Exhibit 1.1

NEOVASC INC.
ANNUAL INFORMATION FORM
FOR THE FISCAL YEAR ENDED DECEMBER 31, 2015
March 29, 2016
| GLOSSARY | 1 |
| TERMS OF REFERENCE | 2 |
| CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND RISK FACTORS | 3 |
| CORPORATE STRUCTURE | 4 |
| Intercorporate Relationships | 4 |
| GENERAL DEVELOPMENT AND DESCRIPTION OF THE BUSINESS | 5 |
| Three Year Development | 6 |
| Additional Products and Third-Party Sales | 13 |
| Product Development | 13 |
| Specialized Skill & Knowledge | 13 |
| Intangible Property | 14 |
| New Products/Components/Cycles | 14 |
| Economic Dependence | 15 |
| Foreign Operations | 16 |
| Lending | 16 |
| Reorganizations | 16 |
| Employees | 17 |
| Social or Environmental Policies | 17 |
| RISK FACTORS | 17 |
| DIVIDEND POLICY | 31 |
| DESCRIPTION OF CAPITAL STRUCTURE AND MARKET FOR SECURITIES | 31 |
| PRIOR SALES | 32 |
| ESCROWED SECURITIES | 33 |
| DIRECTORS AND OFFICERS | 33 |
| CEASE TRADE ORDERS, BANKRUPTCIES, PENALTIES OR SANCTIONS | 37 |
| Cease Trade Orders and Bankruptcies | 37 |
| Penalties and Sanctions | 38 |
| Individual Bankruptcies | 38 |
| CONFLICTS OF INTEREST | 38 |
| AUDIT COMMITTEE INFORMATION | 38 |
| INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS | 40 |
| MATERIAL CONTRACTS | 40 |
| LEGAL PROCEEDINGS | 40 |
| NAMES AND INTEREST OF EXPERTS | 41 |
| TRANSFER AGENT AND REGISTRAR | 41 |
| ADDITIONAL INFORMATION | 41 |
| ADDITIONAL FINANCIAL INFORMATION | 41 |
| SCHEDULE “A” AUDIT COMMITTEE CHARTER | A-1 |
GLOSSARY
This glossary contains general terms used in the discussion of the cardiovascular medical device industry, as well as specific technical terms used in the descriptions of the Company’s technology and business.
Angioplasty: a procedure for the elimination of areas of narrowing in blood vessels.
Aortic: of or pertaining to the aorta or aortic heart valve.
Artery: blood vessel that carries oxygenated blood from the heart to the body’s organs.
Atrium: chamber in the heart.
Balloon catheter: hollow tube with a tiny balloon on its tip, used for gaining access to the arteries; once the catheter is in position, the balloon is inflated in order to push open a section of artery that is obstructed (see Angioplasty).
Biocompatible: materials that can be implanted or used in a patient without the body reacting adversely to the material.
Bovine: of or derived from or pertaining to a cow.
Cardiac reconstruction: procedure to repair damaged portions of the heart in order to improve its function.
Cardiovascular: system encompassing the heart, veins and arteries.
Cardiovascular disease: disease that restricts blood flow within the arteries, generally due to a build-up of Plaque; may refer to coronary or peripheral arteries, or both.
Catheter: hollow tube used for gaining access to the arteries, either to deliver medications or devices, or to withdraw fluids or samples from the body.
CE Mark: designation used to signify regulatory approval for the sale of a product in the European Union.
Coronary Artery: artery that supplies oxygen-rich blood to the heart muscle.
Coronary Artery Disease: disease that affects the Coronary Arteries (the arteries that provide oxygenated blood to the heart muscle); also called cardiovascular disease. (See Cardiovascular Disease).
COSIRA: the Company’s Coronary Sinus Reducer for Treatment of Refractory Angina clinical trial - a multi-center, double blinded sham controlled study intended to assess the safety and efficacy of the Reducer in a rigorous, controlled manner.
FDA: U.S. Food and Drug Administration; governing body that regulates approval for the sale of medical devices in the United States.
French: The French size is a measure of the external diameter of a catheter, a catheter of 1 French has a diameter of ⅓ mm.
Health Canada: the federal department of health of Canada responsible for the regulation of drugs, natural health products, cosmetics and medical devices and includes the Therapeutic Products Directorate, which in turn includes the Medical Devices Bureau.
Interventional Cardiology: practice of treating Coronary Artery Disease intravascularly; that is, through the arterial system using minimally invasive techniques, rather than with open-heart surgery.
Investigational Device Exemption (IDE): allows the investigational device to be used in a U.S. clinical study in order to collect safety and effectiveness data required to support a Premarket Approval (PMA) application or a Premarket Notification 510(k) submission to the FDA. All clinical evaluations of investigational devices in the United States, unless exempt, must have an approved IDE before the study is initiated.
Mitral: of or pertaining to the mitral heart valve.
Mitral Regurgitation (MR): inadequate function of the mitral valve allowing blood to leak back through the closed valve. This is a severe and debilitating medical condition.
Nasdaq: the NASDAQ Stock Market.
Pericardium: sac in the chest cavity that contains the heart; pericardial tissue is the soft tissue that forms the sac.
PeripatchTM: tissue material made from bovine or Porcine pericardium; used to repair damaged/diseased vessels or organs by working as an internal bandage or as a component in the manufacture of heart valves.
Plaque: deposit of fats, cholesterol and other substances on artery walls that eventually causes arteries to become narrowed, restricting proper blood flow.
Porcine: of or derived from or pertaining to a swine or pig.
Reducer™: Neovasc’s proprietary technology for the treatment of refractory angina.
Stent: expandable, metallic tube inserted into a diseased artery to hold vessel open and maintain proper blood flow; may be used to deliver medication to the artery wall (a “drug-eluting stent”).
Tiara™: Neovasc’s proprietary transcatheter mitral valve system in development for the transcatheter treatment of mitral valve disease.
TIARA-I: the Company’s multinational, multicenter early feasibility study being conducted to assess the safety and performance of the Tiara valve system in high risk surgical contexts.
Transcatheter: implanted or completed via a catheter or small tube instead of surgically.
Transcatheter heart valves: specialized artificial heart valves which are implanted via a catheter rather than a traditional surgical approach.
TSX: the Toronto Stock Exchange.
Vein: blood vessel that carries de-oxygenated blood from the body organs to the heart.
Vessel: artery, vein or duct that carries blood through the body.
TERMS OF REFERENCE
The information set forth in this Annual Information Form is as of March 29, 2016, unless another date is indicated. All references to dollars ($) in this document are expressed in U.S. funds, unless otherwise indicated.
| 2 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS AND RISK FACTORS
This Annual Information Form contains forward-looking statements within the meaning of applicable Canadian securities legislation and U.S. securities legislation that may not be based on historical fact, including, without limitation, statements containing the words “believe”, “may”, “plan”, “will”, “estimate”, “continue”, “anticipate”, “intend”, “expect” and similar expressions. Forward-looking statements are necessarily based on estimates and assumptions made by us in light of our experience and perception of historical trends, current conditions and expected future developments, as well as the factors we believe are appropriate. Forward-looking statements in this Annual Information Form include, but are not limited to, statements relating to:
| · | our intention to expand the indications for which we may market TiaraTM (which does not have regulatory approval and is not commercialized) and ReducerTM (which has CE Mark approval for sale in the European Union); |
| · | our plans to develop and commercialize products and the timing of these development programs; |
| · | our ability to replace declining revenues from the tissue business with revenues from the Reducer and Tiara in a timely manner; |
| · | whether we will receive, and the timing and costs of obtaining, regulatory approvals; |
| · | the cost of post-market regulation if we receive necessary regulatory approvals; |
| · | clinical development of our products, including the results of current and future clinical trials and studies; |
| · | our ability to enroll patients in our clinical trials, studies and compassionate use cases in Canada, the United States and in Europe; |
| · | our ability to get our products approved for use; |
| · | the benefits and risks of our products as compared to others; |
| · | our ability to establish, maintain and defend intellectual property rights in our products; |
| · | our need for additional financing and our estimates regarding our capital requirements and future revenues, expenses and profitability; |
| · | our estimates of the size of the potential markets for our products; |
| · | our potential relationships with distributors and collaborators with acceptable development, regulatory and commercialization expertise and the benefits to be derived from such collaborative efforts; |
| · | sources of revenues and anticipated revenues, including contributions from distributors and other third parties, product sales, license agreements and other collaborative efforts for the development and commercialization of products; |
| · | our creation of an effective direct sales and marketing infrastructure for approved products we elect to market and sell directly; |
| · | the rate and degree of market acceptance of our products; |
| · | the timing and amount of reimbursement for our products; |
| · | the success and pricing of other competing therapies that may become available; |
| · | our retention and hiring of qualified employees in the future; |
| · | the manufacturing capacity of third-party manufacturers for our products; |
| · | the competition we face from other companies, research organizations, academic institutions and government agencies, and the risks such competition pose to our products; |
| · | the confidential information we possess about patients, customers and core business functions, and the information technologies we use to protect it; |
| · | the conduct or possible outcomes of any actual or threatened legal proceedings; |
| · | our intention to continue directing a significant portion of our resources into sales expansion; |
| · | government legislation in all countries that we already, or hope to, sell our products in, and its effect on our ability to set prices, enforce patents and obtain product approvals or reimbursements; and |
| · | risks related to lawsuits that could direct our resources and result in the payment of significant damages and other remedies. |
| 3 |
Such statements reflect our current views with respect to future events and are subject to risks and uncertainties and are necessarily based upon a number of estimates and assumptions that, while considered reasonable by us, are inherently subject to significant business, economic, competitive, political and social uncertainties and contingencies, many of which, with respect to future events, are subject to change. The material factors and assumptions used by us to develop such forward-looking statements include, but are not limited to:
| · | our regulatory and clinical strategies will continue to be successful; |
| · | our current positive interactions with regulatory agencies will continue; |
| · | recruitment to clinical trials and studies will continue; |
| · | the time required to enroll, analyze and report the results of our clinical studies will be consistent with projected timelines; |
| · | current and future clinical trials and studies will generate the supporting clinical data necessary to achieve approval of marketing authorization applications; |
| · | the regulatory requirements for approval of marketing authorization applications will be maintained; |
| · | our current good relationships with our suppliers and service providers will be maintained; |
| · | our estimates of market size and reports reviewed by us are accurate; |
| · | our efforts to develop markets and generate revenue from Reducer will be successful; |
| · | genericisation of markets for Tiara and Reducer will develop; and |
| · | capital will be available on terms that are favourable to us. |
By their very nature, forward-looking statements or information involve known and unknown risks, uncertainties and other factors that may cause our actual results, events or developments, or industry results, to be materially different from any future results, events or developments expressed or implied by such forward-looking statements or information. In evaluating these statements, prospective purchasers should specifically consider various factors, including the risks outlined herein, under the heading “Risk Factors”. Should one or more of these risks or uncertainties or a risk that is not currently known to us materialize, or should assumptions underlying the forward-looking statements prove incorrect, actual results may vary materially from those described herein. These forward-looking statements are made as of the date of this Annual Information Form and we do not intend, and do not assume any obligation, to update these forward-looking statements, except as required by law. Investors are cautioned that forward-looking statements are not guarantees of future performance and investors are cautioned not to put undue reliance on forward-looking statements due to their inherent uncertainty.
CORPORATE STRUCTURE
Neovasc Inc. (“Neovasc” or the “Company”) was incorporated on November 2, 2000 under the laws of the Province of British Columbia and was continued to federal jurisdiction under the Canada Business Corporations Act on April 19, 2002.
On July 1, 2008, the Company completed the acquisition of two Israel-based vascular device development companies, concurrently raising C$8.3 million in equity financing in a non-brokered private placement, completing a 20 for 1 share consolidation and changing its name from Medical Ventures Inc. to Neovasc Inc.
The registered and records office of the Company is located at Suite 2600, 595 Burrard Street, Three Bentall Center, Vancouver, British Columbia, V7X 1L3, and its head office and principal place of business is located at Suite 5138 – 13562 Maycrest Way, Richmond, British Columbia, V6V 2J7.
Intercorporate Relationships
The Company has five wholly-owned subsidiaries as follows:
| Name: | Date of Incorporation: | Jurisdiction of Incorporation: |
| Neovasc Medical Inc. (formerly PM Devices Inc.) |
May 7, 1998 | British Columbia |
| 4 |
| Name: | Date of Incorporation: | Jurisdiction of Incorporation: |
| Neovasc Tiara Inc. | March 11, 2013 | Canada (federal) |
| Neovasc Medical Ltd. | September 9, 2002 | Israel |
| Neovasc (US) Inc. (formerly Medical Ventures (US) Inc.) |
July 2, 2007 | United States |
| B-Balloon Ltd. | March 30, 2004 | Israel |
B-Balloon Ltd. is in the process of being voluntarily liquidated. Angiometrx Inc., a wholly-owned subsidiary of Neovasc, amalgamated with Neovasc Medical Inc. (NMI) on January 1, 2015.
GENERAL DEVELOPMENT AND DESCRIPTION OF THE BUSINESS
Neovasc is a specialty medical device company that develops, manufactures and markets products for the rapidly growing cardiovascular marketplace. Its products include the Tiara technology in development for the transcatheter treatment of mitral valve disease, the Neovasc Reducer for the treatment of refractory angina.
In 2009, Neovasc started initial activities to develop novel technologies for catheter-based treatment of mitral valve disease. Based on the early positive results of these activities, the Company formally launched a program to develop the Tiara. Neovasc established a separate entity, Neovasc Tiara Inc. (NTI), in March 2013 to develop and own the intellectual property related to the Tiara (Neovasc has transferred all intellectual property related to Tiara to NTI). On February 3, 2014, Neovasc announced the first human implant of the Tiara under special access compassionate use exemptions. Subsequently eleven additional patients have been implanted with the Tiara (nine under compassionate use approvals in Vancouver, Canada and in Europe and two in the TIARA-I study) bringing the total number of patients treated with the device to twelve as of this date. In December 2014, the Company announced that it had received approval from the FDA to initiate the TIARA-I study in the United States. The TIARA-I study is a multinational, multicenter early feasibility study being conducted to assess the safety and performance of the Tiara valve system in high risk surgical patients. The study will include up to 15 patients enrolled at centers in the United States and up to 15 patients at centers in Canada and Europe. The first European patient was enrolled in the study in Antwerp, Belgium in late-2014 and the first patient in the United States was enrolled in mid-2015. The Tiara is currently available in two sizes; additional sizes are under development. Following completion of the TIARA-I study the Company intends to continue advancing the Tiara to commercialization and will be undertaking additional studies to support authorization to affix the CE Mark and FDA approval as appropriate.
In July 2008, Neovasc acquired Neovasc Medical Ltd. (NML), a pre-commercial vascular device company based in Israel. NML developed and owned intellectual property related to a novel catheter-based treatment for refractory angina, a debilitating condition resulting from inadequate blood flow to the heart muscle. The Company estimates that there are approximately 620,000 refractory angina patients in the United States who are potential candidates for this treatment. The Company has completed development of the Reducer and obtained authorization to affix the CE Mark, which allows for marketing of the Reducer product in the European marketplace. The Company initiated commercial sales of the Reducer product in early-2015. In March 2014 the Company announced that results of its COSIRA trial had been presented at the ACC.14 medical conference. The COSIRA trial was a sham-controlled randomized, double-blinded study of the Reducer device in 104 patients with moderate to severe refractory angina. The results presented at ACC.14 confirmed that the COSIRA trial had met its primary endpoint demonstrating the efficacy of the Reducer device with statistical significance. The COSIRA trial results were published in the New England Journal of Medicine in February 2015.
| 5 |
Neovasc’s business operations started in March 2002, with the acquisition of NMI. NMI manufactures a line of collagen-based surgical patch products made for use in cardiac reconstruction and vascular repair procedures as well as other surgeries. Neovasc, through NMI, also sells biological tissue to industry partners and other customers who incorporate this tissue into their own products such as transcatheter heart valves. Neovasc’s biological products are made from chemically treated biocompatible pericardial tissue. In 2012, Neovasc sold the rights to manufacture a specific line of conventional surgical patch products to LeMaitre Vascular, Inc. (“LeMaitre”) for $4.6 million. Neovasc has refocused its use of this treated pericardial tissue to constitute key components in third-party medical products, such as transcatheter heart valves. The Company also provides customers with consulting services related to the development of these products with specific expertise related to the transcatheter heart valve field as well as contract manufacturing services for these valves at all stages of development through to commercial scale production.
Neovasc’s Strategy
The Company’s core strategy is to focus on the continued development and commercialization of its products, the Tiara and Reducer, providing minimally invasive medical devices for a cardiovascular market that the Company believes is both growing and under-served by current treatment solutions.
