as a condition
to licensing a product.
As for all pharmaceutical products, the use of our products
sometimes produces undesirable side effects or adverse reactions
or events, which are referred to collectively as “adverse
events.” For the most part, these adverse events are known,
are expected to occur at some frequency and are described in the
products’ labeling. Known adverse events of a number of our
products include allergic or anaphylactic reactions including
shock and the transmission of infective agents. The use of
albumin sometimes produces the following adverse events:
hypervolaemia, circulatory overload, pulmonary edema and
hyperhydration. The use of Factor XI sometimes produces the
following adverse events: the induction of neutralizing
antibodies (inhibitors), thromboembolism including myocardial
infarction, disseminated intravascular coagulation, venous
thrombosis and pulmonary embolism and nephrotic syndrome (in
case of treatment for immune tolerance induction). The use of
Factor VIII sometimes produces the following adverse events: the
induction of neutralizing antibodies (inhibitors),
thromboembolic events and hemolytic anemia or hemolysis. The use
of IV anti-hepatitis B immunoglobulins sometimes produces
the following adverse events: thromboembolic reactions such as
myocardial infarction, stroke, pulmonary embolism and deep vein
thromboses, aseptic meningitis, hemolytic anemia or hemolysis
and acute renal failure. The use of IVIG sometimes produces the
following adverse events: nausea, vomiting, asthenia, pyrexia,
rigors, injection site reaction, allergic/anaphylactic reaction,
aseptic meningitis, arthralgia, back pain, dizziness, headache,
rash, pruritus, urticaria, hemolysis/hemolytic anemia,
hyperproteinemia, increased serum viscosity and hyponatremia,
thromboembolic reactions such as myocardial infarction, stroke,
pulmonary embolism and deep vein thromboses, transfusion-related
acute lung injury (“TRALI”) and renal dysfunction and
acute renal failure. The use of Plasbumin 5%, 20%, 25% sometimes
produces the following adverse events: allergic manifestations
including urticaria, chills, fever and changes in respiration,
pulse and blood pressure. The use of Plasmanate sometimes
produces the following adverse events: hypotension, flushing,
urticaria, back pain, nausea and headache. The use of Koate DVI,
which we license exclusively in the United States to Kedrion,
sometimes produces the following adverse events: allergic type
reactions; tingling in the arm, ear and face; blurred vision,
headache, nausea, stomach ache and jittery feeling. The use of
Prolastin/Prolastin C sometimes produces the following adverse
events: dyspnea, tachycardia, rash, chest pain, chills,
influenza-like symptoms, hypersensitivity, hypotension,
hypertension.
In addition, the use of our products may be associated with
serious and unexpected adverse events, or with less serious
reactions at a greater than expected frequency. This may be
especially true when our products
51
are used in critically ill patient populations. When these
unexpected events are reported to us, we must make a thorough
investigation to determine causality and implications for
product safety. These events must also be specifically reported
to the applicable regulatory authorities. If our evaluation
concludes, or regulatory authorities perceive, that there is an
unreasonable risk associated with the product, we would be
obligated to withdraw the impacted lot(s) of that product.
Furthermore, an unexpected adverse event of a new product could
be recognized only after extensive use of the product, which
could expose us to product liability risks, enforcement action
by regulatory authorities and damage to our reputation and
public image.
Talecris received reports that some Gamunex patients have
experienced transient hemolysis
and/or
hemolytic anemia, which are known potential side effects for
this class of drugs. Since 2005, a disproportionate number of
these reports have been received from Canada, where Gamunex
accounted for approximately 80% of all IVIG distributed in 2008.
The Canadian product labeling was updated in 2005 after these
hemolysis events were first reported to Health Canada.
Subsequently, Talecris provided annual updates on these events
to Health Canada from 2006 to 2008, but no further action was
recommended by the Canadian regulators. A serious adverse
finding concerning the risk of hemolysis by any regulatory
authority for intravenous immune globulin products in general,
or Gamunex in particular, could adversely affect our business
and financial results.
Once we produce a product, we rely on physicians to prescribe
and administer it as we have directed and for the indications
described on the labeling. It is not, however, unusual for
physicians to prescribe our products for “off-label”
uses or in a manner that is inconsistent with our directions.
For example, a physician may prescribe an infusion rate for our
Flebogamma IVIG product that is greater than our directed
infusion rate, which in turn may reduce its efficacy or result
in some other adverse effect on the patient. Similarly, a
physician may prescribe a higher or lower dosage than the dosage
we have indicated, which may also reduce our product’s
efficacy or result in some other adverse effect on the patient.
To the extent such off-label uses and departures from our
administration directions become pervasive and produce results
such as reduced efficacy or other adverse effects, the
reputation of our products in the marketplace may suffer.
When a new product is approved, the FDA or other regulatory
authorities may require post-approval clinical trials, sometimes
called Phase IV clinical trials. If the results of such
trials are unfavorable, this could result in the loss of the
license to market the product, with a resulting loss of sales.
Our
ability to continue manufacturing and distributing our products
depends on our and our suppliers’ continued adherence to
cGMP regulations.
The manufacturing processes for our products are governed by
detailed written procedures and federal regulations that set
forth cGMP requirements for blood and blood products. Our
quality operations unit monitors compliance with these
procedures and regulations, and the conformance of materials,
manufacturing intermediates and final products to their
specifications. Failure to adhere to established procedures or
regulations, or to meet a specification, could require that a
product or material be rejected and destroyed. There are
relatively few opportunities for us to rework, reprocess or
salvage nonconforming materials or products.
Our adherence to cGMP regulations and the effectiveness of our
quality systems are periodically assessed through inspections of
our facilities by the FDA and analogous regulatory authorities
of other countries. We cannot assure you that we will not be
cited for deficiencies in the future. If deficiencies are noted
during an inspection, we must take action to correct those
deficiencies and to demonstrate to the regulatory authorities
that our corrections have been effective. If serious
deficiencies are noted or if we are unable to prevent
recurrences, we may have to recall product or suspend operations
until appropriate measures can be implemented. We are required
to report some deviations from procedures to the FDA. Even if we
determine that the deviations were not material, the FDA could
require us to take similar measures. Since cGMP reflects
ever-evolving standards, we regularly need to update our
manufacturing processes and procedures to comply with cGMP.
These changes may cause us to incur costs without improving our
profitability or the safety of our products. For example, more
sensitive testing assays may be required (if and when they
become available) or
52
existing procedures or processes may require revalidation, all
of which may be costly and time-consuming and could delay or
prevent the manufacturing of a product or launch of a new
product.
Changes in manufacturing processes, including a change in the
location where the product is manufactured or a change of a
third-party manufacturer, may require prior FDA review and
approval or revalidation of the manufacturing process and
procedures in accordance with cGMP. There may be comparable
foreign requirements.
For example, we are in the process of
start-up and
validation of the new IVIG Flebogamma DIF Facility in Los
Angeles, California. To validate our manufacturing processes and
procedures following completion of upgraded facilities, we must
demonstrate that the processes and procedures at the upgraded
facilities are comparable to those currently in place at our
facilities. In order to provide such a comparative analysis,
both the existing processes and the processes that we expect to
be implemented at our upgraded facilities must comply with the
regulatory standards prevailing at the time that our expected
upgrade is completed. In addition, regulatory requirements,
including cGMP regulations, continually evolve. Failure to
adjust our operations to conform to new standards as established
and interpreted by applicable regulatory authorities would
create a compliance risk that could impair our ability to
sustain normal operations.
In addition, we have completed the process of transferring the
manufacture of our Thrombate III product from Bayer’s
Berkeley, California, biologics manufacturing facility to our
Clayton manufacturing facility that we are currently validating
with regulatory approval expected in 2012. We cannot guarantee
that we have a sufficient inventory of intermediates and
finished product to meet demand until the new facility is
approved and manufacturing can recommence. To validate our
manufacturing processes and procedures following completion of
upgraded facilities, we must demonstrate that the processes and
procedures at the upgraded facilities are comparable to those
currently in place at Bayer’s facilities. In order to
provide such a comparative analysis, both the existing processes
and the processes that we expect to be implemented at our
upgraded facilities must comply with the regulatory standards
prevailing at the time that our expected upgrade is completed.
If the FDA does not approve the transfer, our ability to market
our Thrombate III product will be seriously impaired or
eliminated. In addition, regulatory requirements, including cGMP
regulations, continually evolve. Failure to adjust our
operations to conform to new standards as established and
interpreted by applicable regulatory authorities would create a
compliance risk that could impair our ability to sustain normal
operations.
A number of inspections by the FDA and foreign control
authorities, including the European Medicines Agency, which is
referred to as the EMA, have been conducted or are expected at
our plasma collection centers in 2011. Some of these inspections
are of licensed centers to assess ongoing compliance with cGMP.
If the FDA (or other authorities) finds these centers not to be
in compliance, our ongoing operations
and/or plans
to expand plasma collections would be adversely affected.
We would
become supply-constrained and our financial performance would
suffer if we could not obtain adequate quantities of
FDA-approved source plasma.
In order for plasma to be used in the manufacturing of our
products, the individual centers at which the plasma is
collected must be licensed by the FDA and approved by the
regulatory authorities, such as the EMA, of those countries in
which we sell our products. When a new plasma collection center
is opened, and on an ongoing basis after its licensure, it must
be inspected by the FDA and the EMA for compliance with cGMP and
other regulatory requirements. An unsatisfactory inspection
could prevent a new center from being licensed or risk the
suspension or revocation of an existing license.
In order to maintain a plasma center’s license, its
operations must continue to conform to cGMP and other regulatory
requirements. In the event that we determine that plasma was not
collected in compliance with cGMP, we may be unable to use and
may ultimately destroy plasma collected from that center, which
would be recorded as a charge to cost of goods. Additionally, if
noncompliance in the plasma collection process is identified
after the impacted plasma has been pooled with compliant plasma
from other sources, entire plasma pools, in-process intermediate
materials and final products could be impacted. Consequently, we
could experience significant inventory impairment provisions and
write-offs which could adversely affect our
53
business and financial results. During 2008, Talecris
experienced such an event at one of their plasma collection
centers, which resulted in a charge to cost of goods sold of
$23.3 million, for which they subsequently recovered
$19.4 million through December 31, 2010. In this
particular instance, a portion of the impacted plasma had been
released to manufacturing prior to Talecris’ detection of
the issue.
We plan to obtain our supplies of plasma for use in our
manufacturing processes through collections at our plasma
collection centers and through selective acquisitions or
remodeling and relocations of existing centers. This strategy is
dependent upon our ability to successfully integrate new
centers, to obtain FDA and other necessary approvals for the
remaining unlicensed plasma centers, to maintain a cGMP
compliant environment in all plasma centers, and to expand
production and attract donors to our centers.
Our ability to maintain a cGMP compliant environment in all
plasma collection centers may be challenged as a result of the
implementation of a comprehensive set of new Standard Operating
Procedures (“SOPs”) that have been approved by the
FDA. Implementing the revised SOPs will be a substantial
project, which will temporarily increase cost and reduce plasma
collection volumes. We have completed the conversion of all of
our collection centers to these new SOPs. The change in SOPs,
although intended to improve quality and compliance, could
temporarily lead to an increase in issues and audit findings by
us, the FDA or other regulatory agencies.
Our ability to expand production and increase our plasma
collection centers to more efficient production levels may be
affected by changes in the economic environment and population
in selected regions where we operate plasma centers, by the
entry of competitive plasma centers into regions where we
operate, by misjudging the demographic potential of individual
regions where we expect to expand production and attract new
donors, by unexpected facility related challenges, or by
unexpected management challenges at selected plasma centers.
A
significant disruption in our supply of plasma could have a
material adverse effect on our business and our growth
plans.
The majority of our revenue depends on our access to
U.S. source plasma, the principal raw material for our
plasma derivative products. Our ability to increase revenues
depends substantially on increased access to plasma. We expect
that our plasma needs for 2012 and going forward will be met
through the volumes of collection at our 147 plasma collection
centers in the United States and supplemented by approximately
800,000 liters of plasma per year to be purchased from
third-party suppliers for the next three years pursuant to
multiple plasma purchase agreements assumed in connection with
the acquisition. If we are unable to obtain sufficient
quantities of source plasma, we may be unable to find an
alternative cost-effective source of plasma.
If we are unable to obtain sufficient quantities of source
plasma, we would be limited in our ability to maintain current
manufacturing levels of plasma derivative products. As a result,
we could experience a substantial decrease in net sales or
profit margins, a loss of customers, a negative effect on our
reputation as a reliable supplier of plasma derivative products,
or a substantial delay in our production growth plans.
Our current business plan envisages an increase in the
production of plasma derivative products, which depends on our
ability to increase plasma collections
and/or
improve product yield. The ability to increase plasma
collections may be limited, our supply of plasma could be
disrupted, or the cost of plasma could increase substantially,
as a result of numerous factors, including:
|
|
|
| |
•
|
A reduction in the donor pool. Regulators in
most of the largest markets for plasma derivative products,
including the United States, restrict the use of plasma
collected from specific countries and regions in the manufacture
of plasma derivative products. For example, the appearance of
the variant Creutzfeldt-Jakob disease, commonly referred to as
“mad cow” disease (which resulted in the suspension of
the use of plasma collected from U.K. residents), and concern
over the safety of blood products (which has led to increased
domestic and foreign regulatory control over the collection and
testing of plasma and the disqualification of certain segments
of the population from the donor pool) have significantly
reduced the potential donor pool. The appearance of new viral
strains could further reduce the potential donor pool. Also,
improvements in socio-economic conditions in the areas where
|
54
|
|
|
| |
|
our and our suppliers’ collection centers are located can
reduce the attractiveness of financial incentives for donors,
resulting in increased donor fees
and/or a
reduction in the number of donors.
|
|
|
|
| |
•
|
Regulatory requirements. The collection of
plasma is heavily regulated, and our ability to collect plasma
(or to increase plasma collection) through our collection
centers, or to obtain plasma from other suppliers, may be
limited or disrupted by the inability to obtain or maintain
necessary regulatory licenses to operate plasma collection
centers in a timely manner or at all, or by the temporary or
permanent shutdown of our or our suppliers’ plasma
collection centers as a result of regulatory violations.
|
| |
| |
•
|
Plasma supply sources. In recent years, there
has been vertical integration in the industry as plasma
derivatives manufacturers have been acquiring plasma collectors.
Plasma availability in the United States grew from approximately
13.7 million liters in 2002 to approximately
18.3 million liters in 2010, while the number of plasma
collection centers declined from 407 to 396 during the same
period. Any significant disruption in supply of plasma or an
increased demand for plasma may require plasma from alternative
sources, which may not be available on a timely basis.
|
A
significant portion of our revenue has historically been derived
from sales of our largest product, Flebogamma IVIG, and we
anticipate that the IVIG product acquired from Talecris,
Gamunex-C/Gamunex IVIG, will also comprise a significant portion
of our net sales on a going forward basis. Any adverse market
event with respect to these products would have a material
adverse effect on us.
We have historically derived a significant portion of our net
sales from our product Flebogamma IVIG. Sales of Flebogamma IVIG
comprised approximately 37% of our total net sales in the fiscal
year ended December 31, 2010. In connection with the
acquisition of Talecris, we have acquired their IVIG product,
Gamunex-C/Gamunex IVIG. We anticipate that Gamunex-C/Gamunex
IVIG will comprise a significant portion of our net sales on a
going forward basis. If either Flebogamma IVIG or
Gamunex-C/Gamunex IVIG lost significant sales, or were
substantially or completely displaced in the market, we would
lose a significant and material source of our net revenue.
Similarly, if either Flebogamma IVIG or Gamunex-C/Gamunex IVIG
were to become the subject of litigation
and/or an
adverse governmental ruling requiring us to cease sales of
either product, our business could be adversely affected.
Although we do not currently anticipate any significant decrease
in the sales of any of these products, a significant decrease
could result from plasma procurement and manufacturing issues
resulting in lower product availability for sales and changing
market conditions.
Our
products face increased competition.
Our products have experienced increased competition. Each of
Baxter and CSL Behring have launched 10% liquid IVIG products in
the United States. Octopharma has launched a 5% liquid IVIG and
we expect Octapharma to launch a 10% liquid IVIG within the next
year. Omrix and Biotest are both seeking approval for liquid
IVIG products in the United States, which, if approved, will
further increase competition among liquid IVIG products. In
2010, CSL Behring received FDA approval and launched Hizentra
Immune Globulin Subcutaneous (Human) 20% liquid. Additionally,
Bio Products Laboratory received approval from the FDA for its
5% concentration IVIG for PI. As competition has increased,
competitors have discounted the price of IVIG products.
Furthermore, many customers are increasingly more price
sensitive regarding IVIG products. If customers demand lower
priced products of competitors, we may lose sales or be forced
to lower our prices.
Octapharma’s IGIV products have been off the market during
the fourth quarter of 2010 and all of 2011 in the U.S. and
other parts of the world. Beginning in the third quarter of
2011, Octapharma began selling their IVIG products again in
Europe. When Octapharma further resumes sales of its products,
it may discount prices to regain lost market share. If customers
demand lower priced products of competitors, we may lose sales
or be forced to lower our prices.
The FDA recently approved Gamunex-C for the subcutaneous route
of administration for the PI indication. We believe that our
competitors are developing several new products and technologies
potentially offering an improved route of administration
possibly for indications beyond PI. If these development efforts
55
are successful and our efforts fail, then we will be at a
competitive disadvantage that may impact our Gamunex sales.
Until December 2002, Talecris’ A1PI product, Prolastin
A1PI, was the only plasma product licensed and marketed for
therapy of congenital A1PI deficiency-related emphysema in the
U.S. Baxter and CSL Behring received licenses for Aralast
and Zemaira, respectively, which were launched in the
U.S. in 2003. In addition, Kamada Ltd. received approval of
its BLA for its A1PI, Glassia, on July 1, 2010. In Europe,
we had an 87% share of A1PI sales in 2008 according to the MRB,
and have the only licensed A1PI products that have marketing
authorization in Europe, with the exception of LFB, which sells
its A1PI product, Alfalastin, in France. Our competitors are
currently pursuing licensing trials in Europe. Should our
competitors receive approvals in Europe sooner than expected,
this will impact our unit volumes and share of sales. These and
other future competitors may increase their sales, lower their
prices or change their distribution model that may harm our
product sales and financial condition. Also, if the attrition
rate of our A1PI patient base accelerates faster than we have
forecast, we would have fewer patients and lower sales volume.
New products may reduce demand for plasma-derived A1PI. A
recombinant form of A1PI (recA1PI) could gain market share
through the elimination of the risk of plasma-borne pathogens,
or through a reduced price permitted by significantly decreased
costs (since the recA1PI would not be sourced from plasma).
Arriva and GTC Biotherapeutics are in the early stages of
development for a recombinant form of A1PI. Although we are not
aware of any active clinical trials for a recA1PI product, a
successful recA1PI, prior to our development of a similar
product, could gain first mover advantage and result in a loss
of our A1PI market share. Similarly, if a new formulation of
A1PI is developed that has a significantly improved rate of
administration, such as aerosol inhalation, the market share of
our A1PI products could be negatively impacted. Similarly,
several companies are attempting to develop products which would
be substitution threats in the A1PI sector, including retinoic
acid, oral synthetic elastase inhibitors and gene therapy. While
these products are all in early stages of development, the
potential for successful product development and launch cannot
be ruled out.
In addition, our plasma-derived therapeutics face competition
from non-plasma products and other courses of treatments. For
example, two RhD hyperimmune globulins for intravenous
administration, Cangene’s WinRho SDF and CSL Behring’s
Rhophylac, are now approved for use to treat ITP, and GSK and
Amgen launched thrombopoietin inhibitors targeting ITP patients
in 2008 that may reduce the demand for IVIG to treat this immune
disorder. There is also a risk that indications for which our
products are now used will be susceptible to new treatments,
such as small molecules, monoclonal or recombinant products.
Recombinant Factor VIII products compete with our own
plasma-derived product in the treatment of Hemophilia A and are
perceived by many to have lower risks of disease transmission.
Additional recombinant products or the use of monoclonal
antibodies, small molecules, or stem cell transplantations could
compete with our products and reduce the demand for our
products. Crucell and Sanofi Pasteur have completed
Phase II clinical trials for a monoclonal rabies product to
compete with our rabies hyperimmune product. If successful, we
estimate that the monoclonal product could take a significant
portion of the rabies market in years subsequent to its
introduction. Also, in February 2009, GTC Biotherapeutics
obtained FDA approval of a competitive ATIII product for the
treatment of hereditary antithrombin deficiency, which is
derived from the milk of transgenic goats. This product now
directly competes with our product, Thrombate III (Human),
which had previously been the only FDA approved product.
Since the late 1980s, Talecris (and prior to 2005, Bayer) had
been the “supplier of record” for the Canadian blood
system. Talecris was awarded new five-year contracts with
Canadian Blood Services and Hema Quebec, the Canadian blood
system operators, in December 2007 that became effective
April 1, 2008. We assumed these contracts in connection
with the acquisition of Talecris, making us the largest supplier
of plasma-derived products to these operators. The contracts may
be extended for two one-year terms upon agreement of the
parties. Under these contracts, we fractionate 100% of the
Canadian plasma initially and a majority of the Canadian plasma
throughout the contract period and supply a majority of the
Canadian requirements for IVIG during the contract term as well.
We transport plasma from Canadian Blood Services and Hema Quebec
collection centers to our manufacturing facility in Clayton,
North Carolina for manufacture, and return the finished product,
along with commercial product, for sale to Canadian Blood
Services and
56
Hema Quebec. Pricing for our products and services is set at the
beginning of the contract period, subject to adjustment for
inflation. The U.S. Dollar based contracts are terminable
upon default, or the occurrence of certain events, including a
third party obtaining Canadian regulatory approval to introduce
a significantly superior product or fractionation service, our
products or services becoming obsolete, or if we make certain
nonrelated improvements and Canadian Blood Services or Hema
Quebec do not accept the associated price increase.
Canadian Blood Services has elected to pursue a multi-source
strategy and although we will continue to be the primary
supplier, we anticipate annual volume declines because of this
strategy. Hema Quebec currently has a sole source strategy for
fractionation of their plasma but could switch to a multi-source
strategy. In 2010, Talecris fractionated 71% of Canadian plasma
and supplied 67% of Canadian requirements of IGIV. Talecris had,
and we expect to continue to, derive significant revenue and
profits under these contracts, and a failure to maintain
contracts with the Canadian blood system operators or any
diminution in the volume or price under future contracts could
have a material adverse effect on our financial results.
New
products could render our plasma derivative products less
competitive.
Our plasma derivative products may face intense competition from
alternative products resulting from technological advances. In
particular, recombinant products, which result from the
alteration of the genes of particular cells, are generally
perceived to be safer than non-recombinant ones. Recombinant
substitutes are currently available for Factor VIII and Factor
IX and are widely used in the United States and Europe. In
addition, less expensive alternatives have long existed for
albumin in its application as a plasma volume expander. If an
increased use of alternative products for Factor VIII, Factor IX
or albumin makes it uneconomical to produce our plasma derived
equivalents or if further technological advances improve these
products or create other competitive alternatives to our plasma
derivative products, our financial condition and results of
operations could be materially adversely affected.
We do not currently sell any recombinant products. Although we
are considering developing recombinant versions of Plasmin, A1PI
and Factor VIII, we cannot be certain that any of these products
will ever be approved or commercialized. As a result, our
product offerings may remain plasma-derived, even if our
competitors offer competing recombinant products.
We face
competition from companies with greater financial
resources.
We operate in highly competitive markets. Our principal
competitors include Baxter International, Inc., Octapharma AG,
CSL, Bio-Rad Laboratories, Ortho Clinical Diagnostic, B. Braun
Melsungen AG, Macopharma and Fresenius Medical Care AG, among
others. Some of our competitors have significantly greater
financial resources than us. As a result, they may be able to
devote more funds to research and development and new production
technologies, as well as to the promotion of their products and
business. These competitors may also be able to sustain for
longer periods a deliberate substantial reduction in the price
of their products or services. The development by a competitor
of a similar or superior product or increased pricing
competition may result in a reduction in our net sales or a
decrease in our profit margins.
Our
products have historically been subject to supply-driven price
fluctuations.
Our products, particularly IVIG, have historically been subject
to price fluctuations as a result of changes in the production
capacity available in the industry, the availability and pricing
of plasma, development of competing products and the
availability of alternative therapies. Higher prices for
plasma-derived products have traditionally spurred increases in
plasma production and collection capacity, resulting over time
in increased product supply and lower prices. As demand
continues to grow, if plasma supply and manufacturing capacity
do not commensurately expand, prices tend to increase.
The robust demand for plasma derived products, particularly for
IVIG, over the last few years has resulted in efforts on the
part of companies, including us, to increase manufacturing
capacity and open new plasma collection centers to increase the
availability of source plasma. Some of our competitors have
announced plans to grow product supply at a rate above expected
demand growth. The growth in demand for IVIG has been
57
outpaced by the recent supply growth, as evidenced by increased
supply in the distribution channel. We, or our competitors, may
misjudge demand growth and over-invest in expanding plasma
collection or manufacturing capacity, which ultimately may
result in lower prices for, or inability to sell, our products.
If we are
unable to obtain product licenses, revenue growth will be
negatively affected.
Revenue growth depends, among other things, on our ability to
have new bioscience and diagnostic products approved for sale in
various jurisdictions in a timely manner. The failure to obtain
a product license without significant delay, or at all, could
materially adversely affect our prospects for revenue growth.
Technological
changes in the production of plasma derivative products could
render our production process uneconomical.
Technological advances have accelerated changes in many
bioscience industries in recent years. Future technological
developments could render our production processes for plasma
derivative products uneconomical and may require us to invest
substantial amounts of capital to upgrade our facilities. Such
investments could have a material adverse effect on our
financial condition and results of operations. In addition, we
may not be able to fund such investment from existing funds or
raise sufficient capital to make such investments.
The
discovery of new pathogens could slow down our growth and
adversely affect profit margins.
The possible appearance of new pathogens could trigger the need
for changes in our existing quality control, inactivation and
production methods, including the administration of new
detection tests. Such a development could result in delays in
production until the new methods are in place, as well as
increased costs that may not be readily passed on to our
customers.
Product
liability claims or product recalls involving our products or
products we distribute could have a material adverse effect on
our business.
Our business exposes us to the risk of product liability claims
that are inherent in the manufacturing, distribution and sale of
plasma-derived therapeutic protein products. We face an inherent
risk of product liability exposure related to the testing of our
product candidates in human clinical trials and an even greater
risk when we commercially sell any products. If we cannot
successfully defend ourselves against claims that our product
candidates or products caused injuries, we could incur
substantial liabilities. Regardless of merit or eventual
outcome, liability claims may result in:
|
|
|
| |
•
|
decreased demand for our products and any product candidates
that we may develop;
|
| |
| |
•
|
injury to our reputation;
|
| |
| |
•
|
withdrawal of clinical trial participants;
|
| |
| |
•
|
costs to defend the related litigation;
|
| |
| |
•
|
substantial monetary awards to trial participants or patients;
|
| |
| |
•
|
loss of revenue; and
|
| |
| |
•
|
the inability to commercialize any products that we may develop.
|
Like many fractionators of plasma, we have been, and may in the
future be, involved in product liability claims relating to our
products, including claims alleging the transmission of disease
through the use of such products. For example, since the 1980s,
it has been alleged that hemophiliacs became infected with
hepatitis C
and/or the
HIV virus by using clotting factor concentrates derived from
human plasma, like our Factor VIII products. Plasma is a
biological matter that is capable of transmitting viruses and
pathogens, whether known or unknown. Therefore, our plasma and
plasma derivative products, if not properly collected, tested,
inactivated, processed, stored and transported, could cause
serious disease and possibly death to the patient. Further, even
when we properly affect such steps, there are viral and other
infections of plasma which may escape detection using current
testing methods and which are not susceptible to inactivation
methods. Any
58
transmission of disease through the use of one of our products
or third-party products sold by us could result in claims by
persons allegedly infected by such products.
Our potential product liability also extends to our diagnostic
and hospital products. In particular, a misdiagnosis due to a
defect in the manufacturing of a blood testing or blood
classification machine or of a reagent could result in serious
injury to the patient whose blood was tested. Likewise, a poorly
sealed blood bag or an inadequate sterilization of solutions
that results in contamination of that product could give rise to
product liability claims. In addition, we sell and distribute
third-party products, and the laws of the jurisdictions where we
sell or distribute these products could also expose us to
product liability claims for those products. Furthermore, the
presence of a defect in a product could require us to carry out
a recall of such product.
Bayer is the defendant in continuing litigation alleging that
use of products manufactured at Talecris’ Clayton site in
the 1980s, prior to Talecris’ formation transaction and
carve-out from Bayer, resulted in the transmission of
hepatitis C virus and HIV to patients. Bayer is also a
defendant in litigation alleging that thimerosal, a preservative
that was added to some intra muscular (hyperimmune) immune
globulin products until 1996 (at which time its use was
discontinued), was the cause of autism and other disorders in
children who received these products. While Talecris is not a
party to any of these actions, and Bayer has agreed to fully
indemnify Talecris from any claims or losses arising out of
these actions, we cannot assure you that our products or any of
their constituents or additives may not someday give rise to
similar product liability claims that we will be forced to
defend and which may have a material adverse affect on our
business.
A product liability claim or a product recall could result in
substantial financial losses, negative reputational
repercussions and an inability to retain customers. We have
product liability insurance coverage for up to
€105 million per insurable event and per year (except
for HIV and hepatitis B or C infections, where the maximum
aggregate amount covered is €13.0 million), although
we have elected to self-insure the first €10.0 million
per claim per year through the purchase by one of our
subsidiaries of such portion of the insurance policy. Claims
made against our insurance policies could exceed our limits of
coverage. We intend to expand our insurance coverage as our
sales grow. However, as product liability insurance is expensive
and can be difficult to obtain, a product liability claim could
decrease our access to product liability insurance on acceptable
terms. In turn, we may not be able to maintain insurance
coverage at a reasonable cost and may not be able to obtain
insurance coverage that will be adequate to satisfy any
liability that may arise. See the section entitled
“Business — Insurance Coverage.”
Our
ability to continue to produce safe and effective products
depends on the safety of our plasma supply against transmittable
diseases.
Despite overlapping safeguards, including the screening of
donors and other steps to remove or inactivate viruses and other
infectious disease causing agents, the risk of transmissible
disease through plasma-derived products cannot be entirely
eliminated. For example, since plasma-derived therapeutics
involve the use and purification of human plasma, there has been
concern raised about the risk of transmitting HIV, prions, West
Nile virus, H1N1 virus (commonly known as the “swine
flu”) and other blood-borne pathogens. There are also
concerns about the future transmission of avian flu H5N1 virus
(commonly known as the “bird flu”). In the 1980s,
thousands of hemophiliacs worldwide were infected with HIV
through the use of contaminated Factor VIII.
New infectious diseases emerge in the human population from time
to time. If a new infectious disease has a period during which
time the causative agent is present in the bloodstream but
symptoms are not present, it is possible that that infectious
agent could contaminate plasma donations. Typically, early in an
outbreak of a new disease, tests for the causative agent do not
exist. During this early phase, we must rely on screening of
donors (e.g., for behavioral risk factors or physical
symptoms) to reduce the risk of plasma contamination. Screening
methods are generally less sensitive and specific than a direct
test as a means of identifying potentially contaminated plasma
units.
During the early phase of an outbreak of a new infectious
disease, our ability to manufacture safe products would depend
on the manufacturing process’ capacity to inactivate or
remove the infectious agent. To
59
the extent that a product’s manufacturing process is
inadequate to inactivate or remove an infectious agent, our
ability to manufacture and distribute that product would be
impaired.
If a new infectious disease was to emerge in the human
population, the regulatory and public health authorities could
impose precautions to limit the transmission of the disease that
would impair our ability to procure plasma, manufacture our
products or both. Such precautionary measures could be taken
before there is conclusive medical or scientific evidence that a
disease poses a risk for plasma-derived products.
In recent years, new testing and viral inactivation methods have
been developed that more effectively detect and inactivate
infectious viruses in collected plasma. There can be no
assurance, however, that such new testing and inactivation
methods will adequately screen for, and inactivate, infectious
agents in the plasma used in the production of our products.
Plasma
and plasma derivative products are fragile and improper handling
of our plasma or plasma derivative products could adversely
affect results of operations.
Plasma is a raw material that is susceptible to damage. Almost
immediately after its collection from a donor, plasma is stored
and transported at temperatures that are at least -20 degrees
Celsius. The production of plasma derivative products occurs at
near freezing temperatures. Once we manufacture plasma
derivative products, they must be handled carefully and kept at
appropriate temperatures. Our failure, or the failure of third
parties that supply, ship or distribute our plasma and plasma
derivative products, to properly care for our plasma or plasma
derivative products may require us to destroy some raw materials
or products. If the volume of plasma or plasma derivative
products damaged by such failures were significant, the loss of
that plasma or those plasma derivative products could have a
material adverse effect on our financial condition and results
of operations.
Our
above-average aging of our receivables has in the past
negatively affected and may in the future negatively affect our
working capital levels and increase financial costs.
Our receivables have an aging average of 84 days,
83 days, 83 days and 63 days at December 31,
2008, 2009 and 2010 and the six months ended June 30, 2011,
respectively, which is substantially higher than the receivables
aging average for the industry in the United States. Our high
receivables aging average is primarily due to significant delays
in collection from Spain, Portugal and Italy. The adoption by
Spain, effective December 31, 2004, of a European Union
directive that requires payment of interest on most receivables
from hospitals and clinics that are part of the social security
systems in Spain that are more than 60 days overdue has
resulted in a significant decrease in collection delays from
these hospitals and clinics. However, we cannot assure that this
trend will continue or that the present receivables aging levels
for these hospitals and clinics will not increase again,
particularly if the funding of these hospitals and clinics is
not increased sufficiently by the appropriate governmental
health agencies. Failure to receive timely payments for the sale
of our products negatively affects our working capital levels
and may require us to obtain more short-term financing than
would otherwise be needed.
Our
future success depends on our ability to retain members of our
senior management and to attract, retain and motivate qualified
personnel.
We are highly dependent on the principal members of our
executive and scientific teams. The loss of the services of any
of these persons might impede the achievement of our research,
development, operational and commercialization objectives. In
particular, we believe the loss of the services of any of Victor
Grifols Roura, Juan-Ignacio Twose Roura, Ramon Riera Roca,
Alfredo Arroyo Guerra, Carlos Roura Fernandez, Vicente Blanquer
Torre, Eva Bastida Tubau, Mateo Borras Humbert, Antonio
Viñes Pares, Montserrat Lloveras Calvo, David I. Bell,
Gregory G. Rich, Shinji Wada, Alberto Grifols Roura, Francisco
Javier Jorba Ribes, Nuria Pascual Lapeña, Mary Kuhn or Joel
Abelson would significantly and negatively impact our business.
We do not maintain “key person” insurance on any of
our executive officers.
Recruiting and retaining qualified operations, finance and
accounting, scientific, clinical and sales and marketing
personnel will be critical to our success. We may not be able to
attract and retain these personnel
60
on acceptable terms, given the competition among numerous
pharmaceutical and biotechnology companies for similar
personnel. We also experience competition for the hiring of
scientific and clinical personnel from universities and research
institutions. In addition, we rely on consultants and advisors,
including scientific and clinical advisors, to assist us in
formulating our research and development and commercialization
strategy. Our consultants and advisors may be employed by
employers other than us and may have commitments under
consulting or advisory contracts with other entities that may
limit their availability to us. If we are unable to attract,
retain and motivate qualified and experienced personnel, we
could lose customers and suffer reduced profitability. Even if
we are successful in attracting and retaining such personnel,
competition for such employees may significantly increase our
compensation costs and adversely affect our financial condition
and results of operations.
Federal cGMP regulations also require that the personnel we
employ and hold responsible for the collection, processing,
testing, storage or distribution of blood or blood components be
adequate in number, educational background, training and
experience, including professional training as necessary, or
combination thereof, and have capabilities commensurate with
their assigned functions, a thorough understanding of the
procedures or control operations they perform, the necessary
training or experience, and adequate information concerning the
application of relevant cGMP requirements for their individual
responsibilities. Our failure to attract, retain, and motivate
qualified personnel may result in a regulatory violation, affect
product quality, require recall or market withdrawal of affected
product, or a suspension or termination of our license to market
our products, or any combination thereof.
Our
business requires substantial capital to operate and grow and to
achieve our strategy of realizing increased operating leverage,
including the completion of several large capital
projects.
We intend to undertake several large capital projects that will
allow us to expand our production capabilities and achieve
operating leverage. Capital projects of this magnitude involve
technology and project management risks. Technologies that have
worked well in a laboratory or in a pilot plant may cost more or
not perform as well, or at all, in full-scale operations.
Projects may run over budget or be delayed. We cannot be certain
that these projects will be completed in a timely manner or that
we will maintain our compliance with cGMP, and we may need to
spend additional amounts to achieve compliance. Additionally, by
the time these multi-year projects are completed, market
conditions may differ significantly from our assumptions
regarding the number of competitors, customer demand,
alternative therapies, reimbursement and public policy, and as a
result capital returns might not be realized.
We plan on spending substantial sums in capital and operating
expense over the next five years to obtain FDA approval, and
that of other regulatory agencies, for new indications for
existing products, to enhance the facilities in which and
processes by which we manufacture existing products, to develop
new product delivery mechanisms for existing products, to
strengthen our plasma collection system and to develop
innovative product additions. We face a number of obstacles to
successfully converting these efforts into profitable products
including but not limited to the successful development of an
experimental product for use in clinical trials, the design of
clinical study protocols acceptable to FDA and other regulatory
agencies, the successful outcome of clinical trials, our ability
to scale our manufacturing processes to produce commercial
quantities or successfully transition technology, FDA approval,
and that of other regulatory agencies, of our product or process
and our ability to successfully market an approved product with
our new process or new indication.
Our planned capital spending is expected to be significant over
the next four years. We currently estimate our capital spending
to be in the range of €650 million to
€700 million on a cumulative basis from 2011 through
2015. The amount and timing of future capital spending is
dependent upon a number of factors, including market conditions,
regulatory requirements and the extent and timing of particular
projects, among other things. Our ability to grow our business
is dependent upon the timely completion of these facilities and
obtaining the requisite regulatory approvals.
To finance these various activities, we may need to incur future
debt or issue additional equity if our cash flows and capital
resources are insufficient, and we may not be able to structure
our debt obligations on
61
favorable economic terms. As a result of the acquisition, we
have substantial indebtedness, which may adversely affect our
ability to structure our debt obligations on favorable economic
terms.
We may
not be able to develop some of our international operations
successfully.
We currently conduct sales in over 100 countries. The successful
operation of such geographically dispersed resources requires
considerable management and financial resources. In particular,
we must bridge our business culture to the business culture of
each country in which we operate. In addition, international
operations and the provision of services in foreign markets are
subject to additional risks such as changing market conditions,
currency exchange rate fluctuations, trade barriers, exchange
controls, regulatory changes, changes to the tax regime, foreign
investment limitations, civil disturbances and war. Furthermore,
if an area in which we have significant operations or an area
into which we are looking to expand suffers an economic
recession
and/or
currency devaluation, our net sales and accounts receivable
collections in that region will likely decline substantially or
we may not be able to successfully expand in that region.
We are
susceptible to interest rate variations.
A majority of our interest-bearing debt at December 31,
2010 and June 30, 2011 bore interest at a floating rate, at
a spread over LIBOR for our U.S. Dollar-denominated debt
and at a spread over EURIBOR for our Euro-denominated debt. At
December 31, 2010, we had a total interest-bearing debt of
€837.8 million, of which €399 million bore a
floating rate of interest. At June 30, 2011, we had a total
of $3.6 billion and €458.4 million of long-term
interest-bearing debt outstanding (not including approximately
$50 million, €36.7 million and the
$200 million equivalent in multicurrencies available for
additional borrowing under the Revolving Credit Facilities), of
which $2.5 billion and €458.4 million bore a
floating rate of interest, respectively. Pursuant to mandatory
hedging requirements under the Senior Credit Facilities, 62% of
our U.S. Dollar-denominated debt under the Senior Term
Loans is hedged at a fixed rate. However, our Euro-denominated
debt is not hedged. Any increase in interest rates payable by
us, which could be adversely affected by, among other things,
our inability to meet certain financial ratios, would increase
our interest expense and reduce our cash flow, which could
materially adversely affect our financial condition and results
of operations.
Our
results of operations and financial condition may be affected by
adverse changes in foreign currency exchange rates, especially a
significant shift in the value of the Euro as compared to the
U.S. Dollar.
A significant portion of our business is conducted in currencies
other than our reporting currency, the Euro. For example, we are
exposed to currency fluctuations with respect to other
currencies such as the U.S. Dollar, the British pound, the
Brazilian real, the Canadian Dollar, the Malaysian ringgit and
the Argentine, Mexican and Chilean pesos. The majority of our
net sales for the six months ended June 30, 2011 were
denominated in U.S. Dollars. As a result, currency
fluctuations among the Euro, the U.S. Dollar and the other
currencies in which we do business have caused foreign currency
translation gains and losses in the past and will likely do so
in the future. In particular, a devaluation of the
U.S. Dollar against the Euro would result in (i) a
decrease in net sales in Euro terms for sales denominated in
U.S. Dollars, and (ii) a decrease in costs in Euro
terms for costs denominated in U.S. dollars. A devaluation
of the Euro against the U.S. Dollar would have the opposite
effect. As a result we could incur unanticipated gains and
losses as a result of changes in foreign currency exchange rates.
We are also exposed to risk based on the payment of
U.S. dollar-denominated indebtedness. At December 31,
2010, $549 million of our indebtedness was denominated in
U.S. Dollars. At June 30, 2011, $2.5 billion
under the Senior Term Loans, $50 million of undrawn
availability under the Revolving Credit Facilities (not
including an additional $200 million equivalent in
multicurrencies of undrawn availability under the Revolving
Credit Facilities which can be drawn in either U.S. Dollars or
Euros) and $1.1 billion aggregate principal amount of
8.25% senior notes due 2018 were denominated in
U.S. Dollars.
62
Developments
in the economy may adversely impact our business.
Since the middle of 2007, there has been disruption and turmoil
in financial markets around the world. Throughout many of our
largest markets, including the United States and Spain, there
have been dramatic declines in the housing market, high levels
of unemployment and underemployment, and reduced earnings, or,
in some cases, losses, for businesses across many industries,
with reduced investments in growth.
A recessionary economic environment may adversely affect demand
for our products. Prolastin/Prolastin-C A1P1 is sold directly to
patients in the United States. As a result of loss of jobs,
patients may lose medical insurance and be unable to purchase
needed medical products or may be unable to pay their share of
deductibles or co-payments. IVIG is primarily sold to hospitals
and specialty pharmacies. Hospitals adversely affected by the
economy may steer patients to less costly therapies, resulting
in a reduction in demand, or demand may shift to public health
hospitals, which purchase at a lower government price. While to
date we cannot directly trace any material reduction in demand
to the recession, if economic conditions do not improve, the
impact may become material.
If our
Los Angeles, Barcelona or Clayton facilities were to suffer a
crippling accident, or a force majeure event materially affected
our ability to operate and produce saleable products, a
substantial part of our manufacturing capacity could be shut
down for an extended period.
Substantially all of our revenues are derived from products
manufactured at our plants located in Parets del Vallès
(Barcelona), Clayton, North Carolina and Los Angeles,
California. In addition, a substantial portion of our plasma
supply is stored at facilities in City of Industry, California,
Benson, North Carolina and our Barcelona and Clayton facilities.
If any of these facilities were to be impacted by an accident or
a force majeure event such as an earthquake, major fire or
explosion, major equipment failure or power failure lasting
beyond the capabilities of our backup generators, our revenues
would be materially adversely affected. In this situation, our
manufacturing capacity could be shut down for an extended period
and we could experience a loss of raw materials, work in process
or finished goods inventory. Other force majeure events such as
terrorist acts, influenza pandemic or similar events could also
impede our ability to operate our business. In addition, in any
such event, the reconstruction of our Los Angeles, Barcelona or
Clayton fractionation plants or our plasma storage facilities,
the regulatory approval of the new facilities, and the
replenishment of raw material plasma could be time-consuming.
During this period, we would be unable to manufacture our
products at other plants due to the need for FDA and foreign
regulatory authority inspection and certification of such
facilities and processes. While we maintain property damage and
business interruption insurance with limits of $500 million
for the United States and €360 million for the rest of
the world, these amounts may still be insufficient to mitigate
the losses from any such event. We may also be unable to recover
the value of the lost plasma or
work-in-process
inventories, as well as the sales opportunities from the
products we would be unable to produce.
Many of our plasma collection centers are located near the
U.S. border with Mexico and for the six months ended
June 30, 2011 and the year ended December 31, 2010,
approximately 10% and 8%, respectively, of our internally
sourced plasma came from collection centers located near the
U.S. border with Mexico. Donations at these centers could
be impacted by changes in U.S. visa rules and the recently
enacted healthcare reform legislation. In addition, we have a
number of plasma centers in regions of the southeast which could
be affected by natural disasters such as hurricanes. A
disruption in our source of plasma due to events arising in a
geographic region where many of our collection centers are
located would limit our ability to maintain our current
production levels of plasma-derived products.
If we
experience equipment difficulties or if the suppliers of our
equipment or disposable goods fail to deliver key product
components or supplies in a timely manner, our manufacturing
ability would be impaired and our product sales could
suffer.
We depend on a limited number of companies that supply and
maintain our equipment and provide supplies such as
chromatography resins, filter media, glass and stoppers used in
the manufacture of our products. If our equipment should
malfunction, the repair or replacement of the machinery may
require
63
substantial time and cost, which could disrupt our production
and other operations. Our plasma collection centers rely on
disposable goods supplied by third parties and information
technology systems hosted by third parties. Our plasma
collection centers cannot operate without an uninterrupted
supply of these disposable goods and the operation of these
systems. We have experienced periodic outages of these systems,
but a material outage would affect our ability to operate our
collection centers. Alternative sources for key component parts
or disposable goods may not be immediately available. Any new
equipment or change in supplied materials may require
revalidation by us
and/or
review and approval by the FDA, or foreign regulatory
authorities, including the EMA, which may be time-consuming and
require additional capital and other resources. We may not be
able to find an adequate alternative supplier in a reasonable
time period, or on commercially acceptable terms, if at all. As
a result, shipments of affected products may be limited or
delayed. Our inability to obtain our key source supplies for the
manufacture of products may require us to delay shipments of
products, harm customer relationships and force us to curtail
operations.
If our
shipping or distribution channels were to become inaccessible
due to a crippling accident, an act of terrorism, a strike or
any other force majeure event, our supply, production and
distribution processes could be disrupted.
Plasma must be transported at a temperature of -20 degrees
Celsius to ensure the preservation of its proteins. Not all
shipping or distribution channels are equipped to transport
plasma at these temperatures. If any of our shipping or
distribution channels becomes inaccessible due to a crippling
accident, an act of terrorism, a strike or any other force
majeure event, we may experience disruptions in our continued
supply of plasma and other raw materials, delays in our
production process or a reduction in our ability to distribute
our products directly to our customers.
We rely
in large part on third parties for the sale, distribution and
delivery of our products.
In the United States, we regularly enter into distribution,
supply and fulfillment contracts with group purchasing
organizations, home care companies, alternate infusion sites,
hospital groups and others. We are highly dependent on these
contracts for the successful sale, distribution and delivery of
our products. For example, we rely principally on group
purchasing organizations and on our distributors to sell our
IVIG product. If the parties with which we contract breach,
terminate, or otherwise fail to perform under the agreements,
our ability to effectively distribute our products will be
impaired and our business may be materially and adversely
affected. In addition, through circumstances outside of our
control, such as general economic decline, market saturation or
increased competition, we may be unable to successfully
renegotiate our contracts or secure terms which are as favorable
to us. In addition, we rely in certain countries on distributors
for sales of our products. Disagreements or difficulties with
our distributors supporting our export business could result in
a loss of sales.
We may
not be able to commercialize products in development.
Before obtaining regulatory approval for the sale of our product
candidates or for marketing of existing products for new
indicated uses, we must conduct, at our own expense, extensive
preclinical tests to demonstrate the safety of our product
candidates in animals and clinical trials to demonstrate the
safety and efficacy of our product candidates in humans.
Preclinical and clinical testing is expensive, difficult to
design and implement, can take many years to complete and is
uncertain as to outcome. A failure of one or more of our
clinical trials can occur at any stage of testing. We may
experience numerous unforeseen events during, or as a result of,
preclinical testing and the clinical trial process that could
delay or prevent our ability to receive regulatory approval or
commercialize our product candidates, including:
|
|
|
| |
•
|
regulators or institutional review boards may not authorize us
to commence a clinical trial or conduct a clinical trial within
a country or at a prospective trial site respectively;
|
| |
| |
•
|
the regulatory requirements for product approval may not be
explicit, may evolve over time and may diverge by jurisdiction;
|
64
|
|
|
| |
•
|
our preclinical tests or clinical trials may produce negative or
inconclusive results, and we may decide, or regulators may
require us, to conduct additional preclinical testing or
clinical trials or we may abandon projects that we had expected
to be promising;
|
| |
| |
•
|
the number of patients required for our clinical trials may be
larger than we anticipate, enrollment in our clinical trials may
be slower than we currently anticipate, or participants may drop
out of our clinical trials at a higher rate than we anticipate,
any of which would result in significant delays;
|
| |
| |
•
|
our third-party contractors may fail to comply with regulatory
requirements or meet their contractual obligations to us in a
timely manner;
|
| |
| |
•
|
we might have to suspend or terminate our clinical trials if the
participants are being exposed to unacceptable health risks or
if any participant experiences an unexpected serious adverse
event;
|
| |
| |
•
|
regulators or institutional review boards may require that we
hold, suspend or terminate clinical research for various
reasons, including noncompliance with regulatory requirements;
|
| |
| |
•
|
undetected or concealed fraudulent activity by a clinical
researcher, if discovered, could preclude the submission of
clinical data prepared by that researcher, lead to the
suspension or substantive scientific review of one or more of
our marketing applications by regulatory agencies, and result in
the recall of any approved product distributed pursuant to data
determined to be fraudulent;
|
| |
| |
•
|
the cost of our clinical trials may be greater than we
anticipate;
|
| |
| |
•
|
the supply or quality of our product candidates or other
materials necessary to conduct our clinical trials may be
insufficient or inadequate because we do not currently have any
agreements with third-party manufacturers for the long-term
commercial supply of any of our product candidates;
|
| |
| |
•
|
an audit of preclinical or clinical studies by the FDA or other
regulatory authority may reveal noncompliance with applicable
regulations, which could lead to disqualification of the results
and the need to perform additional studies; and
|
| |
| |
•
|
the effects of our product candidates may not achieve the
desired clinical benefits or may cause undesirable side effects
or the product candidates may have other unexpected
characteristics.
|
If we are required to conduct additional clinical trials or
other testing of our product candidates beyond those that we
currently contemplate, if we are unable to successfully complete
our clinical trials or other testing, if the results of these
trials or tests are not positive or are only modestly positive
or if there are safety concerns, we may:
|
|
|
| |
•
|
be delayed in obtaining marketing approval for our product
candidates;
|
| |
| |
•
|
not be able to obtain marketing approval;
|
| |
| |
•
|
not be able to obtain reimbursement for our products in some
countries;
|
| |
| |
•
|
obtain approval for indications that are not as broad as
intended; or
|
| |
| |
•
|
have the product removed from the market after obtaining
marketing approval.
|
Our product development costs will also increase if we
experience delays in testing or approvals. We do not know
whether any preclinical tests or clinical trials will begin as
planned, will need to be restructured or will be completed on
schedule, if at all. Significant preclinical or clinical trial
delays also could shorten the patent protection period during
which we may have the exclusive right to commercialize our
product candidates or allow our competitors to bring products to
market before we do and impair our ability to commercialize our
products or product candidates.
Even if preclinical trials are successful, we may still be
unable to commercialize the product due to difficulties in
obtaining regulatory approval for the process or problems in
scaling the engineering process to commercial production.
Additionally, if produced, the product may not achieve an
adequate level of market acceptance by physicians, patients,
healthcare payors and others in the medical community to be
profitable.
65
The degree of market acceptance of our product candidates, if
approved for commercial sale, will depend on a number of
factors, some of which are beyond our control, including:
|
|
|
| |
•
|
the prevalence and severity of any side effects;
|
| |
| |
•
|
the efficacy and potential advantages over alternative
treatments;
|
| |
| |
•
|
the ability to offer our product candidates for sale at
competitive prices;
|
| |
| |
•
|
relative convenience and ease of administration;
|
| |
| |
•
|
the willingness of the target patient population to try new
therapies and of physicians to prescribe these therapies;
|
| |
| |
•
|
the strength of marketing and distribution support; and
|
| |
| |
•
|
sufficient third-party coverage or reimbursement.
|
Therefore, we cannot guarantee that any products that we may
seek to develop will ever be successfully commercialized, and to
the extent they are not, such products could be a significant
expense with no reward.
A
breakdown in our information technology systems could result in
a significant disruption to our business.
Our operations are highly dependent on our information
technology systems. If we were to suffer a breakdown in our
systems, storage, distribution or tracing, we could experience
significant disruptions affecting our manufacturing, accounting
and billing processes.
Our
success depends in part on our ability to obtain and maintain
protection in the United States and other countries of the
intellectual property relating to or incorporated into our
technology and products.
Our success depends in large part on our ability to obtain and
maintain protection in the United States and other countries for
the intellectual property covering or incorporated into our
technology and products, especially intellectual property
related to our purification processes. The patent situation in
the field of biotechnology and pharmaceuticals generally is
highly uncertain and involves complex legal and scientific
questions. We may not be able to obtain additional issued
patents relating to our technology or products. Even if issued,
patents issued to our company or our licensors may be
challenged, narrowed, invalidated, held to be unenforceable or
circumvented, which could limit our ability to stop competitors
from marketing similar products or limit the length of term of
patent protection we may have for our products. Additionally,
most of our patents relate to the processes we use to produce
our products, not the products themselves. In many cases, the
plasma-derived products we produce or develop in the future will
not, in and of themselves, be patentable. Since our patents
relate to processes, if a competitor is able to design and
utilize a process that does not rely on our protected
intellectual property, that competitor could sell a
plasma-derived product similar to one we developed or sell.
Changes in either patent laws or in interpretations of patent
laws in the United States and other countries may diminish the
value of our intellectual property or narrow the scope of our
patent protection. In addition, we are a party to a number of
license agreements which may impose various obligations on our
company, including milestone and royalty payments. If we fail to
comply with these obligations, the licensor may terminate the
license, in which event we might not be able to market any
product that is covered by the licensed patents.
Our patents also may not afford us protection against
competitors with similar technology. Because patent applications
in the United States and many other jurisdictions are typically
not published until 18 months after filing, or in some
cases not at all, and because publications of discoveries in the
scientific literature often lag behind actual discoveries,
neither we nor our licensors can be certain that we or they were
the first to make the inventions claimed in our or their issued
patents or pending patent applications, or that we or they were
the first to file for protection of the inventions set forth in
these patent applications. If a third party has also filed a
U.S. patent application covering our product candidates or
a similar invention, we may have to participate in an
adversarial proceeding, known as an interference, declared by
the U.S. Patent and Trademark
66
Office to determine priority of invention in the United States.
The costs of these proceedings could be substantial and it is
possible that our efforts could be unsuccessful, resulting in a
loss of our anticipated U.S. patent position.
Our patents expire at various dates. Our pending and future
patent applications may not issue as patents or, if issued, may
not issue in a form that will provide us with any competitive
advantage. Even if issued, we cannot guarantee that: any of our
present or future patents or patent claims or other intellectual
property rights will not lapse or be invalidated, circumvented,
challenged or abandoned; our intellectual property rights will
provide competitive advantages; our ability to assert our
intellectual property rights against potential competitors or to
settle current or future disputes will not be limited by our
agreements with third parties; any of our pending or future
patent applications will be issued or have the coverage
originally sought; our intellectual property rights will be
enforced in jurisdictions where competition may be intense or
where legal protection may be weak; or we will not lose the
ability to assert our intellectual property rights against, or
to license our technology to, others and collect royalties or
other payments. In addition, our competitors or others may
design around our protected patents or technologies. Effective
protection of our intellectual property rights may be
unavailable, limited or not applied for in some countries.
Changes in patent laws or their interpretation in the United
States and other countries could also diminish the value of our
intellectual property or narrow the scope of our patent
protection. In addition, the legal systems of certain countries
do not favor the aggressive enforcement of patents, and the laws
of foreign countries may not protect our rights to the same
extent as the laws of the United States. As a result, our patent
portfolio may not provide us with sufficient rights to exclude
others from commercializing products similar or identical to
theirs. In order to preserve and enforce our patent and other
intellectual property rights, we may need to make claims or file
lawsuits against third parties. This can entail significant
costs to us and divert our management’s attention from
developing and commercializing our products.
We, like other companies in the pharmaceutical industry, may
become aware of counterfeit versions of our products becoming
available domestically and abroad. Counterfeit products may use
different and possibly contaminated sources of plasma and other
raw materials, and the purification process involved in the
manufacture of counterfeit products may raise additional safety
concerns, over which we have no control. Any reported adverse
events involving counterfeit products that purport to be our
products could harm our reputation and the sale of our products,
in particular, and consumer willingness to use plasma-derived
therapeutics generally.
In
addition to patented technology, we rely on our unpatented
proprietary technology, trade secrets, processes and
know-how.
We generally seek to protect this information by confidentiality
agreements with our employees, consultants, scientific advisors
and third parties. These agreements may not effectively prevent
disclosure of confidential information, may be limited as to
their term, and may not provide an adequate remedy in the event
of unauthorized disclosure of confidential information. In
addition, our trade secrets may otherwise become known or be
independently developed by competitors or other third parties.
To the extent that our employees, consultants or contractors use
intellectual property owned by others in their work for us,
disputes may arise as to the rights in related or resulting
know-how and inventions. Costly and time-consuming litigation
could be necessary to enforce and determine the scope of our
proprietary rights, and failure to obtain or maintain trade
secret protection could adversely affect our competitive
business position. We also rely on contractual protections with
our customers, suppliers, distributors, employees and
consultants, and implement security measures designed to protect
our trade secrets. We cannot assure that these contractual
protections and security measures will not be breached, that we
will have adequate remedies for any such breach or that our
suppliers, employees or consultants will not assert rights to
intellectual property arising out of such contracts. Since we
rely on trade secrets and nondisclosure agreements, in addition
to patents, to protect some of our intellectual property, there
is a risk that third parties may obtain and improperly utilize
our proprietary information to our competitive disadvantage. We
may not be able to detect unauthorized use or take appropriate
and timely steps to enforce our intellectual property rights.
67
We may
infringe or be alleged to infringe intellectual property rights
of third parties.
Our products or product candidates may infringe or be accused of
infringing one or more claims of an issued patent or may fall
within the scope of one or more claims in a published patent
application that may be subsequently issued and to which we do
not hold a license or other rights. Third parties may own or
control these patents or patent applications in the United
States and abroad. These third parties could bring claims
against us or our collaborators that would cause us to incur
substantial expenses and, if successful against us, could cause
us to pay substantial damages. Further, if a patent infringement
suit were brought against us or our collaborators, we or they
could be forced to stop or delay research, development,
manufacturing or sales of the product or product candidate that
is the subject of the suit.
If we are found to be infringing on the patent rights of a third
party, or in order to avoid potential claims, we or our
collaborators may choose or be required to seek a license from a
third party and be required to pay license fees or royalties or
both. These licenses may not be available on acceptable terms,
or at all. Even if we or our collaborators were able to obtain a
license, the rights may be nonexclusive, which could result in
our competitors gaining access to the same intellectual
property. Ultimately, we could be prevented from commercializing
a product, or be forced to cease some aspect of our business
operations, if, as a result of actual or threatened patent
infringement claims, we or our collaborators are unable to enter
into licenses on acceptable terms.
There has been substantial litigation and other proceedings
regarding patent and other intellectual property rights in the
pharmaceutical and biotechnology industries. In addition to
infringement claims against us, we may become a party to other
patent litigation and other proceedings, including interference
proceedings declared by the United States Patent and Trademark
Office and opposition proceedings in the European Patent Office,
regarding intellectual property rights with respect to our
products. The cost to us of any patent litigation or other
proceeding, even if resolved in our favor, could be substantial.
Some of our competitors may be able to sustain the costs of such
litigation or proceedings more effectively than we can because
of their substantially greater financial resources.
Uncertainties resulting from the initiation and continuation of
patent litigation or other proceedings could have a material
adverse effect on our ability to compete in the marketplace.
Patent litigation and other proceedings may also absorb
significant management time.
Many of our employees were previously employed at universities
or other biotechnology or pharmaceutical companies, including
our competitors or potential competitors. We try to ensure that
our employees do not use the proprietary information or know-how
of others in their work for us. We may, however, be subject to
claims that we or these employees have inadvertently or
otherwise used or disclosed intellectual property, trade secrets
or other proprietary information of any such employee’s
former employer. Litigation may be necessary to defend against
these claims and, even if we are successful in defending
ourselves, could result in substantial costs to us or be
distracting to our management. If we fail to defend any such
claims, in addition to paying monetary damages, we may lose
valuable intellectual property rights or personnel.
We have
in-licensed certain patent rights.
The license agreements for such patent rights impose payment and
other material obligations on us. Although we are currently in
compliance with all of our material obligations under these
licenses, if we were to breach any such obligations, our
counterparties may be entitled to terminate the licenses. This
may restrict or delay or eliminate our ability to develop and
commercialize our products, which could adversely affect our
business. We cannot guarantee that the third-party patents and
technology we license will not be licensed to our competitors.
In the future, we may need to obtain additional licenses, renew
existing license agreements or otherwise replace existing
technology. We are unable to predict whether these license
agreements can be obtained or renewed or the technology can be
replaced on acceptable terms, or at all.
Monitoring
unauthorized use of our intellectual property is difficult and
costly.
Unauthorized use of our intellectual property may have occurred
or may occur in the future. Although we have taken steps to
minimize the risk of this occurring, any such failure to
identify unauthorized use and otherwise adequately protect our
intellectual property would adversely affect our business.
Moreover, if we are
68
required to commence litigation, whether as a plaintiff or
defendant, not only would this be time-consuming, but we would
also be forced to incur significant costs and divert our
attention and efforts of our management and other employees,
which could, in turn, result in lower revenue and higher
expenses.
If we are
unable to protect our trademarks from infringement, our business
prospects may be harmed.
We own trademarks that identify our products and have registered
these trademarks in our key markets. Although we monitor the
possible infringement or misuse of our trademarks, it is
possible that third parties may infringe upon our intellectual
property rights. Any unauthorized use of our trademarks could
harm our reputation or commercial interests. In addition, our
enforcement against third-party infringers may be unduly
expensive or time-consuming, or the outcome may be an inadequate
remedy.
The
Grifols family may continue to exercise significant influence
over the conduct of our business.
The Grifols family and Scranton Enterprises B.V. own, directly
and indirectly, 35.3% of our Class A shares. The
Class A shares exercise 100% of voting control of our
company. As a result, the Grifols family and Scranton
Enterprises B.V. may exercise significant influence over matters
requiring shareholders’ approval including, among other
things, the election of the board of directors, dividend policy
and certain fundamental corporate action, such as the issuance
of bonds, a merger or a dissolution. Conflicts may arise between
the interests of the principal shareholders and those of the
other shareholders and the principal shareholders may choose to
resolve the conflict in a way that does not coincide with the
interests of the other shareholders.
We are
investigating potential Foreign Corrupt Practices Act violations
that occurred at Talecris prior to the acquisition.
We are continuing an internal investigation into potential
violations of the FCPA at Talecris that occurred prior to the
acquisition. Talecris became aware of these potential violations
while conducting an unrelated review. The FCPA investigation is
being conducted by outside counsel. The investigation into
certain possible improper payments to individuals and entities
made after Talecris’ formation initially focused on
payments made in connection with sales in certain Eastern
European and Middle Eastern countries, primarily Belarus, Russia
and Iran, but we are also reviewing sales practices in Brazil,
China, Georgia, Turkey and other countries as deemed appropriate.
In July 2009, Talecris voluntarily contacted the
U.S. Department of Justice to advise them of the
investigation and to offer their cooperation in any
investigation that they wanted to conduct or that they wanted
Talecris to conduct. The DOJ has not indicated what action it
may take, if any, against us or any individual, or the extent to
which it may conduct its own investigation. Even though Talecris
self-disclosed this matter to the DOJ, it or other federal
agencies may seek to impose sanctions that may include, among
other things, debarment, injunctive relief, disgorgement, fines,
penalties, appointment of a monitor, appointment of new control
staff or enhancement of existing compliance and training
programs. Other countries in which Talecris had conducted
business may initiate their own investigations and impose
similar penalties. As a result of this investigation, shipments
to some of these countries have been suspended while we put
additional safeguards in place. In some cases, safeguards
involved terminating consultants and suspending relations with
or terminating distributors in countries under investigation as
circumstances warranted. These actions unfavorably affected
Talecris’ revenue from these countries in 2010 and 2009.
Talecris resumed sales in countries where they believed that
they had appropriate safeguards in place and we are reallocating
products to other countries as necessary. To the extent that we
conclude, or the DOJ concludes, that we cannot implement
adequate safeguards or otherwise need to change our business
practices, distributors, or consultants in affected countries or
other countries, this may result in a permanent loss of business
from those countries. We made an initial presentation of some of
the findings of the internal FCPA investigation to the DOJ in
July 2011. We will continue to present our findings from the
investigation to the DOJ. Given the preliminary nature of our
findings, our continuing investigation and the uncertainties
regarding this matter, we are unable to estimate the financial
outcome. Any sanctions or loss of business could have a material
adverse effect on us or our results of operations financial
condition, or cash flows.
69
A pending
investigation relating to Talecris’ compliance with the
terms of the Pharmaceutical Pricing Agreement under the Public
Health Service program may result in our company being barred
from allocating a fixed amount of IVIG as available for sale at
the Public Health Service price.
In November 2009, Talecris received a letter from the United
States Attorney’s Office for the Eastern District of
Pennsylvania, which is referred to as the USAO. The USAO
requested a meeting to review Talecris’ compliance with the
terms of the Pharmaceutical Pricing Agreement, which is referred
to as the PPA, under the Public Health Service program.
Specifically, the USAO asked for information related to the sale
of Talecris’ IVIG product, Gamunex, under that program. In
order to have federal financial participation apply to their
products under the Medicaid program and to obtain Medicare
Part B coverage, manufacturers are required to enter into a
PPA. The PPA obligates manufacturers to charge covered entities
the Public Health Service price for drugs intended for
outpatient use. The Public Health Service price is based on the
Medicaid rebate amount. If the USAO determines that
Talecris’ practices were inconsistent with the terms of the
PPA, the USAO has stated that it may file a civil action against
Talecris under the Anti-fraud Injunction Act and seek a court
order directing Talecris to comply with the PPA or, potentially,
proceed under some other legal theory. An adverse outcome in an
Anti-fraud Injunction Act action could have a material adverse
effect on our results of operation to the extent that we are
barred from allocating a fixed amount of IVIG as available for
sale at the Public Health Service price and is forced to give a
preference to those purchasers over all other customers. We
could also be subject to fines, damages, penalties, appointment
of a monitor, or enhancement of existing compliance and training
programs as a result of government action. We are cooperating
with the investigation and intend to respond to information
requests from the USAO. We believe that Talecris complied with
the terms of the PPA and federal law.
70
USE OF
PROCEEDS
We will not receive any cash proceeds from the issuance of the
exchange notes. In consideration for issuing the exchange notes
as contemplated in this prospectus, we will receive in exchange
existing notes in like principal amount. The existing notes
surrendered in exchange for exchange notes will be retired and
canceled and cannot be reissued. Issuance of the exchange notes
will not result in a change in our aggregate amount of
outstanding debt.
71
CAPITALIZATION
The following table sets forth our cash and cash equivalents and
capitalization as of June 30, 2011, on an actual basis.
This table should be read in conjunction with “Unaudited
Pro Forma Condensed Combined Financial Information,”
“Selected Historical Consolidated Financial Data,”
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations” and the financial
statements and related notes included elsewhere in this
prospectus.
| |
|
|
|
|
|
|
|
As of
|
|
|
|
|
June 30, 2011
|
|
|
|
|
Actual
|
|
|
|
|
€ in thousands
|
|
|
|
|
Cash and cash
equivalents(1)
|
|
|
583,792
|
|
|
Long-term debt:
|
|
|
|
|
|
The
notes(2)
|
|
|
761,088
|
|
|
Senior secured credit facilities:
|
|
|
|
|
|
Revolving Credit
Facilities(3)
|
|
|
—
|
|
|
Senior Term Loan — Tranche A (USD)
|
|
|
830,277
|
|
|
Senior Term Loan — Tranche A (EUR)
|
|
|
213,125
|
|
|
Senior Term Loan — Tranche B (USD)
|
|
|
892,721
|
|
|
Senior Term Loan — Tranche B (EUR)
|
|
|
217,800
|
|
|
Other bank
loans(4)
|
|
|
18,391
|
|
|
Capital lease
obligations(5)
|
|
|
28,947
|
|
|
|
|
|
|
|
|
Total long-term
debt(6)
|
|
|
2,962,349
|
|
|
|
|
|
|
|
|
Short-term debt:
|
|
|
|
|
|
Talecris Old
Notes(7)
|
|
|
427,691
|
|
|
Promissory
Notes(8)
|
|
|
9,990
|
|
|
Senior secured credit facilities:
|
|
|
|
|
|
Senior Term Loan — Tranche A (USD)
|
|
|
6,746
|
|
|
Senior Term Loan — Tranche A (EUR)
|
|
|
6,875
|
|
|
Senior Term Loan — Tranche B (USD)
|
|
|
0
|
|
|
Senior Term Loan — Tranche B (EUR)
|
|
|
2,200
|
|
|
Other bank
loans(4)
|
|
|
74,673
|
|
|
Capital lease
obligations(5)
|
|
|
8,657
|
|
|
|
|
|
|
|
|
Total short-term
debt(9)
|
|
|
536,832
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
1,513,594
|
|
|
|
|
|
|
|
|
Total capitalization
|
|
|
5,012,775
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
At June 30, 2011, cash and
cash equivalents included €428 million held in a
restricted cash account in connection with the redemption of the
7.75% senior unsecured notes due 2016 (the “Talecris
Old Notes”) issued by Talecris in October 2009, which were
subsequently redeemed on July 1, 2011. We paid a
€78 million make-whole premium payment in connection
with the redemption of the Talecris Old Notes. We extinguished
this existing debt in connection with the acquisition and
related refinancing and in accordance with the terms of our
Senior Credit Facilities.
|
| |
|
(2)
|
|
Represents the $1.1 billion
aggregate principal amount of 8.25% senior notes due 2018.
|
| |
|
(3)
|
|
Represents our Revolving Credit
Facilities in the amount of $50 million,
€36.7 million and the $200 million equivalent in
multicurrencies. As of June 30, 2011, there were no
drawings for cash or letters of credit outstanding against the
facilities. See the section in this prospectus entitled
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations— Liquidity and
Capital Resources” for a more detailed discussion of the
terms of the Revolving Credit Facilities.
|
| |
|
(4)
|
|
Represents short and long-term
credit facilities in various currencies with various lenders of
both us and our subsidiaries. We have €189,476,000 of
aggregate availability under our short-term credit facilities.
|
| |
|
(5)
|
|
Represents short and long-term
capital lease obligations in various currencies of both us and
our subsidiaries.
|
| |
|
(6)
|
|
Excludes amortized deferred
expenses in the amount of €299,840,000.
|
| |
|
(7)
|
|
All of the outstanding Talecris Old
Notes were subsequently redeemed on July 1, 2011.
|
| |
|
(8)
|
|
Represents multiple one-year
promissory notes issued by our subsidiary Instituto Grifols,
S.A. to certain of its employees in 2011. These promissory notes
bear interest at a rate of 5.00% per year.
|
| |
|
(9)
|
|
Excludes amortized deferred
expenses and accrued interest in the amount of €29,458,000.
|
72
RATIO OF
EARNINGS TO FIXED CHARGES
The following table sets forth the ratio of earnings to fixed
charges for each of the periods indicated:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
|
Year Ended December 31,
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2007
|
|
|
2006
|
|
|
|
|
|
2.6x
|
|
|
|
3.9x
|
|
|
|
7.8x
|
|
|
|
6.6x
|
|
|
|
6.0x
|
|
|
|
2.5x
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The ratio of earnings to fixed charges has been calculated based
on financial information prepared in accordance with IFRS. For
the purpose of calculating this ratio, earnings consist of
profit/(loss) before tax from continuing operations before our
share of profit or loss of associates, plus fixed charges and
the distributed earnings of associates, less the preference
security dividend requirement. Fixed charges consist of interest
expense, including the amortization of debt issuance costs, plus
an estimate of the interest within rental expenses and, for
fiscal year 2006, the preference security dividend requirements
of consolidated subsidiaries.
73
THE
EXCHANGE OFFER
Purpose
of the Exchange Offer
The existing notes were originally sold to the initial
purchasers by Escrow Corp. on January 21, 2011 in an
offering not registered under the Securities Act. The initial
purchasers subsequently resold the existing notes to qualified
institutional buyers pursuant to Rule 144A under the
Securities Act and to
non-U.S. persons
outside the United States in reliance on Regulation S under
the Securities Act. In connection with the issuance of the
existing notes, Escrow Corp. entered into a registration rights
agreement with the initial purchasers. On June 1, 2011,
upon consummation of the acquisition, Escrow Corp. was merged
with and into the Issuer, and the Issuer assumed all of Escrow
Corp.’s obligations under the existing notes and the
indenture. In addition, upon consummation of the acquisition,
the Issuer, the Parent Guarantor and the initial Subsidiary
Guarantors executed joinders to the registration rights
agreement that had been entered into by Escrow Corp. and the
initial purchasers of the existing notes on January 21,
2011. Pursuant to the registration rights agreement, the Issuer,
the Parent Guarantor and the Subsidiary Guarantors party thereto
agreed, for the benefit of the holders of the existing notes, at
our cost, to file the registration statement of which this
prospectus forms a part and to complete an exchange offer for
the existing notes. The exchange notes will be issued without a
restrictive legend and generally may be resold without
registration under the U.S. federal securities laws. We are
effecting the exchange offer to comply with the registration
rights agreement.
The registration rights agreement requires us to use
commercially reasonable efforts to:
|
|
|
| |
•
|
file a registration statement for the exchange offer with the
SEC and cause the registration statement to become effective
under the Securities Act within 365 days after the issue
date of the outstanding notes;
|
| |
| |
•
|
consummate the exchange offer within 60 days after the
effectiveness date of the registration statement; and
|
| |
| |
•
|
file a shelf registration statement for the resale of the
outstanding notes under certain circumstances and cause such
registration statement to become effective under the Securities
Act within the later of (a) 365 days after the issue
date of the outstanding notes and (b) 90 days
following a request to file such shelf registration statement by
an initial purchaser or certain other holder.
|
If we fail to meet any of these requirements, we must pay
additional interest on the outstanding notes at a rate of 0.25%
per annum for the first
90-day
period and an additional 0.25% per annum with respect to each
subsequent
90-day
period until the applicable requirement has been met, up to a
maximum additional interest rate of 1.0% per annum. We have also
agreed to keep the registration statement for the exchange offer
effective for not less than 20 days (or longer, if required
by applicable law) after the date on which notice of the
exchange offer is mailed to holders.
Under the registration rights agreement, our obligations to
register the exchange notes will terminate upon the completion
of the exchange offer. However, under certain circumstances
specified in the registration rights agreement, we may be
required to file a “shelf” registration statement for
a continuous offer in connection with the outstanding notes
pursuant to Rule 415 under the Securities Act.
This summary includes only the material terms of the
registration rights agreement. For a full description, you
should refer to the complete copy of the registration rights
agreement, which has been filed as an exhibit to the
registration statement of which this prospectus is a part. See
“Where You Can Find More Information.”
Resale of
Exchange Notes
Under existing interpretations of the Securities Act by the
staff of the SEC contained in several no-action letters to third
parties, we believe that the exchange notes will generally be
freely transferable by holders who have validly participated in
the exchange offer without further registration under the
Securities Act (assuming the truth of certain representations
required to be made by each holder of notes, as set forth
below). For additional information on the staff’s position,
we refer you to the following no-action letters: Exxon Capital
Holdings Corporation, available April 13, 1988; Morgan
Stanley & Co. Incorporated, available June 5,
1991;
74
and Shearman & Sterling, available July 2, 1993.
However, any purchaser of existing notes who is one of our
“affiliates” or who intends to participate in the
exchange offer for the purpose of distributing the exchange
notes or who is a broker-dealer who purchased existing notes
from us to resell pursuant to Rule 144A or any other
available exemption under the Securities Act:
|
|
|
| |
•
|
will not be able to tender its existing notes in the exchange
offer;
|
| |
| |
•
|
will not be able to rely on the interpretations of the staff of
the SEC; and
|
| |
| |
•
|
must comply with the registration and prospectus delivery
requirements of the Securities Act in connection with any sale
or transfer of the existing notes unless such sale or transfer
is made pursuant to an exemption from these requirements.
|
If you wish to exchange existing notes for exchange notes in the
exchange offer, you will be required to make representations in
a letter of transmittal that accompanies this prospectus,
including that:
|
|
|
| |
•
|
any exchange notes to be received by you will be acquired in the
ordinary course of your business;
|
| |
| |
•
|
you have no arrangement or understanding with any person to
participate in the distribution of the exchange notes in
violation of the provisions of the Securities Act;
|
| |
| |
•
|
you are not our “affiliate” (within the meaning of
Rule 405 promulgated under the Securities Act);
|
| |
| |
•
|
if you are not a broker-dealer, you are not engaged in, and do
not intend to engage in, a distribution of exchange
notes; and
|
| |
| |
•
|
if you are a broker-dealer, you acquired the existing notes for
your own account as a result of market-making or other trading
activities (and as such, you are a “participating
broker-dealer”), you have not entered into any arrangement
or understanding with us or any of our affiliates to distribute
the exchange notes and you will deliver a prospectus meeting the
requirements of the Securities Act in connection with any resale
of the exchange notes.
|
Rule 405 promulgated under the Securities Act provides that
an “affiliate” of, or person “affiliated”
with, a specified person, is a person that directly, or
indirectly through one or more intermediaries, controls or is
controlled by, or is under common control with, the person
specified.
The SEC has taken the position that participating broker-dealers
may be deemed to be “underwriters” within the meaning
of the Securities Act, and accordingly may fulfill their
prospectus delivery requirements with respect to the exchange
notes, other than a resale of an unsold allotment from the
original sale of the existing notes, with the prospectus
contained in the exchange offer registration statement. Under
the registration rights agreement, we have agreed to use
commercially reasonable efforts to allow participating
broker-dealers and other persons, if any, subject to similar
prospectus delivery requirements, to use this prospectus in
connection with the resale of the exchange notes for a period of
180 days from the issuance of the exchange notes.
Terms of
the Exchange Offer
This prospectus and the accompanying letter of transmittal
contain the terms and conditions of the exchange offer. Upon the
terms and subject to the conditions set forth in this prospectus
and in the accompanying letter of transmittal, we will accept
for exchange all existing notes that are properly tendered and
not withdrawn on or prior to 5:00 p.m., New York City time,
on the expiration date. After authentication of the exchange
notes by the trustee or an authentication agent, we will issue
and deliver $1,000 principal amount of exchange notes in
exchange for each $1,000 principal amount of outstanding
existing notes accepted in the exchange offer. Holders may
tender some or all of their existing notes in the exchange offer
in denominations of $2,000 and integral multiples of $1,000
thereof.
75
The form and terms of the exchange notes are identical in all
material respects to the form and terms of the existing notes,
except that:
|
|
|
| |
•
|
the offering of the exchange notes has been registered under the
Securities Act;
|
| |
| |
•
|
the exchange notes generally will not be subject to transfer
restrictions or have registration rights; and
|
| |
| |
•
|
certain provisions relating to special interest on the existing
notes provided for under certain circumstances will be
eliminated.
|
The exchange notes will evidence the same debt as the existing
notes. The exchange notes will be issued under and entitled to
the benefits of the indenture.
In connection with the issuance of the existing notes, we made
arrangements for the existing notes to be issued and
transferable in book-entry form through the facilities of DTC,
acting as a depositary. The exchange notes will also be issuable
and transferable in book-entry form through DTC.
The exchange offer is not conditioned upon any minimum aggregate
principal amount of existing notes being tendered. However, our
obligation to accept existing notes for exchange pursuant to the
exchange offer is subject to certain customary conditions that
we describe under “— Conditions” below.
Holders who tender existing notes in the exchange offer will not
be required to pay brokerage commissions or fees or, subject to
the instructions in the letter of transmittal, transfer taxes
with respect to the exchange of existing notes pursuant to the
exchange offer. We will pay all charges and expenses, other than
certain applicable taxes, in connection with the exchange offer.
See “— Solicitation of Tenders; Fees and
Expenses” for more detailed information regarding the
expenses of the exchange offer.
By executing or otherwise becoming bound by the letter of
transmittal, you will be making the representations described
under “— Procedures for Tendering” below.
Expiration
Date; Extensions; Amendments
The term “expiration date” will mean 5:00 p.m.,
New York City time,
on ,
2011, unless we, in our sole discretion, extend the exchange
offer, in which case the term “expiration date” will
mean the latest date and time to which we extend the exchange
offer.
To extend the exchange offer, we will:
|
|
|
| |
•
|
notify the exchange agent of any extension orally (confirmed in
writing) or in writing; and
|
| |
| |
•
|
notify the registered holders of the existing notes by means of
a press release or other public announcement, each before
9:00 a.m., New York City time, on the next business day
after the previously scheduled expiration date.
|
We reserve the right, in our reasonable discretion:
|
|
|
| |
•
|
to delay accepting any existing notes;
|
| |
| |
•
|
to extend the exchange offer; or
|
| |
| |
•
|
if any conditions listed below under
“— Conditions” are not satisfied, to
terminate the exchange offer by giving oral or written notice of
the delay, extension or termination to the exchange agent.
|
We will follow any delay in acceptance, extension or termination
as promptly as practicable by oral (confirmed in writing) or
written notice to the exchange agent and the registered holders.
If we amend the exchange offer in a manner we determine
constitutes a material change, we will promptly disclose the
amendment in a prospectus supplement that we will distribute to
the registered holders.
76
Interest
on the Exchange Notes
Interest on the exchange notes will accrue from the last
interest payment date on which interest was paid on the existing
notes surrendered in exchange for exchange notes. Interest on
the exchange notes will be payable semi-annually on February 1
and August 1 of each year, commencing on February 1, 2012.
Procedures
for Tendering
Only you may tender your existing notes in the exchange offer.
Except as stated under “— Book-Entry
Transfer,” to tender your existing notes in the exchange
offer, you must:
|
|
|
| |
•
|
complete, sign and date the enclosed letter of transmittal, or a
copy of it;
|
| |
| |
•
|
have the signature on the letter of transmittal guaranteed if
required by the letter of transmittal or transmit an
agent’s message in connection with a book-entry
transfer; and
|
| |
| |
•
|
mail, fax or otherwise deliver the letter of transmittal or copy
to the exchange agent before the expiration date.
|
In addition, either:
|
|
|
| |
•
|
the exchange agent must receive a timely confirmation of a
book-entry transfer of your existing notes, if that procedure is
available, into the account of the exchange agent at DTC, the
“book-entry transfer facility,” under the procedure
for book-entry transfer described below before the expiration
date;
|
| |
| |
•
|
the exchange agent must receive certificates for your existing
notes, the letter of transmittal and other required documents
before the expiration date; or
|
| |
| |
•
|
you must comply with the guaranteed delivery procedures
described below.
|
For your existing notes to be tendered effectively, the exchange
agent must receive a valid agent’s message through
DTC’s Automated Tender Offer Program, or ATOP, or a letter
of transmittal and other required documents before the
expiration date. Delivery of the existing notes shall be made by
book-entry transfer in accordance with the procedures described
below. Confirmation of the book-entry transfer must be received
by the exchange agent before the expiration date.
The term “agent’s message” means a message,
transmitted by a book-entry transfer facility to, and received
by, the exchange agent forming a part of a confirmation of a
book-entry, which states that the book-entry transfer facility
has received an express acknowledgment from the participant in
the book-entry transfer facility tendering the outstanding
securities that the participant has received and agrees:
|
|
|
| |
•
|
to participate in ATOP;
|
| |
| |
•
|
to be bound by the terms of the letter of transmittal; and
|
| |
| |
•
|
that we may enforce the agreement against the participant.
|
THE METHOD OF DELIVERY OF YOUR EXISTING NOTES, A LETTER OF
TRANSMITTAL AND ALL OTHER REQUIRED DOCUMENTS TO THE EXCHANGE
AGENT IS AT YOUR ELECTION AND RISK. INSTEAD OF DELIVERY BY MAIL,
WE RECOMMEND THAT YOU USE AN OVERNIGHT OR HAND DELIVERY SERVICE.
IN ALL CASES, YOU SHOULD ALLOW SUFFICIENT TIME TO ASSURE
DELIVERY TO THE EXCHANGE AGENT BEFORE THE EXPIRATION DATE. DO
NOT SEND A LETTER OF TRANSMITTAL OR EXISTING NOTES DIRECTLY
TO US. YOU MAY REQUEST YOUR RESPECTIVE BROKERS, DEALERS,
COMMERCIAL BANKS, TRUST COMPANIES OR NOMINEES TO MAKE THE
EXCHANGE ON YOUR BEHALF.
Each broker-dealer that receives exchange notes for its own
account in exchange for existing notes, where the existing notes
were acquired by such broker-dealer as a result of market-making
activities or other trading activities, must acknowledge that it
will deliver a prospectus in connection with any resale of such
exchange notes. See “Plan of Distribution.”
77
Procedure
if the Existing Notes Are Not Registered in Your Name
If you are a beneficial owner whose existing notes are
registered in the name of a broker, dealer, commercial bank,
trust company or other nominee and you want to tender your
existing notes, you should contact the registered holder
promptly and instruct the registered holder to tender on your
behalf. If you want to tender on your own behalf, you must,
before completing and executing a letter of transmittal and
delivering your existing notes, either make appropriate
arrangements to register ownership of the existing notes in your
name or obtain a properly completed bond power or other proper
endorsement from the registered holder. We urge you to act
immediately since the transfer of registered ownership may take
considerable time.
Book-Entry
Transfer
The Exchange Agent will make requests to establish accounts at
the book-entry transfer facility for purposes of the exchange
offer within two business days after the date of this
prospectus. If you are a financial institution that is a
participant in the book-entry transfer facility’s systems,
you may make book-entry delivery of your existing notes being
tendered by causing the book-entry transfer facility to transfer
your existing notes into the exchange agent’s account at
the book-entry transfer facility in compliance with the
appropriate procedures for transfer. However, although you may
deliver your existing notes through book-entry transfer at the
book-entry transfer facility, you must transmit, and the
exchange agent must receive, a letter of transmittal or copy of
the letter of transmittal, with any required signature
guarantees and any other required documents, except as discussed
in the following paragraph, on or before the expiration date or
the guaranteed delivery procedures outlined below must be
complied with.
DTC’s ATOP is the only method of processing the exchange
offer through DTC. To accept the exchange offer through ATOP,
participants in DTC must send electronic instructions to DTC
through DTC’s communication system instead of sending a
signed, hard copy letter of transmittal. DTC is obligated to
communicate those electronic instructions to the exchange agent.
To tender your existing notes through ATOP, the electronic
instructions sent to DTC and transmitted by DTC to the exchange
agent must contain the participant’s acknowledgment of its
receipt of and agreement to be bound by the letter of
transmittal for your existing notes.
Beneficial
Owner Instructions to Holders of Existing Notes
Only a holder whose name appears on a DTC security position
listing as a holder of existing notes, or the legal
representative or attorney-in-fact of such holder, may execute
and deliver the letter of transmittal.
Holders of existing notes who are not registered holders of, and
who seek to tender, existing notes should (1) obtain a
properly completed letter of transmittal for such existing notes
from the registered holder with signatures guaranteed by an
Eligible Institution and obtain and include with such letter of
transmittal existing notes properly endorsed for transfer by the
registered holder thereof or accompanied by a written instrument
or instruments of transfer or exchange from the registered
holder with signatures on the endorsement or written instrument
or instruments of transfer or exchange guaranteed by an Eligible
Institution or (2) effect a record transfer of such
existing notes and comply with the requirements applicable to
registered holders for tendering existing notes before
5:00 p.m., New York City time, on the expiration date. Any
existing notes properly tendered before 5:00 p.m., New York
City time, on the expiration date accompanied by a properly
completed letter of transmittal will be transferred of record by
the registrar either prior to or as of the expiration date at
our discretion. We have no obligation to transfer any existing
notes from the name of the registered holder of the note if we
do not accept these existing notes for exchange.
Tendering holders should indicate in the applicable box in the
letter of transmittal the name and address to which payment of
accrued and unpaid interest on the existing notes, certificates
evidencing exchange notes
and/or
certificates evidencing existing notes for amounts not accepted
for tender, each as appropriate, are to be issued or sent, if
different from the name and address of the person signing the
letter of transmittal. In the case of issuance in a different
name, the employer identification or social security number of
the person named must also be indicated and a substitute
Form W-9
for this recipient must be completed. If these instructions are
not given, the payments, including accrued and unpaid interest
in cash on the existing notes, exchange
78
notes or existing notes not accepted for tender, as the case may
be, will be made or returned, as the case may be, to the
registered holder of the existing notes tendered.
Issuance of exchange notes in exchange for existing notes will
be made only against deposit of the tendered existing notes.
We will decide all questions as to the validity, form,
eligibility, acceptance and withdrawal of tendered existing
notes, and our determination will be final and binding on you.
We reserve the absolute right to reject any and all existing
notes not properly tendered or reject any existing notes which
would be unlawful in the opinion of our counsel. We also reserve
the right to waive any defects, irregularities or conditions of
tender as to particular existing notes. Our interpretation of
the terms and conditions of the exchange offer, including the
instructions in a letter of transmittal, will be final and
binding on all parties. You must cure any defects or
irregularities in connection with tenders of existing notes as
we determine. Although we intend to notify you of defects or
irregularities with respect to tenders of your existing notes,
we, the exchange agent or any other person will not incur any
liability for failure to give any notification. Your tender of
existing notes will not be deemed to have been made until any
defects or irregularities have been cured or waived. Any of your
existing notes received by the exchange agent that are not
properly tendered and as to which the defects or irregularities
have not been cured or waived will be returned by the exchange
agent to you, unless otherwise provided in the letter of
transmittal, as soon as practicable following the expiration
date.
Guaranteed
Delivery Procedures
If you wish to tender your existing notes but your existing
notes are not immediately available, or time will not permit
your existing notes or other required documents to reach the
exchange agent before the expiration date, or the procedure for
book-entry transfer cannot be completed on a timely basis, you
may affect a tender if:
|
|
|
| |
•
|
the tender is made through an Eligible Institution (as defined
in the Letter of Transmittal),
|
| |
| |
•
|
prior to the expiration date, the exchange agent receives from
such Eligible Institution a properly completed and duly executed
notice of guaranteed delivery, by facsimile transmittal, mail or
hand delivery,
|
| |
| |
•
|
stating the name and address of the holder, the certificate
number or numbers of such holder’s existing notes and the
principal amount of such existing notes tendered;
|
| |
| |
•
|
stating that the tender is being made thereby;
|
| |
| |
•
|
guaranteeing that, within three New York Stock Exchange trading
days after the expiration date, the letter of transmittal, or a
facsimile thereof, together with the certificate(s) representing
the existing notes to be tendered in proper form for transfer,
or an agent’s message and confirmation of a book-entry
transfer into the exchange agent’s account at DTC of
existing notes delivered electronically, and any other documents
required by the letter of transmittal, will be deposited by the
Eligible Institution with the exchange agent; and
|
| |
| |
•
|
such properly completed and executed letter of transmittal, or a
facsimile thereof, together with the certificate(s) representing
all tendered existing notes in proper form for transfer, or an
agent’s message and confirmation of a book-entry transfer
into the exchange agent’s account at DTC of existing notes
delivered electronically and all other documents required by the
letter of transmittal are received by the exchange agent within
three New York Stock Exchange trading days after the expiration
date.
|
Upon request, the exchange agent will send to you a notice of
guaranteed delivery if you wish to tender your existing notes
according to the guaranteed delivery procedures described above.
Withdrawal
of Tenders
Except as otherwise provided in this prospectus, you may
withdraw tenders of existing notes at any time prior to the
expiration date.
79
For a withdrawal to be effective, the exchange agent must
receive a written or facsimile transmission notice of withdrawal
at its address set forth this prospectus prior to the expiration
date. Any such notice of withdrawal must:
|
|
|
| |
•
|
specify the name of the person who deposited the existing notes
to be withdrawn;
|
| |
| |
•
|
identify the existing notes to be withdrawn, including the
certificate number or number and principal amount of such
existing notes or, in the case of existing notes transferred by
book-entry transfer, the name and number of the account at DTC
to be credited; and
|
| |
| |
•
|
be signed in the same manner as the original signature on the
letter of transmittal by which such existing notes were
tendered, including any required signature guarantee.
|
We will determine in our sole discretion all questions as to the
validity, form and eligibility, including time of receipt, of
such withdrawal notices, and our determination shall be final
and binding on all parties. We will not deem any properly
withdrawn existing notes to have been validly tendered for
purposes of the exchange offer, and we will not issue exchange
notes with respect those existing notes unless you validly
retender the withdrawn existing notes. You may retender properly
withdrawn existing notes following one of the procedures
described above under ‘‘— Procedures for
Tendering” at any time prior to the expiration date.
Conditions
Notwithstanding any other term of the exchange offer, we will
not be required to accept for exchange, or exchange the exchange
notes for, any existing notes, and may terminate the exchange
offer as provided in this prospectus before the acceptance of
the existing notes, if:
|
|
|
| |
•
|
the exchange offer violates applicable law, rules or regulations
or an applicable interpretation of the staff of the SEC;
|
| |
| |
•
|
an action or proceeding has been instituted or threatened in any
court or by any governmental agency that might materially impair
our ability to proceed with the exchange offer;
|
| |
| |
•
|
there has been proposed, adopted or enacted any law, rule or
regulation that, in our reasonable judgment, would impair
materially our ability to consummate the exchange offer; or
|
| |
| |
•
|
all governmental approvals that we deem necessary for the
completion of the exchange offer have not been obtained.
|
If we determine in our reasonable discretion that any of these
conditions are not satisfied, we may:
|
|
|
| |
•
|
refuse to accept any existing notes and return all tendered
existing notes to you;
|
| |
| |
•
|
extend the exchange offer and retain all existing notes tendered
before the exchange offer expires, subject, however, to your
rights to withdraw the existing notes; or
|
| |
| |
•
|
waive the unsatisfied conditions with respect to the exchange
offer and accept all properly tendered existing notes that have
not been withdrawn.
|
If the waiver constitutes a material change to the exchange
offer, we will promptly disclose the waiver by means of a
prospectus supplement that we will distribute to the registered
holders of the existing notes.
Exchange
Agent
We have appointed The Bank of New York Mellon Trust Company,
N.A., the trustee under the Indenture, as exchange agent for the
exchange offer. You should send all executed letters of
transmittal to the exchange agent at one of the addresses set
forth below. You should direct questions, requests for
assistance and requests
80
for additional copies of this prospectus or of the letter of
transmittal and requests for a notice of guaranteed delivery to
the exchange agent addressed as follows:
By
Certified or Registered Mail or Overnight
Delivery:
The Bank of New York Mellon Trust Company N.A.
c/o The Bank of New York Mellon Corporation
Corporate Trust Operations — Reorganization Unit
101 Barclay St., Floor 7 East
New York, NY 10286
Attention: Mr. William Buckley
By
Facsimile (eligible institutions only):
(212) 298-1915
Telephone
Inquiries:
(212) 815-5788
Delivery to an address or facsimile number other than those
listed above will not constitute a valid delivery.
Solicitation
of Tenders; Fees and Expenses
We will pay all expenses of soliciting tenders pursuant to the
exchange offer. We are making the principal solicitation by
mail. Our officers and regular employees may make additional
solicitations in person or by telephone or facsimile.
We have not retained any dealer-manager in connection with the
exchange offer and will not make any payments to brokers,
dealers or other persons soliciting acceptances of the exchange
offer. We will, however, pay the exchange agent reasonable and
customary fees for its services and will reimburse the exchange
agent for its reasonable
out-of-pocket
costs and expenses in connection therewith.
We also may pay brokerage houses and other custodians, nominees
and fiduciaries the reasonable
out-of-pocket
expenses incurred by them in forwarding copies of this
prospectus, letters of transmittal and related documents to the
beneficial owners of the existing notes and in handling or
forwarding tenders for exchange.
We will pay the expenses to be incurred in connection with the
exchange offer, including fees and expenses of the exchange
agent and trustee and accounting and legal fees and printing
costs.
We will pay all transfer taxes, if any, applicable to the
exchange of existing notes for exchange notes pursuant to the
exchange offer. If, however, certificates representing exchange
notes or existing notes for principal amounts not tendered or
accepted for exchange are to be delivered to, or are to be
registered or issued in the name of, any person other than the
registered holder of the existing notes tendered, or if tendered
existing notes are registered in the name of any person other
than the person signing the letter of transmittal, or if a
transfer tax is imposed for any reason other than the exchange
of existing notes pursuant to the exchange offer, then the
amount of any such transfer taxes, whether imposed on the
registered holder or any other persons, will be payable by the
tendering holder. If satisfactory evidence of payment of such
taxes or exemption therefrom is not submitted with the letter of
transmittal, the amount of such transfer taxes will be billed by
us directly to such tendering holder.
Consequences
of Failure to Exchange
Participation in the exchange offer is voluntary. We urge you to
consult your financial and tax advisors in making your decisions
on what action to take. Existing notes that are not exchanged
for exchange notes
81
pursuant to the exchange offer will remain restricted
securities. Accordingly, those existing notes may be resold only:
|
|
|
| |
•
|
to a person whom the seller reasonably believes is a qualified
institutional buyer in a transaction meeting the requirements of
Rule 144A promulgated under the Securities Act;
|
| |
| |
•
|
in a transaction meeting the requirements of Rule 144
promulgated under the Securities Act;
|
| |
| |
•
|
outside the United States to a foreign person in a transaction
meeting the requirements of Rule 903 or 904 of
Regulation S promulgated under the Securities Act;
|
| |
| |
•
|
in accordance with another exemption from the registration
requirements of the Securities Act and based upon an opinion of
counsel if we so request;
|
| |
| |
•
|
to us; or
|
| |
| |
•
|
pursuant to an effective registration statement.
|
In each case, the existing notes may be resold only in
accordance with any applicable securities laws of any state of
the United States or any other applicable jurisdiction.
82
MANAGEMENT’S
DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF
OPERATIONS
You are encouraged to read the following discussion and
analysis of our financial condition and results of operations,
together with our consolidated financial statements and related
footnotes included at the end of this prospectus. This
discussion and analysis contains forward-looking statements that
involve risks and uncertainties. See the section entitled
“Risk Factors” included elsewhere in this prospectus
for a discussion of some of the important factors that could
cause actual results to differ materially from those described
or implied by the forward-looking statements contained in the
following discussion and analysis. See the section entitled
“Cautionary Statement Regarding Forward-Looking
Statements” included elsewhere in this prospectus.
All tabular disclosures are presented in thousands of Euros
except share and per share amounts and percentages. Percentages
and amounts presented herein may not calculate or sum precisely
due to rounding.
Business
Overview
We are a leading global specialty biopharmaceutical company that
develops, manufactures and distributes a broad range of plasma
derivative products and also specializes in providing infusion
solutions, nutrition products, blood bags and diagnostic
instrumentation and reagents for use in hospitals and clinics.
Plasma derivatives are proteins found in human plasma, which
once isolated and purified, have therapeutic value. Plasma
derivative products are used to treat patients with hemophilia,
immune deficiencies, infectious diseases and a range of other
severe and often life threatening medical conditions. Our
products and services are used by healthcare providers in more
than 100 countries to diagnose and treat patients with
hemophilia, immune deficiencies, infectious diseases and a range
of other medical conditions.
Our plasma derivative products are manufactured at our plasma
fractionation plant near Barcelona, Spain, which has a capacity
of 2.1 million liters per year, and our plant in Los
Angeles, California, United States, which currently has a
capacity of 2.2 million liters per year. In addition, our
Clayton, North Carolina site, acquired in the acquisition of
Talecris, is one of the world’s largest integrated protein
manufacturing sites, including fractionation, purification and
aseptic filling and finishing of plasma-derived proteins and has
a capacity of 2.6 million liters per year. The Melville,
New York site, which we lease and operate as a result of the
acquisition of Talecris, is an intermediate processing facility
and has a capacity of 1.6 million liters per year.
We organize our business into four divisions: Bioscience,
Hospital, Diagnostic and Raw Materials. Subsequent to the
acquisition, Talecris’ operations have been incorporated
into our existing Bioscience division.
Bioscience. The Bioscience division includes
activities relating to the manufacture of plasma derivatives for
therapeutic use, including the reception, analysis, quarantine,
classification, fractionation and purification of plasma, and
the sale and distribution of end products. The main types of
plasma products manufactured by us are IVIG, Factor VIII, A1PI
and albumin. We also manufacture hyperimmune immunoglobulins,
Antithrombin III, Factor IX and PTC. The Bioscience division,
which accounts for a majority of our total net sales, accounted
for €773.4 million, or 78.1%, and
€521.5 million, or 82.1%, and of our total net sales
for the year ended December 31, 2010 and the six months
ended June 30, 2011, respectively.
Hospital. The Hospital division manufactures
and, in certain instances installs, products that are used by
and in hospitals, such as parenteral solutions and enteral and
parenteral nutritional fluids, which are sold almost exclusively
in Spain and Portugal, and which accounted for
€89.6 million, or 9.0%, and €49.3 million,
or 7.8%, of our total net sales for the year ended
December 31, 2010 and the six months ended June 30,
2011 respectively. We believe we are the leading provider of
intravenous therapy in Spain, with a 34% market share.
Diagnostic. The Diagnostic division focuses on
researching, developing, manufacturing and marketing in vitro
diagnostics products including analytical instruments and
reagents for diagnostics, as well as blood bank products. We
concentrate our Diagnostic business in three areas:
immunohematology, hemostasis and immunology. The Diagnostic
division’s main customers are blood donation centers,
clinical analysis laboratories and hospital immunohematology
services. The division also manufactures and distributes blood
collection
83
bags and other disposables. The Diagnostic division accounted
for €109.1 million, or 11.0% and
€56.8 million, or 8.9%, of our total net sales for the
year ended December 31, 2010 and the six months ended
June 30, 2011, respectively.
Raw Materials. The Raw Materials division
includes the sale of intermediate pastes and plasma to third
parties, which accounted for €4.8 million, or 0.5%,
and €1.7 million, or 0.3%, of our total net sales for
the year ended December 31, 2010 and the six months ended
June 30, 2011, respectively. Sales of the Raw Materials
division are used to optimize inventory levels with the aim of
striking a better balance between plasma collections and
fractionation needs.
Subsequent
events
In August 2011, we acquired the remaining 51% outstanding
capital stock of Woolloomooloo Holdings Pty Ltd. the holding
company of the Australian-Swiss group, Lateral-Medion, of which
we had acquired 49% of the capital stock and 100% of the voting
rights on March 2009 which will not impact goodwill. The total
sum paid for the acquisition of the remaining 51% of the capital
stock amounted to AUD 12.5 million (€9.5 million).
Presentation
of Financial Information
IFRS
Our consolidated financial statements for the years ended
December 31, 2010, 2009, and 2008 and the six months ended
June 30, 2011 and June 30, 2010 have been prepared in
accordance with IFRS as issued by the IASB and IAS 34,
Interim Financial Reporting, respectively.
Factors
Affecting the Comparability of Our Results of
Operations
The
Acquisition
On June 1, 2011, we completed our acquisition of 100% of
the share capital of Talecris, for a total of $3.7 billion.
The acquisition consideration consisted of a combination of cash
consideration of $2.5 billion and non-cash consideration,
through the issuance of our new Class B shares, of
$1.2 billion. The acquisition has been accounted for using
the acquisition method pursuant to IFRS 3 (revised), Business
Combinations. Under the acquisition method, assets and
liabilities are recorded at their fair value on the date of
purchase and the total purchase price is allocated to the
tangible and intangible assets acquired and liabilities assumed.
As of June 30, 2011, the valuation studies necessary to finalize
the fair values of the assets acquired and liabilities assumed
and the related allocation of the purchase price had not been
completed. A final determination of these fair values will
reflect, among other things, our consideration of a final
valuation based on the actual net tangible and intangible
assets, such as acquired in-process research and development,
customer relationships, developed and core technology,
intellectual property, patents and trade names and contingent
liabilities, that exist as of the closing date of the
acquisition. We expect to adjust the fair value of certain of
Talecris’ assets and liabilities.
Costs incurred in connection with the acquisition in the amount
of €55 million have been expensed as incurred and are
reflected in our “Other operating expenses” in the
amounts of €17 million and €38 million, in
the year ended December 31, 2010 and the six months ended
June 30, 2011, respectively.
Additionally, we incurred significant indebtedness in connection
with the consummation of the acquisition, including the
assumption of the existing notes and the closing of the Senior
Credit Facilities, and our total indebtedness and related
interest expenses will be significantly higher than in previous
periods. Financial expenses increased from
€25.3 million in the six months ended June 30,
2010 to €55.5 million in the six months ended
June 30, 2011.
Additional information with respect to the acquisition is set
forth in Note 3 of our interim unaudited consolidated
financial statements for six months ended June 30, 2011,
included elsewhere in this prospectus.
84
Change
in Classification of Albumin for Non-Therapeutic Uses and
Intermediates
During 2009, the results of the Raw Materials division were
streamlined and income and costs from sales of albumin for
non-therapeutic use and intermediate products were reclassified
into the Bioscience division. The sales and cost of sales were
also reclassified for 2008 and 2007. In 2008, the effect of such
reclassification on sales and cost of sales, respectively, was
€11.7 million and €5.7 million and in 2007,
was €14.5 million and €7.6 million,
respectively.
Factors
Affecting Our Financial Condition and Results of
Operations
Price
Controls
Certain healthcare products, including plasma derivative
products, are subject to price controls in many of the markets
where they are sold, including Spain and other countries in the
European Union. The existence of price controls over these
products has adversely affected, and may continue to adversely
affect, our ability to maintain or increase our prices and gross
margins.
As a result of the acquisition, we have significantly expanded
our presence in the United States. The United States is the
principal market in the world for plasma derivative products and
prices for plasma derivative products are currently not
regulated, with the exception of certain government healthcare
programs, such as the 340B/PHS program (although prices are
subject to price pressures from GPOs and insurance companies).
Plasma
Supply Constraints
Plasma, the principal raw material required for the manufacture
of plasma derivative products, is a scarce resource. Our ability
to continue to increase our revenue depends substantially on
increased access to plasma.
We have increased the number of our plasma collection facilities
by 67 centers as a result of the acquisition. We expect that our
plasma needs for 2012 and going forward will be met through the
volumes of collection at our 147 plasma collection centers in
the United States and supplemented by approximately 800,000
liters of plasma per year to be purchased from third-party
suppliers for the next three years pursuant to multiple plasma
purchase agreements assumed in connection with the acquisition.
In addition, we process recovered plasma received from Spanish,
Czech and Slovak hospitals and fractionate plasma for Canadian
Blood Services and Hema Quebec under manufacturing agreements.
In 2010, our plasma collection centers collected approximately
2.6 million liters of plasma. Our expanded network of
plasma collection centers is capable of increasing the annual
plasma collection capacity to 6.5 million liters of plasma
per year. The actual volume of plasma that we are able to
collect in the future may be less or more than these amounts.
See the section of this prospectus entitled “Cautionary
Statement Regarding Forward-Looking Statements.”
In addition, the acquisition of Talecris has allowed us to
significantly expand our fractionation capacity. As a result of
the acquisition, we have four fractionation facilities located
in the United States and Spain, allowing for the fractionation
of up to 8.5 million liters of plasma per year in the
aggregate.
Product
Licensing Requirements
The marketing and sale of pharmaceutical and biological
products, such as our plasma derivatives and parenteral
solutions, is subject to the prior registration of such product
with the competent authorities of the jurisdiction where the
product is to be marketed and sold. The registration processes
are complex and time-consuming. Our ability to increase revenue
by expanding our products into new markets depends substantially
on the successful and timely completion of the registration
processes in such markets.
Certain costs related to the product licensing process, such as
fees payable to the medical personnel who conduct the clinical
trials, fees payable to trial volunteers, the product used
during the trials, product licensing fees and insurance premiums
related to the trials, are capitalized. Expenses related to the
product licensing process include primarily personnel (which are
recorded under personnel expenses) and other materials used in
85
the clinical trials (which are recorded under cost of material
consumed). There is generally a lag time of several months from
the time that we obtain the approval until we begin sales, as we
must then put in place sales, marketing and distribution
infrastructure.
We have obtained product licenses for our three principal
products, Flebogamma/Flebogamma DIF IVIG, Fanhdi Factor VIII and
Grifols Albumin, in all of our principal European markets
(Germany, Italy, United Kingdom and Spain). We have also
obtained product licenses for Flebogamma DIF IVIG and Grifols
Albumin in the United States. In addition, Alphanate Factor
VIII, Albutein, Alphanine Factor IX and Profilnine PTC, products
that we previously acquired from Alpha, have been licensed by
the regulatory authorities in the principal European markets,
the United States and Asia. The two key products acquired from
Talecris, Gamunex and Prolastin A1PI, have also been licensed in
the United States and in their principal European markets.
See the section entitled “Business — Our
Divisional Structure — The Bioscience
Division — Bioscience Products and Services” for
a discussion of the licensing process and of our principal
product licenses.
Past-Due
Receivables
For sales of our products to hospitals and clinics that are part
of the social security systems of Spain, Portugal, Italy and
certain other countries, we depend upon government health
agencies for payment. We have faced significant delays in the
collection of payment for our products in such countries. The
adoption by Spain, effective December 31, 2004, of a
European Union directive that requires payment of interest on
receivables that are more than 60 days overdue has resulted
in a significant decrease in collection delays from these
hospitals and clinics. However, we cannot assure that this trend
will continue or that the present receivables aging levels for
these hospitals and clinics will not increase again,
particularly if the funding of these hospitals and clinics is
not increased sufficiently by the appropriate governmental
health agencies. The failure to receive timely payments for the
sale of our products negatively affects our working capital
levels and may require us to obtain more short-term financing
than we would otherwise need. These significant delays
contributed to our receivables aging average of 84 days,
83 days, 83 days and 63 at December 31, 2008,
2009 and 2010 and June 30, 2011, respectively.
Interest
and Currency Risk
A significant portion of our interest-bearing debt at
December 31, 2010 and June 30, 2011 bore interest at a
floating rate, at a spread over the London Interbank Offered
Rate (“LIBOR”) for our U.S. Dollar-denominated
debt and at a spread over the Euro Interbank Offered Rate
(“EURIBOR”) for our Euro-denominated debt. See the
section below entitled “— Quantitative and
Qualitative Disclosures about Market Risk — Interest
Rate Risk.” As a result, increases in the applicable
floating interest rates would increase our interest expense and
reduce our net cash flow. Pursuant to mandatory hedging
requirements under the Senior Credit Facilities, 62% of our
U.S. Dollar-denominated debt under the Senior Term Loans is
hedged at a fixed rate. However, our Euro-denominated debt is
not hedged. For a further discussion of our hedging
transactions, see the section below entitled
“— Financial Derivatives.”
Our functional currency is the Euro and a majority of our net
sales for the six months ended June 30, 2011 was
denominated in U.S. Dollars. Accordingly, our principal
foreign currency exposure relates to the U.S. Dollar. A
devaluation of the U.S. Dollar against the Euro would
result in (i) a decrease in net sales in Euro terms for
sales denominated in U.S. Dollars, and (ii) a decrease
in costs in Euro terms for costs denominated in
U.S. dollars. A devaluation of the Euro against the
U.S. Dollar would have the opposite effect. See the section
below entitled“— Quantitative and Qualitative
Disclosures about Market Risk — Foreign Currency
Risk.”
We are also exposed to risk based on the payment of
U.S. dollar-denominated indebtedness. At December 31,
2010, $549 million of our indebtedness was denominated in
U.S. Dollars. At June 30, 2011, $2.5 billion
under the Senior Term Loans, $50 million of undrawn
availability under the Revolving Credit Facilities (not
including an additional $200 million equivalent in
multicurrencies of undrawn availability under the Revolving
Credit Facilities which can be drawn in either U.S. Dollars or
Euros) and $1.1 billion aggregate
86
principal amount of 8.25% senior notes due 2018 were
denominated in U.S. Dollars. A devaluation of the
U.S. Dollar against the Euro would result in a decrease in
Euro terms in the amount of debt owed and the related interest
expense for our U.S. Dollar-denominated, interest-bearing
debt. A devaluation of the Euro against the U.S. Dollar
would have the opposite effect.
We are also exposed to currency fluctuations with respect to
other currencies such as the Canadian dollar, British pound,
Brazilian real, Malaysian ringgit and the Argentine, Mexican and
Chilean pesos, although to a significantly lesser degree than
the U.S. Dollar.
Other
Factors
Our financial and operating prospects can also be significantly
affected by a number of other internal and external factors,
such as unfavorable changes in governmental regulation or
interpretation; increased competition; the inability to hire or
retain qualified personnel necessary to sustain planned growth;
the loss of key senior managers; problems in developing some of
the international operations; and lack of sufficient capital,
among others.
Critical
Accounting Policies under IFRS
The preparation of consolidated financial statements in
accordance with IFRS as issued by the IASB requires us to make
estimates and judgments in certain circumstances that affect the
reported amounts of assets, liabilities, revenue, expenses and
the related disclosures of contingent assets and liabilities. A
detailed description of our significant accounting policies is
included in the footnotes to our audited consolidated financial
statements.
We believe that certain of our accounting policies are critical
because they are the most important to the preparation of our
consolidated financial statements. These policies require our
most subjective and complex judgments, often requiring the use
of estimates about the effects of matters that are inherently
uncertain. We apply estimation methodologies consistently from
year to year. Other than changes required due to the issuance of
new accounting guidance, there have been no significant changes
in our application of critical accounting policies during the
periods presented. We periodically review our critical
accounting policies and estimates with the audit committee of
our board of directors. The following is a summary of accounting
policies that we consider critical to our consolidated financial
statements.
(a) Business combinations
As permitted by IFRS 1: First-time Adoption of International
Financial Reporting Standards, we have recognized only business
combinations that occurred on or after January 1, 2004, the
date of transition to IFRS, using the acquisition method.
Entities acquired prior to that date were recognized in
accordance with accounting principles prevailing at that time,
taking into account the necessary corrections and adjustments at
the transition date.
We apply the revised IRS 3 “Business combinations” in
transactions made subsequent to January 1, 2010. We apply
the acquisition method for business combinations. The
acquisition date is the date on which we obtain control of the
acquiree.
The cost of the business combination is calculated as the sum of
the acquisition-date fair values of the assets transferred, the
liabilities incurred or assumed, and equity instruments issued
by us in exchange for control of the acquiree, plus any costs
directly attributable to the business combination. Any
additional consideration contingent on future events or the
fulfillment of certain conditions is included in the cost of the
combination provided that it is probable that an outflow of
resources embodying economic benefits will be required and the
amount of the obligation can be reliably estimated.
Where the cost of the business combination exceeds our interest
in the fair value of the identifiable net assets of the entity
acquired, the difference is recognized as goodwill. If the
acquirer’s interest in the fair value of net assets exceeds
the cost of the business combination, the difference remaining
after reassessment is recognized by the acquirer in profit or
loss.
87
(b) Useful lives of property, plant and equipment and
intangible assets
Property, plant and equipment are depreciated by allocating the
depreciable amount of an asset on a systematic basis over its
useful life. The depreciable amount is the cost or deemed cost
less its residual value. We determine the depreciation charge
separately for each component of property, plant and equipment
with a cost that is significant in relation to the total cost of
the asset.
Depreciation of property, plant and equipment is determined
based on the criteria outlined below:
| |
|
|
|
|
|
|
|
Depreciation
|
|
|
|
|
|
Method
|
|
Rates
|
|
|
|
Buildings
|
|
Straight line
|
|
1%-3%
|
|
Plant and machinery
|
|
Straight line
|
|
8%-10%
|
|
Other installations, equipment and furniture
|
|
Straight line
|
|
10%-30%
|
|
Other property, plant and equipment
|
|
Straight line
|
|
16%-25%
|
We assess whether the useful life of each intangible asset
acquired is finite or indefinite. An intangible asset is
regarded by us as having an indefinite useful life when there is
no foreseeable limit to the period over which the asset will
generate net cash inflows.
Intangible assets with indefinite useful lives and goodwill are
not amortized but tested for impairment at least annually or
more frequently if events indicate a potential impairment loss.
Intangible assets with finite useful lives are amortized by
allocating the depreciable amount of an asset on a systematic
basis over its useful life, by applying the following criteria:
| |
|
|
|
|
|
|
|
Amortization
|
|
Estimated Years of
|
|
|
|
Method
|
|
Useful Life
|
|
|
|
Development expenses
|
|
Straight line
|
|
3 - 5
|
|
Concessions, patents, licenses, trademarks and similar
|
|
Straight line
|
|
5 - 15
|
|
Software
|
|
Straight line
|
|
3 - 6
|
We review residual values, useful lives and depreciation methods
at each financial year-end. Changes to initially established
criteria are accounted for as a change in accounting estimates.
(c) Internally generated intangible assets
Any research and development expenditure incurred during the
research phase of projects is recognized as an expense when
incurred.
Costs related with development activities are capitalized when:
|
|
|
| |
•
|
we have technical studies justifying the feasibility of the
production process;
|
| |
| |
•
|
we have undertaken a commitment to complete production of the
asset whereby it is in condition for sale or internal use;
|
| |
| |
•
|
the asset will generate sufficient future economic benefits;
|
| |
| |
•
|
we have sufficient financial and technical resources to complete
development of the asset and have developed budget and cost
accounting control systems that allow budgeted costs, introduced
changes and costs actually assigned to different projects to be
monitored.
|
The cost of internally generated assets is calculated using the
same criteria established for determining production costs of
inventories. The production cost is capitalized by allocating
the costs attributable to the asset to self-constructed assets
in the consolidated income statement.
Costs incurred in the course of activities which contribute to
increasing the value of the different businesses in which we
operate are expensed as they are incurred. Replacements or
subsequent costs incurred on intangible assets are generally
recognized as an expense, except where they increase the future
economic benefits expected to be generated by the assets.
88
(d) Impairment of goodwill and intangible assets with
indefinite useful lives
We test for possible impairment of goodwill and intangible
assets with indefinite useful lives at least annually.
The recoverable amount is the higher of an asset’s fair
value less costs to sell and its value in use. An asset’s
value in use is calculated based on an estimate of the future
cash flows expected to derive from the use of the asset,
expectations about possible variations in the amount or timing
of those future cash flows, the time value of money, the price
for bearing the uncertainty inherent in the asset and other
factors that market participants would reflect in pricing the
future cash flows deriving from the asset.
Recoverable amount is determined for each individual asset,
unless the asset does not generate cash inflows that are largely
independent of those from other assets or groups of assets. If
this is the case, recoverable amount is determined for the
cash-generating unit (“CGU”) to which the asset
belongs.
Impairment losses recognized for cash-generating units are first
allocated to reduce, where applicable, the carrying amount of
goodwill allocated to the CGU and then to the other assets of
the CGU pro rata on the basis of the carrying amount of each
asset. The carrying amount of each asset may not be reduced
below the highest of its fair value less costs to sell, its
value in use and zero.
At the end of each reporting period, we asses whether there is
any indication that an impairment loss recognized in prior
periods may no longer exist or may have decreased. Impairment
losses on goodwill are not reversible. Impairment losses for
other assets are only reversed if there has been a change in the
estimates used to calculate the recoverable amount of the asset.
A reversal of an impairment loss is recognized in consolidated
profit or loss. The increase in the carrying amount of an asset
attributable to a reversal of an impairment loss may not exceed
the carrying amount that would have been determined, net of
depreciation or amortization, had no impairment loss been
recognized.
The reversal of an impairment loss for a CGU is allocated to its
assets, except for goodwill, pro rata with the carrying amounts
of those assets, with the limit per asset of the lower of its
recoverable value and the carrying amount which would have been
obtained, net of depreciation, had no impairment loss been
recognized.
Details of and movement in goodwill for the six months ended
June 30, 2011 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
December 31,
|
|
|
Business
|
|
|
|
|
|
Translation
|
|
|
June 30,
|
|
|
|
|
2010
|
|
|
combinations
|
|
|
Impairment
|
|
|
Differences
|
|
|
2011
|
|
|
|
|
Net value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK, Ltd.
|
|
|
7,982
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(370
|
)
|
|
|
7,612
|
|
|
Grifols Italia, S.p.A.
|
|
|
6,118
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
6,118
|
|
|
Biomat USA, Inc.
|
|
|
113,052
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(8,534
|
)
|
|
|
104,518
|
|
|
Plasmacare, Inc.
|
|
|
38,464
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(2,903
|
)
|
|
|
35,561
|
|
|
Woolloomooloo Holdings Pty Ltd. (Australia)
|
|
|
23,832
|
|
|
|
0
|
|
|
|
(13,000
|
)
|
|
|
(415
|
)
|
|
|
10,417
|
|
|
Talecris Biotherapeutics,
Inc.(1)
|
|
|
0
|
|
|
|
2,124,082
|
|
|
|
0
|
|
|
|
(6,612
|
)
|
|
|
2,117,470
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
189,448
|
|
|
|
2,124,082
|
|
|
|
(13,000
|
)
|
|
|
(18,834
|
)
|
|
|
2,281,696
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1)
|
|
The name of Talecris
Biotherapeutics, Inc. was changed to Grifols Therapeutics Inc.
in August 2011.
|
Goodwill resulting from the Talecris acquisition is still
provisional as the estimation of the fair value of assets,
liabilities and contingent liabilities of the business acquired
is in progress
Goodwill has been allocated to each of our CGUs in accordance
with their respective business segment and on a geographical
basis, those being the lowest level at which goodwill is
controlled for management purpose and lower than operating
segments. Plasmacare, Inc. is integrated into the management of
Biomat USA, Inc. for the purpose of impairment testing.
89
Goodwill has been allocated to the cash generating units as
follows:
− UK: bioscience segment
− Italy: bioscience segment
− United States: bioscience segment
− Australia: mainly to the diagnostics segment.
The recoverable amount of a CGU is determined based on its value
in use. These calculations use cash flow projections based on
the financial budgets approved by management. Cash flows as of
the year in which stable growth has been reached are
extrapolated using the estimated growth rates indicated below.
At June 30, 2011, there are no indications that the
goodwill of the CGUs belonging to the Bioscience division has
been impaired.
For the six months ended June 30, 2011, there was an
impairment indicator for the Australia CGU and therefore
goodwill impairment was prepared. The CGU’s market
performance was lower than expected. As a result of the
impairment test performed, an impairment of the CGU’s
goodwill (diagnostic) of €13,000 thousand has been
accounted for at June 30, 2011.
The key assumptions used in calculating values in use for the
year ended December 31, 2010 and for the six months ended
June 30, 2011 were as follows:
| |
|
|
|
|
|
|
|
12/31/2010
|
|
|
|
Growth rate
|
|
Pre-tax discount rate
|
|
|
|
Bioscience
|
|
2.0% - 3.0%
|
|
10.5% - 10.9%
|
|
Diagnostic
|
|
2.0%
|
|
10.4%
|
| |
|
|
|
|
|
|
|
06/30/2011
|
|
|
|
Growth rate
|
|
Pre-tax discount rate
|
|
|
|
Bioscience
|
|
N/A
|
|
N/A
|
|
Diagnostic
|
|
2.0%
|
|
11.5%
|
Management determined budgeted gross margins based on past
experience and forecast market development. Average weighted
growth rates are consistent with the forecasts included in
industry reports. The discount rate used reflects specific risks
related to the CGU.
(e) Inventories
Inventories are measured at the lower of cost and net realizable
value. The cost of inventories comprises all costs of purchase,
costs of conversion and other costs incurred in bringing the
inventories to their present location and condition.
The costs of conversion of inventories include costs directly
related to the units of production and a systematic allocation
of fixed and variable production overheads that are incurred in
converting. Fixed production overheads are allocated based on
the higher of normal production capacity or actual level of
production.
The cost of raw materials and other supplies, the cost of
merchandise and costs of conversion are allocated to each
inventory unit on a
first-in,
first-out (“FIFO”) basis. We use the same cost model
for all inventories of the same nature and with a similar use.
Volume discounts extended by suppliers are recognized as a
reduction in the cost of inventories when it is probable that
the conditions for discounts to be received will be met.
Discounts for prompt payment are recognized as a reduction in
the cost of the inventories acquired.
90
The cost of inventories is adjusted against profit and loss when
cost exceeds the net realizable value. Net realizable value is
considered as follows:
|
|
|
| |
•
|
Raw materials and other supplies: replacement cost.
Nevertheless, raw materials are not written down below cost if
the finished goods into which they will be incorporated are
expected to be sold at or above cost of production.
|
| |
| |
•
|
Goods for resale and finished goods: estimated selling price,
less costs to sell.
|
| |
| |
•
|
Work in progress: the estimated selling price of related
finished goods, less the estimated costs of completion and the
estimated costs necessary to make the sale.
|
The previously recognized reduction in value is reversed against
profit and loss when the circumstances that previously caused
inventories to be written down no longer exist or when there is
clear evidence of an increase in net realizable value because of
changed economic circumstances. The reversal of the reduction in
value is limited to the lower of the cost and revised net
realizable value of the inventories. Write-downs may be reversed
with a credit to inventories of finished goods and work in
progress and supplies.
(f) Revenue recognition
We recognize revenue when earned, which is generally at the time
of delivery to the customer. Recognition of revenue also
requires reasonable assurance of collection of sales proceeds, a
fixed and determinable price, persuasive evidence that an
arrangement exists and completion of all other performance
obligations. Allowances against revenues for estimated discounts
and rebates are established by us concurrently with the
recognition of revenue.
We participate in state government-managed Medicaid programs in
the United States. We account for Medicaid rebates by
establishing an accrual at the time the sale is recorded in an
amount equal to our estimate of the Medicaid rebate claims
attributable to such sale. We determine the estimate of the
Medicaid rebates accrual primarily based on historical
experience regarding Medicaid rebates, legal interpretations of
the applicable laws related to the Medicaid program and any new
information regarding changes in the Medicaid programs’
regulations and guidelines that would impact the amount of the
rebates. We consider outstanding Medicaid claims, Medicaid
payments, and levels of inventory in the distribution channel
and adjust the accrual periodically to reflect actual
experience. While these rebate payments to the states generally
occur on a one- to two-quarter lag, any adjustments for actual
experience have not been material.
Group Purchasing Organizations or other customers in the United
States that have entered into contracts with us for purchases of
Flebogamma are eligible for a pricing discount based upon a
minimum purchase quantity of Flebogamma each month. These
rebates are recorded as a reduction of sales and accounts
receivable in the same month the sales are invoiced based upon a
combination of actual customer purchase data and on historical
experience when the actual customer purchase data is reported
later in time.
91
Results
of Operations
We have included information regarding our results of operations
in the following table. The subsequent discussion and analysis
provides information that our management believes is relevant to
an assessment and understanding of our consolidated results of
operations. You are encouraged to read the following discussion
and analysis of our financial condition and results of
operations together with our consolidated financial statements
and related footnotes included elsewhere in this prospectus.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
|
|
|
|
|
|
|
|
June 30,
|
|
|
Years Ended December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Revenues
|
|
|
635,341
|
|
|
|
487,809
|
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
814,311
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
2,757
|
|
|
|
41,209
|
|
|
|
45,749
|
|
|
|
73,093
|
|
|
|
31,058
|
|
|
Self-constructed non-current assets
|
|
|
32,346
|
|
|
|
16,051
|
|
|
|
33,513
|
|
|
|
41,142
|
|
|
|
25,794
|
|
|
Supplies
|
|
|
(175,142
|
)
|
|
|
(157,107
|
)
|
|
|
(304,818
|
)
|
|
|
(286,274
|
)
|
|
|
(206,738
|
)
|
|
Other operating income
|
|
|
1,009
|
|
|
|
631
|
|
|
|
1,196
|
|
|
|
1,443
|
|
|
|
1,289
|
|
|
Personnel expenses
|
|
|
(183,727
|
)
|
|
|
(141,972
|
)
|
|
|
(289,008
|
)
|
|
|
(273,168
|
)
|
|
|
(238,159
|
)
|
|
Other operating expenses
|
|
|
(155,532
|
)
|
|
|
(98,279
|
)
|
|
|
(205,260
|
)
|
|
|
(203,381
|
)
|
|
|
(192,288
|
)
|
|
Amortisation and depreciation
|
|
|
(28,156
|
)
|
|
|
(21,434
|
)
|
|
|
(45,776
|
)
|
|
|
(39,554
|
)
|
|
|
(33,256
|
)
|
|
Transaction costs of Talecris business combination
|
|
|
(38,607
|
)
|
|
|
(2,019
|
)
|
|
|
(16,999
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Non-financial and other capital grants
|
|
|
742
|
|
|
|
550
|
|
|
|
728
|
|
|
|
1,188
|
|
|
|
2,941
|
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(22,302
|
)
|
|
|
681
|
|
|
|
(372
|
)
|
|
|
(1,147
|
)
|
|
|
(1,991
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
68,729
|
|
|
|
126,120
|
|
|
|
209,683
|
|
|
|
226,528
|
|
|
|
202,961
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
1,761
|
|
|
|
2,179
|
|
|
|
4,526
|
|
|
|
7,067
|
|
|
|
2,682
|
|
|
Finance expenses
|
|
|
(55,546
|
)
|
|
|
(25,285
|
)
|
|
|
(49,660
|
)
|
|
|
(27,087
|
)
|
|
|
(29,305
|
)
|
|
Change in fair value of financial instruments
|
|
|
13,945
|
|
|
|
(15,404
|
)
|
|
|
(7,593
|
)
|
|
|
(587
|
)
|
|
|
(l,268
|
)
|
|
Gains/(losses) on disposal of financial instruments
|
|
|
—
|
|
|
|
—
|
|
|
|
91
|
|
|
|
(245
|
)
|
|
|
—
|
|
|
Exchange gains/(losses)
|
|
|
(2,122
|
)
|
|
|
1,970
|
|
|
|
1,616
|
|
|
|
(1,733
|
)
|
|
|
(2,825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance expense
|
|
|
(41,962
|
)
|
|
|
(36,540
|
)
|
|
|
(51,020
|
)
|
|
|
(22,585
|
)
|
|
|
(30,716
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of equity accounted investees
|
|
|
(807
|
)
|
|
|
(728
|
)
|
|
|
(879
|
)
|
|
|
51
|
|
|
|
24
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
25,960
|
|
|
|
88,852
|
|
|
|
157,784
|
|
|
|
203,994
|
|
|
|
172,269
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
(7,347
|
)
|
|
|
(23,022
|
)
|
|
|
(42,517
|
)
|
|
|
(56,424
|
)
|
|
|
(50,153
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the period
|
|
|
18,613
|
|
|
|
65,830
|
|
|
|
115,267
|
|
|
|
147,570
|
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six
Months Ended June 30, 2011 Compared to Six Months Ended
June 30, 2010
Our results of operations for the six months ended June 30,
2011 include Talecris beginning on June 1, 2011, the date
of the consummation of the acquisition.
The following discussion and analysis contains information
regarding our results of operations for the six months ended
June 30, 2011 as compared to the six months ended
June 30, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
|
|
|
|
|
|
|
|
June 30,
|
|
|
Change
|
|
|
|
|
2011
|
|
|
2010
|
|
|
€
|
|
|
%
|
|
|
|
|
Revenues
|
|
|
635,341
|
|
|
|
487,809
|
|
|
|
147,532
|
|
|
|
30.2
|
%
|
|
Changes in inventories of finished goods and work in progress
|
|
|
2,757
|
|
|
|
41,209
|
|
|
|
(38,452
|
)
|
|
|
(93.3
|
)%
|
|
Self-constructed non-current assets
|
|
|
32,346
|
|
|
|
16,051
|
|
|
|
16,295
|
|
|
|
101.5
|
%
|
|
Supplies
|
|
|
(175,142
|
)
|
|
|
(157,107
|
)
|
|
|
(18,035
|
)
|
|
|
11.5
|
%
|
|
Other operating income
|
|
|
1,009
|
|
|
|
631
|
|
|
|
378
|
|
|
|
59.9
|
%
|
|
Personnel expenses
|
|
|
(183,727
|
)
|
|
|
(141,972
|
)
|
|
|
(41,755
|
)
|
|
|
29.4
|
%
|
|
Other operating expenses
|
|
|
(155,532
|
)
|
|
|
(98,279
|
)
|
|
|
(57,253
|
)
|
|
|
58.3
|
%
|
|
Amortisation and depreciation
|
|
|
(28,156
|
)
|
|
|
(21,434
|
)
|
|
|
(6,722
|
)
|
|
|
31.4
|
%
|
|
Transaction costs of Talecris business combination
|
|
|
(38,607
|
)
|
|
|
(2,019
|
)
|
|
|
(36,588
|
)
|
|
|
1812.2
|
%
|
|
Non-financial and other capital grants
|
|
|
742
|
|
|
|
550
|
|
|
|
192
|
|
|
|
34.9
|
%
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(22,302
|
)
|
|
|
681
|
|
|
|
(22,983
|
)
|
|
|
(3374.9
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
68,729
|
|
|
|
126,120
|
|
|
|
(57,391
|
)
|
|
|
(45.5
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
92
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
|
|
|
|
|
|
|
|
June 30,
|
|
|
Change
|
|
|
|
|
2011
|
|
|
2010
|
|
|
€
|
|
|
%
|
|
|
|
|
Finance income
|
|
|
1,761
|
|
|
|
2,179
|
|
|
|
(418
|
)
|
|
|
(19.2
|
)%
|
|
Finance expenses
|
|
|
(55,546
|
)
|
|
|
(25,285
|
)
|
|
|
(30,261
|
)
|
|
|
119.7
|
%
|
|
Change in fair value of financial instruments
|
|
|
13,945
|
|
|
|
(15,404
|
)
|
|
|
29,349
|
|
|
|
190.5
|
%
|
|
Exchange gains/(losses)
|
|
|
(2,122
|
)
|
|
|
1,970
|
|
|
|
(4,092
|
)
|
|
|
(207.7
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance expense
|
|
|
(41,962
|
)
|
|
|
(36,540
|
)
|
|
|
(5,422
|
)
|
|
|
14.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of equity accounted investees
|
|
|
(807
|
)
|
|
|
(728
|
)
|
|
|
(79
|
)
|
|
|
10.9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
25,960
|
|
|
|
88,852
|
|
|
|
(62,892
|
)
|
|
|
(70.8
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
(7,347
|
)
|
|
|
(23,022
|
)
|
|
|
15,675
|
|
|
|
(68.1
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the period
|
|
|
18,613
|
|
|
|
65,830
|
|
|
|
(47,217
|
)
|
|
|
(71.7
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues.
Our revenues increased by 30.2% from €487.8 million in
the six months ended June 30, 2010 to
€635.3 million in the six months ended June 30,
2011. This increase is primarily attributable to the acquisition
of Talecris in June 2011 and related increase in sales volume
and revenues in our Bioscience division. If we excluded Talecris
revenues, our revenue would have increased by 8% in the six
months ended June 30, 2011 as compared to the same period
in 2010.
Our results of operations for the six months ended June 30,
2011 reflect changes in the revenue of each of our business
divisions as a percentage of our total revenues as a result of
the acquisition. Revenues for the Bioscience division grew by
37.2% to €521.5 million in the six months ended
June 30, 2011, representing 82.1% of total revenue. The
Diagnostic division increased its revenue by 4.4% to
€56.8 million in the six months ended June 30,
2011 and the Hospital division increased its revenues by 9.2% to
€49.3 million in the six months ended June 30,
2011. As a result of the acquisition and the increased size of
our Bioscience division, revenues from the Diagnostic and
Hospital divisions as a percentage of total revenue decreased
from 11.2% and 9.3%, respectively, in the six months ended
June 30, 2010 to 8.9% and 7.8%, respectively, in the six
months ended June 30, 2011.
The acquisition has also modified the geographic mix of our
sales. In the six months ended June 30, 2011, 42% of our
revenues, totaling €266.5 million, were generated in
the United States and Canada, while 38.7% of revenues were
generated in the European Union. This was an increase of 69.1%
over the same period in 2010 and makes North America our largest
market. For the six months ended June 30, 2011, 80% of our
activities were generated outside of Spain, with revenues from
Spain as a percentage of total revenues decreasing to 19% in the
six months ended June 30, 2011 as compared to 24.5% in the
same period in 2010. In the Europe Union, sales increased by
10.4% and reached €246.1 million, which included
increases in market share in Germany, Portugal. We also
experienced significant growth in Australia.
The following tables reflect a summary of revenues by each of
our divisions and geographical regions for the six months ended
June 30, 2011 as compared to the six months ended
June 30, 2010:
Summary of Revenues by Division
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
|
% of total
|
|
|
June 30,
|
|
|
% of total
|
|
|
|
|
|
|
|
|
|
|
2011
|
|
|
revenues
|
|
|
2010
|
|
|
revenues
|
|
|
% var
|
|
|
% var CC(a)
|
|
|
|
|
Bioscience
|
|
|
521,538
|
|
|
|
82.1
|
|
|
|
380,081
|
|
|
|
77.9
|
|
|
|
37.2
|
|
|
|
40.9
|
|
|
Diagnostic
|
|
|
56,831
|
|
|
|
8.9
|
|
|
|
54,413
|
|
|
|
11.2
|
|
|
|
4.4
|
|
|
|
3.8
|
|
|
Hospital
|
|
|
49,289
|
|
|
|
7.8
|
|
|
|
45,146
|
|
|
|
9.3
|
|
|
|
9.2
|
|
|
|
8.8
|
|
|
Raw Materials
|
|
|
1,658
|
|
|
|
0.3
|
|
|
|
1,835
|
|
|
|
0.4
|
|
|
|
(9.6
|
)
|
|
|
(6.8
|
)
|
|
Others
|
|
|
6,025
|
|
|
|
1.0
|
|
|
|
6,334
|
|
|
|
1.3
|
|
|
|
(4.9
|
)
|
|
|
(4.9
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
635,341
|
|
|
|
100.0
|
|
|
|
487,809
|
|
|
|
100.0
|
|
|
|
30.2
|
|
|
|
33.0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
93
|
|
|
|
(a)
|
|
Revenue variance in constant
currency is determined by comparing adjusted current period
revenue, calculated using prior period monthly average exchange
rates, to the prior period revenue. This measure is considered
to be a non-IFRS financial measure. Revenue variance in constant
currency calculates revenue variance without the impact of
foreign exchange fluctuations. We believe that constant currency
revenue variance is an important measure of our operations
because it neutralizes foreign exchange impact and better
illustrates the underlying change in revenues from one year to
the next. We believe that this presentation provides a more
useful
period-over-period
comparison as changes due solely to changes in exchange rates
are eliminated. Revenue variance in constant currency, as
defined and presented by us, may not be comparable to similar
measures reported by other companies. Revenue variance in
constant currency has limitations, particularly because the
currency effects that are eliminated constitute a significant
element of our revenue and expenses and could impact our
performance significantly. We do not evaluate our results and
performance without considering revenue variances in constant
currency on the one hand and changes in revenue prepared in
accordance with IFRS on the other. We caution the readers of
this prospectus to follow a similar approach by considering data
regarding constant currency
period-over-period
revenue variance only in addition to, and not as a substitute
for or superior to, other measures of financial performance
prepared in accordance with IFRS.
|
Summary of Revenues by Region
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
|
|
|
Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
|
% of total
|
|
|
June 30,
|
|
|
% of total
|
|
|
|
|
|
|
|
|
|
|
2011
|
|
|
revenues
|
|
|
2010
|
|
|
revenues
|
|
|
% var
|
|
|
% var
CC*
|
|
|
|
|
European Union
|
|
|
246,144
|
|
|
|
38.7
|
|
|
|
223,019
|
|
|
|
45.7
|
|
|
|
10.4
|
|
|
|
10.1
|
|
|
United States and Canada
|
|
|
267,124
|
|
|
|
42.0
|
|
|
|
157,948
|
|
|
|
32.4
|
|
|
|
69.1
|
|
|
|
79.3
|
|
|
Rest of World
|
|
|
122,073
|
|
|
|
19.0
|
|
|
|
106,842
|
|
|
|
21.9
|
|
|
|
14.3
|
|
|
|
12.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
635,341
|
|
|
|
100.0
|
|
|
|
487,809
|
|
|
|
100.0
|
|
|
|
30.2
|
|
|
|
33.0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Please see footnote (a) to the
table above. |
Bioscience. Revenue for the Bioscience
division, which included Talecris’ June 2011 sales,
increased by 37.2% from €380.1 million in the six
months ended June 30, 2010 to €521.5 million in
the six months ended June 30, 2011. If we excluded
Talecris’ revenues, our revenue for the Bioscience division
would have increased by 9.7% in the six months ended
June 30, 2011 as compared to the same period in 2010. An
increase in the sales volumes of all of our key plasma
derivative products resulting from our acquisition of Talecris
was the main driver of the division’s growth, partially
offset by a decline in prices. As a result of the acquisition,
we expanded the breadth of our portfolio of plasma derivative
products and brand names and diversified our end markets.
Hospital. Revenues from the Hospital division
increased by 9.2% from €45.1 million in the six months
ended June 30, 2010 to €49.3 million in the six
months ended June 30, 2011. This increase is attributable
primarily to a 13.4% increase in revenues from the intravenous
therapies subdivision, a 10.7% increase in revenues from the
medical devices subdivision and a 7.8% increase in revenues from
the hospital logistics subdivision. This growth in sales volume
reflects our ongoing product and geographical market
diversification strategy, which includes increased contract
manufacturing of intravenous therapies and the development of
new medical devices. Revenue from contract manufacturing has
increased by 91.8% to €2.4 million in the six months
ended June 30, 2011. Our revenue from Misterium
installations has increased by 196.7% to €1.4 million
in the six months ended June 30, 2011. In addition, sales
of our new BlisPack product have brought in
€0.3 million of revenues to date.
Sales in our Hospital division are primarily in Spain and have
been affected by the current economic crisis. In particular, the
weakened Spanish economy has affected the prices of our products
in the intravenous therapy line and has led to decreased capital
investments by hospitals.
Diagnostic. Our Diagnostic division increased
revenues by 4.4% to from €54.4 million in the six
months ended June 30, 2010 to €56.8 million in
the six months ended June 30, 2011. The growth in the
Diagnostic division was driven primarily by a 10.1% increase in
revenues from sales to blood banks period over period. By
region, blood bank growth is primarily occurring in the European
Union with a 33.3% increase. In
94
addition, pathogen inactivation of blood components experienced
a 28.4% increase in revenues in the European Union.
This growth was offset by stable revenues in our
immunohematology sector, the largest subdivision of our Hospital
division. Immunohematology experienced only modest growth of
1.1% period over period primarily as a result of stable sales
under our custom manufacturing agreement with Ortho Clinical
Diagnostics, Inc., which has led to stable sales, and a decline
in revenues from Australia by 53.7% to €1.6 million in
the six months ended June 30, 2011.
Raw Materials. Revenues from our Raw Materials
division decreased from €1.8 million in the six months
ended June 30, 2010 to €1.7 million in the six
months ended June 30, 2011.
Changes
in inventories of finished goods and work in progress and
materials consumed and Supplies.
The cost of supplies increased by 11.5%, from €157.1 in the
six months ended June 30, 2010 to €175.1 in the six
months ended June 30, 2011. The increase in cost of
supplies is primarily the result of the acquisition of Talecris,
which caused a €19.0 million increase. Excluding the
acquisition of Talecris cost of supplies decreased from
€157.1 million in the six months ended June 30,
2010 to €156.1 in the six months ended June 30, 2011.
In addition, €2.8 million corresponds to change in
inventories of finished goods and work in progress. This
decrease was the result of measures implemented by us to control
inventory levels. This trend, which started in the first quarter
of 2011, will be reinforced throughout the year as a result of
the acquisition of Talecris.
Self-constructed
non-current assets.
Self-constructed non-current assets increased by 101.5%, from
€16.1 million in the six months ended June 30,
2010 to €32.3 million in the six months ended
June 30, 2011, primarily as a result of a
€16.8 million increase attributable to the integration
of Talecris.
Personnel
expenses.
Personnel expenses increased by 29.4%, from
€141.0 million in the six months ended June 30,
2010 to €183.7 million in the six months ended
June 30, 2011. The increase in personnel expenses reflects
the acquisition of Talecris and a related expense of
€30.4 million. As a result of the acquisition, our
headcount increased from 5,968 as of December 31, 2010 to
11,174 as of June 30, 2011.
Other
operating expenses.
Other operating expenses increased by 58.3%, from
€98.3 million in the six months ended June 30,
2010 to €155.5 million in the six months ended
June 30, 2011. The acquisition of Talecris led to a
€40.0 million increase in other operating expenses.
Excluding Talecris operations, our other operating expenses
increased by €17.2 million in the six months ended
June 30, 2011. In addition, we incurred
€38.6 million in costs in connection with the
acquisition.
Amortisation
and depreciation.
Depreciation and amortization of fixed assets increased by
31.4%, from €21.4 million in the six months ended
June 30, 2010 to €28.2 million in the six months
ended June 30, 2011. This increase was primarily due to the
increase in our fixed asset base as a result of the acquisition
of Talecris.
Net
Finance expense.
Net finance expense increased by 14.8% from
€36.5 million in the six months ended June 30,
2010 to €42.0 million in the six months ended
June 30, 2011. This increase was primarily a result of an
increase in finance expense by 119.4%, from
€25.3 million in the six months ended June 30,
2010 to €55.5 million in the six months ended
June 30, 2011, due to the entry into the Senior Credit
Facilities and the issuance of our 8.25% senior notes due
2018 in order to finance the acquisition. The net finance
expense also includes a non-
95
realized gain of €14.2 million for the six months
ended June 30, 2011 arising from futures contracts with
respect to our Class A shares (a loss of
€15.8 million for the six months ended June 30,
2010).
Income
tax expense.
In the six months ended June 30, 2011, we had a profit
before income tax of €26.0 million and income tax
expense of €7.3 million, which represents an effective
tax rate of 28.3%. This amount includes deductions of
€4.1 million relating to research and development
deductions and other deductions. Our effective tax rate
increased from 25.9% for the six month period ended
June 30, 2010 primarily due to a greater portion of our
revenues being taxed at a higher tax rate as a result of the
acquisition.
Year
Ended December 31, 2010 Compared to Year Ended
December 31, 2009
The following table contains information regarding our results
of operations for the year ended December 31, 2010 as
compared to the year ended December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
Change
|
|
|
|
|
2010
|
|
|
2009
|
|
|
€
|
|
|
%
|
|
|
|
|
Revenues
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
77,544
|
|
|
|
8.5
|
%
|
|
Changes in inventories of finished goods and work in progress
|
|
|
45,749
|
|
|
|
73,093
|
|
|
|
(27,344
|
)
|
|
|
(37.4
|
)%
|
|
Self-constructed non-current assets
|
|
|
33,513
|
|
|
|
41,142
|
|
|
|
(7,629
|
)
|
|
|
(18.5
|
)%
|
|
Supplies
|
|
|
(304,818
|
)
|
|
|
(286,274
|
)
|
|
|
(18,544
|
)
|
|
|
6.5
|
%
|
|
Other operating income
|
|
|
1,196
|
|
|
|
1,443
|
|
|
|
(247
|
)
|
|
|
(17.1
|
)%
|
|
Personnel expenses
|
|
|
(289,008
|
)
|
|
|
(273,168
|
)
|
|
|
(15,840
|
)
|
|
|
5.8
|
%
|
|
Other operating expenses
|
|
|
(205,260
|
)
|
|
|
(203,381
|
)
|
|
|
(1,879
|
)
|
|
|
0.9
|
%
|
|
Amortisation and depreciation
|
|
|
(45,776
|
)
|
|
|
(39,554
|
)
|
|
|
(6,222
|
)
|
|
|
15.7
|
%
|
|
Transaction costs of Talecris business combination
|
|
|
(16,999
|
)
|
|
|
0
|
|
|
|
(16,999
|
)
|
|
|
100
|
%
|
|
Non-financial and other capital grants
|
|
|
728
|
|
|
|
1,188
|
|
|
|
(460
|
)
|
|
|
(38.7
|
)%
|
|
Impairment and gains/(losses) on disposal of fixed assets
|
|
|
(372
|
)
|
|
|
(1,147
|
)
|
|
|
775
|
|
|
|
(67.6
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
209,683
|
|
|
|
226,528
|
|
|
|
(16,845
|
)
|
|
|
(7.4
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
4,526
|
|
|
|
7,067
|
|
|
|
(2,541
|
)
|
|
|
(36.0
|
)%
|
|
Finance expenses
|
|
|
(49,660
|
)
|
|
|
(27,087
|
)
|
|
|
(22,573
|
)
|
|
|
83.3
|
%
|
|
Change in fair value of financial instruments
|
|
|
(7,593
|
)
|
|
|
(587
|
)
|
|
|
(7,006
|
)
|
|
|
1193.5
|
%
|
|
Gains/(losses) on disposal of financial instruments
|
|
|
91
|
|
|
|
(245
|
)
|
|
|
336
|
|
|
|
137.1
|
%
|
|
Exchange gains/(losses)
|
|
|
1,616
|
|
|
|
(1,733
|
)
|
|
|
3,349
|
|
|
|
193.2
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance expense
|
|
|
(51,020
|
)
|
|
|
(22,585
|
)
|
|
|
(28,435
|
)
|
|
|
125.9
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of equity accounted investees
|
|
|
(879
|
)
|
|
|
51
|
|
|
|
(930
|
)
|
|
|
(1823.5
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
157,784
|
|
|
|
203,994
|
|
|
|
(46,210
|
)
|
|
|
(22.7
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
(42,517
|
)
|
|
|
(56,424
|
)
|
|
|
13,907
|
|
|
|
(24.6
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
115,267
|
|
|
|
147,570
|
|
|
|
(32,303
|
)
|
|
|
(21.9
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Revenues.
Overall revenues increased by 8.5%, from
€913.2 million in 2009 to €990.7 million in
2010, driven mainly by increased revenues from our Bioscience
division.
96
The following tables reflect a summary of revenues by each of
our divisions and geographical regions for the year ended
December 31, 2010 as compared to the year ended
December 31, 2009:
Summary of Revenues by Division
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
% of total
|
|
|
December 31,
|
|
|
% of total
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
revenues
|
|
|
2009
|
|
|
revenues
|
|
|
% var
|
|
|
% var CC*
|
|
|
|
|
Bioscience
|
|
|
773,372
|
|
|
|
78.1
|
|
|
|
694,969
|
|
|
|
76.1
|
|
|
|
11.3
|
|
|
|
7.7
|
|
|
Diagnostic
|
|
|
109,088
|
|
|
|
11.0
|
|
|
|
103,091
|
|
|
|
11.3
|
|
|
|
5.8
|
|
|
|
3.4
|
|
|
Hospital
|
|
|
89,552
|
|
|
|
9.0
|
|
|
|
86,328
|
|
|
|
9.5
|
|
|
|
3.7
|
|
|
|
2.9
|
|
|
Raw Materials
|
|
|
4,815
|
|
|
|
0.5
|
|
|
|
22,665
|
|
|
|
2.5
|
|
|
|
(78.8
|
)
|
|
|
(79.8
|
)
|
|
Others
|
|
|
13,903
|
|
|
|
1.4
|
|
|
|
6,133
|
|
|
|
0.7
|
|
|
|
126.7
|
|
|
|
126.6
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
990,730
|
|
|
|
100.0
|
|
|
|
913,186
|
|
|
|
100.0
|
|
|
|
9.6
|
|
|
|
6.5
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Please see footnote (a) to the
table on page 93. |
Summary of Revenues by Region
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
% of total
|
|
|
December 31,
|
|
|
% of total
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
revenues
|
|
|
2009
|
|
|
revenues
|
|
|
% var
|
|
|
% var CC*
|
|
|
|
|
European Union
|
|
|
432,191
|
|
|
|
43.6
|
|
|
|
424,591
|
|
|
|
46.5
|
|
|
|
1.8
|
|
|
|
1.1
|
|
|
United States and Canada
|
|
|
339,018
|
|
|
|
34.2
|
|
|
|
296,659
|
|
|
|
32.5
|
|
|
|
14.3
|
|
|
|
10.0
|
|
|
Rest of World
|
|
|
219,521
|
|
|
|
22.2
|
|
|
|
191,936
|
|
|
|
21.0
|
|
|
|
14.4
|
|
|
|
7.7
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
990,730
|
|
|
|
100.0
|
|
|
|
913,186
|
|
|
|
100.0
|
|
|
|
8.5
|
|
|
|
5.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Please see footnote (a) to the
table on page 93. |
Bioscience. Revenues from our Bioscience
division increased by 11.3%, from €695.0 million in
2009 to €773.4 million in 2010. The increase in
revenues is primarily due to increases in sales volume for each
of our three main product lines. Increases in sales volume were
partially offset by decreases in prices across our product
lines. Revenues from sales of IVIG, albumin and Factor VIII
increased 20.6%, 6.4% and 7.6%, respectively. IVIG sales growth
was primarily due to increases in revenues from the United
States (a 38.2% increase) and the Rest of the World (a 13.8%
increase), with revenues from the European Union remaining
stable.
Hospital. Revenues from our Hospital division
increased by 3.7%, from €86.3 million in 2009 to
€89.6 million in 2010. In 2010, we recorded increases
in revenues from each of our main product lines: intravenous
therapy, medical devices, hospital logistics and nutrition. The
key growth drivers in the Hospital division are
(i) intravenous therapies, which increased 6.6% and
(ii) medical devices, which increased 8.4%. The increase in
revenue from intravenous therapies is primarily attributable to
a 4% increase in revenues in the European Union, while growth in
the medical devices product line is primarily attributable to
increased sales in the European Union.
Diagnostic. Revenues from our Diagnostic
division increased by 5.8%, from €103.1 million in
2009 to €109.1 million in 2010. The growth in the
Diagnostic division was driven primarily by: a 17.2% increase in
blood bank revenue and a 18.4% increase in hemostasis revenues.
The increase in revenue from blood banks is primarily
attributable to an increase in sales volume in the European
Union and the Rest of the World, while growth in hemostatis is
primarily attributable expanded sales in the European Union.
Immunohematology experienced only modest growth of 2.4% in 2010,
primarily as a result of stable sales under our custom
manufacturing agreement with Ortho Clinical Diagnostics, Inc.,
and a decline in revenues from Australia by 23.1% to
€5.1 million in 2010.
Raw Materials. Revenues decreased by 78.8%,
from €22.7 million in 2009 to €4.8 million
in 2010, primarily due to the fact that 2009 sales comprised of
one-off plasma sales, while in 2010 we experienced only
recurrent sales.
97
Changes
in inventories of finished goods and work in progress and
Supplies.
Supplies increased by 7.2%, from €286.3 million in
2009 to €306.9 million in 2010. From these supplies,
€282.5 million corresponds to purchases of raw
materials (€325.4 million in 2009) and goods for
resale and €24.4 corresponds to change in inventories of
raw materials and goods for resale (€(39.1) million in
2009). In addition, €45.7 million corresponds to
change in inventories of finished goods and work in progress.
This increase was primarily because we intended to build up
inventories to secure future growth.
Personnel
expenses.
Personnel expenses increased by 5.8%, from
€273.2 million in 2009 to €289.0 million in
2010, primarily due to increased sales personnel and an increase
in variable remuneration paid.
Other
operating expenses.
Other operating expenses increased by 0.9%, from
€203.4 million in 2009 to €205.3 million in
2010. In addition, we incurred costs in connection with the
proposed acquisition of Talecris in the amount of
€17.0 million.
Amortisation
and depreciation.
Amortisation and depreciation increased by 15.7%, from
€39.6 million in 2009 to €45.8 million in
2010. This increase was primarily due to the continued increase
in our fixed asset base, including a new sterile filling and
purification area at our Los Angeles plant and our new corporate
offices in Sant Cugat del Vallès, Barcelona.
Net
finance expense.
Net finance expense increased by 125.9% from
€22.6 million in 2009 to €51 million in
2010. This significant increase was mainly due to a 83.3%
increase in finance expenses, from €27.1 million in
2009 to €49.7 million in 2010, as a result of
(i) the full-year impact of the interest expense on the
Grifols Old Notes which amounted to €31.9 million in
2010 as compared to €6.8 million in 2009 and
(ii) a non-realized loss of €8.4 million arising
from futures contracts with respect to our Class A shares.
In addition, finance income decreased by 36.0%, from
€7.1 million in 2009 to €4.5 million in 2010
primarily due to a 55.8% decrease in interest from Spanish
Social Security, from €6.5 million in 2009 to
€2.9 million in 2010.
Income
tax expense.
In 2010, we had a consolidated profit before income tax of
€157.8 million and income tax expense of
€42.5 million, which represents an effective tax rate
of 26.9%. This amount includes deductions of
€10.8 million relating to research and development and
other deductions. The effective tax rate decreased from 27.7% in
2009 primarily due to a lower profit before taxes in 2010 and a
reduction in the amount of deductions year over year.
Profit
after income tax from continuing operations
As a result of the above, profit after income tax from
continuing operations decreased 21.9%, from
€147.6 million in 2009 to €115.3 million in
2010.
98
Year
Ended December 31, 2009 Compared to Year Ended
December 31, 2008
The following table contains information regarding our results
of operations for the year ended December 31, 2009 as
compared to the year ended December 31, 2008:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
Change
|
|
|
|
|
2009
|
|
|
2008
|
|
|
€
|
|
|
%
|
|
|
|
|
Revenues
|
|
|
913,186
|
|
|
|
814,311
|
|
|
|
98,875
|
|
|
|
12.1
|
%
|
|
Changes in inventories of finished goods and work in progress
|
|
|
73,093
|
|
|
|
31,058
|
|
|
|
42,035
|
|
|
|
135.3
|
%
|
|
Self-constructed non-current assets
|
|
|
41,142
|
|
|
|
25,794
|
|
|
|
15,348
|
|
|
|
59.5
|
%
|
|
Supplies
|
|
|
(286,274
|
)
|
|
|
(206,738
|
)
|
|
|
(79,536
|
)
|
|
|
38.5
|
%
|
|
Other operating income
|
|
|
1,443
|
|
|
|
1,289
|
|
|
|
154
|
|
|
|
11.9
|
%
|
|
Personnel expenses
|
|
|
(273,168
|
)
|
|
|
(238,159
|
)
|
|
|
(35,009
|
)
|
|
|
14.7
|
%
|
|
Other operating expenses
|
|
|
(203,381
|
)
|
|
|
(192,288
|
)
|
|
|
(11,093
|
)
|
|
|
5.8
|
%
|
|
Amortisation and depreciation
|
|
|
(39,554
|
)
|
|
|
(33,256
|
)
|
|
|
(6,298
|
)
|
|
|
18.9
|
%
|
|
Non-financial and other capital grants
|
|
|
1,188
|
|
|
|
2,941
|
|
|
|
(1,753
|
)
|
|
|
(59.6
|
)%
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
(1,147
|
)
|
|
|
(1,991
|
)
|
|
|
844
|
|
|
|
(42.4
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
226,528
|
|
|
|
202,961
|
|
|
|
23,567
|
|
|
|
11.6
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
7,067
|
|
|
|
2,682
|
|
|
|
4,385
|
|
|
|
163.5
|
%
|
|
Finance expenses
|
|
|
(27,087
|
)
|
|
|
(29,305
|
)
|
|
|
2,218
|
|
|
|
(7.6
|
)%
|
|
Change in fair value of financial instruments
|
|
|
(587
|
)
|
|
|
(1,268
|
)
|
|
|
681
|
|
|
|
(53.7
|
)%
|
|
Gains/(losses) on disposal of financial instruments
|
|
|
(245
|
)
|
|
|
—
|
|
|
|
(245
|
)
|
|
|
—
|
|
|
Exchange gains/(losses)
|
|
|
(1,733
|
)
|
|
|
(2,825
|
)
|
|
|
1,092
|
|
|
|
(38.7
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Finance expense
|
|
|
(22,585
|
)
|
|
|
(30,716
|
)
|
|
|
8,131
|
|
|
|
(26.5
|
)%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of (profit)/loss of equity accounted investees
|
|
|
51
|
|
|
|
24
|
|
|
|
27
|
|
|
|
112.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
203,994
|
|
|
|
172,269
|
|
|
|
31,725
|
|
|
|
18.4
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
(56,424
|
)
|
|
|
(50,153
|
)
|
|
|
(6,271
|
)
|
|
|
12.5
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
147,570
|
|
|
|
122,116
|
|
|
|
25,454
|
|
|
|
20.8
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
Sales
Net sales increased by 12.1%, from €814.3 million in
2008 to €913.2 million in 2009.
The following tables reflect a summary of net sales by each of
our divisions and geographical regions for the year ended
December 31, 2009 as compared to the year ended
December 31, 2008:
Summary of Revenues by Division
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
% of total
|
|
|
December 31,
|
|
|
% of total
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
revenues
|
|
|
2008
|
|
|
revenues
|
|
|
% var
|
|
|
% var CC*
|
|
|
|
|
Bioscience
|
|
|
694,969
|
|
|
|
76.1
|
|
|
|
617,918
|
|
|
|
78.6
|
|
|
|
12.5
|
|
|
|
11.9
|
|
|
Diagnostic
|
|
|
103,091
|
|
|
|
11.3
|
|
|
|
85,705
|
|
|
|
10.9
|
|
|
|
20.3
|
|
|
|
20.9
|
|
|
Hospital
|
|
|
86,328
|
|
|
|
9.5
|
|
|
|
82,566
|
|
|
|
10.5
|
|
|
|
4.6
|
|
|
|
5.0
|
|
|
Raw Materials
|
|
|
22,665
|
|
|
|
2.5
|
|
|
|
22,794
|
|
|
|
2.9
|
|
|
|
4.6
|
|
|
|
(14.0
|
)
|
|
Others
|
|
|
6,133
|
|
|
|
0.7
|
|
|
|
5,328
|
|
|
|
0.7
|
|
|
|
(0.6
|
)
|
|
|
15.3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
913,186
|
|
|
|
100.0
|
|
|
|
814,311
|
|
|
|
100.0
|
|
|
|
12.5
|
|
|
|
12.1
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Please see footnote (a) to
the table on page 93. |
99
Summary of Revenues by Region
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
Year Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
% of total
|
|
|
December 31,
|
|
|
% of total
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
revenues
|
|
|
2008
|
|
|
revenues
|
|
|
% var
|
|
|
% var CC*
|
|
|
|
|
European Union
|
|
|
424,591
|
|
|
|
46.5
|
|
|
|
404,099
|
|
|
|
49.6
|
|
|
|
5.1
|
|
|
|
7.3
|
|
|
United States and Canada
|
|
|
296,659
|
|
|
|
32.5
|
|
|
|
290,666
|
|
|
|
35.7
|
|
|
|
2.1
|
|
|
|
(4.9
|
)
|
|
Rest of World
|
|
|
191,936
|
|
|
|
21.0
|
|
|
|
119,546
|
|
|
|
14.7
|
|
|
|
60.6
|
|
|
|
65
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
913,186
|
|
|
|
100.0
|
|
|
|
814,311
|
|
|
|
100.0
|
|
|
|
12.1
|
|
|
|
11.4
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Please see footnote (a) to
the table on page 93. |
Bioscience. Net sales increased by 12.5%, from
€618.0 million in 2008 to €695.0 million in
2009. The increase in net sales was primarily due to increases
in sales of our three main product lines: IVIG, albumin and
Factor VIII. The 5.5% increase in IVIG sales was driven by a
€5.5 million increase in volume, a
€6.6 million increase in price and a positive foreign
exchange rate which resulted in a €3.6 million
increase. Revenues from the sale of Factor VIII grew by 19.8%
primarily due to an increase in sales volume of
€43.9 million, a price decrease of
€18.4 million as a sales mix (tenders awarded at low
prices compared to regular sales) and a negative foreign
exchange effect of €1.7 million. By region, the United
States, which had a €6.3 million increase in net
sales, and Latin America, which had a €12.7 million
increase, were the main contributors to growth. The increase in
sales of Albumin resulted mainly from an increase in overall
sales volume of €18.7 million, and a price increase of
€2.9 million, and a positive foreign exchange rate
effect of €0.7 million. By region, the main changes
are a €8.8 million increase in net sales in the United
States (from €33.3 million in 2008 to
€42.1 million in 2009) and a
€11.5 million increase in net sales in the rest of the
world (from €41.4 million in 2008 to
€52.9 million in 2009).
Hospital. Net sales increased by 4.6%, from
€82.6 million in 2008 to €86.3 million in
2009. In 2009, we recorded increases in each of our main product
lines. The growth drivers in the Hospital division are the
medical devices and hospital logistics product lines. Growth in
the medical devices product line is primarily attributable to
increased sales in Spain, while growth in the hospital logistics
product line is primarily attributable to increased sales in
Latin America.
Diagnostic. Net sales increased by 20.3%, from
€85.7 million in 2008 to €103.1 million in
2009. The 20.3% growth in the Diagnostic division was driven
primarily by: (i) the March 2009 acquisition of a majority
voting interest in Woolloomooloo Holdings Pty Ltd., an
Australian-Swiss company that is a distributor of diagnostic
products in Australia and Switzerland, whose revenues amounted
to a total of €11.2 million. Excluding this
acquisition, sales growth in the Diagnostic division was 7.3%;
(ii) a 1.9% decrease in immunohematology (excluding the
above mentioned sales from the Australian-Swiss company of
€11.2 million), mainly due to a €5.1 million
planned sales reduction in OEM instruments sales. Excluding the
OEM sales, the growth is 16.3%, from €26.7 million in
2008 to €31.1 million in 2009 which is mainly due to
gel cards sales growth; (iii) a €2.9 million
increase in PIBC (pathogen inactivation of blood components),
from €4.8 million to €7.8 million, mainly
due to the incorporation of a new product line for distribution
by Cerus Corporation beginning in the fourth quarter 2008; and
(iv) a €1.8 million increase in blood bank, from
€15.5 million to €17.3 million and a
€1.7 million increases in hemostasis sales for
€6.5 million to €8.2 million, including the
launch of the Q-Coagulometer, an instrument developed in-house.
Raw Materials. Sales in 2009 compared to 2008
remained flat; however, 2009 sales were comprised of plasma
sales, while 2008 sales were comprised both of the manufacturing
service plus intermediate paste contract with Baxter Healthcare
Corporation, which was terminated in October 2009, as well as
plasma sales.
Changes
in inventories of finished goods and work in progress and
materials consumed.
Supplies increased by 38.5%, from €206.7 million in
2008 to €286.3 million in 2009. From these supplies,
an amount of €325.4 million in 2009 corresponds to
purchases of raw materials and goods for resale and an amount of
€39.1 million corresponds to change in inventories of
raw materials and goods for resale. In addition, an amount of
€73.1 million corresponds to change in inventories of
finished goods and work in
100
progress. This increase was primarily because we intended to
build up inventories in 2009 to secure future growth.
