Use these links to rapidly review the document
TABLE OF CONTENTS
VERACYTE, INC. Index to Audited Financial Statements Years Ended December 31, 2011 and 2012
VERACYTE, INC. Index to Unaudited Interim Condensed Financial Statements
Confidential Draft Submission No. 2 as confidentially submitted to the Securities and Exchange Commission on August 30, 2013. This draft registration statement has not been publicly filed with the Securities and Exchange Commission and all information herein remains strictly confidential.
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form S-1
REGISTRATION STATEMENT
Under
THE SECURITIES ACT OF 1933
VERACYTE, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 8071 | 20-5455398 | ||
| (State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
7000 Shoreline Court, Suite 250
South San Francisco, California 94080
(650) 243-6300
(Address, including zip code, and telephone number, including area code, of registrant's principal executive offices)
Bonnie H. Anderson
President and Chief Executive Officer
7000 Shoreline Court, Suite 250
South San Francisco, California 94080
(650) 243-6300
(Name, address, including zip code, and telephone number, including area code, of agent for service)
| Copies to: | ||||
Stanton D. Wong Gabriella A. Lombardi Heidi E. Mayon Pillsbury Winthrop Shaw Pittman LLP Four Embarcadero Center, 22nd Floor San Francisco, California 94111 |
Shelly D. Guyer Chief Financial Officer Veracyte, Inc. 7000 Shoreline Court, Suite 250 South San Francisco, California 94080 |
William H. Hinman Simpson Thacher & Bartlett LLP 2475 Hanover Street Palo Alto, California 94304 |
||
Approximate date of commencement of proposed sale to the public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. o
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer" and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer ý (Do not check if a smaller reporting company) |
Smaller reporting company o |
CALCULATION OF REGISTRATION FEE
|
||||
| Title of each class of securities to be registered |
Proposed maximum aggregate offering price(1)(2) |
Amount of registration fee |
||
|---|---|---|---|---|
Common Stock, par value $0.001 per share |
||||
|
||||
- (1)
- Estimated
solely for the purpose of calculating the registration fee pursuant to Rule 457(o) under the Securities Act of 1933.
- (2)
- Includes the offering price of shares that the underwriters have the option to purchase to cover over-allotments, if any.
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and we are not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
PROSPECTUS (Subject to Completion)
Issued , 2013
Shares

COMMON STOCK
Veracyte, Inc. is offering shares of its common stock. This is our initial public offering and no public market currently exists for our shares. We anticipate that the initial public offering price of our common stock will be between $ and $ per share.
We intend to apply to list our common stock on The NASDAQ Global Market under the symbol "VCYT".
We are an "emerging growth company" under applicable Securities and Exchange Commission rules and will be subject to reduced public company reporting requirements for this prospectus and future filings.
Investing in our common stock involves risks. Please see "Risk Factors" beginning on page 10.
PRICE $ A SHARE
| |
Price to Public |
Underwriting Discounts and Commissions |
Proceeds to Company |
|||
|---|---|---|---|---|---|---|
Per Share |
$ | $ | $ | |||
Total |
$ | $ | $ |
We have granted the underwriters the right to purchase up to an additional shares of common stock to cover over-allotments.
The Securities and Exchange Commission and state securities regulators have not approved or disapproved these securities, or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriters expect to deliver the shares of common stock to purchasers on , 2013.
| MORGAN STANLEY | LEERINK SWANN |
| WILLIAM BLAIR | COWEN AND COMPANY |
, 2013

You should rely only on the information contained in this prospectus and any free writing prospectus we have prepared. We have not, and the underwriters have not, authorized anyone to provide you with information or make any representations different from or in addition to those contained in this prospectus or any free writing prospectus we have prepared. We and the underwriters take no responsibility for and can provide no assurance as to the reliability of any other information that others may give you. We are offering to sell shares of common stock and seeking offers to buy shares of our common stock only in jurisdictions where offers and sales are permitted. The information contained in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of the common stock. Our business, financial condition, results of operations and prospectus may have changed since that date.
Until , 2013 (25 days after commencement of this offering), all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers' obligation to deliver a prospectus when acting as underwriters and with respect to their unsold allotments or subscriptions.
For investors outside the United States: We have not, and the underwriters have not, done anything that would permit this offering or possession or distribution of this prospectus or any free writing prospectus we may provide to you in connection with this offering in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about and to observe any restrictions relating to this offering and the distribution of this prospectus and any such free writing prospectus outside of the United States.
This summary highlights information contained in greater detail elsewhere in this prospectus. This summary is not complete and does not contain all of the information you should consider in making your investment decision. You should read the entire prospectus carefully before making an investment in our common stock. You should carefully consider, among other things, our financial statements and the related notes and the sections entitled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" included elsewhere in this prospectus.
Overview
We are a diagnostics company pioneering the field of molecular cytology to improve patient outcomes and lower healthcare costs. We specifically target diseases that often require invasive procedures for an accurate diagnosis – diseases where many healthy patients undergo costly interventions that ultimately prove unnecessary. We improve the accuracy of diagnosis at an earlier stage of patient care by deriving clinically actionable genomic information from cytology samples collected in an outpatient setting. Our first commercial solution, the Afirma Thyroid FNA Analysis, includes as its centerpiece our Gene Expression Classifier, which we refer to as the GEC. The GEC helps physicians reduce the number of unnecessary surgeries by employing a proprietary 142-gene signature to preoperatively determine whether thyroid nodules previously classified by cytopathology as indeterminate can be reclassified as benign. We have demonstrated the clinical utility and cost effectiveness of the GEC in studies published in peer-reviewed journals and established the clinical validity of the GEC in a study published in The New England Journal of Medicine in 2012.
Since we commercially launched Afirma in January 2011, we have processed over 50,000 fine needle aspiration, or FNA, samples for evaluation using Afirma and performed more than 10,000 GECs to resolve indeterminate cytopathology results. We have obtained positive coverage decisions from Aetna, Humana, Medicare and UnitedHealthcare. Collectively, these payers represent more than 100 million covered lives. Additionally, we have entered into a global co-promotion agreement with Genzyme Corporation, a subsidiary of Sanofi. Our revenue has increased from $2.6 million in 2011 to $17.1 million for the trailing twelve months ending June 30, 2013.
For decades, pathologists have diagnosed complex diseases by evaluating cells taken from a surgical tissue sample. More recently, molecular diagnostic tests that analyze the genomic material in these samples have emerged as an important complement to surgical pathology by predicting outcomes and guiding treatment decisions. Both approaches, however, typically require relatively large quantities of tissue that must be obtained through an invasive surgical procedure. Cytopathology, which relies on small samples such as FNAs collected in an outpatient setting, is often the first step in the diagnostic process because it offers a minimally invasive and cost-effective alternative to surgery. However, cytology samples tend to be small and non-uniform, which contributes to a relatively high rate of diagnostic ambiguity and results in many patients undergoing surgery to obtain an accurate diagnosis. Molecular diagnostics broadly used today are not designed to reduce this ambiguity.
We are building our molecular cytology business by developing molecular diagnostics that yield clinically actionable genomic information from cytology samples, as opposed to surgical tissue samples. Molecular cytology identifies genomic signatures from cytology samples to inform clinical decisions pre-operatively. We believe molecular cytology has the potential to improve patient care while simultaneously lowering costs to the healthcare system in a broad range of areas including thyroid, pulmonology, dermatology and reproductive endocrinology. Based on our internal analysis and third-party data, we believe molecular diagnostic solutions to address these markets could represent an approximately $4.0 billion opportunity.
Our strategy is to focus on diseases where a large number of patients undergo invasive and costly diagnostic procedures that could be avoided with a more accurate diagnosis from a cytology sample taken pre-operatively. In prioritizing our opportunities, we develop a detailed understanding of the unmet
1
clinical need and the shortcomings of the current standard of care. We define the precise clinical question in these diseases that, if informed by genomic information, would alter the standard of care in a way that improves patient outcomes while reducing costs in both the short- and long-term. Only then do we deploy our scientific expertise in biomarker discovery and algorithm development to derive a genomic signature that provides meaningful diagnostic information.
We developed our first commercial offering, Afirma, to address a significant unmet need in thyroid nodule diagnosis. Thyroid nodules, or bumps under the skin of the neck around the thyroid gland, are usually benign; however, patients with nodules are generally referred to an endocrinologist for evaluation. Endocrinologists typically collect cells from the nodule for cytopathology with an FNA and send these samples to a cytopathologist for analysis. Approximately 525,000 thyroid FNAs were performed in the United States in 2011. Typically, 15% to 30% of FNAs yield indeterminate results, meaning they cannot be diagnosed as definitively benign or malignant by cytopathology alone. Because the risk of malignancy is approximately 25% for an indeterminate diagnosis, clinical practice guidelines have historically recommended patients with indeterminate cytopathology results undergo surgery to remove part or all of their thyroid to obtain an accurate pathology diagnosis. However, in 70% to 80% of these cases, the thyroid nodule proves to be benign for cancer. We estimate the average cost of this surgery to be $15,000, and surgery can result in complications and leave a patient in need of hormone replacement therapy for life. We believe Afirma, if fully adopted, could result in over $500 million in direct cost savings to the healthcare system over five years.
Afirma is a comprehensive solution that consists of cytopathology and the GEC. The GEC reduces the number of unnecessary diagnostic surgeries by analyzing the genomic signature of FNA samples judged to be indeterminate by cytopathology and reclassifies about 50% of those nodules to a benign diagnosis. In The New England Journal of Medicine clinical validation study for the GEC, the study authors concluded that the GEC could be useful to physicians in making important patient care decisions, such as recommending watchful waiting in lieu of diagnostic surgery for patients who receive a GEC benign result following indeterminate cytopathology findings. A subsequent clinical utility study published in Thyroid covered 368 patients from 51 different endocrinologists. Each of these patients had both a cytopathology indeterminate result and a GEC benign result. The study found that physicians recommended surgery in only 7.6% of these cases, representing a 90% reduction in surgeries when compared to the historical average for patients with cytopathology indeterminate results alone. We believe the GEC is currently the only diagnostic test that meets the criteria of the National Comprehensive Cancer Network, or NCCN, for safely monitoring patients with indeterminate cytopathology results in lieu of surgery.
In addition to thyroid cancer, there are many other complex diseases in which cytology samples play a critical role in clinical decision making. As with thyroid nodule diagnosis, inherent ambiguity in evaluation of cytopathology samples often results in unnecessary costs and procedures that would be avoidable if a molecular diagnostic test could refine diagnoses reached by cytopathology alone. We are currently developing the Afirma Malignant GEC test for rare forms of thyroid cancer or metastases to the thyroid that is intended to better inform surgical strategy. We are also in late biomarker discovery in interstitial lung disease, a group of lung diseases affecting the tissue and space around the microscopic air sacs of the lungs that are difficult to diagnose prior to surgery. Specifically, we intend to improve the accuracy of diagnosis of idiopathic pulmonary fibrosis, one of the more progressive, often fatal, interstitial lung diseases, and to provide critical information to physicians and patients as they decide whether to pursue potentially lifesaving treatments or participate in clinical studies.
Company Highlights
- •
- Clinically validated solution with demonstrated utility and significant payer adoption. We have demonstrated the benefits of Afirma in multiple clinical studies that have been published in leading peer-reviewed publications. As a result of Afirma's demonstrated utility and our managed care
2
- •
- Large, underserved specialty markets. Approximately
525,000 thyroid FNAs were performed in the United States in 2011, by an estimated 3,500 endocrinologists whom we believe specialize in thyroid disease. We estimate the thyroid nodule diagnostic market
to be approximately $500 million per year in the United States and approximately $300 million outside of the United States. We believe we can effectively market Afirma with a small
specialty sales force, in part because Afirma represents a significant innovation in the underserved thyroid cancer diagnostic market. Because Afirma represents a significant innovation for this
underserved and relatively concentrated base of physicians, we believe we can effectively market Afirma with a small specialty sales force.
- •
- Turnkey solution that drives customer retention. We market
Afirma as a comprehensive offering that combines cytopathology with the GEC. Afirma simplifies the diagnostic process for physicians while optimizing utilization of our molecular diagnostic to
maximize clinical benefits for patients and cost savings for payers. We believe these characteristics are key drivers of a physician's decision to convert their existing FNA protocol to Afirma. Since
we commercially launched Afirma in 2011, more than 80% of physicians who ordered five or more Afirma tests in 2011 remain customers today. As a result, our targeted sales force devotes fewer resources
to maintaining business with our existing base of physicians and instead focuses on driving adoption of Afirma among new customers. We intend to duplicate this model with solutions we develop for
other diseases.
- •
- Demonstrated core competencies leverageable across multiple
products. We successfully advanced Afirma from the concept stage in early 2008 to a commercial product with broad physician and payer
adoption today. We believe our expertise in disease selection, genomic signature discovery, clinical study design, commercialization and managed care, all of which we have demonstrated with the
success of Afirma, will allow us to establish molecular cytology solutions in a range of diseases.
- •
- Product pipeline with multiple high-value solutions. We believe we are well-positioned to introduce multiple new products in the near- and medium-term. In the second quarter of 2014, we plan to introduce the Afirma Malignant GEC, our first product line extension for Afirma, to guide surgical strategy for the treatment of medullary thyroid cancer and other rare and metastatic forms of thyroid cancer. We plan to commercialize our first product for interstitial lung disease in 2016 and believe this product will serve as the foundational application to expand our molecular cytology platform to the treatment of lung disease.
expertise, we have obtained positive coverage decisions from a range of payers, including Aetna, Humana, Medicare and UnitedHealthcare.
Our Solution
We are pioneering the field of molecular cytology by developing molecular diagnostics that yield clinically actionable genomic information from cytology samples. Molecular cytology combines the screening benefits of a minimally invasive cytology sample with genomic information to inform disease diagnosis or treatment decisions pre-operatively. We focus on diseases in which a large number of patients undergo invasive and costly diagnostic procedures that could be avoided with a more accurate diagnosis from a cytology sample taken prior to surgery. Positioning our test as an alternative to an invasive procedure allows us to efficiently validate the accuracy of our diagnostic test by comparing our test results to those obtained using the more invasive approach. Armed with clinical data that supports the use of molecular cytology in lieu of a more invasive or costly procedure, we believe we are well-positioned to support clinical studies that demonstrate how our products change the standard of care, improve patient outcomes and reduce costs.
In contrast to molecular diagnostics developed for surgical tissue, we have developed the expertise to solve many of the technical challenges associated with generating analytically valid and clinically relevant genomic information from smaller, heterogeneous cytology samples. To this end, we use a whole-genome approach for gene selection and proprietary machine-learning algorithms with statistical methods to identify the genomic signature that achieves the desired performance.
3
Afirma is our first commercial solution based on our molecular cytology platform. We drive physician adoption and retention by marketing Afirma as the centerpiece of a comprehensive solution for improved disease diagnosis, which allows our offering to seamlessly integrate into a physician's practice workflow. We offer Afirma to physicians as a turnkey solution that combines cytopathology for every patient with our molecular diagnostic test when cytopathology yields ambiguous results. Our solution includes a complete patient report that guides decision making. By integrating disparate diagnostic procedures into one comprehensive offering, we can simplify and improve the diagnostic process for physicians and their patients while optimizing utilization of our molecular diagnostics to maximize clinical benefits and cost savings. We intend to duplicate this model with solutions we develop for other diseases.
Our capabilities in managed care and claims adjudication are essential to our success in obtaining positive coverage decisions and reimbursement. Our integrated team combines expertise in advocating for positive coverage decisions with specific insights into what tactical steps will maximize reimbursement from each payer. As a result, we have developed detailed knowledge of the intricacies of specific payer practices and requirements, which informs our strategy across disease selection, clinical study design, marketing and sales.
Advantages of Afirma FNA Analysis for Stakeholders
- •
- Benefits for patients. With the GEC, approximately half of
the patients with indeterminate cytology results may avoid unnecessary, invasive diagnostic surgery. Patients who obtain an Afirma benign result avoid the potential for surgery-related complications,
the effects of life-long hormone replacement therapy and the associated costs. We estimate that approximately 115,000 FNAs performed in the United States in 2011 yielded an indeterminate result.
- •
- Benefits for physicians. Afirma enables every physician,
regardless of practice setting, to offer his or her patients access to advanced technology for the diagnosis and management of thyroid nodules. Afirma does not introduce any new steps into the
physician's patient-care routine and often simplifies their workflow. In addition, our cytopathology provider, Thyroid Cytology Partners, is a specialized practice focused solely on performing thyroid
FNAs and meets high-quality standards with short turnaround times.
- •
- Benefits for payers. Payers differentiate themselves by offering their insured the most advanced care available in medicine. However, payers are also under increased pressure to contain rising healthcare costs. Afirma allows payers to provide advanced care at a cost lower than the current standard of care. The first peer-reviewed economic impact study, published in the Journal of Clinical Endocrinology and Metabolism, concluded that routine use of the GEC in the United States would prevent tens of thousands of surgeries each year. Based on our estimate of the average cost of surgery of $15,000 as well as clinical utility studies, we believe full adoption of Afirma would result in over $500 million in direct cost savings to the healthcare system over five years.
Our Strategy
Our goal is to resolve diagnostic ambiguity pre-operatively, allowing patients to avoid unnecessary procedures and generating significant cost savings for the healthcare system.
Our strategy includes the following key elements:
- •
- Accelerate the growth of Afirma by expanding our base of prescribing physicians and achieving broader reimbursement.
- •
- Market our novel molecular diagnostic tests as the centerpiece of a comprehensive patient-care solution.
- •
- Drive cost and capital efficiencies by offering turnkey solutions to physicians in specialty markets.
4
- •
- Broaden our addressable market in endocrinology by leveraging our thyroid expertise to introduce new products.
- •
- Capitalize on our demonstrated core competencies to expand molecular cytology to additional diseases.
Risks Associated with Our Business
Our business is subject to numerous risks and uncertainties, including those identified in "Risk Factors" immediately following this prospectus summary. You should read these risks before you invest in our common stock. We may be unable, for many reasons, including those that are beyond our control, to implement our business strategy. In particular, risks associated with our business include:
- •
- We are an early-stage company with a history of losses, and we expect to incur net losses for the foreseeable future and
may never achieve or sustain profitability.
- •
- Our financial results depend solely on sales of Afirma, and we will need to generate sufficient revenue from this and
other diagnostic solutions to grow our business.
- •
- We depend on Medicare, Aetna and UnitedHealthcare for a significant portion of our revenue and if one or more significant
payers stop providing reimbursement or decrease the amount of reimbursement for our tests, our revenue could decline.
- •
- If payers do not provide reimbursement, rescind or modify their reimbursement policies or delay payments for our tests, or
if we are unable to successfully negotiate reimbursement contracts, our commercial success could be compromised.
- •
- We may experience limits on our revenue if physicians decide not to order Afirma.
- •
- The success of our relationship with Genzyme to co-promote Afirma may have a significant effect on our business.
- •
- Because we do not recognize a significant portion of our revenue on an accrual basis, our quarterly operating results are
likely to fluctuate.
- •
- We rely on sole suppliers for some of the reagents, equipment, chips and other materials used in Afirma, and we may not be
able to find replacements or transition to alternative suppliers.
- •
- We depend on a specialized cytopathology practice to perform the cytopathology component of Afirma, and our ability to
perform our diagnostic solution would be harmed if we were required to secure a replacement.
- •
- If we are unable to support demand for Afirma or any of our future products or solutions, our business could suffer.
- •
- If the FDA were to begin regulating our tests, we could incur substantial costs and delays associated with trying to obtain premarket clearance or approval.
Corporate Information
We were incorporated in Delaware as Calderome, Inc. in August 2006. Calderome operated as an incubator until early 2008. We changed our name to Veracyte, Inc. in March 2008. Our principal executive offices are located at 7000 Shoreline Court, Suite 250, South San Francisco, California 94080 and our telephone number is (650) 243-6300. Our website address is www.veracyte.com. We do not incorporate the information on, or accessible through, our website into this prospectus, and you should not consider any information on, or accessible through, our website as part of this prospectus.
Unless the context indicates otherwise, as used in this prospectus, the terms "Veracyte," "Company," "we," "us" and "our" refer to Veracyte, Inc. Veracyte and Afirma are our trademarks. This prospectus also contains trademarks and trade names that are the property of their respective owners. We do not intend our use or display of other companies' trade names, trademarks or service marks to imply relationships with, or endorsements or sponsorship of us by, these other companies.
5
Common stock offered by us |
shares | |
Common stock to be outstanding after this offering |
shares ( shares if the underwriters exercise their over-allotment option in full) |
|
Over-allotment option |
We have granted to the underwriters the option, exercisable for 30 days from the date of this prospectus, to purchase up to additional shares of common stock. |
|
Use of proceeds |
We intend to use the net proceeds received by us from this offering for working capital and general corporate purposes, including continued investments in research and development and in the growth of our business. In addition, we may use a portion of the net proceeds from this offering for acquisitions of complementary businesses, technologies or other assets. However, we do not have agreements for any material acquisitions at this time. See "Use of Proceeds". |
|
Risk factors |
See "Risk Factors" beginning on page 10 and the other information included in this prospectus for a discussion of factors you should consider carefully before deciding to invest in our common stock. |
|
Proposed NASDAQ Global Market symbol |
VCYT |
The number of shares of common stock that will be outstanding after this offering is based on 63,704,170 shares outstanding as of June 30, 2013, on an as-converted basis, and excludes:
- •
- 9,681,245 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2013, at a
weighted average exercise price of $0.70 per share;
- •
- 99,206 shares of common stock issuable upon the exercise of warrants to purchase Series C preferred stock, which
will become exercisable for shares of common stock upon conversion of our Series C preferred stock into common stock immediately prior to the completion of this offering, with an exercise price
of $1.89 per share; and
- •
- 574,821 shares of common stock reserved for future issuance under our 2008 Stock Plan and shares of common stock, subject to increase on an annual basis, reserved for future issuance under our 2013 Stock Incentive Plan, which will become effective in connection with this offering.
Unless otherwise indicated, all information in this prospectus assumes:
- •
- that our restated certificate of incorporation, which we will file in connection with the completion of this offering, is in effect;
6
- •
- the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 59,989,268 shares of
common stock immediately prior to the completion of this offering; and
- •
- no exercise by the underwriters of their over-allotment option to purchase up to additional shares of common stock from us.
7
The following summary financial data should be read together with our financial statements and related notes, "Selected Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" appearing elsewhere in this prospectus. The summary statements of operations data for the years ended December 31, 2011 and 2012 and the six months ended June 30, 2012 and 2013, and the balance sheet data as of June 30, 2013 have been derived from our audited financial statements and unaudited interim condensed financial statements included elsewhere in this prospectus. Historical results are not necessarily indicative of the results that may be expected in the future and results of interim periods are not necessarily indicative of the results for the entire year.
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | 2012 | 2013 | |||||||||
| |
(In thousands, except share and per share data) |
||||||||||||
| |
|
|
(Unaudited) |
||||||||||
Statements of Operations Data: |
|||||||||||||
Revenue |
$ | 2,645 | $ | 11,628 | $ | 3,947 | $ | 9,452 | |||||
Operating expenses: |
|||||||||||||
Cost of revenue(1) |
2,925 | 7,584 | 3,000 | 6,004 | |||||||||
Research and development(1) |
6,680 | 6,608 | 3,158 | 3,912 | |||||||||
Selling and marketing(1) |
2,934 | 8,447 | 3,045 | 5,318 | |||||||||
General and administrative(1) |
5,372 | 7,918 | 3,618 | 5,528 | |||||||||
Total operating expenses(1) |
17,911 | 30,557 | 12,821 | 20,762 | |||||||||
Loss from operations |
(15,266 | ) | (18,929 | ) | (8,874 | ) | (11,310 | ) | |||||
Interest income |
2 | 2 | — | — | |||||||||
Interest expense |
— | — | — | (5 | ) | ||||||||
Other income (expense), net |
819 | 278 | — | (2,070 | ) | ||||||||
Net loss |
$ | (14,445 | ) | $ | (18,649 | ) | $ | (8,874 | ) | $ | (13,385 | ) | |
Net loss per common share, basic and diluted |
$ | (6.23 | ) | $ | (7.17 | ) | $ | (3.48 | ) | $ | (4.12 | ) | |
Shares used in computing net loss per common share, basic and diluted |
2,320,252 | 2,601,352 | 2,553,287 | 3,250,863 | |||||||||
Other Operating Data: |
|||||||||||||
Fine needle aspirations (FNAs) received |
6,402 | 25,890 | 9,535 | 23,181 | |||||||||
- (1)
- Includes stock-based compensation as follows:
| |
Year Ended December 31, |
Six Months Ended June 30, |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | 2012 | 2013 | ||||||||||
| |
(In thousands) |
|||||||||||||
| |
|
|
(Unaudited) |
|||||||||||
Cost of revenue |
$ | 32 | $ | 26 | $ | 16 | $ | 13 | ||||||
Research and development |
130 | 131 | 48 | 103 | ||||||||||
Selling and marketing |
77 | 111 | 52 | 76 | ||||||||||
General and administrative |
227 | 407 | 174 | 297 | ||||||||||
Total stock-based compensation |
$ | 466 | $ | 675 | $ | 290 | $ | 489 | ||||||
8
| |
As of June 30, 2013 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro Forma | Pro Forma As Adjusted |
||||||||
| |
(In thousands) |
||||||||||
| |
(Unaudited) |
||||||||||
Balance Sheet Data: |
|||||||||||
Cash and cash equivalents |
$ | 20,683 | |||||||||
Working capital |
14,049 | ||||||||||
Total assets |
27,159 | ||||||||||
Convertible preferred stock |
79,025 | ||||||||||
Accumulated deficit |
(73,455 | ) | |||||||||
Total stockholders' (deficit) equity |
(70,788 | ) | |||||||||
The preceding table presents a summary of our unaudited balance sheet data as of June 30, 2013:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to the conversion of all outstanding shares of our convertible preferred stock into
common stock immediately prior to the completion of this offering; and
- •
- on a pro forma as adjusted basis to give further effect to the receipt of the estimated net proceeds from the sale of shares of common stock in this offering at a price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders' equity (deficit) by $ million, assuming that the number of shares offered as set forth on the cover page of this prospectus remains the same, and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us. Each increase (decrease) of 1.0 million shares in the number of shares of common stock offered by us would increase (decrease) each of cash and cash equivalents, working capital, total assets and total stockholders' equity (deficit) by approximately $ million, assuming a price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
9
Investing in our common stock involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including our financial statements and related notes included elsewhere in this prospectus, before making an investment decision. If any of the following risks is realized, our business, financial condition, results of operations and prospects could be materially and adversely affected. In that event, the trading price of our common stock could decline and you could lose part or all of your investment.
Risks Related to Our Business
We are an early-stage company with a history of losses, and we expect to incur net losses for the foreseeable future and may never achieve or sustain profitability.
We have incurred net losses since our inception. For the years ended December 31, 2011 and 2012 and for the six months ended June 30, 2013, we had a net loss of $14.4 million, $18.6 million and $13.4 million, respectively, and we expect to incur additional losses this year and in future years. As of June 30, 2013, we had an accumulated deficit of $73.5 million. To date, we have generated only limited revenue, and we may never achieve revenue sufficient to offset our expenses. Over the next several years, we expect to continue to devote substantially all of our resources to increase adoption of, and reimbursement for, Afirma and to develop future diagnostic solutions. We may never achieve or sustain profitability, and our failure to achieve and sustain profitability in the future could cause the market price of our common stock to decline.
Our financial results depend solely on sales of Afirma, and we will need to generate sufficient revenue from this and other diagnostic solutions to grow our business.
All of our historical revenue has been derived from the sale of Afirma, which we commercially launched in January 2011. For the foreseeable future, we expect to derive substantially all of our revenue from sales of Afirma. We are in various stages of research and development for other diagnostic solutions that we may offer, but there can be no assurance that we will be able to identify other diseases that can be effectively addressed with our molecular cytology platform or, if we are able to identify such diseases, whether or when we will be able to successfully commercialize these solutions. If we are unable to increase sales of Afirma, expand reimbursement for Afirma, or successfully develop and commercialize other solutions, our revenue and our ability to achieve and sustain profitability would be impaired, and the market price of our common stock could decline.
We depend on Medicare, Aetna and UnitedHealthcare for a significant portion of our revenue and if one or more significant payers stop providing reimbursement or decrease the amount of reimbursement for our tests, our revenue could decline.
Reimbursement on behalf of patients covered by Medicare accounted for 34% and 35% of our revenue for the year ended December 31, 2012 and for the six months ended June 30, 2013, respectively. UnitedHealthcare accounted for 12% and 14% of our revenue for the year ended December 31, 2012 and for the six months ended June 30, 2013, respectively. Aetna accounted for 13% and 7% of our revenue for the year ended December 31, 2012 and for the six months ended June 30, 2013, respectively. Effective January 2012, Palmetto GBA, the regional Medicare administrative contractor, or MAC, that handled claims processing for Medicare services with jurisdiction at that time, issued coverage and payment determinations on the GEC. On a five-year rotational basis, Medicare requests bids for its regional MAC services. In mid-September 2013, Noridian Administrative Services is scheduled to succeed Palmetto as the MAC for our region. The change in the MAC processing Medicare claims for the GEC could result in a change in the coverage or reimbursement rates for the GEC, or the loss of coverage. The transition to Noridian could also result in delays in payments made to us on behalf of Medicare patients.
10
We do not have a contracted rate of reimbursement with Aetna, Humana or UnitedHealthcare. Payers may suspend or discontinue reimbursement at any time, may require or increase co-payments from patients, or may reduce the reimbursement rates paid to us. Any such actions could have a negative effect on our revenue.
If payers do not provide reimbursement, rescind or modify their reimbursement policies or delay payments for our tests, or if we are unable to successfully negotiate reimbursement contracts, our commercial success could be compromised.
Physicians may not order our tests unless payers reimburse a substantial portion of the test price. There is significant uncertainty concerning third-party reimbursement of any test incorporating new technology, including the GEC. Reimbursement by a payer may depend on a number of factors, including a payer's determination that tests such as the GEC are:
- •
- not experimental or investigational;
- •
- appropriate for the specific patient;
- •
- cost-effective;
- •
- supported by peer-reviewed publications; and
- •
- included in clinical practice guidelines.
Since each payer makes its own decision as to whether to establish a policy or enter into a contract to reimburse our test, seeking these approvals is a time-consuming and costly process.
We do not have a contracted rate of reimbursement with most payers. Without a contracted rate for reimbursement, our claims are often denied upon submission, and we must appeal the claims. The appeals process is time consuming and expensive, and may not result in payment. In cases where there is not a contracted rate for reimbursement, there is typically a greater co-insurance or co-payment requirement which may result in further delay or decreased likelihood of collection.
We expect to continue to focus substantial resources on increasing adoption of and coverage and reimbursement for Afirma. We believe it may take several years to achieve coverage and contracted reimbursement with a majority of third-party payers. However, we cannot predict whether, under what circumstances, or at what payment levels payers will reimburse for our test. If we fail to establish broad adoption of and reimbursement for our products, or if we are unable to maintain existing reimbursement from payers, our ability to generate revenue could be harmed and our future prospects and our business could suffer.
We may experience limits on our revenue if physicians decide not to order Afirma.
If we are unable to create or maintain demand for Afirma in sufficient volume, we may not become profitable. To generate demand, we will need to continue to educate physicians about the benefits and cost-effectiveness of Afirma through published papers, presentations at scientific conferences and one-on-one education by our sales force. In addition, our ability to obtain and maintain adequate reimbursement from third-party payers will be critical to generating revenue.
Several existing guidelines and historical practices in the United States regarding indeterminate thyroid nodule FNA results recommend a full or partial surgical thyroidectomy in most cases. Accordingly, physicians may be reluctant to order a diagnostic solution that may suggest surgery is unnecessary where several current guidelines and historical practice have typically led to such procedures. Moreover, our diagnostic services are performed at our clinical reference laboratory rather than by a pathologist in a local laboratory, so pathologists may be reluctant to support our services. In addition, guidelines for the diagnosis and treatment of thyroid nodules may subsequently be revised to recommend another type of
11
treatment protocol, and these changes may result in medical practitioners deciding not to use Afirma. These facts may make physicians reluctant to convert to using Afirma, which could limit our ability to generate revenue and our ability to achieve profitability. To the extent international markets have existing practices and standards of care that are different than those in the United States, we may face challenges with the adoption of Afirma outside the United States.
The success of our relationship with Genzyme to co-promote Afirma may have a significant effect on our business.
We sell Afirma in the United States through our internal sales team and through our co-promotion agreement with Genzyme Corporation. We are also working with Genzyme to begin selling Afirma in certain countries outside of the United States. Under the agreement, we are required to pay Genzyme a co-promotion fee that is equal to a percentage of our cash receipts from Afirma. The percentage is currently 40% and will decrease to 32% in March 2014 and thereafter. Our agreement with Genzyme expires in 2027 and either party may terminate the agreement at any time without cause and with six months prior notice. If we were to terminate the agreement without cause prior to January 2014, we would be required to repay 50% of the $10.0 million fee we received from Genzyme. Such percentage would be reduced to 40% of such fee if we were to terminate the agreement between January 2014 and January 2015, and 30% of such fee if we were to terminate the agreement between January 2015 and January 2016. We have also granted Genzyme a right of first offer to co-promote any future thyroid cancer product that we commercialize. If Genzyme does not commit the necessary resources to market and sell Afirma to the level of our expectations, or if they terminate the agreement, we may not realize the benefits of this relationship, and our ability to generate revenue in the future may be harmed. If our agreement with Genzyme were terminated, we would have to hire additional sales personnel to support the growth of Afirma and any other thyroid product we agree to co-promote with Genzyme. Any such termination may also delay our entry into international markets.
Because we do not recognize a significant portion of our revenue on an accrual basis, our quarterly operating results are likely to fluctuate.
We currently recognize the majority of our revenue upon the earlier of receipt of third-party payer notification of payment or when cash is received. We have little visibility as to when we will receive payment for our diagnostic test, and we must appeal negative payment decisions, which delays collections. These factors will likely result in fluctuations in our quarterly revenue. As a result, comparing our operating results on a period-to-period basis may not be meaningful. You should not rely on our past results as an indication of our future performance. In addition, these fluctuations in revenue may make it difficult for us, research analysts and investors to accurately forecast our revenue and operating results. If our revenue or operating results fall below expectations, the price of our common stock would likely decline.
We rely on sole suppliers for some of the reagents, equipment, chips and other materials used in Afirma, and we may not be able to find replacements or transition to alternative suppliers.
We rely on sole suppliers, such as NuGEN Technologies, Inc. and Affymetrix, Inc., for critical supply of reagents, equipment, chips and other materials that we use to perform the GEC. All of these items are unique to these suppliers. While we have developed alternate sourcing strategies for these materials, we cannot be certain whether these strategies will be effective or the alternative sources will be available when we need them. If these suppliers can no longer provide us with the reagents, equipment, chips, and other materials used in processing the GEC, if the materials do not meet our quality specifications, or if we cannot obtain acceptable substitute materials, an interruption in test processing could occur. Any such interruption may significantly affect our future revenue and harm our customer relations and reputation. In addition, in order to mitigate these risks, we may need to maintain inventories of these supplies at higher levels than would be the case if multiple sources of supply were available.
12
We depend on a specialized cytopathology practice to perform the cytopathology component of Afirma, and our ability to perform our diagnostic solution would be harmed if we were required to secure a replacement.
We rely on Thyroid Cytology Partners, P.A., or TCP, with whom we have entered into an agreement to provide cytopathology professional diagnoses on thyroid FNA samples at a fixed price per test. We have also agreed to allow TCP to co-locate in a portion of our facilities in Austin, Texas. Our agreement with TCP is effective until December 2015 and thereafter automatically renews every year unless either party provides notice of intent not to renew.
If TCP were not able to support our current test volume or future increases in test volume or to provide the quality of services we require, or if we are unable to agree on commercial terms and our relationship with TCP were to terminate, our business would be harmed until we are able to secure the services of another cytopathology provider. There can be no assurance that we would be successful in finding a replacement that would be able to conduct cytopathology diagnoses at the same volume or with the same high-quality results as TCP. Locating another suitable cytopathology provider could be time consuming and would result in delays in processing tests until a replacement was fully integrated with our test processing operations.
If we are unable to support demand for Afirma or any of our future products or solutions, our business could suffer.
As demand for Afirma or any of our future products or solutions grows, we will need to continue to scale our testing capacity and processing technology, expand customer service, billing and systems processes and enhance our internal quality assurance program. We will also need additional certified laboratory scientists and other scientific and technical personnel to process higher volumes of our tests. We cannot assure you that any increases in scale, related improvements and quality assurance will be successfully implemented or that appropriate personnel will be available. Failure to implement necessary procedures, transition to new processes or hire the necessary personnel could result in higher costs of processing tests or inability to meet demand. There can be no assurance that we will be able to perform our testing on a timely basis at a level consistent with demand, or that our efforts to scale our operations will not negatively affect the quality of test results. If we encounter difficulty meeting market demand or quality standards, our reputation could be harmed and our future prospects and our business could suffer.
If the FDA were to begin regulating our tests, we could incur substantial costs and delays associated with trying to obtain premarket clearance or approval.
Clinical laboratory tests like Afirma are regulated under the Clinical Laboratory Improvement Amendments of 1988, or CLIA, as well as by applicable state laws. Most laboratory developed tests, or LDTs, are not currently subject to FDA regulation, although reagents, instruments, software or components provided by third parties and used to perform LDTs may be subject to regulation. We believe that Afirma is an LDT. As a result, we believe Afirma should not be subject to regulation in accordance with the FDA's current policy of exercising enforcement discretion regarding LDTs.
From time to time, the FDA has indicated that it was revisiting its current policy of enforcement discretion and planned to issue guidance that, when finalized, would adopt a risk-based framework that would increase FDA oversight of LDTs. In July 2010, the FDA convened a public meeting to discuss such a risk-based framework. Legislative proposals addressing oversight of LDTs were introduced in the previous two Congresses and we expect that new legislative proposals will be introduced from time to time. We cannot provide any assurance that FDA regulation, including premarket review, will not be required in the future for our tests, whether through additional guidance issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. It is possible that legislation will be enacted into law or guidance could be issued by the FDA which may result in increased regulatory burdens for us to continue to offer our tests or to develop and introduce new tests. We cannot predict the timing or content of future legislation enacted or guidance issued regarding LDTs, or how it will affect our business.
13
In June 2011, the FDA issued draft guidance regarding "Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only". To date, the FDA has not issued final research-use only guidance. We cannot predict the ultimate timing or form of any such guidance or regulation and or the potential effect on Afirma, our tests in development or the materials used to perform our tests. While we qualify all materials used in our tests according to CLIA regulations, we cannot be certain that the FDA would not promulgate rules or issue guidance documents that could affect our ability to purchase materials necessary for the performance of our tests. Should any of the reagents, instruments, software or components obtained by us from suppliers and used in conducting our tests be affected by future regulatory actions, our business could be adversely affected by those actions, including increasing the cost of testing or delaying, limiting or prohibiting the purchase of reagents, instruments, software or components necessary to perform testing.
If FDA premarket review or approval is required for Afirma or any of our future tests we may develop, or we decide to voluntarily pursue FDA review or approval, we may be forced to stop selling our tests or we may be allowed to keep selling our tests while we work to obtain FDA approval. Our business would be negatively affected until such review is completed and clearance to market or approval is obtained. The regulatory approval process may involve, among other things, successfully completing additional clinical studies and submitting premarket notification or filing a premarket approval application with the FDA. If premarket review is required by the FDA or if we decide to voluntarily pursue FDA premarket review of our tests, there can be no assurance that Afirma or any tests we may develop in the future will be cleared or approved on a timely basis, if at all, nor can there be assurance that labeling claims will be consistent with our current claims or adequate to support continued adoption of and reimbursement for our tests. If our tests are allowed to remain on the market but there is uncertainty in the marketplace about our tests, if they are labeled investigational by the FDA, or if labeling claims the FDA allows us to make are limited, orders may decline and reimbursement may be adversely affected. Ongoing compliance with FDA regulations would increase the cost of conducting our business, and subject us to heightened regulation by the FDA and penalties for failure to comply with these requirements.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
In addition to the need to scale our testing capacity, future growth will impose significant added responsibilities on management, including the need to identify, recruit, train and integrate additional employees. In addition, rapid and significant growth may place strain on our administrative and operational infrastructure. Our ability to manage our business and growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We have only recently installed a new, internally developed data warehouse, which is critical to our ability to track our diagnostic services and patient reports delivered to physicians, as well as to support our financial reporting systems. The time and resources required to optimize these systems is uncertain, and failure to complete optimization in a timely and efficient manner could adversely affect our operations. If we are unable to manage our growth effectively, it may be difficult for us to execute our business strategy and our business could be harmed.
Billing for our diagnostic solution is complex, and we must dedicate substantial time and resources to the billing process to be paid for our tests.
Billing for clinical laboratory testing services is complex, time consuming and expensive. Depending on the billing arrangement and applicable law, we bill various payers, including Medicare, insurance companies and patients, all of which have different billing requirements. We generally bill third-party payers for our diagnostic solution and pursue reimbursement on a case-by-case basis where pricing contracts are not in place. To the extent laws or contracts require us to bill patient co-payments or co-insurance, we must also comply with these requirements. We may also face increased risk in our
14
collection efforts, including potential write-offs of doubtful accounts and long collection cycles, which could adversely affect our business, results of operations and financial condition.
Several factors make the billing process complex, including:
- •
- differences between the list price for Afirma and the reimbursement rates of payers;
- •
- compliance with complex federal and state regulations related to billing Medicare;
- •
- disputes among payers as to which party is responsible for payment;
- •
- differences in coverage among payers and the effect of patient co-payments or co-insurance;
- •
- differences in information and billing requirements among payers;
- •
- incorrect or missing billing information; and
- •
- the resources required to manage the billing and claims appeals process.
Additionally, our billing activities require us to implement compliance procedures and oversight, train and monitor our employees, challenge coverage and payment denials, assist patients in appealing claims, and undertake internal audits to evaluate compliance with applicable laws and regulations as well as internal compliance policies and procedures. Payers also conduct external audits to evaluate payments, which add further complexity to the billing process. These billing complexities, and the related uncertainty in obtaining payment for our diagnostic solution, could negatively affect our revenue and cash flow, our ability to achieve profitability, and the consistency and comparability of our results of operations.
We rely on a third party to transmit claims to payers, and any delay in transmitting claims could have an adverse effect on our revenue.
While we manage the overall processing of claims, we rely on a third-party provider to transmit the actual claims to payers based on the specific payer billing format. We have previously experienced delays in claims processing when our third-party provider made changes to its invoicing system, and again when it did not submit claims to payers within the timeframe we require. If claims for Afirma are not submitted to payers on a timely basis, or if we are required to switch to a different provider to handle claim submissions, we may experience delays in our ability to process these claims and receipt of payments from payers, which would have an adverse effect on our revenue and our business.
International expansion of our business exposes us to business, regulatory, political, operational, financial and economic risks associated with doing business outside of the United States.
Our business strategy includes international expansion, primarily through our co-promotion agreement with Genzyme, and may include establishing and maintaining physician outreach and education capabilities outside of the United States and expanding our relationships with international payers. Doing business internationally involves a number of risks, including:
- •
- multiple, conflicting and changing laws and regulations such as tax laws, privacy laws, export and import restrictions,
employment laws, regulatory requirements and other governmental approvals, permits and licenses;
- •
- failure by us to obtain regulatory approvals where required for the use of our solution in various countries;
- •
- complexities associated with managing multiple payer reimbursement regimes, government payers or patient self-pay systems;
- •
- logistics and regulations associated with shipping tissue samples, including infrastructure conditions and transportation delays;
15
- •
- limits on our ability to penetrate international markets if we are not able to process tests locally;
- •
- financial risks, such as longer payment cycles, difficulty collecting accounts receivable, the effect of local and
regional financial crises on demand and payment for our solution and exposure to foreign currency exchange rate fluctuations;
- •
- natural disasters, political and economic instability, including wars, terrorism, and political unrest, outbreak of
disease, boycotts, curtailment of trade and other business restrictions; and
- •
- regulatory and compliance risks that relate to maintaining accurate information and control over activities that may fall within the purview of the Foreign Corrupt Practices Act of 1977, its books and records provisions or its anti-bribery provisions.
Any of these factors could significantly harm our future international expansion and operations and, consequently, our revenue and results of operations.
If we are unable to compete successfully, we may be unable to increase or sustain our revenue or achieve profitability.
Our principal competition for Afirma comes from traditional methods used by physicians to diagnose thyroid cancer. Practice guidelines in the United States have historically recommended that patients with indeterminate diagnoses from cytopathology results be considered for surgery to remove all or part of the thyroid to rule out cancer. This practice has been the standard of care in the United States for many years, and we need to educate physicians about the benefits of Afirma to change clinical practice.
We also face competition from commercial laboratories, such as Laboratory Corporation of America Holdings and Quest Diagnostics Incorporated, with strong infrastructure to support the commercialization of diagnostic services. We face potential competition from companies such as Life Technologies Corporation, which is currently expected to be acquired by Thermo Fisher Scientific Inc., and Illumina, Inc., both of which have recently announced their intention to enter the clinical diagnostics market. Other potential competitors include companies that develop diagnostic products, such as Roche Diagnostics, a division of Roche Holding Ltd, Siemens AG and Qiagen N.V. We also face competition from Asuragen Inc. and other companies that measure mutational markers such as BRAF and KRAS to identify nodules that are malignant instead of benign. In the future, we may also face competition from companies developing new products or technologies.
In addition, competitors may develop their own versions of our solution in countries where we do not have patents or where our intellectual property rights are not recognized and compete with us in those countries, including encouraging the use of their solution by physicians in other countries.
To compete successfully we must be able to demonstrate, among other things, that our diagnostic test results are accurate and cost effective, and we must secure a meaningful level of reimbursement for our products.
Many of our potential competitors have widespread brand recognition and substantially greater financial, technical and research and development resources and selling and marketing capabilities than we do. Others may develop products with prices lower than ours that could be viewed by physicians and payers as functionally equivalent to our solution, or offer solutions at prices designed to promote market penetration, which could force us to lower the list price of our solution and affect our ability to achieve profitability. If we are unable to change clinical practice in a meaningful way or compete successfully against current and future competitors, we may be unable to increase market acceptance and sales of our products, which could prevent us from increasing our revenue or achieving profitability and could cause the market price of our common stock to decline.
16
Developing new products involves a lengthy and complex process, and we may not be able to commercialize on a timely basis, or at all, other products we are developing.
We have enhancements to our current Afirma offering and other diagnostic solutions under development that will require us to devote considerable resources to research and development. There can be no assurance that we will be able to identify other diseases that can be effectively addressed with our molecular cytology platform. In addition, if we identify such diseases, we may not be able to develop products with the diagnostic accuracy necessary to be clinically useful and commercially successful. We are in the process of developing the Afirma Malignant GEC and a product for interstitial lung disease. These products may not be fully developed and introduced as planned in 2014 and 2016, respectively. In the longer term, we may face challenges obtaining sufficient numbers of samples to validate a genomic signature for a molecular diagnostic product. In order to develop and commercialize diagnostic products, we need to:
- •
- expend significant funds to conduct substantial research and development;
- •
- conduct analytical and clinical studies;
- •
- scale our laboratory processes to accommodate new tests; and
- •
- build the commercial infrastructure to market and sell new products.
Our product development process involves a high degree of risk and may take several years. Our product development efforts may fail for many reasons, including:
- •
- failure to identify a genomic signature in biomarker discovery;
- •
- inability to secure sufficient numbers of samples to conduct analytical and clinical studies; or
- •
- failure of clinical validation studies to support the effectiveness of the test.
Typically, few research and development projects result in commercial products, and success in early clinical studies often is not replicated in later studies. At any point, we may abandon development of a product candidate or we may be required to expend considerable resources repeating clinical studies, which would adversely affect the timing for generating potential revenue from a new product and our ability to invest in other products in our pipeline. If a clinical validation study fails to demonstrate the prospectively defined endpoints of the study, we might choose to abandon the development of the product, which could harm our business. In addition, competitors may develop and commercialize competing products or technologies faster than us or at a lower cost.
We may acquire businesses or assets, form joint ventures or make investments in other companies or technologies that could harm our operating results, dilute our stockholders' ownership, increase our debt or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of complementary businesses or assets, as well as technology licensing arrangements. We also may pursue strategic alliances that leverage our core technology and industry experience to expand our offerings or distribution, or make investments in other companies. To date, we have not acquired other companies and have limited experience with respect to the formation of strategic alliances and joint ventures. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions by us also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could harm our operating results. Integration of an acquired company or business also may require management resources that otherwise would be available for ongoing development of our existing business. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, joint venture or investment.
17
To finance any acquisitions or investments, we may choose to issue shares of our stock as consideration, which would dilute the ownership of our stockholders. Once we become a public company, if the price of our common stock is low or volatile, we may not be able to acquire other companies for stock. Alternatively, it may be necessary for us to raise additional funds for these activities through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
If we are unable to develop products to keep pace with rapid technological, medical and scientific change, our operating results and competitive position could be harmed.
In recent years, there have been numerous advances in technologies relating to diagnostics, particularly diagnostics that are based on genomic information. These advances require us to continuously develop our technology and work to develop new solutions to keep pace with evolving standards of care. Our solutions could become obsolete unless we continually innovate and expand our product offerings to include new clinical applications. If we are unable to develop new products or to demonstrate the applicability of our products for other diseases, our sales could decline and our competitive position could be harmed.
If we fail to comply with federal, state and foreign laboratory licensing requirements, we could lose the ability to perform our tests or experience disruptions to our business.
We are subject to CLIA, a federal law that regulates clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention or treatment of disease. CLIA regulations mandate specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management and quality assurance. CLIA certification is also required in order for us to be eligible to bill state and federal healthcare programs, as well as many private third-party payers, for Afirma. To renew these certifications, we are subject to survey and inspection every two years. Moreover, CLIA inspectors may make random inspections of our clinical reference laboratories.
We are also required to maintain state licenses to conduct testing in our laboratories. California law establishes standards for day-to-day operation of our clinical reference laboratory in South San Francisco, including the training and skills required of personnel and quality control matters. In addition, our clinical reference laboratories are required to be licensed on a test-specific basis by New York State. New York law also mandates proficiency testing for laboratories licensed under New York state law, regardless of whether such laboratories are located in New York. We have obtained a license from New York for our South San Francisco laboratory and have applied for a license for our Austin laboratory. If New York State does not license our Texas laboratory, we would not be able to prepare samples for cytopathology on FNAs from patients in New York in that laboratory. Moreover, several other states require that we hold licenses to test samples from patients in those states. Other states may have similar requirements or may adopt similar requirements in the future.
If we were to lose our CLIA certificate or state license for our South San Francisco laboratory, whether as a result of revocation, suspension or limitation, we would no longer be able to perform the GEC, which would eliminate our primary source of revenue and harm our business. If we were to lose our CLIA certificate for our Austin laboratory, we would need to move the receipt and storage of FNAs, as well as the slide preparation for cytopathology, to South San Francisco, which could result in a delay in processing tests during that transition and increased costs. If we were to lose our license issued by New York or by other states where we are required to hold licenses, we would not be able to test specimens from those states.
Finally, we may be subject to regulation in foreign jurisdictions as we pursue offering Afirma internationally. Other limitations, such as prohibitions on the import of tissue necessary for us to perform our tests or restrictions on the export of tissue imposed by countries outside of the United States or the import of tissue into the United States, may limit our ability to offer Afirma internationally in the future.
18
Changes in healthcare policy, including legislation reforming the U.S. healthcare system, may have a material adverse effect on our financial condition and operations.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, collectively, the PPACA, enacted in March 2010, makes changes that are expected to significantly affect the pharmaceutical and medical device industries and clinical laboratories. Beginning in 2013, each medical device manufacturer must pay a sales tax in an amount equal to 2.3% of the price for which such manufacturer sells its medical devices that are listed with the FDA. The FDA has asserted that clinical laboratory tests such as Afirma are medical devices. However, consistent with the FDA's policy of exercising enforcement discretion for LDTs, Afirma is not currently listed as a medical device with the FDA. We cannot assure you that the tax will not be extended to services such as ours in the future if Afirma were to be regulated as a device. The PPACA also mandates a reduction in payments for clinical laboratory services paid under the Medicare Clinical Laboratory Fee Schedule, or CLFS, of 1.75% for the years 2011 through 2015 and a productivity adjustment to the CLFS which would affect our cytopathology billings.
Other significant measures contained in the PPACA include, for example, coordination and promotion of research on comparative clinical effectiveness of different technologies and procedures, initiatives to revise Medicare payment methodologies, such as bundling of payments across the continuum of care by providers and physicians, and initiatives to promote quality indicators in payment methodologies. The PPACA also includes significant new fraud and abuse measures, including required disclosures of financial arrangements with physician customers, lower thresholds for violations and increasing potential penalties for such violations. In addition, the PPACA establishes an Independent Payment Advisory Board, or IPAB, to reduce the per capita rate of growth in Medicare spending. The IPAB has broad discretion to propose policies to reduce expenditures, which may have a negative effect on payment rates for services. The IPAB proposals may affect payments for clinical laboratory services beginning in 2016 and for hospital services beginning in 2020. We are monitoring the effect of the PPACA to determine the trends and changes that may be necessitated by the legislation, any of which may potentially affect our business.
In addition to the PPACA, the effect of which on our business cannot presently be fully quantified, various healthcare reform proposals have also emerged from federal and state governments. For example, in February 2012, Congress passed the Middle Class Tax Relief and Job Creation Act of 2012, which in part reduced the potential future cost-based increases to the Medicare CLFS by 2%. Overall the expected total fee cut to the CLFS for 2013 is 2.95% not considering a further reduction of 2% anticipated from implementation of the automatic expense reductions (sequester) under the Budget Control Act of 2011, which went into effect for dates of service on or after April 1, 2013. Reductions resulting from the Congressional sequester are applied to total claims payment made; however, they do not currently result in a rebasing of the negotiated or established Medicare or Medicaid reimbursement rates.
