PART II — INFORMATION REQUIRED IN OFFERING CIRCULAR
An Offering Statement pursuant to Regulation A relating to these securities has been filed with the Securities and Exchange Commission. Information contained in this Preliminary Offering Circular is subject to completion or amendment. These securities may not be sold nor may offers to buy be accepted before the Offering Statement filed with the Commission is qualified. This Preliminary Offering Circular shall not constitute an offer to sell or the solicitation of an offer to buy nor may there be any sales of these securities in any state in which such offer, solicitation or sale would be unlawful before registration or qualification under the laws of any such state. We may elect to satisfy our obligation to deliver a Final Offering Circular by sending you a notice within two business days after the completion of our sale to you that contains the URL where the Final Offering Circular or the Offering Statement in which such Final Offering Circular was filed may be obtained.
Preliminary Offering Circular, Dated October 4, 2019
Subject to Completion

REMSLEEP HOLDINGS, INC.
$3,000,000/50,000,000 SHARES OF COMMON STOCK
$0.06 PER SHARE
We are offering up to 50 million (50,000,000) shares of our common stock on a “best efforts” basis at a fixed offering price of $0.06 per share for aggregate maximum gross proceeds of $3 million ($3,000,000). The minimum purchase requirement per investor is $6,000 (100,000 shares); however, we can waive the minimum purchase requirement on a case-by-case basis in our sole discretion. See “Description of Securities” beginning on page 55.
Investing in this offering involves high degree of risk, and you should not invest unless you can afford to lose your entire investment. See “Risk Factors” beginning on page 8 for a discussion of certain risks that you should consider in connection with an investment in our securities.
Our common stock currently trades on the OTC Market’s Pinks under the symbol “RMSL” and the closing price of our common stock on September 27, 2019 was $0.02. Our common stock currently trades on a sporadic and limited basis. Our Board of Directors used its business judgment in setting the $0.06 purchase price per share to the Company as consideration for the stock to be issued in this offering. The purchase price per share bears no relationship to our book value or any other measure of our current value or worth.
The proposed sale will begin as soon as practicable after this offering statement has been qualified by the Securities and Exchange Commission (the “SEC”) and the relevant state regulators, as necessary. This offering will terminate upon the earlier to occur of: (i) the sale of all 50 million shares being offered; (ii) 365 days after this offering statement is qualified by the SEC; (iii) the Company terminates this offering for any reason.
This offering is being conducted on a “best efforts” basis pursuant to Regulation A of Section 3(b) of the Securities Act of 1933, as amended (the “Securities Act”), for Tier 2 offerings and there is no minimum offering amount we must sell before we close. We have made no arrangements to place subscription proceeds or funds in an escrow, trust or similar account, which means that the proceeds or funds from the sale of common stock will be immediately available to us for use in our operations and once received and accepted are irrevocable. See “Plan of Distribution” and “Description of Securities” for a description of our capital stock.
| Price to Public | Underwriting Discount and Commissions(1) | Proceeds to Issuer(2) | Proceeds to Other Persons | |||||||||||||
| Per share | $ | 0.06 | $ | 0.00 | $ | 0.06 | $ | 0.00 | ||||||||
| Total Minimum | N/A | N/A | N/A | N/A | ||||||||||||
| Total Maximum | $ | 3,000,000 | $ | 0.00 | $ | 3,000,000 | $ | 0.00 | ||||||||
| (1) | We do not intend to use commissioned sales agents or underwriters. |
| (2) | The amounts shown are before deducting offering costs to us, which include legal, accounting, printing, due diligence, marketing, consulting, selling and other costs incurred in this offering, estimated to be $75,000. |
Our executive offices are located at 637 N. Orange Ave., Suite 609, Orlando, FL 32789 and our telephone number is (515) 490-9067. the Company’s website is www.remsleep.com, the contents of which is not incorporated by reference into this Offering Circular.
THE UNITED STATES SECURITIES AND EXCHANGE COMMISSION DOES NOT PASS UPON THE MERITS OF OR GIVE ITS APPROVAL TO ANY SECURITIES OFFERED OR THE TERMS OF THE OFFERING, NOR DOES IT PASS UPON THE ACCURACY OR COMPLETENESS OF ANY OFFERING CIRCULAR OR OTHER SOLICITATION MATERIALS. THESE SECURITIES ARE OFFERED PURSUANT TO AN EXEMPTION FROM REGISTRATION WITH THE COMMISSION; HOWEVER, THE COMMISSION HAS NOT MADE AN INDEPENDENT DETERMINATION THAT THE SECURITIES OFFERED ARE EXEMPT FROM REGISTRATION.
THE SHARES OF COMMON STOCK HAVE NOT BEEN REGISTERED UNDER THE SECURITIES ACT OF 1933, AS AMENDED, OR APPLICABLE STATE SECURITIES LAWS, AND ARE BEING OFFERED AND SOLD IN RELIANCE ON EXEMPTIONS FROM THE REGISTRATION REQUIREMENTS OF THESE LAWS. THE SHARES OF COMMON STOCK HAVE NOT BEEN APPROVED OR DISAPPROVED BY THE SECURITIES AND EXCHANGE COMMISSION OR ANY STATE REGULATORY AUTHORITY NOR HAS THE COMMISSION OR ANY STATE REGULATORY AUTHORITY PASSED UPON OR ENDORSED THE MERITS OF THE OFFERING OR THE ACCURACY OR ADEQUACY OF THIS OFFERING CIRCULAR. ANY REPRESENTATION TO THE CONTRARY IS UNLAWFUL.
GENERALLY, NO SALE MAY BE MADE TO YOU IN THIS OFFERING IF THE AGGREGATE PURCHASE PRICE YOU PAY IS MORE THAN 10% OF THE GREATER OF YOUR ANNUAL INCOME OR NET WORTH. DIFFERENT RULES APPLY TO ACCREDITED INVESTORS AND NON-NATURAL PERSONS. BEFORE MAKING ANY REPRESENTATION THAT YOUR INVESTMENT DOES NOT EXCEED APPLICABLE THRESHOLDS, WE ENCOURAGE YOU TO REVIEW RULE 251(D)(2)(I)(C) OF REGULATION A. FOR GENERAL INFORMATION ON INVESTING, WE ENCOURAGE YOU TO REFER TO WWW.INVESTOR.GOV.
This Offering Circular follows the disclosure format of Part I of Form S-1 pursuant to the general instructions of Part II(a)(1)(ii) of Form 1-A.
The approximate date of commencement of proposed sale to the public is _________, 2019.
TABLE OF CONTENTS
i
We are offering to sell, and seeking offers to buy, our securities only in jurisdictions where such offers and sales are permitted. You should rely only on the information contained in this Offering Circular. We have not authorized anyone to provide you with any information other than the information contained in this Offering Circular. The information contained in this Offering Circular is accurate only as of its date, regardless of its delivery or of any sale or delivery of our securities. Neither the delivery of this Offering Circular nor any sale or delivery of our securities shall, under any circumstances, imply that there has been no change in our affairs since the date of this Offering Circular. This Offering Circular will be updated and made available for delivery to the extent required by the federal securities laws.
Unless otherwise specified, the information in this Offering Circular is set forth as of the date of this Offering Circular, and we anticipate that changes in our affairs will occur after such date. We have not authorized any person to give any information or to make any representations, other than as contained in this Offering Circular, in connection with the offer contained in this Offering Circular. If any person gives you any information or makes representations in connection with this Offer, do not rely on it as information we have authorized. This Offering Circular is not an offer to sell our common stock in any state or other jurisdiction to any person to whom it is unlawful to make such offer.
In this Offering Circular, unless the context indicates otherwise, references to “REMSleep,” “RMSL,” “REMSleep Holdings,” the “Company,” “we,” “our,” “us” and similar expressions refer to the activities of and the assets and liabilities of the business and operations of REMSleep Holdings, Inc., a Nevada corporation.
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Some of the statements under “Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Our Business” and elsewhere in this Offering Circular constitute forward-looking statements. Forward-looking statements relate to expectations, beliefs, projections, future plans and strategies, anticipated events or trends and similar matters that are not historical facts. In some cases, you can identify forward-looking statements by terms such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “should,” “will” and “would” or the negatives of these terms or other comparable terminology.
You should not place undue reliance on forward looking statements. The cautionary statements set forth in this Offering Circular, including in “Risk Factors” and elsewhere, identify important factors which you should consider in evaluating our forward-looking statements. These factors include, among other things:
| ● | The speculative nature of the business we intend to develop; |
| ● | Our ability to successfully develop material revenue streams from our developmental activities, many of which are close to start up and not operating at the present time. |
| ● | Our dependence upon external sources for the financing of our operations. |
| ● | Our ability to effectively execute our business plan; |
| ● | Our ability to manage our expansion, growth and operating expenses; |
| ● | Our ability to finance our business; and |
| ● | Our ability to promote our business. |
Although the forward-looking statements in this Offering Circular are based on our beliefs, assumptions and expectations, taking into account all information currently available to us, we cannot guarantee future transactions, results, performance, achievements or outcomes. No assurance can be made to any investor by anyone that the expectations reflected in our forward-looking statements will be attained, or that deviations from them will not be material and adverse. We undertake no obligation, other than as may be required by law, to re-issue this Offering Circular or otherwise make public statements updating our forward-looking statements.
1
This prospectus includes our trademarks and trade names, including, without limitation, DeltaWave, DeltaWave CPAP and our logo, which are our property and are protected under applicable intellectual property laws. This prospectus also contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. Solely for convenience, trademarks, trade names and service marks referred to in this prospectus may appear without the ®, ™ or SM symbols, but such references are not intended to indicate, in any way, that we or the applicable owner will not assert, to the fullest extent permitted under applicable law, our or its rights or the right of any applicable licensor to these trademarks, trade names and service marks. We do not intend our use or display of other parties’ trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties.
Unless otherwise indicated, information contained in this prospectus concerning our industry and the markets in which we operate, including our general expectations, market position and market opportunity, is based on our management’s estimates and research, as well as industry and general publications and research, surveys and studies conducted by third parties. We believe that the information from these third-party publications, research, surveys and studies included in this prospectus is reliable. Management’s estimates are derived from publicly available information, their knowledge of our industry and their assumptions based on such information and knowledge, which we believe to be reasonable. This data involves a number of assumptions and limitations which are necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in “Risk Factors” starting on page 8 of this Offering Circular. These and other factors could cause our future performance to differ materially from our assumptions and estimates.
2
This summary highlights selected information contained elsewhere in this Offering Circular. This summary is not complete and does not contain all the information that you should consider before deciding whether to invest in our common stock. You should carefully read the entire Offering Circular, including the risks associated with an investment in our company discussed in the “Risk Factors” section of this Offering Circular beginning on page 8 and our financial statements and the related notes appearing at the end of this Offering Circular, before making an investment decision. Some of the statements in this Offering Circular are forward-looking statements. See the section entitled “Cautionary Statement Regarding Forward-Looking Statements.”
Unless the context requires otherwise, references to “REMSleep,” the “Company,” “we,” “us,” and “our,” refer to REMSleep Holdings, Inc. and its wholly-owned subsidiary, REMSleep LLC.
Overview
We are a medical technology company focused on the development and commercialization of innovative and minimally invasive solutions for patients with obstructive sleep apnea. Our officers have 35 years of sleep-industry experience, including having been employed at sleep industry companies. Our goal is to develop sleep products that achieve optimum compliance and comfort for CPAP patients.
In May 2017, we applied for a patent with the US Patent and Trademark Office for our proprietary DeltaWave CPAP interface (“DeltaWave”), a new, innovative sleep apnea product to act as an interface for the delivery of CPAP therapy and other respiratory needs. DeltaWave is a nasal-pillow type interface designed to offer better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally.
Market Opportunity
Sleep apnea is a serious and chronic disease that negatively impacts a patient’s sleep, health and quality of life. According to the World Health Organization, sleep apnea affects more than 100 million people worldwide. Left untreated, sleep apnea increases the risk of high blood pressure, hypertension, heart failure, stroke, coronary artery disease and other life-threatening diseases.
Ventilation is commonly used in the treatment of respiratory conditions such as sleep apnea. On such form of ventilation is a continuous positive air pressure system often referred to as a CPAP system which typically includes a mask that interfaces with a user’s mouth or nasal passages, supplying air pressure from an air flow generator that is typically located in the proximity of the user. The mask directs the flow of air into the breathing passage of the user while allowing the user to exhale.
If a user suffers from claustrophobia, a mask that fits over the mouth might be upsetting. Also, men with facial hair may have trouble getting a seal with a standard CPAP mask that fits over the nose or around the mouth. Nasal pillows eliminate these concerns by applying CPAP pressure directly into the user’s nostrils. Nasal pillows are a type of CPAP mask consisting of plastic inserts that look like headphone earbuds that slip directly into the nostrils. The prescribed pressure used to keep the airway open is delivered through this mask. Furthermore, some people prefer nasal pillows because they do not leave marks on the face from either a mask interface or the straps needed to keep the mask in place.
Many people find nasal pillows to be a favorable option for the administration of CPAP to treat their sleep apnea. However, it is important that nasal pillows are properly sized. If they are too small, air may leak out around them and reduce the effectiveness of treatment. Conversely, if the nasal pillows are too large, they may uncomfortably stretch the nostrils. Proper fitting will address most of the issues.
For the treatment of sleep apnea, the CPAP system is worn while the user sleeps. In CPAP systems where the mask interfaces with the user’s nostrils, the nostril interface need be held in place and maintain a seal so that, as the user moves during sleep, the mask/nostril interface remains intact and sealed to provide positive airway pressure. The mask/nostril interface is often referred to as pillow interfaces that are inserted partially into each nostril, maintaining an air-tight seal. As the pillow interfaces are worn while the user sleeps, it is important that the pillow interfaces be as comfortable as possible. If the seal is not air-tight, less positive airway pressure is delivered and, the break in the seal will often create noise that may impact the user’s sleep.
3
Another issue with continuous positive air pressure system is the flow velocity of the positive airway pressure. In providing an adequate volume of air for each breath the patient takes (inhalation), the CPAP system must provide a minimum volume per time period. Tradeoffs are made between providing greater volumes by using larger orifices or by using smaller orifices and increasing the velocity of the provided air. Unfortunately, when the velocity of the provided air is increased, several user issues occur including burning sensations in the user’s sinuses, drying of the user’s sinuses, a compromised therapeutic index, and increased noise that is undesirable when the user is trying to sleep.
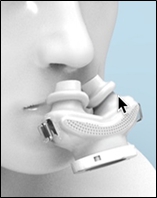
Existing CPAP systems use nasal pillow interfaces that are round. These pillow interfaces cause discomfort when worn as the nasal septum is typically more sensitive to pressure and is typically relatively flat in areas of the nasal openings. Round cross-sectional shapes do not interface well with flat walls and, as an accommodation, prior pillow interfaces are typically made of a soft material that flattens when inserted. This flattening reduces the cross-sectional area of these prior nasal pillows, resulting in an acceleration of the air velocity and noise. The increase in air flow velocity leads to dryness, a sudden burning sensation in the nostrils, and stuffiness in the sinuses. Higher air flow velocity and lower volume of incoming air is less efficient at correcting apnea and often interrupts normal breathing patterns. DeltaWave is designed to deliver more air volume with less driving pressure. Therefore, the patient gets more air coming into the upper airways at a slower air velocity. Also, the pillows that fit into the nostrils are designed to better fit the shape of the nostrils for better comfort and better airtight seal.
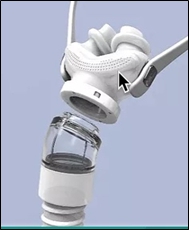
Further, most prior interface pillow designs included turning points for the incoming air, some as much as 90 degrees, resulting in turbulence, pressure drop, and a decrease in the therapeutic index of the treatment. As a result of this, more pressure is typically required to deliver the required increased flow rate which partially compensates for inadequate air volume needed for treatment. This increase in air velocity increases unwanted side effects. An increase in air flow velocity is not preferred over supplying adequate air volume. Another issue with existing CPAP systems is disposal of extra pressure, either from the air flow generator or from the user’s exhalation. Many existing CPAP systems have inadequate exit venting or exit venting that is directed toward the user’s body, creating discomfort as the user exhales. DeltaWave is designed to deliver air flow to the nostrils without using any sharp turning points for the incoming air. DeltaWave is also designed to avoid any choke points or areas where the air flow would be restricted, which would cause pressure drop in the system. Avoiding air flow turning points and avoiding restriction (choke) points create and assure laminar flow.
Our DeltaWave CPAP system is designed to provide interface pillows that seal within nostrils while retaining comfort and proper air flow.
Target Market
Our target market includes:
| ● | Sleep product distributors that will distribute our product; | |
| ● | Home care dealers; | |
| ● | Private sleep labs; |
4
| ● | Internet providers; | |
| ● | Product end users; | |
| ● | Physicians, particularly sleep physicians; | |
| ● | Medical groups; | |
| ● | Hospitals; and | |
| ● | Medical associations, such as the American Academy of Sleep Medicine and the American Sleep Association. |
We expect that most of our revenues will be in the home care dealers and hospital target market.
Marketing
We plan to sell and market our DeltaWave device in the U.S. through the following activities:
| ● | Negotiate and secure agreements with industry distributor partners; | |
| ● | Secure agreements with Internet retailers for online sales; | |
| ● | Market DeltaWave at respiratory trade shows, social media, press releases; | |
| ● | Market and generate online sales through our website supplemented by search engine optimization; | |
| ● | Disseminate press releases to media outlets and publications that reach sleep medical practices and DME managers/distributors, including trade publications like Sleep Medicine, Sleep Review, Sleep, The Sleep Magazine; and | |
| ● | Attend sleep and healthcare, respiratory industry trade shows. |
Manufacturing
Our product will be manufactured by mold makers. We presently have our molds made in China; however, we are considering relocating the manufacturing of our molds to the United States.
Company Information
We were incorporated in the state of Nevada on June 6, 2007. On August 2, 2010, we changed our name from Bella Viaggio, Inc. to Kat Gold Holdings Corp. Effective January 1, 2015, we completed an exchange agreement to purchase 100% of the outstanding interests of REMSleep LLC in exchange for 50,000,000 shares of our common stock, at which time REMSleep LLC became our wholly-owned subsidiary and we adopted their business of developing and distributing sleep apnea products. On January 5, 2015, we changed our name to REMSleep Holdings, Inc. to reflect our new business model.
Our website is http://www.remsleep.com. No information from our website is incorporated herein. REMSleep Holding, Inc. is referred to herein as “we,” “us,” “our” and similar terms.
Penny Stock Rules
We are subject to the provisions of Section 15(g) and Rule 15g-9 of the Exchange Act, commonly referred to as the “penny stock rule.” Section 15(g) sets forth certain requirements for transactions in penny stock, and Rule 15g-9(d) incorporates the definition of “penny stock” that is found in Rule 3a51-1 of the Exchange Act. The SEC generally defines a penny stock to be any equity security that has a market price less than $5.00 per share, subject to certain exceptions. We will be subject to the SEC’s penny stock rules.
5
Since our common stock may be deemed to be a penny stock, trading in the shares of our common stock is subject to additional sales practice requirements on broker-dealers who sell penny stock to persons other than established customers and accredited investors. “Accredited investors” are persons with assets in excess of $1,000,000 (excluding the value of such person’s primary residence) or annual income exceeding $200,000 or $300,000 together with their spouse. For transactions covered by these rules, broker-dealers must make a special suitability determination for the purchase of such security and must have the purchaser’s written consent to the transaction prior to the purchase. Additionally, for any transaction involving a penny stock, unless exempt the rules require the delivery, prior to the first transaction of a risk disclosure document, prepared by the SEC, relating to the penny stock market. A broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information for the penny stocks held in an account and information to the limited market in penny stocks. Consequently, these rules may restrict the ability of broker-dealer to trade and/or maintain a market in our common stock and may affect the ability of the Company’s stockholders to sell their shares of common stock.
There can be no assurance that our shares of common stock will qualify for exemption from the Penny Stock Rule. In any event, even if our common stock was exempt from the Penny Stock Rule, we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the SEC the authority to restrict any person from participating in a distribution of penny stock if the SEC finds that such a restriction would be in the public interest.
In addition to the “penny stock” rules described above, FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low-priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low-priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
6
| Issuer: | REMSleep Holdings, Inc., a Nevada corporation | |
| Securities Offered: | Up to 50 million (50,000,000) shares of common stock, par value $0.001 per share (the “Shares”), on a “best effort offering” basis by the officers and directors of the Company. | |
| Offering Price: | $0.06 per Share | |
| Minimum Purchase: | $6,000 (100,000 Shares) although the Company reserves the right to accept subscriptions for lesser amounts. | |
| Capitalization: | Common Stock, par value $0.001 per share
● 1 billion (1,000,000,000) authorized, 73,139,1651 issued and outstanding as of the date of this Offering Circular.
Preferred Stock, par value $0.001 per share2
● Series A Preferred Stock, 5,000,000 shares authorized, 4,000,000 issued and outstanding as of June 30, 2019; ● Series B Preferred Stock, 5,000,000 shares authorized, none issued as of June 30, 2019; and ● Series C Preferred Stock, 5,000,000 shares authorized, none issued as of June 30, 2019. | |
| Number of Shares Outstanding Upon Completion of the Offering: | 123,139,165 shares of common stock, assuming all of the Shares being offered are sold3. | |
| Placement Agent: | None | |
| Trading Market: | OTC PINKS: RMSL | |
| Use of Proceeds: | If we sell all of the Shares being offered, our net proceeds (after our estimated Offering expenses of $75,000) will be $2,925,000. We intend use these net proceeds for acquisitions and working capital and other general corporate purposes. See “Use of Proceeds.” | |
| Dividends: | The Company has not declared or paid a cash dividend to stockholders since it was organized and does not intend to pay dividends in the foreseeable future. The Board of Directors presently intends to retain any earnings to finance our operations and does not expect to authorize cash dividends in the foreseeable future. Any payment of cash dividends in the future will depend upon the Company’s earnings, capital requirements and other factors. | |
| Risk Factors: | Investing in our common stock involves a high degree of risk, including, but not limited to:
● Speculative nature of our business; ● Competition; ● Our ability to continue as a going concern; ● Our need for more capital; ● Immediate and substantial dilution; and ● Limited market for our common stock.
Investors are advised to read and pay careful attention to the section “Risk Factors” starting on page 8 of this Offering Circular. |
1 Does not include (i) 4,000,000 shares of common stock issuable upon the conversion of 4,000,000 shares of Series A Preferred Stock issued and outstanding as of the date of this Offering Circular, (ii) 1,500,000 shares of common stock issuable upon the exercise of warrants issued in conjunction with convertible debt on May 30, 2019 and exercisable for 3 years at $0.045 per share, and (iii) any common stock issuable upon the conversion of outstanding convertible debt.
2 Each share of Series A Preferred Stock is convertible into one share of common stock. Each share of Series B Preferred Stock is convertible into 100 shares of common stock. Each share of Series C Preferred Stock is convertible into 50 shares of common stock.
3 Does not include the shares of common stock reflected in footnote 1 above.
7
An investment in our common stock involves a high degree of risk. You should carefully consider the following risk factors, together with the other information contained in this Offering Circular, before purchasing our common stock. Any of the following factors could harm our business, financial condition, results of operations or prospects, and could result in a partial or complete loss of your investment. Some statements in this Offering Circular, including statements in the following risk factors, constitute forward-looking statements. Please refer to the section entitled “Cautionary Statement Regarding Forward-Looking Statements.”
Risks Related to our Business
Going Concern
The Company has an accumulated deficit of $4,440,510 at June 30, 2019, had a net loss of $2,938,488 and net cash used in operating activities of $98,395 for the six months ended June 30, 2019. The Company’s ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. These conditions and the ability to successfully resolve these factors over the next twelve months raise substantial doubt about the Company’s ability to continue as a going concern.
If we are unable to raise enough capital in this offering or obtain additional financing, we may not be able to fulfill our business plan.
At June 30, 2019, we only had $181,763 cash on hand. Our entire business plan, including our ability to conduct manufacturing, marketing, generate sales and further develop products, are entirely dependent upon adequate financing. Should we fail to obtain adequate financing: (a) our financial condition will be negatively affected; (b) we will be unable to conduct the essential aspects of our business plan, including marketing; (c) investments in our common stock will be negatively impacted; (d) we will be forced to liquidate our business and file for bankruptcy protection.
We currently have only patent-pending protection for our DeltaWave device.
We have filed a patent application for our DeltaWave device with the USPTO; however, as yet, it has not granted yet and there can be no assurances that one will be issued.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for noncompliance with these requirements.
Periodic maintenance fees on any issued patent are due to be paid to the USPTO and foreign patent agencies in several stages over the lifetime of the patent and, in some jurisdictions, during the pendency of a patent application. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. While an inadvertent lapse can in many cases be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, our competitors might be able to enter the market, which would have a material adverse effect on our business.
8
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
Changes in either the patent laws or interpretation of the patent laws in the United States could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. Assuming that other requirements for patentability are met, prior to March 2013, in the United States, the first to invent the claimed invention was entitled to the patent, while outside the United States, the first to file a patent application was entitled to the patent. After March 2013, under the Leahy-Smith America Invents Act, or the America Invents Act, enacted in September 2011, the United States transitioned to a first inventor to file system in which, assuming that other requirements for patentability are met, the first inventor to file a patent application will be entitled to the patent on an invention regardless of whether a third party was the first to invent the claimed invention. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application.
The America Invents Act also includes a number of significant changes that affect the way patent applications will be prosecuted and also may affect patent litigation. These include allowing third party submission of prior art to the USPTO during patent prosecution and additional procedures to attack the validity of a patent by USPTO administered post-grant proceedings, including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. Therefore, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our owned or in-licensed patent applications and the enforcement or defense of our owned or in-licensed issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, the patent positions of companies in the development and commercialization of biologics and pharmaceuticals are particularly uncertain. Recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. This combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could have a material adverse effect on our existing patent portfolio and our ability to protect and enforce our intellectual property in the future.
If patients or physicians are not willing to change current practices to adopt our DeltaWave device to treat moderate to severe sleep apnea, our DeltaWave device may fail to gain increased market acceptance, and our business will be adversely affected.
Our primary strategy to generate and grow our revenue is to drive the adoption of our DeltaWave device to treat patients with moderate to severe sleep apnea who are unable to use or get consistent benefit from their current CPAP masks. Physicians and users may choose not to use our DeltaWave device for a number of reasons, including:
| ● | lack of availability of adequate third-party payor coverage or reimbursement; |
| ● | lack of experience with our products; |
| ● | our inability to convince key opinion leaders to provide recommendations regarding our DeltaWave device, or to convince physicians, patients and healthcare payors that our DeltaWave device is an attractive alternative to other treatment devices; |
| ● | perceived inadequacy of evidence supporting benefits of our DeltaWave device over existing alternatives; |
| ● | liability risks generally associated with the use of new products and procedures; and |
| ● | the training required to use new products. |
If additional physicians or other medical professionals do not adopt our DeltaWave device for any reason, including those listed above, our ability to execute our growth strategy will be impaired, and our business may be adversely affected.
In addition, patients may choose not to use our DeltaWave device if, among other potential reasons, their airway anatomy would not allow for effective treatment with DeltaWave device, they are worried about potential adverse effects of our DeltaWave device, such as infection, discomfort or they are unable to obtain adequate third-party coverage or reimbursement.
9
We currently compete and will in the future continue to compete against other companies, some of which have longer operating histories, more established products or greater resources than we do, which may prevent us from achieving increased market penetration and improved operating results.
The medical technology industry is highly competitive, subject to change and significantly affected by new product introductions and other activities of industry participants. Our competitors have historically dedicated and will continue to dedicate significant resources to promoting their products or developing new products or methods to treat moderate to severe sleep apnea. We also believe other emerging businesses are in the early stages of developing devices designed to treat sleep apnea. In addition, we also compete, both within and outside of the United States with invasive surgical treatment options which are primarily used in the treatment of mild to moderate sleep apnea.
In addition, our DeltaWave device is designed in the treatment of sleep apnea in patients who cannot use or obtain consistent benefit from current CPAP system devices. If one or more CPAP device manufacturers successfully develop a CPAP device that is more effective, better tolerated or otherwise results in better compliance by patients, or if improvements in other devices make them more effective, cost effective, easier to use or otherwise more attractive than our DeltaWave device, sales of our DeltaWave device could be significantly and adversely affected, which could have a material adverse effect on our business, financial condition and results of operations. In addition, if other companies are successful in developing devices that are approved for a broader range of indications than our DeltaWave device, we will be at a further competitive disadvantage, which could also affect our business, financial condition and results of operations.