Self-constructed
non-current assets.
Self-constructed non-current assets increased by 59.5%, from
€25.8 million in 2008 to €41.1 million in
2009 primarily due to the continued increase in our fixed assets
based on the capital expenditures expansion plan.
Personnel
expenses.
Personnel expenses increased by 14.7%, from
€238.2 million in 2008 to €273.2 million in
2009, primarily due to increased personnel in the production
area, specifically in the plasma collection companies.
Other
operating expenses.
Other operating expenses increased by 5.8%, from
€192.3 million in 2008 to €203.4 million in
2009, primarily due to our overall increase in operations.
Depreciation
and amortization of fixed assets.
Depreciation and amortization of fixed assets increased by
18.9%, from €33.3 million in 2008 to
€39.6 million in 2009. This increase was primarily due
to the continued increase in our fixed asset base, including a
new sterile filling and purification area at our Los Angeles
plant and our new corporate offices in Sant Cugat del
Vallès, Barcelona.
Net
finance expense.
Net finance expense decreased by 26.5%, from
€30.7 million in 2008 to €22.6 million in
2009. This decrease was primarily due to increased interest
income from Spanish Social Security by 195.5%, from
€2.2 million in 2008 to €6.5 million in
2009. Additionally, financial expenses decreased by 7.6%, from
€29.3 million in 2008 to €27.1 million in
2009 mainly due to lower interest rates.
Income
tax.
In 2009, we had a consolidated profit before income tax of
€204.0 million and income tax of
€56.4 million, which represents an effective tax rate
of 27.6%. This amount includes deductions of
€12.7 million relating to research and development and
others.
Liquidity
and Capital Resources
Uses
and Sources of Funds
Our principal liquidity and capital requirements consist of the
following:
|
|
|
| |
•
|
costs and expenses relating to the operation of our business,
including working capital for inventory purchases and accounts
receivable financing;
|
| |
| |
•
|
capital expenditures for existing and new operations; and
|
| |
| |
•
|
debt service requirements relating to our existing and future
debt.
|
Historically, we have financed our liquidity and capital
requirements through internally generated cash flows, debt
financings and capital infusions.
Historical
Cash Flows
Below are our consolidated statements of cash flow for the years
ended December 31, 2008, 2009 and 2010 and the six months
ended June 30, 2010 and 2011 prepared under IFRS.
101
Statements
of Cash Flows
For the Years Ended December 31, 2010, 2009, and 2008 and
the Six Months Ended
June 30, 2011 and 2010
(In thousands of Euros)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
6/30/11
|
|
|
6/30/10
|
|
|
12/31/10
|
|
|
12/31/09
|
|
|
12/31/08
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before tax
|
|
|
25,960
|
|
|
|
88,852
|
|
|
|
157,784
|
|
|
|
203,994
|
|
|
|
172,269
|
|
|
Adjustments for:
|
|
|
92,638
|
|
|
|
53,782
|
|
|
|
92,351
|
|
|
|
61,800
|
|
|
|
66,034
|
|
|
Amortisation and depreciation
|
|
|
28,156
|
|
|
|
21,434
|
|
|
|
45,776
|
|
|
|
39,554
|
|
|
|
33,256
|
|
|
Other adjustments:
|
|
|
64,482
|
|
|
|
32,348
|
|
|
|
46,575
|
|
|
|
22,246
|
|
|
|
32,778
|
|
|
(Profit) /losses on equity accounted investments
|
|
|
807
|
|
|
|
728
|
|
|
|
879
|
|
|
|
(51
|
)
|
|
|
(24
|
)
|
|
Exchange differences
|
|
|
2,122
|
|
|
|
(1,970
|
)
|
|
|
(1,616
|
)
|
|
|
1,733
|
|
|
|
2,825
|
|
|
Net provision charges
|
|
|
14,454
|
|
|
|
129
|
|
|
|
913
|
|
|
|
53
|
|
|
|
1,994
|
|
|
(Profit) /loss on disposal of fixed assets
|
|
|
9,416
|
|
|
|
(681
|
)
|
|
|
(276
|
)
|
|
|
1,147
|
|
|
|
2,001
|
|
|
Government grants taken to income
|
|
|
(742
|
)
|
|
|
(550
|
)
|
|
|
(728
|
)
|
|
|
(1,188
|
)
|
|
|
(2,943
|
)
|
|
Finance expense/income
|
|
|
37,130
|
|
|
|
33,386
|
|
|
|
47,442
|
|
|
|
17,551
|
|
|
|
27,891
|
|
|
Other adjustments
|
|
|
1,295
|
|
|
|
1,306
|
|
|
|
(39
|
)
|
|
|
3,001
|
|
|
|
1,034
|
|
|
Changes in capital and assets
|
|
|
(65,159
|
)
|
|
|
13,700
|
|
|
|
(78,767
|
)
|
|
|
(104,127
|
)
|
|
|
(86,550
|
)
|
|
Change in inventories
|
|
|
752
|
|
|
|
(11,982
|
)
|
|
|
(18,306
|
)
|
|
|
(113,104
|
)
|
|
|
(98,520
|
)
|
|
Change in trade and other receivables
|
|
|
(66,961
|
)
|
|
|
20,239
|
|
|
|
(23,546
|
)
|
|
|
(12,549
|
)
|
|
|
(7,951
|
)
|
|
Change in current financial assets and other current assets
|
|
|
(451
|
)
|
|
|
(3,875
|
)
|
|
|
(73,022
|
)
|
|
|
(1,287
|
)
|
|
|
405
|
|
|
Change in current trade and other payables
|
|
|
1,501
|
|
|
|
9,318
|
|
|
|
36,107
|
|
|
|
22,813
|
|
|
|
19,516
|
|
|
Other cash flows from operating activities
|
|
|
(36,745
|
)
|
|
|
(34,465
|
)
|
|
|
(67,116
|
)
|
|
|
(73,487
|
)
|
|
|
(77,310
|
)
|
|
Interest paid
|
|
|
(34,021
|
)
|
|
|
(19,801
|
)
|
|
|
(40,129
|
)
|
|
|
(14,719
|
)
|
|
|
(25,972
|
)
|
|
Interest recovered
|
|
|
999
|
|
|
|
3,861
|
|
|
|
5,436
|
|
|
|
2,509
|
|
|
|
2,213
|
|
|
Income tax paid/(recovered)
|
|
|
(3,723
|
)
|
|
|
(18,525
|
)
|
|
|
(32,423
|
)
|
|
|
(61,277
|
)
|
|
|
(53,551
|
)
|
|
Net cash from operating activities
|
|
|
16,694
|
|
|
|
121,869
|
|
|
|
104,252
|
|
|
|
88,180
|
|
|
|
74,443
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(1,669,390
|
)
|
|
|
(56,997
|
)
|
|
|
(108,588
|
)
|
|
|
(136,626
|
)
|
|
|
(130,923
|
)
|
|
Group companies and business units
|
|
|
(1,615,417
|
)
|
|
|
(3,727
|
)
|
|
|
(1,474
|
)
|
|
|
(15,385
|
)
|
|
|
(632
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(52,838
|
)
|
|
|
(49,151
|
)
|
|
|
(103,402
|
)
|
|
|
(118,770
|
)
|
|
|
(129,568
|
)
|
|
Property, plant and equipment
|
|
|
(42,841
|
)
|
|
|
(43,146
|
)
|
|
|
(86,800
|
)
|
|
|
(103,415
|
)
|
|
|
(119,824
|
)
|
|
Intangible assets
|
|
|
(9,997
|
)
|
|
|
(6,005
|
)
|
|
|
(16,602
|
)
|
|
|
(15,355
|
)
|
|
|
(9,744
|
)
|
|
Other financial assets
|
|
|
(1,135
|
)
|
|
|
(4,119
|
)
|
|
|
(3,712
|
)
|
|
|
(2,471
|
)
|
|
|
(723
|
)
|
|
Proceeds from the sale of investments
|
|
|
69,151
|
|
|
|
2,863
|
|
|
|
4,532
|
|
|
|
673
|
|
|
|
157
|
|
|
Property, plant and equipment
|
|
|
69,151
|
|
|
|
2,863
|
|
|
|
3,911
|
|
|
|
673
|
|
|
|
157
|
|
|
Associates
|
|
|
0
|
|
|
|
0
|
|
|
|
621
|
|
|
|
0
|
|
|
|
0
|
|
|
Net cash used in investing activities
|
|
|
(1,600,239
|
)
|
|
|
(54,134
|
)
|
|
|
(104,056
|
)
|
|
|
(135,953
|
)
|
|
|
(130,766
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(2,264
|
)
|
|
|
(1,250
|
)
|
|
|
(1,250
|
)
|
|
|
26,655
|
|
|
|
(4,212
|
)
|
|
Issue
|
|
|
(2,264
|
)
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
(76
|
)
|
|
|
0
|
|
|
Acquisition of treasury shares
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,250
|
)
|
|
|
(25,186
|
)
|
|
|
(4,880
|
)
|
|
Disposal of treasury shares
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
51,917
|
|
|
|
668
|
|
|
Proceeds from and payments for financial liability instruments
|
|
|
2,235,339
|
|
|
|
(8,671
|
)
|
|
|
(1,066
|
)
|
|
|
344,413
|
|
|
|
96,349
|
|
|
Issue
|
|
|
2,982,877
|
|
|
|
51,067
|
|
|
|
118,238
|
|
|
|
525,078
|
|
|
|
394,109
|
|
|
Redemption and repayment
|
|
|
(747,538
|
)
|
|
|
(59,738
|
)
|
|
|
(119,304
|
)
|
|
|
(180,665
|
)
|
|
|
(297,760
|
)
|
|
Dividends and interest on other equity instruments paid
|
|
|
0
|
|
|
|
(53
|
)
|
|
|
(27,282
|
)
|
|
|
(80,913
|
)
|
|
|
(34,792
|
)
|
|
Other cash flows from financing activities
|
|
|
(287,203
|
)
|
|
|
323
|
|
|
|
323
|
|
|
|
741
|
|
|
|
0
|
|
|
Transaction costs of financial instruments issued in the
acquisition of Talecris
|
|
|
(287,550
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Other amounts received from financing activities
|
|
|
347
|
|
|
|
323
|
|
|
|
323
|
|
|
|
741
|
|
|
|
0
|
|
|
Net cash (used in)/from financing activities
|
|
|
1,945,872
|
|
|
|
(9,651
|
)
|
|
|
(29,275
|
)
|
|
|
290,896
|
|
|
|
57,345
|
|
|
Effect of exchange rate fluctuations on cash
|
|
|
(18,184
|
)
|
|
|
42,684
|
|
|
|
19,356
|
|
|
|
(119
|
)
|
|
|
(344
|
)
|
|
Net increase in cash and cash equivalents
|
|
|
344,143
|
|
|
|
100,768
|
|
|
|
(9,723
|
)
|
|
|
243,004
|
|
|
|
678
|
|
|
Cash and cash equivalents at beginning of the period
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
249,372
|
|
|
|
6,368
|
|
|
|
5,690
|
|
|
Cash and cash equivalents at end of the period
|
|
|
583,792
|
|
|
|
350,140
|
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
6,368
|
|
In the fiscal years ended December 31, 2008, 2009 and 2010,
we generated net cash flow of €0.7 million,
€243.0 million and €(9.7) million,
respectively. In the six months ended June 30, 2010 and
June 30, 2011, we generated net cash flow of
€100.8 million and €344.1 million,
respectively.
102
Net
Cash from Operating Activities
In 2008, we generated net cash from operating activities of
€74.4 million. The €161.0 million of cash
flow generated by our operations was offset in part by the
€86.6 million of cash used for working capital
requirements, which principally included a
€98.5 million increase in inventory levels, with a
stock turnover of 327 days. The important increase in
inventories was primarily due to a
build-up of
inventory in plasma and intermediates to secure future growth.
In 2009, we generated net cash from operating activities of
€88.2 million. The €192.3 million of cash
flow generated by operations was offset in part by the
€104.1 million of cash used for working capital
requirements. The principal effects on working capital were:
|
|
|
| |
•
|
a €12.5 million increase in receivables, with the days
sales outstanding ratio remaining at 83 days, which was
similar to 2008;
|
| |
| |
•
|
a €113.1 million increase in inventories, which
represented a significant increase from 2008, with the days
sales outstanding ratio at 377 days, due to increased
plasma collection activity and
slower-than-budgeted
sales, which resulted in remaining inventory at the stage of
intermediate or finished goods; and
|
| |
| |
•
|
a €22.8 million increase in current liabilities, with
a day payable outstanding ratio of 64 days.
|
In 2010, we generated net cash from operating activities of
€104.3 million. The principal effects on working
capital were:
|
|
|
| |
•
|
a €23.5 million increase in receivables, with the days
sales outstanding ratio at 83 days, which was similar to
2009;
|
| |
| |
•
|
a €18.3 million increase in inventories with the
stocks turnover ratio at 364 days, which is lower than 2009;
|
| |
| |
•
|
a €73.0 million increase in current financial assets
and other current assets due to the costs incurred related to
the issue of debt and equity that are expected in connection
with the acquisition of Talecris included in other current
assets; and
|
| |
| |
•
|
a €36.1 million increase in current trade and other
payables, with a day payable outstanding ratio of 76 days.
|
In the six months ended June 30, 2011, we generated net
cash from operating activities of €16.7 million. The
impact of non-recurring effects were the following:
|
|
|
| |
•
|
a decrease in profit before tax due to the transaction costs
incurred during the six month period ended June 30, 2011
amounting to €39 million (€2 million for the
six months ended at June 30, 2010) that have been paid
in this period; and
|
| |
| |
•
|
the change in current trade and other payables includes
€20 million corresponding to business combination
costs accrued by Talecris companies prior to acquisition date
and paid during June 2011.
|
Net
Cash Used in Investing Activities
Net cash used in investing activities amounted to
€130.8 million in 2008, €136.0 million in
2009, €104.1 million in 2010 and
€1.60 billion in the six months ended June 30,
2011. A significant part of the investments made between 2008
and December 31, 2010 were related to
“non-recurring” capital expenditures, primarily
investments for capacity expansion. For the six months ended
June 30, 2011, most of the cash used in investing
activities related to the acquisition of Talecris.
At June 30, 2011, we had carried out the following
investing operations which did not require the use of cash or
cash equivalents: (i) we sold properties in Spain amounting
to €80.4 million which together with the related
mortgage loan of €53.5 million resulted in a net cash
inflow of €26.9 million, and (ii) we paid part of
103
the consideration for the acquisition of Talecris through the
issuance and delivery of our Class B shares. The issuance
of our Class B shares had no cash impact.
Net
Cash from (Used in) Financing Activities
We generated net cash from financing activities of
€57.3 million in 2008. The principal factor was
€96.3 million in additional loans incurred mainly to
finance capital expenditures and to finance inventory.
Net cash from financing activities amounted to
€290.9 million in 2009, mainly from net disposals of
owned shares of €26.7 million and proceeds from the
private placement of the Grifols Old Notes. For a further
discussion of the Grifols Old Notes, see the section below
entitled “— Indebtedness.”
Net cash used in financing activities amounted to
€29.3 million in 2010, mainly due to the payment of
dividends.
Net cash from financing activities was €1.95 billion
in the six months ended June 30, 2011, primarily due to the
issuance of $1.1 billion aggregate principal amount of our
8.25% senior notes due 2018 and borrowings under the Senior
Term Loans in the amount of $2.5 billion and
€440 million (which does not include an additional
$50 million, €36.7 million and the
$200 million equivalent in multicurrencies of availability
under the undrawn Revolving Credit Facilities). For a further
discussion of the 8.25% senior notes due 2018 and the
Senior Credit Facilities, see the section below entitled
“— Indebtedness.”
Pursuant to the terms of the Senior Credit Facilities and in
connection with the consummation of the acquisition we
refinanced substantially all of our and Talecris’ existing
debt. On June 1, 2011, we redeemed all of the Grifols Old
Notes and repaid existing bank loans amounting to
€297 million. We paid a €112 million
make-whole premium payment in connection with the redemption of
the Grifols Old Notes. On June 13, 2011, we redeemed 10% of
the $600 million aggregate principal amount of Talecris Old
Notes. At June 30, 2011, our cash and cash equivalents
included €428 million held in a restricted cash
account in connection with the redemption of the remaining
Talecris Old Notes, which were subsequently redeemed on
July 1, 2011. As of the date hereof, there are no Talecris
Old Notes outstanding. We paid a €78 million
make-whole premium payment in connection with the redemption of
the Talecris Old Notes.
Working
Capital
We believe that we have sufficient working capital for the next
12 months from the date of this prospectus, although we can
not assure you that this will be the case.
The following table presents, for the periods indicated, our
inventory, trade receivables and trade payables and their aging:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
June 30,
|
|
|
|
|
2008
|
|
|
2009
|
|
|
2010
|
|
|
2011
|
|
|
|
|
€
|
|
|
Days(1)
|
|
|
€
|
|
|
Days(1)
|
|
|
€
|
|
|
Days(1)
|
|
|
€
|
|
|
Days(1)
|
|
|
|
|
Inventory(2)
|
|
|
373,098
|
|
|
|
327
|
|
|
|
484,462
|
|
|
|
377
|
|
|
|
527,865
|
|
|
|
364
|
|
|
|
997,826
|
|
|
|
300
|
|
|
Receivables(3)
|
|
|
186,324
|
|
|
|
84
|
|
|
|
207,840
|
|
|
|
83
|
|
|
|
224,355
|
|
|
|
83
|
|
|
|
390,979
|
|
|
|
63
|
|
|
Payables(4)
|
|
|
95,396
|
|
|
|
65
|
|
|
|
108,274
|
|
|
|
64
|
|
|
|
120,326
|
|
|
|
76
|
|
|
|
—
|
(5)
|
|
|
—
|
(5)
|
|
|
|
|
(1)
|
|
At the last day of the period.
|
| |
|
(2)
|
|
Aging of inventory is calculated by
dividing total inventories, as the case may be, at the end of
each period by the total cost of sales for such period and
multiplying the result by 365.
|
| |
|
(3)
|
|
We have calculated the average age
of receivables by Total Receivables * 365/Sales (last
12 months).
|
| |
|
(4)
|
|
We have calculated the average age
of payables by Total Payables * 365/Purchases + External
services + Acquisitions fixed assets third parties (last
12 months). Payables include only the concepts that are
also included as purchases, external services and acquisitions
fixed assets.
|
| |
|
(5)
|
|
Due to the timing of the
acquisition, at June 30, 2011, we were unable to determine
accounts payable reflecting Talecris operations.
|
104
Inventory. Inventory aging average has
increased from 2008 to 2010 primarily because we intended to
build up inventories in 2008 to secure future growth, and this
trend continued through 2010. A decrease in sales in certain
products and markets resulted in the ratio of 364 days in
2010, from 327 days in 2008. From December 31, 2010 to
June 30, 2011 the ratio has decreased to 300 days.
Accounts receivable. Accounts receivable aging
has maintained the level of turnover from 84 days in 2008
to 83 days in 2010. From December 31, 2010 to
June 30, 2011 the ratio has decreased to 63 days,
mainly due to an increase in revenues from North America as a
result of the acquisition. In certain markets, particularly in
Southern Europe (e.g., Spain and Italy), it is common practice
for government or local authority-backed entities to pay
suppliers well after the
30-60-day
period normally applied, with payments occurring very often
after one year. As a result, we require significant investments
in working capital to accommodate certain clients’ payment
terms. In order to reduce our working capital funding needs, our
recent policy has been to sell, to the extent necessary,
receivables with a maturity beyond 30 days. Certain
receivables are sold to financial institutions at the end of
each quarter without recourse.
Accounts payable. The ratio remained stable at
the level of 65 days for the period 2008 to 2009, and
increased to 76 days in 2010. Due to the timing of the
acquisition, as of June 30, 2011, we were unable to
determine such figures incorporating Talecris operations.
Capital
Expenditures
The following table presents our capital expenditures in the six
months ended June 30, 2011 and the years ended December 31,
2010, 2009 and 2008, by division.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months Ended
|
|
|
Years Ended
|
|
|
|
|
June 30,
|
|
|
December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Facilities(1)
|
|
|
30,027
|
|
|
|
62,287
|
|
|
|
65,076
|
|
|
|
62,292
|
|
|
Development
cost(2)
|
|
|
1,675
|
|
|
|
3,057
|
|
|
|
5,626
|
|
|
|
3,662
|
|
|
Bioscience division
|
|
|
31,702
|
|
|
|
65,344
|
|
|
|
70,702
|
|
|
|
65,954
|
|
|
Facilities(1)
|
|
|
8,597
|
|
|
|
12,559
|
|
|
|
7,524
|
|
|
|
9,266
|
|
|
Development
cost(2)
|
|
|
—
|
|
|
|
573
|
|
|
|
—
|
|
|
|
—
|
|
|
Hospital division
|
|
|
8,597
|
|
|
|
13,132
|
|
|
|
7,524
|
|
|
|
9,266
|
|
|
Facilities(1)
|
|
|
4,153
|
|
|
|
12,914
|
|
|
|
11,547
|
|
|
|
12,485
|
|
|
Development
cost(2)
|
|
|
1,885
|
|
|
|
2,983
|
|
|
|
2,520
|
|
|
|
1,593
|
|
|
Diagnostic division
|
|
|
6,038
|
|
|
|
15,897
|
|
|
|
14,067
|
|
|
|
14,078
|
|
|
Raw materials division
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
516
|
|
|
Shared infrastructure
|
|
|
6,762
|
|
|
|
11,424
|
|
|
|
26,477
|
|
|
|
39,879
|
|
|
Total
|
|
|
53,098
|
|
|
|
105,797
|
|
|
|
118,770
|
|
|
|
129,693
|
|
|
|
|
|
(1)
|
|
Facilities include manufacturing
and other facilities.
|
| |
|
(2)
|
|
Development cost includes the
capitalized portion only. Development expenses are capitalized
only when the conditions of IAS 38 for such capitalization are
met and are subsequently depreciated over an estimated useful
life, as permitted under IFRS. Otherwise, research and
development expenses are expensed as they are incurred. For
2008, 2009, 2010 and the six months ended June 30, 2011,
Grifols had total development expenses of
€25.6 million, €35.2 million,
€36.6 million and €25.7 million,
respectively, and had amortizations on development cost of
€4.6 million, €5.6 million,
€3.7 million and €4.8 million, respectively.
|
January
2008 through December 2010
Facilities. The most important capital
projects relating to the expansion and improvement of our
manufacturing facilities were:
|
|
|
| |
•
|
the acquisition of Novartis’ former industrial facilities
in Parets del Vallès in Barcelona, Spain for
€17.5 million;
|
105
|
|
|
| |
•
|
the acquisition of our headquarters for €35 million in
2008;
|
| |
| |
•
|
the opening of a new building in Barcelona to house the raw
material storage unit for €2.5 million in 2008;
|
| |
| |
•
|
the establishment of research and development quality control
laboratories and the installation of new manufacturing lines for
parenteral nutrition products for the Hospital division for
€2.5 million in 2008;
|
| |
| |
•
|
the establishment of a new preparation area to increase the
production of DG Gel cards completed in 2008 for
€1.7 million;
|
| |
| |
•
|
investment at the Los Angeles plant to fund its expansion,
improvements and specialized machinery for €5 million;
|
| |
| |
•
|
capital expenditures for the Los Angeles plant in the amount of
€29.2 million in 2009;
|
| |
| |
•
|
refurbishment work at our headquarters for
€14.2 million in 2009;
|
| |
| |
•
|
investments in the Los Angeles IVIG plant of approximately
€18.1 million in 2010;
|
| |
| |
•
|
refurbishment costs and information technology-related
expenditures at our headquarters of approximately
€7.7 million in 2010;
|
| |
| |
•
|
costs related to the expansion of the new fractionation plant in
Parets del Vallès of approximately €9.4 million
in 2010;
|
| |
| |
•
|
investments for the new laboratories in San Marcos, Texas
of approximately €10.2 million in 2010; and
|
| |
| |
•
|
investments for new plants and machinery in our Hospital
division of approximately €10.0 million in 2010.
|
Development Cost. Our important projects included:
|
|
|
| |
•
|
completion of the FDA licensing process for the sale of
Flebogamma DIF IVIG in the United States in early 2007, which
consists of a new method for obtaining IVIG that could
significantly increase the protein yield and would provide
increased safety through the use of a new method of viral
elimination known as nanofiltration;
|
| |
| |
•
|
completion of the development of Erytra in early 2010, which is
a fully automated instrument with a high processing capacity for
pre-transfusion compatibility tests using the gel agglutination
technique;
|
| |
| |
•
|
improvements to the Triturus and Wadiana automated analyzers;
|
| |
| |
•
|
pursuit of Albumin usage in the treatment of Alzheimer’s
disease;
|
| |
| |
•
|
conducting clinical trials for the sale of Flebogamma DIF 10% in
ITP in the European Union; and
|
| |
| |
•
|
continuation of clinical trials for the sale of fibrin glue,
which is a fibrin clot preparation to control bleeding during
surgery, in the United States.
|
January
2011 through December 2012
Our business plan calls for capital expenditures for the period
from January 2011 through December 2012 of approximately
€373.5 million, with €211.4 million
anticipated for 2011 and €162.1 million anticipated
for 2012. We plan to finance our projected capital expenditures
with internally generated cash flow and debt financing. Our
principal planned capital expenditures for the period from
January 2011 through December 2012 are the following:
Facilities. The most important capital
projects relating to the expansion and improvement of our
manufacturing facilities that we plan to make are:
|
|
|
| |
•
|
investments related to the Parets del Vallès plant in Spain
of approximately €1.3 million in 2011 and
€11.9 million in 2012, mainly related to fractionation
capacity expansion;
|
106
|
|
|
| |
•
|
incur further headquarters-related expenses and IT projects of
approximately €11.7 million in 2011 and
€22.2 million in 2012;
|
| |
| |
•
|
investments related to the Clayton, North Carolina plant of
approximately €134.2 million in 2011 and
€50.6 million in 2012, mainly related to fractionation
capacity expansion; and
|
| |
| |
•
|
further expenditures at our new plant in Murcia, Spain of
approximately €8.9 million in 2011 and
€2.1 million in 2012.
|
Development Cost. We are undertaking research
and development projects in all of our major product areas.
Indebtedness
Senior
Credit Facilities
In order to fund the acquisition, on November 23, 2010, we
entered into a credit and guaranty agreement, which provided for
senior term loans aggregating $2.5 billion and
€440 million (the “Senior Term Loans”), and
revolving credit facilities in the amounts of $50 million,
€36.7 million and the $200 million equivalent in
multicurrencies (the “Revolving Credit Facilities,”
and together with the Senior Term Loans, the “Senior Credit
Facilities”), from a syndicate of lenders led by Deutsche
Bank Securities Inc., Nomura International plc, Banco Bilbao
Vizcaya Argentaria, S.A., BNP Paribas, HSBC Securities (USA)
Inc. and Morgan Stanley Senior Funding, Inc.
The Senior Term Loans consist of two tranches, Tranche A
and Tranche B. The Tranche A Senior Term Loans, which
amount to $1.2 billion and €220 million, mature
five years after the closing of the acquisition and have a
repayment schedule with quarterly amortization equal to 0%, 10%,
10% and 15% per annum of the original principal amount in years
one through four, with the balance due after the fourth
anniversary of the closing of the acquisition to the maturity
date. The Tranche B Senior Term Loans, which amount to
$1.3 billion and €220 million, mature six years
after the closing of the acquisition and will have a repayment
schedule with quarterly amortization equal to 1% per annum of
the original principal amount, with the remainder to be paid at
maturity. The Senior Term Loans were fully funded at the closing
of the acquisition.
The Revolving Credit Facilities, which amount to
$50 million, €36.7 million and the
$200 million equivalent in multicurrencies, will be
available during the period commencing on the closing of the
acquisition and ending on the fifth anniversary of the closing
of the acquisition. As of June 30, 2011, no amounts were
drawn against the Revolving Credit Facilities.
The interest rates on the Senior Term Loans and the Revolving
Credit Facilities are based on (a) in the case of United
States Dollar-denominated debt the base rate (the greatest of
(i) the prime rate, (ii) the federal funds rate plus
0.50% and (iii) the applicable LIBOR rate plus 1.00%) with
a floor of 2.75%, plus an applicable margin or (b) the
applicable LIBOR rate with a floor of 1.75%, plus an applicable
margin. The applicable margin will be (a) 4.00% for the
foreign currency-denominated Revolving Credit Facilities and the
foreign Tranche A Senior Term Loan, (b) 4.50% for the
foreign Tranche B Senior Term Loan, (c) for the United
States Dollar-denominated Revolving Credit Facilities, the
United States Dollar-denominated multicurrency Revolving Credit
Facilities and the United States Dollar-denominated
Tranche A Senior Term Loan, (i) 1.75% for base rate
loans and (ii) 3.75% for LIBOR rate loans, and (d) for
the United States Dollar-denominated Tranche B Senior Term
Loan, (i) 3.25% for base rate loans and (ii) 4.25% for
LIBOR rate loans.
Borrowings under the Senior Credit Facilities are subject to
mandatory prepayment upon the occurrence of certain events,
including the incurrence of certain debt and the sale or other
disposition of certain assets. In addition, a portion of the
borrowings under the Senior Credit Facilities are subject to
mandatory prepayment in the event we have excess cash flow, as
defined therein.
The Senior Credit Facilities are guaranteed by Grifols, S.A. and
certain subsidiaries of Grifols, S.A. that together with
Grifols, S.A. represent, in the aggregate, at least 85% of the
consolidated assets, consolidated EBITDA and consolidated
turnover of Grifols, S.A. and its subsidiaries, and are secured
by a perfected first priority security interest (subject to
permitted liens, as defined in the credit and guaranty
agreement) in all of
107
the tangible and intangible assets of the credit parties, as
defined therein (subject to certain exclusions and limitations).
The Senior Credit Facilities contain customary affirmative and
negative covenants and events of default. Negative covenants
include, among other limitations, limitations on additional
debt, liens, asset sales and affiliate transactions. Events of
defaults include, among other events, violation of covenants,
material breaches of representations, cross-default to other
material debt, bankruptcy and insolvency and material judgments.
Furthermore, Grifols is required to comply with certain ratios
and financial maintenance covenants. At June 30, 2011, we
were in compliance with the financial covenants under the Senior
Credit Facilities. We are required to provide financial
information to the lending banks within the six-month period
subsequent to December 31 of each year while the loan is
outstanding.
The terms of the Senior Credit Facilities limit our ability to
pay ordinary dividends. If our leverage ratio exceeds 3.75x, we
may not pay more than $10 million of dividends in any
fiscal year. If our leverage ratio is 3.75x or less, then we may
pay dividends in an amount not to exceed an amount equal to
(a) the sum of (i) 40% of the consolidated net income
of Grifols, S.A. and its subsidiaries accrued since the most
recently ended fiscal quarter prior to the closing date of the
acquisition to the end of the most recently ended fiscal quarter
of Grifols, S.A. for which financial statements have been
delivered to the lenders (or, in case such consolidated net
income shall be a deficit, minus 100% of such deficit) and
(ii) the net cash proceeds received from the issuance or
sale of equity interests in Grifols, S.A. and its subsidiaries
after the closing date that are not applied to prepay the loans,
less (b) the sum of amounts used to make (i) certain
permitted restricted payments, (ii) below par purchases of
senior term loans and (iii) certain permitted capital
expenditures.
8.25% Senior
Notes due 2018
On January 21, 2011, Escrow Corp., an escrow company formed
solely for the purpose of issuing such notes, completed the sale
of $1.1 billion aggregate principal amount of
8.25% senior notes due 2018 (the “Notes”) to the
initial purchasers thereof in an offering not registered under
the Securities Act. The initial purchasers subsequently resold
the Notes to qualified institutional buyers pursuant to Rule
144A under the Securities Act and to non-U.S. persons outside
the United States in reliance on Regulation S under the
Securities Act. The proceeds of the offering of the Notes were
held in an escrow account pending completion of the acquisition
of Talecris and the satisfaction of other conditions. On
June 1, 2011, upon completion of the acquisition, Escrow
Corp. was merged with and into our subsidiary, Grifols Inc., and
Grifols Inc. assumed all of Escrow Corp.’s obligations
under the Notes and the indenture governing the Notes and the
proceeds from the issuance of the Notes were released from the
escrow account. The proceeds from the Notes together with funds
drawn on the Senior Credit Facilities were used to finance the
cash portion of the consideration for the acquisition.
The Notes yield 8.25% to maturity and pay interest semi-annually
on February 1 and August 1 to holders of record on the
immediately preceding January 15 and July 15, respectively.
The Notes are guaranteed on a senior unsecured basis by existing
and future subsidiaries of Grifols, S.A. that guarantee the
Senior Credit Facilities, other than foreign subsidiaries of
Grifols Inc. As of the date of this prospectus, the Notes are
guaranteed by Grifols Biologicals Inc., Biomat USA, Inc.,
Grifols Therapeutics Inc., Talecris Plasma Resources, Inc.,
Instituto Grifols, S.A., Diagnostic Grifols, S.A., Movaco, S.A.,
Laboratorios Grifols, S.A., Grifols Italia, S.p.A. and Grifols
Deutschland GmbH.
Grifols Inc. may redeem the Notes in whole or in part, at any
time on and after February 1, 2014, at the redemption
prices (expressed as percentages of principal amount) set forth
below plus accrued and unpaid interest and additional interest,
if any, on the Notes redeemed, to the applicable redemption
date, if redeemed during the twelve-month period beginning on
February 1 of the years indicated below:
| |
|
|
|
|
|
Fiscal Year
|
|
Percentage
|
|
|
|
|
2014
|
|
|
106.188
|
%
|
|
2015
|
|
|
104.125
|
%
|
|
2016
|
|
|
102.063
|
%
|
|
2017 and thereafter
|
|
|
100.000
|
%
|
108
Grifols Inc. may redeem up to 35% of the outstanding Notes with
money raised in one or more equity offerings by Grifols, S.A. at
any time (which may be more than once) prior to February 1,
2014, as long as at least 65% of the aggregate principal amount
of Notes issued remains outstanding immediately following any
such offerings.
Grifols Inc. may redeem some or all of the Notes at any time
prior to February 1, 2014 at a price equal to 100% of the
principal plus a premium as defined under the indenture
(computed using a discount rate equal to the U.S. Treasury
rate as of such redemption date plus 0.50%), plus accrued and
unpaid interest and additional interest, if any.
Grifols Inc. is not required to make mandatory redemption or
sinking fund payments with respect to the Notes.
If we experience a change of control, Grifols Inc. must give
holders of the Notes the opportunity to sell Grifols Inc. their
Notes at 101% of their face amount, plus accrued and unpaid
interest.
Grifols, S.A. and its restricted subsidiaries may incur
additional indebtedness if our Fixed Charge Coverage Ratio for
our most recently ended four full fiscal quarters immediately
preceding the date on which such additional indebtedness is
incurred would have been at least 2.00 to 1.00, determined on a
pro forma basis.
The indenture governing the Notes contains certain covenants
limiting, subject to exceptions, carve-outs and qualifications,
Grifols, S.A.’s ability and its restricted
subsidiaries’ ability to: (i) pay dividends or make
certain other restricted payments or investments;
(ii) incur additional indebtedness or provide guarantees of
indebtedness and issue disqualified stock; (iii) create
liens on assets; (iv) merge, consolidate, or sell all or
substantially all of our and our restricted subsidiaries’
assets; (v) enter into certain transactions with
affiliates; (vi) create restrictions on dividends or other
payments by our restricted subsidiaries; and (vii) create
guarantees of indebtedness by restricted subsidiaries. The
indenture also contains certain customary events of default.
Other
bank debt
We are party to a number of other short- and long-term credit
facilities. These facilities are with various lenders and
consist of long-term and short-term indebtedness of both us and
our subsidiaries. At December 31, 2010 we had
€220.8 million outstanding under long-term credit
facilities which have maturity dates that range from 2012 to
2020, and €173.1 million outstanding under short-term
credit facilities. Most of our short-term credit facilities were
repaid on June 2, 2011 in connection with the acquisition
and related refinancing. At June 30, 2011, we had
€18.4 million outstanding under long-term credit
facilities, which have maturity dates that range from 2012 to
2020, and €74.7 million outstanding under short-term
credit facilities. The short-term credit facilities have
maturity dates occurring in the next 12 months. We have
€189.5 million of aggregate availability under
short-term credit facilities. The majority of the facilities
provide that the lenders may terminate such facilities at will
or change the terms unilaterally.
Grifols
Old Notes
On September 21, 2009, Grifols Inc. completed a private
placement of $200.0 million aggregate principal amount of
notes maturing in 12 years, $245.0 million,
£25.0 million and €10 million aggregate
principal amount of notes maturing in 10 years and
$100.0 million aggregate principal amount of notes maturing
in seven years (together, the “Grifols Old Notes”).
The Grifols Old Notes were the general unsecured obligations of
Grifols Inc. and were guaranteed fully and unconditionally on a
joint and several basis by Grifols, S.A., Laboratorios Grifols,
S.A., Instituto Grifols, S.A., Diagnostic Grifols, S.A., Biomat,
S.A., Movaco, S.A., Biomat USA, Inc. and Grifols Biologicals Inc.
The interest rate for the Grifols Old Notes was based on a
spread over the applicable U.S. Treasury Note Yield at the
time of issue corresponding to the average life of the Grifols
Old Notes. The spread on the
12-year
notes was 370 basis points over the United States Treasury
Note Yield, the spread on the
10-year
notes was 350 basis points over the United States Treasury
Note Yield and the spread on the
7-year notes
was 335 basis points over the United States Treasury Note
Yield.
109
The note purchase agreement entered into in connection with the
Grifols Old Notes contained financial maintenance covenants and
customary affirmative and negative covenants, including among
other things, restrictions on indebtedness, investments, sales
of assets, mergers and consolidations, prepayments of
subordinated indebtedness, liens, and dividends and
distributions, as well as customary events of default.
In connection with the acquisition of Talecris and related
refinancing and in accordance with the terms of the Senior
Credit Facilities, on June 1, 2011, we redeemed 100% of the
aggregate principal amount of the Grifols Old Notes. We paid a
make-whole premium in connection with the redemption of the
Grifols Old Notes in the amount of €112 million, which
has been capitalized as a deferred expense.
Contractual
Obligations
The following table presents our principal existing contractual
obligations as of December 31, 2010 requiring future
payments. This table does not give effect to the acquisition of
Talecris consummated on June 1, 2011.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments Due by Period
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
After
|
|
|
|
|
Total
|
|
|
2011
|
|
|
2012
|
|
|
2013
|
|
|
2014
|
|
|
2014
|
|
|
|
|
Operating
leases(1)
|
|
|
52,628
|
|
|
|
13,769
|
|
|
|
10,897
|
|
|
|
8,427
|
|
|
|
7,201
|
|
|
|
12,334
|
|
|
Financial debt
obligations(2)
|
|
|
857,021
|
|
|
|
191,635
|
|
|
|
85,171
|
|
|
|
51,582
|
|
|
|
17,936
|
|
|
|
510,697
|
|
|
Interest — financial debt
obligations(3)
|
|
|
300,669
|
|
|
|
34,895
|
|
|
|
36,300
|
|
|
|
34,497
|
|
|
|
33,603
|
|
|
|
161,374
|
|
|
Licenses and
royalties(4)
|
|
|
7,188
|
|
|
|
3,289
|
|
|
|
3,215
|
|
|
|
684
|
|
|
|
0
|
|
|
|
0
|
|
|
Total
|
|
|
1,217,506
|
|
|
|
243,588
|
|
|
|
135,583
|
|
|
|
95,190
|
|
|
|
58,740
|
|
|
|
684,405
|
|
|
|
|
|
(1)
|
|
Operating leases include primarily
leases for our plasma collection centers and marketing offices
worldwide. These amounts reflect only our contractual
obligations as of December 31, 2010, and therefore assume
that these operating leases will not be renewed or replaced with
new operating leases upon expiration. Investors are cautioned
that our operating lease expenses will likely be substantially
higher than the amounts provided in this table because our
operations will require us to either renew or replace our
operating leases.
|
| |
|
(2)
|
|
Includes principal amortization for
short and long-term debt including, among other things,
capitalized lease obligations. The financial debt primarily
relates to €857 million outstanding as of
December 31, 2010 including $600.0 million of the
Grifols Old Notes, a €165.7 million syndicated loan
facility that bore interest at an annual rate of EURIBOR plus
0.80% for Euro-denominated debt and LIBOR plus 0.80% for United
States Dollar-denominated debt. The remaining financial debt was
made up largely of working capital facilities that bore interest
at market rate.
|
| |
|
(3)
|
|
Computed using interest rates in
effect as of December 31, 2010
|
| |
|
(4)
|
|
License and royalty payment
formulas are generally based on volume of sales. The amounts
presented in the table are calculated based on the net sales of
2011 without assuming any growth in sales. Additionally, the
columns “After 2014” and “Total” only
include one year of payments under the license agreement with
Marca Grifols, S.L. which expires in January 2092. See
“Certain Relationships and Related Party Transactions.”
|
Off-balance
Sheet Arrangements
We do not have any off-balance sheet arrangements.
Financial
Derivatives
During the fiscal year ended December 31, 2009, we entered
into two unquoted futures contracts, the notional underlying of
which consists of our shares, with a solvent financial
institution. The contracts are settled by differences between
the market value of the notional underlying and the exercise
price. On May 30, 2011, we sold 500,000 futures and
realized a gain of €1 million. In June 2011, we
extended the remaining future contracts until December 2011.
To cover the interest rate risk related to the Grifols Old Notes
issued in 2009, we entered into a swap to hedge the interest
rate of the
10-year
United States government bonds, with a nominal amount of
$200.0 million maturing on September 21, 2009,
swapping a variable interest rate for a fixed one. At the date
of redemption, the valuation resulted in a financial cost of
€3.275 million, which was recognized in equity, net of
110
the tax effect, under “Cash Flow Hedges” and deferred
over the term of the ten-year corporate bond. As consequence of
redemption of the Grifols Old Notes in connection with the
acquisition, the cash flow hedge has been expensed.
Pursuant to the terms of the Senior Credit Facilities, we are
required to enter into hedging transactions with respect to at
least 50% of our borrowings thereunder. In June 2011, we entered
into two derivatives transactions in order to comply with this
mandatory hedging obligation: (i) a
step-up
interest rate swap and (ii) a swap floor, which have a
notional value of $1.55 billion each. The interest rate
swap complies with the criteria required for hedge accounting.
The swap floor value at June 30, 2011 is included in
non-current financial assets. The last maturity date of the swap
floor is 2016.
As the floor included in Tranche A and Tranche B
Senior Term Loans is in the money, embedded derivatives exist in
those contracts, which have been fair valued and separated from
the loans.
Additional information regarding our derivative instruments is
set forth in Note 12(c) of our interim unaudited
consolidated financial statements for six months ended
June 30, 2011, included elsewhere in this prospectus.
Quantitative
and Qualitative Disclosures about Market Risk
Interest
Rate Risk
Borrowings at variable interest rates expose us to interest rate
risk. A significant portion of our interest bearing debt at
December 31, 2010 and June 30, 2011 bore interest at a
floating rate, at a spread over LIBOR for our
U.S. Dollar-denominated debt and at a spread over EURIBOR
for our Euro-denominated debt. At December 31, 2010, we had
a total interest bearing debt of €837.8 million, of
which €399 million bore a floating rate of interest.
At June 30, 2011, we had a total of $3.6 billion and
€458.4 million of long-term interest-bearing debt
outstanding (not including approximately $50 million,
€36.7 million and the $200 million equivalent in
multicurrencies available for additional borrowing under the
Revolving Credit Facility), of which $2.5 billion and
€458.4 million bore a floating rate of interest. As a
result, increases in the applicable floating interest rates
would increase our interest expense and reduce our net cash flow.
We estimate that a 100 basis point variation in interest
rates at December 31, 2010 would have increased/decreased
equity and consolidated profit after income tax by €3,794
thousand. This analysis assumes that all other variables are
held constant, especially that exchange rates remain constant.
Pursuant to mandatory hedging requirements under the Senior
Credit Facilities, 62% of our U.S. Dollar-denominated debt
under the Senior Term Loans is hedged at a fixed rate. However,
our Euro-denominated debt is not hedged. For a further
discussion of our hedging transactions see the section above
entitled “— Financial Derivatives.”
Foreign
Exchange Risk
We operate internationally and are exposed to currency risk when
operating in foreign currencies. Our functional currency is the
Euro and the majority of our net sales as of the six months
ended June 30, 2011 were denominated in U.S. Dollars.
Accordingly, our principal foreign currency exposure relates to
the U.S. Dollar. A devaluation of the U.S. Dollar
against the Euro would result in (i) a decrease in net
sales in Euro terms for sales denominated in U.S. Dollars,
and (ii) a decrease in costs in Euro terms for costs
denominated in U.S. dollars. A devaluation of the Euro
against the U.S. Dollar would have the opposite effect.
We estimate that a 10% increase in the value of the
U.S. Dollar against the Euro at December 31, 2010,
would have increased our equity by €34,973 thousand
(€35,795 thousand at December 31, 2009) and would
have decreased profit by €3,066 thousand (€1,626
thousand at December 31, 2009). This analysis assumes that
all other variables are held constant, especially that interest
rates remain constant. A 10% weakening of the U.S. Dollar
against the Euro at December 31, 2010 would have had the
opposite effect, all other variables held constant.
We are also exposed to risk based on the payment of
U.S. dollar-denominated indebtedness. At the year ended
December 31, 2010 $549 million of our indebtedness was
denominated in U.S. Dollars. At June 30, 2011,
$2.55 billion under the Senior Term Loans, $50 million
of undrawn availability under the Revolving
111
Credit Facilities (not including an additional $200 million
equivalent in multicurrencies of undrawn availability under the
Revolving Credit Facilities which can be drawn in either Euros
or U.S. Dollars) and $1.1 billion aggregate principal
amount of 8.25% senior notes due 2018 were denominated in
U.S. Dollars. A devaluation of the U.S. Dollar against
the Euro would result in a decrease in Euro terms in the amount
of debt owed and the related interest expense for our
U.S. Dollar-denominated, interest-bearing debt. A
devaluation of the Euro against the U.S. Dollar would have
the opposite effect.
112
BUSINESS
History
of Grifols
We were founded in 1940 in Barcelona, Spain by
Dr. José Antonio Grifols i Roig, a specialist and
pioneer in blood transfusions and clinical analysis and the
grandfather of our current chairman. We have been making and
selling plasma derivative products for more than 70 years.
Over the last 25 years, we have grown from a predominantly
domestic Spanish company into a global company by expanding both
organically and through acquisitions throughout Europe, the
United States, Latin America and Asia.
We are a vertically integrated global producer of plasma
derivatives, with 147 FDA licensed plasma collection centers
located across the United States, including over 27 new
collection centers acquired in the last five years. We have
expanded our plasma collection network through acquisitions and
opening new centers. In 2006, we acquired PlasmaCare, Inc., a
group of companies with 14 plasma collection centers and also
acquired an additional eight plasma collection centers from a
subsidiary of Baxter Healthcare Corporation. We also acquired 67
plasma collection centers in our acquisition of Talecris.
In May 2006, we completed our initial public offering in Spain
and our common stock is listed on the Barcelona, Madrid,
Valencia and Bilbao stock exchanges (the “Spanish Stock
Exchanges”) and quoted on the Automated Quotation System of
the Spanish Stock Exchanges. In January 2008, we became part of
the IBEX-35 Index, which comprises the top 35 listed Spanish
companies by liquidity and market capitalization.
On June 1, 2011 we acquired all of the issued and
outstanding shares of Talecris Biotherapeutics Holdings Corp., a
Delaware corporation, furthering our position as a diversified,
global provider of life-saving and life-enhancing plasma protein
therapeutics and making us the world’s third largest
producer of plasma derivative products. For a further discussion
of our acquisition of Talecris, see the section below entitled
“The Acquisition and Related Financing Events.”
Our Class A and Class B shares are listed on the
Spanish Stock Exchanges and quoted on the Automated Quotation
System of the Spanish Stock Exchanges under the symbol
“GRF” and “GRF.P,” respectively. Our
Class B shares were issued as part of the consideration for
the acquisition of Talecris and are traded in the U.S. on
the NASDAQ Global Select Market in the form of ADSs, evidenced
by ADRs, under the symbol “GRFS.” Each ADS represents
one-half (0.5) of one of our Class B shares. Our ADSs are
currently traded in U.S. Dollars.
We were incorporated in Spain as a limited liability company on
June 22, 1987. Our principal executive office is located at
Avinguda de la Generalitat, 152 Parque Empresarial Can Sant
Joan, 08174 Sant Cugat del Vallès, Barcelona, Spain and our
telephone number is +34 93 571 0500. Our registered office is
located at
c/Jesús
y María, 6, Barcelona, Spain.
Company
Overview
We are a leading global specialty biopharmaceutical company that
develops, manufactures and distributes a broad range of plasma
derivative products and also specializes in providing infusion
solutions, nutrition products, blood bags and diagnostic
instrumentation and reagents for use in hospitals and clinics.
Plasma derivatives are proteins found in human plasma, which
once isolated and purified, have therapeutic value. Plasma
derivative products are used to treat patients with hemophilia,
immune deficiencies, infectious diseases and a range of other
severe and often life threatening medical conditions. Our
products and services are used by healthcare providers in more
than 100 countries to diagnose and treat patients with
hemophilia, immune deficiencies, infectious diseases and a range
of other medical conditions.
Our plasma derivative products are manufactured at our plasma
fractionation plant near Barcelona, Spain, which has a capacity
of 2.1 million liters per year, and our plant in Los
Angeles, California, United States, which currently has a
capacity of 2.2 million liters per year. In addition, our
Clayton, North Carolina site, acquired in the acquisition of
Talecris, is one of the world’s largest integrated protein
manufacturing sites, including fractionation, purification and
aseptic filling and finishing of plasma-derived proteins and has
a
113
capacity of 2.6 million liters per year. The Melville, New
York site, which we lease and operate as a result of the
acquisition of Talecris, is an intermediate processing facility
and has a capacity of 1.6 million liters per year.
We organize our business into four divisions: Bioscience,
Hospital, Diagnostic and Raw Materials. Subsequent to the
acquisition, Talecris’ operations have been incorporated
into our existing Bioscience division.
Bioscience. The Bioscience division includes
activities relating to the manufacture of plasma derivatives for
therapeutic use, including the reception, analysis, quarantine,
classification, fractionation and purification of plasma, and
the sale and distribution of end products. The main types of
plasma products manufactured by us are IVIG, Factor VIII, A1PI
and albumin. We also manufacture hyperimmune immunoglobulins,
Antithrombin III, Factor IX and PTC. The Bioscience division,
which accounts for a majority of our total net sales, accounted
for €773.4 million, or 78.1%, and
€521.5 million, or 82.1%, and of our total net sales
for the year ended December 31, 2010 and the six months
ended June 30, 2011, respectively.
Hospital. The Hospital division manufactures
and, in certain instances installs, products that are used by
and in hospitals, such as parenteral solutions and enteral and
parenteral nutritional fluids, which are sold almost exclusively
in Spain and Portugal, and which accounted for
€89.6 million, or 9.0%, and €49.3 million,
or 7.8%, of our total net sales for the year ended
December 31, 2010 and the six months ended June 30,
2011 respectively. We believe we are the leading provider of
intravenous therapy in Spain, with a 34% market share.
Diagnostic. The Diagnostic division focuses on
researching, developing, manufacturing and marketing in vitro
diagnostics products including analytical instruments and
reagents for diagnostics, as well as blood bank products. We
concentrate our Diagnostic business in three areas:
immunohematology, hemostasis and immunology. The Diagnostic
division’s main customers are blood donation centers,
clinical analysis laboratories and hospital immunohematology
services. The division also manufactures and distributes blood
collection bags and other disposables. The Diagnostic division
accounted for €109.1 million, or 11.0% and
€56.8 million, or 8.9%, of our total net sales for the
year ended December 31, 2010 and the six months ended
June 30, 2011, respectively.
Raw Materials. The Raw Materials division
includes the sale of intermediate pastes and plasma to third
parties, which accounted for €4.8 million, or 0.5%,
and €1.7 million, or 0.3%, of our total net sales for
the year ended December 31, 2010 and the six months ended
June 30, 2011, respectively. Sales of the Raw Materials
division are used to optimize inventory levels with the aim of
striking a better balance between plasma collections and
fractionation needs.
Competitive
Strengths
We believe we have a number of competitive strengths, including:
Global
Player with an Established Presence in the Two Largest Plasma
Derivatives Markets
We are the third largest producer of plasma products with
operations in more than 100 countries with a direct presence in
24 countries. We have an established presence in North America
and Europe, which are the two largest plasma derivatives sales
regions in the world, accounting for approximately
$8.6 billion or 73% of the $11.8 billion in total
worldwide sales in 2008. North America and the European Union
accounted for 81.8% of our total revenues in the Bioscience
division in the six months ended June 30, 2011. Our
acquisition of Talecris has allowed us to further expand our
presence in the United States. We also have a presence in fast
growing sales regions including Asia (Malaysia, China and
Thailand), Japan, Canada, Australia and Latin America (Mexico,
Colombia, Argentina, Chile and Brazil). In addition, we operate
nine manufacturing facilities located in the United States,
Spain, Mexico, Switzerland and Australia.
114
Vertically
Integrated Business Model with a Secure Supply of United States
Source Plasma
We are a vertically integrated global producer of plasma
derivatives. Our activities include sourcing raw material,
manufacturing various plasma derivative products and sales and
distribution of the final products to healthcare providers.
We have expanded our plasma collection network through a
combination of organic growth and acquisitions and the opening
of new plasma collection centers. Our acquisitions of SeraCare
(now renamed Biomat USA) in 2002, PlasmaCare, Inc. in 2006,
eight plasma collection centers from a subsidiary of Baxter
Healthcare Corporation in 2006, four plasma collection centers
from Bio-Medics, Inc. in 2007, and one plasma collection center
from Amerihealth Plasma LLC in 2008 have given us reliable
access to United States source plasma. In 2010, we collected
2.6 million liters of plasma from our plasma centers. Our
acquisition of Talecris in June 2011 expanded our network by an
additional 67 centers. Following the acquisition, we have 147
plasma collection centers in the United States, which in the six
months ended June 30, 2011, collected 2.8 million
liters of plasma combined.
By decreasing our reliance on third parties for plasma and
having a secure supply of United States source plasma, a
critical operational requirement in our business, we are able to
better ensure the availability of plasma for our manufacturing
needs, assure the quality of the plasma throughout the
manufacturing process, better control plasma costs and improve
our margins.
State-of-the-Art,
FDA-Approved Manufacturing Facilities in Spain and the United
States
We have
state-of-the-art
plasma derivatives manufacturing facilities that are highly
efficient and safe. All of our fractionation facilities, other
than the Melville facility, have European Medicine Agency
(“EMEA”) certification. Our plasma fractionation plant
located in Parets del Vallès, near Barcelona, Spain, is
licensed by the FDA for the production of albumin and IVIG. The
Parets del Vallès facility has a unique design that
separates the maintenance area from the clean areas required for
the fractionation and purification procedures. This design,
which we developed in-house, minimizes the risk of contamination
and reduces maintenance costs.
In July 2003, we acquired Alpha Therapeutic Corporation’s
plasma fractionation business, which included a plasma
fractionation plant in Los Angeles, California, certain
inventories, patents, FDA approved licenses for plasma
derivative products in the United States and product licenses
and marketing and distribution structures in Europe and Asia.
This acquisition helped us increase our fractionation capacity
and expand our presence in the United States market. We have
made significant capital investments in the plant, including the
construction of purification and aseptic filling areas for
coagulation factors and albumin, which were completed in 2006
and 2009, and an increase of the fractionation capacity by
0.7 million liters to 2.2 million liters, which was
approved by the FDA during 2009.
As a result of the acquisition of Talecris we obtained an
additional fractionation facility in Clayton, North Carolina.
The Clayton facility is one the world’s largest integrated
protein manufacturing sites, including fractionation,
purification and aseptic filling and finishing of plasma-derived
proteins with a fractionation capacity of approximately
2.6 million liters of plasma per year. Over the last
15 years, the facility in Clayton has benefited from
roughly $699 million of capital investment, including
compliance enhancements, general site infrastructure upgrades,
capacity expansions and new facilities, such as its
chromatographic purification facilities and its high-capacity
sterile filling facility.
In connection with the acquisition and pursuant to the Consent
Agreement (as defined below), Talecris’ Melville, New York
facility was sold to Kedrion S.p.A. We now lease and operate the
Melville facility to produce intermediate pastes which are
further purified into final products at the Clayton facility.
See “Business — The Acquisition and Related
Financing Events.” The Melville facility has a
fractionation capacity of approximately 1.6 million liters
of plasma per year, which, when combined with the added capacity
from the Clayton facility, has increased our fractionation
capacity by approximately 98%.
Our current production processes for IVIG and Factor VIII have
been approved by the FDA as has the use of the intermediate
pastes created as raw material at the Barcelona and Los Angeles
plants, giving us
115
increased production efficiency and flexibility. On
July 21, 2011, we obtained FDA approval for the utilization
of the intermediate product Fraction II+III made at our Los
Angeles’ plant to be further purified at the Clayton
facility to obtain Gamunex, a product we acquired in the
acquisition of Talecris. We are pursuing additional
opportunities to use intermediates produced at our Grifols
plants in the previously Talecris-owned facilities as well as
intermediates from the previously Talecris-owned facilities in
our Grifols facilities.
Strong
Reputation for Safety and Reliable Service
We have never experienced a recall of any of our finished
biological products, although certain of our other products have
been subject to non-material recalls. Prior to the acquisition,
Talecris had four recalls of its finished biological products.
Our philosophy is that the health of the plasma donor and the
patient recipients of our drugs is the paramount consideration.
We believe that our safety philosophy is consistent with our
business objective of generating profit. We also believe that we
have a strong reputation for safety, making our products
particularly attractive to customers. Our vertically integrated
business model allows us to assure the safety and quality of our
plasma derivative products through the implementation of our own
safety standards throughout each stage of production.
We adopted and maintain rigorous safety standards that exceed
those required by health authorities in Europe and the United
States and actively invest in the continued improvement of our
manufacturing facilities and plasma fractionation process. In
2006, we developed a new sterile filling and purification area
for our Los Angeles, California plant, and developed a
nanofiltration area for our Parets del Vallès plant.
Additionally, we developed the nanofiltration method of viral
elimination of our IVIG and Antithrombin III products.
We require our management to adhere to a formal code of ethical
conduct. By signing the formal code of ethical conduct, our
managers commit to making our products the safest and most
effective on the market. The code imposes the obligation on each
manager to report any ethical concerns directly to our Board of
Directors. Our high safety standards and reliability have helped
us establish and maintain successful long-term relationships
with key customers and physicians worldwide. We believe the
strength of our reputation positions us favorably as we continue
to expand our business.
We maintain standards consistent with other industry
participants with regard to infectious disease screening and
quarantine of units. For example, source plasma inventory is
held for not less than 60 days. Some of our additional
safety policies include look-back procedures for seroconversion
and ongoing testing of donations for a
12-month
period after a negative donation. We have also introduced
innovative methods such as the PediGri system. This system
allows the physician to track the origin of the fractionated
product prescribed to patients back to the source donor,
providing full traceability of plasmatic raw material throughout
the plasma supply-chain.
High-Quality,
Industry-Leading Plasma Derivative and Diagnostic
Products
Our plasma derivative product portfolio includes a broad range
of reliable, high-quality products that improve patient care.
Our Factor VIII/von Willebrand Factor product is used both for
the treatment of hemophilia and von Willebrand disease. We
believe that the von Willebrand market segment will grow at a
higher rate than the overall Factor VIII market. In addition, we
offer our albumin product with a reduced aluminum content,
meeting European requirements and making our albumin product
more attractive to biotechnology companies and genetic labs, as
well as hospitals and physicians.
Our acquisition of Talecris expanded our portfolio of IVIG and
A1PI products. Gamunex IVIG, which was launched in North America
in 2003 as a premium
ready-to-use
liquid IVIG product, is one of the leading products in the IVIG
segment with a 24% share of United States sales in 2010
according to MRB. We believe Gamunex IVIG is considered to be
the industry benchmark due to a comprehensive set of
differentiated product characteristics that have positioned it
as the premium product in its category since its launch. We
manufacture the only IVIG products approved for CIDP in the
United States and Canada and, through mutual recognition
procedures, in 16 European countries. The CIDP indication
approval makes certain of the IVIG products that we manufacture
the only IVIG products approved for use in a neurological
indication in North America. According to an independent survey
by Harris Interactive, CIDP is the largest IVIG segment in the
United States, representing 29% of total unit volume. This same
survey estimated that the FDA’s approval of
116
IVIG for CIDP may double Gamunex’s licensed market access
to 61% of total United States IVIG unit volume. Further, the FDA
granted Gamunex IVIG orphan drug status, which provides
marketing exclusivity for the CIDP indication in the United
States through September 2015. In addition, following the
acquisition of Talecris we are the world’s largest producer
of A1PI, which is used for the treatment of A1PI
deficiency-related emphysema. In 2010, Prolastin A1PI had a 64%
share of sales in the United States. In 2008, it had an 87%
share of sales in Europe, where it is licensed in
15 countries, and it only competes with another licensed
A1PI product in France. Prolastin-C is also the only licensed
A1PI product in Canada.
We have focused our product offerings in the diagnostic market
in three distinct submarkets, immunohematology, hemostasis and
immunology. Our Diagnostic division has developed
state-of-the-art
automated analyzers for each of these submarkets. In the
immunohematology field, our Wadiana and Erytra analyzers are
fully automated instruments with high processing capacities for
pre-transfusion compatibility tests using the gel agglutination
technique. In the hemostasis field, our Q Coagulometer is an
automated instrument used for coagulation tests. In the
immunology field, our Triturus analyzers completely automate
enzyme immunoassays for hospital laboratories.
Over
70-Year
History of Successful Innovation
We have a strong track record as an innovator in the industry,
focusing on three areas: (i) discovering and developing new
products, (ii) researching new applications for existing
products and (iii) improving our manufacturing processes to
increase yields, safety and efficiency. For example, we
developed a unique fractionation design that reduces the risk of
contamination, reduces maintenance costs and increases the
amount of product extracted per liter of plasma. We have also
developed the first centrifugation unit for the automated
cleaning of blood cells, known as the Coombs test. In addition,
we were one of the first fractionators to conduct double viral
inactivation processes for Factor VIII and have designed and
implemented a new process for the sterile filling of vials that
reduces exposure to potential contaminants as compared to other
existing processes. We have recently developed a nanofiltration
method of viral inactivation for our IVIG and
Antithrombin III products. We believe that adoption of
novel policies and methodologies have raised industry standards
and made us a leader in safety and product quality.
Talecris was the developer of the first
ready-to-use
10% liquid IVIG product in North America and the first A1PI
product in the world. Talecris applied new developments in
protein purification, including caprylate and chromatography
technologies, to produce and sell a third-generation IVIG
product. Talecris’ next generation A1PI product,
Prolastin-C A1PI, was approved by the FDA and Health Canada. In
March 2010, Talecris launched Prolastin-C A1PI in the United
States and in the third quarter of 2010 in Canada. Talecris
completed the conversion of its existing U.S. and Canadian
Prolastin A1PI patients to Prolastin-C A1PI during 2010.
Presently, additional clinical trials are being required by
European authorities as a precursor to Prolastin-C A1PI approval
in Europe. In October 2010, the FDA approved Gamunex-C for the
subcutaneous route of administration for the treatment of
primary immunodeficiency. Additionally, Talecris received
approval for and we are proceeding with a proof of concept trial
for plasma-derived Plasmin to treat ischemic stroke in six
countries outside of the United States.
Experienced
and Committed Management Team
We have an experienced and committed management team. The
President and Chief Executive Officer, Victor Grifols Roura, is
the grandson of our founder and has held his current office for
over 26 years. The President of our Global Industrial
Division, Juan Ignacio Twose Roura, has been associated with
Grifols and our predecessor for more than 37 years. The
President of our Global Commercial Division, Ramón Riera,
has been associated with Grifols and our predecessor for more
than 33 years. Our Chief Financial Officer, Alfredo Arroyo,
has been associated with Grifols for five years. The President
of U.S. Operations and Chief Executive Officer of Grifols
Inc., Gregory Gene Rich, has been in the industry for nearly
31 years.
117
Our
Business Strategy
Our objective is to consolidate and expand our leadership
position in the plasma derivative product industry by employing
the following strategies:
Expand
Our Presence Internationally, Including in Emerging
Markets
Geographical diversification is a cornerstone of our strategy.
We currently operate in more than 100 countries with a direct
presence in 24 countries.
Certain sales regions are expected to experience significant
growth driven by enhanced socioeconomic conditions and more
informed patients who are demanding better quality medical care,
as well as increasing government healthcare spending on plasma
derivative products in some of these markets. In Latin America
and the Asia-Pacific region, we are expanding our presence by
establishing and strengthening relationships with distributors
as well as obtaining additional product licenses. Our presence
and experience in Latin America, in countries such as Mexico,
Colombia, Argentina, Chile and Brazil, where we have been
marketing and selling products for over 20 years, has
positioned us to benefit from this potential growth in both the
Bioscience and Diagnostic divisions. In addition, our
acquisition of product licenses and marketing and distribution
structures in Asia has helped accelerate the development of our
presence in that region. In the Asia-Pacific region, we have
established a presence through our subsidiaries and
representative offices in Malaysia, China, Thailand, Singapore
and Japan. Additionally, we have a license to market the latest
generation IVIG in Australia, which gives us the opportunity to
reach a country that has one of the highest levels of IVIG
consumption per capita.
Additionally, in March 2009, in a €25 million
transaction, we acquired 49% of the profit-sharing rights and
99% of the voting rights in Woolloomooloo Holdings Pty Ltd., the
holding company of an Australian-Swiss group that distributes
diagnostic products in Australia and Switzerland and has a
manufacturing facility in Switzerland, demonstrating our
continued focus on international expansion and acquisitions that
generate synergies. In August 2011, we acquired the remaining
outstanding capital stock and now hold 100% of the stock and
voting rights of the company.
Continue
Investment in Research and Development, Innovation and New
Facilities
Research and development is a significant aspect of our
business. We have recently increased investments in research and
development, in particular to develop the possible use of
albumin in treating Alzheimer’s disease, as well as other
projects relating to future biotechnological developments.
Recent product developments include Niuliva, an anti-hepatitis B
intravenous immunoglobin, launched in Italy, Spain and Latin
America at the start of 2010, and Flebogamma DIF, the latest
generation intravenous immunoglobulin, with licenses for
marketing in the United States and Europe, with additional
licenses expected in Latin America and Asia. We spent more than
€36.6 million in 2010 on research and development, a
3.7% increase in comparison to 2009. As of June 30, 2011,
we had approximately 706 scientists and support staff dedicated
to research and development.
We are currently implementing a €690 million
investment plan over 2011 to 2015 of which we have approximately
€300 million still outstanding under the plan. As part
of the plan, we are constructing new fractionation facilities in
Clayton, North Carolina and Parets del Vallès, Barcelona.
Construction of and the receipt of the necessary approvals for
the new Clayton facility is expected to be completed in 2015. We
expect this new facility to increase our fractionation capacity
by six million liters. Construction of and the receipt of the
necessary approvals for the new Parets del Vallès facility
is expected to be completed in 2014 and will increase our
fractionation capacity by an additional one million liters. We
also began construction on a new plant in Murcia, Spain in 2009
that will enable us to increase the production capacity of
non-pvc parenteral solutions by 15 million units per year.
The facility is expected to be operational in 2012.
We are also focused on expanding our existing facilities. In
2010, we completed the construction of a new IVIG plant in Los
Angeles, United States, which we believe will be operational
within the next year. In 2009, we completed construction of a
new raw materials warehouse and new research and development and
control laboratories at our industrial complex in Parets del
Vallès, Barcelona and new corporate offices in Sant
118
Cugat, Barcelona. In 2010, we completed construction on a new
production plant in Barcelona for “fibrin glue”, a new
product combining human plasma proteins, fibrinogen and thrombin
which, when combined, act as a biological glue. Also in 2010, we
completed construction on a new plasma analysis laboratory in
the United States in San Marcos, Texas. This new laboratory
near our existing facilities will enable us to cope with the
increasing volumes of plasma that we must analyze.
Expand
Our Product Offerings
Our research and development team, whose work is primarily
concentrated on the Bioscience division, will continue to seek
to develop new plasma derivative products as well as new
applications for our existing plasma derivative products. We
also seek to leverage our plasma derivative product portfolio by
offering diagnostic and hospital products developed by our
research and development team or by premier healthcare companies
with which we maintain distribution agreements. We believe that
by increasing the number of products we offer, we can generate
higher revenues, diversify our product base and facilitate our
entry into new markets. In addition, we also believe that a
one-stop shopping approach offering a broader range of
complementary, high-quality products is particularly attractive
to our existing and potential customers.
We are pursuing new plasma products, including two versions of
Plasmin. We recently completed a Phase I clinical trial for
the use of one version of Plasmin for the treatment of
peripheral arterial occlusive disease (“aPAO”). In
addition we are applying a commercial process to another version
of Plasmin in order to produce a recombinant form of Plasmin to
treat ischemic stroke. Additionally, we are evaluating whether
to continue Talecris’ research into recombinant versions of
Factor VIII and A1PI through the use of human cell lines. If
successful, the development of these therapies could
significantly improve our revenue and profitability.
The integration of Talecris gave us (i) a broad range of
key products addressing a variety of therapeutic areas such as
neurology, immunology, pulmonology and hematology, among others
and (ii) enhanced our research and development pipeline
with complementary products and new recombinant projects that we
are evaluating, all of which we expect will further expand our
broad product offerings.
The
Acquisition and Related Financing Events
On June 1, 2011, pursuant to the Merger Agreement, we
completed our acquisition of Talecris, for a total of
$3.7 billion. The acquisition was effected through
(i) the merger of Talecris with and into Merger Sub, and
(ii) the immediately subsequent merger of Grifols Inc., a
Delaware corporation and wholly owned subsidiary of Grifols,
S.A., with and into Merger Sub, with Merger Sub continuing as
the surviving corporation and a wholly owned subsidiary of
Grifols, S.A. Merger Sub was subsequently renamed Grifols Inc.
Each share of Talecris common stock, par value $0.01 per share,
was converted into the right to receive $19 in cash and ADSs
representing 0.6485 (or 0.641 for Talecris directors and
Talecris Holdings, LLC) of a non-voting
(Class B) share of Grifols, S.A. and cash in lieu of
fractional Class B shares and any cash representing
dividends or other distributions payable in accordance with the
Merger Agreement. ADSs representing the Class B shares are
listed on the NASDAQ Global Select Market under the symbol
“GRFS.”
On April 29, 2011, Grifols, S.A. and Talecris entered into
the Consent Agreement with the staff of the Bureau of
Competition of the FTC which provided for certain conditions to
the closing of the acquisition. The FTC accepted the Consent
Agreement for public comment on May 31, 2011. The Consent
Agreement required us to divest certain assets to Kedrion
S.p.A., a corporation organized under the laws of Italy
(“Kedrion”). Specifically, we agreed to sell Kedrion
(i) Talecris’ fractionation facility located in
Melville, New York; (ii) two plasma collection centers
located in Mobile, Alabama, and Winston Salem, North Carolina;
(iii) an agreed quantity of plasma; and (iv) the
exclusive right to sell, in the United States, the Factor VIII
product previously sold under Talecris’ brand name Koate.
The Consent Agreement permits us to lease the Melville facility
back from Kedrion for up to four years. In addition, the Consent
Agreement required us to enter into various agreements with
Kedrion to implement the Consent Agreement (collectively, the
“Divestiture Agreements”), including (i) a
contract manufacturing agreement under which for seven years we
will manufacture at least 300,000 plasma liter equivalents of
(x) Koate, (y) private label IVIG and (z) private
label albumin, for sale by
119
Kedrion in the United States; and (ii) a five-year option
for Kedrion to purchase a non-exclusive license to Koate-related
intellectual property for use in the United States. The Consent
Agreement also required the appointment of an independent
monitor to oversee our compliance and requires us to submit
periodic reports to the FTC setting forth in detail the manner
and form in which we intend to comply, are complying, and have
complied with the Consent Agreement. As required by the Consent
Agreement we satisfied all necessary conditions within ten days
of the completion of the acquisition. Our next compliance report
to the FTC is due in 2012.
In order to finance the cash portion of the acquisition
consideration and repay certain indebtedness, on
November 23, 2010, we entered into a credit and guaranty
agreement, which provided for the Senior Term Loans aggregating
$2.5 billion and €440 million, and the Revolving
Credit Facilities in the amounts of $50 million,
€36.7 million and the $200 million equivalent in
multicurrencies (together with the Senior Term Loans, the
“Senior Credit Facilities”), from a syndicate of
lenders led by Deutsche Bank Securities Inc., Nomura
International plc, Banco Bilbao Vizcaya Argentaria, S.A., BNP
Paribas, HSBC Securities (USA) Inc. and Morgan Stanley Senior
Funding, Inc. On June 1, 2011, upon completion of the
acquisition, we closed the Senior Credit Facilities and the
lenders funded their commitments thereunder. As of June 30,
2011, no amounts have been drawn on the Revolving Credit
Facilities. For a summary of the material terms of the Senior
Credit Facilities, see the section of this prospectus entitled
“Management’s Discussion and Analysis of Financial
Condition and Results of Operations —
Indebtedness — Senior Credit Facilities.”
In addition, on January 21, 2011, Escrow Corp., an escrow
company formed solely for the purpose of issuing the existing
notes, completed a private offering of the existing notes to
Deutsche Bank Securities Inc., Nomura International plc, BBVA
Securities Inc., BNP Paribas Securities Corp., HSBC Securities
(USA) Inc., Morgan Stanley & Co. Incorporated, Scotia
Capital (USA) Inc., as the initial purchasers. The proceeds of
the offering of the existing notes were held in an escrow
account pending completion of the acquisition and the
satisfaction of other conditions. On June 1, 2011, upon
consummation of the acquisition, Escrow Corp. was merged with
and into the Issuer, and the Issuer assumed all of Escrow
Corp.’s obligations under the existing notes and the
indenture. The proceeds of the offering of the existing notes
were disbursed from the escrow account and used to pay a portion
of the acquisition purchase price. In addition, upon
consummation of the acquisition, the Issuer, the Parent
Guarantor and the initial Subsidiary Guarantors executed
joinders to a registration rights agreement that had been
entered into by Escrow Corp. and the initial purchasers of the
existing notes on January 21, 2011. Pursuant to the
registration rights agreement, the Issuer, the Parent Guarantor
and the initial Subsidiary Guarantors agreed, for the benefit of
the holders of the existing notes, at our cost, to file the
registration statement of which this prospectus forms a part and
to complete an exchange offer for the existing notes. For a
summary of the material terms of the notes, see the section of
this prospectus entitled “Management’s Discussion and
Analysis of Financial Condition and Results of
Operations — Indebtedness —
8.25% Senior Notes due 2018.”
Pursuant to the terms of the Senior Credit Facilities and in
connection with the consummation of the acquisition we
refinanced substantially all of our and Talecris’ existing
debt. On June 1, 2011, we redeemed all of the Grifols Old
Notes and repaid existing bank loans amounting to
€297 million. We paid a €112 million
make-whole premium payment in connection with the redemption of
the Grifols Old Notes. On June 13, 2011, we redeemed 10% of
the $600 million aggregate principal amount of Talecris Old
Notes. At June 30, 2011, our cash and cash equivalents
included €428 million held in a restricted cash
account in connection with the redemption of the remaining
Talecris Old Notes, which were subsequently redeemed on
July 1, 2011. As of the date hereof, there are no Talecris
Old Notes outstanding. We paid a €78 million
make-whole premium payment in connection with the redemption of
the Talecris Old Notes.
Our
Divisional Structure
The
Bioscience Division
The Bioscience division is responsible for the research and
development, production and marketing of plasma derivative
products. In the six months ended June 30, 2011, the
Bioscience division accounted for over 82.1% of our revenue.
120
Operational Structure. The following chart
illustrates our operational structure:
From plasma/blood donation to therapeutic application, there are
four major steps in the industry value chain process:
(i) plasma collection, (ii) transport and logistics,
(iii) manufacturing process (known as
“fractioning”) and (iv) marketing and
distribution. We are present at all levels of the value chain,
from collection centers to distribution of the final products.
This integration enables us to leverage our position at each
stage to control the overall process, to benefit from lower
prices and to introduce complementary products to our customers,
such as those offered through the Hospital division and the
Diagnostic division.
Plasma Collection. Plasma is the key raw
material used in the production of plasma-derived products. We
obtain our plasma mainly from the United States through our 147
plasma collection centers and, to a much lesser extent, through
agreements with third parties, although 100% of our commercial
products are created using source plasma. We gathered
approximately 2.6 million liters of plasma in 2010, prior
to the acquisition of the 67 centers from Talecris, and an
estimated 2.8 million liters of plasma were gathered from
both Grifols’ and Talecris’ plasma collection centers
combined in the six months ended June 30, 2011. As we
source approximately 90% of our plasma internally, we have been
able to ensure the availability of plasma for our manufacturing
needs, to assure the quality of the plasma throughout our
manufacturing process, to have better control over our plasma
costs and to improve our margins.
We estimate that our plasma requirements for 2012 and going
forward will be covered with the following:
|
|
|
| |
•
|
Plasma collected through our 147 plasma collection centers in
the United States; and
|
| |
| |
•
|
approximately 800,000 liters of plasma per year to be purchased
from third-party suppliers for the next three years pursuant to
multiple plasma purchase agreements assumed from Talecris in
connection with the acquisition.
|
At the plasma collection stage, we focus on the well-being of
the donor. For this reason, we have implemented mechanisms to
ensure that the donor meets the guidelines set forth by
applicable regulations regarding, among other things, health,
age and frequency of donations. Once the plasma donation is
completed, we shift our focus towards the well-being of the
ultimate user of our plasma-derived products. As required by
applicable United States and European regulations, we test every
single donation for pathogens such as HIV, hepatitis A, B and C,
parvovirus B19 and syphilis. If we discover a unit of plasma
that is contaminated, we notify the donor and remove all plasma
previously donated by such donor from our inventory.
Transport and Logistics. Once the plasma has
been collected it is frozen at the collection center and sent to
fractionation centers. One essential aspect of this process is
the safety procedures put in place to guarantee the quality and
safety of the donated plasma. To ensure the preservation of the
proteins found in
121
plasma, plasma must be kept at a temperature of -20 degrees
Celsius. In accordance with European and United States
requirements, we store our plasma at a temperature of -30
degrees Celsius. During transportation, plasma is kept at a
temperature of at least -20 degrees Celsius. Our frozen plasma
is transported by one of two transport companies which are the
same used industry wide.
Although the law only requires a two-month hold, plasma we
collect from our donors is held in inventory in Spain for three
months because viruses may not be detectable until they reach a
certain minimum mass. Therefore, if plasma collected from a
donor is found to be contaminated, we can remove all plasma
previously donated by the same donor during the previous
three-month period from our inventory. We maintain a library of
samples of every single donation of plasma we have collected
worldwide since 1987. We have begun to add the donations
received at the Talecris-owned plasma centers to our library.
This gives us a valuable database for traceability purposes.
Manufacturing (“Fractionation and
Purification”). Once the plasma has been
obtained, it may be used immediately for blood transfusions. It
may also be frozen (as fresh frozen plasma) and then
manufactured into plasma derivatives by separating the plasma
into component proteins through a process called fractionation.
The fractionation process consists of the separation of specific
proteins through temperature and pH changes, as well as the use
of filtration and centrifugation techniques. This process also
includes a phase of administration of various viral inactivation
procedures. The fractionation occurs in tanks at near freezing
temperatures to maintain the integrity of the proteins. All
known plasma derivative products can be fractionated from the
same batch of plasma. As a result, the development of a new or
higher yielding plasma derivative product would likely generate
incremental sales without increasing the requirement for
additional plasma.
We currently operate manufacturing facilities located in the
United States, Spain, Mexico, Switzerland and Australia. The
American and Spanish facilities have plasma fractionation and
purification capabilities.
The Parets del Vallès plant in Barcelona, Spain is a
state-of-the-art
manufacturing and fractionation facility with a high degree of
efficiency and safety. In addition to licenses from the European
Union and other authorities for the production of various plasma
derivative products, the plant is licensed by the FDA for the
production of albumin and IVIG. We believe we are one of the few
European plasma derivatives plants to be licensed by the FDA.
The plant has a fractionation capacity of 2.1 million
liters per year.
We acquired our fractionation facility in Los Angeles,
California from Alpha Therapeutic Corporation
(“Alpha”) in July 2003. This plant currently has a
fractionation capacity of approximately 2.2 million liters
per year, following an increase in capacity of 700,000 liters
per year, which began in 2010. We have significantly improved
the manufacturing standards at the Los Angeles plant since its
acquisition, including adding a
state-of-the-art
sterile filling and purification area for the plant. Also, we
have instituted a no rework policy and implemented a process for
the videotaping and laser identification of every product, as
well as the traceability of products through our PediGri
program. This same program is also used in our Barcelona
facilities and is being instituted in our Clayton facility. The
Los Angeles plant is subject to regulation by the FDA and is
currently operating under a consent decree from the FDA and the
United States Department of Justice dating to the time the plant
was owned and operated by Alpha. See the section entitled
“— Legal Proceedings — Alpha Consent
Decree.”
The manufacturing site in Clayton, North Carolina that we
acquired from Talecris is one of the world’s largest fully
integrated facilities for plasma-derived therapies. The site
includes plasma receiving, fractionation, purification,
filling/freeze-drying and packaging capabilities as well as
freezer storage, testing laboratories and a cGMP (current good
manufacturing practice) pilot plant for clinical supply
manufacture. The Clayton plant has a fractionation capacity of
approximately 2.6 million liters per year.
The manufacturing site in Melville, New York, which we lease
from Kedrion as a result of the Divestiture Agreements, provides
us with additional manufacturing capacity. We operate the
Melville facility to produce intermediate pastes which are
further purified into final products at the Clayton facility.
The Melville plant has a fractionation capacity of approximately
1.6 million liters per year.
122
The Spanish and American manufacturing facilities currently have
an aggregate fractionation capacity of 8.5 million liters
of plasma. In 2010, Grifols and Talecris combined fractionated
an aggregate of approximately 7.0 million liters of plasma.
We are constructing new fractionation facilities in Clayton,
North Carolina and Parets del Vallès, Barcelona.
Construction of and the receipt of the necessary approvals for
the new Clayton facility is expected to be completed in 2015. We
expect this new facility to increase our fractionation capacity
by six million liters. Construction of and the receipt of the
necessary approvals for the new Parets del Vallès facility
is expected to be completed in 2014 and will increase our
fractionation capacity by an additional one million liters.
We are also undertaking to expand our existing facilities. We
recently completed construction of a new production plant for
“fibrin glue”, a biological sealant, as well as new
corporate offices in our industrial complex in Parets del
Vallès. The new production plant will enable us to expand
the Albumin and Factor VIII purification areas, two of our main
plasma products. We have applied for a license and expect to
have the plant online within the year. We also recently
completed construction on a new analysis laboratory in the
U.S. in San Marcos, Texas. The new laboratory will
enable us to cope with increasing volumes of plasma to be
analyzed and its proximity to our existing facility will enable
us to get it operating and staffed with experienced staff
quickly. Additionally, we have finished the construction of a
new plant in Los Angeles for the production of Flebogamma DIF
and we have begun the validation process with the appropriate
regulatory authorities.
Currently, the Parets del Vallès and Los Angeles plants are
equipped and licensed to produce certain plasma derivative
products for both the United States and European markets. For
example, we produce our Flebogamma IVIG product for all of our
markets in Parets del Vallès. In addition, the FDA and the
European Union authorities have authorized the use of cryopaste
fractionated at our Parets del Vallès plant for the
production of Factor VIII at our Los Angeles plant, and the use
of intermediate pastes II and III fractionated at our
Los Angeles plant for the production of Flebogamma IVIG at our
Parets del Vallès plant. This flexibility allows us to
increase production efficiency and to address changes in demand
between the United States and the European markets. Subsequent
to the closing of the first half of 2011, we obtained FDA
approval for the utilization of the intermediate product
Fraction II+III made at the Los Angeles’ plant to be
further purified at our Clayton plant to obtain Gamunex, a
product we acquired in the acquisition of Talecris.
Safety. We have never experienced a recall of
any of our finished biological products, although certain of our
other products have been subject to non-material recalls. Before
its acquisition by us, Talecris had experienced four recalls of
its finished biological products. Our philosophy is that the
health of the plasma donor and the patient are the paramount
considerations. We strongly believe that our safety philosophy
is consistent with the business objective of generating profit.
We also believe that we have a strong reputation for safety in
our markets, thus making our products particularly attractive to
customers. Our vertically integrated business model allows us to
assure the safety and quality of our plasma derivative products
through the implementation of our safety standards throughout
the value chain.
The plasma collection, fractionation and purification process is
long, complex and highly regulated. We have adopted and maintain
rigorous safety standards that we believe exceed those required
by health authorities in Europe and the United States and we
actively invest in the continued improvement of our
manufacturing facilities and plasma fractionation process.
Fractionation plants must be cleaned and sterilized frequently.
Our fractionation plant at Parets del Vallès was designed
in a way that reduces the clean area required for the
fractionation tanks and separates them from the room-temperature
work area. This allows us to perform all maintenance work from
outside the room-temperature area, thereby decreasing the risk
of contamination. We believe that none of our competitors have
similar designs.
We voluntarily shut down all of our manufacturing facilities for
an aggregate of 45 days every year to perform maintenance
work, expansion projects and other capital investments. Our
manufacturing facilities have never been shut down because of
regulatory noncompliance while under our operation. We believe
that our voluntary shutdown procedure lowers the risk of any
mandatory shutdown.
After the plasma derivatives are processed, we inspect each
bottle for irregularities such as imperfect seals, bottle
cracks, volume mismeasurements and the presence of foreign
objects.
123
We have also developed and installed in our Parets del
Vallès plant our own proprietary process of sterile filling
of bottles designed to reduce the risk of contamination. Under
the sterile filling process of other fractionators, bottles and
stoppers are sterilized independently, leaving the inside of the
bottles exposed to potential contaminants in the environment for
several minutes. Our process sterilizes both the bottle and
stopper. The bottle is reopened in a small sterile room for only
two seconds in order to insert the product and then resealed,
greatly reducing exposure to the environment and reducing the
risk of contamination. We are not aware of any competitor having
a similar process.
Since January 1999, we have videotaped the filling process to
enable us to identify the cause of, and rectify more easily, any
related problem. Our policy is to maintain each videotape for
six years. We also imprint an identification number on each of
our bottles with a laser for easier identification in the event
of a recall and to reduce the risk of tampering. This allows us
to protect the integrity of our manufacturing process.
Distribution Process. With each batch of
plasma derivatives, we deliver electronic information regarding
the origin, characteristics and controls of each of the units of
plasma that we used in the preparation of the batch to our
customers. This feature, called PediGri, allows for full
traceability of the human plasma raw material in the event of a
problem with a specific product. This is of utmost importance
for containing the transmission of diseases in the event of a
potential product recall. We have had this system in place since
1996, and we believe we are the only fractionator that provides
this feature to customers.
We have our own sales and distribution networks covering
substantially all of our markets, staffed with highly trained
personnel. A majority of our net sales in 2010 were made through
our own distribution network, which is experienced in the proper
handling of our products. This network provides for greater
safety because it allows us to know at all times where our
products are located, thus enabling us to act immediately in the
case of a potential product recall. In countries where we do not
have our own distribution network, we have carefully selected
distributors who follow all of our safety standards.
Additionally, outside of the United States we are working on
changing some of Talecris’ products from their existing
distribution channels into our own, reducing their distribution
costs. We expect to have them fully transferred by 2017.
In addition, we have an agreement with Catalent Pharma Solutions
pursuant to which they provide packaging, labeling and testing
services for us in connection with the distribution of our
products in Europe. The agreement expires in 2013, though we
have the option to extend it twice, each time for a period of
two years. We may terminate the agreement upon 12 months
notice, and Catalent may terminate the agreement upon
24 months notice, beginning in 2011. Either party may
terminate the agreement upon the bankruptcy of the other party
or if there is a material breach which is not cured within 30
business days.
Bioscience Products and Services. Collected
plasma, whether source or recovered, is fractionated into
different component proteins. We fractionate and purify a broad
range of plasma derivative products, however, IVIG, A1PI, Factor
VIII and albumin, our principal products, accounted for 41%,
13%, 12% and 4.4% of the Bioscience division net sales for the
six months ended June 30, 2011. Our principal plasma
derivative products and their respective applications are:
| |
|
|
|
Product Description
|
|
Main Applications
|
|
|
Flebogamma IVIG. Human intravenous immunoglobulin,
liquid, pasteurized and solvent detergent inactivation.
Flebogamma DIF IVIG. Human intravenous immunoglobulin,
liquid, pasteurized, solvent detergent inactivation and
nanofiltration.
Gamunex IVIG. Human intravenous immunoglobulin, liquid
|
|
IVIG assists in the treatment of primary and secondary
immunological deficiencies, the treatment of immune-mediated
idiopathic thrombocytopenic purpura (“ITP”), Guillain
Barré syndrome, Kawasaki disease, allogeneic bone marrow
transplants, CPI, and on an off-label application basis,
multiple sclerosis, skin disease, asthma and CIDP, a
neurological indication. IVIG is also currently being
investigated for use in the treatment of Alzheimer’s
disease and other neurological conditions.
|
|
|
|
|
|
Gamunex-C. Human immunoglobulin, liquid, injection
|
|
|
124
| |
|
|
|
Product Description
|
|
Main Applications
|
|
|
|
Our IVIG products have a 5% and 10% concentration.
|
|
|
|
|
|
|
|
Prolastin/Prolastin-C A1PI. Human proteinase inhibitor
|
|
Used to treat congenital A1PI deficiency-related emphysema.
|
|
|
|
|
|
Trypsone A1PI. Human proteinase inhibitor
|
|
|
|
|
|
|
|
Fanhdi Factor VIII and Alphanate Factor VIII. High purity
anti-hemophilic Factor VIII containing von Willebrand factor.
|
|
Used for the prevention and control of bleeding in Factor VIII
deficiency (hemophilia A), and indication in the United States
for von Willebrand congenital hemorrhagic disease.
|
|
|
|
|
|
Koate DVI. Anti-hemophilic Factor VIII
|
|
|
|
|
|
|
|
Grifols Albumin. Pasteurized sterile aqueous solutions
containing 25%, 20% or 5% human serum albumin. This albumin has
a low aluminum content, a requirement in Europe but not in the
United States, that makes it particularly attractive for
biotechnology companies. We also offer Albutein Albumin,
a product containing 25%, 20% or 5% human serum that we obtained
in our acquisition of Alpha and Talecris’ Plasbumin
a product containing 25%, 20% or 5% human serum
|
|
Used to re-establish and maintain circulation volume in the
treatment of traumatic or hemorrhagic shock and severe burns.
Also used for liver disease and increasingly by biotechnology
companies as a stabilizer.
|
In addition to the products described above, we also produce
hyperimmune immunoglobulins, which are used for the treatment of
tetanus, hepatitis A and B, and RH complications during birth;
Anbin, Thrombate and Antithrombin III, which are used in the
treatment of thrombotic diseases; Alphanine/Novix Factor IX,
which is used in the prevention and control of bleeding in
patients with hemophilia B; Niuliva, Anti-hepatitis B IVIG used
in liver transplantations.
To sell plasma derivative products, we must first register the
products with the competent authorities of the jurisdiction of
the market where the product is to be marketed and sold.
To comply with the regulatory requirements in a given
jurisdiction, we have a core team in Spain and the United States
that prepares, files and coordinates the registration process
with the technical personnel at the subsidiary assigned to that
jurisdiction. We have approximately 624 hemoderivative product
licenses registered in over 90 countries. Our most significant
government issued licenses for plasma derivative products are:
|
|
|
| |
•
|
Flebogamma/Gamunex IVIG. We have 93 licenses
for marketing and sale of one or both of these IVIG products: 32
in the European Union, 4 in the United States, 26 in Latin
America, 12 in Asia and 19 in the rest of the world;
|
| |
| |
•
|
Fanhdi/Alphanate/Koate Factor VIII. We have 97
licenses for the marketing and sale of one or more of these
Factor VIII products: 22 in the European Union, 31 in Latin
America, 2 in the United States, 22 in Asia and 20 in the rest
of the world;
|
| |
| |
•
|
Albumin Grifols/Albutein/Plasbumin Albumin. We
have 195 licenses for the marketing and sale of one or more of
these albumin products in its various concentrations: 37 in the
European Union, 13 in the United States and Canada, 52 in Latin
America, 67 in Asia and 26 in the rest of the world; and
|
| |
| |
•
|
Prolastin/Trypsone A1PI. We have 23 licenses
for the marketing and sale of one or both of these A1PI
products: 16 in the European Union, 5 in Latin America, and 2 in
the United States.
|
We market and sell our bioscience products through our own sales
and distribution network in the United States, the European
Union and most of Latin America and Asia. We believe that having
knowledgeable sales representation and our own distribution
network staffed with highly trained personnel is critical to a
successful marketing and sales effort. See the sections entitled
“— Marketing and Sales” and
“— Distribution.”
125
In addition to the sale of the products described above, we have
entered into a series of arrangements with some Spanish
transfusion organizations to fractionate recovered plasma from
such organizations and manufacture plasma derivatives under our
own brand name for use by hospitals. We charge the transfusion
centers for the fractionation and manufacturing service. We have
similar, albeit smaller, arrangements with Czech and Slovak
organizations. We also provide virus photo-inactivation of
transfusion plasma to hospitals and clinics in Spain. The plasma
is inactivated at our manufacturing facilities and then sent
back to the clinic or hospital at which it was collected, where
it is used for transfusions.
Under the Talecris contracts with Canadian Blood Services and
Hema Quebec, the Canadian blood system operators, we fractionate
100% of the Canadian plasma initially and a majority of the
Canadian plasma throughout the contract period and supply a
majority of the Canadian requirements for IVIG during the
contract term as well. We transport plasma from Canadian Blood
Services and Hema Quebec collection centers to our manufacturing
facility in Clayton, North Carolina for manufacture, and return
the finished product, along with commercial product, for sale to
Canadian Blood Services and Hema Quebec. For additional detail,
see the subsection below entitled
“— Customers.”
The
Hospital Division
The Hospital division manufactures primarily intravenous
solutions and nutrition products for sale in Spain and Portugal.
In addition to the above-mentioned solutions, we manufacture
accessories such as feeding tubes for nutrition, bags for the
preparation of liquid diets, and Nutribag, a parenteral
nutritional bag made of ethyl vinyl acetate. In recent years, we
have developed a business called Grifols Partnership, by which
we manufacture customized IV solutions for third parties
based on the specific requirements of each customer. Our
Hospital division accounted for 9.0% and 7.8% of our net sales
in the year ended December 31, 2010 and in the six months
ended June 30, 2011, respectively. We believe we are the
leader in the Spanish intravenous therapy segment.
We also offer medical devices such as disposable sterile
therapeutic medical products for urology, radiology,
hemodynamics and anesthesia, as well as urodynamics and
lithotripsy and radiological diagnosis instruments. All of these
products are manufactured by third parties and complement our
portfolio of hospital products.
Finally, we offer products manufactured by third parties related
to the logistical organization of the pharmacies and general
warehouses of hospitals, including furniture, transport carts,
bottling instruments and software programs for hospital
management, admissions and accounting. We have an exclusive
license through June 2013 for Spain, Portugal, Italy, Latin
America and portions of Africa, to distribute logistics products
manufactured by the Cardinal Health Group under the trademark
“Pyxis.”
We manufacture parenteral solutions in glass bottles at our
manufacturing plant in Parets del Vallès, which has an
estimated capacity of 45 million bottles per year. We
manufacture parenteral solutions in flexible plastic containers
at our Las Torres de Cotillas manufacturing facility in Murcia,
Spain, which has an estimated capacity of 27 million
flexible plastic containers per year. Construction work began on
a new plant at the Las Torres de Cotillas facility, which will
increase the production capacity of parenteral solutions in
flexible plastic containers by 15 million units per year.
As of December 31, 2010, we had spent approximately
€7.4 million and we expect to spend an additional
€11.0 million on this facility in 2011 and 2012.
The principal raw materials for our intravenous therapy products
are plastic and glass bottles, which we purchase from various
European suppliers. None of our hospital products suppliers
accounted for more than 8% of our total hospital products supply
purchases in each of the year ended December 31, 2010 and
the six months ended June 30, 2011.
126
The following table describes our principal hospital products
and their respective applications:
| |
|
|
|
Product Description
|
|
Main Applications
|
|
|
|
Intravenous therapy:
|
|
|
|
|
|
|
|
Intravenous fluid and electrolyte solutions. Main product
groups include hypotonic solutions, isotonic solutions,
hypertonic solutions and plasma volume expander solutions.
|
|
Fluid and electrolyte replacement and conduit for the
administration of medicines.
|
|
|
|
|
|
Intravenous washing solutions. Washing solutions in
specially designed containers.
|
|
Cleaning of injury and operation areas and urological irrigation.
|
|
|
|
|
|
Intravenous mixtures.
Ready-to-use
intravenous mixtures of potassium, antibiotics, gastroprotective
agents, levofloxacine and paracetamol for various purposes. We
complement this product by offering Grifill, a system for the
preparation of intravenous mixtures at in-hospital pharmacies
using the principle of sterile filtration. We obtained a product
license for Grifill in December 2003 from the FDA, which allows
us to market and sell this product in the United States.
|
|
Increases safety and efficiency by rendering unnecessary the
mixing of solutions at in-hospital pharmacies.
|
|
|
|
|
|
Nutrition:
|
|
|
|
|
|
|
|
Soyacal fat emulsion. Fat emulsion at 10% and 20%,
administered intravenously.
|
|
The fat emulsion is the main energy source for the patient in
parenteral nutrition, providing calories and acid fats.
|
|
|
|
|
|
Glucose solution. High glucose concentrate (5%, 15%, 30%
and 50%).
|
|
Offers carbohydrate support for a patient’s diet that is
administered intravenously.
|
|
|
|
|
|
Dietgrif enteral liquid diets. Oral diets with all the
requirements for balanced nutrition. Different diets include
standard, standard fiber, polypeptidic, hyperproteic and
energetic.
|
|
For patients who are unable to eat enough to maintain a
nutritious diet, administered through feeding tubes as well as
orally.
|
|
|
|
|
|
Amino acid solutions. Solutions at 8% and 10% and
hyper-nitrogenated amino acid solutions.
|
|
Offers amino acid support for a patient’s diet.
|
|
|
|
|
|
Medical devices. Disposable sterile therapeutic medical
products and radiological diagnosis instruments.
|
|
The products are used in uruology, radiology, cardiology,
neurology hemodynamics, anesthesia, urodynamics and lithotripsy.
|
|
|
|
|
|
Hospital logistics. Products, some manufactured by
third-parties, like furniture, transport carts, bottling
instruments and software programs, including our own Pyxis
system.
|
|
Used in the logistical organization of the pharmacy and general
warehouse of hospitals as well as in hospital management,
admissions and accounting.
|
The production, marketing and sale of our parenteral solutions
are subject to the prior registration of such products with the
competent authorities of the jurisdiction where the product is
to be marketed and sold. We have approximately 77 licenses for
our hospital products registered in eight European Union
countries and eight countries in Latin America. Our sales
representatives sell primarily to pharmacy, nutrition and
gastroenterology units in hospitals and other units in hospitals
that use our medical devices, using our own distribution
network. We also sell a product called the Misterium, a mini
sterile modular room, a “cleanroom,” which we uniquely
design for each order and then install where the customer
requests.
127
Our marketing strategy focuses mainly on further penetration of
the United States market with our Grifill product for the
preparation of intravenous mixtures and of the Spanish and Latin
American markets through the sale of the Pyxis logistics
products.
In addition, we have initiated an international and the
geographical diversification strategy for the division through
third-party agreements, including a five-year agreement with
CareFusion, a global leader in medical technology, to distribute
the BlisPack system throughout several countries of Europe,
Middle East, Africa and Asia. BlisPack is a system designed by
us to automate the cutting of prescription pill blister packs,
and identification of specific drugs for individual patients to
be used by hospitals.
The
Diagnostic Division
Our Diagnostic division focuses on researching, developing,
manufacturing and marketing of in vitro diagnostics products
including analytical instruments and reagents for diagnostics,
as well as blood bank products. We believe we are the leader in
Spain in the automated immunology market and are second in the
blood bank and immunohematology markets. The Diagnostic division
accounted for 11.0% and 8.9% of our net sales in the year ended
December 31, 2010 and the six months ended June 30,
2011, respectively.
We assemble our machines individually at our Parets del
Vallès facility. We manufacture our blood bags at our Las
Torres de Cotillas facility which has an estimated capacity of
eight million blood bags per year. See the section entitled
“— Property, Plant and Equipment.”
Our principal diagnostic products are:
| |
|
|
|
Product Description
|
|
Main Applications
|
|
|
|
Immunohematology:
|
|
|
|
|
|
|
|
Wadiana/Erytra analyzers. Automated immunohematology
analyzers which use gel technology.
|
|
Used to perform routine pre-transfusion testing and
immunohematology tests in general.
|
|
|
|
|
|
Immunology:
|
|
|
|
|
|
|
|
Triturus analyzers. Fully automated analyzer with open
system for any ELISA test offering multi-test/multi-batch
capability.
|
|
Allows hospitals to automate the enzyme immunoassays in
microtiter plate format and to process several batches of
samples simultaneously.
|
|
|
|
|
|
Reagents, instrumentation and software. Instruments,
reagents and software for coagulation testing.
|
|
Used to establish the coagulation status of patients and to
handle the corresponding results.
|
|
|
|
|
|
Hemostasis:
|
|
|
|
|
|
|
|
Q-Coagulometer analyzers. Fully automated hemostasis
analyzer which uses reagents to measure coagulation levels.
|
|
Used to diagnose and measure coagulation status of patients with
coagulation-related and hemorrhagic disorders.
|
|
|
|
|
|
Blood banks:
|
|
|
|
|
|
|
|
Leucored and Standard Blood bags. Blood bags configured
according to all blood bank separation protocols. Leucored blood
bags incorporate an in-line filtration system.
|
|
Used for collection and transfusion of blood.
|
In addition to our own products, we offer our customers products
from outside sourcing to complement our existing product lines
such as immunology products from Meridian Diagnostics, Inc.,
IMMCO Diagnostics, Inc., AESKU Diagnostics GmbH and DIESSE
Diagnostica Senese S.p.A., and hemostasis products from Alere
Inc.
The production, marketing and sale of our diagnostic products
are subject to the prior registration of such products with the
competent authorities of the jurisdictions where the product is
to be marketed and sold. In
128
particular, we obtained an FDA license to sell the Wadiana
analyzer in the United States in May 2003. We have approximately
746 diagnostic product licenses registered in a total of 45
countries in Europe, the United States, Latin America and Asia.
Our marketing strategy for the diagnostic division focuses
mainly on continuing the penetration of our Wadiana/Erytra
analyzers and related gel cards in the principal world markets
directly or through marketing partners. In particular, we are
building a diagnostics sales team focused on the United States
market.
Geographic
Markets
The following chart presents our total revenues by geographic
market:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Six Months
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Ended
|
|
|
|
|
|
|
|
|
|
|
|
|
|
June 30,
|
|
|
Years Ended December 31,
|
|
|
|
|
2011
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands of Euros)
|
|
|
|
|
European Union
|
|
|
246,144
|
|
|
|
432,191
|
|
|
|
424,591
|
|
|
|
404,099
|
|
|
United States and Canada
|
|
|
267,124
|
|
|
|
339,018
|
|
|
|
296,659
|
|
|
|
290,666
|
|
|
Rest of the World
|
|
|
122,073
|
|
|
|
219,521
|
|
|
|
191,936
|
|
|
|
119,546
|
|
|
Total
|
|
|
635,341
|
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
814,311
|
|
Research
and Development
Research and development is a significant aspect of our business
and is mostly concentrated in the Bioscience division. We also
collaborate from time to time with governmental authorities in
our research and development projects and receive grants
and/or
interest-free preferential loans from Spanish governmental
authorities and
not-for-profit
organizations for use on such projects. As a result of our past
and ongoing investment in research and development, we believe
that we are positioned to continue as a leader in the
plasma-derived therapies industry. Innovation by research and
development operations is critical to our future growth and
ability to remain competitive in our industry. We have a strong
commitment to science and technology with a track record of
accomplishments and pipeline opportunities. In 2010, we spent
more than €36.6 million (3.7% of net revenues) in
research and development. In the six months ended June 30,
2011, Grifols and Talecris combined spent more than
€25.7 million (4.0% of net revenues) in research and
development and had approximately 706 scientists and support
staff dedicated to research and development.
Our principal research and development objectives are developing
new products, researching new applications for existing products
and improving our manufacturing processes to improve yields,
safety and efficiency. The following are our Bioscience division
initiatives:
|
|
|
| |
•
|
Developing new products. We constantly obtain,
purify and inactivate proteins whose therapeutic purpose is
known, such as its use as a fibrin sealant. Fibrin sealant is a
combination of two plasma proteins (fibrogen and thrombin) that
is being developed as an adjunct to surgical hemostasis. When
locally applied, both proteins mix and coagulate producing a
biological adhesive that mimics natural fibrin blood clot. We
are currently carrying out clinical trials for this protein in
vascular, organ and soft-tissue surgery and we estimate launch
of this product in 2014. A Phase I safety study regarding the
safety and efficacy of Plasmin for the treatment of aPAO was
completed. A Phase II study is currently ongoing. We are
also preparing a new catheter design for improved administration
of Plasmin. We are thereby using the experience of our Hospital
and Diagnostic divisions to deliver potential synergies. Among
the additional products under development, the most relevant
are: a topical Thrombin to help stop bleeding during surgery;
Prothrombin Complex with potential indications on reversal of
warfarin overdose in anticoagulated patients or treatment of
Factor VIII inhibitors in hemophilia A; Intravenous fibrinogen,
with indication in congenital and acquired deficiency and,
potentially, in massive bleedings; and a human derived
supplement for cell culture for use in research and,
potentially, for cGMP applications related to, for example:
biotechnology (recombinant protein production), stem cell based
regenerative medicine or gene therapy;
|
129
|
|
|
| |
•
|
Researching new applications for existing
products. We have been conducting clinical trials
for the use of Fanhdi and Alphanate, anti-hemophilic products
that consist of the Factor VIII protein and use the von
Willebrand coagulation factor, on patients who have a von
Willebrand factor deficiency. We have launched this product in
Italy and in the United States and we expect to seek its license
in other countries. We have undertaken several studies to
explore alternative uses for albumin. We began a line of
research focused on the systematic practice of therapeutic
plasmapheresis with albumin in the treatment of Alzheimer’s
disease in 2005 with the participation of the ACE Foundation
(Catalan Institute of Applied Neuroscience) and Hospital Vall
d’Hebron in Barcelona, Hospital Gregorio Marañón
in Madrid, Howard University in Washington, D.C., and the
Mid Atlantic Geriatric Association in New Jersey. The positive
interim results obtained from the first studies were published
in 2009 and led to the design of a further medical study
combining plasmapheresis and intravenous immunoglobulins that
began in 2011. In addition to the Alzheimer’s research, we
signed a collaboration agreement with the Fundacio Clinic per a
la Recerca Biomedica (the Clinic Foundation for Biomedical
Research) to finance two additional lines of albumin research.
The first is an ongoing multicenter trial that uses albumin on
patients with liver cirrhosis and ascites to prevent the
complications inherent to this illness. The second trial
includes conducting plasma replacements in patients with
acute-on-chronic
liver failure and it is expected that the first patient will be
treated during 2010. We are also conducting a pilot randomized
clinical trial with Anbinex (antithrombin) in cardiac surgery
with cardiopulmonary bypass, as well as a study of the efficacy
and safety of Anbinex treatment in patients suffering from
severe burns. We plan to include Talecris’ Thrombate to
these programs.
|
We also have other ongoing clinical trials to expand the range
of indications for existing products, including two in
idiopathic thrombocytopenic purpura with Flebogamma 10% DIF, one
in children with primary immunodeficiency with Flebogamma 5% DIF
and one with Niuliva (hepatitis B intravenous immunoglobulin) in
liver transplantation. There are other clinical trials to extend
our current licenses to other countries in antithrombin III
congenital deficiency, emphysema due to congenital deficiency of
Alpha-1 Antitrypsin and hemophilia A and B.
At a much more basic and earlier (non-clinical) research phase,
we are active with several of our proteins in relevant fields
such as Oncology, Chronic Obstructive Pulmonary Disease,
Regenerative Medicine, Coagulation and some specific potential
applications such as prolongation of the half-life of
coagulation factors or treatment of drug overdoses.
|
|
|
| |
•
|
Improving our manufacturing processes to improve yields,
safety and efficiency. Among the projects related
to improving yields, safety and efficiency, we are developing a
nanofiltered Factor VIII/VWF concentrate that will increase the
already excellent safety margin of our current products while
simplifying process steps, which will improve efficiency without
significantly impacting yields. Research is being done also in
liquid formulations for Alpha-1 Antitrypsin, which would
represent an advantage for the user and reduce production costs
associated with freeze-drying the product. Alternative packaging
materials are being developed for albumin and IVIG, which would
represent an advantage to the user because of ease of use. Also
in development is a nanofiltered albumin preparation that would
improve the already well established safety profile of albumin
and might be of interest for certain end users, like vaccine
manufacturers.
|
Research is also ongoing to improve plasma management and
monitoring worldwide. We completed the plasma bottle sampling
system for use in all of our blood donor centers in the United
States. Additionally, there are ongoing trials with radio
frequency identification in the Los Angeles warehouse and the
collection centers. During the year, pilot tests have been
carried out to study the possible thermodynamic effect of radio
frequency identification on plasma temperature.
The research and development team in the Hospital division
primarily focuses on developing complementary products and on
improving the safety and efficiency of existing ones. In the
fluid therapy market, work continues on the study of stabilities
of various
ready-to-use
mixtures in polypropylene packaging, in order to increase the
range of mixtures available for hospital use. In 2009, we also
began developing physiological
130
saline and 5% glucose electrolyte solutions packaged in
polypropylene bags. These bags are partially filled at different
volumes for the purposes of adding medication.
Research and development in the Diagnostic division primarily
focuses on the development of reagents and equipment for
pretransfusional testing and hemostasis diagnosis. The following
are the principal research and development projects that we are
currently undertaking in the Diagnostic division:
|
|
|
| |
•
|
the continued development of technology associated with the
classification of blood types through the use of gel technology;
|
| |
| |
•
|
a research project to enable us to work with frozen blood bags
to improve the availability and quality of the reagent red cells
manufactured therefrom is ongoing, as diluted red cells are an
important reagent in the screening process of antibodies and
antibody identification. It is estimated the project will be
completed by the end of 2011;
|
| |
| |
•
|
a new stand-alone digital reader will be launched during the
last quarter of 2011 in order to automate the reading and
interpretation process of the gel cards we manufacture and sell;
|
| |
| |
•
|
a new auto analyzer to perform ELISA techniques in microplates.
This analyzer is set to replace the current Triturus, of which
over one thousand units have been sold and placed worldwide.
This new instrument will increase the output and sample and
reagents capacity in comparison to the Triturus, adding some new
features such as continuous loading and unloading of samples and
an innovative simplified fluidic system;
|
| |
| |
•
|
a new Hemostasis Analyzer, complementary to the Q-Coagulometer,
that will be able to serve medium to large laboratories. It will
have about three times the capacity and output of the
Q-Coagulometer;
|
| |
| |
•
|
a newly formulated thromboplastin reagent is close to
completion. Additionally, a new liquid Thrombin reagent of human
origin is in the final stage of development. We are also working
on a new activated cephaline reagent based on synthetic
phospholipids; and
|
| |
| |
•
|
an instrument called Stat, which will be able to read the
results shown by the multicards (rapid blood typing test), is
being developed by our Swiss subsidiary acquired in 2009.
|
While our current protein products are derived from human
plasma, we expect that recombinant technologies will be a major
source of new protein therapies commercialized in the future. We
have initiated recombinant protein development programs for
Plasmin, Factor VIII and A1PI. Recombinant or recPlasmin is a
patentable form of Plasmin that retains key properties of
Plasmin that make it a candidate for development as a therapy
for ischemic stroke. We have filed composition of matter patents
covering recPlasmin. We are conducting pre-clinical development
of recombinant Factor VIII and A1PI utilizing advanced
protein-production technology licensed from Crucell N.V.
Marketing
and Sales
General
We currently sell Bioscience, Hospital and Diagnostic products
to hospitals and clinics, GPOs, governments and other
distributors in over 100 countries.
In the United States, group purchasing organizations, which are
referred to as GPOs, are entities that act as purchasing
intermediaries for their members, which are primarily hospitals,
nursing homes and other healthcare providers. GPOs negotiate the
price and volume of supplies, equipment and pharmaceutical
products, including plasma derivatives, used by their members.
Hospitals report that GPOs save them 10% to 15% on their
purchases. The GPOs’ large market position and their
substantial purchasing volume provide them with significant
negotiating power, resulting in price pressures for
manufacturers like us. Approximately 54% and 45% of our sales in
the United States were made through GPOs in the year ended
December 31, 2010 and the six months ended June 30,
2011, respectively.
131
We market our products to the GPOs’ members and their
clients through focused sales presentations. Although price and
volume are negotiated by the GPO, the actual sales are made to
the GPO’s authorized distributor(s) at the contract price,
and the distributor then sells the products to the GPO’s
members. For safety and post-sale service reasons, the
distributor is required to provide us with the specifics of the
ultimate delivery to the client.
The sales, marketing and distribution process is different in
Europe, where the bulk of sales are generally made directly to
hospitals, with private fractionation companies meeting most of
European demand. We have developed long-standing relationships
with major hospitals in most of our European markets, and we
believe that hospitals are loyal customers that recognize the
high quality and safety of our products, our reliability as a
supplier and the strong product expertise and service provided
by our sales representatives. Due to the nature of our customer
base and the prevalence of repeat sales in the industry, we
market our products through focused sales presentations rather
than by advertising campaigns. In addition, we provide plasma
fractionation and viral inactivation services to Spanish, Czech
and Slovak hospitals and clinics.
Sales to Eastern Europe, the Middle East and Japan are made
mostly by third parties outside of our sales network. Our sales
in Latin America are made mainly by our sales network.
Sales
Representatives
We require our sales representatives to be able to highlight the
technical differences between our products and those of our
competitors. This requires a high degree of training as the
salesperson has to be able to interact and discuss the
differences with doctors, pharmacists and other medical staff.
Sales representatives call on departmental heads, purchasing
agents, senior hospital directors and managers. We compensate
our sales representatives by means of a fixed salary and a bonus
component based on sales. We divide our sales efforts along the
lines of our main product categories. Our sales personnel are
primarily located in Europe and the United States, but we also
have sales personnel in Latin America and Asia.
As of June 30, 2011, we had approximately 372 sales
personnel in the Bioscience division, approximately 104 sales
personnel in the Hospital division, approximately 110 sales
personnel in the Diagnostic division and approximately 50 sales
support personnel. Additionally, we have approximately 121
post-sale service personnel that install diagnostic equipment,
train users and provide post-sale assistance to customers.
Customers
No single customer accounted for more than 4% of our total
revenue in both the year ended December 31, 2010 and the
six months ended June 30, 2011. Our top ten customers
accounted for approximately 22% and 21% of our total revenue in
each of the year ended December 31, 2010 and the six months
ended June 30, 2011, respectively. However, we anticipate
that we will experience an increase in customer concentration as
our net sales in the United States grow as a percentage of our
total sales.
Since the late 1980s Talecris has been the “supplier of
record” for the Canadian blood system. Under existing
contracts, we are the largest supplier of plasma-derived
products to the Canadian blood system operators, Canadian Blood
Services and Hema Quebec. We transport plasma from Canadian
Blood Services and Hema Quebec collection centers to our
manufacturing facility in Clayton, North Carolina for
manufacture, and return the finished product, along with
commercial product, for sale to Canadian Blood Services and Hema
Quebec. Pricing for our products and services is set at the
beginning of the contract period, subject to adjustment for
inflation. The U.S. dollar based contracts are terminable
upon default, or the occurrence of certain events, including a
third party obtaining Canadian regulatory approval to introduce
a significantly superior product or fractionation service, our
products or services becoming obsolete, or if we make certain
nonrelated improvements and Canadian Blood Services or Hema
Quebec do not accept the associated price increase. Talecris
awarded new five year contracts in December 2007, which became
effective April 1, 2008. The contracts may be extended for
two one-year terms upon agreement of the parties. Under these
contracts, we fractionate 100% of the Canadian plasma initially
and a majority of the Canadian plasma throughout the contract
period and supply a majority of the Canadian requirements for
IGIV during the contract term as well. Canadian Blood Services
has elected to pursue a multi-source strategy and although we
will continue to be the
132
primary supplier, we anticipate annual volume declines because
of their strategy. Hema Quebec currently has a sole source
strategy for fractionation of their plasma but could switch to a
multi-source strategy. In 2010, Talecris fractionated 71% of
Canadian plasma and supplied 67% of Canadian requirements of
IGIV.
Advertising
We do not conduct any widespread advertising. Instead, we
participate in medical conferences and fairs and occasionally
publish advertisements in medical magazines.
Distribution
We believe that having our own distribution network staffed with
highly trained personnel is a critical element of a successful
sales and marketing effort. Through this network, we are able to
provide high-quality pre and post-sales service, which we
believe enhances brand recognition and customer loyalty. Our
distribution network is experienced in the proper handling of
our products and allows us to know where our products are
located, enabling us to act quickly in the event of a suspected
problem or product recall.
Our sales, marketing and distribution network includes
approximately 128 employees. Our distribution network
personnel are located in Europe, Latin America, the United
States and Asia and handle the distribution of our
biopharmaceutical and other medical products as well as goods
manufactured by other premier healthcare companies which
complement our own products.
During 2010, we distributed products through our own
distribution network. In some cases, particularly in the field
of Diagnostics, we distribute products through marketing
partners and third-party distributors. We have a direct presence
in 24 countries and we have carefully selected distributors in
more than 100 countries around the world. We have a responsive,
effective logistics organization that is able to meet the needs
of hospital centers throughout the world punctually.
Each of our commercial subsidiaries is responsible for the
requirements of the local market. It is our goal that each
commercial subsidiary is recognizably one of our companies by
its quality of service, ethical standards and knowledge of
customer’s needs. This strong local knowledge enables us to
build and maintain long term relationships with customers in the
hospital to earn their trust and confidence.
Patents
and Trademarks
General
As of June 30, 2011, Grifols owned approximately 673
patents and patent applications, of which approximately 184 are
in the process of final approval. These patents are granted a
20-year
protection period. Only approximately 222 of these patents are
set to expire in the next 10 years. As of June 30,
2011, we also owned approximately 1,568 trademarks, of which
approximately 158 are in the process of final approval. We are
in the process of including the Talecris intellectual property
into our portfolio and therefore, its intellectual property is
not included herein.
We maintain a department with personnel in Spain and in the
United States to handle the patent and trademark approval and
maintenance process and to monitor possible infringements. We
are not aware of any infringements of our patents and trademarks
and we do not license any of our patents to third parties.
Plasma
Derivative Products
As of June 30, 2011, we owned approximately 438 patents and
patent applications related to plasma derivatives. The most
important of these patents include:
|
|
|
| |
•
|
Process for the production of virus-inactivated human
Gammaglobulin G. We have patents for this process in Argentina,
Austria, Belgium, Chile, Czech Republic, Finland, France,
Germany, Greece, Hong Kong, Hungary, Ireland, Italy, Japan,
Mexico, Netherlands, Portugal, Slovakia, Spain, Sweden,
Switzerland/Liechtenstein, Turkey, United Kingdom, Uruguay and
the United States which are either pending registration or are
set to expire between 2022 and 2024;
|
133
|
|
|
| |
•
|
Use of Therapeutic Human Albumin for the preparation of a drug
for the treatment of patients suffering from cognitive
disorders. We have patents for this process in Argentina,
Australia, Brazil, Canada, Chile, China, the European Union,
Hong Kong, Japan, Mexico, New Zealand, Russia, Spain, Uruguay
and the United States which are either pending registration or
are set to expire between 2027 and 2028; and
|
| |
| |
•
|
Process for removing viruses in fibrinogen solutions. We have
patents for this process in Argentina, Australia, Austria,
Belgium, Brazil, Canada, Chile, Czech Republic, Finland, France,
Germany, Greece, Hungary, Ireland, Italy, Japan, Mexico,
Netherlands, New Zealand, Poland, Portugal, Slovakia, Spain,
Sweden, Switzerland/Liechtenstein, Turkey, United Kingdom and
the United States which are either pending registration or are
set to expire in 2024.
|
Hospital
and Diagnostic Products
As of June 30, 2011, we owned approximately 235 patents and
patent applications related to our Hospital and Diagnostic
products. The most important of these patents include:
|
|
|
| |
•
|
Process for the sterile filling of flexible material bags
(Grifill). We have patents for this apparatus in Austria,
Belgium, Chile, Denmark, France, Germany, Italy, Japan, Mexico,
Netherlands, Portugal, Spain, Sweden, Switzerland/Liechtenstein,
United Kingdom and the United States which are set to expire
between 2014 and 2022;
|
| |
| |
•
|
Bags with filtration system (Gribag). We have patents for this
vessel in France, Germany, Italy, Japan, Portugal, Spain,
Switzerland/Liechtenstein and the United Kingdom which are set
to expire between 2012 and 2014;
|
| |
| |
•
|
Instrument for the precise low rate administration of parenteral
solutions without the need for mechanical equipment (Griflow).
We have patents for this process in Austria, Belgium, Brazil,
France, Germany, Italy, Japan, Mexico, Portugal,
Switzerland/Liechtenstein, United Kingdom and the United States
which are set to expire between 2011 and 2014;
|
| |
| |
•
|
Wadiana machine for clinical analysis. We have patents for this
apparatus in Argentina, Austria, Belgium, Brazil, Chile, France,
Germany, Italy, Japan, Mexico, Netherlands, Portugal, Slovakia,
Spain, Switzerland/Liechtenstein, United Kingdom and the United
States which are set to expire between 2018 and 2023;
|
| |
| |
•
|
Triturus machine for automated laboratory tests. We have patents
for this apparatus in Argentina, France, Germany, Italy, Japan,
Mexico, Spain, United Kingdom and the United States which are
set to expire in 2018;
|
| |
| |
•
|
Centrifuge machine for clinical analysis, which we have a patent
for in Spain and which is set to expire in 2018;
|
| |
| |
•
|
Blister handling machine (Blispack). We have patents for this
apparatus in Argentina, Brazil, Chile, Spain, the European Union
and Mexico which are either pending registration or are set to
expire in 2028;
|
| |
| |
•
|
Q Coagulometer. We have patents for this apparatus in Spain, the
European Union, Japan and the United States which are either
pending registration or are set to expire in 2025; and
|
| |
| |
•
|
Erytra apparatus for the automatic analysis of samples on gel
cards. We have patents for this apparatus in Argentina,
Australia, Brazil, Canada, Chile, China, Spain, the European
Union, Hong Kong, Japan, Mexico and the United States which are
either pending registration or are set to expire in 2029.
|
Licenses
from Third Parties
We use certain technologies developed by third parties through
licensing arrangements that provide for our payment of royalties
to the patent holder. We currently use only one non-exclusive
license for the viral inactivation of plasma for transfusions
through the individual treatment of plasma units granted by the
German Red Cross and covering the territories of Spain,
Portugal, the Czech Republic, Argentina, Chile and Mexico.
134
The method used is photo-inactivation in the presence of
methylene blue and light radiation. Exclusivity rights under the
related patent and, consequently, our obligation to pay
royalties for this license expired in August 2011.
We license from Bayer HealthCare LLC certain intellectual
property rights. Under the licensing agreement with Bayer
Healthcare, we were granted a royalty-free, worldwide and
perpetual license covering certain intellectual properties not
acquired by us in connection with our formation transaction. As
amended on August 10, 2007, the agreement permits us,
subject to specified limitations, to grant a sublicense to
Baxter International Inc., Baxter Healthcare Corporation, and
certain of their affiliates relating to certain Japanese patent
rights not acquired by us in connection with our formation
transaction.
Property,
Plant and Equipment
Our headquarters are located in Barcelona, Spain. As of
June 30, 2011, we leased or owned facilities in 24
countries. We currently own or lease nine manufacturing
facilities in seven locations, four of which have plasma
fractionation capabilities. The table below shows the geographic
location of each manufacturing facility, the products
manufactured, the number of units manufactured and current
manufacturing capacity, as well as other facilities of the
company.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010 Actual
|
|
Current Installed
|
|
Complex
|
|
Location and Size
|
|
Products(1)
|
|
Production(2)
|
|
Annual Capacity(2)
|
|
|
|
Industrial Complex One Parets
|
|
Barcelona, Spain 58,285 square meters of which we own
45,647 square meters
|
|
Fanhdi Factor VIII
Albumin Grifols
Anbin - Antithrombin III
Flebogamma IVIG
Intramuscular
Gammaglobulins
Trypsone - A1PI
Novix Factor IX
|
|
1,933 liters
1,888 liters
70 liters
2,585 liters
54 liters
283 liters
40 liters
|
|
2,450 liters
2,200 liters
400 liters
4,100 liters
450 liters
450 liters
60 liters
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex Two Parets
|
|
Barcelona, Spain 35,525 square meters of which we own
19,853 square meters
|
|
Parenteral Solutions
Gel Cards
|
|
21.3 million rigid bottles
12 million units
|
|
45 million rigid
bottles
18 million units
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex Three Parets
|
|
Barcelona, Spain 40,113 square meters which we rent
|
|
Plasma Storage
|
|
0.6 million liters
|
|
0.8 million liters
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex USA
|
|
Los Angeles, California 93,078 square meters which we own
|
|
Albutein Albumin
Alphanate Factor VIII
Alphanine IX
Profilnine
|
|
1,340 liters
1,223 liters
125 liters
51 liters
|
|
1,500 liters
2,000 liters
500 liters
100 liters
|
|
|
|
|
|
|
|
|
|
|
|
Clayton Facility
|
|
Clayton, North Carolina 69,203 square meters of which we
own 60,771 square meters
|
|
Koate Factor VIII
Plasbumin/Plasmanate-Fraction V
Antithrombin III
Gamunex-C IVIG
Intramuscular
Gammaglobulins Prolastin/
Prolastin-C A1PI
|
|
2,200 liters
2,376 liters
220 liters
3,381 liters
60 liters
3,012 liters
|
|
2,700 liters
2,400 liters
1,700 liters
5,200 liters
350 liters
3,503 liters
|
135
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010 Actual
|
|
Current Installed
|
|
Complex
|
|
Location and Size
|
|
Products(1)
|
|
Production(2)
|
|
Annual Capacity(2)
|
|
|
|
City of Industry USA
|
|
Temple, California 5,000 square meters which we rent
|
|
Plasma storage
|
|
400 liters
|
|
1,200 liters
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex One Murcia
|
|
Murcia, Spain 10,285 square meters which we rent
|
|
Blood bags
|
|
6 million equivalent units
|
|
8 million equivalent units
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex Two Murcia
|
|
Murcia, Spain 26,873 square meters which we own
|
|
Parenteral and intravenous solutions
|
|
21.5 million flexible containers
|
|
27 million flexible containers
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex Switzerland
|
|
Fribourg, Switzerland 12,000 square meters which we rent
|
|
Multicards
|
|
0.2 million units
|
|
2.5 million units
|
|
|
|
|
|
|
|
|
|
|
|
Industrial Complex Australia
|
|
Melbourne, Australia 3,838 square meters which we own
|
|
Gel Cards
|
|
1 million units
|
|
7.0 million units
|
|
|
|
|
|
|
|
|
|
|
|
Plasma Testing Lab Austin, TX
|
|
Austin, TX, USA 2,235 square meters which we rent
|
|
Plasma testing
|
|
N/A
|
|
N/A
|
|
|
|
|
|
|
|
|
|
|
|
Plasma Testing Lab San Marcos, TX
|
|
San Marcos, TX, USA
7,670 square meters which we own
|
|
Plasma testing
|
|
N/A
|
|
N/A
|
|
|
|
|
|
|
|
|
|
|
|
Headquarters Sant Cugat Barcelona
|
|
Sant Cugat, Spain 32,211 square meters which we rent
|
|
Headquarters
|
|
N/A
|
|
N/A
|
|
|
|
|
|
|
|
|
|
|
|
Plasma Testing Lab Raleigh, North Carolina
|
|
Raleigh, North Carolina 7,061 square meters which we rent
|
|
Plasma testing
|
|
N/A
|
|
N/A
|
|
|
|
|
|
|
|
|
|
|
|
Melville Facility
|
|
Melville, New York 9,562 square meters which we rent
|
|
Plasma fractionation
|
|
1,286 liters
|
|
1,550 liters
|
136
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010 Actual
|
|
Current Installed
|
|
Complex
|
|
Location and Size
|
|
Products(1)
|
|
Production(2)
|
|
Annual Capacity(2)
|
|
|
|
Benson
|
|
Benson, North Carolina 3,642 square meters which we rent
|
|
Plasma storage
|
|
758 liters
|
|
1,200 liters
|
|
|
|
|
(1)
|
|
For diagnostic products, other than
blood bags, there is not a meaningful installed capacity
measurement as each unit is built and assembled individually.
|
| |
|
(2)
|
|
In thousands of liters, except as
indicated.
|
The Parets del Vallès plant has an estimated fractionation
capacity of 2.1 million liters per year, while the Los
Angeles plant has an estimated fractionation capacity of
2.2 million liters per year. The Clayton facility has an
estimated fractionation capacity of 2.6 million liters per
year. The Melville facility has an estimated fractionation
capacity of 1.6 million liters per year. In 2010, we
fractionated 1.9 million liters, 1.2 million liters,
2.5 million liters and 1.3 million liters of plasma at
the Parets del Vallès, Los Angeles, Clayton and Melville
plants, respectively. In the six months ended June 30,
2011, we fractionated 1.0 million liters, 0.6 million
liters, 1.1 million liters and 0.7 million liters of
plasma at the Parets del Vallès, Los Angeles, Clayton and
Melville plants, respectively.
In addition to the plasma fractionation facilities, the Parets
del Vallès plant also has energy generation, research and
development, packaging and storage facilities for the Bioscience
division and the Hospital division.
The manufacturing facilities in Parets del Vallès and Las
Torres de Cotillas, Spain meet all the regulations and standards
of the European health authorities. In addition, the Instituto
Grifols plant in Parets del Vallès holds an establishment
license granted by the FDA in 1995, as well as an ISO 9001
certification for its parenteral solutions and diagnostic
manufacturing facilities. The manufacturing facilities in Los
Angeles, California are subject to regulation by the FDA and are
currently operating under a consent decree obtained by the FDA
and the United States Department of Justice. See the section
entitled “— Legal Proceedings — Alpha
Consent Decree.”
We lease most of our plasma collection centers as well as our
main laboratory facility located in Austin, Texas. We believe
that we maintain licenses with the appropriate regulatory
authorities, including the FDA, for all of these locations.
Insurance
Coverage
The following describes the most significant risks that we face
in our operations and our insurance coverage contracted to
mitigate such risks.
General
and Product Liability
We have a program of insurance policies designed to protect us
and our subsidiaries from product liability claims. The program
expires in May 2012. Our protection from product liability
exposure includes worldwide coverage against claims brought by
persons who, because of their use of our products, become
infected by any product we sold. The maximum amount of coverage
for product liability claims is €105 million per claim
per year. However, for claims for HIV and hepatitis B or C
infections, the maximum aggregate amount covered is up to
€13.0 million. See the subsection below entitled
“— Self-insurance.”
Our master liability program also protects us and our
affiliates, except for our United States operations, from
liability for environmental damages. This risk is covered up to
a maximum of €13.0 million.
Biomat USA, PlasmaCare and Talecris Plasma Resources are not
covered by our master liability insurance program and they
maintain a separate liability insurance policy with Beazley
Insurance Company.
137
The policy covers the plasmapheresis business activities and
expires in May 2012. The maximum amount of coverage for
liability claims under the policy is $10.0 million per
claim per year.
Property
Damage and Business Interruption
Our property damage and business interruption master insurance
policy covers us and our subsidiaries (including the United
States subsidiaries) and expires in May 2012. This master policy
covers damages suffered by plants and buildings, equipment,
machinery, raw materials, supplies, semi-finished products and
finished products. Under the terms of this master policy, the
insurer will cover damages produced by fire, smoke, lightning
and explosions, among others, for up to $500.0 million in
the United States and up to €360.0 million for the
rest of the world. It also covers material damages or losses
produced by equipment or machinery breakdown, flooding and
robbery or looting, among others, for up to
€40.0 million, €20.0 million, and
€2.0 million, respectively.
In addition, this policy covers loss of profit for a period of
indemnity of 24 months with a deductible equivalent to up
to 5 business days of lost profits for our Spanish and United
States subsidiaries. Pursuant to the loss of profit benefit, in
the event that any or all of our plants stop production due to
an event not excluded under the policy, the insurer must cover
fixed expenses, in addition to net profits we did not earn
during the term of coverage.
Self-insurance
We are self-insuring part of the risks described above through
the purchase of a portion of the relevant insurance policies by
Squadron Reinsurance., Ltd., one of our wholly owned
subsidiaries. We self-insure the first €10.0 million
per claim per year of our product liability policy and the first
€200,000 per loss for property damage and the first
10 days of lost profits. These amounts are in addition to
the deductibles for each of the policies that make up our
insurance coverage programs.
Employees
As of June 30, 2011, we had 11,174 employees. The
table below shows the number of employees as of such date by
geographic location and the function they perform.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
|
Marketing
|
|
|
|
|
|
R&D and
|
|
|
|
|
|
Senior
|
|
|
Location
|
|
Employees
|
|
|
and Sales
|
|
|
Production
|
|
|
Technical
|
|
|
Administrative
|
|
|
Management
|
|
|
|
|
Spain
|
|
|
2,383
|
|
|
|
255
|
|
|
|
1,466
|
|
|
|
246
|
|
|
|
374
|
|
|
|
42
|
|
|
Rest of European Union
|
|
|
275
|
|
|
|
151
|
|
|
|
28
|
|
|
|
27
|
|
|
|
53
|
|
|
|
17
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total European Union
|
|
|
2,658
|
|
|
|
406
|
|
|
|
1,493
|
|
|
|
273
|
|
|
|
427
|
|
|
|
59
|
|
|
United States
|
|
|
8,282
|
|
|
|
306
|
|
|
|
7,032
|
|
|
|
420
|
|
|
|
452
|
|
|
|
71
|
|
|
Rest of the World
|
|
|
234
|
|
|
|
143
|
|
|
|
23
|
|
|
|
13
|
|
|
|
43
|
|
|
|
11
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
11,174
|
|
|
|
855
|
|
|
|
8,548
|
|
|
|
706
|
|
|
|
922
|
|
|
|
142
|
|
Our employees, other than those added in the Talecris
acquisition, have an average of 6 years with Grifols.
We actively train our employees. The Grifols Academy of
Plasmapheresis opened in Spain during the second quarter of
2011. It is a meeting point for advanced training on all
processes related to the preparation and production of
plasma-derived medicines. In addition, the “Grifols
Academy” will be a dissemination center for scientific and
business knowledge, fostering a continued exchange among experts
and other external bodies, such as healthcare professional
associations, hospitals, schools and universities, among others.
Our commitment to fostering training initiatives is consistent
with the degree of specialization required by the hemoderivative
industry. In 2009, we opened the “Grifols Academy of
Plasmapheresis” in Phoenix (Arizona, USA), which has been
attended by 905 participants and at which over 8,628 hours
of training were taught in 2010.
Our Spanish employees are represented by two labor unions, the
Workers’ Commissions (Comisiones Obreras) and the
Workers General Union (Unión General de Trabajadores).
The remainder of our employees
138
are not represented by labor unions. Only the employees of our
subsidiaries in Spain, Germany, Italy, France, Argentina and
Brazil are covered by collective bargaining agreements. We have
not experienced any significant work stoppages in the last
15 years, except for a
one-day
general strike in Spain in June 2002. We consider our employee
relations to be good.
We subscribe to an insurance policy that covers death or
permanent disability of employees caused by work accidents. All
of our employees are covered under this policy. We implemented a
pension plan in all our Spanish entities beginning on
January 1, 2002, which excludes top management and which
requires us to make matching payments to these employees.
Legal
Proceedings
General
We are involved in various legal proceedings in the ordinary
course of our business. In the event of adverse outcomes of
these proceedings, we believe that resulting liabilities will
either be covered by insurance or not have a material adverse
effect on our financial condition or results of operations.
Alpha
Consent Decree
The Los Angeles fractionation plant has been operating since
1998 under a consent decree agreed to by Alpha, the operator of
the plant at that time, the FDA and the United States Department
of Justice as a result of certain quality deficiencies. Namely,
Alpha was noncompliant with FDA mandated good manufacturing
practices. We acquired this plant from Alpha in 2003 with the
consent decree in place. The consent decree provides for annual
inspection of the plant by the FDA. On February 17, 2006,
the FDA advised us that they would no longer require third party
review and approval of product lots prior to their respective
releases, as originally required under the consent decree.
We have worked with the FDA to reconcile any deficiencies
identified by the FDA and believe that we currently maintain
licenses with all appropriate regulatory authorities, including
the FDA, for this location. We believe that we have
significantly improved manufacturing standards at the Los
Angeles plant. We have invested in the upgrade of the sterile
filling areas of the plant and are planning further capital
investments with a view toward the removal of the consent decree
in the medium term. However, we cannot assure you that the
consent decree will be removed in such period or at all, and
while the consent decree remains in place we will continue to
incur increased costs and expend administrative resources
related to it. Although we cannot guarantee if or when the
consent decree will be lifted, based on the current level of
compliance, there are no commercial activities that are
prohibited or limited by the consent decree.
Hemophilia
Associations
Since the 1980s, it has been alleged that hemophiliacs became
infected with hepatitis C
and/or the
HIV virus by using clotting factor concentrates derived from
human plasma, like our Factor VIII products. Beginning in 1997
the Spanish Hemophilia Association (Federación
Española de Hemofilia) has periodically approached us
seeking contributions on behalf of Spanish hemophiliacs who had
been infected during the 1980s with hepatitis C
and/or the
HIV virus. Neither the Spanish Hemophilia Association nor any of
its members has ever commenced formal legal proceedings against
us (except for three cases brought by members that were
adjudicated in our favor). We have been involved in several
legal proceedings (acto de conciliación) relating to
such matters.
In February 2005, a claimant brought a claim against the Health
Board of Castilla y Leon claiming €180,000 in damages due
to the alleged contraction of hepatitis C and the health
authorities requested that this claim be extended to include us.
We contested this claim and the claim was rejected by the
Administrative Court in February 2011. The claimant has filed
another appeal before the Supreme Court which is pending
resolution.
In 2007, we were notified of a claim for maximum damages of
€12,690,000 filed by a group of 100 Catalan hemophiliacs
against all plasma fractionation companies. During 2008, this
claim was rejected. The ruling was appealed but the claim was
rejected by the Appeals Court in January 2011. The claimant has
filed another appeal before the Supreme court which is pending
resolution.
139
Plasma
Centers of America, LLC and G&M Crandall Limited Family
Partnership
Talecris had a three year Amended and Restated Plasma
Sale/Purchase Agreement with Plasma Centers of America, LLC
(“PCA”) under which Talecris was required to purchase
annual minimum quantities of plasma from plasma collection
centers approved by Talecris, including the prepayment of 90%
for unlicensed plasma. Talecris was also committed to finance
the development of up to eight plasma collection centers, which
were to be used to source plasma for Talecris. Under the terms
of the agreement, Talecris had a conditional obligation to
purchase such centers under certain conditions for a sum
determined by a formula set forth in the agreement. Talecris
provided approximately $4 million (excluding accrued
interest) in financing related to the development of such
centers and advanced payments for unlicensed plasma. Talecris
recorded a provision within SG&A during 2008 related to
loans and advances provided.
In August 2008, Talecris notified PCA that they were in breach
of the Amended and Restated Plasma Sale/Purchase Agreement.
Talecris terminated the agreement in September 2008. In November
2008, TPR filed suit in federal court in Raleigh, North Carolina
against the G&M Crandall Limited Family Partnership and its
individual partners as guarantors of obligations of PCA.
Talecris was served in January 2009 in a parallel state action
by PCA, alleging breach of contract by TPR. The federal case has
been stayed. On December 13, 2010, a jury in the state
court case rendered a verdict in the amount of $37 million
in favor of PCA against TPR in a breach of contract claim.
Talecris is evaluating its response to this verdict, including
post-trial motions and appeal. Interest on the verdict, if
sustained, will accrue at 8% simple interest from the date of
breach.
Foreign
Corrupt Practices Act Investigation
We are continuing an internal investigation into potential
violations of the FCPA at Talecris which occurred prior to the
acquisition. In July 2009, Talecris voluntarily contacted the
DOJ to advise them that they were conducting an internal
investigation into potential violations of the FCPA. The FCPA
investigation is being conducted by outside counsel under the
direction of our Board of Directors. The investigation into
possible improper payments to individuals and entities made
after Talecris’ formation initially focused on payments
made in connection with sales in certain Eastern European and
Middle Eastern countries, primarily Belarus, Russia, and Iran,
but we are also reviewing sales practices in Brazil, Bulgaria,
China, Georgia, Libya, Poland, Turkey, Ukraine, and other
countries as deemed appropriate. The DOJ has not indicated what
action it may take, if any, against us or any individual, or the
extent to which it may conduct its own investigation. Even
though Talecris self-disclosed this matter to the DOJ and we
continue to cooperate with the DOJ on this matter, it or other
federal agencies may seek to impose sanctions on us that may
include, among other things, debarment, injunctive relief,
disgorgement, fines, penalties, appointment of a monitor,
appointment of new control staff, or enhancement of existing
compliance and training programs. Other countries in which we do
business may initiate their own investigations and impose
similar penalties. As a result of this investigation, shipments
to some of these countries have been suspended while we put
additional safeguards in place. In some cases, safeguards
involved terminating consultants and suspending relations with
or terminating distributors in countries under investigation as
circumstances warranted. Talecris resumed sales in countries
where they believed that they had appropriate safeguards in
place and we are reallocating products to other countries as
necessary. We made an initial presentation of some of the
findings of the internal FCPA investigation to the DOJ in July
2011. We will continue to present our findings from the
investigation to the DOJ.
Compliance
with Pharmaceutical Pricing Agreement
In November 2009, Talecris received a letter from the USAO for
the Eastern District of Pennsylvania. The USAO requested a
meeting to review the Talecris’ compliance with the terms
of the PPA under the Public Health Service program.
Specifically, the USAO asked for information related to the sale
of Talecris’ IGIV product, Gamunex, under that program. In
order to have federal financial participation apply to their
products under the Medicaid program and to obtain Medicare
Part B coverage, manufacturers are required to enter into a
PPA. The PPA obligates manufacturers to charge covered entities
the Public Health Service price for drugs intended for
outpatient use. The Public Health Service price is based on the
Medicaid rebate amount. We believe that we have complied with
the terms of the PPA and federal law. If the USAO determines
that Talecris’ practices were inconsistent with the terms
of the PPA, the USAO has stated that it may file a civil action
against Talecris under
140
the Anti-fraud Injunction Act and seek a court order directing
Talecris to comply with the PPA or, potentially, proceed under
some other legal theory. We could also be subject to fines,
damages, penalties, appointment of a monitor, or enhancement of
existing compliance and training programs as a result of
government action. We are cooperating with the investigation and
intend to respond to information requests from the USAO. We
believe that Talecris complied with the terms of the PPA and
federal law.
Antitrust
approval of Talecris-Grifols Merger
On April 29, 2011, Grifols, S.A. and Talecris entered into
a Consent Agreement with the staff of the Bureau of Competition
of the FTC which provided for certain conditions to the closing
of the acquisition. The FTC accepted the Consent Agreement for
public comment on May 31, 2011. The Consent Agreement
required us to divest certain assets to Kedrion. Specifically,
we agreed to sell Kedrion (i) Talecris’ fractionation
facility located in Melville, New York; (ii) two plasma
collection centers located in Mobile, Alabama, and Winston
Salem, North Carolina; (iii) an agreed quantity of plasma;
and (iv) the exclusive right to sell, in the United States,
the Factor VIII product previously sold under Talecris’
brand name Koate. The Consent Agreement permits us to lease the
Melville facility back from Kedrion for up to four years. In
addition, the Consent Agreement required us to enter into
various Divestiture Agreements with Kedrion to implement the
Consent Agreement, including (i) a contract manufacturing
agreement under which for seven years we will manufacture at
least 300,000 plasma liter equivalents of (x) Koate,
(y) private label IVIG and (z) private label albumin,
for sale by Kedrion in the United States; and (ii) a
five-year option for Kedrion to purchase a non-exclusive license
to Koate-related intellectual property for use in the United
States. The Consent Agreement also required the appointment of
an independent monitor to oversee our compliance and requires us
to submit periodic reports to the FTC setting forth in detail
the manner and form in which we intend to comply, are complying,
and have complied with the Consent Agreement. As required by the
Consent Agreement we satisfied all necessary conditions within
ten days of the completion of the acquisition. Our next
compliance report to the FTC is due in 2012.
Shareholder
Litigation Regarding the Talecris-Grifols Merger
Four purported class action lawsuits were filed by Talecris
stockholders challenging the acquisition. Two of the lawsuits
were filed in the Court of Chancery of the State of Delaware and
have been consolidated under the caption In re Talecris
Biotherapeutics Holdings Shareholder Litigation, Consol. C.A.
No. 5614-VCL.
The other two lawsuits were filed in the Superior Court of the
State of North Carolina and are captioned Rubin v. Charpie,
et al., No. 10 CV 004507 (North Carolina Superior Court,
Durham County), and Kovary v. Talecris Biotherapeutics
Holdings Corp., et al., No. 10 CV 011638 (North Carolina
Superior Court, Wake County). The lawsuits name as defendants
Talecris, the members of the Talecris Board of Directors,
Grifols, S.A. and its subsidiary, Grifols Inc., and, in the
Delaware consolidated action, Talecris Holdings and Stream
Merger Sub, Inc. The two North Carolina actions have been stayed.
All of the lawsuits allege that the individual defendants (and,
in the consolidated Delaware action, Talecris Holdings) breached
their fiduciary duties to the Talecris stockholders in
connection with the proposed transaction with Grifols, and that
Grifols (and, in one of the North Carolina cases, Talecris, and
in the Delaware action, Grifols Inc.) aided and abetted those
breaches. The Delaware complaint alleges, among other things,
that the consideration offered to Talecris stockholders pursuant
to the proposed transaction was inadequate; that the Talecris
Board of Directors failed to take steps to maximize stockholder
value; that Talecris’ initial public offering and debt
refinancing in 2009 were intended to facilitate a sale of
Talecris; that Cerberus and Talecris Holdings arranged the
proposed merger for the benefit of Cerberus, without regard to
the interests of other stockholders; that the voting agreements
impermissibly locked up the transaction; that the Merger
Agreement contained terms, including a termination fee, that
favor Grifols and deter alternative bids; and that the
preliminary
Form F-4
filed on August 10, 2010 contained material misstatements
and/or
omissions, including with respect to the availability of
appraisal rights in the merger; the purpose and effects of the
Virginia reincorporation merger; the antitrust risks of the
proposed transaction; the financial advisors’ analyses
regarding the Grifols’ non-voting stock to be issued in
connection with the transaction; and the fees to be paid to
Morgan Stanley by Talecris and Grifols in connection with the
proposed transaction. The Delaware complaint also alleges that
Talecris stockholders are entitled to appraisal rights in
connection with the transaction pursuant to Section 262 of
the Delaware General Corporation
141
Law, and that the transaction violates the Delaware General
Corporation Law by failing to provide such rights. The Delaware
action seeks equitable and injunctive relief, including a
determination that the stockholders have appraisal rights in
connection with the merger, and damages.
On October 29, 2010, the parties to the Delaware litigation
entered into a Memorandum of Understanding (“MOU”),
reflecting an agreement in principle to settle that litigation.
On January 24, 2011, the parties filed a settlement
agreement with the Delaware Chancery Court, providing for the
complete settlement of the Delaware and the North Carolina
actions. The settlement provides, among other things, for the
provision of appraisal rights in accordance with DGCL 262 in
connection with the transaction; for an increase in the merger
consideration by an additional 500,000 shares of Grifols
non-voting shares to holders of Talecris common stock other than
the Talecris specified affiliates; and for certain additional
disclosures provided therein. The settlement also provides for a
dismissal of the Delaware and North Carolina actions with
prejudice and a release of claims. The Delaware Chancery Court
approved the settlement agreement on October 17, 2011. The
Delaware litigation was thereby formally settled and dismissed
and the North Carolina actions are in the process of being
dismissed.
142
INDUSTRY
OVERVIEW
The
Plasma Industry
We operate within the plasma industry. We refer to our
operations pertaining to the plasma industry as our
“Bioscience Division.” We derive most of our industry
related data from the Marketing Research Bureau
(“MRB”) reports. The MRB publishes a study of the
worldwide plasma-derivative product market once every three
years. They also publish a study of the U.S. market once a
year. The information included in this prospectus is from the
worldwide study of the year 2008, which was published in 2009
and the U.S. study of the year 2010, which was published in
2011.
Plasma
Source and Collection
Plasma derivatives are proteins that are found in human plasma
and that, once isolated and purified, have therapeutic value.
Plasma, a liquid that accounts for approximately 50% of blood,
is obtained after separation via centrifugation of red blood
cells, white blood cells and platelets. Proteins are the key
component of plasma, accounting for 7% of plasma’s
composition (water accounts for 90% of plasma’s
composition). Proteins are made of albumin, which accounts for
60% of protein volume, globulins (IVIG), which account for 15%,
coagulation factors, which account for 1%, and other proteins,
which account for the remaining 24%. There are hundreds of
proteins present in plasma; however, only a handful of these
proteins have been developed for therapeutic applications to
date.
The plasma industry is characterized by essential raw materials
(representing greater than 50% of costs on average), with access
to raw materials important to growth. Plasma can be obtained
from three main sources: long-term blood supply agreements with
blood donation organizations, plasma collection centers and
third-party suppliers. There are two main methods for obtaining
plasma, the “plasmapheresis” method, which is the main
source of plasma for the United States and internationally, and
the traditional method.
Plasmapheresis was invented by Dr. Grifols in 1949. Plasma
obtained through plasmapheresis is referred to as “source
plasma.” Through this method, plasma is mechanically
separated from the cellular elements of blood (such as red and
white cells and platelets) through centrifugation or membrane
filtration at the time the donation is made. These cellular
elements are then returned to the donor as part of the same
procedure. Because blood cells are returned, it is possible for
individuals to donate plasma up to twice per week, making this
method more viable than the traditional method for obtaining
plasma. The traditional method is through the separation of
plasma from blood obtained from a blood donation, referred to as
“recovered plasma.” Although recovered plasma may be
used in the production of plasma derivatives, the amount of
plasma obtained through this method is insufficient to cover the
existing demand for plasma because donors are limited to making
one donation every three months.
In order to prevent the deterioration of coagulation factors,
plasma is typically frozen as soon as possible after collection.
Source plasma is generally frozen within six hours following
donation, whereas recovered plasma must first be separated from
the blood cells and frozen within 24 to 72 hours if
intended for the fractionation and purification of proteins.
According to the MRB, the human plasma-derived products industry
has demonstrated revenue growth at a compound annual rate of
approximately 6.8% from 1994 through 2008 with worldwide sales
of approximately $11.8 billion in 2008. Sales in the United
States have grown at a compound annual rate of approximately 9%
from 1993 through 2010, with sales of $4.8 billion in 2010,
representing a 3.4% increase over 2009, according to the MRB.
Although the industry has experienced consistent worldwide
growth in demand, a more balanced supply and demand dynamic has
moderated price increases. Demand for plasma derivatives has
grown substantially through active management of disease, the
discovery of new therapeutic applications, the development of
new products and the increase in prophylactic use. The two main
regions for sale of plasma derivatives in 2008 were North
America and Europe, which together represent 73% of global sales
of plasma-derived therapies.
143
The largest sales region is North America, estimated to be
$4.33 billion in 2008, followed by Europe, estimated to be
$4.27 billion. Although prices are not regulated in the
United States, the presence of large GPOs, which are entities
that act as purchasing intermediaries for hospitals and
physicians, may create pricing pressure as they command
substantial purchasing volumes. Prices in Europe are subject to
regulations that fix maximum prices in certain countries.
The policy of the World Health Organization and many European
jurisdictions is based on a recommendation that blood and its
derivatives be obtained from voluntary, altruistic donors.
Payment to donors is prohibited in most European countries;
however, the United States permits payment to donors. Because of
this limitation, most European countries are unable to meet
their supply requirements and rely on the United States paid
donations to fill the supply gap. The United States supplies
approximately 60% of the world’s plasma.
Effectively, the United States only permits the sale of plasma
derivative products that have been manufactured with plasma
collected in the United States. Plasma collected in the United
States can be used in plasma derivative products sold in most
world markets, whereas plasma collected in Europe is generally
used only in the country where it is obtained.
The plasma collection industry is heavily regulated in the
United States. Federal, state and local regulations are designed
to protect the health of the donors as well as the integrity and
safety of the plasma. In the United States, the opening of a
plasma collection center is subject to a licensing and
certification process by the FDA and periodic inspections of
facilities and processes. Normally it takes approximately
12 months from the time a collection center begins to
operate until a plasma collection center receives FDA approval.
The FDA regulates the characteristics, operation and
qualification of personnel of plasma collection centers.
According to FDA rules, a donor of plasma can donate plasma up
to twice a week. Failure to comply with FDA, state or local
regulations, may ultimately result in the forced closure of a
collection center or monetary fines or both, depending on the
issues involved.
United States and European regulatory authorities impose
stringent requirements to avoid the transmission of blood-borne
diseases. Each donation is typically tested for the following
infections: hepatitis A, hepatitis B, hepatitis C,
parvovirus B19 and HIV. Then it is sent to a fractionator, where
it undergoes additional viral marker testing as well as nucleic
acid testing in the production environment. Thereafter, it is
broken down into its constituent parts, or
“fractions.” “Bulk” fractions are further
refined into final products through various purification
processes, formulation and aseptic filling.
Entry into the plasma derivatives manufacturing business
requires an understanding of the operationally complex nature of
the business, which requires a highly skilled workforce with
specialized know-how; significant intellectual property,
including trade secrets relating to purification of products and
pathogen safety; the need to develop recognized and trusted
brands as well as sales, marketing and distribution
infrastructures and relationships; and the ability to comply
with extensive regulation by the FDA and comparable authorities
worldwide. Additionally, the construction and maintenance,
including regular improvements necessitated by evolving
standards of cGMP, of production facilities requires extensive
capital expenditures and may involve long lead times to obtain
necessary governmental approval. Further, unlike small molecule
pharmaceutical products, which are often subject to patent
expirations on a defined date, plasma-derived protein therapies
are usually protected through intellectual property relating to
process, including trade secrets, which may not have a scheduled
expiration. New entrants may, however, develop and market
competing products by subcontracting portions of the
manufacturing process, such as fractionation or purification,
from existing plasma derivative manufacturers. Also, existing
fractionators with operations in one region are increasingly
entering other regional areas. In addition, new competitors in
the United States would need to secure an adequate supply of
United States plasma.
Principal
Plasma Derivative Products
Collected plasma, whether source or recovered, is fractionated
to isolate component proteins, which are then purified. The
fractionation occurs in tanks at near freezing temperatures to
maintain the integrity of the proteins.
144
The four largest selling plasma proteins, which together
constituted approximately 74% of plasma-derived product sales in
the world in 2008 and 78% in the United States in 2010 and their
therapeutic properties are:
|
|
|
| |
•
|
IVIG is the part of the plasma that contains antibodies.
IVIG assists in the treatment of primary and secondary
immunological deficiencies, idiopathic thrombocytopenic purpura
(“ITP”), Guillain-Barré syndrome, Kawasaki
disease, Allogeneic bone marrow transplant, and Chronic
Inflammatory Demyelinating Polyneuropathy (“CIDP”). In
addition, physicians prescribe IVIG for a variety of diseases,
including multiple sclerosis, skin disease and asthma, even
though these uses are not described in the product’s
labeling and differ from those tested in clinical studies and
approved by the FDA or similar regulatory authorities in other
countries. These unapproved, or “off-label,” uses are
common across medical specialties, and physicians may believe
such off-label uses constitute the preferred standard of care or
treatment of last resort for many patients in varied
circumstances. IVIG is also currently being investigated for use
in the treatment of Alzheimer’s disease and other
neurological conditions. Industry participants believe that,
because IVIG is a complex mixture of antibody molecules, it is
unlikely that a recombinant (or synthetic) alternative will be
developed within the foreseeable future. IVIG has global sales
of $5.1 billion, which represents 43.4% of the total plasma
derivatives sales. IVIG sales experienced significant growth in
recent years driven by improving usages, physician awareness and
a strong reimbursement environment, and it now represents the
largest plasma-derived product by sales value. It is one of the
key growth drivers of the industry largely due to the increasing
number of medical conditions for which IVIG is used;
|
| |
| |
•
|
Factor VIII is a blood coagulation factor which ensures
that blood coagulates correctly after hemorrhage. Persons born
with Factor VIII deficiency or who acquire this deficiency over
time through the formation of antibodies that inactivate it,
require administration of Factor VIII in determined situations
(before surgery or after injury or serious hemorrhage). Factor
VIII is also often used for the treatment of hemophilia A, a
disease that is suffered by one out of every 10,000 men (women
are not susceptible to this disease). Factor VIII used in these
cases is either extracted from human plasma or is genetically
modified into a recombinant substitute from bovine, mouse or
hamster cells. Recombinant products account for most sales in
the Factor VIII market. In 2008, worldwide plasma-derived Factor
VIII and von Willebrand Factor annual sales were approximately
$1.8 billion, comprising 15.5% of total plasma derivatives
sales. Plasma-derived Factor VIII and von Willebrand Factor had
a compound annual growth rate of 8.7% over the past ten years.
Growth in Factor VIII is being driven by increased patient
identification and treatment in developing countries. The
current per capita Factor VIII utilization is significantly
higher in the United States and Europe than in developing
countries;
|
| |
| |
•
|
Albumin is the most commonly found protein in plasma and
represents the biggest product by volume but has low unitary
prices given its commoditized nature. One of albumin’s main
functions is to carry and store a wide variety of small
molecules such as bilirubin, cortisol, sex hormones, free fatty
acids and some medicines. Albumin is used in the treatment of
burns, severe hemorrhage, sepsis, hemodialyzed patients with
hypotension, nephritic syndrome and necrotizing pancreatitis,
among others. Biotechnology companies also use high-purity
albumin as a stabilizer for their products. Clinical trials are
currently underway for new applications for this product,
including, among others, for the treatment of stroke and liver
cirrhosis. Albumin has global sales of $1.7 billion,
comprising 14.4% of the total plasma derivatives industry. The
demand for albumin has increased since 2000 and is projected to
grow moderately over the next few years; and
|
| |
| |
•
|
A1PI is a naturally occurring, self-defensive protein
produced in the liver. A1PI is used to treat congenital A1PI
deficiency-related emphysema. This deficiency may predispose an
individual to several illnesses but most commonly appears as
emphysema in adults. U.S. sales of A1PI have experienced a
compound annual growth rate of 16% between 1997 and 2010. We
believe there are approximately 11,000 individuals currently
identified with A1PI deficiency in North America and Europe with
5,500 of those individuals currently undergoing A1PI treatment,
based on internal estimates. There are an estimated 200,000
individuals with A1PI deficiency at high risk for development of
emphysema in North America and Europe. Many individuals with
symptoms are misdiagnosed before receiving a diagnosis of A1PI
deficiency-related emphysema. Based on patient registries in
many European countries, we believe that severe A1PI deficiency
is also prevalent in Europe, and that European
|
145
|
|
|
| |
|
patients may represent approximately 30% of potential global
sales. Epidemiological surveys have demonstrated that there is
significant latent demand for A1PI as only approximately 10% of
all patients in need of treatment have been identified (source:
Alpha-1-antitrypsin deficiency. High prevalence in the
St. Louis area determined by direct population screening.
Silverman EK, et al. Am Rev Respir Dis. 1989;
140:961-966). Even fewer patients are being treated using an
A1PI product due to the limited number of countries with
licensed product.
|
Plasma
Derivative Worldwide Sales by Category
The following table presents a breakdown of global sales by
plasma derivative product in 2008:
| |
|
|
|
|
|
|
|
Percentage of
|
|
|
Product
|
|
Global Sales
|
|
|
|
|
IVIG
|
|
|
43.4
|
%
|
|
Factor
VIII(1)
|
|
|
15.5
|
%
|
|
Albumin
|
|
|
14.4
|
%
|
|
Factor IX
|
|
|
2.7
|
%
|
|
Hyperimmunes
|
|
|
7.6
|
%
|
|
Alpha 1 Proteinase Inhibitor (“A1PI”)
|
|
|
3.6
|
%
|
|
Fibrin glue
|
|
|
3.7
|
%
|
|
Antithrombin III
|
|
|
2.9
|
%
|
|
Others
|
|
|
6.2
|
%
|
Source: Marketing Research Bureau
|
|
|
|
(1)
|
|
Including sales of von Willebrand
Factor
|
Plasma-Derived
Products Sales by Geographic Region
Due to the cost of plasma-derived therapies, the majority of
plasma sales are derived from the more economically developed
regions in the world. Compared to the United States and Canada,
where the industry is open, though highly regulated, Europe is
characterized by local fractionators, considerable government
control and divergent health care systems.
The following table presents a breakdown of 2008 global sales
for plasma derivatives by region:
| |
|
|
|
|
|
|
|
Percentage of
|
|
|
Region
|
|
Global Sales
|
|
|
|
|
North America
|
|
|
36.7
|
%
|
|
Europe
|
|
|
36.2
|
%
|
|
Asia Pacific
|
|
|
15.3
|
%
|
|
Latin America
|
|
|
5.7
|
%
|
|
Middle East
|
|
|
2.6
|
%
|
|
Others
|
|
|
3.5
|
%
|
Source: Marketing Research Bureau
Historical
Market Growth of Plasma-Derived Products
|
|
|
| |
•
|
IVIG. According to the MRB, worldwide sales
for IVIG have grown at a 12.5% compound annual rate between 1994
and 2008, although current growth is materially lower. This
growth has been driven by increased evidence that IVIG is
effective in treating a broader universe of ailments than
previously considered and increased incidence of acquired
autoimmune and other ailments due to an increase in life
expectancy.
|
| |
| |
•
|
Factor VIII. According to the MRB, the
worldwide sales of plasma-derived Factor VIII, including von
Willebrand factor sales, have grown at a 5.1% compound
annual rate between 1994 and 2008, and
|
146
|
|
|
| |
|
Grifols and Talecris believe that demand growth will continue.
The United States Factor VIII market is supplied primarily by
recombinant products. We believe that continued plasma-derived
Factor VIII growth worldwide will be driven by the following
therapeutic indications:
|
|
|
|
| |
•
|
Treatment of von Willebrand disease. The
treatment of von Willebrand disease requires a Factor VIII
product containing von Willebrand factor. Von Willebrand factor
is not present in recombinant and monoclonal Factor VIII
products; and
|
| |
| |
•
|
Immune Tolerance Therapy
(“ITT”). Plasma-derived ITT is used
principally as a second attempt at treatment when an initial
course of recombinant ITT has failed. The daily administration
of a high dose of either recombinant or plasma-derived Factor
VIII for six months to a year is an increasingly popular
treatment to combat inhibitors, which are substances that
restrict the activity of Factor VIII. Doses in the second
attempt at ITT tend to be significantly higher than in the
initial course of treatment.
|
|
|
|
| |
•
|
Albumin. According to MRB, the worldwide sales
demand for albumin has grown at a 0.6% compound annual rate
between 1994 and 2008. This slow growth is due to a perception
that less expensive alternatives such as saline are as effective
as albumin in the treatment of traumatic or hemorrhagic shock
and severe burns.
|
| |
| |
•
|
Alpha 1 Proteinase Inhibitor
(“A1PI”). A1PI is a fourth protein that
is growing in sales. According to the MRB, the worldwide sales
demand for Alpha-1 has grown at a 14.6% compound annual rate
between 1994 and 2008.
|
Production
of Plasma-Derived Products (Fractionation)
Three principle techniques are used to separate proteins into
bulk fractions: the Cohn, Kistler-Nitschmann and Chromatography
techniques.
Cohn. Cohn, the most widely employed technique
in the industry, which we utilize, subjects plasma to varying
conditions of alcohol concentration, pH level and temperature to
separate specific protein fractions from the plasma. The
fractions are then collected using centrifugation or filtration.
Following fractionation, the protein pastes are purified using
steps such as solvent detergent treatment, caprylate incubation,
column chromatography, and various methods of filtration.
Kistler-Nitschmann. Kistler-Nitschmann is
derived from the Cohn process and is often used in smaller
fractionation facilities. This technique produces a limited
product range, primarily consisting of immunoglobulins and
albumin.
Chromatography. Chromatography separates
plasma proteins by specifically targeting the unique
characteristics of each protein, which include: molecular size,
using gel filtration; charge, using ion exchange chromatography;
and known reactions with specific molecules, using affinity
chromatography. Chromatography has higher product purity and
superior product yields compared to the Cohn technique. However,
regulatory hurdles, including the approval process for the
procedure and the type of production facility required, have
made the cost of switching to chromatography very expensive. As
a result, few plasma fractionators have adopted this technique
for fractionation, although many use it for purification.
Once the plasma has been broken down into bulk fractions using
one of these separation techniques, each fraction undergoes a
series of production steps including purification, filling,
freeze-drying (for those products requiring lyophilisation),
packaging and distribution. Purification involves the further
isolation of the fraction, as well as viral removal/inactivation
steps, using a variety of technologies. The specific procedures
used differentiate the end product and are generally proprietary
to each fractionator.
Plasma
Supply
Plasma-derived product manufacturers secure human plasma in the
United States from either third-party supply contracts (e.g.,
with a blood bank or with an independent plasma collection
company) or from vertically integrated plasma collection
centers. Historically, several of the largest global
fractionators relied on
147
smaller, independently owned United States source plasma
collection companies to supply a portion of their plasma supply.
Over time, fractionators chose to vertically integrate and
acquire many of these suppliers. Currently, all four of the
largest global fractionators are either fully integrated or have
a significant percent of their total plasma collection
internalized as a result of vertical integration.
We believe the growth in United States source plasma collections
over the past several years has been higher than in other
geographic areas. Such belief is based on our view that the
growth of source plasma collection in the United States is
primarily due to (i) the desire of fractionators to have
the flexibility to export United States source plasma for the
manufacture of products outside the United States, (ii) the
favorable collection environment for source plasma centers in
the United States, and (iii) the decreasing availability of
recovered plasma worldwide.