State legislation on reimbursement applies to Medicaid reimbursement and Managed Medicaid reimbursement rates within that state. Some states have passed or proposed legislation that would revise reimbursement methodology for clinical laboratory payment rates under those Medicaid programs. Recent changes to reimbursement methodologies have not changed the payment rate for Afirma; however, we cannot predict whether future healthcare initiatives will be implemented at the federal or state level or in countries outside of the United States in which we may do business, or the effect any future legislation or regulation will have on us. The taxes imposed by the new federal legislation, cost reduction measures and the expansion in the role of the U.S. government in the healthcare industry may result in decreased revenue, lower reimbursement by payers for our tests or reduced medical procedure volumes, all of which may adversely affect our business, financial condition and results of operations. In addition, sales of our tests outside the United States will subject our business to foreign regulatory requirements and cost-reduction measures, which may also change over time.
19
Ongoing calls for deficit reduction at the Federal government level and reforms to programs such as the Medicare program to pay for such reductions may affect the pharmaceutical, medical device and clinical laboratory industries. In particular, recommendations by the Simpson-Bowles Commission called for the combination of Medicare Part A (hospital insurance) and Part B (physician and ancillary service insurance) into a single co-insurance and co-payment structure. Currently, clinical laboratory services are excluded from the Medicare Part B co-insurance and co-payment as preventative services. Combining Parts A and B may require clinical laboratories to collect co-payments from patients which may increase our costs and reduce the amount ultimately collected.
Complying with numerous statutes and regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
We are subject to other regulation by both the federal government and the states in which we conduct our business, including:
- •
- Medicare billing and payment regulations applicable to clinical laboratories;
- •
- the Federal anti-kickback law and state anti-kickback prohibitions;
- •
- the Federal physician self-referral prohibition, commonly known as the Stark Law, and state equivalents;
- •
- the Federal Health Insurance Portability and Accountability Act of 1996;
- •
- the Medicare civil money penalty and exclusion requirements;
- •
- the Federal False Claims Act civil and criminal penalties and state equivalents; and
- •
- the Foreign Corrupt Practices Act of 1977, which applies to our international activities.
We have adopted policies and procedures designed to comply with these laws and regulations. In the ordinary course of our business, we conduct internal reviews of our compliance with these laws. Our compliance is also subject to governmental review. The growth of our business and sales organization and our expansion outside of the United States may increase the potential of violating these laws or our internal policies and procedures. The risk of our being found in violation of these or other laws and regulations is further increased by the fact that many have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Any action brought against us for violation of these or other laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management's attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including civil and criminal penalties, damages and fines, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
If we are sued for product liability or errors and omissions liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of Afirma could lead to product liability claims if someone were to allege that the GEC failed to perform as it was designed. We may also be subject to liability for errors in the results we provide to physicians or for a misunderstanding of, or inappropriate reliance upon, the information we provide. Our GEC is performed on FNA samples that are diagnosed as indeterminate by standard cytopathology review. We report results as benign or suspicious to the prescribing physician. Under certain circumstances, we might report a result as benign that later proves to have been malignant. This could be the result of the physician having poor nodule sampling in collecting the FNA, performing the FNA on a different nodule than the one that is malignant or failure of the GEC to perform as intended. We may also be subject to similar types of claims related to products we may develop in the
20
future. A product liability or errors and omissions liability claim could result in substantial damages and be costly and time consuming for us to defend. Although we maintain product liability and errors and omissions insurance, we cannot assure you that our insurance would fully protect us from the financial impact of defending against these types of claims or any judgments, fines or settlement costs arising out of any such claims. Any product liability or errors and omissions liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could cause injury to our reputation or cause us to suspend sales of our products and solutions. The occurrence of any of these events could have an adverse effect on our business and results of operations.
The loss of members of our senior management team or our inability to attract and retain key personnel could adversely affect our business.
Our success depends largely on the skills, experience and performance of key members of our executive management team and others in key management positions. The efforts of each of these persons together will be critical to us as we continue to develop our technologies and test processes and focus on our growth. If we were to lose one or more of these key employees, we may experience difficulties in competing effectively, developing our technologies and implementing our business strategy.
In addition, our research and development programs and commercial laboratory operations depend on our ability to attract and retain highly skilled scientists, including licensed clinical laboratory scientists and biostatisticians. We may not be able to attract or retain qualified scientists and technicians in the future due to the intense competition for qualified personnel among life science businesses, particularly in the San Francisco Bay Area. Because it is expected that there will be a shortage of clinical laboratory scientists in coming years, it may become more difficult to hire sufficient numbers of qualified personnel. We also face competition from universities and public and private research institutions in recruiting and retaining highly qualified scientific personnel. Additionally, our success depends on our ability to attract and retain qualified salespeople. We may have difficulties locating, recruiting or retaining qualified salespeople, which could cause a delay or decline in the rate of adoption of our solution. If we are not able to attract and retain the necessary personnel to accomplish our business objectives, we may experience constraints that could adversely affect our ability to support our research and development, clinical laboratory and sales efforts. All of our employees are at-will, which means that either we or the employee may terminate their employment at any time. We do not carry key man insurance for any of our employees.
If our laboratory in South San Francisco becomes inoperable due to an earthquake or either of our laboratories becomes inoperable for any other reason, we will be unable to perform our testing services and our business will be harmed.
We perform all of the GEC testing at our laboratory in South San Francisco, California. Our laboratory in Austin, Texas accepts and stores substantially all FNA samples pending transfer to our California laboratory for GEC processing. The equipment we use to perform the GEC would be costly to replace and could require substantial lead time to replace and qualify for use. Either of our facilities may be harmed or rendered inoperable by natural or man-made disasters, including earthquakes, flooding and power outages, which may render it difficult or impossible for us to perform our testing services for some period of time or to receive and store samples. The inability to perform GEC testing or the backlog of GEC tests that could develop if our California facility is inoperable for even a short period of time may result in the loss of customers or harm our reputation, and we may be unable to regain those customers in the future. Although we maintain insurance for damage to our property and the disruption of our business, this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
21
If we cannot enter into new clinical study collaborations, our product development and subsequent commercialization could be delayed.
In the past, we have entered into clinical study collaborations, and our success in the future depends in part on our ability to enter into additional collaborations with highly regarded institutions. This can be difficult due to internal and external constraints placed on these organizations. Some organizations may limit the number of collaborations they have with any one company so as to not be perceived as biased or conflicted. Organizations may also have insufficient administrative and related infrastructure to enable collaborations with many companies at once, which can extend the time it takes to develop, negotiate and implement a collaboration. Additionally, organizations often insist on retaining the rights to publish the clinical data resulting from the collaboration. The publication of clinical data in peer-reviewed journals is a crucial step in commercializing and obtaining reimbursement for a diagnostic solution such as Afirma, and our inability to control when and if results are published may delay or limit our ability to derive sufficient revenue from any solution.
If we use hazardous materials in a manner that causes contamination or injury, we could be liable for resulting damages.
We are subject to federal, state and local laws, rules and regulations governing the use, discharge, storage, handling and disposal of biological material, chemicals and waste. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, remediation costs and any related penalties or fines, and any liability could exceed our resources or any applicable insurance coverage we may have. The cost of compliance with these laws and regulations may become significant, and our failure to comply may result in substantial fines or other consequences, and either could negatively affect our operating results.
Security breaches, loss of data and other disruptions to us or our third-party service providers could compromise sensitive information related to our business or prevent us from accessing critical information and expose us to liability, which could adversely affect our business and our reputation.
In the ordinary course of our business, we and our third-party service providers collect and store sensitive data, including legally protected health information, personally identifiable information about our patients, credit card information, intellectual property, and our proprietary business and financial information. We manage and maintain our applications and data utilizing a combination of on-site systems, managed data center systems and cloud-based data center systems. We face a number of risks relative to our protection of, and our service providers' protection of, this critical information, including loss of access, inappropriate disclosure and inappropriate access, as well as risks associated with our ability to identify and audit such events.
The secure processing, storage, maintenance and transmission of this critical information is vital to our operations and business strategy, and we devote significant resources to protecting such information. Although we take measures to protect sensitive information from unauthorized access or disclosure, our information technology and infrastructure may be vulnerable to attacks by hackers or viruses or otherwise breached due to employee error, malfeasance or other activities. While we have not experienced any such attack or breach, if such event would occur and cause interruptions in our operations, our networks would be compromised and the information we store on those networks could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the Health Insurance Portability and Accountability Act of 1996, and regulatory penalties. Unauthorized access, loss or dissemination could also disrupt our operations, including our ability to process tests, provide test results, bill payers or patients, process claims and appeals, provide customer assistance services, conduct research and development activities, collect, process and prepare company
22
financial information, provide information about our solution and other patient and physician education and outreach efforts through our website, manage the administrative aspects of our business and damage our reputation, any of which could adversely affect our business.
In addition, the interpretation and application of consumer, health-related and data protection laws in the United States, Europe and elsewhere are often uncertain, contradictory and in flux. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. If so, this could result in government-imposed fines or orders requiring that we change our practices, which could adversely affect our business. Complying with these various laws could cause us to incur substantial costs or require us to change our business practices, systems and compliance procedures in a manner adverse to our business.
If we cannot license rights to use technologies on reasonable terms, we may not be able to commercialize new products in the future.
In the future, we may license third-party technology to develop or commercialize new products. In return for the use of a third party's technology, we may agree to pay the licensor royalties based on sales of our solutions. Royalties are a component of cost of services and affect the margins on our solutions. We may also need to negotiate licenses to patents and patent applications after introducing a commercial product. Our business may suffer if we are unable to enter into the necessary licenses on acceptable terms, or at all, if any necessary licenses are subsequently terminated, if the licensors fail to abide by the terms of the license or fail to prevent infringement by third parties, or if the licensed patents or other rights are found to be invalid or unenforceable.
If we are unable to protect our intellectual property effectively, our business would be harmed.
We rely on patent protection as well as trademark, copyright, trade secret and other intellectual property rights protection and contractual restrictions to protect our proprietary technologies, all of which provide limited protection and may not adequately protect our rights or permit us to gain or keep any competitive advantage. If we fail to protect our intellectual property, third parties may be able to compete more effectively against us and we may incur substantial litigation costs in our attempts to recover or restrict use of our intellectual property.
We apply for patents covering our products and technologies and uses thereof, as we deem appropriate, however we may fail to apply for patents on important products and technologies in a timely fashion or at all, or we may fail to apply for patents in potentially relevant jurisdictions. As of June 30, 2013, we had six pending United States non-provisional patent applications and one allowed patent application. It is possible that none of our pending patent applications will result in issued patents in a timely fashion or at all, and even if patents are granted, they may not provide a basis for intellectual property protection of commercially viable products, may not provide us with any competitive advantages, or may be challenged and invalidated by third parties. It is possible that others will design around our current or future patented technologies. We may not be successful in defending any challenges made against our patents or patent applications. Any successful third-party challenge to our patents could result in the unenforceability or invalidity of such patents and increased competition to our business. The outcome of patent litigation can be uncertain and any attempt by us to enforce our patent rights against others may not be successful, or, if successful, may take substantial time and result in substantial cost, and may divert our efforts and attention from other aspects of our business.
The patent positions of life sciences companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in such companies' patents has emerged to date in the United States or elsewhere. Courts frequently render opinions in the biotechnology field that may affect the patentability of certain inventions or discoveries, including opinions that may affect the patentability of methods for analyzing or comparing DNA.
23
In particular, the patent positions of companies engaged in the development and commercialization of genomic diagnostic tests, like Afirma, are particularly uncertain. Various courts, including the U.S. Supreme Court, have recently rendered decisions that affect the scope of patentability of certain inventions or discoveries relating to certain diagnostic tests and related methods. These decisions state, among other things, that patent claims that recite laws of nature (for example, the relationship between blood levels of certain metabolites and the likelihood that a dosage of a specific drug will be ineffective or cause harm) are not themselves patentable. What constitutes a law of nature is uncertain, and it is possible that certain aspects of genetic diagnostics tests would be considered natural laws. Accordingly, the evolving case law in the United States may adversely affect our ability to obtain patents and may facilitate third-party challenges to any owned and licensed patents. The laws of some foreign countries do not protect intellectual property rights to the same extent as the laws of the United States, and we may encounter difficulties protecting and defending such rights in foreign jurisdictions. The legal systems of many other countries do not favor the enforcement of patents and other intellectual property protection, particularly those relating to biotechnology, which could make it difficult for us to stop the infringement of our patents in such countries. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business.
Changes in either the patent laws or in interpretations of patent laws in the United States or other countries may diminish the value of our intellectual property. We cannot predict the breadth of claims that may be allowed or enforced in our patents or in third-party patents. We may not develop additional proprietary products, methods and technologies that are patentable.
In addition to pursuing patents on our technology, we take steps to protect our intellectual property and proprietary technology by entering into agreements, including confidentiality agreements, non-disclosure agreements and intellectual property assignment agreements, with our employees, consultants, academic institutions, corporate partners and, when needed, our advisors. Such agreements may not be enforceable or may not provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure or other breaches of the agreements, and we may not be able to prevent such unauthorized disclosure. If we are required to assert our rights against such party, it could result in significant cost and distraction.
Monitoring unauthorized disclosure is difficult, and we do not know whether the steps we have taken to prevent such disclosure are, or will be, adequate. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, it would be expensive and time consuming, and the outcome would be unpredictable. In addition, courts outside the United States may be less willing to protect trade secrets.
We may also be subject to claims that our employees have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers, or to claims that we have improperly used or obtained such trade secrets. Litigation may be necessary to defend against these claims. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights and face increased competition to our business. A loss of key research personnel work product could hamper or prevent our ability to commercialize potential products, which could harm our business. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to management.
Further, competitors could attempt to replicate some or all of the competitive advantages we derive from our development efforts, willfully infringe our intellectual property rights, design around our protected technology or develop their own competitive technologies that fall outside of our intellectual property rights. Others may independently develop similar or alternative products and technologies or replicate any of our products and technologies. If our intellectual property does not adequately protect us against competitors' products and methods, our competitive position could be adversely affected, as could our business.
24
We have not yet registered certain of our trademarks, including Afirma, in all of our potential markets. If we apply to register these trademarks, our applications may not be allowed for registration in a timely fashion or at all, and our registered trademarks may not be maintained or enforced. In addition, opposition or cancellation proceedings may be filed against our trademark applications and registrations, and our trademarks may not survive such proceedings. If we do not secure registrations for our trademarks, we may encounter more difficulty in enforcing them against third parties than we otherwise would.
To the extent our intellectual property offers inadequate protection, or is found to be invalid or unenforceable, we would be exposed to a greater risk of direct competition. If our intellectual property does not provide adequate coverage of our competitors' products, our competitive position could be adversely affected, as could our business. Both the patent application process and the process of managing patent disputes can be time consuming and expensive.
We may be involved in litigation related to intellectual property, which could be time-intensive and costly and may adversely affect our business, operating results or financial condition.
We may receive notices of claims of direct or indirect infringement or misappropriation or misuse of other parties' proprietary rights from time to time. Some of these claims may lead to litigation. We cannot assure you that we will prevail in such actions, or that other actions alleging misappropriation or misuse by us of third-party trade secrets, infringement by us of third-party patents and trademarks or other rights, or the validity of our patents, trademarks or other rights, will not be asserted or prosecuted against us.
We might not have been the first to make the inventions covered by each of our pending patent applications and we might not have been the first to file patent applications for these inventions. To determine the priority of these inventions, we may have to participate in interference proceedings, derivation proceedings, or other post-grant proceedings declared by the United States Patent and Trademark Office that could result in substantial cost to us. No assurance can be given that other patent applications will not have priority over our patent applications. In addition, recent changes to the patent laws of the United States allow for various post-grant opposition proceedings that have not been extensively tested, and their outcome is therefore uncertain. Furthermore, if third parties bring these proceedings against our patents, we could experience significant costs and management distraction.
Litigation may be necessary for us to enforce our patent and proprietary rights or to determine the scope, coverage and validity of the proprietary rights of others. The outcome of any litigation or other proceeding is inherently uncertain and might not be favorable to us, and we might not be able to obtain licenses to technology that we require on acceptable terms or at all. Further, we could encounter delays in product introductions, or interruptions in product sales, as we develop alternative methods or products. In addition, if we resort to legal proceedings to enforce our intellectual property rights or to determine the validity, scope and coverage of the intellectual property or other proprietary rights of others, the proceedings could be burdensome and expensive, even if we were to prevail. Any litigation that may be necessary in the future could result in substantial costs and diversion of resources and could have a material adverse effect on our business, operating results or financial condition.
As we move into new markets and applications for our products, incumbent participants in such markets may assert their patents and other proprietary rights against us as a means of slowing our entry into such markets or as a means to extract substantial license and royalty payments from us. Our competitors and others may now and, in the future, have significantly larger and more mature patent portfolios than we currently have. In addition, future litigation may involve patent holding companies or other adverse patent owners who have no relevant product revenue and against whom our own patents may provide little or no deterrence or protection. Therefore, our commercial success may depend in part on our non-infringement of the patents or proprietary rights of third parties. Numerous significant intellectual property issues have been litigated, and will likely continue to be litigated, between existing and
25
new participants in our existing and targeted markets and competitors may assert that our products infringe their intellectual property rights as part of a business strategy to impede our successful entry into or growth in those markets. Third parties may assert that we are employing their proprietary technology without authorization. In addition, our competitors and others may have patents or may in the future obtain patents and claim that making, having made, using, selling, offering to sell or importing our products infringes these patents. We could incur substantial costs and divert the attention of our management and technical personnel in defending against any of these claims. Parties making claims against us may be able to obtain injunctive or other relief, which could block our ability to develop, commercialize and sell products, and could result in the award of substantial damages against us. In the event of a successful claim of infringement against us, we may be required to pay damages and ongoing royalties, and obtain one or more licenses from third parties, or be prohibited from selling certain products. We may not be able to obtain these licenses on acceptable terms, if at all. We could incur substantial costs related to royalty payments for licenses obtained from third parties, which could negatively affect our financial results. In addition, we could encounter delays in product introductions while we attempt to develop alternative methods or products to avoid infringing third-party patents or proprietary rights. Defense of any lawsuit or failure to obtain any of these licenses could prevent us from commercializing products, and the prohibition of sale of any of our products could materially affect our business and our ability to gain market acceptance for our products.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, during the course of this kind of litigation, there could be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
In addition, our agreements with some of our customers, suppliers or other entities with whom we do business require us to defend or indemnify these parties to the extent they become involved in infringement claims, including the types of claims described above. We could also voluntarily agree to defend or indemnify third parties in instances where we are not obligated to do so if we determine it would be important to our business relationships. If we are required or agree to defend or indemnify third parties in connection with any infringement claims, we could incur significant costs and expenses that could adversely affect our business, operating results, or financial condition.
Our inability to raise additional capital on acceptable terms in the future may limit our ability to develop and commercialize new solutions and technologies and expand our operations.
We expect capital expenditures and operating expenses to increase over the next several years as we expand our infrastructure, commercial operations and research and development activities. We may seek to raise additional capital through equity offerings, debt financings, collaborations or licensing arrangements. Additional funding may not be available to us on acceptable terms, or at all. If we raise funds by issuing equity securities, dilution to our stockholders could result. Any equity securities issued also may provide for rights, preferences or privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings could impose significant restrictions on our operations. The incurrence of additional indebtedness or the issuance of certain equity securities could result in increased fixed payment obligations and could also result in restrictive covenants, such as limitations on our ability to incur additional debt or issue additional equity, limitations on our ability to acquire or license intellectual property rights, and other operating restrictions that could adversely affect our ability to conduct our business. In addition, the issuance of additional equity securities by us, or the possibility of such issuance, may cause the market price of our common stock to decline. In the event that we enter into collaborations or licensing arrangements to raise capital, we may be required to accept unfavorable terms. These agreements may require that we relinquish or license to a third party on unfavorable terms our rights to
26
technologies or product candidates that we otherwise would seek to develop or commercialize ourselves, or reserve certain opportunities for future potential arrangements when we might be able to achieve more favorable terms. If we are not able to secure additional funding when needed, we may have to delay, reduce the scope of or eliminate one or more research and development programs or selling and marketing initiatives. In addition, we may have to work with a partner on one or more of our products or market development programs, which could lower the economic value of those programs to our company.
Risks Related to Being a Public Company
We will incur increased costs and demands on management as a result of compliance with laws and regulations applicable to public companies, which could harm our operating results.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company, including costs associated with public company reporting requirements. In addition, the Sarbanes-Oxley Act of 2002 and the Dodd-Frank Act of 2010, as well as rules implemented by the Securities and Exchange Commission, or the SEC, and The NASDAQ Stock Market, impose a number of requirements on public companies, including with respect to corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these compliance and disclosure obligations. Moreover, these rules and regulations will increase our legal, accounting and financial compliance costs and will make some activities more time-consuming and costly. We also expect that it will be more expensive for us to obtain director and officer liability insurance.
If we are unable to implement and maintain effective internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our reported financial information and the market price of our common stock may be negatively affected.
As a public company, we will be required to maintain internal control over financial reporting and to report any material weaknesses in such internal control. Section 404 of the Sarbanes-Oxley Act of 2002 requires that we evaluate and determine the effectiveness of our internal control over financial reporting and, beginning with our annual report for the year ending December 31, 2014, provide a management report on the internal control over financial reporting. If we have a material weakness in our internal control over financial reporting, we may not detect errors on a timely basis and our financial statements may be materially misstated. We are in the process of compiling the system and processing documentation necessary to perform the evaluation needed to comply with Section 404 of the Sarbanes-Oxley Act. We may not be able to complete our evaluation, testing and any required remediation in a timely fashion. During the evaluation and testing process, if we identify one or more material weaknesses in our internal control over financial reporting, our management will be unable to conclude that our internal control over financial reporting is effective. Moreover, when we are no longer an emerging growth company, our independent registered public accounting firm will be required to issue an attestation report on the effectiveness of our internal control over financial reporting. Even if our management concludes that our internal control over financial reporting is effective, our independent registered public accounting firm may conclude that there are material weaknesses with respect to our internal controls or the level at which our internal controls are documented, designed, implemented or reviewed.
If we are unable to conclude that our internal control over financial reporting is effective, or when we are no longer an emerging growth company, if our auditors were to express an adverse opinion on the effectiveness of our internal control over financial reporting because we had one or more material weaknesses, investors could lose confidence in the accuracy and completeness of our financial disclosures, which could cause the price of our common stock to decline. Internal control deficiencies could also result in a restatement of our financial results in the future.
27
We are an emerging growth company and may elect to comply with reduced public company reporting requirements applicable to emerging growth companies, which could make our common stock less attractive to investors.
We are an emerging growth company, as defined under the Securities Act of 1933, or the Securities Act. We will remain an emerging growth company for up to five years, although if our revenue exceeds $1 billion in any fiscal year before that time, we would cease to be an emerging growth company as of the end of that fiscal year. In addition, if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our second fiscal quarter of any fiscal year before the end of that five-year period, we would cease to be an emerging growth company as of December 31 of that year. As an emerging growth company, we may choose to take advantage of exemptions from various reporting requirements applicable to certain other public companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced financial statement and financial-related disclosures, reduced disclosure obligations regarding executive compensation in our periodic reports and proxy statements, and exemptions from the requirement of holding a nonbinding advisory vote on executive compensation and obtaining stockholder approval of any golden parachute payments not previously approved by our stockholders. We cannot predict whether investors will find our common stock less attractive if we choose to rely on any of these exemptions. If some investors find our common stock less attractive as a result of any choices to reduce future disclosure we may make, there may be a less active trading market for our common stock and our stock price may be more volatile.
Risks Related to this Offering and Our Common Stock
Our stock price may be volatile, and you may not be able to sell shares of our common stock at or above the price you paid.
Prior to this offering, there has been no public market for our common stock, and an active public market for our stock may not develop or be sustained after this offering. We and the representatives of the underwriters will determine the initial public offering price of our common stock through negotiation. This price will not necessarily reflect the price at which investors in the market will be willing to buy and sell our stock following this offering. In addition, the trading price of our common stock following this offering is likely to be highly volatile and could be subject to wide fluctuations in response to various factors, some of which are beyond our control. These factors include:
- •
- actual or anticipated variations in our and our competitors' results of operations;
- •
- announcements by us or our competitors of new products, commercial relationships or capital commitments;
- •
- changes in reimbursement by current or potential payers;
- •
- issuance of new securities analysts' reports or changed recommendations for our stock;
- •
- periodic fluctuations in our revenue, due in part to the way in which we recognize revenue;
- •
- actual or anticipated changes in regulatory oversight of our products;
- •
- developments or disputes concerning our intellectual property or other proprietary rights;
- •
- commencement of, or our involvement in, litigation;
- •
- announced or completed acquisitions of businesses or technologies by us or our competitors;
- •
- any major change in our management; and
- •
- general economic conditions and slow or negative growth of our markets.
28
In addition, the stock market in general, and the market for stock of life sciences companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to the operating performance of those companies. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance. These fluctuations may be even more pronounced in the trading market for our stock shortly following this offering. In addition, in the past, following periods of volatility in the overall market and the market price of a particular company's securities, securities class action litigation has often been instituted against these companies. This litigation, if instituted against us, could result in substantial costs and a diversion of our management's attention and resources.
If securities or industry analysts issue an adverse opinion regarding our stock or do not publish research or reports about our company, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that equity research analysts publish about us and our business. We do not control these analysts or the content and opinions included in their reports. Securities analysts may elect not to provide research coverage of our company after the completion of this offering, and such lack of research coverage may adversely affect the market price of our common stock. The price of our common stock could also decline if one or more equity research analysts downgrade our common stock or if those analysts issue other unfavorable commentary or cease publishing reports about us or our business. If one or more equity research analysts cease coverage of our company, we could lose visibility in the market, which in turn could cause our stock price to decline.
Future sales of shares by existing stockholders could cause our stock price to decline.
If our existing stockholders sell, or indicate an intention to sell, substantial amounts of our common stock in the public market after the lock-up and other legal restrictions on resale discussed in this prospectus lapse, the trading price of our common stock could decline. Based on shares outstanding as of , 2013, upon completion of this offering, we will have outstanding a total of shares of common stock. Of these shares, only of the shares of common stock sold in this offering will be freely tradable, without restriction, in the public market immediately after the offering. Each of our directors and officers and substantially all of our other stockholders has entered into a lock-up agreement with the underwriters that restricts their ability to sell or transfer their shares. The lock-up agreements pertaining to this offering will expire 180 days from the date of this prospectus. The underwriters, however, may, in their sole discretion, waive the contractual lock-up prior to the expiration of the lock-up agreements. After the lock-up agreements expire, based on shares outstanding as of , 2013, up to an additional shares of common stock will be eligible for sale in the public market, of which are held by directors, executive officers and other affiliates and will be subject to volume limitations under Rule 144 under the Securities Act, and various vesting agreements. In addition, shares of common stock that are subject to outstanding options as of , 2013 will become eligible for sale in the public market to the extent permitted by the provisions of various vesting agreements, the lock-up agreements and Rules 144 and 701 under the Securities Act. We intend to file a registration statement on Form S-8 under the Securities Act covering all of the shares of common stock subject to options outstanding and reserved for issuance under our stock plans. This registration statement will become effective immediately upon filing, and shares covered by this registration statement will be eligible for sale in the public markets, subject to Rule 144 limitations applicable to affiliates and any lock-up agreements described above. If these additional shares are sold, or if it is perceived that they will be sold, in the public market, the trading price of our common stock could decline.
29
Insiders have substantial control over us and will be able to influence corporate matters.
As of , 2013, directors and executive officers and their affiliates beneficially owned, in the aggregate, % of our outstanding capital stock. As a result, these stockholders will be able to exercise significant influence over all matters requiring stockholder approval, including the election of directors and approval of significant corporate transactions, such as a merger or other sale of our company or its assets. This concentration of ownership could limit stockholders' ability to influence corporate matters and may have the effect of delaying or preventing a third party from acquiring control over us.
Anti-takeover provisions in our charter documents and under Delaware law could discourage, delay or prevent a change in control and may affect the trading price of our common stock.
Provisions in our restated certificate of incorporation and our amended and restated bylaws to become effective upon completion of this offering may have the effect of delaying or preventing a change of control or changes in our management. Our restated certificate of incorporation and amended and restated bylaws include provisions that:
- •
- authorize our board of directors to issue, without further action by the stockholders, up to shares of
undesignated preferred stock;
- •
- require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by
written consent;
- •
- specify that special meetings of our stockholders can be called only by our board of directors, our chairman of the board,
or our chief executive officer;
- •
- establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our
stockholders, including proposed nominations of persons for election to our board of directors;
- •
- establish that our board of directors is divided into three classes, Class I, Class II and Class III,
with each class serving staggered terms;
- •
- provide that our directors may be removed only for cause;
- •
- provide that vacancies on our board of directors may, except as otherwise required by law, be filled only by a majority of
directors then in office, even if less than a quorum;
- •
- specify that no stockholder is permitted to cumulate votes at any election of directors; and
- •
- require a super-majority of votes to amend certain of the above-mentioned provisions.
In addition, we are subject to the provisions of Section 203 of the Delaware General Corporation Law regulating corporate takeovers. Section 203 generally prohibits us from engaging in a business combination with an interested stockholder subject to certain exceptions.
Our management will have broad discretion in the use of the net proceeds from this offering and may not use them effectively.
Our management will have broad discretion in the application of the net proceeds from this offering and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Our failure to apply these funds effectively could have a material adverse effect on our business, delay the development of new tests and cause the price of our common stock to decline.
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
The initial public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock immediately after this offering. Therefore, if you purchase our
30
common stock in this offering, you will incur an immediate dilution of $ in net tangible book value per share from the price you paid, based on an assumed initial public offering price of $ per share. In addition, new investors who purchase shares in this offering will contribute approximately % of the total amount of equity capital raised by us through the date of this offering, but will only own approximately % of the outstanding equity capital. The exercise of outstanding options and warrants will result in further dilution. For a detailed description of the dilution that you will experience immediately after this offering, see "Dilution".
We have never paid dividends on our capital stock, and we do not anticipate paying dividends in the foreseeable future.
We have never paid dividends on any of our capital stock and currently intend to retain any future earnings to fund the growth of our business. In addition, our loan and security agreement restricts our ability to pay cash dividends on our common stock and we may also enter into credit agreements or other borrowing arrangements in the future that will restrict our ability to declare or pay cash dividends on our common stock. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. As a result, capital appreciation, if any, of our common stock will be the sole source of gain for the foreseeable future.
31
INFORMATION REGARDING FORWARD-LOOKING STATEMENTS
This prospectus includes forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, strategy and plans, and our expectations for future operations, are forward-looking statements. The words "believe," "may," "will," "estimate," "continue," "anticipate," "design," "intend," "expect" or the negative version of these words and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements on our current expectations and projections about future events and trends that we believe may affect our financial condition, results of operations, strategy, short- and long-term business operations and objectives, and financial needs. These forward-looking statements are subject to a number of risks, uncertainties and assumptions, including those described in "Risk Factors". In light of these risks, uncertainties and assumptions, the forward-looking events and circumstances discussed in this prospectus may not occur, and actual results could differ materially and adversely from those anticipated or implied in the forward-looking statements. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Forward-looking statements include, but are not limited to, statements about:
- •
- our ability to continue to increase adoption of and obtain reimbursement for Afirma;
- •
- anticipated trends and challenges in our business and the competition that we face;
- •
- the execution of our business plan and our growth strategy;
- •
- our expectations regarding the size of and growth in potential markets;
- •
- changes in laws or regulations applicable to our business, including potential regulation by the FDA;
- •
- our strategic relationships, collaboration and co-promotion efforts;
- •
- our ability to develop and commercialize new products and the timing of commercialization;
- •
- the outcome or success of clinical studies;
- •
- our liquidity and working capital requirements;
- •
- our expectations regarding future revenue and expenses; and
- •
- our expectations regarding the use of proceeds from this offering.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, level of activity, performance or achievements. In addition, neither we nor any other person assumes responsibility for the accuracy and completeness of any of these forward-looking statements. Any forward-looking statement made by us in this prospectus speaks only as of the date on which it is made. We disclaim any duty to update any of these forward-looking statements after the date of this prospectus to confirm these statements to actual results or revised expectations.
You may rely only on the information contained in this prospectus. You should read this prospectus and the documents that we reference in this prospectus and have filed with the Securities and Exchange Commission as exhibits to the registration statement of which this prospectus is a part completely and with the understanding that our actual future results, levels of activity, performance and events and circumstances may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements.
This prospectus also contains statistical data and estimates that we obtained from industry publications and reports. These publications typically indicate that they have obtained their information from sources they believe to be reliable, but do not guarantee the accuracy and completeness of their information. Although we have assessed the information in the publications and found it to be reasonable and believe the publications are reliable, we have not independently verified their data.
32
We estimate that the net proceeds from the sale of shares of our common stock in this offering will be approximately $ , based on an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. If the underwriters' over-allotment option to purchase additional shares from us is exercised in full, we estimate that our net proceeds will be approximately $ . A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the estimated net proceeds to us by $ million, assuming that the number of shares offered by us as set forth on the cover page of this prospectus remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) the net proceeds to us by $ million, assuming a price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds received by us from this offering for working capital and general corporate purposes, including continued investments in research and development and in the growth of our business. In addition, we may use a portion of the net proceeds received by us from this offering for acquisitions of complementary businesses, technologies or other assets. We have no agreements with respect to any material acquisitions at this time, and we have not allocated specific amounts of net proceeds for any of these purposes.
We cannot specify with certainty the particular amounts or uses for the net proceeds to be received by us from this offering. Accordingly, our management will have broad discretion in using the net proceeds to be received by us from this offering.
Pending the use of proceeds from this offering as described above, we plan to invest the net proceeds in short- and intermediate-term, interest-bearing obligations, investment-grade instruments, certificates of deposit or direct or guaranteed obligations of the U.S. government.
We have never declared or paid any cash dividend on our capital stock. We currently intend to retain any future earnings and do not expect to pay any dividends in the foreseeable future. Our loan and security agreement restricts our ability to pay cash dividends on our common stock, and we may also enter into credit agreements or other borrowing arrangements in the future that will further restrict our ability to declare or pay cash dividends on our common stock. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant.
33
The following table sets forth our cash and cash equivalents and capitalization as of June 30, 2013, as follows:
- •
- on an actual basis;
- •
- on a pro forma basis to give effect to the conversion of all outstanding shares of our convertible preferred stock into
common stock immediately prior to the completion of this offering; and
- •
- on a pro forma as adjusted basis to give further effect to the receipt of the estimated net proceeds from the sale of shares of common stock in this offering at a price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated expenses payable by us.
You should read this table in conjunction with "Selected Financial Data" and "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements and related notes included elsewhere in this prospectus.
| |
As of June 30, 2013 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Actual | Pro Forma | Pro Forma as Adjusted |
|||||||
| |
(In thousands, except share and per share data) |
|||||||||
| |
(Unaudited) |
|||||||||
Cash and cash equivalents |
$ | 20,683 | $ | $ | ||||||
Long-term debt, net of discount |
4,826 | |||||||||
Preferred stock warrant liability |
175 | |||||||||
Convertible preferred stock, par value $0.001 per share: 60,187,700 shares authorized, 59,989,268 issued and outstanding, actual; no shares authorized, issued or outstanding, pro forma and pro forma as adjusted |
79,025 | |||||||||
Stockholders' (deficit) equity: |
||||||||||
Common stock, par value $0.001 per share: 77,000,000 shares authorized, 3,714,902 shares issued and outstanding, actual; shares authorized, 63,704,170 shares issued and outstanding, pro forma; shares issued and outstanding, pro forma as adjusted |
4 | |||||||||
Additional paid-in capital |
2,663 | |||||||||
Accumulated deficit |
(73,455 | ) | ||||||||
Total stockholders' (deficit) equity |
(70,788 | ) | ||||||||
Total capitalization |
$ | 13,238 | $ | $ | ||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) each of cash and cash equivalents, additional paid-in capital, total capitalization and total stockholders' (deficit) equity by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) cash and cash equivalents, additional paid-in capital, total capitalization and total stockholders' (deficit) equity by approximately $ million, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting
34
discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
If the underwriters' over-allotment option were exercised in full, pro forma as adjusted cash and cash equivalents, common stock, additional paid-in capital, total stockholders' deficit and shares issued and outstanding as of June 30, 2013 would be $ , $ , $ , $ and , respectively.
The number of shares of common stock in the table above excludes:
- •
- 9,681,245 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2013, at a
weighted average exercise price of $0.70 per share;
- •
- 99,206 shares of common stock issuable upon the exercise of warrants to purchase Series C preferred stock, which
will become exercisable for shares of common stock upon conversion of our Series C preferred stock into common stock immediately prior to the completion of this offering, with an exercise price
of $1.89 per share; and
- •
- 574,821 shares of common stock reserved for future issuance under our 2008 Stock Plan and shares of common stock, subject to increase on an annual basis, reserved for future issuance under our 2013 Stock Incentive Plan, which will become effective in connection with this offering.
35
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the initial public offering price per share of our common stock and the pro forma as adjusted net tangible book value per share of our common stock immediately after this offering.
Net tangible book value per share is determined by dividing our total tangible assets less our total liabilities by the number of shares of common stock outstanding. Our historical net tangible book value (deficit) as of June 30, 2013, was $ , or $ per share of common stock. Our pro forma net tangible book value (deficit) as of June 30, 2013, was $ , or $ per share of common stock, based on the total number of shares of our common stock outstanding as of June 30, 2013, after giving effect to the conversion of all outstanding shares of our convertible preferred stock into common stock.
After giving effect to the sale of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, our pro forma as adjusted net tangible book value as of June 30, 2013 would have been $ , or $ per share. This represents an immediate increase in pro forma net tangible book value of $ per share to existing stockholders and an immediate dilution in net tangible book value of $ per share to purchasers of common stock in this offering, as illustrated in the following table:
Assumed initial public offering price per share |
$ | ||||||
Pro forma net tangible book value (deficit) per share as of June 30, 2013 |
$ | ||||||
Increase in pro forma net tangible book value (deficit) per share attributable to new investors |
|||||||
Pro forma as adjusted net tangible book value (deficit) per share after this offering |
|||||||
Dilution per share to investors participating in this offering |
$ | ||||||
Each $1.00 increase (decrease) in the assumed public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) our pro forma as adjusted net tangible book value by approximately $ million, or approximately $ per share, and the dilution per share to investors in this offering by approximately $ per share, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. We may also increase or decrease the number of shares we are offering. An increase (decrease) of 1.0 million shares in the number of shares offered by us would (decrease) our pro forma as adjusted net tangible book value by approximately $ million, or approximately $ per share, and the pro forma dilution per share to investors in this offering by approximately $ per share, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
If the underwriters' over-allotment option to purchase additional shares is exercised in full, the pro forma as adjusted net tangible book value per share after this offering would be $ per share, the increase in pro forma as adjusted net tangible book value per share to existing stockholders would be $ per share and the dilution to new investors purchasing shares in this offering would be $ per share.
36
The following table presents, on a pro forma as adjusted basis as of June 30, 2013, the differences between existing stockholders and purchasers of shares in this offering with respect to the number of shares purchased from us, the total consideration paid or to be paid and the average price paid per share assuming with respect to the purchasers of shares in this offering an initial public offering price of $ per share, the midpoint of the price range on the cover of this prospectus before deducting estimated underwriting discounts and commissions and estimated expenses payable by us:
| |
Total Shares | Total Consideration | |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Average Price per Share |
||||||||||||||
| |
Number | Percent | Amount | Percent | |||||||||||
Existing stockholders before this offering |
% | $ | % | $ | |||||||||||
Investors participating in this offering |
|||||||||||||||
Total |
100 | % | $ | 100 | % | ||||||||||
Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, would increase (decrease) the total consideration paid to us by new investors and total consideration paid to us by all stockholders by $ million, assuming that the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase (decrease) of 1.0 million shares in the number of shares offered by us would increase (decrease) the total consideration paid to us by new investors and total consideration paid to us by all stockholders by $ million, assuming an initial public offering price of $ per share, the midpoint of the price range set forth on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us.
If the underwriters' over-allotment option to purchase additional shares is exercised in full, existing stockholders would own % and new investors would own % of the total number of shares of our common stock outstanding immediately after this offering.
The calculations above are based on shares outstanding as of June 30, 2013 after giving effect to the conversion of all outstanding shares of convertible preferred stock into common stock and exclude:
- •
- 9,681,245 shares of common stock issuable upon the exercise of options outstanding as of June 30, 2013, at a
weighted average exercise price of $0.70 per share;
- •
- 99,206 shares of common stock issuable upon the exercise of warrants to purchase Series C preferred stock, which
will become exercisable for shares of common stock upon conversion of our Series C preferred stock into common stock immediately prior to the completion of this offering, with an exercise price
of $1.89 per share; and
- •
- 574,821 shares of common stock reserved for future issuance under our 2008 Stock Plan and shares of common stock, subject to increase on an annual basis, reserved for future issuance under our 2013 Stock Incentive Plan, which will become effective in connection with this offering.
To the extent that any outstanding options or warrants are exercised or new options are issued under our incentive plans, there will be further dilution to investors participating in this offering.
37
We derived the selected statements of operations data for the years ended December 31, 2011 and 2012 and the selected balance sheets data as of December 31, 2011 and 2012 from our audited financial statements included elsewhere in this prospectus. We derived the selected statements of operations data for the six months ended June 30, 2012 and 2013 and the selected balance sheets data as of June 30, 2013 from our unaudited interim condensed financial statements and related notes included elsewhere in this prospectus. Our unaudited interim condensed financial statements were prepared on the same basis as our audited financial statements and include, in our opinion, all adjustments, consisting of normal recurring adjustments that we consider necessary for a fair presentation of the financial information set forth in those financial statements. Historical results are not necessarily indicative of the results that may be expected in the future. You should read the selected financial data together with "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our financial statements, related notes and other financial information included elsewhere in this prospectus. The selected financial data is qualified in its entirety by the financial statements and related notes included elsewhere in this prospectus.
| |
Year Ended December 31, | Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | 2012 | 2013 | |||||||||
| |
(In thousands, except share and per share data) |
||||||||||||
| |
|
|
(Unaudited) |
||||||||||
Statements of Operations Data: |
|||||||||||||
Revenue |
$ | 2,645 | $ | 11,628 | $ | 3,947 | $ | 9,452 | |||||
Operating expenses: |
|||||||||||||
Cost of revenue(1) |
2,925 | 7,584 | 3,000 | 6,004 | |||||||||
Research and development(1) |
6,680 | 6,608 | 3,158 | 3,912 | |||||||||
Selling and marketing(1) |
2,934 | 8,447 | 3,045 | 5,318 | |||||||||
General and administrative(1) |
5,372 | 7,918 | 3,618 | 5,528 | |||||||||
Total operating expenses(1) |
17,911 | 30,557 | 12,821 | 20,762 | |||||||||
Loss from operations |
(15,266 | ) | (18,929 | ) | (8,874 | ) | (11,310 | ) | |||||
Interest income |
2 | 2 | — | — | |||||||||
Interest expense |
— | — | — | (5 | ) | ||||||||
Other income (expense), net |
819 | 278 | — | (2,070 | ) | ||||||||
Net loss |
$ | (14,445 | ) | $ | (18,649 | ) | $ | (8,874 | ) | $ | (13,385 | ) | |
Net loss per common share, basic and diluted |
$ | (6.23 | ) | $ | (7.17 | ) | $ | (3.48 | ) | $ | (4.12 | ) | |
Shares used in computing net loss per common share, basic and diluted |
2,320,252 | 2,601,352 | 2,553,287 | 3,250,863 | |||||||||
Other Operating Data: |
|||||||||||||
FNAs received |
6,402 | 25,890 | 9,535 | 23,181 | |||||||||
- (1)
- Includes employee stock-based compensation as follows:
| |
Year Ended December 31, |
Six Months Ended June 30, |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | 2012 | 2013 | ||||||||||
| |
(In thousands) |
|||||||||||||
| |
|
|
(Unaudited) |
|||||||||||
Cost of revenue |
$ | 32 | $ | 26 | $ | 16 | $ | 13 | ||||||
Research and development |
130 | 131 | 48 | 103 | ||||||||||
Selling and marketing |
77 | 111 | 52 | 76 | ||||||||||
General and administrative |
227 | 407 | 174 | 297 | ||||||||||
Total stock-based compensation |
$ | 466 | $ | 675 | $ | 290 | $ | 489 | ||||||
| |
As of December 31, | |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
As of June 30, 2013 |
|||||||||
| |
2011 | 2012 | ||||||||
| |
(In thousands) |
|||||||||
| |
|
|
(Unaudited) |
|||||||
Balance Sheets Data: |
||||||||||
Cash and cash equivalents |
$ | 7,566 | $ | 14,002 | $ | 20,683 | ||||
Working capital |
6,707 | 7,390 | 14,049 | |||||||
Total assets |
10,451 | 19,067 | 27,159 | |||||||
Convertible preferred stock |
49,296 | 63,372 | 79,025 | |||||||
Accumulated deficit |
(41,420 | ) | (60,069 | ) | (73,455 | ) | ||||
Total stockholders' (deficit) equity |
(40,766 | ) | (58,471 | ) | (70,788 | ) | ||||
38
MANAGEMENT'S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed below. Factors that could cause or contribute to such differences include, but are not limited to, those identified below and those discussed in "Risk Factors" included elsewhere in this prospectus.
Overview
We are a diagnostics company pioneering the field of molecular cytology to improve patient outcomes and lower healthcare costs. We specifically target diseases that often require invasive procedures for an accurate diagnosis – diseases where many healthy patients undergo costly interventions that ultimately prove unnecessary. We improve the accuracy of diagnosis at an earlier stage of patient care by deriving clinically actionable genomic information from cytology samples collected in an outpatient setting. Our first commercial solution, the Afirma Thyroid FNA Analysis, includes as its centerpiece our Gene Expression Classifier, which we refer to as the GEC. The GEC helps physicians reduce the number of unnecessary surgeries by employing a proprietary 142-gene signature to preoperatively determine whether thyroid nodules previously classified by cytopathology as indeterminate can be reclassified as benign. We have demonstrated the clinical utility and cost effectiveness of the GEC in studies published in peer-reviewed journals and established the clinical validity of the GEC in a study published in The New England Journal of Medicine in 2012. Since we commercially launched Afirma in January 2011, we have processed over 50,000 fine needle aspiration, or FNA, samples for evaluation using Afirma and performed more than 10,000 GECs to resolve indeterminate cytopathology results.
We market and sell our solution with a sales force consisting of our own sales professionals and members of the Genzyme endocrinology sales team. In January 2012, we entered into a co-promotion agreement with Genzyme for the co-exclusive right to promote and market Afirma in the United States and in 40 countries pursuant to which we received a $10.0 million fee from Genzyme. Under the agreement, we are required to pay Genzyme a co-promotion fee that is equal to a percentage of our cash receipts from Afirma.
As of August 2013, the list price for the GEC is $4,275. We invoice for routine cytopathology at a standard price of $490. We obtained Medicare coverage for the GEC effective in January 2012 which provides reimbursement at an agreed upon rate. In addition, we received positive coverage decisions for the GEC from UnitedHealthcare in March 2013, Aetna in June 2013 and Humana in July 2013, and have also received positive coverage decisions from a number of other smaller payers. Collectively, these payers represent more than 100 million covered lives. Reimbursement rates vary by payer.
Our revenue increased from $2.6 million in 2011 to $11.6 million in 2012. Our revenue increased from $3.9 million for the six months ended June 30, 2012 to $9.5 million for six months ended June 30, 2013. We incurred a net loss of $14.4 million and $18.6 million for the years ended December 31, 2011 and 2012, respectively, and $13.4 million for the six months ended June 30, 2013. As of June 30, 2013, we had an accumulated deficit of $73.5 million.
Financial Overview
Revenue
We generate revenue from the sale of our Afirma solution. We generally invoice third-party payers upon delivery of a patient report to the prescribing physician. As such, we take the assignment of benefits and the risk of collection from the third-party payer and individual patients.
39
For tests performed where an agreed upon reimbursement rate and a predictable history of collections exists, such as in the case of Medicare, we recognize revenue upon delivery of a patient report to the prescribing physician. In all other situations, as we do not have sufficient history of collection and are not able to determine a predictable pattern of payment, we recognize revenue upon the earlier of receipt of third-party payer notification of payment or when cash is received. Our ability to increase our revenue will depend on our ability to penetrate the market, obtain contracted reimbursement from additional third-party payers and increase our collection rate for tests performed.
Cost of Revenue
The components of our cost of revenue are materials and service costs, including stock-based compensation expense, direct labor costs, equipment and infrastructure expenses associated with testing samples, shipping charges to transport samples, and allocated overhead including rent, information technology, equipment depreciation and utilities. Costs associated with performing tests are recorded as the test is processed regardless of whether and when revenue is recognized with respect to that test. As a result, our cost of revenue as a percentage of revenue may vary significantly from period to period because we do not recognize all revenue in the period in which the associated costs are incurred. We expect cost of revenue in absolute dollars to increase as the number of tests we perform increases. However, we expect that the cost per test will decrease over time due to the efficiencies we may gain as test volume increases and from automation and other cost reductions.
Research and Development
Research and development expenses include costs incurred to develop our technology, collect clinical samples and conduct clinical studies to develop and support our products. These costs consist of personnel costs, including stock-based compensation expense, prototype materials, laboratory supplies, consulting costs, costs associated with setting up and conducting clinical studies at domestic and international sites and allocated overhead including rent, information technology, equipment depreciation and utilities. We expense all research and development costs in the periods in which they are incurred. We expect our research and development expenses will increase in absolute dollars in future periods as we continue to invest in research and development activities related to developing additional products.
Selling and Marketing
Selling and marketing expenses consist of personnel costs, including stock-based compensation expense, direct marketing expenses, consulting costs, and allocated overhead including rent, information technology, equipment depreciation and utilities. In addition, up-front co-promotion fees paid to Genzyme, net of amortization, are included in selling and marketing expenses. We expect our selling and marketing expenses to increase in future periods primarily driven by the co-promotion fees to Genzyme, the hiring of additional internal sales personnel and marketing and education expenses to drive market penetration and reimbursement.
General and Administrative
General and administrative expenses include executive, finance and accounting, human resources, billing and client services, and quality and regulatory functions. These expenses include personnel costs, including stock-based compensation expense, audit and legal expenses, consulting costs, and allocated overhead, including rent, information technology, equipment depreciation and utilities. We expect to incur additional expenses as a result of operating as a public company, including expenses related to compliance with the rules and regulations of the Securities and Exchange Commission and The NASDAQ Stock Market, additional insurance expenses, investor relations activities and other administrative and professional services. We also expect our general and administration expenses will increase in absolute dollars as we expand our billing and client services functions.
40
Interest Income
Interest income is from interest on our cash equivalents.
Interest Expense
Interest expense is attributable to our borrowings under the loan agreement entered into in June 2013.
Other Income (Expense), Net
Other income (expense), net is related primarily to the change in value of the preferred stock liability associated with our obligation to issue additional shares of Series B and Series C convertible preferred stock. In June 2010, we entered into a tranched Series B convertible preferred stock purchase agreement. In November 2012, we entered into a tranched Series C convertible preferred stock purchase agreement. In connection with the initial closing of each of these agreements, we agreed to issue to the purchasers, and the purchasers agreed to purchase, additional shares of the Series B and Series C convertible preferred stock within a specified timeframe. We determined that the liability to issue additional Series B and Series C convertible preferred stock at a future date was a freestanding instrument that should be accounted for as a liability. Accordingly, we recorded a liability related to this instrument at the time of each initial close in June 2010 and November 2012 and remeasure the liabilities at each reporting period with the corresponding gain or loss from the adjustment recorded as other income (expense), net. The Series B liability expired in July 2011. The Series C liability expired in June 2013.
In addition, other income (expense), net in 2011 includes $0.1 million we received from Genzyme in exchange for exclusive rights to negotiate a co-promotion agreement.
Critical Accounting Polices and Estimates
Our management's discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with United States generally accepted accounting principles, or U.S. GAAP. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements, as well as the reported revenue generated and expenses incurred during the reporting periods. Our estimates are based on our historical experience and on various other factors that we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions and any such differences may be material. We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management's judgments and estimates.
Revenue Recognition
Our revenue is generated from the sale of Afirma, a diagnostic solution for the assessment of thyroid nodules. We generally bill third-party payers upon delivery of a patient report to the prescribing physician. As such, we take assignment of benefits and risk of collections from the third-party payer and individual patients.
Revenue is recognized when persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed or determinable; and collectability is reasonably assured. The assessment of the fixed or determinable nature of the fees charged for testing performed and the collectability of those fees require significant judgment by management. When evaluating these criteria, we consider whether we have sufficient history to reliably estimate a payer's payment pattern. We review the
41
number of tests paid against the number of tests billed and the payer's outstanding balance for unpaid tests to determine whether payments are being made at a consistently high percentage of tests billed and at appropriate amounts given the amount billed by us. To date, except for third-party payers with contracted reimbursement coverage, we have not been able to demonstrate a predictable pattern of collectability from third-party payers. Some patients have out-of-pocket costs for amounts not covered by their insurance carrier and we may bill the patient directly for these amounts in the form of co-payments and co-insurance in accordance with their insurance carrier and health plans. Some payers may not cover our test as ordered by the physician under their reimbursement policies. In such situations, we pursue reimbursement from the patients on a case-by-case basis. To date, we have not been able to demonstrate a predictable pattern of collectability directly from patients. In the absence of contracted reimbursement and/or a predictable pattern of collectability at consistent payment amounts, we believe that all the revenue recognition criteria are met upon the earlier of receipt of third-party payer notification of payment or when cash is received and accordingly, we recognize revenue at that time.
We use judgment in our assessment of whether the fee is fixed or determinable and whether collectability is reasonably assured in determining when to recognize revenue in the future as we continue to gain payment experience with third-party payers and patients.
Allowance for Doubtful Accounts
We accrue an allowance for doubtful accounts against our accounts receivable based on estimates consistent with historical payment experience. Our allowance for doubtful accounts is evaluated on a regular basis and adjusted when trends or significant events indicate that a change in estimate is appropriate. Historically, the amounts of uncollectible accounts receivable that have been written off have been consistent with management's expectations. Accounts receivable are written off against the allowance when the appeals process is exhausted or when there is other substantive evidence that the account will not be paid.
If the financial conditions of our customers were to deteriorate, resulting in an impairment of their ability to make payments, additional allowances may be required.
