false2021FY0001388658http://fasb.org/us-gaap/2021-01-31#AccountingStandardsUpdate201613MemberP3Y00013886582021-01-012021-12-3100013886582021-06-30iso4217:USD00013886582022-02-18xbrli:shares00013886582021-12-3100013886582020-12-31iso4217:USDxbrli:shares00013886582020-01-012020-12-3100013886582019-01-012019-12-310001388658us-gaap:CommonStockMember2018-12-310001388658us-gaap:AdditionalPaidInCapitalMember2018-12-310001388658us-gaap:RetainedEarningsMember2018-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2018-12-3100013886582018-12-310001388658us-gaap:CommonStockMember2019-01-012019-12-310001388658us-gaap:AdditionalPaidInCapitalMember2019-01-012019-12-310001388658us-gaap:RetainedEarningsMember2019-01-012019-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-01-012019-12-310001388658us-gaap:CommonStockMember2019-12-310001388658us-gaap:AdditionalPaidInCapitalMember2019-12-310001388658us-gaap:RetainedEarningsMember2019-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2019-12-3100013886582019-12-310001388658us-gaap:CommonStockMember2020-01-012020-12-310001388658us-gaap:AdditionalPaidInCapitalMember2020-01-012020-12-310001388658us-gaap:RetainedEarningsMembersrt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-12-310001388658srt:CumulativeEffectPeriodOfAdoptionAdjustmentMember2019-12-310001388658us-gaap:RetainedEarningsMember2020-01-012020-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-01-012020-12-310001388658us-gaap:CommonStockMember2020-12-310001388658us-gaap:AdditionalPaidInCapitalMember2020-12-310001388658us-gaap:RetainedEarningsMember2020-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2020-12-310001388658us-gaap:CommonStockMember2021-01-012021-12-310001388658us-gaap:AdditionalPaidInCapitalMember2021-01-012021-12-310001388658us-gaap:RetainedEarningsMember2021-01-012021-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-01-012021-12-310001388658us-gaap:CommonStockMember2021-12-310001388658us-gaap:AdditionalPaidInCapitalMember2021-12-310001388658us-gaap:RetainedEarningsMember2021-12-310001388658us-gaap:AccumulatedOtherComprehensiveIncomeMember2021-12-3100013886582019-09-102019-09-1000013886582019-09-1000013886582020-08-212020-08-2100013886582020-08-210001388658irtc:ProductAndServicesZioXTServiceCode93243Memberus-gaap:SubsequentEventMember2022-01-100001388658us-gaap:SubsequentEventMemberirtc:ProductAndServicesZioXTServiceCode93247Member2022-01-100001388658srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccountingStandardsUpdate201613Member2020-12-310001388658srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccountingStandardsUpdate201613Member2019-12-310001388658srt:CumulativeEffectPeriodOfAdoptionAdjustmentMemberus-gaap:AccountingStandardsUpdate201613Member2018-12-310001388658us-gaap:CustomerConcentrationRiskMemberirtc:FederalGovernmentAgenciesMemberus-gaap:SalesRevenueNetMember2021-01-012021-12-31xbrli:pure0001388658us-gaap:CustomerConcentrationRiskMemberirtc:FederalGovernmentAgenciesMemberus-gaap:SalesRevenueNetMember2020-01-012020-12-310001388658us-gaap:CustomerConcentrationRiskMemberirtc:FederalGovernmentAgenciesMemberus-gaap:SalesRevenueNetMember2019-01-012019-12-310001388658us-gaap:CreditConcentrationRiskMemberus-gaap:AccountsReceivableMemberirtc:FederalGovernmentAgenciesMember2021-01-012021-12-310001388658us-gaap:CreditConcentrationRiskMemberus-gaap:AccountsReceivableMemberirtc:FederalGovernmentAgenciesMember2020-01-012020-12-310001388658srt:MinimumMember2021-01-012021-12-310001388658srt:MaximumMember2021-01-012021-12-310001388658us-gaap:SoftwareDevelopmentMember2021-01-012021-12-310001388658irtc:PrintedCircuitBoardAssembliesMember2021-12-310001388658irtc:PrintedCircuitBoardAssembliesMember2020-12-310001388658irtc:CommercialPayorsMember2021-01-012021-12-310001388658irtc:CommercialPayorsMember2020-01-012020-12-310001388658irtc:CommercialPayorsMember2019-01-012019-12-310001388658irtc:NonContractedThirdPartyPayorsMember2021-01-012021-12-310001388658irtc:NonContractedThirdPartyPayorsMember2020-01-012020-12-310001388658irtc:NonContractedThirdPartyPayorsMember2019-01-012019-12-310001388658irtc:CentersForMedicareAndMedicaidMember2021-01-012021-12-310001388658irtc:CentersForMedicareAndMedicaidMember2020-01-012020-12-310001388658irtc:CentersForMedicareAndMedicaidMember2019-01-012019-12-310001388658irtc:HealthcareInstitutionsMember2021-01-012021-12-310001388658irtc:HealthcareInstitutionsMember2020-01-012020-12-310001388658irtc:HealthcareInstitutionsMember2019-01-012019-12-310001388658us-gaap:MoneyMarketFundsMember2021-12-310001388658us-gaap:USGovernmentCorporationsAndAgenciesSecuritiesMember2021-12-310001388658us-gaap:CorporateNoteSecuritiesMember2021-12-310001388658us-gaap:CommercialPaperMember2021-12-310001388658us-gaap:MoneyMarketFundsMember2020-12-310001388658us-gaap:USGovernmentCorporationsAndAgenciesSecuritiesMember2020-12-310001388658us-gaap:CorporateNoteSecuritiesMember2020-12-310001388658us-gaap:CommercialPaperMember2020-12-31irtc:investment_security0001388658us-gaap:CarryingReportedAmountFairValueDisclosureMember2021-12-310001388658us-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Member2021-12-310001388658us-gaap:CarryingReportedAmountFairValueDisclosureMember2020-12-310001388658us-gaap:EstimateOfFairValueFairValueDisclosureMemberus-gaap:FairValueInputsLevel2Member2020-12-310001388658us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMember2021-12-310001388658us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMember2021-12-310001388658us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel3Member2021-12-310001388658us-gaap:MoneyMarketFundsMember2021-12-310001388658us-gaap:FairValueInputsLevel1Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2021-12-310001388658us-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2021-12-310001388658us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2021-12-310001388658us-gaap:USGovernmentAgenciesDebtSecuritiesMember2021-12-310001388658us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2021-12-310001388658us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2021-12-310001388658us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2021-12-310001388658us-gaap:CorporateDebtSecuritiesMember2021-12-310001388658us-gaap:FairValueInputsLevel1Memberus-gaap:CommercialPaperMember2021-12-310001388658us-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMember2021-12-310001388658us-gaap:FairValueInputsLevel3Memberus-gaap:CommercialPaperMember2021-12-310001388658us-gaap:CommercialPaperMember2021-12-310001388658us-gaap:FairValueInputsLevel1Member2021-12-310001388658us-gaap:FairValueInputsLevel2Member2021-12-310001388658us-gaap:FairValueInputsLevel3Member2021-12-310001388658us-gaap:FairValueInputsLevel1Memberus-gaap:MoneyMarketFundsMember2020-12-310001388658us-gaap:FairValueInputsLevel2Memberus-gaap:MoneyMarketFundsMember2020-12-310001388658us-gaap:MoneyMarketFundsMemberus-gaap:FairValueInputsLevel3Member2020-12-310001388658us-gaap:MoneyMarketFundsMember2020-12-310001388658us-gaap:FairValueInputsLevel1Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2020-12-310001388658us-gaap:FairValueInputsLevel2Memberus-gaap:USGovernmentAgenciesDebtSecuritiesMember2020-12-310001388658us-gaap:USGovernmentAgenciesDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2020-12-310001388658us-gaap:USGovernmentAgenciesDebtSecuritiesMember2020-12-310001388658us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel1Member2020-12-310001388658us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel2Member2020-12-310001388658us-gaap:CorporateDebtSecuritiesMemberus-gaap:FairValueInputsLevel3Member2020-12-310001388658us-gaap:CorporateDebtSecuritiesMember2020-12-310001388658us-gaap:FairValueInputsLevel1Memberus-gaap:CommercialPaperMember2020-12-310001388658us-gaap:FairValueInputsLevel2Memberus-gaap:CommercialPaperMember2020-12-310001388658us-gaap:FairValueInputsLevel3Memberus-gaap:CommercialPaperMember2020-12-310001388658us-gaap:CommercialPaperMember2020-12-310001388658us-gaap:FairValueInputsLevel1Member2020-12-310001388658us-gaap:FairValueInputsLevel2Member2020-12-310001388658us-gaap:FairValueInputsLevel3Member2020-12-310001388658irtc:RawMaterialsMember2021-12-310001388658irtc:RawMaterialsMember2020-12-310001388658irtc:FinishedGoodsMember2021-12-310001388658irtc:FinishedGoodsMember2020-12-310001388658irtc:PrintedCircuitBoardAssembliesMember2021-01-012021-12-310001388658irtc:PrintedCircuitBoardAssembliesMember2020-01-012020-12-310001388658irtc:LaboratoryAndManufacturingEquipmentMember2021-12-310001388658irtc:LaboratoryAndManufacturingEquipmentMember2020-12-310001388658irtc:ComputerEquipmentAndSoftwareMember2021-12-310001388658irtc:ComputerEquipmentAndSoftwareMember2020-12-310001388658us-gaap:FurnitureAndFixturesMember2021-12-310001388658us-gaap:FurnitureAndFixturesMember2020-12-310001388658us-gaap:LeaseholdImprovementsMember2021-12-310001388658us-gaap:LeaseholdImprovementsMember2020-12-310001388658us-gaap:SoftwareDevelopmentMember2021-12-310001388658us-gaap:SoftwareDevelopmentMember2020-12-310001388658irtc:SanFranciscoMemberirtc:OfficeLeaseMember2018-10-04utr:sqft0001388658irtc:SanFranciscoMemberirtc:OfficeLeaseMember2018-10-042018-10-04irtc:optionToExtend0001388658irtc:LincolnshireLeaseMember2009-10-290001388658irtc:CypressLeaseMember2021-03-180001388658irtc:SanFranciscoMemberirtc:OfficeLeaseMember2021-03-182021-03-1800013886582021-03-180001388658irtc:SanFranciscoMemberirtc:DeerfieldLeaseMember2021-12-310001388658irtc:VerilyLifeSciencesLLCMember2019-09-032019-09-030001388658irtc:VerilyLifeSciencesLLCMember2019-12-310001388658irtc:VerilyLifeSciencesLLCMember2019-09-030001388658us-gaap:SubsequentEventMember2022-02-250001388658irtc:SiliconValleyBankTermLoanMemberirtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMember2018-10-310001388658irtc:PharmakonLoanAgreementMember2018-10-012018-10-310001388658us-gaap:PrimeRateMemberirtc:SiliconValleyBankTermLoanMemberirtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMember2018-10-012018-10-310001388658irtc:SiliconValleyBankTermLoanMembersrt:MinimumMemberirtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMember2018-10-310001388658irtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMemberus-gaap:RevolvingCreditFacilityMember2018-10-310001388658irtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMemberus-gaap:StandbyLettersOfCreditMember2018-10-310001388658irtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMemberus-gaap:StandbyLettersOfCreditMember2018-10-012018-10-310001388658srt:MinimumMemberirtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMemberus-gaap:RevolvingCreditFacilityMember2018-10-310001388658srt:MaximumMemberirtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMemberus-gaap:RevolvingCreditFacilityMember2018-10-012018-10-310001388658irtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMemberus-gaap:RevolvingCreditFacilityMember2021-12-310001388658irtc:ThirdAmendedAndRestatedSiliconValleyBankLoanAgreementMember2021-12-310001388658us-gaap:DomesticCountryMember2021-12-310001388658us-gaap:StateAndLocalJurisdictionMember2021-12-310001388658irtc:StockOptionsIssuedAndOutstandingMember2021-12-310001388658irtc:StockOptionsIssuedAndOutstandingMember2020-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2021-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2020-12-310001388658irtc:GrantUnderFutureStockPlansMember2021-12-310001388658irtc:GrantUnderFutureStockPlansMember2020-12-310001388658irtc:TwoThousandSixEquityIncentivePlanMembersrt:MaximumMember2021-01-012021-12-310001388658irtc:TwoThousandSixEquityIncentivePlanMemberus-gaap:ShareBasedCompensationAwardTrancheOneMember2021-01-012021-12-310001388658irtc:TwoThousandSixEquityIncentivePlanMember2021-01-012021-12-310001388658irtc:IncentiveStockOptionsMemberirtc:TwoThousandSixEquityIncentivePlanMembersrt:MaximumMember2021-01-012021-12-310001388658irtc:TwoThousandSixEquityIncentivePlanMembersrt:MinimumMember2021-01-012021-12-310001388658irtc:TwoThousandSixEquityIncentivePlanMemberirtc:OtherStockOptionMembersrt:MaximumMember2021-01-012021-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2016-10-012016-10-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2016-10-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2021-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMembersrt:MinimumMember2016-10-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMembersrt:MaximumMember2021-01-012021-12-310001388658irtc:EmployeeStockPurchasePlanMember2016-10-310001388658irtc:EmployeeStockPurchasePlanMember2016-10-302016-10-310001388658irtc:EmployeeStockPurchasePlanMember2016-10-012016-10-31irtc:numberOfPurchasePeriod0001388658irtc:EmployeeStockPurchasePlanMember2021-01-012021-12-310001388658irtc:EmployeeStockPurchasePlanMember2021-12-310001388658srt:MinimumMember2020-01-012020-12-310001388658srt:MaximumMember2020-01-012020-12-310001388658irtc:EmployeeStockPurchasePlanMember2020-01-012020-12-310001388658srt:MinimumMember2019-01-012019-12-310001388658srt:MaximumMember2019-01-012019-12-310001388658irtc:EmployeeStockPurchasePlanMember2019-01-012019-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2018-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2019-01-012019-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2019-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2020-01-012020-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2020-12-310001388658irtc:TwoThousandSixteenEquityIncentivePlanMember2021-01-012021-12-3100013886582018-01-012018-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2019-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-310001388658us-gaap:EmployeeStockOptionMember2019-01-012019-12-310001388658irtc:MarketConditionAwardsMember2020-01-012020-12-310001388658us-gaap:CostOfSalesMember2021-01-012021-12-310001388658us-gaap:CostOfSalesMember2020-01-012020-12-310001388658us-gaap:CostOfSalesMember2019-01-012019-12-310001388658us-gaap:ResearchAndDevelopmentExpenseMember2021-01-012021-12-310001388658us-gaap:ResearchAndDevelopmentExpenseMember2020-01-012020-12-310001388658us-gaap:ResearchAndDevelopmentExpenseMember2019-01-012019-12-310001388658us-gaap:SellingGeneralAndAdministrativeExpensesMember2021-01-012021-12-310001388658us-gaap:SellingGeneralAndAdministrativeExpensesMember2020-01-012020-12-310001388658us-gaap:SellingGeneralAndAdministrativeExpensesMember2019-01-012019-12-310001388658us-gaap:EmployeeStockMember2021-12-310001388658us-gaap:EmployeeStockMember2021-01-012021-12-310001388658us-gaap:PerformanceSharesMemberirtc:A2019AwardsMember2020-01-012020-03-310001388658us-gaap:PerformanceSharesMemberirtc:A2019AwardsMember2020-06-192020-06-19irtc:employee0001388658us-gaap:PerformanceSharesMemberirtc:A2019AwardsMember2021-01-012021-12-310001388658us-gaap:PerformanceSharesMemberirtc:A2020AwardsMembersrt:MinimumMember2020-02-012020-02-290001388658us-gaap:PerformanceSharesMemberirtc:A2020AwardsMembersrt:MaximumMember2020-02-012020-02-290001388658us-gaap:PerformanceSharesMemberirtc:A2020AwardsMember2020-02-012020-02-290001388658us-gaap:PerformanceSharesMemberirtc:A2020AwardsMember2020-02-290001388658us-gaap:PerformanceSharesMemberirtc:A2020AwardsMember2021-01-012021-12-310001388658irtc:AwardedJanuary2021Memberus-gaap:PerformanceSharesMembersrt:MaximumMemberirtc:A2021AwardsMember2021-01-012021-01-310001388658irtc:AwardedJanuary2021Memberus-gaap:PerformanceSharesMemberirtc:A2021AwardsMember2021-01-012021-01-310001388658irtc:AwardedJanuary2021Memberus-gaap:PerformanceSharesMemberirtc:A2021AwardsMember2021-01-310001388658irtc:AwardedJanuary2021Memberus-gaap:PerformanceSharesMemberirtc:A2021AwardsMember2021-01-012021-12-310001388658us-gaap:PerformanceSharesMemberirtc:AwardedFebruary2021Membersrt:MinimumMemberirtc:A2021AwardsMember2021-02-012021-02-280001388658us-gaap:PerformanceSharesMemberirtc:AwardedFebruary2021Membersrt:MaximumMemberirtc:A2021AwardsMember2021-02-012021-02-280001388658us-gaap:PerformanceSharesMemberirtc:AwardedFebruary2021Memberirtc:A2021AwardsMember2021-02-012021-02-280001388658us-gaap:PerformanceSharesMemberirtc:AwardedFebruary2021Memberirtc:A2021AwardsMember2021-02-280001388658us-gaap:PerformanceSharesMemberirtc:AwardedFebruary2021Memberirtc:A2021AwardsMember2021-01-012021-12-310001388658irtc:ConsultingAndProfessionalServicesAgreementCPSAMember2020-01-012020-12-310001388658irtc:ConsultingAndProfessionalServicesAgreementCPSAMember2021-01-012021-12-310001388658us-gaap:EmployeeStockOptionMember2021-01-012021-12-310001388658us-gaap:EmployeeStockOptionMember2020-01-012020-12-310001388658us-gaap:EmployeeStockOptionMember2019-01-012019-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2021-01-012021-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2020-01-012020-12-310001388658us-gaap:RestrictedStockUnitsRSUMember2019-01-012019-12-31
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
________________________________________________________________________________________________________
FORM 10-K
________________________________________________________________________________________________________
(Mark One) | | | | | |
| ☒ | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2021
OR | | | | | |
| ☐ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 FOR THE TRANSITION PERIOD FROM TO |
Commission File Number 001-37918
________________________________________________________________________________________________________
iRhythm Technologies, Inc.
(Exact name of Registrant as specified in its Charter)
________________________________________________________________________________________________________ | | | | | |
| Delaware | 20-8149544 |
(State or other jurisdiction of incorporation or organization) | (I.R.S. Employer Identification No.) |
699 8th Street, Suite 600 San Francisco, California (Address of principal executive offices) | 94103 (Zip Code) |
Registrant’s telephone number, including area code: (415) 632-5700
________________________________________________________________________________________________________
Securities registered pursuant to Section 12(b) of the Act: Common Stock, Par Value $.001 Per Share, Common Stock traded on the NASDAQ Stock Market
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☒ No ☐
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the Registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the Registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the Registrant was required to submit and post such files). Yes ☒ No ☐
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405) is not contained herein, and will not be contained, to the best of Registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☐
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definition of “large accelerated filer”, “accelerated filer”, “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
| | | | | | | | | | | |
| Large accelerated filer | ☒ | Accelerated filer | ☐ |
| Non-accelerated filer | ☐ | Small reporting company | ☐ |
| Emerging growth company | ☐ | | |
If an emerging growth company, indicate by check mark if the Registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☒
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the Registrant, based on the closing price of the shares of common stock on The NASDAQ Stock Market on June 30, 2021, was approximately $1.9 billion.
The number of shares of Registrant’s Common Stock outstanding as of February 18, 2022 was 29,513,264.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the information called for by Part III of this Form 10-K is hereby incorporated by reference from the definitive Proxy Statements for our annual meeting of stockholders, which will be filed with the Securities and Exchange Commission not later than 120 days after December 31, 2021. | | | | | | | | |
| Title of each class | Trading Symbol | Name of each exchange on which registered |
| Common Stock, Par Value $.001 Per Share | IRTC | The Nasdaq Stock Market |
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K contains forward-looking statements concerning our business, operations and financial performance and condition, as well as our plans, objectives and expectations for our business, operations and financial performance and condition. Any statements contained herein that are not statements of historical facts may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “anticipate,” “assume,” “believe,” “contemplate,” “continue,” “could,” “due,” “estimate,” “expect,” “goal,” “intend,” “may,” “objective,” “plan,” “predict,” “potential,” “positioned,” “seek,” “should,” “target,” “will,” “would” and other similar expressions that are predictions of or indicate future events and future trends, or the negative of these terms or other comparable terminology. These forward-looking statements include, but are not limited to, statements about:
•the impact of the COVID-19 pandemic on our operations and financial results;
•plans to conduct further clinical studies;
•our plans to modify our current products, or develop new products, to address additional indications;
•the expected growth of our business and our organization;
•our expectations regarding government and third-party payor coverage and reimbursement;
•our expectations regarding the size of our sales organization and expansion of our sales and marketing efforts in international geographies;
•our expectations regarding revenue, cost of revenue, cost of service per device, operating expenses, including research and development expense, sales and marketing expense and general and administrative expenses;
•worsening economic conditions, including inflation, in the United States or internationally;
•our ability to retain and recruit key personnel, including the continued development of a sales and marketing infrastructure;
•our ability to obtain and maintain intellectual property protection for our products;
•our estimates of our expenses, ongoing losses, future revenue, capital requirements and our needs for, or ability to obtain, additional financing;
•our ability to identify and develop new and planned products and acquire new products;
•our financial performance; and
•developments and projections relating to our competitors or our industry.
We believe that it is important to communicate our future expectations to our investors. However, there may be events in the future that we are not able to accurately predict or control and that may cause our actual results to differ materially from the expectations we describe in our forward-looking statements. These forward-looking statements are based on management’s current expectations, estimates, forecasts and projections about our business and the industry in which we operate and management’s beliefs and assumptions and are not guarantees of future performance or development and involve known and unknown risks, uncertainties and other factors that are in some cases beyond our control. As a result, any or all of our forward-looking statements in this Annual Report on Form 10-K may turn out to be inaccurate. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this Annual Report on Form 10-K. Potential investors are urged to consider these factors carefully in evaluating the forward-looking statements. These forward-looking statements speak only as of the date of this Annual Report on Form 10-K. We assume no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future.
You should not rely upon forward-looking statements as predictions of future events. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward-looking statements will be achieved or occur. We undertake no obligation to update publicly any forward-looking statements for any reason after the date of this Annual Report on Form 10-K to conform these statements to actual results or to changes in our expectations.
You should read this Annual Report on Form 10-K and the documents that we reference in this Annual Report on Form 10-K and have filed with the SEC as exhibits to the Annual Report on Form 10-K with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect.
PART I
Item 1. Business.
Overview
We are a digital healthcare company redefining the way cardiac arrhythmias are clinically diagnosed by combining our wearable biosensing technology with cloud-based data analytics and deep-learning capabilities. Our goal is to be the leading provider of ambulatory electrocardiogram (“ECG”) monitoring for patients at risk for arrhythmias. We have created a full portfolio of ambulatory cardiac monitoring services on a unique platform, called the Zio service, which combines an easy-to-wear and unobtrusive biosensor that can be worn for up to 14 consecutive days with powerful proprietary algorithms that distill data from millions of heartbeats into clinically actionable information. We believe that the Zio service allows physicians to diagnose many arrhythmias more quickly and efficiently than traditional technologies and avoid multiple indeterminate tests. Early detection of heart rhythm disorders, such as atrial fibrillation (“AF”) and other clinically relevant arrhythmias, allows for appropriate medical intervention and helps avoid more serious downstream medical events, including stroke. Since receiving clearance from the Food and Drug Administration (“FDA”) in 2009, we have provided the Zio service to over four million patients and have collected over one billion hours of curated heartbeat data, creating what we believe to be the world’s largest repository of ambulatory ECG patient data. This data provides us with a competitive advantage by informing our proprietary deep-learned algorithms, which may enable operating efficiencies, gross margin improvement and business scalability. We believe the Zio service is well aligned with the goals of the U.S. healthcare system: improving population health, enhancing the patient care experience, reducing per-capita cost, and improving the clinician experience.
According to the Centers for Disease Control and Prevention, approximately 11 million patients in the United States have a heart rhythm disorder, or arrhythmia. The most common sustained type of arrhythmia is AF. The American Heart Association (“AHA”), estimates that as many as six million people in the United States have AF with at least one-third of these patients being asymptomatic at the time of their diagnosis. Individuals with AF are five times more likely to suffer a stroke; however, the National Stroke Association (“NSA”) estimates that up to 80% of strokes suffered by people with AF are preventable with early detection and proper treatment.
The ambulatory cardiac monitoring market is well-established with an estimated 5.6 million diagnostic tests performed annually in the United States, which we believe to be an existing $2.0 billion market opportunity for our Zio service. Traditional ambulatory cardiac monitoring tools used by physicians for diagnosing patients with suspected arrhythmias, such as Holter and cardiac event monitors, are constrained by one or more of the following: short prescribed monitoring times, non-continuous data collection and reporting, cumbersome equipment and low patient compliance. As an example of these traditional constraints, patients often remove these traditional monitors when sleeping, showering or exercising, leading to failure to capture critical data. These limitations contribute to incomplete diagnoses and repeat testing, which in turn result in suboptimal patient care and higher costs to the health system.
We believe the Zio service provides a comprehensive solution that addresses all of these limitations and offers a clear value proposition to patients, providers, and payors by providing an easy-to-use, clinically proven, cost-effective platform solution. Our Zio service is prescribed by physicians for both identifying arrhythmias as well as for identifying risk factors which may be associated with a previously-identified arrhythmia. It improves physician management and diagnosis of arrhythmias by providing a patient-friendly wearable biosensor, curating and analyzing voluminous uninterrupted continuous ECG data, and ultimately creating a concise report that is used by the physician to make a diagnosis that can be integrated into a patient’s electronic health record. We believe our Zio service can continue taking significant market share from the existing ambulatory cardiac monitoring market and expanding the market for new clinical use cases and indications. We believe the Zio service has the potential to supplant traditional technology and become the primary monitoring option for patients who are candidates for ambulatory cardiac monitoring due to its ability to detect more arrhythmias with a high degree of clinical accuracy, which allows for earlier changes in clinical patient management.
The Zio service consists of:
•wearable patch-based biosensors, Zio XT and Zio AT monitors, which continuously records and stores ECG data from every patient heartbeat for up to 14 consecutive days. Zio AT offers the option of timely transmission of data during the prescribed wear period;
•cloud-based analysis of the recorded cardiac rhythms using our proprietary, deep-learned algorithms;
•a final quality assessment review of the data by our certified cardiographic technicians;
•a scalable digital platform that includes an easy-to-read Zio report, which is a curated summary of findings that includes high-quality and clinically-actionable information which is sent directly to a patient’s physician and can be integrated into a patient’s electronic health record; and
•a proprietary cloud-based digital information system, ZioSuite, that allows clinicians to connect via a web browser or mobile application for convenient online report interpretation and streamlined clinical workflows.
We refer to both the Zio AT monitor, and the Zio XT monitor herein as our Zio monitor(s), unless otherwise specified.
We have reviewed a body of clinical evidence which we interpret to show, among other advantages, that the Zio service helps reduce healthcare costs and improves arrhythmia detection, characterization and diagnosis by prescribing physicians. These improvements have the potential to change clinical management of patients. Our clinical evidence is helping to drive physician adoption. We interpreted one study of the Zio service, published in The American Journal of Cardiology in August 2013, to show that among 16,142 consecutive Zio service patients in whom an arrhythmia was detected, over 50% of symptomatic arrhythmias detected by the Zio service occurred more than 48 hours into the wear period. Although this study did not directly compare the Zio service to Holter monitoring performance, it should be noted that 48 hours is outside of the typical wear period for Holter monitors. Furthermore, an internal analysis of 500,000 consecutive Zio records concluded that the first incidence of certain critical arrhythmias, specifically ventricular tachycardia, pause, AV block and atrial fibrillation, occurred beyond 7 days in 22%, 18%, 16%, and 13% of patients, respectively. Based upon our review of another prospective comparative study against Holter monitor, published in The American Journal of Medicine in January 2014, we concluded that the Zio service detected 96 arrhythmia events compared to 61 arrhythmia events detected by the Holter monitor (P < 0.001), providing a 57% improvement in diagnostic yield, which is the percentage of patients in whom an arrhythmia was detected during the monitoring period. In summary, we interpreted the clinical results to show that the Zio service is preferred by patients and allows for significantly longer continuous monitoring, improved clinical accuracy, increased detection of arrhythmias by physicians, and meaningful changes in clinical management.
Over four million patients have utilized the Zio service since its commercialization, we have achieved both policy coverage and, where applicable, contracts with the majority of payors in the United States, including the Centers for Medicare & Medicaid Services (“CMS”) and other government agencies. Over 95% of patients in the U.S. are able to access reimbursed Zio services through our third-party payor contracts, our independent diagnostic testing facilities ("IDTF") participation status with CMS, and self-pay programs when excluding state Medicaid programs. We have designed a comprehensive strategy to allow us to compete favorably in the ambulatory cardiac monitoring market, which includes capturing market share from existing monitoring devices in the United States and international markets as well as expanding the market through new indications. We expect to drive sales and margin growth in our business by expanding our sales organization, securing additional contracts with commercial payors, maintaining technology leadership through research and development, and continuing to build clinical evidence supporting the benefits of the Zio service.
We have collected over one billion hours of curated heartbeat data, creating what we believe to be the world’s largest repository of annotated, continuous ambulatory ECG recordings with contextual patient information. This extensive database, along with our proprietary analytic platform, differentiates the Zio service and gives us a competitive advantage. We will continue to seek opportunities to capitalize on our product design, proprietary analytic capabilities and data repository to capture additional opportunities in the digital healthcare market.
We are a vertically-integrated company headquartered in San Francisco, California, and we have additional commercial operations and facilities in Lincolnshire, Illinois, Houston, Texas, and the United Kingdom. We manufacture our devices in Cypress, California. Our revenue was $322.8 million and $265.2 million for the years ended December 31, 2021 and 2020, respectively and we incurred a net loss of $101.4 million and $43.8 million for those same periods.
Market Opportunity
Every year, millions of patients experience symptoms potentially associated with cardiac arrhythmias, a condition in which the electrical impulses that coordinate heartbeats do not occur properly, causing the heart to beat too quickly, too slowly or irregularly. Examples of arrhythmias include supra ventricular arrhythmias, which are fast heart rates that originate from the upper chambers of the heart, atrial tachycardia, atrial flutter and AF. The symptoms of arrhythmias include palpitations or a skipped heartbeat, rapid heartbeat, shortness of breath, dizziness, light-headedness, fainting spells, vertigo, anxiety and fatigue or no symptoms at all. Early detection is essential in order to obtain early treatment and help avoid more serious medical conditions, such as stroke, and additional medical costs.
Atrial Fibrillation and Stroke
In patients with AF, the upper chambers of the heart beat irregularly and blood does not flow properly to the lower chambers of the heart. The AHA estimates that AF affects as many as six million patients in the United States and 33.5 million patients worldwide. The NSA estimates that one-third of AF patients are asymptomatic and still undiagnosed. More than 750,000 hospitalizations occur each year because of AF, and the condition contributes to an estimated 130,000 deaths each year. Since AF is more common among people over the age of 60, these numbers are expected to increase as the U.S. population ages.
In addition, AF is the leading risk factor for stroke because AF can cause blood to collect in the heart and potentially form a clot, which can travel to the brain potentially resulting in an ischemic stroke. While individuals with AF are approximately five times more likely to suffer a stroke, the NSA estimates that up to 80% of strokes in people with AF can be prevented through early detection and proper treatment. According to the AHA, stroke costs the United States an estimated $34 billion each year in healthcare costs and lost productivity, and is a leading cause of serious long-term disability. The AHA estimates that ischemic strokes represent 87% of all strokes in the United States and that between 15% and 20% of the estimated 690,000 ischemic strokes are attributable to AF.
Currently, the Zio service is prescribed by physicians primarily for symptomatic patients. However, we believe that high-risk asymptomatic patients represent an additional market opportunity for the Zio service. Monitoring high-risk asymptomatic patients may lead to increased diagnoses and earlier treatment and potentially avoid more severe downstream conditions, because, as the Framingham Study published in Stroke in September 1995 demonstrated, 18% of AF-related strokes present with asymptomatic AF that is only detected at the time of stroke.
Early detection of AF is critical in optimizing patient care, delivering earlier treatment to help avoid further adverse clinical events, managing symptoms caused by AF, and reducing the total public health burden of treating stroke. The AHA and American Stroke Association (“ASA”) have published treatment guidelines for patients diagnosed with AF to manage heart rhythm and rate and prevent stroke. These early treatments include:
•medications such as oral anticoagulants
•treatment with anti-arrhythmic drugs
•interventions such as cardiac ablation therapy to help control heart rhythm and rate
Atrial fibrillation burden, the amount of time a patient spends in AF during a monitoring period, has been identified in the clinical community as an important measure for determining appropriate and effective therapeutic interventions to manage patients with AF and assessing stroke risk. The calculated AF burden is only as good as the data available for analysis during the monitoring period. Since the most common type of AF occurs intermittently, long-term continuous patch-based monitoring, such as the Zio service, more accurately measures AF burden because every heartbeat is recorded without interruption during the entire monitoring period. A study to determine the correlation between AF burden, as measured by the Zio service, and the risk of stroke in patients was published in JAMA Cardiology in May 2018. Using this data in combination with electronic health record data from 1,965 patients at two large integrated health care delivery systems, the researchers concluded that an increase in the burden of AF is independently associated with a higher risk of ischemic stroke and arterial thromboembolism (“TE”) in patients who are not taking anticoagulant medication. An AF burden of 11.4% or higher was associated with more than three-fold increased risk for stroke or TE event after adjusting for either CHA2DS2-VASc or ATRIA scores, two tools physicians use to assess stroke risk.
Heart Block post Transcatheter Aortic Valve Replacement ("TAVR")
TAVR is now the preferred strategy for aortic valve replacement in most patients with severe symptomatic aortic stenosis. Due to the less invasive strategy, TAVR provides treatment for patients who would have been declined surgical replacement. According to the American College of Cardiology ("ACC"), it is estimated 100,000 patients undergo TAVR procedure annually in the United States, and expected to increase with over 250,000 procedures conducted worldwide by 2025. One out of ten patients experiences injury to the conduction system during TAVR resulting in high-degree atrioventricular block (“HAVB”) necessitating permanent pacemaker implantation. While most HAVB occurs within 48 hours of TAVR, there is a growing number of patients developing HAVB after initial TAVR hospitalization due to the trend for early discharge post-TAVR.
Well-established risk factors can be used to identify and advise high-risk patients and guide management. As the population of patients at increased risk for late presentation of HAVB grows, development of algorithms for mobile outpatient cardiac telemetry monitoring post-TAVR, particularly in the first 2 weeks after discharge, may be needed to reduce the risk of adverse events.
Ambulatory Cardiac Monitoring Overview
Arrhythmia symptoms are generally monitored either in a physician’s office or healthcare facility or remotely with the use of ambulatory cardiac monitoring devices. Typically, physicians will administer a resting ECG in their offices to record and analyze the electrical impulses of patients’ hearts. If physicians determine that patients require monitoring for a longer period of time to generate a diagnosis, they have historically prescribed an ambulatory cardiac monitoring device such as a Holter monitor. If the diagnosis is not definitive following the first monitoring period, physicians may prescribe a repeat Holter monitoring period, or alternatively, prescribe event monitors, mobile cardiac telemetry or implantable loop recorders. Physicians use frequency and acuity of symptoms to determine which monitoring device to prescribe. Some physicians own their own ambulatory cardiac monitoring devices and provide ambulatory monitoring services directly to their patients, while others outsource these services to third-party providers.
Holter Monitors
Holter monitors are non-invasive, ambulatory, battery-operated monitoring products that continuously record the ECG data of a patient, during a typical prescribed wear period of 24 to 48 hours. A Holter monitor consists of a recorder, electrodes that are attached to the patient’s chest and wires, or electrode leads, connecting the electrodes to the recorder. After the prescribed wear period, the data recorded by the device is delivered by hand, mail or internet for processing and analysis by the physician’s office or a third-party provider. Holter monitors are typically prescribed for patients who experience daily symptoms. For patients with suspected arrhythmias, Holter monitors have a relatively low diagnostic yield of approximately 24% due to a limited prescribed wear period of typically no more than 48 hours and low patient compliance, likely resulting from bulky equipment and cumbersome electrode leads. The low diagnostic yield is also attributable to missing data, because patients typically remove the electrodes and disconnect their Holter monitors in order to shower, sleep and exercise.
Cardiac Event Monitors and Mobile Cardiac Telemetry
Cardiac event monitoring is another type of non-invasive, ambulatory monitoring. Event monitoring differs from Holter monitoring in that the monitor is prescribed and worn for a longer period of time, up to 30 days, and the data recorded during the wear period are symptom driven. Event monitors generally record several minutes of activity at a time and then start over, a process referred to as memory loop recording. There are many types of event recorders available with a range of features including patient-triggered or auto-detected symptom recording, and manual data transmission or auto-send. Typically, physicians prescribe event monitors for patients with lower acuity symptoms. Mobile cardiac telemetry ("MCT"), is another form of event monitor that usually uses wireless technology, such as a cell phone network, to transmit event data during the wear period for both patient-triggered and auto-detected events to a monitoring facility where the ECG data is analyzed and the facility determines if physicians should be notified of significant events. Typically, physicians prescribe MCT for patients with higher acuity symptoms such as syncope, or fainting, that require more timely notification and actions.
Event and mobile cardiac monitors have several limitations, including limited data storage, the lack of trend data, and poor patient compliance due to electrode replacement, bulky equipment and the fact the patient must both activate and transmit events in some cases. Additionally, MCT technology has unique limitations including the need for patients to keep the transmitter close at all times and frequently change the battery or recharge the device to ensure timely transmissions as well as notifications sent to physicians of non-actionable events. These limitations can severely impact a physician’s ability to provide
a timely diagnosis and result in a lower diagnostic yield. More recently, there have been new MCT products introduced to the market that include patch-based or combined recorder-transmitter features to try and address some of these limitations.
Implantable Loop Recorders
A separate segment of ambulatory cardiac monitoring consists of implantable diagnostic products such as implantable loop recorders, also known as insertable cardiac monitors. Implantable loop recorders are implanted underneath a patient’s skin during a hospital-based, minimally-invasive procedure. These devices remain implanted in a patient for up to three years, capturing data in a looping manner for patient-triggered or automatically-detected events. Limitations of this monitoring option include the semi-permanent nature of the implant, infection risks during insertion and removal, non-continuous data collection, under- or over-sensing which may exhaust the memory of the loop recorder, risk of missing events due to the looping nature of the recording, and the high cost of the device.
Limitations of Traditional Ambulatory Cardiac Monitors
Limitations of the various types of traditional ambulatory cardiac monitors can include the following:
•short prescribed monitoring periods leading to low diagnostic yield;
•non-continuous and interrupted data collection, resulting in an incomplete picture of a patient’s arrhythmia experience;
•bulky monitoring equipment with dangling electrode leads causing discomfort and low patient compliance;
•the need to use multiple, often times costly, diagnostic options that would not be necessary if initial tests had produced a higher diagnostic yield;
•the generation of excessive and uncurated data for the physician to analyze; and
•over notification of non-actionable events.
We believe there is a significant opportunity for a disruptive arrhythmia monitoring solution that offers a portfolio of ambulatory cardiac monitoring services on a single platform that is cost effective and provides certainty in a single test obtained through uninterrupted continuous monitoring, combined with patient-friendly design, to enhance compliance and simplify the monitoring experience while maximizing diagnostic yield.
Our Solution
We have developed an uninterrupted, long-term continuous ambulatory cardiac monitoring platform known as the Zio service that provides continuous ambulatory cardiac monitoring through both the Zio XT monitor and Zio AT monitor. The Zio service combines an FDA-cleared and CE-marked wire-free, patch-based, 14-day wearable biosensor with a proprietary cloud-based data analytic platform to help physicians monitor patients and diagnose arrhythmias with a high degree of accuracy and confidence. Since commercialization, over four million patients have utilized the Zio service, and we have collected over one billion hours of heartbeats, creating what we believe to be the world’s largest repository of curated ambulatory ECG patient data.
While wearing either the Zio XT or Zio AT monitor, patients have the ability to mark when symptoms occur by pressing a trigger button on the device, and separately recording contextual data like activities and circumstances in a written symptom diary or digitally via the myZio application. This allows physicians to match symptoms and activity with ECG data. Following the wear period, the monitor is returned and data are uploaded to our secure cloud and run through our proprietary, deep-learned algorithms. A concise report of preliminary findings is prepared by our certified cardiographic technicians and made available on our proprietary cloud-based portal, ZioSuite, that allows clinicians to connect via a web browser or mobile application. Zio AT offers the additional capability of actionable transmissions during the wear period to assist physicians in diagnosing and treating the small percentage of the population requiring more timely action. During the Zio AT wear period, physicians will receive notifications if there are significant events that meet pre-determined arrhythmia detection criteria.

We believe the Zio service is a disruptive option for ambulatory cardiac monitoring. The Zio service addresses patient compliance, continuously monitors patients without requiring patient maintenance for up to 14 consecutive days and produces easy-to-read, comprehensive digital reports that provide the information physicians need to make accurate and timely clinical decisions. Clinical studies have shown that our innovative digital healthcare solution improves physicians’ abilities to detect arrhythmias by increasing diagnostic yield, and potentially allows them to change the course of treatment. Our proprietary deep-learned algorithms give us a competitive advantage due to the depth and breadth of ECG data available from the over one billion hours of curated and annotated ECG data collected to date. Additionally, we believe we have the first mover advantage in the long-term continuous market, particularly related to our efforts to secure both policy coverage and, where applicable, contracts with the majority of payors in the United States, including CMS and other government agencies. Over 95% of patients in the U.S. are able to access reimbursed Zio services through our third-party payor contracts, our IDTF participation status with CMS, and self-pay programs when excluding state Medicaid programs.
We are actively working to make the Zio service the standard of care for patients who require ambulatory cardiac monitoring. Our solution helps reduce healthcare costs and improves arrhythmia detection, characterization and diagnosis by providing simple, seamless integration of heart rhythm data from patient to cloud to physician. We believe we offer a high value, low cost, disruptive solution to a market ready for innovative technology.
Key Benefits
Value to Patients
We designed the Zio monitor specifically to address patient compliance issues common to other ambulatory cardiac monitors. Our wire-free wearable biosensor is easy to apply, comfortable, lightweight and unobtrusive. The Zio monitor requires no patient management or manipulation during the wear period because no battery changes, patch changes, adhesive changes or lead wire / electrode management is required. Patients wear it discreetly during activities of daily life including exercising and showering for up to 14 consecutive days. For a small percentage of patients, an additional Zio AT monitor is provided for longer monitoring prescriptions. We interpreted a clinical study by Barrett et al published in The American Journal of Medicine in January 2014, or the Barrett Study, to confirm that the Zio service is a patient-friendly monitoring option, and the study noted that 94% of patients found the Zio monitor comfortable to wear. The Zio monitor allows patients to mark when a symptom occurs by pressing a button on the Zio monitor and logging the surrounding circumstances into a written or digital symptom diary, thus allowing physicians to link symptoms with the ECG data. Additionally, patients have access to our professional 24/7 customer service team and dedicated financial counselors to address any product, service, enrollment or billing questions.
Value to Providers
Providers, such as physicians, receive high-quality, easy-to-read, actionable digital reports that help them diagnose patients and streamline clinical workflow. The Zio service has been shown in multiple peer-reviewed published clinical studies to detect more arrhythmias compared to Holter monitoring during their respective prescribed wear periods confirming the Zio service's added benefit of higher diagnostic yield beyond 48 hours of wear time. We analyze and generate patient reports at our CMS-certified IDTFs staffed with our certified cardiographic technicians who specialize in advanced arrhythmia interpretation to help ensure high accuracy and quality of reports before delivering them to the prescribing physician. Due to high patient compliance, the reports include up to 14 days of non-interrupted data correlated with patient-triggered and diary symptom events. Physicians can use this continuous correlated data to more conclusively diagnose arrhythmias.
Accurate detection and higher diagnostic yield allow physicians to more quickly prescribe the appropriate treatment options for patients, while minimizing the need for repeat testing. From our review, we determined that in 28% of cases observed in a clinical study by Rosenberg et al published in Pacing and Clinical Electrophysiology in March 2013, or the Rosenberg Study, the physician changed the patient’s clinical management after prescribing the Zio service as compared to a Holter monitor.
Additionally, the Zio service allows clinical staff to focus on more value-added activities by not requiring electrode changes or battery recharging during use, device cleaning and maintenance following use, and by reducing physician and hospital staff time needed to review and curate ECG data. Our 24/7 customer service team provides troubleshooting for patient-related issues, removing this burden from the physician practice.
Value to Payors
The graph above compares the costs of monitoring to the diagnostic yield of various ambulatory cardiac monitors in the United States. The analysis, completed by Decision Drivers Analytics and commissioned by us, uses cost data from the Centers for Medicare & Medicaid Services (“CMS”) published diagnostic yields, and our internal database, and demonstrates that the Zio service has a diagnostic yield on par with much more expensive devices but superior to less expensive options. This implies that it is the most cost-effective modality among its peer group, optimizing the cost, time, and reliability of reaching a timely diagnosis.
Patients who use traditional Holter monitors often do not receive a diagnosis after one monitoring period. A retrospective, longitudinal study conducted by Arnold et al published in the Journal of Health Economics and Outcomes Research in February 2015, evaluated the clinical consequences and costs of CMS patients who had no previous evidence of a cardiac arrhythmia and were undergoing their first Holter monitoring test. Our review of data from this study indicates that there was no diagnosis reached for 70% of patients after an initial Holter test. The Zio service has been shown to have a low cost per diagnosis compared to existing monitoring modalities due to its high diagnostic yield.
We believe that the Zio service is the best test for most patients requiring ambulatory cardiac monitoring because it allows physicians to identify a timely course of treatment and avoids healthcare costs associated with additional monitoring. The Zio monitor is patient friendly and allows significantly longer and more continuous monitoring, resulting in improved clinical accuracy and a potentially meaningful change in clinical management. Better diagnostic yield results in decreased costs due to fewer additional tests. We believe that the Zio service could reduce the need for multiple consecutive tests because it offers certainty in a single test.
Early detection of arrhythmias allows physicians to assess a patient’s risk factors and decide on the best treatment course for avoiding potentially more severe downstream conditions. Specifically, the early detection of AF allows physicians to consider strategies to mitigate the risk of stroke. According to multiple studies, preventative treatments, such as oral anticoagulants, have been shown to reduce stroke rates by 80%, thereby potentially avoiding the patient effects of stroke and the high costs associated with post-stroke management.