Many of the companies against which we compete may have competitive advantages with respect to primary competitive factors in the sleep apnea treatment market, including:
| ● | greater company, product and brand recognition; |
| ● | superior product safety, reliability and durability; |
| ● | more effective marketing to and education of patients, physicians and sleep centers; |
| ● | greater product ease of use and patient comfort; |
| ● | more sales force experience and greater market access; |
| ● | better product support and service; |
| ● | more advanced technological innovation, product enhancements and speed of innovation; |
| ● | more effective pricing and revenue strategies; and |
| ● | more effective sales teams and strategies. |
We also compete with other medical technology companies to recruit and retain qualified sales, training and other personnel.
In addition, though there are currently no pharmacologic therapies approved to treat sleep apnea, we may in the future face competition from pharmaceutical companies that develop such therapies. We also expect to experience increased competition in the future as other companies develop and commercialize competing devices. Any of these companies may also have the competitive advantages described above.
Our long-term growth depends on our ability to enhance our DeltaWave device, expand our indications and develop and commercialize additional products.
It is important to our business that we continue to enhance our DeltaWave device and develop and introduce new products. Developing products is expensive and time-consuming and could divert management’s attention away from our core business. The success of any new product offering or product enhancements to our DeltaWave device will depend on several factors, including our ability to:
| ● | properly identify and anticipate physician and patient needs |
| ● | develop and introduce new products and product enhancements in a timely manner; |
| ● | avoid infringing upon the intellectual property rights of third-parties; |
| ● | demonstrate, if required, the safety and efficacy of new products with data from preclinical studies and clinical trials; |
| ● | obtain the necessary regulatory clearances or approvals for expanded indications, new products or product modifications; |
10
| ● | be fully FDA-compliant with marketing of new devices or modified products; |
| ● | provide adequate training to potential users of our products; |
| ● | receive adequate coverage and reimbursement for procedures performed with our products; and |
| ● | develop an effective and dedicated sales and marketing team. |
If we are not successful in expanding our indications and developing and commercializing new products and product enhancements, our ability to increase our revenue may be impaired, which could have a material adverse effect on our business, financial condition and results of operations.
Our financial results may fluctuate significantly and may not fully reflect the underlying performance of our business.
Our quarterly and annual results of operations may vary significantly in the future, and period-to-period comparisons of our operating results may not be meaningful. Accordingly, the results of any one quarter or period should not be relied upon as an indication of future performance. Our quarterly and annual financial results may fluctuate as a result of a variety of factors, many of which are outside our control and, as a result, may not fully reflect the underlying performance of our business. One such factor includes seasonal variations in our sales.
Other factors that may cause fluctuations in our quarterly and annual results include:
| ● | patient and physician adoption of our DeltaWave device; |
| ● | changes in coverage policies by third-party payors that affect the reimbursement of procedures using our products; |
| ● | timing of new product offerings, acquisitions, licenses or other significant events by us or our competitors; |
| ● | unanticipated pricing pressure; |
| ● | the hiring, retention and continued productivity of our sales representatives; |
| ● | our ability to expand the geographic reach of our sales and marketing efforts; |
| ● | our ability to obtain regulatory clearance or approval for any products in development or for our current products for additional indications or in additional countries outside the United States; |
| ● | results of clinical research and trials on our existing products and products in development; |
| ● | delays in receipt of anticipated purchase orders; |
| ● | delays in, or failure of, component and raw material deliveries by our suppliers; and |
| ● | positive or negative coverage in the media or clinical publications of our products or products of our competitors or our industry. |
Because our quarterly and annual results may fluctuate, period-to-period comparisons may not be the best indication of the underlying results of our business and should only be relied upon as one factor in determining how our business is performing. These fluctuations may also increase the likelihood that we will not meet our forecasted performance, which could negatively affect the market price for our common stock.
Our results of operations could be materially harmed if we are unable to accurately forecast customer demand for our DeltaWave device and manage our inventory.
To ensure adequate inventory supply, we must forecast inventory needs and place orders with our suppliers based on our estimates of future demand for our DeltaWave device. Our ability to accurately forecast demand for our DeltaWave device could be negatively affected by many factors, including our failure to accurately manage our expansion strategy, product introductions by competitors, an increase or decrease in customer demand for our DeltaWave device or for products of our competitors, our failure to accurately forecast customer acceptance of new products, unanticipated changes in general market conditions or regulatory matters and weakening of economic conditions or consumer confidence in future economic conditions. Inventory levels in excess of customer demand may result in inventory write-downs or write-offs, which would cause our gross margin to be adversely affected and could impair the strength of our brand. Conversely, if we underestimate customer demand for our DeltaWave device, our third-party contract manufacturers may not be able to deliver products to meet our requirements, and this could result in damage to our reputation and customer relationships. In addition, if we experience a significant increase in demand, additional supplies of raw materials or additional manufacturing capacity may not be available when required on terms that are acceptable to us, or at all, or suppliers or our third-party manufacturers may not be able to allocate sufficient capacity in order to meet our increased requirements, which could have an adverse effect on our ability to meet customer demand for our DeltaWave device and our results of operations.
11
We seek to maintain sufficient levels of inventory in order to protect ourselves from supply interruptions. As a result, we are subject to the risk that a portion of our inventory will become obsolete or expire, which could have a material adverse effect on our earnings and cash flows due to the resulting costs associated with the inventory impairment charges and costs required to replace such inventory.
We will rely on a limited number of third-party suppliers and contract manufacturers for the manufacture and assembly of our products, and a loss or degradation in performance of these suppliers and contract manufacturers could have a material adverse effect on our business, financial condition and results of operations.
We will rely on third-party suppliers and contract manufacturers for the raw materials and components used in our DeltaWave device and to manufacture and assemble our products. Any of our suppliers or our third-party contract manufacturers may be unwilling or unable to supply the necessary materials and components or manufacture and assemble our products reliably and at the levels we anticipate or that are required by the market. Our ability to supply our products commercially and to develop any future products depends, in part, on our ability to obtain these materials, components and products in accordance with regulatory requirements and in sufficient quantities for commercialization and clinical testing. Our manufacturers may decide in the future to discontinue or reduce the level of business they conduct with us. If we are required to change contract manufacturers due to any change in or termination of our relationships with these third parties, or if our manufacturers are unable to obtain the materials they need to produce our products at consistent prices or at all, we may lose sales, experience manufacturing or other delays, incur increased costs or otherwise experience impairment to our customer relationships. We cannot guarantee that we will be able to establish alternative relationships on similar terms, without delay or at all.
Establishing additional or replacement suppliers for any of these materials, components or services, if required, could be time-consuming and expensive, may result in interruptions in our operations and product delivery, may affect the performance specifications of our DeltaWave device or could require that we modify its design. Even if we are able to find replacement suppliers or third-party contract manufacturers, we will be required to verify that the new supplier or third-party manufacturer maintains facilities, procedures and operations that comply with our quality expectations and applicable regulatory requirements. Furthermore, our contract manufacturers could require us to move to another one of their production facilities or use alternative materials or components. Any of these events could require that we obtain a new regulatory authority approval before we implement the change, which could result in further delay and which may not be obtained at all. While we seek to maintain sufficient levels of inventory as discussed above, those inventories may not fully protect us from supply interruptions.
If our third-party suppliers fail to deliver the required commercial quantities of materials on a timely basis and at commercially reasonable prices, and we are unable to find one or more replacement suppliers capable of production at a substantially equivalent cost in substantially equivalent volumes and quality on a timely basis, the continued commercialization of our DeltaWave device, the supply of our products to customers and the development of any future products will be delayed, limited or prevented, which could have material adverse effect on our business, financial condition and results of operations.
Performance issues, service interruptions or price increases by our shipping carriers could adversely affect our business and harm our reputation and ability to provide our services on a timely basis.
Expedited, reliable shipping will be essential to our operations. We will rely heavily on providers of transport services for reliable and secure point-to-point transport of our DeltaWave device to our customers and for tracking of these shipments. Should a carrier encounter delivery performance issues such as loss, damage or destruction of any systems, it would be costly to replace such systems in a timely manner and such occurrences may damage our reputation and lead to decreased demand for our DeltaWave device and increased cost and expense to our business. In addition, any significant increase in shipping rates could adversely affect our operating margins and results of operations. Similarly, strikes, severe weather, natural disasters or other service interruptions affecting delivery services we use would adversely affect our ability to process orders for our DeltaWave device on a timely basis.
12
Consolidation in the healthcare industry or group purchasing organizations could lead to demands for price concessions, which may affect our ability to sell our products at prices necessary to support our current business strategies.
Healthcare costs have risen significantly over the past decade, which has resulted in or led to numerous cost reform initiatives by legislators, regulators and third-party payors. Cost reform has triggered a consolidation trend in the healthcare industry to aggregate purchasing power, which may create more requests for pricing concessions in the future. Additionally, group purchasing organizations, independent delivery networks and large single accounts may continue to use their market power to consolidate purchasing decisions for hospitals and ASCs. We expect that market demand, government regulation, third-party coverage and reimbursement policies and societal pressures will continue to change the healthcare industry worldwide, resulting in further business consolidations and alliances among our customers, which may exert further downward pressure on the prices of our products.
We have limited experience marketing and selling our DeltaWave device, and if we are unable to expand, manage and maintain our direct sales and marketing organization we may not be able to generate revenue growth.
We have not commenced sales of our DeltaWave device and as a result, we have no experience in marketing and selling our DeltaWave device. We intend to sell our DeltaWave device through a direct sales force that targets ENT physicians and sleep centers in the United States and Europe, and also utilize various direct-to-patient marketing initiatives, including paid search, radio, social media and online videos. Our operating results will be directly dependent upon the efforts of these employees. If our direct sales force fails to adequately promote, market and sell our DeltaWave device and obtain reimbursement for it, our revenue may be adversely affected.
In order to generate revenue, we plan to engage a direct sales organization. This may require us to split or adjust sales territories, which may adversely affect our ability to retain customers in those territories. Additionally, our future success will depend largely on our ability to continue to hire, train, retain and motivate skilled sales and reimbursement personnel with significant industry experience and technical knowledge of sleep apnea devices and related products. Because the competition for their services is high, we cannot assure you we will be able to hire and retain additional personnel on favorable or commercially reasonable terms, if at all. Failure to hire or retain qualified sales and reimbursement personnel would prevent us from expanding our business and generating revenue. If we are unable to expand our sales and marketing capabilities, we may not be able to effectively commercialize our DeltaWave device, which could have an adverse effect on our business, financial condition and results of operations.
Our ability to maintain our competitive position depends on our ability to attract and retain senior management and other highly qualified personnel.
Our success will depend in part on our ability to attract, retain and motivate highly qualified management and other personnel. We are highly dependent upon our management team, Thomas J. Wood, our Chief Executive Officer, and Russell F. Bird, our President. The replacement of any of our management team likely would involve significant time and costs and may significantly delay or prevent the achievement of our business objectives and could therefore have an adverse effect on our business. In addition, we do not carry any “key person” insurance policies that could offset potential loss of service under applicable circumstances.
We face the risk of product liability claims that could be expensive, divert management’s attention and harm our reputation and business. We may not be able to maintain adequate product liability insurance.
Our business exposes us to the risk of product liability claims that are inherent in the testing, manufacturing and marketing of medical devices. This risk exists even if a device is cleared or approved for commercial sale by the FDA and manufactured in facilities licensed and regulated by the FDA or an applicable foreign regulatory authority. Our DeltaWave device is designed to affect, and any future products will be designed to affect, important bodily functions and processes. Any side effects, manufacturing defects, misuse or abuse associated with our DeltaWave device could result in patient injury or death. The medical device industry has historically been subject to extensive litigation over product liability claims, and we cannot offer any assurance that we will not face product liability suits. We may be subject to product liability claims if our DeltaWave device causes, or merely appears to have caused, patient injury or death. In addition, an injury that is caused by the activities of our suppliers, such as those who provide us with components and raw materials, may be the basis for a claim against us. Product liability claims may be brought against us by patients, healthcare providers or others selling or otherwise coming into contact with our DeltaWave device, among others. If we cannot successfully defend ourselves against product liability claims, we will incur substantial liabilities and reputational harm. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| ● | costs of litigation; |
| ● | distraction of management’s attention from our primary business; |
13
| ● | the inability to commercialize our DeltaWave device or new products; |
| ● | decreased demand for our DeltaWave device; |
| ● | damage to our business reputation; |
| ● | product recalls or withdrawals from the market; |
| ● | withdrawal of clinical trial participants; |
| ● | substantial monetary awards to patients or other claimants; or |
| ● | loss of sales. |
While we may attempt to manage our product liability exposure by proactively recalling or withdrawing from the market any defective products, any recall or market withdrawal of our products may delay the supply of those products to our customers and may impact our reputation. We can provide no assurance that we would be successful in initiating appropriate market recall or market withdrawal efforts that may be required in the future or that these efforts will have the intended effect of preventing product malfunctions and the accompanying product liability that may result. Such recalls and withdrawals may also be used by our competitors to harm our reputation for safety or be perceived by patients as a safety risk when considering the use of our products, either of which could have a material adverse effect on our business, financial condition and results of operations.
If we are unable to obtain insurance at an acceptable cost or on acceptable terms or otherwise protect against potential product liability claims, we could be exposed to significant liabilities. A product liability claim, recall or other claim with respect to uninsured liabilities or for amounts in excess of insured liabilities could have a material adverse effect on our business, financial condition and results of operations.
If the quality of our DeltaWave device does not meet the expectations of physicians or patients, then our brand and reputation or our business could be adversely affected.
In the course of conducting our business, we must adequately address quality issues that may arise with our DeltaWave device, including defects in third-party components included in our DeltaWave device. In addition, even in the absence of quality issues, we may be subject to claims and liability if the performance of our DeltaWave device does not live up to the expectations of physicians or patients as a result of the patient’s use of the product. If the quality of our DeltaWave device does not meet the expectations of physicians or patients, then our brand and reputation with those physicians or patients, or our business, financial condition and results of operations, could be adversely affected.
If we choose to acquire new and complementary businesses, products or technologies, we may be unable to complete these acquisitions or to successfully integrate them in a cost-effective and non-disruptive manner.
Our success depends, in part, on our ability to continually enhance and broaden our product offerings in response to changing customer demands, competitive pressures and advances in technologies. Accordingly, although we have no current commitments with respect to any acquisition or investment, we may in the future pursue the acquisition of, or joint ventures relating to, complementary businesses, products or technologies instead of developing them ourselves. We do not know if we will be able to successfully complete any future acquisitions or joint ventures, or whether we will be able to successfully integrate any acquired business, product or technology or retain any key employees related thereto. Integrating any business, product or technology we acquire could be expensive and time-consuming, disrupt our ongoing business and distract our management. If we are unable to integrate any acquired businesses, products or technologies effectively, our business will be adversely affected. In addition, any amortization or charges resulting from the costs of acquisitions could increase our expenses.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. The global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the global financial crisis, could result in a variety of risks to our business, including weakened demand for our DeltaWave device, and our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also strain our manufacturers or suppliers, possibly resulting in supply disruption, or cause our customers to delay making payments for our services. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the economic climate and financial market conditions could adversely affect our business.
14
Failure of a key information technology system, process or site could have an adverse effect on our business.
We will rely extensively on information technology systems to conduct our business. These systems affect, among other things, ordering and managing materials from suppliers, shipping products to customers, processing transactions, summarizing and reporting results of operations, complying with regulatory, legal or tax requirements, data security and other processes necessary to manage our business. If our systems are damaged or cease to function properly due to any number of causes, ranging from catastrophic events to power outages to security breaches, and our business continuity plans do not effectively compensate on a timely basis, we may experience interruptions in our operations, which could have an adverse effect on our business.
In addition, we accept payments for many of our sales through credit and debit card transactions, which are handled through a third-party payment processor. As a result, we are subject to a number of risks related to credit and debit card payments. As a result of these transactions, we pay interchange and other fees, which may increase over time and could require us to either increase the prices we charge for our DeltaWave device or experience an increase in our costs and expenses. In addition, as part of the payment processing process, we transmit our customers’ credit and debit card information to our third-party payment processor. We may in the future become subject to lawsuits or other proceedings for purportedly fraudulent transactions arising out of the actual or alleged theft of our customers’ credit or debit card information if the security of our third-party credit card payment processor is breached. We and our third-party credit card payment processor are also subject to payment card association operating rules, certification requirements and rules governing electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. If we or our third-party credit card payment processor fail to comply with these rules or requirements, we may be subject to fines and higher transaction fees and lose our ability to accept credit and debit card payments from our customers, and there may be an adverse effect on our business.
We are subject to anti-bribery, anti-corruption, and anti-money laundering laws, including the U.S. Foreign Corrupt Practices Act, as well as export control laws, customs laws, sanctions laws and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures and legal expenses, which could adversely affect our business, results of operations and financial condition.
As we grow our international presence and global operations, we will have increasing obligations to comply with trade and economic sanctions and other restrictions imposed by the United States, the European Union and other governments and organizations. The U.S. Departments of Justice, Commerce, State and Treasury and other federal agencies and authorities have a broad range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of economic sanctions laws, export control laws, the U.S. Foreign Corrupt Practices Act, or the FCPA, and other federal statutes and regulations, including those established by the Office of Foreign Assets Control, or OFAC. In addition, the U.K. Bribery Act of 2010, or the Bribery Act, prohibits both domestic and international bribery, as well as bribery across both private and public sectors. An organization that “fails to prevent bribery” by anyone associated with the organization can be charged under the Bribery Act unless the organization can establish the defense of having implemented “adequate procedures” to prevent bribery. Under these laws and regulations, as well as other anti-corruption laws, anti-money laundering laws, export control laws, customs laws, sanctions laws and other laws governing our operations, various government agencies may require export licenses, may seek to impose modifications to business practices, including cessation of business activities in sanctioned countries or with sanctioned persons or entities and modifications to compliance programs, which may increase compliance costs, and may subject us to fines, penalties and other sanctions. A violation of these laws or regulations would negatively affect our business, financial condition and results of operations.
We bear the risk of warranty claims on our DeltaWave device.
We bear the risk of warranty claims on our DeltaWave device. We may not be successful in claiming recovery under any warranty or indemnity provided to us by our suppliers or vendors in the event of a successful warranty claim against us by a customer or that any recovery from such vendor or supplier would be adequate. In addition, warranty claims brought by our customers related to third-party components may arise after our ability to bring corresponding warranty claims against such suppliers expires, which could result in costs to us.
15
We may need substantial additional funding beyond our existing cash resources and the proceeds from this offering and may be unable to raise capital when needed, which could force us to delay or reduce our commercialization efforts or product development programs.
We believe that the net proceeds from this offering will be sufficient to meet our capital requirements and fund our operations for at least 12 months. However, we have based these estimates on assumptions that may prove to be incorrect, and we could spend our available financial resources much faster than we currently expect. Any future funding requirements will depend on many factors, including:
| ● | patient, physician and market acceptance of our DeltaWave device; |
| ● | the cost of filing and prosecuting patent applications and defending and enforcing our patent or other intellectual property rights; |
| ● | the cost of defending, in litigation or otherwise, any claims that we infringe third-party patents or other intellectual property rights; |
| ● | the cost and timing of additional regulatory clearances or approvals; |
| ● | the cost and timing of establishing additional sales and marketing capabilities; |
| ● | costs associated with any product recall that may occur; |
| ● | the effect of competing technological and market developments; |
| ● | the extent to which we acquire or invest in products, technologies and businesses, although we currently have no commitments or agreements relating to any of these types of transactions; and |
| ● | the costs of operating as a public company. |
Any additional equity or debt financing that we raise may contain terms that are not favorable to us or our stockholders. If we raise additional funds by selling additional shares of our common stock or other securities convertible into or exercisable or exchangeable for shares of our common stock after this offering (including through the exercise by the underwriters of their option to purchase additional shares of our common stock), the issuance of such securities will result in dilution to our stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible into or exercisable or exchangeable for shares of our common stock, in future transactions may be higher or lower than the price per share paid by investors in this offering. Furthermore, investors purchasing any securities we may issue in the future may have rights superior to your rights as a holder of our common stock.
In addition, any future debt financing into which we enter may impose upon us covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our common stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. If we raise additional funds through collaboration and licensing arrangements with third-parties, it may be necessary to relinquish some rights to our technologies or our products, or grant licenses on terms that are not favorable to us.
Furthermore, we cannot be certain that additional funding will be available on acceptable terms, if at all. If we do not have, or are not able to obtain, sufficient funds, we may have to delay development or commercialization of our products or license to third-parties the rights to commercialize products or technologies that we would otherwise seek to commercialize. We also may have to reduce marketing, customer support or other resources devoted to our products or cease operations. Any of these factors could harm our business, financial condition and results of operations.
If we fail to successfully manage our new product development or if we fail to anticipate the issues associated with such development or expansion, our business may suffer.
We have one patent pending for DeltaWave. Our ability to anticipate and manage a variety of issues associated with any new product development or market expansion, such as:
| ● | market acceptance; |
| ● | effective management of our applications and other products |
Our business would suffer if we fail to successfully anticipate and manage these issues associated with product development publishing and you may lose all or part of your investment.
16
If we cannot attract customers, we will not generate revenues and our business will fail.
As of the date of this Offering Circular, we have not generated any revenue. If our business fails, you will lose all or part of your investment.
We may encounter difficulties managing our planned growth, which would adversely affect our business and could result in increasing costs as well as a decrease in our stock price.
We intend to establish a customer base and develop new products for them. To manage our anticipated growth, we must continue to improve our operational and financial systems and expand, train, retain and manage our employee base to meet new opportunities. Because of the registration of our securities, we are subject to reporting and disclosure obligations, and we anticipate that we will hire additional finance and administrative personnel to address these obligations. In addition, the anticipated growth of our business will place a significant strain on our existing managerial and financial resources. If we cannot effectively manage our growth, our business may be harmed.
Because we do not have an audit committee, shareholders will have to rely on the directors, who are not independent, to perform these functions.
We do not have an audit or compensation committee comprised of independent directors. These functions are performed by the board of directors as a whole. The members of the Board of Directors are not independent directors. Thus, there is a potential conflict in that the board members are also engaged in management and participate in decisions concerning management compensation and audit issues that may affect management performance.
Failure to protect our proprietary technology and intellectual property rights could substantially harm our business and results of operations.
Our success depends to a significant degree on our ability to protect our proprietary technology, methodologies, know-how and our brand. We will rely on a combination of contractual restrictions, and other intellectual property laws and confidentiality procedures to establish and protect our proprietary rights. However, the steps we will take to protect our intellectual property may be inadequate. We will not be able to protect our intellectual property if we are unable to enforce our rights or if we do not detect unauthorized use of our intellectual property. If we fail to protect our intellectual property rights adequately, our competitors may gain access to our technology and our business may be harmed. In addition, defending our intellectual property rights might entail significant expense. We may be unable to prevent third parties from acquiring domain names or trademarks that are similar to, infringe upon, or diminish the value of our trademarks and other proprietary rights.
As we grow our business, our plan is to enter into confidentiality and invention assignment agreements with our employees and consultants and enter into confidentiality agreements with other parties. No assurance can be given that these agreements will be effective in controlling access to and distribution of our proprietary information. Further, these agreements may not prevent our competitors from independently developing technologies that are substantially equivalent or superior to our products.
In order to protect our intellectual property rights, we may be required to spend significant resources to monitor and protect our intellectual property rights. Litigation may be necessary in the future to enforce our intellectual property rights and to protect our trade secrets. Litigation brought to protect and enforce our intellectual property rights could be costly, time-consuming, and distracting to management, and could result in the impairment or loss of portions of our intellectual property. Further, our efforts to enforce our intellectual property rights may be met with defenses, counterclaims, and countersuits attacking the validity and enforceability of our intellectual property rights. Our inability to protect our proprietary technology against unauthorized copying or use, as well as any costly litigation or diversion of our management’s attention and resources, could delay further sales or the implementation of our products, impair the functionality of our products, delay introductions of new products, result in our substituting inferior or more costly technologies into our products, or injure our reputation.
17
We could incur substantial costs as a result of any claim of infringement of another party’s intellectual property rights.
In recent years, there has been significant litigation involving patents and other intellectual property rights in the software industry. Companies providing software are increasingly bringing and becoming subject to suits alleging infringement of proprietary rights, particularly patent rights, we face a higher risk of being the subject of intellectual property infringement claims. We do not currently have a patent portfolio, which could prevent us from deterring patent infringement claims through our own patent portfolio, and our competitors and others may now and in the future have significantly larger and more mature patent portfolios than we have. The risk of patent litigation has been amplified by the increase in the number of a type of patent holder, which we refer to as a non-practicing entity, whose sole business is to assert such claims and against whom our own intellectual property portfolio may provide little deterrent value. We could incur substantial costs in prosecuting or defending any intellectual property litigation. If we sue to enforce our rights or are sued by a third party that claims that our solution infringes its rights, the litigation could be expensive and could divert our management resources. As of the date of this offering circular, we have not received any written notice of an infringement claim, invitation to license, or other intellectual property infringement action.
Any intellectual property litigation to which we might become a party, or for which we are required to provide indemnification, may require us to do one or more of the following:
| ● | Cease selling or using products that incorporate the intellectual property that we allegedly infringe; | |
| ● | Make substantial payments for legal fees, settlement payments or other costs or damages; | |
| ● | Obtain a license, which may not be available on reasonable terms or at all, to sell or use the relevant technology; or | |
| ● | Redesign the allegedly infringing products to avoid infringement, which could be costly, time-consuming or impossible. |
If we are required to make substantial payments or undertake any of the other actions noted above as a result of any intellectual property infringement claims against us or any obligation to indemnify our customers for such claims, such payments or actions could harm our business.
Our Articles of Incorporation and Bylaws and certain provisions of Nevada law make it more difficult for a third party to acquire us and make a takeover more difficult to complete, even if such a transaction were in the stockholders’ interest.
Our Articles of Incorporation and Bylaws and certain provisions of Nevada State law could have the effect of making it more difficult or more expensive for a third party to acquire, or from discouraging a third party from attempting to acquire, control of the Company, even when these attempts may be in the best interests of our stockholders. For example, we are governed by Section 203 of the Nevada General Corporation Law. In general, Section 203 prohibits a public Nevada corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes mergers, asset sales or other transactions resulting in a financial benefit to the stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years, did own, fifteen percent (15%) or more of the corporation’s outstanding voting stock. These provisions may have the effect of delaying, deferring or preventing a change in our control.
Limitations of director liability and indemnification of directors, officers and employees.
Our Articles of Incorporation limits the liability of directors to the maximum extent permitted by Nevada law. Nevada law provides that directors of a corporation will not be personally liable for monetary damages for breach of their fiduciary duties as directors, except for liability for any:
| ● | breach of their duty of loyalty to us or our stockholders; | |
| ● | act or omission not in good faith or that involves intentional misconduct or a knowing violation of law; | |
| ● | unlawful payments of dividends or unlawful stock repurchases or redemptions as provided n Section 174 of the Nevada General Corporation Law; or | |
| ● | transactions for which the directors derived an improper personal benefit. |
18
These limitations of liability do not apply to liabilities arising under the federal or state securities laws and do not affect the availability of equitable remedies such as injunctive relief or rescission. Our corporate bylaws (“Bylaws”) provide that we will indemnify our directors, officers and employees to the fullest extent permitted by law. Our Bylaws also provide that we are obligated to advance expenses incurred by a director or officer in advance of the final disposition of any action or proceeding. We believe that these Bylaw provisions are necessary to attract and retain qualified persons as directors and officers. The limitation of liability in our Articles of Incorporation and Bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might provide a benefit to us and our stockholders. Our results of operations and financial condition may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions.
Risks Related to Government Regulation
Our products are subject to extensive government regulation and oversight both in the United States and abroad, and our failure to comply with applicable requirements could harm our business.
We and our products are subject to extensive regulation in the United States and elsewhere, including by the FDA and its foreign counterparts. The FDA and foreign regulatory agencies regulate, among other things, with respect to medical devices: design, development and manufacturing; testing, labeling, content and language of instructions for use and storage; clinical trials; product safety; establishment registration and device listing; marketing, sales and distribution; pre-market clearance and approval; record keeping procedures; advertising and promotion; recalls and field safety corrective actions; post-market surveillance, including reporting of deaths or serious injuries and malfunctions that, if they were to recur, could lead to death or serious injury; post-market approval studies; and product import and export.