Market estimates continue to point to new growth in United
States source plasma, as new centers are developed in the United
States and individual plasma center productivity improves.
Despite the growth in United States source plasma supply, a
continued increase in demand for plasma products in recent years
has led to industry supply constraints, which stimulated the
addition of new plasma collection centers to meet the increased
need for source plasma.
In response to IVIG demand, we and certain of our competitors
and independent suppliers opened a significant number of new
plasma collection centers. We believe that worldwide plasma
collection is increasing and will continue to increase in future
years, primarily driven by increased plasma collection in the
United States. As a result, the supply of IVIG inventory has
increased throughout the distribution channel.
Fractionation
and Purification
Currently, product production capacity may be limited by
fractionation capacity or purification capacity. We, along with
certain of our competitors, have announced plans to invest in
the development of additional fractionation and purification
capacity.
Manufacturing
and Sale of Plasma Derivative Products
The manufacture and sale of plasma derivative products is
heavily regulated. Manufacturing facilities and processes must
be licensed by the FDA to manufacture medicinal products to be
sold in the United States. Likewise, manufacturing facilities
and products are also subject to strict European regulations to
manufacture medicines intended for distribution in the European
Union.
The plasma derivative product, like medicinal products, is also
subject to prior licensing by the competent authorities of the
jurisdiction where the product is to be marketed and sold. The
licensing process generally requires the applicant to conduct
clinical trials and submit information certifying the safety,
efficacy and quality of the product. The requirements,
formalities and timetables for the registration process
generally vary from jurisdiction to jurisdiction.
In the European Union, the licensing requirements of the
different member countries have been largely unified for
pharmaceutical products. However, in the area of biological
products this trend has been slower. Today, mutual recognition
for cGMP inspections and licensing procedures through mutual
recognition or centralized procedure at the EMA are in place and
fully operational.
United
States Plasma Products Distribution
Historically, manufacturers of plasma-derived products sought to
distribute their finished product through the same distribution
channels as pharmaceuticals, typically through wholesalers,
which purchased products at fixed prices, re-sold them at
contract prices and charged the difference to the manufacturer.
The plasma therapeutics market, however, has evolved from
wholesalers to highly specialized plasma distributors, including:
|
|
|
| |
•
|
“Group Purchasing Organizations,” which are referred
to as GPOs, which are umbrella buying groups representing
inpatient and outpatient hospitals and non-acute members who
benefit through consolidated
|
148
|
|
|
| |
|
supply contracts. GPOs do not purchase products directly,
rather, they select authorized distributors which purchase
inventory and handle all product logistics for their members;
|
|
|
|
| |
•
|
Wholesalers/Distributors either provide product directly to, or
enter into distribution agreements with, hospitals, GPOs, and
physician offices. The distributor is generally paid service
fees for “encumbered” products on a GPO contract, or
they purchase “unencumbered” products directly from
manufacturers which are not part of a GPO contract;
|
| |
| |
•
|
Homecare and specialty pharmacy providers are a growing segment
which provides patient treatment in the home, either through
self-medication or with the assistance of a nurse. These
providers either purchase products direct from manufacturers or
through GPOs; and
|
| |
| |
•
|
Manufacturer Direct programs distribute products directly to a
physician’s office or a patient’s home.
|
The distribution by product line and type are summarized as
follows:
|
|
|
| |
•
|
According to the MRB, it is estimated that 45% of the IVIG sold
in the United States in 2010 was purchased by hospitals;
alternate infusion sites, including physician offices
represented about 20% of IVIG volume; and homecare companies
represented 30% of the IVIG volume;
|
| |
| |
•
|
A1PI is generally distributed by homecare companies and
specialty pharmacies and administered by a nurse at home or at a
hospital infusion suite;
|
| |
| |
•
|
Albumin is generally used in surgical and trauma settings and is
generally sold to hospital groups; and
|
| |
| |
•
|
Clotting factors, such as Factor VIII, generally are
self-administered by patients and are mainly channeled from
manufacturers to patients through home care companies and
similar agencies.
|
The
Hospital Pharmacy Sector
In addition to the plasma industry, our “Hospital
Division” operates in the hospital pharmacy sector. In
order to be marketed and sold, hospital products must comply
with local regulations that generally require that these
products be shown to be safe and effective. Competition is
primarily based on price and quality of service. Since freight
costs can affect profitability significantly, sales of
intravenous therapy products, such as parenteral solutions
(fluid therapy), are generally made to markets that are
relatively near manufacturing facilities.
The Spanish and Portuguese markets for intravenous therapy have
experienced stable growth. According to IMS Health, a leading
provider of information to the pharmaceutical and healthcare
industries, the intravenous therapy market in Spain was
€127 million in 2010. According to AENE, the Spanish
market for enteral nutrition products was €244 million
in 2010. In addition, the Spanish market for parenteral
nutrition fluids consisted of total sales of
€25 million in 2010, according to IMS Health.
The In
Vitro Diagnostic Market
We also operate a “Diagnostic Division.” The three
most important sectors of the in vitro diagnostic market in
which we sell our diagnostic products are the following:
|
|
|
| |
•
|
immunohematology, which is the diagnosis of blood type and the
screening of antibodies, accounting for 2.6%, or
$1.11 million, of the 2010 world market for in vitro
diagnostic products according to the business information
company Global Data;
|
| |
| |
•
|
immunology, which is the study of defense mechanisms against
antigens, accounting for 47.9%, or $20.4 million, of the
2010 world market; and
|
| |
| |
•
|
hemostasis, which is the analysis of processes related to blood
coagulation, accounting for 4.0%, or $1.70 million, of the
2010 world market.
|
The diagnostic products market encompasses mainly products
related to the analytical testing of biological samples to
determine the presence and characteristics of pathogens, to
study defense mechanisms
149
against antigens and to analyze processes related to blood
coagulation. The testing is performed in vitro, that is,
outside the body, with samples of blood, urine or other bodily
fluids and tissues. These tests are generally carried out in
laboratories.
The in vitro diagnostic market has grown significantly over
the past few years as a result of the introduction of new
technologies, increasing test volumes and favorable pricing
environments. Significant technological progress and automation
have resulted in specific and precise diagnoses. This
improvement in diagnosis translates into a better application
and monitoring of therapies and an improvement in disease
prevention.
In order to be marketed and sold, diagnostic products must
comply with local regulations that generally require that these
products be shown to be safe and effective. These are products
that, even though they are not pharmaceutical, are in contact
with the human body or its fluids. Competition for diagnostic
products is based on reputation for quality and safety, the
particular features of the product and, to a lesser extent,
price.
150
REGULATORY
MATTERS
Government
Regulation
Government authorities in the United States, at the federal,
state and local level, and in other countries extensively
regulate, among other things, the research, development,
testing, approval, manufacturing, labeling, post-approval
monitoring and reporting, packaging, promotion, storage,
advertising, distribution, marketing and export and import of
healthcare products such as those we collect, manufacture, sell
and/or are
currently developing. The process of obtaining regulatory
approvals and the subsequent substantial compliance with
appropriate federal, state, local and foreign statutes and
regulations require the expenditure of substantial time and
financial resources. The following is a summary of the overall
regulatory landscape for our business.
United States Government Regulation. In the
United States, the FDA regulates drugs, biologics and plasma
collection under the Federal Food, Drug, and Cosmetic Act and
implementing regulations. Failure to comply with the applicable
FDA requirements at any time during the product-development
process, approval process or after approval may result in
administrative or judicial sanctions. These sanctions could
include, as applicable, the FDA’s imposition of a clinical
hold on trials for drugs, devices or biologics, refusal to
approve pending applications, withdrawal of an approval, warning
letters, product recalls, product seizures, total or partial
suspension of production or distribution, injunctions, fines,
civil penalties or criminal prosecution or any combination of
these sanctions. Any agency or judicial enforcement action could
have a material adverse effect on us.
The BLA Approval Process. Drugs that are also
biological products must also satisfy the requirements of the
Public Health Service Act and its implementing regulations. In
order for a biological drug product to be legally marketed in
the United States, the product must have a Biologic License
Application (“BLA”), approved by the FDA.
The steps for obtaining FDA approval of a BLA to market a
biological product in the United States include:
|
|
|
| |
•
|
completion of preclinical laboratory tests, animal studies and
formulation studies under the FDA’s good laboratory
practices regulations;
|
| |
| |
•
|
submission to the FDA of an Investigational New Drug Application
(“IND”), for human clinical testing, which must become
effective before human clinical trials may begin and which must
include approval by an independent Institutional Review Board,
which is referred to as an IRB, at each clinical site before the
trials may be initiated;
|
| |
| |
•
|
performance of adequate and well-controlled clinical trials in
accordance with Good Clinical Practices to establish the safety
and efficacy of the product for each indication;
|
| |
| |
•
|
submission to the FDA of a BLA, which contains detailed
information about the chemistry, manufacturing and controls for
the product, reports of the outcomes and full data sets of the
clinical trials and proposed labeling and packaging for the
product;
|
| |
| |
•
|
satisfactory review of the contents of the BLA by the FDA,
including the satisfactory resolution of any questions raised
during the review;
|
| |
| |
•
|
satisfactory completion of an FDA Advisory Committee review, if
applicable;
|
| |
| |
•
|
satisfactory completion of an FDA inspection of the
manufacturing facility or facilities at which the product is
produced to assess compliance with cGMP to assure that the
facilities, methods and controls are adequate to ensure the
product’s identity, strength, quality and purity; and
|
| |
| |
•
|
FDA approval of the BLA including agreement on post-marketing
commitments, if applicable.
|
Preclinical tests include laboratory evaluations of product
chemistry, toxicity and formulation, as well as animal studies.
An IND sponsor must submit the results of the preclinical tests,
together with manufacturing information and analytical data, to
the FDA as part of the IND. Some preclinical testing may
continue after
151
the IND is submitted. The IND must become effective before human
clinical trials may begin. An IND will automatically become
effective 30 days after receipt by the FDA, unless before
that time the FDA raises concerns or questions about issues such
as the conduct of the trials
and/or
supporting preclinical data as outlined in the IND. In that
case, the IND sponsor and the FDA must resolve any outstanding
FDA concerns or questions before clinical trials can proceed. In
other words, submission of an IND may not result in the FDA
allowing clinical trials to commence. Further, recently enacted
healthcare reform law introduced a biosimilar pathway, which
will permit companies to obtain FDA approval of generic versions
of existing biologics based upon reduced documentation and data
requirements deemed sufficient to demonstrate safety and
efficacy than are required for the pioneer biologic.
Clinical trials involve the administration of the
investigational product to human subjects under the supervision
of qualified investigators. Clinical trials are conducted under
strict requirements to ensure the protection of human subjects
participating in the trial and protocols detailing, among other
things, the objectives of the study, the parameters to be used
in monitoring safety and the effectiveness criteria to be
evaluated. A protocol for each clinical trial and any subsequent
protocol amendments must be submitted to the FDA as part of the
IND. In addition, an IRB (usually, but not necessarily specific
to each study site), must approve the protocol, subject consent
form and any amendments. All research subjects must be informed,
among other things, about the risks and benefits of the
investigational product and provide their informed consent in
writing. Federal regulations governing the protection of human
subjects in clinical trials have remained generally consistent
for many years, subject to certain amendments. In July 2011, HHS
and the FDA issued an advance notice of proposed rulemaking
seeking comments on proposals to substantially change aspects of
these regulations, seeking comments, for example, on mandating
the use of a single IRB for multi-site trials, imposing
specified data security and information regulations on trials,
imposing new consent requirements with respect to the use of
biospecimens that have been stripped of patient-identifiers, and
the requiring the use of standardized consent forms. The outcome
of this regulatory review is not yet certain, and accordingly
its impact on our operations is not clear.
Clinical trials typically are conducted in three sequential
phases, but the phases may overlap or be combined.
Phase I trials usually involve the initial introduction of the
investigational drug into a small group of healthy volunteers
(e.g., 10 to 20) to evaluate the product’s safety,
dosage tolerance and pharmacokinetics and, if possible, to gain
an early indication of its effectiveness.
Phase II trials usually involve controlled trials in a
larger but limited patient population (e.g., a few hundred) to:
|
|
|
| |
•
|
evaluate dosage tolerance and appropriate dosage;
|
| |
| |
•
|
identify possible adverse effects and safety risks; and
|
| |
| |
•
|
provide a preliminary evaluation of the efficacy of the drug for
specific indications.
|
Phase III trials usually further evaluate clinical efficacy
and test further for safety in an expanded patient population
(e.g., several hundred to several thousand). Phase III
trials usually involve comparison with placebo, standard
treatments or other active comparators. Usually two
well-controlled large Phase III or pivotal trials
demonstrating safety and efficacy are required. These trials are
intended to establish the overall risk-benefit profile of the
product and provide an adequate basis for physician labeling.
Phase III trials are usually larger, more time consuming,
more complex and more costly than Phase I and Phase II
trials. Since most of our products are aimed at very small
populations where it is not always possible to conduct two large
studies, regulators may accept one study on a smaller number of
patients than would typically be required for pharmaceutical
products in general, provided the data are sufficiently robust.
Phase I, Phase II and Phase III testing may not
be completed successfully within any specified period, if at
all. Furthermore, the FDA, or the companies may suspend or
terminate clinical trials at any time on various grounds,
including a finding that the subjects or patients are being
exposed to an unacceptable health risk, have experienced a
serious and unexpected adverse event, or that continued use in
an investigational setting
152
may be unethical. Similarly, an IRB can suspend or terminate
approval of research if the research is not being conducted in
accordance with the IRB’s requirements or if the research
has been associated with unexpected serious harm to patients.
Assuming successful completion of the required clinical testing,
the results of the preclinical studies and of the clinical
trials, together with other detailed information, including
information on the chemistry, manufacture and composition of the
product, are submitted to the FDA in the form of a BLA
requesting approval to market the product for one or more
indications. In most cases, the BLA must be accompanied by a
substantial user fee. The FDA will initially review the BLA for
completeness before it accepts the BLA for filing. After the BLA
submission is accepted for filing, the FDA reviews the BLA to
determine, among other things, whether a product is safe and
effective for its intended use and whether the product is being
manufactured in accordance with cGMP to assure and preserve the
product’s identity, strength, quality, purity and potency.
Under the Pediatric Research Equity Act of 2003, which is
referred to as the PREA, BLAs, or supplements to BLAs, must
contain data to assess the safety and effectiveness of the drug
for the claimed indications in all relevant pediatric
subpopulations and to support dosing and administration for each
pediatric subpopulation for which the drug is safe and
effective. The FDA may grant deferrals for submission of data or
full or partial waivers. Unless otherwise required by
regulation, PREA does not apply to any drug for an indication
for which orphan designation has been granted.
Before approving a BLA, the FDA generally will inspect the
facility or the facilities at which the product is manufactured.
The FDA will not approve the product if it finds that the
facility does not appear to be in cGMP compliance. If the FDA
determines the application, manufacturing process or
manufacturing facilities are not acceptable, it will either
disapprove the application or issue a Complete Response letter
in which it will outline the deficiencies in the BLA and provide
the applicant an opportunity to meet with FDA representatives
and subsequently to submit additional information or data to
address the deficiencies. Notwithstanding the submission of any
requested additional information, the FDA ultimately may decide
that the application does not satisfy the regulatory criteria
for approval.
The testing and approval processes require substantial time,
effort and financial resources, and each may take several years
to complete. Data obtained from clinical activities are not
always conclusive and may be susceptible to varying
interpretations, which could delay, limit or prevent regulatory
approval. The FDA may not grant approval on a timely basis, or
at all. We may encounter difficulties or unanticipated costs in
their efforts to secure necessary governmental approvals, which
could delay or preclude us from marketing their products. The
FDA may limit the indications for use or place other conditions
on any approvals that could restrict the commercial application
of the products. After approval, some types of changes to the
approved product, such as adding new indications, manufacturing
changes and additional labeling claims, are subject to further
testing requirements and FDA review and approval.
Post-Approval Requirements. After regulatory
approval of a product is obtained, we are required to comply
with a number of post-approval requirements. For example, as a
condition of approval of a BLA, the FDA may require
post-marketing testing and surveillance to monitor the
product’s safety or efficacy. In addition, holders of an
approved BLA are required to keep extensive records, to report
certain adverse reactions and production problems to the FDA, to
provide updated safety and efficacy information and to comply
with requirements concerning advertising and promotional
labeling for their products. Also, quality control and
manufacturing procedures must continue to conform to cGMP
regulations and practices, as well as the manufacturing
conditions of approval set forth in the BLA. The FDA
periodically inspects manufacturing facilities to assess
compliance with cGMP, which imposes certain procedural,
substantive and recordkeeping requirements. Accordingly,
manufacturers must continue to expend time, money and effort in
the area of production and quality control to maintain
compliance with cGMP and other aspects of regulatory compliance.
Future FDA inspections may identify compliance issues at our
facilities or at the facilities of our contract manufacturers
that may disrupt production or distribution, or require
substantial resources to correct and prevent recurrence of any
deficiencies, and could result in fines or penalties by
regulatory authorities. In addition, discovery of problems with
a product or the failure to comply with applicable requirements
may
153
result in restrictions on a product, manufacturer or holder of
an approved BLA, including withdrawal or recall of the product
from the market or other voluntary, FDA-initiated or judicial
action that could delay or prohibit further marketing. Newly
discovered or developed safety or efficacy data may require
changes to a product’s approved labeling, including the
addition of new warnings and contraindications. The recently
enacted healthcare reform law established and provided
significant funding for a Patient-Centered Outcomes Research
Institute to coordinate and fund Comparative Effectiveness
Research. Also, new government requirements, including those
resulting from new legislation, may be established that could
delay or prevent regulatory approval our products under
development.
Orphan Drug Designation. The FDA may grant
orphan drug designation to drugs intended to treat a “rare
disease or condition” that affects fewer than 200,000
individuals in the United States, or more than 200,000
individuals in the United States and for which there is no
reasonable expectation that the cost of developing and making
available in the United States a drug for this type of disease
or condition will be recovered from sales in the United States
for that drug. Orphan drug designation must be requested before
submitting an application for marketing approval. Orphan drug
designation does not convey any advantage in, or shorten the
duration of, the regulatory review and approval process. Orphan
drug designation can provide opportunities for grant funding
towards clinical trial costs, tax advantages and FDA user-fee
exemptions. In addition, if a product which has an orphan drug
designation subsequently receives the first FDA approval for the
indication for which it has such designation, the product is
entitled to orphan drug exclusivity, which means the FDA may not
approve any other application to market the same drug for the
same indication for a period of seven years, except in limited
circumstances, such as a showing of clinical superiority to the
product with orphan exclusivity or a meaningfully different mode
of administration. Competitors may receive approval of different
drugs or biologics for the indications for which the orphan
product has exclusivity. However, if a company with orphan drug
exclusivity is not able to supply the market, the FDA could
allow another company with the same drug a license to market for
said indication.
Fast Track Designation. The FDA’s fast
track programs, one of which is fast track designation, are
designed to facilitate the development and review of new drugs
that are intended to treat serious or life-threatening
conditions and that demonstrate the potential to address unmet
medical needs for the conditions. Fast track designation applies
to a combination of the product and the specific indication for
which it is being studied. Thus, it is the development program
for a specific drug for a specific indication that receives fast
track designation. The sponsor of a product designated as being
in a fast track drug development program may engage in close
early communication with the FDA, including through timely
meetings and feedback on clinical trials. Products in fast track
drug development programs also may receive Priority Review or
accelerated approval (i.e., where the review cycle is set with a
six-month review clock instead of 10- or
12-month
review clock). Sponsors may also be able to submit completed
portions of an application before the entire application is
completed; however, the review clock will not officially begin
until the entire completed BLA is submitted to and filed by the
FDA. The FDA may notify a sponsor that its program is no longer
classified as a fast track development program if the fast track
designation is no longer supported by emerging data, the
designated drug development program is no longer being pursued,
or another product that meets the unmet medical need for the
same indication is approved first.
Plasma Collection. The FDA requires a
licensing and certification process for each plasma collection
center prior to opening and conducts periodic inspections of
facilities and processes. Many states also regulate plasma
collection, imposing similar obligations and additional
inspections and audits. Collection centers are subject to
periodic inspections by regulatory authorities, which if
noncompliance is alleged, may result in fines, citations, the
temporary closing of the centers, loss or suspension of
licenses,
and/or
recall of finished products.
Anti-Fraud and Abuse Regulation. Since we
supply products and services that are reimbursed by
U.S. federally funded programs such as Medicare and
Medicaid, our activities are also subject to regulation by CMS
and enforcement by the OIG within the U.S. Department of
Health and Human Services (“HHS”). A provision of the
U.S. Social Security Act known as the “Anti-Kickback
Law” prohibits providers and others from directly or
indirectly soliciting, receiving, offering or paying any
remuneration with the intent of generating referrals or orders
for services or items covered by a government health care
program. Many states have similar laws. Courts have interpreted
this law very broadly, including holding that a violation has
154
occurred if even one purpose of the remuneration is to generate
referrals, even if there are other lawful purposes. There are
statutory and regulatory exceptions (known as safe harbors) that
outline arrangements that are deemed lawful. However, the fact
that an arrangement does not fall within a safe harbor does not
necessarily render the conduct illegal under the Anti-Kickback
Law. In sum, even legitimate business arrangements between the
companies and referral sources could lead to scrutiny by
government enforcement agencies, and require extensive company
resources to respond to government investigations. Violations of
the Anti-Kickback Law may be punished by civil and criminal
penalties
and/or
exclusion from participation in federal health care programs,
including Medicare and Medicaid. The recent U.S. healthcare
reform law strengthened provisions of the Anti-Kickback Law.
The federal False Claims Act (“FCA”) is violated by
any entity that “presents or causes to be presented”
knowingly false claims for payment to the federal government. In
addition, the recently enacted healthcare reform law amended the
FCA to create a cause of action against any person who knowingly
makes a false statement material to an obligation to pay money
to the government, or knowingly conceals or improperly decreases
an obligation to pay or transmit money or property to the
government. For the purposes of these recent amendments, an
“obligation” includes an overpayment, which is defined
broadly to include “any funds that a person receives or
retains under [Medicare and Medicaid] to which the person, after
applicable reconciliation, is not entitled . . . .”
The FCA is commonly used to sue those who submit allegedly false
Medicare or Medicaid claims, as well as those who induce or
assist others to submit a false claim. Courts and government
officials have found that “false claims” can result
not only from noncompliance with the express requirements of
applicable governmental reimbursement programs, such as Medicaid
or Medicare, but also from noncompliance with other laws, such
as provisions of the Food, Drug and Cosmetic Act that prohibit
off-label promotion, or laws that require quality care in
service delivery. The qui tam or whistleblower provisions of the
FCA allow private individuals to bring actions on behalf of the
government alleging that the government was defrauded, with
tremendous potential financial gain to private citizens in the
event they prevail. When a private party brings a whistleblower
action under the FCA, the defendant is not made aware of the
lawsuit until the government starts its own investigation or
makes a decision on whether it will intervene. Many states have
enacted similar laws that also apply to claims submitted to
commercial insurance companies. The bringing of any FCA action
could require us to devote resources to investigate and defend
the action. Violations of the FCA could result in penalties for
each separate false claim.
Regulation Outside the United States. In
addition to regulations in the United States, we are subject to
a variety of regulations in other jurisdictions governing
clinical trials and commercial sales and distribution of its
products. Whether or not we obtain FDA approval for a product,
we must obtain approval of a product by the comparable
regulatory authorities of countries outside the United States
before it can commence the marketing of the product in those
countries. The approval process varies from country to country,
and the time may be longer or shorter than that required for FDA
approval. The requirements governing the conduct of clinical
trials, product licensing, pricing and reimbursement vary
greatly from country to country. Also, in addition to approval
of final products, U.S. plasma centers collecting plasma
for manufacture into products to be distributed in the European
Union must also be approved by the competent European Health
Authority.
Medicines can be authorized in the European Union by using
either the centralized authorization procedure or national
authorization procedures. The EMA is responsible for the
centralized authorization procedure.
Centralized Authorization Procedure. The EMA
is responsible for the centralized procedure, also known as the
“Community authorization procedure”, for human
medicines. This procedure results in a single marketing
authorization called a “Community marketing
authorization” that is valid across the European Union, as
well as in the EEA/EFTA states Iceland, Liechtenstein and
Norway. The centralized procedure is compulsory for human
medicines that are:
|
|
|
| |
•
|
derived from biotechnology processes, such as genetic
engineering;
|
| |
| |
•
|
advanced-therapy medicines, such as gene-therapy, somatic
cell-therapy or tissue-engineered medicines;
|
155
|
|
|
| |
•
|
intended for the treatment of HIV/Aids, cancer, diabetes,
neurodegenerative disorders or autoimmune diseases and other
immune dysfunctions; and
|
| |
| |
•
|
officially designated “orphan medicines” (medicines
used for rare diseases).
|
For medicines that do not fall within these categories or the
“mandatory scope”, companies have the option of
submitting an application for a centralized marketing
authorization to the Agency, as long as the medicine concerned
is a significant therapeutic, scientific or technical
innovation, or if its authorization would be in the interest of
public health.
Applications through the centralized procedure are submitted
directly to the Agency. Evaluation by the Agency’s relevant
scientific committee takes up to 210 days, at the end of
which the committee adopts an opinion on whether the medicine
should be marketed or not. This opinion is then transmitted to
the European Commission, which has the ultimate authority for
granting marketing authorizations in the European Union.
Once a Community marketing authorization has been granted, the
marketing-authorization holder can begin to make the medicine
available to patients and healthcare professionals in all
European Union countries.
National Authorization Procedures. Each
European Union Member State has its own procedures for the
authorization, within their own territory, of medicines that
fall outside the scope of the centralized procedure.
There are also two possible routes available to companies for
the authorization of such medicines in several countries
simultaneously:
Decentralized procedure. Using the
decentralized procedure, companies may apply for simultaneous
authorization in more than one European Union country of
medicines that have not yet been authorized in any European
Union country and that do not fall within the mandatory scope of
the centralized procedure.
Mutual-recognition procedure. In the
mutual-recognition procedure, a medicine is first authorized in
one European Union Member State, in accordance with the national
procedures of that country. Following this, further marketing
authorizations can be sought from other European Union countries
in a procedure whereby the countries concerned agree to
recognize the validity of the original, national marketing
authorization.
In some cases, disputes arising in these procedures can be
referred to the EMA for arbitration as part of a “referral
procedure.”
Orphan Designation. Applications for
designation of orphan medicines are reviewed by the EMA through
the Committee for Orphan Medicinal Products (“COMP”).
The criteria for orphan designation are:
|
|
|
| |
•
|
the medicinal product is intended for the diagnosis, prevention
or treatment of a life-threatening or chronically debilitating
condition affecting no more than five in 10,000 persons in
the European Union at the time of submission of the designation
application (prevalence criterion); or
|
| |
| |
•
|
the medicinal product is intended for the diagnosis, prevention
or treatment of a life-threatening, seriously debilitating or
serious and chronic condition and without incentives it is
unlikely that the revenue after marketing of the medicinal
product would cover the investment in its development; and
|
| |
| |
•
|
either no satisfactory method of diagnosis, prevention or
treatment of the condition concerned is authorized, or, if such
method exists, the medicinal product will be of significant
benefit to those affected by the condition.
|
Companies with an orphan designation for a medicinal product
benefit from incentives such as:
|
|
|
| |
•
|
protocol assistance (scientific advice for orphan medicines
during the product-development phase);
|
| |
| |
•
|
direct access to centralized marketing authorization and
10-year
marketing exclusivity;
|
| |
| |
•
|
financial incentives (fee reductions or exemptions); and
|
| |
| |
•
|
national incentives detailed in an inventory made available by
the European Commission.
|
156
Since February 1, 2009, orphan medicinal products are
eligible for the following level of fee reductions:
|
|
|
| |
•
|
full (100%) reduction for protocol assistance and
follow-up;
|
| |
| |
•
|
full (100%) reduction for pre-authorization inspections 50%
reduction for new applications for marketing authorization to
applicants other than small and medium-sized enterprises;
|
| |
| |
•
|
full (100%) reduction for new applications for marketing
authorization only to small and medium-sized
enterprises; and
|
| |
| |
•
|
full (100%) reduction for post authorization activities
including annual fees only to small and medium sized enterprises
in the first year after granting a marketing authorization.
|
The funds made available by the Community for fee exemptions for
orphan medicinal products amounted to €6,000,000 in 2008.
Canadian
Regulatory Process
Authorization to Market: Therapeutic products
can be marketed in Canada after they have been subject to a
review to assess their safety, efficacy and quality. A New Drug
Submission (“NDS”) must be submitted to Health Canada
for review, and a Notice of Compliance (“NOC”) and a
Drug Identification Number (“DIN”) received by the
sponsor prior to marketing a product in Canada. Responsibility
for review of pharmaceutical drug products resides with Health
Canada’s Therapeutic Products Directorate
(“TPD”), while responsibility for review of biological
products are under the Biologics, Radiopharmaceuticals and
Genetic Therapies Directorate (“BGTD”). An active DIN
is required for any product being marketed in Canada.
Changes to Market Authorization: There are
four classes of changes to existing market authorizations in
Canada. Level 1 changes are considered “significantly
different” and have the potential to impact safety,
efficacy, quality or effectiveness of the product. These require
the filing of a Supplemental New Drug Submission
(“SNDS”), and an NOC must be issued by Health Canada
prior to implementation of the change. Level 2 changes are
not considered “significant”, but a Notifiable Change
(“NC”) submission must be filed to Health Canada for
review, and approval is provided via a “No Objection”
letter to the sponsor. Level 3 changes have minimal
potential to impact safety, quality or effectiveness, and can be
made without prior approval of Health Canada; a summary of these
changes is reported to Health Canada with the sponsor’s
Annual Drug Notification. Level 4 changes are implemented
without any notification to Health Canada, based on no
expectation of risk.
Clinical Trials: A Clinical Trial Application
(“CTA”) must be submitted to Health Canada prior to
conducting any study protocol that proposes the use of a new
product, or the use of an existing product, where the
indication, target population, route of administration or dosing
differs from the current market authorization. The CTA provides
summaries of pre-clinical and clinical studies conducted and (if
applicable) chemistry, manufacturing and control data, and is
submitted to either TPD (for drug products) or BGTD (for
biological products) for review. The TPD or BGTD are responsible
for assessing protection and safety of the participants as well
as quality of the product; they will issue a “No
Objection” letter to sponsors for studies deemed
acceptable. Research ethics board approval at each site is also
required prior to conduct of the study.
Establishment Licensing: All establishments in
Canada which are involved in the fabrication,
packaging/labeling, testing, import, distribution or warehousing
of drug products, must have a current establishment license
(licenses are issued for a one-year period, and applications
need to be re-filed every year). As an importer/distributor,
part of the licensing requirements include demonstration that
any foreign (non-Canadian) facilities involved in fabrication,
packaging/labeling or testing of products imported/distributed
under the license comply with Good Manufacturing Practices
(“GMP”).
Post-Approval Requirements: The Health
Products and Food Branch Inspectorate (“HPFBI”) of
Health Canada periodically inspects licensed establishments in
Canada to verify compliance with GMP. Manufacturers and
importers are required to monitor the safety and quality of
their products and must report adverse reactions to the Marketed
Health Products Directorate (“MHPD”) in accordance
with a prescribed timeline and format.
157
Regulatory
Process for Markets outside North America and
Europe
The majority of regulatory authorities in countries outside
North America and Europe require that a product first be
approved by the FDA or European authority prior to granting the
market authorization in their country. There are a limited
number of countries (Bahamas, Bermuda, Guam, Oman and Qatar)
that do not require further local product registration for
products and products may be distributed based on the existing
FDA approval. In addition to requiring the submission of a
license application containing documentation supporting the
safety, efficacy and quality of the product, many countries
require the submission of FDA Export Certificates for products
to provide assurance that such products can be legally marketed
in the US. The Certificate of Pharmaceutical Product
(“CPP”)
and/or the
Certificate to Foreign Government (“CFG”) are issued
by the FDA at the request of the manufacturer seeking licensure
in the country outside the US. The CPP conforms to the format
established by the World Health Organization (“WHO”)
and is intended for use by the importing country when
considering whether to license the product in question for sale
in that country. The CFG serves to document that the product can
be legally marketed in the US and the manufacturer is in
compliance with GMP. A limited number of regulatory authorities
in countries outside North America and Europe conduct
on-site
inspections to verify GMP compliance. Failure to maintain and
document GMP compliance could result in withdrawal of marketing
authorization. In addition changes to manufacturing or testing
procedures for the product require approval of the change in the
US prior to the submission of the variation to the registration
in the international market. These changes may require approval
in each market in order to maintain product distribution.
Furthermore, any changes in the distributors supporting our
export business could result in a loss of sales.
Pharmaceutical
Pricing and Reimbursement
In the United States and markets in other countries, sales of
any products for which we receive regulatory approval for
commercial sale will depend in part on the availability of
reimbursement from third-party payors. Third-party payors
include government health programs, managed care providers,
private health insurers and other organizations. These
third-party payors are increasingly challenging the price and
examining the cost-effectiveness of medical products and
services. In addition, significant uncertainty exists as to the
reimbursement status of newly approved healthcare products. Our
products may not be considered cost-effective. Adequate
third-party reimbursement may not be available to enable us to
maintain price levels sufficient to realize an appropriate
return on its investment in product development.
In the United States, our products are reimbursed or purchased
under several government programs, including, Medicaid, Medicare
Parts B and D, the 340B/Public Health Service (“PHS”)
program, and pursuant to our contract with the Department of
Veterans Affairs. Medicaid is a joint state and federal
government health plan that provides covered outpatient
prescription drugs for low-income individuals. Under Medicaid,
drug manufacturers pay rebates to the states based on
utilization data provided by the states. The rebate amount for
branded drugs had been equal to a minimum of 15.1% of the
Average Manufacturer Price (“AMP”) or AMP less Best
Price (“BP”), whichever is greater. The recently
enacted healthcare reform law increased the size of the Medicaid
rebates paid by drug manufacturers for most brand drugs to a
minimum of 23.1% of the AMP, with limitation of this increase on
certain drugs, including, for example, certain clotting factors,
to a minimum of 17.1%, effective for drugs purchased by Medicaid
programs on or after January 1, 2010. In 2010, the
healthcare reform law also newly extended this rebate obligation
to prescription drugs covered by Medicaid managed care
organizations. In addition, the statutory definition of AMP
changed in 2010 as a result of the new healthcare reform law,
and in November 2010 CMS withdrew previously issued regulations
defining this critical pricing term and has not yet issued new
regulations. We believe we are making reasonable assumptions
regarding our reporting obligations with respect to this new
definition, but the adequacy of our assumptions is not certain.
Medicare Part B reimburses providers for drugs provided in
the outpatient setting based upon Average Sales Price
(“ASP”). Recent federal government reforms to Medicare
Part B have reduced the reimbursement rates for IVIG.
Beginning January 1, 2008, CMS reduced the reimbursement
for separately covered outpatient drugs and biologics, including
IVIG in the hospital outpatient setting, from ASP +6% to ASP
+5%, using 2006 Medicare claims data as a reference for this
reduction. CMS reduced this reimbursement further in 2009
158
to ASP +4%, using aggregate hospital cost report data as a
reference for the reduction. For 2010, the rate remained as
ASP+4%, based on a cost-based methodology that also involved
reallocating certain overhead costs from packaged drugs to
unpackaged drugs. In 2011, relying on the 2010 methodology, CMS
increased the rate to ASP +5%. For 2012, as part of a July 2011
proposed rule issued in connection with the Medicare hospital
outpatient prospective payment system, CMS has proposed
continuing to rely on the
2010-2011
methodology, which according to CMS would result in a rate of
ASP +4% or lower as of January 1, 2012. The proposed
decrease in reimbursement rates could restrict access to our
products.
Medicare Part D is a partial, voluntary prescription drug
benefit created by the federal government primarily for persons
65 years old and over. The Part D drug program is
administered through private insurers that contract with CMS.
Government payment for some of the costs of prescription drugs
may increase demand for any products for which we receive
marketing approval. However, to obtain payments under this
program, we are required to negotiate prices with private
insurers operating pursuant to federal program guidance. These
prices may be lower than we might otherwise obtain. In addition,
beginning in 2011, the recently enacted healthcare reform law
generally requires that in order for a drug manufacturer’s
products to be reimbursed under Medicare Part D, the
manufacturer must enter into a Medicare Coverage Gap Discount
Program agreement with the Secretary of the United States
Department of Health and Human Services, and reimburse each
Medicare Part D plan sponsor an amount equal to 50% savings
for the manufacturer’s brand name drugs and biologics which
the Part D plan sponsor has provided to its Medicare
Part D beneficiaries who are in the “donut hole”
(or a gap in Medicare Part D coverage for beneficiaries who
have expended certain amounts for drugs).
Some payors, including Medicare Part D plans, some state
Medicaid programs, and many private health insurers and
self-insured health plans reimburse providers for drugs based
upon a discount off of the Average Wholesale Price
(“AWP”). AWP is a list price determined by third-party
publishers, which does not reflect actual transactions in the
distribution chain. We do not publish an AWP for any of our
products. We may be at a competitive disadvantage where
providers are reimbursed on an AWP basis and competitors’
products are reimbursed at higher rates than their corresponding
products.
The availability of federal funds to pay for our products under
the Medicaid and Medicare Part B programs requires that we
extend discounts under the 340B/PHS drug pricing program. The
340B drug pricing program extends discounts to a variety of
community health clinics and other specified entities that
receive health services grants from the PHS, as well as
hospitals that serve a disproportionate share of certain low
income individuals. The PHS price (also known as the
“ceiling price”) cannot exceed the AMP (as reported to
CMS under the Medicaid drug rebate program) less the Medicaid
unit rebate amount. We have entered into a PPA with the
government in which we agree to participate in the 340B/PHS
program by charging eligible entities no more than the PHS
ceiling price for drugs intended for outpatient use. The
recently enacted healthcare reform law expands the number of
qualified 340B entities eligible to purchase the products for
outpatient use. The healthcare reform law also imposes a
“must sell” obligation on manufacturers that will
require manufacturers to offer their products to eligible
entities at legally-mandated discount prices if such products
are “made available to any other purchaser at any other
price.” The healthcare reform law imposes this obligation
by requiring HHS to add the requirement to the 340B/PHS program
agreements that manufacturers must execute with HHS. Rulemaking
to implement this obligation is not yet complete, and as a
result the full impact of the changes is not yet certain,
however the new provision could be implemented in a manner that
requires us to allocate more of our products for sale under the
340B program in order to maintain the availability of federal
funds to pay for their products under Medicaid and Medicare
Part B coverage. Further regulatory rule making is required
to define this new requirement. Additional legislative changes
to the 340B program have been proposed, though it is too early
to determine which changes will be adopted, or what their impact
will be.
We make our products available for purchase by authorized
government users of the Federal Supply Schedule
(“FSS”), pursuant to their FSS contracts with the
Department of Veterans Affairs. Under the Veterans Health Care
Act of 1992, which is referred to as the VHC Act, the companies
are required to offer discounted FSS contract pricing to four
Federal agencies — the Department of Veterans Affairs,
the Department of Defense, the Coast Guard and the Public Health
Service (including the Indian Health Service) — for
federal
159
funding to be made available for reimbursement of any of our
products under the Medicaid program and for our products to be
eligible to be purchased by those four Federal agencies. FSS
pricing to those four Federal agencies must be equal to or less
than the “Federal Ceiling Price,” which is, at a
minimum, 24% off the Non-Federal Average Manufacturer Price,
which is referred to as “Non-FAMP,” for the prior
fiscal year.
The recently enacted United States healthcare reform law imposes
a fee on manufacturers and importers of branded drugs and
biologics based on their sales to United States government
health programs. An aggregate fee of $2.5 billion will be
imposed on all covered entities for 2011. The aggregate fee will
be allocated among applicable manufacturers and importers based
on their relative sales to government health programs. The
aggregate fee will increase to $4.1 billion for 2018 and is
scheduled to be reduced to $2.8 billion for 2019. Beginning
in 2013, the healthcare reform law also imposes a new excise tax
on many medical devices, equal to 2.3% of the sales price, and
excludes devices generally purchased by the general public at
retail for individual use. In addition, the Prescription Drug
User Fee Act (“PDUFA”), first enacted in 1992, sets
forth user fees that pharmaceutical and biological companies pay
to the FDA for certain applications for approvals of drugs and
biologicals, as well as on the establishments where the products
are made, and on the products themselves. The fees under PDUFA
cover a substantial portion of FDA’s operating budget, and
the measure also addresses aspects of the regulatory approval
process, such as timing and procedures. PDUFA is subject to
reauthorization by Congress in 2012, and while reauthorization
is likely it is not yet certain if the measure will remain
substantially unchanged, or be amended in a manner that is
favorable or unfavorable to our operations.
The marketability of any products for which we receive
regulatory approval for commercial sale may suffer if the
government and third-party payors fail to provide adequate
coverage and reimbursement. Federal, state and local governments
in the United States have enacted and continue to consider
additional legislation to limit the growth of healthcare costs,
including the cost of prescription drugs. Existing and future
legislation could limit payments for biologics such as the drug
candidates that we are developing, including possibly permitting
the federal government to negotiate prices directly with
manufacturers. In addition, an increasing emphasis on managed
care in the United States has increased and will continue to
increase the pressure on pharmaceutical pricing. For a
discussion of certain risks related to reimbursement and
pricing, please see “Risk Factors — Risks
Relating to the Healthcare Industry — The
implementation of the 2010 health care reform law in the
United States may adversely affect our business.
Other
Governmental Regulation
Our operations and many of the products that we manufacture or
sell are subject to extensive regulation by numerous other
governmental agencies, both within and outside the United
States. In the United States, apart from the agencies discussed
above, our facilities, operations, employees, products (their
manufacture, sale, import and export) and services are regulated
by the Drug Enforcement Agency, the Environmental Protection
Agency, the Occupational Health & Safety
Administration, the Department of Agriculture, the Department of
Labor, Customs and Border Protection, the Transportation
Security Administration, the Department of Commerce, the
Department of Treasury, the Department of Justice, the
U.S. Office of Foreign Assets Control and others. State
agencies also regulate our facilities, operations, employees,
products and services within their respective states. Government
agencies outside the United States also regulate public health,
product registration, manufacturing, environmental conditions,
labor, exports, imports and other aspects of our global
operations. For further discussion of the impact of regulation
on our business, see “Risk Factors — Risks
Relating to the Healthcare Industry — Certain of
our business practices are subject to scrutiny by regulatory
authorities, as well as to lawsuits brought by private citizens
under federal and state laws. Failure to comply with applicable
law or an adverse decision in lawsuits may result in adverse
consequences to us.”
160
DIRECTORS
AND EXECUTIVE OFFICERS
Board of
Directors
Pursuant to our Bylaws, we are managed by a Board of Directors
(Consejo de Administración) (the “Board”),
which may be composed of not less than three and not more than
15 directors. Our current Board has eleven directors.
Directors may be either individuals or legal entities. Under
Spanish law, our Board is responsible for our management,
administration and representation in all matters concerning the
business, subject to the provisions of our Bylaws and the powers
conferred at the general shareholders’ meeting.
Appointment
and Dismissal
Pursuant to our Bylaws, directors are elected by our
shareholders to serve for a term of five years and may be
re-elected to serve for an unlimited number of terms, except in
the case of independent directors, who pursuant to our Board of
Director’s Regulations (reglamento de funcionamiento
interno del consejo de administración) (the “Board
Regulations”) shall not serve as such for a period
exceeding 12 years. A director may serve any number of
consecutive five-year terms. A director may be either an
individual or an entity represented by an individual. If a
director ceases to hold office prior to the expiration of his or
her term, our Board may fill the vacancy by appointing, from
among our shareholders, a new director to replace the outgoing
director. Any director so appointed will hold office until the
next general shareholders’ meeting when the appointment may
be (i) confirmed or (ii) revoked by our shareholders.
Any such appointment will be only for the remainder of the term
of the outgoing director, without prejudice to such
director’s eventual election. A director may resign, or be
removed, from office by a resolution of our general
shareholders’ meeting at any time. A director who is also
our shareholder may vote freely on any of our shareholders’
resolutions relating to the appointment and dismissal of
directors (including the appointment or dismissal of that
director).
In addition, pursuant to our Board Regulations, directors must
tender their resignation to the Board and the Board may accept
such resignation, in its discretion, under the following
circumstances: (i) when the director ceases to hold the
executive position to which such director’s appointment to
the Board was related; (ii) when the director becomes
unable to hold the office due to a legal cause of ineligibility
or incompatibility; (iii) when the director has been
formally charged with certain crimes (including, but not limited
to, crimes against personal freedom, economic crimes, crimes
against the justice administration) or a formal inquiry is
opened against him or her by a regulator; (iv) when the
director has been severely admonished by the Audit Committee for
having breached his or her duties as director; (v) when the
director’s participation on the Board may jeopardize our
interests or when the reasons for his or her appointment cease
to exist; and (vi) in the case of a proprietary director
(consejero dominical), when the relevant shareholder
ceases to hold its stake in us, or reduces its stake below the
level that reasonably justified the appointment of such director.
In addition, under Spanish corporate law, a holder of voting
shares (or group of shareholders of voting shares acting
together) may, subject to availability of seats on the Board,
appoint a number of directors proportionate that
shareholder’s (or group of shareholders’) interest in
our voting capital. If the voting capital stock represented by
the shares held by such shareholder (or group of shareholders)
is equal to or greater than the result of dividing our total
voting capital stock by the number of directors, such
shareholder (or group of shareholders) shall have the right to
appoint a proportionate number of directors. For example, a
shareholder holding 20 voting shares out of a total of 100
voting shares in a company with five directors will be entitled
to appoint one director. Should this power be exercised, shares
so pooled shall not participate in the voting for the other
members of the Board. However, they may exercise their voting
rights with respect to the removal of existing directors. Since
such rights apply only to voting shares or non-voting shares
that have recovered their voting rights, our Class B
shares, and the ADS which represent them in the US, do not count
towards the proportional representation right.
Our Board must appoint a Chairman from among its members.
Mr. Víctor Grifols Roura is the current Chairman of
our Board. The Board shall also designate one or more
Vice-Chairmen, who shall be numbered consecutively, and who
shall replace the Chairman in the event of impossibility to act
or absence.
161
Our Board may also appoint a Secretary and a Vice-Secretary.
Neither the Secretary nor the Vice-Secretary is required to be a
member of the Board of Directors; however, the Secretary or the
Vice-Secretary will not be entitled to vote on matters before
our Board unless he or she is a member of our Board.
Mr. Raimon Grifols Roura is the current Secretary
non-member of our Board and Ms. Nuria Martín
Barnés is the current Vice-Secretary non-member of our
Board.
Meetings
of the Board of Directors
Pursuant to our Bylaws, a meeting of our Board may be called by
the Chairman whenever he considers such a meeting necessary or
suitable. The Chairman of the Board is also required to call a
meeting at the request of one-third of the directors. Meetings
of the Board are called through a letter sent by certified mail
with requested return receipt, at least 20 days before the
date of the meeting. Such notice of a meeting of the Board must
state the place, date and time as well as the issues to be
discussed. The Board generally holds a meeting at least every
three months and is required to meet at least once a year. The
Spanish Companies Law (Ley de Sociedades de Capital) and
our Bylaws provide that a majority of the directors (half plus
one of the directors present at a meeting) of the Board
(represented in person or by proxy by another director on the
Board) constitutes a quorum. Except as otherwise provided by law
or specified in our Bylaws, resolutions of the Board must be
passed by an absolute majority of the directors present or
represented at a meeting, with the Chairman having the right to
cast a deciding vote in the event of a tie.
Delegation
of Powers
Pursuant to Spanish law, a board of directors may delegate its
powers either to an Executive Committee (Comision
Ejecutiva) or to one or more Chief Executive Officers
(Consejeros Delegados). Spanish corporate law provides
that resolutions appointing an Executive Committee, any Chief
Executive Officer or authorizing the permanent delegation of
all, or part of, such board of directors’ powers, requires
a two-thirds majority of the members of such board of directors
and the registration of such resolution in the Commercial
Registry. Pursuant to our Bylaws, our Board may delegate all or
part of its powers to one or more directors, or to an Executive
Committee. Our Board may also revoke such powers at any time.
Under Spanish corporate law, a board of directors may also grant
general or specific powers of attorney to any person whether or
not that person is a director or a shareholder. General powers
of attorney must be registered in the Commercial Registry.
However, Spanish law provides that the following powers may not
be delegated: (i) the formulation and submission for
approval of the yearly financial statements at the general
shareholders’ meeting; and (ii) those powers granted
to the board of directors by a general shareholders’
meeting (unless otherwise provided in the relevant
shareholders’ resolution).
Mr. Víctor Grifols Roura is currently the Chairman of
the Board and Chief Executive Officer (Consejero
Delegado) of Grifols, S.A.
162
Set forth below are the names and current positions of Grifols,
S.A.’s directors:
| |
|
|
|
|
|
|
|
Name
|
|
Title
|
|
Type
|
|
Director Since
|
|
|
|
Víctor Grifols Roura
|
|
Director, Chairman of the Board
and Chief Executive Officer
(Consejero Delegado)
|
|
Executive
|
|
July
1991(1)
|
|
Juan Ignacio Twose Roura
|
|
Director
|
|
Executive
|
|
April
2000(2)
|
|
Ramón Riera Roca
|
|
Director
|
|
Executive
|
|
April
2000(3)
|
|
Tomás Dagá Gelabert
|
|
Director
|
|
Other External
|
|
April 2000
|
|
Thorthol Holdings B.V. (represented
by Mr. José Antonio Grifols Gras)
|
|
Director
|
|
Proprietary
|
|
January
2000(4)
|
|
Thomas H. Glanzmann
|
|
Director
|
|
Other External
|
|
April 2006
|
|
Edgar Dalzell Jannotta
|
|
Director
|
|
Independent
|
|
December 2006
|
|
Anna Veiga Lluch
|
|
Director
|
|
Independent
|
|
December 2008
|
|
William Brett Ingersoll
|
|
Director
|
|
Independent
|
|
June 2011
|
|
Luis Isasi Fernandez De Bobadilla
|
|
Director
|
|
Independent
|
|
May 2011
|
|
Steven Francis Mayer
|
|
Director
|
|
Independent
|
|
June 2011
|
|
Raimon Grifols Roura
|
|
Secretary non-member
|
|
—
|
|
July 2001
|
|
Nuria Martín Barnés
|
|
Vice Secretary non-member
|
|
—
|
|
July 2001
|
|
|
|
|
(1)
|
|
Between July 8, 1991 and
May 30, 2002, Mr. Víctor Grifols Roura was not a
director but sat on the Board as representative of our then
director Deria, S.A.
|
| |
|
(2)
|
|
Between May 25, 2001 and
May 30, 2002, Mr. Juan Ignacio Twose Roura was not a
director but sat on the Board as representative of our then
director Grifols Engineering, S.A.
|
| |
|
(3)
|
|
Between May 25, 2001 and
May 30, 2002, Mr. Ramón Riera Roca was not a
director but sat on the Board as representative of our then
director Grifols International, S.A.
|
| |
|
(4)
|
|
Thorthol Holdings B.V. is
represented on the Board of Directors by Mr. José
Antonio Grifols Gras. Between January 20, 2000 and
June 1, 2002 Thorthol Holdings B.V. was not a director but
its current representative on the Board, Mr. José
Antonio Grifols Gras, sat on the Board as director.
|
Director
Biographies
Víctor
Grifols Roura
Mr. Grifols Roura, who in 1985 succeeded his father as
Chief Executive Officer of our predecessor, headed the 1987
reorganization that created the company that we are today.
Mr. Grifols Roura originally joined our predecessor in 1973
as an Export Manager and later served as Sales Manager.
Mr. Grifols Roura earned a business administration degree
from the University of Barcelona.
Juan
Ignacio Twose Roura
Mr. Twose has served as a director of our predecessor, and
now Grifols, S.A., since 1973. He has also served as our
Vice-President of Manufacturing since 1988. Mr. Twose has
been responsible for our industrial division since our creation.
Mr. Twose received a degree in Industrial Engineering from
the Escuela Técnica Superior of Barcelona.
Ramón
Riera Roca
Mr. Riera has served as our director since 2000. He also
serves as our Vice-President of Marketing and Sales.
Mr. Riera joined our predecessor in 1977, became the Vice
President of Marketing & Sales in 1988 and Managing
Director of Grifols International in 1997. Mr. Riera earned
a degree in Chemical Sciences from the Autonomous University of
Barcelona.
163
Tomás
Dagá Gelabert
Mr. Dagá has served as our director since April 2000.
Mr. Dagá is also a member of the board of directors of
Scranton Enterprises B.V., Zambon, S.A., Pharmazam S.A. and
StoraEnso Barcelona, S.A. Mr. Dagá is also a trustee
of the Joaquim Molins Figueras Foundation in Barcelona.
Mr. Dagá is the managing partner of the Barcelona
office of the law firm Osborne Clarke Spain. Prior to joining
Osborne Clarke, Mr. Dagá worked in the corporate and
tax department of Peat Marwick Mitchell & Co. in
Barcelona from December 1979 to September 1986.
Mr. Dagá earned a law degree from the University of
Barcelona.
José
Antonio Grifols Gras
Dr. Grifols has served as a director of Grifols, S.A.
representing Thorthol Holdings B.V. since June 2002.
Dr. Grifols has been a professor of Theoretical Physics at
the Autonomous University of Barcelona since September 1990 and
the head of the Physics Department of the university since
September 2002. Dr. Grifols’ activities involve both
teaching undergraduate and graduate courses (that include
quantum mechanics, general relativity and cosmology) and doing
research in high energy physics, astrophysics and cosmology.
Dr. Grifols trained as a physicist in many European and
American Institutions including Max-Planck-Institut (Munich,
1971-1974,
1981), Stanford University
(1976-1978),
CERN (Geneva, 1983, 1984, 1987, 1995), Deutsches Elektronen
Synchrotron (Hamburg, 1986, 1987, 1988), Oxford University
(1984), University of Florida (Gainesville, 1985), Lawrence
Berkeley Laboratory (Berkeley, 1987) and Fermi National
Laboratory (Chicago, 1996).
Thomas H.
Glanzmann
Mr. Glanzmann has served as our director since April 2006.
Following the acquisition, Mr. Glanzmann was appointed
Chairman of Grifols Inc. and was hired by Grifols, S.A. to lead
the integration of Talecris. In addition, he is currently the
Chief Executive Officer and President of Gambro AB. Prior to his
current position, Mr. Glanzmann was the CEO and Managing
Director of HemaCue. He also was a Senior Advisor to the
Executive Chairman and Acting Managing Director at The World
Economic Forum. Between 1988 and 2004 he held various positions
at Baxter Healthcare: Senior Vice President and Corporate
Officer of Baxter Healthcare Corporation; President of Baxter
Bioscience; Chief Executive Officer of Immuno International; and
President of the European Biotech Group, among other positions.
Between 1984 and 1988 he worked at Philip Morris where he
amongst other positions was the country manager for Norway,
Denmark and Iceland. Mr. Glanzmann holds a M.B.A. from IMD
in Switzerland and a B.A. in Political Science from Dartmouth
College, USA.
Edgar
Dalzell Jannotta
Mr. Jannotta has served as our independent director since
December 2006. In March 2001, he was named Chairman of William
Blair & Company, L.L.C., an international investment
banking firm. Mr. Jannotta joined William Blair &
Company in 1959 as an Associate, became a Partner in January
1965 and was Managing Partner from 1977 to 1995. Before being
appointed Managing Partner, Mr. Jannotta worked on
investment banking and private equity transactions in the
corporate finance department. He was Chairman of the Securities
Industry Association (1982) and has served as a director of
the New York Stock Exchange Inc. He serves as a director on the
boards of Aon Corporation, Commonwealth Edison Company, Molex
Incorporated, and Sloan Valve Company. Mr. Jannotta
completed his undergraduate studies at Princeton University and
received his M.B.A. from Harvard Business School.
Anna
Veiga Lluch
Ms. Veiga has served as our director since 2008. She
graduated in Biology
(1974-1979)
and received her Ph.D. at Universidad Autonoma de Barcelona in
1991. She has been the IVF laboratory Director at the
Reproductive Medicine Service, Institut Universitari Dexeus from
1982 to 2005. She is the Director of the Barcelona Stem Cell
Bank at the Centre for Regenerative Medicine in Barcelona, the
Scientific Director at the Reproductive Medicine Service,
Institut Universitari Dexeus and Associate professor at the
Departament de
164
Ciències Experimentals i de la Vida, Universitat Pompeu
Fabra. Her main areas of interest are Clinical Embryology,
Reproductive Genetics, Embryonic and Pluripotent Stem Cell
research and Bioethics.
W. Brett
Ingersoll
Mr. Ingersoll has served as our independent director since
June 2011. Prior to such time Mr. Ingersoll served as a
member of the board of directors of Talecris Biotherapeutics
Holdings Corp. from April 2005. Mr. Ingersoll has served as
managing director of Cerberus Capital Management, L.P. and
predecessor entities since November 2002 and co-head of private
equity of Cerberus globally. From 1993 until 2002,
Mr. Ingersoll served as a partner for J.P. Morgan
Partners. In addition, Mr. Ingersoll is also a member of
the boards of directors of the following companies: Steward
Healthcare System, LLC, DynCorp International, EntreCap
Financial, LLC, ACE Aviation Holdings, Inc., AerCap Holdings
N.V. y EnduraCare Therapy Management, LLC. Mr. Ingersoll
received his BA in Economics from Brigham Young University and
his MBA from Harvard Business School.
Steven F.
Mayer
Mr. Mayer has served as our independent director since June
2011. Prior to such time Mr. Mayer served as a member of
the board of directors of Talecris Biotherapeutics Holdings
Corp. from April 2005. Mr. Mayer is the managing director
of Cerberus California, LLC and predecessor entities since
November 2002 and co-head of private equity of Cerberus
globally. Mr. Mayer is also member of the boards of
directors of BlueLinx Holdings, Inc., DecisionOne Corporation,
LNR Property Holdings, Ltd. and Spyglass Entertainment, LLC.
Mr. Mayer received his AB, cum laude, from Princeton
University and his JD, magna cum laude, from Harvard Law School.
Luis
Isasi Fernandez De Bobadilla
Mr. Isasi has served as our independent director since May
2011. He is president and managing director of Morgan Stanley
España, country head for Spain, and board member of the
Madrid Stock Exchange. He joined Morgan Stanley in London in
1987. Prior to that, he served as executive director at First
Chicago Ltd. in London and, previously, worked in New York for
the Latin American department of Morgan Guaranty Trust Co.
Mr. Isasi started his professional career in Abengoa, in
Seville (Spain) in 1977. Mr. Isasi has a Bachelors Degree
in Business by the University of Seville, and holds a M.B.A.
from Columbia Business School in New York, United States,
obtained in 1982.
Biographies
of the Secretary Non-Members of the Board of
Directors
Raimon
Grifols Roura
Mr. Grifols has served as Secretary non-member to the Board
of Directors since August 2001. Mr. Grifols is also a
member of the board of directors of Squadron Reinsurance Ltd.,
Marca Grifols, S.L., Arrahona Optimus, S.A., patron of the
Probitas Fundación Privada, and secretary to the board of
directors of Instituto Grifols, S.A., Xantic Spain, S.A.
Mr. Grifols is a partner at Osborne Clarke Spain.
Mr. Grifols earned his law degree from the University of
Barcelona.
Nuria
Martín Barnés
Ms. Martín has served as Vice-Secretary non-member to
the Board of Directors since 2001. She is also a member of the
Board of Directors of Compañía General de Inversiones,
S.A., S.I.C.A.V., Gesiuris S.G.I.I.C., S.A., CAT Patrimonis,
S.I.C.A.V., S.A., URC Patrimonis, S.I.C.A.V., S.A. and Technetix
Spain, S.L. Ms. Martín is a Partner at Osborne Clarke
Spain. Prior to joining Osborne Clarke she worked in the
Corporate and Tax Department of KPMG Peat Marwick from 1982 to
1986. Ms. Martín earned her law degree from the
University of Barcelona.
165
Executive
Officers
Our principal executive officers are:
| |
|
|
|
|
|
|
|
Name
|
|
Title
|
|
Since
|
|
|
|
Víctor Grifols Roura
|
|
President and Chief Executive Officer
|
|
|
1985
|
|
|
Juan Ignacio Twose Roura
|
|
President of Global Industrial Division
|
|
|
1988
|
|
|
Ramón Riera Roca
|
|
President of Global Commercial Division
|
|
|
1988
|
|
|
Alfredo Arroyo Guerra
|
|
Chief Financial Officer
|
|
|
2007
|
|
|
Montserrat Lloveras Calvo
|
|
Director of Corporate Accounting and Reporting
|
|
|
1991
|
|
|
Antonio Viñes Páres
|
|
Director of Corporate Planning and Control
|
|
|
1994
|
|
|
Eva Bastida Tubau
|
|
Director of Scientific Affairs
|
|
|
2007
|
|
|
Vicente Blanquer Torre
|
|
Technical Director of Biological Industrial Group
|
|
|
1993
|
|
|
Mateo Florencio Borrás Humbert
|
|
Director of Global Human Resources
|
|
|
2008
|
|
|
Carlos Roura Fernández
|
|
Co-President of Global Industrial Division
|
|
|
1987
|
|
|
Francisco Javier Jorba Ribes
|
|
President of Biological Industrial Group
|
|
|
1995
|
|
|
Gregory Gene Rich
|
|
President and Chief Executive Officer of Grifols Inc.
|
|
|
2001
|
|
|
David Ian Bell
|
|
Vice President of Grifols Inc.
|
|
|
2003
|
|
|
Albert Grifols Roura
|
|
Co-President of Instituto Grifols, S.A.
|
|
|
1999
|
|
|
Nuria Pascual Lapeña
|
|
Director of Corporate Investor Relations Office
|
|
|
1997
|
|
|
Shinji Wada
|
|
President of Plasma Centers of Grifols Inc.
|
|
|
2003
|
|
|
Mary Kuhn
|
|
Executive Vice President of Grifols Inc.
|
|
|
2011
|
|
|
Joel Abelson
|
|
President of North America Commercial Division
|
|
|
2011
|
|
Executive
Officer Biographies
The following are the biographies of our principal executive
officers who are not also directors:
Alfredo
Arroyo Guerra
Mr. Arroyo has served as Chief Financial Officer of
Grifols, S.A. since January 2007. Previously, Mr. Arroyo
served as a CFO and in various Senior Finance positions in
companies such as KPMG, Carrefour, Chupa Chups, Reckitt
Benckiser and Winterthur. Mr. Arroyo received a degree in
Economics and is a Certified Public Accountant.
Montserrat
Lloveras Calvo
Mrs. Lloveras has served as the Director of Corporate
Accounting and Reporting (previously the Administration Director
and Controller) since 1991. She joined our predecessor in 1984
as the Costs Analyst of the Financial Department and in 1988 was
promoted to the position of Administration Director.
Mrs. Lloveras received a degree and a M.B.A. from the
Escuela Superior de Administración y Dirección de
Empresas in Barcelona.
Antonio
Viñes Páres
Mr. Viñes has served as the Director of Corporate
Planning and Control (previously the Planning and Control
Director) at Grifols, S.A. since 1994. He joined us in 1978,
occupying several positions in the Commercial and Marketing
Departments. Mr. Viñes received a degree in Biology
from the Autonomous University of Barcelona.
Eva
Bastida Tubau
Mrs. Bastida joined us in 2004 as the Medical Marketing
Director of Grifols International, S.A. and took on the position
of Director of Scientific Affairs (previously Executive
Scientific Director of Grifols, S.A.) in 2007. Previously,
Mrs. Bastida worked as a Clinical Scientist in the
Hemostasis Department of the Hospital
166
Clinic in Barcelona, Spain. In
1993-1998
she was the Head of Clinical Development at Sanofi in Barcelona,
Spain and from
1999-2003
she was responsible for Worldwide Clinical Development at
Sanofi-Synthelabo in Paris, France. Mrs. Bastida has
obtained her Pharmacy & Medical Pharmacology Degree
from the University of Barcelona and a PhD in Cell Biology at
the American Red Cross in Bethesda, Maryland. She holds a PDD by
IESE and she has more than 24 years of experience, six of
them with us.
Vicente
Blanquer Torre
Mr. Blanquer has served as the Technical Director of the
Biological Industrial Group (previously the Pharmaceutical
Technical Director) at Grifols, S.A. since 1993, and is
responsible for both Bioscience’s Quality Assurance and
Quality Control. From 1987 until 1993, he was the Deputy
Technical Director, responsible for Process Quality Control
concerning the Plasma derivatives manufacturing.
Mr. Blanquer received a Degree in Pharmacy from the
University of Barcelona.
Mateo
Florencio Borrás Humbert
Mr. Borrás has served as the Director of Global Human
Resources (previously Human Resources Director) at Grifols, S.A.
since 2008. Previously, he served as a HR Director at different
companies such as EMAYA, Nissan Motor Ibérica and others.
He is a member of AEDIPE and he is an Arbitrator at the
Arbitrator Corps of Catalonian Labour Court.
Mr. Borrás received a degree in Psychology and a
Postgraduate on Labour and Social Security, both at Universidad
Central de Barcelona.
Carlos
Roura Fernández
Mr. Roura has served as the co-president of the Global
Industrial Division (previously the General Manager of Hospital
Operations) since 1987. Mr. Roura joined us in 1977 and has
held several positions since that time. Beginning in 2002, he
has served as President of Farmafluid, a Spanish association of
medical parenteral nutritional fluid laboratories. Since 2008,
Mr. Roura is deputy Vice President of the Industrial
Division. Mr. Roura is an Industrial Engineer.
Francisco
Javier Jorba Ribes
Mr. Jorba has served as President of the Biological
Industrial Group (previously the General Manager of Bioscience
Operations) since 1995. He joined us in 1979 as Director of
Plasma Procurement and Director of the A.I.P.H. Program. He was
also General Manager of Biomat, S.A. from 1991 until 1995 and
Managing Director of Instituto Grifols, S.A. until the
consummation of the acquisition. At present, Mr. Jorba is
Co-President of the Global Industrial Division. Mr. Jorba
received a degree in General Medicine and Surgery in 1975 from
the University of Barcelona and completed his Residency in
Pediatrics in 1978 from the same University.
Gregory
Gene Rich
Mr. Rich has served as the President of
U.S. Operations and Chief Executive Officer and Chairman of
the Grifols Inc. Board of Directors, since December 2001.
Previously, Mr. Rich worked for Grupo Picking Pack, as
Chief Operating Officer from December 2000 to December 2001 and
from July 1997 to August 2000, as Senior Vice President for
Green Cross International, the then parent of Alpha Therapeutic
Corporation. Mr. Rich also worked for Alpha Therapeutic
Corporation as Vice President and General Manager of
International Operations from October 1995 to July 1997. In
between his two terms at Alpha Therapeutic Corporation,
Mr. Rich worked for us from January 1983 to October 1995
and served as our co-President for the period December 1985
through his departure in 1995. Mr. Rich earned a Bachelors
of Science degree from California Polytechnic University, Pomona.
David Ian
Bell
Mr. Bell joined us as a Vice President of Grifols Inc. in
July 2003 and has since been responsible for Corporate
Operations and Development. He also serves as General Counsel
and is a member of our Executive Committee in Spain.
Mr. Bell is responsible for all legal activities of our
U.S. Operations, including litigation,
167
mergers and acquisitions, real estate transactions, intellectual
property and contracts. He is also responsible for regulatory,
registrations and licensing, governmental and public affairs and
human resources. Prior to joining us, Mr. Bell was Vice
President and General Counsel for Alpha Therapeutic Corporation.
Additionally, he was a partner in the U.S. law firm of
Knapp, Petersen & Clarke where he specialized in
complex litigation involving healthcare, pharmaceutical and
biotechnology regulation and liability. Mr. Bell attended
the University of California, Irvine, Southwestern University
School of Law and a postgraduate program at Harvard Law School.
He is a member of the California State Bar and is admitted to
practice before the United States Supreme Court and numerous
Federal Appellate and District Courts.
Albert
Grifols Roura
Mr. Grifols joined us in September 1985. From
1995-1999,
he served as the Director of the international subsidiaries,
from
1999-2008,
he served as the Managing Director of Biomat, S.A., and from
2009-2011 he served as Managing Director of Laboratorios
Grifols, S.A. From January 1, 2011 through the consummation
of the acquisition Mr. Grifols was the Managing Director of
the Diagnostic Industrial Division. Following the consummation
of the acquisition Mr. Grifols serves as Co-President of
Institutio Grifols, S.A. Mr. Grifols is an Industrial
Engineer. He received his degree from the Higher Technical
School of Engineers of Terrassa (Polytechnic University of
Catalonia).
Nuria
Pascual Lapeña
Ms. Pascual joined us in 1996. She currently serves as a
Director of the Corporate Investor Relations Office. Prior to
joining us, she served in different positions at various banking
institutions (Deutsche Bank and Banco Santander de Negocios).
She is a member of the board of directors of several companies
related to her family’s businesses. Ms. Pascual
received a degree in Economics & Business
Administration and received a Masters of Sciences in Economics
from the London School of Economics and Political Sciences.
Shinji
Wada
Mr. Wada started his career in the plasma industry in 1981
working for a Japanese plasma fractionation company, the Green
Cross Corporation, parent company of Alpha therapeutic in Los
Angeles. He assumed various positions at Alpha Therapeutic
including M&A, International Sales and Marketing. After our
acquisition of Alpha’s plasma fractionation business, he
was assigned to manage Biomat USA, Inc., the US plasma
collection arm of Grifols, S.A. and he has been CEO of Biomat
USA, Inc., since 2005.
Mary
Kuhn
Ms. Kuhn joined Grifols as Executive Vice President of
Grifols Inc. in June 2011 and is responsible for the North
American Manufacturing Division. Prior to joining Grifols Inc.,
Ms. Kuhn held various positions within Bayer
Pharmaceuticals/Biologics and then Talecris, most recently as
Executive Vice President of Operations. Ms. Kuhn received a
degree in Pharmacy from Purdue University and an EMBA from the
University of New Haven.
Joel
Abelson
Mr. Abelson joined Grifols Inc. in June 2011 as President
of North American Commercial Operations. He also serves as
Corporate Vice President of Grifols Inc. Mr. Abelson worked
for Talecris from March 2006-June 2011, serving most recently as
Senior Vice President and General Manager, Portfolio Management
and International Business. Prior to joining Talecris,
Mr. Abelson worked for Bayer Healthcare
(1994-2005)
where he held several senior management positions in Canada and
the US, including Vice President, Global Strategic Marketing,
Biological Products Division. Prior to joining the private
sector, Mr. Abelson held various policy and management
positions with the Government of Ontario, Canada
(1984-1994)
including service in the Cabinet Office as a Senior Policy
Advisor to the Premier’s Policy and Priorities Board.
Mr. Abelson has a Bachelor of Arts (Honours) degree from
Carleton University in Ottawa, Canada and a Master’s degree
in Public Administration from the University of Toronto.
168
Committees
of our Board of Directors
Our Board has an Audit Committee (Comité de
Auditoría) and an Appointments and Remuneration
Committee (Comisión de Nombramientos y
Retribuciones). The following is a brief description of such
committees.
Audit
Committee
Our Board established an Audit Committee in compliance with
Article 24.bis of our Bylaws and Article 14 of the
Board Regulations.
The regulations applicable to the Audit Committee are set forth
in the provisions referred to above, as well as the Bylaws of
the Audit Committee, which were approved by the Board and the
Audit Committee on December 9, 2008. In connection with the
acquisition, at a Board meeting held on May 24, 2011 our
Bylaws and Board Regulations were amended to conform to NASDAQ
Listing Rules and to facilitate the listing of our ADSs
representing our Class B shares on NASDAQ.
The Audit Committee consists of a minimum of three directors and
a maximum of five directors who are appointed by the Board based
on such directors’ knowledge, competence and experience in
accounting, audit and risk management matters. The majority of
the members of the Audit Committee must be external directors
(consejeros externos), which includes independent
directors (consejeros independientes) and proprietary
directors (consejeros dominicales). In addition, all
members of the Audit Committee, including the chairman, must
meet the independence, experience and other requirements set
forth in the Exchange Act and the NASDAQ Listing Rules.
The responsibilities of the Audit Committee include:
|
|
|
| |
•
|
Report to the shareholders at general shareholders meetings
regarding matters for which the Audit Committee is responsible;
|
| |
| |
•
|
Have sole authority to recommend to the Board the appointment,
hiring and replacement of the external auditor regardless of the
faculties vested in the general shareholders’ meeting and
the Board with regard to the approval of such resolutions under
Spanish law;
|
| |
| |
•
|
Monitor the internal audit services and propose the selection,
appointment, reelection and resignation of the manager of the
Internal Audit Department; propose the budget for the Internal
Audit Department; receive periodic information on the Internal
Audit Department’s activities (including the annual work
plan and annual activities reports prepared by the manager); and
verify that the top management takes the conclusions and
recommendations of their reports into account;
|
| |
| |
•
|
Set up and supervise procedures for the receipt, retention and
treatment of complaints regarding accounting, internal controls
or auditing matters, as well as the confidential and anonymous
submission by employees of concerns regarding questionable
accounting or auditing matters;
|
| |
| |
•
|
Know the process for gathering financial information and the
internal control system; review the annual accounts and the
periodic financial statements that should be submitted to the
securities regulatory authorities and make sure that the
appropriate accounting standards are followed; report to the
Board on any change in the accounting standards and on balance
sheet and off balance sheet risks;
|
| |
| |
•
|
Receive information from the auditors regarding matters that
could impair their independence, or any other matters relating
to conduct of audits of the financial statements as well as any
other communications provided for in the legislation governing
audits of financial statements and in technical auditing
regulations;
|
| |
| |
•
|
Supervise any transactions entered into with significant
shareholders as set forth in the Board Regulations; and
|
| |
| |
•
|
Ensure compliance with the Internal Code of Conduct for
Securities Market Issues, the Board Regulations, the rules of
conduct set out in the “Code of Ethics for Grifols
Executives” and the “Code of Conduct for Grifols’
Employees” and, in general, any other corporate
regulations; Make the necessary proposals to improve such
regulations.
|
169
The Audit Committee currently consists of Messrs. Luis
Isasi Fernandez De Bobadilla, Steven F. Mayer and W. Brett
Ingersoll. Mr. Tomás Dagá Gelabert serves as
secretary non-member.
Appointments
and Remuneration Committee
Pursuant to Article 15 of the Board Regulations, the
Appointments and Remuneration Committee is required to consist
of between three and five members, the majority of which must be
external directors (consejeros externos), which includes
independent directors (consejeros independientes) and
proprietary directors (consejeros dominicales).
The responsibilities of the Appointments and Remuneration
Committee include:
|
|
|
| |
•
|
assisting in the nomination of directors, including evaluating
potential nominees in light of the level of knowledge,
competence and experience necessary to serve on the Board;
|
| |
| |
•
|
reporting and making proposals to the Board on the appointment
of members to the various committees of the Board and on the
persons who should hold the office of Secretary and
Vice-secretary of the Board;
|
| |
| |
•
|
making proposals for the orderly and planned succession of the
Chairman of the Board and the Chief Executive Officer;
|
| |
| |
•
|
reporting on proposals for the appointment and removal of any
members of senior management made by the Chief Executive Officer;
|
| |
| |
•
|
making proposals on the remuneration plans for the Board and
senior management;
|
| |
| |
•
|
periodically reviewing the remuneration plans of senior
management, including considering their suitability and
performance; and
|
| |
| |
•
|
reporting on transactions in which directors may have a conflict
of interest.
|
The Appointments and Remuneration Committee currently consists
of Messrs. Víctor Grifols Roura, Edgar Dalzell
Jannotta and Anna Veiga Lluch. Mr. Raimon Grifols Roura
serves as secretary non-member.
NASDAQ
Corporate Governance Requirements
Pursuant to NASDAQ Listing Rules, as a foreign private issuer,
we may elect to follow our home country practice in lieu of the
corporate governance requirements of the Rule 5600 Series,
with the exception of those rules that are required to be
followed pursuant to the provisions of Listing
Rule 5615(a)(3). We have elected to follow Spanish
practices in lieu of the requirements of the Rule 5600
Series to the extent permitted under NASDAQ Listing
Rule 5615(a)(3). We have disclosed on our website each
requirement that we do not follow and describe the Spanish
practices followed by us in lieu of such requirements. Our
website is
http://www.grifols.com.
The information provided on our website is not part of this
prospectus and is not incorporated herein by reference.
Family
Relationships
Mr. Víctor Grifols Roura, the Chairman of our Board
and our Chief Executive Officer, and Mr. Raimon Grifols
Roura, the Secretary non-member of the Board of Directors, are
brothers.
Messrs. Víctor Grifols Roura, Raimon Grifols Roura,
and José Antonio Grifols Gras are the grandchildren of
Mr. José Antonio Grifols i Roig, our founder.
Mr. Juan Ignacio Twose Roura, one of our directors and
Vice-President of Manufacturing, is a cousin of
Messrs. Víctor Grifols Roura and Raimon Grifols Roura.
Mr. Francisco Javier Jorba Ribes is the
brother-in-law
of Mr. Víctor Grifols Roura.
170
COMPENSATION
OF DIRECTORS AND EXECUTIVE OFFICERS
Compensation
of Members of the Board of Directors
Our directors are entitled to receive compensation for serving
on our Board. Our Bylaws generally set forth the processes for
the determination of the compensation paid to the members of our
Board. Article 20 of our Bylaws provides that the maximum
aggregate amount to be paid to our directors will be established
each year or for the period of time decided by our shareholders
at our shareholders’ meetings. The Board then determines,
pursuant to Article 26 of the Board Regulations, how much
of the shareholder-approved aggregate compensation amount will
be allocated to each director as compensation, taking into
account the recommendations of the Appointments and Remuneration
Committee and their dedication to our business.
Our director compensation philosophy, as set forth in
Article 27 of our Board Regulations, provides that the
remuneration of external directors shall be established in a
manner that provides incentives for our directors to be
dedicated and involved while not creating an obstacle to their
independence. To that end, Article 27 further establishes
that the Board, following the advice of the Appointments and
Remuneration Committee, shall take the necessary measures to
ensure that external directors’ remuneration adheres to the
following guidelines: (a) their remuneration is linked to
their dedication, abilities and functions; and (b) they are
excluded from any plans (x) consisting in the delivery of
equity awards or options or other instruments linked to the
value of our shares; (y) linked to our performance or
(z) including retirement benefits. However, external
directors may be remunerated with our shares only if they agree
to hold them for the duration of the term that they hold their
office.
As discussed above, our Appointments and Remuneration Committee
is required, pursuant to Article 15 of the Board
Regulations, to consist of between three and five members, the
majority of which must be external directors (consejeros
externos). In accordance with Spanish corporate governance
requirements and consistent with NASDAQ Listing Rules for
foreign private issuers, our Appointments and Remuneration
Committee currently consists of Messrs. Víctor Grifols
Roura, Edgar Dalzell Jannotta and Anna Veiga Lluch, with
Mr. Dalzell and Ms. Viega in their capacity as
external independent directors.
In accordance with the compensation policy outlined in our
Bylaws, at the shareholders’ meeting on May 24, 2011,
the shareholders set the maximum annual amount available for
compensation to the external independent members of the Board of
Directors at €60,000 per external director. On such date,
four members of the Board were serving as external independent
directors, Thomas Glanzmann, Edgar Dalzell Jannotta, Anna Veiga
Lluch and Luís Isasi Fernández de Bobadilla.
Messrs. Ingersoll and Mayer became external independent
directors in June 2011. As of the date of this prospectus, Edgar
Dalzell Jannotta, Anna Veiga Lluch, Luís Isasi
Fernández de Bobadilla, W. Brett Ingersoll and Steven F.
Mayer are our external independent directors.
The total compensation paid to directors in 2010, in the
aggregate, amounted to approximately €2,246,000. Of the
total director compensation amount, executive directors
(consejeros ejecutivos) received €1,679,000 in fixed
compensation and €386,000 in variable compensation and
external independent directors received €180,000. The
external proprietary directors do not receive any compensation
for their service on the Board. In addition, none of our
directors received compensation in the form of pensions or life
insurance in 2010, nor did they receive equity compensation.
None of our directors received attendance fees for meetings of
the Board or committees of the Board.
Finally, pursuant to Article 20 of our Bylaws, our
directors were reimbursed for all expenses incurred in
connection with their service as a director.
In addition, subsequent to the acquisition of Talecris, one of
our directors entered into a consultancy agreement for a term of
three years pursuant to which he will receive compensation in
the amount of $1.0 million per year with an additional
$2.0 million payable upon the fulfilment of certain
conditions.
171
Compensation
of Executive Officers
In 2010, our principal executive officers (excluding those who
also served as members of the Board) were paid compensation
amounting to €4,672,000 in the aggregate. The breakdown of
the aggregate amount paid to such executive officers for
discharging their executive duties in 2010 is set forth in the
table below.
| |
|
|
|
|
|
|
|
Amount Paid
|
|
Component
|
|
in 2010
|
|
|
|
(In thousands of Euros)
|
|
|
|
Salaries
|
|
|
3,779
|
|
|
Variable Compensation
|
|
|
893
|
|
|
Stock options and/or other securities
|
|
|
N/A
|
|
|
Other — e.g., life and health insurance
|
|
|
N/A
|
|
|
Other — e.g., pensions/savings
|
|
|
N/A
|
|
Salaries paid in U.S. dollars have been calculated at the
exchange rate between the U.S. Dollar and the Euro as of
December 31, 2010 of US$1.3362 to €1.00.
Equity
and Other Incentive Programs
In 2010, no compensation was paid pursuant to a profit-sharing
plan or any stock option and no other equity compensation was
awarded to any of our directors or executive officers.
Pension
and Retirement Compensation Programs
In 2010, no amounts were set aside or accrued by us or our
subsidiaries to provide pension, retirement or similar benefits
for our directors or executive officers.
172
SECURITY
OWNERSHIP OF MAJOR SHAREHOLDERS, DIRECTORS AND
EXECUTIVE OFFICERS
The following table sets forth certain information, including
information regarding beneficial ownership of our Class A
shares as of October 21, 2011, for (i) our major
shareholders, including, in accordance with applicable Spanish
regulations, each person or entity that is known to us to be the
beneficial owner of more than 3% of our Class A shares,
(ii) each of our directors, and (iii) each of our
executive officers. As of that date, there were a total of
213,064,899 Class A shares issued and outstanding.
Since our Class A shares are represented through book
entries, their exact ownership structure cannot be known, except
through the information that the shareholders provide
voluntarily or in compliance with applicable regulations, and
information provided by Iberclear and its participating entities.
Beneficial ownership is determined in accordance with applicable
Spanish regulations.
| |
|
|
|
|
|
|
|
|
|
|
|
Number of
|
|
Percentage of
|
|
Name of Beneficial Owner
|
|
Class A Shares
|
|
Class A Shares
|
|
|
|
Major Shareholders
|
|
|
|
|
|
|
|
|
|
Capital Research and Management
Company(1)
|
|
|
31,995,474
|
|
|
|
15.017
|
|
|
Deria
S.A.(2)
|
|
|
18,706,988
|
|
|
|
8.771
|
|
|
Scranton Enterprises
B.V.(3)
|
|
|
16,149,937
|
|
|
|
7.580
|
|
|
Thorthol Holdings
B.V.(4)
|
|
|
15,042,766
|
|
|
|
7.060
|
|
|
Víctor Grifols
Lucas(5)
|
|
|
13,112,187
|
|
|
|
6.154
|
|
|
American Funds Insurance Series Growth
Fund(6)
|
|
|
6,400,370
|
|
|
|
3.004
|
|
|
Directors
|
|
|
|
|
|
|
|
|
|
José Antonio Grifols
Gras(4)
|
|
|
15,042,766
|
|
|
|
7.060
|
|
|
Víctor Grifols Roura
|
|
|
440,450
|
|
|
|
*
|
|
|
Edgar Dalzell Jannotta
|
|
|
254,127
|
|
|
|
*
|
|
|
Ramón Riera
Roca(7)
|
|
|
169,085
|
|
|
|
*
|
|
|
Juan Ignacio Twose Roura
|
|
|
119,274
|
|
|
|
*
|
|
|
Thomas H.
Glanzmann(8)
|
|
|
79,561
|
|
|
|
*
|
|
|
Tomás Dagá Gelabert
|
|
|
51,898
|
|
|
|
*
|
|
|
Anna Veiga Lluch
|
|
|
100
|
|
|
|
*
|
|
|
Luis Isasi Fernandez De Bobadilla
|
|
|
100
|
|
|
|
*
|
|
|
Steven F. Mayer
|
|
|
0
|
|
|
|
|
|
|
W. Brett Ingersoll
|
|
|
0
|
|
|
|
*
|
|
|
Executive Officers
|
|
|
|
|
|
|
|
|
|
Antonio Viñes Parés
|
|
|
111,115
|
|
|
|
*
|
|
|
Gregory Gene Rich
|
|
|
71,598
|
|
|
|
*
|
|
|
Carlos Roura Fernández
|
|
|
48,314
|
|
|
|
*
|
|
|
Francisco Javier Jorba Ribes
|
|
|
47,364
|
|
|
|
*
|
|
|
Montserrat Lloveras Calvo
|
|
|
35,309
|
|
|
|
*
|
|
|
Vicente Blanquer Torre
|
|
|
22,377
|
|
|
|
*
|
|
|
Alberto Grifols Roura
|
|
|
13,000
|
|
|
|
*
|
|
|
David Ian Bell
|
|
|
10,000
|
|
|
|
*
|
|
|
Nuria Pascual Lapeña
|
|
|
9,796
|
|
|
|
*
|
|
|
Mateo Florencio Borrás Humbert
|
|
|
491
|
|
|
|
*
|
|
|
Alfredo Arroyo Guerra
|
|
|
—
|
|
|
|
—
|
|
|
Eva Bastida Tubau
|
|
|
—
|
|
|
|
—
|
|
|
Shinji Wada
|
|
|
—
|
|
|
|
—
|
|
|
Mary Kuhn
|
|
|
—
|
|
|
|
—
|
|
|
Joel Abelson
|
|
|
—
|
|
|
|
—
|
|
173
|
|
|
|
(1)
|
|
Capital Research and Management
Company has indirect voting rights over all 31,995,474 our
Class A shares. American Funds Insurance Series Growth
Fund has direct voting rights over 8,771,882 of our Class A
shares reported by Capital Research and Management Company.
|
| |
|
(2)
|
|
The various members of the Grifols
Roura family hold their respective shares indirectly through
Deria S.A.
|
| |
|
(3)
|
|
Scranton Enterprises B.V. is a
corporation whose shares are owned by certain of our directors
and by William Blair & Co. L.L.C. Some Grifols family
members who are directors or executive officers hold part of
their shares indirectly through Scranton Enterprises B.V.
|
| |
|
(4)
|
|
The various members of the Grifols
Gras family hold their respective shares indirectly through
Thorthol Holdings B.V., which is represented on the Board of
Directors by José Antonio Grifols Gras.
|
| |
|
(5)
|
|
13,112,187 Class A shares are
held directly by Rodellar Amsterdam B.V., through which
Víctor Grifols Lucas exercises indirect voting rights.
|
| |
|
(6)
|
|
American Funds Insurance
Series Growth Fund has direct voting rights over all
6,400,370 of our Class A shares. American Funds Insurance
Series Growth Fund has delegated the right to vote its
proxies to Capital Research and Management Company, its
investment advisor.
|
| |
|
(7)
|
|
8,000 Class A shares are held
indirectly.
|
| |
|
(8)
|
|
61,000 Class A shares are held
indirectly through Kolholmen Investments AB.
|
Significant
Changes in Ownership
In accordance with Spanish reporting requirements, the following
transfers of shares were reported to the Spanish Comisión
Nacional del Mercado de Valores, or the CNMV, as of
October 21, 2011: (i) April 9, 2009, FMR LLC
disposed of a number of Class A shares that reduced its
percentage of ownership of voting share capital below 3%;
(ii) on December 4, 2009, Victor Grifols Lucas
acquired a number of Class A shares that increased his
percentage of ownership of voting share capital above 5%;
(iii) on April 12, 2010, American Funds Insurance
Series Growth Fund acquired a number of Class A shares
that increased its percentage of ownership of voting share
capital above 3%; (iv) on November 12, 2010, The Bank
of New York Mellon Corporation disposed of a number of
Class A shares that decreased its percentage of ownership
of voting share capital below 3%; (v) on May 25, 2011,
Fidelity International Limited disposed of a number of
Class A shares that decreased its percentage of ownership
of voting share capital below 1%; (vi) on June 30,
2011, Capital Research and Management Company acquired a number
of Class A shares that increased its percentage of
ownership of voting share capital above 15%; and (vii) on
August 4, 2011, Blackrock, Inc. disposed of a number of
Class A shares that decreased its percentage of ownership
of voting share capital below 3%.
174
CERTAIN
RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Patent
and Trademark Use
Mr. José Antonio Grifols Gras, a member of our Board,
is the manager of Marca Grifols, S.L., which owns the trademark
“Grifols.” In addition, the Grifols family is the
controlling shareholder of Marca Grifols, S.L. Grifols entered
into a license agreement with Marca Grifols, S.L. on
January 26, 1993 that permits us to use the
“Grifols” trademark for 99 years. The license
agreement provides that we must pay an annual fee for the
license, which is based on inflation and our net sales. In 2008,
2009 and 2010, the fees for this license were €1,200,741,
€1,229,559 and €1,278,462 respectively.
Certain patents that we use were originally registered in the
names of Mr. Víctor Grifols Lucas, our former Chief
Executive Officer. We have previously freely used many of these
patents without any formal licensing agreements and without
paying any consideration. In June 2004, Mr. Grifols Lucas
and Mr. Grifols Roura acknowledged in writing that such
patents belong to us and in February 2006, they formally
completed the transfer of these patents to us for an immaterial
amount.
Sale and
Lease Back Transactions
On May 10, 2011 we sold five properties in Spain for an
aggregate amount of €80.4 million to Gridpan Invest,
S.L., a company owned by Scranton Enterprises, B.V., one of our
major shareholders and a related party. These properties related
primarily to non-core assets such as offices and warehouses and
a factory premise. Two of the premises were sold together with
their related mortgage loans for a total of
€53.5 million. As a result of the sale, we recognized
a net loss of €7.4 million. The prices paid for the
properties were established based on appraisals made by
independent appraisers.
Simultaneous with the sale, we entered into operating lease
agreements with Gridpan Invest, S.L. with respect to the
aforementioned properties. The key terms of the operating lease
agreements are:
|
|
|
| |
•
|
Compulsory initial term of five years;
|
| |
| |
•
|
Initial rent established at market prices, to be reviewed
annually, based on the percentage variation in the Spanish
Consumer Price Index;
|
| |
| |
•
|
Automatic extensions for five-year periods that can be
terminated by either party by advance six month notice; and
|
| |
| |
•
|
Upon vacating the premises, the lessor will reimburse us for the
remaining value of any leasehold improvements which we have made.
|
In addition, we entered into a free of charge purchase option
with respect to the shares of Gridpan Invest, S.L. exercisable
between May 10, 2016 and May 10, 2017. The strike
price will be at market value at the date of exercise, based on
appraisals made by independent appraisers. The rental expense
recognized by us for the six months period ended June 30,
2011 in connection with the operating lease agreements amounted
to €1,084 thousand, which related to the minimum
contractual payments.
Legal
Services
Mr. Tomás Dagá Gelabert, a member of our Board,
Mr. Raimon Grifols Roura, Secretary of the Board, and
Ms. Nuria Martín Barnés, Vice-Secretary of the
Board of Directors, are partners at the Barcelona office of the
Osborne Clarke, S.L.P. This law firm is advising us with regard
to Spanish law in connection with the exchange offer and renders
various other legal services to us. We believe that the fees we
pay for these services are comparable to those we would have had
to pay to a third-party law firm for similar services.
175
Charitable
Contributions
We currently contribute to two charitable foundations, the
Mr. Víctor Grifols i Lucas Foundation and the Probitas
Private Foundation. Both foundations were formed by us, and
certain of our current officers and directors serve as patrons
of the Probitas Private Foundation.
We contribute to the Mr. Víctor Grifols i Lucas
Foundation, an entity that provides grants to further the study
of bioethics. It was created in 1998 with the mission of
promoting bioethics through dialogue between specialists in a
range of areas. The Víctor Grifols i Lucas Foundation seeks
to foster ethical attitudes in organizations, companies and
individuals active in the field of human health, offering a
discussion platform which provides a forum for the exchange of
different perspectives on the ethics. Mr. Víctor
Grifols i Lucas is our former Chief Executive Officer and the
father of Mr. Víctor Grifols Roura, our Chairman of
the Board and Chief Executive Officer and Mr. Raimon
Grifols Roura, the Secretary non-member of the Board. In 2008,
2009, and 2010, we contributed €145,000, €251,000 and
€250,500, respectively, to the Víctor Grifols i Lucas
Foundation.
In addition, we contribute to the Probitas Private Foundation,
an entity that provides medical and sanitary assistance to
international communities that lack medical and sanitary
resources or that have an urgent and essential need for such
services due to catastrophes. The Probitas Private Foundation
was founded by us in 2008. Messrs. Raimon Grifols Roura, a
Secretary non-member of our Board of Directors, and Tomás
Dagá Gelabert, one of our directors, are patrons of the
Probitas Private Foundation. In 2008, 2009 and 2010, we
contributed €1,250,000, €1,449,000 and
€1,253,217, respectively. We have committed to contribute
to Probitas Private Foundation an amount equal to 0.7% of our
profits before tax each year.
176
DESCRIPTION
OF THE NOTES
On January 21, 2011, Giant Funding Corp. (the “Escrow
Issuer”), an escrow company formed solely for the purpose
of issuing the notes, completed a private offering of the
existing notes. The existing notes were issued under an
indenture (the “Indenture”) between the Escrow Issuer
and The Bank of New York Mellon Trust Company, N.A., a
national banking association (the “Trustee”). The
proceeds of the offering of the existing notes were held in
escrow pending consummation of the acquisition of Talecris and
the satisfaction of other conditions. Upon initial issuance, the
existing notes were obligations of the Escrow Issuer and were
not obligations of the Grifols Inc. (the “Company”).
On June 1, 2011 (the “Completion Date”), upon
consummation of the acquisition of Talecris and the satisfaction
of certain additional conditions described further below, the
Escrow Issuer was merged with and into the Company, the Company
assumed all of the Escrow Issuer’s obligations under the
existing notes and the Indenture and the Guarantors became
parties to the Indenture. The exchange notes will be issued by
the Company under the Indenture.
You will find the definitions of capitalized terms used in this
description under the heading “— Certain
definitions.” For purposes of this description: the word
“Company” refers only to Grifols Inc. and not
to any of its subsidiaries, the word “Parent”
refers to Grifols, S.A. and not to any of its subsidiaries; the
words “we,” “us” and
“our” each refer to Grifols, S.A. together with
its consolidated subsidiaries. In addition, the term
“Notes” has the meaning set forth in the Indenture and
refers to the existing notes, the exchange notes and any
additional notes issued under the Indenture from time to time
after this exchange offer, all of which will be treated as a
single class of securities under the Indenture. Certain defined
terms used in this description but not defined herein have the
meanings assigned to them in the Indenture. The terms of the
Notes include those expressly set forth in the Indenture and
those made part of the Indenture by reference to the
Trust Indenture Act of 1939, as amended (the
“Trust Indenture Act”).
This description of the notes (including the exchange notes) is
intended to be a useful overview of the material provisions of
the Notes and the Indenture. Since this Description of the Notes
is only a summary, it does not contain all of the details found
in the full text of, and is qualified in its entirety by the
provisions of, the Notes and the Indenture. We urge you to read
the Indenture, and any supplements thereto, because it, not this
description, defines your rights as Holders of these Notes. The
Company will make a copy of the Indenture available to the
Holders upon request and a copy of the Indenture is filed as an
exhibit to the registration statement of which this prospectus
forms a part. You may request copies of the Indenture at our
address set forth under the heading “Where You Can Find
More Information.”
Except as the context otherwise requires, the provisions
described below in this description of the Notes refer to the
provisions of the Indenture as in effect beginning on the
Completion Date.
Brief
Description of the Notes and the Guarantees
The
Notes
The Notes (including any exchange notes issued in the exchange
offer) are:
|
|
|
| |
•
|
general unsecured obligations of the Company;
|
| |
| |
•
|
senior in right of payment to all of the Company’s existing
and any future Subordinated Indebtedness;
|
| |
| |
•
|
pari passu in right of payment with all of the
Company’s existing and any future unsecured Indebtedness
that is not by its terms expressly subordinated to the Notes;
|
| |
| |
•
|
effectively junior in right of payment to the Company’s
existing and future secured Indebtedness, including the
Company’s Obligations under the Credit Agreement, to the
extent of the value of the collateral securing such Indebtedness;
|
| |
| |
•
|
unconditionally guaranteed by Parent and its Restricted
Subsidiaries that Guarantee the Obligations under the Credit
Agreement (other than any Foreign Subsidiary of the
Company); and
|
| |
| |
•
|
structurally subordinated to Indebtedness of Subsidiaries of
Parent that are not Guarantors.
|
177
The
Guarantees
Each Guarantee of the Notes is:
|
|
|
| |
•
|
a senior unsecured obligation of each Guarantor;
|
| |
| |
•
|
senior in right of payment to all existing and any future
subordinated Indebtedness of such Guarantor;
|
| |
| |
•
|
pari passu in right of payment with all existing and any
future Indebtedness of that Guarantor that is not by its terms
expressly subordinated to its Guarantee of the Notes;
|
| |
| |
•
|
effectively junior in right of payment to the existing and
future secured Indebtedness of that Guarantor, including such
Guarantor’s obligations under the Credit Agreement, to the
extent of the value of the collateral securing such
Indebtedness; and
|
| |
| |
•
|
structurally subordinated to Indebtedness of any Subsidiaries of
that Guarantor that are not Guarantors.
|
Principal,
Maturity and Interest
The Company initially issued $1,100,000,000 aggregate principal
amount of existing notes. The Company may issue additional Notes
under the Indenture from time to time. Any offering of
additional Notes is subject to compliance with the covenant
described below under the caption “Certain
Covenants — Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock.” Any additional
Notes will be identical in all respects to the Notes offered
hereby, except that additional Notes will have different
issuance dates and may have different issuance prices. The
existing notes, the exchange notes and any additional Notes
subsequently issued under the Indenture will be treated as a
single class for all purposes under the Indenture, including,
without limitation, waivers, amendments, redemptions and offers
to purchase. The Company will issue Notes in denominations of
$2,000 and integral multiples of $1,000. The Notes will mature
on February 1, 2018.
Interest on the Notes will accrue at the rate of 8.25% per annum
and will be payable semi-annually in arrears on February 1 and
August 1. Payments on the existing notes commenced on
August 1, 2011. Payments on the exchange notes will
commence on February 1, 2012. The Company will make each
interest payment to the Holders of record on the immediately
preceding January 15 and July 15.
Interest on the Notes will accrue from the date of original
issuance or, if interest has already been paid, from the date it
was most recently paid. Interest will be computed on the basis
of a 360-day
year comprised of twelve
30-day
months.
Methods
of Receiving Payments on the Notes
If a Holder has given the Company wire transfer instructions,
the Company will pay all principal, interest, premium, if any,
and Additional Interest under the registration rights agreement,
if any, on that Holder’s Notes in accordance with those
instructions. All other payments on Notes will be made at the
office or agency of the paying agent and registrar within the
City and State of New York unless the Company elects to make
interest payments by check mailed to the Holders at their
address set forth in the register of Holders.
Paying
Agent and Registrar for the Notes
The Trustee will initially act as paying agent and registrar.
The Company may change the paying agent or registrar without
prior notice to the Holders of the Notes, and Parent or any of
our Subsidiaries may act as paying agent or registrar.
Escrow
Arrangements until the Completion Date
The following is a summary of the escrow arrangements with
respect to the proceeds of the initial private offering of the
existing notes through the Completion Date. Upon initial
issuance, the existing notes were the obligations of the Escrow
Issuer and were not obligations of the Company or any
Guarantors. The Escrow Issuer was formed solely to issue the
existing notes.
178
The Indenture provides that the Escrow Issuer was required to
deposit into an account (the “Escrow Account”)
the net proceeds of the offering, plus an amount (either in cash
or Eligible Escrow Investments) sufficient to pay an amount
equal to 101% of the aggregate principal amount of the Notes,
plus accrued and unpaid interest, if any, on the Notes, through
March 9, 2011. The Escrow Amount was pledged to the
Trustee, for the benefit of the Holders of the Notes, and
invested in certain Eligible Escrow Investments in which the
Trustee, for the benefit of Holders of the Notes, had a valid
and perfected security interest. Pursuant to the Indenture, the
funds in the Escrow Account were to be released upon receipt by
the escrow agent of an officers’ certificate from the
Company to the effect that, substantially concurrently with the
release from escrow:
|
|
|
| |
•
|
the acquisition of Talecris Biotherapeutics Holdings Corp. by
the Parent and its Subsidiaries had been consummated in
accordance with the Merger Agreement without any waiver or
amendment thereof or any consent thereunder, in any case which
was materially adverse to the Holders of Notes;
|
| |
| |
•
|
(a) Parent and its other Subsidiaries party thereto had
entered into the Credit Agreement on the terms described in the
offering memorandum with such changes as were not in the
aggregate materially adverse to the Holders of Notes and
(b) no Default or Event of Default shall have occurred and
be continuing thereunder (after giving effect to the
Transactions) that shall not have been waived;
|
| |
| |
•
|
the Escrow Issuer had merged with and into the Company (with the
Company continuing as the surviving corporation), the Company
had assumed all of the obligations of the Escrow Issuer under
the Notes and the Indenture and the Guarantors had become
parties to the Indenture; and
|
| |
| |
•
|
no Default of Event of Default had occurred or was continuing
under the Indenture.
|
In the event that satisfaction of such conditions (the date of
such satisfaction, the “Completion Date”) had
not taken place on or prior to the earlier to occur of
(i) the determination by the Company’s Board of
Directors, in its good faith judgment, that the Completion Date
would not occur by March 4, 2011 (the “Outside
Date”) or, in the event an Escrow Extension Election
has been made, the Extended Outside Date or (ii) the
Outside Date or, in the event an Escrow Extension Election has
been made, the Extended Outside Date (such earlier date, the
“Date of Determination”), the funds in the
Escrow Account would have been released on the Special
Redemption Date for the purpose of effecting the mandatory
redemption (the “Special Redemption”) of the
Notes in accordance with the requirements of the Indenture (see
“— Special Redemption”). Notwithstanding the
foregoing, the Company could by notice to the Trustee and the
Escrow Agent (an “Escrow Extension Election”)
delivered not later than February 25, 2010, make a one-time
election to extend the Outside Date to a date (the
“Extended Outside Date”) specified by the
Company that was not later than September 30, 2011 so long
as concurrently with the provision of such notice, the Company
deposited an amount sufficient, when taken together with the
amount of Eligible Escrow Investments then on deposit in the
Escrow Account, to pay an amount equal to 101% of the aggregate
principal amount of the Notes plus all accrued interest on the
Notes through the Extended Outside Date. The Company delivered
notice to the Trustee and the escrow agent on February 24,
2011 and exercised its right to make an Escrow Extension
Election. The Extended Outside Date was June 30, 2011. Any
excess funds remaining in the Escrow Account after the Special
Redemption would be released to the Company.
Funds held in the Escrow Account, pending release for the uses
set forth above, were permitted to be invested at the direction
of the Escrow Issuer in Eligible Escrow Investments maturing
prior to the date three business days after the Extended Outside
Date; provided that such Eligible Escrow Investments were
required to be held in the Escrow Account and that the Trustee,
for the benefit of the Holders, was required to have a valid and
perfected security interest in such Eligible Escrow Investments
until their liquidation or conversion to cash for release for
the uses set forth above. See “— Special
Redemption.”
Prior to the Completion Date, the Escrow Issuer was required to
have no business or activities (other than as contemplated
above, including the merger into the Company on the Completion
Date) or assets or liabilities other than the Notes, but the
other restrictive covenants in the Indenture generally did not
apply. Following the merger of the Escrow Issuer with and into
the Company and the assumption by the Company and the Guarantors
of their obligations under the Indenture and the Notes, all
restrictive covenants were deemed to
179
have been applicable to the Parent and its Restricted
Subsidiaries beginning on the Issue Date and, to the extent that
the Parent and its Restricted Subsidiaries took any action or
inaction after the Issue Date and prior to the Completion Date
that was prohibited by the Indenture, the Company shall have
been in Default on such date. For the avoidance of doubt, and
notwithstanding anything to the contrary set forth in the
Indenture, consummation of the Transactions, including the entry
into the Credit Agreement and borrowings thereunder and the
merger of the Escrow Issuer with and into the Company and the
Guarantee of the Notes by the Guarantors, in each case to occur
on the Completion Date substantially as described in the
offering memorandum, were permitted.
On the Completion Date, the proceeds from the offering of the
existing notes were disbursed from the Escrow Account and were
used to pay a portion of the acquisition purchase price. The
Company succeeded to the assets of the Escrow Issuer, and all
accrued but undistributed income on the Eligible Escrow
Investments was for the account of the Company and not the
Holders.
Guarantees
The Notes are Guaranteed by Parent and the Restricted
Subsidiaries that guarantee the obligations under the Credit
Agreement (other than any Foreign Subsidiary of the Company and
any Domestic Subsidiary that is an Immaterial Subsidiary). These
Guarantees are joint and several obligations of the Guarantors.
The obligations of each Guarantor under its Guarantee will be
limited as necessary to prevent that Guarantee from constituting
a fraudulent conveyance under applicable law, after giving
effect to all other obligations of that Guarantor, including its
Guarantee of all obligations under the Credit Agreement. If a
Guarantee were to be rendered voidable, it could be subordinated
by a court to all other debt, including Guarantees and
contingent liabilities, of the applicable Guarantor and,
depending on the amount of such debt, a Guarantor’s
liability in respect of its Guarantee could be reduced to zero.
See “Risk Factors — A guarantee could be
voided if it constitutes a fraudulent transfer under
U.S. bankruptcy or similar state law, which would prevent
the holders of the notes from relying on that Guarantor to
satisfy claims.”
A Guarantor may not sell or otherwise dispose of all or
substantially all of its assets to, or consolidate with or merge
with or into (whether or not such Guarantor is the surviving
Person), another Person, other than us or another Guarantor,
unless:
(1) immediately after giving effect to that transaction, no
Default or Event of Default exists; and
(2) either:
(a) the Person acquiring the property in any such sale or
disposition or the Person formed by or surviving any such
consolidation or merger (if other than a Guarantor) assumes all
the obligations of that Guarantor under the Indenture and its
Guarantee and the registration rights agreement pursuant to a
supplemental Indenture and other documents satisfactory to the
Trustee; or
(b) except in the case of Parent, the Net Proceeds of such
sale or other disposition are applied in accordance with the
provisions of the Indenture relating to Asset Sales.
The Guarantee of a Guarantor will be released:
(1) except in the case of Parent, in connection with
(a) any sale or other disposition of all of the assets of
that Guarantor (including by way of merger or consolidation) to
a Person that is not (either before or after giving effect to
such transaction) a Restricted Subsidiary of Parent, if the sale
or other disposition complies with the provisions of the
Indenture relating to Asset Sales or (b) any sale of all of
the Capital Stock of a Guarantor to a Person that is not (either
before or after giving effect to such transaction) Parent or a
Restricted Subsidiary of Parent, if the sale complies with the
provisions of the Indenture relating to Asset Sales, in each
case as provided below under the caption “Repurchase at the
Option of Holders — Asset Sales”;
(2) if the Company designates any Restricted Subsidiary
that is a Guarantor as an Unrestricted Subsidiary in accordance
with the applicable provisions of the Indenture;
180
(3) upon Legal Defeasance or Covenant Defeasance as
provided below under the heading “Legal Defeasance and
Covenant Defeasance” and upon a discharge of the Indenture
as provided under the heading “Satisfaction and
Discharge”; or
(4) except in the case of Parent, if such Guarantor shall
not Guarantee any Indebtedness under any Credit Facility (other
than if such Guarantor no longer Guarantees any such
Indebtedness as a result of payment under any Guarantee of any
such Indebtedness by any Guarantor); provided that a
Guarantor shall not be permitted to be released from its
Guarantee pursuant to this clause (4) if it is an obligor
with respect to Indebtedness that would not, under “Certain
Covenants — Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock,” be permitted to be
incurred by a Restricted Subsidiary that is not a Guarantor
(unless it is also designated as an Unrestricted Subsidiary).
Optional
Redemption
Except as set forth below, the Notes are not redeemable at the
Company’s option prior to February 1, 2014.
On or prior to February 1, 2014, the Company may on one or
more occasions redeem up to 35% of the aggregate principal
amount of Notes issued under the Indenture at a redemption price
of 108.250% of the principal amount thereof, plus accrued and
unpaid interest and Additional Interest, if any, to the
redemption date, with the net cash proceeds of any Qualified
Equity Offering; provided that:
(1) at least 65% of the aggregate principal amount of Notes
originally issued under the Indenture remains outstanding
immediately after the occurrence of such redemption (excluding
Notes held by Parent and its Subsidiaries); and
(2) the redemption occurs within 90 days of the date
of the closing of such Qualified Equity Offering.
On or prior to February 1, 2014, the Company may redeem all
or a part of the Notes, upon not less than 30 nor more than
60 days’ prior notice mailed by first-class mail to
the registered address of each Holder of Notes or otherwise in
accordance with the procedures of The Depository
Trust Company, at a redemption price equal to 100% of the
principal amount of the Notes redeemed plus the Applicable
Premium as of, and accrued and unpaid interest and Additional
Interest, if any, to the redemption date, subject to the rights
of Holders of Notes on the relevant record date to receive
interest due on the relevant interest payment date.
Except pursuant to the prior paragraphs, the Notes are not
redeemable at the Company’s option prior to
February 1, 2014. The Company is not prohibited by the
terms of the Indenture, however, from acquiring the Notes by
means other than a redemption, whether pursuant to an issuer
tender offer, in open market transactions or otherwise, assuming
such acquisition does not otherwise violate the terms of the
Indenture.
On or after February 1, 2014, the Company may redeem all or
a part of the Notes, upon not less than 30 nor more than
60 days’ notice, at the redemption prices (expressed
as percentages of principal amount) set forth below plus accrued
and unpaid interest and Additional Interest, if any, on the
Notes redeemed, to the applicable redemption date, if redeemed
during the twelve-month period beginning on February 1 of the
years indicated below:
| |
|
|
|
|
|
Fiscal Year
|
|
Percentage
|
|
|
|
2014
|
|
|
106.188
|
%
|
|
2015
|
|
|
104.125
|
%
|
|
2016
|
|
|
102.063
|
%
|
|
2017 and thereafter
|
|
|
100.000
|
%
|
Unless the Company defaults in the payment of the redemption
price, interest will cease to accrue on the Notes or portions
thereof called for redemption on the applicable redemption date.
181
Special
Redemption
As described under the caption, “— Escrow
Arrangements through the Completion Date,” in the event
that the Completion Date had not occurred on or prior to the
Date of Determination, the Escrow Issuer would have been
required to redeem the Notes, on the date that was three
business days after the Date of Determination (the
“Special Redemption Date”), at a cash
redemption price of 101% of the aggregate principal amount of
the Notes, plus accrued and unpaid interest, if any, to the date
of redemption. The Indenture provided that upon the receipt of
written instruction from the Escrow Issuer and an officers’
certificate, the Trustee would send a notice of such redemption
on behalf of the Escrow Issuer to the Holders of the Notes of
such Special Redemption on the Date of Determination if the
Completion Date had not occurred on or prior to such Date of
Determination.
Mandatory
Redemption
The Company is not required to make sinking fund payments with
respect to the Notes. However, under certain circumstances, the
Company may be required to offer to purchase the Notes as
described under the captions “— Special
Redemption” and “— Repurchase at the Option
of Holders.”
Offers to
Purchase; Open Market Purchases
The Company and its Subsidiaries may acquire Notes by means
other than a redemption or required repurchase, whether by
tender offer, open market purchases, negotiated transactions or
otherwise, in accordance with applicable securities laws, so
long as such acquisition does not otherwise violate the terms of
the Indenture. However, other existing or future agreements of
the Company or its Subsidiaries may limit the ability of the
Company or its Subsidiaries to purchase Notes prior to maturity.
Additional
Amounts
All payments made by any Guarantor that is not formed or
incorporated under the laws of the United States or any
State of the United States or the District of Columbia (each, a
“non-U.S. Guarantor”)
under or with respect to such
non-U.S. Guarantor’s
Guarantee will be made free and clear of and without withholding
or deduction for or on account of any present or future Taxes
imposed or levied by or on behalf of any Taxing Authority within
Spain or other jurisdiction in which such
non-U.S. Guarantor
is organized, resident or doing business for tax purposes or
within or through which payment is made or any political
subdivision or taxing authority or agency thereof or therein
(any of the aforementioned being a “Taxing
Jurisdiction”), unless such
non-U.S. Guarantor
is required to withhold or deduct Taxes by law or by the
interpretation or administration thereof. If any
non-U.S. Guarantor
is required to withhold or deduct any amount for or on account
of Taxes imposed by a Taxing Authority within Spain, or within
any other Taxing Jurisdiction, from any payment made under or
with respect to the Guarantee of such
non-U.S. Guarantor,
such
non-U.S. Guarantor
will pay such additional amounts (“Additional
Amounts”) as may be necessary so that the net amount
received by each Holder of Notes (including Additional Amounts)
after such withholding or deduction will equal the amount the
holder would have received if such Taxes had not been withheld
or deducted; provided, however, that no Additional
Amounts will be payable with respect to:
(1) any Tax imposed by the United States or by any
political subdivision or Taxing Authority thereof or therein;
(2) any Taxes that would not have been so imposed, deducted
or withheld but for the existence of any connection between the
Holder or beneficial owner of a Note (or between a fiduciary,
settlor, beneficiary, member or shareholder of, or possessor of
power over, the Holder or beneficial owner of such Note, if the
holder or beneficial owner is an estate, nominee, trust,
partnership or corporation) and the relevant Taxing Jurisdiction
(other than the mere receipt of such payment or the ownership or
holding of the execution, delivery, registration or enforcement
of such Note);
(3) any estate, inheritance, gift, sales, excise, transfer
or personal property Tax or similar Tax, assessment or
governmental charge, subject to the last paragraph of this
covenant;
182
(4) any Taxes payable otherwise than by deduction or
withholding from payments under or with respect to the Guarantee
by any
non-U.S. Guarantor
of such Note;
(5) any Taxes that would not have been so imposed, deducted
or withheld if the Holder or beneficial owner of a Note or
beneficial owner of any payment on the Guarantee of such Note
had (i) made a declaration of non-residence, or any other
claim or filing for exemption, to which it is entitled or
(ii) complied with any certification, identification,
information, documentation or other reporting requirement
concerning the nationality, residence, identity or connection
with the relevant Taxing Jurisdiction of such holder or
beneficial owner of such Note or any payment on such Note
(provided that (x) such declaration of non-residence
or other claim or filing for exemption or such compliance is
required by the applicable law of the Taxing Jurisdiction as a
precondition to exemption from, or reduction in the rate of the
imposition, deduction or withholding of, such Taxes and
(y) at least 30 days prior to the first payment date
with respect to which such declaration of non-residence or other
claim or filing for exemption or such compliance is required
under the applicable law of the Taxing Jurisdiction, holders at
that time have been notified by such Guarantor or any other
Person through whom payment may be made that a declaration of
non-residence or other claim or filing for exemption or such
compliance is required to be made);
(6) any Taxes that would not have been so imposed, deducted
or withheld if the beneficiary of the payment had presented the
Note for payment within 30 days after the date on which
such payment or such Note became due and payable or the date on
which payment thereof is duly provided for, whichever is later
(except to the extent that the Holder would have been entitled
to Additional Amounts had the Note been presented on the last
day of such
30-day
period);
(7) any payment under or with respect to a Note to any
Holder that is a fiduciary or partnership or any Person other
than the sole beneficial owner of such payment or Note, to the
extent that a beneficiary or settlor with respect to such
fiduciary, a member of such partnership or the beneficial owner
of such payment or Note would not have been entitled to the
Additional Amounts, or to a reduced amount of Additional
Amounts, had such beneficiary, settlor, member or beneficial
owner been the actual Holder of such Note; or
(8) any combination of items (1) through
(7) above.
The foregoing provisions shall survive any termination or
discharge of the Indenture and payment of the Notes and shall
apply mutatis mutandis to any Taxing Jurisdiction with
respect to any successor Person to a
non-U.S. Guarantor.
Each applicable
non-U.S. Guarantor
will also make any applicable withholding or deduction and remit
the full amount deducted or withheld to the relevant authority
in accordance with applicable law. Each applicable
non-U.S. Guarantor
will furnish to the Trustee, within 60 days after the date
the payment of any Taxes deducted or withheld is due pursuant to
applicable law, certified copies of tax receipts or, if such tax
receipts are not reasonably available to such
non-U.S. Guarantor,
such other documentation that provides reasonable evidence of
such payment by such
non-U.S. Guarantor.
Copies of such tax receipts or, if such tax receipts are not
reasonably available, such other documentation will be made
available to the Holders or the paying agent, as applicable,
upon request.
At least 30 days prior to each date on which any payment
under or with respect to any Guarantee is due and payable, if
any
non-U.S. Guarantor
will be obligated to pay Additional Amounts with respect to such
payment, such
non-U.S. Guarantor
will deliver to the Trustee and the paying agent an
officers’ certificate stating the fact that such Additional
Amounts will be payable and the amounts so payable and will set
forth such other information necessary to enable such Trustee
and paying agent to pay such Additional Amounts to Holders of
such Notes on the payment date, unless such obligation to pay
Additional Amounts arises after the 30th day prior to such
date, in which case it shall be promptly paid thereafter.
Whenever in the Indenture or in this “Description of the
Notes” there is mentioned, in any context, the payment of
principal, premium, if any, interest or of any other amount
payable under or with respect to any
183
Note, such mention shall be deemed to include mention of the
payment of Additional Amounts to the extent that, in such
context, Additional Amounts are, were or would be payable in
respect thereof.
The
non-U.S. Guarantors
will pay any present or future stamp, court or documentary taxes
or any other excise or property taxes, charges or similar levies
that arise in any jurisdiction from the execution, delivery,
enforcement or registration of their respective Guarantees of
the Notes, the Indenture or any other document or instrument in
relation thereto, excluding all such taxes, charges or similar
levies imposed by any jurisdiction outside the United States in
which any
non-U.S. Guarantor
or any successor Person is organized or resident for tax
purposes or any jurisdiction in which a paying agent is located,
and the
non-U.S. Guarantors
will agree to indemnify the Holders of the Notes for any such
non-excluded taxes paid by such Holders.
Repurchase
at the Option of Holders
Change
of Control
Upon the occurrence of a Change in Control, the Company shall be
obligated to make an offer to purchase (a “Change of
Control Offer”) and each Holder of Notes will have the
right to require the Company to repurchase all or any part
(equal to $1,000 or an integral multiple of $1,000) of that
Holder’s Notes pursuant to a Change of Control Offer on the
terms set forth in the Indenture. In the Change of Control
Offer, the Company will offer a Change of Control payment in
cash equal to 101% of the aggregate principal amount of Notes
repurchased plus accrued and unpaid interest and Additional
Interest, if any, on the Notes repurchased, to the date of
purchase. The Company shall be required to purchase all Notes
tendered pursuant to the Change of Control Offer and not
withdrawn. Subject to compliance with the provisions of the
third succeeding paragraph, within 30 days following any
Change of Control, the Company will mail a notice to the Trustee
and each Holder describing the transaction or transactions that
constitute the Change of Control and offering to repurchase
Notes on the Change of Control payment date specified in the
notice, which date will be no earlier than 30 days and no
later than 60 days from the date such notice is mailed,
pursuant to the procedures required by the Indenture and
described in such notice. The Company will comply with the
requirements of
Rule 14e-1
under the Exchange Act and any other securities laws and
regulations thereunder to the extent those laws and regulations
are applicable in connection with the repurchase of the Notes as
a result of a Change of Control. To the extent that the
provisions of any securities laws or regulations conflict with
the Change of Control provisions of the Indenture, the Company
will comply with the applicable securities laws and regulations
and will not be deemed to have breached its obligations under
the Change of Control provisions of the Indenture by virtue of
such conflict.
On the Change of Control payment date, the Company will, to the
extent lawful:
(1) accept for payment all Notes or portions of Notes
validly and properly tendered and not withdrawn pursuant to the
Change of Control Offer;
(2) deposit with the paying agent an amount equal to the
Change of Control payment in respect of all Notes or portions of
Notes validly and properly tendered and not withdrawn; and
(3) deliver or cause to be delivered to the Trustee the
Notes properly accepted together with an officers’
certificate stating the aggregate principal amount of Notes or
portions of Notes being purchased by the Company.
The paying agent will promptly mail (or wire) to each Holder of
Notes validly and properly tendered and not withdrawn the Change
of Control payment for such Notes, and the Trustee will promptly
authenticate and mail (or cause to be transferred by book entry)
to each Holder a new Note equal in principal amount to any
unpurchased portion of the Notes surrendered, if any;
provided that each new Note will be in a principal amount
of $2,000 or an integral multiple of $1,000 in excess thereof.
The Company will publicly announce the results of a Change of
Control Offer on or as soon as practicable after the Change of
Control payment date.
The provisions described above that require the Company to make
a Change of Control Offer following a Change of Control will be
applicable whether or not any other provisions of the Indenture
are applicable,
184
except as described below under “Legal Defeasance and
Covenant Defeasance.” Except as described above with
respect to a Change of Control, the Indenture does not contain
provisions that permit the Holders of the Notes to require that
the Company repurchase or redeem the Notes in the event of a
takeover, recapitalization, spin-off or similar transaction.
The Company will not be required to make a Change of Control
Offer upon a Change of Control if (i) a third party makes
the Change of Control Offer in the manner, at the times and
otherwise in compliance with the requirements set forth in the
Indenture applicable to a Change of Control Offer made by the
Company and purchases all Notes validly and properly tendered
and not withdrawn under the Change of Control Offer,
(ii) notice of redemption of all of the Notes has been
given pursuant to the Indenture as described above under the
caption “Optional Redemption,” unless and until there
is a default in payment of the applicable redemption price, or
(iii) in connection with or in contemplation of any Change
of Control for which a definitive agreement is in place the
Company or a third party has made an offer to purchase (an
“Alternate Offer”) any and all Notes validly
and properly tendered at a cash price equal to or higher than
the Change of Control Payment and has purchased all Notes
validly and properly tendered and not withdrawn in accordance
with the terms of such Alternate Offer; provided that the
terms of such Alternate Offer shall not require Holders to
irrevocably tender Notes and such Alternate Offer shall not
close unless and until the Change of Control is actually
consummated.
The provisions under the Indenture relative to the
Company’s obligation to make a Change of Control Offer may,
prior to the occurrence of a Change of Control, be waived or
modified with the consent of the Holders of at least a majority
in principal amount of the then outstanding Notes issued under
the Indenture. Following the occurrence of a Change of Control,
any change, amendment or modification in any material respect of
the obligation of the Company to make and consummate a Change of
Control Offer may only be effected with the consent of each
holder affected thereby.
The definition of Change of Control includes a phrase relating
to the direct or indirect sale, lease, transfer, conveyance or
other disposition of “all or substantially all” of the
properties or assets of the Company and its Restricted
Subsidiaries taken as a whole. Although there is a limited body
of case law interpreting the phrase “substantially
all,” there is no precise established definition of the
phrase under applicable law. Accordingly, the ability of a
Holder of Notes to require the Company to repurchase its Notes
as a result of a sale, lease, transfer, conveyance or other
disposition of less than all of the assets of the Company and
its Restricted Subsidiaries taken as a whole to another Person
or group may be uncertain.
Asset
Sales
Parent will not, and will not permit any of the Restricted
Subsidiaries to, make any Asset Sale unless:
(1) Parent (or the Restricted Subsidiary, as the case may
be) receives consideration at the time of the Asset Sale at
least equal to the fair market value of the assets sold, leased,
transferred, conveyed or otherwise disposed of; and
(2) at least 75% of the consideration received in the Asset
Sale by Parent or such Restricted Subsidiary is in the form of
cash, Cash Equivalents or Replacement Assets, or a combination
thereof.
For purposes of this provision, each of the following will be
deemed to be cash:
(a) any liabilities of Parent or any of the Restricted
Subsidiaries, as shown on Parent’s or such Restricted
Subsidiary’s most recent balance sheet (other than
contingent liabilities and liabilities that are by their terms
subordinated to the Notes or any Guarantee), that are assumed by
the transferee of any such assets and with respect to which
Parent or such Restricted Subsidiary is released from further
liability;
(b) any securities, Notes or other obligations received by
Parent or such Restricted Subsidiary from such transferee that
are converted by Parent or such Restricted Subsidiary into cash
within 365 days of the consummation of such Asset Sale
(subject to ordinary settlement periods), to the extent of the
cash received in that conversion;
185
(c) any Voting Stock or assets referred to in
clauses (2) and (3) of the following
paragraph; and
(d) any Designated Non-Cash Consideration received by
Parent or such Restricted Subsidiary in such Asset Sale having
an aggregate fair market value (as determined in good faith by
Parent’s Board of Directors), taken together with all other
Designated Non-Cash Consideration received pursuant to this
clause (d) that is at such time outstanding, not to exceed
an amount equal to the greater of (x) $80 million and
(y) 2.0% of Total Assets at the time of the receipt of such
Designated Non-Cash Consideration, with the fair market value of
each item of Designated Non-Cash Consideration being measured at
the time received and without giving effect to subsequent
changes in value.
Within 365 days after the receipt of any Net Proceeds from
an Asset Sale, Parent or such Restricted Subsidiary may apply
those Net Proceeds at our option:
(1) to repay Indebtedness and other Obligations under any
Credit Facility;
(2) to acquire all or substantially all of the assets of,
or a majority of the Voting Stock of, another Permitted Business;
(3) to make any capital expenditures or to acquire other
long-term assets that are used or useful in a Permitted
Business; or
(4) any combination of the foregoing.
In the case of each of clauses (2), (3) and (4) above,
the entry into a definitive agreement to acquire such assets
within 365 days after the receipt of any Net Proceeds from
an Asset Sale shall be treated as a permitted application of the
Net Proceeds from the date of such agreement so long as Parent
or such Restricted Subsidiary enters into such agreement with
the good faith expectation that such Net Proceeds will be
applied to satisfy such commitment within 180 days of such
agreement and such Net Proceeds are actually so applied within
such period.
Pending the final application of any Net Proceeds, we may
temporarily reduce revolving credit borrowings under our Credit
Agreement or otherwise invest the Net Proceeds in any manner
that is not prohibited by the Indenture.
Any Net Proceeds from Asset Sales that are not applied or
invested as provided in the second paragraph of this covenant
will constitute “Excess Proceeds.” When the
aggregate amount of Excess Proceeds exceeds $50 million,
the Company will make an Asset Sale Offer to all Holders of
Notes and all Holders of other Indebtedness of Parent or any of
the Restricted Subsidiaries that is pari passu with the
Notes containing provisions similar to those set forth in the
Indenture with respect to offers to purchase or redeem with the
proceeds of sales of assets to purchase the maximum principal
amount of Notes and such other pari passu Indebtedness
that may be purchased out of the Excess Proceeds. The offer
price in any Asset Sale Offer will be equal to 100% of the
principal amount thereof plus accrued and unpaid interest and
Additional Interest, if any, to the date of purchase, and will
be payable in cash. If any Excess Proceeds remain after
consummation of an Asset Sale Offer, we may use those Excess
Proceeds for any purpose not otherwise prohibited by the
Indenture. If the aggregate principal amount of Notes and other
pari passu Indebtedness validly and properly tendered and
not withdrawn into such Asset Sale Offer exceeds the amount of
Excess Proceeds, the Trustee will select the Notes and the
Company or the Trustee, agent or other similar party with
respect to such other pari passu Indebtedness will select
such Indebtedness to be purchased as described below under
“Selection and Notice.” Upon completion of each Asset
Sale Offer, the amount of Excess Proceeds will be reset at zero.
The Company will comply with the requirements of
Rule 14e-1
under the Exchange Act and any other securities laws and
regulations thereunder to the extent those laws and regulations
are applicable in connection with each repurchase of Notes
pursuant to an Asset Sale Offer. To the extent that the
provisions of any securities laws or regulations conflict with
the Asset Sale provisions of the Indenture, the Company will
comply with the applicable securities laws and regulations and
will not be deemed to have breached its obligations under the
Asset Sale provisions of the Indenture by virtue of such
compliance.
186
Parent’s and the Restricted Subsidiaries’ existing and
future Indebtedness may contain limitations on certain events
that would constitute a Change of Control or Asset Sale or
require such Indebtedness to be repurchased upon a Change of
Control or Asset Sale. Moreover, the exercise by Holders of
Notes of their right to require us to repurchase such Notes
could cause a default under Parent’s and the Restricted
Subsidiaries’ existing or future Indebtedness, even if the
Change of Control or Asset Sale itself does not, due to the
financial effect of such purchases on us. In the event that a
Change of Control or Asset Sale occurs at a time when the
Company is prohibited from purchasing Notes, the Company could
seek the consent of the applicable lenders to the purchase of
Notes or could attempt to refinance the borrowings that contain
such prohibition. If the Company does not obtain a consent or
repay those borrowings, the Company will remain prohibited from
purchasing Notes. In addition, the Company’s ability to pay
cash to Holders of Notes upon a repurchase may be limited by the
Company’s then existing financial resources. The Company
cannot assure you that sufficient funds will be available when
necessary to make any required repurchases. The Company’s
failure to repurchase Notes in connection with a Change of
Control or Asset Sale would result in a default under the
Indenture. Such a default would, in turn, constitute a default
under Parent’s existing Indebtedness and may constitute a
default under future Indebtedness as well. The Company’s
obligation to make an offer to repurchase the Notes as a result
of a Change of Control may be waived or modified at any time
prior to the occurrence of such Change of Control with the
written consent of the Holders of at least a majority in
aggregate principal amount of the Notes then outstanding. See
“Amendment, Supplement and Waiver.”
Selection
and Notice
If less than all of the Notes are to be redeemed or purchased at
any time, the Trustee will select Notes for redemption or
purchase as follows:
(1) if the Notes are listed on any national securities
exchange, in compliance with the requirements of the principal
national securities exchange on which the Notes are
listed; or
(2) if the Notes are not listed on any national securities
exchange, on a pro rata basis, by lot or by such method as the
Trustee deems fair and appropriate.
No Notes of $2,000 or less can be redeemed in
part. Notices of purchase or redemption will be
mailed by first class mail at least 30 but not more than
60 days before the redemption date to each Holder of Notes
to be redeemed at its registered address, except that redemption
notices may be mailed more than 60 days prior to a
redemption date if the notice is issued in connection with a
defeasance of the Notes or a satisfaction and discharge of the
Indenture. Any inadvertent defect in the notice of redemption,
including an inadvertent failure to give notice, to any Holder
selected for redemption will not impair or affect the validity
of the redemption of any other Note redeemed in accordance with
the provisions of the Indenture. Notices of redemption may not
be conditional.
If any Note is to be redeemed in part only, the notice of
redemption that relates to that Note will state the portion of
the principal amount of that Note that is to be redeemed. A new
Note in principal amount equal to the unredeemed portion of the
original Note will be issued in the name of the Holder of Notes
upon cancellation of the original Note. Notes called for
redemption become due on the date fixed for redemption. Notes
held in certificated form must be surrendered to the paying
agent in order to collect the redemption price. On and after the
redemption date, interest ceases to accrue on Notes or portions
of them called for redemption.
Certain
Covenants
Set forth below are summaries of certain covenants that are
contained in the Indenture. If on any date following the Issue
Date (i) the Notes have Investment Grade Ratings from both
Rating Agencies, and (ii) no Default has occurred and is
continuing under the Indenture (the occurrence of the events
described in the foregoing clauses (i) and (ii) being
collectively referred to as a “Covenant Suspension
Event”), then Parent
187
and the Restricted Subsidiaries will not be subject to the
following covenants (collectively, the “Suspended
Covenants”):
(1) “Repurchase at the Option of Holders —
Asset Sales”;
(2) “— Restricted Payments”;
(3) “— Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock”;
(4) clause (d) of the first paragraph of
“— Merger, Consolidation or Sale of Assets”;
(5) “— Transactions with Affiliates”;
(6) “— Dividend and Other Payment Restrictions
Affecting Restricted Subsidiaries”; and
(7) “— Designation of Restricted and Unrestricted
Subsidiaries.”
In the event that Parent and the Restricted Subsidiaries are not
subject to the Suspended Covenants under the Indenture for any
period of time as a result of the foregoing, and on any
subsequent date (the “Reversion Date”) one or
both of the Rating Agencies withdraw their Investment Grade
Rating or downgrade the rating assigned to the Notes below an
Investment Grade Rating, then Parent and the Restricted
Subsidiaries will thereafter again be subject to the Suspended
Covenants under the Indenture with respect to future events,
including, without limitation, a proposed transaction described
in clause (b) above.
The period of time between the Suspension Date and the Reversion
Date is referred to in this description as the
“Suspension Period.” Additionally, upon the
occurrence of a Covenant Suspension Event, the amount of Excess
Proceeds from Net Proceeds shall be reset at zero. In the event
of any such reinstatement of the Suspended Covenants, no action
taken or omitted to be taken by Parent or any of the Restricted
Subsidiaries prior to such reinstatement will give rise to a
Default or Event of Default under the Indenture; provided
that (1) with respect to Restricted Payments made after
any such reinstatement, the amount of Restricted Payments made
will be calculated as though the covenant described under the
caption “— Restricted Payments” had been in
effect prior to, but not during, the Suspension Period,
provided that no Subsidiaries may be designated as
Unrestricted Subsidiaries during the Suspension Period) and
(2) all Indebtedness incurred, or Disqualified Stock or
Preferred Stock issued, during the Suspension Period will be
classified to have been incurred or issued pursuant to
clause (2) of the second paragraph of
“— Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock.”
There can be no assurance that the Notes will ever achieve or
maintain Investment Grade Ratings.
Restricted
Payments
Parent will not, and will not permit any of the Restricted
Subsidiaries to, directly or indirectly:
(1) declare or pay any dividend or make any other payment
or distribution on account of Parent’s or any Restricted
Subsidiaries’ Equity Interests (including, without
limitation, any payment in connection with any merger or
consolidation involving Parent or any Restricted Subsidiary) or
to the direct or indirect holders of Parent’s or any
Restricted Subsidiaries’ Equity Interests in their capacity
as such (in each case other than dividends or distributions
payable in Parent’s Equity Interests (other than
Disqualified Stock) or to Parent or any Restricted Subsidiary);
(2) purchase, redeem, defease or otherwise acquire or
retire for value (including, without limitation, in connection
with any merger or consolidation involving Parent or any of the
Restricted Subsidiaries) any of Parent’s or the Restricted
Subsidiaries’ Equity Interests (in each case other than any
of the Restricted Subsidiaries’ Equity Interests owned by
Parent or another Restricted Subsidiary or for consideration
consisting solely of Parent’s Equity Interests other than
Disqualified Stock);
(3) make any payment on or with respect to, or purchase,
redeem, repurchase, defease or otherwise acquire or retire for
value any of Parent’s or the Restricted Subsidiaries’
Subordinated Indebtedness (other than Subordinated Indebtedness
owed to Parent or any of the Restricted Subsidiaries), except
(i) a payment of interest or principal at the Stated
Maturity thereof, (ii) the purchase, repurchase or other
188
acquisition of any such Indebtedness in anticipation of
satisfying a sinking fund obligation, principal installment or
final maturity, in each case, due within one year of the date of
such purchase, repurchase or other acquisition, or
(iii) for consideration consisting solely of Parent’s
Equity Interests other than Disqualified Stock; or
(4) make any Restricted Investment
(all such payments and other actions set forth in these
clauses (1) through (4) above being collectively
referred to as “Restricted Payments”), unless,
at the time of and after giving effect to such Restricted
Payment:
(1) no Default or Event of Default has occurred and is
continuing or would occur as a consequence of such Restricted
Payment;
(2) Parent would, at the time of such Restricted Payment
and after giving pro forma effect thereto as if such Restricted
Payment had been made at the beginning of the applicable
four-quarter period, have been permitted to incur at least $1.00
of additional Indebtedness pursuant to the Fixed Charge Coverage
Ratio test set forth in the first paragraph of
“— Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock”; and
(3) such Restricted Payment, together with the aggregate
amount of all other Restricted Payments made by Parent and the
Restricted Subsidiaries after the Issue Date (excluding
Restricted Payments permitted by clauses (3)(i), (5), (6), (10),
(11) and (12) of the next paragraph), is less than the
sum, without duplication, of:
(A) 50% of the Consolidated Net Income of Parent for the
period (taken as one accounting period) from the beginning of
the first full fiscal quarter of Parent commencing immediately
prior to the Issue Date to the end of Parent’s most
recently ended fiscal quarter for which internal financial
statements are available at the time of such Restricted Payment
(or, if such Consolidated Net Income for such period is a
deficit, less 100% of such deficit), plus
(B) 100% of the aggregate net cash proceeds or the fair
value (as determined in good faith by the Board of Directors) of
property or assets received by Parent or a Restricted Subsidiary
since the date following the Issue Date as a contribution to the
common equity capital of Parent or from the issue or sale of
Equity Interests of Parent (other than Disqualified Stock) or
from the issue or sale of convertible or exchangeable
Disqualified Stock or convertible or exchangeable debt
securities of Parent that have been converted into or exchanged
for such Equity Interests (other than Equity Interests or
Disqualified Stock or debt securities sold to a Subsidiary of
Parent and other than proceeds from the BBVA ‘B’ Share
Transaction), together with the aggregate net cash and Cash
Equivalents received by Parent or any Restricted Subsidiaries at
the time of such conversion or exchange, plus
(C) to the extent that any Restricted Investment that was
made after the Issue Date is sold for cash or otherwise
liquidated or repaid for cash, the proceeds realized from the
sale of such Restricted Investment and proceeds representing the
return of the capital with respect to such Restricted
Investment, in each case to Parent or any Restricted Subsidiary,
less the cost of the disposition of such Restricted Investment,
plus
(D) to the extent that any Unrestricted Subsidiary is
redesignated as a Restricted Subsidiary after the Issue Date,
the portion (proportionate to Parent’s interest in such
Unrestricted Subsidiary) of the fair market value of the net
assets of the Unrestricted Subsidiary at the time such
Unrestricted Subsidiary is designated a Restricted Subsidiary;
plus
(E) 50% of any dividends received by the Parent or any
Restricted Subsidiary from any Unrestricted Subsidiary to the
extent the Parent’s or such Restricted Subsidiary’s
Investment in such Unrestricted Subsidiary was a Restricted
Investment, and to the extent such dividends were not otherwise
included in the Consolidated Net Income of Parent for such
period.
189
So long as no Default has occurred and is continuing or would be
caused thereby (except with respect to clauses (1), (4), (5),
(7), (9) and (10) below), the preceding provisions
will not prohibit:
(1) the payment of any dividend (or other distribution) or
the consummation of any irrevocable redemption within
90 days after the date of declaration of the dividend (or
other distribution) or giving of the redemption notice, as the
case may be, if at the date of declaration or notice the
dividend (or other distribution) payment or redemption would
have complied with the provisions of the Indenture;
(2) the making of any Restricted Payment in exchange for,
or out of the net cash proceeds of the substantially concurrent
sale (other than to any Restricted Subsidiary) of, Parent’s
Equity Interests (other than Disqualified Stock) or from the
substantially concurrent contribution of common equity capital
to Parent; provided that the amount of any such net cash
proceeds that are utilized to make any such Restricted Payment
will be excluded from clause (3)(B) of the preceding paragraph;
(3) the purchase, defeasance, redemption, repurchase or
other acquisition or retirement of Subordinated Indebtedness of
Parent or any Restricted Subsidiary with (i) the net cash
proceeds from an incurrence of Permitted Refinancing
Indebtedness or (ii) in exchange for, or out of the
proceeds of a substantially concurrent Qualified Equity Offering;
(4) in the case of a Restricted Subsidiary, the payment of
dividends (or in the case of any partnership or limited
liability company, any similar distribution) to the holders of
its Capital Stock on a pro rata basis;
(5) repurchases of Equity Interests deemed to occur upon
the exercise of stock options, warrants or other convertible
securities if such Equity Interests represent a portion of the
exercise price thereof and repurchases of Equity Interests
deemed to occur upon the withholding of a portion of the Equity
Interests granted or awarded to an employee to pay for the taxes
payable by such employee upon such grant or award, or the
vesting thereof, in an amount not to exceed $20 million;
(6) cash payments, in lieu of issuance of fractional shares
in connection with the exercise of warrants, options or other
securities convertible into or exchangeable for Equity Interests
of Parent or a Restricted Subsidiary;
(7) the repurchase, redemption or other acquisition or
retirement for value of any Subordinated Indebtedness following
a Change of Control after the Company shall have complied with
the provisions of the covenants described above under the
captions “Repurchase at the Option of Holders —
Change of Control” and “Asset Sales,” including
the payment of the applicable purchase price;
(8) the declaration and payment of regularly scheduled or
accrued dividends to holders of any class or series of
Disqualified Stock of Parent or any preferred stock of any
Restricted Subsidiary of Parent issued on or after the Issue
Date in accordance with the Fixed Charge Coverage Ratio test
described below under the caption “— Incurrence
of Indebtedness and Issuance of Preferred Stock”,
(9) payments made in accordance with the Merger Agreement
as disclosed under “Use of Proceeds”,
(10) to the extent constituting a Restricted Payment, the
BBVA ‘B’ Share Transaction;
(11) the repurchase, redemption or other acquisition of the
Equity Interests of Parent or any Restricted Subsidiary from
Persons who are, or were formerly, employees, officers and
directors of the Parent and its Subsidiaries and their
Affiliates, heirs and executors; provided that the
aggregate amount of all such repurchases pursuant to this
clause (11) shall not exceed $5.0 million in any
twelve month period; and
(12) other Restricted Payments in an aggregate amount since
the Issue Date not to exceed $75 million.
The amount of all Restricted Payments (other than cash) will be
the fair market value on the date of the Restricted Payment of
the asset(s), property or securities proposed to be transferred
or issued by Parent or such Restricted Subsidiary, as the case
may be, pursuant to the Restricted Payment. The fair market
value of
190
any assets or securities that are required to be valued by this
covenant will be determined by the Company’s Board of
Directors, whose resolutions with respect thereto will be
delivered to the Trustee. The Board of Directors’
determination must be based upon an opinion or appraisal issued
by an accounting, appraisal or investment banking firm of
national standing if the fair market value exceeds
$25 million.
Incurrence
of Indebtedness and Issuance of Disqualified Stock and Preferred
Stock
Parent will not, and will not permit any of the Restricted
Subsidiaries to, directly or indirectly, create, incur, issue,
assume, guarantee or otherwise become directly or indirectly
liable, contingently or otherwise, with respect to
(collectively, “incur”) any Indebtedness
(including Acquired Debt), and Parent will not issue any
Disqualified Stock and will not permit any of the Restricted
Subsidiaries to issue any shares of preferred stock;
provided, however, that Parent may incur
Indebtedness (including Acquired Debt) or issue Disqualified
Stock, and the Company and any of the Guarantors may incur
Indebtedness (including Acquired Debt) or issue preferred stock
if the Fixed Charge Coverage Ratio for the most recently ended
four full fiscal quarters for which internal financial
statements are available immediately preceding the date on which
such additional Indebtedness is incurred or such Disqualified
Stock or such preferred stock is issued, as the case may be,
would have been at least 2.00 to 1.00, determined on a pro forma
basis (including a pro forma application of the net proceeds
therefrom including to refinance other indebtedness), as if the
additional indebtedness had been incurred or the preferred stock
or Disqualified Stock had been issued, as the case may be, at
the beginning of such four-quarter period.
The first paragraph of this covenant will not prohibit the
incurrence of any of the following items of Indebtedness
(collectively, “Permitted Debt”):
(1) Indebtedness incurred by Parent and the Restricted
Subsidiaries pursuant to Credit Facilities and any Qualified
Securitization Financing, including the Credit Agreement, in an
amount outstanding at any time not to exceed the sum of
(x) $2,750 million plus (y) €478 million
minus (z) the aggregate amount of Net Proceeds applied
pursuant to clause (1) of the third paragraph under
“Repurchase at the Option of Holders — Asset
Sales”;
(2) the incurrence by Parent and the Restricted
Subsidiaries of the Existing Indebtedness (provided that all
Existing Indebtedness to be Repaid is either repaid, discharged
or defeased on the Issue Date);
(3) the incurrence by Parent and any Guarantor of
Indebtedness represented by the Notes to be issued on the Issue
Date and the Guarantees thereof (and any Notes and Guarantees
issued in exchange for the Notes and Guarantees pursuant to the
registration rights agreement);
(4) the incurrence by Parent or any Restricted Subsidiary
of Indebtedness represented by Capital Lease Obligations,
mortgage financings, purchase money obligations, industrial
development or similar bonds, or tax-advantaged governmental or
quasi-governmental financing, including without limitation the
sale and leaseback arrangements described under clause (5)
under the exclusions set forth under the definition of Asset
Sale, in each case incurred for the purpose of financing all or
any part of the purchase price or cost of design, development,
construction, installation or improvement (including at any
point subsequent to the purchase) of real or personal property,
plant or equipment used in the business of Parent or such
Restricted Subsidiary (whether through the direct acquisition or
otherwise of such assets or the acquisition of Equity Interests
of any Person owning such assets), in an aggregate principal
amount, including all Indebtedness incurred to refund, refinance
or replace any Indebtedness incurred pursuant to this clause
(4), not to exceed the greater of (x) $200 million and
(y) 3.0% of Total Assets, at any time outstanding;
(5) the incurrence by Parent or any Restricted Subsidiary
of Permitted Refinancing Indebtedness in exchange for, or the
net proceeds of which are used to renew, refund, refinance,
replace, defease or discharge Indebtedness (other than
intercompany Indebtedness) that was incurred under the first
paragraph of this covenant or clauses (3) through
(7) and (16) of this paragraph;
191
(6) the incurrence by Parent or any Restricted Subsidiary
of intercompany Indebtedness owed to Parent or any Restricted
Subsidiary; provided, however, that:
(a) if the Company is the obligor on any such Indebtedness
owed to any Restricted Subsidiary that is not a Guarantor, such
Indebtedness must be expressly subordinated to the prior payment
in full in cash of all Obligations then due with respect to the
Notes;
(b) if a Guarantor is the obligor on any such Indebtedness
owed to any Restricted Subsidiary that is not the Company or a
Guarantor, such Indebtedness is expressly subordinated to the
prior payment in full in cash of all Obligations then due with
respect to such Guarantor’s Guarantee; and
(c) (i) any subsequent issuance or transfer of Equity
Interests that results in any such Indebtedness being held by a
Person other than Parent or a Restricted Subsidiary and
(ii) any sale or other transfer of any such Indebtedness
(other than the creation of a Permitted Lien upon such
intercompany Indebtedness to a Person that is not either Parent
or a Restricted Subsidiary shall be deemed, in each case, to
constitute an incurrence of such Indebtedness by Parent or such
Restricted Subsidiary, as the case may be, that was not
permitted by this clause (6);
(7) the incurrence by Parent or any Restricted Subsidiary
of Hedging Obligations or entry into derivative transactions, in
each case, in the normal course of business and so long as such
obligations and transactions are not entered into for
speculative purposes;
(8) the incurrence of Guarantees by Parent, the Company or
any Guarantors of Indebtedness of Parent or any Restricted
Subsidiary that was permitted to be incurred by another
provision of this covenant;
(9) the incurrence of Guarantees by any Restricted
Subsidiary that is not a Guarantor of Indebtedness of a
Restricted Subsidiary that is not a Guarantor that was permitted
to be incurred by another provision of this covenant;
(10) the incurrence by Parent and the Restricted
Subsidiaries of Indebtedness in respect of workers’
compensation claims, self-retention or self-insurance
obligations, unemployment insurance, performance, bid, release,
appeal, surety and similar bonds and related reimbursement
obligations and completion guarantees provided or incurred by
Parent and the Restricted Subsidiaries in the ordinary course of
business, guarantees for the account of suppliers in the
ordinary course of business and consistent with past practice,
and obligations in connection with participation in government
reimbursement or other programs or other similar requirements;
(11) the incurrence by Parent and the Restricted
Subsidiaries of Indebtedness arising from Parent’s and the
Restricted Subsidiaries’ agreements providing for
indemnification, contribution, earnout, adjustment of purchase
price or similar obligations, in each case, incurred or assumed
in connection with the sale of goods or acquisition or
disposition of any business, assets or Capital Stock of a
Restricted Subsidiary; provided that the maximum
aggregate liability in respect of all such Indebtedness shall at
no time exceed the gross proceeds actually received by Parent
and the Restricted Subsidiaries in connection with such
acquisition or disposition;
(12) the incurrence by Parent and the Restricted
Subsidiaries of Indebtedness arising from the honoring by a bank
or other financial institution of a check, draft or similar
instrument inadvertently drawn against insufficient funds in the
ordinary course of business, provided, however, that such
Indebtedness is extinguished within five business days of
incurrence;
(13) the incurrence by Parent or any Restricted Subsidiary
of Indebtedness to the extent the net proceeds thereof are
promptly deposited to defease the Notes as described below under
the caption “Legal Defeasance and Covenant Defeasance”;
(14) the incurrence by Foreign Subsidiaries of Parent
(other than any Guarantor) of Indebtedness in an aggregate
principal amount at any time outstanding not to exceed
$200 million;
192
(15) the incurrence of Indebtedness consisting of
(i) the financing of insurance premiums or
(ii) take-or-pay
obligations contained in supply arrangements, in each case, in
the ordinary course of business;
(16) the incurrence by Parent or any of its Restricted
Subsidiaries of Acquired Debt; provided that
(i) such Indebtedness existed prior to the consummation of
the related acquisition and was not created in contemplation
thereof and (ii) to the extent the aggregate amount of
Indebtedness incurred in reliance on this clause (16)
following the Issue Date exceeds $50 million, then on a pro
forma basis, either (a) the Company would be permitted to
incur at least $1.00 of additional Indebtedness pursuant to the
Fixed Charge Coverage Ratio test set forth in the first
paragraph of this covenant or (b) the Fixed Charge Coverage
Ratio would be greater than immediately prior to such
transactions;
(17) Indebtedness of Parent or any Restricted Subsidiary
constituting reimbursement obligations with respect to letters
of credit or trade Guarantees issued in the ordinary course of
business to the extent that such letters of credit or trade
Guarantees are not drawn upon or, if drawn upon, to the extent
such drawing is reimbursed no later than the 30 days
following receipt by Parent or such Restricted Subsidiary of a
demand for reimbursement;
(18) Guarantees in the ordinary course of business of the
obligations of suppliers, customers, franchisees and licensees
of Parent or any Restricted Subsidiary;
(19) to the extent constituting Indebtedness,
(i) deferred compensation to employees of Parent and the
Restricted Subsidiaries in the ordinary course of business,
(ii) unfunded pension fund and other employee benefit plan
obligations and liabilities to the extent that they are
permitted to remain unfunded under applicable law,
(iii) contingent liabilities arising out of endorsements of
checks and other negotiable instruments for deposit or
collection in the ordinary course of business,
(iv) reserves established by Parent or any Restricted
Subsidiary for litigation or tax contingencies and (v) the
BBVA ‘B’ Share Transaction;
(20) Indebtedness in an amount not to exceed
$20 million issued in lieu of cash payments of Restricted
Payments permitted by clause (5) of the covenant described
under “— Restricted Payments”; and
(21) the incurrence by Parent or any Restricted Subsidiary
of additional Indebtedness or the issuance by Parent of
Disqualified Stock or preferred stock in an aggregate principal
amount (or accreted value, as applicable) at any time
outstanding, including all Indebtedness incurred to refund,
refinance or replace any Indebtedness incurred pursuant to this
clause (21), not to exceed $350 million.
For purposes of determining compliance with this covenant, in
the event that an item of proposed Indebtedness meets the
criteria of more than one of the categories of Permitted Debt
described in clauses (1) through (21) above as of the
date of incurrence thereof or is entitled to be incurred
pursuant to the first paragraph of this covenant, the Company
shall, in its sole discretion, (x) at the time the proposed
Indebtedness is incurred, classify all or a portion of that item
of Indebtedness on the date of its incurrence under either the
first paragraph of this covenant or under such category of
Permitted Debt, as the case may be, (y) reclassify at a
later date all or a portion of that or any other item of
Indebtedness as being or having been incurred in any manner that
complies with this covenant (so long as the Indebtedness being
reclassified could have been incurred under the first paragraph
or under such category of Permitted Debt on the date of its
incurrence) and (z) elect to comply with this covenant and
the applicable definitions in any order. The accrual of
interest, the accretion or amortization of original issue
discount, the payment of interest on any Indebtedness in the
form of additional Indebtedness with the same terms, the
reclassification of preferred stock as Indebtedness due to a
change in accounting principles, and the payment of dividends on
Disqualified Stock in the form of additional shares of the same
class of Disqualified Stock will not be deemed to be an
incurrence of Indebtedness or an issuance of Disqualified Stock
for purposes of this covenant; provided, in each such
case, that the amount of any such accrual, accretion or payment
is included in Parent’s Fixed Charges as accrued.
Notwithstanding any other provision of this covenant, the
maximum amount of Indebtedness that Parent or the Restricted
Subsidiaries may incur pursuant to this covenant shall not be
deemed to be exceeded solely as a result of fluctuations in
exchange rates or currency values.
193
The Company will not incur any Indebtedness that is
contractually subordinate or junior in right of payment to any
Senior Debt of the Company and subordinate or junior in right of
payment to the Notes; provided, however, that no
Indebtedness of the Company will be deemed to be contractually
subordinated in right of payment solely by virtue of being
unsecured or secured by a junior Lien or by virtue of being
structurally subordinated. No Guarantor will incur any
Indebtedness that is subordinate or junior in right of payment
to the Senior Debt of such Guarantor and subordinate or junior
in right of payment to such Guarantor’s Guarantee;
provided, however, that no Indebtedness of a
Guarantor will be deemed to be contractually subordinated in
right of payment solely by virtue of being unsecured or secured
by a junior Lien.
Parent will not permit any Unrestricted Subsidiary to incur any
Indebtedness other than Non-recourse Debt; provided,
however, that if any such Indebtedness ceases to be
Non-recourse Debt of an Unrestricted Subsidiary, such event
shall be deemed to be an incurrence of Indebtedness by the
obligors of such Indebtedness.
Liens
Parent will not, and will not permit any of the Restricted
Subsidiaries to, directly or indirectly, create, incur, assume
or suffer to exist any Lien of any kind securing Indebtedness,
Attributable Debt or trade payables on any property, asset, or
any proceeds therefrom (“Primary Lien”), now
owned or hereafter acquired, except Permitted Liens, unless:
(1) in the case of Liens securing Subordinated
Indebtedness, the Notes and related Guarantees are secured by a
Lien on such property (including Capital Stock of a Restricted
Subsidiary) or assets that are senior in priority to such
Liens; and
(2) in the case of Liens securing Senior Debt, the Notes
and related Guarantees are equally and ratably secured by a Lien
on such property (including Capital Stock of a Restricted
Subsidiary) or assets.
Any Lien created for the benefit of the Holders of the Notes
pursuant to the immediately preceding paragraph shall
automatically and unconditionally be released and discharged
upon the release and discharge of the Primary Lien, without any
further action on the part of any Person.
Dividend
and Other Payment Restrictions Affecting Restricted
Subsidiaries
Parent will not, and will not permit any of the Restricted
Subsidiaries to, directly or indirectly, create or permit to
exist or become effective any consensual encumbrance or
restriction on the ability of any Restricted Subsidiary to:
(1) pay dividends or make any other distributions on or in
respect of its Capital Stock to Parent or any Restricted
Subsidiary, or with respect to any other interest or
participation in, or measured by, its profits, or pay any
Indebtedness owed to Parent or any other Restricted Subsidiary;
(2) make any loans or advances to Parent or any other
Restricted Subsidiary;
(3) transfer any of its properties or assets to Parent or
any other Restricted Subsidiary; or
(4) guarantee Parent’s or any Restricted
Subsidiary’s Indebtedness.
However, the preceding restrictions will not apply to
encumbrances or restrictions existing under or by reason of:
(1) the Credit Agreement and any other agreements as in
effect on the Issue Date or subsequent agreements relating to
Indebtedness of Parent or any Restricted Subsidiary and any
amendments, modifications, restatements, renewals, increases,
supplements, refundings, replacements or refinancings of those
agreements; provided that the amendments, modifications,
restatements, renewals, increases, supplements, refundings,
replacement or refinancings are not materially more restrictive,
taken as a whole, with respect to such dividend and other
payment restrictions than those contained in those agreements on
the Issue Date unless in the good faith determination of the
Board of Directors, such restrictions are not
194
likely to result in the Company being unable to make scheduled
payments of principal and interest on the Notes as they come due;
(2) the Indenture, the Notes and the Guarantees;
(3) applicable law, rules, regulations and orders;
(4) any instrument governing Indebtedness or Capital Stock
of a Person acquired by Parent or any Restricted Subsidiary as
in effect at the time of such acquisition (except to the extent
such Indebtedness or Capital Stock was incurred in connection
with or in contemplation of such acquisition), which encumbrance
or restriction is not applicable to any Person, or the
properties or assets of any Person, other than the Person, or
the property or assets of the Person, so acquired; provided
that, in the case of Indebtedness, such Indebtedness was
permitted by the terms of the Indenture to be incurred;
(5) customary non-assignment provisions in contracts,
licenses and leases entered into in the ordinary course of
business;
(6) purchase money obligations for property acquired in the
ordinary course of business and Capital Lease Obligations that
impose restrictions on the property purchased or leased of the
nature described in clause (3) of the preceding paragraph;
(7) any agreement for the sale or other disposition of a
Restricted Subsidiary or of all or substantially all of its
assets that restricts distributions of assets by, or Equity
Interests of, that Restricted Subsidiary pending its sale or
other disposition;
(8) Permitted Refinancing Indebtedness; provided
that the restrictions contained in the agreements governing
such Permitted Refinancing Indebtedness are not materially more
restrictive, taken as a whole, than those contained in the
agreements governing the Indebtedness being refinanced;
(9) Liens permitted to be incurred under the provisions of
the “— Liens” covenant that limit the right
of the debtor to dispose of the assets subject to such Liens;
(10) restrictions on cash or other deposits or net worth
imposed by customers (including governmental entities) under
contracts entered into in the ordinary course of business;
(11) provisions limiting the disposition or distribution of
assets or property in joint venture agreements, asset sale
agreements, sale and leaseback transactions, stock sale
agreements and other similar agreements entered into in the
ordinary course of business or with the approval of the
Company’s Board of Directors, which limitation is
applicable only to the assets that are the subject of such
agreements;
(12) any encumbrance or restriction on our ability or the
ability of any Restricted Subsidiary to transfer its interest in
any Investment not prohibited under “— Restricted
Payments”;
(13) customary restrictions imposed on the transfer of, or
in licenses related to, copyrights, patents or other
intellectual property and contained in agreements entered into
in the ordinary course of business;
(14) any other agreement governing Indebtedness or
Disqualified Stock entered into after the Issue Date that
contains encumbrances and restrictions that are not more
restrictive than would be permitted by clause (1) of this
paragraph;
(15) restrictions created in connection with any Qualified
Securitization Financing that, in the good faith determination
of the Board of Directors of the Issuer, are necessary or
advisable to effect such Qualified Securitization
Financing; and
(16) agreements pursuant to any tax sharing arrangement
between Parent and any one or more of its direct or indirect
Subsidiaries.
195
Merger,
Consolidation or Sale of Assets
The Company may not, directly or indirectly:
(1) consolidate or merge with or into another Person
(whether or not the Company is the surviving corporation) or
(2) sell, assign, transfer, lease, convey (not including
any conveyance, if any, resulting solely from the creation of
any Lien, unless remedies are exercised in connection therewith)
or otherwise dispose of all or substantially all of the
properties and assets of the Company and its Restricted
Subsidiaries, taken as a whole, in one or more related
transactions, to another Person or Persons; unless:
(a) either: (x) the Company is the surviving entity;
or (y) the Person formed by or surviving any such
consolidation or merger (if other than the Company) or to which
such sale, assignment, transfer, lease, conveyance or other
disposition has been made is a corporation, limited partnership
or limited liability company organized or existing under the
laws of the United States, any state of the United States or the
District of Columbia;
(b) the Person formed by or surviving any such
consolidation or merger (if other than the Company) or the
Person to which such sale, assignment, transfer, conveyance or
other disposition has been made assumes all obligations of the
Company under the Notes and the Indenture pursuant to an
agreement in a form reasonably satisfactory to the Trustee;
(c) immediately after such transaction no Default or Event
of Default exists; and
(d) the Company or the Person formed by or surviving any
such consolidation or merger (if other than the Company), or to
which such sale, assignment, transfer, conveyance or other
disposition has been made would, on the date of such transaction
after giving pro forma effect thereto and any related financing
transactions as if the same had occurred at the beginning of the
applicable four-quarter period, (i) be permitted to incur
at least $1.00 of additional Indebtedness pursuant to the Fixed
Charge Coverage Ratio test set forth in the
“— Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock” covenant or
(ii) Parent’s Fixed Charge Coverage Ratio would not be
less than the Parent’s Fixed Charge Coverage Ratio
immediately prior to such transaction or series of transactions.
In addition, the Company and its Restricted Subsidiaries may
not, directly or indirectly, lease all or substantially all of
the Company’s and its Restricted Subsidiaries’
properties and assets, in one or more related transactions, to
any other Person.
The Person formed by or surviving any consolidation or merger
(if other than the Company) will succeed to, and be substituted
for, and may exercise every right and power of the Company under
the Indenture; provided that the Company shall not be
released in the case of a lease of all or substantially all of
its assets.
Clauses (c) and (d) of the first paragraph of this
“Merger, Consolidation or Sale of Assets” covenant
will not apply to:
(1) a merger of the Company with an Affiliate solely for
the purpose of reincorporating us in another jurisdiction;
(2) a merger of the Escrow Issuer with and into the Company
on the Completion Date; or
(3) any consolidation or merger, or any sale, assignment,
transfer, conveyance, lease or other disposition of assets
between or among the Company and its Restricted Subsidiaries.
Parent may not, directly or indirectly: (1) consolidate or
merge with or into another Person (whether or not Parent is the
surviving corporation) or (2) sell, assign, transfer,
convey (not including any conveyance, if any, resulting solely
from the creation of any Lien, unless remedies are exercised in
connection therewith) or otherwise dispose of all or
substantially all of the properties and assets of Parent and its
Restricted Subsidiaries, taken as a whole, in one or more
related transactions, to another Person or Persons; unless:
(a) the Person formed by or surviving any such
consolidation or merger (if other than Parent) or the Person to
which such sale, assignment, transfer, conveyance or other
disposition has been made assumes
196
all obligations of Parent under its Guarantee and the Indenture
pursuant to an agreement in a form reasonably satisfactory to
the Trustee;
(b) immediately after such transaction no Default or Event
of Default exists; and
(c) Parent or the Person formed by or surviving any such
consolidation or merger (if other than Parent), or to which such
sale, assignment, transfer, conveyance or other disposition has
been made would, on the date of such transaction after giving
pro forma effect thereto and any related financing transactions
as if the same had occurred at the beginning of the applicable
four-quarter period, (i) be permitted to incur at least
$1.00 of additional Indebtedness pursuant to the Fixed Charge
Coverage Ratio test set forth in the
“— Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock” covenant or
(ii) Parent’s Fixed Charge Coverage Ratio would not be
less than the Parent’s Fixed Charge Coverage Ratio
immediately prior to such transaction or series of transactions.
In addition, Parent and the Restricted Subsidiaries may not,
directly or indirectly, lease all or substantially all of
Parent’s and the Restricted Subsidiaries’ properties
and assets, in one or more related transactions, to any other
Person.
The Person formed by or surviving any consolidation or merger
(if other than Parent) will succeed to, and be substituted for,
and may exercise every right and power of Parent under the
Indenture; provided that Parent shall not be released in
the case of a lease of all or substantially all of its assets.
Clauses (b) and (c) of the fifth paragraph of this
“Merger, Consolidation or Sale of Assets” covenant
will not apply to:
(1) a merger of Parent with an Affiliate solely for the
purpose of reincorporating Parent in another
jurisdiction; or
(2) any consolidation or merger, or any sale, assignment,
transfer, conveyance, lease or other disposition of assets
between or among Parent and the Restricted Subsidiaries.
Designation
of Restricted and Unrestricted Subsidiaries
The Company’s Board of Directors may designate any
Restricted Subsidiary (other than the Company) to be an
Unrestricted Subsidiary if that designation would not cause a
Default. If a Restricted Subsidiary is designated as an
Unrestricted Subsidiary, the aggregate fair market value of all
outstanding Investments owned by Parent and the Restricted
Subsidiaries in the Subsidiary properly designated will be
deemed to be an Investment made as of the time of the
designation and will reduce the amount available for Restricted
Payments under the first paragraph of the
“— Restricted Payments” covenant or
Permitted Investments, as determined by the Company. That
designation will only be permitted if the Investment would be
permitted at that time and if the Restricted Subsidiary
otherwise meets the definition of an Unrestricted Subsidiary.
The Company’s Board of Directors may redesignate any
Unrestricted Subsidiary to be a Restricted Subsidiary if the
redesignation would not cause a Default.
Transactions
with Affiliates
Parent will not, and will not permit any of the Restricted
Subsidiaries to, make any payment to, or sell, lease, transfer
or otherwise dispose of any of Parent’s or the Restricted
Subsidiaries’ respective properties or assets to, or
purchase any property or assets from, or enter into or make or
amend any transaction, contract, agreement, understanding, loan,
advance or guarantee with, or for the benefit of, any Affiliate
involving aggregate payments of consideration in excess of
$10 million (each, an “Affiliate
Transaction”), unless:
(1) the Affiliate Transaction is on terms that taken as a
whole are no less favorable to Parent or the relevant Restricted
Subsidiary than those that would have been obtained in a
comparable transaction by Parent or such Restricted Subsidiary
with an unrelated Person; and
197
(2) the Company delivers to the Trustee:
(a) with respect to any Affiliate Transaction or series of
related Affiliate Transactions involving aggregate consideration
in excess of $20 million, a resolution of the Board of
Directors of the Company set forth in an officers’
certificate certifying that such Affiliate Transaction complies
with this covenant and that such Affiliate Transaction has been
approved by a majority of the Company’s Board of Directors
(and, if any, a majority of the disinterested members of the
Company’s Board of Directors with respect to such
transaction); and
(b) with respect to any Affiliate Transaction or series of
related Affiliate Transactions involving aggregate consideration
in excess of $50 million, an opinion as to the fairness to
the Holders of such Affiliate Transaction from a financial point
of view issued by an accounting, appraisal or investment banking
firm of national standing.