Derivative Liability
We account for derivative financial instruments as either equity or liabilities based upon the characteristics and provisions of each instrument. We recorded the preferred stock liability incurred in connection with our Series B and Series C convertible preferred stock and the preferred stock warrant liability related to the issuance of a warrant for Series C convertible preferred stock, each as a derivative financial instrument liability at their fair value on the date of issuance, and we remeasure them on each subsequent balance sheet date. The changes in fair value are recognized as a gain or loss from the adjustment to other income (expense), net in the statements of operations and comprehensive loss. We estimate the fair value of this liability using option-pricing models that include assumptions for future financings, expected volatility, expected life, yield and risk-free interest rate.
Deferred Tax Assets
We file U.S. federal income tax returns and tax returns in California, Texas and other states. To date, we have not been audited by the Internal Revenue Service or any state income tax authority.
As of December 31, 2012, our gross deferred tax assets were $24.9 million. The deferred tax assets were primarily comprised of federal and state tax net operating loss and tax credit carryforwards. Utilization of the net operating loss and tax credit carryforwards may be subject to annual limitation due to historical or future ownership percentage change rules provided by the Internal Revenue Code of 1986, and similar state provisions. The annual limitation may result in the expiration of certain net operating loss and tax credit carryforwards before their utilization.
42
We are required to reduce our deferred tax assets by a valuation allowance if it is more likely than not that some or all of our deferred tax assets will not be realized. We must use judgment in assessing the potential need for a valuation allowance, which requires an evaluation of both negative and positive evidence. The weight given to the potential effect of negative and positive evidence should be commensurate with the extent to which it can be objectively verified. In determining the need for and amount of our valuation allowance, if any, we assess the likelihood that we will be able to recover our deferred tax assets using historical levels of income, estimates of future income and tax planning strategies. As a result of historical cumulative losses and, based on all available evidence, we believe it is more likely than not that our recorded net deferred tax assets will not be realized. Accordingly, we recorded a valuation allowance against all of our net deferred tax assets at December 31, 2012. We will continue to maintain a full valuation allowance on our deferred tax assets until there is sufficient evidence to support the reversal of all or some portion of this allowance.
Stock-based Compensation
We recognize compensation costs related to stock options granted to employees based on the estimated fair value of the awards on the date of grant, net of estimated forfeitures. We estimate the grant date fair value, and the resulting stock-based compensation expense, using the Black-Scholes option-pricing model. The grant date fair value of stock-based awards is expensed on a straight-line basis over the vesting period of the respective award. Performance-based awards vest and are expensed over the performance period when the related performance goal is probable of being achieved.
We account for stock-based compensation arrangements with non-employees using a fair value approach. The fair value of these options is measured using the Black-Scholes option-pricing model reflecting the same assumptions as applied to employee options in each of the reported periods, other than the expected life, which is assumed to be the remaining contractual life of the option. The compensation costs of these arrangements are subject to remeasurement over the vesting terms as earned.
We recorded stock-based compensation expense of $0.5 million, $0.7 million and $0.5 million for the years ended December 31, 2011, and 2012, and the six months ended June 30, 2013, respectively. We expect to continue to grant stock options and other equity-based awards in the future, and to the extent that we do, our stock-based compensation expense recognized in future periods will likely increase.
The Black-Scholes option-pricing model requires the use of highly subjective and complex assumptions, which determine the fair value of stock-based awards. Our assumptions are as follows:
- •
- Expected term. The expected term represents the period
that the stock-based awards are expected to be outstanding. Our historical share option exercise experience does not provide a reasonable basis upon which to estimate an expected term because of a
lack of sufficient data. Therefore we estimate the expected term by using the simplified method provided by the SEC. The simplified method calculates the expected term as the average of the
time-to-vesting and the contractual life of the options.
- •
- Expected volatility. As our common stock has never been
publicly traded, the expected volatility is derived from the average historical volatilities of publicly traded companies within our industry that we consider to be comparable to our business over a
period approximately equal to the expected term.
- •
- Risk-free interest rate. The risk-free interest rate is
based on the U.S. Treasury yield in effect at the time of grant for zero coupon U.S. Treasury notes with maturities approximately equal to the expected term.
- •
- Expected dividend. The expected dividend is assumed to be zero as we have never paid dividends and have no current plans to pay any dividends on our common stock.
43
In addition to the assumptions used in the Black-Scholes option-pricing model, we also estimate a forfeiture rate to calculate the stock-based compensation for our equity awards. We will continue to use judgment in evaluating the expected volatility, expected terms and forfeiture rates utilized for our stock-based compensation calculations on a prospective basis.
Significant factors, assumptions and methodologies used in determining the estimated fair value of our common stock
We are also required to estimate the fair value of the common stock underlying our stock-based awards when performing the fair value calculations using the Black-Scholes option-pricing model. Our board of directors, with the assistance of management, determined the fair value of our common stock on each grant date. Option grants are based on the estimated fair value of our common stock on the date of grant, which is determined by taking into account several factors, including the following:
- •
- important developments in our operations, in particular coverage policies or contracts with third-party payers;
- •
- valuations performed by an independent third party;
- •
- the prices at which we sold our convertible preferred stock and the rights, preferences, and privileges of the convertible
preferred stock relative to those of our common stock, including the liquidation preferences of the convertible preferred stock;
- •
- our actual operating results and financial performance;
- •
- conditions in our industry and the economy in general;
- •
- stock price performance of comparable public companies;
- •
- the estimated likelihood of achieving a liquidity event, such as an IPO or an acquisition of our company, given prevailing
market conditions; and
- •
- the illiquidity of the common stock underlying stock options.
In determining the estimated fair value of our common stock, our board of directors, with the assistance of management, used the market approach to estimate the enterprise value of our company in accordance with the American Institute of Certified Public Accountants Accounting and Valuation Guide, Valuation of Privately-Held Company Equity Securities Issued as Compensation. The market approach, comprised of the Guideline Publicly Traded Company and the M&A Transaction methodologies, estimates the value of a company by comparing it to a peer group of similar publicly traded companies. When selecting the peer group to be used for the market multiple approaches, we focused on companies within the molecular diagnostics industry. The criteria we used to select comparable companies included the stage of development of their product candidates, their position in the industry and their overall risk profile. The peer group in the Guideline Publicly Traded Company was reviewed at each valuation date to assess whether to add or remove companies to maintain the relevance of the peer group; our peer group's composition has changed over time based upon this continuing evaluation. In connection with our November 2012 contemporaneous valuation, we removed two of the peer group companies we deemed no longer comparable to us, either as they were acquired or their business model was no longer similar to ours, and replaced them with two other companies that we believe are comparable to us. Based on these considerations, we believe that our peer group of comparable companies has been a representative group for purposes of performing valuations.
Once a group of comparable publicly traded companies is selected, market multiples are calculated using each company's stock price and other financial data. Typically, a company's value is estimated by applying selected market multiples of selected peer group companies to a company's forecasted financial results. We used revenue multiples in the Guideline Publicly Traded Company methodology and in the
44
M&A Transaction methodology. As part of the Guideline Public Company methodology used in the January 2012, April 2013 and June 2013 valuations, we took into consideration the revenue multiples and enterprise value of select companies that had completed IPOs in the molecular diagnostic industry in the prior twelve months. For the November 2012, April 2013 and June 2013 valuations, we also used the OPM Backsolve method, a form of the market approach to valuation, which derives the implied equity value for a company from a recent transaction involving the company's own securities.
The initial estimated enterprise value was then allocated to the common stock using the Option Pricing Method, the Probability Weighted Expected Return Method or the Hybrid Method.
The Option Pricing Method, or OPM, treats the enterprise as a call option, to be distributed among the common and convertible preferred security classes, with exercise prices based on the liquidation preference of the convertible preferred stock. Therefore, by extension, the common stock has value only if the funds available for distribution to the stockholders exceed the value of the liquidation preference at the time of a liquidity event such as a merger, sale or IPO, assuming the enterprise has funds available to make a liquidation preference meaningful and collectible by the stockholders. The common stock is modeled to be a call option with a claim on the enterprise at an exercise price equal to the remaining value immediately after the convertible preferred stock is liquidated. The OPM uses the Black-Scholes option-pricing model to price the call option. The OPM is appropriate to use when the range of possible future outcomes is so difficult to predict that forecasting discrete exit events would be highly speculative.
The Probability Weighted Expected Return Method, or PWERM, is a scenario-based analysis that estimates the value per share based on the probability-weighted present value of expected future investment returns, considering each of the possible outcomes available to us, as well as the rights of each share class. PWERM estimates the common stock value to our stockholders under possible future scenarios which includes various IPO outcomes and liquidation. The value per share under each scenario is then probability weighted and the resulting weighted values per share are summed to determine the fair value per share of our common stock. In the liquidation scenario, the value per share is allocated taking into account the liquidation preferences and participation rights of our convertible preferred stock consistent with the method outlined in the American Institute of Certified Public Accountants Practice Guide, Valuation of Privately-Held Company Equity Securities Issued as Compensation. In the IPO scenarios, it is assumed that all outstanding shares of our convertible preferred stock will convert into common stock. Over time, as we achieve certain company-related milestones, the probability of each scenario is evaluated and adjusted accordingly.
The Hybrid Method employs the concepts of the PWERM and OPM in a single framework. The PWERM estimates the future equity value under a range of IPO exits, and allocates the same in each scenario according to the subject company's capital structure, probability-weighting each exit and discounting the value to a present value equivalent using a risk-adjusted discount rate. The Option Pricing Model frames the scenario where the Company remains private, and is modeled over a weighted average term to exit using a financing round or external comparable benchmarks as the basis for fair market value determination.
In determining the estimated fair value of our common stock, our board of directors also considers the fact that our common stock is not freely tradable in the public market. The estimated fair value of our common stock at each grant date reflects a non-marketability discount partially based on the anticipated likelihood and timing of a future liquidity event.
45
Common stock valuations
Information regarding our stock option grants to our employees and non-employees, along with the estimated fair value per share of the underlying common stock, for stock options granted since January 1, 2012 is summarized as follows:
Grant Date
|
Number of Common Shares Underlying Options Granted |
Exercise Price per Common Share |
Estimated Fair Value per Share of Common Stock |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
March 10, 2012 |
1,814,628 | $ | 0.67 | $ | 0.67 | |||||
April 11, 2012 |
924,000 | 0.67 | 0.67 | |||||||
June 6, 2012 |
450,000 | 0.67 | 0.67 | |||||||
July 25, 2012 |
270,000 | 0.67 | 0.67 | |||||||
December 6, 2012 |
269,167 | 1.00 | 1.00 | |||||||
February 5, 2013 |
1,709,007 | 1.00 | 1.00 | |||||||
June 20, 2013 |
801,625 | 1.51 | 1.98 | |||||||
The intrinsic value of all outstanding options as of June 30, 2013 was $ million based on the mid-point of the estimated price range set forth on the cover of this prospectus, of which approximately $ million related to vested options and approximately $ million related to unvested options.
The estimated fair value per share of the common stock in the table above represents the determination by our board of directors of the estimated fair value of our common stock as of the date of the grant, taking into consideration various objective and subjective factors, including the conclusions, if applicable, of the most recent valuation of our common stock, as discussed below.
March 2012. We granted options to purchase 1,814,628 shares of our common stock on March 10, 2012. Our board of directors set an exercise price of $0.67 per share. We had obtained a contemporaneous independent valuation of our common stock as of January 19, 2012. The valuation was prepared on a minority, non-marketable interest basis. We used the Guideline Publicly Traded Company methodology to determine an enterprise value. The valuation used a non-marketability discount of 35% and a discount rate of 20% based on our risk-adjusted cost of capital. Our considerations of the form, timing and probability of a particular future liquidity event or outcome were based on the business outlook as of January 19, 2012. We estimated a 30% probability of an initial public offering with a high valuation, a 30% probability of an initial public offering with a low valuation, and a 40% probability of liquidation. The estimated time to a liquidity event assumed a timeline of either an IPO of 2.5 years or dissolution in 0.7 years. We allocated the enterprise value using the PWERM and these three scenarios. This valuation indicated a fair value of $0.67 per share for our common stock as of January 19, 2012. In the judgment of our board of directors, there were no internal or external developments that would indicate that the fair value of our common stock would have increased from January 19, 2012. Accordingly, the board of directors determined that the estimated fair value of our common stock at March 10, 2012 as $0.67 per share.
April to July 2012. We granted options to purchase 924,000, 450,000 and 270,000 shares of our common stock on April 11, 2012, June 6, 2012, and July 25, 2012. Our board of directors set an exercise price of $0.67 per share. Although our revenues increased as compared to the same period in the prior year, we had only been generating revenues since January 2011. During this time we had not obtained coverage from any new third-party payers, and continued to recognize the majority of our revenues upon the earlier of receipt of third-party payer notification of payment or when cash is received due to the absence of contracted reimbursement or a predictable pattern and history of collectability in connection with our billings. In the judgment of our board of directors, there were no internal or external developments that would indicate that the fair value of our common stock would have increased from January 19, 2012. Accordingly, the board of directors determined that the estimated fair value of our common stock at April 11, 2012, June 6, 2012, and July 25, 2012 was $0.67 per share.
46
December 2012. We granted options to purchase 269,167 shares of our common stock on December 6, 2012. Our board of directors set an exercise price of $1.00 per share. We had obtained a contemporaneous independent valuation of our common stock as of November 1, 2012. The valuation was prepared on a minority, non-marketable interest basis. In November 2012, we issued 7,936,508 shares of Series C convertible preferred stock at a purchase price of $1.89 per share, resulting in gross proceeds to us of $15.0 million. The preferred stock has rights, preferences, and privileges that are significantly different from those of our common stock, including liquidation preferences. For purposes of the November 1, 2012 valuation, we determined that the OPM was the most appropriate valuation methodology to estimate the fair value of our common stock given the uncertainty of determining various exit scenarios and due to the recently completed financing. We utilized the OPM Backsolve method to estimate the equity value based on the November 2012 Series C preferred stock financing, at a price of $1.89 per share, which we believed to be the most indicative of our value as of November 1, 2012. The estimated time to a liquidity event assumed a timeline of either an IPO or dissolution of two years. The valuation used a non-marketability discount of 20%. This valuation indicated a fair value of $1.00 per share for our common stock as of November 1, 2012.
In the judgment of our board of directors, there were no internal or external developments that would indicate that the fair value of our common stock would have increased from November 1, 2012. Accordingly, the board of directors determined that the estimated fair value of our common stock at December 6, 2012 was $1.00 per share.
February 2013. We granted options to purchase 1,709,007 shares of our common stock on February 5, 2013. Our board of directors set an exercise price of $1.00 per share. In the judgment of our board of directors, there were no internal or external developments that would indicate that the fair value of our common stock would have increased from December 6, 2012. Accordingly, the board of directors determined that the estimated fair value of our common stock at February 5, 2013 was $1.00 per share.
April 2013. We obtained a contemporaneous independent valuation of our common stock as of April 30, 2013. Our considerations of the form, timing and probability of a particular future liquidity event or outcome were based on the business outlook at the time of the valuation. We estimated a 50% probability of an IPO and a 50% probability that we would continue as a private company. Accordingly, we used a hybrid method of the OPM and the PWERM in allocating the equity value, weighting the fair values estimated under these methods equally. The hybrid methodology was applied to reflect the uncertainties associated with growth-stage companies, especially in the medical diagnostics sector. Many medical diagnostic companies seeking an IPO in the past 12 months had to either offer their shares at a substantial discount to the proposed offering range or withdrew their filings. This supports the application of the hybrid model as of April 30, 2013.
For the IPO scenario, we determined the equity value using the Guideline Public Company methodology. The valuation used a discount rate of 20% based on our risk-adjusted cost of capital. The common stock value based on the PWERM method incorporates probability estimates for a potential future IPO in six months with low, mid, and high valuation scenarios at 30%, 60%, and 10% probability, respectively.
For the stay private scenario, we determined the equity value utilizing the Backsolve method based on the second closing of the Series C preferred stock financing, with a purchase price of $1.89 per share, which closed in June 2013 and resulted in gross proceeds to us of $13.0 million. The preferred stock has rights, preferences, and privileges that are significantly different from those of our common stock, including the liquidation preferences of the convertible preferred stock. We allocated the equity value to the various classes of securities using the OPM.
We applied equal weighting to the results under the OPM and the PWERM methodologies to arrive at a pre-discounted value and then applied a non-marketability discount of 20% which resulted in an
47
estimated common stock value of $1.51 per share on a non-marketable, minority interest basis as of April 30, 2013.
June 2013. We granted options to purchase 801,625 shares of our common stock on June 20, 2013. Our board of directors set an exercise price of $1.51 per share for these options based in part on a contemporaneous third-party valuation prepared as of April 30, 2013. Subsequent to the granting of these options, we obtained a third-party valuation as of June 30, 2013 which determined a fair value of our common stock of $1.98 per share on that date.
Our considerations of the form, timing and probability of a particular future liquidity event or outcome were based on the business outlook at the time of the June 30, 2013 valuation. We estimated a 70% probability of an IPO and a 30% probability that we would continue as a private company. Accordingly, we continued to use a hybrid method of the OPM and the PWERM in allocating the equity value, weighting the fair values estimated under these methods based on our estimates of the probability of each scenario.
For the IPO scenario, we determined the equity value using the Guideline Public Company methodology and applied a non-marketability discount of 10%. The common stock value based on the PWERM method incorporates probability estimates for a potential future IPO in six months with low, mid, and high valuation scenarios at 25%, 60%, and 15% probability, respectively.
For the stay private scenario, we determined the equity value utilizing the Backsolve method based on our outstanding equity securities as of June 30, 2013. The preferred stock has rights, preferences, and privileges that are significantly different from those of our common stock, including the liquidation preferences of the convertible preferred stock. We allocated the equity value to the various classes of securities using the OPM and applied a non-marketability discount of 20%.
We applied a 30% and 70% weighting to the values determined under the OPM and the PWERM methodologies, respectively, which resulted in an estimated common stock value of $1.98 per share on a non-marketable, minority interest basis as of June 30, 2013.
The increase from the April 2013 valuation to the June 2013 valuation primarily resulted from an increase in the enterprise value as a result of continued growth in our business, including increases in FNA volume, obtaining a coverage decision from Aetna, the successful completion of our financings and an increase in the PWERM weighting from 50% to 70%, reflecting a higher probability of an IPO liquidity event. As noted above, the board granted stock options in June 2013 with an exercise price of $1.51 per share based in part on the fair value of our common stock determined in the April 30, 2013 valuation. However, for financial reporting purposes, we reassessed the fair value of the underlying common stock on the June 20, 2013 grant date and determined that the fair value should be based on the June 30, 2013 valuation. This valuation indicated a fair value of our common stock of $1.98 per share and accordingly, for financial reporting purposes, we have recorded stock-based compensation expense based on the reassessed fair value.
Factors Affecting Our Performance
The number of FNAs we receive and test
The growth in our business is tied to the number of FNAs we receive. Generally 5%-10% of the FNA samples we receive have insufficient cellular material from which to render a cytopathology diagnosis. We do not bill for these tests. For results that are benign or suspicious/malignant, we bill for the cytopathology portion of the test. If the sample is indeterminate, we perform the GEC. Historically, approximately 14%-17% of samples we have received for cytopathology have been diagnosed as indeterminate. We also perform GEC testing on a small number of samples referred by physicians where prior cytopathology testing has resulted in an indeterminate result. Of the FNA samples sent for GEC testing, approximately 5%-10% have insufficient RNA from which to render a finding. We issue a patient report classifying the
48
sample as GEC Benign, GEC Suspicious or GEC No Result. We bill for the GEC Benign and GEC Suspicious results only. At this time, we also issue the cytopathology report for the indeterminate samples, and bill for the cytopathology portion of the test. We incur costs of collecting and shipping the FNAs and a portion of the costs of performing tests where we cannot ultimately issue a patient report. Because we cannot bill for all samples received, the number of FNAs received does not directly correlate to the total number of patient reports issued and thus potential revenue generated.
Continued adoption of and reimbursement for Afirma
As of August 2013, the list price for the GEC is $4,275. To date only a portion of payers have reimbursed us at full list price. Revenue growth depends on our ability to achieve broader reimbursement at increased levels from third-party payers and to expand our base of prescribing physicians. To drive increased adoption of Afirma, we plan to increase our marketing efforts and to selectively increase our internal sales force in high-volume geographies domestically and to leverage our relationship with Genzyme to accelerate Afirma growth both in the United States and internationally. Because many payers consider the GEC experimental and investigational, we may not receive payment on many tests and payments may not be at acceptable levels compared to what we have billed. We expect our revenue growth will increase as more payers make a positive coverage decision, which should enhance our collections. If we are unable to expand the base of prescribing physicians at an acceptable rate, or if we are not able to execute our strategy for increasing reimbursement, we may not be able to effectively increase our revenue.
How we recognize revenue
A significant portion of our revenue is recognized when cash is received. Medicare is the only payer with agreed upon reimbursement rates and a predictable history of collections, which allows us to recognize the related revenue on an accrual basis. Until we achieve a predictable pattern of collections and a consistent payment amount from a larger number of payers, we will recognize a large portion of our revenue upon the earlier of notification of payment or when cash is received. Additionally, as we commercialize new products, we will need to achieve a predictable pattern of collections and a consistent payment amount for each payer for each new product offering prior to being able to recognize the related revenue on an accrual basis. Because the timing and amount of cash payments received from payers is difficult to predict, we expect that our revenue will fluctuate significantly in any given quarter. In addition, even if we begin to accrue larger amounts of revenue related to Afirma, when we introduce new products we do not expect we will be able to recognize revenue from new products on an accrual basis for some period of time. This may result in continued fluctuations in our revenue.
Impact of Genzyme co-promotion agreement
The $10.0 million fee we received from Genzyme under our co-promotion agreement is being amortized over a four-year period beginning in 2012, and is recorded as a reduction of selling and marketing expenses. Under the agreement, we pay a significant portion of our cash receipts to Genzyme for co-promoting Afirma, and such amounts are recorded in selling and marketing expense. The co-promotion agreement requires that we pay a certain percentage of our cash receipts to Genzyme, which percentage decreases over time. As of January 2013, the percentage is 40%, and it decreases to 32% in March 2014 and thereafter. As our cash collections grow, both from volume growth as well as from increased reimbursement rates and collections for Afirma, the total amount we pay to Genzyme will increase in absolute dollars although the percentage of revenue we are required to pay Genzyme decreases over time. We believe our relationship with Genzyme will accelerate sales of Afirma. As a result, our selling and marketing expense may be higher than what we would have incurred if we alone were marketing and promoting Afirma.
We also may receive up to an additional $3.0 million from Genzyme, consisting of $0.6 million for each of up to five countries outside of the United States in which we obtain regulatory authorization to
49
market Afirma and achieve a specified level of reimbursement. Genzyme has also agreed to spend $0.5 million to support clinical development expenses required for entry into the international markets covered by our agreement. This obligation expires in July 2014.
Our agreement with Genzyme expires in 2027 and either party may terminate the agreement at any time without cause and with six months' prior notice. If we terminate the agreement without cause prior to January 2014, we will be required to repay 50% of the $10.0 million fee we received. The percentage decreases to 40% of such fee if we were to terminate the agreement between January 2014 and January 2015, and 30% of such fee if we were to terminate the agreement between January 2015 and January 2016. Subsequent to January 2016, we are not required to repay any portion of the fee in the event we terminate the agreement without cause.
Development of additional products
We rely on sales of Afirma to generate all of our revenue. Our product development pipeline includes the Afirma Malignant GEC, a test that we believe will serve our current base of prescribing physicians. We also plan to pursue development of products for additional diseases to increase and diversify our revenue. For example, we are pursuing a solution for interstitial lung disease, or ILD, that will offer an alternative to surgery by developing a genomic signature to classify samples collected through less invasive bronchoscopy techniques. Accordingly, we expect to continue to invest heavily in research and development in order to expand the capabilities of our solution and to develop additional products. Our success in developing new products will be important in our efforts to grow our business by expanding the potential market for our products and diversifying our sources of revenue.
Timing of our research and development expenses
We deploy state-of-the-art and costly genomic technologies in our biomarker discovery experiments, and our spending on these technologies may vary substantially from quarter to quarter. We also spend a significant amount to secure clinical samples that can be used in discovery and product development as well as clinical validation studies. The timing of these research and development activities is difficult to predict, as is the timing of sample acquisitions. If a substantial number of clinical samples are acquired in a given quarter or if a high-cost experiment is conducted in one quarter versus the next, the timing of these expenses can affect our financial results. We conduct clinical studies to validate our new products as well as on-going clinical studies to further the published evidence to support our commercialized test, Afirma. As these studies are initiated, start-up costs for each site can be significant and concentrated in a specific quarter. Spending on research and development, for both experiments and studies, may vary significantly by quarter depending on the timing of these various expenses.
Seasonal fluctuations in FNA volume and collections
Our business is subject to fluctuations in FNA volume throughout the year as a result of physician practices being closed for holidays or endocrinology and thyroid-related industry meetings which are widely attended by our prescribing physicians. Like other companies in our field, vacations by physicians and patients tend to negatively affect our volumes more during the summer months and during the end of year holidays compared to other times of the year. Our reimbursed rates and cash collections are also subject to seasonality. Medicare normally makes downward adjustments in its fee schedules at the beginning of the year which may negatively affect our reimbursement. Additionally, patient deductibles generally reset at the beginning of each year which means that patients early in the year are responsible for a greater portion of the cost of our tests, and we have lower collection rates from individuals than from Medicare and third-party payers. Later in the year, particularly in the fourth quarter, we experience better payment results as third-party payers tend to clear pending claims toward year end. This trend historically has increased our cash collections in the fourth quarter and decreased cash collections for the subsequent
50
first quarter of the succeeding year. The effects of these seasonal fluctuations in prior periods may have been obscured by the growth of our business.
Results of Operations
Comparison of the Six Months Ended June 30, 2012 and 2013
| |
Six Months Ended June 30, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Dollar Change |
% Change |
|||||||||||
| |
2012 | 2013 | |||||||||||
| |
(In thousands) |
|
|||||||||||
| |
(Unaudited) |
|
|
||||||||||
Revenue |
$ | 3,947 | $ | 9,452 | $ | 5,505 | 139 | % | |||||
Operating expenses: |
|||||||||||||
Cost of revenue |
3,000 | 6,004 | 3,004 | 100 | % | ||||||||
Research and development |
3,158 | 3,912 | 754 | 24 | % | ||||||||
Selling and marketing |
3,045 | 5,318 | 2,273 | 75 | % | ||||||||
General and administrative |
3,618 | 5,528 | 1,910 | 53 | % | ||||||||
Total operating expenses |
12,821 | 20,762 | 7,941 | 62 | % | ||||||||
Loss from operations |
(8,874 | ) | (11,310 | ) | (2,436 | ) | 27 | % | |||||
Interest expense |
— | (5 | ) | (5 | ) | N/M | |||||||
Other income (expense), net |
— | (2,070 | ) | (2,070 | ) | N/M | |||||||
Net loss |
$ | (8,874 | ) | $ | (13,385 | ) | $ | (4,511 | ) | 51 | % | ||
Revenue
Revenue increased $5.5 million, or 139%, for the six months ended June 30, 2013 compared to the same period in 2012 primarily due to a $3.8 million increase in revenue from increased adoption of Afirma, resulting in increased collections, and a $1.7 million increase in revenue from Medicare.
Cost of revenue
Cost of revenue increased $3.0 million, or 100%, for the six months ended June 30, 2013 compared to the same period in 2012 primarily due to the increase in the number of FNAs received for analysis from 9,535 for the six months ended June 30, 2012 to 23,181 in the same period in 2013.
Research and development
Research and development expenses increased $0.8 million, or 24%, for the six months ended June 30, 2013 compared to the same period in 2012. The increase was primarily driven by a $0.5 million increase in costs to support our product pipeline and ongoing support for Afirma and a $0.4 million increase in personnel expenses related to headcount increase.
Selling and marketing
Selling and marketing expenses increased $2.3 million, or 75%, for the six months ended June 30, 2013 compared to the same period in 2012. The increase was primarily due to a $1.9 million increase in net expense recognized under our co-promotion agreement with Genzyme, which was entered into in January 2012. The net expense of $1.9 million is comprised of the co-promotion fee to Genzyme offset in part by amortization of the deferred upfront fee paid to us by Genzyme. In addition, there was an increase of $0.2 million in personnel expenses for additional sales representatives hired in the six months ended June 30, 2013 and a $0.2 million increase in marketing and promotional materials.
51
General and administrative
General and administrative expenses increased $1.9 million, or 53%, for the six months ended June 30, 2013 compared to the same period in 2012. The increase is primarily related to a $1.0 million increase in personnel expenses resulting from an increase in headcount and employee severance, a $0.5 million increase in professional fees and a $0.3 million increase in facility, equipment, and information technology expenses.
Other income (expense), net
Other income (expense), net, was ($2.1) million for the six months ended June 30, 2013 and is primarily related to the increase in value of the preferred stock liability associated with our obligation to issue additional shares of Series C convertible preferred stock.
Comparison of the Years Ended December 31, 2011 and 2012
| |
Year Ended December 31, |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Dollar Change |
% Change |
|||||||||||
| |
2011 | 2012 | |||||||||||
| |
(In thousands) |
|
|||||||||||
Revenue |
$ | 2,645 | $ | 11,628 | $ | 8,983 | 340 | % | |||||
Operating expenses: |
|||||||||||||
Cost of revenue |
2,925 | 7,584 | 4,659 | 159 | % | ||||||||
Research and development |
6,680 | 6,608 | (72 | ) | (1 | )% | |||||||
Selling and marketing |
2,934 | 8,447 | 5,513 | 188 | % | ||||||||
General and administrative |
5,372 | 7,918 | 2,546 | 47 | % | ||||||||
Total operating expenses |
17,911 | 30,557 | 12,646 | 71 | % | ||||||||
Loss from operations |
(15,266 | ) | (18,929 | ) | (3,663 | ) | 24 | % | |||||
Interest income |
2 | 2 | — | — | % | ||||||||
Other income (expense), net |
819 | 278 | (541 | ) | 66 | % | |||||||
Net loss |
$ | (14,445 | ) | $ | (18,649 | ) | $ | (4,204 | ) | 29 | % | ||
Revenue
Revenue increased $9.0 million, or 340%, in 2012 compared to 2011 primarily due to a $6.4 million increase in revenue from increased Afirma adoption, resulting in increased collections, and a $2.6 million increase in revenue from Medicare.
Cost of revenue
Cost of revenue increased $4.7 million, or 159%, in 2012 compared to 2011 primarily due to the increase in the number of FNAs received for analysis from 6,402 in 2011 to 25,890 in 2012.
Research and development
Research and development expenses were essentially flat in 2012 compared to 2011. Our research and development expenses in 2011 reflect the conclusion of clinical studies and other research and development activities supporting the commercial launch of Afirma. In 2012, our research and development expenses shifted to the development of our product pipeline as well as the continued support of Afirma.
52
Selling and marketing
Selling and marketing expenses increased $5.5 million, or 188%, in 2012 compared to 2011. This increase was primarily due to $3.1 million in net expense recognized under our co-promotion agreement with Genzyme, partially offset by amortization of the deferred fee. The remaining $2.4 million increase included a $1.4 million increase in personnel expenses as we hired a vice president of sales and additional sales representatives in 2012, a $0.4 million increase in marketing and promotional materials, a $0.3 million increase in allocated information technology, facilities and other costs and a $0.3 million increase in travel and meetings related expenses.
General and administrative
The $2.5 million, or 47%, increase in general and administrative expenses for 2012 compared to 2011 was due to a $1.8 million increase in personnel expenses primarily from increased headcount, higher bonus payments and higher stock-based compensation expense, a $0.3 million increase in professional fees and a $0.3 million increase in occupancy and equipment expenses.
Other income (expense), net
Other income (expense), net was $0.8 million for the year ended December 31, 2011, and is primarily comprised of $0.7 million related to the decrease in value of the preferred stock liability associated with our obligation to issue additional shares of Series B convertible preferred stock. In addition, $0.1 million represents a payment made to us by Genzyme in connection with the right to negotiate an exclusive co-promotion arrangement. Other income (expense), net was $0.3 million for the year ended December 31, 2012, which represents the decrease in value of the preferred stock liability associated with our obligation to issue additional shares of Series C convertible preferred stock.
Quarterly Results of Operations Data
The following table sets forth our unaudited quarterly statements of operations data and other data for each of the six most recent quarters in the period ended June 30, 2013. We have prepared the quarterly results of operations data on a consistent basis with the audited financial statements included elsewhere in this prospectus. In the opinion of management, the quarterly results of operations data reflects all necessary adjustments, consisting only of normal recurring adjustments, necessary for a fair statement of this data. The statements of operations data should be read in conjunction with the financial statements
53
and related notes included elsewhere in this prospectus. The results of historical periods are not necessarily indicative of results for a full year or for any future period.
| |
Three Months Ended, | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Mar 31, 2012 |
June 30, 2012 |
Sept 30, 2012 |
Dec 31, 2012 |
Mar 31, 2013 |
June 30, 2013 |
|||||||||||||
| |
(In thousands) |
||||||||||||||||||
Statements of Operations Data: |
|||||||||||||||||||
Revenue |
$ | 1,468 | $ | 2,479 | $ | 3,224 | $ | 4,457 | $ | 4,384 | $ | 5,068 | |||||||
Operating expenses: |
|||||||||||||||||||
Cost of revenue |
1,254 | 1,746 | 1,984 | 2,600 | 2,773 | 3,231 | |||||||||||||
Research and development |
1,481 | 1,677 | 1,729 | 1,721 | 2,010 | 1,902 | |||||||||||||
Selling and marketing |
1,215 | 1,830 | 2,347 | 3,055 | 2,703 | 2,615 | |||||||||||||
General and administrative |
1,766 | 1,852 | 2,103 | 2,197 | 2,791 | 2,737 | |||||||||||||
Total operating expenses |
5,716 | 7,105 | 8,163 | 9,573 | 10,277 | 10,485 | |||||||||||||
Loss from operations |
(4,248 | ) | (4,626 | ) | (4,939 | ) | (5,116 | ) | (5,893 | ) | (5,417 | ) | |||||||
Interest income |
— | — | 1 | 1 | — | — | |||||||||||||
Interest expense |
— | — | — | — | — | (5 | ) | ||||||||||||
Other income (expense), net |
— | — | — | 278 | (1,002 | ) | (1,068 | ) | |||||||||||
Net loss |
$ | (4,248 | ) | $ | (4,626 | ) | $ | (4,938 | ) | $ | (4,837 | ) | $ | (6,895 | ) | $ | (6,490 | ) | |
Other Operating Data: |
|||||||||||||||||||
FNAs received |
3,925 | 5,610 | 7,052 | 9,303 | 10,757 | 12,424 | |||||||||||||
Revenue increased quarter over quarter through December 31, 2012 due to increased collections which resulted from increased adoption of Afirma. In the quarter ended March 31, 2013, the coding for the GEC changed to a miscellaneous code for certain diagnostic tests, including the GEC. This change resulted in longer collection times as payers had to change their internal systems, and we had to appeal more claims under the new coding. While the number of FNAs received continued to grow in the first quarter of 2013, revenue decreased from the quarter ended December 31, 2012 to the quarter ended March 31, 2013 due to several factors, including: Medicare's downward adjustment to the cytopathology fee schedule, the effect of the implementation of the automatic expense reductions under the Budget Control Act of 2011, the resetting of patient deductibles in the first quarter and third-party payers clearing pending claims before year end.
Operating expenses generally increased consistently with the growth of the business. Cost of revenue increases are directly related to the increasing volume of tests received during the quarters in 2012. During the quarters ended March 31 and June 30, 2013, we experienced increased costs due to the implementation of automation in our California laboratory that is expected to yield future cost efficiencies per test. We expect our cost of revenue to increase in a non-linear manner in the next several quarters as our Austin, Texas laboratory becomes fully operational. Our expenditures in research and development were lower in the quarter ended December 31, 2012 due to the timing of some large studies and experiments which were delayed and occurred in the quarter ended March 31, 2013. Our selling and marketing expenses decreased from the fourth quarter of 2012 to the first quarter of 2013, primarily due to contractual rate reductions under our co-promotion agreement with Genzyme which decreases take effect in the first quarter of each year. The continued decrease of our selling and marketing expenses from the quarter ended March 31, 2013 to the quarter ended June 30, 2013 was primarily due to the reduction of direct marketing and consulting expenses. Our general and administrative expenses increased from the quarter ended December 31, 2012 to the quarter ended March 31, 2013, primarily due to building out our Austin, Texas laboratory. The quarter ended March 31, 2013 also included non-recurring severance costs. General and administrative expenses remained relatively flat in the quarter ended June 30, 2013 due in part to the continued build out of the Austin facility, which began processing cytology samples in May, as well as
54
increases in professional and other expenses related to the growth of our business. We expect our general and administrative expenses will increase in the future as we continue to grow our business.
Liquidity and Capital Resources
Since inception, our operations have been financed primarily by net proceeds of $78.6 million from sales of our preferred stock and a $10.0 million payment from our co-promotion agreement with Genzyme, and since June 2013, borrowings under our loan and security agreement. As of December 31, 2012 and June 30, 2013, we had $14.0 million and $20.7 million of cash and cash equivalents, respectively.
In June 2013, we entered into a loan and security agreement with a financial institution. This agreement provides for term loans of up to an aggregate of $10.0 million. On entering into the agreement, we drew down an initial $5.0 million term loan. We may request a second term loan of up to $5.0 million on or prior to March 31, 2014. Loans drawn under the loan and security agreement will be used for working capital and general corporate purposes.
The initial term loan bears interest at a fixed rate equal to 6.06%. The second term loan, if drawn, will bear interest at a fixed rate equal to the greater of (a) 5.88% or (b) the three-year U.S. Treasury note rate, plus 5.40%. We are required to repay any outstanding principal amounts of each loan in 30 equal monthly installments beginning 18 months after the date of each borrowing. In each case, on the date of our final principal payment, we must also pay an end-of-term payment equal to 4.45% of the amount borrowed. We may, at our option, prepay the term loan borrowings by paying the lender a prepayment premium.
Our obligations under the loan and security agreement are secured by a security interest on substantially all of our assets, excluding our intellectual property and certain other assets. The loan and security agreement contains customary conditions to borrowing, events of default, and covenants, including covenants limiting our ability to dispose of assets, undergo a change in control, merge with or acquire other entities, incur debt, incur liens, pay dividends or other distributions to holders of our capital stock, repurchase stock and make investments, in each case subject to certain exceptions. The loan and security agreement does not require that we comply with any financial covenants.
In connection with the drawdown of the initial $5.0 million term loan under the loan and security agreement, we issued the lender a warrant to purchase 99,206 shares of our Series C preferred stock, which will become exercisable for the same number of shares of our common stock following completion of this offering. The warrant will expire on the seventh anniversary of this offering. If we draw down the second term loan under the loan and security agreement, we will issue the lender a second warrant with identical terms.
Our primary uses of cash are to fund our operations as we continue to grow our business. Cash used to fund operating expenses is impacted by the timing of when we pay expenses, as reflected in the change in our outstanding accounts payable and accrued expenses.
We believe that our existing cash and cash equivalents as of June 30, 2013, together with amounts available under our loan and security agreement and the estimated net proceeds from this offering, will be sufficient to meet our anticipated cash requirements for at least the next 12 months. Management may elect, however, to finance operations by utilizing available borrowings under our loan and security agreement or selling equity securities. If additional funding is required or desired, there can be no assurance that additional funds will be available to us on acceptable terms on a timely basis, if at all, or that we will generate sufficient cash from operations to adequately fund our operating needs or achieve or sustain profitability. If we are unable to raise additional capital or generate sufficient cash from operations to adequately fund our operations, we will need to curtail planned activities to reduce costs. Doing so will likely have an unfavorable effect on our ability to execute on our business plan.
55
The following table summarizes our cash flows for the periods indicated:
| |
Year Ended December 31, |
Six Months Ended June 30, |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | 2012 | 2013 | |||||||||
| |
(In thousands) |
||||||||||||
| |
|
|
(Unaudited) |
||||||||||
Cash provided by (used in) operating activities |
$ | (13,524 | ) | $ | (7,167 | ) | $ | 1,985 | $ | (10,623 | ) | ||
Cash used in investing activities |
(331 | ) | (1,462 | ) | (642 | ) | (891 | ) | |||||
Cash provided by financing activities |
18,646 | 15,065 | 66 | 18,195 | |||||||||
Cash Flows from Operating Activities
Cash used in operating activities for the six months ended June 30, 2013 was $10.6 million. The net loss of $13.4 million reflects non-cash charges of $2.1 million for the change in the value of the preferred stock liability, $1.3 million in amortization of the deferred fee received from Genzyme, $0.4 million of depreciation and amortization, $0.5 million of stock-based and equity-based compensation and $0.1 million of bad debt expense. The increase in net operating assets of $0.9 million was primarily due to a $1.7 million increase in accrued liabilities due to timing of payments and a $0.3 million decrease in supply inventory due to the increase in volume of testing performed, offset by a $0.5 million increase in accounts receivable due to increased revenues from Medicare and a $0.6 million increase in prepaid expenses and other assets primarily related to costs for our anticipated initial public offering.
Cash provided by operating activities for the six months ended June 30, 2012 was $2.0 million. The net loss of $8.9 million reflects non-cash charges of $1.1 million in amortization of the deferred fee from Genzyme, $0.3 million of depreciation and amortization and $0.4 million of stock-based and equity-based compensation. The increase in net operating assets of $11.1 million was primarily due to the $10.0 million we received from Genzyme. Accounts payable and accrued liabilities increased $2.1 million due to the growth in our operations and the timing of our payments. Accounts receivable increased by $0.4 million due to the increase in accrued revenue in 2012 as we had only begun to sell Afirma in 2011. In addition, there was a $0.4 million increase in supplies inventory related to increased test volume.
Cash used in operating activities for the year ended December 31, 2012 was $7.2 million. The net loss of $18.6 million was offset by non-cash charges of $0.9 million of stock-based and equity-based compensation, $0.7 million for depreciation and amortization, $0.3 million for the change in value of the preferred stock liability and $0.2 million of bad debt expense. The increase in net operating assets of $12.3 million was primarily due to the $10.0 million deferred payment from Genzyme, of which we amortized $2.4 million as of December 31, 2012. Accounts payable and accrued liabilities increased $3.9 million due to the growth in our operations and the timing of our payments. Accounts receivable increased by $0.6 million due to the increase in accrued revenue in 2012 as we had only begun to sell Afirma in 2011. In addition, there was an $0.8 million increase in supplies inventory related to increased test demand.
Cash used in operating activities for the year ended December 31, 2011 was $13.5 million. The net loss of $14.4 million was offset by non-cash charges of $0.7 million of stock-based and equity-based compensation, $0.7 million for the change in value of the preferred stock liability, $0.6 million of depreciation and amortization, $0.2 million of bad debt expense and a $0.2 million loss on the disposal of property and equipment. The decrease in net operating assets of $0.1 million was primarily due to the increase in accounts receivable as 2011 was our first year with revenue, and an increase of $0.1 million in supplies inventory, offset by an increase in accounts payable and accrued liabilities of $0.6 million due to the growth in our operations and the timing of payments.
56
Cash Flows from Investing Activities
Cash used in investing activities is primarily related to the acquisition of property and equipment totaling $0.6 million and $0.9 million for the six months ended June 30, 2012 and 2013, respectively. Purchases of property and equipment were primarily related to research and development and laboratory equipment.
Cash used in investing activities is related to the acquisition of property and equipment totaling $0.3 million and $1.5 million for the years ended December 31, 2011 and 2012, respectively, and the change in restricted cash balance totaling $55,000 and $0 for the years ended December 31, 2011 and 2012, respectively. Purchases of property and equipment were primarily related to research and development and laboratory equipment.
Cash Flows from Financing Activities
Cash from financing activities for the six months ended June 30, 2013 primarily is from net proceeds of $4.9 million from the loan and security agreement we entered into in June 2013 and net proceeds of $13.0 million from the sale of our convertible preferred stock.
Cash from financing activities for the six months ended June 30, 2012 consists of proceeds of $66,000 from the exercise of options to purchase common stock.
Cash from financing activities for the years ended December 31, 2011 and 2012 of $18.6 million and $15.1 million, respectively, were primarily due to the net proceeds from the sale of our convertible preferred stock.
Contractual Obligations
The following table summarizes our contractual obligations as of December 31, 2012 (in thousands):
| |
Payments Due by Period | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Less Than 1 Year |
1 to 3 Years |
3 to 5 Years |
More Than 5 Years |
Total | |||||||||||
Operating leases |
$ | 816 | $ | 1,927 | $ | 635 | $ | 130 | $ | 3,508 | ||||||
In February 2010, we entered into a non-cancellable lease agreement to lease our headquarters and laboratory space in South San Francisco, California. The lease expires in March 2016.
In November 2012, we entered into a non-cancellable lease agreement commencing February 2013 to lease laboratory space in Austin, Texas. The lease expires in July 2018.
In June 2013, we entered into a $10.0 million loan and security agreement with a financial institution, and drew down an initial term loan of $5.0 million. We are required to pay interest only on this loan for the first 18 months and then will begin paying principal and interest over the subsequent 30-month period.
Off-Balance Sheet Arrangements
We have not entered into any off-balance sheet arrangements.
Quantitative and Qualitative Disclosures about Market Risk
We are exposed to market risks in the ordinary course of our business. These risks primarily relate to interest rates. We had cash and cash equivalents of $14.0 million and $20.7 million as of December 31, 2012 and June 30, 2013, respectively, which consist of bank deposits and money market funds. Such interest-bearing instruments carry a degree of risk; however, we have not been exposed to, nor do we anticipate being exposed to, material risks due to changes in interest rates. A hypothetical 10% change in
57
interest rates during any of the periods presented would not have had a material impact on our financial statements.
JOBS Act Accounting Election
We are an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. Under the JOBS Act, emerging growth companies can delay adopting new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies.
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board, or FASB, issued authoritative guidance to achieve common fair value measurement and disclosure requirements between U.S. GAAP and International Financial Reporting Standards. This new literature amends current fair value measurement and disclosure guidance to include increased transparency around valuation inputs and investment categorization. The guidance is effective for fiscal years and interim periods beginning after December 15, 2011. As this guidance provides only presentation requirements, its adoption did not impact our financial condition or results of operations.
In June 2011, the FASB issued authoritative guidance requiring companies to present items of net income, items of other comprehensive income and total comprehensive income in one continuous statement or two consecutive statements. This guidance eliminates the option for companies to present other comprehensive income in the statement of stockholders' equity. We adopted this standard in January 2012. As this guidance provides only presentation requirements, its adoption did not impact our financial condition or results of operations.
In February 2013, the FASB issued Accounting Standards Update (ASU) No. 2013-02, Reporting of Amounts Reclassified Out of Accumulated Other Comprehensive Income. This ASU requires reporting and disclosure about changes in accumulated other comprehensive income balances and reclassifications out of accumulated other comprehensive income. We adopted this guidance as of January 1, 2013 on a prospective basis and the adoption did not have a material effect on our financial statements as we do not have comprehensive income (loss).
58
Overview
We are a diagnostics company pioneering the field of molecular cytology to improve patient outcomes and lower healthcare costs. We specifically target diseases that often require invasive procedures for an accurate diagnosis – diseases where many healthy patients undergo costly interventions that ultimately prove unnecessary. We improve the accuracy of diagnosis at an earlier stage of patient care by deriving clinically actionable genomic information from cytology samples collected in an outpatient setting. Our first commercial solution, the Afirma Thyroid FNA Analysis, includes as its centerpiece our Gene Expression Classifier, which we refer to as the GEC. The GEC helps physicians reduce the number of unnecessary surgeries by employing a proprietary 142-gene signature to preoperatively determine whether thyroid nodules previously classified by cytopathology as indeterminate can be reclassified as benign. We have demonstrated the clinical utility and cost effectiveness of the GEC in studies published in peer-reviewed journals and established the clinical validity of the GEC in a study published in The New England Journal of Medicine in 2012.
Since we commercially launched Afirma in January 2011, we have processed over 50,000 fine needle aspiration, or FNA, samples for evaluation using Afirma and performed more than 10,000 GECs in order to resolve indeterminate cytopathology results. We have received positive coverage decisions from Aetna, Humana, Medicare and UnitedHealthcare. Collectively, these payers represent more than 100 million covered lives. Additionally, we have entered into a global co-promotion agreement with Genzyme Corporation, a subsidiary of Sanofi. Our revenue has increased from $2.6 million in 2011 to $17.1 million for the trailing twelve months ending June 30, 2013.
For decades, pathologists have diagnosed complex diseases by evaluating cells taken from a surgical tissue sample. More recently, molecular diagnostic tests that analyze the genomic material in these samples have emerged as an important complement to surgical pathology by predicting outcomes and guiding treatment decisions. Both approaches, however, typically require relatively large quantities of tissue that must be obtained through an invasive surgical procedure. Cytopathology, which relies on small samples such as FNAs, collected in an outpatient setting, is often the first step in the diagnostic process because it offers a minimally invasive and cost effective alternative to surgery. However, cytology samples tend to be small and non-uniform, which contributes to a relatively high rate of diagnostic ambiguity, which results in many patients undergoing surgery to obtain an accurate diagnosis. Molecular diagnostics broadly used today are not designed to reduce this ambiguity.
We are building our molecular cytology business by developing molecular diagnostics that yield clinically actionable genomic information from cytology samples, as opposed to surgical tissue samples. Molecular cytology identifies genomic signatures from cytology samples to inform clinical decisions prior to surgery. We believe molecular cytology has the potential to improve patient care while simultaneously lowering costs to the healthcare system in a broad range of areas including thyroid, pulmonology, dermatology and reproductive endocrinology. Based on our internal analysis and third-party data, we believe molecular diagnostic solutions to address these markets could represent an approximately $4.0 billion opportunity.
Our strategy is to focus on diseases in which a large number of patients undergo invasive and costly diagnostic procedures that could be avoided with a more accurate diagnosis from a cytology sample taken pre-operatively. In prioritizing our opportunities, we develop a detailed understanding of the unmet clinical need and the shortcomings of the current standard of care. We precisely define the clinical question in these diseases that, if informed by genomic information, would alter the standard of care in a way that improves patient outcomes while reducing costs in both the short- and long-term. Only then do we deploy our expertise in biomarker discovery and algorithm development to derive a genomic signature that provides meaningful diagnostic information. We position our diagnostic solution as an alternative to an invasive procedure and attempt to efficiently validate the accuracy of our diagnostic tests during product development by comparing our results to those obtained using the more invasive approach.
59
We developed our first commercial offering, Afirma, to address a significant unmet need in thyroid nodule diagnosis. Thyroid nodules, or bumps under the skin of the neck around the thyroid gland, are usually benign, however, patients with thyroid nodules larger than one centimeter are often referred to an endocrinologist for evaluation. Endocrinologists typically collect cells from the nodule for cytopathology with an FNA and send these samples to a cytopathologist for analysis. Approximately 525,000 thyroid FNAs were performed in the United States in 2011. Typically 15% to 30% of FNAs yield indeterminate results, meaning they cannot be diagnosed as definitively benign or malignant by cytopathology alone. Because the risk of malignancy is approximately 25% for an indeterminate diagnosis, clinical practice guidelines have historically recommended that patients with indeterminate cytopathology results undergo surgery to remove part or all of their thyroid to obtain an accurate pathology diagnosis. However, in 70%-80% of these cases, the thyroid nodule proves to be benign for cancer. We estimate the average cost of surgery to be $15,000, and surgery can result in complications and leave a patient in need of hormone replacement therapy for life.
Afirma is a comprehensive solution that consists of cytopathology and the GEC. The GEC reduces the number of unnecessary diagnostic surgeries by analyzing the genomic signature of FNA samples judged to be indeterminate by cytopathology and reclassifies about 50% of those nodules to a benign diagnosis. In The New England Journal of Medicine clinical validation study for the GEC, the study authors concluded that the GEC could be useful to physicians in making important patient care decisions, such as recommending watchful waiting in lieu of diagnostic surgery for patients who receive a GEC benign result following indeterminate cytopathology findings. A subsequent clinical utility study published in Thyroid covered 368 patients from 51 different endocrinologists. Each of these patients had both a cytopathology indeterminate result and a GEC-benign result. The study found that physicians recommended surgery in only 7.6% of these cases, representing a 90% reduction in surgeries when compared to the historical average for patients with cytopathology indeterminate results alone. We believe the GEC is currently the only diagnostic test that meets the criteria of the National Comprehensive Cancer Network, or NCCN, for safely monitoring patients with indeterminate cytopathology results in lieu of surgery.
The graphic below illustrates how Afirma changes the traditional method of thyroid nodule diagnosis.

In addition to thyroid cancer, there are many other complex diseases in which cytology samples play a critical role in clinical decision making. As with thyroid nodule diagnosis, inherent ambiguity in evaluation
60
of cytopathology samples often results in unnecessary costs and procedures that would be avoidable if a molecular diagnostic test could refine diagnoses reached by cytopathology alone. We are currently developing the Afirma Malignant GEC test to identify rare forms of thyroid cancer or metastases to the thyroid that is intended to better inform surgical strategy. We are also in late biomarker discovery in interstitial lung disease, a group of lung diseases affecting the tissue and space around the microscopic air sacs of the lungs that are difficult to diagnose prior to surgery. Specifically, we intend to improve the accuracy of diagnosis of idiopathic pulmonary fibrosis, one of the more progressive, often fatal, interstitial lung diseases, and to provide critical information to physicians and patients as they decide whether to pursue potentially lifesaving treatments and participate in clinical studies.
Limitations of Disease Diagnosis Today
Surgical pathology has long been part of the standard of care for diagnosis in many complex diseases, including the diagnosis of many kinds of cancer and lung diseases. Samples collected from surgeries allow multiple slices, or sections, of the tissue to be stained, permitting a pathologist to evaluate the shape and structure of the cells in question, or cellular morphology, that diagnostically classify the sample. However, surgical pathology by definition requires an invasive procedure. Cytopathology, or the analysis of small numbers of cells obtained by minimally invasive needle biopsies, scrapings or smears, what we refer to as cytology samples, is designed to provide a pathologic diagnosis using a small biopsy, obviating the need for surgery. However, cytology samples often have small numbers of cells for microscopic analysis which can make it difficult to make a definitive diagnosis. Even when tissue samples are obtained through a diagnostic surgery, there are limitations of microscopic review to guide patient care and treatment decisions. Cells that structurally appear the same by pathology review under a microscope may function differently over the course of disease progression. Predicting aggressiveness of disease, the likelihood of recurrence, which patients are likely to respond to treatment and which therapies would be most likely to improve outcomes is difficult. Even in cases in which pathology provides a definitive benign diagnosis, patient care would be meaningfully improved with lower costs if that diagnosis could be provided without surgery.