Our Technology Platform
The Zio service is built on a proven technology platform that is designed to integrate seamlessly across the health system to provide long-term continuous ambulatory cardiac monitoring. It consists of (1) a patient-friendly, patch-based biosensor that maximizes patient comfort and compliance, (2) a deep-learned neural network algorithm that provides accurate analytics and clinical results, (3) skilled clinical and operational staff who utilize proprietary software to ensure quality in clinical results and are available 24/7 to support physician and patient needs, and (4) a cloud-based portal, ZioSuite, through which physicians can access and interpret the Zio reports via a web browser or mobile application.
The platform typically collects approximately 1.5 million heartbeats of data for each patient during a single application of up to 14 consecutive days. Our Zio service delivers a curated, concise, and clinically actionable report to the prescribing physician. Through the Zio AT offering, physicians can access the additional capability of timely transmissions during the wear period. During the wear period, physicians will receive notifications and reports if there are significant events that meet pre-determined arrhythmia detection criteria.
Zio XT and AT monitors
The Zio monitor is a single-use, wire-free, wearable patch-based biosensor that records a patient’s heartbeats and ECG data. The Zio monitor was specifically designed with the patient and physician in mind. The Zio monitor includes the following features:
•patented clear, flexible, lightweight, wire-free design;
•unobtrusive and inconspicuous profile;
•proprietary adhesive backing designed to keep the Zio monitor securely in place for the duration of the prescribed wear period;
•water-resistant functionality, allowing patients to shower, sleep, and perform normal daily activities, including moderate exercise;
•hydrogel electrodes and a compliant mechanical design to deliver a clear ECG with minimal artifact from movement;
•large symptom button, or patient trigger, that is easy to find and press;
•indicated single application wear period of up to 14 days (for longer monitoring prescriptions, additional Zio AT monitors will be provided); and
•sufficient battery power for the entire wear period, without the need to recharge or replace batteries.
Symptoms can be logged through a paper symptom log or through the myZio App (iOS and Android).
The Zio XT service monitors continuously for up to 14 days, delivering a comprehensive report after return and analysis. The Zio AT service delivers the same comprehensive final report, but also provides physicians with actionable notifications during the wear period. These timely alerts are provided via a Bluetooth capability in the Zio AT monitor that sends data to a wireless gateway. The wireless gateway, slightly larger than a smart phone, is provided to the patient at the time of monitor application and will collect and transmit data from the monitor to the cloud via a long term evolution ("LTE”) protocol.
Enrollment and Initiation of the Zio service
Once a physician determines a patient is a candidate for the Zio service, the patient is enrolled through our online portal, ZioSuite. The wire-free Zio monitor is applied to the patient’s chest by the clinical staff, and monitoring is initiated. There is also an option for physicians to enroll patients remotely. With this option, the physician enrolls the patient and the patient receives the Zio monitor in the mail along with a detailed set of self-application instructions. Additionally, the Zio service can be ordered directly through the physicians’ electronic health record system through the use of the Company's Electronic Health Record (“EHR”) integration service.
Monitoring
The Zio monitor is worn continuously by the patient for up to 14 days without the need for patient maintenance. The Zio monitor can be worn in the shower, while sleeping, and during moderate exercise. During the wear period, the device continuously records and stores ECG data. The Zio monitor features a patient trigger button for marking any symptoms during the wear period; the patient is instructed to push the button when a symptom occurs and make a corresponding entry into the written or digital symptom diary. At the end of the prescribed wear period, the patient removes the device and places it and the written diary (if applicable) into a pre-paid postal box, which ships to one of our clinical centers.
Data Analysis and Assessment
At one of our clinical centers, the returned device is validated with patient identifiers that are compliant with the Health Insurance Portability and Accountability Act of 1996, (“HIPAA”), and up to 14 days of heartbeat data is uploaded to be processed through our cloud-based, FDA-cleared proprietary deep-learned algorithms for highly accurate ECG analysis. When complete, a preliminary curated report is created. Our process can take the equivalent of 30,000 pages of ECG strips and distill it into an actionable summary report of about 10 to 15 pages, summarizing the key findings and providing supporting details on clinically relevant events and metrics during the wear period. Our certified cardiographic technicians play a critical role in report curation by providing a quality review of the data before the final Zio report is electronically delivered to the patient’s physician for final interpretation and diagnosis.
Final Zio Report and the ZioSuite Web Portal
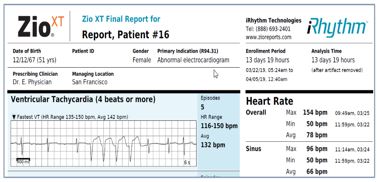
The final Zio report, which is curated for both Zio XT and Zio AT patients, provides information in a concise format for review and interpretation by the patient’s physician. Data provided includes total analysis time, AF burden, AF duration, comprehensive symptom/rhythm correlation, detailed findings per day, and arrhythmia type. If pre-determined physician notification criteria for symptoms are met, the prescribing physician is notified by phone of the serious findings prior to the Zio report being made available electronically. The Zio report is delivered through our secure, HIPAA compliant web portal, ZioSuite. ZioSuite is an easy to use, intuitive, and comprehensive portal for clinicians that streamlines clinical workflows to enable cardiac care. By enabling streamlined workflows, ZioSuite reduces administrative burden and helps to create more time for patient management. Physicians can open the Zio report and add their interpretation into the report file. These reports can be uploaded into the patients’ electronic medical record for storage and are available for use by the patient’s other physicians. Excerpts of these reports are included below to highlight the key features.
ZioSuite Web portal via desktop or mobile application
| | | | | | | | | | | |
| Up to 14-days continuous recording and storage | | | |
| | Up to 20,000 minutes
of continuous ECG
data, equivalent to
approximately 1.5
million heartbeats. | |
| Easy-to-read summary | | | |
| | Preliminary findings
based on both the
proprietary algorithms
and certified cardiographic
technicians.
Final interpretation by
a patient’s physician. | |
| Comprehensive symptom/rhythm correlation | | | |
| | | | | | | | | | | |
| | Patient-triggered and
symptom logged events
mapped to arrhythmia. | |
| AF Burden | | | |
| | Total AF during
wear period and
daily AF burden. | |
| AF Duration | | | |
| | Total number of AF
episodes categorized
by duration. | |
Reporting Events During the Wear Period with Zio AT
The Zio service with Zio AT includes a wireless gateway that provides connectivity between the Zio AT monitor and our monitoring center which enables symptomatic and asymptomatic data transmission during the prescribed wear period. The Zio AT monitor, in conjunction with the wireless gateway and the unique Zio arrhythmia detection algorithm, has arrhythmia auto-detection capabilities that produce actionable event data for physician review. The definition of an actionable arrhythmia event is customized by the physician to meet his or her needs, with the intent to reduce the number of over-notifications of data that do not require more timely medical action. Additionally, patients have the option of pressing a trigger button which marks the continuous record and initiates a wireless transfer of a 90-second ECG strip to our monitoring center. In addition to the final Zio report, physicians receive daily symptomatic and auto-detected arrhythmia event reports.
ECG strip of auto-detected actionable arrhythmia event from Zio AT Transmission Report
Zio AT improves the speed and accuracy of diagnosis relative to traditional mobile cardiac telemetry (“MCT”) devices and services. The patient-friendly design of the Zio monitor enables 98% patient compliance over prescribed wear times. We believe a comparative review of published literature with our clinical data indicates that the Zio AT solution is able to detect critical arrhythmias up to five days sooner than the leading competitor. Further analysis indicates that Zio AT achieves a higher diagnostic yield in half the time of leading competitive MCT services (83% diagnostic yield in just 14 days vs. 61% of a competitive service over a 30 day prescription period.) To date, 99.6% of interpreting physicians have agreed with the findings of the final Zio AT patient report, which we believe demonstrates efficiency and time-savings gains for interpreting physicians.
Our Collaboration with Verily
On September 3, 2019, we entered into a Development Collaboration Agreement (the “Development Agreement”) with Verily Life Sciences LLC (an Alphabet Company and referred to as “Verily”). Pursuant to the terms of the Development Agreement, the parties will develop certain next-generation AF screening, detection, or monitoring products, which involve combining Verily and our technology platforms and capabilities. The solution combines a long-term, patient-compliant, non-invasive, low-cost Zio Watch with a diagnostic-grade, AI-based approach to AF detection and burden to address an unmet AF monitoring need by leveraging the Zio service infrastructure. Under the terms of the Development Agreement, we paid Verily an upfront fee of $5.0 million in cash. In addition, we agreed to pay Verily up to an aggregate of $12.75 million in additional milestone payments upon achievement of various development and regulatory milestones over the duration of the Development Agreement, which payments will be made in cash directly to Verily.
Through December 31, 2021, we have achieved milestones and related payment obligations totaling, $11.0 million, including a $3.0 million obligation incurred during our most recent quarter. We have agreed to make additional payments up to an aggregate of $1.75 million, subject to the achievement of certain development and regulatory milestones including provision of the Zio Watch and completion of market evaluation scheduled to begin between late 2022 and early 2023.
The Development Agreement provides each party with certain licenses to use certain intellectual property of the other party for development activities in the field of AF screening, detection, or monitoring. Ownership of developed intellectual property will be allocated to the parties depending on the subject matter of the underlying developed intellectual property, and, for certain subject matter, shall be jointly owned.
During the term of the Agreement, the parties agreed not to collaborate with certain parties to develop or commercialize products on a disease management platform for certain AF patients, and the parties agreed to certain exclusivity provisions on development and commercialization of products for certain AF patients, subject to exceptions, including contractual rights and rights with respect to pre-existing product offerings.
Business Strategy
Our goal is to be the leading ambulatory cardiac monitoring option for patients at risk for arrhythmias. The key elements of our strategy include:
•Further penetrating the existing ambulatory cardiac monitoring market. We intend to expand our market penetration by targeting the large existing ambulatory cardiac monitoring market in the United States and driving broader awareness of its advantages. We will leverage our platform of products, Zio XT and Zio AT, as a way of meeting the ambulatory cardiac monitoring needs of targeted large integrated delivery networks (“IDN”). We will continue to position the Zio service as providing certainty in a single test due to high patient compliance and superior quality of uninterrupted data. Zio XT will be positioned as the workhorse service while Zio AT is appropriate for the smaller percentage of the population that requires timely notification. Marketing and education throughout the medical community are key to bringing awareness and communicating the strong clinical evidence backing the Zio service. In addition, we expect to continue developing and publishing clinical evidence to demonstrate the advantages of the Zio service. Also, within existing accounts, we will continue to introduce our Zio service beyond cardiology and electrophysiology into other departments, including neurology, emergency rooms and primary care offices. To enable this broader adoption within a hospital system, we have successfully interfaced the Zio ordering and report posting processes into a number of large health systems’ EHR systems. This seamless integration of Zio workflow processes into those already used within the IDN has proven to be a key factor in spurring growth within existing and new accounts and is an important part of our ongoing market penetration strategy.
•Pursuing international expansion opportunities. While our initial commercial focus is the U.S. market, we have initiated efforts that will allow for future expansion into international geographies. We have an initial presence in the United Kingdom with efforts underway to pursue national reimbursement. In September 2020, we were named a winner of the Artificial Intelligence in Health and Care Award run by the Accelerated Access Collaborate as part of the NHS AI Lab. This funding will bring the Zio service to selected NHS sites over a 3-year program measuring clinical, pathway and economic outcomes. We also received positive guidance from the National Institute for Health and Care Excellence ("NICE") in December 2020 for the adoption of the Zio XT service which may facilitate for future support of the Zio service through the MedTech Funding Mandate. We are also conducting diligence and prioritizing other geographies based on market size, regulatory pathway and reimbursement opportunity. We estimate the total addressable market in our initial selected countries of the U.K. and Japan, where we have started regulatory and reimbursement efforts is at least five million existing ambulatory monitoring tests annually.
•Expanding indications and clinical use cases. We intend to continue expanding indications and clinical use cases for the Zio service in untapped patient populations at risk for arrhythmias through our clinical and market development efforts. We believe these additional indications and clinical use cases represent a significant opportunity for us. This market development initiative includes expanding use for our Zio service into the following patient populations:
◦patients at high risk for asymptomatic (silent) AF, estimated to be at least ten million patients at any given time;
◦ongoing management of paroxysmal atrial fibrillation patients, estimated to be one million at any given time;
◦post-ischemic stroke patients, with an annual incidence of 690,000 patients;
◦post-cardiac catheter ablation patients, estimated to be 200,000 annual procedures; and
◦post-transcatheter aortic valve patients, estimated to be 100,000 annual procedures
We believe there is a significant near-term opportunity for us to expand the Zio service into adjacent markets such as silent AF. Initial efforts to proactively monitor this asymptomatic population with Zio XT, including end-to-end care pathway pilots, are planned in 2022.
In addition, we believe there is potential to increase the core symptomatic total addressable market by moving further upstream in the care pathway to the primary care physician call point. We believe that nearly eight million patients with palpitations in the United States visit their primary care physicians annually and that we can educate these primary care physicians on the benefits of the Zio service for this patient population.
•Advancing our product portfolio and core technology offering. We continue to invest in building a unique, innovative product portfolio and digital platform that addresses unmet needs in the ambulatory cardiac monitoring market. We will continue to invest in research and development efforts to further differentiate our
biosensor, data analytics and reporting, information system and digital platform. In 2021, we received FDA clearance for our third-generation biosensor, the Zio Monitor and second-generation deep-learned ECG detection algorithm.
•Expanding our footprint in digital healthcare. We believe that we have collected the world’s largest repository of ECG data from ambulatory patients, and we will continue to look for ways to utilize our proprietary data to create value-driving opportunities in digital healthcare, such as expansion of indications for the Zio service, new clinical insights, new therapeutic discoveries, payor and provider decision support, as well as internal operating improvements, We will also pursue development of our analytical engine for ambulatory consumer and other medical data, including the curation of third-party biosensor data in existing and new indications.
Reimbursement and Revenue from the Zio service
We receive revenue for the Zio service primarily from third-party payors, which include commercial payors and government agencies, such as CMS and the military. In addition, a small percentage of institutions, which are typically hospitals or private physician practices, purchase the Zio service from us directly.
Third-party payors require us to identify the service for which we are seeking reimbursement by using a Current Procedural Terminology (“CPT”) code set maintained by the AMA. For the year ended December 31, 2021, we received 82% of our revenue through third-party payors. As we continue to contract with more commercial insurers and the patient population ages and becomes eligible for CMS programs, we believe more of our revenue will convert to third-party payor billing.
Our clinical centers, where we conduct the analysis of ECG data captured by the Zio XT monitor and the Zio AT monitor, are CMS-certified IDTFs that qualify us as a provider and allow us to bill CMS directly for the Zio service. We meet CMS requirements, including having an independent medical director for oversight and certified cardiographic technicians for quality assurance of our Zio reports.
Clinical Results and Studies
The Zio service has been the subject of over 35 peer-reviewed publications on its effectiveness to date. This body of clinical evidence is driving clinical adoption and clinical use case expansion. The following sections summarize a few of the key clinical studies which have been driving adoption of the Zio service. In our discussion of the results of these publications, we have indicated changes in percentage terms, regardless of sample size, and the statistical significance is demonstrated by the relevant p-values, all of which are less than 0.05, which is the commonly accepted threshold for statistical significance. This follows the convention used by the authors of the study as well as standard clinical practice.
Benefit of 14-Day Continuous Monitoring
A retrospective study by Turakhia et al, published in The American Journal of Cardiology in August 2013, analyzed data from 26,751 patients using the Zio service for the first time between January 1, 2011 and December 31, 2011. While there was not a direct comparison of the Zio service to Holter monitoring performance, we interpreted results from the study to show that among the 16,142 patients with detected, clinically relevant arrhythmias, over 50% of the first-diagnosed symptomatic arrhythmias occurred after 48 hours of monitoring, suggesting that these arrhythmias could have been missed by traditional Holter monitoring during the typical maximum prescribed monitoring time.
Diagnostic Yield and Monitoring Preference
In the Barrett study, a prospective head-to-head study comparing the detection of arrhythmias between a 24-hour Holter monitor, which has a typical prescribed wear period of 24-48 hours, and the 14-day Zio service, a total of 146 patients referred for evaluation of cardiac arrhythmias between April 2012 and July 2012 underwent simultaneous ambulatory ECG recording with both devices. The purpose of the Barrett study was to determine the number of arrhythmia events and the percentage of patients in whom an arrhythmia was detected, known as “diagnostic yield,” during the comparative prescribed wear periods. Our interpretation of the results of the Barrett study are that over the total wear period of each device, the Zio service detected 96 arrhythmia events compared with 61 arrhythmia events by the Holter monitor (P < 0.001) providing a 57% improvement in diagnostic yield. An increase in diagnostic yield provides increased data for the prescribing physician to use when making a diagnosis. In addition, we interpreted survey results to show that 94% of patients found the Zio XT monitor comfortable to wear, whereas only 52% patients found the Holter monitor comfortable to wear. Of the 102 physicians surveyed, from our review, we concluded that 90% thought a definitive diagnosis was achieved using data from the Zio service, as opposed to 64% using data from the 24-hour Holter monitor. This clinical trial, however, was a single center study with a relatively small sample size which did not compare the Zio service with any product except the Holter monitor.
A prospective, randomized study by Eysenck et al, published in the Journal of Interventional Cardiac Electrophysiology in February 2019, compared the accuracy of AF burden detection across four different categories of external cardiac monitors (“ECMs”) to implanted pacemakers (“PPM”), the ‘gold standard’ of rhythm monitoring. The study enrolled 21 patients previously implanted with a PPM, each acting as their own control subject, who wore every ECM, including Zio XT, for two weeks in randomized order. Zio XT had an R-squared assessment of fit value of 0.99 compared to the PPM. Zio XT was the only monitor to detect 100% of clinically relevant AF, outperforming the other ECM monitors in the study.
Changing Clinical Management for AF
In the Rosenberg Study, a prospective single center study of 74 patients undergoing management of AF, patients received both the Zio XT monitor and a 24-hour Holter monitor simultaneously to determine the pattern of AF, to document a response to therapy and to potentially diagnose other arrhythmias. From our review, we concluded that the Zio service identified AF events in 24% more patients (18 patients) than Holter monitors (P < 0.0001) and the diagnosed pattern of AF was changed in 28% of patients (21 patients) after Zio service monitoring.
Based on our review of the study, we concluded that 28% of patients (21 patients) had a change in their clinical management. The most common changes included a change in antiarrhythmic medication, initiation or discontinuation of anticoagulation medication, recommendation of pacemaker placement, atrioventricular junction ablation, pulmonary vein isolation procedure and cardioversion. This clinical trial, however, was also a single center study with a relatively small sample size which did not compare the Zio service with any product except the Holter monitor.
AF Burden as a Predictor of Stroke Risk
The KP-RHYTHM Study, a retrospective study of 1,965 Kaiser Permanente patients with paroxysmal AF who were monitored with the Zio service between October 2011 and October 2016, examined the independent association between AF burden, which is the amount of time that a patient spends in AF during the monitoring period as measured by the Zio service, and the risk of ischemic stroke. The findings were derived by linking detailed clinical outcome data from Kaiser Permanente’s electronic medical records with our database of analyzed ECG recordings. We interpreted the study results to show that an AF burden of more than 11% of the total time their heart rhythm was monitored was found to be associated with a three-fold increase in stroke risk, independent of other known risk factors in patients who were not taking medication to prevent blood clots. We concluded that these results suggest that information on AF burden, which is measured by the Zio service, may help patients and providers better evaluate treatment options for reducing risk of stroke. This clinical study was limited to Kaiser Permanente’s patients from the Northern and Southern California regions.
Monitoring of Asymptomatic AF in High Risk Patients
Currently, the Zio service is prescribed by physicians primarily for symptomatic patients. However, the NSA estimates that one-third of the AF population suffers from asymptomatic, or silent, AF. We see a future opportunity in proactively monitoring the approximately ten million patients who are at high risk of asymptomatic AF to identify those with the illness.
STUDY-AF was a single-center, single-arm prospective study by Turakhia et al published in Clinical Cardiology in May 2015 that enrolled 75 high-risk but previously undiagnosed AF patients from May 2012 to August 2013. Patients were 55 years of age or older and considered high risk with two or more of the following risk factors: coronary disease, heart failure, hypertension, diabetes or sleep apnea, but had no prior documented AF or history of blood clots causing blockage in blood vessels. We interpreted the results to show that long-term continuous monitoring with the Zio service identified 11% of patients with previously undiagnosed AF or atrial tachycardia, a rapid heartbeat where electrical signals initiate abnormally in the upper chamber of the heart. We concluded from our review that in patients with AF, 75% of patients experienced the longest AF episode after the first 48 hours of monitoring and there was also a high prevalence of asymptomatic atrial tachycardia and frequent supraventricular ectopic complexes identified, which may be relevant to development of AF or stroke. This clinical trial, however, was also a single center study with a relatively small sample size.
The mHealth Screening to Prevent Strokes, or mSToPS study, led by researchers at the Scripps Research Translational Institute (“SRTI”) and in collaboration with Aetna Inc., Healthagen LLC, Janssen Research and Development, LLC and Johnson & Johnson, utilized a web-based platform to remotely recruit from 5,214 eligible patients from the Aetna Commercial Fully Insured and Medicare Advantage programs. Women over the age of 65 and men over 55 with certain risk factors were selected to participate based on information derived from claims data that placed them at a potentially increased risk of undiagnosed asymptomatic AF. Three peer-reviewed articles were published on the mSToPS study between 2018 and 2021. The study design was published in the Journal of the American Medical Association in July 2018. The one-year results were published in Heart Rhythm O2 in December 2020. We interpreted the results to show that at one-year, AF was newly diagnosed in 6.6 percent of patients who were actively monitored by the Zio service versus 2.4 percent in the observational control group receiving routine care. In addition to this primary endpoint to evaluate the difference in new AF diagnoses, active monitoring in an asymptomatic, moderate-risk population with the Zio service was associated with an increase in cardiology outpatient visits but also significantly lower rates of emergency department visits and hospitalizations over the one year following monitoring. We concluded that these results suggest active monitoring with the Zio service can significantly reduce high-cost healthcare resource utilization and shift toward appropriate care settings at one year. The three-year follow-up intended to assess whether screening asymptomatic individuals for AF and other arrhythmias with the Zio service can improve clinical outcomes. The three-year study results were published in PLOS One in October 2021. Over the course of the study:
•AF was newly diagnosed in 11.4% of those actively monitored with the Zio service versus only 7.7% of the control group (a statistically significant 48% improvement).
•Active monitoring with the Zio service led to a statistically significant reduction in the combined primary endpoint of cardiac event (death, stroke, systemic emboli or myocardial infarction) versus clinical care. The study found the incidence rate of a cardiac event was 3.6 per 100 person-years in people diagnosed with AF who underwent active monitoring, compared to the control group incidence rate of 4.5 per 100 person-years.
•Active monitoring with the Zio service also led to fewer hospitalizations for bleeding, the primary safety endpoint for the study (incidence rate of 0.32 per 100 person-years versus 0.71 per 100 person-years).
•An additional finding showed a clinical event was common in the four weeks surrounding a clinical diagnosis of AF not detected by screening. Cumulatively, 42.9% of participants in the control group were hospitalized in the four weeks surrounding the AF diagnosis; however, for those diagnosed utilizing data provided by the Zio service, only one person experienced a negative clinical outcome in the period surrounding his or her diagnosis.
We concluded the mSToPS study found that active screening for AF, as part of a prospective, pragmatic, direct-to-participant and nationwide study, was associated with a significant improvement in clinical outcomes and safety at three years relative to routine care.
An additional study examining early detection of AF using the Zio service in high risk patients is the Home-based Screening for Early Detection of AF, or SCREEN-AF was published in the Journal of the American Medical Association (“JAMA”) in February 2021. This randomized control trial recruited 856 participants 75 years or older with hypertension and no known AF from 48 primary care clinics across Canada and Germany between 2015 and 2019. The intervention group included ambulatory screening for AF for two weeks with the Zio service utilized at baseline and again at three months, in addition to standard care for six months. We interpreted the results to show AF was newly diagnosed in the screening group in 5.3% of subjects at six months compared to 0.5% in the standard of care control group. A secondary outcome showed oral anticoagulation (“OAC”) therapy was prescribed for 75% of participants who had screen-detected AF. We concluded that proactive screening and monitoring using the Zio service could increase AF detection by 10-fold over standard of care potentially prompting anticoagulation use.
In November 2019, we announced our participation in a randomized, controlled study, GUARD-AF (Reducing stroke by screening for undiagnosed atrial fibrillation in elderly individuals), sponsored by the Bristol-Myers Squibb-Pfizer Alliance. The study seeks to determine if earlier detection of AF through screening in previously undiagnosed men and women ultimately impacts the rate of stroke, compared to usual standard medical care. The screening arm will utilize the Zio XT service. The GUARD-AF study population (n=52,000) will include men and women at least 70 years of age visiting their primary care physician for usual follow-up care. The primary outcome measures will be stroke and bleeding events leading to hospitalization. The trial will identify outcome events using insurance claims from a healthcare claims database which, although subject to certain limitations, is expected to provide evidence on health outcomes associated with AF detection intervention that may help inform future guidelines.
Research and Development
Our research and development activities are focused on:
•Improvements and extensions to existing products and services. We are continuously working to improve the Zio service to increase patient comfort, product quality, operational scalability and security.
•Advancing our technology offering. Our product pipeline includes patch-based solutions that combine continuous monitoring for extended periods with accelerated notification of significant events through mobile transmission capabilities.
•Customer workflow optimization. We have initiatives that aim to increase customer productivity by optimizing workflow through easier patient enrollment, report access and interpretation, and integration of Zio reports directly into electronic health records.
•Data analytics. We are focused on improving and enhancing our backend deep-learned analytic platform, building on our core competency in data analytics.
•Developing clinical evidence. We are involved in clinical studies to further support the benefits of the Zio service and expand indications for use.
•Continuing to solidify our footprint in digital healthcare. Using our repository of ambulatory ECG patient data, we will continue to look for ways to create value-driving opportunities in digital healthcare, such as expansion of indications for the Zio service, new therapeutic discoveries, development of an analytical engine for ambulatory consumer and other medical data and payor and provider decision support.
Our research and development activities consist of software development, algorithm and product development, regulatory affairs, and clinical research. Our research and development expense was $38.7 million, $41.3 million and $37.3 million for the years ended December 31, 2021, 2020 and 2019, respectively.
Sales and Marketing
We market our ambulatory cardiac monitoring solution in the United States through a direct sales organization comprised of sales management, field billing specialists, quota-carrying sales representatives, and a customer experience team. Our sales representatives focus on initial introduction into new accounts, penetration across a sales region, driving adoption within existing accounts and conveying our message of clinical and economic value to service line managers, hospital administrators, and other clinical departments. We continue to optimize the structure of our U.S. sales organization to expand the current customer account base and increase utilization of our Zio service. In addition, we will continue exploring sales and marketing expansion opportunities in international geographies.
We market our Zio service to a variety of physician specialties including general cardiologists, electrophysiologists, neurologists, primary care physicians and other physician specialists who diagnose and manage care for patients with arrhythmias. We have found success focusing on IDNs, in which large networks of facilities and providers work together to offer a continuum of care to a specific geographic area or market. Focusing on sales to IDNs gives us the opportunity to conduct a holistic sale for health systems interested in making value-based purchasing decisions.
Competition
We operate in a highly competitive and fragmented industry, subject to rapid change and significantly affected by new product introductions, results of clinical research, corporate combinations and other factors. Large competitors in the ambulatory cardiac market include companies that sell standard Holter monitors including GE Healthcare, Philips Healthcare, Mortara Instrument, Inc., Spacelabs Healthcare Inc. and Welch Allyn Holdings, Inc. (acquired by Hill-Rom Holdings, Inc.). Additional competitors, such as BioTelemetry, Inc. (acquired by Royal Philips), Preventice Solutions, Inc. (acquired by Boston Scientific, Inc.) and Bardy Diagnostics, Inc. (acquired by Hill-Rom Holdings, Inc. which was recently acquired by Baxter International, Inc.) offer ambulatory cardiac monitoring services and also function as service providers. Recently, there has been increased acquisition activity and consolidation in our industry. Many of our competitors have substantially greater financial, manufacturing, marketing and technical resources than we do. Furthermore, many of our competitors have well-established brands, widespread distribution channels, broader product offerings and an established customer base.
These competitors have also developed patch-based cardiac monitors that have received FDA and foreign regulatory clearances. We are also aware of some small start-up companies entering the patch-based cardiac monitoring market. Large medical device companies may continue to acquire or form alliances with these smaller companies in order to diversify their product offering and participate in the digital health space.
Future competition could come from manufacturers of wearable fitness products or large information technology companies focused on improving healthcare. For example, Apple Inc. has added capabilities on its watch platform to measure non-continuous ECG and to alert users to the potential presence of irregular heartbeats suggestive of asymptomatic AF. These competitors and potential competitors may introduce new products and services that more directly compete with our Zio service.
We believe the principal competitive factors in our market include:
•ease of use, comfort and unobtrusiveness of the device for the patient;
•quality and clinical validation of the deep-learned algorithms used to detect arrhythmias;
•concise and comprehensive reports supporting efficient physician interpretation;
•ease of use of service workflow for physicians and supporting clinicians;
•digital tools for data management including mobile app, website tools and EHR integration;
•contracted rates with third-party payors;
•government reimbursement rates associated with our products and services;
•quality of clinical data and publications in peer-reviewed journals;
•size, experience, knowledge and training of sales and marketing teams;
•availability and reliability of sales representatives and customer support services;
•workflow protocols for solution implementation in existing care pathways;
•reputation of existing device manufacturers and service providers; and
•relationships with physicians, hospitals, administrators, and other third-party payors.
Intellectual Property
To protect our proprietary rights, we rely on a combination of trademark, copyright, patent, trade secret and other intellectual property laws, employment, confidentiality and invention assignment agreements and protective contractual provisions with our employees, contractors, consultants, suppliers, partners and other third parties. In addition, we have entered into exclusive and non-exclusive licenses in the ordinary course of business relating to a wide array of technologies or other intellectual property rights or assets.
Our patent portfolio includes numerous issued and pending patent applications in the U.S. and other parts of the world, which in the aggregate, we believe to be of material importance in the operation of our business. U.S. patents, as well as most foreign patents, are generally effective for 20 years from the date the earliest application was filed. In some cases, the patent term may be extended. As of December 31, 2021 we owned, or retained an exclusive license to, twenty-three issued patents from the U.S. Patent Office, eight issued patents from the Japanese Patent Office, two issued patents from the Australian Patent Office, four issued patents from the Canadian Patent Office, five issued patents from the European Patent Offices, three issued patents from the Korean Patent Office, and one issued patent from the Chinese Patent Office. Our U.S. issued patents as of December 31, 2021 are set to expire over a range of years, from November 2028, with respect to some of our earlier patents, to February 2041, subject to any extensions.
As of December 31, 2021, we had twenty-six pending patent applications globally, including twelve in the United States, three in the European Patent Office, four in Japan, three Patent Cooperation Treaty (“PCT”) international applications, and one in each of Australia, Korea, China and India.
As of December 31, 2021, our trademark portfolio contained a U.S. trademark registration for the mark My ZIO, ZIO and a pending U.S. application for Zio AT. It also contained registered trademarks for the mark IRHYTHM in Australia and the European Union and pending applications for that mark in Australia, Austria, Canada, China, Denmark, Finland, France, Germany, Japan, Norway, Sweden, Switzerland, United Kingdom and United States. It further contained pending applications for the mark ZIO in Australia, Canada, China, Japan, Norway and Switzerland and a registered trademark for ZIO in the European Union.
Our patents and patent applications seek to protect aspects of our core technologies and our product concepts for continuous cardiac monitoring. We believe that our patent position provides us with sufficient rights to protect our current and proposed commercial products. However, our patent applications may not result in issued patents, and any patents that have been issued or might be issued may not protect our intellectual property rights. We also rely on trade secrets, technical know-how and continuing innovation to develop and maintain our competitive position. We seek to protect our proprietary information and other intellectual property by generally requiring our employees, consultants, contractors, suppliers, outside scientific collaborators and other advisors to execute non-disclosure and assignment of invention agreements on commencement of their employment or engagement. Agreements with our employees also forbid them from bringing the proprietary rights of third parties to us. We also generally require confidentiality or material transfer agreements from third parties that receive our confidential data or materials.
Manufacturing and Supply
We manufacture our ambulatory cardiac monitors, Zio XT and Zio AT, in our leased facilities in Cypress, California. This 17,558 square foot facility provides space for our assembly and production operations, including packaging, storage and shipping. We believe our manufacturing facilities will be sufficient to meet our manufacturing needs for at least the next year, but plan to move into a new, larger facility during 2022.
Our manufacturing operations are subject to regulatory requirements of the FDA’s Quality System Regulation (“QSR”) for medical devices sold in the United States, set forth at 21 CFR part 820, the Medical Devices Directive 93/42/EEC (“MDD”) and the Medical Device Regulations 2017/745 of the European Parliament and of the Council (“MDR”), which is required for doing business in the European Union (“EU”), and the UK Medical Device Regulations 2002 (as amended) (“UK MDR”). We are also subject to applicable requirements relating to the environment, waste management and health and safety matters, including measures relating to the release, use, storage, treatment, transportation, discharge, disposal, sale, labeling, collection, recycling, treatment and remediation of hazardous substances.
Our failure or the failure of our suppliers to maintain compliance with either the QSR, MDR, MDD, or UK MDR requirements could result in the shutdown of our manufacturing operations, recall of our products, and various enforcement actions such as warning letters or loss of clearance or certification, which would harm our business. In the event that one of our suppliers fails to maintain compliance with our or governmental quality requirements, we may have to qualify a new supplier and could experience manufacturing delays as a result.
Our quality control management programs have earned us a number of quality-related designations. Our manufacturing facilities, have received EN ISO 13485:2016 certification. The NSAI most recently conducted an ISO 13485 surveillance audit in 2021 and a renewed ISO certification was received in August 2021. We have been an FDA-registered medical device manufacturer since 2008 and have been a California-licensed medical device manufacturer since 2009. The most recent FDA inspection of our manufacturing facility occurred in October 2021, and no FDA Form 483 observations resulted. The FDA also performed a Remote Regulatory Assessment (“RRA”) of our design facilities in San Francisco, California, which concluded in May 2021. Unlike on-site inspections, the FDA does not issue formal 483 observations at the conclusion of RRAs.
Circuit board components of the Zio XT and Zio AT monitors are provided by contract electronic manufacturers, Cal-Comp USA, and Veris Manufacturing, Inc. We have manufacturing service agreements with both providers that allow either party to terminate the agreement with 90 days prior written notice. There are a number of additional critical components and sub-assemblies sourced by other vendors. The vendors for these materials are qualified through stringent evaluation and testing of their performance. We implement a strict no-change policy with our contract manufacturers to ensure that no components are changed without our approval. Our production group in Cypress, California performs inspection, assembly, testing and product release.
Order quantities and lead times for components purchased from suppliers are based on our forecasts derived from historical demand and anticipated future demand. Lead times for components may vary significantly depending on the size of the order, time required to fabricate and test the components, specific supplier requirements and current market demand for the components and subassemblies. To date, we have not experienced unmanageable delays in obtaining any of our components or subassemblies.
The COVID-19 pandemic has significantly impacted the global economy and has contributed to cost increases, supply constraints and increasing inflationary pressures. We continuously monitor the effects of inflationary factors and supply chain constraints, such as increases in the cost of our components and increasing labor costs due to high wage inflation and challenging labor market conditions. Competitive and regulatory conditions may restrict our ability to fully recover these costs through price increases. As a result, it may be difficult to fully offset the impact of persistent inflation.
Government Regulation
United States Food & Drug Administration (FDA)
The Zio XT and Zio AT monitors are considered medical devices subject to extensive and ongoing regulation by the FDA under the Federal Food, Drug, and Cosmetic Act (“FD&C Act”) and its implementing regulations, as well as other federal and state regulatory bodies in the United States. The laws and regulations govern, among other things, product design and development, pre-clinical and clinical testing, manufacturing, packaging, labeling, storage, recordkeeping and reporting, clearance or approval, marketing, distribution, promotion, import and export, and post-marketing surveillance and associated regulatory reporting.
The FDA regulates the medical device market to ensure the safety and efficacy of these products. The FDA allows for two primary pathways for a medical device to gain approval for commercialization: a successful premarket approval (“PMA”), application or 510(k) clearance pursuant to Section 510(k) of the FD&C Act. A novel product must go through the more rigorous PMA process if it cannot receive authorization through a 510(k) clearance. FDA has established three different classes of medical devices that indicate the level of risk associated with using a device and the consequent degree of regulatory controls needed to govern its safety and efficacy. Most Class I devices are exempt from 510(k) requirements. Most Class II devices, including the Zio XT and Zio AT monitors, and the Zio ECG Utilization Service System (“ZEUS System”), require 510(k) clearance from the FDA in order to be marketed in the United States. A 510(k) submission must demonstrate that the device is substantially equivalent to a device legally in commercial distribution in the United States: (1) before May 28, 1976; or (2) to another device that has been cleared through the 510(k) process and determined by FDA to be substantially equivalent. To be substantially equivalent, the proposed device must have the same intended use as the predicate device and either have the same technological characteristics as the predicate device or have different technological characteristics and not raise different questions of safety or effectiveness than the predicate device. Clinical data is sometimes required to support substantial equivalence. In some instances, data from human clinical trials must also be submitted in support of a 510(k) submission. If so, this data must be collected in a manner that conforms with specific requirements in federal regulations. Most Class III devices are high-risk devices that pose a significant risk of illness or injury or devices found not to be substantially equivalent to Class I and II predicate devices through the 510(k) process and require PMA. The PMA process for Class III devices is more involved and includes the submission of clinical data to support claims made for the device.
The Zio XT and Zio AT monitors maintain FDA 510(k) clearance as Class II devices, with the Zio monitor receiving a new 510(k) clearance in May 2021. In addition, the ZEUS System's most recent update received FDA 510(k) clearance in May 2021 as a Class II Software as a Medical Device. The ZEUS System is the combination of proprietary algorithms and software tools that our certified cardiac technicians utilize to curate the ECG data and create the Zio Report electronically. Significant modifications made to the ZEUS System since its original clearance have been regularly evaluated by the FDA.
Pervasive and Continuing Regulation
After a device is placed on the market, numerous regulatory requirements continue to apply. These include:
•the FDA’s QSR, which requires manufacturers, including their suppliers, to follow stringent design, testing, control, documentation and other quality assurance procedures during all aspects of the product lifecycle, including the manufacturing process;
•labeling regulations and FDA prohibitions against the promotion of products for un-cleared, unapproved or off-label uses;
•medical device reporting (“MDR”) regulations, which require that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to recur;
•medical device recalls, which require that manufacturers report to the FDA any recall of a medical device, provided the recall was initiated to either reduce a risk to health posed by the device, or to remedy a violation of the FD&C Act caused by the device that may present a risk to health; and
•post-market surveillance regulations, which apply when necessary to protect the public health or to provide additional safety and effectiveness data for the device.
After a device receives 510(k) clearance or PMA approval, any modification that could significantly affect its safety or effectiveness, or that would constitute a major change in its intended use, performance or functionality may require a new clearance or approval. The FDA requires each manufacturer to make this determination initially, but the FDA can review any such decision and can disagree with a manufacturer’s determination. If the FDA disagrees with the determination not to seek a
new 510(k) clearance or PMA, the FDA may retroactively require a new 510(k) clearance or premarket approval. The FDA could also require a manufacturer to cease marketing and distribution and/or recall the modified device until 510(k) clearance or premarket approval is obtained. Also, in these circumstances, the manufacturer may be subject to significant regulatory fines, penalties, and other enforcement actions, such as warning letters.
We have registered our facilities with the FDA as a medical device manufacturer and have obtained a manufacturing license from the California Department of Public Health, or CDPH. The FDA and CDPH have broad post-market and regulatory enforcement powers. We are subject to unannounced inspections by the FDA and the Food and Drug Branch of CDPH to determine our compliance with the QSR and other regulations, and these inspections may include the manufacturing facilities of our suppliers. Additionally, the EU Notified Body regularly inspects our manufacturing, design and operational facilities to ensure ongoing ISO 13485 compliance and periodically reviews technical design files in accordance with the Medical Device Regulations in order to maintain our CE mark or issue CE mark for new or updated devices.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include any of the following sanctions:
•warning letters, fines, injunctions, consent decrees and civil penalties;
•repair, replacement, refunds, recall or seizure of our products;
•operating restrictions, partial suspension or total shutdown of production;
•refusing our requests for 510(k) clearance or premarket approval of new products, new intended uses or modifications to existing products;
•withdrawing 510(k) clearance or premarket approvals that have already been granted; and
•criminal prosecution
European Union and United Kingdom
The Zio XT monitor is currently regulated in the European Union as a medical device per the European Union Directive 93/42/EEC, also known as the Medical Device Directive (“MDD”). The MDD sets out the basic regulatory framework for medical devices in the European Union. In May 2021, the MDR, a more comprehensive regulatory framework, replaced the MDD.
The system of regulating medical devices operates by way of a certification for each medical device. Each certified device is marked with the CE mark which shows that the device has a certificate of conformance. There are national bodies known as Competent Authorities in each member state which oversee the implementation of the MDD and MDR within their jurisdiction. The means for achieving the requirements for the CE mark vary according to the nature of the device. Devices are classified in accordance with their perceived risks, similarly to the U.S. system. The class of a product determines the conformity assessment required before the CE mark can be placed on a product. Conformity assessments for our products are carried out as required. Each member state can appoint Notified Bodies within its jurisdiction. If a Notified Body of one member state has issued a CE mark, the device can be sold throughout the European Union without further conformance tests being required in other member states. The CE mark is contingent upon continued compliance with the applicable regulations and the quality system requirements of the ISO 13485 standard. Our current CE mark for Zio XT is issued by the National Standards Authority of Ireland, or NSAI under the MDD. We will need to pursue CE mark under the new MDR.
Due to United Kingdom’s departure from the European Union, the UK Medicines & Healthcare products Regulatory Agency (“MHRA”) has issued new requirements associated with the UK Conformity Assessed (“UKCA”) mark. The UKCA marking is a new UK product marking that is used for goods being placed on the market in Great Britain. It covers most goods which previously required the CE marking. The UKCA requirement became effective on January 1, 2021. However, to allow businesses time to adjust to the new requirements, we will still be able to use the CE marking until 2023. Additionally, MHRA has indicated that additional new or modified regulations are planned in the future beyond those outlined in UK MDR 2002. We will need to pursue UKCA marking under this new requirement.
Health Insurance Portability and Accountability Act
The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), established comprehensive federal protection for the privacy and security of health information. Under HIPAA, the Department of Health and Human Services (“HHS”), has issued regulations to protect the privacy and security of protected health information used or disclosed by Covered Entities, including healthcare providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in healthcare transactions and standardization of identifiers for health plans and providers. The privacy regulations protect medical records and other protected health information by limiting their use and release, giving patients the right to access their medical records and limiting most disclosures of health information to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures. HIPAA requires Covered Entities to execute Business Associate Agreements with individuals and organizations, or Business Associates, who provide services to Covered Entities and who need access to protected health information. We are a Covered Entity and a provider under HIPAA and subject to HIPAA regulations.
In 2009, Congress enacted Subtitle D of the Health Information Technology for Economic and Clinical Health Act (“HITECH”). HITECH amends HIPAA and, among other things, creates new targets for enforcement, imposes new penalties for noncompliance and establishes new breach notification requirements for Covered Entities and Business Associates.
Under HITECH’s breach notification requirements, Covered Entities must report breaches of protected health information that has not been encrypted or otherwise secured in accordance with guidance from HHS. Required breach notices must be made as soon as is reasonably practicable, but no later than 60 days following discovery of the breach. Reports must be made to affected individuals and to HHS, and in some cases, they must be reported through local and national media, depending on the size of the breach. We are subject to audit under HHS’s HITECH-mandated audit program. We may also be audited in connection with a privacy complaint. We are subject to prosecution and/or administrative enforcement and increased civil and criminal penalties for non-compliance, including a new, four-tiered system of monetary penalties adopted under HITECH. We are also subject to enforcement by state attorneys general who were given authority to enforce HIPAA under HITECH. To mitigate the risk of penalties under the HITECH breach notification provisions, we must ensure that breaches of protected health information are promptly detected and reported within the company, so that we can make all required notifications on a timely basis. However, even if we make required reports on a timely basis, we may still be subject to penalties for the underlying breach.
In addition to the federal privacy regulations, there are a number of state privacy laws, such as the California Consumer Privacy Act (“CCPA”) and the California Privacy Rights Act ("CPRA”), that apply to us. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely, and new privacy and security laws in this area are evolving. Requirements of these laws and penalties for violations vary widely. These new legislative and regulatory developments may impact our privacy and security strategy and our web-based and mobile assets.
If we or our operations are found to be in violation of HIPAA, HITECH, or their implementing regulations, we may be subject to penalties, including civil and criminal penalties, fines, and exclusion from participation in federal or state healthcare programs, and the curtailment or restructuring of our operations. HITECH increased the civil and criminal penalties that may be imposed against Covered Entities, their Business Associates and possibly other persons, and gave state attorneys general new authority to file civil actions for damages or injunctions in federal courts to enforce the federal HIPAA laws and seek attorney’s fees and costs associated with pursuing federal civil actions.
Federal, State and Foreign Fraud and Abuse Laws
Because of the significant federal funding involved in CMS programs such as Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws to eliminate fraud and abuse in federal healthcare programs. Our business is subject to compliance with these laws. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Healthcare and Education Affordability Reconciliation Act, which we refer to collectively as the Affordable Care Act, was enacted in the United States. The Affordable Care Act expands the government’s investigative and enforcement authority and increases the penalties for fraud and abuse, including amendments to both the Anti-Kickback Statute and the False Claims Act, to make it easier to bring suit under these statutes. The Affordable Care Act also allocates additional resources and tools for the government to police healthcare fraud, with expanded subpoena power for HHS, additional funding to investigate fraud and abuse across the healthcare system and expanded use of recovery audit contractors for enforcement.
Anti-Kickback Statute
The federal Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid.
The definition of “remuneration” has been broadly interpreted to include anything of value, including, for example, gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payment of cash and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered businesses, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition, some kickback allegations have been claimed to violate the federal False Claims Act.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are otherwise lawful in businesses outside of the healthcare industry. Recognizing that the Anti- Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Office of Inspector General (“OIG”) of the HHS to issue a series of regulations known as “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of recipients for healthcare products or services reimbursed by any source, not only CMS programs.
Government officials have focused their enforcement efforts on the marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain individual sales, marketing and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Federal False Claims Act
Another development affecting the healthcare industry is the increased use of the federal False Claims Act (“FCA”) and in particular, action brought pursuant to the FCA’s “whistleblower” or “qui tam” provisions. The FCA imposes liability on any person or entity that, among other things, knowingly presents, or causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the FCA allow a private individual to bring actions on behalf of the federal government alleging that the defendant has violated the FCA and to share in any monetary recovery. As a result, in recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted false claims laws analogous to the FCA, and many of these state laws apply where a claim is submitted to any third-party payor and not only a federal healthcare program.