The regulations to which we are subject are complex and have tended to become more stringent over time. Regulatory changes could result in restrictions on our ability to carry on or expand our operations, higher than anticipated costs or lower than anticipated sales. The FDA enforces these regulatory requirements through periodic unannounced inspections. We do not know whether we will pass any future FDA inspections. Failure to comply with applicable regulations could jeopardize our ability to sell our products and result in enforcement actions such as: warning letters; fines; injunctions; civil penalties; termination of distribution; recalls or seizures of products; delays in the introduction of products into the market; total or partial suspension of production; refusal to grant future clearances or approvals; withdrawals or suspensions of current approvals, resulting in prohibitions on sales of our products; and in the most serious cases, criminal penalties.
We may not receive the necessary clearances or approvals for our future products or expanded indications, and failure to timely obtain necessary clearances or approvals for our future products or expanded indications would adversely affect our ability to grow our business.
An element of our strategy is to continue to upgrade our products, add new features and expand the indications and uses for our current products. In the United States, before we can market a new medical device, or a new use of, new claim for or significant modification to an existing product, we must first receive either clearance under Section 510(k) of the Federal Food, Drug, and Cosmetic Act, or the FDCA, or approval of a pre-market approval application, or PMA, from the FDA, unless an exemption applies. In the 510(k) clearance process, before a device may be marketed, the FDA must determine that a proposed device is “substantially equivalent” to a legally-marketed “predicate” device, which includes a device that has been previously cleared through the 510(k) process, a device that was legally marketed prior to May 28, 1976 (pre-amendments device), a device that was originally on the U.S. market pursuant to an approved PMA and later down-classified, or a 510(k)-exempt device. To be “substantially equivalent,” the proposed device must have the same intended use as the predicate device, and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data are sometimes required to support substantial equivalence. In the process of obtaining PMA approval, which was required for our DeltaWave device, the FDA must determine that a proposed device is safe and effective for its intended use based, in part, on extensive data, including, but not limited to, technical, pre-clinical, clinical trial, manufacturing and labeling data. The PMA process is typically required for devices that are deemed to pose the greatest risk, such as life-sustaining, life-supporting or implantable devices.
19
Modifications to products that are approved through a PMA application generally require FDA approval. Similarly, certain modifications made to products cleared through a 510(k) may require a new 510(k) clearance. Both the PMA approval and the 510(k) clearance process can be expensive, lengthy and uncertain. The FDA’s 510(k) clearance process usually takes from three to 12 months, but can last longer. The process of obtaining a PMA is much more costly and uncertain than the 510(k) clearance process and generally takes from one to three years, or even longer, from the time the application is filed with the FDA. In addition, a PMA generally requires the performance of one or more clinical trials. Despite the time, effort and cost, a device may not be approved or cleared by the FDA. Any delay or failure to obtain necessary regulatory approvals could harm our business. Furthermore, even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses for the device, which may limit the market for the device.
In the United States, we have obtained approval of our DeltaWave device through the PMA pathway. Any modification to the DeltaWave device that has not been previously approved may require us to submit a new PMA or PMA supplement and obtain FDA approval prior to implementing the change. If the FDA requires us to go through a lengthier, more rigorous examination for future products or modifications to existing products than we had expected, product introductions or modifications could be delayed or canceled, which could adversely affect our ability to grow our business.
The FDA can delay, limit or deny clearance or approval of a device for many reasons, including:
| ● | our inability to demonstrate to the satisfaction of the FDA or the applicable regulatory entity or notified body that our products are safe or effective for their intended uses; |
| ● | the disagreement of the FDA or the applicable foreign regulatory body with the design or implementation of our clinical trials or the interpretation of data from pre-clinical studies or clinical trials; |
| ● | serious and unexpected adverse device effects experienced by participants in our clinical trials; |
| ● | the data from our pre-clinical studies and clinical trials may be insufficient to support clearance or approval, where required; |
| ● | our inability to demonstrate that the clinical and other benefits of the device outweigh the risks; |
| ● | the manufacturing process or facilities we use may not meet applicable requirements; and |
| ● | the potential for approval policies or regulations of the FDA or applicable foreign regulatory bodies to change significantly in a manner rendering our clinical data or regulatory filings insufficient for clearance or approval. |
In addition, the FDA may change its clearance and approval policies, adopt additional regulations or revise existing regulations, or take other actions, which may prevent or delay approval or clearance of our future products under development or impact our ability to modify our currently cleared products on a timely basis. Such policy or regulatory changes could impose additional requirements upon us that could delay our ability to obtain new approvals, increase the costs of compliance or restrict our ability to maintain our current approval. For example, as part of the Food and Drug Administration Safety and Innovation Act, or FDASIA, enacted in 2012, Congress reauthorized the Medical Device User Fee Amendments with various FDA performance goal commitments and enacted several “Medical Device Regulatory Improvements” and miscellaneous reforms, which are further intended to clarify and improve medical device regulation both pre- and post-clearance and approval. Some of these proposals and reforms could impose additional regulatory requirements upon us that could delay our ability to obtain new approvals, increase the costs of compliance or restrict our ability to maintain our current approval.
In order to sell our products in member countries of the EEA our products must comply with the essential requirements of the European Union Medical Devices Directive (Council Directive 93/42/EEC) and the Active Implantable Medical Devices Directive (Council Directive 90/385/EEC). Compliance with these requirements is a prerequisite to be able to affix the Conformité Européene, or CE, mark to our products, without which they cannot be sold or marketed in the EEA. To demonstrate compliance with the essential requirements we must undergo a conformity assessment procedure, which varies according to the type of medical device and its classification. Except for low-risk medical devices (Class I non-sterile, non-measuring devices), where the manufacturer can issue an EC Declaration of Conformity based on a self-assessment of the conformity of its products with the essential requirements of the European Union Medical Devices Directive, a conformity assessment procedure requires the intervention of an organization accredited by a member state of the EEA to conduct conformity assessments, or a Notified Body. Depending on the relevant conformity assessment procedure, the Notified Body would typically audit and examine the technical file and the quality system for the manufacture, design and final inspection of our devices. The Notified Body issues a certificate of conformity following successful completion of a conformity assessment procedure conducted in relation to the medical device and its manufacturer and their conformity with the essential requirements. This certificate entitles the manufacturer to affix the CE mark to its medical devices after having prepared and signed a related EC Declaration of Conformity.
20
As a general rule, demonstration of conformity of medical devices and their manufacturers with the essential requirements must be based, among other things, on the evaluation of clinical data supporting the safety and performance of the products during normal conditions of use. Specifically, a manufacturer must demonstrate that the device achieves its intended performance during normal conditions of use, that the known and foreseeable risks, and any adverse events, are minimized and acceptable when weighed against the benefits of its intended performance, and that any claims made about the performance and safety of the device are supported by suitable evidence. If we fail to remain in compliance with applicable European laws and directives, we would be unable to continue to affix the CE mark to our products, which would prevent us from selling them within the EEA.
Modifications to our products may require us to obtain new PMA approvals or approvals of a PMA supplement, and if we market modified products without obtaining necessary approvals, we may be required to cease marketing or recall the modified products until required approvals are obtained.
Certain modifications to a PMA-approved device may require approval of a new PMA or a PMA supplement, or alternatively a notification or other submission to the FDA. The FDA may not agree with our decisions regarding whether a new PMA or PMA supplement is necessary. We may make modifications to our approved devices in the future that we believe do not require approval of a new PMA or PMA supplement. If the FDA disagrees with our determination and requires us to submit a new PMA or PMA supplement for modifications to our previously approved products, we may be required to cease marketing or to recall the modified product until we obtain approval, and we may be subject to significant regulatory fines or penalties. In addition, the FDA may not approve our products for the indications that are necessary or desirable for successful commercialization or could require clinical trials to support any modifications. Any delay or failure in obtaining required approvals would adversely affect our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth.
Failure to comply with post-marketing regulatory requirements could subject us to enforcement actions, including substantial penalties, and might require us to recall or withdraw a product from the market.
Even though we have obtained approval for the DeltaWave device, we are subject to ongoing and pervasive regulatory requirements governing, among other things, the manufacture, marketing, advertising, medical device reporting, sale, promotion, registration, and listing of devices. For example, we must submit periodic reports to the FDA as a condition of PMA approval. These reports include safety and effectiveness information about the device after its approval. Failure to submit such reports, or failure to submit the reports in a timely manner, could result in enforcement action by the FDA. Following its review of the periodic reports, the FDA might ask for additional information or initiate further investigation.
In addition, the PMA approval for our DeltaWave device was subject to several conditions of approval, including a post-market long-term study and extended follow-up of the pre-market study cohort. Though we believe we have complied with these conditions to date, any failure to comply with the conditions of approval could result in the withdrawal of PMA approval and the inability to continue to market the device. Failure to conduct the required studies in accordance with institutional review board, or IRB, and informed consent requirements, or adverse findings in these studies, could also be grounds for withdrawal of approval of the PMA.
The regulations to which we are subject are complex and have become more stringent over time. Regulatory changes could result in restrictions on our ability to continue or expand our operations, higher than anticipated costs, or lower than anticipated sales. Even after we have obtained the proper regulatory approval to market a device, we have ongoing responsibilities under FDA regulations and applicable foreign laws and regulations. The FDA, state and foreign regulatory authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by the FDA, state or foreign regulatory authorities, which may include any of the following sanctions:
| ● | untitled letters or warning letters; |
21
| ● | fines, injunctions, consent decrees and civil penalties; |
| ● | recalls, termination of distribution, administrative detention, or seizure of our products; |
| ● | customer notifications or repair, replacement or refunds; |
| ● | operating restrictions or partial suspension or total shutdown of production; |
| ● | delays in or refusal to grant our requests for future PMA approvals or foreign regulatory approvals of new products, new intended uses, or modifications to existing products; |
| ● | withdrawals or suspensions of our current PMA or foreign regulatory approvals, resulting in prohibitions on sales of our products; |
| ● | FDA refusal to issue certificates to foreign governments needed to export products for sale in other countries; and |
| ● | criminal prosecution. |
Any of these sanctions could result in higher than anticipated costs or lower than anticipated sales and have a material adverse effect on our reputation, business, financial condition and results of operations.
Our products must be manufactured in accordance with federal and state regulations, and we or any of our suppliers or third-party manufacturers could be forced to recall our installed systems or terminate production if we fail to comply with these regulations.
The methods used in, and the facilities used for, the manufacture of our products must comply with the FDA’s Quality System Regulation, or QSR, which is a complex regulatory scheme that covers the procedures and documentation of the design, testing, production, process controls, quality assurance, labeling, packaging, handling, storage, distribution, installation, servicing and shipping of medical devices. Furthermore, we are required to verify that our suppliers maintain facilities, procedures and operations that comply with our quality standards and applicable regulatory requirements. The FDA enforces the QSR through periodic announced or unannounced inspections of medical device manufacturing facilities, which may include the facilities of subcontractors. Our products are also subject to similar state regulations and various laws and regulations of foreign countries governing manufacturing.
Our third-party manufacturers may not take the necessary steps to comply with applicable regulations, which could cause delays in the delivery of our products. In addition, failure to comply with applicable FDA requirements or later discovery of previously unknown problems with our products or manufacturing processes could result in, among other things: warning letters or untitled letters; fines, injunctions or civil penalties; suspension or withdrawal of approvals; seizures or recalls of our products; total or partial suspension of production or distribution; administrative or judicially imposed sanctions; the FDA’s refusal to grant pending or future clearances or approvals for our products; clinical holds; refusal to permit the import or export of our products; and criminal prosecution of us or our employees.
Any of these actions could significantly and negatively affect supply of our products. If any of these events occurs, our reputation could be harmed, we could be exposed to product liability claims and we could lose customers and experience reduced sales and increased costs.
If treatment guidelines for sleep apnea change or the standard of care evolves, we may need to redesign and seek new marketing authorization from the FDA for one or more of our products.
If treatment guidelines for sleep apnea changes or the standard of care for this condition evolves, we may need to redesign the applicable product and seek new approvals from the FDA. Our PMA approvals from the FDA will be based on the then current treatment guidelines. If treatment guidelines change so that different treatments become desirable, the clinical utility of one or
22
Our products may cause or contribute to adverse medical events or be subject to failures or malfunctions that we are required to report to the FDA, and if we fail to do so, we would be subject to sanctions that could harm our reputation, business, financial condition and results of operations. The discovery of serious safety issues with our products, or a recall of our products either voluntarily or at the direction of the FDA or another governmental authority, could have a negative impact on us.
We are subject to the FDA’s medical device reporting regulations and similar foreign regulations, which require us to report to the FDA when we receive or become aware of information that reasonably suggests that one or more of our products may have caused or contributed to a death or serious injury or malfunctioned in a way that, if the malfunction were to recur, it could cause or contribute to a death or serious injury. The timing of our obligation to report is triggered by the date we become aware of the adverse event as well as the nature of the event. We may fail to report adverse events of which we become aware within the prescribed timeframe. We may also fail to recognize that we have become aware of a reportable adverse event, especially if it is not reported to us as an adverse event or if it is an adverse event that is unexpected or removed in time from the use of the product. If we fail to comply with our reporting obligations, the FDA could take action, including warning letters, untitled letters, administrative actions, criminal prosecution, imposition of civil monetary penalties, revocation of our device approval, seizure of our products or delay in clearance or approval of future products.
The FDA and foreign regulatory bodies have the authority to require the recall of commercialized products in the event of material deficiencies or defects in design or manufacture of a product or in the event that a product poses an unacceptable risk to health. The FDA’s authority to require a recall must be based on a finding that there is reasonable probability that the device could cause serious injury or death. We may also choose to voluntarily recall a product if any material deficiency is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, component failures, malfunctions, manufacturing defects, labeling or design deficiencies, packaging defects or other deficiencies or failures to comply with applicable regulations. Product defects or other errors may occur in the future.
Depending on the corrective action we take to redress a product’s deficiencies or defects, the FDA may require, or we may decide, that we will need to obtain new approvals for the device before we may market or distribute the corrected device. Seeking such approvals may delay our ability to replace the recalled devices in a timely manner. Moreover, if we do not adequately address problems associated with our devices, we may face additional regulatory enforcement action, including FDA warning letters, product seizure, injunctions, administrative penalties or civil or criminal fines.
Companies are required to maintain certain records of recalls and corrections, even if they are not reportable to the FDA. We may initiate voluntary withdrawals or corrections for our products in the future that we determine do not require notification of the FDA. If the FDA disagrees with our determinations, it could require us to report those actions as recalls and we may be subject to enforcement action. A future recall announcement could harm our reputation with customers, potentially lead to product liability claims against us and negatively affect our sales. Any corrective action, whether voluntary or involuntary, as well as defending ourselves in a lawsuit, will require the dedication of our time and capital, distract management from operating our business and may harm our reputation and financial results.
If we do not obtain and maintain international regulatory registrations or approvals for our products, we will be unable to market and sell our products outside of the United States.
Sales of our products outside of the United States are subject to foreign regulatory requirements that vary widely from country to country. In addition, the FDA regulates exports of medical devices from the United States. While the regulations of some countries may not impose barriers to marketing and selling our products or only require notification, others require that we obtain the approval of a specified regulatory body. Complying with foreign regulatory requirements, including obtaining registrations or approvals, can be expensive and time-consuming, and we may not receive regulatory approvals in each country in which we plan to market our products or we may be unable to do so on a timely basis. The time required to obtain registrations or approvals, if required by other countries, may be longer than that required for FDA approval, and requirements for such registrations, clearances or approvals may significantly differ from FDA requirements. If we modify our products, we may need to apply for additional regulatory approvals before we are permitted to sell the modified product. In addition, we may not continue to meet the quality and safety standards required to maintain the authorizations that we have received. If we are unable to maintain our authorizations in a particular country, we will no longer be able to sell the applicable product in that country.
23
Regulatory approval by the FDA does not ensure registration, clearance or approval by regulatory authorities in other countries, and registration, clearance or approval by one or more foreign regulatory authorities does not ensure registration, clearance or approval by regulatory authorities in other foreign countries or by the FDA. However, a failure or delay in obtaining registration or regulatory clearance or approval in one country may have a negative effect on the regulatory process in others.
Legislative or regulatory reforms in the United States or the European Union, or EU, may make it more difficult and costly for us to obtain regulatory clearances or approvals for our products or to manufacture, market or distribute our products after clearance or approval is obtained.
From time to time, legislation is drafted and introduced in the United States Congress that could significantly change the statutory provisions governing the regulation of medical devices. In addition, FDA regulations and guidance are often revised or reinterpreted by the FDA in ways that may significantly affect our business and our products. Any new statutes, regulations or revisions or reinterpretations of existing regulations may impose additional costs or lengthen review times of any future products or make it more difficult to obtain approval for, manufacture, market or distribute our products. We cannot determine what effect changes in regulations, statutes, legal interpretation or policies, when and if promulgated, enacted or adopted may have on our business in the future. Such changes could, among other things, require additional testing prior to obtaining clearance or approval; changes to manufacturing methods; recall, replacement or discontinuance of our products; or additional record keeping.
On April 5, 2017, the European Parliament passed the Medical Devices Regulation (Regulation 2017/745), which repeals and replaces the EU Medical Devices Directive and the Active Implantable Medical Devices Directive. Unlike directives, which must be implemented into the national laws of the EEA member states, the regulations would be directly applicable, i.e., without the need for adoption of EEA member state laws implementing them, in all EEA member states and are intended to eliminate current differences in the regulation of medical devices among EEA member States. The Medical Devices Regulation, among other things, is intended to establish a uniform, transparent, predictable and sustainable regulatory framework across the EEA for medical devices and ensure a high level of safety and health while supporting innovation.
The Medical Devices Regulation will become applicable three years after publication (in 2020). Once applicable, the new regulations will among other things:
| ● | strengthen the rules on placing devices on the market and reinforce surveillance once they are available; |
| ● | establish explicit provisions on manufacturers’ responsibilities for the follow-up of the quality, performance and safety of devices placed on the market; |
| ● | improve the traceability of medical devices throughout the supply chain to the end-user or patient through a unique identification number; |
| ● | set up a central database to provide patients, healthcare professionals and the public with comprehensive information on products available in the EU; |
| ● | strengthened rules for the assessment of certain high-risk devices, such as implants, which may have to undergo an additional check by experts before they are placed on the market. |
These modifications may have an effect on the way we conduct our business in the EEA.
We are subject to certain federal, state and foreign fraud and abuse laws, health information privacy and security laws and transparency laws, which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
There are numerous U.S. federal and state, as well as foreign, laws pertaining to healthcare fraud and abuse, including anti-kickback, false claims and physician transparency laws. Our business practices and relationships with providers are subject to scrutiny under these laws. We may also be subject to privacy and security regulation related to patient, customer, employee and other third-party information by both the federal government and the states and foreign jurisdictions in which we conduct our business. The healthcare laws and regulations that may affect our ability to operate include, but are not limited to:
| ● | The federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce either the referral of an individual or furnishing or arranging for a good or service, for which payment may be made, in whole or in part, under federal healthcare programs, such as Medicare and Medicaid. A person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation. The U.S. government has interpreted this law broadly to apply to the marketing and sales activities of manufacturers. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act. Violations of the federal Anti-Kickback Statute may result in civil monetary penalties up to $100,000 for each violation, plus up to three times the remuneration involved. Civil penalties for such conduct can further be assessed under the federal False Claims Act. Violations can also result in criminal penalties, including criminal fines of up to $100,000 and imprisonment of up to 10 years. Similarly, violations can result in exclusion from participation in government healthcare programs, including Medicare and Medicaid; |
24
| ● | The federal civil and criminal false claims laws and civil monetary penalties laws, including the federal civil False Claims Act, which prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, claims for payment from Medicare, Medicaid or other federal healthcare programs that are false or fraudulent. These laws can apply to manufacturers who provide information on coverage, coding, and reimbursement of their products to persons who bill third-party payers. Private individuals can bring False Claims Act “qui tam” actions, on behalf of the government and such individuals, commonly known as “whistleblowers,” may share in amounts paid by the entity to the government in fines or settlement. When an entity is determined to have violated the federal civil False Claims Act, the government may impose civil fines and penalties ranging from $11,181 to $22,363 for each false claim, plus treble damages, and exclude the entity from participation in Medicare, Medicaid and other federal healthcare programs; |
| ● | The federal Civil Monetary Penalties Law, which prohibits, among other things, offering or transferring remuneration to a federal healthcare beneficiary that a person knows or should know is likely to influence the beneficiary’s decision to order or receive items or services reimbursable by the government from a particular provider or supplier; |
| ● | The Health Insurance Portability and Accountability Act of 1996, or HIPAA, which created additional federal criminal statutes that prohibit, among other things, executing a scheme to defraud any healthcare benefit program and making false statements relating to healthcare matters. Similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge of the statute or specific intent to violate it to have committed a violation; |
| ● | The federal Physician Sunshine Act under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, collectively referred to as the Affordable Care Act, which require certain applicable manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, or CHIP, to report annually to the DHHS Centers for Medicare and Medicaid Services, or CMS, information related to payments and other transfers of value to physicians, certain other healthcare providers, and teaching hospitals, and applicable manufacturers and group purchasing organizations, to report annually ownership and investment interests held by physicians and their immediate family members. Applicable manufacturers are required to submit annual reports to CMS. Failure to submit required information may result in civil monetary penalties of $11,052 per failure up to an aggregate of $165,786 per year (or up to an aggregate of $1.105 million per year for “knowing failures”), for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission, and may result in liability under other federal laws or regulations; |
| ● | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH Act, and their respective implementing regulations, which impose requirements on certain covered healthcare providers, health plans and healthcare clearinghouses as well as their business associates that perform services for them that involve individually identifiable health information, relating to the privacy, security and transmission of individually identifiable health information without appropriate authorization, including mandatory contractual terms as well as directly applicable privacy and security standards and requirements. Failure to comply with the HIPAA privacy and security standards can result in civil monetary penalties up to $55,910 per violation, not to exceed $1.68 million per calendar year for non-compliance of an identical provision, and, in certain circumstances, criminal penalties with fines up to $250,000 per violation and/or imprisonment. State attorneys general can also bring a civil action to enjoin a HIPAA violation or to obtain statutory damages on behalf of residents of his or her state; and |
25
| ● | Analogous state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers or patients; state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the applicable compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm customers, foreign and state laws, including the EU General Data Protection Regulation, or GDPR, governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect, thus complicating compliance efforts; and state laws related to insurance fraud in the case of claims involving private insurers. |
These laws and regulations, among other things, constrain our business, marketing and other promotional activities by limiting the kinds of financial arrangements, including sales programs, we may have with hospitals, physicians or other potential purchasers of our products. Due to the breadth of these laws, the narrowness of statutory exceptions and regulatory safe harbors available, and the range of interpretations to which they are subject, it is possible that some of our current or future practices might be challenged under one or more of these laws.
To enforce compliance with the healthcare regulatory laws, certain enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Responding to investigations can be time-and resource-consuming and can divert management’s attention from the business. Additionally, as a result of these investigations, healthcare providers and entities may have to agree to additional compliance and reporting requirements as part of a consent decree or corporate integrity agreement. Any such investigation or settlement could increase our costs or otherwise have an adverse effect on our business. Even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to. If our operations are found to be in violation of any of the healthcare laws or regulations described above or any other healthcare regulations that apply to us, we may be subject to penalties, including administrative, civil and criminal penalties, damages, fines, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, imprisonment, contractual damages, reputational harm, disgorgement and the curtailment or restructuring of our operations.
We may be subject to, or may in the future become subject to, U.S. federal, state, and foreign laws and regulations imposing obligations on how we collect, store and process personal information. Our actual or perceived failure to comply with such obligations could harm our business. Ensuring compliance with such laws could also impair our efforts to maintain and expand our customer base, and thereby decrease our revenue.
In the conduct of our business, we may at times process personal data, including health-related personal data. The U.S. federal government and various states have adopted or proposed laws, regulations, guidelines and rules for the collection, distribution, use and storage of personal information of individuals. We may also be subject to U.S. federal rules, regulations and guidance concerning data security for medical devices, including guidance from the FDA. State privacy and security laws vary from state to state and, in some cases, can impose more restrictive requirements than U.S. federal law. Where state laws are more protective, we must comply with the stricter provisions. In addition to fines and penalties that may be imposed for failure to comply with state law, some states also provide for private rights of action to individuals for misuse of personal information.
26
The EU also has laws and regulations dealing with the collection, use and processing of personal data obtained from individuals in the EU, which are often more restrictive than those in the United States and which restrict transfers of personal data to the United States unless certain requirements are met. These obligations may be interpreted and applied in a manner that is inconsistent from one jurisdiction to another and may conflict with other requirements or our practices. In addition, these rules are constantly under scrutiny. For example, following a decision of the Court of Justice of the European Union in October 2015, transferring personal data to U.S. companies that had certified as members of the U.S. Safe Harbor Scheme was declared invalid. In July 2016 the European Commission adopted the U.S.-EU Privacy Shield Framework which replaces the Safe Harbor Scheme. However, this Framework is under review and there is currently litigation challenging other EU mechanisms for adequate data transfers (i.e., the standard contractual clauses). It is uncertain whether the Privacy Shield Framework and/or the standard contractual clauses will be similarly invalidated by the European courts. We rely on a mixture of mechanisms to transfer personal data from our EU business to the United States, and could be impacted by changes in law as a result of a future review of these transfer mechanisms by European regulators under the GDPR, as well as current challenges to these mechanisms in the European courts.
Any actual or perceived failure by us or the third parties with whom we work to comply with privacy or security laws, policies, legal obligations or industry standards, or any security incident that results in the unauthorized release or transfer of personally identifiable information, may result in governmental enforcement actions and investigations including by European Data Protection Authorities and U.S. federal and state regulatory authorities, fines and penalties, litigation and/or adverse publicity, including by consumer advocacy groups, and could cause our customers, their patients and other healthcare professionals to lose trust in us, which could harm our reputation and have a material adverse effect on our business, financial condition and results of operations.
Healthcare policy changes, including recently enacted legislation reforming the U.S. healthcare system, could harm our business, financial condition and results of operations.
In the United States, there have been and continue to be a number of legislative initiatives to contain healthcare costs. In March 2010, the Affordable Care Act was enacted in the United States, which made a number of substantial changes in the way healthcare is financed by both governmental and private insurers. Among other ways in which it may affect our business, the Affordable Care Act:
| ● | imposed an annual excise tax of 2.3% on any entity that manufactures or imports medical devices offered for sale in the United States, with limited exceptions (described in more detail below), although the effective rate paid may be lower. Through a series of legislative amendments, the tax was suspended for 2016 through 2019. Absent further legislative action, the device excise tax will be reinstated on medical device sales starting January 1, 2020; |
| ● | established a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in comparative clinical effectiveness research in an effort to coordinate and develop such research; |
| ● | implemented payment system reforms including a national pilot program on payment bundling to encourage hospitals, physicians and other providers to improve the coordination, quality and efficiency of certain healthcare services through bundled payment models; and |
| ● | expanded the eligibility criteria for Medicaid programs. |
We do not yet know the full impact that the Affordable Care Act will have on our business. The taxes imposed by the Affordable Care Act and the expansion in the government’s role in the U.S. healthcare industry may result in decreased profits to us, lower reimbursement by payors for our DeltaWave device, and/or reduced medical procedure volumes, all of which may have a material adverse effect on our business, financial condition and results of operations. The Trump Administration and the U.S. Congress may take further action regarding the Affordable Care Act, including, but not limited to, repeal or replacement. Most recently, the Tax Cuts and Jobs Act of 2017 was enacted, which, among other things, removes penalties for not complying with the individual mandate to carry health insurance. Additionally, all or a portion of the Affordable Care Act and related subsequent legislation may be modified, repealed or otherwise invalidated through judicial challenge, which could result in lower numbers of insured individuals, reduced coverage for insured individuals and adversely affect our business.
We expect additional state and federal healthcare policies and reform measures to be adopted in the future, any of which could limit reimbursement for healthcare products and services or otherwise result in reduced demand for our DeltaWave device or additional pricing pressure and have a material adverse effect on our industry generally and on our customers. Any changes of, or uncertainty with respect to, future coverage or reimbursement rates could affect demand for our DeltaWave device, which in turn could impact our ability to successfully commercialize our DeltaWave device and could have a material adverse effect on our business, financial condition and results of operations.
27
The clinical trial process required to obtain regulatory approvals is lengthy and expensive with uncertain outcomes. If clinical studies of our future products do not produce results necessary to support regulatory clearance or approval in the United States or, with respect to our current or future products, elsewhere, we will be unable to expand the indications for or commercialize these products and may incur additional costs or experience delays in completing, or ultimately be unable to complete, the commercialization of those products.