The role of genomic information in medical practice is evolving rapidly and has affected the diagnosis of disease as well as treatment decisions. Over the past decade, molecular diagnostic tests that analyze genomic material from surgical tissue samples have emerged as an important complement to evaluations performed by pathologists. Information at the molecular level enables one to understand more fully the makeup and specific subtype of disease to improve diagnosis. In many cases, the genomic information derived from these samples can guide treatment decisions as part of the standard of care. However, due to limitations of available technologies, many of these molecular tests require relatively large quantities of tissue with known levels of cellularity that most often must be obtained through an invasive surgical procedure.
Cytology samples offer a more attractive alternative for early, less invasive and less costly diagnosis. These samples are commonly obtained using minimally invasive methods, such as FNA biopsies, washings, brushings, lavages or bronchoscopy biopsies, from which to diagnose various diseases. Physicians typically collect these samples in an outpatient setting, without surgery, and therefore have the potential to offer a lower cost and less invasive approach to disease diagnosis. Cytology samples, however, are challenging for both traditional cytopathology, as well as molecular cytology, due to the small amount of cellular material obtained in the collection process and the often non-uniform nature of the collected tissue. The high rate of ambiguity in diagnosis on cytology samples today results in many patients undergoing other subsequent invasive procedures, often including surgery, to obtain an accurate diagnosis.
Extracting clinically meaningful genomic information from these small, heterogeneous cytology samples offers the potential to reduce ambiguity in diagnosis prior to surgery and inform treatment decisions at a much lower cost to the healthcare system.
61
Our Solution
We are pioneering the field of molecular cytology by developing molecular diagnostics that yield clinically actionable genomic information from cytology samples. Molecular cytology combines the screening benefits of a minimally invasive cytology sample with genomic information to inform disease diagnosis or treatment decisions pre-operatively. Our approach begins by developing a detailed understanding of the unmet clinical need and the current standard of care. We precisely define the clinical question in a disease area that, if informed by genomic information, would alter the standard of care in a way that reduces costs and improves patient outcomes. Only then do we deploy our scientific expertise in biomarker discovery and algorithm development to derive a genomic signature that provides meaningful diagnostic information. We focus on diseases in which a large number of patients undergo invasive and costly diagnostic procedures that could be avoided with a more accurate diagnosis from a cytology sample taken pre-operatively. Positioning our test as an alternative to an invasive procedure allows us to efficiently validate the accuracy of our test by comparing our test results to those obtained using the more invasive approach. Armed with clinical data that supports the use of molecular cytology in lieu of a more invasive or costly procedure, we believe we are well-positioned to support clinical studies that demonstrate how our products change the standard of care, improve patient outcomes and reduce costs.
We take an integrated team approach in identifying a large, unmet need and carefully defining the relevant clinical question and performance specifications we believe must be achieved to alter patient care. We then leverage the expertise we have developed in biomarker discovery and algorithm development to derive a genomic signature that provides an answer to that clinical question. In contrast to molecular diagnostics developed for surgical tissue, our solution solves many of the technical challenges associated with generating analytically valid and clinically relevant genomic information from smaller, heterogeneous cytology samples. To this end, we use a whole-genome approach for gene selection and machine-learning algorithms with statistical methods to identify the genomic signature that achieves the desired performance. Once we have a feasible genomic signature to move forward in product development, we partner with key opinion leaders to design and execute clinical studies that specifically validate the key attributes we believe will be required for broad adoption and reimbursement of our products.
In order to achieve broad clinical adoption and consistent reimbursement, we believe stakeholders in the healthcare system are increasingly demanding that a molecular diagnostic not only meet a rigorous standard of evidence supporting a test's ability to detect disease, but also provide information to physicians that affects clinical decisions, improves patient outcomes and favorably affects cost. Our clinical studies are designed to demonstrate that by deploying our solutions, physicians can safely avoid or delay a more invasive diagnostic procedure for a meaningful proportion of a patient population. Our studies are also designed to confirm that our diagnostic solution materially affects the standard of care and to quantify the resulting costs savings and benefits to patient care. The clinical evidence supporting the GEC is sufficiently robust to reduce diagnostic surgery on patients with cytology indeterminate results by approximately 90% as measured by our published clinical utility and clinical validity data.
We drive physician adoption and retention by marketing Afirma as the centerpiece of a comprehensive solution for improved disease diagnosis, which allows our solution to seamlessly integrate into a physician's practice workflow. We offer Afirma to physicians as a turnkey solution that combines cytopathology for every patient with the GEC when cytopathology yields ambiguous results. Our solution includes a complete patient report that guides decision making. By integrating disparate diagnostic procedures into one comprehensive offering, we can simplify and improve the diagnostic process for physicians and their patients while optimizing utilization of our molecular diagnostics to maximize clinical benefits and cost savings. We intend to duplicate this model with solutions we develop for other diseases.
Our capabilities in managed care and claims adjudication are essential to our success in obtaining positive coverage decisions and reimbursement. Our integrated team combines expertise in advocating for positive coverage decisions with specific insights into what tactical steps will maximize reimbursement from each payer. As a result, we have developed detailed knowledge of the intricacies of specific payer practices
62
and requirements, which informs our strategy across disease selection, clinical study design, marketing and sales.
Thyroid Cancer Diagnostic Market
Afirma addresses a large and growing thyroid nodule diagnostic market where significant ambiguity in cytopathology offers the potential to reduce the rate of surgery needed to diagnose or treat thyroid cancers. These dynamics offer an attractive opportunity for diagnostic improvement:
- •
- Large, growing market. Thyroid cancer is the fastest
growing cancer in the United States according to the American Cancer Society, and screening of nodules suspicious for cancer is rapidly increasing the number of thyroid FNAs performed. Approximately
525,000 thyroid FNAs were performed in the United States in 2011. We estimate the thyroid nodule diagnostic market opportunity today is approximately $500 million per year in the United States,
consisting of an estimated $100 million of cytopathology testing, $350 million of GECs performed on indeterminate cytopathology samples and an additional $50 million related to a
molecular cytology test for malignant thyroid FNA samples. Our market research indicates that there is an estimated $300 million market opportunity for the GEC internationally. We believe we
can effectively market Afirma with a small specialty sales force in part because Afirma represents a significant innovation in the underserved thyroid cancer diagnostic market. Because Afirma
represents a significant innovation for this underserved and relatively concentrated base of physicians, we believe we can effectively market Afirma with a small specialty sales force.
- •
- High costs of unnecessary surgery for patients and
payers. The biology of thyroid cells is complex. Microscopic analysis by a cytopathologist typically results in 15% to 30% of diagnoses
being deemed indeterminate, meaning they cannot be diagnosed as definitively benign or malignant by cytopathology alone. This ambiguity results in confusion for doctors and patients. The 2011 NCCN
Clinical Practice Guidelines in Oncology recommend these patients undergo a diagnostic surgery, which we estimate costs $15,000 on average. Post-surgical diagnosis indicates a benign condition in 70%
to 80% of these surgeries but surgery can result in complications and leave a patient in need of hormone replacement therapy for life.
- •
- Concentrated base of customers. We estimate that
approximately 3,500 endocrinologists specialize in thyroid disease. While endocrinologists are responsible for diagnosing patients and referring them to surgery when necessary, endocrinologists
generally do not perform the surgeries themselves. Afirma represents a new solution that endocrinologists can employ to better identify patients with benign results, where watchful waiting is the
appropriate standard of care rather than referral to a surgeon.
- •
- Highly fragmented thyroid FNA cytopathology market. We believe the analysis of thyroid FNAs is highly fragmented among local cytopathologists and a number of local, regional and national laboratories. As a result, turnaround times and analysis quality can vary between laboratories and cytopathologists. Because an ambiguous diagnosis often leads patients to opt for thyroid surgery, cytopathology practices that meet standards comparable to those found in leading academic settings have the potential to reduce the frequency of indeterminate diagnoses and subsequent thyroid surgeries.
Afirma Thyroid FNA Analysis
Afirma Thyroid FNA Analysis is our comprehensive solution for thyroid nodule diagnosis. Our customers, primarily endocrinologists, radiologists and head and neck specialists, can implement Afirma in their practice without any meaningful changes to their workflow. Samples for both cytology and the GEC are collected during one FNA procedure on the patient using well accepted techniques.
63
The majority of our customers practice in the community setting. Our community-based customers send both the cytopathology and the GEC samples overnight to our licensed CLIA laboratory for analysis. After we receive samples and accession them into our laboratory information system, the GEC samples are stored in a freezer while the cytopathology samples are prepared and stained for review by Thyroid Cytology Partners, or TCP, a practice solely focused on thyroid FNA analysis. When cytopathology results are indeterminate, we perform the GEC on the patient's sample collected from the same FNA procedure. Approximately 14% to 17% of thyroid FNA biopsies to date from TCP have been classified as indeterminate and have been reflexed to the GEC. This rate is at the low end of the 15% to 30% range cited in the 2009 American Thyroid Association Guidelines, suggesting TCP's specialized focus on thyroid cytopathology offers results more consistent with academic settings. Through our relationship with TCP, the high quality of care historically only accessible to patients in academic settings is now broadly available.
By using a single thyroid-specialty laboratory to offer consistent cytopathology analysis, we can optimize quality and manage appropriate utilization, ensuring that the GEC is not run on cytologically benign or malignant samples, or where the FNA contains insufficient cellular material for diagnosis. Our ability to manage utilization is attractive to payers looking to capture the value we promise in patient care.
Physicians based in academic settings generally conduct cytopathology in their own laboratory. With Afirma, the GEC sample is preserved until they have processed the cytopathology results. The GEC samples from patients with a cytopathology indeterminate diagnosis are then sent overnight to our laboratory for analysis.
Whether the final result is rendered by cytopathology alone or a combination of cytopathology and the GEC, physicians receive an actionable answer based on samples collected in a single patient visit.
The graphic below illustrates the Afirma workflow:
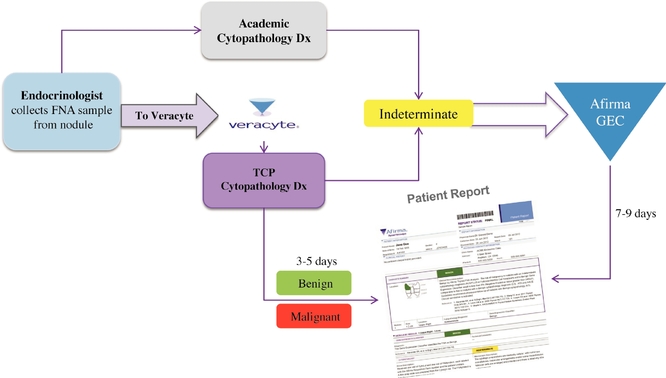
Advantages of Afirma for Stakeholders
Patients
With the GEC, approximately half of the patients with indeterminate cytology results may avoid unnecessary, invasive diagnostic surgery. Patients who obtain an Afirma benign result avoid the potential
64
for surgery-related complications, the effects of life-long hormone replacement therapy and the associated costs. We estimate that approximately 115,000 FNAs performed in the United States in 2011 yielded an indeterminate result. With Afirma, patients benefit from access to high-quality cytopathology services delivered as part of our comprehensive solution. Samples for both cytopathology and the GEC can be collected during one routine FNA procedure, delivering to patients a comprehensive assessment of their health status from the first office visit.
Physicians
Afirma enables every physician, regardless of practice setting, to offer his or her patients access to advanced technology for the diagnosis and management of thyroid nodules. We believe the GEC is the only test available today to reclassify an indeterminate thyroid diagnosis as benign with a risk of malignancy similar to that of a benign diagnosis by cytopathology alone. Afirma does not introduce any new steps into the physician's patient-care routine and eliminates the step of preparing slides for cytopathology. In addition, TCP, our cytopathology provider, is a specialized practice focused solely on performing thyroid FNAs and meets high quality standards with short turnaround times. According to a market research study commissioned by Genzyme, a survey of 229 endocrinologists indicated that 86% of 102 Afirma users reported that they were either extremely satisfied or very satisfied with the services of TCP.
Payers
Payers differentiate themselves by offering their insured the most advanced care available in medicine, however, payers are also under increased pressure to contain rising healthcare costs. Afirma allows payers to provide advanced care at a cost lower than the current standard of care. The first peer-reviewed and independent economic impact study, published in the Journal of Clinical Endocrinology and Metabolism, concluded that routine use of the GEC in the United States would prevent tens of thousands of surgeries each year. Based on our estimate of the average cost of surgery of $15,000 as well as clinical utility studies, we believe full adoption of Afirma would result in over $500 million in direct cost savings to the healthcare system over five years.
Our Strategy
Our goal is to resolve diagnostic ambiguity pre-operatively, allowing patients to avoid unnecessary procedures and generate significant cost savings for the healthcare system.
Key initiatives driving our strategy include:
- •
- Accelerate the growth of Afirma. We will continue to drive
rapid adoption of Afirma by expanding our base of prescribing physicians and achieving broader reimbursement. We plan to selectively grow our sales force in high-volume geographies domestically and
leverage our marketing relationship with Genzyme to accelerate Afirma growth both in the Unites States and internationally. We intend to increase the body of clinical and pharmacoeconomic evidence to
support Afirma's inclusion in additional clinical practice guidelines. We will use our inclusion in guidelines and the extensive data published on Afirma to date, coupled with our core expertise in
managed care, claims adjudication, and billing to drive broader reimbursement.
- •
- Market our novel molecular diagnostic tests as the centerpiece of a comprehensive patient-care solution. In each disease area we pursue, we intend to offer one comprehensive solution that integrates our tests with the disparate diagnostic procedures recommended by clinical practice guidelines. By applying a consistent, evidenced-based diagnostic framework to every patient that fits seamlessly within the physician's practice workflow, we reduce complexity for our customers and optimize utilization of our molecular diagnostics to maximize patient benefit and cost savings.
65
- •
- Drive cost and capital efficiencies by offering turnkey solutions to physicians in specialty
markets. The infrastructure we have built to make Afirma commercially available is designed to support a rapid acceleration in patient
volumes as we drive broader adoption. Because we market Afirma in a specialty market as part of a turnkey solution, our targeted sales force is able to devote fewer resources to maintaining business
with our existing base of prescribing physicians and instead focus on driving adoption of Afirma among new customers. As a result, we believe we are well-positioned to drive rapid margin improvements
and achieve scale in Afirma with only incremental capital investments. We intend to target diseases that are well suited to this sales model whenever possible.
- •
- Broaden our addressable market in endocrinology. Our
product development pipeline includes additional genomic tests to complement Afirma that will serve our current base of physician customers. The large volumes of thyroid FNA samples we receive in the
course of performing Afirma provides us with access to patient FNAs from rare malignancies or cancers that have metastasized to the thyroid gland. For example, in the second quarter of 2014, we plan
to introduce the Afirma Malignant GEC, our first product line extension to guide surgical strategy for the treatment of medullary thyroid cancer and other rare and metastatic forms of thyroid cancer.
- •
- Expand molecular cytology to additional diseases. We intend to apply our core competencies we have developed in disease selection, genomic discovery, clinical development, and managed care strategy to additional areas of unmet need. For example, we are pursuing a solution for ILD diagnosis that will offer an alternative to surgery by developing genomic signatures derived from cytology samples collected through less invasive bronchoscopy techniques. We intend to commercialize our first lung product in 2016 and believe this product will serve as the foundational application to expand our molecular cytology platform within the pulmonology vertical.
The Afirma Gene Expression Classifier
Development
For the GEC, we used a whole-genome approach to identify gene expression patterns that could best identify a benign thyroid nodule signature in thyroid FNA samples diagnosed as indeterminate by cytopathology. We utilized microarray technology to perform whole-genome analyses on hundreds of thyroid samples, producing a rich database of more than one billion genomic measurements of thyroid biology. We initially measured mRNA expression in over 247,000 transcripts before selecting the target genes to be measured. We acquired large numbers of FNA samples taken from endocrinology practices across the United States in the early development of the GEC. Because thyroid cancer is a complex disease with multiple, sometimes rare, subtypes, this approach provided the diversity of clinical samples that would be encountered both during clinical validation and in commercial practice. Our scientists then developed machine-learning algorithms using sophisticated statistical approaches to distill the large amount of genomic data, and to address FNA sample variability, dilution effects and RNA quantity and quality challenges. The development of the GEC first on thyroid surgical tissue and then on thyroid FNA samples was first published in 2010 in the Journal of Clinical Endocrinology and Metabolism.
Clinical Validation
We collaborated with clinicians across the country to demonstrate the clinical validity of the GEC in a range of practice settings. Clinical validity refers to the accuracy of the results from the GEC against diagnosis from expert pathological review of surgical tissue samples.
Preoperative Diagnosis of Benign Thyroid Nodules with Indeterminate Cytology (The New England Journal of Medicine, 2012)
In this study, our gene expression classifier exhibited a negative predictive value, or NPV, of 95% for indeterminate results in the atypia or follicular lesion of undetermined clinical significance category (AUS/FLUS) and 94% for indeterminate results in the suspicious for follicular or Hürthle cell neoplasm category
66
(SFN/SHN) and reclassified as benign over half of the true benign FNA samples that had indeterminate cytopathology diagnoses, which the authors defined to include any results suspicious for malignancy in addition to AUS/FLUS and SFN/SHN. This pivotal validation study employed a prospective, multicenter, double-blind study design to validate the accuracy of pre-operative GEC benign results compared to post-operative expert pathology review. It was the second prospective multicenter study validating the GEC approach. The study supported the consideration of a more conservative approach than surgery for most patients with thyroid nodules that are cytologically indeterminate but benign according to GEC results.
This large multicenter study included 49 academic and community practices across 26 states over 19 months. The study involved patients with ultrasonographically confirmed thyroid nodules one centimeter or larger in diameter. 4,812 thyroid FNA samples were prospectively collected from 3,789 patients. In the independent validation set of 265 nodules that were indeterminate by cytopathology, 85 were subsequently determined malignant by surgical pathology, equivalent to a 32% risk of malignancy. The GEC correctly identified 78 of the 85 malignant nodules as suspicious, a 92% sensitivity (95% confidence interval, or CI, 84 to 97). The GEC achieved a 52% specificity (95% CI 44 to 59) and reclassified as benign over half of the true benign FNA samples that had indeterminate cytopathology diagnoses. The authors concluded that a benign GEC result has a post-test probability of malignancy that is similar to the probability for operated nodules with cytologically benign features on an FNA, making watchful waiting a safe and effective clinical option for these patients.
Molecular Classification of Thyroid Nodules using High-Dimensionality Genomic Data (Journal of Clinical Endocrinology and Metabolism, 2010)
In this study, our FNA trained classifier exhibited an NPV of 96% on a modest sized test set of indeterminate FNA samples, demonstrating an NPV similar to operated nodules with benign FNA cytology. In this study, the authors defined indeterminate results to include any cytological results suspicious for malignancy in addition to AUS/FLUS and SFN/SHN. This prospective, multicenter, double-blind study was the first study on an independent modest-sized set of FNA samples to clinically validate the gene expression classifier approach. In addition, this study demonstrated that even with substantial degradation of RNA and in the presence of blood, in some cases with dilution of up to 80%, the GEC correctly recognized benign nodules and did not miss malignancy in the majority of FNA samples.
In this study, the GEC was prospectively validated on an independent test set of 48 FNA samples, one-half of which had indeterminate cytopathology. The GEC exhibited an NPV of 96% and a specificity of 84%. The reference gold standard in this outcome study was the post-operative determination of whether the thyroid nodule was benign or malignant by expert endocrine surgical pathologists who were blinded to the GEC results. The authors concluded that the GEC performance and validation conducted on an independent validation set demonstrated a high enough specificity to reclassify over half of indeterminate FNAs as benign and that the observed NPV indicated that those nodules classified as benign by the GEC carry a similar risk of malignancy as a benign diagnosis by thyroid nodule FNA cytopathology alone.
Clinical Utility and Cost Effectiveness
We collaborated with clinicians to demonstrate the clinical utility of the GEC, which refers to the effect of the GEC result on treatment decision-making and patient outcomes. The clinical utility of the GEC is based on preventing surgery on cytologically indeterminate but benign thyroid nodules that would otherwise be referred for a diagnostic thyroid surgery. Because thyroid nodules with indeterminate FNA cytopathology have an approximately 25% risk of malignancy when resected, approximately 75% of these operations will likely be on nodules determined to be benign post-operatively. Thyroid surgery is associated with potential complications, including temporary and permanent hypocalcemia, recurrent laryngeal nerve injury (with voice change, dysphagia, and potentially airway compromise), and bleeding, with an incidence as high as approximately 2% to 10%. Hypothyroidism is an expected consequence of
67
thyroid surgery, with patients requiring life-long thyroid hormone supplementation or replacement therapy. We believe the most appropriate metric for evaluating the clinical utility of the GEC is the reduction of surgeries performed on patients with benign nodules that are diagnosed as cytologically indeterminate. We believe the impact of the GEC on the physician and patient decision making is immediate and measurable from both the perspective of avoidance of unnecessary surgery and cost savings.
Clinical utility
The Impact of Benign Gene Expression Classifier Test Results on the Endocrinologist-patient Decision to Operate in Patients with Thyroid Nodules with Indeterminate Fine Needle Aspiration Cytopathology (Thyroid, 2012)
This study found that approximately one surgery was avoided for every two GECs run on thyroid FNAs with indeterminate cytopathology, which the authors defined to include any results suspicious for malignancy in addition to AUS/FLUS and SFN/SHN. This study evaluated the clinical utility of the GEC in a multicenter, cross-sectional survey of the endocrinologists' decision to operate on patients with a cytopathology indeterminate FNA and a benign GEC result. The study reviewed the first 2,040 GEC tests performed on samples that were classified as indeterminate by cytopathology, of which the GEC reclassified 52.3% of these results as benign. In the study, a cohort of 51 endocrinologists (46 community-based; 5 academic based) at 21 practice sites in 11 states completed case report forms on whether surgery was recommended for their Afirma benign patients. Of 368 unique patients (395 cytopathology indeterminate FNAs) for whom data was collected, physicians and patients opted for watchful waiting in lieu of diagnostic thyroid surgery 92.4% of the time when the GEC result reclassified the patient's indeterminate nodule as benign. Surgery was performed on only 7.6% (CI 5.1 to 10.8) of patients, compared to the 74% rate of surgery on indeterminate thyroid nodules previously reported by Thyroid in 2011, a 90% reduction in the decision to operate (p < 0.001). The study demonstrates the effect of the GEC on clinical decision making for patients with indeterminate thyroid nodules. The graph below sets forth the results of the study:
Afirma Gene Expression Classifier:
Proven Clinical Utility

68
In addition, such results were consistent with results from an earlier unpublished study presented at the American Thyroid Association annual scientific meeting in 2011, which reported the results of a web-and mail-based opinion survey of 32 physician practices, with a mean of 89% of physicians reporting that they recommended watchful waiting for patients with cytologically indeterminate FNAs but benign GEC results.
Health economics
Cost-effectiveness of a Novel Molecular Test for Cytologically Indeterminate Thyroid Nodules (Journal of Clinical Endocrinology and Metabolism, 2011)
This clinical study was conducted by researchers from the Johns Hopkins University School of Medicine. Supported with a research grant from us, the authors found that use of the GEC can potentially avoid almost three-fourths of currently performed surgeries in patients with benign nodules but indeterminate cytopathology results, which the authors defined to include any results suspicious for malignancy in addition to AUS/FLUS and SFN/SHN.
Researchers modeled the direct cost savings of utilizing the GEC in clinical practice. They developed a 16-state Markov decision model based upon the 2009 American Thyroid Association Guidelines for the treatment of adult patients with thyroid nodules with an FNA cytopathology indeterminate diagnosis. The decision model was based on clinical validation study results and expert opinion though model variables necessarily require a substantial degree of judgment. One million patient simulations were run through the decision model to represent five years of treatment and follow-up for patients who first presented with cytologically indeterminate thyroid nodules. Utilization of the GEC yielded an estimated direct cost savings of $1,453 and an increase of 0.07 quality adjusted life years, or QALYs, per patient, a modest increase in the quality of life. A Monte Carlo simulation of 10,000 trials testing the sensitivity of all variables across a range of values resulted in the GEC being both less costly and more effective in improving care quality 92.5% of the time. A Monte Carlo simulation is the repeated sampling of random outcomes to predict likely outcomes. Additionally, the authors found no difference in cancers left untreated between the current care paradigm of sending patients with indeterminate nodules to surgery versus clinical observation following a benign GEC result. The authors concluded that if the GEC were to be universally adopted in routine clinical practice in the United States, every year 74% fewer surgeries would be performed on patients with benign nodules that cytopathology would have classified as indeterminate.
The cost savings estimate in the Johns Hopkins model was based on an estimated 14% rate of surgery on a GEC benign nodule, which rate is almost double the 7.6% subsequently reported in Thyroid as
69
described above. Based on the rate of surgery on GEC benign nodules reported in Thyroid, we estimate that each GEC test would save approximately $2,600. The graph below sets forth the results of the study:
Impact on Patient Quality-Adjusted Life Years (QALY) and Cost
Effectiveness of Incorporating GEC into Practice

Analytical Validity
Analytical Performance Verification of a Molecular Diagnostic for Cytology-Indeterminate Thyroid Nodules (Journal of Clinical Endocrinology and Metabolism, 2012)
We conducted extensive analytical performance studies to validate the performance of the GEC to ensure our ability to offer a robust, accurate and reproducible assay result on patient samples. Over 40 sub-studies were performed on a large number of FNA samples. In the above study, the GEC was subjected to an analytical verification study in our clinical laboratory.
This study found that the RNA content in an FNA sample that is preserved in our proprietary FNAProtect is stable for up to six days at room temperature with no changes in RNA yield or quality. Additionally, the GEC results were found to be stable over the range of shipping conditions expected in community practice. Analytic sensitivity studies demonstrated tolerance to variation in RNA input (5-25ng) and to the dilution of malignant FNA material down to 20%. Analytic specificity studies using malignant samples mixed with blood up to 83% and genomic DNA up to 30% demonstrated negligible assay interference with respect to false-negative results, although benign FNA samples mixed with relatively high proportions of blood demonstrated a potential for false-positive results. The GEC results were shown to be reproducible across operators, runs, reagent lots, and in inter-laboratory comparisons (standard deviation of 0.158 for scores on a >6 unit scale), demonstrating the highest level of evidence for analytic validity based on the Evaluation of Genomic Applications in Practice and Prevention, or EGAPP, criteria. Analytical sensitivity, analytical specificity, robustness, and quality control of the GEC were successfully verified, indicating its suitability for clinical use.
70
The table below summarizes the Afirma clinical studies that have been performed to date:
| Study |
Publication/ Presentation |
Main Findings |
||
|---|---|---|---|---|
| Clinical Validity | ||||
| Preoperative Diagnosis of Benign Thyroid Nodules with Indeterminate Cytology | The New England Journal of Medicine (August 2012) |
• Pivotal clinical validation study (prospective, multicenter, double-blind) • A GEC benign result is comparable in accuracy to a benign cytology result |
||
| Molecular Classification of Thyroid Nodules Using High-Dimensionality Genomic Data | Journal of Clinical Endocrinology and Metabolism (December 2010) |
• First prospective, multicenter, double-blind validation study • Even in the presence of degraded RNA, bloody samples, or malignant samples diluted up to 80% with aspirate material from benign nodules, the GEC correctly recognizes benign nodules and does not miss malignancy in the majority of FNA samples |
||
| Clinical Utility | ||||
| The Impact of Benign Gene Expression Classifier Test Results on the Endocrinologist-Patient Decision to Operate on Patients with Thyroid Nodules with Indeterminate Fine-Needle Aspiration Cytopathology | Thyroid (October 2012) |
• Large multicenter study of endocrinologists' practices • Approximately one surgery was avoided for every two GEC tests run on thyroid FNAs with indeterminate cytology |
||
| Clinical Practice Impact of a Novel mRNA–based Gene Expression Classifier in Thyroid Nodules with Indeterminate Fine Needle Aspiration Cytopathology | American Thyroid Association (Abstract Poster Presentation) (October 2011) |
• Assessed clinical utility by surveying physicians' treatment decisions(1) • Applying the survey results to 540 patients with indeterminate cytopathology, physicians recommended watchful waiting and sonographic follow up in lieu of surgery in 89% (234 of 263) of patients with a benign GEC result |
||
| Health Economics | ||||
| Cost-Effectiveness of a Novel Molecular Test for Cytologically Indeterminate Thyroid Nodules | Journal of Clinical Endocrinology and Metabolism (August 2011) |
• Use of Afirma can potentially avoid almost three-fourths of currently performed surgeries in patients with benign nodules |
||
| Analytical Validity | ||||
| Analytical Performance Verification of a Molecular Diagnostic for Cytology-Indeterminate Thyroid Nodules | Journal of Clinical Endocrinology and Metabolism (October 2012) |
• Analytical sensitivity, analytical specificity, robustness, and quality control of the GEC were successfully verified, indicating its suitability for clinical use |
||
| Other Studies | ||||
| A Large Multicenter Correlation Study of Thyroid Nodule Cytopathology and Histopathology | Thyroid (March 2011) |
• Prospective multicenter study and meta-review of 11 recently published U.S. based pathology series • Two-thirds of cytologically indeterminate nodules(1) were found to be benign post-operatively • Operated cytology benign nodules were found to have an 11% risk of malignancy in the prospective study and 6% risk of malignancy in the meta-review (range 2%-18%) |
||
- (1)
- Indeterminate results were defined to include any cytological results suspicious for malignancy in addition to AUS/FLUS and SFN/SHN.
71
The table below summarizes review articles related to Afirma that have been published to date:
| Title |
Publication |
Summary |
||
|---|---|---|---|---|
| Use of the Afirma Gene Expression Classifier for Preoperative Identification of Benign Thyroid Nodules with Indeterminate Fine Needle Aspiration Cytopathology | PLoS Currents: Evidence on Genomic Tests (February 2013) |
• Studies reviewed regarding clinical validity, analytic validity, and clinical utility support recommendation for offering patients the alternative of using the GEC in lieu of thyroid resection in the specific case of thyroid FNAs with indeterminate cytopathology |
||
| Minimizing Unnecessary Surgery for Thyroid Nodules | The New England Journal of Medicine (August 2012) |
• Clinical algorithm recommending monitoring in lieu of diagnostic surgery in patients with indeterminate FNA cytopathology results |
||
| Diagnostic Use of Molecular Markers in the Evaluation of Thyroid Nodules | Endocrine Practice (September/October 2012) |
• Genomic tests exhibit variable performance characteristics and require clinical validation in prospective, multicenter, blinded studies before widespread adoption • Prospective, large scale validation of Afirma provides the broadest available data among any of the thyroid nodule diagnostic tests |
||
| Molecular Biomarkers in Thyroid FNA Samples | Journal of Clinical Endocrinology & Metabolism (December 2012) |
• Clinical implementation of genomic tests requires robust demonstration of analytic validity, as reported for Afirma in Walsh et al JCEM 2012 • As many as 30-40% of thyroid carcinomas do not display known somatic oncogene mutations and may harbor novel genetic alterations • The mutation assessment test may serve best as a diagnostic algorithm to identify suspected malignancy with an NPV of up to 95%, Afirma may serve to exclude malignancy |
||
| Diagnosis and Management of Differentiated Thyroid Cancer using Molecular Biology | Laryngoscope (April 2013) |
• Molecular markers can be classified broadly into those with high positive predictive value (BRAF, RET/PTC, PAX8/PPARc) and those with potentially high negative predictive value (gene expression microarrays) • Gene expression microarrays may eliminate the need for unnecessary diagnostic lobectomy in 60% to 90% of cases |
||
| Molecular markers in the diagnosis of thyroid nodules | Brazilian Archives of Endocrinology and Metabolism (March 2013) |
• The Afirma GEC raises specificity on indeterminate cytology thyroid nodules from 0% to 52%, effectively reducing the need to operate by one-half |
||
| Progress in Molecular-based Management of Differentiated Thyroid Cancer | The Lancet (March 2013) |
• The GEC performed best on the atypia of undetermined significance (AUS) or follicular lesion of undetermined significance (FLUS) and follicular neoplasm or suspicious for follicular neoplasm lesions (SFN/SHN) (sensitivity 90%, NPV 94-95%), whereas the NPV was lower for the suspicious for malignancy lesions (85%), which have a higher prevalence of malignancy |
||
72
Practice Guidelines
We believe inclusion of new products in practice guidelines is essential to drive their broad adoption and reimbursement. In order to change patient care, tests must carry a high level of published evidence demonstrating clinical validity, analytic validity, clinical utility and cost effectiveness. When studies with such evidence are published in peer-reviewed journals, the authors of practice guidelines may assess the level of evidence and determine whether modifying existing guidelines to include new technology is warranted. In January 2013, the NCCN modified its thyroid cancer guidelines to recommend that physicians consider molecular testing for those patients with cytopathology indeterminate thyroid nodules who have a low risk of cancer. The revised NCCN guidelines further suggest that if a molecular diagnostic test predicts a risk of malignancy comparable to the risk of malignancy of a benign cytopathology result, observation in lieu of a diagnostic surgery is recommended. Based on published evidence, the GEC meets these criteria. We believe our published evidence provides a basis for the American Thyroid Association and the American Association of Clinical Endocrinologists to consider inclusion of the GEC in their treatment guidelines. Additionally, UpToDate, a leading evidence-based clinical decision support resource for physicians, recommended the GEC in its February 2013 review.
Marketing and Sales
Marketing
Our marketing strategy focuses on the comprehensive nature of the Afirma Thyroid FNA Analysis which includes as its centerpiece our proprietary GEC. Our comprehensive solution reduces the number of unnecessary diagnostic surgeries for patients with thyroid nodules. We believe our solution-based approach differentiates us in the marketplace because we serve as a one-stop provider–Afirma integrates disparate diagnostic procedures into one comprehensive offering, simplifying and improving the diagnostic process for physicians. Our approach can deliver a number of benefits to physicians, payers, and patients, including:
- •
- reduction of unnecessary thyroid surgeries;
- •
- lower healthcare costs; and
- •
- actionable information from a single patient visit.
We employ diverse marketing programs to inform key stakeholders of the value of our solution in order to drive adoption and reimbursement. As part of our marketing strategy, we educate physicians, healthcare professionals and managed care executives about our unique value proposition, which is supported by numerous peer-reviewed publications demonstrating the analytical and clinical validity, clinical utility and cost-effectiveness of Afirma. We primarily achieve this through national and regional clinical meetings focused on thyroid and endocrine disease and disorders. We also sponsor physician speaker programs and continuing medical education where both academic and community physicians educate their peers on the benefits of Afirma and provide personal testimony of the value they have provided to their patients using Afirma. We market to patient advocacy organizations and managed care organizations directly through meetings, phone calls and direct educational efforts. Finally, our website serves as a portal for educational material for healthcare professionals, payers and patients.
Sales
Pursuant to our co-promotion agreement with Genzyme, we engage in joint marketing efforts with sales professionals from Genzyme. Our primary target market for Afirma is the approximately 3,500 endocrinologists in the United States whom we believe perform the majority of FNAs in community-based practice settings. To address this concentrated market, we deploy a team of our internal sales professionals and professionals from Genzyme that specialize in endocrinology sales. Our sales team is organized into eight regions, with each region having a Veracyte sales person complemented by Genzyme sales professionals. We have designed sales goals and financial incentives to align the interests of all sales
73
representatives, regardless of company affiliation, to drive Afirma adoption and growth. Our combined sales team has significant experience selling sophisticated diagnostic services to physicians and deep expertise working with endocrinologists who diagnose and treat patients with thyroid cancer.
We have experienced a high level of customer retention. Of the physicians who ordered five or more tests in 2011, more than 80% remain customers today.
We, together with Genzyme, are in the early stages of commercializing Afirma internationally. We intend to selectively target attractive markets for entry beginning in 2014.
Strategic Relationship with Genzyme
On January 18, 2012, we entered into a co-promotion agreement with Genzyme Corporation, a subsidiary of Sanofi, whereby we granted Genzyme the co-exclusive right to market Afirma in the United States and in 40 countries pursuant to which we received a $10.0 million up-front fee from Genzyme. Genzyme is an established leader in endocrinology globally, developing and commercializing Thyrogen®(thyrotropin alfa for injection) in over 42 countries worldwide. Thyrogen is an adjunctive diagnostic agent used in follow up of patients with well-differentiated thyroid cancer, and an adjunctive treatment for ablation or destruction of thyroid remnants in patients who have had their thyroid removed for the treatment of well-differentiated thyroid cancer. Afirma offers the Genzyme endocrinology sales force a diagnostic solution that can be promoted as part of a comprehensive solution aimed at improving the quality of care for patients with suspected or confirmed thyroid cancer. We began joint marketing under the agreement in June 2012. We manage the relationship through a steering committee that oversees tactical and strategic planning activities.
Under the agreement, we are required to pay Genzyme a co-promotion fee that is equal to a percentage of our cash receipts from Afirma. As of January 18, 2013, the percentage is 40%, but it will decrease to 32% in March 2014 and thereafter. We may receive up to an additional $3.0 million from Genzyme consisting of $0.6 million for each country outside of the United States in which we obtain regulatory authorization to market Afirma and achieve a specified level of reimbursement, for up to five countries. Genzyme has also agreed to spend $0.5 million to support clinical development expenses required for entry into the international markets covered by our agreement. This obligation expires in July 2014. We record the Genzyme co-promotion fees, net of amortization related to the upfront fee, within selling and marketing expense in our statements of operations.
Our agreement with Genzyme expires January 18, 2027 and either party may terminate the agreement at any time without cause and with six months prior notice. If we terminate the agreement without cause prior to January 18, 2014, we will be required to repay 50% of the $10.0 million up-front fee, with such percentage being reduced to 40% of such fee if we were to terminate the agreement between January 18, 2014 and January 18, 2015, and 30% of such fee if we were to terminate between January 18, 2015 and January 18, 2016. After January 18, 2016, we are not required to return any portion of the fee if we terminate the agreement without cause. In addition, either party may terminate the agreement upon the occurrence of certain events or cause. We have also granted Genzyme a right of first offer to co-promote any future thyroid cancer product that we commercialize.
Reimbursement
Revenue for Afirma comes from several sources, including commercial third-party payers, such as insurance companies and health maintenance organizations, government payers, such as Medicare and Medicaid, and patients.
Payer Landscape for Afirma
Reimbursement for Afirma is comprised of two separate components: routine cytopathology and, when cytopathology yields an indeterminate result, reimbursement for the GEC. Substantially all patient
74
samples are assessed with cytopathology for which we bill both the technical and professional component using established CPT codes. We bill payers directly for the GEC using either a unique code or a miscellaneous code. Payers generally assign the GEC its own specific code once a contracting decision is made by the payer.
Effective January 2012, Palmetto GBA, a Medicare administrative contractor with jurisdiction at that time over reimbursement coverage determinations for our products, completed and published an independent technology assessment of Afirma. The review determined that Afirma met criteria for analytical and clinical validity, and clinical utility as a reasonable and necessary Medicare benefit. This coverage decision provided approximately 50 million Medicare participants with access to Afirma.
As of July 2013, more than 100 million lives are covered for Afirma and hundreds of payers have reimbursed one or more GEC tests. We obtained a positive coverage decision from UnitedHealthcare in March 2013, Aetna in June 2013 and Humana in July 2013.
Reimbursement Strategy
We employ a multi-pronged strategy designed to achieve broad coverage and reimbursement for Afirma:
Meet the evidence standards necessary to be consistent with leading clinical guidelines. We believe inclusion in leading clinical practice guidelines plays a critical role in payers' coverage decisions. The data published on the GEC to date is consistent with the requirements of the widely-recognized NCCN clinical practice guidelines. We believe that our data provides compelling evidence for inclusion in the American Thyroid Association and the American Association of Clinical Endocrinologists guidelines as well.
Execute an internal managed care policy and claims adjudication function as part of our core business operations. We believe that obtaining adequate and widespread reimbursement is a critical factor in our long-term success. We employ a team of in-house claims processing and reimbursement specialists who work with patients and payers to obtain maximum reimbursement. In parallel, a managed care team collaborates with our reimbursement specialists to ensure our payer outreach strategy reacts and anticipates the changing needs of our customer base. Our customer service team is an integral part of our reimbursement strategy, working with patients and physician practices to navigate the claims process.
Cultivate a network of key opinion leaders. Key opinion leaders are able to influence clinical practice by publishing research and determining whether new tests should be integrated into practice guidelines. We collaborate with key opinion leaders early in the development process to ensure our clinical studies are designed and executed in a way that clearly demonstrates the benefits of our tests to physicians and payers.
Compile a growing library of peer-reviewed studies that demonstrate the test is effective. To date, several peer-reviewed articles and review papers have been published and have helped support our efforts aimed at widespread adoption and reimbursement of Afirma. In each disease area we pursue, we intend to conduct studies in order to develop similar supporting literature.
Our Product Pipeline
We are continuously evaluating substantial unmet clinical needs in large, addressable markets where we can leverage our molecular cytology platform to commercialize comprehensive solutions that improve quality of life for patients by reducing unnecessary surgeries and costs. Today, minimally invasive cytology biopsies are routinely collected from numerous organs such as breast, cervix, endometrium and others. Similar to thyroid, these often generate ambiguous results that lead to invasive procedures including surgery.
75
Afirma Malignant GEC
Our product development pipeline includes additional molecular cytology tests to complement Afirma that can serve our current customer physician base. We believe we can add value to physicians, payers, and patients by characterizing thyroid nodule FNAs classified as suspicious or malignant by cytopathology with genomic information that determines subclass or suspected malignant diagnosis that could influence the choice of surgery. Several clinical manifestations that may present as a malignant thyroid nodule, such as a recurrent metastatic cancer from another organ or parathyroid conditions, would not be treated by removing the thyroid. Additionally, medullary thyroid cancer, a rare and aggressive form of thyroid cancer, requires a full central neck and lymph node surgery for treatment. Today, many of these remain undiagnosed until thyroid surgery is performed, requiring a second and more invasive surgery. We believe the only way to positively affect patient care and costs is to diagnose these conditions from the FNA. Our Afirma Malignant GEC test is being developed to inform on surgical strategy using the FNA and direct the patient to the right surgery the first time. We intend to introduce this product in the second quarter of 2014, which will expand the number of patients for which we can perform testing using the Afirma solution.
Idiopathic Pulmonary Fibrosis and Nodules Suspicious for Lung Cancer
We believe the lung disease market provides several opportunities to expand our molecular cytology platform to improve patient care and reduce costs. We have chosen ILDs as our entry into the lung vertical, as it is a large and often overlooked disease area in need of diagnostics that would meaningfully improve the standard of care. We estimate that over 200,000 patients present each year with an ILD for whom accurate diagnosis is crucial in order to develop optimal treatment plans and accurately communicate prognosis. Bronchoscopy, a minimally invasive procedure often used to diagnose lung cancer, is typically inadequate for definitive diagnosis of ILDs. As a result, tens of thousands of patients undergo expensive and invasive diagnostic surgeries.
We are in late stage biomarker discovery for IPF, one of the more challenging ILDs to diagnose. Based on our results, we are now investing in the collection of prospective samples and advancing the program into product development. We also have early biomarker discovery efforts underway to help resolve the diagnosis of nodules found on imaging modalities that are suspicious for lung cancer.

76
Research and Development
Our technology platform offers a number of key attributes:
- •
- Core expertise in whole genome analysis. Our team of
bioinformatics and computational scientists possess extensive knowledge of both existing computational methods as well as the capacity to develop proprietary methods as needed for algorithm design. We
demonstrated our ability to make sense of large amounts of genomic data with machine learning algorithms in the development of the GEC.
- •
- Proprietary capabilities in analyzing small, heterogeneous cytology
samples. We have developed proprietary technology, intellectual property and know-how for optimized methods for extraction and analysis
of nanogram quantities of RNA from small biopsy samples. Although others can extract RNA from FNAs, we believe their process has not been optimized and scaled for high-throughput clinical testing and
large-scale clinical development studies involving amplification and hybridization to high-density microarrays. Our process uses commercially available reagents and instruments with our own
proprietary process and protocols, which results in RNA extraction from the range of FNAs used in our clinical development studies and our commercial laboratory test.
- •
- Precision and reproducibility. We have in place standard
operating procedures governing reagents, materials, instruments and controls and extensive experience from numerous verification studies performed for the GEC. We are applying the same high-quality
control methods that were developed for our reagents and processes, along with our proprietary software for automation, sample tracking, data quality control and statistical analysis, to our
development process in interstitial lung disease and expect to do so for other diseases in the future.
- •
- Technology agnostic discovery platform. We are not reliant on specific formats and are able to take advantage of a multitude of genomic technologies in developing future tests. When we developed the GEC in 2008, microarray technologies were a cost-effective discovery technology compared to other approaches that were nascent at the time. More recently, the rapid cost reductions achieved in next generation sequencing platforms has allowed us to pursue our whole genome approach to biomarker discovery using a range of technologies, including gene expression and DNA methylation, as well as DNA and RNA sequencing.
Our research and development expenses for the years ended December 31, 2011 and 2012 and for the six months ended June 30, 2013 were $6.7 million, $6.6 million and $3.9 million, respectively.
Laboratory Operations
Our laboratory operations are headquartered at our CLIA-certified laboratory in South San Francisco, California, where we perform all GEC testing. Beginning in May 2013, our customers began shipping samples to our laboratory in Austin, Texas. Once received, samples are processed through our automated accessioning system, prepared for cytopathology review, and delivered to TCP for cytopathology diagnosis. If an FNA sample is diagnosed as indeterminate following cytopathology, the sample is transferred to South San Francisco where we perform GEC testing. Our South San Francisco facility is responsible for quality assurance oversight, licensing and regulation compliance and maintenance for both of our laboratories to ensure data integrity and consistent, validated processes.
Quality Assurance
Our quality assurance function oversees quality of the our laboratories as well as the quality systems used in research and development, client services, billing operations and sales and marketing. We have established a quality system implementation and maintenance, document control, supplier qualification, corrective or preventive actions oversight, and employee training processes that we believe achieves excellence in operations across the entire business. We continuously monitor and improve our quality over
77
time and believe our implementation of these processes has supported our achievement of product performance, customer satisfaction and retention and a philosophy of continuous improvement.
Competition
We believe the principal competitive factors in our target market include:
- •
- quality and strength of clinical and analytical validation data;
- •
- confidence in diagnostic results;
- •
- the extent of reimbursement;
- •
- inclusion in practice guidelines;
- •
- cost-effectiveness; and
- •
- ease of use.
We believe we compete favorably on the factors described above.
Our principal competition for Afirma comes from traditional methods used by physicians to diagnose thyroid cancer. Practice guidelines in the United States have historically recommended that patients with indeterminate diagnoses from cytopathology results be considered for surgery to remove all or part of the thyroid to rule out cancer. This practice has been the standard of care in the United States for many years, and we will need to educate physicians about the benefits of our test in order to change clinical practice.
We also face competition from commercial laboratories, such as Laboratory Corporation of America Holdings and Quest Diagnostics Incorporated, with strong infrastructure to support the commercialization of diagnostic services. We face potential competition from companies, such as Life Technologies Corporation, which is currently expected to be acquired by Thermo Fisher Scientific Inc., and Illumina, Inc., both of which have recently announced their intention to enter the clinical diagnostics market. Other potential competitors include companies that develop diagnostic tests such as Roche Diagnostics, a division of Roche Holding Ltd, Siemens AG and Qiagen N.V. We also face competition from Asuragen Inc. and other companies that measure mutational markers such as BRAF and KRAS to identify nodules that are malignant instead of benign. In the future, we may also face competition from companies developing new products or technologies.
In addition, competitors may develop their own versions of our solution in countries where we do not have patents or where our intellectual property rights are not recognized and compete with us in those countries.
Many of our potential competitors have widespread brand recognition and substantially greater financial, technical and research and development resources and selling and marketing capabilities than we do. Others may develop products with prices lower than ours that could be viewed by physicians and payers as functionally equivalent to our solution, or offer solutions at prices designed to promote market penetration, which could force us to lower the list price of our solutions and affect our ability to achieve profitability. If we are unable to change clinical practice in a meaningful way or compete successfully against current and future competitors, we may be unable to increase market acceptance and sales of our products, which could prevent us from increasing our revenue or achieving profitability and could cause our stock price to decline.
Intellectual Property
In order to remain competitive, we must develop and maintain protection of the proprietary aspects of our technologies. To that end, we rely on a combination of patents, copyrights and trademarks, as well as contracts, such as confidentiality, invention assignment and licensing agreements. We also rely upon trade secret laws to protect unpatented know-how and continuing technological innovation. In addition, we have
78
what we consider to be reasonable security measures in place to maintain confidentiality. Our intellectual property strategy is intended to develop and maintain our competitive position.
As of June 30, 2013, we had six pending United States nonprovisional patent applications and one allowed patent application related to methods that are used in the Afirma diagnostic and one pending United States provisional patent application relating to our lung disease product under development. Many of these patent applications have also been filed in one or more foreign countries.
We intend to file additional patent applications in the United States and abroad to strengthen our intellectual property rights; however, our patent applications (including the patent applications listed above) may not result in issued patents in a timely fashion or at all, and we cannot assure investors that any patents that have issued or might issue will protect our technology. We may receive notices of claims of potential infringement from third parties in the future. For additional information, see the section of this prospectus captioned "Risk Factors–Risks Related to Intellectual Property".
We hold registered trademarks in the United States for "Veracyte" and "Afirma".
We require all employees and technical consultants working for us to execute confidentiality agreements, which provide that all confidential information received by them during the course of the employment, consulting or business relationship be kept confidential, except in specified circumstances. Our agreements with our research employees provide that all inventions, discoveries and other types of intellectual property, whether or not patentable or copyrightable, conceived by the individual while he or she is employed by us are assigned to us. We cannot provide any assurance, however, that employees and consultants will abide by the confidentiality or assignment terms of these agreements. Despite measures taken to protect our intellectual property, unauthorized parties might copy aspects of our technology or obtain and use information that we regard as proprietary.
Regulation
Clinical Laboratory Improvement Amendments of 1988, or CLIA
As a clinical reference laboratory, we are required to hold certain federal, state and local licenses, certifications and permits to conduct our business. Under CLIA, we are required to hold a certificate applicable to the type of laboratory examinations we perform and to comply with standards covering personnel, facilities administration, quality systems and proficiency testing.
We have current certificates under CLIA to perform testing at each of our locations. To renew our CLIA certificates, we are subject to survey and inspection every two years to assess compliance with program standards. The regulatory and compliance standards applicable to the testing we perform may change over time, and any such changes could have a material effect on our business.
If one of our clinical reference laboratories is out of compliance with CLIA requirements, we may be subject to sanctions such as suspension, limitation or revocation of our CLIA certificate, as well as directed plan of correction, state on-site monitoring, civil money penalties, civil injunctive suit or criminal penalties. We must maintain CLIA compliance and certification to be eligible to bill for diagnostic services provided to Medicare beneficiaries. If we were to be found out of compliance with CLIA requirements and subjected to sanction, our business could be harmed.
U.S. Food and Drug Administration: Diagnostic Kits
Diagnostic kits that are sold and distributed through interstate commerce are regulated as medical devices by the FDA. Devices subject to FDA regulation must undergo premarket review prior to commercialization unless the device is of a type exempted from such review. In addition, manufacturers of medical devices must comply with various regulatory requirements under the Federal Food, Drug, and Cosmetic Act, or FDC Act, and implementing regulations promulgated under that Act. Entities that fail to comply with FDA requirements may be subject to issuance of notice of observations, untitled or warning
79
letters, and can be liable for criminal or civil penalties, such as recalls, import detentions, seizures, or injunctions, including orders to cease manufacturing.
The FDC Act classifies medical devices into one of three categories based on the risks associated with the device and the level of control necessary to provide reasonable assurance of safety and effectiveness. Class I devices are deemed to be low risk and are subject to the fewest regulatory controls. Many Class I devices are exempt from FDA premarket review requirements. Class III devices are generally the highest risk devices and are subject to the highest level of regulatory control to provide reasonable assurance of the device's safety and effectiveness. Class III devices must typically be approved by the FDA before they are marketed. For Class II devices, the FDA generally requires clearance through the premarket notification, or 510(k) clearance, process.
Generally, establishments that manufacture or distribute devices, including manufacturers, repackagers and relabelers, specification developers, and initial importers, are required to register their establishments with the FDA and provide the FDA a list of the devices that they handle at their facilities.
After a device is placed on the market, numerous regulatory requirements apply. These include: all of the relevant elements of the Quality System Regulation, or QSR, labeling regulations, restrictions on promotion and advertising, the Medical Device Reporting regulation (which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury if it were to recur), and the Reports of Corrections and Removals regulation (which requires manufacturers to report certain recalls and field actions to the FDA).
The FDA has issued a regulation outlining specific requirements for "specimen transport and storage containers." "Specimen transport and storage containers" are medical devices "intended to contain biological specimens, body waste, or body exudate during storage and transport" so that the specimen can be used effectively for diagnostic examination. A specimen transport and storage container is a Class I device so long as no sterility claims are made. It is subject to MDR requirements, the reporting of corrections and removals, registration and listing. It is exempt from premarket review, and from QSR requirements except for recordkeeping and complaint handling requirements. Our facility is registered with the FDA and the container we provide for collection and transport of FNA samples from a physician to our clinical reference laboratory is listed with the FDA as a Class I medical device and is subject to regulation by the FDA.
The FDA enforces the requirements described above by various means, including inspection and market surveillance. If the FDA finds a violation, it can institute a wide variety of enforcement actions, ranging from an Untitled Letter or Warning Letter to more severe sanctions such as:
- •
- fines, injunctions, and civil penalties;
- •
- recall or seizure of products;
- •
- operating restrictions, partial suspension or total shutdown of production; and
- •
- criminal prosecution.