The following items will not be deemed to be Affiliate
Transactions and, therefore, will not be subject to the
provisions of the prior paragraph:
(1) any customary consulting or employment agreement or
arrangement, benefit arrangement or plan, incentive compensation
plan, stock option or stock ownership plan, employee benefit
plan, severance or termination arrangements, expense
reimbursement arrangements, officer or director indemnification
agreement or any similar arrangement entered into by Parent or
any of the Restricted Subsidiaries for the benefit of their
directors, officers, employees and consultants and payments and
transactions pursuant thereto, in each case, in the ordinary
course of business;
(2) transactions between or among Parent
and/or the
Restricted Subsidiaries;
(3) payment of reasonable directors compensation and
indemnification costs permitted by Parent’s and the
Restricted Subsidiaries’ organizational documents for the
benefit of directors, officers and employees, in each case, in
the ordinary course of business;
(4) Permitted Investments or Restricted Payments that are
permitted by the “— Restricted Payments”
covenant;
(5) any agreement (including any certificate of
designations relating to Capital Stock) as in effect as of the
Issue Date or any amendment thereto or any transaction
contemplated thereby (including pursuant to any amendment
thereto) in any replacement agreement thereto so long as any
such amendment or replacement agreement is not more
disadvantageous to the Holders in any material respect than the
original agreement as in effect on the Issue Date;
(6) the granting or performance of customary registration
rights in respect of restricted Equity Interests held or
acquired by Affiliates;
(7) loans and advances to employees in the ordinary course
of business not to exceed $10 million in the aggregate
amount at any one time outstanding;
(8) the consummation of the Transactions and the payment of
all fees, expenses and other amounts, and the performance of all
obligations of Parent and the Restricted Subsidiaries, in
connection therewith;
(9) transactions with customers, clients, suppliers or
purchasers or sellers of goods or services, in each case, in the
ordinary course of business and consistent with past practice
and on terms that are not materially less favorable to Parent or
such Restricted Subsidiary, as the case may be, determined in
good faith by Parent, that those that could be obtained in a
comparable arm’s-length transaction with a Person that is
not an Affiliate of Parent;
(10) the issuance or repurchase of Equity Interests (other
than Disqualified Stock) of Parent to any Affiliate of
Parent; and
(11) licenses of, or other grants of rights to use,
intellectual property granted by Parent or any Restricted
Subsidiary in the ordinary course of business.
198
Additional
Guarantees
If Parent or any Restricted Subsidiary acquires or creates
another Restricted Subsidiary (other than a Foreign Subsidiary
of the Company or any Immaterial Subsidiary) after the Issue
Date that guarantees any Indebtedness of the Company or any of
its Domestic Subsidiaries, then that newly acquired or created
Restricted Subsidiary will execute and deliver to the Trustee a
supplemental Indenture providing for a Guarantee and deliver an
opinion of counsel satisfactory to the Trustee as to the due
authorization, execution and delivery and the enforceability of
such Guarantee within 45 business days of the date on which it
was acquired or created.
Payments
for Consent
Parent will not, and will not permit any of the Restricted
Subsidiaries to, directly or indirectly, pay or cause to be paid
any consideration, to or for the benefit of any Holder of Notes
for or as an inducement to any consent, waiver or amendment of
any of the terms or provisions of the Indenture or the Notes
unless such consideration is offered to be paid and is paid to
all Holders of the Notes that consent, waive or agree to amend
in the time frame set forth in the solicitation documents
relating to such consent, waiver or agreement.
Reports
Whether or not required by rules and regulations of the SEC, so
long as any Notes are outstanding, Parent will furnish to the
Holders of Notes or cause the Trustee to furnish to the Holders
of Notes:
(1) within the time periods specified in the SEC’s
rules and regulations, all annual financial information that
would be required to be contained in a filing with the SEC on
Form 20-F
if Parent were required to file such Form pursuant to
Section 13(a) or 15(d) of the Exchange Act or any successor
provision thereto, including a “Management’s
Discussion and Analysis of Financial Condition and Results of
Operations” and a report on Parent’s consolidated
annual financial statements by Parent’s certified
independent accountants; and
(2) within 45 days of the first three fiscal quarters
of each fiscal year of Parent, quarterly financial information
prepared on the same basis as the audited financial information
referred to in clause (1) above which shall have been the
subject of a SAS 100 (or equivalent) review by the
Company’s independent auditors together with a
“Management’s Discussion of Analysis of Financial
Condition and Results of Operations” for such fiscal
quarter.
Parent will be deemed to have furnished such reports to the
Trustee and the Holders if Parent has filed such information or
reports with the SEC via the EDGAR filing system and such
information or reports are publicly available.
Delivery of such reports, information and documents to the
Trustee shall be for informational purposes only and the
Trustee’s receipt of such shall not constitute constructive
notice of any information contained therein or determinable from
information contained therein, including Parent’s
compliance with any of the covenants contained in the Indenture
(as to which the Trustee will be entitled to conclusively rely
upon an officer’s certificate).
In addition, following the consummation of the exchange offer
contemplated by the registration rights agreement, whether or
not required by the SEC, Parent will file a copy of all of the
information and reports referred to in clauses (1) and
(2) above with the SEC for public availability within the
time periods specified in the SEC’s rules and regulations
(unless the SEC will not accept such a filing). In addition,
Parent, the Company and the other Guarantors have agreed that,
for so long as any Notes remain outstanding, if at any time
Parent is not required to file with the SEC the information and
reports required by clauses (1) and (2) above, Parent
will furnish to the Holders and to securities analysts and
prospective investors, upon their request, the information
required to be delivered pursuant to Rule 144A(d)(4) under
the Securities Act.
Notwithstanding anything herein to the contrary, Parent will not
be deemed to have failed to comply with any of its agreements
hereunder for purposes of clause (4) under “Events of
Default and Remedies” until
199
120 days after the date any information or report hereunder
is required to be furnished to Holders of Notes or filed with
the SEC pursuant to this covenant.
Events of
Default and Remedies
Each of the following is an “Event of Default”:
(1) default for 30 days in the payment when due of
interest on or Additional Interest with respect to the Notes;
(2) default in payment when due of the principal of or
premium, if any, on the Notes;
(3) failure by Parent or any Restricted Subsidiary to
comply with the “— Merger, Consolidation or Sale
of Assets” covenant or with the provision described under
the heading “Repurchase at the Option of
Holders — Change of Control”;
(4) failure by Parent or any Restricted Subsidiary for
60 days after notice to comply with any other covenant or
agreement in the Indenture or the Notes after written notice
thereof is given to the Company by the Trustee or to Parent and
the Restricted Subsidiaries and to the Trustee by Holders of at
least 25% in aggregate principal amount of the then outstanding
Notes voting as a single class;
(5) default under any agreement, bond, mortgage, Indenture
or instrument under which there may be issued or by which there
may be secured or evidenced any Indebtedness for money borrowed
by any Parent or any Restricted Subsidiary (or the payment of
which is guaranteed by Parent or any Restricted Subsidiary)
whether such Indebtedness or Guarantee now exists, or is created
after the Issue Date, if that default:
(a) is caused by a failure to pay any scheduled installment
of principal on such Indebtedness prior to the expiration of the
grace period provided in such Indebtedness on the date of such
default (a “Payment Default”); or
(b) results in the acceleration of such Indebtedness prior
to its express maturity,
and, in each case, the principal amount of any such
Indebtedness, together with the principal amount of any other
such Indebtedness under which there has been a Payment Default
or the maturity of which has been so accelerated, aggregates
$100 million or more; provided, however,
where (i) neither Parent nor any Restricted Subsidiary has
general liability with respect to such Indebtedness, and
(ii) the creditor has agreed in writing that such
creditor’s recourse is solely to specified assets or
Unrestricted Subsidiaries, the amount of such Indebtedness shall
be deemed to be the lesser of (x) the principal amount of
such Indebtedness, and (y) the fair market value of such
specified assets to which the creditor has recourse;
(6) failure by Parent, the Company or any Significant
Subsidiary or any group of Restricted Subsidiaries that, taken
together, would constitute a Significant Subsidiary to pay final
and non-appealable judgments entered by a court or courts of
competent jurisdiction aggregating in excess of
$100 million (net of any amounts covered by insurance),
which judgments are not paid, discharged or stayed for a period
of 60 days;
(7) except as permitted by the Indenture, any Guarantee of
a Significant Subsidiary, or any group of Restricted
Subsidiaries that, taken together, would constitute a
Significant Subsidiary, shall be held in any judicial proceeding
to be unenforceable or invalid or shall cease for any reason to
be in full force and effect or any Guarantor that is a
Significant Subsidiary, or any group of Restricted Subsidiaries
that, taken together, would constitute a Significant Subsidiary,
or any Person acting on behalf of any Guarantor that is a
Significant Subsidiary, or any group of Restricted Subsidiaries
that, taken together, would constitute a Significant Subsidiary,
shall deny or disaffirm in writing its obligations under its
Guarantee; and
(8) certain events of bankruptcy or insolvency described in
the Indenture with respect to Parent, the Company or any
Restricted Subsidiary that is a Significant Subsidiary or any
group of Restricted Subsidiaries that, taken together, would
constitute a Significant Subsidiary.
200
In the case of an Event of Default arising from certain events
of bankruptcy or insolvency, with respect to the Company or
Parent, all outstanding Notes will become due and payable
immediately without further action or notice. If any other Event
of Default occurs and is continuing, the Trustee or the Holders
of at least 25% in aggregate principal amount of the then
outstanding Notes may declare all the Notes to be due and
payable immediately.
Holders of the Notes may not enforce the Indenture or the Notes
except as provided in the Indenture. Subject to certain
limitations, Holders of a majority in aggregate principal amount
of the then outstanding Notes may direct the Trustee in its
exercise of any trust or power. The Trustee may withhold from
Holders of the Notes notice of any continuing Default or Event
of Default if it determines that withholding notices is in their
interest, except a Default or Event of Default relating to the
payment of principal or interest or Additional Interest.
Subject to the provisions of the Indenture relating to the
duties of the Trustee, in case an Event of Default occurs and is
continuing, the Trustee will be under no obligation to exercise
any of the rights or powers under the Indenture at the request
or direction of any Holders of Notes unless such Holders have
offered to the Trustee reasonable indemnity or security against
any loss, liability or expense. Except to enforce the right to
receive payment of principal, premium, if any, or interest or
Additional Interest, if any, when due, no Holder of a Note may
pursue any remedy with respect to the Indenture or the Notes
unless:
(1) such Holder has previously given the Trustee notice
that an Event of Default is continuing;
(2) Holders of at least 25% in aggregate principal amount
of the then outstanding Notes have requested the Trustee to
pursue the remedy;
(3) such Holders have offered, and, if requested, have
provided, the Trustee security or indemnity reasonably
satisfactory to it against any loss, liability or expense;
(4) the Trustee has not complied with such request within
60 days after the receipt of the request and the offer of
security or indemnity; and
(5) Holders of a majority in aggregate principal amount of
the then outstanding Notes have not given the Trustee a
direction inconsistent with such request within such
60-day
period.
The Holders of a majority in aggregate principal amount of the
Notes then outstanding by notice to the Trustee may on behalf of
the Holders of all of the Notes rescind an acceleration or waive
any existing Default or Event of Default and its consequences
under the Indenture except a continuing Default or Event of
Default in the payment of interest or Additional Interest on, or
the principal of, the Notes.
The Company is required to deliver to the Trustee annually a
statement regarding compliance with the Indenture. Within 5
business days of an executive officer becoming actually aware of
any Default or Event of Default, the Company is required to
deliver to the Trustee a statement specifying such Default or
Event of Default.
No
Personal Liability of Directors, Officers, Employees and
Stockholders
No past, present or future director, officer, employee, partner,
manager, agent, member, incorporator (or Person forming any
limited liability company) or stockholder of the Company or of
any Guarantor, as such, will have any liability for any
obligations of the Company or of the Guarantors under the Notes,
the Indenture, the Guarantees, or for any claim based on, in
respect of, or by reason of, such obligations or their creation.
Each Holder of Notes by accepting a Note and guarantee waives
and releases all such liability. The waiver and release are part
of the consideration for issuance of the Notes and guarantees.
The waiver may not be effective to waive liabilities under the
federal securities laws.
201
Legal
Defeasance and Covenant Defeasance
The Company may, at its option and at any time, elect to have
all of the Company’s obligations discharged with respect to
the outstanding Notes and all obligations of the Guarantors
discharged with respect to their Guarantees (“Legal
Defeasance”) except for:
(1) the rights of Holders of outstanding Notes to receive
payments in respect of the principal of, or interest or premium
and Additional Interest, if any, on, such Notes when such
payments are due from the trust referred to below;
(2) the Company’s obligations with respect to the
Notes concerning issuing temporary Notes, registration of Notes,
mutilated, destroyed, lost or stolen Notes and the maintenance
of an office or agency for payment and money for security
payments held in trust;
(3) the rights, powers, trusts, duties and immunities of
the Trustee, and the Company’s and the Guarantors’
obligations in connection therewith; and
(4) the Legal Defeasance and Covenant Defeasance provisions
of the Indenture.
In addition, the Company’s may, at its option and at any
time, elect to have the Company’s obligations and the
obligations of the Guarantors released with respect to certain
covenants (including the obligation to make Change of Control
Offers and Asset Sale Offers) that are described in the
Indenture (“Covenant Defeasance”) and
thereafter any omission to comply with those covenants will not
constitute a Default or Event of Default with respect to the
Notes. In the event Covenant Defeasance occurs, certain events
(not including non-payment, bankruptcy, receivership,
rehabilitation and insolvency events) described under the
heading “Events of Default and Remedies” will no
longer constitute an Event of Default with respect to the Notes.
In order to exercise either Legal Defeasance or Covenant
Defeasance:
(1) the Company must irrevocably deposit with the Trustee,
in trust, for the benefit of the Holders of the Notes, cash in
United States dollars, non-callable Government Securities, or a
combination of cash in United States dollars and non-callable
Government Securities, in amounts as will be sufficient, in the
opinion of a nationally recognized investment bank, appraisal
firm or firm of independent public accountants as selected by
Parent, to pay the principal of, or interest and premium and
Additional Interest, if any, on the outstanding Notes on the
Stated Maturity or on the applicable redemption date, as the
case may be, and the Company must specify whether the Notes are
being defeased to maturity or to a particular redemption date;
(2) in the case of Legal Defeasance, the Company must
deliver to the Trustee an opinion of counsel reasonably
acceptable to the Trustee confirming that (a) the Company
has received from, or there has been published by, the Internal
Revenue Service a ruling or (b) since the Issue Date, there
has been a change in the applicable federal income tax law, in
either case to the effect that, and based thereon such opinion
of counsel will confirm that, the Holders of the outstanding
Notes will not recognize income, gain or loss for federal income
tax purposes as a result of such Legal Defeasance and will be
subject to federal income tax on the same amounts, in the same
manner and at the same times as would have been the case if such
Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Company must
deliver to the Trustee an opinion of counsel reasonably
acceptable to the Trustee confirming that the Holders of the
outstanding Notes will not recognize income, gain or loss for
federal income tax purposes as a result of such Covenant
Defeasance and will be subject to federal income tax on the same
amounts, in the same manner and at the same times as would have
been the case if such Covenant Defeasance had not occurred;
(4) no Default or Event of Default has occurred and is
continuing on the date of such deposit (other than a Default or
Event of Default resulting from the borrowing of funds to be
applied to such deposit);
(5) such Legal Defeasance or Covenant Defeasance will not
result in a breach or violation of, or constitute a default
under, any material agreement or instrument (including, without
limitation, the Credit
202
Agreement, but excluding the Indenture) to which the Company or
any Guarantor is a party or by which the Company or any
Guarantor is bound;
(6) the Company must deliver to the Trustee an
officers’ certificate stating that the deposit was not made
by the Company with the intent of preferring the Holders of
Notes over Parent’s or any Restricted Subsidiary’s
other creditors with the intent of defeating, hindering,
delaying or defrauding the Company’s or any Restricted
Subsidiary’s creditors or others; and
(7) the Company must deliver to the Trustee an
officers’ certificate and an opinion of counsel, each
stating that all conditions precedent relating to the Legal
Defeasance or the Covenant Defeasance have been complied with.
Amendment,
Supplement and Waiver
Except as provided in the next three succeeding paragraphs, the
Indenture or the Notes or the Guarantees may be amended or
supplemented with the consent of the Holders of at least a
majority in aggregate principal amount of the Notes then
outstanding (including, without limitation, consents obtained in
connection with a purchase of, or tender offer or exchange offer
for, Notes), and any existing Default or Event of Default or
compliance with any provision of the Indenture or the Notes or
the Guarantees may be waived with the consent of the Holders of
a majority in aggregate principal amount of the then outstanding
Notes (including, without limitation, consents obtained in
connection with a purchase of, or tender offer or exchange offer
for, Notes).
Without the consent of each Holder adversely affected, an
amendment, supplement or waiver may not (with respect to any
Notes held by a non-consenting Holder):
(1) reduce the principal amount of Notes whose Holders must
consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed maturity of
any Note or alter the provisions with respect to the redemption
of the Notes (other than provisions relating to the covenants
described above under the caption “Repurchase at the Option
of Holders” or the minimum notice provisions required with
respect to redemption of the Notes);
(3) reduce the rate of or change the time for payment of
interest on any Note;
(4) waive a Default or Event of Default in the payment of
principal of, or interest or premium, or Additional Interest, if
any, on the Notes (except a rescission of acceleration of the
Notes by the Holders of at least a majority in aggregate
principal amount of the then outstanding Notes and a waiver of
the Payment Default that resulted from such acceleration);
(5) make any Note payable in currency other than that
stated in the Notes;
(6) make any change in the provisions of the Indenture
relating to waivers of past Defaults or the rights of Holders of
Notes to receive payments of principal of, or interest or
premium, or Additional Interest, if any, on the Notes;
(7) waive a redemption payment with respect to any Note
(other than a payment required by one of the covenants);
(8) make any change in the preceding amendment and waiver
provisions;
(9) release Parent from its Guarantee or release all or
substantially all of the Guarantors from their Guarantees, in
each case, except in accordance with the Indenture; or
(10) release the Lien on the Escrow Amount or any funds or
investment property therein otherwise then in accordance with
the terms of the Indenture and the applicable escrow agreement.
203
Notwithstanding the preceding, without the consent of any Holder
of Notes, the Company, the Guarantors and the Trustee may amend
or supplement the Indenture, the Notes or the Guarantees:
(1) to cure any ambiguity, mistake, defect or inconsistency;
(2) to provide for uncertificated Notes in addition to or
in place of certificated Notes;
(3) to provide for the assumption by a successor
corporation of the Company’s or a Guarantor’s
obligations under the Notes, the Indenture
and/or a
Guarantee in the case of a merger or consolidation or sale of
all or substantially all of the Company’s or such
Guarantor’s assets;
(4) to make any change that would provide any additional
rights or benefits to the Holders of Notes or that does not
adversely affect the legal rights under the Indenture of any
such Holder;
(5) to comply with any requirement of the SEC in order to
effect or maintain the qualification of the Indenture under the
Trust Indenture Act;
(6) to add covenants for the benefit of the Holders or to
surrender any right or power conferred upon the Company or any
Guarantor;
(7) to add a Guarantor under the Indenture;
(8) to conform the text of the Indenture, the Guarantees or
the Notes to any provision of this “Description of the
Notes” to the extent that such provision in this
“Description of the Notes” was intended to be a
verbatim recitation of a provision of the Indenture, Guarantee
or the Notes;
(9) to provide for the issuance of additional Notes in
accordance with the limitations as set forth in the Indenture;
(10) to provide for a successor Trustee in accordance with
the terms of the Indenture or to otherwise comply with any
requirement of the Indenture; or
(11) to comply with the rules of any applicable securities
depositary.
Where the consent of the Holders of the Notes is required to
approve an amendment, supplement, waiver or consent under the
Indenture, it is not necessary for the consent of the Holders of
Notes to approve the particular form of any proposed amendment,
supplement, waiver and consent, but it is sufficient if such
consent approves the substance thereof.
Satisfaction
and Discharge
The Indenture will be discharged and will cease to be of further
effect as to all Notes issued thereunder, when:
(1) either:
(a) all Notes that have been authenticated, except lost,
stolen or destroyed Notes that have been replaced or paid and
Notes for whose payment money has been deposited in trust, have
been delivered to the Trustee for cancellation; or
(b) all Notes that have not been delivered to the Trustee
for cancellation have become due and payable by reason of the
mailing of a notice of redemption or otherwise or will become
due and payable within one year, and the Company has irrevocably
deposited or caused to be deposited with the Trustee as trust
funds in trust solely for the benefit of the Holders, cash in
United States dollars, non-callable Government Securities, or a
combination of cash and non-callable Government Securities, in
such amounts as will be sufficient without consideration of any
reinvestment of interest, to pay and discharge the entire
indebtedness on the Notes not delivered to the Trustee for
cancellation for principal, premium, and Additional Interest, if
any, and accrued interest to the date of maturity or redemption;
204
(2) no Default or Event of Default has occurred and is
continuing on the date of the deposit (other than a Default or
Event of Default resulting from the borrowing of funds to be
applied to such deposit) and the deposit will not result in a
breach or violation of, or constitute a default under, any other
instrument to which the Company or any Guarantor is a party or
by which the Company or any Guarantor is bound;
(3) the Company or any Guarantor has paid or caused to be
paid all sums payable by the Company under the
Indenture; and
(4) the Company has delivered irrevocable instructions to
the Trustee under the Indenture to apply the deposited money
and/or
non-callable Government Securities toward the payment of the
Notes at maturity or the redemption date, as the case may be.
In addition, the Company must deliver an officers’
certificate and an opinion of counsel to the Trustee stating
that all conditions precedent to satisfaction and discharge have
been satisfied.
Concerning
the Trustee
The Indenture provides that, except during the continuance of an
event of default, the Trustee thereunder will perform only such
duties as are specifically set forth in the Indenture. If an
event of default has occurred and is continuing, the Trustee
will exercise such rights and powers vested in it under the
Indenture and use the same degree of care and skill in its
exercise as a prudent person would exercise under the
circumstances in the conduct of such person’s own affairs.
If the Trustee becomes a creditor of the Company or of any
Guarantor, the Indenture limits its right to obtain payment of
claims in certain cases, or to realize on certain property
received in respect of any such claim as security or otherwise.
The Trustee will be permitted to engage in other transactions;
however, if it acquires any conflicting interest, it must
(i) eliminate such conflict within 90 days,
(ii) apply to the SEC for permission to continue as Trustee
(if the Indenture has been qualified under the
Trust Indenture Act) or (iii) resign.
The Holders of a majority in aggregate principal amount of the
then outstanding Notes will have the right to direct the time,
method and place of conducting any proceeding for exercising any
remedy available to the Trustee, subject to certain exceptions.
The Indenture provides that in case an Event of Default occurs
and is continuing, the Trustee will be required, in the exercise
of its power, to use the degree of care of a prudent man in the
conduct of his own affairs. Subject to such provisions, the
Trustee will be under no obligation to exercise any of its
rights or powers under the Indenture at the request of any
Holder of Notes, unless such Holder has offered to the Trustee
security and indemnity satisfactory to it against any loss,
liability or expense.
Governing
Law
The Indenture and the Notes are governed by the laws of the
State of New York, without regard to the principles of conflicts
of law.
Certain
Definitions
Set forth below are certain defined terms used in the Indenture.
Reference is made to the Indenture for a full disclosure of all
such terms, as well as any other capitalized terms used herein
for which no definition is provided.
“Acquired Debt” means, with respect to any
specified Person:
(1) Indebtedness of any other Person existing at the time
such other Person is merged with or into or became a Subsidiary
of such specified Person, whether or not such Indebtedness is
incurred in connection with, or in contemplation of, such other
Person merging with or into, or becoming a Subsidiary of, such
specified Person; and
205
(2) Indebtedness secured by a Lien encumbering any asset
acquired by such specified Person.
“Additional Amounts” has the meaning set forth
under “— Additional Amounts.”
“Affiliate” of any specified Person means any
other Person directly or indirectly controlling or controlled by
or under direct or indirect common control with such specified
Person. For purposes of this definition, “control,” as
used with respect to any Person, means the possession, directly
or indirectly, of the power to direct or cause the direction of
the management or policies of such Person, whether through the
ownership of voting securities, by agreement or otherwise;
provided that beneficial ownership of 10% or more of the
Voting Stock of a Person will be deemed to be control. For
purposes of this definition, the terms “controlling,”
“controlled by” and “under common control
with” have correlative meanings.
“Applicable Premium” means, as determined by
the Company, with respect to any Note on any redemption date,
the greater of:
(1) 1.0% of the principal amount of such Note; and
(2) the excess, if any, of (a) the present value at
such redemption date of (i) the redemption price of such
Note at February 1, 2014 (such redemption price being set
forth in the tables appearing above under the fourth paragraph
under the caption “Optional Redemption”), plus
(ii) all required interest payments due on such Note
through February 1, 2014 (excluding accrued but unpaid
interest to the redemption date), computed using a discount rate
equal to the Treasury Rate as of such redemption date plus
50 basis points; over (b) the principal amount of such
Note.
“Asset Sale” means the sale, lease (as lessor),
conveyance or other disposition of any assets or rights;
provided that the sale, lease, conveyance or other
disposition of all or substantially all of the assets of the
Parent and the Restricted Subsidiaries taken as a whole or the
Company and its Restricted Subsidiaries taken as a whole will be
governed by the provisions of the Indenture described above
under “Repurchase at the Option of Holders —
Change of Control”
and/or the
provisions described above under “Certain
Covenants — Merger, Consolidation or Sale of
Assets” and not by the provisions of “Repurchase at
the Option of Holders — Asset Sales.”
Notwithstanding the preceding, the following items will not be
deemed to be Asset Sales:
(1) any single transaction or series of related
transactions that involves assets or rights having a fair market
value of less than $10 million;
(2) a transfer of assets or rights between or among Parent
and the Restricted Subsidiaries or between or among the
Restricted Subsidiaries;
(3) the sale, lease, conveyance or other disposition of
equipment, inventory (including, but not limited to, raw
materials,
work-in-progress
and finished goods) or other assets or rights in the ordinary
course of business, or if excess, obsolete, damaged, worn-out,
scrap or surplus or no longer used or useful in the conduct of
business as then being conducted;
(4) a Restricted Payment that is permitted by “Certain
Covenants — Restricted Payments” or a Permitted
Investment;
(5) the sale, lease, conveyance or other disposition of
property or assets acquired within the twelve month period prior
to such sale, lease, conveyance or disposition in preparation
for a sale and leaseback transaction relating to such property
or assets;
(6) an issuance of Equity Interests by a Restricted
Subsidiary to Parent or another Restricted Subsidiary;
(7) the sale or other disposition of cash or Cash
Equivalents;
(8) the license or
sub-license
of patents, trademarks, copyrights, know how, process technology
or other intellectual property to third Persons by Parent or a
Restricted Subsidiary, so long as Parent or such Restricted
Subsidiary retain the right to use such licensed property;
206
(9) the granting or assumption of a Lien permitted by
“Certain Covenants — Liens,” including a
Permitted Lien;
(10) any sale or disposition of Securitization Assets to a
Securitization Subsidiary in connection with a Qualified
Securitization Financing;
(11) the sale or disposition of accounts receivable in
connection with the collection or compromise thereof in the
ordinary course of business; and
(12) Project Dispositions.
“Asset Sale Offer” has the meaning assigned to
that term in the Indenture governing the Notes.
“Attributable Debt” in respect of a sale and
leaseback transaction means, at the time of determination, the
present value of the obligation of the lessee for net rental
payments during the remaining term of the lease included in such
sale and leaseback transaction, including any period for which
such lease has been extended or may, at the option of the
lessor, be extended. Such present value shall be calculated
using a discount rate equal to the rate of interest implicit in
such transaction, determined in accordance with IFRS.
“BBVA” means Banco Bilbao Vizcaya Argentaria,
S.A.
“BBVA ‘B’ Share Transaction” means
(a) the purchase by BBVA of certain non-voting Class B
common stock issued by Parent, (b) the contribution of the
purchase price of such shares by Parent to the Company and
(c) the subsequent purchase of such shares from BBVA by the
Company in connection with the Merger, in each case, as
described in the offering memorandum.
“Board of Directors” means:
(1) with respect to a corporation, the board of directors
of the corporation or any committee thereof duly authorized to
act on behalf of such board of directors;
(2) with respect to a partnership, the board of directors
of the general partner of the partnership;
(3) with respect to a limited liability company, the
managing member or members or any controlling committee of
managing members thereof; and
(4) with respect to any other Person, the board or
committee of such Person serving a similar function.
“Business Day” means each day that is not a
Saturday, Sunday or other day on which banking institutions in
New York, New York are authorized or required by law to close.
“Capital Lease Obligation” of any Person means
the obligations of such Person to pay rent or other amounts
under any lease of (or other arrangement conveying the right to
use) real or personal property, or a combination thereof, which
obligations are required to be classified and accounted for as
capital leases on a balance sheet of such Person in accordance
with IFRS, and the amount of such obligations shall be the
capitalized amount thereof required to be set forth on a balance
sheet of such Person in accordance with IFRS.
“Capital Stock” means:
(1) in the case of a corporation, any and all shares,
including common stock and preferred stock;
(2) in the case of an association or business entity, any
and all shares, interests, participations, rights or other
equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability
company, partnership or membership interests (whether general or
limited); and
(4) any other interest or participation that confers on a
Person the right to receive a share of the profits and losses
of, or distributions of assets of, the issuing Person, but
excluding from all of the foregoing any debt securities
convertible into Capital Stock, whether or not such debt
securities include any right of participation with Capital Stock.
207
“Cash Equivalents” means:
(1) marketable securities (a) issued or directly and
unconditionally guaranteed as to interest and principal by the
United States Government or (b) issued by any agency or
instrumentality of the United States the obligations of
which are backed by the full faith and credit of the United
States, in each case maturing within one year after such date
and having, at the time of the acquisition thereof, a rating of
at least A-1
from S&P or at least
P-1 from
Moody’s;
(2) marketable direct obligations issued by any state of
the United States of America or any political subdivision of any
such state or any public instrumentality thereof, in each case
maturing one year after such date and having, at the time of the
acquisition thereof, a rating of at least
A-1 from
S&P or at least
P-1 from
Moody’s;
(3) certificates of deposit or bankers’ acceptances
maturing within 6 months after its date of issuance or
acceptance by any Lender or by any commercial bank organized
under the laws of the United States of America or any state
thereof or the District of Columbia that (a) is at least
“adequately capitalized” (as defined in the
regulations of its primary Federal banking regulator),
(b) has Tier 1 capital of not less than
$1,000 million and (c) has a rating of at least AA-
from S&P and Aa3 from Moody’s;
(4) any repurchase agreement entered into with any Lender
or any commercial banking institution satisfying the criteria of
clause (3) herein which (a) is secured by a fully
perfected security interest in any obligation of the type
described in clause (1)(a) and (b) has a market value at
the time such repurchase agreement is entered into of not less
than 100% of the repurchase obligation of such commercial
banking institution thereunder;
(5) commercial paper and variable fixed rate Notes issued
by any commercial banking institution satisfying the criteria of
clause (3) herein or any variable or fixed rate Note issued
by, or guaranteed by, a corporation (other than structured
investment vehicles and other than corporations used in
structured financing transactions) rated
A-1 (or the
equivalent thereof) or better by S&P or
P-1 (or the
equivalent thereof) or better by Moody’s, in each case with
average maturities of not more than one year from the date of
acquisition thereof;
(6) shares of any money market mutual fund that
(a) has substantially all of its assets invested
continuously in the types of investments referred to in
clauses (1) through (5) above, (b) has net assets
of not less than $5,000 million, and (c) has the
highest rating obtainable from either S&P or
Moody’s; and
(7) instruments equivalent to those referred to in
clauses (1) through (6) above denominated in Euros or
any other foreign currency comparable in credit quality and
tenor to those referred to above and customarily used by
corporations for short term cash management purposes in any
jurisdiction outside the United States to the extent reasonably
required in connection with any business conducted by any
Subsidiary organized in such jurisdiction, in each case which
instruments or obligors (or the parents of such obligors) have
comparable tenor and ratings described in such clauses or
equivalent ratings from comparable foreign ratings agencies;
provided, that, in the case of any Investment by the
Parent or a Foreign Subsidiary, “Cash Equivalents”
shall also include: (x) direct obligations of the sovereign
nation (or any agency thereof) in which such Foreign Subsidiary
is organized and is conducting business or in obligations fully
and unconditionally guaranteed by such sovereign nation (or any
agency thereof), in each case maturing within 12 months
after such date, (y) investments of the type and maturity
described in clauses (1) through (7) above of Foreign
Subsidiaries, which Investments have ratings described in such
clauses or equivalent ratings from comparable foreign rating
agencies and (z) shares of money market mutual or similar
funds which invest exclusively in assets otherwise satisfying
the requirements of this definition (including this proviso).
“Change of Control” means the occurrence of any
of the following:
(1) any sale, lease, exchange or other transfer (in one
transaction or a series of related transactions) of all or
substantially all of the property and assets of Parent and the
Restricted Subsidiaries, taken as a whole, to any Person or
group of related Persons for purposes of Section 13(d) of
the Exchange Act (a
208
“Group”), together with any Affiliates thereof
(whether or not otherwise in compliance with the provisions of
the Indenture), other than to the Company or one or more
Guarantors;
(2) the adoption of any plan or proposal for the
liquidation or dissolution of Parent or the Company (whether or
not otherwise in compliance with the provisions of the
Indenture);
(3) (a) any Person or Group (other than a Permitted
Holder Group) shall be or become the owner, directly or
indirectly, beneficially or of record, of shares representing
more than 35% of the aggregate ordinary voting power represented
by Parent’s issued and outstanding Capital Stock or
(b) the Permitted Holder Group becomes the owner, directly
or indirectly, beneficially or of record, of shares representing
more than 50% of the aggregate ordinary voting power represented
by our issued and outstanding Capital Stock;
(4) the Company shall cease to be a Subsidiary of
Parent; or
(5) the replacement of a majority of Parent’s Board of
Directors over a two-year period from the directors who
constituted our Board of Directors at the beginning of such
period, and such replacement shall not have been approved by a
vote of at least a majority of the Board of Directors then still
in office who either were members of such Board of Directors at
the beginning of such period or whose election as a member of
such Board of Directors was previously so approved.
“Change of Control Offer” has the meaning
assigned to that term in the Indenture governing the Notes.
“Consolidated Cash Flow” means, with respect to
any specified Person for any period, the Consolidated Net Income
of such Person for such period plus (without duplication):
(1) an amount equal to any extraordinary loss plus any net
loss realized by such Person or any of its Subsidiaries in
connection with an Asset Sale, to the extent such losses were
deducted in computing such Consolidated Net Income; plus
(2) provision or expenses for taxes based on income or
profits of such Person and its Restricted Subsidiaries for such
period, to the extent that such provision or expenses for taxes
were deducted in computing such Consolidated Net Income;
plus
(3) the amount of any franchise or similar taxes paid or
accrued, to the extent that such provision or expenses for taxes
were deducted in computing such Consolidated Net Income;
plus
(4) the Fixed Charges of such Person and its Restricted
Subsidiaries for such period, to the extent that any such
expense was deducted in computing such Consolidated Net Income;
plus
(5) depreciation, amortization (including amortization of
goodwill, financing costs and other intangibles but excluding
amortization of prepaid cash expenses that were paid in a prior
period) of such Person and its Restricted Subsidiaries for such
period to the extent that such depreciation, amortization, and
other non-cash expenses were deducted in computing such
Consolidated Net Income; plus
(6) any expenses (including fees) or charges relating to
any public or private sale of Capital Stock of such Person,
Investment, acquisition (including integration charges and
expenses identified at the time of closing of any acquisition as
resulting from such acquisition), recapitalization, disposition,
listing or discharge of securities registration obligations, the
Merger Agreement (including ratings agencies fees paid and other
transaction costs arising in connection with the offering of the
Notes and the Merger) or Indebtedness (including the discharge,
extinguishment or repayment thereof) permitted to be incurred
under the Indenture (in each case whether or not consummated) or
to the Transactions and, in each case, deducted in such period
in computing Consolidated Net Income; plus
(7) the amount of any minority interest expense deducted in
such period in calculating Consolidated Net Income; plus
(8) any non-cash compensation charge in such period arising
from any grant of stock, stock options or other equity-based
award; plus
209
(9) gains (or losses) in respect of returned surplus assets
of any employee benefit plan (in the case of gains) or increased
funding obligations in excess of actual cash expenditures for
such period (in the case of losses), it being understood that
the actual cash expenditure in respect of any such increased
funding obligations shall be given effect for the purpose of
calculating Consolidated Cash Flow in each future period during
which such an actual cash expenditure is made); plus
(10) any non-cash pension and other post-employment benefit
expense deducted in such period in computing Consolidated Net
Income; plus
(11) any non-cash decrease in consolidated IFRS revenue
resulting from purchase accounting in connection with any
acquisitions permitted hereunder less any non-cash increase in
consolidated IFRS revenue resulting from purchase accounting in
connection with acquisitions permitted hereunder; plus
(12) any extraordinary, unusual, or non-recurring losses,
charges and expenses deducted in such period in calculating
Consolidated Net Income; plus
(13) any other non-cash charges, including any write off or
write downs, reducing Consolidated Net Income for such period
(provided that if any such non-cash charges represent an
accrual or re serve for potential cash items in any future
period, the cash payment in respect thereof in such future
period shall be subtracted from Consolidated Cash Flow to such
extent, and excluding amortization of a prepaid cash item that
was paid in a prior period and the reversal of any accrual of,
or cash reserve for, anticipated charges in any period where
such accrual or reserve is no longer required); plus
(14) any Exceptional Items, without duplication, resulting
in a loss in accordance with IFRS; minus
(15) any non-cash items increasing such Consolidated Net
Income for such period, other than the accrual of revenue in the
ordinary course of business, in each case, on a consolidated
basis (without duplication) and determined in accordance with
IFRS; minus
(16) any Exceptional Items, without duplication resulting
in a gain in accordance with IFRS; minus
(17) any extraordinary, unusual, or non-recurring gains
increasing Consolidated Net Income during such period.
“Consolidated Net Income” means, with respect
to any specified Person for any period, the aggregate of the Net
Income of such Person and its Subsidiaries that are Restricted
Subsidiaries for such period, on a consolidated basis,
determined in accordance with IFRS; provided that:
(1) the Net Income (but not loss, except to the extent of
any cash capital contribution) of any Person that is not a
Restricted Subsidiary or that is accounted for by the equity
method of accounting will be included only to the extent of the
amount of dividends or distributions paid in cash to the
specified Person or a Restricted Subsidiary of the Person;
(2) the Net Income of any Restricted Subsidiary will be
excluded to the extent that the declaration or payment of
dividends or similar distributions by that Restricted Subsidiary
to that specified Person or another Restricted Subsidiary of
such Person of that Net Income is not at the date of
determination permitted without any prior governmental approval
(that has not been obtained) or, directly or indirectly, by
operation of the terms of its charter or any agreement,
instrument, judgment, decree, order, statute, rule or
governmental regulation applicable to that Restricted Subsidiary
or its stockholders;
(3) the following non-cash items will be excluded:
(i) the cumulative effect of a change in accounting
principles;
(ii) the write-off of any debt issuance costs;
(iii) any non-cash impairment charges relating to goodwill;
(iv) any non-cash income (or loss) related to
non-speculative hedging activities;
(v) any income (or loss) from discontinued operations;
210
(vi) any extraordinary gain, loss or charge (including in
connection with any Asset Sale);
(vii) all deferred financing costs written off, premiums
paid and other net gains or losses in connection with any early
extinguishment of Indebtedness;
(viii) any non-cash impairment charges resulting from the
impairment or disposal of long-lived assets and the amortization
of intangibles arising in connection with business combinations;
(ix) accruals and reserves that are established within
twelve months after the Issue Date, provided that any
such accruals or reserves paid in cash shall be deducted from
Consolidated Net Income for the period in which paid unless
excluded pursuant to another clause of this definition;
(x) any non-cash expense or gain related to recording of
the fair market value of interest rate or currency agreements
and commodity agreements entered into, in each case, in the
ordinary course of business and not for speculative purposes;
(xi) unrealized gains and losses relating to
non-speculative hedging transactions and
mark-to-market
of Indebtedness denominated in foreign currencies;
(xii) the amount of non-cash charges relating to the
exercise of options or the grant of stock, stock options or
other equity based awards; and
(xiii) any non-cash expense related to the establishment of
allowances or reserves attributable to the non-recognition of
deferred tax assets.
“Credit Agreement” means that certain credit
and guaranty agreement of Parent and certain of its Subsidiaries
with Deutsche Bank AG New York Branch, as administrative agent,
and the other parties thereto, dated on or about the Assumption
Date, including any related Notes, Guarantees, instruments and
agreements executed in connection therewith, and, in each case,
as amended, modified, renewed, refunded, replaced (whether after
or upon termination or otherwise), restructured, restated or
refinanced (including any agreement to extend the maturity
thereof and adding additional borrowers or guarantors and
including by means of sales of debt securities) in whole or in
part under such agreement or agreements or any successor
agreement or agreements from time to time under the same or any
other agent, lender or group of lenders and including increasing
the amount of available borrowings thereunder; provided
that such increase is permitted under “Certain
Covenants — Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock.”
“Credit Facilities” means one or more debt
facilities or agreements (including, without limitation, the
Credit Agreement) or commercial paper facilities or Indentures,
in each case with banks or other institutional lenders providing
for, or acting as initial purchasers of, revolving credit loans,
term loans, Notes, debentures, securities, receivables financing
(including through the sale of receivables to such lenders or to
special purpose entities formed to borrow from such lenders
against such receivables) or letters of credit, in each case, as
amended, restated, modified, renewed, refunded, replaced
(whether after or upon termination or otherwise), restructured,
restated or refinanced (including any agreement to extend the
maturity thereof and adding additional borrowers or guarantors
and including by means of sales of debt securities to
institutional investors) in whole or in part from time to time
and including increasing the amount of available borrowings
thereunder; provided that such increase is permitted
under “Certain Covenants — Incurrence of
Indebtedness and Issuance of Disqualified Stock and Preferred
Stock.”
“Default” means any event that is, or with the
passage of time or the giving of notice or both would be, an
Event of Default.
“Designated Non-Cash Consideration” means the
fair market value of non-cash consideration received by Parent
or any Restricted Subsidiary in connection with an Asset Sale
that is so designated as Designated Non-Cash Consideration
pursuant to an officer’s certificate, setting forth the
basis of such valuation, less the amount of cash or Cash
Equivalents received in connection with a subsequent sale,
redemption or payment of, on or with respect to, such Designated
Non-Cash Consideration.
“Disqualified Stock” means any Capital Stock
that, by its terms (or by the terms of any security into which
it is convertible, or for which it is exchangeable, in each case
at the option of the holder of the Capital
211
Stock), or upon the happening of any event, matures or is
mandatorily redeemable, pursuant to a sinking fund obligation or
otherwise, or redeemable at the option of the holder of the
Capital Stock, in whole or in part, on or prior to the date that
is 91 days after the date on which the Notes mature.
Notwithstanding the preceding sentence, any Capital Stock that
would constitute Disqualified Stock solely because the holders
of the Capital Stock have the right to require Parent or any of
its Restricted Subsidiaries to repurchase such Capital Stock
upon the occurrence of a Change of Control or an Asset Sale will
not constitute Disqualified Stock if the terms of such Capital
Stock provide that Parent or such Restricted Subsidiary may not
repurchase or redeem any such Capital Stock pursuant to such
provisions unless such repurchase or redemption complies with
“Certain Covenants — Restricted Payments.”
The amount of Disqualified Stock deemed to be outstanding at any
time for purposes of the Indenture will be the maximum amount
that Parent and the Restricted Subsidiaries may become obligated
to pay upon the maturity of, or pursuant to any mandatory
redemption provisions of, such Disqualified Stock, exclusive of
accrued dividends.
“Domestic Subsidiary” means any Subsidiary that
is not a Foreign Subsidiary.
“Eligible Escrow Investments” means
(1) Government Securities maturing no later than the
Business Day preceding the Special Redemption Date and
(2) securities representing an interest or interests in
money market funds registered under the Investment Company Act
of 1940 whose shares are registered under the Securities Act as
investing exclusively in direct obligations of the United States
of America.
“Equity Interests” means Capital Stock and all
warrants, options, restricted stock units, performance units or
other rights to acquire Capital Stock (but excluding any debt
security that is convertible into, or exchangeable for, Capital
Stock).
“Exceptional Items” means one-off cash gains or
losses incurred by Parent or any of its Subsidiaries during the
relevant period and to include one-off restructuring costs
related to the Transactions.
“Exchange Act” means the Securities Exchange
Act of 1934, as amended, and the rules and regulations of the
SEC promulgated thereunder.
“Existing Indebtedness” means Indebtedness of
Parent and its Restricted Subsidiaries (without duplication) in
existence on the Issue Date (other than Indebtedness under the
Credit Agreement or in respect of the Notes), until such amounts
are repaid.
“Existing Indebtedness to be Repaid” means all
Indebtedness of Parent and its Restricted Subsidiaries other
than €100 million of Indebtedness outstanding on the
Issue Date.
“Fixed Charge Coverage Ratio” means, with
respect to any specified Person for any period, the ratio of the
Consolidated Cash Flow of such Person for such period to the
Fixed Charges of such Person for such period. In the event that
the specified Person or any of its Restricted Subsidiaries
incurs, assumes, Guarantees, repays, repurchases or redeems any
Indebtedness (other than ordinary working capital borrowings) or
issues, repurchases or redeems preferred stock subsequent to the
commencement of the period for which the Fixed Charge Coverage
Ratio is being calculated and on or prior to the date on which
the event for which the calculation of the Fixed Charge Coverage
Ratio is made (the “Calculation Date”), then
the Fixed Charge Coverage Ratio will be calculated giving pro
forma effect to such incurrence, assumption, Guarantee,
repayment, repurchase or redemption of Indebtedness, or such
issuance, repurchase or redemption of preferred stock, and the
use of the proceeds therefrom (including use on the Calculation
Date) as if the same had occurred at the beginning of the
applicable four-quarter reference period; provided,
however, that the Fixed Charges of such Person
attributable to interest on any Indebtedness under a revolving
credit facility computed on a pro forma basis will be computed
based on the average daily balance of such Indebtedness during
the four-quarter reference period and using the interest rate in
effect at the end of such period (taking into account any
interest rate option, swap, cap or similar agreement applicable
to such Indebtedness). In addition, for purposes of calculating
the Fixed Charge Coverage Ratio:
(1) acquisitions that have been made or are, on the
Calculation Date, being made by the specified Person or any of
its Restricted Subsidiaries, including through mergers or
consolidations, or any Person or any of its Restricted
Subsidiaries acquired by (including acquisitions on the
Calculation Date) the
212
specified Person or any of its Restricted Subsidiaries, and
including any related financing transactions and including any
increase in ownership of Restricted Subsidiaries, during the
four-quarter reference period or subsequent to such reference
period and on or prior to the Calculation Date will be given pro
forma effect as if they had occurred on the first day of the
four-quarter reference period and Consolidated Cash Flow for
such reference period will be calculated without giving effect
to clause (3) of the proviso set forth in the definition of
Consolidated Net Income;
(2) the Consolidated Cash Flow attributable to discontinued
operations, as determined in accordance with IFRS, and
operations or businesses (and ownership interests therein)
disposed of prior to the Calculation Date, will be
excluded; and
(3) the Fixed Charges attributable to discontinued
operations, as determined in accordance with IFRS, and
operations or businesses (and ownership interests therein)
disposed of prior to the Calculation Date, will be excluded, but
only to the extent that the obligations giving rise to such
Fixed Charges will not be obligations of the specified Person or
any of its Restricted Subsidiaries following the Calculation
Date;
provided that whenever pro forma effect is to be given to
an acquisition or a disposition, the amount of income or
earnings related thereto (including the incurrence of any
Indebtedness and any pro forma expense and cost reductions that
have occurred or are reasonably expected to occur, regardless of
whether those expense and cost reductions could then be
reflected in pro forma financial statements in accordance with
Regulation S-X
promulgated under the Securities Act or any regulation or policy
of the SEC related thereto) shall be reasonably determined in
good faith by one of the Company’s responsible senior
financial or accounting officers so long as such cost savings
are actually expected to be achieved within 12 months of
such acquisition or disposition.
“Fixed Charges” means, with respect to any
specified Person for any period, the sum, without duplication,
of:
(1) the consolidated interest expense of such Person and
its Restricted Subsidiaries for such period, whether paid or
accrued (including, without limitation, amortization of debt
issuance costs and original issue discount, non-cash interest
payments, the interest component of any deferred payment
obligations, the interest component of all payments associated
with Capital Lease Obligations, imputed interest with respect to
Attributable Debt, commissions, discounts and other fees and
charges incurred in respect of letter of credit or bankers’
acceptance financings, and net of the effect of all payments
made or received pursuant to Hedging Obligations in respect of
interest rates); plus
(2) the consolidated interest expense of such Person and
its Restricted Subsidiaries that was capitalized during such
period; plus
(3) any interest expense on Indebtedness of another Person
that is Guaranteed by such Person or one of its Restricted
Subsidiaries or secured by a Lien on assets of such Person or
one of its Restricted Subsidiaries, whether or not such
Guarantee or Lien is called upon; plus
(4) the product of (a) all dividends, whether paid or
accrued and whether or not in cash, on any series of preferred
stock of such Person or any of its Restricted Subsidiaries,
other than dividends on Equity Interests payable solely in
Equity Interests of such Person (other than Disqualified Stock)
or to such Person or one of its Restricted Subsidiaries, times
(b) a fraction, the numerator of which is one and the
denominator of which is one minus the then current combined
federal, state and local statutory tax rate of such Person,
expressed as a decimal, in each case, on a consolidated basis
and in accordance with IFRS.
“Foreign Subsidiary” means any Subsidiary that
is organized under the laws of a jurisdiction other than the
United States of America, a State thereof or the District of
Columbia.
213
“Government Securities” means securities that
are:
(1) direct obligations of the United States of America for
the timely payment of which its full faith and credit is
pledged; or
(2) obligations of a Person controlled or supervised by and
acting as an agency or instrumentality of the United States of
America the timely payment of which is unconditionally
guaranteed as a full faith and credit obligation by the United
States of America, which, in either case, are not callable or
redeemable at the option of the issuers thereof, and shall also
include a depository receipt issued by a bank (as defined in
Section 3(a)(2) of the Securities Act), as custodian with
respect to any such Government Securities or a specific payment
of principal of or interest on any such Government Securities
held by such custodian for the account of the holder of such
depository receipt; provided, however, that
(except as required by law) such custodian is not authorized to
make any deduction from the amount payable to the holder of such
depository receipt from any amount received by the custodian in
respect of the Government Securities or the specific payment of
principal of or interest on the Government Securities evidenced
by such depository receipt.
“Guarantee” means a guarantee other than by
endorsement of negotiable instruments for collection in the
ordinary course of business, direct or indirect, in any manner
including, without limitation, by way of a pledge of assets or
through letters of credit or reimbursement agreements in respect
thereof, of all or any part of any Indebtedness.
“Guarantor” means each Person that Guarantees
the Notes in accordance with the terms of the Indenture
governing the Notes.
“Hedging Obligations” means, with respect to
any specified Person, the obligations of such Person under:
(1) interest rate swap agreements (whether from fixed to
floating or floating to fixed), interest rate cap agreements and
interest rate collar agreements;
(2) other agreements or arrangements designed to manage
interest rates or interest rate risk; and
(3) foreign exchange contracts, currency swap agreements or
other agreements or arrangements designed to protect such Person
against fluctuations in currency exchange rates or commodity
prices.
“Holder” means a Person in whose name a Note is
registered.
“IFRS” means the International Financial
Reporting Standards, as promulgated by the International
Accounting Standards Board (or any successor board or agency),
as in effect on the Issue Date.
“Immaterial Subsidiary” means, as of any date,
any Restricted Subsidiary whose total assets, as of that date,
are less than $10 million and whose total revenues for the
most recent
12-month
period do not exceed $10 million; provided that a
Restricted Subsidiary will not be considered to be an Immaterial
Subsidiary if it, directly or indirectly, guarantees or
otherwise provides direct credit support for any Indebtedness of
Parent or any of its other Restricted Subsidiaries.
“Indebtedness” means, with respect to any
specified Person, any indebtedness (excluding accrued expenses
or trade payables), of such Person, whether or not contingent:
(1) in respect of borrowed money;
(2) evidenced by bonds, Notes, debentures or similar
instruments or letters of credit (or reimbursement agreements in
respect thereof);
(3) in respect of banker’s acceptances;
(4) representing Capital Lease Obligations;
214
(5) representing the balance deferred and unpaid of the
purchase price of any property due more than six months after
such property is acquired, except any such balance that
constitutes an accrued expense or trade payable; or
(6) representing the net amount of any Hedging Obligations,
if and to the extent any of the preceding items (other than
letters of credit and Hedging Obligations) would appear as a
liability upon a balance sheet of the specified Person prepared
in accordance with IFRS. In addition, the term
“Indebtedness” includes all Indebtedness of others
secured by a Lien on any asset of the specified Person (whether
or not such Indebtedness is assumed by the specified Person)
and, to the extent not otherwise included, the Guarantee by the
specified Person of any Indebtedness of any other Person.
The amount of any Indebtedness outstanding as of any date will
be (without duplication):
(1) the accreted value of the Indebtedness, in the case of
any Indebtedness issued with original issue discount;
(2) the principal amount of the Indebtedness, together with
any interest on the Indebtedness that is more than 30 days
past due, in the case of any other Indebtedness; and
(3) in respect of Indebtedness of another Person secured by
a Lien on the assets of the specified Person, the lesser of:
(a) the fair market value of such assets that are subject
to such Lien at the date of determination; and
(b) the amount of the Indebtedness of the other Person
secured by such assets.
“Investment Grade Rating” means a rating equal
to or higher than Baa3 (or the equivalent) by Moody’s and
BBB- (or the equivalent) by S&P, or an equivalent rating by
any other Rating Agency.
“Investments” means, with respect to any
Person, all direct or indirect investments by such Person in
other Persons (including Affiliates) in the forms of loans
(including Guarantees or other obligations), advances or capital
contributions (excluding commission, travel and similar advances
to officers and employees made in the ordinary course of
business), purchases or other acquisitions for consideration of
Indebtedness, Equity Interests or other securities, together
with all items that are or would be classified as investments on
a balance sheet prepared in accordance with IFRS (it being
understood that capital expenditures shall not be deemed to be
“Investments”). If Parent or any of its Restricted
Subsidiaries sells or otherwise disposes of any Equity Interests
of any direct or indirect Subsidiary of Parent such that, after
giving effect to any such sale or disposition, such Person is no
longer a Subsidiary of Parent, Parent will be deemed to have
made an Investment on the date of any such sale or disposition
equal to the fair market value of the Equity Interests of such
Subsidiary not sold or disposed of in an amount determined as
provided in the final paragraph of “Certain
Covenants — Restricted Payments.” The acquisition
by Parent or any of its Restricted Subsidiaries of a Person that
holds an Investment in a third Person will be deemed to be an
Investment by Parent or such Restricted Subsidiary in such third
Person in an amount equal to the fair market value of the
Investment held by the acquired Person in such third Person in
an amount determined as provided in the final paragraph of
“Certain Covenants — Restricted Payments.”
Except as otherwise provided in the Indenture, the amount of an
Investment will be determined at the time the Investment was
made and without giving effect to subsequent changes in value.
“Issue Date” means January 21, 2011.
“Lien” means, with respect to any asset, any
mortgage, lien, pledge, charge, security interest or encumbrance
of any kind in respect of such asset, whether or not filed,
recorded or otherwise perfected under applicable law, including
any conditional sale or other title retention agreement, any
lease in the nature thereof, any option or other agreement to
sell or give a security interest in and any filing of or
agreement to give any financing statement under the Uniform
Commercial Code (or equivalent statutes) of any jurisdiction.
“Mergers” shall have the meaning assigned to
such term in the Merger Agreement.
215
“Merger Agreement” means the Agreement and Plan
of Merger, dated as of June 6, 2010, among the Grifols,
S.A., Grifols Inc. and Talecris Biotherapeutics Holdings Corp.,
as the same may be amended prior to the Issue Date.
“Moody’s” means Moody’s Investors
Service, Inc. and any successor to its rating agency business.
“Net Income” means, with respect to any
specified Person, the net income (loss) of such Person,
determined in accordance with IFRS and before any reduction in
respect of preferred stock dividends, excluding, however:
(1) any gain (but not loss), together with any related
provision for taxes on such gain (but not loss), realized in
connection with: (a) any Asset Sale or (b) the
disposition of any securities by such Person or any of its
Restricted Subsidiaries or the extinguishment of any
Indebtedness of such Person or any of its Restricted
Subsidiaries;
(2) any extraordinary gain (but not loss), together with
any related provision for taxes on such extraordinary gain (but
not loss); and
(3) any merger termination fee received by Parent or a
Restricted Subsidiary.
“Net Proceeds” means the aggregate cash
proceeds received by Parent or any Restricted Subsidiary in
respect of any Asset Sale (including, without limitation, any
cash received upon the sale or other disposition of any non-cash
consideration received in any Asset Sale), net of (i) the
direct costs directly attributable to such Asset Sale,
including, without limitation, legal, accounting and investment
banking fees, and sales commissions, (ii) taxes paid or
payable as a result of the Asset Sale, in each case, after
taking into account any available tax credits or deductions and
any tax sharing arrangements, (iii) amounts required to be
applied to the repayment of Indebtedness secured by a Lien on
the asset or assets that were the subject of such Asset Sale,
(iv) any reserve for adjustment in respect of the sale
price of such asset or assets established in accordance with
IFRS (unless such reserve is not used) against any liabilities
associated with such Asset Sale and retained by Parent or any
Restricted Subsidiary, as the case may be, after such Asset
Sale, including, without limitation, pension and other
post-employment benefit liabilities, liabilities related to
environmental matters and liabilities under any indemnification
obligations (whether fixed or contingent) associated with such
Asset Sale.
“Non-recourse Debt” means Indebtedness:
(1) as to which neither Parent nor any of the Restricted
Subsidiaries (a) provides credit support of any kind
(including any undertaking, agreement or instrument that would
constitute Indebtedness) or (b) is directly or indirectly
liable as a guarantor or otherwise;
(2) no default with respect to which (including any rights
that the holders thereof may have to take enforcement action
against an Unrestricted Subsidiary) would permit upon notice,
lapse of time or both any holder of any other Indebtedness of
Parent or any of the Restricted Subsidiaries to declare a
default on such other Indebtedness or cause the payment thereof
to be accelerated or payable prior to its Stated
Maturity; and
(3) as to which the lenders have been notified in writing
that they will not have any recourse to the stock or assets of
Parent or any of the Restricted Subsidiaries.
“non-U.S. Guarantor”
has the meaning set forth under “— Additional
Amounts.”
“Obligations” means any principal, interest,
penalties, fees, indemnifications, reimbursements, damages and
other liabilities payable under the documentation governing any
Indebtedness.
“Parent” means Grifols, S.A.
“Permitted Business” means healthcare products
and services (including the lines of business conducted by
Parent, Talecris and the Restricted Subsidiaries on the date of
the Indenture) and any businesses ancillary, complementary or
reasonably related thereto.
216
“Permitted Holder Group” means any group
comprised solely of the Grifols family, holding directly or
indirectly (the “Existing Holders”), or
(ii) a person or group of related persons for purposes of
Section 13(d) of the Exchange Act that includes the
Existing Holders where the Existing Holders control (whether
through exercise of voting rights, by contract or otherwise) the
Parent.
“Permitted Investments” means:
(1) any Investment in Parent or in a Restricted Subsidiary;
(2) any Investment cash and Cash Equivalents and
Investments that were Cash Equivalents when made;
(3) loans and advances to employees, officers, consultants
and directors of Parent or a Restricted Subsidiary in the
ordinary course of business for bona fide business purposes not
in excess of $5 million at any one time outstanding;
(4) any Investment by Parent or a Restricted Subsidiary in
a Person, if as a result of such Investment:
(a) such Person becomes a Restricted Subsidiary; or
(b) such Person is merged, consolidated or amalgamated with
or into, or transfers or conveys substantially all of its assets
to, or is liquidated into, Parent or a Restricted Subsidiary;
(5) any Investment made as a result of the receipt of
non-cash consideration from an Asset Sale that was made pursuant
to and in compliance under “Repurchase at the Option of
Holders — Asset Sales”;
(6) any acquisition of assets or Capital Stock solely in
exchange for the issuance of Parent’s Equity Interests
(other than Disqualified Stock);
(7) any Investments received (A) in compromise of
obligations of trade creditors or customers that were incurred
in the ordinary course of business of Parent or the Restricted
Subsidiaries, including pursuant to any plan of reorganization
or similar arrangement upon the bankruptcy or insolvency or
other reorganization of any trade creditor or customer or
(B) in resolution of litigation, arbitration or other
disputes or (C) as a result of foreclosure, perfection or
enforcement of any Lien;
(8) Hedging Obligations;
(9) Investments in Permitted Joint Ventures, together with
all other Investments pursuant to this clause (9) in an
aggregate amount at the time of such Investment not to exceed
$75 million (with the fair market value of each Investment
being measured at the time made and without giving effect to
subsequent changes in value);
(10) payroll, travel, moving and similar advances to cover
matters that are expected at the time of such advances
ultimately to be treated as expenses for accounting purposes and
that are made in the ordinary course of business;
(11) repurchases of the Notes;
(12) Notes, chattel paper and accounts receivable owing to
Parent or the Restricted Subsidiaries created or acquired in the
ordinary course of business (including concessionary trade terms
we deem reasonable under the circumstances);
(13) Investments in existence or made pursuant to legally
binding written commitments in existence on the Issue Date, and
any extension, modification, replacement, refunding, refinancing
or renewal thereof in whole or in part;
(14) Guarantees of Indebtedness issued in accordance with
the covenant described under the heading “Certain
Covenants — Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock,” and performance or
completion Guarantees in the ordinary course of business;
217
(15) Investments of a Restricted Subsidiary acquired after
the Issue Date, or of an entity acquired by, merged into,
amalgamated with, or consolidated with a Restricted Subsidiary
in a transaction that is not prohibited by the covenant
described under the heading “Certain Covenants —
Merger, Consolidation or Sale of Assets” after the Issue
Date, to the extent that such Investments were not made in
contemplation of such acquisition, merger, amalgamation or
consolidation and were in existence on the date of such
acquisition, merger, amalgamation or consolidation;
(16) Investments consisting of purchases and acquisitions
of inventory, supplies, material or equipment, including
pre-payments therefor;
(17) deposits, prepayments and other credits to suppliers
in the ordinary course of business consistent with past practice;
(18) Investments representing amounts held for employees of
Parent and the Restricted Subsidiaries under deferred
compensation plans; provided that the amount of such
Investments (excluding income earned thereon) shall not exceed
the amount otherwise payable to such employees the payment of
which was deferred under such plan and any amounts matched by
Parent or the Restricted Subsidiaries under such plan;
(19) Investments consisting of the licensing or
contribution of intellectual property pursuant to development,
marketing or manufacturing agreements or arrangements or similar
agreements or arrangements with other Persons in the ordinary
course of business;
(20) any Investment in exchange for, or out of the net
proceeds of the substantially concurrent sale (other than to a
Subsidiary of Parent or a Restricted Subsidiary or an employee
stock ownership plan or similar trust) of Capital Stock (other
than Disqualified Stock) of Parent; provided that the
amount of any net cash proceeds that are utilized for such
Investment will be excluded from clause 3(B) of the second
part of the first paragraph set forth under “Certain
Covenants — Restricted Payments”;
(21) Investments consisting of advances or loans to Persons
building, developing or overseeing the construction of plasma
collection centers expected to supply principally Parent or the
Restricted Subsidiaries in the ordinary course of business and
consistent with past practice;
(22) Investments relating to any Securitization Subsidiary
of Parent or any Restricted Subsidiary organized in connection
with a Qualified Securitization Financing that, in the good
faith determination of the Board of Directors and Parent, are
necessary or advisable to effect such Qualified Securitization
Financing;
(23) Investments in the ordinary course of business
consisting of UCC Article 3 endorsements for collection or
deposit and UCC Article 4 customary trade arrangements with
customers consistent with past practices; and
(24) other Investments in any Person having an aggregate
fair market value (measured on the date each such Investment was
made and without giving effect to subsequent changes in value),
when taken together with all other Investments made pursuant to
this clause (24) that are at the time outstanding, not to
exceed $200 million.
“Permitted Joint Venture” means any joint
venture that Parent or any Restricted Subsidiary is a party to
that is engaged in a Permitted Business.
“Permitted Liens” means:
(1) Liens to secure Obligations in respect of (i) any
Indebtedness incurred under clause (1) of the second
paragraph of “Certain Covenants — Incurrence of
Indebtedness and Issuance of Disqualified Stock and Preferred
Stock” and (ii) Indebtedness in excess of the maximum
amount of Indebtedness permitted to be secured by Liens pursuant
to the foregoing subclause (i), so long as, in the case of this
subclause (ii), immediately after giving effect to the
incurrence of such Indebtedness on the date such Indebtedness is
incurred (or, in the case of Indebtedness incurred pursuant to
revolving commitments under any Credit
218
Facility, on the date such revolving commitments are provided)
on a pro forma basis the Secured Leverage Ratio would not
exceed 3.0 to 1.0;
(2) Liens in favor of Parent or any Restricted Subsidiary;
(3) Liens and deposits to secure the performance of bids,
trade contracts, leases, statutory obligations, letters of
credit or trade guarantees, surety or appeal bonds, performance
bonds or other obligations of a like nature, in each case in the
ordinary course of business;
(4) Liens to secure Indebtedness (including Capital Lease
Obligations) permitted by clause (4) of the second
paragraph of “Certain Covenants — Incurrence of
Indebtedness and Issuance of Disqualified Stock and Preferred
Stock” covering only the assets acquired, or financed, with
such Indebtedness;
(5) Liens existing on the date of the Indenture and any
extensions, renewals or replacements thereof;
(6) Liens for taxes, assessments or governmental charges or
claims that are not yet delinquent or that are being contested
in good faith by appropriate proceedings promptly instituted and
diligently concluded; provided that any reserve or other
appropriate provision as is required in conformity with IFRS has
been made therefor and Liens for taxes assessed on real estate
assets that are not delinquent;
(7) Liens, pledges or deposits in the ordinary course of
business to secure workers’ compensation claims,
self-retention or self-insurance obligations, unemployment
insurance, performance, bid, release, appeal, surety and similar
bonds and related reimbursement obligations and completion
guarantees provided or incurred by Parent and the Restricted
Subsidiaries in the ordinary course of business, lease
obligations or nondelinquent obligations under social security
laws and obligations in connection with participation in
government insurance, benefits, reimbursement or other programs
or other similar requirements, return of money bonds and other
similar obligations, including obligations to secure health and
safety and environmental obligations (exclusive of obligations
for the payment of borrowed money or Indebtedness);
(8) Liens imposed by law, such as carrier’s,
supplier’s, workmen’s, warehousemen’s,
landlord’s, materialmen’s, repairmen’s and
mechanic’s Liens and other similar Liens arising in the
ordinary course of business or are being contested in good faith;
(9) easements,
rights-of-way,
restrictions, encroachments, minor defects or irregularities in
title and other similar charges or encumbrances not interfering
in any material respect with the ordinary conduct of our and our
Restricted Subsidiaries’ business or assets taken as a
whole;
(10) Liens securing Hedging Obligations so long as the
related Indebtedness is, and is permitted to be under the
Indenture, secured by the same property securing the Hedging
Obligations;
(11) Liens securing Permitted Refinancing Indebtedness
(other than Permitted Refinancing Indebtedness in respect of the
Existing Indebtedness to be Repaid), provided that such
Liens do not extend to any property or assets other that the
property or assets that secure the Indebtedness being refinanced;
(12) Liens created for the benefit of or securing the Notes
and the Guarantees;
(13) Liens arising from judgments in circumstances not
constituting an Event of Default as described under the heading
“Events of Default and Remedies”;
(14) Liens arising out of conditional sale, title
retention, consignment or similar arrangements for sale of goods
in the ordinary course of business;
(15) Liens in favor of customs or revenue authorities
arising as a matter of law to secure payment of customs duties
in connection with the importation of goods;
(16) bankers’ Liens, rights of setoff or similar
rights and remedies as to deposit accounts;
(17) Liens on specific items of inventory or other goods
and proceeds of any Person securing such Person’s
obligations in respect of bankers’ acceptances issued or
created for the account of such Person to facilitate the
purchase, shipment or storage of such inventory or other goods;
219
(18) Liens on insurance policies and proceeds thereof, or
other deposits, to secure insurance premium financings in the
ordinary course of business;
(19) Liens on accounts receivable and related assets of a
Securitization Subsidiary incurred in connection with a
Qualified Securitization Financing;
(20) Liens on property (including Capital Stock) of a
Person existing at the time such Person becomes a Restricted
Subsidiary of the Parent or is merged with or into or
consolidated with the Parent or any of its Restricted
Subsidiaries; provided that such Liens were in existence
prior to the contemplation of such Person becoming a Restricted
Subsidiary of the Parent or such merger or consolidation, were
not incurred in contemplation thereof and do not extend to any
assets other than those of the Person that becomes a Restricted
Subsidiary of the Parent or is merged with or into or
consolidated with the Parent or any of its Restricted
Subsidiaries;
(21) filing of Uniform Commercial Code financing statements
under U.S. state law (or similar filings under applicable
jurisdiction) in connection with operating leases in the
ordinary course of business;
(22) operating leases, licenses, subleases and sublicenses
of assets (including real property and intellectual property
rights), in each case entered into in the ordinary course of
business;
(23) Liens (including put and call arrangements) on Capital
Stock or other securities of any Unrestricted Subsidiary that
secure Indebtedness of such Unrestricted Subsidiary;
(24) limited recourse Liens in respect of the ownership
interests in, or assets owned by, any joint ventures which are
not Restricted Subsidiaries securing obligations of such joint
ventures;
(25) Liens incurred by the Parent or any Restricted
Subsidiary with respect to obligations that do not exceed
$250 million at any one time outstanding;
(26) Liens on the assets of any Restricted Subsidiary
(other than the Company or any Guarantor) to secure Indebtedness
of such Restricted Subsidiary;
(27) Liens solely on cash earnest money deposits made by
Parent or any Restricted Subsidiary in connection with any
letter-of-intent
or purchase agreement entered into in connection with any
Investment permitted under the Indenture;
(28) any interest of a lessor or sublessor under any lease
of real estate permitted hereunder and covering only the assets
so leased any Liens encumbing such lessor’s or
sublessor’s interest or title; and
(29) any zoning or similar law or right reserved in any
governmental office or agency to control or regulate the use of
any real property.
“Permitted Refinancing Indebtedness” means any
Indebtedness of Parent or any of the Restricted Subsidiaries
issued in exchange for, or the net proceeds of which are used to
extend, refinance, renew, replace, defease, refund or discharge
other Indebtedness of Parent or any of the Restricted
Subsidiaries (other than intercompany Indebtedness); provided
that:
(1) the principal amount (or accreted value, if applicable)
of such Permitted Refinancing Indebtedness does not exceed the
principal amount (or accreted value, if applicable) of the
Indebtedness extended, refinanced, renewed, replaced, defeased,
refunded or discharged (plus all accrued interest on the
Indebtedness and the amount of all fees, expenses and premiums
incurred in connection therewith);
(2) such Permitted Refinancing Indebtedness has a final
maturity date later than the final maturity date of, and has a
Weighted Average Life to Maturity equal to or greater than the
Weighted Average Life to Maturity of, the Indebtedness being
extended, refinanced, renewed, replaced, defeased, refunded or
discharged;
(3) if the Indebtedness being extended, refinanced,
renewed, replaced, defeased, refunded or discharged is
subordinated in right of payment to the Notes, such Permitted
Refinancing Indebtedness is
220
subordinated in right of payment to, the Notes on terms at least
as favorable to the Holders of Notes as those contained in the
documentation governing the Indebtedness being extended,
refinanced, renewed, replaced, defeased, refunded or
discharged; and
(4) such Indebtedness is incurred either by Parent, a
Guarantor or by the Restricted Subsidiary who is the obligor on
the Indebtedness being extended, refinanced, renewed, replaced,
defeased, refunded or discharged.
“Person” means any individual, corporation,
partnership, joint venture, association, joint-stock company,
trust, unincorporated organization, limited liability company or
government or other entity.
“Project Disposition” means any sale,
assignment, conveyance, transfer or other disposition of
facilities under construction of Parent and its Restricted
Subsidiaries as of the Issue Date (including the real estate
related thereto) which are intended by Parent upon completion of
construction to be repurchased or leased by Parent or one of its
Restricted Subsidiaries or any business related, ancillary or
complementary thereto; provided, that the consideration
received for such assets shall be cash in an amount at least
equal to the book value.
“Qualified Equity Offering” means any public or
any private offering of Parent’s Capital Stock (excluding
Disqualified Stock).
“Qualified Securitization Financing” means any
transaction or series of transactions entered into by Parent or
any of its Restricted Subsidiaries pursuant to which Parent or
such Restricted Subsidiary sells, conveys, contributes, assigns,
grants an interest in or otherwise transfers to a Securitization
Subsidiary, Securitization Assets (and/or grants a security
interest in such Securitization Assets transferred or purported
to be transferred to such Securitization Subsidiary), and which
Securitization Subsidiary funds the acquisition of such
Securitization Assets (a) with cash, (b) through the
issuance to Parent’s or such Seller’s Retained
Interests or an increase in Parent’s or such Seller’s
Retained Interests,
and/or
(c) with proceeds from the sale, pledge or collection of
Securitization Assets.
“Rating Agencies” means Moody’s and
S&P or if Moody’s or S&P or both shall not make a
rating on the Notes publicly available, a nationally recognized
statistical rating agency or agencies, as the case may be,
selected by the Company which shall be substituted for
Moody’s or S&P or both, as the case may be.
“Replacement Assets” means any properties or
assets used or useful in a Permitted Business.
“Restricted Investment” means an Investment
other than a Permitted Investment.
“Restricted Subsidiary” means, at any time,
each direct and indirect Subsidiary of Parent (including,
without limitation, the Company) that is not then an
Unrestricted Subsidiary; provided, however, that
upon the occurrence of an Unrestricted Subsidiary ceasing to be
an Unrestricted Subsidiary, such Subsidiary shall be included in
the definition of “Restricted Subsidiary.”
“S&P” means Standard &
Poor’s, a division of The McGraw-Hill Companies, Inc., and
any successor to its rating agency business.
“SEC” means the Securities and Exchange
Commission.
“Secured Indebtedness” means any Indebtedness
for borrowed money of Parent or any of its Restricted
Subsidiaries secured by a Lien on any assets of Parent or any of
its Restricted Subsidiaries.
“Secured Leverage Ratio,” as of any date of
determination, means the ratio of:
(1) the outstanding principal amount of Secured
Indebtedness of Parent and its Restricted Subsidiaries as of
such date on a consolidated basis in accordance with IFRS
(provided, that for purposes of this definition, any undrawn
revolving commitments under a secured credit facility shall be
deemed to be Secured Indebtedness in the full amount of such
undrawn revolving commitments for so long as such commitments
are outstanding); to
221
(2) Consolidated Cash Flow of Parent and its Restricted
Subsidiaries for the period of the most recent four consecutive
fiscal quarters ending prior to such date for which financial
statements prepared on a consolidated basis in accordance with
IFRS are available; provided, however, that
Consolidated Cash Flow shall be determined for purposes of this
definition with such pro forma adjustment consistent with
the definition of Fixed Charge Coverage Ratio.
“Securities Act” means the Securities Act of
1933, as amended, and the rules and regulations of the SEC
promulgated thereunder.
“Securitization Assets” means any accounts
receivable owed to Parent or any of its Subsidiaries (whether
now existing or arising or acquired in the future) arising in
the ordinary course of business from the sale of goods or
services, all collateral securing such accounts receivable, all
contracts and contract rights and all guarantees or other
obligations in respect of such accounts receivable, all proceeds
of such accounts receivable and other assets (including contract
rights) which are of the type customarily transferred or in
respect of which security interests are customarily granted in
connection with securitizations of accounts receivable and which
are sold, conveyed, contributed, assigned, pledged or otherwise
transferred by such Parent or any of its Subsidiaries to a
Securitization Subsidiary.
“Securitization Repurchase Obligation” means
any obligation of a seller of Securitization Assets in a
Qualified Securitization Financing to repurchase Securitization
Assets arising as a result of a breach of a representation,
warranty or covenant with respect to such Securitization Assets,
including as a result of a receivable or portion thereof
becoming subject to any asserted defense, dispute, off set,
counterclaim or other dilution of any kind as a result of any
action taken by, any failure to take action by or any other
event relating to the seller, but in each case, not as a result
of such receivable being or becoming uncollectible for credit
reasons.
“Securitization Subsidiary” means a Restricted
Subsidiary of the Parent that engages in no activities other
than in connection with the acquisition
and/or
financing of Securitization Assets, all proceeds thereof and all
rights (contingent and other), collateral and other assets
relating thereto, and any business or activities incidental or
related to such business, and which is designated by the Board
of Directors of the Parent (or a duly authorized committee
thereof) or such other Person (as provided below) as a
Securitization Subsidiary and (a) no portion of the
Indebtedness or any other obligations (contingent or otherwise)
of which (i) is guaranteed by Parent or any of its
Subsidiaries, other than another Securitization Subsidiary
(excluding guarantees of obligations (other than the principal
of, and interest on, Indebtedness) pursuant to Standard
Securitization Undertakings), (ii) is recourse to or
obligates Parent or any of its Subsidiaries, other than another
Securitization Subsidiary, in any way other than pursuant to
Standard Securitization Undertakings or (iii) subjects any
property or asset (other than Securitization Assets) of Parent
or any of its Subsidiaries, other than another Securitization
Subsidiary, directly or indirectly, contingently or otherwise,
to the satisfaction thereof, other than pursuant to Standard
Securitization Undertakings, (b) with which none of Parent
nor any of its Subsidiaries, other than another Securitization
Subsidiary, has any material contract, agreement, arrangement or
understanding other than (i) the applicable receivables
purchase agreements and related agreements, in each case, having
reasonably customary terms, or (ii) on terms which the
Parent reasonably believes to be no less favorable to Parent or
the applicable Subsidiary than those that might be obtained at
the time from Persons that are not Affiliates of Parent or any
of its Subsidiaries and (c) to which neither Parent nor any
of its Subsidiaries other than another Securitization
Subsidiary, has any obligation to maintain or preserve such
entity’s financial condition or cause such entity to
achieve certain levels of operating results. Any such
designation by the Board of Directors of Parent (or a duly
authorized committee thereof) or such other Person shall be
evidenced to the Trustee by delivery to the Trustee of a
certified copy of the resolution of the board of directors of
Parent or such other Person giving effect to such designation
and a certificate executed by an authorized officer certifying
that such designation complied with the foregoing conditions.
“Seller’s Retained Interests” means the
debt or equity interests held by Parent or any of its
Subsidiaries in a Securitization Subsidiary to which
Securitization Assets have been transferred, including any such
debt or equity received as consideration for or as a portion of
the purchase price for the Securitization Assets
222
transferred, or any other instrument through Parent or such
Subsidiary has rights to or receives distributions in respect of
any residual or excess interest in the Securitization Assets.
“Significant Subsidiary” means any Subsidiary
that would be a “significant subsidiary” as defined in
Article 1,
Rule 1-02
of
Regulation S-X,
promulgated pursuant to the Securities Act, as in effect on the
Issue Date.
“Standard Securitization Undertakings” means
representations, warranties, covenants, Securitization
Repurchase Obligations and indemnities entered into by Parent or
any of its Subsidiaries that are reasonably customary in
accounts receivable securitization transactions.
“Stated Maturity” means, with respect to any
installment of interest or principal on any series of
Indebtedness, the date on which the payment of interest or
principal was scheduled to be paid in the documentation
governing such Indebtedness as of the Issue Date, and will not
include any contingent obligations to repay, redeem or
repurchase any such interest or principal prior to the date
originally scheduled for the payment thereof.
“Subordinated Indebtedness” means all
Indebtedness (whether outstanding on the Issue Date or
thereafter incurred) that is subordinated or junior in right of
payment to the Notes pursuant to a written agreement, executed
by the Person to whom such Indebtedness is owed, to that effect.
“Subsidiary” means, with respect to any
specified Person:
(1) any corporation, association or other business entity
of which more than 50% of the total voting power of shares of
Capital Stock entitled (without regard to the occurrence of any
contingency and after giving effect to any voting agreement or
stockholders’ agreement that transfers voting power) to
vote in the election of directors, managers or Trustees of the
corporation, association or other business entity is at the time
owned or controlled, directly or indirectly, by that Person or
one or more of the other Subsidiaries of that Person (or a
combination thereof); and
(2) any partnership (a) the sole general partner or
the managing general partner of which is such Person or a
Subsidiary of such Person or (b) the only general partners
of which are that Person or one or more Subsidiaries of that
Person (or any combination thereof).
Unless otherwise specified herein, all references to any
“Subsidiary” shall refer to a Subsidiary of Parent.
“Tax” means any tax, duty, levy, impost,
assessment or other governmental charge (including penalties,
interest and any other liabilities related thereto).
“Taxing Authority” means any government or
political subdivision or territory or possession of any
government or any authority or agency therein or thereof having
power to impose or collect any Tax.
“Taxing Jurisdiction” has the meaning set forth
under “— Additional Amounts.”
“Total Assets” means the total consolidated
assets of Parent and the Restricted Subsidiaries, as shown on
the most recent internal balance sheet of the Parent prepared on
a consolidated basis (excluding Unrestricted Subsidiaries) in
accordance with IFRS.
“Transactions” means (i) the Mergers,
(ii) the repayment of certain of Parent’s and the
Restricted Subsidiaries’ existing Indebtedness,
(iii) the closing of the Credit Agreement and the issuance
and sale of the Notes and the other transactions described in
the offering memorandum.
“Treasury Rate” means, as obtained by the
Company, as of any redemption date, the yield to maturity as of
such redemption date of United States Treasury securities with a
constant maturity (as compiled and published in the most recent
Federal Reserve Statistical Release H.15 (519) that has
become publicly available at least two business days prior to
the redemption date (or, if such Statistical Release is no
longer published, any publicly available source of similar
market data)) most nearly equal to the period from the
redemption date to February 1, 2014; provided,
however, that if the period from the redemption date to
February 1, 2014 is less than one year, the weekly average
yield on actually traded United States Treasury securities
adjusted to a constant maturity of one year will be used.
223
“Trust Indenture Act” means the
Trust Indenture Act of 1939, as amended (15 U.S.C.
§§ 77aaa-77bbbb).
“Unrestricted Subsidiary” means any Subsidiary
(or any successor to any of them) that is designated by the
Company’s Board of Directors as an Unrestricted Subsidiary
pursuant to a board resolution, but only to the extent that such
Subsidiary:
(1) has no Indebtedness other than Non-recourse Debt;
(2) except as permitted by the covenant described under the
heading “Certain Covenants — Transactions with
Affiliates,” is not party to any agreement, contract,
arrangement or understanding with Parent or any Restricted
Subsidiary unless the terms of any such agreement, contract,
arrangement or understanding are no less favorable to Parent or
such Restricted Subsidiary than those that might be obtained at
the time from Persons who are not Affiliates of Parent
and/or the
Restricted Subsidiaries;
(3) is a Person with respect to which neither Parent nor
any Restricted Subsidiary has any direct or indirect obligation
(a) to subscribe for additional Equity Interests or
(b) to maintain or preserve such Person’s financial
condition or to cause such Person to achieve any specified
levels of operating results;
(4) has not Guaranteed or otherwise directly or indirectly
provided credit support for any Indebtedness of Parent or any
Restricted Subsidiary; and
(5) has at least one director on its Board of Directors
that is not a director or executive officer of Parent or any
Restricted Subsidiary and has at least one executive officer
that is not a director or executive officer of Parent or any
Restricted Subsidiary.
Any designation of a Subsidiary as an Unrestricted Subsidiary
will be evidenced to the Trustee by filing with the Trustee a
certified copy of the board resolution giving effect to such
designation and an officers’ certificate certifying that
such designation complied with the preceding conditions and was
permitted under “Certain Covenants — Restricted
Payments.” If, at any time, any Unrestricted Subsidiary
would fail to meet the preceding requirements as an Unrestricted
Subsidiary, it will thereafter cease to be an Unrestricted
Subsidiary for purposes of the Indenture and any Indebtedness of
such Subsidiary will be deemed to be incurred by a Restricted
Subsidiary as of such date and, if such Indebtedness is not
permitted to be incurred as of such date under “Certain
Covenants — Incurrence of Indebtedness and Issuance of
Disqualified Stock and Preferred Stock,” the Company will
be in default of such covenant. The Company’s Board of
Directors may at any time designate any Unrestricted Subsidiary
to be a Restricted Subsidiary; provided that such
designation will be deemed to be an incurrence of Indebtedness
by a Restricted Subsidiary of any outstanding Indebtedness of
such Unrestricted Subsidiary and such designation will only be
permitted if (1) such Indebtedness is permitted under
“Certain Covenants — Incurrence of Indebtedness
and Issuance of Disqualified Stock and Preferred Stock,”
calculated on a pro forma basis as if such designation had
occurred at the beginning of the four-quarter reference period;
(2) no Default or Event of Default would be in existence
following such designation; and (3) such Subsidiary
executes and delivers to the Trustee a supplemental Indenture
providing for a Guarantee.
“Voting Stock” of any Person as of any date
means the Capital Stock of such Person that is at the time
entitled to vote in the election of the Board of Directors of
such Person.
“Weighted Average Life to Maturity” means, when
applied to any Indebtedness at any date, the number of years
obtained by dividing:
(1) the sum of the products obtained by multiplying
(a) the amount of each then remaining installment, sinking
fund, serial maturity or other required payments of principal,
including payment at final maturity, in respect of the
Indebtedness, by (b) the number of years (calculated to the
nearest one-twelfth) that will elapse between such date and the
making of such payment; by
(2) the then outstanding principal amount of such
Indebtedness.
224
BOOK-ENTRY
SETTLEMENT AND CLEARANCE
The
Global Notes
The exchange notes will be issued in the form of one or more
registered notes in global form, without interest coupons, the
“global notes.”
Upon issuance, each of the global notes will be deposited with
the trustee as custodian for The Depository Trust Company
(“DTC”), and registered in the name of
Cede & Co., as nominee of DTC.
Ownership of beneficial interests in each global note will be
limited to persons who have accounts with DTC (“DTC
Participants”), or persons who hold interests through DTC
Participants. We expect that under procedures established by DTC:
|
|
|
| |
•
|
upon deposit of each global note with DTC’s custodian, DTC
will credit portions of the principal amount of each global note
to the accounts of the DTC Participants; and
|
| |
| |
•
|
ownership of beneficial interests in each global note will be
shown on, and transfer of ownership of those interests will be
effected only through, records maintained by DTC (with respect
to interests of DTC Participants) and the records of DTC
Participants (with respect to other owners of beneficial
interests in any of the global notes).
|
Beneficial interests in the global notes may not be exchanged
for notes in physical, certificated form except in the limited
circumstances described below.
Book-Entry
Procedures for the Global Notes
All interests in the global notes will be subject to the
operations and procedures of DTC, Euroclear and Clearstream. We
provide the following summaries of those operations and
procedures solely for the convenience of investors. Each
settlement system controls its own operations and procedures and
may change them at any time. Neither we nor the trustee are
responsible for those operations or procedures.
DTC has advised us that it is:
|
|
|
| |
•
|
a limited purpose trust company organized under the laws of the
State of New York;
|
| |
| |
•
|
a “banking organization” within the meaning of the New
York State Banking Law;
|
| |
| |
•
|
a member of the Federal Reserve System;
|
| |
| |
•
|
a “clearing corporation” within the meaning of the
Uniform Commercial Code; and
|
| |
| |
•
|
a “clearing agency” registered under Section 17A
of the Exchange Act.
|
DTC was created to hold securities for its participants and to
facilitate the clearance and settlement of securities
transactions between its participants through electronic
book-entry changes to the accounts of its participants.
DTC’s participants include securities brokers and dealers,
banks, trust companies, clearing corporations and other
organizations. Indirect access to DTC’s system is also
available to others such as banks, brokers, dealers and trust
companies; these indirect participants clear through or maintain
a custodial relationship with a DTC Participant, either directly
or indirectly. Investors who are not DTC Participants may
beneficially own securities held by or on behalf of DTC only
through DTC Participants or indirect participants in DTC.
So long as DTC’s nominee is the registered owner of a
global note, that nominee will be considered the sole owner or
holder of the notes represented by that global note for all
purposes under the Indentures. Except as provided below, owners
of beneficial interests in a global note:
|
|
|
| |
•
|
will not be entitled to have notes represented by the global
note registered in their names;
|
| |
| |
•
|
will not receive or be entitled to receive physical,
certificated notes; and
|
|
|
|
| |
•
|
will not be considered the owners or holders of the notes under
the Indentures for any purpose, including with respect to the
giving of any direction, instruction or approval to the trustee
under the Indentures.
|
225
As a result, each investor who owns a beneficial interest in a
global note must rely on the procedures of DTC to exercise any
rights of a holder of notes under the Indentures (and, if the
investor is not a participant or an indirect participant in DTC,
on the procedures of the DTC Participant through which the
investor owns its interest).
Payments of principal, premium (if any) and interest with
respect to the notes represented by a global note will be made
by the trustee to DTC’s nominee as the registered holder of
the global note. Neither we nor the trustee will have any
responsibility or liability for the payment of amounts to owners
of beneficial interests in a global note, for any aspect of the
records relating to or payments made on account of those
interests by DTC, or for maintaining, supervising or reviewing
any records of DTC relating to those interests.
Payments by participants and indirect participants in DTC to the
owners of beneficial interests in a global note will be governed
by standing instructions and customary industry practice and
will be the responsibility of those participants or indirect
participants and DTC.
Transfers between participants in DTC will be effected under
DTC’s procedures and will be settled in
same-day
funds. Transfers between participants in Euroclear or
Clearstream will be effected in the ordinary way under the rules
and operating procedures of those systems.
Cross-market transfers between DTC Participants, on the one
hand, and Euroclear or Clearstream participants, on the other
hand, will be effected within DTC through the DTC Participants
that are acting as depositaries for Euroclear and Clearstream.
To deliver or receive an interest in a global note held in a
Euroclear or Clearstream account, an investor must send transfer
instructions to Euroclear or Clearstream, as the case may be,
under the rules and procedures of that system and within the
established deadlines of that system. If the transaction meets
its settlement requirements, Euroclear or Clearstream, as the
case may be, will send instructions to its DTC depositary to
take action to effect final settlement by delivering or
receiving interests in the relevant global notes in DTC, and
making or receiving payment under normal procedures for
same-day
funds settlement applicable to DTC. Euroclear and Clearstream
participants may not deliver instructions directly to the DTC
depositaries that are acting for Euroclear or Clearstream.
Because of time zone differences, the securities account of a
Euroclear or Clearstream participant that purchases an interest
in a global note from a DTC Participant will be credited on the
business day for Euroclear or Clearstream immediately following
the DTC settlement date. Cash received in Euroclear or
Clearstream from the sale of an interest in a global note to a
DTC Participant will be received with value on the DTC
settlement date but will be available in the relevant Euroclear
or Clearstream cash account as of the business day for Euroclear
or Clearstream following the DTC settlement date.
DTC, Euroclear and Clearstream have agreed to the above
procedures to facilitate transfers of interests in the global
notes among participants in those settlement systems. However,
the settlement systems are not obligated to perform these
procedures and may discontinue or change these procedures at any
time. Neither we nor the trustee will have any responsibility
for the performance by DTC, Euroclear or Clearstream or their
participants or indirect participants of their obligations under
the rules and procedures governing their operations.
Certificated
Notes
Except as set forth above, notes in physical, certificated form
will be issued and delivered to each person that DTC identifies
as a beneficial owner of the related notes only if:
|
|
|
| |
•
|
DTC notifies us at any time that it is unwilling or unable to
continue as depositary for the global notes and a successor
depositary is not appointed within 90 days;
|
| |
| |
•
|
DTC ceases to be registered as a clearing agency under the
Exchange Act and a successor depositary is not appointed within
90 days;
|
| |
| |
•
|
we, at our option, notify the trustee that we elect to cause the
issuance of certificated notes; or
|
| |
| |
•
|
certain other events provided in the indenture should occur.
|
226
CERTAIN
MATERIAL UNITED STATES FEDERAL INCOME TAX
CONSIDERATIONS
CIRCULAR
230 DISCLOSURE:
TO ENSURE COMPLIANCE WITH U.S. TREASURY DEPARTMENT
CIRCULAR 230, WE HEREBY NOTIFY YOU THAT:
(A) ANY DISCUSSION OF U.S. FEDERAL TAX ISSUES IN
THIS PROSPECTUS IS NOT INTENDED OR WRITTEN TO BE RELIED UPON,
AND CANNOT BE RELIED UPON, BY YOU FOR THE PURPOSE OF AVOIDING
PENALTIES THAT MAY BE IMPOSED UNDER THE U.S. INTERNAL
REVENUE CODE;
(B) SUCH DISCUSSION IS WRITTEN TO SUPPORT THE PROMOTION
OR MARKETING OF THE TRANSACTIONS OR MATTERS ADDRESSED IN THIS
PROSPECTUS; AND
(C) YOU SHOULD SEEK ADVICE BASED ON YOUR PARTICULAR
CIRCUMSTANCES FROM AN INDEPENDENT TAX ADVISOR.
General
The following general discussion summarizes certain material
U.S. federal income tax consequences applicable to
beneficial owners of the existing notes who:
1. acquired such existing notes in the original offering
and at the original offering price for cash,
2. exchange such existing notes in this exchange offer for
exchange notes, and
3. hold the existing notes and will hold the exchange notes
as capital assets within the meaning of the Internal Revenue
Code of 1986, as amended (the “Code”).
This summary does not include all of the rules which may affect
the U.S. tax treatment of your investment in the exchange
notes, and does not consider special rules applicable to certain
taxpayers, including a dealer in securities or currencies, bank
or other financial institution, regulated investment company,
real estate investment trust, tax-exempt entity, insurance
company, U.S. expatriate, person who or that holds notes as
part of a hedging, integrated, conversion or constructive sale
transaction or as part of a straddle, trader in securities that
elects to use a
mark-to-market
method of accounting for their securities holdings, person
liable for alternative minimum tax, investor who holds through
partnerships or other pass-through entities, or U.S. holder
whose “functional currency” for tax purposes is not
the U.S. Dollar. This summary does not consider state,
local or foreign tax laws.
This discussion is based upon the Code, existing and proposed
Treasury Regulations thereunder, and judicial decisions and
administrative interpretations thereof, in each case in effect
as of the date hereof, all of which are subject to change,
possibly with retroactive effect. We cannot assure holders that
the Internal Revenue Service (the “IRS”) will not
challenge one or more of the tax considerations described below.
We have not obtained, and do not intend to obtain, a ruling from
the IRS or an opinion of counsel with respect to the
U.S. federal tax considerations resulting from acquiring,
holding or disposing of the existing notes or the exchange notes.
For purposes of this discussion, the term
“U.S. holder” means a beneficial owner of an
existing note who is:
1. an individual who is a citizen or resident of the United
States for U.S. federal income tax purposes;
2. a corporation, or an entity taxable as a corporation for
U.S. federal income tax purposes, created or organized in
or under the laws of the United States, any state therein or the
District of Columbia;
3. an estate, the income of which is subject to
U.S. federal income taxation regardless of its
source; or
227
4. a trust (i) if a court within the U.S. is able
to exercise primary supervision over such trust’s
administration and one or more U.S. persons have the
authority to control all substantial decisions of such trust or
(ii) that has a valid election in effect under applicable
Treasury regulations to be treated as a U.S. person.
As used herein, the term
“non-U.S. holder”
means an individual, corporation, estate or trust that is a
beneficial owner of an existing note who is not a
U.S. holder. If a partnership or other entity or
arrangement taxable as a partnership holds the existing notes,
the tax treatment of a partner in such a partnership will
generally depend on the status of the partner and on the
activities of the partnership. If you are a partner in a
partnership which holds existing notes, you should consult your
own tax advisors.
THIS DISCUSSION IS FOR INFORMATION PURPOSES ONLY AND IS NOT
INTENDED AS TAX ADVICE. YOU SHOULD CONSULT YOUR OWN TAX ADVISORS
AS TO THE PARTICULAR TAX CONSIDERATIONS TO YOU OF PARTICIPATING
IN THIS EXCHANGE OFFER AND HOLDING AND DISPOSING OF THE EXCHANGE
NOTES, INCLUDING THE EFFECT AND APPLICABILITY OF STATE, LOCAL OR
FOREIGN TAX LAWS OR ANY TAX TREATY.
Exchange
of Existing Notes for Exchange Notes
The exchange of an existing note for an exchange note pursuant
to this exchange offer will not be a taxable exchange for
U.S. federal income tax purposes. Accordingly, holders
participating in this exchange offer will not recognize any
income, gain or loss for U.S. federal income tax purposes
upon the receipt of an exchange note, and holders will be
required to continue to include interest on the exchange note in
gross income in the manner and to the extent described herein. A
holder’s holding period for an exchange note will include
the holding period for the existing note exchanged therefor. A
holder’s basis in the exchange note immediately after the
exchange will be the same as its basis in the existing note
exchanged in this exchange offer immediately before the exchange.
The
Exchange Notes
U.S.
Holders
This section applies only to U.S. Holders.
Non-U.S. Holders
should consult the discussion below under the heading
“Non-U.S. Holders.”
Payment
of Stated Interest
Payment of stated interest on the exchange notes generally will
be includable in the gross income of a U.S. holder as
ordinary interest income at the time such payments are received
or accrued in accordance with such U.S. holder’s
method of accounting for U.S. federal income tax purposes.
Sale,
Exchange, Redemption or Other Taxable Disposition of the
Exchange Notes
Upon the sale, exchange, redemption or other taxable disposition
of an exchange note, a U.S. holder generally will recognize
gain or loss in an amount equal to the difference between:
(i) the amount realized on the sale, exchange, redemption
or other taxable disposition (other than amounts attributable to
accrued but unpaid stated interest which, if not previously
included in income, will be treated as interest paid on the
exchange notes) and (ii) a U.S. holder’s adjusted
U.S. federal income tax basis in the exchange note. A
U.S. holder’s adjusted U.S. federal income tax
basis in an exchange note generally will equal the amount the
U.S. holder paid for the existing note exchanged for such
exchange note, decreased by the amount of any payments other
than qualified stated interest previously received by the
U.S. holder on such note (either prior to or after such
note was exchanged in the exchange offer).
Any gain or loss recognized will be capital gain or loss and
will be long-term capital gain or loss if, on the date of the
sale, the U.S. holder has a holding period in the exchange
note (including the U.S. holder’s holding period for
the existing note exchanged therefor) of more than one year.
Non-corporate taxpayers are
228
generally subject to tax on net long-term capital gains at a
preferential rate. The deductibility of capital losses is
subject to limitations.
Medicare
Contribution Tax on Unearned Income
Recently-enacted legislation requires certain U.S. holders
who are individuals, estates or trusts to pay an additional 3.8%
tax on, among other things, interest on and capital gains from
the sale or other disposition of instruments such as the
existing notes and the exchange notes for taxable years
beginning after December 31, 2012. U.S. holders should
consult their tax advisors regarding the effect of this
legislation, if any, on their ownership and disposition of the
exchange notes.
Non-U.S.
Holders
The following general discussion is limited to the
U.S. federal income tax consequences relevant to a
non-U.S. holder,
as defined above. If you are not a
non-U.S. holder,
this section does not apply to you.
U.S.
Federal Tax Withholding
The 30% U.S. federal tax withholding will not apply to any
payment of interest on the exchange notes provided that the
non-U.S. holder:
|
|
|
| |
•
|
does not actually (or constructively) own 10% or more of the
total combined voting power of all classes of our voting stock
within the meaning of the Code and the Treasury Regulations;
|
| |
| |
•
|
is not a controlled foreign corporation that is related,
directly or indirectly, to us through stock ownership;
|
| |
| |
•
|
is not a bank whose receipt of interest on the exchange notes is
pursuant to a loan agreement entered into in the ordinary course
of business; and
|
| |
| |
•
|
has fulfilled the certification requirements set forth below.
|
The certification requirements referred to above will be
fulfilled if the
non-U.S. holder
certifies on a properly complete and duly executed IRS
Form W-8BEN,
or such successor form as the IRS may designate, under penalties
of perjury, that it is not a U.S. person for
U.S. federal income tax purposes and provides its name and
address, and (i) the
non-U.S. holder
files such form or successor form with the withholding agent or
(ii) in the case of an exchange note held on the
non-U.S. holder’s
behalf by a securities clearing organization, bank or other
financial institution holding customers’ securities in the
ordinary course of its trade or business, the foreign financial
institution fulfills the certification requirement by filing IRS
Form W-8IMY
(or successor form) if it has entered into an agreement with the
IRS to be treated as a qualified intermediary. With respect to
exchange notes held by a
non-U.S. partnership
and certain other
non-U.S. entities,
unless the
non-U.S. partnership
or entity has entered into a withholding agreement with the IRS,
the
non-U.S. partnership
or entity generally will be required to provide on IRS
Form W-8IMY
(or successor form) and to associate with such form an
appropriate certification or other appropriate documentation
from each partner, other member or beneficial owner of the
exchange note. A
non-U.S. holder
should consult its own tax advisor regarding possible additional
reporting requirements.
If a
non-U.S. holder
cannot satisfy the requirements described above, payments of
interest made to it will be subject to the 30% U.S. federal
tax withholding described above, unless the
non-U.S. holder
provides us with a properly executed (i) IRS
Form W-8BEN
(or successor form) claiming an exemption from (or a reduction
of) withholding under the benefit of a tax treaty and stating
its taxpayer identification number or (ii) IRS
Form W-8ECI
(or successor form) stating that payments on the exchange notes
are not subject to withholding of tax because such payments are
effectively connected with its conduct of a trade or business in
the United States, as discussed below.
229
U.S.
Federal Income Tax
If a
non-U.S. holder
is engaged in a trade or business in the United States and
interest on the exchange notes is treated as effectively
connected with the conduct of that trade or business, such
non-U.S. holder
will be subject to U.S. federal income tax on the interest
on a net income basis in the same manner as if it were a
U.S. holder, unless an applicable tax treaty provides
otherwise. In such a case, such a
non-U.S. holder
will not be subject to the 30% U.S. federal tax withholding
if it provides to the withholding agent a properly executed IRS
Form W-8ECI
or other applicable form. In addition, a
non-U.S. holder
that is a corporation for U.S. federal income tax purposes
may be subject to a branch profits tax with respect to such
non-U.S. holder’s
effectively connected earnings and profits at a rate of 30% (or
at a reduced rate under an applicable income tax treaty).
Sale,
Exchange, Redemption or Other Taxable Disposition of the
Exchange Notes
Any gain realized on the sale, exchange, redemption or other
taxable disposition of exchange notes generally will not be
subject to U.S. federal income tax unless:
|
|
|
| |
•
|
that gain is effectively connected with the conduct of a trade
or business in the United States by the
non-U.S. holder,
(and, if a tax treaty applies, such gain is attributable to a
permanent establishment in the United States); or
|
| |
| |
•
|
the
non-U.S. holder
is an individual who is present in the United States for
183 days or more in the taxable year of that disposition,
and certain other conditions are met.
|
A holder described in the first bullet point above will be
required to pay U.S. federal income tax on the net gain
derived from the sale in the same manner as a U.S. holder,
except as otherwise required by an applicable tax treaty, and if
such holder is a foreign corporation, it may also be required to
pay a branch profits tax equal to 30% of its effectively
connected earnings and profits attributable to such gain, or a
lower rate provided by an applicable income tax treaty. A holder
described in the second bullet point above will be subject to a
30% U.S. federal income tax on the gain derived from the
sale, which may be offset by certain U.S. source capital
losses. To the extent that the amount realized on any sale,
exchange, redemption or other taxable disposition of the
exchange notes is attributable to accrued but unpaid interest
not previously included in income, such amount would be treated
as interest and subject to tax as described above.
Information
Reporting and Backup Withholding
U.S.
Holders
Under the Code, a U.S. holder may be subject, under certain
circumstances, to information reporting
and/or
backup withholding at the prevailing statutory rate provided in
the Code with respect to certain payments made on or with
respect to the exchange notes. This withholding applies only if
a U.S. holder (i) fails to furnish the
U.S. holder’s taxpayer identification number
(“TIN”), which for an individual is a social security
number, within a reasonable time after a request therefor,
(ii) furnishes an incorrect TIN, (iii) is notified by
the IRS that the U.S. holder failed to report interest or
dividends properly, or (iv) fails, under certain
circumstances, to provide a certified statement, signed under
penalty of perjury, that the TIN provided is correct and that
the U.S. holder has not been notified by the IRS that the
U.S. holder is subject to backup withholding. To prevent
backup withholding, the U.S. holder or other payee is
required to properly complete IRS
Form W-9.
These requirements generally do not apply with respect to
certain holders, including tax exempt organizations and certain
financial institutions.
Backup withholding is not an additional federal income tax. Any
amount withheld from a payment under the backup withholding
rules is allowable as a credit against a U.S. holder’s
U.S. federal income tax liability (and may entitle such
U.S. holder to a refund), provided that the required
information is timely furnished to the IRS. A U.S. holder
should consult the U.S. holder’s own tax advisor as to
the U.S. holder’s qualification for exemption from
backup withholding and the procedure for obtaining such
exemption.
230
Non-U.S.
Holders
If a
non-U.S. holder
provides the applicable IRS
Form W-8BEN,
IRS
Form W-8IMY
or other applicable form, together with all appropriate
attachments and signed under penalties of perjury, identifying
the
non-U.S. holder
and stating that the
non-U.S. holder
is not a U.S. person, the
non-U.S. holder
will not be subject to IRS reporting requirements or
U.S. backup withholding with respect to interest payments.
Under current Treasury Regulations, payments on the sale,
exchange, redemption or other taxable disposition of an exchange
note made to or through a U.S. office of a broker generally
will be subject to information reporting and backup withholding
unless the holder either certifies its status as a
non-U.S. holder
under penalties of perjury on the applicable IRS
Form W-8BEN,
IRS
Form W-8IMY
or other applicable form (as described above) or otherwise
establishes an exemption. The payment of the proceeds on the
disposition of an exchange note by a
non-U.S. holder
to or through a
non-U.S. office
of a
non-U.S. broker
will not be subject to backup withholding or information
reporting unless the
non-U.S. broker
is a “U.S. Related Person” (as defined below).
The payment of proceeds on the disposition of an exchange note
by a
non-U.S. holder
to or through a
non-U.S. office
of a U.S. broker or a U.S. Related Person generally
will not be subject to backup withholding but will be subject to
information reporting unless the holder certifies its status as
a
non-U.S. holder
under penalties of perjury or the broker has certain documentary
evidence in its files as to the
non-U.S. holder’s
foreign status and has no actual knowledge or reason to know
that such holder is a U.S. person.
For this purpose, a “U.S. Related Person” is:
(i) a “controlled foreign corporation” for
U.S. federal income tax purposes, (ii) a foreign
person 50% or more of whose gross income from all sources for a
specified three-year period is derived from activities that are
effectively connected with the conduct of a U.S. trade or
business or (iii) a foreign partnership with certain
connections to the United States.
Backup withholding is not an additional tax and may be refunded
(or credited against the holder’s U.S. federal income
tax liability, if any), provided that certain required
information is timely furnished to the IRS. The information
reporting requirements may apply regardless of whether
withholding is required. Copies of the information returns
reporting such interest (including any OID) and withholding also
may be made available to the tax authorities in the country in
which a
non-U.S. holder
is a resident under the provisions of an applicable income tax
treaty or agreement.
231
EXCHANGE
CONTROLS AND LIMITATIONS AFFECTING SECURITY HOLDERS
Restrictions
on Foreign Investment
Under present regulations, foreign investors may transfer
invested capital, capital gains and dividends out of Spain
without limitation on the amount other than applicable taxes.
Law 19/2003 (July 4, 2003) updated Spanish exchange
control and money laundering prevention provisions, by
recognizing the principle of freedom of the movement of capital
between Spanish residents and nonresidents.
The law establishes procedures for the declaration of capital
movements for purposes of administrative or statistical
information and authorizes the Spanish government to take
measures which are justified on grounds of public policy or
public security. It also provides the mechanism to take
exceptional measures with regard to third countries if such
measures have been approved by the European Union or by an
international organization to which Spain is a party.
The Spanish Stock Exchanges and securities markets are open to
foreign investors. Royal Decree
664/1999, on
Foreign Investments (April 23, 1999), established a new
framework for the regulation of foreign investments in Spain
which, on a general basis, does no longer require any prior
consents or authorizations from authorities in Spain (without
prejudice to specific regulations for several specific sectors,
such as television, radio, mining, telecommunications, etc.).
Royal Decree 664/1999 requires notification of all foreign
investments in Spain and liquidations of such investments upon
completion of such investments to the Investments Registry of
the Ministry of Economy, strictly for administrative statistical
and economical purposes. Where the investment or divestiture is
made in shares of a Spanish company listed on any of the Spanish
Stock Exchanges, the duty to provide notice of a foreign
investment or divestiture lies with the relevant entity with
whom the shares (in book-entry form) have been deposited or
which has acted as an intermediary in connection with the
investment or divestiture.
Only investments from “tax haven” countries (as they
are defined in Royal Decree 1080/1991), shall require notice
before and after execution of the investment, except that no
prior notice shall be required for: (i) investments in
listed or publicly negotiable securities or in participations in
collective investment schemes that are registered with the CNMV,
and (ii) investments that do not increase the foreign
ownership of the share capital of a Spanish company to over 50%.
In specific instances, the Council of Ministers may agree to
suspend, all or part of, Royal Decree 664/1999 following a
proposal of the Ministry of Economy, or, in some cases, a
proposal by the head of the government department with authority
for such matters and a report of the Foreign Investment Body.
These specific instances include a determination that the
investments, due to their nature, form or condition, affect
activities, or may potentially affect activities relating to the
exercise of public powers, national security or public health.
Royal Decree 664/1999 is currently suspended for investments
relating to national defense. In those cases in respect of which
Royal Decree 664/1999 is suspended, the affected investor must
obtain prior administrative authorization in order to carry out
the investment.
Exchange
Controls
Payments or transfers between non-residents and residents of
Spain must be made through a registered entity, such as a bank
or other financial institution registered with the Bank of Spain
and/or the
CNMV (entidades registradas), through bank accounts
opened abroad with a foreign bank or a foreign branch of a
registered entity, in cash, or by check payable to bearer. All
charges, payments or transfers which exceed €6,010, if made
in cash or by check payable to bearer, must be notified to the
Spanish exchange control authorities.
232
PLAN OF
DISTRIBUTION
Each broker-dealer that receives exchange notes for its own
account in connection with the exchange offer must acknowledge
that it will deliver a prospectus in connection with any resale
of such exchange notes. This prospectus, as it may be amended or
supplemented from time to time, may be used by a broker-dealer
in connection with resales of exchange notes received in
exchange for existing notes where such notes were acquired as a
result of market-making activities or other trading activities.
We have agreed that, for a period of up to 180 days after
the consummation of the exchange offer, we will make this
prospectus available to any broker-dealer, at such
broker-dealer’s request, for use in connection with any
such resale. In addition, all dealers effecting transactions in
the exchange notes may be required to deliver a prospectus
during the time periods prescribed by applicable securities laws.
We will not receive any proceeds from the issuance of exchange
notes in the exchange offer or from any sale of exchange notes
by broker-dealers. Exchange notes received by broker-dealers for
their own accounts pursuant to the exchange offer may be sold
from time to time in one or more transactions in the
over-the-counter
market, in negotiated transactions, through the writing of
options on the exchange notes or a combination of such methods
of resale, at market prices prevailing at the time of resale, at
prices related to such prevailing market prices or at negotiated
prices. Any such resale may be made directly to purchasers or to
or through brokers or dealers who may receive compensation in
the form of commissions or concessions from any such
broker-dealer
and/or the
purchasers of any such exchange notes. Any broker-dealer that
resells exchange notes that were received by it for its own
account pursuant to the exchange offer and any broker or dealer
that participates in a distribution of such exchange notes may
be deemed to be an “underwriter” within the meaning of
the Securities Act, and any profit on any such resale of
exchange notes and any commissions or concessions received by
any such persons may be deemed to be underwriting compensation
under the Securities Act. The letter of transmittal states that
by acknowledging that it will deliver and by delivering a
prospectus, a broker-dealer will not be deemed to admit that it
is an “underwriter” within the meaning of the
Securities Act.
For a period of 180 days after the expiration date, we will
promptly send additional copies of this prospectus and any
amendment or supplement to this prospectus to any broker-dealer
that requests such documents in the letter of transmittal. We
have agreed to pay all expenses incident to the exchange offer
(including the expenses of one counsel for the holder of the
notes) other than commissions or concessions of any brokers or
dealers and will indemnify the holders of the exchange notes,
including any broker-dealers, against certain liabilities,
including liabilities under the Securities Act.
233
VALIDITY
OF SECURITIES
The validity of the exchange notes and the guarantees offered
hereby, except for the authorization of the guarantees issued by
Grifols, S.A., Instituto Grifols, S.A., Diagnostic Grifols,
S.A., Movaco, S.A., Laboratorios Grifols, S.A., Grifols Italia,
S.p.A., Grifols Deutschland GmbH, will be passed upon for us by
Proskauer Rose LLP, New York, New York. The authorization of the
guarantees issued by Grifols, S.A., Instituto Grifols, S.A.,
Diagnostic Grifols, S.A., Movaco, S.A., Laboratorios Grifols,
S.A., will be passed upon for us by Osborne Clarke S.L.P. The
authorization of the guarantee issued by Grifols Italia, S.p.A.
will be passed upon for us by SLA Studio Legale Associato. The
authorization of the guarantee issued by Grifols Deutschland
GmbH will be passed upon for us by Osborne Clarke Germany.
EXPERTS
The consolidated balance sheets of Grifols, S.A. and its
subsidiaries as of December 31, 2010 and 2009 and the
related consolidated income statements, consolidated statements
of comprehensive income, statements of changes in consolidated
equity and consolidated statements of cash flows for each of the
years in the three-year period ended December 31, 2010,
have been included herein in reliance upon the report of KPMG
Auditores, S.L., independent registered public accounting firm,
appearing elsewhere herein, and upon the authority of the said
firm as experts in accounting and auditing.
The consolidated financial statements of Talecris
Biotherapeutics Holdings Corp. as of December 31, 2010 and
2009 and for each of the three years in the period ended
December 31, 2010 included in this prospectus have been so
included in reliance on the report of PricewaterhouseCoopers
LLP, an independent registered public accounting firm, given on
the authority of such firm as experts in auditing and accounting.
WHERE YOU
CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on
Form F-4
(Reg.
No. 333- )
with respect to the securities being offered hereby. This
prospectus does not contain all of the information contained in
the registration statement, including the exhibits and
schedules. You should refer to the registration statement,
including the exhibits and schedules, for further information
about use and the securities being offered hereby. Statements we
make in this prospectus about certain contracts or other
documents are not necessarily complete. When we make such
statements, we refer you to the copies of the contracts or
documents that are filed as exhibits to the registration
statement because those statements are qualified in all respects
by reference to those exhibits. As described below, the
registration statement, including exhibits and schedules is on
file at the offices of the SEC and may be inspected without
charge.
We file reports and other information with the SEC. You can
inspect and copy these reports, and other information at the
Public Reference Room of the SEC, 100 F Street, N.E.,
Washington, D.C. 20549. You can obtain copies of these
materials from the Public Reference Section of the SEC,
100 F Street, N.E., Washington, D.C. 20549, at
prescribed rates. Please call the SEC at 1-202-551-8090 for
further information on the operation of the public reference
room. Our SEC filings will also be available to you on the
SEC’s web site. The address of this site is
http://www.sec.gov.
In addition, we make available, free of charge, on or through
our web site, copies of such reports and other information. We
maintain a web site at
http://www.grifols.com.
The information contained in or connected to our web site is not
part of this prospectus unless expressly provided otherwise
herein. This prospectus summarizes documents that are not
delivered herewith. Copies of such documents are available upon
your request, without charge, by writing or telephoning us at:
| |
|
Grifols, S.A.
Avinguda de la Generalitat,
152-158
Parc de Negocis Can Sant Joan
08174 Sant Cugat del Vallès, 08174, Barcelona, Spain
|
|
Attention: Investor Relations Telephone: (+34)
935-710-500
|
To ensure timely delivery, please make your request as soon as
practicable and, in any event, no later than five business days
prior to the expiration of the exchange offer.
You may read and copy any document we file with the SEC at the
SEC’s public reference room at 100 F Street, NE,
Washington, D.C. 20549. Please call the SEC at
1-800-SEC-0330
for further information on the public reference room. Our SEC
filings are also available to the public at the SEC’s web
site at
http://www.sec.gov.
234
INDEX TO
FINANCIAL STATEMENTS
Grifols,
S.A. and Subsidiaries
Unaudited Condensed Consolidated Interim Financial
Statements:
| |
|
|
|
|
|
|
|
|
F-2
|
|
|
|
|
|
F-4
|
|
|
|
|
|
F-5
|
|
|
|
|
|
F-6
|
|
|
|
|
|
F-7
|
|
|
|
|
|
F-8
|
|
|
|
|
|
F-29
|
|
|
|
|
|
|
|
|
Audited Consolidated Financial Statements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-37
|
|
|
|
|
|
F-38
|
|
|
|
|
|
F-40
|
|
|
|
|
|
F-41
|
|
|
|
|
|
F-42
|
|
|
|
|
|
F-43
|
|
|
|
|
|
F-45
|
|
|
|
|
|
F-127
|
|
|
|
|
|
F-129
|
|
|
|
|
|
F-132
|
|
|
|
|
|
F-135
|
|
|
|
|
|
F-138
|
|
|
|
|
|
|
|
|
Talecris Biotherapeutics Holdings Corp.
|
|
|
|
|
|
|
|
|
|
|
|
Audited Consolidated Financial Statements:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-148
|
|
|
|
|
|
F-149
|
|
|
|
|
|
F-150
|
|
|
|
|
|
F-151
|
|
|
|
|
|
F-152
|
|
|
|
|
|
F-153
|
|
|
|
|
|
F-206
|
|
F-1
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
At 30 June 2011 and 31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
31/12/10
|
|
|
|
|
(Unaudited)
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
ASSETS
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
Goodwill (note 6)
|
|
|
2,281,696
|
|
|
|
189,448
|
|
|
Other intangible assets (note 7)
|
|
|
128,474
|
|
|
|
78,299
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
2,410,170
|
|
|
|
267,747
|
|
|
Property, plant and equipment (note 7)
|
|
|
639,735
|
|
|
|
434,131
|
|
|
Investments in equity accounted investees
|
|
|
3,546
|
|
|
|
598
|
|
|
Non-current financial assets (note 12)
|
|
|
41,667
|
|
|
|
7,535
|
|
|
Deferred tax assets
|
|
|
139,435
|
|
|
|
34,889
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
3,234,553
|
|
|
|
744,900
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
|
997,826
|
|
|
|
527,865
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
|
|
|
Trade receivables (note 8)
|
|
|
405,450
|
|
|
|
224,355
|
|
|
Other receivables
|
|
|
48,971
|
|
|
|
44,032
|
|
|
Current income tax assets
|
|
|
41,029
|
|
|
|
14,607
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
|
495,450
|
|
|
|
282,994
|
|
|
Other current financial assets
|
|
|
19,254
|
|
|
|
12,946
|
|
|
Other current assets (note 9)
|
|
|
13,344
|
|
|
|
80,628
|
|
|
Cash and cash equivalents (note 10)
|
|
|
583,792
|
|
|
|
239,649
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
2,109,666
|
|
|
|
1,144,082
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
5,344,219
|
|
|
|
1,888,982
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the unaudited
condensed consolidated interim financial statements.
F-2
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
31/12/10
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
EQUITY AND LIABILITIES
|
|
Equity
|
|
|
|
|
|
|
|
|
|
Share capital (note 11)
|
|
|
114,914
|
|
|
|
106,532
|
|
|
Share premium (note 11)
|
|
|
890,355
|
|
|
|
121,802
|
|
|
Reserves (note 11)
|
|
|
569,682
|
|
|
|
403,604
|
|
|
Own shares (note 11)
|
|
|
(1,927
|
)
|
|
|
(1,927
|
)
|
|
Profit for the period/year attributable to the Parent
|
|
|
19,269
|
|
|
|
115,513
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
1,592,293
|
|
|
|
745,524
|
|
|
Available-for-sale
financial assets
|
|
|
(575
|
)
|
|
|
—
|
|
|
Cash flow hedges
|
|
|
(2,331
|
)
|
|
|
(1,751
|
)
|
|
Translation differences
|
|
|
(88,734
|
)
|
|
|
(50,733
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income
|
|
|
(91,640
|
)
|
|
|
(52,484
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Equity attributable to the Parent
|
|
|
1,500,653
|
|
|
|
693,040
|
|
|
Non-controlling interests
|
|
|
12,941
|
|
|
|
14,350
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
1,513,594
|
|
|
|
707,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
1,815
|
|
|
|
2,088
|
|
|
Provisions
|
|
|
10,461
|
|
|
|
1,378
|
|
|
Non-current financial liabilities
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
2,642,944
|
|
|
|
665,385
|
|
|
Other financial liabilities
|
|
|
72,400
|
|
|
|
10,474
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities (note 12)
|
|
|
2,715,344
|
|
|
|
675,859
|
|
|
Deferred tax liabilities
|
|
|
140,075
|
|
|
|
79,141
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
2,867,695
|
|
|
|
758,466
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
Provisions
|
|
|
35,828
|
|
|
|
4,365
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
507,374
|
|
|
|
191,635
|
|
|
Other financial liabilities
|
|
|
17,336
|
|
|
|
18,236
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities (note 12)
|
|
|
524,710
|
|
|
|
209,871
|
|
|
Debts with associates
|
|
|
2,352
|
|
|
|
1,162
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
266,393
|
|
|
|
160,678
|
|
|
Other payables
|
|
|
25,618
|
|
|
|
11,928
|
|
|
Current income tax liabilities
|
|
|
27,227
|
|
|
|
4,172
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables
|
|
|
319,238
|
|
|
|
176,778
|
|
|
Other current liabilities
|
|
|
80,802
|
|
|
|
30,950
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
962,930
|
|
|
|
423,126
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
3,830,625
|
|
|
|
1,181,592
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity and liabilities
|
|
|
5,344,219
|
|
|
|
1,888,982
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the unaudited
condensed consolidated interim financial statements.
F-3
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Income Statements
For the Six Month Period Ended 30 June 2011 and
2010
| |
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
30/06/10
|
|
|
|
|
(Unaudited)
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Continuing Operations
|
|
|
|
|
|
|
|
|
|
Revenues (note 5)
|
|
|
635,341
|
|
|
|
487,809
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
2,757
|
|
|
|
41,209
|
|
|
Self-constructed non-current assets
|
|
|
32,346
|
|
|
|
16,051
|
|
|
Supplies
|
|
|
(175,142
|
)
|
|
|
(157,107
|
)
|
|
Other operating income
|
|
|
1,009
|
|
|
|
631
|
|
|
Personnel expenses
|
|
|
(183,727
|
)
|
|
|
(141,972
|
)
|
|
Other operating expenses
|
|
|
(155,532
|
)
|
|
|
(98,279
|
)
|
|
Amortisation and depreciation (note 7)
|
|
|
(28,156
|
)
|
|
|
(21,434
|
)
|
|
Transaction costs of Talecris business combination (note 3
& 9)
|
|
|
(38,607
|
)
|
|
|
(2,019
|
)
|
|
Non-financial and other capital grants
|
|
|
742
|
|
|
|
550
|
|
|
Impairment and gains/(losses) on disposal of fixed assets
(notes 6 & 7)
|
|
|
(22,302
|
)
|
|
|
681
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
68,729
|
|
|
|
126,120
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
1,761
|
|
|
|
2,179
|
|
|
Finance expenses (notes 8 & 13)
|
|
|
(55,546
|
)
|
|
|
(25,285
|
)
|
|
Change in fair value of financial instruments (note 13)
|
|
|
13,945
|
|
|
|
(15,404
|
)
|
|
Exchange gains/(losses)
|
|
|
(2,122
|
)
|
|
|
1,970
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance expense
|
|
|
(41,962
|
)
|
|
|
(36,540
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of equity accounted investees
|
|
|
(807
|
)
|
|
|
(728
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
25,960
|
|
|
|
88,852
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (note 14)
|
|
|
(7,347
|
)
|
|
|
(23,022
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the period
|
|
|
18,613
|
|
|
|
65,830
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
19,269
|
|
|
|
66,408
|
|
|
Loss attributable to non-controlling interests
|
|
|
(656
|
)
|
|
|
(578
|
)
|
|
Basic earnings per share (Euros)
|
|
|
0.09
|
|
|
|
0.31
|
|
|
Diluted earnings per share (Euros)
|
|
|
0.09
|
|
|
|
0.31
|
|
The accompanying notes form an integral part of the unaudited
condensed consolidated interim financial statements.
F-4
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statement of Comprehensive Income
For the Six Month Period Ended 30 June 2011 and
2010
| |
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
30/06/10
|
|
|
|
|
(Unaudited)
|
|
|
|
|
(Expressed in
|
|
|
|
|
thousands of Euros)
|
|
|
|
|
Consolidated profit for the period
|
|
|
18,613
|
|
|
|
65,830
|
|
|
Other comprehensive income
|
|
|
|
|
|
|
|
|
|
Income and expenses generated during the period
|
|
|
|
|
|
|
|
|
|
Measurement of financial instruments
|
|
|
(575
|
)
|
|
|
0
|
|
|
Available-for-sale
financial assets
|
|
|
(822
|
)
|
|
|
0
|
|
|
Tax effect
|
|
|
247
|
|
|
|
0
|
|
|
Cash flow hedges
|
|
|
(2,331
|
)
|
|
|
0
|
|
|
Cash flow hedges
|
|
|
(3,829
|
)
|
|
|
0
|
|
|
Tax effect
|
|
|
1,498
|
|
|
|
0
|
|
|
Translation differences
|
|
|
(38,541
|
)
|
|
|
74,874
|
|
|
|
|
|
|
|
|
|
|
|
|
Income and expenses generated during the period
|
|
|
(41,447
|
)
|
|
|
74,874
|
|
|
|
|
|
|
|
|
|
|
|
|
Income and expense recognised in the income statement:
|
|
|
|
|
|
|
|
|
|
Cash flow hedges
|
|
|
1,751
|
|
|
|
99
|
|
|
Cash flow hedges
|
|
|
2,870
|
|
|
|
159
|
|
|
Tax effect
|
|
|
(1,119
|
)
|
|
|
(60
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Income and expense recognised in the income statement:
|
|
|
1,751
|
|
|
|
99
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income and expenses for the period
|
|
|
(39,697
|
)
|
|
|
74,973
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income and expenses for the period
|
|
|
(21,083
|
)
|
|
|
140,803
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income/(losses) attributable to the Parent
|
|
|
(19,887
|
)
|
|
|
139,935
|
|
|
Total comprehensive income/(losses) attributable to
non-controlling interests
|
|
|
(1,196
|
)
|
|
|
868
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period
|
|
|
(21,083
|
)
|
|
|
140,803
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the unaudited
condensed consolidated interim financial statements.
F-5
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Statement of Changes in Consolidated Equity
For the Six Month Period Ended 30 June 2011
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable to Equity Holders of the Parent
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Comprehensive Income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Available-for
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale
|
|
|
Attributable
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share
|
|
|
Share
|
|
|
|
|
|
to
|
|
|
Interim
|
|
|
Own
|
|
|
Translation
|
|
|
Cash Flow
|
|
|
Financial
|
|
|
to
|
|
|
Non-Controlling
|
|
|
|
|
|
|
|
|
|
|
Capital
|
|
|
Premium
|
|
|
Reserves (*)
|
|
|
Parent
|
|
|
Dividend
|
|
|
Shares
|
|
|
Differences
|
|
|
Hedges
|
|
|
Assets
|
|
|
Parent
|
|
|
Interests
|
|
|
Equity
|
|
|
|
|
|
|
|
(Unaudited)
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Balances at 31 December 2009
|
|
|
106,532
|
|
|
|
121,802
|
|
|
|
314,903
|
|
|
|
147,972
|
|
|
|
(31,960
|
)
|
|
|
(677
|
)
|
|
|
(90,253
|
)
|
|
|
(1,948
|
)
|
|
|
0
|
|
|
|
566,371
|
|
|
|
12,157
|
|
|
|
578,528
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation differences
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
73,428
|
|
|
|
—
|
|
|
|
—
|
|
|
|
73,428
|
|
|
|
1,446
|
|
|
|
74,874
|
|
|
|
|
|
|
Cash flow hedges
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
99
|
|
|
|
—
|
|
|
|
99
|
|
|
|
—
|
|
|
|
99
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the period
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
73,428
|
|
|
|
99
|
|
|
|
0
|
|
|
|
73,527
|
|
|
|
1,446
|
|
|
|
74,973
|
|
|
|
|
|
|
Profit/(loss) for the period
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
66,408
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
66,408
|
|
|
|
(578
|
)
|
|
|
65,830
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
66,408
|
|
|
|
0
|
|
|
|
0
|
|
|
|
73,428
|
|
|
|
99
|
|
|
|
0
|
|
|
|
139,935
|
|
|
|
868
|
|
|
|
140,803
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operations with own shares
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,250
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,250
|
)
|
|
|
—
|
|
|
|
(1,250
|
)
|
|
|
|
|
|
Other changes
|
|
|
—
|
|
|
|
—
|
|
|
|
1
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1
|
|
|
|
—
|
|
|
|
1
|
|
|
|
|
|
|
Distribution of 2009 profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves
|
|
|
—
|
|
|
|
—
|
|
|
|
88,783
|
|
|
|
(88,783
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0
|
|
|
|
—
|
|
|
|
0
|
|
|
|
|
|
|
Dividends
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(27,229
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(27,229
|
)
|
|
|
(53
|
)
|
|
|
(27,282
|
)
|
|
|
|
|
|
Interim dividend
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(31,960
|
)
|
|
|
31,960
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0
|
|
|
|
—
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operations with equity holders or owners
|
|
|
0
|
|
|
|
0
|
|
|
|
88,784
|
|
|
|
(147,972
|
)
|
|
|
31,960
|
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(28,478
|
)
|
|
|
(53
|
)
|
|
|
(28,531
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 30 June 2010
|
|
|
106,532
|
|
|
|
121,802
|
|
|
|
403,687
|
|
|
|
66,408
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
|
(16,825
|
)
|
|
|
(1,849
|
)
|
|
|
0
|
|
|
|
677,828
|
|
|
|
12,972
|
|
|
|
690,800
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 31 December 2010
|
|
|
106,532
|
|
|
|
121,802
|
|
|
|
403,604
|
|
|
|
115,513
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
|
(50,733
|
)
|
|
|
(1,751
|
)
|
|
|
0
|
|
|
|
693,040
|
|
|
|
14,350
|
|
|
|
707,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation differences
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(38,001
|
)
|
|
|
|
|
|
|
—
|
|
|
|
(38,001
|
)
|
|
|
(540
|
)
|
|
|
(38,541
|
)
|
|
|
|
|
|
Cash flow hedges
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(580
|
)
|
|
|
—
|
|
|
|
(580
|
)
|
|
|
—
|
|
|
|
(580
|
)
|
|
|
|
|
|
Available-for-sale
financial assets Gains/(losses)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(575
|
)
|
|
|
(575
|
)
|
|
|
—
|
|
|
|
(575
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the period
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(38,001
|
)
|
|
|
(580
|
)
|
|
|
(575
|
)
|
|
|
(39,156
|
)
|
|
|
(540
|
)
|
|
|
(39,696
|
)
|
|
|
|
|
|
Profit/(loss) for the period
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
19,269
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
19,269
|
|
|
|
(656
|
)
|
|
|
18,613
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the period
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
19,269
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(38,001
|
)
|
|
|
(580
|
)
|
|
|
(575
|
)
|
|
|
(19,887
|
)
|
|
|
(1,196
|
)
|
|
|
(21,083
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other changes
|
|
|
—
|
|
|
|
—
|
|
|
|
(35
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(35
|
)
|
|
|
(213
|
)
|
|
|
(248
|
)
|
|
|
|
|
|
Capital Increase (note 11)
|
|
|
8,382
|
|
|
|
768,553
|
|
|
|
(2,264
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
774,671
|
|
|
|
—
|
|
|
|
774,671
|
|
|
|
|
|
|
Other movements (note 11)
|
|
|
—
|
|
|
|
—
|
|
|
|
52,864
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
52,864
|
|
|
|
—
|
|
|
|
52,864
|
|
|
|
|
|
|
Distribution of 2010 profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves
|
|
|
—
|
|
|
|
—
|
|
|
|
115,513
|
|
|
|
(115,513
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0
|
|
|
|
—
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operations with equity holders or owners
|
|
|
8,382
|
|
|
|
768,553
|
|
|
|
166,078
|
|
|
|
(115,513
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
827,500
|
|
|
|
(213
|
)
|
|
|
827,287
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 30 June 2011
|
|
|
114,914
|
|
|
|
890,355
|
|
|
|
569,682
|
|
|
|
19,269
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
|
(88,734
|
)
|
|
|
(2,331
|
)
|
|
|
(575
|
)
|
|
|
1,500,653
|
|
|
|
12,941
|
|
|
|
1,513,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(*) |
|
Reserves include accumulated earnings and other reserves |
The accompanying notes form an integral part of the unaudited
condensed consolidated interim financial statements.
F-6
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statement of Cash Flows
For the Six Month Period Ended 30 June 2011 and
2010
| |
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
30/06/10
|
|
|
|
|
(Unaudited)
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
Profit before tax
|
|
|
25,960
|
|
|
|
88,852
|
|
|
Adjustments for:
|
|
|
92,638
|
|
|
|
53,782
|
|
|
Amortisation and depreciation
|
|
|
28,156
|
|
|
|
21,434
|
|
|
Other adjustments:
|
|
|
64,482
|
|
|
|
32,348
|
|
|
Losses on equity accounted investments
|
|
|
807
|
|
|
|
728
|
|
|
Exchange differences
|
|
|
2,122
|
|
|
|
(1,970
|
)
|
|
Net provision charges
|
|
|
14,454
|
|
|
|
129
|
|
|
(Profit)/loss on disposal of fixed assets
|
|
|
9,416
|
|
|
|
(681
|
)
|
|
Government grants taken to income
|
|
|
(742
|
)
|
|
|
(550
|
)
|
|
Finance expense/income
|
|
|
37,130
|
|
|
|
33,386
|
|
|
Other adjustments
|
|
|
1,295
|
|
|
|
1,306
|
|
|
Changes in capital and assets
|
|
|
(65,159
|
)
|
|
|
13,700
|
|
|
Change in inventories
|
|
|
752
|
|
|
|
(11,982
|
)
|
|
Change in trade and other receivables
|
|
|
(66,961
|
)
|
|
|
20,239
|
|
|
Change in current financial assets and other current assets
|
|
|
(451
|
)
|
|
|
(3,875
|
)
|
|
Change in current trade and other payables
|
|
|
1,501
|
|
|
|
9,318
|
|
|
Other cash flows from operating activities
|
|
|
(36,745
|
)
|
|
|
(34,465
|
)
|
|
Interest paid
|
|
|
(34,021
|
)
|
|
|
(19,801
|
)
|
|
Interest recovered
|
|
|
999
|
|
|
|
3,861
|
|
|
Income tax recovered
|
|
|
(3,723
|
)
|
|
|
(18,525
|
)
|
|
Net cash from operating activities
|
|
|
16,694
|
|
|
|
121,869
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(1,669,390
|
)
|
|
|
(56,997
|
)
|
|
Group companies and business units (note 3)
|
|
|
(1,615,417
|
)
|
|
|
(3,727
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(52,838
|
)
|
|
|
(49,151
|
)
|
|
Property, plant and equipment
|
|
|
(42,841
|
)
|
|
|
(43,146
|
)
|
|
Intangible assets
|
|
|
(9,997
|
)
|
|
|
(6,005
|
)
|
|
Other financial assets
|
|
|
(1,135
|
)
|
|
|
(4,119
|
)
|
|
Proceeds from the sale of property, plant and equipment
|
|
|
69,151
|
|
|
|
2,863
|
|
|
Property, plant and equipment
|
|
|
69,151
|
|
|
|
2,863
|
|
|
Net cash used in investing activities
|
|
|
(1,600,239
|
)
|
|
|
(54,134
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(2,264
|
)
|
|
|
(1,250
|
)
|
|
Issue
|
|
|
(2,264
|
)
|
|
|
(1,250
|
)
|
|
Proceeds from and payments for financial liability instruments
|
|
|
2,235,339
|
|
|
|
(8,671
|
)
|
|
Issue
|
|
|
2,982,877
|
|
|
|
51,067
|
|
|
Redemption and repayment
|
|
|
(747,538
|
)
|
|
|
(59,738
|
)
|
|
Dividends and interest on other equity instruments paid
|
|
|
0
|
|
|
|
(53
|
)
|
|
Other cash flows from financing activities
|
|
|
(287,203
|
)
|
|
|
323
|
|
|
Transaction costs of financial instruments issued in the
acquisition of Talecris
|
|
|
(287,550
|
)
|
|
|
0
|
|
|
Other amounts received from financing activities
|
|
|
347
|
|
|
|
323
|
|
|
Net cash from/(used in) financing activities
|
|
|
1,945,872
|
|
|
|
(9,651
|
)
|
|
Effect of exchange rate fluctuations on cash
|
|
|
(18,184
|
)
|
|
|
42,684
|
|
|
Net increase in cash and cash equivalents
|
|
|
344,143
|
|
|
|
100,768
|
|
|
Cash and cash equivalents at beginning of the period
|
|
|
239,649
|
|
|
|
249,372
|
|
|
Cash and cash equivalents at end of period
|
|
|
583,792
|
|
|
|
350,140
|
|
The accompanying notes form an integral part of the unaudited
condensed consolidated interim financial statements.
F-7
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial Statements
for the
six month period ended 30 June 2011
Grifols, S.A. (hereinafter, the Company or the Parent Company)
was founded in Spain on 22 June 1987 as a limited liability
company for an indefinite period of time. Its registered and
fiscal address is in Barcelona (Spain). The Company’s
statutory activity is the provision of corporate administrative,
management and control services and investment in real and
personal property. Its main activity consists on the provision
of corporate administrative, management and control services to
its subsidiaries.
All the Company’s shares are listed in the Barcelona,
Madrid, Valencia, and Bilbao stock exchanges and on the Spanish
electronic market. Class B shares issued in May 2011, began
quotation on the NASDAQ (United States) and on the Automated
Quotation System in Spain on 2 June 2011 (see note 11).
Grifols, S.A. is the parent company of a Group (hereinafter the
Group) which acts on an integrated basis under a common
management and whose main activity is the procurement,
manufacture, preparation, and sale of therapeutic products,
particularly haemoderivatives.
The main manufacturing facilities of the Spanish companies of
the Group are located in Barcelona, Parets del Vallés
(Barcelona) and Torres de Cotillas (Murcia), while those of the
North American companies are located in Los Angeles (California,
USA), Clayton (North Carolina, USA) and Melville (New York, USA).
|
|
|
(2)
|
Basis of
Presentation and Accounting Principles Applied
|
These condensed consolidated interim financial statements have
been prepared in accordance with IAS 34 Interim Financial
Reporting. They do not include all of the information
required for full annual financial statements, and should be
read in conjunction with the consolidated financial statements
of the Group for the year ended 31 December 2010 prepared
in accordance with IFRS as issued by the International
Accounting Standard Board (IASB).
The figures in these condensed consolidated interim financial
statements are expressed in thousands of Euros.
The condensed consolidated interim financial statements of the
Grifols Group for the six month period ended 30 June 2011
have been prepared based on the accounting records kept by
Grifols and its subsidiaries.
Accounting
principles and basis of consolidation applied
The accounting principles and basis of consolidation applied in
the preparation of these condensed consolidated interim
financial statements are the same as those applied by the Group
in its consolidated financial statements as at and for the year
ended 31 December 2010.
In addition, the following standards that entered into force in
2011 have, accordingly, been taken into account for the
preparation of these condensed consolidated interim financial
statements:
|
|
|
| |
•
|
IAS 24 Revised Related Party Disclosures (effective date:
1 January 2011).
|
| |
| |
•
|
Amendment to IFRIC 14: Prepayment of a minimum funding
requirement (effective date: 1 January 2011).
|
| |
| |
•
|
IFRS 7 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 January 2011).
|
| |
| |
•
|
Amendment to IFRIC 13 Customer Loyalty Programmes (effective
date: 1 January 2011).
|
| |
| |
•
|
IAS 34 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 January 2011).
|
| |
| |
•
|
IAS 1 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 January 2011).
|
F-8
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The application of these standards has not had a significant
impact on the Group’s condensed consolidated interim
financial statements or has not been applicable.
The IASB also issued the following standards that are effective
for reporting periods beginning after 1 July 2011:
|
|
|
| |
•
|
Amendment to IAS 12 Deferred tax: recovery of underlying
assets (effective date: 1 January 2012)
|
| |
| |
•
|
Amendment to IFRS 1 Severe Hyperinflation and Removal of Fixed
Dates for First-time Adopters (effective date: 1 July 2011)
|
| |
| |
•
|
Amendment to IFRS 7 Financial
Instrument: Disclosures — Transfer of Financial
Assets (effective date: 1 July 2011)
|
| |
| |
•
|
IFRS 9 Financial instruments (effective
date: 1 January 2013)
|
| |
| |
•
|
IFRS 10 Consolidated Financial Statements (effective date:
1 January 2013)
|
| |
| |
•
|
IFRS 11 Joint Arrangements (effective date: 1 January
2013)
|
| |
| |
•
|
IFRS 12 Disclosures of Interests in Other Entities (effective
date: 1 January 2013)
|
| |
| |
•
|
IFRS 13 Fair Value Measurement (effective
date: 1 January 2013)
|
| |
| |
•
|
IAS 27 Separate Financial Statements (effective
date: 1 January 2013)
|
| |
| |
•
|
IAS 28 Investments in Associates and Joint Ventures (effective
date: 1 January 2013)
|
| |
| |
•
|
IAS 19 Employee Benefits (effective date: 1 January
2013)
|
The Group has not applied any of the standards or
interpretations issued prior to their effective date. The
Company’s directors do not expect that any of the above
amendments will have a significant effect on the consolidated
financial statements.
Responsibility
regarding information, estimates, hypotheses, and relevant
judgments in the application of accounting
policies
The information contained in these condensed consolidated
interim financial statements for the six month period ended
30 June 2011 is the responsibility of the Directors of the
Parent Company. The preparation of condensed consolidated
interim financial statements requires management to make
judgements, estimates and assumptions that affect the
application of accounting policies and the reported amounts of
assets and liabilities, income and expense. Actual results may
differ from these estimates.
These estimates are made based on the best information available
and refer to:
|
|
|
| |
•
|
The corporate tax expense which, according to IAS 34, is
recognised in interim periods based on the best estimate of the
average tax rate that the Group expects for the annual period.
|
| |
| |
•
|
The useful lives of property, plant, and equipment and
intangible assets.
|
| |
| |
•
|
Measurement of assets and goodwill to determine any related
impairment losses.
|
| |
| |
•
|
Evaluation of the capitalisation of development costs.
|
| |
| |
•
|
Evaluation of provisions and contingencies.
|
| |
| |
•
|
The assumptions used for calculation of the fair value of
financial instruments.
|
| |
| |
•
|
Evaluation of the effectiveness of hedging.
|
| |
| |
•
|
Evaluation of the nature of leases (operating or financial).
|
F-9
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The estimates, hypotheses and relevant judgements used in the
preparation of these condensed consolidated interim financial
statements do not differ from those applied in the preparation
of the consolidated financial statements as at and for the year
ended 31 December 2010.
Seasonality
of transactions during this period
Given the nature of the activities conducted by the Group, there
are no factors that determine any significant seasonality in the
Group’s operations that could affect the interpretation of
these condensed consolidated interim financial statements for
the six months period ended 30 June 2011 in comparison with
the financial statements for a full fiscal year.
Relative
importance
When determining the information to be disclosed in these Notes,
in accordance with IAS 34, the relative importance in relation
to these condensed consolidated interim financial statements has
been taken into account.
|
|
|
(3)
|
Changes
in the composition of the Group
|
For the preparation of its condensed consolidated interim
financial statements, the Group has included its investments in
all subsidiaries, associates and joint ventures. Note 1
(b) of the consolidated financial statements as at
31 December 2010 lists the subsidiaries, associates and
joint ventures in which Grifols, S.A. holds a direct or indirect
stake and that were included in the scope of consolidation at
that date.
The main variations in the scope of consolidation during the
interim period ended 30 June 2011 are detailed below:
Talecris
Biotherapeutics Holdings Corp. and
subsidiaries
On 2 June 2011 the Group acquired 100% of the share capital
of the American company Talecris Biotherapeutics Holdings Corp.
(hereinafter Talecris), which also specialises in the production
of plasma-derived biological medication, for a total of Euros
2,593 million (US Dollars 3,736 million).
The operation was performed through a combined offer of cash and
a new issue of Grifols non — voting shares
(hereinafter Class B shares) (see note 11).
The offer was made in relation to all Talecris shares and the
price offered per share amounted to US Dollars 19 in cash
(totaling US Dollar 2,541 million) and 0.641
Grifols’s Class B shares for each Talecris share
issued held by Talecris LLC and directors of Talecris and 0.6485
Grifols’s Class B shares for each Talecris share
issued (totaling US Dollar 1,195 million).
On 2 May 2011, the Group signed a “Consent
Agreement” with the Staff of the Bureau of Competition of
the US Federal Trade Commission (FTC) by means of which the
conditions for the merger transaction between both companies
were agreed.
To satisfy the Consent Agreement conditions, the Group has
signed agreements for the sale of assets and entered into
certain commercial, lease and manufacturing agreements with the
Italian company Kedrion, for up to seven years.
These agreements refer to the following areas:
|
|
|
| |
•
|
Kedrion and Grifols entered into a contract manufacturing
agreement to fractionate and purify Kedrion’s plasma to
deliver IVIG and Albumin under Kedrion’s private label, and
Factor VIII under the trade name Koate, all of them for sale
only in the United States.
|
F-10
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
|
|
|
| |
•
|
Grifols is committed to sell to Kedrion the Melville
fractionation facility. Grifols lease from Kedrion the Melville
fractionation facility being the lease term 3 years with an
optional extension of up to 1 year at Grifols request.
|
| |
| |
•
|
Grifols transfer to Kedrion all Koate (factor
VIII) technology and commercial agreements for the US
market. Grifols will produce Koate for Kedrion up to a period of
7 years.
|
| |
| |
•
|
Grifols is committed to sell to Kedrion two plasma collection
centers. In addition Grifols committed to sell 200.000 liters of
source plasma to Kedrion at a fixed price.
|
| |
| |
•
|
Grifols authorizes Kedrion to market and sell in the US, IVIG
and albumin manufactured by Grifols for Kedrion.
|
These conditions established in the Consent Agreement have been
executed on 3 June 2011.
At the date of publication of these Condensed Consolidated
Interim Financial Statements, taking into account that the
transaction is recent and not all the information necessary to
adequately determine the fair value of the assets, liabilities
and contingent liabilities, the Group has not made any fair
value adjustments to book values of Talecris at acquisitions
date, prepared under IFRS. The areas under analysis are mainly
tangible and intangible assets, acquired in-process research and
development, customer relationships, developed and core
technology, intellectual property, patents and trade names and
contingent liabilities.
Details of the aggregate business combination cost and
provisional fair value of the net assets acquired and
provisional goodwill at the acquisition date (or excess of the
cost of the business combination over the fair value of
identifiable net assets acquired) follows. The values shown in
the below table should therefore be considered as provisional
amounts.
| |
|
|
|
|
|
|
|
|
|
|
|
Thousands of
|
|
|
Thousands
|
|
|
|
|
Euros
|
|
|
of USD
|
|
|
|
|
Cost of business combination (valuation of Class B Shares)
|
|
|
829,799
|
|
|
|
1,195,574
|
|
|
Cash paid (19 USD per share)
|
|
|
1,763,601
|
|
|
|
2,540,997
|
|
|
Total cost of business combination
|
|
|
2,593,400
|
|
|
|
3,736,571
|
|
|
Book value of net assets acquired (provisional)
|
|
|
469,318
|
|
|
|
676,193
|
|
|
Goodwill (excess of the cost of the business combination over
the fair value of identifiable net assets acquired)
|
|
|
2,124,082
|
|
|
|
3,060,378
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(see note 6
|
)
|
|
|
|
|
|
Cash paid
|
|
|
1,763,601
|
|
|
|
2,540,996
|
|
|
Cash and cash equivalents of the acquired company
|
|
|
(149,693
|
)
|
|
|
(215,678
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash outflow for the acquisition
|
|
|
1,613,908
|
|
|
|
2,325,319
|
|
|
|
|
|
|
|
|
|
|
|
The fair value of Class B shares has been determined at the
average price of the first weeks of quotation price on the stock
exchange, being considered as a representative period for
determining the fair value as they started quotation on
2 June.
Costs incurred in the acquisition amounting to Euros
55 million have been expensed as incurred and are included
in Other operating expenses for an amount of Euros
38 million in the six month period ended 30 June 2011,
Euros 2 million in the first half of the year 2010, and
Euros 15 million in the second half of the year 2010.
Goodwill generated in the acquisition is attributed to the
workforce, synergies and other expected benefits from the
business combination of the assets and activities of the Group.
F-11
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The acquisition of Talecris will consolidate the Group as the
world’s third largest producer of plasma products,
significantly expanding its presence in the United States. Among
other aspects, it will increase product availability in the
market to the benefit of patients, through higher collection
capacity and plasma fractionation, as well as with complementary
R&D projects.
Had the acquisition taken place at 1 January 2011, the
Group’s revenue for the period would be Euros 503,625
thousand higher and consolidated profit for the period,
excluding exceptional items as transaction costs and stock
options cancellation costs derived from the change of control,
would be Euros 75,478 thousand higher. Revenues and profits
corresponding to Talecris from the date of acquisition to
30 June 2011 amount to Euros 104,730 thousand and Euros
17,926 thousand.
At the date of acquisition, the amounts of recognized assets,
liabilities and contingent liabilities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Book Value
|
|
|
|
|
Thousands of
|
|
|
Thousands of
|
|
|
|
|
Euros
|
|
|
USD
|
|
|
|
|
Intangible assets (note 7)
|
|
|
50,621
|
|
|
|
72,936
|
|
|
Property, plant and equipment (note 7)
|
|
|
306,401
|
|
|
|
441,462
|
|
|
Non — current financial assets
|
|
|
3,720
|
|
|
|
5,359
|
|
|
Deferred tax assets
|
|
|
80,115
|
|
|
|
115,429
|
|
|
Inventories
|
|
|
490,976
|
|
|
|
707,398
|
|
|
Trade and other receivables
|
|
|
126,772
|
|
|
|
182,653
|
|
|
Other assets
|
|
|
3,683
|
|
|
|
5,307
|
|
|
Cash and cash equivalents
|
|
|
149,693
|
|
|
|
215,678
|
|
|
Total assets
|
|
|
1,211,981
|
|
|
|
1,746,222
|
|
|
Non — current provisions
|
|
|
9,250
|
|
|
|
13,327
|
|
|
Non — current financial liabilities
|
|
|
6,289
|
|
|
|
9,061
|
|
|
Current financial liabilities
|
|
|
473,085
|
|
|
|
681,621
|
|
|
Current provisions
|
|
|
31,180
|
|
|
|
44,924
|
|
|
Trade and other payables
|
|
|
158,113
|
|
|
|
227,809
|
|
|
Other current liabilities
|
|
|
44,055
|
|
|
|
63,475
|
|
|
Deferred tax liabilities
|
|
|
20,691
|
|
|
|
29,812
|
|
|
Total liabilities and contingent liabilities
|
|
|
742,663
|
|
|
|
1,070,029
|
|
|
Total net assets acquired
|
|
|
469,318
|
|
|
|
676,193
|
|
The figures showed above correspond to the book value as at the
date of publication of these Condensed Consolidated Interim
Financial Statements. The fair value of the assets, liabilities
and contingent liabilities was not finally determined.
|
|
|
(4)
|
Financial
Risk Management Policy
|
At 30 June 2011 the Group’s financial risk management
objectives and policies are consistent with those disclosed in
the consolidated financial statements for the year ended
31 December 2010.
F-12
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The distribution by business segments of the Group’s net
revenues and consolidated income for the six month periods ended
30 June 2011 and 30 June 2010 is as follow:
| |
|
|
|
|
|
|
|
|
|
|
|
Net Revenues
|
|
|
|
|
Six Months Ended
|
|
|
Six Months Ended
|
|
|
Segments
|
|
30 June 2011
|
|
|
30 June 2010
|
|
|
|
|
(Thousands of Euros)
|
|
|
|
|
Bioscience
|
|
|
521,538
|
|
|
|
380,081
|
|
|
Hospital
|
|
|
49,289
|
|
|
|
45,146
|
|
|
Diagnostic
|
|
|
56,831
|
|
|
|
54,413
|
|
|
Raw materials + Other
|
|
|
7,683
|
|
|
|
8,169
|
|
|
TOTAL
|
|
|
635,341
|
|
|
|
487,809
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
Consolidated
|
|
|
|
|
Income/(loss)
|
|
|
|
|
Six Months Ended
|
|
|
Six Months Ended
|
|
|
Segments
|
|
30 June 2011
|
|
|
30 June 2010
|
|
|
|
|
(Thousands of Euros)
|
|
|
|
|
Bioscience
|
|
|
186,521
|
|
|
|
162,938
|
|
|
Hospital
|
|
|
4,786
|
|
|
|
5,196
|
|
|
Diagnostic
|
|
|
(11,264
|
)
|
|
|
4,798
|
|
|
Raw materials + Other
|
|
|
3,694
|
|
|
|
4,763
|
|
|
Total income of reported segments
|
|
|
183,737
|
|
|
|
177,695
|
|
|
Unallocated expenses plus net financial result
|
|
|
(157,777
|
)
|
|
|
(88,843
|
)
|
|
Profit before income tax from continuing operations
|
|
|
25,960
|
|
|
|
88,852
|
|
The variation in the Diagnostic profit is mainly due to the
goodwill impairment recognized in this period (see note 6).
The variation in the Bioscience segment profit reflects mainly
the incorporation of one month of Talecris companies amounting
to Euros 35,592 thousand.
The main variation in unallocated expenses plus net financial
result is mainly due to the transaction costs from the
acquisition of Talecris Biotherapeutics Holdings Corp.
F-13
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
Details and movement in goodwill during the six months ended
30 June 2011 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
|
Business
|
|
|
|
|
|
Translation
|
|
|
Balance at
|
|
|
|
|
31/12/10
|
|
|
Combination
|
|
|
Impairment
|
|
|
Differences
|
|
|
30/06/11
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Net value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK,Ltd. (UK)
|
|
|
7,982
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(370
|
)
|
|
|
7,612
|
|
|
Grifols Italia,S.p.A. (Italy)
|
|
|
6,118
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
6,118
|
|
|
Biomat USA, Inc. (USA)
|
|
|
113,052
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(8,534
|
)
|
|
|
104,518
|
|
|
Plasmacare, Inc. (USA)
|
|
|
38,464
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(2,903
|
)
|
|
|
35,561
|
|
|
Woolloomooloo Holdings Pty Ltd. (Australia)
|
|
|
23,832
|
|
|
|
0
|
|
|
|
(13,000
|
)
|
|
|
(415
|
)
|
|
|
10,417
|
|
|
Talecris Biotherapeutics
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(USA)
|
|
|
0
|
|
|
|
2,124,082
|
|
|
|
0
|
|
|
|
(6,612
|
)
|
|
|
2,117,470
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
189,448
|
|
|
|
2,124,082
|
|
|
|
(13,000
|
)
|
|
|
(18,834
|
)
|
|
|
2,281,696
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill has been allocated to each of the Group’s
cash-generating units (CGUs) in accordance with their respective
business segments and on a geographical basis, this being the
lowest level at which goodwill is controlled for management
purpose and lower than the operating segments. Plasmacare, Inc.
is integrated into the management of Biomat USA, Inc. for the
purpose of impairment testing.
Goodwill has been allocated to the cash generating units as
follows:
|
|
|
| |
•
|
UK: bioscience segment
|
| |
| |
•
|
Italy: bioscience segment
|
| |
| |
•
|
USA: bioscience segment
|
| |
| |
•
|
Australia: mainly to diagnostic segment.
|
Goodwill resulting from the Talecris acquisition is still
provisional as the estimation of the fair value of assets,
liabilities and contingent liabilities of the business acquired
is in progress (see note 3).
The recoverable amount of a CGU is determined based on its value
in use. These calculations use cash flow projections based on
the financial budgets approved by management. Cash flows
estimated as of the year in which stable growth has been reached
are extrapolated using the estimated growth rates indicated
below.
At 30 June 2011, on the basis of the profits generated
during the six-month period ended 30 June 2011, there are
no indications that the goodwill of the CGUs belonging to the
Bioscience segment has been impaired.
For the six months ended 30 June 2011, there was an
impairment indicator for the Australia CGU and therefore
goodwill impairment was prepared. The CGU’s market
performance was lower than expected. As a result of the
impairment test performed, an impairment of the CGU’s
goodwill (diagnostic) of Euros 13,000 thousand has been
accounted for at 30 June 2011.
F-14
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The key assumptions used in calculating values in use for the
year ended 31 December 2010 and for the six month period
ended 30 June 2011 were as follows:
| |
|
|
|
|
|
|
|
31/12/2010
|
|
|
|
Growth Rate
|
|
Pre- Tax Discount Rate
|
|
|
|
Bioscience
|
|
2.0% - 3.0%
|
|
10.5% - 10.9%
|
|
Diagnostic
|
|
2.0%
|
|
10.4%
|
| |
|
|
|
|
|
|
|
30/06/2011
|
|
|
|
Growth Rate
|
|
Pre - Tax Discount Rate
|
|
|
|
Bioscience
|
|
N/A
|
|
N/A
|
|
Diagnostic
|
|
2.0%
|
|
11.5%
|
Management determined budgeted gross margins based on past
experience and forecast market development. Average weighted
growth rates are coherent with the forecasts included in
industry reports. The discount rate used reflects specific risks
related to the CGU.
|
|
|
(7)
|
Other
Intangible Assets and Property, Plant, and Equipment
|
Movement of Other Intangible Assets and Property, Plant and
Equipment during the six months ended 30 June 2011 is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other
|
|
|
|
|
|
|
|
|
|
|
Intangible
|
|
|
Property, Plant
|
|
|
|
|
|
|
|
Assets
|
|
|
and Equipment
|
|
|
Total
|
|
|
|
|
Total Cost at 31/12/2010
|
|
|
151,861
|
|
|
|
656,295
|
|
|
|
808,156
|
|
|
Total dep. & amort. At 31/12/2010
|
|
|
(73,562
|
)
|
|
|
(221,515
|
)
|
|
|
(295,077
|
)
|
|
Impairment at 31/12/2010
|
|
|
0
|
|
|
|
(649
|
)
|
|
|
(649
|
)
|
|
Balance at 31/12/2010
|
|
|
78,299
|
|
|
|
434,131
|
|
|
|
512,430
|
|
|
Cost
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additions
|
|
|
9,997
|
|
|
|
43,101
|
|
|
|
53,098
|
|
|
Business Combination
|
|
|
50,621
|
|
|
|
306,401
|
|
|
|
357,022
|
|
|
Disposals
|
|
|
(588
|
)
|
|
|
(123,965
|
)
|
|
|
(124,553
|
)
|
|
Transfers
|
|
|
(126
|
)
|
|
|
(885
|
)
|
|
|
(1,011
|
)
|
|
Translation differences
|
|
|
(2,515
|
)
|
|
|
(19,327
|
)
|
|
|
(21,842
|
)
|
|
Total Cost at 30/06/2011
|
|
|
209,250
|
|
|
|
861,620
|
|
|
|
1,070,870
|
|
|
Depreciation & amortization
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additions
|
|
|
(8,630
|
)
|
|
|
(19,526
|
)
|
|
|
(28,156
|
)
|
|
Disposals
|
|
|
0
|
|
|
|
13,727
|
|
|
|
13,727
|
|
|
Transfers
|
|
|
600
|
|
|
|
411
|
|
|
|
1,011
|
|
|
Translation differences
|
|
|
816
|
|
|
|
5,553
|
|
|
|
6,369
|
|
|
Total dep. & amort. At 30/06/2011
|
|
|
(80,776
|
)
|
|
|
(221,350
|
)
|
|
|
(302,126
|
)
|
|
Impairment
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additions
|
|
|
0
|
|
|
|
114
|
|
|
|
114
|
|
|
Impairment at 30/06/2011
|
|
|
0
|
|
|
|
(535
|
)
|
|
|
(535
|
)
|
|
Balance at 30/06/2011
|
|
|
128,474
|
|
|
|
639,735
|
|
|
|
768,209
|
|
Additions in property, plant and equipment mainly relates to the
Bioscience segment, Talecris contributing an amount of Euros
16 million.
F-15
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
At 30 June 2011 there are no indications that these assets
have been impaired.
The key assumptions used in calculating value in use for
intangible assets with indefinite useful lives for the year 2010
were as follows:
Growth rate used to extrapolate projections: 3.0%
Pre-tax discount rate: 10.9%
|
|
|
(a)
|
Sale
of Spanish properties and lease back
|
On 10 May, 2011 the Group sold five properties based in
Spain mainly related to non-core assets such as offices and
warehouses and a factory premise, by an aggregated amount of
Euros 80.4 million to Gridpan Invest, S.L., a company fully
owned by Scranton Enterprises, B.V., a related party of Grifols,
S.A. Two of the premises were sold together with its related
mortgage loans amounting in total to Euros 53.5 million. As
a result of the transactions the Group has recognized a net loss
of Euros 7.4 million. The prices paid for the properties
were established based on the appraisals made by independent
appraisers.
At the same time, operating lease agreements for the
aforementioned properties were entered into with Gridpan Invest,
S.L., the main terms of the agreements being as follows:
|
|
|
| |
•
|
Compulsory initial term of five years,
|
| |
| |
•
|
Initial rent established at market prices and will be reviewed
annually, based on the percentage variation in the Spanish
Consumer Price Index (CPI),
|
| |
| |
•
|
Automatic extensions of five-year periods that can be avoided by
both parties by a six month anticipated notice.
|
| |
| |
•
|
Upon vacating the premises, the lessor will reimburse Grifols
for the remaining value of leasehold improvements Grifols made.
|
In addition, the Group entered into a free of charge purchase
option over the shares of Gridpan Invest, S.L. exercisable
between 10 May 2016 and 10 May 2017. The strike price
will be at market value at the date of exercise, based on
independent appraisers.
The rental expense recognized by the Group for the six months
period ended 30 June 2011 in connection with these
agreements amounted to Euros 1,084 thousand, which related in
full to the minimum contractual payments.
|
|
|
(b)
|
Sale
of properties and equipment in the USA and lease
back
|
On 9 June 2011 the Group entered into several agreements
for the sale and lease back of a manufacturing building and
related equipment to third party companies California Biogrif
330, LP and LA 300 Biologicals Financing, LP respectively. In
addition, a lease was entered into for the piece of land on
which the building sold is constructed, for a term of
99 years, to the same party. The sales price received for
the building amounted to US Dollars 35.4 million
(Euros 24.6 million) and the sales price for the equipment
US Dollars 23.8 million (Euros 16.5 million).
The lease of the building has been designed as operating, while
the lease of the equipment is considered as finance considering
the terms of the purchase option. As a result of the sale of the
building, the Group has recognized a net loss of US Dollars
2.4 million (Euros 1.3 million) mainly due to the
expenses incurred on the transaction.
F-16
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The main terms of the operating lease agreement over the
building are as follows:
|
|
|
| |
•
|
Compulsory initial term of 20 years.
|
| |
| |
•
|
Initial rent has been established at market prices and will be
reviewed annually with a 3% increase. On the first day of the
sixth year, the remaining rents until year twenty will be paid
in advance in a lump sum.
|
| |
| |
•
|
Renewal option to extend for a ten-year period at Grifols Group
election.
|
| |
| |
•
|
Purchase options granted during the sixth year and in year
twenty (20) at market value, to be estimated by independent
appraisers.
|
The main terms of the finance lease agreement over the equipment
are a compulsory term of five years, and sixty (60) monthly
rent instalments of Dollars 529 thousand (Euros 369 thousand).
The lease agreement is not renewable and provides for the
repurchase of the equipment at the end of the term for $1.
The rental expense recognized by the Group for the six month
period ended 30 June 2011 in connection with the operating
lease agreement amounted to US Dollars 148 thousand (Euros
103 thousand) , which related in full to the minimum contractual
payments.
Future minimum non — cancellable payments of new
operating leases derived from the above mentioned operating
leases and Talecris business acquisition are as follows:
| |
|
|
|
|
|
|
|
30/06/11
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Maturity:
|
|
|
|
|
|
Up to 1 year
|
|
|
20,990
|
|
|
Between 1 and 5 years
|
|
|
87,742
|
|
|
More than 5 years
|
|
|
17,293
|
|
|
|
|
|
|
|
|
Total future minimum payments
|
|
|
126,025
|
|
|
|
|
|
|
|
Details of minimum payments and the current finance lease
liabilities incurred on the financial lease transaction over the
equipment in the US described above, by maturity date, are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
|
|
Current
|
|
|
Non-current
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Minimum payments
|
|
|
4,659
|
|
|
|
17,295
|
|
|
Interest
|
|
|
(1,932
|
)
|
|
|
(3,553
|
)
|
|
Present value
|
|
|
2,727
|
|
|
|
13,742
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30/06/11
|
|
|
|
|
Minimum
|
|
|
|
|
|
|
|
|
|
|
Payments
|
|
|
Interest
|
|
|
Present Value
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Maturity at:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less than one year
|
|
|
4,659
|
|
|
|
1,932
|
|
|
|
2,727
|
|
|
Two years
|
|
|
4,391
|
|
|
|
1,486
|
|
|
|
2,905
|
|
|
Three years
|
|
|
4,391
|
|
|
|
1,119
|
|
|
|
3,272
|
|
|
Four years
|
|
|
4,391
|
|
|
|
706
|
|
|
|
3,685
|
|
|
Five years
|
|
|
4,122
|
|
|
|
242
|
|
|
|
3,880
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
21,954
|
|
|
|
5,485
|
|
|
|
16,469
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-17
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
At 30 June 2011, some Group companies had signed purchase
agreements for credit rights without recourse with certain
financial institutions.
The total sum of credit rights sold without recourse, for which
ownership was transferred to financial entities pursuant to the
aforementioned agreements, amounts to Euros 73,116 thousand for
the six month period ended at 30 June 2011 (Euros 88,747
thousand for the six month period ended 30 June 2010).
The financial expenses of these operations incurred by the Group
for the six month period ended 30 June 2011 amounted to
approximately Euros 2,194 thousand (Euros 3,958 thousand for the
six month period ended at 30 June 2010) which are
recorded under the “Finance Expenses” caption in the
condensed consolidated income statement.