We will need to obtain PMA approval for our DeltaWave device. In order to obtain PMA approval for a device, the sponsor must conduct well-controlled clinical trials designed to assess the safety and efficacy of the product candidate. Conducting clinical trials is a complex and expensive process, can take many years, and outcomes are inherently uncertain. We incur substantial expense for, and devote significant time to, clinical trials but cannot be certain that the trials will ever result in commercial revenue. We may experience significant setbacks in clinical trials, even after earlier clinical trials showed promising results, and failure can occur at any time during the clinical trial process. Any of our products may malfunction or may produce undesirable adverse effects that could cause us or regulatory authorities to interrupt, delay or halt clinical trials. We, the FDA, or another regulatory authority may suspend or terminate clinical trials at any time to avoid exposing trial participants to unacceptable health risks.
Successful results of pre-clinical studies are not necessarily indicative of future clinical trial results, and predecessor clinical trial results may not be replicated in subsequent clinical trials. Additionally, the FDA may disagree with our interpretation of the data from our pre-clinical studies and clinical trials, or may find the clinical trial design, conduct or results inadequate to prove safety or efficacy, and may require us to pursue additional pre-clinical studies or clinical trials, which could further delay the clearance or approval of our products. The data we collect from our pre-clinical studies and clinical trials may not be sufficient to support FDA clearance or approval, and if we are unable to demonstrate the safety and efficacy of our future products in our clinical trials, we will be unable to obtain regulatory clearance or approval to market our products.
In addition, we may estimate and publicly announce the anticipated timing of the accomplishment of various clinical, regulatory and other product development goals, which are often referred to as milestones. These milestones could include the obtainment of the right to affix the CE mark in the European Union; the submission to the FDA of an investigational device exemption, or IDE, application to commence a pivotal clinical trial for a new product candidate; the enrollment of patients in clinical trials; the release of data from clinical trials; and other clinical and regulatory events. The actual timing of these milestones could vary dramatically compared to our estimates, in some cases for reasons beyond our control. We cannot assure you that we will meet our projected milestones and if we do not meet these milestones as publicly announced, the commercialization of our products may be delayed and, as a result, our stock price may decline.
Clinical trials are necessary to support PMA applications and may be necessary to support PMA supplements for modified versions of our marketed device products. This would require the enrollment of large numbers of suitable subjects, which may be difficult to identify, recruit and maintain as participants in the clinical trial.
Patient enrollment in clinical trials and completion of patient follow-up depend on many factors, including the size of the patient population, the nature of the trial protocol, the proximity of patients to clinical sites, the eligibility criteria for the clinical trial, patient compliance, competing clinical trials and clinicians’ and patients’ perceptions as to the potential advantages of the product being studied in relation to other available therapies, including any new treatments that may be approved for the indications we are investigating. For example, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive post-treatment procedures or follow-up to assess the safety and efficacy of a product candidate, or they may be persuaded to participate in contemporaneous clinical trials of a competitor’s product candidate. In addition, patients participating in our clinical trials may drop out before completion of the trial or experience adverse medical events unrelated to our products. Delays in patient enrollment or failure of patients to continue to participate in a clinical trial may delay commencement or completion of the clinical trial, cause an increase in the costs of the clinical trial and delays, or result in the failure of the clinical trial.
28
Clinical trials must be conducted in accordance with the laws and regulations of the FDA and other applicable regulatory authorities’ legal requirements, regulations or guidelines, and are subject to oversight by these governmental agencies and IRBs at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted with supplies of our devices produced under current good manufacturing practice, or cGMP, requirements and other regulations. Furthermore, we rely on CROs, and clinical trial sites to ensure the proper and timely conduct of our clinical trials and while we have agreements governing their committed activities, we have limited influence over their actual performance. We depend on our collaborators and on medical institutions and CROs to conduct our clinical trials in compliance with good clinical practice, or GCP, requirements. To the extent our collaborators or the CROs fail to enroll participants for our clinical trials, fail to conduct the study to GCP standards or are delayed for a significant time in the execution of trials, including achieving full enrollment, we may be affected by increased costs, program delays or both. In addition, clinical trials that are conducted in countries outside the United States may subject us to further delays and expenses as a result of increased shipment costs, additional regulatory requirements and the engagement of non-U.S. CROs, as well as expose us to risks associated with clinical investigators who are unknown to the FDA, and different standards of diagnosis, screening and medical care.
Failure can occur at any stage of clinical testing. Our clinical studies may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical and non-clinical testing in addition to those we have planned. Our failure to adequately demonstrate the safety and efficacy of our system or any product we may develop in the future would prevent receipt of regulatory clearance or approval and, ultimately, the commercialization of that product or indication for use. Even if our future products are cleared or approved in the United States, commercialization of our products in foreign countries would require approval by regulatory authorities in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials. Any of these occurrences could have an adverse effect on our business, financial condition and results of operations.
Risks Related to this Offering and Our Common Stock
There is no minimum offering.
We do not have a minimum offering and we may use the proceeds from this Offering immediately following our acceptance of the corresponding subscription agreements. It is possible we may only raise a minimum amount of capital, which could leave us with insufficient capital to implement our business plan, potentially resulting in greater operating losses unless we are able to raise the required capital from alternative sources. There is no assurance that alternative capital, if needed, would be available on terms acceptable to us, or at all.
You will incur immediate and substantial dilution as a result of this Offering.
After giving effect to the sale by us of 50,000,000 shares offered in this Offering at the public offering price of $0.06 per Share, and after estimated offering expenses payable by us, if we sell 25% of the shares in this Offering, investors in this Offering will suffer immediate and substantial dilution per share in the net tangible book value of the common stock they purchased in this Offering. Investors will experience more dilution in the net book value per share the less we sell. See “Dilution” on page 32 for a more detailed discussion of the dilution you will incur if you purchase shares of our common stock in this Offering.
Management will have broad discretion as to the use of the proceeds from this Offering, and may not use the proceeds effectively.
Our management will have broad discretion in the application of the net proceeds from this Offering and could spend the proceeds in ways that may not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively could have a material adverse effect on our business and cause the price of our common stock to decline.
29
We have the ability to issue additional shares of our common stock and shares of preferred stock without asking for stockholder approval, which could cause your investment to be diluted.
Our Articles of Incorporation, as amended, authorizes the Board of Directors to issue up to 1 billion shares of common stock and up to 5 million shares of each of Series A Preferred Stock, Series B Preferred Stock and Series C Preferred Stock. Each share of Series A Preferred Stock is convertible into one share of common stock and has the voting equivalency of 25 shares of common stock. Each share of Series B Preferred Stock is convertible into 100 shares of common stock and has the voting equivalency of 100 shares of common stock. Each share of Series C Preferred Stock is convertible into 50 shares of common stock and has the voting equivalency of 50 shares of common stock. Currently, our officers and directors own 4,000,000 shares of Series A Preferred Stock and there are no shares of Series B Preferred Stock or Series C Preferred Stock outstanding. The power of the Board of Directors to issue shares of common stock, preferred stock or warrants or options to purchase shares of common stock or preferred stock is generally not subject to stockholder approval. Accordingly, any additional issuance of our common stock, or preferred stock that may be convertible into common stock, may have the effect of diluting your investment, and the new securities may have rights, preferences and privileges senior to those of our common stock.
Substantial sales of our stock may impact the market price of our common stock.
Future sales of substantial amounts of our common stock, including shares that we may issue upon exercise of options and warrants, could adversely affect the market price of our common stock. Further, if we raise additional funds through the issuance of common stock or securities convertible into or exercisable for common stock, the percentage ownership of our stockholders will be reduced and the price of our common stock may fall.
Our common stock is thinly traded, and investors may be unable to sell some or all of their shares at the price they would like, or at all, and sales of large blocks of shares may depress the price of our common stock.
Our common stock has historically been sporadically or “thinly-traded,” meaning that the number of persons interested in purchasing shares of our common stock at prevailing prices at any given time may be relatively small or nonexistent. As a consequence, there may be periods of several days or more when trading activity in shares of our common stock is minimal or non-existent, as compared to a seasoned issuer that has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. This could lead to wide fluctuations in our share price. Investors may be unable to sell their common stock at or above their purchase price, which may result in substantial losses. Also, as a consequence of this lack of liquidity, the trading of relatively small quantities of shares by our stockholders may disproportionately influence the price of shares of our common stock in either direction. The price of shares of our common stock could, for example, decline precipitously in the event a large number of shares of our common stock are sold on the market without commensurate demand, as compared to a seasoned issuer that could better absorb those sales without adverse impact on its share price.
Resales of our common stock in the public market during this Offering by our stockholders may cause the market price of our common stock to fall.
This issuance of shares of common stock in this Offering could result in resales of our common stock by our current stockholders concerned about the potential dilution of their holdings. In turn, these resales could have the effect of depressing the market price for our common stock.
We do not intend to pay any cash dividends on our shares of common stock in the near future, so our stockholders will not be able to receive a return on their shares unless they sell their shares.
We intend to retain any future earnings to finance the development and expansion of our business. We do not anticipate paying any cash dividends on our common stock in the foreseeable future. There is no assurance that future dividends will be paid, and if dividends are paid, there is no assurance with respect to the amount of any such dividend. Unless we pay dividends, our stockholders will not be able to receive a return on their shares unless they sell such shares.
30
Material weaknesses in our internal control over financial reporting may adversely affect our Common Stock.
As an SEC reporting company, we are subject to the reporting requirements of the Exchange Act and governance requirements of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition, proxy statement, and other information. The Sarbanes-Oxley Act requires, among other things, that we establish and maintain effective disclosure controls and procedures and internal controls and procedures for financial reporting. Section 404 of the Sarbanes-Oxley Act requires that we include a report of management on our internal control over financial reporting in our annual report on Form 10-K. That report must contain an assessment by management of the effectiveness of our internal control over financial reporting and must include disclosure of any material weaknesses in internal control over financial reporting that we have identified. Effective internal control is necessary for us to provide reliable financial reports and prevent fraud. If we cannot provide reliable financial reports or prevent fraud, we may not be able to manage our business as effectively as we would if an effective control environment existed, and our business and reputation with investors may be harmed. As a result, our small size and any current internal control deficiencies may adversely affect our financial condition, results of operation and access to capital. We have not performed an in-depth analysis to determine if historical un-discovered failures of internal controls exist, and may in the future discover areas of our internal control that need improvement. Any inability to report and file our financial results accurately and timely could harm our reputation and adversely impact the trading price of our common stock.
“Penny stock” rules may make buying or selling our securities difficult which may make our stock less liquid and make it harder for investors to buy and sell our shares.
Trading in our securities is subject to the SEC’s “penny stock” rules and it is anticipated that trading in our securities will continue to be subject to the penny stock rules for the foreseeable future. The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. These rules require that any broker-dealer who recommends our securities to persons other than prior customers and accredited investors must, prior to the sale, make a special written suitability determination for the purchaser and receive the purchaser’s written agreement to execute the transaction. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated with trading in the penny stock market. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by these requirements may discourage broker-dealers from recommending transactions in our securities, which could severely limit the liquidity of our securities and consequently adversely affect the market price for our securities.
The Financial Industry Regulatory Authority (FINRA) sales practice requirements may also limit a stockholder’s ability to buy and sell our Common Stock.
In addition to the “penny stock” rules described above, the Financial Industry Regulatory Authority, which we refer to as FINRA, has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, the FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our Common Stock, which may limit your ability to buy and sell our Common Stock and have an adverse effect on the market for shares of our Common Stock.
Our officers and directors hold a significant percentage of our outstanding voting securities, which could reduce the ability of minority stockholders to effect certain corporate actions.
Our officers and directors collectively own 52,188,188 shares of common stock and 4,000,000 shares of Series A Preferred Stock. Each share of common stock is entitled to one vote and each share of Series A Preferred Stock is entitled to 25 votes which means that our officers and directors currently own or control approximately 87.39% of the voting power of the Company. Upon the completion of the Offering and assuming we sell all 50,000,000 shares, our officers and directors will own or control approximately 69.13% of the voting power of the Company. As a result of this ownership, they possess and can continue to possess significant influence over our Board of Directors and corporate transactions. Their ownership and control may also have the effect of delaying or preventing a future change in control, impeding a merger, consolidation, takeover or other business combination or discourage a potential acquirer from making a tender offer. In the event we do not sell a sufficient amount of Shares in this Offering, they will continue to own a significant portion of our outstanding common stock and may have significant influence on our Company.
If securities industry analysts do not publish research reports on us, or publish unfavorable reports on us, then the market price and market trading volume of our common stock could be negatively affected.
Any trading market for our common stock will be influenced in part by any research reports that securities industry analysts publish about us. We do not currently have and may never obtain research coverage by securities industry analysts. If no securities industry analysts commence coverage of us, the market price and market trading volume of our common stock could be negatively affected. In the event we are covered by analysts, and one or more of such analysts downgrade our securities, or otherwise reports on us unfavorably, or discontinues coverage or us, the market price and market trading volume of our common stock could be negatively affected.
31
If you purchase Shares in this Offering, your ownership interest in our common stock will be diluted immediately, to the extent of the difference between the price to the public charged for each Share in this Offering and the net tangible book value per share of our common stock after this Offering.
Our historical net tangible book value as of June 30, 2019 was $(0.02) per then-outstanding share of our common stock. Historical net tangible book value per share equals the amount of our total tangible assets less total liabilities, divided by the total number of shares of our common stock outstanding, all as of the date specified.
The following table illustrates the per share dilution to new investors discussed above, assuming the sale of, respectively, 100%, 75%, 50% and 25% of the shares offered for sale in this Offering (after deducting estimated offering expenses of $75,000):
| Percentage of shares offered that are sold | 100% | 75% | 50% | 25% | ||||||||||||
| Price to the public charged for each share in this offering | $ | 0.06 | $ | 0.06 | $ | 0.06 | $ | 0.06 | ||||||||
| Shares issued | 50,000,000 | 37,500,000 | 25,000,000 | 12,500,000 | ||||||||||||
| Capital raised | $ | 3,000,000 | $ | 2,225,000 | $ | 1,500,000 | $ | 750,000 | ||||||||
| Less: estimated offering costs | $ | (75,000 | ) | $ | (75,000 | ) | $ | (75,000 | ) | $ | (75,000 | ) | ||||
| Net offering proceeds | $ | 2,925,000 | $ | 2,175,000 | $ | 1,425,000 | $ | 675,000 | ||||||||
| Historical net tangible book value pre-offering (1) | $ | (1,071,623 | ) | $ | (1,071,623 | ) | $ | (1,071,623 | ) | $ | (1,071,623 | ) | ||||
| Pro-forma net tangible book value post-offering (2) | $ | 1,853,377 | $ | 1,103,377 | $ | 353,377 | $ | (396,623 | ) | |||||||
| Pre-offering issued and outstanding shares of common stock | 73,139,165 | 73,139,165 | 73,139,165 | 73,139,165 | ||||||||||||
| Pro-forma post-offering shares issued and outstanding (3) | 123,139,165 | 110,639,165 | 98,139,165 | 85,639,165 | ||||||||||||
| Historical net tangible book value per share as of June 30, 2019 | $ | (0.02 | ) | $ | (0.02 | ) | $ | (0.02 | ) | $ | (0.02 | ) | ||||
| Net tangible book value per share, after this offering | $ | 0.014 | $ | 0.01 | $ | 0.004 | $ | (0.005 | ) | |||||||
| Increase in net tangible book value per share attributable to new investors in this offering | $ | 0.035 | $ | 0.03 | $ | 0.024 | $ | 0.015 | ||||||||
| Dilution per share to new investors | $ | 0.045 | $ | 0.05 | $ | 0.056 | $ | 0.065 |
| (1) | Historical net tangible book value pre-offering is based on the net tangible equity of the Company as of June 30, 2019. This consists of the total assets (excluding intangible assets) of the Company less liabilities. |
| (2) | Pro-forma net tangible book value post-offering equity is the Company’s net book value plus expected net offering proceeds. |
| (3) | Consists of 73,139,165 shares of common stock issued and outstanding at of the date of this Offering Circular plus the number of shares sold in this offering. |
32
This Offering Circular is part of an Offering Statement that we filed with the SEC, using a continuous offering process. Periodically, as we have material developments, we will provide an Offering Circular supplement that may add, update or change information contained in this Offering Circular. Any statement that we make in this Offering Circular will be modified or superseded by any inconsistent statement made by us in a subsequent Offering Circular supplement. The Offering Statement we filed with the SEC includes exhibits that provide more detailed descriptions of the matters discussed in this Offering Circular. You should read this Offering Circular and the related exhibits filed with the SEC and any Offering Circular supplement, together with additional information contained in our annual reports, semi-annual reports and other reports and information statements that we will file periodically with the SEC. See the section entitled “Additional Information” below for more details.
This Offering is being made on a “best efforts,” self-underwritten basis without the use of an exclusive placement agent. As there is no minimum number of Shares we have to sell in this Offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit said proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds.
This Offering Circular is part of an exemption under Regulation A that permits our officers and directors to sell the Shares directly to the public in those jurisdictions where the Offering Circular is approved, with no commission or other remuneration payable for any Shares sold. There are no present plans or arrangements to enter into any contracts or agreements to sell the Shares with a broker or dealer. In offering the Shares on our behalf, our officers and directors will rely on the safe harbor from broker dealer registration set out in Rule 3a4-1 under the Exchange Act. The Offering will be conducted primarily by the Company’s President, Mr. Thomas J. Wood. Mr. Wood meets the requirements of Rule 3a4-1 because he is not subject to a statutory disqualification, as defined in Section 3(a)(39) of the Exchange Act, he will not be compensated in connection with the sale of the issuer’s securities by the payment of commissions, bonus, or other remuneration based either directly or indirectly on transactions in securities. He is not an associated person of a broker or dealer. Further, his sales duties are limited in frequency and proportion. He is serving and will serve as President of the Company and will be selling securities only in connection with this Offering. Mr. Wood also intends to rely heavily on advertising, social media, email campaigns, and other such avenues of communication and to identify and contact prospective purchasers by such means.
Exchange Listing
Our common stock is traded on OTC Pinks Current Information under the symbol “RMSL.”
Pricing of the Offering
Prior to the Offering, there has been a limited public market for our common stock. The $0.06 Offering Price was arbitrarily determined by us. The principal factors considered in determining the Offering Price include:
| ● | the information set forth in this Offering Circular and otherwise available; |
| ● | our history and prospects and the history of and prospects for the industry in which we compete; |
| ● | our past and present financial performance; |
| ● | our prospects for future earnings and the present state of our development; |
| ● | the general condition of the securities markets at the time of this Offering; |
| ● | the recent market prices of, and demand for, publicly traded common stock of generally comparable companies; and |
| ● | other factors deemed relevant by us. |
33
Offering Period and Expiration Date
This Offering will start on or after the date the Offering Statement of which this Offering Circular is a part is qualified by the SEC (the “Qualification Date”) and will terminate upon the earlier of (i) the anniversary of the Qualification Date; (ii) when all of the Shares are sold; (iii) we terminate this Offering. The Company will offer and sell all of the Shares in the Offering at the price of $0.06 per Share for the duration of the Offering.
Selling Security Holders
There are no selling security holders in this Offering.
Procedures for Subscribing
If you decide to subscribe for Shares in this Offering, you should:
| 1. | Electronically receive, review, execute and deliver to us a subscription agreement; and |
| 2. | Deliver funds directly by credit card, wire or electronic funds transfer via ACH to the specified account maintained by us. |
Any potential investor will have ample time to review the subscription agreement, along with their counsel, prior to making any final investment decision. We shall only deliver such subscription agreement upon request after a potential investor has had ample opportunity to review this Offering Circular.
Minimum Purchase
The minimum amount established for investors is $6,000 (100,000 shares), unless such minimum is waived by the Company, in its sole discretion. All money we receive from the offering will be immediately appropriated by us for the uses set forth in the Use of Proceeds section of this Offering Circular.
No Escrow
The proceeds of this Offering will not be placed into an escrow account. We will offer our common stock on a “best efforts” basis. As there is no minimum offering, upon the approval of any subscription to this Offering Circular, the Company shall immediately deposit said proceeds into the bank account of the Company and may dispose of the proceeds in accordance with the Use of Proceeds.
Right to Reject Subscriptions
After we receive your complete, executed subscription agreement and the funds required under the subscription agreement have been transferred to the Company, we have the right to review and accept or reject your subscription in whole or in part, for any reason or for no reason. We will return all monies from rejected subscriptions immediately to you, without interest or deduction.
Acceptance of Subscriptions
Subscriptions are irrevocable and the purchase price is non-refundable as expressly stated in this Offering Circular. The Company, by determination of the Board of Directors, in its sole discretion, may issue the Shares in this Offering for cash, promissory notes, services, and/or other consideration without notice to subscribers. All proceeds received by the Company from subscribers for this Offering will be available for use by the Company upon acceptance of subscriptions for the Shares by the Company.
Upon our acceptance of a subscription agreement, we will countersign the subscription agreement and issue the Shares subscribed at closing. Once you submit the subscription agreement and it is accepted, you may not revoke or change your subscription or request your subscription funds. All accepted subscription agreements are irrevocable.
Other Selling Restrictions
Other than in the United States, no action has been taken by us that would permit a public offering of our common stock in any jurisdiction where action for that purpose is required. Our common stock may not be offered or sold, directly or indirectly, nor may this Offering Circular or any other Offering material or advertisements in connection with the offer and sale of shares of our common stock be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this Offering Circular comes are advised to inform themselves about and to observe any restrictions relating to this Offering and the distribution of this Offering Circular. This Offering Circular does not constitute an offer to sell or a solicitation of an offer to buy our common stock in any jurisdiction in which such an offer or solicitation would be unlawful.
34
The Use of Proceeds is an estimate based on the Company’s current business plan. We may find it necessary or advisable to reallocate portions of the net proceeds reserved for one category to another, or to add additional categories, and we will have broad discretion in doing so.
The maximum gross proceeds from the sale of the Shares in this Offering are $3,000,000. The net proceeds from the offering, assuming it is fully subscribed, are expected to be approximately $2,925,000 after the payment of offering costs of $75,000 including printing, mailing, marketing, legal and accounting costs, and other compliance and professional fees that may be incurred. The estimate of the budget for offering costs is an estimate only and the actual offering costs may differ from those expected by management.
Management of the Company has wide latitude and discretion in the use of proceeds from this Offering. At present, management’s best estimate of the use of proceeds, at various funding milestones, is set out in the chart below. However, potential investors should note that this chart contains only the best estimates of the Company’s management based upon information available to them at the present time, and that the actual use of proceeds is likely to vary from this chart based upon circumstances as they exist in the future, various needs of the Company at different times in the future, and the discretion of the Company’s management at all times.
We intend to use the net proceeds in the event 25%, 50%, 75% and 100% of the Shares being offered in this Offering are sold as indicated below:
If 25% of the Shares offered (net proceeds of $675,000) are sold:
| Item | Amount | Percentage | ||||||
| Marketing and Advertising | $ | 253,846 | 38 | % | ||||
| Inventory | 92,308 | 14 | % | |||||
| Lab Studies/Testing | 69,231 | 10 | % | |||||
| Professional Fees | 57,692 | 9 | % | |||||
| Research and Development | 46,154 | 7 | % | |||||
| Regulatory | 28,846 | 4 | % | |||||
| Working Capital | 126,923 | 18 | % | |||||
| Total | $ | 675,000 | 100 | % | ||||
If 50% of the Shares offered (net proceeds of $1,425,000) sold:
| Item | Amount | Percentage | ||||||
| Marketing and Advertising | $ | 535,897 | 38 | % | ||||
| Inventory | 194,872 | 14 | % | |||||
| Lab Studies/Testing | 146,154 | 10 | % | |||||
| Professional Fees | 121,795 | 9 | % | |||||
| Research and Development | 97,436 | 7 | % | |||||
| Regulatory | 60,897 | 4 | % | |||||
| Working Capital | 267,949 | 18 | % | |||||
| Total | $ | 1,425,000 | 100 | % | ||||
35
If 75% of the Shares offered (net proceeds of $2,175,000) are sold:
| Item | Amount | Percentage | ||||||
| Marketing and Advertising | $ | 817,949 | 38 | % | ||||
| Inventory | 297,436 | 14 | % | |||||
| Lab Studies/Testing | 223,077 | 10 | % | |||||
| Professional Fees | 185,897 | 9 | % | |||||
| Research and Development | 148,718 | 7 | % | |||||
| Regulatory | 92,949 | 4 | % | |||||
| Working Capital | 408,974 | 18 | % | |||||
| Total | $ | 2,175,000 | 100 | % | ||||
If 100% of the Shares offered (net proceeds of $2,925,000) are sold:
| Item | Amount | Percentage | ||||||
| Marketing and Advertising | $ | 1,100,000 | 38 | % | ||||
| Inventory | 400,000 | 14 | % | |||||
| Lab Studies/Testing | 300,000 | 10 | % | |||||
| Professional Fees | 250,000 | 9 | % | |||||
| Research and Development | 200,000 | 7 | % | |||||
| Regulatory | 125,000 | 4 | % | |||||
| Working Capital | 550,000 | 18 | % | |||||
| Total | $ | 2,925,000 | 100 | % | ||||
The precise amounts that we will devote to each of the foregoing items, and the timing of expenditures, will vary depending on numerous factors. As indicated in the table above, if we sell only 75%, or 50%, or 25% of the Shares offered for sale in this Offering, we would expect to use the net proceeds for the same purposes as we would use the net proceeds from a sale of 100% of the Shares, and in approximately the same proportions, until such time as such use of proceeds would leave us without working capital reserve. At that point we would expect to modify our use of proceeds by the implementation of our business plan, leaving us with the working capital reserve indicated.
In the event we do not sell all of the Shares being offered, we may seek additional financing from other sources in order to support the intended use of proceeds indicated above. If we secure additional equity funding, investors in this Offering would be diluted. In all events, there can be no assurance that additional financing would be available to us when wanted or needed and, if available, on terms acceptable to us.
36
Corporate History
We were incorporated in the State of Nevada on June 6, 2007. On August 2, 2010, we changed our name from Bella Viaggio, Inc. to Kat Gold Holdings Corp. Effective January 1, 2015, we completed an exchange agreement to purchase 100% of the outstanding interests of REMSleep LLC in exchange for 50,000,000 common shares of REMSleep Holdings, Inc.’s stock, at which time REMSleep LLC became our wholly-owned subsidiary and adopted their business of developing and distributing our sleep apnea products. On January 5, 2015, we changed our name to REMSleep Holdings, Inc. to reflect our new business model.
Overview
Our officers have 35 years of sleep-industry experience, including having been employed at sleep industry companies. Our officers invented our DeltaWave CPAP interface (the “DeltaWave”) as an innovative new device to treat patients with sleep apnea. The patent-pending DeltaWave device is a nasal-pillows type interface that will result in better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally. A survey that appeared in DME Business found that 89% of patients stated that mask-interface comfort was their primary concern. The primary issue that we have addressed with the DeltaWave is the “work of breathing” component. We believe that our DeltaWave is designed to effectively address the stubborn issues that continue to affect a patient’s ability to comply with treatment, as follows:
| ● | Does not disrupt normal breathing mechanics; | |
| ● | Is not claustrophobic; | |
| ● | Causes zero work of breathing (WOB); | |
| ● | Minimizes or eliminates drying of the sinuses; | |
| ● | Uses less driving pressure; and | |
| ● | Allows users to feel safe and secure while sleeping. |
Pending adequate financing, we plan to conduct clinical trials to test product effectiveness.
On June 28, 2016, we applied for a patent for a new, innovative sleep apnea product that serves as an interface for the delivery of CPAP therapy and other respiratory needs. Our goal is to develop sleep products that achieve optimum compliance and comfort for CPAP patients.
Our website is located at: http://www.remsleep.com. No information from our website is incorporated herein. REMSleep Holding, Inc. is referred to herein as “we,” “us,” or “our.”
Industry Background
The market for sleep treatment and equipment was $7.96 billion in 2011 and will reach a projected $19.72 billion by 2017, with North America accounting for a majority of the market. More than 8 million CPAP interfaces are sold annually in the U.S., with another 2.5 million globally. There are also an estimated 80 million people with undiagnosed sleep apnea. Sleep apnea is a condition that affects millions of people in the United States alone. An increasingly sedentary lifestyle and bad working habits has led to obesity and otherwise poor cardiac and aerobic health. This has led to a fast-growing epidemic of obstructive sleep apnea (sleep apnea), which greatly reduces the quality of sleep one gets and can ultimately result in hypertension, heart failure, stroke, and at the least, reduced performance in everyday life. The market for sleep treatment and equipment was $7.96 billion in 2011 and will reach a projected $19.72 billion by 2017, with North America accounting for a majority of the market. More than 8 million CPAP interfaces are sold annually in the U.S., with another 2.5 million globally. There are also an estimated 80 million people with undiagnosed sleep apnea. Sleep apnea results in numerous afflictions that affect people’s day-to-day lives and can eventually contribute to serious health conditions. While people’s knowledge of this affliction has grown strongly in recent years, and the market is expanding fast nationwide, up to 80% of people with sleep apnea may be undiagnosed 1 – a market of millions of new potential users. Even those who are tested and prescribed a sleep apnea machine often give up after a short time due to discomfort or what is called the “work of breathing” with traditional machines. In fact, over 50% of patients give up on using CPAP therapy after 6 months. This is a major waste of resources and a very telling statistic.