When an entity is determined to have violated the FCA, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between approximately $12,000 and $24,000 for each separate instance of false claim. As part of any settlement, the government will usually require the entity to enter into a corporate integrity agreement, which imposes certain compliance, certification and reporting obligations. There are many potential bases for liability under the FCA. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The federal government has used the FCA to assert liability on the basis of inadequate care, kickbacks and other improper referrals, and improper use of CMS billing numbers, in addition to the more predictable allegations of misrepresentations with respect to the services rendered. In addition, the federal government has prosecuted companies under the FCA in connection with off-label promotion of products. Activities relating to the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products and services and the sale and marketing of our products and services may be subject to scrutiny under these laws.
While we are unaware of any current matters, we are unable to predict whether we will be subject to actions under the FCA or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly affect our financial performance.
Open Payments
The Physician Payment Sunshine Act, known as “Open Payments” and enacted as part of the Affordable Care Act, requires all pharmaceutical and medical device manufacturers of products covered by Medicare, Medicaid or the Children’s Health Insurance Program to report annually to HHS: (i) payments and transfers of value to teaching hospitals and licensed physicians, (ii) physician ownership in the manufacturer, and (iii) research payments. The payments required to be reported include the cost of meals provided to a physician, travel reimbursements and other transfers of value, including those provided as part of contracted services such as speaker programs, advisory boards, consultation services and clinical trial services. The statute requires the federal government to make reported information available to the public. Failure to comply with the reporting requirements can result in significant civil monetary penalties ranging from $1,000 to $10,000 for each payment or other transfer of value that is not reported (up to a maximum per annual report of $150,000) and from $10,000 to $100,000 for each knowing failure to report (up to a maximum per annual report of $1.0 million). We are subject to Open Payments and the information we disclose may lead to greater scrutiny, which may result in modifications to established practices and additional costs. Additionally, similar reporting requirements have also been enacted on the state level domestically, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with healthcare professionals.
Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act (“FCPA”) prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party or candidate for the purpose of influencing any act or decision of the foreign entity in order to assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring us to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, if any, and to devise and maintain an adequate system of internal accounting controls for international operations.
International Laws
In Europe, various countries have adopted anti-bribery laws providing for severe consequences in the form of criminal penalties and significant fines for individuals or companies committing a bribery offense. Violations of these anti-bribery laws, or allegations of such violations, could have a negative impact on our business, results of operations and reputation.
For instance, in the United Kingdom, under the U.K. Bribery Act 2010, a bribery occurs when a person offers, gives or promises to give a financial or other advantage to induce or reward another individual to improperly perform certain functions or activities, including any function of a public nature. Bribery of foreign public officials also falls within the scope of the U.K. Bribery Act 2010. An individual found in violation of the U.K. Bribery Act 2010, faces imprisonment of up to 10 years. In addition, the individual can be subject to an unlimited fine, as can commercial organizations for failure to prevent bribery.
There are also international privacy laws, such as the General Data Protection Regulation (“GDPR”), that impose restrictions on the access, use, and disclosure of health information. All of these laws may impact our business. Our failure to comply with these privacy laws or significant changes in the laws, or judicial rulings interpreting these laws, may impact our ability to obtain required patient information and therefore could significantly impact our business and our future business plans.
U.S. Centers for Medicare and Medicaid Services (“CMS”)
Medicare is a federal program administered by CMS through fiscal intermediaries and carriers. Available to individuals age 65 or over, and certain other individuals, the Medicare program provides, among other things, healthcare benefits that cover, within prescribed limits, the major costs of most medically necessary care for such individuals, subject to certain deductibles and copayments.
CMS has established guidelines for the coverage and reimbursement of certain products, supplies and services. In general, in order to be reimbursed by Medicare, a healthcare product or service furnished to a Medicare beneficiary must be reasonable and necessary for the diagnosis or treatment of an illness or injury, or to improve the functioning of a malformed body part. The methodology for determining coverage status and the amount of Medicare reimbursement varies based upon, among other factors, the setting in which a Medicare beneficiary received healthcare products and services. Any changes in federal legislation, regulations and policy affecting Medicare coverage and reimbursement relative to our Zio service could have a material effect on our performance.
CMS also administers the Medicaid program, a cooperative federal/state program that provides medical assistance benefits to qualifying low income and medically needy persons. State participation in Medicaid is optional, and each state is given discretion in developing and administering its own Medicaid program, subject to certain federal requirements pertaining to payment levels, eligibility criteria and minimum categories of services. The coverage, method and level of reimbursement varies from state to state and is subject to each state’s budget restraints. Changes to the coverage, method or level of reimbursement for our Zio service may affect future revenue negatively if reimbursement amounts are decreased or discontinued.
All CMS programs are subject to statutory and regulatory changes, retroactive and prospective rate adjustments, administrative rulings, interpretations of policy, intermediary determinations, and government funding restrictions, all of which may materially increase or decrease the rate of program payments to healthcare facilities and other healthcare providers, including those paid for our Zio service.
Our facilities in Illinois, California and Texas are enrolled as independent diagnostic testing facilities (“IDTFs”) defined by CMS as entities independent of a hospital or physician’s office in which diagnostic tests are performed by licensed or certified non-physician personnel under appropriate physician supervision. CMS has set certain performance standards that every IDTF must meet in order to obtain or maintain its billing privileges.
United States Healthcare Reform
Changes in healthcare policy could increase our costs and subject us to additional regulatory requirements that may interrupt commercialization of our current and future solutions. Changes in healthcare policy could increase our costs, decrease our revenue and impact sales of and reimbursement for our current and future products and services. The Affordable Care Act (“ACA”) substantially changes the way healthcare is financed by both governmental and private insurers, and significantly impacts our industry. The ACA contains a number of provisions that impact our business and operations, some of which in ways we cannot currently predict, including those governing enrollment in federal healthcare programs and reimbursement changes.
It is uncertain how modification or repeal of any of the provisions of the ACA, including as a result of current and future executive orders and legislative actions, will impact us and the medical device industry as a whole. Any changes to, or repeal of, the ACA may have a material adverse effect on our results of operations.
Effective as of January 1, 2022, the U.S. federal No Surprises Act, prohibits providers from charging patients an amount beyond the in-network cost sharing amount for services rendered by out-of-network providers, subject to limited exceptions. Further, the No Surprises Act requires providers to send an insured patient’s health plan a good faith estimate of expected charges, including billing and diagnostic codes, prior to when the patient is scheduled to receive the item or service.
We cannot predict what other health care programs and regulations will ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the United States may have on our business.
Human Capital
As of December 31, 2021, we have approximately 1,700 employees globally. None of our employees are represented by a collective bargaining agreement, and we believe our employee relations are good. Although the COVID-19 pandemic has disrupted how and where we work, our employees have demonstrated ongoing resilience. During 2021, employees continued to effectively navigate strict safety protocols for those working onsite, primarily in manufacturing, and the majority of our employees were not required to be onsite and worked from home.
Diversity, Equity & Inclusion
We believe in the richness and quality of a working environment that is informed by people from all walks of life and strive to create a genuinely inclusive environment. We are proud that, overall, our workforce is more than 60% female and greater than 50% of employees are ethnically diverse. To further build on our commitment to diversity, equity and inclusion, we have formed a Diversity Council of representatives from across our company that is working to coordinate efforts as we define our corporate strategy. In addition, we have an active Employee Resource Group (“ERG”) with plans to increase the number of ERGs to reflect the diversity of our workforce, and we are building external partnerships to expand the diversity of our talent outreach. We value our differences, recognizing that from those differences comes our strengths, and we are committed to continuing to build a culture of diversity, equity, and inclusion.
Health & Safety
We are committed to maintaining a healthy, safe, and secure work environment that protects our employees from harm. We comply with applicable health, safety, and environmental laws as well as related company policies and procedures, and provide necessary training as appropriate by role and location.
Total Rewards
We offer an attractive mix of compensation and benefit plans to support our employees and their families’ physical, mental, and financial well-being. We believe that we employ a fair and merit-based total compensation system, and we evaluate our compensation programs to ensure our employees are compensated fairly for the important work they do, and that we reward outstanding performance. We are also committed to achieving internal pay equity.
We offer our employees competitive benefits that follow local standards and support physical, mental and financial wellness. In support of employee wellness, we provide a variety of benefits options, resources, programs, online tools and regular webinars. We generally offer to full time employees globally health benefits, retirement plan, paid time off and family leave, an Employee Stock Purchase Plan (“ESPP”), and an employee assistance program that is available to employees and their families with a variety of support services.
It is important that all employees have an opportunity to have an ownership interest in our company, and there are several programs that provide the ability to own our stock. Generally, more than 75% of our employees participates in at least one of our stock programs. During their tenure with our company, all employees have an opportunity to receive an equity award, either upon hire and/or during an annual review process to recognize those with significant impact on achieving our goals. Another program offered to all employees, whether part or full time, is the ability to participate in our ESPP. Participants in the ESPP may purchase our stock at a 15% discount to market price. We believe our discounted stock purchase program helps to build an ownership mentality amongst participating employees.
Workforce Development
The growth and success of our employees is a top priority, as it impacts our overall company performance. We are investing heavily to build in-house tools and resources to support managers and employees on the road to success and ongoing growth. Our core competencies are the foundation for programs and tools being developed to identify top talent, prepare future managers and leaders, and provide equal access to growth opportunities.
We offer a variety of training opportunities, whether focused on building vocational or management and leadership skills. We use tools like Linkedin Learning to expand readiness of content and options for skill building. Despite the ongoing challenges of COVID-19, we continued to facilitate sessions around our core competencies, interview skills and coaching practices, and we rolled out a toolbox on our intranet with resources for employees and managers across the employee lifecycle.
ITEM 1A. RISK FACTORS
Investing in our common stock involves a high degree of risk. You should carefully consider the risks and uncertainties described below, together with all of the other information contained in this Annual Report on Form 10-K, including our financial statements and the related notes thereto, before making a decision to invest in our common stock. The realization of any of the following risks could materially and adversely affect our business, financial condition, operating results and prospects. In that event, the price of our common stock could decline, and you could lose part or all of your investment.
Summary of Risk Factors
Our business is subject to numerous risks and uncertainties that you should consider before investing in our company, as more fully described below. The principal factors and uncertainties that make investing in our company risky include, among others:
•changes in public health insurance coverage and CMS reimbursement rates for the Zio service could affect the adoption and profitability of our Zio service;
•if third-party commercial payors do not provide any or adequate reimbursement, rescind or modify their reimbursement policies or delay payments for our products, including as a result of the CMS and Medicare Administrative Contractor (“MAC”) reimbursement rates, including Novitas Solutions' reimbursement rates for our Zio XT service, or if we are unable to successfully negotiate reimbursement contracts, our commercial success could be compromised;
•the COVID-19 pandemic and efforts to reduce its spread have adversely impacted, and is expected to continue to materially and adversely impact, our business and operations;
•we have a history of net losses, which we expect to continue, and we may not be able to achieve or sustain profitability in the future;
•our business is dependent upon physicians adopting our Zio service and if we fail to obtain broad adoption, our business would be adversely affected;
•we plan to introduce new products and services and our business will be harmed if we are not successful in selling these new products and services to our existing customers and new customers;
•the market for ambulatory cardiac monitoring solutions is highly competitive. If our competitors are able to develop or market monitoring products and services that are more effective, or gain greater acceptance in the marketplace, than any products and services we develop, our commercial opportunities will be reduced or eliminated;
•we have recently experienced management turnover, which creates uncertainties and could harm our business;
•we may become a party to intellectual property litigation or administrative proceedings that could be costly and could interfere with our ability to provide the Zio service; and
•changes in the regulatory environment may constrain or require us to restructure our operations, which may harm our revenue and operating results.
Risks Related to Our Business
The COVID-19 pandemic and efforts to reduce its spread have adversely impacted, and is expected to continue to materially and adversely impact, our business and operations.
The COVID-19 pandemic has had, and is expected to continue to have, an adverse impact on our operations, particularly as a result of preventive and precautionary measures that we, other businesses, and governments are taking. The spread of
COVID-19 in the United States has resulted in travel restrictions impacting all areas of our business, most notably our sales representatives. Many hospitals have also limited access for non-patients, including our sales professionals, which has negatively impacted our access to physicians and their patients. New hospital sanitization and social distancing protocols, as well as increased competition for resources within hospitals that have dedicated certain resources to COVID patients, have impacted and may continue to impact our business and operations. We have also noted staffing shortage at providers in certain regions in the United States and we believe that the status of provider capacity and resource prioritization, vaccination levels and other pandemic-related effects could materially impact our revenue in future periods.
Additionally, we anticipate that an increase in the unemployment rate due to the impact of COVID-19 would decrease the number of potential patients with access to health insurance, which may result in fewer diagnostic procedures. As hospitals cancel and defer diagnostic and elective procedures, it reduces their revenue and impacts their financial results, which could result in pricing pressure on our services as they seek cost savings. Prolonged restrictions relating to COVID-19 could adversely affect our revenue, results of operations, cash flows, and financial position.
Although we saw recovery of patient registrations for the Zio service in 2021, we expect these challenges of the COVID-19 pandemic to continue to impact the volume of Zio services provided for the duration of the pandemic, but its extent cannot be quantified at this time. Our customers’ patients are also experiencing the economic impact of the current pandemic. Even an important diagnostic procedure like the Zio service may be less of a priority than other items for those patients who have lost their jobs, are furloughed, have reduced work hours or are worried about the continuation of their medical insurance. Such economic effects on patients may delay or disrupt our ability to both provision our service and collect co-payments or out-of-pocket costs, which would have an adverse effect on our revenue and cash flow. Patients may also be reluctant to visit their physicians or hospitals due to fear of contracting COVID-19. Physicians are not performing as many diagnostic tests for their patients and the facilities where these tests are performed may not be open, adequately staffed or open the entire day. Even where physicians continue to treat symptomatic patients, treatment of asymptomatic patients is being deferred in many cases. The reduction in diagnostic testing and physician visits, the increase in deferred treatment, and changes in patient behaviors are translating into fewer Zio services being prescribed.
Governmental mandates related to the COVID-19 pandemic have impacted, and are expected to continue to impact, our personnel and personnel at third-party manufacturing facilities in the United States and other countries, and the availability or cost of materials, which could disrupt our supply chain and reduce margins. For the employees who continue to support essential operations, including those at its manufacturing facilities, we have instituted protective equipment policies and, to the degree practical, social distancing measures to protect the safety of our employees. While we have continued to deliver our Zio service by operating with remote employees and essential employees on site, an extended implementation of these governmental mandates could further impact our ability to effectively provide our Zio service, and could impede progress of all ongoing initiatives. Appropriate social distancing techniques and other measures at our facilities have been implemented for the limited number of employees who have returned to work to support essential operations, and will not return until the risk to employee health has meaningfully diminished.
Our ongoing operations may be impacted as a result of employees assuming additional roles and responsibilities within our organization and we would have fewer resources available to run our operations, which could negatively impact our business operations and revenue as a result. We may also encounter voluntary departures of key employees due to any of the foregoing actions that we undertake. If key personnel or large groups of our employees contract the virus, that may also impact our business and operations. For example, an outbreak in our manufacturing facility could cause us to have to shut down our manufacturing operations, which would in turn present risk to the ongoing supply of devices required to continue our business operations. In the meantime, we have taken steps to support our employees, including providing the ability for the majority of employees to work remotely and implementing policies to enhance employee safety, including appropriate social distancing techniques, for those employees required to work in our facilities. We are also assessing our business continuity plans in the context of this pandemic.
Additionally, due to COVID-19, we have experienced delays in receiving Zio XT monitors back from patients, with some patients not returning the device at all. As a result, the increased prevalence of COVID-19 could result in negative changes to historical expectations around device return timelines and loss rates, both of which would negatively impact our finances. Finally, we anticipate that the COVID-19 pandemic may impact clinical and regulatory matters. COVID-19 is delaying enrollment in new clinical trials across the medical device industry and may affect any new trials we decide to pursue. Regulatory clearances may take extended duration of time as agencies focus on COVID-19-related priorities which, in turn, would delay our introduction of new products and services.
The COVID-19 pandemic has also created global supply chain shortages and delays, particularly for some electronic components, such as those contained in our PCBAs. We have experienced longer lead times, sometimes in excess of 52 weeks,
and increased broker fees during the COVID-19 pandemic, which has increased our cost of revenue. We have also had to increase our inventory levels as a result of these potential shortages. If we are unable to secure our typical supply of components on a timely basis, it may affect our ability to provide the Zio service on a timely basis, which would affect our cash flows and ability to operate on a timely basis.
Any of these occurrences may significantly harm our business, financial condition, cash flows and prospects. The ultimate impact of the COVID-19 pandemic is highly uncertain and subject to change. This impact is having a material, adverse impact on our liquidity, capital resources, operations and business and those of the third parties on which we rely, and could worsen over time. The extent to which the COVID-19 pandemic impacts our results will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of COVID-19 and the actions to contain COVID-19 or treat its impact, among others. We do not yet know the full extent of potential delays or impacts on our business, financial condition, cash flows and results of operations. Additionally, while the potential economic impact brought by, and the duration of, the COVID-19 pandemic is difficult to assess or predict, the COVID-19 pandemic has resulted in, and may continue to result in, significant disruption of global financial markets, reducing our ability to raise additional capital, which could negatively impact our short-term and long-term liquidity and our ability to operate on a timely basis, or at all.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. The COVID-19 pandemic has caused extreme volatility and disruptions in the global capital and credit markets. A severe or prolonged economic downturn, could result in a variety of risks to our business, including driving hospitals to tighten budgets and curtail spending, which would negatively impact our sales and business. In addition, higher unemployment or reductions in employer-provided benefits plans could result in fewer commercially insured patients, which could negatively impact our revenue and business as a result. A weak or declining economy could also strain our third party manufacturers or suppliers, possibly resulting in supply disruptions, or cause our customers to delay making payments for our Zio service. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the economic climate and financial market conditions could adversely affect our business.
We have a history of net losses, which we expect to continue, and we may not be able to achieve or sustain profitability in the future.
We have incurred net losses since our inception in September 2006. The losses and accumulated deficit were primarily due to the substantial investments we made to develop and improve our technology and products and improve our business and the Zio service through research and development efforts and infrastructure improvements. Over the next several years, we expect to continue to devote substantially all of our resources to increase adoption of and reimbursement for our Zio service, which includes Zio XT and Zio AT, and to develop additional arrhythmia detection and management products and services. These efforts may prove more expensive than we currently anticipate and we may not succeed in increasing our revenue sufficiently to offset these higher expenses or at all. Accordingly, we cannot assure you that we will achieve profitability in the future or that, if we do become profitable, we will sustain profitability. Our failure to achieve and sustain profitability in the future could cause the market price of our common stock to decline.
Our business is dependent upon physicians adopting our Zio service and if we fail to obtain broad adoption, our business would be adversely affected.
Our success will depend on our ability to bring awareness to the Zio brand and educate physicians regarding the benefits of our Zio service over existing products and services, such as Holter monitors and event monitors, and to persuade them to prescribe the Zio service as the diagnostic product for their patients. We do not know if the Zio service will be successful over the long term and market acceptance may be hindered if physicians are not presented with compelling data demonstrating the efficacy of our service compared to alternative technologies. Any studies we, or third parties which we sponsor, may conduct comparing our Zio service with alternative technologies will be expensive, time consuming and may not yield positive results. Additionally, adoption will be directly influenced by a number of financial factors, including the ability of providers to obtain sufficient reimbursement from third-party commercial payors, and the Centers for Medicare & Medicaid Services (“CMS”), for the professional services they provide in applying the Zio monitor and analyzing the Zio report. The efficacy, safety, performance and cost-effectiveness of our Zio service, on a stand-alone basis and relative to competing services, will determine the availability and level of reimbursement received by us and providers. Some payors do not have pricing contracts with us setting forth the Zio service reimbursement rates for us and providers. Physicians may be reluctant to prescribe the Zio service to patients covered by such non-contracted insurance policies because of the uncertainty surrounding reimbursement rates and
the administrative burden of interfacing with patients to answer their questions and support their efforts to obtain adequate reimbursement for the Zio service. If physicians do not adopt and prescribe our Zio service, our revenue will not increase and our financial condition will suffer as a result.
If we are unable to keep up with demand for the Zio service, our revenue could be impaired, market acceptance for the Zio service could be harmed and physicians may instead prescribe our competitors’ products and services.
As demand for the Zio service increases, we may encounter production or service delays or shortfalls. Such production or service delays or shortfalls may be caused by many factors, including the following:
•while we intend to continue to expand our manufacturing capacity, our production processes may have to change to accommodate this growth, potentially involving significant capital expenditures;
•key components of the Zio monitors are provided by a single supplier or limited number of suppliers, and we do not maintain large inventory levels of these components; if we experience a shortage or quality issues in any of these components, we would need to identify and qualify new supply sources, which could increase our expenses and result in manufacturing delays;
•global demand and supply factors concerning commodity components common to all electronic circuits, including Zio monitors, could result in shortages that manifest as extended lead times for circuit boards, which could limit our ability to sustain and/or grow our business;
•shelter-in-place orders in effect in California and elsewhere due to the COVID-19 outbreak;
•we may experience a delay in completing validation and verification testing for new production processes and/or equipment at our manufacturing facilities;
•we are subject to state, federal and international regulations, including the FDA’s Quality System Regulation (“QSR”), the EU’s Medical Device Directive (“MDD”) and, as of May 2021, the EU’s Medical Device Regulation (“MDR”), and the developing regulations by Medicines & Healthcare Regulatory Agency (“MHRA”) post Brexit in the United Kingdom for both the manufacture of the Zio monitor and the provision of the Zio service, noncompliance with which could cause an interruption in our manufacturing and services;
•to increase our manufacturing output significantly and scale our services, we will have to attract and retain qualified employees for our operations; and
•in response to unexpectedly rapid growth of our business, clinical operations capacity may not meet demand while new resources are being recruited and trained, which could delay the return of Zio reports to our customers.
Our inability to successfully manufacture our Zio monitors in sufficient quantities, or provide the Zio service in a timely manner, would materially harm our business.
Our manufacturing facilities and processes and those of our third-party suppliers are subject to unannounced FDA, state and Notified Body regulatory inspections for compliance with the QSR, MDD, MDR, UK MDR requirements. Developing and maintaining a compliant quality system is time consuming and investment intensive. Failure to maintain compliance with, or not fully complying with the requirements of the FDA and state regulators could result in enforcement actions against us or our third-party suppliers, which could include the issuance of warning letters, adverse publicity, seizures, prohibitions on product sales, recalls and civil and criminal penalties, any one of which could significantly impact our manufacturing supply and provision of services and impair our financial results. Failure to maintain compliance with, or not fully complying with the requirements of, MDD, MDR, and UK MDR could result in similar disruptions in these markets.
We depend on third-party vendors to manufacture some of our components, which could make us vulnerable to supply shortages and price fluctuations that could harm our business.
We rely on third-party vendors for components and sub-assemblies used in our Zio monitors. Our reliance on third-party vendors subjects us to a number of risks, including:
•inability to obtain adequate supply in a timely manner or on commercially reasonable terms;
•interruption of supply resulting from modifications to, or discontinuation of, a supplier’s operations, including those caused by pandemics such as COVID-19;
•production delays related to the evaluation and testing of products from alternative suppliers and corresponding regulatory qualifications;
•inability of the manufacturer or supplier to comply with the QSR, state regulatory authorities, and in some cases the Notified Body audits;
•miscommunication of design specifications due to errors/omissions by either the vendor or our company, resulting delayed delivery of acceptable product;
•delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s failure to consistently produce quality components;
•an outbreak of disease or similar public health threat, such as the existing threat of coronavirus, particularly as it may impact our supply chain;
•price fluctuations due to a lack of long-term supply arrangements with our suppliers for key components;
•inability to control the quality of products manufactured by third parties;
•delays in delivery by our suppliers due to changes in demand from us or their other customers; and
•delays in obtaining required materials and components that are in short supply within the time frames we require, at an affordable cost, or at all.
Any significant delay or interruption in the supply of components or sub-assemblies, or our inability to obtain substitute components, sub-assemblies or materials from alternate sources at acceptable prices and in a timely manner could impair our ability to meet the demand for our Zio service and harm our business.
Our revenue relies substantially on our Zio service, which is currently our only offering. If the Zio service or future product offerings fail to gain, or lose, market acceptance, our business will suffer.
Our current revenue is dependent on prescriptions of the Zio service, and we expect that sales of the Zio service will account for substantially all of our revenue for the foreseeable future. We are in various stages of research and development for other diagnostic solutions and new indications for our technology and the Zio service; however, there can be no assurance that we will be able to successfully develop and commercialize any new products or services. Any new products may not be accepted by physicians or may merely replace revenue generated by our Zio service and not generate additional revenue. If we have difficulty launching new products, our reputation may be harmed and our financial results adversely affected. In order to substantially increase our revenue, we will need to target physicians other than cardiologists, such as emergency room doctors, primary care physicians and other physicians with whom we have had little contact and may require a different type of selling effort. If we are unable to increase prescriptions of the Zio service, expand reimbursement for the Zio service, or successfully develop and commercialize new products and services, our revenue and our ability to achieve and sustain profitability would be impaired.
Our quarterly and annual results may fluctuate significantly and may not fully reflect the underlying performance of our business.
Our quarterly and annual results of operations, including our revenue, profitability and cash flow, may vary significantly in the future and period-to-period comparisons of our operating results may not be meaningful. Accordingly, the results of any one quarter or period should not be relied upon as an indication of future performance. Our quarterly and annual financial results may fluctuate as a result of a variety of factors, many of which are outside our control and, as a result, may not fully reflect the underlying performance of our business. Fluctuation in quarterly and annual results may decrease the value of our common stock. Factors that may cause fluctuations in our quarterly and annual results include, without limitation:
•market awareness and acceptance of the Zio service;
•our ability to get payors under contract at acceptable reimbursement rates;
•the availability of reimbursement for the Zio service at acceptable rates through government programs;
•our ability to attract new customers and improve our business with existing customers;
•results of our clinical trials and publication of studies by us, competitors or third parties;
•the timing and success of new product introductions by us or our competitors or any other change in the competitive dynamics of our industry, including consolidation among competitors, customers or strategic partners;
•the amount and timing of costs and expenses related to the maintenance and expansion of our business and operations;
•changes in our pricing policies or those of our competitors;
•general economic, industry and market conditions;
•the impact of the COVID-19 pandemic on our operations and financial results;
•the regulatory environment;
•expenses or loss of sales associated with unforeseen product quality issues;
•timing of physician prescriptions and demand for our Zio service;
•seasonality factors, such as patient and physician vacation schedules, severe weather conditions and insurance deductibles, that hamper or otherwise restrict when a patient seeking diagnostic services such as the Zio service visits the prescribing physician;
•the hiring, training and retention of key employees, including our ability to expand our sales team and clinical operations team;
•litigation or other claims against us for intellectual property infringement or otherwise;
•our ability to obtain additional financing as necessary; and
•advances and trends in new technologies and industry standards.
Because our quarterly results may fluctuate, period-to-period comparisons may not be the best indication of the underlying results of our business and should only be relied upon as one factor in determining how our business is performing.
We have noticed seasonality in the use of our Zio service which, along with other factors such as severe weather, may cause quarterly fluctuations in our revenue.
During the summer months and the holiday season, we have observed that the use of our Zio service decreases, which reduces our revenue during those periods. We believe that the decrease in demand may result from physicians or their patients taking vacations. Severe weather conditions or natural disasters also may hamper or otherwise restrict when patients seeking diagnostic services, such as the Zio service, visit prescribing physicians. Similarly, we generally experience some effects of seasonality due to the renewal of insurance deductibles at the beginning of the calendar year. These factors may cause our results of operations to vary from quarter to quarter.
Reimbursement by CMS is highly regulated and subject to change; our failure to comply with applicable regulations could result in decreased revenue and may subject us to penalties or have an adverse impact on our business.
For the year ended December 31, 2021, we received approximately 14% of our revenue from reimbursement for our Zio service by CMS. Under CMS guidelines for participation in the Medicare program CMS designates us as an independent diagnostic treatment facility (“IDTF”). CMS imposes extensive and detailed requirements on IDTFs, including but not limited to, rules that govern how we structure our relationships with physicians, how and when we submit reimbursement claims, how we operate our monitoring facilities and how and where we provide our monitoring solutions. Our failure to comply with applicable CMS rules could result in a discontinuation of our reimbursement under the CMS payment programs, our being required to return funds already paid to us, civil monetary penalties, criminal penalties and/or exclusion from the CMS programs.
Changes in public health insurance coverage and CMS reimbursement rates for the Zio service could affect the adoption and profitability of our Zio service.
Government payors may change their coverage and reimbursement policies, as well as payment amounts, in a way that would prevent or limit reimbursement for our Zio service, which would significantly harm our business. Government and other third-party payors require us to report the service for which we are seeking reimbursement by using a Current Procedural Terminology (“CPT”) code-set maintained by the American Medical Association (“AMA”). For Zio XT, we had historically utilized temporary CPT codes (or Category III CPT codes), used for newly introduced technologies specific to our category of diagnostic monitoring. The process to convert Category III CPT codes to Category I CPT codes is governed by the AMA and CMS. On October 25, 2019, the AMA’s CPT Editorial Panel established two new Category I CPT codes which are applicable to the Zio service and took effect on January 1, 2021.
In November 2021, CMS published its Calendar Year 2022 Medicare Physician Fee Schedule Final Rule (the “2022 Final Rule”). In the Final Rule, CMS did not issue national pricing and continued carrier pricing for calendar year 2022 on Category I CPT codes 93241, 93243, 93245 and 93247 for extended external ECG monitoring, the relevant codes for our Zio XT service. These codes were designated by CMS for contractor pricing in calendar 2022, which means that prices will be determined regionally by each Medicare Administrative Contractor (“MAC” or “MACs”).
Determinations of which products or services will be reimbursed under Medicare can be developed at the national level through a national coverage determination (“NCD”) by CMS, or at the local level through a local coverage determination (“LCD”), by one or more of the regional MACs who are private contractors that process and pay claims on behalf of CMS for different regions. In the absence of a specific NCD, as is the case with Zio XT historically and for Calendar Year 2022 following the Final Rule, the MAC with jurisdiction over a specific geographic region will have the discretion to make an LCD. The Company is seeking to establish LCD pricing with one or more MACs to establish pricing for 2022 and will be subject to LCD pricing until such time CMS establishes a NCD.
In January 2022, Novitas Solutions, the MAC which covers the region where our Independent Diagnostic Testing Facility (“IDTF”) in Houston, Texas is located and where almost all of our Medicare services for Zio XT are processed, updated reimbursement rates for CPT codes 93243 and 93247 for the Houston jurisdiction to $223 and $233, respectively. These updated rates are retroactive to January 1, 2022. These rates were higher than the rates posted by Novitas Solutions in 2021, but continue to be significantly below our historical Medicare rates for our Zio XT service. We are continuing to negotiate with Novitas Solutions and other MACs to establish higher pricing for the Category I CPT Codes but can offer no assurances as to the timing or outcome of those discussions.
We remain engaged with all of the MACs and are working with other industry participants to submit additional cost data on long-term continuous electrocardiogram (“ECG”) monitoring for consideration to establish appropriate local rates. We cannot provide certainty at this time on the potential outcome of the discussions with the MACs or on the timing of any action to be taken.
Determinations of which products or services will be reimbursed under Medicare can be developed at the national level through a national coverage determination (“NCD”) by CMS, or at the local level through a local coverage determination (“LCD”), by one or more of the regional MACs who are private contractors who establish and write LCD policies and process and pay claims on behalf of CMS for different regions. In the absence of a specific NCD, the MAC with jurisdiction over a specific geographic region will have the discretion to make an LCD. If Novitas Solutions were to change, limit or cease to cover the Zio service by altering the existing LCD, it would negatively impact our revenue from CMS.
Given the evolving nature of the healthcare industry and on-going healthcare cost reforms, we are and will continue to be subject to changes in the level of Medicare coverage for our products, and unfavorable coverage determinations at the national or local level could adversely affect our business and results of operations.
Further, a reduction in coverage by Medicare could cause some commercial third-party payors to implement similar reductions in their coverage or level of reimbursement of the Zio service. Given the evolving nature of the healthcare industry and on-going healthcare cost reforms, we will continue to be subject to changes in the level of Medicare coverage for its products, and unfavorable coverage determinations at the national or local level could adversely affect its results of operations. Although a large majority of commercial customers have re-contracted the Zio XT service since the establishment of the Category I codes on January 1, 2021 matching to pre-existing rates, if we are unsuccessful in improving the Medicare rates, we believe that commercial rates may begin to be more negatively impacted.
As a result of the CPT code changes that took effect January 1, 2021, the number of claims from the first half of 2021 which contained differences between the submitted price and reimbursement rate and overall denials increased significantly compared to our historical experience as a result of CPT code transition issues with the payors. We continue to work with the payors to collect on these claims and the collection cycle for these claims is significantly longer than usual and may lead to higher write-offs of doubtful accounts for those periods and negatively impact our results of operations.
If the Company is unable to achieve a level of revenues adequate to support its cost structure, or is unable to reduce its overall cost structure, this would raise substantial doubts about the Company's ability to continue as a going concern.
Controls imposed by CMS and commercial third-party payors designed to reduce costs, commonly referred to as “utilization review”, may affect our operations. Federal law contains numerous provisions designed to ensure that services rendered to CMS patients meet professionally recognized standards and are medically necessary, appropriate for the specific patient and cost-effective. These provisions include a requirement that a sampling of CMS patients must be reviewed by quality improvement organizations, which review the appropriateness of product prescriptions, the quality of care provided, and the appropriateness of reimbursement costs. Quality improvement organizations may deny payment for services or assess fines and also have the authority to recommend to the U.S. Department of Health and Human Services, that a provider which is in substantial noncompliance with the standards of the quality improvement organization be excluded from participation in the Medicare program. The Patient Protection and Affordable Care Act, as amended by the Health Care and Education
Affordability Reconciliation Act (the “Affordable Care Act”), potentially expands the use of prepayment review by Medicare contractors by eliminating statutory restrictions on their use, and, as a result, efforts to impose more stringent cost controls are expected to continue. Utilization review is also a requirement of most non-governmental managed care organizations and other third-party payors. To date these controls have not had a significant effect on our operations, but significant limits on the scope of services reimbursed and on reimbursement rates and fees could have a material, adverse effect on our business, financial position and results of operations in the future.
Each state’s Medicaid program has its own coverage determinations related to our services, and some state Medicaid programs do not provide their recipients with coverage for our Zio service. Even if our Zio service is covered by a state Medicaid program, we must be recognized as a Medicaid provider by the state in which the Medicaid recipient receiving the services resides in order for us to be reimbursed by a state’s Medicaid program. Even if we are recognized as a provider in a state, Medicare’s rate for our Zio service may be low, and the Medicaid reimbursement amounts are sometimes as low, or lower, than the Medicare reimbursement rate. In addition, and as noted above, each state’s Medicaid program has its own coverage determinations related to our Zio service, and many state Medicaid programs do not provide their recipients with coverage for our Zio service. As a result of all of these factors, our Zio service is not reimbursed or only reimbursed at a very low dollar amount by many state Medicaid programs. In some cases, a state Medicaid program’s reimbursement rate for our Zio service might be zero dollars. Additionally, certain states may require Medicaid recipients to pay for part of the Zio Service, and since the recipients of Medicaid are low income individuals, we are often unable to collect any amounts directly from individual recipients of the Zio service covered by Medicaid. Low or zero dollar Medicaid reimbursement rates for our Zio service would have an adverse effect on our business, gross margins and revenues. Most of the Zio services we provide are reimbursed through Medicare or private third party payors and not Medicaid, but if that were to change in the future, or the percentage of Zio services provided to Medicaid recipients were to increase, our gross margins would be adversely affected as a result.
Also, healthcare reform legislation or regulation may be proposed or enacted in the future that may adversely affect such policies and amounts. Changes in the healthcare industry directed at controlling healthcare costs or perceived over-utilization of ambulatory cardiac monitoring products and services could reduce the volume of Zio services prescribed by physicians. If more healthcare cost controls are broadly instituted throughout the healthcare industry, the volume of cardiac monitoring solutions prescribed could decrease, resulting in pricing pressure and declining demand for our Zio service. We cannot predict whether and to what extent existing coverage and reimbursement will continue to be available. If physicians, hospitals and clinics are unable to obtain adequate coverage and government reimbursement of the Zio service, they are significantly less likely to use the Zio service and our business and operating results would be harmed.
In addition, any changes to, or repeal of, the Affordable Care Act or its implementing regulations may have a material adverse effect on our results of operations. We cannot predict what other healthcare programs and regulations will ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the United States may have on our business.
If third-party commercial payors do not provide any or adequate reimbursement, including as a result of the CMS and MAC reimbursement rates for our Zio XT service, rescind or modify their reimbursement policies or delay payments for our products, including the Zio service, or if we are unable to successfully negotiate reimbursement contracts, our commercial success could be compromised.
We receive a substantial portion of our revenue from third-party private commercial payors, such as medical insurance companies. These commercial payors may reimburse our products, including the Zio service, at inadequate rates, suspend or discontinue reimbursement at any time, impose requirements that may result in a greater number of denied claims, or require or increase co-payments from patients. The recent actions taken by CMS and MACs to reduce the reimbursement rates for use of the Zio XT service by Medicare patients could influence the price that commercial payors are willing to pay for our Zio service. Contracts with commercial payors, which set forth the Zio XT service reimbursement rates for us and prescribing physicians, could be terminated or payors could seek to renegotiate such contracts at any time to try to obtain pricing at reduced amounts at or near the Novitas Solutions reimbursement rates. Some payors do not have contracts with us and others that are already in the process of negotiating contracts may look to negotiate lower reimbursement rates for the Zio XT service in response to actions taken by Novitas Solutions. Any such actions could have a significant and adverse effect on our revenue and the revenue of physicians who prescribe our Zio service. Physicians may not prescribe our Zio service unless payors reimburse a substantial portion of the submitted costs, including the physician’s, hospital’s or clinic’s charges related to the application of certain products, including the Zio monitor and the interpretation of results which may inform a diagnosis. Additionally, certain payors may require that physicians prescribe another arrhythmia diagnostic monitoring option prior to prescribing the Zio service. There is significant uncertainty concerning third-party reimbursement of any new product or service until a contracted rate is established. Reimbursement by a commercial payor may depend on a number of factors, including, but not limited to, a payor’s determination that the prescribed service is:
•not experimental or investigational;
•appropriate for the specific patient;
•cost effective;
•supported by peer-reviewed publications; and
•accepted and used by physicians within their provider network.
Since each payor makes its own decision as to whether to establish a policy concerning reimbursement or enter into a contract with us to set the price of reimbursement, seeking reimbursement on a payor-by-payor basis is a time consuming and costly process to which we dedicate substantial resources. If we do not dedicate sufficient resources to establishing contracts with third-party commercial payors, or continue to validate the clinical value of Zio services through studies and physician adoption, the amount that we are reimbursed for our products may decline, our revenue may become less predictable, and we will need to expend more efforts on a claim-by-claim basis to obtain reimbursement for our products.
A substantial portion of our revenue is derived from third-party commercial payors who have pricing contracts with us, which means that the payor has agreed to a defined reimbursement rate for our services. These contracts provide a high degree of certainty to us, physicians, clinics and hospitals with respect to the rate at which our services will be reimbursed. These contracts also impose a number of obligations regarding billing and other matters, and our noncompliance with a material term of such contracts may result in termination of the contract and loss of any associated revenue. We expect to continue to dedicate resources to maintaining compliance with these contracted payors, to ensure payors acknowledge and are aware of the clinical and economic value of our services and the interest on the part of physicians, clinics and hospitals who use our services and participate in their provider networks; however, we can provide no assurance that we will retain any given contractual payor relationship. A loss of these pricing contracts can increase the uncertainty of reimbursement of claims from third-party payors.
A portion of our revenue is derived from third-party commercial payors without such contracts in place. Without a contracted rate, reimbursement claims for our products are often denied upon submission, and we or our billing partner, XIFIN, Inc. (“XIFIN”), must appeal the denial. The appeals process is time-consuming and expensive, and may not result in full or any payment. In cases where there is no contracted rate for reimbursement it may be more difficult for us to acquire new accounts with physicians, clinics and hospitals. In addition, in the absence of a contracted rate, there is typically a greater out-of-network, co-insurance or co-payment requirement which may result in payment delays or decreased likelihood of full collection. In some cases involving non-contracted insurance companies, we may not be able to collect any amount or only a portion of the invoiced amount for our services.
We expect to continue to dedicate resources to establishing pricing contracts with non-contracted insurance companies; however, we can provide no assurance that we will be successful in obtaining such pricing contracts or that such pricing contracts will contain reimbursement for our services at rates that are favorable to us. If we fail to establish these contracts, we
will be able to recognize revenue only based on an estimated average collection rate per historical cash collections. In addition, XIFIN may need to expend significant resources obtaining reimbursement on a claim-by-claim basis and in adjudicating claims which are denied altogether or not reimbursed at acceptable rates. We currently pay XIFIN a percentage of the amounts it collects on our behalf and this percentage may increase in the future if it needs to expend more resources in adjudicating such claims. We sometimes informally engage physicians, hospitals and clinics to help establish contracts with third-party payors who insure their patients. We cannot provide any assurance that such physicians, hospitals and clinics will continue to help us establish contracts in the future. If we fail to establish contracts with more third-party payors it may adversely affect our ability to increase our revenue. In addition, a failure to enter into contracts could affect a physician’s willingness to prescribe our services because of the administrative work involved in interacting with patients to answer their questions and help them obtain reimbursement for our services. If physicians are unwilling to prescribe our services due to the lack of certainty and administrative work involved with patients covered by non-contracted insurance companies, or patients covered by non-contracted insurance companies are unwilling to risk that their insurance may charge additional out-of-pocket fees, our revenue could decline or fail to increase.
Our continued rapid growth could strain our personnel resources and infrastructure, and if we are unable to manage the anticipated growth of our business, our future revenue and operating results may be harmed.
We have experienced rapid growth in our headcount and in our operations. Any growth that we experience in the future will provide challenges to our organization, requiring us to expand our sales personnel, manufacturing, clinical, customer care and billing operations and general and administrative infrastructure. In addition to the need to scale our operational and service capacity, future growth will impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. Rapid expansion in personnel could mean that less experienced people manufacture our Zio monitors, market, sell and support our Zio service, and analyze the data to produce Zio reports, which could result in inefficiencies and unanticipated costs, reduced quality in either our Zio reports or manufactured devices, and disruptions to our service operations. Additionally, rapid expansion could require us to rely on overtime to increase capacity that could, in turn, result in greater employee attrition and a loss in productivity during the process of recruiting and training additional resources and add to our operating expenses.
As we seek to gain greater efficiency, we may expand the automated portion of our Zio service and require productivity improvements from our certified cardiographic technicians. Such improvements could compromise the quality of our Zio reports. In addition, rapid and significant growth may strain our administrative and operational infrastructure. Our ability to manage our business and growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our business could be harmed.
If we are unable to support demand for the Zio service or any of our future products or services, our business could suffer.
As demand for the Zio service or any of our future products or services increases, we will need to continue to scale our manufacturing capacity and algorithm processing technology, expand customer service, billing and systems processes and enhance our internal quality assurance program. We will also need additional certified cardiographic technicians and other personnel to process higher volumes of data. We cannot assure you that, with any increases in scale, required improvements will be successfully implemented, quality assurance will be maintained, or that appropriate personnel will be available to facilitate growth of our business. Failure to implement necessary procedures, transition to new processes or hire the necessary personnel could result in higher costs of processing data or inability to meet increased demand. There can be no assurance that we will be able to perform our data analysis on a timely basis at a level consistent with demand, quality standards and physician expectations. If we encounter difficulty meeting market demand, quality standards or physician expectations, our reputation could be harmed and our future prospects and business could suffer.
We plan to introduce new products and services and our business will be harmed if we are not successful in selling these new products and services to our existing customers and new customers
We have received FDA clearance for our Zio AT ECG Monitoring System, (“Zio AT”), which is designed to provide timely transmission of data during the wear period. However, we do not yet know whether Zio AT or any other new products and services will be well received and broadly adopted by physicians and their patients or whether sales will be sufficient for us to offset the costs of development, implementation, support, operation, sales and marketing. Although we have performed extensive testing of our new products and services, their broad-based implementation may require more support than we anticipate, which would further increase our expenses. Additionally, new products and services may subject us to additional risks of product performance, customer complaints and litigation. If sales of our new products and services are lower than we
expect, or if we expend additional resources to fix unforeseen problems and develop modifications, our operating margins are likely to decrease.
We rely on single suppliers for some of the materials used in our products, and if any of those suppliers are unable or unwilling to produce these materials or supply them in the quantities that we need at the quality we require, we may not be able to find replacements or transition to alternative suppliers before our business is materially impacted.
We rely on single suppliers for the supply of our adhesive sub-assembly, disposable plastic housings, instruments and other materials that we use to manufacture and label our Zio monitors. These components and materials are critical and, in some cases, there are relatively few alternative sources of supply. We have not qualified additional suppliers for some of these components and materials and we do not carry a significant inventory of these items. While we believe that alternative sources of supply may be available, we cannot be certain whether they will be available if and when we need them and that any alternative suppliers would be able to provide the quantity and quality of components and materials that we would need to manufacture our Zio monitors if our existing suppliers were unable to satisfy our supply requirements. To utilize other supply sources, we would need to identify and qualify new suppliers to our quality standards, which could result in manufacturing delays and increase our expenses. Any supply interruption, such as those that we have experienced during the COVID-19 pandemic, could limit our ability to manufacture our products and could therefore harm our business, financial condition and results of operations. If our current suppliers and any alternative suppliers do not provide us with the materials we need to manufacture our products or perform our services, if the materials do not meet our quality specifications, or if we cannot obtain acceptable substitute materials, an interruption in our Zio service could occur. Any such interruption may significantly affect our future revenue and harm our relations and reputation with physicians, hospitals, clinics and patients.
If our manufacturing facility becomes damaged or inoperable, or if we are required to vacate the facility, we may be unable to manufacture and ship our Zio monitors, or we may experience delays in production or an increase in costs which could adversely affect our results of operations.
We currently manufacture and assemble the Zio monitors in only one location. Our products are comprised of components sourced from a variety of contract manufacturers, with final assembly completed at our facility in Cypress, California. Our facility and equipment, or those of our suppliers, could be harmed or rendered inoperable by natural or man-made disasters, including fire, earthquake, terrorism, pandemic outbreaks, flooding and power outages. Any of these may render it difficult or impossible for us to manufacture new products, ship assembled products, and/or receive returned units for some period of time. If our Cypress facility is inoperable for even a short period of time, the inability to manufacture, ship and receive our Zio monitors, and the interruption in research and development of any future products, may result in harm to our reputation, increased costs, the loss of orders and lower revenue. Furthermore, it could be costly and time consuming to repair or replace our facilities and the equipment we use to perform our research and development work and manufacture our products.