Other current assets corresponding to the costs incurred in
connection with the issuance of new share capital increase have
been taken to equity when the capital increase has been
performed while other current assets corresponding to the
issuance of senior debt and High Yield bonds, have been deducted
from the financial liability when the debt has been issued
(2 June 2011) (see note 12). Expenses amounting to
Euros 38,607 thousand, for the six month period ended
30 June 2011, incurred related to the business combination
have been expensed (Euros 2,019 thousand for the six month
period ended at 30 June 2010).
|
|
|
(10)
|
Cash and
Cash equivalents
|
At 30 June 2011, cash and cash equivalents includes Euros
428 million in a restricted cash account in order to pay
the bonds proceeding from Talecris, which have been subsequently
paid on 1 July 2011 (see note 12).
The Group has carried out the following investing
and/or
financing operations which have not required the use of cash or
cash equivalents:
|
|
|
| |
•
|
The Group has sold properties in Spain amounting to Euros
80.4 million which together with its related mortgage loan
of Euros 53.5 million resulted in a net cash inflow of
Euros 26.9 million (see note 7).
|
| |
| |
•
|
Part of the consideration paid in the acquisition of Talecris
Group has been realized by delivery of Class B shares (see
note 3). The issue of Class B shares has had no cash
impacts.
|
At 30 June 2011 net cash from operating activities
amounts to Euros 16,694 thousand. The impact of non-recurring
effects are the following:
|
|
|
| |
•
|
This amount includes a decrease in profit before tax due to the
transaction costs incurred by the Group during the six month
period ended 30 June 2011 amounting to Euros 38,607
thousand (2,019 thousand for the six months ended at
30 June 2010) that have been paid in this period.
|
| |
| |
•
|
Change in current trade and other payables includes Euros 19,516
thousand corresponding to business combination costs accrued by
Talecris companies prior to acquisition date and paid during
June 2011.
|
|
|
|
(11)
|
Capital
and Reserves
|
Details of consolidated equity and changes are shown in the
condensed consolidated interim statement of changes in equity,
which forms part of the condensed consolidated interim financial
statements.
F-18
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
|
|
|
(a)
|
Share
Capital and Share Premium
|
As authorised by the shareholders at their extraordinary
shareholders’ general meeting held on 25 January 2011,
the Parent Company agreed to increase share capital through the
issue of 83,811,688 new non-voting shares (Class B shares),
which have been used in its acquisition of Talecris. These
shares are listed on the NASDAQ Global Market (United States)
and the Automated Quotation System (“mercado
continuo”) (Spain).
At 30 June 2011 the Company’s share capital currently
stands at 114,913,618 Euros, represented by:
|
|
|
| |
•
|
Class A Shares: 213,064,899 ordinary shares of 0.50
Euros nominal value each, fully subscribed and paid up, of the
same class and series being the ordinary shares of the Company.
|
| |
| |
•
|
Class B Shares: 83,811,688 preference non-voting
shares of 0.10 Euros nominal value each, of the same class and
series, and with the preferential rights set forth in the
Company’s by laws.
|
On 1 June 2011 Grifols, S.A. informed that the “Nota
sobre Acciones” (Securities Note) requested for the
admission to trading of Class B Shares was registered.
Grifols has requested the admission to trading of the
Class B Shares on the Stocks Exchanges of Madrid,
Barcelona, Bilbao and Valencia as well as on Automated Quotation
System (“mercado continuo”) and, through the American
Depositary Shares (ADSs), on the National Association of
Securities Dealers Automated Quotation (NASDAQ). The trading of
Class B Shares on the Stock Market Interconnection System
and the ADSs on the NASDAQ started on 2 June 2011.
The fair value of the Class B Shares has been estimated as
its market value on the first weeks of quotation, as they began
quotation on 2 June 2011. The positive difference amounting
to Euros 52,864 thousand arising between the value assigned in
the deeds of the share increase (Euros 776,935 thousand) and the
fair value (Euros 829,799 thousand) has been presented as
reserves.
The main characteristics of the Class B shares are as
follows:
|
|
|
| |
•
|
Each Class B share entitles its holder to receive a minimum
annual preferred dividend out of the distributable profits at
the end of each fiscal year equal to a 0.01 Euros per
Class B share if the aggregate preferred dividend does not
exceed the distributable profits of that fiscal year. This
preferred dividend is not cumulative if no sufficient
distributable profits are obtained in the year.
|
| |
| |
•
|
Each Class B share is entitled to receive, in addition to
the preferred dividend referred to above, the same dividends and
other distributions as one Grifols ordinary share.
|
| |
| |
•
|
Each Class B share entitles its holder to have it redeemed
under certain circumstances, if a tender offer for all or part
of the shares in the Company is made and settled except if
holders of Class B shares have been entitled to participate
in such offer and have their shares acquired in such offer
equally and on the same terms as holders of Class A shares.
Terms and conditions of redemption incorporated in by laws limit
the amounts to be redeemed to the existence of distributable
reserves and limit the percentage of shares to be redeemed to a
relation to the ordinary shares to which the offer is addressed.
|
| |
| |
•
|
Each Class B shares has the right to receive prior to the
ordinary shares, upon the
winding-up
and liquidation of Grifols, an amount equal to the sum of
(i) the nominal value of each Class B share, and
(ii) the share premium
paid-up for
such Class B share when it was subscribed for. Each holder
is entitled to receive, in addition to the Class B
liquidation amount, the same liquidation amount that is paid to
each Grifols ordinary share.
|
The availability of the reserves for distribution is subject to
legislation applicable to each of the Group companies. At
30 June 2011, an amount of Euros 28,811 thousand which is
equivalent to the carrying amount of development costs pending
amortisation of certain Spanish companies (Euros 28,876 thousand
at
F-19
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
31 December 2010) are, in accordance with applicable
legislation, restricted reserves which cannot be distributed
until these development costs have been amortised.
Companies in Spain are obliged to transfer 10% of each
year’s profits to a legal reserve until this reserve
reaches an amount equal to 20% of share capital. This reserve is
not distributable to shareholders and may only be used to offset
losses if no other reserves are available. Under certain
conditions it may be used to increase share capital provided
that the balance left on the reserve is at least equal to 10% of
the nominal value of the total share capital after the increase.
At 30 June 2011 and 31 December 2010 the legal reserve
of the Parent Company amounts to Euros 21,306 thousand.
Distribution of the legal reserves of other Spanish companies is
subject to the same restrictions as those of the Parent Company
and at 30 June 2011 and 31 December 2010 the balance
of the legal reserves of the other Spanish companies amounts to
Euros 2,106 thousand.
Other foreign Group companies have a legal reserve amounting to
Euros 692 thousand at 30 June 2011 and 31 December
2010.
The Parent Company has executed the following transactions with
its own shares during the six month period ended 30 June
2010. There were no movements in own shares from 30 June
2010 through 30 June 2011.
| |
|
|
|
|
|
|
|
|
|
|
|
Num. of Shares
|
|
|
Thousand Euros
|
|
|
|
|
Balance at 1 January 2010
|
|
|
53,326
|
|
|
|
677
|
|
|
Acquisitions
|
|
|
105,000
|
|
|
|
1,250
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 30 June 2010 and 30 June 2011
|
|
|
158,326
|
|
|
|
1,927
|
|
|
|
|
|
|
|
|
|
|
|
The Parent holds own shares equivalent to 0.05% of its capital
at 30 June 2011 (0.07% at 31 December 2010).
The profits of Grifols, S.A. and subsidiaries will be
distributed as agreed by respective shareholders of each company
at their general meetings.
There were no dividends payments during the six month period
ended 30 June 2011 and 2010. With regard to the results of
the annual period 2009, the dividend approved at the Shareholder
General Assembly in 2010 was paid in July 2010.
F-20
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
|
|
|
(12)
|
Financial
Liabilities
|
The detail of non-current financial liabilities at 30 June
2011 and 31 December 2010 is as follow:
| |
|
|
|
|
|
|
|
|
|
Non-current Financial Liabilities
|
|
30/06/11
|
|
|
31/12/10
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Issue of Corporate bonds(a)
|
|
|
0
|
|
|
|
446,918
|
|
|
Issue High Yield Bonds(a)
|
|
|
761,088
|
|
|
|
0
|
|
|
Transaction costs on bonds
|
|
|
(110,542
|
)
|
|
|
(5,715
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Non-current promissory notes(a)
|
|
|
650,546
|
|
|
|
441,203
|
|
|
Tranche A (USD)
|
|
|
830,277
|
|
|
|
0
|
|
|
Tranche B (USD)
|
|
|
892,721
|
|
|
|
0
|
|
|
Tranche A (EUR)
|
|
|
213,125
|
|
|
|
0
|
|
|
Tranche B (EUR)
|
|
|
217,800
|
|
|
|
0
|
|
|
Implicit Floor and swap floor
|
|
|
(19,565
|
)
|
|
|
0
|
|
|
Transaction costs on loans and borrowings
|
|
|
(185,314
|
)
|
|
|
(1,365
|
)
|
|
Club Deal
|
|
|
0
|
|
|
|
100,000
|
|
|
Other loans
|
|
|
18,391
|
|
|
|
120,813
|
|
|
Finance lease liabilities
|
|
|
24,963
|
|
|
|
4,734
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings(b)
|
|
|
1,992,398
|
|
|
|
224,182
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings and bonds or other non current marketable
securities
|
|
|
2,642,944
|
|
|
|
665,385
|
|
|
Financial derivatives
|
|
|
61,685
|
|
|
|
0
|
|
|
Other non-current financial liabilities
|
|
|
10,715
|
|
|
|
10,474
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,715,344
|
|
|
|
675,859
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
High
Yield Senior Unsecured Notes
|
On 13 January 2011, the Group closed its scheduled issue of
High Yield Senior Unsecured Notes for an amount of
US Dollars 1,100 million, with a seven year maturity
period (2018) and an annual coupon of 8.25%. This issuance,
together with the already completed syndicated loan disclosed in
the following paragraphs, allowed the Company to obtain
necessary funds to pay the acquisition of Talecris (see
note 3) on 2 June 2011.
As requested by this new credit agreement, on 2 June 2011
the Group has cancelled the US Private Placement (corporate
bonds) totaling US Dollar 600 million and has expensed
all associated transaction costs. The make — whole
premium payment related to the required extinguishment of the US
Private Placement amounting to Euros 112 million has been
included as transaction costs as the payment was a requirement
for obtaining new credit agreement. These costs together with
other debt issuance costs (underwriting fees, ticking fees,
closing fees, etc.) amounting to further Euros 239 million
have been deferred as transaction costs based on the allocation
to the associated liabilities.
F-21
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
On 23 November 2010 the Group signed loan agreements
amounting to US Dollars 3,400 million for the purchase
of Talecris. Details of this collateralized senior debt are as
follows:
|
|
|
| |
•
|
Non-current syndicated financing
Tranche A: Senior Debt Loan repayable in five
years divided into two tranches: U.S. Tranche A and Foreign
Tranche A.
|
|
|
|
| |
•
|
Aggregate Principal Amount of US 1,200 million.
|
| |
| |
•
|
Applicable margin of 375 basic points (bp) linked to US Libor.
|
| |
| |
•
|
Floor over US Libor of 1.75%
|
|
|
|
| |
•
|
Aggregate Principal Amount of EUR 220 million.
|
| |
| |
•
|
Applicable margin of 400 basic points (bp) linked to Euribor.
|
| |
| |
•
|
Floor over Euribor of 1.75%
|
The detail of the Tranche A by maturity is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US Tranche A
|
|
|
Foreign Tranche A
|
|
|
|
|
|
|
|
Amortization in
|
|
|
Amortization in
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thousands of US
|
|
|
Thousands of
|
|
|
|
|
|
Amortization in
|
|
|
|
|
Currency
|
|
|
Dollar
|
|
|
Euros
|
|
|
Currency
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2012
|
|
|
USD
|
|
|
|
112,500
|
|
|
|
77,839
|
|
|
|
EUR
|
|
|
|
20,625
|
|
|
2013
|
|
|
USD
|
|
|
|
127,500
|
|
|
|
88,217
|
|
|
|
EUR
|
|
|
|
23,375
|
|
|
2014
|
|
|
USD
|
|
|
|
180,000
|
|
|
|
124,542
|
|
|
|
EUR
|
|
|
|
33,000
|
|
|
2015
|
|
|
USD
|
|
|
|
585,000
|
|
|
|
404,760
|
|
|
|
EUR
|
|
|
|
107,250
|
|
|
2016
|
|
|
USD
|
|
|
|
195,000
|
|
|
|
134,920
|
|
|
|
EUR
|
|
|
|
35,750
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
USD
|
|
|
|
1,200,000
|
|
|
|
830,277
|
|
|
|
EUR
|
|
|
|
220,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
•
|
Non-current syndicated financing
Tranche B: six year loan (payment of whole
principal upon maturity) divided into two tranches: U.S.
Tranche B and Foreign Tranche B.
|
|
|
|
| |
•
|
Aggregate Principal Amount of US 1,300 million.
|
| |
| |
•
|
Applicable margin of 425 basic points (bp) linked to US Libor.
|
| |
| |
•
|
Floor over US Libor of 1.75%
|
|
|
|
| |
•
|
Aggregate Principal Amount of EUR 220 million.
|
| |
| |
•
|
Applicable margin of 450 basic points (bp) linked to Euribor.
Floor over Euribor of 1.75%
|
F-22
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The detail of the Tranche B by maturity is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US Tranche B
|
|
|
Foreign Tranche B
|
|
|
|
|
|
|
|
Amortization in
|
|
|
Amortization in
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thousands of US
|
|
|
Thousands of
|
|
|
|
|
|
Amortization in
|
|
|
|
|
Currency
|
|
|
Dollar
|
|
|
Euros
|
|
|
Currency
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2011
|
|
|
USD
|
|
|
|
6,500
|
|
|
|
4,497
|
|
|
|
EUR
|
|
|
|
1,100
|
|
|
2012
|
|
|
USD
|
|
|
|
13,000
|
|
|
|
8,995
|
|
|
|
EUR
|
|
|
|
2,200
|
|
|
2013
|
|
|
USD
|
|
|
|
13,000
|
|
|
|
8,995
|
|
|
|
EUR
|
|
|
|
2,200
|
|
|
2014
|
|
|
USD
|
|
|
|
13,000
|
|
|
|
8,995
|
|
|
|
EUR
|
|
|
|
2,200
|
|
|
2015
|
|
|
USD
|
|
|
|
13,000
|
|
|
|
8,995
|
|
|
|
EUR
|
|
|
|
2,200
|
|
|
2016
|
|
|
USD
|
|
|
|
9,750
|
|
|
|
6,746
|
|
|
|
EUR
|
|
|
|
1,650
|
|
|
2017
|
|
|
USD
|
|
|
|
1,231,750
|
|
|
|
852,245
|
|
|
|
EUR
|
|
|
|
208,450
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
USD
|
|
|
|
1,300,000
|
|
|
|
899,467
|
|
|
|
EUR
|
|
|
|
220,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
•
|
Senior revolving credit facility amounting to
US Dollars 300 million. No amounts have been drawn
against the credit facility as of 30 June 2011.
|
|
|
|
| |
•
|
U.S. Revolving Credit Facility :
|
|
|
|
| |
•
|
Committed Amount : US 50 million
|
| |
| |
•
|
Applicable margin of 375 basis point (bp).
|
|
|
|
| |
•
|
U.S. Multicurrency Revolving Credit Facility:
|
|
|
|
| |
•
|
Committed Amount : US 200 million
|
| |
| |
•
|
Applicable margin of 375 basis point (bp)
|
|
|
|
| |
•
|
Foreign Revolving Credit Facility :
|
|
|
|
| |
•
|
Committed Amount : US 50 million.
|
| |
| |
•
|
Applicable margin of 400 basis point (bp).
|
The total amortization plus interests of the High Yield Bond and
Tranche A & B Senior Loan is detailed as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tranche A and B Senior
|
|
|
|
|
High Yield Bond
|
|
|
Loan
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity
|
|
|
|
|
|
|
|
|
|
2011
|
|
|
59,301
|
|
|
|
80,474
|
|
|
2012
|
|
|
62,790
|
|
|
|
235,792
|
|
|
2013
|
|
|
62,790
|
|
|
|
241,228
|
|
|
2014
|
|
|
62,790
|
|
|
|
279,372
|
|
|
2015
|
|
|
62,790
|
|
|
|
613,129
|
|
|
2016
|
|
|
62,790
|
|
|
|
248,557
|
|
|
2017
|
|
|
62,790
|
|
|
|
1,087,608
|
|
|
2018
|
|
|
763,994
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
1,200,034
|
|
|
|
2,786,160
|
|
|
|
|
|
|
|
|
|
|
|
F-23
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The issue of the High Yield Bond and Credit Agreement are
subject to compliance with certain covenants. At 30 June
2011 the Group is in compliance with these covenants.
Grifols, S.A., Grifols Inc. and significant subsidiaries are
guarantors of the new debt. Significant subsidiaries are those
that meet 85% of earnings before interests, tax, depreciation
and amortization, 85% of total consolidated assets and 85% of
aggregated turnover of the Group or represents more than 3% of
the above measures.
Club Deal and bilateral loans amounting to Euros
297 million have been cancelled on 2 June 2011. All
deferred costs associated with them and the remaining cash flow
hedge related to the US Private Placement carried out in October
2009 (totally amounting to Euros 9.3 million) have been
expensed.
As the floor included in Tranche A and Tranche B loans
is in the money, embedded derivatives exist in those contracts,
which have been fair valued and separated from the loans.
In June 2011, the Group subscribed two derivatives in order to
comply with the mandatory hedging according to the Credit
Agreement, a
step-up
interest rate swap and a swap floor, which have a notional of
US Dollars 1,550 million each. The interest rate swap
complies with the criteria required for hedge accounting.
The detail of derivatives at 30 June 2011 and
31 December 2010 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Notional at
|
|
|
Notional at
|
|
|
Value at
|
|
|
Value at
|
|
|
Financial Derivatives
|
|
30/06/11
|
|
|
31/12/10
|
|
|
30/06/11
|
|
|
31/12/10
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Interest Rate Swap
|
|
|
50,000
|
|
|
|
50,000
|
|
|
|
(1,146
|
)
|
|
|
(1,809
|
)
|
|
Interest Rate Swap (Cash flow hedge)
|
|
|
1,072,442
|
|
|
|
—
|
|
|
|
(3,829
|
)
|
|
|
—
|
|
|
Implicit Floor
|
|
|
3,113,540
|
|
|
|
—
|
|
|
|
(54,364
|
)
|
|
|
—
|
|
|
Currency Rate Swap
|
|
|
47,800
|
|
|
|
—
|
|
|
|
(2,346
|
)
|
|
|
—
|
|
|
Liability
|
|
|
4,283,782
|
|
|
|
50,000
|
|
|
|
(61,685
|
)
|
|
|
(1,809
|
)
|
|
Unquoted future
|
|
|
17,416
|
|
|
|
23,221
|
|
|
|
3,344
|
|
|
|
(2,821
|
)
|
|
Unquoted future
|
|
|
26,370
|
|
|
|
26,370
|
|
|
|
4,078
|
|
|
|
(3,930
|
)
|
|
Swap floor
|
|
|
1,072,442
|
|
|
|
—
|
|
|
|
32,558
|
|
|
|
—
|
|
|
Assets
|
|
|
1,116,227
|
|
|
|
49,591
|
|
|
|
39,980
|
|
|
|
(6,751
|
)
|
The swap floor value at 30 June 2011 is included in
non-current financial assets. The last maturity date of the swap
floor is 2016.
F-24
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
The detail of current financial liabilities 30 June 2011
and 31 December 2010 is as follows:
| |
|
|
|
|
|
|
|
|
|
Current Financial Liabilities
|
|
30/06/11
|
|
|
31/12/10
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Talecris bonds (note 10)
|
|
|
427,691
|
|
|
|
0
|
|
|
Transaction costs High Yield Bonds
|
|
|
(18,032
|
)
|
|
|
0
|
|
|
Interest accrued on bonds
|
|
|
27,907
|
|
|
|
7,207
|
|
|
Promisory notes
|
|
|
9,586
|
|
|
|
8,235
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonds
|
|
|
447,152
|
|
|
|
15,442
|
|
|
Tranche A (USD)
|
|
|
0
|
|
|
|
0
|
|
|
Tranche B (USD)
|
|
|
6,746
|
|
|
|
0
|
|
|
Tranche A (EUR)
|
|
|
6,875
|
|
|
|
0
|
|
|
Tranche B (EUR)
|
|
|
2,200
|
|
|
|
0
|
|
|
Transaction costs on loans and borrowings
|
|
|
(37,216
|
)
|
|
|
(708
|
)
|
|
Club Deal
|
|
|
0
|
|
|
|
66,667
|
|
|
Other loans
|
|
|
75,079
|
|
|
|
106,954
|
|
|
Finance lease liabilities
|
|
|
6,538
|
|
|
|
3,280
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings
|
|
|
60,222
|
|
|
|
176,193
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings and bonds or other current marketeable
securities
|
|
|
507,374
|
|
|
|
191,635
|
|
|
Financial derivatives
|
|
|
7,320
|
|
|
|
8,560
|
|
|
Other current financial liabilities
|
|
|
10,016
|
|
|
|
9,676
|
|
|
|
|
|
|
|
|
|
|
|
|
Other current financial liabilities
|
|
|
17,336
|
|
|
|
18,236
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
524,710
|
|
|
|
209,871
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(13)
|
Financial
Income and Expenses
|
In relation to futures contracts with a creditworthy financial
entity the underlying asset of which is Company shares, the
financial income/(loss) for the six month period ended
30 June 2011 reflects an unrealised gain of Euros
14.2 million (loss of Euros 15.8 million for the six
month period ended at 30 June 2010). On 30 May 2011
the Company has sold 500,000 futures and realized a gain of
Euros 1 million. In June 2011 the remaining future
contracts were extended until December 2011.
Income tax expense is recognised based on management’s best
estimate of the weighted average annual income tax rate expected
for the full financial year applied to the pre-tax income of the
interim period. The Group’s consolidated effective tax rate
has increased from 25.9% for the six month period ended
30 June 2010 to 28.3% for the six month period ended
30 June 2011 mainly due to a greater portion of earnings
being taxed at a higher tax rate due to the inclusion of
Talecris.
|
|
|
(15)
|
Discontinued
Operations
|
The Group does not consider any operations as discontinued for
the six month period ended 30 June 2011.
F-25
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
(16) Commitments
and Contingencies.
There have been no significant changes to the Group’s
commercial commitments during the first half of 2011. We have
included information regarding significant litigation matters
and other contingencies related to Talecris below.
At 30 June 2011 the Group has commitments and open purchase
orders for capital spending from Talecris of approximately
US Dollars 114.2 million.
|
|
|
(b)
|
Plasma
Centers of America, LLC and G&M Crandall Limited Family
Partnership
|
On 13 December 2010, a jury in the state court case
rendered a verdict in the amount of US Dollar
37.0 million in favor of Plasma Centers of America, LLC
(PCA) against Talecris Plasma Resources Inc. (TPR) in a breach
of contract claim, which was confirmed by the court in post
trial motions. The Talecris management filed an appeal to the
North Carolina Court of Appeals to review the judgment entered
in this case. The jury verdict, if sustained, will bear simple
interest at 8% per statute from the date of breach, which totals
approximately US Dollars 8.2 million at 30 June
2011, of which US Dollars 1.5 million was accrued
during the six month period ended 30 June 2011 and
US Dollars 6.7 million was accrued during the year
ended 31 December 2010. The acquired net assets of Talecris
Group included US Dollars 45.2 million within current
provisions in the consolidated balance sheet related to the PCA
judgment.
During the first quarter of 2011, the Talecris Group secured an
appeal bond from a surety company in the amount of
US Dollars 25.0 million in regard to this litigation.
|
|
|
(c)
|
Foreign
Corrupt Practices Act
|
The Talecris Group is conducting an internal investigation into
potential violations of the Foreign Corrupt Practices Act (FCPA)
that they became aware of during the conduct of an unrelated
review. The FCPA investigation is being conducted by outside
counsel. The investigation initially focused on sales to certain
Eastern European and Middle Eastern countries, primarily
Belarus, Russia, and Iran, but they are also reviewing sales
practices in Brazil, Bulgaria, China, Georgia, Libya, Poland,
Turkey, Ukraine, and other countries as deemed appropriate.
In July 2009, the Talecris Group voluntarily contacted the
U.S. Department of Justice (DOJ) to advise them of the
investigation and to offer our cooperation in any investigation
that they want to conduct or they want us to conduct. The DOJ
has not indicated what action it may take, if any, against us or
any individual, or the extent to which it may conduct its own
investigation. Even though they self-disclosed this matter to
the DOJ, it or other federal agencies may seek to impose
sanctions on us that may include, among other things, debarment,
injunctive relief, disgorgement, fines, penalties, appointment
of a monitor, appointment of new control staff, or enhancement
of existing compliance and training programs. Other countries in
which the Talecris Group does business may initiate their own
investigations and impose similar penalties. As a result of this
investigation, we suspended shipments to some of these countries
while the Talecris Group put additional safeguards in place. In
some cases, safeguards involved terminating consultants and
suspending relations with or terminating distributors in
countries under investigation as circumstances warranted. The
Talecris Group has resumed sales in countries where the Talecris
Group believes they have appropriate safeguards in place and are
reallocating product to other countries as necessary. To the
extent that they conclude, or the DOJ concludes, that they
cannot implement adequate safeguards or otherwise need to change
our business practices, distributors, or consultants in affected
countries or other countries, this may result in a permanent
loss of business from those countries. The Talecris Group
completed their internal FCPA investigation during the first
quarter of 2011 and made an initial presentation of some of
their findings to the DOJ in July 2011. The
F-26
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
preliminary findings of this investigation indicate that it is
probable that there were FCPA violations by persons associated
with the Talecris Group that the DOJ or other regulators may
assert are attributable to the Group.
Any sanctions or related loss of business could have a material
adverse effect on the Group or our results of operations. It is
possible; however, that any sanctions that DOJ or other federal
agencies might otherwise consider imposing would be reduced, if
not eliminated, in light of the comprehensive compliance
measures that they have implemented. Given the preliminary
nature of our findings, the continuing investigation and the
uncertainties regarding this matter, the Group is unable to
estimate the financial outcome and consequently, has not accrued
any amounts related to the outcome of this matter.
|
|
|
(d)
|
Compliance
with Pharmaceutical Pricing Agreement
|
In November 2009, the Talecris Group received a letter from the
United States Attorney’s Office for the Eastern District of
Pennsylvania (USAO). The USAO requested a meeting to review our
compliance with the terms of the Pharmaceutical Pricing
Agreement (PPA) under the Public Health Service program.
Specifically, the USAO asked for information related to the sale
of our IGIV product, Gamunex, under that program. In order to
have federal financial participation apply to their products
under the Medicaid program and to obtain Medicare Part B
coverage, manufacturers are required to enter into a PPA. The
PPA obligates manufacturers to charge covered entities the
Public Health Service price for drugs intended for outpatient
use. The Public Health Service price is based on the Medicaid
rebate amount. The Group believes that they have complied with
the terms of the PPA and federal law. If the USAO determines
that the Talecris practices are inconsistent with the terms of
the PPA, the USAO has stated that it may file a civil action
against us under the Anti-fraud Injunction Act and seek a court
order directing the company to comply with the PPA or,
potentially, proceed under some other legal theory. The Group
could also be subject to fines, damages, penalties, appointment
of a monitor, or enhancement of existing compliance and training
programs as a result of government action. The Group is
cooperating with the investigation and intend to respond to
information requests from the USAO. Based on the information
obtained to date, the Group have not determined that any
potential liability that may result is probable or can be
reasonably estimated. Therefore, the Group has not made any
accrual in our unaudited condensed consolidated interim
financial statements at 30 June 2011.
Transactions with related parties have been performed as part of
the Groups’ ordinary trade and have been performed at
arm’s length. The sale and lease back transaction with
related parties described in note 7 a) and has
been made at arm’s length.
Group transactions with related parties during the six months
ended 30 June 2011 were as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key Management
|
|
|
Other Related
|
|
|
Board of Directors
|
|
|
|
|
Associates
|
|
|
Personnel
|
|
|
Parties
|
|
|
of the Company
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Net sales
|
|
|
21
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other service expenses
|
|
|
(1,690
|
)
|
|
|
|
|
|
|
(15,045
|
)
|
|
|
(120
|
)
|
|
Personnel expenses
|
|
|
—
|
|
|
|
(3,250
|
)
|
|
|
—
|
|
|
|
(1,168
|
)
|
|
Sales of Property
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Plant and Equipment
|
|
|
—
|
|
|
|
—
|
|
|
|
80,393
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1,669
|
)
|
|
|
(3,250
|
)
|
|
|
65,348
|
|
|
|
(1,288
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-27
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
Condensed Consolidated Interim Financial
Statements — (Continued)
“Other services expenses” include costs for
professional services with related companies amounting to Euros
9,239 thousand. These costs correspond to those incurred in
increasing share capital and the issuance of debt and are
deducted from equity and from financial liabilities.
A director signed a consultancy agreement for a three years
period for which fees amount to US Dollar 1 million
per year and an additional bonus fee of US Dollar
2 million payable upon the fulfilment of certain conditions.
Trade and other receivables at 30 June 2011 include an
amount of Euros 14,471 thousand with related companies.
Group transactions with related parties during the six months
ended 30 June 2010 were as follow:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key Management
|
|
|
Other Related
|
|
|
Board of Directors
|
|
|
|
|
Personnel
|
|
|
Parties
|
|
|
of the company
|
|
|
|
|
Thousand Euros
|
|
|
|
|
Other service expenses
|
|
|
—
|
|
|
|
(5,912
|
)
|
|
|
(90
|
)
|
|
Personnel expenses
|
|
|
(2,931
|
)
|
|
|
—
|
|
|
|
(1,033
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(2,931
|
)
|
|
|
(5,912
|
)
|
|
|
(1,123
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-executive board members representing shareholders interests
have received no remuneration during the six month period ended
on 30 June 2011 and 2010.
The Group has not extended any advances or loans to the members
of the board of directors or key management personnel nor has it
assumed any guarantee commitments on their behalf. It has also
not assumed any pension or life insurance obligations on behalf
of former or current members of the board of directors or key
management personnel.
|
|
|
(18)
|
Condensed
Consolidating Financial Information
|
The High Yield Senior Unsecured Notes mentioned in
note 12(a) were issued by Grifols Inc., which is a
wholly-owned subsidiary of Grifols, S.A., and are jointly and
severally, irrevocably and fully and unconditionally guaranteed
by Grifols, S.A. and certain other of its wholly-owned
subsidiaries (‘the Guarantors’). Supplemental
condensed consolidating financial information is presented in
Appendix I comprising the Group’s income statements
and cash flow statements, both consolidated, for the six month
period ended June 30, 2011 and June 30, 2010 and its
consolidated balance sheets as at June 30, 2011 and
December 31, 2010 showing the amounts attributable to
Grifols, S.A., Grifols Inc. and those of its other subsidiaries
that were Guarantors as at June 30, 2011 separately from
the amounts attributable to those of its subsidiaries that were
not Guarantors. The condensed consolidated financial information
has been prepared and presented pursuant to SEC
Regulation S-X,
Rule 3-10,
“Financial Statements of Guarantors and Issuers of
Guaranteed Securities Registered or Being Registered”,
which is included in Appendix I.
In August 2011 Grifols acquired the remaining
51% outstanding capital stock of Woolloomooloo Holdings Pty
Ltd. the holding company of the Australian-Swiss group,
Lateral-Medion, of which the Company had acquired 49% of the
capital stock and 100% of the voting rights on March 2009 which
will not impact the goodwill. The total sum paid for the
acquisition of the remaining 51% of the capital stock amounts to
AUD 12.5 million (Euros 9.5 million).
The Board of Directors of Grifols, S.A. authorised for issue
these Condensed Consolidated Interim Financial Statements at
their meeting held on 4 October 2011.
F-28
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Assets
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
0
|
|
|
|
0
|
|
|
|
29,895
|
|
|
|
15,123
|
|
|
|
144,430
|
|
|
|
189,448
|
|
|
Other intangible assets
|
|
|
5,731
|
|
|
|
3,162
|
|
|
|
49,120
|
|
|
|
4,725
|
|
|
|
15,561
|
|
|
|
78,299
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
5,731
|
|
|
|
3,162
|
|
|
|
79,015
|
|
|
|
19,848
|
|
|
|
159,991
|
|
|
|
267,747
|
|
|
Property, plant and equipment
|
|
|
95,452
|
|
|
|
28,150
|
|
|
|
231,728
|
|
|
|
78,801
|
|
|
|
0
|
|
|
|
434,131
|
|
|
Investments in Subsidiaries
|
|
|
345,025
|
|
|
|
222,273
|
|
|
|
2,532
|
|
|
|
938
|
|
|
|
(570,768
|
)
|
|
|
0
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
0
|
|
|
|
21,005
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(21,005
|
)
|
|
|
0
|
|
|
Investments in equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
598
|
|
|
|
598
|
|
|
Non-current financial assets
|
|
|
709
|
|
|
|
5,804
|
|
|
|
734
|
|
|
|
288
|
|
|
|
0
|
|
|
|
7,535
|
|
|
Deferred tax assets
|
|
|
1,091
|
|
|
|
2,076
|
|
|
|
9,534
|
|
|
|
2,932
|
|
|
|
19,256
|
|
|
|
34,889
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
448,008
|
|
|
|
282,470
|
|
|
|
323,543
|
|
|
|
102,807
|
|
|
|
(411,928
|
)
|
|
|
744,900
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
|
796
|
|
|
|
0
|
|
|
|
538,311
|
|
|
|
59,401
|
|
|
|
(70,643
|
)
|
|
|
527,865
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade receivables
|
|
|
8,946
|
|
|
|
11,561
|
|
|
|
209,758
|
|
|
|
100,719
|
|
|
|
(106,629
|
)
|
|
|
224,355
|
|
|
Other receivables
|
|
|
3,157
|
|
|
|
201
|
|
|
|
27,612
|
|
|
|
11,971
|
|
|
|
1,091
|
|
|
|
44,032
|
|
|
Current income tax assets
|
|
|
6,168
|
|
|
|
6,071
|
|
|
|
297
|
|
|
|
2,071
|
|
|
|
0
|
|
|
|
14,607
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
|
18,271
|
|
|
|
17,833
|
|
|
|
237,667
|
|
|
|
114,761
|
|
|
|
(105,538
|
)
|
|
|
282,994
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
238,262
|
|
|
|
(1,311
|
)
|
|
|
14,699
|
|
|
|
22,860
|
|
|
|
(274,510
|
)
|
|
|
0
|
|
|
Other current financial assets
|
|
|
267
|
|
|
|
224
|
|
|
|
8
|
|
|
|
12,447
|
|
|
|
0
|
|
|
|
12,946
|
|
|
Other current assets
|
|
|
13,460
|
|
|
|
60,568
|
|
|
|
5,150
|
|
|
|
1,450
|
|
|
|
0
|
|
|
|
80,628
|
|
|
Cash and cash equivalents
|
|
|
25
|
|
|
|
227,456
|
|
|
|
1,444
|
|
|
|
10,724
|
|
|
|
0
|
|
|
|
239,649
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
271,081
|
|
|
|
304,770
|
|
|
|
797,279
|
|
|
|
221,643
|
|
|
|
(450,691
|
)
|
|
|
1,144,082
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
719,089
|
|
|
|
587,240
|
|
|
|
1,120,822
|
|
|
|
324,450
|
|
|
|
(862,619
|
)
|
|
|
1,888,982
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-29
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Equity and Liabilities
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
106,532
|
|
|
|
0
|
|
|
|
21,497
|
|
|
|
36,340
|
|
|
|
(57,837
|
)
|
|
|
106,532
|
|
|
Share premium
|
|
|
121,802
|
|
|
|
72,932
|
|
|
|
106,854
|
|
|
|
5,703
|
|
|
|
(185,489
|
)
|
|
|
121,802
|
|
|
Reserves
|
|
|
73,076
|
|
|
|
190,844
|
|
|
|
175,221
|
|
|
|
59,636
|
|
|
|
(95,173
|
)
|
|
|
403,604
|
|
|
Own shares
|
|
|
(1,927
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
Interim dividend
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(152
|
)
|
|
|
152
|
|
|
|
0
|
|
|
Profit for the year attributable to the Parent
|
|
|
63,226
|
|
|
|
(10,726
|
)
|
|
|
112,853
|
|
|
|
23,906
|
|
|
|
(73,746
|
)
|
|
|
115,513
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
362,709
|
|
|
|
253,050
|
|
|
|
416,425
|
|
|
|
125,433
|
|
|
|
(412,093
|
)
|
|
|
745,524
|
|
|
Cash flow hedges
|
|
|
0
|
|
|
|
(1,751
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,751
|
)
|
|
Translation differences
|
|
|
0
|
|
|
|
20,449
|
|
|
|
(18,344
|
)
|
|
|
11,123
|
|
|
|
(63,961
|
)
|
|
|
(50,733
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
0
|
|
|
|
18,698
|
|
|
|
(18,344
|
)
|
|
|
11,123
|
|
|
|
(63,961
|
)
|
|
|
(52,484
|
)
|
|
Equity attributable to the Parent
|
|
|
362,709
|
|
|
|
271,748
|
|
|
|
398,081
|
|
|
|
136,556
|
|
|
|
(476,054
|
)
|
|
|
693,040
|
|
|
Non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
14,350
|
|
|
|
14,350
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
362,709
|
|
|
|
271,748
|
|
|
|
398,081
|
|
|
|
136,556
|
|
|
|
(461,704
|
)
|
|
|
707,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
142
|
|
|
|
187
|
|
|
|
1,683
|
|
|
|
76
|
|
|
|
0
|
|
|
|
2,088
|
|
|
Provisions
|
|
|
0
|
|
|
|
0
|
|
|
|
1,127
|
|
|
|
251
|
|
|
|
0
|
|
|
|
1,378
|
|
|
Non-current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
133,982
|
|
|
|
441,203
|
|
|
|
40,350
|
|
|
|
49,695
|
|
|
|
155
|
|
|
|
665,385
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
15,875
|
|
|
|
0
|
|
|
|
0
|
|
|
|
5,130
|
|
|
|
(21,005
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
200
|
|
|
|
0
|
|
|
|
9,596
|
|
|
|
678
|
|
|
|
0
|
|
|
|
10,474
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities
|
|
|
150,057
|
|
|
|
441,203
|
|
|
|
49,946
|
|
|
|
55,503
|
|
|
|
(20,850
|
)
|
|
|
675,859
|
|
|
Deferred tax liabilities
|
|
|
11,907
|
|
|
|
2,860
|
|
|
|
62,718
|
|
|
|
1,519
|
|
|
|
137
|
|
|
|
79,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
162,106
|
|
|
|
444,250
|
|
|
|
115,474
|
|
|
|
57,349
|
|
|
|
(20,713
|
)
|
|
|
758,466
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provisions
|
|
|
257
|
|
|
|
0
|
|
|
|
30
|
|
|
|
4,078
|
|
|
|
0
|
|
|
|
4,365
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
other marketable securities
|
|
|
103,131
|
|
|
|
7,364
|
|
|
|
41,433
|
|
|
|
39,862
|
|
|
|
(155
|
)
|
|
|
191,635
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
42,863
|
|
|
|
(162,772
|
)
|
|
|
385,947
|
|
|
|
9,017
|
|
|
|
(275,055
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
8,830
|
|
|
|
0
|
|
|
|
9,316
|
|
|
|
90
|
|
|
|
0
|
|
|
|
18,236
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities
|
|
|
154,824
|
|
|
|
(155,408
|
)
|
|
|
436,696
|
|
|
|
48,969
|
|
|
|
(275,210
|
)
|
|
|
209,871
|
|
|
Debts with associates
|
|
|
1,162
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
1,162
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
33,426
|
|
|
|
24,766
|
|
|
|
146,861
|
|
|
|
60,617
|
|
|
|
(104,992
|
)
|
|
|
160,678
|
|
|
Other payables
|
|
|
1,141
|
|
|
|
12
|
|
|
|
5,288
|
|
|
|
3,627
|
|
|
|
1,860
|
|
|
|
11,928
|
|
|
Current income tax liabilities
|
|
|
0
|
|
|
|
0
|
|
|
|
2,369
|
|
|
|
3,663
|
|
|
|
(1,860
|
)
|
|
|
4,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables
|
|
|
34,567
|
|
|
|
24,778
|
|
|
|
154,518
|
|
|
|
67,907
|
|
|
|
(104,992
|
)
|
|
|
176,778
|
|
|
Other current liabilities
|
|
|
3,464
|
|
|
|
1,872
|
|
|
|
16,023
|
|
|
|
9,591
|
|
|
|
0
|
|
|
|
30,950
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
194,274
|
|
|
|
(128,758
|
)
|
|
|
607,267
|
|
|
|
130,545
|
|
|
|
(380,202
|
)
|
|
|
423,126
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
356,380
|
|
|
|
315,492
|
|
|
|
722,741
|
|
|
|
187,894
|
|
|
|
(400,915
|
)
|
|
|
1,181,592
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity and liabilities
|
|
|
719,089
|
|
|
|
587,240
|
|
|
|
1,120,822
|
|
|
|
324,450
|
|
|
|
(862,619
|
)
|
|
|
1,888,982
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-30
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
30 June 2011
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Assets
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of euros)
|
|
|
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
0
|
|
|
|
0
|
|
|
|
147,240
|
|
|
|
14,884
|
|
|
|
2,119,572
|
|
|
|
2,281,696
|
|
|
Other intangible assets
|
|
|
4,856
|
|
|
|
4,668
|
|
|
|
97,924
|
|
|
|
5,638
|
|
|
|
15,388
|
|
|
|
128,474
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
4,856
|
|
|
|
4,668
|
|
|
|
245,164
|
|
|
|
20,522
|
|
|
|
2,134,960
|
|
|
|
2,410,170
|
|
|
Property, plant and equipment
|
|
|
60,159
|
|
|
|
25,515
|
|
|
|
517,262
|
|
|
|
36,799
|
|
|
|
0
|
|
|
|
639,735
|
|
|
Investments in Subsidiaries
|
|
|
1,132,109
|
|
|
|
2,790,820
|
|
|
|
19,856
|
|
|
|
1,654
|
|
|
|
(3,944,439
|
)
|
|
|
0
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
0
|
|
|
|
0
|
|
|
|
92,597
|
|
|
|
0
|
|
|
|
(92,597
|
)
|
|
|
0
|
|
|
Investments in equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
2,283
|
|
|
|
0
|
|
|
|
1,263
|
|
|
|
3,546
|
|
|
Non-current financial assets
|
|
|
756
|
|
|
|
37,830
|
|
|
|
2,261
|
|
|
|
820
|
|
|
|
0
|
|
|
|
41,667
|
|
|
Deferred tax assets
|
|
|
1,538
|
|
|
|
28,162
|
|
|
|
91,897
|
|
|
|
3,149
|
|
|
|
14,689
|
|
|
|
139,435
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
1,199,418
|
|
|
|
2,886,995
|
|
|
|
971,320
|
|
|
|
62,944
|
|
|
|
(1,886,124
|
)
|
|
|
3,234,553
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
|
861
|
|
|
|
0
|
|
|
|
992,913
|
|
|
|
72,362
|
|
|
|
(68,310
|
)
|
|
|
997,826
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade receivables
|
|
|
22,331
|
|
|
|
38,346
|
|
|
|
485,460
|
|
|
|
105,548
|
|
|
|
(246,235
|
)
|
|
|
405,450
|
|
|
Other receivables
|
|
|
1,452
|
|
|
|
171
|
|
|
|
32,683
|
|
|
|
14,665
|
|
|
|
0
|
|
|
|
48,971
|
|
|
Current income tax assets
|
|
|
7,054
|
|
|
|
29,059
|
|
|
|
2,340
|
|
|
|
2,576
|
|
|
|
0
|
|
|
|
41,029
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
|
30,837
|
|
|
|
67,576
|
|
|
|
520,483
|
|
|
|
122,789
|
|
|
|
(246,235
|
)
|
|
|
495,450
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
457,222
|
|
|
|
384,790
|
|
|
|
15,364
|
|
|
|
21,536
|
|
|
|
(878,912
|
)
|
|
|
0
|
|
|
Other current financial assets
|
|
|
7,422
|
|
|
|
211
|
|
|
|
10
|
|
|
|
11,611
|
|
|
|
0
|
|
|
|
19,254
|
|
|
Other current assets
|
|
|
1,929
|
|
|
|
235
|
|
|
|
12,201
|
|
|
|
2,154
|
|
|
|
(3,175
|
)
|
|
|
13,344
|
|
|
Cash and cash equivalents
|
|
|
46,027
|
|
|
|
40,056
|
|
|
|
486,482
|
|
|
|
11,228
|
|
|
|
(1
|
)
|
|
|
583,792
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
544,298
|
|
|
|
492,868
|
|
|
|
2,027,453
|
|
|
|
241,680
|
|
|
|
(1,196,633
|
)
|
|
|
2,109,666
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
1,743,716
|
|
|
|
3,379,863
|
|
|
|
2,998,773
|
|
|
|
304,624
|
|
|
|
(3,082,757
|
)
|
|
|
5,344,219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
accompanying note forms an integral part of the condensed
consolidated interim financial statements
F-31
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
30 June 2011
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Equity and liabilities
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of euros)
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
114,914
|
|
|
|
0
|
|
|
|
45,570
|
|
|
|
36,340
|
|
|
|
(81,910
|
)
|
|
|
114,914
|
|
|
Share premium
|
|
|
890,355
|
|
|
|
849,867
|
|
|
|
623,197
|
|
|
|
6,125
|
|
|
|
(1,479,189
|
)
|
|
|
890,355
|
|
|
Reserves
|
|
|
134,038
|
|
|
|
232,983
|
|
|
|
310,167
|
|
|
|
77,088
|
|
|
|
(184,594
|
)
|
|
|
569,682
|
|
|
Own shares
|
|
|
(1,927
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
Profit for the period/ year attributable to the Parent
|
|
|
31,802
|
|
|
|
(22,950
|
)
|
|
|
81,268
|
|
|
|
(505
|
)
|
|
|
(70,346
|
)
|
|
|
19,269
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
1,169,182
|
|
|
|
1,059,900
|
|
|
|
1,060,202
|
|
|
|
119,048
|
|
|
|
(1,816,039
|
)
|
|
|
1,592,293
|
|
|
Available-for-sale
financial assets
|
|
|
(575
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(575
|
)
|
|
Cash flow hedges
|
|
|
0
|
|
|
|
(2,331
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(2,331
|
)
|
|
Translation differences
|
|
|
0
|
|
|
|
(2,219
|
)
|
|
|
(40,085
|
)
|
|
|
5,838
|
|
|
|
(52,268
|
)
|
|
|
(88,734
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income
|
|
|
(575
|
)
|
|
|
(4,550
|
)
|
|
|
(40,085
|
)
|
|
|
5,838
|
|
|
|
(52,268
|
)
|
|
|
(91,640
|
)
|
|
Equity attributable to the Parent
|
|
|
1,168,607
|
|
|
|
1,055,350
|
|
|
|
1,020,117
|
|
|
|
124,886
|
|
|
|
(1,868,307
|
)
|
|
|
1,500,653
|
|
|
Non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
12,941
|
|
|
|
12,941
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
1,168,607
|
|
|
|
1,055,350
|
|
|
|
1,020,117
|
|
|
|
124,886
|
|
|
|
(1,855,366
|
)
|
|
|
1,513,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
159
|
|
|
|
170
|
|
|
|
1,408
|
|
|
|
78
|
|
|
|
0
|
|
|
|
1,815
|
|
|
Provisions
|
|
|
0
|
|
|
|
0
|
|
|
|
10,211
|
|
|
|
250
|
|
|
|
0
|
|
|
|
10,461
|
|
|
Non-current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
other marketable securities
|
|
|
405,262
|
|
|
|
2,200,801
|
|
|
|
36,728
|
|
|
|
279
|
|
|
|
(126
|
)
|
|
|
2,642,944
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
0
|
|
|
|
0
|
|
|
|
479,210
|
|
|
|
0
|
|
|
|
(479,210
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
4,869
|
|
|
|
57,016
|
|
|
|
9,792
|
|
|
|
725
|
|
|
|
(2
|
)
|
|
|
72,400
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities
|
|
|
410,131
|
|
|
|
2,257,817
|
|
|
|
525,730
|
|
|
|
1,004
|
|
|
|
(479,338
|
)
|
|
|
2,715,344
|
|
|
Deferred tax liabilities
|
|
|
8,625
|
|
|
|
45,540
|
|
|
|
83,551
|
|
|
|
2,218
|
|
|
|
141
|
|
|
|
140,075
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
418,915
|
|
|
|
2,303,527
|
|
|
|
620,900
|
|
|
|
3,550
|
|
|
|
(479,197
|
)
|
|
|
2,867,695
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provisions
|
|
|
341
|
|
|
|
0
|
|
|
|
30
|
|
|
|
4,204
|
|
|
|
31,253
|
|
|
|
35,828
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
13,330
|
|
|
|
(20,002
|
)
|
|
|
469,601
|
|
|
|
44,445
|
|
|
|
0
|
|
|
|
507,374
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
36,901
|
|
|
|
22,625
|
|
|
|
390,957
|
|
|
|
41,816
|
|
|
|
(492,299
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
1,330
|
|
|
|
3,829
|
|
|
|
11,937
|
|
|
|
240
|
|
|
|
0
|
|
|
|
17,336
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities
|
|
|
51,561
|
|
|
|
6,452
|
|
|
|
872,495
|
|
|
|
86,501
|
|
|
|
(492,299
|
)
|
|
|
524,710
|
|
|
Debts with associates
|
|
|
2,352
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
2,352
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
82,788
|
|
|
|
14,222
|
|
|
|
357,739
|
|
|
|
67,540
|
|
|
|
(255,896
|
)
|
|
|
266,393
|
|
|
Other payables
|
|
|
13,511
|
|
|
|
10
|
|
|
|
6,911
|
|
|
|
3,466
|
|
|
|
1,720
|
|
|
|
25,618
|
|
|
Current income tax liabilities
|
|
|
2,327
|
|
|
|
(976
|
)
|
|
|
24,144
|
|
|
|
3,452
|
|
|
|
(1,720
|
)
|
|
|
27,227
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables
|
|
|
98,626
|
|
|
|
13,256
|
|
|
|
388,794
|
|
|
|
74,458
|
|
|
|
(255,896
|
)
|
|
|
319,238
|
|
|
Other current liabilities
|
|
|
3,314
|
|
|
|
1,278
|
|
|
|
96,437
|
|
|
|
11,025
|
|
|
|
(31,252
|
)
|
|
|
80,802
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
156,194
|
|
|
|
20,986
|
|
|
|
1,357,756
|
|
|
|
176,188
|
|
|
|
(748,194
|
)
|
|
|
962,930
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
575,109
|
|
|
|
2,324,513
|
|
|
|
1,978,656
|
|
|
|
179,738
|
|
|
|
(1,227,391
|
)
|
|
|
3,830,625
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity and liabilities
|
|
|
1,743,716
|
|
|
|
3,379,863
|
|
|
|
2,998,773
|
|
|
|
304,624
|
|
|
|
(3,082,757
|
)
|
|
|
5,344,219
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The
accompanying note forms an integral part of the condensed
consolidated interim financial statements
F-32
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Income Statements
for the
Six Month Period Ended 30 June 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Profit and loss
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
34,477
|
|
|
|
10,887
|
|
|
|
684,918
|
|
|
|
218,344
|
|
|
|
(460,817
|
)
|
|
|
487,809
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
0
|
|
|
|
0
|
|
|
|
16,172
|
|
|
|
(1,200
|
)
|
|
|
26,237
|
|
|
|
41,209
|
|
|
Self-constructed non-current assets
|
|
|
259
|
|
|
|
529
|
|
|
|
4,340
|
|
|
|
(500
|
)
|
|
|
11,423
|
|
|
|
16,051
|
|
|
Supplies
|
|
|
(80
|
)
|
|
|
0
|
|
|
|
(369,205
|
)
|
|
|
(123,511
|
)
|
|
|
335,689
|
|
|
|
(157,107
|
)
|
|
Other operating income
|
|
|
41
|
|
|
|
0
|
|
|
|
535
|
|
|
|
55
|
|
|
|
0
|
|
|
|
631
|
|
|
Personnel expenses
|
|
|
(11,439
|
)
|
|
|
(6,398
|
)
|
|
|
(93,855
|
)
|
|
|
(30,280
|
)
|
|
|
0
|
|
|
|
(141,972
|
)
|
|
Other operating expenses
|
|
|
(15,026
|
)
|
|
|
(3,470
|
)
|
|
|
(132,985
|
)
|
|
|
(37,136
|
)
|
|
|
90,337
|
|
|
|
(98,279
|
)
|
|
Amortisation and depreciation
|
|
|
(3,634
|
)
|
|
|
(475
|
)
|
|
|
(13,231
|
)
|
|
|
(3,917
|
)
|
|
|
(177
|
)
|
|
|
(21,434
|
)
|
|
Transaction costs of Talecris business combination
|
|
|
(322
|
)
|
|
|
(1,678
|
)
|
|
|
(14
|
)
|
|
|
(4
|
)
|
|
|
0
|
|
|
|
(2,019
|
)
|
|
Non-financial and other capital grants
|
|
|
323
|
|
|
|
0
|
|
|
|
227
|
|
|
|
0
|
|
|
|
0
|
|
|
|
550
|
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
10
|
|
|
|
(1
|
)
|
|
|
(68
|
)
|
|
|
(362
|
)
|
|
|
1,102
|
|
|
|
681
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
4,609
|
|
|
|
(606
|
)
|
|
|
96,834
|
|
|
|
21,489
|
|
|
|
3,794
|
|
|
|
126,120
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
1,875
|
|
|
|
7,158
|
|
|
|
1,468
|
|
|
|
355
|
|
|
|
(8,677
|
)
|
|
|
2,179
|
|
|
Dividends
|
|
|
56,774
|
|
|
|
0
|
|
|
|
0
|
|
|
|
159
|
|
|
|
(56,933
|
)
|
|
|
0
|
|
|
Finance expense
|
|
|
(3,868
|
)
|
|
|
(15,610
|
)
|
|
|
(11,955
|
)
|
|
|
(2,534
|
)
|
|
|
8,682
|
|
|
|
(25,285
|
)
|
|
Change in fair value of financial instruments
|
|
|
(15,540
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
3
|
|
|
|
133
|
|
|
|
(15,404
|
)
|
|
Gains/ (losses) on disposal of financial instruments
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(720
|
)
|
|
|
720
|
|
|
|
0
|
|
|
Exchange gains/ (losses)
|
|
|
120
|
|
|
|
(910
|
)
|
|
|
37
|
|
|
|
2,723
|
|
|
|
0
|
|
|
|
1,970
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Finance expense
|
|
|
39,361
|
|
|
|
(9,362
|
)
|
|
|
(10,450
|
)
|
|
|
(14
|
)
|
|
|
(56,075
|
)
|
|
|
(36,540
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(728
|
)
|
|
|
(728
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
43,970
|
|
|
|
(9,968
|
)
|
|
|
86,384
|
|
|
|
21,475
|
|
|
|
(53,009
|
)
|
|
|
88,852
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
4,510
|
|
|
|
3,820
|
|
|
|
(22,296
|
)
|
|
|
(5,699
|
)
|
|
|
(3,357
|
)
|
|
|
(23,022
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
48,480
|
|
|
|
(6,148
|
)
|
|
|
64,088
|
|
|
|
15,776
|
|
|
|
(56,366
|
)
|
|
|
65,830
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
48,480
|
|
|
|
(6,148
|
)
|
|
|
64,175
|
|
|
|
15,776
|
|
|
|
(55,875
|
)
|
|
|
66,408
|
|
|
Loss attributable to non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
(87
|
)
|
|
|
0
|
|
|
|
(491
|
)
|
|
|
(578
|
)
|
The
accompanying note forms an integral part of the condensed
consolidated interim financial statements
F-33
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Income Statements
for the
Six Month Period Ended 30 June 2011
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Profit and loss
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
28,108
|
|
|
|
11,547
|
|
|
|
872,666
|
|
|
|
218,750
|
|
|
|
(495,730
|
)
|
|
|
635,341
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
0
|
|
|
|
0
|
|
|
|
(907
|
)
|
|
|
2,598
|
|
|
|
1,066
|
|
|
|
2,757
|
|
|
Self-constructed non-current assets
|
|
|
288
|
|
|
|
792
|
|
|
|
23,826
|
|
|
|
2,492
|
|
|
|
4,948
|
|
|
|
32,346
|
|
|
Supplies
|
|
|
(234
|
)
|
|
|
0
|
|
|
|
(438,787
|
)
|
|
|
(136,825
|
)
|
|
|
400,704
|
|
|
|
(175,142
|
)
|
|
Other operating income
|
|
|
39
|
|
|
|
0
|
|
|
|
911
|
|
|
|
59
|
|
|
|
0
|
|
|
|
1,009
|
|
|
Personnel expenses
|
|
|
(13,045
|
)
|
|
|
(7,390
|
)
|
|
|
(130,255
|
)
|
|
|
(33,037
|
)
|
|
|
0
|
|
|
|
(183,727
|
)
|
|
Other operating expenses
|
|
|
(17,211
|
)
|
|
|
(4,642
|
)
|
|
|
(176,397
|
)
|
|
|
(38,666
|
)
|
|
|
81,384
|
|
|
|
(155,532
|
)
|
|
Amortisation and depreciation
|
|
|
(3,417
|
)
|
|
|
(506
|
)
|
|
|
(19,737
|
)
|
|
|
(4,129
|
)
|
|
|
(367
|
)
|
|
|
(28,156
|
)
|
|
Transaction costs of Talecris business combination
|
|
|
(38,462
|
)
|
|
|
(145
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(38,607
|
)
|
|
Non-financial and other capital grants
|
|
|
333
|
|
|
|
0
|
|
|
|
409
|
|
|
|
0
|
|
|
|
0
|
|
|
|
742
|
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
574
|
|
|
|
0
|
|
|
|
(4,379
|
)
|
|
|
(5,497
|
)
|
|
|
(13,000
|
)
|
|
|
(22,302
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
(43,027
|
)
|
|
|
(344
|
)
|
|
|
127,350
|
|
|
|
5,745
|
|
|
|
(20,995
|
)
|
|
|
68,729
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
3,446
|
|
|
|
7,804
|
|
|
|
1,407
|
|
|
|
697
|
|
|
|
(11,593
|
)
|
|
|
1,761
|
|
|
Dividends
|
|
|
53,352
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(357
|
)
|
|
|
(52,995
|
)
|
|
|
0
|
|
|
Finance expense
|
|
|
(8,949
|
)
|
|
|
(40,034
|
)
|
|
|
(14,223
|
)
|
|
|
(3,408
|
)
|
|
|
11,068
|
|
|
|
(55,546
|
)
|
|
Change in fair value of financial instruments
|
|
|
16,023
|
|
|
|
(2,492
|
)
|
|
|
414
|
|
|
|
0
|
|
|
|
0
|
|
|
|
13,945
|
|
|
Gains/ (losses) on disposal of financial instruments
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(772
|
)
|
|
|
772
|
|
|
|
0
|
|
|
Exchange gains/ (losses)
|
|
|
824
|
|
|
|
(1,155
|
)
|
|
|
402
|
|
|
|
(2,193
|
)
|
|
|
0
|
|
|
|
(2,122
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Finance expense
|
|
|
64,696
|
|
|
|
(35,877
|
)
|
|
|
(12,000
|
)
|
|
|
(6,033
|
)
|
|
|
(52,748
|
)
|
|
|
(41,962
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of loss of equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
37
|
|
|
|
0
|
|
|
|
(844
|
)
|
|
|
(807
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
21,669
|
|
|
|
(36,221
|
)
|
|
|
115,387
|
|
|
|
(288
|
)
|
|
|
(74,587
|
)
|
|
|
25,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
10,133
|
|
|
|
13,271
|
|
|
|
(34,119
|
)
|
|
|
(217
|
)
|
|
|
3,585
|
|
|
|
(7,347
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
31,802
|
|
|
|
(22,950
|
)
|
|
|
81,268
|
|
|
|
(505
|
)
|
|
|
(71,002
|
)
|
|
|
18,613
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
31,802
|
|
|
|
(22,950
|
)
|
|
|
81,268
|
|
|
|
(505
|
)
|
|
|
(70,346
|
)
|
|
|
19,269
|
|
|
Loss attributable to non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(656
|
)
|
|
|
(656
|
)
|
The
accompanying note forms an integral part of the condensed
consolidated interim financial statements
F-34
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statement of Cash Flows
for the
Six Month Ended 30 June 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before tax
|
|
|
43,970
|
|
|
|
(9,968
|
)
|
|
|
86,384
|
|
|
|
21,475
|
|
|
|
(53,009
|
)
|
|
|
88,852
|
|
|
Adjustments for:
|
|
|
(36,258
|
)
|
|
|
53,117
|
|
|
|
(14,924
|
)
|
|
|
992
|
|
|
|
50,855
|
|
|
|
53,782
|
|
|
Amortisation and depreciation
|
|
|
3,634
|
|
|
|
475
|
|
|
|
13,231
|
|
|
|
3,917
|
|
|
|
177
|
|
|
|
21,434
|
|
|
Other adjustments:
|
|
|
(39,892
|
)
|
|
|
52,642
|
|
|
|
(28,155
|
)
|
|
|
(2,925
|
)
|
|
|
50,678
|
|
|
|
32,348
|
|
|
Losses on equity accounted investments
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
728
|
|
|
|
728
|
|
|
Exchange differences
|
|
|
(120
|
)
|
|
|
910
|
|
|
|
(37
|
)
|
|
|
(2,723
|
)
|
|
|
0
|
|
|
|
(1,970
|
)
|
|
Net provision charges
|
|
|
0
|
|
|
|
0
|
|
|
|
222
|
|
|
|
611
|
|
|
|
(704
|
)
|
|
|
129
|
|
|
(Profit) / loss on disposal of fixed assets
|
|
|
(10
|
)
|
|
|
1
|
|
|
|
68
|
|
|
|
362
|
|
|
|
(1,102
|
)
|
|
|
(681
|
)
|
|
Government grants taken to income
|
|
|
(323
|
)
|
|
|
0
|
|
|
|
(227
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(550
|
)
|
|
Finance expense / income
|
|
|
(39,453
|
)
|
|
|
15,292
|
|
|
|
(1,275
|
)
|
|
|
1,924
|
|
|
|
56,898
|
|
|
|
33,386
|
|
|
Other adjustments
|
|
|
14
|
|
|
|
36,439
|
|
|
|
(26,906
|
)
|
|
|
(3,099
|
)
|
|
|
(5,142
|
)
|
|
|
1,306
|
|
|
Changes in capital and assets
|
|
|
(10,057
|
)
|
|
|
(5,934
|
)
|
|
|
(153,089
|
)
|
|
|
(5,655
|
)
|
|
|
188,435
|
|
|
|
13,700
|
|
|
Change in inventories
|
|
|
(185
|
)
|
|
|
0
|
|
|
|
(15,656
|
)
|
|
|
1,417
|
|
|
|
2,442
|
|
|
|
(11,982
|
)
|
|
Change in trade and other receivables
|
|
|
1,174
|
|
|
|
178,830
|
|
|
|
84,421
|
|
|
|
13,813
|
|
|
|
(257,999
|
)
|
|
|
20,239
|
|
|
Change in current financial assets and other current assets
|
|
|
(14,769
|
)
|
|
|
(154,124
|
)
|
|
|
(6,038
|
)
|
|
|
(15,249
|
)
|
|
|
186,305
|
|
|
|
(3,875
|
)
|
|
Change in current trade and other payables
|
|
|
3,723
|
|
|
|
(30,640
|
)
|
|
|
(215,816
|
)
|
|
|
(5,636
|
)
|
|
|
257,687
|
|
|
|
9,318
|
|
|
Other cash flows from operating activities
|
|
|
59,972
|
|
|
|
(10,669
|
)
|
|
|
(18,577
|
)
|
|
|
(7,085
|
)
|
|
|
(58,106
|
)
|
|
|
(34,465
|
)
|
|
Interest paid
|
|
|
(2,978
|
)
|
|
|
(15,528
|
)
|
|
|
(686
|
)
|
|
|
(1,153
|
)
|
|
|
544
|
|
|
|
(19,801
|
)
|
|
Interest recovered
|
|
|
1,876
|
|
|
|
540
|
|
|
|
3,301
|
|
|
|
20
|
|
|
|
(1,876
|
)
|
|
|
3,861
|
|
|
Dividends received
|
|
|
56,774
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(56,774
|
)
|
|
|
0
|
|
|
Income tax recovered
|
|
|
4,300
|
|
|
|
4,319
|
|
|
|
(21,192
|
)
|
|
|
(5,952
|
)
|
|
|
0
|
|
|
|
(18,525
|
)
|
|
Net cash from operating activities
|
|
|
57,627
|
|
|
|
26,546
|
|
|
|
(100,206
|
)
|
|
|
9,727
|
|
|
|
128,175
|
|
|
|
121,869
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(5,294
|
)
|
|
|
(10,008
|
)
|
|
|
(33,253
|
)
|
|
|
(10,198
|
)
|
|
|
1,757
|
|
|
|
(56,997
|
)
|
|
Group companies and business units
|
|
|
(2,263
|
)
|
|
|
0
|
|
|
|
(535
|
)
|
|
|
(1,472
|
)
|
|
|
543
|
|
|
|
(3,727
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(3,013
|
)
|
|
|
(5,915
|
)
|
|
|
(32,734
|
)
|
|
|
(8,702
|
)
|
|
|
1,214
|
|
|
|
(49,151
|
)
|
|
Property, plant and equipment
|
|
|
(2,014
|
)
|
|
|
(4,997
|
)
|
|
|
(29,785
|
)
|
|
|
(7,519
|
)
|
|
|
1,169
|
|
|
|
(43,146
|
)
|
|
Intangible assets
|
|
|
(999
|
)
|
|
|
(918
|
)
|
|
|
(2,950
|
)
|
|
|
(1,183
|
)
|
|
|
45
|
|
|
|
(6,005
|
)
|
|
Other financial assets
|
|
|
(18
|
)
|
|
|
(4,093
|
)
|
|
|
16
|
|
|
|
(24
|
)
|
|
|
0
|
|
|
|
(4,119
|
)
|
|
Proceeds from the sale of property, plant and equipment
|
|
|
382
|
|
|
|
0
|
|
|
|
(1,467
|
)
|
|
|
3,980
|
|
|
|
(32
|
)
|
|
|
2,863
|
|
|
Group companies and business units
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,464
|
)
|
|
|
1,423
|
|
|
|
41
|
|
|
|
0
|
|
|
Property, plant and equipment
|
|
|
382
|
|
|
|
0
|
|
|
|
(3
|
)
|
|
|
2,557
|
|
|
|
(73
|
)
|
|
|
2,863
|
|
|
Net cash used in investing activities
|
|
|
(4,912
|
)
|
|
|
(10,008
|
)
|
|
|
(34,720
|
)
|
|
|
(6,218
|
)
|
|
|
1,725
|
|
|
|
(54,134
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
540
|
|
|
|
(540
|
)
|
|
|
(1,250
|
)
|
|
Issue
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
540
|
|
|
|
(540
|
)
|
|
|
0
|
|
|
Acquisition of own shares
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,250
|
)
|
|
Proceeds from and payments for financial liability instruments
|
|
|
(26,358
|
)
|
|
|
3,364
|
|
|
|
191,273
|
|
|
|
9,182
|
|
|
|
(186,132
|
)
|
|
|
(8,671
|
)
|
|
Issue
|
|
|
(299
|
)
|
|
|
(184
|
)
|
|
|
29,580
|
|
|
|
21,970
|
|
|
|
0
|
|
|
|
51,067
|
|
|
Redemption and repayment
|
|
|
(28,016
|
)
|
|
|
(5,417
|
)
|
|
|
(24,265
|
)
|
|
|
(2,040
|
)
|
|
|
0
|
|
|
|
(59,738
|
)
|
|
Debts with group companies
|
|
|
1,957
|
|
|
|
8,965
|
|
|
|
185,958
|
|
|
|
(10,748
|
)
|
|
|
(186,132
|
)
|
|
|
0
|
|
|
Dividends and interest on other equity instruments paid
|
|
|
0
|
|
|
|
0
|
|
|
|
(51,079
|
)
|
|
|
(5,748
|
)
|
|
|
56,774
|
|
|
|
(53
|
)
|
|
Other cash flows from financing activities
|
|
|
324
|
|
|
|
0
|
|
|
|
(1
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
323
|
|
|
Transaction costs of financial instruments issued in the
acquisition of Talecris
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Other amounts received from financing activities
|
|
|
324
|
|
|
|
0
|
|
|
|
(1
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
323
|
|
|
Net cash from / (used in) financing activities
|
|
|
(27,284
|
)
|
|
|
3,364
|
|
|
|
140,193
|
|
|
|
3,974
|
|
|
|
(129,898
|
)
|
|
|
(9,651
|
)
|
|
Effect of exchange rate fluctuations on cash
|
|
|
0
|
|
|
|
41,374
|
|
|
|
928
|
|
|
|
384
|
|
|
|
(2
|
)
|
|
|
42,684
|
|
|
Net increase in cash and cash equivalents
|
|
|
25,431
|
|
|
|
61,276
|
|
|
|
6,195
|
|
|
|
7,866
|
|
|
|
0
|
|
|
|
100,768
|
|
|
Cash and cash equivalents at beginning of the period
|
|
|
144
|
|
|
|
237,804
|
|
|
|
7,191
|
|
|
|
4,233
|
|
|
|
0
|
|
|
|
249,372
|
|
|
Cash and cash equivalents at end of period
|
|
|
25,575
|
|
|
|
299,080
|
|
|
|
13,386
|
|
|
|
12,099
|
|
|
|
0
|
|
|
|
350,140
|
|
The
accompanying note forms an integral part of the condensed
consolidated interim financial statements
F-35
Appendix I
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statement of Cash Flows
for the
Six Month Ended 30 June 2011
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before tax
|
|
|
21,669
|
|
|
|
(36,221
|
)
|
|
|
115,387
|
|
|
|
(288
|
)
|
|
|
(74,587
|
)
|
|
|
25,960
|
|
|
Adjustments for:
|
|
|
(64,381
|
)
|
|
|
43,007
|
|
|
|
(5,099
|
)
|
|
|
29,999
|
|
|
|
89,112
|
|
|
|
92,638
|
|
|
Amortisation and depreciation
|
|
|
3,417
|
|
|
|
506
|
|
|
|
19,737
|
|
|
|
4,129
|
|
|
|
367
|
|
|
|
28,156
|
|
|
Other adjustments:
|
|
|
(67,798
|
)
|
|
|
42,501
|
|
|
|
(24,836
|
)
|
|
|
25,870
|
|
|
|
88,745
|
|
|
|
64,482
|
|
|
Losses / (Profit) on equity accounted investments
|
|
|
0
|
|
|
|
0
|
|
|
|
(36
|
)
|
|
|
0
|
|
|
|
843
|
|
|
|
807
|
|
|
Exchange differences
|
|
|
(824
|
)
|
|
|
1,155
|
|
|
|
(402
|
)
|
|
|
2,193
|
|
|
|
0
|
|
|
|
2,122
|
|
|
Net provision charges
|
|
|
0
|
|
|
|
0
|
|
|
|
1,121
|
|
|
|
333
|
|
|
|
13,000
|
|
|
|
14,454
|
|
|
(Profit) / loss on disposal of fixed assets
|
|
|
(574
|
)
|
|
|
0
|
|
|
|
10,006
|
|
|
|
(16
|
)
|
|
|
0
|
|
|
|
9,416
|
|
|
Government grants taken to income
|
|
|
(333
|
)
|
|
|
0
|
|
|
|
(409
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(742
|
)
|
|
Finance expense / income
|
|
|
(66,067
|
)
|
|
|
36,497
|
|
|
|
(32,416
|
)
|
|
|
24,542
|
|
|
|
74,574
|
|
|
|
37,130
|
|
|
Other adjustments
|
|
|
0
|
|
|
|
4,849
|
|
|
|
(2,700
|
)
|
|
|
(1,182
|
)
|
|
|
328
|
|
|
|
1,295
|
|
|
Changes in capital and assets
|
|
|
(173,540
|
)
|
|
|
(392,604
|
)
|
|
|
(58,615
|
)
|
|
|
(17,028
|
)
|
|
|
576,628
|
|
|
|
(65,159
|
)
|
|
Change in inventories
|
|
|
(63
|
)
|
|
|
0
|
|
|
|
29,076
|
|
|
|
(14,981
|
)
|
|
|
(13,280
|
)
|
|
|
752
|
|
|
Change in trade and other receivables
|
|
|
(5,535
|
)
|
|
|
(27,638
|
)
|
|
|
(62,348
|
)
|
|
|
(16,166
|
)
|
|
|
44,726
|
|
|
|
(66,961
|
)
|
|
Change in current financial assets and other current assets
|
|
|
(222,035
|
)
|
|
|
(373,208
|
)
|
|
|
(729
|
)
|
|
|
5,604
|
|
|
|
589,917
|
|
|
|
(451
|
)
|
|
Change in current trade and other payables
|
|
|
54,094
|
|
|
|
8,242
|
|
|
|
(24,614
|
)
|
|
|
8,515
|
|
|
|
(44,735
|
)
|
|
|
1,501
|
|
|
Other cash flows from operating activities
|
|
|
58,291
|
|
|
|
(13,635
|
)
|
|
|
(26,294
|
)
|
|
|
(420
|
)
|
|
|
(54,687
|
)
|
|
|
(36,745
|
)
|
|
Interest paid
|
|
|
(5,498
|
)
|
|
|
(26,733
|
)
|
|
|
(2,727
|
)
|
|
|
(124
|
)
|
|
|
1,061
|
|
|
|
(34,021
|
)
|
|
Interest recovered
|
|
|
2,395
|
|
|
|
126
|
|
|
|
874
|
|
|
|
0
|
|
|
|
(2,396
|
)
|
|
|
999
|
|
|
Dividends received
|
|
|
53,352
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(53,352
|
)
|
|
|
0
|
|
|
Income tax recovered / (paid)
|
|
|
8,042
|
|
|
|
12,972
|
|
|
|
(24,441
|
)
|
|
|
(296
|
)
|
|
|
(0
|
)
|
|
|
(3,723
|
)
|
|
Net cash from operating activities
|
|
|
(157,961
|
)
|
|
|
(399,453
|
)
|
|
|
25,378
|
|
|
|
12,263
|
|
|
|
536,467
|
|
|
|
16,694
|
|
|
Cash flows from investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(4,366
|
)
|
|
|
(1,765,855
|
)
|
|
|
132,900
|
|
|
|
(31,298
|
)
|
|
|
(771
|
)
|
|
|
(1,669,390
|
)
|
|
Group companies and business units
|
|
|
(411
|
)
|
|
|
(1,763,601
|
)
|
|
|
149,693
|
|
|
|
(327
|
)
|
|
|
(771
|
)
|
|
|
(1,615,417
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(3,374
|
)
|
|
|
(2,342
|
)
|
|
|
(16,696
|
)
|
|
|
(30,426
|
)
|
|
|
(0
|
)
|
|
|
(52,838
|
)
|
|
Property, plant and equipment
|
|
|
(2,345
|
)
|
|
|
(576
|
)
|
|
|
(10,876
|
)
|
|
|
(29,044
|
)
|
|
|
(0
|
)
|
|
|
(42,841
|
)
|
|
Intangible assets
|
|
|
(1,029
|
)
|
|
|
(1,766
|
)
|
|
|
(5,820
|
)
|
|
|
(1,382
|
)
|
|
|
0
|
|
|
|
(9,997
|
)
|
|
Other financial assets
|
|
|
(581
|
)
|
|
|
88
|
|
|
|
(97
|
)
|
|
|
(545
|
)
|
|
|
0
|
|
|
|
(1,135
|
)
|
|
Proceeds from the sale of investments
|
|
|
26,472
|
|
|
|
(1
|
)
|
|
|
41,069
|
|
|
|
1,611
|
|
|
|
0
|
|
|
|
69,151
|
|
|
Property, plant and equipment
|
|
|
26,472
|
|
|
|
(1
|
)
|
|
|
41,069
|
|
|
|
1,611
|
|
|
|
0
|
|
|
|
69,151
|
|
|
Net cash used in investing activities
|
|
|
22,106
|
|
|
|
(1,765,856
|
)
|
|
|
173,969
|
|
|
|
(29,687
|
)
|
|
|
(771
|
)
|
|
|
(1,600,239
|
)
|
|
Cash flows from financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(2,264
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(2,264
|
)
|
|
Issue
|
|
|
(2,264
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(2,264
|
)
|
|
Proceeds from and payments for financial liability instruments
|
|
|
206,299
|
|
|
|
2,259,181
|
|
|
|
333,225
|
|
|
|
25,217
|
|
|
|
(588,583
|
)
|
|
|
2,235,339
|
|
|
Issue
|
|
|
465,722
|
|
|
|
2,517,783
|
|
|
|
9,907
|
|
|
|
(10,535
|
)
|
|
|
(0
|
)
|
|
|
2,982,877
|
|
|
Redemption and repayment
|
|
|
(243,297
|
)
|
|
|
(431,704
|
)
|
|
|
(80,975
|
)
|
|
|
8,438
|
|
|
|
(0
|
)
|
|
|
(747,538
|
)
|
|
Debts with group companies
|
|
|
(16,126
|
)
|
|
|
173,102
|
|
|
|
404,293
|
|
|
|
27,314
|
|
|
|
(588,583
|
)
|
|
|
0
|
|
|
Dividends and interest on other equity instruments paid
|
|
|
0
|
|
|
|
0
|
|
|
|
(47,421
|
)
|
|
|
(5,931
|
)
|
|
|
53,352
|
|
|
|
0
|
|
|
Other cash flows from financing activities
|
|
|
(22,178
|
)
|
|
|
(264,102
|
)
|
|
|
(486
|
)
|
|
|
(437
|
)
|
|
|
0
|
|
|
|
(287,203
|
)
|
|
Deferred acquisition costs of financial instruments related to
acquisition of Talecris
|
|
|
(22,540
|
)
|
|
|
(264,099
|
)
|
|
|
(486
|
)
|
|
|
(425
|
)
|
|
|
0
|
|
|
|
(287,550
|
)
|
|
Other amounts received from financing activities
|
|
|
362
|
|
|
|
(3
|
)
|
|
|
0
|
|
|
|
(12
|
)
|
|
|
0
|
|
|
|
347
|
|
|
Net cash from financing activities
|
|
|
181,857
|
|
|
|
1,995,079
|
|
|
|
285,318
|
|
|
|
18,849
|
|
|
|
(535,231
|
)
|
|
|
1,945,872
|
|
|
Effect of exchange rate fluctuations on cash
|
|
|
0
|
|
|
|
(17,170
|
)
|
|
|
373
|
|
|
|
(921
|
)
|
|
|
(466
|
)
|
|
|
(18,184
|
)
|
|
Net increase / (decrease) in cash and cash equivalents
|
|
|
46,002
|
|
|
|
(187,400
|
)
|
|
|
485,038
|
|
|
|
504
|
|
|
|
(1
|
)
|
|
|
344,143
|
|
|
Cash and cash equivalents at beginning of the period
|
|
|
25
|
|
|
|
227,456
|
|
|
|
1,444
|
|
|
|
10,724
|
|
|
|
0
|
|
|
|
239,649
|
|
|
Cash and cash equivalents at end of period
|
|
|
46,027
|
|
|
|
40,056
|
|
|
|
486,482
|
|
|
|
11,228
|
|
|
|
(1
|
)
|
|
|
583,792
|
|
The
accompanying note forms an integral part of the condensed
consolidated interim financial statements
F-36
Report
of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
Grifols, S.A.
Barcelona, Spain
We have audited the accompanying consolidated balance sheets of
Grifols, S.A. and subsidiaries (“the Company”) as of
December 31, 2010 and 2009, and the related consolidated
income statements, consolidated statements of comprehensive
income, statements of changes in consolidated equity and
consolidated statements of cash flows for each of the years in
the three-year period ended December 31, 2010. These
consolidated financial statements are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on these consolidated financial statements based on our
audits.
We conducted our audits in accordance with the standards of the
Public Company Accounting Oversight Board (United States). Those
standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are
free of material misstatement. An audit includes examining, on a
test basis, evidence supporting the amounts and disclosures in
the financial statements. An audit also includes assessing the
accounting principles used and significant estimates made by
management, as well as evaluating the overall financial
statement presentation. We believe that our audits provide a
reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred
to above present fairly, in all material respects, the financial
position of Grifols, S.A. and subsidiaries as of
December 31, 2010 and 2009, and the results of their
operations and their cash flows for each of the years in the
three-year period ended December 31, 2010, in conformity
with International Financial Reporting Standards as issued by
the International Accounting Standards Board.
/s/ KPMG Auditores, S.L.
Barcelona, Spain, October 5, 2011
F-37
GRIFOLS,
S.A. AND SUBSIDIARIES
At
31 December 2010 and 2009
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
ASSETS
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
Intangible assets
Goodwill (note 7)
|
|
|
189,448
|
|
|
|
174,000
|
|
|
Other intangible assets (note 8)
|
|
|
78,299
|
|
|
|
69,385
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
267,747
|
|
|
|
243,385
|
|
|
Property, plant and equipment (note 9)
|
|
|
434,131
|
|
|
|
371,705
|
|
|
Investments in equity accounted investees (note 10)
|
|
|
598
|
|
|
|
383
|
|
|
Non-current financial assets (note 11)
|
|
|
7,535
|
|
|
|
3,731
|
|
|
Deferred tax assets (note 29)
|
|
|
34,889
|
|
|
|
33,395
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
744,900
|
|
|
|
652,599
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
Inventories (note 12)
|
|
|
527,865
|
|
|
|
484,462
|
|
|
Trade and other receivables
Trade receivables
|
|
|
224,355
|
|
|
|
207,840
|
|
|
Other receivables
|
|
|
44,032
|
|
|
|
39,540
|
|
|
Current income tax assets
|
|
|
14,607
|
|
|
|
7,802
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables (note 13)
|
|
|
282,994
|
|
|
|
255,182
|
|
|
Other current financial assets (note 14)
|
|
|
12,946
|
|
|
|
8,217
|
|
|
Other current assets (note 15)
|
|
|
80,628
|
|
|
|
7,345
|
|
|
Cash and cash equivalents (note 16)
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
1,144,082
|
|
|
|
1,004,578
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
1,888,982
|
|
|
|
1,657,177
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the consolidated
financial statements.
F-38
GRIFOLS,
S.A. AND SUBSIDIARIES
Consolidated
Balance Sheets — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
EQUITY AND LIABILITIES
|
|
Equity
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
106,532
|
|
|
|
106,532
|
|
|
Share premium
|
|
|
121,802
|
|
|
|
121,802
|
|
|
Reserves
|
|
|
403,604
|
|
|
|
314,903
|
|
|
Own shares
|
|
|
(1,927
|
)
|
|
|
(677
|
)
|
|
Interim dividend
|
|
|
0
|
|
|
|
(31,960
|
)
|
|
Profit for the year attributable to the Parent
|
|
|
115,513
|
|
|
|
147,972
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
745,524
|
|
|
|
658,572
|
|
|
Cash flow hedges
|
|
|
(1,751
|
)
|
|
|
(1,948
|
)
|
|
Translation differences
|
|
|
(50,733
|
)
|
|
|
(90,253
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
(52,484
|
)
|
|
|
(92,201
|
)
|
|
Equity attributable to the Parent (note 17)
|
|
|
693,040
|
|
|
|
566,371
|
|
|
Non-controlling interests (note 19)
|
|
|
14,350
|
|
|
|
12,157
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
707,390
|
|
|
|
578,528
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
Grants (note 20)
|
|
|
2,088
|
|
|
|
2,311
|
|
|
Provisions (note 21)
|
|
|
1,378
|
|
|
|
1,232
|
|
|
Non-current financial liabilities
Loans and borrowings, bonds and other marketable securities
|
|
|
665,385
|
|
|
|
703,186
|
|
|
Other financial liabilities
|
|
|
10,474
|
|
|
|
12,552
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities (note 22)
|
|
|
675,859
|
|
|
|
715,738
|
|
|
Deferred tax liabilities (note 29)
|
|
|
79,141
|
|
|
|
60,325
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
758,466
|
|
|
|
779,606
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
Provisions (note 21)
|
|
|
4,365
|
|
|
|
4,702
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
191,635
|
|
|
|
113,991
|
|
|
Other financial liabilities
|
|
|
18,236
|
|
|
|
12,230
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities (note 22)
|
|
|
209,871
|
|
|
|
126,221
|
|
|
Debts with associates (note 33)
|
|
|
1,162
|
|
|
|
0
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
160,678
|
|
|
|
120,909
|
|
|
Other payables
|
|
|
11,928
|
|
|
|
17,832
|
|
|
Current income tax liabilities
|
|
|
4,172
|
|
|
|
3,258
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables (note 23)
|
|
|
176,778
|
|
|
|
141,999
|
|
|
Other current liabilities (note 24)
|
|
|
30,950
|
|
|
|
26,121
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
423,126
|
|
|
|
299,043
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
1,181,592
|
|
|
|
1,078,649
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity and liabilities
|
|
|
1,888,982
|
|
|
|
1,657,177
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the consolidated
financial statements.
F-39
GRIFOLS,
S.A. AND SUBSIDIARIES
For
the Years Ended 31 December 2010, 2009 and
2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues (note 25)
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
814,311
|
|
|
Changes in inventories of finished goods and work in progress
(note 12)
|
|
|
45,749
|
|
|
|
73,093
|
|
|
|
31,058
|
|
|
Self-constructed non-current assets (notes 8 and 9)
|
|
|
33,513
|
|
|
|
41,142
|
|
|
|
25,794
|
|
|
Supplies (note 12)
|
|
|
(306,859
|
)
|
|
|
(286,274
|
)
|
|
|
(206,738
|
)
|
|
Other operating income (note 27)
|
|
|
1,196
|
|
|
|
1,443
|
|
|
|
1,289
|
|
|
Personnel expenses (note 26)
|
|
|
(289,008
|
)
|
|
|
(273,168
|
)
|
|
|
(238,159
|
)
|
|
Other operating expenses (note 27)
|
|
|
(220,218
|
)
|
|
|
(203,381
|
)
|
|
|
(192,288
|
)
|
|
Amortisation and depreciation (notes 8 and 9)
|
|
|
(45,776
|
)
|
|
|
(39,554
|
)
|
|
|
(33,256
|
)
|
|
Non-financial and other capital grants (note 20)
|
|
|
728
|
|
|
|
1,188
|
|
|
|
2,941
|
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
(372
|
)
|
|
|
(1,147
|
)
|
|
|
(1,991
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
209,683
|
|
|
|
226,528
|
|
|
|
202,961
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
4,526
|
|
|
|
7,067
|
|
|
|
2,682
|
|
|
Finance expenses
|
|
|
(49,660
|
)
|
|
|
(27,087
|
)
|
|
|
(29,305
|
)
|
|
Change in fair value of financial instruments (note 32)
|
|
|
(7,593
|
)
|
|
|
(587
|
)
|
|
|
(1,268
|
)
|
|
Gains/(losses) on disposal of financial instruments
|
|
|
91
|
|
|
|
(245
|
)
|
|
|
—
|
|
|
Exchange gains/(losses)
|
|
|
1,616
|
|
|
|
(1,733
|
)
|
|
|
(2,825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net finance expense (note 28)
|
|
|
(51,020
|
)
|
|
|
(22,585
|
)
|
|
|
(30,716
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit/(loss) of equity accounted investees
(note 10)
|
|
|
(879
|
)
|
|
|
51
|
|
|
|
24
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
157,784
|
|
|
|
203,994
|
|
|
|
172,269
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense (note 29)
|
|
|
(42,517
|
)
|
|
|
(56,424
|
)
|
|
|
(50,153
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
115,267
|
|
|
|
147,570
|
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
115,267
|
|
|
|
147,570
|
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
115,513
|
|
|
|
147,972
|
|
|
|
121,728
|
|
|
Profit/(loss) attributable to non-controlling interests
(note 19)
|
|
|
(246
|
)
|
|
|
(402
|
)
|
|
|
388
|
|
|
Basic earnings per share (Euros) (note 18)
|
|
|
0.54
|
|
|
|
0.71
|
|
|
|
0.58
|
|
|
Diluted earnings per share (Euros) (note 18)
|
|
|
0.54
|
|
|
|
0.71
|
|
|
|
0.58
|
|
The accompanying notes form an integral part of the consolidated
financial statements.
F-40
GRIFOLS,
S.A. AND SUBSIDIARIES
For
the Years Ended 31 December 2010, 2009 and
2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Consolidated profit for the year
|
|
|
115,267
|
|
|
|
147,570
|
|
|
|
122,116
|
|
|
Income and expenses generated during the year
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Measurement of financial instruments (note 11)
|
|
|
0
|
|
|
|
(14
|
)
|
|
|
(6
|
)
|
|
Available-for-sale
financial assets
|
|
|
0
|
|
|
|
(18
|
)
|
|
|
(9
|
)
|
|
Tax effect
|
|
|
0
|
|
|
|
4
|
|
|
|
3
|
|
|
Cash flow hedges (note 17 (f))
|
|
|
0
|
|
|
|
(1,998
|
)
|
|
|
0
|
|
|
Cash flow hedges
|
|
|
0
|
|
|
|
(3,275
|
)
|
|
|
0
|
|
|
Tax effect
|
|
|
0
|
|
|
|
1,277
|
|
|
|
0
|
|
|
Translation differences
|
|
|
42,225
|
|
|
|
(4,145
|
)
|
|
|
13,955
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income and expenses generated during the year
|
|
|
42,225
|
|
|
|
(6,157
|
)
|
|
|
13,949
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income and expense recognised in the income statement:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Measurement of financial instruments (note 11)
|
|
|
0
|
|
|
|
172
|
|
|
|
0
|
|
|
Available-for-sale
financial assets
|
|
|
0
|
|
|
|
245
|
|
|
|
0
|
|
|
Tax effect
|
|
|
0
|
|
|
|
(73
|
)
|
|
|
0
|
|
|
Cash flow hedges (note 17 (f))
|
|
|
197
|
|
|
|
50
|
|
|
|
0
|
|
|
Cash flow hedges
|
|
|
324
|
|
|
|
80
|
|
|
|
0
|
|
|
Tax effect
|
|
|
(127
|
)
|
|
|
(30
|
)
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income and expense recognised in the income statement:
|
|
|
197
|
|
|
|
222
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the year
|
|
|
157,689
|
|
|
|
141,635
|
|
|
|
136,065
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income attributable to the Parent
|
|
|
155,230
|
|
|
|
140,386
|
|
|
|
135,781
|
|
|
Total comprehensive income attributable to non-controlling
interests
|
|
|
2,459
|
|
|
|
1,249
|
|
|
|
284
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the year
|
|
|
157,689
|
|
|
|
141,635
|
|
|
|
136,065
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying notes form an integral part of the consolidated
financial statements.
F-41
GRIFOLS,
S.A. AND SUBSIDIARIES
For
the Years Ended 31 December 2010, 2009 and
2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from/(used in) operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
157,784
|
|
|
|
203,994
|
|
|
|
172,269
|
|
|
Adjustments for:
|
|
|
92,351
|
|
|
|
61,800
|
|
|
|
66,034
|
|
|
Amortisation and depreciation (notes 8 and 9)
|
|
|
45,776
|
|
|
|
39,554
|
|
|
|
33,256
|
|
|
Other adjustments:
|
|
|
46,575
|
|
|
|
22,246
|
|
|
|
32,778
|
|
|
(Profit)/losses on equity accounted investments (note 10)
|
|
|
879
|
|
|
|
(51
|
)
|
|
|
(24
|
)
|
|
Exchange differences
|
|
|
(1,616
|
)
|
|
|
1,733
|
|
|
|
2,825
|
|
|
Impairment of assets and net provision charges
|
|
|
913
|
|
|
|
53
|
|
|
|
1,994
|
|
|
(Profits)/losses on disposal of fixed assets
|
|
|
(276
|
)
|
|
|
1,147
|
|
|
|
2,001
|
|
|
Government grants taken to income (note 20)
|
|
|
(728
|
)
|
|
|
(1,188
|
)
|
|
|
(2,943
|
)
|
|
Net finance expense
|
|
|
47,442
|
|
|
|
17,551
|
|
|
|
27,891
|
|
|
Other adjustments
|
|
|
(39
|
)
|
|
|
3,001
|
|
|
|
1,034
|
|
|
Change in operating assets and liabilities
|
|
|
(78,767
|
)
|
|
|
(104,127
|
)
|
|
|
(86,550
|
)
|
|
Change in inventories
|
|
|
(18,306
|
)
|
|
|
(113,104
|
)
|
|
|
(98,520
|
)
|
|
Change in trade and other receivables
|
|
|
(23,546
|
)
|
|
|
(12,549
|
)
|
|
|
(7,951
|
)
|
|
Change in current financial assets and other current assets
|
|
|
(73,022
|
)
|
|
|
(1,287
|
)
|
|
|
405
|
|
|
Change in current trade and other payables
|
|
|
36,107
|
|
|
|
22,813
|
|
|
|
19,516
|
|
|
Other cash flows used in operating activities
|
|
|
(67,116
|
)
|
|
|
(73,487
|
)
|
|
|
(77,310
|
)
|
|
Interest paid
|
|
|
(40,129
|
)
|
|
|
(14,719
|
)
|
|
|
(25,972
|
)
|
|
Interest received
|
|
|
5,436
|
|
|
|
2,509
|
|
|
|
2,213
|
|
|
Income tax paid
|
|
|
(32,423
|
)
|
|
|
(61,277
|
)
|
|
|
(53,551
|
)
|
|
Net cash from operating activities
|
|
|
104,252
|
|
|
|
88,180
|
|
|
|
74,443
|
|
|
Cash flows from/(used in) investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(108,588
|
)
|
|
|
(136,626
|
)
|
|
|
(130,923
|
)
|
|
Group companies and business units
|
|
|
(1,474
|
)
|
|
|
(15,385
|
)
|
|
|
(632
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(103,402
|
)
|
|
|
(118,770
|
)
|
|
|
(129,568
|
)
|
|
Property, plant and equipment
|
|
|
(86,800
|
)
|
|
|
(103,415
|
)
|
|
|
(119,824
|
)
|
|
Intangible assets
|
|
|
(16,602
|
)
|
|
|
(15,355
|
)
|
|
|
(9,744
|
)
|
|
Other financial assets
|
|
|
(3,712
|
)
|
|
|
(2,471
|
)
|
|
|
(723
|
)
|
|
Proceeds from the sale of investments
|
|
|
4,532
|
|
|
|
673
|
|
|
|
157
|
|
|
Property, plant and equipment
|
|
|
3,911
|
|
|
|
673
|
|
|
|
157
|
|
|
Associates (note 2 (c))
|
|
|
621
|
|
|
|
0
|
|
|
|
0
|
|
|
Net cash used in investing activities
|
|
|
(104,056
|
)
|
|
|
(135,953
|
)
|
|
|
(130,766
|
)
|
|
Cash flows from/(used in) financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(1,250
|
)
|
|
|
26,655
|
|
|
|
(4,212
|
)
|
|
Issue
|
|
|
0
|
|
|
|
(76
|
)
|
|
|
0
|
|
|
Acquisition of own shares (note 17 (d))
|
|
|
(1,250
|
)
|
|
|
(25,186
|
)
|
|
|
(4,880
|
)
|
|
Disposal of treasury shares
|
|
|
0
|
|
|
|
51,917
|
|
|
|
668
|
|
|
Proceeds from and payments for financial liability instruments
|
|
|
(1,066
|
)
|
|
|
344,413
|
|
|
|
96,349
|
|
|
Issue
|
|
|
118,238
|
|
|
|
525,078
|
|
|
|
394,109
|
|
|
Redemption and repayment
|
|
|
(119,304
|
)
|
|
|
(180,665
|
)
|
|
|
(297,760
|
)
|
|
Dividends and interest on other equity instruments paid
|
|
|
(27,282
|
)
|
|
|
(80,913
|
)
|
|
|
(34,792
|
)
|
|
Other cash flows from financing activities
|
|
|
323
|
|
|
|
741
|
|
|
|
0
|
|
|
Other amounts received from financing activities
|
|
|
323
|
|
|
|
741
|
|
|
|
0
|
|
|
Net cash from/(used in) financing activities
|
|
|
(29,275
|
)
|
|
|
290,896
|
|
|
|
57,345
|
|
|
Effect of exchange rate fluctuations on cash
|
|
|
19,356
|
|
|
|
(119
|
)
|
|
|
(344
|
)
|
|
Net increase/(decrease) in cash and cash equivalents
|
|
|
(9,723
|
)
|
|
|
243,004
|
|
|
|
678
|
|
|
Cash and cash equivalents at beginning of the year
|
|
|
249,372
|
|
|
|
6,368
|
|
|
|
5,690
|
|
|
Cash and cash equivalents at end of year
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
6,368
|
|
The accompanying notes form an integral part of the consolidated
financial statements
F-42
GRIFOLS,
S.A. AND SUBSIDIARIES
For
the Years Ended 31 December 2010, 2009 and
2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable to Equity Holders of the Parent
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated Other Comprehensive Income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Available-for
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale
|
|
|
Attributable
|
|
|
Non-
|
|
|
|
|
|
|
|
Share
|
|
|
Share
|
|
|
|
|
|
to
|
|
|
Interim
|
|
|
Own
|
|
|
Translation
|
|
|
Cash Flow
|
|
|
Financial
|
|
|
to
|
|
|
Controlling
|
|
|
|
|
|
|
|
Capital
|
|
|
Premium
|
|
|
Reserves*
|
|
|
Parent
|
|
|
Dividend
|
|
|
Shares
|
|
|
Differences
|
|
|
Hedges
|
|
|
Assets
|
|
|
Parent
|
|
|
Interests
|
|
|
Equity
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Balances at 31 December 2007
|
|
|
106,532
|
|
|
|
131,832
|
|
|
|
184,608
|
|
|
|
87,774
|
|
|
|
0
|
|
|
|
(28,893
|
)
|
|
|
(98,516
|
)
|
|
|
0
|
|
|
|
(152
|
)
|
|
|
383,185
|
|
|
|
981
|
|
|
|
384,166
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation differences
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
14,059
|
|
|
|
—
|
|
|
|
—
|
|
|
|
14,059
|
|
|
|
(104
|
)
|
|
|
13,955
|
|
|
Available-for-sale
financial assets Gains/(losses)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
(6
|
)
|
|
|
(6
|
)
|
|
|
—
|
|
|
|
(6
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
14,059
|
|
|
|
0
|
|
|
|
(6
|
)
|
|
|
14,053
|
|
|
|
(104
|
)
|
|
|
13,949
|
|
|
Profit for the year
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
121,728
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
121,728
|
|
|
|
388
|
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
121,728
|
|
|
|
0
|
|
|
|
0
|
|
|
|
14,059
|
|
|
|
0
|
|
|
|
(6
|
)
|
|
|
135,781
|
|
|
|
284
|
|
|
|
136,065
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net movement in own shares
|
|
|
—
|
|
|
|
—
|
|
|
|
24
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,194
|
)
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
(4,170
|
)
|
|
|
—
|
|
|
|
(4,170
|
)
|
|
Other changes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
0
|
|
|
|
(15
|
)
|
|
|
(15
|
)
|
|
Distribution of 2007 profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves
|
|
|
—
|
|
|
|
—
|
|
|
|
63,037
|
|
|
|
(63,037
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0
|
|
|
|
—
|
|
|
|
0
|
|
|
Dividends
|
|
|
—
|
|
|
|
(10,030
|
)
|
|
|
—
|
|
|
|
(24,737
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(34,767
|
)
|
|
|
—
|
|
|
|
(34,767
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transaction with owners of the Company
|
|
|
0
|
|
|
|
(10,030
|
)
|
|
|
63,061
|
|
|
|
(87,774
|
)
|
|
|
0
|
|
|
|
(4,194
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(38,937
|
)
|
|
|
(15
|
)
|
|
|
(38,952
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at 31 December 2008
|
|
|
106,532
|
|
|
|
121,802
|
|
|
|
247,669
|
|
|
|
121,728
|
|
|
|
0
|
|
|
|
(33,087
|
)
|
|
|
(84,457
|
)
|
|
|
0
|
|
|
|
(158
|
)
|
|
|
480,029
|
|
|
|
1,250
|
|
|
|
481,279
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation differences
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(5,796
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(5,796
|
)
|
|
|
1,651
|
|
|
|
(4,145
|
)
|
|
Cash flow hedges
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,948
|
)
|
|
|
—
|
|
|
|
(1,948
|
)
|
|
|
—
|
|
|
|
(1,948
|
)
|
|
Gains/(Losses) on
available-for-sale
financial assets
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
158
|
|
|
|
158
|
|
|
|
—
|
|
|
|
158
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(5,796
|
)
|
|
|
(1,948
|
)
|
|
|
158
|
|
|
|
(7,586
|
)
|
|
|
1,651
|
|
|
|
(5,935
|
)
|
|
Profit/(loss) for the year
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
147,972
|
|
|
|
0
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
147,972
|
|
|
|
(402
|
)
|
|
|
147,570
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
147,972
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(5,796
|
)
|
|
|
(1,948
|
)
|
|
|
158
|
|
|
|
140,386
|
|
|
|
1,249
|
|
|
|
141,635
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-43
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable to Equity Holders of the Parent
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated Other Comprehensive Income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Available-for
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Attributable
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Sale
|
|
|
Attributable
|
|
|
Non-
|
|
|
|
|
|
|
|
Share
|
|
|
Share
|
|
|
|
|
|
to
|
|
|
Interim
|
|
|
Own
|
|
|
Translation
|
|
|
Cash Flow
|
|
|
Financial
|
|
|
to
|
|
|
Controlling
|
|
|
|
|
|
|
|
Capital
|
|
|
Premium
|
|
|
Reserves*
|
|
|
Parent
|
|
|
Dividend
|
|
|
Shares
|
|
|
Differences
|
|
|
Hedges
|
|
|
Assets
|
|
|
Parent
|
|
|
Interests
|
|
|
Equity
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Net movement in own shares
|
|
|
—
|
|
|
|
—
|
|
|
|
(5,679
|
)
|
|
|
—
|
|
|
|
|
|
|
|
32,410
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
26,731
|
|
|
|
—
|
|
|
|
26,731
|
|
|
Other changes
|
|
|
—
|
|
|
|
—
|
|
|
|
(124
|
)
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(124
|
)
|
|
|
44
|
|
|
|
(80
|
)
|
|
Business combinations
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0
|
|
|
|
9,876
|
|
|
|
9,876
|
|
|
Distribution of 2008 profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves
|
|
|
—
|
|
|
|
—
|
|
|
|
73,037
|
|
|
|
(73,037
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
0
|
|
|
|
—
|
|
|
|
0
|
|
|
Dividends
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(48,691
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(48,691
|
)
|
|
|
(54
|
)
|
|
|
(48,745
|
)
|
|
Interim dividend
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(31,960
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(31,960
|
)
|
|
|
(208
|
)
|
|
|
(32,168
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transaction with owners of the Company
|
|
|
0
|
|
|
|
0
|
|
|
|
67,235
|
|
|
|
(121,728
|
)
|
|
|
(31,960
|
)
|
|
|
32,410
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(54,044
|
)
|
|
|
9,658
|
|
|
|
(44,386
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2009
|
|
|
106,532
|
|
|
|
121,802
|
|
|
|
314,903
|
|
|
|
147,972
|
|
|
|
(31,960
|
)
|
|
|
(677
|
)
|
|
|
(90,253
|
)
|
|
|
(1,948
|
)
|
|
|
0
|
|
|
|
566,371
|
|
|
|
12,157
|
|
|
|
578,528
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Translation differences
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
39,520
|
|
|
|
0
|
|
|
|
0
|
|
|
|
39,520
|
|
|
|
2,705
|
|
|
|
42,225
|
|
|
Cash flow hedges
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
197
|
|
|
|
0
|
|
|
|
197
|
|
|
|
0
|
|
|
|
197
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
39,520
|
|
|
|
197
|
|
|
|
0
|
|
|
|
39,717
|
|
|
|
2,705
|
|
|
|
42,422
|
|
|
Profit/(loss) for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
115,513
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
115,513
|
|
|
|
(246
|
)
|
|
|
115,267
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total comprehensive income for the year
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
115,513
|
|
|
|
0
|
|
|
|
0
|
|
|
|
39,520
|
|
|
|
197
|
|
|
|
0
|
|
|
|
155,230
|
|
|
|
2,459
|
|
|
|
157,689
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net movement in own shares
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,250
|
)
|
|
|
|
|
|
|
(1,250
|
)
|
|
Other changes
|
|
|
0
|
|
|
|
0
|
|
|
|
(82
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(82
|
)
|
|
|
(213
|
)
|
|
|
(295
|
)
|
|
Distribution of 2009 profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reserves
|
|
|
0
|
|
|
|
0
|
|
|
|
88,783
|
|
|
|
(88,783
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
0
|
|
|
Dividends
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(27,229
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(27,229
|
)
|
|
|
(53
|
)
|
|
|
(27,282
|
)
|
|
Interim dividend
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(31,960
|
)
|
|
|
31,960
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Transaction with owners of the Company
|
|
|
0
|
|
|
|
0
|
|
|
|
88,701
|
|
|
|
(147,972
|
)
|
|
|
31,960
|
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(28,561
|
)
|
|
|
(266
|
)
|
|
|
(28,827
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2010
|
|
|
106,532
|
|
|
|
121,802
|
|
|
|
403,604
|
|
|
|
115,513
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
|
(50,733
|
)
|
|
|
(1,751
|
)
|
|
|
0
|
|
|
|
693,040
|
|
|
|
14,350
|
|
|
|
707,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
* |
|
Reserves include accumulated earnings, legal reserves and other
reserves |
The accompanying notes form an integral part of the consolidated
financial statements
F-44
GRIFOLS,
S.A. AND SUBSIDIARIES
|
|
|
(1)
|
Nature,
Principal Activities and Subsidiaries
|
Grifols, S.A. (hereinafter the Company) was incorporated with
limited liability under Spanish law on 22 June 1987. Its
registered and tax offices are in Barcelona. The Company’s
statutory activity consists of providing corporate and business
administrative, management and control services, as well as
investing in assets and property. The Company’s principal
activity consists of rendering administrative, management and
control services to its subsidiaries.
On 17 May 2006 the Company completed its flotation on the
Spanish stock market which was conducted through the public
offering of 71,000,000 ordinary shares of Euros 0.50 par
value each and a share premium of Euros 3.90 per share. The
total capital increase (including the share premium) amounted to
Euros 312.4 million, equivalent to a price of Euros 4.40
per share.
With effect as of 2 January 2008 the Company’s shares
were floated on the Spanish stock exchange’s IBEX-35 index.
All of the Company’s shares are listed on the Barcelona,
Madrid, Valencia and Bilbao stock exchanges and on the
electronic stock market.
Grifols, S.A. is the parent company of the subsidiaries listed
in section 1(b) of these Notes.
Grifols, S.A. and subsidiaries (hereinafter the Group) act on an
integrated basis and under common management and their principal
activity is the procurement, manufacture, preparation and sale
of therapeutic products, especially haemoderivatives.
The main business locations of the Group’s Spanish
companies are in Barcelona, Parets del Vallés (Barcelona)
and Torres de Cotilla (Murcia), while the American
companies’ installations are located in Los Angeles,
California (USA).
The Group companies are grouped into three areas: industrial,
commercial and services.
The following companies are included:
Diagnostic Grifols, S.A. which has registered offices in
Parets del Vallès (Barcelona), Spain and was incorporated
into the Group on 24 March 1987, and is engaged in the
development and manufacture of diagnostic equipment,
instrumentation and reagents.
Instituto Grifols, S.A. which has registered offices in
Parets del Vallès (Barcelona), Spain, and was incorporated
into the Group on 21 September 1987, carries out its
activities in the area of bioscience and is engaged in plasma
fractioning and the manufacture of haemoderivative
pharmaceutical products.
Laboratorios Grifols, S.A., with registered offices in
Parets del Vallès (Barcelona), Spain, was incorporated into
the Group on 18 April 1989 and is engaged in the production
of glass- and plastic-packaged parenteral solutions, parenteral
and enteral nutrition products and blood extraction equipment
and bags. Its production facilities are in Barcelona and Murcia.
Biomat, S.A., with registered offices in Parets del
Vallès (Barcelona), Spain, was incorporated into the Group
on 30 July 1991. It operates in the field of bioscience and
basically engages in analysis and certification of the quality
of plasma used by Instituto Grifols, S.A. It also provides
transfusion centres with plasma virus inactivation services.
F-45
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Grifols Engineering, S.A., with registered offices in
Parets del Vallès (Barcelona), Spain, was incorporated into
the Group on 14 December 2000 and is engaged in the design
and development of the Group’s manufacturing installations
and part of the equipment and machinery used at these premises.
The company also renders engineering services to third parties.
Logister, S.A. was incorporated with limited liability
under Spanish law on 22 June 1987 and its registered
offices are at Polígono Levante, calle Can Guasch, s/n,
08150 Parets del Vallés, Barcelona. Its activity comprises
the manufacture, sale and purchase, marketing and distribution
of all types of computer products and materials. 99.985% of this
company is solely-owned directly by Movaco, S.A., another
subsidiary.
Biomat USA, Inc., with registered offices at 1209, Orange
Street, Wilmington, New Castle (Delaware Corporation) (USA), was
incorporated into the Group on 1 March 2002 and carries out
its activities in the area of bioscience, procuring human
plasma. Since 1 November 2007, this company’s share
capital is held by Instituto Grifols, S.A. and Grifols, Inc.
Grifols Biologicals, Inc., with registered offices at 15
East North Street, Dover, (Delaware) (USA), was incorporated
into the Group on 15 May 2003 and is exclusively engaged in
plasma fractioning and the production of haemoderivatives.
Grifols, Inc. directly owns 100% of this company.
PlasmaCare, Inc., with registered offices at 1209 Orange
Street, County of New Castle, Wilmington, Delaware 19801, was
incorporated into the Group on 3 March 2006 and carries out
its activities in the area of bioscience, procuring human
plasma. Since 1 November 2007, this company’s share
capital is held by Instituto Grifols, S.A. and Grifols, Inc.
Plasma Collection Centers, Inc., with registered offices
at 1209 Orange Street, County of New Castle, Wilmington,
Delaware 19801 (USA) and incorporated on 2 March 2007. Its
activity, developed in the bioscience area, consists of
procuring human plasma. 100% of this company’s share
capital is held directly by Biomat USA, Inc. In January 2010
Plasma Collection Centers, Inc. merged with Biomat USA, Inc.,
having no impact on the Group.
Lateral Grifols Pty Ltd. (formerly Diamed Australia Pty
Ltd.), with registered offices at Unit 5/80 Fairbank, Clayton
South, Victoria 3149 (Australia), was incorporated into the
Group on 3 March 2009. Its activity consists of the
distribution of pharmaceutical products and the development and
manufacture of reagents for diagnostics. This company is
directly and fully owned by Woolloomooloo Holdings Pty Ltd.
Medion Grifols Diagnostic AG, with registered offices at
Bonnstrasse, 9, 3186 Düdingen, Switzerland, was
incorporated into the Group on 3 March 2009. The
Company’s statutory activity consists of development and
production in the biotechnology and diagnostic sectors. 80% of
this company is directly held by Saturn Investments AG.
The companies responsible for the marketing and distribution of,
mainly, products manufactured by the industrial area companies
are all grouped in the commercial area.
Movaco, S.A. was incorporated with limited liability
under Spanish law on 21 July 1987 and its registered
offices are at Polígono Levante, calle Can Guasch, s/n,
08150 Parets del Vallés, Barcelona. Its principal activity
is the distribution and sale of reagents, chemical products and
other pharmaceutical specialities, and of medical-surgical
materials, equipment and instruments for use in laboratories and
healthcare centres.
Grifols International, S.A., with registered offices in
Parets del Vallès (Barcelona), Spain, was incorporated into
the Group on 4 June 1997. This company directs and
coordinates the marketing, sales and logistics
F-46
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
for all the Group’s commercial subsidiaries. Products are
marketed through subsidiaries operating in different countries.
These subsidiaries, their registered offices and date of
incorporation into the Group, are listed below.
Grifols Portugal Productos Farmacéuticos e Hospitalares,
Lda., was incorporated with limited liability under
Portuguese law on 10 August 1988. Its registered offices
are at Jorge Barradas, 30—c R/C, 1500 Lisbon (Portugal) and
it imports, exports and markets pharmaceutical and hospital
equipment and products particularly Grifols products. 99.99% of
this company is owned directly by Movaco, S.A.
Grifols Chile, S.A. was incorporated under limited
liability in Chile on 2 July 1990. Its registered offices
are at calle Avda. Americo Vespucio 2242, Comuna de Conchali,
Santiago de Chile (Chile). Its statutory activity comprises the
development of pharmaceutical businesses, which can involve the
import, production, marketing and export of related products.
Grifols Argentina, S.A. was incorporated with limited
liability in Argentina on 1 November 1991 and its
registered offices are at Bartolomé Mitre 1371, fifth floor
office “P” (CP 1036), Buenos Aires (Argentina). Its
statutory activity consists of clinical and biological research,
the preparation of reagents and therapeutic and diet products,
the manufacture of other pharmaceutical specialities and the
marketing thereof.
Grifols s.r.o. was incorporated with limited liability
under Czech Republic law on 15 December 1992. Its
registered offices are at Zitná 2, Praga (Czech Republic)
and its statutory activity consists of the purchase, sale and
distribution of chemical-pharmaceutical products, including
human plasma.
Logistica Grifols, S.A. de C.V. (formerly Grifols
México, S.A. de C.V.) was incorporated with limited
liability under Mexican law on 9 January 1970, with
registered offices at calle Eugenio Cuzin
no 909,
Parque Industrial Belenes Norte, 45150 Zapopan, Jalisco
(Mexico). Its statutory activity comprises the manufacture and
marketing of pharmaceutical products for human and veterinary
use. On 6 May 2008 Grifols Mexico S.A. de C.V. was spun off
into two companies and its name was changed to Logística
Grifols, S.A. de C.V.
Grifols México, S.A. de C.V. was incorporated with
limited liability under Mexican law on 6 May 2008, as a
result of the spin off of the former company Grifols Mexico,
S.A. de C.V. Its registered offices are at calle Eugenio Cuzin
no 909,
Parque Industrial Belenes Norte, 45150 Zapopan, Jalisco
(Mexico). Its statutory activity comprises the production,
manufacture, adaptation, conditioning, sale and purchase,
commissioning, representation and consignment of all kinds of
pharmaceutical products and the acquisition of machinery,
equipment, raw materials, tools, assets and property for the
aforementioned purposes.
Grifols USA, LLC. was incorporated in the State of
Florida (USA) on 19 April 1990. Its registered offices are
at 8880 N.W. 18 Terrace, Miami, Florida (USA) and its statutory
activity is any activity permitted by US legislation. This
company is 100% directly owned by Grifols Biologicals, Inc.
Grifols Italia S.p.A. has its registered offices at Via
Carducci 62 d, 56010 Ghezzano, Pisa (Italy) and its statutory
activity comprises the purchase, sale and distribution of
chemical-pharmaceutical products. 66.66% of this company was
acquired on 9 June 1997 and the remaining 33.34% on
16 June 2000.
Grifols UK Ltd., the registered offices of which are at
72, St. Andrew’s Road, Cambridge CB4 1G
(United Kingdom), is engaged in the distribution and sale
of therapeutic and other pharmaceutical products, especially
haemoderivatives. 66.66% of this company was acquired on
9 June 1997 and the remaining 33.34% on 16 June 2000.
Grifols Deutschland GmbH was incorporated with limited
liability under German law on 21 May 1997, with registered
offices at Siemensstrasse 32, D-63225 Langen (Germany). Its
statutory activity consists of the import, export, distribution
and sale of reagents, chemical and pharmaceutical products,
especially to laboratories and healthcare centres, and medical
and surgical materials, equipment and instruments for laboratory
use.
F-47
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Grifols Brasil, Ltda. was incorporated with limited
liability in Brazil on 4 May 1998. Its registered offices
are at Rua Marechal Hermes 247, Centro Cívico, CEP
80530-230,
Curitiba (Brazil). Its statutory activity consists of the import
and export, preparation, distribution and sale of pharmaceutical
and chemical products for laboratory and hospital use, and
medical-surgical equipment and instrumentation.
Grifols France, S.A.R.L. was incorporated with limited
liability under French law on 2 November 1999, with
registered offices at Centre d’affaires auxiliares system,
Bat. 10, Parc du Millenaire — 125, Rue Henri
Becquerel, 34036, Montpellier (France). Its statutory activity
is the marketing of chemical and healthcare products.
Alpha Therapeutic Italia, S.p.A. was incorporated on
3 July 2000, with registered offices at Piazza Meda 3,
20121 Milan (Italy), and engages in the distribution and sale of
therapeutic products, especially haemoderivatives.
Grifols Asia Pacific Pte, Ltd was incorporated on
10 September 1986 , with registered offices at 501 Orchard
Road #20-01 Wheelock Place, Singapore, and its activity
consists of the distribution and sale of medical and
pharmaceutical products.
Grifols Malaysia Sdn Bhd is partly owned (30%) by Grifols
Asia Pacific Pte, Ltd. The registered offices of this company
are in Selangor (Malaysia) and it engages in the distribution
and sale of pharmaceutical products.
Grifols (Thailand) Ltd was incorporated on
1 September 1995 and its registered offices are at 287
Liberty Square Level 8, Silom Road, Bangkok. Its activity
comprises the import, export and distribution of pharmaceutical
products. 48% of this company is directly owned by Grifols Asia
Pacific Pte., Ltd.
Grifols Polska Sp.z.o.o. was incorporated on
12 December 2003, with registered offices at
UL. Nowogrodzka, 68, 00-116,
Warsaw, Poland, and engages in the distribution and sale of
pharmaceutical, cosmetic and other products.
Australian Corporate Number 073 272 830 Pty Ltd.
(formerly Lateral Grifols Diagnostics Pty Ltd.), with registered
offices at Unit 5/80 Fairbank, Clayton South, Victoria 3149
(Australia) was incorporated into the Group on 3 March
2009. Its activity comprises the distribution of pharmaceutical
products and reagents for diagnostics. This company is 100%
directly held by Woolloomooloo Holdings Pty Ltd.
Medion Diagnostics GmbH with registered offices at
Lochhamer Schlag 12 D-82166 Gräfelfing (Germany), was
incorporated into the Group on 3 March 2009. The
Company’s statutory activity consists of the distribution
and sale of biotechnological and diagnostic products. This
company is directly and fully owned by Medion Grifols Diagnostic
AG.
Grifols Nordic, AB (formerly Xepol, AB) with registered
offices in Engelbrekts Kyrkogata 7B 114 26 Stockhom, Sweden, was
incorporated into the Group on 3 June 2010. Its activity
consists of research and development, production and marketing,
either directly or through subsidiaries, of pharmaceutical
products, medical devices and any other asset deriving from the
aforementioned activities. This company is 100% directly owned
by Grifols, S.A.
Grifols Colombia, Ltda, with registered offices at Cra 7
71-52 TBP 9
Cundinamarca, Bogota, Colombia, was incorporated on 3 June
2010. Its activity consists of the sale, commercialisation and
distribution of medicines, pharmaceutical (including but not
limited to haemoderivatives) and hospital products, medical
devices, biomedical equipment, laboratory instruments and
reactives for diagnosis
and/or
sanitary software.
F-48
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The following companies are included in this area:
Grifols, Inc. was incorporated on 15 May 2003 with
registered offices at 15 East North Street, Dover (Delaware,
USA). Its principal activity is the holding of investments in
Group companies.
Grifols Viajes, S.A., with registered offices in
Barcelona, Spain, was incorporated into the Group on
31 March 1995 and operates as a retail travel agency
exclusively serving Group companies.
Squadron Reinsurance Ltd., with registered offices in
Dublin, Ireland, was incorporated into the Group on
25 April 2003 and engages in the reinsurance of Group
companies’ insurance policies.
Arrahona Optimus, S.L., with registered offices in
Barcelona, Spain, was incorporated into the Group on
28 August 2008. The Company’s statutory activity is
the development and construction of offices and business
premises. Its only asset is the office complex located in the
municipality of Sant Cugat del Vallés.
Gri-Cel, S.A., with registered offices at Avenida de la
Generalitat 152, Sant Cugat del Vallés (Barcelona), was
incorporated on 9 November 2009. The Company’s
statutory activity consists of research and development in the
field of regenerative medicine, awarding of research grants,
subscription to collaboration agreements with entities and
participation in projects in the area of regenerative medicine.
Saturn Australia Pty Ltd., with registered offices at
Unit 5/80 Fairbank, Clayton South, Victoria 3149 (Australia),
was incorporated into the Group on 3 March 2009. Its
activity consists of holding shares and investments. This
company is directly and fully owned by Woolloomooloo Holdings
Pty Ltd.
Saturn Investments AG, with registered offices at
c/o Dr. Christoph
Straub, Hanibuel 8, CH 6300 Zug (Switzerland) was incorporated
into the Group on 3 March 2009. Its activity consists of
the holding of shares. This company is directly and fully owned
by Saturn Australia Pty Ltd.
Woolloomooloo Holdings Pty Ltd., with registered offices
at Unit 5/80 Fairbank, Clayton South, Victoria 3149 (Australia),
was incorporated into the Group on 3 March 2009. Its
activity consists of holding shares. 49% of this holding company
is directly held by Grifols, S.A.
|
|
|
(c)
|
Associates
and other participations
|
Quest Internacional, Inc, 35% owned by Diagnostic
Grifols, S.A., with registered offices in Miami, Florida (USA),
engages in the manufacture and marketing of reagents and
clinical analysis instruments. On 9 November 2010 the Group
sold the interest it held in this company.
UTE Salas Blancas, a joint venture participated in 50% by
Grifols Engineering, S.A. was formed in 2009 and is domiciled at
calle Mas Casanovas 46, Barcelona. Its statutory activity
consists of the drafting of the project, execution of works and
installation of clean rooms and other facilities in the Banc de
Sang i Teixits (blood and tissue bank) wing of a hospital.
Nanotherapix, S.L. was incorporated on 25 June 2009
and is 51% owned by Gri-Cel, S.A. through a share capital
increase carried out on 9 March 2010. This company is
domiciled at Avenida Generalitat 152, San Cugat del Valles,
Barcelona and its activity consists of the development,
validation and production of the technology required to
implement the use of genetic and cellular therapy for the
treatment of human and animal pathologies.
F-49
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(2)
|
Basis of
Presentation of the Consolidated Financial Statements
|
The accompanying consolidated financial statements have been
authorised for issue by the directors of Grifols, S.A. on the
basis of the accounting records of Grifols, S.A. and of the
Group companies. The accompanying consolidated financial
statements for 2010, 2009 and 2008 have been prepared under
International Financial Reporting Standards, as issued by the
International Accounting Standard Board (IFRS-IASB) to present
fairly the consolidated equity and consolidated financial
position of Grifols, S.A. and subsidiaries at 31 December
2010 and 2009, as well as the consolidated results from their
operations, consolidated comprehensive income, consolidated cash
flows and changes in consolidated equity for the three-year
period then ended.
After having received the required authorizations from the
Federal Trade Commission (FTC) of the United States of America,
the acquisition of the American Talecris Biotherapeutics
Holdings Corp (Talecris) by Grifols was completed on
2 June, 2011 and the financing arrangements which were held
on stand-by were released. In order to comply with the
Registration rules of the Securities and Exchange Commission and
in connection with the filing of a F-4 Registration Statement,
Grifols is required to disclose, as additional and updated
information, consolidating financial information for each of the
annual periods ended 31 December 2010, 2009 and 2008 for
its subsidiaries which act as guarantors to the financing
arrangements, and also to update post-balance sheet date events,
in respect to the financial statements for the same periods
issued on 29 March 2011.
The Board of Directors of Grifols have therefore, issued these
consolidated financial statements to comply with the SEC rules
and include the additional disclosures of the guarantors and
updated post-balance sheet date events. The additional
disclosures are addressed in the notes 35 and 36.
The Group adopted EU-IFRS for the first time on 1 January
2004 and has been preparing its consolidated annual accounts
under International Financial Reporting Standards, as adopted by
the European Union (IFRS-EU) as required by Capital markets
regulations governing the presentation of financial statements
by companies whose debt or own equity instruments are listed on
a regulated market.
The Group’s consolidated financial statements for 2010,
2009 and 2008 have been prepared under International Financial
Reporting Standards (IFRS) as issued by the International
Accounting Standard Board, and in the case of Grifols, S.A. and
subsidiaries do not differ from IFRS as adopted by the European
Union taking into account all mandatory accounting policies and
rules and measurement bases with material effect, as well as the
alternative treatments permitted by the relevant standards in
this connection.
|
|
|
(a)
|
Changes
to IFRS in 2010, 2009 and 2008
|
In accordance with IFRSs, the following should be noted in
connection with the scope of application of IFRS and the
preparation of these consolidated financial statements of the
Group.
Effective
date in 2008
Standards
that have not affected the Group
|
|
|
| |
•
|
Amendment to IAS 39 Reclassification of Financial Assets:
Effective Date and Transition (effective date 1 July 2008).
|
| |
| |
•
|
IFRIC 16 Hedges of a Net Investment in a Foreign Operation
(effective date 1 October 2008).
|
| |
| |
•
|
IFRIC 12 Service Concession Arrangements (effective date
1 January 2008).
|
| |
| |
•
|
IFRIC 14 IAS 19 The Limit of a Defined Benefit Asset, Minimum
Funding Requirements and their Interaction (effective date
1 January 2008)
|
F-50
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
•
|
Amendments to IAS 39 and IFRS 7: Reclassification of Financial
Instruments (effective date 1 July 2008).
|
Effective
date in 2009
|
|
|
a)
|
Standards
effective as of 1 January 2009 that have required changes
to accounting policies and presentation
|
|
|
|
| |
•
|
IAS 1 Presentation of Financial Statements (revised 2007)
(annual periods beginning on or after 1 January 2009). This
standard modifies the requirements for presentation of the
financial statements, introducing the statement of comprehensive
income, which comprises income and other comprehensive income.
Entities may also present two separate statements, an income
statement showing profit or loss for the year and a statement of
other comprehensive income presenting profit or loss for the
year and other comprehensive income. When an entity changes an
accounting policy retrospectively or makes a retrospective
reclassification of items in its financial statements, it must
also present a statement of financial position (balance sheet)
as at the beginning of the earliest comparative period.
|
| |
| |
•
|
IFRS 8 Operating Segments (annual periods beginning on or after
1 January 2009). The impact of this standard mainly relates
to the disclosure of financial information by segment. See
note 6.
|
| |
| |
•
|
IAS 23 Borrowing Costs (revised 2007) (annual periods beginning
on or after 1 January 2009). This is a change in accounting
policy. The Group applies this standard to borrowing costs
related to qualifying assets capitalised on or subsequent to the
date this standard became effective. The standard eliminates the
possibility of recognising these borrowing costs as an expense,
stipulating that borrowing costs that are directly attributable
to the acquisition, construction or production of a qualifying
asset form part of the cost of that asset. Since 1 January
2009 the Group has capitalised interest amounting to Euros 1,278
thousand (see note 26).
|
| |
| |
•
|
Amendments to IFRS 7: “Improving Disclosures about
Financial Instruments” (applicable for years beginning on
or after 1 January 2009).
|
b) Standards
effective as of 1 January 2009 that have not affected the
Group
|
|
|
| |
•
|
IFRIC 13 Customer Loyalty Programmes (annual periods beginning
after 31 December 2008).
|
| |
| |
•
|
IFRS 2 Share-Based Payment: Modifications to vesting
conditions and cancellations (applied retrospectively to annual
periods beginning on or after 1 January 2009).
|
| |
| |
•
|
IAS 32 Financial Instruments: Presentation and IAS 1:
Presentation of Financial Statements: Changes to puttable
financial instruments and obligations arising on liquidation
(effective as of 1 January 2009).
|
| |
| |
•
|
Improvements to IFRSs. This document modifies various standards
and is effective for years beginning on or after 1 July
2009.
|
| |
| |
•
|
IFRS 1 First-time Adoption of International Financial Reporting
Standards and IAS 27 Consolidated and Separate Financial
Statements: These changes relate to the measurement of
investments in separate financial statements. This standard is
applied prospectively for years started on or after
1 January 2009.
|
| |
| |
•
|
Embedded derivatives: Amendments to IFRIC 9 and IAS 39
(applicable for years started after 31 December 2008).
|
| |
| |
•
|
IFRS 1 First-time Adoption of International Financial Reporting
Standards (applicable to annual periods beginning after
31 December 2009). This change does not affect the Group.
|
| |
| |
•
|
IFRIC 15 Agreements for the Construction of Real Estate.
|
F-51
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
•
|
IFRIC 17 Distribution of Non-Cash Assets to Owners (effective
date: 1 July 2009).
|
| |
| |
•
|
IFRIC 18 Transfers of Assets from Customers (effective date:
1 July 2009).
|
| |
| |
•
|
IAS 39 Financial Instruments: Recognition and Measurement.
Changes to the items that can be classified as hedged. The
amendment clarifies the types of risks that can be classified as
hedged when applying hedge accounting (effective date:
1 July 2009).
|
Effective
date in 2010
|
|
|
| |
•
|
Amendment to IFRS 2 Group Cash-settled Share Based Payment
Transactions (effective date: 1 January 2010).
|
| |
| |
•
|
2009 improvements to IFRSs (effective date: 1 January 2010).
|
| |
| |
•
|
Amendment to IAS 32 Financial Instruments: Presentation,
Classification of Rights Issues (effective date: 1 February
2010).
|
| |
| |
•
|
IFRIC 19 Extinguishing financial liabilities with equity
instruments (effective date: 1 July 2010).
|
| |
| |
•
|
Amendment to IFRS 1 Limited Exemption from Comparative IFRS 7
Disclosures for First-time Adopters (effective date: 1 July
2010).
|
| |
| |
•
|
IFRS 3 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 July 2010).
|
| |
| |
•
|
IAS 27 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 July 2010).
|
In accordance with the new IFRS 3 (mandatory application in
years starting after 1 July 2009), transaction costs, which
differ from costs of issuing debt or equity instruments, are
recognised as an expense as incurred. The application of this
new standard has an impact on the consolidated financial
statements of the Grifols Group (see note 15).
The application of the other standards has not had a significant
impact on the Group’s consolidated financial statements or
has not been applicable.
Standards
issued but not effective on 2010
|
|
|
| |
•
|
IAS 24 Revised Related Party Disclosures
|
| |
| |
•
|
Amendment to IFRIC 14: Prepayment of a minimum funding
requirement (effective date: 1 January 2011).
|
| |
| |
•
|
IFRS 7 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 January 2011).
|
| |
| |
•
|
Amendment to IFRIC 13 Customer Loyalty Programmes (effective
date: 1 January 2011).
|
| |
| |
•
|
IAS 34 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 January 2011).
|
| |
| |
•
|
IAS 1 Amendments resulting from May 2010 Annual Improvements
(effective date: 1 January 2011).
|
The Group has not applied any of the standards or
interpretations issued prior to their deadline. The
Company’s directors do not expect that the entry into force
of these modifications will have a significant effect on the
consolidated financial statements.
|
|
|
(b)
|
Relevant
accounting estimates, assumptions and judgements used when
applying accounting principles
|
The preparation of consolidated financial statements in
conformity with IFRS requires management to make judgements,
estimates and assumptions that affect the application of Group
accounting policies. A
F-52
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
summary of the items requiring a greater degree of judgement or
complexity, or where the assumptions and estimates made are
significant to the preparation of the consolidated financial
statements, are as follows:
|
|
|
| |
•
|
The assumptions used for calculation of the fair value of
financial instruments (see note 4 (k)).
|
| |
| |
•
|
Measurement of assets and goodwill to determine any related
impairment losses (see note 4(i)).
|
| |
| |
•
|
Useful lives of property, plant and equipment and intangible
assets (see notes 4(g) and 4(h)).
|
| |
| |
•
|
Evaluation of the capitalisation of development costs (see
note 4(h)).
|
| |
| |
•
|
Evaluation of provisions and contingencies (see note 4(r)).
|
| |
| |
•
|
Evaluation of the effectiveness of hedging (see note 4(l)
and 17 (f)).
|
| |
| |
•
|
The application of the definition of a business (see
note 4(b)).
|
F-53
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The percentages of direct or indirect ownership of subsidiaries
by the Company at 31 December 2010, 2009 and 2008, as well
as the consolidation method used in each case for preparation of
the accompanying consolidated financial statements, are detailed
below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
31/12/09
|
|
31/12/08
|
|
|
|
Percentage Ownership
|
|
Percentage Ownership
|
|
Percentage Ownership
|
|
|
|
Direct
|
|
Indirect
|
|
Direct
|
|
Indirect
|
|
Direct
|
|
Indirect
|
|
|
|
Parent
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols, S.A.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fully-consolidated companies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Laboratorios Grifols, S.A.
|
|
|
99.998
|
|
|
|
0.002
|
|
|
|
99.998
|
|
|
|
0.002
|
|
|
|
99.998
|
|
|
|
0.002
|
|
|
Instituto Grifols, S.A.
|
|
|
99.998
|
|
|
|
0.002
|
|
|
|
99.998
|
|
|
|
0.002
|
|
|
|
99.998
|
|
|
|
0.002
|
|
|
Movaco, S.A.
|
|
|
99.999
|
|
|
|
0.001
|
|
|
|
99.999
|
|
|
|
0.001
|
|
|
|
99.999
|
|
|
|
0.001
|
|
|
Grifols Portugal Productos Farmacéuticos e Hospitalares,
Lda.
|
|
|
0.010
|
|
|
|
99.990
|
|
|
|
0.015
|
|
|
|
99.985
|
|
|
|
0.015
|
|
|
|
99.985
|
|
|
Diagnostic Grifols, S.A.
|
|
|
99.998
|
|
|
|
0.002
|
|
|
|
99.998
|
|
|
|
0.002
|
|
|
|
99.998
|
|
|
|
0.002
|
|
|
Logister, S.A.
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
Grifols Chile, S.A.
|
|
|
99.000
|
|
|
|
—
|
|
|
|
99.000
|
|
|
|
—
|
|
|
|
99.000
|
|
|
|
—
|
|
|
Biomat, S.A.
|
|
|
99.900
|
|
|
|
0.100
|
|
|
|
99.900
|
|
|
|
0.100
|
|
|
|
99.900
|
|
|
|
0.100
|
|
|
Grifols Argentina, S.A.
|
|
|
99.260
|
|
|
|
0.740
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols, s.r.o.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Logistica Grifols S.A. de C.V.
|
|
|
99.990
|
|
|
|
0.010
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols México, S.A. de C.V.
|
|
|
99.990
|
|
|
|
0.010
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols Viajes, S.A.
|
|
|
99.900
|
|
|
|
0.100
|
|
|
|
99.900
|
|
|
|
0.100
|
|
|
|
99.900
|
|
|
|
0.100
|
|
|
Grifols USA, LLC.
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
Grifols International, S.A.
|
|
|
99.900
|
|
|
|
0.100
|
|
|
|
99.900
|
|
|
|
0.100
|
|
|
|
99.900
|
|
|
|
0.100
|
|
|
Grifols Italia, S.p.A.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols UK, Ltd.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols Deutschland, GmbH
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols Brasil, Ltda.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols France, S.A.R.L.
|
|
|
99.000
|
|
|
|
1.000
|
|
|
|
99.000
|
|
|
|
1.000
|
|
|
|
99.000
|
|
|
|
1.000
|
|
|
Grifols Engineering, S.A.
|
|
|
99.950
|
|
|
|
0.050
|
|
|
|
99.950
|
|
|
|
0.050
|
|
|
|
99.950
|
|
|
|
0.050
|
|
|
Biomat USA, Inc.
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
Squadron Reinsurance Ltd.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols Inc.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols Biologicals Inc.
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
Alpha Therapeutic Italia, S.p.A.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Grifols Asia Pacific Pte., Ltd.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
F-54
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
31/12/09
|
|
31/12/08
|
|
|
|
Percentage Ownership
|
|
Percentage Ownership
|
|
Percentage Ownership
|
|
|
|
Direct
|
|
Indirect
|
|
Direct
|
|
Indirect
|
|
Direct
|
|
Indirect
|
|
|
|
Grifols Malaysia Sdn Bhd
|
|
|
—
|
|
|
|
30.000
|
|
|
|
—
|
|
|
|
30.000
|
|
|
|
—
|
|
|
|
30.000
|
|
|
Grifols (Thailand) Ltd.
|
|
|
—
|
|
|
|
48.000
|
|
|
|
—
|
|
|
|
48.000
|
|
|
|
—
|
|
|
|
48.000
|
|
|
Grifols Polska Sp.z.o.o.
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Plasmacare, Inc.
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
Plasma Collection Centers, Inc.
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
Arrahona Optimus S.L.
|
|
|
99.995
|
|
|
|
0.005
|
|
|
|
100.000
|
|
|
|
—
|
|
|
|
100.000
|
|
|
|
—
|
|
|
Woolloomooloo Holdings Pty Ltd.
|
|
|
49.000
|
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Lateral Grifols Pty Ltd.
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
—
|
|
|
Australian Corporate Number 073 272 830 Pty Ltd
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
—
|
|
|
Saturn Australia Pty Ltd.
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
—
|
|
|
Saturn Investments AG
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
49.000
|
|
|
|
—
|
|
|
|
—
|
|
|
Medion Grifols Diagnostic AG
|
|
|
—
|
|
|
|
39.200
|
|
|
|
—
|
|
|
|
39.200
|
|
|
|
—
|
|
|
|
—
|
|
|
Medion Diagnostics GmbH
|
|
|
—
|
|
|
|
39.200
|
|
|
|
—
|
|
|
|
39.200
|
|
|
|
—
|
|
|
|
—
|
|
|
Gri-Cel, S.A.
|
|
|
0.001
|
|
|
|
99.999
|
|
|
|
0.001
|
|
|
|
99.999
|
|
|
|
—
|
|
|
|
—
|
|
|
Grifols Colombia, Ltda.
|
|
|
99.000
|
|
|
|
1.000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Grifols Nordic AB
|
|
|
100.000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
31/12/09
|
|
31/12/08
|
|
|
|
Percentage Ownership
|
|
Percentage Ownership
|
|
Percentage Ownership
|
|
|
|
Direct
|
|
Indirect
|
|
Direct
|
|
Indirect
|
|
Direct
|
|
Indirect
|
|
|
|
Companies accounted for using the equity method
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quest International, Inc.
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
35.000
|
|
|
|
—
|
|
|
|
35.000
|
|
|
Nanotherapix, S.L
|
|
|
—
|
|
|
|
51.000
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
Subsidiaries in which the Company directly or indirectly owns
the majority of equity or voting rights have been fully
consolidated. Associates in which the Company owns between 20%
and 50% of share capital and has no power to govern the
financial or operating policies of these companies have been
accounted for under the equity method.
Although the Group holds 30% of the shares with voting rights of
Grifols Malaysia Sdn Bhd, it controls the majority of the
profit-sharing and voting rights of Grifols Malaysia Sdn Bhd
through a contract with the other shareholder and a pledge on
its shares.
Grifols (Thailand) Ltd. has two classes of shares and it grants
the majority of voting rights to the class of shares held by the
Group.
F-55
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The Group holds 99% of the voting rights in its Australian and
Swiss subsidiaries.
On 9 March 2010 one of the Group companies acquired 51% of
Nanotherapix, S.L., a technologically based company which
engages in advisory services, training of researchers, design
and development of technologies, services, know-how, molecules
and products applied to biotechnology, biomedicine and
pharmaceutical fields. The investment has been made through a
share capital increase of Euros 1,474 thousand in 2010 and
between 2011 and 2014 successive contributions will be made
through additional yearly share capital increases amounting to
Euros 1,472 thousand. These contributions are dependent on
certain shareholders of Nanotherapix, S.L. performing research
advisory and management tasks for this company. The acquisition
of Nanotherapix, S.L. has been treated as an equity-accounted
joint venture, as the company’s strategic and operational
decisions require unanimous consent of the parties sharing
control and Grifols does not have the majority vote in the board
of directors. Due to the losses incurred by Nanotherapix, S.L.,
impairment has been made for part of the investment in 2010 (see
note 10).
On 3 June 2010 the Group acquired 100% of Xepol AB (now
Grifols Nordic AB) which holds the intellectual property rights
for the treatment of the post-polio syndrome which includes
patents for the USA, Europe and Japan for a specific method of
treatment for this syndrome using intravenous immunoglobulin
(haemoderivative). The sum paid for this acquisition amounted to
Euros 2,255 thousand. The assets acquired and liabilities
assumed do not constitute a business pursuant to the definition
provided in IFRS 3 and, therefore, the transaction has been
recognised as the acquisition of an intangible asset.
On 9 November 2010 the Group sold the 35% interest it held
in the US company Quest International, Inc. for a sales price of
Euros 621 thousand.
|
|
|
(3)
|
Business
Combinations
|
|
|
|
(a)
|
Acquisition
of plasma collection centre from AmeriHealth Plasma
LLC.
|
On 1 April 2008 the Group acquired through Biomat USA, Inc.
a plasma collection centre in the USA from AmeriHealth Plasma
LLC.
The business combination cost included a contingent price of
Euros 1,328 thousand based on the number of litres of certain
products obtained during the following three years. The
contingent price has been determined based on the present value
of the estimated payments during the aforementioned period. In
2009 the estimated contingent price increased by Euros 225
thousand.
Details of the aggregate business combination cost and fair
value of the net assets acquired and goodwill at the acquisition
date are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Cost of the business combination
|
|
|
|
|
|
|
|
|
|
Cash paid
|
|
|
632
|
|
|
|
632
|
|
|
Fair value of deferred payment
|
|
|
1,968
|
|
|
|
1,743
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,600
|
|
|
|
2,375
|
|
|
Fair value of net assets acquired
|
|
|
3
|
|
|
|
3
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
2,597
|
|
|
|
2,372
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(see note 7
|
)
|
|
|
(see note 7
|
)
|
Goodwill generated in the acquisition is attributed to the blood
donors list of the plasma centre, an intangible which is not a
contractual or separable asset and other expected benefits from
the business combination related with the assets and activities
of the Group.
F-56
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Had the acquisition taken place at 1 January 2008, the
Group’s revenue and consolidated profit for the year would
not have varied significantly. The profit generated between the
acquisition date and 31 December 2008 is immaterial.
|
|
|
(b)
|
Acquisition
of Australian-Swiss group
|
On 3 March 2009 the Group acquired 49% of the economic
rights and 99% of the voting rights in a holding company of the
Australian-Swiss group Woolloomooloo Holdings Pty Ltd, thereby
gaining control of this group, for Euros 25 million through
a share capital increase fully subscribed by Grifols, S.A.
Details of the aggregate business combination cost, the fair
value of the net assets acquired and goodwill at the acquisition
date were follows:
| |
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Cost of the business combination
|
|
|
|
|
|
Cash paid
|
|
|
25,000
|
|
|
Fair value of deferred payment
|
|
|
497
|
|
|
|
|
|
|
|
|
Total cost of the business combination
|
|
|
25,497
|
|
|
Fair value of net assets acquired
|
|
|
9,307
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
16,190
|
|
|
|
|
|
|
|
|
|
|
|
(see note 7
|
)
|
Although at 3 March 2009 not all the information necessary
to allocate the purchase price correctly between the different
balance sheet captions used in the business combination was
available to the Group, further information was obtained at
31 December 2009 which made it possible to allocate assets
and liabilities more accurately in accordance with the amounts
indicated in the table above. Upon completion of the analysis,
no changes have arisen to the estimate made at 31 December
2009.
Goodwill generated in the acquisition is attributed to the
synergies and other expected benefits from the business
combination of the assets and activities of the Group.
The Australian-Swiss Group provides the commercial strength
required by Grifols to consolidate and increase its presence in
the diagnostic markets of Australia and New Zealand, which until
this acquisition consisted only of the sale of instruments
through distributors.
After obtaining the licence for Flebogamma DIF in Australia
(next generation IVIG), Grifols haemoderivatives began to be
commercialised in this country.
Grifols’s investment also included the acquisition under
the same terms, of Medion, located in Switzerland, which has
developed new technology for determining blood groups,
supplementary to that used by Grifols.
Had the acquisition taken place at 1 January 2009, the
Group’s revenue and consolidated profit for the period
would not have varied significantly. Accumulated losses incurred
by the Australian-Swiss group attributable to the Group results
from the date of acquisition to 31 December 2009 amounted
to Euros 652 thousand.
F-57
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
At the date of acquisition the amounts of recognised assets,
liabilities and contingent liabilities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
Book Value
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Intangible assets (note 8)
|
|
|
6,525
|
|
|
|
476
|
|
|
Property, plant and equipment (note 9)
|
|
|
2,307
|
|
|
|
3,113
|
|
|
Deferred tax assets (note 29)
|
|
|
500
|
|
|
|
258
|
|
|
Inventories (note 12)
|
|
|
3,549
|
|
|
|
3,549
|
|
|
Trade and other receivables
|
|
|
2,096
|
|
|
|
2,096
|
|
|
Other assets
|
|
|
293
|
|
|
|
293
|
|
|
Cash and cash equivalents
|
|
|
10,112
|
|
|
|
10,112
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
25,382
|
|
|
|
19,897
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other payables
|
|
|
3,165
|
|
|
|
3,165
|
|
|
Other liabilities
|
|
|
1,273
|
|
|
|
1,272
|
|
|
Deferred tax liabilities (note 29)
|
|
|
1,761
|
|
|
|
551
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and contingent liabilities
|
|
|
6,199
|
|
|
|
4,988
|
|
|
Total net assets
|
|
|
19,183
|
|
|
|
14,909
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-controlling interests (note 19)
|
|
|
(9,876
|
)
|
|
|
|
|
|
Total net assets acquired
|
|
|
9,307
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill (note 7)
|
|
|
16,190
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash paid
|
|
|
25,497
|
|
|
|
|
|
|
Cash and cash equivalents of the acquired company
|
|
|
(10,112
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash outflow for the acquisition
|
|
|
15,385
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets were measured at fair value. The royalty
relief method has been used to measure certain patents acquired
by the Group. An 8% royalty was considered, together with a
discount rate after tax of 10%. Patents were measured on the
basis of projected sales for a fifteen-year period.
|
|
|
(4)
|
Accounting
and Valuation Principles Applied
|
Subsidiaries are entities, including special purpose entities
(SPE), over which the Group exercises control, either directly
or indirectly through other subsidiaries. Control is the power
to govern the financial and operating policies of an entity so
as to obtain benefits from its activities. In assessing control,
potential voting rights held by the Group or other entities that
are exercisable or convertible at the end of each reporting
period are considered.
Information on subsidiaries forming the consolidated Group is
included in note 2 (c).
The income, expenses and cash flows of subsidiaries are included
in the consolidated financial statements from the date of
acquisition, which is when the Group takes control, until the
date that control ceases.
All significant balances and transactions as well as any
unrealised gains or losses are eliminated in the consolidation
process.
F-58
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The accounting principles and criteria used by subsidiaries have
been consistent with those applied by the Company in the
preparation of the consolidated financial statements.
The financial statements of subsidiaries refer to the same date
and the same reporting period as the financial statements of
Grifols, S.A.
|
|
|
(b)
|
Business
combinations
|
As permitted by IFRS 1: First-time Adoption of International
Financial Reporting Standards, the Group has recognized only
business combinations that occurred on or after 1 January
2004, date of transition to IFRS, using the acquisition method.
Entities acquired prior to that date were recognised in
accordance with accounting principles prevailing at that time,
taking into account the necessary corrections and adjustments at
the transition date.
The Group applies the revised IFRS 3: Business Combinations in
transactions made subsequent to 1 January 2010.
The Company applies the acquisition method for business
combinations.
The acquisition date is the date on which the Company obtains
control of the acquiree.
Business
combinations made subsequent to 1 January 2010
The consideration transferred in a business combination is
determined at acquisition date and calculated as the sum of the
fair values of the assets transferred, the liabilities incurred
or assumed, the equity interests issued and any asset or
liability contingent consideration depending on future events or
the compliance of certain conditions in exchange for the control
of the business acquired.
The consideration transferred excludes any payment that does not
form part of the exchange for the acquired business.
Acquisition-related costs are accounted for as expenses when
incurred. Share increase costs are recognised as equity when the
increase takes place and borrowing costs are deducted from the
financial liability when it is recognised.
At the acquisition date the Group recognizes, at fair value, the
assets acquired and liabilities assumed. Liabilities assumed
include contingent liabilities provided that they represent
present obligations that arise from past events and their fair
value can be measured reliably. The Group also recognises
indemnification assets transferred by the seller at the same
time and following the same measurement criteria as the item
that is subject to indemnification from the acquired business
taking into consideration, where applicable, the insolvency risk
and any contractual limit on the indemnity amount.
This criteria does not include non-current assets or disposable
groups of assets which are classified as held for sale,
long-term defined benefit employee benefit liabilities,
share-based payment transactions, deferred tax assets and
liabilities and intangible assets arising from the acquisition
of previously transferred rights.
Assets and liabilities assumed are classified and designated for
subsequent measurement in accordance with the contractual terms,
economic conditions, operating or accounting policies and other
factors that exist at the acquisition date, except for leases
and insurance contracts.
The excess between the consideration transferred and the value
of net assets acquired and liabilities assumed, less the value
assigned to minority interests, is recognised as goodwill. Where
the excess is negative, a bargain purchase gain is recognized
immediately in profit and loss.
Any contingent consideration payable is recognized at fair value
at the acquisition date. If the contingent consideration is
classified as equity, it is not remeasured and settlement is
accounted for within equity.
F-59
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Otherwise, subsequent changes to the fair value of the
contingent consideration are recognized in profit and loss.
Business
combinations made prior to 1 January 2010
The cost of the business combination is calculated as the sum of
the acquisition-date fair values of the assets transferred, the
liabilities incurred or assumed, and equity instruments issued
by the Group, in exchange for control of the acquiree, plus any
costs directly attributable to the business combination. Any
additional consideration contingent on future events or the
fulfilment of certain conditions is included in the cost of the
combination provided that it is probable that an outflow of
resources embodying economic benefits will be required and the
amount of the obligation can be reliably estimated. Subsequent
recognition of contingent considerations or subsequent
variations to contingent considerations are recognised as a
prospective adjustment to the cost of the business combination.
Where the cost of the business combination exceeds the
Group’s interest in the fair value of the identifiable net
assets of the entity acquired, the difference is recognised as
goodwill. If the acquirer’s interest in the fair value of
net assets exceeds the cost of the business combination, the
difference remaining after reassessment is recognised by the
acquirer in profit and loss.
|
|
|
(c)
|
Non-controlling
interests
|
Prior to adoption of IFRS 3 (Revised) non-controlling interests
in subsidiaries acquired after 1 January 2004 were
recognised at the acquisition date at the proportional part of
the fair value of the identifiable net assets. Non-controlling
interests in subsidiaries acquired prior to the transition date
were recognised at the proportional part of the equity of the
subsidiaries at the date of first consolidation.
Non-controlling interests are disclosed in the consolidated
balance sheet under equity separately from equity attributable
to the parent. Non-controlling interests’ share in
consolidated profit or loss for the year (and in consolidated
comprehensive income for the year) is disclosed separately in
the consolidated income statement (consolidated statement of
comprehensive income).
Consolidated comprehensive income and changes in equity of the
subsidiaries attributable to the Group and non-controlling
interests after consolidation adjustments and eliminations, is
determined in accordance with the percentage ownership at year
end, without considering the possible exercise or conversion of
potential voting rights and after discounting the effect of
dividends, agreed or otherwise, on preference shares with
cumulative rights classified in equity accounts. However, Group
and non-controlling interests are calculated taking into account
the possible exercise of potential voting rights and other
derivative financial instruments which, in substance, currently
allow access to the economic benefits associated with the
interests held, such as entitlement to a share in future
dividends and changes in the value of subsidiaries.
The excess of losses attributable to non-controlling interests,
which cannot be attributed to the latter as such losses exceed
their interest in the equity of the Company, is recognised as a
decrease in the equity of the Company, except when the
non-controlling interests are obliged to assume part or all of
the losses and are in a position to make the necessary
additional investment. Subsequent profits obtained by the Group
are attributed to the Company until the non-controlling
interests’s share in prior years’ losses is recovered.
As of 1 January 2010 and in accordance with IFRS 3
(Revised), profit and loss and each component of other
comprehensive income are assigned to equity attributable to
shareholders of the Parent company and to non-controlling
interests in proportion to their interest, although this implies
a balance receivable from non-controlling interests. Agreements
signed between the Group and the non-controlling interests are
recognised as a separate transaction.
F-60
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(d)
|
Associates
and joint ventures
|
Associates
Associates are entities over which the Company has significant
direct or indirect influence through subsidiaries. Significant
influence is the power to participate in the financial and
operating policy decisions of the investee but is not control or
joint control over those policies. The existence of potential
voting rights that are currently exercisable or convertible,
including potential voting rights held by the Group or other
entities, are considered when assessing whether an entity has
significant influence.
Investments in associates are accounted for using the equity
method from the date that significant influence commences until
the date that significant influence ceases.
Details of investments accounted for using the equity method are
included in note 2 (c).
Purchases of shareholdings in associates are recognised applying
the acquisition method, as described for subsidiaries. Any
excess of cost of acquisition over the part of fair value of the
identifiable net assets acquired is considered as goodwill,
which is included in the fair value of the investment. If the
cost of acquisition is less than the fair value of identifiable
net assets acquired, the difference is recognised when
determining the investor’s share in the profit of the
associate in the period of acquisition.
The Group’s share in the profit or loss of the associates
from the date of acquisition is recognised as an increase or
decrease in the value of the investments, with a credit or debit
to profit or loss of associates accounted for using the equity
method of the consolidated income statement (consolidated
statement of comprehensive income). The Group’s share in
other comprehensive income of the associate obtained from the
date of acquisition is recognised as an increase or decrease in
the investment in the associate with a balancing entry on a
separate line in other comprehensive income. The distribution of
dividends is recognised as a decrease in the value of the
investment. The Group’s share of profit and loss, including
impairment losses recognised by the associates, is calculated
based on income and expenses arising from application of the
purchase method.
The Group’s share in the profit or loss of an associate and
changes in equity are calculated to the extent of the
Group’s interest in the associate at year end and does not
reflect the possible exercise or conversion of potential voting
rights. However, the Group’s share is calculated taking
into account the possible exercise of potential voting rights
and other derivative financial instruments which, in substance,
currently allow access to the economic benefits associated with
the interests held, such as entitlement to a share in future
dividends and changes in the value of associates.
Losses of an associate attributable to the Group are limited to
the extent of its interest, except where the Group has legal or
implicit obligations or when payments have been made on behalf
of the associate. For the purpose of recognising losses in
associates, net investments are considered as the carrying
amount of the investment after application of the equity method
plus any other item which in substance forms part of the
investment in the associate. Subsequent profits attributable to
those associates for which impairment losses are limited are
recognised to the extent of the previously unrecognised losses.
Unrealised gains and losses on transactions between the Group
and associates are only recognised when they relate to interests
of other unrelated investors, except in the case of unrealised
losses evidencing the impairment of the transferred asset.
The accounting policies of associates have been harmonised in
terms of timing and measurement, applying the policies described
for subsidiaries.
F-61
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Joint
ventures
Joint ventures are those in which there is a contractual
agreement to share the control over an economic activity, in
such a way that strategic financial and operating decisions
relating to the activity require the unanimous consent of the
Group and the remaining venturers.
Investments in joint ventures are accounted for using the equity
method.
The acquisition cost of investments in joint ventures is
determined consistently with that established for investments in
associates.
|
|
|
(e)
|
Foreign
currency transactions
|
|
|
|
(i)
|
Functional
currency and presentation currency
|
The consolidated financial statements are presented in thousands
of Euros, which is the functional and presentation currency of
the Company.
|
|
|
(ii)
|
Transactions,
balances and cash flows in foreign currency
|
Foreign currency transactions are translated into the functional
currency using the previous month’s exchange rate for all
transactions performed during the current month. This method
does not differ significantly from applying the exchange rate at
the date of the transaction.
Monetary assets and liabilities denominated in foreign
currencies have been translated into thousands of Euros at the
closing rate, while non-monetary assets and liabilities measured
at historical cost have been translated at the exchange rate
prevailing at the transaction date. Non-monetary assets measured
at fair value have been translated into thousands of Euros at
the exchange rate at the date that the fair value was determined.
In the consolidated statement of cash flows, cash flows from
foreign currency transactions have been translated into
thousands of Euros at the exchange rates prevailing at the dates
the cash flows occur. The effect of exchange rate fluctuations
on cash and cash equivalents denominated in foreign currencies
is recognised separately in the statement of cash flows as
“Effect of exchange rate fluctuations on cash and cash
equivalents”.
Exchange gains and losses arising on the settlement of foreign
currency transactions and the translation into thousands of
Euros of monetary assets and liabilities denominated in foreign
currencies are recognised in profit or loss.
|
|
|
(iii)
|
Translation
of foreign operations
|
The translation into thousands of Euros of foreign operations
for which the functional currency is not the currency of a
hyperinflationary economy is based on the following criteria:
|
|
|
| |
•
|
Assets and liabilities, including goodwill and net asset
adjustments derived from the acquisition of the operations,
including comparative amounts, are translated at the closing
rate at each balance sheet date.
|
| |
| |
•
|
Income and expenses, including comparative amounts, are
translated into thousands of Euros using the previous
month’s exchange rate for all transactions performed during
the current month. This method does not differ significantly
from using the exchange rate at the date of the transaction;
|
| |
| |
•
|
All resulting exchange differences are recognised as translation
differences in equity.
|
F-62
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
In the consolidated statement of cash flows, cash flows,
including comparative balances, of the subsidiaries and foreign
joint ventures are translated into thousands of Euros applying
the exchange rates prevailing at the transaction date.
In accordance with IAS 23: Borrowing Costs, since 1 January
2009 the Group recognises interest cost directly attributable to
the purchase, construction or production of qualifying assets as
an increase in the value of these assets. Qualifying assets are
those which require a substantial period of time before they can
be used or sold. To the extent that funds are borrowed
specifically for the purpose of obtaining a qualifying asset,
the amount of borrowing costs eligible for capitalisation is
determined as the actual borrowing costs incurred, less any
investment income on the temporary investment of those funds.
Capitalised borrowing costs corresponding to general borrowing
are calculated as the weighted average of the qualifying assets
without considering specific funds. The amount of borrowing
costs capitalised cannot exceed the amount of borrowing costs
incurred during that period. The capitalised interest cost
includes adjustments to the carrying amount of financial
liabilities arising from the effective portion of hedges entered
into by the Group.
The Group begins capitalising borrowing costs as part of the
cost of a qualifying asset when it incurs expenditures for the
asset, interest is accrued, and it undertakes activities that
are necessary to prepare the asset for its intended use or sale,
and ceases capitalising borrowing costs when all or
substantially all the activities necessary to prepare the
qualifying asset for its intended use or sale are complete.
Nevertheless, capitalisation of borrowing costs is suspended
when active development is interrupted for extended periods.
|
|
|
(g)
|
Property,
plant and equipment
|
Property, plant and equipment are recognised at cost or deemed
cost, less accumulated depreciation and any accumulated
impairment losses. The cost of self-constructed assets is
determined using the same principles as for an acquired asset,
while also considering the criteria applicable to production
costs of inventories. Capitalised production costs are
recognised by allocating the costs attributable to the asset to
“self-constructed non-current assets” in the
consolidated income statement.
At 1 January 2004, upon their first application of IFRS-EU,
the Group opted to apply the exemption regarding fair value and
revaluation as deemed cost as permitted by IFRS 1: First-time
Adoption of IFRS.
Property, plant and equipment are depreciated by allocating the
depreciable amount of an asset on a systematic basis over its
useful life. The depreciable amount is the cost or deemed cost
less its residual value. The Group determines the depreciation
charge separately for each component of property, plant and
equipment with a cost that is significant in relation to the
total cost of the asset.
Depreciation of property, plant and equipment is determined
based on the following criteria outlined belows:
| |
|
|
|
|
|
|
|
|
|
|
|
Depreciation
|
|
|
|
|
|
|
Method
|
|
|
Rates
|
|
|
|
Buildings
|
|
|
Straight line
|
|
|
|
1% — 3%
|
|
|
Plant and machinery
|
|
|
Straight line
|
|
|
|
8% — 10%
|
|
|
Other installations, equipment and furniture
|
|
|
Straight line
|
|
|
|
10% — 30%
|
|
|
Other property, plant and equipment
|
|
|
Straight line
|
|
|
|
16% — 25%
|
|
F-63
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The Group reviews residual values, useful lives and depreciation
methods at each financial year end. Changes to initially
established criteria are accounted for as a change in accounting
estimates.
(iii) Subsequent
recognition
Subsequent to initial recognition of the asset, only those costs
incurred which will probably generate future economic benefits
and for which the amount may reliably be measured are
capitalised. Costs of
day-to-day
servicing are recognised in profit and loss as incurred.
The Group recognises in the carrying amount of an item of
property, plant and equipment the cost of replacing part of such
an item when that cost is incurred if the recognition criteria
are met. The carrying amount of those parts that are replaced is
derecognised in accordance with the derecognition provisions of
IAS 16.
(iv) Impairment
The Group tests for impairment and reversals of impairment
losses on property, plant and equipment based on the criteria
set out in section (i) of this note.
Goodwill is generated on business combinations. As permitted by
IFRS 1: First-time Adoption of International Financial Reporting
Standards, the Group has recognised only business combinations
that occurred on or after 1 January 2004, the date of
transition to IFRS-EU, using the acquisition method. Entities
acquired prior to that date were recognised in accordance with
accounting principles prevailing at that time, taking into
account the necessary corrections and adjustments at the
transition date.
Goodwill is not amortised, but tested for impairment annually or
more frequently if events indicate a potential impairment loss.
Goodwill acquired in business combinations is allocated to the
cash-generating units (CGUs) or groups of CGUs which are
expected to benefit from the synergies of the business
combination and the criteria described in note 7 are
applied. After initial recognition, goodwill is measured at cost
less any accumulated impairment losses.
|
|
|
(ii)
|
Internally
generated intangible assets
|
Any research and development expenditure incurred during the
research phase of projects is recognised as an expense when
incurred.
Costs related with development activities are capitalised when:
|
|
|
| |
•
|
The Group has technical studies justifying the feasibility of
the production process.
|
| |
| |
•
|
The Group has undertaken a commitment to complete production of
the asset whereby it is in condition for sale or internal use.
|
| |
| |
•
|
The asset will generate sufficient future economic benefits.
|
| |
| |
•
|
The Group has sufficient financial and technical resources to
complete development of the asset and has developed budget and
cost accounting control systems which allow budgeted costs,
introduced changes and costs actually assigned to different
projects to be monitored.
|
The cost of internally generated assets is calculated using the
same criteria established for determining production costs of
inventories. The production cost is capitalised by allocating
the costs attributable to the asset to self-constructed assets
in the consolidated income statement.
F-64
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Costs incurred in the course of activities which contribute to
increasing the value of the different businesses in which the
Group as a whole operates are expensed as they are incurred.
Replacements or subsequent costs incurred on intangible assets
are generally recognised as an expense, except where they
increase the future economic benefits expected to be generated
by the assets.
|
|
|
(iii)
|
Other
intangible assets
|
Other intangible assets are carried at cost, less accumulated
amortisation and impairment losses.
Emission rights, which are recognised when the Group becomes
entitled to such rights, are carried at cost less accumulated
impairment. Rights acquired free of charge or at a price
substantially lower than fair value, are recognised at fair
value, which is generally the market value of the rights at the
start of the calendar year. The difference between fair value
and, where appropriate, the amount received, is recognised under
“government grants”. Government grants are recognised
in profit or loss in line with the emission of gases in
proportion to total emissions foreseen for the complete period
for which the emission rights have been received, irrespective
of whether the rights previously received have been sold or
impaired.
Under the terms of Law 1 of 9 March 2005 governing
greenhouse gas emission rights, emission rights deriving from a
certified reduction in emissions or from a unit created to
reduce emissions through clean development mechanisms or a
pooling of rights, are carried at cost of production using the
same criteria as for inventories.
Emission rights are not amortised. The Group derecognises
emission rights on a weighted average cost basis.
|
|
|
(v)
|
Useful
life and amortisation rates
|
The Group assesses whether the useful life of each intangible
asset acquired is finite or indefinite. An intangible asset is
regarded by the Group as having an indefinite useful life when
there is no foreseeable limit to the period over which the asset
will generate net cash inflows.
Intangible assets with indefinite useful lives are not amortised
but tested for impairment at least annually.
Intangible assets with finite useful lives are amortised by
allocating the depreciable amount of an asset on a systematic
basis over its useful life, by applying the following criteria:
| |
|
|
|
|
|
|
|
|
|
|
|
|
Estimated
|
|
|
|
Amortisation
|
|
|
Years of
|
|
|
|
Method
|
|
|
Useful Life
|
|
|
|
Development expenses
|
|
|
Straight line
|
|
|
3 — 5
|
|
Concessions, patents, licences, trademarks and similar
|
|
|
Straight line
|
|
|
5 — 15
|
|
Software
|
|
|
Straight line
|
|
|
3 — 6
|
The depreciable amount is the cost or deemed cost of an asset
less its residual value.
The Group reviews the residual value, useful life and
amortisation method for intangible assets at each financial year
end. Changes to initially established criteria are accounted for
as a change in accounting estimates.
F-65
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(i)
|
Impairment
of non-financial assets subject to depreciation or
amortisation
|
The Group evaluates whether there are indications of possible
impairment losses on non-financial assets subject to
amortisation or depreciation to verify whether the carrying
amount of these assets exceeds the recoverable amount.
Irrespective of any indication of impairment, the Group tests
for possible impairment of goodwill, intangible assets with
indefinite useful lives, and intangible assets with finite
useful lives not yet available for use, at least annually.
The recoverable amount is the higher of an asset’s fair
value less costs to sell and its value in use. An asset’s
value in use is calculated based on an estimate of the future
cash flows expected to derive from the use of the asset,
expectations about possible variations in the amount or timing
of those future cash flows, the time value of money, the price
for bearing the uncertainty inherent in the asset and other
factors that market participants would reflect in pricing the
future cash flows deriving from the asset.
Negative differences arising from comparison of the carrying
amounts of the assets with their recoverable amounts are
recognised in the consolidated income statement.
Recoverable amount is determined for each individual asset,
unless the asset does not generate cash inflows that are largely
independent of those from other assets or groups of assets. If
this is the case, recoverable amount is determined for the
cash-generating unit (CGU) to which the asset belongs.
Impairment losses recognised for cash-generating units are first
allocated to reduce, where applicable, the carrying amount of
goodwill allocated to the CGU and then to the other assets of
the CGU pro rata on the basis of the carrying amount of each
asset. The carrying amount of each asset may not be reduced
below the highest of its fair value less costs to sell, its
value in use and zero.