37
A major challenge in the current market is not only to get more patients diagnosed but to also increase CPAP compliance. According to market analyst Frost & Sullivan, “The development of finer and ergonomic CPAP devices will help increase patient ability to adhere to sleep therapy. The market is also seeing a rise in newer technologies that replace elaborate practices, target patient comfort to improve compliance, and help drive acceptance of sleep monitoring devices.”
A growing knowledge of sleep apnea and its treatment has helped to increase awareness with the public. In addition to making the use of a CPAP or related device less intimidating, a move toward affordable and prescription-based technology can greatly expand the market “Evolving technologies will also influence patient preferences for products, treatment modalities, and diagnostic locations,” states Frost & Sullivan 2. “As such, the global sleep apnea treatment market is expected to shift to home-based diagnostics for early identification and treatment of patients as well as portable devices that can reduce sleep apnea with minimal inconvenience.”
Sleep apnea causes breathing interruptions of between 10 to 20 seconds that can occur hundreds of times during a night, disrupting the natural sleep rhythm and depriving people of the restorative sleep they need to be energetic, mentally sharp, and productive the next day. CPAP can be a very effective method used to treat sleep apnea, but as noted, noncompliance remains a stubborn issue for both physicians and patients. CPAP technology therefore is constantly being updated and improved, and the new CPAP devices are lighter, quieter, and more comfortable.
Health care spending continues to grow rapidly on an annual basis in the United States. Spending was $2.7 trillion in 2011 and, in 2013, it reached over $3.6 trillion. By 2022, spending is projected to reach $5 trillion, or around 20% of GDP, according to the Centers for Medicare and Medicaid Services 3. Growing alongside this market is the U.S. life science industry, which will grow an estimated 2.2% in 2014 to $93 billion. This includes R&D spending, with growth primarily from smaller biopharmaceutical innovators and medical device manufacturers.
Within this market, sleep apnea products have experienced rapid growth. In the past couple of decades there has been a rapid increase in the technological developments in the field of sleep apnea diagnosis and treatment. The result has been strong growth for sleep apnea devices globally. Demand for new and innovative treatment methodologies is driving growth, helping to provide patients with a healthy lifestyle. “Obstructive sleep apnea is destroying the health of millions of Americans, and the problem has only gotten worse over the last two decades,” according to American Academy of Sleep Medicine President Dr. Timothy Morgenthaler 5. “The effective treatment of sleep apnea is one of the keys to success as our nation attempts to reduce health care spending and improve chronic disease management.”
Sleep problems are considered a “global epidemic,” with sleep apnea as a major contributor to the disorder. An estimated 100 million people worldwide have sleep apnea, though more than 80% of these people are undiagnosed. The market for sleep apnea diagnostic and therapeutic devices on a global level was $7.96 billion in 2011 and will reach a projected $19.72 billion by 2017, according to a study from Markets & Markets.6 Nationwide in the U.S., there are more than 1,600 businesses in the Sleep Disorder Clinics market, according to research firm IBISWorld. These businesses have combined annual revenue of $7 billion and have maintained a combined annual growth rate (CAGR) of 9.8% from 2008 to 2013. “Sleep clinics have gained exposure during the period due to the rising number of sleep disorders,” states IBISWorld. “Moreover, health insurance policies are increasingly covering all or at least part of the costs of tests and, as more patients have been able to gain greater access to specialized sleep clinics, industry revenue grows.”
There are also more than 972,000 physicians and 365,000 doctors’ offices, as well as nearly 5,800 hospitals. In addition, the market for U.S. home healthcare is served by about 30,000 businesses with combined annual revenue of $59 billion. The market includes medical and skilled nursing services; medical equipment, supplies, and medication services; personal care; and therapeutic services (like physical and respiratory therapy).
38
Sources:
1. Markets & Markets. “Global Sleep Apnea Diagnostics & Therapeutic Devices Market.” http://www.marketsandmarkets.com/PressReleases/sleep-apnea-devices.asp
2. Frost & Sullivan. “Sleep apnea market is in need of finer, ergonomic treatments.” June 4, 2014. http://www.frost.com/prod/servlet/press-release.pag?docid=290951848
3. Forbes. “Annual U.S. Healthcare Spending Hits $3.8 Trillion.” Feb. 2, 2014. http://www.forbes.com/sites/danmunro/2014/02/02/annual-u-s-healthcare-spending-hits-3-8-trillion/
5. American Academy of Sleep Medicine. “Rising prevalence of sleep apnea in U.S. threatens public health.” Sept. 2014. http://www.aasmnet.org/articles.aspx?id=5043
6. Markets & Markets. “Global Sleep Apnea Diagnostics & Therapeutic Devices Market.” http://www.marketsandmarkets.com/PressReleases/sleep-apnea-devices.asp
Marketing
We plan to market the DeltaWave device in the U.S., as follows:
| ● | Submit manufacture orders to our manufacturer according to market demand; | |
| ● | Negotiate and secure agreements with industry distributor partners; | |
| ● | Secure agreements with Internet retailers for online sales; | |
| ● | Market DeltaWave at respiratory trade shows, social media, press releases; | |
| ● | Market and generate online sales through our website supplemented by search engine optimization; | |
| ● | Disseminate press releases to media outlets and publications that reach sleep medical practices and DME managers/distributors, including trade publications like Sleep Medicine, Sleep Review, Sleep, The Sleep Magazine; and | |
| ● | Attend sleep and healthcare, respiratory industry trade shows. |
Target Market
Our target market includes:
| ● | Sleep product distributors that will distribute our product; | |
| ● | Home care dealers; | |
| ● | Private sleep labs; | |
| ● | Internet providers; | |
| ● | Product end users; | |
| ● | Physicians, particularly sleep physicians; | |
| ● | Medical groups; | |
| ● | Hospitals; and | |
| ● | Medical associations, such as the American Academy of Sleep Medicine and the American Sleep Association. |
We expect that most of our revenues will be in the home care dealers and hospital target market.
39
Manufacturing
Our product will be manufactured by mold makers. We presently have molds made in China; however, we are considering relocating the manufacturing of our molds to the United States.
Operations Contingent Upon Adequate Financing
Our entire business plan, including our ability to conduct manufacturing, marketing, generate sales and further develop products, are entirely dependent upon adequate financing. Should we fail to obtain adequate financing: (a) our financial condition will be negatively affected; (b) we will be unable to conduct the essential aspects of our business plan, including marketing as reflected above; (c) investments in our common stock will be negatively impacted; (d) we will be forced to liquidate our business and file for bankruptcy protection.
Competition
The sleep apnea devices market is highly consolidated, with primary competitors being:
| ● | ResMed; |
| ● | Philips Respironics; |
| ● | Naus Medical; |
| ● | Fisher & Paykel Healthcare; |
| ● | DeVilbiss Healthcare; |
| ● | CareFusion; |
| ● | InnoMed; |
| ● | DeVilbiss; and |
| ● | TAP. |
ResMed is the market leader (45% of market share), followed by Philips (30%), and Fisher/Paykel (12%). Our competitors offer a full range of sleep products.
Our competitors have greater financial, operational and personnel resources than we do. We will attempt to overcome our competitors’ competitive advantages by emphasizing the advantages of our Delta Wave product.
Government Regulations
FDA
Our products are subject to extensive regulation particularly as to safety, efficacy and adherence to FDA Quality System Regulation, and related manufacturing standards. Medical device products are subject to rigorous FDA and other governmental agency regulations in the United States and similar regulations of foreign agencies abroad. The FDA regulates the design, development, research, preclinical and clinical testing, introduction, manufacture, advertising, labeling, packaging, marketing, distribution, import and export, and record keeping for such products, to ensure that medical products distributed in the United States are safe and effective for their intended use. In addition, the FDA is authorized to establish special controls to provide reasonable assurance of the safety and effectiveness of most devices. Non-compliance with applicable requirements can result in import detentions, fines, civil and administrative penalties, injunctions, suspensions or losses of regulatory approvals, recall or seizure of products, operating restrictions, refusal of the government to approve product export applications or allow us to enter supply contracts, and criminal prosecution.
40
Unless an exemption applies, the FDA requires that a manufacturer introducing a new medical device or a new indication for use of an existing medical device obtain either a Section 510(k) premarket notification clearance or a premarket approval, or PMA, before introducing it into the U.S. market. The type of marketing authorization is generally linked to the classification of the device. The FDA classifies medical devices into one of three classes (Class I, II or III) based on the degree of risk the FDA determines to be associated with a device and the level of regulatory control deemed necessary to ensure the device’s safety and effectiveness.
Our products currently marketed in the United States are marketed in reliance on 510(k) pre-marketing clearances as either Class I or Class II devices. The process of obtaining a Section 510(k) clearance generally requires the submission of performance data and often clinical data, which in some cases can be extensive, to demonstrate that the device is “substantially equivalent” to a device that was on the market before 1976 or to a device that has been found by the FDA to be “substantially equivalent” to such a pre-1976 device, a predecessor device is referred to as “predicate device.” As a result, FDA clearance requirements may extend the development process for a considerable length of time. In addition, in some cases, the FDA may require additional review by an advisory panel, which can further lengthen the process. The PMA process, which is reserved for new devices that are not substantially equivalent to any predicate device and for high-risk devices or those that are used to support or sustain human life, may take several years and requires the submission of extensive performance and clinical information.
Medical devices can be marketed only for the indications for which they are cleared or approved. After a device has received 510(k) clearance for a specific intended use, any change or modification that significantly affects its safety or effectiveness, such as a significant change in the design, materials, method of manufacture or intended use, may require a new 510(k) clearance or PMA approval and payment of an FDA user fee. The determination as to whether a modification could significantly affect the device’s safety or effectiveness is initially left to the manufacturer using available FDA guidance; however, the FDA may review this determination to evaluate the regulatory status of the modified product at any time and may require the manufacturer to cease marketing and recall the modified device until 510(k) clearance or PMA approval is obtained. The manufacturer may also be subject to significant regulatory fines or penalties. The FDA is currently reviewing its guidance describing when it believes a manufacturer is obligated to submit a new 510(k) for modifications or changes to a previously cleared device. The FDA is expected to issue revised guidance to assist device manufacturers in making this determination. It is unclear whether the FDA’s approach in this new guidance will result in substantive changes to existing policy and practice regarding the assessment of whether a new 510(k) is required for changes or modifications to existing devices.
Any devices we manufacture and distribute pursuant to clearance or approval by the FDA are subject to pervasive and continuing regulation by the FDA and certain state agencies. These include product listing and establishment registration requirements, which help facilitate FDA inspections and other regulatory actions. As a medical device manufacturer, our manufacturing facilities are subject to inspection on a routine basis by the FDA. We are required to adhere to applicable regulations setting forth detailed cGMP requirements, as set forth in the QSR, which require, manufacturers, including third-party manufacturers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all phases of the design and manufacturing process. Noncompliance with these standards can result in, among other things, fines, injunctions, civil penalties, recalls or seizures of products, total or partial suspension of production, refusal of the government to grant 510(k) clearance or PMA approval of devices, withdrawal of marketing approvals and criminal prosecutions. We believe that our design, manufacturing and quality control procedures are in compliance with the FDA’s regulatory requirements.
We must also comply with post-market surveillance regulations, including medical device reporting, or MDR, requirements which require that we review and report to the FDA any incident in which our products may have caused or contributed to a death or serious injury. We must also report any incident in which our product has malfunctioned if that malfunction would likely cause or contribute to a death or serious injury if it were to recur.
Labeling and promotional activities are subject to scrutiny by the FDA and, in certain circumstances, by the Federal Trade Commission. Medical devices approved or cleared by the FDA may not be promoted for unapproved or un-cleared uses, otherwise known as “off-label” promotion. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses, and a company that is found to have improperly promoted off-label uses may be subject to significant liability, including substantial monetary penalties and criminal prosecution.
41
Other Healthcare Laws
Even though we do not submit claims or bill governmental programs and other third-party payers directly for reimbursement for our products sold in the United States, we are still subject to laws and regulations that may restrict our business practices, including, without limitation, anti-kickback, false claims, physician payment transparency and data privacy and security laws. The government has interpreted these laws broadly to apply to the marketing and sales activities of manufacturers and distributors like us.
The federal Anti-Kickback Statute prohibits, among other things, persons or entities from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the purchase, lease, order or recommendation of, any good, facility, item or service for which payment may be made, in whole or in part, under federal healthcare programs such as Medicare and Medicaid. In addition, a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the federal civil False Claims Act.
The federal civil False Claims Act prohibits, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. A claim includes “any request or demand” for money or property presented to the U.S. government. The civil False Claims Act also applies to false submissions that cause the government to be paid less than the amount to which it is entitled, such as a rebate. Intent to deceive is not required to establish liability under the civil False Claims Act.
The Federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, created federal criminal statutes that prohibit among other actions, knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors, knowingly and willfully embezzling or stealing from a healthcare benefit program, willfully obstructing a criminal investigation of a healthcare offense, and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. Like the Anti-Kickback Statute, a person or entity does not need to have actual knowledge of these statutes or specific intent to violate them to have committed a violation.
Also, many states and countries outside the U.S. have similar fraud and abuse statutes or regulations that may be broader in scope and may apply regardless of payor, in addition to items and services reimbursed under Medicaid and other state programs.
Under HIPAA, the Department of Health and Human Services, or HHS, has issued regulations to protect the privacy and security of protected health information used or disclosed by covered entities including health care providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties. In addition to federal privacy and security regulations, there are state laws governing confidentiality and security of health information that are applicable to our business. New laws governing privacy may be adopted in the future as well. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Additionally, there has been a recent trend of increased federal and state regulation of payments and transfers of value provided to healthcare professionals or entities. The Physician Payment Sunshine Act was enacted in law as part of PPACA, which imposed new annual reporting requirements on device manufacturers for payments and other transfers of value provided by them, directly or indirectly, to physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their family members. A manufacturer’s failure to submit timely, accurately and completely the required information for all payments, transfers of value or ownership or investment interests may result in civil monetary penalties. Certain states also mandate implementation of commercial compliance programs, impose restrictions on device manufacturer marketing practices and/or require the tracking and reporting of gifts, compensation and other remuneration to healthcare professionals and entities.
42
The shifting commercial compliance environment and the need to build and maintain robust systems to comply with different compliance or reporting requirements in multiple jurisdictions increase the possibility that a healthcare company may fail to comply fully with one or more of these requirements. If our operations are found to be in violation of any of the health regulatory laws described above or any other laws that apply to us, we may be subject to penalties, including potentially significant criminal and civil and administrative penalties, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
Environmental Regulation
Our operations are not subject to environmental regulation.
Employees
We have the following employees:
| ● | Thomas J. Wood, Chief Executive Officer (full-time) |
| ● | Jonathan B. Lane, Vice President and Chief Technology Officer (full-time) |
| ● | Russell F. Bird, President (part-time) |
We believe that our relations with our employees are good. All other services to the Company are provided by contractors who are primarily paid with stock-based compensation. Personnel will be added on an as-needed basis and based on available funds.
Legal Proceedings
On October 24, 2017, the Company was notified that a petition had been filed in the Iowa District Court for Polk County by Mr. John M. Wesson for failure to repay a loan. Mr. Wesson had loaned the Company $30,000 and $20,000 on October 24, 2012 and June 12, 2013, respectively. The loans were to accrue interest at 5%. While the Company was under previous management, the loans were removed from the books in first quarter of 2015. On April 26, 2018, the Company agreed to repay the loan in full including accrued interest and $5,000 for legal fees. The $50,000 plus $7,341 was booked to retained earnings in 2016 as a correction of an error. As of June 30, 2019, there is $45,000 and $16,101 of principal and interest, respectively, due on this loan.
We may from time to time be involved in various claims and legal proceedings of a nature we believe are normal and incidental to temporary employee staffing business. These matters may include product liability, intellectual property, employment, personal injury cause by our employees, and other general claims. We will accrue for contingent liabilities when it is probable that a liability has been incurred and the amount can be reasonably estimated. We are not presently a party to any legal proceedings that, in the opinion of our management, are likely to have a material adverse effect on our business. Regardless of outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
We have an executive office at 637 N. Orange Ave., Suite 609, Orlando, FL 32789 where we have access to a conference room for meetings.
43
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of our operations together with our consolidated financial statements and the notes thereto appearing elsewhere in this Offering Circular. This discussion contains forward-looking statements reflecting our current expectations, whose actual outcomes involve risks and uncertainties. Actual results and the timing of events may differ materially from those stated in or implied by these forward-looking statements due to a number of factors, including those discussed in the sections entitled “Risk Factors,” “Cautionary Statement Regarding Forward-Looking Statements” and elsewhere in this Offering Circular. Please see the notes to our Financial Statements for information about our Critical Accounting Policies and Recently Issued Accounting Pronouncements.
Overview
We were incorporated in the State of Nevada on June 6, 2007. On August 2, 2010, we changed our name from Bella Viaggio, Inc. to Kat Gold Holdings Corp. Effective January 1, 2015, we completed an exchange agreement to purchase 100% of the outstanding interests of REMSleep LLC in exchange for 50,000,000 common shares of REMSleep Holdings, Inc.’s stock, at which time REMSleep LLC became our wholly-owned subsidiary and adopted their business of developing and distributing our sleep apnea products. On January 5, 2015, we changed our name to REMSleep Holdings, Inc. to reflect our new business model.
On June 28, 2016, we applied for a patent for DeltaWave, a new, innovative sleep apnea nasal pillow device that serves as an interface for the delivery of CPAP therapy and other respiratory needs. Our goal is to develop sleep products that achieve optimum compliance and comfort for CPAP patients.
Our officers have 35 years of sleep-industry experience, including having been employed at sleep industry companies. Our officers invented our DeltaWave CPAP interface (the “DeltaWave”) as an innovative new device to treat patients with sleep apnea. The patent-pending DeltaWave device is a nasal-pillows type interface that will result in better comfort and, therefore, better compliance since it was specifically designed with unique airflow characteristics to enable patients with sleep apnea to breathe normally. The primary issue that we have addressed with the DeltaWave is the “work of breathing” component. We believe that our DeltaWave is designed to effectively address the stubborn issues that continue to affect a patient’s ability to comply with treatment, as follows:
| ● | Does not disrupt normal breathing mechanics; |
| ● | Is not claustrophobic; |
| ● | Causes zero work of breathing (WOB); |
| ● | Minimizes or eliminates drying of the sinuses; |
| ● | Uses less driving pressure; and |
| ● | Allows users to feel safe and secure while sleeping. |
Pending adequate financing, we plan to conduct clinical trials to test product effectiveness.
Our website is located at: http://www.remsleep.com, the contents of which are not incorporated into this Offering Circular.
Results of Operations
Fiscal year ended December 31, 2018 compared to the fiscal year ended December 31, 2017
Revenues
We generated no revenues during our fiscal years ending December 31, 2018 and 2017.
44
Operating Expenses
For the year ended December 31, 2018, professional fees decreased $42,312, or 49% to $43,900 compared to $86,212 for the year ended December 31, 2017. Professional fees consist mostly of accounting, audit and legal fees. The decrease of $42,312 in the current year is attributed to a decrease in legal fees. In 2017, $21,250 of the legal expense was non-cash expense from the issuances of common stock.
Consulting expense was $175,582 compared to $516,396 for the years ended December 31, 2018 and 2017, respectively. During the fiscal year ended December 31, 2017, we granted 1,574,761 shares of common stock to consultants for total non-cash expense of $709,168, $173,282 of which was booked to prepaids as of December 31, 2017. During the fiscal year ended December 31, 2018, we granted common stock to consultants for total non-cash expense of $175,282.
Officer compensation was $38,650 and $24,000 for the years ended December 31, 2018 and 2017, respectively, an increase of $14,650, or 61%. There was an increase to the monthly compensation for our CEO.
General and administrative expense was $36,249 and $24,857 for the years ended December 31, 2018 and 2017, respectively, an increase of $11,392 or 45.8%. The increase in fiscal year ended December 31, 2018 can be largely attributed to an increase in depreciation, development and other fee expense.
Net Loss
For the year ended December 31, 2018, we had a net loss of $412,702 as compared to a net loss of $653,965 for the year ended December 31, 2017. Our net loss was lower in the fiscal year ended December 31, 2018 primarily due to a decrease in stock issued for services.
Three months ended June 30, 2019 compared to the three months ended June 30, 2018
Operating Expenses
Professional fees were $9,900 compared to $19,950 for the three months ended June 30, 2019 and 2018, respectively, a decrease of $10,050, or 50%. Professional fees consist mostly of accounting, audit and legal fees. The decrease in the three month period ended June 30, 2019 is due to lower audit and legal expense.
Consulting expense was $17,720 compared to $266,278 for the three months ended June 30, 2019 and 2018, respectively. A majority of the consulting expense is due to the granting of common stock. In the three month period ended June 30, 2019, there was a significant decrease in the number of shares issued for consulting services, thus decreasing the expense.
Compensation expense was $2,044,000 and $12,000 for the three months ended June 30, 2019 and 2018, respectively. In the three month period ended June 30, 2019, we issued 25,000,000 shares each to both our President and CEO for services for total non-cash expense of $2,000,000. We also issued our President 500,000 shares of Series A preferred stock for total non-cash compensation expense of $20,000 on June 12, 2019.
General and administrative expense was $17,759 and $8,762 for the three months ended June 30, 2019 and 2018, respectively, an increase of $8,999, or 102.6%. The increase can be largely attributed to an increase in depreciation, development and web design expense.
Total other expense for the three months ended June 30, 2019, was $257,377. Other expense includes $58,106 of debt discount amortization, a $925,498 loss on the issuance of convertible debt and a gain in the change of fair value of $737,905. These are all expenses related to convertible debt. We also incurred $11,678 of interest expense. In the three month period ended June 30, 2018, we had $623 of interest expense.
Net Loss
For the three months ended June 30, 2019, we had a net loss of $2,346,756 as compared to a net loss of $307,613 for the three months ended June 30, 2018. Our net loss was higher for the three month period ended June 30, 2019 compared to the three month period ended June 30, 2018 primarily due to the expense associated with the other non-cash expense from the issuance of convertible debt and common stock issued for services.
45
Six months ended June 30, 2019 compared to the six months ended June 30, 2018
Operating Expenses
Professional fees were $25,400 compared to $36,100 for the six months ended June 30, 2019 and 2018, respectively, a decrease of $10,700, or 29.6%. Professional fees consist mostly of accounting, audit and legal fees. The decrease for the six month period ended June 30, 2019 compared to the six month period ended June 30, 2018 was due to lower audit and legal expense.
Consulting expense was $17,720 compared to $383,799 for the six months ended June 30, 2019 and 2018, respectively. A majority of the consulting expense is due to the granting of common stock. In the six month period ended June 30, 2019, there was a significant decrease in the number of shares issued for consulting services, thus decreasing the expense.
Compensation expense was $2,062,000 and $18,000 for the six months ended June 30, 2019 and 2018, respectively. In the six month period ended June 30, 2019, we issued 25,000,000 shares each to both our President and CEO for services for total non-cash expense of $2,000,000. We also issued our President 500,000 shares of Series A Preferred tock for total non-cash compensation expense of $20,000.
General and administrative expense was $34,128 and $13,701 for the six months ended June 30, 2019 and 2018, respectively, an increase of $20,427, or 149%. The increase in the six month period ended June 30, 2019 period can be largely attributed to an increase in depreciation, development and web design expense.
Total other expense for the six months ended June 30, 2019, was $799,240. Other expense includes $102,059 of debt discount amortization, a $1,051,207 loss on the issuance of convertible debt and a loss in the change of fair value of $376,191. These are all expenses related to convertible debt. We also incurred $22,165 of interest expense. In the six month period ended June 30, 2018, we had $1,239 of interest expense and a loss in the change of fair value of $16,895.
Net Loss
For the six months ended June 30, 2019, we had a net loss of $2,938,488 as compared to a net loss of $469,734 for the six months ended June 30, 2018. Our net loss was higher in the six month period ended June 30, 2019 primarily due to the expense associated with the other non-cash expense from the issuance of convertible debt and common stock issued for services.
Liquidity and Capital Resources; Going Concern
At June 30, 2019, we had $181,763 cash on hand and $18,719 in prepaid expenses. We have suffered recurring losses from operations since our inception. In addition, we have yet to generate an internal cash flow from our business operations or successfully raised the financing required to develop our proposed business. As a result of these and other factors, our independent auditor has expressed substantial doubt about our ability to continue as a going concern. Our future success and viability, therefore, are dependent upon our ability to generate capital financing. The failure to generate sufficient revenues or raise additional capital may have a material and adverse effect upon us and our shareholders.
We do not have sufficient capital to fund our operations for the next 12 months. Management’s plans with regard to these matters encompass the following actions: (i) obtaining funding from new investors to alleviate our working capital deficiency, and (ii) implementing a plan to generate sales. Our continued existence is dependent upon our ability to resolve our liquidity problems and increase profitability in our current business operations. However, the outcome of management’s plans cannot be ascertained with any degree of certainty. Our financial statements do not include any adjustments that might result from the outcome of these risks and uncertainties.
46
The Company’s ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations.
To date, we have funded our operations primarily through the issuances of convertible debt and from support from parties related through common ownership and directorship. We do not have any written or oral agreements with our officers or directors obligating them to advance the Company any money. Related party loans are unsecured, non-interest bearing and due on demand. As of June 30, 2019 and December 31, 2018, the balance due on these loans is $179,191 and $179,191, respectively. Beginning on January 1, 2019, the balance began accruing interest at the rate of 12.5% per annum. As of June 30, 2019, total accrued interest is $11,107.