Key elements of this strategy include:
| · | continuing the Company’s initial clinical experience of the Tiara, completing enrollment of a multi-center feasibility study in 2016, and if the enrollment and results of the feasibility study are successful, initiating a CE Mark study in 2016. |
| · | continuing development of the Reducer, initiating a U.S. FDA IDE study in 2016 and supporting the successful COSIRA trial with additional experience through the Company’s targeted commercial launch of Reducer in Europe; and |
| · | continuing efforts to support Neovasc customers through their regulatory pathways and commercialization of their products, with the view to ultimately supplying pericardial tissue and engaging in full scale manufacturing of their sub-components or complete transcatheter heart valves for commercial sale. |
Three Year Development
Recent Developments Subsequent to December 31, 2015
On January 11, 2016, the Company announced that the U.S. FDA had granted approval for participating physicians to treat patients with its 40mm Tiara in the TIARA-I study.
Year Ended December 31, 2015 Developments
On February 3, 2015, the Company closed a previously announced equity financing for gross proceeds to the Company of $74,883,850. The financing was underwritten by Leerink Partners LLC as sole book-running manager for a syndicate of underwriters, which placed 10,415,000 common shares of Neovasc from treasury and 1,660,000 common shares sold by certain directors, officers and employees of the Company each at a price per common share of $7.19. Neovasc is using the net proceeds received by the Company (i) to enroll patients in the TIARA-I study; (ii) to initiate a CE Mark study and U.S. IDE study for Tiara; (iii) to further develop and refine Tiara; (iv) to advance the commercialization of Reducer in Europe; (v) to initiate a U.S. IDE study for Reducer; and (vi) for general corporate purposes.
On February 5, 2015, the final results from the Company’s COSIRA trial assessing the efficacy and safety of the Reducer for treatment of Refractory Angina, were published in the New England Journal of Medicine.
In October 2015, the Company’s Tiara was featured in a “live case” broadcast to the 27th Annual Transcatheter Cardiovascular Therapeutics scientific symposium, the world's largest educational meeting specializing in interventional cardiovascular medicine. During the live broadcast, Dr. Anson Cheung and Dr. John Webb of St. Paul’s Hospital (Vancouver, Canada) implanted a 35mm Tiara in a patient suffering from severe mitral regurgitation.
| 6 |
Year Ended December 31, 2014 Developments
On February 3, 2014, Neovasc announced the first human implant of the Tiara and, on February 20, 2014, a second human implant was completed, both under special compassionate use exemptions. Subsequently ten additional patients have been implanted with Tiara in early feasibility and compassionate use cases bringing the total number of patients treated with the device to twelve as of the date of this Annual Information Form.
On March 26, 2014, the Company closed a previously announced bought deal equity financing for gross cash proceeds of C$25,152,000. The financing was underwritten by Cormark Securities Inc., which placed 4,192,000 common shares of Neovasc at a price of C$6.00 per common share.
The Company submitted applications for listing of its common shares on the TSX and the Nasdaq Capital Market. Our Common Shares began trading on the TSX on June 23, 2014 and on Nasdaq on May 21, 2014.
On October 9, 2014, the Company received conditional IDE approval from the U.S. FDA to initiate the U.S. arm of its TIARA-I study for the Company’s Tiara and on November 27, 2014, the first patient was enrolled in the European arm of the study.
Year Ended December 31, 2013 Developments
On February 28, 2013, the Company announced that the clinical protocol for its COSIRA trial had been published in the peer-reviewed journal Trials (Jolicoeur et al. Trials, 2013, 14:46).
On May 13, 2013, the Company completed patient enrollment in its COSIRA trial designed to further assess the efficacy and safety of the Reducer.
On May 21, 2013, the Company presented initial data from open label patient Registries that are tracking the progress of refractory angina patients implanted with the Reducer at EuroPCR 2013. The data presented six-month follow up data from fifteen Registry patients showing that their angina and physical disability were significantly improved after Reducer implantation. Among other measures, patients were assessed on the Canadian Cardiovascular Society (CCS) grading scale, which is widely used to describe and classify the severity of effort-related angina. Despite the small size of this sample, the change in CCS scores was statistically significant compared to baseline (average CCS score was 3.07 at baseline and 1.64 at follow up (n= 14, p=<0.0001)). Patient subsets who received stress thallium and echo dobutamine testing also showed significant improvement with the Reducer (p=0.042 (n=10) and p=0.004 (n=13), respectively).
On May 23, 2013, the Company presented positive animal data from its Tiara at EuroPCR 2013. Data on long-term implantation of the Tiara in an animal model of mitral regurgitation showed that all seven animals implanted with the Tiara were thriving at the end of the 150-day study, with no signs of inflammation or other adverse reactions to the implanted valve. The Tiara was well incorporated into the native anatomy. At 150 days, valve leaflets were functioning well with no significant degradation in hydrodynamic performance or calcification. In all of the animals, left ventricular outflow was unimpeded, and the aortic valve continued to function normally.
On October 29, 2013, the Company announced that a review of results from preclinical studies of the Tiara had been published in the Journal of the American College of Cardiology: Cardiovascular Interventions (J Am Coll Cardiol Intv. 2014;7(2):154-162). The review concludes that implantation of the Tiara is technically feasible, has an encouraging safety profile and results in a stable and well-functioning mitral bioprosthesis.
| 7 |
On November 6, 2013, the Company reported topline results for its COSIRA trial assessing the efficacy and safety of the Reducer. As discussed in more detail below, the trial demonstrates that the Reducer achieved its primary endpoint, significantly improving the symptoms and functioning of patients disabled by previously untreatable refractory angina. The COSIRA trial also confirmed that the Reducer is well tolerated, with no reports of device-related serious adverse events. The safety and efficacy data from the randomized, controlled COSIRA trial is consistent with results seen in previous non-randomized pilot studies of the Reducer.
On November 13, 2013, the Company announced that the first patent covering the Tiara has been issued by the U.S. Patent and Trademark Office. The new patent protects key aspects of the Tiara. It is the first patent to issue from a portfolio of U.S. and international patent applications Neovasc has filed aimed at establishing an extensive intellectual property estate covering the entire Tiara, including the prosthesis, delivery system, associated accessories and methods of use.
Neovasc’s Products
Tiara
In the second quarter of 2011, the Company formally initiated a new project to develop the Tiara, a product for treating mitral valve disease. The Tiara is in preclinical / early clinical stage development to provide a minimally invasive transcatheter device for the millions of patients who experience mitral regurgitation as a result of mitral heart valve disease (in 2014 it was estimated that mitral regurgitation affects approximately 4.1 million people in the United States and the European Union). Mitral regurgitation is often severe and can lead to heart failure and death. Unmet medical need in these patients is high. Currently, a significant percentage of patients with severe mitral regurgitation are not good candidates for conventional surgical repair or replacement due to frailty or comorbidities. There are approximately 1.7 million patients suffering from significant mitral regurgitation in the United States. Currently there is no transcatheter mitral valve replacement device approved for use in any market.
Clinical experience to date has been primarily with the 35mm Tiara valve and the 32 French delivery system. First clinical use of the 40mm Tiara occurred in the fourth quarter of 2015 and first use of the 45mm Tiara is targeted for 2016. The additional sizes will allow Neovasc to expand treatment to a broader population of patients.
To date, twelve patients have been implanted with Tiara in early feasibility and compassionate use cases and Neovasc believes that early results have been encouraging. The 30-day survival rate for the first twelve patients implanted with Tiara is 73% with one patient now over two years post implant. The Tiara has been successfully implanted in both functional and degenerative MR patients, as well as patients with pre-existing prosthetic aortic valves and mitral surgical rings.
The results from these early feasibility and compassionate use cases have been instrumental in helping to demonstrate the potential of the Tiara as well as refining the implantation procedure, patient selection criteria and the device itself. Careful patient selection continues to be critical as the Company and clinical community continue to learn more about treating this population of very sick patients.
While many challenges remain prior to achieving commercial production (including, but not limited to, positive clinical trial and study results and obtaining regulatory approval from the relevant authorities), the Company believes the Tiara is being widely recognized as one of the leading devices exploring this new treatment option for patients who are unable or unsuited to receive an open heart surgical valve replacement or repair. There are several other transcatheter mitral valve replacement devices in development by third parties; some of which have been implanted in early feasibility type studies with varying results.
Neovasc believes that there are several unique attributes of the Tiara that may provide advantages over other approaches to mitral valve replacement. There is no certainty that the Tiara will successfully proceed through clinical testing and ultimately receive regulatory approval to treat these patients, nor is it possible to determine at this time if any of the other development stage devices will succeed in obtaining regulatory approval.
| 8 |
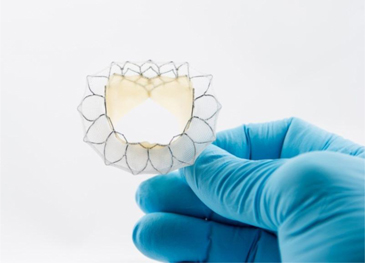
The Tiara valve is made up of two major components: the leaflets and skirt, which are made from the Company’s Peripatch tissue, and the nitinol frame (to which the leaflets and skirt are attached), which is manufactured by a well-established specialty manufacturer in the medical device industry. If this supplier were unable to provide the nitinol frame in the future, it would seriously impact the further development of the Tiara. The Tiara delivery system is manufactured in-house by the Company using components that are readily available.
Regulatory Status
The Tiara is an early-stage development product without regulatory approvals in any country. The Company intends to continue to fund development of the product as cash flow allows and anticipates applying for CE Mark approval in Europe in the next two to three years. As at December 31, 2015, the Company has spent approximately $25.5 million developing the product and anticipates that it may require an additional $25-30 million as it moves forward to achieve CE Mark. There is no assurance that European regulatory approval will be granted in the time frame anticipated by management, or granted at any time in the future. There is no expectation that this product will be revenue-generating in the near term, although management believes that the product is addressing an important unmet clinical need and that the demand for the product is high.
On October 9, 2014 Neovasc announced that it has received conditional IDE approval from the U.S. FDA to initiate the U.S. arm of its TIARA-I study for the Company’s Tiara. The TIARA-I study is a multinational, multicenter early feasibility study being conducted to assess the safety and performance of Neovasc’s Tiara mitral valve system and implantation procedure in high-risk surgical patients suffering from severe MR. Severe MR is a critical condition that affects millions of patients and, if left untreated, can lead to heart failure or death. This FDA conditional approval allows clinical investigators to begin enrolling patients at participating U.S. medical centers once local hospital and related approvals are in place. This is an important step towards Tiara becoming one of the first transcatheter mitral valve replacement devices available for treating U.S. patients. The TIARA-I study will enroll up to 30 patients globally and is being overseen by a multidisciplinary committee of internationally recognized physicians. Tiara has also been implanted under compassionate use approvals in Canada and implantations under similar approvals are anticipated in other countries in the future.
Reducer
The Reducer is a treatment for patients with refractory angina, a painful and debilitating condition that occurs when the coronary arteries deliver an inadequate supply of blood to the heart muscle, despite treatment with standard revascularization or cardiac drug therapies. It affects approximately 620,000 individuals in the United States who are not eligible for conventional treatments and typically lead severely restricted lives as a result of their disabling symptoms, and its incidence is growing. The Reducer provides relief of angina symptoms by altering blood flow in the heart’s venous system, thereby increasing the perfusion of oxygenated blood to ischemic areas of the heart muscle.
| 9 |
The pain associated with refractory angina can make it difficult for patients to engage in routine activities, such as walking or climbing stairs. Using a catheter-based procedure, the Reducer is implanted in the coronary sinus, the major blood vessel that sends de-oxygenated blood from the heart muscle back to the right atrium of the heart. Pilot clinical studies demonstrate that the Reducer provides significant relief of chest pain in refractory angina patients. There are approximately 620,000 refractory angina patients in the United States who are potential candidates for the Reducer, either because they cannot be revascularized or because they are otherwise poorly managed using conventional medical therapies. These patients represent a substantial market opportunity for the Reducer product. If physicians adopt the Reducer for use in these refractory patients, it is expected that there will be a natural spillover into the broader recurrent angina market, which represents a substantially larger patient population.
The Reducer is targeting a currently untreatable patient population. A refractory patient by definition is resistant to other therapies. A patient who has refractory angina is not a surgical candidate, cannot benefit from existing interventional cardiology therapies and is not receiving adequate relief from available drug regimens to manage their chest pain. As such there are currently no direct competitors to the Reducer as the patient will have exhausted all other treatment options before a Reducer is considered. Once the Reducer is established as a standard of care for the refractory angina patient, Neovasc believes that the Reducer may also be considered for use in the larger population of recurrent angina patients (patients who are receiving repeat treatments for angina pain) and thus increase its market potential.
The Company has completed a COSIRA trial to assess the efficacy of the Reducer device. The COSIRA trial’s primary endpoint was a two-class improvement six months after implantation in patients’ ratings on the CCS angina grading scale, a four-class functional classification that is widely used to characterize the severity of angina symptoms and disability. Only patients with severe angina, CCS Class 3 or 4, were enrolled in the COSIRA trial. The COSIRA trial analysis showed that the study met the primary endpoint, with patients receiving the Reducer achieving a statistically significant improvement in CCS scores (two classes or better) compared to patients receiving a sham control (18 of 52 (34.6%) of the Reducer patients improved ≥ 2 CCS classes compared to 8 of 52 (15.4%) of the control patients (p-value = 0.024)). The analysis also showed that patients treated with the Reducer showed a statistically significant improvement of one or more CCS classes compared to the sham control patients (37 of 52 (71.2%) of the Reducer patients showed this improvement compared to 22 of 52 (42.3%) of the control patients (p-value = 0.003)). The COSIRA trial results were published in the New England Journal of Medicine in February 2015.
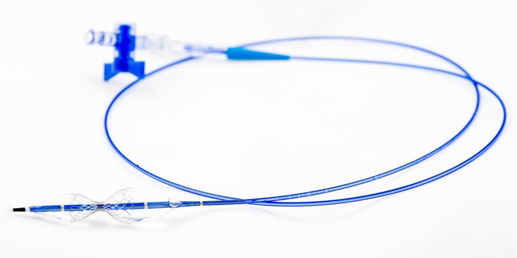
| 10 |
The Reducer is an hourglass-shaped, balloon-expandable, stainless steel, bare metal device, which is implanted in the coronary sinus, creating a restriction in venous outflow from the myocardium (the muscular layer of the heart wall). It is implanted using conventional percutaneous, or needle puncture, techniques. The Reducer is provided sterile and pre-loaded on a balloon catheter system. The system is 9 French sheath compatible and operates over a .035 inch guide wire. The implantation procedure is quick and requires minimal training. Once guide wire access to the coronary sinus is achieved, implantation typically takes less than 20 minutes.
Following implantation, the Reducer is incorporated into the endothelial tissue and creates a permanent (but reversible) narrowing in the coronary sinus. The coronary sinus is narrowed from a typical diameter of 10-12mm to approximately 3mm at the site of implantation. This narrowing slightly elevates the venous outflow pressure, which restores a more normal ratio of epicardial to endocardial blood flow between the outer and inner layers of the ischemic areas of the heart muscle. This results in improved perfusion of the endocardium, which helps relieve ischemia and chest pain. The physiological mechanism behind this effect is well documented in medical literature.
The clinical utility of this approach was demonstrated by a number of analogous approaches used in the past that achieved positive clinical outcomes for angina patients by constricting or intermittently blocking the coronary sinus to improve perfusion to the heart muscle. However, these therapies required the use of highly invasive surgery, or leaving a catheter in the heart for a prolonged period, making them impractical or clinically unacceptable for use in modern medical practice. The Reducer was developed to deliver this therapy in a safe, simple and effective manner via a minimally invasive catheter that is consistent with contemporary medical practice.
The Reducer has demonstrated excellent results in multiple animal studies and in a clinical trial of fifteen patients suffering from chronic refractory angina who were followed for three years after implantation. The six-month results from this clinical trial were published in the Journal of the American College of Cardiology and three-year follow-up data was presented at the annual scientific meeting of the American College of Cardiology in March 2010. In this clinical trial, implantation of the Reducer resulted in significant clinical improvements in stress test and perfusion measurements, as well as in overall quality of life in the majority of the patients. These improvements were maintained for the three years of the study. During this period, the Reducer appeared safe and well tolerated in these patients. More recently, the Company completed the COSIRA trial – a multi-center, double blinded sham controlled study intended to assess the safety and efficacy of the Reducer in a rigorous, controlled manner. The results of COSIRA trial were positive and are discussed in more detail below.
Following this positive data from the COSIRA trial, the Company initiated a pilot launch of the Reducer in select European markets in early 2015. The Company has signed distribution agreements in Italy, Switzerland, the United Kingdom and Saudi Arabia and has initial sales into these countries. Launch of the product is also underway in select centers in Germany via direct sales. Based on the initial results from the targeted launch, Neovasc is presently developing an expanded sales plan and strategy for 2016 and beyond. It is anticipated that sales of the product in the United States would follow obtaining U.S. regulatory approval, if such approval is granted, as described further below.