If we fail to increase our sales and marketing capabilities and develop broad brand awareness in a cost effective manner, our growth will be impeded and our business may suffer.
We plan to continue to expand and optimize our sales and marketing infrastructure in order to increase our prescribing physician base and our business. Identifying and recruiting qualified personnel and training them in the application of the Zio service, on applicable federal and state laws and regulations and on our internal policies and procedures requires significant time, expense and attention. It often takes several months or more before a sales representative is fully trained and productive. Our business may be harmed if our efforts to expand and train our sales force do not generate a corresponding increase in revenue. In particular, if we are unable to hire, develop and retain talented sales personnel or if new sales personnel are unable to achieve desired productivity levels in a reasonable period of time, we may not be able to realize the expected benefits of this investment or increase our revenue.
Our ability to increase our customer base and achieve broader market acceptance of our products will depend, to a significant extent, on our ability to expand our marketing efforts. We plan to dedicate significant resources to our marketing programs. Our business may be harmed if our marketing efforts and expenditures do not generate a corresponding increase in revenue.
In addition, we believe that developing and maintaining broad awareness of our brand in a cost effective manner is critical to achieving broad acceptance of the Zio service and penetrating new accounts. Brand promotion activities may not generate patient or physician awareness or increase revenue, and even if they do, any increase in revenue may not offset the costs and expenses we incur in building our brand. If we fail to successfully promote, maintain and protect our brand, we may fail to attract or retain the physician acceptance necessary to realize a sufficient return on our brand building efforts, or to achieve the level of brand awareness that is critical for broad adoption of the Zio service.
Billing for our Zio service is complex, and we must dedicate substantial time and resources to the billing process.
Billing for IDTF services is complex, time consuming and expensive. Depending on the billing arrangement and applicable law, we bill several types of payors, including CMS, third-party commercial payors, institutions and patients, which may have different billing requirements procedures or expectations. We also must bill patient co-payments, co-insurance and deductibles. We face risk in our collection efforts, including potential write-offs of doubtful accounts and long collection cycles, which could adversely affect our business, financial condition and results of operations.
Several factors make the billing and collection process uncertain, including:
•differences between the submitted price for our Zio service and the reimbursement rates of payors;
•compliance with complex federal and state regulations related to billing CMS;
•differences in coverage among payors and the effect of patient co-payments, co-insurance and deductibles;
•differences in information and billing requirements among payors; and
•incorrect or missing patient history, indications or billing information.
As a result of the CPT code changes that took effect January 1, 2021, the number of claims from the first half of 2021 which contained differences between the submitted price and reimbursement and overall denials increased significantly compared to our historical experience as a result of CPT code transition issues with the payors. We continue to work with the payors to collect on these claims and the collection cycle for these claims is significantly longer than usual and may lead to higher write-offs of doubtful accounts for those periods and negatively impact our results of operations.
Additionally, our billing activities require us to implement compliance procedures and oversight, train and monitor our employees and undertake internal review procedures to evaluate compliance with applicable laws, regulations and internal policies. Payors also conduct audits to evaluate claims, which may add further cost and uncertainty to the billing process. These billing complexities, and the related uncertainty in obtaining payment for our Zio service, could negatively affect our revenue and cash flow, our ability to achieve profitability, and the consistency and comparability of our results of operations.
The operation of our call centers and monitoring facilities is subject to rules and regulations governing IDTFs; failure to comply with these rules could prevent us from receiving reimbursement from CMS and some commercial payors.
In order to be a participating provider in the Medicare program, and to be reimbursed by CMS under the program, we established an independent diagnostic treatment facility (or “IDTF”). An IDTF is a “provider-type” designation under Medicare, defined by CMS as an entity(ies) independent of a hospital or physician’s office in which diagnostic tests are performed by licensed or certified nonphysician personnel under appropriate physician supervision. Our IDTFs are staffed by certified cardiographic technicians, who are overseen by a medical director who reviews the accuracy of the data we curate and from which we prepare reports. The existence of an IDTF allows us to bill a government payor for the Zio service through one or more MACs, such as Novitas Solutions, Noridian Healthcare Solutions and Palmetto GBA. MACs are companies that operate on behalf of the federal government to process Medicare claims for reimbursement and allow us to obtain reimbursement for our Zio service at CMS defined rates. Certification as an IDTF requires that we follow strict regulations governing how the center operates, such as requirements regarding the experience and certifications of the certified cardiographic technicians. In addition, many commercial payors require our IDTFs to maintain accreditation and certification with the Joint Commission of American Hospitals. To do so we must demonstrate a specified quality standard and are subject to routine inspection and audits. These rules and regulations vary from location to location and are subject to change. If they change, we may have to change the operating procedures at our IDTFs, which could increase our costs significantly. If we fail to obtain and maintain IDTF certification, our Zio service may no longer be reimbursed by CMS and some commercial payors, which would have a material adverse impact on our business.
During the year ended December 31, 2021, we recognized approximately eight percent of our revenue from non-contracted third-party payors, and as a result, our quarterly operating results are difficult to predict.
We have limited visibility as to when we will receive payment for our Zio service with non-contracted payors and we or XIFIN must appeal any negative payment decisions, which often delay collections further. Additionally, a portion of the revenue from non-contracted payors is received from patient co-pays, which we may not receive for several months following delivery of service or at all. For revenue related to non-contracted payors, we estimate an average collection rate based on factors including historical cash collections. Subsequent adjustments, if applicable, are recorded as an adjustment to revenue. Fluctuations in revenue may make it difficult for us, research analysts and investors to accurately forecast our revenue and operating results or
to assess our actual performance. If our revenue or operating results fall below expectations, the price of our common stock would likely decline.
We rely on a third-party billing company, XIFIN, to transmit and pursue claims with payors. A delay in transmitting or pursuing claims could have an adverse effect on our revenue.
While we manage the overall processing of claims, we rely on XIFIN to transmit substantially all of our claims to payors, and pursue most claim denials. If claims for our Zio service are not submitted to payors on a timely basis, not properly adjudicated upon a denial, or if we are required to switch to a different claims processor, we may experience delays in our ability to process receipt of payments from payors, which would have an adverse effect on our revenue and our business.
The market for ambulatory cardiac monitoring solutions is highly competitive. If our competitors are able to develop or market monitoring products and services that are more effective, or gain greater acceptance in the marketplace, than any products and services we develop, our commercial opportunities will be reduced or eliminated.
The market for ambulatory cardiac monitoring products and services is evolving rapidly and becoming increasingly competitive. Our Zio service competes with a variety of products and services that provide alternatives for ambulatory cardiac monitoring, including Holter monitors and mobile cardiac telemetry monitors. Our industry is highly fragmented and characterized by a small number of large manufacturers and a large number of smaller regional service providers. These third parties compete with us in marketing to payors and prescribing physicians, recruiting and retaining qualified personnel, acquiring technology and developing products and services that compete with the Zio service. Our ability to compete effectively depends on our ability to distinguish our company and the Zio service from our competitors and their products, and includes such factors as:
•safety and efficacy;
•acute and long term outcomes;
•ease of use;
•price;
•physician, hospital and clinic acceptance; and
•third-party reimbursement.
Our industry is subject to rapid change and significantly affected by new product introductions, results of clinical research, corporate combinations and other factors. Large competitors in the ambulatory cardiac market include companies that sell standard Holter monitors including GE Healthcare, Philips Healthcare, Mortara Instrument, Inc., Spacelabs Healthcare Inc. and Welch Allyn Holdings, Inc. (acquired by Hill-Rom Holdings, Inc.). Additional competitors, such as BioTelemetry, Inc. (acquired by Royal Philips), Preventice Solutions, Inc. (acquired by Boston Scientific, Inc.) and Bardy Diagnostics, Inc. (acquired by Hill-Rom Holdings, Inc. which was recently acquired by Baxter International, Inc.) offer ambulatory cardiac monitoring services and also function as service providers. These companies have also developed other patch-based cardiac monitors that have received FDA and foreign regulatory clearances. There are also several small start-up companies trying to compete in the patch-based cardiac monitoring space, as well as several entering the patch-based cardiac monitoring market. Large medical device companies may continue to acquire or form alliances with these smaller companies in order to diversify their product offering and participate in the digital health space.
We have seen a trend in the market for large medical device companies to acquire, invest in or form alliances with these smaller companies in order to diversify their product offerings and participate in the digital health space. Future competition could come from makers of wearable fitness products or large information technology companies focused on improving healthcare. For example, Apple Inc. has added capabilities on its watch platform to measure non-continuous ECG and to alert users to the potential presence of irregular heartbeats suggestive of asymptomatic AF. These competitors and potential competitors may introduce new products and services that more directly compete with our Zio service. Recently, there has been increased acquisition activity and consolidation in our industry. Many of our competitors and potential competitors have significantly greater financial and other resources than we do and have well-established reputations, broader product offerings, and worldwide distribution channels that are significantly larger and more effective than ours. If our competitors and potential competitors are better able to develop new ambulatory cardiac monitoring solutions than us, or develop more effective or less expensive cardiac monitoring solutions, they may render our current Zio service obsolete or non-competitive. Competitors may also be able to deploy larger or more effective sales and marketing resources than we currently have. Competition with these companies could result in price cutting, reduced profit margins and loss of market share, any of which would harm our business, financial condition and results of operations.
Our ability to compete depends on our ability to innovate successfully.
The market for medical devices, including the ambulatory cardiac monitoring segment, is competitive, dynamic, and marked by rapid and substantial technological development and product innovation. While there are barriers that would challenge new entrants or existing competitors from developing products that compete directly with ours, these barriers can be overcome. Demand for the Zio service and future related products or services could be diminished by equivalent or superior products and technologies offered by competitors. If we are unable to innovate successfully, our products and services could become obsolete and our revenue would decline as our customers purchase our competitors’ products and services.
In order to remain competitive, we must continue to develop new product offerings and enhancements to the Zio service. We can provide no assurance that we will be successful in fully recognizing the strategic value of our ECG database, expanding the indications for our Zio service, developing new products or commercializing them in ways that achieve market acceptance. In addition, if we develop new products, sales of those products may reduce revenue generated from our existing products. Maintaining adequate research and development personnel and resources to meet the demands of the market is essential. If we are unable to develop new products, applications or features or improve our algorithms due to constraints, such as insufficient cash resources, high employee turnover, inability to hire personnel with sufficient technical skills or a lack of other research and development resources, we may not be able to maintain our competitive position compared to other companies. Furthermore, many of our competitors devote a considerably greater amount of funds to their research and development programs than we do, and those that do not may be acquired by larger companies that would allocate greater resources to research and development programs. Our failure or inability to devote adequate research and development resources or compete effectively with the research and development programs of our competitors could harm our business.
We have entered into a development agreement with a third party that may not result in the development of commercially viable products or the generation of significant future revenues.
We have entered into a development agreement with Verily Life Sciences LLC (an Alphabet Company, referred to as “Verily”) to develop certain next-generation AF screening, detection, or monitoring products, which involve combining Verily and our technology platforms and capabilities (the “Development Agreement”). As part of the Development Agreement, we paid Verily an up-front fee of $5.0 million in cash, and $4.0 million in milestone payments during the year ended December 31, 2020 with an additional $4.0 million in milestone payments made in January of 2021. Through December 31, 2021, we have achieved milestones and related payment obligations totaling $11.0 million, including a $3.0 million obligation incurred during our most recent quarter. We have agreed to make additional payments up to an aggregate of $1.75 million, subject to the achievement of certain development and regulatory milestones. The success of our collaboration with Verily is highly dependent on the efforts provided to the collaboration by Verily and us and the skill sets of our respective employees. Support of these development efforts requires significant resources, including research and development, manufacturing, quality assurance, and clinical and regulatory personnel. Even if our development and clinical trial efforts succeed, the FDA may not clear the developed products or may require additional product testing and clinical trials before clearing the developed products, which would result in product launch delays and additional expense. If cleared by the FDA, the developed products may not be accepted in the marketplace, and there is no assurance that adequate reimbursement will be available, or that an alternative payment model can be developed.
After the initial term and scope of the Development Agreement, and in order to commercialize any developed products with Verily, we will need to enter into a commercialization agreement. There is no guarantee that we will be able to enter into such an agreement on commercially reasonable terms or at all. If we are unable to reach agreement with Verily on terms, the up-front fee and regulatory and development milestone payments and our internal development costs would not be recovered and the licenses to use Verily’s technology will expire.
This collaboration may not result in the development of products that achieve commercial success and could be terminated prior to developing any products. In the event of any termination or expiration of the Development Agreement, we may be required to devote additional resources to product development and we may face increased competition, including from Verily. Verily may use the experience and insights it develops in the course of the collaboration with us to initiate or accelerate their development of products that compete with our products, which may create competitive disadvantages for us. Accordingly, we cannot provide assurance that our collaboration with Verily or any other third party will result in the successful development of commercially viable products or result in significant additional future revenues for our company.
The continuing clinical acceptance of the Zio service depends upon maintaining strong working relationships with physicians.
The development, marketing, and sale of the Zio service depends upon our ability to maintain strong working relationships with physicians and other key opinion leaders. We rely on these professionals’ knowledge and experience for the development, marketing and sale of our products. Among other things, physicians assist us in clinical trials and product development matters and provide public presentations at trade conferences regarding the Zio service. If we cannot maintain our strong working relationships with these professionals and continue to receive their advice and input, the development and marketing of the Zio service could suffer, which could harm our business, financial condition and results of operations.
The medical device industry’s relationship with physicians is under increasing scrutiny by the Health and Human Services Office of the Inspector General, (“OIG”), the Department of Justice (“DOJ”), state attorneys general, and other foreign and domestic government agencies. Our failure to comply with laws, rules and regulations governing our relationships with physicians, or an investigation into our compliance by the OIG, DOJ, state attorneys general or other government agencies, could significantly harm our business.
We have a significant amount of debt, which may affect our ability to operate our business and secure additional financing in the future.
As of December 31, 2021, we had $21.4 million outstanding under our term loan provided by of our loan agreement with Silicon Valley Bank (“SVB”). We must make significant annual debt payments under the loan agreement which will divert resources from other activities. Our debt with SVB is collateralized by substantially all of our assets and contains customary financial and operating covenants limiting our ability to, among other things, dispose of assets, undergo a change in control, merge or consolidate, enter into certain transactions with affiliates, make acquisitions, incur debt, incur liens, pay dividends, repurchase stock and make investments, in each case subject to certain exceptions. The covenants in the loan agreement, as well as in any future financing agreements into which we may enter, may restrict our ability to finance our operations and engage in, expand or otherwise pursue our business activities and strategies. Our ability to comply with these covenants may be affected by events beyond our control and future breaches of any of these covenants could result in a default under the loan agreement. If not waived, future defaults could cause all of the outstanding indebtedness under the loan agreement to become immediately due and payable and terminate commitments to extend further credit. If we do not have or are unable to generate sufficient cash available to repay our debt obligations when they become due and payable, either upon maturity or in the event of a default, we may not be able to obtain additional debt or equity financing on favorable terms, if at all, which may negatively impact our ability to operate and continue our business as a going concern.
We are also exposed to the risk that our earnings and cash flows could be adversely impacted by fluctuations in interest rates. Our policy is to manage interest costs using the mix of fixed- and floating-rate debt, which we cannot guarantee will mitigate the risk of interest rate fluctuation.
We may experience inflationary pressures, caused by the COVID-19 pandemic or as a result of general macroeconomic factors, which could increase our manufacturing costs and operating expenses and have a material adverse impact on our results of operations.
We continuously monitor the effects of inflationary factors, such as increases in our cost of goods sold and selling and operating expenses, which may adversely affect our results of operations. Specifically, may experience inflationary pressure affecting the cost of the components for our Zio service and in the wages that we pay our employees due to challenging labor market conditions. Competitive and regulatory conditions may restrict our ability to fully recover these costs through price increases. As a result, it may be difficult to fully offset the impact of persistent inflation. Our inability or failure to do so could have a material adverse effect on our business, financial condition and results of operations or cause us to need to obtain additional capital in future earlier than anticipated.
We have recently experienced management turnover, which creates uncertainties and could harm our business.
We have experienced significant changes in our executive leadership in the past twenty four months. In September 2021, we announced the appointment of Quentin S. Blackford as our President and Chief Executive Officer, effective October 4, 2021. In June 2021, our former Chief Executive Officer, Michael J. Coyle, announced he was resigning and our Chief Financial Officer, Douglas J. Devine, assumed the position of Interim Chief Executive Officer. In December 2020, our prior Chief Executive Officer, Kevin M. King, announced his retirement as our Chief Executive Officer. In June 2020, our Chief Financial Officer, Matthew C. Garrett, announced that he was resigning for personal reasons and in March 2020, our Chief Operating Officer, Karim Karti, resigned.
Changes to strategic or operating goals, which can often times occur with the appointment of new executives, can create uncertainty, may negatively impact our ability to execute quickly and effectively, and may ultimately be unsuccessful. In addition, executive leadership transition periods are often difficult as the new executives gain detailed knowledge of our operations, and friction can result from changes in strategy and management style. Management turnover inherently causes some loss of institutional knowledge, which can negatively affect strategy and execution. If we do not integrate new executives successfully, we may be unable to manage and grow our business, and our financial condition and profitability may suffer as a result. In addition, to the extent we experience additional management turnover, competition for top management is high and it may take months to find a candidate that meets our requirements. If we are unable to attract and retain qualified management personnel, our business could suffer.
We depend on our senior management team and the loss of one or more key employees or an inability to attract and retain highly skilled employees could harm our business.
Our success depends largely on the continued services of key members of our executive management team and others in key management positions. For example, the services of Quentin S. Blackford, our President and Chief Executive Officer, and Douglas J. Devine, our Chief Financial Officer and Chief Operating Officer, are essential to formulating and executing on corporate strategy and to ensuring the continued operations and integrity of financial reporting within our company. In addition, the services provided by David A. Vort, our Executive Vice President of Sales, are critical to the growth that we have experienced in the sales of our Zio service. The services of Patrick Murphy, our General Counsel, are critical for managing our legal and compliance functions. The services of Mark Day, our Executive Vice President of Research and Development are critical for managing our research and development programs. Our employees may terminate their employment with us at any time. If we lose one or more key employees, we may experience difficulties in competing effectively, developing our technologies and implementing our business strategy. We do not currently maintain key person life insurance policies on these or any of our employees.
In addition, our research and development programs and clinical operations depend on our ability to attract and retain highly skilled engineers and certified cardiographic technicians. We may not be able to attract or retain qualified engineers and certified cardiographic technicians in the future due to the competition for qualified personnel. We have from time to time experienced, and we expect to continue to experience, difficulty in hiring and retaining employees with appropriate qualifications. Many of the companies with which we compete for experienced personnel have greater resources than us. If we hire employees from competitors or other companies, their former employers may attempt to assert that these employees or we have breached legal obligations, resulting in a diversion of our time and resources and, potentially, damages. In addition, job candidates and existing employees, particularly in the San Francisco Bay Area, often consider the value of the stock awards they receive in connection with their employment. If the perceived value of our stock awards declines, it may harm our ability to recruit and retain highly skilled employees. If we fail to attract new personnel or fail to retain and motivate our current personnel, our business and future growth prospects would be harmed.
International expansion of our business exposes us to market, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
Our business strategy includes international expansion. Doing business internationally involves a number of risks, including:
•multiple, conflicting and changing laws and regulations such as tax laws, privacy laws, export and import restrictions, employment laws, regulatory requirements and other governmental approvals, permits and licenses;
•obtaining and sustaining regulatory approvals, certifications, and regulatory compliance where required for the sale of our products and services in various countries;
•requirements to maintain data and the processing of that data on servers located within such countries;
•complexities associated with managing multiple payor reimbursement regimes, government payors or patient self-pay systems;
•logistics and regulations associated with shipping and returning our Zio monitors following use;
•limits on our ability to penetrate international markets if we are required to process the Zio service locally;
•financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the effect of local and regional financial pressures on demand and payment for our products and services and exposure to foreign currency exchange rate fluctuations;
•natural disasters, political and economic instability, including wars, terrorism, political unrest, outbreak of disease, boycotts, curtailment of trade and other market restrictions;
•regulatory and compliance risks that relate to maintaining accurate information and control over activities subject to regulation under the United States Foreign Corrupt Practices Act of 1977 (“FCPA”), U.K. Bribery Act of 2010 and comparable laws and regulations in other countries; and
•compliance risks associated with General Data Protection Regulation (“GDPR”) enacted to protect the privacy of all individuals in the European Union and addresses export of the data outside of the European Union.
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenue and results of operations.
Our relationships with business partners in new international markets may subject us to an increased risk of litigation.
As we expand our business internationally, if we cannot successfully manage the unique challenges presented by international markets and our relationships with new business partners within those markets, our expansion activities may be adversely affected and we may become subject to an increased risk of litigation.
We may become involved in disputes relating to our products, contracts and business relationships. Such disputes include litigation against persons whom we believe have infringed on our intellectual property, infringement litigation filed against us, litigation against a competitor or litigation filed against us by distributors or service providers resulting from a breach of contract or other claim. Any of these disputes may result in substantial costs to us, judgments, settlements and diversion of our management’s attention, which could adversely affect our business, financial condition or operating results. There is also a risk of adverse judgments, as the outcome of litigation in foreign jurisdictions can be inherently uncertain.
We could be adversely affected by violations of the FCPA, and similar worldwide anti-bribery laws which could have a material adverse effect on our business.
The FCPA and similar worldwide anti-bribery laws generally prohibit companies and their intermediaries from corruptly providing any benefits to government officials for the purpose of obtaining or retaining business. We are in the process of designing and implementing policies and procedures intended to help ensure compliance with these laws. In the future, we may operate in parts of the world that have experienced governmental corruption to some degree. We cannot assure you that our internal control policies and procedures will protect us from improper acts committed by our employees or agents. Violations of these laws, or allegations of such violations, could disrupt our business and have a material adverse effect on our business and operations.
In addition, the DOJ or other governmental agencies could impose a broad range of civil and criminal sanctions under the FCPA and other laws and regulations including, but not limited to, injunctive relief, disgorgement, fines, penalties, modifications to business practices including the termination or modification of existing business relationships, the imposition of compliance programs and the retention of a monitor to oversee compliance with the FCPA. The imposition of any of these sanctions or remedial measures could have a material adverse effect on our business and results of operations.
Our proprietary data analytics engine may not operate properly, which could damage our reputation, give rise to claims against us or divert application of our resources from other purposes, any of which could harm our business and operating results.
The ECG data that is gathered through our Zio monitors is curated by algorithms that are part of our Zio service and a Zio report is delivered to the prescribing physician for diagnosis. The continuous development, maintenance and operation of our deep-learned backend data analytics engine is expensive and complex, and may involve unforeseen difficulties including material performance problems, undetected defects or errors. We may encounter technical obstacles, and it is possible that we may discover additional problems that prevent our proprietary algorithms from operating properly. We may also attempt to develop new capabilities and incorporate new technologies, including artificial intelligence, which could impact our data analytics platform’s performance. If our data analytics platform does not function reliably or fails to meet physician or payor expectations in terms of performance, physicians may stop prescribing the Zio service and payors could attempt to cancel their contracts with us.
Any unforeseen difficulties we encounter in our existing or new software, cloud-based applications, telecommunication service providers, and analytics services, and any failure by us to identify and address them could result in loss of revenue or market share, diversion of development resources, injury to our reputation and increased service and maintenance costs. Correction of
defects or errors could prove to be impossible or impracticable. The costs incurred in correcting any defects or errors may be substantial and could adversely affect our operating results.
Provision of the Zio service is dependent upon third-party vendors who are subject to disruptions, which could directly or indirectly harm our business and operating results.
The analysis we perform to create the diagnostic report for the Zio service is dependent upon a recording made by each device, which requires the physical return of the Zio monitor to one of our clinical centers. We predominantly rely on the U.S. Postal Service (“USPS”) to perform this delivery service. Delivery of the Zio monitor to one of our clinical centers may be subject to disruption by natural disasters such as earthquake or flooding, labor disagreements or errors on behalf of USPS staff, operational and funding reductions negatively impacting USPS service capabilities, structural issues timely processing in some geographies, or other disruption to the USPS delivery infrastructure. Further, for the Zio AT monitor, we rely on the provision of cellular communication services for the timely transmission of patient information and reportable events. Once received, all data from both Zio XT and AT monitors is processed, curated and reported on through cloud-computing resources. The reliability of these communication and cloud services is also subject to natural disasters, labor disruptions, human error, and infrastructure failure.
Any of these disruptions may render it difficult or temporarily impossible for us to provide some or all of the Zio service, adversely affecting our operating results, causing significant distraction for management, and negatively impacting our business reputation.
Security breaches, loss of data and other disruptions could compromise sensitive information related to our business or patients, or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we and our third-party billing and collections provider, XIFIN, collect, process, and store sensitive data, including legally-protected personally identifiable health information about patients in the United States and in the United Kingdom. This personally identifiable information may include, among other information, names, addresses, phone numbers, email addresses, insurance account information, age, gender, and heart rate data. We also process and store, and use additional third parties to process and store, sensitive intellectual property and other proprietary business information, including that of our customers, payors and collaborative partners. Our patient information is encrypted but not de-identified. We manage and maintain our applications and data utilizing a combination of on-site systems, managed data center systems and cloud-based computing center systems. These applications and data encompass a wide variety of business critical information, including research and development information, commercial information and business and financial information.
We are highly dependent on information technology networks and systems, including the internet and services hosted by Amazon Web Services and other third party service providers, to securely process, transmit and store this critical information. Security breaches of this infrastructure, including physical or electronic break-ins, computer viruses, attacks by hackers and similar breaches, can create system disruptions, shutdowns, or unauthorized disclosure or modifications of confidential information involving patient health information to become publicly available. The secure processing, storage, maintenance and transmission of this critical information are vital to our operations and business strategy, and we devote significant resources to protecting such information, including executing Business Associates Agreements with applicable vendors. Although we take measures to protect sensitive information from unauthorized access or disclosure, cyber-attacks are becoming more sophisticated and frequent, and our information technology and infrastructure, and that of XIFIN and other third parties we utilize to process or store data, may be vulnerable to viruses and worms, phishing attacks, denial-of-service attacks, physical or electronic break-ins, attacks by hackers, breaches due to employee error, malfeasance, or misuse, or similar disruptions from unauthorized tampering. We have in the past been subject to cyber-attacks and data breaches and expect that we will be subject to additional cyber-attacks in the future and may experience future data breaches. While we have implemented data privacy and security measures that we believe are compliant with applicable privacy laws and regulations, some confidential and protected health information, is transmitted to us by third parties, who may not implement adequate security and privacy measures. Further, if third party service providers that process or store data on our behalf experience security breaches or violate applicable laws, agreements, or our policies, such events may also put our information at risk and could in turn have an adverse effect on our business.
A security breach or privacy violation that leads to disclosure or modification of, or prevents access to, patient information, including protected health information, could harm our reputation, compel us to comply with disparate state breach notification laws, require us to verify the correctness of database contents and otherwise subject us to liability under laws that protect personal data, resulting in increased costs or loss of revenue. If we are unable to prevent such security breaches or privacy violations or implement satisfactory remedial measures in a timely manner, the market perception of the effectiveness of our
security measures could be harmed, our operations could be disrupted, our brand could be adversely affected, demand for our products and services may decrease, we may be unable to provide the Zio service, we may lose sales and customers, and we may suffer loss of reputation, financial loss and other regulatory penalties because of lost or misappropriated information, including sensitive patient data. We may be required to expend significant capital and financial resources to invest in security measures, protect against such threats or to alleviate problems caused by breaches in security. In addition, these breaches and other inappropriate access can be difficult to detect, and any delay in identifying them may lead to increased harm. Although we have invested in our systems and the protection of our data to reduce the risk of an intrusion or interruption, and we monitor our systems on an ongoing basis for any current or potential threats, we can give no assurances that these measures and efforts will prevent all intrusions, interruptions, or breakdowns.
Because techniques used to obtain unauthorized access or to sabotage systems change frequently and generally are not recognized until launched, we may be unable to anticipate these techniques or to implement adequate preventive measures.
In the event that patients or physicians authorize or enable third parties to access their data on our systems, we cannot ensure the complete integrity or security of such data in our systems as we would not control that access. Third parties may also attempt to fraudulently induce our employees, or patients or physicians who use our technology, into disclosing sensitive information such as user names, passwords or other information. Third parties may also otherwise compromise our security measures in order to gain unauthorized access to the information we store. This could result in significant legal and financial exposure, a loss in confidence in the security of our service, interruptions or malfunctions in our service, and, ultimately, harm to our future business prospects and revenue.
Any such breach or interruption of our systems, or those of XIFIN or any of our third party information technology partners, could compromise our networks or data security processes and sensitive information could be inaccessible or could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such interruption in access, improper access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of patient information, such as the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), the General Data Protection Regulation, and the European Union Data Protection Directive, and regulatory penalties. Regardless of the merits of any such claim or proceeding, defending it could be costly and divert management’s attention from leading our business. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to perform our services, bill payors or patients, process claims and appeals, provide customer assistance services, conduct research and development activities, collect, process and prepare company financial information, provide information about our current and future solutions and engage in other patient and clinician education and outreach efforts. Any such breach could also result in the compromise of our trade secrets and other proprietary information, which could adversely affect our business and competitive position.
Depending on the nature of the information compromised, in the event of a data breach or other unauthorized access to or acquisition of our user data, we may also have obligations to notify users about the incident and we may need to provide some form of remedy for the individuals affected by the incident. A growing number of legislative and regulatory bodies have adopted consumer notification requirements in the event of unauthorized access to or acquisition of certain types of personal data. Such breach notification laws continue to evolve and may be inconsistent from one jurisdiction to another. Complying with these obligations could cause us to incur substantial costs and could increase negative publicity surrounding any incident that compromises user data. In addition, the interpretation and application of consumer, health-related and data protection laws, rules and regulations in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. It is possible that these laws, rules and regulations may be interpreted and applied in a manner that is inconsistent with our practices or those of our distributors and partners. If we or these third parties are found to have violated such laws, rules or regulations, it could result in government-imposed fines, orders requiring that we or these third parties change our or their practices, or criminal charges, which could adversely affect our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices, systems and compliance procedures in a manner adverse to our business. In addition, California recently enacted the California Consumer Privacy Act (“CCPA”), which became effective on January 1, 2020, and will, among other things, require new disclosures to California consumers and afford such consumers new abilities to opt out of certain sales of personal information. It remains unclear how various provisions of the CCPA will be interpreted and enforced. The effects of the CCPA are potentially significant and may require us to modify our data processing practices and policies and to incur substantial costs and expenses in an effort to comply with this legislation.
The use, misuse or off-label use of the Zio service may result in injuries that lead to product liability suits, which could be costly to our business.
The use, misuse or off-label use of the Zio service may in the future result in outcomes and complications potentially leading to product liability claims. For example, we are aware that physicians have prescribed the Zio service off-label for pediatric patients. We have also received and may in the future receive product liability or other claims with respect to the Zio service, including claims related to skin irritation and alleged burns. In addition, if the Zio monitor is defectively designed, manufactured or labeled, contains defective components or is misused, we may become subject to costly litigation initiated by physicians, or the hospitals and clinics where physicians prescribing our Zio service work, or their patients. Product liability claims are especially prevalent in the medical device industry and could harm our reputation, divert management’s attention from our core business, be expensive to defend and may result in sizable damage awards against us.
Although we maintain product liability insurance, we may not have sufficient insurance coverage for future product liability claims. We may not be able to obtain insurance in amounts or scope sufficient to provide us with adequate coverage against all potential liabilities. Any product liability claims brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing continuing coverage, harm our reputation, significantly increase our expenses, and reduce product sales. Product liability claims in excess of our insurance coverage would be paid out of cash reserves, harming our financial condition and operating results.
Our forecasts of market growth may prove to be inaccurate, and even if the markets in which we compete achieve the forecasted growth, our business may not increase at similar rates, if at all.
Growth forecasts are subject to significant uncertainty and are based on assumptions and estimates that may not prove to be accurate. Our forecasts relating to, among other things, the expected growth in the ambulatory cardiac monitoring solutions market may prove to be inaccurate.
Our growth is subject to many factors, including whether the market for first-line ambulatory cardiac monitoring solutions continues to improve, the rate of market acceptance of the Zio service as compared to the products of our competitors and our success in implementing our business strategies, each of which is subject to many risks and uncertainties. If our Zio service works as anticipated to provide a correct first-line diagnosis, it may lead to a decrease in the amount of ambulatory cardiac monitoring prescriptions each year in the United States. This outcome would result if our Zio service is proven to produce the right diagnosis the first time, thereby reducing the need for additional testing. Accordingly, our forecasts of market opportunity should not be taken as indicative of our future growth.
We may acquire other companies or technologies, or enter into joint ventures or other strategic alliances, which could divert our management’s attention, result in additional dilution to our stockholders and otherwise disrupt our operations and harm our operating results.
We may in the future seek to acquire or invest in businesses, applications or technologies that we believe could complement or expand our ambulatory cardiac monitoring solutions portfolio, enhance our technical capabilities or otherwise offer growth opportunities. The pursuit of potential acquisitions may divert the attention of management and cause us to incur various costs and expenses in identifying, investigating and pursuing suitable acquisitions, whether or not they are consummated. We may not be able to identify desirable acquisition targets or be successful in entering into an agreement with any particular target or obtain the expected benefits of any acquisition or investment. In addition, any of these transactions could be material to our financial condition and operating results and expose us to many risks, including:
•disruption in our relationships with existing strategic partners or suppliers as a result of such a transaction;
•unanticipated liabilities related to acquired companies;
•difficulties integrating acquired personnel, technologies and operations into our existing business;
•retention of key employees;
•diversion of management time and focus from operating our business to management of strategic alliances or joint ventures or acquisition integration challenges;
•increases in our expenses and reductions in our cash available for operations and other uses;
•possible write-offs or impairment charges relating to acquired businesses; and
•possible compliance, regulatory, or product issues.
To date, the growth of our operations has been largely organic, and we have limited experience in acquiring other businesses or technologies or entering into joint ventures or strategic alliances. Acquisitions, joint ventures or strategic alliances could also result in dilutive issuances of equity securities, the use of our available cash, or the incurrence of debt, which could harm our operating results. In addition, if an acquired business, joint venture or strategic alliance fails to materialize or fails to meet our expectations, our operating results, business and financial condition may suffer.
Consolidation of commercial payors could result in payors eliminating coverage or reducing reimbursement rates for our Zio service.
When payors combine their operations, the combined company may elect to reimburse our Zio service at the lowest rate paid by any of the participants in the consolidation or use its increased size to negotiate reduced rates. If one of the payors participating in the consolidation does not reimburse for the Zio service at all, the combined company may elect not to reimburse for the Zio service, which would adversely impact our operating results. While attempts by Aetna Inc. to acquire Humana Inc. and Anthem Inc. to acquire Cigna Corp. have been largely abandoned due to antitrust challenges by the DOJ, it is possible that these or other payor consolidations may occur in the future.
Our ability to utilize our net operating loss carryovers may be limited.
As of December 31, 2021, we had federal and state net operating loss carryforwards (“NOLs”) of $464.3 million and $279.1 million, respectively, which if not utilized will begin to expire in 2027 for federal purposes and have begun expiring for state purposes. We may use these NOLs to offset against taxable income for U.S. federal and state income tax purposes. However, Section 382 of the Internal Revenue Code, as amended, may limit the NOLs we may use in any year for U.S. federal income tax purposes in the event of certain changes in ownership of our company. A Section 382 “ownership change” generally occurs if one or more stockholders or groups of stockholders who own at least 5% of a company’s stock increase their ownership by more than 50 percentage points (by value) over their lowest ownership percentage within a rolling three-year period. Similar rules may apply under state tax laws. Future issuances or sales of our stock, including certain transactions involving our stock that are outside of our control, could cause an “ownership change.” If an “ownership change” has occurred in the past or occurs in the future, Section 382 would impose an annual limit on the amount of pre-ownership change NOLs and other tax attributes we can use to reduce our taxable income, potentially increasing and accelerating our liability for income taxes, and also potentially causing those tax attributes to expire unused. Any limitation on using NOLs could, depending on the extent of such limitation and the NOLs previously used, result in our retaining less cash after payment of U.S. federal and state income taxes during any year in which we have taxable income, rather than losses, than we would be entitled to retain if such NOLs were available as an offset against such income for U.S. federal and state income tax reporting purposes, which could adversely impact our operating results.
We have identified a material weakness in our internal control over financial reporting which could, if not remediated, result in material misstatements in our financial statements.
We are responsible for establishing and maintaining adequate internal control over our financial reporting, as defined in Rule 13a-15(f) under the Securities Exchange Act. As disclosed in Item 9A of this Annual Report on Form 10-K, we identified a material weakness in our internal control over financial reporting. A material weakness is defined as a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. As a result of this material weakness, we concluded that our internal control over financial reporting was not effective based on criteria set forth by the Committee of Sponsoring Organization of the Treadway Commission in Internal Control-An Integrated Framework (2013).
To implement remedial measures as disclosed in Item 9A of our Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on February 26, 2021, we committed additional resources, hired additional staff, and provided additional management oversight. If our remedial measures are insufficient to address the material weaknesses, or if additional material weaknesses or significant deficiencies in our internal control over financial reporting are discovered or occur in the future, our consolidated financial statements may contain material misstatements, and we could be required to restate our financial results. In addition, if we are unable to successfully remediate the material weakness that continues to exist and if we are unable to produce accurate and timely financial statements, our stock price may be adversely affected.
Risks Related to Our Intellectual Property
We may become a party to intellectual property litigation or administrative proceedings that could be costly and could interfere with our ability to provide the Zio service.
The medical device industry has been characterized by extensive litigation regarding patents, trademarks, trade secrets, and other intellectual property rights, and companies in the industry have used intellectual property litigation to gain a competitive advantage. It is possible that U.S. and foreign patents and pending patent applications or trademarks controlled by third parties, especially those held by our competitors, may be alleged to cover our products or services, or that we may be accused of misappropriating third parties’ trade secrets. Additionally, our products include hardware and software components that we purchase from vendors, and may include design components that are outside of our direct control. Our competitors, many of which have substantially greater resources and have made substantial investments in patent portfolios, trade secrets, trademarks, and competing technologies, may have applied for or obtained, or may in the future apply for or obtain, patents or trademarks that will prevent, limit or otherwise interfere with our ability to make, use, sell and/or export our products and services or to use product names. Moreover, in recent years, individuals and groups that are non-practicing entities, commonly referred to as “patent trolls,” have purchased patents or otherwise obtained rights to other intellectual property assets for the purpose of making claims of infringement in order to extract settlements. From time to time, we may receive threatening letters, notices or “invitations to license,” or may be the subject of claims that our products and business operations infringe or violate the intellectual property rights of others. The defense of these matters can be time-consuming, costly to defend in litigation, divert management’s attention and resources, damage our reputation and brand, and cause us to incur significant expenses or make substantial payments to satisfy judgments or settle claims. Vendors from which we purchase hardware or software may not indemnify or defend us in the event that such hardware or software is accused of infringing a third-party’s patent or trademark or of misappropriating a third-party’s trade secrets.
Further, if such patents, trademarks, or trade secrets are successfully asserted against us, this may harm our business and result in injunctions preventing us from selling our products, license fees, damages and the payment of attorney’s fees and court costs. In addition, if we are found to have willfully infringed third-party patents or trademarks or to have misappropriated trade secrets, we could be required to pay treble damages in addition to other penalties. Although patent, trademark, trade secret, and other intellectual property disputes in the medical device and services area have often been settled through licensing or similar arrangements, costs associated with such arrangements may be substantial and could include ongoing royalties. We may be unable to obtain necessary licenses on satisfactory terms, if at all. If we do not obtain necessary licenses, we may not be able to redesign our Zio monitors or our Zio service to avoid infringement and our product development efforts may be negatively affected as a result.
Similarly, interference or derivation proceedings provoked by third parties or brought by the U.S. Patent and Trademark Office (“USPTO”) may be necessary to determine priority with respect to our patents, patent applications, trademarks or trademark applications. We may also become involved in other proceedings, such as reexamination, inter partes review, derivation or opposition proceedings before the USPTO or other jurisdictional body relating to our intellectual property rights or the intellectual property rights of others. Adverse determinations in a judicial or administrative proceeding or failure to obtain necessary licenses could prevent us from manufacturing the Zio monitors and selling the Zio service or using product names, which would have a significant adverse impact on our business.
Additionally, we may need to commence proceedings against others to enforce our patents or trademarks, to protect our trade secrets or know how, or to determine the enforceability, scope and validity of the proprietary rights of others. These proceedings would result in substantial expense to us and significant diversion of effort by our technical and management personnel. We may not prevail in any lawsuits that we initiate, a scenario that could also result in the invalidation of our asserted patents, and the damages or other remedies awarded, if any, may not be commercially meaningful. We may not be able to stop a competitor from marketing and selling products that are the same or similar to our products and services or from using product or service names that are the same or similar to ours, and our business may be harmed as a result.
We use certain open source software in the infrastructure supporting the Zio service. Licensees of open source software may be required to make public and use certain source code, to license proprietary software for free or to make certain derivative works available to others. As a result, we may face claims from companies that incorporate open source software into their products or from open source licensors, claiming ownership of, or demanding release of, the source code, the open source software or derivative works that were developed using such software, or otherwise seeking to enforce the terms of the applicable open source license. These claims could result in litigation and could require us to cease offering the Zio service unless and until we can re-engineer it to avoid infringement. This re-engineering process could require significant additional research and development resources, and we may not be able to complete it successfully. While we monitor and control the use of open source software in the Zio service and in any third party software that is incorporated into the Zio service, and we try to ensure
that no open source software is used in such a way as to require us to disclose the source code underlying the Zio service, there can be no guarantee that such use could not inadvertently occur. These risks could be difficult to eliminate or manage, and, if not addressed, could harm our business, intellectual property protection, financial condition and operating results.
Intellectual property rights may not provide adequate protection, which may permit third parties to compete against us more effectively.
In order to remain competitive, we must develop and maintain protection of the proprietary aspects of our technologies. We rely on a combination of patents, copyrights, trademarks, trade secret laws and confidentiality and invention assignment agreements with employees and third parties to protect our intellectual property rights. As of December 31, 2021, we owned, or retained exclusive license to, twenty-three issued U.S. patents, the earliest of which will expire in 2028. As of December 31, 2021, we also owned, or retained an exclusive license to, eight issued patents from the Japanese Patent Office, two issued patents from the Australian Patent Office, four issued patents from the Canadian Patent Office, five issued patents from the European Patent Office, three issued patents from the Korean Patent Office, and one issued patent from the Chinese Patent Office. The earliest expiration date of these international patents is 2027. As of December 31, 2021, we had twenty-six pending patent applications globally, including twelve in the United States, three in the European Patent Office, four in Japan, three Patent Cooperation Treaty (“PCT”) international applications, and one in each of Australia, Korea, China and India. Our patents and patent applications are directed to covering key aspects of the design, manufacture and use of the Zio monitor and the Zio service.
We rely, in part, on our ability to obtain and maintain patent protection for our proprietary products and processes. The process of applying for and obtaining a patent is expensive, time-consuming and complex, and we may not be able to file, prosecute, maintain, enforce or license all necessary or desirable patent applications at a reasonable cost, in a timely manner, or in all jurisdictions where protection may be commercially advantageous, or we may not be able to protect our proprietary rights at all. Despite our efforts to protect our proprietary rights, unauthorized parties may be able to obtain and use information that we regard as proprietary. In addition, the issuance of a patent does not ensure that it is valid or enforceable, so even if we obtain patents, they may not be valid or enforceable against third parties. Our patent applications may not result in issued patents and our patents may not be sufficiently broad to protect our technology. Issued international patents may carry a requirement to “work” a patent in the applicable geography; failure to do so could lead to loss of the patent or the requirement to accept licensing terms, both of which would be favorable to our competitors. Furthermore, the issuance of a patent does not give us the right to practice the patented invention. Third parties may have blocking patents that could prevent us from marketing our own products and practicing our own technology. Alternatively, third parties may seek approval to market their own products similar to or otherwise competitive with our products. In these circumstances, we may need to defend and/or assert our patents, including by filing lawsuits alleging patent infringement. In any of these types of proceedings, a court or agency with jurisdiction may find our patents invalid or unenforceable; competitors may then be able to market products and use manufacturing and analytical processes that are substantially similar to ours. Even if we have valid and enforceable patents, these patents still may not provide protection against competing products or processes sufficient to achieve our business objectives. Litigation is time-consuming and expensive and would divert our resources.
If we are unable to protect the confidentiality of our trade secrets and other proprietary information, our business and competitive position may be harmed.
We rely heavily on trade secrets as well as invention assignment and confidentiality provisions that we have in contracts with our employees, consultants, collaborators and others to protect our algorithms and other aspects of our Zio service. We may not be able to prevent the unauthorized disclosure or use of our technical knowledge or other trade secrets by consultants, vendors or former or current employees, despite the existence generally of these confidentiality agreements and other contractual restrictions. These agreements may not provide meaningful protection for our trade secrets, know-how, or other proprietary information in the event of any unauthorized use, misappropriation, or disclosure of such trade secrets, know-how, or other proprietary information. There can be no assurance that employees, consultants, vendors and clients have executed such agreements or have not breached or will not breach their agreements with us, that we will have adequate remedies for any breach, or that our trade secrets will not otherwise become known or independently developed by competitors. Despite the protections we do place on our intellectual property, monitoring unauthorized use and disclosure of our intellectual property is difficult, and we do not know whether the steps we have taken to protect our intellectual property will be adequate. In addition, the laws of many foreign countries will not protect our intellectual property rights to the same extent as the laws of the United States. Consequently, we may be unable to prevent our proprietary technology from being exploited abroad, which could affect our ability to expand to international markets or require costly efforts to protect our technology.
We may also employ individuals who were previously or are concurrently employed at research institutions or other medical device companies, including our competitors or potential competitors. We may be subject to claims that these employees, or
we, have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former or concurrent employers, or that patents and applications we have filed to protect inventions of these employees, even those related to one or more of our products, are rightfully owned by their former or concurrent employer. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
To the extent our intellectual property protection is incomplete, we are exposed to a greater risk of direct competition. A third party could, without authorization, copy or otherwise obtain and use our products or technology, or develop similar technology. Our competitors could purchase our products and attempt to replicate some or all of the competitive advantages we derive from our development efforts or design around our protected technology. Our failure to secure, protect and enforce our intellectual property rights could substantially harm the value of our Zio service, brand and business. The theft or unauthorized use or publication of our trade secrets and other confidential business information could reduce the differentiation of our products and harm our business, the value of our investment in development or business acquisitions could be reduced and third parties might make claims against us related to losses of their confidential or proprietary information. Any of the foregoing could materially and adversely affect our business.