We have issued the following convertible debt:
| ● | On July 9, 2018, the Company issued a Convertible Promissory Note in favor of Power Up Lending Group LTD (“Power Up”). The principal amount of the Note was $45,000 with an original issue discount of $3,000 and carried an interest rate of 12% per annum. It became due and payable with accrued interest on July 9, 2019. Power Up had the option to convert the Note plus accrued interest into common shares of the Company after 180 days. The conversion rate was a 39% discount to the average of the lowest two trading price for twenty days prior to the date of conversion. During the six months June 30, 2019, PowerUp Lending Group LTD converted $45,000 of principal into 5,599,447 shares of common stock. |
| ● | On August 30, 2018, the Company issued a Convertible Promissory Note in favor of LG Capital Funding LLC (“LG”). The principal amount of the Note was $32,000 with an original issue discount of $2,000 and carries an interest rate of 10% per annum. It became due and payable with accrued interest on August 30, 2019. During the first six months LG has the option to convert the Note plus accrued interest at a fixed price of $0.10 per share. After the 6-month anniversary, the Conversion Price was equal to 60% of the lowest closing bid price for the eighteen prior trading days including the day of conversion. Using the stock price on the date the Note was issued of $0.185 and the conversion price of $0.10, the Company valued each share at $0.085 for an additional expense of $27,200. The Company has accounted for the $27,200 has debt discount with a credit to additional paid in capital. The discount will be amortized over the term of the note. During the six months June 30, 2019, LG Capital Funding LLC converted $26,310 and $1,689 of principal and interest, respectively, into 4,050,340 shares of common stock. |
| ● | On January 23, 2019, the Company issued a Convertible Promissory Note in favor of One44 Capital LLC (“One44”). The principal amount of the Note is $100,000 with an interest rate of 12% per annum. It becomes due and payable with accrued interest on January 23, 2020. During the six months June 30, 2019, One44 Capital LLC converted $4,500 and $195 of principal and interest, respectively, into 755,477 shares of common stock. |
| ● | On May 3, 2019, the Company issued a Convertible Promissory Note in favor of Odyssey Capital Funding, LLC (“OCF”) for the principal amount of $100,000. The Note bears interest at a rate of 12% per annum and matures on May 3, 2020. OCF or its assignee (“Holder”) is entitled, at its option, at any time, to convert all or any amount of the principal face amount of the Note then outstanding into shares of the Company’s common stock at a price (“Conversion Price”) for each share of Common Stock equal to 55% of the lowest trading price of the Common Stock as reported on the OTC Marketplace exchange which the Company’s shares are traded or any exchange upon which the Common Stock may be traded in the future (“Exchange”), for the twenty prior trading days including the day upon which a Notice of Conversion is received by the Company or its transfer agent. In no event shall the Holder be allowed to effect a conversion if such conversion, along with all other shares of Company Common Stock beneficially owned by the Holder and its affiliates would exceed 4.99% of the outstanding shares of the Common Stock of the Company (which may be increased up to 9.9% upon 60 days’ prior written notice by the Investor). The Note may be prepaid or assigned with certain penalties/premiums: (i) If 60 days or less after issuance, the Company shall pay 130% of the principal plus accrued interest; (ii) from the 61st th to the 120th day after issuance, 140% of the principal plus accrued interest and (iii) from the 121st to the 180th day after issuance, 145% of the principal plus accrued interest. The Note may not be prepaid after the 180th day after issuance. Such redemption must be closed and funded within 3 days of giving notice of redemption of the right to redeem shall be null and void. |
47
Upon (i) a transfer of all or substantially all of the assets of the Company to any person in a single transaction or series of related transactions, (ii) a reclassification, capital reorganization or other change or exchange of outstanding shares of the Common Stock, other than a forward or reverse stock split or stock dividend, or (iii) any consolidation or merger of the Company with or into another person or entity in which the Company is not the surviving entity (other than a merger which is effected solely to change the jurisdiction of incorporation of the Company and results in a reclassification, conversion or exchange of outstanding shares of Common Stock solely into shares of Common Stock) (each of items (i), (ii) and (iii) being referred to as a “Sale Event”), then, in each case, the Company shall, upon request of the Holder, redeem this Note in cash for 150% of the principal amount, plus accrued but unpaid interest through the date of redemption, or at the election of the Holder, such Holder may convert the unpaid principal amount of this Note (together with the amount of accrued but unpaid interest) into shares of Common Stock immediately prior to such Sale Event at the Conversion Price.
| ● | On May 30, 2019, the Company sold a convertible promissory note to each of Armada Investment Fund, LLC, a Delaware limited liability company (“AIF”), BHP Capital NY Inc., a New York corporation (“BHP”), and Jefferson Street Capital LLC, a New Jersey limited liability company (“JSC”), for $35,000. Each note was in the principal amount of $36,750, bears interest at the rate of 12% per annum and matures on February 29, 2020. Any outstanding principal and accrued interest not paid at maturity shall bear interest at the rate of 22% per annum. |
The holder of each note has the right to convert such note, from time to time and in whole or in part, into shares of common stock of the Company; provided, however, in no event shall the holder be entitled to convert any portion of its note which would result in the holder and its affiliates to be deemed the beneficial owner of more than 4.99% of the outstanding shares of the Company’s common stock. The conversion price of the notes shall be equal to the lesser of (i) 55% multiplied by the lowest traded price of the common stock during the previous twenty (20) trading day period before the issue date of the note, or (ii) the “Variable Conversion Price” defined as 55% of lowest trading price for the common stock during the twenty (20) trading day period ending on the latest complete trading day prior to the conversion date. If the trading price cannot be calculated for the common stock on such date in the manner provided above, the trading price shall be the fair market value as reasonably determined by the Company. The notes may be prepaid or assigned with certain penalties/premiums: (i) if before the 30th after issuance, the Company shall pay 115% of the principal plus accrued interest; (ii) from the 30th to the 60th day after issuance, 120% of the principal plus accrued interest; (iii) from the 61st to the 90th day after issuance, 125% of the principal plus accrued interest; (iv) from the 91st to the 120th day after issuance, 130% of the principal plus accrued interest; (v) from the 121st to the 150th day after issuance, 140% of the principal plus accrued interest; and (vi) from the 151st to the 180th day after issuance, 145% of the principal plus accrued interest. Such prepayment must be closed and funded within 3 days of giving notice.
At the option of the holders, the sale, conveyance or disposition of all or substantially all of the assets of the Company, the effectuation by the Company of a transaction or series of related transactions in which more than 50% of the voting power of the Company is disposed of, or the consolidation, merger or other business combination of the Company with or into any other person or persons when the Company is not the survivor shall be deemed to be an event of default pursuant to which the Company shall be required to pay to the respective holders, upon the consummation of and as a condition to such transaction, an amount equal to the default amount.
While each note is outstanding, the Company is required to notify the respective holder of any future financing transaction with a third party investor and if requested by such holder, amend and restate the note to be identical to the instruments evidencing the subsequent investment. The foregoing does not apply in respect of (i) an underwritten public offering of the Company’s common stock or and (ii) shares of common stock or options to pursuant to any stock or option plan duly adopted for such purpose by a majority of the members of the Board, (b) securities upon the exercise or exchange of or conversion of the notes and/or other securities exercisable or exchangeable for or convertible into shares of common stock issued and outstanding on the date thereof, and (c) securities issued pursuant to acquisitions or strategic transactions approved by a majority of the disinterested directors of the Company.
In connection with each of the notes, on May 30, 2019, the Company issued to each of AIF, BHP and JSC a warrant to purchase 500,000 shares of common stock from the date of issuance until the third anniversary date thereof for $0.045 per share. In the event that there is not effective registration statement registering the shares of common stock issuable upon the exercise of such warrant under the Securities Act, the warrant maybe exercised in whole or in part by means of a “cashless exercise”. The Company may redeem the warrant for $0.001 per share in certain circumstances.
48
Cash Flow from Operations
Cash used in operating activities for the six months ended June 30, 2019 was $98,395 as compared to $64,313 cash used in operating activities for the six months ended June 30, 2018.
Cash Flows from Investing
Cash used in investing activities for the six months ended June 30, 2019 was $26,927 as compared to $6,648 of cash used in investing activities for the six months ended June 30, 2018.
Cash Flows from Financing
For the six months ended June 30, 2019, we received $292,000 from the issuance of convertible debt and repaid $1,555 on our auto loan. For the six months ended June 30, 2018, we received $77,500 from the sale of common stock and repaid $8,000 on other loan payables.
As of June 30, 2019, we owe $311,440 to our convertible debt holders.
Critical Accounting Estimates and Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Note 1 to the Financial Statements describes the significant accounting policies and methods used in the preparation of the Financial Statements. Estimates are used for, but not limited to, contingencies and taxes. Actual results could differ materially from those estimates. The following critical accounting policies are impacted significantly by judgments, assumptions, and estimates used in the preparation of the Financial Statements.
We are subject to various loss contingencies arising in the ordinary course of business. We consider the likelihood of loss or impairment of an asset or the incurrence of a liability, as well as our ability to reasonably estimate the amount of loss in determining loss contingencies. An estimated loss contingency is accrued when management concludes that it is probable that an asset has been impaired, or a liability has been incurred and the amount of the loss can be reasonably estimated. We regularly evaluate current information available to us to determine whether such accruals should be adjusted.
We recognize deferred tax assets (future tax benefits) and liabilities for the expected future tax consequences of temporary differences between the book carrying amounts and the tax basis of assets and liabilities. The deferred tax assets and liabilities represent the expected future tax return consequences of those differences, which are expected to be either deductible or taxable when the assets and liabilities are recovered or settled. Future tax benefits have been fully offset by a 100% valuation allowance as management is unable to determine that it is more likely than not that this deferred tax asset will be realized.
49
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources and would be considered material to investors.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). ASU 2016-02 requires lessees to recognize lease assets and lease liabilities on the balance sheet and requires expanded disclosures about leasing arrangements. The new standard supersedes the present U.S. GAAP standard on leases and requires substantially all leases to be reported on the balance sheet as right-of-use assets and lease obligations. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018 and interim periods in fiscal years beginning after December 15, 2018, with early adoption permitted. At this point in time we do not expect any material impact on our financial statements as a result of adopting this standard.
Topic 606, Revenue from Contracts with Customers, of the Financial Accounting Standards Board’s (FASB) Accounting Standards Codification (ASC). The guidance in ASC 606 was originally issued by the FASB in May 2014 in Accounting Standards Update (ASU) 2014-09, Revenue from Contracts with Customers (Topic 606). Since then, the FASB has issued several ASUs that have revised or clarified the guidance in ASC 606. The Company has evaluated the impact of this accounting standard update and noted that it has had no material impact.
On June 20, 2018, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2018-07, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 is intended to reduce cost and complexity and to improve financial reporting for share-based payments to nonemployees (for example, service providers, external legal counsel, suppliers, etc.). Under the new standard, companies will no longer be required to value non-employee awards differently from employee awards. Meaning that companies will value all equity classified awards at their grant-date under ASC718 and forgo revaluing the award after this date. The Company has chosen to early adopt this standard.
In January 2017, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Update (“ASU”) 2017-01, Business Combinations (Topic 805) Clarifying the Definition of a Business. The amendments in this update clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions or disposals of assets or businesses. The definition of a business affects many areas of accounting including acquisitions, disposals, goodwill, and consolidation. The guidance is effective for interim and annual periods beginning after December 15, 2017 and should be applied prospectively on or after the effective date. The Company is in the process of evaluating the impact of this accounting standard update.
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
50
DIRECTORS, EXECUTIVE OFFICERS AND SIGNIFICANT EMPLOYEES
The following table sets forth information regarding our executive officers, directors and significant employees, including their ages as of the date of this Offering Circular:
The names of our director and executive officers as of the date of this Offering Circular, their respective ages, positions, and biographies are set forth below. Our executive officers are appointed by, and serve at the discretion of, our board of directors.
| Name | Age | Position(s) | ||||
| Russell F. Bird | 70 | President and Chairman of the Board | ||||
| Thomas J. Wood | 73 | Chief Executive Officer and Director | ||||
| Jonathan B. Lane | 59 | Vice President and Chief Technology Officer | ||||
Professional Experience
The biographies of each executive officer below contain information regarding the person’s service as an executive officer, business experience, director positions held currently or at any time during the last five years, and information regarding involvement in certain legal or administrative proceedings, if applicable.
Russell F. Bird has been our Chairman and Director since January 1, 2015 and our President since January 1, 2019. From year to year, he operated businesses that offered sleep apnea interfaces, devices, and other respiratory equipment and supplies. From May 23, 2013 to the present, he has been the Managing Member of REMSleep, LLC, a Iowa limited liability company. In 1979, he founded Medical Gases Australia, a medical manufacturing and distribution firm that specializing in respiratory and other health products. Medical Gases Australia placed the first patients on CPAP therapy. He then started Medical Industries of America in 1985, a medical manufacturing and distribution firm specializing in respiratory and other health products.
Thomas J. Wood has been our Chief Executive Officer and Director effective January 1, 2015. From May 23, 2013 to the present, he has been the Managing Member of REMSleep, LLC, a Iowa limited liability company. Thomas J. Wood has been awarded several U.S. patents in the area of sleep apnea. He is the inventor and developer of Nasal Aire, which won the 2004 Frost and Sullivan Award for Product Innovation. His US Patents also include the Nasal Aire II and Petite Nasal Aire. Tom has 25 years of experience as a respiratory therapist in the ICU at Baylor Medical Center and Parkland Memorial hospitals in Dallas, Texas. He also worked for two years with the Muscular Dystrophy Association, responsible for respiratory care for patients with Amyotrophic Lateral Sclerosis.
Jonathan B. Lane has been serving as the Chief Technology Officer of the Company since July 2018 and Vice President since January 2019. Mr. Lane has 35 years of design and engineering experience. He was the CEO/Founder of Badencorp from 1992 to 2018, and Director of Engineering/Co-founder of Searchmont Engine Company from 2006 to 2009. Jonathan worked twelve years for various fortune 500 companies including Boeing, General Dynamics and Bell Helicopter. From there, he went on to form his own company, Badencorp, which specialized in providing engineering and design services across all disciplines within Aerospace, Automotive, Biomedical, Consumer Products and Heavy Industries.
Family Relationships
There are no family relationships between any of our officers and directors.
Significant Employees
We do not have any significant employees other than our current executive officers named in this Offering Circular.
51
Involvement in Certain Legal Proceedings
Our directors and executive officers have not been personally involved in any of the following events during the past ten years:
| ● | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; | |
| ● | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); | |
| ● | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; | |
| ● | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; | |
| ● | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or | |
| ● | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Conflicts of Interest
Investors should be aware of the following potential conflicts of interest:
| ● | None of our officers and directors is required to commit their full time to our affairs and, accordingly, they may have conflicts of interest in allocating their time among various business activities. |
52
Board Composition
Our Board of Directors currently consists of two members, Thomas J. Wood and Russell F. Bird. Each director of the Company serves until the next annual meeting of stockholders and until his successor is elected and duly qualified, or until his earlier death, resignation or removal. Our board is authorized to appoint persons to the offices of Chairman of the Board of Directors, President, Chief Executive Officer, one or more vice presidents, a Treasurer or Chief Financial Officer and a Secretary and such other offices as may be determined by the board.
Director Independence
We currently do not have any independent directors, as the term “independent” is defined in Section 803A of the NYSE Amex LLC Company Guide. Since the OTC Markets does not have rules regarding director independence, the Board makes its determination as to director independence based on the definition of “independence” as defined under the rules of the New York Stock Exchange (“NYSE”) and American Stock Exchange (“Amex”).
Board Committees
Our board does not currently have a standing Audit Committee, Compensation Committee or Nominating/Corporate Governance Committee due the board’s limited size and the Company’s limited operations. The entire Board of Directors performs all functions that would otherwise be performed by committees. Given the present size of our Board, it is not practical for us to have committees other than those described above, or to have more than two directors on such committees. If we are able to grow our business and increase our operations, we intend to expand the size of our board and our committees and allocate responsibilities accordingly.
Board Leadership Structure and Risk Oversight
The Board of Directors oversees our business and considers the risks associated with our business strategy and decisions. The board currently implements its risk oversight function as a whole. Each of the board committees, when established, will provide risk oversight in respect of its areas of concentration and report material risks to the board for further consideration.
Code of Ethics
We have not adopted a code of ethics due to our limited size. We intend to adopt a code of ethics when warranted.
COMPENSATION OF DIRECTORS AND EXECUTIVE OFFICERS
Summary Compensation
The following table provides information as to the compensation of our “named executive officers” for the past two fiscal years under Item 402(m) of Regulation S-K.
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||||||
| Nonqualified | ||||||||||||||||||||||||||||||||||||
| Non-Equity | Deferred | |||||||||||||||||||||||||||||||||||
| Stock | Option | Incentive Plan | Compensation | All Other | ||||||||||||||||||||||||||||||||
| Name and | Salary | Bonus | Awards | Awards | Compensation | Earnings | Compensation | Total | ||||||||||||||||||||||||||||
| principal position | Year | ($) | ($) | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||||||||
| Russell F. Bird | 2018 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||||||||||||
| (President and Chairman) | 2017 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||||||||||||
| Thomas J. Wood | 2018 | $ | 38,850 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 38,850 | |||||||||||||||||||
| (Executive Officer) | 2017 | $ | 21,150 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 21,150 | |||||||||||||||||||
| Jonathan B. Lane | 2018 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||||||||||||
| (VP, CTO) | 2017 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | 0 | |||||||||||||||||||
Outstanding Equity Awards at Fiscal Year End
There were no outstanding equity awards as of December 31, 2018.
Employment Agreements
The Company executed an employment agreement with its CEO, Thomas J. Wood, on January 1, 2018. Per the terms of the agreement, Mr. Wood is to be compensated $3,000 per month. The agreement expired on January 2, 2019. The Company executed a new employment agreement with Mr. Wood on April 1, 2019. Per the terms of the agreement, Mr. Wood is to be compensated $4,000 per month. The agreement expires on April 1, 2020.
The Company executed an director agreement with its President, Russell F. Bird, on January 1, 2019. Per the terms of the agreement Mr. Bird is to be compensated $3,000 per month for serving as the President of the Board. The agreement expires on January 1, 2020.
Director Compensation
We did not provide any cash compensation to directors for their service as directors during the last fiscal year.
53
SECURITY OWNERSHIP OF MANAGEMENT AND CERTAIN SECURITY HOLDERS
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. In accordance with SEC rules, shares of our common stock which may be acquired upon exercise of stock options or warrants which are currently exercisable or which become exercisable within 60 days of the date of the applicable table below are deemed beneficially owned by the holders of such options and warrants and are deemed outstanding for the purpose of computing the percentage of ownership of such person, but are not treated as outstanding for the purpose of computing the percentage of ownership of any other person. Subject to community property laws, where applicable, the persons or entities named in the tables below have sole voting and investment power with respect to all shares of our common stock indicated as beneficially owned by them.
The following table sets forth information with respect to the beneficial ownership of our common stock as of the date of this Offering Circular, by (i) each stockholder known by us to be the beneficial owner of more than 5% of our outstanding voting capital stock, (ii) each of our directors and executive officers, and (iii) all of our directors and executive officers as a group. To the best of our knowledge, except as otherwise indicated, each of the persons named in the table has sole voting and investment power with respect to the shares of our capital stock beneficially owned by such person, except to the extent such power may be shared with a spouse. To our knowledge, none of the shares listed below are held under a voting trust or similar agreement, except as noted. To our knowledge, there is no arrangement, including any pledge by any person of securities of the Company or any of its parents, the operation of which may at a subsequent date result in a change in control of the Company.
Name and Address of Beneficial Owner (1)(2) | Shares of Common Stock | Percent of Class | ||||||
| Russell F. Bird, President and Chairman (3) | 28,219,494 | 37.56 | % | |||||
| Thomas J. Wood, CEO (4) | 27,969,494 | 37.22 | % | |||||
| Jonathan B. Lane, VP/CTO | 1,000,000 | 1.37 | % | |||||
| All Officers and Directors as a Group (2 persons) | 57,188,988 | 74.14 | % | |||||
| (1) | Beneficial ownership is calculated based on 73,139,165 shares of common stock issued and outstanding as of the date hereof, together with securities exercisable or convertible into such shares within sixty (60) days of the date hereof for each stockholder. The shares of common stock issuable pursuant to those convertible securities, options or warrants are deemed outstanding for computing the percentage ownership of the person holding such convertible securities, options or warrants but are not deemed outstanding for the purposes of computing the percentage ownership of any other person. |
| (2) | The address for each of the officers and directors is c/o REMSleep Holdings, Inc, 637 North Orange Ave., Suite 609, Orlando, FL 32789. |
| (3) | Includes 2,000,000 shares of common stock issuable upon the conversion of 2,000,000 shares of Series A Preferred Stock held by Russell F. Bird. Each share of Series A Preferred Stock is convertible into one share of common stock and has 25 votes. |
| (4) | Includes 2,000,000 shares of common stock issuable upon the conversion of 2,000,000 shares of Series A Preferred Stock held by Thomas J. Wood. Each share of Series A Preferred Stock is convertible into one share of common stock and has 25 votes. |
54
Disclosure of Commission Position of Indemnification for Securities Act Liabilities
In accordance with the provisions in our Articles of Incorporation, we will indemnify an officer, director, or former officer or director, to the full extent permitted by law.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended (the “Act”) may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of us in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
SEC rules require us to disclose any transaction or currently proposed transaction in which the Company is a participant and in which any related person has or will have a direct or indirect material interest involving the lesser of $120,000 or one percent (1%) of the average of the Company’s total assets as of the end of last two completed fiscal years. A related person is any executive officer, director, nominee for director, or holder of 5% or more of the Company’s common stock, or an immediate family member of any of those persons.
The Company has received support from parties related through common ownership and directorship. All of the expenses herein have been borne by these individuals on behalf of the Company and are treated as shareholder loans. These loans are unsecured, non-interest bearing and due on demand. As of June 30, 2019 and December 31, 2018, the balance due on these loans is $179,191 and $179,191, respectively. Beginning on January 1, 2019, the balance due will accrue interest at 12.5%. As of June 30, 2019, total accrued interest is $11,107.
General
This Offering Circular relates to the sale of up to 50,000,000 shares of our common stock by the Company on a “best efforts” basis at an offering price of $0.06 per share, for a gross offering proceeds of $3,000,000 if all shares are sold. There is no minimum aggregate offering amount and no provision to escrow or return investor funds if any minimum number of shares is not sold. The minimum amount established for investors is $6,000 (100,000 shares), unless such minimum is waived by the Company, in its sole discretion. All money we receive from the offering will be immediately appropriated by us for the uses set forth in the Use of Proceeds section of this Offering Circular.
| ● | On June 6, 2007, our Articles of Incorporation were filed with the Secretary of State of Nevada under the name Bella Viaggio, Inc. Our authorized capital stock consisted of 70 million shares of common stock, par value $0.001 per share, and 5 million shares of “blank check” preferred stock, par value $0.001 per share. |
| ● | On August 2, 2010, a Certificate of Amendment was filed with the Secretary of State of Nevada thereby changing the Company’s name to Kat Gold Holdings Corp. and increasing the number of authorized common stock to 500 million shares. |
| ● | On December 26, 2012, a Certificate of Amendment was filed with the Secretary of State of the State of Nevada to effectuate an increase in the Company’s authorized number of shares of common stock to 1,000,000,000. |
| ● | On January 5, 2015, a Certificate of Amendment was filed with the Secretary of State of Nevada thereby changing the Company’s name to REMSleep Holdings, Inc. |
| ● | On March 30, 2015, REMSleep Holdings, Inc. entered into an exchange agreement to purchase 100% of the outstanding interests of REMSleep LLC in exchange for 50,000,000 shares of REMSleep Holdings, Inc.’s common stock. REMSleep LLC is now a wholly-owned subsidiary of REMSleep Holdings, Inc. and REMSleep Holdings, Inc. has acquired the business and operations of REMSleep LLC. |
55
| ● | On June 15, 2017, the Company filed a Certificate of Amendment to its Articles of Incorporation (the “Certificate of Amendment”), with the Secretary of State of the State of Nevada to affect a 1-for-20 reverse stock split of its common stock, whereby every twenty shares of existing common stock will be converted into one share of new common stock. |
| ● | On June 29, 2017, FINRA approved the Company’s Reverse Stock Split. The Reverse Stock Split took effect at the open of business on June 30, 2017. All shares through these financial statements have been retroactively adjusted to reflect the reverse. |
As of the date of this Offering Circular, the Company had 73,139,165 shares of common stock and 4,000,000 shares of Series A Preferred Stock outstanding. There were no shares of Series B Preferred Stock or Series C Preferred Stock outstanding.
Common Stock
Each share of common stock entitles the holder to one vote, either in person or by proxy, at meetings of stockholders. The holders are not permitted to vote their shares cumulatively. Accordingly, the stockholders of our common stock who hold, in the aggregate, more than fifty percent of the total voting rights can elect all of our directors and, in such event, the holders of the remaining minority shares will not be able to elect any of such directors. The vote of the holders of a majority of the issued and outstanding shares of common stock entitled to vote thereon is sufficient to authorize, affirm, ratify or consent to such act or action, except as otherwise provided by law. Stockholders may take action by written consent of over 50% of the issued and outstanding common stock of the Company.
Holders of common stock are entitled to receive ratably such dividends, if any, as may be declared by the Board of Directors out of funds legally available. We have not paid any dividends since our inception, and we presently anticipate that all earnings, if any, will be retained for development of our business. Any future disposition of dividends will be at the discretion of our Board of Directors and will depend upon, among other things, our future earnings, operating and financial condition, capital requirements, and other factors.
Holders of our common stock have no pre-emptive rights or other subscription rights, conversion rights, redemption or sinking fund provisions. Upon our liquidation, dissolution or winding up, the holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities. There are not any provisions in our Articles of Incorporation, as amended, or our Bylaws that would prevent or delay change in our control.
Preferred Stock
Our Articles of Incorporation, as amended, authorizes the Board of Directors to issue up to 1 billion shares of common stock and up to 5 million shares of each of Series A Preferred Stock, Series B Preferred Stock and Series C Preferred Stock. Our Board of Directors has the discretion to determine the rights, preferences, privileges and restrictions, including voting rights, dividend rights, conversion rights, redemption privileges and liquidation preferences, of each series of Preferred Stock.
The purpose of authorizing our Board of Directors to issue Preferred Stock and determination its rights and preferences is to eliminate delays associated with a stockholder vote on specific issuances. The issuance of Preferred Stock, while providing flexibility in connection with possible acquisitions, future financings and other corporate purposes, could have the effect of making it more difficult for a third party to acquire, or could discourage a third party from seeking to acquire, a majority of our outstanding voting stock.
| ● | The Company is currently authorized to issue 5,000,000 Series A Preferred Stock, par value $0.001 per share. Each share of Series A Preferred Stock is convertible into one share of common stock and has the voting power of 25 shares of common stock. The Series A Preferred Stock ranks equal to the common stock on liquidation and pays no dividend. Our current officers and directors own an aggregate of 4,000,000 shares of Series A Preferred Stock. |
| ● | The Company is currently authorized to issue 5,000,000 Series B Preferred Stock, par value $0.001 per share. Each share of Series B Preferred Stock has a 1:100 voting right and is convertible into 100 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are no shares of Series B Preferred Stock issued and outstanding. |
56
| ● | The Company is currently authorized to issue 5,000,000 Series C Preferred Stock, par value $0.001 par share. Each share of Series C Preferred Stock has a 1:50 voting right and is convertible into 50 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are no shares of Series C Preferred Stock issued and outstanding. |
Market Price, Dividends, and Related Stockholder Matters
Our common stock currently trades on the OTC Pinks under the symbol “RMSL” and the closing price of our common stock on September 27, 2019 was $0.02.
Our common stock currently trades on a sporadic and limited basis.
As of the date of this Offering Circular, there were 155 stockholders of record.
We do not have an equity incentive plan.
We have not declared any cash dividends on our common stock in the past two years and do not anticipate paying such dividends in the foreseeable future. We plan to retain any future earnings for use in our business. Any decisions as to future payments of dividends will depend on our earnings and financial position and such other facts, as the Board of Directors deems relevant.
Anti-takeover Provisions
Certain provisions of our Articles of Incorporation and By-Laws may make it more difficult and time-consuming to acquire the Company, thereby reducing our vulnerability to an unsolicited proposal for our takeover. These provisions are outlined below.
Preferred Stock
Our Series A Preferred Stock is convertible into 1 share of common stock per share and has the voting equivalency of 25 votes per share. Our Series B Preferred Stock is convertible into 100 shares of common stock per share and has the voting equivalency of 100 votes per share. Our Series C Preferred Stock is convertible into 50 shares of common stock per share and has the voting equivalency of 50 votes per share. Currently, there are 4,000,000 shares of Series A Preferred Stock outstanding, all of which are owned by our officers and directors, and no shares of Series B Preferred Stock and Series C Preferred Stock are outstanding. The issuance of any unissued Series A Preferred Stock, Series B Preferred Stock or Series C Preferred Stock might tend to discourage or render more difficult a merger or other change of control.
Nevada Law
Certain provisions of Nevada law may have an anti-takeover effect and may delay or prevent a tender offer or other acquisition transaction that a stockholder might consider to be in his or her best interest. The summary of the provisions of Nevada law set forth below does not purport to be complete and is qualified in its entirety by reference to Nevada law.
The issuance of shares of preferred stock, the issuance of rights to purchase such shares, and the imposition of certain other adverse effects on any party contemplating a takeover could be used to discourage an unsolicited acquisition proposal. For instance, the issuance of our Preferred Stock if the option to acquire such shares is exercised would impede a business combination by the voting and conversion rights that would enable a holder to block such a transaction. In addition, under certain circumstances, the issuance of Preferred Stock could adversely affect the voting power of holders of our common stock.
57
Under Nevada law, a director, in determining what he reasonably believes to be in or not opposed to the best interests of the corporation, does not need to consider only the interests of the corporation’s stockholders in any takeover matter but may also, in his discretion, may consider any of the following:
| ● | The interests of the corporation’s employees, suppliers, creditors and customers; |
| ● | The economy of the state and nation; |
| ● | The impact of any action upon the communities in or near which the corporation’s facilities or operations are located; |
| ● | The long-term interests of the corporation and its stockholders, including the possibility that those interests may be best served by the continued independence of the corporation; and |
| ● | Any other factors relevant to promoting or preserving public or community interests. |
Because our Board of Directors is not required to make any determination on matters affecting potential takeovers solely based on its judgment as to the best interests of our stockholders, our board could act in a manner that would discourage an acquisition attempt or other transaction that some, or a majority, of our stockholders might believe to be in their best interests or in which such stockholders might receive a premium for their stock over the then market price of such stock. Our board presently does not intend to seek stockholder approval prior to the issuance of currently authorized stock, unless otherwise required by law or applicable stock exchange rules.
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES LIABILITIES
In accordance with the provisions in our Articles of Incorporation, we will indemnify an officer, director, or former officer or director, to the full extent permitted by law.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to our directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by us of expenses incurred or paid by a director, officer or controlling person of us in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this Offering, there has been a limited market for our common stock. Future sales of substantial amounts of our common stock, or securities or instruments convertible into our common stock, in the public market, or the perception that such sales may occur, could adversely affect the market price of our common stock prevailing from time to time. Furthermore, because there will be limits on the number of shares available for resale shortly after this Offering due to contractual and legal restrictions described below, there may be resales of substantial amounts of our common stock in the public market after those restrictions lapse. This could adversely affect the market price of our common stock prevailing at that time.