Regulatory Status
The Reducer is approved for sale in Europe, having received CE Mark designation in November 2011. In preparation for product launch, Neovasc has completed development of the commercial-generation Reducer and the product is currently being transferred to commercial scale manufacture. The Company has completed the COSIRA trial that is expected to provide data to support broad commercialization of the Reducer product. The COSIRA trial is a double-blinded, randomized, sham controlled, multi-center trial of 104 patients at eleven clinical investigation sites. The study completed enrollment in early 2013 and on November 6, 2013, the Company reported topline results for its COSIRA trial assessing the efficacy and safety of the Reducer. In February 2015, the COSIRA trial results were published in the New England Journal of Medicine. As discussed above, the data shows that the Reducer achieved its primary endpoint, significantly improving the symptoms and functioning of patients disabled by previously untreatable refractory angina. The COSIRA trial also confirmed that the Reducer is safe and well tolerated. The safety and efficacy data from the randomized, controlled COSIRA trial is consistent with results seen in previous non-randomized pilot studies of the Reducer. Placement of the Reducer is performed using a minimally-invasive transvenous procedure that is similar to implanting a coronary stent and takes approximately 20 minutes. Neovasc has begun discussions with the FDA on the development of a randomized IDE trial in the United States. The Company expects to begin this trial in 2016. U.S marketing approval is expected about two to four years after the clinical trial begins. There is no assurance that U.S regulatory approval will be granted in the time frame anticipated by management, or granted at any time in the future. The cost of the U.S. clinical trial is expected to be $15-20 million.
| 11 |
Tissue Products
Neovasc produces Peripatch, an advanced biological tissue product that is manufactured from pericardium, which is the protective sac that surrounds the heart of an animal. Neovasc uses its proprietary processes to convert raw pericardial tissue from animal sources into sheets of implantable tissue that can be incorporated into third-party medical devices (for example, for use as the material for artificial heart valve leaflets). Peripatch tissue retains the mechanical characteristics of natural tissue and is readily incorporated into the body without rejection. Peripatch tissue was originally developed to fabricate artificial heart valves and has a 25-year history of successful implantation for heart valve and other surgical applications. Peripatch tissue can be manufactured to meet the mechanical and biological characteristics required for a wide variety of applications, such as heart valve leaflets.
The product line includes Peripatch surgical patches, which are rectangular patches made from bovine tissue, applied as internal bandages to repair weak or damaged organs or vessels. On October 31, 2012, Neovasc amended its agreement with LeMaitre allowing LeMaitre to exercise its option to purchase certain specific rights to Neovasc’s biological vascular surgical patch technology on an accelerated basis. Under the terms of the amendment, LeMaitre is permitted to use the Peripatch technology for the sole purpose of manufacturing surgical patches that it markets as its XenoSure™ surgical patch product line. Neovasc ceased manufacturing surgical patches for LeMaitre in the second quarter of 2015.
The Company also provides a range of custom Peripatch products to industry customers for incorporation into their own products, such as transcatheter heart valves and other specialty cardiovascular devices. These include Peripatch tissue fabricated from bovine and porcine sources and offered in a wide variety of shapes and sizes. Neovasc works closely with its industry customers to develop and supply tissue to meet their specific needs, such as for transcatheter heart valve leaflets. This often includes providing tissue in custom shapes or molded to three dimensional configurations. The Company also provides product development and specialized manufacturing services related to Peripatch tissue-based products such as transcatheter heart valves. The Company actively consults with a range of heart valve programs in order to refine their products and provide tissue to meet their needs and also provides transcatheter valve prototyping, pilot manufacture and commercial manufacture services to a range of customers.
Although the generic method of processing tissue in a way similar to the Peripatch is widely used, the Company’s competitive position stems from its own proprietary process that is supported by a 25-year implant history for use as a surgical heart valve. A company that establishes its own process will have to go through a significant and costly series of studies to prove that their process produces tissue that is suitable as a medical device. The Peripatch product has already met these requirements and has already been validated through many years of successful use in multiple applications. Neovasc’s customers make the decision to use the Company’s tissue rather than take on the demanding and lengthy process of developing their own tissue processing operation. As stated elsewhere herein, Neovasc is not aware of any other company in the world that both provides such tissue and partners with customers to provide specialized heart valve development and manufacturing services.
The basic Peripatch technology was established over 25 years ago by a third party that was a predecessor company to NMI, when the material was used to fashion the leaflets and other components in surgical heart valves. Neovasc’s processing of the material is a trade secret and proprietary to the Company. However, the use of the product in transcatheter minimally invasive heart valves and other medical devices such as artificial hearts are new uses for the technology. Appropriate testing is conducted to ensure the appropriateness and durability of the tissue for a new application before the medical device can be approved for use, and there is some additional risk when applying the technology to a new product or when amending to, or adding to, the fixation process to meet a new demand, such as for three dimensional shape setting of the tissue.
| 12 |
The supply of Peripatch products and the associated product development, consulting and specialized manufacturing services related to Peripatch tissue-based products represents 95% of the Company’s current revenues.
Regulatory Status
While the Company does not maintain stand-alone marketing approval for its tissue products, a number of third-party products which incorporate Peripatch tissue are approved for sale (i.e. such products have obtained regulatory approval, such as a CE Mark or Canadian medical device license) or have pending approvals in various markets. There is no assurance that further regulatory approvals for third-party products will be obtained.
Additional Products and Third-Party Sales
Neovasc provides consulting and original equipment manufacturing services to other medical device companies when these services fall within the scope of the Company’s expertise and capabilities. These activities are substantially focused on providing specialized development and manufacturing services for industry customers who incorporate the Company’s Peripatch tissue into their vascular device products such as heart valves. The goal of these activities is to drive near-term revenues as well as support development of a long-term revenue stream through the ongoing provision of tissue and manufacturing services to customers with commercially successful devices that incorporate Neovasc tissue. Revenue earned from various contract agreements varies throughout the year depending on customer needs.
Product Development
Product development at the Company is currently focused on completing commercialization of the Reducer as well as clinical stage and pre-commercialization development work on the Tiara. The Company may also investigate other potential new internal or external projects that leverage the Company’s existing technologies, infrastructure and expertise.
Specialized Skill & Knowledge
Tiara
The team that developed the Tiara has extensive background experience developing medical devices and heart valves. As the product has progressed through the clinical and regulatory pathways, and to broaden its specialized staff, the Company opened an office in Minnesota. Since opening this office, the Company has been successful in attracting talent and filling out the team supporting the Tiara. The office in Minnesota now employs the majority of our clinical and regulatory affairs groups, as well as an engineering and project management group. The opening of this office was an important milestone in the ongoing development of the Tiara.
Reducer
The manufacture of the Reducer requires basic catheter and stent manufacturing techniques that are common in the industry. The one component that is more complex is the manufacture of the hour glass shaped balloon and the Company works closely with an industry partner who manufactures this component. The Reducer is assembled and sterilized by well-known medical device contract manufacturers. While the device is not manufactured in-house, the product is supported by a team with specialized background knowledge of refractory angina and the clinical and regulatory requirements for the device. Our Medical Director, Shmuel Banai, has been involved in the Reducer since its inception.
| 13 |
Peripatch
The Company employs a proprietary process to manufacture Peripatch. The process has been improved over time and can be adapted to meet the needs of our customers. The Company has an engineering team that is skilled and knowledgeable in the process and the product has a 25-year implant history that differentiates it from other processing techniques.
The Company’s management team has developed specific skills and knowledge based on each individual’s experiences. A description of each of their qualifications can be found under the heading “Directors and Officers”, below.
Intangible Property
Patents
The Company’s ability to protect its products from unauthorized or infringing use by third parties depends substantially on its ability to obtain and maintain valid and enforceable patents. Neovasc has issued and pending patent applications in Canada, the United States, Europe and other select countries covering certain aspects of the technology that Neovasc intends to commercialize (including the Reducer and the Tiara). Accordingly, rights under any of Neovasc’s issued patents may provide it with commercially meaningful protection for its products or afford it a commercial advantage against the Company’s competitors or their competitive products or processes. However, patents that have been issued to Neovasc or that may be issued in the future may not be valid upon challenge or enforceable. Further, even if valid and enforceable, Neovasc’s issued patents may not be sufficiently broad to prevent others from marketing products like the Company’s own, despite these patent rights. In addition, patents are country/jurisdiction specific, i.e. the rights afforded under a patent are limited to the jurisdiction of the government which granted the patent. Thus the rights afforded by a U.S. patent are limited to the United States or its territories, and are unenforceable elsewhere. In general, the exclusive rights provided by a patent begin on the date the patent issues and expires 20 years from the filing date of the application, though this term may differ slightly depending on the specific patent laws in the applicable jurisdiction (for example, U.S. patents may have a patent term adjustment granted by the U.S. Patent and Trademark Office to compensate for certain delays in examining an application). The Company also relies on trade secret protection to protect its interests in proprietary know-how and for processes for which patents are difficult to obtain or enforce.
Trademarks
The Company owns registrations and/or pending applications for, the trademark NEOVASC, TIARA, NEOVASC REDUCER, and PERIPATCH in the United States, Canada, and the European Union.
New Products/Components/Cycles
Tiara
The Tiara is an early stage development product that will require several more years of development before it obtains regulatory approval, if ever, for use in any jurisdiction. A first-in-human implantation of Tiara was successfully performed on January 30, 2014 by physicians at St. Paul’s Hospital in Vancouver, British Columbia. The transapical procedure resulted in the elimination of mitral regurgitation and significantly improved heart function in the patient, without the need for cardiac bypass support and with no procedural complications. Subsequently eleven additional patients have been successfully implanted with Tiara (ten under compassionate use approvals in Vancouver, Canada and in Europe and two in the TIARA-I study) bringing the total number of patients treated with the device to twelve as of this date. In December 2014, the Company announced that it had received approval from the FDA to initiate the TIARA-I study in the United States. The TIARA-I study is a multinational, multicenter early feasibility study being conducted to assess the safety and performance of the Tiara valve system in high risk surgical patients. The study will include up to 15 patients enrolled at centers in the United States and up to 15 patients at centers in Canada and Europe. The first European patient was enrolled in the study in Antwerp, Belgium in late-2014 and the first U.S. patient was enrolled in mid-2015. The Tiara is currently available in two sizes; additional sizes are under development. Following completion of the TIARA-I study the Company intends to continue advancing the Tiara to commercialization and will be undertaking additional studies to support authorization to affix the CE Mark and FDA approval as appropriate. Further information about Tiara can be found above under the heading “Neovasc’s Products”.
| 14 |
Reducer
The Reducer is a late-stage product with European CE Mark approval. The Company initiated a pilot launch of the Reducer in select European markets in 2015. The Company has also been exploring initiation of Reducer sales in other non-US markets and has signed distribution agreements in several countries. It is anticipated that sales of the product in the United States would follow once U.S. regulatory approval has been granted, as described further below.
A well-known and well-established medical device contract manufacturer will manufacture the Reducer for the Company. The Reducer is already in pilot production in preparation for commercial launch. The majority of the components that make up the Reducer are readily available; however, two critical components of the device are not. The balloon portion of the delivery system is technically challenging to manufacture and the Reducer device, whilst a basic technology, must be manufactured in Israel due to restrictions on the transfer of intellectual property and manufacturing out of Israel stemming from certain research grants received by NML prior to the acquisition in July 2008. Further information about Reducer can be found above under the heading “Neovasc’s Products”.
Peripatch Products
The basic Peripatch technology was established over 25 years ago, when the material was used to fashion the leaflets and other components in surgical heart valves. Neovasc’s processing of the material is a trade secret and proprietary to the Company.
Neovasc sources its porcine tissue from one abattoir in Canada. The bovine tissue is sourced from abattoirs in the United States for sale of tissue destined for the U.S. market and from abattoirs in both Australia and New Zealand for the sale of tissue destined for Europe. There is a degree of capacity constraint related to the supply of raw tissue but with multiple approved suppliers for each type of tissue the risk of disruption is minimized.
While a definitive pattern of demand has not yet been established and the effect is expected to be minimal, the cyclical nature of the meat industry could conceivably have an impact on the quality and availability of raw tissue and could potentially impact the yields and margins for the product over the course of any given year. Further information about Peripatch can be found above under the heading “Neovasc’s Products”.
Economic Dependence
For the year ended December 31, 2015, revenues from the Company’s four largest customers accounted for approximately 30%, 29%, 18% and 10% of the Company’s sales. Some of these customers are either development stage companies or do not have established markets for their products. In addition, 58% of the Company’s revenues for the year ended December 31, 2015 are derived from consulting services. The Company’s consulting service revenues are contract-driven and they can fluctuate from quarter to quarter and year to year as current projects are completed and new projects start.
The Company anticipates that it will be able to convert more of its consulting services customers, whose products are currently in product development and clinical trials, into contract manufacturing customers as they commercialize their own products. This process is dependent on the product development and regulatory success of these existing customers, so revenues are therefore difficult to project. A change in the economic outlook of these four largest customers could have a material adverse impact on the anticipated revenues of the Company. Factors that may impact these customers may include, among others, the stage of development; additional capital requirements; the impact of any global economic downturn; the ability to develop, manufacture and commercialize its products in a cost-effective manner; the ability to integrate newly-acquired businesses and the ability to protect their intellectual property.
| 15 |
Foreign Operations
While the Company’s headquarters are in Vancouver, British Columbia and a large part of all its operations are in Vancouver, the Company is exposed to factors that influence its revenue from customers located in foreign locations and revenues that are denominated in foreign currencies. The majority of the Company’s revenues are derived from product sales in the United States and Europe, primarily denominated in U.S. dollars and Euros, while the majority of the Company’s costs are denominated in Canadian dollars. The Company expects that foreign currency denominated international sales will continue to account for a significant portion of its revenues. Consequently, a decrease in the value of a relevant foreign currency in relation to the Canadian dollar will have an adverse effect on the Company’s results of operations, with lower than expected revenue amounts and gross margins being reported in the Company’s Canadian dollar financial statements prior to translation into the U.S. dollar presentation currency. In addition, any decrease in the value of the U.S. dollar or Euro occurring in between the time a sale is consummated and the time payment is received by Neovasc will lead to a foreign exchange loss being recognized on the foreign-currency denominated trade account receivable. The fluctuation of foreign exchange may impose an adverse effect on the Company’s results of operations and cash flows in the future. The Company does not conduct any hedging activities to mitigate these foreign exchange risks.
Additionally, Neovasc may be materially and adversely affected by increases in duty rates, exchange or price controls, repatriation restrictions, or other restrictions on foreign currencies. The Company’s international operations are subject to certain other risks common to international operations, including, without limitation: government regulations, import restrictions and, in certain jurisdictions, reduced protection for the Company’s intellectual property rights. Foreign currency translation gains and losses arising from normal business operations are credited to or charged to operations in the period incurred. To date, the Company has not entered into any foreign exchange forward contracts. For the year ended December 31, 2015, approximately 50% of the Company’s revenue was generated from customers in the United States, 48% from customers in the European Union and 2% from customers in Israel. Approximately 58% of the Company’s revenue was denominated in U.S. dollars and 42% was denominated in Euros. Substantially all of the Company’s long-lived assets are located in Canada.
Lending
The Company’s cash management policy is to maintain sufficient cash on hand to meet forecast expenditures and to invest any excess capital according to the Company’s investment policy. The Company’s investment policy for these excess cash balances will follow a conservative investment philosophy based on three fundamentals: preservation of capital, liquidity, and best available net return on invested capital.
The Company prohibits speculation on currencies. If there are insufficient foreign funds, foreign currencies will be purchased on an ad hoc basis at the spot rate to fund expenditures. If there are surplus foreign funds, foreign currencies will be converted to Canadian dollars.
The Company has not been involved in any bankruptcy, receivership or similar proceedings within the three most recent completed financial years.
Reorganizations
As described under the heading “Corporate Structure” above, the Company acquired two Israeli companies on July 1, 2008; B-Balloon Ltd. is in the process of being voluntarily liquidated and NML continues to operate as an intellectual property holding company. On September 30, 2013, Neovasc transferred the intangible assets, including patents, trademarks and other know-how, for the Tiara device to its 100% wholly-owned subsidiary NTI. On January 1, 2015, Angiometrx Inc. was amalgamated with NMI.
| 16 |
The Company and its subsidiaries now operate as follows: Neovasc Inc. is the Canadian public company and 100% owner of each of the subsidiary entities. NMI is the operating company for the group. It holds all the tangible assets and the Peripatch tissue technology and employs all the employees of the Company. NTI holds all the intangible assets related to the Tiara and NML holds all the intangible assets related to the Reducer program. NMI charges both NTI and NML for the development services performed by its employees to develop the Tiara and Reducer products respectively. NML received a royalty based on Reducer revenues generated by NMI.
Employees
The Company has seen rapid growth in its employee numbers. The Company has added additional staff in production to meet growing demand for the Company’s services and products, in research and development and clinical and regulatory affairs to accelerate the development of the Tiara and Reducer and in support areas such as biology, chemistry, risk management, human resources and quality affairs to better assist the commercial and development activities. At the end of each of the last five years and as at March 29, 2016 the number of employees was as follows:
| Year ended December 31: | Number of Employees: |
| 2011 | 60 |
| 2012 | 77 |
| 2013 | 119 |
| 2014 | 146 |
| 2015 | 197 |
| Current | 205 |
Social or Environmental Policies
The Company’s processing of its pericardial tissue involves the use of some controlled and/or hazardous materials. The use and disposal of these materials is controlled by the Company’s quality control procedures and systems. Environmental factors are considered when disposing of these materials and the Company takes steps to ensure it is in compliance with the appropriate regulations surrounding disposal of these materials.