Further, it is possible that others will independently develop the same or similar technology or otherwise obtain access to our unpatented technology, and in such cases we could not assert any trade secret rights against such parties. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our trade secret rights and related confidentiality and nondisclosure provisions. If we fail to obtain or maintain trade secret protection, or if our competitors obtain our trade secrets or independently develop technology similar to ours or competing technologies, our competitive market position could be materially and adversely affected. In addition, some courts inside and outside the United States are less willing or unwilling to protect trade secrets, and agreement terms that address non-competition are difficult to enforce in many jurisdictions, and might not be enforceable in certain cases.
If our trademarks and tradenames are not adequately protected, then we may not be able to build name recognition in our markets and our business may be adversely affected.
We rely on trademarks, service marks, trade names and brand names, such as our registered trademark “ZIO,” to distinguish our products from the products of our competitors, and have registered or applied to register these trademarks. We cannot assure you that our trademark applications will be approved. During trademark registration proceedings, we may receive rejections. Although we are given an opportunity to respond to those rejections, we may be unable to overcome such rejections. In addition, in proceedings before the USPTO and in proceedings before comparable agencies in many foreign jurisdictions, third parties are given an opportunity to oppose pending trademark applications and to seek to cancel registered trademarks. Opposition or cancellation proceedings may be filed against our trademarks, and our trademarks may not survive such proceedings. In the event that our trademarks are successfully challenged, we could be forced to rebrand our products, which could result in loss of brand recognition and could require us to devote resources towards advertising and marketing new brands. Further, we cannot assure you that competitors will not infringe our trademarks or that we will have adequate resources to enforce our trademarks. Additionally, we are aware of at least one third party that has registered the “IRHYTHM” mark in the European Union in connection with computer software for controlling and managing patient medical information, heart rate monitors, and heart rate monitors to be worn during moderate exercise, among other uses. We and the third party are involved in adversary proceedings before the Trademark Office in the European Union, and those proceedings could impact our ability to obtain a European Union trade mark registration for the “IRHYTHM” mark, although we already own many national registrations for IRHYTHM in Europe.
Changes in patent law could diminish the value of patents in general, thereby impairing our ability to protect our existing and future products.
Recent patent reform legislation could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of issued patents. In 2011, the Leahy-Smith America Invents Act (“Leahy-Smith Act”) was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications are prosecuted and also may affect patent litigation. These also include provisions that switched the United States from a “first-to-invent” system to a “first-to-file” system, allow third-party submission of prior art to the USPTO during patent prosecution and set forth additional procedures to attack the validity of a patent by the USPTO, administered post grant proceedings. Under a first-to-file system, assuming the other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to the patent on an invention regardless of whether another inventor had made the invention earlier. Under the new post grant provisions of the Leahy-Smith Act, the USPTO introduced procedures that provide additional administrative pathways for third parties to challenge issued patents. Inter partes review (“IPR”) is one of these procedures. The number of IPR challenges filed is increasing, and in many cases, the USPTO is canceling or significantly narrowing issued patent claims. Accordingly, even if a patent is granted by the USPTO, there is risk that it may not withstand an IPR challenge. Accordingly, it is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. The Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents, all of which could have a material adverse effect on our business, financial condition, results of operations and prospects.
In addition, patent reform legislation may pass in the future that could lead to additional uncertainties and increased costs surrounding the prosecution, enforcement and defense of our patents and applications. Furthermore, the U.S. Supreme Court and the U.S. Court of Appeals for the Federal Circuit have made, and will likely continue to make, changes in how the patent laws of the United States are interpreted. Recent case law has increased uncertainty regarding the availability of patent protection for certain technologies and the costs associated with obtaining patent protection for those technologies. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. In particular, the 2014 decision by the U.S. Supreme Court in Alice Corp. v. CLS Bank International has increased the difficulty of obtaining new software patents and enforcing existing software patents. Similarly, foreign courts have made, and will likely continue to make, changes in how the patent laws in their respective jurisdictions are interpreted. We cannot predict future changes in the interpretation of patent laws or changes to patent laws that might be enacted into law by U.S. and foreign legislative bodies. Those changes may materially affect our patents or patent applications and our ability to obtain additional patent protection in the future.
Risks Related to Government Regulation
Changes in the regulatory environment may constrain or require us to restructure our operations, which may harm our revenue and operating results.
Healthcare laws and regulations change frequently and may change significantly in the future. We may not be able to adapt our operations to address every new regulation, and new regulations may adversely affect our business. We cannot assure you that a review of our business by courts or regulatory authorities would not result in a determination that adversely affects our revenue and operating results, or that the healthcare regulatory environment will not change in a way that restricts our operations. In addition, there is risk that the U.S. Congress may implement changes in laws and regulations governing healthcare service providers, including measures to control costs, or reductions in reimbursement levels, which may adversely affect our business and results of operations.
Government payors, such as CMS, as well as insurers, have increased their efforts to control the cost, utilization and delivery of healthcare services. From time to time, the U.S. Congress has considered and implemented changes in the CMS fee schedules in conjunction with budgetary legislation. Further reductions of reimbursement by CMS for services or changes in policy regarding coverage of tests or other requirements for payment, such as prior authorization or a physician or qualified practitioner’s signature on test requisitions, may be implemented from time to time. Reductions in the reimbursement rates and changes in payment policies of other third-party payors may occur as well. Similar changes in the past have resulted in reduced payments as well as added costs and have added more complex regulatory and administrative requirements. For example, on January 29, 2021, Novitas Solutions, a MAC that we, physicians and hospitals rely on to process Medicare reimbursement claims related to our Zio service recently published reimbursement rates that were considerably lower than those than expected. On January 10, 2022, Novitas Solutions published rates for 2022 that were retroactive to the January 1, 2022 and replaced the rates that it had published in 2021. These revised rates were higher that the rates posted in 2021, but continue to be significantly below the historical Medicare rates for our Zio XT service. Further changes in federal, state, local and third-party payor
regulations or policies may have a material adverse impact on our business. Actions by agencies regulating insurance or changes in other laws, regulations, or policies may also have a material adverse effect on our business.
If we fail to comply with healthcare and other governmental regulations, we could face substantial penalties and our business, results of operations and financial condition could be adversely affected.
The products and services we offer are highly regulated, and there can be no assurance that the regulatory environment in which we operate will not change significantly and adversely in the future. Our arrangements with physicians, hospitals and clinics may expose us to broadly applicable fraud and abuse and other laws and regulations that may restrict the financial arrangements and relationships through which we market, sell and distribute our products and services. Our employees, consultants, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements. Federal and state healthcare laws and regulations that may affect our ability to conduct business, include, without limitation:
•federal and state laws and regulations regarding billing and claims payment applicable to our Zio service and regulatory agencies enforcing those laws and regulations;
•the federal Anti-Kickback Statute, which prohibits, among other things, any person from knowingly and willfully offering, soliciting, receiving or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any good or service for which payment may be made under federal healthcare programs, such as the CMS programs;
•the federal False Claims Act, which prohibits, among other things, individuals or entities from knowingly presenting, or causing to be presented, false claims, or knowingly using false statements, to obtain payment from the federal government;
•federal criminal laws that prohibit executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters;
•the FCPA, the U.K. Bribery Act of 2010, and other local anti-corruption laws that apply to our international activities;
•the federal Physician Payment Sunshine Act, or Open Payments, created under the Affordable Care Act, and its implementing regulations, which requires manufacturers of drugs, medical devices, biologicals and medical supplies for which payment is available under Medicare, Medicaid, or the Children’s Health Insurance Program to report annually to the U.S. Department of Health and Human Services, information related to payments or other transfers of value made to licensed physicians and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members;
•HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act, and its implementing regulations, which impose certain requirements relating to the privacy, security and transmission of individually identifiable health information; HIPAA also created criminal liability for knowingly and willfully falsifying or concealing a material fact or making a materially false statement in connection with the delivery of or payment for healthcare benefits, items or services;
•the GDPR, which replaces the 1995 Data Protection Directive known as Directive 95/46/EC;
•the federal physician self-referral prohibition, commonly known as the Stark Law;
•the FDA’s Code of Federal Regulations, including but not limited to, 21 CFR Parts 820, 803, 806, and 801 that outlines requirements for medical device design, manufacturing, labeling, distribution, and post-market surveillance requirements;
•the European Union’s Medical Device Directives and Medical Device Regulations (“MDR”) that outline requirements for medical device CE marking; and
•state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws which may apply to items or services reimbursed by any third-party payor, including commercial insurers, and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
The Affordable Care Act was enacted in 2010. The Affordable Care Act, among other things, amends the intent requirement of the federal Anti-Kickback Statute and criminal healthcare fraud statutes. A person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, the Affordable Care Act provides that the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the False Claims Act.
Because of the breadth of these laws and the narrowness of available statutory and regulatory exemptions, it is possible that some of our activities could be subject to challenge under one or more of such laws. Any action brought against us for violations of these laws or regulations, even successfully defended, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. We may be subject to private “qui tam” actions brought by individual whistleblowers on behalf of the federal or state governments, with potential liability under the federal False Claims Act including mandatory treble damages and significant per-claim penalties, which were increased to $11,665 to $23,331 per false claim in 2020.
Although we have adopted policies and procedures designed to comply with these laws and regulations and conduct internal reviews of our compliance with these laws, our compliance is also subject to governmental review. The growth of our business and sales organization and our expansion outside of the United States may increase the potential of violating these laws or our internal policies and procedures. The risk of our being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of the federal, state and foreign laws described above or any other current or future fraud and abuse or other healthcare laws and regulations that apply to us, we may be subject to penalties, including significant criminal, civil, and administrative penalties, damages, fines, imprisonment, for individuals, exclusion from participation in government programs, such as Medicare and Medicaid, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
If we fail to obtain and maintain necessary regulatory clearances or approvals for the Zio monitors and Zio service, or if clearances or approvals for future products and indications are delayed or not issued, our commercial operations would be harmed.
The Zio monitors, including the associated software and algorithm, and the Zio service are subject to extensive regulation by the FDA in the United States, by the Competent Authorities in the European Union and the United Kingdom. Such regulations are wide ranging and govern, among other things:
•product design, development, manufacture, and release;
•laboratory, preclinical and clinical testing, labeling, packaging, storage and distribution;
•premarketing clearance or approval;
•service operations
•record keeping;
•product marketing, promotion and advertising, sales and distribution; and
•post-market surveillance, including reporting of deaths or serious injuries and certain categories of field correction and removals.
Before a new medical device or service, or a new intended use for an existing product or service, can be marketed in the United States, a company must first submit and receive either 510(k) clearance or premarketing approval from the FDA, unless an exemption applies. Either process can be expensive, lengthy and unpredictable. We may not be able to obtain the necessary clearances or approvals or may be unduly delayed in doing so, which could harm our business. Furthermore, even if we are granted regulatory clearances or approvals, they may include significant limitations on the indicated uses for the product, which may limit the market for the product. Although we have obtained 510(k) clearance to market the Zio monitors and the Zio service (Software as a Medical Device elements), our clearance can be revoked if safety, efficacy, or significant regulatory compliance problems develop.
In addition, we are required to file various reports with the FDA, and European or U.K. regulators, including reports required by the medical device reporting regulations that require that we report to the regulatory authorities if our Zio service may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or
serious injury if the malfunction were to recur. If these reports are not filed in a timely manner, regulators may impose sanctions and we may be subject to product liability or regulatory enforcement actions, all of which could harm our business.
If we initiate a correction or removal for our Zio service to reduce a risk to health posed by the Zio service, we would be required to submit a publicly available Correction and Removal report to the FDA and, in many cases, similar reports to other regulatory agencies. This report could be classified by the FDA as a device recall which could lead to increased scrutiny by the FDA, other international regulatory agencies and our customers regarding the quality and safety of our Zio service. Furthermore, the submission of these reports are public and could be used by competitors against us and cause physicians to delay or cancel prescriptions, which could harm our reputation.
If we assess a potential quality issue or complaint as not requiring either field action or notification, respectively, regulators may review documentation of that decision during a subsequent audit. If regulators disagree with our decision, or take issue with either our investigation process or the resulting documentation, regulatory agencies may impose sanctions and we may be subject to regulatory enforcement actions, including warning letters, all of which could harm our business.
The FDA and the Federal Trade Commission (“FTC”) also regulate the advertising and promotion of our products and services to ensure that the claims we make are consistent with our regulatory clearances, that there is adequate and reasonable data to substantiate the claims and that our promotional labeling and advertising is neither false nor misleading. If the FDA or FTC determines that any of our advertising or promotional claims are misleading, not substantiated or not permissible, we may be subject to enforcement actions, including warning letters, and we may be required to revise our promotional claims and make other corrections or restitutions. Similar to the FDA and FTC, the European Union under the Medical Device Regulations has similar requirements and enforcement action regarding promotional material.
The FDA and state and international authorities have broad enforcement powers. Our failure to comply with applicable regulatory requirements could result in enforcement action by any such agency, which may include any of the following sanctions:
•adverse publicity, warning letters, fines, injunctions, consent decrees and civil penalties;
•repair, replacement, refunds, recall or seizure of our products;
•operating restrictions, partial suspension or total shutdown of either production, distribution or service operation;
•denial of our requests for regulatory clearance or premarket approval of new products or services, new intended uses or modifications to existing products or services;
•withdrawal of regulatory clearance or premarket approvals that have already been granted; and
•criminal prosecution.
If any of these events were to occur, our business and financial condition could be harmed.
Material modifications to the Zio monitors, labelling of the Zio monitors, or Zio service may require new 510(k) clearances, CE Mark or other premarket approvals or may require us to recall or cease marketing our products and services until clearances are obtained.
Material modifications to the intended use or technological characteristics of the Zio monitors or Zio service will require new 510(k) clearances, premarket approvals or CE Mark certification, or require us to recall or cease marketing the modified devices until these clearances or approvals are obtained. Based on FDA published guidelines, the FDA requires device manufacturers to initially make and document a determination of whether or not a modification requires a new clearance or approval; however, the FDA can review a manufacturer’s decision. Any modification to an FDA cleared device or service that would significantly affect its safety or efficacy or that would constitute a major change in its intended use would require a new 510(k) clearance or possibly a premarket approval. We may not be able to obtain additional 510(k) clearances or premarket approvals for new products or for modifications to, or additional indications for, the Zio monitors or Zio service in a timely fashion, or at all. Delays in obtaining required future clearances would harm our ability to introduce new or enhanced products in a timely manner, which in turn would harm our future growth. We have made modifications to the Zio monitors and Zio service in the past that we believe do not require additional clearances or approvals, and we may make additional modifications in the future. If the FDA or a Notified Body disagrees and requires new clearances or approvals for any of these modifications, we may be required to recall and to stop selling or marketing the Zio monitors and Zio service as modified, which could harm our operating results and require us to redesign our products or services. In these circumstances, we may be subject to significant enforcement actions.
If we or our suppliers fail to comply with the FDA’s QSR or the European Union’s Medical Device Directive and Medical Device Regulations, or United Kingdom’s Medical Device Regulations, our manufacturing or distribution operations could be delayed or shut down and our revenue could suffer.
Our manufacturing and design processes and those of our third-party suppliers are required to comply with the FDA’s Quality System Regulation (“QSR”) and the EU’s Medical Device Directive (“MDD”), through May 2021, after which time compliance with the MDR transitional provisions will be required until full transition to MDR compliance is achieved. Additionally, the UK will require the UKCA marking per new policies released by MHRA. All of these regulations cover procedures and documentation requirements for the design, testing, production, control, quality assurance, labeling, packaging, storage, shipping, and post-market surveillance of Zio monitors and associated software. We are also subject to similar state requirements and licenses, and to ongoing ISO 13485:2016 compliance in all operations to maintain our CE Mark. In addition, we must engage in extensive recordkeeping and reporting and must make available our facilities and records for periodic unannounced inspections by governmental agencies, including the FDA, state authorities, Notified Bodies and comparable agencies in other countries. If we perform poorly in a regulatory inspection, our operations could be disrupted and our manufacturing interrupted. Failure to take adequate corrective action in response to an adverse regulatory inspection could result in, among other things, a shutdown of our manufacturing or product distribution operations, significant fines, suspension of marketing clearances and approvals, seizures or recalls of our device, operating restrictions, warning letters, and criminal prosecutions, any of which would cause our business to suffer. Furthermore, our key component suppliers may not currently be or may not continue to be in compliance with applicable regulatory requirements, which may result in manufacturing delays for our product and cause our revenue to decline.
We are registered with the FDA as a medical device specifications developer and manufacturer. The FDA has broad post-market and regulatory enforcement powers. We are subject to announced or unannounced inspections by the FDA and the Food and Drug Branch of the California Department of Public Health (“CDPH”) to determine our compliance with the QSR and other regulations at both our design and manufacturing facilities, and these inspections may include the manufacturing facilities of our suppliers. The most recent FDA inspection of our manufacturing facility occurred in October 2021 and no FDA Form 483 observations were issued. The FDA also performed a Remote Regulatory Assessment (“RRA”) of our design facilities in San Francisco, California, which concluded in May 2021. Unlike on-site inspections, the FDA does not issue formal 483 observations at the conclusion of RRAs.
We are also registered with the EU as a medical device developer, manufacturer, distributor, and service operator through the National Standard Authority of Ireland (“NSAI”) our European Notified Body. Most recently, the NSAI completed an ISO 13485 surveillance audit of our design, manufacturing and service operations in May 2021 and a renewal of our ISO 13485 certification was issued in August 2021.
We can provide no assurance that we will continue to remain in compliance with the QSR or MDD, MDR, or UK MDR. If the FDA, CDPH, NSAI, or other EU or UK Notified Bodies inspect any of our facilities and discover compliance problems, we may have to cease manufacturing and product distribution until we can take the appropriate remedial steps to correct the audit findings. Taking corrective action may be expensive, time consuming and a distraction for management and if we experience a delay at our manufacturing facility we may be unable to produce Zio monitors, which would harm our business.
Zio monitors may in the future be subject to product recalls that could harm our reputation.
The FDA and similar governmental authorities in other countries have the authority to require the recall of commercialized products in the event of material regulatory deficiencies or device defects. A government mandated or voluntary recall by us could occur as a result of component failures, manufacturing errors, design issues, labeling issues, or various regulatory compliance topics. Recalls of Zio monitors and associated software would divert managerial attention, be expensive, harm our reputation with customers and harm our financial condition and results of operations. A recall announcement would also negatively affect our stock price.
Healthcare reform measures could hinder or prevent the Zio service’s commercial success.
In the United States, there have been, and we expect there will continue to be, a number of legislative and regulatory changes to the healthcare system in ways that could harm our future revenues and profitability and the demand for the Zio service. Federal and state lawmakers regularly propose and, at times, enact legislation that would result in significant changes to the healthcare system, some of which are intended to contain or reduce the costs of medical products and services. The Affordable Care Act contains a number of provisions, including those governing enrollment in federal healthcare programs, reimbursement changes and fraud and abuse measures, all of which will impact existing government healthcare programs and will result in the development of new programs. We face uncertainties that might result from modifications or repeal of any of the provisions of
the Affordable Care Act, including as a result of current and future executive orders and legislative actions. The impact of those changes on us and potential effect on the medical device industry as a whole is currently unknown. Any changes to the Affordable Care Act are likely to have an impact on our results of operations, and may have a material adverse effect on our results of operations. We cannot predict what other health care programs and regulations will ultimately be implemented at the federal or state level or the effect of any future legislation or regulation in the United States may have on our business.
The continuing efforts of the government, insurance companies, managed care organizations and other payors of healthcare services to contain or reduce costs of healthcare may harm:
•our ability to set a price that we believe is fair for our Zio service;
•our ability to generate revenue and achieve or maintain profitability; and
•the availability of capital.
Compliance with environmental laws and regulations could be expensive, and failure to comply with these laws and regulations could subject us to significant liability.
Our research and development and manufacturing operations may involve the use of hazardous substances and are subject to a variety of federal, state, local and foreign environmental laws and regulations relating to the storage, use, discharge, disposal, remediation of, and human exposure to, hazardous substances and the sale, labeling, collection, recycling, treatment and disposal of products containing hazardous substances. Liability under environmental laws and regulations can be joint and several and without regard to fault or negligence. Compliance with environmental laws and regulations may be expensive and noncompliance could result in substantial liabilities, fines and penalties, personal injury and third-party property damage claims and substantial investigation and remediation costs. Environmental laws and regulations could become more stringent over time, imposing greater compliance costs and increasing risks and penalties associated with violations. We cannot assure you that violations of these laws and regulations will not occur in the future or have not occurred in the past as a result of human error, accidents, equipment failure or other causes. The expense associated with environmental regulation and remediation could harm our financial condition and operating results.
Exposure to United Kingdom political developments, including the outcome of its withdrawal from membership in the European Union, could be costly and difficult to comply with and could seriously harm our business.
We have based a significant portion of our non-U.S. operations in the United Kingdom. In June 2016, a referendum was held in the U.K. which resulted in a majority voting in favor of the U.K. withdrawing from the E.U. (commonly referred to as "Brexit"). Pursuant to legislation approved by the U.K. Parliament and the E.U. Parliament in January 2020, the U.K. withdrew from the E.U. with effect from 11 p.m. (GMT) on January 31, 2020 on the terms of a withdrawal agreement agreed between the U.K. and the E.U. in October 2019. On December 24, 2020, the U.K. and E.U. agreed to a trade deal (the “Trade and Cooperation Agreement”) which was ratified by the U.K. on December 30, 2020. The Trade and Cooperation Agreement is subject to formal approval by the European Parliament and the Council of the European Union before it comes into effect and has been applied provisionally since January 1, 2021. There are still a number of areas of uncertainty in connection with the future of the U.K. and its relationship with the E.U. and the application and interpretation of the Trade and Cooperation Agreement, and Brexit related matters may take several years to be clarified and resolved. For example, because a significant proportion of the regulatory framework in the U.K. is currently derived from E.U. directives and regulations, Brexit could result in material changes to the regulatory regime applicable to many of our current operations. Although the Trade and Cooperation Agreement offers U.K. and E.U. companies preferential access to each other’s markets, ensuring imported goods will be free of tariffs and quotas, economic relations between the U.K. and the E.U. will now be on more restricted terms than existed previously. Therefore, at this time, we cannot predict the impact that the Trade and Cooperation Agreement and any future agreements contemplated under the terms of the Trade and Cooperation Agreement will have on our future business efforts to commercialize our Zio service in the U.K. and E.U. Accordingly, it is possible that new terms of the Trade and Cooperation Agreement may adversely affect our operations and financial results. We are currently in the process of evaluating our own risks and uncertainties to ascertain what financial, trade, regulatory and legal implications the Trade and Cooperation Agreement could have on our operations in the U.K. and otherwise. Finally, uncertainty surrounding Brexit has contributed to recent fluctuations in the U.K. economy as a whole which could experience future disruptions. As a result, Brexit could cause financial and capital markets within and outside the U.K. or the E.U. to constrict, thereby negatively impacting our ability to finance our U. K. operations which could also have an adverse effect on our results of operations and financial condition.
Risks Related to Our Common Stock
Future sales and issuances of securities could negatively affect our stock price and dilute the ownership interest of our existing investors.
Our expected future capital requirements may depend on many factors, including expanding our customer base, the expansion of our salesforce, and the timing and extent of spending on the development of our technology to increase our product offerings. If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Additionally, new investors could gain rights, preferences and privileges senior to those of existing holders of our common stock. Any future debt financing into which we enter may impose upon us additional covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our common stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders.
Sales or issuances of a substantial amount of securities, or the perception that such sales could occur, may cause a decline in the price of our common stock. Future resales of our common stock by our existing stockholders could cause the market price of our common stock to decline. In addition, the shares of common stock subject to outstanding options and restricted stock units under our 2016 Equity Incentive Plan and our 2016 Employee Stock Purchase Plan and the shares reserved for future issuance under both such plans may become eligible for sale in the public markets in the future, subject to certain legal and control limitations.
We may sell shares or other securities in any offering at a price per share that is less than the price per share paid by existing investors, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our common stock, or securities convertible or exchangeable into common stock, in future transactions may be higher or lower than the price per share paid by existing investors.
The market price of our common stock may fluctuate substantially, and you could lose all or part of your investment.
The market price of our common stock may continue to fluctuate substantially in response to, among other things, the risk factors described in this Annual Report on Form 10-K and other factors, many of which are beyond our control, including:
•changes in analysts’ estimates, investors’ perceptions, recommendations by securities analysts or our failure to achieve analysts’ estimates;
•quarterly variations in our or our competitors’ results of operations;
•the impact or anticipated impact of the COVID-19 pandemic on us;
•periodic fluctuations in our revenue, due in part to the way in which we recognize revenue;
•the financial projections we may provide to the public, any changes in these projections or our failure to meet these projections;
•general market conditions and other factors unrelated to our operating performance or the operating performance of our competitors, including deteriorating market conditions due to investor concerns regarding inflation and potential hostilities between Russia and Ukraine;
•changes in reimbursement coverage and rates by current or potential payors;
•changes in CPT codes or the establishment of new CPT codes applicable to the Zio service;
•changes in operating performance and stock market valuations of other technology companies generally, or those in the medical device industry in particular;
•actual or anticipated changes in regulatory oversight of our products;
•the results of our clinical trials;
•the loss of key personnel, including changes in our board of directors and management;
•legislation or regulation affecting our market;
•lawsuits threatened or filed against us;
•the announcement of new products or product enhancements by us or our competitors;
•announced or completed acquisitions of businesses or technologies by us or our competitors;
•announcements related to patents issued to us or our competitors and to litigation; and
•developments in our industry.
Fluctuations in our stock price, volume of shares traded, and changes in our market valuations may make our stock less attractive to certain investors. Stockholders may file securities class action litigation following periods of market volatility. Securities litigation, like the current action we are subject to in the District Court for the Northern District of California, could subject us to substantial costs, divert resources and the attention of management from our business, and adversely affect our business, results of operations, financial condition, reputation and cash flows. These factors may materially and adversely affect the market price of our common stock.
If securities or industry analysts do not publish research or reports about our business, or if they issue adverse or misleading opinions regarding our stock, our stock price and trading volume could decline.
The trading market for our common stock will be influenced by the research and reports that industry or securities analysts publish about us or our business. If any of the analysts who cover us issue an adverse or misleading opinion regarding us, our business model, our intellectual property or our stock performance, or if our target studies and operating results fail to meet the expectations of analysts, our stock price would likely decline. If one or more of these analysts cease coverage of us or fail to publish reports on us regularly, we could lose visibility in the financial markets, which in turn could cause our stock price or trading volume to decline.
The requirements of being a public company may strain our resources, divert management’s attention and affect our ability to attract and retain executive management and qualified board members.
As a public company, we are subject to the reporting requirements of the Exchange Act, the Sarbanes-Oxley Act, the Dodd Frank Act, the listing requirements of The NASDAQ Stock Market and other applicable securities laws, rules and regulations. Compliance with these laws, rules and regulations will increase our legal and financial compliance costs, make some activities more difficult, time consuming or costly and increase demand on our systems and resources. The Exchange Act requires, among other things, that we file annual, quarterly and current reports with respect to our business and operating results. The Sarbanes-Oxley Act requires, among other things, that we maintain effective disclosure controls and procedures and internal control over financial reporting. In order to maintain and, if required, improve our disclosure controls and procedures and internal control over financial reporting to meet this standard, significant resources and management oversight may be required. As a result, our management and other personnel divert attention from operational and other business matters to devote substantial time to these public company requirements. In particular, we incur significant expenses and devote substantial management effort toward ensuring compliance with the requirements of Section 404. We continue to hire additional accounting and financial staff with appropriate public company experience and technical accounting knowledge. We cannot predict or estimate the amount of additional costs we will incur in order to remain compliant with our public company reporting requirements or the timing of such costs. Additional compensation costs and any future equity awards will increase our compensation expense, which will increase our general and administrative expense and could adversely affect our profitability.
In addition, changing laws, regulations and standards relating to corporate governance and public disclosure are creating uncertainty for public companies, increasing legal and financial compliance costs and making some activities more time consuming. These laws, regulations and standards are subject to varying interpretations, in many cases due to their lack of specificity and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices. We intend to invest resources to comply with evolving laws, regulations and standards, and this investment may result in increased general and administrative expenses and a diversion of management’s time and attention from revenue generating activities to compliance activities. If our efforts to comply with new laws, regulations and standards differ from the activities intended by regulatory or governing bodies due to ambiguities related to their application and practice, regulatory authorities may initiate legal proceedings against us and our business may be harmed.
As a public company, it is more expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These factors could also make it more difficult for us to attract and retain qualified executive officers and members of our board of directors, particularly to serve on our audit committee and compensation committee. In addition, compliance with applicable rules and regulations for public companies is generally more expensive than it is for private companies.
As a result of disclosure of information in this filing and in other filings required of a public company, our business and financial condition is more visible, which could be advantageous to our competitors and other third parties and could result in threatened or actual litigation. If such claims are successful, our business and operating results could be harmed, and even if the claims are resolved in our favor, these claims, and the time and resources necessary to resolve them, could divert the resources of our management and harm our business and operating results.
Anti-takeover provisions in our amended and restated certificate of incorporation and bylaws, and Delaware law, could discourage a change in control of our company or a change in our management.
Our amended and restated certificate of incorporation and bylaws contain provisions that might enable our management to resist a takeover. These provisions include:
•a classified board of directors;
•advance notice requirements applicable to stockholders for matters to be brought before a meeting of stockholders and requirements as to the form and content of a stockholders’ notice;
•a supermajority stockholder vote requirement for amending certain provisions of our amended and restated certificate of incorporation and bylaws;
•the right to issue preferred stock without stockholder approval, which could be used to dilute the stock ownership of a potential hostile acquirer;
•allowing stockholders to remove directors only for cause;
•a requirement that the authorized number of directors may be changed only by resolution of the board of directors;
•allowing all vacancies, including newly created directorships, to be filled by the affirmative vote of a majority of directors then in office, even if less than a quorum, except as otherwise required by law;
•a requirement that our stockholders may only take action at annual or special meetings of our stockholders and not by written consent;
•limiting the forum to Delaware for certain litigation against us; and
•limiting the persons that can call special meetings of our stockholders to our board of directors, the chairperson of our board of directors, the chief executive officer or the president (in the absence of a chief executive officer).
These provisions might discourage, delay or prevent a change in control of our company or a change in our management. The existence of these provisions could adversely affect the voting power of holders of common stock and limit the price that investors might be willing to pay in the future for shares of our common stock. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which generally prohibits a Delaware corporation from engaging in any of a broad range of business combinations with any “interested” stockholder for a period of three years following the date on which the stockholder became an “interested” stockholder.
Our amended and restated certificate of incorporation and bylaws provide that the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, another State court in Delaware or the federal district court for the District of Delaware) will be the sole and exclusive forum for substantially all disputes between us and our stockholders, which could limit our stockholders’ abilities to obtain a favorable judicial forum for disputes with us or our directors, officers or employees.
Our amended and restated certificate of incorporation and bylaws provide that, unless we consent to the selection of an alternative forum, the Court of Chancery of the State of Delaware (or, if the Court of Chancery does not have jurisdiction, another State court in Delaware or the federal district court for the District of Delaware) is the sole and exclusive forum for (i) any derivative action or proceeding brought on our behalf, (ii) any action asserting a claim of breach of fiduciary duty owed by any of our directors, officers or other employees to us or to our stockholders, (iii) any action asserting a claim against the company or any director or officer of the company arising pursuant to the Delaware General Corporation Law, our amended and restated certificate of incorporation or bylaws, (iv) any action to interpret, apply, enforce or determine the validity of our amended and restated certificate of incorporation or bylaws, or (v) any action asserting a claim against us governed by the internal affairs doctrine, in each such case subject to said Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein. This provision would not apply to suits brought to enforce a duty or liability created by the Exchange Act or any other claim for which the U.S. federal courts have exclusive jurisdiction. Our investors cannot waive compliance with the federal securities laws and the rules and regulations thereunder. Our bylaws further provide that the federal district courts of the United States will be the exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. The enforceability of similar exclusive federal forum provisions in other companies’ organizational documents has been challenged in legal proceedings, and while the Delaware Supreme Court has ruled that this type of exclusive federal forum provision is facially valid under Delaware law, there is uncertainty as to whether other courts would enforce such provisions. The choice of forum provision may limit a stockholder’s ability to bring a claim in a judicial forum that it finds favorable for disputes with us or our directors, officers or other employees, which may discourage such lawsuits
against us and our directors, officers and other employees. Alternatively, if a court were to find the choice of forum provision contained in our amended and restated certificate of incorporation or bylaws to be inapplicable or unenforceable in an action, we may incur additional costs associated with resolving such action in other jurisdictions, which could harm our business, financial condition and operating results.
We have not paid dividends in the past and do not expect to pay dividends in the future, and, as a result, any return on investment may be limited to the value of our stock.
We have never paid dividends and do not anticipate paying dividends in the foreseeable future. The payment of dividends will depend on our earnings, capital requirements, financial condition, prospects and other factors our board of directors may deem relevant. In addition, our loan agreement with Silicon Valley Bank limits our ability to, among other things, pay dividends or make other distributions or payments on account of our common stock, subject to certain exceptions. If we do not pay dividends, our stock may be less valuable because a return on your investment will only occur if our stock price appreciates and you sell our common stock thereafter.
Item 1B. Unresolved Staff Comments.
Not applicable.
Item 2. Properties.
We currently lease 117,560 square feet for our corporate headquarters located in San Francisco, California under a twelve-year lease term, which will expire on August 31, 2031. We also lease 44,616 square feet in Deerfield, Illinois, for our corporate office under a lease agreement which will expire on June 30, 2033. The Deerfield, Illinios lease commenced in January 2022.
We lease 41,500 square feet for our clinical center in Lincolnshire, Illinois under a lease agreement that expires in July 2022. We also lease 20,276 square feet in Houston, Texas for another clinical center under a lease agreement that expires in October 2027.
We lease 17,558 square feet for our manufacturing and distribution facilities in Cypress, California under an agreement that expires in September 2022. We also lease 66,983 square feet in Cypress, California for our office and warehouse facilities under a lease agreement which will expire in April 2032.
We believe that these facilities are sufficient to meet our current and anticipated future needs.
Item 3. Legal Proceedings.
From time to time, we are involved in claims and legal proceedings or investigations, that arise in the ordinary course of business. Such matters could have an adverse impact on our reputation, business and financial condition and divert the attention of our management from the operation of our business. These matters are subject to many uncertainties and outcomes that are not predictable.
On February 1, 2021, a putative class action lawsuit was filed in the United States District Court for the Northern District of California alleging that we and our former Chief Executive Officer, Kevin M. King, violated Sections 10(b) and 20(a) of the Exchange Act and SEC Rule 10b-5 promulgated thereunder. On August 2, 2021, the lead plaintiff filed an amended complaint, and filed a further amended complaint on September 24, 2021. The amended complaint names as defendants, in addition to us and Mr. King, our former Chief Executive Officer, Michael J. Coyle, and current Chief Financial Officer, Douglas J. Devine. The purported class in the amended complaint includes all persons who purchased or acquired our common stock between August 4, 2020 and July 13, 2021, and seeks unspecified damages purportedly sustained by the class. On October 27, 2021, we filed a motion to dismiss the amended complaint. The motion to dismiss was fully briefed and the Court held a hearing on the motion on February 4, 2022, after which the Court took the matter under submission. The Court has not yet issued a ruling. We believe the class action to be without merit and plan to vigorously defend ourselves.
On March 26, 2021, we received a grand jury subpoena from the U.S. Attorney’s Office for the Northern District of California requesting information related to communications with the Food and Drug Administration and our products. On September 14, 2021, we received a second subpoena requesting additional information. We are cooperating fully and are providing the requested information.
Item 4. Mine Safety Disclosures.
Not applicable.
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Holders of Common Stock
As of February 18, 2022 there were 22 holders of record of our common stock. Certain shares are held in “street” name and, accordingly, the number of beneficial owners of such shares is not known or included in the foregoing number.
Dividend Policy
We have never declared or paid cash dividends on our capital stock. We intend to retain all available funds and any future earnings, if any, to fund the development and expansion of our business and we do not anticipate paying any cash dividends in the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our board of directors.
Performance Graph
This graph is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference into any filing of iRhythm Technologies, Inc. under the Securities Act or the Exchange Act, whether made before or after the date hereof and irrespective of any general incorporation language in any such filing.
The following graph shows the total stockholder return of an investment of $100 in cash at market close on December 31, 2016, through December 31, 2021 for (i) our common stock, (ii) the NASDAQ Composite Index (U.S.) and (iii) the NASDAQ Biotechnology Index. Pursuant to applicable Securities and Exchange Commission rules, all values assume reinvestment of the full amount of all dividends, however no dividends have been declared on our common stock to date. The stockholder return shown on the graph below is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future stockholder returns.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 12/31/2016 | | 12/31/2017 | | 12/31/2018 | | 12/31/2019 | | 12/31/2020 | | 12/31/2021 |
| iRhythm Technologies, Inc. | $ | 100 | | | $ | 187 | | | $ | 232 | | | $ | 227 | | | $ | 791 | | | $ | 392 | |
| NASDAQ Composite | $ | 100 | | | $ | 128 | | | $ | 123 | | | $ | 167 | | | $ | 239 | | | $ | 291 | |
| NASDAQ Biotechnology | $ | 100 | | | $ | 121 | | | $ | 110 | | | $ | 137 | | | $ | 172 | | | $ | 171 | |
Issuer Purchases of Equity Securities
None.
Item 6. [Reserved].
Data responsive to Item 6 have not been presented in accordance with amendments to Item 301 of Regulation S-K.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
You should read the following discussion and analysis of our financial condition and results of operations together with the financial statements and related notes included elsewhere in Item 8 of Part II of this Annual Report on Form 10-K. This discussion and other parts of this Annual Report on Form 10-K contain forward-looking statements that involve risks and uncertainties, such as statements of our plans, objectives, expectations and intentions. Our actual results could differ materially from those discussed in these forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed in the section of this Annual Report on Form 10-K entitled “Risk Factors.”
Overview
The objectives of our Management’s Discussion and Analysis of Financial Condition and Results of Operations (“MD&A”) are to provide users of our consolidated financial statements with the following
•a narrative explanation of the financial statements from the perspective of management that includes our financial condition, results of operations, cash flows, liquidity and certain other factors that may affect future results; and
•a discussion on matters that are reasonably likely to have a material impact on future operations.
We are a digital healthcare company redefining the way cardiac arrhythmias are clinically diagnosed by combining our wearable biosensing technology with cloud-based data analytics and deep-learning capabilities. Our goal is to be the leading provider of ambulatory electrocardiogram (“ECG”) monitoring for patients at risk for arrhythmias. We have created a full portfolio of ambulatory cardiac monitoring services on a unique platform, called the Zio service, which combines an easy-to-wear and unobtrusive biosensor that can be worn for up to 14 consecutive days with powerful proprietary algorithms that distill data from millions of heartbeats into clinically actionable information. The Zio service consists of:
•wearable patch-based biosensors, Zio XT and Zio AT monitors, which continuously record and store ECG data from every patient heartbeat for up to 14 consecutive days; Zio AT offers the option of timely, detection based transmission of data during the prescribed wear period;
•cloud-based analysis of the recorded cardiac rhythms using our proprietary, deep-learned algorithms;
•a final quality assessment review of the data by our certified cardiographic technicians; and
•an easy-to-read Zio report, a curated summary of findings that includes high quality and clinically-actionable information which is sent directly to a patient’s physician through ZioSuite and can be integrated into a patient’s electronic health record.
We receive revenue for the Zio service primarily from third-party payors, which include commercial payors and government agencies, such as CMS, Veterans Administration, and the military. In addition, a small percentage of institutions, which are typically hospitals or private physician practices, purchase the Zio service from us directly. Our revenue in the third-party commercial payor category is primarily contracted, which means we have entered into pricing contracts with these payors. Third-party contracted payors accounted for approximately 60%, 51% and 47% of our revenue for the years ended December 31, 2021, 2020 and 2019, respectively. Approximately 14%, 27% and 27% of our total revenue for the years ended December 31, 2021, 2020 and 2019, respectively, is received from Centers for Medicare and Medicaid Services (“CMS”), which is under established reimbursement codes. Healthcare institutions, which are typically hospitals or private physician practices accounted for approximately 18%, 16% and 20% of our revenue for the years ended December 31, 2021, 2020, and 2019 respectively. Non-contracted third-party payors and self-pay accounted for 8%, 6%, and 5% of our total revenue for the years ended December 31, 2021, 2020, and 2019, respectively. We rely on a third-party billing partner, XIFIN, Inc., to submit patient claims and collect from commercial payors, certain government agencies, and patients.
Since our Zio service was cleared by the U.S. Food and Drug Administration (“FDA”), we have provided the Zio service to over four million patients and have collected over one billion hours of curated heartbeat data. We believe the Zio service is well-positioned to penetrate an already-established $1.8 billion U.S. ambulatory cardiac monitoring market by offering a user-friendly device to patients, actionable information to physicians and value to payors.
We market our ambulatory cardiac monitoring solution in the United States through a direct sales organization comprised of sales management, field billing specialists, quota-carrying sales representatives, and a customer service team. Our sales representatives focus on initial introduction into new customers, penetration across a sales region, driving adoption within existing accounts and conveying our message of clinical and economic value to service line managers and hospital
administrators and other clinical departments. In addition, we will continue exploring sales and marketing expansion opportunities in international geographies.
COVID-19 Impact
Beginning in mid-March 2020, we experienced decreasing levels in the Zio service patient registrations which impacted our revenues during the years ended December 31, 2021 and 2020. This decrease in revenue is due to a variety of challenges associated with the COVID-19 pandemic in the United States, including, among others:
•a reduction in physician prescriptions for our Zio service due to:
◦a reduction in diagnostic testing outside of those tests related to severe respiratory distress;
◦reduction in the hours of most physicians’ offices;
◦physicians and hospitals prioritizing the treatment of critically ill patients; and
◦patient reluctance to visit physicians or hospitals for fear of contracting COVID-19;
•cancellation and reduction of physician attendance at professional medical society meetings and trade shows and our decision not to attend them;
•travel restrictions and changing hospital policies that have limited access of our sales professionals to hospitals where the Zio services are prescribed and where patients have historically been enrolled;
•delays in receiving Zio XT back from patients with some patients not returning the device at all; and patients who have lost jobs, been furloughed, have reduced work hours or are worried about the continuation of medical insurance being unable to afford the Zio service.
Government mandates related to the COVID-19 pandemic have impacted, and are expected to continue to impact, our personnel and personnel at third-party manufacturing facilities in the United States and other countries, and the availability or cost of materials, which could disrupt our supply chain and reduce margins. For instance, on or about March 16, 2020, the Health Officers for the counties of San Francisco (where our headquarters are located), Santa Clara, San Mateo, Marin, Contra Costa and Alameda, where many employees are located, issued mandatory shelter-in-place orders and all employees transitioned to a remote work environment. We are also subject to orders in Southern California that temporarily shut down its manufacturing and distribution facilities in Cypress, California. For a limited number of employees who continue to support essential operations, including those at our manufacturing facilities, the we have instituted protective equipment policies and, to the degree practical, social distancing measures to protect the safety of its employees. While we have continued to deliver the Zio service by operating with remote employees and essential employees on site, an extended implementation of these government mandates could further impact the Company's ability to effectively provide its Zio service, and could impede progress of all ongoing initiatives. Appropriate social distancing techniques and other measures at our facilities have been implemented for the limited number of employees who have returned to work to support essential operations, and will not return until the risk to employee health has meaningfully diminished.
While hospital systems and healthcare facilities shift their focus and resources to treating COVID-19 patients and combating the spread of the coronavirus, we have adapted our service to meet the immediate needs of physicians, customers, and patients and significantly increased the utilization of its home enrollment service which allows patients to receive and wear the single-use Zio device without going to a healthcare facility.
Government mandates related to the COVID-19 pandemic have impacted, and are expected to continue to impact, payor processing times of our claims and appeals. This increase in response times may be due, in part, to staffing shortages at the payors.
During the second half of fiscal year 2020, we saw recovery of patient registrations for the Zio service to pre-COVID levels of the first quarter of 2020. During the six months ended June 30, 2021, we experienced an increase in the level of Zio service patient registration. This increase may be due in part to re-opening of certain regions within the United States and increases in the vaccination status of patients and providers. During the six months ended December 31, 2021, we experienced a decrease in the level of Zio service patient registrations. This decrease may be due, in part, to staffing shortages at providers in certain regions of the United States. The status of provider capacity and resource prioritization, vaccination levels and other pandemic-related effects could materially impact the volume of our patient registrations in future periods.
We are taking a variety of measures to promote the safety and security of our employees and customers. Our response to COVID-19 is focused on:
•Protecting and supporting the health and well-being of our employees, our communities and our customers by limiting the transmission of COVID-19. Following recommendations from federal and local government and healthcare agencies, we transitioned employees to a remote work environment beginning in early March 2020. For a small number of our employees who continue to support essential operations at our facilities, we have instituted social distancing and other measures to ensure the safety of our employees. We rapidly implemented business continuity protocols and have been able to transition to a remote operating environment while continuing to deliver our Zio service. We will continue to follow local and national guidelines to determine the appropriate time to resume in-office functions.
•Delivering uninterrupted patient care for both Zio XT and Zio AT and supporting efforts to monitor COVID-19 patients. While hospital systems and healthcare facilities shift their focus and resources to treating COVID-19 patients and combating the spread of COVID-19, we have adapted our service to meet the immediate needs of our physician customers and patients. Our digital service platform enables physician ordering, results reporting, data curation and patient support independent of location, across virtual or in-office care models. As an example, we have significantly increased the utilization of our “Home Enrollment” service. This service allows patients to receive and wear the single-use Zio monitor without going to a healthcare facility. Physicians can prescribe the Zio service for their patients, either in-office or through a virtual care setting, and we ship the Zio monitor directly to the patient’s residence. We pay for the costs of shipping the Zio monitor, which represents additional expense for us. We also guide patients through the patch application process and inform them of instructions for wear. Home enrollment also eliminates clinical staff exposure to patients, as well as application, cleaning or reusing traditional Holter and event monitors that may have been exposed to viruses or other pathogens.
•Adjusting our operating plan as appropriate to ensure continued financial strength. We continue to maintain a strong cash position and have taken initiatives to adjust our operating plan to ensure we maintain appropriate liquidity during these uncertain times. In addition, we raised $206.8 million in proceeds from a follow-on public offering in August 2020 to fund growth initiatives and for working capital and other general corporate purposes.
Components of Results of Operations
Revenue
The majority of our revenue is derived from provision of our Zio service to customers in the United States. We earn revenue from the provision of our Zio service primarily from contracted third-party payors, CMS and healthcare institutions. In addition, a small percentage of institutions, which are typically hospitals or private physician practices, purchase the Zio service from us directly, and a very small percentage of commercial non-contracted payors.