Federal Oversight of Laboratory Developed Tests and Research Use Only Products
Clinical laboratory tests like Afirma are regulated under CLIA, as administered by the Centers for Medicare & Medicaid Services, or CMS, as well as by applicable state laws. Clinical laboratory tests that are developed and validated by a laboratory for its own use, which are referred to as laboratory developed tests, or LDTs, currently are generally not subject to FDA regulation, although reagents, instruments, software or components provided by third parties and used to perform LDTs may be subject to regulation. We believe that the Afirma GEC is an LDT. As a result, we believe our diagnostic services should not be subject to regulation under established FDA policies. Beginning in 1992, the FDA began expressing its view that all LDTs were subject to FDA regulation as devices; however, it stated that it would generally
80
exercise enforcement discretion and not apply the regulatory requirements for medical devices to LDTs. In June 2010, the FDA announced that it was revisiting its policy of exercising enforcement discretion with respect to LDTs. The FDA held a public meeting in July 2010, and FDA officials subsequently indicated that the FDA is interested in developing a risk-based application of oversight for LDTs and that it plans to issue draft guidance on the regulation of LDTs that would more stringently regulate LDTs that met criteria that would be established by the FDA. On June 5, 2013, FDA Commissioner Margaret A. Hamburg reiterated calls made by other Agency officials for increased FDA oversight of LDTs. Two days later, a laboratory association petitioned the FDA to refrain from issuing any such LDT guidance. Meanwhile, the Food and Drug Administration Safety and Innovation Act requires the FDA to notify Congress at least 60 days prior to issuing a draft or final guidance on the regulation of LDTs. The notice must include anticipated details of the action. Draft guidance has not yet been issued with respect to this proposed oversight of LDTs.
Some products are for research use only, or RUO. An RUO product is not intended for human clinical use and must be labeled "For Research Use Only. Not for use in diagnostic procedures." RUOs are a separate regulatory category and are not considered medical devices. They are therefore not subject to the FDA regulatory requirements discussed above. They cannot make any claims related to safety, effectiveness, or diagnostic utility or be intended for human clinical diagnostic or prognostic use. In June 2011, the FDA issued draft guidance regarding "Commercially Distributed In Vitro Diagnostic Products Labeled for Research Use Only or Investigational Use Only." Aspects of this draft guidance, which has not been finalized, are controversial.
We cannot predict the ultimate form of any such RUO or LDT guidance and the potential effect on our solutions or materials used to perform our diagnostic services. While we qualify all materials used in our diagnostic services according to CLIA regulations, we cannot be certain that the FDA might not promulgate rules or issue guidance documents that could affect our ability to purchase materials necessary for the performance of our diagnostic services. Should any of the reagents obtained by us from vendors and used in conducting our diagnostic services be affected by future regulatory actions, our business could be adversely affected by those actions, including increasing the cost of service or delaying, limiting or prohibiting the purchase of reagents necessary to perform the service.
We cannot provide any assurance that FDA regulation, including premarket review, will not be required in the future for our diagnostic services, whether through additional guidance or regulations issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. Legislative proposals addressing oversight of LDTs were introduced in recent years and we expect that new legislative proposals will be introduced from time to time. It is possible that legislation could be enacted into law or guidance could be issued by the FDA which may result in new or increased regulatory requirements for us to continue to offer our diagnostic services or to develop and introduce new services.
If premarket review is required, our business could be negatively affected until such review is completed and clearance to market or approval is obtained, and the FDA could require that we stop selling our diagnostic services pending premarket clearance or approval. If our diagnostic services are allowed to remain on the market but there is uncertainty about our services, if they are labeled investigational by the FDA, or if labeling claims the FDA allows us to make are limited, order levels may decline and reimbursement may be adversely affected. The regulatory approval process may involve, among other things, successfully completing additional clinical studies and submitting a premarket notification or filing a PMA application with the FDA. If premarket review is required by the FDA, there can be no assurance that our diagnostic services will be cleared or approved on a timely basis, if at all, nor can there any be assurance that labeling claims will be consistent with our current claims or adequate to support continued adoption of and reimbursement for our solution. Ongoing compliance with FDA regulations would increase the cost of conducting our business, and subject us to heightened requirements of the FDA and penalties for failure to comply with these requirements. We may also decide voluntarily to pursue FDA premarket review of our diagnostic services if we determine that doing so would be appropriate.
81
Health Insurance Portability and Accountability Act
Under the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, the Department of Health and Human Services, or HHS, has issued regulations to protect the privacy and security of protected health information used or disclosed by health care providers, such as us. HIPAA also regulates standardization of data content, codes and formats used in health care transactions and standardization of identifiers for health plans and providers. Penalties for violations of HIPAA regulations include civil and criminal penalties.
We have developed and implemented policies and procedures designed to comply with these regulations. The requirements under these regulations may change periodically and could have an effect on our business operations if compliance becomes substantially more costly than under current requirements.
In addition to federal privacy regulations, there are a number of state laws governing confidentiality of health information that are applicable to our business.
New laws governing privacy may be adopted in the future as well. We have taken steps to comply with health information privacy requirements to which we are aware that we are subject. However, we can provide no assurance that we are or will remain in compliance with diverse privacy requirements in all of the jurisdictions in which we do business. Failure to comply with privacy requirements could result in civil or criminal penalties, which could have a materially adverse effect on our business.
Federal and State Physician Self-referral Prohibitions
We are subject to the federal physician self-referral prohibitions, commonly known as the Stark Law, and to similar restrictions under California's Physician Ownership and Referral Act, or PORA. Together these restrictions generally prohibit us from billing a patient or any governmental or private payer for any diagnostic services when the physician ordering the service, or any member of such physician's immediate family, has an investment interest in or compensation arrangement with us, unless the arrangement meets an exception to the prohibition.
Both the Stark Law and PORA contain an exception for compensation paid to a physician for personal services rendered by the physician. We have compensation arrangements with a number of physicians for personal services, such as speaking engagements and consulting activities. We have structured these arrangements with terms intended to comply with the requirements of the personal services exception to Stark and PORA.
However, we cannot be certain that regulators would find these arrangements to be in compliance with Stark, PORA or similar state laws. We would be required to refund any payments we receive pursuant to a referral prohibited by these laws to the patient, the payer or the Medicare program, as applicable.
Sanctions for a violation of the Stark Law include the following:
- •
- denial of payment for the services provided in violation of the prohibition;
- •
- refunds of amounts collected by an entity in violation of the Stark Law;
- •
- a civil penalty of up to $15,000 for each service arising out of the prohibited referral;
- •
- possible exclusion from federal healthcare programs, including Medicare and Medicaid; and
- •
- a civil penalty of up to $100,000 against parties that enter into a scheme to circumvent the Stark Law's prohibition.
These prohibitions apply regardless of the reasons for the financial relationship and the referral. No finding of intent to violate the Stark Law is required for a violation. In addition, knowing violations of the Stark Law may also serve as the basis for liability under the Federal False Claims Act.
82
Further, a violation of PORA is a misdemeanor and could result in civil penalties and criminal fines. Finally, other states have self-referral restrictions with which we have to comply that differ from those imposed by federal and California law. While we have attempted to comply with the Stark Law, PORA and similar laws of other states, it is possible that some of our financial arrangements with physicians could be subject to regulatory scrutiny at some point in the future, and we cannot provide assurance that we will be found to be in compliance with these laws following any such regulatory review.
Federal and State Anti-kickback Laws
The Federal health care program Anti-kickback Law makes it a felony for a person or entity , including a laboratory, to knowingly and willfully offer, pay, solicit or receive remuneration, directly or indirectly, in order to induce business that is reimbursable under any federal health care program. A violation of the Anti-kickback Law may result in imprisonment for up to five years and fines of up to $250,000 in the case of individuals and $500,000 in the case of organizations. Convictions under the Anti-kickback Law result in mandatory exclusion from federal health care programs for a minimum of five years. In addition, HHS has the authority to impose civil assessments and fines and to exclude health care providers and others engaged in prohibited activities from Medicare, Medicaid and other federal health care programs. Actions which violate the Anti-kickback Law also incur liability under the Federal False Claims Act, which prohibits knowingly presenting, or causing to be presented, a false or fraudulent claim for payment to the U.S. Government.
Although the Anti-kickback Law applies only to federal health care programs, a number of states, including California, have passed statutes substantially similar to the Anti-kickback Law pursuant to which similar types of prohibitions are made applicable to all other health plans and third-party payers. Both California's fee-splitting statute, Business and Professions Code Section 650, and its Medi-Cal anti-kickback statute, Welfare and Institutions Code Section 14107.2, have been interpreted by the California Attorney General and California courts in substantially the same way as HHS and the courts have interpreted the Anti-kickback Law. A violation of Section 650 is punishable by imprisonment and fines of up to $50,000. A violation of Section 14107.2 is punishable by imprisonment and fines of up to $10,000.
Federal and state law enforcement authorities scrutinize arrangements between health care providers and potential referral sources to ensure that the arrangements are not designed as a mechanism to induce patient care referrals or induce the purchase or prescribing of particular products or services. The law enforcement authorities, the courts and Congress have also demonstrated a willingness to look behind the formalities of a transaction to determine the underlying purpose of payments between health care providers and actual or potential referral sources. Generally, courts have taken a broad interpretation of the scope of the Anti-kickback Law, holding that the statute may be violated if merely one purpose of a payment arrangement is to induce referrals or purchases.
In addition to statutory exceptions to the Anti-kickback Law, regulations provide for a number of safe harbors. If an arrangement meets the provisions of a safe harbor, it is deemed not to violate the Anti-kickback Law. An arrangement must fully comply with each element of an applicable safe harbor in order to qualify for protection. There are no regulatory safe harbors to California's Section 650.
Among the safe harbors that may be relevant to us is the discount safe harbor. The discount safe harbor potentially applies to discounts provided by providers and suppliers, including laboratories, to physicians or institutions. If the terms of the discount safe harbor are met, the discounts will not be considered prohibited remuneration under the Anti-kickback Law. California does not have a discount safe harbor. However, as noted above, Section 650 has generally been interpreted consistent with the Anti-kickback Law.
The personal services safe harbor to the Anti-kickback Law provides that remuneration paid to a referral source for personal services will not violate the Anti-kickback Law provided all of the elements of that safe harbor are met. One element is that if the agreement is intended to provide for the services of the
83
physician on a periodic, sporadic or part-time basis, rather than on a full-time basis for the term of the agreement, the agreement specifies exactly the schedule of such intervals, their precise length, and the exact charge for such intervals. Our personal services arrangements with some physicians may not meet the specific requirement of this safe harbor that the agreement specify exactly the schedule of the intervals of time to be spent on the services because the nature of the services, such as speaking engagements, does not lend itself to exact scheduling and therefore meeting this element of the personal services safe harbor is impractical. Failure to meet the terms of the safe harbor does not render an arrangement illegal. Rather, the government may evaluate such arrangements on a case-by-case basis, taking into account all facts and circumstances.
While we believe that we are in compliance with the Anti-kickback Law and Section 650, there can be no assurance that our relationships with physicians, academic institutions and other customers will not be subject to investigation or challenge under such laws. If imposed for any reason, sanctions under the Anti-kickback Law and Section 650 could have a negative effect on our business.
Other Federal and State Fraud and Abuse Laws
In addition to the requirements discussed above, several other health care fraud and abuse laws could have an effect on our business. For example, provisions of the Social Security Act permit Medicare and Medicaid to exclude an entity that charges the federal health care programs substantially in excess of its usual charges for its services. The terms "usual charge" and "substantially in excess" are ambiguous and subject to varying interpretations.
Further, the Federal False Claims Act prohibits a person from knowingly submitting a claim, making a false record or statement in order to secure payment or retaining an overpayment by the federal government. In addition to actions initiated by the government itself, the statute authorizes actions to be brought on behalf of the federal government by a private party having knowledge of the alleged fraud. Because the complaint is initially filed under seal, the action may be pending for some time before the defendant is even aware of the action. If the government is ultimately successful in obtaining redress in the matter or if the plaintiff succeeds in obtaining redress without the government's involvement, then the plaintiff will receive a percentage of the recovery. Finally, the Social Security Act includes its own provisions that prohibit the filing of false claims or submitting false statements in order to obtain payment. Violation of these provisions may result in fines, imprisonment or both, and possible exclusion from Medicare or Medicaid programs. California has an analogous state false claims act applicable to all payers, as do many other states.
California Laboratory Licensing
In addition to federal certification requirements of laboratories under CLIA, licensure is required and maintained for our South San Francisco clinical reference laboratory under California law. Such laws establish standards for the day-to-day operation of a clinical reference laboratory, including the training and skills required of personnel and quality control. In addition, California laws mandate proficiency testing, which involves testing of specimens that have been specifically prepared for the laboratory.
If our clinical reference laboratory is out of compliance with California standards, the California Department of Health Services, or DHS, may suspend, restrict or revoke our license to operate our clinical reference laboratory, assess substantial civil money penalties, or impose specific corrective action plans. Any such actions could materially affect our business. We maintain a current license in good standing with DHS. However, we cannot provide assurance that DHS will at all times in the future find us to be in compliance with all such laws.
84
New York Laboratory Licensing
Because we receive specimens from New York State, our clinical reference laboratories are required to be licensed by New York, under New York laws and regulations, which establish standards for:
- •
- day-to-day operation of a clinical laboratory, including training and skill levels required of laboratory personnel;
- •
- physical requirements of a facility;
- •
- equipment; and
- •
- validation and quality control.
New York law also mandates proficiency testing for laboratories licensed under New York state law, regardless of whether or not such laboratories are located in New York. If a laboratory is out of compliance with New York statutory or regulatory standards, the New York State Department of Health, or DOH, may suspend, limit, revoke or annul the laboratory's New York license, censure the holder of the license or assess civil money penalties. Statutory or regulatory noncompliance may result in a laboratory's operator being found guilty of a misdemeanor under New York law. DOH also must approve the LDT before the test is offered in New York. Should we be found out of compliance with New York laboratory requirements, we could be subject to such sanctions, which could harm our business. We maintain a current license in good standing with DOH for our South San Francisco laboratory. We have applied to the DOH for a license for our Austin laboratory. We cannot provide assurance that our Austin laboratory will obtain a license from the State of New York or that the DOH will at all times find us to be in compliance with applicable laws.
Other States' Laboratory Licensing
In addition to New York and California, other states including Florida, Maryland, Pennsylvania and Rhode Island, require licensing of out-of-state laboratories under certain circumstances. We have obtained licenses from states where we believe we are required to be licensed, and believe we are in compliance with applicable licensing laws.
From time to time, we may become aware of other states that require out-of-state laboratories to obtain licensure in order to accept specimens from the state, and it is possible that other states do have such requirements or will have such requirements in the future. If we identify any other state with such requirements or if we are contacted by any other state advising us of such requirements, we intend to comply with such requirements.
Corporate Practice of Medicine
Numerous states, including California and Texas, have enacted laws prohibiting corporations such as us from practicing medicine and employing or engaging physicians to practice medicine. These laws are designed to prevent interference in the medical decision-making process by anyone who is not a licensed physician. This prohibition is generally referred to as the prohibition against the corporate practice of medicine. Violation of this prohibition may result in civil or criminal fines, as well as sanctions imposed against us or the professional through licensing proceedings. The pathologists who review and classify thyroid FNA cytopathology results for Afirma are employed by Thyroid Cytology Partners, a Texas professional association, pursuant to services agreement between us and TCP. Pursuant to the agreement, we pay TCP a monthly fee on a per FNA basis, and TCP manages and supervises the pathologists who perform the cytopathology services as a component of Afirma. TCP is managed by Pathology Resources Consultants, or PRC, which provides management and other services to medical practitioners. We have entered into a services agreement with PRC in connection with our arrangement with TCP, pursuant to which we engaged PRC exclusively to manage the pathology services being provided by TCP. Our
85
agreement with PRC is effective until December 2015 and automatically renews on an annual basis unless either party provides notice of intent not to renew.
Employees
As of June 30, 2013, we had 98 employees, of which 24 work in laboratory operations, 19 in research and development and clinical development, 15 in selling and marketing, 40 in general and administrative including 16 in billing and client services, seven in information technology, and two in quality and regulatory affairs. None of our employees are the subject of collective bargaining arrangements, and our management considers its relationships with employees to be good.
Facilities
We lease 24,000 square feet of office and laboratory space at our headquarters in South San Francisco, California, under a lease that expires in 2016, with an option for us to extend the lease for an additional three years. We also lease approximately 10,400 square feet of office and laboratory space in Austin, Texas, under a lease that expires in 2018, with an option for us to extend the lease for an additional five years. We believe that our existing facilities are adequate to meet our business requirements for the near-term and that additional space will be available on commercially reasonable terms, if required.
Environmental Matters
Our operations require the use of hazardous materials (including biological materials) which subject us to a variety of federal, state and local environmental and safety laws and regulations. Some of these regulations provide for strict liability, holding a party potentially liable without regard to fault or negligence. We could be held liable for damages and fines as a result of our, or others', business operations should contamination of the environment or individual exposure to hazardous substances occur. We cannot predict how changes in laws or new regulations will affect our business, operations or the cost of compliance.
Legal Proceedings
From time to time, we may be party to lawsuits in the ordinary course of business. We are currently not a party to any legal proceedings.
86
Executive Officers and Directors
Our executive officers, directors and key employees, and their ages and positions as of August 15, 2013, are as set forth below:
Name
|
Age | Position | |||
|---|---|---|---|---|---|
Executive Officers and Key Employees |
|||||
Bonnie H. Anderson |
55 | President, Chief Executive Officer and Director | |||
Shelly D. Guyer |
53 | Chief Financial Officer and Secretary | |||
Christopher M. Hall |
45 | Chief Commercial Officer | |||
Giulia C. Kennedy, Ph.D. |
54 | Chief Scientific Officer | |||
Richard B. Lanman, M.D. |
58 | Chief Medical Officer | |||
Directors |
|||||
Brian G. Atwood(1)(3) |
60 | Chairman of Board and Director | |||
Brook H. Byers(2)(3) |
68 | Director | |||
Fred E. Cohen, M.D., D.Phil.(1) |
56 | Director | |||
Samuel D. Colella(2) |
73 | Director | |||
Karin Eastham(1) |
63 | Director | |||
Evan Jones(2) |
56 | Director | |||
Jesse I. Treu, Ph.D(3) |
66 | Director | |||
- (1)
- Member
of the Audit Committee
- (2)
- Member
of the Compensation Committee
- (3)
- Member of the Nominating and Corporate Governance Committee
Bonnie H. Anderson has served as our Chief Executive Officer and as a member of our board of directors since February 2008. In August 2013, she was appointed as our President. Prior to joining us, Ms. Anderson was an independent strategic consultant from April 2006 to January 2008, including as a strategic consultant for us from July 2007 to January 2008. Ms. Anderson was a Vice President at Beckmam Coulter, Inc., a manufacturer of biomedical testing instrument systems, tests and supplies, from September 2000 to March 2006. She currently serves as a member of the Board of Trustees of the Keck Graduate Institute of Applied Life Sciences. Ms. Anderson holds a B.S. in Medical Technology from Indiana University of Pennsylvania. Our board of directors has concluded that Ms. Anderson should serve on our board of directors due to her extensive industry experience, strategic perspective of our development, historic knowledge of our company and key leadership position as our President and Chief Executive Officer.
Shelly D. Guyer has served as our Chief Financial Officer and Secretary since April 2013. Prior to joining us, Ms. Guyer served as Chief Financial Officer and Executive Vice President of Finance and Administration of iRhythm Technologies, Inc., a medical device and service company, from April 2008 to December 2012. From March 2006 to August 2007, Ms. Guyer served as Vice President of Business Development and Investor Relations of Nuvelo Inc., a biopharmaceutical company. Prior to joining Nuvelo, Ms. Guyer worked at J.P. Morgan Securities and its predecessor companies for over 17 years, serving in a variety of roles including in healthcare investment banking. Ms. Guyer holds a A.B. in Politics from Princeton University and an M.B.A. from the Haas School of Business at the University of California, Berkeley.
Christopher M. Hall has served as our Chief Commercial Officer since March 2010. Prior to joining us, Mr. Hall served as Chief Business Officer of Celera Corporation, a diagnostics company focusing on personalized disease management, from October 2008 to February 2010. From August 2002 to February
87
2010, Mr. Hall served in various executive and senior positions at Berkeley HeartLab, Inc., a cardiovascular disease management company that was acquired by Celera in October 2007, including Chief Clinical Operations Officer and Vice President of Marketing. Mr. Hall holds a B.A. in Economics and Political Science from DePauw University and an M.B.A. from Harvard University.
Giulia C. Kennedy, Ph.D., has served as our Chief Scientific Officer since September 2008 and served as our Senior Vice President of Research and Development from April 2008 to September 2008. Prior to joining us, Dr. Kennedy was a Senior Director at Affymetrix, Inc., a microarray technology company, where she served from January 2000 to March 2008. Prior to joining Affymetrix, Dr. Kennedy served in scientific roles at Chiron Corporation and Millennium Pharmaceuticals, Inc., both of which were biotechnology companies. Dr. Kennedy holds a B.S. in Applied Science from Youngstown State University and a Ph.D. in Biochemistry from Case Western Reserve University School of Medicine and completed postdoctoral training in the Biochemistry Department and Hormone Research Institute at the University of California, San Francisco.
Richard B. Lanman, M.D., has served as our Chief Medical Officer since July 2008. Prior to joining us, Dr. Lanman served as Executive Vice President and Chief Medical Officer of diaDexus Inc., a medical diagnostics company, from April 2005 to July 2008. From November 2000 until March 2005, Dr. Lanman served as Chief Medical Officer and Executive Vice President, Business Development, of Atherotech, Inc., a laboratory test and medical device company. Prior to Atherotech, Dr. Lanman was Founder and Chief Executive Officer of Adesso Healthcare Technology Services, Inc., an application service provider profiling quality and utilization for specialist physician networks. Earlier in his career, he was in physician practice management roles as Senior Vice President and Medical Director for San Jose Medical Group, and as a Chief of Quality at The Permanente Medical Group. Dr. Lanman holds a B.S. in Chemistry from Stanford University and an M.D. from Northwestern University, Feinberg School of Medicine, and completed internship and residency at the University of California, San Francisco.
Brian G. Atwood has served as Chairman of our board of directors since February 2008 and as a director since December 2006. Since 1999, Mr. Atwood has served as a Managing Director of Versant Ventures, a healthcare-focused venture capital firm that he co-founded. Prior to founding Versant Ventures, Mr. Atwood served as a general partner of Brentwood Associates, a venture capital firm. He was also founder, President and Chief Executive Officer of Glycomed, Inc., a biopharmaceutical company. Mr. Atwood is currently a director of Cadence Pharmaceuticals, Inc., Clovis Oncology, Inc. and Trius Therapeutics, Inc. and a number of privately held companies. Mr. Atwood served as a director of Helicos BioSciences Corporation from 2003 until September 2011 and Pharmion Corporation from January 2000 until its acquisition in March 2008. Mr. Atwood holds a B.S. in Biological Sciences from the University of California, Irvine, an M.S. in Ecology from the University of California, Davis and an M.B.A. from Harvard University. Our board of directors has concluded that Mr. Atwood should serve on our board of directors due to his experience in the venture capital industry, his experience as a director of numerous publicly traded and privately held companies, as well as his experience founding and serving as President and Chief Executive Officer of a publicly traded biopharmaceutical company.
Brook H. Byers has served as a member of our board of directors since January 2007. Mr. Byers is a Managing Partner of Kleiner Perkins Caufield & Byers, a venture capital firm which he joined in 1977. Mr. Byers currently serves as a director of Pacific Biosciences of California, Inc. and a number of privately held companies and served as a director of Genomic Health, Inc. from January 2001 to June 2011. Mr. Byers holds a B.S. in Electrical Engineering from the Georgia Institute of Technology and an M.B.A. from the Stanford Graduate School of Business. Our board of directors has concluded that Mr. Byers should serve on our board of directors due to his expertise and background as a founder and chairman of numerous publicly traded and privately held life sciences companies, his service as a director of numerous companies in the life sciences and molecular diagnostics industry, and his leadership in personalized medicine initiatives.
88
Fred E. Cohen, M.D., D.Phil., has served as a member our board of directors since January 2007. Dr. Cohen is a partner at TPG, a private equity firm he joined in 2001, and serves as co-head of TPG's biotechnology group. Dr. Cohen is also an Adjunct Professor of Cellular and Molecular Pharmacology at the University of California, San Francisco, where he has taught since 1988. Dr. Cohen currently serves as a director of Aptalis Holdings Inc., a privately held company, BioCryst Pharmaceuticals, Inc., Genomic Health, Inc., and Quintiles Transnational Holdings Inc., and a number of other privately held companies. Dr. Cohen holds a B.S. in Molecular Biophysics and Biochemistry from Yale University, a D.Phil. in Molecular Biophysics from Oxford University and an M.D. from Stanford University. Our board of directors has concluded that Dr. Cohen should serve on our board of directors due to his significant leadership experience in the medical and finance fields through his background as an M.D. and a venture capitalist, his extensive technical expertise relevant to our business, and his experience as an investor in and on the boards of numerous life sciences and healthcare companies.
Samuel D. Colella has served as a member our board of directors since December 2006. Since 1999, Mr. Colella has served as a Managing Director of Versant Ventures, a healthcare-focused venture capital firm venture capital firm that he co-founded. Mr. Colella is also a general partner of Institutional Venture Partners, a venture capital firm he joined in 1984. Mr. Colella currently serves as the Chairman of the Board of Fluidigm Corporation and as a director of Genomic Health, Inc. and a number of privately held companies. Mr. Colella served as a director of Alexza Pharmaceuticals, Inc. from September 2002 to June 2012 and Jazz Pharmaceuticals, Inc. from April 2003 to January 2012. Mr. Colella holds a B.S. in Business and Engineering from the University of Pittsburgh and an M.B.A. from the Stanford Graduate School of Business. Our board of directors has concluded that Mr. Colella should serve on our board of directors due to his significant leadership in the life sciences industry, having founded, invested in and served on the boards of numerous publicly and privately held life sciences and healthcare companies. He also brings extensive senior management experience in a broad array of diverse businesses.
Karin Eastham has served as a member our board of directors since December 2012. Ms. Eastham serves on the boards of directors of several life sciences companies. From May 2004 to September 2008, Ms. Eastham served as Executive Vice President and Chief Operating Officer, and as a member of the Board of Trustees, of the Burnham Institute for Medical Research, a non-profit corporation engaged in biomedical research. From April 1999 to May 2004, Ms. Eastham served as Senior Vice President, Chief Financial Officer and Secretary of Diversa Corporation, a biotechnology company. She previously held similar positions with CombiChem, Inc., a computational chemistry company, and Cytel Corporation, a biopharmaceutical company. Ms. Eastham also held several positions, including Vice President, Finance, at Boehringer Mannheim Corporation, a diagnostics company, from 1976 to 1988. Ms. Eastham currently serves as a director of Geron Corporation, Illumina, Inc., MorphoSys AG, and Trius Therapeutics, Inc. Ms. Eastham served as a director of Amylin Pharmaceuticals, Inc. from September 2005 until its acquisition in August 2012, Genoptix, Inc. from August 2008 until its acquisition in March 2011, and Tercica, Inc. from December 2003 until its acquisition in October 2008. Ms. Eastham received a B.S. in Accounting and an M.B.A. from Indiana University and is a Certified Public Accountant. Our board of directors has concluded that Ms. Eastham should serve on our board of directors due to her experience as a director of numerous life sciences companies, as well as her extensive senior management experience in the biopharmaceutical industry, particularly in key corporate finance and accounting positions.
Evan Jones has served as a member of our board of directors since February 2008. Mr. Jones has served since 2007 as Managing Member of jVen Capital, LLC, a life sciences investment company. He also serves as executive chairman of Opgen, Inc., a privately held genetic analysis company. He was a co-founder of Digene Corporation, a publicly-traded biotechnology company focused on women's health and molecular diagnostic testing, serving as Chairman of the Board from 1995 until its acquisition in 2007 and serving as Chief Executive Officer from 1990 to 2006 and as President from 1990 to 1999. Mr. Jones is a director of CAS Medical Systems, Inc. and Fluidigm Corporation. Mr. Jones received a B.A. in Biotechnology from the University of Colorado and an M.B.A. from The Wharton School at the University
89
of Pennsylvania. Our board of directors has concluded that Mr. Jones' knowledge of the life sciences industry and his experience as a chief executive officer and as a board member of other publicly traded and privately held life sciences companies qualifies him to serve on our board of directors.
Jesse I. Treu, Ph.D., has served as a member our board of directors since June 2010. Dr. Treu has been a partner at Domain Associates, a venture capital firm, since its inception in 1985. Dr. Treu currently serves as a director of Regado Biosciences, Inc., a biopharmaceutical company, Tandem Diabetes Care, Inc., a privately held company, and a number of other privately held life sciences and biopharmaceutical companies. He served as a director of SenoRx, Inc. from October 1999 until June 2008 and Somaxon Pharmaceuticals, Inc. from December 2003 to June 2010. Prior to the formation of Domain Associates, Dr. Treu was vice president of the predecessor organization to The Wilkerson Group, and its venture capital arm, CW Ventures. Previous to that, Dr. Treu held a number of management and corporate staff positions in the medical industry, including positions at General Electric Company and Technicon Instruments. Dr. Treu holds a B.S. in Physics from Rensselaer Polytechnic Institute and an M.A. and a Ph.D. in Physics from Princeton University. Our board of directors has concluded that Dr. Treu should serve on our board of directors due to his extensive management and board experience in the healthcare industry.
Board Composition
Our board of directors currently consists of eight members. The restated certificate of incorporation that will become effective upon completion of this offering provides that the authorized number of directors on our board will consist of not fewer than and not more than directors, as the board of directors may from time to time determine. Our board of directors will initially consist of eight directors. The authorized number of directors may be changed by resolution of our board of directors. Vacancies on our board of directors can be filled by resolution of our board of directors. Upon the completion of this offering, our board of directors will be divided into three classes, each serving staggered, three-year terms:
- •
- Our Class I directors will
be and
and their terms will expire at the first annual
meeting of stockholders following the date of this prospectus;
- •
- Our Class II directors will
be and
and their terms will expire at the second annual
meeting of stockholders following the date of this prospectus; and
- •
- Our Class III directors will be and and their terms will expire at the third annual meeting of stockholders following the date of this prospectus.
As a result, only one class of directors will be elected at each annual meeting of stockholders, with the other classes continuing for the remainder of their respective terms.
Code of Business Conduct and Ethics
We have adopted a code of business conduct and ethics that applies to each of our directors, officers and employees, including our Chief Executive Officer, our Chief Financial Officer and other employees who perform financial or accounting functions. We have also adopted a code of ethics for senior financial officers applicable to our Chief Executive Officer, Chief Financial Officer and other key management employees. Upon completion of this offering, the code of business conduct and ethics and the code of ethics for senior financial officers will each be posted on our website.
Director Independence
Our board of directors determined that and are "independent directors" as defined under the rules of The NASDAQ Stock Market.
90
Role of the Board in Risk Oversight
Our board of directors is responsible for overseeing the overall risk management process at the company. The responsibility for managing risk rests with executive management while the committees of our board of directors and our board of directors as a whole participate in the oversight process. Our board of directors' risk oversight process builds upon management's risk assessment and mitigation processes, which include reviews of long-term strategic and operational planning, executive development and evaluation, regulatory and legal compliance, and financial reporting and internal controls.
Board Committees
We have established an audit committee, compensation committee and nominating and corporate governance committee. We believe that the composition of these committees meets the criteria for independence under, and the functioning of these committees complies with the applicable requirements of, the Sarbanes-Oxley Act, the current rules of The NASDAQ Stock Market and SEC rules and regulations. We intend to comply with future requirements as they become applicable to us. Each committee has the composition and responsibilities described below:
Audit committee. Mr. Atwood, Dr. Cohen and Ms. Eastham serve on our audit committee. Ms. Eastham is the chairperson of this committee. Our audit committee oversees our corporate accounting and financial reporting process and assists our board of directors in oversight of the integrity of our financial statements, our compliance with financial and regulatory requirements, our independent auditor's qualifications, independence and performance, and our internal accounting and financial controls. Our audit committee is responsible for the appointment, compensation, retention and oversight of our independent auditors. Our board of directors has determined that is an audit committee financial expert, as defined by the rules promulgated by the SEC, and has the requisite financial sophistication as defined under the applicable rules and regulations of The NASDAQ Stock Market.
Compensation committee. Messrs. Byers, Colella and Jones serve on our compensation committee. Mr. Jones is the chairperson of this committee. Our compensation committee oversees our compensation policies, plans and benefits programs and assists our board of directors in meeting its responsibilities with regard to oversight and determination of executive compensation. In addition, our compensation committee reviews and makes recommendations to our board of directors with respect to our major compensation plans, policies and programs and assesses whether our compensation structure establishes appropriate incentives for officers and employees.
Nominating and corporate governance committee. Messrs. Atwood and Byers and Dr. Treu serve on our nominating and corporate governance committee. Dr. Treu is the chairperson of this committee. Our nominating and corporate governance committee is responsible for making recommendations to our board of directors regarding candidates for directorships and the size and composition of the board of directors and its committees. In addition, our nominating and corporate governance committee is responsible for reviewing and making recommendations to our board of directors on matters concerning corporate governance and conflicts of interest.
Compensation Committee Interlocks and Insider Participation
In the past three years, none of the members of our compensation committee is or has in the past served as an officer or employee of our company. None of our executive officers currently serves, or in the past year has served, as a member of a board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
91
Director Compensation
Directors who are employees do not receive any additional compensation for their service on our board of directors. We reimburse our non-employee directors for their reasonable out-of-pocket costs and travel expenses in connection with their attendance at board of directors and committee meetings.
The following table sets forth the compensation accrued or paid by us to certain non-employee directors during the year ended December 31, 2012, for service on our board of directors. We did not pay or accrue any compensation for Messrs. Atwood, Byers and Colella or for Drs. Cohen and Treu during the year ended December 31, 2012.
Name
|
Fees Earned or Paid in Cash ($) |
Option Awards ($)(1)(2) | Total ($) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Karin Eastham |
$ | 1,712 | (3) | 16,621 | 18,333 | |||||
Evan Jones |
— | 13,297 | 13,297 | |||||||
- (1)
- Amounts
represent the aggregate fair value of the option awards computed as of the grant date of each option award in accordance with FASB ASC Topic 718,
rather than amounts paid to or realized by the named individual. Our assumptions with respect to the calculation of these values are set forth above in "Management's Discussion and Analysis of
Financial Condition and Results of Operations–Critical Accounting Policies–Stock-based Compensation". There can be no assurance that option awards will be exercised (in which
case no value will be realized by the individual) or that the value on exercise will approximate the fair value as computed in accordance with FASB ASC Topic 718.
- (2)
- The following table sets forth outstanding equity awards held by non-employee directors as of December 31, 2012:
Name(1)
|
Option Grant Date |
Number of Securities Underlying Unexercised Options Exercisable |
Number of Securities Underlying Unexercised Options Unexercisable |
Option Exercise Price(2)($/sh) |
Option Expiration Date |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Karin Eastham |
12/06/12 | (3) | — | 25,000 | $ | 1.00 | 12/05/22 | |||||||||
Evan Jones |
07/08/08 | (4) | 160,000 | — | $ | 0.02 | 07/07/18 | |||||||||
|
05/17/11 | (3) | 20,000 | — | $ | 0.59 | 05/16/21 | |||||||||
|
12/06/12 | (5) | 13,334 | 6,666 | $ | 1.00 | 12/05/22 | |||||||||
- (1)
- Messrs. Atwood,
Byers and Colella and Drs. Cohen and Treu did not hold any outstanding options as of December 31, 2012.
- (2)
- The
grant date fair value of the common stock underlying these option awards is equal to the option exercise price on the date of grant.
- (3)
- This
option vests ratably over 12 months from the grant date.
- (4)
- This
option vests as to 25% of the underlying shares on the one year anniversary of the grant date, and the remainder ratably over 36 months
thereafter.
- (5)
- This option vests ratably over 12 months from the vesting commencement date. The vesting commencement date is May 1, 2012.
- (3)
- We have agreed to pay Ms. Eastham an annual cash retainer of $20,000 for her service as director and $5,000 for her service as chairperson of our audit committee. The amount above reflects the pro rated portion of Ms. Eastham's cash retainer from the day she joined our board of directors through December 31, 2012.
92
Summary Compensation Table
The following table sets forth information concerning the total compensation of our Chief Executive Officer and two other highest paid executive officers, who we refer to as our named executive officers, earned for services rendered to us in all capacities during the year ended December 31, 2012:
Name and Principal Position
|
Fiscal Year | Salary ($) | Option Awards ($)(1)(2) |
Non-Equity Incentive Plan Compensation ($)(3) |
All Other Compensation ($) |
Total ($) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Bonnie H. Anderson |
2012 | 355,000 | 302,631 | (4) | 62,500 | — | 720,131 | ||||||||||||
Chief Executive Officer |
|||||||||||||||||||
Mark E. Spring(5) |
2012 |
296,250 |
288,417 |
(6) |
30,000 |
54,913 |
(7) |
669,580 |
|||||||||||
Former Chief Financial Officer |
|||||||||||||||||||
Christopher M. Hall |
2012 |
304,148 |
93,082 |
(8) |
30,500 |
— |
427,730 |
||||||||||||
Chief Commercial Officer |
|||||||||||||||||||
- (1)
- Amounts
represent the aggregate fair value of the option awards computed as of the grant date of each option award in accordance with FASB ASC Topic 718,
rather than amounts paid to or realized by the named individual. Our assumptions with respect to the calculation of these values are set forth above in "Management's Discussion and Analysis of
Financial Condition and Results of Operations–Critical Accounting Policies–Stock-based Compensation". There can be no assurance that option awards will be exercised (in which
case no value will be realized by the individual) or that the value on exercise will approximate the fair value as computed in accordance with FASB ASC Topic 718.
- (2)
- Includes
fully vested options granted in February 2013 for service in 2012 pursuant to our Executive Bonus Plan, under which our executive officers are
eligible to receive annual cash and equity incentives if the company achieves certain annual corporate goals as determined for such year by our board of directors. Such bonuses may be paid partly in
cash and partly in fully vested stock options or restricted stock at the discretion of our board of directors.
- (3)
- Reflects
the amount approved by our board of directors as cash incentive under our Executive Bonus Plan.
- (4)
- Includes
the grant of a fully vested option to purchase 96,451 shares of our common stock granted on February 5, 2013 pursuant to our Executive Bonus
Plan.
- (5)
- Mr. Spring's
employment with us ended in February 2013. Shelly D. Guyer was appointed our Chief Financial Officer in April 2013.
- (6)
- Includes
the grant of a fully vested option to purchase 46,296 shares of our common stock granted on February 5, 2013 pursuant to our Executive Bonus
Plan.
- (7)
- Consisted
of $36,765 for reimbursement of relocation expenses and a tax gross-up for such expenses of $18,148.
- (8)
- Includes the grant of a fully vested option to purchase 47,068 shares of our common stock granted on February 5, 2013 pursuant to our Executive Bonus Plan.
93
Outstanding Equity Awards at Fiscal Year End
The following table sets forth certain information with respect to outstanding equity awards held by each of our named executive officers as of December 31, 2012:
| |
Option Awards | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Name
|
Number of Securities Underlying Unexercised Options–Exercisable(1) |
Number of Securities Underlying Unexercised Options– Unexercisable(1) |
Exercise Price of Options |
Expiration Date of Options |
|||||||||
Bonnie H. Anderson |
52,613 | (2) | — | $ | 0.20 | 02/02/2020 | |||||||
|
400,000 | (3) | — | (5) | $ | 0.59 | 09/27/2020 | ||||||
|
72,500 | (4) | — | $ | 0.59 | 02/22/2021 | |||||||
|
131,131 | (4) | — | $ | 0.67 | 03/09/2022 | |||||||
|
575,000 | (3) | — | (6) | $ | 0.67 | 03/09/2022 | ||||||
Mark E. Spring |
610,000 |
(3) |
— |
(7) |
$ |
0.67 |
04/10/2022 |
||||||
Christopher M. Hall |
450,000 |
(3) |
— |
(8) |
$ |
0.20 |
03/29/2020 |
||||||
|
50,000 | (3) | — | (5) | $ | 0.59 | 09/27/2020 | ||||||
|
44,000 | (4) | — | $ | 0.59 | 02/22/2021 | |||||||
|
150,000 | (3) | — | (6) | $ | 0.67 | 03/09/2022 | ||||||
|
67,708 | (4) | — | $ | 0.67 | 03/09/2022 | |||||||
- (1)
- Each
option award listed in the table may be exercised in full prior to the vesting of the shares underlying the option. Vesting of each option is subject
to continued service on the applicable vesting date. All options listed in this table were granted pursuant to the Company's 2008 Stock Plan.
- (2)
- Option
award vests at a rate of 1/24th of the total number of shares subject to the option each month following the vesting commencement date. The
vesting commencement date is January 1, 2010.
- (3)
- All
option awards vest as to 25% of the total number of shares subject to the option one year after the vesting commencement date, and the remaining shares
vest at a rate of 1/48th of the total number of shares subject to the options each month thereafter. If an option holder is terminated without Cause or resigns for Good Reason (each as defined
in the applicable option agreement) within 12 months of a change in control, 100% of the shares subject to the option shall vest immediately prior to such termination or resignation.
- (4)
- Options
were fully vested on the date of grant.
- (5)
- The
vesting commencement date is September 28, 2010.
- (6)
- The
vesting commencement date is March 10, 2012.
- (7)
- The
vesting commencement date is January 5, 2012.
- (8)
- The vesting commencement date is March 15, 2010.
Employment Arrangements
Bonnie H. Anderson
On February 15, 2008, we entered into an employment agreement with Bonnie H. Anderson, our President and Chief Executive Officer. The agreement provided Ms. Anderson with an initial base salary at an annual rate of $300,000 and provided that Ms. Anderson was eligible to receive an annual bonus targeted at 20% of her base salary. Since entering into this agreement, our board of directors has adjusted Ms. Anderson's base salary to $350,000 and pursuant to an amendment to the employment agreement dated March 11, 2009, increased her target bonus percentage to 30% of her base salary. Ms. Anderson is entitled to participate in all employee benefit plans, including group health care plans and all fringe benefit plans. Ms. Anderson's employment agreement provides that she is an at-will employee and her employment may be terminated at any time by her or us.
94
On August 24, 2012, we entered into a Change of Control and Severance Agreement with Ms. Anderson, with an initial term of four years, which term automatically renews for additional one year periods unless either party provides written notice of non-renewal at least 60 days prior to the date of automatic renewal and which term extends for one year from a "change of control," as defined in the agreement, if such change of control occurs within the final twelve months of the initial term or the term as extended through automatic renewal. Pursuant to the agreement, if Ms. Anderson is terminated by us without "cause" (as defined in the agreement), or Ms. Anderson terminates her employment for "good reason" (as defined in the agreement), each during a period not within two months prior to and ending 12 months following a change of control, or the Change of Control Period, Ms. Anderson is entitled to (i) 12 months of salary continuation from the termination date, (ii) a lump sum payment equal to her pro-rated annual bonus for performance up to the end of the applicable performance period and (iii) accelerated vesting equal to 50% of any outstanding equity awards along with the extension of the post-termination exercise period of such awards to 24 months after the termination date.
Further, if Ms. Anderson is terminated by us without cause, or Ms. Anderson terminates her employment for good reason each during the Change of Control Period, Ms. Anderson is entitled to (i) a lump sum severance payment equal to 12 months of salary from the termination date, (ii) a lump sum payment equal to 100% of the higher of her (A) annual target bonus for the year in which the change of control occurs, (B) annual target bonus for the year in which the termination occurs, or (C) actual bonus for the year prior to the year in which the termination occurs and (iii) accelerated vesting equal to 100% of any outstanding equity awards along with the extension of the post-termination exercise period of such awards to 24 months after the termination date.
In either of the above situations, receipt of the above-described benefits are subject to Ms. Anderson executing a release of certain claims against us. Further, in either of the above situations Ms. Anderson will also be reimbursed (or receive payments in lieu of such reimbursements) if she elects and pays to continue health insurance under the Consolidated Omnibus Budget Reconciliation Act of 1985, or COBRA, for any premiums paid for continued health benefits for Ms. Anderson and her eligible dependents until the earlier of (i) 12 months after the termination date or (ii) the date upon which Ms. Anderson or her eligible dependents become covered under similar plans.
Pursuant to the employment agreement and Change of Control and Severance Agreement with Ms. Anderson, following her termination, she will maintain the confidentiality of our confidential information and will not solicit any of our employees for a 12 month period.
Shelly D. Guyer
On April 8, 2013, we entered into a Change of Control and Severance Agreement with Ms. Guyer, with an initial term of four years, which term automatically renews for additional one year periods unless either party provides written notice of non-renewal at least 60 days prior to the date of automatic renewal and which term extends for one year from a "change of control," as defined in the agreement, if such change of control occurs within the final twelve months of the initial term or the term as extended through automatic renewal. Pursuant to the agreement, if Ms. Guyer is terminated by us without "cause" (as defined in the agreement), or Ms. Guyer terminates her employment for "good reason" (as defined in the agreement), each during a period not within two months prior to and ending 12 months following a change of control, or the Change of Control Period, Ms. Guyer is entitled to six months of salary continuation from the termination date.
Further, if Ms. Guyer is terminated by us without cause, or Ms. Guyer terminates her employment for good reason, each during the Change of Control Period, Ms. Guyer is entitled to (i) a lump sum severance payment equal to six months of salary from the termination date, (ii) a lump sum payment equal to 50% the higher of her (A) annual target bonus for the year in which the change of control occurs, (B) annual target bonus for the year in which the termination occurs, or (C) actual bonus for the year prior to the year
95
in which the termination occurs and (iii) accelerated vesting equal to 100% of any outstanding equity awards along with the extension of the post-termination exercise period of such awards to 18 months after the termination date
In either of the above situations, receipt of the above-described benefits are subject to Ms. Guyer executing a release of certain claims against us. Further, in either of the above situations, Ms. Guyer will also be reimbursed (or receive payments in lieu of such reimbursements) if she elects and pays to continue health insurance under COBRA for any premiums paid for continued health benefits for Ms. Guyer and her eligible dependents until the earlier of (i) six months after the termination date or (ii) the date upon which Ms. Guyer or her eligible dependents become covered under similar plans.
Pursuant to the Change of Control and Severance Agreement with Ms. Guyer, following her termination, she will maintain the confidentiality of our confidential information.
Christopher M. Hall
On August 24, 2012, we entered into a Change of Control and Severance Agreement with Christopher M. Hall, with an initial term of four years, which term automatically renews for additional one year periods unless either party provides written notice of non-renewal at least 60 days prior to the date of automatic renewal and which term extends for one year from a "change of control," as defined in the agreement, if such change of control occurs within the final twelve months of the initial term or the term as extended through automatic renewal. Pursuant to the agreement, if Mr. Hall is terminated by us without "cause" (as defined in the agreement), or Mr. Hall terminates his employment for "good reason" (as defined in the agreement), each during a period not within two months prior to and ending 12 months following a change of control, or the Change of Control Period, Mr. Hall is entitled to six months of salary continuation from the termination date.
Further, if Mr. Hall is terminated by us without cause, or Mr. Hall terminates his employment for good reason, each during the Change of Control Period, Mr. Hall is entitled to (i) a lump sum severance payment equal to six months of salary from the termination date, (ii) a lump sum payment equal to 50% of the higher of his (A) annual target bonus for the year in which the change of control occurs, (B) annual target bonus for the year in which the termination occurs, or (C) actual bonus for the year prior to the year in which the termination occurs and (iii) accelerated vesting equal to 100% of any outstanding equity awards along with the extension of the post-termination exercise period of such awards to 18 months after the termination date.
In either of the above situations, receipt of the above-described benefits are subject to Mr. Hall executing a release of certain claims against us. Further, in either of the above situations, Mr. Hall will also be reimbursed (or receive payments in lieu of such reimbursements) if he elects and pays to continue health insurance under COBRA for any premiums paid for continued health benefits for Mr. Hall and his eligible dependents until the earlier of (i) six months after the termination date or (ii) the date upon which Mr. Hall or his eligible dependents become covered under similar plans.
Pursuant to the Change of Control and Severance Agreement with Mr. Hall, following his termination, he will maintain the confidentiality of our confidential information.
Employee Benefit Plans
2008 Stock Plan
Our 2008 Stock Plan was adopted by our board of directors in February 2008 and was subsequently approved by our stockholders. The purpose of the 2008 Stock Plan is to attract and retain the best personnel, provide incentives to our employees, directors and consultants and to promote the success of the Company's business.
96
Our 2008 Stock Plan provides for the grant of nonstatutory stock options and restricted stock awards to our employees, directors and consultants, and incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, which may be granted only to our employees.
Share reserve. As of June 30, 2013, 11,800,873 shares of common stock have been authorized for issuance under the 2008 Stock Plan. As of June 30, 2013 options to purchase a total of 9,681,245 shares of common stock were outstanding under the 2008 Stock Plan. If an option or an award to purchase restricted stock expires or is cancelled for any reason, the shares allocable to the unexercised portion of such option or award will become available for future award under the 2008 Stock Plan. If a share previously issued under the 2008 Stock Plan is reacquired pursuant to a forfeiture provision, then such a share will again become available for award under the 2008 Stock Plan.
Administration. Our board of directors administers the 2008 Stock Plan. The board of directors may delegate any of its administrative functions to a committee. Subject to the provisions of our 2008 Stock Plan, the administrator may take all actions it deems necessary or advisable for the administration of the 2008 Stock Plan. All actions of the administrator are final and binding on all persons.
Stock options. The administrator may grant incentive or nonstatutory stock options under our 2008 Stock Plan; provided that incentive stock options are only granted to employees. The exercise price of options granted under the plan must be equal to or greater than 100% of the fair market value of our common stock on the date of grant and the term of an option may not exceed ten years; provided, however, that an incentive stock option held by an optionee who owns more than 10% of the total combined voting power of all classes of our stock may not have a term in excess of five years and must have an exercise price of at least 110% of the fair market value of our common stock on the grant date. The exercise price for an option may be paid in cash or cash equivalents. In addition, the administrator may allow for payment by surrender of shares, promissory note, cashless exercise, pledge of shares or other forms of payment as may be permitted by our board of directors. Subject to the provisions of our 2008 Stock Plan, the administrator determines the remaining terms of the options (e.g., exercisability and vesting). The administrator may permit an optionee to exercise his or her option as to shares that have not vested, subject to the Company's right to repurchase any shares unvested as of the optionee's termination of service at the lower of the original exercise price or the then-current fair market value of the shares. After an optionee's termination of service, the optionee may exercise his or her option, to the extent vested as of the date of termination, for a period of three months (or twelve months in the case of termination due to death or disability) following such termination. However, in no event may an option be exercised later than the expiration of its term.
Restricted shares. The administrator may award restricted shares or grant stock purchase rights under our 2008 Stock Plan. The terms of the award of restricted shares will be set forth in a restricted share agreement between the purchaser and us. Any right to acquire shares, other than options, shall automatically expire if not exercised by the purchaser within thirty days after we communicate the grant of such right to the purchaser. Awards of restricted shares or shares received upon the exercise of a stock purchase right may be subject to forfeiture conditions, rights of repurchase, rights of first refusal and other restrictions as set forth in the applicable restricted share agreement. Once a stock purchase right is exercised, the purchaser will generally have all of the rights of a stockholder with respect to such shares, other than the right to transfer such shares before vesting.
Transferability. Our 2008 Stock Plan generally does not allow for options to be transferred in any manner other than by will or the laws of descent and distribution. Notwithstanding the foregoing, to the extent permitted by our board of directors, a nonstatutory stock option may be transferred to a family member or trust to the extent permitted by applicable laws.
Adjustments. If any change is made in our common stock subject to the 2008 Stock Plan including a subdivision, stock dividend, dividend payable in a form other than stock that has a material effect on our
97
shares, a combination or consolidation, a recapitalization, a spin-off or a similar occurrence, then equitable adjustments will be made to one or more of the following: the number of shares available under the 2008 Stock Plan, the number of shares covered by each outstanding option or the exercise price under each outstanding option and the price of shares subject to our right to repurchase.
Corporate transaction. If the Company is a party to a merger or other change of control event, outstanding awards under the 2008 Stock Plan will be treated as the administrator determines, including, without limitation, that each award be assumed or substituted by the successor; provided, that if the successor did does not assume or substitute the award, such award shall fully vest and be exercisable and the administrator shall notify the participant that the award shall be fully vested and exercisable for a period of time as determined by the administrator in its sole discretion.
Plan amendments and termination. Our board of directors may at any time amend, suspend or terminate the 2008 Stock Plan. Certain amendments which materially alter or impair the rights of existing option holders require an optionee's consent. Our 2008 Stock Plan will automatically terminate on , unless we terminate it sooner.
Upon the completion of this offering, the 2008 Stock Plan will be terminated and no shares of our common stock will remain available for future issuance under the 2008 Stock Plan.
2013 Stock Incentive Plan
General. Our 2013 Stock Incentive Plan was adopted by our board of directors and approved by our stockholders in , 2013.
The 2013 Stock Incentive Plan provides for the granting of incentive stock options within the meaning of Section 422 of the Internal Revenue Code to employees and the granting of nonstatutory stock options to employees, non-employee directors, advisors and consultants. The 2013 Stock Incentive Plan also provides for the grants of restricted stock, stock appreciation rights and stock unit awards to employees, non-employee directors, advisors and consultants.
Administration. The compensation committee of our board of directors will administer the 2013 Stock Incentive Plan, including the determination of the recipient of an award, the number of shares subject to each award, whether an option is to be classified as an incentive stock option or nonstatutory option, and the terms and conditions of each award, including the exercise and purchase prices and the vesting or duration of the award.