At the end of each reporting period the Group assesses whether
there is any indication that an impairment loss recognised in
prior periods may no longer exist or may have decreased.
Impairment losses on goodwill are not reversible. Impairment
losses for other assets are only reversed if there has been a
change in the estimates used to calculate the recoverable amount
of the asset.
A reversal of an impairment loss is recognised in consolidated
profit or loss. The increase in the carrying amount of an asset
attributable to a reversal of an impairment loss may not exceed
the carrying amount that would have been determined, net of
depreciation or amortisation, had no impairment loss been
recognised.
The reversal of an impairment loss for a CGU is allocated to its
assets, except for goodwill, pro rata with the carrying amounts
of those assets, with the limit per asset of the lower of its
recoverable value and the carrying amount which would have been
obtained, net of depreciation, had no impairment loss been
recognised.
(j) Leases
|
|
|
(i)
|
Lessee
accounting records
|
The Group has the right to use certain assets through lease
contracts.
Leases in which the Group assumes substantially all the risks
and rewards incidental to ownership are classified as finance
leases, otherwise they are classified as operating leases.
• Finance
leases
At the commencement of the lease term, the Group recognises
finance leases as assets and liabilities at the lower of the
fair value of the leased asset and the present value of the
minimum lease payments. Initial direct costs are added to the
asset’s carrying amount. Minimum lease payments are
apportioned
F-66
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
between the finance charge and the reduction of the outstanding
liability. The finance charge is allocated to each period during
the lease term so as to produce a constant periodic rate of
interest on the remaining balance of the liability. Contingent
rents are recognised as an expense in the years in which they
are incurred.
• Operating
leases
Lease payments under an operating lease (excluding insurance and
maintenance) are recognised as an expense on a straight-line
basis unless another systematic basis is representative of the
time pattern of the user’s benefit.
(ii) Leasehold
improvements
Non-current investments in properties leased from third parties
are classified using the same criteria as for property, plant
and equipment. Investments are amortised over the lower of their
useful lives and the term of the lease contract. The lease term
is consistent with that established for recognition of the lease.
(k) Financial
instruments
|
|
|
(i)
|
Classification
of financial instruments
|
Financial instruments are classified on initial recognition as a
financial asset, a financial liability or an equity instrument
in accordance with the substance of the contractual arrangement
and the definitions of a financial liability, a financial asset
and an equity instrument set out in IAS 32: Financial
Instruments — Presentation.
Financial instruments are classified into the following
categories: financial assets and financial liabilities at fair
value through profit and loss, loans and receivables,
held-to-maturity
investments,
available-for-sale
financial assets and financial liabilities. The Group classifies
financial instruments into different categories based on the
nature of the instruments and management’s intentions on
initial recognition.
Regular way purchases and sales of financial assets are
recognised at trade date, when the Group undertakes to purchase
or sell the asset.
a) Financial
assets at fair value through profit or loss
Financial assets and financial liabilities at fair value through
profit or loss are those which are classified as held for
trading or which the Group designated as such on initial
recognition.
A financial asset or liability is classified as held for trading
if:
|
|
|
| |
•
|
it is acquired or incurred principally for the purpose of
selling or repurchasing it in the near term
|
| |
| |
•
|
it forms part of a portfolio of identified financial instruments
that are managed together and for which there is evidence of a
recent pattern of short-term profit-taking, or
|
| |
| |
•
|
it is a derivative, except for a derivative which has been
designated as a hedging instrument and complies with conditions
for effectiveness or a derivative that is a financial guarantee
contract.
|
Financial assets and financial liabilities at fair value through
profit or loss are initially recognised at fair value.
Transaction costs directly attributable to the acquisition or
issue are recognised as an expense.
The Group does not reclassify any financial assets or
liabilities from or to this category while they are recognised
in the consolidated balance sheet.
F-67
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
b) Loans
and receivables
Loans and receivables are non-derivative financial assets with
fixed or determinable payments that are not quoted in an active
market, other than those classified in other financial asset
categories. These assets are recognised initially at fair value,
including transaction costs, and are subsequently measured at
amortised cost using the effective interest method.
c) Available-for-sale
financial assets
Available-for-sale
financial assets are non-derivative financial assets that are
either designated specifically to this category or do not comply
with requirements for classification in the above categories.
Available-for-sale
financial assets are initially recognised at fair value, plus
any transaction costs directly attributable to the purchase.
After initial recognition, financial assets classified in this
category are measured at fair value and any gain or loss is
accounted for in other comprehensive income recognised in
equity. On disposal of the financial assets, amounts recognised
in other comprehensive income or the impairment losses are
reclassified to profit or loss.
d) Financial
assets and liabilities carried at cost
Investments in equity instruments whose fair value cannot be
reliably measured and derivative instruments that are linked to
and must be settled by delivery of such unquoted equity
instruments, are measured at cost.
e) Financial
assets and liabilities at fair value through profit or
loss
Financial assets and financial liabilities at fair value through
profit or loss, which comprise derivatives, are initially
recognised at fair value and after initial recognition are
recognised at fair value through profit and loss.
(ii) Offsetting
principles
A financial asset and a financial liability can only be offset
when the Group currently has a legally enforceable right to set
off the recognised amounts and intends either to settle on a net
basis, or to realise the asset and settle the liability
simultaneously.
(iii) Fair
value
The fair value is the amount for which an asset can be
exchanged, or a liability settled, between knowledgeable,
willing parties in an arm’s length transaction. The Group
generally applies the following systematic hierarchy to
determine the fair value of financial assets and financial
liabilities:
|
|
|
| |
•
|
Firstly, the Group applies the quoted prices of the most
advantageous active market to which the entity has immediate
access, adjusted where appropriate to reflect any differences in
counterparty credit risk between instruments traded in that
market and the one being valued. The quoted market price for an
asset held or liability to be issued is the current bid price
and, for an asset to be acquired or liability held, the asking
price. If the Group has assets and liabilities with offsetting
market risks, it uses mid-market prices as a basis for
establishing fair values for the offsetting risk positions and
applies the bid or asking price to the net open position as
appropriate.
|
| |
| |
•
|
When current bid and asking prices are unavailable, the price of
the most recent transactions is used, adjusted to reflect
changes in economic circumstances.
|
| |
| |
•
|
Otherwise, the Group applies generally accepted measurement
techniques using, insofar as is possible, market data and, to a
lesser extent, specific Group data.
|
F-68
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
(iv) Amortised
cost
The amortised cost of a financial asset or liability is the
amount at which the asset or liability was measured at initial
recognition, minus principal repayments, plus or minus the
cumulative amortisation using the effective interest method of
any difference between that initial amount and maturity amount
and minus any reduction for impairment or uncollectibility.
(v) Impairment
of financial assets carried at cost
The amount of the impairment loss on assets carried at cost is
measured as the difference between the carrying amount of the
financial asset and the present value of estimated future cash
flows discounted at the current market rate of return for a
similar financial asset. Such impairment losses cannot be
reversed and are therefore recognised directly against the value
of the asset and not as an allowance account.
(vi) Impairment
of
available-for-sale
financial assets
When a decline in the fair value of an
available-for-sale
financial asset at fair value through profit or loss has been
accounted for in other comprehensive income, the accumulative
loss is reclassified from equity to profit or loss when there is
objective evidence that the asset is impaired, even though the
financial asset has not been derecognised. The impairment loss
recognised in profit and loss is calculated as the difference
between the acquisition cost, net of any reimbursements or
repayment of the principal, and the present fair value, less any
impairment loss previously recognised in profit and loss for the
year.
Impairment losses relating to investments in equity instruments
are not reversible and are therefore recognised directly against
the value of the asset and not as a corrective provision.
If the fair value of debt instruments increases and the increase
can be objectively related to an event occurring after the
impairment loss was recognised, the increase is recognised in
profit and loss up to the amount of the previously recognised
impairment loss and any excess is accounted for in other
comprehensive income recognised in equity.
(vii) Financial
liabilities
Financial liabilities, including trade and other payables, which
are not classified at fair value through profit or loss, are
initially recognised at fair value less any transaction costs
that are directly attributable to the issue of the financial
liability. After initial recognition, liabilities classified
under this category are measured at amortised cost using the
effective interest method.
(viii) Derecognition
of financial assets
The Group applies the criteria for derecognition of financial
assets to part of a financial asset or part of a group of
similar financial assets or to a financial asset or group of
similar financial assets.
Financial assets are derecognised when the contractual rights to
the cash flows from the financial asset expire or have been
transferred and the Group has transferred substantially all the
risks and rewards of ownership. Where the Group retains the
contractual rights to receive cash flows, it only derecognises
financial assets when it has assumed a contractual obligation to
pay the cash flows to one or more recipients and if the
following requirements are met:
|
|
|
| |
•
|
Payment of the cash flows is conditional on their prior
collection.
|
| |
| |
•
|
The Group is unable to sell or pledge the financial asset.
|
| |
| |
•
|
The cash flows collected on behalf of the eventual recipients
are remitted without material delay and the Group is not
entitled to reinvest the cash flows. This criterion is not
applicable to investments in
|
F-69
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
|
cash or cash equivalents made by the Group during the settlement
period from the collection date to the date of required
remittance to the eventual recipients, provided that interest
earned on such investments is passed on to the eventual
recipients.
|
If the Group neither transfers nor retains substantially all the
risks and rewards of ownership of the financial asset, it
determines whether it has retained control of the financial
asset. In this case:
|
|
|
| |
•
|
If the Group has not retained control, it derecognises the
financial asset and recognises separately as assets or
liabilities any rights and obligations created or retained in
the transfer.
|
| |
| |
•
|
If the Group has retained control, it continues to recognise the
financial asset to the extent of its continuing involvement in
the financial asset and recognises an associated liability. The
extent of the Group’s continuing involvement in the
transferred asset is the extent to which it is exposed to
changes in the value of the transferred asset. The transferred
asset and the associated liability are measured on a basis that
reflects the rights and obligations that the Group has retained.
The associated liability is measured in such a way that the
carrying amount of the transferred asset and the associated
liability is equal to the amortised cost of the rights and
obligations retained by the Group, if the transferred asset is
measured at amortised cost, or to the fair value of the rights
and obligations retained by the Group, if the transferred asset
is measured at fair value. The Group continues to recognise any
income arising on the transferred asset to the extent of its
continuing involvement and recognises any expense incurred on
the associated liability. Recognised changes in the fair value
of the transferred asset and the associated liability are
accounted for consistently with each other in profit and loss or
equity, following the general recognition criteria described
previously, and are not offset.
|
If the Group retains substantially all the risks and rewards of
ownership of a transferred financial asset, the consideration
received is recognised in equity. Transaction costs are
recognised in profit and loss using the effective interest
method.
Hedging financial instruments are initially recognised using the
same criteria as those described for financial assets and
financial liabilities. Hedging financial instruments that do not
meet the hedge accounting requirements are classified and
measured as financial assets and financial liabilities at fair
value through profit and loss. Derivative financial instruments
which qualify for hedge accounting are initially measured at
fair value.
At the inception of the hedge the Group formally designates and
documents the hedging relationships and the objective and
strategy for undertaking the hedges. Hedge accounting is only
applicable when the hedge is expected to be highly effective at
the inception of the hedge and in subsequent years in achieving
offsetting changes in fair value or cash flows attributable to
the hedged risk, throughout the period for which the hedge was
designated (prospective analysis) and the actual effectiveness,
which can be reliably measured, is within a range of 80%-125%
(retrospective analysis).
Cash flow
hedges
The Group recognises the portion of the gain or loss on the
measurement at fair value of a hedging instrument that is
determined to be an effective hedge in other comprehensive
income. The ineffective portion and the specific component of
the gain or loss or cash flows on the hedging instrument,
excluding the measurement of the hedge effectiveness, are
recognised with a debit or credit to finance expenses or finance
income.
If a hedge of a forecast transaction subsequently results in the
recognition of a financial asset or a financial liability, the
associated gains or losses that were recognised in other
comprehensive income are
F-70
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
reclassified from equity to profit or loss in the same period or
periods during which the asset acquired or liability assumed
affects profit or loss and under the same caption of the
consolidated income statement (consolidated statement of
comprehensive income).
The Group’s acquisition of equity instruments of the
Company is recognised separately at cost of acquisition in the
consolidated balance sheet as a reduction in equity, regardless
of the motive of the purchase. Any gains or losses on
transactions with own equity instruments are not recognised in
consolidated profit or loss.
The subsequent redemption of Company shares, where applicable,
leads to a reduction in share capital in an amount equivalent to
the par value of such shares. Any positive or negative
difference between the cost of acquisition and the par value of
the shares is debited or credited to accumulated gains.
Transaction costs related with own equity instruments, including
the issue costs related with a business combination, are
accounted for as a deduction from equity, net of any tax effect.
Transactions realized in instruments of the Company’s own
equity are shown under equity and any gains or losses are also
credited or debited against reserves.
Inventories are measured at the lower of cost and net realisable
value. The cost of inventories comprises all costs of purchase,
costs of conversion and other costs incurred in bringing the
inventories to their present location and condition.
The costs of conversion of inventories include costs directly
related to the units of production and a systematic allocation
of fixed and variable production overheads that are incurred in
converting. Fixed production overheads are allocated based on
the higher of normal production capacity or actual level of
production.
The cost of raw materials and other supplies, the cost of
merchandise and costs of conversion are allocated to each
inventory unit on a
first-in,
first-out (FIFO) basis.
The Group uses the same cost model for all inventories of the
same nature and with a similar use within the Group.
Volume discounts extended by suppliers are recognised as a
reduction in the cost of inventories when it is probable that
the conditions for discounts to be received will be met.
Discounts for prompt payment are recognised as a reduction in
the cost of the inventories acquired.
The cost of inventories is adjusted against profit and loss when
cost exceeds the net realisable value. Net realisable value is
considered as follows:
|
|
|
| |
•
|
Raw materials and other supplies: replacement cost.
Nevertheless, raw materials are not written down below cost if
the finished goods into which they will be incorporated are
expected to be sold at or above cost of production.
|
| |
| |
•
|
Goods for resale and finished goods: estimated selling price,
less costs to sell.
|
| |
| |
•
|
Work in progress: the estimated selling price of related
finished goods, less the estimated costs of completion and the
estimated costs necessary to make the sale.
|
The previously recognised reduction in value is reversed against
profit and loss when the circumstances that previously caused
inventories to be written down no longer exist or when there is
clear evidence of an
F-71
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
increase in net realisable value because of changed economic
circumstances. The reversal of the reduction in value is limited
to the lower of the cost and revised net realisable value of the
inventories. Write-downs may be reversed with a credit to
inventories of finished goods and work in progress and supplies.
|
|
|
(o)
|
Cash
and cash equivalents
|
Cash and cash equivalents include cash on hand and demand
deposits in financial institutions. They also include other
short-term, highly liquid investments that are readily
convertible to known amounts of cash and which are subject to an
insignificant risk of changes in value. An investment normally
qualifies as a cash equivalent when it has a maturity of less
than three months from the date of acquisition.
The Group classifies cash flows relating to interest received
and paid as operating activities, and dividends received and
distributed by the Company are classified under investing and
financing activities, respectively.
Government grants are recognised in the balance sheet when there
is reasonable assurance that they will be received and that the
Group will comply with the conditions attached.
Outright capital grants are initially recognised as deferred
income in the consolidated balance sheet. Income from capital
grants is recognised as other income in the consolidated income
statement in line with the depreciation of the corresponding
financed assets.
Operating grants received to offset expenses or losses already
incurred, or to provide immediate financial support not related
to future disbursements, are recognised as other income in the
consolidated income statement.
|
|
|
(iii)
|
Interest
rate grants
|
Financial liabilities comprising implicit assistance in the form
of below market interest rates are initially recognised at fair
value. The difference between this value, adjusted where
necessary for the emission costs of the financial liability and
the amount received, is recognised as an official grant based on
the nature of the grant awarded.
|
|
|
(i)
|
Defined
contribution plans
|
The Group recognises the contributions payable to a defined
contribution plan in exchange for a service in the period in
which contributions are accrued. Accrued contributions are
recognised as an employee benefit expense in the corresponding
consolidated income statement in the year that the contribution
was made.
|
|
|
(ii)
|
Termination
benefits
|
Termination benefits payable that do not relate to restructuring
processes in progress are recognised when the Group is
demonstrably committed to terminating the employment of current
employees prior to retirement date. The Group is demonstrably
committed to terminating the employment of current employees
when a detailed formal plan has been prepared and there is no
possibility of withdrawing or changing the decisions made.
F-72
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(iii)
|
Short-term
employee benefits
|
The Group recognises the expected cost of short-term employee
benefits in the form of accumulating compensated absences when
the employees render service that increases their entitlement to
future compensated absences. In the case of non-accumulating
compensated absences, the expense is recognised when the
absences occur.
The Group recognises the expected cost of profit-sharing and
bonus payments when it has a present legal or constructive
obligation to make such payments as a result of past events and
a reliable estimate of the obligation can be made.
Provisions are recognised when the Group has a present
obligation (legal or implicit) as a result of a past event; it
is probable that an outflow of resources embodying economic
benefits will be required to settle the obligation; and a
reliable estimate can be made of the amount of the obligation.
The amount recognised as a provision is the best estimate of the
expenditure required to settle the present obligation at the end
of the reporting period, taking into account all risks and
uncertainties surrounding the amount to be recognised as a
provision and, where the time value of money is material, the
financial effect of discounting provided that the expenditure to
be made each period can be reliably estimated. The discount rate
is a pre-tax rate that reflects current market assessments of
the time value of money and the risks specific to the liability.
The discount rate does not reflect risks for which future cash
flow estimates have been adjusted.
If it is no longer probable that an outflow of resources
embodying economic benefits will be required to settle the
obligation, the provision is reversed against the consolidated
income statement item where the corresponding expense was
recognised.
Revenue is measured at the fair value of the consideration
received or receivable for the sale of goods and services, net
of VAT and any other amounts or taxes which are effectively
collected on the behalf of third parties. Volume or other types
of discounts for prompt payment are recognised as a reduction in
revenues if considered probable at the time of revenue
recognition.
The Group recognises revenue from the sale of goods when:
|
|
|
| |
•
|
the Group has transferred to the buyer the significant risks and
rewards of ownership of the goods.
|
| |
| |
•
|
the Group retains neither continuing managerial involvement to
the degree usually associated with ownership nor effective
control over the goods sold;
|
| |
| |
•
|
the amount of revenue can be measured reliably;
|
| |
| |
•
|
it is probable that the economic benefits associated with the
transaction will flow to the Group; and
|
| |
| |
•
|
the costs incurred or to be incurred in respect of the
transaction can be measured reliably.
|
|
|
|
(ii)
|
Rendering
of services
|
Revenues associated with the rendering of service transactions
are recognised by reference to the stage of completion at the
consolidated balance sheet date when the outcome of the
transaction can be estimated reliably, i.e., when revenues, the
stage of completion, the costs incurred and the costs to
complete the transaction can be estimated reliably and it is
probable that the economic benefits derived from the transaction
will flow to the Group.
F-73
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
When the outcome of the transaction involving the rendering of
services cannot be estimated reliably, revenue is recognised
only to the extent of the expenses recognised that are
recoverable.
|
|
|
(iii)
|
Revenue
from dividends
|
Revenue from dividends is recognised when the Group’s right
to receive payment is established.
|
|
|
(iv)
|
Revenue
from interest on delayed collections
|
Under European legislation governing credit periods allowed by
government entities (Social Security, in the case of the Group)
to pay suppliers under government contracts, certain
subsidiaries of the Group recover delay interest prescribed by
legislation, after forward claims have been made in courts of
law. To the extent that such delay interest claims are
recognized by the courts and collected, the Group accrues
interest on the basis of its historical experience.
The income tax expense and tax income for the year comprises
current tax and deferred tax.
Current tax is the amount of income taxes payable or recoverable
in respect of the consolidated taxable profit or consolidated
tax loss for the year. Current tax assets or liabilities are
measured at the amount expected to be paid to or recovered from
the taxation authorities, using the tax rates and tax laws that
have been enacted or substantially enacted at the balance sheet
date.
Deferred tax liabilities are the amounts of income taxes payable
in future periods in respect of taxable temporary differences,
whereas deferred tax assets are the amounts of income taxes
recoverable in future periods in respect of deductible temporary
differences, the carryforward of unused tax losses, and the
carryforward of unused tax credits. Temporary differences are
differences between the carrying amount of an asset or liability
in the balance sheet and its tax base.
Current and deferred tax are recognised as income or an expense
and included in profit or loss for the year except to the extent
that the tax arises from a transaction or event which is
recognised, in the same or a different year, directly in equity,
or a business combination.
|
|
|
(i)
|
Taxable
temporary differences
|
Taxable temporary differences are recognised in all cases except
where:
|
|
|
| |
•
|
They arise from the initial recognition of goodwill or an asset
or liability in a transaction which is not a business
combination and at the time of the transaction, affects neither
accounting profit nor taxable income;
|
| |
| |
•
|
They are associated with investments in subsidiaries over which
the Group is able to control the timing of the reversal of the
temporary difference and it is not probable that the temporary
difference will reverse in the foreseeable future.
|
|
|
|
(ii)
|
Deductible
temporary differences
|
Deductible temporary differences are recognised provided that:
|
|
|
| |
•
|
It is probable that taxable profit will be available against
which the deductible temporary difference can be utilised,
unless the differences arise from the initial recognition of an
asset or liability in a transaction which is not a business
combination and at the time of the transaction, affects neither
accounting profit nor taxable profit.
|
F-74
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
•
|
The temporary differences are associated with investments in
subsidiaries to the extent that the difference will reverse in
the foreseeable future and sufficient taxable income is expected
to be generated against which the temporary difference can be
offset.
|
Tax planning opportunities are only considered on evaluation of
the recoverability of deferred tax assets and if the Group
intends to use these opportunities or it is probable that they
will be utilised.
Deferred tax assets and liabilities are measured at the tax
rates that are expected to apply to the years when the asset is
realised or the liability is settled, based on tax rates and tax
laws that have been enacted or substantively enacted. The tax
consequences that would follow from the manner in which the
Group expects to recover or settle the carrying amount of its
assets or liabilities are also reflected in the measurement of
deferred tax assets and liabilities.
At year end the Group reviews the carrying amount of deferred
tax assets to write down the balance if it is not probable that
sufficient taxable income will be available to apply the tax
asset.
Deferred tax assets which do not meet the above conditions are
not recognised in the consolidated balance sheet. At year end
the Group assesses whether deferred tax assets which were
previously not recognised currently meet the conditions for
recognition.
|
|
|
(iv)
|
Offset
and recognition
|
The Group only offsets current tax assets and current tax
liabilities if it has a legally enforceable right to set off the
recognised amounts and intends either to settle on a net basis,
or to realise the asset and settle the liability simultaneously.
The Group only offsets deferred tax assets and liabilities where
it has a legally enforceable right, where these relate to income
taxes levied by the same taxation authority and where the
taxation authority permits the entity to settle on a net basis,
or to realise the asset and settle the liability simultaneously
for each of the future years in which significant amounts of
deferred tax assets or liabilities are expected to be settled or
recovered.
Deferred tax assets and liabilities are recognised in the
consolidated balance sheet under non-current assets or
liabilities, irrespective of the expected date of recovery or
settlement.
An operating segment is a component of the Group that engages in
business activities from which it may earn revenues and incur
expenses, whose operating results are regularly reviewed by the
Group’s chief operating decision maker to make decisions
about resources to be allocated to the segment and assess its
performance, and for which discrete financial information is
available.
|
|
|
(v)
|
Classification
of assets and liabilities as current and
non-current
|
The Group classifies assets and liabilities in the consolidated
balance sheet as current and non-current. Current assets and
liabilities are determined as follows:
|
|
|
| |
•
|
Assets are classified as current when they are expected to be
realised, or are intended for sale or consumption in the
Group’s normal operating cycle within twelve months after
the balance sheet date and they are held primarily for the
purpose of trading. Cash and cash equivalents are also
classified as current, except where they may not be exchanged or
used to settle a liability, at least within twelve months after
the balance sheet date.
|
F-75
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
•
|
Liabilities are classified as current when they are expected to
be settled in the Group’s normal operating cycle within
12 months after the balance sheet date and they are held
primarily for the purpose of trading, or where the Group does
not have an unconditional right to defer settlement of the
liability for at least 12 months after the balance sheet
date.
|
| |
| |
•
|
Financial liabilities are classified as current when they are
due to be settled within twelve months after the reporting
period, even if the original term was for a period longer than
twelve months, and an agreement to refinance, or to reschedule
payments, on a long-term basis is completed after the reporting
period and before the consolidated financial statements are
authorised for issue.
|
|
|
|
(5)
|
Financial
Risk Management Policy
|
The Group is exposed to the following risks associated with the
use of financial instruments:
|
|
|
| |
•
|
Credit risk
|
| |
| |
•
|
Liquidity risk
|
| |
| |
•
|
Market risk
|
This note provides information on the Group’s exposure to
each of these risks, the Group’s objectives and procedures
to measure and mitigate this risk, and the Group’s capital
management strategy. More exhaustive quantitative information is
disclosed in note 32.
The Group’s risk management policies are established in
order to identify and analyse the risks to which the Group is
exposed, establish suitable risk limits and controls, and
control risks and compliance with limits. Risk management
procedures and policies are regularly reviewed to ensure they
take into account changes in market conditions and in the
Group’s activities. The Group’s management procedures
and rules are designed to create a strict and constructive
control environment in which all employees understand their
duties and obligations.
The Group’s Audit Committee supervises how management
controls compliance with the Group’s risk management
procedures and policies and reviews whether the risk management
policy is suitable considering the risks to which the Group is
exposed. This committee is assisted by Internal Audit which acts
as supervisor. Internal Audit performs regular and ad hoc
reviews of the risk management controls and procedures and
reports its findings to the Audit Committee.
Credit
risk
Credit risk is the risk to which the Group is exposed in the
event that a customer or counterparty to a financial instrument
fails to discharge a contractual obligation, and mainly results
from trade receivables and the Group’s investments in
financial assets.
Trade
receivables
The Group is not exposed to significant credit risk because most
of the customers belong to the public healthcare system. The
risk to which receivables from these entities are exposed is a
risk of delays in payment. Group companies mitigate this risk by
exercising their right to receive legal interest.
Furthermore, no significant bad debt issues have been detected
in the markets in which it sells to private entities.
In the US market, the Group sells the majority of its products
to distributors who in turn sell them to retail pharmacies,
hospitals, homecare companies, and other health care facilities.
Prices and quantities are
F-76
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
contracted between the Group and the dispensing entities, either
individually or through Group Purchase Organizations (GPOs).
Group Purchasing Organizations are buying alliances of large
numbers of hospitals and allied organizations that deliver
cost-savings and efficiency benefits by leveraging their
combined purchasing power. Other channels of distribution are
primarily focused on sales directly to specialty pharmacies,
haemophilia treatment centres and government entities. No
significant bad debt issues have been detected with the
customers the Group sells to.
The Group recognises valuation adjustments for impairment
equivalent to its best estimate of the losses incurred in
relation to trade and other receivables. The main valuation
adjustments made are based on specific losses related with
identified risks that are individually significant, while the
bad debt risk in the Group is low because a significant
proportion of receivables are due from public entities.
Financial
instruments and deposits
The Group has invested part of the resources generated by the
issue of bonds in the United States in deposits with financial
institutions with sound credit ratings.
Liquidity
risk
Liquidity risk is the risk that the Group cannot meet its
financial obligations as they fall due. The Group’s
approach to managing liquidity is to ensure where possible, that
it always has sufficient liquidity to settle its obligations at
the maturity date, both in normal conditions and in times of
tension, to avoid incurring unacceptable losses or tarnishing
the Group’s reputation.
The Group ensures the availability of financing through a
sufficient amount of committed credit facilities to meet its
payment obligations at due dates.
The Group issued bonds in the United States during 2009. The
resources generated will enable the Group to extend the life of
its debt from current to non-current and ensure that the
necessary financial resources are available to implement its
future plans. The resources generated have therefore been used
to pay current and non-current liabilities, with the remaining
amount, totalling Euros 211,539 thousand recognised as a current
investment under “Cash and cash equivalents” at
31 December 2010 (Euros 237,777 thousand at
31 December 2009) (see note 22).
In the balance sheet at 31 December 2010, 22% of the
financial liabilities is current and 78% non-current, while at
31 December 2009, 14% was current and 86% non-current.
Market
risk
Market risk comprises the risk of changes in market prices, for
example, exchange rates, interest rates, or the prices of equity
instruments affecting the Group’s revenues or the value of
financial instruments it holds. The objective of managing market
risk is to manage and control the Group’s exposure to this
risk within reasonable parameters at the same time as optimising
returns.
(i) Currency
risk
The Group operates internationally and is therefore exposed to
currency risks when operating with foreign currencies,
especially with regard to the US Dollar. Currency risk is
associated with future commercial transactions, recognised
assets and liabilities, and net investments in foreign
operations.
The Group holds several investments in foreign operations, the
net assets of which are exposed to currency risk. Currency risk
affecting net assets of the Group’s foreign operations in
US Dollars are mitigated primarily through borrowings in
the corresponding foreign currencies.
F-77
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The Group’s main exposure to currency risk is due to the
US Dollar, which is used in a significant percentage of
transactions in foreign currencies. Since the Company had
US Dollar revenues that were, as a proportion, 96.9% of
US Dollar expenses during 2010, the Group has a natural
hedge against US Dollar fluctuations and therefore the
risks associated with such exchange-rate fluctuations are
minimal.
Details of the Group’s exposure to currency risk at
31 December 2010, 2009 and 2008 of principal financial
instruments are shown in note 32.
(ii) Interest-rate
risk
The Group’s interest rate risks arise from current and
non-current borrowings. Borrowings at variable interest rates
expose the Group to cash flow interest rate risks. During 2010
and because of the issue of bonds in 2009 (see note 22
(a.1.1)), a significant portion of liabilities bear fixed
interest rates, whereas the rest of the financial liabilities
with banks bear variable interest rates. Nevertheless, the Group
has a variable to fixed interest-rate swap for loans of Euros
50,000 thousand maturing in 2013 (see note 32).
(iii) Market
price risk
The Group has signed two unquoted futures contracts, the
underlying asset of which is shares in Grifols, S.A. It is
therefore exposed to risk of value fluctuations.
Price risk affecting raw materials is mitigated by the vertical
integration of the haemoderivatives business in a sector which
is highly concentrated.
The directors’ policy is to maintain a solid capital base
in order to ensure investor, creditor and market confidence and
sustain future business development. The directors control
capital performance using rates of returns on equity (ROE) and
returns on invested capital (ROIC). The board of directors also
controls the level of dividends paid to shareholders.
In 2010, the ROE stood at 16.7% (26.1% in December 2009 and
25.3% in December 2008) and the ROIC at 11.2% (13.9% in
December 2009 and 15.3% in December 2008). The ROE is calculated
by dividing profit attributable to the Company by the equity
attributable to the Company. The ROIC is calculated by dividing
operating profit after income tax by invested capital, which is
equal to total assets less cash, less other current financial
assets and less current and non-current financial liabilities
excluding current and non-current borrowings.
Compared with these rates, the weighted average finance expense
for interest-bearing liabilities (excluding liabilities with
implicit interest) has been 4.8% in 2010 (3.9% in 2009 and 5.2%
in 2008). Considering the September 2009 issue of bonds in the
USA, the weighted average finance expense for interest-bearing
liabilities for the fourth quarter of 2009 was 5.1%.
The Group has no share-based payment schemes for employees.
At 31 December 2010 the Group holds own shares equivalent
to 0.07% of its share capital (0.03% at 31 December 2009).
The Group does not have a formal plan for repurchasing shares.
In accordance with IFRS 8: Operating Segments, financial
information for operating segments is reported in the
accompanying Appendix I, which forms an integral part of
this note to the consolidated financial statements.
F-78
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Group companies are divided into three areas: companies from the
industrial area, companies from the commercial area and
companies from the services area. Within each of these areas,
activities are organised based on the nature of the products and
services manufactured and marketed.
Assets, liabilities, income and expenses for segments include
directly and reliably attributable items. Items which are not
attributed to segments by the Group are:
|
|
|
| |
•
|
Balance sheet: cash and cash equivalents,
receivables, public entities, deferred tax assets and
liabilities, loans and borrowings and certain payables.
|
| |
| |
•
|
Income statement: general administration
expenses, other operating income/expenses, finance
income/expense and income tax.
|
There have been no inter-segment sales.
The operating segments defined by the Group are as follows:
|
|
|
| |
•
|
Bioscience: including all activities related
with products deriving from human plasma for therapeutic use.
|
| |
| |
•
|
Hospital: comprising all non-biological
pharmaceutical products and medical supplies manufactured by
Group companies earmarked for hospital pharmacy. Products
related with this business which the Group does not manufacture
but markets as supplementary to its own products are also
included.
|
| |
| |
•
|
Diagnostic: including the marketing of
diagnostic testing equipment, reagents and other equipment,
manufactured by Group or other companies.
|
| |
| |
•
|
Raw materials: including sales of intermediate
biological products and the rendering of manufacturing services
to third party companies.
|
Details of net sales by groups of products for 2010, 2009 and
2008 as a percentage of net sales are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
% of Sales
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Hemoderivatives
|
|
|
77.9
|
%
|
|
|
76.0
|
%
|
|
|
75.6
|
%
|
|
Other hemoderivatives
|
|
|
0.2
|
%
|
|
|
0.2
|
%
|
|
|
0.3
|
%
|
|
Transfusional medicine
|
|
|
7.9
|
%
|
|
|
8.2
|
%
|
|
|
7.3
|
%
|
|
In vitro diagnosis
|
|
|
3.1
|
%
|
|
|
3.1
|
%
|
|
|
3.2
|
%
|
|
Fluid therapy and nutrition
|
|
|
5.0
|
%
|
|
|
5.2
|
%
|
|
|
5.7
|
%
|
|
Hospital supplies
|
|
|
4.1
|
%
|
|
|
4.2
|
%
|
|
|
4.4
|
%
|
|
Raw materials
|
|
|
0.5
|
%
|
|
|
2.5
|
%
|
|
|
2.8
|
%
|
|
Other
|
|
|
1.3
|
%
|
|
|
0.6
|
%
|
|
|
0.7
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b)
|
Geographical
information
|
Geographical information is grouped into four areas:
|
|
|
| |
•
|
Spain
|
| |
| |
•
|
Rest of the European Union
|
F-79
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
•
|
United States of America
|
| |
| |
•
|
Rest of the world
|
The financial information reported for geographical areas is
based on sales to third parties in these markets as well as the
location of assets.
No entity represents 10% or more of the Group’s sales.
Details of and movement in goodwill in the year 2008 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
Business
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/07
|
|
|
|
Combinations
|
|
|
Differences
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Net value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK, Ltd.
|
|
|
9,369
|
|
|
|
|
—
|
|
|
|
(2,156
|
)
|
|
|
7,213
|
|
|
Grifols Italia,S.p.A.
|
|
|
6,118
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,118
|
|
|
Biomat USA, Inc.
|
|
|
85,390
|
|
|
|
|
2,372
|
|
|
|
5,256
|
|
|
|
93,018
|
|
|
Plasmacare, Inc.
|
|
|
34,912
|
|
|
|
|
—
|
|
|
|
2,017
|
|
|
|
36,929
|
|
|
Plasma Collection Centers, Inc.
|
|
|
14,454
|
|
|
|
|
—
|
|
|
|
835
|
|
|
|
15,289
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
150,243
|
|
|
|
|
2,372
|
|
|
|
5,952
|
|
|
|
158,567
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3(a))
|
|
|
|
|
|
|
|
|
|
Details of and movement in goodwill in the year 2009 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
Business
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/08
|
|
|
Combinations
|
|
|
Differences
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Net value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK, Ltd.
|
|
|
7,213
|
|
|
|
—
|
|
|
|
523
|
|
|
|
7,736
|
|
|
Grifols Italia, S.p.A.
|
|
|
6,118
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,118
|
|
|
Biomat USA, Inc.
|
|
|
93,018
|
|
|
|
225
|
|
|
|
(3,154
|
)
|
|
|
90,089
|
|
|
Plasmacare, Inc.
|
|
|
36,929
|
|
|
|
—
|
|
|
|
(1,253
|
)
|
|
|
35,676
|
|
|
Plasma Collection Centers, Inc.
|
|
|
15.289
|
|
|
|
—
|
|
|
|
(519
|
)
|
|
|
14,770
|
|
|
Woolloomooloo Holdings Pty Ltd.
|
|
|
—
|
|
|
|
16,190
|
|
|
|
3,421
|
|
|
|
19,611
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
158,567
|
|
|
|
16,415
|
|
|
|
(982
|
)
|
|
|
174,000
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3(a)
|
and 3(b))
|
|
|
|
|
|
|
|
|
|
F-80
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Details of and movement in goodwill in the year 2010 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/09
|
|
|
Transfers
|
|
|
Differences
|
|
|
31/12/10
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Net value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grifols UK, Ltd.
|
|
|
7,736
|
|
|
|
—
|
|
|
|
246
|
|
|
|
7,982
|
|
|
Grifols Italia,S.p.A.
|
|
|
6,118
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,118
|
|
|
Biomat USA, Inc.
|
|
|
90,089
|
|
|
|
14,770
|
|
|
|
8,193
|
|
|
|
113,052
|
|
|
Plasmacare, Inc.
|
|
|
35,676
|
|
|
|
—
|
|
|
|
2,788
|
|
|
|
38,464
|
|
|
Plasma Collection Centers, Inc.
|
|
|
14,770
|
|
|
|
(14,770
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Woolloomooloo Holdings Pty Ltd.
|
|
|
19,611
|
|
|
|
—
|
|
|
|
4,221
|
|
|
|
23,832
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
174,000
|
|
|
|
—
|
|
|
|
15,448
|
|
|
|
189,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Impairment
testing:
Goodwill has been allocated to each of the Group’s
cash-generating units (CGUs) in accordance with their respective
business segments and on a geographical basis, this being the
lowest level at which goodwill is controlled by management for
management purposes and lower than the operating segments.
Plasma Collection Centers, Inc. and Plasmacare, Inc. are
integrated into the management of Biomat USA, Inc. for the
purpose of impairment analysis.
Goodwill has been allocated to the cash generating units as
follows:
|
|
|
| |
•
|
UK: bioscience segment
|
| |
| |
•
|
Italy: bioscience segment
|
| |
| |
•
|
USA: bioscience segment
|
| |
| |
•
|
Australia: mainly to the Diagnostics segment.
|
The recoverable amount of a CGU is determined based on its value
in use. These calculations are based on cash flow projections
from the financial budgets approved by management. Cash flows
estimated as of the year in which stable growth in the CGU has
been reached are extrapolated using the estimated growth rates
indicated below.
The key assumptions used in calculating value in use of the CGUs
have been as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Growth Rate
|
|
Discount Rate After Tax
|
|
|
|
2010
|
|
2009
|
|
2010
|
|
2009
|
|
|
|
Bioscience
|
|
|
2% — 3%
|
|
|
|
3%
|
|
|
|
8% — 8.5%
|
|
|
|
8%
|
|
|
Diagnostic
|
|
|
2%
|
|
|
|
2%
|
|
|
|
8.30%
|
|
|
|
8.7%
|
|
Management determines budgeted gross margins based on past
experience and forecast market development. Average weighted
growth rates are coherent with the forecasts included in
industry reports. The discount rates used reflect specific risks
related to the CGUs.
F-81
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Paragraph A20 of IAS 36 requires the discount rate used to
be a pre-tax rate and establishes that when the basis used to
estimate the discount rate is post-tax, that basis is adjusted
to reflect a pre-tax rate. The following pre-tax discount rates
have been used:
| |
|
|
|
|
|
|
|
|
|
|
|
Discount Rate Before Tax
|
|
|
|
2010
|
|
2009
|
|
|
|
Bioscience
|
|
|
10.5% — 10.9%
|
|
|
|
9.5% — 13%
|
|
|
Diagnostic
|
|
|
10.40%
|
|
|
|
9.8%
|
|
The use of post-tax discount rates adjusted to reflect pre-tax
discount rates has not given rise to any values in use which
differ significantly from those which would have arisen had the
discount rates been pre-tax.
|
|
|
(8)
|
Other
Intangible Assets
|
Details of other intangible assets and movement during the years
ended 31 December 2010, 2009 and 2008 are included in
Appendix II, which forms an integral part of these notes to
the consolidated financial statements.
The cost of
fully-amortised
intangible assets in use at 31 December 2010 and 2009 is
Euros 57,203 thousand and Euros 38,183 thousand, respectively.
The Group has recognised Euros 9,963 thousand in 2010 (Euros
11,823 thousand in 2009 and Euros 7,644 thousand in
2008) as self-constructed assets.
At 31 December 2010 the Group has recognised licenses with
indefinite useful lives under intangible assets for a carrying
amount of Euros 24,691 thousand (Euros 23,379 thousand at
31 December 2009). The Group has also recognised Euros
11,492 thousand as costs of development in progress (Euros
21,943 thousand at 31 December 2009).
At 31 December 2010 the Group has recognised
CO2
emission rights for Euros 534 thousand (Euros 493 thousand at
31 December 2009) (see note 4(h (iv))).
During 2010 the Group signed a distribution agreement for a new
blood genotype test developed by Progenika Biopharma, acquiring
a customer portfolio of Euros 1,358 thousand and which is
recognised under “Other intangible assets”.
Impairment
testing:
Indefinite-lived intangible assets have been allocated to the
Group’s Plasmacare, Inc. and Biomat USA, Inc.
cash-generating units (CGUs), which belong to the Bioscience
segment.
The recoverable amount of a CGU is determined based on its value
in use. These calculations are based on cash flow projections
from the financial budgets approved by management. Cash flows
estimated as of the year in which stable growth in the CGU has
been reached are extrapolated using the estimated growth rates
indicated below.
The key assumptions used in calculating value in use are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Discount Rate After Tax
|
|
|
|
2010
|
|
2009
|
|
|
|
Growth rate used to extrapolate projections
|
|
|
3%
|
|
|
|
3%
|
|
|
Discount rate after tax
|
|
|
8.5%
|
|
|
|
8%
|
|
F-82
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Paragraph A20 of IAS 36 requires the discount rate used to
be a pre-tax rate and establishes that when the basis used to
estimate the discount rate is post-tax, that basis is adjusted
to reflect a pre-tax rate. The pre-tax rate in 2010 is 10.9%
(9.5% in 2009). The use of a post-tax discount rate adjusted to
reflect the pre-tax discount rate has not given rise to any
values in use which differ significantly from those which would
have arisen had the discount rates been pre-tax.
|
|
|
(9)
|
Property,
Plant and Equipment
|
Details of property, plant and equipment and movement in the
consolidated balance sheet at 31 December 2010, 2009 and
2008 are included in Appendix III, which forms an integral
part of these notes to the consolidated financial statements.
The main investments during the years 2010 and 2009 correspond
to the construction of the production plants in Los Angeles and
Parets del Vallès.
The main investments during the year 2008 have been as follows:
|
|
|
| |
•
|
Purchase of land and buildings in Parets del Vallès,
Barcelona with a value of Euros 19.4 million, financed
through a mortgage loan from Caixa Catalunya.
|
| |
| |
•
|
Purchase of land and buildings under construction in Sant Cugat
del Vallès, Barcelona through the acquisition of the real
estate company Arrahona Optimus, S.L. for Euros 33 million
at 31 December 2008, financed through a mortgage loan from
BBVA.
|
Property, plant and development under construction at
31 December 2010 and 2009 mainly comprises investments made
to extend the companies’ installations and to increase
their productive capacity.
|
|
|
a)
|
Mortgaged
property, plant and equipment
|
At 31 December 2010 certain land and buildings have been
mortgaged for Euros 49,316 thousand (Euros 45,382 thousand
at 31 December 2009) to secure payment of certain
loans (see note 22).
|
|
|
b)
|
Official
capital grants received
|
During 2010, the Group has received capital grants totalling
Euros 323 thousand (Euros 742 thousand at 31 December 2009)
(see note 20).
Group policy is to contract sufficient insurance coverage for
the risk of damage to property, plant and equipment. At
31 December 2010 and 2009 the Group has a combined
insurance policy for all Group companies, which adequately
covers the carrying amount of all the Group’s assets.
At 1 January 2004, date of first adopting IFRS-EU, the
Group opted to apply the exemption regarding fair value and
revaluation as deemed cost as permitted by IFRS 1: First-time
Adoption of IFRS. In accordance with this exemption, the
Group’s land and buildings were revalued based on
independent expert appraisals at 1 January 2004. Appraisals
were performed based on market values.
F-83
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
e)
|
Assets
under finance lease
|
The Group had contracted the following types of property, plant
and equipment under finance leases at 31 December 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
Asset
|
|
Cost
|
|
|
Depreciation
|
|
|
Net value
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Technical installations and other property, plant and equipment
|
|
|
19,641
|
|
|
|
(5,507
|
)
|
|
|
14,134
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The Group has contracted the following types of property, plant
and equipment under finance leases at 31 December 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
Asset
|
|
Cost
|
|
|
Depreciation
|
|
|
Net value
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Technical installations and other property, plant and equipment
|
|
|
15,264
|
|
|
|
(4,782
|
)
|
|
|
10,482
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Details of minimum lease payments and the present value of
finance lease liabilities, disclosed by maturity date, are
detailed in note 22 (a.1.3).
|
|
|
f)
|
Fully-depreciated
assets
|
The cost of fully-depreciated property, plant and equipment in
use at 31 December 2010 and 2009 is Euros 98,978 thousand
and Euros 73,370 thousand, respectively.
|
|
|
g)
|
Self-constructed
property, plant and equipment
|
At 31 December 2010 the Group has recognised Euros 23,550
thousand as self-constructed property, plant and equipment
(Euros 29,319 thousand at 31 December 2009 and Euros 18,150
thousand at 31 December 2008).
h) Purchase
commitments
At 31 December 2010 the Group has property, plant and
equipment purchase commitments amounting to Euros 6,148 thousand.
|
|
|
(10)
|
Investments
Accounted for Using the Equity Method
|
At 31 December 2009 and 2008 equity accounted investments
comprised the investment held by Diagnostic Grifols, S.A. in the
company Quest International, Inc. This company is located in
Miami, Florida (USA) and its activity consists of the
manufacture and commercialisation of reagents and clinical
analysis instruments. On 9 November 2010 the Group sold its
interest in the company for a sale price of Euros 621 thousand.
Because the Group had significant influence over these
companies, the consolidation method used was the equity method.
Details of and movement in this caption in year 2008 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/07
|
|
|
Gains
|
|
|
Differences
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Equity accounted investments
|
|
|
243
|
|
|
|
24
|
|
|
|
107
|
|
|
|
374
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-84
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Details of and movement in this caption in year 2009 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/08
|
|
|
Gains
|
|
|
Differences
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Equity accounted investments
|
|
|
374
|
|
|
|
51
|
|
|
|
(42
|
)
|
|
|
383
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Details of and movement in this caption in year 2010 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
Gain/(Losses &
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/09
|
|
|
Impairment)
|
|
|
Disposals
|
|
|
Acquisitions
|
|
|
Differences
|
|
|
31/12/10
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Equity accounted investments
|
|
|
383
|
|
|
|
(879
|
)
|
|
|
(463
|
)
|
|
|
1,472
|
|
|
|
85
|
|
|
|
598
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The balance at 31 December 2010 relates to the investment
(acquired in 2010) which Gri-cel, S.A. holds in
Nanotherapix, S.L. (see note 2 (c), a joint venture which
has been accounted for using the equity method).
Summarised financial information on the equity accounted
investments is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Percentage
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Country
|
|
|
Ownership
|
|
|
Assets
|
|
|
Liabilities
|
|
|
Equity
|
|
|
Result
|
|
|
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
31/12/2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quest International, Inc
|
|
|
USA
|
|
|
|
35
|
%
|
|
|
1,736
|
|
|
|
667
|
|
|
|
1,069
|
|
|
|
69
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quest International, Inc
|
|
|
USA
|
|
|
|
35
|
%
|
|
|
1,664
|
|
|
|
580
|
|
|
|
1,084
|
|
|
|
145
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Nanotherapix, S.L.
|
|
|
Spain
|
|
|
|
51
|
%
|
|
|
2,375
|
|
|
|
1,212
|
|
|
|
1,163
|
|
|
|
(312
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(11)
|
Non-Current
Financial Assets
|
Details of this caption of the consolidated balance sheet at
31 December 2010, 2009 and 2008 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Non-current guarantee deposits
|
|
|
1,217
|
|
|
|
1,142
|
|
|
|
1,113
|
|
|
Assets available for sale
|
|
|
535
|
|
|
|
501
|
|
|
|
523
|
|
|
Loans to third parties
|
|
|
5,783
|
|
|
|
2,088
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current financial assets
|
|
|
7,535
|
|
|
|
3,731
|
|
|
|
1,636
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During 2010, the Group has extended two new mortgage loans
totalling Euros 3,723 thousand to the owners of two plasma
centres in the USA occupied by group companies. These loans have
a term of 20 years, bear interest at a fixed rate of 4.5%
and are secured by the property and by a personal security. This
interest rate does not differ from a mortgage market interest
rate. In 2009 the Group extended a similar mortgage loan for an
amount of Euros 2,174 thousand.
At 31 December 2010,
available-for-sale
assets relate to the following:
|
|
|
| |
•
|
The interest of less than 1% that the Group holds in Northfield
Laboratories, Inc. (USA). At 31 December 2010, 2009 and
2008 provision has been made for the full amount of this
investment, based on its fair value.
|
F-85
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
| |
•
|
The interest of less than 2% in the share capital of
biotechnology company, Cardio 3 Bioscience (with registered
offices in Belgium) acquired by Grifols, S.A. in December 2008
for an amount of Euros 500 thousand. The activity of this
company involves research into and the development of biological
therapies using stem cells for the treatment of cardiovascular
diseases. The Group has measured this asset at cost, as its fair
value cannot be reliably determined.
|
Details of inventories at 31 December are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Goods for resale
|
|
|
63,050
|
|
|
|
65,718
|
|
|
Raw materials and other supplies
|
|
|
160,326
|
|
|
|
170,987
|
|
|
Work in progress and semi-finished goods
|
|
|
203,971
|
|
|
|
146,612
|
|
|
Finished goods
|
|
|
100,518
|
|
|
|
101,145
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
527,865
|
|
|
|
484,462
|
|
|
|
|
|
|
|
|
|
|
|
Movement in inventories of finished products, work in progress
and materials consumed was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
*
|
|
|
*
|
|
|
*
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Inventories of goods for resale
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net purchases
|
|
|
56,542
|
|
|
|
50,886
|
|
|
|
79,902
|
|
|
Changes in inventories
|
|
|
6,911
|
|
|
|
(9,201
|
)
|
|
|
(22,700
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
63,453
|
|
|
|
41,685
|
|
|
|
57,202
|
|
|
Raw materials and supplies
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net purchases
|
|
|
225,994
|
|
|
|
274,537
|
|
|
|
190,667
|
|
|
Changes in inventories
|
|
|
17,412
|
|
|
|
(29,948
|
)
|
|
|
(41,131
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
243,406
|
|
|
|
244,589
|
|
|
|
149,536
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Materials consumed
|
|
|
306,859
|
|
|
|
286,274
|
|
|
|
206,738
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in inventories of finished products and work in progress
|
|
|
(45,749
|
)
|
|
|
(73,093
|
)
|
|
|
(31,058
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Changes in inventories of finished products , work in progress
and materials consumed
|
|
|
261,110
|
|
|
|
213,181
|
|
|
|
175,680
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-86
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Reconciliation of goods for resale during 2010, 2009 and 2008
has been as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Inventories of goods for resale at 1 January
|
|
|
65,718
|
|
|
|
54,509
|
|
|
|
37,138
|
|
|
Business combinations
|
|
|
—
|
|
|
|
158
|
|
|
|
—
|
|
|
Net cancellations for the year
|
|
|
—
|
|
|
|
(568
|
)
|
|
|
(515
|
)
|
|
Increase/(decrease) of goods for resale
|
|
|
(6,911
|
)
|
|
|
9,201
|
|
|
|
22,700
|
|
|
Translation differences
|
|
|
4,243
|
|
|
|
2,418
|
|
|
|
(4,814
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories of goods for resale at 31 December
|
|
|
63,050
|
|
|
|
65,718
|
|
|
|
54,509
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of inventories of raw materials and materials
consumed during 2010, 2009 and 2008 has been as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Inventories of raw materials at 1 January
|
|
|
170,987
|
|
|
|
142,209
|
|
|
|
96,044
|
|
|
Business combinations
|
|
|
—
|
|
|
|
824
|
|
|
|
—
|
|
|
Increase/(decrease) in raw materials
|
|
|
(17,412
|
)
|
|
|
29,948
|
|
|
|
41,131
|
|
|
Translation differences
|
|
|
6,751
|
|
|
|
(1,994
|
)
|
|
|
5,034
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories of raw materials at 31 December
|
|
|
160,326
|
|
|
|
170,987
|
|
|
|
142,209
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reconciliation of inventories of finished goods and work in
progress during 2010, 2009 and 2008 has been as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Inventories of finished goods and work in progress at
1 January
|
|
|
247,757
|
|
|
|
176,939
|
|
|
|
138,226
|
|
|
Business combinations
|
|
|
—
|
|
|
|
2,567
|
|
|
|
—
|
|
|
Increase in inventories of finished goods and work in progress
|
|
|
45,749
|
|
|
|
73,093
|
|
|
|
31,058
|
|
|
Translation differences
|
|
|
10,983
|
|
|
|
(4,842
|
)
|
|
|
7.655
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories of finished goods and work in progress at
31 December
|
|
|
304,489
|
|
|
|
247,757
|
|
|
|
176,939
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net purchases include purchases made in the following foreign
currencies:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Currency
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US Dollar
|
|
|
145,584
|
|
|
|
196,936
|
|
|
|
168,037
|
|
|
Other currencies
|
|
|
6,569
|
|
|
|
4,498
|
|
|
|
7,315
|
|
F-87
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(13)
|
Trade and
Other Receivables
|
Details at 31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Trade receivables
|
|
|
224,355
|
|
|
|
207,840
|
|
|
Other receivables
|
|
|
31,012
|
|
|
|
27,210
|
|
|
Associates
|
|
|
5
|
|
|
|
812
|
|
|
Personnel
|
|
|
366
|
|
|
|
395
|
|
|
Advances for fixed assets
|
|
|
494
|
|
|
|
1,103
|
|
|
Other advances
|
|
|
3,265
|
|
|
|
1,844
|
|
|
Public entities, other receivables
|
|
|
8,890
|
|
|
|
8,176
|
|
|
|
|
|
|
|
|
|
|
|
|
Other receivables
|
|
|
44,032
|
|
|
|
39,540
|
|
|
Current income tax assets
|
|
|
14,607
|
|
|
|
7,802
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
282,994
|
|
|
|
255,182
|
|
|
|
|
|
|
|
|
|
|
|
Trade
receivables
Trade receivables, net of the provision for bad debts, include
notes receivable discounted at banks at 31 December 2010,
which amount to Euros 1,396 thousand (Euros 1,298 thousand at
31 December 2009) (see note 22).
Trade receivables include balances in the following foreign
currencies:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Currency
|
|
|
|
|
|
|
|
|
|
US Dollar
|
|
|
52,466
|
|
|
|
45,297
|
|
|
Chilean Peso
|
|
|
17,008
|
|
|
|
12,778
|
|
|
Mexican Peso
|
|
|
10,583
|
|
|
|
7,986
|
|
|
Argentinean Peso
|
|
|
4,075
|
|
|
|
3,404
|
|
|
Brazilian Real
|
|
|
4,616
|
|
|
|
3,225
|
|
|
Czech Crown
|
|
|
3,030
|
|
|
|
3,217
|
|
|
Pound Sterling
|
|
|
3,116
|
|
|
|
2,849
|
|
|
Thai Baht
|
|
|
1,842
|
|
|
|
1,366
|
|
|
Polish Zloty
|
|
|
2,379
|
|
|
|
1,292
|
|
|
Australian Dollar
|
|
|
3,769
|
|
|
|
1,101
|
|
|
Other currencies
|
|
|
2,412
|
|
|
|
1,644
|
|
Other
receivables
Other receivables at 31 December 2010 and 2009 include
Euros 6,639 thousand (Euros 8,089 thousand at 31 December
2009) reflecting interest receivable from social
security-affiliated entities.
During 2010, 2009 and 2008 certain Grifols Group companies have
sold receivables without recourse from several public entities
to Deutsche Bank, S.A.E. According to these contracts, the Group
receives an initial payment which usually amounts to
approximately 90% of the nominal amount of the receivables.
Receipt of the deferred amount (remainder of the nominal amount)
is collected by the Group once Deutsche
F-88
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Bank has collected the nominal amount of the receivables and
until then the pending amount is recognised in the balance sheet
as a receivable. Because the receivables are with public
entities it is considered that there is very low credit risk. At
31 December 2010, Euros 19,504 thousand is receivable for
this deferred amount (Euros 13,675 thousand at 31 December
2009). Initial payment is made when the sale is completed and
therefore, the bad debt risk associated with this part of the
nominal amount of the receivables is transferred. The Group has
transferred control of the receivables to Deutsche Bank and
therefore, the Group has derecognised the total initial payment
on its balance sheet, since all risks and rewards have been
transferred.
Certain foreign group companies and one Spanish company have
also entered into contracts to sell receivables without recourse
to financial institutions.
Total balances receivable without recourse sold to financial
institutions through the aforementioned contracts amount to
Euros 185.2 million at 31 December 2010 (Euros
116.3 million at 31 December 2009).
The finance cost of these operations for the Group totals
approximately Euros 5,378 thousand which has been recognised
under finance costs in the 2010 consolidated income statement
(Euros 2,531 thousand in 2009 and Euros 2,128 thousand in 2008)
(see note 28).
Details of balances with related parties are shown in
note 33.
Receivables from public entities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Taxation authorities, VAT
|
|
|
8,191
|
|
|
|
7,451
|
|
|
Social Security
|
|
|
85
|
|
|
|
107
|
|
|
Other public entities
|
|
|
614
|
|
|
|
618
|
|
|
|
|
|
|
|
|
|
|
|
|
Public entities, other receivables
|
|
|
8,890
|
|
|
|
8,176
|
|
|
|
|
|
|
|
|
|
|
|
Current
tax assets
Current tax assets are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Recoverable income tax:
|
|
|
|
|
|
|
|
|
|
Current year
|
|
|
9,352
|
|
|
|
7,188
|
|
|
Prior years
|
|
|
5,255
|
|
|
|
614
|
|
|
|
|
|
|
|
|
|
|
|
|
Current tax assets
|
|
|
14,607
|
|
|
|
7,802
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(14)
|
Other
Current Financial Assets
|
Details of this caption of the consolidated balance sheet at
31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Current investments
|
|
|
12,387
|
|
|
|
5,943
|
|
|
Guarantee deposits
|
|
|
44
|
|
|
|
209
|
|
|
Current loans to third parties
|
|
|
515
|
|
|
|
395
|
|
|
Financial derivatives (note 32)
|
|
|
—
|
|
|
|
1,670
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other current financial assets
|
|
|
12,946
|
|
|
|
8,217
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-89
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
“Current investments” comprise current
guarantee deposits held in financial institutions with maturity
greater than three months from the date of acquisition.
|
|
|
(15)
|
Other
Current Assets
|
Details of this caption of the consolidated balance sheet at
31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Prepaid expenses — professional services
|
|
|
72,983
|
|
|
|
1,703
|
|
|
Prepaid expenses — insurance
|
|
|
3,508
|
|
|
|
3,403
|
|
|
Royalties and rentals
|
|
|
2,589
|
|
|
|
611
|
|
|
Other prepaid expenses
|
|
|
1,548
|
|
|
|
1,628
|
|
|
|
|
|
|
|
|
|
|
|
|
Total other current assets
|
|
|
80,628
|
|
|
|
7,345
|
|
|
|
|
|
|
|
|
|
|
|
At 31 December 2010 professional services include an amount
of Euros 71,174 thousand relating to costs incurred for
professional services directly relating to the share capital
increase and the debt issue expected to be made in relation to
the acquisition of Talecris (see note 31 (f)).
Costs related to the capital increase will be taken to equity
when the capital increase is performed. Costs relating to the
issue of debt will be deducted from the financial liability when
it is recognised.
Costs incurred in relation to the business combination,
amounting to Euros 16,999 thousand, have been recognised as
expenses for 2010 (see note 27).
|
|
|
(16)
|
Cash and
Cash Equivalents
|
Details of this caption of the consolidated balance sheet at
31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Current deposits
|
|
|
211,564
|
|
|
|
237,801
|
|
|
Cash at banks
|
|
|
28,085
|
|
|
|
11,571
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cash and cash equivalents
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
|
|
|
|
|
|
|
|
Current deposits mainly include the surplus of funds from the
issue of bonds in the USA during 2009 (see note 5 (a)).
Details of cash and cash equivalents at 31 December 2010
and 2009 by currency are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Currency
|
|
|
|
|
|
|
|
|
|
Euro
|
|
|
4,268
|
|
|
|
2,153
|
|
|
US Dollar
|
|
|
202,942
|
|
|
|
208,800
|
|
|
Other currency
|
|
|
32,439
|
|
|
|
38,419
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
|
|
|
|
|
|
|
|
Details of consolidated equity and changes are shown in the
consolidated statement of changes in equity, which forms an
integral part of the consolidated financial statements.
F-90
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
At 31 December 2010 and 2009 the Company’s share
capital is represented by 213,064,899 ordinary shares of Euros
0.50 par value each, which are subscribed and fully paid
and have the same voting and profit-sharing rights.
These shares are freely transferable.
The Company only has information on the identity of its
shareholders when this information is provided voluntarily or to
comply with prevailing legislation. Based on the information
available to the Company, its most significant shareholders at
31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
Percentage Ownership
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Scranton Enterprises,B.V.
|
|
|
7.58
|
%
|
|
|
10.65
|
%
|
|
Capital Research and Management Company
|
|
|
10.02
|
%
|
|
|
—
|
|
|
Other
|
|
|
82.40
|
%
|
|
|
89.35
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100.00
|
%
|
|
|
100.00
|
%
|
|
|
|
|
|
|
|
|
|
|
There have been no movements in share premium during 2010 and
2009. In 2008 dividends were paid from share premium amounting
to Euros 10,030 thousand.
The availability of the reserves for distribution is subject to
legislation applicable to each of the Group companies. At
31 December 2010, Euros 28,876 thousand equivalent to the
carrying amount of development costs pending amortisation of
certain Spanish companies (Euros 25,987 thousand at
31 December 2009) (see note 8) are, in accordance
with applicable legislation, restricted reserves which cannot be
distributed until these development costs have been amortised.
Companies in Spain are obliged to transfer 10% of each
year’s profits to a legal reserve until this reserve
reaches an amount equal to 20% of share capital. This reserve is
not distributable to shareholders and may only be used to offset
losses if no other reserves are available. Under certain
conditions it may be used to increase share capital provided
that the balance left on the reserve is at least equal to 10% of
the nominal value of the total share capital after the increase.
At 31 December 2010 the legal reserve of the Parent has
been fully appropriated and amounts to Euros 21,306 thousand
(Euros 18,657 thousand at 31 December 2009).
Distribution of the legal reserves of Spanish companies is
subject to the same restrictions as those of the Parent Company
and at 31 December 2010 and 2009 the balance of the legal
reserve of other Spanish companies amounts to Euros 2,106
thousand.
Other foreign Group companies have a legal reserve amounting to
Euros 692 thousand (Euros 654 thousand at 31 December 2009).
F-91
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
During the year ended 31 December 2009 the Company has
carried out the following operations with own shares:
| |
|
|
|
|
|
|
|
|
|
|
|
No. of
|
|
|
Thousands of
|
|
|
|
|
Shares
|
|
|
Euros
|
|
|
|
|
Balance at 1 January 2009
|
|
|
2,411,622
|
|
|
|
33,087
|
|
|
Acquisitions
|
|
|
2,176,929
|
|
|
|
25,186
|
|
|
Disposals
|
|
|
(4,535,225
|
)
|
|
|
(57,596
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2009
|
|
|
53,326
|
|
|
|
677
|
|
|
|
|
|
|
|
|
|
|
|
During the year ended 31 December 2010 the Company has
carried out the following operations with own shares:
| |
|
|
|
|
|
|
|
|
|
|
|
No. of
|
|
|
Thousands of
|
|
|
|
|
Shares
|
|
|
Euros
|
|
|
|
|
Balance at 1 January 2010
|
|
|
53,326
|
|
|
|
677
|
|
|
Acquisitions
|
|
|
105,000
|
|
|
|
1,250
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December 2010
|
|
|
158,326
|
|
|
|
1,927
|
|
|
|
|
|
|
|
|
|
|
|
As a result, the Company holds own shares equivalent to 0.07% of
its capital at 31 December 2010 (0.03% at 31 December
2009).
|
|
|
(e)
|
Distribution
of profits
|
The profits of Grifols, S.A. and subsidiaries will be
distributed as agreed by respective shareholders of each company
at their general meetings.
The Board of directors of Grifols, S.A. will propose to the
shareholders at their annual general meeting that the profit of
Grifols, S.A. for the year ended 31 December 2010,
amounting to Euros 63,548 thousand, be transferred to reserves.
The distribution of the Company’s profit for the year ended
31 December 2009 is presented in the consolidated statement
of changes in equity.
The dividend per share distributed at 30 June 2009 is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
30/06/2009
|
|
|
|
|
|
|
|
|
|
|
Amount
|
|
|
|
|
% of par
|
|
|
Euro per
|
|
|
(Thousands of
|
|
|
|
|
Value
|
|
|
Share
|
|
|
Euros)
|
|
|
|
|
Ordinary shares
|
|
|
46
|
|
|
|
0.23
|
|
|
|
48,691
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total dividends paid in June 2009
|
|
|
46
|
|
|
|
0.23
|
|
|
|
48,691
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends with a charge to profits
|
|
|
46
|
|
|
|
0.23
|
|
|
|
48,691
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total dividends paid in June 2009
|
|
|
46
|
|
|
|
0.23
|
|
|
|
48,691
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-92
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
The dividend per share (interim dividend) distributed in
December 2009 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/2009
|
|
|
|
|
|
|
|
|
|
|
Amount
|
|
|
|
|
% of par
|
|
|
Euro per
|
|
|
(Thousands of
|
|
|
|
|
Value
|
|
|
Share
|
|
|
Euros)
|
|
|
|
|
Ordinary shares
|
|
|
30
|
|
|
|
0.15
|
|
|
|
31,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total dividends paid in December 2009
|
|
|
30
|
|
|
|
0.15
|
|
|
|
31,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interim dividend
|
|
|
30
|
|
|
|
0.15
|
|
|
|
31,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total dividends paid in December 2009
|
|
|
30
|
|
|
|
0.15
|
|
|
|
31,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The dividend per share distributed in July 2010 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/07/2010
|
|
|
|
|
|
|
|
|
|
|
Amount
|
|
|
|
|
% of par
|
|
|
Euro per
|
|
|
(Thousands of
|
|
|
|
|
Value
|
|
|
Share
|
|
|
Euros)
|
|
|
|
|
Total dividends paid in July 2010 (ordinary shares)
|
|
|
26
|
|
|
|
0.13
|
|
|
|
27,229
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
To cover the interest rate risk related to the planned issuance
of corporate bonds by Grifols Inc. (see note 22) a
swap was contracted in July 2009 to hedge the interest rate of
10-year US
government bonds, with a nominal amount of US Dollars
200 million and maturity on 21 September 2009 (date of
issuance of the bonds), swapping a variable interest rate for a
fixed rate. The Group has recognised this derivative as hedging
of cash flows from a highly probable transaction. At the date of
redemption, the valuation resulted in a financial cost of Euros
3,275 thousand, which has been recognised in equity, net of the
tax effect under “Cash flow hedges” and deferred over
the term of the ten-year corporate bond (see notes 22 and
32).
The calculation of basic earnings per share is based on the
profit for the year attributable to the shareholders of the
Company divided by the weighted average number of ordinary
shares in circulation throughout the year, excluding own shares.
Details of the calculation of basic earnings per share are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Profit for the year attributable to equity holders of the
Company (thousands of Euros)
|
|
|
115,513
|
|
|
|
147,972
|
|
|
|
121,728
|
|
|
Weighted average number of ordinary shares in circulation
|
|
|
212,909,162
|
|
|
|
209,451,806
|
|
|
|
210,707,597
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic earnings per share (Euros per share)
|
|
|
0.54
|
|
|
|
0.71
|
|
|
|
0.58
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The weighted average number of ordinary shares issued is
determined as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of Shares
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Issued ordinary shares at 1 January
|
|
|
213,011,573
|
|
|
|
210,653,277
|
|
|
|
210,964,436
|
|
|
Effect of own shares
|
|
|
(102,411
|
)
|
|
|
(1,201,471
|
)
|
|
|
(256,839
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
212,909,162
|
|
|
|
209,451,806
|
|
|
|
210,707,597
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-93
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Diluted earnings per share are calculated by dividing profit
attributable to shareholders of the Company by the weighted
average number of ordinary shares in circulation considering the
diluting effects of potential ordinary shares. At
31 December 2010, 2009 and 2008 basic and diluted earnings
per share are the same as no potential diluting effects exist.
|
|
|
(19)
|
Non-controlling
Interests
|
Details of non-controlling interests and movement during the
year ended 31 December 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Business
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/08
|
|
|
Additions
|
|
|
Combinations
|
|
|
Dividends
|
|
|
Differences
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Grifols (Thailand) Pte Ltd
|
|
|
977
|
|
|
|
308
|
|
|
|
—
|
|
|
|
(112
|
)
|
|
|
30
|
|
|
|
1,203
|
|
|
Grifols Malaysia Sdn Bhd
|
|
|
273
|
|
|
|
35
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(5
|
)
|
|
|
303
|
|
|
Woolloomooloo Holdings Pty Ltd.
|
|
|
—
|
|
|
|
(745
|
)
|
|
|
9,876
|
|
|
|
(106
|
)
|
|
|
1,626
|
|
|
|
10,651
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,250
|
|
|
|
(402
|
)
|
|
|
9,876
|
|
|
|
(218
|
)
|
|
|
1,651
|
|
|
|
12,157
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3
|
(b))
|
|
|
|
|
|
|
|
|
|
|
|
|
Details of non-controlling interests and movement during the
year ended 31 December 2010 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/09
|
|
|
Additions
|
|
|
Dividends
|
|
|
Differences
|
|
|
31/12/10
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Grifols (Thailand) Pte Ltd
|
|
|
1,203
|
|
|
|
367
|
|
|
|
(108
|
)
|
|
|
255
|
|
|
|
1,717
|
|
|
Grifols Malaysia Sdn Bhd
|
|
|
303
|
|
|
|
302
|
|
|
|
—
|
|
|
|
76
|
|
|
|
681
|
|
|
Woolloomooloo Holdings Pty Ltd.
|
|
|
10,651
|
|
|
|
(915
|
)
|
|
|
(158
|
)
|
|
|
2,374
|
|
|
|
11,952
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
12,157
|
|
|
|
(246
|
)
|
|
|
(266
|
)
|
|
|
2,705
|
|
|
|
14,350
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Capital grants
|
|
|
1,830
|
|
|
|
2,025
|
|
|
Interest-rate grants (preference loans)
|
|
|
258
|
|
|
|
286
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
2,088
|
|
|
|
2,311
|
|
|
|
|
|
|
|
|
|
|
|
F-94
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Details of capital grants are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Total amount of capital grant:
|
|
|
|
|
|
|
|
|
|
Prior to 1995
|
|
|
330
|
|
|
|
330
|
|
|
1995
|
|
|
627
|
|
|
|
627
|
|
|
1996
|
|
|
54
|
|
|
|
54
|
|
|
1997
|
|
|
426
|
|
|
|
426
|
|
|
1998
|
|
|
65
|
|
|
|
65
|
|
|
1999
|
|
|
42
|
|
|
|
42
|
|
|
2000
|
|
|
181
|
|
|
|
181
|
|
|
2001
|
|
|
214
|
|
|
|
214
|
|
|
2002
|
|
|
626
|
|
|
|
626
|
|
|
2004
|
|
|
1,940
|
|
|
|
1,940
|
|
|
2005
|
|
|
35
|
|
|
|
35
|
|
|
2006
|
|
|
35
|
|
|
|
35
|
|
|
2007
|
|
|
33
|
|
|
|
33
|
|
|
2008
|
|
|
124
|
|
|
|
124
|
|
|
2009
|
|
|
742
|
|
|
|
742
|
|
|
Current period
|
|
|
323
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
5,797
|
|
|
|
5,474
|
|
|
Less, revenues recognised:
|
|
|
|
|
|
|
|
|
|
Prior years
|
|
|
(3,140
|
)
|
|
|
(2,444
|
)
|
|
Current year
|
|
|
(612
|
)
|
|
|
(696
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(3,752
|
)
|
|
|
(3,140
|
)
|
|
Translation differences
|
|
|
(215
|
)
|
|
|
(309
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net value of capital grants
|
|
|
1,830
|
|
|
|
2,025
|
|
|
|
|
|
|
|
|
|
|
|
At 31 December 2010 interest-rate grants (preference loans)
include Euros 258 thousand (Euros 286 thousand at
31 December 2009) of implicit interest on loans
extended by the Spanish Ministry of Science and Technology as
these are interest free.
Movement during 2008 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Transfers to
|
|
|
Balances at
|
|
|
|
|
31/12/07
|
|
|
Additions
|
|
|
Profit or Loss
|
|
|
31/12/08
|
|
|
|
|
Interest-rate grants (preference loans)
|
|
|
2,463
|
|
|
|
561
|
|
|
|
(2,686
|
)
|
|
|
338
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Movement during 2009 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Transfers to
|
|
|
Balances at
|
|
|
|
|
31/12/08
|
|
|
Additions
|
|
|
Profit or Loss
|
|
|
31/12/09
|
|
|
|
|
Interest-rate grants (preference loans)
|
|
|
338
|
|
|
|
440
|
|
|
|
(492
|
)
|
|
|
286
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-95
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Movement during 2010 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Transfers to
|
|
|
Balances at
|
|
|
|
|
31/12/09
|
|
|
Additions
|
|
|
Profit or Loss
|
|
|
31/12/10
|
|
|
|
|
Interest-rate grants (preference loans)
|
|
|
286
|
|
|
|
88
|
|
|
|
(116
|
)
|
|
|
258
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Details of provisions at 31 December 2010 and 2009 are as
follows:
| |
|
|
|
|
|
|
|
|
|
Non-Current Provisions(a)
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Provisions for pensions and similar obligations
|
|
|
787
|
|
|
|
595
|
|
|
Other provisions
|
|
|
591
|
|
|
|
637
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current provisions
|
|
|
1,378
|
|
|
|
1,232
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
Current Provisions(b)
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Trade provisions
|
|
|
4,365
|
|
|
|
4,702
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a)
|
Non-current
provisions
|
At 31 December 2010 and 2009 provisions for pensions and
similar obligations mainly comprise a provision made by certain
foreign subsidiaries in respect of labour commitments with
certain employees.
Movement in non-current provisions during 2009 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
Business
|
|
|
|
|
|
Translation
|
|
Balances at
|
|
|
|
31/12/08
|
|
Combination
|
|
Reversal
|
|
Cancellation
|
|
Differences
|
|
31/12/09
|
|
|
|
Thousands of Euros
|
|
|
|
Non-current provisions
|
|
|
3,045
|
|
|
|
102
|
|
|
|
(1,411
|
)
|
|
|
(457
|
)
|
|
|
(47
|
)
|
|
|
1,232
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Movement in non-current provisions during 2010 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Translation
|
|
Balances at
|
|
|
|
31/12/09
|
|
Charge
|
|
Cancellation
|
|
Differences
|
|
31/12/10
|
|
|
|
Thousands of Euros
|
|
|
|
Non-current provisions
|
|
|
1,232
|
|
|
|
140
|
|
|
|
(71
|
)
|
|
|
77
|
|
|
|
1,378
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Movement in trade provisions during 2009 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
Business
|
|
|
|
Translation
|
|
Balances at
|
|
|
|
31/12/08
|
|
Combination
|
|
Charge
|
|
Differences
|
|
31/12/09
|
|
|
|
Thousands of Euros
|
|
|
|
Trade provisions
|
|
|
3,830
|
|
|
|
198
|
|
|
|
636
|
|
|
|
38
|
|
|
|
4,702
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Movement in trade provisions during 2010 is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Translation
|
|
Balances at
|
|
|
|
31/12/09
|
|
Charge
|
|
Cancellation
|
|
Differences
|
|
31/12/10
|
|
|
|
Thousands of Euros
|
|
|
|
Trade provisions
|
|
|
4,702
|
|
|
|
41
|
|
|
|
(414
|
)
|
|
|
36
|
|
|
|
4,365
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-96
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(22)
|
Financial
Liabilities
|
This note provides information on the contractual conditions of
the loans obtained by the Group, which are measured at amortised
cost, except for the financial derivative, which is measured at
fair value. For further information on exposure to interest rate
risk, currency risk and liquidity risk and the fair values of
financial liabilities, please refer to note 32.
|
|
|
a)
|
Non-current
financial liabilities
|
Details at 31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Current Financial Liabilities
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Corporate bonds (a.1.1)
|
|
|
441,203
|
|
|
|
410,552
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonds
|
|
|
441,203
|
|
|
|
410,552
|
|
|
|
—
|
|
|
Club Deal (a.1.2)
|
|
|
99,408
|
|
|
|
195,471
|
|
|
|
225,320
|
|
|
Other loans (a.1.2)
|
|
|
120,040
|
|
|
|
90,961
|
|
|
|
79,069
|
|
|
Finance lease liabilities (a.1.3)
|
|
|
4,734
|
|
|
|
6,202
|
|
|
|
7,124
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings
|
|
|
224,182
|
|
|
|
292,634
|
|
|
|
311,513
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings and bonds or other non-current
marketable securities (a.1)
|
|
|
665,385
|
|
|
|
703,186
|
|
|
|
311,513
|
|
|
Preference loans extended by the Spanish Ministry of Science and
Technology (a.2)
|
|
|
9,744
|
|
|
|
11,135
|
|
|
|
10,685
|
|
|
Debt on the acquisition of the plasma centre (a.2)
|
|
|
530
|
|
|
|
1,050
|
|
|
|
1,098
|
|
|
Other
|
|
|
200
|
|
|
|
367
|
|
|
|
759
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other non-current financial liabilities (a.2)
|
|
|
10,474
|
|
|
|
12,552
|
|
|
|
12,542
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
675,859
|
|
|
|
715,738
|
|
|
|
324,055
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current loans and borrowing arrangements are shown net of
the loan arrangement expenses which are pending amortisation:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
31/12/09
|
|
31/12/08
|
|
|
|
Thousands of Euros
|
|
|
|
Loan arrangement expenses
|
|
|
1,365
|
|
|
|
2,105
|
|
|
|
2,245
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(a.1)
|
Loans and
borrowings and bonds or other non-current marketable
securities
|
(a.1.1) Corporate
Bonds
On 21 September 2009 the Group, through Grifols, Inc.,
concluded the first private placement of corporate bonds in the
USA totalling US Dollars 600 million. The issue was
subscribed by 22 qualified investors, 90% in US Dollars and
the remaining 10% in Pounds Sterling and Euros. The issue was
structured in three tranches: US Dollars 200 million
at 12 years, US Dollars 300 million at
10 years and US Dollars 100 million at
7 years, with spreads over the yields of the 10 year
US Treasury bond of 370 basis points for those issued at
12 years, 350 basis points for those issued at
10 years and 335 basis points for 7 year bonds.
F-97
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
A summary of corporate bonds at 31 December 2010 is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fixed
|
|
|
|
|
|
Duration
|
|
Interest
|
|
Amount
|
|
(Years)
|
|
Rate
|
|
|
|
|
100,000
|
|
|
Thousands of USD
|
|
|
7
|
|
|
|
6.42
|
%
|
|
|
245,000
|
|
|
Thousands of USD
|
|
|
10
|
|
|
|
6.94
|
%
|
|
|
200,000
|
|
|
Thousands of USD
|
|
|
12
|
|
|
|
7.14
|
%
|
|
|
10,000
|
|
|
Thousands of EUR
|
|
|
10
|
|
|
|
6.94
|
%
|
|
|
25,000
|
|
|
Thousands of GBP
|
|
|
10
|
|
|
|
6.94
|
%
|
Funds raised have enabled the Group to extend the term of its
financial borrowings from current to non-current, at the same
time ensuring the availability of financial resources required
to consolidate its plans for the future. Funds raised have
therefore been used to settle current and non-current
liabilities and the remaining amount has been used in current
investments classified under “Cash and cash
equivalents” for an equivalent amount of Euros 211,539
thousand at 31 December 2010 (Euros 237,777 thousand at
31 December 2009). This amount has been invested mainly in
US Dollar deposits with financial institutions of
recognised solvency.
With the issuance of the bonds, an interest rate hedge was
contracted for the interest on the
10-year loan
from the US government (see notes 17 (f) and 32).
This issue of corporate bonds is subject to compliance with
certain financial ratio covenants. At 31 December 2010 and
2009 the Group complies with these financial ratio covenants.
Details of and movements related to the issue of corporate bonds
are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Opening balance
|
|
|
|
|
|
|
|
|
|
Issuance of corporate bonds in the USA
|
|
|
416,465
|
|
|
|
409,411
|
|
|
Transaction costs
|
|
|
(5,913
|
)
|
|
|
(5,967
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
410,552
|
|
|
|
403,444
|
|
|
|
|
|
|
|
|
|
|
|
|
Movements
|
|
|
|
|
|
|
|
|
|
Transferred to profit and loss
|
|
|
660
|
|
|
|
150
|
|
|
Corporate bonds issued in the USA, exchange differences
|
|
|
(1,772
|
)
|
|
|
338
|
|
|
Translation differences
|
|
|
31,763
|
|
|
|
6,620
|
|
|
Closing balance
|
|
|
|
|
|
|
|
|
|
Corporate bonds issued in the USA
|
|
|
446,918
|
|
|
|
416,465
|
|
|
Transaction costs
|
|
|
(5,715
|
)
|
|
|
(5,913
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
441,203
|
|
|
|
410,552
|
|
|
|
|
|
|
|
|
|
|
|
(a.1.2) Other
non-current loans and borrowings
Details of the terms and conditions of non-current loans and
borrowings at 31 December 2010 and 2009 are included in
Appendix IV, which forms an integral part of these notes to
the consolidated financial statements.
At 26 May 2008 a Club Deal refinancing agreement was signed
with 24 financial entities for Euros 350 million
(including the option to draw down a tranche of the loan in US
Dollars), in order to
F-98
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
refinance the non-current syndicated loan then existing. This
loan provided the Group with a significant margin for leverage
to carry out planned investment programmes.
This syndicated loan, which matures on 26 May 2013, is
subject to compliance with certain financial ratio covenants. In
accordance with the
agreed-upon
conditions, the level of compliance with financial ratios and
levels is determined at year end. The Company is required to
provide financial information to the lending banks within the
six-month period subsequent to 31 December of each year for
the duration of the contract.
In 2009 the 24 financial entities and the Company unanimously
agreed to a novation of the syndicated loan. The net financial
debt/equity ratio was replaced by the minimum equity ratio. This
replacement unifies all syndicated loan ratios with the bond
issuance carried out by the Group in the USA.
At 31 December 2010 and 2009 the Group fulfilled the
financial covenants established in the syndicated loan contract.
(a.1.3) Finance
lease liabilities
Details of minimum payments and the current finance lease
liabilities, by maturity date, are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Current
|
|
|
Non-Current
|
|
|
Current
|
|
|
Non-Current
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Minimum payments
|
|
|
3,552
|
|
|
|
5,089
|
|
|
|
5,088
|
|
|
|
6,675
|
|
|
Interest
|
|
|
(272
|
)
|
|
|
(355
|
)
|
|
|
(354
|
)
|
|
|
(473
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Present value
|
|
|
3,280
|
|
|
|
4,734
|
|
|
|
4,734
|
|
|
|
6,202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Minimum
|
|
|
|
|
|
Present
|
|
|
Minimum
|
|
|
|
|
|
Present
|
|
|
|
|
Payments
|
|
|
Interest
|
|
|
Value
|
|
|
Payments
|
|
|
Interest
|
|
|
Value
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity at:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Less than one year
|
|
|
3,552
|
|
|
|
272
|
|
|
|
3,280
|
|
|
|
5,088
|
|
|
|
354
|
|
|
|
4,734
|
|
|
Two years
|
|
|
2,411
|
|
|
|
161
|
|
|
|
2,250
|
|
|
|
3,364
|
|
|
|
200
|
|
|
|
3,164
|
|
|
Three years
|
|
|
1,271
|
|
|
|
96
|
|
|
|
1,175
|
|
|
|
1,382
|
|
|
|
114
|
|
|
|
1,268
|
|
|
Four years
|
|
|
763
|
|
|
|
50
|
|
|
|
713
|
|
|
|
831
|
|
|
|
72
|
|
|
|
759
|
|
|
Five years
|
|
|
314
|
|
|
|
23
|
|
|
|
291
|
|
|
|
577
|
|
|
|
41
|
|
|
|
536
|
|
|
More than five years
|
|
|
330
|
|
|
|
25
|
|
|
|
305
|
|
|
|
521
|
|
|
|
46
|
|
|
|
475
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
8,641
|
|
|
|
627
|
|
|
|
8,014
|
|
|
|
11,763
|
|
|
|
827
|
|
|
|
10,936
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-99
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
(a.1.4) Maturity
of non-current loans and borrowings and bonds
Details of maturity of non-current loans and borrowings and
bonds at 31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity at:
|
|
|
|
|
|
|
|
|
|
Two years
|
|
|
85,171
|
|
|
|
81,388
|
|
|
Three years
|
|
|
51,582
|
|
|
|
79,696
|
|
|
Four years
|
|
|
17,936
|
|
|
|
75,905
|
|
|
Five years
|
|
|
17,548
|
|
|
|
12,506
|
|
|
More than five years
|
|
|
493,148
|
|
|
|
453,691
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
665,385
|
|
|
|
703,186
|
|
|
|
|
|
|
|
|
|
|
|
(a.2) Other
non-current financial liabilities
Details of the interest-free preference loans extended by the
Spanish Ministry of Science and Technology to various group
companies are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Date
|
|
|
Amount
|
|
|
Non-
|
|
|
|
|
|
Non-
|
|
|
|
|
|
Company
|
|
Awarded
|
|
|
Awarded
|
|
|
Current
|
|
|
Current
|
|
|
Current
|
|
|
Current
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Instituto Grifols S.A.
|
|
|
31/01/2001
|
|
|
|
637
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
86
|
|
|
Instituto Grifols S.A.
|
|
|
13/02/2002
|
|
|
|
691
|
|
|
|
—
|
|
|
|
94
|
|
|
|
89
|
|
|
|
94
|
|
|
Instituto Grifols S.A.
|
|
|
17/01/2003
|
|
|
|
1,200
|
|
|
|
157
|
|
|
|
165
|
|
|
|
307
|
|
|
|
165
|
|
|
Instituto Grifols S.A.
|
|
|
13/11/2003
|
|
|
|
2,000
|
|
|
|
520
|
|
|
|
279
|
|
|
|
762
|
|
|
|
279
|
|
|
Instituto Grifols S.A.
|
|
|
17/01/2005
|
|
|
|
2,680
|
|
|
|
1,031
|
|
|
|
375
|
|
|
|
1,345
|
|
|
|
375
|
|
|
Instituto Grifols S.A.
|
|
|
29/12/2005
|
|
|
|
2,100
|
|
|
|
1,025
|
|
|
|
288
|
|
|
|
1,253
|
|
|
|
288
|
|
|
Instituto Grifols S.A.
|
|
|
29/12/2006
|
|
|
|
1,700
|
|
|
|
1,015
|
|
|
|
234
|
|
|
|
1,190
|
|
|
|
234
|
|
|
Instituto Grifols S.A.
|
|
|
27/12/2007
|
|
|
|
1,700
|
|
|
|
1,164
|
|
|
|
232
|
|
|
|
1,324
|
|
|
|
—
|
|
|
Instituto Grifols S.A.
|
|
|
31/12/2008
|
|
|
|
1,419
|
|
|
|
1,175
|
|
|
|
—
|
|
|
|
1,131
|
|
|
|
—
|
|
|
Instituto Grifols S.A.
|
|
|
16/01/2009
|
|
|
|
1,540
|
|
|
|
1,294
|
|
|
|
—
|
|
|
|
1,249
|
|
|
|
—
|
|
|
Laboratorios Grifols, S.A.
|
|
|
20/03/2001
|
|
|
|
219
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
30
|
|
|
Laboratorios Grifols, S.A.
|
|
|
29/01/2002
|
|
|
|
210
|
|
|
|
—
|
|
|
|
29
|
|
|
|
27
|
|
|
|
29
|
|
|
Laboratorios Grifols, S.A.
|
|
|
15/01/2003
|
|
|
|
220
|
|
|
|
29
|
|
|
|
30
|
|
|
|
56
|
|
|
|
30
|
|
|
Laboratorios Grifols, S.A.
|
|
|
26/09/2003
|
|
|
|
300
|
|
|
|
76
|
|
|
|
41
|
|
|
|
111
|
|
|
|
41
|
|
|
Laboratorios Grifols, S.A.
|
|
|
22/10/2004
|
|
|
|
200
|
|
|
|
77
|
|
|
|
28
|
|
|
|
100
|
|
|
|
28
|
|
|
Laboratorios Grifols, S.A.
|
|
|
20/12/2005
|
|
|
|
180
|
|
|
|
88
|
|
|
|
25
|
|
|
|
107
|
|
|
|
25
|
|
|
Laboratorios Grifols, S.A.
|
|
|
29/12/2006
|
|
|
|
400
|
|
|
|
233
|
|
|
|
54
|
|
|
|
273
|
|
|
|
54
|
|
|
Laboratorios Grifols, S.A.
|
|
|
27/12/2007
|
|
|
|
360
|
|
|
|
212
|
|
|
|
42
|
|
|
|
242
|
|
|
|
—
|
|
|
Laboratorios Grifols, S.A.
|
|
|
31/12/2008
|
|
|
|
600
|
|
|
|
497
|
|
|
|
—
|
|
|
|
478
|
|
|
|
—
|
|
|
Diagnostic Grifols, S.A.
|
|
|
27/11/2008
|
|
|
|
857
|
|
|
|
358
|
|
|
|
129
|
|
|
|
468
|
|
|
|
129
|
|
|
Diagnostic Grifols, S.A.
|
|
|
25/05/2010
|
|
|
|
203
|
|
|
|
116
|
|
|
|
31
|
|
|
|
—
|
|
|
|
—
|
|
|
Grifols Engineering, S.A.
|
|
|
21/04/2009
|
|
|
|
524
|
|
|
|
427
|
|
|
|
34
|
|
|
|
447
|
|
|
|
—
|
|
|
Grifols Engineering, S.A.
|
|
|
21/04/2009
|
|
|
|
203
|
|
|
|
165
|
|
|
|
13
|
|
|
|
176
|
|
|
|
—
|
|
|
Grifols Engineering, S.A.
|
|
|
28/01/2010
|
|
|
|
100
|
|
|
|
85
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
20,243
|
|
|
|
9,744
|
|
|
|
2,123
|
|
|
|
11,135
|
|
|
|
1,887
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-100
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
During 2010 the implicit borrowing costs taken to profit and
loss amount to Euros 567 thousand (Euros 616 thousand in
2009 and Euros 516 thousand in 2008) (see note 28).
At 31 December 2010, this caption also includes Euros 555
thousand (Euros 1,133 thousand at 31 December
2009) comprising the Euros equivalent of the debt in
US Dollars payable in the long term to Amerihealth Plasma,
LLC for the plasma centre acquired in the USA. Deferred finance
expenses resulting from this transaction amount to Euros 25
thousand (Euros 83 thousand at 31 December 2009) and
are deducted from the aforementioned amount. Other current
financial liabilities include the current portion of this debt
which amounts to Euros 637 thousand (Euros 442 thousand at
31 December 2009).
Details of the maturity of other non-current financial
liabilities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity at:
|
|
|
|
|
|
|
|
|
|
Two years
|
|
|
2,964
|
|
|
|
2,632
|
|
|
Three years
|
|
|
2,159
|
|
|
|
2,883
|
|
|
Four years
|
|
|
1,989
|
|
|
|
2,026
|
|
|
Five years
|
|
|
1,266
|
|
|
|
1,867
|
|
|
More than five years
|
|
|
2,096
|
|
|
|
3,144
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10,474
|
|
|
|
12,552
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
b)
|
Current
Financial Liabilities
|
Details at 31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
Current financial liabilities
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Bonds (b.1.1)
|
|
|
8,235
|
|
|
|
6,407
|
|
|
Interest of issue corporate bonds in the USA (b.1.1)
|
|
|
7,207
|
|
|
|
6,716
|
|
|
|
|
|
|
|
|
|
|
|
|
Bonds
|
|
|
15,442
|
|
|
|
13,123
|
|
|
Club Deal (b.1.2)
|
|
|
66,250
|
|
|
|
33,014
|
|
|
Other loans (b.1.2)
|
|
|
106,663
|
|
|
|
63,120
|
|
|
Finance lease liabilities (a.1.3)
|
|
|
3,280
|
|
|
|
4,734
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings
|
|
|
176,193
|
|
|
|
100,868
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings and bonds and other marketable
securities (b.1)
|
|
|
191,635
|
|
|
|
113,991
|
|
|
Financial derivatives (note 32)
|
|
|
8,560
|
|
|
|
3,333
|
|
|
Preference loans extended by the Spanish Ministry of Science and
Technology (a.2)
|
|
|
2,123
|
|
|
|
1,887
|
|
|
Receivables from social security affiliated entities transferred
to a financial institution (b.2)
|
|
|
6,503
|
|
|
|
5,459
|
|
|
Debt on the acquisition of the plasma centre (a.2)
|
|
|
637
|
|
|
|
442
|
|
|
Debt with Novartis (b.2)
|
|
|
—
|
|
|
|
779
|
|
|
Guarantee deposits received
|
|
|
149
|
|
|
|
59
|
|
|
Other current financial liabilities
|
|
|
264
|
|
|
|
271
|
|
|
|
|
|
|
|
|
|
|
|
|
Other current financial liabilities (b.2)
|
|
|
18,236
|
|
|
|
12,230
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
209,871
|
|
|
|
126,221
|
|
|
|
|
|
|
|
|
|
|
|
F-101
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Current loans and borrowing arrangements are shown net of the
loan arrangement expenses which are pending amortization:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Loan arrangement expenses
|
|
|
707
|
|
|
|
825
|
|
|
|
|
|
|
|
|
|
|
|
Current loans and borrowing arrangements are shown include
accrued interest amounting to Euros 483 thousand (Euros 538
thousand at 31 December 2009).
(b.1) Loans
and borrowings and bonds or other current marketable
securities
Details at 31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Promissory notes issued to bearer
|
|
|
8,373
|
|
|
|
6,510
|
|
|
Interest pending accrual on promissory notes issued to bearer
|
|
|
(138
|
)
|
|
|
(103
|
)
|
|
Interest accrued on corporate bonds
|
|
|
7,207
|
|
|
|
6,716
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
15,442
|
|
|
|
13,123
|
|
|
|
|
|
|
|
|
|
|
|
During 2010 and 2009 a Group company has issued bearer
promissory notes at one year of Euros 3,000 nominal amount each
and an interest rate of 5.00% and 4.75%, respectively, which
were earmarked for Group employees.
Details of the issue of bearer promissory notes to group
employees are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/09
|
|
|
|
|
|
|
|
|
|
|
|
Promissory
|
|
Interest
|
|
|
|
|
|
|
|
Nominal
|
|
|
|
Notes
|
|
Pending
|
|
|
|
|
|
|
|
Amount
|
|
|
|
Subscribed
|
|
Accrual
|
|
|
|
|
|
Maturity
|
|
(Thousands
|
|
Interest
|
|
(Thousands
|
|
(Thousands
|
|
|
|
Issue Date
|
|
Date
|
|
of Euros)
|
|
Rate
|
|
of Euros)
|
|
of Euros)
|
|
|
|
Issue of bearer promissory notes
|
|
|
05/05/09
|
|
|
|
05/05/10
|
|
|
|
3.000
|
|
|
|
4.75
|
%
|
|
|
6,510
|
|
|
|
(103
|
)
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
|
|
|
|
|
|
|
|
|
Promissory
|
|
Interest
|
|
|
|
|
|
|
|
Nominal
|
|
|
|
Notes
|
|
Pending
|
|
|
|
|
|
|
|
Amount
|
|
|
|
Subscribed
|
|
Accrual
|
|
|
|
|
|
Maturity
|
|
(Thousands
|
|
Interest
|
|
(Thousands
|
|
(Thousands
|
|
|
|
Issue Date
|
|
Date
|
|
of Euros)
|
|
Rate
|
|
of Euros)
|
|
of Euros)
|
|
|
|
Issue of bearer promissory notes
|
|
|
05/05/10
|
|
|
|
05/05/11
|
|
|
|
3.000
|
|
|
|
5.00
|
%
|
|
|
8,373
|
|
|
|
(138
|
)
|
F-102
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(b.1.2)
|
Other
current loans and borrowings
|
Details of current loans and borrowings are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest Rate (*)
|
|
Drawn Down
|
|
|
|
|
Min - Max
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Loans in:
|
|
|
|
|
|
|
|
|
|
|
|
US Dollars
|
|
5.00%
|
|
|
1,384
|
|
|
|
3,010
|
|
|
Euros
|
|
1.17% — 6%
|
|
|
143,990
|
|
|
|
73,664
|
|
|
Other currencies
|
|
TIIE+2% — 15%
|
|
|
26,368
|
|
|
|
18,449
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
171,742
|
|
|
|
95,123
|
|
|
Discounted trade notes (note 13)
|
|
1.4-4.69%
|
|
|
1,396
|
|
|
|
1,298
|
|
|
Current interest on loans and borrowings
|
|
|
|
|
483
|
|
|
|
538
|
|
|
Finance lease payables
|
|
|
|
|
3,552
|
|
|
|
5,088
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
177,173
|
|
|
|
102,047
|
|
|
Less, current portion of deferred finance expenses for leasing
|
|
|
|
|
(272
|
)
|
|
|
(354
|
)
|
|
Less, current portion of loan arrangement expenses
|
|
|
|
|
(708
|
)
|
|
|
(825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
176,193
|
|
|
|
100,868
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(*) |
|
Loans accrue variable interest rates. |
At 31 December 2010 the Group has a drawable borrowing
limit of Euros 704,315 thousand (Euros 703,231 thousand at
31 December 2009).
(b.2)
Other current financial liabilities
At 31 December 2010 and 2009 other current financial
liabilities also include approximately Euros 6,503 thousand and
Euros 5,459 thousand, respectively, which were paid directly by
social security affiliated entities and are transferable to
Deutsche Bank, S.A.E. under contracts (see note 13).
At 31 December 2009 this caption included an outstanding
receivable of Euros 779 thousand from Novartis Vaccines and
Diagnostics, Inc. for the licence contract signed by a Group
company during 2006. At 31 December 2010 this debt has been
fully repaid.
F-103
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
|
|
|
(23)
|
Trade and
Other Payables
|
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Suppliers and trade payables
|
|
|
160,678
|
|
|
|
120,887
|
|
|
Other
|
|
|
—
|
|
|
|
22
|
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
160,678
|
|
|
|
120,909
|
|
|
Public entities, other payables
|
|
|
11,928
|
|
|
|
17,832
|
|
|
|
|
|
|
|
|
|
|
|
|
Other trade payables
|
|
|
11,928
|
|
|
|
17,832
|
|
|
Current income tax liabilities
|
|
|
4,172
|
|
|
|
3,258
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
176,778
|
|
|
|
141,999
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
Details of related parties are shown in note 33.
Balances with suppliers include the following payables in
foreign currencies:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Currency
|
|
|
|
|
|
|
|
|
|
US Dollar
|
|
|
58,932
|
|
|
|
31,377
|
|
|
Chilean Peso
|
|
|
1,490
|
|
|
|
894
|
|
|
Swiss Franc
|
|
|
897
|
|
|
|
686
|
|
|
Czech Crown
|
|
|
568
|
|
|
|
380
|
|
|
Brazilian Real
|
|
|
428
|
|
|
|
621
|
|
|
Pound Sterling
|
|
|
405
|
|
|
|
266
|
|
|
Other currencies
|
|
|
665
|
|
|
|
1,419
|
|
The Group’s exposure to currency risk and liquidity risk
associated with trade and other payables is described in
note 32.
Public
entities, other payables
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Taxation authorities, VAT/Canary Islands Tax
|
|
|
3,472
|
|
|
|
3,292
|
|
|
Taxation authorities, withholdings
|
|
|
3,119
|
|
|
|
8,184
|
|
|
Social Security
|
|
|
3,246
|
|
|
|
3,027
|
|
|
Other public entities
|
|
|
2,091
|
|
|
|
3,329
|
|
|
|
|
|
|
|
|
|
|
|
|
Public entities, other payables
|
|
|
11,928
|
|
|
|
17,832
|
|
|
|
|
|
|
|
|
|
|
|
At 31 December 2010, Other public entities include a Euros
1,860 thousand provision (Euros 2,781 thousand at
31 December 2009) recognised as a result of a
different interpretation of a specific tax situation which could
be made by the current tax inspection (see note 29 (c)).
F-104
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Current
tax liabilities
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Taxation authorities, income tax:
|
|
|
|
|
|
|
|
|
|
Current year
|
|
|
4,161
|
|
|
|
3,185
|
|
|
Prior years
|
|
|
11
|
|
|
|
73
|
|
|
|
|
|
|
|
|
|
|
|
|
Current tax liabilities
|
|
|
4,172
|
|
|
|
3,258
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(24)
|
Other
Current Liabilities
|
Details at 31 December are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Salaries payable
|
|
|
28,321
|
|
|
|
24,367
|
|
|
Other payables
|
|
|
2,629
|
|
|
|
1,754
|
|
|
|
|
|
|
|
|
|
|
|
|
Other current liabilities
|
|
|
30,950
|
|
|
|
26,121
|
|
|
|
|
|
|
|
|
|
|
|
Revenues are mainly generated by the sale of goods.
The distribution of net consolidated revenues for 2010, 2009 and
2008, by segment, was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
%
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Bioscience
|
|
|
78
|
%
|
|
|
76
|
%
|
|
|
76
|
%
|
|
Diagnostics
|
|
|
11
|
%
|
|
|
10
|
%
|
|
|
10
|
%
|
|
Hospital
|
|
|
9
|
%
|
|
|
10
|
%
|
|
|
10
|
%
|
|
Raw materials
|
|
|
1
|
%
|
|
|
3
|
%
|
|
|
3
|
%
|
|
Others
|
|
|
1
|
%
|
|
|
1
|
%
|
|
|
1
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The geographical distribution of net consolidated revenues is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
%
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Spain
|
|
|
23
|
%
|
|
|
25
|
%
|
|
|
24
|
%
|
|
European Union
|
|
|
21
|
%
|
|
|
22
|
%
|
|
|
26
|
%
|
|
United States
|
|
|
34
|
%
|
|
|
32
|
%
|
|
|
36
|
%
|
|
Rest of the world
|
|
|
22
|
%
|
|
|
21
|
%
|
|
|
14
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
100
|
%
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-105
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Net consolidated revenues include net sales made in the
following foreign currencies:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Currency
|
|
|
|
|
|
|
|
|
|
|
|
|
|
US Dollar
|
|
|
405,439
|
|
|
|
349,064
|
|
|
|
304,445
|
|
|
Pound Sterling
|
|
|
36,199
|
|
|
|
33,668
|
|
|
|
36,668
|
|
|
Chilean Peso
|
|
|
28,760
|
|
|
|
21,083
|
|
|
|
16,047
|
|
|
Mexican Peso
|
|
|
25,652
|
|
|
|
36,472
|
|
|
|
29,182
|
|
|
Brazilian Real
|
|
|
21,949
|
|
|
|
21,262
|
|
|
|
15,916
|
|
|
Australian Dollar
|
|
|
13,950
|
|
|
|
6,387
|
|
|
|
—
|
|
|
Czech Crown
|
|
|
13,698
|
|
|
|
12,863
|
|
|
|
12,568
|
|
|
Argentinean Peso
|
|
|
13,122
|
|
|
|
11,323
|
|
|
|
9,145
|
|
|
Polish Zloty
|
|
|
11,668
|
|
|
|
13,525
|
|
|
|
—
|
|
|
Other currency
|
|
|
18,989
|
|
|
|
18,013
|
|
|
|
13,062
|
|
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Wages and salaries
|
|
|
232,174
|
|
|
|
219,803
|
|
|
|
191,644
|
|
|
Contributions to pension plans (note 31)
|
|
|
1,615
|
|
|
|
1,571
|
|
|
|
1,365
|
|
|
Other social charges
|
|
|
8,615
|
|
|
|
8,072
|
|
|
|
6,310
|
|
|
Social Security
|
|
|
46,604
|
|
|
|
43,722
|
|
|
|
38,840
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
289,008
|
|
|
|
273,168
|
|
|
|
238,159
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(27)
|
Other
Operating Income and Expenses
|
Other
operating expenses
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Changes in trade provisions (notes 21 (b) and 32)
|
|
|
398
|
|
|
|
1,348
|
|
|
|
561
|
|
|
Professional services (note 15)
|
|
|
40,530
|
|
|
|
25,266
|
|
|
|
22,874
|
|
|
Commissions
|
|
|
8,038
|
|
|
|
7,711
|
|
|
|
7,075
|
|
|
Supplies and other materials
|
|
|
30,544
|
|
|
|
28,859
|
|
|
|
26,874
|
|
|
Operating leases (note 30 (a))
|
|
|
19,272
|
|
|
|
17,364
|
|
|
|
16,583
|
|
|
Freight
|
|
|
20,956
|
|
|
|
20,518
|
|
|
|
19,485
|
|
|
Repairs and maintenance costs
|
|
|
22,480
|
|
|
|
21,365
|
|
|
|
17,642
|
|
|
Advertising
|
|
|
14,708
|
|
|
|
15,580
|
|
|
|
16,872
|
|
|
Insurance
|
|
|
10,807
|
|
|
|
10,803
|
|
|
|
10,367
|
|
|
Royalties and service charges
|
|
|
884
|
|
|
|
4,954
|
|
|
|
8,760
|
|
|
Travel expenses
|
|
|
12,742
|
|
|
|
11,935
|
|
|
|
14,210
|
|
|
External services
|
|
|
24,603
|
|
|
|
25,024
|
|
|
|
21,891
|
|
|
Others
|
|
|
14,256
|
|
|
|
12,654
|
|
|
|
9,094
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other operating expenses
|
|
|
220,218
|
|
|
|
203,381
|
|
|
|
192,288
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-106
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Research and development expenses incurred by the Group
(including personnel expenses and amortization of intangible
assets and property, plant and equipment) amount to Euros
36.6 million in 2010 (Euros 35.2 million in 2009 and
Euros 25.6 million in 2008).
Other
operating income
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Income from insurance claims
|
|
|
771
|
|
|
|
807
|
|
|
|
584
|
|
|
Grants
|
|
|
307
|
|
|
|
378
|
|
|
|
497
|
|
|
Other income
|
|
|
118
|
|
|
|
258
|
|
|
|
208
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other operating income
|
|
|
1,196
|
|
|
|
1,443
|
|
|
|
1,289
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(28)
|
Finance
Income and Expense
|
Details are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Interest from Social Security
|
|
|
2,876
|
|
|
|
6,510
|
|
|
|
2,212
|
|
|
Other finance income
|
|
|
1,650
|
|
|
|
557
|
|
|
|
470
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
4,526
|
|
|
|
7,067
|
|
|
|
2,682
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Syndicated loan (other finance expenses)
|
|
|
(1,172
|
)
|
|
|
(747
|
)
|
|
|
(1,849
|
)
|
|
Syndicated loan (interest)
|
|
|
(3,303
|
)
|
|
|
(6,289
|
)
|
|
|
(12,152
|
|
|
Finance expenses from sale of receivables (note 13)
|
|
|
(5,378
|
)
|
|
|
(2,531
|
)
|
|
|
(2,128
|
)
|
|
Interests costs of Corporate bonds issued in the USA
(note 22)
|
|
|
(31,923
|
)
|
|
|
(6,766
|
)
|
|
|
—
|
|
|
Implicit interest on preference loans (note 22 (a2))
|
|
|
(567
|
)
|
|
|
(616
|
)
|
|
|
(516
|
)
|
|
Capitalised interest
|
|
|
2,399
|
|
|
|
1,278
|
|
|
|
—
|
|
|
Other finance expenses
|
|
|
(9,716
|
)
|
|
|
(11,416
|
)
|
|
|
(12,660
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance expenses
|
|
|
(49,660
|
)
|
|
|
(27,087
|
)
|
|
|
(29,305
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value of financial derivatives (note 32)
|
|
|
(7,593
|
)
|
|
|
(587
|
)
|
|
|
(1,268
|
)
|
|
Impairment and profit/(losses) on disposal of financial
instruments
|
|
|
91
|
|
|
|
(245
|
)
|
|
|
—
|
|
|
Exchange differences
|
|
|
1,616
|
|
|
|
(1,733
|
)
|
|
|
(2,825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net finance income and expense
|
|
|
(51,020
|
)
|
|
|
(22,585
|
)
|
|
|
(30,716
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
During 2010 the Group has capitalised interest at a rate of
between 2.6% and 7.1% based on the financing received (between
3% and 4% during 2009) (see note 4 (f)).
Companies present annual income tax returns. The standard rate
of tax is 30% for Spanish companies, which may be reduced by
certain credits.
Grifols, S.A. is authorised to present a consolidated tax return
with Diagnostic Grifols, S.A., Movaco, S.A., Laboratorios
Grifols, S.A., Instituto Grifols, S.A., Logister, S.A., Biomat,
S.A., Grifols Viajes, S.A., Grifols International, S.A., Grifols
Engineering, S.A., Arrahona Optimus, S.L. and Gri-Cel, S.A.
(since 2009).
F-107
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Grifols, S.A., in its capacity as parent, is responsible for the
presentation and payment of the consolidated tax return.
The
North-American
company Grifols, Inc. is also authorised to present consolidated
tax returns in the USA with Grifols Biologicals, Inc., Grifols
USA, LLC, Biomat USA, Inc. and Plasmacare, Inc.
|
|
|
a)
|
Reconciliation
of accounting and taxable income
|
Details of the income tax expense are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Profit for the year before income tax
|
|
|
157,784
|
|
|
|
203,994
|
|
|
|
172,269
|
|
|
Tax at 30%
|
|
|
47,335
|
|
|
|
61,198
|
|
|
|
51,680
|
|
|
Permanent differences
|
|
|
2,300
|
|
|
|
1,935
|
|
|
|
2,678
|
|
|
Effect of different tax rates
|
|
|
3,346
|
|
|
|
5,159
|
|
|
|
4,366
|
|
|
Tax credits for research and development
|
|
|
(7,281
|
)
|
|
|
(8,106
|
)
|
|
|
(5,403
|
)
|
|
Other tax credits (deductions)
|
|
|
(3,516
|
)
|
|
|
(4,548
|
)
|
|
|
(4,199
|
)
|
|
Other income tax expenses
|
|
|
333
|
|
|
|
786
|
|
|
|
1,031
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total income tax expense
|
|
|
42,517
|
|
|
|
56,424
|
|
|
|
50,153
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred tax expenses
|
|
|
15,547
|
|
|
|
8,832
|
|
|
|
6,987
|
|
|
Current income tax
|
|
|
26,970
|
|
|
|
47,592
|
|
|
|
43,166
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
42,517
|
|
|
|
56,424
|
|
|
|
50,153
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred
tax assets and liabilities
Details of deferred tax assets and liabilities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax Effect
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax credits (deductions)
|
|
|
4,830
|
|
|
|
5,992
|
|
|
|
13,215
|
|
|
Tax loss carryforwards
|
|
|
1,233
|
|
|
|
88
|
|
|
|
163
|
|
|
Fixed assets, amortisation and depreciation
|
|
|
998
|
|
|
|
728
|
|
|
|
299
|
|
|
Unrealised margins on inventories
|
|
|
19,256
|
|
|
|
19,814
|
|
|
|
17,222
|
|
|
Provision for bad debts
|
|
|
395
|
|
|
|
444
|
|
|
|
281
|
|
|
Inventories
|
|
|
235
|
|
|
|
225
|
|
|
|
1,004
|
|
|
Cash flow hedges
|
|
|
1,120
|
|
|
|
1,247
|
|
|
|
—
|
|
|
Other provisions
|
|
|
4,297
|
|
|
|
2,439
|
|
|
|
825
|
|
|
Others
|
|
|
2,525
|
|
|
|
2,418
|
|
|
|
1,187
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
34,889
|
|
|
|
33,395
|
|
|
|
34,297
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
17,948
|
|
|
|
15,186
|
|
|
|
12,423
|
|
|
Revaluations of assets
|
|
|
15,210
|
|
|
|
15,011
|
|
|
|
15,345
|
|
|
Fixed assets, amortisation and depreciation
|
|
|
40,520
|
|
|
|
23,873
|
|
|
|
14,028
|
|
|
Finance leases
|
|
|
3,396
|
|
|
|
3,634
|
|
|
|
3,647
|
|
|
Inventories
|
|
|
—
|
|
|
|
—
|
|
|
|
2,041
|
|
|
Provision for investments
|
|
|
696
|
|
|
|
873
|
|
|
|
2,322
|
|
|
Others
|
|
|
1,371
|
|
|
|
1,748
|
|
|
|
2,163
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
79,141
|
|
|
|
60,325
|
|
|
|
51,969
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-108
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Movement in deferred tax assets and liabilities is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred Tax Assets
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Balance at 1 January
|
|
|
33,395
|
|
|
|
34,297
|
|
|
|
34,110
|
|
|
Movements during the year
|
|
|
865
|
|
|
|
(1,478
|
)
|
|
|
687
|
|
|
Business combinations (note 3)
|
|
|
—
|
|
|
|
500
|
|
|
|
—
|
|
|
Adjustments for changes in tax rate through profit and loss
|
|
|
—
|
|
|
|
69
|
|
|
|
(514
|
)
|
|
Translation differences
|
|
|
629
|
|
|
|
7
|
|
|
|
14
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December
|
|
|
34,889
|
|
|
|
33,395
|
|
|
|
34,297
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred Tax Liabilities
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Balance at 1 January
|
|
|
60,325
|
|
|
|
51,969
|
|
|
|
43,794
|
|
|
Movements during the year
|
|
|
16,537
|
|
|
|
7,423
|
|
|
|
6,721
|
|
|
Business combinations (note 3)
|
|
|
—
|
|
|
|
1,761
|
|
|
|
—
|
|
|
Adjustments for changes in tax rate through profit and loss
|
|
|
—
|
|
|
|
—
|
|
|
|
439
|
|
|
Translation differences
|
|
|
2,279
|
|
|
|
(828
|
)
|
|
|
1,015
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at 31 December
|
|
|
79,141
|
|
|
|
60,325
|
|
|
|
51,969
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
As permitted by Royal Decree — Law 3/1993 governing
urgent tax and financial measures and Royal Decrees —
Law 7/1994 and Law 2/1995 governing accelerated depreciation of
property, plant and equipment for investments which generate
employment, the Spanish companies have opted to apply
accelerated depreciation to certain additions to property, plant
and equipment, which has resulted in the corresponding deferred
tax liability.
Details of deferred tax assets and liabilities on items directly
debited and credited to equity during the year are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Tax Effect
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Available-for-sale
financial assets
|
|
|
—
|
|
|
|
(69
|
)
|
|
|
3
|
|
|
Cash flow hedges (note 17 (g))
|
|
|
127
|
|
|
|
1,247
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
127
|
|
|
|
1,178
|
|
|
|
3
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The remaining assets and liabilities recognised in 2010, 2009
and 2008 were recognised on the income statement.
The Spanish consolidated companies have deductions pending
application at 31 December 2010 and 2009 mainly in respect
of research and development, which are detailed below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Applicable
|
|
|
Year of Origin
|
|
2010
|
|
|
2009
|
|
|
Through
|
|
|
|
|
2008
|
|
|
101
|
|
|
|
417
|
|
|
|
2023
|
|
|
2009
|
|
|
500
|
|
|
|
5,575
|
|
|
|
2024
|
|
|
2010
|
|
|
4,229
|
|
|
|
—
|
|
|
|
2025
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,830
|
|
|
|
5,992
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-109
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
At 31 December 2010 the Group recognised a tax credit of
Euros 4,830 thousand (Euros 5,992 thousand at 31 December
2009) from the deductions pending application, as its
future recovery was reasonably assured.
At 31 December 2010 the Group has future tax deductions of
Euros 23,685 thousand (Euros 25,806 thousand at 31 December
2009) pending application as a result of goodwill generated
on the acquisition of Biomat USA, Inc. This amount will be
deducted annually from the taxable profits until 2022, without
being limited by the amount of tax payable in any one year. The
amount that will be deducted in 2010 at the rate of 30% will be
Euros 2,121 thousand. The Group has recognised a deferred tax
liability of Euros 14,848 thousand in respect of this item at
31 December 2010 (Euros 12,727 thousand at 31 December
2009).
At 31 December 2010 the Group also has future tax
deductions of Euros 9,727 thousand (Euros 10,368 thousand at
31 December 2009) pending application as a result of
goodwill generated on the acquisition of Plasmacare, Inc. This
amount will be deducted annually from the taxable profits until
2026, without being limited by the amount of tax payable in any
one year. The amount deducted in 2010 at the rate of 30% has
been Euros 641 thousand. The Group has recognised a deferred tax
liability of Euros 3,100 thousand in respect of this item at
31 December 2010 (Euros 2,459 thousand at 31 December
2009).
At 31 December 2010 the Group has recognised loss
carryforwards of Euros 1,233 thousand (Euros 88 thousand at
31 December 2009 and Euros 163 at 31 December 2008),
of which, Euros 1,187 thousand correspond to the Australian
company Woolloomooloo Holdings Pty. Ltd., whilst Euros 46
thousand relate to the US company Grifols USA, LLC.
The Group has not recognised the tax effect of loss
carryforwards of Euros 1,231 thousand (Euros 1,117 thousand at
31 December 2009 and Euros 635 thousand at 31 December
2008) from Grifols Portugal as deferred tax assets. The
remaining companies do not have significant loss carryforwards
which have not been recognised.
|
|
|
c)
|
Years
open to inspection
|
In accordance with current legislation, taxes cannot be
considered definitive until they have been inspected and agreed
by the taxation authorities or before the prescribed inspection
period has elapsed.
During 2010 the following events arose in relation to the tax
inspections performed in Group companies:
|
|
|
| |
•
|
On 30 June 2010, in relation to the inspection underway on
Grifols, S.A., Instituto Grifols, S.A., Laboratorios Grifols,
S.A. and Movaco, S.A., the Group has received assessments for
income tax, value added tax (VAT), personal income tax and
withholding tax on investment income. The total amount settled
was Euros 586 thousand and the income tax expense amounts to
Euros 1,257 thousand.
|
| |
| |
•
|
During 2010 the tax inspection on the income tax, VAT and
withholdings for 2006 of Grifols Italia, S.p.A. was concluded,
implying no significant payment for the Group.
|
| |
| |
•
|
Logística Grifols, S.A. de CV: Tax ruling on the financial
statements for 2005 and 2006. Group management does not expect
any significant liabilities to arise as a result of this
inspection.
|
| |
| |
•
|
At 30 June 2010 Grifols, Inc. and subsidiaries received
notification of income tax inspection for the years closed
31 December 2006, 2007 and 2008. Due to, among other
reasons, differences in the interpretation of prevailing tax
legislation, the Group’s directors have set up a provision
of Euros 1,860 thousand, which is recognised under “income
tax” in the income statement and under “public
entities, other” in the balance sheet (see note 23).
|
| |
| |
•
|
Grifols Brasil, Lda.: Tax on circulation of goods and services
(ICMS) for 2006 to 2010. Group management does not expect that
any significant liability will arise from this inspection.
|
F-110
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
During 2009 the following events arose in relation to the tax
inspections:
|
|
|
| |
•
|
Notification of the completion of the inspection of Biomat USA,
Inc., resulting in a favourable conclusion.
|
During 2008 the following events had arisen in relation to the
tax inspections performed in Group companies:
|
|
|
| |
•
|
Notification of the favourable completion of the inspection of
Grifols Deutschland, except for Euros 150 thousand which was
taken to profit and loss in 2008.
|
| |
| |
•
|
Notification of the favourable completion of the inspection of
Grifols, Inc., Grifols Biologicals, Inc., Grifols USA, Inc. and
Plasmacare, Inc.
|
|
|
|
(a)
|
Operating
leases (as lessee)
|
At 31 December 2010 and 2009 the Group leased buildings
from third parties under operating leases.
The Group has warehouses and buildings contracted under
operating lease. The duration of these lease contracts ranges
from between 1 to 30 years. Contracts may be renewed on
termination. Lease instalments are adjusted periodically in
accordance with the price index established in each contract.
One Group company has entered into lease contracts which include
contingent rents. These contingent rents have been based on
production capacity, surface area used and the real estate
market and are expensed on a straight line basis.
Operating lease instalments of Euros 19,272 thousand have been
recognised as an expense for the year at 31 December 2010
(Euros 17,364 thousand at 31 December 2009 and 16,583
thousand at 31 December 2008) (see note 27).
Future minimum payments on non-cancellable operating leases at
31 December are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Up to 1 year
|
|
|
13,769
|
|
|
|
10,098
|
|
|
|
9,575
|
|
|
Between 1 and 5 years
|
|
|
31,003
|
|
|
|
25,943
|
|
|
|
24,919
|
|
|
More than 5 years
|
|
|
7,856
|
|
|
|
8,084
|
|
|
|
7,192
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total future minimum payments
|
|
|
52,628
|
|
|
|
44,125
|
|
|
|
41,686
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(b)
|
Operating
leases (as lessor)
|
The Group has a building leased to third parties under an
operating lease at 31 December 2010, 2009 and 2008. Future
minimum payments receivable under non-cancellable operating
leases are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Maturity:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Up to 1 year
|
|
|
64
|
|
|
|
91
|
|
|
|
69
|
|
|
Between 1 and 5 years
|
|
|
21
|
|
|
|
56
|
|
|
|
50
|
|
|
More than 5 years
|
|
|
—
|
|
|
|
10
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total future minimum payments
|
|
|
85
|
|
|
|
157
|
|
|
|
119
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-111
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
This contract does not include contingent rents or purchase
options. Income of Euros 96 thousand has been recognised in 2010
(Euros 85 thousand in 2009 and Euros 70 thousand in 2008).
|
|
|
(31)
|
Other
Commitments with Third Parties and Other Contingent
Liabilities
|
The Group has not extended any security or bank guarantees to
third parties.
|
|
|
(b)
|
Obligations
with personnel
|
As described in note 4 (q) section (i), Spanish
companies of the Group are obliged to contribute to a defined
contribution pension plan. Contributions made by the Group
amounted to Euros 460 thousand in 2010 (Euros 416 thousand at
31 December 2009).
In successive years this contribution will be defined through
labour negotiations.
Some foreign subsidiaries of the Group have made contributions
of Euros 1,155 thousand to complementary pension schemes (Euros
1,155 thousand at 31 December 2009).
|
|
|
(c)
|
Judicial
procedures and arbitration
|
Details of legal proceedings in which the Company or Group
companies are involved are as follows:
Instituto
Grifols, S.A.
|
|
|
| |
•
|
Litigation was initiated in February 2000. Proceedings have been
brought jointly against the Company and another plasma
fractioning company.
|
The claimant (an individual) claimed Euros 542 thousand in
damages due to the alleged contraction of HIV and Hepatitis C.
The first instance court in Cadiz fully rejected the claim
against Instituto Grifols, S.A. on 25 November 2005.
An appeal was filed, which was rejected by the Cádiz
Provincial Court in April 2007, thereby confirming the
company’s line of defense. Notification was published on
3 February 2011 that on 19 January 2011 the Spanish
High Court had fully rejected the appeal against the Cádiz
Provincial Court’s decision to reject the claim against
Instituto Grifols, S.A.
|
|
|
| |
•
|
A claim brought against the Health Board of Castilla y León
in February 2005.
|
The defendant (an individual) claimed Euros 180 thousand in
damages due to the alleged contraction of Hepatitis C. The
health authorities requested that this claim be extended to
include the Company.
Notification was published on 2 February 2011 that this
appeal has been rejected on 30 December 2010. This ruling
is pending confirmation, unless it is appealed in the Spanish
High Court.
|
|
|
| |
•
|
The Company was notified in 2007 of a claim for maximum damages
of Euros 12,960 thousand filed by a group of 100 Catalan
haemophiliacs against all plasma fractionation companies. During
2008 this claim was rejected by the Courts, and the ruling
appealed by the group of haemophiliacs. Notification was
published on 21 January 2011 that on 18 January 2011
the Barcelona Provincial Court had rejected the
haemophiliacs’ claim. This ruling is pending confirmation,
unless it is appealed in the Spanish High Court.
|
F-112
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Grifols
Biologicals, Inc.
|
|
|
| |
•
|
Legal proceedings (consent decree) which were brought against
the plasma fractioning centre in Los Angeles.
|
|
|
|
| |
|
The blood plasma fractioning centre in Los Angeles is managed
through consent decree which was applied for in January 1998 to
the Courts by the FDA and US Department of Justice as a result
of an infringement of FDA regulations committed by the former
owner of the centre (Alpha Therapeutic Corporation, hereinafter
ATC). As a result of this consent decree, the Los Angeles centre
is subject to strict FDA audits and may only sell products
manufactured in the centre subsequent to prior authorisation.
|
| |
| |
|
The Company cannot guarantee if or when the consent decree will
be lifted.
|
| |
| |
|
In March 2004 as a result of improvements to the centre made by
the Group, the FDA awarded several free sales certificates for
the former ATC products manufactured in this centre.
|
| |
| |
|
Based on the current level of compliance, there are no
commercial activities that are prohibited or limited by the
consent decree.
|
No provision has been made for these legal issues as the Group
considers that these will not have a probable adverse impact.
|
|
|
(d)
|
Long-term
materials supply contract
|
The long-term supply contract for plasma signed by the Group in
2008 was terminated by the Group in 2009 on the grounds of
failure by the supplier to meet certain contractual terms. The
supplier has not accepted the arguments of the Group and both
are presently holding negotiations to settle the dispute in
arbitration proceedings, the Directors of the Group being of the
opinion that the eventual settlement will not involve any
significant additional costs.
|
|
|
(e)
|
Agreement
for the acquisition of Talecris Biotherapeutics Holdings Corp
(Talecris)
|
On 6 June 2010 the Company entered into an agreement to
acquire the American company Talecris Biotherapeutics, which
also specialises in the production of plasma-derived biological
medication, for a total of US Dollars 3,400 million.
This agreement will become effective subject to approval by the
Defence of Competition authorities. In the event that this
approval is not obtained, the Company will be required to pay
US Dollars 375 million as indemnity for the damages
caused.
The operation will be performed through a combined offer of cash
and Grifols shares which would not carry the right to vote on
new share issues.
The offer is made in relation to all Talecris shares and the
price offered per share amounts to US Dollars 19 in cash
and 0.641 shares in Grifols without the right to vote on
new share issues. As a result of the ruling on the claim filed
by certain shareholders of Talecris in the State of Delaware
against Talecris, Cerburus, Grifols and the Agreement and
Plan of Merger, appraisal rights have been granted to those
Talecris shareholders who have requested them and Grifols has
undertaken to issue 500,000 shares without additional
voting rights which will be distributed amongst all of the
shareholders of Talecris, except for Talecris Holdings LLC and
the directors of Talecris. As a result of this additional share
issue, the share exchange equation stands at
(a) 0.641 shares without voting rights of Grifols for
each Talecris share issued, at the closing date of the
transaction, held by Talecris LLC and the directors of Talecris
and (b) 0.6485 shares without voting rights of Grifols
for each Talecris share issued, at the transaction closing date,
held by the remaining shareholders.
F-113
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
On 6 June 2010 and in relation to this potential
acquisition, the Company obtained financing commitments from six
financial institutions for a total of US Dollars
4,500 million. This financing would be used to cover the
cash payment of the acquisition and to refinance the existing
debt.
On 23 November 2010 the Company signed loan agreements
amounting to US Dollars 3,400 million for the purchase
of Talecris. This amount forms part of the US Dollars
4,500 million collateralised on 6 June 2010. Details
of this collateralised senior debt are as follows:
|
|
|
| |
•
|
Non-current syndicated financing with financial institutions:
Loan repayable in 5 years totalling US Dollars
1,500 million. Margin of 375 basis points (bp) linked
to US Libor and 400 bp linked to Euribor. BB and Ba3 rating.
|
| |
| |
•
|
Non-current syndicated financing with institutional investors:
6 year bullet loan (payment of whole principal upon
maturity) amounting to US Dollars 1,600 million.
Margin of 425 bp linked to US Libor and 450 bp linked
to Euribor. BB and Ba3 rating.
|
| |
| |
•
|
Senior revolving credit facility amounting to US Dollars
300 million. BB and Ba3 rating.
|
This debt will be effective once the Talecris purchase
transaction has been completed.
|
|
|
(32)
|
Financial
Instruments
|
Classification
Disclosure of financial instruments by nature and category is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/09
|
|
|
|
|
|
|
|
|
|
|
Financial
|
|
|
|
|
|
|
|
Available-for-
|
|
|
|
|
|
Assets/
|
|
|
|
|
|
|
|
Sale Financial
|
|
|
Loans and
|
|
|
(Liabilities) Held
|
|
|
Debts and
|
|
|
|
|
Assets
|
|
|
Receivables
|
|
|
for Trading
|
|
|
Payables
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Non-current financial assets
|
|
|
501
|
|
|
|
3,230
|
|
|
|
—
|
|
|
|
—
|
|
|
Other current financial assets
|
|
|
—
|
|
|
|
6,547
|
|
|
|
—
|
|
|
|
—
|
|
|
Interest-rate swap
|
|
|
—
|
|
|
|
—
|
|
|
|
(3,333
|
)
|
|
|
—
|
|
|
Unquoted futures
|
|
|
—
|
|
|
|
—
|
|
|
|
1,670
|
|
|
|
—
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
239,204
|
|
|
|
—
|
|
|
|
—
|
|
|
Bank loans
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(382,566
|
)
|
|
Other financial liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(21,449
|
)
|
|
Bonds and other securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(423,675
|
)
|
|
Finance lease liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(10,936
|
)
|
|
Trade and other payables
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(120,909
|
)
|
|
Other current liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,754
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
501
|
|
|
|
248,981
|
|
|
|
(1,663
|
)
|
|
|
(961,289
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-114
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
|
|
|
|
|
|
|
|
Financial
|
|
|
|
|
|
|
|
Available-for-
|
|
|
|
|
|
Assets/
|
|
|
|
|
|
|
|
Sale Financial
|
|
|
Loans and
|
|
|
(Liabilities) Held
|
|
|
Debts and
|
|
|
|
|
Assets
|
|
|
Receivables
|
|
|
for Trading
|
|
|
Payables
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Non-current financial assets
|
|
|
535
|
|
|
|
7,000
|
|
|
|
—
|
|
|
|
—
|
|
|
Other current financial assets
|
|
|
—
|
|
|
|
12,946
|
|
|
|
—
|
|
|
|
—
|
|
|
Interest-rate swap
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,809
|
)
|
|
|
—
|
|
|
Unquoted futures
|
|
|
—
|
|
|
|
—
|
|
|
|
(6,751
|
)
|
|
|
—
|
|
|
Trade and other receivables
|
|
|
—
|
|
|
|
259,497
|
|
|
|
—
|
|
|
|
—
|
|
|
Bank loans
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(392,361
|
)
|
|
Other financial liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(20,150
|
)
|
|
Bonds and other securities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(456,645
|
)
|
|
Finance lease liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(8,014
|
)
|
|
Trade and other payables
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(160,678
|
)
|
|
Payables for Group companies
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,162
|
)
|
|
Other current liabilities
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,629
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
535
|
|
|
|
279,443
|
|
|
|
(8,560
|
)
|
|
|
(1,041,639
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net
losses and gains by financial instrument category
Details are as follows:
Financial
assets
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/09
|
|
|
|
|
|
|
|
|
|
|
Available-
|
|
|
|
|
|
|
|
Assets at Fair
|
|
|
|
|
|
for-Sale
|
|
|
|
|
|
|
|
Value through
|
|
|
Loans And
|
|
|
Financial
|
|
|
|
|
|
|
|
Profit or Loss
|
|
|
Receivables
|
|
|
Assets
|
|
|
Total
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Finance income at amortised cost
|
|
|
—
|
|
|
|
7,067
|
|
|
|
—
|
|
|
|
7,067
|
|
|
Change in fair value
|
|
|
2,015
|
|
|
|
—
|
|
|
|
—
|
|
|
|
2,015
|
|
|
Reclassification of equity to profit or loss
|
|
|
—
|
|
|
|
—
|
|
|
|
(172
|
)
|
|
|
(172
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net gains/(losses) in profit and loss
|
|
|
2,015
|
|
|
|
7,067
|
|
|
|
(172
|
)
|
|
|
8,910
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value
|
|
|
—
|
|
|
|
—
|
|
|
|
14
|
|
|
|
14
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net gains in equity
|
|
|
—
|
|
|
|
—
|
|
|
|
14
|
|
|
|
14
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
2,015
|
|
|
|
7,067
|
|
|
|
(158
|
)
|
|
|
8,924
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-115
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
|
|
|
|
|
|
|
|
Available-
|
|
|
|
|
|
|
|
Assets at Fair
|
|
|
|
|
|
for-Sale
|
|
|
|
|
|
|
|
Value through
|
|
|
Loans and
|
|
|
Financial
|
|
|
|
|
|
|
|
Profit or Loss
|
|
|
Receivables
|
|
|
Assets
|
|
|
Total
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Finance income at amortised cost
|
|
|
—
|
|
|
|
4,526
|
|
|
|
—
|
|
|
|
4,526
|
|
|
Change in fair value
|
|
|
1,601
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,601
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net gains in profit and loss
|
|
|
1,601
|
|
|
|
4,526
|
|
|
|
—
|
|
|
|
6,127
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
1,601
|
|
|
|
4,526
|
|
|
|
—
|
|
|
|
6,127
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Financial
liabilities
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/09
|
|
|
|
|
Liabilities at
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
through Profit
|
|
|
Debts and
|
|
|
Hedging
|
|
|
|
|
|
|
|
or Loss
|
|
|
Payables
|
|
|
Derivatives
|
|
|
Total
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Finance expenses at amortised cost
|
|
|
—
|
|
|
|
(27,087
|
)
|
|
|
—
|
|
|
|
(27,087
|
)
|
|
Change in fair value
|
|
|
(2,602
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,602
|
)
|
|
Reclassification of equity to profit or loss
|
|
|
—
|
|
|
|
—
|
|
|
|
(50
|
)
|
|
|
(50
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net losses in profit and loss
|
|
|
(2,602
|
)
|
|
|
(27,087
|
)
|
|
|
(50
|
)
|
|
|
(29,739
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Change in fair value
|
|
|
—
|
|
|
|
—
|
|
|
|
1,998
|
|
|
|
1,998
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net gains in equity
|
|
|
—
|
|
|
|
—
|
|
|
|
1,998
|
|
|
|
1,998
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(2,602
|
)
|
|
|
(27,087
|
)
|
|
|
1,948
|
|
|
|
(27,741
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
|
|
Liabilities at
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair Value
|
|
|
|
|
|
|
|
|
|
|
|
|
|
through Profit
|
|
|
Debts and
|
|
|
Hedging
|
|
|
|
|
|
|
|
or Loss
|
|
|
Payables
|
|
|
Derivatives
|
|
|
Total
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Finance expenses at amortised cost
|
|
|
—
|
|
|
|
(49,660
|
)
|
|
|
—
|
|
|
|
(49,660
|
)
|
|
Change in fair value
|
|
|
(9,194
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(9,194
|
)
|
|
Reclassification of equity to profit or loss
|
|
|
—
|
|
|
|
—
|
|
|
|
(197
|
)
|
|
|
(197
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net losses in profit and loss
|
|
|
(9,194
|
)
|
|
|
(49,660
|
)
|
|
|
(197
|
)
|
|
|
(59,051
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(9,194
|
)
|
|
|
(49,660
|
)
|
|
|
(197
|
)
|
|
|
(59,051
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Fair
value
The fair value of corporate bonds amounts to Euros
496 million at 31 December 2010. The valuation has
been made based on observable market data. The interest rate
swap, unquoted futures contract and hedging derivative are also
measured at fair value using observable market data. These fair
values are level 2 in fair value hierarchy, using other
than quoted prices (level 1) that are observable for
the assets/liabilities, either directly or indirectly.
The fair value of financial assets and the remaining financial
liabilities does not differ significantly from their carrying
amount.
F-116
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Financial
derivatives
|
|
|
a)
|
Derivative
financial instruments at fair value through profit or
loss
|
Derivative financial instruments that do not meet the hedge
accounting requirements are classified and measured as financial
assets or financial liabilities at fair value through profit and
loss.
The Group recognized the following swaps at 31 December
2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Value at
|
|
|
|
|
|
Derivatives
|
|
Par
|
|
|
31/12/09
|
|
|
Maturity
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
|
|
|
Interest rate swap
|
|
|
50,000
|
|
|
|
(3,333
|
)
|
|
|
26/07/2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 22
|
)
|
|
|
|
|
|
Unquoted future
|
|
|
23,221
|
|
|
|
1,189
|
|
|
|
30/12/2010
|
|
|
Unquoted future
|
|
|
26,370
|
|
|
|
481
|
|
|
|
30/12/2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
49,591
|
|
|
|
1,670
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 14
|
)
|
|
|
|
|
The Group has recognized the following derivatives at
31 December 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Value at
|
|
|
|
|
|
Derivatives
|
|
Par
|
|
|
31/12/10
|
|
|
Maturity
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
|
|
|
Interest rate swap
|
|
|
50,000
|
|
|
|
(1,809
|
)
|
|
|
26/07/2013
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unquoted future
|
|
|
23,221
|
|
|
|
(2,821
|
)
|
|
|
31/03/2011
|
|
|
Unquoted future
|
|
|
26,370
|
|
|
|
(3,930
|
)
|
|
|
31/03/2011
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
49,591
|
|
|
|
(6,751
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
8,560
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 22
|
)
|
|
|
|
|
During 2009 the Company contracted two unquoted futures
contracts, the notional underlying of which consists of the
Company’s shares, with a solvent financial institution. The
two contracts have shares 2 million and 2.2 million
underlying with an exercise price of Euros 11.6107 and Euros
11.9864, respectively. The contracts were to expire on
30 December 2010, although the Company could terminate them
prior to this date. On 30 December 2010 it was agreed to
extend the futures contract to 31 March 2011, through a
novation without liquidation under the same terms and
conditions. The contracts are settled by differences between the
market value of the notional underlying and the exercise price.
|
|
|
b)
|
Bond
issue hedging derivative financial instruments
|
See explanation in note 17 (f).
F-117
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Credit
risk
Exposure
to credit risk
The carrying amount of financial assets represents the maximum
exposure to credit risk. At 31 December 2010 and 2009 the
maximum level of exposure to credit risk is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying Amount
|
|
Note
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Non-current financial assets
|
|
|
11
|
|
|
|
7,535
|
|
|
|
3,731
|
|
|
Other current financial assets
|
|
|
14
|
|
|
|
12,946
|
|
|
|
6,547
|
|
|
Financial derivatives
|
|
|
14
|
|
|
|
—
|
|
|
|
1,670
|
|
|
Trade receivables
|
|
|
13
|
|
|
|
224,355
|
|
|
|
207,840
|
|
|
Other receivables
|
|
|
13
|
|
|
|
35,142
|
|
|
|
31,364
|
|
|
Cash and cash equivalents
|
|
|
16
|
|
|
|
239,649
|
|
|
|
249,372
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
519,627
|
|
|
|
500,524
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The maximum level of exposure to risk associated with
receivables at 31 December 2010 and 2009, by geographical
area, is as follows.
| |
|
|
|
|
|
|
|
|
|
Carrying Amount
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Domestic
|
|
|
70,517
|
|
|
|
70,521
|
|
|
EU countries
|
|
|
46,787
|
|
|
|
47,755
|
|
|
United States of America
|
|
|
43,833
|
|
|
|
29,130
|
|
|
United Kingdom
|
|
|
3,423
|
|
|
|
3,054
|
|
|
Other European countries
|
|
|
3,162
|
|
|
|
5,454
|
|
|
Other regions
|
|
|
56,633
|
|
|
|
51,926
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
224,355
|
|
|
|
207,840
|
|
|
|
|
|
|
|
|
|
|
|
Impairment
losses
Details of the maturity of trade receivables, net of impairment
provisions are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Not due
|
|
|
148,838
|
|
|
|
120,339
|
|
|
Overdue less than 1 month
|
|
|
21,860
|
|
|
|
38,278
|
|
|
Overdue from 1 to 4 months
|
|
|
32,729
|
|
|
|
25,597
|
|
|
Overdue from 4 months to 1 year
|
|
|
14,812
|
|
|
|
17,357
|
|
|
Overdue more than a year
|
|
|
6,116
|
|
|
|
6,269
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
224,355
|
|
|
|
207,840
|
|
|
|
|
|
|
|
|
|
|
|
Unimpaired overdue receivables mainly relate to public entities.
F-118
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Movement in the provision for bad debts was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
31/12/08
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Opening balance
|
|
|
4,038
|
|
|
|
3,172
|
|
|
|
3,285
|
|
|
Net provisions for the year
|
|
|
357
|
|
|
|
712
|
|
|
|
317
|
|
|
Net cancellations for the year
|
|
|
(796
|
)
|
|
|
(42
|
)
|
|
|
(249
|
)
|
|
Translation differences
|
|
|
178
|
|
|
|
196
|
|
|
|
(181
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Closing balance
|
|
|
3,777
|
|
|
|
4,038
|
|
|
|
3,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
An analysis of the concentration of credit risk is provided in
note 5.
Liquidity
risk
Details of the contracted maturity date of financial
liabilities, including borrowing costs and excluding the effects
of offsetting agreements, are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
More
|
|
|
|
|
|
|
|
Amount at
|
|
|
Contractual
|
|
|
6 Months
|
|
|
6-12
|
|
|
1-2
|
|
|
2-5
|
|
|
Than
|
|
|
Carrying Amount
|
|
Note
|
|
|
31/12/09
|
|
|
Flows
|
|
|
or Less
|
|
|
Months
|
|
|
Years
|
|
|
Years
|
|
|
5 Years
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Non-derivative financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank loans
|
|
|
22
|
|
|
|
382,566
|
|
|
|
412,390
|
|
|
|
88,707
|
|
|
|
15,087
|
|
|
|
83,772
|
|
|
|
177,413
|
|
|
|
47,411
|
|
|
Other financial liabilities
|
|
|
22
|
|
|
|
21,449
|
|
|
|
27,420
|
|
|
|
6,927
|
|
|
|
2,582
|
|
|
|
4,417
|
|
|
|
10,076
|
|
|
|
3,418
|
|
|
Bonds and other securities
|
|
|
22
|
|
|
|
423,675
|
|
|
|
687,798
|
|
|
|
27,440
|
|
|
|
14,317
|
|
|
|
28,634
|
|
|
|
85,903
|
|
|
|
531,504
|
|
|
Finance lease liabilities
|
|
|
22
|
|
|
|
10,936
|
|
|
|
11,334
|
|
|
|
230
|
|
|
|
4,751
|
|
|
|
3,294
|
|
|
|
2,586
|
|
|
|
473
|
|
|
Suppliers
|
|
|
23
|
|
|
|
120,909
|
|
|
|
120,909
|
|
|
|
120,550
|
|
|
|
359
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other current liabilities
|
|
|
24
|
|
|
|
1,754
|
|
|
|
1,754
|
|
|
|
1,754
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Derivative financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest rate swap
|
|
|
22
|
|
|
|
3,333
|
|
|
|
3,333
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
3,333
|
|
|
|
—
|
|
|
Unquoted futures
|
|
|
14
|
|
|
|
(1,670
|
)
|
|
|
(1,670
|
)
|
|
|
—
|
|
|
|
(1,670
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
962,952
|
|
|
|
1,263,268
|
|
|
|
245,608
|
|
|
|
35,426
|
|
|
|
120,117
|
|
|
|
279,311
|
|
|
|
582,806
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
More
|
|
|
|
|
|
|
|
Amount at
|
|
|
Contractual
|
|
|
6 Months
|
|
|
6-12
|
|
|
1-2
|
|
|
2-5
|
|
|
Than
|
|
|
Carrying Amount
|
|
Note
|
|
|
31/12/10
|
|
|
Flows
|
|
|
or Less
|
|
|
Months
|
|
|
Years
|
|
|
Years
|
|
|
5 Years
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Non-derivative financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bank loans
|
|
|
22
|
|
|
|
392,361
|
|
|
|
420,168
|
|
|
|
117,256
|
|
|
|
66,428
|
|
|
|
87,986
|
|
|
|
92,561
|
|
|
|
55,937
|
|
|
Other financial liabilities
|
|
|
22
|
|
|
|
20,150
|
|
|
|
22,361
|
|
|
|
8,150
|
|
|
|
2,134
|
|
|
|
3,467
|
|
|
|
6,283
|
|
|
|
2,327
|
|
|
Bonds and other securities
|
|
|
22
|
|
|
|
456,645
|
|
|
|
728,893
|
|
|
|
23,771
|
|
|
|
15,537
|
|
|
|
31,073
|
|
|
|
93,220
|
|
|
|
565,292
|
|
|
Finance lease liabilities
|
|
|
22
|
|
|
|
8,014
|
|
|
|
8,629
|
|
|
|
2,034
|
|
|
|
1,505
|
|
|
|
2,412
|
|
|
|
2,348
|
|
|
|
330
|
|
|
Debts with associates
|
|
|
33
|
|
|
|
1,162
|
|
|
|
1,162
|
|
|
|
1,162
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Suppliers
|
|
|
23
|
|
|
|
160,678
|
|
|
|
160,678
|
|
|
|
160,657
|
|
|
|
21
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other current liabilities
|
|
|
24
|
|
|
|
2,629
|
|
|
|
2,629
|
|
|
|
2,629
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Derivative financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest rate swap
|
|
|
22
|
|
|
|
1,809
|
|
|
|
1,809
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
1,809
|
|
|
|
—
|
|
|
Unquoted futures
|
|
|
22
|
|
|
|
6,751
|
|
|
|
6,751
|
|
|
|
6,751
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
|
|
|
|
1,050,199
|
|
|
|
1,353,080
|
|
|
|
322,410
|
|
|
|
85,625
|
|
|
|
124,938
|
|
|
|
196,221
|
|
|
|
623,886
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-119
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Currency
risk
The Group’s exposure to currency risk is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/09
|
|
|
|
|
EUR (*)
|
|
|
USD (**)
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Trade receivables
|
|
|
1,839
|
|
|
|
7,308
|
|
|
Loans to Group companies
|
|
|
16,854
|
|
|
|
—
|
|
|
Trade payables
|
|
|
(252
|
)
|
|
|
(2,339
|
)
|
|
Payables to Group companies
|
|
|
(10,365
|
)
|
|
|
(17,946
|
)
|
|
Loans with Group companies
|
|
|
—
|
|
|
|
(10,431
|
)
|
|
Non-current bank loans
|
|
|
(6,854
|
)
|
|
|
—
|
|
|
Non-current bonds
|
|
|
(10,000
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance sheet exposure
|
|
|
(8,778
|
)
|
|
|
(23,408
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(*) |
|
balances in Euros in subsidiaries with USD local currency |
| |
|
(**) |
|
Balances in USD in subsidiaries with Euro local currency |
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
|
|
EUR (*)
|
|
|
USD (**)
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Trade receivables
|
|
|
67
|
|
|
|
3,938
|
|
|
Receivables from Group companies
|
|
|
12
|
|
|
|
—
|
|
|
Loans to Group companies
|
|
|
16,852
|
|
|
|
—
|
|
|
Cash
|
|
|
415
|
|
|
|
45
|
|
|
Trade payables
|
|
|
(533
|
)
|
|
|
(6,200
|
)
|
|
Payables for Group companies
|
|
|
(6,828
|
)
|
|
|
(21,455
|
)
|
|
Current bank loans
|
|
|
(5,875
|
)
|
|
|
—
|
|
|
Non-current bank loans
|
|
|
(979
|
)
|
|
|
(262
|
)
|
|
Non-current bonds
|
|
|
(9,860
|
)
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance sheet exposure
|
|
|
(6,729
|
)
|
|
|
(23,934
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(*) |
|
balances in Euros in subsidiaries with USD local currency |
| |
|
(**) |
|
Balances in USD in subsidiaries with Euro local currency |
The most significant exchange rates applied during the years
ended 31 December 2010 and 2009 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average Exchange Rate
|
|
Closing Exchange Rate
|
|
Euro
|
|
2010
|
|
2009
|
|
31/12/10
|
|
31/12/09
|
|
|
|
USD
|
|
|
1.34
|
|
|
|
1.38
|
|
|
|
1.34
|
|
|
|
1.44
|
|
A sensitivity analysis for foreign exchange fluctuations is as
follows:
Had the US Dollar strengthened by 10% against the Euro at
31 December 2010, equity would have increased by Euros
34,973 thousand (Euros 35,795 thousand at
31 December 2009) and profit would have decreased by
Euros 3,066 thousand (at 31 December 2009 it would have
decreased by Euros 1,626 thousand).
F-120
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
This analysis assumes that all other variables are held
constant, especially that interest rates remain constant. This
analysis has been performed using the same criteria as in 2009.
A 10% weakening of the US Dollar against the Euro at
31 December 2010 and 2009 would have had the opposite
effect for the amounts shown above, all other variables being
held constant.
Interest-rate
risk
Interest-rate
profile
To date, the profile of interest on interest-bearing financial
instruments is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Fixed-interest financial instruments
|
|
|
|
|
|
|
|
|
|
Financial assets
|
|
|
19,220
|
|
|
|
9,674
|
|
|
Financial liabilities
|
|
|
(457,521
|
)
|
|
|
(423,675
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(438,301
|
)
|
|
|
(414,001
|
)
|
|
Variable-interest financial instruments Financial liabilities
|
|
|
(399,499
|
)
|
|
|
(393,502
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(399,499
|
)
|
|
|
(393,502
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(837,800
|
)
|
|
|
(807,503
|
)
|
|
|
|
|
|
|
|
|
|
|
Sensitivity
analysis
A 100 basis point variation in interest rates at the
presentation date of 31 December 2010 would have
increased/decreased
equity and consolidated profit after income tax by Euros
3,794 thousand. This analysis assumes that all other
variables are held constant, especially that exchange rates
remain constant.
A 100 basis point variation in interest rates at the
presentation date of 31 December 2009 would have
increased/decreased equity and consolidated profit after income
tax by Euros 4,732 thousand.
|
|
|
(33)
|
Balances
and Transactions with Related Parties
|
Details of balances with related parties are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
31/12/10
|
|
|
31/12/09
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Receivables from associates
|
|
|
5
|
|
|
|
812
|
|
|
Debts with associates
|
|
|
(1,162
|
)
|
|
|
—
|
|
|
Payables to associates
|
|
|
—
|
|
|
|
(22
|
)
|
|
Payables to members of the board of directors
|
|
|
(62
|
)
|
|
|
(121
|
)
|
|
Payables to other related parties
|
|
|
(4,641
|
)
|
|
|
(3,322
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(5,860
|
)
|
|
|
(2,653
|
)
|
|
|
|
|
|
|
|
|
|
|
Payables are included in suppliers and trade payables (see
note 23).
Transactions
with related parties
Transactions with related parties have been performed as part of
the group’s ordinary trade and have been performed at
arm’s length.
F-121
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Group transactions with related parties during 2008 were as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key
|
|
|
|
|
|
Board of
|
|
|
|
|
|
|
|
Management
|
|
|
Other Related
|
|
|
Directors of
|
|
|
|
|
Associates
|
|
|
Personnel
|
|
|
Parties
|
|
|
the Company
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Net purchases
|
|
|
125
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other service expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
4,981
|
|
|
|
180
|
|
|
Personnel expenses
|
|
|
—
|
|
|
|
4.253
|
|
|
|
—
|
|
|
|
1,995
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
125
|
|
|
|
4,253
|
|
|
|
4,981
|
|
|
|
2,175
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends received by the Board of directors of the Company
amounted to Euros 2,600 thousand in 2008.
Group transactions with related parties during 2009 were as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key
|
|
|
Other
|
|
|
Board of
|
|
|
|
|
|
|
|
Management
|
|
|
Related
|
|
|
Directors of
|
|
|
|
|
Associates
|
|
|
Personnel
|
|
|
Parties
|
|
|
the Company
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Net purchases
|
|
|
86
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net sales
|
|
|
(700
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other service expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
7,257
|
|
|
|
240
|
|
|
Personnel expenses
|
|
|
—
|
|
|
|
5,849
|
|
|
|
—
|
|
|
|
2,148
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(614
|
)
|
|
|
5,849
|
|
|
|
7,257
|
|
|
|
2,388
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends received by the Board of directors of the Company
amounted to Euros 6,152 thousand in 2009.
Group transactions with related parties during 2010 are as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Key
|
|
|
Other
|
|
|
Board of
|
|
|
|
|
|
|
|
Management
|
|
|
Related
|
|
|
Directors of
|
|
|
|
|
Associates
|
|
|
Personnel
|
|
|
Parties
|
|
|
the Company
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
Net purchases
|
|
|
505
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Net sales
|
|
|
(14
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Other service expenses
|
|
|
—
|
|
|
|
—
|
|
|
|
12,506
|
|
|
|
180
|
|
|
Personnel expenses
|
|
|
—
|
|
|
|
5,839
|
|
|
|
—
|
|
|
|
2,066
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
491
|
|
|
|
5,839
|
|
|
|
12,506
|
|
|
|
2,246
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dividends received by the Board of directors of the Company
amounted to Euros 2,062 thousand in 2010.
“Other service expenses” include costs for
professional services with related companies amounting to Euros
7,590 thousand. These costs correspond to those incurred related
to the share capital increase and the issuance of debt which is
expected to be carried out relating to the acquisition of
Talecris (see note 15).
Non-employee board member representing shareholders interests
have received no remuneration during 2010, 2009 or 2008.
The Group has not extended any advances or loans to the members
of the board of directors or key management personnel nor has it
assumed any guarantee commitments on their behalf. It has also
not assumed any pension or life insurance obligations on behalf
of former or current members of the board of directors or key
management personnel.
F-122
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
On 13 January 2011, the Group closed its scheduled issue of
High Yield Senior Unsecured Notes for an amount of
US Dollars 1,100 million, with a 7 year maturity
period and an annual coupon of 8.25%. This issue, together with
the already completed syndicated loan for an amount of
US Dollars 3,400 million, enabled the Company to
obtain US Dollars 4,500 million, the estimated maximum
financing requirement for the acquisition of Talecris.
At the extraordinary general shareholders’ meeting held on
25 January 2011, the Company agreed to increase share
capital through the issue of 87 million new non-voting
shares, which it will use in its acquisition of Talecris. These
shares are scheduled to be listed on the NASDAQ Global Market
(United States) and the Automated Quotation System
(“mercado continuo”) (Spain).
The Group has therefore completed all the tranches of the
proposed financing structure to conclude the transaction, which
is still pending approval by the U.S. Federal Trade
Commission (FTC).
On 5 March 2011 Grifols has extended the deadline for
closing the acquisition of Talecris Biotherapeutics Holdings
Corp. and the financing and commitments with those extending the
financing to 30 June 2011.
|
|
|
(35)
|
Subsequent
Events (2)
|
Acquisition
of Talecris Biotherapeutics Holdings Corp. and
subsidiaries
On 2 June 2011 the Group finalized the acquisition of 100%
of the share capital of Talecris, which also specialises in the
production of plasma-derived biological medication, for a total
consideration of Euros 2,593 million (US Dollars
3,736 million).
As described in note 31 e) the operation was performed
through a combined offer of cash, and a new issue of Grifols
non-voting shares (hereinafter Class B shares).
On 2 May 2011, the Group signed a “Consent
Agreement” with the Staff of the Bureau of Competition of
the US Federal Trade Commission (FTC) by means of which the
conditions for the merger transaction between both companies
were agreed.
To satisfy the Consent Agreement conditions, the Group has
signed agreements for the sale of assets and entered into
certain commercial, lease and manufacturing agreements with the
Italian company Kedrion, for up to seven years.
These agreements refer to the following areas:
|
|
|
| |
•
|
Kedrion and Grifols entered into a contract manufacturing
agreement to fractionate and purify Kedrion’s plasma to
deliver IVIG and Albumin under Kedrion’s private label, and
Factor VIII under the trade name Koate, all of them for sale
only in the United States.
|
| |
| |
•
|
Grifols is committed to sell to Kedrion the Melville
fractionation facility. Grifols lease from Kedrion the Melville
fractionation facility being the lease term 3 years with an
optional extension of up to 1 year at Grifols request.
|
| |
| |
•
|
Grifols transfer to Kedrion all Koate (factor
VIII) technology and commercial agreements for the US
market. Grifols will produce Koate for Kedrion up to a period of
7 years.
|
| |
| |
•
|
Grifols is committed to sell to Kedrion two plasma collection
centers. In addition Grifols committed to sell 200.000 liters of
source plasma to Kedrion at a fixed price.
|
| |
| |
•
|
Grifols authorizes Kedrion to market and sell in the US, IVIG
and albumin manufactured by Grifols for Kedrion.
|
F-123
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
After approval of the acquisition by the FTC, these conditions
established in the Consent Agreement have been executed on
3 June 2011.
At the date of publication of these Consolidated Financial
Statements, taking into account that the transaction is recent
and not all the information necessary to adequately determine
the fair value of the assets, liabilities and contingent
liabilities, the Group has not made any fair value adjustments
to book values of Talecris at acquisitions date, prepared under
IFRS. The areas under analysis are mainly tangible and
intangible assets, acquired in-process research and development,
customer relationships, developed and core technology,
intellectual property, patents and trade names and contingent
liabilities.
Details of the aggregate business combination cost and
provisional fair value of the net assets acquired and
provisional goodwill at the acquisition date (or excess of the
cost of the business combination over the fair value of
identifiable net assets acquired) follows. The values shown in
the below table should therefore be considered as provisional
amounts.
| |
|
|
|
|
|
|
|
|
|
|
|
Thousands of
|
|
|
Thousands
|
|
|
|
|
Euros
|
|
|
of USD
|
|
|
|
|
Cost of business combination (valuation of Class B Shares)
|
|
|
829,799
|
|
|
|
1,195,574
|
|
|
Cash paid (19 USD per share)
|
|
|
1,763,601
|
|
|
|
2,540,997
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of business combination
|
|
|
2,593,400
|
|
|
|
3,736,571
|
|
|
Book value of net assets acquired (provisional)
|
|
|
469,318
|
|
|
|
676,193
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill (excess of the cost of the business combination over
the fair value of identifiable net assets acquired)
|
|
|
2,124,082
|
|
|
|
3,060,378
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(see note 6
|
)
|
|
|
|
|
|
Cash paid
|
|
|
1,763,601
|
|
|
|
2,540,996
|
|
|
Cash and cash equivalents of the acquired company
|
|
|
(149,693
|
)
|
|
|
(215,678
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Cash outflow for the acquisition
|
|
|
1,613,908
|
|
|
|
2,325,319
|
|
|
|
|
|
|
|
|
|
|
|
The fair value of Class B shares has been determined at the
average price of the first weeks of quotation price on the stock
exchange, being considered as a representative period for
determining the fair value as they started quotation on
2 June 2011.
Costs incurred in the acquisition amounting to Euros
55 million have been expensed as incurred (thereof Euros
17 million already incurred in the year 2010).
Goodwill generated in the acquisition is attributed to the
workforce, synergies and other expected benefits from the
business combination of the assets and activities of the Group.
The acquisition of Talecris will consolidate the Group as the
world’s third largest producer of plasma products,
significantly expanding its presence in the United States. Among
other aspects, it will increase product availability in the
market to the benefit of patients, through higher collection
capacity and plasma fractionation, as well as with complementary
R&D projects.
Sale
of Spanish properties and lease back
On 10 May, 2011 the Group sold five properties based in
Spain mainly related to non-core assets such as offices and
warehouses and a factory premise, by an aggregated amount of
Euros 80.4 million to Gridpan Invest, S.L., a company fully
owned by Scranton Enterprises, B.V., a related party of Grifols,
S.A. Two of the premises were sold together with its related
mortgage loans amounting in total to Euros 53.5 million. As
a result of the transactions the Group has recognized a net loss
of Euros 7.4 million. The prices paid for the properties
were established based on the appraisals made by independent
appraisers.
F-124
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
At the same time, operating lease agreements for the
aforementioned properties were entered into with Gridpan Invest,
S.L., the main terms of the agreements being as follows:
|
|
|
| |
•
|
Compulsory initial term of five years,
|
| |
| |
•
|
Initial rent established at market prices and will be reviewed
annually, based on the percentage variation in the Spanish
Consumer Price Index (CPI),
|
| |
| |
•
|
Automatic extensions of five-year periods that can be avoided by
both parties by a six month anticipated notice.
|
| |
| |
•
|
Upon vacating the premises, the lessor will reimburse Grifols
for the remaining value of leasehold improvements Grifols made.
|
In addition, the Group entered into a free of charge purchase
option over the shares of Gridpan Invest, S.L. exercisable
between 10 May 2016 and 10 May 2017. The strike price
will be at market value at the date of exercise, based on
independent appraisers.
The rental expense recognized by the Group for the six months
period ended 30 June 2011 in connection with these
agreements amounted to Euros 1,084 thousand, which related in
full to the minimum contractual payments.
Sale
of properties and equipment in the USA and lease
back
On 9 June 2011 the Group entered into several agreements
for the sale and lease back of a manufacturing building and
related equipment to third party companies California Biogrif
330, LP and LA 300 Biologicals Financing, LP respectively. In
addition, a lease was entered into for the piece of land on
which the building sold is constructed, for a term of
99 years, to the same party. The sales price received for
the building amounted to US Dollars 35.4 million
(Euros 24.6 million) and the sales price for the equipment
US Dollars 23.8 million (Euros 16.5 million).
The lease of the building has been designed as operating, while
the lease of the equipment is considered as finance considering
the terms of the purchase option. As a result of the sale of the
building, the Group has recognized a net loss of US Dollars
2.4 million (Euros 1.3 million) mainly due to the
expenses incurred on the transaction.
The main terms of the operating lease agreement over the
building are as follows:
|
|
|
| |
•
|
Compulsory initial term of 20 years.
|
| |
| |
•
|
Initial rent has been established at market prices and will be
reviewed annually with a 3% increase. On the first day of the
sixth year, the remaining rents until year twenty will be paid
in advance in a lump sum.
|
| |
| |
•
|
Renewal option to extend for a ten-year period at Grifols Group
election.
|
| |
| |
•
|
Purchase options granted during the sixth year and in year
twenty (20) at market value, to be estimated by independent
appraisers.
|
The main terms of the finance lease agreement over the equipment
are a compulsory term of five years, and sixty (60) monthly
rent instalments of Dollars 529 thousand (Euros 369 thousand).
The lease agreement is not renewable and provides for the
repurchase of the equipment at the end of the term for $1.
The rental expense recognized by the Group for the six month
period ended 30 June 2011 in connection with the operating
lease agreement amounted to US Dollars 148 thousand (Euros
103 thousand) , which related in full to the minimum contractual
payments.
F-125
GRIFOLS,
S.A. AND SUBSIDIARIES
Notes to
the Consolidated Financial
Statements — (Continued)
Woolloomooloo
Holdings Pty Ltd
In August 2011 Grifols acquired the remaining
51% outstanding capital stock of Woolloomooloo Holdings Pty
Ltd. the holding company of the Australian-Swiss group,
Lateral-Medion, of which the Company had acquired 49% of the
capital stock and 100% of the voting rights on March 2009. The
total sum paid for the acquisition of the remaining 51% of the
capital stock amounts to AUD 12.5 million (Euros
9.5 million).
|
|
|
(36)
|
Condensed
Consolidated Financial Information
|
The High Yield Senior Unsecured Notes (note 34) were
issued by Grifols Inc., which is a wholly-owned subsidiary of
Grifols, S.A., and are jointly and severally, irrevocably and
fully and unconditionally guaranteed by Grifols, S.A. and
certain other of its wholly-owned subsidiaries (“the
Guarantors”). Supplemental condensed consolidating
financial information is presented in Appendix V comprising
the Group’s income statements and cash flow statements,
both consolidated, for Fiscal 2010, Fiscal 2009 and Fiscal 2008
and its consolidated balance sheets as at December 31, 2010
and December 31, 2009, showing the amounts attributable to
Grifols, S.A., Grifols Inc. and those of its other subsidiaries
that were Guarantors as at December 31, 2010 separately
from the amounts attributable to those of its subsidiaries that
were not Guarantors. The condensed consolidated financial
information has been prepared and presented pursuant to SEC
Regulation S-X,
Rule 3-10,
“Financial Statements of Guarantors and Issuers of
Guaranteed Securities Registered or Being Registered”,
which is included in Appendix V.
The Board of Directors of Grifols, S.A. approved these
Consolidated Financial Statements on 4 October 2011.