Upon completion of this Offering, assuming the maximum number of shares of common stock offered in this Offering are sold, there will be 123,139,165 shares of our common stock outstanding.
58
Rule 144
The shares of common stock sold in this Offering are not considered “restricted securities” under Securities Act Rule 144. As a result, sales of the securities by persons who are not affiliates of the Company would not be subject to any transfer restrictions under Rule 144. Affiliates will continue to be subject to the limitations of Rule 144, other than the holding period requirement. A person who is an affiliate of ours at such time or during the prior 90 days would be entitled to sell within any three-month period only a number of shares that does not exceed 1% of the number of shares of our common stock then outstanding.
Transfer Agent
Below is the name, mailing address, phone and fax numbers and website of our transfer agent:
Action Stock Transfer
2469 E. Fort Union Blvd, Suite 214
Salt Lake City, UT 84121
T: (801) 274-1088
F: (801) 274-1099
www.actionstocktransfer.com
Certain legal matters with respect to the shares of common stock offered hereby will be passed upon by Philip Magri of Magri Law, LLC, Fort Lauderdale, FL 33334.
The financial statements of the Company appearing elsewhere in this Offering Circular have been audited by Fruci & Associates II, PLLC, an independent accounting firm.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a Regulation A Offering Statement on Form 1-A under the Securities Act with respect to the Shares of common stock offered hereby. This Offering Circular, which constitutes a part of the Offering Statement, does not contain all of the information set forth in the Offering Statement or the exhibits and schedules filed therewith. For further information about us and the common stock offered hereby, we refer you to the Offering Statement and the exhibits and schedules filed therewith. Statements contained in this Offering Circular regarding the contents of any contract or other document that is filed as an exhibit to the Offering Statement are not necessarily complete, and each such statement is qualified in all respects by reference to the full text of such contract or other document filed as an exhibit to the Offering Statement. Upon the completion of this Offering, we will be required to file periodic reports, proxy statements, and other information with the SEC pursuant to the Securities Exchange Act of 1934. You may read and copy this information at the SEC’s Public Reference Room, 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements and other information about issuers, including us, that file electronically with the SEC. The address of this site is www.sec.gov.
59
REMSLEEP HOLDINGS, INC
60

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of REMSleep Holdings, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of REMSleep Holdings, Inc. (“the Company”) as of December 31, 2018 and 2017, and the related statements of operations, stockholders’ equity (deficit), and cash flows for each of the years in the two-year period ended December 31, 2018, and the related notes (collectively referred to as the financial statements). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2018 and 2017, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2018, in conformity with accounting principles generally accepted in the United States of America.
Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has an accumulated deficit, net losses, and negative cash flows from operations. These factors raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audit. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audit, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audit also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides a reasonable basis for our opinion.
/s/ Fruci & Associates II, PLLC
We have served as the Company’s auditor since 2018.
Spokane, Washington
March 28, 2019
61
BALANCE SHEETS
December 31, 2018 | December 31, 2017 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 16,640 | $ | 2,014 | ||||
| Prepaid expenses | 2,000 | 173,282 | ||||||
| Total current assets | 18,640 | 175,296 | ||||||
| Property and equipment, net | 38,436 | 8,486 | ||||||
| Total Assets | $ | 57,076 | $ | 183,782 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable | $ | 240,399 | $ | 239,878 | ||||
| Accrued compensation | - | 2,850 | ||||||
| Accrued interest | 18,508 | 12,341 | ||||||
| Accrued stock to be issued | - | 194,068 | ||||||
| Convertible Notes, net of discount of $33,759 | 43,241 | - | ||||||
| Derivative Liability | 96,110 | - | ||||||
| Loan payable – related party | 179,191 | 182,191 | ||||||
| Loans payable | 59,712 | 50,000 | ||||||
| Total Liabilities | 637,161 | 681,328 | ||||||
| Commitments and Contingencies | - | - | ||||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Series A preferred stock, no par value, 5,000,000 shares authorized, 3,500,000 and 3,500,000 issued and outstanding, respectively | 105,000 | 105,000 | ||||||
| Series B preferred stock, no par value, 5,000,000 shares authorized, no shares issued | - | - | ||||||
| Series C preferred stock, no par value, 5,000,000 shares authorized, no shares issued | - | - | ||||||
| Common stock, $.001 par value, 1,000,000,000 shares authorized, 4,315,894 and 3,610,751 shares issued and outstanding, respectively | 4,316 | 3,611 | ||||||
| Common stock to be issued | 228,604 | 58,225 | ||||||
| Additional paid in capital | 584,017 | 424,938 | ||||||
| Accumulated Deficit | (1,502,022 | ) | (1,089,320 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | (580,085 | ) | (497,546 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 57,076 | $ | 183,782 | ||||
The accompanying notes are an integral part of these financial statements.
62
STATEMENTS OF OPERATIONS
| For the Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Operating Expenses: | ||||||||
| Professional fees | $ | 43,900 | $ | 86,212 | ||||
| Consulting | 175,582 | 516,396 | ||||||
| Officer compensation | 38,650 | 24,000 | ||||||
| General and administrative | 36,249 | 24,857 | ||||||
| Total operating expenses | 294,381 | 651,465 | ||||||
| Loss from operations | (294,381 | ) | (651,465 | ) | ||||
| Other expense: | ||||||||
| Interest expense | (6,875 | ) | (2,500 | ) | ||||
| Discount amortization | (40,441 | ) | - | |||||
| Loss on issuance of convertible debt | (47,020 | ) | - | |||||
| Change in fair value of derivative | (23,985 | ) | - | |||||
| Total other expense | (118,321 | ) | (2,500 | ) | ||||
| Loss before income taxes | (412,702 | ) | (653,965 | ) | ||||
| Provision for income taxes | - | - | ||||||
| Net Loss | $ | (412,702 | ) | $ | (653,965 | ) | ||
| Net loss per share, basic and diluted | $ | (0.10 | ) | $ | (0.19 | ) | ||
| Weighted average common shares outstanding, basic and diluted | 4,065,317 | 3,391,703 | ||||||
The accompanying notes are an integral part of these financial statements.
63
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE YEARS ENDED DECEMBER 31, 2018 AND 2017
| Series A Preferred Shares | Series A Preferred Stock Amount | Common Shares | Common Stock Amount | Common stock to be issued | Additional Paid-in Capital | Accumulated Deficit | Total | |||||||||||||||||||||||||
| Balance, December 31, 2016 | 5,000,000 | $ | 105,000 | 3,273,111 | $ | 3,273 | $ | - | $ | (31,599 | ) | $ | (435,355 | ) | $ | (358,681 | ) | |||||||||||||||
| Cancellation of outstanding stock | (1,500,000 | ) | - | (150,000 | ) | (150 | ) | - | 150 | - | - | |||||||||||||||||||||
| Common stock issued for services | - | - | 487,640 | 488 | 58,225 | 456,387 | - | 515,100 | ||||||||||||||||||||||||
| Net loss for the year ended December 31, 2017 | - | - | - | - | - | - | (653,965 | ) | (653,965 | ) | ||||||||||||||||||||||
| Balance, December 31, 2017 | 3,500,000 | 105,000 | 3,610,751 | 3,611 | 58,225 | 424,938 | (1,089,320 | ) | (497,546 | ) | ||||||||||||||||||||||
| Common stock sold for cash | - | - | 477,143 | 477 | - | 89,523 | - | 90,000 | ||||||||||||||||||||||||
| Common stock issued for services | - | - | 228,000 | 228 | 170,379 | 42,356 | 212,963 | |||||||||||||||||||||||||
| Beneficial Conversion feature | - | - | - | - | - | 27,200 | - | 27,200 | ||||||||||||||||||||||||
| Net loss for the year ended December 31, 2018 | - | - | - | - | - | - | (412,702 | ) | (412,702 | ) | ||||||||||||||||||||||
| Balance, December 31, 2018 | 3,500,000 | $ | 105,000 | 4,315,894 | $ | 4,316 | $ | 228,604 | $ | 584,017 | $ | (1,502,022 | ) | $ | (580,085 | ) | ||||||||||||||||
The accompanying notes are an integral part of these financial statements.
64
STATEMENTS OF CASH FLOWS
| For the Years Ended December 31, | ||||||||
| 2018 | 2017 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (412,702 | ) | $ | (653,965 | ) | ||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation expense | 12,576 | 4,359 | ||||||
| Stock based compensation | 175,282 | 535,886 | ||||||
| Change in fair value of derivative | 23,985 | - | ||||||
| Discount amortization | 40,441 | - | ||||||
| Loss on issuance of convertible debt | 47,020 | - | ||||||
| Changes in Operating Assets and Liabilities: | ||||||||
| Accounts payable | 520 | 13,480 | ||||||
| Accrued officer compensation | (2,850 | ) | 2,850 | |||||
| Accrued interest | 6,167 | 2,500 | ||||||
| Prepaid expenses | (2,000 | ) | - | |||||
| Net cash used by operating activities | (111,561 | ) | (94,890 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of property and equipment | (42,526 | ) | - | |||||
| Net cash used by investing activities | (42,526 | ) | - | |||||
| Cash Flows from Financing Activities: | ||||||||
| Proceeds/repayments – related party | (3,000 | ) | 96,904 | |||||
| Proceeds from loan payable | 16,963 | - | ||||||
| Repayment of loans | (7,250 | ) | - | |||||
| Proceeds from convertible notes payable | 72,000 | - | ||||||
| Proceeds from sale of common stock | 90,000 | - | ||||||
| Net cash provided by financing activities | 168,713 | 96,904 | ||||||
| Net increase in cash | 14,626 | 2,014 | ||||||
| Cash at beginning of the year | 2,014 | - | ||||||
| Cash at end of the year | $ | 16,640 | $ | 2,014 | ||||
| Supplemental cash flow information: | ||||||||
| Interest paid in cash | $ | - | $ | - | ||||
| Taxes paid | $ | - | $ | - | ||||
| Supplemental non-cash disclosure: | ||||||||
| Loan payable for purchase of automobile | $ | 16,936 | $ | - | ||||
The accompanying notes are an integral part of these financial statements.
65
NOTES TO FINANCIAL STATEMENTS
December 31, 2018
NOTE 1 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business Activity
REMSleep Holdings, Inc., (the “Company”) was incorporated in the State of Nevada on June 6, 2007. On January 5, 2015 the name of the Company was changed to REMSleep Holdings, Inc. and the business model was changed to reflect the new direction of the Company; to develop and distribute products to help people affected by sleep apnea. On May 30, 2015 REMSleep LLC was formally merged into REMSleep Holdings, Inc.
Basis of Presentation
The Company’s financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Concentrations of Credit Risk
We maintain our cash in bank deposit accounts, the balances of which at times may exceed federally insured limits. We continually monitor our banking relationships and consequently have not experienced any losses in our accounts. We believe we are not exposed to any significant credit risk on cash.
Cash equivalents
The Company considers all highly liquid investments with a maturity of three months or less when purchased to be cash equivalents. There were no cash equivalents for the year ended December 31, 2018 or 2017.
Fair value of financial instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three (3) broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three (3) levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
Level 1: Quoted market prices available in active markets for identical assets or liabilities as of the reporting date.
Level 2: Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date.
Level 3: Pricing inputs that are generally unobservable inputs and not corroborated by market data.
The carrying amount of the Company’s financial assets and liabilities, such as cash, prepaid expenses and accrued expenses approximate their fair value because of the short maturity of those instruments. The Company’s notes payable approximates the fair value of such instruments based upon management’s best estimate of interest rates that would be available to the Company for similar financial arrangements at December 31, 2018.
The following table presents assets and liabilities that are measured and recognized at fair value as of December 31, 2018 on a recurring basis:
| Description | Level 1 | Level 2 | Level 3 | Total Gains and (Losses) | ||||||||||||
| Derivative | - | - | 96,110 | (23,985 | ) | |||||||||||
| Total | $ | - | $ | - | $ | 96,110 | $ | (23,985 | ) | |||||||
66
Fixed Assets
Fixed assets are carried at the lower of cost or net realizable value. All fixed assets with a cost of $2,000 or greater are capitalized. Major betterments that extend the useful lives of assets are also capitalized. Normal maintenance and repairs are charged to expense as incurred. When assets are sold or otherwise disposed of, the cost and accumulated depreciation are removed from the accounts and any resulting gain or loss is recognized in operations.
Depreciation is computed using the straight-line method over the estimated useful lives of three years.
Income taxes
The Company follows Section 740-10-30 of the FASB Accounting Standards Codification, which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are based on the differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the fiscal year in which the differences are expected to reverse. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the fiscal years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the Statements of Income in the period that includes the enactment date.
The Company adopted section 740-10-25 of the FASB Accounting Standards Codification (“Section 740-10-25”) with regards to uncertainty income taxes. Section 740-10-25 addresses the determination of whether tax benefits claimed or expected to be claimed on a tax return should be recorded in the financial statements. Under Section 740-10-25, the Company may recognize the tax benefit from an uncertain tax position only if it is more likely than not that the tax position will be sustained on examination by the taxing authorities, based on the technical merits of the position. The tax benefits recognized in the financial statements from such a position should be measured based on the largest benefit that has a greater than fifty percent (50%) likelihood of being realized upon ultimate settlement. Section 740-10-25 also provides guidance on de-recognition, classification, interest and penalties on income taxes, accounting in interim periods and requires increased disclosures. The Company had no material adjustments to its liabilities for unrecognized income tax benefits according to the provisions of Section 740-10-25.
Stock-based Compensation
We account for equity-based transactions with nonemployees under the provisions of ASC Topic No. 505-50, Equity-Based Payments to Non-Employees (“ASC 505-50”). ASC 505-50 establishes that equity-based payment transactions with nonemployees shall be measured at the fair value of the consideration received or the fair value of the equity instruments issued, whichever is more reliably measurable. The fair value of common stock issued for payments to nonemployees is measured at the market price on the date of grant. The fair value of equity instruments, other than common stock, is estimated using the Black-Scholes option valuation model. In general, we recognize the fair value of the equity instruments issued as deferred stock compensation and amortize the cost over the term of the contract.
We account for employee stock-based compensation in accordance with the guidance of FASB ASC Topic 718, Compensation—Stock Compensation, which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on their fair values. The fair value of the equity instrument is charged directly to compensation expense and credited to additional paid-in capital over the period during which services are rendered.
Basic and Diluted Earnings Per Share
Net income (loss) per common share is computed pursuant to section 260-10-45 of the FASB Accounting Standards Codification. Basic net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock outstanding during the period. Diluted net income (loss) per common share is computed by dividing net income (loss) by the weighted average number of shares of common stock and potentially outstanding shares of common stock during the period. The weighted average number of common shares outstanding and potentially outstanding common shares assumes that the Company incorporated as of the beginning of the first period presented.
The Company’s diluted loss per share is the same as the basic loss per share for the years ended December 31, 2018 and 2017, as the inclusion of any potential shares would have had an anti-dilutive effect due to the Company generating a loss.
Recent Accounting Pronouncements
In February 2016, the FASB issued ASU 2016-02, Leases (Topic 842). ASU 2016-02 requires lessees to recognize lease assets and lease liabilities on the balance sheet and requires expanded disclosures about leasing arrangements. The new standard supersedes the present U.S. GAAP standard on leases and requires substantially all leases to be reported on the balance sheet as right-of-use assets and lease obligations. ASU 2016-02 is effective for fiscal years beginning after December 15, 2018 and interim periods in fiscal years beginning after December 15, 2018, with early adoption permitted. At this point in time we do not expect any material impact on our financial statements as a result of adopting this standard.
67
Topic 606, Revenue from Contracts with Customers, of the Financial Accounting Standards Board’s (FASB) Accounting Standards Codification (ASC). The guidance in ASC 606 was originally issued by the FASB in May 2014 in Accounting Standards Update (ASU) 2014-09, Revenue from Contracts with Customers (Topic 606). Since then, the FASB has issued several ASUs that have revised or clarified the guidance in ASC 606. The Company has evaluated the impact of this accounting standard update and noted that it has had no material impact.
On June 20, 2018, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2018-07, Compensation—Stock Compensation (Topic 718): Improvements to Nonemployee Share-Based Payment Accounting. ASU 2018-07 is intended to reduce cost and complexity and to improve financial reporting for share-based payments to nonemployees (for example, service providers, external legal counsel, suppliers, etc.). Under the new standard, companies will no longer be required to value non-employee awards differently from employee awards. Meaning that companies will value all equity classified awards at their grant-date under ASC718 and forgo revaluing the award after this date. The Company has chosen to early adopt this standard.
In January 2017, the Financial Accounting Standards Board (“FASB”) issued an Accounting Standards Update (“ASU”) 2017-01, Business Combinations (Topic 805) Clarifying the Definition of a Business. The amendments in this update clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions or disposals of assets or businesses. The definition of a business affects many areas of accounting including acquisitions, disposals, goodwill, and consolidation. The guidance is effective for interim and annual periods beginning after December 15, 2017 and should be applied prospectively on or after the effective date. The Company is in the process of evaluating the impact of this accounting standard update.
The Company has implemented all new accounting pronouncements that are in effect. These pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
NOTE 2 - GOING CONCERN
The accompanying financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has an accumulated deficit of $1,502,022 at December 31, 2018, had a net loss of $412,702 and net cash used in operating activities of $111,560 for the year ended December 31, 2018. The Company’s ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. These conditions and the ability to successfully resolve these factors over the next twelve months raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
NOTE 3 - PROPERTY & EQUIPMENT
Property and Equipment are first recorded at cost. Depreciation is computed using the straight-line method over the estimated useful lives of the various classes of assets as follows between three and five years.
Long lived assets, including property and equipment, to be held and used by the Company are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Impairment losses are recognized if expected future cash flows of the related assets are less than their carrying values. Measurement of an impairment loss is based on the fair value of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Maintenance and repair expenses, as incurred, are charged to expense. Betterments and renewals are capitalized in plant and equipment accounts. Cost and accumulated depreciation applicable to items replaced or retired are eliminated from the related accounts with any gain or loss on the disposition included as income.
Property and equipment stated at cost, less accumulated depreciation consisted of the following:
| December 31, 2018 | December 31, 2017 | |||||||
| Furniture/fixtures | $ | 14,904 | $ | 14,904 | ||||
| Office equipment | 2,458 | - | ||||||
| Automobile | 16,963 | - | ||||||
| Tooling/Molds | 23,105 | - | ||||||
| Less: accumulated depreciation | (18,994 | ) | (6,418 | ) | ||||
| Fixed assets, net | $ | 38,436 | $ | 8,486 | ||||
Depreciation expense
Depreciation expense for the years ended December 31, 2018 and 2017 was $12,576 and $4,359, respectively.
68
NOTE 4 - LOANS PAYABLE
On October 24, 2017, the Company was notified that a petition had been filed in the Iowa District Court for Polk County by a Mr. John M. Wesson for failure to repay a loan. Mr. Wesson had loaned the Company $30,000 and $20,000 on October 24, 2012 and June 12, 2013, respectively. The loans were to accrue interest at 5%. While the Company was under previous management the loans were removed from the books in Q1 of 2015. On April 26, 2018, the Company agreed to repay the loan in full including accrued interest and $5,000 for legal fees. The $50,000 plus $7,341 was booked to retained earnings in 2016 as a correction of an error. As of December 31, 2018, there is $14,841 of interest accrued on the loan.
On March 23, 2018, the Company purchased an automobile. The purchase price was $16,963.46. The interest rate on the loan is 5.8% and matures on April 7, 2023. Payments on the loan, consisting of principal and interest, are $327 per month.
NOTE 5 - CONVERTIBLE NOTES
On July 9, 2018, the Company issued a Convertible Promissory Note in favor of Power Up Lending Group LTD (“Power Up”). The principal amount of the Note is $45,000 with an original issue discount of $3,000 and carries an interest rate of 12% per annum. It becomes due and payable with accrued interest on July 9, 2019. Power Up has the option to convert the Note plus accrued interest into common shares of the Company, after 180 days. The conversion rate is a 39% discount to the average of the lowest two trading price for twenty days prior to the date of conversion. The company bifurcated the conversion feature and accounted for it as a derivative liability. The Company recorded the derivative liability at its fair value of $89,020 based on the Black Scholes Merton pricing model and a corresponding debt discount of $42,000 to be amortized utilizing the interest method of accretion over the term of the note. As of December 31, 2018, the Company fair valued the derivative at $96,110. In addition, $21,575 of the debt discount has been amortized to interest expense.
A summary of the activity of the derivative liability for the notes above is as follows:
| Balance at December 31, 2017 | $ | - | ||
| Increase to derivative due to new issuances | $ | 89,020 | ||
| Derivative loss due to mark to market adjustment | $ | 7,090 | ||
| Balance at December 31, 2018 | $ | 96,110 |
A summary of quantitative information about significant unobservable inputs (Level 3 inputs) used in measuring the Company’s derivative liability that are categorized within Level 3 of the fair value hierarchy for the year ended December 31, 2018 is as follows:
| Inputs | December 31, 2018 | Initial Valuation | ||||||
| Stock price | $ | .06 | $ | .55 | ||||
| Conversion price | $ | .0244 | $ | .244 | ||||
| Volatility (annual) | 342.93 | % | 261.04 | % | ||||
| Risk-free rate | 2.56 | % | 2.34 | % | ||||
| Years to maturity | .52 | 1 | ||||||
The development and determination of the unobservable inputs for Level 3 fair value measurements and fair value calculations are the responsibility of the Company’s management. The Company noted that there was no material difference between the results of the Black Scholes Pricing Model and that of the Binomial Option Pricing Model.
On August 30, 2018, the Company issued a Convertible Promissory Note in favor of LG Capital Funding LLC (“LG”). The principal amount of the Note is $32,000 with an original issue discount of $2,000 and carries an interest rate of 10% per annum. It becomes due and payable with accrued interest on August 30, 2019. During the first six months LG has the option to convert the Note plus accrued interest at a fixed price of $0.10 per share. After the 6-month anniversary, the Conversion Price shall be equal to 60% of the lowest closing bid price for the eighteen prior trading days including the day of conversion. The Company accounted for the initial conversion feature as a beneficial conversion feature. A beneficial conversion feature arises when the conversion price of a convertible instrument is below the per share fair value of the underlying stock into which it is convertible. If LG were to convert at the price of $0.10, they could convert the full $32,000 into 320,000 shares of common stock. Using the stock price on the date the note was issued of $.185 and the conversion price of $.10, the Company valued each share at $.085 for an additional expense of $27,200. The Company has accounted for the $27,200 has debt discount with a credit to additional paid in capital. The discount will be amortized over the term of the note. As of December 31, 2018, $13,659 has been amortized to interest expense.
69
NOTE 6 - RELATED PARTY TRANSACTIONS
The Company has received support from parties related through common ownership and directorship. All of the expenses herein have been borne by these individuals on behalf of the Company and are treated as shareholder loans. These loans are unsecured, non-interest bearing and due on demand. As of December 31, 2018, and, 2017, the balance due on these loans is $179,191 and $182,191, respectively.
NOTE 7 - COMMON STOCK
On January 15, 2017, the Company issued, as compensation for services provided, 5,000 common shares with a fair value of $4.25 ($0.2125 pre-split) for total non-cash expense of $21,250. The $21,250 was recognized over the six-month term of the contract. As of December 31, 2017, all $21,250 has been expensed.
On March 6, 2017, the Company issued, as compensation for services provided, 32,500 common shares with a fair value of $4.25 ($0.2125 pre-split) for total non-cash expense of $138,125.
On June 15, 2017, the Company filed a Certificate of Amendment to its Articles of Incorporation (the “Certificate of Amendment”), with the Secretary of State of the State of Nevada to affect a 1-for-20 reverse stock split of its common stock, whereby every twenty shares of existing common stock will be converted into one share of new common stock.
On April 1, 2017, the Company entered into a Fee Agreement with Frederick M. Lehrer to provide legal services to the Company. Per the terms of that agreement Mr. Lehrer was granted 5,000 shares of common stock with a fair value of $4.25 for total non-cash expense of $21,250. As of December 31, 2018, the shares have not yet been issued by the transfer agent; so therefore, have been credited to common stock to be issued.
On April 10, 2017, the Company issued, as compensation for services provided, 50,000 common shares with a fair value of $4.25 for total non-cash expense of $212,500.
In April 2017, with the agreement of the executive of the Company’s previous management, the Company cancelled 150,000 common shares that had been previously issued to him.
On June 29, 2017, FINRA approved the Company’s Reverse Stock Split. The Reverse Stock Split took effect at the open of business on June 30, 2017. All shares through these financial statements have been retroactively adjusted to reflect the reverse.
On August 1, 2017, the Company issued, as compensation for services provided, 150,000 common shares with a fair value of $0.2125 for total non-cash expense of $31,875.
On August 11, 2017, the Company issued, as compensation for services provided, 250,000 common shares with a fair value of $0.2125 for total non-cash expense of $53,125.
On November 16, 2017, the Company issued, as compensation for services provided, 1,087,261 common shares with a fair value of $0.2125 per share for total non-cash expense of $231,043. The expense is being recognized over the six-month term of the contract. As of December 31, 2017, $57,761 has been debited to consulting expense. In addition, as of December 31, 2017, the shares have not yet been issued; as a result, $36,975 has been credited to common stock to be issued and the remaining $194,068 to accruals. As of September 30, 2018, the shares were re-valued at $0.231 for a change in value of $16,895. In addition, $173,282 was amortized to stock for services expense and the accrual for stock to be issued was reclassed to common stock to be issued in equity, which was reduced $40,584 for the issuance of 178,000 shares by the transfer agent. As of December 31, 2018, the full amount has been amortized to stock compensation expense.
During the year ended December 31, 2018, the Company sold 477,143 shares of common stock for total cash proceeds of $90,000.
During the nine months ended September 30, 2018, the Company granted 1,760,000 shares of common stock for services at $0.25 per share for total non-cash expense of $440,000. Subsequent to the year ended December 31, 2018 all of the shares were returned. As the expense that was recorded was material to the financial statements, the fact that the services agreed upon were not performed and that all shares were returned to the Company, the previous $440,000 of expense that was recorded as of September 30, 2018, has been reversed as of December 31, 2018.
70
NOTE 8 - PREFERRED STOCK
The Company is currently authorized to issue 5,000,000 Class A preferred shares, $0.001 par value with 1:25 voting rights. The Series A Preferred Stock ranks equal to the common stock on liquidation and pays no dividend.
In April 2017, with the agreement of the executive of the Company’s previous management, the Company cancelled 1,500,000 Class A Preferred Shares that had been previously issued to him in 2015.
The Company is currently authorized to issue 5,000,000 Class B Preferred Shares, $0.001 par value. Each share of Series B Preferred Stock has a 1:100 voting right and is convertible into 100 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are no shares of Series B Preferred Stock issued and outstanding.
The Company is currently authorized to issue 5,000,000 Class C Preferred Shares, $0.001 par value. Each share of Series C Preferred Stock has a 1:50 voting right and is convertible into 50 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are no shares of Series C Preferred Stock issued and outstanding.
NOTE 9 - INCOME TAX
Deferred taxes are provided on a liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carry forwards and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of changes in tax laws and rates on the date of enactment. The U.S. federal income tax rate is 21%.
The provision for Federal income tax consists of the following December 31:
| 2018 | 2017 | |||||||
| Federal income tax benefit attributable to: | ||||||||
| Current Operations | $ | 87,000 | $ | 137,000 | ||||
| Less: valuation allowance | (87,000 | ) | (137,000 | ) | ||||
| Net provision for Federal income taxes | $ | - | $ | - | ||||
The cumulative tax effect at the expected rate of 21% of significant items comprising our net deferred tax amount is as follows:
| 2018 | 2017 | |||||||
| Deferred tax asset attributable to: | ||||||||
| Net operating loss carryover | $ | 315,000 | $ | 229,000 | ||||
| Less: valuation allowance | (315,000 | ) | (229,000 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
At December 31, 2018, the Company had net operating loss carry forwards of approximately $315,000 that maybe offset against future taxable income. No tax benefit has been reported in the December 31, 2018 or 2017 financial statements since the potential tax benefit is offset by a valuation allowance of the same amount. The change in the valuation allowance for the year ended December 31, 2018 was a decrease of $50,000.
On December 22, 2017, the U.S. government enacted comprehensive tax legislation commonly referred to as the Tax Cut and Jobs Act (the “Tax Act”). The Tax Act establishes new tax laws that affects 2018 and future years, including a reduction in the U.S. federal corporate income tax rate to 21% effective January 1, 2018. For certain deferred tax assets and deferred tax liabilities.