We recognize revenue on an accrual basis based on estimates of the amount that will ultimately be realized, which is the difference between the amount submitted for payment and the amount received. These estimates require significant judgment by management. In determining the amount to accrue for a delivered report, and Zio service provided, we consider factors such as claim payment history from both payors and patient out-of-pocket costs, payor coverage, whether there is a contract between the payor or healthcare institution and the Company, historical amount received for the service, and any current developments or changes that could impact reimbursement and healthcare institution payments.
We are subject to seasonality similar to other companies in our field, as vacations by physicians and patients tend to affect enrollment in the Zio service more during the summer months and during the end of calendar year holidays compared to other times of the year. Revenue may be impacted by the outcome of adjudications with contracted and non-contracted payors, as well as changes in the CMS reimbursement rates. Clinical capacity limitations may also restrict our ability to complete the performance obligations to achieve revenue recognition.
Cost of Revenue and Gross Margin
Cost of revenue includes direct labor, material costs, equipment and infrastructure expenses, device scrap and loss, amortization of internal-use software, allocated overhead, and shipping and handling. Direct labor includes payroll and personnel-related costs including stock-based compensation involved in manufacturing, clinical data curation, and customer
service. Material costs include both the disposable materials costs of the Zio monitors and amortization of the re-usable printed circuit board assemblies (“PCBAs”). Each Zio XT monitor includes a PCBA, and each Zio AT monitor includes a PCBA and gateway board, the cost of which is amortized over the anticipated number of uses of the board. We expect cost of revenue to increase in absolute dollars as our revenue increases due to increased direct labor, direct materials, and variable spending, partially offset by economies of scale in relation to fixed costs such as overhead, depreciation and amortization, and facilities costs.
We calculate gross margin as gross profit divided by revenue. Our gross margin has been and will continue to be affected by a variety of factors, including increased contracting with third-party payors and institutional providers. We have in the past been able to increase our pricing as third-party payors become more familiar with the benefits of the Zio service and move to contracted pricing arrangements. We expect to continue to decrease the cost of revenue per device by obtaining volume purchase discounts for our material costs, implementing scan-time algorithm and process improvements, automating manufacturing assembly and packaging, and software-driven and other workflow enhancements to reduce labor costs. These decreases may be offset by increases to materials and electronics components pricing, labor rates, shipping rates, depreciation and amortization of investments, and increases in the general level of inflation.
Gross margin for the year ended December 31, 2021 was negatively impacted due to the decrease in reimbursement rates from Novitas Solutions, increased costs associated with capacity constraints, and COVID-related labor costs, offset by volume benefits, with limited impact of higher Zio AT volumes, and improved payor adjudications.
Although a large majority of our commercial customers have renewed their contracts for the Zio XT service since the establishment of the Category I codes on January 1, 2021 matching to pre-existing rates, if we are unsuccessful in improving the Medicare rates, we believe that commercial rates may begin to be more negatively impacted, which would have a negative impact on our gross margins.
Research and Development Expenses
We expense research and development costs as they are incurred. Research and development expenses include payroll and personnel-related costs, including stock-based compensation, consulting services, clinical studies, laboratory supplies and allocated facility overhead costs. We expect our research and development costs to increase in absolute dollars as we hire additional personnel to develop new product and service offerings, product enhancements and clinical evidence.
Selling, General and Administrative Expenses
Our sales and marketing expenses consist of payroll and personnel-related costs, including stock-based compensation, sales commissions, travel expenses, consulting, public relations costs, direct marketing, tradeshow and promotional expenses and allocated facility overhead costs.
Our general and administrative expenses consist primarily of payroll and personnel-related costs for executive, finance, legal and administrative personnel, including stock-based compensation. Other significant expenses include professional fees for legal and accounting services, consulting fees, recruiting fees, bad debt expense, third-party patient claims processing fees and travel expenses.
Interest Expense
Interest expense is attributable to borrowings under our loan agreements. See Note 7. Debt, for further information on our loan agreements.
Other Income, Net
Other income, net consists primarily of interest income which consists of interest received on our cash equivalents and investments.
Results of Operations
Comparison of the Years Ended December 31, 2021, and 2020 | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | | | |
| 2021 | | 2020 | | $ Change | | % Change |
| (dollars in thousands) |
| Revenue | $ | 322,825 | | | $ | 265,166 | | | $ | 57,659 | | | 22 | % |
| Cost of revenue | 109,258 | | | 70,277 | | | 38,981 | | | 55 | % |
| Gross profit | 213,567 | | | 194,889 | | | 18,678 | | | 10 | % |
| Gross margin | 66 | % | | 73 | % | | | | |
| Operating expenses: | | | | | | | |
| Research and development | 38,671 | | | 41,329 | | | (2,658) | | | 6 | % |
| Selling, general and administrative | 274,839 | | | 197,233 | | | 77,606 | | | 39 | % |
| Total operating expenses | 313,510 | | | 238,562 | | | 74,948 | | | 31 | % |
| Loss from operations | (99,943) | | | (43,673) | | | (56,270) | | | 129 | % |
| Interest expense | (1,169) | | | (1,519) | | | 350 | | | 23 | % |
| Other income, net | 118 | | | 1,591 | | | (1,473) | | | 93 | % |
| Loss before income taxes | (100,994) | | | (43,601) | | | (57,393) | | | 132 | % |
| Income tax provision | 367 | | | 229 | | | 138 | | | 60 | % |
| Net loss | $ | (101,361) | | | $ | (43,830) | | | $ | (57,531) | | | 131 | % |
| | | | | | | |
Revenue
Revenue increased $57.7 million, or 22%, to $322.8 million during the year ended December 31, 2021 from $265.2 million during the year ended December 31, 2020. The increase in revenue was primarily attributable to the increase in volume of the Zio services as a result of increased demand from our customers, partially offset by a decrease in revenue recognized from CMS, based on the updated 2021 published rates.
Cost of Revenue and Gross Margin
Cost of revenue increased $39.0 million, or 55%, to $109.3 million during the year ended December 31, 2021 from $70.3 million during the year ended December 31, 2020. The increase in cost of revenue was primarily due to increased Zio service volume, increase in costs resulting from capacity limitations associated with clinical operations, as well as costs incurred to support a larger cost structure in 2021.
Gross margin for the year ended December 31, 2021 decreased to 66%, compared to 73% for the year ended December 31, 2020. The decrease in gross margin is primarily due to the updated reimbursement rates announced by Novitas Solutions in April 2021 with an effective date of January 1, 2021, increased costs associated with capacity constraints, with limited impact of higher Zio AT volumes and COVID-related labor costs, offset by volume benefits.
Research and Development Expenses
Research and development expenses decreased $2.7 million, or 6%, to $38.7 million during the year ended December 31, 2021 from $41.3 million during the year ended December 31, 2020. The decrease was primarily attributable to a $4.0 million decrease of Verily milestone expense offset by a $1.7 million increase in payroll and employee stock-based compensation.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased $77.6 million, or 39%, to $274.8 million during the year ended December 31, 2021 from $197.2 million during the year ended December 31, 2020. The increase was primarily attributable to a $64.6 million increase in payroll and employee stock-based compensation as a result of increased headcount and executive hires to support the growth in our operations, a $5.1 million increase in facilities and rent due to increased facility maintenance costs, and an increase of $4.5 million in professional services fees.
In February 2022, the our Board of Directors approved a decision to pursue reducing our leased space for our headquarters in San Francisco, California. We expect these decisions will result in an impairment of the right of use asset lease and we are assessing the impact to our Consolidated Financial Statements for the quarter ending March 31, 2022.
Interest Expense
Interest expense decreased $0.4 million to $1.2 million during the year ended December 31, 2021 from $1.5 million during the year ended December 31, 2020 due to principal re-payments on the SVB Loan starting in November 2020.
Other Income, Net
Other income, decreased $1.5 million to $0.1 million for the year ended December 31, 2021, compared to $1.6 million for year ended December 31, 2020. The decrease in other income is primarily due to a decrease in interest income and value added tax reclaims.
Comparison of the Year Ended December 31 2020, and 2019 | | | | | | | | | | | | | | | | | | | | | | | |
| Years Ended December 31, | | | | |
| 2020 | | 2019 | | $ Change | | % Change |
| (dollars in thousands) |
| Revenue | $ | 265,166 | | | $ | 214,552 | | | $ | 50,614 | | | 24 | % |
| Cost of revenue | 70,277 | | | 52,485 | | | 17,792 | | | 34 | % |
| Gross profit | 194,889 | | | 162,067 | | | 32,822 | | | 20 | % |
| Gross margin | 73 | % | | 76 | % | | | | |
| Operating expenses: | | | | | | | |
| Research and development | 41,329 | | | 37,299 | | | 4,030 | | | 11 | % |
| Selling, general and administrative | 197,233 | | | 179,523 | | | 17,710 | | | 10 | % |
| Total operating expenses | 238,562 | | | 216,822 | | | 21,740 | | | 10 | % |
| Loss from operations | (43,673) | | | (54,755) | | | 11,082 | | | 20 | % |
| Interest expense | (1,519) | | | (1,643) | | | 124 | | | 8 | % |
| Other income, net | 1,591 | | | 1,895 | | | (304) | | | 16 | % |
| | | | | | | |
| Loss before income taxes | (43,601) | | | (54,503) | | | 10,902 | | | 20 | % |
| Income tax provision | 229 | | | 65 | | | 164 | | | 252 | % |
| Net loss | $ | (43,830) | | | $ | (54,568) | | | $ | 10,738 | | | 20 | % |
Revenue
Revenue increased $50.6 million, or 24%, to $265.2 million during the year ended December 31, 2020 from $214.6 million during the year ended December 31, 2019. The increase in revenue was primarily attributable to the increase in volume of the Zio services as a result of increased in demand from our customers.
Cost of Revenue and Gross Margin
Cost of revenue increased $17.8 million, or 34%, to $70.3 million during the year ended December 31, 2020 from $52.5 million during the year ended December 31, 2019. The increase in cost of revenue was primarily due to increased Zio service volume and costs incurred to support a larger cost structure in 2020.
Gross margin for the year ended December 31, 2020 decreased to 73%, compared to 76% for the year ended December 31, 2019. The decrease in gross margin was primarily driven by product mix, as well as fixed costs that we continue to incur, partially offset by increased volume.
Research and Development Expenses
Research and development expenses increased $4.0 million, or 11%, to $41.3 million during the year ended December 31, 2020 from $37.3 million during the year ended December 31, 2019. The increase was primarily attributable to a $8.3
million increase in payroll and employee stock-based compensation as a result of increased headcount and an increase of $1.0 million in Verily milestone expense. This was partially offset by $4.7 million increase in capitalization of internal use software.
Selling, General and Administrative Expenses
Selling, general and administrative expenses increased $17.7 million, or 10%, to $197.2 million during the year ended December 31, 2020 from $179.5 million during the year ended December 31, 2019. The increase was primarily attributable to a $29.5 million increase in payroll and employee stock-based compensation as a result of increased headcount to support the growth in our operations, a $2.9 million increase in facilities and rent due to expansion of the San Francisco headquarters and increased facility related costs, partially offset by decrease of $8.9 million in travel, entertainment and office meals as a result of the COVID-19 pandemic and a decrease of $4.4 million in professional services fees.
A significant amount of selling, general, and administrative incremental spend can be directly attributed to our continued focus on salesforce expansion and its support infrastructure to support our growth strategy.
Interest Expense
Interest expense decreased $0.1 million to $1.5 million during the year ended December 31, 2020 from $1.6 million during the year ended December 31, 2019 due to principal payments on the SVB Loan starting in November 2020 and a decrease in the interest rate in 2020.
Other Income, Net
Other income, decreased $0.3 million to $1.6 million for the year ended December 31, 2020, compared to $1.9 million for year ended December 31, 2019. The decrease in other income is primarily due to a decrease in amortization of investments, partially offset by an increase in interest income, and prior year value added tax reclaims.
Liquidity and Capital Expenditures
Overview
We are continuously reviewing our liquidity and anticipated capital requirements in light of the significant uncertainty created by the COVID-19 global pandemic. We believe we will have adequate liquidity over the next twelve months to operate our business and to meet our cash requirements.
As of December 31, 2021, we had cash and cash equivalents of $127.6 million, short-term investments of $111.6 million, and an accumulated deficit of $406.0 million.
Our expected future capital requirements may depend on many factors including expanding our customer base, the expansion of our salesforce, and the timing and extent of spending on the development of our technology to increase our product offerings. If we raise additional funds by issuing equity securities, our stockholders may experience dilution. Any future debt financing into which we enter may impose upon us additional covenants that restrict our operations, including limitations on our ability to incur liens or additional debt, pay dividends, repurchase our common stock, make certain investments and engage in certain merger, consolidation or asset sale transactions. Any debt financing or additional equity that we raise may contain terms that are not favorable to us or our stockholders.
Cash Flows
The following table summarizes our cash flows for the periods indicated (in thousands): | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Net cash (used in) provided by: | | | | | |
| Operating activities | $ | (37,753) | | | $ | (13,759) | | | $ | (21,863) | |
| Investing activities | 105,264 | | | (132,391) | | | (89,274) | |
| Financing activities | (28,577) | | | 214,316 | | | 111,576 | |
| Net increase in cash, cash equivalents, and restricted cash | $ | 38,934 | | | $ | 68,166 | | | $ | 439 | |
Cash Used in Operating Activities
During the year ended December 31, 2021, cash used in operating activities was $37.8 million which consisted of a net loss of $101.4 million, adjusted by non-cash charges of $109.8 million and a net change of $46.2 million in our net operating assets and liabilities. The non-cash charges are primarily comprised of stock-based compensation expense of $54.5 million, as well as depreciation and amortization expense of $9.8 million and a change in allowance for doubtful accounts and contractual allowance of $37.1 million and amortization of right of use assets of $6.8 million. The change in our net operating assets and liabilities compared to December 31, 2020 was primarily due to an increase of $53.6 million in the change in accounts receivable as a result of the CPT code changes that took effect January 1, 2021. The number of claims from the first half of 2021 which contained differences between the submitted price and reimbursement and overall denials increased significantly compared to our historical experience as a result of CPT code transition issues with the payors. We continue to work with the payors to collect on these claims and the collection cycle for these claims is significantly longer than usual. While we believe we have properly estimated the impact to our contractual allowances and allowance for doubtful accounts, the inherent uncertainty caused by longer collection cycle and claims adjudication process could result in additional provisions for contractual allowances and doubtful accounts which would negatively impact our results of operations in future periods. We believe we have adequate balance sheet liquidity to manage through these delays.
Accrued liabilities increased $7.9 million primarily due to increased compensation and benefit accruals as a result of increased head count, and an increase in accounts payable of $6.1 million, primarily due to milestone achievements on our collaboration with Verily, partially offset by an increase of $5.0 million in inventory.
During the year ended December 31, 2020, cash used in operating activities was $13.8 million which consisted of a net loss of $43.8 million, adjusted by non-cash charges of $86.3 million and a net change of $56.2 million in our net operating assets and liabilities. The non-cash charges are primarily comprised of stock-based compensation expense of $41.5 million, as well as depreciation and amortization expense of $6.9 million and a change in allowance for doubtful accounts and contractual allowance of $31.4 million and amortization of right of use assets of $6.0 million. The change in our net operating assets and liabilities compared to December 31, 2019 was primarily due to an increase of $38.0 million in the change in accounts receivable as a result of the increase in our revenue, an increase of $6.1 million in other assets, primarily related to an increase in PCBA boards due to increased sales, a decrease of $4.8 million in operating lease liability and a decrease of $3.9 million in accounts payable.
During the year ended December 31, 2019 cash used in operating activities was $21.9 million, which consisted of a net loss of $54.6 million, adjusted by non-cash charges of $62.4 million and a net change of $29.7 million in our net operating assets and liabilities. The non-cash charges are primarily comprised of stock-based compensation expense of $26.2 million, as well as depreciation and amortization expense of $3.4 million and a change in allowance for doubtful accounts and contractual allowance of $24.6 million. The change in our net operating assets and liabilities compared to December 31, 2018 was primarily due to an increase of $28.7 million in the change in accounts receivable as a result of the increase in our revenue, and an increase of $6.0 million in accrued liabilities, primarily related to increased accrued payroll and related compensation accruals.
Cash From Investing Activities
Cash provided by investing activities during the year ended December 31, 2021 was $105.3 million, which consisted primarily of $122.2 million in purchases of available for sale investments partially offset by $28.1 million of capital expenditures to purchase property and equipment and $255.5 million in cash received from the maturities of available for sale investments.
Cash used in investing activities during the year ended December 31, 2020 was $132.4 million, which consisted primarily of $277.5 million in purchases of available for sale investments, $13.6 million of capital expenditures to purchase property and equipment, partially offset by $144.1 million cash received from the maturities of available for sale investments and $14.5 million in cash received from sales of available for sale investments.
Cash used in investing activities during the year ended December 31, 2019 was $89.3 million, which consisted primarily of $165.9 million in purchases of available for sale investments and $20.5 million of capital expenditures to purchase property and equipment, partially offset by $95.6 million in cash received from the maturities of available for sale investments and $1.5 million in cash received from sales of available for sale investments.
Cash From Financing Activities
During the year ended December 31, 2021, cash used in financing activities was $28.6 million, primarily due to $25.9 million in tax withholding upon the vesting of RSUs and $11.7 million in repayment of long term debt. This was partially offset by $8.9 million in proceeds from the issuance of common stock in connection with employee option exercises and our Employee Stock Purchase Plan.
During the year ended December 31, 2020, cash provided by financing activities was $214.3 million, primarily due to $206.0 million from the issuance of common stock in a public offering, net of discounts and issuance costs and $20.2 million in proceeds from the issuance of common stock in connection with employee option exercises and our Employee Stock Purchase Plan. This amount was partially offset by $10.0 million in tax withholding upon the vesting of RSUs and $1.9 million in re-payment of long term debt.
During the year ended December 31, 2019, cash provided by financing activities was $111.6 million, primarily due to $107.4 million from the issuance of common stock in a public offering, net of discounts and issuance costs and $9.5 million in proceeds from the issuance of common stock in connection with employee option exercises and our Employee Stock Purchase Plan. This was partially offset by $5.3 million in tax withholding upon the vesting of RSUs.
Indebtedness
Bank Debt
In October 2018, we entered into the Third Amended and Restated Loan and Security Agreement with SVB (“Third Amended and Restated SVB Loan Agreement”). This Agreement amends and restates the Second Amended and Restated Loan and Security Agreement between the Company and SVB dated December 4, 2015, as amended by the First Loan Modification Agreement between the Company and SVB dated August 22, 2016.
Pursuant to the Third Amended and Restated SVB Loan Agreement, we obtained a term loan (“SVB Term Loan”) for $35.0 million. Total proceeds from the SVB Term Loan were used to pay off the loan agreement with Biopharma Secured Investments III Holdings Cayman LP (“Pharmakon”), totaling $35.8 million. We made interest-only payments through October 31, 2020, followed by 36 monthly payments of principal plus interest on the SVB Term Loan. Interest charged on the SVB Term Loan will be the greater of (a) a floating rate based on the “Prime Rate” published by The Wall Street Journal minus 0.75%, or (b) 4.25%.
Under the Third Amended and Restated SVB Loan Agreement, we may borrow, repay, and reborrow under a revolving credit line, but not in excess of the maximum loan amount of $25.0 million, which includes an $11.0 million standby letter of credit sublimit availability. In October 2018, a $6.9 million standby letter of credit was obtained in connection with a lease for our San Francisco headquarters. Any principal amount outstanding under the Third Amended and Restated SVB Loan Agreement revolving credit line shall bear interest at an amount that is the greater of (a) a floating rate per annum equal to the rate published by The Wall Street Journal as the “Prime Rate” or (b) 5.00%. We may borrow up to 75% of eligible accounts receivable, up to the maximum of $25.0 million.
The Third Amended and Restated Loan Agreement requires us to maintain a minimum consolidated liquidity ratio or minimum adjusted Earnings Before Interest, Tax, Depreciation, and Amortization during the term of the loan facility. In addition, the SVB Loan Agreement contains customary affirmative and negative covenants and events of default. We were in compliance with loan covenants as of December 31, 2021. The obligations under the Third Amended and Restated Loan Agreement are collateralized by substantially all of our assets.
Critical Accounting Policies and Estimates
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with United States generally accepted accounting principles (“U.S. GAAP”). The preparation of these financial statements requires our management to make judgments and estimates that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these judgments and estimates under different assumptions or conditions and any such differences may be material. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
All policies described below have not significantly changed over the last fiscal year. In addition, the policies below are critical because they require management to make difficult, subjective and complex judgments about matters that are inherently uncertain and it is likely that materially different amounts would be reported under different conditions or using different assumptions.
Revenue Recognition
Our Zio XT monitor, a wearable patch-based biosensor, continuously records and stores ECG data from every patient heartbeat for up to 14 consecutive days. The Zio XT monitor is returned to our monitoring facility and the heartbeat data is curated and analyzed by our proprietary algorithms and reviewed by our certified cardiac technicians. The final step in the Zio service is the delivery of an electronic Zio Report to the prescribing physician with a summary of findings. Our Zio XT service is generally billable when the Zio Report is issued to the physician.
Our Zio AT mobile cardiac telemetry monitor, a wearable patch-based biosensor, offers what our Zio XT offers plus the additional capability of transmissions during the wear period to assist physicians in diagnosing and treating the small percentage of the population requiring more timely action. During the wear period, physicians will receive notifications if there are significant events that meet predetermined arrhythmia detection criteria. Our Zio AT service revenue is recognized over the prescription period and delivery of an electronic Zio Report.
We account for contract revenue with a customer when there is a legally enforceable contract between us and the customer, the rights of the parties are identified, the contract has commercial substance, and collectability of the contract consideration is probable. Our revenue is measured based on consideration specified in the contract with each customer. A unique aspect of healthcare is the involvement of multiple parties to the service transaction. In addition to the patient, often a third-party, for example a commercial or governmental payor or healthcare institution, like a hospital or clinic, will pay us for some or all of the service on the patient’s behalf. Separate contractual arrangements exist between us and many third-party payors that establish amounts the third-party payor will pay on behalf of a patient for covered services rendered and should be considered in determining collectability and the transaction price for services provided to a patient covered by that third-party payor.
We recognize revenue on an accrual basis based on estimates of the amount that will ultimately be realized, which is the difference between the amount submitted for payment and the amount received. Revenue recognition is subject to uncertainty because these estimates require significant judgment by management. In determining the amount to accrue for a delivered report, we consider factors such as claim payment history from both payors and patient out-of-pocket costs, payor coverage, whether there is a contract between the payor or healthcare institution and us, historical amount received for the service, and any current developments or changes that could impact reimbursement and healthcare institution payments.
A summary of the payment arrangements with third-party payors and healthcare institutions is as follows:
•Contracted third-party payors – We have contracts with negotiated prices for services provided for patients with commercial healthcare insurance carriers
•Centers for Medicare and Medicaid Services (“CMS”) – We have received independent diagnostic testing facility approval from regional Medicare Administrative Contractors and will receive reimbursement per the relevant Current Procedural Terminology (“CPT”) code rate for the services rendered to the patient covered by CMS.
•Non-contracted third-party payors: Non-contracted commercial and government payors often reimburse out-of-network rates provided under the relevant CPT codes on a case-by-case basis. The transaction price used for determining revenue recognition is based on factors including an average of our historical collection experience for our non-contracted services. This rate is reviewed at least quarterly.
•Healthcare institutions – Healthcare institutions are typically hospitals or physician practices in which we have negotiated amounts for its monitoring services, including certain governmental agencies such as the Veteran’s Administration and Department of Defense.
We are utilizing the portfolio approach practical expedient under ASC 606 for revenue recognition. We account for the contracts within each portfolio as a collective group, rather than individual contracts. Based on history with these portfolios and the similar nature and characteristics of the patients within each portfolio, we have concluded that the financial statement effects are not materially different than if accounting for revenue on a contract-by-contract basis.
For the healthcare institutions, we have historical experience of collecting substantially all of the negotiated contractual rates and determined at contract inception that these customers, and or their related third-party payor that pays us on their behalf, have the intention and ability to pay the promised consideration. As such, we have not provided an implicit price concession but, rather, have chosen to accept the risk of default, and adjustments to the transaction price are recorded as bad debt expense.
For contracted and CMS portfolios, we are providing an implicit price concession because, while we have a contract with the underlying payor, we expect to accept a lower amount of consideration when claims are adjudicated and allowable claims are determined by the commercial payor. The implicit price concession is recorded as variable consideration to the transaction price and recorded as an adjustment to revenue as a contractual allowance. Historical cash collection indicates that it is probable that substantially all of the contracted claim amount will be received. We provide for estimates of uncollectible patient accounts receivable, based upon historical experience, at the time revenue is recognized, with such provisions presented as bad debt expense within the selling, general and administrative line item of the consolidated statement of operations. Adjustments to these estimates for actual experience are also recorded as an adjustment to bad debt expense.
For non-contracted portfolios, we are providing an implicit price concession because we do not have a contract with the underlying payor, the result of which requires us to estimate transaction price based on historical cash collections utilizing the expected value method. Subsequent adjustments to the transaction price are recorded as an adjustment to revenue and not as bad debt expense.
Allowance for Doubtful Accounts and Contractual Allowance
Allowance for doubtful accounts and contractual allowance is subject to uncertainty because we establish an allowance for doubtful accounts for estimated uncollectible receivables based on our historical collections, review of specific outstanding claims, consideration of relevant qualitative factors and an established allowance percentage by aging category, all of which are inherently uncertain. We write off outstanding accounts against the allowance for doubtful accounts when they are deemed to be uncollectible. Increases and decreases in the allowance for doubtful accounts are included as a component of general and administrative expenses. We record reductions in revenue for estimated uncollectible amounts as contractual allowances.
We review and update our estimates for the allowance for doubtful accounts and the contractual allowance periodically to reflect our experience regarding historical collections. If we were to make different judgments or utilize different estimates in the allowance for doubtful accounts and the contractual allowance, differences in both the amount of reported general and administrative expenses and revenue could result.
Estimated Usage of the Printed Circuit Board Assembly
We use a printed circuit board assembly (“PCBA”) in each Zio XT and Zio AT wearable device and a gateway board in Zio AT device. These boards are reused numerous times in multiple patients. Each time the PCBA and gateway board is used in a wearable Zio XT and Zio AT monitor, a portion of the cost of the PCBA and gateway board is recorded as a cost of revenue. Estimated usage of PCBAs is subject to uncertainty because we base our estimates of how many times a PCBA and gateway board can be used on testing in research and development, loss rates, product obsolescence, and the amount of time it takes the device to go through the manufacturing, shipping, customer shelf and patient wear time and upload process. These estimates can be subjective, complex, and inherently uncertain. We periodically evaluate the use estimate.
Stock-Based Compensation
We recognize compensation costs related to stock option grants, restricted stock unit grants (“RSUs”), and shares under the employee stock purchase plan (“ESPP”), and market condition awards based on the estimated fair value of the awards on the date of grant, net of estimated forfeitures. We estimate the grant date fair value, and the resulting stock-based compensation expense for options and shares under the ESPP, using the Black-Scholes option pricing model. We estimate the grant date fair value, and the resulting stock-based compensation expense for market condition awards using the Monte-Carlo option pricing model. The RSU grant date fair value is based on the closing price on the date of the grant. The grant date fair value of stock-based awards is expensed on a straight-line basis over the period during which the employee is required to provide service in exchange for the award (generally the vesting period).
We estimate the fair value of our stock-based awards using the Black-Scholes and Monte-Carlo option-pricing models, which requires the input of highly subjective assumptions. Our assumptions are as follows:
•Expected term. The expected term represents the period that the stock-based awards are expected to be outstanding. We use the simplified method to determine the expected term, which is calculated as the average of the time to vesting and the contractual life of the options.
•Expected volatility. The expected volatility is derived from the average implied volatility of our common stock. As our common stock has been publicly traded for a limited time, in some cases expected volatility is derived from the average historical volatilities of publicly traded companies within our industry that we consider to be comparable to our business over a period approximately equal to the expected term for employees’ options and the remaining contractual life for nonemployees’ options.
•Risk-free interest rate. The risk-free interest rate is based on the U.S. Treasury yield with a maturity equal to the expected term of the option in effect at the time of grant.
•Dividend yield. The expected dividend is assumed to be zero as we have never paid dividends and have no current plans to pay any dividends on our common stock.
In addition to the assumptions used in the Black-Scholes and Monte-Carlo option-pricing model, we also estimate a forfeiture rate to calculate the stock-based compensation for our equity awards. We will continue to use judgment in evaluating the expected volatility, expected terms and forfeiture rates utilized for our stock-based compensation calculations on a prospective basis.
We recognize compensation expense related to RSUs based on the grant date fair value on a straight-line basis over the period during which the employee is required to provide service in exchange for the award (generally the vesting period). Where there is a minimum performance condition required to vest in RSUs, we perform a probability assessment to estimate the number of shares that are probable to vest.
We recognize compensation expense related to the ESPP based on the estimated fair value on the date of grant, net of estimated forfeitures. We estimate the grant date fair value, and the resulting stock-based compensation expense, using the Black-Scholes option pricing model for each purchase period. The grant date fair value is expensed on a straight-line basis over the offering period.
Stock-based compensation is subject to uncertainty due to management estimates used in monte-carlo and black scholes valuations, as well as probability assessments performed to estimated numbers of shares probable to vest where awards have minimum performance conditions.
We recorded stock-based compensation expense of $54.5 million, $41.5 million, and $26.2 million for the years ended December 31, 2021, 2020 and 2019, respectively. We expect to continue to grant RSUs and other equity-based awards in the future, and to the extent that we do, our stock-based compensation expense recognized in future periods will likely increase.
Material Cash Requirements
The Company's material cash requirements include the following contractual and other obligations.
•Debt interest and principal payments - In October 2018, we entered into the Third Amended and Restated Loan and Security Agreement with Silicon Valley Bank, Pursuant to which we obtained a term loan (“SVB Term Loan”) for $35.0 million. We made interest-only payments through October 31, 2020, and began making monthly payments of principal plus interest in November 2020.
•Operating leases - We lease our facilities under non-cancelable operating leases that have remaining lease terms of up to 9.75 years. As of December 31, 2021, we had fixed lease payment obligations of $136.5 million, with $12.6 million payable within 12 months.
•Purchase obligations - Purchase obligations represent an estimate of open purchase orders and contractual obligations, in the ordinary course of business for which we have not received the goods or services. As of December 31, 2021, we had $53.5 million of such purchase obligations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk.
We are exposed to market risks in the ordinary course of our business. These risks primarily include risk related to interest rate sensitivities and foreign currency exchange rate sensitivity.
Interest Rate Sensitivity
We had cash, cash equivalents and investments of $239.1 million as of December 31, 2021; which consisted of bank deposits, money market funds and U.S. government securities, corporate notes, and commercial paper. Such interest-earning instruments carry a degree of interest rate risk; however, historical fluctuations in interest income have not been significant.
We do not enter into investments for trading or speculative purposes and have not used any derivative financial instruments to manage our interest rate risk exposure. We have not been exposed nor do we anticipate being exposed to material risks due to changes in interest rates. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
For the years ended December 31, 2021 and 2020 we had total outstanding debt of $21.4 million and $33.0 million, respectively, which is net of debt issuance costs. The Third Amended and Restated SVB Loan Agreement Note carries a variable interest rate based on the “Prime Rate” published by The Wall Street Journal. A hypothetical 10% change in interest rates during any of the periods presented would not have had a material impact on our consolidated financial statements.
Foreign Currency Exchange Rate Sensitivity
We face foreign exchange risk as a result of entering into transactions denominated in currencies other than U.S. dollars, particularly in British Pound Sterling. As of December 31, 2021, we do not consider this risk to be material. We do not utilize any forward foreign exchange contracts. All foreign transactions settle on the applicable spot exchange basis at the time such payments are made.
Item 8. Financial Statements and Supplementary Data.
IRHYTHM TECHNOLOGIES, INC.
Index to Financial Statements
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of iRhythm Technologies, Inc.
Opinions on the Financial Statements and Internal Control over Financial Reporting
We have audited the accompanying consolidated balance sheets of iRhythm Technologies, Inc. and its subsidiary (the “Company”) as of December 31, 2021 and 2020, and the related consolidated statements of operations, of comprehensive loss, of stockholders' equity and of cash flows for each of the three years in the period ended December 31, 2021, including the related notes (collectively referred to as the “consolidated financial statements”). We also have audited the Company's internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO).
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the three years in the period ended December 31, 2021 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company did not maintain, in all material respects, effective internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control - Integrated Framework (2013) issued by the COSO because a material weakness in internal control over financial reporting existed as of that date related to an ineffective control environment commensurate with the Company's financial reporting requirements, due to an insufficient number of professionals with an appropriate level of accounting and internal control knowledge, training and experience.
A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis. The material weakness referred to above is described in Management’s Annual Report on Internal Control Over Financial Reporting appearing under Item 9A. We considered this material weakness in determining the nature, timing, and extent of audit tests applied in our audit of the 2021 consolidated financial statements, and our opinion regarding the effectiveness of the Company’s internal control over financial reporting does not affect our opinion on those consolidated financial statements.
Basis for Opinions
The Company's management is responsible for these consolidated financial statements, for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting, included in management's report referred to above. Our responsibility is to express opinions on the Company’s consolidated financial statements and on the Company's internal control over financial reporting based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud, and whether effective internal control over financial reporting was maintained in all material respects.
Our audits of the consolidated financial statements included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
Definition and Limitations of Internal Control over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit
preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Critical Audit Matters
The critical audit matter communicated below is a matter arising from the current period audit of the consolidated financial statements that was communicated or required to be communicated to the audit committee and that (i) relates to accounts or disclosures that are material to the consolidated financial statements and (ii) involved our especially challenging, subjective, or complex judgments. The communication of critical audit matters does not alter in any way our opinion on the consolidated financial statements, taken as a whole, and we are not, by communicating the critical audit matter below, providing a separate opinion on the critical audit matter or on the accounts or disclosures to which it relates.
Contractual Allowance – Contracted Third-Party Payors
As described in Note 2 to the consolidated financial statements, a large portion of the Company’s transactions are covered by third-party payors with whom there is a contractual agreement or established amount the third-party payor will pay (contracted third-party payors). These contracts impose a number of obligations regarding billing and other matters, and the Company’s noncompliance with a material term of such contracts may result in a denial of the claim. The number of claims from the first half of 2021 which had differences between the submitted price and reimbursement, and the overall denials, increased significantly compared to the Company’s historical experience as a result of the Current Procedural Terminology (CPT) code transition issues with certain payors. The Company recognizes revenue from contracted third-party payors, net of contractual allowances. As of December 31, 2021, the Company’s contractual allowance balance was $31.3 million, a significant portion of which relates to revenue from services provided to patients where contracted third-party payors pay for the service on the patient's behalf. As disclosed by management, management accounts for denied claims as a form of variable consideration that is included as a reduction to the transaction price and records as an adjustment to revenue as a contractual allowance. The contractual allowance requires judgment by management and is based on historical collections, review of specific outstanding claims and consideration of relevant qualitative factors.
The principal considerations for our determination that performing procedures relating to the contractual allowance for contracted third-party payors is a critical audit matter are the significant judgment by management when developing the estimate of the contractual allowance; this in turn led to a high degree of auditor judgment, subjectivity, and effort in performing procedures and evaluating audit evidence related to the contractual allowance based on historical collections, review of specific outstanding claims and consideration of relevant qualitative factors.
Addressing the matter involved performing procedures and evaluating audit evidence in connection with forming our overall opinion on the consolidated financial statements. These procedures included testing the effectiveness of controls relating to the contractual allowance for contracted third party payors, including controls over the historical collections, review of specific outstanding claims and consideration of relevant qualitative factors. These procedures also included, among others (i) testing management’s process for developing the estimate of the contractual allowance; (ii) testing the completeness and accuracy of the underlying data used in the estimate; (iii) testing, on a sample basis, the accuracy of revenue transactions and collections from the historical billing and collection data used in management’s analysis; (iv) performing a retrospective comparison of actual collections subsequent to year-end to evaluate the reasonableness of certain of the estimates; and (v) evaluating the reasonableness of adjustments made by management to contractual allowances.
/s/ PricewaterhouseCoopers LLP
San Jose, California
February 28, 2022
We have served as the Company’s auditor since 2009.
IRHYTHM TECHNOLOGIES, INC.
Consolidated Balance Sheets
(In thousands, except par value and share data) | | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Assets | | | |
| Current assets: | | | |
| Cash and cash equivalents | $ | 127,562 | | | $ | 88,628 | |
| Short-term investments | 111,569 | | | 246,589 | |
| Accounts receivable, net | 46,430 | | | 29,932 | |
| Inventory | 10,268 | | | 5,313 | |
| Prepaid expenses and other current assets | 9,693 | | | 7,363 | |
| Total current assets | 305,522 | | | 377,825 | |
| Property and equipment, net | 55,944 | | | 34,247 | |
| Operating lease right-of-use assets | 84,587 | | | 84,714 | |
| Goodwill | 862 | | | 862 | |
| Other assets | 16,052 | | | 14,091 | |
| Total assets | $ | 462,967 | | | $ | 511,739 | |
| Liabilities and Stockholders’ Equity | | | |
| Current liabilities: | | | |
| Accounts payable | $ | 10,509 | | | $ | 4,365 | |
| Accrued liabilities | 51,486 | | | 40,532 | |
| Deferred revenue | 3,049 | | | 930 | |
| Debt, current portion | 11,667 | | | 11,667 | |
| | | |
| Operating lease liabilities, current portion | 11,142 | | | 8,171 | |
| Total current liabilities | 87,853 | | | 65,665 | |
| Debt, noncurrent portion | 9,690 | | | 21,339 | |
| Other noncurrent liabilities | 697 | | | 1,830 | |
| Operating lease liabilities, noncurrent portion | 85,212 | | | 81,293 | |
| Total liabilities | 183,452 | | | 170,127 | |
| Commitments and contingencies (Note 6) | | | |
| Stockholders’ equity: | | | |
Preferred stock, $0.001 par value – 5,000,000 shares authorized at December 31, 2021 and 2020; and none issued and outstanding at December 31, 2021 and 2020, respectively | — | | | — | |
Common stock, $0.001 par value – 100,000,000 shares authorized at December 31, 2021 and 2020; 29,493,726 and 29,019,350 shares issued and outstanding at December 31, 2021 and 2020, respectively | 27 | | | 27 | |
| Additional paid-in capital | 685,594 | | | 646,258 | |
| Accumulated other comprehensive income (loss) | (61) | | | 11 | |
| Accumulated deficit | (406,045) | | | (304,684) | |
| Total stockholders’ equity | 279,515 | | | 341,612 | |
| Total liabilities and stockholders’ equity | $ | 462,967 | | | $ | 511,739 | |
The accompanying notes are an integral part of these consolidated financial statements.
IRHYTHM TECHNOLOGIES, INC.
Consolidated Statements of Operations
(In thousands, except share and per share data) | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Revenue | $ | 322,825 | | | $ | 265,166 | | | $ | 214,552 | |
| Cost of revenue | 109,258 | | | 70,277 | | | 52,485 | |
| Gross profit | 213,567 | | | 194,889 | | | 162,067 | |
| Operating expenses: | | | | | |
| Research and development | 38,671 | | | 41,329 | | | 37,299 | |
| Selling, general and administrative | 274,839 | | | 197,233 | | | 179,523 | |
| Total operating expenses | 313,510 | | | 238,562 | | | 216,822 | |
| Loss from operations | (99,943) | | | (43,673) | | | (54,755) | |
| Interest expense | (1,169) | | | (1,519) | | | (1,643) | |
| Other income, net | 118 | | | 1,591 | | | 1,895 | |
| Loss before income taxes | (100,994) | | | (43,601) | | | (54,503) | |
| Income tax provision | 367 | | | 229 | | | 65 | |
| Net loss | $ | (101,361) | | | $ | (43,830) | | | $ | (54,568) | |
| Net loss per common share, basic and diluted | $ | (3.46) | | | $ | (1.58) | | | $ | (2.16) | |
| Weighted-average shares, basic and diluted | 29,331,010 | | | 27,754,404 | | | 25,265,918 | |
The accompanying notes are an integral part of these consolidated financial statements.
IRHYTHM TECHNOLOGIES, INC.
Consolidated Statements of Comprehensive Loss
(In thousands) | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Net loss | $ | (101,361) | | | $ | (43,830) | | | $ | (54,568) | |
| Other comprehensive income (loss): | | | | | |
| Net change in unrealized gains (losses) on available-for-sale securities | (72) | | | (71) | | | 98 | |
| Comprehensive loss | $ | (101,433) | | | $ | (43,901) | | | $ | (54,470) | |
The accompanying notes are an integral part of these consolidated financial statements.
IRHYTHM TECHNOLOGIES, INC.
Consolidated Statements of Stockholders’ Equity
(In thousands, except share and per share data) | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | Common Stock | | Additional
Paid-In
Capital | | Accumulated
Deficit | | Accumulated
Other
Comprehensive
Income (Loss) | | Total
Stockholders’
Equity |
| | Shares | | Amount | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| | | | | | | | | | | | |
| Balances at December 31, 2018 | | 24,368,073 | | | $ | 23 | | | $ | 257,955 | | | $ | (205,825) | | -205825000 | $ | (16) | | | $ | 52,137 | |
| Issuance of common stock in connection with employee equity incentive plans, net | | 734,453 | | | — | | | 9,495 | | | — | | | — | | | 9,495 | |
Warrants exercised | | 4,852 | | | | | | | | | | | |
| Issuance of common stock in connection with follow-on public offering, net of issuance costs | | 1,575,342 | | | 2 | | | 107,292 | | | — | | | | | 107,294 | |
Tax withholding upon vesting of restricted stock awards | | — | | | — | | | (5,288) | | | — | | | — | | | (5,288) | |
Stock-based compensation expense | | — | | | — | | | 26,241 | | | — | | | — | | | 26,241 | |
Net loss | | — | | | — | | | — | | | (54,568) | | | — | | | (54,568) | |
Net change in unrealized loss on investments | | — | | | — | | | — | | | — | | | 98 | | | 98 | |
| Balances at December 31, 2019 | | 26,682,720 | | | 25 | | | 395,695 | | | (260,393) | | | 82 | | | 135,409 | |
| Issuance of common stock in connection with employee equity incentive plans | | 1,079,488 | | | — | | | 20,244 | | | — | | | — | | | 20,244 | |
| Issuance of common stock in connection with follow-on public offering, net of issuance costs | | 1,257,142 | | | 2 | | | 206,023 | | | — | | | — | | | 206,025 | |
| Tax withholding upon vesting of restricted stock awards | | — | | | | | (10,009) | | | | | | | (10,009) | |
| Stock-based compensation expense | | — | | | — | | | 34,305 | | | — | | | — | | | 34,305 | |
| Accounting Standards Codification 326 cumulative effect adjustment upon adoption | | | | | | | | (461) | | | | | (461) | |
| Net loss | | — | | | — | | | — | | | (43,830) | | | — | | | (43,830) | |
Net change in unrealized loss on investments | | — | | | — | | | — | | | — | | | (71) | | | (71) | |
| Balances at December 31, 2020 | | 29,019,350 | | 27 | | | 646,258 | | | (304,684) | | | 11 | | | 341,612 | |
| Issuance of common stock in connection with employee equity incentive plans | | 474,376 | | | — | | | 8,943 | | | — | | | — | | | 8,943 | |
| Tax withholding upon vesting of restricted stock awards | | — | | | | | (25,853) | | | | | | | (25,853) | |
| Stock-based compensation expense | | — | | | — | | | 56,246 | | | — | | | — | | | 56,246 | |
| Net loss | | — | | | — | | | — | | | (101,361) | | | — | | | (101,361) | |
| Net change in unrealized loss on investments | | — | | | — | | | — | | | — | | | (72) | | | (72) | |
| Balances at December 31, 2021 | | 29,493,726 | | $ | 27 | | | $ | 685,594 | | | $ | (406,045) | | | $ | (61) | | | $ | 279,515 | |
The accompanying notes are an integral part of these consolidated financial statements.
IRHYTHM TECHNOLOGIES, INC.
Consolidated Statements of Cash Flows
(In thousands) | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Cash flows from operating activities | | | | | |
| Net loss | $ | (101,361) | | | $ | (43,830) | | | $ | (54,568) | |
| Adjustments to reconcile net loss to net cash used in operating activities: | | | | | |
| Depreciation and amortization | 9,842 | | | 6,900 | | | 3,445 | |
| Stock-based compensation | 54,527 | | | 41,515 | | | 26,241 | |
| Amortization of debt discount and issuance costs | — | | | — | | | 37 | |
| Accretion of discounts on investments, net of premium amortization | 1,641 | | | 430 | | | (884) | |
| Provision for bad debt and contractual allowance | 37,074 | | | 31,431 | | | 24,647 | |
| Cost of operating lease right-of-use assets | 6,752 | | | 6,030 | | | 8,953 | |
| Changes in operating assets and liabilities: | | | | | |
| Accounts receivable | (53,572) | | | (37,957) | | | (28,725) | |
| Inventory | (4,960) | | | (1,389) | | | (1,974) | |
| Prepaid expenses and other current assets | (2,329) | | | (3,027) | | | (696) | |
| Other assets | (1,831) | | | (6,149) | | | (4,732) | |
| Accounts payable | 6,135 | | | (3,881) | | | 5,604 | |
| Accrued liabilities | 7,946 | | | 1,308 | | | 6,002 | |
| Deferred revenue | 2,119 | | | (321) | | | 28 | |
| Operating lease liabilities | 264 | | | (4,819) | | | (5,241) | |
| Net cash used in operating activities | (37,753) | | | (13,759) | | | (21,863) | |
| Cash flows from investing activities | | | | | |
| Purchases of property and equipment | (28,067) | | | (13,551) | | | (20,457) | |
| Purchases of available-for-sale investments | (122,184) | | | (277,510) | | | (165,915) | |
| Sales of available-for-sale investments | — | | | 14,525 | | | 1,498 | |
| Maturities of available-for-sale investments | 255,515 | | | 144,145 | | | 95,600 | |
| Net cash provided by (used in) investing activities | 105,264 | | | (132,391) | | | (89,274) | |
| Cash flows from financing activities | | | | | |
Issuance of common stock in connection with follow-on public offering, net | — | | | 206,025 | | | 107,369 | |
Proceeds from issuance of common stock in connection with employee
equity incentive plans | 8,943 | | | 20,244 | | | 9,495 | |
| Tax withholding upon vesting of restricted stock awards | (25,853) | | | (10,009) | | | (5,288) | |
| Repayments of long-term debt | (11,667) | | | (1,944) | | | — | |
| Net cash (used in) provided by financing activities | (28,577) | | | 214,316 | | | 111,576 | |
| Net increase in cash and cash equivalents | 38,934 | | | 68,166 | | | 439 | |
| Cash and cash equivalents, beginning of year | 88,628 | | | 20,462 | | | 20,023 | |
| Cash and cash equivalents, end of year | $ | 127,562 | | | $ | 88,628 | | | $ | 20,462 | |
| Supplemental disclosures of cash flow information | | | | | |
| Interest paid | $ | 1,194 | | | $ | 1,502 | | | $ | 1,644 | |
| Non-cash investing and financing activities | | | | | |
| Property and equipment included in accounts payable and accrued liabilities | $ | 9 | | | $ | 3 | | | $ | 293 | |
| Deferred offering costs included in accounts payable and accrued liabilities | $ | — | | | $ | — | | | $ | 77 | |
| Right-of-use assets obtained in exchange for new operating lease liabilities | $ | 6,625 | | | $ | 621 | | | $ | 88,860 | |
| Capitalized stock-based compensation | $ | 3,593 | | | $ | 1,129 | | | $ | — | |
The accompanying notes are an integral part of these consolidated financial statements.