F-126
APPENDIX
I
GRIFOLS,
S.A. AND SUBSIDIARIES
OPERATING
SEGMENTS
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Bioscience
|
|
|
Hospital
|
|
|
Diagnostics
|
|
|
Raw Materials
|
|
|
Others/Unallocated
|
|
|
Consolidated
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
773,372
|
|
|
|
694,969
|
|
|
|
617,918
|
|
|
|
89,552
|
|
|
|
86,328
|
|
|
|
82,566
|
|
|
|
109,088
|
|
|
|
103,091
|
|
|
|
85,705
|
|
|
|
4,815
|
|
|
|
22,665
|
|
|
|
22,794
|
|
|
|
13,903
|
|
|
|
6,133
|
|
|
|
5,328
|
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
814,311
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total revenues
|
|
|
773,372
|
|
|
|
694,969
|
|
|
|
617,918
|
|
|
|
89,552
|
|
|
|
86,328
|
|
|
|
82,566
|
|
|
|
109,088
|
|
|
|
103,091
|
|
|
|
85,705
|
|
|
|
4,815
|
|
|
|
22,665
|
|
|
|
22,794
|
|
|
|
13,903
|
|
|
|
6,133
|
|
|
|
5,328
|
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
814,311
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit/(Loss) for the segment
|
|
|
306,091
|
|
|
|
297,584
|
|
|
|
262,229
|
|
|
|
7,401
|
|
|
|
8,374
|
|
|
|
8,534
|
|
|
|
6,793
|
|
|
|
12,136
|
|
|
|
13,603
|
|
|
|
2,110
|
|
|
|
3,850
|
|
|
|
7,369
|
|
|
|
7,785
|
|
|
|
6,133
|
|
|
|
5,328
|
|
|
|
330,180
|
|
|
|
328,077
|
|
|
|
297,063
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Unallocated expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(120,497
|
)
|
|
|
(101,549
|
)
|
|
|
(94,102
|
)
|
|
|
(120,497
|
)
|
|
|
(101,549
|
)
|
|
|
(94,102
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating profit
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
209,683
|
|
|
|
226,528
|
|
|
|
202,961
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income/expenses
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(51,020
|
)
|
|
|
(22,585
|
)
|
|
|
(30,716
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit/(loss) of equity accounted investees
|
|
|
(879
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
51
|
|
|
|
24
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(879
|
)
|
|
|
51
|
|
|
|
24
|
|
|
Income tax expense
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(42,517
|
)
|
|
|
(56,424
|
)
|
|
|
(50,153
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit for the year after tax
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
115,267
|
|
|
|
147,570
|
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment assets
|
|
|
1,062,464
|
|
|
|
994,245
|
|
|
|
798,843
|
|
|
|
85,992
|
|
|
|
68,214
|
|
|
|
63,660
|
|
|
|
129,824
|
|
|
|
82,202
|
|
|
|
67,087
|
|
|
|
954
|
|
|
|
1,312
|
|
|
|
4,379
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
1,279,234
|
|
|
|
1,145,973
|
|
|
|
933,969
|
|
|
Equity accounted investments
|
|
|
516
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
383
|
|
|
|
374
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
516
|
|
|
|
383
|
|
|
|
374
|
|
|
Unallocated assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
609,232
|
|
|
|
510,821
|
|
|
|
245,896
|
|
|
|
609,232
|
|
|
|
510,821
|
|
|
|
245,896
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,888,982
|
|
|
|
1,657,177
|
|
|
|
1,180,239
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Segment liabilities
|
|
|
74,489
|
|
|
|
79,988
|
|
|
|
75,120
|
|
|
|
14,486
|
|
|
|
12,579
|
|
|
|
11,909
|
|
|
|
12,573
|
|
|
|
10,763
|
|
|
|
9,066
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
101,548
|
|
|
|
103,330
|
|
|
|
96,095
|
|
|
Unallocated liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,080,044
|
|
|
|
975,319
|
|
|
|
602,865
|
|
|
|
1,080,044
|
|
|
|
975,319
|
|
|
|
602,865
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
1,181,592
|
|
|
|
1,078,649
|
|
|
|
698,960
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Amortisation and depreciation
|
|
|
21,630
|
|
|
|
21,893
|
|
|
|
21,644
|
|
|
|
4,719
|
|
|
|
3,808
|
|
|
|
3,725
|
|
|
|
8,265
|
|
|
|
5,261
|
|
|
|
5,000
|
|
|
|
0
|
|
|
|
0
|
|
|
|
67
|
|
|
|
11,162
|
|
|
|
8,592
|
|
|
|
2,820
|
|
|
|
45,776
|
|
|
|
39,554
|
|
|
|
33,256
|
|
|
Expenses that do not require cash payments
|
|
|
(526
|
)
|
|
|
(2,059
|
)
|
|
|
(1,744
|
)
|
|
|
(12
|
)
|
|
|
(70
|
)
|
|
|
32
|
|
|
|
0
|
|
|
|
(1
|
)
|
|
|
15
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(7
|
)
|
|
|
0
|
|
|
|
(26
|
)
|
|
|
(275
|
)
|
|
|
(538
|
)
|
|
|
(2,156
|
)
|
|
|
(1,979
|
)
|
|
Additions for the year of property, plant & equipment
and intangible assets
|
|
|
65,344
|
|
|
|
70,702
|
|
|
|
65,954
|
|
|
|
13,132
|
|
|
|
7,524
|
|
|
|
9,266
|
|
|
|
15,897
|
|
|
|
14,067
|
|
|
|
14,078
|
|
|
|
0
|
|
|
|
0
|
|
|
|
516
|
|
|
|
11,424
|
|
|
|
26,477
|
|
|
|
39,879
|
|
|
|
105,797
|
|
|
|
118,770
|
|
|
|
129,693
|
|
This Appendix forms an integral part of note 6 to the
consolidated financial statements
F-127
APPENDIX I
GRIFOLS,
S.A. AND SUBSIDIARIES
GEOGRAPHICAL
INFORMATION
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Spain
|
|
|
European Union
|
|
|
United States
|
|
|
Rest of the World
|
|
|
Consolidated
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
227,947
|
|
|
|
225,759
|
|
|
|
190,809
|
|
|
|
204,244
|
|
|
|
198,832
|
|
|
|
213,290
|
|
|
|
339,018
|
|
|
|
296,659
|
|
|
|
290,666
|
|
|
|
219,521
|
|
|
|
191,936
|
|
|
|
119,546
|
|
|
|
990,730
|
|
|
|
913,186
|
|
|
|
814,311
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Assets by geographic areas
|
|
|
682,473
|
|
|
|
632,537
|
|
|
|
532,392
|
|
|
|
117,706
|
|
|
|
82,245
|
|
|
|
96,845
|
|
|
|
936,030
|
|
|
|
821,641
|
|
|
|
502,797
|
|
|
|
152,773
|
|
|
|
120,754
|
|
|
|
48,205
|
|
|
|
1,888,982
|
|
|
|
1,657,177
|
|
|
|
1,180,239
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other information:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additions for the year of property, plant & equipment
and intangible assets
|
|
|
50,319
|
|
|
|
65,046
|
|
|
|
93,005
|
|
|
|
3,972
|
|
|
|
2,341
|
|
|
|
999
|
|
|
|
43,847
|
|
|
|
43,726
|
|
|
|
33,475
|
|
|
|
7,659
|
|
|
|
7,657
|
|
|
|
2,214
|
|
|
|
105,797
|
|
|
|
118,770
|
|
|
|
129,693
|
|
This
Appendix forms an integral part of note 6 to the
consolidated financial statements
F-128
APPENDIX II
GRIFOLS,
S.A. AND SUBSIDIARIES
Changes
in Other Intangible Assets
For the
Year Ended 31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/2009
|
|
|
Additions
|
|
|
Transfers
|
|
|
Disposals
|
|
|
Differences
|
|
|
31/12/2010
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Development costs
|
|
|
55,414
|
|
|
|
6,614
|
|
|
|
0
|
|
|
|
(26
|
)
|
|
|
69
|
|
|
|
62,071
|
|
|
Concessions, patents, licenses brands & similar
|
|
|
46,259
|
|
|
|
2,410
|
|
|
|
847
|
|
|
|
0
|
|
|
|
3,227
|
|
|
|
52,743
|
|
|
Software
|
|
|
28,597
|
|
|
|
5,455
|
|
|
|
318
|
|
|
|
(20
|
)
|
|
|
352
|
|
|
|
34,702
|
|
|
Other intangible assets
|
|
|
513
|
|
|
|
2,121
|
|
|
|
0
|
|
|
|
(299
|
)
|
|
|
10
|
|
|
|
2,345
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of intangible assets
|
|
|
130,783
|
|
|
|
16,600
|
|
|
|
1,165
|
|
|
|
(345
|
)
|
|
|
3,658
|
|
|
|
151,861
|
|
|
Accum. amort. of development costs
|
|
|
(29,427
|
)
|
|
|
(3,699
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(69
|
)
|
|
|
(33,195
|
)
|
|
Accum. amort of concessions, patents, licenses,
brands & similar
|
|
|
(15,526
|
)
|
|
|
(1,603
|
)
|
|
|
(845
|
)
|
|
|
0
|
|
|
|
(654
|
)
|
|
|
(18,628
|
)
|
|
Accum. amort. of software
|
|
|
(16,430
|
)
|
|
|
(4,965
|
)
|
|
|
1
|
|
|
|
20
|
|
|
|
(172
|
)
|
|
|
(21,546
|
)
|
|
Accum. amort. of other intangible assets
|
|
|
0
|
|
|
|
(189
|
)
|
|
|
(4
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(193
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accum. amort intangible assets
|
|
|
(61,383
|
)
|
|
|
(10,456
|
)
|
|
|
(848
|
)
|
|
|
20
|
|
|
|
(895
|
)
|
|
|
(73,562
|
)
|
|
Impairment of other intangible assets
|
|
|
(15
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
15
|
|
|
|
0
|
|
|
|
0
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount of intangible assets
|
|
|
69,385
|
|
|
|
6,144
|
|
|
|
317
|
|
|
|
(310
|
)
|
|
|
2,763
|
|
|
|
78,299
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 8 to the
consolidated financial statements
F-129
APPENDIX II
GRIFOLS,
S.A. AND SUBSIDIARIES
Changes
in Other Intangible Assets
For the
Year Ended 31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Business
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/2008
|
|
|
Additions
|
|
|
Combinations
|
|
|
Transfers
|
|
|
Disposals
|
|
|
Differences
|
|
|
31/12/2009
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Development costs
|
|
|
47,299
|
|
|
|
8,146
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(31
|
)
|
|
|
55,414
|
|
|
Concessions, patents, licenses brands & similar
|
|
|
40,461
|
|
|
|
1
|
|
|
|
6,525
|
|
|
|
(5
|
)
|
|
|
0
|
|
|
|
(723
|
)
|
|
|
46,259
|
|
|
Software
|
|
|
22,272
|
|
|
|
6,700
|
|
|
|
0
|
|
|
|
1
|
|
|
|
(240
|
)
|
|
|
(136
|
)
|
|
|
28,597
|
|
|
Other intangible assets
|
|
|
0
|
|
|
|
508
|
|
|
|
0
|
|
|
|
5
|
|
|
|
0
|
|
|
|
0
|
|
|
|
513
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of intangible assets
|
|
|
110,032
|
|
|
|
15,355
|
|
|
|
6,525
|
|
|
|
1
|
|
|
|
(240
|
)
|
|
|
(890
|
)
|
|
|
130,783
|
|
|
Accum. amort. of development costs
|
|
|
(23,878
|
)
|
|
|
(5,580
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
31
|
|
|
|
(29,427
|
)
|
|
Accum. amort of concessions, patents, licenses,
brands & similar
|
|
|
(14,881
|
)
|
|
|
(806
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
161
|
|
|
|
(15,526
|
)
|
|
Accum. amort. of software
|
|
|
(13,517
|
)
|
|
|
(3,097
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
132
|
|
|
|
52
|
|
|
|
(16,430
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accum. amort intangible assets
|
|
|
(52,276
|
)
|
|
|
(9,483
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
132
|
|
|
|
244
|
|
|
|
(61,383
|
)
|
|
Impairment of other intangible assets
|
|
|
0
|
|
|
|
(15
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(15
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount of intangible assets
|
|
|
57,756
|
|
|
|
5,857
|
|
|
|
6,525
|
|
|
|
1
|
|
|
|
(108
|
)
|
|
|
(646
|
)
|
|
|
69,385
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 8 to the
consolidated financial statements
F-130
APPENDIX II
GRIFOLS,
S.A. AND SUBSIDIARIES
Changes
in Other Intangible Assets
For the
Year Ended 31 December 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/2007
|
|
|
Additions
|
|
|
Transfers
|
|
|
Disposals
|
|
|
Differences
|
|
|
31/12/2008
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Development costs
|
|
|
43,141
|
|
|
|
5,255
|
|
|
|
0
|
|
|
|
(1,146
|
)
|
|
|
49
|
|
|
|
47,299
|
|
|
Concessions, patents, licenses brands and similar
|
|
|
40,790
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,618
|
)
|
|
|
1,289
|
|
|
|
40,461
|
|
|
Software
|
|
|
17,704
|
|
|
|
4,489
|
|
|
|
(59
|
)
|
|
|
(8
|
)
|
|
|
146
|
|
|
|
22,272
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total cost of intangible assets
|
|
|
101,635
|
|
|
|
9,744
|
|
|
|
(59
|
)
|
|
|
(2,772
|
)
|
|
|
1,484
|
|
|
|
110,032
|
|
|
Accum. amort. of development costs
|
|
|
(18,916
|
)
|
|
|
(4,634
|
)
|
|
|
(287
|
)
|
|
|
0
|
|
|
|
(41
|
)
|
|
|
(23,878
|
)
|
|
Accum. amort of concessions, patents, licenses,
brands & similar
|
|
|
(14,110
|
)
|
|
|
(2,322
|
)
|
|
|
287
|
|
|
|
1,616
|
|
|
|
(352
|
)
|
|
|
(14,881
|
)
|
|
Accum. amort. of software
|
|
|
(11,386
|
)
|
|
|
(2,124
|
)
|
|
|
59
|
|
|
|
10
|
|
|
|
(76
|
)
|
|
|
(13,517
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Accum. amort intangible assets
|
|
|
(44,412
|
)
|
|
|
(9,080
|
)
|
|
|
59
|
|
|
|
1,626
|
|
|
|
(469
|
)
|
|
|
(52,276
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount of intangible assets
|
|
|
57,223
|
|
|
|
664
|
|
|
|
0
|
|
|
|
(1,146
|
)
|
|
|
1,015
|
|
|
|
57,756
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 8 to the
consolidated financial statements
F-131
APPENDIX III
GRIFOLS,
S.A. AND SUBSIDIARIES
Changes
in Property, Plant and Equipment
For the
Year Ended 31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/09
|
|
|
Additions
|
|
|
Transfers
|
|
|
Disposals
|
|
|
Differences
|
|
|
31/12/10
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land and buildings
|
|
|
142,600
|
|
|
|
10,594
|
|
|
|
28,930
|
|
|
|
(1,085
|
)
|
|
|
3,703
|
|
|
|
184,742
|
|
|
Plant and machinery
|
|
|
344,030
|
|
|
|
35,356
|
|
|
|
21,857
|
|
|
|
(8,242
|
)
|
|
|
12,268
|
|
|
|
405,269
|
|
|
Under construction
|
|
|
70,781
|
|
|
|
43,247
|
|
|
|
(49,694
|
)
|
|
|
0
|
|
|
|
1,950
|
|
|
|
66,284
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
557,411
|
|
|
|
89,197
|
|
|
|
1,093
|
|
|
|
(9,327
|
)
|
|
|
17,921
|
|
|
|
656,295
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Buildings
|
|
|
(9,502
|
)
|
|
|
(1,890
|
)
|
|
|
(16
|
)
|
|
|
0
|
|
|
|
(139
|
)
|
|
|
(11,547
|
)
|
|
Plant and machinery
|
|
|
(176,204
|
)
|
|
|
(33,430
|
)
|
|
|
(1,394
|
)
|
|
|
6,016
|
|
|
|
(4,956
|
)
|
|
|
(209,968
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(185,706
|
)
|
|
|
(35,320
|
)
|
|
|
(1,410
|
)
|
|
|
6,016
|
|
|
|
(5,095
|
)
|
|
|
(221,515
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Impairment of other property, plant and equipment
|
|
|
0
|
|
|
|
(649
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(649
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount
|
|
|
371,705
|
|
|
|
53,228
|
|
|
|
(317
|
)
|
|
|
(3,311
|
)
|
|
|
12,826
|
|
|
|
434,131
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 9 to the
consolidated financial statements
F-132
APPENDIX III
GRIFOLS,
S.A. AND SUBSIDIARIES
Changes
in Property, Plant and Equipment
For the
Year Ended 31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Business
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/08
|
|
|
Additions
|
|
|
Combinations
|
|
|
Transfers
|
|
|
Disposals
|
|
|
Differences
|
|
|
31/12/09
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land and buildings
|
|
|
111,067
|
|
|
|
9,729
|
|
|
|
0
|
|
|
|
22,905
|
|
|
|
0
|
|
|
|
(1,101
|
)
|
|
|
142,600
|
|
|
Plant and machinery
|
|
|
287,761
|
|
|
|
33,994
|
|
|
|
2,307
|
|
|
|
27,784
|
|
|
|
(5,881
|
)
|
|
|
(1,935
|
)
|
|
|
344,030
|
|
|
Under construction
|
|
|
63,620
|
|
|
|
59,692
|
|
|
|
0
|
|
|
|
(50,882
|
)
|
|
|
(757
|
)
|
|
|
(892
|
)
|
|
|
70,781
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
462,448
|
|
|
|
103,415
|
|
|
|
2,307
|
|
|
|
(193
|
)
|
|
|
(6,638
|
)
|
|
|
(3,928
|
)
|
|
|
557,411
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Buildings
|
|
|
(8,049
|
)
|
|
|
(1,514
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
61
|
|
|
|
(9,502
|
)
|
|
Plant and machinery
|
|
|
(153,390
|
)
|
|
|
(28,557
|
)
|
|
|
0
|
|
|
|
192
|
|
|
|
4,942
|
|
|
|
609
|
|
|
|
(176,204
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(161,439
|
)
|
|
|
(30,071
|
)
|
|
|
0
|
|
|
|
192
|
|
|
|
4,942
|
|
|
|
670
|
|
|
|
(185,706
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount
|
|
|
301,009
|
|
|
|
73,344
|
|
|
|
2,307
|
|
|
|
(1
|
)
|
|
|
(1,696
|
)
|
|
|
(3,258
|
)
|
|
|
371,705
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 9 to the
consolidated financial statements
F-133
APPENDIX III
GRIFOLS,
S.A. AND SUBSIDIARIES
Changes
in Property, Plant and Equipment
For the
Year Ended 31 December 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balances at
|
|
|
|
|
|
Business
|
|
|
|
|
|
|
|
|
Translation
|
|
|
Balances at
|
|
|
|
|
31/12/07
|
|
|
Additions
|
|
|
Combinations
|
|
|
Transfers
|
|
|
Disposals
|
|
|
Differences
|
|
|
31/12/08
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cost:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Land and buildings
|
|
|
79,845
|
|
|
|
29,142
|
|
|
|
0
|
|
|
|
641
|
|
|
|
0
|
|
|
|
1,439
|
|
|
|
111,067
|
|
|
Plant and machinery
|
|
|
233,812
|
|
|
|
35,408
|
|
|
|
3
|
|
|
|
22,423
|
|
|
|
(5,939
|
)
|
|
|
2,054
|
|
|
|
287,761
|
|
|
Under construction
|
|
|
30,079
|
|
|
|
55,399
|
|
|
|
0
|
|
|
|
(23,948
|
)
|
|
|
(128
|
)
|
|
|
2,218
|
|
|
|
63,620
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
343,736
|
|
|
|
119,949
|
|
|
|
3
|
|
|
|
(884
|
)
|
|
|
(6,067
|
)
|
|
|
5,711
|
|
|
|
462,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated depreciation:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Buildings
|
|
|
(6,735
|
)
|
|
|
(1,234
|
)
|
|
|
0
|
|
|
|
(39
|
)
|
|
|
29
|
|
|
|
(70
|
)
|
|
|
(8,049
|
)
|
|
Plant and machinery
|
|
|
(135,669
|
)
|
|
|
(22,942
|
)
|
|
|
0
|
|
|
|
923
|
|
|
|
5,027
|
|
|
|
(729
|
)
|
|
|
(153,390
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(142,404
|
)
|
|
|
(24,176
|
)
|
|
|
0
|
|
|
|
884
|
|
|
|
5,056
|
|
|
|
(799
|
)
|
|
|
(161,439
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Carrying amount
|
|
|
201,332
|
|
|
|
95,773
|
|
|
|
3
|
|
|
|
0
|
|
|
|
(1,011
|
)
|
|
|
4,912
|
|
|
|
301,009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(note 3
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 9 to the
consolidated financial statements
F-134
APPENDIX IV
GRIFOLS,
S.A. AND SUBSIDIARIES
Non-current
Loans and Borrowings
For the
Year Ended 31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Initial Loan
|
|
|
|
|
|
|
|
|
|
|
Interest
|
|
Concession
|
|
|
Maturity
|
|
|
Face
|
|
|
Arrangement
|
|
|
Carrying
|
|
|
Loan
|
|
Currency
|
|
|
Rate
|
|
Date
|
|
|
Date
|
|
|
Amount
|
|
|
Expenses
|
|
|
Amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Syndicated loan — Club deal
|
|
|
EUR
|
|
|
Euribor + 0,8%
|
|
|
01/05/2008
|
|
|
|
26/05/2013
|
|
|
|
350,000
|
|
|
|
(2,427
|
)
|
|
|
99,408
|
|
|
Instituto de crédito Oficial
|
|
|
EUR
|
|
|
Euribor + 1%
|
|
|
01/06/2006
|
|
|
|
26/05/2016
|
|
|
|
30,000
|
|
|
|
(210
|
)
|
|
|
17,955
|
|
|
Caixa Catalunya — Mortgage loan
|
|
|
EUR
|
|
|
Euribor + 0,9%
|
|
|
01/02/2008
|
|
|
|
01/02/2018
|
|
|
|
14,000
|
|
|
|
(294
|
)
|
|
|
10,115
|
|
|
Banco Santander
|
|
|
EUR
|
|
|
ICO + 1,8%
|
|
|
01/06/2009
|
|
|
|
01/06/2016
|
|
|
|
6,000
|
|
|
|
—
|
|
|
|
5,400
|
|
|
Caja de Madrid
|
|
|
EUR
|
|
|
Euribor + 1%
|
|
|
05/06/2009
|
|
|
|
05/06/2016
|
|
|
|
6,000
|
|
|
|
—
|
|
|
|
5,400
|
|
|
Banco Guipuzcoano
|
|
|
EUR
|
|
|
Euribor + 1%
|
|
|
25/03/2010
|
|
|
|
25/03/2020
|
|
|
|
8,500
|
|
|
|
—
|
|
|
|
8,500
|
|
|
Banco Sabadell
|
|
|
EUR
|
|
|
Euribor + 1%
|
|
|
11/06/2010
|
|
|
|
30/06/2012
|
|
|
|
1,465
|
|
|
|
—
|
|
|
|
1,413
|
|
|
SCH
|
|
|
EUR
|
|
|
1.75%
|
|
|
13/10/2010
|
|
|
|
13/10/2017
|
|
|
|
900
|
|
|
|
—
|
|
|
|
876
|
|
|
Ibercaja
|
|
|
EUR
|
|
|
Euribor + 1,99%
|
|
|
30/07/2009
|
|
|
|
31/07/2016
|
|
|
|
1,800
|
|
|
|
—
|
|
|
|
1,664
|
|
|
Caja de Madrid
|
|
|
|
|
|
Euribor + 2%
|
|
|
09/03/2010
|
|
|
|
25/03/2020
|
|
|
|
10,000
|
|
|
|
—
|
|
|
|
10,000
|
|
|
SCH
|
|
|
|
|
|
Euribor + 1%
|
|
|
18/11/2010
|
|
|
|
31/01/2012
|
|
|
|
169
|
|
|
|
—
|
|
|
|
169
|
|
|
BBVA — Mortgage loan
|
|
|
EUR
|
|
|
Euribor + 1,2%
|
|
|
21/10/2008
|
|
|
|
31/12/2024
|
|
|
|
45,000
|
|
|
|
(676
|
)
|
|
|
39,201
|
|
|
Caixa Catalunya
|
|
|
EUR
|
|
|
ICO + 1,99%
|
|
|
30/07/2009
|
|
|
|
25/08/2016
|
|
|
|
1,440
|
|
|
|
—
|
|
|
|
1,353
|
|
|
Caixa Galicia
|
|
|
EUR
|
|
|
Euribor + 1%
|
|
|
11/06/2010
|
|
|
|
25/06/2020
|
|
|
|
1,180
|
|
|
|
—
|
|
|
|
1,003
|
|
|
Banca Toscana
|
|
|
EUR
|
|
|
6 months Euribor + 1%
|
|
|
08/05/2008
|
|
|
|
30/06/2013
|
|
|
|
3,000
|
|
|
|
—
|
|
|
|
939
|
|
|
Cofides
|
|
|
EUR
|
|
|
6 months Euribor + 0,45%
|
|
|
01/08/2008
|
|
|
|
20/08/2017
|
|
|
|
6,854
|
|
|
|
—
|
|
|
|
5,875
|
|
|
Cofides
|
|
|
EUR
|
|
|
Euribor + 2%
|
|
|
20/09/2011
|
|
|
|
20/03/2017
|
|
|
|
10,745
|
|
|
|
—
|
|
|
|
10,177
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
497,053
|
|
|
|
(3,607
|
)
|
|
|
219,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current finance lease creditors (see note 22)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
4,734
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
497,053
|
|
|
|
(3,607
|
)
|
|
|
224,182
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 22 to the
consolidated financial statements.
F-135
APPENDIX IV
GRIFOLS, S.A. AND SUBSIDIARIES
Non-current Loans and Borrowings
For the Year Ended 31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Initial Loan
|
|
|
|
|
|
|
|
|
|
Interest
|
|
Concession
|
|
|
Maturity
|
|
|
Face
|
|
|
Arrangement
|
|
|
Carrying
|
|
|
Loan
|
|
Currency
|
|
Rate
|
|
Date
|
|
|
Date
|
|
|
Amount
|
|
|
Expenses
|
|
|
Amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Syndicated loan — Club deal
|
|
EUR
|
|
Euribor + 0,8%
|
|
|
01/05/2008
|
|
|
|
26/05/2013
|
|
|
|
350,000
|
|
|
|
(2,427
|
)
|
|
|
195,471
|
|
|
Instituto de crédito Oficial
|
|
EUR
|
|
Euribor + 1%
|
|
|
01/06/2006
|
|
|
|
26/05/2016
|
|
|
|
30,000
|
|
|
|
(210
|
)
|
|
|
21,933
|
|
|
Caixa Catalunya — Mortgage loan
|
|
EUR
|
|
Euribor + 0,9%
|
|
|
01/02/2008
|
|
|
|
01/02/2018
|
|
|
|
14,000
|
|
|
|
(294
|
)
|
|
|
11,733
|
|
|
Banco Santander
|
|
EUR
|
|
ICO + 1,8%
|
|
|
01/06/2009
|
|
|
|
01/06/2016
|
|
|
|
6,000
|
|
|
|
—
|
|
|
|
6,000
|
|
|
Caja de Madrid
|
|
EUR
|
|
Euribor + 1%
|
|
|
05/06/2009
|
|
|
|
05/06/2016
|
|
|
|
6,000
|
|
|
|
—
|
|
|
|
6,000
|
|
|
Ibercaja
|
|
EUR
|
|
Euribor + 1,9%
|
|
|
30/07/2009
|
|
|
|
31/07/2016
|
|
|
|
1,800
|
|
|
|
—
|
|
|
|
1,800
|
|
|
BBVA — Mortgage loan
|
|
EUR
|
|
Euribor + 1,2%
|
|
|
21/10/2008
|
|
|
|
31/12/2024
|
|
|
|
45,000
|
|
|
|
(676
|
)
|
|
|
33,649
|
|
|
Caixa Catalunya
|
|
EUR
|
|
ICO + 1,99%
|
|
|
30/07/2009
|
|
|
|
25/08/2016
|
|
|
|
1,440
|
|
|
|
—
|
|
|
|
1,440
|
|
|
Banca Toscana
|
|
EUR
|
|
6 months Euribor + 1%
|
|
|
08/05/2008
|
|
|
|
30/06/2013
|
|
|
|
3,000
|
|
|
|
—
|
|
|
|
1,552
|
|
|
Cofides
|
|
EUR
|
|
6 months Euribor + 0,45%
|
|
|
01/08/2008
|
|
|
|
20/08/2017
|
|
|
|
6,854
|
|
|
|
—
|
|
|
|
6,854
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
464,094
|
|
|
|
(3,607
|
)
|
|
|
286,432
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current finance lease creditors (see note 22)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
6,202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
464,094
|
|
|
|
(3,607
|
)
|
|
|
292,634
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This
appendix forms an integral part of note 22 to the
consolidated financial statements.
F-136
APPENDIX IV
GRIFOLS,
S.A. AND SUBSIDIARIES
Non-current
Loans and Borrowings
For the
Year Ended 31 December 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Initial Loan
|
|
|
|
|
|
|
|
|
|
Interest
|
|
Concession
|
|
|
Maturity
|
|
|
Face
|
|
|
Arrangement
|
|
|
Carrying
|
|
|
Loan
|
|
Currency
|
|
Rate
|
|
Date
|
|
|
Date
|
|
|
Amount
|
|
|
Expenses
|
|
|
Amount
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Thousands of Euros
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Syndicated loan — Club deal
|
|
EUR/USD
|
|
Euribor + 0,8%
|
|
|
01/05/2008
|
|
|
|
26/05/2013
|
|
|
|
350,000
|
|
|
|
(1,984
|
)
|
|
|
225,320
|
|
|
Institut Catalá de Finances
|
|
EUR
|
|
5.70%
|
|
|
27/01/2005
|
|
|
|
28/02/2010
|
|
|
|
6,247
|
|
|
|
(62
|
)
|
|
|
312
|
|
|
Instituto de Crédito Oficial
|
|
EUR
|
|
4.94%
|
|
|
01/06/2006
|
|
|
|
26/05/2016
|
|
|
|
30,000
|
|
|
|
(210
|
)
|
|
|
25,907
|
|
|
Caixa Catalunya — Mortgage loan
|
|
EUR
|
|
5.25%
|
|
|
01/02/2008
|
|
|
|
01/02/2018
|
|
|
|
14,000
|
|
|
|
(294
|
)
|
|
|
13,350
|
|
|
BBVA — Mortgage loan
|
|
EUR
|
|
6.50%
|
|
|
21/10/2008
|
|
|
|
31/12/2024
|
|
|
|
45,000
|
|
|
|
(676
|
)
|
|
|
30,463
|
|
|
Banca Toscana
|
|
EUR
|
|
5.33%
|
|
|
08/05/2008
|
|
|
|
30/06/2013
|
|
|
|
3,000
|
|
|
|
—
|
|
|
|
2,183
|
|
|
Cofides
|
|
EUR
|
|
5.61%
|
|
|
01/08/2008
|
|
|
|
20/08/2017
|
|
|
|
6,854
|
|
|
|
—
|
|
|
|
6,854
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
448,247
|
|
|
|
(3,226
|
)
|
|
|
304,389
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current finance lease creditors (see note 22)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,124
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
448,247
|
|
|
|
(3,226
|
)
|
|
|
311,513
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
This appendix forms an integral part of note 22 to the
consolidated financial statements.
F-137
APPENDIX V
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Balance Sheets
at 31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Assets
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Expressed in thousands of euros)
|
|
|
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
0
|
|
|
|
0
|
|
|
|
27,165
|
|
|
|
12,465
|
|
|
|
134,370
|
|
|
|
174,000
|
|
|
Other intangible assets
|
|
|
8,111
|
|
|
|
950
|
|
|
|
44,251
|
|
|
|
3,405
|
|
|
|
12,668
|
|
|
|
69,385
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
8,111
|
|
|
|
950
|
|
|
|
71,416
|
|
|
|
15,870
|
|
|
|
147,038
|
|
|
|
243,385
|
|
|
Property, plant and equipment
|
|
|
94,683
|
|
|
|
18,508
|
|
|
|
187,213
|
|
|
|
72,299
|
|
|
|
(998
|
)
|
|
|
371,705
|
|
|
Investments in Subsidiaries
|
|
|
342,810
|
|
|
|
206,165
|
|
|
|
16,356
|
|
|
|
273
|
|
|
|
(565,604
|
)
|
|
|
0
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
0
|
|
|
|
21,748
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(21,748
|
)
|
|
|
0
|
|
|
Investments in equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
383
|
|
|
|
383
|
|
|
Non-current financial assets
|
|
|
650
|
|
|
|
2,125
|
|
|
|
727
|
|
|
|
229
|
|
|
|
0
|
|
|
|
3,731
|
|
|
Deferred tax assets
|
|
|
953
|
|
|
|
1,774
|
|
|
|
9,218
|
|
|
|
1,604
|
|
|
|
19,846
|
|
|
|
33,395
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
447,207
|
|
|
|
251,270
|
|
|
|
284,930
|
|
|
|
90,275
|
|
|
|
(421,083
|
)
|
|
|
652,599
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
|
704
|
|
|
|
0
|
|
|
|
495,542
|
|
|
|
62,006
|
|
|
|
(73,790
|
)
|
|
|
484,462
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade receivables
|
|
|
9,419
|
|
|
|
182,073
|
|
|
|
270,117
|
|
|
|
118,660
|
|
|
|
(372,429
|
)
|
|
|
207,840
|
|
|
Other receivables
|
|
|
8,344
|
|
|
|
51
|
|
|
|
23,989
|
|
|
|
7,156
|
|
|
|
0
|
|
|
|
39,540
|
|
|
Current income tax assets
|
|
|
4,178
|
|
|
|
2,139
|
|
|
|
508
|
|
|
|
2,476
|
|
|
|
(1,499
|
)
|
|
|
7,802
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
|
21,940
|
|
|
|
184,263
|
|
|
|
294,613
|
|
|
|
128,292
|
|
|
|
(373,926
|
)
|
|
|
255,182
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
222,829
|
|
|
|
44,522
|
|
|
|
13,522
|
|
|
|
21,840
|
|
|
|
(302,713
|
)
|
|
|
0
|
|
|
Other current financial assets
|
|
|
1,921
|
|
|
|
123
|
|
|
|
1
|
|
|
|
6,172
|
|
|
|
0
|
|
|
|
8,217
|
|
|
Other current assets
|
|
|
2,707
|
|
|
|
318
|
|
|
|
3,156
|
|
|
|
1,164
|
|
|
|
0
|
|
|
|
7,345
|
|
|
Cash and cash equivalents
|
|
|
144
|
|
|
|
237,804
|
|
|
|
7,191
|
|
|
|
4,233
|
|
|
|
0
|
|
|
|
249,372
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
250,245
|
|
|
|
467,030
|
|
|
|
814,025
|
|
|
|
223,707
|
|
|
|
(750,429
|
)
|
|
|
1,004,578
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
697,452
|
|
|
|
718,300
|
|
|
|
1,098,955
|
|
|
|
313,982
|
|
|
|
(1,171,512
|
)
|
|
|
1,657,177
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-138
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Equity and liabilities
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Unaudited)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(Expressed in thousands of euros)
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
106,532
|
|
|
|
0
|
|
|
|
21,497
|
|
|
|
36,080
|
|
|
|
(57,577
|
)
|
|
|
106,532
|
|
|
Share premium
|
|
|
121,802
|
|
|
|
72,932
|
|
|
|
123,317
|
|
|
|
5,703
|
|
|
|
(201,952
|
)
|
|
|
121,802
|
|
|
Reserves
|
|
|
59,342
|
|
|
|
193,822
|
|
|
|
92,666
|
|
|
|
52,597
|
|
|
|
(83,524
|
)
|
|
|
314,903
|
|
|
Own shares
|
|
|
(677
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(677
|
)
|
|
Interim dividend
|
|
|
(31,960
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(208
|
)
|
|
|
208
|
|
|
|
(31,960
|
)
|
|
Profit for the year attributable to the Parent
|
|
|
72,923
|
|
|
|
(2,976
|
)
|
|
|
137,853
|
|
|
|
26,664
|
|
|
|
(86,492
|
)
|
|
|
147,972
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
327,962
|
|
|
|
263,778
|
|
|
|
375,333
|
|
|
|
120,836
|
|
|
|
(429,337
|
)
|
|
|
658,572
|
|
|
Cash flow hedges
|
|
|
0
|
|
|
|
(1,948
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,948
|
)
|
|
Translation differences
|
|
|
0
|
|
|
|
(44
|
)
|
|
|
(34,796
|
)
|
|
|
(478
|
)
|
|
|
(54,935
|
)
|
|
|
(90,253
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
0
|
|
|
|
(1,992
|
)
|
|
|
(34,796
|
)
|
|
|
(478
|
)
|
|
|
(54,935
|
)
|
|
|
(92,201
|
)
|
|
Equity attributable to the Parent
|
|
|
327,962
|
|
|
|
261,786
|
|
|
|
340,537
|
|
|
|
120,358
|
|
|
|
(484,272
|
)
|
|
|
566,371
|
|
|
Non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
86
|
|
|
|
12,071
|
|
|
|
12,157
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
327,962
|
|
|
|
261,786
|
|
|
|
340,537
|
|
|
|
120,444
|
|
|
|
(472,201
|
)
|
|
|
578,528
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
77
|
|
|
|
174
|
|
|
|
2,000
|
|
|
|
60
|
|
|
|
0
|
|
|
|
2,311
|
|
|
Provisions
|
|
|
0
|
|
|
|
0
|
|
|
|
989
|
|
|
|
243
|
|
|
|
0
|
|
|
|
1,232
|
|
|
Non-current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
236,733
|
|
|
|
410,550
|
|
|
|
21,577
|
|
|
|
34,327
|
|
|
|
0
|
|
|
|
703,187
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
16,854
|
|
|
|
0
|
|
|
|
0
|
|
|
|
7,089
|
|
|
|
(23,943
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
367
|
|
|
|
0
|
|
|
|
11,565
|
|
|
|
620
|
|
|
|
0
|
|
|
|
12,552
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities
|
|
|
253,954
|
|
|
|
410,550
|
|
|
|
33,142
|
|
|
|
42,036
|
|
|
|
(23,943
|
)
|
|
|
715,739
|
|
|
Deferred tax liabilities
|
|
|
11,231
|
|
|
|
1,349
|
|
|
|
44,503
|
|
|
|
4,172
|
|
|
|
(931
|
)
|
|
|
60,324
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
265,262
|
|
|
|
412,073
|
|
|
|
80,634
|
|
|
|
46,511
|
|
|
|
(24,874
|
)
|
|
|
779,606
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provisions
|
|
|
291
|
|
|
|
0
|
|
|
|
30
|
|
|
|
4,381
|
|
|
|
0
|
|
|
|
4,702
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
35,579
|
|
|
|
6,907
|
|
|
|
42,988
|
|
|
|
28,517
|
|
|
|
0
|
|
|
|
113,991
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
43,768
|
|
|
|
0
|
|
|
|
234,177
|
|
|
|
22,576
|
|
|
|
(300,521
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
3,594
|
|
|
|
0
|
|
|
|
7,496
|
|
|
|
1,140
|
|
|
|
0
|
|
|
|
12,230
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities
|
|
|
82,941
|
|
|
|
6,907
|
|
|
|
284,661
|
|
|
|
52,233
|
|
|
|
(300,521
|
)
|
|
|
126,221
|
|
|
Debts with associates
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
10,583
|
|
|
|
35,919
|
|
|
|
371,086
|
|
|
|
75,744
|
|
|
|
(372,423
|
)
|
|
|
120,909
|
|
|
Other payables
|
|
|
7,464
|
|
|
|
8
|
|
|
|
7,116
|
|
|
|
3,244
|
|
|
|
0
|
|
|
|
17,832
|
|
|
Current income tax liabilities
|
|
|
0
|
|
|
|
0
|
|
|
|
946
|
|
|
|
3,805
|
|
|
|
(1,493
|
)
|
|
|
3,258
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables
|
|
|
18,047
|
|
|
|
35,927
|
|
|
|
379,148
|
|
|
|
82,793
|
|
|
|
(373,916
|
)
|
|
|
141,999
|
|
|
Other current liabilities
|
|
|
2,949
|
|
|
|
1,607
|
|
|
|
13,945
|
|
|
|
7,620
|
|
|
|
0
|
|
|
|
26,121
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
104,228
|
|
|
|
44,441
|
|
|
|
677,784
|
|
|
|
147,027
|
|
|
|
(674,437
|
)
|
|
|
299,043
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
369,490
|
|
|
|
456,514
|
|
|
|
758,418
|
|
|
|
193,538
|
|
|
|
(699,311
|
)
|
|
|
1,078,649
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity and liabilities
|
|
|
697,452
|
|
|
|
718,300
|
|
|
|
1,098,955
|
|
|
|
313,982
|
|
|
|
(1,171,512
|
)
|
|
|
1,657,177
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-139
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Assets
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Non-current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intangible assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Goodwill
|
|
|
0
|
|
|
|
0
|
|
|
|
29,895
|
|
|
|
15,123
|
|
|
|
144,430
|
|
|
|
189,448
|
|
|
Other intangible assets
|
|
|
5,731
|
|
|
|
3,162
|
|
|
|
49,120
|
|
|
|
4,725
|
|
|
|
15,561
|
|
|
|
78,299
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total intangible assets
|
|
|
5,731
|
|
|
|
3,162
|
|
|
|
79,015
|
|
|
|
19,848
|
|
|
|
159,991
|
|
|
|
267,747
|
|
|
Property, plant and equipment
|
|
|
95,452
|
|
|
|
28,150
|
|
|
|
231,728
|
|
|
|
78,801
|
|
|
|
0
|
|
|
|
434,131
|
|
|
Investments in Subsidiaries
|
|
|
345,025
|
|
|
|
222,273
|
|
|
|
2,532
|
|
|
|
938
|
|
|
|
(570,768
|
)
|
|
|
0
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
0
|
|
|
|
21,005
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(21,005
|
)
|
|
|
0
|
|
|
Investments in equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
598
|
|
|
|
598
|
|
|
Non-current financial assets
|
|
|
709
|
|
|
|
5,804
|
|
|
|
734
|
|
|
|
288
|
|
|
|
0
|
|
|
|
7,535
|
|
|
Deferred tax assets
|
|
|
1,091
|
|
|
|
2,076
|
|
|
|
9,534
|
|
|
|
2,932
|
|
|
|
19,256
|
|
|
|
34,889
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current assets
|
|
|
448,008
|
|
|
|
282,470
|
|
|
|
323,543
|
|
|
|
102,807
|
|
|
|
(411,928
|
)
|
|
|
744,900
|
|
|
Current assets
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Inventories
|
|
|
796
|
|
|
|
0
|
|
|
|
538,311
|
|
|
|
59,401
|
|
|
|
(70,643
|
)
|
|
|
527,865
|
|
|
Trade and other receivables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade receivables
|
|
|
8,946
|
|
|
|
11,561
|
|
|
|
209,758
|
|
|
|
100,719
|
|
|
|
(106,629
|
)
|
|
|
224,355
|
|
|
Other receivables
|
|
|
3,157
|
|
|
|
201
|
|
|
|
27,612
|
|
|
|
11,971
|
|
|
|
1,091
|
|
|
|
44,032
|
|
|
Current income tax assets
|
|
|
6,168
|
|
|
|
6,071
|
|
|
|
297
|
|
|
|
2,071
|
|
|
|
0
|
|
|
|
14,607
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trade and other receivables
|
|
|
18,271
|
|
|
|
17,833
|
|
|
|
237,667
|
|
|
|
114,761
|
|
|
|
(105,538
|
)
|
|
|
282,994
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
238,262
|
|
|
|
(1,311
|
)
|
|
|
14,699
|
|
|
|
22,860
|
|
|
|
(274,510
|
)
|
|
|
0
|
|
|
Other current financial assets
|
|
|
267
|
|
|
|
224
|
|
|
|
8
|
|
|
|
12,447
|
|
|
|
0
|
|
|
|
12,946
|
|
|
Other current assets
|
|
|
13,460
|
|
|
|
60,568
|
|
|
|
5,150
|
|
|
|
1,450
|
|
|
|
0
|
|
|
|
80,628
|
|
|
Cash and cash equivalents
|
|
|
25
|
|
|
|
227,456
|
|
|
|
1,444
|
|
|
|
10,724
|
|
|
|
0
|
|
|
|
239,649
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
271,081
|
|
|
|
304,770
|
|
|
|
797,279
|
|
|
|
221,643
|
|
|
|
(450,691
|
)
|
|
|
1,144,082
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
|
719,089
|
|
|
|
587,240
|
|
|
|
1,120,822
|
|
|
|
324,450
|
|
|
|
(862,619
|
)
|
|
|
1,888,982
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-140
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Balance Sheets
at
31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Equity and Liabilities
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Equity
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share capital
|
|
|
106,532
|
|
|
|
0
|
|
|
|
21,497
|
|
|
|
36,340
|
|
|
|
(57,837
|
)
|
|
|
106,532
|
|
|
Share premium
|
|
|
121,802
|
|
|
|
72,932
|
|
|
|
106,854
|
|
|
|
5,703
|
|
|
|
(185,489
|
)
|
|
|
121,802
|
|
|
Reserves
|
|
|
73,076
|
|
|
|
190,844
|
|
|
|
175,221
|
|
|
|
59,636
|
|
|
|
(95,173
|
)
|
|
|
403,604
|
|
|
Own shares
|
|
|
(1,927
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,927
|
)
|
|
Interim dividend
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(152
|
)
|
|
|
152
|
|
|
|
0
|
|
|
Profit for the year attributable to the Parent
|
|
|
63,226
|
|
|
|
(10,726
|
)
|
|
|
112,853
|
|
|
|
23,906
|
|
|
|
(73,746
|
)
|
|
|
115,513
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
362,709
|
|
|
|
253,050
|
|
|
|
416,425
|
|
|
|
125,433
|
|
|
|
(412,093
|
)
|
|
|
745,524
|
|
|
Cash flow hedges
|
|
|
0
|
|
|
|
(1,751
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,751
|
)
|
|
Translation differences
|
|
|
0
|
|
|
|
20,449
|
|
|
|
(18,344
|
)
|
|
|
11,123
|
|
|
|
(63,961
|
)
|
|
|
(50,733
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive income
|
|
|
0
|
|
|
|
18,698
|
|
|
|
(18,344
|
)
|
|
|
11,123
|
|
|
|
(63,961
|
)
|
|
|
(52,484
|
)
|
|
Equity attributable to the Parent
|
|
|
362,709
|
|
|
|
271,748
|
|
|
|
398,081
|
|
|
|
136,556
|
|
|
|
(476,054
|
)
|
|
|
693,040
|
|
|
Non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
14,350
|
|
|
|
14,350
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity
|
|
|
362,709
|
|
|
|
271,748
|
|
|
|
398,081
|
|
|
|
136,556
|
|
|
|
(461,704
|
)
|
|
|
707,390
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grants
|
|
|
142
|
|
|
|
187
|
|
|
|
1,683
|
|
|
|
76
|
|
|
|
0
|
|
|
|
2,088
|
|
|
Provisions
|
|
|
0
|
|
|
|
0
|
|
|
|
1,127
|
|
|
|
251
|
|
|
|
0
|
|
|
|
1,378
|
|
|
Non-current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and other marketable securities
|
|
|
133,982
|
|
|
|
441,203
|
|
|
|
40,350
|
|
|
|
49,695
|
|
|
|
155
|
|
|
|
665,385
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
15,875
|
|
|
|
0
|
|
|
|
0
|
|
|
|
5,130
|
|
|
|
(21,005
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
200
|
|
|
|
0
|
|
|
|
9,596
|
|
|
|
678
|
|
|
|
0
|
|
|
|
10,474
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current financial liabilities
|
|
|
150,057
|
|
|
|
441,203
|
|
|
|
49,946
|
|
|
|
55,503
|
|
|
|
(20,850
|
)
|
|
|
675,859
|
|
|
Deferred tax liabilities
|
|
|
11,907
|
|
|
|
2,860
|
|
|
|
62,718
|
|
|
|
1,519
|
|
|
|
137
|
|
|
|
79,141
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total non-current liabilities
|
|
|
162,106
|
|
|
|
444,250
|
|
|
|
115,474
|
|
|
|
57,349
|
|
|
|
(20,713
|
)
|
|
|
758,466
|
|
|
Current liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provisions
|
|
|
257
|
|
|
|
0
|
|
|
|
30
|
|
|
|
4,078
|
|
|
|
0
|
|
|
|
4,365
|
|
|
Current financial liabilities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Loans and borrowings, bonds and
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
other marketable securities
|
|
|
103,131
|
|
|
|
7,364
|
|
|
|
41,433
|
|
|
|
39,862
|
|
|
|
(155
|
)
|
|
|
191,635
|
|
|
Advances and notes between parent and subsidiaries
|
|
|
42,863
|
|
|
|
(162,772
|
)
|
|
|
385,947
|
|
|
|
9,017
|
|
|
|
(275,055
|
)
|
|
|
0
|
|
|
Other financial liabilities
|
|
|
8,830
|
|
|
|
0
|
|
|
|
9,316
|
|
|
|
90
|
|
|
|
0
|
|
|
|
18,236
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current financial liabilities
|
|
|
154,824
|
|
|
|
(155,408
|
)
|
|
|
436,696
|
|
|
|
48,969
|
|
|
|
(275,210
|
)
|
|
|
209,871
|
|
|
Debts with associates
|
|
|
1,162
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
1,162
|
|
|
Trade and other payables
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Suppliers
|
|
|
33,426
|
|
|
|
24,766
|
|
|
|
146,861
|
|
|
|
60,617
|
|
|
|
(104,992
|
)
|
|
|
160,678
|
|
|
Other payables
|
|
|
1,141
|
|
|
|
12
|
|
|
|
5,288
|
|
|
|
3,627
|
|
|
|
1,860
|
|
|
|
11,928
|
|
|
Current income tax liabilities
|
|
|
0
|
|
|
|
0
|
|
|
|
2,369
|
|
|
|
3,663
|
|
|
|
(1,860
|
)
|
|
|
4,172
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total trade and other payables
|
|
|
34,567
|
|
|
|
24,778
|
|
|
|
154,518
|
|
|
|
67,907
|
|
|
|
(104,992
|
)
|
|
|
176,778
|
|
|
Other current liabilities
|
|
|
3,464
|
|
|
|
1,872
|
|
|
|
16,023
|
|
|
|
9,591
|
|
|
|
0
|
|
|
|
30,950
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
194,274
|
|
|
|
(128,758
|
)
|
|
|
607,267
|
|
|
|
130,545
|
|
|
|
(380,202
|
)
|
|
|
423,126
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
356,380
|
|
|
|
315,492
|
|
|
|
722,741
|
|
|
|
187,894
|
|
|
|
(400,915
|
)
|
|
|
1,181,592
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total equity and liabilities
|
|
|
719,089
|
|
|
|
587,240
|
|
|
|
1,120,822
|
|
|
|
324,450
|
|
|
|
(862,619
|
)
|
|
|
1,888,982
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-141
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Income Statements
for the
year ended 31 December 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Profit and Loss
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
54,725
|
|
|
|
17,424
|
|
|
|
1,211,130
|
|
|
|
320,481
|
|
|
|
(789,449
|
)
|
|
|
814,311
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
0
|
|
|
|
0
|
|
|
|
64,328
|
|
|
|
482
|
|
|
|
(33,752
|
)
|
|
|
31,058
|
|
|
Self-constructed non-current assets
|
|
|
1,731
|
|
|
|
0
|
|
|
|
9,823
|
|
|
|
2,395
|
|
|
|
11,845
|
|
|
|
25,794
|
|
|
Supplies
|
|
|
(288
|
)
|
|
|
0
|
|
|
|
(670,916
|
)
|
|
|
(177,875
|
)
|
|
|
642,341
|
|
|
|
(206,738
|
)
|
|
Other operating income
|
|
|
68
|
|
|
|
0
|
|
|
|
1,171
|
|
|
|
50
|
|
|
|
0
|
|
|
|
1,289
|
|
|
Personnel expenses
|
|
|
(20,483
|
)
|
|
|
(8,708
|
)
|
|
|
(162,901
|
)
|
|
|
(46,067
|
)
|
|
|
0
|
|
|
|
(238,159
|
)
|
|
Other operating expenses
|
|
|
(31,786
|
)
|
|
|
(8,305
|
)
|
|
|
(243,732
|
)
|
|
|
(53,113
|
)
|
|
|
144,648
|
|
|
|
(192,288
|
)
|
|
Amortisation and depreciation
|
|
|
(4,245
|
)
|
|
|
(497
|
)
|
|
|
(24,178
|
)
|
|
|
(4,270
|
)
|
|
|
(66
|
)
|
|
|
(33,256
|
)
|
|
Non-financial and other capital grants
|
|
|
0
|
|
|
|
0
|
|
|
|
2,941
|
|
|
|
0
|
|
|
|
0
|
|
|
|
2,941
|
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
(31
|
)
|
|
|
0
|
|
|
|
(1,960
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,991
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
(309
|
)
|
|
|
(86
|
)
|
|
|
185,706
|
|
|
|
42,083
|
|
|
|
(24,433
|
)
|
|
|
202,961
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
11,184
|
|
|
|
1
|
|
|
|
2,411
|
|
|
|
1,151
|
|
|
|
(12,065
|
)
|
|
|
2,682
|
|
|
Dividends
|
|
|
72,172
|
|
|
|
25,035
|
|
|
|
10,382
|
|
|
|
48
|
|
|
|
(107,637
|
)
|
|
|
0
|
|
|
Finance expense
|
|
|
(18,626
|
)
|
|
|
(1,892
|
)
|
|
|
(18,443
|
)
|
|
|
(2,597
|
)
|
|
|
12,253
|
|
|
|
(29,305
|
)
|
|
Change in fair value of financial instruments
|
|
|
(1,195
|
)
|
|
|
0
|
|
|
|
(73
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,268
|
)
|
|
Exchange gains/(losses)
|
|
|
(1,335
|
)
|
|
|
752
|
|
|
|
2,395
|
|
|
|
(4,637
|
)
|
|
|
0
|
|
|
|
(2,825
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Finance expense
|
|
|
62,200
|
|
|
|
23,896
|
|
|
|
(3,328
|
)
|
|
|
(6,035
|
)
|
|
|
(107,449
|
)
|
|
|
(30,716
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit/(loss) of equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
24
|
|
|
|
24
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
61,891
|
|
|
|
23,810
|
|
|
|
182,378
|
|
|
|
36,048
|
|
|
|
(131,858
|
)
|
|
|
172,269
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
2,755
|
|
|
|
448
|
|
|
|
(50,673
|
)
|
|
|
(10,295
|
)
|
|
|
7,612
|
|
|
|
(50,153
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
64,646
|
|
|
|
24,258
|
|
|
|
131,705
|
|
|
|
25,753
|
|
|
|
(124,246
|
)
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
64,646
|
|
|
|
24,258
|
|
|
|
131,705
|
|
|
|
25,753
|
|
|
|
(124,246
|
)
|
|
|
122,116
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
64,646
|
|
|
|
24,258
|
|
|
|
131,705
|
|
|
|
25,753
|
|
|
|
(124,634
|
)
|
|
|
121,728
|
|
|
Profit/(loss) attributable to non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
388
|
|
|
|
388
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-142
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Income Statements
for the
year ended 31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Profit and Loss
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
64,981
|
|
|
|
19,074
|
|
|
|
1,367,208
|
|
|
|
373,372
|
|
|
|
(911,449
|
)
|
|
|
913,186
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
0
|
|
|
|
0
|
|
|
|
103,193
|
|
|
|
989
|
|
|
|
(31,089
|
)
|
|
|
73,093
|
|
|
Self-constructed non-current assets
|
|
|
2,406
|
|
|
|
571
|
|
|
|
11,605
|
|
|
|
6,973
|
|
|
|
19,587
|
|
|
|
41,142
|
|
|
Supplies
|
|
|
(449
|
)
|
|
|
0
|
|
|
|
(809,640
|
)
|
|
|
(215,708
|
)
|
|
|
739,523
|
|
|
|
(286,274
|
)
|
|
Other operating income
|
|
|
59
|
|
|
|
0
|
|
|
|
678
|
|
|
|
706
|
|
|
|
0
|
|
|
|
1,443
|
|
|
Personnel expenses
|
|
|
(23,337
|
)
|
|
|
(12,781
|
)
|
|
|
(181,298
|
)
|
|
|
(55,763
|
)
|
|
|
11
|
|
|
|
(273,168
|
)
|
|
Other operating expenses
|
|
|
(31,633
|
)
|
|
|
(5,727
|
)
|
|
|
(258,789
|
)
|
|
|
(67,898
|
)
|
|
|
160,666
|
|
|
|
(203,381
|
)
|
|
Amortisation and depreciation
|
|
|
(5,659
|
)
|
|
|
(850
|
)
|
|
|
(27,319
|
)
|
|
|
(5,726
|
)
|
|
|
0
|
|
|
|
(39,554
|
)
|
|
Non-financial and other capital grants
|
|
|
431
|
|
|
|
0
|
|
|
|
637
|
|
|
|
120
|
|
|
|
0
|
|
|
|
1,188
|
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
(148
|
)
|
|
|
(2
|
)
|
|
|
(843
|
)
|
|
|
(154
|
)
|
|
|
0
|
|
|
|
(1,147
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
6,651
|
|
|
|
285
|
|
|
|
205,432
|
|
|
|
36,911
|
|
|
|
(22,751
|
)
|
|
|
226,528
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
9,395
|
|
|
|
6,054
|
|
|
|
3,699
|
|
|
|
1,384
|
|
|
|
(13,465
|
)
|
|
|
7,067
|
|
|
Dividends
|
|
|
72,226
|
|
|
|
0
|
|
|
|
0
|
|
|
|
165
|
|
|
|
(72,391
|
)
|
|
|
0
|
|
|
Finance expense
|
|
|
(10,660
|
)
|
|
|
(10,267
|
)
|
|
|
(16,787
|
)
|
|
|
(3,762
|
)
|
|
|
14,389
|
|
|
|
(27,087
|
)
|
|
Change in fair value of financial instruments
|
|
|
(979
|
)
|
|
|
0
|
|
|
|
146
|
|
|
|
0
|
|
|
|
246
|
|
|
|
(587
|
)
|
|
Gains/(losses) on disposal of financial instruments
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,482
|
)
|
|
|
0
|
|
|
|
1,237
|
|
|
|
(245
|
)
|
|
Exchange gains/(losses)
|
|
|
(723
|
)
|
|
|
(858
|
)
|
|
|
(242
|
)
|
|
|
90
|
|
|
|
0
|
|
|
|
(1,733
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Finance expense
|
|
|
69,259
|
|
|
|
(5,071
|
)
|
|
|
(14,666
|
)
|
|
|
(2,123
|
)
|
|
|
(69,984
|
)
|
|
|
(22,585
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit/(loss) of equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
51
|
|
|
|
51
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
75,910
|
|
|
|
(4,786
|
)
|
|
|
190,766
|
|
|
|
34,788
|
|
|
|
(92,684
|
)
|
|
|
203,994
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
(2,987
|
)
|
|
|
1,810
|
|
|
|
(52,913
|
)
|
|
|
(8,124
|
)
|
|
|
5,790
|
|
|
|
(56,424
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
72,923
|
|
|
|
(2,976
|
)
|
|
|
137,853
|
|
|
|
26,664
|
|
|
|
(86,894
|
)
|
|
|
147,570
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
72,923
|
|
|
|
(2,976
|
)
|
|
|
137,853
|
|
|
|
26,664
|
|
|
|
(86,894
|
)
|
|
|
147,570
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
72,923
|
|
|
|
(2,976
|
)
|
|
|
137,853
|
|
|
|
26,664
|
|
|
|
(86,492
|
)
|
|
|
147,972
|
|
|
Profit/(loss) attributable to non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(402
|
)
|
|
|
(402
|
)
|
The accompanying note forms an integral part of the consolidated
financial statements
F-143
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Income Statements
for the
year ended 31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
Profit and Loss
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Revenues
|
|
|
66,966
|
|
|
|
21,313
|
|
|
|
1,351,397
|
|
|
|
428,832
|
|
|
|
(877,778
|
)
|
|
|
990,730
|
|
|
Changes in inventories of finished goods and work in progress
|
|
|
0
|
|
|
|
0
|
|
|
|
25,189
|
|
|
|
(1,872
|
)
|
|
|
22,432
|
|
|
|
45,749
|
|
|
Self-constructed non-current assets
|
|
|
580
|
|
|
|
1,208
|
|
|
|
10,165
|
|
|
|
2,672
|
|
|
|
18,888
|
|
|
|
33,513
|
|
|
Supplies
|
|
|
(464
|
)
|
|
|
0
|
|
|
|
(732,316
|
)
|
|
|
(247,988
|
)
|
|
|
673,909
|
|
|
|
(306,859
|
)
|
|
Other operating income
|
|
|
93
|
|
|
|
0
|
|
|
|
1,014
|
|
|
|
89
|
|
|
|
0
|
|
|
|
1,196
|
|
|
Personnel expenses
|
|
|
(23,931
|
)
|
|
|
(13,651
|
)
|
|
|
(189,100
|
)
|
|
|
(62,326
|
)
|
|
|
0
|
|
|
|
(289,008
|
)
|
|
Other operating expenses
|
|
|
(43,753
|
)
|
|
|
(7,417
|
)
|
|
|
(260,686
|
)
|
|
|
(77,041
|
)
|
|
|
168,679
|
|
|
|
(220,218
|
)
|
|
Amortisation and depreciation
|
|
|
(7,384
|
)
|
|
|
(992
|
)
|
|
|
(28,623
|
)
|
|
|
(8,222
|
)
|
|
|
(555
|
)
|
|
|
(45,776
|
)
|
|
Non-financial and other capital grants
|
|
|
259
|
|
|
|
0
|
|
|
|
470
|
|
|
|
(1
|
)
|
|
|
0
|
|
|
|
728
|
|
|
Impairment and net losses on disposal of fixed assets
|
|
|
(2
|
)
|
|
|
(139
|
)
|
|
|
(756
|
)
|
|
|
(578
|
)
|
|
|
1,103
|
|
|
|
(372
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Results from operating activities
|
|
|
(7,636
|
)
|
|
|
322
|
|
|
|
176,754
|
|
|
|
33,565
|
|
|
|
6,678
|
|
|
|
209,683
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Finance income
|
|
|
4,054
|
|
|
|
15,172
|
|
|
|
2,856
|
|
|
|
1,243
|
|
|
|
(18,799
|
)
|
|
|
4,526
|
|
|
Dividends
|
|
|
76,491
|
|
|
|
0
|
|
|
|
0
|
|
|
|
160
|
|
|
|
(76,651
|
)
|
|
|
0
|
|
|
Finance expense
|
|
|
(8,124
|
)
|
|
|
(32,274
|
)
|
|
|
(22,759
|
)
|
|
|
(5,304
|
)
|
|
|
18,801
|
|
|
|
(49,660
|
)
|
|
Change in fair value of financial instruments
|
|
|
(7,670
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
77
|
|
|
|
0
|
|
|
|
(7,593
|
)
|
|
Gains/ (losses) on disposal of financial instruments
|
|
|
0
|
|
|
|
0
|
|
|
|
1,573
|
|
|
|
(879
|
)
|
|
|
(603
|
)
|
|
|
91
|
|
|
Exchange gains/(losses)
|
|
|
75
|
|
|
|
(455
|
)
|
|
|
(389
|
)
|
|
|
2,385
|
|
|
|
0
|
|
|
|
1,616
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Finance expense
|
|
|
64,826
|
|
|
|
(17,557
|
)
|
|
|
(18,719
|
)
|
|
|
(2,318
|
)
|
|
|
(77,252
|
)
|
|
|
(51,020
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share of profit/(loss) of equity accounted investees
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(879
|
)
|
|
|
(879
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax from continuing operations
|
|
|
57,190
|
|
|
|
(17,235
|
)
|
|
|
158,035
|
|
|
|
31,247
|
|
|
|
(71,453
|
)
|
|
|
157,784
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income tax expense
|
|
|
6,036
|
|
|
|
6,509
|
|
|
|
(45,182
|
)
|
|
|
(7,445
|
)
|
|
|
(2,435
|
)
|
|
|
(42,517
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit after income tax from continuing operations
|
|
|
63,226
|
|
|
|
(10,726
|
)
|
|
|
112,853
|
|
|
|
23,802
|
|
|
|
(73,888
|
)
|
|
|
115,267
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Consolidated profit for the year
|
|
|
63,226
|
|
|
|
(10,726
|
)
|
|
|
112,853
|
|
|
|
23,802
|
|
|
|
(73,888
|
)
|
|
|
115,267
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit attributable to equity holders of the Parent
|
|
|
63,226
|
|
|
|
(10,726
|
)
|
|
|
112,853
|
|
|
|
23,906
|
|
|
|
(73,746
|
)
|
|
|
115,513
|
|
|
Profit/(loss) attributable to non-controlling interests
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(104
|
)
|
|
|
(142
|
)
|
|
|
(246
|
)
|
The accompanying note forms an integral part of the consolidated
financial statements
F-144
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statements of Cash Flows
for the
year ended 31 December 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from/(used in) operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
61,891
|
|
|
|
23,810
|
|
|
|
182,378
|
|
|
|
36,048
|
|
|
|
(131,858
|
)
|
|
|
172,269
|
|
|
Adjustments for:
|
|
|
(56,619
|
)
|
|
|
(16,796
|
)
|
|
|
35,898
|
|
|
|
13,009
|
|
|
|
90,542
|
|
|
|
66,034
|
|
|
Amortisation and depreciation
|
|
|
4,245
|
|
|
|
497
|
|
|
|
24,178
|
|
|
|
4,270
|
|
|
|
66
|
|
|
|
33,256
|
|
|
Other adjustments:
|
|
|
(60,864
|
)
|
|
|
(17,293
|
)
|
|
|
11,720
|
|
|
|
8,739
|
|
|
|
90,476
|
|
|
|
32,778
|
|
|
(Profit)/losses on equity accounted investments
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(24
|
)
|
|
|
(24
|
)
|
|
Exchange differences
|
|
|
1,335
|
|
|
|
(752
|
)
|
|
|
(2,395
|
)
|
|
|
4,637
|
|
|
|
0
|
|
|
|
2,825
|
|
|
Impairment of assets and net provision charges
|
|
|
0
|
|
|
|
0
|
|
|
|
1,800
|
|
|
|
194
|
|
|
|
0
|
|
|
|
1,994
|
|
|
(Profits)/losses on disposal of fixed assets
|
|
|
31
|
|
|
|
0
|
|
|
|
1,960
|
|
|
|
10
|
|
|
|
0
|
|
|
|
2,001
|
|
|
Government grants taken to income
|
|
|
0
|
|
|
|
0
|
|
|
|
(2,941
|
)
|
|
|
(2
|
)
|
|
|
0
|
|
|
|
(2,943
|
)
|
|
Net finance expense
|
|
|
(62,437
|
)
|
|
|
(23,139
|
)
|
|
|
4,416
|
|
|
|
1,587
|
|
|
|
107,464
|
|
|
|
27,891
|
|
|
Other adjustments
|
|
|
207
|
|
|
|
6,598
|
|
|
|
8,880
|
|
|
|
2,313
|
|
|
|
(16,964
|
)
|
|
|
1,034
|
|
|
Change in operating assets and liabilities
|
|
|
(112,558
|
)
|
|
|
(138,087
|
)
|
|
|
(15,842
|
)
|
|
|
(4,178
|
)
|
|
|
184,115
|
|
|
|
(86,550
|
)
|
|
Change in inventories
|
|
|
(117
|
)
|
|
|
0
|
|
|
|
(120,261
|
)
|
|
|
(18,933
|
)
|
|
|
40,791
|
|
|
|
(98,520
|
)
|
|
Change in trade and other receivables
|
|
|
4,579
|
|
|
|
(133,431
|
)
|
|
|
36,498
|
|
|
|
(12,003
|
)
|
|
|
96,406
|
|
|
|
(7,951
|
)
|
|
Change in current financial assets and other current assets
|
|
|
(123,479
|
)
|
|
|
27,683
|
|
|
|
(49,701
|
)
|
|
|
2,913
|
|
|
|
142,989
|
|
|
|
405
|
|
|
Change in current trade and other payables
|
|
|
6,459
|
|
|
|
(32,339
|
)
|
|
|
117,622
|
|
|
|
23,845
|
|
|
|
(96,071
|
)
|
|
|
19,516
|
|
|
Other cash flows used in operating activities
|
|
|
66,654
|
|
|
|
22,420
|
|
|
|
(40,647
|
)
|
|
|
(18,107
|
)
|
|
|
(107,630
|
)
|
|
|
(77,310
|
)
|
|
Interest paid
|
|
|
(17,206
|
)
|
|
|
(1,818
|
)
|
|
|
(16,641
|
)
|
|
|
(1,478
|
)
|
|
|
11,171
|
|
|
|
(25,972
|
)
|
|
Interest received
|
|
|
11,171
|
|
|
|
0
|
|
|
|
2,213
|
|
|
|
0
|
|
|
|
(11,171
|
)
|
|
|
2,213
|
|
|
Dividends received
|
|
|
72,172
|
|
|
|
25,035
|
|
|
|
10,382
|
|
|
|
41
|
|
|
|
(107,630
|
)
|
|
|
0
|
|
|
Income tax paid
|
|
|
517
|
|
|
|
(797
|
)
|
|
|
(36,601
|
)
|
|
|
(16,670
|
)
|
|
|
0
|
|
|
|
(53,551
|
)
|
|
Net cash from operating activities
|
|
|
(40,632
|
)
|
|
|
(108,653
|
)
|
|
|
161,787
|
|
|
|
26,772
|
|
|
|
35,169
|
|
|
|
74,443
|
|
|
Cash flows from/(used in) investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(36,693
|
)
|
|
|
(8,282
|
)
|
|
|
(48,123
|
)
|
|
|
(39,752
|
)
|
|
|
1,927
|
|
|
|
(130,923
|
)
|
|
Group companies and business units
|
|
|
(1,927
|
)
|
|
|
0
|
|
|
|
(632
|
)
|
|
|
0
|
|
|
|
1,927
|
|
|
|
(632
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(34,212
|
)
|
|
|
(8,250
|
)
|
|
|
(47,434
|
)
|
|
|
(39,672
|
)
|
|
|
0
|
|
|
|
(129,568
|
)
|
|
Other financial assets
|
|
|
(554
|
)
|
|
|
(32
|
)
|
|
|
(57
|
)
|
|
|
(80
|
)
|
|
|
0
|
|
|
|
(723
|
)
|
|
Proceeds from the sale of investments
|
|
|
1,740
|
|
|
|
0
|
|
|
|
(6
|
)
|
|
|
102
|
|
|
|
(1,679
|
)
|
|
|
157
|
|
|
Property, plant and equipment
|
|
|
61
|
|
|
|
0
|
|
|
|
(6
|
)
|
|
|
102
|
|
|
|
0
|
|
|
|
157
|
|
|
Other financial assets
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Associates
|
|
|
1,679
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,679
|
)
|
|
|
0
|
|
|
Net cash used in investing activities
|
|
|
(34,953
|
)
|
|
|
(8,282
|
)
|
|
|
(48,129
|
)
|
|
|
(39,650
|
)
|
|
|
248
|
|
|
|
(130,766
|
)
|
|
Cash flows from/(used in) financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(4,212
|
)
|
|
|
41,179
|
|
|
|
(1,619
|
)
|
|
|
1,926
|
|
|
|
(41,486
|
)
|
|
|
(4,212
|
)
|
|
Issue
|
|
|
0
|
|
|
|
41,179
|
|
|
|
(1,619
|
)
|
|
|
1,926
|
|
|
|
(41,486
|
)
|
|
|
0
|
|
|
Acquisition of own shares
|
|
|
(4,880
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(4,880
|
)
|
|
Disposal of treasury shares
|
|
|
668
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
668
|
|
|
Proceeds from and payments for financial liability instruments
|
|
|
114,565
|
|
|
|
76,084
|
|
|
|
(29,551
|
)
|
|
|
38,775
|
|
|
|
(103,524
|
)
|
|
|
96,349
|
|
|
Issue
|
|
|
306,325
|
|
|
|
27,058
|
|
|
|
21,262
|
|
|
|
39,223
|
|
|
|
241
|
|
|
|
394,109
|
|
|
Redemption and repayment
|
|
|
(250,641
|
)
|
|
|
0
|
|
|
|
(41,516
|
)
|
|
|
(5,603
|
)
|
|
|
0
|
|
|
|
(297,760
|
)
|
|
Debts with group companies
|
|
|
58,881
|
|
|
|
49,026
|
|
|
|
(9,297
|
)
|
|
|
5,155
|
|
|
|
(103,765
|
)
|
|
|
0
|
|
|
Dividends and interest on other equity instruments paid
|
|
|
(34,767
|
)
|
|
|
0
|
|
|
|
(81,473
|
)
|
|
|
(28,141
|
)
|
|
|
109,589
|
|
|
|
(34,792
|
)
|
|
Other cash flows from financing activities
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Other amounts received from financing activities
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Net cash from/(used in) financing activities
|
|
|
75,586
|
|
|
|
117,263
|
|
|
|
(112,643
|
)
|
|
|
12,560
|
|
|
|
(35,421
|
)
|
|
|
57,345
|
|
|
Effect of exchange rate fluctuations on cash
|
|
|
0
|
|
|
|
(18
|
)
|
|
|
(90
|
)
|
|
|
(236
|
)
|
|
|
0
|
|
|
|
(344
|
)
|
|
Net increase/(decrease) in cash and cash equivalents
|
|
|
1
|
|
|
|
310
|
|
|
|
925
|
|
|
|
(554
|
)
|
|
|
(4
|
)
|
|
|
678
|
|
|
Cash and cash equivalents at beginning of the year
|
|
|
72
|
|
|
|
(310
|
)
|
|
|
991
|
|
|
|
4,938
|
|
|
|
0
|
|
|
|
5,691
|
|
|
Cash and cash equivalents at end of year
|
|
|
73
|
|
|
|
0
|
|
|
|
1,916
|
|
|
|
4,384
|
|
|
|
(5
|
)
|
|
|
6,368
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-145
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statements of Cash Flows
for the
year ended 31 December 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from/(used in) operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
75,910
|
|
|
|
(4,786
|
)
|
|
|
190,766
|
|
|
|
34,788
|
|
|
|
(92,684
|
)
|
|
|
203,994
|
|
|
Adjustments for:
|
|
|
(64,395
|
)
|
|
|
6,960
|
|
|
|
34,101
|
|
|
|
15,848
|
|
|
|
69,287
|
|
|
|
61,800
|
|
|
Amortisation and depreciation
|
|
|
5,659
|
|
|
|
850
|
|
|
|
27,319
|
|
|
|
5,726
|
|
|
|
0
|
|
|
|
39,554
|
|
|
Other adjustments:
|
|
|
(70,054
|
)
|
|
|
6,110
|
|
|
|
6,782
|
|
|
|
10,122
|
|
|
|
69,287
|
|
|
|
22,246
|
|
|
(Profit) /losses on equity accounted investments
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(51
|
)
|
|
|
(51
|
)
|
|
Exchange differences
|
|
|
723
|
|
|
|
858
|
|
|
|
242
|
|
|
|
(90
|
)
|
|
|
(0
|
)
|
|
|
1,733
|
|
|
Impairment of assets and net provision charges
|
|
|
15
|
|
|
|
0
|
|
|
|
(378
|
)
|
|
|
857
|
|
|
|
(441
|
)
|
|
|
53
|
|
|
(Profits) / losses on disposal of fixed assets
|
|
|
148
|
|
|
|
2
|
|
|
|
843
|
|
|
|
154
|
|
|
|
0
|
|
|
|
1,147
|
|
|
Government grants taken to income
|
|
|
(431
|
)
|
|
|
0
|
|
|
|
(637
|
)
|
|
|
(120
|
)
|
|
|
0
|
|
|
|
(1,188
|
)
|
|
Net finance expense
|
|
|
(71,094
|
)
|
|
|
9,104
|
|
|
|
2,801
|
|
|
|
7,650
|
|
|
|
69,091
|
|
|
|
17,552
|
|
|
Other adjustments
|
|
|
585
|
|
|
|
(3,854
|
)
|
|
|
3,911
|
|
|
|
1,671
|
|
|
|
688
|
|
|
|
3,001
|
|
|
Change in operating assets and liabilities
|
|
|
64,484
|
|
|
|
(70,131
|
)
|
|
|
(42,265
|
)
|
|
|
(21,021
|
)
|
|
|
(35,195
|
)
|
|
|
(104,127
|
)
|
|
Change in inventories
|
|
|
31
|
|
|
|
0
|
|
|
|
(119,451
|
)
|
|
|
(14,745
|
)
|
|
|
21,061
|
|
|
|
(113,104
|
)
|
|
Change in trade and other receivables
|
|
|
14,276
|
|
|
|
(44,627
|
)
|
|
|
(71,598
|
)
|
|
|
(20,188
|
)
|
|
|
109,587
|
|
|
|
(12,549
|
)
|
|
Change in current financial assets and other current assets
|
|
|
57,822
|
|
|
|
(52,577
|
)
|
|
|
57,541
|
|
|
|
(6,128
|
)
|
|
|
(57,945
|
)
|
|
|
(1,287
|
)
|
|
Change in current trade and other payables
|
|
|
(7,645
|
)
|
|
|
27,073
|
|
|
|
91,243
|
|
|
|
20,040
|
|
|
|
(107,898
|
)
|
|
|
22,813
|
|
|
Other cash flows used in operating activities
|
|
|
51,198
|
|
|
|
(1,187
|
)
|
|
|
(39,676
|
)
|
|
|
(11,597
|
)
|
|
|
(72,225
|
)
|
|
|
(73,487
|
)
|
|
Interest paid
|
|
|
(9,889
|
)
|
|
|
(2,362
|
)
|
|
|
(2,265
|
)
|
|
|
(7,135
|
)
|
|
|
6,932
|
|
|
|
(14,719
|
)
|
|
Interest received
|
|
|
6,932
|
|
|
|
0
|
|
|
|
2,256
|
|
|
|
253
|
|
|
|
(6,932
|
)
|
|
|
2,509
|
|
|
Dividends received
|
|
|
72,226
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(72,226
|
)
|
|
|
0
|
|
|
Income tax paid
|
|
|
(18,070
|
)
|
|
|
1,175
|
|
|
|
(39,667
|
)
|
|
|
(4,715
|
)
|
|
|
0
|
|
|
|
(61,277
|
)
|
|
Net cash from operating activities
|
|
|
127,197
|
|
|
|
(69,144
|
)
|
|
|
142,926
|
|
|
|
18,018
|
|
|
|
(130,817
|
)
|
|
|
88,180
|
|
|
Cash flows from/(used in) investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(45,238
|
)
|
|
|
(6,092
|
)
|
|
|
(72,920
|
)
|
|
|
(19,379
|
)
|
|
|
7,003
|
|
|
|
(136,626
|
)
|
|
Group companies and business units
|
|
|
(32,497
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
10,109
|
|
|
|
7,003
|
|
|
|
(15,385
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(12,490
|
)
|
|
|
(4,081
|
)
|
|
|
(72,770
|
)
|
|
|
(29,429
|
)
|
|
|
(0
|
)
|
|
|
(118,770
|
)
|
|
Other financial assets
|
|
|
(251
|
)
|
|
|
(2,011
|
)
|
|
|
(150
|
)
|
|
|
(59
|
)
|
|
|
(0
|
)
|
|
|
(2,471
|
)
|
|
Proceeds from the sale of investments
|
|
|
39
|
|
|
|
(1
|
)
|
|
|
182
|
|
|
|
453
|
|
|
|
(0
|
)
|
|
|
673
|
|
|
Property, plant and equipment
|
|
|
39
|
|
|
|
(1
|
)
|
|
|
182
|
|
|
|
453
|
|
|
|
0
|
|
|
|
673
|
|
|
Other financial assets
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(0
|
)
|
|
|
0
|
|
|
Associates (note 2 (c))
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Net cash used in investing activities
|
|
|
(45,199
|
)
|
|
|
(6,093
|
)
|
|
|
(72,738
|
)
|
|
|
(18,926
|
)
|
|
|
7,003
|
|
|
|
(135,953
|
)
|
|
Cash flows from/(used in) financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
26,730
|
|
|
|
0
|
|
|
|
6,923
|
|
|
|
60
|
|
|
|
(7,058
|
)
|
|
|
26,655
|
|
|
Issue
|
|
|
0
|
|
|
|
0
|
|
|
|
6,923
|
|
|
|
60
|
|
|
|
(7,059
|
)
|
|
|
(76
|
)
|
|
Acquisition of own shares
|
|
|
(25,186
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(25,186
|
)
|
|
Disposal of treasury shares
|
|
|
51,917
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
51,917
|
|
|
Proceeds from and payments for financial liability instruments
|
|
|
(28,061
|
)
|
|
|
318,419
|
|
|
|
(14,808
|
)
|
|
|
10,136
|
|
|
|
58,727
|
|
|
|
344,413
|
|
|
Issue
|
|
|
78,933
|
|
|
|
406,807
|
|
|
|
22,614
|
|
|
|
16,724
|
|
|
|
(0
|
)
|
|
|
525,078
|
|
|
Redemption and repayment
|
|
|
(95,331
|
)
|
|
|
(39,510
|
)
|
|
|
(34,533
|
)
|
|
|
(11,291
|
)
|
|
|
0
|
|
|
|
(180,665
|
)
|
|
Debts with group companies
|
|
|
(11,662
|
)
|
|
|
(48,878
|
)
|
|
|
(2,889
|
)
|
|
|
4,703
|
|
|
|
58,726
|
|
|
|
0
|
|
|
Dividends and interest on other equity instruments paid
|
|
|
(80,651
|
)
|
|
|
(5,552
|
)
|
|
|
(57,404
|
)
|
|
|
(9,456
|
)
|
|
|
72,150
|
|
|
|
(80,913
|
)
|
|
Other cash flows from financing activities
|
|
|
54
|
|
|
|
174
|
|
|
|
431
|
|
|
|
82
|
|
|
|
0
|
|
|
|
741
|
|
|
Other amounts received from financing activities
|
|
|
54
|
|
|
|
174
|
|
|
|
431
|
|
|
|
82
|
|
|
|
0
|
|
|
|
741
|
|
|
Net cash from/(used in) financing activities
|
|
|
(81,928
|
)
|
|
|
313,041
|
|
|
|
(64,858
|
)
|
|
|
822
|
|
|
|
123,818
|
|
|
|
290,896
|
|
|
Effect of exchange rate fluctuations on cash
|
|
|
0
|
|
|
|
0
|
|
|
|
(54
|
)
|
|
|
(65
|
)
|
|
|
0
|
|
|
|
(119
|
)
|
|
Net increase / (decrease) in cash and cash equivalents
|
|
|
71
|
|
|
|
237,804
|
|
|
|
5,276
|
|
|
|
(151
|
)
|
|
|
4
|
|
|
|
243,004
|
|
|
Cash and cash equivalents at beginning of the year
|
|
|
73
|
|
|
|
0
|
|
|
|
1,915
|
|
|
|
4,384
|
|
|
|
(4
|
)
|
|
|
6,368
|
|
|
Cash and cash equivalents at end of year
|
|
|
144
|
|
|
|
237,804
|
|
|
|
7,191
|
|
|
|
4,233
|
|
|
|
0
|
|
|
|
249,372
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-146
APPENDIX V
GRIFOLS,
S.A. AND SUBSIDIARIES
Condensed
Consolidated Statements of Cash Flows
for the
year ended 31 December 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Parent
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
(Expressed in thousands of Euros)
|
|
|
|
|
Cash flows from/(used in) operating activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Profit before income tax
|
|
|
57,190
|
|
|
|
(17,235
|
)
|
|
|
158,035
|
|
|
|
31,247
|
|
|
|
(71,453
|
)
|
|
|
157,784
|
|
|
Adjustments for:
|
|
|
(59,557
|
)
|
|
|
49,580
|
|
|
|
23,077
|
|
|
|
6,508
|
|
|
|
72,743
|
|
|
|
92,351
|
|
|
Amortisation and depreciation
|
|
|
7,384
|
|
|
|
992
|
|
|
|
28,623
|
|
|
|
8,222
|
|
|
|
555
|
|
|
|
45,776
|
|
|
Other adjustments:
|
|
|
(66,941
|
)
|
|
|
48,588
|
|
|
|
(5,546
|
)
|
|
|
(1,714
|
)
|
|
|
72,188
|
|
|
|
46,575
|
|
|
(Profit)/losses on equity accounted investments
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
879
|
|
|
|
879
|
|
|
Exchange differences
|
|
|
(75
|
)
|
|
|
455
|
|
|
|
389
|
|
|
|
(2,385
|
)
|
|
|
0
|
|
|
|
(1,616
|
)
|
|
Impairment of assets and net provision charges
|
|
|
(1,097
|
)
|
|
|
0
|
|
|
|
(160
|
)
|
|
|
518
|
|
|
|
1,652
|
|
|
|
913
|
|
|
(Profits)/losses on disposal of fixed assets
|
|
|
2
|
|
|
|
139
|
|
|
|
756
|
|
|
|
578
|
|
|
|
(1,751
|
)
|
|
|
(276
|
)
|
|
Government grants taken to income
|
|
|
(259
|
)
|
|
|
0
|
|
|
|
(470
|
)
|
|
|
1
|
|
|
|
0
|
|
|
|
(728
|
)
|
|
Net finance expense
|
|
|
(65,578
|
)
|
|
|
30,875
|
|
|
|
2,513
|
|
|
|
2,731
|
|
|
|
76,901
|
|
|
|
47,442
|
|
|
Other adjustments
|
|
|
66
|
|
|
|
17,119
|
|
|
|
(8,574
|
)
|
|
|
(3,157
|
)
|
|
|
(5,493
|
)
|
|
|
(39
|
)
|
|
Change in operating assets and liabilities
|
|
|
2,233
|
|
|
|
145,612
|
|
|
|
(199,272
|
)
|
|
|
7,902
|
|
|
|
(35,242
|
)
|
|
|
(78,767
|
)
|
|
Change in inventories
|
|
|
(92
|
)
|
|
|
0
|
|
|
|
(23,919
|
)
|
|
|
8,854
|
|
|
|
(3,149
|
)
|
|
|
(18,306
|
)
|
|
Change in trade and other receivables
|
|
|
4,915
|
|
|
|
170,362
|
|
|
|
54,428
|
|
|
|
13,110
|
|
|
|
(266,361
|
)
|
|
|
(23,546
|
)
|
|
Change in current financial assets and other current assets
|
|
|
(20,490
|
)
|
|
|
(13,649
|
)
|
|
|
(2,979
|
)
|
|
|
(1,263
|
)
|
|
|
(34,641
|
)
|
|
|
(73,022
|
)
|
|
Change in current trade and other payables
|
|
|
17,900
|
|
|
|
(11,101
|
)
|
|
|
(226,802
|
)
|
|
|
(12,799
|
)
|
|
|
268,909
|
|
|
|
36,107
|
|
|
Other cash flows used in operating activities
|
|
|
77,492
|
|
|
|
(26,404
|
)
|
|
|
(28,009
|
)
|
|
|
(13,704
|
)
|
|
|
(76,491
|
)
|
|
|
(67,116
|
)
|
|
Interest paid
|
|
|
(5,891
|
)
|
|
|
(31,361
|
)
|
|
|
(4,404
|
)
|
|
|
(2,525
|
)
|
|
|
4,052
|
|
|
|
(40,129
|
)
|
|
Interest received
|
|
|
4,052
|
|
|
|
1,110
|
|
|
|
4,326
|
|
|
|
0
|
|
|
|
(4,052
|
)
|
|
|
5,436
|
|
|
Dividends received
|
|
|
76,491
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(76,491
|
)
|
|
|
0
|
|
|
Income tax paid
|
|
|
2,840
|
|
|
|
3,847
|
|
|
|
(27,931
|
)
|
|
|
(11,179
|
)
|
|
|
0
|
|
|
|
(32,423
|
)
|
|
Net cash from operating activities
|
|
|
77,358
|
|
|
|
151,553
|
|
|
|
(46,169
|
)
|
|
|
31,953
|
|
|
|
(110,443
|
)
|
|
|
104,252
|
|
|
Cash flows from/(used in) investing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Payments for investments
|
|
|
(7,854
|
)
|
|
|
(16,042
|
)
|
|
|
(67,863
|
)
|
|
|
(19,020
|
)
|
|
|
2,191
|
|
|
|
(108,588
|
)
|
|
Group companies and business units
|
|
|
(8
|
)
|
|
|
0
|
|
|
|
(884
|
)
|
|
|
(1,523
|
)
|
|
|
941
|
|
|
|
(1,474
|
)
|
|
Property, plant and equipment and intangible assets
|
|
|
(7,788
|
)
|
|
|
(12,405
|
)
|
|
|
(67,006
|
)
|
|
|
(17,453
|
)
|
|
|
1,250
|
|
|
|
(103,402
|
)
|
|
Other financial assets
|
|
|
(58
|
)
|
|
|
(3,637
|
)
|
|
|
27
|
|
|
|
(44
|
)
|
|
|
0
|
|
|
|
(3,712
|
)
|
|
Proceeds from the sale of investments
|
|
|
109
|
|
|
|
946
|
|
|
|
(2,169
|
)
|
|
|
5,555
|
|
|
|
91
|
|
|
|
4,532
|
|
|
Property, plant and equipment
|
|
|
109
|
|
|
|
946
|
|
|
|
(1,289
|
)
|
|
|
4,054
|
|
|
|
91
|
|
|
|
3,911
|
|
|
Other financial assets
|
|
|
0
|
|
|
|
0
|
|
|
|
(880
|
)
|
|
|
1,501
|
|
|
|
0
|
|
|
|
621
|
|
|
Associates
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Net cash used in investing activities
|
|
|
(7,745
|
)
|
|
|
(15,096
|
)
|
|
|
(70,032
|
)
|
|
|
(13,465
|
)
|
|
|
2,282
|
|
|
|
(104,056
|
)
|
|
Cash flows from/(used in) financing activities
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from and payments for equity instruments
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
890
|
|
|
|
(890
|
)
|
|
|
(1,250
|
)
|
|
Issue
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
890
|
|
|
|
(890
|
)
|
|
|
0
|
|
|
Acquisition of own shares
|
|
|
(1,250
|
)
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
(1,250
|
)
|
|
Disposal of treasury shares
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
|
0
|
|
|
Proceeds from and payments for financial liability instruments
|
|
|
(41,495
|
)
|
|
|
(165,388
|
)
|
|
|
165,287
|
|
|
|
8,437
|
|
|
|
32,093
|
|
|
|
(1,066
|
)
|
|
Issue
|
|
|
63,226
|
|
|
|
(2,616
|
)
|
|
|
30,546
|
|
|
|
27,082
|
|
|
|
0
|
|
|
|
118,238
|
|
|
Redemption and repayment
|
|
|
(99,505
|
)
|
|
|
0
|
|
|
|
(16,672
|
)
|
|
|
(3,127
|
)
|
|
|
0
|
|
|
|
(119,304
|
)
|
|
Debts with group companies
|
|
|
(5,216
|
)
|
|
|
(162,772
|
)
|
|
|
151,413
|
|
|
|
(15,518
|
)
|
|
|
32,093
|
|
|
|
0
|
|
|
Dividends and interest on other equity instruments paid
|
|
|
(27,229
|
)
|
|
|
0
|
|
|
|
(55,298
|
)
|
|
|
(21,713
|
)
|
|
|
76,958
|
|
|
|
(27,282
|
)
|
|
Other cash flows from financing activities
|
|
|
242
|
|
|
|
0
|
|
|
|
56
|
|
|
|
25
|
|
|
|
0
|
|
|
|
323
|
|
|
Other amounts received from financing activities
|
|
|
242
|
|
|
|
0
|
|
|
|
81
|
|
|
|
0
|
|
|
|
0
|
|
|
|
323
|
|
|
Net cash from/(used in) financing activities
|
|
|
(69,732
|
)
|
|
|
(165,388
|
)
|
|
|
110,045
|
|
|
|
(12,361
|
)
|
|
|
108,161
|
|
|
|
(29,275
|
)
|
|
Effect of exchange rate fluctuations on cash
|
|
|
0
|
|
|
|
18,583
|
|
|
|
409
|
|
|
|
364
|
|
|
|
0
|
|
|
|
19,356
|
|
|
Net increase/(decrease) in cash and cash equivalents
|
|
|
(119
|
)
|
|
|
(10,348
|
)
|
|
|
(5,747
|
)
|
|
|
6,491
|
|
|
|
0
|
|
|
|
(9,723
|
)
|
|
Cash and cash equivalents at beginning of the year
|
|
|
144
|
|
|
|
237,804
|
|
|
|
7,191
|
|
|
|
4,233
|
|
|
|
0
|
|
|
|
249,372
|
|
|
Cash and cash equivalents at end of year
|
|
|
25
|
|
|
|
227,456
|
|
|
|
1,444
|
|
|
|
10,724
|
|
|
|
0
|
|
|
|
239,649
|
|
The accompanying note forms an integral part of the consolidated
financial statements
F-147
REPORT OF
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Stockholders and Board of Directors of
Talecris Biotherapeutics Holdings Corp:
In our opinion, the consolidated financial statements listed in
the accompanying index present fairly, in all material respects,
the financial position of Talecris Biotherapeutics Holdings
Corp. and its subsidiaries at December 31, 2010 and 2009,
and the results of their operations and their cash flows for
each of the three years in the period ended December 31,
2010 in conformity with accounting principles generally accepted
in the United States of America. In addition, in our opinion,
the financial statement schedule listed in the accompanying
index presents fairly, in all material respects, the information
set forth therein when read in conjunction with the related
consolidated financial statements. These financial statements
and financial statement schedule are the responsibility of the
Company’s management. Our responsibility is to express an
opinion on these financial statements and financial statement
schedule based on our audits. We conducted our audits in
accordance with the standards of the Public Company Accounting
Oversight Board (United States). Those standards require that we
plan and perform the audits to obtain reasonable assurance about
whether the financial statements are free of material
misstatement. An audit includes examining, on a test basis,
evidence supporting the amounts and disclosures in the financial
statements, assessing the accounting principles used and
significant estimates made by management, and evaluating the
overall financial statement presentation. We believe that our
audits provide a reasonable basis for our opinion.
/s/ PricewaterhouseCoopers
LLP
PricewaterhouseCoopers LLP
Raleigh, North Carolina
February 23, 2011
F-148
Talecris
Biotherapeutics Holdings Corp.
Consolidated
Balance Sheets
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
(In thousands, except share and per share amounts)
|
|
|
|
|
ASSETS
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
197,876
|
|
|
$
|
65,239
|
|
|
Accounts receivable, net of allowances of $3,253 and $3,461,
respectively
|
|
|
134,842
|
|
|
|
136,978
|
|
|
Inventories
|
|
|
694,499
|
|
|
|
644,054
|
|
|
Deferred income taxes
|
|
|
96,593
|
|
|
|
88,652
|
|
|
Prepaid expenses and other
|
|
|
29,662
|
|
|
|
31,466
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
1,153,472
|
|
|
|
966,389
|
|
|
Property, plant, and equipment, net
|
|
|
382,793
|
|
|
|
267,199
|
|
|
Investment in affiliate
|
|
|
2,926
|
|
|
|
1,935
|
|
|
Intangible assets
|
|
|
10,880
|
|
|
|
10,880
|
|
|
Goodwill
|
|
|
172,860
|
|
|
|
172,860
|
|
|
Deferred income taxes
|
|
|
—
|
|
|
|
5,848
|
|
|
Other
|
|
|
15,522
|
|
|
|
19,894
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
1,738,453
|
|
|
$
|
1,445,005
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
59,975
|
|
|
$
|
71,046
|
|
|
Accrued expenses and other liabilities
|
|
|
251,726
|
|
|
|
170,533
|
|
|
Current portion of capital lease obligations
|
|
|
860
|
|
|
|
740
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
312,561
|
|
|
|
242,319
|
|
|
Long-term debt and capital lease obligations
|
|
|
605,301
|
|
|
|
605,267
|
|
|
Deferred income taxes
|
|
|
14,432
|
|
|
|
—
|
|
|
Other
|
|
|
11,795
|
|
|
|
15,265
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
944,089
|
|
|
|
862,851
|
|
|
Commitments and contingencies (Note 14)
|
|
|
|
|
|
|
|
|
|
Stockholders’ equity:
|
|
|
|
|
|
|
|
|
|
Common stock, $0.01 par value; 400,000,000 shares
authorized; 125,577,877 and 122,173,274 shares issued and
outstanding, respectively
|
|
|
1,253
|
|
|
|
1,212
|
|
|
Additional paid-in capital
|
|
|
813,783
|
|
|
|
767,032
|
|
|
Accumulated deficit
|
|
|
(20,378
|
)
|
|
|
(186,446
|
)
|
|
Accumulated other comprehensive (loss) income, net of tax
|
|
|
(294
|
)
|
|
|
356
|
|
|
|
|
|
|
|
|
|
|
|
|
Total stockholders’ equity
|
|
|
794,364
|
|
|
|
582,154
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity
|
|
$
|
1,738,453
|
|
|
$
|
1,445,005
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these consolidated financial statements.
F-149
Talecris
Biotherapeutics Holdings Corp.
Consolidated
Income Statements
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands, except per share amounts)
|
|
|
|
|
Net revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Product
|
|
$
|
1,576,936
|
|
|
$
|
1,507,754
|
|
|
$
|
1,334,550
|
|
|
Other
|
|
|
24,683
|
|
|
|
25,455
|
|
|
|
39,742
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
1,601,619
|
|
|
|
1,533,209
|
|
|
|
1,374,292
|
|
|
Cost of goods sold
|
|
|
911,976
|
|
|
|
901,077
|
|
|
|
882,157
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
689,643
|
|
|
|
632,132
|
|
|
|
492,135
|
|
|
Operating expenses:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling, general, and administrative
|
|
|
287,011
|
|
|
|
289,929
|
|
|
|
227,524
|
|
|
Research and development
|
|
|
69,649
|
|
|
|
71,223
|
|
|
|
66,006
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
356,660
|
|
|
|
361,152
|
|
|
|
293,530
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
332,983
|
|
|
|
270,980
|
|
|
|
198,605
|
|
|
Other non-operating (expense) income
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Interest expense, net
|
|
|
(45,837
|
)
|
|
|
(74,491
|
)
|
|
|
(96,640
|
)
|
|
PCA judgment
|
|
|
(43,690
|
)
|
|
|
—
|
|
|
|
—
|
|
|
CSL merger termination fee
|
|
|
—
|
|
|
|
75,000
|
|
|
|
—
|
|
|
Loss on extinguishment of debt
|
|
|
—
|
|
|
|
(43,033
|
)
|
|
|
—
|
|
|
Equity in earnings of affiliate
|
|
|
991
|
|
|
|
441
|
|
|
|
426
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
|
(88,536
|
)
|
|
|
(42,083
|
)
|
|
|
(96,214
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
244,447
|
|
|
|
228,897
|
|
|
|
102,391
|
|
|
Provision for income taxes
|
|
|
(78,379
|
)
|
|
|
(75,008
|
)
|
|
|
(36,594
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
|
166,068
|
|
|
|
153,889
|
|
|
|
65,797
|
|
|
Less dividends to preferred stockholders and other non-common
stockholders’ charges
|
|
|
—
|
|
|
|
(11,744
|
)
|
|
|
(14,672
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income available to common stockholders
|
|
$
|
166,068
|
|
|
$
|
142,145
|
|
|
$
|
51,125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income per common share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
1.35
|
|
|
$
|
4.56
|
|
|
$
|
39.01
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted
|
|
$
|
1.29
|
|
|
$
|
1.50
|
|
|
$
|
0.71
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these consolidated financial statements.
F-150
Talecris
Biotherapeutics Holdings Corp.
Consolidated
Statements of Cash Flows
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
(In thousands)
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
166,068
|
|
|
$
|
153,889
|
|
|
$
|
65,797
|
|
|
Adjustments to reconcile net income to net cash provided by
operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation and amortization
|
|
|
36,030
|
|
|
|
28,936
|
|
|
|
20,269
|
|
|
Amortization of deferred loan fees and debt discount
|
|
|
4,262
|
|
|
|
3,785
|
|
|
|
3,764
|
|
|
Share-based compensation expense
|
|
|
16,966
|
|
|
|
47,546
|
|
|
|
38,707
|
|
|
Amortization of deferred compensation
|
|
|
1,983
|
|
|
|
5,714
|
|
|
|
5,922
|
|
|
Write-off of unamortized debt issuance costs
|
|
|
—
|
|
|
|
12,141
|
|
|
|
—
|
|
|
Asset impairment
|
|
|
595
|
|
|
|
3,061
|
|
|
|
4,282
|
|
|
Provision for doubtful receivables and advances
|
|
|
3,519
|
|
|
|
2,858
|
|
|
|
4,978
|
|
|
Recognition of previously deferred revenue
|
|
|
(230
|
)
|
|
|
(230
|
)
|
|
|
(4,784
|
)
|
|
Equity in earnings of affiliate
|
|
|
(991
|
)
|
|
|
(441
|
)
|
|
|
(426
|
)
|
|
Loss on disposal of property, plant, and equipment
|
|
|
896
|
|
|
|
1,196
|
|
|
|
48
|
|
|
Decrease (increase) in deferred tax assets
|
|
|
12,339
|
|
|
|
1,215
|
|
|
|
(5,488
|
)
|
|
Excess tax benefits from share-based payment arrangements
|
|
|
(13,481
|
)
|
|
|
(13,406
|
)
|
|
|
—
|
|
|
Changes in assets and liabilities, excluding the effects of
business acquisitions
|
|
|
27,526
|
|
|
|
(12,109
|
)
|
|
|
(100,055
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by operating activities
|
|
|
255,482
|
|
|
|
234,155
|
|
|
|
33,014
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant, and equipment
|
|
|
(152,849
|
)
|
|
|
(75,163
|
)
|
|
|
(86,212
|
)
|
|
Business acquisitions, net of cash acquired
|
|
|
—
|
|
|
|
(30,431
|
)
|
|
|
(10,272
|
)
|
|
Financing arrangements with third party suppliers, net of
repayments
|
|
|
—
|
|
|
|
—
|
|
|
|
(16,335
|
)
|
|
Other
|
|
|
765
|
|
|
|
976
|
|
|
|
880
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash used in investing activities
|
|
|
(152,084
|
)
|
|
|
(104,618
|
)
|
|
|
(111,939
|
)
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Borrowings under revolving credit facility
|
|
|
915
|
|
|
|
1,201,749
|
|
|
|
1,430,092
|
|
|
Repayments of borrowings under revolving credit facility
|
|
|
(915
|
)
|
|
|
(1,381,690
|
)
|
|
|
(1,363,188
|
)
|
|
Repayments of borrowings under term loans
|
|
|
—
|
|
|
|
(1,016,000
|
)
|
|
|
(7,000
|
)
|
|
Repayments of capital lease obligations
|
|
|
(751
|
)
|
|
|
(574
|
)
|
|
|
(1,192
|
)
|
|
Proceeds from issuance of 7.75% Notes
|
|
|
—
|
|
|
|
600,000
|
|
|
|
—
|
|
|
Discount on 7.75% Notes
|
|
|
—
|
|
|
|
(4,074
|
)
|
|
|
—
|
|
|
Financing transaction costs
|
|
|
(394
|
)
|
|
|
(14,879
|
)
|
|
|
—
|
|
|
Proceeds from initial public offering, net of issuance costs
|
|
|
—
|
|
|
|
519,749
|
|
|
|
—
|
|
|
Costs related to initial public offering
|
|
|
—
|
|
|
|
(2,557
|
)
|
|
|
—
|
|
|
Repurchases of common stock
|
|
|
(4,917
|
)
|
|
|
(4,183
|
)
|
|
|
(36,118
|
)
|
|
Proceeds from exercises of stock options
|
|
|
22,333
|
|
|
|
7,581
|
|
|
|
—
|
|
|
Excess tax benefits from share-based payment arrangements
|
|
|
13,481
|
|
|
|
13,406
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
|
29,752
|
|
|
|
(81,472
|
)
|
|
|
22,594
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
(513
|
)
|
|
|
195
|
|
|
|
(157
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase (decrease) in cash and cash equivalents
|
|
|
132,637
|
|
|
|
48,260
|
|
|
|
(56,488
|
)
|
|
Cash and cash equivalents at beginning of year
|
|
|
65,239
|
|
|
|
16,979
|
|
|
|
73,467
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of year
|
|
$
|
197,876
|
|
|
$
|
65,239
|
|
|
$
|
16,979
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these consolidated financial statements.
F-151
Talecris
Biotherapeutics Holdings Corp.
Consolidated
Statements of Stockholders’ Equity (Deficit)
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Additional
|
|
|
|
|
|
Other
|
|
|
|
|
|
|
|
Common Stock
|
|
|
Paid-in
|
|
|
Accumulated
|
|
|
Comprehensive
|
|
|
|
|
|
|
|
Shares
|
|
|
Amount
|
|
|
Capital
|
|
|
Deficit
|
|
|
Income (Loss)
|
|
|
Total
|
|
|
|
|
(In thousands, except share amounts)
|
|
|
|
|
Balance at December 31, 2007
|
|
|
5,317,232
|
|
|
$
|
—
|
|
|
$
|
27,010
|
|
|
$
|
(406,132
|
)
|
|
$
|
(11,635
|
)
|
|
$
|
(390,757
|
)
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
65,797
|
|
|
|
—
|
|
|
|
65,797
|
|
|
Other comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(11,772
|
)
|
|
|
(11,772
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
54,025
|
|
|
Share-based compensation cost
|
|
|
—
|
|
|
|
—
|
|
|
|
29,258
|
|
|
|
—
|
|
|
|
—
|
|
|
|
29,258
|
|
|
Issuance of restricted stock
|
|
|
42,720
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Forfeitures of restricted stock
|
|
|
(287,784
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Repurchases and retirement of common stock
|
|
|
(2,215,880
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Fair value adjustment on common stock with put/call feature
|
|
|
—
|
|
|
|
—
|
|
|
|
(8,942
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(8,942
|
)
|
|
Interest accretion on IBR put option
|
|
|
—
|
|
|
|
—
|
|
|
|
(309
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(309
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2008
|
|
|
2,856,288
|
|
|
|
—
|
|
|
|
47,017
|
|
|
|
(340,335
|
)
|
|
|
(23,407
|
)
|
|
|
(316,725
|
)
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
153,889
|
|
|
|
—
|
|
|
|
153,889
|
|
|
Other comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
476
|
|
|
|
476
|
|
|
Reclassification of unrealized loss on derivatives to earnings
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
23,287
|
|
|
|
23,287
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
177,652
|
|
|
Share-based compensation cost
|
|
|
—
|
|
|
|
—
|
|
|
|
39,206
|
|
|
|
—
|
|
|
|
—
|
|
|
|
39,206
|
|
|
Issuance of restricted stock
|
|
|
14,464
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Forfeitures of restricted stock
|
|
|
(16,368
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Repurchases and retirement of common stock
|
|
|
(251,108
|
)
|
|
|
—
|
|
|
|
(51
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(51
|
)
|
|
Series A and B preferred stock dividends declared
|
|
|
—
|
|
|
|
—
|
|
|
|
(45,250
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(45,250
|
)
|
|
Conversion of Series A and B preferred stock to common stock
|
|
|
88,227,868
|
|
|
|
882
|
|
|
|
154,903
|
|
|
|
—
|
|
|
|
—
|
|
|
|
155,785
|
|
|
Initial public offering
|
|
|
28,947,368
|
|
|
|
289
|
|
|
|
519,460
|
|
|
|
—
|
|
|
|
—
|
|
|
|
519,749
|
|
|
Costs related to initial public offering
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,557
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(2,557
|
)
|
|
Fair value adjustment on common stock with put/call feature
|
|
|
—
|
|
|
|
—
|
|
|
|
(6,585
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(6,585
|
)
|
|
Reclassification of mezzanine equity to permanent equity upon
cancellation of common stock put/call feature
|
|
|
—
|
|
|
|
17
|
|
|
|
39,926
|
|
|
|
—
|
|
|
|
—
|
|
|
|
39,943
|
|
|
Stock option exercises
|
|
|
2,394,762
|
|
|
|
24
|
|
|
|
7,557
|
|
|
|
—
|
|
|
|
—
|
|
|
|
7,581
|
|
|
Excess tax benefit from share-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
13,406
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,406
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
|
122,173,274
|
|
|
|
1,212
|
|
|
|
767,032
|
|
|
|
(186,446
|
)
|
|
|
356
|
|
|
|
582,154
|
|
|
Net income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
166,068
|
|
|
|
—
|
|
|
|
166,068
|
|
|
Other comprehensive loss
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(650
|
)
|
|
|
(650
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Comprehensive income
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
165,418
|
|
|
Share-based compensation cost
|
|
|
—
|
|
|
|
—
|
|
|
|
15,895
|
|
|
|
—
|
|
|
|
—
|
|
|
|
15,895
|
|
|
Repurchases and retirement of common stock
|
|
|
(246,823
|
)
|
|
|
(3
|
)
|
|
|
(4,914
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,917
|
)
|
|
Stock option exercises
|
|
|
3,650,579
|
|
|
|
37
|
|
|
|
22,296
|
|
|
|
—
|
|
|
|
—
|
|
|
|
22,333
|
|
|
Excess tax benefit from share-based compensation
|
|
|
—
|
|
|
|
—
|
|
|
|
13,481
|
|
|
|
—
|
|
|
|
—
|
|
|
|
13,481
|
|
|
Shares issued upon RSU vesting
|
|
|
847
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
Vesting of restricted common stock
|
|
|
—
|
|
|
|
7
|
|
|
|
(7
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at December 31, 2010
|
|
|
125,577,877
|
|
|
$
|
1,253
|
|
|
$
|
813,783
|
|
|
$
|
(20,378
|
)
|
|
$
|
(294
|
)
|
|
$
|
794,364
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The accompanying Notes to Consolidated Financial Statements are
an integral part of these consolidated financial statements.
F-152
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial Statements
|
|
|
1.
|
Description
of Business
|
We are a biopharmaceutical company that researches, develops,
manufactures, markets, and sells protein-based therapies that
extend and enhance the lives of individuals who suffer from
chronic and acute, often life-threatening, conditions, such as
primary immune deficiencies, chronic inflammatory demyelinating
polyneuropathy (CIDP), alpha-1 antitrypsin deficiency-related
emphysema, bleeding disorders, infectious diseases, and severe
trauma. Our primary products have orphan drug designation to
serve populations with rare, chronic diseases. Our products are
derived from human plasma, the liquid component of blood, which
is sourced from our plasma collection centers or purchased from
third parties, located in the United States. Plasma contains
many therapeutic proteins, which we extract through the process
of fractionation at our Clayton, North Carolina and Melville,
New York facilities. The fractionated intermediates are
then purified, formulated into final bulk, and aseptically
filled into final containers for sale. We also sell the
fractionated intermediate products.
The majority of our sales are concentrated in two key
therapeutic areas of the plasma business: Immunology/Neurology,
through our intravenous immune globulin (IGIV) product for the
treatment of primary immune deficiency and autoimmune diseases,
such as CIDP, and Pulmonology, through our alpha-1 proteinase
inhibitor (A1PI) product for the treatment of alpha-1
antitrypsin deficiency-related emphysema. These therapeutic
areas are served by our products, Gamunex, Immune Globulin
Intravenous (Human), 10% Caprylate/Chromatography Purified
(Gamunex, Gamunex IGIV) and Prolastin Alpha-1 Proteinase
Inhibitor (Human) (Prolastin, Prolastin A1PI, Prolastin-C A1PI
). In March 2010, we launched Prolastin-C A1PI, our next
generation A1PI product, in the United States, and in the third
quarter of 2010, we launched Prolastin-C A1PI in Canada. As of
December 31, 2010, we have completed the conversion of our
existing U.S. and Canadian Prolastin patients to
Prolastin-C A1PI. During 2010, Gamunex-C was approved for the
subcutaneous route of administration for the PI indication in
Canada and the U.S. Sales of Gamunex-C/Gamunex IGIV and
Prolastin/Prolastin-C A1PI together comprised 76.4%, 74.7%, and
72.3% of our net revenue for the years ended December 31,
2010, 2009, and 2008, respectively. We also have a line of
hyperimmune therapies that provides treatment for tetanus,
rabies, hepatitis A, hepatitis B, and Rh factor control during
pregnancy and at birth. In addition, we provide plasma-derived
therapies for critical care/hemostasis, including the treatment
of hemophilia, an anti-coagulation factor (Thrombate III), as
well as albumin to expand blood volume. We sell our products
worldwide, but 81% of our sales were in the United States and
Canada in 2010.
We are headquartered in Research Triangle Park, North Carolina
and our primary manufacturing facilities are a short distance
away in Clayton, North Carolina. Our Clayton site is one of the
world’s largest plasma protein processing facilities whose
operations include fractionation, purification, filling, and
finishing. We have an integrated plasma collection center
platform, which as of December 31, 2010, consisted of
69 operating centers, of which 67 were FDA licensed and two
were unlicensed. In addition to the United States, we have
operations in Germany and Canada to support our international
sales and marketing activities.
On October 6, 2009, we completed our initial public
offering (IPO), which resulted in net proceeds to us of
$519.7 million. In addition, during October 2009, we
amended our revolving credit facility and completed the issuance
of $600.0 million, 7.75% Unsecured Senior Notes, due
November 15, 2016, at a price of 99.321% of par, in a
private placement to certain qualified institutional buyers. The
issuance of the 7.75% Notes resulted in net proceeds to us
of $583.9 million. Proceeds from these transactions were
used to repay and terminate our then existing First and Second
Lien Term Loans, settle and terminate certain interest rate swap
contracts, and repay amounts outstanding under our revolving
credit facility. On July 19, 2010, we exchanged all of our
then existing 7.75% Senior Notes due 2016 for
7.75% Senior Notes due 2016 that have been registered under
the Securities Act of 1933, as amended. Additional information
regarding our IPO and refinancing transactions are included in
Note 4, “Initial Public Offering and Use of
Proceeds,” and Note 12, “Long-Term Debt and
Capital Lease Obligations,” respectively.
F-153
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Until January 21, 2010, a majority of our outstanding
common stock was owned by Talecris Holdings, LLC. Talecris
Holdings, LLC is owned by (i) Cerberus-Plasma Holdings LLC,
the managing member of which is Cerberus Partners, L.P., and
(ii) limited partnerships affiliated with Ampersand
Ventures. Substantially all rights of management and control of
Talecris Holdings, LLC are held by Cerberus-Plasma Holdings LLC.
As of December 31, 2010, Talecris Holdings, LLC owned
approximately 48.7% of our outstanding common stock.
As discussed in Note 3, we entered into a definitive merger
agreement with Grifols S.A. and Grifols, Inc. (Grifols) on
June 6, 2010.
|
|
|
2.
|
Summary
of Significant Accounting Policies
|
Throughout our consolidated financial statements, references to
“Talecris Biotherapeutics Holdings Corp.,”
“Talecris,” “the Company,” “we,”
“us,” and “our” are references to Talecris
Biotherapeutics Holdings Corp. and its wholly-owned subsidiaries.
All tabular disclosures of dollar amounts are presented in
thousands. All share and per share amounts are presented at
their actual amounts.
A
seven-for-one
share dividend on our common stock was paid on
September 10, 2009. All share and per-share amounts have
been retroactively adjusted for all periods presented to reflect
the share dividend.
Principles
of Consolidation
The accompanying consolidated financial statements include the
accounts of Talecris Biotherapeutics Holdings Corp. and its
wholly-owned subsidiaries. All significant intercompany
transactions and balances have been eliminated upon
consolidation.
Use of
Estimates
The preparation of financial statements in conformity with
U.S. generally accepted accounting principles
(U.S. GAAP) requires us to make estimates and judgments in
certain circumstances that affect the reported amounts of
assets, liabilities, revenues and expenses, and the related
disclosures of contingent assets and liabilities. The most
significant judgments we have made include, but are not limited
to, estimates used in determining values of inventories,
allowances for doubtful accounts and notes receivable,
long-lived and indefinite-lived assets, litigation accruals and
related settlements, losses under contractual obligations,
leasehold impairments, deferred income taxes, income tax
provisions, accruals for uncertain income tax positions,
self-insurance accruals, share-based payment transactions,
derivative instruments, and other operating allowances and
accruals. We also use significant judgments in applying purchase
accounting to business acquisitions.
We periodically evaluate estimates used in the preparation of
the financial statements for reasonableness, including estimates
provided by third parties. Appropriate adjustments to the
estimates are made prospectively, as necessary, based on such
periodic evaluations. We base our estimates on, among other
things, currently available information, market conditions, and
industry and historical experience, which collectively form the
basis of making judgments about the carrying values of assets
and liabilities that are not readily apparent from other
sources. Although we believe that our assumptions are reasonable
under the circumstances, actual future results could differ
materially. In addition, if we had used different estimates and
assumptions, our financial position and results of operations
could have differed materially from that which is presented.
Cash
and Cash Equivalents
All highly liquid investments with original maturities of three
months or less when purchased are considered cash equivalents
and are carried at cost due to the short period of time to
maturity.
F-154
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Accounts
Receivable, net
Accounts receivable, net, consists of amounts owed to us by our
customers on credit sales with terms generally ranging from 30
to 150 days from date of invoice and are presented net of
an allowance for doubtful accounts receivable on our
consolidated balance sheets.
We maintain an allowance for doubtful accounts receivable for
estimated losses resulting from our inability to collect from
customers. In extending credit, we assess our customers’
creditworthiness by, among other factors, evaluating our
customers’ financial condition, credit history, and the
amount involved, both initially and on an ongoing basis.
Collateral is generally not required. In evaluating the adequacy
of our allowance for doubtful accounts receivable, we primarily
analyze accounts receivable balances, the percentage of accounts
receivable by aging category, and historical bad debts. We also
consider, among other things, customer concentrations and
changes in customer payment terms or payment patterns.
If the financial conditions of our customers were to
deteriorate, resulting in an impairment of their ability to make
payments or our ability to collect, an increase to the allowance
may be required. Also, should actual collections of accounts
receivable be different than our estimates included in
determining the allowance, the allowance would be adjusted
through charges or credits to selling, general, and
administrative expenses (SG&A) in our consolidated income
statements in the period in which such changes in collection
become known. If conditions were to change in future periods,
additional allowances or reversals may be required. Such
allowances or reversals could be significant.
Concentrations
of Credit Risk
Customer
Concentration
Our accounts receivable, net, includes amounts due from
pharmaceutical wholesalers and distributors, buying groups,
hospitals, physicians’ offices, patients, and others. Our
concentrations with customers that represented more than 10% of
our accounts receivable, net, were:
|
|
|
| |
•
|
At December 31, 2010: Amerisource Bergen- 12.8%
|
| |
| |
•
|
At December 31, 2009: FFF Enterprise, Inc.- 14.6%
|
The following table summarizes our concentrations with customers
that represented more than 10% of our total net revenue:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
2010
|
|
2009
|
|
2008
|
|
|
|
FFF Enterprise, Inc.
|
|
|
13.9
|
%
|
|
|
14.4
|
%
|
|
|
12.8
|
%
|
|
Amerisource Bergen
|
|
|
13.1
|
%
|
|
|
12.3
|
%
|
|
|
12.0
|
%
|
|
Canadian Blood Services
|
|
|
<10
|
%
|
|
|
<10
|
%
|
|
|
10.6
|
%
|
Counterparty
Risk
As discussed further in Note 27, “Subsequent
Events,” we initiated a foreign currency hedging program in
the first quarter of 2011 for the purpose of managing the
economic effects of the volatility associated with short-term
changes in euro/U.S. dollar exchange rates on our earnings
and cash flows. These derivative financial instruments present
certain market and counterparty risks. We seek to manage the
counterparty risks associated with these contracts by limiting
transactions to counterparties with which we have established
banking relationships and limit the duration of the contracts to
less than one year. We are exposed to potential losses if a
counterparty fails to perform according to the terms of the
agreement. We do not require collateral or other security to be
furnished by counterparties to our derivative financial
instruments. There can be no
F-155
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
assurance, however, that our practice effectively mitigates
counterparty risk. A number of financial institutions similar to
those that serve or may serve as counterparties to our hedging
arrangements were adversely affected by the global credit
crisis. The failure of any of the counterparties to our hedging
arrangements to fulfill their obligations to us could adversely
affect our results of operations and cash flows.
Inventories
Inventories consist of raw materials,
work-in-process,
and finished goods held for sale and are stated at the lower of
cost or market, which approximates actual costs determined on
the
first-in,
first-out method. In evaluating whether inventory is stated at
the lower of cost or market, we consider such factors as the
amount of inventory on hand and in the distribution channel, the
estimated time required to sell such inventory, remaining shelf
life, and current and expected market conditions, including
levels of competition. As appropriate, provisions are recorded
to reduce inventories to their net realizable value. We record
provisions for
work-in-process
inventory when we believe the inventory does not meet all
criteria to permit release to the market. Provisions are
recorded for finished goods that do not have sufficient
remaining shelf lives. We record recoveries directly to cost of
goods sold after the impacted material is determined to be
usable and is sold to third parties.
Property,
Plant, and Equipment, net
Property, plant, and equipment are recorded at cost, less
accumulated depreciation and amortization. Internal labor costs
directly related to asset additions are capitalized. Major
renewals and betterments are capitalized. All feasibility
studies and maintenance and repair costs are expensed as
incurred. Certain interest costs incurred by us during the
construction period, based on our weighted average borrowing
rates of debt, are capitalized and included in the cost of the
related asset.
We generally depreciate and amortize property, plant, and
equipment using the straight-line method over the useful lives
presented in the following table:
| |
|
|
|
Asset Type
|
|
Useful Life (Years)
|
|
|
|
Buildings
|
|
10 to 45
|
|
Building improvements
|
|
10 to 20
|
|
Machinery and equipment
|
|
3 to 20
|
|
Furniture and fixtures
|
|
5 to 10
|
|
Computer hardware and software
|
|
3 to 7
|
|
Leasehold improvements
|
|
the estimated useful life of the improvement or, if shorter, the
life of the lease
|
We lease various property and equipment. Leased property and
equipment that meet certain criterion are capitalized and the
present values of the related lease payments are recorded as
liabilities. Capital lease payments are allocated between a
reduction of the lease obligation and interest expense using the
interest rate implicit in the lease. All other leases are
accounted for as operating leases and the related payments are
expensed ratably over the rental period. Amortization of assets
under capital leases is computed using the straight-line method
over the shorter of the remaining lease term or the estimated
useful life.
Business
Acquisitions
Results of business acquisitions are included in our results of
operations as of the respective acquisition dates. The purchase
price of each acquisition is allocated to the net assets
acquired based on estimates of their fair values at the date of
the acquisition. Any purchase price in excess of the fair value
of net assets acquired is recorded as goodwill. The accounting
for business acquisitions requires us to make estimates and
F-156
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
assumptions related to the estimated fair values of the net
assets acquired. Significant judgments are used during this
process, particularly with respect to intangible assets.
Generally, definite-lived intangible assets are amortized over
their estimated useful lives. Goodwill and other
indefinite-lived intangible assets are not amortized, but are
annually assessed for impairment. Therefore, the purchase price
allocation to intangible assets and goodwill could have a
significant impact on future operating results.
Identifiable
Intangible Assets
Identifiable intangible assets are recorded at cost, or when
acquired as part of a business acquisition, at estimated fair
value. Definite-lived intangible assets are amortized over their
useful lives. Indefinite-lived intangible assets, such as
regulatory licenses, are not amortized, but are annually
assessed for impairment.
Impairment
Reviews
We evaluate the recoverability of recorded goodwill and other
indefinite-lived intangible asset amounts annually as of
December 31 or when events or changes in circumstances indicate
that evidence of potential impairment exists, using a fair value
based test. This test requires us to make estimates of factors
that include, but are not limited to, projected future operating
results and business plans, economic projections, anticipated
future cash flows, comparable marketplace data from a consistent
industry group, and the cost of capital. Any applicable
impairment loss is the amount, if any, by which the implied fair
value is less than the carrying value.
We review the carrying amounts of other long-lived assets for
potential impairment whenever events or changes in circumstances
indicate that the carrying amount of the asset may not be
recoverable. We periodically evaluate whether events or changes
in circumstances have occurred that may warrant revision of the
estimated useful lives of our long-lived assets or whether the
remaining carrying amount of long-lived assets should be
evaluated for possible impairment. An example of such a change
in circumstances includes a significant adverse change in the
extent or manner in which an asset is being used.
Debt
Issuance Costs and Debt Discount
We capitalize costs associated with the issuance of our debt and
amortize these costs to interest expense, net, over the term of
the related debt agreement using an effective yield amortization
method, or similar method. Unamortized debt issuance costs are
written off within total other non-operating expense, net, in
our consolidated income statements when indebtedness under the
related credit facility is repaid or restructured prior to
maturity.
We record debt discounts as a reduction of the face amount of
the related debt. Debt discounts are amortized to interest
expense, net, over the term of the related debt agreement using
an effective yield amortization method, or similar method.
Revenue
Recognition and
Gross-to-Net
Revenue Adjustments
We recognize revenue when earned, which is generally at the time
of delivery to the customer. Recognition of revenue also
requires reasonable assurance of collection of sales proceeds, a
fixed and determinable price, persuasive evidence that an
arrangement exists, and completion of all other performance
obligations. The recognition of revenue is deferred if there are
significant post-delivery obligations, such as customer
acceptance.
Allowances against revenue for estimated discounts, rebates,
administrative fees, chargebacks, and shelf-stock adjustments
are established by us concurrently with the recognition of
revenue. The standard terms and conditions under which products
are shipped to our customers generally do not allow a right of
return. In the
F-157
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
rare instances in which we grant a right of return, revenue is
reduced at the time of sale to reflect expected returns and
deferred until all conditions of revenue recognition are met.
We have supply agreements with our major distributors, which
require them to purchase minimum quantities of our products. We
regularly review the supply levels of our products on hand at
major distributors, primarily by analyzing inventory reports
supplied by these distributors, available data regarding the
sell-through of our products, our internal data, and other
available information. When we believe distributor inventory
levels have increased relative to underlying demand, we evaluate
the need for sales return allowances. Factors that influence the
allowance include historical sales return activity, levels of
inventory in the distribution network, inventory turnover,
demand history, demand projections, estimated product
shelf-life, pricing, and competition. Sales returns have not
been material during the periods presented.
Revenue from milestone payments for which we have no continuing
performance obligations is recognized upon achievement of the
related milestone. When we have continuing performance
obligations, the milestone payments are deferred and recognized
as revenue over the term of the arrangement as we complete our
performance obligations.
Gross product sales are subject to a variety of deductions that
are generally estimated and recorded in the same period that the
revenue is recognized, and primarily represent rebates to
government agencies, chargebacks to wholesalers and
distributors, and customer prompt payment discounts. These
gross-to-net
revenue adjustments are described below.
We offer rebates to certain classes of trade, which we account
for by establishing an accrual at the time the sale is recorded
in an amount equal to our estimate of rebates attributable to
each sale. We determine our estimate of the rebates primarily
based on historical experience and current contract
arrangements. We consider the sales performance of products
subject to rebates and the levels of inventory in the
distribution channel and adjust the accrual periodically to
reflect actual experience. Rebates accrued upon sale are settled
based on actual experience. Due to the limited classes of trade
that participate in rebate programs and our visibility of
inventories in the channel, adjustments for actual experience
have not been material.
We participate in state government-managed Medicaid programs. We
account for Medicaid rebates by establishing an accrual at the
time the sale is recorded in an amount equal to our estimate of
the Medicaid rebate claims attributable to such sale. We
determine our estimate of the Medicaid rebates accrual primarily
based on historical experience regarding Medicaid rebates, legal
interpretations of the applicable laws related to the Medicaid
program and any new information regarding changes in the
Medicaid programs’ regulations and guidelines that would
impact the amount of the rebates. We consider outstanding
Medicaid claims, Medicaid payments, and levels of inventory in
the distribution channel and adjust the accrual periodically to
reflect actual experience. Adjustments for actual experience
have not been material.
Sales allowances are established based upon consideration of a
variety of factors, including, but not limited to, our sales
terms which generally provide for up to a 2% prompt pay discount
on domestic and international sales, contractual agreements with
customers, estimates of the amount of product in the pipeline,
and prescribing patterns. We believe that our sales allowance
accruals are reasonably determinable and are based on the
information available at the time to arrive at our best estimate
of the accruals. Actual sales allowances incurred are dependent
upon future events. We periodically monitor the factors that
influence sales allowances and make adjustments to these
provisions when we believe that the actual sales allowances may
differ from prior estimates. If conditions in future periods
change, revisions to previous estimates may be required,
potentially in significant amounts. As these prompt pay
discounts are typically settled within 30 to 45 days of the
sale, adjustments for actual experience have not been material.
Our estimates for discounts, customer and government rebates,
and administrative fees are by their nature more predictable and
less subjective. Estimates for chargebacks are more subjective
and, consequently, may be more variable. We enter into
agreements with certain customers to establish contract pricing
for our products,
F-158
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
which these entities purchase from the wholesaler or distributor
(collectively, wholesalers) of their choice. Consequently, when
our products are purchased from wholesalers by these entities at
the contract price which is less than the price charged by us to
the wholesaler, we provide the wholesaler with a credit referred
to as a chargeback. The allowance for chargebacks is based on
our estimate of the wholesaler inventory levels, and the
expected sell-through of our products by the wholesalers at the
contract price based on historical chargeback experience and
other factors. Our estimates of inventory levels at the
wholesalers are subject to inherent limitations, as our
estimates rely on third party data, and their data may itself
rely on estimates, and be subject to other limitations. We
periodically monitor the factors that influence our provision
for chargebacks, and make adjustments when we believe that
actual chargebacks may differ from established allowances. These
adjustments occur in a relatively short period of time.
Shelf-stock adjustments are credits issued to our customers to
reflect decreases in the selling prices of our products.
Agreements to provide this form of price protection are
customary in our industry and are intended to reduce a
customer’s inventory cost to better reflect current market
prices. Shelf-stock adjustments are based upon the amount of
product that our customers have remaining in their inventories
at the time of the price reduction. Decreases in our selling
prices are discretionary decisions made by us to reflect market
conditions. Amounts recorded for estimated price adjustments are
based upon specified terms with customers, estimated declines in
market prices, and estimates of inventory held by customers. Our
estimates of inventory levels at the customer are subject to
inherent limitations, as our estimates may rely on third party
data, and their data may itself rely on estimates, and be
subject to other limitations. We regularly monitor these factors
and evaluate our reserves for shelf-stock adjustments. We have
not experienced significant shelf-stock adjustments during the
periods presented.
Shipping
and Handling
Shipping and handling costs incurred for inventory purchases are
included in cost of goods sold in our consolidated income
statements. Shipping and handling costs incurred to warehouse,
pick, pack, and prepare inventory for delivery to customers are
included in selling, general and administrative expenses
(SG&A) in our consolidated income statements. Shipping and
handling costs included in SG&A amounted to
$3.7 million, $3.6 million, and $3.5 million for
the years ended December 31, 2010, 2009, and 2008,
respectively.
Advertising
Costs
The costs of advertising are expensed as incurred within
SG&A in our consolidated income statements. Our advertising
costs consist primarily of product samples, print media, online
advertising, and promotional material. We incurred advertising
costs totaling $11.3 million, $10.2 million, and
$10.5 million for the years ended December 31, 2010,
2009, and 2008, respectively.
Research
and Development Expenses
Research and development (R&D) expenses include the costs
directly attributable to the conduct of research and development
programs for new products and extensions or improvements of
existing products and the related manufacturing processes. Such
costs include salaries and related employee benefit costs,
payroll taxes, materials (including the material required for
clinical trials), supplies, depreciation on and maintenance of
R&D equipment, services provided by outside contractors for
clinical development and clinical trials, regulatory services,
and fees. R&D also includes the allocable portion of
facility costs such as rent, depreciation, utilities, insurance,
and general support services. All costs associated with R&D
are expensed as incurred.
F-159
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Share-Based
Compensation
We value share-based compensation at the grant date using a fair
value model and recognize this value as expense over the
employees’ requisite service period, typically the period
over which the share-based compensation vests. We classify
share-based compensation costs consistent with each
grantee’s salary. We record income tax benefits which
result from realizing a tax deduction in excess of previously
recognized compensation expense as additional paid-in capital.
The fair value of our common stock on the grant date is a
significant factor in determining the fair value of share-based
compensation awards and the ultimate non-cash compensation cost
that we will be required to record over the vesting period.
Given the absence of a trading market for our common stock on
grant dates prior to October 1, 2009, our board of
directors, or special dividend committee or compensation
committee designated by our board of directors, estimated the
fair value of our common stock contemporaneously with each grant
using numerous objective and subjective factors. These factors
included: (i) our stage of development, our efforts to
become independent from Bayer, and revenue growth; (ii) the
timing of the anticipated launch of new products and new
indications; (iii) business conditions and business
challenges at the time; (iv) available market data,
including observable market transactions, and valuations for
comparable companies; (v) the illiquid nature of our stock
options and stock grants; and (vi) the likelihood of
achieving a liquidity event for the shares of common stock
underlying the options, such as an initial public offering or
sale of our company, given prevailing market conditions at the
grant date. In making the assessment of common stock fair value
on each award date, our board of directors or designated
committee of our board of directors considered the guidance in
American Institute of Certified Public Accountants Technical
Practice Aid, “Valuation of Privately-Held Company Equity
Securities Issued as Compensation.” The valuations were
completed utilizing the market
and/or an
income approach and then the enterprise value was allocated
using the “Probability-Weighted Expected Return
Method,” which provides different probability weights of
various likely scenarios (distressed; remain private; private
sale; IPO), and develops valuations by determining the present
value of the future expected common stock value under each of
these scenarios. For option awards granted on October 1,
2009, the fair value of our common stock was determined to be
the IPO price per share of $19.00. For option awards granted
subsequent to our IPO, we consider the fair value of our common
stock to be the closing share price as reported by The NASDAQ
Global Select Market on the grant date.
We estimate the fair value of stock options at the grant date
using the Black-Scholes pricing model, which requires the use of
a number of assumptions related to the risk-free interest rate,
average life of options (expected term), expected volatility,
and dividend yield. There was no trading market for our common
stock or stock options on grant dates prior to October 1,
2009. Therefore, our application of the Black-Scholes pricing
model incorporates historical volatility measures of similar
public companies. A forfeiture rate based on historical
attrition rates of award holders is used in estimating the
granted awards not expected to vest. If actual forfeitures
differ from the expected rate, we may be required to make
additional adjustments to compensation expense in future
periods. The Black-Scholes option pricing model was developed
for use in estimating the fair value of traded options that have
no vesting restrictions and are fully transferable,
characteristics that are not present in our option grants. If
the model permitted consideration of the unique characteristics
of employee stock options, the resulting estimate of the fair
value of the stock options, and resulting compensation expense,
could be different.
The stock options that we granted to employees typically have
service-based and performance-based components. The performance
stock unit (PSU) awards that we grant to employees vest based on
the achievement of pre-established objective performance goals,
which are generally financial in nature. The restricted stock
and restricted stock unit (RSU) awards that we grant to
employees are typically service-based only. Stock option grants,
restricted stock, and RSU awards to non-employee directors are
service-based only. Service-based awards vest annually in equal
amounts over the vesting period. The performance-based component
of the stock options vests annually upon the achievement of
corporate performance objectives
F-160
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
which are established by our board of directors. We make
assessments as to whether the performance conditions related to
the performance-based stock options will be achieved. We record
compensation cost for awards with performance conditions based
on the probable outcome of the performance conditions.
Litigation
Accruals
We record an accrual for our exposures to our various litigation
matters as a charge to our consolidated income statements when
it becomes probable and can be reasonably estimated. The
exposure to legal matters is evaluated and estimated, if
possible, following consultation with legal counsel. Such
estimates are based on currently available information and,
given the subjective nature and complexities inherent in making
these estimates, the ultimate outcome of our legal matters may
be significantly different than the amounts estimated.
Additional information regarding our possible litigation
exposures is included in Note 14, “Commitments and
Contingencies.”
Environmental
Costs
We record liabilities when our environmental assessments
indicate that remediation efforts are probable, and the costs
can be reasonably estimated. We recognize a current period
expense for the liability when
clean-up
efforts do not benefit future periods. We capitalize costs that
benefit more than one accounting period. Estimates, when
applicable, of our liabilities are based on currently available
facts, existing technology, and presently enacted laws and
environmental regulations taking into consideration the likely
effects of inflation and other societal and economic factors,
and include estimates of associated legal costs. The amounts
also consider prior experience in remediating contaminated
sites, other companies’
clean-up
experience, and data released by the Environmental Protection
Agency (EPA) or other organizations. The estimates are subject
to revision in future periods based on actual costs or new
circumstances. We evaluate recoveries from insurance coverage or
government sponsored programs separately from our liability, and
when recovery is assured, we record and report an asset
separately from the associated liability. At December 31,
2010 and 2009, no environmental related assets or liabilities
are reflected on our consolidated balance sheets as no amounts
are probable or estimable.
Other
Contingencies
We recognize liabilities for other contingencies when we have an
exposure, that, when analyzed, indicates it is both probable
that an asset has been impaired or a liability incurred, and the
amount of impairment or loss can be reasonably estimated. Funds
spent to remedy these contingencies are charged against the
accrued liability, if one exists, or expensed, if no liability
was previously established. When a range of probable loss can be
estimated, we accrue the most likely amount within the range of
probable losses.
Self-Insurance
Programs
We maintain self-insured retentions and deductibles for some of
our insurance programs and limit our exposure to claims by
maintaining stop-loss
and/or
aggregate liability coverage under which the insurer is the
primary obligor to the insured. The estimate of our claims
liability is subject to inherent limitations as it relies on our
judgment of the likely ultimate costs that will be incurred to
settle reported claims and unreported claims for incidents
incurred but not reported as of the balance sheet date. When
estimating our liability for such claims, we consider a number
of factors, including, but not limited to, self-insured
retentions, deductibles, claim experience, demographic factors,
severity factors, and maximum claims exposure. If actual claims
exceed these estimates, additional charges may be required.
F-161
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Income
Taxes
We calculate a provision for, or benefit from, income taxes
using the asset and liability method, under which deferred tax
assets and liabilities are recorded based on the difference
between the financial statement and tax bases of assets and
liabilities using enacted tax rates in effect for the year in
which the differences are expected to reverse. A reduction in
the carrying amounts of deferred tax assets by a valuation
allowance is required, if, based on the available evidence, it
is more likely than not that the assets will not be realized.
Accordingly, we periodically assess the need to establish
valuation allowances for deferred tax assets based on the
more-likely-than-not realization threshold criterion. In
assessing the need for a valuation allowance, we consider all
available evidence, both positive and negative, including
historical levels of income, expectations and risks associated
with future taxable income, and ongoing prudent and feasible tax
planning strategies.
We establish reserves for uncertain income tax positions, based
on the technical support for the positions, our past audit
experience with similar situations, and potential interest and
penalties related to the matters. Our recorded reserves
represent our best estimate of the amount, if any, that we will
ultimately be required to pay to settle such matters. The
resolution of our uncertain income tax positions is dependent on
uncontrollable factors such as law changes, new case law and the
willingness of the income tax authorities to settle, including
the timing thereof and other factors. Although we do not
anticipate significant changes to our uncertain income tax
positions in the next twelve months, items outside of our
control could cause our uncertain income tax positions to change
in the future, which would be recorded within (provision)
benefit for income taxes in our consolidated income statements.
Interest and penalties related to unrecognized tax benefits are
recognized as a component of our income tax provision.
Interest
Costs
We capitalize a portion of the interest costs we incur during
the construction of long-lived assets, primarily plant and
equipment, as an additional cost of the related asset. The
amount of interest capitalized is determined by applying our
weighted average borrowing rate to the related capital spending
during the construction period. We incurred interest costs
related to our debt, including imputed interest on capital lease
obligations, and interest rate swap contracts of
$48.3 million, $72.8 million, and $97.2 million
for the years ended December 31, 2010, 2009, and 2008,
respectively, of which $5.9 million, $2.0 million, and
$2.3 million, respectively, were capitalized related to the
construction of property and equipment. Our interest rate swap
contracts were settled and terminated during 2009.
Derivative
Financial Instruments
All derivative financial instruments are recorded on our
consolidated balance sheets as assets or liabilities and
measured at fair value, which considers the instrument’s
term, notional amount, discount rate, credit risk, and other
factors. For derivatives designated as hedges of the fair value
of assets or liabilities, the changes in fair values of both the
derivatives and the hedged items are recorded in current
earnings. For derivatives designated as cash flow hedges, the
effective portion of the changes in fair value of the
derivatives are recorded in other comprehensive income (loss)
and subsequently recognized in earnings when the hedged items
impact income. Changes in the fair value of derivatives not
designated as hedges and the ineffective portion of cash flow
hedges are recorded in current earnings. When determining the
fair value of our derivative financial instruments, we analyze
the instruments from a market participant’s perspective to
determine a hypothetical exit price to the counterparty. At
December 31, 2009, our derivative financial instruments
consisted of two interest rate cap contracts with an aggregate
notional amount of $175.0 million for which the cap rate of
6.00% was significantly higher than prevailing market interest
rates; therefore, the fair market value was zero. At
December 31, 2010, we did not have any derivative financial
instruments. We initiated a foreign currency hedging program in
the first quarter of 2011 as discussed in Note 27,
“Subsequent Events.”
F-162
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Fair
Value of Financial Instruments
At December 31, 2010, we had no financial assets or
liabilities which were required to be measured at fair value. At
December 31, 2009, we had two interest rate cap contracts
with an aggregate notional amount of $175.0 million for
which the cap rate of 6.00% was significantly higher than
prevailing market interest rates; therefore, the fair market
value was zero.
At December 31, 2010 and 2009, the estimated fair value of
our 7.75% Notes was $648.8 million and
$607.9 million, which was calculated by reference to open
bid/ask quotations of our 7.75% Notes. We had no amounts
outstanding under our variable rate revolving credit facility at
December 31, 2010 and 2009. At December 31, 2010 and
2009, we have notes receivable outstanding, which bear interest
at market rates, and consequently, the recorded amounts
approximate fair value. The recorded amounts of all other
financial instruments, which consist of cash and cash
equivalents, accounts receivable, net, accounts payable, accrued
expenses and other liabilities, approximate fair value due to
the short duration of the instruments.
Comprehensive
Income
Comprehensive income is defined as the change in equity
resulting from recognized transactions and other events and
circumstances from non-owner sources. Comprehensive income
includes net income as currently reported under U.S. GAAP
and other comprehensive income (loss). Other comprehensive
income (loss) considers the effect of additional economic events
that are not required to be recorded in determining net income,
but rather are reported as a separate component of
stockholders’ equity (deficit).
The following table includes information regarding our other
comprehensive income (loss):
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross
|
|
|
Tax
|
|
|
Net
|
|
|
|
|
Amount
|
|
|
Effect
|
|
|
Amount
|
|
|
|
|
Year ended December 31, 2010
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustments
|
|
$
|
(576
|
)
|
|
$
|
—
|
|
|
$
|
(576
|
)
|
|
Additional minimum pension liability
|
|
|
(74
|
)
|
|
|
—
|
|
|
|
(74
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss
|
|
$
|
(650
|
)
|
|
$
|
—
|
|
|
$
|
(650
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2009
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustments
|
|
$
|
232
|
|
|
$
|
—
|
|
|
$
|
232
|
|
|
Additional minimum pension liability
|
|
|
244
|
|
|
|
—
|
|
|
|
244
|
|
|
Reclassification of unrealized loss on derivative financial
instruments
|
|
|
37,513
|
|
|
|
(14,226
|
)
|
|
|
23,287
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive income
|
|
$
|
37,989
|
|
|
$
|
(14,226
|
)
|
|
$
|
23,763
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2008
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation adjustments
|
|
$
|
(216
|
)
|
|
$
|
—
|
|
|
$
|
(216
|
)
|
|
Net unrealized loss on derivative financial instruments
|
|
|
(18,477
|
)
|
|
|
6,973
|
|
|
|
(11,504
|
)
|
|
Additional minimum pension liability
|
|
|
(52
|
)
|
|
|
—
|
|
|
|
(52
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other comprehensive loss
|
|
$
|
(18,475
|
)
|
|
$
|
6,973
|
|
|
$
|
(11,772
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-163
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
The following table includes information regarding our
accumulated other comprehensive (loss) income:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Foreign currency translation adjustments
|
|
$
|
(412
|
)
|
|
$
|
164
|
|
|
Additional minimum pension liability
|
|
|
118
|
|
|
|
192
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated other comprehensive (loss) income
|
|
$
|
(294
|
)
|
|
$
|
356
|
|
|
|
|
|
|
|
|
|
|
|
During the year ended December 31, 2009, we settled and
terminated our interest rate swap contracts, which resulted in a
loss of $30.9 million. Our accumulated other comprehensive
loss at December 31, 2008 included $23.3 million, net
of taxes, related to unrealized losses associated with our
interest rate swap contracts. As a result of their settlement
and termination, we reclassified $23.3 million out of
accumulated other comprehensive loss to loss on extinguishment
of debt within total other non-operating expense, net, in our
consolidated income statement for the year ended
December 31, 2009.
Foreign
Currency Translation
For our international operations, local currencies have been
determined to be the functional currencies. We translate the
financial statements of international subsidiaries to their
U.S. dollar equivalents at
end-of-period
currency exchange rates for assets and liabilities and at
average currency exchange rates for revenues and expenses. We
record these translation adjustments as a component of other
comprehensive income (loss) within stockholders’ equity
(deficit). We recognize transaction gains and losses arising
from fluctuations in currency exchange rates on transactions
denominated in currencies other than the functional currency as
incurred within SG&A in our consolidated income statements.
We incurred foreign currency transaction (losses) gains of
$(4.7) million, $1.9 million, and $(1.0) million
for the years ended December 31, 2010, 2009, and 2008,
respectively. As discussed in Note 27, “Subsequent
Events,” we initiated a foreign currency hedging program in
the first quarter of 2011 in order to reduce the impact of the
volatility of foreign exchange rates and improve predictability.
Business
Segments
We operate our plasma-derived protein therapeutics business as a
single reportable business segment since all operating
activities are directed from our North Carolina headquarters and
all of our products result from a common manufacturing process
based on a single feedstock.
Earnings
per Share
We calculate basic earnings per share based upon the weighted
average number of common shares outstanding. We calculate
diluted earnings per share based upon the weighted average
number of common shares outstanding plus the dilutive effect of
common share equivalents calculated using the treasury stock
method.
Recent
Accounting Pronouncements
In April 2010, the Financial Accounting Standards Board (FASB)
issued new accounting guidance which clarifies questions
surrounding the accounting implications of the different signing
dates of the Health Care and Education Reconciliation Act
(signed March 30, 2010) and the Patient Protection and
Affordable Care Act (signed March 23, 2010). The new
guidance states that the FASB and the Office of the Chief
Accountant at the SEC would not be opposed to viewing the two
Acts together for accounting purposes. The adoption of this
guidance did not have a material impact on our consolidated
financial statements.
F-164
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
In February 2010, the FASB issued new accounting guidance
regarding disclosures related to subsequent events. An entity
that is a U.S. Securities and Exchange Commission (SEC)
filer is not required to disclose the date through which
subsequent events have been evaluated. This change alleviates
potential conflicts between the Accounting Standards
Codification (ASC) and the SEC’s requirement. The adoption
of this guidance did not have a material impact on our
consolidated financial statements.
In January 2010, the FASB issued guidance that requires new
disclosures for fair value measurements and provides clarity for
existing disclosures. This update requires new disclosures for
(1) transfers in and out of levels 1 and 2, and
(2) activity in level 3, by requiring the
reconciliation to present separate information about purchases,
sales, issuance, and settlements. Also, this update clarifies
the disclosures related to the fair value of each class of
assets and liabilities and the input and valuation techniques
for both recurring and nonrecurring fair value measurements in
levels 2 and 3. The effective date for the disclosures and
clarifications is for the interim and annual reporting periods
beginning after December 15, 2009 except for the
disclosures about purchases, sales, issuances and settlements,
which is effective for fiscal years beginning after
December 15, 2010. This update is not expected to have a
material impact on our consolidated financial statements or
related disclosures.
In October 2009, the FASB issued new accounting guidance
regarding multiple-deliverable revenue arrangements. The new
guidance addresses the accounting for multiple-deliverable
arrangements to enable vendors to account for products or
services (deliverables) separately rather than as a combined
unit. This guidance establishes a hierarchy for determining the
selling price of a deliverable, which is based on:
(a) vendor-specific objective evidence;
(b) third-party evidence; or (c) estimates. This
guidance also eliminates the residual method of allocation and
requires that arrangement consideration be allocated at the
inception of the arrangement to all deliverables using the
relative selling price method. In addition, this guidance
significantly expands required disclosures related to a
vendor’s multiple-deliverable revenue arrangements. The
guidance is effective prospectively for revenue arrangements
entered into or materially modified in fiscal years beginning on
or after June 15, 2010 and early adoption is permitted. A
company may elect, but will not be required, to adopt the
guidance retrospectively for all prior periods. We do not
anticipate that the adoption of this guidance will have a
material impact on our consolidated financial statements or
related disclosures.
In August 2009, the FASB released new accounting guidance
concerning measuring liabilities at fair value. The new guidance
provides clarification that in circumstances in which a quoted
price in an active market for the identical liability is not
available, a reporting entity is required to measure fair value
using certain valuation techniques. Additionally, it clarifies
that a reporting entity is not required to adjust the fair value
of a liability for the existence of a restriction that prevents
the transfer of the liability. This new guidance is effective
for the first reporting period after its issuance; however,
earlier application is permitted. The adoption of this guidance
did not have a material impact on our consolidated financial
statements or related disclosures.
On January 30, 2009, the SEC released the final rules
requiring all registered companies to use extensible Business
Reporting Language (XBRL) when submitting financial statements
to the SEC. The new rules initially will require interactive
data reporting only by domestic and foreign large accelerated
filers that prepare their financial statements in accordance
with U.S. GAAP and have a worldwide public common equity
float above $5.0 billion for their first quarterly period
ending after June 15, 2009 and all reporting periods
thereafter. We will be required to file using XBRL beginning
with our quarterly reporting period ending March 31, 2011.
|
|
|
3.
|
Definitive
Merger Agreement with Grifols S.A. and Grifols, Inc.
(Grifols)
|
We entered into a definitive merger agreement with Grifols on
June 6, 2010, as amended by Amendment 1 on November 4,
2010. Under the terms of the agreement, Grifols will acquire,
through merger transactions, all of the common stock of Talecris
for a combination of $19.00 in cash and 0.6485 (or 0.641 for
Talecris
F-165
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
directors and Talecris Holdings, LLC) of a newly-issued
non-voting Grifols’ (Class B) ordinary share for
each outstanding Talecris share (the merger consideration).
Under the terms of the agreement, completion of the transaction
is subject to obtaining certain regulatory approvals,
shareholder approvals, as well as other customary conditions.
The 0.641 exchange ratio and the additional exchange ratio of
0.0075 (which together comprise the 0.6485 exchange ratio) are
generally fixed but the 0.641 exchange ratio will be adjusted if
the 0.641 exchange ratio would result in Grifols issuing in
excess of 86.5 million Grifols non-voting shares and the
additional 0.0075 exchange ratio will be adjusted if such
additional exchange ratio would result in Grifols issuing in
excess of 0.5 million Grifols non-voting shares. The
Grifols non-voting shares will be listed on NASDAQ in the form
of American Depositary Shares and the Madrid, Barcelona, Bilbao
and Valencia stock exchanges and quoted on the Automated
Quotation System of the Spanish Stock Exchanges. Grifols
non-voting shares will carry the same economic rights as Grifols
ordinary shares. Additionally, Talecris share-based
compensation, whether vested or unvested, generally will be
converted into the right to receive or acquire the merger
consideration, or, in the case of employee stock options, the
right to acquire the merger consideration, as described in the
merger agreement in lieu of Talecris common stock. The merger
agreement provides that if the merger agreement is terminated
under specified circumstances Grifols will be required to pay
Talecris a termination fee of either $100 million or
$375 million, depending on the specified circumstances. If
the merger agreement is terminated under other specified
circumstances, Talecris will be required to pay Grifols a
termination fee of $100 million. Generally, except as noted
above, all fees and expenses incurred in connection with the
merger agreement and the transactions contemplated by the merger
agreement will be paid by the party incurring those expenses. We
have incurred and will continue to incur significant costs
related to investment banking, legal, and accounting activities,
as well as retention expenses, related to this merger
transaction. The leading shareholders of Grifols have entered
into an agreement with us, subject to conditions, to vote their
Grifols shares in favor of the transaction and, separately,
Talecris Holdings, LLC, an affiliate of Cerberus Capital
Management, L.P., which owns approximately 49% of the
outstanding Talecris common stock, has entered into an agreement
with Grifols, subject to conditions, to vote its Talecris shares
in favor of the transaction.
Under the terms of the definitive merger agreement with Grifols,
we are permitted to offer retention amounts up to a total of
$15.0 million to employees. As of December 31, 2010,
we have offered retention amounts totaling approximately
$10.2 million to employees, of which $2.9 million was
paid during 2010 and the remaining amounts are expected to be
paid in 2011, subject to the terms of the retention agreements.
We incurred retention expenses, including fringe benefits, of
$6.9 million during the year ended December 31, 2010.
The remaining retention amounts will likely be recognized
ratably through the second quarter of 2011.
We have entered into agreements with investment bankers related
to our definitive merger agreement with Grifols. We incurred
fees totaling $2.5 million under these agreements during
2010. During the year ended December 31, 2010, we also
incurred legal, accounting, and other fees of $18.3 million
associated with the merger. We are obligated to pay additional
fees totaling $21.3 million upon successful closing of the
merger transaction.
|
|
|
4.
|
Initial
Public Offering and Use of Proceeds
|
On October 6, 2009, we completed our IPO of
56,000,000 shares of our common stock, par value $0.01 per
share, at an offering price of $19.00 per share. Our IPO
included 28,947,368 shares newly issued and sold by us and
27,052,632 shares sold by the selling stockholder, Talecris
Holdings, LLC, including 6,000,000 shares sold by the
selling stockholder pursuant to the underwriters’ option to
purchase additional shares. After deducting the payment of
underwriters’ discounts and commissions, the net primary
proceeds to us from the sale of shares in our IPO were
approximately $519.7 million, which we used to repay
$389.8 million and $129.9 million of principal under
our First and Second Lien Term Loans, respectively. We did not
receive any proceeds from the sale of shares by the selling
stockholder. In addition to the $30.3 million of
underwriters’ discounts and commissions deducted from the
offering proceeds, we incurred other offering
F-166
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
costs of $3.9 million, of which $1.3 million is
included in SG&A in our consolidated income statement for
the year ended December 31, 2009 and $2.6 million is
included as a reduction of additional paid-in capital on our
December 31, 2009 consolidated balance sheet. At
December 31, 2009, approximately $0.2 million of
accrued offering expenses were payable to underwriters.
|
|
|
5.
|
Definitive
Merger Agreement with CSL Limited (CSL)
|
On August 12, 2008, we entered into a definitive merger
agreement with CSL, under which CSL agreed to acquire us for
cash consideration of $3.1 billion, less net debt, as
defined. The closing of the transaction was subject to the
receipt of certain regulatory approvals as well as other
customary conditions. The U.S. Federal Trade Commission
filed an administrative complaint before the Commission
challenging the merger and a complaint in Federal district court
seeking to enjoin the merger during the administrative process.
On June 8, 2009, the merger parties agreed to terminate the
definitive merger agreement. CSL paid us a merger termination
fee of $75.0 million, which is included as other
non-operating income in our consolidated income statement for
the year ended December 31, 2009. The U.S. Federal
Trade Commission’s complaints were subsequently dismissed.
In consideration of the definitive merger agreement with CSL,
our board of directors approved a retention program in August
2008 for an amount up to $20.0 million. We recorded
retention expense of $8.2 million and $5.1 million,
excluding fringe benefit, during the years ended
December 31, 2009 and 2008, respectively. We classified the
cost of this retention program consistent with each
recipient’s salary. We made payments of approximately
$13.3 million under this retention program during 2009. No
further payments are due.
In November 2006, we entered into an Asset Purchase Agreement
(APA) with International BioResources, L.L.C. and affiliated
entities (IBR) pursuant to which we acquired certain assets and
assumed certain liabilities from IBR. The APA was subsequently
amended in June 2007 to provide for the acceleration of all
milestones and other amounts owed to IBR under the contingent
consideration provision of the APA, and as a result, we issued
2,146,232 shares of our common stock to IBR in June 2007.
IBR had the right to put the shares of our common stock back to
us for cash ($15.61 per common share) under certain
circumstances prior to June 30, 2008. IBR was entitled to
interest at a rate of 8% per annum from the issuance date of the
shares through December 31, 2007. In January 2008, IBR
exercised their put right, as amended, for 1,185,232 common
shares, which we repurchased in February 2008. In March 2008,
IBR exercised their put right, as amended, for the remaining
961,000 common shares, which we repurchased in April 2008. The
repurchased shares were retired and the embedded put feature was
cancelled.
The following table summarizes our purchase accounting for
plasma collection centers acquired from IBR under the June 2007
Agreement. The plasma collection centers were acquired to
support our plasma
F-167
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
supply vertical integration strategy. The plasma collection
centers’ results of operations have been included in our
consolidated financial statements from their respective date of
acquisition.
| |
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Payments at closing
|
|
$
|
5,181
|
|
|
$
|
2,147
|
|
|
Notes receivable and other advances
|
|
|
44,540
|
|
|
|
10,430
|
|
|
Performance incentive payments
|
|
|
837
|
|
|
|
843
|
|
|
Allocable portion of accelerated contingent consideration
|
|
|
6,020
|
|
|
|
2,580
|
|
|
Transaction costs
|
|
|
—
|
|
|
|
56
|
|
|
|
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
56,578
|
|
|
$
|
16,056
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents
|
|
$
|
62
|
|
|
$
|
21
|
|
|
Inventory
|
|
|
5,416
|
|
|
|
1,778
|
|
|
Other current assets
|
|
|
183
|
|
|
|
—
|
|
|
Property, plant, and equipment
|
|
|
10,181
|
|
|
|
1,814
|
|
|
Intangible assets- regulatory licenses
|
|
|
3,860
|
|
|
|
840
|
|
|
Goodwill
|
|
|
37,060
|
|
|
|
11,643
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets acquired
|
|
|
56,762
|
|
|
|
16,096
|
|
|
Current liabilities assumed
|
|
|
(184
|
)
|
|
|
(40
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total purchase price
|
|
$
|
56,578
|
|
|
$
|
16,056
|
|
|
|
|
|
|
|
|
|
|
|
|
Number of plasma collection centers acquired
|
|
|
12
|
|
|
|
3
|
|
|
|
|
|
|
|
|
|
|
|
The purchase price for the plasma collection centers acquired
from IBR during 2009 and 2008 consisted of various loans and
advances made to IBR and performance incentive payments for
achieving certain milestones related to the timing of plasma
collection center openings. The purchase price also includes the
allocable portion of accelerated contingent consideration due to
IBR as discussed above. We have no further financing commitments
to IBR under the terms of our June 2007 Agreement.
|
|
|
7.
|
Goodwill
and Intangible Assets
|
There were no changes to the carrying amount of goodwill for the
year ended December 31, 2010. Changes to the carrying
amount of goodwill for the year ended December 31, 2009
were as follows:
| |
|
|
|
|
|
Balance at December 31, 2008
|
|
$
|
135,800
|
|
|
Acquisitions of plasma collection centers from IBR
|
|
|
37,060
|
|
|
|
|
|
|
|
|
Balance at December 31, 2009
|
|
$
|
172,860
|
|
|
|
|
|
|
|
Additional information regarding our business acquisitions is
included in Note 6, “Business Acquisitions.”
We assess goodwill for impairment annually as of
December 31, or more frequently if events and circumstances
indicate that impairment may have occurred. The impairment test
requires us to allocate goodwill to our reporting units and
estimate the fair value of the reporting units that contain
goodwill. If the carrying value of a reporting unit exceeds its
fair value, the goodwill of that reporting unit is potentially
impaired and we proceed to step two of the impairment analysis.
In step two of the analysis, we would record
F-168
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
an impairment loss determined by the excess of the carrying
amount of the reporting unit’s goodwill over its implied
fair value.
We have assessed goodwill at the reporting unit level. We
allocated our Company’s enterprise value to our reporting
units based upon their relative contributions to one of our
principal operating performance measures, adjusted EBITDA. We
determined that the allocated fair value of the reporting unit
exceeded its carrying value, and as a result, no adjustment to
our recorded goodwill was required at December 31, 2010.
Additional information regarding the use of non-GAAP financial
measures is included in Note 12, “Long-Term Debt and
Capital Lease Obligations.”
At December 31, 2010 and 2009, we had $10.9 million of
intangible assets recorded on our consolidated balance sheet,
all of which were indefinite-lived regulatory licenses
associated with our plasma collection centers. We performed our
annual impairment testing of indefinite-lived intangible assets
as of December 31, 2010, which resulted in no impairment of
the recorded amounts.
|
|
|
8.
|
Collaborative
and Other Agreements
|
Supply
and Service Agreement
We have a Supply and Service Agreement, as amended, through 2012
to provide albumin to an unaffiliated third party, which is used
in conjunction with a proprietary product manufactured by them.
We earn a commission on sales of the third party’s product
at a fixed rate which depends on the territory where the product
is sold, as defined in the agreement. We also provide regulatory
support as required. We earned commissions of $6.6 million,
$5.5 million, and $8.6 million under this agreement
for the years ended December 31, 2010, 2009, and 2008,
respectively, which have been recorded in other net revenue in
our consolidated income statements.
Settlement
Agreement
We were co-plaintiff along with Bayer Healthcare (Bayer) in
patent litigation in the United States District Court for the
District of Delaware against Baxter International Inc. and
Baxter Healthcare (collectively, Baxter) in which, we, as
exclusive licensee of Bayer’s U.S. Patent
No. 6,686,191 (the ‘191 patent), alleged that Baxter
by its manufacture and importation of its liquid IGIV product,
Gammagard Liquid, had infringed the ‘191 patent. Pursuant
to a Settlement Agreement with Baxter, Baxter will pay us an
amount comprising 1.2% of Baxter’s net sales in the United
States of Gammagard Liquid and any other product sold by Baxter
or an affiliate in the United States under a different
brand name that is a liquid intravenous immunoglobulin through
August 2011. Thereafter, until expiration of the ‘191
patent, Baxter will continue to owe the same amount in royalties
if Baxter continues to use the licensed technologies under a
separate Sublicense Agreement. During the years ended
December 31, 2010, 2009, and 2008, we recorded
$10.0 million, $10.6 million, and $8.7 million,
respectively, of fees from Baxter within other net revenue in
our consolidated income statements.
Licensed
Technology
We licensed certain technology related to the formulation of one
of our products to an unaffiliated third party. As consideration
for the technology transfer, we received an upfront licensing
fee of $4.0 million during 2007, of which 33% is refundable
under certain conditions. We recognized $2.6 million of the
licensing fee during 2008 as a result of the completion of a
portion of our performance obligations, which we recorded within
other net revenue in our consolidated income statement. The
remaining portion has been deferred on our consolidated balance
sheets at December 31, 2010 and 2009. We will recognize the
remaining portion of the deferred licensing fee once our
remaining performance obligations have been completed. Under the
terms of this agreement, we will also receive royalty payments
from this third party, which escalates with volume.
F-169
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
During the years ended December 31, 2010, 2009 and 2008, we
recorded $2.0 million, $1.6 million and
$1.1 million of royalties under this agreement within other
net revenue in our consolidated income statements.
|
|
|
9.
|
Inventories
and Cost of Goods Sold
|
Inventories consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Raw material
|
|
$
|
184,664
|
|
|
$
|
171,866
|
|
|
Work-in-process
|
|
|
346,086
|
|
|
|
312,178
|
|
|
Finished goods
|
|
|
163,749
|
|
|
|
160,010
|
|
|
|
|
|
|
|
|
|
|
|
|
Total inventories
|
|
$
|
694,499
|
|
|
$
|
644,054
|
|
|
|
|
|
|
|
|
|
|
|
Our raw material inventories include unlicensed plasma and
related testing costs of $2.6 million and $7.6 million
at December 31, 2010 and 2009, respectively, which we
believe are realizable.
Unabsorbed
Talecris Plasma Resources, Inc. (TPR) Infrastructure and
Start-Up
Costs
Our cost of goods sold includes $6.6 million,
$44.0 million, and $98.5 million for the years ended
December 31, 2010, 2009, and 2008, respectively, related to
unabsorbed TPR infrastructure and
start-up
costs associated with the development of our plasma collection
center platform. The reduction in unabsorbed TPR infrastructure
and start-up
costs resulted primarily from the maturation of our plasma
collection center platform.
Plasma
Center current Good Manufacturing Practices (cGMP)
Issue
During the first and second quarters of 2008, we incurred
charges to cost of goods sold of $16.3 million and
$7.0 million, respectively, due to deviations from our
standard operating procedures and cGMP at one of our plasma
collection centers. Our preliminary investigations concluded
that the deviations from our standard operating procedures and
cGMP resulted in impairments to the related raw material and
work-in-process
inventories as we concluded there was no probable future
economic benefit related to the impacted inventories.
Subsequently, due to further investigations and new facts and
circumstances, we determined that certain impacted inventories
were saleable. We record recoveries directly to cost of goods
sold after the impacted material is converted into final
products and sold to third parties. During the years ended
December 31, 2009 and 2008, we recorded recoveries of
$1.9 million and $17.5 million, respectively. For the
year ended December 31, 2008, recoveries totaled
$17.5 million, resulting in a net provision of
$5.8 million for 2008. During the year ended
December 31, 2010, recoveries were not significant.
Customer
Settlement
We settled a dispute with a customer in September 2007 regarding
intermediate material manufactured by us, which is used by this
customer in their manufacturing process. We recorded a charge to
cost of goods sold of $7.9 million during the year ended
December 31, 2007 for inventory impairment related to this
material, which we recovered in its entirety during 2008 as the
related material was determined to be saleable, converted into
final product, and sold to other customers. During 2008, we
recorded an additional inventory provision of $2.6 million
related to this dispute for products held in Europe, for which
we recovered $0.8 million and $1.8 million during 2009
and 2008, respectively, as the impacted material was determined
to be saleable, converted into final product, and sold to other
customers.
F-170
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
10.
|
Property,
Plant, and Equipment, net
|
Property, plant, and equipment, net, consisted of the following:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Land
|
|
$
|
4,136
|
|
|
$
|
4,136
|
|
|
Buildings and improvements
|
|
|
88,652
|
|
|
|
68,417
|
|
|
Machinery and equipment
|
|
|
143,813
|
|
|
|
102,887
|
|
|
Furniture and fixtures
|
|
|
7,377
|
|
|
|
5,492
|
|
|
Computer hardware and software
|
|
|
64,729
|
|
|
|
54,761
|
|
|
Capital leases of buildings
|
|
|
8,704
|
|
|
|
8,374
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
317,411
|
|
|
|
244,067
|
|
|
Less: accumulated depreciation and amortization
|
|
|
(95,319
|
)
|
|
|
(62,463
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
222,092
|
|
|
|
181,604
|
|
|
Construction in progress
|
|
|
160,701
|
|
|
|
85,595
|
|
|
|
|
|
|
|
|
|
|
|
|
Total property, plant, and equipment, net
|
|
$
|
382,793
|
|
|
$
|
267,199
|
|
|
|
|
|
|
|
|
|
|
|
Depreciation expense was $36.0 million, $28.8 million,
and $20.1 million for the years ended December 31,
2010, 2009, and 2008, respectively.
During 2009 and 2008, we recorded impairment charges of
$3.1 million and $3.6 million, respectively, primarily
within cost of goods sold in our consolidated income statements
related primarily to capital lease assets and leasehold
improvements at certain of our plasma collection centers which
were closed or were under development and we no longer plan to
open. No material impairment charges related to property, plant,
and equipment were recorded during 2010.
|
|
|
11.
|
Investment
in Affiliate
|
We have a 30% interest in the Class 1 common stock of
Centric Health Resources, Inc. (Centric). Our investment in
Centric is accounted for using the equity method of accounting
based on our assessment that our interest allows us to exercise
significant influence, but not control. Under the equity method,
our investment, originally recorded at cost, is adjusted to
recognize our share of net earnings or losses of Centric as they
occur. Our recognition of losses is limited to the extent of our
investment in, advances to, and commitments for the investment.