At the discretion of our board of directors, our compensation committee may consist solely of two or more "non-employee directors." To the extent required by our board of directors, the composition of our compensation committee may satisfy the requirements for plans intended to qualify for exemption under Rule 16b-3 of the Exchange Act and Section 162(m) of the Internal Revenue Code. Our board of directors may appoint one or more separate committees of our board of directors, each consisting of one or more members of our board of directors, to administer our 2013 Stock Incentive Plan with respect to employees who are not subject to Section 16 of the Exchange Act. Subject to applicable law, our board of directors may also authorize one or more officers to designate employees, other than employees who are subject to Section 16 of the Securities Exchange Act of 1934, as amended, or the Exchange Act, to receive awards under our 2013 Stock Incentive Plan or determine the number of such awards to be received by such employees subject to limits specified by our board of directors.
Authorized shares. Under our 2013 Stock Incentive Plan, shares of our common stock have been authorized for issuance. In addition, the number of shares that have been authorized for issuance under the 2013 Stock Incentive Plan will be automatically increased on the first day of each year beginning in fiscal 2014 and ending in 2023, in an amount equal to the least of (i) shares, (ii) % of the outstanding shares of our common stock on the last day of the immediately preceding year or (iii) another
98
amount determined by our board of directors. Shares subject to awards granted under the 2013 Stock Incentive Plan that are forfeited or terminated before being exercised or settled, or are not delivered to the participant because such award is settled in cash, will again become available for issuance under the 2013 Stock Incentive Plan. Shares withheld to satisfy the grant, exercise price or tax withholding obligation related to an award will again become available for issuance under the 2013 Stock Incentive Plan. However, shares that have actually been issued shall not again become available unless forfeited. No more than shares may be delivered upon the exercise of incentive stock options granted under the 2013 Stock Incentive Plan plus, to the extent allowable under applicable law, any shares that again become available for issuance under the 2013 Stock Incentive Plan. In addition, shares originally reserved for issuance under our 2008 Stock Plan but which are not issued or subject to outstanding grants on the effective date of the 2013 Stock Incentive Plan, and shares subject to outstanding options under our 2008 Stock Plan on the effective date of the 2013 Stock Incentive Plan that are subsequently forfeited or terminated for any reason before being exercised, and shares subject to vesting restrictions under the 2008 Stock Plan that are subsequently forfeited, up to a number of additional shares not to exceed an aggregate of shares, will again become available for awards under our 2013 Stock Incentive Plan on the date the 2013 Stock Incentive Plan becomes effective.
No participant in the 2013 Stock Incentive Plan can receive option grants, restricted shares, stock appreciation rights or stock units totaling more than an aggregate of shares in any calendar year, except in the participant's first year of employment in which the participant may receive equity awards totaling up to shares. No participant in the 2013 Stock Incentive Plan may be paid more than an aggregate of in cash during any calendar year with respect to equity awards that are payable in cash.
Types of Awards
- •
- Stock options. A stock option is the right to purchase a certain number of shares of stock, at a certain exercise price, in the future. Under our 2013 Stock Incentive Plan, incentive stock options and nonstatutory options must be granted with an exercise price of at least 100% of the fair market value of our common stock on the date of grant. Incentive stock options granted to any holder of more than 10% of the voting shares of our company must have an exercise price of at least 110% of the fair market value of our common stock on the date of grant. No incentive stock option can be granted to an employee if as a result of the grant, the employee would have the right in any calendar year to exercise for the first time one or more incentive stock options for shares having an aggregate fair market value in excess of $100,000. The stock option agreement specifies the date when all or any installment of the option is to become exercisable. We expect that 1/4th of the total number of shares subject to the options will vest and become exercisable 12 months after the vesting commencement date for options granted, and the remaining options will vest and become exercisable at a rate of 1/48th of the total number of shares subject to the options each month thereafter. Each stock option agreement sets forth the term of the options, which is prohibited from exceeding ten years (five years in the case of an incentive stock option granted to any holder of more than 10% of our voting shares), and the extent to which the optionee will have the right to exercise the option following termination of the optionee's service with the company. Payment of the exercise price may be made in cash or cash equivalents or, if provided for in the stock option agreement evidencing the award, (i) by surrendering, or attesting to the ownership of, shares which have already been owned by the optionee, (ii) by delivery of an irrevocable direction to a securities broker to sell shares and to deliver all or part of the sale proceeds to us in payment of the aggregate exercise price, (iii) by delivery of an irrevocable direction to a securities broker or lender to pledge shares and to deliver all or part of the loan proceeds to us in payment of the aggregate exercise price, (iv) by a "net exercise" arrangement, (v) by delivering a full-recourse promissory note or (vi) by any other form that is consistent with applicable laws, regulations and rules.
99
- •
- Restricted stock. Restricted stock is a share award that
may be subject to vesting conditioned upon continued service, the achievement of performance objectives or the satisfaction of any other condition as specified in a restricted stock agreement.
Participants who are granted restricted stock awards generally have all of the rights of a stockholder with respect to such stock, other than the right to transfer such stock prior to vesting. Subject
to the terms of the 2013 Stock Incentive Plan, our compensation committee will determine the terms and conditions of any restricted stock award, including any vesting arrangement, which will be set
forth in a restricted stock agreement to be entered into between us and each recipient. Restricted stock may be awarded for such consideration as our compensation committee may determine, including
without limitation cash, cash equivalents, full-recourse promissory notes, future services or services rendered prior to the award, without payment by the recipient.
- •
- Stock units. Stock units give recipients the right to
acquire a specified number of shares of stock at a future date upon the satisfaction of certain conditions, including any vesting arrangement, established by our compensation committee and as set
forth in a stock unit agreement. Unlike restricted stock, the stock underlying stock units will not be issued until the stock units have vested and are settled, and recipients of stock units generally
will have no voting or dividend rights prior to the time the vesting conditions are satisfied and the award is settled. Our compensation committee may elect to settle vested stock units in cash or in
common stock or in a combination of cash and common stock. Subject to the terms of the 2013 Stock Incentive Plan, our compensation committee will determine the terms and conditions of any stock unit
award, which will be set forth in a stock unit agreement to be entered into between us and each recipient.
- •
- Stock appreciation rights. Stock appreciation rights typically will provide for payments to the recipient based upon increases in the price of our common stock over the exercise price of the stock appreciation right. The exercise price of a stock appreciation right will be determined by our compensation committee, which shall not be less than the fair market value of our common stock on the date of grant. Our compensation committee may elect to pay stock appreciation rights in cash or in common stock or in a combination of cash and common stock.
Other Plan Features
Under the 2013 Stock Incentive Plan:
- •
- Unless the agreement evidencing an award expressly provides otherwise, no award granted under the plan may be transferred
in any manner (prior to the vesting and lapse of any and all restrictions applicable to shares issued under such award), other than by will or the laws of descent and distribution.
- •
- Nondiscretionary, automatic grants of nonstatutory stock options will be made to outside directors. Any outside director who first joins our board of directors on or after the effective date, will be automatically granted an initial nonstatutory option to purchase shares of our common stock that have a value of $ , calculated using the fair market value of our common stock on the date of grant, upon first becoming a member of our board of directors. The initial option will vest and become exercisable over four years in equal monthly installments. On the first business day after each of our regularly scheduled annual meetings of stockholders, each outside director will be automatically granted an option to purchase shares of our common stock that have a value of $ , calculated using the fair market value of our common stock on the date of grant, provided that the outside director has served on our board of directors for at least six months. Each annual option will vest and become exercisable on the first anniversary of the date of grant, or immediately prior to the next regular annual meeting of the company's stockholders following the date of grant if the meeting occurs prior to the first anniversary date. The options granted to outside directors will have a per share exercise price equal to 100% of the fair market value of the underlying shares on
100
- •
- In the event of a recapitalization, stock split or similar capital transaction, our compensation committee will make
appropriate and equitable adjustments to the number of shares reserved for issuance under the 2008 Stock Plan, including the share number in the formula for automatic annual increases, the limitation
regarding the total number of shares underlying awards given to an individual participant in any calendar year, the number of shares that can be issued as incentive stock options and other adjustments
in order to preserve the benefits of outstanding awards under the 2013 Stock Incentive Plan.
- •
- Generally, if we merge with or into another corporation, we will provide for full exercisability or vesting and
accelerated expiration of outstanding awards or settlement of the intrinsic value of the outstanding awards in cash or cash equivalents followed by cancellation of such awards unless the awards are
continued if we are the surviving entity, or assumed or substituted for by any surviving entity or a parent or subsidiary of the surviving entity.
- •
- If we are involved in an asset acquisition, stock acquisition, merger or similar transaction with another entity, our
compensation committee may make awards under the 2013 Stock Incentive Plan by the assumption, substitution or replacement of awards granted by another entity. The terms of such assumed, substituted or
replaced awards will be determined by our compensation committee in its discretion.
- •
- Awards under our 2013 Stock Incentive Plan may be made subject to the attainment of performance criteria including cash
flows, earnings per share, earnings before interest, taxes and amortization, return on equity, total stockholder return, share price performance, return on capital, return on assets or net assets,
revenue, income or net income, operating income or net operating income, operating profit or net operating profit, operating margin or profit margin, return on operating revenue, return on invested
capital, market segment shares, costs, expenses, regulatory body approval for commercialization of a product or implementation or completion of critical projects.
- •
- The 2013 Stock Incentive Plan terminates ten years after its initial adoption, unless terminated earlier by our board of directors. Our board of directors may amend or terminate the plan at any time, subject to stockholder approval where required by applicable law. Any amendment or termination may not materially impair the rights of holders of outstanding awards without their consent.
the date of grant and will become fully vested if we are subject to a change of control. In addition, such options will terminate on the earlier of (i) the day before the tenth anniversary of the date of grant or (ii) the date 12 months after the termination of the outside director's termination of service for any reason.
Limitation on Liability and Indemnification Matters
Our certificate of incorporation contains provisions that limit the personal liability of our directors for monetary damages to the fullest extent permitted by the General Corporation Law of the State of Delaware, or the DGCL. Consequently, our directors will not be personally liable to us or our stockholders for monetary damages for any breach of fiduciary duties as directors, except liability for:
- •
- any breach of the director's duty of loyalty to us or our stockholders;
- •
- any act or omission not in good faith or that involves intentional misconduct or a knowing violation of law;
- •
- unlawful payments of dividends or unlawful stock repurchases or redemptions as provided in Section 174 of the DGCL;
or
- •
- any transaction from which the director derived an improper personal benefit.
101
Our certificate of incorporation and bylaws provide that we are required to indemnify our directors, in each case to the fullest extent permitted by the DGCL. Our bylaws also provide that we shall advance expenses incurred by a director in advance of the final disposition of any action or proceeding, and permit us to secure insurance on behalf of any officer, director, employee or other agent for any liability arising out of his or her actions in that capacity regardless of whether we would otherwise be permitted to indemnify him or her under the provisions of the DGCL. Prior to the closing of the offering, we plan to enter into indemnification agreements with each of our officers and directors. With certain exceptions, these agreements will provide for indemnification for related expenses including, among other things, attorneys' fees, judgments, fines and settlement amounts incurred by any of our directors in any action or proceeding. We believe that these bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons to serve as directors and officers. We also maintain directors' and officers' liability insurance.
The limitation of liability and indemnification provisions in our certificate of incorporation and bylaws may discourage stockholders from bringing a lawsuit against our directors for breach of their fiduciary duty of care. They may also reduce the likelihood of derivative litigation against our directors and officers, even though an action, if successful, might benefit us and other stockholders. Further, a stockholder's investment may be adversely affected to the extent that we pay the costs of settlement and damage awards against directors and officers. At present, there is no pending litigation or proceeding involving any of our directors, officers or employees for which indemnification is sought, and we are not aware of any threatened litigation that may result in claims for indemnification.
102
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
In addition to the cash and equity compensation arrangements of our directors and named executive officers discussed above under "Management–Director Compensation" and "Executive Compensation," the following is a description of transactions since January 1, 2010, to which we have been a party in which the amount involved exceeded or will exceed $120,000 and in which any of our directors, executive officers, beneficial holders of more than 5% of our capital stock, or entities affiliated with or immediate family members of any of the foregoing, had or will have a direct or indirect material interest.
Sales of Convertible Preferred Stock
The following table summarizes purchases of our convertible preferred stock since January 1, 2010 by our directors, executive officers and holders of more than 5% of our capital stock and their affiliated entities. Each outstanding share of our convertible preferred stock is convertible into one share of our common stock upon the completion of this offering. As of August 15, 2013, all of our outstanding convertible preferred stock will convert into 59,989,268 shares of our common stock assuming the conversion immediately upon the closing of this offering.
| |
Shares of Preferred Stock | |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Aggregate Purchase Price ($) |
|||||||||
Purchaser
|
Series B | Series C | ||||||||
Entities affiliated with Domain Partners(1) |
9,600,000 | 2,744,101 | 17,186,351 | |||||||
Entities affiliated with Versant Ventures(2) |
4,016,000 | 3,215,553 | 11,097,395 | |||||||
TPG Biotechnology Partners II, L.P.(3) |
4,016,000 | 3,158,385 | 10,989,348 | |||||||
KPCB Holdings, Inc.(4) |
4,016,000 | 3,158,385 | 10,989,348 | |||||||
jVen Capital, LLC(5) |
552,000 | 443,629 | 1,528,459 | |||||||
Karin Eastham Defined Benefit Plan(6) |
— | 52,910 | 100,000 | |||||||
Total |
22,200,000 | 12,772,963 | 51,890,901 | |||||||
- (1)
- The
purchasers were Domain Partners VIII, L.P. and DP VIII Associates, L.P. Jesse I. Treu, a director of our company, is affiliated with these
entities.
- (2)
- The
purchasers were Versant Venture Capital III, L.P. and Versant Side Fund III, L.P. Brian G. Atwood and Samuel D. Colella,
directors of our company, are affiliated with these entities.
- (3)
- Fred
E. Cohen, a director of our company, is affiliated with this entity.
- (4)
- Brook
H. Byers, a director of our company, is affiliated with this entity.
- (5)
- Evan
Jones, a director of our company, is affiliated with this entity.
- (6)
- Karin Eastham, a director of our company, is affiliated with this entity.
Investor Rights Agreement
Holders of our convertible preferred stock are entitled to certain registration rights following this offering with respect to the common stock issued or issuable upon conversion of the convertible preferred stock. See "Description of Capital Stock–Investor Rights Agreement" for additional information.
Indemnification Agreements
Prior to the closing of the offering, we plan to enter into indemnification agreements with our directors and officers. The indemnification agreements and our certificate of incorporation and bylaws require us to indemnify these individuals to the fullest extent permitted by Delaware law. See "Management–Limitation on Liability and Indemnification Matters".
103
Related Party Transaction Policy
We intend to adopt a formal policy that our executive officers, directors, holders of more than 5% of any class of our voting securities, and any member of the immediate family of and any entity affiliated with any of the foregoing persons, are not permitted to enter into a related party transaction with us without the prior consent of our audit committee, or other independent members of our board of directors in the event it is inappropriate for our audit committee to review such transaction due to a conflict of interest. Any request for us to enter into a transaction with an executive officer, director, principal stockholder, or any of their immediate family members or affiliates, in which the amount involved exceeds $120,000 must first be presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, our audit committee is to consider the relevant facts and circumstances available and deemed relevant to our audit committee, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related party's interest in the transaction. All of the transactions described above were entered into prior to the adoption of such policy.
Although we have not had a written policy for the review and approval of transactions with related persons prior to 2013, our board of directors has historically reviewed and approved any transaction where a director or officer had a financial interest, including all of the transactions described above. Prior to approving such a transaction, the material facts as to a director's or officer's relationship or interest as to the agreement or transaction were disclosed to our board of directors. Our board of directors would take this information into account when evaluating the transaction and in determining whether such a transaction was fair to the company and in the best interests of all of our stockholders. In addition, for each related party transaction described above, the disinterested directors in the context of each such transaction approved the applicable agreement and transaction.
104
The following table sets forth information regarding the number of shares of common stock beneficially owned on August 15, 2013, and immediately following consummation of this offering, by:
- •
- each person who is known by us to beneficially own 5% or more of our common stock;
- •
- each of our named executive officers and directors; and
- •
- all of our executive officers and directors as a group.
We have determined beneficial ownership in accordance with SEC rules. Except as indicated by the footnotes below, we believe, based on the information furnished to us, that the persons and entities named in the table below have sole voting and investment power with respect to all shares of common stock that they beneficially own, subject to applicable community property laws.
Applicable percentage ownership is based on 63,712,607 shares of common stock outstanding at August 15, 2013 and assumes the conversion of all outstanding shares of our convertible preferred stock into shares of our common stock. For purposes of the table below, we have assumed that shares of common stock will be outstanding upon completion of this offering. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed to be outstanding all shares of common stock subject to options held by that person or entity that are currently exercisable or exercisable within 60 days of August 15, 2013. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person.
Except as otherwise set forth below, the address of each beneficial owner is 7000 Shoreline Court, Suite 250, South San Francisco, California 94080.
| |
|
Percentage of Shares Beneficially Owned |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of Shares Beneficially Owned(1) |
|||||||||
Name and Address of Beneficial Owner
|
Before Offering |
After Offering |
||||||||
5% Stockholders: |
||||||||||
Entities affiliated with Domain Partners(2) |
12,344,101 | 19.37 | % | |||||||
KPCB Holdings, Inc.(3) |
14,207,718 | 22.30 | % | |||||||
TPG Biotechnology Partners II, L.P(4) |
14,207,718 | 22.30 | % | |||||||
Entities affiliated with Versant Ventures(5) |
14,464,886 | 22.70 | % | |||||||
Directors and Executive Officers: |
||||||||||
Bonnie H. Anderson(6) |
3,224,036 | 4.92 | % | |||||||
Brian G. Atwood(5) |
14,464,886 | 22.70 | % | |||||||
Brook H. Byers(3) |
14,207,718 | 22.30 | % | |||||||
Fred E. Cohen, M.D., D.Phil.(7) |
- | |||||||||
Samuel D. Colella(5) |
14,464,886 | 22.70 | % | |||||||
Karin Eastham(8) |
77,910 | * | ||||||||
Evan Jones(9) |
2,195,629 | 3.44 | % | |||||||
Jesse I. Treu, Ph.D.(2) |
12,344,101 | 19.37 | % | |||||||
Shelly D. Guyer(10) |
600,000 | * | ||||||||
Christopher M. Hall(11) |
933,776 | 1.44 | % | |||||||
All directors and executive officers as a group (10 persons)(12) |
62,512,942 | 92.87 | % | |||||||
- *
- Less
than 1%
- (1)
- Unless otherwise indicated, includes shares owned by a spouse, minor children and relatives sharing the same home, as well as entities owned or controlled by the named person. Also includes options to
105
purchase shares of common stock exercisable within 60 days of August 15, 2013. Unless otherwise indicated, shares are owned of record and beneficially by the named person.
- (2)
- Consists
of 12,253,179 shares held by Domain Partners VIII, L.P. and 90,922 shares held by DP VIII Associates, L.P. The managing members of
One Palmer Square Associates VIII, L.L.C., the general partner of Domain Partners VIII, L.P. and DP VIII Associates, L.P., share voting and dispositive power with respect to these
shares. The managing members of One Palmer Square Associates VIII, L.L.C. are Jesse I. Treu, a member of our board of directors, James C. Blair, Brian H. Dovey, Brian K. Halak, Kathleen K. Schoemaker
and Nicole Vitullo. Each of Jesse I. Treu, James C. Blair, Brian H. Dovey, Brian K. Halak, Kathleen K. Schoemaker and Nicole Vitullo disclaims beneficial ownership of these shares except to the extent
of his or her pecuniary interest therein. The address for these entities is One Palmer Square, Suite 515, Princeton, New Jersey 08542.
- (3)
- Includes
12,697,935 shares of common stock beneficially owned by Kleiner Perkins Caufield & Byers XII, LLC, or KPCB XII; 182,782 shares
of common stock beneficially owned by KPCB XII Founders Fund, LLC, or KPCB XII FF; 245,741 shares of common stock beneficially owned by Brook H. Byers, a member of our board of
directors; and 1,081,260 shares of common stock beneficially owned by individuals and entities associated with Kleiner Perkins Caufield & Byers. All shares are held for convenience in the name
of "KPCB Holdings, Inc. as nominee," for the accounts of such individuals and entities who each exercise their own voting and dispositive power over such shares. The managing member of
KPCB XII and KPCB XII FF is KPCB XII Associates, LLC ("KPCB XII Associates"). Brook H. Byers, L. John Doerr, Joseph Lacob, Raymond J. Lane,
Theodore E. Schlein and Russ Siegelman, the managers of KPCB XII Associates, exercise shared voting and dispositive power over the shares directly held by KPCB XII and
KPCB XII FF. The principal business address for all entities and individuals affiliated with Kleiner Perkins Caufield & Byers is 2750 Sand Hill Road, Menlo Park, California 94025.
- (4)
- Consists
of 14,207,718 shares held by TPG Biotechnology Partners II, L.P., a Delaware limited partnership whose general partner is TPG Biotechnology
GenPar II, L.P., a Delaware limited partnership, whose general partner is TPG Biotechnology GenPar II Advisors, LLC, a Delaware limited liability company, whose sole member is TPG
Holdings I, L.P. whose general partner is TPG Holdings I-A, LLC, a Delaware limited liability company, whose sole member is TPG Group Holdings (SBS), L.P., a Delaware limited
partnership, whose general partner is TPG Group Holdings (SBS) Advisors, Inc., a Delaware corporation. David Bonderman and James G. Coulter are directors, officers and sole shareholders of TPG
Group Holdings (SBS) Advisors, Inc. and may therefore be deemed to be the beneficial owners of the shares held by TPG Biotechnology Partners II, L.P. Messrs. Bonderman and Coulter
disclaim beneficial ownership of the shares held by TPG Biotechnology Partners II, L.P. except to the extent of their pecuniary interest therein. The address of TPG Group Holdings (SBS)
Advisors, Inc. and Messrs. Bonderman and Coulter is c/o TPG Global, LLC, 301 Commerce Street, Suite 3300, Fort Worth, Texas 76102.
- (5)
- Consists of 14,379,957 shares held by Versant Venture Capital III, L.P. and 84,929 shares held by Versant Side Fund III, L.P. Versant Ventures III, LLC, the sole general partner of Versant Venture Capital III, L.P. and Versant Side Fund III, L.P., has voting and dispositive power with respect to these shares. The individual managing members of Versant Ventures III, LLC are Brian G. Atwood, Bradley J. Bolzon, Samuel D. Colella, Ross A. Jaffe, William J. Link, Kirk G. Nielsen, Rebecca B. Robertson, and Charles M. Warden, all of whom share voting and investment power with respect to these shares. Messrs. Atwood and Colella are members of our board of directors. Each individual managing member disclaims beneficial ownership of these shares, except to the extent of their pecuniary interest in such shares. The address of each entity affiliated with Versant Ventures is 3000 Sand Hill Road, Building Four, Suite 210, Menlo Park, California 94025.
106
- (6)
- Includes
options to purchase 1,827,695 shares of our common stock which are immediately exercisable, 985,514 of which are subject to the company's right of
repurchase on or prior to 60 days after August 15, 2013 and 50,000 of which are subject to the company's right of repurchase, which right lapses upon the closing of this offering if this
offering closes in 2013.
- (7)
- Does
not include 14,207,718 shares held by TPG Biotechnology Partners II, LP. Dr. Cohen is a partner at TPG Biotechnology
Partners II, LP. Dr. Cohen does not have voting or dispositive power with respect to the shares held by TPG Biotechnology Partners II, LP and disclaims beneficial
ownership of such shares. The address of Dr. Cohen is c/o TPG Global, LLC, 301 Commerce Street, Suite 3300, Fort Worth, Texas 76102.
- (8)
- Includes
options to purchase 25,000 shares of our common stock which are immediately exercisable, 4,167 of which are subject to the company's right of
repurchase on or prior to 60 days after August 15, 2013, which right lapses over time. Also includes 52,910 shares held by Karin Eastham Defined Benefit Plan.
- (9)
- Includes
options to purchase 200,000 shares of our common stock which are immediately exercisable. Also includes 1,995,629 shares held by jVen
Capital, LLC, of which Mr. Jones is Managing Member.
- (10)
- Consists
of options to purchase 600,000 shares of our common stock which are immediately exercisable, all of which are subject to the company's right of
repurchase on or prior to 60 days after August 15, 2013, which right lapses over time.
- (11)
- Consists
of options to purchase 933,776 shares of our common stock which are immediately exercisable, 273,959 of which are subject to the company's right
of repurchase on or prior to 60 days after August 15, 2013, which right lapses over time.
- (12)
- Includes options to purchase 3,586,471 shares of our common stock which are immediately exercisable, 1,863,640 of which are subject to the company's right of repurchase on or prior to 60 days after August 15, 2013, which right lapses over time and 50,000 of which are subject to the company's right of repurchase, which right lapses upon the closing of this offering.
107
General
The following is a summary of the rights of our common stock and preferred stock and certain provisions of our restated certificate of incorporation and amended and restated bylaws, as they will be in effect upon the completion of this offering. For more detailed information, please see our restated certificate of incorporation and amended and restated bylaws, which are filed as exhibits to the registration statement of which this prospectus is part.
Immediately following the completion of this offering, our authorized capital stock will consist of shares, with a par value of $0.001 per share, of which:
- •
- shares will be designated as common stock; and
- •
- shares will be designated as preferred stock.
As of August 15, 2013, we had outstanding 63,712,607 shares of common stock held of record by 51 stockholders, assuming the automatic conversion of all outstanding shares of preferred stock into common stock immediately prior to the closing of this offering. Upon completion of this offering, no shares of preferred stock will be outstanding.
Common Stock
Each holder of common stock is entitled to one vote per share on all matters submitted to a vote of stockholders. We have not provided for cumulative voting in the election of directors. Subject to preferences that may apply to shares of preferred stock outstanding at the time, the holders of outstanding shares of our common stock are entitled to receive dividends out of assets legally available at the times and in the amounts that our board of directors may determine from time to time. Upon our liquidation, dissolution or winding-up, the holders of common stock are entitled to share ratably in all assets remaining after payment of all liabilities and the liquidation preferences of any outstanding preferred stock. Holders of common stock have no preemptive or conversion rights or other subscription rights. There are no redemption or sinking fund provisions applicable to our common stock.
Preferred Stock
Upon the closing of this offering, our board of directors will have the authority, without further action by our stockholders, to issue from time to time up to shares of preferred stock in one or more series, and to establish the number of shares to be included in each series and fix the powers, preferences and rights of the shares of each wholly unissued series and any of its qualifications, limitations or restrictions. Our board of directors will also be able to increase or decrease the number of shares of any series, but not below the number of shares of that series then outstanding, without any further vote or action by the stockholders.
The issuance of preferred stock could decrease the amount of earnings and assets available for distribution to the holders of common stock or adversely affect the rights and powers, including voting rights, of the holders of common stock. The issuance of preferred stock, while providing flexibility in connection with possible acquisitions and other corporate purposes, could, among other things, have the effect of delaying, deferring or preventing a change in control of our company, which could depress the market price of our common stock. We have no current plans to issue any shares of preferred stock.
Warrants
As of August 15, 2013, we had a warrant outstanding to purchase 99,206 shares of our Series C preferred stock at an exercise price of $1.89 per share, which will become exercisable for the same number of shares of our common stock upon completion of this offering. This warrant has a net exercise provision
108
under which its holder may, in lieu of payment of the exercise price in cash, surrender the warrant and receive a net amount of shares based on the fair market value of our stock at the time of exercise of the warrants after deduction of the aggregate exercise price. This warrant contain provisions for adjustment of the exercise price and number of shares issuable upon the exercise of the warrant in the event of certain stock dividends, stock splits, reorganizations, reclassifications and consolidations. This warrant will expire on the seventh anniversary of this offering.
Registration Rights
After this offering, the holders of 59,989,268 shares of our common stock issued upon the conversion of our preferred stock will be entitled to contractual rights to require us to register those shares under the Securities Act. These rights are provided under the terms of our amended and restated investor rights agreement. If we propose to register any of our securities under the Securities Act for our own account, holders of shares having registration rights are entitled to include their shares in our registration statement, provided, among other conditions, that the underwriters of any such offering have the right to limit the number of shares included in the registration. These holders have waived their rights to include their shares in this offering. Holders of shares having registration rights may also require us to file up to two additional registration statements on Form S-3 or similar short-form registration statement, if we are eligible to use Form S-3 or similar short-form registration statement, and the value of the securities to be registered is at least $1,500,000.
We will pay all expenses relating to any demand, piggyback or Form S-3 registration described below, other than underwriting discounts and selling commissions. The registration rights terminate upon the earlier of the third anniversary of this offering, a change of control, or with respect to the registration rights of an individual holder, when that holder can sell all of the holder's shares covered by registration rights pursuant to Rule 144 under the Securities Act in any 90-day period.
Demand Registration Rights
After the expiration of the 180-day lock-up agreements referred to under "Shares Eligible for Future Sale," and subject to limitations and conditions specified in the investor rights agreement, holders of a majority of the shares covered by registration rights may require us to prepare and file a registration statement under the Securities Act covering all shares they request that we register. We are not obligated to effect more than two of these stockholder-initiated registrations.
Piggyback Registration Rights
If we propose to register any of our securities under the Securities Act either for our own account or for the account of other stockholders, the holders of shares having registration rights will, subject to certain exceptions, be entitled to include their shares in our registration statement. These registration rights are subject to specified conditions and limitations, including the right of the underwriters to limit the number of shares included in any such registration statement under certain circumstances, but not below 25% of the total number of shares covered by the registration statement.
Form S-3 Registration Rights
At any time after we are qualified to file a registration statement on Form S-3, and subject to limitations and conditions specified in the investor rights agreement, the holders of shares having registration rights may require us to prepare and file a registration statement on Form S-3 under the Securities Act covering their shares, so long as the aggregate offering price of the shares to be registered is at least $1,500,000. We not obligated to effect more than two of these Form S-3 registrations.
109
Anti-takeover Effects of Delaware Law and Our Restated Certificate of Incorporation and Bylaws
Certain provisions of Delaware law, our restated certificate of incorporation and our amended and restated bylaws to become effective upon completion of this offering could have the effect of delaying, deferring or discouraging another party from acquiring control of us. These provisions, which are summarized below, are expected to discourage certain types of coercive takeover practices and inadequate takeover bids. These provisions are also designed, in part, to encourage persons seeking to acquire control of us to first negotiate with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging such proposals, including proposals that are priced above the then-current market value of our common stock, because, among other reasons, the negotiation of such proposals could result in an improvement of their terms.
Certificate of Incorporation and Bylaws
Our restated certificate of incorporation and amended and restated bylaws to become effective upon completion of this offering include provisions that:
- •
- authorize our board of directors to issue, without further action by the stockholders, up to shares of
undesignated preferred stock;
- •
- require that any action to be taken by our stockholders be effected at a duly called annual or special meeting and not by
written consent;
- •
- specify that special meetings of our stockholders can be called only by our board of directors, our chairman of the board,
or our chief executive officer;
- •
- establish an advance notice procedure for stockholder approvals to be brought before an annual meeting of our
stockholders, including proposed nominations of persons for election to our board of directors;
- •
- establish that our board of directors is divided into three classes, Class I, Class II and Class III,
with each class serving staggered terms;
- •
- provide that our directors may be removed only for cause;
- •
- provide that vacancies on our board of directors may, except as otherwise required by law, be filled only by a majority of
directors then in office, even if less than a quorum;
- •
- specify that no stockholder is permitted to cumulate votes at any election of directors; and
- •
- require a super-majority of votes to amend certain of the above-mentioned provisions.
Delaware Law
We are subject to the provisions of Section 203 of the General Corporation Law of the State of Delaware regulating corporate takeovers. In general, these provisions prohibit a publicly-held Delaware corporation from engaging in a business combination with an interested stockholder for a period of three years following the date the person became an interested stockholder unless:
- •
- prior to the date of the transaction, the board of directors of the corporation approved either the business combination
or the transaction that resulted in the stockholder becoming an interested stockholder;
- •
- upon completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned at least 85% of the voting stock of the corporation outstanding at the time the transaction commenced, excluding for purposes of determining the voting stock outstanding, (1) shares owned by persons who are directors and also officers and
110
- •
- on or after the date the business combination is approved by the board of directors of the corporation and authorized at a meeting of stockholders by at least 662/3% of the outstanding voting stock that is not owned by the interested stockholder.
(2) shares owned by employee stock plans in which employee participants do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or exchange offer; or
Section 203 defines "business combination" to include the following:
- •
- any merger or consolidation involving the corporation and the interested stockholder;
- •
- any sale, transfer, pledge or other disposition of 10% or more of the assets of the corporation involving the interested
stockholder;
- •
- subject to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of
the corporation to the interested stockholder;
- •
- any transaction involving the corporation that has the effect of increasing the proportionate share of the stock of any
class or series of the corporation beneficially owned by the interested stockholder; or
- •
- the receipt by the interested stockholder of the benefit of any loans, advances, guarantees, pledges or other financial benefits provided by or through the corporation.
In general, Section 203 defines an interested stockholder as any entity or person beneficially owning 15% or more of the outstanding voting stock of the corporation and any entity or person affiliated with or controlling or controlled by any of these entities or persons.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is . The transfer agent's address is .
Listing
We have applied to list our common stock on The NASDAQ Global Market under the symbol "VCYT".
111
SHARES ELIGIBLE FOR FUTURE SALE
Before this offering, there has not been a public market for shares of our common stock. Future sales of substantial amounts of shares of our common stock, including shares issued upon the exercise of outstanding options, in the public market after this offering, or the possibility of these sales occurring, could cause the market price for our common stock to fall or impair our ability to raise equity capital in the future.
Based on the number of shares outstanding as of , 2013, upon the completion of this offering a total of shares of common stock will be outstanding, assuming that there are no exercises of options or warrants after , 2013 and no exercise of the underwriters' over-allotment option. Of these shares, all shares of common stock sold in this offering, and any shares sold upon exercise of the underwriters' over-allotment option, will be freely tradable in the public market without restriction or further registration under the Securities Act of 1933, unless these shares are held by "affiliates," as that term is defined in Rule 144 under the Securities Act.
The remaining shares of common stock will be "restricted securities," as that term is defined in Rule 144. These restricted securities are eligible for public sale only if they are registered under the Securities Act or if they qualify for an exemption from registration under Rules 144 or 701 under the Securities Act, which are summarized below.
Subject to the lock-up agreements described below and the provisions of Rules 144 and 701, these restricted securities will be available for sale in the public market as follows:
Date
|
Number of Shares | |||
|---|---|---|---|---|
On the date of this prospectus |
— | |||
Between 90 and 180 days after the date of this prospectus |
— | |||
At various times beginning more than 180 days after the date of this prospectus |
||||
In addition, of the shares of our common stock that were subject to options outstanding as of , 2013, options to purchase shares of common stock were vested as of , 2013 and will be eligible for sale at various times beginning more than 180 days following the effective date of this offering.
Rule 144
In general, under Rule 144, beginning 90 days after the date of this prospectus, a person who is not deemed to be our affiliate and has not been our affiliate at any time during the three months preceding a sale will be entitled to sell any shares of our common stock that such person has beneficially owned for at least six months, including the holding period of any prior owner other than one of our affiliates, without regard to manner of sale, volume limitations or notice provisions of Rule 144. Sales of our common stock by any such person would be subject to the availability of current public information about us if the shares to be sold were beneficially owned by such person, including the holding period of any prior owner other than one of our affiliates, for less than one year.
In addition, under Rule 144, a person may sell shares of our common stock acquired from us immediately upon the closing of this offering, without regard to volume limitations or the availability of public information about us, if:
- •
- the person is not our affiliate and has not been our affiliate at any time during the preceding three months; and
- •
- the person has beneficially owned the shares to be sold for at least one year, including the holding period of any prior owner other than one of our affiliates.
112
Beginning 90 days after the date of this prospectus, our affiliates who have beneficially owned shares of our common stock for at least six months, including the holding period of any prior owner other than one of our affiliates, would be entitled to sell within any three-month period a number of shares that does not exceed the greater of:
- •
- 1% of the number of shares of our common stock then-outstanding, which will equal approximately shares
immediately after this offering; and
- •
- the average weekly trading volume in our common stock during the four calendar weeks preceding the date of filing of a notice on Form 144 with respect to the sale.
Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
In general, under Rule 701, any of our employees, consultants or advisors who purchase shares from us in connection with a compensatory stock or option plan or other written agreement in a transaction before the date of this offering that was completed in reliance on Rule 701 and complied with the requirements of Rule 701 will, subject to the lock-up restrictions described below, be eligible to resell such shares 90 days after the date of this offering in reliance on Rule 144, but without compliance with certain restrictions, including the holding period, contained in Rule 144.
Lock-up Agreements
In connection with this offering we and our officers, directors, substantially all of our stockholders and optionholders have agreed with the underwriters, subject to certain exceptions, not to dispose of or hedge any of our common stock or securities convertible into or exchangeable for shares of common stock, enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of the common stock or such other securities, file or cause to be filed a registration statement covering shares of common stock or any securities that are convertible into, exchangeable for, or represent the right to receive, common stock or any substantially similar securities, or publicly disclose the intention to do any of the foregoing, during the period from the date of this prospectus continuing through the date 180 days after the date of this prospectus, except with the prior written consent of Morgan Stanley & Co. LLC and Leerink Swann LLC. This agreement does not apply to the issuance by us of shares under any existing employee benefit plans. These agreements are subject to certain exceptions, as set forth in "Underwriters".
Registration Rights
After this offering, the holders of shares of common stock will be entitled to rights to cause us to register the sale of those shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares, other than shares purchased by our affiliates, becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration. See "Description of Capital Stock–Registration Rights" for additional information.
Stock Plans
We intend to file a registration statement on Form S-8 under the Securities Act covering all of the shares of common stock subject to options outstanding or reserved for issuance under our stock plans. We expect to file this registration statement as soon as practicable after this offering. However, none of the shares registered on Form S-8 will be eligible for resale until the expiration of the lock-up agreements to which they are subject.
113
MATERIAL UNITED STATES TAX CONSIDERATIONS TO NON-U.S. HOLDERS
The following is a summary of certain material U.S. federal income and estate tax consequences applicable to non-U.S. holders (as defined below) with respect to the purchase, ownership and disposition of our common stock, but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Internal Revenue Code, Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income tax consequences different from those set forth below. This summary is limited to the tax consequences to those persons that purchase our common stock in this offering and will hold our common stock as capital assets within the meaning of Section 1221 of the Internal Revenue Code.
This summary does not address the tax considerations arising under the laws of any U.S. state or local jurisdiction or any non-U.S. jurisdiction or under U.S. federal gift, generation-skipping and, except to the extent specifically set forth below, estate tax laws or the potential application of certain provisions of the Internal Revenue Code relating to what is known as the Medicare Contribution tax. In addition, this discussion does not address tax considerations applicable to an investor's particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
- •
- banks, insurance companies or other financial institutions;
- •
- persons subject to the alternative minimum tax;
- •
- tax-exempt organizations;
- •
- dealers in securities or currencies;
- •
- "controlled foreign corporations," or "passive foreign investment companies," each as defined for U.S. federal income tax
purposes;
- •
- partnerships or entities classified as partnerships for U.S. federal income tax purposes, or any investors in such
entities;
- •
- traders in securities that elect to use a mark-to-market method of accounting for their securities holdings;
- •
- persons that own, or are deemed to own, more than five percent of our common stock (except to the extent specifically set
forth below);
- •
- certain former citizens or long-term residents of the United States;
- •
- persons who hold our common stock as a position in a hedging transaction, "straddle," "conversion transaction" or other
risk reduction transaction;
- •
- persons who acquire our common stock through the exercise of employee stock options or otherwise as compensation for
services; or
- •
- persons deemed to sell our common stock under the constructive sale provisions of the Internal Revenue Code.
If a partnership or entity classified as a partnership for U.S. federal income tax purposes is a beneficial owner of our common stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership and disposition of our common stock arising under the U.S. federal estate, generation-skipping or gift tax rules
114
or under the laws of any U.S. state or local or any non-U.S. or other taxing jurisdiction or under any applicable tax treaty.
Non-U.S. Holder Defined
For purposes of this discussion, you are a non-U.S. holder if you are a beneficial owner of our common stock (other than a partnership or entity classified as a partnership for U.S. federal income tax purposes) that for U.S. federal income tax purposes is not:
- •
- an individual citizen or resident of the United States;
- •
- a corporation or other entity taxable as a corporation for U.S. federal income tax purposes created or organized in the
U.S. or under the laws of the U.S. or any political subdivision thereof;
- •
- an estate whose income is subject to U.S. federal income tax regardless of its source; or
- •
- a trust (i) whose administration is subject to the primary supervision of a U.S. court and which has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (ii) which has made a valid election to be treated as a U.S. person.
A foreign individual may be treated as a resident instead of a nonresident of the United States in any calendar year for U.S. federal income tax purposes if the individual was present in the United States for at least 31 days in that calendar year and for an aggregate of at least 183 days during the three-year period ending with the current calendar year. Subject to certain exceptions, for purposes of this calculation, all of the days present in the current year, one-third of the days present in the immediately preceding year, and one-sixth of the days present in the second preceding year are counted.
Distributions
If we make distributions on our common stock, those payments will constitute dividends for U.S. federal income tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero. Any excess will be treated as gain from the sale or other disposition of the common stock and will be treated as described below under "–Gain on Disposition of Common Stock".
Any dividend paid to you generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty, unless the dividends are effectively connected with your conduct of a U.S. trade or business, as discussed below. In order to receive a reduced treaty rate, you must provide us or the relevant paying agent with an IRS Form W-8BEN or other appropriate version of IRS Form W-8 prior to the distribution date properly certifying qualification for the reduced rate.
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income tax treaty, attributable to a permanent establishment maintained by you in the United States) generally will be subject to U.S. federal income tax at the same graduated rates applicable to U.S. persons, net of certain deductions and credits. If you are a corporate non-U.S. holder, you also may be subject to a branch profits tax equal to 30% (or such lower rate specified by an applicable income tax treaty) of your earnings and profits for the taxable year, subject to certain adjustments, that are effectively connected with your conduct of a trade or business in the United States. Payment of effectively connected dividends that are included in your gross income generally will be exempt from withholding tax if you provide us or the relevant paying agent with an IRS Form W-8ECI or other applicable IRS Form W-8 prior to the distribution date properly certifying such exemption.
115
If you are eligible for a reduced rate of withholding tax pursuant to a tax treaty, you may be able to obtain a refund of any excess amounts currently withheld if you timely file an appropriate claim for refund with the IRS.
Gain on Disposition of Common Stock
Subject to the discussion below under "Backup Withholding and Information Reporting" and "Foreign Account Tax Compliance," you generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
- •
- the gain is effectively connected with your conduct of a U.S. trade or business (and, if required by an applicable income
tax treaty, the gain is attributable to a permanent establishment maintained by you in the United States), in which case you will be required to pay tax on the net gain derived from the sale (net of
certain deductions or credits) under regular graduated U.S. federal income tax rates, and if you are a non-U.S. holder that is a corporation, you may also be subject to a branch profits tax at a 30%
rate (or such lower rate as may be specified by an applicable income tax treaty) of your earnings and profits for the taxable year, subject to certain adjustments, that are effectively connected with
your conduct of a trade or business in the United States;
- •
- you are an individual who is present in the United States for a period or periods aggregating 183 days or more
during the calendar year in which the sale or disposition occurs and certain other conditions are met, in which case you will be required to pay a flat 30% tax on the gain derived from the sale, which
gain may be offset by U.S. source capital losses (even though you are not considered a resident of the United States) subject to applicable income tax or other treaties providing otherwise; or
- •
- our common stock constitutes a U.S. real property interest by reason of our status as a "U.S. real property holding corporation" for U.S. federal income tax purposes, or a USRPHC , at any time within the shorter of the five-year period preceding the disposition or your holding period for our common stock. In general, a corporation is a USRPHC if the fair market value of its U.S. real property interests (as defined in the Internal Revenue Code and applicable Treasury regulations) equals or exceeds 50% of the sum of the fair market value of its worldwide (U.S. and foreign) real property interests and its other assets used or held for use in a trade or business.
We believe that we are not currently and will not become a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC , however, as long as our common stock is regularly traded on an established securities market, such common stock will be treated as a U.S. real property interest only if you actually or constructively hold more than five percent of such regularly traded common stock at any time during the five year (or shorter) period that is described above.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address, and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
Payments of dividends or of proceeds on the disposition of common stock made to you will be subject to additional information reporting and backup withholding at a current rate of 28% unless you establish an exemption, for example by properly certifying your non-U.S. status on a Form W-8BEN or another appropriate version of IRS Form W-8. Notwithstanding the foregoing, backup withholding and information reporting will apply if the relevant paying agent has actual knowledge, or reason to know, that
116
you are a U.S. person. Payment of the proceeds from a disposition of our common stock by a non-U.S. holder effected through a non-U.S. office of a non-U.S. broker generally will not be subject to information reporting or backup withholding if the payment is not received in the United States. However, information reporting, but generally not backup withholding, will apply to such a payment if the broker has certain connections with the U.S. unless the broker has documentary evidence in its records that the beneficial owner thereof is a non-U.S. holder and specified conditions are met or an exemption is otherwise established.
Backup withholding is not an additional tax; rather, the U.S. income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner.
Foreign Account Tax Compliance
Legislation commonly referred to as the Foreign Accounts Tax Compliance Act, or FATCA, generally will impose a 30% U.S. withholding tax on dividends on our common stock and the gross proceeds from a disposition of our common stock if paid to a foreign entity, regardless of whether such foreign entity is the beneficial owner or an intermediary, unless (i) if the entity is a "foreign financial institution," the foreign entity undertakes certain due diligence, reporting, withholding and certification obligations, (ii) if the foreign entity is not a "foreign financial institution," the foreign entity identifies certain of its U.S. investors, or (iii) the foreign entity is otherwise exempted under FATCA. The obligation to withhold under FATCA is currently expected to apply to dividends paid on or after July 1, 2014 and to gross proceeds from sales or other dispositions of our common stock after December 31, 2016. You are encouraged to consult with your own tax advisor regarding the possible implications of this legislation on your investment in our common stock.
U.S. Federal Estate Tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for U.S. federal estate tax purposes) at the time of death generally will be includable in the decedent's gross estate for U.S. federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Each prospective investor should consult its own tax advisor regarding the particular U.S. federal, state and local and non-U.S. tax consequences of the purchase, ownership and disposition of our common stock, including the consequences of any proposed change in applicable laws.
117
Under the terms and subject to the conditions in an underwriting agreement dated the date of this prospectus, the underwriters named below, for whom Morgan Stanley & Co. LLC and Leerink Swann LLC are acting as representatives, have severally agreed to purchase, and we have agreed to sell to them, severally, the number of shares indicated below:
Name
|
Number of Shares | |
|---|---|---|
Morgan Stanley & Co. LLC |
||
Leerink Swann LLC |
||
William Blair & Company, L.L.C. |
||
Cowen and Company, LLC |
||
Total |
||
The underwriters and the representatives are collectively referred to as the "underwriters" and the "representatives," respectively. The underwriters are offering the shares of common stock subject to their acceptance of the shares from us and subject to prior sale. The underwriting agreement provides that the obligations of the several underwriters to pay for and accept delivery of the shares of common stock offered by this prospectus are subject to the approval of certain legal matters by their counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares of common stock offered by this prospectus if any such shares are taken. However, the underwriters are not required to take or pay for the shares covered by the underwriters' over-allotment option described below.
The underwriters initially propose to offer part of the shares of common stock directly to the public at the offering price listed on the cover page of this prospectus and part to certain dealers. After the initial offering of the shares of common stock, the offering price and other selling terms may from time to time be varied by the representatives.
We have granted to the underwriters an option, exercisable for 30 days from the date of this prospectus, to purchase up to additional shares of common stock at the public offering price listed on the cover page of this prospectus, less underwriting discounts and commissions. The underwriters may exercise this option solely for the purpose of covering over-allotments, if any, made in connection with the offering of the shares of common stock offered by this prospectus. To the extent the option is exercised, each underwriter will become obligated, subject to certain conditions, to purchase about the same percentage of the additional shares of common stock as the number listed next to the underwriter's name in the preceding table bears to the total number of shares of common stock listed next to the names of all underwriters in the preceding table.
The following table shows the per share and total public offering price, underwriting discounts and commissions, and proceeds before expenses to us. These amounts are shown assuming both no exercise and full exercise of the underwriters' option to purchase up to an additional shares of common stock.
| |
|
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Per Share | No Exercise | Full Exercise | |||||||
Public offering price |
$ | $ | $ | |||||||
Underwriting discounts and commissions to be paid by us |
$ | $ | $ | |||||||
Proceeds, before expenses, to us |
$ | $ | $ | |||||||
The estimated offering expenses payable by us, exclusive of the underwriting discounts and commissions, are approximately $ . We have agreed to reimburse the underwriters for certain expenses in an amount up to $ .
118
The underwriters have informed us that they do not intend sales to discretionary accounts to exceed 5% of the total number of shares of common stock offered by them.
We have applied to list our common stock on The NASDAQ Global Market under the trading symbol "VCYT".
We and all directors and officers and the holders of all of our outstanding stock and stock options have agreed that, without the prior written consent of Morgan Stanley & Co. LLC and Leerink Swann LLC on behalf of the underwriters, we and they will not, during the period ending 180 days after the date of this prospectus (the "restricted period"):
- •
- offer, pledge, sell, contract to sell, sell any option or contract to purchase, purchase any option or contract to sell,
grant any option, right or warrant to purchase, lend or otherwise transfer or dispose of, directly or indirectly, any shares of common stock or any securities convertible into or exercisable or
exchangeable for shares of common stock;
- •
- file any registration statement with the Securities and Exchange Commission relating to the offering of any shares of
common stock or any securities convertible into or exercisable or exchangeable for common stock; or
- •
- enter into any swap or other arrangement that transfers to another, in whole or in part, any of the economic consequences of ownership of common stock.
whether any such transaction described above is to be settled by delivery of common stock or such other securities, in cash or otherwise. In addition, we and each such person agrees that, without the prior written consent of Morgan Stanley & Co. LLC and Leerink Swann LLC on behalf of the underwriters, we or such other person will not, during the restricted period, make any demand for, or exercise any right with respect to, the registration of any shares of common stock or any security convertible into or exercisable or exchangeable for common stock.
The restrictions described in the immediately preceding paragraph are subject to certain customary exceptions.
Morgan Stanley & Co. LLC and Leerink Swann LLC, in their sole discretion, may release the common stock and other securities subject to the lock-up agreements described above in whole or in part at any time with or without notice.
In order to facilitate the offering of the common stock, the underwriters may engage in transactions that stabilize, maintain or otherwise affect the price of the common stock. Specifically, the underwriters may sell more shares than they are obligated to purchase under the underwriting agreement, creating a short position. A short sale is covered if the short position is no greater than the number of shares available for purchase by the underwriters under the over-allotment option. The underwriters can close out a covered short sale by exercising the over-allotment option or purchasing shares in the open market. In determining the source of shares to close out a covered short sale, the underwriters will consider, among other things, the open market price of shares compared to the price available under the over-allotment option. The underwriters may also sell shares in excess of the over-allotment option, creating a naked short position. The underwriters must close out any naked short position by purchasing shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there may be downward pressure on the price of the common stock in the open market after pricing that could adversely affect investors who purchase in this offering. As an additional means of facilitating this offering, the underwriters may bid for, and purchase, shares of common stock in the open market to stabilize the price of the common stock. These activities may raise or maintain the market price of the common stock above independent market levels or prevent or retard a decline in the market price of the common stock. The underwriters are not required to engage in these activities and may end any of these activities at any time.
119
We and the underwriters have agreed to indemnify each other against certain liabilities, including liabilities under the Securities Act.
A prospectus in electronic format may be made available on websites maintained by one or more underwriters, or selling group members, if any, participating in this offering. The representative may agree to allocate a number of shares of common stock to underwriters for sale to their online brokerage account holders. Internet distributions will be allocated by the representative to underwriters that may make Internet distributions on the same basis as other allocations.
The underwriters and their respective affiliates are full service financial institutions engaged in various activities, which may include securities trading, commercial and investment banking, financial advisory, investment management, principal investment, hedging, financing and brokerage activities. Certain of the underwriters and their respective affiliates have, from time to time, performed, and may in the future perform, various financial advisory and investment banking services for us or our affiliates, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriters and their respective affiliates may make or hold a broad array of investments and actively trade debt and equity securities (or related derivative securities) and financial instruments (including bank loans) for their own account and for the accounts of their customers and may at any time hold long and short positions in such securities and instruments. Such investment and securities activities may involve our securities and instruments. The underwriters and their respective affiliates may also make investment recommendations or publish or express independent research views in respect of such securities or instruments and may at any time hold, or recommend to clients that they acquire, long or short positions in such securities and instruments.
Pricing of the Offering
Prior to this offering, there has been no public market for our common stock. The initial public offering price was determined by negotiations between us and the representative. Among the factors considered in determining the initial public offering price were our future prospects and those of our industry in general, our sales, earnings and certain other financial and operating information in recent periods, and the price-earnings ratios, price-sales ratios, market prices of securities, and certain financial and operating information of companies engaged in activities similar to ours.
Selling Restrictions
European Economic Area
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a "Relevant Member State") an offer to the public of any shares of our common stock may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any shares of our common stock may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
- •
- to any legal entity which is a qualified investor as defined in the Prospectus Directive;
- •
- to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending
Directive, 150, natural or legal persons (other than qualified investors as defined in the Prospectus Directive), as permitted under the Prospectus Directive, subject to obtaining the prior consent of
the representatives for any such offer; or
- •
- in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares of our common stock shall result in a requirement for the publication by us or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
120
For the purposes of this provision, the expression an "offer to the public" in relation to any shares of our common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares of our common stock to be offered so as to enable an investor to decide to purchase any shares of our common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State, the expression "Prospectus Directive" means Directive 2003/71/EC (and amendments thereto, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State), and includes any relevant implementing measure in the Relevant Member State, and the expression "2010 PD Amending Directive" means Directive 2010/73/EU.
United Kingdom
Each underwriter has represented and agreed that:
- •
- it has only communicated or caused to be communicated and will only communicate or cause to be communicated an invitation
or inducement to engage in investment activity (within the meaning of Section 21 of the Financial Services and Markets Act 2000 ("FSMA") received by it in connection with the issue or sale of
the shares of our common stock in circumstances in which Section 21(1) of the FSMA does not apply to us; and
- •
- it has complied and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of our common stock in, from or otherwise involving the United Kingdom.