Due to the change in ownership provisions of the Tax Reform Act of 1986, net operating loss carry forwards for Federal income tax reporting purposes are subject to annual limitations. Should a change in ownership occur, net operating loss carry forwards may be limited as to use in future years.
ASC Topic 740 provides guidance on the accounting for uncertainty in income taxes recognized in a company’s financial statements. Topic 740 requires a company to determine whether it is more likely than not that a tax position will be sustained upon examination based upon the technical merits of the position. If the more-likely-than-not threshold is met, a company must measure the tax position to determine the amount to recognize in the financial statements.
The Company includes interest and penalties arising from the underpayment of income taxes in the statements of operations in the provision for income taxes. As of December 31, 2018, the Company had no accrued interest or penalties related to uncertain tax positions.
71
NOTE 10 - SUBSEQUENT EVENTS
In accordance with SFAS 165 (ASC 855-10) management has performed an evaluation of subsequent events through the date that the financial statements were available to be issued and has determined that it does not have any material subsequent events to disclose in these financial statements other then the following.
Subsequent to December 31, 2018, PowerUp converted $7,940 into 303,053 shares of common stock.
On January 23, 2019, the Company issued a Convertible Promissory Note in favor of One44 Capital LLC (“One44”). The principal amount of the Note is $100,000 with an interest rate of 12% per annum. It becomes due and payable with accrued interest on January 23, 2020. Power Up has the option to convert the Note plus accrued interest into common shares of the Company, after 180 days. The conversion rate is a 55% of the lowest two trading prices for twenty days prior to the date of conversion.
72
CONDENSED BALANCE SHEETS
| June
30, 2019 | December
31, 2018 | |||||||
| (Unaudited) | ||||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 181,763 | $ | 16,640 | ||||
| Prepaid expenses | 18,719 | 2,000 | ||||||
| Total current assets | 200,482 | 18,640 | ||||||
| Other asset | 10,000 | - | ||||||
| Property and equipment, net | 62,559 | 38,436 | ||||||
| Total Assets | $ | 273,041 | $ | 57,076 | ||||
| LIABILITIES AND STOCKHOLDERS’ DEFICIT | ||||||||
| Current Liabilities: | ||||||||
| Accounts payable | $ | 240,754 | $ | 240,399 | ||||
| Accrued compensation | 14,500 | - | ||||||
| Accrued interest | 24,770 | 18,508 | ||||||
| Accrued interest – related party | 11,107 | - | ||||||
| Convertible Notes, net of discount of $241,950 and $33,759 | 69,490 | 43,241 | ||||||
| Derivative Liability | 746,695 | 96,110 | ||||||
| Loan payable – related party | 179,191 | 179,191 | ||||||
| Loans payable | 58,157 | 59,712 | ||||||
| Total Current Liabilities | 1,344,664 | 637,161 | ||||||
| Total Liabilities | 1,344,664 | 637,161 | ||||||
| Commitments and Contingencies | - | - | ||||||
| STOCKHOLDERS’ DEFICIT: | ||||||||
| Series A preferred stock, no par value, 5,000,000 shares authorized, 4,000,000 and 3,500,000 issued and outstanding, respectively | 125,000 | 105,000 | ||||||
| Series B preferred stock, no par value, 5,000,000 shares authorized, no shares issued | - | - | ||||||
| Series C preferred stock, no par value, 5,000,000 shares authorized, no shares issued | - | - | ||||||
| Common stock, $.001 par value, 1,000,000,000 shares authorized, 66,030,419 and 4,315,894 shares issued and outstanding, respectively | 66,030 | 4,316 | ||||||
| Common stock to be issued | - | 228,604 | ||||||
| Additional paid in capital | 3,177,857 | 584,017 | ||||||
| Accumulated Deficit | (4,440,510 | ) | (1,502,022 | ) | ||||
| TOTAL STOCKHOLDERS’ DEFICIT | (1,071,623 | ) | (580,085 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS’ DEFICIT | $ | 273,041 | $ | 57,076 | ||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
73
CONDENSED STATEMENTS OF OPERATIONS
(UNAUDITED)
| For the Three Months Ended June 30, | For the Six Months Ended June 30, | |||||||||||||||
| 2019 | 2018 | 2019 | 2018 | |||||||||||||
| Operating Expenses: | ||||||||||||||||
| Professional fees | $ | 9,900 | $ | 19,950 | $ | 25,400 | $ | 36,100 | ||||||||
| Consulting | 17,720 | 266,278 | 17,720 | 383,799 | ||||||||||||
| Compensation – related party | 2,044,000 | 12,000 | 2,062,000 | 18,000 | ||||||||||||
| General and administrative | 17,759 | 8,762 | 34,128 | 13,701 | ||||||||||||
| Total operating expenses | 2,089,379 | 306,990 | 2,139,248 | 451,600 | ||||||||||||
| Loss from operations | (2,089,379 | ) | (306,990 | ) | (2,139,248 | ) | (451,600 | ) | ||||||||
| Other expenses: | ||||||||||||||||
| Interest expense | (11,678 | ) | (623 | ) | (22,165 | ) | (1,239 | ) | ||||||||
| Discount amortization | (58,106 | ) | - | (102,059 | ) | - | ||||||||||
| Loss on issuance of convertible debt | (925,498 | ) | - | (1,051,207 | ) | - | ||||||||||
| Change in fair value | 737,905 | - | 376,191 | (16,895 | ) | |||||||||||
| Total other expense | (257,377 | ) | (623 | ) | (799,240 | ) | (18,134 | ) | ||||||||
| Loss before income taxes | (2,346,756 | ) | (307,613 | ) | (2,938,488 | ) | (469,734 | ) | ||||||||
| Provision for income taxes | - | - | - | - | ||||||||||||
| Net Loss | $ | (2,346,756 | ) | $ | (307,613 | ) | $ | (2,938,488 | ) | $ | (469,734 | ) | ||||
| Basic and fully diluted net loss per share | $ | (0.11 | ) | $ | (0.05 | ) | $ | (0.22 | ) | $ | (0.10 | ) | ||||
| Weighted average common shares outstanding, basic and diluted | 20,993,944 | 5,759,828 | 13,108,972 | 4,666,617 | ||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
74
STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT)
FOR THE SIX MONTHS ENDED JUNE 30, 2018
(UNAUDITED)
| Series A Preferred Shares | Series A Preferred Stock Amount | Common Shares | Common Stock Amount | Common stock to be issued | Additional Paid-in Capital | Accumulated Deficit | Total | |||||||||||||||||||||||||
| Balance, December 31, 2017 | 3,500,000 | $ | 105,000 | 3,610,751 | $ | 3,611 | $ | 58,225 | $ | 424,938 | $ | (1,089,320 | ) | $ | (497,546 | ) | ||||||||||||||||
| Common stock sold for cash | - | - | 327,143 | 327 | - | 77,173 | - | 77,500 | ||||||||||||||||||||||||
| Common stock issued for services | - | - | - | - | 210,963 | - | - | 210,963 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (162,121 | ) | (162,121 | ) | ||||||||||||||||||||||
| Balance, March 31, 2018 | 3,500,000 | 105,000 | 3,937,894 | 3,938 | 269,188 | 502,111 | (1,251,441 | ) | (371,204 | ) | ||||||||||||||||||||||
| Common stock issued | - | - | 178,000 | 178 | (40,584 | ) | 40,406 | - | - | |||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (307,613 | ) | (307,613 | ) | ||||||||||||||||||||||
| Balance, June 30, 2018 | 3,500,000 | $ | 105,000 | 4,115,894 | $ | 4,116 | $ | 228,604 | $ | 542,517 | $ | (1,559,054 | ) | $ | (678,817 | ) | ||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
75
REMSLEEP HOLDINGS, INC. STATEMENT OF STOCKHOLDERS’ EQUITY (DEFICIT) FOR THE SIX MONTHS ENDED JUNE 30, 2019 (UNAUDITED) |
| Series A Preferred Shares | Series A Preferred Stock Amount | Common Shares | Common Stock Amount | Common stock to be issued | Additional Paid-in Capital | Accumulated Deficit | Total | |||||||||||||||||||||||||
| Balance, December 31, 2018 | 3,500,000 | $ | 105,000 | 4,315,894 | $ | 4,316 | $ | 228,604 | $ | 584,017 | $ | (1,502,022 | ) | $ | (580,085 | ) | ||||||||||||||||
| Common stock issued for conversion of debt | - | - | 1,523,291 | 1,523 | - | 49,604 | - | 51,127 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (591,732 | ) | (591,732 | ) | ||||||||||||||||||||||
| Balance, March 31, 2019 | 3,500,000 | 105,000 | 5,839,185 | 5,839 | 228,604 | 633,621 | (2,093,754 | ) | (1,120,690 | ) | ||||||||||||||||||||||
| Common stock issued for services – related party | - | - | 50,000,000 | 50,000 | - | 1,950,000 | - | 2,000,000 | ||||||||||||||||||||||||
| Preferred stock issued for services - related party | 500,000 | 20,000 | - | - | - | - | - | 20,000 | ||||||||||||||||||||||||
| Common stock issued for services | 1,309,260 | 1,309 | (228,604 | ) | 244,615 | - | 17,320 | |||||||||||||||||||||||||
| Common stock issued for conversion of debt | - | - | 8,881,974 | 8,882 | - | 307,768 | - | 316,650 | ||||||||||||||||||||||||
| Warrants issued with convertible debt | - | - | - | - | - | 41,853 | - | 41,853 | ||||||||||||||||||||||||
| Net loss | - | - | - | - | - | - | (2,346,756 | ) | (2,346,756 | ) | ||||||||||||||||||||||
| Balance, June 30, 2019 | 4,000,000 | $ | 125,000 | 66,030,419 | $ | 66,030 | $ | - | $ | 3,177,857 | $ | (4,440,510 | ) | $ | (1,071,623 | ) | ||||||||||||||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
76
|
CONDENSED STATEMENTS OF CASH FLOWS (UNAUDITED) |
| For
the Six Months Ended June 30, | ||||||||
| 2019 | 2018 | |||||||
| Cash Flows from Operating Activities: | ||||||||
| Net loss | $ | (2,938,488 | ) | $ | (469,734 | ) | ||
| Adjustments to reconcile net loss to net cash used in operations: | ||||||||
| Depreciation expense | 2,804 | 1,032 | ||||||
| Stock compensation expense | 17,320 | 383,499 | ||||||
| Stock compensation expense – related party | 2,020,000 | - | ||||||
| Change in fair value of derivative | (376,191 | ) | 16,895 | |||||
| Discount amortization | 102,059 | - | ||||||
| Loss on issuance of convertible debt | 1,051,207 | - | ||||||
| Changes in Operating Assets and Liabilities | ||||||||
| Prepaids | (3,914 | ) | - | |||||
| Other asset | (10,000 | ) | - | |||||
| Accounts Payable | 356 | 3,256 | ||||||
| Accrued compensation – related party | 14,500 | (500 | ) | |||||
| Accrued interest | 10,845 | 1,239 | ||||||
| Accrued interest – related party | 11,107 | - | ||||||
| Net cash used in operating activities | (98,395 | ) | (64,313 | ) | ||||
| Cash Flows from Investing Activities: | ||||||||
| Purchase of equipment | (26,927 | ) | (6,648 | ) | ||||
| Net Cash used in investing activities | (26,927 | ) | (6,648 | ) | ||||
| Cash Flows from Financing Activities: | ||||||||
| Repayment of loans | (1,555 | ) | (8,000 | ) | ||||
| Proceeds from convertible notes payable | 292,000 | - | ||||||
| Proceeds from sale of common stock | - | 77,500 | ||||||
| Net cash provided by financing activities | 290,445 | 69,500 | ||||||
| Net increase (decrease) in cash | 165,123 | (1,461 | ) | |||||
| Cash at beginning of the period | 16,640 | 2,014 | ||||||
| Cash at end of the period | $ | 181,763 | $ | 553 | ||||
| Supplemental cash flow information: | ||||||||
| Interest paid in cash | $ | - | $ | - | ||||
| Taxes paid | $ | - | $ | - | ||||
| Supplemental non-cash disclosure: | ||||||||
| Common stock issued for conversion of note payable principal and accrued interest | $ | 80,395 | $ | - | ||||
The accompanying notes are an integral part of these unaudited condensed financial statements.
77
NOTES TO CONDENSED FINANCIAL STATEMENTS
June 30, 2019
(Unaudited)
NOTE 1 - BACKGROUND
Business Activity
REMSleep Holdings, Inc., (the “Company”) was incorporated in the State of Nevada on June 6, 2007. On January 5, 2015 the name of the Company was changed to REMSleep Holdings, Inc. and the business model was changed to reflect the new direction of the Company; to develop and distribute products to help people affected by sleep apnea. On May 30, 2015 REMSleep LLC was formally merged into REMSleep Holdings, Inc.
NOTE 2 - SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These unaudited condensed financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”) and the rules and regulations of the Securities and Exchange Commission (“SEC”). These financial statements and the notes attached hereto should be read in conjunction with the financial statements and notes included in the Company’s 10-K for its fiscal year ended December 31, 2018. In the opinion of the Company, all adjustments, including normal recurring adjustments necessary to present fairly the financial position of the Company, as of June 30, 2019 and the results of its operations and cash flows for the three months, then ended have been included. The results of operations for the interim period are not necessarily indicative of the results for the full year ending December 31, 2019.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. Actual results could differ from those estimates.
Fair Value of Financial Instruments
The Company follows paragraph 825-10-50-10 of the FASB Accounting Standards Codification for disclosures about fair value of its financial instruments and paragraph 820-10-35-37 of the FASB Accounting Standards Codification (“Paragraph 820-10-35-37”) to measure the fair value of its financial instruments. Paragraph 820-10-35-37 establishes a framework for measuring fair value in accounting principles generally accepted in the United States of America (U.S. GAAP), and expands disclosures about fair value measurements. To increase consistency and comparability in fair value measurements and related disclosures, Paragraph 820-10-35-37 establishes a fair value hierarchy which prioritizes the inputs to valuation techniques used to measure fair value into three broad levels. The fair value hierarchy gives the highest priority to quoted prices (unadjusted) in active markets for identical assets or liabilities and the lowest priority to unobservable inputs. The three levels of fair value hierarchy defined by Paragraph 820-10-35-37 are described below:
| Level 1: | Quoted market prices available in active markets for identical assets or liabilities as of the reporting date. |
| Level 2: | Pricing inputs other than quoted prices in active markets included in Level 1, which are either directly or indirectly observable as of the reporting date. |
| Level 3: | Pricing inputs that are generally unobservable inputs and not corroborated by market data. |
The carrying amount of the Company’s financial assets and liabilities, such as cash, prepaid expenses and accrued expenses approximate their fair value because of the short maturity of those instruments. The Company’s notes payable approximates the fair value of such instruments as the notes bear interest rates that are consistent with current market rates.
The following table classifies the Company’s liabilities measured at fair value on a recurring basis into the fair value hierarchy as of:
June 30, 2019:
Description | Level 1 | Level 2 | Level 3 | Total Gains and (Losses) | ||||||||||||
| Derivative | $ | - | $ | - | $ | 746,695 | $ | 376,191 | ||||||||
December 31, 2018:
| Description | Level 1 | Level 2 | Level 3 | Total Gains and (Losses) | ||||||||||||
| Derivative | $ | - | $ | - | $ | 96,110 | $ | (23,985 | ) | |||||||
78
Recently Adopted Accounting Pronouncements
In February 2016, the FASB issued Accounting Standard Update (“ASU”) 2016-02, Leases (Topic 842). This ASU requires that a lessee recognize the assets and liabilities that arise from operating leases. A lessee should recognize in the statement of financial position a liability to make lease payments (the lease liability) and a right-of-use asset representing its right to use the underlying asset for the lease term. For leases with a term of 12 months or less, a lessee is permitted to make an accounting policy election by class of underlying asset not to recognize lease assets and lease liabilities. This new guidance will be effective for annual reporting periods beginning after December 15, 2018, including interim periods within those annual reporting periods, and early adoption is permitted. In transition, lessees and lessors are required to recognize and measure leases at the beginning of the earliest period presented using a modified retrospective approach. The Company has elected to not recognize lease assets and liabilities for leases with a term less than twelve months.
We have reviewed other recently issued accounting pronouncements and plan to adopt those that are applicable to us. We do not expect the adoption of any other pronouncements to have an impact on our results of operations or financial position.
NOTE 3 - GOING CONCERN
The accompanying unaudited financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company has an accumulated deficit of $4,440,510 at June 30, 2019, had a net loss of $2,938,488 ($2,814,395 of which was non-cash expense for stock issued for services and convertible debt derivatives) and net cash used in operating activities of $98,395 for the six months ended June 30, 2019. The Company’s ability to raise additional capital through the future issuances of common stock and/or debt financing is unknown. The obtainment of additional financing, the successful development of the Company’s contemplated plan of operations, and its transition, ultimately, to the attainment of profitable operations are necessary for the Company to continue operations. These conditions and the ability to successfully resolve these factors over the next twelve months raise substantial doubt about the Company’s ability to continue as a going concern. The financial statements of the Company do not include any adjustments that may result from the outcome of these aforementioned uncertainties.
NOTE 4 - PROPERTY & EQUIPMENT
Long lived assets, including property and equipment and certain intangible assets to be held and used by the Company are reviewed for impairment whenever events or changes in circumstances indicate that the carrying value of the assets may not be recoverable. Impairment losses are recognized if expected future cash flows of the related assets are less than their carrying values. Measurement of an impairment loss is based on the fair value of the asset. Long-lived assets and certain identifiable intangibles to be disposed of are reported at the lower of carrying amount or fair value less cost to sell.
Property and Equipment and intangible assets are first recorded at cost. Depreciation and/or amortization is computed using the straight-line method over the estimated useful lives of the various classes of assets as follows between three and five years.
Maintenance and repair expenses, as incurred, are charged to expense. Betterments and renewals are capitalized in plant and equipment accounts. Cost and accumulated depreciation applicable to items replaced or retired are eliminated from the related accounts with any gain or loss on the disposition included as income.
Assets stated at cost, less accumulated depreciation consisted of the following:
| June 30, 2019 | December 31, 2018 | |||||||
| Equipment | $ | 14,904 | $ | 14,904 | ||||
| Office equipment | 2,458 | 2,458 | ||||||
| Automobile | 16,979 | 16,963 | ||||||
| Tooling / Molds | 50,016 | 23,105 | ||||||
| Less: accumulated depreciation | (21,798 | ) | (18,994 | ) | ||||
| Fixed assets, net | $ | 62,559 | $ | 38,436 | ||||
Depreciation expense
Depreciation expense for the six months ended June 30, 2019 and 2018 was $2,804 and $1,032, respectively.
79
NOTE 5 - LOANS PAYABLE
On October 24, 2017, the Company was notified that a petition had been filed in the Iowa District Court for Polk County by a Mr. John M. Wesson for failure to repay a loan. Mr. Wesson had loaned the Company $30,000 and $20,000 on October 24, 2012 and June 12, 2013, respectively. The loans were to accrue interest at 5%. While the Company was under previous management the loans were removed from the books in Q1 of 2015. On April 26, 2018, the Company agreed to repay the loan in full including accrued interest and $5,000 for legal fees. The $50,000 plus $7,341 was booked to retained earnings in 2016 as a correction of an error. As of June 30, 2019, there is $45,000 and $16,101 of principal and interest due on this loan. As of December 31, 2018, there is $45,000 and $14,841 of principal and interest due on this loan.
On March 23, 2018, the Company purchased an automobile. The purchase price was $16,963 The interest rate on the loan is 5.8% and matures on April 7, 2023. Payments on the loan, consisting of principal and interest, are $327 per month. As of June 30, 2019, the balance on this loan is $13,157.
NOTE 6 - CONVERTIBLE NOTES
The following table summarizes the convertible notes and related activity as of June 30, 2019:
| Note Holder | Date | Maturity Date | Interest | Balance December 31, 2018 | Additions | Conversions | Balance June 30, 2019 | |||||||||||||||||
| PowerUp Lending Group LTD | 7/9/18 | 7/9/19 | 12 | % | $ | 45,000 | $ | - | $ | (45,000 | ) | $ | - | |||||||||||
| LG Capital Funding LLC | 8/30/18 | 8/30/2019 | 10 | % | 32,000 | - | (26,310 | ) | 5,690 | |||||||||||||||
| ONE44 Capital LLC | 1/23/2019 | 1/23/2020 | 12 | % | - | 100,000 | (4,500 | ) | 95,500 | |||||||||||||||
| Odyssey Capital Funding, LLC | 5/3/2019 | 5/3/2020 | 12 | % | - | 100,000 | - | 100,000 | ||||||||||||||||
| Armada Investment Fund LLC | 5/30/2020 | 2/29/2020 | 12 | % | - | 36,750 | - | 36,750 | ||||||||||||||||
| BHP Capital NY Inc. | 5/30/2020 | 2/29/2020 | 12 | % | - | 36,750 | - | 36,750 | ||||||||||||||||
| Jefferson Street Capital LLC | 5/30/2020 | 2/29/2020 | 12 | % | - | 36,750 | - | 36,750 | ||||||||||||||||
| Total | $ | 77,000 | $ | 310,250 | $ | 75,810 | $ | 311,440 | ||||||||||||||||
| Less debt discount | (33,759 | ) | (241,950 | ) | ||||||||||||||||||||
| $ | 43,241 | $ | 69,490 | |||||||||||||||||||||
A summary of the activity of the derivative liability for the notes above is as follows:
| Balance at December 31, 2017 | $ | - | ||
| Increase to derivative due to new issuances | 89,020 | |||
| Derivative loss due to mark to market adjustment | 7,090 | |||
| Balance at December 31, 2018 | 96,110 | |||
| Increase to derivative due to new issuances | 1,314,354 | |||
| Decrease to derivative due to conversion | (287,578 | ) | ||
| Derivative loss due to mark to market adjustment | (376,191 | ) | ||
| Balance at June 30, 2019 | $ | 746,695 |
A summary of quantitative information about significant unobservable inputs (Level 3 inputs) used in measuring the Company’s derivative liability that are categorized within Level 3 of the fair value hierarchy for the six months ended June 30, 2019 is as follows:
| Inputs | June
30, 2019 |
Initial Valuation |
||||||
| Stock price | $ | .0433 | $ | .55 - .0248 | ||||
| Conversion price | $ | .006 - .0186 | $ | .244 - .0055 | ||||
| Volatility (annual) | 402.56 – 487.05 | % | 261.04% - 410.61 | |||||
| Risk-free rate | 2.09% - 2.4 | % | 2.34% - 2.58 | |||||
| Years to maturity | .25 - .84 | .75 - 1 | ||||||
The development and determination of the unobservable inputs for Level 3 fair value measurements and fair value calculations are the responsibility of the Company’s management
80
NOTE 7 - RELATED PARTY TRANSACTIONS
The Company has received support from parties related through common ownership and directorship. These loans are unsecured, non-interest bearing and due on demand. As of June 30, 2019 and December 31, 2018, the balance due on these loans is $179,191 and $179,191, respectively. Beginning on January 1, 2019, the balance due will accrue interest at 12.5%. As of June 30, 2019, total accrued interest is $11,107.
The Company executed an employment agreement with its CEO, Tom Wood, on January 1, 2018. Per the terms of the agreement Mr. Wood is to be compensated $3,000 per month. The agreement expired on January 2, 2019. The Company executed a new employment agreement with Mr. Wood on April 1, 2019. Per the terms of the agreement Mr. Wood is to be compensated $4,000 per month. The agreement expires on April 1, 2020.
The Company executed an employment agreement with its Chairman, Russell Bird, on January 1, 2019. Per the terms of the agreement Mr. Wood is to be compensated $3,000 per month.
On June 14, 2019, the Company granted 25,000,000 shares each to Mr. Wood and Mr. Bird for services provided. The shares were valued at $0.04, the closing stock price on the date of grant, for total non-cash compensation expense of $2,000,000.
On June 14, 2019, the Company granted 500,000 shares of Series A preferred stock to Mr. Bird for services provided. The shares were valued at $0.04, the closing stock price of the Company’s common shares on the date of grant, for total non-cash compensation expense of $20,000. The closing price for common stock was deemed an acceptable method for valuation as one share of preferred is convertible into one share of common.
NOTE 8 - COMMON STOCK
During the six months June 30, 2019, PowerUp Lending Group LTD converted $45,000 of principal into 5,599,447 shares of common stock.
During the six months June 30, 2019, LG Capital Funding LLC converted $26,310 and $1,689 of principal and interest, respectively, into 4,050,340 shares of common stock.
During the six months June 30, 2019, One44 Capital LLC converted $4,500 and $195 of principal and interest, respectively, into 755,477 shares of common stock.
During the six months June 30, 2019, the Company granted 400,000 shares of common stock for services. The shares were valued at $0.04, the closing stock price on the date of grant, for total non-cash expense of $17,320. In addition, 909,261 shares were issued by the transfer agent for stock granted in a prior period. The stock was debited to common stock to be issued for $228,604.
See Note 7 for stock issued to related parties.
NOTE 9 - PREFERRED STOCK
The Company is currently authorized to issue 5,000,000 Class A preferred shares, $0.001 par value with 1:25 voting rights. The Series A Preferred Stock ranks equal to the common stock on liquidation, pays no dividend and is convertible to common stock for one shares of common for one share of preferred.
See Note 7 for preferred stock issued to a related party.
The Company is currently authorized to issue 5,000,000 Class B Preferred Shares, $0.001 par value. Each share of Series B Preferred Stock has a 1:100 voting right and is convertible into 100 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are no shares of Series B Preferred Stock issued and outstanding.
The Company is currently authorized to issue 5,000,000 Class C Preferred Shares, $0.001 par value. Each share of Series C Preferred Stock has a 1:50 voting right and is convertible into 50 shares of common stock. No dividends will be paid and in the event of liquidation all shares of Series B will automatically convert into common stock. There are no shares of Series C Preferred Stock issued and outstanding.
81
NOTE 10 - WARRANTS
On May 30, 2019, the Company issued 1,500,000 warrants in conjunction with convertible debt. The warrants are exercisable for 3 years at $0.045 per share. The warrants were evaluated for purposes of classification between liability and equity. The warrants do not contain features that would require a liability classification and are therefore considered equity. The Black Scholes pricing model was used to estimate the fair value of the Warrants issued with the following inputs:
| Warrants | 1,500,000 | |||
| Exercise Price | $ | 0.045 | ||
| Term | 3 years | |||
| Volatility | 406 | % | ||
| Risk Free Interest Rate | 2.0 | % | ||
| Fair Value | $ | 41,853 |
Using the fair value calculation, the relative fair value between the debt issued and the warrants was calculated to determine the warrants recorded equity amount of $41,853, accounted for in additional paid in capital.
Activity for the six months ended June 30, 2019 is as follows:
| Number of Warrants | Weighted Average Exercise Price | Weighted Average Remaining Contract Term | ||||||||||
| Outstanding at December 31, 2018 | - | $ | - | - | ||||||||
| Granted | 1,500,000 | 0.045 | 2.90 | |||||||||
| Expired | - | - | - | |||||||||
| Exercised | - | - | - | |||||||||
| Exercisable at June 30, 2018 | 1,500,000 | $ | 0.045 | 2.90 | ||||||||
NOTE 11 - SUBSEQUENT EVENTS
In accordance with SFAS 165 (ASC 855-10) management has performed an evaluation of subsequent events through the date that the financial statements were available to be issued and has determined that it does not have any material subsequent events to disclose in these financial statements other then the following.
Subsequent to June 30, 2019, LG Capital converted $5,690 and $466, of principal and interest, respectively, into 306,274 shares of common stock.
82
PART III – EXHIBITS
Item 16. Index to Exhibits
83
SIGNATURES
Pursuant to the requirements of Regulation A, the issuer certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form 1-A and has duly caused this Offering Statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Orlando, State of Florida, on October 4, 2019.
| REMSLEEP HOLDINGS, INC. | ||
| By: | /s/ Thomas J. Wood | |
| Thomas J. Wood | ||
| Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| (Principal Financial and Accounting Officer) | ||
This Offering Statement has been signed by the following persons in the capacities and on the dates indicated.
| Signature | Title | Date | ||
/s/ Thomas J. Wood |
Chief Executive Officer and Director |
October 4, 2019 | ||
| Thomas J. Wood | (Principal Executive Officer) | |||
| (Principal Financial and Accounting Officer) | ||||
/s/ Russell F. Bird |
President and Chairman of the Board of Directors | October 4, 2019 | ||
| Russell F. Bird |
84