RISK FACTORS
This document contains forward-looking statements regarding the Company, business, prospects and results of operations that involve risks and uncertainties. Neovasc’s actual results could differ materially from the results that may be anticipated by such forward-looking statements and discussed elsewhere in this Annual Information Form. Factors that could cause or contribute to such differences include, but are not limited to, those discussed below, as well as those discussed elsewhere in this Annual Information Form. If any of the following risks occur, the Company’s business, financial condition or operating results could be harmed. In that case, the trading price of Neovasc common shares could decline.
Investment in the common shares of the Company is speculative and involves a high degree of risk, is subject to the following specific risks among others, and should be undertaken only by purchasers whose financial resources are sufficient to enable them to assume such risks. The common shares of the Company should not be purchased by persons who cannot afford the possibility of the loss of their entire investment. Prospective purchasers should review these risks as well as other matters disclosed elsewhere in this Annual Information Form with their professional advisors.
The Company is subject to lawsuits that could divert its resources and result in the payment of significant damages and other remedies.
The Company is engaged as a defendant in lawsuits filed by CardiAQ Valve Technologies, Inc. (“CardiAQ”), as further described below. Litigation resulting from CardiAQ’s claims could be costly and time-consuming and could divert the attention of management and key personnel from our business operations. We cannot assure that we will succeed in defending any of these claims and that judgments will not be entered against us with respect to the litigation resulting from such claims. If we are unsuccessful in our defense of these claims or unable to settle the claims in a manner satisfactory to us, we may be faced with significant monetary damages, loss of intellectual property rights, or injunctive relief against us that could have a material adverse effect on our business and financial condition.
| 17 |
On June 6, 2014, Neovasc was named in a lawsuit filed by CardiAQ in the U.S. District Court for the District of Massachusetts concerning intellectual property rights ownership, unfair trade practices and a breach of contract relating to Neovasc’s transcatheter mitral valve technology, including the Tiara. On June 23, 2014, CardiAQ also filed a complaint against Neovasc in Germany requesting that Neovasc assign its right to one of its European patent applications to CardiAQ. On July 7, 2014, the Company was made aware through a press release issued by CardiAQ of a stay in proceedings for Neovasc’s European patent application that is the subject of the German lawsuit. This stay of proceedings was granted without an opportunity for Neovasc to respond to CardiAQ’s allegations. The Company requested that the stay be lifted, but the request was denied by the European Patent office pending resolution of the German lawsuit. Neovasc filed its response to the German lawsuit in December 2014.
The Company intends to vigorously defend itself in both lawsuits. After motion practice and discovery, the District of Massachusetts case is currently scheduled to go to trial on May 2, 2016. The court in Munich is expected to render its decision after a hearing currently scheduled for August 2016.
The outcome of these matters is not currently determinable nor is it possible to accurately predict the outcome or quantum of these proceedings to the Company at this time. Until this matter has been resolved by the appropriate Courts, the Company cannot give any assurances as to such outcome.
When the Company assesses that it is more likely that no present obligation exists at the end of the reporting period and that the possibility of an outflow of economic resources embodying economic benefits is possible, but not probable, no provision is recognized and contingent liability disclosure is required. The Company has applied the disclosure exemption and not provided an estimate of the contingent liability disclosure as it may seriously prejudice the Company’s position in the dispute.
Third parties may claim we are infringing their intellectual property and we could suffer significant litigation or licensing expenses or be prevented from selling products.
We may be involved in substantial litigation regarding patent and other intellectual property rights in the medical device industry. We may be subject to challenges by third parties regarding our intellectual property, including, among others, claims regarding validity, enforceability, scope and effective term. From time to time, we have been and may in the future be forced to defend against claims and legal actions alleging infringement of the intellectual property rights of others, and such intellectual property litigation is typically costly and time-consuming. Adverse determinations in any such litigation could result in significant liabilities to third parties or injunctions, or could require us to seek licenses from third parties and, if such licenses are not available on commercially reasonable terms, prevent us from manufacturing, selling or using certain products, any one of which could have a material adverse effect on us. In addition, some licenses may be non-exclusive, which could provide our competitors access to the same technologies.
Third parties could also obtain patents that may require us to either redesign products or, if possible, negotiate licenses from such third parties. Such licenses may materially increase our expenses. If we are unable to redesign products or obtain a license, we might have to exit a particular product offering.
The success of our business depends in part on our ability to obtain and maintain intellectual property protection for our technology and know-how, and operate without infringing the intellectual property rights of other companies. It is possible that as a result of future litigation our products currently marketed or under development may be found to infringe or otherwise violate third party intellectual property rights. A former customer has alleged that we have wrongfully used its confidential information in connection with the Tiara. While we believe this allegation to be without merit, the ultimate outcome of a litigation depends on numerous factors and our success in the event of any such litigation is not guaranteed. Intellectual property litigation proceedings, if instituted against us, could result in substantial costs, inability to market our products including the Tiara, loss of our proprietary rights and diversion of our management’s and technical team’s attention and resources.
| 18 |
Our inability to protect our intellectual property could have a material adverse effect on our business.
Our success and competitive position are dependent in part upon our proprietary intellectual property. We rely on a combination of patents and trade secrets to protect our proprietary intellectual property, and we expect to continue to do so. Although we seek to protect our proprietary rights through a variety of means, we cannot guarantee that the protective steps we have taken are adequate to protect these rights. Patents issued to or licensed by us in the past or in the future may be challenged and held invalid. The scope of our patent claims also may vary between countries, as individual countries have distinctive patent laws. In addition, as our patents expire, we may be unsuccessful in extending their protection through patent term extensions. The expiration of, or the failure to maintain or extend our patents, could have a material adverse effect on us.
We also rely on confidentiality agreements with certain employees, consultants and other third parties to protect, in part, trade secrets and other proprietary information. These agreements could be breached and we may not have adequate remedies for such a breach. In addition, others could independently develop substantially equivalent proprietary information or gain access to our trade secrets or proprietary information.
We may spend significant resources to enforce our intellectual property rights and such enforcement could result in litigation. Intellectual property litigation is complex and can be expensive and time-consuming. However, our efforts in this regard may not be successful. We also may not be able to detect infringement. In addition, competitors may design around our technology or develop competing technologies. Patent litigation can result in substantial cost and diversion of effort. Intellectual property protection may also be unavailable or limited in some foreign countries, enabling our competitors to capture increased market position. The invalidation of key intellectual property rights or an unsuccessful outcome in lawsuits filed to protect our intellectual property could have a material adverse effect on our financial condition, results of operations or prospects.
Our products are continually the subject of clinical trials conducted by us, our competitors, or other third parties, the results of which may be unfavorable, or perceived as unfavorable, and could have a material adverse effect on our business, financial condition, and results of operations.
The regulatory approval process for new products and new indications for existing products requires extensive clinical trials and procedures, including early clinical experiences and regulatory studies. Unfavorable or inconsistent clinical data from current or future clinical trials or procedures conducted by us, our competitors, or third parties, or perceptions regarding this clinical data, could adversely affect our ability to obtain necessary approvals and the market's view of our future prospects. Such clinical trials and procedures are inherently uncertain and there can be no assurance that these trials or procedures will be completed in a timely or cost-effective manner or result in a commercially viable product. Failure to successfully complete these trials or procedures in a timely and cost-effective manner could have a material adverse effect on our prospects. Clinical trials or procedures may experience significant setbacks even after earlier trials have shown promising results. Further, preliminary results from clinical trials or procedures may be contradicted by subsequent clinical analysis. In addition, results from our clinical trials or procedures may not be supported by actual long-term studies or clinical experience. If preliminary clinical results are later contradicted, or if initial results cannot be supported by actual long-term studies or clinical experience, our business could be adversely affected. Clinical trials or procedures may be suspended or terminated by us or regulatory authorities at any time if it is believed that the trial participants face unacceptable health risks.
A number of companies in the medical device industry have suffered significant setbacks in advanced clinical trials, even after positive results in earlier trials. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. Negative or inconclusive results or adverse medical events during a clinical trial could cause a clinical trial to be delayed, repeated or terminated. In addition, failure to construct appropriate clinical trial protocols could result in the test or control group experiencing a disproportionate number of adverse events and could cause a clinical trial to be repeated or terminated.
| 19 |
Moreover, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and receive compensation in connection with such services. Under certain circumstances, the Company may be required to report some of these relationships to the FDA. FDA may conclude that a financial relationship between the company and a principal investigator has created a conflict of interest or otherwise affected interpretation of the study. FDA may therefore question the integrity of the data generated at the applicable clinical trial site and the utility of the clinical trial itself may be jeopardized. This could result in a delay in approval, or rejection, of our marketing applications by the FDA and may ultimately lead to the denial of regulatory approval of one or more of our product candidates.
We have significant additional future capital needs and there are uncertainties as to our ability to raise additional funding.
We require significant additional capital resources to expand our business, in particular the further development of our medical devices. Technical innovations often require substantial time and investment before we can determine commercial viability. Advancing our products, market expansion of our currently marketed products or acquisition and development of any new products or medical devices will require considerable resources and additional access to capital markets. In addition, our future cash requirements may vary materially from those now expected. For example, our future capital requirements may increase if:
| · | we experience more competition for our medical devices from other medical device companies or in more markets than anticipated; |
| · | we experience delays or unexpected increases in costs in connection with obtaining regulatory approvals for our products in the various markets where we hope to sell our products; |
| · | we experience unexpected or increased costs relating to preparing, filing, prosecuting, maintaining, defending and enforcing patent claims, or other lawsuits, brought by either us or our competition; |
| · | we experience scientific progress sooner than expected in our discovery, research and development projects, if we expand the magnitude and scope of these activities, or if we modify our focus as a result of our discoveries; |
| · | we experience setbacks in our progress with pre-clinical studies and clinical trials are delayed; |
| · | we are required to perform additional pre-clinical studies and clinical trials; or |
| · | we elect to develop, acquire or license new technologies, products or businesses. |
We could potentially seek additional funding through corporate collaborations and licensing arrangements, through public or private equity or debt financing, or through other transactions. However, if sales are slow to increase or if capital market conditions in general, or with respect medical device companies such as ours, are unfavourable, our ability to obtain significant additional funding on acceptable terms, if at all, will be negatively affected. Additional financing that we may pursue may involve the sale of our common shares or financial instruments that are exchangeable for, or convertible into, our common shares which could result in significant dilution to our shareholders.
If sufficient capital is not available, we may be required to delay our business expansion or our research and development projects, either of which could have a material adverse effect on our business, financial condition, prospects or results of operations.
We have a history of significant losses and a significant accumulated deficit.
We may incur losses in the future and our losses may increase. We have incurred net losses in each fiscal year since inception. In the year ended December 31, 2015, we had a net loss of $26,730,490 and at December 31, 2014, we had an accumulated deficit of $115,288,713. We have increased our research and development expenses in recent periods and we plan further increases in the future as cash flows allow. The planned increases in research and development expenses may result in larger losses in future periods. As a result, we will need to generate significantly greater revenues than we have to date to achieve and maintain profitability. There can be no assurance that revenues will increase. Our business strategies may not be successful and we may not be profitable in any future period. Our operating results have varied in the past and they may continue to fluctuate in the future. In addition, our operating results may not follow any past trends.
| 20 |
We are subject to the risks associated with product liability claims, insurance and recalls.
Prior to patient use, our products undergo extensive clinical testing and are approved by the applicable regulatory authorities. However, despite all reasonable efforts to ensure safety, it is possible that we or our partners may sell products which are defectively manufactured or labeled, contain defective components or are misused. Our products may also fail to meet patient expectations or produce harmful side effects. Such unexpected quality, safety or efficacy issues may be caused by a number of factors, including manufacturing defects, failure to adhere to good clinical practices, failure to adhere to good manufacturing practices, non-compliance with clinical protocols or the presence of other harmful conditions in a clinical trial inadequacies of product-related information conveyed to physicians or patients, or other factors or circumstances unique to the patient. Whether or not scientifically justified, such unexpected safety or efficacy concerns can arise and may lead to product recalls, loss of or delays in market acceptance, market withdrawals, or declining sales, as well as product liability, consumer fraud and/or other claims. Additionally, we may be exposed to product liability claims from patients in clinical trials. Such liability might result from claims made directly by consumers or by medical device companies or others selling such products. It is impossible to predict the scope of injury or liability from such defects or unexpected reactions, or the impact on the market for such products of any allegations of these claims, even if unsupported, or the measure of damages which might be imposed as a result of any claims or the cost of defending such claims. Substantial damage awards and/or settlements have been handed down – notably in the United States and other common law jurisdictions – against medical device companies based on claims for injuries allegedly caused by the use of their products. Although our shareholders would not have personal liability for such damages, the expenses of litigation or settlements, or both, in connection with any such injuries or alleged injuries and the amount of any award imposed on us in excess of existing insurance coverage, if any, may have a material adverse impact on us and on the price of our common shares. In addition, we may not be able to avoid significant product liability exposure even if we take appropriate precautions, including maintaining product liability coverage (subject to deductibles and maximum payouts). Any liability that we may have as a result could have a material adverse effect on our business, financial condition and results of operations, to the extent insurance coverage for such liability is not available. Product liability claims in the future, regardless of their ultimate outcome, could have a material adverse effect on our reputation and on our ability to attract and retain customers for our products.
Use of our products in unapproved circumstances could expose us to liabilities.
The marketing approval from the FDA and other regulators of certain of our products are, or are expected to be, limited to specific indications. We are prohibited by law from marketing or promoting any unapproved use of our products. Physicians, however, in most jurisdictions, can use these products in ways or circumstances other than those strictly within the scope of the regulatory approval. Although the product training we provide to physicians and other health care professionals is limited to approved uses or for clinical trials, no assurance can be given that claims might not be asserted against us if our products are used in ways or for procedures that are not approved.
We have substantial competition in the medical device industry and with respect to our products.
The medical device industry is highly competitive and is characterized by extensive research and development and rapid technological change. Many companies, as well as research organizations, currently engage in, or have in the past engaged in, efforts related to the development of medical devices in the same therapeutic areas as we do. Due to the size of the cardiovascular market and the large unmet medical need for products that treat cardiovascular illnesses, a number of the world’s largest medical device companies are developing, or could potentially develop, products that could compete with ours.
| 21 |
Many of the companies developing competing technologies and products may have significantly greater financial resources and expertise in discovery, research and development, manufacturing, pre-clinical studies and clinical testing, obtaining regulatory approvals and marketing than we do. Other smaller companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. Academic institutions, government agencies and other public and private research organizations may also conduct research, seek patent protection and establish collaborative arrangements for discovery, research, clinical development and marketing of products similar to ours. There is a risk that one or more of our competitors may develop more effective or more affordable products than us and that such competitors will commercialize products that will render our medical devices obsolete. We face competition with respect to product efficacy and safety, ease of use and adaptability to various modes of administration, acceptance by physicians, the timing and scope of regulatory approvals, availability of resources, reimbursement coverage, price and patent positions of others. In addition, these companies and institutions also compete with us in recruiting and retaining qualified personnel. If we fail to develop new products or enhance our existing products in the face of such strong competition, such competition could have a material adverse effect on our business, financial condition or results of operations.
Our approved products may not achieve or maintain expected levels of market acceptance, which could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our securities to decline.
Even if we are able to obtain regulatory approvals for our products, the success of those products is dependent upon achieving and maintaining market acceptance. New medical devices that appear promising in development may fail to reach the market or may have only limited or no commercial success. Levels of market acceptance for our products could be impacted by several factors, many of which are not within our control, including but not limited to:
| · | safety, efficacy, convenience and cost-effectiveness of our products compared to products of our competitors; |
| · | scope of approved uses and marketing approval; |
| · | timing of market approvals and market entry; |
| · | difficulty in, or excessive costs to, manufacture; |
| · | infringement or alleged infringement of the patents or intellectual property rights of others; |
| · | availability of alternative products from our competitors; |
| · | acceptance of the price of our products; and |
| · | ability to market our products effectively at the retail level. |
In addition, the success of any new product will depend on our ability to either successfully build our in-house sales capabilities or to secure new, or to realize the benefits of existing arrangements with third-party marketing or distribution partners. Seeking out, evaluating and negotiating marketing or distribution agreements may involve the commitment of substantial time and effort and may not ultimately result in an agreement. In addition, the third-party marketing or distribution partners may not be as successful in promoting our products as we had anticipated. If we are unable to commercialize new products successfully, whether through a failure to achieve market acceptance, a failure to build our own in-house sales capabilities, a failure to secure new marketing partners or to realize the benefits of our arrangements with existing marketing partners, there may be a material adverse effect on our business, financial condition and results of operations and it could cause the market value of our securities to decline.
In addition, by the time any products are ready to be commercialized, the proposed market for these products may have changed. Our estimates of the number of patients who have received or might have been candidates to use a specific product may not accurately reflect the true market or market prices for such products or the extent to which such products, if successfully developed, will actually be used by patients. Our failure to successfully introduce and market our products that are under development would have a material adverse effect on our business, financial condition, and results of operations.
| 22 |
If we are not able to convince public payors and hospitals to include our products on their approved product lists, our revenues may not meet expectations and our business, results of operations and financial condition may be adversely affected.