121
The validity of the shares of common stock offered by this prospectus will be passed upon for us by Pillsbury Winthrop Shaw Pittman LLP, San Francisco and Palo Alto, California. Simpson Thacher & Bartlett LLP, Palo Alto, California is representing the underwriters in this offering.
The financial statements as of December 31, 2011 and 2012 and for each of the two years in the period ended December 31, 2012 included in this prospectus have been so included in reliance on the report (which contains an explanatory paragraph relating to the Company's experience of recurring operating losses and negative cash flows from operations as described in Note 2 to the financial statements) of PricewaterhouseCoopers LLP, an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the Securities and Exchange Commission a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement or the exhibits and schedules to the registration statement. Please refer to the registration statement, exhibits and schedules for further information with respect to the common stock offered by this prospectus. Statements contained in this prospectus regarding the contents of any contract or other document are only summaries. With respect to any contract or document that is filed as an exhibit to the registration statement, you should refer to the exhibit for a copy of the contract or document, and each statement in this prospectus regarding that contract or document is qualified by reference to the exhibit. You may read and copy the registration statement and its exhibits and schedules at the SEC's public reference room, located at 100 F Street, N.E., Room 1580, Washington D.C. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. The SEC also maintains a website that contains reports, proxy and information statements and other information regarding companies, such as ours, that file documents electronically with the SEC. The address of that website is www.sec.gov. The information on the SEC's web site is not part of this prospectus, and any references to this web site or any other web site are inactive textual references only.
Upon completion of this offering, we will become subject to the information and reporting requirements of the Securities Exchange Act of 1934 and, in accordance with this law, will be required to file periodic reports, proxy statements and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the SEC's public reference facilities and the website of the SEC referred to above.
122
VERACYTE, INC.
Index to Audited Financial Statements
Years Ended December 31, 2011 and 2012
F-1
Report of Independent Registered Public Accounting Firm
To
the Board of Directors and Stockholders of
Veracyte, Inc.
In our opinion, the accompanying balance sheets and the related statements of operations and comprehensive loss, statements of convertible preferred stock and stockholders' deficit, and statements of cash flows present fairly, in all material respects, the financial position of Veracyte, Inc. at December 31, 2011 and 2012, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2012 in conformity with accounting principles generally accepted in the United States of America. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits. We conducted our audits of these statements in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
As discussed in Note 2 to the financial statements, the Company has experienced recurring operating losses and negative cash flows from operations. Management's plans with regard to its liquidity are also discussed in Note 2.
/s/ PricewaterhouseCoopers LLP
San Jose, California
August 12, 2013
F-2
VERACYTE, INC.
Balance Sheets
(In thousands, except share and per share amounts)
| |
As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Assets |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 7,566 | $ | 14,002 | |||
Accounts receivable, net of allowance of $235 and $222 as of December 31, 2011 and 2012 |
229 | 569 | |||||
Supplies inventory |
279 | 1,050 | |||||
Prepaid expenses and other current assets |
519 | 710 | |||||
Restricted cash |
– | 50 | |||||
Total current assets |
8,593 | 16,381 | |||||
Property and equipment, net |
1,687 | 2,446 | |||||
Restricted cash |
168 | 118 | |||||
Other assets |
3 | 122 | |||||
Total assets |
$ | 10,451 | $ | 19,067 | |||
Liabilities, Convertible Preferred Stock, and Stockholders' Deficit |
|||||||
Current liabilities: |
|||||||
Accounts payable |
$ | 550 | $ | 1,888 | |||
Accrued liabilities |
1,336 | 4,020 | |||||
Deferred Genzyme co-promotion fee |
– | 2,500 | |||||
Preferred stock liability |
– | 583 | |||||
Total current liabilities |
1,886 | 8,991 | |||||
Deferred rent, net of current portion |
35 | 61 | |||||
Deferred Genzyme co-promotion fee, net of current portion |
– | 5,114 | |||||
Total liabilities |
1,921 | 14,166 | |||||
Commitments and contingencies (Note 8) |
|||||||
Convertible preferred stock, $0.001 par value; 45,147,999 and 59,147,999 shares authorized, 45,147,999 and 53,084,507 shares issued and outstanding as of December 31, 2011 and December 31, 2012; aggregate liquidation value of $50,835 and $65,835 as of December 31, 2011 and 2012 |
49,296 |
63,372 |
|||||
Stockholders' deficit: |
|||||||
Common stock, $0.001 par value; 60,000,000 and 77,000,000 shares authorized, 2,379,782 and 2,670,767 shares issued and outstanding as of December 31, 2011 and 2012 |
2 | 3 | |||||
Additional paid-in capital |
652 | 1,595 | |||||
Accumulated deficit |
(41,420 | ) | (60,069 | ) | |||
Total stockholders' deficit |
(40,766 | ) | (58,471 | ) | |||
Total liabilities, convertible preferred stock, and stockholders' deficit |
$ | 10,451 | $ | 19,067 | |||
The accompanying notes are an integral part of these financial statements.
F-3
VERACYTE, INC.
Statements of Operations and Comprehensive Loss
(In thousands, except share and per share amounts)
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Revenue |
$ | 2,645 | $ | 11,628 | |||
Operating expenses: |
|||||||
Cost of revenue |
2,925 | 7,584 | |||||
Research and development |
6,680 | 6,608 | |||||
Selling and marketing |
2,934 | 8,447 | |||||
General and administrative |
5,372 | 7,918 | |||||
Total operating expenses |
17,911 | 30,557 | |||||
Loss from operations |
(15,266 | ) | (18,929 | ) | |||
Interest income |
2 | 2 | |||||
Other income (expense), net |
819 | 278 | |||||
Net loss and comprehensive loss |
$ | (14,445 | ) | $ | (18,649 | ) | |
Net loss per common share, basic and diluted |
$ | (6.23 | ) | $ | (7.17 | ) | |
Shares used to compute net loss per common share, basic and diluted |
2,320,252 | 2,601,352 | |||||
Pro forma net loss per common share, basic and diluted (unaudited) |
$ | (0.38 | ) | ||||
Shares used to compute pro forma net loss per common share, basic and diluted (unaudited) |
48,961,439 | ||||||
The accompanying notes are an integral part of these financial statements.
F-4
VERACYTE, INC.
Statements of Convertible Preferred Stock and Stockholders' Deficit
(In thousands, except share and per share amounts)
| |
|
|
|
|
|
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Convertible Preferred Stock |
|
|
|
|
|
||||||||||||||||
| |
Common Stock | |
|
|
||||||||||||||||||
| |
Additional Paid-in Capital |
Accumulated Deficit |
Total Stockholders' Deficit |
|||||||||||||||||||
| |
Shares | Amount | Shares | Amount | ||||||||||||||||||
Balance–January 1, 2011 |
30,249,334 | $ | 30,674 | 2,227,387 | $ | 2 | $ | 162 | $ | (26,975 | ) | $ | (26,811 | ) | ||||||||
Issuance of Series B convertible preferred stock in February 2011 for cash at $1.25 per share, net of issuance costs of $1 |
7,449,335 |
9,311 |
– |
– |
– |
– |
– |
|||||||||||||||
Issuance of Series B convertible preferred stock in July 2011 for cash at $1.25 per share, net of issuance costs of $1 |
7,449,330 | 9,311 | – | – | – | – | – | |||||||||||||||
Common stock issued on exercise of common stock options |
– | – | 152,395 | – | 24 | – | 24 | |||||||||||||||
Stock-based compensation expense (employee) |
– | – | – | – | 378 | – | 378 | |||||||||||||||
Stock-based compensation expense (non-employee) |
– | – | – | – | 88 | – | 88 | |||||||||||||||
Net loss and comprehensive loss |
– | – | – | – | – | (14,445 | ) | (14,445 | ) | |||||||||||||
Balance–December 31, 2011 |
45,147,999 | 49,296 | 2,379,782 | 2 | 652 | (41,420 | ) | (40,766 | ) | |||||||||||||
Issuance of Series C convertible preferred stock in November and December 2012 for cash at $1.89 per share, net of issuance costs of $63 and $861 preferred stock liability |
7,936,508 |
14,076 |
– |
– |
– |
– |
– |
|||||||||||||||
Common stock issued on exercise of common stock options |
– | – | 290,985 | 1 | 75 | – | 76 | |||||||||||||||
Stock-based compensation expense (employee) |
– | – | – | – | 590 | – | 590 | |||||||||||||||
Stock-based compensation expense (non-employee) |
– | – | – | – | 85 | – | 85 | |||||||||||||||
Equity-based compensation |
– | – | – | – | 193 | – | 193 | |||||||||||||||
Net loss and comprehensive loss |
– | – | – | – | – | (18,649 | ) | (18,649 | ) | |||||||||||||
Balance–December 31, 2012 |
53,084,507 | $ | 63,372 | 2,670,767 | $ | 3 | $ | 1,595 | $ | (60,069 | ) | $ | (58,471 | ) | ||||||||
|
||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
VERACYTE, INC.
Statements of Cash Flows
(In thousands)
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Operating activities |
|||||||
Net loss |
$ | (14,445 | ) | $ | (18,649 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||
Depreciation and amortization |
611 | 706 | |||||
Bad debt expense |
235 | 225 | |||||
Loss on write-off of property and equipment |
215 | – | |||||
Genzyme co-promotion fee amortization |
– | (2,386 | ) | ||||
Stock-based compensation |
466 | 675 | |||||
Equity-based compensation |
193 | 259 | |||||
Change in value of preferred stock liability |
(719 | ) | (278 | ) | |||
Changes in operating assets and liabilities: |
|||||||
Accounts receivable |
(463 | ) | (565 | ) | |||
Supplies inventory |
(143 | ) | (771 | ) | |||
Prepaid expenses and current other assets |
(117 | ) | (191 | ) | |||
Other assets |
(1 | ) | (119 | ) | |||
Accounts payable |
116 | 1,348 | |||||
Accrued liabilities and deferred rent |
528 | 2,579 | |||||
Deferred Genzyme co-promotion fee |
– | 10,000 | |||||
Net cash used in operating activities |
(13,524 | ) | (7,167 | ) | |||
Investing activities |
|||||||
Purchases of property and equipment |
(276 | ) | (1,462 | ) | |||
Change in restricted cash |
(55 | ) | – | ||||
Net cash used in investing activities |
(331 | ) | (1,462 | ) | |||
Financing activities |
|||||||
Proceeds from issuance of convertible preferred stock, net of issuance costs |
18,622 | 14,989 | |||||
Proceeds from the exercise of common stock options |
24 | 76 | |||||
Net cash provided by financing activities |
18,646 | 15,065 | |||||
Net increase in cash and cash equivalents |
4,791 | 6,436 | |||||
Cash and cash equivalents at beginning of period |
2,775 | 7,566 | |||||
Cash and cash equivalents at end of period |
$ | 7,566 | $ | 14,002 | |||
Supplementary cash flow information of non-cash investing and financing activities: |
|||||||
Purchases of property and equipment included in accounts payable and accrued liabilities |
$ | 106 | $ | 109 | |||
Preferred stock liability |
$ | – | $ | 861 | |||
Convertible preferred stock issuance costs included in accounts payable |
$ | – | $ | 52 | |||
Transfer of equity-based compensation from liabilities to equity |
$ | – | $ | 193 | |||
The accompanying notes are an integral part of these financial statements.
F-6
VERACYTE, INC.
Notes to Audited Financial Statements
1. Organization and Description of Business
Veracyte, Inc. (the "Company") was incorporated in the state of Delaware on August 15, 2006 as Calderome, Inc. Calderome operated as an incubator until early 2008. On March 4, 2008, the Company changed its name to Veracyte, Inc. Veracyte is a diagnostics company pioneering the field of molecular cytology to improve patient outcomes and lower healthcare costs. The Company specifically targets diseases that often require invasive procedures for an accurate diagnosis – diseases where many healthy patients undergo costly interventions that ultimately prove unnecessary. The Company improves the accuracy of diagnosis at an earlier stage of patient care by deriving clinically actionable genomic information from cytology samples collected in an outpatient setting. The Company's first commercial solution, the Afirma Thyroid FNA Analysis, includes as its centerpiece the Gene Expression Classifier ("GEC"). The GEC helps physicians reduce the number of unnecessary surgeries by employing a proprietary 142-gene signature to preoperatively determine whether thyroid nodules previously classified by cytopathology as indeterminate can be reclassified as benign. The Company's operations are based in South San Francisco, California and Austin, Texas, and it operates in one segment.
2. Summary of Significant Accounting Policies
Basis of Presentation
The Company's financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America ("U.S. GAAP").
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company has incurred significant losses and negative cash flows from operations. At December 31, 2012, the Company had an accumulated deficit of $60.1 million and cash and cash equivalents of $14.0 million. As discussed in Note 14–Subsequent Events, the Company raised $13.0 million in gross proceeds from the issuance of Series C Preferred Stock in June 2013 and entered into a $10.0 million loan and security agreement under which the Company has drawn $5.0 million. The Company's management believes that its currently available resources, including the funds obtained from the preferred stock and debt transactions, will provide sufficient funds to enable the Company to meet its obligations through at least December 31, 2013. However, if the Company's anticipated operating results are not achieved in future periods, planned expenditures may need to be reduced in order to extend the time period over which the then-available resources would be able to fund the Company's operations. The Company will need to raise additional capital to fully implement its business plan. Additional funding may not be available to the Company on acceptable terms, or at all.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Significant items subject to such estimates include: revenue recognition; allowance for doubtful accounts; the useful lives of property and equipment; the recoverability of long-lived assets; the determination of fair value of the Company's common stock, stock options, preferred stock liability; income tax uncertainties, including a valuation allowance for deferred tax assets; and contingencies. The Company bases these estimates on historical and anticipated results, trends, and various other assumptions that the Company believes are reasonable under the circumstances, including assumptions as to future events. These estimates form the basis for making judgments about the
F-7
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
carrying values of assets and liabilities and recorded revenue and expenses that are not readily apparent from other sources. Actual results could differ from those estimates and assumptions.
Concentrations of Credit Risk and Other Risks and Uncertainties
The Company's cash and cash equivalents are deposited with one major financial institution in the United States of America. Deposits in this institution may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
Several of the components of the Company's sample collection kit and test reagents are obtained from single-source suppliers. If these single-source suppliers fail to satisfy the Company's requirements on a timely basis, it could suffer delays in being able to deliver its diagnostic solution, a possible loss of revenue, or incur higher costs, any of which could adversely affect its operating results.
The Company is also subject to credit risk from its accounts receivable related to its sales of Afirma. The Company generally does not perform evaluations of customers' financial condition and generally does not require collateral. All of the Company's accounts receivables are derived from sales of Afirma in the United States.
As of December 31, 2012, all of the Company's revenue is derived from the sale of Afirma. The Company's solution to date has been delivered primarily to physicians in the United States. The Company's significant third-party payers and their related revenue as a percentage of revenue are as follows:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Medicare |
38% | 34% | |||||
Aetna |
14% | 13% | |||||
UnitedHealthcare |
13% | 12% | |||||
|
65% | 59% | |||||
Accounts receivable from Medicare amounted to 34% and 87% of gross accounts receivable as of December 31, 2011 and 2012. No other third-party payer represented more than 10% of the Company's revenue or accounts receivable balances for these periods.
Cash Equivalents
Cash equivalents consist of short-term, highly liquid investments with original maturities of three months or less from the date of purchase. Cash equivalents consist primarily of amounts invested in money market accounts.
Restricted Cash
As of December 31, 2011 and 2012, deposits of $168,000 were restricted from withdrawal and held by a bank in the form of certificates of deposit and collateral for letters of credit. The balance as of December 31, 2011 and 2012 consists of a certificate of deposit of $50,000 held as collateral for payment of
F-8
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
the Company's credit cards and a letter of credit totaling $118,000 which is related to security for the lease of the Company's office space.
Allowance for Doubtful Accounts
The Company accrues an allowance for doubtful accounts against its accounts receivable based on estimates consistent with historical collection experience in relation to the amounts billed. Bad debt expense is included in general and administrative expense on the Company's statements of operations and comprehensive loss. Accounts receivable are written off against the allowance when the claims appeals process is exhausted or when there is other substantive evidence that the account will not be paid.
| |
As of December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
| |
(In thousands) |
||||||
Beginning balance |
$ | – | $ | 235 | |||
Charged to expense |
235 | 225 | |||||
Write-offs, net of recoveries |
– | (238 | ) | ||||
Ending balance |
$ | 235 | $ | 222 | |||
Supplies Inventory
Supplies inventory consists of test reagents and other consumables used in the sample collection kits and in the GEC and are valued at the lower of cost or market value. Cost is determined using actual costs on a first-in, first-out basis.
Property and Equipment
Property and equipment are stated at cost less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally between three and five years. Leasehold improvements are amortized using the straight-line method over the shorter of the estimated useful life of the asset or the term of the lease. Maintenance and repairs are charged to expense as incurred, and improvements and betterments are capitalized. When assets are retired or otherwise disposed of, the cost and accumulated depreciation are removed from the balance sheet and any resulting gain or loss is reflected in the statements of operations and comprehensive loss in the period realized.
Internal-use Software
The Company capitalizes third-party costs incurred in the application development stage to design and implement the software used in the GEC. Costs incurred in the development of application of the software are capitalized and amortized over an estimated useful life of three years on a straight line basis.
During the years ended December 31, 2011 and 2012, the Company capitalized $0 and $173,000 of software development costs. During the years ended December 31, 2011 and 2012, the Company wrote-off $215,000 and $0 of capitalized software costs to research and development expenses. Amortization expense totaled $16,000 and $47,000, for the years ended December 31, 2011 and 2012, respectively. Capitalized
F-9
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
software is included in property and equipment, and had a net book value of $58,000 and $184,000 and as of December 31, 2011 and 2012, respectively.
Long-lived Assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. An impairment loss is recognized when the total of estimated future undiscounted cash flows, expected to result from the use of the asset and its eventual disposition, are less than its carrying amount. Impairment, if any, would be assessed using discounted cash flows or other appropriate measures of fair value. There were no impairments for the years ended December 31, 2011 and 2012.
Bonus Accruals
The Company accrues for liabilities under discretionary employee and executive bonus plans. These estimated compensation liabilities are based on progress against corporate objectives approved by the Board of Directors, compensation levels of eligible individuals, and target bonus percentage levels. The Board of Directors and the Compensation Committee of the Board of Directors review and evaluate the performance against these objectives and ultimately determine what discretionary payments are made. As of December 31, 2011 and 2012, the Company accrued $407,000 and $671,000, respectively, for liabilities associated with these employee and executive bonus plans. As more fully discussed in Note 11 to the financial statements, a portion of the bonus accruals was settled with equity awards issued subsequent to year end.
Fair Value of Financial Instruments
The carrying amounts of certain financial instruments including cash and cash equivalents, accounts receivable, prepaid expenses and other current assets, accounts payable and accrued liabilities approximate fair value due to their relatively short maturities.
Revenue Recognition
The Company's revenue is generated from the provision of diagnostic services using its Afirma solution; the Company's service is completed upon the delivery of test results to the prescribing physician which triggers the billing for the service. The Company recognizes revenue related to billings for commercial carriers or governmental programs subject to contractual arrangements and when there is a predictable pattern of collectability on an accrual basis, net of contractual adjustments. These contractual adjustments represent the difference between the list price (the billing rate) and the reimbursement rate set by commercial or governmental payers. Until a contract has been negotiated with a commercial carrier or governmental program, the Afirma solution may or may not be covered by these entities' existing reimbursement policies. In addition, patients do not enter into direct agreements with us that commit them to pay any portion of the cost of the tests in the event that their insurance declines to reimburse the Company. In the absence of an agreement or other clearly enforceable legal right to demand payment, when test services are provided to patients with non-contracted insurance carriers or no insurance, the related revenue is only recognized upon the earlier of payment notification, if applicable, or cash receipt.
F-10
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
For all services performed, the Company considers whether or not the following revenue recognition criteria are met: persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed or determinable; and collectability is reasonably assured.
Persuasive evidence of an arrangement exists and delivery is deemed to have occurred upon delivery of a patient report to the prescribing physician. The assessment of the fixed or determinable nature of the fees charged for diagnostic testing performed and the collectability of those fees require significant judgment by management. Management believes that these two criteria have been met when there is contracted reimbursement coverage and/or a predictable pattern of collectability with individual third-party payers and accordingly, recognizes revenue upon delivery of the patient report. Some patients have out-of-pocket costs for amounts not covered by their insurance carrier, and the Company may bill the patient directly for these amounts in the form of co-payments and co-insurance in accordance with their insurance carrier and health plans. Some payers may not cover the Company's GEC as ordered by the prescribing physician under their reimbursement policies. The Company pursues reimbursement from such patients on a case-by-case basis. In the absence of contracted reimbursement coverage or a predictable pattern and history of collectability, the Company believes that the fee is fixed or determinable and collectability is reasonably assured only upon receipt of third-party payer notification of payment or when cash is received and accordingly, recognizes revenue at that time.
Cost of Revenue
Cost of revenue is expensed as incurred and includes material and service costs related to the initial cytopathology testing performed by a third-party pathology group, direct labor costs, equipment and infrastructure expenses associated with testing tissue samples, shipping charges to transport samples, and allocated overhead including rent, information technology, equipment depreciation and utilities.
Research and Development
Research and development costs are charged to operations as incurred. Research and development costs include, but are not limited to, payroll and personnel-related expenses, stock-based compensation expense, prototype materials, laboratory supplies, consulting costs, and allocated overhead including rent, information technology, equipment depreciation and utilities.
Income Taxes
The Company accounts for income taxes under the liability method. Under this method, deferred tax assets and liabilities are determined based on the difference between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to affect taxable income. Valuation allowances are established when necessary to reduce deferred tax assets to the amounts expected to be realized.
The Company assesses all material positions taken in any income tax return, including all significant uncertain positions, in all tax years that are still subject to assessment or challenge by relevant taxing authorities. The Company's assessment of an uncertain tax position begins with the initial determination of the position's sustainability and is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. As of each balance sheet date, unresolved uncertain tax positions must be reassessed, and the Company will determine whether (i) the factors underlying the sustainability assertion have changed and (ii) the amount of the recognized tax benefit is still appropriate.
F-11
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
2. Summary of Significant Accounting Policies (Continued)
The recognition and measurement of tax benefits requires significant judgment. Judgments concerning the recognition and measurement of a tax benefit may change as new information becomes available.
Stock-based Compensation
Stock-based compensation expense for equity instruments issued to employees is measured based on the grant-date fair value of the awards. The fair value of each employee stock option is estimated on the date of grant using the Black-Scholes option-pricing valuation model. The Company recognizes compensation costs on a straight-line basis for all employee stock based compensation awards that are expected to vest over the requisite service period of the awards, which is generally the awards' vesting period. Forfeitures are required to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from those estimates.
Equity instruments issued to non-employees are valued using the Black-Scholes option-pricing valuation model and are subject to remeasurement as the underlying equity instruments vest.
Net Loss and Unaudited Pro Forma Net Loss per Common Share
Basic net loss per common share is calculated by dividing net loss attributable to common stockholders by the weighted-average number of common shares outstanding during the period, without consideration of common stock equivalents. Diluted net loss per common share is computed by dividing net loss attributable to common stockholders by the weighted-average number of common share equivalents outstanding for the period determined using the treasury stock method. Potentially dilutive securities consisting of convertible preferred stock and options to purchase common stock are considered to be common stock equivalents and were excluded from the calculation of diluted net loss per common share because their effect would be antidilutive for all periods presented. In contemplation of an initial public offering, the Company has presented the unaudited pro forma basic and diluted net loss per common share which has been computed to give effect to the conversion of the convertible preferred stock into common stock.
Recent Accounting Pronouncements
In June 2011, the Financial Accounting Standards Board ("FASB") issued authoritative guidance requiring companies to present items of net income, items of other comprehensive income and total comprehensive income in one continuous statement or two consecutive statements. This guidance eliminates the option for companies to present other comprehensive income in the statement of stockholders' equity. The Company adopted this guidance as of January 1, 2012. As this guidance provides only presentation requirements, the adoption of this guidance did not impact the Company's financial condition or results of operations.
In May 2011, the FASB issued authoritative guidance to achieve common fair value measurement and disclosure requirements between U.S. GAAP and International Financial Reporting Standards. This new literature amends current fair value measurement and disclosure guidance to include increased transparency around valuation inputs and investment categorization. The guidance is effective for fiscal years and interim periods beginning after December 15, 2011. The Company adopted this standard in January 2012, as reflected in Note 5 to the financial statements.
F-12
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
3. Net Loss Per Common Share
The following table presents the calculation of basic and diluted net loss per common share for the years ended December 31, 2011 and 2012 (in thousands, except share and per share amounts):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Net loss |
$ | (14,445 | ) | $ | (18,649 | ) | |
Shares used to compute net loss per common share, basic and diluted |
2,320,252 | 2,601,352 | |||||
Net loss per common share, basic and diluted |
$ | (6.23 | ) | $ | (7.17 | ) | |
The following outstanding shares of common stock equivalents have been excluded from diluted net loss per common share for the years ended December 31, 2011 and 2012 because their inclusion would be anti-dilutive:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Shares of common stock subject to outstanding options |
5,718,952 | 8,910,706 | |||||
Shares of common stock subject to conversion from preferred stock |
45,147,999 | 53,084,507 | |||||
Total shares of common stock equivalents |
50,866,951 | 61,995,213 | |||||
The following table sets forth the computation of the Company's unaudited pro forma basic and diluted net loss per common share after giving effect to the conversion of convertible preferred stock using the as-if converted method into common stock as though the conversion had occurred at the beginning of the year ended December 31, 2012 (in thousands, except share and per share amounts):
| |
Year Ended December 31, 2012 |
|||
|---|---|---|---|---|
| |
(Unaudited) |
|||
Net loss |
$ | (18,649 | ) | |
Shares used to compute net loss per common share, basic and diluted |
2,601,352 | |||
Pro forma adjustments to reflect assumed conversion of convertible preferred stock |
46,360,087 | |||
Shares used to compute pro forma net loss per common share, basic and diluted |
48,961,439 | |||
Pro forma net loss per common share, basic and diluted |
$ | (0.38 | ) | |
F-13
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
4. Balance Sheet Components
Property and Equipment, Net
Property and equipment consisted of the following (in thousands):
| |
As of December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Leasehold improvements |
$ | 328 | $ | 341 | |||
Laboratory equipment |
1,658 | 2,061 | |||||
Computer equipment |
371 | 526 | |||||
Software, including software developed for internal use |
302 | 554 | |||||
Furniture and fixtures |
54 | 81 | |||||
Construction-in-process |
84 | 699 | |||||
Total property and equipment, gross |
2,797 | 4,262 | |||||
Accumulated depreciation and amortization |
(1,110 | ) | (1,816 | ) | |||
Total property and equipment, net |
$ | 1,687 | $ | 2,446 | |||
Depreciation and amortization expense was $611,000 and $706,000 for the years ended December 31, 2011 and 2012, and was recorded in the statements of operations and comprehensive loss as follows (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Cost of revenue |
$ | 397 | $ | 401 | |||
Research and development |
162 | 184 | |||||
Selling and marketing |
21 | 46 | |||||
General and administrative |
31 | 75 | |||||
Total depreciation and amortization expense |
$ | 611 | $ | 706 | |||
Accrued Liabilities
Accrued liabilities consisted of the following (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Accrued compensation expenses |
$ | 787 | $ | 1,360 | |||
Accrued consulting fees |
93 | 28 | |||||
Accrued legal and professional fees |
123 | 84 | |||||
Accrued other |
213 | 373 | |||||
Accrued Genzyme co-promotion fees |
– | 2,175 | |||||
Deferred rent–short-term |
120 | – | |||||
Total accrued liabilities |
$ | 1,336 | $ | 4,020 | |||
F-14
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
5. Fair Value Measurements
The Company records its financial assets and liabilities at fair value. The carrying amounts of certain financial instruments of the Company, including cash and cash equivalents, prepaid expenses and other current assets, accounts payable and accrued liabilities, approximate fair value due to their relatively short maturities. The accounting guidance for fair value provides a framework for measuring fair value, clarifies the definition of fair value, and expands disclosures regarding fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The accounting guidance establishes a three-tiered hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value as follows:
- •
- Level I: Inputs which include quoted prices in active markets for identical assets and liabilities.
- •
- Level II: Inputs other than Level I that are observable, either directly or indirectly, such as quoted
prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full
term of the assets or liabilities.
- •
- Level III: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The following table sets forth the fair value of the Company's financial assets and liabilities measured on a recurring basis, as of December 31, 2011 and 2012 (in thousands):
| |
As of December 31, 2011 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level I | Level II | Level III | Total | |||||||||
Financial Assets: |
|||||||||||||
Money market funds |
$ | 7,344 | $ | – | $ | – | $ | 7,344 | |||||
Total financial assets |
$ | 7,344 | $ | – | $ | – | $ | 7,344 | |||||
| |
As of December 31, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level I | Level II | Level III | Total | |||||||||
Financial Assets: |
|||||||||||||
Money market funds |
$ | 12,830 | $ | – | $ | – | $ | 12,830 | |||||
Total financial assets |
$ | 12,830 | $ | – | $ | – | $ | 12,830 | |||||
Financial Liabilities: |
|||||||||||||
Preferred stock liability |
$ | – | $ | – | $ | 583 | $ | 583 | |||||
Total financial liabilities |
$ | – | $ | – | $ | 583 | $ | 583 | |||||
F-15
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
5. Fair Value Measurements (Continued)
The Company's Level 3 liabilities consist of a preferred stock liability (see Note 9). The following table sets forth a summary of the changes in the fair value of the Company's Level 3 financial liabilities, which are measured on a recurring basis:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
| |
(In thousands) |
||||||
Beginning balance |
$ | 719 | $ | – | |||
Fair value of preferred stock liability |
– | 861 | |||||
Change in fair value of preferred stock liability recorded in other income (expense), net |
(719 | ) | (278 | ) | |||
Ending balance |
$ | – | $ | 583 | |||
6. Genzyme Co-promotion Agreement
In May 2011, the Company received $100,000 from Genzyme Corporation ("Genzyme") in connection with an extension of an exclusive right to negotiate a co-promotion agreement.
In January 2012, the Company and Genzyme executed a co-promotion agreement for the co-exclusive rights and license to promote and market the Company's Afirma thyroid cancer solution in the United States and in 40 named countries. In exchange, the Company received a $10.0 million co-promotion fee from Genzyme in February 2012. The Company may receive an additional $3.0 million in payments, $600,000 for each country outside of the United States in which the Company obtains marketing authorization and achieves a specified level of reimbursement, for up to five countries. Under the terms of the agreement, Genzyme will receive a percentage of cash receipts that the Company has received related to Afirma as co-promotion fees. The percentage was 50% in 2012 and will decrease to 40% in January 2013 and will further decrease to 32% in March 2014 and thereafter. Genzyme will also spend up to $500,000 for qualifying clinical development activities in countries that require additional testing for approval. This obligation expires in July 2014. The agreement expires in January 2027 and either party may terminate the agreement at any time and with six months prior notice. The Company is amortizing the co-promotion fee over a four-year period, which is management's best estimate of the life of the agreement, in part because after that period either party may terminate the agreement without penalty. The Company amortized $2.4 million in the year ended December 31, 2012, which is reflected as a reduction to selling and marketing expenses in the statements of operations and comprehensive loss. The unamortized balance of the co-promotion fee is $7.6 million as of December 31, 2012.
7. Thyroid Cytology Partners
In 2010, the Company entered into an arrangement with Pathology Resource Consultants, P.A. ("PRC") to set-up and manage a specialized pathology practice to provide testing services to the Company. There is no direct monetary compensation from the Company to PRC as a result of this arrangement. The Company's service agreement with the specialized pathology practice, Thyroid Cytology Partners ("TCP"), is effective through December 31, 2015, unless terminated earlier, and renews annually thereafter. Under the service agreement, Veracyte pays TCP based on a fixed price per test schedule, which is reviewed periodically for changes in market pricing. Subsequent to December 2012, an amendment to the service agreement allows TCP to use a portion of Veracyte's facility in Austin, Texas. TCP will reimburse the Company for a proportionate share of the Company's rent and related operating expense costs for the
F-16
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
7. Thyroid Cytology Partners (Continued)
leased facility. The Company does not have an ownership interest in or provide any form of financial or other support to TCP.
The Company has concluded that TCP represents a variable interest entity and that the Company is not the primary beneficiary as it does not have the ability to direct the activities that most significantly impact TCP's economic performance. Therefore, the Company does not consolidate TCP. All amounts paid to TCP under the service agreement are expensed as incurred and included in cost of revenue. All amounts to be received from TCP will be recorded in the same period as the corresponding lease costs.
TCP provided $434,000 and $1.8 million in cytopathology testing and evaluation services in the years ended December 31, 2011 and 2012, respectively. The Company also reimbursed TCP for licensure fees of $83,000 and $137,000 in the years ended December 31, 2011 and 2012, respectively. Expenses for testing and evaluation services and reimbursed professional licensure fees are included in cost of revenue in the statements of operations and comprehensive loss. The Company's outstanding obligations to TCP were $134,000 and $458,000 as of December 31, 2011 and 2012, respectively, which were included in accounts payable in the Company's balance sheets.
8. Commitments and Contingencies
Operating Leases
The Company leases its headquarters and South San Francisco laboratory facilities under a non-cancelable lease agreement that expired March 31, 2013. The lease was amended in July 2012 to extend the term to March 31, 2016 and to provide tenant improvement allowances of up to $253,000. The Company provided security deposits in the form of irrevocable standby letters of credit secured with restricted cash deposits at the Company's primary bank. The Company deposited $118,000 in restricted cash accounts as collateral for the lease which is included in restricted cash in the Company's balance sheets as of December 31, 2011 and 2012.
In November 2012, the Company entered into a non-cancelable lease agreement commencing February 1, 2013 to lease laboratory space in Austin, Texas. The lease expires on July 31, 2018. The Company paid a cash security deposit of $75,000, which is included in other assets in the Company's balance sheet as of December 31, 2012.
Future minimum lease payments under non-cancellable operating leases as of December 31, 2012 are as follows (in thousands):
Year Ending December 31,
|
Amounts | |||
|---|---|---|---|---|
2013 |
$ | 816 | ||
2014 |
938 | |||
2015 |
989 | |||
2016 |
413 | |||
2017 |
222 | |||
Thereafter |
130 | |||
Total minimum lease payments |
$ | 3,508 | ||
The Company recognizes rent expense on a straight-line basis over the non-cancellable lease period. Facilities rent expense was $570,000 and $711,000 and for the years ended December 31, 2011 and 2012, respectively.
F-17
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
8. Commitments and Contingencies (Continued)
Contingencies
From time to time, the Company may be involved in legal proceedings arising in the ordinary course of business. The Company believes there is no litigation pending that could have, individually or in the aggregate, a material adverse effect on the financial position, results of operations or cash flows.
9. Convertible Preferred Stock
Convertible preferred stock as of December 31, 2011 and 2012 consists of the following (in thousands, except for share data):
| |
Shares Authorized | Original Issue Price |
Shares Issued and Outstanding |
Aggregate Liquidation Amount |
Proceeds Net of Issuance Costs and Preferred Stock Liability |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Series A |
22,399,999 | $ | 1.00 | 22,399,999 | $ | 22,400 | $ | 22,328 | ||||||||
Series B |
22,748,000 | 1.25 | 22,748,000 | 28,435 | 26,968 | |||||||||||
Balance at December 31, 2011 |
45,147,999 | 45,147,999 | 50,835 | 49,296 | ||||||||||||
Series A |
22,399,999 |
$ |
1.00 |
22,399,999 |
22,400 |
22,328 |
||||||||||
Series B |
22,748,000 | 1.25 | 22,748,000 | 28,435 | 26,968 | |||||||||||
Series C |
14,000,000 | 1.89 | 7,936,508 | 15,000 | 14,076 | |||||||||||
Balance at December 31, 2012 |
59,147,999 | 53,084,507 | $ | 65,835 | $ | 63,372 | ||||||||||
In June 2010, the Company recorded a preferred stock liability as the investors received the right to purchase from the Company, on the same terms, additional shares of Series B convertible preferred stock, in a second and third tranche. As the investors hold a majority of the seats on the Board of Directors, the decision to complete the second and third tranche were deemed to be outside the control of the Company. The Company recorded a preferred stock liability of $1.4 million for the fair value of the Company's obligation to sell the convertible preferred stock for the second and third tranche of Series B convertible preferred stock. The preferred stock liability was valued using the option-pricing method with the following assumptions: 100% probability of success of the second and third tranches, a term of 0.75 years for the second tranche and 1.59 years for the third tranche, a risk-free rate of 0.3% for the second tranche and 0.7% for the third tranche, and volatility of 43.8% for the second tranche and 45.1% for the third tranche. This resulted in an initial fair value of $0.5 million for the second tranche and $0.9 million for the third tranche for the Company's obligation to sell the convertible preferred stock. At year end 2010, a change in value of the liability of $0.7 million was recorded to other income (expense), net. In February 2011 and June 2011, the Company issued 7,449,335 and 7,449,330 shares of Series B convertible preferred stock, respectively, at $1.25 per share for aggregate net proceeds of $18.6 million, in the second and third tranche of the Series B financing. With the issuance of the Series B convertible preferred stock, the Company recorded $0.7 million to other income (expense) related to the change in value of the preferred stock liability before retirement of the preferred stock liability in 2011.
In November 2012, the Company entered into a Series C Preferred Stock Purchase Agreement (the "Series C Agreement"). Under the Series C Agreement, the Company authorized the issuance and sale of an aggregate of 13,227,513 shares of its Series C convertible preferred stock, which may be sold in three
F-18
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
9. Convertible Preferred Stock (Continued)
closings: 7,910,053 shares in the initial purchase (the "Initial Closing"), 5,291,005 shares in the second closing ("the Second Closing"), and 26,455 shares in an additional closing (the "First Additional Closing").
The Initial Closing of the Series C convertible preferred stock occurred in November 2012 and the First Additional Closing in December 2012. In the Initial Closing and the First Additional Closing, the Company issued an aggregate of 7,936,508 shares of its Series C convertible preferred stock at a price per share of $1.89 for gross proceeds of $15.0 million.
Following the written confirmation from the Company and the holders of at least 662/3% of the then outstanding shares of Series C convertible preferred stock purchased pursuant to the Series C Agreement, a Second Closing will take place provided that the written confirmation of the Second Closing occurs on or before the 12 month anniversary of the Initial Closing. The total number of shares that may be sold in the second closing is 5,291,005, which at a price per share of $1.89 would result in total gross proceeds of $10.0 million. In November 2012, the Company recorded a preferred stock liability as the investors received the right to purchase from the Company, on the same terms, additional shares of Series C convertible preferred stock, in a second tranche. As the investors hold a majority of the board seats, the decision to complete the second tranche was deemed to be outside the control of the Company. The preferred stock liability was valued using the option-pricing method with the following assumptions: 100% probability of success of the second tranche, fair value of Series C preferred stock of $1.78, a term of 0.67 years and expected volatility of 44%. This resulted in an initial fair value of $0.9 million for the Company's obligation to sell the convertible preferred stock. At December 31, 2012, the Company revalued the preferred stock liability to $0.6 million and recorded other income (expense), net of $0.3 million related to the change in value of the liability through that date.
In June 2013, the Company completed the second tranche, see Note 14–Subsequent Events.
The rights, preferences and privileges of the Series A, Series B and Series C convertible preferred stock are as follows:
Dividends
The holders of the outstanding shares of Series A, Series B and Series C convertible preferred stock are entitled to receive, when and if declared by the Board of Directors, a non-cumulative cash dividend at the rate of eight percent (8%) of the applicable original issue price per annum on each outstanding share of Series A, Series B and Series C convertible preferred stock. Such dividends are payable in preference to any dividends for common stock declared by the Board of Directors. No dividends have been declared to date.
Conversion Rights
Each share of Series A, Series B and Series C convertible preferred stock is, at the option of the holder, convertible into the number of fully paid and non-assessable shares of common stock as determined by dividing the original issue price applicable to such convertible preferred stock by the conversion price in effect at that time. The conversion price for each series preferred stock shall initially be the original issue price of such series of preferred stock and shall be adjusted in accordance with conversion provision contained in the Company's Amended and Restated Certificate of Incorporation.
Each share of convertible preferred stock will be automatically be converted into shares of common stock based on the then effective conversion price (i) upon the affirmative election of the holders of at
F-19
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
9. Convertible Preferred Stock (Continued)
least a majority of the outstanding shares of the convertible preferred stock or (ii) immediately upon the closing of a firmly underwritten public offering filed under the Securities Act of 1933, as amended, covering the offer and sale of common stock for the account of the Company in which the gross cash proceeds to the Company are at least $40 million.
Voting Rights
Each holder is entitled to the number of votes equal to the number of shares of common stock into which the shares of preferred stock could be converted.
Liquidation Rights
Upon liquidation, dissolution, or winding down of the Company, the holders of the convertible preferred stock shall be entitled to receive, prior and in preference to any distribution of any of the assets or surplus funds of the Company to the holders of shares of common stock, an amount equal to the per share issue price of such series of preferred stock ($1.00 per share for Series A convertible preferred stock, $1.25 per share for Series B convertible preferred stock, and $1.89 per share for Series C convertible preferred stock), plus all declared and unpaid dividends on such shares (the "liquidation preference"). If available assets are insufficient to pay the full liquidation preference, the available assets will be distributed among the holders of the convertible preferred stock, on a pari passu and pro rata basis. After the payment of the liquidation preference, all remaining assets available for distribution will be distributed ratably among the holders of the common stock.
Other
The Company recorded the convertible preferred stock at fair value on the dates of issuance, net of issuance costs. The Company classifies the convertible preferred stock outside of stockholders' equity because the shares contain liquidation features that are not solely within its control. During the years ended December 31, 2011 and 2012, the Company did not adjust the carrying values of the convertible preferred stock to the deemed redemption values of such shares since a liquidation event was not probable. Subsequent adjustments to increase the carrying values to the ultimate redemption values will be made only when it becomes probable that such a liquidation event will occur.
10. Stockholders' Deficit
Common Stock
The Company's Certificate of Incorporation, as amended November 5, 2012, authorizes the Company to issue 77,000,000 shares of common stock with a par value of $0.001 per share. The holder of each share of common stock shall have one vote for each share of stock. The common stockholders are also entitled to receive dividends whenever funds and assets are legally available and when declared by the Board of Directors, subject to the prior rights of holders of all series of convertible preferred stock outstanding. No dividends have been declared as of December 31, 2012.
F-20
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
10. Stockholders' Deficit (Continued)
As of December 31, 2011 and 2012, the Company had reserved shares of common stock, on an as-if converted basis, for issuance as follows:
| |
As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Conversion of Series A convertible preferred stock |
22,399,999 | 22,399,999 | |||||
Conversion of Series B convertible preferred stock |
22,748,000 | 22,748,000 | |||||
Conversion of Series C convertible preferred stock |
– | 7,936,508 | |||||
Conversion of Series C convertible preferred stock reserved for issuance |
– | 5,291,005 | |||||
Options issued and outstanding |
5,718,952 | 8,910,706 | |||||
Options available for grant under stock option plan |
1,899,834 | 1,389,495 | |||||
Total |
52,766,785 | 68,675,713 | |||||
11. Stock Incentive Plan
Stock Option Plan
On February 15, 2008, the Company adopted the 2008 Stock Plan (the "2008 Plan"). The 2008 Plan provides for the granting of options to purchase common stock and common stock to employees, directors and consultants of the Company. The Company may grant incentive stock options ("ISOs"), non-statutory stock options ("NSOs") or restricted stock under the 2008 Plan. ISOs may only be granted to Company employees (including directors who are also considered employees). NSOs and restricted stock may be granted to Company employees, directors and consultants.
Options under the 2008 Plan may be granted for terms of up to ten years from the date of grant, as determined by the Board of Directors, provided however, that with respect to an ISO granted to a person who owns stock representing more than 10% of the voting power of all classes of stock of the Company, the term shall be for no more than five years from the date of grant.
The exercise price of options granted under the 2008 Plan must be at a price no less than 100% of the estimated fair value of the shares on the date of grant, as determined by the Board of Directors, provided however, that with respect to an ISO granted to an employee who at the time of grant of such option owns stock representing more than 10% of the voting power of all classes of stock of the Company, the exercise price shall not be less than 110% of the estimated fair value of the shares on the date of grant.
Options granted under the 2008 Plan to newly hired employees generally vest over four years (generally 25% after one year and monthly thereafter). Options granted to employees as part of annual bonus compensation are generally fully vested at the grant date.
F-21
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
11. Stock Incentive Plan (Continued)
Activity under the Company's 2008 Plan is set forth below:
| |
Shares Available for Grant |
Stock Options Outstanding |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
|
|
|
|
(In thousands) |
|||||||||||
Balance–January 1, 2011 |
2,824,999 | 4,946,182 | $ | 0.31 | 8.82 | $ | 1,387 | |||||||||
Granted |
(1,474,500 |
) |
1,474,500 |
0.60 |
||||||||||||
Cancelled |
549,335 | (549,335 | ) | 0.32 | ||||||||||||
Exercised |
– | (152,395 | ) | 0.16 | ||||||||||||
Balance–December 31, 2011 |
1,899,834 | 5,718,952 | 0.39 | 8.22 | 1,221 | |||||||||||
Additional options authorized |
2,972,400 |
– |
||||||||||||||
Granted |
(3,727,795 | ) | 3,727,795 | 0.69 | ||||||||||||
Cancelled |
245,056 | (245,056 | ) | 0.49 | ||||||||||||
Exercised |
– | (290,985 | ) | 0.26 | ||||||||||||
Balance–December 31, 2012 |
1,389,495 | 8,910,706 | 0.52 | 8.17 | 4,311 | |||||||||||
Options exercisable–December 31, 2012 |
4,166,004 | $ | 0.37 | 7.32 | $ | 2,631 | ||||||||||
Options vested and expected to vest–December 31, 2012 |
8,472,770 | $ | 0.51 | 8.13 | $ | 4,156 | ||||||||||
Outstanding and exercisable stock options as of December 31, 2012 are summarized as follows:
| |
Options Outstanding | Options Vested and Exercisable | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exercise Prices
|
Number of Options Outstanding |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
Number of Options Exercisable |
Weighted Average Remaining Contractual Life (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| |
|
|
(In thousands) |
|
|
(In thousands) |
|||||||||||||
$0.02 |
1,183,500 | 5.67 | $ | 1,160 | 1,176,312 | 5.66 | $ | 1,153 | |||||||||||
$0.20 |
973,557 | 7.07 | 779 | 778,711 | 7.04 | 623 | |||||||||||||
$0.59 |
2,091,479 | 7.84 | 857 | 1,313,971 | 7.85 | 539 | |||||||||||||
$0.60 |
934,375 | 8.68 | 374 | 428,382 | 8.68 | 171 | |||||||||||||
$0.67 |
3,458,628 | 9.27 | 1,141 | 438,628 | 9.19 | 145 | |||||||||||||
$1.00 |
269,167 | 9.93 | – | 30,000 | 9.93 | – | |||||||||||||
|
8,910,706 | 8.17 | $ | 4,311 | 4,166,004 | 7.32 | $ | 2,631 | |||||||||||
The aggregate intrinsic value is calculated as the difference between the exercise price of the underlying stock options and the fair value of the Company's common stock for stock options that were in-the-money.
The weighted average fair value of options to purchase common stock granted was $0.42 and $0.49 in the years ended December 31, 2011 and 2012, respectively.
The weighted average fair value of options to purchase common stock vested was $0.25 and $0.35 per share in the years ended December 31, 2011 and 2012. The total estimated grant date fair value of
F-22
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
11. Stock Incentive Plan (Continued)
employee options to purchase common stock vested during the years ended December 31, 2011 and 2012 was $466,000 and $583,000 respectively.
The weighted average fair value of options to purchase common stock exercised was $0.16 and $0.22 in the years ended December 31, 2011 and 2012, respectively. The intrinsic value of options to purchase common stock exercised was $68,000 and $215,000 in the years ended December 31, 2011 and 2012, respectively. The estimated fair value of the Company's common stock as of December 31, 2011 and 2012 was $0.60 and $1.00 per share, respectively.
In February 2008, the Company entered into a restricted stock purchase agreement with a founder. The Company issued 1,396,341 shares of restricted common stock at $0.005 per share, of which 62,060 shares were unvested as of January 1, 2011. These shares had a grant date fair value of $0.015 per share and became fully vested in 2011.
Stock-based Compensation
The Company uses the grant date fair market value of its common stock to value both employee and non-employee options when granted. The Company revalues non-employee options each reporting period using the fair market value of the Company's common stock as of the last day of each reporting period.
Determining Fair Value of Stock Options
The fair value of the shares of common stock underlying stock options has historically been determined by the Board of Directors. Because there has been no public market for the Company's common stock, the Board of Directors has determined fair value of the common stock at the time of grant of the option by considering a number of objective and subjective factors including important developments in the Company's operations, valuations performed by an independent third party, sales of convertible preferred stock, actual operating results and financial performance, the conditions in our industry and the economy in general, the stock price performance of comparable public companies, and the lack of liquidity of the Company's common stock, among other factors. The fair value of the underlying common stock shall be determined by the Board of Directors until such time as the Company's common stock is listed on national stock exchange.
The Black-Scholes option-pricing valuation model is used to determine the fair value of stock options. The input assumptions used to estimate fair value of these awards include the exercise price of the award, the expected option term, the expected volatility of the Company's stock over the option's expected term, the risk-free interest rate over the option's expected term, and the Company's expected dividend yield, if any.
The estimated expected term of options granted is determined by taking the average of the vesting term and the contractual term of each option. As the Company has limited stock price history from which to forecast stock price volatility, it estimates common stock price volatility by calculating the actual average volatility of the common stock of a selected peer group whose share price is publicly available. The Company uses a look-back period commensurate with the expected life of each option award. The risk-free interest rates used in the valuation model are based on U.S. Treasury issues with remaining terms similar to the expected term of the options. The Company does not anticipate paying any dividends in the foreseeable future and therefore used an expected dividend yield of zero.
F-23
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
11. Stock Incentive Plan (Continued)
Summary of Assumptions
The fair value of share-based payments for option granted to employees and directors was estimated on the date of grant using the Black-Scholes option-pricing valuation model based on the following weighted average assumptions:
| |
Year Ended December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Expected term (in years) |
5.00 - 6.08 | 5.00 - 6.08 | |||||
Expected volatility |
70.78 - 80.92% | 82.07 - 84.33% | |||||
Risk-free interest rate |
1.19 - 2.51% | 0.65 - 1.19% | |||||
Dividend yield |
– | – | |||||
Stock-based compensation related to stock options granted to non-employees is recognized as the stock options are earned. The fair value of the stock options granted is calculated at each reporting date using the Black-Scholes option-pricing model with the following assumptions: expected life is equal to the remaining contractual term of the award as of the measurement date ranging from 6.52 years to 9.84 years as of December 31, 2011 and 8.23 years to 9.93 years as of December 31, 2012; risk free rate is 1.23% to 1.86% for the year ended December 31, 2011 and 1.43% to 1.77% for the year ended December 31, 2012; expected dividend yield of 0%; and volatility ranging from 79.35% to 81.62% as of December 31, 2011 and 81.14% to 82.11% as of December 31, 2012.
The following table summarizes stock-based compensation expense related to stock options for the years ended December 31, 2011 and 2012 included in the statements of operations and comprehensive loss as follows (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Cost of revenue |
$ | 32 | $ | 26 | |||
Research and development |
130 | 131 | |||||
Selling and marketing |
77 | 111 | |||||
General and administrative |
227 | 407 | |||||
Total stock-based compensation expense |
$ | 466 | $ | 675 | |||
If all of the remaining non-vested and outstanding stock option awards that have been granted vested, the Company would recognize approximately $1.6 million in compensation expense over a weighted average remaining period of 2.8 years. No compensation expense will be recognized for any stock options that do not vest.
Equity-based Compensation
For the years ended December 31, 2011 and 2012, the Company paid a portion of its executive bonuses through the grant of stock options. The equity transaction associated with these bonuses is classified as equity-based compensation expense. Accruals for the anticipated grants were $193,000 and
F-24
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
11. Stock Incentive Plan (Continued)
$259,000 in the years ended December 31, 2011 and 2012, respectively, and are included in accrued liabilities in the balance sheets. The expenses were determined as follows:
- •
- In March 2012, the Company's Board of Directors authorized the grant of 438,628 fully vested stock options at a fair value
of $0.44 resulting in $193,000 in expense in the year ended December 31, 2011. The option fair value was determined using the Black-Scholes option-pricing valuation model. The option exercise
price was $0.67 as determined by the Company's Board of Directors, the risk free rate was 0.88%, the expected life was 5.0 years, the volatility was determined to be 83.52% and expected
dividend yield of 0%. Upon issuance of the fully vested options, the liability was reclassified into additional paid-in capital.
- •
- In February 2013, the Company's Board of Directors authorized the grant of 402,007 fully vested stock options at a fair value of $0.65 resulting in $259,000 in expense in the year ended December 31, 2012. The fair value of the options was determined using the Black-Scholes option-pricing valuation model with the following assumptions: fair market value of common stock of $1.00 as determined by the Company's Board of Directors, risk-free rate of 0.88%, expected term of 5.0 years, expected volatility of 81.41% and expected dividend yield of 0%. Upon issuance of the fully vested options, the liability was reclassified into additional paid-in capital.