IRHYTHM TECHNOLOGIES, INC.
Notes to Consolidated Financial Statements
1. Organization and Description of Business
iRhythm Technologies, Inc. (the “Company”) was incorporated in the state of Delaware in September 2006. The Company is a digital healthcare company redefining the way cardiac arrhythmias are clinically diagnosed by combining wearable biosensing technology with cloud-based data analytics and deep-learning capabilities. The Company began commercial operations in the United States in 2009 following clearance by the U.S. Food and Drug Administration.
The Company is headquartered in San Francisco, California, which also serves as a clinical center. The Company has additional clinical centers in Lincolnshire, Illinois and Houston, Texas and a manufacturing facility in Cypress, California. In March 2016, the Company formed a wholly-owned subsidiary in the United Kingdom. Additionally, in June 2021, the Company formed a wholly-owned subsidiary in Singapore. The Company manages its operations as a single operating segment. Substantially all of the Company’s assets are maintained in the United States. The Company derives substantially all of its revenue from the provision of services to customers in the United States.
On September 10, 2019, the Company issued and sold an aggregate of 1,575,342 shares (the "2019 Shares") of common stock, in a public offering at a price of $73.00 per share. The 2019 Shares included the full exercise of the underwriters’ option to purchase an additional 205,479 shares of common stock. Total proceeds received from the offering were $107.3 million, after deducting discounts and issuance costs.
On August 21, 2020, the Company issued and sold an aggregate of 1,257,142 shares (the "Shares") of common stock, in a public offering at a price of $175.00 per share. The Shares included the full exercise of the underwriters’ option to purchase an additional 163,975 shares of common stock. Total proceeds received from the offering were $206.8 million, after deducting discounts and issuance costs.
2. Summary of Significant Accounting Policies
Basis of Presentation
The accompanying consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America (“U.S. GAAP”) and include the accounts of iRhythm Technologies, Inc. and its wholly-owned subsidiary. All intercompany accounts and transactions have been eliminated. The financial statements of the Company’s subsidiary use the U.S. dollar as the functional currency. For all non-functional currency balances, the remeasurement of such balances to functional currency results in a foreign exchange transaction gain or loss, which is recorded in the consolidated statements of operations.
Risks and Uncertainties
COVID-19
As a result of the COVID-19 pandemic, the Company has experienced significant business disruptions, restrictions on its ability to travel, reduction in access to customers due to diverted resources at hospitals, slower response times from payors who are also facing business interruptions, and shortened business hours as governments institute prolonged shelter-in-place and/or self-quarantine mandates.
Government mandates related to the COVID-19 pandemic have impacted, and are expected to continue to impact, Company personnel and personnel at third-party manufacturing facilities in the United States and other countries, and the availability or cost of materials, which could disrupt our supply chain and reduce margins. For instance, on or about March 16, 2020, the Health Officers for the counties of San Francisco (where the Company's headquarters is located), Santa Clara, San Mateo, Marin, Contra Costa and Alameda, where many employees are located, issued mandatory shelter-in-place orders and all employees transitioned to a remote work environment. The Company is also subject to orders in Southern California that temporarily shut down its manufacturing and distribution facilities in Cypress, California. For a limited number of employees who continue to support essential operations, including those at our manufacturing facilities, the Company has instituted protective equipment policies and, to the degree practical, social distancing measures to protect the safety of its employees. While the Company has continued to deliver its Zio service by operating with remote employees and essential employees on site, an extended implementation of these government mandates could further impact the Company's ability to effectively provide its Zio service, and could impede progress of all ongoing initiatives. Appropriate social distancing techniques and other
measures at the Company's facilities have been implemented for the limited number of employees who have returned to work to support essential operations, and will not return until the risk to employee health has meaningfully diminished.
While hospital systems and healthcare facilities shift their focus and resources to treating COVID-19 patients and combating the spread of the coronavirus, the Company has adapted its service to meet the immediate needs of physicians, customers, and patients and significantly increased the utilization of its home enrollment service which allows patients to receive and wear the single-use Zio device without going to a healthcare facility.
Government mandates related to the COVID-19 pandemic have impacted, and are expected to continue to impact, payor processing times of our claims and appeals. This increase in response times may be due, in part, to staffing shortages at the payors.
During the six months ended June 30, 2021, the Company experienced an increase in the level of Zio service patient registration. This increase may be due in part to re-opening of certain regions within the United States and increases in the vaccination status of patients and providers. During the six months ended December 31, 2021, the Company experienced a decrease in the level of Zio service patient registrations. This decrease may be due, in part, to staffing shortages at providers in certain regions within the United States. The status of provider capacity and resource prioritization, vaccination levels and other pandemic-related effects could meaningfully impact the volume of our patient registrations in future periods.
The Company is continuously reviewing its liquidity and anticipated capital requirements in light of the significant uncertainty created by the COVID-19 pandemic. The Company believes it will have adequate liquidity over the next 12 months to operate its business and to meet its cash requirements. As of December 31, 2021, the Company is in compliance with its debt covenants.
The ultimate impact of the COVID-19 pandemic is highly uncertain and subject to change. This impact is having a material, adverse impact on liquidity, capital resources, supply chain, operations and business and those of the third parties on which the Company relies, and could worsen over time. The extent to which the COVID-19 pandemic impacts the Company’s results will depend on future developments, which are highly uncertain and cannot be predicted, including new information which may emerge concerning the severity of the COVID-19 pandemic and the actions to contain the COVID-19 pandemic or treat its impact, among others. The full extent of potential delays or impacts on the business, financial condition, cash flows and results of operations remains unknown. Additionally, while the potential economic impact brought by, and the duration of, the COVID-19 pandemic is difficult to assess or predict, the COVID-19 pandemic has resulted in, and may continue to result in, significant disruption of global financial markets, reducing the Company’s ability to raise additional capital through equity, equity-linked or debt financings, which could negatively impact short-term and long-term liquidity and the ability to operate on a timely basis, or at all.
Reimbursement
Government payors may change their coverage and reimbursement policies, as well as payment amounts, in a way that would prevent or limit reimbursement for the Zio service, which would significantly harm the Company. Government and other third-party payors require the Company to report the service for which it is seeking reimbursement by using a Current Procedural Terminology (“CPT”), code-set maintained by the American Medical Association (“AMA”). For Zio XT, the Company had historically utilized temporary CPT codes (or Category III CPT codes) used for newly introduced technologies and specific to our category of diagnostic monitoring. The process to convert Category III CPT codes to Category I CPT codes is governed by the AMA and Centers for Medicare and Medicaid (“CMS”). On October 25, 2019, the AMA’s CPT Editorial Panel established two new Category I CPT codes which are applicable to the Zio service and took effect on January 1, 2021. In November 2021, CMS published the Calendar Year 2022 Medicare Physician Fee Schedule Final Rule (the “2022 Final Rule”). In the Final Rule, CMS did not issue national pricing and continued carrier pricing for calendar year 2022 on Category I CPT codes 93241, 93243, 93245 and 93247 for extended external ECG monitoring, the relevant codes for the Company’s Zio XT service. These codes were designated by CMS for contractor pricing in calendar 2022, which means that prices will be determined regionally by each Medicare Administrative Contractor (“MAC” or “MACs”).
Determinations of which products or services will be reimbursed under Medicare can be developed at the national level through a national coverage determination (“NCD”) by CMS, or at the local level through a local coverage determination (“LCD”), by one or more of the regional Medicare Administrative Contractors (“MACs”) who are private contractors that process and pay claims on behalf of CMS for different regions. In the absence of a specific NCD, as is the case with Zio XT historically and for Calendar Year 2022 following the Final Rule, the MAC with jurisdiction over a specific geographic region will have the discretion to make an LCD. The Company is seeking to establish LCD pricing with one or more MACs to establish pricing for 2022 and will be subject to LCD pricing until such time CMS establishes a NCD.
In January 2022, Novitas Solutions, the MAC which covers the region where our Independent Diagnostic Testing Facility (“IDTF”) in Houston, Texas is located and where almost all of our Medicare services for Zio XT are processed, updated reimbursement rates for CPT codes 93243 and 93247 for the Houston jurisdiction to $223 and $233, respectively. These updated rates are retroactive to January 1, 2022. These rates were higher than the rates posted by Novitas in 2021, but continue to be significantly below our historical Medicare rates for our Zio XT service. We are continuing to negotiate with Novitas and other MACs to establish higher pricing for the Category I CPT Codes but can offer no assurances as to the timing or outcome of those discussions.
If the published Medicare reimbursement rates are not adequate to support our cost structure, or we are unable to reduce our overall cost structure, the Company may be unable to provide the Zio service or would experience a significant loss of revenue, either of which would have a material adverse effect on our cash flows, results of operations, and financial condition.
Further, a reduction in coverage by Medicare could cause some commercial third-party payors to implement similar reductions in their coverage or level of reimbursement of the Zio service. Given the evolving nature of the healthcare industry and on-going healthcare cost reforms, the Company will continue to be subject to changes in the level of Medicare coverage for its products, and unfavorable coverage determinations at the national or local level could adversely affect its results of operations. Although a large majority of commercial customers have re-contracted the Zio XT service since the establishment of the Category I codes on January 1, 2021 matching to pre-existing rates, if the Company is unsuccessful in improving the Medicare rates, the Company believes that commercial rates may begin to be more negatively impacted next year.
If the Company is unable to achieve a level of revenues adequate to support its cost structure, or is unable to reduce its overall cost structure, this would raise substantial doubts about the Company's ability to continue as a going concern.
Use of Estimates
The preparation of the consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, management evaluates its estimates, including those related to revenue recognition, contractual allowances, allowance for doubtful accounts, the useful lives of property and equipment, the recoverability of long-lived assets including the estimated usage of the printed circuit board assemblies (“PCBAs”), the incremental borrowing rate for operating leases, accounting for income taxes, and various inputs used in estimating stock-based compensation. Certain of these estimates are impacted by uncertainties surrounding COVID-19 such as revenue recognition, contractual allowances for revenue, allowance for doubtful accounts, and stock based compensation. The Company bases these estimates on historical and anticipated results, trends, and various other assumptions that management believes are reasonable under the circumstances, including assumptions as to future events. Actual results may differ from those estimates.
Fair Value of Financial Instruments
The carrying amounts of certain of the Company’s financial instruments, which include cash equivalents, short-term investments, long-term investments, accounts receivable, accounts payable and accrued liabilities, approximate fair value due to their short maturities.
Cash Equivalents
Cash equivalents consist of short-term, highly liquid investments with original maturities of three months or less from the date of purchase.
Investments
Short-term investments consist of debt securities classified as available-for-sale and have maturities less than one year as of the balance sheet date. Long-term investments have maturities greater than one year as of the balance sheet date. All investments are carried at fair value based upon quoted market prices.
The Company periodically assesses its portfolio of debt investments for impairment. For debt securities in an unrealized loss position, this assessment first takes into account the intent to sell, or whether it is more likely than not that the Company will be required to sell the security before recovery of its amortized cost basis. If either of these criteria are met, the debt security’s amortized cost basis is written down to fair value through interest and other, net. For debt securities in an unrealized loss position that do not meet the aforementioned criteria, the Company assesses whether the decline in fair value has resulted from credit losses or other factors.
The Company evaluates expected credit losses by considering factors such as historical experience, market data, issuer-specific factors, and current economic conditions. Expected credit losses on available-for-sale debt securities are recognized in other income, net in the condensed consolidated statements of operations, and any remaining unrealized losses, net of taxes, are reported as a component of accumulated other comprehensive loss. The Company did not recognize any credit losses on its available-for-sale securities during the year ended December 31, 2021 and there were no impairment charges for unrealized losses in the periods presented.
The cost of available-for-sale securities sold is based on the specific-identification method and realized gains and losses are included in earnings. Amortization of premiums and accretion of discounts are reported as a component of other income, net.
Accounts Receivable, Allowance for Doubtful Accounts and Contractual Allowances
Accounts receivable includes amounts due to the Company from healthcare institutions, third-party payors, and government payors and their related patients, as a result of the Company's normal business activities. Accounts receivable is reported on the consolidated balance sheets net of an estimated allowance for doubtful accounts and contractual allowances.
The Company establishes an allowance for doubtful accounts for estimated uncollectible receivables based on its assessment of the collectability of customer accounts and recognizes the provision as a component of selling, general and administrative expenses. The Company records a provision for contractual allowances based on the estimated differences between contracted amounts and expected collection rates. Such provisions are based on the Company's historical experience and are reported as a reduction of revenue.
The Company regularly reviews the allowances by considering factors such as historical experience, credit quality, the age of the accounts receivable balances, and current economic conditions that may affect a customer’s ability to pay.
The Company submitted the majority of Zio XT claims from the first and second quarter of 2021 on a delayed basis due to the CPT code transition. Claims were being held due to administrative delays with payors and a combination of negotiations with payors. Most of the held claims were released and submitted by the end of the second quarter of 2021. For the contracted portfolio, once submitted, the number of claims from the first half of 2021 which contained differences between the submitted price and reimbursement and overall denials increased significantly compared to our historical experience as a result of CPT code transition issues with the payors. We continue to work with the payors to collect on these claims and the collection cycle for these claims is significantly longer than usual. While we believe we have properly estimated the impact to our contractual allowances and allowance for doubtful accounts, inherent uncertainty caused by the longer-collection cycle and claims adjudication process could result in additional provisions for contractual allowances and doubtful accounts which would negatively impact our results of operations in future periods.
The following table presents the changes in the allowance for doubtful accounts (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2021 | | 2020 | | 2019 |
| Balance, beginning of year | $ | 12,711 | | | $ | 9,049 | | | $ | 7,296 | |
| Add: adoption of ASC 326 | — | | | 461 | | | — | |
| Add: provision for doubtful accounts | 9,615 | | | 10,515 | | | 9,129 | |
| Less: write-offs, net of recoveries and other adjustments | (8,314) | | | (7,314) | | | (7,376) | |
| Balance, end of year | $ | 14,012 | | | $ | 12,711 | | | $ | 9,049 | |
The following table presents the changes in the contractual allowance (in thousands):
| | | | | | | | | | | | | | | | | |
| Year ended December 31, |
| 2021 | | 2020 | | 2019 |
| Balance, beginning of year | $ | 21,281 | | | $ | 15,433 | | | $ | 9,205 | |
| Add: allowance for contractual adjustments | 27,459 | | | 20,916 | | | 15,518 | |
| Less: contractual adjustments | (17,466) | | | (15,068) | | | (9,290) | |
| Balance, end of year | $ | 31,274 | | | $ | 21,281 | | | $ | 15,433 | |
Concentrations of Risk
Credit Risk
Financial instruments that potentially subject the Company to a concentration of credit risk consist primarily of cash and cash equivalents, investments and accounts receivable. Cash balances are deposited in financial institutions which, at times, may be in excess of federally insured limits. Cash equivalents are invested in highly rated money market funds. The Company invests in a variety of financial instruments, such as, but not limited to, U.S. government securities, corporate notes, commercial paper and, by policy, limits the amount of credit exposure with any one financial institution or commercial issuer. The Company has not experienced any material losses on its deposits of cash and cash equivalents or investments.
Concentrations of credit risk with respect to accounts receivable are limited due to the large number of customers comprising the Company’s customer base and their dispersion across many geographies. The Company does not require collateral. The Company records an allowance for doubtful accounts based on the assessment of the collectability of customer accounts, considering factors such as historical experience, credit quality, the age of the accounts receivable balances, and current economic conditions that may affect a customer’s ability to pay. Centers for Medicare and Medicaid Services (“CMS”), accounted for approximately 14%, 27% and 27% of the Company’s revenue for the years ended December 31, 2021, 2020 and 2019, respectively. CMS accounted for 8% and 20% of accounts receivable as of December 31, 2021 and 2020, respectively.
Inflationary Risk
The Company continuously monitors the effects of inflationary factors, such as increases in cost of goods sold and selling and operating expenses, which may adversely affect its results of operations. Specifically, the Company may experience inflationary pressure affecting the cost of the components for our Zio service and in the wages paid to its employees due to challenging labor market conditions. Competitive and regulatory conditions may restrict the Company's ability to fully recover these costs through price increases. As a result, it may be difficult to fully offset the impact of persistent inflation. The Company's inability or failure to do so could have a material adverse effect on our business, financial condition and results of operations or cause us to need to obtain additional capital in future earlier than anticipated.
Supply Risk
The Company relies on single-source vendors to supply some of its disposable housings, instruments and other materials used to manufacture the Zio monitor and the adhesive that binds the Zio monitor to a patient’s body. These components and materials are critical, and there could be a considerable delay in finding alternative sources of supply.
A global semiconductor supply shortage is having wide-ranging effects across multiple industries. The supply shortage has impacted multiple suppliers that provide the printed circuit board assemblies to the Company. The semiconductor supply shortage may have an impact on the Company until global supply is sufficient for global demand.
Supply Chain Constraints
Economies worldwide have also seen other indirect COVID-19 pandemic related disruptions including supply chain impacts, material inflation, and labor constraints in certain markets and geographies. Such economic disruption has had an adverse effect on the Company's business environment as increased lead times and component shortages have resulted in higher inventory costs. While the Company has increased inventory safety stock levels to help mitigate the delays and disruptions in supply, the Company cannot be certain that any prolonged, intensified or worsened effect from the COVID-19 pandemic would not further impact its supply chain.
Inventory
Inventory owned by the Company is valued at cost, on the first in, first out (“FIFO”) basis, or the lower of cost or net realizable value. The Company records write-downs of inventory that is obsolete or in excess of anticipated demand. The Company also records market value based write-downs on consideration of product lifecycle stage, technology trends, product development plans and assumptions about future demand and market conditions. Actual demand may differ from forecasted demand, and such differences may have a material effect on recorded inventory values. Inventory write-downs are charged to cost of revenue and establish a new cost basis for the inventory.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation and amortization is computed using the straight-line method over the estimated useful lives of the assets, ranging from three to five years. Leasehold improvements are amortized over the shorter of the lease term or the estimated useful lives of the assets. Maintenance and repairs are charged to expense as incurred, and improvements and betterments are capitalized.
Internal-Use Software
The Company capitalizes costs related to internal-use software during the application development stage. Costs related to planning and post implementation activities are expensed as incurred. Capitalized internal-use software is amortized, and recognized as cost of revenue, on a straight-line basis over the estimated useful life, which is up to five years. The Company evaluates the useful lives of these assets on an annual basis, and tests for impairment whenever events or changes in circumstances occur that could impact the recoverability of these assets. Capitalized internal-use software costs are classified as a component of property and equipment.
Goodwill
Goodwill represents the excess of the purchase price paid over the fair value of tangible and identifiable intangible net assets acquired in business combinations. Goodwill is tested for impairment on an annual basis and at any other time if events occur or circumstances indicate that the carrying amount of goodwill may not be recoverable. Such events or circumstances may include significant adverse changes in the general business climate, among other things. The impairment test is performed by determining the enterprise fair value of the Company, which is primarily based on the Company’s market capitalization. If the Company’s carrying value, as a one reporting unit entity, is less than its fair value, then the fair value is allocated to all of its assets and liabilities (including any unrecognized intangible assets) as if the fair value was the purchase price to acquire the Company. The excess of the fair value over the amounts assigned to the Company’s assets and liabilities is the implied fair value of the goodwill. If the carrying amount of goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. The Company performs its annual evaluation of goodwill during the fourth quarter of each fiscal year. The Company did not record any charges related to goodwill impairment in any of the periods presented in these consolidated financial statements.
Impairment of Long-Lived Assets
The Company annually reviews long-lived assets for impairment or whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparison of the carrying amount to the future net cash flows which the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the assets exceeds the projected discounted future net cash flows arising from the asset. To date, there have been no such impairments of long-lived assets.
In February 2022, the Company's Board of Directors approved a decision to pursue reducing its leased space for its headquarters in San Francisco, California. The Company expects this decision will result in an impairment of the right of use asset for the San Francisco office lease and is assessing the impact to its Consolidated Financial Statements for the quarter ending March 31, 2022. Refer to Note 13. Subsequent Events for further details.
Other Assets
The Company uses Printed Circuit Board Assemblies (“PCBAs”), in each wearable Zio XT and Zio AT monitor as well as the wireless gateway used in conjunction with the ZIO AT monitor. The PCBAs are used numerous times and have useful lives beyond one year. Each time a PCBA is used in a wearable Zio XT or Zio AT monitor, or a wireless gateway is used with a Zio AT monitor a portion of the cost of the PCBA and/or gateway is recorded as a cost of revenue. The PCBAs are recorded as other assets and were $13.9 million and $12.6 million as of December 31, 2021 and 2020, respectively. The Company has based its estimates of how many times a PCBA can be used on testing in research and development, loss rates, product obsolescence, and the amount of time it takes the device to go through the manufacturing, shipping, customer shelf and patient wear time and upload process. The Company periodically evaluates the use estimate.
Comprehensive Loss
Comprehensive loss represents all changes in stockholders’ equity during the period from non-owner sources. The Company’s unrealized gains and losses on available-for-sale securities represent the only component of other comprehensive loss that are excluded from the reported net loss and that are presented in the consolidated statements of comprehensive loss.
Revenue Recognition
The Company’s revenue is generated primarily from the provision of its cardiac rhythm monitoring service, the Zio XT service. The Zio XT is a cardiac rhythm monitoring service that has a patient wear period of up to 14 days and is billable when the monitoring reports are delivered to the healthcare provider, which is also when the service is complete and the Company recognizes revenue. The time from when the patient has the Zio XT device applied to the time the report is posted is generally around 25 days. The Company has concluded that the Zio XT service is one performance obligation on the basis that the customer cannot benefit from each component of the service on its own or together with other resources that are readily available to the customer.
The Zio AT mobile cardiac telemetry monitor, a wearable patch-based biosensor, offers what the Zio XT offers plus the additional capability of transmissions during the wear period to assist physicians in diagnosing and treating patients that require more timely action. During the wear period, physicians will receive notifications if there are significant events that meet predetermined arrhythmia detection criteria. The Zio AT service revenue is recognized over the prescription period and delivery of an electronic Zio Report with two performance obligations.
The Company recognizes as revenue the amount of consideration to which it expects to be entitled in exchange for performing the service. The consideration the Company is entitled to varies by portfolio, as further defined below, and includes estimates that require significant judgment by management. A unique aspect of healthcare is the involvement of multiple parties to the service transaction. In addition to the patient, often a third-party, for example a commercial or governmental payor or healthcare institution, will pay the Company for some or all of the service on the patient’s behalf. Separate contractual arrangements exist between the Company and third-party payors that establish amounts the third-party payor will pay on behalf of a patient for covered services rendered.
A small portion of the Company’s transactions are covered by third-party payors with whom there is neither a contractual agreement nor an established amount that the third-party payor will pay. In determining the collectability and transaction price for its service, the Company considers factors such as insurance claims which are adjudicated as allowable under the applicable policy and payment history from both payors and patient out-of-pocket costs, payor coverage, whether there is a contract between the payor or healthcare institution and the Company, historical amount received for the service, and any current developments or changes that could impact reimbursement and healthcare institution payments. Certain of these factors are forms of variable consideration which are only included in the transaction price to the extent it is probable that a significant reversal of cumulative revenue will not occur when the uncertainty associated with the variable consideration is subsequently resolved.
A summary of the payment arrangements with third-party payors and healthcare institutions is as follows:
•Contracted third-party payors – The Company has contracts with negotiated prices for services provided to patients with commercial healthcare insurance coverage.
•CMS – The Company has received independent diagnostic testing facility approval from regional Medicare Administrative Contractors and will receive reimbursement per the relevant CPT code rates for the services rendered to the patient covered by CMS.
•Non-contracted third-party payors – Non-contracted commercial and government payors often reimburse out-of-network rates provided under the relevant CPT codes on a case-by-case basis. The transaction price used
for determining revenue recognition is based on factors including an average of the Company’s historical collection experience for its non-contracted services. This rate is reviewed at least quarterly.
•Healthcare institutions – Healthcare institutions are typically hospitals or physician practices in which the Company has negotiated amounts for its monitoring services, including certain governmental agencies such as the Veterans Administration and Department of Defense.
The Company is utilizing the portfolio approach practical expedient under ASC 606 for revenue recognition whereby services provided under each of the above payor types form a separate portfolio. The Company accounts for the contracts within each portfolio as a collective group, rather than individual contracts. Based on history with these portfolios and the similar nature and characteristics of the patients within each portfolio, the Company has concluded that the financial statement effects are not materially different than if accounting for revenue on a contract-by-contract basis.
For contracted and CMS portfolios, the Company recognizes revenue, net of contractual allowances, and recognizes an allowance for doubtful accounts for uncollectible patient accounts receivable. The transaction price is determined based on negotiated rates, and the Company has historical experience of collecting substantially all of these contracted rates. These contracts also impose a number of obligations regarding billing and other matters, and the Company’s noncompliance with a material term of such contracts may result in a denial of the claim. The Company accounts for denied claims as a form of variable consideration that is included as a reduction to the transaction price recognized as revenue. The Company estimates the denied claims which require management judgment. The estimated denied claims are based on historical information and judgement includes the historical period utilized. The Company monitors the estimated denied claims against the latest available information, and subsequent changes to the estimated denied claims are recorded as an adjustment to revenue in the periods during which such changes occur. Delays in claims submissions could lead to an increase in denials if we miss the payors’ filing deadlines and could result in a reduction in the Company’s receipt of payments. Historical cash collection indicates that it is probable that substantially all of the transaction price, less the estimate of denied claims, will be received. Contracted payors may require that we bill patient co-payments and deductibles and from time to time we may not be able to collect such amounts due to credit risk. The Company provides for estimates of uncollectible patient accounts receivable, based upon historical experience where judgment includes the historical period utilized, at the time revenue is recognized, with such provisions presented as bad debt expense within the selling, general and administrative line item of the consolidated statement of operations. Adjustments to these estimates for actual experience are also recorded as an adjustment to bad debt expense.
We submitted the majority of Zio XT claims from the first and second quarter of 2021 on a delayed basis due to the CPT code transition. Claims were being held due to a combination of negotiations with payors and administrative delays with payors. Most of the held claims were released and submitted by the end of the second quarter of 2021. For the contracted portfolio, once submitted, the number of claims from the first half of 2021 which contained differences between the submitted price and reimbursement and overall denials increased significantly compared to our historical experience as a result of CPT code transition issues with the payors. We continue to work with the payors to collect on these claims and the collection cycle for these claims is significantly longer than usual. While we believe we have properly estimated the impact to our contractual allowances and allowance for doubtful accounts, the inherent uncertainty caused by longer-collection cycle and claims adjudication process could result in additional provisions for contractual allowances and doubtful accounts which would negatively impact our results of operations in future periods.
For non-contracted portfolios, the Company is providing an implicit price concession due to the lack of a contracted rate with the underlying payor, the result of which requires the Company to estimate the transaction price based on historical cash collections utilizing the expected value method. All subsequent adjustments to the transaction price are recorded as an adjustment to revenue.
For healthcare institutions, the transaction price is determined based on negotiated rates, and the Company has historical experience collecting substantially all of these contracted rates. Historical cash collection indicates that it is probable that substantially all of the transaction price will be received. As such, the Company is not providing an implicit price concession but, rather, has chosen to accept the risk of default, and any subsequent uncollected amounts are recorded as bad debt expense.
Disaggregation of Revenue
The Company disaggregates revenue from contracts with customers by payor type. The Company believes these categories aggregate the payor types by nature, amount, timing and uncertainty of its revenue streams. Disaggregated revenue by payor type and major service line for the years ended December 31, 2021 and December 31, 2020 were as follows (in thousands): | | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Contracted third-party payors | $ | 193,871 | | | $ | 135,939 | | | $ | 101,845 | |
| Non-contracted third-party payors | 26,929 | | | 15,295 | | | 10,770 | |
| Centers for Medicare & Medicaid | 44,529 | | | 72,536 | | | 58,918 | |
| Healthcare institutions | 57,496 | | | 41,396 | | | 43,019 | |
| Total | $ | 322,825 | | | $ | 265,166 | | | $ | 214,552 | |
Contract Liabilities
ASC 606 requires an entity to present a revenue contract as a contract liability when the Company has an obligation to transfer goods or services to a customer for which the Company has received consideration from the customer, or an amount of consideration from the customer is due and unconditional (whichever is earlier).
Certain of the Company’s customers pay the Company directly for the Zio XT service upon shipment of devices. Such advance payments are contract liabilities and are recorded as deferred revenue on the Consolidated Balance Sheets and revenue is recognized when reports are delivered to the healthcare provider. During the year ended December 31, 2021, $0.9 million relating to the contract liability balance at the beginning of 2021 was recognized as revenue. Total revenue recognized during the year ended December 30, 2020 that was included in the contract liability balance at the beginning of 2020 was $1.2 million.
Contract Costs
Under ASC 340, the incremental costs of obtaining a contract with a customer are recognized as an asset. Incremental costs of obtaining a contract are those costs that an entity incurs to obtain a contract with a customer that it would not have incurred if the contract had not been obtained.
The Company’s current commission programs are considered incremental. However, as a practical expedient, ASC 340 permits the Company to immediately expense contract acquisition costs, as the asset that would have resulted from capitalizing these costs will be amortized in one year or less.
Cost of Revenue
Cost of revenue includes direct labor, material costs, overhead, data analysis, customer care, equipment and infrastructure expenses, amortization of internal-use software, and shipping and handling. Direct labor includes payroll and personnel-related costs involved in manufacturing. Material costs include both the disposable costs of the device and amortization of the PCBAs. Each time the PCBA is used in a wearable Zio XT monitor, a portion of the cost of the PCBA is recorded as a cost of revenue.
Research and Development
The Company’s research and development costs are expensed as incurred. Research and development costs include, but are not limited to, payroll and personnel-related expenses, laboratory supplies, consulting costs and overhead charges.
Income Taxes
The Company uses the asset and liability method to account for income taxes in accordance with the authoritative guidance for income taxes. Under this method, deferred tax assets and liabilities are determined based on future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and tax loss and credit carryforwards. Deferred tax assets and liabilities are measured using enacted tax rates applied to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is established when necessary to reduce deferred tax assets to the amount expected to be realized.
The Company recognizes the effect of income tax positions only if those positions are more likely than not of being sustained. Recognized income tax positions are measured at the largest amount that has a greater than 50% likelihood of being realized. Changes in recognition or measurement are reflected in the period in which the change in judgment occurs. The Company records interest and penalties related to unrecognized tax benefits in income tax expense. To date, there have been no interest or penalties charged in relation to the unrecognized tax benefits.
The Company recognize taxes on Global Intangible Low-Taxed Income as a current period expense when incurred.
Stock-based Compensation
The Company measures its stock-based awards made to employees based on the estimated fair values of the awards as of the grant date. The fair value of market condition awards is determined using the Monte-Carlo option pricing model and the fair value of stock options is determined using the Black-Scholes option pricing model. Stock-based compensation expense is recognized over the requisite service period using the straight-line method and is based on the value of the portion of stock-based payment awards that is ultimately expected to vest. As such, the Company’s stock-based compensation is reduced for the estimated forfeitures at the date of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates. For restricted stock, the compensation cost for these awards is based on the closing price of the Company’s common stock on the date of grant, and recognized as compensation expense on a straight-line basis over the requisite service period.
The Company recognizes compensation expense related to the Employee Stock Purchase Plan (“ESPP”) based on the estimated fair value of the options on the date of grant, net of estimated forfeitures. The Company estimates the grant date fair value, and the resulting stock-based compensation expense, using the Black-Scholes option pricing model for each purchase period. The grant date fair value is expensed on a straight-line basis over the offering period.
Net Loss per Common Share
Basic net loss per common share is calculated by dividing the net loss by the weighted average number of shares of common stock outstanding during the period, without consideration of potentially dilutive securities. Diluted net loss per common share is the same as basic net loss per common share for all periods presented, since the effect of potentially dilutive securities are anti-dilutive.
Leases
Identifying a lease
The Company determines whether a contract contains a lease at the inception of a contract. If the contract conveys the right to control the use of an identified asset for a period of time in exchange for consideration, the Company considers the contract to contain a lease. The Company determines whether a contract conveys the right to control the use of an identified asset for a period of time if the contract contains both of the following terms:
| | | | | | | | | | | | | | |
| | |
• | The right to obtain substantially all of the economic benefits from use of the identified asset; and | |
| | | |
• | The right to direct the use of the identified asset. |
Discount rate for leases
As the rate implicit in the lease is not readily determinable for our operating leases, the Company generally uses an incremental borrowing rate based on information available at the commencement date such as a synthetic credit rating and current cost of borrowing to determine the present value of future lease payments.
Lease term
The lease term is generally the minimum noncancellable period of each lease. The Company does not include option periods in determining the right-of-use asset and operating lease liability at inception unless it is reasonably certain that the Company will exercise the option at inception or when a triggering event occurs. As of December 31, 2021, no renewal options were included in the determination of lease terms.
Lease Modification
The San Francisco Lease is in the same building with the same landlord as the lease for the Company’s prior headquarters in San Francisco (“existing lease”). Upon the commencement of the San Francisco Lease, the existing lease which had an original expiration date of February 2020, was modified to expire in September 2019 and accordingly the right-of-use asset and lease liability was remeasured as of the modification date.
3. Cash Equivalents and Investments
The fair value of cash equivalents and available-for-sale investments at December 31, 2021 and 2020, were as follows (in thousands): | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2021 |
| Amortized Cost | | Gross Unrealized | | Estimated Fair Value |
| | Gains | | Losses | |
| Money market funds | $ | 110,137 | | | $ | — | | | $ | — | | | $ | 110,137 | |
| U.S. government securities | 50,490 | | | — | | | (46) | | | 50,444 | |
| Corporate notes | 31,158 | | | — | | | (15) | | | 31,143 | |
| Commercial paper | 29,982 | | | — | | | — | | | 29,982 | |
| Total cash equivalents and available-for-sale investments | $ | 221,767 | | | $ | — | | | $ | (61) | | | $ | 221,706 | |
| Classified as: | | | | | | | |
| Cash equivalents | | | | | | | $ | 110,137 | |
| Short-term investments | | | | | | | 111,569 | |
| | | | | | | |
| Total cash equivalents and available-for-sale investments | | | | | | | $ | 221,706 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 |
| Amortized
Cost | | Gross Unrealized | | Estimated
Fair Value |
| | Gains | | Losses | |
| Money market funds | $ | 59,823 | | | $ | — | | | $ | — | | | $ | 59,823 | |
| U.S. government securities | 190,663 | | | 16 | | | (2) | | | 190,677 | |
| Corporate notes | 26,426 | | | 2 | | | (5) | | | 26,423 | |
| Commercial paper | 29,489 | | | — | | | — | | | 29,489 | |
| Total cash equivalents and available-for-sale investments | $ | 306,401 | | | $ | 18 | | | $ | (7) | | | $ | 306,412 | |
| Classified as: | | | | | | | |
| Cash equivalents | | | | | | | $ | 59,823 | |
| Short-term investments | | | | | | | 246,589 | |
| | | | | | | |
| Total cash equivalents and available-for-sale investments | | | | | | | $ | 306,412 | |
The following table summarizes the fair value of the Company's cash equivalents, short-term and long-term marketable securities classified by maturity (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Due within one year | $ | 221,706 | | | $ | 306,412 | |
| Due after one year through three years | — | | | — | |
| Total cash equivalents and available-for-sale investments | $ | 221,706 | | | $ | 306,412 | |
The following tables present the Company's available-for-sale securities that were in an unrealized loss position as of December 31, 2021 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2021 |
| Less than 12 months | | 12 Months or Greater | | Total |
| Assets | Fair Value | | Unrealized Loss | | Fair Value | | Unrealized Loss | | Fair Value | | Unrealized Loss |
| U.S. government securities | $ | 50,445 | | | $ | (46) | | | $ | — | | | $ | — | | | $ | 50,445 | | | $ | (46) | |
| Corporate notes | 31,143 | | | (15) | | | — | | | — | | | 31,143 | | | (15) | |
| Total | $ | 81,588 | | | $ | (61) | | | $ | — | | | $ | — | | | $ | 81,588 | | | $ | (61) | |
| | | | | | | | | | | |
Unrealized losses as of December 31, 2021 were not material. Available-for-sale securities held as of December 31, 2021 had a weighted average maturity of 95 days. At December 31, 2021, 13 investments were in an unrealized loss position and no investments have been in an unrealized loss position for more than one year.
4. Fair Value Measurements
The Company discloses and recognizes the fair value of its assets and liabilities using a hierarchy that prioritizes the inputs to valuation techniques used to measure fair value. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The hierarchy gives the highest priority to valuations based upon unadjusted quoted prices in active markets for identical assets or liabilities (Level 1 measurements) and the lowest priority to valuations based upon unobservable inputs that are significant to the valuation (Level 3 measurements). The guidance establishes three levels of the fair value hierarchy as follows:
Level 1—Inputs are unadjusted quoted prices in active markets for identical assets or liabilities at the measurement date.
Level 2—Inputs (other than quoted market prices included in Level 1) are either directly or indirectly observable for the asset or liability through correlation with market data at the measurement date and for the duration of the instrument’s anticipated life.
Level 3—Inputs reflect management’s best estimate of what market participants would use in pricing the asset or liability at the measurement date. Consideration is given to the risk inherent in the valuation technique and the risk inherent in the inputs to the model.
Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. The Company’s assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability. The corporate notes, commercial paper and government securities are classified as Level 2 as they were valued based upon quoted market prices for similar instruments in active markets, quoted prices for identical or similar instruments in markets that are not active and model-based valuation techniques for which all significant inputs are observable in the market or can be corroborated by observable market data for substantially the full term of the assets.
The fair value of the Company’s outstanding interest-bearing obligations is estimated using the net present value of the future payments, discounted at an interest rate that is consistent with market interest rates, which is a Level 2 input. The carrying amount and the estimated fair value of the Company’s outstanding interest-bearing obligations at December 31, 2021 were $21.4 million and $21.7 million, respectively. The carrying amount and the estimated fair value of the Company’s outstanding interest-bearing obligations at December 31, 2020 were $33.0 million and $33.9 million, respectively.
The Company had no transfers between levels of the fair value hierarchy of its assets measured at fair value.
The following tables present the fair value of the Company’s financial assets determined using the inputs defined above (in thousands).
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2021 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Assets | | | | | | | |
| Money market funds | $ | 110,137 | | | $ | — | | | $ | — | | | $ | 110,137 | |
| U.S. government securities | — | | | 50,444 | | | — | | | 50,444 | |
| Corporate notes | — | | | 31,143 | | | — | | | 31,143 | |
| Commercial paper | — | | | 29,982 | | | — | | | 29,982 | |
| Total | $ | 110,137 | | | $ | 111,569 | | | $ | — | | | $ | 221,706 | |
| | | | | | | | | | | | | | | | | | | | | | | |
| December 31, 2020 |
| Level 1 | | Level 2 | | Level 3 | | Total |
| Assets | | | | | | | |
| Money market funds | $ | 59,823 | | | $ | — | | | $ | — | | | $ | 59,823 | |
| U.S. government securities | — | | | 190,677 | | | — | | | 190,677 | |
| Corporate notes | — | | | 26,423 | | | — | | | 26,423 | |
| Commercial paper | — | | | 29,489 | | | — | | | 29,489 | |
| Total | $ | 59,823 | | | $ | 246,589 | | | $ | — | | | $ | 306,412 | |
5. Balance Sheet Components
Inventory and Other Assets
Inventory consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Raw materials | $ | 5,101 | | | $ | 2,469 | |
| Finished goods | 5,167 | | | 2,844 | |
| Total | $ | 10,268 | | | $ | 5,313 | |
The Company uses PCBAs in each wearable Zio XT and Zio AT monitor as well as the wireless gateway used in conjunction with the Zio AT monitor. The PCBAs are used numerous times and have useful lives beyond one year. Each time a PCBA is used in a wearable Zio XT monitor or Zio AT monitor, a portion of the cost of the PCBA is recorded as a cost of revenue. Each time a wireless gateway is used with a Zio AT monitor, a portion of the gateway is recorded as a cost of revenue. PCBAs which are recorded as other assets were $13.9 million and $12.6 million as of December 31, 2021, and December 31, 2020, respectively. The amortization was $4.4 million and $3.0 million for the years ending December 31, 2021 and 2020 respectively.
Property and Equipment, Net
Property and equipment, net consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Laboratory and manufacturing equipment | $ | 5,029 | | | $ | 4,667 | |
| Computer equipment and software | 2,353 | | | 2,005 | |
| Furniture and fixtures | 4,174 | | | 3,794 | |
| Leasehold improvements | 20,431 | | | 9,215 | |
| Internal-use software | 46,661 | | | 28,416 | |
| Total property and equipment, gross | 78,648 | | | 48,097 | |
| Less: accumulated depreciation and amortization | (22,704) | | | (13,850) | |
| Total property and equipment, net | $ | 55,944 | | | $ | 34,247 | |
Depreciation and amortization expense for the years ended December 31, 2021 and 2020 was $9.8 million and $6.9 million, respectively.
Accrued Liabilities
Accrued liabilities consisted of the following (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Accrued vacation | $ | 7,431 | | | $ | 6,007 | |
| Accrued payroll and related expenses | 34,484 | | | 19,709 | |
| Accrued ESPP Contributions | 1,002 | | | 851 | |
| Accrued professional services fees | 1,724 | | | 1,709 | |
| Accrued interest | 78 | | | 121 | |
| Claims payable | 2,988 | | | 4,757 | |
| Other | 3,779 | | | 7,378 | |
| Total accrued liabilities | $ | 51,486 | | | $ | 40,532 | |
6. Commitments and Contingencies
Lease Arrangements
The Company leases office, manufacturing, and clinical centers under non-cancelable operating leases which expire on various dates through 2031. These leases generally contain scheduled rent increases or escalation clauses and renewal options. Operating lease right-of-use assets and lease liabilities are recognized based on the present value of the future minimum lease payments over the lease term at commencement date. The operating lease right-of-use assets also include any lease payments made to the lessor at or before the commencement date as well as variable lease payments which are based on a consumer price index. The Company is also subject to variable lease payments related to janitorial services and electricity which are not included in the operating lease right-of-use asset as they are based on actual usage. The Company recognizes operating lease expense on a straight-line basis over the lease period. The total operating lease cost recognized during the year ended December 31, 2021 was $13.5 million which primarily consisted of lease payments and common area maintenance costs. Cash paid for operating leases during the year ended December 31, 2021 was $12.2 million.
On October 4, 2018, the Company entered into an office lease (“San Francisco Lease”) to rent approximately 117,560 rentable square feet in San Francisco, California, which became the Company’s new headquarters in October 2019. The term of the San Francisco Lease began on May 13, 2019, and expires on August 31, 2031. The Company is entitled to one option to extend the San Francisco Lease for a five-year term, subject to certain requirements.
On October 29, 2009, the Company entered into a lease for a clinic (“Lincolnshire Lease”) to rent approximately 41,500 rentable square feet in Lincolnshire, Illinois, which became the Company’s clinic facilities. The term of the Lincolnshire Lease began on November 1, 2009, and expires on July 31, 2022.
On March 18, 2021, the Company entered into an office/industrial lease (“Cypress Lease”) to rent approximately 68,933 rentable square feet in Cypress, California, which became the Company’s new office and warehouse facilities. The term of the Cypress Lease began on October 25, 2021, and expires on April 30, 2032. The Company is entitled to one option to extend the Cypress Lease for a five-year term, subject to certain requirements.
In February 2022, the Company's Board of Directors approved a decision to pursue reducing its leased space for its headquarters in San Francisco, California. The Company expects these decisions will result in an impairment of the right of use asset for the San Francisco office lease and is assessing the impact to its Consolidated Financial Statements for the quarter ending March 31, 2022.
As of December 31, 2021, maturities of operating lease liabilities were as follows (in thousands):
| | | | | |
| Year Ended December 31: | |
| 2022 | $ | 12,617 | |
| 2023 | 12,912 | |
| 2024 | 13,304 | |
| 2025 | 13,696 | |
| 2026 | 14,100 | |
| Thereafter | 69,898 | |
| 136,527 | |
| Less: imputed interest | (40,173) | |
| Total lease liabilities | $ | 96,354 | |
The weighted average remaining lease term of the Company's operating leases as of December 31, 2021 was 9.62 years. The weighted average discount rate of the Company's operating leases was 7.27% as of December 31, 2021.
In December 2021, the Company entered into a lease for 44,616 rentable square feet in Deerfield Illinois ("Deerfield Lease"). The Deerfield Lease commenced in January 2022, and will be recorded as a right of use asset to the Company's financial statements in the first quarter of 2022. The Company is currently assessing the impact to its consolidated financial statements.
Legal Proceedings
From time to time, the Company is involved in claims and legal proceedings or investigations, that arise in the ordinary course of business. Such matters could have an adverse impact on its reputation, business and the financial condition and divert the attention of its management from the operation of its business. These matters are subject to many uncertainties and outcomes that are not predictable.
On February 1, 2021, a putative class action lawsuit was filed in the United States District Court for the Northern District of California alleging that the Company and its former Chief Executive Officer, Kevin M. King, violated Sections 10(b) and 20(a) of the Exchange Act and SEC Rule 10b-5 promulgated thereunder. On August 2, 2021, the lead plaintiff filed an amended complaint, and filed a further amended complaint on September 24, 2021. The amended complaint names as defendants, in addition to the Company and Mr. King, the Company's former Chief Executive Officer, Michael J. Coyle, and current Chief Financial Officer, Douglas J. Devine. The purported class in the amended complaint includes all persons who purchased or acquired the Company's common stock between August 4, 2020 and July 13, 2021, and seeks unspecified damages purportedly sustained by the class. On October 27, 2021, the Company filed a motion to dismiss the amended complaint. The motion to dismiss was fully briefed and the Court held a hearing on the motion on February 4, 2022, after which the Court took the matter under submission. The Court has not yet issued a ruling. The Company believes the class action to be without merit and plans to vigorously defend itself.
On March 26, 2021, the Company received a grand jury subpoena from the U.S. Attorney’s Office for the Northern District of California requesting information related to communications with the Food and Drug Administration and the Company’s products. On October 14, 2021, the Company received a second subpoena requesting additional information. The Company is cooperating fully and is providing the requested information.