The direct cost of implanting or using our medical devices is seldom paid by individual patients. Successful commercialization of such devices will depend largely upon the availability of reimbursement for the surgery and medical costs associated with the product from third-party payors. We expect that our products will be purchased by health-care providers, clinics, and hospitals that will subsequently bill various third-party payors such as government programs and private insurance plans. These expectant payors carefully review and increasingly challenge the prices charged for medical devices and services. Provincial government sponsored health programs in Canada and similar programs in the United States reimburse hospitals a pre-determined fixed amount for the costs associated with a particular procedure based on the patient’s discharge diagnosis and similarly reimburse the surgeon or physician based on the procedure performed, without taking into consideration the actual costs incurred by either party or the actual cost of the device. New products are being scrutinized increasingly with respect to whether or not they will be covered by the various health plans and at what level of reimbursement. Third-party payors may determine that our products are unnecessary, not cost-effective, too experimental, or are primarily intended for non-approved indications.
Our business may be materially adversely affected by new legislation, new regulatory requirements, and the continuing efforts of governmental and third party payors to contain or reduce the costs of healthcare through various means, including the U.S. healthcare reform legislation signed in 2010.
The government and regulatory authorities in Canada, the United States, Europe and other markets in which we sell our products may propose and adopt new legislation and regulatory requirements relating to medical product approval criteria and manufacturing requirements. Such legislation or regulatory requirements, or the failure to comply with such, could adversely impact our operations and could have a material adverse effect on our business, financial condition and results of operations.
The growth of overall healthcare costs as a percentage of gross domestic product in many countries means that governments and payors are under intense pressure to control healthcare spending even more tightly. These pressures are particularly strong given the ongoing effects of the recent global economic and financial crisis, including the continuing debt crisis in certain countries in Europe, and the risk of a similar crisis in the United States. As a result, our businesses and the healthcare industry in general are operating in an ever more challenging environment with very significant pricing pressures. In recent years, national, federal, provincial, state, and local officials and legislators have proposed, or are reportedly considering proposing, a variety of price based reforms to the healthcare systems in the European Union, the United States and other countries. Some proposals include measures that would limit or eliminate payments for certain medical procedures and treatments or subject pricing to government control. Furthermore, in certain foreign markets, the pricing or profitability of healthcare products is subject to government controls and other measures that have been prepared by legislators and government officials. While we cannot predict whether any such legislative or regulatory proposals or reforms will be adopted, the adoption of any such proposals or reforms could adversely affect the commercial viability of our existing and potential products.
In March 2010, U.S. President Obama signed into law the Patient Protection and Affordable Care Act and the Health Care and Education Affordability Reconciliation Act of 2010. Certain provisions of the law will not be effective for a number of years and there are many programs and requirements for which the details have not yet been fully established or consequences not fully understood, and it is unclear what the full impacts will be from the law. The legislation imposes new taxes on medical device makers in the form of a 2.3% excise tax on all U.S. medical device sales. Under the legislation, the total cost to the medical device industry is expected to be approximately $20 billion over 10 years. The new tax could materially and adversely affect our business, cash flows and results of operations. The law also focuses on a number of Medicare provisions aimed at improving quality and decreasing costs. It is uncertain at this point what negative unintended consequences these provisions will have on patient access to new technologies. The Medicare provisions include value-based payment programs, increased funding of comparative effectiveness research, reduced hospital payments for avoidable readmissions and hospital acquired conditions, and pilot programs to evaluate alternative payment methodologies that promote care coordination (such as bundled physician and hospital payments). Additionally, the law includes a reduction in the annual rate of inflation for Medicare payments to hospitals that began in 2011 and the establishment of an independent payment advisory board to recommend ways of reducing the rate of growth in Medicare spending beginning in 2014. We cannot predict what health care programs and regulations will be ultimately implemented at the federal or state level, or the effect of any future legislation or regulation. However, any changes that lower reimbursement for our products or reduce medical procedure volumes could adversely affect our business and results of operations.
| 23 |
Our industry is the subject of numerous governmental investigations into marketing and other business practices. These investigations could result in the commencement of civil and/or criminal proceedings, substantial fines, penalties, and/or administrative remedies, divert the attention of our management, and have an adverse effect on our financial condition and results of operations.
Our industry is the subject of numerous governmental investigations into marketing and other business practices. This has included increased regulation, enforcement, inspections, and governmental investigations of the medical device industry and disclosure of financial relationships with health care professionals. In the United States, the laws in which we are subject to include:
| · | the federal Anti-Kickback Statute, which prohibits, among other things, soliciting, receiving, offering or providing remuneration intended to induce the purchase or recommendation of an item or service reimbursable under a federal healthcare program, such as the Medicare or Medicaid programs. This statute has been applied to medical device manufacturer marketing practices, educational programs, pricing policies and relationships with healthcare providers. A person or entity does not need to have actual knowledge of this statute or specific intent to violate it to have committed a violation; |
| · | federal civil and criminal false claims laws and civil monetary penalty laws, including civil whistleblower or qui tam actions that prohibit, among other things, knowingly presenting, or causing to be presented, claims for payment or approval to the federal government that are false or fraudulent, knowingly making a false statement material to an obligation to pay or transmit money or property to the federal government or knowingly concealing or knowingly and improperly avoiding or decreasing an obligation to pay or transmit money or property to the federal government. The government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes; |
| · | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, and its implementing regulations, which created federal criminal laws that prohibit, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| · | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, also imposes certain regulatory and contractual requirements regarding the privacy, security and transmission of individually identifiable health information; |
| · | federal “sunshine” requirements imposed by the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, on device manufacturers regarding any “transfer of value” made or distributed to physicians and teaching hospitals; and |
| · | state and foreign law equivalents of each of the above federal laws, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; state laws that require device companies to comply with the industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state laws that require device manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state laws governing the privacy and security of certain health information, many of which differ from each other in significant ways and often are not preempted by HIPAA. |
| 24 |
Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available under such laws, it is possible that some of our business activities, including certain sales and marketing practices and financial arrangements with physicians, could be subject to challenge under one or more of such laws. Any action against us, even if we successfully defend against it, could result in the commencement of civil and/or criminal proceedings, exclusion from governmental health care programs, substantial fines, penalties, and/or administrative remedies, divert the attention of our management, and have an adverse effect on our financial condition and results of operations. We anticipate that the government will continue to scrutinize our industry closely, and that additional regulation by governmental authorities, both foreign and domestic, may increase compliance costs, exposure to litigation and other adverse effects to our operations.
Our products are subject to extensive regulation, which can be costly and time consuming, cause unanticipated delays, or prevent the receipt of the required approvals to commercialize products.
The pre-clinical and clinical trials of any products developed by us and the manufacturing, labeling, sale, distribution, export or import, marketing, advertising and promotion of any of those products are subject to rigorous regulation by federal, provincial, state and local governmental authorities. Our medical devices are principally regulated in the United States by the FDA, in Canada by the Health Canada (particularly, the Therapeutic Products Directorate), in the European Union by the European Medicines Agency (EMA), and by other similar regulatory authorities in other jurisdictions. Government regulation substantially increases the cost and risk of researching, developing, manufacturing and selling products. Following several widely publicized issues in recent years, the FDA and similar regulatory authorities in other jurisdictions have become increasingly focused on product safety. This development has led to requests for more clinical trial data, for the inclusion of a significantly higher number of patients in clinical trials and for more detailed analysis of trial results. Consequently, the process of obtaining regulatory approvals, particularly from the FDA, has become more costly, time consuming and challenging than in the past. Any product developed by us or our future collaborative partners, if any, must receive all relevant regulatory approvals or clearances from the applicable regulatory authorities before it may be marketed and sold in a particular country.
Any of our products that receive regulatory approval could be subject to extensive post-market regulation that can affect sales, marketing and profitability.
With respect to any products for which we obtain regulatory approval, we will be subject to post-marketing regulatory obligations, including the requirements by the FDA, EMA and similar agencies in other jurisdictions to maintain records regarding product safety and to report to regulatory authorities serious or unexpected adverse events. The occurrence of unanticipated serious adverse events or other safety problems could cause the governing agencies to impose significant restrictions on the indicated uses for which the product may be marketed, impose other restrictions on the distribution or sale of the product or require potentially costly post-approval studies. In addition, post-market discovery of previously unknown safety problems or increased severity or significance of a pre-existing safety signal could result in withdrawal of the product from the market and product recalls. Compliance with extensive post-marketing record keeping and reporting requirements requires a significant commitment of time and funds, which may limit our ability to successfully commercialize approved products.
Our industry is subject to health and safety risks.
We produce products for human implantation and use. While we take substantial precautions such as laboratory and clinical testing, clinical studies, quality control and assurance testing and controlled production methods, the associated health and safety risks cannot be eliminated. Our products may be found to be, or to contain substances that are harmful to the health of our patients and customers and which, in extreme cases, may cause serious health conditions or death. This sort of finding may expose us to substantial risk of litigation and liability.
| 25 |
Further, we could be forced to discontinue production of certain products, which would harm our profitability. Neovasc maintains product liability insurance coverage; however, there is no guarantee that our current coverage will be sufficient or that we can secure insurance coverage in the future at commercially viable rates or with the appropriate limits.
We may face risks associated with our manufacturing operations.
Manufacturing operations are subject to numerous unanticipated technological problems and delays. Our manufacturing processes, products and their various components are, and will be, subject to regulations specified by the various regulatory bodies such as Health Canada and the FDA. There can be no assurance that we will be able to comply with all stated manufacturing regulations. Failure or delay by the Company to comply with such regulations or to satisfy regulatory inspections could have an adverse effect on the Company’s business and operations.
Additionally, two critical components of the Reducer are not readily available. The balloon portion of the delivery system is technically challenging to manufacture and the Reducer device, while a basic technology, must be manufactured in Israel due to restrictions on the transfer of intellectual property and manufacturing out of Israel stemming from certain research grants received by NML prior to the acquisition in July 2008.
Use of our products may increase the risk of animal disease.
Our critical raw material used in most of our customers’ devices is animal derived pericardial tissue. As this raw material is derived from an animal, it is subject to many inconsistencies and potential risks. The most notable risk is the disease Bovine Spongiform Encephalopathy (BSE), also known as mad cow disease which can arise from bovine tissue. Although the tissue originates from the United States where strict controls are in place to prevent diseased animals from being processed, it cannot be assured that the livestock in the United States will remain free from BSE. There is also no assurance that our supplier will regularly deliver tissue with the specifications required to manufacture its products.
The manufacture of our products is highly regulated and complex and we may experience supply interruptions that could harm our ability to manufacture products.
We use a broad range of raw and organic materials and other items in the design and manufacture of our products. Our products are manufactured from treated natural animal tissue and man-made materials. Our non-implantable products are manufactured from man-made raw materials including resins, chemicals, electronics and metals. We purchase certain of the materials and components used in the manufacture of our products from external suppliers, and we purchase certain supplies from single sources for reasons of quality assurance, cost-effectiveness, availability or constraints resulting from regulatory requirements. General economic conditions could adversely affect the financial viability of our suppliers, resulting in their inability to provide materials and components used in the manufacture of our products. While we work closely with suppliers to monitor their financial viability and to assure continuity of supply and maintain high quality and reliability, these efforts may not be successful. In addition, due to the rigorous regulations and requirements of regulatory authorities regarding the manufacture of our products (including the need for approval of any change in supply arrangements), we may have difficulty establishing additional or replacement sources on a timely basis or at all if the need arises. Although alternative supplier options are considered and identified, we typically do not pursue regulatory qualification of alternative sources due to the strength of our existing supplier relationships and the time and expense associated with the regulatory validation process. A change in suppliers could require significant effort or investment in circumstances where the items supplied are integral to product performance or incorporate unique technology, and the loss of any existing supply contract could have a material adverse effect on us.
In particular, the Tiara valve is made up of two major components: the leaflets and skirt, which are made from the Peripatch and the nitinol frame, which is manufactured by a well-established specialty manufacturer in the medical device industry. However, if this supplier were unable to provide the nitinol frame in the future, it would seriously impact the further development of the Tiara.
| 26 |
Regulatory agencies from time to time have limited or banned the use of certain materials used in the manufacture of medical device products. In these circumstances, transition periods typically provide time to arrange for alternative materials.
We are dependent on limited products for substantially all of our current revenues. If the volume or price of these products decline or the costs of related manufacturing, distribution or marketing increase, it could have a material adverse effect on our business, financial condition and results of operations and could cause the market value of our securities to decline.
Sales of a limited number of our products represent substantially all of our current revenues. If the volume or pricing of our existing significant products decline in the future, or our cost to manufacture, distribute our existing significant products increase in the future, our market our business, financial condition and results of operations could be materially adversely affected and this could cause the market value of our securities to decline. In addition, if these products were to become subject to any other issues, such as material adverse changes in prescription growth rates, unexpected side effects, regulatory proceedings, material product liability litigation, publicity affecting doctor or patient confidence or pressure from competitive products, the adverse impact on our business, financial condition, results of operations and the market value of our securities could be significant.
We may face exposure to adverse movements in foreign currency exchange rates.
Our business has expanded internationally and as a result, a significant portion of our revenues, expenses, current assets and current liabilities are preliminary denominated in U.S. dollars, Euros and other foreign currencies. The functional currency of Neovasc and its subsidiaries is the Canadian dollar and the presentation currency of our financial statements is U.S. dollars. A decrease in the value of such foreign currencies relative to the Canadian dollar could result in losses in revenues from currency exchange rate fluctuations. To date, we have not hedged against risks associated with foreign exchange rate exposure.
If we were to lose our foreign private issuer status under U.S. federal securities laws, we would likely incur additional expenses associated with compliance with the U.S. securities laws applicable to U.S. domestic issuers.
As a foreign private issuer, as defined in Rule 3b-4 under the Exchange Act, we are exempt from certain of the provisions of the U.S. federal securities laws. For example, the U.S. proxy rules and the Section 16 reporting and “short swing” profit rules do not apply to foreign private issuers. However, if we were to lose our status as a foreign private issuer, these regulations would immediately apply and we would also be required to commence reporting on forms required of U.S. companies, such as Forms 10-K, 10-Q and 8-K, rather than the forms currently available to us, such as Forms 40-F and 6-K. Compliance with these additional disclosure and timing requirements under these securities laws would likely result in increased expenses and would require our management to devote substantial time and resources to comply with new regulatory requirements. Further, to the extent that we were to offer or sell our securities outside of the United States, we would have to comply with the more restrictive Regulation S requirements that apply to U.S. companies, and we would no longer be able to utilize the multijurisdictional disclosure system forms for registered offerings by Canadian companies in the United States, which could limit our ability to access the capital markets in the future.
Failure to comply with the U.S. Foreign Corrupt Practices Act (FCPA), as well as the anti-bribery laws of the nations in which we conduct business (such as the UK’s Bribery Act or the Corruption of Foreign Public Officials Act of Canada (CFPOA), could subject us to penalties and other adverse consequences.
Our business is subject to the FCPA which generally prohibits companies and company employees from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. The FCPA also requires companies to maintain accurate books and records and internal controls, including at foreign-controlled subsidiaries. In addition, we are subject to other anti-bribery laws of the nations in which we conduct business that apply similar prohibitions as the FCPA (e.g., the UK’s Bribery Act, the CFPOA and the OECD Anti-Bribery Convention). Our employees or other agents may, without our knowledge and despite our efforts, engage in prohibited conduct under our policies and procedures and the FCPA or other anti-bribery laws that we may be subject to for which we may be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
| 27 |
Legislative actions, potential new accounting pronouncements, and higher insurance costs are likely to impact our future financial position or results of operations.
Future changes in financial accounting standards may cause adverse, unexpected revenue fluctuations and affect our financial position or results of operations. New pronouncements and varying interpretations of pronouncements have occurred with greater frequency and are expected to occur in the future. Compliance with changing regulations of corporate governance and public disclosure may result in additional expenses. All of these uncertainties are leading generally toward increasing insurance costs, which may adversely affect our business, results of operations and our ability to purchase any such insurance, at acceptable rates or at all, in the future.
We are dependent upon our key personnel to achieve our business objectives.
As a technology-driven company, intellectual input from key management and personnel is critical to achieve our business objectives. Consequently, our ability to retain these individuals and attract other qualified individuals is critical to our success. The loss of the services of key individuals might significantly delay or prevent achievement of our business objectives. In addition, because of a relative scarcity of individuals with the high degree of education and scientific achievement required for our business, competition among medical device companies for qualified employees is intense and, as a result, we may not be able to attract and retain such individuals on acceptable terms, or at all. In addition, other than our lead engineer on Tiara, we do not maintain “key person” life insurance on any of our officers, employees, or consultants, and so any delay in replacing such persons, or an inability to replace them with persons of similar expertise, would have a material adverse effect on our business, financial condition and results of operations.
We also have relationships with scientific collaborators at academic and other institutions, some of whom conduct research at our request or assist us in formulating our research and development strategies. These scientific collaborators are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. In addition, even though our collaborators are required to sign confidentiality agreements prior to working with us, they may have arrangements with other companies to assist such other companies in developing technologies that may prove competitive to us.