The following table summarizes equity-based compensation expense for the years ended December 31, 2011 and 2012, which were included in the statements of operations and comprehensive loss as follows (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Cost of revenue |
$ | 2 | $ | 2 | |||
Research and development |
80 | 100 | |||||
Selling and marketing |
41 | 39 | |||||
General and administrative |
70 | 118 | |||||
Total equity-based compensation expense |
$ | 193 | $ | 259 | |||
12. Income Taxes
The Company operates in only one jurisdiction, United States. The Company did not record a provision or benefit for income taxes during the years ended December 31, 2011 and 2012. The following table presents a reconciliation of the tax expense computed at the statutory federal rate and the Company's tax expense for the period presented (in thousands):
| |
Year Ended December, 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
U.S. federal taxes at statutory rate |
$ | (4,911 | ) | $ | (6,341 | ) | |
State taxes (net of federal benefit) |
(843 | ) | (1,074 | ) | |||
Permanent differences |
(108 | ) | 261 | ||||
Tax credits |
(181 | ) | (113 | ) | |||
Change in valuation allowance |
6,043 | 7,267 | |||||
Total |
$ | – | $ | – | |||
F-25
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
12. Income Taxes (Continued)
The tax effects of temporary differences and carryforwards that give rise to significant portions of the deferred tax assets are as follows (in thousands):
| |
As of December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Deferred tax assets: |
|||||||
Net operating loss carryforwards |
$ | 16,547 | $ | 20,536 | |||
Research and development credit |
723 | 954 | |||||
Stock-based compensation |
50 | 154 | |||||
Genzyme co-promotion agreement |
3,049 | ||||||
Accruals, depreciation and deferred rent |
197 | 157 | |||||
Gross deferred tax assets |
17,517 | 24,850 | |||||
Valuation allowance |
(17,469 | ) | (24,767 | ) | |||
Net deferred tax assets |
48 | 83 | |||||
Deferred tax liabilities: |
|||||||
Property and equipment |
$ | (48 | ) | $ | (83 | ) | |
Gross deferred tax liabilities |
(48 | ) | (83 | ) | |||
Net deferred tax liabilities |
$ | – | $ | – | |||
The Company has established a full valuation allowance against its deferred tax assets due to the uncertainty surrounding realization of such assets. The valuation allowance increased $6.0 million and $7.3 million during the years ended December 31, 2011 and 2012, respectively.
The guidance for accounting for income taxes prescribes certain realization requirements for stock compensation. The table above does not include certain deferred tax assets at December 31, 2011 and 2012 that could arise directly from tax deductions for equity compensation expense incurred in the periods to the extent it exceeds equity compensation expense recognized for financial reporting purposes in those periods. If and when such benefits are ultimately realized, additional paid in capital would be increased and taxes payable would be reduced.
As of December 31, 2012, the Company had net operating loss carryforwards of approximately $52.0 million and $49.6 million available to reduce future taxable income, if any, for Federal and state income tax purposes, respectively. The U.S. federal net operating loss carryforwards will begin to expire in 2026 while for state purposes, the net operating losses will begin to expire in 2018.
As of December 31, 2012, the Company had credit carryforwards of approximately $0.9 million and $0.7 million available to reduce future taxable income, if any, for Federal and California state income tax purposes, respectively. The Federal credit carryforwards begin to expire in 2028. California credits have no expiration date.
The Internal Revenue Code of 1986, as amended, imposes restrictions on the utilization of net operating losses and tax credits in the event of an "ownership change" of a corporation. Accordingly, a company's ability to use net operating losses and tax credits may be limited as prescribed under Internal Revenue Code Section 382 and 383 ("IRC Section 382"). Events which may cause limitations in the amount of the net operating losses or tax credits that the Company may use in any one year include, but
F-26
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
12. Income Taxes (Continued)
are not limited to, a cumulative ownership change of more than 50% over a three-year period. Utilization of the federal and state net operating losses may be subject to substantial annual limitation due to the ownership change limitations provided by the IRC Section 382 rules and similar state provisions. The Company has not had any ownership changes from inception to March 31, 2013. In the event the Company has subsequent changes in ownership, net operating losses and research and development credit carryovers could be limited and may expire unutilized.
As of December 31, 2012, the Company had unrecognized tax benefits of $0.5 million, all of which would not currently affect the Company's effective tax rate if recognized due to the Company's deferred tax assets being fully offset by a valuation allowance. The Company does not anticipate that the amount of unrecognized tax benefits relating to tax positions existing at December 31, 2012 will significantly increase or decrease within the next twelve months.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2012 | |||||
Unrecognized tax benefits, beginning of period |
$ | 263 | $ | 341 | |||
Gross increases–tax position in prior period |
– | 67 | |||||
Gross decrease–tax position in prior period |
– | – | |||||
Gross increases–current period tax positions |
78 | 73 | |||||
Lapse of statute of limitations |
– | – | |||||
Unrecognized tax benefits, end of period |
$ | 341 | $ | 481 | |||
It is the Company's policy to include penalties and interest expense related to income taxes as a component of other expense and interest expense, respectively, as necessary. There was no interest expense or penalties related to unrecognized tax benefits recorded through December 31, 2012.
The Company's major tax jurisdictions are the United States and California. All of the Company's tax years will remain open for examination by the Federal and state tax authorities for three and four years, respectively, from the date of utilization of the net operating loss or research and development credit. The Company does not have any tax audits pending.
13. 401(k) Plan
The Company sponsors a 401(k) defined contribution plan covering all employees. There were no employer contributions to the plan in the years ended December 31, 2011 and 2012.
14. Subsequent Events
In February 2013, the Company granted its Chief Executive Officer an incentive stock option to purchase 50,000 shares of its common stock with an exercise price of $1.00 per share and a contractual term of 10 years. This option will only vest if an initial public offering or merger occurs in 2013.
In June 2013, the Company amended its Amended and Restated Certificate of Incorporation to increase the number of authorized shares of Series C convertible preferred stock from 14,000,000 to
F-27
VERACYTE, INC.
Notes to Audited Financial Statements (Continued)
14. Subsequent Events (Continued)
14,852,001 and amended the Series C Agreement to increase the number of shares that may be sold in additional closings from 26,455 to a total of 1,640,212. The Company completed the Second Closing and two additional closings under the Series C Agreement and received gross proceeds of $10.0 million from existing investors and $3.0 million from a new investor for the issuance of 6,904,761 shares.
In June 2013, the Company entered into a loan and security agreement with a financial institution to fund its working capital and other general corporate needs. The agreement provided for term loans of up to $10.0 million in aggregate. The Company drew down $5.0 million in funds under the agreement in June 2013. The Company is required to pay interest only on the $5.0 million loan for the first 18 months and then will begin paying principal and interest over a 30 month period. The loan bears interest at a rate of 6.06% per annum. In addition, the Company issued the financial institution a warrant to purchase 99,206 shares of Series C convertible preferred stock at $1.89 per share. The warrant expires on the earlier of (i) June 26, 2023 or (ii) the seventh anniversary of the Company's initial public offering.
The Company may request a second term loan of up to $5.0 million on or prior to March 31, 2014. The Company's obligations under the loan and security agreement are secured by a security interest on substantially all of its assets, excluding it's intellectual property and certain other assets. The loan and security agreement contains customary conditions to borrowing, events of default, and covenants, including covenants limiting the Company's ability to dispose of assets, undergo a change in control, merge with or acquire other entities, incur debt, incur liens, pay dividends or other distributions to holders of our capital stock, repurchase stock and make investments, in each case subject to certain exceptions. The loan and security agreement does not require that the Company comply with any financial covenants.
The Company has evaluated subsequent events through August 12, 2013, the date the audited financial statements were issued.
F-28
VERACYTE, INC.
Index to Unaudited Interim Condensed Financial Statements
Six Months Ended June 30, 2012 and 2013
F-29
VERACYTE, Inc.
Condensed Balance Sheets
(In thousands, except share and per share amounts)
| |
December 31, 2012 |
June 30, 2013 |
Pro Forma Stockholders' Equity as of June 30, 2013 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
|
(Unaudited) |
(Unaudited) |
|||||||
Assets |
||||||||||
Current assets: |
||||||||||
Cash and cash equivalents |
$ | 14,002 | $ | 20,683 | ||||||
Accounts receivable, net of allowance of $222 and $318 as of December 31, 2012 and June 30, 2013 |
569 | 991 | ||||||||
Supplies inventory |
1,050 | 770 | ||||||||
Prepaid expenses and other current assets |
710 | 1,398 | ||||||||
Restricted cash |
50 | – | ||||||||
Total current assets |
16,381 | 23,842 | ||||||||
Property and equipment, net |
2,446 | 3,025 | ||||||||
Restricted cash |
118 | 118 | ||||||||
Other assets |
122 | 174 | ||||||||
Total assets |
$ | 19,067 | $ | 27,159 | ||||||
Liabilities, Convertible Preferred Stock, and Stockholders' (Deficit) Equity |
||||||||||
Current liabilities: |
||||||||||
Accounts payable |
$ | 1,888 | $ | 1,906 | ||||||
Accrued liabilities |
4,020 | 5,387 | ||||||||
Deferred Genzyme co-promotion fee |
2,500 | 2,500 | ||||||||
Preferred stock liability |
583 | – | ||||||||
Total current liabilities |
8,991 | 9,793 | ||||||||
Long-term debt, net of discount |
– | 4,826 | ||||||||
Deferred rent, net of current portion |
61 | 264 | ||||||||
Preferred stock warrant liability |
– | 175 | $ | – | ||||||
Deferred Genzyme co-promotion fee, net of current portion |
5,114 | 3,864 | ||||||||
Total liabilities |
14,166 | 18,922 | ||||||||
Commitments and Contingencies |
||||||||||
Convertible preferred stock; $0.001 par value, 59,147,999 and 60,187,700 shares authorized at December 31, 2012 and June 30, 2013 (unaudited), respectively; 53,084,507 and 59,989,268 shares issued and outstanding at December 31, 2012 and June 30, 2013 (unaudited), respectively; no shares authorized, issued and outstanding, pro forma (unaudited); aggregate liquidation value of $65,835 and $78,885 at December 31, 2012 and June 30, 2013 (unaudited), respectively |
63,372 |
79,025 |
$ |
– |
||||||
Stockholders' (deficit) equity: |
||||||||||
Common stock, $0.001 par value; 77,000,000 shares authorized; 2,670,767 and 3,714,902 shares issued and outstanding at December 31, 2012 and June 30, 2013 (unaudited), respectively; shares authorized and 63,704,170 shares issued and outstanding, pro forma (unaudited) |
3 |
4 |
64 |
|||||||
Additional paid-in capital |
1,595 |
2,663 |
81,803 |
|||||||
Accumulated deficit |
(60,069 |
) |
(73,455 |
) |
(73,455 |
) |
||||
Total stockholders' (deficit) equity |
(58,471 |
) |
(70,788 |
) |
$ |
8,412 |
||||
Total liabilities, convertible preferred stock, and stockholders' (deficit) equity |
$ |
19,067 |
$ |
27,159 |
||||||
The accompanying notes are an integral part of these condensed financial statements.
F-30
VERACYTE, INC.
Condensed Statements of Operations and Comprehensive Loss
(Unaudited)
(In thousands, except share and per share
amounts)
| |
Six Months Ended June 30, | ||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Revenue |
$ | 3,947 | $ | 9,452 | |||
Operating expenses: |
|||||||
Cost of revenue |
3,000 | 6,004 | |||||
Research and development |
3,158 | 3,912 | |||||
Selling and marketing |
3,045 | 5,318 | |||||
General and administrative |
3,618 | 5,528 | |||||
Total operating expenses |
12,821 | 20,762 | |||||
Loss from operations |
(8,874 | ) | (11,310 | ) | |||
Interest expense |
– | (5 | ) | ||||
Other income (expense), net |
– | (2,070 | ) | ||||
Net loss and comprehensive loss |
$ | (8,874 | ) | $ | (13,385 | ) | |
Net loss per common share, basic and diluted |
$ | (3.48 | ) | $ | (4.12 | ) | |
Shares used to compute net loss per common share, basic and diluted |
2,553,287 | 3,250,863 | |||||
Pro forma net loss per common share, basic and diluted |
$ | (0.24 | ) | ||||
Shares used to compute pro forma net loss per common share, basic and diluted |
56,781,744 | ||||||
The accompanying notes are an integral part of these condensed financial statements.
F-31
VERACYTE, INC.
Condensed Statements of Cash Flows
(Unaudited)
(In thousands)
| |
Six Months Ended June 30, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Operating activities |
|||||||
Net loss |
$ | (8,874 | ) | $ | (13,385 | ) | |
Adjustments to reconcile net loss to net cash used in operating activities: |
|||||||
Depreciation and amortization |
349 | 428 | |||||
Bad debt expense |
85 | 117 | |||||
Genzyme co-promotion fee amortization |
(1,136 | ) | (1,250 | ) | |||
Stock-based compensation |
290 | 489 | |||||
Equity-based compensation |
126 | – | |||||
Amortization of debt discount and issuance costs |
– | 2 | |||||
Change in value of preferred stock liability |
– | 2,070 | |||||
Changes in operating assets and liabilities: |
|||||||
Accounts receivables |
(437 | ) | (539 | ) | |||
Supplies inventory |
(448 | ) | 280 | ||||
Prepaid expenses and current other assets |
(67 | ) | (646 | ) | |||
Other assets |
(24 | ) | 28 | ||||
Accounts payable |
753 | 35 | |||||
Accrued liabilities and deferred rent |
1,368 | 1,748 | |||||
Deferred Genzyme co-promotion fee |
10,000 | – | |||||
Net cash provided by (used in) operating activities |
1,985 | (10,623 | ) | ||||
Investing activities |
|||||||
Purchases of property and equipment |
(642 | ) | (941 | ) | |||
Change in restricted cash |
– | 50 | |||||
Net cash used in investing activities |
(642 | ) | (891 | ) | |||
Financing activities |
|||||||
Proceeds from the issuance of long-term debt, net of debt issuance costs |
– | 4,877 | |||||
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs |
– | 12,998 | |||||
Proceeds from the exercise of common stock options |
66 | 320 | |||||
Net cash provided by financing activities |
66 |
18,195 |
|||||
Net increase in cash and cash equivalents |
1,409 |
6,681 |
|||||
Cash and cash equivalents at beginning of period |
7,566 |
14,002 |
|||||
Cash and cash equivalents at end of period |
$ |
8,975 |
$ |
20,683 |
|||
The accompanying notes are an integral part of these condensed financial statements.
F-32
VERACYTE, INC.
Notes to Condensed Financial Statements
1. Summary of Significant Accounting Policies
Unaudited Interim Financial Statements
The interim balance sheet as of June 30, 2013, and the statements of operations and comprehensive loss and cash flows for the six months ended June 30, 2012 and 2013 are unaudited. The unaudited interim financial statements have been prepared on the same basis as the annual financial statements and, in the opinion of management, reflect all adjustments, which include only normal recurring adjustments, necessary to present fairly the Company's financial position as of June 30, 2013 and its results of operations and cash flows for the six months ended June 30, 2012 and 2013. The financial data and the other financial information contained in these notes to the financial statements related to the three month periods are also unaudited. The results of operations for the six months ended June 30, 2013 are not necessarily indicative of the results to be expected for the year ending December 31, 2013 or for any other future annual or interim period. These financial statements should be read in conjunction with the Company's audited financial statements included elsewhere in this prospectus.
Unaudited Pro Forma Stockholders' Equity
The pro forma stockholders' equity as of June 30, 2013 presents the Company's stockholders' equity as though all of the Company's convertible preferred stock outstanding had automatically converted into 59,989,268 shares of common stock upon the completion of a qualifying initial public offering ("IPO") of the Company's common stock. In addition, the pro forma stockholders' equity assumes the reclassification of the preferred stock warrant liability to additional paid-in capital upon a qualifying initial public offering of the Company's common stock, as the warrants upon an initial public offering become common stock warrants that are not subject to remeasurement. The shares of common stock issuable and the proceeds expected to be received in the IPO are excluded from such pro forma financial information.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities as of the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Significant items subject to such estimates include: revenue recognition; allowance for doubtful accounts; the useful lives of property and equipment; the recoverability of long-lived assets; the determination of fair value of the Company's common stock, stock options, preferred stock liability; income tax uncertainties, including a valuation allowance for deferred tax assets; and contingencies. The Company bases these estimates on historical and anticipated results, trends, and various other assumptions that the Company believes are reasonable under the circumstances, including assumptions as to future events. These estimates form the basis for making judgments about the carrying values of assets and liabilities and recorded revenue and expenses that are not readily apparent from other sources. Actual results could differ from those estimates and assumptions.
Concentrations of Credit Risk and Other Risks and Uncertainties
The Company's cash and cash equivalents are deposited with one major financial institution in the United States of America. Deposits in this institution may exceed the amount of insurance provided on such deposits. The Company has not experienced any losses on its deposits of cash and cash equivalents.
Several of the components of the Company's sample collection kit and test reagents are obtained from single source suppliers. If these single source suppliers fail to satisfy the Company's requirements on a
F-33
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
1. Summary of Significant Accounting Policies (Continued)
timely basis, it could suffer delays in being able to deliver Afirma, a possible loss of revenue, or incur higher costs, any of which could adversely affect its operating results.
The Company is also subject to credit risk from its accounts receivable related to its sales of Afirma. The Company generally does not perform evaluations of customers' financial condition and generally does not require collateral. All of the Company's accounts receivables are derived from sales of Afirma in the United States.
As of December 31, 2012 and June 30, 2013, all of the Company's revenue is derived from the sale of Afirma. To date, Afirma has been available only to physicians in the United States. The Company's significant third-party payers and percentage of revenue as a percentage of revenue were as follows:
| |
Six Months Ended June 30, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Medicare |
40 | % | 35 | % | |||
Aetna |
16 | % | 7 | % | |||
United Healthcare |
11 | % | 14 | % | |||
|
67 | % | 56 | % | |||
Accounts receivable from Medicare amounted to 87% and 86% of gross receivables as of December 31, 2012 and June 30, 2013, respectively. No other third-party payer represented more than 10% of the Company's service revenues or accounts receivable balances for these periods.
Cash and Cash Equivalents
Cash and cash equivalents consist of all highly liquid investments with original maturities of three months or less at the date of purchase. Cash equivalents consist primarily of amounts invested in money market accounts.
Restricted Cash
At December 31, 2012 and June 30, 2013, deposits of $168,000 and $118,000 were restricted from withdrawal and held by a bank in the form of certificates of deposit and collateral for letters of credit. The balance at December 31, 2012 and June 30, 2013 consists of a certificate of deposit of $50,000 and $0, respectively, held as collateral for payment of the Company's credit cards and a letter of credit totaling $118,000 and $118,000, respectively, which is related to security for the lease of office space.
Allowance for Doubtful Accounts
The Company accrues an allowance for doubtful accounts against its accounts receivable based on estimates consistent with historical payment experience. Bad debt expense is included in general and administrative expense on the Company's statements of operations and comprehensive loss. Accounts receivable are written off against the allowance when the appeals claims process is exhausted or when there is other substantive evidence that the account will not be paid. The Company's allowance for doubtful accounts as of December 31, 2012 and June 30, 2013 was $222,000 and $318,000, respectively. The provision for bad debt expense was $85,000 and $117,000 for the six months ended June 30, 2012 and 2013,
F-34
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
1. Summary of Significant Accounting Policies (Continued)
respectively. There were no write-offs and $21,000 in write-offs for doubtful accounts against the allowance during the six months ended June 30, 2012 and 2013, respectively.
Supplies Inventory
Supplies inventory consists of test reagents and other consumables used in the sample collection kits and in the GEC and are valued at the lower of cost or market value. Cost is determined using actual costs on a first-in, first-out basis.
Internal-use Software
Capitalized software costs consist of third-party costs incurred in the application development stage to design and implement the software that is used in the GEC. Costs incurred in the development of application of the software are capitalized and amortized over an estimated useful life of three years on a straight line basis. During the six months ended June 30, 2012 and 2013, the Company capitalized $0 and $166,000 of software development costs, respectively. Capitalized software is classified as part of property and equipment, and had a net book value of $184,000 and $311,000 as of December 31, 2012 and June 30, 2013, respectively.
Bonus Accruals
The Company accrues for liabilities under discretionary employee and executive bonus plans. These estimated compensation liabilities are based on progress against corporate objectives approved by the Board of Directors, compensation levels of eligible individuals, and target bonus percentage levels. The Board of Directors and the Compensation Committee of the Board of Directors review and evaluate the performance against these objectives and ultimately determine what discretionary payments are made. At December 31, 2012 and June 30, 2013, the Company accrued $671,000 and $410,000, respectively, for liabilities associated with these employee and executive bonus plans.
Revenue Recognition
The Company's revenue is generated from the provision of diagnostic services using its Afirma solution; the Company's service is completed upon the delivery of test results to the prescribing physician which triggers the billing for the service. The Company recognizes revenue related to billings for commercial carriers or governmental programs subject to contractual arrangements and when there is a predictable pattern of collectability on an accrual basis, net of contractual adjustments. These contractual adjustments represent the difference between the list price (the billing rate) and the reimbursement rate set by commercial or governmental payers. Until a contract has been negotiated with a commercial carrier or governmental program, the Afirma solution may or may not be covered by these entities' existing reimbursement policies. In addition, patients do not enter into direct agreements with us that commit them to pay any portion of the cost of the tests in the event that their insurance declines to reimburse the Company. In the absence of an agreement or other clearly enforceable legal right to demand payment, when test services are provided to patients with non-contracted insurance carriers or no insurance the related revenue is only recognized upon the earlier of payment notification, if applicable, or cash receipt.
For all services performed, the Company considers whether or not the following revenue recognition criteria are met: persuasive evidence of an arrangement exists; delivery has occurred or services have been rendered; the fee is fixed or determinable; and collectability is reasonably assured.
F-35
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
1. Summary of Significant Accounting Policies (Continued)
Persuasive evidence of an arrangement exists and delivery is deemed to have occurred upon delivery of a patient report to the prescribing physician. The assessment of the fixed or determinable nature of the fees charged for testing performed and the collectability of those fees require significant judgment by management. Management believes that these two criteria have been met when there is contracted reimbursement coverage and/or a predictable pattern of collectability with individual third-party payers and accordingly, recognizes revenue upon delivery of the patient report. Some patients have out-of-pocket costs for amounts not covered by their insurance carrier, and the Company may bill the patient directly for these amounts in the form of co-payments and co-insurance in accordance with their insurance carrier and health plans. Some payers may not cover the GEC as ordered by the prescribing physician under their reimbursement policies. The Company pursues reimbursement from such patients on a case-by-case basis. In the absence of contracted reimbursement coverage or a predictable pattern and history of collectability, the Company believes that the fee is fixed or determinable and collectability is reasonably assured only upon receipt of third-party payer notification of payment or when cash is received and accordingly, recognizes revenue at that time.
Net Loss per Common Share
Basic net loss per common share is calculated by dividing net loss for the period by the weighted-average number of common shares outstanding during the period, without consideration of common stock equivalents. Diluted net loss per common share is computed by dividing the loss for the period by the weighted-average number of common share equivalents outstanding for the period determined using the treasury stock method. Potentially dilutive securities consisting of convertible preferred stock and options to purchase common stock are considered to be common stock equivalents and were excluded from the calculation of diluted net loss per common share because their effect would be antidilutive for all periods presented.
Unaudited Pro Forma Net Loss per Common Share
Pro forma basic and diluted net loss per common share has been computed to give effect to the conversion of all of the outstanding shares of convertible preferred stock into common stock.
2. Accrued Liabilities
Accrued liabilities consist of the following (in thousands):
| |
December 31, 2012 | June 30, 2013 | |||||
|---|---|---|---|---|---|---|---|
Accrued compensation expenses |
$ | 1,360 | $ | 1,121 | |||
Accrued consulting fees |
28 | – | |||||
Accrued legal and professional fees |
84 | 215 | |||||
Accrued Genzyme co-promotion fees |
2,175 | 3,668 | |||||
Accrued other |
373 | 383 | |||||
Accrued liabilities |
$ | 4,020 | $ | 5,387 | |||
F-36
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
3. Fair Value Measurements
The Company records its financial assets and liabilities at fair value. The carrying amounts of certain financial instruments of the Company, including cash and cash equivalents, prepaid expenses and other current assets, accounts payable and accrued liabilities, approximate fair value due to their relatively short maturities. The carrying value of long-term debt approximates its fair value because the interest rate approximates market rates that the Company could obtain for debt with similar terms. The accounting guidance for fair value provides a framework for measuring fair value, clarifies the definition of fair value, and expands disclosures regarding fair value measurements. Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date. The accounting guidance establishes a three-tiered hierarchy, which prioritizes the inputs used in the valuation methodologies in measuring fair value as follows:
- •
- Level I: Inputs which include quoted prices in active markets for identical assets and liabilities.
- •
- Level II: Inputs other than Level I that are observable, either directly or indirectly, such as quoted
prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full
term of the assets or liabilities.
- •
- Level III: Unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The Company's financial assets and liabilities subject to fair value measurements on a recurring basis and the level of inputs used in such measurements were as follows (in thousands):
| |
December 31, 2012 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level I | Level II | Level III | Total | |||||||||
Financial Assets: |
|||||||||||||
Money market funds |
$ | 12,830 | $ | – | $ | – | $ | 12,830 | |||||
Total financial assets |
$ | 12,830 | $ | – | $ | – | $ | 12,830 | |||||
Financial Liabilities: |
|||||||||||||
Preferred stock liability |
$ | – | $ | – | $ | 583 | $ | 583 | |||||
Total financial liabilities |
$ | – | $ | – | $ | 583 | $ | 583 | |||||
| |
June 30, 2013 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Level I | Level II | Level III | Total | |||||||||
Financial Assets: |
|||||||||||||
Money market funds |
$ | 11,896 | $ | – | $ | – | $ | 11,896 | |||||
Total financial assets |
$ | 11,896 | $ | – | $ | – | $ | 11,896 | |||||
Financial Liabilities: |
|||||||||||||
Preferred stock warrant liability |
$ | – | $ | – | $ | 175 | $ | 175 | |||||
Total financial liabilities |
$ | – | $ | – | $ | 175 | $ | 175 | |||||
F-37
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
3. Fair Value Measurements (Continued)
The Company's Level III liabilities consist of a preferred stock liability and a preferred stock warrant liability (see Note 5). The following table sets forth a summary of the changes in the fair value of the Company's Level III financial liabilities, which are measured on a recurring basis (in thousands):
Balance as of December 31, 2012 |
$ | 583 | ||
Change in fair value of preferred stock liability recorded in other income (expense), net |
2,070 | |||
Settlement of preferred stock liability |
(2,653 | ) | ||
Fair value of preferred stock warrant liability |
175 | |||
Balance as of June 30, 2013 |
$ | 175 | ||
In November 2012, the Company recorded a preferred stock liability as investors received the right to purchase from the Company, on the same terms, additional shares of Series C convertible preferred stock, in a second tranche. As the investors hold a majority of the board seats, the decision to complete the second tranche was deemed to be outside the control of the Company. The preferred stock liability was valued using the option-pricing method, which resulted in an initial fair value of $0.9 million for the Company's obligation to sell the convertible preferred stock. In June 2013, the Company settled the preferred stock liability upon completion of the sale of the second tranche of Series C convertible preferred stock. Immediately prior to settlement, the Company revalued the preferred stock liability to $2.7 million and recorded other expense, net of $2.1 million related to the change in value of the liability through that date. The preferred stock liability was valued using the option-pricing method with the following assumptions: 100% probability of success of the second tranche, fair value of Series C preferred stock of $2.39, a term of 0.003 years and expected volatility of 36.4%.
4. Debt
In June 2013, the Company entered into a loan and security agreement with a financial institution to fund its working capital and other general corporate needs. The agreement provided for term loans of up to $10.0 million in aggregate. The Company drew down $5.0 million in funds under the agreement in June 2013. The Company is required to repay the outstanding principal in 30 equal installments beginning 18 months after the date of the borrowing. The loan bears interest at a rate of 6.06% per annum. The loan carries prepayment penalties of 2.25% and 1.5% for prepayment within one and two years, respectively, of the loan origination and 0.75% thereafter.
Upon execution of the loan and security agreement, the Company issued the financial institution a warrant to purchase shares of Series C convertible preferred stock at $1.89 per share (See Note 5). At the time of issuance, the aggregate fair value of the warrant for the 99,206 shares exercisable under the warrant was $175,000. The fair value of the warrant was carved out from total proceeds, resulting in a debt discount to be amortized to interest expense over 48 months, through the maturity date of the initial loan, using the effective interest rate method, and was recorded as a preferred stock warrant liability. The end of term payment of $223,000 representing 4.45% of the total outstanding principal balance will be accreted over the life of the loan as interest expense. As a result of the debt discount and the end of term payment, the effective interest rate for the loan differs from the contractual rate. The Company's interest expense related to the amortization of the debt discount and accretion of the end of term payment was not material for the six months ended June 30, 2013.
F-38
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
4. Debt (Continued)
The Company may request a second term loan of up to $5.0 million on or prior to March 31, 2014. The Company's obligations under the loan and security agreement are secured by a security interest on substantially all of its assets, excluding its intellectual property and certain other assets. The loan and security agreement contains customary conditions related to borrowing, events of default, and covenants, including covenants limiting the Company's ability to dispose of assets, undergo a change in control, merge with or acquire other entities, incur debt, incur liens, pay dividends or other distributions to holders of its capital stock, repurchase stock and make investments, in each case subject to certain exceptions. The agreement also allows the lender to call the debt in the event there is a material adverse change in the Company's business or financial condition. The loan and security agreement does not require that the Company comply with any financial covenants.
5. Convertible Preferred Stock Warrants
In June 2013, in conjunction with the execution of the loan and security agreement (Note 4), the Company issued to the lender a warrant to purchase up to 198,412 shares of Series C convertible preferred stock with an exercise price of $1.89 per share. Upon the draw down of the $5.0 million term loan, the warrant became exercisable for 99,206 shares. If the Company draws the second term loan, the remaining 99,206 shares will become exercisable under the warrant. The warrant expires at the earlier of (i) June 26, 2023 or (ii) the seventh anniversary of the Company's initial public offering. The warrant is exercisable in cash or through a cashless exercise provision. Under the cashless exercise provision, the holder may, in lieu of payment of the exercise price in cash, surrender the warrant and receive a net amount of shares based on the fair market value of the Company's Series C convertible preferred stock at the time of exercise of the warrant after deducting the aggregate exercise price. In the event that all outstanding shares of the Series C convertible preferred stock are converted into common stock, the warrant will be exercisable for the same number of shares of common stock.
The fair value of the currently exercisable portion of the warrant in the amount of $175,000 was recorded as a preferred stock warrant liability upon issuance and is subject to remeasurement at each reporting period. The fair value of the warrant upon issuance was calculated using the Black-Scholes option-pricing valuation model with the following assumptions: Series C preferred stock value of $2.40 per share, contractual term of 7.3 years, risk-free interest rate of 2.1%, expected volatility of 73.7%, and expected dividend yield of 0%. The fair value of the preferred stock warrant liability did not change from issuance to June 30, 2013.
6. Convertible Preferred Stock
In June 2013, the Company amended its Amended and Restated Certificate of Incorporation to increase the number of authorized shares of Series C convertible preferred stock from 14,000,000 to 14,852,001 and amended the Series C stock purchase agreement to increase the number of shares that may be sold in additional closings from 26,455 to a total of 1,640,212. The Company completed the second closing and two additional closings under the agreement, and received gross proceeds of $13.0 million for the issuance of an aggregate of 6,904,761 shares of Series C convertible preferred stock.
F-39
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
7. Stock Incentive Plan
The following table summarizes activity under the Company's 2008 Stock Plan, including grants to non-employees and restricted stock issued (in thousands, except per share amounts):
| |
Shares Available for Grant |
Options Outstanding |
Weighted Average Exercise Price per Share |
Aggregate Intrinsic Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Balances at December 31, 2012 |
1,389,495 | 8,910,706 | $ | 0.52 | $ | 4,311 | |||||||
Additional options authorized |
1,000,000 | – | – | ||||||||||
Options granted |
(2,510,632 | ) | 2,510,632 | 1.16 | |||||||||
Options exercised |
– | (1,044,135 | ) | 0.31 | |||||||||
Options forfeited |
695,958 | (695,958 | ) | 0.66 | |||||||||
Balances at June 30, 2013 |
574,821 | 9,681,245 | $ | 0.70 | $ | 12,431 | |||||||
Vested–June 30, 2013 |
4,704,914 | $ | 0.50 | $ | 6,957 | ||||||||
Expected to vest–June 30, 2013 |
9,124,394 | $ | 0.68 | $ | 11,819 | ||||||||
The aggregate intrinsic value was calculated as the difference between the exercise price of the options to purchase common stock and the estimated fair value of the Company's common stock of $1.98 per share as of June 30, 2013.
Outstanding and exercisable stock options at June 30, 2013 are summarized as follows:
| |
Options Outstanding | Options Vested and Exercisable | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Exercise Price
|
Number | Weighted-Average Remaining Contractual Life (in Years) |
Number | Weighted-Average Remaining Contractual Life (in Years) |
|||||||||
$0.02 |
723,500 | 5.15 | 723,500 | 5.15 | |||||||||
$0.20 |
784,778 | 6.65 | 674,881 | 6.64 | |||||||||
$0.59 |
1,825,125 | 7.31 | 1,327,307 | 7.33 | |||||||||
$0.60 |
895,000 | 8.19 | 509,216 | 8.20 | |||||||||
$0.67 |
2,750,506 | 8.78 | 1,060,133 | 8.73 | |||||||||
$1.00 |
1,904,211 | 9.58 | 409,877 | 9.58 | |||||||||
$1.51 |
798,125 | 9.97 | – | – | |||||||||
$0.02-1.51 |
9,681,245 | 8.26 | 4,704,914 | 7.50 | |||||||||
The weighted average fair value of stock options granted was $0.47 and $0.78 per share in the six months ended June 30, 2012 and 2013, respectively.
The weighted average fair value of stock options vested was $0.35 and $0.52 per share in the six months ended June 30, 2012 and 2013, respectively.
The weighted average fair value of stock options exercised was $0.24 and $0.21 per share in the three months ended June 30, 2012 and 2013, respectively. The intrinsic value of stock options exercised was $109,000 and $1.7 million in the six months ended June 30, 2012 and 2013, respectively.
F-40
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
7. Stock Incentive Plan (Continued)
Stock-based Compensation
Stock-based compensation expense recognized was as follows (in thousands):
| |
Six Months Ended June 30, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Cost of revenue |
$ | 16 | $ | 13 | |||
Research and development |
48 | 103 | |||||
Selling and marketing |
52 | 76 | |||||
General and administrative |
174 | 297 | |||||
Total |
$ | 290 | $ | 489 | |||
As of June 30, 2013, the Company had $2.6 million of unrecognized compensation expense related to unvested stock options, which is expected to be recognized over an estimated weighted-average period of 3.0 years.
The estimated grant date fair value of employee stock options was calculated using the Black-Scholes option-pricing valuation model, based on the following assumptions:
| |
Six Months Ended June 30, |
|||
|---|---|---|---|---|
| |
2012 | 2013 | ||
Weighted-average volatility |
83.06 - 83.69% | 80.42 - 81.41% | ||
Weighted-average expected term (years) |
5.0 - 6.08 | 5.0 - 6.08 | ||
Risk-free interest rate |
0.90 - 1.19% | 0.88 - 1.60% | ||
Expected dividend yield |
0% | 0% | ||
Stock-based compensation related to stock options granted to non-employees is recognized as the stock options are earned. The fair value of the stock options granted is calculated at each reporting date using the Black-Scholes option-pricing model with the following assumptions: expected life is the equal to the remaining contractual term of the award as of the measurement date ranging from 8.73 years to 9.69 years as of June 30, 2012 and 8.22 years to 9.43 years as of June 30, 2013; risk free rate is based on the U.S. Treasury Constant Maturity rate with a term similar to the expected life of the option at the measurement date ranging from 1.43%-1.61% as of June 30, 2012 and 2.19%-2.41% as of June 30, 2013; expected dividend yield of 0%; and volatilities ranging from 82.48% to 82.96% as of June 30, 2012 and 79.01% to 79.58% as of June 30, 2013.
Equity-based Compensation
The Company paid 50% of 2012 executive bonuses through the grant of stock options. The equity transaction associated with these bonuses is classified as equity-based compensation expense. The accrual for the anticipated grants was $259,000 and $0 at December 31, 2012 and June 30, 2013, respectively, and is included in accrued liabilities in the balance sheet.
In February 2013, the Company's Board of Directors authorized the grant of 402,007 fully vested stock options at a fair value of approximately $0.65 resulting in $259,000 in expense in the year ended
F-41
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
7. Stock Incentive Plan (Continued)
December 31, 2012. The fair value of the stock options was determined using the Black-Scholes option-pricing valuation model. The grant date fair market value was $1.00 as determined by the Company's Board of Directors, the risk free rate was 0.88%, the expected life was 5.0 years, the volatility was determined to be 81.41% and there was no dividend yield.
In February 2013, the Company granted its Chief Executive Officer an incentive stock option to purchase 50,000 shares of common stock with an exercise price of $1.00 per share and a contractual term of 10 years. The option will only vest if an initial public offering or merger occurs in 2013. The Company has not recorded any compensation expense related to this option grant as the vesting event is not deemed probable of occurring as of June 30, 2013.
The following table summarizes equity-based compensation expense for the six months ended June 30, 2012 and 2013, which were included in the statements of operations and comprehensive loss as follows:
| |
Six Months Ended June 30, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Cost of revenue |
$ | 1 | $ | – | |||
Research and development |
44 | – | |||||
Selling and marketing |
21 | – | |||||
General and administrative |
60 | – | |||||
Total |
$ | 126 | $ | – | |||
8. Genzyme Co–promotion Agreement
In January 2012, Veracyte and Genzyme Corporation ("Genzyme") executed a co-promotion agreement for the co-exclusive rights and license to promote and market the Company's Afirma thyroid cancer solution in the United States and in 40 named countries. In exchange, the Company received a $10.0 million co-promotion fee from Genzyme. The Company may receive an additional $3.0 million in payments, $600,000 for each country outside of the United States in which the Company obtains marketing authorization and achieves a specified level of reimbursement, for up to five countries. Under the terms of the agreement, Genzyme will receive a percentage of cash receipts that the Company has received related to Afirma as co-promotion fees. The percentage was 50% in 2012 and decreased to 40% in January 2013 and will further decrease to 32% in March 2014 and thereafter. Genzyme will also spend up to $500,000 for qualifying clinical development activities in countries that require additional testing for approval. This obligation expires in July 2014. The agreement expires in January 2027 and either party may terminate the agreement at any time and with six months prior notice. The Company is amortizing the co-promotion fee over a four-year period, which is management's best estimate of the life of the arrangement, in part because after that period either party may terminate the agreement without penalty. The Company amortized $1.1 million and $1.3 million in the six months ended June 30, 2012 and 2013, respectively, which are reflected as a reduction to selling and marketing expenses in the statements of operations and comprehensive loss. The unamortized balance of the co-promotion fee is $6.4 million as of June 30, 2013.
F-42
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
9. Thyroid Cytology Partners
In 2010, the Company entered into an arrangement with Pathology Resource Consultants, P.A. ("PRC") to establish and manage a specialized pathology practice to provide cytopathology testing services to the Company. There is no direct monetary compensation from the Company to PRC as a result of this arrangement. The Company's services agreement with the specialized pathology practice, Thyroid Cytology Partners ("TCP"), is effective through December 31, 2015, unless terminated earlier, and renews annually thereafter. Under the services agreement, the Company pays TCP based on a fixed price per test schedule, which is reviewed periodically for changes in market pricing. Subsequent to December 2012, an amendment to the services agreement allows TCP to use a portion of the Company's facility in Austin, Texas. TCP will reimburse the Company for a proportionate share of the Company's rent and related operating expense costs for the leased facility. The Company does not have an ownership interest in or provide any form of financial or other support to TCP.
The Company has concluded that TCP represents a variable interest entity and that the Company is not the primary beneficiary as it does not have the ability to direct the activities that most significantly impact TCP's economic performance. Therefore, the Company does not consolidate TCP. All amounts paid to TCP under the services agreement are expensed as incurred. All amounts to be received from TCP will be recorded in the same period as the corresponding lease costs.
TCP provided $643,000 and $1.5 million in cytopathology testing and evaluation services in the six months ended June 30, 2012 and 2013, respectively. The Company also reimbursed TCP for licensure fees of $58,000 and $0 in six months ended June 30, 2012 and 2013, respectively. Expenses for testing and evaluation services and reimbursed professional licensure fees are included in cost of revenue in the statements of operations and comprehensive loss. The Company's outstanding obligations to TCP were $458,000 and $536,000 as of December 31, 2012 and June 30, 2013, respectively, which were included in accounts payable in the Company's balance sheets.
10. Net Loss per Common Share and Pro Forma Net Loss Per Common Share
The following table presents the calculation of basic and diluted net loss per common share for the six months ended June 30, 2012 and 2103 (in thousands, except share and per share amounts):
| |
Six Months Ended June 30, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Net loss |
$ | (8,874 | ) | $ | (13,385 | ) | |
Shares used to compute net loss per common share, basic and diluted |
2,553,287 | 3,250,863 | |||||
Net loss per common share, basic and diluted |
$ | (3.48 | ) | $ | (4.12 | ) | |
F-43
VERACYTE, INC.
Notes to Condensed Financial Statements (Continued)
10. Net Loss per Common Share and Pro Forma Net Loss Per Common Share (Continued)
The following outstanding common stock equivalents were excluded from the computation of diluted net loss per common share for the periods presented because including them would have been antidilutive:
| |
Six Months Ended June 30, |
||||||
|---|---|---|---|---|---|---|---|
| |
2012 | 2013 | |||||
Convertible preferred stock |
45,147,999 | 59,989,268 | |||||
Options to purchase common stock |
8,489,352 | 9,681,245 | |||||
Warrants to purchase convertible preferred stock |
– | 99,206 | |||||
|
53,637,351 | 69,769,719 | |||||
The following table sets forth the computation of the Company's pro forma basic and diluted net loss per common share during the six months ended June 30, 2013 (in thousands, except share and per share amounts):
| |
Six Months Ended June 30, 2013 |
|||
|---|---|---|---|---|
Pro forma net loss: |
||||
Net loss used in computing pro forma net loss per common share, basic and diluted |
$ | (13,385 | ) | |
Shares used in computing net loss per common share, basic and diluted |
3,250,863 | |||
Pro forma adjustments to reflect assumed conversion of convertible preferred stock |
53,530,881 | |||
Shares used in computing pro forma net loss per common share, basic and diluted |
56,781,744 | |||
Pro forma net loss per common share, basic and diluted |
$ | (0.24 | ) | |
11. Subsequent Events
The Company has evaluated subsequent events through August 30, 2013, the date the unaudited interim financial statements for the six months ended June 30, 2013 were issued.
F-44


Part II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the various expenses expected to be incurred by the Registrant in connection with the sale and distribution of the securities being registered hereby, other than underwriting discounts and commissions. All amounts are estimated except the Securities and Exchange Commission registration fee and the Financial Industry Regulatory Authority, Inc. filing fee.
Securities and Exchange Commission registration fee |
* | |
Financial Industry Regulatory Authority, Inc. filing fee |
* | |
NASDAQ Stock Market filing fee |
* | |
Blue Sky fees and expenses |
* | |
Accounting fees and expenses |
* | |
Legal fees and expenses |
* | |
Printing and engraving expenses |
* | |
Registrar and transfer agent fees |
* | |
Miscellaneous fees and expenses |
* | |
Total |
$ * | |
- *
- To be filed by amendment
Item 14. Indemnification of Directors and Officers
Section 145 of the General Corporation Law of the State of Delaware (the "DGCL") provides for the indemnification of officers, directors, and other corporate agents in terms sufficiently broad to indemnify such persons under certain circumstances for liabilities (including reimbursement for expenses incurred) arising under the Securities Act of 1933. Article of the Registrant's Restated Certificate of Incorporation (Exhibit 3.1(b) hereto), and Article of the Registrant's Amended and Restated Bylaws (Exhibit 3.2(b) hereto), provide for indemnification of the Registrant's directors, officers, employees and other agents to the extent and under the circumstances permitted by the DGCL. The Registrant has also entered into agreements with its directors and officers that will require the Registrant, among other things, to indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers to the fullest extent not prohibited by law.
The Underwriting Agreement (Exhibit 1.1 hereto) provides for indemnification by the Underwriters of us and our directors and officers for certain liabilities, including liabilities arising under the Securities Act of 1933 (the "Securities Act"), and affords certain rights of contribution with respect thereto.
Item 15. Recent Sales of Unregistered Securities
The following sets forth information regarding all unregistered securities sold since January 1, 2010 through August 15, 2013:
From June 4, 2010 to July 26, 2011, the Registrant issued and sold an aggregate of 22,748,000 shares of its Series B convertible preferred stock at $1.25 per share to 10 accredited investors for aggregate consideration of $28,435,000.(1)
From November 6, 2012 to June 27, 2013, the Registrant issued and sold an aggregate of 14,841,269 shares of its Series C convertible preferred stock at $1.89 per share to 11 accredited investors for aggregate consideration of $28,049,998.(1)
II-1
The Registrant has granted to its directors, officers and employees options to purchase 10,748,984 shares of common stock under the Registrant's 2008 Stock Plan, as amended, with per share exercise prices ranging from $0.20 to $1.51, and issued 1,553,224 shares of common stock upon exercise of such options for aggregate consideration of $434,351, at exercise prices ranging from $0.02 to $1.00.(2)
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. The Registrant believes that each transaction was exempt from the registration requirements of the Securities Act in reliance on the following exemptions:
- (1)
- These
transactions were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(2) of the Securities Act or
Regulation D promulgated thereunder as transactions by an issuer not involving any public offering. The recipients of the securities in each of these transactions represented their intentions
to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in
these transactions. All recipients had adequate information about the Registrant or had adequate access, through their relationships with the Registrant, to information about the Registrant.
- (2)
- These transactions were deemed to be exempt from registration under the Securities Act in reliance upon Rule 701 promulgated under Section 3(b) of the Securities Act pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had adequate information about the Registrant or had adequate access, through their relationships with the Registrant, to information about the Registrant.
Item 16. Exhibits and Financial Statement Schedules
- (a)
- Exhibits
| Exhibit Number | Description | ||
|---|---|---|---|
| 1.1 | * | Form of Underwriting Agreement. | |
| 3.1 | (a)+ | Fourth Amended and Restated Certificate of Incorporation of the Registrant, as amended. | |
| 3.1 | (b)* | Form of Restated Certificate of Incorporation of the Registrant, to be in effect upon the completion of this offering. | |
| 3.2 | (a)+ | Bylaws of the Registrant. | |
| 3.2 | (b)* | Form of Amended and Restated Bylaws of the Registrant, to be in effect upon the completion of this offering. | |
| 4.1 | * | Form of Common Stock Certificate. | |
| 4.2 | + | Second Amended and Restated Investors Rights Agreement, dated November 6, 2012, between the Registrant and certain investors. | |
| 4.3 | + | Amendment to Second Amended and Restated Investors Rights Agreement, dated June 14, 2013, between the Registrant and certain investors. | |
| 4.4 | + | Warrant to Purchase Series C Preferred Stock dated June 26, 2013. | |
| 5.1 | * | Opinion of Pillsbury Winthrop Shaw Pittman LLP. | |
| 10.1 | * | Form of Indemnification Agreement between the Registrant and its officers and directors. | |
| 10.2 | #+ | 2008 Stock Plan and forms of agreements thereunder. | |
| 10.3 | #* | 2013 Stock Incentive Plan and forms of agreements thereunder. | |
| 10.4 | + | Lease Agreement dated as of February 10, 2010 between ARE-San Francisco No 17, LLC and the Registrant. | |
II-2
| Exhibit Number | Description | ||
|---|---|---|---|
| 10.5 | + | First Amendment to Lease Agreement entered into as of July 11, 2012 between ARE-San Francisco No 17, LLC and the Registrant. | |
| 10.6 | + | Lease Agreement between Riata Holdings, L.P., as landlord, and the Registrant, as tenant, dated November 28, 2012. | |
| 10.7 | † | Co-Promotion Agreement dated as of January 18, 2012 between Genzyme Corporation and the Registrant. | |
| 10.8 | Amendment to Co-Promotion Agreement, effective April 9, 2013, between Genzyme Corporation and the Registrant. | ||
| 10.9 | + | Loan and Security Agreement dated as of June 26, 2013 between Silicon Valley Bank and the Registrant. | |
| 10.10 | #+ | Employment Agreement, dated as of February 15, 2008, between Bonnie Anderson and the Registrant. | |
| 10.11 | #+ | Amendment to Bonnie Anderson Employment Agreement, dated as of December 22, 2008, between Bonnie Anderson and the Registrant | |
| 10.12 | #+ | Amendment No. 2 to Bonnie Anderson Employment Agreement, effective as of March 11, 2009, between Bonnie Anderson and the Registrant. | |
| 10.13 | #+ | Change of Control and Severance Agreement, effective as of August 24, 2012, between Bonnie Anderson and the Registrant. | |
| 10.14 | #+ | Change of Control and Severance Agreement, effective as of August 24, 2012, between Christopher Hall and the Registrant. | |
| 10.15 | #+ | Change of Control and Severance Agreement, effective as of April 8, 2013, between Shelly Guyer and the Registrant. | |
| 10.16 | #+ | Executive Bonus Plan | |
| 23.1 | * | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | |
| 23.2 | * | Consent of Pillsbury Winthrop Shaw Pittman LLP (included in Exhibit 5.1) | |
| 24.1 | Power of Attorney (see page II-5 of this Registration Statement) | ||
- *
- To
be filed by amendment.
- †
- Confidential
treatment requested.
- #
- Management
contract or compensatory arrangement.
- +
- Previously filed.
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
II-3
The undersigned registrant hereby undertakes that:
- (1)
- For
purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration
statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be
deemed to be part of this registration statement as of the time it was declared effective.
- (2)
- For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-4
Pursuant to the requirements of the Securities Act of 1933, the Registrant has duly caused this Registration Statement to be signed on its behalf by the undersigned, thereunto duly authorized in the City of South San Francisco, State of California, on the day of August, 2013.
| VERACYTE, INC. | ||||
By |
Bonnie H. Anderson President and Chief Executive Officer |
|||
KNOW ALL PERSONS BY THESE PRESENTS that each person whose signatures appears below constitutes and appoints Bonnie H. Anderson and Shelly D. Guyer, and each of them, his or her true and lawful attorneys-in-fact and agents, each with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments, including post-effective amendments, to this Registration Statement, and any registration statement relating to the offering covered by this Registration Statement and filed pursuant to Rule 462(b) under the Securities Act of 1933, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that each of said attorney-in-fact and agents or their substitute or substitutes may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities and on the dates indicated.
Name
|
Title
|
Date
|
|||
|---|---|---|---|---|---|
| Bonnie H. Anderson |
President, Chief Executive Officer (Principal Executive Officer) and Director | August , 2013 | |||
| Shelly D. Guyer |
Chief Financial Officer (Principal Financial and Accounting Officer) | August , 2013 | |||
| Brian G. Atwood |
Chairman of Board of Directors | August , 2013 | |||
| Brook H. Byers |
Director | August , 2013 | |||
| Fred E. Cohen, M.D., D.Phil. |
Director | August , 2013 | |||
| Samuel D. Colella |
Director | August , 2013 | |||
| Karin Eastham |
Director | August , 2013 | |||
| Evan Jones |
Director | August , 2013 | |||
| Jesse I. Treu, Ph.D. |
Director | August , 2013 |
II-5
| Exhibit Number | Description | |
|---|---|---|
1.1* |
Form of Underwriting Agreement. | |
3.1(a)+ |
Fourth Amended and Restated Certificate of Incorporation of the Registrant, as amended. | |
3.1(b)* |
Form of Restated Certificate of Incorporation of the Registrant, to be in effect upon the completion of this offering. | |
3.2(a)+ |
Bylaws of the Registrant. | |
3.2(b)* |
Form of Amended and Restated Bylaws of the Registrant, to be in effect upon the completion of this offering. | |
4.1* |
Form of Common Stock Certificate. | |
4.2+ |
Second Amended and Restated Investors Rights Agreement, dated November 6, 2012, between the Registrant and certain investors. | |
4.3+ |
Amendment to Second Amended and Restated Investors Rights Agreement, dated June 14, 2013, between the Registrant and certain investors. | |
4.4+ |
Warrant to Purchase Series C Preferred Stock dated June 26, 2013. | |
5.1* |
Opinion of Pillsbury Winthrop Shaw Pittman LLP. | |
| 10.1* | Form of Indemnification Agreement between the Registrant and its officers and directors. | |
| 10.2#+ | 2008 Stock Plan and forms of agreements thereunder. | |
| 10.3#* | 2013 Stock Incentive Plan and forms of agreements thereunder. | |
| 10.4+ | Lease Agreement dated as of February 10, 2010 between ARE-San Francisco No 17, LLC and the Registrant. | |
| 10.5+ | First Amendment to Lease Agreement entered into as of July 11, 2012 between ARE-San Francisco No 17, LLC and the Registrant. | |
| 10.6+ | Lease Agreement between Riata Holdings, L.P., as landlord, and the Registrant, as tenant, dated November 28, 2012. | |
| 10.7† | Co-Promotion Agreement dated as of January 18, 2012 between Genzyme Corporation and the Registrant. | |
| 10.8 | Amendment to Co-Promotion Agreement, effective April 9, 2013, between Genzyme Corporation and the Registrant. | |
| 10.9+ | Loan and Security Agreement dated as of June 26, 2013 between Silicon Valley Bank and the Registrant. | |
| 10.10#+ | Employment Agreement, dated as of February 15, 2008, between Bonnie Anderson and the Registrant. | |
| 10.11#+ | Amendment to Bonnie Anderson Employment Agreement, dated as of December 22, 2008, between Bonnie Anderson and the Registrant | |
| 10.12#+ | Amendment No. 2 to Bonnie Anderson Employment Agreement, effective as of March 11, 2009, between Bonnie Anderson and the Registrant. | |
| 10.13#+ | Change of Control and Severance Agreement, effective as of August 24, 2012, between Bonnie Anderson and the Registrant. | |
| 10.14#+ | Change of Control and Severance Agreement, effective as of August 24, 2012, between Christopher Hall and the Registrant. |
| Exhibit Number | Description | |
|---|---|---|
| 10.15#+ | Change of Control and Severance Agreement, effective as of April 8, 2013, between Shelly Guyer and the Registrant. | |
| 10.16#+ | Executive Bonus Plan | |
| 23.1* | Consent of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm. | |
| 23.2* | Consent of Pillsbury Winthrop Shaw Pittman LLP (included in Exhibit 5.1) | |
| 24.1 | Power of Attorney (see page II-5 of this Registration Statement) |
- *
- To
be filed by amendment.
- †
- Confidential
treatment requested.
- #
- Management
contract or compensatory arrangement.
- +
- Previously filed.