Development Agreement
On September 3, 2019, the Company entered into a Development Collaboration Agreement (the “Development Agreement”) with Verily Life Sciences LLC (“Verily”). The Development Agreement, which is over a 24 month term, involves joint development and production of intellectual property between the Company and Verily. Each participant has primary responsibility for certain aspects of development and approval, with all processes to be performed at each respective party’s own cost. Costs incurred by the Company in connection with the Development Agreement will be expensed as research and development expense in accordance with ASC 730, Research and Development.
The Company and Verily will develop certain next-generation atrial fibrillation (“AF”) screening, detection, or monitoring products pursuant to the Development Agreement, which products will involve combining Verily and the Company's technology platforms and capabilities. Under the terms of the Development Agreement, the Company paid Verily an upfront fee of $5.0 million in 2019. In addition, the Company has agreed to make additional payments to Verily up to an aggregate of $12.75 million in milestone payments upon achievement of various development and regulatory milestones over the 24 months of the Development Agreement, which payments will be made in cash to Verily.
We have achieved milestones tied to payments totaling $11.0 million to date, $3.0 million of which is associated with a milestone achieved during the quarter ended December 31, 2021 and is included in Accounts Payable on December 31, 2021. We have agreed to make additional payments over the term of the Development Agreement up to an aggregate of $1.75 million, subject to the achievement of certain development and regulatory milestones including provision Zio Watch and completion of market evaluation scheduled to begin between late 2022 and early 2023.
The Development Agreement provides each party with licenses to use certain intellectual property of the other party for development activities in the field of AF screening, detection, or monitoring. Ownership of developed intellectual property will be allocated to the Company or Verily depending on the subject matter of the underlying developed intellectual property, and, for certain subject matter, shall be jointly owned.
Indemnifications
In the ordinary course of business, the Company enters into agreements pursuant to which it agrees to indemnify customers, vendors, lessors, business partners, and other parties with respect to certain matters, including losses arising out of the breach of such agreements, services to be provided by the Company, or from intellectual property infringement claims made by third parties. Pursuant to such agreements, the Company may indemnify, hold harmless and defend an indemnified party for losses suffered or incurred by the indemnified party. Some of the provisions will limit losses to those arising from third-party actions. In some cases, the indemnification will continue after the termination of the agreement. The maximum potential amount of future payments the Company could be required to make under these provisions is not determinable. The Company has also entered into indemnification agreements with its directors and officers that may require the Company to indemnify its directors and officers against liabilities that may arise by reason of their status or service as directors or officers to the fullest extent permitted by applicable law. The Company currently has directors’ and officers’ insurance. The Company has never incurred material costs to defend lawsuits or settle claims related to these indemnification provisions, and believes that the estimated fair value of these indemnification obligations is not material and it has not accrued any amounts for these obligations.
7. Debt
Bank Debt
In October 2018, the Company entered into the Third Amended and Restated Loan and Security Agreement with SVB (“Third Amended and Restated SVB Loan Agreement”). This Agreement amends and restates the Second Amended and Restated Loan and Security Agreement between the Company and SVB dated December 4, 2015, as amended by the First Loan Modification Agreement between the Company and SVB dated August 22, 2016.
Pursuant to the Third Amended and Restated SVB Loan Agreement, the Company obtained a term loan (“SVB Term Loan”) for $35.0 million. Total proceeds from the SVB Term Loan were used to pay off the loan agreement with Biopharma Secured Investments III Holdings Cayman LP (“Pharmakon”), totaling $35.8 million. The Company made interest-only payments through October 31, 2020. Beginning in November 2020, the Company began monthly payments of principal plus interest, which will continue through October 31, 2023. Interest charged on the SVB Term Loan will be the greater of (a) a floating rate based on the “Prime Rate” published by The Wall Street Journal minus 0.75%, or (b) 4.25%. The weighted average interest rate was 4.25% and 4.25% for the years ended December 31, 2021 and 2020, respectively.
Under the Third Amended and Restated SVB Loan Agreement, the Company may borrow, repay, and reborrow under a revolving credit line, but not in excess of the maximum loan amount of $25.0 million, which includes an $11.0 million standby letter of credit sublimit availability. In October 2018, a $6.9 million standby letter of credit was obtained in connection with a lease for the Company’s San Francisco headquarters. Any principal amount outstanding under the Third Amended and Restated SVB Loan Agreement revolving credit line shall bear interest at an amount that is the greater of (a) a floating rate per annum equal to the rate published by The Wall Street Journal as the “Prime Rate” or (b) 5.00%. The Company may borrow up to 75% of eligible accounts receivable, up to the maximum of $25.0 million. As of December 31, 2021, the Company was eligible to borrow up to $13.8 million and no amount was outstanding under the revolving credit line.
The Third Amended and Restated Loan Agreement requires the Company to maintain a minimum consolidated liquidity ratio or minimum adjusted Earnings Before Interest, Tax, Depreciation, and Amortization during the term of the loan facility. In addition, the SVB Loan Agreement contains customary affirmative and negative covenants and events of default. The Company was in compliance with loan covenants as of December 31, 2021. The obligations under the Third Amended and Restated Loan Agreement are collateralized by substantially all assets of the Company.
Future minimum payments
Future minimum payments under the Third Amended and Restated Loan and Security Agreement with Silicon Valley Bank at December 31, 2021 are as follows (in thousands):
| | | | | |
| Year Ending December 31, | |
| 2022 | $ | 12,357 | |
| 2023 | 9,914 | |
| |
| Total | 22,271 | |
| Less: Amount representing interest | (883) | |
| Less: Debt Issuance Costs | (31) | |
| Total Carrying Value | $ | 21,357 | |
| |
| Reported as: | |
| Short-term debt | $ | 11,667 | |
| Long-term debt | 9,690 | |
| Total | $ | 21,357 | |
8. Income Taxes
The following table presents components of the Company’s provision for income taxes as for the period presented (in thousands):
| | | | | | | | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 | | 2019 |
| Current expense (benefit): | | | | | |
| Federal | $ | — | | | $ | — | | | $ | — | |
| State | 223 | | | 181 | | | — | |
| Foreign | 150 | | | 59 | | | 68 | |
| Total current tax expense (benefit) | 373 | | | 240 | | | 68 | |
| | | | | |
| Deferred expense (benefit): | | | | | |
| Federal | — | | | — | | | — | |
| State | — | | | — | | | — | |
| Foreign | (6) | | | (11) | | | (3) | |
| Total deferred tax expense (benefit) | (6) | | | (11) | | | (3) | |
| | | | | |
| Total Tax Expense (benefit) | $ | 367 | | | $ | 229 | | | $ | 65 | |
The following table presents a reconciliation of the tax expense computed at the statutory federal rate and the Company’s tax expense for the period presented (in thousands):
| | | | | | | | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 | | 2019 |
| Tax at statutory federal rate | $ | (21,198) | | | $ | (9,172) | | | $ | (11,446) | |
| State income taxes, net of federal benefit | 223 | | | 181 | | | — | |
| Stock-based compensation | (7,049) | | | (20,762) | | | (5,560) | |
| Meals and Entertainment | 182 | | | 177 | | | 409 | |
Section 162(m) limitation - officers compensation | 4,856 | | | 544 | | | — | |
Other | (347) | | | 89 | | | 614 | |
| Tax credits | (381) | | | (1,426) | | | (1,128) | |
| Foreign Rate Differential | (30) | | | (9) | | | — | |
| Change in valuation allowance | 24,111 | | | 30,607 | | | 17,176 | |
| Provision for income taxes | $ | 367 | | | $ | 229 | | | $ | 65 | |
Deferred income taxes reflect the net effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
Significant components of the Company’s deferred tax assets and liabilities are as follows (in thousands):
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Deferred tax assets: | | | |
| Net operating loss carryforwards | $ | 114,856 | | | $ | 91,034 | |
| Tax credit carryforwards | 9,174 | | | 7,110 | |
| Share-based compensation | 9,812 | | | 8,831 | |
| Allowances and other | 20,988 | | | 14,996 | |
| Lease obligation | 23,868 | | | 22,624 | |
| Total deferred tax assets | 178,698 | | | 144,595 | |
| Valuation allowance | (154,734) | | | (123,803) | |
| Net deferred tax assets | 23,964 | | | 20,792 | |
| | | |
| Deferred Tax Liabilities: | | | |
| Depreciation and Amortization | (3,212) | | | (108) | |
| Right of use asset | (20,696) | | | (20,634) | |
| Total deferred tax liability | (23,908) | | | (20,742) | |
| Total deferred tax assets | $ | 56 | | | $ | 50 | |
Due to the uncertainties surrounding the realization of deferred tax assets through future taxable income, the Company has provided a full valuation allowance against its U.S. deferred tax assets, and, therefore, no benefit has been recognized for the net operating loss carryforwards and other deferred tax assets. The U.S. valuation allowance increased by $30.9 million and $35.4 million for the years ended December 31, 2021 and December 31, 2020, respectively. The current year change in the U.S. valuation allowance is primarily related to the increase in net operating loss carryforwards generated during the year. The Company recorded an immaterial deferred tax asset related to the Company’s foreign operations in the United Kingdom.
The valuation allowance for deferred tax assets consisted of the following activity for the years ended December 31, 2021, 2020 and 2019 (in thousands):
| | | | | | | | | | | | | | | | | | | | | | | |
| Balance at beginning of year | | Additions | | Deductions | | Balance at end of year |
| Year Ended December 31, 2021 | $ | 123,803 | | | $ | 30,931 | | | $ | — | | | $ | 154,734 | |
| Year Ended December 31, 2020 | $ | 88,433 | | | $ | 35,370 | | | $ | — | | | $ | 123,803 | |
| Year Ended December 31, 2019 | $ | 66,435 | | | $ | 21,998 | | | $ | — | | | $ | 88,433 | |
As of December 31, 2021, the Company had approximately $464.3 million of federal and $279.1 million of state net operating loss carryforwards available to offset future taxable income which expires in varying amounts beginning in 2027 and 2020 respectively.
As of December 31, 2021, the Company had research tax credit carryforwards of approximately $7.4 million, and $6.1 million available to reduce future taxable income, if any, for both federal and state purposes, respectively. The federal tax credit carryforwards expire beginning in 2028 and the state tax credits can be carried forward indefinitely.
Section 382 of the Internal Revenue Code, and similar state provisions, limits the use of net operating loss and tax credit carryforwards in certain situations where equity transactions result in a change of ownership as defined by Internal Revenue Code Section 382. In the event the Company should experience an ownership change, as defined, utilization of its net operating loss carryforwards and tax credits could be limited.
Effective January 1, 2019, the Company adopted ASC 740-10 (formerly known as FIN 48), Accounting for Income Taxes, guidance that addresses the recognition, measurement, and disclosure of uncertain tax positions.
A reconciliation of the Company’s unrecognized tax benefit amount is as follows (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Balance at beginning of year | $ | 2,302 | | | $ | 1,842 | | | $ | 1,459 | |
| Additions for tax positions taken in current year | 772 | | | 488 | | | 383 | |
| Increases in balance related to prior year tax positions | 236 | | | — | | | — | |
| Decreases in balance related to prior year tax positions | — | | | (28) | | | — | |
| Balance at end of year | $ | 3,310 | | | $ | 2,302 | | | $ | 1,842 | |
The total amount of gross unrecognized tax benefits was $3.3 million, $2.3 million, and $1.8 million as of December 31, 2021, 2020, and 2019 respectively. None of the Company’s unrecognized tax benefits that, if recognized, would affect its effective tax rate. The Company does not anticipate the total amounts of unrecognized tax benefits will significantly increase or decrease in the next 12 months. The Company’s policy is to include interest and penalties related to unrecognized tax benefits within the provision for taxes. Management determined that no accrual for interest or penalties was required as of December 31, 2021, 2020 and 2019.
The Company files income tax returns in the U.S. and UK jurisdictions. All of the Company's tax years are open to examination by the US federal and state tax authorities. The UK is open to examination for tax years starting 2017 and forward. The Company currently has no federal, state or foreign tax examinations in progress, nor has it had any federal or state examinations since inception.
9. Stockholders’ Equity
Common stock
The Company’s amended and restated certificate of incorporation dated October 25, 2016, as amended, authorizes the Company to issue 100,000,000 shares of common stock with a par value of $0.001 per share and 5,000,000 shares of preferred stock with a par value of $0.001 per share. The holders of common stock are entitled to receive dividends whenever funds and assets are legally available and when declared by the board of directors, subject to the prior rights of holders of all series of convertible preferred stock outstanding. No dividends were declared through December 31, 2021.
The Company had reserved shares of common stock for issuance as follows:
| | | | | | | | | | | |
| December 31, |
| 2021 | | 2020 |
| Options issued and outstanding | 504,106 | | | 609,881 | |
| Unvested restricted stock units | 1,649,561 | | | 1,114,159 | |
| Shares available for grant under future stock plans | 9,011,213 | | | 8,016,517 | |
| Shares available for future issuance | 11,164,880 | | | 9,740,557 | |
10. Stock Incentive Plans
2006 Plan
In October 2006, the Company adopted the 2006 Equity Incentive Plan, as amended, (the “2006 Plan”). The Plan provided for the granting of stock options to employees and non-employees of the Company. Options granted under the Plan were either incentive stock options or nonqualified stock options. Incentive stock options (“ISO”) were granted only to employees (including officers and directors who are also employees). Nonqualified stock options (“NSO”) may be granted to employees and non-employees. The board of directors had the authority to determine to whom options will be granted, the number of options, the term and the exercise price.
Options under the Plan were granted for periods of up to ten years and at the fair value of the shares on the date of grant as determined by the board of directors. In general, options become exercisable at a rate of 25% after the first anniversary of the grant and then monthly vesting for an additional three years from date of grant. The term for options is no longer than five years for ISOs for which the grantee owns greater than 10% of the voting power of all classes of stock and no longer than ten years for all other options. The Company issues new shares upon the exercise of options.
2016 Plan
In October 2016, the Company adopted the 2016 Equity Incentive Plan, (the “2016 Plan”). The 2016 Plan was subsequently approved by the Company’s stockholders and became effective on October 19, 2016, immediately before the effective date of the IPO. Following the effectiveness of the 2016 Plan, no additional options will be granted under the 2006 Plan. In addition, to the extent that any awards outstanding or subject to vesting restrictions under the 2006 Plan are subsequently forfeited or terminated for any reason before being exercised or settled, the shares of common stock reserved for issuance pursuant to such awards as of the closing of the IPO will become available for issuance under the 2016 Plan. The remaining shares available for grant under the 2006 Plan became available for issuance under the 2016 Plan upon the closing of the IPO. On the first day of each year, the 2016 Plan authorizes an annual increase of the least of 3,865,000 shares, 5% of outstanding shares on the last day of the immediately preceding fiscal year or an amount as determined by the Company's Board of Directors. As of December 31, 2021, the Company has reserved 7,159,947 shares of common stock for issuance under the 2016 Plan.
Pursuant to the 2016 Plan, stock options, restricted shares, stock units, including restricted stock units and stock appreciation rights may be granted to employees, consultants, and outside directors of the Company. Options granted may be either ISOs or NSOs.
Stock options are governed by stock option agreements between the Company and recipients of stock options. ISOs and NSOs may be granted under the 2016 Plan at an exercise price of not less than 100% of the fair market value of the common stock on the date of grant, determined by the Compensation Committee of the Board of Directors. Options become exercisable and expire as determined by the Compensation Committee, provided that the term of ISOs may not exceed ten years from the date of grant.
Employee Stock Purchase Plan (“ESPP”)
In October 2016, the Company’s Board of Directors and stockholders approved the Employee Stock Purchase Plan (the “ESPP”). Under the ESPP, the Company initially reserved 483,031 shares of common stock for issuance as of its effective date of October 19, 2019. On the first day of each calendar year, the number of shares reserved increases by the least of 966,062 shares, 1.5% of the shares of the Company’s common stock outstanding on the last day of the immediately preceding fiscal year, or an amount as determined by the Company’s Board of Directors. The ESPP allows eligible employees to purchase shares of the Company’s common stock at a discount through payroll deductions of up to 15% of their eligible compensation, subject to any plan limitations. The ESPP provides for 12 month offering periods that each contain two 6 month purchase periods. At the end of each purchase period, employees are able to purchase shares at 85% of the lower of the fair market value of the Company’s common stock on the first trading day of the offering period or on the last day of the purchase period.
As of December 31, 2021, 515,231 shares of common stock have been issued to employees participating in the ESPP and 1,851,538 shares were available for issuance under the ESPP.
The Company used the following assumptions to estimate the fair value of the ESPP offered for the year ended December 31, 2021: expected term of 0.5 – 1 year, volatility of 93.99% - 106.96%, risk-free interest rate of 0.04% - 0.18% and expected dividend yield of zero.
The Company used the following assumptions to estimate the fair value of the ESPP offered for the year ended December 31, 2020: expected term of 0.5 – 1 year, volatility of 68.72% - 75.78%, risk-free interest rate of 0.11% - 0.18% and expected dividend yield of zero.
The Company used the following assumptions to estimate the fair value of the ESPP offered for the year ended December 31, 2019: expected term of 0.5 – 1 year, volatility of 43.61% - 48.05%, risk-free interest rate of 1.60% - 2.35% and expected dividend yield of zero.
Equity Incentive Plan Activity
A summary of share-based awards available for grant under the 2016 Equity Incentive Plan is as follows:
| | | | | |
| Shares Available
for Grant |
| Balance at December 31, 2018 | 4,717,292 | |
| Additional options authorized | 1,218,402 | |
| Awards granted | (649,911) | |
| Awards forfeited | 181,513 | |
| Awards withheld for tax purposes | 60,836 | |
| Balance at December 31, 2019 | 5,528,132 | |
| Additional awards authorized | 1,333,928 | |
| Awards granted | (595,915) | |
| Awards forfeited | 156,623 | |
| Awards withheld for tax purposes | 82,622 | |
| Balance at December 31, 2020 | 6,505,390 | |
| Additional awards authorized | 1,450,967 | |
| Awards granted | (1,289,567) | |
| Awards forfeited | 356,999 | |
| Awards withheld for tax purposes | 135,886 | |
| Balance at December 31, 2021 | 7,159,675 | |
During the year ended December 31, 2021, 1,009,457 restricted stock units ("RSUs") were granted, 285,331 RSUs vested, and 200,019 RSUs were forfeited.
The following table summarizes stock option activity under the 2006 and 2016 Equity Incentive Plans:
| | | | | | | | | | | | | | | | | | | | | | | |
| | | Options Outstanding |
Options Outstanding | | Weighted- Average Exercise Price Per Share | | Weighted- Average Remaining Contractual Life (years) | | Aggregate Intrinsic Value (in thousands) |
| Balance at December 31, 2018 | 2,094,137 | | | $ | 23.20 | | | 7.02 | | $ | 97,976 | |
| Options granted | 20,010 | | | $ | 82.77 | | | | | |
| Options exercised | (540,307) | | | $ | 9.59 | | | | | |
| Options forfeited | (70,593) | | | $ | 54.54 | | | | | |
| Balance at December 31, 2019 | 1,503,247 | | | $ | 27.40 | | | 6.43 | | $ | 62,401 | |
| Options granted | — | | | $ | — | | | | | |
| Options exercised | (868,614) | | | $ | 17.31 | | | | | |
| Options forfeited | (24,752) | | | $ | 66.89 | | | | | |
| Balance at December 31, 2020 | 609,881 | | | $ | 40.18 | | | 6.24 | | 120,163 | |
| Options granted | — | | | $ | — | | | | | |
| Options exercised | (90,939) | | | $ | 31.15 | | | | | |
| Options forfeited | (14,836) | | | $ | 68.81 | | | | | |
| Balance at December 31, 2021 | 504,106 | | | $ | 40.97 | | | 5.20 | | 38,675 | |
| Options exercisable – December 31, 2021 | 483,548 | | | $ | 39.61 | | | 5.15 | | 37,756 | |
| Options vested and expected to vest – December 31, 2021 | 503,884 | | | $ | 40.95 | | | 5.20 | | 38,667 | |
The aggregate intrinsic values of options outstanding, exercisable, vested and expected to vest were calculated as the difference between the exercise price of the options and the closing price of the Company’s common stock.
During the year ended December 31, 2019, the Company granted options with a weighted-average grant date fair value of $38.29 per share. The Company did not grant any options during the year ended December 31, 2020 or during the year ended December 31, 2021 .
The aggregate intrinsic value of options exercised was $10.9 million, $109.2 million and $36.9 million for the years ended December 31, 2021, 2020 and 2019, respectively. The total estimated grant date fair value of options vested during the period was $2.8 million, $4.9 million and $7.8 million for the years ended December 31, 2021, 2020 and 2019, respectively.
The fair value of non-vested restricted stock units (“RSUs”) is based on the Company’s closing stock price on the date of grant. A summary for the year ended December 31, 2021, is as follows:
| | | | | | | | | | | | | | | | | | | | | | | |
| Shares Underlying RSUs | | Weighted Average Grant Date Fair Value | | Weighted Remaining Vesting Period (in years) | | Aggregate Intrinsic Value (in thousands) |
| Non-vested as of December 31, 2019 | 886,030 | | | $ | 77.92 | | | 1.39 | | $ | 60,330 | |
| Granted | 595,915 | | | $ | 104.09 | | | | | |
| Vested | (235,915) | | | $ | 69.01 | | | | | |
| Forfeited | (131,871) | | | $ | 59.85 | | | | | |
| Non-vested as of December 31, 2020 | 1,114,159 | | | $ | 94.25 | | | 1.38 | | $ | 264,290 | |
| Granted | 1,289,567 | | | $ | 98.80 | | | | | |
| Vested | (412,002) | | | $ | 104.94 | | | | | |
| Forfeited | (342,163) | | | $ | 113.95 | | | | | |
| Non-vested as of December 31, 2021 | 1,649,561 | | | $ | 91.01 | | | 1.64 | | $ | 194,137 | |
11. Stock-Based Compensation
Employee Options Valuation
The fair value of employee and director stock options was estimated at the date of grant using the Black-Scholes option pricing model with the weighted average assumptions below.
| | | | | |
| Year Ended
December 31, |
| 2019 |
| Expected term (in years) | 6.1 |
| Expected volatility | 45.0 | % |
| Risk-free interest rate | 2.39 | % |
| Dividend yield | — | % |
The Company did not grant stock options during the years ended December 31, 2021 and December 31, 2020.
Market-based RSU Valuation
The fair value of market based RSUs was estimated at the date of grant using the Monte-Carlo option pricing model with the assumptions below. Additional details on the Company's market based RSUs are included below.
| | | | | |
| Year Ended
December 31, |
| 2020 |
| Expected term (in years) | 0.74 |
| Expected volatility | 63.0 | % |
| Risk-free interest rate | 0.17 | % |
| Dividend yield | — | % |
Fair Value of Common Stock— The Company’s Board of Directors determined the fair value of each share of underlying common stock based on the closing price of the Company’s common stock as reported on the date of grant.
Expected Term—The expected term represents the period that the share-based awards are expected to be outstanding. As the Company has very limited historical information to develop reasonable expectations about future exercise patterns and post-vesting employment termination behavior for its stock-option grants the Company has elected to use the “simplified method” as prescribed by authoritative guidance to compute expected term.
Expected Volatility—Since the Company does not have sufficient trading history for its common stock, the expected volatility was estimated based on the average volatility for comparable publicly traded companies over a period equal to the expected term of the stock option grants. When selecting comparable publicly traded companies in a similar industry on which it has based its expected stock price volatility, the Company selected companies with comparable characteristics to it, including enterprise value, risk profiles, position within the industry, and with historical share price information sufficient to meet the expected life of the stock-based awards. The Company will continue to apply this process until a sufficient amount of historical information regarding the volatility of its own stock price becomes available.
Risk-Free Interest Rate—The risk-free interest rate is based on the U.S. Treasury yield curve in effect on the date of grant for zero coupon U.S. Treasury notes with maturities approximately equal to expected term of the option award.
Expected Dividend Yield—The Company has never paid dividends on its common stock and has no plans to pay dividends on its common stock. Therefore, the Company used an expected dividend yield of zero.
In addition to the assumptions used in the Black-Scholes option-pricing model, the Company also estimates a forfeiture rate to calculate the stock-based compensation for the Company’s equity awards. The Company will continue to use judgment in evaluating the expected volatility, expected terms and forfeiture rates utilized for the Company’s stock-based compensation calculations on a prospective basis.
The following table summarizes the total stock-based compensation expense included in the statements of operations and comprehensive loss for all periods presented (in thousands):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31 |
| 2021 | | 2020 | | 2019 |
| Cost of revenue | $ | 1,896 | | | $ | 27 | | | $ | 658 | |
| Research and development | 5,565 | | | 7,727 | | | 4,462 | |
| Selling, general and administrative | 47,066 | | | 33,761 | | | 21,121 | |
| Total stock-based compensation expense | $ | 54,527 | | | $ | 41,515 | | | $ | 26,241 | |
As of December 31, 2021, there was total unamortized compensation costs of $0.8 million, net of estimated forfeitures, related to unvested stock options, which the Company expects to recognize over a period of approximately 0.4 years and $113.5 million, net of estimated forfeitures, related to unrecognized RSU expense, which the Company expects to recognize over a period of 2.4 years, and $3.1 million unrecognized ESPP expense, which the Company will recognize over 0.9 years.
Performance based RSUs (“PRSU”) and Market-based RSUs
The Company grants PRSUs to key executives of the Company. PRSUs can be earned in accordance with the performance equity program for each respective grant
2019 Awards
In February 2019, the company granted PRSU's (“2019 awards”) to be earned based on the compound annual growth rate (“CAGR”) of fiscal year 2020's revenue compared to fiscal year 2018's revenue.
Due to the impact of the COVID-19 pandemic, management determined that the Company’s achievement of its performance targets described above, was not probable in the first quarter of fiscal year 2020. PRSU expense of $4.8 million recognized in fiscal year 2019 related to the 2019 awards was reversed in the first quarter of fiscal year 2020.
On June 19, 2020, the Company modified the terms of the 2019 awards to vest based on the Company’s average stock price for the 60 days preceding January 1, 2021. The modification impacted all active recipients of the 2019 awards, a total of ten recipients. The total incremental compensation cost resulting from the modification of $13.6 million was recognized ratably through March 31, 2021. The Company recognized $2.2 million of compensation cost for the year ended December 31, 2021 in connection with the 2019 awards.
February 2020 Awards
In February 2020, the company granted PRSU's (“February 2020 awards”) for fiscal year 2022's annual unit volume CAGR compared to fiscal year 2019's annual unit volume CAGR, measuring a minimum performance threshold of 19.7% to earn 50% of target, and a maximum threshold of 29% achieved to earn 200% of target. A total of 133,834 PRSU shares were granted with grant date fair value of $11.0 million. The 2020 awards also include a service-based component.
Compensation cost in connection with the probable number of shares that vested was recognized ratably through March 31, 2021. During the year ended December 31, 2021, the Company determined that it was probable that the February 2020 Awards would vest and recognized $0.8 million of compensation cost in connection with the February 2020 awards.
January 2021 Awards
In January 2021, the Company granted PRSUs (“January 2021 awards”) for fiscal year 2021's annual consolidated revenue compared to fiscal year 2020's annual consolidated revenue, measuring a performance threshold of 10.0% to earn 100% of target. A total of 53,862 PRSU shares were granted with a grant date fair value of $13.9 million. The January 2021 awards also include a service-based component.
Compensation cost in connection with the probable number of shares that will vest will be recognized ratably through March 31, 2022. During the year ended December 31, 2021, the Company determined that it was probable that the January 2021 Awards would vest and recognized $9.9 million of compensation cost in connection with the January 2021 awards.
February 2021 Awards
In February 2021, the Company granted PRSU's (“February 2021 awards”) for fiscal year 2023's annual unit volume Compound Annual Growth Rate (“CAGR”) compared to fiscal year 2020's annual unit volume CAGR, measuring a minimum performance threshold of 19.7% to earn 50% of target, and a maximum threshold of 29% achieved to earn 200% of target. A total of 112,872 PRSU shares were granted with grant date fair value of $17.3 million. The February 2021 awards also include a service-based component.
Compensation cost in connection with the probable number of shares that will vest will be recognized ratably through March 31, 2024. During the year ended December 31, 2021, the Company determined that it was probable that the February 2021 Awards would vest and recognized $2.4 million of compensation cost in connection with the February 2021 awards.
Non employee Stock-Based Compensation
On July 3, 2020, the Company’s Chief Financial Officer (“CFO”) resigned and entered into a Consulting and Professional Services Agreement (“CPSA”) with the Company to provide consulting services through July 2, 2021. Pursuant to the original terms of the awards, the CFO will continue to vest in outstanding awards as long as services are provided to the Company under the CPSA as a non-employee consultant. In accordance with ASC 718, the Company recognized expense related to all awards expected to vest over the duration of the CPSA in 2020 as an equity-based severance cost as the consulting services are not substantive. Total expense related to non-employee stock-based compensation recognized for the year ended December 31, 2020, was $1.8 million.
On January 12, 2021, the Company's Chief Executive Officer (“CEO”) resigned and entered into a CPSA with the Company. Pursuant to the original terms of the awards, the CEO will continue to vest in outstanding awards as long as services are provided to the Company under the CPSA as a non-employee consultant or a member of the Company's Board of Directors. In accordance with ASC 718, the Company recognized expense related to all awards expected to vest over the duration of the CPSA in the three months ended March 31, 2021, as an equity-based severance cost as the consulting services are not substantive. Total expense related to non-employee stock-based compensation recognized for the year ended December 31, 2021 was $5.4 million.
12. Net Loss Per Common Share
As the Company had net losses for the years ended December 31, 2021, 2020 and 2019, all potential common shares were determined to be anti-dilutive. The following table sets forth the computation of the basic and diluted net loss per share during the years ended December 31, 2021, 2020 and 2019 (in thousands, except share and per share data):
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Numerator: | | | | | |
| Net loss | $ | (101,361) | | | $ | (43,830) | | | $ | (54,568) | |
| Denominator: | | | | | |
| Weighted-average shares used to compute net loss per common share, basic and diluted | 29,331,010 | | | 27,754,404 | | | 25,265,918 | |
| Net loss per common share, basic and diluted | $ | (3.46) | | | $ | (1.58) | | | $ | (2.16) | |
The following outstanding shares of potentially dilutive securities have been excluded from diluted net loss per common share for the years ended December 31, 2021, 2020 and 2019 because their inclusion would be anti-dilutive:
| | | | | | | | | | | | | | | | | |
| Year Ended December 31, |
| 2021 | | 2020 | | 2019 |
| Options to purchase common stock | 504,106 | | | 609,881 | | | 1,503,247 | |
| RSUs issued and unvested | 1,649,561 | | | 1,114,159 | | | 886,030 | |
| Total | 2,153,667 | | | 1,724,040 | | | 2,389,277 | |
13. Subsequent Events
Novitas Announcement
In November 2021, CMS published its Calendar Year 2022 Medicare Physician Fee Schedule Final Rule (the “Final Rule”). In the Final Rule, CMS did not finalize national pricing for the extended external ECG patch, medical magnetic tape recorder (SD339) supply, and ruled to contractor pricing for CPT codes 93241, 93243, 93245 and 93247. The Company has been working with Medicare Administrative Contractors (MACs”) to establish pricing for these codes.
On January 10, 2022, Novitas Solutions, the MAC which covers the region where the Company's Independent Diagnostic Testing Facility in Houston, Texas where almost all Medicare services for Zio XT are processed, published reimbursement rates for CPT codes 93243 and 93247 for the Houston jurisdiction to $223 and $233, respectively. These updated rates are retroactive to January 1, 2022.
Leases
In December 2021, the Company entered into a lease for 44,616 rentable square feet in Deerfield Illinois (“Deerfield Lease”). The Deerfield lease commenced in January 2022, the Company will recognize a related right of use asset and obligation in its Consolidated Financial Statements for the quarter ending March 31, 2022. The amount of such asset and obligation has not yet been determined.
In February 2022, the Company's Board of Directors approved a decision to pursue reducing its leased space for its headquarters in San Francisco, California. The Company plans to consolidate operations and will begin marketing the excess space for sublease by the end of the first quarter of 2022. Depending on the outcome of marketing the sublease, the Company believes these decisions could result in an impairment of the right of use asset for the San Francisco office lease and is assessing the impact to its Consolidated Financial Statements for the quarter ending March 31, 2022.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to provide reasonable assurance that information required to be disclosed by us in reports that we file or submit under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our Chief Executive Officer (“CEO”) (principal executive officer) and Chief Financial Officer (“CFO”) (principal financial officer), as appropriate to allow for timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures.
As required by Rule 13a-15(b) under the Exchange Act, our management, including our CEO and CFO, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rule 13a-15(e) under the Exchange Act) as of the end of the period covered by this report. Based on that evaluation, our CEO and our CFO have concluded that as of December 31, 2021 our disclosure controls and procedures were not effective at the reasonable assurance level because of the material weakness in internal controls previously disclosed and discussed below. Notwithstanding the material weakness, our management, including our CEO and CFO, has concluded that our consolidated financial statements, included in the 2021 Annual Report on Form 10-K, fairly present, in all material respects, our financial condition, results of operations and cash flows for the periods presented in conformity with generally accepted accounting principles.
In light of the material weakness described below, the Company performed additional analysis and other post-closing procedures to determine its consolidated financial statements are prepared in accordance with generally accepted accounting principles. Accordingly, management concluded that the financial statements included in this report fairly present in all material respects the Company’s financial condition, results of operations and cash flows for the periods presented.
Management’s Annual Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Rule 13a-15(f) of the Exchange Act. Our management has assessed the effectiveness of our internal control over financial reporting as of December 31, 2021 using the criteria described in Internal Control—Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO"). A material weakness is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
We did not design or maintain an effective control environment commensurate with our financial reporting requirements. Given the rapid growth in the size and complexity of the business, we failed to maintain a sufficient number of professionals with an appropriate level of accounting and internal control knowledge, training and experience to appropriately analyze, record and disclose accounting matters timely and accurately. This material weakness contributed to additional material weaknesses, previously disclosed and remediated. In aggregate, these material weaknesses (including the previously remediated material weaknesses) resulted in the misstatement of our revenues, revenue reserves, bad debt expense, property and equipment, research and development expense and related financial disclosures, and in the revision of the Company’s consolidated financial statements for the years ended December 31, 2017, December 31, 2018, and each interim period therein as well as the quarters ended March 31, 2019, June 30, 2019, and September 30, 2019. Additionally, this material weakness could result in a misstatement of account balances or disclosures that would result in a material misstatement to the annual or interim consolidated financial statements that would not be prevented or detected. Because of these deficiencies, and in spite of our remediation progress, described below, we concluded that the Company's internal control over financial reporting was not effective as of December 31, 2021.
The effectiveness of the Company’s internal control over financial reporting as of December 31, 2021 has been audited by PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which is included in Part II, Item 8 of this Annual Report on Form 10-K.
Remediation Plan Activities
As it relates to the material weakness that exists as of December 31, 2021, we performed the following actions:
•Increased the depth and experience of our Finance organization by increasing the number of management and staff members, expanding our technical experience in accounting, auditing and reporting matters, and performing internal controls training for management and staff members.
•Hired two Internal Audit Directors (one with broad Finance experience and one with relevant control experience over information technology environments). These individuals are focused on the development, maintenance and monitoring of our overall control environment and system of key internal controls over financial reporting.
•Hired several IT compliance experts including a Sr. Director of Cybersecurity with 20+ years of experience in cybersecurity, a Healthcare IT Compliance director with 15 years’ experience in technology risk management compliance and assurance experience, and a senior IT compliance analyst with 20 years’ experience in information security, IT governance and IT asset management.
These investments in resources have significantly improved the stability of our accounting organization and our IT compliance expertise. While significant progress has been made in response to the material weakness in the control environment, key remediation plan activities include additional training programs, automation efforts, and hiring of additional resources in support of certain control activities in the IT Compliance area. Additionally, time is needed to demonstrate sustainability as it relates to our internal control over financial reporting and improvements made to our complement of resources, including demonstrating sustained operating effectiveness of our internal controls.
Changes in Internal Control Over Financial Reporting
There have been no changes in internal control over financial reporting during the quarter ended December 31, 2021, other than as described above, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Internal control over financial reporting has inherent limitations. Internal control over financial reporting is a process that involves human diligence and compliance and is subject to lapses in judgment and breakdowns resulting from human failures. Internal control over financial reporting can also be circumvented by collusion or improper management override of the controls. Projections of any evaluation of controls effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or deterioration in the degree of compliance with policies or procedures.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this item is incorporated by reference from our definitive Proxy Statement to be filed with the SEC in connection with our 2022 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2021.
Item 11. Executive Compensation.
The information required by this item is incorporated by reference from our definitive Proxy Statement to be filed with the SEC in connection with our 2022 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2021.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this item is incorporated by reference from our definitive Proxy Statement to be filed with the SEC in connection with our 2022 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2021.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
The information required by this item is incorporated by reference from our definitive Proxy Statement to be filed with the SEC in connection with our 2022 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2021.
Item 14. Principal Accounting Fees and Services.
Our independent registered public accounting firm is PricewaterhouseCoopers LLP, San Jose, CA, PCAOB ID: 238.
The information required by this item is incorporated by reference from our definitive Proxy Statement to be filed with the SEC in connection with our 2022 Annual Meeting of Stockholders within 120 days after the end of the fiscal year ended December 31, 2021.
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a)List the following documents filed as a part of this Annual Report on Form 10-K:
i.All financial statements;
ii.Those financial statement schedules required to be filed by Item 8 of this form, and by paragraph (b) below. All financial statement schedules are omitted because they are not applicable or the amounts are immaterial or the required information is presented in the consolidated financial statements and notes thereto in Part II, Item 8 above.
iii.Those exhibits required by Item 601 of Regulation S-K (§ 229.601 of this chapter) and by paragraph (b) below. Identify in the list each management contract or compensatory plan or arrangement required to be filed as an exhibit to this form pursuant to Item 15(b) of this report.
(b)Registrants shall file, as exhibits to this form, the exhibits required by Item 601 of Regulation S-K (§ 229.601 of this chapter).
(c)Registrants shall file, as financial statement schedules to this form, the financial statements required by Regulation S-X (17 CFR 210) which are excluded from the annual report to shareholders by Rule 14a-3(b) including (1) separate financial statements of subsidiaries not consolidated and fifty percent or less owned persons; (2) separate financial statements of affiliates whose securities are pledged as collateral; and (3) schedules.
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| Exhibit Index |
| | | | Incorporated by Reference |
Exhibit Number | | Exhibit Title | | Form | | File No. | | Exhibit | | Filing Date |
| 3.1 | | | | 8-K | | 001-37918 | | 3.1 | | October 26, 2016 |
| 3.2 | | | | 8-K | | 001-37918 | | 3.2 | | October 26, 2016 |
| 4.1 | | | | S-1 | | 333-213773 | | 4.1 | | September 23, 2016 |
| 4.2 | | | | S-1/A | | 333-213773 | | 4.2 | | October 7, 2016 |
| 4.3 | | | | 10-K | | 001-37918 | | 4.3 | | March 2, 2020 |
| 4.4 | | | | 10-K | | 001-37918 | | 4.4 | | March 2, 2020 |
| 4.8 | | | | S-1 | | 333-213773 | | 4.8 | | September 23, 2016 |
| 10.1+ | | | | S-1 | | 333-213773 | | 10.1 | | September 23, 2016 |
| 10.2+ | | | | S-1 | | 333-213773 | | 10.2 | | September 23, 2016 |
| 10.3+ | | | | S-1/A | | 333-213773 | | 10.3 | | October 7, 2016 |
| 10.4+ | | | | S-1/A | | 333-213773 | | 10.4 | | October 7, 2016 |
| 10.5+ | | | | S-1/A | | 333-213773 | | 10.5 | | October 7, 2016 |
| 10.6 | | | | S-1 | | 333-213773 | | 10.6 | | September 23, 2016 |
| 10.7 | | | | S-1 | | 333-213773 | | 10.7 | | September 23, 2016 |
| 10.8 | | | | S-1 | | 333-213773 | | 10.8 | | September 23, 2016 |
| 10.9 | | | | S-1/A | | 333-213773 | | 10.9 | | October 7, 2016 |
| 10.10 | | | | S-1 | | 333-213773 | | 10.10 | | September 23, 2016 |
| 10.11 | | | | S-1 | | 333-213773 | | 10.11 | | September 23, 2016 |
| 10.12 | | | | S-1 | | 333-213773 | | 10.12 | | September 23, 2016 |
| 10.13 | | | | S-1 | | 333-213773 | | 10.13 | | September 23, 2016 |
| 10.14 | | | | S-1 | | 333-213773 | | 10.14 | | September 23, 2016 |
| 10.15 | | | | S-1 | | 333-213773 | | 10.15 | | September 23, 2016 |
| 10.16 | | | | S-1/A | | 333-213773 | | 10.16 | | October 7, 2016 |
| 10.17 | | | | S-1/A | | 333-213773 | | 10.17 | | October 7, 2016 |
| 10.18 | | | | S-1 | | 333-213773 | | 10.18 | | September 23, 2016 |
| 10.19 | | | | S-1 | | 333-213773 | | 10.19 | | September 23, 2016 |
| 10.20± | | | | S-1 | | 333-213773 | | 10.20 | | September 23, 2016 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 10.21 | | | | S-1/A | | 333-213773 | | 10.21 | | October 7, 2016 |
| 10.22 | | | | S-1 | | 333-213773 | | 10.22 | | September 23, 2016 |
| 10.23 | | | | S-1 | | 333-213773 | | 10.23 | | September 23, 2016 |
| 10.24 | | | | S-1/A | | 333-213773 | | 10.24 | | October 7, 2016 |
| 10.25+ | | | | S-1 | | 333-213773 | | 10.25 | | September 23, 2016 |
| 10.26+ | | | | S-1 | | 333-213773 | | 10.26 | | September 23, 2016 |
| 10.27+ | | | | S-1 | | 333-213773 | | 10.27 | | September 23, 2016 |
| 10.28+ | | | | S-1 | | 333-213773 | | 10.28 | | September 23, 2016 |
| 10.29 | | | | 10-Q | | 001-37918 | | 10.29 | | November 14, 2017 |
| 10.30 | | | | S-1 | | 333-213773 | | 10.30 | | September 23, 2016 |
| 10.31 | | | | S-1/A | | 333-213773 | | 10.31 | | October 7, 2016 |
| 10.32 | | | | 10-Q | | 001-37918 | | 10.32 | | August 7, 2017 |
| 10.33 | | | | 10-Q | | 001-37918 | | 10.33 | | August 3, 2018 |
| 10.34 | | | | 10-Q | | 001-37918 | | 10.34 | | August 3, 2018 |
| 10.35 | | | | | | | | | | |
| 10.36 | | | | 8-K | | 001-37918 | | 10.1 | | October 29, 2018 |
| 10.37 | | | | 10-Q | | 001-37918 | | 10.37 | | December 23, 2019 |
| 10.38 | | | | 10-Q | | 001-37918 | | 10.38 | | December 23, 2019 |
| 10.39± | | | | 10-Q | | 001-37918 | | 10.39 | | December 23, 2019 |
| 10.40 | | | | 10-Q | | 001-37918 | | 10.40 | | August 7, 2020 |
| 10.41 | | | | 8-K | | 001-37918 | | 10.41 | | August 21, 2020 |
| 10.42 | | | | 10-Q | | 001-37918 | | 10.42 | | May 5, 2021 |
| 10.43± | | | | 10-K | | 001-37918 | | 10.43 | | February 25, 2022 |
| 10.44± | | | | 10-K | | 001-37918 | | 10.44 | | February 25, 2022 |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| 21.1 | | | | S-1 | | 333-213773 | | 21.1 | | September 23, 2016 |
| 23.1 | | | | | | | | | | |
| 31.1† | | | | | | | | | | |
| 31.2† | | | | | | | | | | |
| 32.1† | | | | | | | | | | |
| 101.INS | | XBRL Instance Document | | | | | | | | |
| 101.SCH | | XBRL Taxonomy Extension Schema Document | | | | | | | | |
| 101.CAL | | XBRL Taxonomy Extension Calculation Linkbase Document | | | | | | | | |
| 101.DEF | | XBRL Taxonomy Extension Definition Linkbase Document | | | | | | | | |
| 101.LAB | | XBRL Taxonomy Extension Label Linkbase Document | | | | | | | | |
| 101.PRE | | XBRL Taxonomy Extension Presentation Linkbase Document | | | | | | | | |
________________________________________________________________________________________________
†The certifications attached as Exhibit 31.1, 31.2 and 32.1 that accompany this Annual Report on Form 10-K, are deemed furnished and not filed with the Securities and Exchange Commission and are not to be incorporated by reference into any filing of iRhythm Technologies, Inc. under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended, whether made before or after the date of this Annual Report on Form 10-K, irrespective of any general incorporation language contained in such filing.
+ Indicates management contract or compensatory plan.
± Confidential treatment has been requested for portions of this exhibit. These portions have been omitted and have been filed
separately with the Securities and Exchange Commission.
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| | | | | | | | | | | |
| | iRhythm Technologies, Inc. |
| | | |
| Date: February 28, 2022 | | By: | /s/ Quentin S. Blackford |
| | | Quentin S. Blackford
President and Chief Executive Officer
(Principal Executive Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
| | | | | | | | | | | | | | |
| Name | | Title | | Date |
| | | | |
| /s/ Quentin S. Blackford | | President, Chief Executive Officer and Director (Principal Executive Officer) | | February 28, 2022 |
| Quentin S. Blackford | | | | |
| | | | |
| /s/ Douglas J. Devine | | Chief Financial Officer and Chief Operating Officer
(Principal Financial Officer) | | February 28, 2022 |
| Douglas J. Devine | | | | |
| | | | |
| /s/ Bruce G. Bodaken | | Director | | February 28, 2022 |
| Bruce G. Bodaken | | | | |
| | | | |
| /s/ Ralph Snyderman M.D. | | Director | | February 28, 2022 |
| Ralph Snyderman M.D. | | | | |
| | | | |
| /s/ C. Noel Bairey Merz, M.D. | | Director | | February 28, 2022 |
| C. Noel Bairey Merz, M.D. | | | | |
| | | | |
| /s/ Mark J. Rubash | | Director | | February 28, 2022 |
| Mark J. Rubash | | | | |
| | | | |
| /s/ Karen Ling | | Director | | February 28, 2022 |
| Karen Ling | | | | |
| | | | |
| /s/ Renee Budig | | Director | | February 28, 2022 |
| Renee Budig | | | | |
| | | | |
| /s/ Kevin M. King | | Director | | February 28, 2022 |
| Kevin M. King | | | | |
| | | | |
| /s/ Abhijit Y. Talwalkar | | Director and Chairman of the Board | | February 28, 2022 |
| Abhijit Y. Talwalkar | | | | |