Incentive provisions for our key executives include the granting of stock options that vest over time, designed to encourage such individuals to stay with us. However, a low share price, whether as a result of disappointing progress in our sales or development programs or as a result of market conditions generally, could render such agreements of little value to our key executives. In such event, our key executives could be susceptible to being hired away by our competitors who could offer a better compensation package. If we are unable to attract and retain key personnel our business, financial conditions and results of operations may be adversely affected.
The continuing development of many of our products depends upon us maintaining strong relationships with physicians.
If we fail to maintain our working relationships with physicians, many of our products may not be developed and marketed in line with the needs and expectations of the professionals who use and support our products, which could cause a decline in our earnings and profitability. The research, development, marketing, and sales of our new and improved products is dependent upon our maintaining working relationships with physicians. We rely on these professionals to provide us with considerable knowledge and experience regarding the development, marketing, and sale of our products. Physicians assist us as researchers, marketing and product consultants, inventors, and public speakers. If we are unable to maintain our strong relationships with these professionals and continue to receive their advice and input, the development and marketing of our products could suffer, which could have a material adverse effect on our consolidated earnings, financial condition, and/or cash flows.
| 28 |
A period of significant growth can place a strain on management systems.
If we experience a period of significant growth in the number of personnel, this could place a strain upon its management systems and resources. Our future will depend in part on the ability of its officers and other key employees to implement and improve its financial and management controls, reporting systems and procedures on a timely basis and to expand, train and manage its employee workforce. There can be no assurance that we will be able to effectively manage such growth. Our failure to do so could have a material adverse effect upon our business, prospects, results of operation and financial condition.
Consolidation in the health care industry could have an adverse effect on our revenues and results of operations.
Many health care industry companies, including health care systems, are consolidating to create new companies with greater market power. Organizations such as GPOs, independent delivery networks, and large single accounts such as the U.S. Veterans Administration, continue to consolidate purchasing decisions for many of our health care provider customers. As a result, transactions with customers are larger, more complex, and tend to involve more long-term contracts. The purchasing power of these larger customers has increased, and may continue to increase, causing downward pressure on product pricing. If we are not one of the providers selected by one of these organizations, we may be precluded from making sales to its members or participants. Even if we are one of the selected providers, we may be at a disadvantage relative to other selected providers that are able to offer volume discounts based on purchases of a broader range of medical equipment and supplies. Further, we may be required to commit to pricing that has a material adverse effect on our revenues and profit margins, business, financial condition and results of operations. We expect that market demand, governmental regulation, third-party reimbursement policies and societal pressures will continue to change the worldwide health care industry, resulting in further business consolidations and alliances, which may exert further downward pressure on the prices of our products and could adversely impact our business, financial condition, and results of operations.
We may or may not successfully identify and complete corporate transactions on favorable terms or achieve anticipated synergies relating to any acquisitions or alliances, and such acquisitions could result in unforeseen operating difficulties and expenditures, require significant management resources and require significant charges.
As a part of our growth strategy, we regularly explore potential acquisitions of complementary businesses, technologies, services or products as well as potential strategic alliances or divestitures of assets or a sale of the Company. We may be unable to find suitable acquisition candidates or appropriate partners with which to form alliances. Even if we identify appropriate acquisition or alliance candidates, we may be unable to complete the acquisitions or alliances on favorable terms, if at all. Acquisition activities can be thwarted by overtures from competitors for the targeted candidates, government regulation and replacement product developments in our industry. In addition, the process of integrating an acquired business, technology, service or product into our existing operations could result in unforeseen difficulties and expenditures. Integration of an acquired company often requires significant expenditures as well as significant management resources that otherwise would be available for ongoing development of our other businesses. Moreover, we may not realize the anticipated financial or other benefits of an acquisition or alliance.
We may be required to take charges or write-downs in connection with acquisitions. In particular, acquisitions of businesses engaged in the development of new products may give rise to in-process research and development assets. To the extent that the value of these assets declines, we may be required to write down the value of the assets. Also, in connection with certain asset acquisitions, we may be required to take an immediate charge related to acquired in-process research and development. Either of these situations could result in substantial charges, which could adversely affect our results of operations.
| 29 |
Future acquisitions could also involve the issuance of equity securities, the incurrence of debt, contingent liabilities or amortization of expenses related to other intangible assets, any of which could adversely impact our financial condition or results of operations. In addition, equity or debt financing required for such acquisitions may not be available.
Any corporate transaction will be accompanied by certain risks including but not limited to:
| · | exposure to unknown liabilities of acquired companies and the unknown issues with any associated technologies or research; |
| · | higher than anticipated acquisition costs and expenses; |
| · | exposure to other companies’ shares that shareholders could receive as consideration for our shares in a corporate transaction; |
| · | the difficulty and expense of integrating operations, systems, and personnel of acquired companies; |
| · | disruption of our ongoing business; |
| · | inability to retain key customers, distributors, vendors and other business partners of the acquired company; and |
| · | diversion of management’s time and attention. |
We may not be able to successfully overcome these risks and other problems associated with acquisitions and this may adversely affect our business, financial condition or results of operations.
Anti-takeover provisions could discourage a third party from making a takeover offer that could be beneficial to our shareholders.
Some of the provisions in our articles of incorporation and by-laws could delay or prevent a third party from acquiring us or replacing members of our board of directors, even if the acquisition or the replacements would be beneficial to our shareholders. Such provisions include the following:
| · | shareholders cannot amend our articles of incorporation unless at least two-thirds of the shares entitled to vote approve the amendment; and |
| · | our board of directors can, without shareholder approval, issue preferred shares having any terms, conditions, rights and preferences that the board determines. |
These provisions could also reduce the price that certain investors might be willing to pay for our securities and result in the market price for our securities, including the market price for our common shares, being lower than it would be without these provisions.
Conflicts of interest may arise among the Company’s officers and directors as a result of their involvement with other issuers.
Certain of our directors and officers serve as directors, officers, promoters and members of management of other companies and, therefore, it is possible that a conflict may arise between their duties as a director or officer, and their duties as a director or officer of such other companies. There can be no assurance that in the carrying out of their duties with respect to us, these persons will not find themselves in situations which could give rise to conflicts of interest. There can be no assurance that if conflicts do arise, they will be resolved in a manner favourable to us. There can be no assurance that future transactions or arrangements between the companies and any of such entities will be advantageous to us.
Significant shareholders of the Company could influence our business operations and sales of our shares by such significant shareholders could influence our share price.
Frost Gamma Investments Trust (the “Frost Group”) is a significant shareholder of the Company holding approximately 22% of our common shares. The exercise of voting rights associated with shares held by the Frost Group at meetings of shareholders may have significant influences on our business operations. Also, the Frost Group owns shares for the purpose of investment, and therefore, if the Frost Group sells those shares in the market in the future, it could have significant influences on our share price, depending on the market environment at the time of such sale.
| 30 |
DIVIDEND POLICY
Neovasc has not declared or paid any dividends on the outstanding common shares since its inception and does not anticipate that it will do so in the foreseeable future. The declaration of dividends on Neovasc’s common shares is within the discretion of the Board of Directors and will depend on the assessment of, among other factors, earnings, capital requirements and the Company’s operating and financial condition. At the present time, anticipated capital requirements are such that the Company intends to follow a policy of retaining earnings in order to finance the further development of the business.
DESCRIPTION OF CAPITAL STRUCTURE AND MARKET FOR SECURITIES
The Company is authorized to issue an unlimited number of common shares without par value of which 66,836,745 were issued and outstanding as of March 29, 2016. The common shares all have equal voting rights and are entitled to receive notice of any shareholders meeting at which they have the right to vote. Subject to the rights of any other class of shares, upon any liquidation, dissolution, winding-up or other distribution of the Company’s assets, the holders of common shares are entitled to participate equally.
The Company is also authorized to issue an unlimited number of preferred shares, which do not have voting rights and are not entitled to receive notice of any shareholders meetings. Upon liquidation, dissolution, winding-up or other distribution of the Company’s assets, the holders of preferred shares are entitled to participate in priority to the holders of common shares. The preferred shares may be issued in series and the Company’s board of directors may attach special rights, privileges, restrictions or conditions to any preferred shares. There were no preferred shares issued and outstanding as of March 29, 2016.
Our Common Shares are listed on the TSX in Canada (trading symbol: NVC) and on Nasdaq in the United States (trading symbol: NVCN). Our Common Shares began trading on the TSX on June 23, 2014. The following table sets forth, for the periods indicated, the reported high, low and closing prices (in Canadian dollars) and volume traded on the TSX.
| 2015 | Max | Min | End | Total Volume |
| January | C$12.75 | C$7.50 | C$11.90 | 661,969 |
| February | C$12.42 | C$10.60 | C$12.27 | 364,889 |
| March | C$12.49 | C$11.10 | C$11.40 | 83,327 |
| April | C$11.35 | C$9.76 | C$9.76 | 68,657 |
| May | C$10.33 | C$7.58 | C$8.43 | 146,245 |
| June | C$9.15 | C$8.07 | C$8.55 | 38,258 |
| July | C$8.96 | C$6.55 | C$7.92 | 41,755 |
| August | C$7.69 | C$5.00 | C$7.38 | 37,678 |
| September | C$8.63 | C$6.96 | C$7.13 | 54,212 |
| October | C$8.48 | C$5.23 | C$7.19 | 78,667 |
| November | C$7.35 | C$5.31 | C$6.70 | 102,061 |
| December | C$6.89 | C$4.81 | C$6.25 | 53,172 |
Our Common Shares began trading on Nasdaq on May 21, 2014. The following table sets forth, for the periods indicated, the reported high, low and closing prices (in U.S. dollars) and volume traded on Nasdaq.
| 2015 | Max | Min | End | Total Volume |
| January | $10.12 | $6.45 | $9.35 | 3,683,828 |
| February | $9.90 | $8.48 | $9.90 | 1,492,519 |
| March | $9.90 | $8.29 | $9.00 | 1,425,413 |
| 31 |
| 2015 | Max | Min | End | Total Volume |
| April | $9.38 | $8.09 | $8.36 | 2,416,512 |
| May | $8.48 | $6.28 | $7.00 | 1,895,965 |
| June | $7.63 | $6.55 | $6.84 | 1,310,214 |
| July | $6.94 | $5.03 | $5.90 | 852,264 |
| August | $6.33 | $3.66 | $5.67 | 1,161,751 |
| September | $6.57 | $4.60 | $4.91 | 928,040 |
| October | $6.48 | $3.79 | $5.55 | 1,326,278 |
| November | $5.74 | $4.00 | $4.98 | 740,632 |
| December | $5.03 | $3.41 | $4.50 | 1,035,303 |
PRIOR SALES
The following table sets forth information in respect of our common shares that we issued upon the exercise of options granted under our incentive stock option plan during the 2015 financial year.
| Exercise Date | Number of Shares | Exercise Price |
| January 7, 2015 | 68,868 | C$0.010 |
| January 7, 2015 | 65,000 | C$0.355 |
| January 12, 2015 | 10,600 | C$0.355 |
| January 12, 2015 | 2,000 | C$1.000 |
| January 12, 2015 | 3,600 | C$1.450 |
| January 19, 2015 | 48,438 | C$0.010 |
| January 23, 2015 | 27,900 | C$0.355 |
| January 23, 2015 | 1,000 | C$0.970 |
| January 23, 2015 | 3,600 | C$1.000 |
| January 23, 2015 | 7,200 | C$1.450 |
| January 28, 2015 | 1,600,000 | C$0.355 |
| January 28, 2015 | 124,000 | C$1.000 |
| January 28, 2015 | 6,000 | C$1.450 |
| February 6, 2015 | 70,375 | C$0.355 |
| February 6, 2015 | 31,000 | C$0.970 |
| February 6, 2015 | 400 | C$2.700 |
| February 13, 2015 | 8,000 | C$0.355 |
| February 13, 2015 | 26,500 | C$0.970 |
| February 13, 2015 | 11,000 | C$2.490 |
| February 20, 2015 | 8,000 | C$0.355 |
| February 26, 2015 | 3,500 | C$0.970 |
| February 26, 2015 | 4,000 | C$1.000 |
| February 26, 2015 | 3,000 | C$1.450 |
| February 26, 2015 | 3,000 | C$2.490 |
| March 9, 2015 | 18,770 | C$0.010 |
| March 9, 2015 | 600 | C$2.490 |
| March 9, 2015 | 600 | C$2.500 |
| March 9, 2015 | 400 | C$2.700 |
| March 9, 2015 | 20 | C$6.500 |
| March 31, 2015 | 89,500 | C$1.450 |
| March 31, 2015 | 16,600 | C$2.490 |
| April 21, 2015 | 1,500 | C$0.970 |
| May 12, 2015 | 2,000 | C$1.450 |
| May 12, 2015 | 4,000 | C$2.490 |
| May 12, 2015 | 400 | C$6.500 |
| May 20, 2015 | 11,264 | C$0.010 |
| June 12, 2015 | 2,688 | C$0.010 |
| June 12, 2015 | 200 | C$1.000 |
| June 12, 2015 | 1,600 | C$1.450 |
| June 12, 2015 | 280 | C$6.500 |
| 32 |
| Exercise Date | Number of Shares | Exercise Price |
| July 9, 2015 | 6,000 | C$1.000 |
| July 24, 2015 | 700 | C$2.490 |
| August 6, 2015 | 900 | C$2.490 |
| August 21, 2015 | 1,250 | C$1.000 |
| October 16, 2015 | 12,000 | C$2.490 |
| October 16, 2015 | 2,000 | C$2.500 |
| November 10, 2015 | 200 | C$0.970 |
| November 23, 2015 | 4,500 | C$0.970 |
| November 23, 2015 | 100,000 | C$1.000 |
| December 10, 2015 | 19,450 | C$0.970 |
| December 14, 2015 | 66,000 | C$1.000 |
| December 14, 2015 | 7,200 | C$1.450 |
| Total | 2,507,603 |
The following table sets forth information in respect of options to acquire our common shares that we granted under our incentive stock option plan during the 2015 financial year.
| Grant Date | Number of Options | Grant Price |
| February 24, 2015 | 200,000 | C$11.76 |
| March 3, 2015 | 25,000 | C$12.16 |
| March 17, 2015 | 50,000 | C$12.02 |
| April 16, 2015 | 332,047 | C$10.61 |
| May 14, 2015 | 4,500 | C$8.45 |
| June 1, 2015 | 70,500 | C$8.43 |
| July 1, 2015 | 97,500 | C$8.55 |
| August 1, 2015 | 103,500 | C$7.92 |
| September 1, 2015 | 28,500 | C$7.38 |
| October 1, 2015 | 68,500 | C$7.13 |
| November 2, 2015 | 12,000 | C$7.19 |
| December 1, 2015 | 75,000 | C$6.70 |
| December 28, 2015 | 356,630 | C$5.19 |
| Total | 1,423,677 |
The following table sets forth information in respect of our common shares that we issued, other than on exercise of stock options as set out above, during the 2015 financial year.
| Issuance Date | Number of Shares | Issue Price |
| February 3, 2015 | 10,415,000 | $7.19 |
| Total | 10,415,000 |
No other common shares, preferred shares, debt securities or warrants, or securities exchangeable or convertible into common shares, preferred shares, debt securities or warrants have been issued during the 2015 financial year.
ESCROWED SECURITIES
The Company does not have any escrowed securities or securities subject to contractual restrictions on transfer.
DIRECTORS AND OFFICERS
All directors hold office until the next annual general meeting of the Company’s shareholders or until they resign or are removed from office in accordance with the Company’s articles and the Canada Business Corporations Act.
Each director has formally consented to serve as a director with Neovasc.
| 33 |
Neovasc’s current directors and officers, their business background and principal occupations during the five preceding years and the periods during which each has served in their positions as directors or officers are as follows:
Paul Geyer – Chairman of the Board and Director
Mr. Geyer is Chairman of the Board. On July 1, 2008, he resigned as President and Chief Executive Officer of the Company. Mr. Geyer has served on the Company’s board since November 2, 2000 and is a resident of British Columbia, Canada. In addition, Mr. Geyer is a member of the Company’s audit committee.
Since June 2009, Mr. Geyer has been Chief Executive Officer of LightIntegra Technology Inc., a private medical device company focused on the development of the ThromboLux technology, used as a point of care device to determine platelet quality for blood transfusions.
Mr. Geyer is an active angel investor and supporter of local technology and life sciences firms. He is on the board of directors of the BC Technology Industry Association, and is a director for several private companies. He served previously on the board of directors for the Medical Device Development Center and MEDEC, the association representing medical device companies across Canada. Mr. Geyer is Chairman of the Board of BC Social Venture Partners. In April 2011, Mr. Geyer was awarded the LifeSciences BC Leadership Award.
Mr. Geyer graduated with a B.A.Sc. in electrical engineering from the University of British Columbia and has taken post graduate programs in finance and biomedical engineering. Mr. Geyer has completed a Certified Director program at the Anderson Graduate School of Management at the University of California, Los Angeles.