Centric provides services in the management of our Prolastin and
Gamunex Direct programs. In this capacity, Centric provides
warehousing, order fulfillment, distribution, home infusion, and
customer relationship services for us primarily related to our
U.S. sales of Prolastin/Prolastin-C A1PI. Centric maintains
inventory on our behalf which they utilize to fill customer
orders. Centric also provides services to us in collecting
accounts receivable for sales made under the Prolastin and
Gamunex Direct programs. We provide Centric a fee for each unit
of product provided to patients which escalates with volume. The
total fees for such services for the years ended
December 31, 2010, 2009, and 2008 were $22.9 million,
$20.3 million, $17.5 million, respectively. The
majority of these fees are recorded within cost of goods sold in
our consolidated income statements. The value of the finished
goods inventories that Centric held on our behalf was
$8.0 million and $7.1 million at December 31,
2010 and 2009, respectively.
F-171
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
12.
|
Long-Term
Debt and Capital Lease Obligations
|
We were obligated under the following debt instruments:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
7.75% Notes
|
|
$
|
600,000
|
|
|
$
|
600,000
|
|
|
Discount on 7.75% Notes
|
|
|
(3,379
|
)
|
|
|
(3,954
|
)
|
|
Revolving credit facility
|
|
|
—
|
|
|
|
—
|
|
|
Capital lease obligations
|
|
|
9,540
|
|
|
|
9,961
|
|
|
|
|
|
|
|
|
|
|
|
|
Total debt and capital lease obligations
|
|
|
606,161
|
|
|
|
606,007
|
|
|
Less: current maturities
|
|
|
(860
|
)
|
|
|
(740
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Long-term debt and capital lease obligations, net of current
maturities
|
|
$
|
605,301
|
|
|
$
|
605,267
|
|
|
|
|
|
|
|
|
|
|
|
7.75%
Unsecured Senior Notes, due November 15, 2016
On October 21, 2009, we completed the issuance of
$600.0 million, 7.75% Senior Notes, due
November 15, 2016, at a price of 99.321% of par, in a
private placement to certain qualified institutional buyers. The
7.75% Notes yield 7.875% to maturity and pay interest
semi-annually on May 15 and November 15 to holders of record on
the immediately preceding May 1 and November 1,
respectively. The 7.75% Notes are guaranteed on a senior
unsecured basis by our existing and future domestic
subsidiaries. Except as described below, we will not be entitled
to redeem the 7.75% Notes at our option prior to
November 12, 2012.
We may redeem some or all of the 7.75% Notes, at our
option, at any time on or after November 12, 2012, at the
redemption prices (expressed as percentages of principal amount)
set forth below plus accrued and unpaid interest and additional
interest, if any, on the 7.75% Notes redeemed, to the
applicable redemption date, if redeemed during the twelve-month
period beginning on November 15 of the years indicated below:
| |
|
|
|
|
|
Fiscal Year
|
|
Percentage
|
|
|
|
|
2012
|
|
|
103.875
|
%
|
|
2013
|
|
|
102.583
|
%
|
|
2014
|
|
|
101.292
|
%
|
|
2015 and thereafter
|
|
|
100.000
|
%
|
In addition, at any time during each twelve-month period ending
on November 15, 2010, 2011, and 2012, we may redeem up to
10% of the originally issued principal amount of the
7.75% Notes at a redemption price of 103% of the principal
amount of the 7.75% Notes redeemed plus accrued and unpaid
interest and additional interest, if any, to the redemption
date, subject to the rights of the holders of the
7.75% Notes on the relevant record date to receive interest
due on the relevant interest payment date. No principal amounts
were redeemed during 2010.
At any time, or from time to time, on or prior to
November 15, 2012, we may, at our option, redeem up to 35%
of the aggregate principal amount of the 7.75% Notes issued
under the indenture with the net cash proceeds to us of certain
equity offerings at a redemption price equal to 107.75% of the
principal amount of the 7.75% Notes plus accrued and unpaid
interest and additional interest, if any, to the applicable
redemption date, provided that at least 65% of the aggregate
principal amount of the 7.75% Notes originally issued
remains outstanding immediately after such redemption and the
redemption occurs within 90 days of the date of the closing
of such equity offering.
Under the Make-Whole redemption feature, we may redeem 100% of
the principal plus a premium as defined under the indenture
(computed using a discount rate equal to the U.S. Treasury
rate as of such
F-172
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
redemption date plus 0.50%), plus accrued and unpaid interest
and additional interest, if any, prior to November 15,
2012, with respect to some or all of the 7.75% Notes,
subject to the rights of the holders on the relevant record date
to receive interest due on the relevant interest payment date.
We are not required to make mandatory redemption or sinking fund
payments with respect to the 7.75% Notes.
Upon a change of control, the 7.75% Notes are puttable at
101% of principal plus accrued and unpaid interest and
additional interest, if any.
We may incur additional indebtedness and our subsidiary
guarantors may also incur additional indebtedness if our Fixed
Charge Coverage Ratio for our most recently ended four full
fiscal quarters immediately preceding the date on which such
additional indebtedness is incurred would have been at least
2.00 to 1.00, determined on a pro forma basis.
The indenture contains certain covenants limiting, subject to
exceptions, carve-outs and qualifications, our ability and our
restricted subsidiaries’ ability to: (i) sell assets;
(ii) pay distributions on, redeem or repurchase its capital
stock or redeem or repurchase its subordinated debt;
(iii) make certain investments; (iv) incur or
guarantee additional indebtedness or issue preferred stock;
(v) create or incur certain liens; (vi) enter into
agreements that restrict distributions or other payments from
our restricted subsidiaries to us; (vii) engage in certain
sale and leaseback transactions; (viii) engage in certain
transactions with affiliates; (ix) transfer or dispose of
the capital stock of the restricted subsidiary to persons other
than us or our restricted subsidiaries; and (x) create
unrestricted subsidiaries. The indenture also contains certain
customary events of default.
On July 19, 2010, we exchanged all of our then outstanding
7.75% Notes for similar 7.75% Notes that were
registered under the Securities Act. This exchange did not
impact our capitalization.
Revolving
Credit Facility
We have a $325.0 million asset-based credit agreement
administered by Wachovia Bank, N.A., an affiliate of Wells Fargo
Securities, which was amended on October 15, 2009 as
described below. We use our available cash balances to repay
amounts outstanding under our revolving credit facility. We
deposit any excess amounts into an overnight investment account.
Outstanding principal under this facility is due and payable on
the maturity date of December 6, 2011. As such, any future
outstanding balances will likely be recorded as current
liabilities. At December 31, 2010, $2.4 million was
being utilized for letters of credit and $322.6 million was
unused and available. The letters of credit were used as
security for utilities, insurance, and third party warehousing.
Borrowings under this facility bear interest at a rate based
upon either the ABR or LIBOR, at our option, plus applicable
margins based upon borrowing availability. The ABR represents
the greater of the Federal Funds Effective Rate plus 0.50% or
the Prime Rate. Interest accrues on the revolving credit
facility at the ABR plus 0.25-0.75% or LIBOR plus 1.50-2.00%.
For the years ended December 31, 2010, 2009, and 2008, the
weighted average interest rates of our revolving credit facility
were 3.50%, 2.79%, and 4.79%, respectively. No amounts were
outstanding under the revolving credit facility at
December 31, 2010 and 2009.
The revolving credit facility is secured by a Pledge and
Security Agreement dated December 6, 2006 under which
substantially all of our personal property, including real
estate, manufacturing equipment, accounts receivable, inventory,
and stock are pledged as security, each as defined within the
agreement.
The revolving credit facility contains default provisions, and,
pursuant to the October 15, 2009 amendment described below,
imposes restrictions on annual capital expenditures if our
leverage ratio is 2.00 to 1.00 or less, and contains a financial
covenant which requires us to maintain a fixed charge coverage
ratio of at least 1.10 to 1.00 if our borrowing availability
based on eligible collateral is less than $48.75 million.
F-173
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
The revolving credit facility defines certain terms in
calculating covenant ratios, including adjusted EBITDA and
Indebtedness.
The borrowing base under our revolving credit facility is based
on our accounts receivable and inventory, and is calculated as
(i) 85% of our eligible accounts receivable plus
(ii) the lesser of (a) 65% of our eligible inventory
(valued on a
first-in-first-out
basis), (b) 85% of the net orderly liquidation value of our
eligible inventory as determined by a recent appraisal, and
(c) $300 million. Only up to $100 million may be
advanced to us based on the value of our
work-in-process
inventory (with “filled-not-packed” and
“packed-not-released” inventory being considered
finished goods inventory). From time to time, the collateral
agent under the revolving credit facility may modify our
eligibility standards, establish or adjust reserves, or reduce
one or more of the other elements used in computing the
borrowing base.
On October 15, 2009, we entered into an amendment to the
revolving credit facility dated as of October 12, 2009. The
revolving credit facility, as amended, permitted the
7.75% Notes, described above, to be issued as long as the
First and Second Lien Term Loan Credit Agreements were
terminated in connection with the offering of the
7.75% Notes. The amendment also (i) increases the
covenant baskets for permitted acquisitions to
$250 million, (ii) permits the payment of cash
dividends commencing with the first fiscal quarter of 2010 if
certain conditions are met as described below, and
(iii) increases our capital expenditure baskets so that we
will be permitted to make capital expenditures of up to
$225 million in each of 2010 and 2011. Moreover, pursuant
to the amendments, we are not subject to any limitation on our
capital expenditures in any fiscal year if our leverage ratio,
as defined, as of the end of the fiscal year most recently ended
was less than or equal to 2.00 to 1.00. Minimum availability
tests under the revolving credit facility were also increased
from $32.5 million to $48.75 million in connection
with the amendment.
Our revolving credit facility, as amended, permits the payment
of cash dividends to holders of our common stock commencing with
the first fiscal quarter of 2010, so long as (i) the
leverage ratio determined as of the end of the immediately
preceding fiscal quarter for the then most recently completed
four fiscal quarters, is equal to or less than 2.00 to 1.00 and
(ii) the minimum pro forma availability as of the date of
such dividend (after giving effect to such cash dividend, the
funding of all revolving loans, and the issuance of all letters
of credit to be funded or issued as of such date) is not less
than $48.75 million; provided that, the aggregate amount of
restricted payments shall not exceed 50% of Net Income during
the period from October 1, 2009 to the end of the most
recently ended fiscal quarter as of the date of the restricted
payment.
First
and Second Lien Term Loans
Our First and Second Lien Term Loans were repaid in full and
terminated as a result of the application of the net proceeds to
us from our October 6, 2009 IPO and the issuance of our
7.75% Notes on October 21, 2009. The weighted average
annualized interest rates on the First Lien Term Loan were 4.66%
and 6.60% for the years ended December 31, 2009 and 2008,
respectively, and the weighted average annualized interest rates
on the Second Lien Term Loan were 7.68% and 9.63% for the years
ended December 31, 2009 and 2008, respectively.
Financial
Impact of IPO and Refinancing Transactions
The following table summarizes the changes to our indebtedness
during 2009, including the impact from the application of the
net proceeds to us of $519.7 million from our IPO discussed
in Note 4, “Initial Public
F-174
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Offering and Use of Proceeds,” and the net proceeds to us
of $583.9 million from the refinancing transactions
discussed below:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
October 21,
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
2009 Net
|
|
|
October 6,
|
|
|
2009
|
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
Repayments
|
|
|
2009 IPO
|
|
|
Refinancing
|
|
|
Amortization
|
|
|
2009
|
|
|
|
|
Revolving Credit Facility
|
|
$
|
179,941
|
|
|
$
|
(124,348
|
)
|
|
$
|
—
|
|
|
$
|
(55,593
|
)
|
|
$
|
—
|
|
|
$
|
—
|
|
|
First Lien Term Loan
|
|
|
686,000
|
|
|
|
(5,250
|
)
|
|
|
(389,812
|
)
|
|
|
(290,938
|
)
|
|
|
—
|
|
|
|
—
|
|
|
Second Lien Term Loan
|
|
|
330,000
|
|
|
|
—
|
|
|
|
(129,937
|
)
|
|
|
(200,063
|
)
|
|
|
—
|
|
|
|
—
|
|
|
7.75% Notes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
600,000
|
|
|
|
—
|
|
|
|
600,000
|
|
|
Discount on 7.75% Notes
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(4,074
|
)
|
|
|
120
|
|
|
|
(3,954
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total indebtedness
|
|
$
|
1,195,941
|
|
|
$
|
(129,598
|
)
|
|
$
|
(519,749
|
)
|
|
$
|
49,332
|
|
|
$
|
120
|
|
|
$
|
596,046
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition to the debt principal repayments in the preceding
table, we used $28.7 million of the net proceeds to us from
the issuance of the 7.75% Notes to settle and terminate
certain interest rate swap contracts with a notional amount of
$390.0 million and $8.6 million to pay accrued
interest associated with our then outstanding First and Second
Lien Term Loans. In addition to the $4.1 million of
discounts on the 7.75% Notes disclosed in the table above,
approximately $12.0 million of commissions were deducted
from the gross issuance proceeds. Subsequently, we paid
$6.1 million to settle and terminate our remaining interest
rate swap contract with a notional amount of $50.0 million.
As a result of the IPO and refinancing transactions, we
recognized a charge during the fourth quarter of 2009 of
$12.1 million to write-off previously deferred debt
issuance costs related to our First and Second Lien Term Loans
and $30.9 million related to costs associated with the
settlement and termination of our interest rate swap contracts.
These charges, which totaled $43.0 million, were recorded
within other non-operating expense, net, in our consolidated
income statement for the year ended December 31, 2009. We
capitalized $14.9 million of debt issuance costs associated
with the issuance of the 7.75% Notes and the revolving
credit facility amendment. We incurred other costs related to
our IPO of $3.9 million, of which $1.3 million is
included within SG&A in our consolidated income statement
for the year ended December 31, 2009 and $2.6 million
is included as a reduction of additional paid-in capital on our
December 31, 2009 consolidated balance sheet. The following
table summarizes changes in deferred debt issuance costs during
the year ended December 31, 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Newly
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capitalized
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
|
Debt Issuance
|
|
|
|
|
|
December 31,
|
|
|
|
|
2008
|
|
|
Charges
|
|
|
Costs
|
|
|
Amortization
|
|
|
2009
|
|
|
|
|
Revolving Credit Facility
|
|
$
|
3,014
|
|
|
$
|
—
|
|
|
$
|
1,545
|
|
|
$
|
(1,041
|
)
|
|
$
|
3,518
|
|
|
First Lien Term Loan
|
|
|
9,629
|
|
|
|
(8,054
|
)
|
|
|
—
|
|
|
|
(1,575
|
)
|
|
|
—
|
|
|
Second Lien Term Loan
|
|
|
4,744
|
|
|
|
(4,087
|
)
|
|
|
—
|
|
|
|
(657
|
)
|
|
|
—
|
|
|
7.75% Notes
|
|
|
—
|
|
|
|
—
|
|
|
|
13,334
|
|
|
|
(392
|
)
|
|
|
12,942
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred debt issuance costs
|
|
$
|
17,387
|
|
|
$
|
(12,141
|
)
|
|
$
|
14,879
|
|
|
$
|
(3,665
|
)
|
|
$
|
16,460
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deferred debt issuance costs are recorded within other long-term
assets on our consolidated balance sheets and are amortized to
interest expense, net, in our consolidated income statements on
a straight-line basis, which approximates the effective yield
amortization method, over the term of the related credit
facility.
F-175
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Interest
Rate Swaps and Caps
During 2009, we used $28.7 million of the net proceeds to
us from the issuance of our 7.75% Notes to settle and
terminate certain interest rate swap contracts with a notional
amount of $390.0 million. Subsequently, we paid
$6.1 million to settle and terminate our remaining interest
rate swap contract with a notional amount of $50.0 million.
As a result of the settlement and termination of these interest
rate swap contracts, we recognized a charge of
$30.9 million (approximately $18.9 million after tax)
during the year ended December 31, 2009 within total other
non-operating expense, net, in our consolidated income
statement. At December 31, 2008, approximately
$23.3 million, net of taxes, was recorded in accumulated
other comprehensive loss, related to our interest rate swap
contracts. As a result of their settlement and termination, we
reclassified $23.3 million out of accumulated other
comprehensive loss to loss on extinguishment of debt within
total other non-operating expense, net, in our consolidated
income statement for the year ended December 31, 2009.
At December 31, 2009, we had two interest rate cap
contracts with a notional principal amount of
$175.0 million outstanding for which the cap rate of 6.00%
was significantly higher than prevailing market interest rates;
therefore, the fair market value was zero. The interest rate
caps matured in February of 2010.
Additional
Information Regarding Our Financial Covenants
The lenders under our revolving credit facility use adjusted
EBITDA as the basis of calculation of our compliance with our
Leverage Ratio (Total Debt divided by the last twelve
months’ adjusted EBITDA) and Interest Coverage Ratio (last
twelve months’ adjusted EBITDA divided by Cash Interest
Expense). Both the Leverage Ratio and the Interest Coverage
Ratio are measures our lenders use to monitor our performance
and ability to generate positive cash flows.
Adjusted EBITDA is defined in our revolving credit facility as
net income plus interest expense, depreciation and amortization,
income taxes, and other adjustments. Other adjustments include,
but are not limited to, the following to the extent that they
are included in net income:
|
|
|
| |
•
|
Write offs, write-downs, asset revaluations and other non-cash
charges, losses, and expenses, including non-cash equity
compensation expense;
|
| |
| |
•
|
Impairments of intangibles and goodwill;
|
| |
| |
•
|
Extraordinary gains and losses;
|
| |
| |
•
|
Fees paid pursuant to our Management Agreement, as amended, with
Talecris Holdings, LLC, which was terminated in connection with
our IPO;
|
| |
| |
•
|
Fees and expenses incurred in connection with transactions and
permitted acquisitions and investments;
|
| |
| |
•
|
Extraordinary, unusual, or non-recurring charges and expenses
including transition, restructuring, and “carve-out”
expenses;
|
| |
| |
•
|
Legal, accounting, consulting, and other expenses relating to
potential or actual issuances of equity interests, including an
initial public offering of common stock;
|
| |
| |
•
|
Costs associated with our Special Recognition Bonuses; and
|
| |
| |
•
|
Other items.
|
In addition to our lenders, adjusted EBITDA is used by our
management and the compensation committee of our Board of
Directors.
Our management uses adjusted EBITDA as one of our primary
financial performance measures in the
day-to-day
oversight of our business to, among other things, allocate
financial and human resources across our
F-176
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
organization, determine appropriate levels of capital investment
and research and development spending, determine staffing needs
and develop hiring plans, manage our plants’ production
plans, and assess appropriate levels of sales and marketing
initiatives. Our management uses adjusted EBITDA in its decision
making because this supplemental operating performance measure
facilitates internal comparisons to historical operating results
and external comparisons to competitors’ historical
operating results by eliminating various income and expense
items which are either not part of operating income or may vary
significantly when comparing our results among the periods
presented to our competitors or other companies.
The compensation committee of our Board of Directors uses
adjusted EBITDA as a financial performance objective because it
is one of our primary financial performance measures used in the
day-to-day
oversight of our business to, among other things, allocate
financial and human resources across our organization, determine
appropriate levels of capital investment and research and
development spending, determine staffing needs and develop
hiring plans, manage our plants’ production plans, and
assess appropriate levels of sales and marketing initiatives. In
order to motivate top performance by our executives, we
establish a target level for each of the various performance
criteria that is high enough that there is no certainty it is
achievable. The target level for any performance criterion
changes from year to year. These target performance levels
reflect challenges with respect to various factors such as sales
volume and pricing, cost control, working capital management,
plasma platform objectives, R&D objectives and sales and
marketing objectives, among others. Our compensation committee
has discretion to adjust the actual results related to the
performance targets positively or negatively for items which, in
the opinion of the compensation committee, were not reasonably
within management’s control. The compensation committee
also evaluates the manner in which actual results were achieved
to determine if unusual actions or risks were taken that would
impact or manipulate the results.
Components of our provision for income taxes are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Current provision:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
$
|
58,907
|
|
|
$
|
68,960
|
|
|
$
|
28,639
|
|
|
State and local
|
|
|
5,418
|
|
|
|
3,421
|
|
|
|
4,590
|
|
|
Foreign
|
|
|
1,793
|
|
|
|
1,348
|
|
|
|
1,776
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current provision
|
|
|
66,118
|
|
|
|
73,729
|
|
|
|
35,005
|
|
|
Deferred provision:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Federal
|
|
|
12,366
|
|
|
|
69
|
|
|
|
7
|
|
|
State and local
|
|
|
(105
|
)
|
|
|
1,210
|
|
|
|
1,582
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred provision
|
|
|
12,261
|
|
|
|
1,279
|
|
|
|
1,589
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
$
|
78,379
|
|
|
$
|
75,008
|
|
|
$
|
36,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-177
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
A reconciliation of expected income tax expense at the
U.S. Federal rate of 35% to actual income tax expense is as
follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Amount computed at statutory rate
|
|
$
|
85,556
|
|
|
$
|
80,114
|
|
|
$
|
35,837
|
|
|
State income taxes (net of Federal benefit)
|
|
|
7,040
|
|
|
|
4,291
|
|
|
|
4,059
|
|
|
Research and development credits
|
|
|
(11,426
|
)
|
|
|
(7,732
|
)
|
|
|
(4,052
|
)
|
|
State tax credits (net of Federal benefit)
|
|
|
(1,607
|
)
|
|
|
(871
|
)
|
|
|
(600
|
)
|
|
Federal benefit of tax deductions for qualified production
activities
|
|
|
(6,080
|
)
|
|
|
(2,764
|
)
|
|
|
(2,037
|
)
|
|
Capitalized transaction costs
|
|
|
6,800
|
|
|
|
(2,352
|
)
|
|
|
584
|
|
|
Nondeductible meals and entertainment expenses
|
|
|
671
|
|
|
|
504
|
|
|
|
425
|
|
|
Other
|
|
|
(2,575
|
)
|
|
|
3,818
|
|
|
|
2,378
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Provision for income taxes
|
|
$
|
78,379
|
|
|
$
|
75,008
|
|
|
$
|
36,594
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We calculate a provision for, or benefit from, income taxes
using the asset and liability method. Deferred tax assets and
liabilities are recognized for future consequences attributable
to differences between the financial statement carrying amounts
of existing assets and liabilities and their respective tax
bases and operating loss and tax credit carry-forwards. Deferred
tax assets and liabilities are measured using enacted tax rates
expected to apply to taxable income in the years in which those
temporary differences are expected to be recovered or settled.
The effect on deferred tax assets and liabilities of a change in
tax rates is recognized in income in the period that includes
the enactment date. The major components of our deferred tax
assets and liabilities are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Current:
|
|
|
|
|
|
|
|
|
|
Deferred income tax assets:
|
|
|
|
|
|
|
|
|
|
Allowances on accounts receivable
|
|
$
|
8,074
|
|
|
$
|
11,020
|
|
|
Inventories
|
|
|
32,026
|
|
|
|
23,928
|
|
|
Revenue recognition
|
|
|
1,455
|
|
|
|
7,857
|
|
|
Stock-based compensation
|
|
|
23,855
|
|
|
|
30,952
|
|
|
Deferred bonuses
|
|
|
4,200
|
|
|
|
4,617
|
|
|
Accrued expenses
|
|
|
21,994
|
|
|
|
4,568
|
|
|
State tax credit carry-forward
|
|
|
3,335
|
|
|
|
3,195
|
|
|
Other
|
|
|
2,341
|
|
|
|
3,543
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred income tax assets
|
|
|
97,280
|
|
|
|
89,680
|
|
|
Deferred income tax liabilities:
|
|
|
|
|
|
|
|
|
|
Other liabilities
|
|
|
(687
|
)
|
|
|
(1,028
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred income tax liabilities
|
|
|
(687
|
)
|
|
|
(1,028
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net current deferred income tax assets
|
|
$
|
96,593
|
|
|
$
|
88,652
|
|
|
|
|
|
|
|
|
|
|
|
F-178
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Non-current:
|
|
|
|
|
|
|
|
|
|
Deferred income tax assets:
|
|
|
|
|
|
|
|
|
|
Property, plant, and equipment
|
|
$
|
—
|
|
|
$
|
14,170
|
|
|
Other
|
|
|
459
|
|
|
|
252
|
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred income tax assets
|
|
|
459
|
|
|
|
14,422
|
|
|
Deferred income tax liabilities:
|
|
|
|
|
|
|
|
|
|
Property, plant, and equipment
|
|
|
(1,292
|
)
|
|
|
—
|
|
|
Intangibles
|
|
|
(13,599
|
)
|
|
|
(8,574
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Total deferred income tax liabilities
|
|
|
(14,891
|
)
|
|
|
(8,574
|
)
|
|
|
|
|
|
|
|
|
|
|
|
Net non-current deferred income tax (liabilities) assets
|
|
$
|
(14,432
|
)
|
|
$
|
5,848
|
|
|
|
|
|
|
|
|
|
|
|
|
Net deferred income tax assets
|
|
$
|
82,161
|
|
|
$
|
94,500
|
|
|
|
|
|
|
|
|
|
|
|
We record a valuation allowance to reduce our deferred tax
assets to the amount that we believe is more likely than not to
be realized. In assessing the need for a valuation allowance, we
consider all available evidence, both positive and negative,
including historical levels of income, expectations and risks
associated with future taxable income, and ongoing prudent and
feasible tax planning strategies.
We have not provided for U.S. Federal income and foreign
withholding taxes on our
non-U.S. subsidiaries’
cumulative undistributed earnings of approximately
$13.2 million as of December 31, 2010 as such earnings
are intended to be reinvested outside of the
U.S. indefinitely. It is not practicable to estimate the
amount of tax that might be payable if some or all of such
earnings were to be remitted, and foreign tax credits would be
available to reduce or eliminate the resulting U.S. income
tax liability.
At December 31, 2010 we had state tax credit carryforwards
of $5.1 million that will start expiring in 2015. Our
ability to offset future taxable income with tax credit
carryforwards may be limited in certain circumstances, including
changes in ownership.
The following table summarizes activity related to our gross
unrecognized tax positions:
| |
|
|
|
|
|
Unrecognized tax benefits at December 31, 2007
|
|
$
|
6,885
|
|
|
Additions for tax positions taken in the current year
|
|
|
3,626
|
|
|
Reductions for tax positions taken in a prior year
|
|
|
(521
|
)
|
|
|
|
|
|
|
|
Unrecognized tax benefits at December 31, 2008
|
|
|
9,990
|
|
|
Additions for tax positions taken during a prior year
|
|
|
3,899
|
|
|
Reductions for tax positions taken in a prior year
|
|
|
(1,642
|
)
|
|
|
|
|
|
|
|
Unrecognized tax benefits at December 31, 2009
|
|
|
12,247
|
|
|
Additions for tax positions taken in the current year
|
|
|
2,485
|
|
|
Reductions for tax positions taken in a prior year
|
|
|
(5,908
|
)
|
|
|
|
|
|
|
|
Unrecognized tax benefits at December 31, 2010
|
|
$
|
8,824
|
|
|
|
|
|
|
|
As of December 31, 2010, our total gross unrecognized tax
benefits were approximately $8.8 million, of which
approximately $5.8 million would reduce our effective
income tax rate if recognized. Interest and penalties related to
unrecognized tax benefits are included in income tax expense. No
material interest or penalties were incurred during the years
presented.
F-179
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
The IRS exam of the 2005, 2006, and 2007 tax years is
effectively settled as the Joint Committee on Taxation has
completed its review of our position and has agreed not to take
exception to the refund approved by the Internal Revenue Service
in its report dated June 2009. The favorable resolution of this
matter resulted in a release of $4.7 million gross
unrecognized tax benefits.
We have analyzed our filing positions for all open years in all
significant Federal, state, and foreign jurisdictions where we
are required to file income tax returns. The periods subject to
examination by the major tax jurisdictions where we conduct
business are tax periods 2005 through 2010.
|
|
|
14.
|
Commitments
and Contingencies
|
Leases
We lease office buildings, plasma collection centers,
refrigerated storage, furniture, machinery, computer equipment,
and miscellaneous equipment under leasing agreements. The
majority of our leases are operating leases. In addition to
rent, certain of our leases require us to pay directly for
taxes, insurance, maintenance, and other operating expenses.
Future minimum lease payments required under our capital leases
and non-cancellable operating leases as of December 31,
2010 are as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Non-Cancellable
|
|
|
|
|
|
|
|
Operating
|
|
|
|
|
Capital
|
|
|
Leases
|
|
|
|
|
2011
|
|
$
|
1,814
|
|
|
$
|
17,427
|
|
|
2012
|
|
|
1,836
|
|
|
|
14,614
|
|
|
2013
|
|
|
1,862
|
|
|
|
10,246
|
|
|
2014
|
|
|
1,830
|
|
|
|
8,921
|
|
|
2015
|
|
|
1,729
|
|
|
|
7,016
|
|
|
Thereafter
|
|
|
4,845
|
|
|
|
27,198
|
|
|
|
|
|
|
|
|
|
|
|
|
Total future minimum lease payments
|
|
|
13,916
|
|
|
$
|
85,422
|
|
|
|
|
|
|
|
|
|
|
|
|
Less: amounts representing interest
|
|
|
(4,376
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Present value of net minimum lease payments
|
|
|
9,540
|
|
|
|
|
|
|
Less: current portion of capital lease obligations
|
|
|
(860
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
8,680
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In the preceding table, the future minimum annual rentals
payable under non-cancellable leases denominated in foreign
currencies have been calculated based upon the December 31,
2010 foreign currency exchange rates. Rental cost was
approximately $18.2 million, $16.6 million, and
$14.8 million for the years ended December 31, 2010,
2009, and 2008, respectively.
Employment
Agreements
We have employment agreements and offer letters with certain of
our employees which require payments generally ranging from 100%
to 150% of the employee’s annual compensation if employment
is terminated not for cause by us, or by the employee, for good
reason, as defined. Certain of these arrangements also include
provisions for payments of bonuses under our annual incentive
plan and the vesting of equity awards, as well as other
customary payments, such as accrued personal days, bonuses,
continuing benefits, and outplacement services. In the event of
a change of control of the Company, our share-based compensation
plans permit accelerated vesting of awards under defined
circumstances.
F-180
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Customer
Commitments
We have supply agreements with some of our customers which
require us to provide certain minimum quantities of our products
for various periods. At December 31, 2010, we currently
anticipate being able to fill these supply agreements in the
foreseeable future and we do not consider our potential exposure
for unfilled customer orders to be material.
Litigation
We are involved in various legal and regulatory proceedings that
arise in the ordinary course of business. We record accruals for
such contingencies to the extent that we conclude that their
occurrence is both probable and estimable. We consider many
factors in making these assessments, including the professional
judgment of experienced members of management and our legal
counsel. We have estimated the likelihood of unfavorable
outcomes and the amounts of such potential losses. In our
opinion, the ultimate outcome of these proceedings and claims is
not anticipated to have a material adverse effect on our
consolidated financial position, results of operations, or cash
flows. However, the ultimate outcome of litigation is
unpredictable and actual results could be materially different
from our estimates. We record anticipated recoveries under
applicable insurance contracts when we are assured of recovery.
National
Genetics Institute/Baxter Healthcare Corporation
Litigation
In May 2008, Baxter Healthcare Corporation (Baxter) and National
Genetics Institute (NGI), a wholly-owned subsidiary of
Laboratory Corporation of America, filed a complaint in the
U.S. District Court for the Eastern District of North
Carolina, alleging that we infringed U.S. Patent Nos.
5,780,222, 6,063,563, and 6,566,052. They subsequently withdrew
and re-filed the case in November 2008. The patents deal
primarily with a method of screening large numbers of biological
samples utilizing various pooling and matrix array strategies,
and the complaint alleges that the patents are owned by Baxter
and exclusively licensed to NGI. In November 2008, we filed our
answer to their complaint, asserting anti-trust and other
counterclaims, and filed a request for re-examination of the
patents with the Patent and Trademark Office (PTO), which was
subsequently granted. The case was settled effective
October 1, 2010, with us paying $3.9 million to NGI,
which was accrued in 2009, and us receiving a
paid-up
license to the technology subject to the disputed patents and
the parties dismissing their claims and counterclaims.
Plasma
Centers of America, LLC and G&M Crandall Limited Family
Partnership
We had a three year Amended and Restated Plasma Sale/Purchase
Agreement with Plasma Centers of America, LLC (PCA) under which
we were required to purchase annual minimum quantities of plasma
from plasma collection centers approved by us, including the
prepayment of 90% for unlicensed plasma. We were also committed
to finance the development of up to eight plasma collection
centers, which were to be used to source plasma for us. Under
the terms of the agreement, we had a conditional obligation to
purchase such centers for a sum determined by a formula set
forth in the agreement. We provided $3.2 million in
financing, including accrued interest, related to the
development of such centers, and we advanced payment of
$1.0 million for unlicensed plasma. We recorded a provision
within SG&A during 2008 related to these loans and advances.
In August 2008, we notified PCA that they were in breach of the
Amended and Restated Plasma Sale/Purchase Agreement. We
terminated the agreement in September 2008. In November 2008,
TPR filed suit in federal court in Raleigh against the G&M
Crandall Limited Family Partnership and its individual partners
as guarantors of obligations of PCA. We were served in January
2009 in parallel state action by PCA, alleging breach of
contract by TPR. The federal case has been stayed. On
December 13, 2010, a jury in the state court case rendered
a verdict in the amount of $37.0 million in favor of PCA
against TPR in a breach of contract claim, which was confirmed
by the court in post trial motions. We intend to appeal. The
jury verdict, if
F-181
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
sustained, will bear simple interest at 8% per statute from the
date of breach, which totals approximately $6.7 million at
December 31, 2010. We have recorded a $43.7 million
charge within other non-operating expense, net, in our
consolidated income statement for the year ended
December 31, 2010 as a result of the judgment.
Foreign
Corrupt Practices Act
We are conducting an internal investigation into potential
violations of the Foreign Corrupt Practices Act (FCPA) that we
became aware of during the conduct of an unrelated review. The
FCPA investigation is being conducted by outside counsel under
the direction of a special committee of our board of directors.
The investigation initially focused on sales to certain Eastern
European and Middle Eastern countries, primarily Belarus,
Russia, and Iran, but we are also reviewing sales practices in
Brazil, Bulgaria, China, Georgia, Libya, Poland, Turkey,
Ukraine, and other countries as deemed appropriate.
In July 2009, we voluntarily contacted the U.S. Department
of Justice (DOJ) to advise them of the investigation and to
offer our cooperation in any investigation that they want to
conduct or they want us to conduct. The DOJ has not indicated
what action it may take, if any, against us or any individual,
or the extent to which it may conduct its own investigation.
Even though we self-disclosed this matter to the DOJ, it or
other federal agencies may seek to impose sanctions on us that
may include, among other things, debarment, injunctive relief,
disgorgement, fines, penalties, appointment of a monitor,
appointment of new control staff, or enhancement of existing
compliance and training programs. Other countries in which we do
business may initiate their own investigations and impose
similar penalties. As a result of this investigation, we
suspended shipments to some of these countries while we put
additional safeguards in place. In some cases, safeguards
involved terminating consultants and suspending relations with
or terminating distributors in countries under investigation as
circumstances warranted. These actions unfavorably affected
revenue from these countries in 2010 and 2009. We have resumed
sales in countries where we believe we have appropriate
safeguards in place and are reallocating product to other
countries as necessary. To the extent that we conclude, or the
DOJ concludes, that we cannot implement adequate safeguards or
otherwise need to change our business practices, distributors,
or consultants in affected countries or other countries, this
may result in a permanent loss of business from those countries.
We expect to complete our internal FCPA investigation and
present our findings to the DOJ in 2011. The preliminary
findings of our investigation indicate that it is probable that
there were FCPA violations by persons associated with us that
the DOJ or other regulators may assert are attributable to us.
Any sanctions or related loss of business could have a material
adverse effect on us or our results of operations. It is
possible, however, that any sanctions that DOJ or other federal
agencies might otherwise consider imposing would be reduced, if
not eliminated, in light of the comprehensive compliance
measures that we have implemented. Given the preliminary nature
of our findings, our continuing investigation and the
uncertainties regarding this matter, we are unable to estimate
the financial outcome and consequently, we have not accrued any
amounts related to the outcome of this matter.
Compliance
with Pharmaceutical Pricing Agreement
In November 2009, we received a letter from the United States
Attorney’s Office for the Eastern District of Pennsylvania
(USAO). The USAO requested a meeting to review our compliance
with the terms of the Pharmaceutical Pricing Agreement (PPA)
under the Public Health Service program. Specifically, the USAO
asked for information related to the sale of our IGIV product,
Gamunex, under that program. In order to have federal financial
participation apply to their products under the Medicaid program
and to obtain Medicare Part B coverage, manufacturers are
required to enter into a PPA. The PPA obligates manufacturers to
charge covered entities the Public Health Service price for
drugs intended for outpatient use. The Public Health Service
price is based on the Medicaid rebate amount. We believe that we
have complied with the terms of
F-182
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
the PPA and federal law. If the USAO determines that our
practices are inconsistent with the terms of the PPA, the USAO
has stated that it may file a civil action against us under the
Anti-fraud Injunction Act and seek a court order directing the
company to comply with the PPA or, potentially, proceed under
some other legal theory. We could also be subject to fines,
damages, penalties, appointment of a monitor, or enhancement of
existing compliance and training programs as a result of
government action. We are cooperating with the investigation and
intend to respond to information requests from the USAO. Based
on the information obtained to date, we have not determined that
any potential liability that may result is probable or can be
reasonably estimated. Therefore, we have not made any accrual in
our consolidated financial statements at December 31, 2010.
Exclusive
License Agreements with Crucell N.V. (Crucell)
During September 2008, we entered into an exclusive commercial
license agreement with Crucell for recombinant technology. In
consideration of the license that Crucell granted us, we paid an
upfront license fee of $2.5 million, which we recorded in
R&D in our consolidated income statement during the year
ended December 31, 2008. During the year ended
December 31, 2010, we paid $1.5 million to Crucell,
which we recorded in R&D in our consolidated income
statement. We could be required to pay up to $28.0 million
of additional development milestones as certain activities are
completed. Upon commercialization of the product, we are
required to pay a royalty at a tiered rate, ranging between 3.5%
and 6%, based on the related net sales of the product.
During December 2008, we entered into another exclusive
commercial license agreement with Crucell for recombinant
technology. In consideration of the license that Crucell granted
us, we paid an upfront license fee of $1.5 million, which
we recorded in R&D in our consolidated income statement for
the year ended December 31, 2008. During the year ended
December 31, 2009, we paid $0.5 million to Crucell,
which is included in R&D in our consolidated income
statement. We could be required to pay up to $18.5 million
of additional development milestones as certain activities are
completed. Upon commercialization of the product, we are
required to pay a royalty at a tiered rate, ranging between 3%
and 5%, based on the related net sales of the product.
Under the terms of both exclusive license agreements with
Crucell, we may terminate either agreement by giving Crucell
90 days prior written notice and payment of all outstanding
amounts owed to Crucell.
Purchase
Commitments
We have purchase agreements that require us to purchase minimum
annual quantities of plasma, other raw materials, and associated
subcontracted manufacturing services and other services. At
December 31, 2010, our purchase commitments, generally
subject to annual price negotiations, are as follows:
| |
|
|
|
|
|
2011
|
|
$
|
201,875
|
|
|
2012
|
|
|
179,850
|
|
|
2013
|
|
|
167,637
|
|
|
2014
|
|
|
127,080
|
|
|
2015
|
|
|
121,308
|
|
|
Thereafter
|
|
|
55,595
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
853,345
|
|
|
|
|
|
|
|
An inability of any of our suppliers to satisfy their
obligations in a timely manner could cause a disruption in our
plasma supply, which could materially adversely affect our
business.
F-183
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
We entered into a contract, effective January 1, 2011, for
subcontract manufacturing services that is substantially similar
to, and replaces, one to which we were a party that expired on
December 31, 2010. The minimum purchase obligations under
this contract, which expires on December 31, 2012, of
approximately $30 million for both 2011 and 2012 are not
included in the table above.
On January 6, 2011, we entered into a contract for
subcontract manufacturing services. The minimum purchase
obligations of approximately $4.0 million for each of the
years 2012 through 2015 are not included in the table above.
In addition to the minimum purchase commitments in the table
above, at December 31, 2010, we have commitments and open
purchase orders for capital spending to be made of
$214.5 million.
Environmental
Matters
Our operations are subject to extensive and evolving federal,
state, and local environmental laws and regulations. Compliance
with such laws and regulations can be costly. Additionally,
governmental authorities may enforce the laws and regulations
with a variety of civil and criminal enforcement measures,
including monetary penalties and remediation requirements. It is
possible that new information or future developments could
require us to reassess our potential exposure related to
environmental matters. We may incur significant costs and
liabilities in order to comply with existing environmental laws
and regulations. It is also possible that other developments,
such as increasingly strict environmental laws and regulations
and claims for damages to property, employees, other persons,
and the environment resulting from current or past operations,
could result in substantial costs and liabilities in the future
as this information becomes available, or other relevant
developments occur. We establish accrued liabilities or adjust
previously accrued amounts accordingly. While there are still
uncertainties relating to the ultimate costs we may incur, based
upon our evaluation and experience to date, we believe that
compliance with all applicable laws and regulations will not
have a material adverse impact on our financial position,
operating results, or cash flows. At December 31, 2010 and
2009, no amounts have been accrued as we are not currently aware
of any probable liabilities.
Other
All pharmaceutical companies, including us, are subject to
periodic inspections by the FDA and other regulatory authorities
of manufacturing and plasma collection facilities, procedures,
and processes. If in the course of an inspection, the FDA or
other regulatory authority notes conditions they believe are
objectionable with respect to cGMP or other applicable
regulations, we must implement effective corrective actions or
face regulatory or enforcement sanctions.
|
|
|
15.
|
Related
Party Transactions
|
Until January 21, 2010, a majority of our outstanding
common stock was owned by Talecris Holdings, LLC. Talecris
Holdings, LLC is owned by (i) Cerberus-Plasma Holdings LLC,
the managing member of which is Cerberus Partners, L.P., and
(ii) limited partnerships affiliated with Ampersand
Ventures. Substantially all rights of management and control of
Talecris Holdings, LLC are held by Cerberus-Plasma Holdings LLC.
As of December 31, 2010, Talecris Holdings, LLC owned
approximately 48.7% of our outstanding common stock. We had a
management agreement with Cerberus-Plasma Holdings LLC and an
affiliate of Ampersand Ventures, which was terminated as of
September 30, 2009 in connection with our IPO. We have a
Master Consulting and Advisory Services Agreement with an
affiliate of Cerberus to provide certain advisory services to us.
We have an equity investment in Centric as further discussed in
Note 11, “Investment in Affiliate;” therefore, we
consider Centric to be a related party during the periods
presented.
F-184
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
The following table summarizes our related party transactions
for the years ended December 31, 2010, 2009, and 2008 and
our related party accounts payable balances at December 31,
2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
Expenses
|
|
2010
|
|
2009
|
|
2008
|
|
|
|
Centric (product distribution and other services)
|
|
$
|
22,865
|
|
|
$
|
20,306
|
|
|
$
|
17,508
|
|
|
Cerberus/Ampersand (management fees)
|
|
$
|
—
|
|
|
$
|
5,715
|
|
|
$
|
6,871
|
|
|
Cerberus (operational support)
|
|
$
|
—
|
|
|
$
|
608
|
|
|
$
|
4,184
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
Payable
|
|
2010
|
|
2009
|
|
|
|
Centric (product distribution and other services)
|
|
$
|
6,406
|
|
|
$
|
5,537
|
|
|
Cerberus (operational support)
|
|
$
|
—
|
|
|
$
|
349
|
|
On October 6, 2009, we completed our IPO of
56,000,000 shares of our common stock, par value $0.01 per
share. Additional information regarding our IPO is included in
Note 4, “Initial Public Offering and Use of
Proceeds.”
A
seven-for-one
share dividend on our common stock was paid on
September 10, 2009. All share and per-share amounts have
been retroactively adjusted for all periods presented to reflect
the share dividend.
On September 30, 2009, 1,000,000 shares of our
Series A preferred stock and 192,310 shares of our
Series B preferred stock were converted into an aggregate
of 85,846,320 shares of our common stock in connection with
our IPO. In addition, on September 30, 2009,
2,381,548 shares of our common stock were issued to settle
$45.3 million of accrued dividends upon the conversion of
our Series A and B preferred stock. Additional information
regarding our Series A and B preferred stock is included in
Note 17, “Redeemable Series A and B Senior
Convertible Preferred Stock.”
During the year ended December 31, 2008, we repurchased
2,146,232 shares of our common stock from IBR for
$35.4 million at a put value of $15.61 per share plus
accrued charges. The shares were issued to IBR during the year
ended December 31, 2007 as a result of the acceleration of
the contingent consideration provision of our November 2006
Asset Purchase Agreement, as amended. Additional information
regarding the shares repurchased from IBR is included in
Note 6 “Business Acquisitions.”
During the year ended December 31, 2008, our board of
directors approved the retirement of 2,212,640 shares of
our common stock held in treasury, and approved that shares of
our common stock repurchased in the future would be immediately
retired by the Company.
Information regarding employee share-based compensation is
included in Note 18, “Share-Based Compensation.”
|
|
|
17.
|
Redeemable
Series A and B Senior Convertible Preferred Stock
|
On September 30, 2009, 1,000,000 shares of our
Series A preferred stock and 192,310 shares of our
Series B preferred stock were converted into an aggregate
of 85,846,320 shares of our common stock in connection with
our IPO. In addition, on September 30, 2009,
2,381,548 shares of our common stock were issued to settle
$45.3 million of accrued dividends upon the conversion of
our Series A and B preferred stock.
F-185
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
18.
|
Share-Based
Compensation
|
In connection with our IPO, we ceased further grants under our
2005 Stock Option and Incentive Plan and 2006 Restricted Stock
Plan. The Talecris Biotherapeutics Holdings Corp. 2009 Long-Term
Incentive Plan (2009 Plan), which was adopted by our board of
directors on August 7, 2009, became effective in connection
with our IPO. The 2009 Plan provides for the grant of awards in
the form of incentive stock options, nonqualified stock options,
share appreciation rights, restricted stock, RSU’s,
unrestricted shares of common stock, deferred share units, and
performance awards. Our employees, directors, and consultants
are eligible to receive awards under the 2009 Plan. The maximum
number of shares that we may issue for all awards under the 2009
Plan is 7,200,000. As of December 31, 2010,
5,798,837 shares remain available for grant under the 2009
Plan.
We value share-based compensation at the grant date using a fair
value model and recognize this value as expense over the
employees’ requisite service period, typically the period
over which the share-based compensation vests. We classify
share-based compensation costs consistent with each
grantee’s salary. In connection with stock option exercise
and restricted share vesting, we recognized net tax benefits of
$26.6 million, $20.4 million, and $3.3 million
for the years ended December 31, 2010, 2009 and 2008,
respectively. We record income tax benefits realized upon
exercise or vesting of an award in excess of that previously
recognized in earnings as additional
paid-in-capital.
We recognized excess tax benefits related to share-based
compensation of $13.5 million and $13.4 million during
the years ended December 31, 2010 and 2009, respectively.
Share-based compensation expense for the years ended
December 31, 2010, 2009, and 2008 was as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
SG&A
|
|
$
|
12,606
|
|
|
$
|
40,968
|
|
|
$
|
33,780
|
|
|
R&D
|
|
|
1,024
|
|
|
|
2,303
|
|
|
|
2,361
|
|
|
Cost of goods sold
|
|
|
3,336
|
|
|
|
4,275
|
|
|
|
2,566
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total expense
|
|
$
|
16,966
|
|
|
$
|
47,546
|
|
|
$
|
38,707
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Capitalized in inventory
|
|
$
|
2,265
|
|
|
$
|
3,574
|
|
|
$
|
3,233
|
|
Amounts capitalized in inventory are recognized in cost of goods
sold in our consolidated income statements primarily within
twelve months.
The following table summarizes the remaining unrecognized
compensation cost related to our share-based compensation awards
as of December 31, 2010 and the weighted average period
over which the non-cash compensation cost is expected to be
recognized:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted-
|
|
|
|
|
Unrecognized
|
|
|
Average
|
|
|
|
|
Compensation
|
|
|
Period
|
|
|
|
|
Cost
|
|
|
(Years)
|
|
|
|
|
Stock options
|
|
$
|
3,965
|
|
|
|
2.10
|
|
|
Restricted share awards
|
|
|
721
|
|
|
|
0.25
|
|
|
RSU’s
|
|
|
6,589
|
|
|
|
2.10
|
|
|
Performance share awards
|
|
|
1,138
|
|
|
|
1.25
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
12,413
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In addition to the unrecognized compensation cost included in
the table above, at December 31, 2010, $1.7 million of
compensation cost was included in inventory on our consolidated
balance sheet, which we
F-186
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
expect to be recognized as non-cash compensation expense in our
consolidated income statement primarily within the next twelve
months. The amount of share-based compensation expense that we
will ultimately be required to record could change in the future
as a result of additional grants, changes in the fair value of
shares for performance-based awards, differences between our
anticipated forfeiture rate and the actual forfeiture rate, the
probability of achieving targets established for performance
award vesting, and other actions by our board of directors or
its compensation committee.
Stock
Options
Stock options, which entitle the holder to purchase, after the
end of a vesting term, a specified number of shares of our
common stock at a price per share equal to the exercise price,
are accounted for at fair value at the date of the grant. Option
awards are granted with an exercise price at least equal to the
fair value of our common stock at the date of grant and
generally vest over periods of three to five years. The exercise
price of stock options is determined by our board of directors.
The stock options that we granted to employees typically have
service-based and performance-based components. The stock
options granted to members of our board of directors are
service-based only. Our stock options generally expire ten years
after the date of grant, or earlier if an option holder ceases
to be employed by the Company. Stock option exercises are
settled with newly issued common stock previously authorized and
available for issuance.
The following is a summary of stock option activity for the
years ended December 31, 2010, 2009, and 2008:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Remaining
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Contractual
|
|
|
Aggregate
|
|
|
|
|
|
|
|
Exercise
|
|
|
Term
|
|
|
Intrinsic
|
|
|
|
|
Shares
|
|
|
Price
|
|
|
(Years)
|
|
|
Value
|
|
|
|
|
Outstanding at December 31, 2007
|
|
|
12,932,344
|
|
|
$
|
6.42
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
2,291,304
|
|
|
$
|
10.98
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(945,232
|
)
|
|
$
|
2.98
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2008
|
|
|
14,278,416
|
|
|
$
|
6.96
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
638,472
|
|
|
$
|
18.91
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(392,688
|
)
|
|
$
|
4.89
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(2,394,762
|
)
|
|
$
|
3.17
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2009
|
|
|
12,129,438
|
|
|
$
|
8.40
|
|
|
|
6.7
|
|
|
$
|
168,235
|
|
|
Granted
|
|
|
73,593
|
|
|
$
|
20.39
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(24,950
|
)
|
|
$
|
19.05
|
|
|
|
|
|
|
|
|
|
|
Exercised
|
|
|
(3,650,579
|
)
|
|
$
|
6.12
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2010
|
|
|
8,527,502
|
|
|
$
|
9.45
|
|
|
|
5.7
|
|
|
$
|
118,090
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2010
|
|
|
7,882,452
|
|
|
$
|
8.66
|
|
|
|
4.8
|
|
|
$
|
115,419
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Vested and expected to vest at December 31, 2010
|
|
|
8,488,004
|
|
|
$
|
9.36
|
|
|
|
5.7
|
|
|
$
|
118,296
|
|
At December 31, 2009 and 2008, stock options with a
weighted average exercise price of $8.16 and $4.02 were
exercisable for 8,711,838 shares and 6,950,872 shares,
respectively. Our estimate of the stock options vested and
expected to vest at December 31, 2010 considers an expected
forfeiture rate of 3%.
The aggregate intrinsic value in the table above represents the
difference between the $23.30 closing price of our common stock
as reported by The NASDAQ Global Select Market on
December 31, 2010 and the weighted average exercise price,
multiplied by the number of options outstanding or exercisable.
The total
F-187
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
intrinsic value and net cash proceeds to us from stock option
exercises during the year ended December 31, 2010 were
$62.7 million and $22.3 million, respectively. We do
not record the aggregate intrinsic value for financial
accounting purposes and the value changes based upon changes in
the fair value of our common stock. The total fair value of
stock options that vested during the years ended
December 31, 2010, 2009, and 2008 were $56.1 million,
$72.5 million, and $24.7 million, respectively.
The weighted average grant date fair value of options granted
during the years ended December 31, 2010, 2009, and 2008
were $9.99, $9.49, and $4.35, respectively. We estimated the
fair value of stock options at their grant date using the
Black-Scholes option pricing model. This model was developed for
use in estimating the fair value of traded options that have no
vesting restrictions and are fully transferable, characteristics
that are not present in our option grants. If the model
permitted consideration of the unique characteristics of
employee stock options, the resulting estimate of the fair value
of stock options could be different. The following
weighted-average assumptions were used to estimate the fair
value of stock options granted during the years ended
December 31, 2010, 2009, and 2008:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
2010
|
|
2009
|
|
2008
|
|
|
|
Risk-free interest rate
|
|
|
2.66
|
%
|
|
|
2.66
|
%
|
|
|
2.65
|
%
|
|
Expected term (years)
|
|
|
5.66
|
|
|
|
5.97
|
|
|
|
5.20
|
|
|
Expected volatility
|
|
|
50
|
%
|
|
|
50
|
%
|
|
|
50
|
%
|
|
Expected dividend yield
|
|
|
0
|
%
|
|
|
0
|
%
|
|
|
0
|
%
|
The risk-free interest rate is based upon the U.S. Treasury
yield curve at the time of grant. The expected life of options
is reflective of our historical experience, vesting schedules,
and contractual terms. There is limited trading history for our
common stock; therefore, our application of the Black-Scholes
option pricing model incorporated historical volatility measures
of similar public companies. We currently do not expect to pay
dividends in the future. We generally apply a 3% forfeiture rate
to the options granted over the term of the award. This rate is
calculated based upon historical attrition rates and represents
an estimate of the granted options not expected to vest. If
actual forfeitures differ from the expected rate, we may be
required to make additional adjustments to compensation expense
in future periods.
Restricted
Stock Units
RSU’s, which entitle the holder to receive, at the end of a
vesting term, a specified number of shares of our common stock,
are accounted for at fair value at the date of grant. We granted
483,100 RSU’s in connection with our IPO. These RSU’s
will vest one-third on each of April 1 of 2011, 2012, and 2013,
subject to the award holder being employed on the vesting date.
The aggregate fair value of the RSU’s was
F-188
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
$8.4 million, which will be recognized as compensation
expense ratably through April of 2013. The following is a
summary of RSU activity for the years ended December 31,
2010 and 2009:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
Remaining
|
|
|
|
|
|
|
|
|
|
|
Average
|
|
|
Contractual
|
|
|
Aggregate
|
|
|
|
|
|
|
|
Grant Date
|
|
|
Term
|
|
|
Intrinsic
|
|
|
|
|
Shares
|
|
|
Fair Value
|
|
|
(Years)
|
|
|
Value
|
|
|
|
|
Outstanding at December 31, 2008
|
|
|
—
|
|
|
|
—
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
483,100
|
|
|
$
|
19.00
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(3,076
|
)
|
|
$
|
19.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2009
|
|
|
480,024
|
|
|
$
|
19.00
|
|
|
|
|
|
|
|
|
|
|
Granted
|
|
|
36,015
|
|
|
$
|
20.40
|
|
|
|
|
|
|
|
|
|
|
Forfeited
|
|
|
(30,299
|
)
|
|
$
|
19.04
|
|
|
|
|
|
|
|
|
|
|
Vested
|
|
|
(1,182
|
)
|
|
$
|
19.00
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Outstanding at December 31, 2010
|
|
|
484,558
|
|
|
$
|
19.10
|
|
|
|
2.16
|
|
|
$
|
11,290
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Restricted
Stock
Restricted shares of our common stock, which entitle the holder
to receive, at the end of a vesting term, a specified number of
shares of our common stock, are accounted for at fair value at
the date of grant. Restricted share awards vest on terms
determined by our board of directors or its compensation
committee at the time of the grant. The majority of our
restricted share awards currently outstanding vest annually over
a four-year period from the date of grant unless accelerated by
the compensation committee upon the event of a change in
control, as defined. Any restricted share awards that have not
vested at the time of termination of service are forfeited
except in the event of death, disability, or a change in
control. The restricted share awards are considered issued and
outstanding and have full voting rights. Any dividends declared
with respect to our common stock will vest at the same time as
the underlying restricted stock award.
The following is a summary of restricted share activity for the
years ended December 31, 2010, 2009, and 2008:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
Grant Date
|
|
|
|
|
Shares
|
|
|
Fair Value
|
|
|
|
|
December 31, 2007 unvested shares outstanding
|
|
|
2,811,000
|
|
|
$
|
13.78
|
|
|
Granted
|
|
|
42,720
|
|
|
$
|
9.88
|
|
|
Forfeited
|
|
|
(287,784
|
)
|
|
$
|
11.00
|
|
|
Vested
|
|
|
(870,432
|
)
|
|
$
|
13.45
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2008 unvested shares outstanding
|
|
|
1,695,504
|
|
|
$
|
14.40
|
|
|
Granted
|
|
|
14,464
|
|
|
$
|
16.63
|
|
|
Forfeited
|
|
|
(16,368
|
)
|
|
$
|
11.00
|
|
|
Vested
|
|
|
(779,744
|
)
|
|
$
|
13.50
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2009 unvested shares outstanding
|
|
|
913,856
|
|
|
$
|
15.27
|
|
|
Vested
|
|
|
(727,256
|
)
|
|
$
|
13.74
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, 2010 unvested shares outstanding
|
|
|
186,600
|
|
|
$
|
21.25
|
|
|
|
|
|
|
|
|
|
|
|
F-189
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
The total fair value of restricted shares that vested during the
years ended December 31, 2010, 2009, and 2008 were
$14.5 million, $13.0 million and $8.6 million,
respectively.
Performance
Share Units (PSU’s)
The following is a summary of performance share unit activity
for the year ended December 31, 2010:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted
|
|
|
|
|
|
|
|
Average
|
|
|
|
|
|
|
|
Grant Date
|
|
|
|
|
Shares
|
|
|
Fair Value
|
|
|
|
|
Outstanding PSU’s outstanding at December 31, 2009
|
|
|
—
|
|
|
$
|
—
|
|
|
Granted
|
|
|
261,327
|
|
|
$
|
21.51
|
|
|
Forfeited
|
|
|
(1,627
|
)
|
|
$
|
21.51
|
|
|
|
|
|
|
|
|
|
|
|
|
Unvested PSU’s outstanding at December 31, 2010
|
|
|
259,700
|
|
|
$
|
21.51
|
|
|
|
|
|
|
|
|
|
|
|
PSU’s are awards that vest based on the achievement of
pre-established objective performance goals, which are generally
financial in nature. For performance awards, the compensation
committee establishes a performance period and the performance
targets for each performance measure that must be achieved at
the end of the performance period for awards to vest. The number
of shares issued upon the vesting of the performance awards
varies based on actual performance in a year relative to a
defined minimum and maximum financial target for that year. The
PSU’s granted on March 8, 2010 will vest annually over
a three-year performance period with the potential for 0% to
125% payout, based on the achievement of annual earnings per
share targets that were established at the time of grant.
Other
Information about our Stock Option Plan
During the third quarter of 2009, we entered into an amended and
restated employment agreement with our Chairman and Chief
Executive Officer which included accelerating the vesting of
options to purchase 1,008,000 shares of our common stock at
an exercise price of $21.25 per common share to August 19,
2009. The acceleration of these options resulted in the
recognition of a non-cash charge of $11.8 million of
compensation expense during the third quarter of 2009. Options
to purchase these shares were previously scheduled to vest in
April of 2010 (504,000 options) and April of 2011 (504,000
options).
During the second quarter of 2008, the compensation committee of
our board of directors amended the exercise price of 570,400
stock options outstanding to certain employees from $21.25 per
share to $11.00 per share and during the second quarter of 2008,
the compensation committee also amended the exercise price of
17,152 stock options outstanding to certain members of our board
of directors from $21.25 per share to $11.00 per share. The
stock options that were re-priced were granted during 2007.
During the first quarter of 2008, our board of directors revised
the 2008 corporate objectives related to the performance-based
component of stock options scheduled to vest on April 1,
2009. In addition, during the second quarter of 2008, we began
recognizing compensation cost related to the performance-based
component of stock options scheduled to vest on April 1,
2010 based on our probability assessment of achieving the
related performance objectives.
|
|
|
19.
|
Employee
Benefit Plans
|
Savings
Plan and Profit Sharing Plan
We have a defined contribution plan (Savings Plan), which
qualifies as a deferred salary arrangement under
Section 401(k) of the Internal Revenue Code (IRC). Once
eligible, employees may elect to contribute a portion of their
wages to the Savings Plan, subject to certain limitations. We
match 100% of the first 3% of
F-190
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
employee contributions and 50% of the next 2% of employee
contributions. Our contributions and the employee contributions
are fully vested when contributed. The plan assets are held in
trust and invested as directed by the plan participants.
Matching contribution cost for the Savings Plan was
$8.9 million for the year ended December 31, 2010 and
$7.8 million for both the years ended December 31,
2009 and 2008, and is recorded consistent with each
participant’s salary.
Under the profit sharing portion of the Savings Plan, we may
elect to contribute to eligible employees’ Savings Plan
accounts up to 3% of their eligible earnings, as defined. The
profit sharing portion of the plan is discretionary, with the
percentage amount determined by the compensation committee of
our board of directors, based upon the attainment of certain
financial targets as established by the compensation committee.
Our cost for the profit sharing portion of the Savings Plan was
$7.2 million, $5.8 million, and $7.9 million for
the years ended December 31, 2010, 2009, and 2008,
respectively, and is recorded consistent with each
participant’s salary.
Supplemental
Savings Plan
We have a Supplemental Savings Plan, which is an unfunded
nonqualified deferred compensation plan in which employees at
certain executive levels are eligible to defer pre-tax earnings
as well as to make additional contributions, subject to certain
limitations. Our matching contribution is similar to the Savings
Plan described above and is fully vested when contributed.
Matching contribution cost for the periods presented were not
material to our consolidated financial statements. At
December 31, 2010 and 2009, we have recorded
$6.5 million and $5.1 million, respectively, within
accrued expenses and other liabilities on our consolidated
balance sheets.
Other
Plans
We provide an unfunded defined benefit pension plan to certain
of our Talecris Biotherapeutics, GmbH employees in Germany as
required by statutory law. Pension cost related to this plan was
not material for the periods presented. At December 31,
2010 and 2009, no material obligations related to this plan were
recorded on our consolidated balance sheets.
|
|
|
20.
|
Deferred
Compensation
|
In October of 2006, the compensation committee of our board of
directors approved a Special Recognition Bonus Plan (Bonus Plan)
as a vehicle to award certain employees, senior executives, and
members of our board of directors for the financial success of
our Company from its inception through the effective date of the
Bonus Plan. We recorded compensation expense of
$0.1 million, $0.6 million, and $0.7 million for
the years ended December 31, 2010, 2009, and 2008,
respectively, related to the bonus plan. We made payments of
$0.9 million in both March of 2010 and 2009, and
$1.2 million in March of 2008. No further payments are due
under the Bonus Plan.
In December of 2006, the compensation committee of our board of
directors approved a restricted share and cash recognition award
to certain employees, senior executives, and members of our
board of directors for the financial success of our Company from
its inception through the effective date of the award. We funded
an irrevocable trust for the cash installments under this award.
We made cash payments of $5.8 million, $6.0 million,
and $7.4 million in March of 2010, 2009, and 2008,
respectively, related to the cash recognition portion of the
award. No further cash payments are due under this plan. We
recorded compensation expense of $2.0 million,
$5.7 million, and $5.9 million for the years ended
December 31, 2010, 2009, and 2008, respectively, related to
this award.
F-191
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
We operate our plasma-derived protein therapeutics business as a
single reportable business segment since all operating
activities are directed from our North Carolina headquarters and
all of our products are derived from a single source and result
from a common manufacturing process. All products are
manufactured from a single raw material source, human plasma,
and are processed in whole, or in part, at our principal
manufacturing facilities located in Clayton, North Carolina. Our
Melville, New York, facility primarily supplies intermediate
plasma fractions to our Clayton facilities. Gamunex-C/Gamunex
IGIV and Prolastin/Prolastin-C A1PI constitute the majority of
our net revenue. Although we sell our products worldwide, the
majority of our net revenue was concentrated in the United
States and Canada for the periods presented.
In the following table, we have presented our net revenue by
significant product category. Our Immunology/Neurology product
category includes the products that are used to provide
antibodies to patients who have a genetic or acquired inability
to produce these antibodies, as well as a treatment for CIDP,
and also products that provide antibodies to counter specific
antigens such as rabies. Our Pulmonology product category is
comprised of our Prolastin/Prolastin-C A1PI product, which is
used to treat patients with a genetic alpha-1 antitrypsin
deficiency. Our Critical Care/Hemostasis product category
includes products that are used to supplement, restore, or
maintain normal plasma parameters such as volume or coagulation
values. Other product net revenue primarily consists of sales of
PPF powder and intermediate products, such as cryoprecipitate.
Other net revenue consists of royalties and licensing fees,
milestones, and revenues related to contracted services
performed for third parties at our Melville, New York facility.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Product net revenue:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Immunology/Neurology
|
|
$
|
964,145
|
|
|
$
|
928,054
|
|
|
$
|
781,408
|
|
|
Pulmonology
|
|
|
351,499
|
|
|
|
319,080
|
|
|
|
316,495
|
|
|
Critical Care/Hemostasis
|
|
|
175,071
|
|
|
|
167,469
|
|
|
|
134,216
|
|
|
Other
|
|
|
86,221
|
|
|
|
93,151
|
|
|
|
102,431
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total product net revenue
|
|
|
1,576,936
|
|
|
|
1,507,754
|
|
|
|
1,334,550
|
|
|
Other revenue
|
|
|
24,683
|
|
|
|
25,455
|
|
|
|
39,742
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net revenue
|
|
$
|
1,601,619
|
|
|
$
|
1,533,209
|
|
|
$
|
1,374,292
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
In the following table, we have presented our net revenue by
geographic region. Net revenue for each region is based on the
geographic location of the customer.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
United States
|
|
$
|
1,098,969
|
|
|
$
|
1,011,468
|
|
|
$
|
906,376
|
|
|
Canada
|
|
|
195,092
|
|
|
|
214,883
|
|
|
|
215,964
|
|
|
Europe
|
|
|
196,571
|
|
|
|
185,297
|
|
|
|
168,081
|
|
|
Other
|
|
|
110,987
|
|
|
|
121,561
|
|
|
|
83,871
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total net revenue
|
|
$
|
1,601,619
|
|
|
$
|
1,533,209
|
|
|
$
|
1,374,292
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
We did not maintain significant long-lived assets outside of the
United States at December 31, 2010 and 2009.
F-192
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
The following table illustrates the calculation of our basic
earnings per common share outstanding for the periods presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Net income
|
|
$
|
166,068
|
|
|
$
|
153,889
|
|
|
$
|
65,797
|
|
|
Less:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A preferred stock undeclared dividends
|
|
|
—
|
|
|
|
(9,602
|
)
|
|
|
(11,745
|
)
|
|
Series B preferred stock undeclared dividends
|
|
|
—
|
|
|
|
(2,142
|
)
|
|
|
(2,619
|
)
|
|
Accretion of common stock put option
|
|
|
—
|
|
|
|
—
|
|
|
|
(308
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income available to common stockholders
|
|
$
|
166,068
|
|
|
$
|
142,145
|
|
|
$
|
51,125
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding
|
|
|
123,323,722
|
|
|
|
31,166,613
|
|
|
|
1,310,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic net income per common share
|
|
$
|
1.35
|
|
|
$
|
4.56
|
|
|
$
|
39.01
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
The following table illustrates the calculation of our diluted
earnings per common share outstanding for the periods presented:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Net income
|
|
$
|
166,068
|
|
|
$
|
153,889
|
|
|
$
|
65,797
|
|
|
Less accretion of common stock put option
|
|
|
—
|
|
|
|
—
|
|
|
|
(308
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income available to common stockholders
|
|
$
|
166,068
|
|
|
$
|
153,889
|
|
|
$
|
65,489
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted average common shares outstanding
|
|
|
123,323,722
|
|
|
|
31,166,613
|
|
|
|
1,310,448
|
|
|
Plus incremental shares from assumed conversions:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Series A preferred stock
|
|
|
—
|
|
|
|
53,654,795
|
|
|
|
72,000,000
|
|
|
Series B preferred stock
|
|
|
—
|
|
|
|
10,318,354
|
|
|
|
13,846,320
|
|
|
Stock options and restricted shares
|
|
|
5,603,331
|
|
|
|
7,374,601
|
|
|
|
5,605,032
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dilutive potential common shares
|
|
|
128,927,053
|
|
|
|
102,514,363
|
|
|
|
92,761,800
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Diluted net income per common share
|
|
$
|
1.29
|
|
|
$
|
1.50
|
|
|
$
|
0.71
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
For the years ended December 31, 2010, 2009, and 2008,
604,914, 2,168,730, and 2,016,000 stock options were excluded
from the calculation of diluted earnings per share due to their
anti-dilutive effect.
F-193
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
|
|
|
23.
|
Other
Consolidated Balance Sheet Information
|
Information regarding other accounts on our consolidated balance
sheets is as follows:
| |
|
|
|
|
|
|
|
|
|
|
|
December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
|
|
Accrued expenses and other liabilities:
|
|
|
|
|
|
|
|
|
|
Accrued goods and services
|
|
$
|
80,769
|
|
|
$
|
45,044
|
|
|
Accrued payroll, bonuses, and employee benefits
|
|
|
80,317
|
|
|
|
73,983
|
|
|
Medicaid, commercial rebates, and chargebacks
|
|
|
29,744
|
|
|
|
30,771
|
|
|
Interest payable
|
|
|
6,011
|
|
|
|
9,111
|
|
|
PCA judgment
|
|
|
43,690
|
|
|
|
—
|
|
|
Other
|
|
|
11,195
|
|
|
|
11,624
|
|
|
|
|
|
|
|
|
|
|
|
|
Total accrued expenses and other liabilities
|
|
$
|
251,726
|
|
|
$
|
170,533
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
24.
|
Cash Flow
Supplemental Disclosures
|
Supplemental
Disclosures of Cash Flow Information
Cash paid for:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
2010
|
|
2009
|
|
2008
|
|
|
|
Interest, net of amounts capitalized(1)
|
|
$
|
44,546
|
|
|
$
|
55,131
|
|
|
$
|
87,213
|
|
|
Income taxes
|
|
$
|
63,025
|
|
|
$
|
56,849
|
|
|
$
|
48,910
|
|
|
|
|
|
(1) |
|
Interest paid in the table above excludes payments related to
our interest rate swap contracts, which amounted to
$17.0 million and $9.2 million for the years ended
December 31, 2009 and 2008, respectively. The interest rate
swap contracts were terminated and settled during the fourth
quarter of 2009 as discussed in Note 12, “Long-Term
Debt and Capital Lease Obligations.” |
Changes in assets and liabilities, excluding the effects of
business acquisitions:
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Years Ended December 31,
|
|
|
|
|
2010
|
|
|
2009
|
|
|
2008
|
|
|
|
|
Changes in:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts receivable
|
|
$
|
(1,382
|
)
|
|
$
|
8,575
|
|
|
$
|
(26,894
|
)
|
|
Inventories
|
|
|
(52,034
|
)
|
|
|
(57,452
|
)
|
|
|
(92,856
|
)
|
|
Prepaid expenses and other assets
|
|
|
653
|
|
|
|
7,987
|
|
|
|
(15,823
|
)
|
|
Accounts payable
|
|
|
(11,071
|
)
|
|
|
16,143
|
|
|
|
16,594
|
|
|
Interest payable
|
|
|
(3,100
|
)
|
|
|
(2,239
|
)
|
|
|
(1,957
|
)
|
|
Accrued expenses and other liabilities
|
|
|
94,460
|
|
|
|
14,877
|
|
|
|
20,881
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total
|
|
$
|
27,526
|
|
|
$
|
(12,109
|
)
|
|
$
|
(100,055
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental
Schedule of Non-Cash Investing and Financing
Activities
For
the Year Ended December 31, 2010
We entered into a capital lease agreement related to a building
with an unaffiliated third party. We recorded $0.3 million
directly to property, plant, and equipment, net, and capital
lease obligations.
F-194
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
We retired 246,823 shares of our common stock, which were
withheld from employees for payment of withholding taxes.
For
the Year Ended December 31, 2009
We entered into a number of capital lease agreements related to
buildings with an unaffiliated third party. We recorded
$4.9 million directly to property, plant, and equipment,
net and capital lease obligations.
The common shares that we have issued to employees and members
of our board of directors under our share-based compensation
plans had an embedded feature that permitted the participant (or
designated beneficiary or estate) to sell, or “put,”
the shares of our common stock back to us at fair market value
in the event of the participant’s termination of service
due to death or disability. In addition, we had the right to
repurchase, or “call,” the shares of our common stock
upon a participant’s termination of continuous service, as
defined. We recorded a fair market value adjustment of
$6.6 million related to the vested shares of our common
stock to increase obligations under common stock put/call option
and decrease additional paid-in capital on our consolidated
balance sheet. Both our redemption rights and the
participants’ put rights were terminated in connection with
the closing of our IPO. As a result, we reclassified the fair
value of vested common stock totaling $39.9 million from
obligations under common stock put/call option to permanent
equity on our consolidated balance sheet.
We declared a dividend of $45.3 million related to our
Series A and B preferred stock. In connection with our IPO,
1,000,000 shares of our Series A preferred stock and
192,310 shares of our Series B preferred stock were
converted into an aggregate of 85,846,320 shares of our
common stock. In addition, 2,381,548 shares of our common
stock were issued to settle the $45.3 million preferred
stock dividend upon conversion of our Series A and B
preferred stock.
We retired 251,108 shares of our common stock, which were
withheld from employees for payment of withholding taxes.
We reclassified a previously unrealized loss related to our
interest rate swap contracts of approximately
$23.3 million, net of income tax benefit of
$14.2 million, to earnings as a result of their settlement
and termination. In addition, we recorded other comprehensive
income of $0.5 million. Additional information regarding
the components of our comprehensive income is included in
Note 2, “Summary of Significant Accounting
Policies.”
We entered into two plasma center development agreements related
to buildings to be leased from an unaffiliated third party
during 2008, for which we made the decision not to open as
plasma collection centers during 2009. As a result, we recorded
a loss on contract obligations of $3.4 million, which
decreased our assets under capital leases.
For
the Year Ended December 31, 2008
We entered into a number of capital lease agreements related to
buildings with an unaffiliated third party. We recorded
$6.0 million directly to property, plant, and equipment,
net and capital lease obligations.
We reclassified $1.6 million of long-lived assets related
to two plasma collection centers, net of impairment charges of
$0.8 million, to assets held for sale within prepaid
expenses and other on our consolidated balance sheet.
As a result of the put feature related to the common shares
issued under our share-based compensation plans described above,
we recorded a fair value adjustment of $8.9 million to
increase obligations under common stock put/call option and
decrease additional paid-in capital on our consolidated balance
sheet.
F-195
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
We issued shares of our common stock, which had an embedded put
option, to IBR in connection with our 2006 business acquisition
as described below. We recorded accretion of $0.3 million
related to the IBR put option directly to obligations under
common stock put/call option and additional paid-in capital on
our consolidated balance sheet.
We retired 2,215,880 shares of our common stock, of which
2,146,232 shares were repurchased from IBR and
69,648 shares were withheld from employees for payment of
withholding taxes.
We recorded other comprehensive loss of $11.8 million,
primarily related to unrealized losses associated with our
interest rate swap contracts, net of taxes.
|
|
|
25.
|
Selected
Unaudited Quarterly Financial Data
|
The following table summarizes our unaudited quarterly financial
results for the years ended December 31, 2010 and 2009. In
our opinion, the quarterly financial results presented below
have been prepared on the same basis as our annual audited
consolidated financial statements.
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2010 Quarter Ended
|
|
|
|
|
March 31
|
|
|
June 30
|
|
|
September 30
|
|
|
December 31
|
|
|
|
|
Net revenue
|
|
$
|
380,961
|
|
|
$
|
402,826
|
|
|
$
|
407,001
|
|
|
$
|
410,831
|
|
|
Cost of goods sold
|
|
|
217,351
|
|
|
|
223,217
|
|
|
|
229,908
|
|
|
|
241,500
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
163,610
|
|
|
|
179,609
|
|
|
|
177,093
|
|
|
|
169,331
|
|
|
Operating expenses
|
|
|
83,891
|
|
|
|
91,892
|
|
|
|
80,056
|
|
|
|
100,821
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
79,719
|
|
|
|
87,717
|
|
|
|
97,037
|
|
|
|
68,510
|
|
|
Total other non-operating expense, net
|
|
|
(11,116
|
)
|
|
|
(11,944
|
)
|
|
|
(11,256
|
)
|
|
|
(54,220
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
68,603
|
|
|
|
75,773
|
|
|
|
85,781
|
|
|
|
14,290
|
|
|
(Provision) benefit for income taxes
|
|
|
(23,264
|
)
|
|
|
(28,150
|
)
|
|
|
(29,729
|
)
|
|
|
2,764
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
45,339
|
|
|
$
|
47,623
|
|
|
$
|
56,052
|
|
|
$
|
17,054
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
0.37
|
|
|
$
|
0.39
|
|
|
$
|
0.45
|
|
|
$
|
0.14
|
|
|
Diluted
|
|
$
|
0.35
|
|
|
$
|
0.37
|
|
|
$
|
0.43
|
|
|
$
|
0.13
|
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2009 Quarter Ended
|
|
|
|
|
March 31
|
|
|
June 30
|
|
|
September 30
|
|
|
December 31
|
|
|
|
|
Net revenue
|
|
$
|
371,795
|
|
|
$
|
375,570
|
|
|
$
|
395,731
|
|
|
$
|
390,113
|
|
|
Cost of goods sold
|
|
|
209,201
|
|
|
|
224,008
|
|
|
|
230,666
|
|
|
|
237,202
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
162,594
|
|
|
|
151,562
|
|
|
|
165,065
|
|
|
|
152,911
|
|
|
Operating expenses
|
|
|
88,963
|
|
|
|
81,023
|
|
|
|
95,655
|
|
|
|
95,511
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating income
|
|
|
73,631
|
|
|
|
70,539
|
|
|
|
69,410
|
|
|
|
57,400
|
|
|
Total other non-operating (expense) income, net
|
|
|
(21,256
|
)
|
|
|
54,582
|
|
|
|
(19,475
|
)
|
|
|
(55,934
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income before income taxes
|
|
|
52,375
|
|
|
|
125,121
|
|
|
|
49,935
|
|
|
|
1,466
|
|
|
Provision for income taxes
|
|
|
(18,940
|
)
|
|
|
(41,849
|
)
|
|
|
(14,125
|
)
|
|
|
(94
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income
|
|
$
|
33,435
|
|
|
$
|
83,272
|
|
|
$
|
35,810
|
|
|
$
|
1,372
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Earnings per share:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic
|
|
$
|
25.09
|
|
|
$
|
47.42
|
|
|
$
|
12.01
|
|
|
$
|
0.01
|
|
|
Diluted
|
|
$
|
0.36
|
|
|
$
|
0.89
|
|
|
$
|
0.38
|
|
|
$
|
0.01
|
|
F-196
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Earnings per share amounts for the 2010 and 2009 quarters and
full years have been computed separately. Accordingly, quarterly
amounts may not add to the annual amounts because of differences
in the weighted average shares outstanding during each quarter
due to the effect of potentially dilutive securities only in the
periods in which such effect would be dilutive.
Our net income for the fourth quarter of 2010 includes a
$43.7 million charge (approximately $26.6 million
after tax) related to the PCA judgment. Additional information
regarding the PCA judgment is included in Note 14,
“Commitments and Contingencies.”
Our net income for the second quarter of 2009 includes a
$75.0 million (approximately $48.8 million after tax)
payment we received from CSL as a result of the termination of
the definitive merger agreement. Our net income for the fourth
quarter of 2009 includes a charge of $43.0 million
(approximately $26.3 million after tax) as a result of the
settlement and termination of our interest rate swap contracts
and the write-off of deferred debt issuance costs associated
with our First and Second Lien Term Loans. Additional
information regarding our terminated merger agreement with CSL
is included in Note 5, “Definitive Merger Agreement
with CSL Limited (CSL)” and additional information
regarding our refinancing transactions is included in
Note 12, “Long-Term Debt and Capital Lease
Obligations.”
|
|
|
26.
|
Condensed
Consolidating Financial Information
|
In October 2009, we completed the issuance of our
7.75% Notes. The 7.75% Notes are guaranteed on a
senior unsecured basis by our existing and future domestic
subsidiaries. The accompanying condensed consolidating financial
information has been prepared and presented pursuant to SEC
Regulation S-X,
Rule 3-10,
“Financial Statements of Guarantors and Issuers of
Guaranteed Securities Registered or Being Registered.” Each
of the subsidiary guarantors are 100% owned, directly or
indirectly, by us, and all guarantees are full and unconditional
and joint and several. Our investments in our consolidated
subsidiaries are presented under the equity method of
accounting. No significant administrative costs are borne by the
Parent.
F-197
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Balance Sheets
December 31,
2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Assets:
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
—
|
|
|
$
|
183,737
|
|
|
$
|
14,139
|
|
|
$
|
—
|
|
|
$
|
197,876
|
|
|
Accounts receivable, net
|
|
|
—
|
|
|
|
248,124
|
|
|
|
37,793
|
|
|
|
(151,075
|
)
|
|
|
134,842
|
|
|
Inventories
|
|
|
—
|
|
|
|
657,493
|
|
|
|
37,006
|
|
|
|
—
|
|
|
|
694,499
|
|
|
Other
|
|
|
—
|
|
|
|
126,684
|
|
|
|
(429
|
)
|
|
|
—
|
|
|
|
126,255
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
—
|
|
|
|
1,216,038
|
|
|
|
88,509
|
|
|
|
(151,075
|
)
|
|
|
1,153,472
|
|
|
Property, plant, and equipment, net
|
|
|
—
|
|
|
|
381,707
|
|
|
|
1,086
|
|
|
|
—
|
|
|
|
382,793
|
|
|
Intangible assets
|
|
|
—
|
|
|
|
10,880
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,880
|
|
|
Goodwill
|
|
|
—
|
|
|
|
172,860
|
|
|
|
—
|
|
|
|
—
|
|
|
|
172,860
|
|
|
Investment in Subsidiaries
|
|
|
826,782
|
|
|
|
(36,573
|
)
|
|
|
—
|
|
|
|
(790,209
|
)
|
|
|
—
|
|
|
Advances and notes between Parent and Subsidiaries
|
|
|
1,397,133
|
|
|
|
826,919
|
|
|
|
—
|
|
|
|
(2,224,052
|
)
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
18,167
|
|
|
|
281
|
|
|
|
—
|
|
|
|
18,448
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
2,223,915
|
|
|
$
|
2,589,998
|
|
|
$
|
89,876
|
|
|
$
|
(3,165,336
|
)
|
|
$
|
1,738,453
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity (Deficit):
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
—
|
|
|
$
|
96,503
|
|
|
$
|
114,547
|
|
|
$
|
(151,075
|
)
|
|
$
|
59,975
|
|
|
Accrued expenses and other liabilities
|
|
|
6,011
|
|
|
|
234,730
|
|
|
|
10,985
|
|
|
|
—
|
|
|
|
251,726
|
|
|
Current portion of capital lease obligations
|
|
|
—
|
|
|
|
860
|
|
|
|
—
|
|
|
|
—
|
|
|
|
860
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
6,011
|
|
|
|
332,093
|
|
|
|
125,532
|
|
|
|
(151,075
|
)
|
|
|
312,561
|
|
|
Long-term debt and capital lease obligations
|
|
|
596,621
|
|
|
|
8,680
|
|
|
|
—
|
|
|
|
—
|
|
|
|
605,301
|
|
|
Advances and notes between Parent and Subsidiaries
|
|
|
826,919
|
|
|
|
1,397,133
|
|
|
|
—
|
|
|
|
(2,224,052
|
)
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
25,310
|
|
|
|
917
|
|
|
|
—
|
|
|
|
26,227
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
1,429,551
|
|
|
|
1,763,216
|
|
|
|
126,449
|
|
|
|
(2,375,127
|
)
|
|
|
944,089
|
|
|
Stockholders’ equity (deficit)
|
|
|
794,364
|
|
|
|
826,782
|
|
|
|
(36,573
|
)
|
|
|
(790,209
|
)
|
|
|
794,364
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity (deficit)
|
|
$
|
2,223,915
|
|
|
$
|
2,589,998
|
|
|
$
|
89,876
|
|
|
$
|
(3,165,336
|
)
|
|
$
|
1,738,453
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-198
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Balance Sheets
December 31,
2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Assets:
|
|
Current assets:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash
|
|
$
|
—
|
|
|
$
|
58,320
|
|
|
$
|
6,919
|
|
|
$
|
—
|
|
|
$
|
65,239
|
|
|
Accounts receivable, net
|
|
|
—
|
|
|
|
222,007
|
|
|
|
64,454
|
|
|
|
(149,483
|
)
|
|
|
136,978
|
|
|
Inventories
|
|
|
—
|
|
|
|
605,324
|
|
|
|
38,730
|
|
|
|
—
|
|
|
|
644,054
|
|
|
Other
|
|
|
—
|
|
|
|
117,670
|
|
|
|
2,448
|
|
|
|
—
|
|
|
|
120,118
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current assets
|
|
|
—
|
|
|
|
1,003,321
|
|
|
|
112,551
|
|
|
|
(149,483
|
)
|
|
|
966,389
|
|
|
Property, plant, and equipment, net
|
|
|
—
|
|
|
|
266,067
|
|
|
|
1,132
|
|
|
|
—
|
|
|
|
267,199
|
|
|
Intangible assets
|
|
|
—
|
|
|
|
10,880
|
|
|
|
—
|
|
|
|
—
|
|
|
|
10,880
|
|
|
Goodwill
|
|
|
—
|
|
|
|
172,860
|
|
|
|
—
|
|
|
|
—
|
|
|
|
172,860
|
|
|
Investment in Subsidiaries
|
|
|
680,459
|
|
|
|
(27,925
|
)
|
|
|
—
|
|
|
|
(652,534
|
)
|
|
|
—
|
|
|
Advances and notes between Parent and Subsidiaries
|
|
|
1,355,631
|
|
|
|
862,406
|
|
|
|
—
|
|
|
|
(2,218,037
|
)
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
27,054
|
|
|
|
623
|
|
|
|
—
|
|
|
|
27,677
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total assets
|
|
$
|
2,036,090
|
|
|
$
|
2,314,663
|
|
|
$
|
114,306
|
|
|
$
|
(3,020,054
|
)
|
|
$
|
1,445,005
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Liabilities and Stockholders’ Equity (Deficit):
|
|
Current liabilities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accounts payable
|
|
$
|
—
|
|
|
$
|
103,460
|
|
|
$
|
117,069
|
|
|
$
|
(149,483
|
)
|
|
$
|
71,046
|
|
|
Accrued expenses and other liabilities
|
|
|
9,111
|
|
|
|
150,936
|
|
|
|
10,486
|
|
|
|
—
|
|
|
|
170,533
|
|
|
Current portion of capital lease obligations
|
|
|
—
|
|
|
|
740
|
|
|
|
—
|
|
|
|
—
|
|
|
|
740
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total current liabilities
|
|
|
9,111
|
|
|
|
255,136
|
|
|
|
127,555
|
|
|
|
(149,483
|
)
|
|
|
242,319
|
|
|
Long-term debt and capital lease obligations
|
|
|
596,046
|
|
|
|
9,221
|
|
|
|
—
|
|
|
|
—
|
|
|
|
605,267
|
|
|
Advances and notes between Parent and Subsidiaries
|
|
|
848,779
|
|
|
|
1,355,626
|
|
|
|
13,632
|
|
|
|
(2,218,037
|
)
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
14,221
|
|
|
|
1,044
|
|
|
|
—
|
|
|
|
15,265
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities
|
|
|
1,453,936
|
|
|
|
1,634,204
|
|
|
|
142,231
|
|
|
|
(2,367,520
|
)
|
|
|
862,851
|
|
|
Stockholders’ equity (deficit)
|
|
|
582,154
|
|
|
|
680,459
|
|
|
|
(27,925
|
)
|
|
|
(652,534
|
)
|
|
|
582,154
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total liabilities and stockholders’ equity (deficit)
|
|
$
|
2,036,090
|
|
|
$
|
2,314,663
|
|
|
$
|
114,306
|
|
|
$
|
(3,020,054
|
)
|
|
$
|
1,445,005
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-199
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Income Statements
Year
Ended December 31, 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Net revenue
|
|
$
|
—
|
|
|
$
|
1,438,162
|
|
|
$
|
163,457
|
|
|
$
|
—
|
|
|
$
|
1,601,619
|
|
|
Cost of goods sold
|
|
|
—
|
|
|
|
783,710
|
|
|
|
128,266
|
|
|
|
—
|
|
|
|
911,976
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
—
|
|
|
|
654,452
|
|
|
|
35,191
|
|
|
|
—
|
|
|
|
689,643
|
|
|
Operating expenses
|
|
|
—
|
|
|
|
315,350
|
|
|
|
41,310
|
|
|
|
—
|
|
|
|
356,660
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income from operations
|
|
|
—
|
|
|
|
339,102
|
|
|
|
(6,119
|
)
|
|
|
—
|
|
|
|
332,983
|
|
|
Equity in earnings (losses) of Subsidiaries
|
|
|
166,068
|
|
|
|
(7,998
|
)
|
|
|
—
|
|
|
|
(158,070
|
)
|
|
|
—
|
|
|
Other non-operating (expense) income, net
|
|
|
—
|
|
|
|
(88,555
|
)
|
|
|
19
|
|
|
|
—
|
|
|
|
(88,536
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes
|
|
|
166,068
|
|
|
|
242,549
|
|
|
|
(6,100
|
)
|
|
|
(158,070
|
)
|
|
|
244,447
|
|
|
Provision for income taxes
|
|
|
—
|
|
|
|
(76,481
|
)
|
|
|
(1,898
|
)
|
|
|
—
|
|
|
|
(78,379
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
166,068
|
|
|
$
|
166,068
|
|
|
$
|
(7,998
|
)
|
|
$
|
(158,070
|
)
|
|
$
|
166,068
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Income Statements
Year
Ended December 31, 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Net revenue
|
|
$
|
—
|
|
|
$
|
1,389,172
|
|
|
$
|
144,037
|
|
|
$
|
—
|
|
|
$
|
1,533,209
|
|
|
Cost of goods sold
|
|
|
—
|
|
|
|
784,635
|
|
|
|
116,442
|
|
|
|
—
|
|
|
|
901,077
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
—
|
|
|
|
604,537
|
|
|
|
27,595
|
|
|
|
—
|
|
|
|
632,132
|
|
|
Operating expenses
|
|
|
5,715
|
|
|
|
321,525
|
|
|
|
33,912
|
|
|
|
—
|
|
|
|
361,152
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) from operations
|
|
|
(5,715
|
)
|
|
|
283,012
|
|
|
|
(6,317
|
)
|
|
|
—
|
|
|
|
270,980
|
|
|
Equity in earnings (losses) of Subsidiaries
|
|
|
108,854
|
|
|
|
(7,466
|
)
|
|
|
—
|
|
|
|
(101,388
|
)
|
|
|
—
|
|
|
Other non-operating (expense) income, net
|
|
|
75,000
|
|
|
|
(117,106
|
)
|
|
|
23
|
|
|
|
—
|
|
|
|
(42,083
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes
|
|
|
178,139
|
|
|
|
158,440
|
|
|
|
(6,294
|
)
|
|
|
(101,388
|
)
|
|
|
228,897
|
|
|
(Provision) benefit for income taxes
|
|
|
(24,250
|
)
|
|
|
(49,586
|
)
|
|
|
(1,172
|
)
|
|
|
—
|
|
|
|
(75,008
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
153,889
|
|
|
$
|
108,854
|
|
|
$
|
(7,466
|
)
|
|
$
|
(101,388
|
)
|
|
$
|
153,889
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-200
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Income Statements
Year
Ended December 31, 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Net revenue
|
|
$
|
—
|
|
|
$
|
1,232,366
|
|
|
$
|
141,926
|
|
|
$
|
—
|
|
|
$
|
1,374,292
|
|
|
Cost of goods sold
|
|
|
—
|
|
|
|
764,952
|
|
|
|
117,205
|
|
|
|
—
|
|
|
|
882,157
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Gross profit
|
|
|
—
|
|
|
|
467,414
|
|
|
|
24,721
|
|
|
|
—
|
|
|
|
492,135
|
|
|
Operating expenses
|
|
|
6,871
|
|
|
|
257,281
|
|
|
|
29,378
|
|
|
|
—
|
|
|
|
293,530
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) from operations
|
|
|
(6,871
|
)
|
|
|
210,133
|
|
|
|
(4,657
|
)
|
|
|
—
|
|
|
|
198,605
|
|
|
Equity in earnings (losses) of Subsidiaries
|
|
|
70,263
|
|
|
|
(5,590
|
)
|
|
|
—
|
|
|
|
(64,673
|
)
|
|
|
—
|
|
|
Other non-operating (expense) income, net
|
|
|
—
|
|
|
|
(96,832
|
)
|
|
|
618
|
|
|
|
—
|
|
|
|
(96,214
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Income (loss) before income taxes
|
|
|
63,392
|
|
|
|
107,711
|
|
|
|
(4,039
|
)
|
|
|
(64,673
|
)
|
|
|
102,391
|
|
|
(Provision) benefit for income taxes
|
|
|
2,405
|
|
|
|
(37,448
|
)
|
|
|
(1,551
|
)
|
|
|
—
|
|
|
|
(36,594
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
65,797
|
|
|
$
|
70,263
|
|
|
$
|
(5,590
|
)
|
|
$
|
(64,673
|
)
|
|
$
|
65,797
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-201
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Statements of Cash Flows
Year
Ended December 31, 2010
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
166,068
|
|
|
$
|
166,068
|
|
|
$
|
(7,998
|
)
|
|
$
|
(158,070
|
)
|
|
$
|
166,068
|
|
|
Undistributed equity in (earnings) losses of Subsidiaries
|
|
|
(166,068
|
)
|
|
|
7,998
|
|
|
|
—
|
|
|
|
158,070
|
|
|
|
—
|
|
|
Adjustments to reconcile net income (loss) to net cash flows
provided by (used in) operating activities
|
|
|
—
|
|
|
|
39,946
|
|
|
|
29,728
|
|
|
|
19,740
|
|
|
|
89,414
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
—
|
|
|
|
214,012
|
|
|
|
21,730
|
|
|
|
19,740
|
|
|
|
255,482
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant, and equipment
|
|
|
—
|
|
|
|
(152,484
|
)
|
|
|
(365
|
)
|
|
|
—
|
|
|
|
(152,849
|
)
|
|
Net advances and notes between Parent and Subsidiaries
|
|
|
(30,897
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
30,897
|
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
14,397
|
|
|
|
(13,632
|
)
|
|
|
—
|
|
|
|
765
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities
|
|
|
(30,897
|
)
|
|
|
(138,087
|
)
|
|
|
(13,997
|
)
|
|
|
30,897
|
|
|
|
(152,084
|
)
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net advances and notes between Parent and Subsidiaries
|
|
|
—
|
|
|
|
50,637
|
|
|
|
—
|
|
|
|
(50,637
|
)
|
|
|
—
|
|
|
Other
|
|
|
30,897
|
|
|
|
(1,145
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
29,752
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
|
30,897
|
|
|
|
49,492
|
|
|
|
—
|
|
|
|
(50,637
|
)
|
|
|
29,752
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
—
|
|
|
|
—
|
|
|
|
(513
|
)
|
|
|
—
|
|
|
|
(513
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
—
|
|
|
|
125,417
|
|
|
|
7,220
|
|
|
|
—
|
|
|
|
132,637
|
|
|
Cash and cash equivalents at beginning of year
|
|
|
—
|
|
|
|
58,320
|
|
|
|
6,919
|
|
|
|
—
|
|
|
|
65,239
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of year
|
|
$
|
—
|
|
|
$
|
183,737
|
|
|
$
|
14,139
|
|
|
$
|
—
|
|
|
$
|
197,876
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-202
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Statements of Cash Flows
Year
Ended December 31, 2009
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
153,889
|
|
|
$
|
108,854
|
|
|
$
|
(7,466
|
)
|
|
$
|
(101,388
|
)
|
|
$
|
153,889
|
|
|
Undistributed equity in (earnings) losses of Subsidiaries
|
|
|
(108,854
|
)
|
|
|
7,466
|
|
|
|
—
|
|
|
|
101,388
|
|
|
|
—
|
|
|
Adjustments to reconcile net income (loss) to net cash flows
provided by (used in) operating activities
|
|
|
(2,007
|
)
|
|
|
64,009
|
|
|
|
4,759
|
|
|
|
13,505
|
|
|
|
80,266
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
43,028
|
|
|
|
180,329
|
|
|
|
(2,707
|
)
|
|
|
13,505
|
|
|
|
234,155
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant, and equipment
|
|
|
—
|
|
|
|
(74,576
|
)
|
|
|
(587
|
)
|
|
|
—
|
|
|
|
(75,163
|
)
|
|
Business acquisitions, net of cash acquired
|
|
|
—
|
|
|
|
(30,431
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(30,431
|
)
|
|
Net advances and notes between Parent and Subsidiaries
|
|
|
(1,172,950
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
1,172,950
|
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
(2,788
|
)
|
|
|
3,764
|
|
|
|
—
|
|
|
|
976
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities
|
|
|
(1,172,950
|
)
|
|
|
(107,795
|
)
|
|
|
3,177
|
|
|
|
1,172,950
|
|
|
|
(104,618
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net repaments of borrowings
|
|
|
—
|
|
|
|
(1,196,515
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(1,196,515
|
)
|
|
Issuance of 7.75% Notes, net of discount
|
|
|
595,926
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
595,926
|
|
|
Proceeds from initial public offering, net of issuance costs
|
|
|
517,192
|
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
517,192
|
|
|
Net advances and notes between Parent and Subsidiaries
|
|
|
—
|
|
|
|
1,186,455
|
|
|
|
—
|
|
|
|
(1,186,455
|
)
|
|
|
—
|
|
|
Other
|
|
|
16,804
|
|
|
|
(14,879
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
1,925
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
|
1,129,922
|
|
|
|
(24,939
|
)
|
|
|
—
|
|
|
|
(1,186,455
|
)
|
|
|
(81,472
|
)
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
—
|
|
|
|
—
|
|
|
|
195
|
|
|
|
—
|
|
|
|
195
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
—
|
|
|
|
47,595
|
|
|
|
665
|
|
|
|
—
|
|
|
|
48,260
|
|
|
Cash and cash equivalents at beginning of year
|
|
|
—
|
|
|
|
10,726
|
|
|
|
6,253
|
|
|
|
—
|
|
|
|
16,979
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of year
|
|
$
|
—
|
|
|
$
|
58,321
|
|
|
$
|
6,918
|
|
|
$
|
—
|
|
|
$
|
65,239
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-203
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Talecris
Biotherapeutics Holdings Corp.
Condensed
Consolidating Statements of Cash Flows
Year
Ended December 31, 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Parent/
|
|
|
Guarantor
|
|
|
Non-Guarantor
|
|
|
Consolidating
|
|
|
|
|
|
|
|
Issuer
|
|
|
Subsidiaries
|
|
|
Subsidiaries
|
|
|
Adjustments
|
|
|
Consolidated
|
|
|
|
|
Cash flows from operating activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net income (loss)
|
|
$
|
65,797
|
|
|
$
|
70,263
|
|
|
$
|
(5,590
|
)
|
|
$
|
(64,673
|
)
|
|
$
|
65,797
|
|
|
Undistributed equity in (earnings) losses of Subsidiaries
|
|
|
(70,263
|
)
|
|
|
5,590
|
|
|
|
—
|
|
|
|
64,673
|
|
|
|
—
|
|
|
Adjustments to reconcile net income (loss) to net cash flows
provided by (used in) operating activities
|
|
|
424
|
|
|
|
14,776
|
|
|
|
(63,115
|
)
|
|
|
15,132
|
|
|
|
(32,783
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) operating activities
|
|
|
(4,042
|
)
|
|
|
90,629
|
|
|
|
(68,705
|
)
|
|
|
15,132
|
|
|
|
33,014
|
|
|
Cash flows from investing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Purchases of property, plant, and equipment
|
|
|
—
|
|
|
|
(86,051
|
)
|
|
|
(161
|
)
|
|
|
—
|
|
|
|
(86,212
|
)
|
|
Business acquisitions, net of cash acquired
|
|
|
—
|
|
|
|
(10,272
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(10,272
|
)
|
|
Net advances and notes between Parent and Subsidiaries
|
|
|
40,160
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(40,160
|
)
|
|
|
—
|
|
|
Other
|
|
|
—
|
|
|
|
(15,455
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
(15,455
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash (used in) provided by investing activities
|
|
|
40,160
|
|
|
|
(111,778
|
)
|
|
|
(161
|
)
|
|
|
(40,160
|
)
|
|
|
(111,939
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash flows from financing activities:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net borrowings
|
|
|
—
|
|
|
|
58,712
|
|
|
|
—
|
|
|
|
—
|
|
|
|
58,712
|
|
|
Net advances and notes between Parent and Subsidiaries
|
|
|
—
|
|
|
|
(34,896
|
)
|
|
|
9,868
|
|
|
|
25,028
|
|
|
|
—
|
|
|
Other
|
|
|
(36,118
|
)
|
|
|
—
|
|
|
|
—
|
|
|
|
—
|
|
|
|
(36,118
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net cash provided by (used in) financing activities
|
|
|
(36,118
|
)
|
|
|
23,816
|
|
|
|
9,868
|
|
|
|
25,028
|
|
|
|
22,594
|
|
|
Effect of exchange rate changes on cash and cash equivalents
|
|
|
—
|
|
|
|
91
|
|
|
|
(248
|
)
|
|
|
—
|
|
|
|
(157
|
)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net increase in cash and cash equivalents
|
|
|
—
|
|
|
|
2,758
|
|
|
|
(59,246
|
)
|
|
|
—
|
|
|
|
(56,488
|
)
|
|
Cash and cash equivalents at beginning of year
|
|
|
—
|
|
|
|
7,968
|
|
|
|
65,499
|
|
|
|
—
|
|
|
|
73,467
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents at end of year
|
|
$
|
—
|
|
|
$
|
10,726
|
|
|
$
|
6,253
|
|
|
$
|
—
|
|
|
$
|
16,979
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
F-204
Talecris
Biotherapeutics Holdings Corp.
Notes to
Consolidated Financial
Statements — (Continued)
Special
Meeting of Stockholders
On February 14, 2011, we held a special meeting at which
holders of a majority of our outstanding common stock approved
the adoption of the Agreement and Plan of Merger, dated as of
June 6, 2010, among Grifols and Talecris Biotherapeutics
Inc. The completion of the transaction is subject to obtaining
certain regulatory approvals and other customary conditions.
Grifols agreed to provide written notice to the FTC staff at
least thirty days prior to closing the transaction and, in any
event, not to close the transaction until after 11:59 p.m.
on March 20, 2011. There can be no assurance that Grifols
will reach resolution with the FTC by March 20, 2011. Under
the pending merger agreement, if this transaction is not closed
by the current “outside date” of March 6, 2011,
then under specified circumstances, either Grifols or we may
elect to cause the “outside date” to be extended to a
date not later than the expiration of Grifols financing for the
transaction, or September 6, 2011, whichever is earlier.
Foreign
Currency Hedging Program
In order to reduce the impact of volatility of foreign exchange
rates on intercompany transactions, we initiated a foreign
currency hedging program in the first quarter of 2011 related to
both known and anticipated intercompany transactions of
approximately one year in duration or less. The effective
portion of the changes in fair value of these instruments is
reported in other comprehensive income and reclassified into
earnings in the same period or periods in which the hedged
transactions affect earnings.
The changes in fair value of the hedges against firm commitments
will be recognized in general and administrative expenses
consistent with the underlying intercompany receivables being
hedged. The changes in the fair value hedges against anticipated
intercompany sales will be recognized as an adjustment to
revenues when the hedged inventory sells through to third
parties.
During the first quarter of 2011 we entered into approximately
$49.5 (€37.1) million in notional value of fair value
hedges against firm commitments and $38.4
(€28.2) million in notional value of cash flow hedges
against anticipated future sales. The weighted average
U.S. dollar to euro exchange rate on these foreign currency
contracts is 1.3461.
These derivative financial instruments present certain market
and counterparty risks. We seek to manage the counterparty risks
associated with these contracts by limiting transactions to
counterparties with which we have established banking
relationships and limit the duration of the contracts to less
than one year. We are exposed to potential losses if a
counterparty fails to perform according to the terms of the
agreement. We do not require collateral or other security to be
furnished by counterparties to our derivative financial
instruments. There can be no assurance, however, that our
practice effectively mitigates counterparty risk. A number of
financial institutions similar to those that serve or may serve
as counterparties to our hedging arrangements were adversely
affected by the global credit crisis. The failure of any of the
counterparties to our hedging arrangements to fulfill their
obligations to us could adversely affect our results of
operations and cash flows.
F-205
Schedule II
Talecris
Biotherapeutics Holdings Corp.
Valuation
and Qualifying Accounts
Years
Ended December 31, 2010, 2009, and 2008
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance at
|
|
Charges to
|
|
Charges to
|
|
|
|
Balance at
|
|
|
|
Beginning of
|
|
Costs and
|
|
Other
|
|
|
|
End of
|
|
|
|
Period
|
|
Expenses
|
|
Accounts
|
|
Deductions
|
|
Period
|
|
|
|
(In thousands)
|
|
|
|
Reserve for doubtful accounts receivable:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2010
|
|
$
|
3,461
|
|
|
$
|
3,519
|
|
|
$
|
—
|
|
|
$
|
(3,727
|
)(1)
|
|
$
|
3,253
|
|
|
Year ended December 31, 2009
|
|
$
|
2,020
|
|
|
$
|
2,858
|
|
|
$
|
—
|
|
|
$
|
(1,417
|
)(1)
|
|
$
|
3,461
|
|
|
Year ended December 31, 2008
|
|
$
|
2,631
|
|
|
$
|
728
|
|
|
$
|
—
|
|
|
$
|
(1,339
|
)(1)
|
|
$
|
2,020
|
|
|
Reserve for doubtful notes receivable and other advances:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2010
|
|
$
|
4,250
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
4,250
|
|
|
Year ended December 31, 2009
|
|
$
|
4,250
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
—
|
|
|
$
|
4,250
|
|
|
Year ended December 31, 2008
|
|
$
|
—
|
|
|
$
|
4,250
|
(3)
|
|
$
|
—
|
|
|
|
|
|
|
$
|
4,250
|
|
|
Inventory reserves:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Year ended December 31, 2010
|
|
$
|
44,377
|
|
|
$
|
55,419
|
|
|
$
|
—
|
|
|
$
|
(39,796
|
)(2)
|
|
$
|
60,000
|
|
|
Year ended December 31, 2009
|
|
$
|
49,766
|
|
|
$
|
21,758
|
|
|
$
|
—
|
|
|
$
|
(27,147
|
)(2)
|
|
$
|
44,377
|
|
|
Year ended December 31, 2008
|
|
$
|
47,534
|
|
|
$
|
36,840
|
|
|
$
|
—
|
|
|
$
|
(34,608
|
)(2)
|
|
$
|
49,766
|
|
|
|
|
|
(1) |
|
Includes write-offs of uncollectible accounts receivable and the
effects of foreign exchange. |
| |
|
(2) |
|
Includes the net of write-offs, reversals of reserved inventory
that was sold or recoverable for other purposes such as testing,
and the effects of foreign exchange. |
| |
|
(3) |
|
Includes a provision for $3.2 million related to notes
receivables and $1.0 million related to advances for
unlicensed plasma from a then existing third party supplier due
to uncertainty regarding collection. |
F-206
PART II
INFORMATION
NOT REQUIRED IN PROSPECTUS
|
|
|
Item 20.
|
Indemnification
of Directors and Officers
|
The following is a summary of the statutes, charter and bylaw
provisions or other arrangements under which the
registrants’ directors and officers are insured or
indemnified against liability in their capacities as such. All
of the directors and officers of the registrants are covered by
insurance policies maintained and held in effect by Grifols,
S.A. against certain losses arising from claims of breach of
duty.
Grifols,
S.A.
Indemnification
under Grifols, S.A.’s bylaws (estatutos) and Spanish
Law
Under Spanish law Grifols, S.A.’s current and former
directors will be liable to the company, the shareholders and
the creditors of the company for any damage they cause through
acts contrary to the law or the bylaws, or acts carried out in
breach of the duties inherent in the discharge of their office.
No provision of Grifols, S.A.’s bylaws provides for the
indemnification of the directors with respect to such
liabilities.
Grifols,
S.A. Directors & Officers Insurance
Grifols, S.A. maintains an insurance policy that protects its
officers, managers and directors as well as all of the officers,
managers and directors of its subsidiaries from certain
liabilities in connection with civil, criminal or administrative
claims that arise as a result of actions taken in their official
capacity.
Insofar as indemnification for liabilities arising under the
Securities Act of 1933, as amended (the “Act”) may be
permitted for directors, officers or persons controlling
Grifols, S.A. pursuant to the foregoing provisions, the
registrants have been informed that in the opinion of the
Securities and Exchange Commission such indemnification is
against public policy as expressed in the Act and is therefore
unenforceable.
Grifols
Inc.
Indemnification
under Grifols Inc.’s articles of incorporation, bylaws and
Virginia Law
The Virginia Stock Corporation Act (the “VSCA”)
permits Grifols Inc. to indemnify its officers and directors in
connection with certain actions, suits and proceedings brought
against them if they conducted themselves in good faith and
believed their conduct to be in the best interests of Grifols
Inc. and, in the case of criminal actions, had no reasonable
cause to believe that the conduct was unlawful. Unless limited
in Grifols Inc.’s articles of incorporation, the VSCA
requires such indemnification when a director entirely prevails
in the defense of any proceeding to which he or she was a party
because he or she is or was a director of Grifols Inc., and
further provides that Grifols Inc. may make any other or further
indemnity (including indemnity with respect to a proceeding by
or in the right of Grifols Inc.), and may make additional
provision for advances and reimbursement of expenses, if
authorized by its articles of incorporation, shareholder-adopted
bylaws or a resolution adopted by the shareholders, except an
indemnity against willful misconduct or a knowing violation of
the criminal law.
The VSCA establishes a statutory limit on liability of officers
and directors of Grifols Inc. for damages assessed against them
in a suit brought by or in the right of Grifols Inc. or brought
by or on behalf of shareholders of Grifols Inc. and authorizes
Grifols Inc., with shareholder approval, to specify a lower
monetary limit on liability in its articles of incorporation or
bylaws; however, the liability of an officer or director shall
not be limited if such officer or director engaged in willful
misconduct or a knowing violation of the criminal law or of any
federal or state securities law.
Grifols Inc.’s articles of incorporation provide that
Grifols Inc. will indemnify and advance expenses to its officers
and directors to the fullest extent permitted by the VSCA.
Grifols Inc.’s articles of incorporation further provide
for the limitation or elimination of the liability of an officer
or director of Grifols Inc. for monetary damages to Grifols Inc.
or its shareholders to the fullest extent permitted by the VSCA.
II-1
Grifols Inc.’s amended and restated bylaws provide that, to
the fullest extent permitted by the VSCA, as it exists on the
date hereof or as hereafter amended, Grifols Inc. will indemnify
and advance expenses to any person who was or is a party to any
proceeding, including a proceeding brought by or in the right of
Grifols Inc. or brought by the shareholders of Grifols Inc., by
reason of the fact that such person is or was an officer or
director of Grifols Inc. Grifols Inc.’s amended and
restated bylaws also provide that for a period of six years from
the merger on June 1, 2011, the corporation shall honor the
exculpation, indemnification and advancement of expenses
provisions in the bylaws of Talecris Biotherapeutics Holdings
Corp. as in effect immediately prior to such time.
Subsidiary
Guarantors
Registrants
incorporated under the Delaware General Corporation
Law
Grifols Biologicals Inc., Biomat USA, Inc., Grifols Therapeutics
Inc. and Talecris Plasma Resources, Inc. are incorporated under
the laws of the State of Delaware.
Section 145(a) of the DGCL provides that a Delaware
corporation shall have the power to indemnify any person who was
or is a party or is threatened to be made a party to any
threatened, pending or completed action, suit or proceeding,
whether civil, criminal, administrative or investigative (other
than an action by or in the right of the corporation) by reason
of the fact that the person is or was a director, officer,
employee or agent of the corporation, or is or was serving at
the request of the corporation as a director, officer, employee
or agent of another corporation, partnership, joint venture,
trust or other enterprise, against expenses (including
attorneys’ fees), judgments, fines and amounts paid in
settlement actually and reasonably incurred by the person in
connection with such action, suit or proceeding if the person
acted in good faith and in a manner the person reasonably
believed to be in or not opposed to the best interests of the
corporation, and, with respect to any criminal action or
proceeding, had no reasonable cause to believe the person’s
conduct was unlawful.
Section 145(b) of the DGCL provides that a Delaware
corporation shall have the power to indemnify any person who was
or is a party or is threatened to be made a party to any
threatened, pending or completed action or suit by or in the
right of the corporation to procure a judgment in its favor by
reason of the fact that such person acted in any of the
capacities set forth above, against expenses (including
attorneys’ fees) actually and reasonably incurred by the
person in connection with the defense or settlement of such
action or suit if the person acted under standards similar to
those discussed above, except that no indemnification shall be
made in respect of any claim, issue or matter as to which such
person shall have been adjudged to be liable to the corporation
unless and only to the extent that the Court of Chancery or the
court in which such action or suit was brought shall determine
that despite the adjudication of liability, such person is
fairly and reasonably entitled to be indemnified for such
expenses which the Court of Chancery or such other court shall
deem proper.
Section 145 of the DGCL further provides that to the extent
a director or officer of a corporation has been successful in
the defense of any action, suit or proceeding referred to in
subsections (a) and (b) or in the defense of any
claim, issue or matter therein, such person shall be indemnified
against expenses (including attorneys’ fees) actually and
reasonably incurred by such person in connection therewith; that
indemnification provided for by Section 145 shall not be
deemed exclusive of any other rights to which the indemnified
party may be entitled; and that the corporation shall have power
to purchase and maintain insurance on behalf of a director or
officer of the corporation against any liability asserted
against such person and incurred by such person in any such
capacity or arising out of such person’s status as such
whether or not the corporation would have the power to indemnify
such person against such liabilities under Section 145.
The certificate of incorporation of each of the above-referenced
Delaware corporation registrants provides for indemnification of
officers and directors to the fullest extent permitted by
Delaware law.
The bylaws of each of the above-referenced Delaware corporation
registrants provide that, to the full extent permitted by the
laws of the State of Delaware, the corporation shall indemnify
any person made or threatened to be made a party to any
threatened, pending, or completed action, suit or proceeding by
reason of
II-2
the fact that such person is or was a director, officer,
employee or agent of the corporation, except that the bylaws of
Grifols Biologicals Inc. do not provide for indemnification of
directors and officers.
Registrants
incorporated under the laws of the Kingdom of
Spain
Instituto Grifols, S.A., Diagnostic Grifols, S.A., Movaco, S.A.
and Laboratorios Grifols, S.A. are incorporated under the laws
of the Kingdom of Spain.
Under Spanish law, all of a company’s (sociedad
anónima) current and former directors will be liable to the
company, the shareholders and the creditors of the company for
any damage they cause through acts contrary to the law or the
bylaws, or acts carried out in breach of the duties inherent in
the discharge of their office. There are no provisions in the
Spanish registrants’ bylaws that provide for the
indemnification of the directors with respect to such
liabilities.
Indemnification
under Grifols Italia, S.p.A.’s bylaws and Italian
Law
Under Italian law, Grifols Italia, S.p.A.’s current and
former directors will be liable to the company, the shareholders
and the creditors of the company for any damage they cause
through acts contrary to the law or the bylaws, or acts carried
out in breach of the duties inherent in the discharge of their
office. No provision of Grifols Italia, S.p.A.’s bylaws
provides for the indemnification of the directors with respect
to such liabilities.
Indemnification
under Grifols Deutschland GmbH’s bylaws and German
Law
Under German law, Grifols Deutschland GmbH’s managing
directors will be liable to the company for any damage caused by
acts contrary to the law or the company’s bylaws, or acts
carried out in breach of the duties inherent to their office, if
and to the extent they are at fault. The managing directors will
be liable for damages caused against third parties by
(i) willful and immoral acts (ii) the violation of
applicable protective law with willful intent or gross
negligence or (iii) the violation of their organizational
duties or accepted standards protecting third parties, if and to
the extent they are at fault. No provision of the company’s
bylaws provides for the indemnification of the managing
directors with respect to such liabilities.
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
2
|
.1
|
|
Agreement and Plan of Merger, dated as of June 6, 2010, by
and among Grifols, S.A., Grifols, Inc. and Talecris
Biotherapeutics Holdings Corp. (incorporated by reference to
Exhibit 2.1 of our Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
2
|
.2
|
|
Amendment No. 1 to the Agreement and Plan of Merger, dated
as of November 4, 2010, by and among Grifols, S.A.,
Grifols, Inc. and Talecris Biotherapeutics Holdings Corp.
(incorporated by reference to Exhibit 2.2 of Amendment
No. 2 to our Registration Statement on
Form F-4
(File
No. 333-168701)
filed on November 5, 2010)
|
|
|
3
|
.1.1*
|
|
By-Laws (Estatutos) of Grifols, S.A.
|
|
|
3
|
.1.2*
|
|
By-Laws (Estatutos) of Grifols, S.A. (English translation)
|
|
|
3
|
.2.1*
|
|
Amended and Restated Articles of Incorporation of Grifols Inc.
|
|
|
3
|
.2.2*
|
|
Amended and Restated By-laws of Grifols Inc.
|
|
|
3
|
.3.1*
|
|
Certificate of Incorporation of Grifols Biologicals Inc.
|
|
|
3
|
.3.2*
|
|
Certificate of Correction of the Certificate of Incorporation of
Grifols Biologicals Inc.
|
|
|
3
|
.3.3*
|
|
By-laws of Grifols Biologicals Inc.
|
|
|
3
|
.4.1*
|
|
Amended and Restated Certificate of Incorporation of Biomat USA,
Inc.
|
|
|
3
|
.4.2*
|
|
By-laws of Biomat USA, Inc.
|
|
|
3
|
.5.1*
|
|
Certificate of Incorporation of Grifols Therapeutics, Inc.
|
|
|
3
|
.5.2*
|
|
Certificate of Amendment to the Certificate of Incorporation of
Grifols Therapeutics, Inc.
|
|
|
3
|
.5.3*
|
|
Certificate of Amendment to the Certificate of Incorporation of
Grifols Therapeutics, Inc.
|
II-3
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
3
|
.5.4*
|
|
By-laws of Grifols Therapeutics, Inc.
|
|
|
3
|
.6.1*
|
|
Certificate of Incorporation of Talecris Plasma Resources, Inc.
|
|
|
3
|
.6.2*
|
|
Certificate of Amendment of Certificate of Incorporation of
Talecris Plasma Resources, Inc.
|
|
|
3
|
.6.3*
|
|
Amended and Restated By-laws of Talecris Plasma Resources, Inc.
|
|
|
3
|
.7.1*
|
|
By-laws (Estatutos) of Instituto Grifols, S.A.
|
|
|
3
|
.7.2*
|
|
By-laws (Estatutos) of Instituto Grifols, S.A. (English
translation)
|
|
|
3
|
.8.1*
|
|
By-laws (Estatutos) of Diagnostic Grifols, S.A.
|
|
|
3
|
.8.2*
|
|
By-laws (Estatutos) of Diagnostic Grifols, S.A. (English
translation)
|
|
|
3
|
.9.1*
|
|
By-laws (Estatutos) of Movaco, S.A.
|
|
|
3
|
.9.2*
|
|
By-laws (Estatutos) of Movaco, S.A. (English translation)
|
|
|
3
|
.10.1*
|
|
By-laws (Estatutos) of Laboratorios Grifols, S.A.
|
|
|
3
|
.10.2*
|
|
By-laws (Estatutos) of Laboratorios Grifols, S.A. (English
translation)
|
|
|
3
|
.11.1*
|
|
By-laws of Grifols Italia, S.p.A.
|
|
|
3
|
.11.2*
|
|
By-laws of Grifols Italia, S.p.A. (English translation)
|
|
|
3
|
.12*
|
|
By-laws of Grifols Deutschland GmbH
|
|
|
4
|
.1*
|
|
Senior Notes Indenture, dated as of January 21, 2011,
relating to the 8.25% Senior Notes due 2018, among Giant
Funding Corp. and The Bank of New York Mellon
Trust Company, N.A., as trustee.
|
|
|
4
|
.2*
|
|
Form of 8.25% Senior Note (included as Exhibit A to
Exhibit 4.1)
|
|
|
4
|
.3*
|
|
Supplemental Indenture, dated June 1, 2011, by and among
Grifols Inc., Grifols, S.A., the subsidiary guarantors party
thereto and The Bank of New York Mellon Trust Company,
N.A., as trustee.
|
|
|
4
|
.4*
|
|
Second Supplemental Indenture, dated as of October 4, 2011,
between Grifols Deutschland GmbH and The Bank of New York Mellon
Trust Company, N.A., as trustee.
|
|
|
4
|
.5*
|
|
Registration Rights Agreement, dated January 21, 2011, by
and among Giant Funding Corp. and Deutsche Bank Securities Inc.,
as representative of the several initial purchasers.
|
|
|
4
|
.6*
|
|
Registration Rights Agreement Joinder, dated as of June 1,
2011, by and among Grifols Inc., Grifols, S.A. and the
subsidiary guarantors party thereto.
|
|
|
5
|
.1*
|
|
Opinion of Proskauer Rose LLP, New York, NY, United States of
America
|
|
|
5
|
.2*
|
|
Opinion of Hunton & Williams LLP, Richmond, VA, United
States of America
|
|
|
5
|
.3*
|
|
Opinion of Osborne Clarke, S.L.P., Barcelona, Spain
|
|
|
5
|
.4*
|
|
Opinion of SLA Studio Legale Associato, Milan, Italy with
respect to Grifols Italia, S.p.A.
|
|
|
5
|
.5*
|
|
Opinion of Osborne Clarke, Munich, Germany with respect to
Grifols Deutschland GmbH
|
|
|
10
|
.1*
|
|
Credit and Guaranty Agreement, dated as of November 23,
2010, among Grifols Inc., Grifols, S.A., certain subsidiaries of
Grifols, S.A., the lenders party thereto, Deutsche Bank
Securities, Inc., Nomura International PLC, Banco Bilbao Vizcaya
Argentaria, S.A., BNP Paribas, HSBC Securities (USA) Inc. and
Morgan Stanley Senior Funding, Inc., as joint lead arrangers and
joint bookrunners and Deutsche Bank AG New York branch, as
administrative agent and collateral agent.
|
|
|
10
|
.2*
|
|
First Amendment to Credit and Guaranty Agreement, dated as of
March 3, 2011, by and among Grifols, Inc., Deutsche Bank AG
New York Branch, as administrative agent.
|
|
|
10
|
.3*
|
|
Second Amendment to Credit and Guaranty Agreement, dated as of
May 31, 2011, by and among Grifols, Inc. and Deutsche Bank
AG New York Branch, as administrative agent.
|
|
|
10
|
.4*
|
|
Counterpart Agreement, dated June 1, 2011, by and among
Grifols Inc., Grifols, S.A. and certain of its subsidiaries, the
Lenders party thereto and Deutsche Bank AG New York Branch, as
administrative agent and collateral agent.
|
|
|
10
|
.5*
|
|
U.S. Pledge and Security Agreement, dated as of June 1,
2011, among the grantors party thereto and Deutsche Bank AG New
York Branch, as collateral agent.
|
II-4
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
10
|
.6*
|
|
Assumption Agreement, dated as of June 1, 2011, by and
between Grifols, Inc. and Deutsche Bank AG New York Branch, as
administrative agent.
|
|
|
10
|
.7
|
|
Voting Agreement between Grifols, S.A. and Talecris Holdings,
LLC (incorporated by reference to Exhibit 10.1 of our
Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
10
|
.8
|
|
Form of Voting Agreement between certain holders of ordinary
shares of Grifols, S.A. and Talecris Biotherapeutics Holdings
Corp. (incorporated by reference to Exhibit 10.2 of our
Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
10
|
.9
|
|
Lock-up
Agreement between Grifols, S.A. and Talecris Holdings, LLC
(incorporated by reference to Exhibit 10.3 of our
Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
10
|
.10
|
|
Appraisal Indemnity Agreement, dated as of November 4,
2010, by and among Talecris Biotherapeutics Holdings Corp.,
Grifols, S.A., and Talecris Holdings, LLC, and solely with
respect to the provisions of Section 9, Cerberus Capital
Management, L.P. (incorporated by reference to Exhibit 10.4
of Amendment No. 2 to our Registration Statement on
Form F-4
(File
No. 333-168701)
filed on November 5, 2010)
|
|
|
10
|
.11
|
|
Toll Manufacturing Agreement for Testing and Packaging, dated
April 4, 2008, by and between Talecris Biotherapeutics,
GmbH and Catalent France Limoges SAS (incorporated by reference
to Exhibit 10.35 of Amendment No. 8 to Talecris’
Registration Statement on
Form S-1
(File
No. 333-144941)
filed on August 19, 2009)+
|
|
|
10
|
.12
|
|
Retained Intellectual Property License Agreement, dated as of
March 31, 2005, by and between Bayer Healthcare LLC and
Talecris Biotherapeutics, Inc. (f/k/a NPS Biotherapeutics, Inc.)
(incorporated by reference to Exhibit 10.31.1 of Amendment
No. 1 to Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 24, 2007)
|
|
|
10
|
.13
|
|
Amendment to Retained Intellectual Property Licensing Agreement,
entered into as of August 10, 2007, by and between Bayer
Healthcare LLC and Talecris Biotherapeutics, Inc. (incorporated
by reference to Exhibit 10.31.2 of Amendment No. 1 to
Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 24, 2007)
|
|
|
10
|
.14
|
|
Fractionation Services and Commercial Products Agreement, dated
as of April 1, 2008, between and amongst Canadian Blood
Services/Societe Canadienne Du Sang, Talecris Biotherapeutics,
Inc. and Talecris Biotherapeutics, Ltd. (incorporated by
reference to Exhibit 10.29 of Amendment No. 9 to
Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 11, 2009)+
|
|
|
10
|
.15
|
|
Fractionation Services and Commercial Products Agreement, dated
as of April 1, 2008, between and amongst
Héma-Québec, Talecris Biotherapeutics, Ltd. and
Talecris Biotherapeutics, Inc. (incorporated by reference to
Exhibit 10.30.1 of Amendment No. 9 to Talecris’
Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 11, 2009)+
|
|
|
10
|
.16
|
|
Amending Agreement No. 1, effective as of May 26,
2008, to Fractionation Services and Commercial Products, dated
as of April 1, 2008, by and among Héma-Québec,
Talecris Biotherapeutics, Ltd. and Talecris Biotherapeutics,
Inc. (incorporated by reference to Exhibit 10.30.2 of
Amendment No. 6 to Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on July 2, 2009)+
|
|
|
12
|
.1*
|
|
Statement of Computation of Ratio of Earnings to Fixed Charges
|
|
|
21
|
.1*
|
|
Subsidiaries of the Registrants
|
|
|
23
|
.1*
|
|
Consent of Proskauer Rose LLP, New York, NY, United States of
America (included in Exhibit 5.1)
|
|
|
23
|
.2*
|
|
Consent of Hunton & Williams LLP, Richmond, VA, United
States of America (included in Exhibit 5.2)
|
|
|
23
|
.3*
|
|
Consent of Osborne Clarke Spain, Barcelona, Spain (included in
Exhibit 5.3)
|
|
|
23
|
.4*
|
|
Consent of SLA Studio Legale Associato, Milan, Italy with
respect to Grifols Italia, S.p.A. (included in Exhibit 5.4)
|
II-5
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
23
|
.5*
|
|
Consent of Osborne Clarke Germany, Munich, Germany with respect
to Grifols Deutschland GmbH (included in Exhibit 5.5)
|
|
|
23
|
.6*
|
|
Consent of KPMG Auditores, S.L., Independent Registered Public
Accounting Firm
|
|
|
23
|
.7*
|
|
Consent of PriceWaterhouseCoopers LLP, Independent Registered
Public Accounting Firm
|
|
|
24
|
.1*
|
|
Power of Attorney (included on the signature pages hereto)
|
|
|
25
|
.1*
|
|
Statement of Eligibility on
Form T-1
of The Bank of New York Mellon Trust Company, N.A., as trustee
under the indenture
|
|
|
99
|
.1*
|
|
Form of Letter of Transmittal
|
|
|
99
|
.2*
|
|
Form of Notice of Guaranteed Delivery
|
|
|
99
|
.3*
|
|
Form of Letter to Registered Holders and Depository
Trust Company Participants
|
|
|
99
|
.4*
|
|
Form of Letter to Clients
|
|
|
|
|
* |
|
Filed herewith. |
| |
|
+ |
|
Portions of the exhibit have been omitted pursuant to an order
granting confidential treatment dated September 30, 2009 by
the Securities and Exchange Commission. |
The undersigned registrants hereby undertake:
(1) To file, during any period in which offers or sales are
being made, a post-effective amendment to this registration
statement:
(i) To include any prospectus required by
Section 10(a)(3) of the Securities Act of 1933, as amended
(the “Securities Act”);
(ii) To reflect in the prospectus any facts or events
arising after the effective date of the registration statement
(or the most recent post-effective amendment thereto) which,
individually or in the aggregate, represent a fundamental change
in the information set forth in the registration statement.
Notwithstanding the foregoing, any increase or decrease in
volume of securities offered (if the total dollar value of
securities offered would not exceed that which was registered)
and any deviation from the low or high end of the estimated
maximum offering range may be reflected in the form of
prospectus filed with the Commission pursuant to
Rule 424(b) if, in the aggregate, the changes in volume and
price represent no more than a 20% change in the maximum
aggregate offering price set forth in the “Calculation of
Registration Fee” table in the effective registration
statement;
(iii) To include any material information with respect to
the plan of distribution not previously disclosed in the
registration statement or any material change to such
information in the registration statement;
provided, however, that paragraphs (1)(i), (1)(ii) and (1)(iii)
above do not apply if the information required to be included in
a post-effective amendment by those paragraphs is contained in
reports filed with or furnished to the Commission by Grifols
Inc.. pursuant to Section 13 or Section 15(d) of the
Securities Exchange Act of 1934 that are incorporated by
reference in the registration statement, or is contained in a
form of prospectus filed pursuant to Rule 424(b) that is
part of the registration statement.
(2) That, for the purpose of determining any liability
under the Securities Act, each such post-effective amendment
shall be deemed to be a new registration statement relating to
the securities offered
II-6
therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a
post-effective amendment any of the securities being registered
which remain unsold at the termination of the offering.
(4) To file a post-effective amendment to the registration
statement to include any financial statements required by
Item 8.A of
Form 20-F
at the start of any delayed offering or throughout a continuous
offering. Financial statements and information otherwise
required by Section 10(a)(3) of the Securities Act need not
be furnished, provided, that the registrants include in the
prospectus, by means of a post-effective amendment, financial
statements required pursuant to this paragraph (4) and
other information necessary to ensure that all other information
in the prospectus is at least as current as the date of those
financial statements. Notwithstanding the foregoing, a
post-effective amendment need not be filed to include financial
statements and information required by Section 10(a)(3) of
the Securities Act or
Rule 3-19
of
Regulation S-X
if such financial statements and information are contained in
periodic reports filed with or furnished to the Commission by
Grifols Inc. pursuant to Section 13 or Section 15(d)
of the Securities Exchange Act of 1934 that are incorporated by
reference in this registration statement.
(5) That, for the purpose of determining liability under
the Securities Act to any purchaser:
(i) Each prospectus filed by the registrants pursuant to
Rule 424(b)(3) shall be deemed to be part of the
registration statement as of the date the filed prospectus was
deemed part of and included in the registration
statement; and
(ii) Each prospectus required to be filed pursuant to
Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration
statement in reliance on Rule 430B relating to an offering
made pursuant to Rule 415(a)(1)(i), (vii), or (x) for
the purpose of providing the information required by
Section 10(a) of the Securities Act shall be deemed to be
part of and included in the registration statement as of the
earlier of the date such form of prospectus is first used after
effectiveness or the date of the first contract of sale of
securities in the offering described in the prospectus. As
provided in Rule 430B, for liability purposes of the issuer
and any person that is at that date an underwriter, such date
shall be deemed to be a new effective date of the registration
statement relating to the securities in the registration
statement to which the prospectus relates, and the offering of
such securities at that time shall be deemed to be the initial
bona fide offering thereof. Provided, however, that no statement
made in a registration statement or prospectus that is part of
the registration statement or made in a document incorporated or
deemed incorporated by reference into the registration statement
or prospectus that is part of the registration statement will,
as to a purchaser with a time of contract of sale prior to such
effective date, supersede or modify any statement that was made
in the registration statement or prospectus that was part of the
registration statement or made in any such document immediately
prior to such effective date.
(6) That, for the purpose of determining liability of the
registrants under the Securities Act to any purchaser in the
initial distribution of the securities, each undersigned
registrant undertakes that in a primary offering of securities
of such undersigned registrant pursuant to this registration
statement, regardless of the underwriting method used to sell
the securities to the purchaser, if the securities are offered
or sold to such purchaser by means of any of the following
communications, such undersigned registrant will be a seller to
the purchaser and will be considered to offer or sell such
securities to such purchaser:
(i) Any preliminary prospectus or prospectus of the
undersigned registrants relating to the offering required to be
filed pursuant to Rule 424;
(ii) Any free writing prospectus relating to the offering
prepared by or on behalf of the undersigned registrants or used
or referred to by the undersigned registrants;
II-7
(iii) The portion of any other free writing prospectus
relating to the offering containing material information about
the undersigned registrants or their securities provided by or
on behalf of the undersigned registrants; and
(iv) Any other communication that is an offer in the
offering made by the undersigned registrants to the purchaser.
(7) That, for purposes of determining any liability under
the Securities Act, each filing of Grifols Inc.’s annual
report pursuant to Section 13(a) or 15(d) of the Securities
Exchange Act of 1934 (and, where applicable, each filing of an
employee benefit plan’s annual report pursuant to
Section 15(d) of the Securities Exchange Act of
1934) that is incorporated by reference in the registration
statement shall be deemed to be a new registration statement
relating to the securities offered therein, and the offering of
such securities at that time shall be deemed to be the initial
bona fide offering thereof.
Insofar as indemnification for liabilities arising under the
Securities Act may be permitted to directors, officers or
persons controlling the registrants pursuant to the foregoing
provisions, or otherwise, the registrants have been advised that
in the opinion of the Securities and Exchange Commission such
indemnification is against public policy as expressed in the
Securities Act and is, therefore, unenforceable. In the event
that a claim for indemnification against such liabilities (other
than the payment by a registrant of expenses incurred or paid by
a director, officer or controlling person of such registrant in
the successful defense of any action, suit or proceeding) is
asserted by such director, officer or controlling person against
any registrant in connection with the securities being
registered, such registrant will, unless in the opinion of its
counsel the matter has been settled by controlling precedent,
submit to a court of appropriate jurisdiction the question
whether such indemnification by it is against public policy as
expressed in the Securities Act and will be governed by the
final adjudication of such issue.
II-8
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Grifols Inc. certifies that it has reasonable grounds
to believe that it meets all of the requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Virginia, on October 24, 2011.
GRIFOLS INC.
Name: David I. Bell
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints David I.
Bell, as his or her true and lawful attorney-in-fact and agent,
with full and several power of substitution, for him or her and
in his or her name, place and stead, in any and all capacities,
to sign any and all amendments (including post-effective
amendments) and supplements to this registration statement or
any registration statement in connection herewith that is to be
effective upon filing pursuant to Rule 462 (b) under
the Securities Act of 1933, as amended, and to file the same,
with all exhibits thereto, and all documents in connection
therewith, with the Securities and Exchange Commission, granting
unto said attorney-in-fact and agent, full power and authority
to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully for all
intents and purposes as he or she might or could do in person,
hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitute, may lawfully do or cause to be
done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
/s/ Gregory
G. Rich
Gregory
G. Rich
|
|
Director and Chief Executive Officer
(principal executive officer)
|
|
|
|
|
|
/s/ Max
de Brouwer
Max
de Brouwer
|
|
Chief Financial Officer (principal
financial officer and principal accounting officer)
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Director
|
|
|
|
|
|
/s/ Ramón
Riera
Ramón
Riera
|
|
Director
|
|
|
|
|
|
/s/ Juan
Ignacio Twose Roura
Juan
Ignacio Twose Roura
|
|
Director
|
|
|
|
|
|
/s/ Alfredo
Arroyo
Alfredo
Arroyo
|
|
Director
|
|
|
|
|
|
Tomás
Dagá Gelabert
|
|
Director
|
|
|
|
|
|
Thomas
Glanzmann
|
|
Chairman of the Board of Directors
|
II-9
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Grifols, S.A. certifies that it has reasonable grounds
to believe that it meets all of the requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Spain, on October 24, 2011.
GRIFOLS, S.A.
|
|
|
| |
By:
|
/s/ Victor
Grifols Roura
|
Name: Victor Grifols Roura
|
|
|
| |
Title:
|
Chairman and Chief Executive Officer
|
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Victor
Grifols Roura, as his or her true and lawful attorney-in-fact
and agent, with full and several power of substitution, for him
or her and in his or her name, place and stead, in any and all
capacities, to sign any and all amendments (including
post-effective amendments) and supplements to this registration
statement or any registration statement in connection herewith
that is to be effective upon filing pursuant to
Rule 462 (b) under the Securities Act of 1933, as
amended, and to file the same, with all exhibits thereto, and
all documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every
act and thing requisite and necessary to be done in and about
the premises, as fully for all intents and purposes as he or she
might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or his substitute, may
lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director, Chairman of the Board of Directors and
Chief Executive Officer
(principal executive officer)
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Vice President and Chief Financial Officer
(principal financial officer)
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Administrative Director and Controller
(principal accounting officer)
|
|
|
|
|
|
/s/ Juan
Ignacio Twose Roura
Juan
Ignacio Twose Roura
|
|
Director and Vice President
|
|
|
|
|
|
/s/ Ramón
Riera Roca
Ramón
Riera Roca
|
|
Director and Vice President
|
|
|
|
|
|
/s/ Tomás
Dagá Gelabert
Tomás
Dagá Gelabert
|
|
Director
|
II-10
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
/s/ José
Antonio Grifols Gras
Thorthol
Holdings B.V. (represented by José Antonio Grifols Gras)
|
|
Director
|
|
|
|
|
/s/ Edgar
Dalzell Jannotta
Edgar
Dalzell Jannotta
|
|
Director
|
|
|
|
|
/s/ Anna
Veiga Lluch
Anna
Veiga Lluch
|
|
Director
|
|
|
|
|
/s/ William
Brett Ingersoll
William
Brett Ingersoll
|
|
Director
|
|
|
|
|
Luis
Isasi Fernández de Bobadilla
|
|
Director
|
|
|
|
|
/s/ Steven
Francis Mayer
Steven
Francis Mayer
|
|
Director
|
|
|
|
|
/s/ Thomas
Glanzmann
Thomas
Glanzmann
|
|
Director
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
II-11
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Grifols Biologicals Inc. certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Delaware, on October 24, 2011.
GRIFOLS BIOLOGICALS INC.
Name: David I. Bell
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints David I.
Bell, as his or her true and lawful attorney-in-fact and agent,
with full and several power of substitution, for him or her and
in his or her name, place and stead, in any and all capacities,
to sign any and all amendments (including post-effective
amendments) and supplements to this registration statement or
any registration statement in connection herewith that is to be
effective upon filing pursuant to Rule 462 (b) under
the Securities Act of 1933, as amended, and to file the same,
with all exhibits thereto, and all documents in connection
therewith, with the Securities and Exchange Commission, granting
unto said attorney-in-fact and agent, full power and authority
to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully for all
intents and purposes as he or she might or could do in person,
hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitute, may lawfully do or cause to be
done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
/s/ Gregory
G. Rich
Gregory
G. Rich
|
|
Director, Chairman of the Board of
Directors and the principal executive officer
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Director
|
|
|
|
|
|
/s/ Willie
Zuniga
Willie
Zuniga
|
|
Director
|
|
|
|
|
|
/s/ Max
de Brouwer
Max
de Brouwer
|
|
Principal financial officer and principal
accounting officer
|
II-12
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Biomat USA, Inc. certifies that it has reasonable
grounds to believe that it meets all of the requirements for
filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Delaware, on October 24, 2011.
BIOMAT USA, INC.
Name: David I. Bell
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints David I.
Bell, as his or her true and lawful attorney-in-fact and agent,
with full and several power of substitution, for him or her and
in his or her name, place and stead, in any and all capacities,
to sign any and all amendments (including post-effective
amendments) and supplements to this registration statement or
any registration statement in connection herewith that is to be
effective upon filing pursuant to Rule 462 (b) under
the Securities Act of 1933, as amended, and to file the same,
with all exhibits thereto, and all documents in connection
therewith, with the Securities and Exchange Commission, granting
unto said attorney-in-fact and agent, full power and authority
to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully for all
intents and purposes as he or she might or could do in person,
hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitute, may lawfully do or cause to be
done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Director and Chairman of the Board of Directors
|
|
|
|
|
|
/s/ Gregory
Gene Rich
Gregory
Gene Rich
|
|
Director and the principal executive officer
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director
|
|
|
|
|
|
/s/ Juan
Ignacio Twose Roura
Juan
Ignacio Twose Roura
|
|
Director
|
|
|
|
|
|
Tomás
Dagá Gelabert
|
|
Director
|
|
|
|
|
|
/s/ Ramón
Riera Roca
Ramón
Riera Roca
|
|
Director
|
|
|
|
|
|
/s/ Shinji
Wada
Shinji
Wada
|
|
Director
|
|
|
|
|
|
/s/ Javier
Jorba
Javier
Jorba
|
|
Director
|
|
|
|
|
|
/s/ Max
de Brouwer
Max
de Brouwer
|
|
Principal financial officer and principal accounting officer
|
II-13
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Grifols Therapeutics Inc. certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Delaware, on October 24, 2011.
GRIFOLS THERAPEUTICS INC.
Name: David I. Bell
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints David I.
Bell, as his or her true and lawful attorney-in-fact and agent,
with full and several power of substitution, for him or her and
in his or her name, place and stead, in any and all capacities,
to sign any and all amendments (including post-effective
amendments) and supplements to this registration statement or
any registration statement in connection herewith that is to be
effective upon filing pursuant to Rule 462 (b) under
the Securities Act of 1933, as amended, and to file the same,
with all exhibits thereto, and all documents in connection
therewith, with the Securities and Exchange Commission, granting
unto said attorney-in-fact and agent, full power and authority
to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully for all
intents and purposes as he or she might or could do in person,
hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitute, may lawfully do or cause to be
done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
/s/ Gregory
Gene Rich
Gregory
Gene Rich
|
|
Director, Chairman of the Board of
Directors and the principal executive officer
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Director
|
|
|
|
|
|
Mary
J. Kuhn
|
|
Director
|
|
|
|
|
|
/s/ Max
de Brouwer
Max
de Brouwer
|
|
Principal financial officer and principal accounting officer
|
II-14
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Talecris Plasma Resources, Inc. certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Delaware, on October 24, 2011.
TALECRIS PLASMA RESOURCES, INC.
Name: David I. Bell
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints David I.
Bell, as his or her true and lawful attorney-in-fact and agent,
with full and several power of substitution, for him or her and
in his or her name, place and stead, in any and all capacities,
to sign any and all amendments (including post-effective
amendments) and supplements to this registration statement or
any registration statement in connection herewith that is to be
effective upon filing pursuant to Rule 462 (b) under
the Securities Act of 1933, as amended, and to file the same,
with all exhibits thereto, and all documents in connection
therewith, with the Securities and Exchange Commission, granting
unto said attorney-in-fact and agent, full power and authority
to do and perform each and every act and thing requisite and
necessary to be done in and about the premises, as fully for all
intents and purposes as he or she might or could do in person,
hereby ratifying and confirming all that said attorney-in-fact
and agent, or his substitute, may lawfully do or cause to be
done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Gregory
G. Rich
Gregory
G. Rich
|
|
Director and the principal executive officer
|
|
|
|
|
|
|
|
|
|
Mary
J. Kuhn
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ Shinji
Wada
Shinji
Wada
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ Max
de Brouwer
Max
de Brouwer
|
|
principal financial officer and principal
accounting officer
|
|
|
II-15
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Instituto Grifols, S.A. certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Spain, on October 24, 2011.
INSTITUTO GRIFOLS, S.A.
|
|
|
| |
By:
|
/s/ Victor
Grifols Roura
|
Name: Victor Grifols Roura
|
|
|
| |
Title:
|
Chairman and Chief Executive Officer
|
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Victor
Grifols Roura, as his or her true and lawful attorney-in-fact
and agent, with full and several power of substitution, for him
or her and in his or her name, place and stead, in any and all
capacities, to sign any and all amendments (including
post-effective amendments) and supplements to this registration
statement or any registration statement in connection herewith
that is to be effective upon filing pursuant to
Rule 462 (b) under the Securities Act of 1933, as
amended, and to file the same, with all exhibits thereto, and
all documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every
act and thing requisite and necessary to be done in and about
the premises, as fully for all intents and purposes as he or she
might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or his substitute, may
lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director, Chairman and Chief Executive Officer
|
|
|
|
|
|
|
|
|
|
/s/ Javier
Jorba Ribes
Biomat,
S.A. (represented by Javier Jorba Ribes)
|
|
Director and Chief Executive Officer
(principal executive officer)
|
|
|
|
|
|
|
|
|
|
José
Antonio Grifols Gras
|
|
Director
|
|
|
|
|
|
|
|
|
|
Edgar
Dalzell Jannotta
|
|
Director
|
|
|
|
|
|
|
|
|
|
Thomas
Glanzmann
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ Juan
Ignacio Twose Roura
Juan
Ignacio Twose Roura
|
|
Director
|
|
|
II-16
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Ramón
Riera Roca
Ramón
Riera Roca
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Chief Financial Officer (principal financial officer)
|
|
|
|
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Controller (principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
|
|
II-17
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Diagnostic Grifols, S.A. certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Spain, on October 24, 2011.
DIAGNOSTIC GRIFOLS, S.A.
|
|
|
| |
By:
|
/s/ Victor
Grifols Roura
|
Name: Victor Grifols Roura
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Victor
Grifols Roura, as his or her true and lawful attorney-in-fact
and agent, with full and several power of substitution, for him
or her and in his or her name, place and stead, in any and all
capacities, to sign any and all amendments (including
post-effective amendments) and supplements to this registration
statement or any registration statement in connection herewith
that is to be effective upon filing pursuant to
Rule 462 (b) under the Securities Act of 1933, as
amended, and to file the same, with all exhibits thereto, and
all documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every
act and thing requisite and necessary to be done in and about
the premises, as fully for all intents and purposes as he or she
might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or his substitute, may
lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director (Administrador Solidario)
|
|
|
|
|
|
|
|
|
|
/s/ Oriol
Duñach Fulla
Oriol
Duñach Fulla
|
|
Director (Administrador Solidario) and
the principal executive officer
|
|
|
|
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Chief Financial Officer (principal financial officer)
|
|
|
|
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Controller (principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
|
|
II-18
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Movaco, S.A. certifies that it has reasonable grounds
to believe that it meets all of the requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Spain, on October 24, 2011.
MOVACO, S.A.
|
|
|
| |
By:
|
/s/ Victor
Grifols Roura
|
Name: Victor Grifols Roura
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Victor
Grifols Roura, as his or her true and lawful attorney-in-fact
and agent, with full and several power of substitution, for him
or her and in his or her name, place and stead, in any and all
capacities, to sign any and all amendments (including
post-effective amendments) and supplements to this registration
statement or any registration statement in connection herewith
that is to be effective upon filing pursuant to
Rule 462 (b) under the Securities Act of 1933, as
amended, and to file the same, with all exhibits thereto, and
all documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every
act and thing requisite and necessary to be done in and about
the premises, as fully for all intents and purposes as he or she
might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or his substitute, may
lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director (Administrador Solidario)
|
|
|
|
|
|
|
|
|
|
/s/ Santiago
González Orti
Santiago
González Orti
|
|
Director (Administrador Solidario) and the principal
executive officer
|
|
|
|
|
|
|
|
|
|
/s/ Miquel
Pascual Montblanch
Miquel
Pascual Montblanch
|
|
Director (Administrador Solidario)
|
|
|
|
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Chief Financial Officer (principal
financial officer)
|
|
|
|
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Controller (principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
|
|
II-19
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Laboratorios Grifols, S.A. certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Spain, on October 24, 2011.
LABORATORIOS GRIFOLS, S.A.
|
|
|
| |
By:
|
/s/ Victor
Grifols Roura
|
Name: Victor Grifols Roura
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Victor
Grifols Roura, as his or her true and lawful attorney-in-fact
and agent, with full and several power of substitution, for him
or her and in his or her name, place and stead, in any and all
capacities, to sign any and all amendments (including
post-effective amendments) and supplements to this registration
statement or any registration statement in connection herewith
that is to be effective upon filing pursuant to
Rule 462 (b) under the Securities Act of 1933, as
amended, and to file the same, with all exhibits thereto, and
all documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every
act and thing requisite and necessary to be done in and about
the premises, as fully for all intents and purposes as he or she
might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or his substitute, may
lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Victor
Grifols Roura
Victor
Grifols Roura
|
|
Director (Administrador Solidario)
|
|
|
|
|
|
|
|
|
|
/s/ Alberto
Grifols Roura
Alberto
Grifols Roura
|
|
Director (Administrador Solidario) and
the principal executive officer
|
|
|
|
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Chief Financial Officer (principal
financial officer)
|
|
|
|
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Controller (principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
|
|
II-20
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Grifols Italia, S.p.A. certifies that it has reasonable
grounds to believe that it meets all of the requirements for
filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Italy, on October 24, 2011.
GRIFOLS ITALIA, S.P.A.
|
|
|
| |
By:
|
/s/ Victor
Grifols Roura
|
Name: Victor Grifols Roura
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Victor
Grifols Roura, as his or her true and lawful attorney-in-fact
and agent, with full and several power of substitution, for him
or her and in his or her name, place and stead, in any and all
capacities, to sign any and all amendments (including
post-effective amendments) and supplements to this registration
statement or any registration statement in connection herewith
that is to be effective upon filing pursuant to
Rule 462 (b) under the Securities Act of 1933, as
amended, and to file the same, with all exhibits thereto, and
all documents in connection therewith, with the Securities and
Exchange Commission, granting unto said attorney-in-fact and
agent, full power and authority to do and perform each and every
act and thing requisite and necessary to be done in and about
the premises, as fully for all intents and purposes as he or she
might or could do in person, hereby ratifying and confirming all
that said attorney-in-fact and agent, or his substitute, may
lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Riccardo
Vanni
Riccardo
Vanni
|
|
Chief Executive Officer (principal
executive officer)
|
|
|
|
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ Ramón
Riera Roca
Ramón
Riera Roca
|
|
Director, Chairman of the Board of Directors
|
|
|
|
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Director
|
|
|
|
|
|
|
|
|
|
/s/ Andrea
Guidi
Andrea
Guidi
|
|
Chief Financial Officer (principal
financial officer and principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
|
|
II-21
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as
amended, Grifols Deutschland GmbH certifies that it has
reasonable grounds to believe that it meets all of the
requirements for filing on
Form F-4
and has duly caused this registration statement to be signed on
its behalf by the undersigned, thereunto duly authorized in
Germany, on October 24, 2011.
GRIFOLS DEUTSCHLAND GMBH
Name: Ramon Riera Roca
|
|
|
| |
By:
|
/s/ Alfredo
Arroyo
Guerra |
Name: Alfredo Arroyo Guerra
POWER OF
ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each of the individuals
whose signature appears below constitutes and appoints Ramon
Riera Roca and Alfredo Arroyo Guerra and each of them, his or
her true and lawful attorneys-in-fact and agent, with full and
several power of substitution, for him or her and in his or her
name, place and stead, in any and all capacities, to sign any
and all amendments (including post-effective amendments) and
supplements to this registration statement or any registration
statement in connection herewith that is to be effective upon
filing pursuant to Rule 462 (b) under the Securities
Act of 1933, as amended, and to file the same, with all exhibits
thereto, and all documents in connection therewith, with the
Securities and Exchange Commission, granting unto said
attorneys-in-fact and agents, and each of them, full power and
authority to do and perform each and every act and thing
requisite and necessary to be done in and about the premises, as
fully for all intents and purposes as he or she might or could
do in person, hereby ratifying and confirming all that said
attorneys-in-fact and agents or any of them, or their
substitutes, may lawfully do or cause to be done by virtue
hereof.
Pursuant to the requirements of the Securities Act of 1933, as
amended, this registration statement has been signed by the
following persons in the capacities and on October 24, 2011.
| |
|
|
|
|
|
|
|
Signature
|
|
Title
|
|
|
|
|
|
|
|
|
|
|
|
/s/ Ramón
Riera Roca
Ramón
Riera Roca
|
|
Managing Director
|
|
|
|
|
|
|
|
|
|
/s/ Alfredo
Arroyo Guerra
Alfredo
Arroyo Guerra
|
|
Managing Director and the principal
executive officer
|
|
|
|
|
|
|
|
|
|
/s/ Thierry
Heirich
Thierry
Heirich
|
|
Managing Director
|
|
|
|
|
|
|
|
|
|
/s/ Montserrat
Lloveras Calvo
Montserrat
Lloveras Calvo
|
|
Managing Director
|
|
|
|
|
|
|
|
|
|
/s/ Ainhoa
Mendizabal
Ainhoa
Mendizabal
|
|
Chief Financial Officer (principal
financial officer and principal accounting officer)
|
|
|
|
|
|
|
|
|
|
/s/ David
I. Bell
David
I. Bell
|
|
Authorized Representative in the United States
|
|
|
II-22
EXHIBIT
INDEX
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
2
|
.1
|
|
Agreement and Plan of Merger, dated as of June 6, 2010, by
and among Grifols, S.A., Grifols, Inc. and Talecris
Biotherapeutics Holdings Corp. (incorporated by reference to
Exhibit 2.1 of our Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
2
|
.2
|
|
Amendment No. 1 to the Agreement and Plan of Merger, dated
as of November 4, 2010, by and among Grifols, S.A.,
Grifols, Inc. and Talecris Biotherapeutics Holdings Corp.
(incorporated by reference to Exhibit 2.2 of Amendment
No. 2 to our Registration Statement on
Form F-4
(File
No. 333-168701)
filed on November 5, 2010)
|
|
|
3
|
.1.1*
|
|
By-Laws (Estatutos) of Grifols, S.A.
|
|
|
3
|
.1.2*
|
|
By-Laws (Estatutos) of Grifols, S.A. (English translation)
|
|
|
3
|
.2.1*
|
|
Amended and Restated Articles of Incorporation of Grifols Inc.
|
|
|
3
|
.2.2*
|
|
Amended and Restated By-laws of Grifols Inc.
|
|
|
3
|
.3.1*
|
|
Certificate of Incorporation of Grifols Biologicals Inc.
|
|
|
3
|
.3.2*
|
|
Certificate of Correction of the Certificate of Incorporation of
Grifols Biologicals Inc.
|
|
|
3
|
.3.3*
|
|
By-laws of Grifols Biologicals Inc.
|
|
|
3
|
.4.1*
|
|
Amended and Restated Certificate of Incorporation of Biomat USA,
Inc.
|
|
|
3
|
.4.2*
|
|
By-laws of Biomat USA, Inc.
|
|
|
3
|
.5.1*
|
|
Certificate of Incorporation of Grifols Therapeutics, Inc.
|
|
|
3
|
.5.2*
|
|
Certificate of Amendment to the Certificate of Incorporation of
Grifols Therapeutics, Inc.
|
|
|
3
|
.5.3*
|
|
Certificate of Amendment to the Certificate of Incorporation of
Grifols Therapeutics, Inc.
|
|
|
3
|
.5.4*
|
|
By-laws of Grifols Therapeutics, Inc.
|
|
|
3
|
.6.1*
|
|
Certificate of Incorporation of Talecris Plasma Resources, Inc.
|
|
|
3
|
.6.2*
|
|
Certificate of Amendment of Certificate of Incorporation of
Talecris Plasma Resources, Inc.
|
|
|
3
|
.6.3*
|
|
Amended and Restated By-laws of Talecris Plasma Resources, Inc.
|
|
|
3
|
.7.1*
|
|
By-laws (Estatutos) of Instituto Grifols, S.A.
|
|
|
3
|
.7.2*
|
|
By-laws (Estatutos) of Instituto Grifols, S.A. (English
translation)
|
|
|
3
|
.8.1*
|
|
By-laws (Estatutos) of Diagnostic Grifols, S.A.
|
|
|
3
|
.8.2*
|
|
By-laws (Estatutos) of Diagnostic Grifols, S.A. (English
translation)
|
|
|
3
|
.9.1*
|
|
By-laws (Estatutos) of Movaco, S.A.
|
|
|
3
|
.9.2*
|
|
By-laws (Estatutos) of Movaco, S.A. (English translation)
|
|
|
3
|
.10.1*
|
|
By-laws (Estatutos) of Laboratorios Grifols, S.A.
|
|
|
3
|
.10.2*
|
|
By-laws (Estatutos) of Laboratorios Grifols, S.A. (English
translation)
|
|
|
3
|
.11.1*
|
|
By-laws of Grifols Italia, S.p.A.
|
|
|
3
|
.11.2*
|
|
By-laws of Grifols Italia, S.p.A. (English translation)
|
|
|
3
|
.12*
|
|
By-laws of Grifols Deutschland GmbH
|
|
|
4
|
.1*
|
|
Senior Notes Indenture, dated as of January 21, 2011,
relating to the 8.25% Senior Notes due 2018, among Giant
Funding Corp. and The Bank of New York Mellon
Trust Company, N.A., as trustee.
|
|
|
4
|
.2*
|
|
Form of 8.25% Senior Note (included as Exhibit A to
Exhibit 4.1)
|
|
|
4
|
.3*
|
|
Supplemental Indenture, dated June 1, 2011, by and among
Grifols Inc., Grifols, S.A., the subsidiary guarantors party
thereto and The Bank of New York Mellon Trust Company,
N.A., as trustee.
|
|
|
4
|
.4*
|
|
Second Supplemental Indenture, dated as of October 4, 2011,
between Grifols Deutschland GmbH and The Bank of New York Mellon
Trust Company, N.A., as trustee.
|
|
|
4
|
.5*
|
|
Registration Rights Agreement, dated January 21, 2011, by
and among Giant Funding Corp. and Deutsche Bank Securities Inc.,
as representative of the several initial purchasers.
|
|
|
4
|
.6*
|
|
Registration Rights Agreement Joinder, dated as of June 1,
2011, by and among Grifols Inc., Grifols, S.A. and the
subsidiary guarantors party thereto.
|
|
|
5
|
.1*
|
|
Opinion of Proskauer Rose LLP, New York, NY, United States of
America
|
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
5
|
.2*
|
|
Opinion of Hunton & Williams LLP, Richmond, VA, United
States of America
|
|
|
5
|
.3*
|
|
Opinion of Osborne Clarke, S.L.P., Barcelona, Spain
|
|
|
5
|
.4*
|
|
Opinion of SLA Studio Legale Associato, Milan, Italy with
respect to Grifols Italia, S.p.A.
|
|
|
5
|
.5*
|
|
Opinion of Osborne Clarke, Munich, Germany with respect to
Grifols Deutschland GmbH
|
|
|
10
|
.1*
|
|
Credit and Guaranty Agreement, dated as of November 23,
2010, among Grifols Inc., Grifols, S.A., certain subsidiaries of
Grifols, S.A., the lenders party thereto, Deutsche Bank
Securities, Inc., Nomura International PLC, Banco Bilbao Vizcaya
Argentaria, S.A., BNP Paribas, HSBC Securities (USA) Inc. and
Morgan Stanley Senior Funding, Inc., as joint lead arrangers and
joint bookrunners and Deutsche Bank AG New York branch, as
administrative agent and collateral agent.
|
|
|
10
|
.2*
|
|
First Amendment to Credit and Guaranty Agreement, dated as of
March 3, 2011, by and among Grifols, Inc., Deutsche Bank AG
New York Branch, as administrative agent.
|
|
|
10
|
.3*
|
|
Second Amendment to Credit and Guaranty Agreement, dated as of
May 31, 2011, by and among Grifols, Inc. and Deutsche Bank
AG New York Branch, as administrative agent.
|
|
|
10
|
.4*
|
|
Counterpart Agreement, dated June 1, 2011, by and among
Grifols Inc., Grifols, S.A. and certain of its subsidiaries, the
Lenders party thereto and Deutsche Bank AG New York Branch, as
administrative agent and collateral agent.
|
|
|
10
|
.5*
|
|
U.S. Pledge and Security Agreement, dated as of June 1,
2011, among the grantors party thereto and Deutsche Bank AG New
York Branch, as collateral agent.
|
|
|
10
|
.6*
|
|
Assumption Agreement, dated as of June 1, 2011, by and
between Grifols, Inc. and Deutsche Bank AG New York Branch, as
administrative agent.
|
|
|
10
|
.7
|
|
Voting Agreement between Grifols, S.A. and Talecris Holdings,
LLC (incorporated by reference to Exhibit 10.1 of our
Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
10
|
.8
|
|
Form of Voting Agreement between certain holders of ordinary
shares of Grifols, S.A. and Talecris Biotherapeutics Holdings
Corp. (incorporated by reference to Exhibit 10.2 of our
Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
10
|
.9
|
|
Lock-up
Agreement between Grifols, S.A. and Talecris Holdings, LLC
(incorporated by reference to Exhibit 10.3 of our
Registration Statement on
Form F-4
(File
No. 333-168701)
filed on August 10, 2010)
|
|
|
10
|
.10
|
|
Appraisal Indemnity Agreement, dated as of November 4,
2010, by and among Talecris Biotherapeutics Holdings Corp.,
Grifols, S.A., and Talecris Holdings, LLC, and solely with
respect to the provisions of Section 9, Cerberus Capital
Management, L.P. (incorporated by reference to Exhibit 10.4
of Amendment No. 2 to our Registration Statement on
Form F-4
(File
No. 333-168701)
filed on November 5, 2010)
|
|
|
10
|
.11
|
|
Toll Manufacturing Agreement for Testing and Packaging, dated
April 4, 2008, by and between Talecris Biotherapeutics,
GmbH and Catalent France Limoges SAS (incorporated by reference
to Exhibit 10.35 of Amendment No. 8 to Talecris’
Registration Statement on
Form S-1
(File
No. 333-144941)
filed on August 19, 2009)+
|
|
|
10
|
.12
|
|
Retained Intellectual Property License Agreement, dated as of
March 31, 2005, by and between Bayer Healthcare LLC and
Talecris Biotherapeutics, Inc. (f/k/a NPS Biotherapeutics, Inc.)
(incorporated by reference to Exhibit 10.31.1 of Amendment
No. 1 to Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 24, 2007)
|
|
|
10
|
.13
|
|
Amendment to Retained Intellectual Property Licensing Agreement,
entered into as of August 10, 2007, by and between Bayer
Healthcare LLC and Talecris Biotherapeutics, Inc. (incorporated
by reference to Exhibit 10.31.2 of Amendment No. 1 to
Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 24, 2007)
|
|
|
10
|
.14
|
|
Fractionation Services and Commercial Products Agreement, dated
as of April 1, 2008, between and amongst Canadian Blood
Services/Societe Canadienne Du Sang, Talecris Biotherapeutics,
Inc. and Talecris Biotherapeutics, Ltd. (incorporated by
reference to Exhibit 10.29 of Amendment No. 9 to
Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 11, 2009)+
|
| |
|
|
|
|
Exhibit
|
|
|
|
Number
|
|
Description of Exhibit
|
|
|
|
|
10
|
.15
|
|
Fractionation Services and Commercial Products Agreement, dated
as of April 1, 2008, between and amongst
Héma-Québec, Talecris Biotherapeutics, Ltd. and
Talecris Biotherapeutics, Inc. (incorporated by reference to
Exhibit 10.30.1 of Amendment No. 9 to Talecris’
Registration Statement on
Form S-1
(File
No. 333-144941)
filed on September 11, 2009)+
|
|
|
10
|
.16
|
|
Amending Agreement No. 1, effective as of May 26,
2008, to Fractionation Services and Commercial Products, dated
as of April 1, 2008, by and among Héma-Québec,
Talecris Biotherapeutics, Ltd. and Talecris Biotherapeutics,
Inc. (incorporated by reference to Exhibit 10.30.2 of
Amendment No. 6 to Talecris’ Registration Statement on
Form S-1
(File
No. 333-144941)
filed on July 2, 2009)+
|
|
|
12
|
.1*
|
|
Statement of Computation of Ratio of Earnings to Fixed Charges
|
|
|
21
|
.1*
|
|
Subsidiaries of the Registrants
|
|
|
23
|
.1*
|
|
Consent of Proskauer Rose LLP, New York, NY, United States of
America (included in Exhibit 5.1)
|
|
|
23
|
.2*
|
|
Consent of Hunton & Williams LLP, Richmond, VA, United
States of America (included in Exhibit 5.2)
|
|
|
23
|
.3*
|
|
Consent of Osborne Clarke Spain, Barcelona, Spain (included in
Exhibit 5.3)
|
|
|
23
|
.4*
|
|
Consent of SLA Studio Legale Associato, Milan, Italy with
respect to Grifols Italia, S.p.A. (included in Exhibit 5.4)
|
|
|
23
|
.5*
|
|
Consent of Osborne Clarke Germany, Munich, Germany with respect
to Grifols Deutschland GmbH (included in Exhibit 5.5)
|
|
|
23
|
.6*
|
|
Consent of KPMG Auditores, S.L., Independent Registered Public
Accounting Firm
|
|
|
23
|
.7*
|
|
Consent of PriceWaterhouseCoopers LLP, Independent Registered
Public Accounting Firm
|
|
|
24
|
.1*
|
|
Power of Attorney (included on the signature pages hereto)
|
|
|
25
|
.1*
|
|
Statement of Eligibility on
Form T-1
of The Bank of New York Mellon Trust Company, N.A., as trustee
under the indenture
|
|
|
99
|
.1*
|
|
Form of Letter of Transmittal
|
|
|
99
|
.2*
|
|
Form of Notice of Guaranteed Delivery
|
|
|
99
|
.3*
|
|
Form of Letter to Registered Holders and Depository
Trust Company Participants
|
|
|
99
|
.4*
|
|
Form of Letter to Clients
|
|
|
|
|
* |
|
Filed herewith. |
| |
|
+ |
|
Portions of the exhibit have been omitted pursuant to an order
granting confidential treatment dated September 30, 2009 by
the Securities and Exchange Commission. |