Alexei Marko – Chief Executive Officer and a Director
Alexei Marko’s 18 years of experience in the medical device field spans product development, sales and marketing and executive management. Mr. Marko has held management positions with Neovasc's predecessor companies since 1999 and assumed the role of CEO in 2008 in conjunction with the company’s expansion and restructuring. Mr. Marko was appointed to the Company’s board of directors on June 12, 2003 and is a resident of British Columbia, Canada.
In October 2007, Mr. Marko was appointed President and Chief Operating Officer of Medical Ventures Corp. (MEV), a predecessor company. Previously, Mr. Marko was the Vice President and Chief Operating Officer and Vice President, Development and Engineering of MEV.
Mr. Marko is a listed inventor on a number of issued or pending patents related to medical technologies. He is also a registered professional engineer, and sits on the board of directors for the Medical Device Development Centre in Vancouver. In 2005, he was named one of Business in Vancouver’s “Top Forty Under 40” in recognition of his achievements.
Mr. Marko completed both his B.A.Sc. (Hons) at Queen’s University and an M.A.Sc. in electrical engineering at the University of British Columbia, specializing in medical device development. He is a registered professional engineer (P.Eng.)
Douglas Janzen - Director
Mr. Janzen has been involved in the life sciences industry for the past 19 years. He is currently the CEO of Northview Ventures, an entity which invests in, and provides strategic advisory services to, a number of technology companies predominately in the life sciences industry. Mr. Janzen has also been a director of iCo Therapeutics Inc., a company listed on the TSXV, since March 2012. Most Recently, Mr. Janzen has taken the position of CEO of Aequus Pharmaceuticals Inc., which listed on the TSXV on March 17, 2015. Mr. Janzen was originally elected to the Company’s Board of Directors on June 2, 2005 and is a resident of British Columbia, Canada. In addition, Mr. Janzen is a member of the Company’s Audit and Compensation Committees.
| 34 |
Previously, he was President and CEO of Cardiome Pharma Corp. (“Cardiome”), a Nasdaq-listed drug development company that completed an C$800 million licensing deal with subsidiaries of Merck & Co. and saw its lead product approved in Europe in 2010. Prior to his involvement with Cardiome, Mr. Janzen was an investment banker with Cormark Securities Inc., a Toronto-based investment bank, acting as Managing Director of Life Sciences. Mr. Janzen is the past Chairman of Life Sciences British Columbia, has served as a director of Biotech Canada, and sits as a director on a number of public and private boards. Mr. Janzen is a past winner of Vancouver’s “Top 40 under 40” award.
Steven Rubin - Director
Mr. Rubin has served as Executive Vice President - Administration since May 2007 and as a director of OPKO Health, Inc. (“OPKO”) since February 2007. Mr. Rubin served as the Senior Vice President, General Counsel and Secretary of IVAX from August 2001 until September 2006. Mr. Rubin currently serves on the Board of Directors of IDI, Inc., (NYSE MKT:IDI), a data and analytics company, Cocrystal Pharma, Inc., a biotechnology company developing antiviral therapeutics for human diseases, Non-Invasive Monitoring Systems, Inc. (OTCBB:NIMU), a medical device company, Sevion Therapeutics, Inc., a company that discovers, develops and acquires innovative product candidates for the treatment of cancer and immunological diseases, Tiger X Medical, Inc. (OTCBB:CDOM), previously an early-stage orthopedic medical device company specializing in designing, developing and marketing reconstructive joint devices and spinal surgical devices, Castle Brands, Inc. (NYSE MKT:ROX), a developer and marketer of premium brand spirits and Kidville, Inc., which operates large, upscale facilities, catering to newborns through five-year-old children and their families and offers a wide range of developmental classes for newborns to five-year-olds . Mr. Rubin previously served on the Board of Directors of Dreams, Inc., a vertically integrated sports licensing and products company (NYSE MKT: DRJ) and as a director of Safestitch Medical, Inc., a medical device Company (OTCQB:SFES), prior to its acquisition by TransEnterix, Inc. and PROLOR Biotech, Inc. prior to its merger with OKPO. Mr. Rubin was elected to the Company’s board of directors on July 1, 2008. He is a resident of the state of Florida, United States. Mr. Rubin is also a member of the Company’s Audit and Governance and Nominating Committees.
Dr. Jane Hsiao - Director
Dr. Hsiao has served as Vice-Chairman and Chief Technical Officer of the Company since May 2007 and as a director since February 2007. Dr. Hsiao served as the Vice Chairman-Technical Affairs of IVAX from 1995 to January 2006. Dr. Hsiao served as Chairman, Chief Executive Officer and President of IVAX Animal Health, IVAX’s veterinary products subsidiary, from 1998 to 2006. Dr. Hsiao has served as Chairman of the Board of Non-Invasive Monitoring Systems, Inc. (OTCBB:NIMU), a medical device company, since October 2008 and was named Interim Chief Executive Officer of Non-Invasive Monitoring Systems, Inc. in February 2012. Dr. Hsiao is also a director of each of TransEnterix, Inc. (OTCBB:TRXC), a medical device company, and Cocrystal Pharma, Inc. (OTCBB: COCP), a publicly traded biotechnology company developing new treatments for viral diseases. Dr. Hsiao previously served as a director for Sorrento Therapeutics, Inc. (OTCBB:SRNE), a development stage biopharmaceutical company, PROLOR Biotech, Inc. prior to its acquisition by the Company in August 2013, and as Chairman of the Board of SafeSitch Medical, Inc. prior to its merger with TransEnterix, Inc. Dr. Hsiao was elected to the Company’s board of directors on July 1, 2008. She is a resident of the state of Florida, United States. Dr. Hsiao is also a member of the Company’s Compensation and Governance and Nominating Committee.
Dr. William O’Neill – Director
Currently, Dr. O’Neill is the Medical Director, Center for Structural Heart Disease, Henry Ford Hospital, Detroit, Michigan. Previously, he was Executive Dean of Clinical Affairs and Chief Medical Officer, University of Miami Health System at the Miller School of Medicine, University of Miami. Prior to this position, from 1987 to 2006, Dr. O’Neill was the director of the division of Cardiovascular Disease at William Beaumont Hospital, Royal Oak, co-director of the Beaumont Heart Center, Royal Oak, and Corporate Chief of Cardiology, William Beaumont Hospitals, Royal Oak and Troy. Dr. O’Neill was named Vice Chair Department of Internal Medicine for Research in January 2003. Prior to joining Beaumont, he was Director of the cardiac catheterization laboratory at the University of Michigan in Ann Arbor and was an Associate Professor of Medicine at the University of Michigan Medical School. Dr. O’Neill is an international leader in the field of interventional cardiology and in the research of new techniques to diagnose and treat obstructed heart arteries.
| 35 |
Dr. O’Neill was originally elected to the Company’s board of directors on July 1, 2008. He is a resident of the state of Michigan, United States. Dr. O’Neill is also a member of the Company’s Compensation and Governance and Nominating Committee.
He is certified in interventional cardiology and cardiovascular disease by the American Board of Internal Medicine. An author of more than 35 book chapters, 230 articles and 330 abstracts, Dr. O’Neill is a graduate of Wayne State University School of Medicine and completed a cardiology fellowship at the University of Michigan Hospital.
Chris Clark – Chief Financial Officer and Corporate Secretary
In April 2007, Mr. Clark was appointed Chief Financial Officer of the Company. Prior to that, Mr. Clark was Director of Finance of Mr. Lube Canada Inc. from 2005 to 2007. Mr. Clark was Director of Finance, Healthpricer Interactive Inc. (formerly One Person Health Services Inc.) from 2004 to 2005 and currently serves as a director of Aequus Pharmaceuticals Inc. (TSXV: AQS). He is a resident of British Columbia, Canada.
Mr. Clark has almost 20 years finance and accounting experience in public practice and in public and private companies, most recently focused in the medical device sector. He received his designation as a Chartered Accountant from the Institute of Chartered Accountants of England and Wales and articled with KPMG before moving to Canada in 1998. He has an honors degree in Economics from Swansea University and a post graduate diploma from Keble College, Oxford.
Brian McPherson – Chief Operating Officer
In June 2009, Mr. McPherson was appointed Chief Operations Officer of the Company. Prior to that Mr. McPherson was Director of Operations from 2008 to 2009. Mr. McPherson was Operations Manager for Pyng Medical from 2003 to 2006, where he also served on the board of directors. Prior to its acquisition by Medtronic, he was a Senior Operations Manager and served on the board of directors of Arterial Vascular Engineering Canada from 1995 to 1999.
Mr. McPherson has more than 20 years’ experience in medical device manufacturing and operations. He holds two diplomas in technology from the British Columbia Institute of Technology, with the most recent in Biomedical Engineering. He is a resident of British Columbia.
Randy Lane, Vice-President, Research and Development
In July 2007, Mr. Lane joined Neovasc, and in May 2014 he was promoted to the position of Vice-President, Research and Development. Prior to joining the Company, Mr. Lane held senior roles at global cardiovascular device firms, including 10 years in product development and manufacturing with Sorin Group Canada Inc.
Mr. Lane has almost 18 years in the medical device industry. Possessing expertise in prosthetic heart valve design and testing, Mr. Lane represents Canada on the ISO 5840 Committee as a technical expert in heart valves and has led teams throughout the complete development program, including the development of process improvements, product development and regulatory testing. Mr. Lane leads a team developing the Tiara.
Mr. Lane holds a Bachelor of Science degree from McGill University, Montreal, Quebec, and is a resident of British Columbia, Canada.
| 36 |
Vicki Bebeau, Vice-President of Clinical Affairs
In May 2014, Ms. Bebeau joined Neovasc as Vice-President of Clinical Affairs. Ms. Bebeau has more than 18 years of experience, fulfilling leadership roles with multinational cardiovascular device firms such as St. Jude Medical, Boston Scientific, and Medtronic. Having planned and directed numerous successful clinical studies of prosthetic heart valves and other cardiovascular devices in support of IDE, PMA, and post market programs to support regulatory approvals, Ms. Bebeau’s efforts have contributed to the adoption of some of the industry’s most novel devices in the United States, Canada, Europe, Australia, and Japan.
Ms. Bebeau is a Registered Nurse whose specific areas of clinical research have included heart valves (open heart and percutaneous), vascular access and closure devices, FFR, OCT, renal denervation, and hypothermia.
Ms. Bebeau holds a Bachelor of Science in Nursing from Bethel College. She represents Canada on the ISO 5840 Committee as a clinical expert in heart valves. Ms. Bebeau is also a MedTech Industry Advisory Board Member for St. Cloud State University. She is a resident of White Bear Lake, Minnesota, USA.
Director & Officer Ownership of Securities
The following table sets out details of the Company’s shares, options and warrants that are directly or indirectly held by directors and executive officers as at the date hereof:
| Name | Number of Common Shares(1) |
Percentage of Outstanding Common Shares |
Number of Common Shares held under Options and Warrants(1) |
| Paul Geyer | 2,144,604 | 3.21% | 335,000 |
| Alexei Marko | 298,236 | 0.45% | 761,250 |
| Doug Janzen | 120,050 | 0.18% | 250,000 |
| Steven Rubin | 206,139 | 0.31% | 315,000 |
| Dr. Jane Hsiao | 2,576,280 | 3.85% | 275,000 |
| Dr. William O’Neill | Nil | 0.00% | 275,000 |
| Chris Clark | 269,335 | 0.40% | 609,000 |
| Brian McPherson | 31,000 | 0.05% | 456,750 |
| Randy Lane | Nil | 0.00% | 567,250 |
| Vicki Bebeau | 10,000 | 0.01% | 200,000 |
| TOTAL | 5,655,644 | 8.47% | 4,044,250 |
Note 1: The information as to shares beneficially owned or over which control or direction is exercised, not being within the knowledge of Neovasc, has been furnished by each director and executive officer individually or from insider reports filed by the individuals and available at www.sedi.ca.
CEASE TRADE ORDERS, BANKRUPTCIES, PENALTIES OR SANCTIONS
Except as disclosed below, no director or executive officer of the Company:
Cease Trade Orders and Bankruptcies
No director of the Company is, as of the date of this Annual Information Form, or has been, within the ten years prior to the date hereof, a director or chief executive officer or chief financial officer of any company (including the Company) that: (i) was subject to an order that was issued while the proposed director was acting as a director, chief executive officer or chief financial officer; or (ii) was subject to an order that was issued after the director ceased to be a director, chief executive officer or chief financial officer and which resulted from an event that occurred while that person was acting in the capacity as director, chief executive officer or chief financial officer.
| 37 |
No director of the Company is, at the date of this Annual Information Form, or has been within ten years before the date of this Annual Information Form, a director or executive officer of any company (including the Company) that, while that person was acting in that capacity, or within a year of that person ceasing to act in that capacity, became bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency or was subject to or instituted any proceedings, arrangement or compromise with creditors or had a receiver, receiver manager or trustee appointed to hold its assets.
Penalties and Sanctions
No director of the Company has been subject to any penalties or sanctions imposed by a court relating to securities legislation or by a securities regulatory authority or has entered into a settlement agreement with a securities regulatory authority, or has been subject to any other penalties or sanctions imposed by a court or regulatory body that would likely be considered important to a reasonable securityholder in deciding whether to vote for a director.
Individual Bankruptcies
No director of the Company has, within the ten years before the date of this Annual Information Form, become bankrupt, made a proposal under any legislation relating to bankruptcy or insolvency, or become subject to or instituted any proceedings, arrangement or compromise with creditors, or had a receiver, receiver manager or trustee appointed to hold the assets of the director.
CONFLICTS OF INTEREST
Some of the Company’s directors and officers are also directors and officers of other reporting companies. It is possible, therefore, that a conflict may arise between their duties as a director or officer of the Company and their duties as a director or officer of such other companies. All such conflicts are disclosed by them in accordance with the Canada Business Corporations Act and they govern themselves in respect thereof to the best of their ability in accordance with the obligations imposed upon them by law.
In the event that any of the Company’s directors or officers has a material interest in any material contract or proposed contract involving the Company they are required to disclose their interest to the board of directors either in writing or in person at a meeting of the directors. Any such contract is then considered and approved by a majority of the disinterested directors. Additionally, any non-arm’s length or related party transaction that requires the approval of the TSX will be subject to more restricted filing and disclosure requirements. Related party transactions are required to be disclosed in the Company’s financial statements.
AUDIT COMMITTEE INFORMATION
Pursuant to National Instrument 52-110, Neovasc is required to have an Audit Committee and make the following disclosure.
The Audit Committee's Charter
See Schedule “A”.
Composition of the Audit Committee
The members of the Audit Committee are Paul Geyer, Steven Rubin and Doug Janzen. Information regarding their relevant education and experience can be found under the heading “Directors and Officers”.
| 38 |
Messrs. Rubin, Geyer and Janzen are independent members(1) of the Audit Committee. All members of the Audit Committee are considered to be financially literate(2).
| (1) | A member of an audit committee is independent if the member has no direct or indirect material relationship with the Company, which could, in the view of the Board of Directors, reasonably interfere with the exercise of a member’s independent judgment. |
| (2) | An individual is financially literate if he has the ability to read and understand a set of financial statements that present a breadth of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by the Company’s financial statements. |
Audit Committee Oversight
The Audit Committee has not made any recommendations to the Board to nominate or compensate any auditor other than Grant Thornton LLP.
Reliance on Certain Exemptions
The Company’s auditor, Grant Thornton LLP, has not provided any material non-audit services during the financial year ended December 31, 2015.
Since the effective date of NI 52-110, the Company has not relied on the exemptions contained in section 2.4 or Part 8 of NI 52-110. Section 2.4 provides an exemption from the requirements that the Audit Committee must pre-approve all non-audit services to be provided by the auditor, where the total amount of fees related to the non-audit services are not expected to exceed 5% of the total fees payable to the auditor in the fiscal year in which the non-audit services were provided. Section 8 permits a company to apply to a securities regulatory authority for an exemption from the requirements of NI 52-110, in whole or in part.
Pre-Approval Policies and Procedures
See Audit Committee Charter for specific policies and procedures for the engagement of non-audit services.
External Auditor Service Fees
The Audit Committee has reviewed the nature and amount of the non-audited services provided by Grant Thornton LLP to the Company to ensure auditor independence. Fees incurred with Grant Thornton LLP for audit and non-audit services in the last two fiscal years for audit fees are outlined in the following table:
| Year ended December 31, 2014 |
Year ended December 31, 2015 | |
| Audit Fees | $63,079 | $58,572 |
| Audit-Related Fees | $93,588 | $59,409 |
| Tax Fees | Nil | Nil |
| All Other Fees | $941 | $773 |
| Total | $157,608 | $118,754 |
Audit fees: All services performed by the Auditors in connection with the review of annual consolidated financial statements of the Company including services performed to comply with generally accepted auditing standards.
| 39 |
Audit Related Fees: All services performed by the Auditors in connection with: the review of quarterly financial statements in accordance with generally accepted standards for a review; equity due diligence required by underwriters, regulators and other parties in connection with raising capital for the Company and internal control reviews.
Tax Fees: All services performed by the Auditors in connection with tax planning, compliance and advice.
Other Fees: All services performed by the Auditors outside of the services described above.
INTEREST OF MANAGEMENT AND OTHERS IN MATERIAL TRANSACTIONS
Other than as set forth herein and other than transactions carried out in the ordinary course of business of the Company or any of its subsidiaries, none of the directors or executive officers of the Company, any shareholder directly or indirectly beneficially owning, or exercising control or direction over, shares carrying more than 10% of the voting rights attached to the shares of the Company, nor an associate or affiliate of any of the foregoing persons has during the three most recently completed financial years, or during the current financial year, had any material interest, direct or indirect, in any transactions that materially affected or is reasonably expected to materially affect the Company or any of its subsidiaries.
MATERIAL CONTRACTS
There are no contracts that are material to the Company, that were entered into other than in the ordinary course of business and not excepted from disclosure and filing requirements, and that were entered into within the financial year ended December 31, 2015.
LEGAL PROCEEDINGS
Except for as otherwise disclosed below, there are no material outstanding legal proceedings or regulatory actions to which the Company is party, nor, to Neovasc’s knowledge, are there any such proceedings or actions contemplated.
The Company is engaged as a defendant in lawsuits filed by CardiAQ, as further described below. Litigation resulting from CardiAQ’s claims could be costly and time-consuming and could divert the attention of management and key personnel from our business operations. We cannot assure that we will succeed in defending any of these claims and that judgments will not be entered against us with respect to the litigation resulting from such claims. If we are unsuccessful in our defense of these claims or unable to settle the claims in a manner satisfactory to us, we may be faced with significant monetary damages, loss of intellectual property rights, or injunctive relief against us that could have a material adverse effect on our business and financial condition.
On June 6, 2014, Neovasc was named in a lawsuit filed by CardiAQ in the U.S. District Court for the District of Massachusetts concerning intellectual property rights ownership, unfair trade practices and a breach of contract relating to Neovasc’s transcatheter mitral valve technology, including the Tiara. On June 23, 2014, CardiAQ also filed a complaint against Neovasc in Germany requesting that Neovasc assign its right to one of its European patent applications to CardiAQ. On July 7, 2014, the Company was made aware through a press release issued by CardiAQ of a stay in proceedings for Neovasc’s European patent application that is the subject of the German lawsuit. This stay of proceedings was granted without an opportunity for Neovasc to respond to CardiAQ’s allegations. The Company requested that the stay be lifted, but the request was denied by the European Patent office pending resolution of the German lawsuit. Neovasc filed its response to the German lawsuit in December 2014.
The Company intends to vigorously defend itself in both lawsuits. After motion practice and discovery, the District of Massachusetts case is currently scheduled to go to trial on May 2, 2016. The court in Munich is expected to render its decision after a hearing currently scheduled for August 2016.
| 40 |
NAMES AND INTEREST OF EXPERTS
No person or company, whose profession or business gives authority to the statement, report, valuation or opinion, who is named as having prepared or certified a statement, report, valuation or opinion described or included in a filing, or referred to in a filing, made under National Instrument 51-102 by the Company during, or relating to, the Company’s most recently completed financial year holds any beneficial interest, direct or indirect, in any securities or property of the Company or of an associate or affiliate of the Company and no such person is expected to be elected, appointed or employed as a director, officer or employee of the Company or of an associate or affiliate of the Company and no such person is a promoter of the Company or an associate or affiliate of the Company. In particular, the current auditors of the Company are Grant Thornton, LLP, Chartered Accountants, 1600 – 333 Seymour St., Vancouver, BC, V6B 5A6. Grant Thornton LLP has reported on Neovasc’s fiscal 2015 audited consolidated financial statements, which have been filed with the securities regulatory authorities. As of March 29, 2016, Grant Thornton LLP has informed the Company that it is independent with respect to the Company within the meaning of the Rules of Professional Conduct of the Institute of Chartered Accountants of British Columbia.
TRANSFER AGENT AND REGISTRAR
Neovasc’s transfer agent and registrar is Computershare Investor Services Inc., 510 Burrard Street, 2nd Floor, Vancouver, British Columbia, Canada, V6C 3B9.
ADDITIONAL INFORMATION
Additional information relating to the Company is available under the Company's profile on the SEDAR website at www.sedar.com and on the website of the U.S. Securities and Exchange Commission at www.sec.gov.
ADDITIONAL FINANCIAL INFORMATION
Additional financial information relating to our Company is provided in our comparative financial statements and management's discussion and analysis for the years ended December 31, 2015 and 2014.
| 41 |
SCHEDULE “A”
AUDIT COMMITTEE CHARTER
Neovasc Inc. (formerly Medical Ventures Corporation)
2007
MEDICAL VENTURES CORPORATION
| 1. | PURPOSE |
The Audit Committee is responsible for assisting the Board of Directors (the “Board”) in fulfilling its oversight responsibilities in relation to:
| · | the integrity of Medical Ventures Corp. (the “Corporation”) financial statements; |
| · | the Corporation’s compliance with legal and financial regulatory requirements; |
| · | the qualifications and independence of the Corporation’s auditor; |
| · | the adequacy and effectiveness of internal controls over financial reporting and disclosure controls; |
| · | the performance of the Corporation’s internal audit function and independent auditor; |
| · | preparing an audit committee report to be included in the Corporation’s management information circular; and |
| · | any additional matters delegated to the Audit Committee by the Board. |
| 2. | MEMBERS |
The Board must appoint a minimum of three directors to be members of the Audit Committee. All of the members of the Audit Committee will meet the criteria for independence contained in applicable laws and stock exchange rules and regulations and at least a majority must be residents of Canada (so long as this is required under applicable law). In addition, every member of the Audit Committee will be Financially Literate and at least one member will have accounting or related financial management expertise, as the Board interprets such qualification in its business judgment. “Financially Literate” means the ability to read and understand a set of financial statements that present a breadth and level of complexity of accounting issues that are generally comparable to the breadth and complexity of the issues that can reasonably be expected to be raised by the Corporation’s financial statements.
| 3. | RESPONSIBILITIES |
The Audit Committee is responsible for performing the duties set out below as well as any other duties delegated to the Audit Committee by the Board.
| (a) | Appointment and Review of the Auditor |
The auditor is ultimately accountable to the Audit Committee and reports directly to the
Audit Committee. Accordingly, the Audit Committee will evaluate and be responsible for the Corporation’s relationship with the auditor. Specifically, the Audit Committee will:
| · | select, evaluate and nominate the auditor to be proposed for appointment or reappointment, as the case may be, by the shareholders; |
| · | review and approve the auditor’s engagement letter; |
| · | after seeking and taking into account the opinions of senior management and the officer in charge of internal audit, review the independence, experience, qualifications and performance of the auditor, including the lead audit partner, in recommending its appointment or reappointment, including considering whether the auditor’s quality controls are adequate and the auditor’s provision of any permitted non-audit services is compatible with maintaining its independence; |
| · | oversee the auditor’s work, including resolving any disagreements between management and the auditor regarding financial reporting; |
| · | at least annually, obtain and review a report by the auditor describing its internal quality-control procedures, any material issues raised by the most recent internal quality-control review, or peer review, of the firm, or by any inquiry or investigation by governmental or professional authorities, within the preceding five years, respecting one or more independent audits carried out by the auditor and any steps taken to deal with any such issues; and |
| · | where appropriate, terminate the auditor. |
| (b) | Confirmation of the Auditor’s Independence |
At least annually, and before the auditor issues its report on the Corporation’s annual financial statements, the Audit Committee will:
| · | confirm that the auditor has submitted a formal written statement describing all of its relationships with the Corporation that in the auditor’s professional judgment may reasonably be thought to bear on its independence; |
| · | discuss with the auditor any disclosed relationships or services that may affect its independence; |
| · | obtain written confirmation from the auditor that it is independent with respect to the Corporation within the meaning of the Rules of Professional Conduct adopted by the Canadian Institute of Chartered Accountants to which it belongs and that it is an independent public accountant with respect to the Corporation within the meaning of federal securities legislation; and |
| · | confirm that the auditor has complied with applicable laws with respect to the rotation of certain members of the audit engagement team for the Corporation. |
| (c) | Pre-Approval of Non-Audit Services |
The Audit Committee will pre-approve the appointment of the auditor for any non-audit service to be provided to the Corporation or its subsidiaries, provided that it will not approve any service that is prohibited under applicable laws, rules and regulations. The Audit Committee may establish policies and procedures, that may be revised from time to time, which pre-approve the appointment of the auditor for certain non-audit services. In addition, the Audit Committee may delegate to one or more independent members the authority to pre-approve the appointment of the auditor for any non-audit service to the extent permitted by applicable law, provided that any pre-approvals granted pursuant to such delegation shall be reported to the full Audit Committee at its next scheduled meeting following such pre-approval.
| A-2 |
| (d) | Communications with the Auditor |
The Audit Committee has the authority to communicate directly with the auditor and will meet privately with the auditor as frequently as the Audit Committee feels is appropriate to fulfill its responsibilities, which will not be less frequently than annually, to discuss any items of concern to the Audit Committee or the auditor, including, without limitation:
| · | planning and staffing of the audit; |
| · | any material written communications between the auditor and management, such as any management letter or schedule of unadjusted differences; |
| · | whether or not the auditor is satisfied with the quality and effectiveness of financial recording procedures and systems; |
| · | the extent to which the auditor is satisfied with the nature and scope of its examination; |
| · | any instances of fraud or other illegal acts involving senior management of the Corporation; |
| · | whether or not the auditor has received the full co-operation of senior management and other employees of the Corporation and whether the auditor has encountered any audit problems or difficulties in the course of its audit work, including any restrictions on the scope of the auditor’s work or access to required information and any significant disagreements with management (along with management’s response); |
| · | the auditor’s opinion of the competence and performance of the Chief Financial Officer and other key financial personnel; and |
| · | the items required to be communicated to the Audit Committee under the Canadian authoritative guidance or under Canadian generally accepted auditing standards. |
| (e) | Review of the Audit Plan |
The Audit Committee will discuss with the auditor the nature of an audit and the responsibility assumed by the auditor when conducting an audit under Canadian generally accepted auditing standards. The Audit Committee will review a summary of the auditor’s audit plan for each audit.
| (f) | Review of Audit Fees |
The Audit Committee will determine the auditor’s fee and the terms of the auditor’s engagement. In determining the auditor’s fee, the Audit Committee will consider, among other things, the number and nature of reports to be issued by the auditor, the quality of the internal controls of the Corporation, the size, complexity and financial condition of the Corporation and the extent of internal audit and other support to be provided to the auditor by the Corporation.
| (g) | Review of Financial Statements |
The Audit Committee will review and discuss with management and the auditor the annual audited financial statements, together with the auditor’s report thereon, and the interim financial statements, before recommending them for approval by the Board. The Audit Committee will also review and discuss with management and the auditor:
| · | management’s discussion and analysis relating to the annual audited financial statements and interim financial statements; |
| A-3 |
| · | all critical accounting policies and practices used or to be used by the Corporation; and |
| · | all alternative treatments of financial information within generally accepted accounting principles that have been discussed with management, ramifications of the use of such alternative disclosures and treatments, and the treatment preferred by the auditor. |
The Audit Committee may also engage the auditor to review the interim financial statements and any reconciliation of the Corporation’s financial statements prior to the Audit Committee’s review of such financial statements or reconciliation.
| (h) | Review of Other Financial Information |
The Audit Committee will:
| · | review annual and interim earnings press releases prior to their public release, as well as financial information and earnings guidance provided to analysts and rating agencies. The Audit Committee will also review the type and presentation of information to be included in such press releases and guidance (including the use of “pro forma” or “adjusted” non-GAAP financial measures); |
| · | ensure that adequate procedures are in place for management’s review of all other financial information extracted or derived from the Corporation’s financial statements that were previously reviewed by the Audit Committee before such information is released to the public, including, without limitation, financial information or statements for use in prospectuses or other offering or public disclosure documents and financial statements required by regulatory authorities, and the Audit Committee shall periodically assess the adequacy of those procedures; |
| · | review major issues regarding accounting principles and financial statement presentations, including any significant changes in the Corporation’s selection or application of accounting principles, and major issues as to the adequacy of the Corporation’s internal controls and any special audit steps adopted in light of any material control deficiencies; |
| · | review analyses prepared by management and/or the auditor setting forth significant financial reporting issues and judgments made in connection with the preparation of the financial statements, including analyses of the effects of alternative GAAP methods of the financial statements; and |
| · | review the effect of regulatory and accounting initiatives as well as off-balance sheet structures on the Corporation’s financial statements. |
| (i) | Relations with Senior Management |
The Audit Committee members will meet privately with senior management as frequently as the Audit Committee feels is appropriate to fulfill its responsibilities, which will not be less frequently than annually to discuss any areas of concern to the Audit Committee or senior management.
| (j) | Oversight of Internal Controls and Disclosure Controls |
The Audit Committee will review with senior management the adequacy of the internal controls that have been adopted by the Corporation to safeguard assets from loss and unauthorized use, to prevent, deter and detect fraud, and to verify the accuracy of the financial records. The Audit Committee will review any special audit steps adopted in light of material weaknesses or significant deficiencies.
| A-4 |
The Audit Committee will review with senior management the controls and procedures that have been adopted by the Corporation to confirm that material information about the Corporation and its subsidiaries that is required to be disclosed under applicable law or stock exchange rules is disclosed within the required time periods. The Audit Committee will also review disclosures made to it by the Chief Executive Officer and Chief Financial Officer during their certification process for applicable securities law filings about any significant deficiencies and material weaknesses in the design or operation of the Corporation’s internal control over financial reporting which are reasonably likely to adversely affect the Corporation’s ability to record, process, summarize and report financial information required to be disclosed by the Corporation in the reports that it files or submits under applicable Canadian federal and provincial legislation and regulations within the required time periods, and any fraud, whether or not material, involving management or other employees who have a significant role in the Corporation’s internal control over financial reporting.
| (k) | Legal and Regulatory Compliance |
The Audit Committee will review with the Corporation’s legal counsel any legal or regulatory matters that could have a significant effect on the Corporation’s financial statements. It will also review with legal counsel material inquiries received from regulators and governmental agencies and advise the Board accordingly.
| (l) | Risk Assessment and Risk Management |
The Audit Committee will review periodically with senior management the Corporation’s guidelines and policies with respect to risk assessment and risk management, including the steps and process taken to monitor and control risks.
| (m) | Taxation Matters |
The Audit Committee will periodically review with senior management the status of significant taxation matters of the Corporation.
| (n) | Hiring Employees of the Auditor |
The Audit Committee has established and will continue to maintain and monitor compliance with policies for hiring partners and employees and former partners and employees of the auditor.
| 4. | COMPLAINTS PROCEDURE |
The Audit Committee has established, and will continue to maintain, procedures for the receipt, retention and treatment of complaints received by the Corporation regarding accounting, internal accounting controls, auditing matters and disclosure controls and procedures for the confidential, anonymous submission of concerns by employees of the Corporation regarding questionable accounting or auditing matters or disclosure controls.
| 5. | REPORTING |
The Audit Committee will regularly report to the Board on:
| · | the auditor’s independence; |
| · | the performance of the auditor and the Audit Committee’s recommendations regarding its reappointment or termination; |
| · | the performance of the internal audit function; |
| A-5 |
| · | the adequacy of the Corporation’s internal controls and disclosure controls; |
| · | its recommendations regarding the annual and interim financial statements of the Corporation and any reconciliation of the Corporation’s financial statements, including any issues with respect to the quality or integrity of the financial statements; |
| · | its review of the annual and interim management’s discussion and analysis; |
| · | any issues that arise with respect to the Corporation’s compliance with legal and regulatory requirements; and |
| · | all other significant matters it has addressed and with respect to such other matters that are within its responsibilities. |
| 6. | REVIEW AND DISCLOSURE |
The Audit Committee will review this Charter at least annually and submit it to the Board together with any proposed amendments. The Board will review the Charter and approve such further amendments as it deems necessary and appropriate.
| 7. | ASSESSMENT |
At least annually, the Corporate Governance Committee will review the effectiveness of the Audit Committee in fulfilling its responsibilities and duties as set out in this Charter and in a manner consistent with the corporate governance guidelines adopted by the Board.
| 8. | CHAIR |
Each year, the Board will appoint one member to be Chair of the Audit Committee. If, in any year, the Board does not appoint a Chair, the incumbent Chair will continue in office until a successor is appointed.
| 9. | REMOVAL AND VACANCIES |
Any member may be removed and replaced at any time by the Board, and will automatically cease to be a member as soon as the member ceases to meet the qualifications set out above. The Board will fill vacancies on the Audit Committee by appointment from among qualified members of the Board. If a vacancy exists on the Audit Committee, the remaining members will exercise all of its powers so long as a quorum remains in office.
| 10. | ACCESS TO INDEPENDENT COUNSEL AND OTHER ADVISORS |
In carrying out its duties, the Audit Committee may retain independent counsel and any other outside advisor at the expense of the Corporation without Board approval at any time and has the authority to determine any such counsel’s or advisor’s fees and other retention terms. The Corporation shall also provide appropriate funding, as determined by the Audit Committee, for the payment of the compensation of the auditor, independent counsel and outside advisors and any ordinary administrative expenses of the Audit Committee that are necessary or appropriate in carrying out its duties.
| A-6 |




